Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/21. The contractual start date was in May 2013. The draft report began editorial review in November 2017 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lynn Rochester reports grants from Newcastle University during the conduct of the study and grants from Parkinson’s UK, the European Union Marie Curie Training Network, the Medical Research Council, the Engineering and Physical Sciences Research Council, the Wellcome Trust and the Stroke Association, outside the submitted work. Claire Ballinger is a member of the Primary Care Community and Preventive Interventions Health Technology Assessment (HTA) group and the associated Methods group. Victoria A Goodwin reports grants from the National Institute for Health Research (NIHR) during the conduct of the study (as a co-applicant), that is the NIHR HTA-funded trial (15/43/07) home-based exercise intervention for older people with frailty as extended rehabilitation following acute illness or injury, (ISRCTN13927531). Sarah E Lamb reports grants from the NIHR HTA programme during the conduct of this study. Furthermore, she was a member of the HTA Additional Capacity Funding Board, HTA End of Life Care and Add-on Studies, HTA Prioritisation Group and HTA Trauma Board during this study.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Ashburn et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Introduction
Parkinson’s disease
Parkinson’s disease (hereafter referred to as Parkinson’s) is a progressive neurological condition characterised by impairments of movement and postural control (balance) and non-motor deficits. 1 There are estimated to be around 127,000 people with Parkinson’s in the UK. 2 The prevalence of Parkinson’s increases steadily with age. 2
Falls in Parkinson’s
Falls among people with Parkinson’s are both common and disabling. 3,4 Approximately 40–70% of people with Parkinson’s fall each year, and one-third fall repeatedly. 5 Overall, people with Parkinson’s are twice as likely to experience falls as a healthy elderly population. 6 There is evidence to show that falls have a major impact on the lives of people with Parkinson’s, including debilitating effects on confidence,7 decreased activities levels8,9 and reduced quality of life. 10 Such falls are financially costly for individuals and health-care systems. Repeat falls are a risk factor for further falls and carry devastating consequences, such as fractures, immobility and fear of falling leading to dependency and social isolation. 4 Although the incidence of falling increases with disease severity, falls are common even in the early stages of the condition. 11
Falling among those with Parkinson’s is associated with a host of risk factors, including disease severity, duration of disease,12,13 self-reported disability12 and impaired mobility,11 as confirmed by Canning et al. 6 The strongest predictor of falling, identified from meta-analysis, is having had a previous fall. 4,6 Loss of motor control [e.g. anticipatory and reactive postural control, reduced leg muscle strength, proprioception and gait speed, increased gait variability and freezing of gait (FoG)] have also been shown to be associated with, and predictors of, falls. 14,15 In addition to the motor symptoms, impaired cognition and orientation,14 as well as misjudgement and distraction,16 have also shown significant association with falling.
Falls prevention
Drugs are the main treatment used to manage the symptoms of Parkinson’s, while research into finding a cure continues. However, reduced postural control and falls do not respond to medication. 3 It has long been accepted that exercise is a fundamental component of treatment for people with Parkinson’s, alongside medical and surgical management, and its positive effects on symptoms are well supported in the literature. There is substantial evidence showing that regular exercise can be particularly beneficial for people with Parkinson’s to maintain postural control, mobility, daily living activities and general symptom management. 17,18 At the conception of this trial, there was no definitive evidence that an exercise programme would be as effective in the population of people with Parkinson’s as it is in the general population. Research has also shown that selecting and targeting each of the symptoms independently may not be enough to influence the falls rate. Instead, a multidimensional model that combines functional exercises with behavioural strategies is likely to provide a greater influence on reducing falls. 19
PDSAFE is an example of a multidimensional programme. 19 It is an individualised, home-based, physiotherapist-delivered, personalised treatment programme of exercises and strategies to help prevent falls in Parkinson’s. The novelty lies in both the content (i.e. disease-specific exercises and strategies for instability, use of motor relearning and cognitive awareness) and delivery [i.e. personalised feedback using a digital versatile disc (DVD) for adherence and self-management]. The programme comprises (1) exercises for postural control, gait and muscle weakness; (2) strategies for reducing freezing and encouraging stability and gait efficacy; and (3) a feedback model to promote learning and adherence. Frequency of intervention sessions is faded over time, 1 hour twice a week for 1 month, then once a week for a further 2 months, then once a month for another 3 months.
Primary aim of the PDSAFE trial
The primary aim of this trial was to determine the clinical effectiveness and cost-effectiveness of a novel personalised exercise and strategy intervention (PDSAFE) as a supplement to usual health-care management in people with Parkinson’s.
Research questions
-
Do fallers with Parkinson’s who undertake PDSAFE with usual care have fewer falls than those who do not undertake the treatment programme during months 0–6 after randomisation?
-
Do fallers with Parkinson’s who undertake PDSAFE with usual care have fewer falls than those who do not undertake the treatment programme during months 6–12 after randomisation?
-
Do fallers with Parkinson’s who undertake PDSAFE have better balance, mobility and quality of life than those who do not undertake the treatment programme?
-
Is the PDSAFE intervention cost-effective, compared with usual care for people with Parkinson’s, from an NHS perspective?
-
What are the personal insights of those who participate in the intervention?
Pilot study
Aims and objectives
Prior to the main trial, a small pilot study was conducted as part of the protocol development work. The aims of the PDSAFE pilot study were to confirm the:
-
content and delivery of the novel intervention
-
implementation of procedures for recording and checking treatment fidelity
-
battery of assessments to be administered at the baseline and follow-up visits.
The objectives of the PDSAFE pilot study were to:
-
recruit a sample of up to 20 people with Parkinson’s: up to 10 in Southampton and up to 10 in Newcastle
-
take informed consent from up to 20 people with Parkinson’s
-
complete screening measures on up to 20 people with Parkinson’s
-
test the collection of falls data through the use of diaries with up to 20 people with Parkinson’s
-
complete baseline assessments with up to 20 people with Parkinson’s
-
assess the time taken to complete assessments
-
determine the feasibility of assessments
-
determine the acceptability to participants of assessments
-
deliver the PDSAFE intervention to up to 20 people with Parkinson’s.
Procedure
Ethics approval was given by the South Central – Hampshire B National Research Ethics Service (reference number 13/SC/0538). Sixteen participants were recruited [six from Newcastle and 10 from Southampton, including one patient and public involvement (PPI) representative]. All participants completed all screening measures [i.e. Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Hoehn and Yahr (H&Y) scale and falls characteristics]. Six participants from each site completed baseline assessments, participated in the intervention for 1 month and completed the battery of assessments post intervention. The remaining four participants in Southampton provided falls diaries only, to test the diary completion required prior to randomisation in the main trial.
Two patterns of treatment delivery were tested to establish the most appropriate way of providing the intensity of treatment in the early stages. In Newcastle, participants had the intervention for two sessions a week for 3 weeks, then once a week for 1 week. In Southampton, participants had treatment for two sessions a week for 2 weeks and then once a week for 2 weeks.
Outcome
Overall, the participants enjoyed the treatment. Participants’ comments included ‘very pleased he participated – he has found it very beneficial and continues to use several strategies’, ‘Doing all home exercises and feels his balance is getting better’ and ‘have enjoyed the exercises and have learned a lot about keeping active’.
The therapists were enthusiastic about the programme and recognised the importance of mapping the house for problem mobility areas and integrating the exercises with video strategies appropriate for each individual. They commented that there was a lot to do in the initial stages around planning the strategies and fitting the weighted vests, so the pattern of treatment of two sessions a week for 3 weeks may be more valuable than two sessions a week for 2 weeks. However, it was agreed that this may not be appropriate for each person and that some flexibility in the way the sessions are delivered should remain. Training was recognised as very important. Some people found the weighted vests difficult to put on and required help from their partner; most people could tolerate only a small percentage of body weight in the vests, yet most people viewed them positively.
The outcomes of the pilot study were discussed at a face-to-face meeting of the grant holders, which was also attended by three PPI members; adjustments to the protocol were suggested and implemented. The pilot study confirmed the content and delivery of the novel intervention for the main trial (including printed versions of the exercises and strategies, video vignettes for play on a tablet or DVD and the use of weighted vests for strengthening exercises). Procedures for recording and checking treatment fidelity were also established, including weekly telephone and Skype™ (Microsoft Corporation, Redmond, WA, USA) consultations with the physiotherapists. As a result of the pilot, the assessment time was shortened and procedures tightened. For example, the handgrip strength measurement test20 was dropped from the main study, but retained as a substudy in the Portsmouth area; and the shorter (15- instead of 30-question) version of Geriatric Depression Scale (GDS) was used.
Chapter 2 Methods
Material in this chapter has been adapted from the trial protocol by Goodwin et al. 21 © Goodwin et al. ;21 licensee BioMed Central. 2015. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Trial design
The PDSAFE trial was a UK, multicentre, community-based, single-blind, randomised controlled trial (RCT) with a 12-month follow-up, with nested economic evaluation and qualitative studies. Ethics approval was given by the South Central – Hampshire B National Research Ethics Service (reference number 14/SC/0039). Local research and development approval was obtained at each participating centre. Figure 1 shows how people with Parkinson’s progressed through the trial.
FIGURE 1.
Diagram showing trial plan.
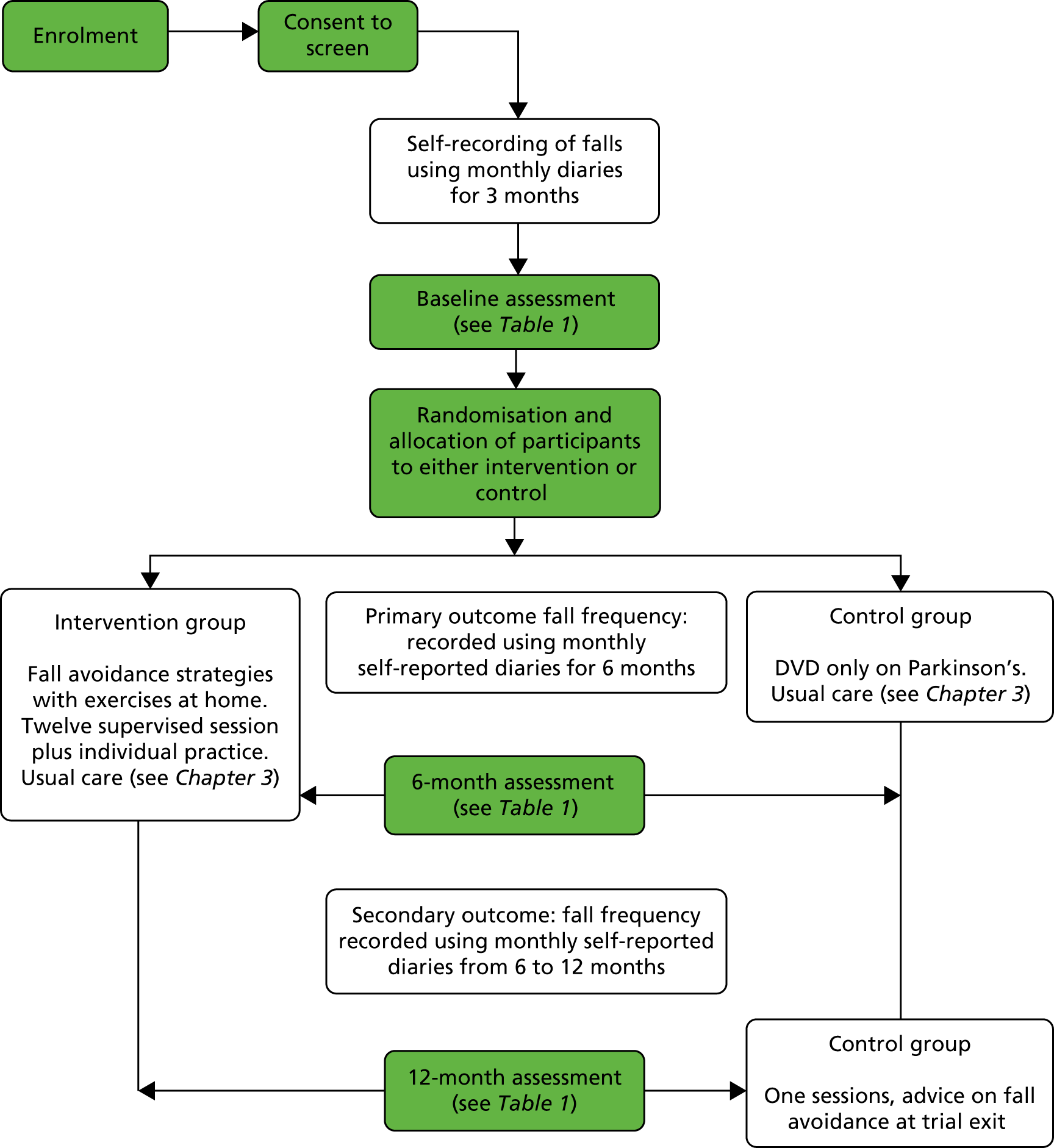
Initially, four recruitment centres were identified: Southampton, Portsmouth, Bournemouth and Poole, and Exeter. The aim was for each of these to recruit 150 people with Parkinson’s to the trial over a 16-month period. Unfortunately, there were several delays in site set-up, and several sites found that they did not have as many people with Parkinson’s as anticipated. Four additional sites were opened: Newcastle, Winchester and Basingstoke, Plymouth and Truro. These centres represent a range of socioeconomic environments. Unfortunately, it was not possible to secure additional funding to follow up participants recruited in these new centres beyond the primary outcome measure at 6 months. Therefore, only participants randomised before 1 May 2016 had a final 12-month assessment.
Participants
Inclusion criteria were broad to allow inclusion of a wide spectrum of Parkinson’s patients in order to provide a generalisable result. Participants were eligible to be included to the trial if they met the following criteria:
-
had a confirmed consultant’s diagnosis of Parkinson’s
-
lived at home
-
had experienced at least one fall in the previous 12 months
-
were able to give informed consent
-
were able to understand and follow commands
-
were able to complete a programme of exercises
-
scored ≥ 24 on the MMSE
-
were willing to participate.
Participants were not eligible to be included in the trial if they:
-
lived in a care home with or without nursing
-
required assistance from another person to walk indoors
-
were wheelchair bound or bedridden unless aided, as defined by H&Y scale stage 5.
Recruitment and selection
Recruitment ran from July 2014 to August 2016. Trial participants were recruited through hospital clinics and Parkinson’s services, through local Parkinson’s UK groups and websites and through word of mouth/the trial website. Research packs comprising an invitation (see Appendix 1), the participant information sheet (see Appendix 2), a response slip (see Appendix 3) and a Freepost pre-addressed envelope were distributed. Once response slips were received by the trial office, trained trial assessors visited potential participants in their own homes to complete consent and screening assessments. The screening assessments included MoCA,22 the MMSE, 23 the H&Y scale,24 demographic information and medical history. Participants were asked to retrospectively recall the number of falls that they had experienced in the previous 12 months. The screening visit was followed by a prospective falls monitoring period of at least 13 weeks.
Three months after a screening visit, the assessor returned to carry out the baseline assessment (October 2014 to November 2016). The assessor checked that the participant was willing to participate in the trial and that they met the criteria to enable them to proceed to the next stage of the trial; the assessor then conducted the baseline assessments with people with Parkinson’s and initiated the randomisation procedure but was blinded to the group allocation. Blinded assessments were repeated 3, 6 and 12 months following randomisation (up to November 2016). Participants recruited during the extension period (those randomised after 1 May 2016) were followed to the primary outcome (6 months) only because of restricted funding.
Randomisation and therapy allocation
Patients were randomised (50 : 50) to receive PDSAFE (intervention group) or usual care plus provision of a Parkinson’s information DVD (control group), using an online randomisation service at the Oxford Clinical Trials Research Unit, University of Oxford. Random allocations were computer-generated, stratified by centre and allocated in blocks with a random size of two, four, six or eight participants. This ensured that the allocated groups within centres were as evenly distributed as possible, while maintaining a system in which allocations were unlikely to be deduced by those needing to remain blinded. The randomisation outcome was e-mailed to the trial co-ordinating centre via the PDSAFE e-mail address and forwarded to the therapy team. The assigned therapist contacted participants within 2 days to inform them of the randomisation outcome and arrange the first visit with them (within 2 weeks for those in the intervention group and within 4 weeks for those in the control group). The same therapist at each site saw control and intervention participants.
Participants in the control group continued to receive their usual care. In addition, they were given a DVD, produced by Parkinson’s UK, containing information about living with Parkinson’s (this was not falls-specific information). A physiotherapist visited the control group participants after randomisation to explain their allocation and the importance of the control group; at the end of the trial, once their final follow-up assessments had been completed, control group participants received guidance on physical activities and strategies for postural control and safety in accordance with their profile of fall events.
For a full description of the PDSAFE intervention refer to Chapter 3.
Outcome measures
The outcome measures are summarised in Table 1. The primary outcome was risk of repeat falling in the first 6 months after randomisation. A fall was defined as an event that resulted in a person coming to rest on the ground or other lower level not as a result of a major intrinsic event or overwhelming hazard. 25 A near-fall was deemed to have occurred when an individual felt that they were going to fall but managed to prevent themselves from doing so. 26 Fall events (falls and near-falls) were recorded using monthly self-completed diaries, which have been used successfully in other studies. 16 Diaries were given to participants by assessors during the visits to conduct screening, baseline and follow-up assessments. The assessors explained to participants how the diaries should be completed and left writing instructions. Participants were asked to return diaries by post each month in a Freepost envelope provided. See Appendix 4 for copies of falls diaries and instructions.
| Screening measure | Source | Time point |
|---|---|---|
| MoCA | Assessor | Screening visit |
| Retrospective recall of falls over the previous 12 months | ||
| MMSE | ||
| H&Y scale | ||
| Demographic information and medical history | ||
| Primary measure | ||
| Fall events 0–6 months (falls) | Monthly self-report diaries | Completed from screening visit to end of participation in trial (maximum of 15 months) |
| Measures | ||
| Fall events 0–6 months (near-falls and fractures) | Monthly self-report diaries | Completed from screening visit to end of participation in trial (maximum of 15 months) |
| Fall events 6–12 months (falls, near-falls and fractures) | Monthly self-report diaries | |
| Mini-BESTest (a test of postural control/balance control) | Assessor | Completed at baseline and at each follow-up assessment (at 3, 6 and 12 months) |
| Timed CST | ||
| Handgrip strength test (substudy in one area only) | ||
| PASE | ||
| PDQ-39 (a quality-of-life measure designed specifically for people with Parkinson’s) | ||
| GDS – 15-question version | Self-report | |
| FES-I | ||
| NFoG test [a questionnaire on FoG (freezing is closely linked to falling)] | ||
| Medication use | ||
| MDS-UPDRS – motor section | ||
| Health professionals and exercise | ||
| Economic measures (people with Parkinson’s) | ||
| Health and social care resource use sheet | Assessor | Completed at baseline and at each follow-up assessment (at 3, 6 and 12 months) |
| EQ-5D | Self-report | |
| Economic Measures (carer) | ||
| Carer demographic information and caring role | Self-report | Completed at baseline and at each follow-up assessment (at 3, 6 and 12 months) |
| CES | Self-report | |
| CSI | Self-report | |
Secondary analysis of the primary outcome measure included rates of falling between 0 and 6 months. Secondary outcomes included risk of repeat falling between 6 and 12 months; rates of falling between 6 and 12 months post randomisation; the Mini-Balance Evaluation Systems Test (Mini-BESTest), a test of balance control;27 the Chair Stand Test (CST); the new freezing of gait test, a questionnaire on FoG;28 the 39-item Parkinson’s Disease Questionnaire (PDQ-39), a quality-of-life measure designed specifically for people with Parkinson’s;29 the short, generic quality-of-life measure known as EuroQol-5 Dimensions (EQ-5D) for economic evaluation;30 the Geriatric Depression Scale (GDS), 15-question version;31 the Falls Efficacy Scale – International (FES-I);32 and the Physical Activity Scale for the Elderly (PASE). 33 The International Parkinson and Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) – motor section,34 MoCA,22 data on medications, use of professionals and exercise activity outside the trial were not regarded as outcome measures, but were measured at all time points for characterisation of the population. The Carer Experience Scale35 and the Caregiver Strain Index36 were administered to carers at baseline and follow-up time points to capture some broader effects of the intervention.
Power and sample size
Primary outcome: risk of repeat falling between 0 and 6 months
The power and sample size calculations were based on the findings of a previous trial by Ashburn et al. 37 This was a rehabilitation trial similar to PDSAFE in design. In the EXSART trial,37 the risk of repeat falling in a 6-month period was 68% in the control group and 56% in the exercise group. However, falls risk was anticipated to be lower in PDSAFE because EXSART was restricted to people who had fallen twice or more often in the previous year. Therefore, it was assumed that the risk of repeat falling between 0 and 6 months would be 63% for the control group and 50% for the intervention group. This required 228 participants per group, with data for analysis of 456 participants in total. Allowing for a 5% drop-out rate between randomisation and 6 months meant that 480 participants needed to be randomised. Furthermore, allowing for a 10% drop-out rate between agreeing to the 3 months pre-randomisation falls collection and randomisation meant that 534 participants needed to be recruited to the pre-randomisation falls collection period. In total, 541 participants were recruited to the pre-randomisation falls collection period. Power calculation scenarios are summarised in Appendix 6, Tables 26 and 27.
Power calculations (see Appendix 6) for rates of falling were based on Tango38 and related to the number of falls during a fixed follow-up period, analysed using negative binomial regression conditioned on baseline counts: specifically, formula 23 in the paper38 was used, assuming equal rates in the baseline and follow-up periods in the control group and a follow-up period of twice the length of the baseline. Anticipating a falls rate ratio (FRR) of 0.8 between 0 and 6 months post randomisation, that is a 20% reduction in the rate of falling in the intervention group compared with the control group, and based on a rate of 2.5 falls in the 3-month baseline period, 197 participants per group were required at analysis, which led to 488 participants being recruited to the pre-randomisation falls collection period.
Other scenarios were considered for the differences in risk of repeat falling or for the FRR (see Appendix 6, Tables 26 and 27) and generally led to recruiting fewer than 600 participants to the pre-randomisation falls collection period. All the calculations aimed for 80% power in 5% two-sided tests between the intervention and control groups.
Embedded substudies
Once people with Parkinson’s were consented to take part in the main trial, participants were asked if they would be willing to take part in the qualitative substudy that ran alongside the main trial. The qualitative researcher contacted suitable participants on the basis of a theoretical sampling strategy (see Chapter 5). Forty–two selected participants from the intervention group agreed to partake in qualitative interviews, before and after receiving treatment (see Chapter 5).
At the point of recruitment, participants were asked if they had a carer. An information sheet was provided for the carer, along with an invitation letter, response slip and pre-paid Freepost envelope; the substudy explored carers’ quality of life. If a carer expressed a wish to take part, they were invited to attend the participant’s (people with Parkinson’s) baseline visit and informed consent was taken from them at that time. There were 463 participants with a carer; 189 carers agreed to take part.
Statistical analysis
A statistical analysis plan was finalised before the data set was unblinded. An intention-to-treat (ITT) approach was used for the main analyses with data analysed on the basis of group allocation. When there were incomplete diaries, a participant was coded as a repeat faller if they reported two or more falls in their incomplete diaries or if there was a report of two or more falls from a retrospective recall at the end of the trial; otherwise, they were included as a non-repeat faller if they had completed ≥ 50% diary days for the period in question. Participants not reporting two or more falls, completing < 50% of diary days and with no other indication of repeat falling during a follow-up period were excluded.
Repeat faller status during 0–6 months (primary outcome) and 6–12 months post randomisation was analysed in logistic regression controlling for site, age, gender, H&Y scale stage as a regressor, the logarithm of retrospectively collected number of falls in the year prior to screening, repeat fall status prior to screening and the logarithm of the prospectively collected rate of falling during the 3-month period prior to randomisation. A small quantity, 0.5, was added to numerators of the rates of falling so that participants with zero falls during the period were included; the results are not sensitive to the quantity added. 39 These controlling variables were finalised in a blind analysis without access to the group indicator, as specified in the analysis plan, and were included in all regression models. Rates of falling during the periods 0–6 months and 6–12 months post randomisation were examined in negative binomial models fitted with command nbreg in Stata® version 11 (StataCorp LP, College Station, TX, USA). The logarithm of a participant’s days of diary follow-up was included in nbreg modelling as an offset.
Odds ratios (ORs) and FRRs are presented with 95% confidence intervals (CIs). The geometric means of individual participant rates (with 0.5 added to the numerator, as before) of falling during 6-month follow-up periods are presented as rates per 6 months to accommodate the skewed distribution across participants. Prespecified subgroup analyses for falling between 0 and 6 months post randomisation were undertaken to identify the differential impact of the intervention according to MoCA status (≤ 25 and ≥ 26) and freezing status, and removing participants with most severe disease by virtue of the MDS-UPDRS (≥ 58) and H&Y scale stage (1–3). Differential effects were further explored on the basis of MoCA, MDS-UPDRS and number of falls in the year prior to screening by creating subgroups defined by tertiles at baseline. All the subgroup analyses involving tertiles were exploratory, that is they were not pre-stated. Both MoCA and MDS-UPDRS had been pre-stated but with different cut-off points and had shown some indication of differential effects.
Retrospective falls recall was examined by Canning et al. 40 as pre-stated subgroups but with different cut-off points; we wished to check the statistically significant interactions that they reported. Therefore, the decision was taken to examine subgroups defined by tertiles, not selected cut-off points. Subgroup analysis started with likelihood ratio tests for interaction in logistic models and Wald tests for interaction in the negative binomial models. Within-subgroup intervention effects are presented with 95% CIs. A secondary per-protocol analysis was carried out, as prespecified, by excluding participants from the PDSAFE group who received fewer than seven of the planned 12 sessions. A similar approach to analysis was followed for continuous secondary outcomes, additionally controlling for the outcome assessed at baseline. Analysis was conducted in SPSS version 24 [(Statistical Product and Service Solutions) SPSS Inc., Chicago, IL, USA] and Stata.
Safety reporting
Following a full risk assessment, PDSAFE was classified as a low-risk trial and, as such, in agreement with the ethics committee and sponsors, proportional safety reporting was implemented. Incidents of hospitalisation and disability, falls or incapacity are expected among this patient population. Therefore, only death, life-threatening events or new disability leading to prolonged hospitalisation attributed to the trial intervention were considered to be serious adverse events (SAEs). Similarly, any hospitalisation that was planned prior to randomisation or that could not be attributed to the PDSAFE intervention or assessments was not recorded as a SAE. Adverse events, such as hospitalisations, changes in health status and falls, were collected routinely as part of follow-up assessments and were monitored for potential SAEs. Falls diaries were also reviewed: if a fall resulting in hospitalisation had happened while someone was participating in the PDSAFE intervention and trial assessments, it would have been recorded as a SAE, but no one had such an event. All suspected SAEs were reviewed by Professor Helen Roberts, a medical physician, who adjudicated whether or not they were related to the trial activity.
An independent Data Monitoring Committee was established. The group met regularly throughout the trial to undertake interim reviews of the trial’s progress, including the review of updated figures on recruitment, data quality, adherence to protocol and follow-up, and main outcomes and safety data. Falls rates in particular were monitored.
Patient and public involvement
Patient and carer involvement was incorporated at all levels of the PDSAFE trial. Several PPI representatives were involved in the design of the study, including the development of participant information sheets, consent forms and intervention resources. Mr John Wood acted as PPI representative on the Trial Steering Committee. The trial was presented to several local Parkinson’s support groups; representatives were invited to the results launch event in September 2017 and contributed to discussion on the interpretation of results and key messages. PPI representatives will also be involved with the dissemination of the findings through existing patient networks, mainly through Parkinson’s UK and its newsletter service.
Chapter 3 The PDSAFE intervention and its delivery
This chapter details the PDSAFE intervention and its delivery. Background information is provided to support the conceptual development of the intervention, the protocol is presented using the TIDieR (Template for Intervention Description and Replication)41 and descriptive statistics demonstrate the content of the intervention delivered over the intervention period.
Background for intervention design
A role for exercise in the treatment of both the physical and cognitive/behavioural symptoms of Parkinson’s has been advocated and is supported by the European Physiotherapy Guidelines for Parkinson’s Disease. 42 In reviewing 70 clinical trials, the guidelines suggest that there is strong evidence that specific physiotherapy interventions help to improve transfers, balance, gait, physical capacity and movement functions,42 all isolated falls risk factors. A recent Cochrane review43 stated that the overall aim of physiotherapy intervention is to optimise independence, safety and well-being, thereby enhancing quality of life; however, the intervention that is most effective at achieving this remains unclear.
Evidence suggests that a multidimensional intervention to reduce falls, incorporating balance, functional strength and strategy training, and thus appreciating the need for motor, cognitive and behavioural training, may be more effective than interventions focusing on independent risk factors such as postural control and/or functional strength alone. 19
The PDSAFE intervention is delivered in the home, tailored to an individual’s specific falls mechanism and functional presentation and personalised to rehabilitate the primary strategy or strategies that contributed to the fall(s) (Figure 2). Not only does this allow the protocol to align with all components of the International Classification of Functioning, Disability and Health (ICF)44 as a person-centred approach, it also follows the consensus-based clinical practice recommendations for falls management in Parkinson’s. 45 From this, personalised exercise prescription, within a menu of exercises, allows an individualised programme to be designed specific to the falls-related risk factors (impairments) that contribute to the primary ‘problematic’ strategy (as recommended by the European Physiotherapy Guidelines for Parkinson’s Disease42). The specific ‘impairment’ training enables physiological improvements in Parkinson’s symptoms and deficits, which allow functional training and strategy task practice in everyday life (see Figure 2). In this way, the rehabilitation of the falls-related strategy and its contributing falls risk factors not only works towards reducing the risk of similar falls again, but also embeds the training in everyday function and, therefore, is more likely to have a greater overall effect across all components of a participant’s life (and, thus, full ICF model).
FIGURE 2.
Conceptual model of the PDSAFE falls prevention protocol intervention.

Intensity is maintained across all aspects of the frequency, intensity, time and type (FITT) principle (as published in the American College of Sports Medicine guidelines)46 to drive physiological adaptation. ‘Frequency’ is regulated to a minimum of three times per week, ‘intensity’ must be perceived as ‘moderately hard/hard’ for all activities of the programme, ‘time’ is set to a maximum of 60 minutes and ‘type’ of exercise is tailored and specific to each individual’s falls mechanism. With the consideration of all factors, it is therefore possible to design a multidimensional programme that does not lose intensity as a result of its many components. In addition, the high intensity, continuous progression and titrated support from intensive to independent practice maintains focus and adherence and encourages personal commitment and investment, as well as fostering an understanding and empowerment of the rehabilitation process for the individual. The addition of visual feedback both in therapy time and as a review through personalised DVDs also aids accurate independent practice and continuation of therapy. Thus, continuous progress and adaption to the neurodegenerative properties of the condition can be made to maintain safety.
In appreciation of the mechanisms of neurorehabilitation and exercise prescription, the PDSAFE intervention protocol is structured in a way that enables intensive, repetitive practice that is salient to an individual and their specific falls profile, thus meeting their needs for effective neuroplastic change. In addition, the embedding of the training in strategy task-related practice across all functional activities enables rehabilitation to take place across all levels of life participation and not just in relation to a specific task, goal or previous fall behaviour.
As a result of its unique structure and delivery, the PDSAFE intervention (see Figure 2) reflects the evidence base for falls prevention in Parkinson’s, meets the holistic recommendations of the ICF framework for practice and facilitates onward progression and independent self-management of the condition by the individual. The novelty lies in both the content (disease-specific exercises and strategies for instability, use of motor relearning and cognitive awareness) and delivery (personalised feedback using a DVD for adherence and self-management).
Intervention protocol
In line with the recommended methods of reporting intervention design, TIDieR41 is detailed in Table 2. The 12 items detailed in the checklist are an extension of the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement – item 547 and the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement – item 1148 and are intended to improve the reporting of interventions.
| TIDieR | Checklist requirement | Protocol description |
|---|---|---|
| 1 | Protocol name | PDSAFE: a personalised falls prevention programme of home exercises for postural control training, muscle strengthening and task-orientated movement strategy training |
| 2 | Protocol rationale and theory of main elements |
|
| 3 | Protocol materials | For the participant:
|
| 4 | Procedures of protocol delivery |
|
| 5 | Protocol providers |
|
| 6 | Mode of protocol delivery |
|
| 7 | Location of protocol delivery |
|
| 8 | Protocol duration, intensity and dose |
Intervention Supervised sessions comprise:Independent practice: |
|
Comparison group Supervised sessions comprise: |
||
| 9 | Protocol personalisation |
|
For a visual representation of the intervention, please refer to the film available at: www.youtube.com/watch?v=emNr0REIm4A&list=PLT3AipgP4l_x7OVNryanVgtcvPZXVJyX1 (accessed 21 February 2019).
Delivery outcomes
Delivery of the PDSAFE intervention was over 2 years and 4 months. The intervention was delivered in a total of eight clinical sites across nine NHS trusts.
Therapists
Physiotherapists were recruited by each site. Trial requirements stated that each therapist should have experience in Parkinson’s or falls rehabilitation. It was initially designed that each site would have one treating therapist and a trained cover for periods of absence. However, owing to clinical workload and logistical delivery, a total of 18 therapists were trained over the study period. One lead therapist co-ordinated the team, delivered the training and development activities, and monitored the fidelity of intervention delivery.
Training, facilitated by the lead therapist, included attendance at one of the compulsory 2-day training events held on three separate occasions. In addition, therapists were asked to attend a virtual weekly meeting by telephone to discuss clinical cases and problem-solve within the boundaries of the intervention protocol. These sessions were chaired by the lead therapist, with a total of 122 telephone contacts made available over the intervention period. To maintain a high standard of clinical reasoning throughout the intervention period, therapists were also asked to attend (either physically or virtually) monthly ‘masterclasses’ on key clinical topics such as cognition and dual tasking, turning, FoG and balance. Alternating with masterclasses, therapists were asked to present case studies on key topics for team discussion. Twelve ‘masterclass’ topics (some were repeated for new therapists) and seven case study reviews were held over the intervention period.
Fidelity of the intervention was a priority to encourage uniformity of practice. The lead therapist observed each therapist in a treatment session with a participant, once a month for the first 3 months of delivery and then once every 3 months for the delivery period. Following the observation, a clinical reasoning discussion was completed and a report written. Therapists could also request additional joint sessions with the lead therapist if they had concerns or queries regarding a particular participant. This ensured that the PDSAFE intervention was uniformly delivered across all sites and by all therapists. A total of 75 fidelity sessions were held over the intervention period, with all therapists being assessed.
Intervention sessions
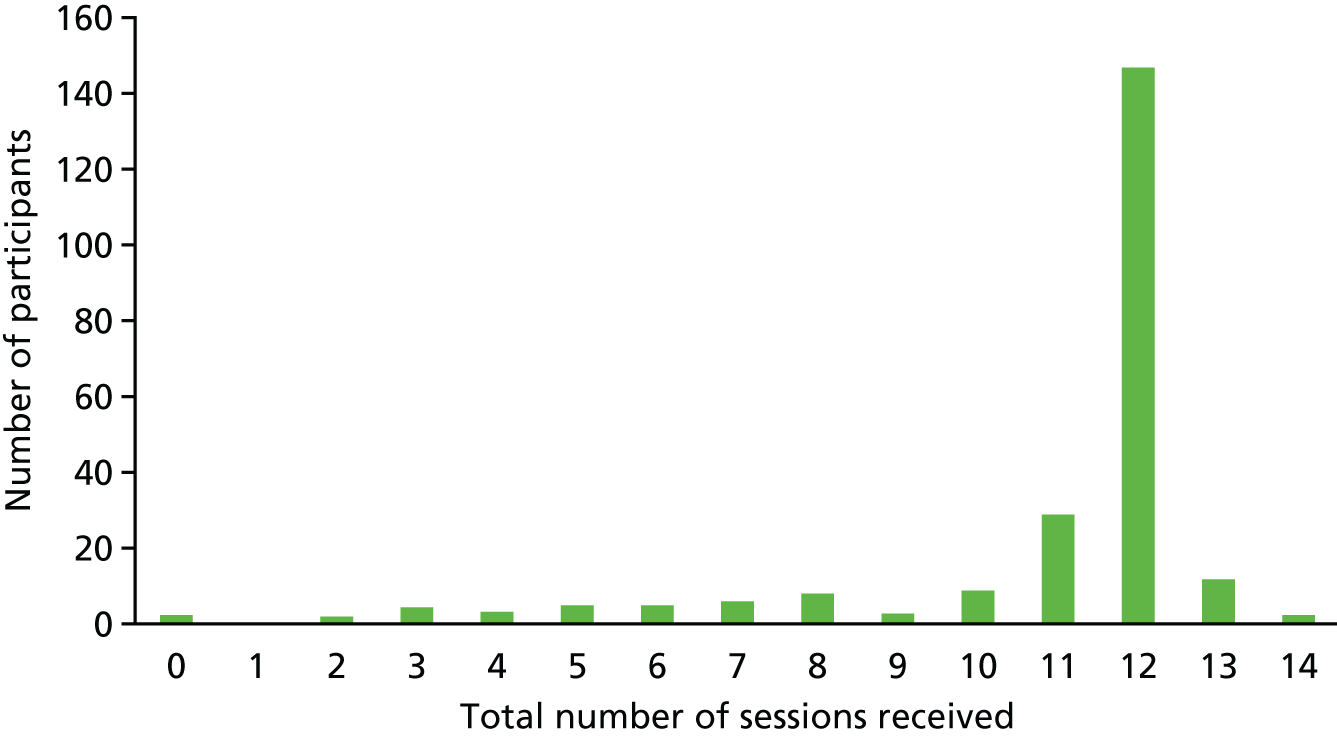
A total of 2587 sessions were delivered to the 291 intervention participants, with the majority of participants receiving the anticipated 12 sessions (mode = 12).
Figure 3 presents the total number of sessions received by participants allocated to the intervention arm of the trial. The majority of participants, 236 out of 238, received the exercise assessment and at least one supervised session. Two participants did not start because they had changed their mind and 19 received fewer than seven sessions; reasons for not fully engaging included admission to a nursing home, deteriorating health, commitment was too much and caring for others; in some cases, no reason was given.
FIGURE 3.
Total number of intervention sessions received by participants.
All interventions sessions included a brief review of falls; warm-up exercises; review, practice and progression of a participant’s individual exercise programme; and strategy training in functional scenarios as a basic structure. Each therapist tailored the strategies treated, exercises prescribed and functional tasks practised from a menu for each participant.
Selection of strategies
Evidence from the literature and from previous studies of falls among patients with Parkinson’s, as well as expert opinion, were used to determine the most frequent falls mechanisms in Parkinson’s. 19 Eight strategies were defined: avoiding tripping, dual tasking, freezing cues, moving in tight spaces, picking up an object, reaching, stepping backwards and turning. As described above, through the process of taking a detailed falls history, clinical assessment and advanced clinical reasoning, therapists determined the most likely ‘fall mechanism’ for each participant (levels 1 and 2 in Figure 2). For example, in the case of a participant who repeatedly reported catching their foot and falling, regardless of the task being undertaken or location, would be most likely to have a falls mechanism of tripping; thus, the strategy ‘avoiding tripping’ would be selected by the therapist.
Over the 291 participants who received the PDSAFE intervention, strategies were selected a total of 440 times, with a strategy being used in treatment a total of 3447 times over the period. This provides a large sample to consider the clinical reasoning process of the therapists selecting the strategies. Figure 4 demonstrates the number of times each strategy was selected as a potential falls mechanism corresponding to the number of times that strategy was used in a treatment session.
FIGURE 4.
Stratification of strategies used in delivery of PDSAFE intervention.
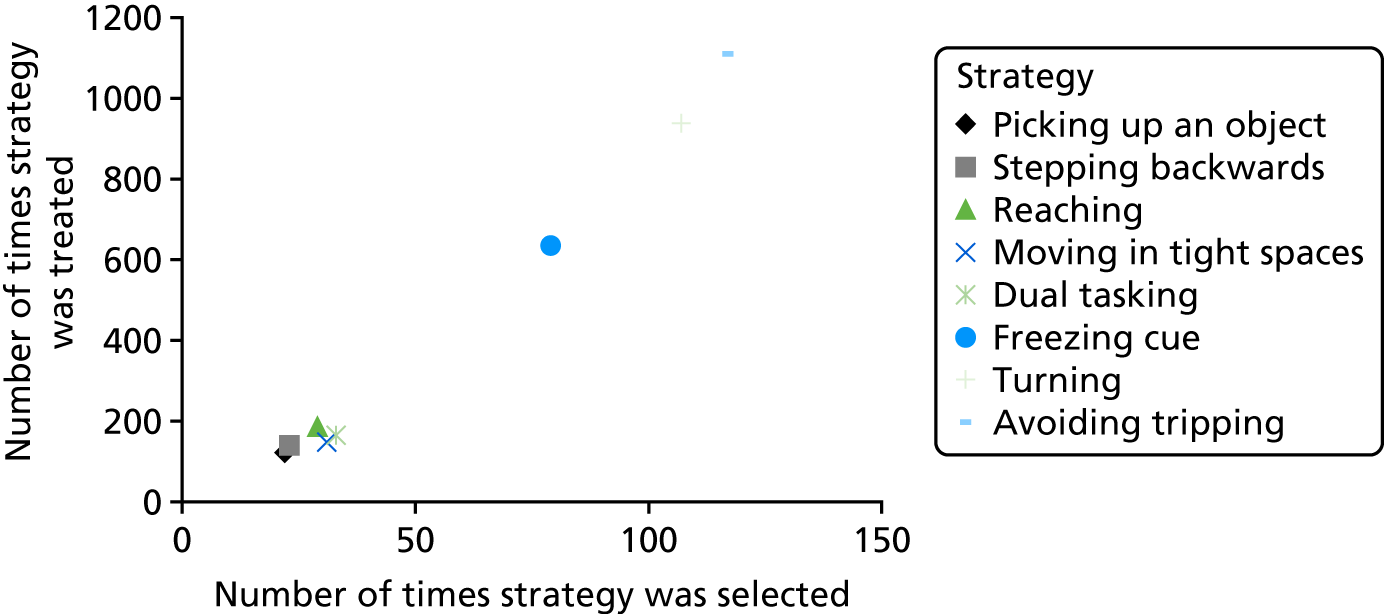
Importantly, this allows description of the strategies selected as primary falls mechanisms and thus treated for the majority of the intervention period versus strategies that may have been selected as a secondary or subsequent strategy and thus treated less frequently or for a shorter period of time.
It is apparent that ‘avoiding tripping’ was the most widely used strategy. It was selected following assessment a total of 116 times, used 1110 times during treatment sessions and accounted for 26% of all strategies selected. The figures for ‘turning’ are similar [selected 107 times and used 938 times during treatment (26% of the total)]. ‘Freezing cues’ was also frequently selected as a strategy [selected 79 times and used 365 times (24% of the total)]. It is clear from Figure 4 that all other strategies were selected and used in treatment with similar frequencies to each other.
Selection of exercise prescription
Once the strategy or strategies most appropriate for addressing a participant’s falls mechanism had been selected, therapists used advanced clinical reasoning and assessment skills to determine the physical impairments and deficits in physical falls risk factors that were most likely to contribute to the fall mechanism (see Figure 2). For example, the participant described above, who frequently caught their foot and subsequently fell, was allocated the ‘avoiding tripping’ strategy. The therapist must consider a number of reasons why the participant has a tendency to catch their foot, such as weakness of the muscles used to lift the toes, failure to transfer weight onto the supporting leg appropriately because of hip weakness or reduced limits of postural control stability, or failure to achieve enough clearance from the ground because of weakness in the hip flexors. Through assessment, the therapist determines the most likely impairment and designs a functional strength and postural control exercise programme from the available menu that treats this impairment.
Evidence from the literature and from previous studies of falls among patients with Parkinson’s, as well as expert opinion, were used to determine what exercises were available on the menu for therapists to select from. 19
Table 3 shows the menu of exercises available for the therapist to select from when putting together an individual participant’s PDSAFE intervention.
| Function | Exercise |
|---|---|
| Balance/postural control | |
| Standing | Standing balance |
| Tandem stand | |
| Reaching | |
| Compensatory step and lunge | |
| Walking | Heel/toe walking |
| Toe/heel walking backwards | |
| Tandem walking | |
| ‘Figure of 8’ walking | |
| Picking up an object | |
| Stepping over an object | |
| Strengthening | Sit to stand |
| Standing toe and heel raises | |
| Forward stepping up and down | |
| Side stepping up and down | |
Exercises were available on six levels; working through the levels enabled progression (level 4 in Figure 2) and maintenance of intensity for each exercise. All programmes had to include at least one balance and one strengthening exercise. A total of 1693 exercises were selected by all therapists over the intervention period with an average of six (range one to eight) exercises prescribed per participant across the period. Figure 5 shows a spread of all exercises being used over the intervention period.
FIGURE 5.
Exercise use frequency across all of the intervention, in all participants.

Figure 5 demonstrates the predominance of the more dynamic postural control (compensatory step and lunge and heel/toe walking) and strengthening exercises over other exercises from the menu. The complex nature of the compensatory step and lunge exercise makes it suitable for the treatment of many of the falls risk factors associated with Parkinson’s; for example, high dynamic stepping actions help with motor control, compensatory stepping helps with the regain of an appropriate base of support from a loss of postural control or a trip by increasing stepping amplitude for those who freeze and expanding limits of stability for those who fall when reaching. The practice of stepping backwards with appropriate postural control and weight distribution will assist those who fall stepping backwards. A common symptom of the Parkinsonian gait is loss of foot clearance, heel strike and step length45 (hence the predominance of tripping as a falls mechanism); thus, the high frequency of the use of heel/toe walking to improve these impairments is also unsurprising. As each exercise programme had to include both strengthening and postural control exercises, the high frequency of selection of strengthening exercises can be attributed to the fact that the postural control menu contained fewer exercises that could be selected. This is less of a clinical reasoning observation and more related to the ratio of exercises in the menu.
Summary
The PDSAFE intervention was deeply rooted in evidence from both rehabilitation after falls and exercise prescription literature. The protocol was a holistic model encompassing all aspects of the ICF and its design allowed a personalised, sophisticated, complex intervention to be prescribed for each participant. ‘Personalised’, ‘intensive’ and ‘progressive’ were key parameters of the intervention, demanding a high level of commitment and collaboration between the therapist and the participant. The intervention was delivered to a high standard. All requirements of fidelity were met and the intervention was comparable across all sites. Delivery was significantly enhanced by the level of training and support provided to the delivery team and a high standard of clinical reasoning and protocol delivery was maintained. The majority of participants received the planned number of sessions. There was a slight rise in the number of participants receiving only seven or eight sessions as this is the point at which the therapists visited less frequently and participants were required to complete longer periods of independent practice. It is likely that this led to some participants withdrawing at this point because of the reduced support/motivation from the therapist. There was a clear predominance of some strategies, which is likely to reflect the demographic of the intervention group (i.e. more frequent fallers and people with Parkinson’s who freeze, plus those who have more advanced disease and thus are more likely to have difficulty turning: with axial rigidity, poor stepping and impaired cognition). The predominance of ‘avoiding tripping’, ‘turning’ and ‘freezing cues’ is in line with the reasons for falling provided by participants most frequently in the previous literature. 26 The design of strategy selection leading to supported exercises to treat falls risk factors provided a protocol design that was deliverable, with all participants receiving both strategies and exercises as planned. Owing to the complexity of the exercises, some exercises are better able to be adapted for multiple impairments and thus are used more frequently. Previous studies19 have provided the same intervention for all participants, regardless of fall mechanism. The use of all the strategies and exercises demonstrates the need for a complex intervention and variability as ‘one size, clearly does not fit all’.
Chapter 4 Statistical trial results
Participants
Recruitment of participants ran from July 2014 to August 2016 (see Appendix 5, Figure 14).
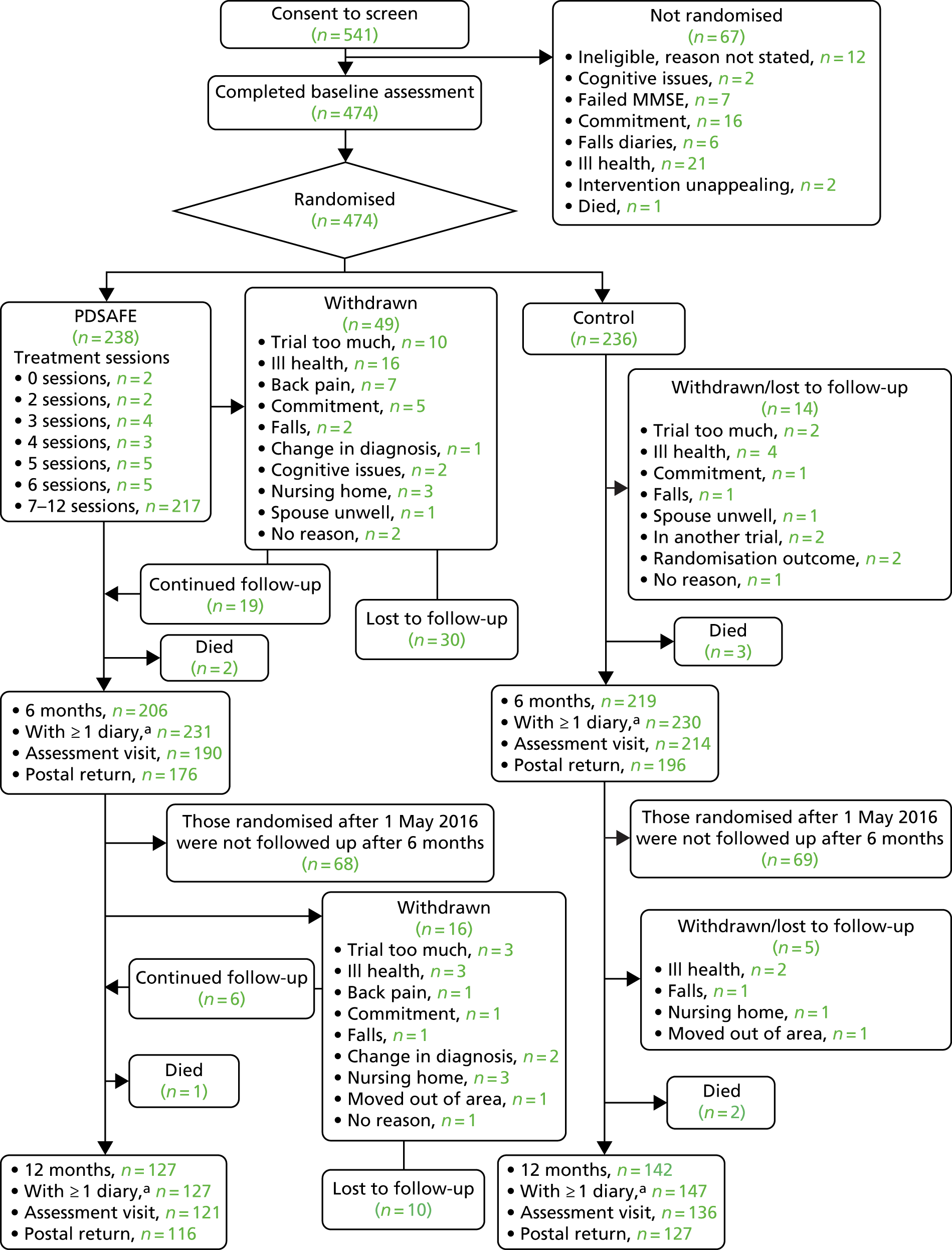
Figure 6 provides the CONSORT flow diagram of participants’ assessments. A total of 640 people with Parkinson’s were invited to participate, but 99 either did not respond or did not meet the eligibility criteria, which left 541 people for consent and completion of a screening visit with a trial assessor in their own home. Of these 541 people, seven did not score the minimum score on the MMSE and were excluded. The remainder went on to complete prospective falls diaries for 3 months (i.e. a minimum of 13 weeks). A further 60 people were excluded during this time [reasons for not being randomised were as follows: medically unfit (n = 21), dislike of completing falls diaries (n = 6), no longer met eligibility criteria (n = 12), reported cognitive issues (n = 2), died (n = 1) or decided not to participate in the trial (n = 18)]. The remaining 474 (88%) people completed a baseline assessment and were randomised into one of two groups: control (n = 236) or the PDSAFE intervention (n = 238). Recruitment and randomisation graphs can be found in the appendices. The groups allocated to PDSAFE and control were similar at baseline (Table 4) in terms of age, gender, disease severity, disease duration, cognitive ability, freezing, medication and coexisting conditions and living status. Retrospective recall of falls was also similar between the groups, but the rate of falling in the 3 months prior to randomisation was greater in those subsequently randomised to the intervention group.
FIGURE 6.
Flow diagram of participants’ progression through the trial. a, Number with any diaries during the preceding 6 months. Adapted from Chivers Seymour et al. 49 © Author(s) [or their employer(s)] 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by the BMJ Publishing Group Ltd. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
| Characteristic | Trial group | |
|---|---|---|
| PDSAFE (n = 238)a | Control (n = 236)b | |
| Gender, n (%) | ||
| Male | 147 (62) | 119 (50) |
| Female | 91 (38) | 117 (50) |
| Age (years) | ||
| Mean (SD) | 71 (7.7) | 73 (7.7) |
| Minimum, maximum | 51, 91 | 46, 88 |
| Disease duration (years) | ||
| Mean (SD) | 8 (6.6) | 8 (5.8) |
| Minimum, maximum | 0, 36 | 0, 29 |
| MMSE score | ||
| Mean (SD) | 28 (1.7) | 29 (1.6) |
| Minimum, maximum | 24, 30 | 24, 30 |
| MoCA score | ||
| Mean (SD) | 26 (2.9) | 26 (3.2) |
| Minimum, maximum | 15, 30 | 9, 30 |
| ≤ 25 (cognitively impaired), n (%) | 91 (38) | 93 (39) |
| Living status, n (%) | ||
| Lived alone | 48 (20) | 59 (25) |
| With a spouse/partner | 174 (73) | 166 (70) |
| With a friend/family | 15 (6) | 10 (4) |
| H&Y scale stage, n (%) | ||
| 1 | 26 (11) | 30 (13) |
| 2 | 78 (33) | 56 (24) |
| 3 | 102 (43) | 112 (48) |
| 4 | 32 (13) | 38 (16) |
| MDS-UPDRS | ||
| Mean (SD) | 32 (15.2) | 33 (17.3) |
| Minimum, maximum | 2, 77 | 4, 92 |
| Phenotype | ||
| TD, n (%) | 21 (9) | 19 (8) |
| PIGD, n (%) | 194 (83) | 206 (88) |
| Indeterminate, n (%) | 20 (8) | 10 (4) |
| FoG in the past month, n (%) | 152 (64) | 139 (59) |
| Number of falls in the 12 months prior to screening | ||
| Median (minimum, maximum) | 3 (1, 1460) | 3 (1, 1095) |
| Mean (SD) | 26 (132.7) | 19 (105.4) |
| Repeat falling in 12 months prior to screening, n (%) | 186 (78) | 189 (80) |
| Rate of falls/person/3 months prior to randomisation | ||
| Median (minimum, maximum) | 1.98 (0, 319) | 0.99 (0, 73) |
| Mean (SD) | 5.9 (22.8) | 3.0 (7.3) |
| Rate of near-falls/person/3 months prior to randomisation | ||
| Median (minimum, maximum) | 4.4 (0 to 440) | 4.3 (0 to 601) |
| Mean (SD) | 13.8 (35.8) | 15.6 (51.4) |
| Medications, n (%) | ||
| Levodopa | 208 (88) | 216 (92) |
| Dopamine agonist | 108 (46) | 106 (45) |
| Monoamine oxidase inhibitor | 52 (22) | 46 (20) |
| Catechol-O-methyltransferase inhibitors | 59 (25) | 41 (17) |
| Other Parkinson’s medication | 19 (8) | 23 (10) |
| GDS score at baseline, n (%) | ||
| > 5 (suggestive of depression) | 147/235 (63) | 164/236 (70) |
| ≥ 10 (indicative of depression) | 50/235 (21) | 49/236 (21) |
| Coexisting conditions, n (%) | ||
| Orthopaedic | 109 (46) | 129 (54) |
| Cardiovascular/respiratory | 85 (36) | 96 (41) |
Delivery of intervention
In the PDSAFE group, 66 participants did not engage with the intervention for a number of reasons, including admission to a care home, deteriorating health, changing their mind about participation, feeling the commitment to be too great, or death; in some cases, no reason was given. The therapy aim was to provide 12 supervised sessions for each participant and, on average, participants had a median of 12 sessions [interquartile range (IQR) 11–12 sessions] or a mean of 11 sessions [standard deviation (SD) 2.4 sessions]. A total of 21 participants received fewer than seven sessions and, along with a further four participants for whom the number of sessions was not available, were excluded from per-protocol analyses.
Falling outcomes
In Table 5, the prospective completion of diaries is described during the period of baseline diary completion prior to randomisation, and in the 12-month period of post-randomisation follow-up. The percentage who returned no diaries was generally low (2–4%) during the period 0 to 6 months; during the final 6 months the percentage who returned no diaries was higher (1–12%). These percentages exclude the number who withdrew or died during the respective periods. Among those returning any diaries for the period in question, the IQR of the numbers of days completed was 90–92 (within target). The target number of days varied between 89 and 92 days across participants, depending on the calendar months covered by their 3-month baseline period.
| Characteristic | Trial group | |
|---|---|---|
| PDSAFE | Control | |
| Randomisation, n | 238 | 236 |
| Baseline | ||
| Number with no diaries | 1/238 | 0/236 |
| Number of diary days among those with diaries | (n = 237) | (n = 236) |
| Median | 92 | 92 |
| IQR | 90–92 | 90–92 |
| Minimum, maximum | 12, 92 | 26, 92 |
| n/N with complete diaries (%) | 196/238 (82) | 198/236 (84) |
| n/N with ≥ 50% diary days (%) | 229/238 (96) | 226/236 (96) |
| 0–6 months post randomisation | ||
| Number entering 0–6 months period | 238 | 236 |
| n/N with no diaries (%) | 7/238 (3) | 6/236 (3) |
| n/N in falls rate ratio analysis (%) | 231/238 (97) | 230/236 (98) |
| n/N exiting (died or withdrawn from follow-up during 0–6 months) (%) | 32/238 (13) | 17/236 (7) |
| n/N exiting with no diaries | 2/206 (1) | 3/219 (1) |
| Number of diary days among those not exiting with diary days | (n = 204) | (n = 216) |
| Median | 182 | 182 |
| IQR | 153–183 | 174–183 |
| Minimum, maximum | 3184 | 29,184 |
| n/N with complete diaries (%) | 130/238 (55) | 158/236 (67) |
| n/N with ≥ 50% diary days (%) | 203/238 (85) | 211/236 (89) |
| Number in follow-up at 6 months | 206 | 219 |
| Number in trial follow-up randomised after 1 May 2016 | ||
| Southampton | 5 | 4 |
| Portsmouth | 7 | 6 |
| Bournemouth | 2 | 2 |
| Newcastle | 15 | 13 |
| Hampshire | 13 | 18 |
| Plymouth | 10 | 11 |
| Cornwall | 16 | 15 |
| Total | 68 | 69 |
| 6–12 months post randomisation | ||
| Number entering 6–12 months period | 138 | 150 |
| n/N with no diaries (%) | 11/138 (8) | 3/150 (2) |
| n/N in falls rate ratio analysis (%) | 127/138 (92) | 147/150 (98) |
| n/N exiting (died or withdrawn from follow-up during 6–12 months) (%) | 11/138 (8) | 7/150 (5) |
| n/N exiting with no diaries (%) | 5/127 (4) | 2/143 (1) |
| Number of diary days among those not exiting with diary days | (n = 122) | (n = 141) |
| Median | 180 | 180 |
| IQR | 151–183 | 153–183 |
| Minimum, maximum | 4184 | 30,184 |
| n/N with complete diaries (%) | 94/138 (68) | 112/150 (75) |
| n/N with ≥ 50% diary days (%) | 114/138 (83) | 132/150 (8) |
There was a trend towards increased repeat falling during the 6 months following randomisation in the PDSAFE group compared with the control group (OR 1.21, 95% CI 0.74 to 1.98; p = 0.447). During the final 6 months of follow-up, there was a trend towards decreased repeat falling (OR 0.86, 95% CI 0.45 to 1.65; p = 0.657). No statistically significant differences between the groups were found (Table 6). These analyses followed the planned treatment for participants with incomplete diaries during a follow-up period, that is participants were classified as a repeat faller if they reported two or more falls in their incomplete diaries or if a check of all PDSAFE records indicated two or more falls in that period. They were classified as a non-repeat faller if fewer than two falls were reported in the incomplete diaries, there was no indication of repeat falling in the period in the PDSAFE records and ≥ 50% of diary days for the period had been completed. Otherwise they were excluded. Two sensitivity analyses are shown in Appendix 7, Table 28, where analysis of repeat falling is also reported, restricted to participants with complete diaries and including all participants with any diaries during the follow-up period in question. Although there were no changes in the statistical significance or otherwise depending on these treatments of missing diaries, there were changes in the magnitude of ORs.
| Period | Trial group | OR (95% CI)a | p-valuea | |
|---|---|---|---|---|
| PDSAFE | Control | |||
| Repeat falling restricted to ≥ 50% diaries, n (%) | ||||
| Baseline | 127/231 (55) | 92/230 (40) | ||
| Baselineb | 112/203 (55) | 80/211 (38) | ||
| 0–6 months | 125/203 (62) | 116/211 (55) | 1.21 (0.74 to 1.98) | 0.447 |
| Baselineb | 55/114 (48) | 47/132 (36) | ||
| 6–12 months | 57/114 (50) | 71/132 (54) | 0.86 (0.45 to 1.65) | 0.657 |
| Fall rates | Falls/person/6 monthsc | FRR (95% CI) | ||
| Baseline | 4.5 | 3.3 | ||
| 0–6 months | 3.4 | 2.7 | 0.98 (0.80 to 1.19) | 0.824 |
| 6–12 months | 2.7 | 2.8 | 0.83 (0.62 to 1.11) | 0.200 |
| Near-fall rates | Near-falls/person/6 monthsc | NFRR (95% CI) | ||
| Baseline | 8.0 | 8.1 | ||
| 0–6 months | 4.7 | 5.6 | 0.67 (0.53 to 0.86) | 0.001 |
| 6–12 months | 3.9 | 3.7 | 1.01 (0.67 to 1.52) | 0.968 |
Participants typically reported rates of falling, expressed per 6 months, of between three and five falls (see Table 6). There was little difference in the rate of falling between the two groups in the first 6 months of follow-up, indicated by the FRR, and a slightly greater difference, with FRR of 0.83 (95% CI 0.62 to 1.11; p = 0.200), during the following 6 months. The analysis of rates of falling is based on participants with any amount of diary completion for the follow-up period in question, with only participants returning no diaries excluded.
Rates of near-falling are also shown in Table 6, expressed per 6-month period. Near-falling was greater in the period prior to randomisation than subsequently. During the 6 months post randomisation, the ratio of near-falling rates between the PDSAFE and control groups was significantly reduced (p = 0.001), with a rate of near-falling in the PDSAFE group of 0.67 (95% CI 0.53 to 0.86) that of the control group. This reduction in near-falling was not maintained during the following 6 months. Like the analysis of falling rates, the analysis of near-falling rates also includes participants irrespective of the amount of diary completion for the period of follow-up in question (as long as any diaries were returned for the period).
In Table 7 a comparison is made between the overall falling (and near-falling) results following the ITT analysis and a per-protocol analysis (excluding participants in the PDSAFE groups receiving fewer than seven sessions). This shows the findings to be similar, there being no suggestion of reduced falling in the PDSAFE group when the analysis is restricted to those receiving seven or more sessions.
| Period | Analysis | |||
|---|---|---|---|---|
| ITT | Per protocol | |||
| OR (95% CI)a | p-valuea | OR (95% CI)a | p-valuea | |
| Repeat falling restricted to ≥ 50% diaries | ||||
| 0–6 months | 1.21 (0.74 to 1.98) | 0.447 | 1.16 (0.71 to 1.92) | 0.538 |
| 6–12 months | 0.86 (0.45 to 1.65) | 0.657 | 0.92 (0.47 to 1.77) | 0.793 |
| Fall rates | FRR (95% CI) | p-valuea | FRR (95% CI) | p-valuea |
| 0–6 months | 0.98 (0.80 to 1.19) | 0.824 | 0.99 (0.81 to 1.22) | 0.982 |
| 6–12 months | 0.83 (0.62 to 1.11) | 0.200 | 0.84 (0.63 to 1.13) | 0.268 |
| Near-fall rates | NFRR (95% CI) | p-valuea | NFRR (95% CI) | p-valuea |
| 0–6 months | 0.67 (0.53 to 0.86) | 0.001 | 0.67 (0.53 to 0.86) | 0.001 |
| 6–12 months | 1.01 (0.67 to 1.52) | 0.968 | 1.01 (0.67 to 1.52) | 0.963 |
Secondary outcomes
Analysis of the secondary outcomes collected by the assessing therapist during home visits (Mini-BESTest, FES-I, FoG and MDS-UPDRS) is reported in Table 8, with CST results reported in Tables 9 and 10. At the 6-month visit, the PDSAFE group had improved mean Mini-BESTest score of 0.95 points (95% CI 0.24 to 1.67 points; p = 0.009), controlled for baseline Mini-BESTest score and other covariates (see Table 7); better falls confidence as assessed by a lower mean FES-I score of 1.60 points (95% CI 3.00 to 0.19 points; p = 0.026), controlled for similar covariates; and improved balance as assessed by the CST (p = 0.041). There were no significant differences at 12 months.
| Outcome measure | Visit | Trial group, mean (SD), n | Mean difference (95% CI)a | p-value | |
|---|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | ||||
| Mini-BESTest (0–28, lower values worse) | Baselineb | 18.3 (5.7), 183 | 17.3 (6.1), 211 | ||
| 6 months | 19.4 (5.9), 183 | 17.5 (6.4), 211 | |||
| 6 months – baseline | 1.1 (3.8), 183 | 0.2 (3.8), 211 | 0.95 (0.24 to 1.67) | 0.009 | |
| Baselineb | 18.5 (5.8), 115 | 17.5 (6.1), 126 | |||
| 12 months | 17.9 (6.5), 115 | 17.4 (6.7), 126 | |||
| 12 months – baseline | –0.7 (4.5), 115 | –0.2 (3.8), 126 | –0.41 (–1.48 to 0.66) | 0.449 | |
| FES-I (16–64, higher values worse) | Baselineb | 34.1 (11.0), 189 | 35.1 (11.5), 211 | ||
| 6 months | 33.4 (10.6), 189 | 36.2 (11.4), 211 | |||
| 6 months – baseline | –0.7 (7.9), 189 | 1.1 (7.2), 211 | –1.6 (–3.0 to –0.19) | 0.026 | |
| Baselineb | 33.4 (10.7), 119 | 33.7 (11.3), 135 | |||
| 12 months | 34.8 (11.2), 119 | 37.2 (11.6), 135 | |||
| 12 months – baseline | 1.3 (8.2), 119 | 3.5 (9.3), 135 | –1.4 (–3.41 to 0.66) | 0.184 | |
| PASE (0–4, lower values worse) | Baselineb | 107.8 (73.5), 153 | 100.1 (67.1), 177 | ||
| 6 months | 110.2 (70.4), 153 | 100.6 (68.0), 177 | |||
| 6 months – baseline | 2.4 (50.8), 153 | 0.5 (49.5), 177 | –1.05 (–11.3 to 9.21) | 0.841 | |
| Baselineb | 108.1 (71.9), 98 | 98.6 (61.1), 115 | |||
| 12 months | 99.4 (72.8), 98 | 87.6 (62.3), 115 | |||
| 12 months – baseline | –8.7 (53.0), 98 | –11.0 (48.5), 115 | –0.55 (–13.9 to 12.8) | 0.935 | |
| PDQ-39 (0–100, higher values worse) | Baselineb | 27.4 (14.3), 126 | 28.7 (15.9), 153 | ||
| 6 months | 28.3 (15.0), 126 | 29.5 (16.5), 153 | |||
| 6 months – baseline | 0.8 (8.3), 126 | 0.9 (9.0), 153 | 0.12 (–2.0 to 2.28) | 0.911 | |
| Baselineb | 27.2 (13.6), 77 | 28.9 (15.9), 100 | |||
| 12 months | 29.1 (15.4), 77 | 31.7 (15.5), 100 | |||
| 12 months – baseline | 1.9 (8.6), 77 | 2.8 (11.2), 100 | 0.48 (–2.53 to 3.49) | 0.754 | |
| GDS (0–15, higher values worse) | Baselineb | 7.7 (2.3), 154 | 7.7 (2.1), 183 | ||
| 6 months | 7.8 (2.5), 154 | 8.0 (2.5), 183 | |||
| 6 months – baseline | 0.3 (1.8), 154 | 0.2 (1.9), 183 | –0.02 (–0.42 to 0.39) | 0.942 | |
| Baselineb | 7.7 (2.1), 96 | 8.0 (2.2), 118 | |||
| 12 months | 7.8 (2.5), 96 | 8.5 (2.4), 118 | |||
| 12 months – baseline | 0.2 (2.0), 96 | 0.4 (1.7), 118 | –0.21 (–0.72 to 0.31) | 0.421 | |
| Period | Trial group, n/N (%) | PDSAFE/control OR (95% CI) | p-valuea | |
|---|---|---|---|---|
| PDSAFE | Control | |||
| Baseline | 45/236 (19) | 45/235 (19) | ||
| Baseline among participants with a 6-month assessment | 29/188 (15) | 35/213 (16) | ||
| 6 months | 27/188 (14) | 47/213 (22) | 0.61 (0.37 to 1.02) | 0.076 |
| Baseline among participants with a 12-month assessment | 15/119 (13) | 21/134 (16) | ||
| 12 months | 23/119 (19) | 38/134 (28) | 0.66 (0.37 to 1.17) | 0.208 |
| Period | Trial group, median (IQR); n | p-valuea | |
|---|---|---|---|
| PDSAFE | Control | ||
| Baseline among participants with 6-month assessment | 14 (11–18); 159 | 14 (11–18); 178 | |
| 6-month assessment | 12 (10–15); 161 | 13 (10–16); 166 | 0.041 (n = 401) |
| Baseline among participants with 12-month assessment | 14 (11–17); 104 | 14 (12–18); 113 | |
| 12-month assessment | 12 (9–14); 96 | 13 (11–15); 96 | 0.163 (n = 253) |
Analysis is shown in Table 11 of the secondary outcomes self-reported by participants after the 6- and 12-month home visits and returned by post. No statistically significant differences between the groups overall were found for these outcomes.
| Assessment and visit | Trial group, mean (SD) | Mean difference (PDSAFE – control) (95% CI)a | p-valuea | |
|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | |||
| PASE (0–400, lower values worse) | ||||
| Baselineb | 107.8 (73.5); n = 153 | 100.1 (67.1); n = 177 | ||
| 6 months | 110.2 (70.4); n = 153 | 100.6 (68.0); n = 177 | ||
| 6 months – baseline | 2.4 (50.8); n = 153 | 0.5 (49.5); n = 177 | –1.05 (–11.3 to 9.21) | 0.841 |
| Baselinec | 108.1 (71.9); n = 98 | 98.6 (61.1); n = 115 | ||
| 12 months | 99.4 (72.8); n = 98 | 87.6 (62.3); n = 115 | ||
| 12 months – baseline | –8.7 (53.0); n = 98 | –11.0 (48.5); n = 115 | –0.55 (–13.9 to 12.8) | 0.935 |
| PDQ-39 (0–100, higher values worse) | ||||
| Baselineb | 27.4 (14.3); n = 126 | 28.7 (15.9); n = 153 | ||
| 6 months | 28.3 (15.0); n = 126 | 29.5 (16.5); n = 153 | ||
| 6 months – baseline | 0.8 (8.3); n = 126 | 0.9 (9.0); n = 153 | 0.12 (–2.0 to 2.28) | 0.911 |
| Baselinec | 27.2 (13.6); n = 77 | 28.9 (15.9); n = 100 | ||
| 12 months | 29.1 (15.4); n = 77 | 31.7 (15.5); n = 100 | ||
| 12 months – baseline | 1.9 (8.6); n = 77 | 2.8 (11.2); n = 100 | 0.48 (–2.53 to 3.49) | 0.754 |
| GDS (0–15, higher values worse) | ||||
| Baselineb | 7.7 (2.3); n = 154 | 7.7 (2.1); n = 183 | ||
| 6 months | 7.8 (2.5); n = 154 | 8.0 (2.5); n = 183 | ||
| 6 months – baseline | 0.3 (1.8); n = 154 | 0.2 (1.9); n = 183 | –0.02 (–0.42 to 0.39) | 0.942 |
| Baselinec | 7.7 (2.1); n = 96 | 8.0 (2.2); n = 118 | ||
| 12 months | 7.8 (2.5); n = 96 | 8.5 (2.4); n = 118 | ||
| 12 months – baseline | 0.2 (2.0); n = 96 | 0.4 (1.7); n = 118 | –0.21 (–0.72 to 0.31) | 0.421 |
Subgroup analysis
In the statistical analysis plan, a subgroup analysis excluding participants with severe disease by virtue of a MDS-UPDRS score of ≥ 59 or H&Y stage 4 was prespecified. These analyses yielded very similar results to those shown in Table 6 for the group as a whole.
In addition, subgroup analyses were prespecified among those with reported freezing of gait or not and among the cognitively impaired (i.e. MoCA score of ≤ 25) and not impaired (i.e. MoCA score of ≥ 26) at baseline. The results of these analyses, relating to the period 0–6-months following randomisation in the case of the falling outcomes, and to the 6-month home visit in the case of the MINI-BESTest and FES-I, are shown in Figure 7. There was a significant interaction between the PDSAFE intervention effect and freezing status (p = 0.025). Among those with Parkinson’s who experienced freezing, the OR of repeat falling was doubled in the PDSAFE group (2.04, 95% CI 1.03 to 2.70; p = 0.042) compared with the control group. With a p-value for interaction of 0.088, there was a trend of differential PDSAFE effect in relation to the rate of falling according to the prespecified MoCA subgroups, with a FRR of 1.19 (95% CI 0.88 to 1.61; p = 0.255) in the cognitively impaired subgroup (i.e. MoCA score of ≤ 25). Subgroup-specific effect sizes and p-values are given in Appendix 8 (Table 29) and Appendix 9 (Table 30) for the analyses of repeat falling and fall rates, respectively.
FIGURE 7.
Overall and subgroup analyses of falling and near-falling outcomes during 0–6 months and secondary outcomes at 6 months. a, Test for interaction of PDSAFE contrast differing across subgroups, controlled for site, age, gender, repeat falling or not in the year prior to screening, log number of falls in the year prior to screening, log rate of falling in the pre-randomisation falls collection period, H&Y scale stage and the outcome in question assessed at baseline. Adapted from Chivers Seymour et al. 49 © Author(s) [or their employer(s)] 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by the BMJ Publishing Group Ltd. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. NFRR = near-falls rate ratio.

The results of further subgroup analyses exploring the differential effects of PDSAFE on the basis of tertiles of MDS-UPDRS, MoCA and the retrospective falls question asked at screening are shown in Figure 7. A further prespecified subgroup analysis excluding the most severe participants according to MDS-UPDRS (≈10% were excluded) did not show statistically significant PDSAFE-to-control effects for falling outcomes. In an exploratory subgroup analysis, only the middle tertile showed a PDSAFE reduction in falls, whereas the most severe tertile showed an increase in falls rate. A similar pattern was found across the tertiles according to the retrospectively reported number of falls in the year prior to screening, with p-value for interaction of 0.050 and only the middle tertile showing a PDSAFE reduction in falls. The analysis of subgroups defined by tertiles of MoCA, MDS-UPDRS and the number of falls, retrospectively, reported in the year prior to screening were not specified in the protocol, but were carried out to explore further the prespecified subgroups in relation to MoCA, and, because Canning et al. 40 report prespecifying subgroup analysis in relation to falls history, the MDS-UPDRS and cognition.
The same subgroup analyses were also explored with respect to near-falls and other secondary outcomes, and results for near-falling, Mini-BESTest and the FES-I are also shown in Figure 7. The effect of PDSAFE did not differ significantly across subgroups for any of these three outcomes, with interaction p-values > 0.05; in general, the PDSAFE effect can be seen to be more consistent across the various parts of the participant group. Details of these analyses are shown in Appendices 8–12 (Tables 29–33).
Appendices 13 and 14 (Tables 34 and 35) repeat the subgroups analyses for the repeat falling and falls rate outcomes shown in Tables 6 and 7, but include corresponding analyses carried on a per-protocol basis. Although there are some differences (including changes in p-values around the cut-off point of 0.05), the overall picture remains similar to that of Figure 7. Appendices 15–17 (Tables 36–39) detail subgroup analyses for the MDS-UPDRS, PASE, PDQ-39 and GDS and these are displayed graphically in Appendix 18 (Figure 13).
Serious adverse events
As described in Chapter 2, Safety reporting, for this trial a SAE was defined as a death, a life-threatening event or a new disability leading to prolonged hospitalisation, attributed to the trial intervention. No SAEs were reported in this trial. From the CONSORT flow diagram (see Figure 6), it can be seen that, in total, three participants died in the PDSAFE group and five in the control group, from causes unrelated to the trial protocol.
Information on hospitalisations was self-reported by participants during the baseline, 3-month, 6-month and 12-month home visits. At each visit, participants were asked about any hospitalisation that occurred since the previous visit and its duration. In the first 6 months following randomisation, nine PDSAFE and 20 control group participants reported hospitalisations; of these, one PDSAFE participant reported two stays. During the 6- to 12-month follow-up period, 18 PDSAFE and 21 control group participants reported hospitalisations; of these, two PDSAFE and four control group participants reported two stays.
Information on fractures was obtained from a variety of sources, including fall-specific information associated with the falls diaries and from self-reported hospitalisations. In the first 6 months following randomisation, five fractures were reported by PDSAFE participants and nine by control group participants; during the period 6–12 months post randomisation, PDSAFE participants reported seven fractures and control group participants reported three fractures.
Chapter 5 Qualitative process evaluation: part 1
The next two chapters describe the two-stage qualitative process evaluation. This chapter explores the expectations and experiences of participants in the PDSAFE intervention in a longitudinal qualitative study.
Aims
-
To explore the expectations of people with Parkinson’s about the intervention.
-
To explore the experiences of people with Parkinson’s about participation in the intervention, including its perceived impact.
-
To gain insight into the barriers to and facilitators of participating in the intervention.
Qualitative methods
Design
A longitudinal qualitative study was conducted alongside the main PDSAFE RCT. The qualitative study drew on the principles of grounded theory50 as follows:
-
gathering rich, in-depth data through ‘intensive interviewing’
-
conducting detailed analyses, which become increasingly theoretical
-
using line-by-line coding to ensure a high level of familiarity with the data
-
writing reflective memoranda to assist with data interpretation
-
employing theoretical sampling.
Theoretical sampling refers to a strategy that ‘allows the researcher to generate theoretical insights by drawing on comparisons among samples of data’. 51 As per the theoretical sampling strategy, participants with a range of different characteristics were included, to confirm that the qualitative findings were broadly applicable to the variety of people with Parkinson’s included in the larger sample. Previous research has suggested that these key characteristics might affect participants’ experiences of the PDSAFE intervention.
Longitudinal qualitative studies are comparatively rare in health research, although they have been identified as particularly useful when exploring conditions of change such as might occur in those with long-term and progressive illnesses, as in this study. 52
Methods and data collection tools
Qualitative semistructured interviews were conducted to explore the experiences of participants in the intervention arm of the PDSAFE trial; they were chosen for their capacity to generate rich, in-depth data about the social realities of participants, in context. 53
Two qualitative interviews were carried out with people from a subset of those who participated in the PDSAFE intervention arm of the trial (see the following section). The initial interview took place after randomisation [time 1 (T1)], but prior to commencement of the intervention. It focused on the impact of Parkinson’s and the participants’ expectations of the intervention. The second interview was carried out 6 months later (T2), and elicited information about participation in the intervention arm of the trial (Table 12). Because the focus was on exploring participants’ experiences of the intervention, the control group were not included in the qualitative substudy.
| Content of interview guide | |
|---|---|
| T1 (after randomisation but prior to start of intervention) | T2 (6 months after initial interview) |
|
|
Initial interview schedules were informed by scoping the relevant literature, which was carried out by referring to study protocols for primary questions of interest. Interview schedules were developed iteratively, in collaboration with the main trial team and stakeholders (including trained physiotherapists, rehabilitation experts and qualitative researchers), other clinical and academic experts (i.e. two qualitative researchers, two social networks experts) and two expert patients. Both interview guides were piloted with expert patients, to ensure that the questions made sense, and revised as necessary. The qualitative Research Fellow employed on the PDSAFE trial completed all the interviews at T1 and T2.
Sample and recruitment
Recruitment for the qualitative study took place from only the first four trial sites (Southampton, Portsmouth, Bournemouth and Poole, and Exeter), so that the qualitative Research Fellow had sufficient time to carry out both sets of interviews and complete analysis.
Participants who were eligible for the PDSAFE trial (see Chapter 2) were invited to ‘opt in’ to qualitative interviews at the time of recruitment to the main trial. Only those participants randomised to the intervention arm were approached to participate.
Theoretical sampling was used to represent the views of participants with a range of characteristics, and included:
-
location (at least 10 people from each site)
-
age (at least two people aged < 80 years and two aged > 80 years in each site)
-
gender (a minimum of two men and two women per site)
-
severity of disease (at least one participant per site with disease severity of H&Y scale stages 1, 2 or 3/4)
-
number of falls [at least one person experiencing a single fall in the previous 12 months, one who had repeat falls (more than one fall and fewer than 10 falls in the last 12 months) and one who had multiple falls (more than 10 falls in the previous 12 months) per site]
-
time since diagnosis [at least one person per site who had been diagnosed for (1) ≤ 5 years, (2) 6–10 years, (3) 11–15 years and (4) ≥ 16 years]
-
living status (at least one person who lived alone per site).
Procedure
During the screening visit for the PDSAFE main trial, assessors asked participants if they would be interested in participating in the qualitative substudy, and left a qualitative study patient information sheet if participants expressed an interest. If a participant was randomised to the intervention arm, the qualitative Research Fellow telephoned them to answer any questions they had and to explore if they were still interested in participating in the qualitative substudy. Once their participation was confirmed, a convenient time for the first qualitative interview (before the treatment with the therapist commenced) was identified.
The Research Fellow then scheduled a second follow-up interview 6 months later. Just prior to the scheduled second interview, the researcher checked with the trial manager and administrator that the participant had not withdrawn from the trial for any reason and then telephoned the patient to confirm that the second scheduled interview was still convenient.
Ethics
Ethics approval and relevant NHS trust governance approvals to carry out this qualitative study were covered within the main trial approvals processes. All data were anonymised, and information about participants was kept separate from the data in locked filing cabinets. The qualitative Research Fellow did not contact potential participants until they had expressed an interest in taking part, and participants also had the opportunity to clarify any queries before confirming participation. Written informed consent was taken immediately before each interview.
Qualitative analysis
Interviews were transcribed verbatim and then checked for accuracy by a member of the research team who had carried out the data collection. Analysis of interview transcripts was carried out using inductive thematic analysis, in which dominant themes were identified through close examination of the data. 54,55 Interview transcripts were read and re-read to ensure a high level of familiarity with the data before line-by-line coding. A coding manual was then created to define emerging codes and themes for initial interviews at T1 and T2, and these codes were then applied to the remaining transcripts. The coding manual was developed iteratively and revised throughout the coding process to ensure that the codes adequately reflected the data. The coding manual was discussed and agreed by core members of the research team at various stages of the coding process.
All interviews were transcribed and analysed by the qualitative Research Fellow. In addition, two T1 and three T2 interview transcripts were independently coded by the senior investigator with qualitative expertise, and discussed with the Research Fellow to promote thorough, careful and reflexive practice in the development of the coding framework. 53
Findings
Participant characteristics
Forty-two participants were recruited at T1. Of these, 37 were interviewed at T2, although two of these participants had withdrawn from the main RCT. Consequently, 35 participants remained in the trial, and completed interviews at T1 and T2. Their demographic characteristics are shown in Table 13.
| Characteristic | Interview at T1 only (n = 42) | Interviewed at T1 and T2 and remained in trial and qualitative study (n = 35) | Interviewed at T1 and T2, but withdrawn from trial (n = 2) | Interviewed at T1 and T2 (n = 37) |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 24 (57) | 18 (51) | 2 (100) | 20 (54) |
| Female | 18 (43) | 17 (49) | 0 (0) | 17 (46) |
| Age range (years) | 57–84 | 57–84 | 68–79 | 57–84 |
| Age group (years), n (%) | ||||
| ≤ 59 | 2 (5) | 2 (6) | 0 (0) | 2 (5) |
| 60–69 | 15 (36) | 11 (31) | 1 (50) | 12 (32) |
| 70–79 | 20 (47) | 17 (49) | 1 (50) | 18 (49) |
| ≥ 80 | 5 (12) | 5 (14) | 0 (0) | 5 (14) |
| Mean age (years) | 71 | 71 | 70 | 71 |
| H&Y scale stage, n (%) | ||||
| 1 | 4 (10) | 4 (11) | 0 (0) | 4 (11) |
| 2 | 12 (28) | 10 (29) | 1 (50) | 10 (27) |
| 3 | 23 (55) | 20 (50) | 1 (50) | 21 (57) |
| 4 | 3 (7) | 1 (3) | 0 (0) | 2 (5) |
| Time since diagnosis (years), n (%) | ||||
| < 5 | 15 (36) | 15 (36) | 0 (0) | 14 (38) |
| 6–10 | 7 (17) | 7 (17) | 0 (0) | 5 (14) |
| 11–15 | 13 (31) | 13 (31) | 1 (50) | 12 (32) |
| ≥ 16 | 8 (19) | 8 (19) | 1 (50) | 6 (16) |
| Time since diagnosis (years) (range) | 1–35 | 1.5–21 | 13–35 | 1.5–25 |
| Mean time since diagnosis (years) | 10 | 9.2 | 24 | 10.4 |
| Falls in previous 12 months, n (%) | ||||
| 1 | 11 (26) | 10 (29) | 1 (50) | 11 (31) |
| 2–10 | 24 (57) | 19 (54) | 1 (50) | 20 (57) |
| > 10 | 3 (7) | 2 (6) | 0 (0) | 2 (6) |
| > 100 | 4 (10) | 4 (11) | 0 (0) | 4 (11) |
| Range of falls in the previous 12 months by groups | 1 to ≥ 200 | 1 to ≥ 200 | 1 to 4 | 1 to ≥ 200 |
| Mean number of falls in the previous 12 months | 32.5 | 37.5 | 2.5 | 35.7 |
| Living status, n (%) | ||||
| Lives alone | 13 (32) | 10 (29) | 2 (100) | 12 (33) |
| Living with partner | 27 (64) | 23 (66) | 0 (0) | 23 (62) |
| Living with relative | 1 (2) | 1 (2.5) | 0 (0) | 1 (2.5) |
| Living with carer | 1 (2) | 1 (2.5) | 0 (0) | 1 (2.5) |
Participant characteristics in the qualitative sample were comparable to the overall trial sample, with the exception of time since diagnosis (the mean was slightly higher in the qualitative sample), number of falls in the previous 12 months (again, the mean was slightly higher in the qualitative sample) and living status (with slightly more participants in the qualitative sample living alone). The higher numbers of people within the last two categories reflect the theoretical sampling, in which we deliberately sampled for these characteristics. All transcripts were included in the thematic analysis.
Findings from the thematic analysis
The transcripts were analysed in two tranches: first, those from the first set of interviews at T1, followed by the second set of interviews at T2.
For the first set of interviews, 13 main themes were generated, including 40 subthemes and 104 codes. The 13 main themes were:
-
Parkinson’s diagnosis
-
Parkinson’s symptoms and experience of Parkinson’s
-
reflections on the forthcoming PDSAFE intervention/therapy
-
previous experience of rehabilitation therapies
-
effect of Parkinson’s on day-to-day routines and life
-
effect of Parkinson’s on mobility
-
losses attributed to Parkinson’s
-
adaptation
-
falls and risk
-
well-being and Parkinson’s
-
other health and social issues
-
trial and study participation
-
importance of social networks.
The second interviews at T2 generated 17 main themes, which included 33 subthemes and 75 codes. The 17 main themes comprised:
-
activities
-
after the sessions ended
-
experience of the assessor sessions
-
barriers to the PDSAFE intervention
-
deteriorating mobility
-
equipment and technology
-
experience of physiotherapy and other exercise outside of PDSAFE
-
facilitators of continuing with the PDSAFE intervention
-
falls
-
impact and treatment of Parkinson’s condition
-
impact of health condition
-
impact of condition
-
impact of PDSAFE intervention
-
PDSAFE physiotherapy sessions
-
strategies
-
therapist input
-
other.
These themes and supporting data have been organised in the rest of this chapter to reflect the three main research aims for the qualitative study. The following four main themes will be discussed below:
-
expectations of participants about the PDSAFE intervention
-
experiences and perceived impact of the PDSAFE intervention for participants
-
barriers to participating in the PDSAFE intervention
-
facilitators of continuing with the PDSAFE intervention.
The following sections include verbatim extracts from interview transcripts. The interviewer was the qualitative Research Fellow.
Expectations of participants about the PDSAFE intervention
The first interview guide included a question enquiring about previous therapies, rehabilitation and experiences of exercise, in order to better understand participants’ expectations about the intervention. Almost all of the participants had experienced some form of physiotherapy in the past, although not necessarily in relation to their Parkinson’s. This previous experience of rehabilitation and exercises appeared to influence participants’ expectations about the intervention, with many anticipating that this would involve a programme of exercises:
Well, as I said, hopefully they will come up with a series of exercises that I can do, hopefully every day, or most days, which will keep me a bit mobile and stop me getting stiff. I dread the thought of being really really stiff and not being able to move and get around; I don’t want that to happen.
Participant with Parkinson’s (21/057)
(Throughout the report, the numbers at the end of the quotation sources indicate anonymised participant identifier/anonymised site identifier.) Some participants described in some detail what they expected, which included tailored exercises, stretching, freezing exercises and exercises for postural control, co-ordination, stiffness and muscle tone:
I’m hoping that they will give me some useful exercises that will deal with, well, the balance; now the question in my mind is whether they will go on from that and talk about also posture, which is something I desperately need and general muscle tone I suppose, so I’m sort of hoping that they will cover all three things.
Participant with Parkinson’s (43/017)
I anticipate that she will do things like check my balance and um, my mobility and coordination and give me exercises to help improve my balance.
Participant with Parkinson’s (54/040)
A few participants highlighted everyday tasks that they anticipated the programme might help with, such as getting dressed, and also wider benefits, such as improvement in confidence and helping to sustain social contacts:
Yes, so, that’s really why I put my name down [for participating in the trial], because I thought, well anything that can help me keep going as long as possible, because I have got one daughter in Dubai and one daughter in Germany, so, and no daughters in England [laughter] and they are not likely to be coming back, so ah, I want to keep going.
Participant with Parkinson’s (43/058)
Participants appeared to value the fact that the intervention was to be delivered at home, citing reasons such as convenience, problems travelling because of poor mobility and relief at not having to visit hospital or clinic sites, or park a car. One person highlighted that, although they were happy for the PDSAFE intervention to take place at home, they felt that appointments outside the home were important to fulfil the need to ‘broaden outlook’ on life.
Most participants talked about anticipated benefits of the PDSAFE intervention. These included general improved mobility, as well as enhanced functional and physical improvements, for example better postural control, stability, posture, co-ordination, muscle tone and pain control, help with FoG, maintenance or improvement of mobility and walking with more confidence:
Well, as I said, hopefully they will come up with a series of exercises that I can do, hopefully every day, or most days which will keep me a bit mobile and stop me getting stiff. I dread the thought of being really really stiff and not being able to move and get around; I don’t want that to happen.
Participant with Parkinson’s (21/057)
Is there anything that you are hoping that you might achieve from the therapy sessions?
Well, maybe that I can walk without, walk with more confidence possibly, and also I have tried using a stick before and I don’t like using a stick because I don’t think it helps your posture. My posture at the moment is pretty good and I’ve seen so many people with sticks, really bent over and I’d like to be taught how to use, if need be, a stick properly.
A few participants highlighted more general, functional tasks and a desire that partaking in the intervention would maintain or improve their general independence. The wish to help others by taking part in a trial was also another major motivator for participation in the study:
Anything that you are hoping it might help with?
Mainly co-ordination and manipulation of such things as my buttons. If I can remain independent so much the better.
OK, so is there anything you think it might help you achieve?
Helping others with Parkinson’s, if they can learn something about physio[therapy] and how it can help people, so that it helps others in the future.
OK, that’s important, anything you would like to achieve personally?
Cure Parkinson’s! I’m joking.
But it can’t do that, can it?! I hope it will help give me my independence back a bit, or some independence, some mobility, I miss my independence. Parkinson’s is so frustrating.
A small minority of people expressed feelings of anxiety or nervousness about the initial therapy sessions; some of this was related to perceived expectations about what it might involve and the intensity of the sessions. Others voiced that they had not previously had success with physiotherapy and so wondered how beneficial it might be:
I will be a bit nervous until I know quite the intensity of it, everybody’s a little bit aware you know that, I mean, what does intense mean?
Participant with Parkinson’s (21/049)
Perceived challenges or barriers to participation included concern about having a ‘bad day’ or an ‘off day’, including anxieties about medication:
I suppose one thing that might be worrying is if the physio[therapist] comes round to do some exercises and I am having an off spell ‘cause I have, you know, sometimes I’ve got a day that’s like that and it makes a difference; sometimes the drugs haven’t kicked in properly for whatever reason and it might be a down spell, an off spell, and I might sort of have to, you know, that might have to be taken into account.
Participant with Parkinson’s (21/039)
Some also had concerns about the time commitment, and expressed ambivalence about or dislike of physical activity:
I mean the thought of doing it every day for 6 months is a bit daunting and I said at the time, I am not absolutely sure I can promise that every day.
Participant with Parkinson’s (21/057)
And anything you’re not looking forward to at all?
Well, the, the further drain on my time and, you know, doing physical exercise is not my favourite thing.
At the time of the second interview, participants were asked if their expectations about the intervention had been fulfilled. The majority were not surprised by what they had been asked to do, and were pleased with the content of the PDSAFE intervention. Those few with unrealistic or inappropriate expectations acknowledged this at the second interview:
Did the physiotherapy meet your expectations, was it what you thought it would be?
Yes, I suppose generally there was not much else she could do really to make sure that everything I did was done correctly and that I understand what it was doing and why it helped.
I knew it would be personal to me but I didn’t expect it to be so personal, I know that sounds a bit ambiguous but she almost assessed how I was doing each time and geared it specifically to what I could and couldn’t do.
Participant with Parkinson’s (54/040)
A number of participants suggested that they had not fully appreciated the time and commitment the intervention programme would take, sometimes suggesting that the programme was onerous or a ‘bind’. This reflected anxieties and concerns from the first interviews about being able to find time in already busy schedules to complete the programme daily:
So it was not quite what you thought it was going to be . . .
No. It was alright in the winter when you, when I have got nothing to do, but when the vegetables started to come, it became a bit of a bind trying to find time to do it, and I wanted to pack it in.
The experiences and perceived impact of the PDSAFE intervention for participants
During follow-up interviews, participants were asked to describe the content of the PDSAFE intervention. All of the participants spoke about the exercises and strategies that they were encouraged to use. Most participants were able to describe these in detail, and described a range of exercises.
Most people found the level of the programme acceptable and usually recognised the progressive nature of the exercises over time:
They got harder [laughs] . . . The expectations were greater, um, for example I could just about put one foot in front of the other and walk the line, like you do if you are drunk, if I did it quickly; so then the next stage was to do it slowly and it is much more difficult and so it was graded.
Participant with Parkinson’s (43/058)
Participants also described a variety of strategies that they had been taught as part of the intervention, including swaying from side to side to promote motion, not cutting corners, walking with one foot in front of the other, using wider steps so as not to shuffle, having feet further apart to prevent falling and picking up feet.
Did the physio[therapist] give you any sort of strategies to use or hints?
Well, if I ground to a halt, because I have been freezing a little bit, just to psych meself up and to count from one to four and then I could go [i.e. move] on the four.
Most participants commented on the expectation that they would engage with the programme daily, or as close to this as possible, and tried to conform to this, although this appeared to be challenging for some, particularly if they had other commitments on the same day:
The idea is to do the exercises every day, she said, if you could do them three or four times a week that would be sufficient, not ideal, but it would be sufficient, so I did try.
Participant with Parkinson’s (21/057)
The PDSAFE intervention involved the use of a range of equipment, including a weighted vest or jacket, the step, the exercise folder and log, a metronome and/or a DVD. Some recognised that the intensity of the exercises increased with the use of equipment:
I would do the exercise, I’d be out the garden doing it out there during the summer and using the step for the legs . . . there in the sunshine, and then I got a weighted jacket, which was harder, more challenging.
Participant with Parkinson’s (21/039)
Experiences of using the equipment were mixed, with many participants commenting particularly on the weighted vest:
I was doing stepping on that [i.e. the step] and that was fine, um, and then she put little feet on it to make it harder, but every time she came, she made it a little bit more difficult and the real shock was when she came with the weighted vest. Have you seen the weighted vest? It’s a torture chamber, honestly, so I picked it up and I said ‘Am I supposed to be wearing this? . . . I can hardly lift it’ and it actually weighed 14 pounds and I had to really sort of struggle to get it on and then do it up and I had to do the stepping with this vest on. Well, I couldn’t believe it, I said ‘Are you sure this is doing me good?’ and she said ‘Yes, [laughter] it definitely is’ and then after I had done that for a few weeks she wanted to put some more weights in it. I said ‘Look I am sorry, but this is really as much as I can cope with’.
Participant with Parkinson’s (21/057)
Although views about use of the equipment were mixed, a small number of participants were disappointed that the vests, steps and folders had to be returned once the physiotherapy visits ceased.
Several people mentioned that the filming and resulting DVD helped them to understand issues with their gait or posture:
There was one of me, my posture, which was, I did not mean to be alarmed because I knew my posture was awful. I did try, as it is one of the things she has helped me with quite a lot actually is the posture and, erm, I suppose it has improved a bit. Well anyway, the DVD was helpful in seeing how awful it was.
Participant with Parkinson’s (21/026)
However, more commonly, participants disliked, or experienced problems with the technology. Some felt that the DVD/metronome was unnecessary or did not help them, some did not use it and some preferred to do exercises to counting or using the beat of music. Some participants described technical problems with the DVD or would have preferred to see the programme on a portable device such as a tablet.
Follow-up interviews also explored the perceived impact and benefits of the PDSAFE intervention for those taking part. The majority of participants felt that they had made some progress in terms of mobility (including improved posture, postural control, functional strength, walking better, falling less frequently and/or enhanced control of freezing symptoms). Some also described improvements in functional activities:
[Laughs] I thought ‘what have I let myself in for?’ initially, but once you start doing the exercises and you know you can do them, you know, like putting my socks on was very difficult and that’s better now, stretching, that helped a lot.
Participant with Parkinson’s (54/074)
In follow-up interviews, over one-third of participants reflected that either their falls had reduced or they were better able to recognise falls triggers since embarking on the PDSAFE intervention:
I think they [i.e. falls] are less frequent because I am thinking in my mind all the time, walk sensibly, open this door sensibly, do this sensibly, because otherwise you are going to fall.
Participant with Parkinson’s (32/014)
Other perceived benefits of the PDSAFE intervention included increased awareness of limitations, for example recognising the need to plan things carefully, increased awareness of safety and recognising poor posture. Linked to this was enhanced recognition of the need to ‘slow down’ and ‘not rush(ing) around’:
I think the most useful thing, probably, is that I have had to learn [pause], to slow down, . . . I mean before I would just dash everywhere, not run I don’t mean, but you know just sort of walk and then I would get my feet in a muddle and you know all that sort of stuff and I think that is probably the thing I have learned most, to control that so that, you know, if I am in a hurry I can still function without fear of tripping.
Participant with Parkinson’s (43/050)
Around half the participants interviewed at T2 also spoke of increased confidence, independence and general well-being:
It made you feel you could do it independent. It was a good help.
Participant with Parkinson’s (32/054)
I feel more confident.
Participant with Parkinson’s (43/027)
Although most participants interviewed at follow-up perceived some improvements, physically, functionally, in falls reduction or more generally (as described above), a small number felt that they had not experienced any benefit from participation in the PDSAFE intervention. Several of these participants simply questioned whether or not the intervention could make any difference to them in the face of a deteriorating condition, even if they felt that the programme was, in itself, good.
Barriers to participating in the PDSAFE intervention
During the follow-up interviews at T2, participants were asked for their views about the barriers to and facilitators of taking part in the PDSAFE intervention. The time commitment to complete the exercise programme was identified as one of the major barriers to continued participation, particularly towards the end of the programme and beyond. Participants often described the programme as being fairly time-consuming or requiring a big commitment. Although for some this was not an problem, others found this challenging, particularly if they already led busy lives, which seemed to be a particular issue for those whose Parkinson’s was not so advanced:
There was nothing difficult about it; the only difficulty about it was me staying on target with the exercises between the visits. I found, because I lead a fairly active life anyhow, I couldn’t always find the time to get to do the regular exercises.
Participant with Parkinson’s (43/055)
A lack of motivation was also commonly offered as the reason for giving up the exercise programme, particularly when visits from the treating physiotherapist became less frequent or stopped altogether:
It would be trying to do it every day [is the most difficult part]. But that day, I know that if I do it I would be better and more flexible and more co-ordinated but it is just motivating myself to do it sometimes. It’s the hardest, that’s . . . it’s the hardest thing to do.
Participant with Parkinson’s (54/040)
However, not all participants ceased the programme at the end of the visits from the physiotherapists (see the following section). Another common barrier alluded to previously, was use of the programme equipment or technology. The two items most often mentioned as problematic were the weighted vest and the DVD with metronome.
For some people, illness, injury and other life events, such as a family death, prevented them from participating fully or at all in the programme. Illnesses and injuries mentioned during the interviews as barriers included problems with knee and shoulder, heart attack, shingles, pain, cancer and high blood pressure. Difficult life events included bereavements and family illness:
I had one week off in March when I was away for the week and then in the beginning of June my husband died and that threw us all out completely.
Participant with Parkinson’s (32/014)
Participants also described their experience of ‘off’ or ‘bad’ days as another barrier. These days were characterised by difficulty in engaging with the programme, particularly at times when medication was not optimised, participants felt particularly ill or symptoms were at their worst:
And sometimes, you know, with Parkinson’s you have your off periods where your drugs haven’t kicked in and there are times when I am having an off evening and I try and do my exercises, but the off spell seemed to be longer than normal, so I might have to miss it then, so it’s just choosing the time of day.
Participant with Parkinson’s (21/039)
Finally, a couple of barriers mentioned by just a few people included fear and forgetting to engage with the programme.
Facilitators of continuing with the PDSAFE intervention
In the same way that a reduction in and eventual cessation of visits from the treating physiotherapist acted as a barrier to continued participation in the programme, the knowledge that the physiotherapist would be visiting again acted as a facilitator of participation for participants. Interviewees commented that they tried harder when they knew that the physiotherapist would be returning and expressed the view that they did not want to disappoint her. Some mentioned the importance of reinforcement for their efforts provided by the therapist, and looked forward to her visits. A few suggested that they engaged in the exercises only when the therapist was present. Even the occasional participant who did not feel that they gained a lot from the programme appeared to value the therapist’s presence:
Also, having the physiotherapist visiting regularly, that gives you something to aim for, like, when you know that she is coming next week and you want to make sure that everything is up to date and that you are doing the exercises properly, so having that sort of check and balance if you like helps to keep . . . and it ran very smoothly.
Carer (21/056)
Support and encouragement from one’s partner, spouse or carer was another important facilitator. This could take several forms, for example the partner or carer completing the exercises alongside the participant, or reminding the participant to complete them:
And it was nice as well because she included me, it was really nice because obviously I would do the exercises with him, but she didn’t make me feel that I shouldn’t be there and she included me, which I thought was really nice. I thought at first did she just want me to go out of the way and think I was interfering, but no she was really good. Obviously, I like to know what is happening, because when I do them with him I would like to know what she is doing. That is quite good.
Spouse (43/059)
Another facilitator mentioned by participants was the written information about the PDSAFE programme provided in a blue folder to those taking part in the intervention arm of the trial:
Well, on every session, whenever she came, we started straight away with the list of exercises which she gave me, which she showed me . . . I had a list, a chart that she gave me, so I could tick off each one as I did it.
Participant with Parkinson’s (43/050)
For some, particularly those with already busy lives, the intervention was easier to engage with if integrated into their daily lives. Participants found novel ways of building the exercises into their daily routines, for example doing them while completing chores, gardening or while in the kitchen. Others would integrate exercises, for example walking or stepping exercises, into leisure activities such as dog walking, playing golf or simply walking into town, or adapted exercises to their environment:
I did them in the morning. I did them while the kettle was boiling. Once you knew what to do it was quite simplistic so I would do my marching you know, and then I would go and put the kettle on and I would do this bit and that and by the time I went out in the morning it had all been done.
Participant with Parkinson’s (43/058)
Other facilitators of the PDSAFE intervention included seeing direct improvements or feeling a sense of progress.
Chapter 6 Qualitative process evaluation: part 2
This second qualitative process evaluation chapter reports on the expectations and experiences of the physiotherapists who delivered the PDSAFE intervention, which were explored using a series of individual interviews.
Overview
The second stage of the qualitative process evaluations involved conducting in-depth, semistructured interviews with a group of the treating therapists. Six of the treating therapists (not those trained to provide cover) were interviewed by an independent researcher. Data were managed and analysed using framework analysis56 and five themes emerged:
-
views on the PDSAFE concept and therapist involvement
-
benefits and limitations of the PDSAFE intervention
-
influences on intervention success
-
perceptions of patients’ experiences and engagement
-
PDSAFE intervention usability and transferability.
Theme 1: views on the PDSAFE concept and therapist involvement
This theme describes therapists’ thoughts on the concept of PDSAFE, their involvement in delivering the programme and the challenges they faced.
Views on the PDSAFE concept and study involvement
On a conceptual basis, therapists responded positively towards PDSAFE; the intervention was described as being holistic, comprehensive and inherently relevant to an individual’s problems and environment. The approach focusing on strategies to enable functional activity was seen as differing from the prevalent approach used in usual practice/the NHS, which was described as being exercise driven:
I thought it was very good, it takes a slightly different tack than a traditional NHS assessment because it’s sort of strategy driven rather than exercise driven so it’s potentially looking at a person at a functional level, trying to kind of really drill down when they are falling and why they are falling but at a much more of a global level and trying to really focus in on that, not address that problem through the provision of exercises but more actually looking at practising functional activities that are linking in with the falls.
Therapist 6
Having PDSAFE in the individual’s environment, ‘where the falls happen’, was seen by all the therapists as the ideal. Being in the person’s home, setting their environment and situation was considered to give much better insight into how to tailor treatment:
It had relevance to them, [. . .] and their problems, they demonstrated their problems by when you look at them and you map the house and you have a really good look at them. So the relevance for them is excellent, so they know that this is their problem and you start working towards trying to solve that problem or a couple of problems that they do have with stuff that is functional, that they need to do for their everyday lives.
Therapist 1
All therapists reported enjoying the experience. One therapist commented that the PDSAFE approach was so different that working with it was initially difficult. Another therapist noted at times feeling constrained/unsupported when patients were unwell and the links back to clinical services seemed less apparent:
I suppose initially actually not approaching it from a training base but approaching it from a strategy training in a functional side was quite hard to embrace to start with but now I’ve got the hang of it, I absolutely love it and I think it’s a really ingenuitive different way of actually looking at how therapists approach the treatments to their patients. I’ve really, really enjoyed it.
Therapist 3
Intervention challenges
There was a consensus that working with patients who had cognitive issues and/or dyskinesia was the most challenging aspect of the delivery of the PDSAFE intervention. This group were noted as challenging to support in usual practice, and one therapist noted needing to seek support during the intervention from a colleague to help their patient progress. This difficulty was attributed to patients with cognitive issues being unable to retain an understanding of the PDSAFE structure, rationale and content covered. Some therapists felt that this group required different support to PDSAFE. Others consider PDSAFE to be capable of helping this group if they had support from a significant other or carer:
We had a fair few that did pass the cognitive screens to be part of the trial but then it is very different to pass a screen like that and then actually be able to participate in the trial, [. . .] It was fine if they had a partner or someone that they were living with to support them, but if they didn’t, that was really hard and we had to use so many different strategies and took lots of our time to try and get them on board and get them familiar with the programme and sometimes it still wouldn’t really work.
Therapist 5
Promoting exercises was seen as more challenging among patients who were unused to exercise/deconditioned, were less physically able and were unwilling to change their routines/habits. There was also comment that many patients struggled to grasp how to progress their exercises:
I think it’s very easy to follow the sort of structured exercises and things but that carry-over into function is the biggest, I found the biggest hurdle and was starting to say to patients at the start of my treatment. I said you know there’s gonna be exercises which support the use of a strategy and the crux of it for you I felt was to have that transferred into function and you know and that’s where the real hard work is for you is to try and change habits, things that you do on a daily basis without really thinking. So I try and sort of prep them, yeah, there was resistance to that I think.
Therapist 2
More individually raised challenges included working with patients who had overly protective spouses/partners and fitting the required number of sessions in around seasonable periods/patients’ busy schedules:
With the higher-level people, only that they had busy lives and getting a schedule to fit in. [. . .] and several of them had really active social lives and travelled and went away and went to rugby matches and so fitting in all the appointments at the appropriate times was difficult.
Therapist 1
Theme 2: benefits and limitations of the PDSAFE intervention
This theme outlines the benefits and limitations attributed to the PDSAFE intervention by therapists.
Ingredients of intervention success
Most therapists associated having minimal/no cognitive difficulties as a key element for intervention success. Cognitive issues were explained as potentially reducing motivation (owing to reduced understanding of intervention intent) and preventing carryover from treatment:
I think the whole thing worked very well as long as they had the cognitive capacity to understand how it all knitted together, which aided their motivation.
Therapist 4
This issue then limited a patient’s ability to progress. However, the belief that cognitive acuity was important for intervention effectiveness was not universal; Therapist 1 did not feel that any group benefited more than any other and reported that all of her patients progressed, including those with cognitive issues:
Everybody progressed, everybody really enjoyed it, all the people I had, even the ones with cognitive impairment were very compliant [. . .] whoever benefits are the ones that are motivated to exercise and I have to say that every participant I saw, mine and (two other therapists) were all motivated. I can’t say that anyone benefited more than anybody else.
Therapist 1
Those described as ‘motivated’ to exercise and to integrate PDSAFE into their routines were thought more likely to derive benefit. Those with mild to moderate Parkinson’s, who were functionally independent, were seen by some as ideal candidates in terms of potential to benefit. Finally, being concerned over one’s personal risk of falls was discussed explicitly by one therapist as important for facilitating the required lifestyle changes:
I think people who have had a significant enough experience of a fall to see why they would want to work on it, so I think sometimes when patients maybe in the trial had had one fall a very long time ago or whose symptoms were so mild that they couldn’t really engage so much with changing their daily habits to prevent them falling, the problem wasn’t really significant enough I suppose.
Therapist 2
Factors associated with reducing intervention effectiveness
In line with therapist comments on the limitations of the PDSAFE intervention, cognitive impairment and/or dyskinesia were noted as limiting intervention success:
There were issues regarding patients with cognitive impairment which made it that much more difficult. [. . .] Patients with dyskinesia which made it again very difficult for anyone who wasn’t really used to cope with some of those issues but I think for the . . . mainly for the person who isn’t that severely affected, I think it had a better outcome.
Therapist 4
One therapist commented that PDSAFE could then become a burden to the patients who, on being given a programme to work on, could not obtain sufficient benefit. Support was noted as a key issue, particularly for those with cognitive problems; patients with no support reportedly struggled to complete all exercises and, as a result, could only partially engage in PDSAFE. The time since a patient’s last fall/the significance of the last fall to the patient was suggested as influencing intervention effectiveness. As PDSAFE was recognised as requiring changes to routines, some therapists commented that, if falling was not a big concern, patients may be less likely to alter these routines.
The extent to which a patient felt able to cope with new commitments was also identified as influential; those with multiple conditions and accompanying appointments, or busy lives generally, were noted as being more likely to withdraw from the treatment because of an inability to take on the substantial commitment posed by PDSAFE:
Where people haven’t progressed is where you’ve got some underlying cognition problems, whether they forget the appointments, where you turn up and they are out and where they just don’t get the intensity, you can’t build them up to that intensity and they have to commit like 6 days or up to 6 days, 5–6 days they have to be doing this for half an hour, so that is quite a commitment to them and so you need to be motivated, you need to be knowing what you’re doing, why you’re doing it and what benefit you are going to get from it and if you haven’t got that type of person, it’s a hard slog.
Therapist 6
Those patients that are so severe that they’ve got so much going on in their life and they’ve got different appointments to attend, they’ve got sort of other illnesses, they’re so tired, they are falling quite frequently and for want of a better phrase ‘in a pickle’ basically. The patients who have got so much on, the idea of doing these, you know, looking at this blue folder and using the blue folder to do anything extra on top of everything else that is going on is just too much for them really. It was quite a struggle and you would go and you would feel like it was just one thing too many at that time.
Therapist 2
Other factors reported as reducing intervention effectiveness included the following:
-
patient attitude/motivation (patients who embraced illness and inactivity were seen as less likely to engage/benefit from PDSAFE)
-
physical ability (e.g. those with walking aids participated at a lower level, which limited the benefit they could achieve)
-
medication (side effects in some in cases hindered participation).
Theme 3: influences on intervention success
This theme highlights the factors and patient characteristics perceived to improve or reduce the likely success of PDSAFE at an individual level.
Strengths associated with the PDSAFE intervention
Several strengths were attributed to the PDSAFE intervention. The focus of PDSAFE on an individual’s functional problems and issues, in their own environment, was seen as a core strength of the programme. One therapist overtly related this patient-centred focus and approach to building patients’ confidence in their functional abilities:
It boosts a lot of people’s confidence, especially the fact that you can take them anywhere, take them outside, [. . .] I did get someone on the beach [. . .] she said to me that her friends had commented periodically, how much better her gait pattern was and how much more confident she was and she felt more confident.
Therapist 1
A number of therapists commented on how helpful the PDSAFE programme structure was not only for them, but also for their patients. This structure was described as being extremely clear and different from usual practice in that there was no ‘wait and see’ element:
The fact that you had very, very clear guidelines to work towards and I thought it was a clear and easy-to-follow intervention programme, [. . .] you had a clear progression and a clear way to lead but you also had a huge amount as a therapist of decision-making yourself and using your clinical reasoning to come to the decisions of what strategy you chose or exercises you chose, so again, it didn’t dumb you down.
Therapist 3
The opportunity afforded by PDSAFE to reinforce functional strategies was reportedly appreciated by therapists. The range of strategies available was also considered to be particularly good; this was seen as giving therapists the ability to respond flexibly and creatively to their patients’ individual needs:
It was a good variety and, from a strategy point of view, you could really open it up and could treat in many different sorts of ways out and about and in their own home and doing anything that they find particularly difficult. So it was quite open at that stage.
Therapist 6
Limitations associated with the PDSAFE intervention
Therapists described a number of limitations associated with the PDSAFE intervention. The three most commonly discussed related to the following:
-
PDSAFE was seen by a number of therapists as less suited to patients with cognitive issues and/or dyskinesia.
-
Some therapists reported that progressions posed ceiling and/or floor effects on exercises, namely exercises could not be made simpler than the lowest-level exercise (even though in usual practice it could be adapted) and, similarly, exercises could not be made more challenging than the uppermost exercise progression.
-
Therapists also reported that being unable to deviate from the set exercise positions inhibited their freedom to clinically reason and adapt the exercise to suit an individual, for example if a patient had a comorbidity, such as knee arthritis, that prevented them from adopting certain positions:
I probably would have treated them in a different position, so somebody for example, if they are, if they’ve got lots of arthritis or if they’ve got any other comorbidities, it’s difficult to then start loading joints if they are a bit arthritic in order to then strengthen. Previously, I would have then maybe looked at strengthening up in lying or on their tummy so I would have done it slightly differently [. . .] rather than on a protocol. I could only, for example, strengthen people up in, say, standing and obviously if you’ve got an arthritic knee or hip, it’s actually quite difficult. So there were some limitations with it but from an exercise point of view.
Therapist 6
Consequently, therapists reported that some patients remained ‘stuck’ at a certain point in the programme. Both of these issues led to therapists suggesting ways to improve the PDSAFE intervention for future implementation (see Theme 5: PDSAFE intervention usability and transferability). Other limitations concerned (1) exercises becoming repetitive for some patients and (2) some progression (specifically involving the metronome) promoted dual tasking at the expense of movement quality:
I definitely think the exercises, I want to be a bit more sort of creative with the patient and I think sometimes they potentially started to find things quite repetitive (Interviewer: yeah, yeah) so I probably would want to involve other exercise ideas, maybe to make them a bit more fun as well. That’s not necessarily a PDSAFE criticism, it’s a general, a lot of exercises we described are often quite boring.
Therapist 2
Yeah I think the variety of exercises and the fact that there is a lower limit and an upper limit to what you can do so you can’t progress further than a certain point. You can’t strip the exercise back past a certain point as well, I suppose.
Therapist 2
Theme 4: perceptions of patients’ experiences and engagement
This theme describes therapist perceptions of patients’ experiences and their engagement with PDSAFE during the programme lifetime and beyond.
Perceptions of patient experience
Most therapists perceived that a majority of patients enjoyed the PDSAFE experience, reporting patients to be engaged and ‘on board with it’. However, one therapist noted occasions when patients had not seemed to enjoy the overall experience, for example when coexisting musculoskeletal conditions were exacerbated by PDSAFE activities or when patients were not open to changing their routines:
I think most people were on board with it [. . .] I don’t think I had any dropouts at all or not due to not wanting to do it. It was they were withdrawn because their diagnosis changed but most people were on board with the programme and wanted to do it and wanted to be part of the trial so that went well.
Therapist 5
There are a couple of patients who had musculoskeletal problems and had problems with pain who probably didn’t enjoy it as much just because they associated movement with discomfort.
Therapist 2
Therapists described a number of particular elements of PDSAFE that they believed patients to have most enjoyed. Three of the most commonly discussed of these were (1) the focus of the programme being on issues most relevant to the individual patient, (2) the clear structure attributed to the programme and (3) the intensity and level of input/contact with therapists to support activity. Other features reported as being positively received by patients related to the novel focus on strategy training, the chance to exercise, the opportunity to use equipment and the confidence regarding managing a fall and the resultant feeling of increased safety developed within the programme:
I think it’s having somebody supervise them, I think it’s somebody coming in and they are not being left with a piece of paper, doing a list of exercises, they are actually having somebody come in and teach them something that they can relate to and for us it was falling but it might have also been getting up out of a chair, rolling over in bed, getting their food out of the boot of the car from shopping, you know.
Therapist 6
The intensity of the programme, that it was centred around them as an individual and the strategy training practice which was different to what they had received before.
Therapist 3
Aspects of the programme that were least enjoyed included (1) completing paperwork, for example falls diaries (although it is worth noting that these were a product of the research trial rather than the intervention itself), (2) reduced contact in later stages of the programme, 3) changing habits/the home environment and (4) exercise. Regarding falls diaries, some therapists attribute this to the tedium of completing paperwork whereas others felt that embarrassment was a factor. Regarding exercise, for some this was due to the difficulty of the exercises whereas for others, more physically able, exercises might become a bore:
It’s always about the documentation, the tick sheets – it’s always a recurring issue so we got to the 6 months’ point and the majority, the ones that had done well without any cognitive impairment, didn’t need the tick sheets, they think ‘I do this every day’ or ‘Monday, Wednesday, Friday is my days’, I’ve got a reference for the exercises and they were glad to see the back of that. Although it’s just a tick sheet and doesn’t take long, it becomes a bit of a nuisance doing it so that’s the thing that I found people moaned about more than anything else.
Therapist 4
I think they probably found it difficult when it went down to monthly and they were used to seeing you more regularly than that and I think probably filling in their forms, none of them liked filling in their fall forms [laughter] and I can understand why because you are focusing in on your . . . falling is . . . whenever I fall over, which thank god is not very often, but if I fall over in the street or trip over the dogs fairly frequently, it’s embarrassing, isn’t it? I really don’t want to dwell on that and I cover up my grazes and I kick my dog out of the way and I carry on my way that nobody sees. If you ask somebody constantly to write down their falls and reminisce about them, I think people found that difficult.
Therapist 1
Patient engagement in the PDSAFE intervention
Therapists perceived most patients to be motivated during the most intensely delivered points of PDSAFE. Patient engagement following intervention tapering was described as variable; some therapists perceived patient engagement to reduce when contact changed to monthly visits, others felt that their patients continued to engage through to programme completion. All therapists raised uncertainty and/or concern regarding the likelihood of their patients continuing the exercise programme. There was a feeling that some would continue, many would ‘ease off’ in terms of intensity/frequency of exercise and some would not continue. Although it was recognised that therapy contact could not continue indefinitely, it was considered that there was a need for some form of follow-on programme/group. Therapist reports of patient feedback support this also:
I did find that a few people, quite a few, lost motivation towards the end. So they started off quite motivated and then towards the end they would lose it so when I was seeing them at a less intense level, they definitely lost motivation and a few of them, I would say to them at the end, will you continue they would say – probably not just doing it themselves and be more likely to go to a group, some of them do tai chi and other exercise classes that are Parkinson’s related or non-Parkinson’s related and they would continue with that.
Therapist 5
They all said ‘is that it?’ so I think my feeling is that with exercise-based programmes you’ve just got to keep seeing people or move them on to something else.
Therapist 1
Theme 5: PDSAFE intervention usability and transferability
This theme discusses therapists’ perceptions of the usability of PDSAFE tools, the training they received, ways to enhance the original programme and its transferability to clinical practice.
Evaluations of therapist training
Training was highly praised, the knowledge of staff was commended and, for the most part, therapists reported feeling supported:
Absolutely fantastic, loved every second of it, comprehensive. I don’t think anything could train you for the completely different way, obviously [name] did the training and then we did the case studies to make us try and think strategy based and I think until you are actually out there and actually doing it, the training couldn’t have been any better and the early fidelity checks were paramount, I think.
Therapist 3
The one instance in which a therapist felt unsupported initially related to part-time working patterns and difficulty synchronising schedules to arrange a telephone conversation. The monthly group discussions and masterclasses were valued as very supportive, inspirational and good sources of continuing professional development. The peer support/double visits were particularly valued as learning and support opportunities. Some therapists noted that although the training in class was good, much learning occurred when implementing the programme, and peer support/double visits were vital to this. There were few negative comments; one therapist reported experiencing a large gap between receiving training and beginning the intervention, which resulted in the therapist struggling/feeling less confident when initially commencing treatment. Another comment was made regarding time spent working through paperwork (e.g. the treatment protocol, which detailed the treatment structure) during initial training, reducing time for practical learning:
The training was delivered and I then had a very long wait before I got my first participant. I can’t remember how long but I can’t remember if I’m honest, but it could have been over a month and I couldn’t . . . it was then difficult to really feel confident with delivering the protocol because you are new, you can’t totally remember, you read the paperwork and you read what’s given to you and you’ve got a reasonable idea but it’s all the conversations, it’s all the scenarios that was discussed so whether somebody could have come and did one . . . our first visit, a few of our first assessments, then that might have worked.
Therapist 6
Tool utility
Therapists commented on the utility of various tools, equipment and documentation used in the PDSAFE intervention. Some of these resources were praised by all participants as exceedingly useful. The use of one-to-one videos, whereby the therapist recorded a patient’s movement and then talked them through an assessment of this, was considered a very useful facility that was, for the most part, appreciated by a majority of patients. There were some therapists who noted some patients struggling to focus on the recorded movement, instead attending to their appearance:
The one thing I did find really useful was when you are doing a strategy with someone and you’ve got them doing a functional task and you video them and then give them immediate feedback, they can see themselves.
Therapist 1
The weighted jacket and the metronome received a more mixed review. The participants reportedly found it very difficult to put on the weighted jacket themselves; some patients found this a considerable struggle and required assistance. Concern was raised regarding being able to use the jacket if people did not have significant others/carers to help. The weighted jacket was also seen as (1) having potential to aggravate musculoskeletal issues and (2) being intimidating/off-putting, especially for those who were unused to exercise/weight training. It was not only patients, but sometimes also partners/spouses, who were intimidated by the jacket:
I would say there was an element of them trying to protect their partner a little bit and not want them to do too much, so things like the weighted vest, even if the participant themselves were quite keen on using it, their wives were not.
Therapist 5
However, some therapists reported that with practice donning the jacket became easier, and some patients responded well/enjoyed wearing it:
I think sometimes with the strengthening part of the programme they may have been quite daunted by the thought of wearing a vest, especially some of the females. The actual, the realisation that they could actually do far more than they ever thought that they were capable of that was really encouraging.
Therapist 4
The metronome was seen as being disliked by many patients because of the associated difficulty of dual tasking, although some therapists reported patients enjoying working with this. There was some suggestion that trying to maintain speed could reduce quality of movement (see Theme 2: benefits and limitations of the PDSAFE intervention).
The pre-recorded DVDs were unanimously reported to be used infrequently by patients. A number of therapists noted technical difficulties associated with this, and reasoned that the effort of setting up the DVD was not justified by the benefit patients derived from watching it. Another explanation was that some patients did not feel the need to remind themselves how to perform the exercises. Therapists noted being surprised by this finding.
Suggestions for improving the PDSAFE intervention
Increasing the number of exercises in the exercise library was a common suggestion/request, as was allowing more flexibility in modifying exercise progressions and postural sets to aid clinical reasoning and individual intervention tailoring. Other suggested improvements included the following:
-
Increase the choice of functional activities and strategies.
-
Allow a more flexible blue folder composition so that it can be simplified for people with cognitive issues. Remove old cards when new ones are issued to reduce confusion.
-
Emphasise video feedback one-to-one sessions with patients and therapists over DVD usage.
-
For exercises, consider having A4 laminated sheets with pictures and few words that can be put up on a patient’s wall.
-
Consider spreading the first two sessions over four sessions, as there is a lot to assimilate.
-
Make computer versions of paperwork more readily available for patients who struggle to complete forms in hardcopy.
-
Add patient demo to PDSAFE training to help therapists understand the flexibility of the programme and how things may be adapted if initial choices fail.
-
Potentially encourage more ‘double’/fidelity sessions and sooner in the programme as these are valuable learning opportunities.
Feasibility and desirability of integrating the PDSAFE in clinical practice
Therapists felt that PDSAFE could be delivered in the community. Although it was recognised that aspects of the programme would work in a hospital setting (e.g. exercises) and that group exercise particularly could be motivational, devoid of vital context, hospital delivery was still seen as a less desirable setting. The need for context in order for patients to learn strategies and attain carry-over was stressed by all. For some, delivering the programme in a hospital, divorced of this context, was possible but less optimal/effective, whereas others considered it infeasible.
There was recognition that PDSAFE, as delivered, amounted to double the therapy sessions usually delivered as part of routine care. This led to therapist uncertainty regarding whether or not PDSAFE would be funded adequately in the current climate; as a result, this issue was noted as being decisive when evaluating the feasibility of delivery of PDSAFE in clinical practice:
I guess it comes down to funding anyway, if the funding is there to deliver it as it is, then yes I imagine that it would be hard to get, just because of the time involved and it’s not just even the intensity of the programme, it’s all that extra stuff in terms of the admin[istration] and the [tele]phone calls to arrange the appointments and things and the yeah, whether it was put into a community service for people with Parkinson’s as a – you are seeing them anyway but we want you to deliver it like this rather than what you would normally do, then yes, I guess, but you would have to have that funding there.
Therapist 5
There was discussion regarding who should be involved in the delivery of PDSAFE; there was a feeling that a qualified physiotherapist would be needed to assess, develop the treatment programme and progress patients, but that therapy assistants could take on some sessions to check in and support patients when they were implementing their personal programmes.
Chapter 7 Economic evaluation
Overview
The cost-effectiveness analysis (CEA) of the PDSAFE physiotherapy intervention compared with usual care in people with Parkinson’s is presented in this chapter. Cost-effectiveness was estimated using a ‘within-trial’ analysis, based on health-care resource use and health-related quality of life (HRQoL) utility data collected over the 12-month trial period. Results are presented using an incremental cost-effectiveness ratio (ICER), estimated by dividing the difference in mean costs between arms by the difference in mean quality-adjusted life-years (QALYs) between arms. ICER estimates were compared with a £20,000- and £30,000-per-QALY threshold applied by the National Institute for Health and Care Excellence (NICE). 57
Methods
Resource use
In line with NICE economic evaluation recommendations, this CEA adopted an NHS and Personal Social Services (PSS) perspective. 58 Data for CEA were collected at baseline and at 3, 6 and 12 months. Costs were evaluated in Great British pounds using a 2016 base year. All analyses were undertaken according to the principle of ITT and in Stata/SE version 12.0. Costs included intervention costs (apportioned per participant) and NHS and social care resource use costs. Intervention costs were collected directly from trial records. For resource use costs, unit costs were obtained from national sources: the Personal Social Services Research Unit’s (PSSRU’s) Unit Costs of Health and Social Care59,60 and NHS Reference Costs 2015 to 2016. 61 Quantities were collected from the trial participants. The resource use questionnaire collected information on (1) primary care services provided in the NHS system [e.g. general practitioner (GP) visits, physiotherapy, occupational therapy], (2) secondary care services provided in the NHS system [e.g. ambulance call outs, accident and emergency (A&E) attendances, hospital stay] and (3) social care services (e.g. home care visits, meals on wheels). Medication use was collected separately. All resource use data were originally collected from participants by interview with an assessor. This was revised after identifying a risk of unblinding of assessors during the interview by the question asking participants how many times they saw a physiotherapist. Therefore, the whole section containing the number of times a physiotherapist was seen was then collected via postal questionnaire, which was completed by participants. Questions on fall-related A&E attendances and hospitalisation admissions were also embedded into the falls diaries for validity checks. Unit costs in the financial year 2015/16 published by the PSSRU59 and NHS Reference Costs 2015 to 2016,61 when information was not available from the PSSRU, were attached to each item of resource use. Table 14 shows the unit costs applied to each resource use item and Table 15 shows details of assumptions varied in each sensitivity analysis.
| Resource use item (unit used in the source) | Unit cost (£) | Source |
|---|---|---|
| NHS resource use | ||
| GP (per contact) | 36.00 | PSSRU 2015/16.59 Per-patient contact lasting 9.22 minutes, with qualifications |
| Practice nurse (per hour) | 43.00 (11.11 per contact) | |
| Parkinson’s nurse (per hour) | 75.00 (19.63 per contact after inflation)a | |
| Health visitor (per contact) | 54 (54.72 after inflation)a | PSSRU 2014/15.62 Cost per face-to-face contact in health visiting services |
| Social worker (per hour) | 79.00 | PSSRU 2015/16.59 Cost per hour of client-related work with qualification. Assume average contact is one hour |
| NHS physiotherapist (per contact) | 49.00 | NHS Reference Costs 2015 to 2016.61 Allied health professionals, physiotherapist, adult, one to one (A08A1). National average unit cost |
| Occupational therapist (per contact) | 79.00 | NHS Reference Costs 2015 to 2016.61 Allied health professionals, occupational therapist, adult, one to one (A06A1). National average unit cost |
| Speech or language therapist (per contact) | 88.00 | NHS Reference Costs 2015 to 2016.61 Allied health professionals, speech and language therapist, adult, one to one (A13A1). National average unit cost |
| A day case at hospital (per case) | 733.00 | NHS Reference Costs 2015 to 2016.61 Table 15: unit costs by point of delivery, 2013–14 to 2015–16. Unit costs per finished consultant episode (time a patient spends in the care of one consultant) for a day case |
| Hospital outpatient attendance (per attendance) | 117.00 | NHS Reference Costs 2015 to 2016.61 Table 15: unit costs by point of delivery, 2013/14 to 2015/16. Unit cost per outpatient attendance |
| A&E by ambulance (per incidence) | 236.00 | NHS Reference Costs 2015 to 2016.61 Table 15: costs by currency for ambulance services between 2013/14 and 2015/16. Unit cost per ‘see and treat and convey’ |
| Ambulance followed by emergency care only (i.e. paramedics, but not conveyed to hospital) (per incidence) | 181.00 | NHS Reference Costs 2015 to 2016.61 Table 15: costs by currency for ambulance services between 2013/14 and 2015/16. Unit cost per ‘see and treat or refer’ |
| A&E by own/public transport (per incidence) | 138.00 | NHS Reference Costs 2015 to 2016.61 Table 15: unit costs by point of delivery, 2013/14 to 2015/16. Unit cost per A&E attendance |
| Hospital stay for treatment (per day) | 373.00 | NHS Reference Costs 2015 to 2016 main schedule.61 Average of cost per elective and non-elective inpatient excess bed-days across all currency codes. Elective inpatient excess bed-days, average across all currency codes: £395. Non-elective inpatient excess bed-days, average across all currency codes: £351 |
| Respite care at hospital (per day) | 264.75 | NHS Reference Costs 2015 to 2016 main schedule.61 Estimated from the day cost for respite care with length of stay of 4 days or fewer (£1059/4 = £264.75) |
| Social care resource use | ||
| Home care/home help (per hour) | 30.75 (15.37 per contact) | PSSRU 2015/16.59 1.5 Home care for older people. Average standard hourly rate for services provided in-house. Assumed 30 minutes per contact |
| Meals on wheels (per time) | 3.00 | South Lanarkshire Council service, social care and health, meals at home (meals on wheels)64 |
| Day centre (per attendance) | 61.00 | PSSRU 2015/16.59 1.4 Local authority own-provision day care for older people. Unit cost per client attendance |
| Lunch club (per time) | 3.09 | Glasgow City Council, social care and health, adults and older people, a lunch club65 |
| Sitting service (per hour) | 21.00 | PSSRU 2015/16.59 6.12 Short-break provision for disabled children and their families. Unit cost per family per hour. Assumed 1 hour per contact |
| Night care (per hour) | 31.83 | PSSRU 2015/16.59 11.6 Home care worker. Applied price multipliers for unsocial hours: 1.035 for an independent sector home care hour. Assumed 1 hour per contact |
| Scenario | Element | Position in base-case analysis | Variation for the sensitivity analysis |
|---|---|---|---|
| 1 | Time horizon | 6 months, in line with the primary end point of the trial and the completion of all the therapist sessions | 12 months, with non-collected data at 12 months being imputed |
| 2 | Cost | 12 therapist sessions in total (12 sessions × 1 hour per session). Intervention cost per person = £649 | 10 actual sessions conducted. Intervention cost per person = £546 |
| 3 | Cost | 12 therapist sessions in total (12 sessions × 1 hour per session). Intervention cost per person = £649 | Total therapist sessions reduced to eight sessions (eight sessions × 1 hour per session). Intervention cost per person = £442.80 |
| 4 | Missing data | Missing data assumed to be missing at random and a mixed strategy of imputation was conducted | Complete-case analysis: missing data assumed to be missing completely at random |
| 5 | Routine visits of physiotherapist | NHS routine use of physiotherapists was included in the total NHS costs | Visits of physiotherapist between baseline and 6 months was not included as there might be bias in the collected data |
Cost of personalised home-based physiotherapy
The cost of implementing the PDSAFE intervention was collected from the trial co-ordinator and lead therapist. The analysis followed an ITT principle; therefore, only the participants allocated to the intervention arm were assumed to incur the cost of treatment and any research-only cost was excluded. This ensures the relevance of the cost estimates if the programme were to be ‘rolled out’ to larger numbers of people.
Therapy session costs included the personnel costs (i.e. salary) of the therapist, travel costs, equipment costs and consumables. The salary cost of the therapist was calculated based on NHS band 6 full-time equivalent (£41,005.91) taken from the 2016 Health Service pay scale. 66 A 2-day training course was carried out with the involved therapists to train them in delivering the home-based personalised physiotherapy intervention. Cost of training included time spent by the trainer (lead therapist), room hire and training materials. During the delivery of the therapy sessions, all participants were provided with printed materials and compact discs (CDs) of the intervention demonstration. In addition, a proportion of participants used assisted equipment including weighted vests, balance pads and steps. This equipment was kept by the physiotherapists and could be used with different participants. Annuitisation was carried out to spread the costs over the anticipated lifespan of this equipment.
Analysis
A cost–utility analysis of the PDSAFE physiotherapy intervention compared with usual care was conducted after imputation. Differences in costs and QALYs between the two groups were estimated using generalised linear models (GLMs), which take into account the typically skewed nature of cost and QALY data. Histograms revealed that the cost data were right-skewed; therefore, gamma distribution was used. For QALYs, as suggested in a previous National Institute for Health Research Health Technology Assessment report,67 decrement of QALY was originally predicted in the regression using a gamma distribution. Decrement of QALY was calculated as the difference between the maximum QALYs that could possibly be accrued within the time frame and the actual QALY gained. However, this analysis cannot proceed with the imputed data; therefore, a Gaussian family was used instead given that the distribution of QALYs was close to a normal distribution. Covariates in the GLM were selected based on statistical significance using regressions with complete cases from the full list of demographics, medical history and screening measures. The chosen covariates included in the model were age, gender, H&Y scale stage, MoCA score, MMSE score, presence of diabetes, history of myocardial infarction, history of ischaemic heart disease, history of deep-brain stimulation and whether or not the participant had an informal carer. Baseline utility score and baseline cost were also included to adjust for any imbalance of utility between groups. 68
Mean costs and QALYs for each group were estimated using the method of recycled predictions. The ICER was estimated from the difference in cost and QALYs from the GLM regression. A 1000-iteration bootstrapping was conducted to investigate the uncertainty surrounding the ICER estimate and the probability that the intervention was cost-effective under a wide range of hypothetical thresholds (£0–200,000). These results were represented in a cost-effectiveness plane and cost-effectiveness acceptability curve (CEAC), respectively.
Utility and quality-adjusted life-year
Effectiveness was expressed as QALYs. QALYs were estimated by the area-under-the-curve approach using the utility index values at each data collection time point, which were generated using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire, with preference weights from the UK general population. 69 EQ-5D-3L questionnaires were completed by participants during an interview conducted by assessors at baseline and at 3, 6 and 12 months. The EQ-5D-3L is a commonly used standardised generic preference-based quality of life (QoL) measure addressing five domains: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. 70 For each dimension, participants indicate the level of problems experienced on a three-level scale: (1) no problem, (2) some problems or (3) extreme problems. These responses were then converted to utility scores using the value set elicited from the UK general population. The EQ-5D-3L is the preferred measure of HRQoL in adults by NICE technology appraisal. 57 Participants who died had their utility score set to zero from the next assessment point after death. The area-under-the-curve method was then used to estimate the QALY score over the 12-month period, following the trapezium rule assuming a linear change in utility between each assessment time point.
Missing data and multiple imputation
Data were missing if participants did not return a questionnaire or returned an incomplete questionnaire. Multiple imputation was conducted at aggregate level for both cost and utility values to avoid convergence issues of the imputation model (the model becomes very statistically demanding when there are too many variables with missing data to predict, which usually leads to model convergence failure, meaning that the model cannot be executed). The EQ-5D-3L scores were imputed as utility values and cost items were imputed as categories, namely NHS cost excluding hospitalisation, hospitalisation cost and social care cost. To maximise the use of the completed data, a single item on resource use in both the interview and postal questionnaire was assumed to indicate that no resource was used for that item during that assessment period. 71 Hospitalisation data in falls diaries were checked and filled up the responses from the resource usage questionnaire if the response was missing. The aggregate-level missing data were imputed with multiple imputation with chained equations and predictive mean matching method for all imputed variables. The intervention and control groups were imputed separately. The multiple-imputed data sets were then used to estimate the difference in QALYs and costs, and the ICER.
Sensitivity analyses
A number of sensitivity analyses were undertaken to assess the robustness of the base-case results to alternative assumptions. These sensitivity analyses are summarised in Table 15.
The time horizon for the base-case analysis was 6 months, as all the participants completed their 6-month data collection and it was the primary end point. Nonetheless, for half of the participants, 12-month data were collected and the effect of intervention may last after the trial period; therefore, the time horizon was varied to 12 months. Sensitivity analyses were carried out on the number of physiotherapy sessions to gain an insight into the most cost-effective level of provision. Complete-case analysis was conducted assuming that data were missing completely at random to assess the impact of imputation strategies on the incremental cost and QALYs. Finally, the participants may have found it difficult to differentiate between the NHS routine physiotherapist and the trial physiotherapist; thus, the reported number of visits may not be accurate. Therefore, the use of an NHS physiotherapist between baseline and 6 months was excluded from the total cost in the sensitivity analysis.
Subgroup analysis
Following on from the statistical analysis plan outlined earlier, three subgroup analyses were undertaken to identify if the intervention was potentially more or less cost-effective than the overall group. The clinical effectiveness analysis found that the PDSAFE intervention reduced the number of falls in the participants who had scored in the middle level of the MDS-UPDRS (i.e. a score of 23–29), were not cognitively impaired (a score of ≥ 26 in the MoCA), did not have freezing symptoms at baseline and had two or three falls during the 12-month monitor period prior to recruitment. Therefore, the ICERs were estimated in the subgroups defined by the above criteria. Interaction variables of the treatment and the group indicator were added into the regression to obtain the incremental effects without splitting the sample. The incremental effects were generated using recycled prediction methods to balance the covariates.
Results
Intervention cost
The PDSAFE intervention costs are detailed in Table 16. The cost per patient for each of the breakdown items is shown in Figure 8. The cost of the intervention was a main cost driver that made up a large portion of the difference between treatment arms. Within the cost of intervention, the therapist time and travel expenses account for > 95% of the total cost; thus, assumptions were varied around the number of sessions.
| Item | Unit cost (£) | Quantity (n) | Sum (£) |
|---|---|---|---|
| Therapists training | |||
| Trainer (lead therapist) | 165 per day | 1.75 days | 288.75 |
| Room hire for 2 days | 150 per day | 2 days | 300.00 |
| Training materials | 3.50 per therapist | 14 therapists | 49.00 |
| Therapy sessions | |||
| Therapist time | 43.62 per visit | 238 participants × 12 | 124,578.72 |
| Consumables (clinical notes) | 1.20 per visit | 238 participants × 12 | 3427.20 |
| Travel expenses | 8.00 per visit | 238 participants × 12 | 22,848.00 |
| Patient equipment during the sessions | |||
| Printed materials | 2.00 per patient | 238 participants | 476.00 |
| CDs | 0.08 per patient | 238 participants | 19.04 |
| Weighted vests | 54.16 each | 23.8a | 1289.01 |
| Balance pads | 19.41 each | 59.5b | 1154.90 |
| Step | 11.99 each (6.31 after annuitisationc) | 15.87d | 100.14 |
| Total cost (per patient) (£) | 649.60 | ||
FIGURE 8.
Cost of PDSAFE home-based personalised physiotherapy intervention. CD, compact disc.

Missing data
The number and percentage of missing data for each collected cost item and EQ-5D-3L questions at the four assessment points are shown in Table 17 for the whole sample, and in Table 18 for the intervention and control (usual-care) groups, respectively. Baseline for the resource use refers to the 3-month monitor period prior to randomisation. Approximately half of the data were missing (primarily attributed to the termination of data collection) for the 12-month follow-up for both the resource use and the EQ-5D-3L data. For the 6-month follow-up, the proportion of data missing for variables collected via postal questionnaires (i.e. NHS resource use excluding hospitalisation) (≈28%) was almost twice that for variables collected through the face-to-face interview (≈15%). The proportions of data missing at each assessment were much higher (≈10% higher for all variables) in the PDSAFE intervention group than in the control group (see Table 18).
| Data items collected from the trial | Time point, n (%) | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| NHS resource use | ||||
| GP | 51 (10.76) | 95 (20.04) | 124 (26.16) | 238 (50.21) |
| Practice nurse | 53 (11.18) | 102 (21.52) | 130 (27.43) | 240 (50.63) |
| Parkinson’s nurse | 52 (10.97) | 98 (20.68) | 129 (27.22) | 241 (50.84) |
| Health visitor | 52 (10.97) | 98 (20.68) | 137 (28.90) | 245 (51.69) |
| Social worker | 52 (10.97) | 100 (21.10) | 138 (29.11) | 247 (52.11) |
| NHS physiotherapist | 52 (10.97) | 99 (20.89) | 136 (28.69) | 244 (51.48) |
| Occupational therapist | 51 (10.76) | 99 (20.89) | 140 (29.54) | 243 (51.27) |
| Speech therapist | 52 (10.97) | 102 (21.52) | 139 (29.32) | 245 (51.69) |
| Day hospital | 52 (10.97) | 96 (20.25) | 138 (29.11) | 245 (51.69) |
| Outpatient clinic | 51 (10.76) | 95 (20.04) | 131 (27.64) | 241 (50.84) |
| A&E by ambulance | 53 (11.18) | 100 (21.10) | 138 (29.11) | 244 (51.48) |
| Ambulance with paramedics only | 132 (27.85) | 124 (26.16) | 144 (30.38) | 243 (51.27) |
| A&E by own transport | 56 (11.81) | 103 (21.73) | 142 (29.96) | 251 (52.95) |
| Hospitalisation | 1 (0.21) | 45 (9.49) | 65 (13.71) | 209 (44.09) |
| Social care resource use | ||||
| Home care help | 13 (2.74) | 61 (12.87) | 79 (16.67) | 230 (48.52) |
| Meals on wheels | 1 (0.21) | 46 (9.70) | 70 (14.77) | 217 (45.78) |
| Day centre | 2 (0.42) | 47 (9.92) | 71 (14.98) | 217 (45.78) |
| Luncheon club | 3 (0.63) | 47 (9.92) | 73 (15.40) | 221 (46.62) |
| Sitting service | 2 (0.42) | 48 (10.13) | 71 (14.98) | 217 (45.78) |
| Night care | 1 (0.21) | 48 (10.13) | 70 (14.77) | 217 (45.78) |
| EQ-5D-3L | ||||
| Mobility | 1 (0.21) | 45 (9.49) | 71 (14.98) | 217 (45.78) |
| Self-care | 1 (0.21) | 45 (9.49) | 71 (14.98) | 217 (45.78) |
| Usual activities | 1 (0.21) | 47 (9.92) | 72 (15.19) | 217 (45.78) |
| Pain and discomfort | 1 (0.21) | 45 (9.49) | 71 (14.98) | 217 (45.78) |
| Anxiety and depression | 4 (0.84) | 46 (9.70) | 70 (14.77) | 217 (45.78) |
| Visual analogue scale | 9 (1.90) | 47 (9.92) | 73 (15.40) | 217 (45.78) |
| Data items collected from the trial | Trial group, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | |||||||
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| NHS resource use | ||||||||
| GP | 31 (13.03) | 59 (24.79) | 77 (32.35) | 126 (52.94) | 20 (8.47) | 36 (15.25) | 47 (19.92) | 112 (47.46) |
| Practice nurse | 31 (13.03) | 60 (25.21) | 79 (33.19) | 126 (52.94) | 22 (9.32) | 42 (17.80) | 51 (21.61) | 114 (48.31) |
| Parkinson’s nurse | 31 (13.03) | 60 (25.21) | 80 (33.61) | 127 (53.36) | 21 (8.90) | 38 (16.10) | 49 (20.76) | 114 (48.31) |
| Health visitor | 32 (13.45) | 58 (24.37) | 84 (35.29) | 130 (54.62) | 20 (8.47) | 40 (16.95) | 53 (22.46) | 115 (48.73) |
| Social worker | 32 (13.45) | 60 (25.21) | 84 (35.29) | 131 (55.04) | 20 (8.47) | 40 (16.95) | 54 (22.88) | 116 (49.15) |
| NHS physiotherapist | 30 (12.61) | 60 (25.21) | 82 (34.45) | 130 (54.62) | 22 (9.32) | 39 (16.53) | 54 (22.88) | 114 (48.31) |
| Occupational therapist | 31 (13.03) | 59 (24.79) | 85 (35.71) | 127 (53.36) | 20 (8.47) | 40 (16.95) | 55 (23.31) | 116 (49.15) |
| Speech therapist | 31 (13.03) | 61 (25.63) | 84 (35.29) | 131 (55.04) | 21 (8.90) | 41 (17.37) | 55 (23.31) | 114 (48.31) |
| Day hospital | 30 (12.61) | 58 (24.37) | 83 (34.87) | 131 (55.04) | 22 (9.32) | 38 (16.10) | 55 (23.31) | 114 (48.31) |
| Outpatient clinic | 31 (13.03) | 59 (24.79) | 79 (33.19) | 127 (53.36) | 20 (8.47) | 36 (15.25) | 52 (22.03) | 114 (48.31) |
| A&E by ambulance | 31 (13.03) | 60 (25.21) | 82 (34.45) | 130 (54.62) | 22 (9.32) | 40 (16.95) | 56 (23.73) | 114 (48.31) |
| Ambulance with paramedics only | 72 (30.25) | 68 (28.57) | 84 (35.29) | 129 (54.20) | 60 (25.42) | 56 (23.73) | 60 (25.42) | 114 (48.31) |
| A&E by own transport | 33 (13.87) | 60 (25.21) | 85 (35.71) | 134 (56.30) | 23 (9.75) | 43 (18.22) | 57 (24.15) | 117 (49.58) |
| Hospitalisation for treatment | 1 (0.42) | 27 (11.34) | 48 (20.17) | 117 (49.16) | 0 (0) | 18 (7.63) | 22 (9.32) | 99 (41.95) |
| Hospitalisation for respite care | 1 (0.42) | 27 (11.34) | 48 (20.17) | 117 (49.16) | 0 (0) | 18 (7.63) | 22 (9.32) | 100 (42.37) |
| Social care resource use | ||||||||
| Home care help | 8 (3.36) | 33 (13.87) | 51 (21.43) | 121 (50.84) | 5 (2.12) | 28 (11.86) | 28 (11.86) | 109 (46.19) |
| Meals on wheels | 1 (0.42) | 27 (11.34) | 48 (20.17) | 117 (49.16) | 0 (0) | 19 (8.05) | 22 (9.32) | 100 (42.37) |
| Day centre | 2 (0.84) | 28 (11.76) | 48 (20.17) | 117 (49.16) | 0 (0) | 19 (8.05) | 23 (9.75) | 100 (42.37) |
| Luncheon club | 3 (1.26) | 28 (11.76) | 48 (20.17) | 119 (50.00) | 0 (0) | 19 (8.05) | 25 (10.59) | 102 (43.22) |
| Sitting service | 1 (0.42) | 28 (11.76) | 49 (20.59) | 117 (49.16) | 1 (0.42) | 20 (8.47) | 22 (9.32) | 100 (42.37) |
| Night care | 1 (0.42) | 28 (11.76) | 48 (20.17) | 117 (49.16) | 0 (0) | 20 (8.47) | 22 (9.32) | 100 (42.37) |
| EQ-5D-3L | ||||||||
| Mobility | 1 (0.42) | 27 (11.34) | 49 (20.59) | 117 (49.16) | 0 (0) | 18 (7.63) | 22 (9.32) | 100 (42.37) |
| Self-care | 1 (0.42) | 27 (11.34) | 49 (20.59) | 117 (49.16) | 0 (0) | 18 (7.63) | 22 (9.32) | 100 (42.37) |
| Usual activities | 1 (0.42) | 27 (11.34) | 50 (21.01) | 117 (49.16) | 0 (0) | 20 (8.47) | 22 (9.32) | 100 (42.37) |
| Pain and discomfort | 1 (0.42) | 27 (11.34) | 48 (20.17) | 117 (49.16) | 0 (0) | 18 (7.63) | 23 (9.75) | 100 (42.37) |
| Anxiety and depression | 3 (1.26) | 28 (11.76) | 48 (20.17) | 117 (49.16) | 1 (0.42) | 18 (7.63) | 22 (9.32) | 100 (42.37) |
| Visual analogue scale | 6 (2.52) | 28 (11.76) | 50 (21.01) | 117 (49.16) | 3 (1.27) | 19 (8.05) | 23 (9.75) | 100 (42.37) |
Resource use and costs
Table 19 reports the use of each resource item (mean number of visits, SD, median, minimum and maximum) accrued over the 6-month primary follow-up period in the intervention and the control groups. The total costs of NHS service use excluding hospitalisation and of hospitalisation and of social care services are presented in Table 20 (before and after the imputation) and Figure 9. Overall, there was no difference between the intervention and control groups in the cost of NHS and social care service use. The total 6-month cost of service use per patient was £3137 (95% CI £2602 to £3673) in the intervention group and £3069 (95% £2621 to £3518) in the control group. Figure 9 shows that resource use was slightly higher in the intervention group than in the control group (but the difference was not statistically significant); however, the total baseline cost (i.e. from 3 months pre baseline to baseline) was also higher in the intervention group. The most commonly used NHS services were GP, NHS physiotherapist, practice nurse, Parkinson’s nurse and outpatient clinic, with at least one visit per patient over the 6-month follow-up period. The intervention group was found to have fewer GP visits, practice nurse visits and home care help visits; none of the differences was statistically significant. The most frequently used social service was home care, of which, over the 6-month follow-up period, each participant in the intervention group had an average of 13 visits and each participant in the control group had an average of 30 visits. Medication was not included in the total costs because of the large number of prescriptions identified, the frequent low cost of medications, identified comparability across arms and poorly reported data.
| Resource use (number of visits) | Trial group | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | ||||||||||
| n | Mean | SD | Median | Minimum, maximum | n | Mean | SD | Median | Minimum, maximum | ||
| NHS | |||||||||||
| GP | 144 | 2.72 | 2.47 | 2 | 0, 16 | 177 | 3.23 | 2.80 | 3 | 0, 15 | 0.09 |
| Practice nurse | 140 | 1.39 | 1.67 | 1 | 0, 9 | 170 | 2.09 | 4.67 | 1 | 0, 43 | 0.09 |
| Parkinson’s nurse | 138 | 1.21 | 1.45 | 1 | 0, 11 | 177 | 1.47 | 1.67 | 1 | 0, 11 | 0.15 |
| Health visitor | 138 | 0.11 | 0.54 | 0 | 0, 5 | 170 | 0.17 | 0.76 | 0 | 0, 8 | 0.42 |
| Social worker | 136 | 0.07 | 0.38 | 0 | 0, 3 | 170 | 0.17 | 0.72 | 0 | 0, 7 | 0.16 |
| NHS physiotherapist | 136 | 2.48 | 3.64 | 0 | 0, 16 | 170 | 1.34 | 2.42 | 0 | 0, 14 | 0.00 |
| Occupational therapist | 136 | 0.35 | 0.93 | 0 | 0, 6 | 169 | 0.50 | 1.44 | 0 | 0, 12 | 0.29 |
| Speech therapist | 135 | 0.89 | 2.09 | 0 | 0, 11 | 167 | 0.59 | 2.03 | 0 | 0, 21 | 0.22 |
| Day hospital | 137 | 0.93 | 1.89 | 0 | 0, 14 | 170 | 0.90 | 1.57 | 0 | 0, 10 | 0.89 |
| Outpatient clinic | 142 | 1.78 | 2.09 | 1 | 0, 13 | 173 | 1.78 | 1.79 | 1 | 0, 13 | 1.00 |
| A&E by ambulance | 137 | 0.14 | 0.42 | 0 | 0, 2 | 167 | 0.11 | 0.33 | 0 | 0, 2 | 0.48 |
| Ambulance with paramedics only | 131 | 0.26 | 0.83 | 0 | 0, 6 | 153 | 0.19 | 0.63 | 0 | 0, 5 | 0.42 |
| A&E by own transport | 135 | 0.16 | 0.45 | 0 | 0, 3 | 165 | 0.22 | 0.59 | 0 | 0, 4 | 0.31 |
| Hospitalisation for treatment | 187 | 0.30 | 2.51 | 0 | 0, 30 | 208 | 0.07 | 0.47 | 0 | 0, 5 | 0.19 |
| Hospitalisation for respite care | 187 | 0 | 0 | 0 | 0, 0 | 208 | 0 | 0 | 0 | 0, 0 | – |
| Social care | |||||||||||
| Home care/home help | 181 | 13.43 | 50.55 | 0 | 0, 390 | 192 | 29.99 | 103.30 | 0 | 0, 910 | 0.05 |
| Meals on wheels | 187 | 1.04 | 12.49 | 0 | 0, 169 | 207 | 1.00 | 12.77 | 0 | 0, 182 | 0.98 |
| Day centre | 186 | 0.49 | 4.35 | 0 | 0, 52 | 206 | 0.63 | 4.59 | 0 | 0, 52 | 0.75 |
| Luncheon club | 186 | 0.21 | 2.86 | 0 | 0, 39 | 204 | 0.51 | 6.43 | 0 | 0, 91 | 0.56 |
| Sitting service | 186 | 0.91 | 7.16 | 0 | 0, 65 | 206 | 1.33 | 7.75 | 0 | 0, 78 | 0.58 |
| Night care | 186 | 0.98 | 13.34 | 0 | 0, 182 | 206 | 0.06 | 0.91 | 0 | 0, 13 | 0.33 |
| Cost categories | Trial group (£) | p-value | |||||
|---|---|---|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | ||||||
| Unadjusted mean (SE) | 95% CI | Unadjusted mean (SE) | 95% CI | OLS | Gamma, log link | Gamma, log link, adjusted for covariates | |
| Complete-case analysis | |||||||
| NHS excluding hospitalisation | 2639.92 (264.95) | 2118.72 to 3161.12 | 2493.00 (202.81) | 2094.05 to 2891.95 | 0.655 | 0.654 | 0.246 |
| Hospitalisation | 111.11 (67.97) | –22.52 to 244.73 | 24.87 (11.95) | 1.37 to 48.36 | 0.189 | 0.052 | Did not converge |
| Social care | 309.36 (81.21) | 149.70 to 469.01 | 515.05 (120.15) | 278.83 to 751.26 | 0.166 | 0.145 | 0.124 |
| Total cost | 2994.41 (303.44) | 2397.48 to 3591.35 | 2871.02 (243.07) | 2392.86 to 3349.18 | 0.748 | 0.748 | 0.167 |
| Total baseline cost (3 months pre baseline to baseline) | 1827.02 (273.80) | 1288.85 to 2365.20 | 1635.20 (200.00) | 1242.08 to 2028.32 | 0.57 | 0.565 | 0.134 |
| After imputation | |||||||
| NHS excluding hospitalisation | 2653.81 (228.53) | 2203.81 to 3103.80 | 2521.66 (188.09) | 2151.88 to 2891.44 | 0.658 | 0.659 | 0.499 |
| Hospitalisation | 107.36 (63.96) | –18.42 to 233.13 | 23.39 (11.17) | 1.44 to 45.34 | 0.197 | 0.00 | Did not converge |
| Social care | 376.23 (85.26) | 208.66 to 543.80 | 524.25 (113.39) | 301.41 to 747.09 | 0.295 | 0.287 | 0.237 |
| Total cost | 3137.39 (272.08) | 2602.18 to 3672.61 | 3069.30 (228.13) | 2620.88 to 3517.72 | 0.849 | 0.849 | Did not converge |
| Total baseline cost (3 months prior to baseline) | 1849.02 (266.16) | 1325.82 to 2372.22 | 1659.16 (194.26) | 1277.38 to 2040.95 | 0.565 | 0.56 | Did not converge |
FIGURE 9.
Mean cost of NHS and social care resources use per patient over the 6-month follow-up period.
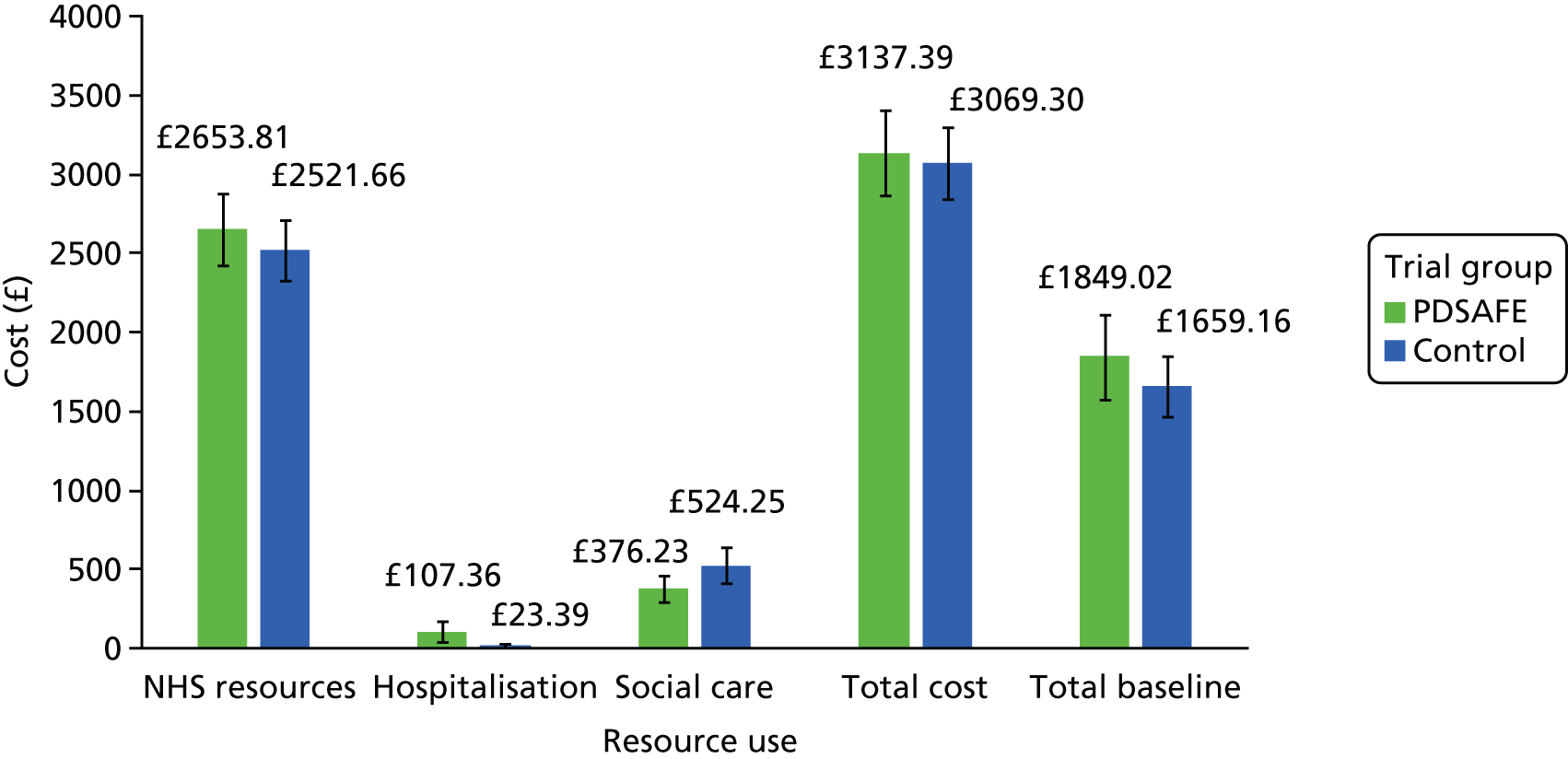
Health-related quality of life
The EQ-5D-3L health utility values for each treatment group at baseline and at 3, 6 and 12 months are shown in Table 21 (complete case), Table 22 (after imputation) and visualised in Figure 10 (after imputation), assuming a linear change between each assessment point. The completeness of the EQ-5D-3L questionnaire at the 6-month follow-up was 79% (188/238) for the intervention group and 90% (213/236) for the control group. The utility values of the intervention group declined slightly less than the control group over the 6-month, as well as the 12-month, period. The differences in utility values between the arms were small (i.e. 0.031 at 6 months and 0.017 at 12 months) given that the full range of the EQ-5D-3L index score was –0.592 (worst) to 1 (full health), and not statistically significant.
| Time point | Trial group | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | ||||||||||
| n | Mean | SD | SE | Minimum, maximum | n | Mean | SD | SE | Minimum, maximum | ||
| Visual analogue scale | |||||||||||
| Baseline | 232 | 60.685 | 23.595 | 1.549 | 3, 100 | 233 | 60.811 | 24.715 | 1.619 | 3, 100 | 0.96 |
| 3-month | 210 | 62.486 | 22.841 | 1.576 | 4, 100 | 217 | 62.465 | 23.350 | 1.585 | 5, 95 | 0.99 |
| 6-month | 188 | 63.750 | 21.808 | 1.590 | 5, 100 | 213 | 63.394 | 21.872 | 1.499 | 3, 100 | 0.87 |
| 12-month | 121 | 64.322 | 17.209 | 1.564 | 25, 99 | 136 | 64.331 | 17.816 | 1.528 | 9, 100 | 1.00 |
| Index score | |||||||||||
| Baseline | 235 | 0.657 | 0.232 | 0.015 | –0.181, 1 | 235 | 0.669 | 0.245 | 0.016 | –0.115, 1 | 0.60 |
| 3-month | 210 | 0.677 | 0.244 | 0.017 | –0.086, 1 | 216 | 0.668 | 0.247 | 0.017 | –0.077, 1 | 0.70 |
| 6-month | 190 | 0.680 | 0.217 | 0.016 | –0.016, 1 | 216 | 0.644 | 0.258 | 0.018 | –0.126, 1 | 0.13 |
| 12-month | 124 | 0.643 | 0.269 | 0.024 | –0.016, 1 | 141 | 0.635 | 0.272 | 0.023 | –0.115, 1 | 0.81 |
| Utility change | |||||||||||
| 6 month – baseline | 188 | 0.008 | 0.239 | 0.017 | –1, 0.795 | 215 | –0.022 | 0.260 | 0.018 | –0.795, 0.672 | 0.2266 |
| 12 month – baseline | 123 | –0.039 | 0.279 | 0.025 | –1, 0.639 | 140 | –0.053 | 0.295 | 0.025 | –0.883, 0.743 | 0.7052 |
| Time point | Trial group | |||
|---|---|---|---|---|
| PDSAFE (N = 238) | Control (N = 236) | |||
| Mean (SE) | 95% CI | Mean (SE) | 95% CI | |
| Baseline | 0.657 (0.015) | 0.628 to 0.687 | 0.669 (0.016) | 0.637 to 0.700 |
| 3 months | 0.672 (0.017) | 0.638 to 0.705 | 0.664 (0.017) | 0.630 to 0.697 |
| 6 months | 0.672 (0.016) | 0.640 to 0.704 | 0.641 (0.017) | 0.607 to 0.676 |
| 12 months | 0.644 (0.027) | 0.590 to 0.697 | 0.626 (0.028) | 0.571 to 0.681 |
FIGURE 10.
The EQ-5D-3L utility values at baseline and at 3, 6 and 12 months (after imputation).
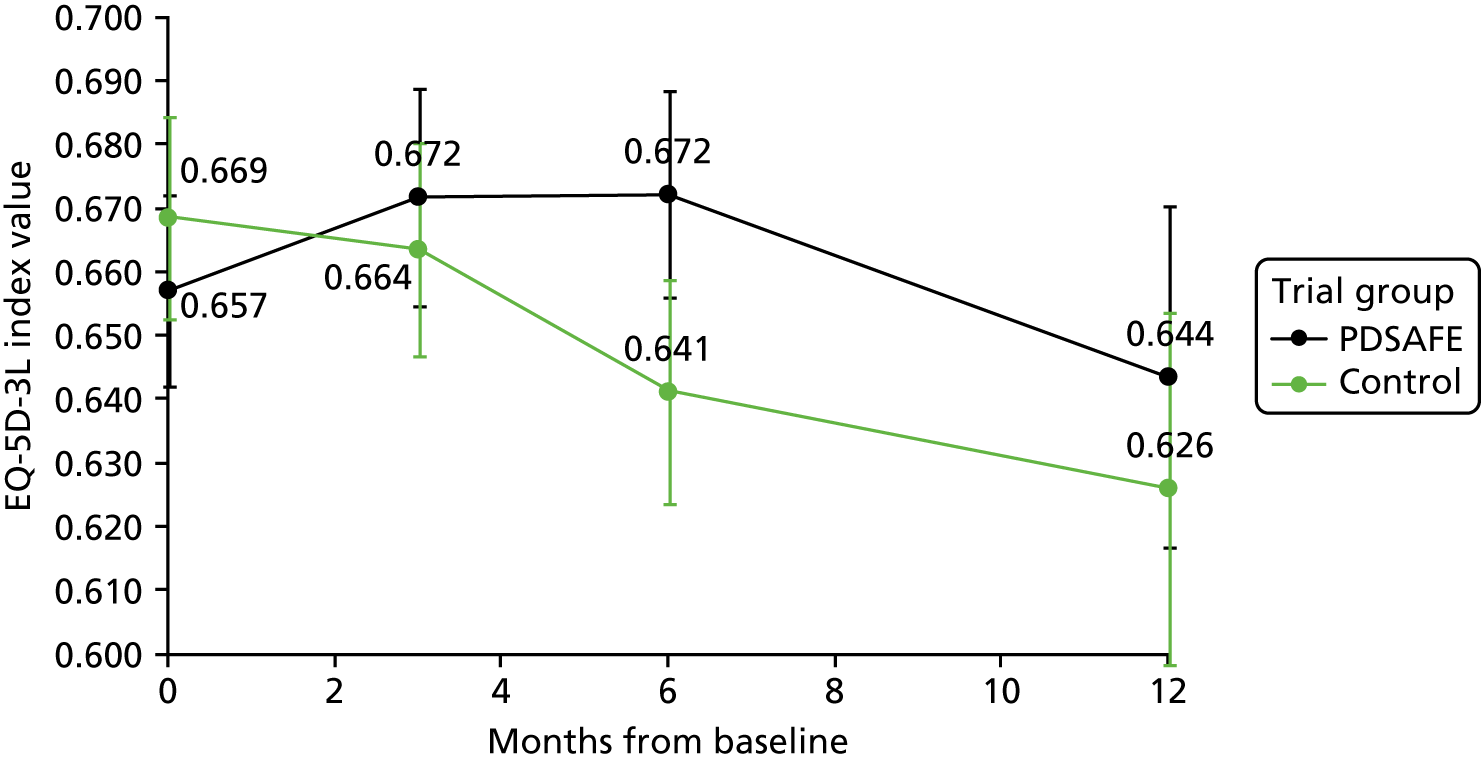
Cost-effectiveness base-case analysis
The cost-effectiveness results for the PDSAFE home-based personalised physiotherapy intervention are presented in Table 23. Overall, the PDSAFE intervention is more costly within the 6-month time horizon but generates greater HRQoL than the control. The average cost per patient was £4020 (95% CI £3531 to £4510) in the intervention group and £3095 (95% CI £2694 to £3496) in the control group, an incremental cost of £925 (95% CI £428 to £1422). The average QALY gain was 0.34 (95% CI 0.326 to 0.345) QALYs in the intervention group and 0.328 (95% CI 0.319 to 0.337) QALYs in the control group, The intervention group had an incremental 0.008 (95% CI –0.006 to 0.021) QALY gain compared with the control group.
| Trial group | Cost (£)a | QALYb | ||||
|---|---|---|---|---|---|---|
| Mean | SE | 95% CI | Mean | SE | 95% CI | |
| PDSAFE (n = 238) | 4020.3 | 249.7 | 3530.7 to 4509.8 | 0.336 | 0.005 | 0.326 to 0.345 |
| Control (n = 236) | 3095.3 | 204.5 | 2694.3 to 3496.2 | 0.328 | 0.005 | 0.319 to 0.337 |
| Difference (95% CI) | 925.0 | 253.5 | 427.8 to 1422.2 | 0.008 | 0.007 | –0.006 to 0.021 |
| ICER | 120,659 per QALY | |||||
| 95% CI for ICER (from bootstrap) | –1,056,012.00 to 434,764.80 | |||||
The cost-effectiveness plane for the base-case analysis is shown in Figure 11. The dyads come from the 1000 bootstrap iterations. The x-axis represents the bootstrapped incremental QALYs between the randomisation groups and the y-axis represents the incremental costs. All of the simulated cost–utility dyads were in the north quadrant, indicating that the intervention was always more expensive than the control. Similarly, the majority of the simulated cost–utility dyads were in the east quadrant, indicating that the intervention was very likely to improve health outcomes compared with the control. The flat oval shape of the cost-effectiveness plane indicates that there is a higher degree of uncertainty surrounding the estimates of the incremental QALYs than the incremental cost, leading to a wide 95% CI for the estimate of ICER.
FIGURE 11.
Cost-effectiveness plane representing 1000 bootstrapped cost difference and QALY difference pairs.
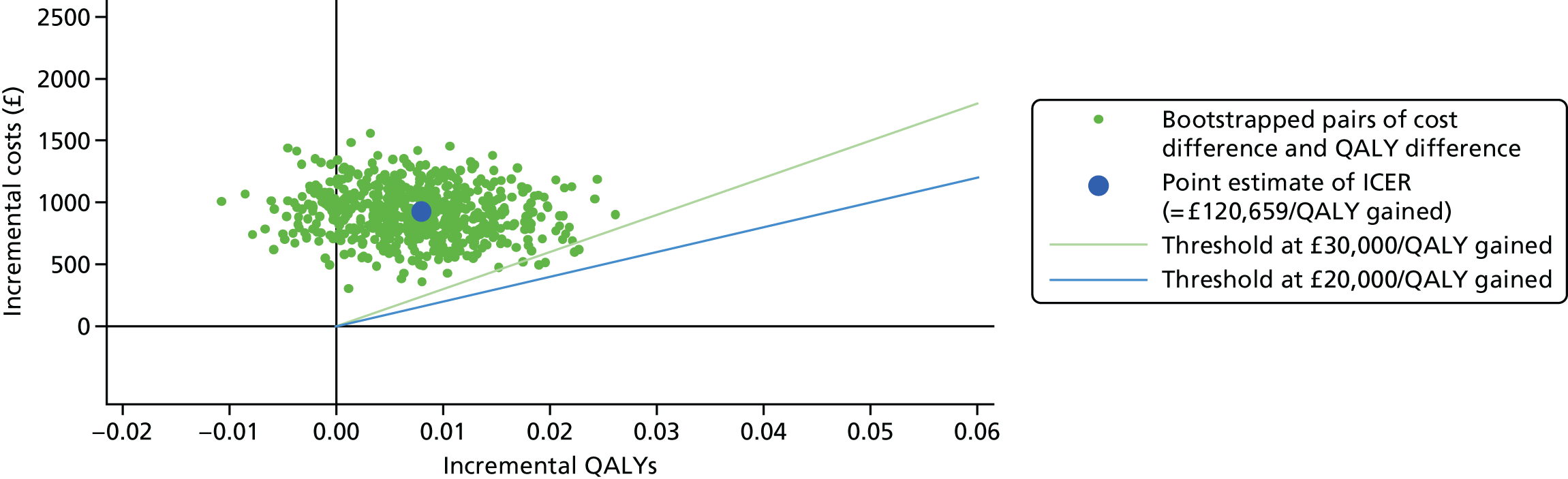
The CEAC for the base-case analysis is shown in Figure 12. The probability that the intervention was cost-effective at the £30,000 per QALY threshold was 0.5%. This corresponds to the very few dyads under the sand line in the cost-effectiveness plane in Figure 11.
FIGURE 12.
Cost-effectiveness acceptability curve.
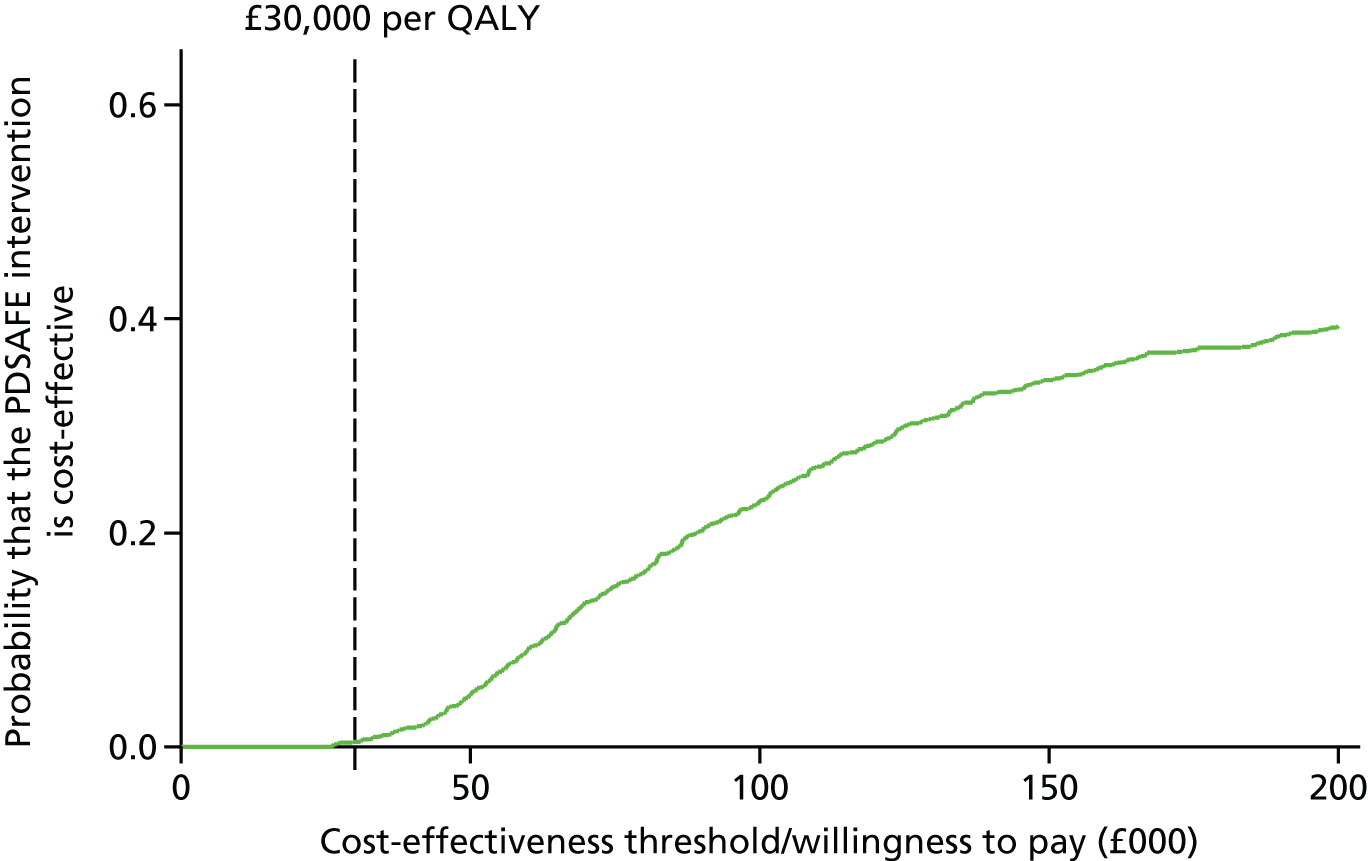
Subgroup cost-effectiveness analysis
The results for the subgroups defined according to cognitive function, presence of freezing symptoms, MDS-UPDRS and retrospective falls at baseline are presented in Table 24. The results indicated that the ICER was lower in two subgroups (although still above NICE’s upper threshold of £30,000): (1) participants who were cognitively impaired (MoCA score of ≤ 25) and (2) participants whose Parkinson’s was of moderate severity (MDS-UPDRS score of between 22 and 39). Compared with the control group, those in the cognitively impaired subgroup of the intervention arm (n = 184) consumed less resource (–£462.80, excluding the intervention cost) and achieved a similar QALY gain to the control group; therefore, the ICER for the PDSAFE intervention versus control was £39,486. In the subgroup including those participants with Parkinson’s moderate severity of (n = 161), the PDSAFE intervention was associated with an incremental cost of £500 and an incremental QALY (0.016) that was double the incremental QALY gain of the overall group, leading to an ICER of £30,731.
| Analysis | Incremental (PDSAFE – control) (95% CI) | ICER (cost per QALY gained) (£) | |
|---|---|---|---|
| Cost (£) | QALYs | ||
| Base-case analysis | |||
| Base-case | 925.0 (427.8 to 1422.2) | 0.008 (–0.006 to 0.021) | 120,659 |
| Subgroup by MoCA (cognitive function) | |||
| Impaired (MoCA score of ≤ 25) (n = 184) | 292.9 (–890.4 to 1476.2) | 0.007 (–0.014 to 0.029) | 39,486 |
| Not impaired (MoCA score of 26 or 27) (n = 122) | 1512.9 (206.9 to 2819.0) | 0.018 (–0.008 to 0.043) | 84,925 |
| Good cognitive function (MoCA score of ≥ 28) (n = 168) | 1513.8 (329.4 to 2698.2) | 0.0006 (–0.020 to 0.021) | 2,613,629 |
| Subgroup by the presence of freezing symptoms | |||
| No freezing (n = 182) | 1284.8 (234.3 to 2335.3) | 0.021 (0.0007 to 0.041) | 61,687 |
| Freezing (n = 291) | 851.5 (–169.9 to 1872.8) | –0.0006 (–0.018 to 0.017) | Control dominates |
| Subgroup by MDS-UPDRS (Parkinson’s severity) | |||
| Lowest disease severity (MDS-UPDRS score of ≤ 22) (n = 153) | 1956.2 (746.9 to 3165.6) | 0.006 (–0.015 to 0.028) | 301,605 |
| Moderate disease severity (MDS-UPDRS score of 23–38) (n = 161) | 500.1 (–760.6 to 1760.8) | 0.016 (–0.006 to 0.039) | 30,731 |
| Highest disease severity (MDS-UPDRS score of ≥ 39) (n = 158) | 600.2 (–639.7 to 1840.0) | –0.004 (–0.027 to 0.019) | Control dominates |
| Subgroup by retrospective falls | |||
| One fall (n = 99) | 1201.3 (–117.3 to 2519.8) | 0.010 (–0.018 to 0.038) | 197,810 |
| Two or three falls (n = 198) | 1121.1 (7.4 to 2234.8) | 0.009 (–0.011 to 0.030) | 53,860 |
| More than three falls (n = 177) | 724.8 (–531.5 to 1981.1) | 0.007 (–0.014 to 0.029) | 82,532 |
Although still above the £30,000 per QALY threshold, compared with the base-case analysis the ICER is lower in the following subgroups: participants with moderate cognitive function (£84,925 per QALY gained), participants who had not experienced freezing symptoms (£61,687 per QALY gained) and participants who had had at least two falls in the 12-month period prior to recruitment (£53,860 per QALY gained for participants who had two or three falls and £82,532 per QALY gained for participants who had more than three falls). In addition, the increase in QALYs gained in the PDSAFE intervention arm for the participants who had not experienced freezing episodes was statistically significant [0.021 (95% CI 0.0007 to 0.041) QALYs]. In contrast, the PDSAFE intervention was found to be less effective than usual care and more costly in two subgroups, the subgroup with more severe Parkinson’s (i.e. those with a MDS-UPDRS score of ≥ 39) and those who had experienced freezing episodes; therefore, usual care dominated PDSAFE in both of these subgroups.
Sensitivity analysis
The results of the sensitivity analysis are presented in Table 25. Although the PDSAFE intervention was cost-ineffective compared with the control in all sensitivity analysis, the ICER decreased by more than half when estimated over a 12-month time horizon with around half of the data imputed based on the observed data. This was due to the linearly interpolated increase in QALYs over the 12-month period.
| Analysis | Incremental (PDSAFE – control) (95% CI) | ICER (cost per QALY gained) (£) | |
|---|---|---|---|
| Cost (£) | QALYs | ||
| Base-case analysis | |||
| Base case (6 months) | 925.0 (427.8 to 1422.2) | 0.008 (–0.006 to 0.021) | 120,659 |
| Time horizon | |||
| 12 months | 1176.2 (248.5 to 2103.9) | 0.021 (–0.011 to 0.054) | 55,176 |
| Cost | |||
| 10 sessions (actual number of sessions) | 824.7 (327.7 to 1321.8) | 0.008 (–0.005 to 0.021) | 105,360 |
| Number of sessions reduced to eight | 719.2 (225.5 to 1212.9) | 0.008 (–0.005 to 0.021) | 91,876 |
| Missing data | |||
| Complete-case analysis (n = 288) | 1138.8 (588.4 to 1689.3) | 0.010 (–0.006 to 0.025) | 117,226 |
| Routine use of physiotherapist | |||
| Routine physiotherapist visit not included in the overall cost | 903.1 (416.4 to 1389.8) | 0.008 (–0.005 to 0.020) | 120,086 |
Discussion
The results showed that the PDSAFE home-based personalised physiotherapy intervention was not likely to be cost-effective for the overall Parkinson’s population from the NHS and PSS perspective over the 6-month time horizon at a £20,000 to £30,000 threshold for the ICER estimated. There was little difference in the mean cost of NHS and social service use between the treatment groups. The utility values declined slightly less in the intervention group than in the control group over the trial period, but the difference was minimal and not statistically significant.
A high degree of uncertainty over the QALYs gained was observed, leading to a wide CI for the estimate of the ICER, which may be a result of the small mean QALY gained observed and large variation among the participants. Another possible explanation might be related to the limitation of the EQ-5D-3L questionnaire in capturing the impact of the PDSAFE physiotherapy intervention on QoL aspects, such as improved falls efficacy and less fear of falling, as identified in the effectiveness results and qualitative study in this report.
Subgroup analyses indicated that the PDSAFE intervention is more likely to be cost-effective in the subgroup of participants who are cognitively impaired. This is due to the slightly lower (albeit non-significant) NHS and social care service costs arising in this subgroup of the intervention arm compared with the control group. The estimated NHS and social resource use costs in the cognitively impaired subgroup were £3916.90 in the control group and £3454.20 in the intervention group, with a marginal £462.70 lower resource use cost in the intervention group. In contrast, the intervention arm was associated with higher resource use in the moderate cognitive function and good cognitive function subgroups (marginal cost £709.20 and £664.20, respectively). In terms of individual service use, among the cognitively impaired subgroup, the intervention group, in comparison with the control group, attended significantly fewer outpatient clinic appointments (1.23 vs. 2.10; p = 0.02), received fewer social worker visits (0.02 vs. 0.14; p = 0.10). However, statistical significance for the differences between groups was not observed for the other health-care resources. The details of the comparison of resource use between the intervention and control groups among the cognitively impaired subpopulation are presented in Appendix 19. The resource use savings arising in this subgroup may be attributed to the unusually high cost of the control arm of this subgroup and the relatively lower cost of the intervention arm as a response to the improved falls efficacy, reduced near-falls and improved balance; however, it could also be due to chance.
The intervention was also more likely to be cost-effective in the subgroup of participants who had a Parkinson’s of moderate severity (MDS-UPDRS score of 23–38), owing to the cost savings arising in service use and the larger magnitude of QALY gain. This is in line with the effectiveness results showing that the PDSAFE intervention was associated with a statistically significantly lower risk of falling in this subgroup in the FRR analysis. In addition, the ICER estimate of the PDSAFE intervention was lower than the overall sample in the participants who had moderate cognitive function, those who had not experienced freezing symptoms and those who had had at least two falls in the year prior to recruitment. In terms of the impact on HRQoL, the PDSAFE intervention was shown to be statistically beneficial in participants who had not experienced freezing episodes, whereas in participants who had experienced freezing episodes or had severe Parkinson’s, it resulted in a lower mean QALY gain than in the control group.
Sensitivity analyses indicated that the intervention was more likely to be cost-effective when a 12-month time horizon was adopted, although only half of the data at the 12-month follow-up were directly observed. This is because of the difference in HRQoL at the 6-month primary end point, as well as the persistent benefit of the intervention beyond the 6-month intervention period. At 12 months, HRQoL in the intervention group was still higher than in the control group with both observed-only data and imputed data; therefore, it is likely that this difference would last beyond the 12-month follow-up point. This means that the probability of the intervention being cost-effective might increase if the results were extrapolated over a longer time horizon; although it would require a longer follow-up study to test the long-term effect because of the large number (50%) of missing data at the 12-month follow-up.
It was found that there was a statistically significantly higher use of routine NHS physiotherapy in the intervention group than in the control group over 0–3 months (1.3 vs. 0.7 times), 3–6 months (1.0 vs. 0.7 times) and 6–12 months (1.4 vs. 0.8), but not during the 3-month period prior to baseline (1.1 vs. 1.0 times). The intervention lasted for 6 months and the higher use of physiotherapists in the intervention group over 0–6 months is likely to be because some participants included trial therapist visits in their health and social care service use data, despite the distinction being made. Originally, the routine service use of physiotherapists was designed to be collected through assessor interview in which the participants could have had more guidance. However, a risk of unblinding the assessors was identified; therefore, the NHS routine resource use questions were sent by post to the participants and filled in by participants themselves at home. Even so, this does not explain the higher use of physiotherapists from 6 to 12 months. In this situation, it is possible that some of the participants in the intervention group found the intervention effective and therefore continued having physiotherapy visits provided by the routine services. Despite the possible inaccuracy in the reported number of visits, the sensitivity analysis assuming no difference between the use of this routine service found that this had little effect on the magnitude of ICER and, therefore, is not likely to cause any bias.
Overall, the cost-effectiveness analysis results reflect the primary outcomes of the trial, in that there was no difference in the risk of repeat falling, but the intervention was associated with a lower risk of near-falling, better balance and an increased confidence in mobility. This mixed result may contribute to the small mean QALY gain (0.008 years over the 6-month trial period) as well as the large degree of uncertainty surrounding the QALY gain, which led to the wide CI of the ICER. The uncertainty analysis (see Figure 12) revealed that the probability of the intervention being cost-effective is 0.5%, which suggested that the PDSAFE intervention was probably not cost-effective from the NHS and PSS perspectives. The large magnitude of ICER was attributed primarily to the very small QALY gains rather than the intervention cost. It was shown in the sensitivity analysis that, even when the number of PDSAFE physiotherapy sessions was reduced from 12 to 8, the ICER (£91,876 per QALY) was still much higher than the NICE threshold (£20,000–30,000 per QALY) because of the unchanged incremental QALY gain. Therefore, cost reduction strategies only, such as changing home-based sessions to group sessions, will not result in the intervention being cost-effective unless the effectiveness improves.
Chapter 8 Discussion and conclusions
Main finding in context
To our knowledge, PDSAFE is the largest trial of physiotherapy for fall prevention among people with Parkinson’s; it was novel, with an intervention that was personalised and conducted in the home. The sample was more than double the size of any previous trial in the field. Despite this, the findings failed to demonstrate that PDSAFE was effective in reducing falls in a heterogeneous sample of people with Parkinson’s. The non-significant result is similar to that found by previous researchers of trials with > 100 participants. 17,37,40,72,73 The exception is the trial by Morris et al. ,72 who in 2015 reported a positive effect of exercise training on fall reduction, largely conducted in an outpatient setting. Interestingly, they did not replicate the finding in 2017 when training was delivered at home and at a lower frequency. 73 Two other research groups found a reduction in falls from interventions outside of normal physiotherapy delivery, with virtual reality and treadmill training74 and tai chi. 75 A mixed and very small response, with short- and long-term effects, to various exercise interventions for fall reduction emerged from the meta-analysis by Shen et al. 76 This PDSAFE trial had a very heterogeneous sample; unlike other fall prevention trials, those with high disease severity or those with more complex symptoms, such as freezing, were not excluded.
Wide spectrum of falling
The wide spectrum of falling experiences among participants in the sample may have contributed to the non-significant finding. People with one fall were included, as were those with multiple falls and those reporting falling every day in the year prior to commencing the trial. This inclusive approach was typical of other trials and a reflection of a Parkinson’s population. The PDSAFE treatment was personalised; for this reason, it was believed that the management of the range of profiles would have been addressed. However, in hindsight, multiple falls are not just about the numbers of falls: the complex and varied nature of falling among people with Parkinson’s may have been the challenge and the key contributing factor to the non-significant treatment effect in this trial. Among this population, falls are indicative of a changing disease process, the pathway from non-falling to near-falling, to falling and to repeat falling can differ, and falls among established repeat fallers are different from the single-faller experience. 77 People with Parkinson’s who have an established pattern of falling will have more global features of decline that may be age- and/or disease-related; Lord et al. 77 suggest that these progressive features create additional challenges for implementing appropriate management and research. Other researchers17,37,40 have previously suggested that those with less severe Parkinson’s have responded positively to fall reduction programmes, but that effect has been counterbalanced with the deterioration experienced among those with more severe disease, leading to non-significant results. The analysis of our trial reflects a similar pattern.
Freezing of gait and worse Parkinson’s
As with any group of people with Parkinson’s, this sample included those with and without FoG. The motor learning potential is reduced in people who freeze78,79 and there is increasing evidence that FoG is an important risk factor of falls and associated with reduced balance22 and impaired cognition. The inclusion of people who did or did not experience freezing illustrates the diverse range of motor problems present in a community sample of people with Parkinson’s and the range of challenges faced when teaching and learning new skills. Interestingly, all participants in the trial showed overall improvement in impairments such as balance, but no overall significant reduction in falls in response to PDSAFE. The complexity of fall avoidance, it seems, resides in the transition between these two features. More supervised therapy focused around the fall avoidance strategies may have been required, even though a specific strategy for FoG was included in the training programme. As with those experiencing FoG, people with greater disease severity (more likely to have additional cognitive impairment) may be more restricted in their ability to implement fall avoidance strategies and may also have had greater difficulty in engaging in unsupervised sessions, thus limiting the intensity of practice and the effects.
Cognitive impairment
Participants were screened for cognitive impairment, namely a score of ≥ 24 on the MMSE scale, to ensure that understanding of and compliance with the exercise programme of the trial was not compromised. In spite of this eligibility procedure, some participants were recruited to the trial with cognitive impairment identified later through a low score on the MoCA. The MoCA is known to be more sensitive to specific cognitive deficits linked to frontal and executive function associated with people who fall,80 but at the time of designing this trial it was still a relatively new test and not widely used. To explore whether or not this was a factor contributing to the lack of benefit overall, we looked at subgroups defined by tertiles of MoCA score at baseline and found a pattern of association between decreasing MoCA score and decreasing PDSAFE benefit with respect to the falling outcomes. As previously highlighted, all participants, even those with cognitive impairments, showed improvements in their balance scores and fall efficacy scales. This is interpreted as indicating that these participants were able to comply with exercise even when their MoCA scores were low but were not able to benefit from the more demanding fall prevention programme, for whatever reason. In future research, including MoCA81 as an eligibility criterion is recommended, although researchers such as Domingos et al. 82 have called for more studies into fall prevention with people with impaired cognition.
Treatment of fall risk
The fall risk factors of participants in the trial (i.e. the secondary outcome measures of balance, falls efficacy and functional strength) were targeted in the intervention; the analysis demonstrated a consistent improvement across the whole PDSAFE group. A positive PDSAFE effect on near-falls, an indicator of instability and reflective of the improvements in balance, was also reported. These results support those of others,77 highlighting the benefits of exercise for balance and gait ability. Interventions in previous trials have been delivered largely outside the home, but the results of this study demonstrate similar findings from interventions within the home environment, which is important for those with fewer opportunities for access, those who are unable to regularly travel for training or those who may suffer from fatigue or apathy.
Therapy
A strength of the trial was the evidence-based therapy programme delivered by skilled trained physiotherapists. The training was structured and practice was rigorously tested for fidelity. Participants received, on average, 11 or 12 face-to-face supervised sessions of 1–1.5 hours’ duration and were encouraged to complete unsupervised daily practice, meeting the recommendations of Sherrington et al. 83 of at least 50 hours of practice. Previous research has used a range of different interventions, often poorly described, with a great deal of diversity of treatment protocols and dosage. 76 This programme was multidimensional, requiring individuals to progress their activities and to integrate their training into everyday functional tasks; although this would have been manageable for those in the moderate to least severe disease group, it may have been too challenging for those at the more severe end of the spectrum, despite treatment being personalised. Active participation and cognitive reasoning were needed to comply with PDSAFE and progress to self-management for falls avoidance. Participants at the severe end of the disease spectrum with cognitive impairment and FoG may have needed more supervised sessions focused on fall prevention strategy training.
In the longitudinal qualitative inquiry into the expectations and experiences of people with Parkinson’s, the first of its type, it was found that many of the participants receiving the treatment had realistic and well-informed expectations about the PDSAFE intervention at the outset. Most had had previous experience of physiotherapy and a variety of other rehabilitation interventions. Participants seemed well motivated to embark on the programme for a variety of personal reasons, including anticipated benefits relating to improved mobility and function, and more general improvements in confidence and independence. People also wanted to participate for altruistic reasons, hoping that the research would benefit others with Parkinson’s. This variety of potential motivations for participating in exercise by people with Parkinson’s, including the altruistic desire to help others, was also found by O’Brien et al. 84 in exploring views of people with Parkinson’s about participation in an exercise group. PDSAFE was valued for its convenience, in being delivered at home, and also for the input from specialist physiotherapists with experience and expertise in Parkinson’s.
Participants recalled the exercise programme well at the time of the second visit, and were able to describe, in some detail, specific components. They recognised that the exercise programme was graded, becoming more intense as it progressed, and acknowledged that their programmes were individually tailored. Several also highlighted the strategies in describing the programme, and understood better the process of discussion and negotiation whereby exercises and strategies were designed to meet individual needs and expectations.
The mixed reactions to the equipment were affected by the value that participants placed on the increased intensity that they afforded. The varied responses to the technology used within PDSAFE reflected a continuum of familiarity and use among participants. However, some who were familiar with computers and smartphones felt that the visual images should be offered in alternative formats (e.g. as an app), which they could download and keep with them (e.g. on a tablet). These findings highlight a need to be creative and flexible when using technology, in order that the means of delivery can likewise be tailored to suit individual participants.
Most participants felt that the PDSAFE intervention had been of some benefit, when reflecting on its impact, whether in mobility, balance, strength or freezing, more general functional activities or improved confidence and independence. Similar views were expressed by people with Parkinson’s in Quinn et al. ,85 who believed that exercise would keep them ‘strong, functional and fit’. A significant minority of our participants mentioned that they experienced fewer falls, or were more aware of triggers for falls. A wide variety of benefits were highlighted, not all of which were captured by the outcome measures used within the main trial.
In terms of barriers to participation, lack of motivation was highlighted as a specific challenge. Participants valued the encouragement, monitoring and feedback provided by the therapists, and missed this once visits became less frequent and eventually stopped. Feedback from physiotherapists was also found by O’Brien et al. 86 to be important to people with Parkinson’s in making decisions about exercise engagement. Another barrier was the time required to complete the individualised programmes; several participants mentioned their other competing commitments and difficulties in carrying out the PDSAFE intervention, either in full or at all. Many participants had busy lives including leisure activities, Parkinson’s groups and family commitments, particularly those whose symptoms were not so severe.
The social support and encouragement provided by both the treating physiotherapists and others such as spouses, carers and partners seemed to be the biggest facilitator for continued engagement with the programme. Ravenek and Schneider,87 in the first study exploring factors influencing participation in physical activity by people with Parkinson’s, highlighted the significance of social support, and described three types: instrumental, information and emotional support. Participants mentioned that they found written information about the PDSAFE intervention useful; this was offered in blue folders to which many made reference.
Fall recording
The most widely adopted and recommended process for recording fall events is the use of self-reported falls diaries,88 as used in the trial. The process is dependent on recall but open to error, most likely under-reporting, particularly when someone has multiple falls. For example, participants who did not receive any treatment (i.e. the control group) had very little interaction with the trial team, and thus received fewer reminders to complete falls diaries than the intervention group. It may be that those in the control group, particularly those with cognitive impairment, were less likely to report falls. Conversely, those in the treatment group may perhaps be more likely to report falls. Near-falls are open to similar errors of recording; they are events that would result in a fall if protective moves such as reaching out or stepping were not taken. 26 Near-falls among people with Parkinson’s have been reported by a number of researchers, such Gazibara et al. 89 and Ashburn et al. ;37 they are often related to stumbling or balance loss, and have been observed to predispose the general older population to falling. 90 Gazibara et al. 89 describe near-falls as common among people with Parkinson’s and particularly among people with FoG. In this trial, participants received guidance on recording fall events, had a 3-month run-in period for recording falls and received reminders to keep records throughout the trial. It is possible that participants in the intervention group were more aware of falling and kept more accurate records. In the future, body-worn sensor technology could be adopted, although the feasibility of this in clinical trials is not yet clear.
Beneficial effects on falls and stratification
As already stated in this discussion, previous researchers17,37,40 have suspected that those with less severe Parkinson’s have responded to fall reduction programmes, whereas, in their trials, deterioration may have occurred among people in the worse categories of Parkinson’s. For that reason, disease severity and FoG were prespecified in a secondary subgroup analysis of PDSAFE; that analysis demonstrated a diverse response to the intervention programme. Those with moderate disease severity benefited most from the intervention, with a fall reduction of 30% over 6 months. Those at the severe end of the disease spectrum and those with FoG experienced an increase in falls of between 43% and 50% over the same period. The overall improvements in fall risks suggested that sufficient therapy for learning and responding to an exercise programme was delivered but fall avoidance by those at the severe end of the disease spectrum fell short. A greater understanding of the complexities of the transition from improved movements to fall avoidance in people who are compromised by a progressive condition is needed. At a simplistic level, PDSAFE may have made people more confident in their daily activities that exposed them to greater fall risk, to which the more able could respond effectively, but the more severely affected were unable to implement strategies for fall avoidance. People with more severe disease (i.e. more likely to have additional cognitive impairment and possibly FoG) may be more restricted in their ability to choose how to respond and may also have greater difficulty in engaging in unsupervised sessions, thus limiting the intensity of practice and the effects of fall prevention. Falls management needs to be stratified with careful consideration of the needs of those with multiple challenging issues that come with a progressive condition. The benefits highlighted by the secondary analysis on those with moderate disease severity need to be confirmed in a further trial.
Health economics
The results of the health economics analysis showed that the PDSAFE home-based personalised physiotherapy intervention was not likely to be cost-effective for the overall Parkinson’s population from the NHS and PSS perspectives over the 6-month time horizon at a £20,000- to £30,000-per-QALY threshold for the ICER estimated. There was little difference in the mean cost of NHS and social service use between the treatment groups. The utility values of the intervention group declined slightly less than in the control group over the trial period, but the difference was minimal and not statistically significant.
Although not cost-effective in the overall population, subgroup analyses indicated that the PDSAFE intervention appears to be cost-effective in the subgroup of participants with more severe disease. This is because of the substantial saving of NHS and social care services arising in this subgroup of the intervention arm. The intervention was also cost-effective in the subgroup of participants who had Parkinson’s of moderate severity (i.e. a MDS-UPDRS score of 23–38), because of the reduction in service use and the larger magnitude of QALY gain. In terms of the impact on HRQoL, the PDSAFE intervention was shown to be statistically significantly beneficial in participants who had not experienced freezing episodes whereas, among participants with severe Parkinson’s, it resulted in a lower mean QALY gain than the control group.
Sensitivity analyses indicated that the intervention was more likely to be cost-effective when a 12-month time horizon was adopted; although only half of the data for participants at the 12-month follow-up were directly observed. This is because of the difference in HRQoL at the 6-month primary end point, as well as the persistent benefit of the intervention beyond the 6-month intervention period. At 12 months, HRQoL in the intervention group was still higher than in the control group, with both observed-only data and imputed data; therefore, it is likely that this difference would last beyond the 12-month follow-up point. This means that the probability of the intervention being cost-effective might increase if extrapolating the results to a longer term.
Limitations
Most participants in the trial were followed to 12 months, with the primary outcome measured at 6 months post randomisation; however, the last 132 participants to be recruited could be followed only to the primary outcome point (i.e. 6 months) because of restricted funding. This meant that the numbers of participants in the trial reduced during the period between 6 and 12 months, creating a limitation in the analysis and interpretation of the long-term outcome of the intervention and the health economic sensitivity analysis.
Participants were asked to complete a number of self-recorded diaries. The most important was the falls diaries for the primary outcome. The trial team invested a great deal in facilitating the completion of the falls diaries. Self-recorded data on unsupervised practice of the intervention was incomplete. The demand on the participant meant that recording of unsupervised practice was not always completed and was less of a priority than the falls record.
Implications for practice
-
The PDSAFE intervention did not reduce falls in a heterogeneous sample of people with Parkinson’s.
-
Secondary analysis supported previous research findings that exercise had a positive effect on fall risk.
Future research
-
Future fall prevention trials should not target heterogeneous samples but specific groups of people with Parkinson’s.
-
Primary trials of fall prevention for disease-specific groups are recommended.
-
Secondary analysis demonstrated that the PDSAFE intervention did have a positive effect on balance, functional strength, falls efficacy and near-falls.
Conclusion
The PDSAFE intervention did not reduce falls in a heterogeneous sample of people with Parkinson’s. There was evidence of improvement in balance, functional strength and falls efficacy, with a reduction in near-falls. Secondary analysis showed diverse responses to the intervention in accordance with disease severity and FoG. Further trials of falls prevention on targeted groups of people with Parkinson’s are recommended.
Acknowledgements
We would especially like to thank all the people with Parkinson’s, and their carers, who agreed to take part in the trial. We also thank the local clinicians and Parkinson’s support groups for their support with recruitment. We are very grateful for the support of the Wessex Clinical Research Network. We would also like to thank Professor Dawn Skelton and Later Life Training for their kind permission to use the Postural Stability Instructors exercise training manual.
Trial management centre
Professor Ann Ashburn (Chief Investigator), Professor Helen C Roberts (Deputy), Dr Kim Chivers Seymour (Trial Manager), Dr Sophia Hulbert (Lead Therapist), Ms Carolyn Fitton (Lead Assessor), Ms Ioanna Marian (Statistician), Dr Claire Ballinger (Qualitative Lead), Ms Alison Roswell (Qualitative Researcher) and Ms Yiqiao Xin (Health Economist).
Administrators
Ms Brenda Colwell, Mr Matt Saxby, Mr Nick Parton, Mr Tim Worth and Mrs Sue McDonald.
PDSAFE Collaborative Group Members
Professor Ann Ashburn, Dr Kim Chivers Seymour, Professor Helen C Roberts, Professor Ruth Pickering, Professor Sarah E Lamb, Dr Claire Ballinger, Dr Victoria A Goodwin, Professor Lynn Rochester, Professor Alice Nieuwboer, Dr Emma McIntosh Dr Dorit Kunkel, Dr Sophia Hulbert, Ms Carolyn Fitton, Ms Ioanna Marian, Ms Yiqiao Xin, Ms Alison Rowsell and Dr Rachel Summer.
Oxford Clinical Trials Research Unit
Professor Sarah E Lamb, Dr Vicki Barber, Dr Joanna Black, Ms Emily Haines and Mr Patrick Julier.
Participating centres
Royal Devon and Exeter NHS Foundation Trust
Mrs Emily Rogers, Dr Ray Sheridan (Principal Investigator), Dr Kathleen Reilly, Mrs Jane Hall, Mr Robert James, Mrs Clare O’Reilly, Ms Kathy Polverino and Mrs Gayle Githens-Mazer.
Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust
Dr Khaled Amar (Principal Investigator), Ms Carly Fricker, Ms Laura Tucker and Ms Chantel Cox.
Hampshire Hospitals NHS Foundation Trust
Mrs Debbie Dellafera, Mrs Trisha Norman, Dr Sam Arianayagam (Principal Investigator) and Mrs Angie Dempster.
Portsmouth Hospitals NHS Trust
Mrs Katie Castle, Mrs Liz Ashdown, Dr Sarah Williams and Professor Helen C Roberts (Principal Investigator).
University Hospital Southampton NHS Foundation Trust
Mrs Veena Agarwal and Professor Helen C Roberts (Principal Investigator).
Poole Hospital NHS Foundation Trust
Mrs Meryl Goddard, Mrs Pippa Collins, Dr Ralph Gregory (Principal Investigator), Mrs Anneke Fox, Mrs Sarah Chessell and Mrs Lynette Corney.
Royal Cornwall Hospitals NHS Trust and Cornwall Partnership NHS Foundation Trust
Dr Christine Schofield (Principal Investigator), Ms Vicky Farrell Mrs Alex Gorree-Wery, Mrs Alison James and Mrs C Stone.
Livewell Southwest
Mrs Bernadette Coles, Mrs Rena Truscott, Dr Jane Stribley (Principal Investigator), Mrs Louise Johnson, Mrs Nicola Thompson, Ms Kyra Higgins and Ms Maria Stevenson.
Southern Health NHS Foundation Trust
Mrs Trisha Norman, Dr Gill Turner (Principal Investigator) and Dr Peter Phiri.
Institute of Neuroscience, Newcastle University
Mr Philip Brown, Mrs Heather Hunter, Ms Rosie Morris and Professor Lynn Rochester (Principal Investigator).
University of Southampton
Mrs Meryl Rowlands, Mrs Tracey McElwaine, Mrs Megan Liddiard and Mrs Vanessa Pressly.
Data Monitoring Committee
Professor Carl Clarke (Chairperson), Dr Rebecca Walwyn and Professor Sarah Tyson.
Trial Steering Committee
Professor Pippa Logan (Chairperson), Mr John Wood (PPI representative), Professor Helen Dawes and Professor Margaret Gosney.
Contributions of authors
Professor Ann Ashburn (Professor of Rehabilitation, Chief Investigator) was a grant holder, designed the study and intervention, conducted fidelity checking and was responsible for data interpretation, chapter writing and critical reviewing.
Professor Ruth Pickering (Professor of Medical Statistics) was a grant holder, designed the study and was responsible for data analysis, data interpretation and chapter writing.
Professor Emma McIntosh (Professor of Health Economics) was a grant holder, designed the study and was responsible for health economics data analysis and interpretation, and chapter writing.
Dr Sophia Hulbert (Senior Research Fellow, clinical physiotherapy) designed the intervention, conducted fidelity checking and was responsible for data analysis, data interpretation and chapter writing.
Professor Lynn Rochester (Professor of Human Movement Science) was a grant holder, designed the study and was responsible for data interpretation and critical reviewing.
Professor Helen C Roberts (Professor and Honorary Consultant, geriatric medicine) was a grant holder, designed the study and was responsible for data interpretation and critical reviewing.
Professor Alice Nieuwboer (Professor of Movement and Rehabilitation Sciences) was a grant holder, designed the study and was responsible for data interpretation and critical reviewing.
Dr Dorit Kunkel (Senior Research Fellow, neurorehabilitation) designed the study and was responsible for trial management, data interpretation and critical reviewing.
Dr Victoria A Goodwin (Senior Research Fellow, frailty) was a grant holder, designed the study and was responsible for data interpretation and critical reviewing.
Professor Sarah E Lamb (Director, Oxford Clinical Trials Research Unit) was a grant holder, designed the study and was responsible for data interpretation and critical reviewing.
Dr Claire Ballinger (Principal Research Fellow, qualitative research and PPI) was a grant holder, designed the study and was responsible for qualitative data analysis and data interpretation, and chapter writing.
Dr Kim Chivers Seymour (Trial Manager/Senior Research Fellow, trials management) designed the study and was responsible for trial management, data interpretation, chapter writing and critical reviewing.
Publications
Goodwin V, Pickering R, Ballinger C, Roberts H, McIntosh E, Lamb S, Nieuwboer A, Rochester L, Ashburn A. A multi-centre, randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s: study protocol. BMC Neurol 2015;15:81.
Hulbert S, Rochester L, Nieuwboer A, Goodwin V, Fitton C, Chivers-Seymour K, Ashburn A. ‘Staying safe’ – a narrative review of falls prevention in people with Parkinson’s – ‘PDSAFE’ [published online ahead of print May 18 2018]. Disabil Rehabil 2018.
Chivers Seymour K, Pickering R, Rochester L, Roberts HC, Ballinger C, Hulbert S, et al. Multicentre, randomised controlled trial of PDSAFE, a physiotherapist-delivered fall prevention programme for people with Parkinson’s. J Neurol Neurosurg Psychiatry 2019;90:774–82.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Jones D, Playfer J, Stokes M. Physical Management in Neurological Rehabilitation. Maryland Heights, MO: Elsevier Mosby; 2004.
- Parkinson’s UK . Parkinson’s UK: Parkinson’s Prevalence in the United Kingdom 2009.
- Bloem BR, Valkenburg VV, Slabbekoorn M, van Dijk JG. The multiple tasks test. Strategies in Parkinson’s disease. Exp Brain Res 2001;137:478-86. https://doi.org/10.1007/s002210000672.
- Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord 2007;22:1892-900. https://doi.org/10.1002/mds.21598.
- Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol 2005;252:1310-15. https://doi.org/10.1007/s00415-005-0855-3.
- Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson’s disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 2014;4:203-21. https://doi.org/10.2217/nmt.14.22.
- Mak MK, Pang MY. Balance confidence and functional mobility are independently associated with falls in people with Parkinson’s disease. J Neurol 2009;256:742-9. https://doi.org/10.1007/s00415-009-5007-8.
- Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J Neurol Sci 2006;248:173-6. https://doi.org/10.1016/j.jns.2006.05.015.
- Nilsson MH, Drake AM, Hagell P. Assessment of fall-related self-efficacy and activity avoidance in people with Parkinson’s disease. BMC Geriatr 2010;10. https://doi.org/10.1186/1471-2318-10-78.
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 2008;23:1428-34. https://doi.org/10.1002/mds.21667.
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology 2010;75:116-24. https://doi.org/10.1212/WNL.0b013e3181e7b688.
- Almeida LR, Sherrington C, Allen NE, Paul SS, Valenca GT, Oliveira-Filho J, et al. Disability is an independent predictor of falls and recurrent falls in people with Parkinson’s disease without a history of falls: a one-year prospective study. J Parkinsons Dis 2015;5:855-64. https://doi.org/10.3233/JPD-150651.
- Paul SS, Thackeray A, Duncan RP, Cavanaugh JT, Ellis TD, Earhart GM, et al. Two-year trajectory of fall risk in people with Parkinson disease: a latent class analysis. Arch Phys Med Rehabil 2016;97:372-9.e1. https://doi.org/10.1016/j.apmr.2015.10.105.
- Paul SS, Allen NE, Sherrington C, Heller G, Fung VS, Close JC, et al. Risk factors for frequent falls in people with Parkinson’s disease. J Parkinsons Dis 2014;4:699-703. https://doi.org/10.3233/JPD-140438.
- Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0096675.
- Ashburn A1, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of falls diaries to facilitate reporting. Disabil Rehabil 2008;30:1205-12. https://doi.org/10.1080/09638280701828930.
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 2011;82:1232-8. https://doi.org/10.1136/jnnp-2011-300919.
- Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol 2013;70:183-90. https://doi.org/10.1001/jamaneurol.2013.646.
- Hulbert S, Rochester L, Nieuwboer A, Goodwin V, Fitton C, Chivers-Seymour K, et al. ‘Staying safe’ – a narrative review of falls prevention in people with Parkinson’s – ‘PDSAFE’. Disabil Rehabil 2018;18:1-10. https://doi.org/10.1080/09638288.2018.1471167.
- Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006;35:409-15. https://doi.org/10.1093/ageing/afl024.
- Goodwin VA, Pickering R, Ballinger C, Roberts H, McIntosh E, Lamb S, et al. A multi-centre, randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s: study protocol. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0332-2.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42. https://doi.org/10.1212/WNL.17.5.427.
- Clark RD, Lord SR, Webster IW. Clinical parameters associated with falls in an elderly population. Gerontology 1993;39:117-23. https://doi.org/10.1159/000213521.
- Stack E, Ashburn A. Fall events described by people with Parkinson’s disease: implications for clinical interviewing and the research agenda. Physiother Res Int 1999;4:190-20. https://doi.org/10.1002/pri.165.
- King L, Horak F. On the mini-BESTest: scoring and the reporting of total scores. Phys Ther 2013;93:571-5. https://doi.org/10.2522/ptj.2013.93.4.571.
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 2009;30:459-63. https://doi.org/10.1016/j.gaitpost.2009.07.108.
- Brown RG, MacCarthy B, Jahanshahi M, Marsden CD. Accuracy of self-reported disability in patients with parkinsonism. Arch Neurol 1989;46:955-9. https://doi.org/10.1001/archneur.1989.00520450025014.
- EuroQol Research Foundation . EQ-5D Home Page 2011. www.euroqol.org.
- Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988;24:709-11.
- Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005;34:614-19. https://doi.org/10.1093/ageing/afi196.
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. https://doi.org/10.1016/0895-4356(93)90053-4.
- Lang A, Fahn S, Munsat TL. Quantification of Neurological Deficit. Oxford: Butterworth-Heinemann; 1989.
- Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making 2011;31:458-68. https://doi.org/10.1177/0272989X10381280.
- Robinson BC. Validation of a caregiver strain index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of an exercise programme for people with Parkinson’s disease who repeatedly fall. J Neuro Neurosurg Psych 2007;78:678-84. https://doi.org/10.1136/jnnp.2006.099333.
- Tango T. Sample size formula for randomized controlled trials with counts of recurrent events. Stats Probabil Letters 2009;79:466-72. https://doi.org/10.1016/j.spl.2008.09.016.
- Zheng H, Kimber A, Goodwin VA, Pickering RM. A comparison of different ways of including baseline counts in negative binomial models for data from falls prevention trials. Biom J 2018;60:66-78. https://doi.org/10.1002/bimj.201700103.
- Canning CG, Sherrington C, Lord SR, Close JC, Heritier S, Heller GZ, et al. Exercise for falls prevention in Parkinson’s disease: a randomized controlled trial. Neurology 2015;84:304-12. https://doi.org/10.1212/WNL.0000000000001155.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Keus SHJ, Munneke M, Graziano M. European Physiotherapy Guidelines for Parkinson’s Disease. Nijmegen: Royal Dutch Society for Physical Therapy (KNGF) and ParkinsonNet; 2015.
- Tomlinson CL, Herd CP, Clarke CE, Meek C, Patel S, Stowe R, et al. Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Syst Rev 2014;6. https://doi.org/10.1002/14651858.CD002815.pub2.
- World Health Organization (WHO) . International Classification of Functioning, Disability and Health (ICF) 2001. www.who.int/classifications/icf/en/.
- van der Marck MA, Klok MP, Okun MS, Giladi N, Munneke M, Bloem BR. NPF Falls Task Force . Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Parkinsonism Relat Disord 2014;20:360-9. https://doi.org/10.1016/j.parkreldis.2013.10.030.
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1423-34. https://doi.org/10.1249/mss.0b013e3180616b27.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 2010;1:100-7. https://doi.org/10.4103/0976-500X.72352.
- The SPIRIT Group . Welcome to the SPIRIT Statement Website 2013. www.spirit-statement.org/.
- Chivers Seymour K, Pickering R, Rochester L, Roberts HC, Ballinger C, Hulbert S, et al. Multicentre, randomised controlled trial of PDSAFE, a physiotherapist-delivered fall prevention programme for people with Parkinson’s [published online ahead of print April 3 2019]. J Neurol Neurosurg Psychiatry 2019. https://doi.org/10.1136/jnnp-2018-319448.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Co.; 1967.
- van den Hoonaard WC, Given LM. The SAGE Encyclopedia of Qualitative Research Methods, Volume 2. Thousand Oaks, CA: Sage Publications Inc.; 2008.
- Murray SA, Kendall M, Carduff E, Worth A, Harris FM, Lloyd A, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339. https://doi.org/10.1136/bmj.b3702.
- Mason J. Qualitative Researching. London: Sage Publications Ltd; 2002.
- Joffe H, Yardley L, Yardley L, Marks DF. Research Methods for Clinical and Health Psychology. London: Sage Publications Ltd; 2004.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2008;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Spencer L, Burgess R, Bryman A. Analyzing Qualitative Data. London: Routlege; 1994.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 6 June 2019).
- National Institute for Health and Care Excellence (NICE) . Methods for the Development of NICE Public Health Guidance: Incorporating Health Economics 2012. www.nice.org.uk/process/pmg4/chapter/incorporating-health-economics (accessed 6 June 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 6 June 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- NHS Digital . 2006/07/UK/General/Practice/Workload/Survey,/Primary/Care/Statistics 2007.
- South Lanarkshire Council . Meals at Home n.d. www.southlanarkshire.gov.uk/info/200227/care_for_the_elderly/485/meals_at_home (accessed 6 June 2019).
- Glasgow City Council . Lunch Clubs 2017. www.glasgow.gov.uk/index.aspx?articleid=17293 (accessed 6 June 2019).
- Royal College of Nursing . Pay Scales for NHS Nursing Staff in England, Wales, Scotland and Northern Ireland from 1 April 2016 n.d. www.rcn.org.uk/employment-and-pay/nhs-pay-scales-2016-17 (accessed 6 June 2019).
- Paton NI, Stöhr W, Oddershede L, Arenas-Pinto A, Walker S, Sculpher M, et al. The Protease Inhibitor Monotherapy Versus Ongoing Triple Therapy (PIVOT) trial: a randomised controlled trial of a protease inhibitor monotherapy strategy for long-term management of human immunodeficiency virus infection. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20210.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results From a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Frew E, Wolstenholme JL, Whynes DK. Willingness-to-pay for colorectal cancer screening. Eur J Cancer 2001;37:1746-51. https://doi.org/10.1016/S0959-8049(01)00200-3.
- McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. A multicentre randomised controlled trial and economic evaluation of continuous positive airway pressure for the treatment of obstructive sleep apnoea syndrome in older people: PREDICT. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19400.
- Morris ME, Menz HB, McGinley JL, Watts JJ, Huxham FE, Murphy AT, et al. A randomized controlled trial to reduce falls in people with Parkinson’s disease. Neurorehabil Neural Repair 2015;29:777-85. https://doi.org/10.1177/1545968314565511.
- Morris ME, Taylor NF, Watts JJ, Evans A, Horne M, Kempster P, et al. A home program of strength training, movement strategy training and education did not prevent falls in people with Parkinson’s disease: a randomised trial. J Physiother 2017;63:94-100. https://doi.org/10.1016/j.jphys.2017.02.015.
- Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet 2016;388:1170-82. https://doi.org/10.1016/S0140-6736(16)31325-3.
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med 2012;366:511-19. https://doi.org/10.1056/NEJMoa1107911.
- Shen X, Wong-Yu IS, Mak MK. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair 2016;30:512-27. https://doi.org/10.1177/1545968315613447.
- Lord S, Galna B, Yarnall AJ, Morris R, Coleman S, Burn D, et al. Natural history of falls in an incident cohort of Parkinson’s disease: early evolution, risk and protective features. J Neurol 2017;264:2268-76. https://doi.org/10.1007/s00415-017-8620-y.
- Heremans E, Nieuwboer A, Spildooren J, Vandenbossche J, Deroost N, Soetens E, et al. Cognitive aspects of freezing of gait in Parkinson’s disease: a challenge for rehabilitation. J Neural Transm 2013;120:543-57. https://doi.org/10.1007/s00702-012-0964-y.
- Heremans E, Nackaerts E, Vervoort G, Broeder S, Swinnen SP, Nieuwboer A. Impaired retention of motor learning of writing skills in patients with Parkinson’s disease with freezing of gait. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0148933.
- Biundo R, Weis L, Antonini A. Cognitive decline in Parkinson’s disease: the complex picture. NPJ Parkinsons Dis 2016;2. https://doi.org/10.1038/npjparkd.2016.18.
- Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, Wadia P, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord 2008;23:297-9. https://doi.org/10.1002/mds.21837.
- Domingos JM, Godinho C, Dean J, Coelho M, Pinto A, Bloem BR, et al. Cognitive impairment in fall-related studies in Parkinson's disease. J Parkinsons Dis 2015;5:453-69.
- Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 2008;56:2234-43. https://doi.org/10.1111/j.1532-5415.2008.02014.x.
- O’Brien, Dodd KJ, Bilney B. A qualitative analysis of a progressive resistance exercise programme for people with Parkinson’s disease. Disabil Rehabil 2008;30:1350-7. https://doi.org/10.1080/09638280701614546.
- Quinn L, Busse M, Khalil H, Richardson S, Rosser A, Morris H. Client and therapist views on exercise programmes for early-mid stage Parkinson’s disease and Huntington’s disease. Disabil Rehabil 2010;32:917-28. https://doi.org/10.3109/09638280903362712.
- O’Brien C, Clemson L, Canning CG. Multiple factors, including non-motor impairments, influence decision making with regard to exercise participation in Parkinson’s disease: a qualitative enquiry. Disabil Rehabil 2016;38:472-81. https://doi.org/10.3109/09638288.2015.1055377.
- Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson’s disease. Disabil Rehabil 2009;31:1925-36. https://doi.org/10.1080/09638280902850261.
- Coote S, Sosnoff JJ, Gunn H. Fall incidence as the primary outcome in multiple sclerosis falls-prevention trials: recommendation from the International MS Falls Prevention Research Network. Int J MS Care 2014;16:178-84. https://doi.org/10.7224/1537-2073.2014-059.
- Gazibara T, Kisic Tepavcevic D, Svetel M, Tomic A, Stankovic I, Kostic VS, et al. Near-falls in people with Parkinson’s disease: circumstances, contributing factors and association with falling. Clin Neurol Neurosurg 2017;161:51-5. https://doi.org/10.1016/j.clineuro.2017.08.008.
- Srygley JM, Herman T, Giladi N, Hausdorff JM. Self-report of missteps in older adults: a valid proxy of fall risk?. Arch Phys Med Rehabil 2009;90:786-92. https://doi.org/10.1016/j.apmr.2008.11.007.
Appendix 1 Participant invitation letter
Appendix 2 Participant information sheet
Appendix 3 Participant reply slip
Appendix 4 Falls diaries
Appendix 5 Recruitment and randomisation
FIGURE 13.
PDSAFE recruitment against target.
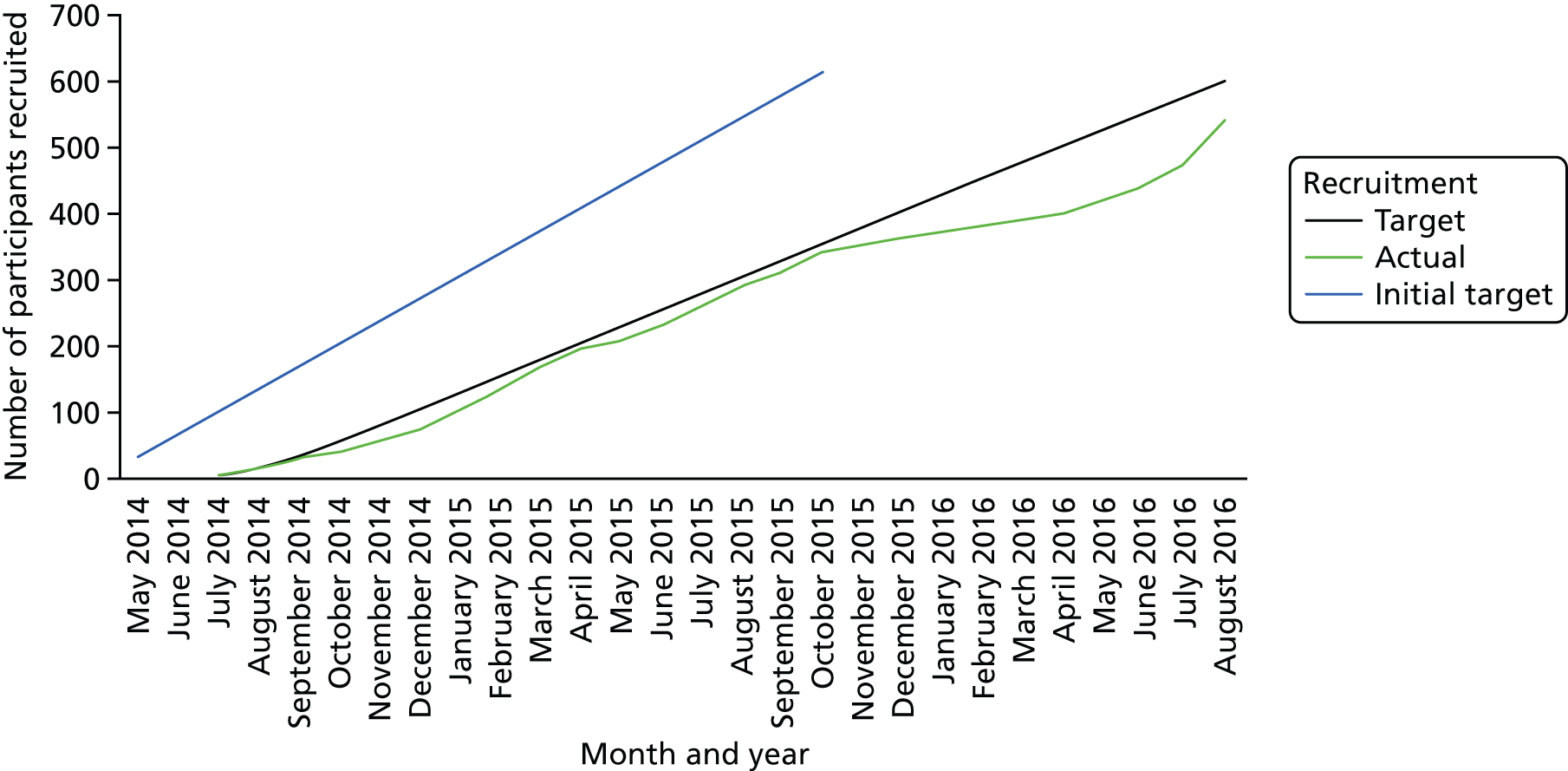
FIGURE 14.
PDSAFE randomisation against target (90% of those recruited).

Appendix 6 Power calculations
| Risk of repeat fallinga | Trial group (%) | Required in analysis (n) | Needed at (n) | |||
|---|---|---|---|---|---|---|
| Control | PDSAFE | Per group | Total | Recruitmentb | Randomisationc | |
| 0–6 months (5% loss to follow-up) | ||||||
| 13% difference | 63 | 50 | 228 | 456 | 534 | 480 |
| 15% difference | 70 | 55 | 163 | 326 | 382 | 344 |
| 15% difference | 60 | 45 | 173 | 346 | 408 | 366 |
| 13% difference | 63 | 50 | 228 | 456 | 534 | 480 |
| 6–12 months (10% loss to follow-up) | ||||||
| 13% difference | 63 | 50 | 228 | 456 | 564 | 508 |
| 15% difference | 70 | 55 | 163 | 326 | 404 | 364 |
| 15% difference | 60 | 45 | 173 | 346 | 430 | 386 |
| Fall ratesa | Baseline rate per 3 months | Required in analysis (n) | Needed at (n) | ||
|---|---|---|---|---|---|
| Per group | Total | Recruitmentb | Randomisationc | ||
| FRR 0–6 months (5% loss to follow-up) | |||||
| 0.75 | 3 | 100 | 200 | 236 | 212 |
| 0.8 | 3 | 164 | 328 | 386 | 346 |
| 0.75 | 2.5 | 120 | 240 | 278 | 254 |
| 0.8 | 2.5 | 197 | 394 | 464 | 416 |
| 0.75 | 2 | 150 | 300 | 352 | 316 |
| 0.8 | 2 | 246 | 492 | 576 | 518 |
| FRR 6–12 months (10% loss to follow-up) | |||||
| 0.75 | 3 | 100 | 200 | 250 | 224 |
| 0.8 | 3 | 164 | 328 | 408 | 366 |
| 0.75 | 2.5 | 120 | 240 | 298 | 268 |
| 0.8 | 2.5 | 197 | 394 | 488 | 438 |
| 0.75 | 2 | 150 | 300 | 372 | 334 |
| 0.8 | 2 | 246 | 492 | 610 | 548 |
Appendix 7 Sensitivity analysis
| Period | Trial group, n/N (%) | PDSAFE/controla OR (95% CI) | p-valuea | |
|---|---|---|---|---|
| PDSAFE | Control | |||
| Repeat falling restricted to ≥ 50% diaries | ||||
| 0–6 months | 125/203 (62) | 116/211 (55) | 1.21 (0.74 to 1.98) | 0.447 |
| 6–12 months | 57/114 (50) | 71/132 (54) | 0.86 (0.45 to 1.65) | 0.657 |
| Repeat fallers restricted to full diary completion | ||||
| 0–6 months | 79/130 (61) | 83/158 (53) | 1.31 (0.72 to 2.39) | 0.374 |
| 6–12 months | 34/64 (53) | 37/76 (49) | 1.28 (0.47 to 3.52) | 0.627 |
| Repeat fallers unrestricted to diary completion | ||||
| 0–6 months | 134/237(57) | 127/236 (54) | 1.07 (0.69 to 1.66) | 0.759 |
| 6–12 months | 63/137 (46) | 78/150 (52) | 0.68 (0.38 to 1.22) | 0.200 |
Appendix 8 Repeat falling
| Participant group | Period (months) | Trial group, n/N (%) | PDSAFE/control OR (95% CI)a | p-valuea | ||
|---|---|---|---|---|---|---|
| PDSAFE | Control | PDSAFE within participant group | Interaction | |||
| All participants | 0 to 6 | 125/203 (62) | 116/211 (55) | 1.21 (0.74 to 1.98) | 0.447 | |
| 6 to 12 | 57/114 (50) | 71/132 (54) | 0.86 (0.45 to 1.65) | 0.657 | ||
| MDS-UPDRS score of ≤ 58 | 0 to 6 | 115/192 (60) | 106/195 (54) | 1.18 (0.71 to 1.95) | 0.521 | |
| 6 to 12 | 55/111 (50) | 66/125 (53) | 0.90 (0.47 to 1.74) | 0.758 | ||
| H&Y scale stage ≤ 3 | 0 to 6 | 104/180 (58) | 92/179 (51) | 1.13 (0.68 to 1.87) | 0.651 | |
| 6 to 12 | 50/106 (47) | 54/113 (48) | 0.91 (47 to 1.76) | 0.785 | ||
| MoCA score | ||||||
| ≤ 25 | 0 to 6 | 49/74 (66) | 45/80 (56) | 2.03 (0.91 to 4.54) | 0.083 | 0.111 |
| ≥ 26 | 0 to 6 | 76/129 (59) | 71/131 (54) | 0.91 (0.49 to 1.67) | 0.754 | |
| ≤ 25 | 6 to 12 | 20/43 (47) | 33/56 (59) | 0.71 (0.25 to 2.02) | 0.525 | 0.606 |
| ≥ 26 | 6 to 12 | 37/71 (52) | 38/76 (50) | 1.01 (0.44 to 2.29) | 0.987 | |
| FoG | ||||||
| No | 0 to 6 | 27/76 (36) | 38/93 (41) | 0.66 (0.32 to 1.38) | 0.269 | 0.025 |
| Yes | 0 to 6 | 98/126 (78) | 78/118 (66) | 2.04 (1.03 to 4.06) | 0.042 | |
| No | 6 to 12 | 12/43 (28) | 22/58 (38) | 0.58 (0.21 to 1.57) | 0.282 | 0.309 |
| Yes | 6 to 12 | 45/70 (64) | 49/74 (66) | 1.14 (0.48 to 2.70) | 0.772 | |
| MoCA score | ||||||
| ≤ 25 | 0 to 6 | 49/74 (66) | 45/80 (56) | 2.04 (0.91 to 4.55) | 0.083 | 0.238 |
| 26 or 27 | 0 to 6 | 32/55 (58) | 27/53 (51) | 1.07 (0.42 to 2.79) | 0.863 | |
| ≥ 28 | 0 to 6 | 44/74 (59) | 44/78 (56) | 0.78 (0.35 to 1.75) | 0.352 | |
| ≤ 25 | 6 to 12 | 20/43 (47) | 33/56 (59) | 0.71 (0.25 to 2.01) | 0.248 | 0.751 |
| 26 or 27 | 6 to 12 | 16/32 (50) | 14/31 (45) | 1.30 (0.38 to 4.43) | 0.680 | |
| ≥ 28 | 6 to 12 | 21/39 (54) | 24/45 (53) | 0.82 (0.27 to 2.45) | 0.720 | |
| MDS-UPDRS score | ||||||
| ≥ 39 | 0 to 6 | 44/61 (72) | 41/74 (55) | 2.55 (1.03 to 6.33) | 0.044 | 0.140 |
| 23–38 | 0 to 6 | 48/77 (62) | 35/60 (58) | 0.80 (0.35 to 1.86) | 0.609 | |
| ≤ 22 | 0 to 6 | 33/65 (51) | 39/76 (51) | 0.97 (0.43 to 2.17) | 0.942 | |
| ≥ 39 | 6 to 12 | 16/29 (55) | 29/46 (63) | 0.80 (0.23 to 2.80) | 0.727 | 0.943 |
| 23–38 | 6 to 12 | 24/49 (50) | 18/38 (47) | 1.02 (0.35 to 2.98) | 0.976 | |
| ≤ 22 | 6 to 12 | 17/36 (47) | 23/47 (49) | 1.02 (0.35 to 2.98) | 0.936 | |
| Retrospective falls | ||||||
| > 3 | 0 to 6 | 75/88 (85) | 49/63 (78) | 1.45 (0.57 to 3.67) | 0.436 | 0.407 |
| 2 or 3 | 0 to 6 | 35/71 (49) | 58/104 (56) | 0.79 (0.39 to 1.61) | 0.523 | |
| 1 | 0 to 6 | 15/44 (34) | 9/44 (20) | 1.68 (0.59 to 4.77) | 0.331 | |
| > 3 | 6 to 12 | 34/45 (76) | 31/41 (76) | 1.18 (0.36 to 3.88) | 0.780 | 0.501 |
| 2 or 3 | 6 to 12 | 17/43 (40) | 35/62 (56) | 0.57 (0.22 to 1.49) | 0.251 | |
| 1 | 6 to 12 | 6/26 (23) | 5/29 (17) | 1.36 (0.32 to 5.73) | 0.672 | |
Appendix 9 Falls rate ratio
| Participant group | Period (months) | Participants contributing diaries, n | PDSAFE/control FRR (95% CI)a | p-valuea | |
|---|---|---|---|---|---|
| PDSAFE within participant group | Interaction | ||||
| All participants | 0–6 | 461 | 0.98 (0.80 to 1.19) | 0.824 | |
| 6–12 | 274 | 0.83 (0.62 to 1.11) | 0.200 | ||
| MDS-UPDRS score of ≤ 58 | 0–6 | 429 | 0.95 (0.77 to 1.18) | 0.648 | |
| 6–12 | 256 | 0.79 (0.58 to 1.08) | 0.142 | ||
| H&Y scale stage ≤ 3 | 0–6 | 395 | 0.99 (0.78 to 1.25) | 0.915 | |
| 6–12 | 240 | 0.80 (0.57 to 1.12) | 0.191 | ||
| MoCA score | |||||
| ≤ 25 | 0–6 | 178 | 1.19 (0.88 to 1.61) | 0.255 | 0.088 |
| ≥ 26 | 0–6 | 283 | 0.85 (0.66 to 1.09) | 0.208 | |
| ≤ 25 | 6–12 | 112 | 1.06 (0.67 to 1.66) | 0.810 | 0.154 |
| ≥ 26 | 6–12 | 162 | 0.69 (0.48 to 1.00) | 0.050 | |
| FoG | |||||
| No | 0–6 | 181 | 0.89 (0.61 to 1.29) | 0.527 | 0.566 |
| Yes | 0–6 | 279 | 1.01 (0.80 to 1.26) | 0.964 | |
| No | 6–12 | 112 | 0.81 (0.47 to 1.38) | 0.766 | 0.956 |
| Yes | 6–12 | 161 | 0.82 (0.58 to 1.15) | 0.251 | |
| MoCA score | |||||
| ≤ 25 | 0–6 | 178 | 1.12 (0.89 to 1.61) | 0.246 | 0.089 |
| 26 or 27 | 0–6 | 119 | 1.06 (0.71 to 1.58) | 0.778 | |
| ≥ 28 | 0–6 | 164 | 0.75 (0.55 to 1.02) | 0.055 | |
| ≤ 25 | 6–12 | 112 | 1.06 (0.67 to 1.67) | 0.800 | 0.361 |
| 26 or 27 | 6–12 | 70 | 0.66 (0.37 to 1.16) | 0.148 | |
| ≥ 28 | 6–12 | 92 | 0.72 (0.45 to 1.17) | 0.191 | |
| MDS-UPDRS score | |||||
| ≥ 39 | 0–6 | 152 | 1.43 (1.04 to 1.95) | 0.028 | 0.009 |
| 23–38 | 0–6 | 155 | 0.70 (0.50 to 0.98) | 0.038 | |
| ≤ 22 | 0–6 | 152 | 0.97 (0.69 to 1.37) | 0.866 | |
| ≥ 39 | 6–12 | 87 | 1.07 (0.67 to 1.72) | 0.773 | 0.231 |
| 23–38 | 6–12 | 95 | 0.60 (0.36 to 1.01) | 0.057 | |
| ≤ 22 | 6–12 | 91 | 1.00 (0.59 to 1.70) | 0.992 | |
| Retrospective falls | |||||
| > 3 | 0–6 | 168 | 1.16 (0.88 to 1.52) | 0.286 | 0.050 |
| 2 or 3 | 0–6 | 196 | 0.70 (0.50 to 0.96) | 0.026 | |
| 1 | 0–6 | 97 | 1.03 (0.59 to 1.80) | 0.916 | |
| > 3 | 6–12 | 96 | 0.98 (0.64 to 1.49) | 0.925 | 0.402 |
| 2 or 3 | 6–12 | 119 | 0.64 (0.40 to 1.04) | 0.072 | |
| 1 | 6–12 | 59 | 0.69 (0.31 to 1.54) | 0.364 | |
Appendix 10 Near-falls rate ratio
| Participant group | Period (months) | Participants contributing diaries, n | PDSAFE/control FRR (95% CI)a | p-valuea | |
|---|---|---|---|---|---|
| PDSAFE within participant group | Interaction | ||||
| All participants | 0–6 | 447 | 0.67 (0.53 to 0.86) | 0.001 | |
| 6–12 | 280 | 1.01 (0.67 to 1.52) | 0.968 | ||
| MDS-UPDRS score of ≤ 58 | 0–6 | 415 | 0.63 (0.49 to 0.80) | 0.000 | |
| 6–12 | 262 | 0.97 (0.64 to 1.46) | 0.881 | ||
| H&Y scale stage ≤ 3 | 0–6 | 386 | 0.65 (0.51 to 0.83) | 0.001 | |
| 6–12 | 243 | 0.92 (0.60 to 1.41) | 0.701 | ||
| MoCA score | |||||
| ≤ 25 | 0–6 | 169 | 0.64 (0.43 to 0.96) | 0.032 | 0.755 |
| ≥ 26 | 0–6 | 281 | 0.69 (0.51 to 0.93) | 0.015 | |
| ≤ 25 | 6–12 | 113 | 1.00 (0.53 to 1.88) | 0.992 | 0.981 |
| ≥ 26 | 6–12 | 168 | 0.99 (0.59 to 1.68) | 0.980 | |
| FoG | |||||
| No | 0–6 | 178 | 0.55 (0.37 to 0.84) | 0.005 | 0.277 |
| Yes | 0–6 | 271 | 0.74 (0.54 to 0.99) | 0.048 | |
| No | 6–12 | 116 | 1.04 (0.54 to 2.03) | 0.899 | 0.903 |
| Yes | 6–12 | 165 | 0.99 (0.59 to 1.66) | 0.976 | |
| MoCA score | |||||
| ≤ 25 | 0–6 | 169 | 0.64 (0.43 to 0.96) | 0.033 | 0.885 |
| 26 or 27 | 0–6 | 119 | 0.74 (0.47 to 1.17) | 0.199 | |
| ≥ 28 | 0–6 | 162 | 0.66 (0.45 to 0.98) | 0.039 | |
| ≤ 25 | 6–12 | 113 | 0.97 (0.52 to 1.81) | 0.930 | 0.407 |
| 26 or 27 | 6–12 | 71 | 1.33 (0.63 to 2.82) | 0.452 | |
| ≥ 28 | 6–12 | 97 | 0.67 (0.33 to 1.35) | 0.266 | |
| MDS-UPDRS score | |||||
| ≥ 39 | 0–6 | 148 | 0.74 (0.49 to 1.12) | 0.152 | 0.364 |
| 23–38 | 0–6 | 150 | 0.56 (0.37 to 0.85) | 0.007 | |
| ≤ 22 | 0–6 | 150 | 0.85 (0.56 to 1.28) | 0.429 | |
| ≥ 39 | 6–12 | 90 | 1.32 (0.65 to 2.70) | 0.446 | 0.567 |
| 23–38 | 6–12 | 97 | 0.77 (0.38 to 1.58) | 0.483 | |
| ≤ 22 | 6–12 | 93 | 1.11 (0.56 to 2.21) | 0.763 | |
| Retrospective falls | |||||
| > 3 | 0–6 | 166 | 0.74 (0.51 to 1.07) | 0.113 | 0.652 |
| 2 or 3 | 0–6 | 187 | 0.70 (0.47 to 1.03) | 0.073 | |
| 1 | 0–6 | 97 | 0.54 (0.31 to 0.95) | 0.032 | |
| > 3 | 6–12 | 98 | 1.17 (0.61 to 2.26) | 0.630 | 0.358 |
| 2 or 3 | 6–12 | 124 | 1.21 (0.65 to 2.27) | 0.545 | |
| 1 | 6–12 | 59 | 0.54 (0.20 to 1.43) | 0.215 | |
Appendix 11 Mini-Balance Evaluation Systems Test
| Participant group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | ||
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | PDSAFE within participant group | Interaction | ||||
| All participants | 6 | 394 | 1.1 (3.8) | 0.2 (3.8) | 0.95 (0.24 to 1.67) | 0.009 | |
| 12 | 241 | –0.7 (4.5) | –0.2 (3.8) | –0.41 (–1.48 to 0.66) | 0.449 | ||
| MDS-UPDRS score of ≤ 58 | 6 | 369 | 1.1 (3.8) | 0.1 (3.6) | 1.04 (0.32 to 1.77) | 0.005 | |
| 12 | 227 | –0.8 (4.4) | –0.1 (3.8) | –0.52 (–1.62 to 0.58) | 0.353 | ||
| H&Y scale stage ≤ 3 | 6 | 345 | 1.1 (3.7) | 0.0 (3.6) | 1.01 (0.35 to 1.82) | 0.004 | |
| 12 | 216 | –0.7 (4.4) | –0.5 (3.8) | –0.30 (–1.43 to 0.84) | 0.607 | ||
| MoCA score | |||||||
| ≤ 25 | 6 | 140 | 0.9 (4.0) | 0.2 (4.5) | 0.73 (–0.46 to 1.91) | 0.228 | 0.654 |
| ≥ 26 | 6 | 254 | 1.2 (3.7) | 0.2 (3.3) | 1.06 (0.18 to 1.95) | 0.019 | |
| ≤ 25 | 12 | 91 | –1.2 (5.0) | 0 (4.1) | –0.76 (–2.47 to 0.95) | 0.384 | 0.570 |
| ≥ 26 | 12 | 150 | –0.3 (4.1) | –0.2 (4.1) | –0.13 (–1.49 to 1.23) | 0.851 | |
| FoG | |||||||
| No | 6 | 157 | 0.7 (3.7) | –0.1 (3.5) | 0.71 (–0.42 to 1.83) | 0.220 | 0.570 |
| Yes | 6 | 236 | 1.4 (3.6) | 0.5 (4.0) | 1.12 (0.20 to 2.04) | 0.017 | |
| No | 12 | 101 | –0.6 (3.8) | –0.4 (4.1) | 0.05 (–1.59 to 1.69) | 0.950 | 0.464 |
| Yes | 12 | 139 | –0.8 (4.9) | 0.1 (3.7) | –0.74 (–2.13 to 0.66) | 0.299 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 140 | 0.9 (4.0) | 0.2 (4.5) | 0.74 (–0.45 to1.92) | 0.225 | 0.892 |
| 12 | 91 | –1.2 (5.0) | 0 (3.4) | –0.75 (–2.47 to 0.97) | 0.391 | 0.786 | |
| 26 or 27 | 6 | 106 | 1.2 (4.4) | 0.1 (3.4) | 1.16 (–0.21 to 2.53) | 0.096 | |
| 12 | 63 | –0.3 (4.6) | –0.6 (5.0) | 0.24 (–1.84 to 2.31) | 0.822 | ||
| ≥ 28 | 6 | 148 | 1.2 (3.2) | 0.3 (3.2) | 1.01 (–0.14 to 2.16) | 0.086 | |
| 12 | 87 | –0.4 (3.8) | 0 (3.5) | –0.35 (–2.12 to 1.43) | 0.701 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 126 | 1.28 (4.2) | 0.6 (4.4) | 0.77 (–0.49 to 2.04) | 0.231 | 0.255 |
| 12 | 73 | 0.1 (4.9) | 1.5 (3.5) | –1.49 (–3.44 to 0.45) | 0.132 | 0.493 | |
| 23–38 | 6 | 126 | 1.9 (3.9) | 0.4 (3.3) | 1.64 (0.40 to 2.87) | 0.010 | |
| 12 | 80 | –0.5 (4.6) | –0.6 (3.4) | 0.07 (–1.74 to 1.88) | 0.939 | ||
| ≤ 22 | 6 | 139 | 0 (3.2) | –0.3 (3.4) | 0.21 (–0.98 to 1.40) | 0.729 | |
| 12 | 85 | –1.46 (3.8) | –1.2 (4.0) | –0.41 (–2.14 to 1.33) | 0.647 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 147 | 1.1 (3.8) | 0.6 (3.5) | 0.70 (–0.46 to 1.86) | 0.236 | 0.162 |
| 12 | 85 | –1.1 (5.2) | 0.1 (3.7) | –1.31 (–3.09 to 0.47) | 0.149 | 0.195 | |
| 2 or 3 | 6 | 168 | 1.2 (4.1) | 0 (4.1) | 1.78 (0.66 to 2.90) | 0.002 | |
| 12 | 104 | –0.4 (3.9) | –0.6 (4.2) | 0.78 (–0.90 to 2.46) | 0.360 | ||
| 1 | 6 | 79 | 0.9 (3.3) | 0.1 (3.4) | 0 (–1.58 to 1.58) | 0.998 | |
| 12 | 52 | –0.2 (4.0) | 0.5 (3.0) | –1.14 (–3.44 to 1.16) | 0.330 | ||
Appendix 12 Falls Efficacy Scale – International
| Participants group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | ||
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | PDSAFE within participant group | Interaction | ||||
| All participants | 6 | 400 | –0.7 (7.9) | 1.1 (7.2) | –1.60 (–3.0 to –0.19) | 0.026 | |
| 12 | 354 | 1.3 (8.2) | 3.5 (9.3) | –1.40 (–3.41 to 0.66) | 0.184 | ||
| MDS-UPDRS score of ≤ 58 | 6 | 376 | –0.8 (7.9) | 1.1 (7.0) | –1.44 (–2.85 to –0.02) | 0.047 | |
| 12 | 241 | 1.3 (8.3) | 3.1 (8.8) | –0.93 (–2.97 to 1.12) | 0.372 | ||
| H&Y scale stage ≤ 3 | 6 | 352 | –0.9 (7.9) | 1.2 (6.7) | –1.5 (–2.97 to 0.04) | 0.045 | |
| 12 | 228 | 0.9 (8.1) | 3.7 (9.1) | –1.50 (–3.63 to 0.65) | 0.170 | ||
| MoCA score | |||||||
| ≤ 25 | 6 | 145 | –0.4 (7.7) | 1.8 (8.3) | –1.89 (–4.16 to 0.37) | 0.101 | 0.724 |
| ≥ 26 | 6 | 255 | –0.9 (8.0) | 0.6 (6.3) | –1.39 (–3.11 to 0.34) | 0.115 | |
| ≤ 25 | 12 | 99 | 1.9 (8.1) | 4.3 (10.3) | –2.20 (–5.41 to 1.02) | 0.180 | 0.534 |
| ≥ 26 | 12 | 155 | 1.0 (8.2) | 2.9 (8.6) | –0.91 (–3.49 to 1.67) | 0.489 | |
| FoG | |||||||
| No | 6 | 159 | 0.1 (6.5) | 0.3 (5.8) | –0.18 (–2.33 to 1.98) | 0.872 | 0.085 |
| Yes | 6 | 240 | –1.2 (8.6) | 1.7 (8.0) | –2.60 (–4.37 to –0.82) | 0.004 | |
| No | 12 | 105 | 1.1 (6.5) | 2.6 (7.6) | –0.89 (–3.96 to 2.18) | 0.569 | 0.580 |
| Yes | 12 | 148 | 1.5 (9.1) | 4.2 (8.6) | –2.01 (–4.63 to 0.61) | 0.132 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 145 | –0.4 (7.7) | 1.8 (8.3) | –1.92 (–4.18 to 0.33) | 0.095 | 0.448 |
| 12 | 99 | 1.9 (8.1) | 4.3 (10.3) | –2.23 (–5.43 to 0.97) | 0.170 | 0.112 | |
| 26 or 27 | 6 | 107 | 0.2 (9.4) | 0.6 (6.9) | –0.21 (–2.83 to 2.41) | 0.873 | |
| 12 | 67 | 2.9 (8.6) | 1.9 (7.4) | 1.93 (–1.92 to 5.77) | 0.325 | ||
| ≥ 28 | 6 | 148 | –1.7 (6.6) | 0.7 (6.0) | –2.33 (–4.56 to -0.09) | 0.041 | |
| 12 | 88 | –0.6 (7.6) | 3.6 (9.4) | –3.26 (–6.66 to 0.13) | 0.060 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 140 | –0.5 (9.3) | 1.5 (5.9) | –1.68 (–3.97 to 0.60) | 0.149 | 0.929 |
| 12 | 86 | 3.4 (7.2) | 2.7 (8.6) | –1.89 (–5.22 to 1.44) | 0.256 | 0.507 | |
| 23–38 | 6 | 132 | –0.3 (7.6) | 0.4 (6.5) | –1.12 (–3.47 to 1.23) | 0.349 | |
| 12 | 88 | 0.6 (8.7) | 2.4 (9.3) | 0.07 (–3.24 to 3.38) | 0.967 | ||
| ≤ 22 | 6 | 124 | –1.6 (6.4) | 0.7 (6.4) | –1.67 (–4.14 to 0.80) | 0.185 | |
| 12 | 77 | 0.7 (9.3) | 4.8 (11.4) | –2.72 (–6.44 to 1.00) | 0.151 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 146 | –1.7 (8.5) | 0.4 (6.5) | –2.15 (–4.44 to 0.14) | 0.065 | 0.834 |
| 12 | 87 | 0.6 (8.7) | 2.4 (9.3) | –1.58 (–5.00 to 1.84) | 0.364 | 0.994 | |
| 2 or 3 | 6 | 170 | –0.3 (6.4) | 1.1 (8.2) | –1.32 (–3.51 to 0.87) | 0.238 | |
| 12 | 110 | 1.17 (7.2) | 3.3 (9.8) | –1.33 (–4.54 to 1.88) | 0.415 | ||
| 1 | 6 | 84 | 0.6 (8.1) | 1.9 (5.4) | –1.21 (–4.20 to 1.78) | 0.426 | |
| 12 | 58 | 2.8 (8.6) | 5.2 (8.3) | –1.43 (–5.67 to 2.82) | 0.509 | ||
Appendix 13 Repeat falling
| Participant group | Period (months) | ITT | Per protocol | ||||
|---|---|---|---|---|---|---|---|
| PDSAFE/control OR (95% CI)a | PDSAFE within participant group p-valuea | Interaction p-valuea | PDSAFE/control OR (95% CI)a | PDSAFE within participant group p-valuea | Interaction p-valuea | ||
| All participants | 0–6 | 1.21 (0.74 to 1.98) | 0.447 | 1.16 (0.71 to 1.92) | 0.538 | ||
| 6–12 | 0.86 (0.45 to 1.65) | 0.657 | 0.92 (0.47 to 1.77) | 0.793 | |||
| MDS-UPDRS score of ≤ 58 | 0–6 | 1.18 (0.71 to 1.95) | 0.521 | 1.14 (0.69 to 1.89) | 0.616 | ||
| 6–12 | 0.90 (0.47 to 1.74) | 0.758 | 0.96 (0.49 to 1.86) | 0.896 | |||
| H&Y scale stage ≤ 3 | 0–6 | 1.13 (0.68 to 1.87) | 0.651 | 1.10 (0.66 to 1.83) | 0.723 | ||
| 6–12 | 0.91 (47 to 1.76) | 0.785 | 0.97 (47 to 1.76) | 0.918 | |||
| MoCA score | |||||||
| ≤ 25 | 0–6 | 2.03 (0.91 to 4.54) | 0.083 | 0.111 | 1.96 (0.88 to 4.41) | 0.101 | 0.113 |
| ≥ 26 | 0–6 | 0.91 (0.49 to 1.67) | 0.754 | 0.87 (0.47 to 1.63) | 0.672 | ||
| ≤ 25 | 6–12 | 0.71 (0.25 to 2.02) | 0.525 | 0.606 | 0.75 (0.26 to 2.12) | 0.583 | 0.561 |
| ≥ 26 | 6–12 | 1.01 (0.44 to 2.29) | 0.987 | 1.10 (0.48 to 2.55) | 0.819 | ||
| FoG | |||||||
| No | 0–6 | 0.66 (0.32 to 1.38) | 0.269 | 0.025 | 0.66 (0.32 to 1.39) | 0.280 | 0.039 |
| Yes | 0–6 | 2.04 (1.03 to 4.06) | 0.042 | 1.90 (0.95 to 3.81) | 0.068 | ||
| No | 6–12 | 0.58 (0.21 to 1.57) | 0.282 | 0.309 | 0.59 (0.22 to 1.63) | 0.311 | 0.304 |
| Yes | 6–12 | 1.14 (0.48 to 2.70) | 0.772 | 1.25 (0.52 to 2.83) | 0.620 | ||
| MoCA score | |||||||
| ≤ 25 | 0–6 | 2.04 (0.91 to 4.55) | 0.083 | 0.238 | 1.96 (0.88 to 4.41) | 0.101 | 0.264 |
| 26 or 27 | 0–6 | 1.07 (0.42 to 2.79) | 0.863 | 0.94 (0.36 to 2.45) | 0.902 | ||
| ≥ 28 | 0–6 | 0.78 (0.35 to 1.75) | 0.352 | 0.81 (0.36 to 1.81) | 0.608 | ||
| ≤ 25 | 6–12 | 0.71 (0.25 to 2.01) | 0.248 | 0.751 | 0.74 (0.25 to 2.01) | 0.575 | 0.820 |
| 26 or 27 | 6–12 | 1.30 (0.38 to 4.43) | 0.680 | 1.23 (0.38 to 4.43) | 0.745 | ||
| ≥ 28 | 6–12 | 0.82 (0.27 to 2.45) | 0.720 | 1.00 (0.27 to 2.45) | 0.994 | ||
| MDS-UPDRS | |||||||
| ≥ 39 | 0–6 | 2.55 (1.03 to 6.33) | 0.044 | 0.140 | 2.45 (0.98 to 6.18) | 0.055 | 0.149 |
| 23–38 | 0–6 | 0.80 (0.35 to 1.86) | 0.609 | 0.79 (0.33 to 1.84) | 0.588 | ||
| ≤ 22 | 0–6 | 0.97 (0.43 to 2.17) | 0.942 | 0.92 (0.41 to 2.08) | 0.839 | ||
| ≥ 39 | 6–12 | 0.80 (0.23 to 2.80) | 0.727 | 0.943 | 0.95 (0.26 to 3.43) | 0.940 | 0.989 |
| 23–38 | 6–12 | 1.02 (0.35 to 2.98) | 0.976 | 1.08 (0.37 to 3.16) | 0.895 | ||
| ≤ 22 | 6–12 | 1.02 (0.35 to 2.98) | 0.936 | 1.01 (0.33 to 3.05) | 0.989 | ||
| Retrospective falls | |||||||
| > 3 | 0–6 | 1.45 (0.57 to 3.67) | 0.436 | 0.407 | 1.38 (0.54 to 3.52) | 0.497 | 0.453 |
| 2 or 3 | 0–6 | 0.79 (0.39 to 1.61) | 0.523 | 0.79 (0.39 to 1.61) | 0.511 | ||
| 1 | 0–6 | 1.68 (0.59 to 4.77) | 0.331 | 1.61 (0.56 to 4.66) | 0.379 | ||
| > 3 | 6–12 | 1.18 (0.36 to 3.88) | 0.780 | 0.501 | 1.12 (0.34 to 3.71) | 0.853 | 0.575 |
| 2 or 3 | 6–12 | 0.57 (0.22 to 1.49) | 0.251 | 0.64 (0.24 to 1.68) | 0.365 | ||
| 1 | 6–12 | 1.36 (0.32 to 5.73) | 0.672 | 1.53 (0.36 to 6.50) | 0.568 | ||
Appendix 14 Falls rate ratio
| Participant group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | ||
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | PDSAFE within participant group | Interaction | ||||
| All participants | 6 | 402 | 1.9 (10.7) | 1.0 (11.7) | 0.16 (–1.83 to 2.15) | 0.876 | |
| 12 | 254 | 7.2 (13.5) | 3.3 (13.4) | 3.86 (0.99 to 6.73) | 0.009 | ||
| MDS-UPDRS score of ≤ 58 | 6 | 378 | 2.4 (10.3) | 2.2 (10.6) | –0.08 (–2.08 to 1.93) | 0.938 | |
| 12 | 241 | 7.6 (13.2) | 4.3 (12.8) | 3.86 (0.94 to 6.78) | 0.010 | ||
| H&Y scale stage ≤ 3 | 6 | 352 | 2.4 (10.4) | 1.7 (10.8) | 0.25 (–1.77 to 2.27) | 0.809 | |
| 12 | 227 | 7.3 (13.2) | 3.9 (14.5) | 3.67 (0.76 to 6.58) | 0.014 | ||
| MoCA score | |||||||
| ≤ 25 | 6 | 145 | 2.1 (10.6) | 1.2 (12.5) | –0.06 (–3.32 to 3.20) | 0.968 | 0.860 |
| ≥ 26 | 6 | 257 | 1.8 (10.8) | 0.9 (11.2) | 0.29 (–2.17 to 2.75) | 0.812 | |
| ≤ 25 | 12 | 98 | 5.3 (13.6) | 3.6 (12.8) | 1.45 (–3.09 to 6.00) | 0.529 | 0.186 |
| ≥ 26 | 12 | 156 | 8.2 (13.4) | 3.1 (13.9) | 5.32 (1.69 to 8.94) | 0.004 | |
| FoG | |||||||
| No | 6 | 160 | 2.9 (11.2) | 2.0 (10.6) | 0.42 (–2.69 to 3.53) | 0.791 | 0.844 |
| Yes | 6 | 241 | 1.4 (10.3) | 0.4 (12.4) | 0.02 (–2.53 to 2.58) | 0.987 | |
| No | 12 | 102 | 6.5 (14.7) | 3.2 (13.0) | 3.34 (–1.03 to 7.72) | 0.133 | 0.846 |
| Yes | 12 | 148 | 7.6 (12.8) | 3.4 (13.8) | 3.90 (0.19 to 7.61) | 0.039 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 145 | 2.0 (10.5) | 1.1 (12.5) | –0.06 (–3.32 to 3.20) | 0.970 | 0.401 |
| 12 | 98 | 5.2 (13.6) | 3.6 (12.7) | 1.40 (–3.10 to 5.90) | 0.540 | 0.448 | |
| 26 or 27 | 6 | 107 | 3.4 (10.7) | 0.1 (11.5) | 2.22 (–1.56 to 5.99) | 0.249 | |
| 12 | 67 | 12.1 (12.6) | 6.2 (14.2) | 5.66 (0.27 to 11.05) | 0.040 | ||
| ≥ 28 | 6 | 150 | 0.5 (10.7) | 1.4 (11.0) | –1.13 (–4.32 to 2.07) | 0.488 | |
| 12 | 89 | 4.9 (13.1) | 0.9 (13.3) | 4.37 (–0.38 to 9.11) | 0.071 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 128 | –1.2 (10.5) | –6.2 (12.5) | 4.20 (0.71 to 7.69) | 0.018 | 0.007 |
| 12 | 79 | –0.6 (13.5) | –6.2 (13.2) | 6.89 (1.70 to 12.09) | 0.009 | 0.295 | |
| 23–38 | 6 | 132 | –0.3 (9.7) | 3.1 (9.1) | –3.47 (–6.83 to –0.11) | 0.043 | |
| 12 | 87 | 6.5 (13.1) | 5.7 (9.7) | 1.36 (–3.4 to 6.12) | 0.574 | ||
| ≤ 22 | 6 | 140 | 7.1 (10.2) | 6.3 (9.2) | 0.12 (–3.15 to 3.40) | 0.941 | |
| 12 | 86 | 14.1 (10.2) | 11.1 (10.3) | 4.07 (–0.69 to 8.82) | 0.093 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 148 | 2.2 (11.9) | –0.9 (12.6) | 1.90 (–1.34 to 5.14) | 0.249 | 0.403 |
| 12 | 87 | 7.3 (14.0) | 0 (16.1) | 7.00 (2.21 to 11.79) | 0.004 | 0.277 | |
| 2 or 3 | 6 | 171 | 2.6 (10.2) | 1.5 (12.1) | –0.68 (–3.80 to 2.44) | 0.668 | |
| 12 | 109 | 9.4 (10.5) | 4.8 (13.5) | 3.07 (–1.41 to 7.54) | 0.179 | ||
| 1 | 6 | 83 | –0.1 (8.6) | 2.9 (8.6) | –1.18 (–5.49 to 3.14) | 0.592 | |
| 12 | 58 | 3.2 (16.2) | 4.3 (7.9) | 1.29 (–4.64 to 7.22) | 0.668 | ||
Appendix 15 Physical Activity Scale for the Elderly
| Participant group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | ||
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | PDSAFE within participant group | Interaction | ||||
| All participants | 6 | 331 | 2.6 (51.0) | 1.3 (49.5) | 1.27 (–9.0 to 11.5) | 0.808 | – |
| 12 | 253 | –8.7 (53.0) | –11.0 (48.5) | 0.52 (–12.8 to 13.8) | 0.939 | – | |
| MDS-UPDRS score of ≤ 58 | 6 | 312 | 2.4 (52.0) | 0.9 (50.0) | 1.19 (–9.52 to 11.89) | 0.828 | – |
| 12 | 200 | –8.1 (54.3) | –10.6 (48.8) | 0.78 (–13.28 to 14.82) | 0.914 | – | |
| H&Y scale stage ≤ 3 | 6 | 292 | 2.4 (52.0) | 3.2 (51.3) | 3.50 (–7.85 to 14.84) | 0.545 | – |
| 12 | 189 | –7.1 (53.7) | –9.3 (50.3) | 0.36 (–13.94 to 14.64) | 0.961 | – | |
| MoCA score | |||||||
| ≤ 25 | 6 | 111 | 5 (46.5) | 5.9 (45.8) | –1.94 (–19.39 to 15.52) | 0.827 | 0.904 |
| ≥ 26 | 6 | 219 | 1.2 (52.9) | –2.5 (50.1) | –0.64 (–13.12 to 11.84) | 0.920 | |
| ≤ 25 | 12 | 79 | –7.6 (44.1) | –2.7 (38.5) | –9.26 (–31.10 to 12.59) | 0.404 | 0.347 |
| ≥ 26 | 12 | 134 | –9.3 (57.8) | –16.2 (53.4) | 3.73 (–12.99 to 20.46) | 0.660 | |
| FoG | |||||||
| No | 6 | 139 | 3.1 (55.7) | 5.7 (48.9) | –2.34 (–17.85 to 13.16) | 0.766 | 0.799 |
| Yes | 6 | 190 | 1.7 (47.8) | –3.5 (48.1) | 0.28 (–13.13 to 13.68) | 0.968 | |
| No | 12 | 96 | –14.6 (61.2) | –11.2 (53.7) | –3.05 (–22.81 to 16.72) | 0.761 | 0.732 |
| Yes | 12 | 116 | –4.4 (46.8) | –10.9 (43.7) | 1.52 (–16.44 to 19.48) | 0.867 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 111 | 5 (46.5) | 5.9 (45.8) | –1.90 (–19.39 to 15.58) | 0.831 | 0.881 |
| 12 | 79 | –7.6 (44.1) | –2.7 (38.5) | –9.26 (–31.1 to 12.59) | 0.404 | 0.501 | |
| 26 or 27 | 6 | 90 | –3.8 (52.9) | 9.3 (45.3) | –5.27 (–24.90 to 14.35) | 0.597 | |
| 12 | 55 | –12.1 (45.1) | –30 (49.5) | 9.29 (–17.0 to 35.18) | 0.480 | ||
| ≥ 28 | 6 | 129 | 4.9 (53) | 1.9 (52.8) | 1.20 (–15.0 to 17.39) | 0.885 | |
| 12 | 79 | –7.3 (66.2) | –7.2 (54.5) | 1.27 (–20.54 to 23.1) | 0.908 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 104 | 7.1 (35.6) | –6.6 (46.2) | 2.31 (–16.16 to 20.71) | 0.809 | 0.387 |
| 12 | 60 | 0.4 (47.8) | –18.1 (51.5) | 4.93 (–21.17 to 30.02) | 0.710 | 0.881 | |
| 23–38 | 6 | 109 | –6.4 (53.6) | 9.6 (55.3) | –10.63 (–28.25 to 7.30) | 0.247 | |
| 12 | 74 | –17.5 (60.9) | –5.3 (56.7) | –3.83 (–15.99 to 20.61) | 0.804 | ||
| ≤ 22 | 6 | 104 | 7.9 (59.5) | –0.3 (45.5) | 5.82 (–12.86 to 21.98) | 0.607 | |
| 12 | 77 | –4.5 (45.6) | –10.8 (39.4) | 0.30 (–22.12 to 22.71) | 0.979 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 118 | 6.1 (50.7) | –6.6 (42) | 10.48 (–6.32 to 27.28) | 0.221 | 0.194 |
| 12 | 72 | –2.4 (47.5) | –11.3 (49.8) | 9.95 (–12.47 to 32.37) | 0.382 | 0.423 | |
| 2 or 3 | 6 | 142 | –0.8 (54.1) | 3.9 (46.8) | –8.48 (–24.43 to 7.47) | 0.296 | |
| 12 | 95 | –10.5 (50.7) | –7 (46.3) | –10.99 (–31.58 to 9.59) | 0.293 | ||
| 1 | 6 | 70 | 2.4 (50.8) | 0.5 (48.6) | –3.99 (–25.84 to 17.84) | 0.719 | |
| 12 | 46 | –18.1 (68.1) | –19 (52.0) | 3.21 (–25.80 to 32.21) | 0.830 | ||
Appendix 16 Parkinson’s Disease Questionnaire
| Participant group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | Participant group | |
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | ||||||
| All participants | 6 | 279 | 0.8 (8.3) | 0.9 (9.0) | –0.12 (–2.28 to 2.0) | 0.911 | – |
| 12 | 177 | 2.0 (8.6) | 2.8 (11.2) | –0.48 (–3.49 to 2.53) | 0.754 | – | |
| MDS-UPDRS score of ≤ 58 | 6 | 268 | 0.9 (8.4) | 0.9 (9.0) | –0.07 (–2.28 to 2.14) | 0.952 | – |
| 12 | 166 | 1.8 (8.5) | 2.9 (11.5) | –0.4 (–3.55 to 2.74) | 0.800 | – | |
| H&Y scale stage ≤ 3 | 6 | 251 | 1.2 (8.0) | 0.2 (8.9.0) | 0.91 (–1.32 to 3.14) | 0.423 | |
| 12 | 159 | 1.7 (8.6) | 2.1 (11.0) | –0.35 (–3.4 to 2.7) | 0.820 | ||
| MoCA score | |||||||
| ≤ 25 | 6 | 90 | 0.7 (9.6) | 0.3 (9.6) | 0.69 (–3.02 to 4.40) | 0.715 | 0.594 |
| ≥ 26 | 6 | 189 | 0.9 (7.7) | 1.1 (8.7) | –0.51 (–3.11 to 2.07) | 0.697 | |
| ≤ 25 | 12 | 61 | 2.2 (10.8) | 4.2 (11.2) | –0.86 (–5.87 to 4.15) | 0.734 | 0.947 |
| ≥ 26 | 12 | 116 | 1.8 (7.3) | 2.0 (11.2) | –0.65 (–4.39 to 3.09) | 0.732 | |
| FoG | |||||||
| No | 6 | 124 | 1.2 (7.5) | 1.4 (8.7) | –0.77 (–4.0 to 2.46) | 0.639 | 0.710 |
| Yes | 6 | 154 | 0.6 (8.9) | 0.3 (9.2) | 0.25 (–2.59 to 3.1) | 0.864 | |
| No | 12 | 79 | 2.8 (8.1) | 4.5 (12.7) | –0.91 (–5.43 to 3.60) | 0.689 | 0.732 |
| Yes | 12 | 97 | 1.3 (9.0) | 1.3 (9.5) | 0.12 (–3.85 to 3.48) | 0.952 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 90 | 0.7 (9.6) | 0.3 (9.6) | 0.67 (–3.02 to 4.36) | 0.721 | 0.441 |
| 12 | 61 | 2.2 (10.8) | 4.2 (11.2) | –0.84 (–5.86 to 4.18) | 0.742 | 0.532 | |
| 26 or 27 | 6 | 76 | 2.3 (7.1) | 1.7 (8) | 1.04 (–3.0 to 5.11) | 0.611 | |
| 12 | 47 | 2 (5.5) | 1 (10.3) | 1.74 (–3.92 to 7.39) | 0.546 | ||
| ≥ 28 | 6 | 103 | –0.2 (8.1) | 0.8 (9.1) | –1.93 (–5.24 to 1.39) | 0.254 | |
| 12 | 69 | 1.7 (8.6) | 2.6 (11.8) | –2.47 (–7.42 to 2.48) | 0.326 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 75 | 1.9 (10.5) | 0.9 (9.8) | –0.85 (–4.32 to 2.62) | 0.631 | 0.492 |
| 12 | 46 | 0.7 (11) | 6 (12.2) | 0.91 (–4.19 to 5.98) | 0.723 | 0.270 | |
| 23–38 | 6 | 95 | –0.1 (7.7) | 0.6 (8.7) | –1.11 (–4.72 to 2.52) | 0.548 | |
| 12 | 63 | 3 (8.1) | 1.3 (11.9) | 1.03 (–3.81 to 5.87) | 0.674 | ||
| ≤ 22 | 6 | 107 | 1.1 (7.3) | 1.3 (8.7) | 2.00 (–2.22 to 6.24) | 0.352 | |
| 12 | 65 | 1.4 (7.4) | 1.6 (10) | –4.73 (–10.76 to 1.29) | 0.123 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 99 | 0.3 (8) | 2 (11.5) | –1.49 (–4.99 to 2.01) | 0.403 | 0.512 |
| 12 | 65 | 0.6 (7.9) | 5.3 (12.5) | –4.77 (–9.50 to –0.37) | 0.048 | 0.090 | |
| 2 or 3 | 6 | 122 | 1 (8) | –0.7 (6.4) | 1.09 (–2.17 to 4.36) | 0.511 | |
| 12 | 75 | 3.7 (9.3) | 1.3 (8.7) | 2.33 (–2.40 to 7.06) | 0.333 | ||
| 1 | 6 | 58 | 1.6 (9.8) | 2.5 (9.1) | –1.23 (–5.84 to 3.39) | 0.601 | |
| 12 | 37 | 1.4 (8.4) | 2.3 (13.6) | 1.10 (–5.3 to 7.52) | 0.736 | ||
Appendix 17 Geriatric Depression Scale
| Participant group | Visit (months) | Participants (n) | Trial group: change from baseline, mean (SD) | PDSAFE – control mean change (95% CI)a | p-valuea | Participant group | |
|---|---|---|---|---|---|---|---|
| PDSAFE | Control | ||||||
| All participants | 6 | 337 | 0.3 (1.8) | 0.2 (1.9) | –0.02 (–0.42 to 0.39) | 0.942 | – |
| 12 | 214 | 0.2 (2.0) | 0.4 (1.7) | –0.21 (–0.72 to 0.31) | 0.421 | – | |
| MDS-UPDRS score of ≤ 58 | 6 | 318 | 0.2 (1.8) | 0.3 (1.9) | –0.10 (–0.52 to 0.32) | 0.636 | – |
| 12 | 200 | 0.2 (2.1) | 0.4 (1.8) | –0.26 (–0.79 to 0.28) | 0.354 | – | |
| H&Y scale stage ≤ 3 | 6 | 301 | 0.3 (2.2) | 0.2 (1.9) | –0.02 (–0.47 to 0.40) | 0.923 | – |
| 12 | 192 | 0.2 (2.0) | 0.5 (1.7) | –0.26 (–0.79 to 0.30) | 0.344 | – | |
| MoCA score | |||||||
| ≤ 25 | 6 | 115 | 0 (1.5) | 0.4 (2.0) | –0.42 (–1.10 to 0.26) | 0.229 | 0.151 |
| ≥ 26 | 6 | 222 | 0.4 (1.9) | 0.1 (1.8) | 0.19 (–0.30 to 0.68) | 0.456 | |
| ≤ 25 | 12 | 79 | 0.2 (2.2) | 0.2 (2.0) | 0.16 (–0.69 to 1.01) | 0.713 | 0.302 |
| ≥ 26 | 12 | 135 | 0.2 (2.0) | 0.6 (1.6) | –0.40 (–1.05 to 0.25) | 0.231 | |
| FoG | |||||||
| No | 6 | 145 | 0.4 (1.9) | 0.2 (1.7) | 0.19 (–0.41 to 0.80) | 0.528 | 0.379 |
| Yes | 6 | 192 | 0.2 (2.2) | 0.3 (2.0) | –0.16 (–0.69 to 0.37) | 0.552 | |
| No | 12 | 99 | 0.7 (2.1) | 0.5 (2.0) | 0.24 (–0.62 to 1.08) | 0.526 | 0.105 |
| Yes | 12 | 113 | –0.2 (1.9) | 0.3 (1.6) | –0.59 (–1.38 to 0.04) | 0.100 | |
| MoCA score | |||||||
| ≤ 25 | 6 | 115 | 0 (1.5) | 0.4 (2.0) | –0.41 (–1.08 to 0.27) | 0.237 | 0.009 |
| 12 | 79 | 0.2 (2.2) | 0.2 (2.0) | 0.16 (–0.70 to 1.02) | 0.715 | 0.450 | |
| 26 or 27 | 6 | 93 | 0.8 (2.1) | –0.2 (2.0) | 0.97 (0.22 to 1.72) | 0.011 | |
| 12 | 56 | 0.2 (2.4) | 0.4 (1.6) | –0.12 (–1.10 to 0.87) | 0.814 | ||
| ≥ 28 | 6 | 130 | 0.1 (1.8) | 0.3 (1.7) | –0.38 (–1.01 to 0.25) | 0.241 | |
| 12 | 78 | 0.2 (1.5) | 0.7 (1.6) | –0.61 (–1.47 to 0.26) | 0.168 | ||
| MDS-UPDRS score | |||||||
| ≥ 39 | 6 | 106 | 0.3 (1.7) | 0.4 (1.9) | –0.06 (–0.74 to 0.63) | 0.868 | 0.841 |
| 12 | 64 | –0.1 (1.8) | 0.4 (1.7) | –0.23 (–1.30 to 0.68) | 0.605 | 0.944 | |
| 23–38 | 6 | 112 | 0.1 (1.4) | 0.1 (1.7) | –0.14 (–0.83 to 0.55) | 0.691 | |
| 12 | 72 | 0.2 (1.9) | 0.2 (1.5) | –0.09 (–0.96 to 0.78) | 0.837 | ||
| ≤ 22 | 6 | 125 | 0.2 (2.2) | 0.1 (1.9) | 0.15 (–0.58 to 0.88) | 0.684 | |
| 12 | 77 | 0.2 (2.2) | 0.5 (0.5 | –0.31 (–1.30 to 0.68) | 0.537 | ||
| Retrospective falls | |||||||
| > 3 | 6 | 62 | 0.3 (2.0) | 0.7 (2.2) | –0.06 (–0.92 to 0.79) | 0.889 | 0.441 |
| 12 | 71 | –0.3 (2.3) | 0.8 (1.6) | –0.01 (–1.10 to 1.08) | 0.987 | 0.019 | |
| 2 or 3 | 6 | 147 | 0.2 (1.8) | –0.1 (1.5) | 0.23 (–0.36 to 1.23) | 0.471 | |
| 12 | 97 | 0.4 (2.0) | 0 (1.6) | 0.44 (–0.65 to 1.48) | 0.278 | ||
| 1 | 6 | 71 | 1.8 (1.8) | 0.3 (1.9) | –0.36 (–1.02 to 0.31) | 0.293 | |
| 12 | 46 | 0.5 (1.2) | 0.8 (1.9) | –1.21 (–2.07 to -0.35) | 0.006 | ||
Appendix 18 Secondary outcomes
FIGURE 15.
Overall and subgroup analysis of the secondary outcomes measured at 6 months (PASE, PDQ-39, GDS and MDS-UPDRS). a, Test for interaction of PDSAFE contrast differing across subgroups controlled for site, age, gender, repeat falling or not in the year prior to screening, log number of falls in the year prior to screening, log rate of falling in the pre-randomisation falls collection period, H&Y scale stage, and the outcome in question assessed at baseline.
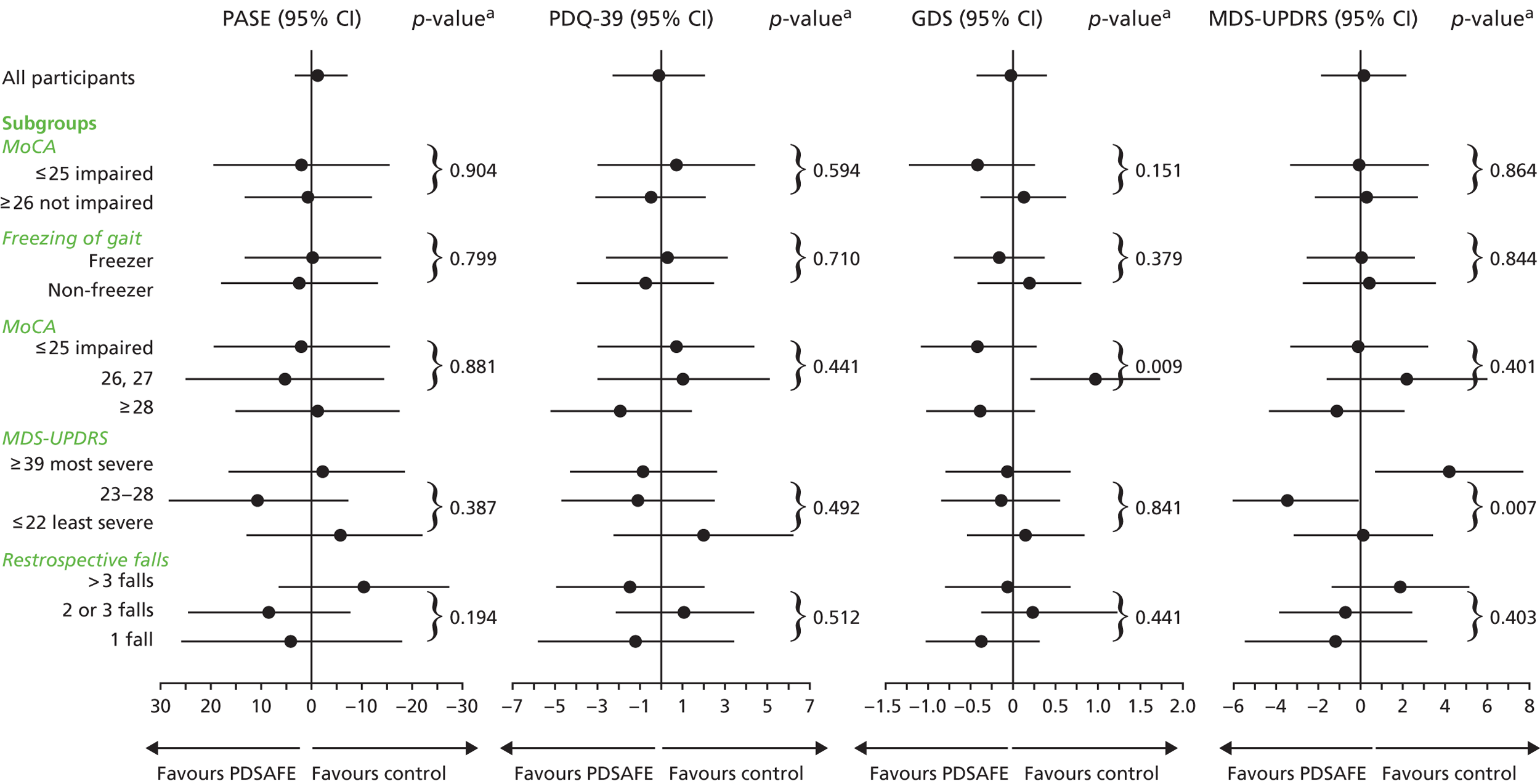
Appendix 19 Further exploration
| Resource | p-value | Trial group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDSAFE (N = 91) | Control (N = 93) | ||||||||||
| Mean | Median | Minimum, maximum | SD | n | Mean | Median | Minimum, maximum | SD | n | ||
| GP | 0.44 | 3.02 | 2.5 | 0, 11 | 2.61 | 44 | 3.45 | 3 | 0, 12 | 2.96 | 62 |
| Practice nurse | 0.23 | 1.73 | 1 | 0, 9 | 2.05 | 44 | 3.11 | 1 | 0, 43 | 7.44 | 61 |
| Parkinson’s nurse | 0.33 | 1.30 | 1 | 0, 11 | 1.87 | 43 | 1.69 | 1 | 0, 11 | 2.11 | 64 |
| Health visitor | 0.56 | 0.20 | 0 | 0, 5 | 0.81 | 45 | 0.13 | 0 | 0, 2 | 0.42 | 62 |
| Social worker | 0.10 | 0.02 | 0 | 0, 1 | 0.15 | 44 | 0.14 | 0 | 0, 2 | 0.47 | 63 |
| NHS physiotherapist | 0.48 | 1.86 | 0 | 0, 12 | 3.52 | 43 | 1.45 | 0 | 0, 9 | 2.32 | 60 |
| Occupational therapist | 0.41 | 0.37 | 0 | 0, 5 | 0.98 | 43 | 0.64 | 0 | 0, 12 | 1.95 | 61 |
| Speech therapist | 0.13 | 0.95 | 0 | 0, 11 | 2.33 | 43 | 0.42 | 0 | 0, 7 | 1.27 | 62 |
| Day hospital | 0.25 | 0.82 | 0 | 0, 14 | 2.31 | 44 | 1.28 | 0.5 | 0, 8 | 1.77 | 60 |
| Outpatient clinic | 0.02 | 1.23 | 1 | 0, 6 | 1.38 | 44 | 2.10 | 2 | 0, 13 | 2.16 | 62 |
| A&E by ambulance | 0.84 | 0.11 | 0 | 0, 2 | 0.44 | 44 | 0.10 | 0 | 0, 2 | 0.35 | 61 |
| Ambulance with paramedics only | 0.58 | 0.26 | 0 | 0, 5 | 0.94 | 42 | 0.37 | 0 | 0, 5 | 0.94 | 54 |
| A&E by own transport | 0.18 | 0.18 | 0 | 0, 3 | 0.54 | 44 | 0.37 | 0 | 0, 4 | 0.81 | 62 |
| Home care/home help | 0.11 | 23.58 | 0 | 0, 390 | 66.44 | 59 | 52.00 | 0 | 0, 728 | 122.76 | 73 |
| Meals on wheels | 0.82 | 3.10 | 0 | 0, 169 | 21.49 | 63 | 2.30 | 0 | 0, 182 | 20.48 | 79 |
| Day centre | 0.54 | 1.44 | 0 | 0, 52 | 7.42 | 63 | 0.83 | 0 | 0, 26 | 4.36 | 78 |
| Luncheon club | 0.37 | 0.00 | 0 | 0, 0 | 0.00 | 63 | 0.17 | 0 | 0, 13 | 1.48 | 77 |
| Sitting service | 0.77 | 1.68 | 0 | 0, 52 | 9.26 | 62 | 2.17 | 0 | 0, 78 | 10.35 | 78 |
| Night care | 0.29 | 2.94 | 0 | 0, 182 | 23.11 | 62 | 0.17 | 0 | 0, 13 | 1.47 | 78 |
| Hospitalisation for treatmenta | 0.14 | 0.71 | 0 | 0, 30 | 4.20 | 63 | 0.03 | 0 | 0, 2 | 0.22 | 80 |
| Hospitalisation for respite carea | / | 0 | 0 | 0, 0 | 0 | 63 | 0 | 0 | 0, 0 | 0 | 80 |
FIGURE 16.
Six months’ resource use of the intervention (n = 91) and control (n = 93) groups in the cognitively impaired group.

Glossary
- Catechol-O-methyltransferase inhibitor
- A drug that inhibits the action of catechol-O-methyltransferase.
List of abbreviations
- A&E
- accident and emergency
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CST
- Chair Stand Test
- DVD
- digital versatile disc
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FES-I
- Falls Efficacy Scale – International
- FoG
- freezing of gait
- FRR
- falls rate ratio
- GDS
- Geriatric Depression Scale
- GLM
- generalised linear model
- GP
- general practitioner
- HRQoL
- health-related quality of life
- H&Y
- Hoehn and Yahr
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- IQR
- interquartile range
- ITT
- intention to treat
- MDS-UPDRS
- International Parkinson and Movement Disorder Society – Unified Parkinson’s Disease Rating Scale
- Mini-BESTest
- Mini-Balance Evaluation Systems Test
- MMSE
- Mini-Mental State Examination
- MoCA
- Montreal Cognitive Assessment
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PASE
- Physical Activity Scale for the Elderly
- PDQ-39
- 39-item Parkinson’s Disease Questionnaire
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- T
- time
- T1
- time 1, prior to commencement of PDSAFE
- T2
- time 2, 6 months later
- TIDieR
- Template for Intervention Description and Replication
