Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/58/15. The contractual start date was in July 2013. The draft report began editorial review in April 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Graham Burns reports personal fees from Boehringer Ingelheim GmbH (Ingelheim am Rhein, Germany), Teva Pharmaceutical Industries Ltd (Petah Tikva, Israel), Chiesi Farmaceutici SpA (Parma, Italy), Pfizer Inc. (New York City, NY, USA) and AstraZeneca plc (Cambridge, UK), and non-financial support from Chiesi and Boehringer Ingelheim, outside the submitted work. Rekha Chaudhuri reports personal fees from AstraZeneca, GlaxoSmithKline (GSK) plc (London, UK), Teva and Novartis International AG (Basel, Switzerland) for advisory board meetings, outside the submitted work. Anthony De Soyza reports grants and non-financial support from AstraZeneca and Chiesi, non-financial support from Boehringer Ingelheim, grants and personal fees from GSK, Bayer AG (Leverkusen, Germany) and Pfizer, and grants from Forest Laboratories (New York City, NY, USA)/Teva, outside the submitted work. He is also a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Elective and Emergency Specialist Care (EESC) Panel. Simon Gompertz reports personal fees from Pfizer and GSK, outside the submitted work. John Norrie reports grants from the NIHR HTA programme during the conduct of the study, was a member of the NIHR HTA Commissioning Board (2010–16) and is currently Deputy Chairperson of the NIHR HTA General Board (2016–present) and a NIHR Journals Library Editor (2014–present). Andrew Wilson reports grants from F. Hoffmann-La Roche (Basel, Switzerland), outside the submitted work. David Price reports grants and personal fees from Aerocrine AB (Stockholm, Sweden), AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan NV (Canonsburg, PA, USA), Mundipharma International Ltd (Cambridge, UK), Napp Pharmaceuticals Ltd (Cambridge, UK), Novartis, Pfizer, Teva, Theravance Biopharma (San Francisco, CA, USA) and Zentiva Group a.s. (Prague, Czech Republic); personal fees from Almirall SA (Barcelona, Spain), Amgen Inc. (Newbury Park, CA, USA), Cipla Ltd (Mumbai, India), GSK, Kyorin Pharmaceutical Co. Ltd (Tokyo, Japan), Merck Sharp & Dohme (Kenilworth, NJ, USA), Skyepharma Production SAS (Saint-Quentin-Fallavier, France); grants from AKL Research and Development Ltd (Stevenage, UK), the British Lung Foundation, the Respiratory Effectiveness Group and the UK NHS; and non-financial support from the NIHR Efficacy and Mechanism Evaluation and HTA programmes, outside the submitted work. He also reports stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals and owns 74% of the social enterprise Optimum Patient Care Ltd (Cambridge, UK, and Australia and Singapore) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Devereux et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Chronic obstructive pulmonary disease (COPD) is defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as:
a common preventable and treatable disease characterised by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to noxious particles or gases. Exacerbations and comorbidities contribute to the overall severity in individual patients.
People with COPD typically present with breathlessness on exertion, a productive cough and wheeze. COPD is usually diagnosed from the age of 40 years onwards and prevalence increases with age. 2 In Westernised countries, COPD is predominantly (80–90%) caused by cigarette smoking,3 but outdoor air pollution and occupational exposure to dusts, vapours and fumes can be significant contributory factors. 4,5 COPD is closely associated with social deprivation, and makes a major contribution to health inequalities in the UK. 6 The progressive airflow limitation of COPD is associated with increasing disability, work absence, long-term morbidity, common physical and psychological comorbidities and premature mortality. People with COPD are more likely to have associated comorbidities,7 including ischaemic heart disease,8 hypertension,9 heart failure,10,11 diabetes mellitus,12 osteoporosis,13 depression14 and lung cancer,15 which increase morbidity and complicate the management of COPD. 7
Acute deteriorations in symptoms, known as exacerbations, are an important clinical feature of COPD. These are usually precipitated by viral/bacterial infection and/or air pollution and are characterised by increasing breathlessness and/or cough, sputum expectoration and malaise. Many exacerbations are severe enough for patients to seek medical help, which usually takes the form of antibiotics and/or corticosteroids from their general practitioner (GP); more severe exacerbations frequently necessitate admission to hospital for more intensive treatment. Exacerbations are associated with accelerated rate of lung function decline,16 reduced physical activity,17 reduced quality of life (QoL),18 increased mortality19 and increased risk of comorbidities such as acute myocardial infarction and stroke. 20
The observational Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study of 2138 COPD patients shed light on factors that influence COPD exacerbations. 21 This study identified a frequent exacerbator (defined as two or more exacerbations in a year) phenotype that affects ≈25% of COPD patients. Patients with this phenotype have an 84% chance of at least one exacerbation in the subsequent year; moreover, this frequent exacerbator phenotype is stable for at least 3 years and can be reliably identified by patient recall. This has been supported by further work demonstrating that the strongest predictor for exacerbations is the number of exacerbations in the preceding year. 22 Frequent exacerbators account for a disproportionate amount of the annual NHS spend on COPD.
The burden of chronic obstructive pulmonary disease on individuals and the NHS
Chronic obstructive pulmonary disease is a major personal and public health burden. 23,24 Data from 591 UK general practices comprising The Health Improvement Network (THIN) indicate that the prevalence of diagnosed COPD in the UK increased from ≈991,000 in 2004 to 1.2 million in 2012. 2 COPD is the fifth leading cause of death in the UK, accounting for ≈5% of all deaths (≈30,000 deaths in 2014). More than 80% of COPD patients, irrespective of disease severity, report a reduced QoL. 24–26 Comorbidities are an important feature of COPD, contributing to ill health and treatment burden. It has been estimated that, in the UK, 33% of people with COPD have hypertension, 19% have ischaemic heart disease, 18% have depression, 11% have diabetes mellitus and 6% have heart failure. 23 Over 50% of people currently diagnosed with COPD in the UK are < 65 years of age, and 24 million working days are lost each year as a result of COPD, with £3.8B per year being lost through reduced productivity. 23
Chronic obstructive pulmonary disease costs the NHS > £1B per year. In 2001, average annual NHS direct costs were £819 (> £1300 in severe COPD) for each COPD patient; 60% of this was accounted for by exacerbations and 19% was due to drug costs. 27 The Hospital Episode Statistics database shows that emergency hospital admissions for exacerbations of COPD in the UK have steadily increased as a percentage of all admissions, from 0.5% in 1991 to 1% in 2000 and to 1.5% in 2008/9. 28 In 2008/9, COPD exacerbations resulted in 164,000 hospital admissions in the UK, with an average length of stay of 7.8 days, accounting for 1.3 million bed-days. 28 COPD is the second leading cause of emergency admission to hospital in the UK and is one of the most costly inpatient conditions treated by the NHS. 23,24 At least 10% of emergency admissions to hospital are as a consequence of COPD, and this proportion is even greater during winter. Approximately 25% of patients who have been diagnosed as having COPD are admitted to hospital at some point, and ≈15% of COPD patients are admitted each year. 23,24 Over 30% of patients admitted to hospital with an exacerbation of COPD are re-admitted within 30 days, and an average of 12% of COPD patients die in the year following admission to hospital. 19
Despite advances in management that have led to the current National Institute for Health and Care Excellence (NICE) COPD guidelines,24 there is still an unmet need for improved pharmacological treatment of COPD, particularly the prevention of exacerbations.
Standard chronic obstructive pulmonary disease therapy
Standard COPD therapy remains suboptimal. At the time when the Theophylline With Inhaled CorticoSteroid (TWICS) trial was conceived, most international COPD management guidelines recommended the use of inhaled corticosteroids (ICSs) – usually in combination with inhaled long-acting β2-agonists (LABAs), known as ICS/LABA – to reduce COPD exacerbation rates and to improve lung function and QoL. 1,24 Although more recent guidelines advocate the use of LABAs in combination with long-acting muscarinic antagonists (LAMAs), ICS/LABA and ICS/LABA/LAMA combinations remain major therapeutic options and continue to be used very widely in the treatment of COPD. 29,30 However, when compared with the marked responses observed in asthma, ICSs in COPD fail to fully suppress airway inflammation and patients continue to have exacerbations despite high ICS doses. Furthermore, little or no positive impact of ICS on mortality or disease progression is evident31,32 and concerns have been raised about long-term sequelae of high-dose ICS use in COPD. 33,34 A relative insensitivity of COPD airway inflammation to the anti-inflammatory effects of high-dose ICS has been demonstrated in induced sputum and airway biopsies of people with COPD. 35–37
In recent years, molecular mechanisms contributing to the reduced corticosteroid sensitivity of COPD have been elucidated. The chronic airway inflammation of COPD is driven by expression of multiple inflammatory genes regulated by acetylation of core histones, which open up the chromatin structure, allowing transcription factors and ribonucleic acid (RNA) polymerase II to bind to deoxyribonucleic acid (DNA), enabling gene transcription and increased synthesis of inflammatory proteins. 38 In COPD, there is increased acetylation of core histones associated with the promoter regions of inflammatory genes, with the degree of acetylation being positively associated with disease severity. 39 Histone acetylation is reversed by histone deacetylase (HDAC) enzymes. Corticosteroids appear to work by reversing histone acetylation through the recruitment of a specific HDAC called HDAC2,38,40,41 thereby switching off activated inflammatory genes. In people with COPD, increased histone acetylation appears to be a consequence of markedly reduced HDAC2 activity/expression in airways, lung tissue and alveolar macrophages. 39 It has been shown that the oxidative stress of COPD activates the enzyme phosphoinositide 3-kinase (PI3K)-δ, which then phosphorylates downstream kinases, resulting in the phosphorylation and inactivation of HDAC2. 41,42 The critical role played by reduced HDAC2 in the corticosteroid resistance of COPD is demonstrated by the finding that the corticosteroid resistance of COPD bronchoalveolar macrophages is completely reversed by overexpressing HDAC2 (using a plasmid vector) to levels seen in control patients without COPD. 40
Low-dose theophylline may have synergistic anti-inflammatory effects with corticosteroids
Oral theophylline has been used in the treatment of COPD for > 70 years, but usually at doses required to achieve relatively high blood concentrations (10–20 mg/l). It has been observed that the reduced HDAC2 activity of COPD can be reversed in a dose-dependent manner by low doses of theophylline; moreover, low-dose theophylline reduces corticosteroid insensitivity in COPD such that there is a marked synergistic interaction between theophylline and corticosteroids in suppressing the release of inflammatory mediators from alveolar macrophages from COPD patients. This in vitro work has shown that at (low) concentrations of 1–5 mg/l theophylline increases HDAC2 activity (sixfold) but at (high) concentrations of over ≈10 mg/l theophylline inhibits rather than stimulates HDAC2 activity. 43,44 These studies show that at concentrations of 1–5 mg/l, there is a marked synergistic effect between theophylline and corticosteroids, with theophylline inducing a 100- to 10,000-fold increase in the suppressive effect of corticosteroids on the release of pro-inflammatory mediators. Such an increase in corticosteroid potency is worthy of clinical interest, particularly if associated with reduced exacerbation rate. An explanation for the ability of low-dose (i.e. 1–5 mg/l) theophylline to increase HDAC activity has been described: it specifically inhibits the enzyme PI3K-δ with consequent restoration of HDAC2 activity to normal in COPD macrophages, rendering them steroid responsive. In mice exposed to cigarette smoke,42 steroid-resistant lung inflammation has also been found to be reduced by low-dose theophylline when given together with steroids. Similarly, rats exposed to cigarette smoke were found to have markedly decreased lung HDAC2 expression, and that reduced HDAC2 expression was correlated with increased lung destruction index. 45 The increased lung destruction index was restored to normal with ICS treatment in combination with low- (but not high-) dose theophylline. It was concluded that low-dose theophylline might provide protection from cigarette smoke damage and improve the anti-inflammatory effects of steroids by increasing HDAC2 activity.
In human peripheral blood mononuclear cells, corticosteroid insensitivity and reduced HDAC2 activity after oxidative stress have been shown to be reversed with low concentrations of theophylline. 46 In a study of human alveolar macrophages extracted from resected lung samples, the addition of hydrogen peroxide reduced HDAC expression and was associated with an increase in interleukin 8 (IL-8) and matrix metalloproteinase-9 (MMP-9) release. 47 The addition of low-dose theophylline restored HDAC expression to levels above that observed with LABA, ICS and ICS/LABA.
These basic research studies suggest that low-dose (i.e. 1–5 mg/l) theophylline could increase HDAC activity and hence reduce corticosteroid resistance in COPD patients, thereby enabling ICS to switch off inflammation and potentially reduce exacerbation rates more effectively. This is supported by findings from two small randomised controlled trials (RCTs) and a population-based health administration database study. The first RCT in 35 patients with acute COPD exacerbations found that low-dose theophylline increased responsiveness to corticosteroids as measured by increased HDAC activity and further reduced concentrations of pro-inflammatory mediators in induced sputum compared with ICSs alone. 48 In the second small (n = 30) pilot RCT of COPD patients, the combination of low-dose theophylline with high-dose ICSs was associated with increased HDAC activity, improved lung function and reduced sputum inflammatory cells and mediators, whereas either drug alone was ineffective. 49 A Canadian health administration database study of 36,492 COPD patients reported that treatment with theophylline alone or in combination with ICS was more protective against exacerbations than treatment with LABA or ICS/LABA [relative risk (RR) 0.89, 95% confidence interval (CI) 0.87 to 0.92]. 50
More recent studies, however, have not replicated the results of earlier studies. Fexer et al. 51 used data from a German ambulatory COPD management programme and closely matched 1496 COPD patients commenced on theophylline with 1496 COPD patients not commenced on theophylline. The use of theophylline was associated with an increased likelihood of exacerbation [hazard ratio (HR) 1.41, 95% CI 1.24 to 1.60] and hospital admission (HR 1.61, 95% CI 1.29 to 2.01). Although it was concluded that theophylline is associated with an increased incidence of exacerbations and hospitalisations, it should be noted that this study did not identify those patients on low-dose theophylline. 51 The Spanish Low-dose Theophylline as Anti-inflammatory Enhancer in Severe Chronic Obstructive Pulmonary Disease (ASSET) trial recruited patients with COPD while hospitalised for a COPD exacerbation and randomised to low-dose theophylline (100 mg twice a day) or matched placebo in addition to usual ICS/LABA treatment. 52 In total, 70 patients were randomised (theophylline, n = 36; placebo, n = 34) and 46 completed the year of treatment (theophylline, n = 23; placebo, n = 23). The addition of theophylline had no effect on the COPD exacerbation rate or plasma/sputum concentrations of HDAC and inflammatory mediators. It should be noted that the study was small and designed to detect a 50% reduction in exacerbations.
Conventionally, oral theophylline has been used as a bronchodilator in COPD; however, to achieve modest clinical effects, relatively high blood concentrations (of 10–20 mg/l) are required. The bronchodilator effect of high-dose theophylline is the consequence of inhibition of phosphodiesterase (PDE) and the consequent relaxation of airway smooth muscle. However, non-specific inhibition of PDE by theophylline is also associated with a wide range of well-recognised side effects that may occur within the conventional therapeutic range of plasma theophylline, namely nausea, gastrointestinal upset, headaches, insomnia, seizures, cardiac arrhythmias and malaise. Theophylline toxicity is dose related, and this is an issue with conventional theophylline use because the therapeutic ratio of theophylline is small and most of the beneficial bronchodilator effect occurs when near-toxic doses are given. 53 Theophylline is metabolised by cytochrome P450 mixed function oxidase; as a consequence, theophylline use is further complicated by significant drug interactions with drugs commonly prescribed to people with COPD, for example clarithromycin or ciprofloxacin. 54 The narrow therapeutic index, modest clinical effect and side effect profile of theophylline, together with drug interactions, the need for blood concentration monitoring and the availability of more effective inhaled therapies, have resulted in current COPD guidelines relegating high-dose theophylline to third-line therapy. 1
The TWICS trial was a pragmatic, double-blind, randomised, placebo-controlled clinical trial that was built on emerging evidence that low-dose (i.e. 1–5 mg/l) theophylline may produce a beneficial synergistic effect in COPD by increasing the corticosteroid sensitivity of the airway inflammation underlying COPD and, as a consequence, reduce the rate of COPD exacerbation when used in conjunction with ICSs.
Hypothesis
The hypothesis being tested was that the addition of low-dose theophylline to ICS therapy in COPD reduces the risk of COPD exacerbation requiring treatment with antibiotics and/or oral corticosteroids (OCSs) during the year of treatment, delivers QoL improvements and is cost-effective.
Objectives
The primary objective of the trial was to determine the clinical effectiveness and cost-effectiveness of adding low-dose theophylline to ICS therapy in patients with COPD and a history of two or more exacerbations treated with antibiotics and/or OCSs in the previous year in relation to the number of exacerbations in the 1-year treatment period requiring therapy with antibiotics and/or OCSs.
The secondary objectives were to compare the following outcomes between participants treated with low-dose theophylline and those treated with placebo:
-
hospital admissions with a primary diagnosis of exacerbation of COPD
-
total number of episodes of pneumonia
-
total number of emergency hospital admissions
-
lung function
-
all-cause and respiratory mortality
-
drug reactions and serious adverse events (SAEs)
-
health-related QoL
-
disease-specific health status
-
total ICS dose/usage
-
health-care utilisation
-
incremental cost per exacerbation avoided
-
lifetime cost-effectiveness based on extrapolation modelling
-
modelled lifetime incremental cost per quality-adjusted life-year (QALY).
An additional secondary objective was the time to the first exacerbation of COPD.
Outcomes
Primary outcomes
The primary outcome was the total number of exacerbations of COPD necessitating changes in management (minimum management change: use of OCSs and/or antibiotics) during the 1-year treatment period, as reported by the participant.
The primary economic outcome was cost per QALY gained during the 1-year treatment period.
Secondary outcomes
-
Total number of COPD exacerbations requiring hospital admission.
-
Total number of episodes of pneumonia.
-
Total number of emergency hospital admissions (all causes).
-
Lung function [forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC)] post bronchodilator, measured using spirometry performed to American Thoracic Society (ATS)/European Respiratory Society (ERS) standards.
-
All-cause and respiratory mortality.
-
Serious adverse events and adverse reactions (ARs).
-
Total dose of ICS.
-
Utilisation of primary or secondary health care for respiratory events.
-
Disease-specific health status measured using the COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnoea scale.
-
Generic health-related QoL measured using the EuroQoL-5 Dimensions, three-level version (EQ-5D-3L) index.
-
Modelled lifetime incremental cost per QALY.
An additional secondary outcome was the time to the first exacerbation of COPD.
Chapter 2 Methods/design
Trial design
The trial protocol has been published in an open-access journal. 55
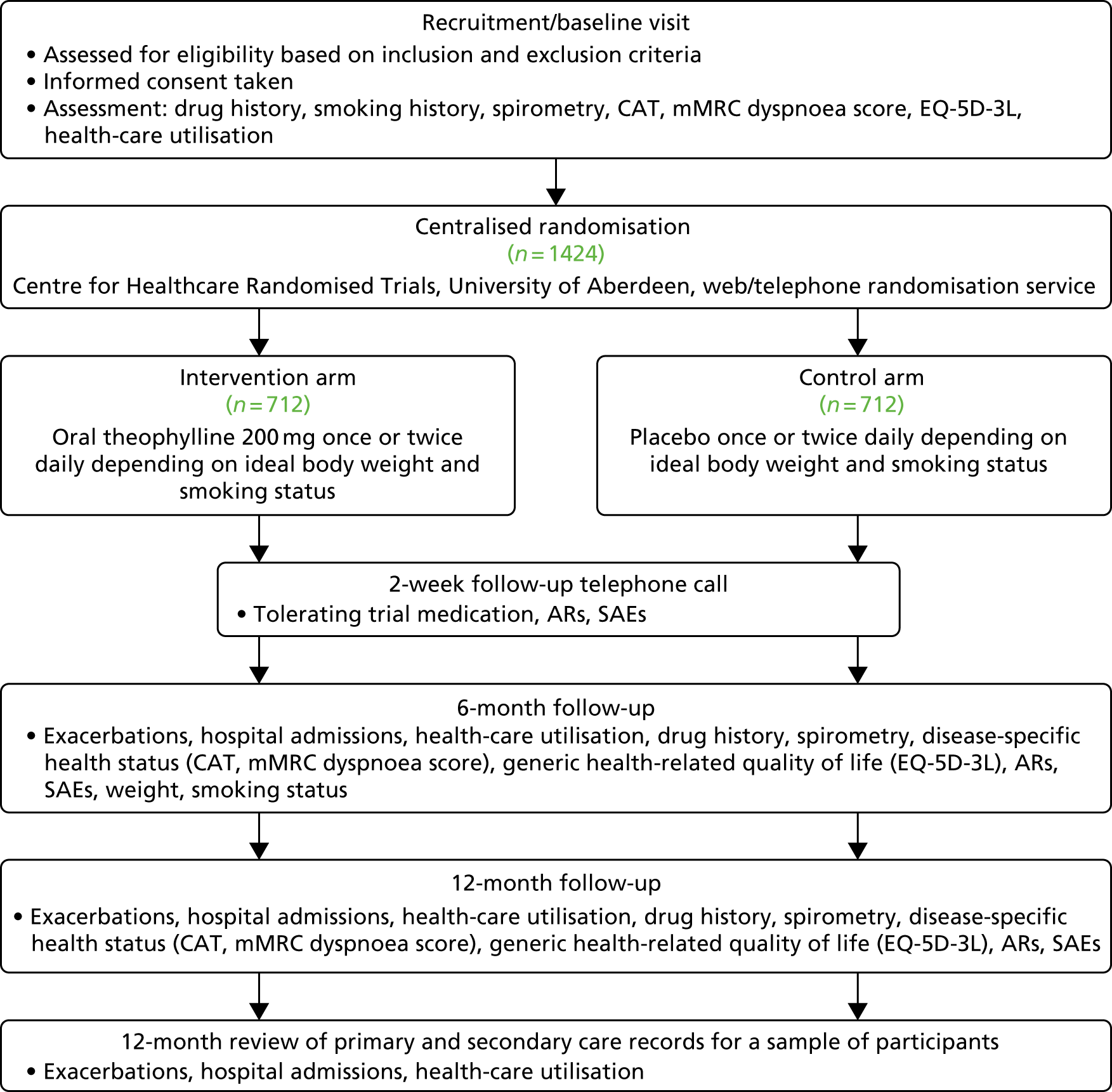
The TWICS trial was a pragmatic, double-blind, randomised, placebo-controlled, parallel-arm, UK multicentre clinical trial that compared the addition of low-dose theophylline or placebo for 52 weeks with current COPD therapy that included ICSs in patients with COPD who had experienced two or more exacerbations of COPD in the previous year treated with OCSs and/or antibiotics. The aim was to recruit 1424 participants, with at least 50% recruited in primary care. The trial was approved by Scotland A Research Ethics Committee (REC) (reference number 13/SS/0081) and the Medicines and Healthcare products Regulatory Agency (MHRA) (EudraCT 2013-001490-25, Clinical Trial Authorisation 21583/0218/001). All participants provided written informed consent, which included consent to inform a participant’s GP of involvement in the trial and consent to pass on a participant’s name and address to a third-party distributer that delivered the trial drug to participants’ homes. Figure 1 provides a schematic representation of the trial design and schedule. Face-to-face trial assessments were carried out at recruitment/baseline and at 6 and 12 months, as shown in Figure 2.
FIGURE 1.
Trial design.

FIGURE 2.
Flow diagram of trial schedule.
The trial was registered on 19 September 2013 as ISRCTN27066620.
Participants
Inclusion criteria
The participants in the TWICS trial were people with COPD who were likely to experience an exacerbation during the 52-week treatment period as evidenced by two or more exacerbations of COPD in the previous year treated with OCSs or antibiotics. Participants had to meet all of the following inclusion criteria, which are typical of studies of people with COPD with exacerbations as the primary end point:
-
aged ≥ 40 years
-
a smoking history of ≥ 10 pack-years
-
an established predominant respiratory diagnosis of COPD (GOLD/NICE guideline definition: post bronchodilator FEV1/FVC of < 0.7)1,2
-
current use of ICS therapy at the baseline/recruitment visit
-
a history of at least two exacerbations requiring treatment with antibiotics and/or OCS use in the previous year, based on patient report
-
clinically stable with no COPD exacerbation for at least the previous 4 weeks
-
able to swallow trial medication
-
able and willing to give informed consent to participate
-
able and willing to participate in the trial procedures, undergo spirometric assessment and complete the trial questionnaire.
Potential participants with COPD who did not fulfil the lung function criterion of FEV1/FVC of < 0.7 at the recruitment/baseline visit were asked to complete a slow vital capacity (SVC) manoeuvre, and FEV1/SVC of < 0.7 was accepted as evidence of airflow obstruction. Historical evidence of FEV1/FVC of < 0.7 was deemed acceptable for those participants who did not achieve FEV1/FVC of < 0.7 or FEV1/SVC of < 0.7 or who were unable to complete spirometry at the recruitment/baseline assessment. Eligibility for inclusion was confirmed by a medically qualified person.
Exclusion criteria
The exclusion criteria for the TWICS trial were typical of studies of people with COPD but also included criteria specific for theophylline, notably concomitant treatment with drugs that were likely to increase plasma theophylline concentration above the low-dose range of 1–5 mg/l. Potential participants were excluded if they fulfilled any of the following criteria:
-
severe or unstable ischaemic heart disease
-
a predominant respiratory disease other than COPD
-
any other significant disease/disorder that, in the investigator’s opinion, put the patient at risk because of trial participation, or might influence the results of the trial or the patient’s ability to participate in the trial
-
previous allocation of a randomisation code in the trial or current participation in another interventional study [Clinical Trial of an Investigational Medicinal Product (CTIMP) or non-CTIMP]
-
women who were pregnant or breastfeeding, or were planning a pregnancy during the trial period
-
current medication includes theophylline
-
known or suspected intolerance to theophylline
-
current use of drugs known to interact with theophylline and/or increase plasma theophylline54 –
-
antimicrobials: aciclovir, clarithromycin, ciprofloxacin, erythromycin, fluconazole, ketoconazole, levofloxacin and norfloxacin
-
cardiovascular drugs: diltiazem, mexiletine, pentoxifylline and verapamil
-
neurological drugs: bupropion, disulfiram, fluvoxamine and lithium
-
hormonal drugs: medroxyprogesterone and oestrogens
-
immunological drugs: methotrexate, peginterferon alpha and tacrolimus
-
miscellaneous: cimetidine, deferasirox, febuxostat, roflumilast and thiabendazole.
-
Patients with COPD as a consequence of alpha-1-antitrypsin deficiency were excluded; however, short- or long-term use of azithromycin56 or use of topical oestrogens or aciclovir were not exclusion criteria.
Identification
Potential participants were recruited from both primary and secondary care sites across the UK. To ensure generalisability, the intention was that the majority of participants (> 50%) would be recruited from primary care. Recruitment strategies differed between centres depending on local geographic and NHS organisational factors.
Primary care and other community-based services
In England, recruitment from general practices was conducted in conjunction with the National Institute for Health Research (NIHR) Clinical Research Network (CRN) at the national and local levels. Practices could participate as independent research sites or as participant identification centres (PICs) for secondary care or other primary care research sites.
In general practices, the local CRN/collaborating recruitment site/trial office liaised directly with practice staff who performed database searches (based on search criteria including the use of inhaled preparations containing corticosteroids, a record of one exacerbation treated with OCSs in the previous year and the use of interacting medications) to identify potential participants. Potentially suitable patients were sent an invitation letter and a participant information leaflet (PIL). For general practices acting as independent research sites, interested potential participants were invited to contact the practice-based trial team for more information and to arrange a recruitment visit. For general practices acting as PICs, interested potential participants were invited to contact the local trial team at the associated secondary or primary care research site for more information and to arrange a recruitment visit. All invitation material, consent forms, trial case report forms and participant-completed questionnaires can be found on the project web page at www.journalslibrary.nihr.ac.uk/programmes/hta/115815/#/ (accessed 23 April 2019).
In Scotland, the Scottish Primary Care Research Network mirrored the role undertaken by the English CRN by identifying potential participants in primary care, with interested patients being invited to make contact with a local trial team based in secondary care.
Potential participants were also identified from other community COPD services, such as pulmonary rehabilitation, COPD community matrons, smoking cessation services and integrated/intermediate care services for patients with COPD. Potentially suitable participants identified by these services were sent an invitation letter and a PIL, and if interested, participants were asked to contact the local trial team (usually in secondary care) for more information and to arrange a recruitment visit.
Secondary care
Potential participants were also identified from patients attending (or who had previously attended) respiratory outpatient appointments or who had been inpatients at the hospitals of the individual recruiting centres. Potentially suitable patients were sent an invitation letter and a PIL from a member of their hospital care team (usually their consultant). Interested patients were invited to contact the local hospital-based trial team for more information and to arrange a recruitment visit.
Recruitment/baseline visit
At the recruitment visit, a participant’s eligibility was confirmed by a medically qualified doctor and fully informed consent was recorded in writing. Baseline data (see Data collection) were also collected.
Randomisation/treatment allocation
Participants were randomised, usually by a research nurse, using a computerised randomisation system available as both an interactive voice response telephone system and an internet-based application; the internet application was used for all randomisations within the trial. The randomisation service was created and administered by the Centre for Healthcare Randomised Trials (CHaRT) in the University of Aberdeen. Consenting participants were stratified by trial centre (for participants recruited in secondary care) or area (for participants recruited in primary care) and by where the participant had been identified (primary or secondary care) and then randomised with equal probability to the intervention (low-dose theophylline) and control (placebo) arms.
The random allocation sequence for the TWICS trial was generated using permuted blocks. This provided randomly generated blocks of entries of varying sizes permuted for each combination of trial centre/area and where the participant had been identified (primary or secondary care). Each entry was assigned a treatment according to a randomly generated sequence utilising block sizes of two or four. Each treatment option was assigned an equal number of times within each block, ensuring that the total number of entries assigned to each treatment remained balanced. The sequence of blocks was also random, so it was not possible for anyone to determine the next treatment to be allocated based on previous allocations made during the randomisation process.
It was possible to randomise a participant only if the relevant eligibility criteria had been met. In addition to trial centre/area and where the participant had been identified (primary or secondary care), sex, height, weight, smoking status (and, for smokers, number of cigarettes per day) and date of birth were captured during the randomisation process to calculate the correct dosage of trial medication for that participant and assign an appropriate drug pack.
With this information captured, the randomisation process assigned a trial number (i.e. a participant identification), allocated a treatment and assigned a drug pack. The user/caller was notified of the trial number and drug pack either on screen or during the randomisation telephone call. The allocated treatment remained blinded throughout, with neither the user/caller nor the participant (or anyone involved in the participant’s care or the assessment of outcomes) made aware of the allocation. All of the data captured or assigned were saved to a secure database.
The random permuted blocks that defined how treatments were allocated to participants were created by the CHaRT programming team during the system development process. The system built to utilise these permuted blocks was tested by a run of simulated randomisations that allowed the outcomes to be cross-checked and validated. Before the randomisation system went ‘live’, enough blocks were created to ensure that entries existed for the maximum expected number of participants across the maximum expected number of trial centres/areas. However, the randomisation system was flexible enough to allow the option to add further permuted blocks to the list if more were required during the lifetime of the trial. In such circumstances, randomly generated sequences in blocks of two and four continued to be utilised.
Intervention
The active intervention was 200-mg tablets of Uniphyllin modified release (MR) taken once or twice a day for 52 weeks. The placebo was manufactured to be visually identical, and was also taken once or twice a day for 52 weeks. The packaging and labelling of active and placebo interventions were identical. The intervention was for 52 weeks of therapy. The 200-mg tablets of Uniphyllin MR and placebo were supplied by Napp Pharmaceuticals Ltd (Cambridge, UK). Napp Pharmaceuticals Ltd is the holder of the marketing authorisation for 200-mg tablets of Uniphyllin MR (marketing authorisation number: PL 16950/0066–0068). Uniphyllin Continus 200 mg, 300 mg and 400 mg is licensed for the treatment and prophylaxis of bronchospasm associated with COPD, asthma and chronic bronchitis; consequently, theophylline was administered within licensed indication. 54 Placebo tablets were manufactured by Mundipharma Research Ltd (Cambridge, UK).
Dosage
The pre-clinical studies outlined in Chapter 1 demonstrate the critical importance of plasma theophylline concentration, with plasma concentrations of 1–5 mg/l having the maximal effect in reducing corticosteroid insensitivity, whereas at concentrations of > 10 mg/l theophylline is inhibitory, augmenting corticosteroid insensitivity. Theophylline dosing in the TWICS trial was based on pharmacokinetic modelling57–66 of theophylline, incorporating the major determinants of theophylline steady-state concentration (Css) [i.e. weight, smoking status and clearance of theophylline (i.e. low, normal, high)], and was designed to achieve a Css plasma theophylline of 1–5 mg/l, and to certainly be < 10 mg/l (> 10 mg is the concentration associated with high-dose theophylline, possible side effects and augmentation of corticosteroid insensitivity). For full details, see Appendix 1.
The dosing of both the interventional arm (200-mg tablets of Uniphyllin MR) and the control arm (placebo tablets) was determined by a participant’s ideal body weight (IBW) and self-reported smoking status:
-
A dose of 200 mg of theophylline MR (one tablet) once daily (or one placebo once daily) was taken by participants who did not smoke, or participants who smoked but had an IBW of ≤ 60 kg.
-
A dose of 200 mg of theophylline MR (one tablet) twice daily (or one placebo twice daily) was taken by participants who smoked and had an IBW of > 60 kg.
Ideal body weight was used unless a participant’s actual weight was lower than the ideal body weight; in such cases, actual body weight was used to determine dose.
Ideal body weight was calculated using the following standard equations:67
For the calculation of dose, to be classed as a ‘non-smoker’ at recruitment, a participant must have abstained from smoking for ≥ 12 weeks. Participants who had given up smoking recently (< 12 weeks ago) were classed as smokers.
Protocol-defined changes in dose during the treatment period
Table 1 summarises changes in dose during the treatment period based on changes in smoking status or weight.
| Characteristics at baseline | Initial dose | Changes to smoking during follow-up | Changes to weight during follow-up | ||||
|---|---|---|---|---|---|---|---|
| IBW (kg) | ABW (kg) | Smoking status | Change to smoking status | Dose change | Change to weight | Dose change | |
| > 60 | > 60 | Smoker | bd | Stop smoking | Reduce to od | Lose; ABW now ≤ 60 kg | Reduce to od |
| > 60 | ≤ 60 | Smoker | od | Stop smoking | No change | Gain; ABW now > 60 kg | Increase to bd |
| ≤ 60 | > 60 | Smoker | od | Stop smoking | No change | Lose; ABW now ≤ 60 kg | No change |
| Gain | No change | ||||||
| ≤ 60 | ≤ 60 | Smoker | od | Stop smoking | No change | Gain | No change |
| > 60 | > 60 | Non-smoker | od | Start smoking | Increase to bd | Lose; ABW now ≤ 60 kg | No change |
| Gain | No change | ||||||
| > 60 | ≤ 60 | Non-smoker | od | Start smoking | No change | Gain | No change |
| ≤ 60 | > 60 | Non-smoker | od | Start smoking | No change | Lose; ABW now ≤ 60 kg | No change |
| Gain | No change | ||||||
| < 60 | ≤ 60 | Non-smoker | od | Start smoking | No change | Gain | No change |
Changes in smoking status
Changes in smoking status are known to influence the pharmacokinetics of theophylline (smokers clear the drug more rapidly). Self-reported smoking status was checked at every contact and participants were advised, in writing and verbally, to contact their trial team if their smoking status changed during the treatment period. Participants who stopped smoking during the treatment period were reclassified as ‘non-smokers’ if they abstained from smoking for ≥ 12 weeks. Smoking participants whose IBW (and actual body weight) was > 60 kg and who stopped smoking had their dose reduced to 200 mg once daily (od) (one tablet once a day); those with an IBW of ≤ 60 kg maintained their 200 mg od dose. Participants who started smoking during the treatment period were reclassified as ‘smokers’ when they had smoked for ≥ 12 weeks. Non-smoking participants whose IBW (and actual body weight) was > 60 kg and who started smoking had their dose increased to 200 mg twice a day (bd) (one tablet twice a day).
Changes in weight
Changes in weight are known to influence the pharmacokinetics of theophylline. Participants who smoked and had an IBW of > 60 kg but an actual body weight of ≤ 60 kg had their dose reduced to 200 mg od. Participants who smoked and had an IBW of > 60 kg but whose actual body weight increased to > 60 kg had their dose increased to 200 mg bd.
Changes in concomitant medication
When informed of their patient’s participation in the trial, GPs were advised to manage their patient for exacerbations as per normal clinical practice but to assume that the participant was taking low-dose theophylline. GPs were advised to avoid, whenever possible, prescribing drugs that were likely to increase plasma theophylline concentrations; they were provided with a list of such drugs. In the event that drugs known to increase theophylline concentration had to be prescribed for ≤ 3 weeks, GPs/participants were asked to suspend the trial medication and recommence it after the course of the interacting drug had been completed, for example prescription of clarithromycin for an exacerbation of COPD. If the interacting drug was to be prescribed for > 3 weeks, GPs/participants were asked to discontinue the trial medication but remain in the trial, and were followed up in accordance with the trial protocol.
Participants were asked to carry a trial card and to show this to anyone prescribing medication for them. This advised the prescriber to assume that the participant was taking low-dose theophylline and included a link to the list of drugs that may increase plasma theophylline concentrations.
Theophylline in the form of intravenous aminophylline is sometimes used in the treatment of severe acute exacerbations of COPD in the hospital setting. It was anticipated that, during the trial, some participants would be hospitalised with life-threatening exacerbations of COPD and that the treating physician may wish to use intravenous aminophylline. The commonly used clinical protocol for intravenous aminophylline was established during the era of high-dose oral theophylline, when patients would be prescribed oral theophylline aiming for a plasma concentration of 10–20 mg/l and a loading dose of aminophylline would raise plasma theophylline concentrations to toxic concentrations (i.e. > 20 mg/l). For a patient not established on oral theophylline, the intravenous protocol comprises a bolus of intravenous aminophylline (usually 250 mg, or 5 mg/kg) followed by a maintenance dose (0.5 mg/kg/hour). For a patient established on oral theophylline, the bolus dose is omitted (because of concerns regarding toxicity) and a maintenance infusion (0.5 mg/kg/hour) is commenced. In the era of high-dose theophylline, it was critical to establish if a patient was taking oral theophylline before a physician started a patient on intravenous aminophylline.
Pharmacokinetic modelling of the low-dose theophylline dosing regimen demonstrated that a 250-mg (or 5 mg/kg if a participant’s weight was < 50 kg) loading dose of aminophylline could be administered to trial participants and their plasma theophylline would remain within the therapeutic high-dose bronchodilating concentration of 10–20 mg/l (see Appendix 1). As per guideline recommendations for plasma theophylline monitoring,24 we advised that plasma theophylline be measured 24 hours after commencing intravenous aminophylline (allocation status would not be discernible from such a concentration). The trial drug was discontinued during intravenous aminophylline therapy, but restarted after discontinuation of intravenous aminophylline therapy.
The advice regarding use of intravenous aminophylline was summarised on a participant’s trial card. In reality, no treating physician contacted the trial team with concerns about intravenous aminophylline.
Supply of trial medication
Each participant received their first bottle of 4 weeks of trial medication (or placebo) from a participating clinical trials pharmacy. For secondary care sites, this was usually the clinical trials pharmacy based at that secondary care site. For participants recruited in primary care study sites, the first bottle of medication was dispensed from the clinical trials pharmacy in NHS Grampian and couriered to a participant’s address.
Each participant also received two further supplies of six bottles (each bottle being a 4-week supply). These supplies were dispatched to participants by a third party (AndersonBrecon, Hereford, UK) and delivered to participants’ addresses via a courier. These shipments were made around weeks 3 and 27 to enable continuity of supply. Receipt of trial medication to a participant’s home address was confirmed by signature on receipt.
Data collection
Baseline, outcome and safety data were collected by face-to-face assessments conducted at recruitment/baseline (week 0), 6 months (week 26) and 12 months (week 52). Participants were telephoned 2 weeks after starting the trial medication to ensure that they were tolerating the medication. The schedule for data collection in the trial is outlined in Table 2. If a participant was unable to attend a scheduled follow-up assessment visit because of an acute illness, for example exacerbation of COPD, or other reasons, the visit was postponed and the participant was assessed within 4 weeks of the scheduled assessment visit. Participants unable to attend a face-to-face assessment at 6 and 12 months were followed up by telephone or home visit, or were sent the questionnaires to complete at home.
| Assessment | Time point | Post-study GP records | |||
|---|---|---|---|---|---|
| Recruitment | 2 weeks (telephone) | Month 6 (face to face) | Month 12 (face to face) | ||
| Assessment of eligibility criteria | ✓ | ||||
| Written informed consent | ✓ | ||||
| Demographic data; contact details | ✓ | ||||
| Clinical history | ✓ | ||||
| Drug history | ✓ | ✓ | ✓ | ||
| Smoking status | ✓ | ✓ | ✓ | ✓ | |
| Height | ✓ | ||||
| Weight | ✓ | ✓ | ✓ | ||
| Total number of COPD exacerbations requiring OCSs/antibiotics | ✓ | ✓ | ✓ | ||
| Hospital admissions | ✓ | ✓ | ✓ | ||
| Health-related quality of life | ✓ | ✓ | ✓ | ||
| Disease-related health status (CAT, mMRC dyspnoea, HARQ) | ✓ | ✓ | ✓ | ||
| Post-bronchodilator lung function | ✓ | ✓ | ✓ | ||
| AEs/drug reactions | ✓ | ✓ | ✓ | ||
| Health-care utilisation | ✓ | ✓ | ✓ | ||
| Patient compliance | ✓ | ✓ | |||
The following data were collected.
Demographic and clinical data
Demographic, contact, clinical history and, if necessary, clinical examination data were captured at the recruitment visit.
Drug history
Regular use of prescription drugs was recorded at recruitment and at the 6- and 12-month assessments. ICS use was checked at recruitment and at 6 and 12 months. Many participants brought their repeat prescription list with them to the assessments. Participants were asked how many times a day they used their ICS preparation and the dose.
Smoking history
Smoking history (i.e. age commenced, age ceased and average number of cigarettes smoked per day) and current smoking status were recorded at recruitment, and pack-year consumption was computed. At the 6- and 12-month assessments, current smoking status was recorded.
Height and weight
Height was measured using clinic stadiometers at baseline. Weight was assessed using clinic scales at recruitment and at the 6- and 12-month assessments.
Number of chronic obstructive pulmonary disease exacerbations
The primary outcome measure of the total number of COPD exacerbations requiring antibiotics/OCSs while on trial medication was ascertained at the 6- and 12-month assessments. Participants were encouraged to record any exacerbations in a space provided on the outer packaging (carton) used to ship medication or on the participant follow-up card, and to bring this to their follow-up assessments. If follow-up at 12 months could not be completed, GPs were contacted and asked to provide information on the number of exacerbations experienced by the participant in the treatment period, and whether or not these resulted in hospital admission.
The ATS/ERS guideline definition of COPD exacerbation was used: a worsening of a patient’s dyspnoea, cough or sputum beyond day-to-day variability sufficient to warrant a change in management. 55,68 The minimum management change was treatment with antibiotics or OCSs. A minimum of 2 weeks between consecutive hospitalisations or the start of a new therapy was necessary to consider events as separate. A modified ATS/ERS operational classification of exacerbation severity was used for each exacerbation:68
-
level I – increased use of a short-acting β2 agonist (SABA) (mild)
-
level II – use of OCSs or antibiotics (moderate)
-
level III – care by services to prevent hospitalisation (moderate)
-
level IV – admitted to hospital (severe).
An exercise to validate patient-reported exacerbations was carried out (see Appendix 2).
Hospital admissions
The number of unscheduled hospital admissions while on trial medication was ascertained at the 6- and 12-month assessments. Emergency admissions consequent on COPD were also identified. Participants were encouraged to record any hospital admissions in the space provided on the outer packaging (carton) used to ship medication or on the participant follow-up card, and to bring this to their follow-up assessments. If follow-up at 12 months could not be completed, participants’ GP or hospital records were checked to ascertain the number of hospital admissions during the treatment period.
Health-related quality of life
Health-related quality-of-life data were captured at recruitment and at the 6- and 12-month assessments by questionnaire using the EQ-5D-3L index,69,70 which has been used widely in studies of COPD. The completed instrument can be translated into quality-of-life utilities suitable for calculation of QALYs through the published UK tariffs. 71
Disease-related health status
Disease-related health status was ascertained at recruitment and at the 6- and 12-month assessments by participant completion of the CAT. 71–74 CAT is an eight-item unidimensional measure of the impact of COPD on a patient’s health. CAT has a scoring interval of 0–40, with 0–5 being the norm for healthy non-smokers and > 30 being indicative of a very high impact of COPD on quality of life. 72 CAT is reliable and responsive, correlates very closely with the St George’s Respiratory Questionnaire and is preferred because it provides a more comprehensive assessment of the symptomatic impact of COPD and is shorter and, thus, easier to complete. 72–74
Participants were also asked to grade their breathlessness using the mMRC dyspnoea scale at recruitment and at the 6- and 12-month assessments. 75 The mMRC dyspnoea scale has been in use for many years to grade the effect of breathlessness on daily activities. The mMRC dyspnoea scale is a single question that assesses breathlessness related to activities; the scoring interval is 0–4, with 0 being ‘not troubled by breathlessness except on strenuous exercise’ and 4 being ‘too breathless to leave the house, or breathless when dressing or undressing’. The mMRC score has been validated against walking test performance and other metrics of COPD health status, for example the St George’s Respiratory Questionnaire. 76
In self-selected recruitment centres, the Hull Airway Reflux Questionnaire (HARQ) was completed by participants at recruitment and at 6 and 12 months. HARQ was used to assess symptoms not elucidated by CAT or the mMRC dyspnoea scale. HARQ is a validated, self-administered questionnaire that is responsive to treatment effects. 77
Post-bronchodilator lung function
Lung function was measured at recruitment and at 6 and 12 months using spirometry performed to ATS/ERS standards. 78 Spirometry is a routine part of the clinical assessment of people with COPD. Post-bronchodilator (LABA within 8 hours, SABA within 2 hours) FEV1 and FVC were measured. If necessary, lung function was measured 15 minutes after administration of a participant’s own SABA. The European Coal and Steel Community predictive equations were used to compute predicted values for FEV1 and FVC. 79 When spirometry was contraindicated, or participants were not able to complete spirometry, it was omitted.
Health-care utilisation
Health-care utilisation during the previous 6 months was ascertained at recruitment and at the 6- and 12-month assessments using a modified version of the Client Service Receipt Inventory (CSRI). 80 CSRI is a research questionnaire for retrospectively collecting cost-related information about a participant’s use of health and social care services.
Adverse reactions and serious adverse events
This trial complied with the UK NHS Health Research Authority guidelines for reporting adverse events (AEs). 81 ARs and SAEs occurring during the 12-month follow-up period were ascertained at the 2-week telephone call and at the 6- and 12-month assessments. Participants were notified of recognised ARs and encouraged to contact their local trial centre if they experienced these.
Hospitalisations for treatment planned prior to randomisation and hospitalisations for elective treatment of pre-existing conditions were not considered, recorded or reported as SAEs. Complications occurring during such hospitalisation were also not considered, recorded or reported as SAEs, unless there was a possibility that the complication arose because of the trial medication (i.e. a possible AR). Exacerbations of COPD, pneumonia or hospital admissions as a consequence of exacerbations of COPD or pneumonia were not considered, recorded or reported as AEs or SAEs because they were primary and secondary outcomes for the trial.
Serious adverse events were assessed as to whether or not the SAE was likely to be related to the treatment using the following definitions:
-
unrelated – when an event is not considered to be related to the trial drug
-
possibly – although a relationship to the trial drug cannot be completely ruled out, the nature of the event, the underlying disease, concomitant medication or temporal relationship make other explanations possible
-
probably – the temporal relationship and absence of a more likely explanation suggest that the event could be related to the trial drug
-
definitely – the known effects of the trial drug or its therapeutic class, or based on challenge testing, suggest that the trial drug is the most likely cause.
The reference safety information used to assess whether or not the event was expected was section 4.8 of the Summary of Product Characteristics for theophylline. 54
Compliance
Compliance/adherence and persistence with trial medication were assessed at the 6- and 12-month assessments. Participants were asked to return empty drug bottles and unused medication; compliance was calculated by pill counting. 82 Participants were deemed to be compliant if they had taken ≥ 70% of the expected doses.
Participant withdrawal
Participants who withdrew from treatment (e.g. because of unacceptable side effects, or because they were prescribed a contraindicated medicine for > 3 weeks) but who agreed to remain in the trial for follow-up were followed up at 6 and 12 months. Those who did not want to attend for clinical follow-up at 6 and 12 months could be followed up by telephone or home visit, or could opt to receive questionnaires at home. Participants who wanted to withdraw from trial follow-up could continue to contribute follow-up data by agreeing to have data extracted from their primary and secondary care medical records.
Sample size
The sample size of 1424 was estimated on the basis of the ECLIPSE study reporting the frequency of COPD exacerbations in 2138 patients. 21 For patients identical to the target population (who in a 1-year period have had at least two self-reported COPD exacerbations requiring antibiotics or OCSs), the mean number of COPD exacerbations within 1 year was 2.22 (SD 1.86). 21 Given a similar rate in the placebo arm, 669 participants were needed in each arm of the trial to detect a clinically important reduction in COPD exacerbations of 15% (i.e. from a mean of 2.22 to 1.89) with 90% power at the two-sided 5% significance level. Allowing for 6% loss to follow-up,83 this was inflated to 712 participants in each trial arm, giving 1424 in total.
The sample size of 1424 included a 6% loss to follow-up based on a Cochrane Review of oral theophylline in COPD. 83 During the present trial, a higher proportion of participants than expected ceased their trial medication (although most were not lost to follow-up). With the appropriate REC and regulatory approvals, recruitment continued beyond 1424 in the time available, with the total number recruited being 1578 to counteract this loss of person-years on medication. Recruitment ended in August 2016.
Statistical analysis
All analyses were prespecified in the statistical analysis plan, which was approved by both the Trial Steering Committee (TSC) and the Data Monitoring Committee (DMC) in advance of analysis. The statistical analysis plan can be found on the project web page at www.journalslibrary.nihr.ac.uk/programmes/hta/115815/#/ (accessed 23 April 2019). Unless prespecified, a 5% two-sided significance level was used to denote statistical significance throughout and estimates are presented alongside their 95% CIs. No adjustments were made for multiple testing. All analyses were conducted in accordance with the intention-to-treat (ITT) principle, with a per-protocol analysis performed as a sensitivity analysis. The per-protocol analysis excluded participants who were not compliant, with compliance being defined as taking ≥ 70% of their expected doses of trial medication. All analyses were undertaken in Stata® version 14 (StataCorp LP, College Station, TX, USA).
Categorical variables are described with number and percentage in each category. Continuous variables are described with mean and SD if normally distributed and median and interquartile range (IQR) if skewed. The number of missing data is reported for each variable.
Primary outcome
The primary outcome (i.e. the number of COPD exacerbations requiring antibiotics and/or OCSs in the 12-month treatment period following randomisation) was compared between randomised groups using a generalised linear model (GLM) with log-link function, overdispersion parameter and length of time in trial as an offset. The estimated treatment effect is presented as unadjusted rate ratio followed by adjusted rate ratio for a set of prespecified baseline variables. The adjustment variables were centre (as a random effect), where the participant was identified (primary or secondary care), age (in years) centred on the mean, sex (male/female), smoking in pack-years, FEV1% predicted, number of COPD exacerbations in the previous year, treatment with LAMA/LABA or a combination, and treatment with long-term antibiotics. Participants who did not provide a full 12 months of follow-up information were included to the point at which they were lost to follow-up, with their time in the trial utilised in the offset variable.
Secondary outcomes
The total number of COPD exacerbations requiring hospital admission and the total number of emergency admissions (all causes) were analysed in the same way as the primary outcome. Occurrence of pneumonia during follow-up was analysed with a mixed-effects logit model. QoL measures (i.e. CAT, EQ-5D-3L, HARQ) and lung function (i.e. FEV1 and FVC) measured at baseline and at the 6- and 12-month follow-ups were compared between groups using a mixed-effects model unadjusted and adjusted for the same prespecified covariate set as described for the primary outcome. Fixed effects included visit number and treatment, with participant and participant–visit interaction fitted as random effects. A treatment–visit interaction was included to assess the differential treatment effect on rate of change in outcome. An autoregressive [AR(1)] correlation structure was used throughout. All participants in the ITT population were included in the analysis and missing outcome data were assumed to be missing at random. Breathlessness as measured by the mMRC dyspnoea scale was analysed using a mixed-effects GLM using a logit link function. All-cause mortality rate and COPD-related mortality and time to first exacerbation were compared between randomised groups using Kaplan–Meier survival curves and Cox regression for adjustment. Total dose of ICS at the end of follow-up and change in total daily dose from baseline were calculated and compared between randomised groups using an independent-samples t-test and linear regression for adjustment. The proportion of participants changing medication during the follow-up period was compared using a chi-squared test.
Sensitivity analyses
To assess the impact of death on the treatment effect for the primary outcome, the total number of exacerbations and the number of exacerbations requiring hospital admission, we undertook a sensitivity analysis excluding those participants who died during the trial period. A sensitivity analysis for QoL and lung function was also undertaken by repeating the mixed-effects models on only those participants who survived until the 12-month follow-up.
Prespecified subgroup analysis
The analysis for the primary outcome was repeated for a number of subgroups. The subgroups were age (< 60, 60–69, ≥ 70 years), sex, body mass index (BMI) (< 18.5, ≥ 18.5–< 25, ≥ 25 kg/m2), smoking status at recruitment (ex-/current smoker), baseline treatment for COPD [triple therapy (ICS/LAMA/LABA), double therapy (ICS/LAMA or ICS/LABA) or single therapy (ICS only)], GOLD stage (I or II, III, IV), number of exacerbations in the 12 months prior to recruitment (2, 3 or 4, ≥ 5), taking OCSs at recruitment (yes/no) and dose of inhaled ICS at recruitment (1600 or ≥ 1600 µg per day of beclomethasone equivalents). A subgroup analysis was undertaken by the addition of a treatment–covariate interaction term and using the ‘lincom’ command in Stata to obtain group-specific estimates. We report observed mean (SD) exacerbations in each subgroup by treatment group, the treatment effect [incidence rate ratio (IRR) and 99% CIs] along with the p-value for the interaction term. We used 99% CIs because of the exploratory nature of the subgroup analysis.
Health economics
Resource use
Health-care resource utilisation during the previous 6 months was collected at the 6- and 12-month assessments using a modified version of the CSRI. 84 The CSRI is a research questionnaire for retrospectively collecting cost-related information about a participant’s use of health and social care services. The main resources whose use was collected during the follow-up period were as follows:
-
theophylline intervention
-
costs of exacerbation treatment; this was broken down into two groups of costs – (1) the location of the treatment, ‘home’, ‘care by services to prevent hospitalisation’ and ‘admitted to hospital’ and (2) the treatment cost of the exacerbations, including medication
-
cost of COPD maintenance medications
-
other health service use (including inpatient, outpatient and primary care use); none of these included exacerbation costs
-
non-COPD emergency hospital admissions
-
regular medication.
Baseline resource use was collected for current use of COPD maintenance treatment and regular medication. For calculating baseline resource use and costs, we have assumed use to be for the 6 months prior to baseline. The number of exacerbations needing treatment in the previous 12 months and the number of exacerbations resulting in hospitalisation in the previous 12 months were also collected.
Unit costs
All resource use was valued in Great British pounds (GBP) and indexed to 2016, using the Health Service Cost Index85 to adjust if necessary.
-
Medication costs were obtained from the British National Formulary (BNF). 86
-
For exacerbations, non-COPD emergency admissions, inpatient stays, outpatient attendances and primary care, costs were obtained from NHS Reference Costs 2015 to 2016,87 Information Services Division (ISD),88 Personal Social Services Research Unit (PSSRU),85 the BNF86 and papers by Oostenbrink et al. 89 and Scott et al. 90
The total cost per participant was calculated by assigning unit costs to resource use for each participant. Total mean costs were calculated using a GLM with a gamma family and clustering for centre number. After multiple imputation, total costs were adjusted for baseline characteristics using standard regression methods, to account for any differences in cost-related variables at baseline. 91
Unit costs and their sources are presented in Table 3.
| Resource | Unit | Unit cost (£) | Source |
|---|---|---|---|
| Intervention | |||
| Theophylline | 200 mg od | 0.05 | BNF86 |
| 200 mg bd | 0.11 | BNF86 | |
| Exacerbation treatment | |||
| Oxygen | Per day | 19 | Oostenbrink et al.89 |
| Medication | Daily dose | Various | BNF86 |
| Inpatient costs | |||
| Ward stay (elective) | Bed-day | 362 | NHS Reference Costs 2015 to 2016 (elective excess bed-day unit cost)87 |
| Ward stay (non-elective) | Bed-day | 298 | NHS Reference Costs 2015 to 2016 (non-elective excess bed-day unit cost)87 |
| COPD-related ward stay | Bed-day | 262 | NHS Reference Costs 2015 to 2016 (weighted average of COPD hospital stays DZ65)87 |
| Long stay on ward | Day | 133 | PSSRU (not-for-profit care home fee: mean £931 per week)85 |
| Outpatient costs | |||
| Day case | Day | 521 | ISD costs book (day cases, all specialties)88 |
| Outpatient appointment | Appointment | 177 | NHS Reference Costs 2015 to 2016 (total outpatient attendances unit cost)87 |
| Primary care costs | |||
| Emergency GP visit | Per contact | 86 | Based on Scott et al.90 for out-of-hours home visit |
| Routine GP visit | Per contact | 31 | PSSRU (including direct care staff costs, without qualifications, 9.22 minutes)85 |
| Community/district nurse | Per contact | 38 | NHS Reference Costs 2015 to 2016 (Community Health Services – N02AF – district nurse)87 |
| Hospital at home team | Per contact | 84 | NHS Reference Costs 2015 to 2016 (Community Health Services – N08AF – specialist nursing asthma and respiratory nursing liaison)87 |
| GP telephone | Per contact | 23.43 | PSSRU (including direct care staff costs, without qualification costs, 7.1 minutes)85 |
| GP home visit | Per contact | 77.22 | PSSRU (including direct care staff costs, without qualification costs, 11.4 minutes visit plus 12 minutes of travelling time)85 |
| Blood test | Per contact | 14.42 | ISD costs book88 (laboratory services, haematology plus practice nurse appointment, PSSRU 201685) |
| Dental service | Per contact | 77 | NHS Reference Costs 2015 to 2016 (general dental service attendance)87 |
| Hearing aid clinic | Per contact | 53 | NHS Reference Costs 2015 to 2016 (audiology)87 |
| Occupational therapist | Per contact | 79 | NHS Reference Costs 2015 to 2016 (occupational therapist)87 |
| Diabetic nurse | Per contact | 71 | NHS Reference Costs 2015 to 2016 (specialist nursing, diabetic)87 |
| Cardiac nurse | Per contact | 81 | NHS Reference Costs 2015 to 2016 (specialist nursing, cardiac)87 |
| Long-term condition nurse/community matron | Per contact | 89 | NHS Reference Costs 2015 to 2016 (active case management)87 |
| Paramedic | Per contact | 181 | NHS Reference Costs 2015 to 2016 (ambulance, see, treat, refer)87 |
| Chiropodist/community clinic/endoscopy | Per contact | 60 | NHS Reference Costs 2015 to 2016 (mean of community health services, no separate chiropodist or community clinic cost)87 |
| Physiotherapist | Per contact | 49 | NHS Reference Costs 2015 to 2016 (physiotherapist)87 |
| Podiatrist | Per contact | 40 | NHS Reference Costs 2015 to 2016 (podiatrist)87 |
| Practice nurse | Per contact | 9.42 | PSSRU (practice nurse, 15.5 minutes per contact)85 |
| Speech therapist | Per contact | 88 | NHS Reference Costs 2015 to 2016 (speech and language therapist)87 |
| Nurse telephone call | Per contact | 6.10 | PSSRU (nurse-led triage)85 |
| Treatment room nurse | Per contact | 27 | NHS Reference Costs 2015 to 2016 (specialist nursing, treatment room)87 |
| Urine sample/sputum test | Per contact | 10.28 | ISD costs book88 (clinical chemistry, plus practice nurse appointment, PSSRU85) |
| Dietitian | Per contact | 81 | NHS Reference Costs 2015 to 2016 (dietitian)87 |
| Influenza vaccination | Per contact | 14.67 | BNF86 plus practice nurse appointment, PSSRU 201685 |
| Early support discharge | Per contact | 124 | NHS Reference Costs 2015 to 2016 (crisis response and early discharge services)87 |
| Diagnostic imaging | Per contact | 37.3 | NHS Reference Costs 2015 to 2016 (total outpatient attendances, diagnostic imaging)87 |
| Optometry | Per contact | 79.19 | NHS Reference Costs 2015 to 2016 (total outpatient attendances, optometry)87 |
| Health-care assistant | Per contact | 6.20 | PSSRU 2016 (band 3 nurse, 15.5 minutes)85 |
| Talking matters | Per contact | 24.06 | PSSRU 2009/10 (counselling services in primary care, telephone consultation 29.7 minutes)92 |
| Community psychiatric nurse/stroke nurse | Per contact | 77 | NHS Reference Costs 2015 to 2016 (other specialist nursing)87 |
| Counselling | Per contact | 78.27 | PSSRU 2009/10 (counselling services in primary care, consultation 96.6 minutes)92 |
| Breast care nurse | Per contact | 59 | NHS Reference Costs 2015 to 2016 (beast care nursing)87 |
| Community mental health team | Per contact | 121 | NHS Reference Costs 2015 to 2016 (other mental health specialist team)87 |
| Pulmonary rehabilitation | Per contact | 78 | NHS Reference Costs 2015 to 2016 (other single condition community rehabilitation teams)87 |
| Emergency costs | |||
| Ambulance | Per attendance | 236 | NHS Reference Costs 2015 to 2016 (see, treat convey)87 |
| Accident and emergency attendance | Per attendance | 138 | NHS Reference Costs 2015 to 2016 (emergency medicine average unit cost)87 |
Health outcomes
The economic outcome used was the QALY, a combination of quality and quantity of life. The QoL measure was generated using completed EQ-5D-3L questionnaires. Participants completed the questionnaire at baseline and at 6 months and 12 months.
Patient-reported health-related QoL obtained from EQ-5D-3L questionnaires was valued in terms of utilities (on a scale from –0.59 to 1, where 1 is full health) using a standard UK value set,71 which were converted into QALYs using standard area under the curve methods; patient utility measurements from each follow-up point were weighted by the time interval between follow-up points. Discrete changes in utility values between follow-up time points were assumed to be linear. After multiple imputation, QALYs were adjusted for baseline characteristics using standard regression methods.
Analysis
The total cost per participant in each intervention was summed and divided by the number of participants in each arm to calculate the total mean cost per participant in each arm, along with the difference in means and a 95% CI.
The mean number of QALYs per participant for each intervention was calculated by summing all participants’ QALYs and dividing by the number of participants in that intervention arm. The differences in the means were also calculated, along with a 95% CI.
The incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in mean costs by the difference in mean QALYs. The NICE threshold of £20,000 to £30,000 per QALY was used when judging whether or not the intervention was cost-effective. 93
Withdrawn participants were included in the analysis; the total time they spent in the trial was used to adjust total costs and QALYs using regression methods.
To explore the uncertainty around the cost and QALY differences and the resulting ICER, a non-parametric bootstrapping technique was employed with 1000 iterations. The results are presented (see Figures 7–10) using a cost-effectiveness plane, showing all 1000 incremental cost-effectiveness pairs, and a cost-effectiveness acceptability curve.
The analysis was carried out using Stata version 14.
Missing data
There was a small amount of multivariate missingness in collected resource data.
Resource use data were not available for some exacerbations because this was not reported by participants or because only limited data were available from GP or hospital records. Missing resource use data on exacerbations were dealt with as follows:
-
For exacerbations with missing length of exacerbation data, the length was assumed to be the mean length of exacerbation specific to that treatment arm.
-
For exacerbations missing a marker to indicate the location of treatment, this was assumed to be at home, as most locations of treatments were at home (> 80%).
-
For exacerbations treated in hospital, missing lengths of stay were assumed to be the length of exacerbation.
-
For exacerbations missing treatment costs, a mean cost of treatment, specific to that treatment arm, was assumed.
At a resource use level, there were small numbers of missing data, which were dealt with as follows:
-
If the length of stay data were missing for emergency hospital admissions, these were imputed using the mean length of stay specific to that treatment arm.
-
If participants had no observations completed to indicate the duration of a COPD maintenance treatment, it was assumed that the treatment duration was the 6 months prior to the date on which the information about the COPD maintenance treatment was collected.
-
If a participant indicated that they had received a COPD maintenance treatment but no medication details were available, a mean cost, specific to that treatment arm, was imputed for that specific maintenance medication.
-
If resource use data were missing for inpatient, outpatient and primary care service use, the participant was assumed not to have used the resource in question.
Complete cases were analysed initially and multiple imputation was used to explore the effect of missing data on the analysis.
The multiple imputation technique used was multiple imputation by chained equations. Multiple imputation assumes that data are missing at random; missing data may depend on observed data.
Assumptions
The following assumptions were made in the health economics analysis: a complete case is defined as a case with data covering resource use for the 12-month follow-up period. In a small number of participants, there was no 6-month data collection; however, the 12-month data collection covered resource use for the whole of the 12-month follow-up period.
Patient and public involvement
A person with COPD was an independent voting member of the TSC. Initially, this was a patient from the Aberdeen Chest Clinic who was nominated by Chest Heart & Stroke Scotland (CHSS) as part of its Voices Scotland initiative. In 2015, this person had to resign from the TSC because of ill health and was replaced by another patient, who is living with COPD, from the Aberdeen Chest Clinic.
Early versions of the trial protocol and PILs were reviewed by a representative from the British Lung Foundation–North Region and by a person who lives with COPD and attends the chest clinic at the Freeman Hospital, Newcastle. They both attended the trial initiation meeting, purposely held in Newcastle in February 2013, and contributed suggestions and changes to the final trial design that were reflected in the protocol and PIL.
The TWICS trial was publicised in 2014 by a press release that included supportive quotations from the British Lung Foundation and CHSS. This publicity resulted in members of the public with COPD volunteering to participate; with their permission, their details were passed on to their local TWICS trial site.
We anticipate that the patient and public involvement (PPI) member of the TSC will comment on the results letter to be sent to trial participants. It is also anticipated that the publication of the trial results will be co-ordinated with press releases from the participating academic/NHS institutions, the British Lung Foundation and CHSS. Members of the trial team will be participating in local public engagement with research activities.
Protocol amendments
There were seven protocol amendments, which are summarised in Table 4.
| Version number, date | Summary of amendments |
|---|---|
| Version 2, 20 June 2013 | Version initially approved by the REC |
| Version 3, 5 August 2013 | To incorporate clarification of the definition of smoker and non-smoker as required by the MHRA |
| Version 4, 5 February 2014 |
|
| Version 5, 2 July 2014 |
|
| Version 6, 4 August 2014 |
|
| Version 7, 11 August 2015 |
|
| Version 8, 19 May 2016 | To amend the protocol to allow for over-recruitment |
| Version 9, 14 April 2017 |
|
Trial oversight
A TSC, with independent members, including PPI members, oversaw the conduct and progress of the trial. An independent DMC oversaw the safety of participants in the trial.
Breaches
Breaches of trial protocol or good clinical practice were recorded and reported to the sponsor. A summary of breaches is included in Appendix 3. Participants who were the subject of a breach remained in the ITT population, the safety population and the per-protocol population (if compliance criteria were met).
Chapter 3 Baseline characteristics
Recruitment
Participants were recruited to the trial between February 2014 and August 2016. During this 31-month period, 141 UK sites were opened to recruitment. Once opened, some sites (n = 20) failed to recruit any participants to the trial. Reasons for this included staff changeover, lack of eligible patients, competing priorities, practice closure and eligible patients who did not agree to take part.
In total, 1578 participants were recruited from 121 sites (Table 5). A detailed summary of recruitment, by site, is given in Appendix 4. In summary, across 33 secondary care sites, 1101 participants were recruited, and, across 88 primary care sites, 477 participants were recruited. Of those recruited in secondary care, 464 were identified in primary care. Overall, 59.6% of participants were identified in primary care.
| Recruitment site based in | Participants identified in | Number of participants |
|---|---|---|
| Secondary care | Secondary care | 637 |
| Secondary care | Primary care | 464 |
| Primary care | Primary care | 477 |
The initial funding included a 24-month recruitment period. Delays in manufacturing and packaging the trial medication led to the projected recruitment being reprofiled across 21 months. After ≈6 months of recruitment, it became clear that we were unlikely to meet the recruitment target within 21 months. To address this, several measures were successfully implemented: ‘second’- and ‘third’-wave sites were opened earlier than planned; additional primary and secondary care sites were identified and a rolling programme of opening these sites was established; a 6-month extension to recruitment was granted by the funder; and additional recruitment time was accommodated within the existing funding. In the 31-month recruitment period we were granted approval to over-recruit beyond the original target of 1424 participants. The justification for this was the higher than anticipated numbers of participants who ceased taking the trial medication (see Chapter 4, Treatment adherence/compliance).
Figure 3 shows the original recruitment targets, the reprofiled recruitment targets (to accommodate the delay in manufacturing/packaging), the revised recruitment targets (after the extension to recruitment was granted) and the actual recruitment.
FIGURE 3.
Recruitment.

Post-randomisation exclusions
Eleven participants were recruited in error and were then excluded. None of these participants took any dose of trial medication and all are excluded from all trial analyses. Reasons for these post-randomisation exclusions are given in Table 6.
| Overarching reason | Specific reason |
|---|---|
| Concomitant medications | Already taking a form of theophylline (n = 1) |
| Concomitant prescription of diltiazem (n = 2) | |
| Concomitant prescription of methotrexate (n = 1) | |
| Not currently prescribed ICS (n = 1) | |
| COPD diagnosis | Diagnosed with right middle lobe collapse, not COPD (n = 1) |
| COPD diagnosis disputed by consultant (n = 1) | |
| Fewer than two exacerbations in the previous year (n = 3) | |
| Spirometry | Did not fulfil spirometric criterion for the trial (n = 1) |
Sixteen participants who were recruited into the TWICS trial were subsequently noted to be ineligible for the trial at the point of recruitment. All 16 participants had taken at least one dose of trial medication and were retained in the follow-up and included in the trial analyses. Seven of these were taking a form of diltiazem at recruitment, and were identified during a review of all baseline medication recorded for participants. Diltiazem can cause a slight increase in serum theophylline concentration; however, any effect is usually clinically insignificant. 94 One of these participants took the trial medication for ≈10 days but stopped because they experienced symptoms considered likely to be related to theophylline. A further participant experienced some symptoms that may have been side effects related to the trial medication and stopped after ≈4 months. One further participant experienced some symptoms that may have been side effects related to the trial medication but did not cease taking the trial medication.
In this same review, a further five participants were noted to have been taking a contraindicated medication at baseline. Three participants were noted to be taking a form of oestrogen. Serum theophylline concentration is slightly increased by concomitant oestrogen but no toxicity has been reported. 94 Two participants who were co-prescribed oestrogen continued to take the trial medication through their 12-month follow-up with no ARs. The third participant experienced symptoms (thought to be related to theophylline) and stopped taking the trial medication after 14 days. One participant was noted to have been taking febuxostat at recruitment. They had taken the trial medication through their 12-month follow-up and experienced symptoms that may have been side effects related to the trial medication. High-dose febuxostat has been reported to possibly increase serum theophylline. 94 One participant was noted to have been taking roflumilast at recruitment. Although roflumilast has no reported effect on serum theophylline concentrations, the two drugs act through phosphodiesterase enzymes,94 albeit theophylline is a phosphodiesterase inhibitor at serum concentrations of 10–20 mg/l when used at ‘high-dose’ levels. This participant had taken trial medication throughout their 12-month follow-up without any ARs.
Three participants were taking a form of theophylline at recruitment. In two cases, this was noted only after the participant had completed their 12-month follow-up (and the participants had taken the trial medication throughout their 12-month follow-up). In the third case, this was noted after the participant had taken the trial medication for 8 days; the trial medication was then stopped. In all three cases, no ARs relating to the trial medication were noted.
One participant was recruited into the TWICS trial when they were already participating in another CTIMP for an unrelated condition. The participant did not disclose this at the time of recruitment and it was not clearly documented in their hospital notes. No interaction between the TWICS trial medication and the medication used in the other trial is likely. The participant continued to take the trial medication for ≈11 months, at which point the participant ceased taking the trial medication as a result of a non-related throat problem.
Baseline characteristics
Baseline characteristics are presented for the 1567 included participants (after exclusion of the 11 post-randomisation exclusions). The theophylline and placebo groups were well balanced in terms of demographic and disease characteristics at baseline.
The mean age of participants was 68.4 years (SD 8.4 years) (Table 7). Just over half of the participants (53.8%) were male. Approximately one-third (31.7%) were current smokers; the remainder were ex-smokers. The median number of pack-years smoked was 42 (IQR 27.7–56.0 pack-years). The mean BMI was 27.2 kg/m2 (SD 6.1 kg/m2).
| Characteristics | Trial arm | Overall | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Sex (N, n, %) | |||
| Male | 788, 425, 53.9 | 779, 418, 53.7 | 1567, 843, 53.8 |
| Female | 788, 363, 46.1 | 779, 361, 46.3 | 1567, 724, 46.2 |
| Age (n, mean, SD) | 788, 68.3, 8.2 | 779, 68.5, 8.6 | 1567, 68.4, 8.4 |
| Smoking status (N, n, %) | |||
| Current smoker | 788, 247, 31.3 | 779, 249, 32.0 | 1567, 496, 31.7 |
| Ex-smoker | 788, 541, 68.7 | 779, 530, 68.0 | 1567, 1071, 68.3 |
| Pack-years (n, mean, SD) | 785, 47.0, 26.3 | 775, 47.1, 30.6 | 1560, 47.1, 28.5 |
| Pack years (n, median, IQR) | 785, 43.0, 28.5–57.0 | 775, 41.0, 27.0–55.0 | 1560, 42.0, 27.7–56.0 |
| BMI (n, mean, SD) | 788, 27.1, 6.2 | 779, 27.3, 6.0 | 1567, 27.2, 6.1 |
| BMI group (N, n, %) | |||
| Underweight | 788, 37, 4.7 | 779, 38, 4.9 | 1567, 75, 4.8 |
| Normal | 788, 285, 36.2 | 779, 246, 31.6 | 1567, 531, 33.9 |
| Overweight | 788, 252, 32.0 | 779, 266, 34.1 | 1567, 518, 33.1 |
| Obese | 788, 214, 27.2 | 779, 229, 29.4 | 1567, 443, 28.3 |
The median number of participant-reported exacerbations in the 12 months prior to recruitment was 3 (IQR 2–4) and the mean number of exacerbations was 3.6 (SD 2.2) (Table 8). The majority of participants (79.9%) were prescribed the ‘triple therapy’ combination of ICS, LABA and LAMA at baseline. Almost one-fifth (16.7%) were prescribed ICS and LABA. The remainder were prescribed ICS only (2.0%) or ICS and LAMA (1.5%).
| Characteristics | Trial arm | Overall | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Exacerbations in the previous 12 months | |||
| n, mean, SD | 785, 3.6, 2.2 | 773, 3.5, 2.1 | 1558, 3.6, 2.2 |
| n, median, IQR | 785, 3, 2–4 | 773, 3, 2–4 | 1558, 3, 2–4 |
| Exacerbations requiring hospitalisation in the previous 12 months | |||
| n, mean, SD | 784, 0.4, 0.8 | 773, 0.4, 1.0 | 1557, 0.4, 0.9 |
| n, median, IQR | 784, 0, 0–1 | 773, 0, 0–0 | 1557, 0, 0–0 |
| GOLD 2011 category (N, n, %) | |||
| C: two or more exacerbations in the previous year, mMRC dyspnoea score of 0–1 and CAT score of < 10 | 779, 37, 4.7 | 768, 45, 5.9 | 1547, 82, 5.3 |
| D: two or more exacerbations in the previous year, mMRC dyspnoea score of ≥ 2 and CAT score of ≥ 10 | 779, 742, 95.3 | 768, 723, 94.1 | 1547, 1465, 94.7 |
| FEV1% predicted (n, mean, SD) | 785, 51.3, 20.1 | 771, 52.2, 19.8 | 1556, 51.7, 20.0 |
| FEV1% predicted category (N, n, %) | |||
| ≥ 80% (GOLD mild) | 785, 70, 8.9 | 771, 73, 9.5 | 1556, 143, 9.2 |
| 50–79.9% (GOLD moderate) | 785, 308, 39.2 | 771, 308, 39.9 | 1556, 616, 39.6 |
| 30–49.9% (GOLD severe) | 785, 291, 37.1 | 771, 295, 38.3 | 1556, 586, 37.7 |
| 0–29.9% (GOLD very severe) | 785, 116, 14.8 | 771, 95, 12.3 | 1556, 211, 13.6 |
| FVC% predicted (n, mean, SD) | 783, 84.3, 22.3 | 770, 86.2, 23.4 | 1553, 85.2, 22.8 |
| FEV1/FVC ratio (n, mean, SD) | 783, 49.0, 19.7 | 770, 48.5, 14.1 | 1553, 48.8, 17.1 |
| Current treatment for COPD (N, n, %) | |||
| ICS | |||
| ICS only | 788, 14, 1.8 | 779, 17, 2.2 | 1567, 31, 2.0 |
| ICS/LABA | 788, 136, 17.3 | 779, 125, 16.0 | 1567, 261, 16.7 |
| ICS/LAMA | 788, 13, 1.6 | 779, 10, 1.3 | 1567, 23, 1.5 |
| ICS/LABA/LAMA | 788, 625, 79.3 | 779, 627, 80.5 | 1567, 1252, 79.9 |
| Oral mucolytic use | 784, 201, 25.6 | 771, 197, 25.6 | 1555, 398, 25.6 |
| Long-term antibiotic use | 784, 51, 6.5 | 771, 48, 6.2 | 1555, 99, 6.4 |
| Comorbidities (N, n, %) | |||
| Asthma | 782, 138, 17.6 | 772, 147, 19.0 | 1554, 285, 18.3 |
| Bronchiectasis | 782, 41, 5.2 | 770, 27, 3.5 | 1552, 68, 4.4 |
| Ischaemic heart disease | 781, 111, 14.2 | 771, 96, 12.5 | 1552, 207, 13.3 |
| Hypertension | 782, 317, 40.5 | 772, 277, 35.9 | 1554, 594, 38.2 |
| Diabetes mellitus | 782, 83, 10.6 | 772, 93, 12.0 | 1554, 176, 11.3 |
| Osteoporosis | 783, 109, 13.9 | 771, 90, 11.7 | 1554, 199, 12.8 |
| Anxiety/depression treated in the previous 5 years | 782, 222, 28.4 | 772, 213, 27.6 | 1554, 435, 28.0 |
| Cerebrovascular event | 783, 46, 5.9 | 772, 58, 7.5 | 1555, 104, 6.7 |
Comorbidities, as reported by participants, were relatively common. Almost one-fifth of participants (18.3%) had a concurrent diagnosis of asthma. Four per cent of participants reported a diagnosis of bronchiectasis. Just over one-third of participants (38.2%) reported a diagnosis of hypertension. Thirteen per cent reported ischaemic heart disease and 6.7% reported a previous cerebrovascular event. Almost one-third (28.0%) reported anxiety or depression in the previous 5 years. Eleven per cent had a diagnosis of diabetes mellitus and 12.8% had a diagnosis of osteoporosis.
Measurement of lung function at baseline revealed that the mean FEV1 was 51.7% (SD 20.0%) predicted. Using the GOLD classification,1 13.6% were classified as having very severe COPD, 37.7% as having severe COPD, 39.6% as having moderate COPD and 9.2% as having mild COPD.
The mean score on the CAT was 22.6 (SD 7.7), indicating that, overall, COPD was having a high impact on the lives of participants (Table 9). Considering the cut-off points used to interpret the scores derived from the CAT, COPD was having a low impact on the lives of 5.3% of participants, a medium impact on the lives of 29.9% of participants, a high impact on the lives of 44.4% of participants and a very high impact on the lives of 20.4% of participants.
| Symptoms and QoL | Trial arm | Overall | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Degree of breathlessness (mMRC dyspnoea)75 [N, n (%)] | |||
| Not troubled by breathlessness except on strenuous exercise | 783, 35 (4.5) | 772, 50 (6.5) | 1555, 85 (5.5) |
| Short of breath when hurrying or walking up a slight hill | 783, 216 (27.6) | 772, 224 (29.0) | 1555, 440 (28.3) |
| Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace | 783, 251 (32.1) | 772, 239 (31.0) | 1555, 490 (31.5) |
| Stops for breath after walking ≈100 m or after a few minutes on level ground | 783, 225 (28.7) | 772, 204 (26.4) | 1555, 429 (27.6) |
| Too breathless to leave the house, or breathless when dressing or undressing | 783, 56 (7.2) | 772, 55 (7.1) | 1555, 111 (7.1) |
| CAT [N, mean (SD)] | 780, 22.8 (7.5) | 771, 22.3 (7.9) | 1551, 22.6 (7.7) |
| CAT group [N, n (%)] | |||
| Low (score of 0–9) | 780, 37 (4.7) | 771, 45 (5.8) | 1551, 82 (5.3) |
| Medium (score of 10–19) | 780, 219 (28.1) | 771, 244 (31.6) | 1551, 463 (29.9) |
| High (score of 20–29) | 780, 361 (46.3) | 771, 328 (42.5) | 1551, 689 (44.4) |
| Very high (score of 30–40) | 780, 163 (20.9) | 771, 154 (20.0) | 1551, 317 (20.4) |
| EQ-5D-3L utility [N, mean (SD)] | 785, 0.62 (0.28) | 772, 0.63 (0.28) | 1557, 0.63 (0.28) |
| EQ-5D-3L VAS [N, mean (SD)] | 785, 59.6 (19.0) | 770, 60.8 (19.1) | 1555, 60.2 (19.1) |
The mean EQ-5D-3L utility score was 0.63 (SD 0.28). The mMRC dyspnoea score revealed that 7.1% of participants were too breathless to leave the house, 27.6% had to stop for breath after walking ≈100 m, 31.5% walked more slowly than contemporaries on level ground because of breathlessness, 28.3% became short of breath when hurrying or walking up a slight hill and only 5.5% of participants were not troubled by breathlessness except on strenuous exercise.
A comparison of the participants recruited in primary and secondary care indicated that those identified in secondary care were slightly younger, were more likely to be ex-smokers and had experienced a greater number of exacerbations in the previous 12 months. Furthermore, a higher proportion of those identified in secondary care had more severe COPD, were on triple (ICS/LAMA/LABA) therapy and long-term antibiotic use, and had a significantly greater prevalence of comorbidities, such as bronchiectasis, ischaemic heart disease and osteoporosis (see Appendix 5, Table 35). Participants recruited in secondary care had a higher CAT score and a slightly lower QoL.
Chapter 4 Clinical effectiveness
Clinical effectiveness of low-dose theophylline compared with placebo
In this chapter, we report the results of people with COPD being treated for 1 year with low-dose theophylline compared with placebo. A total of 1578 participants were randomised to theophylline or placebo; 11 post-randomisation exclusions resulted in 1567 participants being eligible to initiate trial medication and for whom baseline characteristics have been reported (see Chapter 3). Follow-up data were unavailable for 31 (2%) participants (theophylline, n = 16; placebo, n = 15); therefore, the results presented for the ITT analysis are based on 1536 participants (theophylline, n = 772; placebo, n = 764). Figure 4 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the ITT analysis. In total there were 1489 person-years of follow-up data: 747 person-years in the theophylline group and 742 person-years in the placebo group (Table 10).
FIGURE 4.
The CONSORT flow diagram for the ITT analysis. a, Reasons for ineligibility were as follows: 16 did not meet the inclusion criteria for established COPD diagnosis or had a predominant respiratory disease other than COPD, 10 had not had two exacerbations in the previous year, seven did not meet the smoking history criteria, seven were taking contraindicated medication, three were not currently using ICSs, one was not clinically stable, two were participating in another clinical trial, one was currently taking theophylline, one had known or suspected hypersensitivity to theophylline, one was pregnant, two had severe heart disease and 11 did not meet two or more of the inclusion criteria.
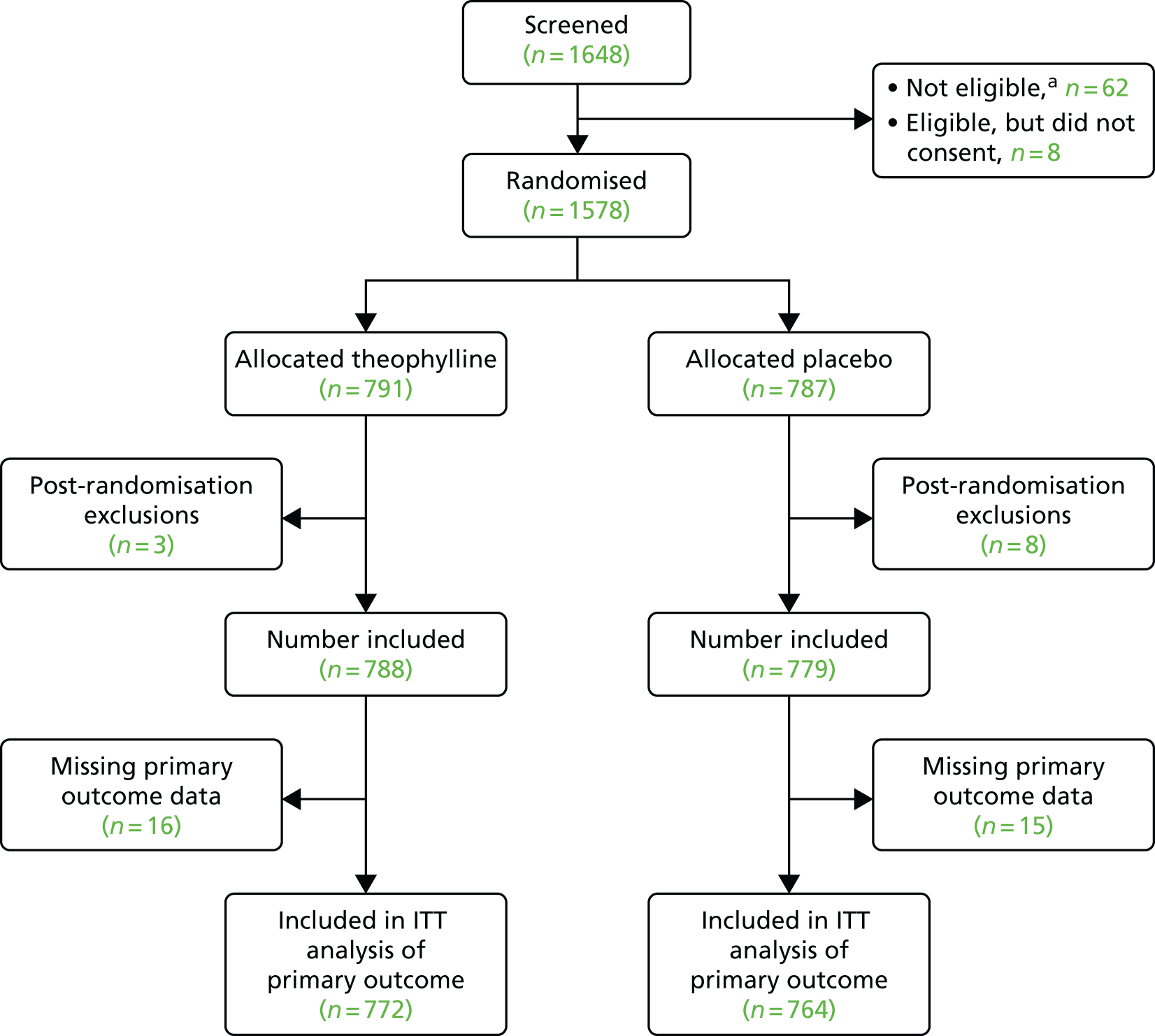
| Exacerbations | Trial arm | Adjusted or unadjusted | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Primary outcome: exacerbations | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Person-years of follow-up | 747.5 | 742.1 | ||||
| Number with at least one exacerbation | 633 | 609 | ||||
| Number of exacerbations | ||||||
| Total | 1727 | 1703 | ||||
| Mean | 2.24 | 2.23 | Unadjusted IRR | 1.00 | 0.92 to 1.09 | 0.965 |
| SD | 1.99 | 1.97 | Adjusted IRRa | 0.99 | 0.91 to 1.08 | 0.840 |
| Exacerbations requiring hospital treatment | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Person-years of follow-up | 747.5 | 742.1 | ||||
| Number with at least one exacerbation | 106 | 130 | ||||
| Number of exacerbations | ||||||
| Total | 134 | 185 | ||||
| Mean | 0.17 | 0.24 | Unadjusted IRR | 0.72 | 0.55 to 0.95 | 0.021 |
| SD | 0.49 | 0.66 | Adjusted IRRa | 0.72 | 0.55 to 0.94 | 0.017 |
| Time to first exacerbation (from randomisation) | ||||||
| Total number included in analysisb | 756 | 753 | ||||
| Number with at least one exacerbation | 617 | 598 | ||||
| % with at least one exacerbation | 81.6 | 79.4 | ||||
| Median time to first exacerbation (days) | 219 | 227 | Unadjusted HR | 1.03 | 0.92 to 1.14 | 0.652 |
| 25th percentile [time to first exacerbation (days)] | 132 | 116 | Adjusted HRa | 1.01 | 0.90 to 1.13 | 0.895 |
| 75th percentile [time to first exacerbation (days)] | 334 | 337 | ||||
Intention-to-treat analysis
Primary outcome: total number of exacerbations of chronic obstructive pulmonary disease necessitating a change in management
In total, 633 out of 772 (82.0%) participants allocated to theophylline had at least one exacerbation, with 1727 exacerbations in the group overall. Among participants allocated to placebo, 609 out of 764 (79.7%) had at least one exacerbation and there were 1703 exacerbations in the group overall. The mean number of exacerbations per participant was 2.24 (SD 1.99) in those allocated to low-dose theophylline and 2.23 (SD 1.97) in those allocated to placebo. The adjusted IRR for exacerbation was 0.99 (95% CI 0.91 to 1.08), indicating no difference in the exacerbation rate during the 12-month follow-up period between those on low-dose theophylline and those on placebo (see Table 10).
The primary outcome was exacerbation treated with antibiotics and/or OCSs, but we also conducted analyses relating treatment with low-dose theophylline to differing levels of treatment for COPD exacerbations, that is antibiotics only, OCSs only or antibiotics and OCSs (see Appendix 5, Table 39). In the adjusted model, for exacerbations treated with antibiotics only, the IRR was 0.94 (95% CI 0.78 to 1.14); for exacerbations treated with OCSs only, the IRR was 0.88 (95% CI 0.62 to 1.25); and for exacerbations treated with antibiotics and OCSs, the IRR was 1.02 (95% CI 0.92 to 1.14).
Among participants allocated to low-dose theophylline, 106 (13.7%) had at least one exacerbation requiring hospital admission, with a total of 134 hospital admissions in the group. Among participants allocated to placebo, 130 (17.0%) had at least one exacerbation requiring hospital admission, and there were 185 admissions in total. A comparison of the proportion of participants with at least one exacerbation requiring hospital admission was not significant at the 5% level (13.7% in the theophylline arm vs. 17.0% in the placebo arm; p = 0.074). In the adjusted model, the IRR for exacerbations of COPD requiring hospital treatment was 0.72 (95% CI 0.55 to 0.94), suggesting that low-dose theophylline resulted in a reduction in the number of exacerbations requiring hospital admission when compared with placebo (see Table 10). However, further exploration of the data showed that, in the theophylline group, only three participants had three or more exacerbations necessitating treatment in hospital (12 exacerbations in total), compared with 13 participants in the placebo group having three or more exacerbations necessitating hospital treatment (51 exacerbations in total). Therefore, a small excess of participants (n = 10) allocated to placebo who had three or more exacerbations requiring treatment in hospital accounted for 39 of the 51 excess admissions in the placebo group (Table 11).
| Number of exacerbations requiring hospital admission | Trial arm, n (%) | |
|---|---|---|
| Theophylline | Placebo | |
| 0 | 666 (86) | 634 (83) |
| 1 | 84 (11) | 100 (13) |
| 2 | 19 (2) | 17 (2) |
| 3 | 0 (0) | 5 (1) |
| 4 | 3 (< 1) | 5 (1) |
| 5 | 0 (0) | 2 (< 1) |
| 6 | 0 (0) | 1 (< 1) |
| Total (n) | 772 | 784 |
Secondary outcomes
Time to first exacerbation
The date of onset of the first exacerbation after commencing trial medication was not available for 27 of the 1242 participants who had at least one exacerbation; therefore, this analysis was based on 1509 participants in the ITT population [294 who had no exacerbation and 1215 (80.5%) who had an exacerbation]. Of the 756 participants allocated to theophylline, 617 (81.6%) had at least one exacerbation, with a median time to first exacerbation of 219 days (7.2 months) after randomisation. In the placebo group, 598 out of 753 (79.4%) participants had at least one exacerbation, with a median time to first exacerbation of 227 days (7.5 months). In a Cox regression analysis, the adjusted HR for the time to first exacerbation was 1.01 (95% CI 0.90 to 1.13), suggesting no significant difference between the treatment groups in terms of the time to first exacerbation (from point of randomisation) during the 12-month follow-up period (see Table 10).
Total number of emergency hospital admissions (including those not related to chronic obstructive pulmonary disease)
Hospital admission data were available for 1517 of the 1536 participants in the ITT population (theophylline, n = 762; placebo, n = 755). A similar proportion of participants had at least one hospital admission for non-COPD-related causes. Among the participants allocated to low-dose theophylline, this proportion was 10.4% (79/762); among those allocated to placebo, it was 12.2% (92/755). In total, there were 116 hospital admissions among participants allocated to theophylline and 119 among those allocated to placebo. The adjusted IRR was 0.99 (95% CI 0.71 to 1.38), suggesting no significant difference in the rate of emergency (unscheduled) hospital admissions between the groups (Table 12).
| Secondary outcome | Trial arm | Adjusted or unadjusted | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Emergency hospital admissions (non-COPD) | ||||||
| Total number included in analysis | 762 | 755 | ||||
| Number with one or more emergency hospital admissions | 79 | 92 | ||||
| Total number of admissions | 116 | 119 | ||||
| Mean admission rate | 0.15 | 0.16 | Unadjusted IRR | 0.96 | 0.69 to 1.35 | 0.830 |
| SD admission rate | 0.56 | 0.47 | Adjusted IRRa | 0.99 | 0.71 to 1.38 | 0.952 |
| All-cause mortality | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number deceased within 12 months | 19 | 14 | Unadjusted HR | 1.35 | 0.68 to 2.69 | 0.398 |
| Percentage deceased within 12 months | 2.5 | 1.8 | Adjusted HRa | 1.38 | 0.69 to 2.76 | 0.369 |
| COPD-/respiratory-related mortality | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number deceased within 12 months | 7 | 8 | Unadjusted HR | 0.87 | 0.31 to 2.39 | 0.785 |
| Percentage deceased within 12 months | 0.9 | 1.0 | Adjusted HRa | 0.85 | 0.30 to 2.40 | 0.762 |
| Pneumonia | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number with pneumonia | 14 | 9 | Unadjusted OR | 1.55 | 0.67 to 3.62 | 0.307 |
| Percentage with pneumonia | 1.8 | 1.2 | ||||
| Total daily dose of ICSs | ||||||
| Total number included in analysis | 770 | 762 | ||||
| Number who changed medication from baseline | 104 | 111 | ||||
| Mean daily dose of ICS at end of follow-up (µg) | 1606 | 1622 | Unadjusted mean difference | –16.3 | –86.8 to 54.2 | 0.650 |
| SD daily dose of ICS at end of follow-up (µg) | 694 | 714 | Adjusted mean differencea | –12.4 | –81.5 to 56.6 | 0.724 |
| Change in daily ICS dose from baseline | ||||||
| Total number included in analysis | 770 | 762 | ||||
| Mean change in daily ICS dose from baseline (µg) | –57 | –58 | Unadjusted mean difference | 1.4 | –36.5 to 39.2 | 0.943 |
| SD change in daily ICS dose from baseline (µg) | 346 | 408 | Adjusted mean differencea | 3.6 | –34.1 to 41.3 | 0.852 |
Mortality (all cause and respiratory related)
There were 33 deaths (from all causes) during the 12-month follow-up period: 19 (2.5%) in the low-dose theophylline group and 14 (1.8%) in the placebo group. Respiratory-related disease accounted for seven deaths in theophylline group and eight deaths in the placebo group. Relative to the placebo group, the adjusted HR for death from all causes in the theophylline group was 1.38 (95% CI 0.69 to 2.76), and for respiratory-related causes it was 0.85 (95% CI 0.30 to 2.40). Therefore, there was no evidence of a significant difference between treatment groups for mortality outcomes (see Table 12).
Total number of episodes of pneumonia
In total, there were 23 episodes of pneumonia reported during the follow-up: 14 in participants allocated to theophylline and nine in participants allocated to placebo (1.8% in the theophylline arm vs. 1.2% in the placebo arm). The proportion of admissions for pneumonia was not found to significantly differ between treatment groups (p = 0.307). The unadjusted odds ratio (OR) was 1.55 (95% CI 0.67 to 3.62); however, in the light of the small event counts, no adjustments were made (see Table 12).
Total dose of inhaled corticosteroids
The total daily dose of ICSs at baseline was available for 1532 of the 1536 members of the ITT population (two missing from each treatment group). The mean total daily beclomethasone-equivalent ICS dose at baseline was 1662 µg (SD 677 µg) in those allocated to theophylline and 1680 µg (SD 691 µg) in those allocated to placebo. During the 12-month follow-up, 215 participants changed their medication: 104 (13.5%) theophylline participants and 111 (14.6%) placebo participants (p = 0.550). The mean total daily beclomethasone-equivalent dose at the end of follow-up was 1606 µg (SD 694 µg) in those allocated to theophylline and 1622 µg (SD 714 µg) in those allocated to placebo, resulting in an adjusted difference of –12.4 µg per day (95% CI –81.4 to 56.6 µg) for theophylline compared with placebo (see Table 12). This lower dose at the end of follow-up in those taking theophylline was not significantly different from the dose for those taking placebo. Both groups showed a slight reduction in total daily dose from baseline to end of follow-up, but a comparison of the adjusted mean dose change between treatment groups was not significant (p = 0.852).
Lung function (% predicted forced expiratory volume in 1 second and forced vital capacity)
In the ITT analysis, lung function was found to be similar between the treatment groups with mean % predicted FEV1 (FEV1%) at the end of the 12-month follow-up of 51.5% (SD 20.4%) for participants allocated to low-dose theophylline (n = 533) and 52.1% (SD 21.7%) for participants allocated to placebo (n = 489). The overall difference in FEV1% predicted (across the 12-month period) was –0.56% (95% CI –2.42% to 1.30%) between the groups. A similar pattern was observed for % predicted FVC, with an overall significant difference of –0.28% (95% CI –2.33% to 1.76%) (Table 13).
| Outcome | Trial arm | Adjusted or unadjusted | Overall mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| % predicted FEV1 | ||||||
| Baseline | ||||||
| Total (n) | 769 | 757 | ||||
| Mean | 51.2 | 52.3 | ||||
| SD | 20.1 | 19.8 | ||||
| 6 months | ||||||
| Total (n) | 553 | 539 | ||||
| Mean | 52.2 | 53.2 | ||||
| SD | 20.5 | 20.9 | ||||
| 12 months | ||||||
| Total (n) | 533 | 489 | ||||
| Mean | 51.5 | 52.1 | Unadjusted | –0.57 | –2.51 to 1.36 | 0.561 |
| SD | 20.4 | 21.7 | Adjusteda | –0.56 | –2.42 to 1.30 | 0.555 |
| % predicted FVC | ||||||
| Baseline | ||||||
| Total (n) | 767 | 756 | ||||
| Mean | 84.3 | 86.3 | ||||
| SD | 22.3 | 23.4 | ||||
| 6 months | ||||||
| Total (n) | 548 | 535 | ||||
| Mean | 83.8 | 84.5 | ||||
| SD | 22.8 | 24.7 | ||||
| 12 months | ||||||
| Total (n) | 525 | 486 | ||||
| Mean | 83.1 | 82.3 | Unadjusted | –0.37 | –2.50 to 1.75 | 0.732 |
| SD | 23.8 | 25.3 | Adjusteda | –0.28 | –2.33 to 1.76 | 0.788 |
Modified Medical Research Council dyspnoea scale
Table 14 details the responses to the mMRC breathlessness scale at baseline and at 6 and 12 months for each treatment group. The proportions of participants in each category are relatively similar in both groups at each time point. The overall adjusted OR (Table 15) from the mixed-effects ordinal logistic regression for theophylline relative to placebo is 1.20 (95% CI 0.88 to 1.63), indicating a slight increase in odds of higher mMRC score in theophylline participants than in the placebo participants, but the increase is not significant.
| mMRC category | Trial arm | |
|---|---|---|
| Theophylline | Placebo | |
| Baseline (N, n, %) | ||
| Not troubled by breathlessness except on strenuous exercise | 767, 35, 4.6 | 757, 50, 6.6 |
| Short of breath when hurrying or walking up a slight hill | 767, 211, 27.5 | 757, 218, 28.8 |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 767, 248, 32.3 | 757, 235, 31.0 |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 767, 219, 28.6 | 757, 201, 26.6 |
| Too breathless to leave house, or breathless when dressing/undressing | 767, 54, 7.0 | 757, 53, 7.0 |
| 6 months (N, n, %) | ||
| Not troubled by breathlessness except on strenuous exercise | 676, 42, 6.2 | 655, 51, 7.8 |
| Short of breath when hurrying or walking up a slight hill | 676, 209, 30.9 | 655, 189, 28.9 |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 676, 197, 29.1 | 655, 179, 27.3 |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 676, 178, 26.3 | 655, 186, 28.4 |
| Too breathless to leave house, or breathless when dressing/undressing | 676, 50, 7.4 | 655, 50, 7.6 |
| 12 months (N, n, %) | ||
| Not troubled by breathlessness except on strenuous exercise | 631, 38, 6.0 | 615, 52, 8.5 |
| Short of breath when hurrying or walking up a slight hill | 631, 186, 29.5 | 615, 158, 25.7 |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 631, 174, 27.6 | 615, 182, 29.6 |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 631, 178, 28.2 | 615, 167, 27.2 |
| Too breathless to leave house, or breathless when dressing/undressing | 631, 55, 8.7 | 615, 56, 9.1 |
| OR | Estimate | 95% CI | p-value |
|---|---|---|---|
| Unadjusted OR | 1.27 | 0.91 to 1.76 | 0.157 |
| Adjusted ORa | 1.20 | 0.88 to 1.63 | 0.244 |
The COPD Assessment Test
The CAT scores were very similar between groups at baseline (Table 16) and remained similar throughout the 12-month treatment period, with a mean score of 21.4 (SD 8.2) for participants allocated to low-dose theophylline (n = 633) and 21.4 (SD 8.6) for participants allocated to placebo (n = 615). A comparison of the profile of the CAT scores across the three time points (i.e. 0, 6 and 12 months) showed an adjusted difference of 0.01 (95% CI –0.65 to 0.68), suggesting no significant difference between the groups of the impact of COPD on the participants’ lives.
| Outcome | Trial arm | Adjusted or unadjusted | Overall mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| CAT score | ||||||
| Baseline | ||||||
| Total (n) | 764 | 756 | ||||
| Mean | 22.7 | 22.3 | ||||
| SD | 7.5 | 7.9 | ||||
| 6 months | ||||||
| Total (n) | 675 | 657 | ||||
| Mean | 21.3 | 21.1 | ||||
| SD | 8.1 | 8.3 | ||||
| 12 months | ||||||
| Total (n) | 633 | 615 | ||||
| Mean | 21.4 | 21.4 | Unadjusted | 0.13 | –0.59 to 0.85 | 0.715 |
| SD | 8.2 | 8.6 | Adjusteda | 0.01 | –0.65 to 0.68 | 0.975 |
| HARQ score | ||||||
| Baseline | ||||||
| Total (n) | 199 | 203 | ||||
| Mean | 24.9 | 25.8 | ||||
| SD | 16.0 | 14.8 | ||||
| 6 months | ||||||
| Total (n) | 191 | 188 | ||||
| Mean | 21.9 | 22.9 | ||||
| SD | 15.1 | 15.7 | ||||
| 12 months | ||||||
| Total (n) | 184 | 172 | ||||
| Mean | 24.1 | 24.2 | Unadjusted | –0.85 | –3.34 to 1.64 | 0.504 |
| SD | 15.7 | 15.9 | Adjusteda | –1.10 | –3.46 to 1.26 | 0.359 |
Hull Airways Reflux Questionnaire
The HARQ assesses respiratory symptoms associated with airway reflux, and was completed by a subset of participants. Participants for whom HARQ data were available were more likely to be female and younger than those who had no HARQ data (see Appendix 5, Table 37). Data were available for 199 (26.0%) participants allocated to theophylline and 203 (26.9%) allocated to placebo at baseline. The HARQ scores were very similar between treatment groups throughout the trial and at the 12-month follow-up; for participants allocated to low-dose theophylline, the mean HARQ score was 24.1 (SD 15.7), based on 184 participants, and for those allocated to placebo, it was 24.2 (SD 15.9), based on 172 participants. A comparison of the profiles of HARQ scores across the three time points (i.e. 0, 6 and 12 months) revealed an adjusted difference of –1.10 (95% CI –3.46 to 1.26), suggesting no significant difference between the groups in reflux-associated respiratory symptoms measured by the HARQ (see Table 15).
Safety outcomes (safety population)
The safety population comprised all participants who were randomised and included in the trial (n = 1567) and initiated their trial medication. Five out of 788 (0.6%) participants allocated theophylline did not initiate medication; 9 out of 779 (1.2%) participants in the placebo group did not initiate placebo. The safety population consisted of 1553 (99.1%) participants (theophylline, n = 783; placebo, n = 770).
Serious adverse events
Overall, 211 (13.6%) participants had at least one SAE, 103 (out of 783, 13.2%) in the low-dose theophylline group and 108 (out of 770, 14.0%) in the placebo group. A total of 276 SAEs were reported in individuals in the safety population. These were balanced between the treatment groups, with 141 in participants allocated to theophylline and 135 in placebo participants. SAEs were classified using System Organ Classes (SOCs). 95 Table 17 details, for each SOC code and for each treatment group, the number of participants experiencing at least one SAE of that code, and the total number of SAEs of that SOC code. No significant differences were observed in the SAE profile of the two treatment groups. The most common SAE SOC code was for ‘cardiac disorders’ [2.8% (2.3% in the theophylline arm and 3.4% in the placebo arm)]. SAEs with a coding of ‘respiratory, thoracic and mediastinal’ occurred in 2.5% of participants (2.3% in the theophylline arm and 2.7% in the placebo arm). A borderline significantly higher proportion of participants in the theophylline group (2.7%) reported a gastrointestinal SAE than in the placebo group (1.3%) (p = 0.051). No pregnancies were reported. Line listings are provided in Appendix 6.
| SAE | Trial arm | p-value | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Total number included in analysis | 783 | 770 | |
| All SAEs | |||
| Number of participants with at least one SAE | 103 | 108 | |
| Percentage of participants with at least one SAE | 13.2 | 14.0 | 0.616 |
| Total number of SAEs | 141 | 135 | |
| Infection and infestations | |||
| Number of participants with at least one SAE of this type | 13 | 9 | |
| Percentage of participants with at least one SAE of this type | 1.7 | 1.2 | 0.413 |
| Total number of SAEs of this type | 13 | 9 | |
| Neoplasms: benign, malignant and unspecified | |||
| Number of participants with at least one SAE of this type | 17 | 11 | |
| Percentage of participants with at least one SAE of this type | 2.2 | 1.4 | 0.272 |
| Total number of SAEs of this type | 18 | 11 | |
| Blood and lymphatic system disorders | |||
| Number of participants with at least one SAE of this type | 0 | 2 | |
| Percentage of participants with at least one SAE of this type | 0 | 0.3 | |
| Total number of SAEs of this type | 0 | 2 | |
| Immune system disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Endocrine disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Metabolism and nutrition disorders | |||
| Number of participants with at least one SAE of this type | 1 | 0 | |
| Percentage of participants with at least one SAE of this type | 0.1 | 0 | |
| Total number of SAEs of this type | 2 | 0 | |
| Nervous system disorders | |||
| Number of participants with at least one SAE of this type | 11 | 7 | |
| Percentage of participants with at least one SAE of this type | 1.4 | 0.9 | 0.361 |
| Total number of SAEs of this type | 13 | 7 | |
| Psychiatric disorders | |||
| Number of participants with at least one SAE of this type | 1 | 2 | |
| Percentage of participants with at least one SAE of this type | 0.1 | 0.3 | |
| Total number of SAEs of this type | 1 | 3 | |
| Eye disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Ear and labyrinth disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Cardiac disorders | |||
| Number of participants with at least one SAE of this type | 18 | 26 | |
| Percentage of participants with at least one SAE of this type | 2.3 | 3.4 | 0.201 |
| Total number of SAEs of this type | 21 | 29 | |
| Vascular disorders | |||
| Number of participants with at least one SAE of this type | 5 | 6 | |
| Percentage of participants with at least one SAE of this type | 0.6 | 0.8 | |
| Total number of SAEs of this type | 6 | 6 | |
| Respiratory, thoracic and mediastinal disorders | |||
| Number of participants with at least one SAE of this type | 18 | 21 | |
| Percentage of participants with at least one SAE of this type | 2.3 | 2.7 | 0.590 |
| Total number of SAEs of this type | 19 | 22 | |
| Hepatobiliary disorders | |||
| Number of participants with at least one SAE of this type | 2 | 4 | |
| Percentage of participants with at least one SAE of this type | 0.3 | 0.5 | |
| Total number of SAEs of this type | 2 | 4 | |
| Gastrointestinal disorders | |||
| Number of participants with at least one SAE of this type | 21 | 10 | |
| Percentage of participants with at least one SAE of this type | 2.7 | 1.3 | 0.051 |
| Total number of SAEs of this type | 22 | 12 | |
| Skin and subcutaneous tissue disorders | |||
| Number of participants with at least one SAE of this type | 1 | 0 | |
| Percentage of participants with at least one SAE of this type | 0.1 | 0 | |
| Total number of SAEs of this type | 1 | 0 | |
| Musculoskeletal and connective tissue disorders | |||
| Number of participants with at least one SAE of this type | 5 | 9 | |
| Percentage of participants with at least one SAE of this type | 0.6 | 1.2 | |
| Total number of SAEs of this type | 5 | 11 | |
| Renal and urinary disorders | |||
| Number of participants with at least one SAE of this type | 6 | 4 | |
| Percentage of participants with at least one SAE of this type | 0.8 | 0.5 | |
| Total number of SAEs of this type | 6 | 4 | |
| Pregnancy, puerperium and perinatal conditions | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Reproductive system and breast disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Congenital, familial and genetic disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| General disorders and administration site disorders | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
| Investigations | |||
| Number of participants with at least one SAE of this type | 0 | 2 | |
| Percentage of participants with at least one SAE of this type | 0 | 0.3 | |
| Total number of SAEs of this type | 0 | 2 | |
| Injury, poisoning and procedural complications | |||
| Number of participants with at least one SAE of this type | 9 | 13 | |
| Percentage of participants with at least one SAE of this type | 1.1 | 1.7 | 0.369 |
| Total number of SAEs of this type | 11 | 13 | |
| Surgical and medical procedures | |||
| Number of participants with at least one SAE of this type | 1 | 0 | |
| Percentage of participants with at least one SAE of this type | 0.1 | 0 | |
| Total number of SAEs of this type | 1 | 0 | |
| Social circumstances | |||
| Number of participants with at least one SAE of this type | 0 | 0 | |
| Percentage of participants with at least one SAE of this type | 0 | 0 | |
| Total number of SAEs of this type | 0 | 0 | |
Adverse reactions
Information on adverse reactions was available for 1408 participants (theophylline, n = 709; placebo, n = 699), with 648 (46%) suffering at least one AR (theophylline, n = 341; placebo; n = 307). There were 1701 ARs in total: 883 in those allocated to low-dose theophylline and 818 in those allocated placebo. Table 18 presents these ARs in more detail, with total number available for analysis for each AR, number of participants with at least one AR of that type and the percentage in each group. The five most common ARs were nausea (10.9% in the theophylline arm and 8.0% in the placebo arm; p = 0.059), insomnia (9.3% in the theophylline arm and 8.9% in the placebo arm; p = 0.790), dizziness (8.1% in the theophylline arm and 9.6% in the placebo arm; p = 0.290), gastro-oesophageal reflux (9.4% in the theophylline arm and 7.5% in the placebo arm; p = 0.217) and headache (9.0% in the theophylline arm and 7.7% in the placebo arm; p = 0.383). In addition, the proportion reporting tachycardia was slightly higher in the placebo group (3.5%) than in the theophylline group (1.9%) (p = 0.058). There were no other observed significant differences in ARs between treatment groups.
| AR | Trial arm | p-value | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Any AR | |||
| Number included in analysis | 709 | 699 | |
| Number with at least one AR | 341 | 307 | |
| Percentage with at least one AR | 48.1 | 43.9 | 0.116 |
| Total number of ARs | 883 | 818 | |
| Anaphylactic/anaphylactoid reaction | |||
| Number included in analysis | 692 | 679 | |
| Number with at least one AR of this type | 0 | 1 | |
| Percentage with at least one AR of this type | 0.0 | 0.1 | |
| Hypersensitivity | |||
| Number included in analysis | 692 | 679 | |
| Number with at least one AR of this type | 5 | 5 | |
| Percentage with at least one AR of this type | 0.7 | 0.7 | > 0.999 |
| Nausea | |||
| Number included in analysis | 695 | 679 | |
| Number with at least one AR of this type | 76 | 54 | |
| Percentage with at least one AR of this type | 10.9 | 8.0 | 0.059 |
| Reflux | |||
| Number included in analysis | 693 | 678 | |
| Number with at least one AR of this type | 65 | 51 | |
| Percentage with at least one AR of this type | 9.4 | 7.5 | 0.217 |
| Diarrhoea | |||
| Number included in analysis | 693 | 680 | |
| Number with at least one AR of this type | 53 | 46 | |
| Percentage with at least one AR of this type | 7.6 | 6.8 | 0.527 |
| Abdominal pain | |||
| Number included in analysis | 692 | 679 | |
| Number with at least one AR of this type | 42 | 34 | |
| Percentage with at least one AR of this type | 6.1 | 5.0 | 0.390 |
| Gastric irritation | |||
| Number included in analysis | 691 | 679 | |
| Number with at least one AR of this type | 38 | 28 | |
| Percentage with at least one AR of this type | 5.5 | 4.1 | 0.235 |
| Vomiting | |||
| Number included in analysis | 693 | 678 | |
| Number with at least one AR of this type | 28 | 22 | |
| Percentage with at least one AR of this type | 4.0 | 3.2 | 0.432 |
| Palpitations | |||
| Number included in analysis | 690 | 678 | |
| Number with at least one AR of this type | 29 | 26 | |
| Percentage with at least one AR of this type | 4.2 | 3.8 | 0.729 |
| Tachycardia | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 13 | 24 | |
| Percentage with at least one AR of this type | 1.9 | 3.5 | 0.058 |
| Insomnia | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 64 | 60 | |
| Percentage with at least one AR of this type | 9.3 | 8.9 | 0.790 |
| Anxiety | |||
| Number included in analysis | 691 | 679 | |
| Number with at least one AR of this type | 52 | 42 | |
| Percentage with at least one AR of this type | 7.5 | 6.2 | 0.327 |
| Rash | |||
| Number included in analysis | 691 | 679 | |
| Number with at least one AR of this type | 35 | 27 | |
| Percentage with at least one AR of this type | 5.1 | 4.0 | 0.332 |
| Pruritus | |||
| Number included in analysis | 692 | 679 | |
| Number with at least one AR of this type | 51 | 63 | |
| Percentage with at least one AR of this type | 7.4 | 9.3 | 0.201 |
| Tremor | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 34 | 38 | |
| Percentage with at least one AR of this type | 4.9 | 5.6 | 0.571 |
| Headache | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 62 | 52 | |
| Percentage with at least one AR of this type | 9.0 | 7.7 | 0.383 |
| Dizziness | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 56 | 66 | |
| Percentage with at least one AR of this type | 8.1 | 9.7 | 0.290 |
| Agitation | |||
| Number included in analysis | 691 | 679 | |
| Number with at least one AR of this type | 22 | 18 | |
| Percentage with at least one AR of this type | 3.2 | 2.6 | 0.558 |
| Convulsions | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 2 | 4 | |
| Percentage with at least one AR of this type | 0.3 | 0.6 | 0.448 |
| Hyperuricemia | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 9 | 7 | |
| Percentage with at least one AR of this type | 1.3 | 1.0 | 0.803 |
| Diuresis | |||
| Number included in analysis | 691 | 678 | |
| Number with at least one AR of this type | 49 | 48 | |
| Percentage with at least one AR of this type | 7.1 | 7.1 | 0.993 |
| Urinary retention | |||
| Number included in analysis | 691 | 677 | |
| Number with at least one AR of this type | 16 | 15 | |
| Percentage with at least one AR of this type | 2.3 | 2.2 | 0.901 |
| Other | |||
| Number included in analysis | 691 | 677 | |
| Number with at least one AR of this type | 82 | 86 | |
| Percentage with at least one AR of this type | 11.9 | 12.7 | 0.638 |
Subgroup analysis (intention to treat)
Appendix 5, Table 40 details the results of the subgroup analysis for the prespecified subgroups. Given the exploratory nature of the analyses, we present 99% CIs. Figure 5 displays this information, alongside the p-values for the interaction in the adjusted model. There was no evidence at the 1% level of statistical significance that any effect of low-dose theophylline differed between subgroups of age, sex, smoking status, BMI, COPD treatments, exacerbation history, COPD severity, baseline ICS dose or use of maintenance OCSs.
FIGURE 5.
Forest plot of estimates from the subgroups. Vertical dashed line represents no effect (IRR = 1), vertical solid line indicates the overall treatment effect for exacerbation (IRR = 0.992). a, Upper limit of CI truncated to 1.5, actual value is 4.09; b, upper limit of CI truncated to 1.5, actual limit is 2.20.

Treatment adherence/compliance
Adherence/compliance was defined as having taken ≥ 70% of expected doses of trial tablets. In the ITT population (n = 1536), 1180 (76.8%) participants fulfilled the definition of adherent/compliant (and made up the per-protocol population). In the theophylline-allocated group, 181 out of 772 (23.4%) participants were classed as non-adherent/non-compliant; three of these never initiated treatment, 171 were non-persistent with (i.e. ceased) trial medication and seven persisted with trial medication but from returned medication it was evident that they were non-adherent/non-compliant (Table 19). In addition, 32 out of 591 low-dose theophylline participants fulfilled the adherent/compliant definition despite not persisting with the trial medication, usually very late in the treatment period (Table 20). In the placebo group, 175 out of 764 (22.9%) participants were classed as non-adherent/non-compliant; six never initiated medication, 159 were non-persistent with trial medication and 10 persisted with trial medication but medication returns demonstrated poor implementation (see Table 19). A further 34 participants were non-persistent with medication but fulfilled the definition of adherent/compliant because they ceased trial medication late into the treatment period (see Table 20). In summary, the per-protocol population comprises 1180 participants (591 from the theophylline arm and 589 from the placebo arm) and there were 1146 person-years of follow-up data (see Table 19). A comparison of the proportion who were non-adherent/non-compliant (23.4% in the theophylline arm vs. 22.9% in the placebo arm) was not significant (p = 0.802). In total, 203 out of 772 participants in the theophylline arm were non-persistent with medication, compared with 193 out of 764 in the placebo arm (unadjusted IRR 1.05, 95% CI 0.84 to 1.32).
| Compliance | Trial arm (n) | |
|---|---|---|
| Theophylline | Placebo | |
| Total | 772 | 764 |
| Not adherent/compliant (took < 70% of doses)a | 181 | 175 |
| Did not start medication (non-initiation) | 3 | 6 |
| Actively ceased medication (non-persistence) | 171 | 159 |
| Did not cease (persistent), but adherence/compliance was < 70% | 7 | 10 |
| Compliant (≥ 70%) | 591 | 589 |
| Stopping medication | Trial arm (n) | |
|---|---|---|
| Theophylline | Placebo | |
| Total | 772 | 764 |
| Did not start medication (non-initiation) | 3 | 6 |
| Actively ceased medication (non-persistent) | 171 | 159 |
| Adherent/compliant but ceased medication (non-persistent) | 32 | 34 |
| Total ceasing medication (who started) (non-persistent)a | 203 | 193 |
| Reason for stopping medication | ||
| Infections: and infestations | 2 | 1 |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 7 | 2 |
| Psychiatric disorders | 2 | 4 |
| Nervous system disorders | 19 | 15 |
| Ear and labyrinth disorders | 3 | 3 |
| Cardiac disorders | 7 | 6 |
| Vascular disorders | 1 | 1 |
| Respiratory, thoracic and mediastinal disorders | 10 | 19 |
| Gastrointestinal disorders | 46 | 32 |
| Hepatobiliary disorders | 0 | 1 |
| Skin and subcutaneous tissue disorders | 9 | 7 |
| Musculoskeletal and connective tissue disorders | 4 | 8 |
| Renal and urinary disorders | 5 | 1 |
| Injury, poisoning and procedural complications | 1 | 1 |
| Surgical and medical procedures | 19 | 21 |
| Social circumstances | 15 | 14 |
| Participant felt no benefit | 25 | 21 |
| No reason given | 28 | 36 |
Reasons for stopping medication
Table 20 presents the reasons for stopping medication among the ITT population by SOC code. The most common reason for stopping medication was gastrointestinal disorders (theophylline, n = 46; placebo, n = 32), followed by surgical and medical procedures (theophylline, n = 19; placebo, n = 21). Although the surgical and medical procedures group included some participants who had discontinued ICSs containing inhalers, the majority of this group comprised participants advised to discontinue the trial drug by a clinician after presenting with a wide range of illnesses. In total, 46 participants discontinued the trial medication because they felt no benefit (theophylline, n = 25; placebo, n = 21), and in 64 cases no reason was given (theophylline, n = 28; placebo, n = 36), with a further 29 ceasing for social circumstances (theophylline, n = 15; placebo, n = 14). There were no obvious differences between the two treatment groups in the reasons why trial medication was discontinued, but no formal statistical testing was undertaken.
Per-protocol analysis
The per-protocol population comprised the 1180 participants of the ITT population who met the trial definition of adherence with the trial medication. The per-protocol analysis comprised 591 participants allocated to low-dose theophylline and 589 allocated to placebo (Figure 6).
FIGURE 6.
The CONSORT flow diagram for the per-protocol analysis.
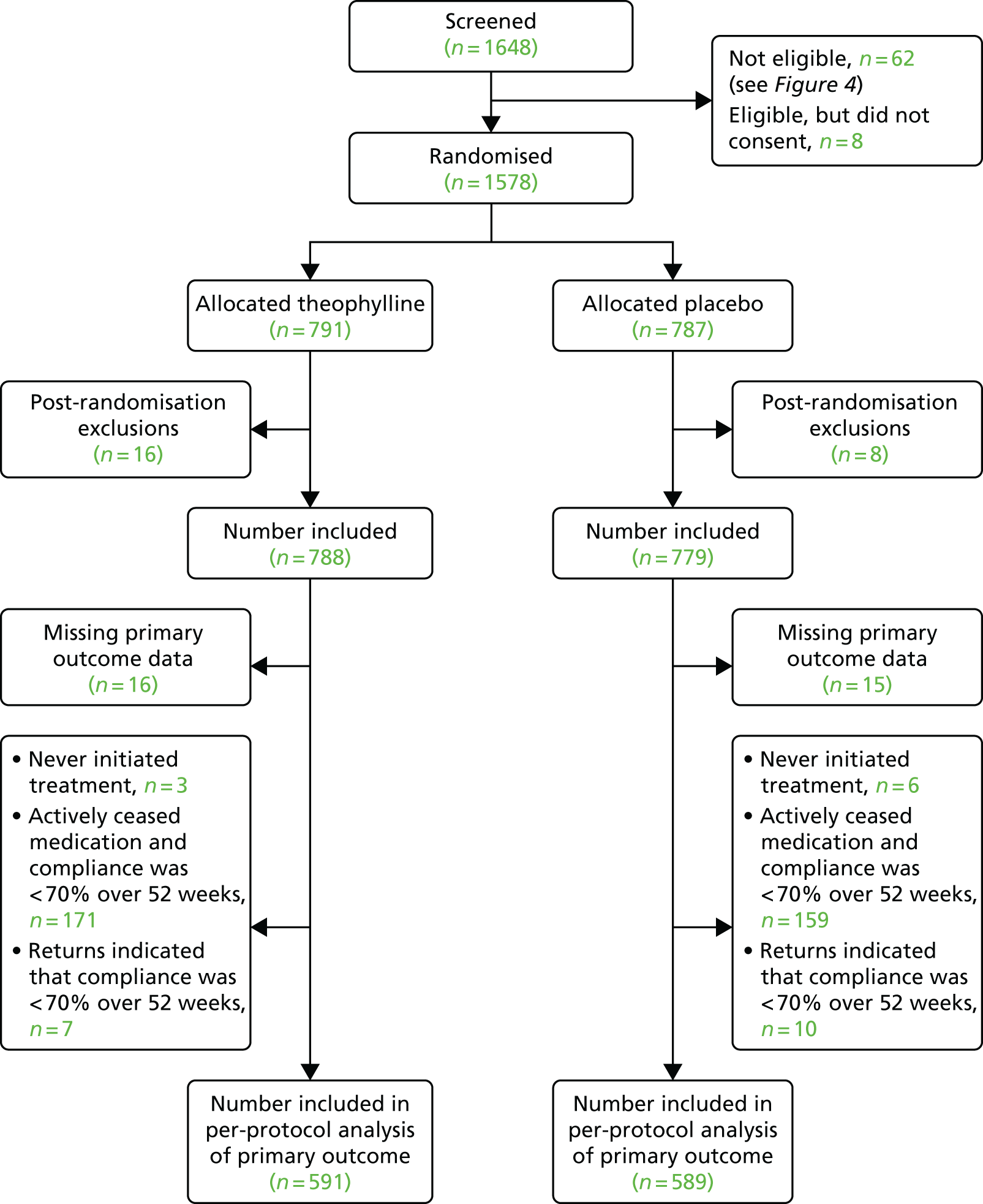
Primary outcome: total number of exacerbations of chronic obstructive pulmonary disease necessitating a change in management
In the per-protocol population, 591 theophylline-allocated participants had a mean of 2.20 (SD 1.96) exacerbations, compared with a mean of 2.14 (SD 1.92) exacerbations among the 589 placebo participants. There were 1298 exacerbations in the theophylline group and 1258 in placebo group. The adjusted IRR for COPD exacerbation was 1.00 (95% CI 0.91 to 1.10), indicating no difference in the exacerbation rate during the 12-month follow-up period for those on low-dose theophylline compared with those receiving placebo who were adherent/compliant with the trial medication (Table 21).
| Exacerbations | Trial arm | Adjusted or unadjusted | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Primary outcome: exacerbations | ||||||
| Total number included in analysis | 591 | 589 | ||||
| Person-years of follow-up | 572.8 | 573.8 | ||||
| Number with at least one exacerbation | 481 | 465 | ||||
| Total number of exacerbations | 1298 | 1258 | ||||
| Mean number of exacerbations | 2.20 | 2.14 | Unadjusted IRR | 1.02 | 0.92 to 1.13 | 0.664 |
| SD (number of exacerbations) | 1.96 | 1.92 | Adjusted IRRa | 1.00 | 0.91 to 1.10 | 0.934 |
| Exacerbations requiring hospital admission | ||||||
| Total number included in analysis | 591 | 589 | ||||
| Number with at least one exacerbation | 76 | 88 | ||||
| Total number of exacerbations | 92 | 126 | ||||
| Mean number of exacerbations | 0.16 | 0.21 | Unadjusted IRR | 0.74 | 0.53 to 1.03 | 0.072 |
| SD (number of exacerbations) | 0.45 | 0.61 | Adjusted IRRa | 0.70 | 0.50 to 0.97 | 0.031 |
| Time to first exacerbation (from randomisation) | ||||||
| Total number included in analysisb | 578 | 583 | ||||
| Number with at least one exacerbation | 468 | 459 | ||||
| Percentage with at least one exacerbation | 81.0 | 78.7 | ||||
| Median time to first exacerbation (days) | 221 | 232 | Unadjusted HR | 1.04 | 0.91 to 1.18 | 0.576 |
| 25th percentile [time to first exacerbation (days)] | 132 | 126 | Adjusted HRa | 1.02 | 0.90 to 1.16 | 0.733 |
| 75th percentile [time to first exacerbation (days)] | 341 | 339 | ||||
Secondary outcomes
Total number of exacerbations of chronic obstructive pulmonary disease resulting in hospital admission
In the per-protocol population, 76 out of 591 (13%) participants allocated to theophylline had at least one COPD exacerbation necessitating hospital admission; there were 92 admissions in the group overall. Among participants allocated to placebo, 88 out of 589 (15%) had at least one admission and there were 126 admissions overall. The mean number of COPD exacerbations necessitating hospital admission was 0.16 (SD 0.45) among the 591 compliant participants in the theophylline group and 0.21 (SD 0.61) among the 589 compliant participants in the placebo group. In the adjusted model, the IRR for COPD exacerbations necessitating hospital admission was 0.70 (95% CI 0.50 to 0.97), suggesting a significant reduction in the number of exacerbations necessitating hospital admission in the low-dose theophylline group compared with the placebo group (see Table 21).
Time to first exacerbation
The information for the time to first exacerbation was missing for 19 of the 1180 per-protocol participants; therefore, this analysis was based on 1161 participants. Among participants allocated to theophylline, 468 out of 578 (81.0%) had at least one exacerbation, with a median time to first exacerbation of 221 days (7.3 months) after randomisation. In the placebo group, 459 out of 583 (78.7%) participants had at least one exacerbation, with a median time to first exacerbation of 232 days (7.7 months). In a Cox regression analysis, the adjusted HR for time to first exacerbation was 1.02 (95% CI 0.90 to 1.16), suggesting no difference between the treatment groups in terms of time to first exacerbation (from point of randomisation) during the 12-month follow-up period (see Table 21).
Total number of emergency hospital admissions (non-chronic obstructive pulmonary disease related)
Hospital admission data were available for 1176 out of 1180 participants in the per-protocol population. Overall, 111 participants had at least one admission (theophylline, n = 45; placebo, n = 66), with 66 admissions in the theophylline group and 85 admissions in the placebo group. The adjusted IRR for admission was 0.82 (95% CI 0.54 to 1.24), suggesting no significant difference in the rate of non-COPD-related emergency hospital admissions between participants compliant with low-dose theophylline and those compliant with placebo (Table 22).
| Non-COPD-related hospital admissions | Trial arm | Adjusted or unadjusted | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Emergency hospital admissions (non-COPD related) | ||||||
| Total number included in analysis | 587 | 589 | ||||
| Number with at least one emergency hospital admission | 45 | 66 | ||||
| Total admissions | 66 | 85 | ||||
| Mean admission rate | 0.11 | 0.14 | Unadjusted IRR | 0.77 | 0.51 to 1.17 | 0.220 |
| SD admission rate | 0.49 | 0.45 | Adjusted IRRa | 0.82 | 0.54 to 1.24 | 0.351 |
| All-cause mortality | ||||||
| Total number included in analysis | 591 | 589 | ||||
| Number deceased within 12 months | 13 | 9 | Unadjusted HR | 1.45 | 0.62 to 3.38 | 0.394 |
| % deceased within 12 months | 2.2 | 1.5 | ||||
| Respiratory related mortality | ||||||
| Total number included in analysis | 591 | 589 | ||||
| Number deceased within 12 months | 5 | 5 | Unadjusted HR | 1.00 | 0.29 to 3.46 | 0.998 |
| Percentage deceased within 12 months | 0.9 | 0.9 | ||||
| Pneumonia | ||||||
| Total number included in analysis | 591 | 589 | ||||
| Number with pneumonia | 9 | 5 | Unadjusted OR | 1.81 | 0.60 to 5.44 | 0.291 |
| Percentage with pneumonia | 1.5 | 0.9 | ||||
| Total daily dose ICS | ||||||
| Total number included in analysis | 589 | 588 | ||||
| Number changed medication from baseline | 78 | 93 | ||||
| Mean ICS daily dose at end of follow-up (µg) | 1617 | 1605 | Unadjusted mean difference | 12.2 | –67.6 to 92.1 | 0.764 |
| SD (ICS daily dose at end of follow-up) (µg) | 693 | 704 | Adjusted mean differencea | 12.5 | –65.9 to 90.9 | 0.754 |
| Change in daily ICS dose from baseline | ||||||
| Total number included in analysis | 589 | 588 | ||||
| Mean change in daily ICS dose from baseline (µg) | –62 | –60 | Unadjusted mean difference | –1.60 | –45.4 to 42.3 | 0.943 |
| SD (change in daily ICS dose from baseline) (µg) | 347 | 417 | Adjusted mean differencea | –0.58 | –44.3 to 43.1 | 0.979 |
Mortality (all cause and respiratory related)
There were 22 deaths (from all causes) during the 12-month follow-up period in the per-protocol population: 13 (2.2%) in participants taking theophylline and nine (1.5%) in participants taking placebo. These deaths were respiratory related in five cases in each of the theophylline and placebo groups. The unadjusted HR for deaths from all causes was 1.45 (95% CI 0.62 to 3.38); for deaths from respiratory-related causes, the HR was 1.00 (95% CI 0.29 to 3.46) for theophylline relative to placebo (see Table 22). Therefore, there was no evidence of a significant difference between treatment groups for mortality outcomes in the per-protocol population. No adjustments were made because of small event counts.
Total number of episodes of pneumonia
There were 14 episodes of pneumonia: nine (out of 591, 1.5%) among low-dose theophylline-adherent/compliant participants and five (out of 589, 0.9%) among placebo-compliant participants. The unadjusted IRR was 1.81 (95% CI 0.60 to 5.44) and no adjustments were made because of small event counts (see Table 22).
Total dose of inhaled corticosteroids
The total daily dose of ICS at baseline was available for 1176 of the 1180 members of the per-protocol population. During the 12-month follow-up, 171 participants changed their medication: 78 (13.2%) theophylline participants and 93 (15.8%) placebo participants (p = 0.210). The mean total daily beclomethasone-equivalent dose at the end of follow-up was 1617 µg (SD 693 µg) in those allocated to theophylline and 1605 µg (SD 704 µg) in those allocated to placebo, resulting in an adjusted daily beclomethasone-equivalent difference of 12.5 µg (95% CI –65.9 to 90.9 µg) between theophylline and placebo (see Table 22); this difference was not significantly different. Both groups showed a slight reduction in total daily dose from baseline to end of follow-up, but a comparison of the adjusted mean dose change between treatment groups revealed that the difference was not significant (p = 0.979).
Lung function (% predicted forced expiratory volume in 1 second and forced vital capacity)
In the per-protocol analysis, lung function profile was similar in both treatment groups. The mean % predicted FEV1 at the end of the 12-month follow-up period was slightly lower in the compliant/adherent theophylline group than in the placebo group [51.3% (SD 20.3%) (n = 455) vs. 52.6% (SD 21.8%) (n = 432)], giving an overall difference between the groups of –1.33% (95% CI –3.47% to 0.80%) (Table 23). A similar pattern was observed for % predicted FVC, with an overall difference of –0.65% (95% CI –2.96% to 1.67%). This was a larger reduction than that observed in the ITT analysis, but remained non-significant.
| Outcome | Trial arm | Adjusted or unadjusted | Overall mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| FEV1% predicted | ||||||
| Baseline | ||||||
| Total (n) | 588 | 583 | ||||
| Mean | 50.7 | 52.8 | ||||
| SD | 20.5 | 20.0 | ||||
| 6 months | ||||||
| Total (n) | 471 | 471 | ||||
| Mean | 52.0 | 53.7 | ||||
| SD | 20.8 | 20.8 | ||||
| 12 months | ||||||
| Total (n) | 455 | 432 | ||||
| Mean | 51.3 | 52.6 | Unadjusted | –1.41 | –3.65 to 0.82 | 0.215 |
| SD | 20.3 | 21.8 | Adjusteda | –1.33 | –3.47 to 0.80 | 0.221 |
| FVC% predicted | ||||||
| Baseline | ||||||
| Total (n) | 586 | 582 | ||||
| Mean | 84.2 | 86.6 | ||||
| SD | 22.9 | 23.5 | ||||
| 6 months | ||||||
| Total (n) | 467 | 467 | ||||
| Mean | 84.3 | 84.6 | ||||
| SD | 23.0 | 24.3 | ||||
| 12 months | ||||||
| Total (n) | 449 | 431 | ||||
| Mean | 83.3 | 82.6 | Unadjusted | –0.84 | –3.25 to 1.56 | 0.492 |
| SD | 23.2 | 25.3 | Adjusteda | –0.65 | –2.96 to 1.67 | 0.584 |
Modified Medical Research Council dyspnoea scale
Tables 24 and 25 detail the responses to the mMRC dyspnoea scale at baseline, 6 months and 12 months for each treatment group in the per-protocol population. In the unadjusted model, the OR for higher mMRC dyspnoea score in theophylline participants compared with placebo participants is 1.54 (95% CI 1.05 to 2.26); the adjusted OR is 1.39 (95% CI 0.97 to 1.98).
| Time point | mMRC category75 | Trial arm (N, n %) | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Baseline | Not troubled by breathlessness except on strenuous exercise | 586, 26, 4.4 | 584, 44, 7.5 |
| Short of breath when hurrying or walking up a slight hill | 586, 160, 27.3 | 584, 176, 30.1 | |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 586, 198, 33.8 | 584, 181, 31.0 | |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 586, 157, 26.8 | 584, 149, 25.5 | |
| Too breathless to leave house, or breathless when dressing/undressing | 586, 45, 7.7 | 584, 34, 5.8 | |
| 6 months | Not troubled by breathlessness except on strenuous exercise | 560, 34, 6.1 | 552, 46, 8.3 |
| Short of breath when hurrying or walking up a slight hill | 560, 182, 32.5 | 552, 160, 29.0 | |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 560, 161, 28.8 | 552, 155, 28.1 | |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 560, 142, 25.4 | 552, 153, 27.7 | |
| Too breathless to leave house, or breathless when dressing/undressing | 560, 41, 7.3 | 552, 38, 6.9 | |
| 12 months | Not troubled by breathlessness except on strenuous exercise | 535, 32, 6.0 | 527, 47, 8.9 |
| Short of breath when hurrying or walking up a slight hill | 535, 167, 31.2 | 527, 149, 28.3 | |
| Walks slower than contemporaries on level ground or has to stop for breath when walking at own pace | 535, 146, 27.3 | 527, 153, 29.0 | |
| Stops for breath after walking about 100 m or after a few minutes on level ground | 535, 147, 27.5 | 527, 135, 25.6 | |
| Too breathless to leave house, or breathless when dressing/undressing | 535, 43, 8.0 | 527, 43, 8.2 | |
| OR | Estimate | 95% CI | p-value |
|---|---|---|---|
| Unadjusted | 1.54 | 1.05 to 2.26 | 0.028 |
| Adjusteda | 1.39 | 0.97 to 1.98 | 0.074 |
The COPD Assessment Test
The CAT scores were very similar between treatment groups at baseline (Table 26) and remained similar through to 12 months, with a mean score of 21.0 (SD 8.2) for theophylline-adherent/compliant participants (n = 534) and 20.9 (SD 8.7) for placebo participants (n = 527) in the per-protocol population. A comparison of the profile of the CAT scores across the three time points (0, 6 and 12 months) showed an adjusted difference of 0.29 (95% CI –0.45 to 1.04), suggesting that the impact of COPD on participants’ lives did not significantly different between the groups of the per-protocol population.
| Time point | Trial arm | Adjusted or unadjusted | Overall mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| CAT score | ||||||
| Baseline | ||||||
| Total (n) | 584 | 583 | ||||
| Mean | 22.7 | 21.8 | ||||
| SD | 7.5 | 7.9 | ||||
| 6 months | ||||||
| Total (n) | 560 | 555 | ||||
| Mean | 21.0 | 20.5 | ||||
| SD | 8.2 | 8.2 | ||||
| 12 months | ||||||
| Total (n) | 534 | 527 | ||||
| Mean | 21.0 | 20.9 | Unadjusted | 0.52 | –0.29 to 1.33 | 0.212 |
| SD | 8.2 | 8.7 | Adjusteda | 0.29 | –0.45 to 1.04 | 0.444 |
| HARQ score | ||||||
| Baseline | ||||||
| Total (n) | 153 | 152 | ||||
| Mean | 25.2 | 26.8 | ||||
| SD | 15.9 | 14.7 | ||||
| 6 months | ||||||
| Total (n) | 160 | 151 | ||||
| Mean | 21.2 | 22.5 | ||||
| SD | 14.8 | 15.6 | ||||
| 12 months | ||||||
| Total (n) | 153 | 141 | ||||
| Mean | 22.9 | 24.4 | Unadjusted | –1.39 | –4.17 to 1.40 | 0.329 |
| SD | 15.6 | 15.8 | Adjusteda | –1.62 | –4.25 to 1.01 | 0.227 |
Hull Airway Reflux Questionnaire
At 12 months, the mean HARQ score was 23.0 (SD 15.6) among 153 theophylline-adherent/compliant participants and 24.4 (SD 15.8) among 141 placebo-adherent/compliant participants. A comparison of the profile of the HARQ scores across the three time points (0, 6 and 12 months) showed an adjusted difference of –1.62 (95% CI –4.25 to 1.01), suggesting no significant difference between the per-protocol treatment groups in reflux-associated respiratory symptoms measured by the HARQ (see Table 26).
Sensitivity analysis
We undertook a sensitivity analysis for the primary outcome and a number of secondary outcomes that excluded the 33 participants who died during the 12-month follow-up period. This left 1503 participants in the ITT population (753 theophylline and 750 placebo). Appendix 5, Tables 41 and 42, give the details of these analyses.
Primary outcome
After excluding participants who died, the adjusted IRR for COPD exacerbations was 0.99 (95% CI 0.91 to 1.07) (see Appendix 5, Table 41), indicating that restricting the result to those who were alive for the full 12-month follow-up did not change the result of the original ITT analysis (IRR 0.99, 95% CI 0.91 to 1.08).
Secondary outcomes
Hospital admissions
Excluding the 33 deaths from the analysis of COPD exacerbations necessitating hospital admission, the adjusted IRR was 0.73 (95% CI 0.55 to 0.97) in the remaining 1503 members of the ITT population, which is very similar to the treatment estimate observed for all 1536 members of the ITT population (IRR 0.72, 95% CI 0.55 to 0.94). Data on admission to hospital for non-COPD reasons were available for 1485 people, after excluding those who died. The adjusted IRR for admission for theophylline relative to placebo was 1.03 (95% CI 0.73 to 1.43), compared with 0.99 (95% CI 0.71 to 1.38) in the full ITT population.
Other
Excluding the 33 deaths made very little difference to the estimates of treatment effect on lung function (FEV1 or FVC) or to the patient-reported outcomes of CAT and HARQ scores (see Appendix 5, Table 42). For FEV1, the adjusted difference was –0.58% (95% CI –2.46% to 1.29%) compared with –0.56% (95% CI –2.42% to 1.30%). For FVC, the adjusted difference was –0.37% (95% CI –2.43% to 1.69%) compared with –0.28% (95% CI –2.33% to 1.76%) for the ITT population. For the CAT score, the treatment difference was 0.02 (95% CI –0.65 to 0.69) compared with 0.01 (95% CI –0.65 to 0.69) in the original ITT population. The HARQ analysis gave an adjusted difference of –0.89 (95% CI –3.27 to 1.50) compared with –1.10 (95% CI –3.46 to 1.26) of the original ITT population. In summary, excluding the 33 deaths made little or no difference to the estimates of treatment effect in the ITT population.
Summary
There was no evidence that, overall, low-dose theophylline significantly reduced the number of COPD exacerbations requiring treatment compared with placebo. There was some evidence that low-dose theophylline reduced exacerbations that necessitated hospital admission, with most benefit being evident in a small [1% (13/1556)] subgroup of patients frequently hospitalised with COPD. The total number of emergency hospital admissions (non-COPD related) did not significantly differ between groups, and nor did total episodes of pneumonia or mortality. Lung function was similar across the 12-month follow-up in the two groups. The impact of the disease on patients was measured by the CAT, mMRC dyspnoea scale and HARQ; no significant differences were found. The safety profile of low-dose theophylline was similar to placebo. There was no evidence that the treatment effect differed in any of the prespecified subgroups.
Chapter 5 Cost-effectiveness
This chapter reports the health economics results from the trial. The objective of the health economics study was to determine the cost-effectiveness of adding low-dose theophylline to ICS therapy over a 12-month period. Mean resource use per participant is presented, along with levels of missing data and mean unadjusted and adjusted costs.
Baseline resource use and costs
Baseline resource use and costs are presented in Table 27.
| Resource use | Trial arm, mean (SD) | |
|---|---|---|
| Theophylline | Placebo | |
| Resource use | ||
| Exacerbations | ||
| Exacerbations requiring treatment in the previous 12 months | 3.63 (2.22); n = 772 | 3.52 (2.08); n = 764 |
| Exacerbations resulting in hospitalisation in the previous 12 months | 0.404 (0.840); n = 768 | 0.358 (0.918); n = 758 |
| Non-exacerbation resource use (mean number of uses per participant in the 6 months prior to randomisation) | ||
| COPD maintenance treatment at baseline | n = 769 | n = 758 |
| Inhaled SABA | 0.967 (0.177) | 0.972 (0.164) |
| Inhaled combined ICS LABA | 0.966 (0.181) | 0.960 (0.195) |
| Inhaled short-acting muscarinic antagonist | 0.068 (0.251) | 0.065 (0.246) |
| Inhaled ICS | 0.043 (0.203) | 0.040 (0.195) |
| Inhaled non-combination LABA | 0.018 (0.134) | 0.029 (0.168) |
| Inhaled LAMA | 0.805 (0.397) | 0.817 (0.387) |
| Nebulised ipratropium | 0.051 (0.291) | 0.041 (0.246) |
| Nebulised SABA | 0.204 (0.536) | 0.185 (0.491) |
| Oral mucolytics | 0.247 (0.432) | 0.248 (0.432) |
| Oral leukotriene antagonists | 0.042 (0.200) | 0.041 (0.198) |
| Long-term antibiotics | 0.066 (0.249) | 0.059 (0.236) |
| Regular medication | ||
| Counta | 4.65 (3.64); n = 772 | 4.41 (3.54); n = 764 |
| Costsb (£) | ||
| Baseline COPD maintenance treatment costsc | n = 769 | n = 758 |
| Inhaled SABA | 17.50 (3.20) | 17.60 (3.00) |
| Inhaled combined ICS and LABA | 325.00 (1897) | 247.00 (486) |
| Inhaled short-acting muscarinic antagonist | 2.77 (10.30) | 2.64 (10.10) |
| Inhaled ICS | 7.28 (50.80) | 8.27 (71.20) |
| Inhaled non-combination LABA | 3.89 (28.60) | 6.20 (35.90) |
| Inhaled LAMA | 164.00 (80.90) | 167.00 (79.00) |
| Nebulised ipratropium | 4.78 (26.30) | 4.19 (24.00) |
| Nebulised SABA | 8.55 (21.30) | 8.14 (20.40) |
| Oral mucolytics | 34.70 (60.70) | 34.90 (60.80) |
| Oral leukotriene antagonists | 0.44 (2.10) | 0.43 (2.08) |
| Long-term antibiotics | 21.00 (88.00) | 25.70 (275) |
| Total baseline COPD maintenance treatment costs | 590.00 (1904) | 522.00 (571) |
There is no significant difference between the arms for any of these baseline resources.
Resource use
Table 28 reports the mean resource use per participant for complete cases, during the 12-month follow-up period.
| Resource use | Trial arm, mean (SD) | |
|---|---|---|
| Theophylline (n = 743) | Placebo (n = 727) | |
| Exacerbation resource usea (mean number of uses per participant in the 12-month follow-up period) | ||
| Increased use of SABA | 1.01 (1.51) | 1.04 (1.60) |
| Increased/started nebulised bronchodilator | 0.288 (0.836) | 0.318 (0.910) |
| OCS | 1.72 (1.87) | 1.68 (1.79) |
| Antibiotics | 2.01 (1.83) | 2.01 (1.84) |
| Oxygen | 0.129 (0.511) | 0.142 (0.541) |
| Other | 0.075 (0.320) | 0.076 (0.354) |
| Treated at home | 2.08 (1.92) | 2.10 (1.90) |
| Care by services to prevent hospitalisation | 0.086 (0.379) | 0.100 (0.416) |
| Admitted to hospital | 0.179 (0.497) | 0.253 (0.676) |
| Non-exacerbation resource use | ||
| COPD maintenance treatment (mean number of uses per participant in the 12-month follow-up period) | ||
| Inhaled SABA | 0.926 (0.262) | 0.934 (0.248) |
| Inhaled combined ICS LABA | 0.918 (0.275) | 0.922 (0.269) |
| Inhaled short-acting muscarinic antagonists | 0.069 (0.253) | 0.062 (0.241) |
| Inhaled ICS | 0.039 (0.194) | 0.044 (0.205) |
| Inhaled non-combination LABA | 0.032 (0.177) | 0.047 (0.211) |
| Inhaled LAMA | 0.817 (0.387) | 0.824 (0.381) |
| Nebulised ipratropium | 0.046 (0.209) | 0.037 (0.189) |
| Nebulised SABA | 0.157 (0.364) | 0.176 (0.381) |
| Oral mucolytics | 0.285 (0.452) | 0.294 (0.456) |
| Oral leukotriene antagonists | 0.046 (0.209) | 0.044 (0.205) |
| Long-term antibiotics | 0.092 (0.289) | 0.085 (0.279) |
| Non-exacerbation health services use | ||
| Inpatient | ||
| General medical ward stays (number of stays) | 0.059 (0.263) | 0.084 (0.406) |
| Long-stay ward stays (number of stays) | 0.004 (0.063) | 0 (0) |
| Other inpatient services (number of contacts) | 0.027 (0.192) | 0.022 (0.173) |
| Outpatient | ||
| Hospital day-case admissions (number of admissions) | 0.187 (0.900) | 0.169 (0.530) |
| Hospital outpatient appointments (number of appointments) | 1.68 (2.63) | 1.58 (2.66) |
| Accident and emergency (no overnight admission; number of visits) | 0.137 (0.490) | 0.128 (0.513) |
| Other inpatient services (number of admissions) | 0.514 (2.87) | 0.476 (2.23) |
| Primary care services | ||
| Emergency GP visit | 1.03 (1.97) | 1.01 (2.10) |
| Routine GP visit | 3.18 (4.33) | 2.84 (3.83) |
| Community district nurse (number of appointments) | 0.801 (9.64) | 0.631 (3.50) |
| Hospital at home team (number of contacts) | 0.101 (1.01) | 0.158 (2.92) |
| Other primary care services (number of contacts) | 2.16 (5.37) | 1.77 (3.68) |
| Non-COPD emergency hospital admissions | ||
| Emergency hospital admissions | 0.150 (0.555) | 0.158 (0.468) |
| Regular medication count | ||
| Regular medication countb | 4.34 (3.55) | 4.32 (3.51) |
As discussed in Chapter 4, the treatment of exacerbations at hospital was significantly different between groups: more exacerbations were treated in hospital in the placebo group than in the theophylline group (p = 0.02).
Missing data
The disaggregated level of missing data affecting resource use is reported below; these are broken down into exacerbations, COPD maintenance treatment and non-COPD emergency hospital admissions.
Exacerbations (length of exacerbation, treatment costs and location of treatment)
A total of 3430 exacerbations were recorded:
-
In 329 participants, data on length of exacerbation were missing (5.9% missing data points).
-
In 210 recorded exacerbations, the location of treatment marker was missing (3.4% missing data points).
-
In 46 participants with exacerbations treated in hospital, length of stay data were missing.
-
In 171 recorded exacerbations, treatment cost was missing (1% missing data points).
Maintenance chronic obstructive pulmonary disease treatment
A total of 82 participants had missing total COPD maintenance costs (5.6% missing data points); these missing data were replaced with a treatment-specific mean.
Non-chronic obstructive pulmonary disease emergency hospital admissions
A total of 235 non-COPD emergency hospital admissions were recorded:
-
Nine participants had missing length of stay data for emergency hospital admissions (2.8% missing data points).
All missing resource data were replaced using pragmatic, naive methods suitable for use when < 10% of data are missing.
Table 29 presents the missing economic data for resource use and EQ-5D-3L completion.
| Data | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Cost | |||
| ITT population | 772 (100) | 764 (100) | 1536 (100) |
| No resource use captured during follow-up | 29 (3.8) | 37 (4.8) | 66 (4.3) |
| Complete cases | 743 (96.2) | 727 (95.2) | 1470 (95.7) |
| EQ-5D-3L | |||
| ITT population | 772 (100) | 764 (100) | 1536 (100) |
| Missing EQ-5D-3L data at baseline/6 months or 12 months | 137 (17.7) | 156 (20.4) | 293 (19.1) |
| Complete cases | 635 (82.3) | 608 (79.6) | 1243 (80.9) |
Resource use data were available for 743 participants in the theophylline arm and 727 in the placebo arm; resource use data during the follow-up period were not captured for 29 (3.8%) participants in the theophylline arm and 37 (4.8%) participants in the placebo arm. Overall, 66 (4.3%) participants were missing resource use data for the whole 12-month follow-up period.
The number of participants with missing EQ-5D-3L data was 137 (17.7%) in the theophylline arm and 156 (20.4%) in the placebo arm. Overall, 293 (19.1%) EQ-5D-3L questionnaires were missing.
Costs
Table 30 reports complete-case costs (unadjusted). Differences between arms are calculated using a GLM with identity link, gamma family and a cluster for centre number. Regular medication was not included in these costs because there was no significant difference between arms in regular medication count.
| Costs | Trial arm, mean (SD) (£) | Difference (£) | 95% CI (£) | |
|---|---|---|---|---|
| Theophylline | Placebo | |||
| Intervention costs | 22 (0.24) | 0 (0) | 22 | 22 to 22 |
| Exacerbation costs | ||||
| Total exacerbation costs | 585 (1682) | 1033 (3383) | –447 | –709 to –186 |
| Total location costs | 535 (1594) | 958 (3185) | –422 | –673 to –171 |
| Location | ||||
| Home | 67 (61) | 68 (60) | –1 | –6 to 4 |
| Services | 33 (145) | 38 (159) | –5 | –23 to 12 |
| Hospital | 436 (1538) | 852 (3142) | –416 | –655 to –177 |
| Treatment | 50 (167) | 75 (296) | –25 | –41 to –8 |
| Non-exacerbation costs | ||||
| COPD maintenance treatment | 974 (379) | 978 (416) | –4 | –45 to 38 |
| Health services resource use (not exacerbation related) | 819 (1,224) | 862 (1812) | –43 | –175 to 89 |
| Non-COPD-related emergency hospital admissions | 282 (1529) | 262 (1136) | 20 | –102 to 143 |
| Total costs | 2684 (2882) | 3136 (4851) | –452 | –771 to –133 |
| Non-intervention, non-exacerbation costs | 2075 (2079) | 2101 (2528) | –26 | –234 to 181 |
There is a significant difference of £452 (95% CI £133 to £771) in the mean total costs between arms, with placebo being more costly than theophylline. This difference is driven by the difference in exacerbation mean costs between arms: £447 (95% CI £186 to £709) higher in the placebo arm. The difference in exacerbation costs is driven by the location of the treatment of an exacerbation. The mean ‘location of exacerbation treatment’ cost is £422 (95% CI £171 to £673) higher in the placebo arm than in the theophylline arm. As presented in Chapter 4, this is driven by a higher number of exacerbations treated in hospital in the placebo arm than in the theophylline arm. This is reflected in the health economics analysis for which the location of treatment costs are further broken down into ‘treatment at home’, ‘care by services to prevent hospitalisation’ and ‘admitted to hospital’. The ‘treatment at home’ and ‘care by services to prevent hospitalisation’ resource use costs show no significant differences between arms; however, the ‘admitted to hospital’ cost is £416 (95% CI £177 to £655) higher in the placebo arm than in the theophylline arm, which is a statistically significant result.
At a per-exacerbation level, this difference can be explored further. The mean cost per exacerbation treated in hospital is £3613 [standard error (SE) £342] in the placebo arm and £2671 (SE £220) in the theophylline arm, a significant difference of £941 (SE £386) (95% CI £140 to £1743). The 10 most costly observations (> £10,000) were all in the placebo arm, and were the result of hospital stays of > 40 days. Because of the lack of treatment effect, we believe this difference to be a chance finding and not a real result of the trial. The distribution for length of hospital stay is similar for both arms apart from a small excess of participants in the placebo arm with longer stays. It is important to note that the proxy for hospital length of stay is length of exacerbation and that this is likely to overestimate length of stay in hospital. In total, 319 exacerbations were treated in hospital: 185 in the placebo arm and 134 in the theophylline arm.
The cost of treatment of exacerbations differed significantly between arms, the mean cost per participant being £25 lower in the theophylline arm. At a per-exacerbation level, this is driven by treatment with oxygen. The mean cost of oxygen use per exacerbation treated with oxygen was £141 (SE £52) (95% CI £40 to £243) lower in the theophylline arm than in the placebo arm. The difference in oxygen treatment is driven by the fact that seven participants, six of whom were in the placebo group, were treated with oxygen for > 51 days, at a cost per exacerbation of > £1000.
The wide SDs for hospitalised exacerbations, treatment of exacerbations, non-COPD emergency hospital admissions and other health services use indicate a wide range of individual participants’ costs in these resource groups.
No other resource use costs are significantly different between arms, which is reflected in the fact that there was no difference between arms for the non-intervention, non-exacerbation costs presented in Table 30.
Economic outcome
Complete-case EQ-5D-3L data and QALYs are reported in Table 31.
| Time point | Trial arm, mean (SD) | Difference (95% CI) | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Baseline | 0.629 (0.280) | 0.643 (0.279) | –0.014 (–0.045 to 0.017) |
| 6 months | 0.630 (0.296) | 0.642 (0.295) | –0.012 (–0.045 to 0.021) |
| 12 months | 0.622 (0.292) | 0.623 (0.308) | –0.001 (–0.034 to 0.032) |
| QALYs over 12 monthsa | 0.626 (0.259) | 0.637 (0.263) | –0.011 (–0.040 to 0.018) |
Utilities from the EQ-5D-3L data at baseline and the 6- and 12-month follow-ups and QALYs are higher in the placebo arm than in the theophylline arm; however, these differences are not significant.
Multiple imputation
Multiple imputation results are presented in Table 32 for costs and QALYs.
| Costs and QALYs | Trial arm, mean (SE) | Difference (95% CI) | |
|---|---|---|---|
| Theophylline | Placebo | ||
| Total costs (£) | 2702 (110) | 3141 (148) | –439 (–846 to –32) |
| Total QALYs | 0.617 (0.010) | 0.621 (0.010) | –0.004 (–0.031 to 0.024) |
Multiple imputation results mirror the complete-case results, with costs significantly higher in the placebo arm (a difference of £439). Total QALYs are higher in the placebo arm; however, this is not a statistically significant result (a difference of 0.004).
Bootstrapping
To explore the robustness of these results, 1000 non-parametric bootstrapped samples were taken from the observed data. The results were plotted using a cost-effectiveness plane to illustrate the mean differences between the arms in incremental costs and QALYs.
Non-adjusted bootstrapped results are presented in Figure 7. This cost-effectiveness plane clearly illustrates that the majority of total mean costs are lower in the theophylline arm than the placebo arm, with the majority of incremental samples falling in the south-east and south-west quadrants of the cost-effectiveness plane (below the horizontal axis of £0). The majority of total mean QALYs are lower in the theophylline arm than in the placebo arm, represented by the majority of bootstrapping samples falling in the south-west quadrant where the placebo arm has higher mean QALYs than the theophylline arm. The cost-effectiveness plane includes an ellipse to illustrate the 95% confidence level.
FIGURE 7.
Cost-effectiveness plane (unadjusted).

This uncertainty is explored further using cost-effectiveness acceptability curves.
The unadjusted bootstrapped results are presented in Figure 8. At a willingness-to-pay threshold of £20,000, there is a 75% chance of theophylline being cost-effective. At £30,000, there is a 64% chance of theophylline being cost-effective. However, these results should be viewed with caution as there is no significant difference in QALYs or clinical effect, and the difference in costs is driven by a very small number of participants with prolonged hospital admissions and the likelihood that the finding of a difference between arms for exacerbations treated in hospital is a chance finding. Moreover, as discussed in Adjusted analysis, the cost benefits of theophylline are not evident in multivariate models.
FIGURE 8.
Cost-effectiveness acceptability curve (unadjusted).
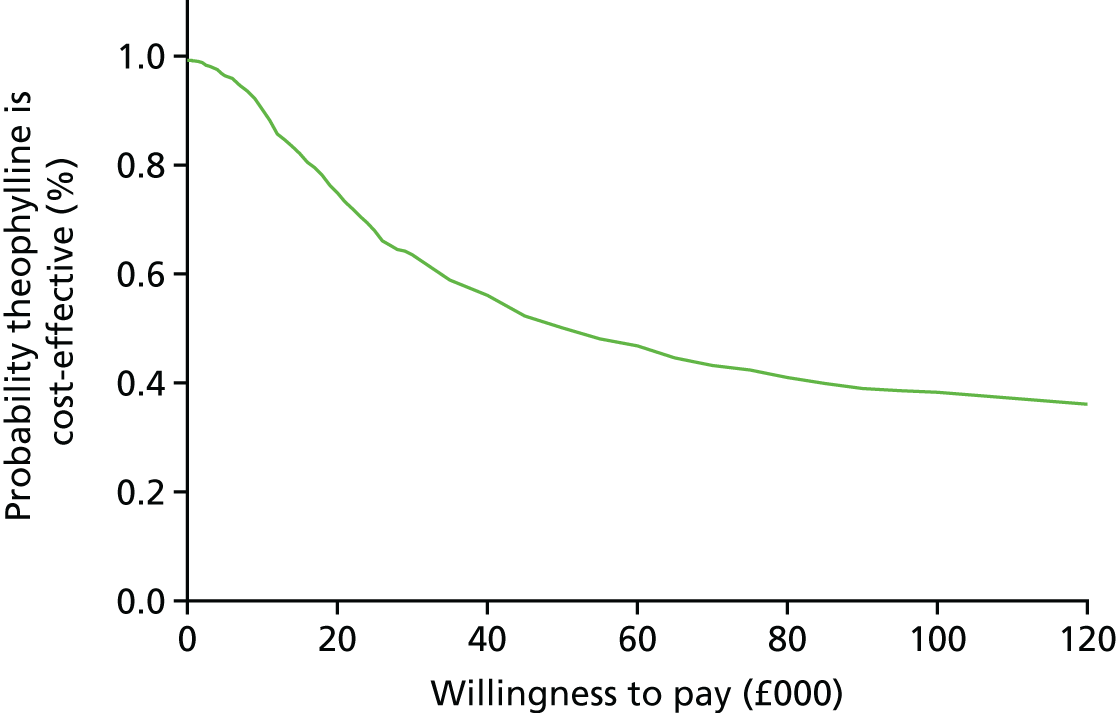
Adjusted analysis
Multiple imputation total mean costs were adjusted for baseline variables that were significant predictors of cost. These were medication count at baseline, EQ-5D-3L data at baseline, offset time (time spent in the trial), age, number of hospitalisations for exacerbations in the 12 months prior to randomisation and number of exacerbations in the 12 months prior to randomisation. A cluster command was used for centre number.
Multiple imputation total mean QALYs were adjusted for baseline variables that were significant predictors of QALYs. These were baseline EQ-5D-3L data, medication count at baseline, offset time, age, sex, hospitalisation for exacerbations in the 12 months prior to randomisation and exacerbations in the 12 months prior to randomisation. A cluster command was used for centre number. These results are presented in Table 33.
| Costs and QALYs | Trial arm, mean (SE) | Difference (95% CI) | Cost-effectiveness | |
|---|---|---|---|---|
| Theophylline | Placebo | |||
| Total costs (£) | 2784 (125) | 3006 (167) | –222 (–472 to 27) | Theophylline dominates: fewer costs and higher QALYs |
| Total QALYs | 0.621 (0.006) | 0.616 (0.007) | 0.005 (–0.015 to 0.025) | |
When multiple imputation total costs are adjusted, there is a trend towards higher costs in the placebo arm; however, this difference is not significant.
Adjusting QALYs for baseline characteristics results in theophylline having higher QALYs than placebo; however, this difference is not significant.
Figure 9 illustrates that, when the results are adjusted for baseline characteristics, the results are more uncertain: the majority of total mean costs in the theophylline arm are still lower than in the placebo arm, although this is now not a significant result. In addition, the QALYs are marginally higher in the theophylline arm, again not a significant result. The ellipse represents the 95% confidence levels.
FIGURE 9.
Cost-effectiveness plane (adjusted).

The adjusted bootstrapped results are presented in a cost-effectiveness acceptability curve in Figure 10. At a willingness-to-pay threshold of £20,000, there is a 90% chance of theophylline being cost-effective. At £30,000, there is an 85% chance of theophylline being cost-effective. Again, these results should be viewed with caution as there was no significant difference between arms for QALYs or treatment effect.
FIGURE 10.
Cost-effectiveness acceptability curve (adjusted).
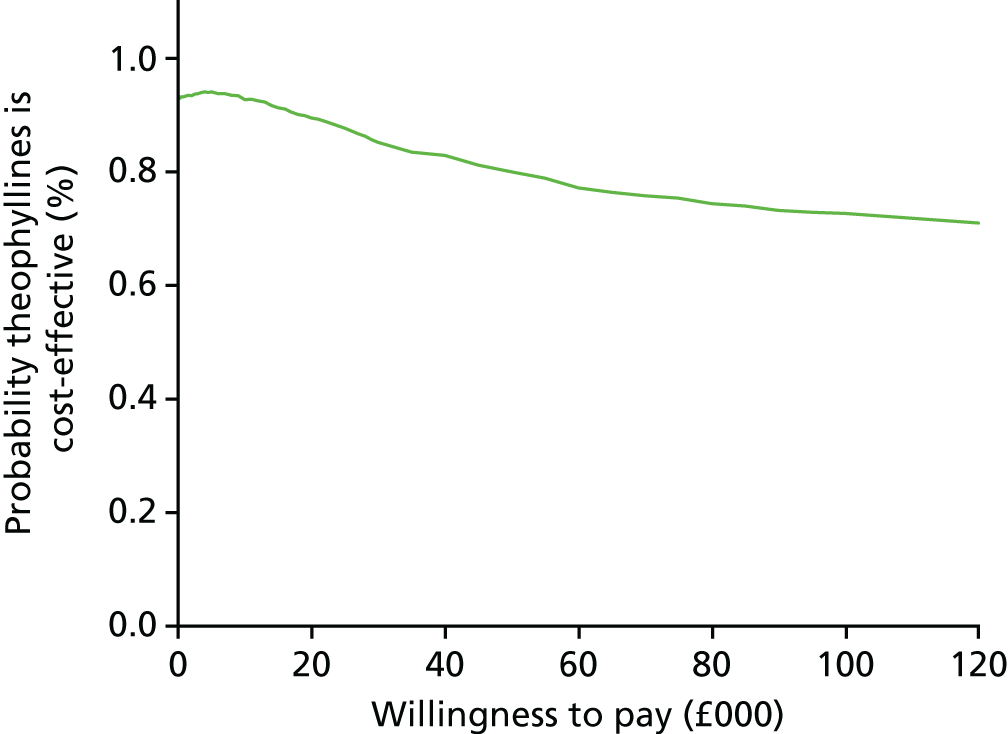
Exacerbation costs were also adjusted separately to explore the adjustment on the significant difference in exacerbation costs between arms. Strong predictors of exacerbation costs were offset time, hospitalisation for exacerbations in the 12 months prior to randomisation and exacerbations in the 12 months prior to randomisation. A cluster command was used for centre number. These results are presented in Table 34 and show that, for adjusted exacerbation costs, although there is a trend for higher costs in the placebo arm, this difference is not significant. The mean cost difference has decreased from £447 to £67.
| Costs | Trial arm, mean (SE) (£) | Difference (£) | 95% CI (£) | |
|---|---|---|---|---|
| Theophylline | Placebo | |||
| Total exacerbation cost | 732 (96) | 799 (71) | –67 | –196 to 61 |
| Location costs | 675 (98) | 735 (72) | –60 | –190 to 68 |
| Treatment costs | 58 (11) | 64 (8) | –6 | –19 to 7 |
Cost-effectiveness
For cost per QALY, the unadjusted results suggest that, although theophylline is cheaper than placebo (significant result), the QALY gain is higher in the placebo arm than in the theophylline arm (non-significant result). The adjusted results suggest that theophylline dominates: it is cheaper than placebo and results in a higher QALY gain. However, this result should be interpreted with caution because the difference in QALYs is not significant. This is mirrored by the trial primary outcome: theophylline is not clinically effective in terms of reducing exacerbations.
Chapter 6 Discussion
Main results
The results of this trial show that, for people with COPD at high risk of exacerbation, the addition of low-dose oral theophylline to a drug regimen that includes an ICS confers no overall clinical or health economic benefit. This result was evident from both the ITT and the per-protocol analyses. The primary outcome measure for this trial was the total number of exacerbations of COPD requiring changes in management (minimum management change: use of OCSs and/or antibiotics) during the 1-year treatment period, as reported by the participant. For the 11 prespecified secondary outcome measures, the addition of low-dose theophylline had no clinical or health economic benefit in 10 of these measures. The addition of low-dose theophylline did reduce the number of COPD exacerbations necessitating hospital admission (often classified as ‘severe’)68 (adjusted IRR 0.72, 95% CI 0.55 to 0.94); however, further inspection of the data indicated that this difference was the consequence of a small excess of participants allocated to placebo (n = 10) having three or more hospital-treated exacerbations, which accounted for 39 of the extra 51 hospital-treated exacerbations in the placebo arm. This effect on hospital admissions was also evident in the per-protocol analysis. Given that adjustments for multiple comparisons were not performed, it is possible that this finding could be due to type I error. However, in the light of a 2018 report96 showing that another PDE inhibitor (roflumilast) is most beneficial in people with prior COPD hospitalisation for exacerbation and greater exacerbation frequency, this finding warrants further investigation. The safety data demonstrated that the addition of low-dose theophylline was not associated with an increase in SAEs or ARs.
Relevance to existing literature
Oral theophylline has been used in the treatment of COPD and asthma for > 70 years. Conventionally, oral theophylline has been used as a bronchodilator in COPD, this effect being mediated by inhibition of PDE. However, in order to achieve modest clinical effects, relatively high blood concentrations (of 10–20 mg/l) are required, but, at these concentrations, non-specific inhibition of PDE is also associated with a wide range of well-recognised side effects, for example nausea, palpitations and headaches. A Cochrane review published in 2002 and updated in 201083 identified 20 randomised placebo-controlled trials of theophylline in COPD, all of crossover design, using dosing schedules to obtain conventional plasma theophylline levels in the therapeutic range (10–20 mg/l), that is conventional high-dose theophylline. The number of participants in these trials ranged from 8 to 60; the total number of participants in the 20 trials was 488. The duration of the studies was 9–90 days, the mean age of participants ranged from 58 to 69 years and four of the studies were graded as being of high quality. The systematic review demonstrated that use of high-dose conventional theophylline resulted in a small but significant increase in FEV1 of 100 ml (95% CI 40 to 160 ml). This was derived from 13 studies with 244 participants. Two studies with a total of 45 participants reported on the incidence of exacerbations, concluding that high-dose conventional theophylline had no effect on the incidence of exacerbations. Three studies with a total of 64 participants reported data on nausea, with the risk of experiencing nausea when on theophylline treatment being significantly increased (RR 7.67, 95% CI 1.47 to 39.94). When compared with previous trials of conventional high-dose theophylline in COPD, the current trial of low-dose theophylline that recruited 1578 participants is clearly somewhat larger and the treatment period is longer in duration. Moreover, in contrast to conventional high-dose theophylline trials with their focus on lung function, the primary outcome of the current study was exacerbations of COPD and the study population comprised participants at high risk of exacerbating. When compared with these trials of high-dose conventional theophylline, the current trial, as expected, showed no effect of low-dose theophylline on lung function (FEV1) and, reassuringly, no increase in side effects. One of the findings from the Cochrane review83 was that very few participants withdrew from intervention trials of high-dose conventional theophylline for any reason. In the review,83 nine studies reported no dropouts, and in the remaining studies the dropout rate was generally very low; the only exception to this low dropout rate was the study of Guyatt et al. ,97 who reported eight withdrawals out of 27 recruited participants (30%). The sample size of the current trial included an estimate of 6% of participants ceasing taking their trial medication, based on the four high-quality studies reported in the Cochrane review,83 in which three out of 51 (6%) participants dropped out. The 26% of participants ceasing trial medication in the current study is greater than anticipated (although balanced across the arms) and more in keeping with the study of Guyatt et al. ,97 probably reflecting the pragmatic nature of the current trial, the fact that participants in the current trial were older than those in the Cochrane review and that the trial lasted longer. 83
The use of high-dose conventional theophylline has declined over the years because of its narrow therapeutic index, modest clinical effect, side effect profile, drug interactions, the need for blood concentration monitoring and the availability of more effective inhaled therapies. 98 High-dose conventional theophylline is now included in current COPD guidelines as a third-line therapy. 1
The concept of using low-dose theophylline to augment the anti-inflammatory effects of corticosteroids on the airway inflammatory processes in COPD originated from in vitro and animal studies investigating the molecular mechanisms contributing to the reduced corticosteroid sensitivity of COPD. 32,38–40,43,46 The key observation was that the reduced HDAC2 activity of COPD can be reversed by low concentrations (1–5 mg/l) of theophylline; moreover, theophylline reduces corticosteroid insensitivity in COPD, such that there is a marked synergistic interaction between theophylline and corticosteroids in suppressing the release of inflammatory mediators from alveolar macrophages obtained from COPD patients. 43,44 These basic research studies suggest that low-dose (i.e. 1–5 mg/l) theophylline could increase HDAC activity and, hence, reduce corticosteroid resistance in COPD patients, thereby enabling ICSs to switch off inflammation and potentially more effectively reduce exacerbation rates.
Prior to commencing the current study, the concept of using low-dose theophylline in conjunction with corticosteroids in COPD had been explored in two small RCTs. The first RCT was in 35 patients admitted to a Spanish hospital with an acute exacerbation of COPD who were treated with a regime that included systemic corticosteroids. 48 Participants were randomised to receive additional low-dose theophylline or nothing in a single-blind design; participants not on ICSs at admission were commenced on ICSs. After 3 months of treatment, low-dose theophylline increased sputum macrophage HDAC activity and reduced sputum concentrations of the pro-inflammatory mediators IL-8 and tumour necrosis factor alpha (TNF-α). There were no clinically significant effects in this small study,48 although fewer participants in the theophylline group than in the control group had a subsequent exacerbation (12.5% vs. 26%). This study48 differed from the current study in the following ways: small sample size, single blinded, no placebo control, 3-month follow-up, participants were recruited only during hospitalisation with exacerbations of COPD, all were male and only 14% had experienced two or more exacerbations in the previous year. Notably, 26% of participants were not followed up at 3 months.
The second small (n = 30) RCT of COPD patients was a double-dummy (low-dose theophylline vs. placebo, standardised dose of ICS vs. placebo), randomised, double-blind, parallel study, based in the UK. 49 After 4 weeks of low-dose theophylline, there was no effect on the primary outcome of absolute number of sputum neutrophils. The combination of low-dose theophylline/ICS significantly reduced a number of secondary end points (e.g. sputum percentage neutrophils, sputum total eosinophil count). In an open-label extension of the trial, the combination of low-dose theophylline/ICS increased peripheral blood mononuclear cell HDAC activity ninefold. The study concluded that the combination of ICS and low-dose theophylline may attenuate airway inflammation in patients with COPD. One of the limitations of this study was that the significant findings were for low-dose theophylline/ICS versus theophylline, rather than low-dose theophylline/ICS versus ICS, suggesting perhaps that the observed effects were a consequence of the ICS and not the low-dose theophylline. This study49 differs from the current study in the following ways: 4-week duration, small numbers, 83% male and younger average age (61 years). Lung function (mean FEV1 54%) was similar.
While the current trial was being conducted, two trials investigating the therapeutic consequences of low-dose theophylline were published. 52,99 The first study,99 from India, was a hospital-based, single-blinded, prospective, randomised, placebo-controlled study that investigated the effects of adding low-dose theophylline to the combination of formoterol plus budesonide. A total of 58 patients with moderate/severe COPD were commenced on a standardised ICS/LABA therapy (budesonide and formoterol) and were randomised to receive in addition either low-dose theophylline or placebo for 60 days. Fifty participants completed the trial and their data were presented. 99 The addition of low-dose theophylline resulted in a greater improvement in total symptom scores, a greater increase in FEV1 and a greater increase in 6-minute walking distance than did the addition of placebo. Of note, however, is that the method of randomisation was not described, the actual number of participants randomised to each treatment group was not presented, the nature of the ‘single blind’ was not explained and there was no ITT analysis. The randomisation appeared not to have eliminated potential sources of bias, as the participants allocated to low-dose theophylline were clearly more severely affected by COPD: their respiratory rate was greater (20.7 vs. 18.7 breathes per minute; p = 0.003), their FEV1 values were lower (49% vs. 57% predicted; p = 0.05), their symptom scores were greater (10.17 vs. 8.37; p = 0.003), their 6-minute walking distance was shorter (373 m vs. 409 m; p = 0.07) and more were classified as having severe COPD (54% vs. 27%; p = 0.09). 99 Moreover, the placebo tablets were described as similar rather than identical. These differences could reflect a bias for the more severely affected participants to be preferentially allocated to the low-dose theophylline arm of the trial. This study99 differs from the current study in the following ways: the sample size was much smaller and hospital based, 92% of participants were male, participants were younger (≈55 years), BMI was lower (≈17 kg/m2), there was a 60-day treatment period and the trial was single blinded. In addition to the issues regarding blinding and randomisation, the results of the trial99 also raise the possibility that, although the intention was to investigate low-dose theophylline, in reality, conventional high-dose theophylline was being tested: an improvement in FEV1 was described with theophylline treatment and the dosing regimen for this study was 400 mg of theophylline for a weight of > 50 kg, 300 mg for a weight of 40–50 kg and 200 mg for a weight of < 40 kg. However, a significant proportion of participants appeared to be underweight, with a mean BMI of ≈17 kg/m2, and theophylline treatment resulted in higher incidences of typical high-dose theophylline toxicity symptoms such as nausea, vomiting, headache, palpitation and insomnia. In the current study, this was avoided by basing theophylline dose on IBW and smoking status.
The second study, the Spanish ASSET trial,52 was a multicentre, randomised, double-blind, placebo-controlled trial that recruited patients with COPD while they were hospitalised for a COPD exacerbation. Participants were randomised to low-dose theophylline (100 mg twice a day) or matched placebo in addition to ICS/LABA treatment; participants not routinely taking ICS/LABA were established on ICS/LABA. In total, 70 patients were randomised (theophylline, n = 36; placebo, n = 34) and 46 completed the year of treatment (theophylline, n = 23; placebo, n = 23). The co-primary outcomes were change in HDAC and exacerbation frequency during the 1-year treatment period. The addition of low-dose theophylline had no effect on plasma/sputum HDAC concentrations and no effect on COPD exacerbation rate [theophylline 0.97 (SD 0.94) vs. placebo 0.88 (SD 0.89)]. This trial52 has some similarities with the current trial: primary outcome of exacerbation, same definition of exacerbation, 1-year treatment period, similar participant age, CAT score and levels of cardiovascular comorbidity at baseline, and no significant difference in ARs between groups. However, there are some important differences between the ASSET trial52 and the current trial (TWICS). The current trial is much larger (n = 1578) than the ASSET trial (n = 70), being designed to detect a 15% reduction in exacerbations with 90% power, whereas the ASSET trial was designed to detect an arguably implausibly large 50% reduction in exacerbations with 80% power. The exacerbation rate in the ASSET trial was about half that observed for the TWICS trial (0.92 vs. 2.23 per year). Perhaps the most plausible explanation for this is that all participants in the ASSET trial were recruited while hospitalised with an exacerbation of COPD, irrespective of exacerbation history, whereas participants in the TWICS trial were clinically stable; 60% were identified from primary care and all had a history of two or more exacerbations in the previous year requiring treatment with antibiotics and/or corticosteroids. The proportion of participants ceasing trial medication was higher in the ASSET trial than in the TWICS trial (34% vs. 26%); however, it should be noted that 14% of participants in the ASSET trial ceased trial medication because their FEV1 improved to > 50% predicted during the 1-year treatment period. This is most likely to be a consequence of the ASSET trial recruiting in the peri-exacerbation period and the TWICS trial recruiting when participants were clinically stable. When compared with the TWICS trial, participants in the ASSET trial were more likely to be male, had more severe COPD (lower FEV1) and were more likely to be hospitalised during the treatment period, but were less likely to be diabetic [probably reflecting the higher mean BMI of the TWICS trial participants (27 vs. 22 kg/m2)].
The GOLD management strategy guideline1 highlights that the clinical relevance of low-dose theophylline has not been fully established and that clinical evidence for an effect of low-dose theophylline, particularly on exacerbations, is limited and contradictory. The TWICS trial is the first large, pragmatic, community-based trial to investigate the effect of adding low-dose theophylline to the treatment regimen of people with COPD who are at a high risk of exacerbating despite a treatment regime that includes maintenance ICSs in COPD.
Pre-clinical work convincingly demonstrates that the combination of low-dose theophylline and corticosteroids has a strong biological effect, increasing HDAC and inhibiting the release of pro-inflammatory mediators. 38,39,41–44 The trials conducted to date have been small (n = 30–70), hospital based and have tended to focus on biological outcomes with short treatment periods. 48,49,52,99 The largest trial52 to date in this field has reported that low-dose theophylline had no effect on HDAC or exacerbations; however, as the authors of the ASSET trial acknowledge, ‘we might have overestimated the potential clinical benefit when we calculated the sample size, which may have precluded us from identifying a clear-cut clinical effect’. 52 The TWICS trial avoids many of the limitations of previous studies and clearly demonstrates that, in a NHS setting, the addition of low-dose oral theophylline to a drug regimen that includes an ICS confers no overall clinical benefit for people with COPD. The participants in the TWICS trial were a group of people with COPD who were at high risk of experiencing an exacerbation based on their history of exacerbating in the previous year. This group was deliberately chosen because of their impact on the NHS and it enabled us to design a trial of realistic (but ambitious) sample size. Although the TWICS trial did not investigate whether or not people with COPD who are at low risk of exacerbation would benefit from low-dose theophylline, the combination of the findings of the TWICS trial and the absence of a biological effect (HDAC concentrations) in the ASSET trial, despite a sample size that was ‘more than enough to demonstrate a biological effect of the intervention’,52 make it highly unlikely that low-dose theophylline would be beneficial in COPD patients who are at low risk of exacerbation. A possible explanation for the disparity between the biological effects observed in previous studies, with short treatment periods, and the absence of beneficial effects in the TWICS trial, with a year-long treatment period, is that any biologically beneficial effect of low-dose theophylline is not sustained in the long term.
Cost-effectiveness
The health economics results indicate that, after adjustment for baseline characteristics, there was no significant difference between the total health economic costs associated with treatment with low-dose theophylline and the costs associated with placebo treatment: adjusted mean difference £222 (95% CI –£27 to £472). With unadjusted complete-case data, the total costs are higher in the placebo arm than in the theophylline arm: a significant difference of £452 (95% CI £132 to £771). This difference was driven by the fact that more participants in the placebo arm than in the theophylline arm received treatment for exacerbations in hospital. The 10 most costly observations (> £10,000) were all in the placebo arm, and were the result of hospital stays of > 40 days. The multiple imputation results mirror the complete-case results with a significant difference in unadjusted costs of £439 (95% CI £32 to £846) higher in the placebo arm.
The difference between arms in total costs is driven solely by the hospital-treated exacerbations and exacerbations treated with oxygen; no other resource group differs significantly between arms. The difference in the number of exacerbations necessitating hospital treatment is likely to be the result of a small number of participants in the placebo arm having very frequent hospital admissions. Therefore, these results should be interpreted with caution.
Exacerbation costs are 22% and 33% of the total costs (theophylline and placebo, respectively), somewhat less than the 60% reported by Britton et al. 27 in 2003, perhaps reflecting differences in management between 2003 and 2015/6, particularly in increased use of preventative drugs, pulmonary rehabilitation and more structured chronic disease management in primary care.
In the unadjusted complete-case analysis, QALY gain was higher in the placebo arm than in the theophylline arm (difference 0.011 QALYs, 95% CI –0.018 to 0.040 QALYs); however, the difference is not significant. Multiple imputation results mirrored the complete-case results; there were no significant differences, with unadjusted results favouring the placebo arm and adjusted results favouring the theophylline arm.
These results reflect the primary outcome of number of exacerbations needing treatment during the 12-month follow-up period; there was no significant difference between arms.
Hettle et al. 100 reported 4-year UK costs in their paper on tiotropium versus usual care in the UK and Belgium. Exacerbation costs ranged from £2295 to £2744 (£574–686 per year) and maintenance costs ranged from £2935 to £3937 (£737–984 per year). This compares to the 1-year costs from this research of exacerbations, ranging from £585 to £1033, and maintenance costs ranging from £2074 to £2101. Although the annual exacerbation costs in the current trial are similar to those of Hettle et al. ,100 the maintenance costs are somewhat higher, reflecting the older average age of participants in the current study (68.4 years vs. 64 years), and the facts that, in the current study, 80% of the participants were prescribed LAMAs (none for the usual care group in the Hettle et al. 100 study), participants with COPD using long-term oxygen were included and participants were more likely to be in the severest GOLD category (14% vs. 8%). Hettle et al. 100 also reported that in Belgium the cost of exacerbations resulting in hospitalisation was 33 times higher than the cost of exacerbations not necessitating hospitalisation, substantiating the increased cost of hospital treatment compared with non-hospital treatment of exacerbations that we found in this research.
The strengths of this research include the fact that there were few participants with no outcome or resource use (low proportion of missing cases: 4.3%), that uncertainty was explored using non-parametric bootstrapping and that, when there was a significant difference in exacerbation costs, this was explored further to identify what was driving this difference.
The two main limitations to the cost-effectiveness analysis are the large number of missing EQ-5D-3L questionnaires (19.1%) and the fact that small numbers of missing data were imputed using naive methods at a disaggregated level.
Strengths and limitations
The main strength of the TWICS trial is that it was a large, pragmatic, predominantly community-based, suitably powered, double-blind, randomised, placebo-controlled, UK multicentre clinical trial with a high follow-up rate for the primary clinical outcome. A total of 1578 individuals were recruited in 121 UK sites; 60% of these were identified in primary care, making it highly likely that the TWICS trial participants reflected normal clinical practice across both primary and secondary care in the UK. The 1-year treatment period allowed for capture of the seasonality of exacerbations. 101
Originally, the TWICS trial aimed to recruit 1424 participants, the sample size being primarily based on the findings of the observational ECLIPSE cohort study of 2138 COPD patients recruited in 46 centres from 12 countries. 21 The ECLIPSE study demonstrated that the best predictor of an exacerbation in a year was a treated exacerbation in the previous year. The ECLIPSE study also identified a frequent exacerbator phenotype, defined as two or more exacerbations in the previous year; moreover, this frequent exacerbator phenotype was relatively stable for 3 years and could be reliably identified by patient report. For the patients experiencing frequent exacerbations recruited into the TWICS trial, data from the ECLIPSE study predicted a mean of 2.22 (SD 1.86) exacerbations in the year of treatment; the sample size for the TWICS trial was based on this. This prediction proved to be remarkably close to what we observed, increasing confidence in the findings, with a mean number of exacerbations in the theophylline arm of 2.24 (SD 1.99) and in the placebo arm of 2.23 (SD 1.97). A notable finding of the TWICS trial was an apparent disparity between the number of exacerbations reported by participants in the year prior to the trial [mean 3.59 (SD 2.15)] and the number of self-reported exacerbations in the treatment year, which was somewhat lower [mean 2.23 (SD 1.99)]. The most likely explanation for this disparity is that we did not ask for dates for the reported exacerbations in the year prior to the trial, whereas, during the trial, we asked for dates and the conventional minimum of 2 weeks between consecutive exacerbation episodes was necessary to consider exacerbations as separate. 68 This resulted in exacerbations that were separated by < 2 weeks being merged. Although further factors contributing to the disparity in exacerbations before and during the trial may include an over-reporting bias by participants and regression to the mean, the exacerbation frequency during the treatment period was remarkably consistent with that predicted by the ECLIPSE study. Although the exacerbation rate observed in the current trial is somewhat higher than in recent explanatory trials,102,103 it is entirely consistent with the recent pragmatic UK Salford Lung Study. 104 The Salford Lung Study, with an inclusion criterion of one or more exacerbations in the previous year, reported exacerbation rates of 1.74–1.90 per year. The slightly higher exacerbation rate in the current trial most probably reflects the participants’ increased propensity to exacerbate (two or more exacerbations in the previous year), as well as the lack of requirement to withhold therapy other than theophylline, meaning that investigators were happier to recruit patients at a higher risk of exacerbation. Although the diagnosis of COPD was confirmed by post-bronchodilator FEV1/FVC of < 0.7, 18.3% of participants reported a concurrent/previous diagnosis of asthma. This may, in part, reflect a diagnostic bias towards the more socially acceptable diagnosis of asthma in the past, but it is possible that the current trial included up to 18% of participants with asthma–COPD overlap syndrome. Although it is possible that these patients may respond differently to the theophylline, whether or not people with asthma–COPD overlap syndrome responded differently to theophylline was not one of the trial objectives.
By recruiting 1578 individuals, 60% of whom were identified in primary care, the TWICS trial exceeded its original recruitment target of 1424 with at least 50% being recruited in primary care. It was initially envisaged that the TWICS trial would recruit from a limited number (seven) of secondary care sites, with primary care sites acting as PIC sites for these secondary care centres. Recruitment to the TWICS trial was delayed by 5 months because of a worldwide shortage of bottle tops for the drug bottles. Initially, recruitment in 16 primary and six secondary care sites was on target and the TWICS trial achieved its recruitment targets for the feasibility phase by recruiting 100 participants in months 7, 8 and 9, with 55% identified in primary care. Within 4 months, it became apparent that it would not be possible to sustain recruitment, with recruitment falling below the required 59 participants per month to a nadir of 26 in month 15 (October 2014). To address this, a change in recruitment strategy was implemented in month 12 (July 2014), with rapid increases in the number and rate of opening up of primary and secondary care sites. Ultimately, 121 recruiting sites were opened up, comprising 88 primary care sites and 33 secondary care sites. Other primary care practices acted as PICs for primary and secondary care sites. In total, 477 participants were recruited and followed up entirely in primary care, 464 participants were identified in primary care but recruited and followed up in secondary care (this was particularly the case in Scotland) and 637 participants were identified, recruited and followed up in secondary care. This change in recruitment strategy was successful, with monthly recruitment remaining above 50 per month from month 19 and reaching a peak of 81 in month 35.
Primary outcome data (number of COPD exacerbations) were collected on 98% of the 1567 participants who commenced the 1-year treatment period (1578 recruited minus 11 post-randomisation exclusions). Several factors contributed to the high follow-up rate. The TWICS trial was designed to be as inclusive as possible by facilitating participation of people with COPD who would normally find it too difficult to participate in a trial because of their ill health. The trial was designed to be relatively ‘light touch’, with three study visits to a local study centre. If participants were unable to attend for assessment, they were visited at home, contacted by telephone or sent the questionnaires to complete at home. Participation and remote follow-up were further facilitated by delivering the trial drug to participants’ homes using a third-party distributor. All participants who ceased taking the trial drug were invited to remain in the trial for follow-up, by face-to-face assessment, telephone assessment or postal questionnaire. If participants could not be followed up directly, for example if they failed to attend follow-up, various methods of follow-up, independent of participant involvement, were used. In the first instance, the participant’s GP was sent a questionnaire enquiring about exacerbations (number, dates, how and where treated); the minimum information requested was the number of exacerbations in the treatment period. Failing this, GP surgeries were contacted by telephone or a request was made for a redacted copy of patient encounter summaries from which the co-chief investigator extracted exacerbation data. The combination of follow-up methods enabled the ITT analysis to include 1489 years of participant follow-up data. Inevitably, there were some participants who did not provide a full 12 months of follow-up data, for example because they died, or for whom 12 months of follow-up data were not available even using a remote follow-up method. A strength of the TWICS trial was that the statistical analytical methods used enabled inclusion of these participants up to the point at which they were lost to follow-up, with their time in study utilised in the offset variable during analysis.
Previous studies investigating the potential anti-inflammatory effects of low-dose theophylline in COPD and asthma (not in conjunction with ICS) have used a ‘one size fits all’ dosing approach, that is all participants received 100 mg bd or 200 mg bd. 43,44,48,99,105–107 In contrast, one of the strengths of the TWICS trial was that theophylline dosing was somewhat personalised, being determined by IBW and smoking status. As noted in the protocol paper,55 population studies have demonstrated that theophylline pharmacokinetics are influenced by weight, COPD disease status (reduced clearance) and smoking (increased clearance). 57–66,108 Smoking increases theophylline clearance by ≈60%, but the plasma theophylline level gradually returns to normal following smoking cessation. This was incorporated into the definition of a non-smoker in the TWICS trial and procedures were implemented to modify, when necessary, the dose of the trial drug in a timely manner if participants changed their smoking status during the treatment period. The use of IBW in preference to actual weight avoided the potential for giving an inappropriately high dose of theophylline to obese participants. In the TWICS trial, theophylline dosing was based on pharmacokinetic modelling, incorporating the major determinants of theophylline Css, namely weight, smoking status and clearance of theophylline (low, normal or high), and was designed to achieve a steady-state plasma theophylline concentration of 1–5 mg/l and certainly < 10 mg/l. 55 Theophylline is metabolised in the liver by the enzyme CYP1A2, which is induced by smoking and inhibited by a number of medications, with a consequent increase in plasma theophylline concentration. For this reason, the exclusion criteria included long-term use of drugs with the potential to increase plasma theophylline concentration;94 conversely, concomitant use of drugs with the potential to lower plasma theophylline concentration was permitted in the trial. Reassuringly, the dosing regimen used for the TWICS trial appeared to be effective in establishing low-dose plasma theophylline concentrations of 1–5 mg/l because there was no evidence of the typical sequelae of conventional high-dose theophylline, such as an improvement in FEV1. In addition, when compared with the placebo group, there was no evidence that participants allocated to low-dose theophylline experienced more SAEs or ARs, nor did they report SAEs or ARs typical of theophylline toxicity, namely gastrointestinal, cardiac, psychological or neurological symptoms. Furthermore, when the reasons for ceasing trial medication were analysed, there were no significant differences between the arms, notably for gastrointestinal, cardiac, psychological or neurological symptoms typical of theophylline toxicity. A consequence of the personalised dosing of the trial drug to achieve a low-dose plasma theophylline concentration well below that associated with typical side effects was that there was no need for blood sampling to monitor plasma theophylline, a necessity that would have greatly increased the complexity of the trial and increased the likelihood of unblinding the participant and/or investigator. The absence of blood testing reduced costs and was extremely popular with primary care sites and contributed to the willingness of many primary care sites to participate in the TWICS trial. The potential limitation of relying on participant-reported smoking status is perhaps less important in this trial, as any smoker claiming to be a non-smoker would have been prescribed the lower dose of theophylline, thereby ensuring that plasma theophylline remained in the low-dose range of 1–5 mg/l.
As with all studies, there are limitations associated with the TWICS trial. The primary outcome for the trial was the number of participant-reported exacerbations during the 1-year treatment period; to facilitate recall, participants were given a diary card to make notes on exacerbations, treatment and health-care usage. The definition of an exacerbation was the widely used ATS/ERS guideline recommendation of a worsening of a patient’s dyspnoea, cough or sputum beyond day-to-day variability sufficient to warrant a change in management. 68 The minimum management change was treatment with antibiotics or OCSs; consequently, the TWICS trial quantified only moderate and severe exacerbations. However, these exacerbations are the ones that are the most burdensome to patients and health-care services. A limitation of the TWICS trial is that the relatively conservative definition of exacerbation probably underestimates the frequency of symptom-defined mild exacerbations that are short-lived and treated by a patient with a temporary increase in bronchodilator therapy. 109 The identification of such mild exacerbations would have required participants to complete daily symptom diary cards, adding to the intrusiveness of the trial and considerably adding to the data-entry burden of research staff. Although the TWICS trial did not quantify mild exacerbations, there were no significant differences between the treatment and placebo arms in QoL/impact on health status as quantified by EQ-5D-3L/CAT, suggesting that either low-dose theophylline had no effect on mild exacerbations or, if there was an effect, it did not impact on health status/health-care usage.
A possible limitation of participant-reported exacerbations is the accuracy of such a report over a 6-month period. Although it would have been possible to obtain such exacerbation data from health-care records, it is well documented that people with COPD do not report all of their exacerbations to health-care professionals. 18,110–112 Patient recall of COPD exacerbations has been shown to be highly reliable over a year: in the London COPD cohort study,112 there was no significant difference between the number of exacerbations recorded on diary cards and patient estimates of their exacerbation number over the same 1-year period [mean 2.4 (SD 2.2) vs. mean 2.3 (SD 2.1)] and there was 93% agreement between patient-recalled and diary-recorded exacerbations. There was, however, a difference between the number of treated exacerbations recorded on diary cards and the number of treated exacerbations remembered by the patient over the same 1-year period [mean 2.3 (SD 2.1) vs. mean 1.8 (SD 1.8)] and there was 88.6% agreement between patient-recalled and diary-recorded treated exacerbations. 112 The patient representatives helping with the TWICS trial were adamant that it was fairly straightforward to recall the number of exacerbations over a 6-month period. A small validation exercise was conducted at two of the largest sites (Aberdeen and Aintree) during the TWICS trial to confirm that participant recall was indeed valid. The validation was done by requesting a care/encounter summary from the GP and comparing this with the participant report. In Aberdeen, 43 records (a 20% sample) were checked; in 37 there was complete agreement between the participant and GP reports. In Aintree, 24 records were checked and in 16 there was complete agreement between the patient and GP reports. Therefore, in a 4% sample of participants, there was 80% agreement. This rate of agreement was slightly lower than that reported by Quint et al. ;112 however, current GP records may not be as reliable a source of exacerbation data as in the past, given that patients have rescue packs at home and can access help for their exacerbations through many non-GP sources, for example pharmacies, emergency and walk-in centres, and accident and emergency departments.
A limitation of the TWICS trial was the proportion of participants who ceased taking their trial drugs (26%) was higher than anticipated (6%), although this was somewhat offset by 10% over-recruitment (n = 154). There was no evidence of bias in ceasing trial medication, with the proportion and the reasons given for ceasing trial medication being equally distributed between those allocated to low-dose theophylline and those allocated to placebo. The original sample size for the TWICS trial (n = 1424) accounted for 6% of participants ceasing their trial medication based on the four high-quality studies reported in a Cochrane review83 of theophylline in COPD, in which 3 out of 51 (6%) participants dropped out. In reality, 413 of the 1578 participants either never started/initiated medication (post-randomisation exclusions, n = 11; non-initiation, n = 8) or ceased taking the trial medication (non-persistence, n = 393). This 26% rate of ceasing trial medication is greater than anticipated, but in keeping with the ASSET trial of low-dose theophylline, which reported a 34% rate of ceasing trial medication. 44 The higher than anticipated rate of ceasing trial medication in the TWICS trial was most likely the consequence of the relatively high rates of comorbidities in participants, giving rise to symptoms that were attributed to the trial medication and a heightened awareness of ARs listed in the PIL and the package insert accompanying the trial medication. This is consistent with 46% of participants reporting ARs typical of high-dose theophylline (but equally distributed between the two trial arms) and why 20% of those ceasing trial medication gave gastrointestinal symptoms as the reason for ceasing trial medication, although there was no significant difference in the incidence of such symptoms in those ceasing low-dose theophylline and those ceasing placebo. Some participants were asked to discontinue trial medication because they had stopped taking an ICS. During the trial, there was an emergent change in prescribing practice away from ICS-containing preparations to LABA/LAMA inhalers; however, this had minimal impact on the trial (certainly < 20 participants), most probably because the participants in this trial were at high risk of exacerbation and were participants in whom there is still a role for ICSs. Although 413 participants ceased trial medication during the TWICS trial, a review of the medication returns indicated that 66 of these participants had > 70% adherence while taking the trial medication when averaged over the 12-month treatment period; for example, they ceased trial medication at 11 months. These individuals were included in the per-protocol analysis. Although per-protocol analyses are biased by their very nature, the per-protocol analysis for this trial included 1142 years of participant data (85% of the 1338 years indicated by the power calculation); therefore, it is not surprising that the results of the per-protocol analysis were almost identical to those of the ITT analysis. Although adherence to the trial medication was quantified through pill counting, it was not practicable to assess adherence to the ICS, as this would have entailed use of non-routine care methodologies such as diaries cards, metered inhalers, etc. The rationale for the use of low-dose theophylline is as an adjunct to ICS therapy; that we were unable to verify adherence to ICS therapy is a limitation of this trial.
Generalisability
This trial has good external validity as it was of a pragmatic design that reflected normal clinical practice across both primary and secondary care in the UK. Participants remained on their existing COPD medications, they were managed in the normal way by their usual health-care teams and the trial recruited from 121 sites (88 primary care and 33 secondary care) that spanned the UK; many of the secondary care sites were district general hospitals. We consider it to be highly probable that the TWICS trial participants are typical of COPD patients in normal clinical practice across both primary and secondary care in the UK and that the findings are generalisable to clinical practice in the UK.
The TWICS trial recruited participants who were highly likely to experience an exacerbation during the 1-year treatment period, as evidenced by the fact that they had experienced two or more treated exacerbations in the previous year. In contrast to many COPD trials, we did not exclude potential participants with mild COPD, as evidenced by FEV1 of > 80% predicted; 9% of the TWICS trial participants had mild COPD based on spirometry criteria, but fulfilled the frequent exacerbator phenotype,21 enhancing the generalisability of the trial. Recruitment to the TWICS trial was limited to frequent exacerbators because, in clinical practice, these are the patients who are usually commenced on this ‘third-line’ therapy;24 moreover, a trial of participants less likely to experience exacerbation (e.g. those experiencing one exacerbation in the previous year) would have been much larger (n = ≈3000) and somewhat more costly. Although we did not test whether or not the addition of low-dose theophylline to ICSs had an effect on people who experienced less frequent exacerbations, there is no scientific or clinical reason why low-dose theophylline should have a differential effect according to the frequency of exacerbations. Therefore, it would seem reasonable to extend the findings of the current study to people with COPD who are at a low risk of experiencing an exacerbation.
Although the results of this trial are generalisable to the UK and probably to other high-income countries, the findings may not be applicable to low- or medium-income countries, with differing pharmacogenetic profiles, where theophylline remains a frequently used therapy in COPD, most probably because it is inexpensive compared with inhaled therapies. 113–116 The randomised, double-blind, placebo-controlled trial of Zhou et al. 117 raises the possibility that, in China at least, there is a therapeutic response to low-dose theophylline in the absence of ICS. In that trial,117 the addition of low-dose theophylline to usual COPD treatment in 110 people with COPD (theophylline, n = 57; placebo, n = 53) for a year significantly reduced the frequency of exacerbations when compared with placebo [mean 0.79 (SD 1.16) vs. 1.70 (SD 2.61); p = 0.047]. The participants in that trial117 differed considerably from those taking part in the TWICS trial: only 30% were taking regular medication prior to the trial, and this was restricted to inhaled salbutamol; use of ICS, LABA and LAMA was excluded; and the target plasma theophylline concentration (5–10 mg/l) was also somewhat higher than the target range (1–5 mg/l) identified for optimum synergistic interaction between corticosteroids and low-dose theophylline. The use of low-dose theophylline in conjunction with corticosteroids in China is being addressed by the ongoing Theophylline And Steroids in COPD Study (TASCS), which is recruiting 2400 people with COPD in China. 118 They are being randomly allocated to low-dose prednisolone (5 mg od) or low-dose theophylline (100 mg bd) with low-dose prednisolone (5 mg od) for 48 weeks. The primary outcome is exacerbation rate over the 48-week treatment period. This study has been presented in abstract form119 and demonstrated that the combination of low-dose theophylline and prednisolone did not reduce the annualised rate of exacerbations. It will be interesting to compare the results of TASCS with the TWICS trial, although it should be noted that the routine use of OCSs as a maintenance treatment for COPD, even though they are cheaper than ICS, would never be contemplated in developed countries for clinical and ethical reasons.
Patient and public involvement
Patient and public involvement in this trial was limited, but effective, and lessons were learnt that have been implemented in a subsequent study funded by the NIHR Health Technology Assessment (HTA) programme (reference 15/130/20),120 for example a person with COPD is a joint grant holder in that study.
A patient with COPD was a voting member of the TWICS trial TSC; recruitment and retention of a patient representative was hindered by ill health. The first patient approached declined because of ill health. The patient representative nominated by CHSS as part of their Voices Scotland initiative had to resign because of ill health. A third patient representative was identified and he made an active contribution to the TWICS trial. Support of the TSC patient representative was actively undertaken by several members of the local study team. In the subsequent trial funded by the NIHR HTA programme,120 we have a patient representative who is supported by CHSS’s Voices Scotland lead who is not only a voting member of the TSC, but also co-ordinator and representative of a panel of 15 COPD patients (as they like to be called).
A representative of the British Lung Foundation and a person with COPD made important contributions to trial design procedures [what was acceptable (e.g. spirometry) and what was not acceptable (e.g. daily diary cards)]. Perhaps the most important suggestions were to deliver trial medication to home addresses and to facilitate follow-up for ill participants by way of home visits and telephone and postal questionnaires. PPI resulted in many changes to the design and content of the ‘short’ PIL (a one-page summary PIL) and the ‘long’ PIL (a more detailed PIL). The importance of these changes is evidenced by the success in recruitment, and there were no changes to the PIL throughout the trial. PPI was particularly insightful during TSC deliberations concerning the validity of patient recall of COPD exacerbations.
The support of the British Lung Foundation and CHSS has been invaluable throughout the trial, identifying volunteers for PPI and publicising the trial.
Conclusions
Main conclusions
To our knowledge, this is the first adequately powered, multicentre, pragmatic, double-blind, randomised, placebo-controlled trial to assess the effectiveness of adding low-dose theophylline to a drug regimen containing ICSs in people with COPD who are at a high risk of exacerbation. The analyses demonstrated that low-dose theophylline has no overall clinical or health economic benefit.
Implications for practice
The trial has shown that low-dose theophylline has no overall clinical impact when added to ICSs in COPD. We anticipate that the results of the trial will be incorporated in an ongoing systematic review of theophylline in COPD. 121 Given that the TWICS trial is one of the largest trials of theophylline to date, we anticipate that it will have a major influence on the meta-analyses and conclusions. National and international COPD guidelines should take the results of the TWICS trial into account when making recommendations on the treatment of COPD and the prevention of exacerbations of COPD. In the meantime, clinical commissioners can now be encouraged to make informed decisions regarding the use theophylline in COPD.
Recommendations for research
The findings from one of the planned secondary analyses was that low-dose theophylline reduces the rate of admission to hospital due to severe COPD exacerbation. Although it is possible that this may be a chance finding, it is consistent with a recent report96 that roflumilast is most beneficial in people with prior COPD hospitalisation for exacerbation and greater exacerbation frequency. A further study investigating the effect of low-dose theophylline in people with COPD who frequently exacerbate and are admitted to hospital is justifiable, given the disproportionate impact on NHS resources of such exacerbations.
Acknowledgements
We would like to thank all the participants who took part in the trial. We are grateful to all the staff at recruitment sites who facilitated identification, recruitment and follow-up of study participants (listed below). We are also grateful to other general practices and organisations that acted as PICs for the trial and practices that provided outcome data for trial participants who were unable to attend for follow-up. We could not have completed the trial without the ongoing support of local and primary care research networks:
-
NHS Research Scotland Primary Care Network (formerly Scottish Primary Care Research Network) – Amanda Cardy, Samantha Holden, Tracy Ibbotson, Yvonne McIlvenna, Marie Pitkethly, Janice Reid, Kim Stringer
-
North of England Commissioning Support (NECS) – Jeanette Dixon, Jill Ducker, Shona Haining, Gillian Johnson, Rachel Nixon, Norah Phipps, Cheryl Rigg
-
NIHR CRN South West Peninsula – Cate Atkins, Helen Clough, Tania Crabb, Patricia Hollway, Sara McNamara, Lisa Treeby, Lorraine Underwood
-
NIHR CRN Eastern – Lynne Baker, Brenda DeBoys, Kim Fell, Fenglin Guo, Emily Ikelle, Helen Jung, Heather Leishman, Rachel Lister, Lynn Mather, Cristina Page, Barbara Stewart
-
NIHR CRN Wessex Primary Care – Christine Brown
-
NIHR CRN Yorkshire and Humber – Carla Bratten
-
NIHR CRN North Thames – Mandy Austin, Carole Bartlett, Carol Keel, Helen McIver, Lucy Peppiatt.
We thank Nadia Lewis-Burke for invaluable assistance in data checking. We are grateful to Georgia Mannion-Krase, Andrea Fraser and Lana Mitchell for their secretarial and data co-ordination support. We are grateful to Kirsty McCormack for her help and advice in developing the grant proposal. We thank Gladys McPherson, Mark Forrest and the programming team in CHaRT for developing and maintaining the trial website. We also thank Juliette Snow, Ruth Speedie and Rachel West for their help with contracting, and Louise Cotterell and Glenys Milton for their help in managing the budget.
We are grateful for the guidance and support of the TSC (chairperson: Bill McNee; independent members: Matt Sydes, Mike Thomas, Alister Laird, Marion Middler) and the DMC (chairperson: Hilary Pinnock; independent members: Chris Weir, Michael Steiner). We are also grateful to Bev Wears (British Lung Foundation) and Jacqueline Waters for helpful comments on early drafts of the trial documentation.
We acknowledge Napp Pharmaceuticals Ltd for providing the trial drug (Uniphyllin, 200-mg modified-release tablets) free of charge for use in the trial.
The Health Services Research Unit and the Health Economics Research Unit are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Staff at recruitment sites
| Site | Staff |
|---|---|
| Secondary care | |
| Aberdeen Royal Infirmary | Ratna Alluri, Faye Annison, David Christie, Michael Christie, Patricia Cooper, Lisa Davidson, Graham Devereux (PI), Margaret Fernie, Vicki Fraser, Amber Johnson, Alison McKay, Celia Meneses, Joy Miller, Beth Robb and Catriona (Tina) Stewart |
| Aintree University Hospital NHS Foundation Trust | Lisa Davies (PI), Nicola Blain, Victoria Hankin, Ben Huson Vlies, Nadia Lewis-Burke, Laura O’Neil, Rachel Powell, Jamie Rylance, Rebecca Tagney, Diane Wood and Dan Wootton |
| Belfast City Hospital | Peter Gray, Kathryn McDowell, Lorcan McGarvey (PI), Jolene Milligan and Brian Wells |
| Queen Elizabeth Hospital Birmingham | Karen Boardman, Joanne Dasgin, Simon Gompertz (PI), Carole Green, Diane Griffiths, Melanie Gunn, Catherine Jones, Salma Kadiri, Heena Khiroya, Emma Low, Rahul Mahida, Mitesh Patel, Sarah Raybould, Julie Richards, Gurpreet Sangha, Elizabeth Sapey, Lydia Sexton, James Stockley, Anita Sullivan (PI), David Thickett, Rebecca Tongue and the NIHR Wellcome Trust Clinical Research Facility, Birmingham |
| Blackpool Victoria Hospital | Charlotte Armer, Adeel Ashraf, Oliver Brennan, Melanie Caswell, Julie Chapman, Stacey Donaldson, Mohamed Etumi, Julie Frudd, Gemma Hatton, Aoife Lillis, Alison Mackle, Karen Pollard, Andrew Potter, Judith Saba, Tarek Saba (PI), Gurkaran Samra, Philomena Shooter and Suzannah Torres |
| Bradford Royal Infirmary | Abid Aziz, Fahtima Begum, Stephen Cox, Umair Hamid, Rizwana Kausser, Leslie Masters, Sujie Mogane, Nabeela Nazir-Ahmed, Karen Regan, Dinesh Saralaya (PI), Kimberley Walker, Laura Walker and Helen Wilson |
| Queen’s Hospital, Burton Hospitals NHS Foundation Trust | Ann Adams, Mosan Ashraf, Gillian Bell, Julie Birch, Elizabeth Kemp, Clare Mewies, Uttam Nanda (PI), Mandy Oakley, Alison Tilley, Louise Wilcox and Clare Williams |
| Calderdale Royal Hospital, Huddersfield Royal Infirmary, Calderdale & Huddersfield NHS Foundation Trust | Annika Graham, Andrew Hardy, James Harris, Alan Hart-Thomas, Lisa Horner, Adam Mawer, Rehan Naseer (PI), Sabiha Ravat, Simone Ryan, Kuljinder Sandhu, Christine Turner and Tracy Wood |
| University Hospital of North Durham | Sarah Clark, Peter Cook (PI), Andrea Kay, Richard Nendick, Neil Munro, Kathryn Potts, Lynsey Stephenson, Anne Sebakungu and Julie Temple |
| Lister Hospital (East and North Hertfordshire) | Hannah Beadle, Kelly Chan, Katie Chong, Angela Cook, Carina Cruz, Sura Dabbagh, Pippa de Sousa, Sunita Gohil, Jodie Graham, Alison McMillan, Victoria Oliver, Mahul Patel, Louise Peacock, Anita Rana, Natalie Rahim, Emma Shinn and Thida Win (PI) |
| Victoria Hospital, Kirkcaldy | Julie Aitken, Sarah Aitken, Laura Beveridge, Keith Boath, Rebecca Cain, Devesh Dhasmana (PI), Sabha Khan, Maria Simpson and Athan Tachtatzis |
| Freeman Hospital, Newcastle | Nicholas Aitken, Angela Bailey, Marion Brooks, Jamie Brown, Gareth Davies, Jade Davison, Margaret Day, Anthony De Soyza (PI), Hazel Douglas, Maureen Foreman, Ben Hood, Rebecca Johnson, Gerry Jones, Karen Martin, Donna McEvoy, Yoko Okada, Jack Oliver, Leeanne Ratcliffe, Sarah Robertson, Therese Small, Graham Soulsby, Julie Stephenson, Hesther Wilson and Sarah Woolcock |
| Glasgow Hospitals (Gartnavel, Glasgow Royal, Southern General, Victoria Infirmary, Western Infirmary) | Jacqueline Anderson, Lindsey Bailey, Anne Benson, Joan Blevings, Christine Bucknall, Rekha Chaudhuri (PI), Brian Choo-Kang, Patricia Clark, Douglas Cowan, Elizabeth Douglas, Tracyanne Grandison, Sharon Grant, Helen Hamilton, John Haughney, June Innes, Jane Lafferty, Nicola Lee, Audrey Lush, Margaret McFadden, Kirsty McLeish, Alison Martin, Lyndsey Meenaghan, Karen Montgomery, Helen Mulholland, Diane Murray, Dominic Rimmer, Colin Rodden, Deborah Stubbings, Joyce Thompson and Nicola Thomson |
| Castle Hill Hospital, Hull | Kayleigh Arnell, William Beswick, Margaret Crookes, Michael Crooks, Laura Douglas, Helen Fowles, Simon Hart, Rhian Horne, Joseph Howard, Victoria Lowthorpe, Alyn Morice (PI), Jackie Mower, Zainab Rai, Susannah Thackray-Nocera, Rachel Thompson, Adam Wolstencroft and Sara Wynn |
| Raigmore Hospital, Inverness | Fiona Barrett, Jim Finlayson, Laura O’Keeffe, Debbie McDonald, Mary McKenzie, Lorna Murray (PI), Gordon Rushworth and Donna Patience |
| University Hospital Wishaw | Angela Brown, Craig Chalmers, Steven Marshall, Louise McGee, Donna Orr, Manish Patel, Fiona Ross and Andrew Smith (PI) |
| Royal Lancaster Infirmary | Mark Wilkinson (PI), Laura Booth, Jayne Craig, Jade Drew, Tim Gatheral, Rebecca Jeffery, Jane Ritchie, Vickie Rose and Andrew Taylor |
| Leighton Hospital, Crewe | Kelly Amor, Duncan Bailey, Christopher Brockelsby, Duncan Fullerton (PI), Nikki Gautam, Gareth Jones, Taya Jones, Syed Kazmi, Diana Lees, Emma Margerun, Julie Meir, Richard Miller, Andy Ritchings and Sarah Tinsley |
| Musgrove Park Hospital | James Allen, Korinna Andrews, Simon Barnes, Oliver Bintcliffe, Eliza Foster, Sarah Foster, Yvonne Moul, Justin Pepperell (PI), Dawn Redwood, Joy Rowe, Dinesh Shrikrishna and Tania Wainwright |
| Norfolk and Norwich University Hospital | Chris Atkins, Mark Baxter, Claire Brockwell, Melissa Crofts, Samantha Fulcher, Gail Heally, Carla Holloway, Divya Jacob, Sanjana Kamath, Jalpa Kotecha, Sue Robinson, Clare Self and Andrew Wilson (PI) |
| University Hospital of North Tees | Nicola Bateman, June Battram, Helen Carey, Julia Fuller, Richard Harrison (PI), Claire Irish, Graham Miller (PI), Lynda Poole, Ben Prudon, Angela Scott-Johnson, Gillian Wallace and Bill Wetherill |
| City Hospital, Nottingham | Tim Harrison (PI), Wendy Gerrard-Tarpey, Sheila Hodgson, Matthew Martin and Catherine Reynolds |
| Derriford Hospital, Plymouth | Julie Alderton, David Derry, Sharon Freeman, Jacinta Hardman, Maggie Kalita, Jennie Kingdon, Mike Marner, Tracy Mynes, Joanne Porter, Judy Sercombe, Caroline Snelgrove, Elizabeth Swanson, Trudy Turner, Neil Ward (PI), Jacqueline Westcott, Gloria Wong and Parag Yajnik |
| South Tyneside District Hospital | Amy Burns, Barrie Duncan, Nadia Elkaram, Liz Fuller (PI), Ben Hood, Paula Madgwick, Claire McBrearty, Sinead McHugh, Rachel Miller, Judith Moore, Asif Shah, Mark Shipley, Ruth Tindle and Michael Walton |
| Torbay Hospital | Gabrielle de Selincourt, Lee Dobson (PI), Lesley Evans, Bianca Hulance, Sally Maddison, Pauline Mercer, Sarah Mills, Andrew Mullinger, Hannah Shiels, Melanie Stone, Natalie Taylor, Christine Tsang, Amanda Vian and Sarah Wright |
| New Cross Hospital, Wolverhampton | Richard Carter, Kay Cash, Lee Dowson (PI), Ahmed Fahim, Clare Hammond, Kelly Kauldhar, Baljinder Kaur, Jonathan Mann, Sarah Milgate, Angela Morgan, Jaynesh Patel, Elizabeth Radford, Gurminder Sahota, Lucy Stelfox, Trevor Thompson and Helen Ward |
| Worcestershire Royal Hospital | Sarah Deacon, Alison Durie, Monica Gauntlett, Kim MacDonald, Terry Martin, Hugh Morrow, Stephen O’Hickey (PI), Heather Perry, Zee Shaan Parvez and Ann White |
| Yeovil District Hospital | Joanna Allison, Sarah Board, Clare Buckley, Sarah Debruijn, Dave Donaldson, Tracey Duckett, Adam Edwards, Alison Lewis, Tressy Pitt-Kerby, Rejendra Sinha (PI), Thikra Al Wattar (PI), Jodhi Wilson and Diane Wood |
| York Hospital, York Teaching Hospital NHS Foundation Trust | Andrew Atherton, Judith Bell, Claire Brookes, Poppy Cottrell, Cheryl Donne, Mark Elliot, Christopher Emms, Richard Evans, Caroline Everett, Mark R Fearnley, Monica Haritakis, Yvonne McGill, Heidi Redfearn, Davina Smith, Mandy Ward, Jacqueline Westmoreland, John White (PI), John Wightman, Paul Wood and Lorraine Wright |
| East of England primary care | |
| Alconbury & Brampton Surgeries | Melanie Fowler, Alyssa Lawford, Duncan Outram (PI) and Caroline Ward |
| Alexandra & Crestview Surgeries | James Atkins (PI), Christina Easter and Barbara Stewart |
| Andaman Surgery | Jane Atkins, Mark Butt (PI), Sarah Butt, Hitesh Kumar, Sue Lock and Laverne Rose |
| Attleborough Surgeries | Sabrina Khalaque (PI), Ruth Mallinson, Lucy McLean and Paul Roebuck |
| Beccles Medical Centre | Kathleen Archer, Charlotte Hawkins (PI), Monica Kettlewell, Julia McLean, Sarah McLennan, Vasilica Munteanu and Charlene Wakefield |
| Bridge Road Surgery | Martin Aylward (PI), Carolyn Harper, Eleanor Schofield, Nicola Shea and Sue Vigus |
| Bridge Street Medical Centre (Cambridge) | Corinne Bakker (PI) and Louise Norman |
| Bridge Street Surgery (Downham Market) | Clare Hambling (PI), Barbara Stewart and Megan Winterbone |
| Campingland Surgery | Mark Holmes (PI), Tracey Sharp, Maxine Smith and Liz Wing |
| Castle Partnership | Penny Atkinson, Richard Gilbert (PI) and Jo Walsh |
| Coltishall Medical Practice | Alison Melton, Angela Norton, Rajesh Selvam, Michele Taylor and Neil Taylor (PI) |
| Comberton and Eversden Surgeries | Will Bailey, Janice Mills and Ian Parker (PI) |
| Cutlers Hill Surgery | Claire Craik (PI), Sarah Caplin and Daniel Treen |
| Davenport House | Jenny Hughes, Anthea Doran and Chas Thenuwara (PI) |
| De Parys Medical Centre | Carolyn Boyd, John Goudling (PI) and Linda Lomax |
| East Norfolk Medical Practice | Liam Steven (PI), Lisa Matcalfe and Maxine Burton |
| Elizabeth Courtauld Surgery | Ali Alsawaf (PI), Sue Cole, Daniela Kreis-Alsayed, Phillipa Oval, David Sneddon and Jeanette Williams |
| Gorleston Medical Centre | Ann Abbott, Dawn Barnham, Lorraine Farrier and Sunder Gopaul (PI) |
| Greyfriars Medical Centre | Patrick Frew (PI), Katrina Kelly, Krystal Lewis-McDonald, Tara Maher and Stephanie Timberlake |
| Harvey Group Practice | Carolyn Downs and Matt Parfitt (PI) |
| Holt Medical Practice | Peter Franklin (PI) and Annie Hughff |
| Hoveton & Wroxham Medical Centre | Carsten Dernedde (PI), Caroline Mansfield and Chris Wright |
| Linton Health Centre | Hayley Haworth, Laurence Kemp (PI), Claire Wade, Donna Watson and Fiona Wharton |
| Long Stratton Medical Partnership | Caroline Dear, Carol Gubby, Helen Mingaye and Mini Nelson (PI) |
| Ludham & Stalham Green Surgeries | Jessica Bane, Elizabeth Christie (PI), Tracey Edwards, Emma Lambon and Jennifer Liu |
| Mount Farm Surgery | Claire Giles (PI), Brian Ainsworth, Julie Friend and Peter Knights |
| Mundesley Medical Centre | Daryl Freeman (PI), Holly Fulcher, Carol Manson, India Mills and Jessica Payne |
| Nuffield Road Medical Centre | Tom Alderson (PI), Janette Bone, Jacqueline Day, Helen Jung and Sally Kaemer |
| Orchard Surgery, Dereham | Dawn Boyce, Stacey Hawkins, Jillian Pewtress, Vanaja Santosh (PI) and Barbara Stewart |
| Peninsula Practice | Lindsey Crockett (PI), Linda Deabill and Ruth Osborne |
| Portmill Surgery | Jehad Aldegather (PI) and Lynne Shoebottom |
| Rosedale Surgery | Amanda Ayers, Jodie Button and Maarten Derks (PI) |
| Roundwell Medical Centre | Chaminda Dooldeniya (PI), Tess Cantan, Denise Steward and Kirsti Withington |
| Salisbury House Surgery | Yasar Khan (PI), Mehar Singh (PI), Carol Bunting, Helen Ingle, Sally Szuca and Paul Vogwell (PI) |
| Sheringham Medical Practice | Pauline Craske, Susan Lees, Ian Smith (PI), Julie Sterry and Nikita Williamson |
| Spinney Surgery | Gill Avery, Reyny Rahman (PI) and Debra Wheatley |
| St Stephens Gate Medical Practice | Frances Scouller (PI), Matthew Butler and Loraine Leggett |
| St Johns Surgery (Terrington) | Susan Atcheson (PI), Barbara Bruce, Jane Coston and Charlotte Walford |
| Staithe Surgery | Diana Hood (PI), Kate Bywater, Sylvia Jackson, Sue Perrott and Sally Ross-Benham |
| The Over Surgery | Lesley Bowring, Judith Davis (PI) and Andrew Kennedy |
| Trinity & Bowthorpe Medical Practice | Gillian Denman, Xanthe Dunthorne and Helene Simper (PI) |
| Vida Healthcare | Ademola Adesanoya (PI), Felicity Bowerman, Audrey Brown, Janeen Henshaw, Lata Motwani and Amanda Pearson |
| Wells Health Centre | Gordon McAnsh (PI), Lisa Palmer and Jan Wright |
| Wellside Surgery | Jacqueline Martindale, Ian Williams (PI) and Anita Willis |
| Woodhall Farm Medical Centre | David Adams, Winnie Chiu, Khalid Mirza (PI) and Lucy Peppiatt |
| Woolpit Health Centre | Jenny Johnson, Karen Norcott, Ruth Osborne, William Smith and Richard West (PI) |
| Wymondham Medical Centre | Louanne Gault, Karen Hamer, Shelina Rajan and Stephen Thurston (PI) |
| York Street Medical Practice | Alistair Brown (PI), Helen Radlett and Stuart Thorpe |
| North of England primary care | |
| Beacon View Medical Centre | Vinod Kumar (PI) and Alison McElvoy |
| Beaumont Park Medical Group | Jill Ducker and Angela McMenzie (PI) |
| Belford Medical Practice | Maureen Birdsall and Sebastian Moss (PI) |
| Bellingham Practice | Jill Ducker and Andrew Sewart (PI) |
| Benfield Park Medical Centre | Valerie Walker and Sian Williams (PI) |
| Burn Brae Medical Group | Anthea Adamson, Louise Chicken, Eleanor Gallagher, Nick Hargreaves (PI) and Alison McClintock |
| Castlegate & Derwent Surgery | Jeanette Dixon, Mary Philipsz (PI), Barbara Robinson and Jackie Smith |
| Corbridge Medical Group | Janet Drinkwater, Jill Ducker, Sally Parkin (PI), Neil Stanley and Anna Townsend-Rose |
| Elvaston Road Surgery | Barbara Bailey, Stephen Hilton and Rachel Nixon |
| Fell Cottage Surgery | Rachel Nixon, Cheryl Rigg and Katherine Woodcock (PI) |
| Grove Medical Group | Alison Carlyle, Guy Clement (PI), Jill Ducker, Ann Hately, Cheryl Rigg and Hannah Smith |
| Guidepost Medical Group | Catherine Bromham (PI), Geraldine Richelle, Sue Rowlands and Geert Van Zon (PI) |
| Haltwhistle Medical Group | Sarah Davies (PI) and Sarah Speed |
| Haydon Bridge & Allendale Medical Practice | Mary Douthwaite, Elaine Fiori, Emily Hadaway (PI) and Mary Henderson |
| Hetton Group Practice | Julia Cook (PI), Jill Ducker, Judith Kirk and Rachel Nixon |
| Humshaugh & Wark Medical Group | Christine Counsell, Katherine Dixon, Louise Shearer and Hayley Wright (PI) |
| Marine Avenue Surgery | Ann Grieves and Justine Norman (PI) |
| Maryport Health Services | Ross Anderson (PI), Janice Cox, Jeanette Dixon and Janet Rasburn |
| Priory Medical Group | Andrew Duggan (PI), Jill Ducker, Tracey Pearson and Christine White |
| Prudhoe Medical Group | Michelle Orton, Margaret Ross and Helen Thornton (PI) |
| Seaton Park Medical Group | Aileen Rose and Emily Watson (PI) |
| Sele Medical Practice | Jill Ducker, Ben Frankel (PI) and Julie Smith |
| Temple Sowerby Medical Practice | Jeanette Dixon and Helen Jervis (PI) |
| The Village Surgery | Jill Ducker, Simon Hartland and Linda Thompson (PI) |
| Waterloo Medical Group | Marie Imlach (PI) and Elaine Sansom |
| West Farm Surgery | Christine Davidson, Kate Grisaffi (PI) and Sally Morrison |
| South West England primary care | |
| Barton Surgery | Elizabeth Alborough (PI), Paula Brison and Ruth Christophers |
| Bovey Tracey & Chudleigh Practice | Carol Gubby, Rachael Minty, Daniel Thomas and Ben Ward (PI) |
| Brunel Medical Practice | Pamela Grills, Rayindra Naidoo, Lisa Van Kuyk and Richard Veale (PI) |
| Claremont Medical Practice | Kevin Douglas (PI), Beth Hawkes, Sonya McGill and Lucinda Ralph |
| Coleridge Medical Centre | Nigel De-Sousa (PI), Jane Stewart and Stacy Wilson |
| Helston Medical Centre | Gary Crocker, Linda Davies (PI) and Linda Quinn |
| Ide Lane Surgery | Jackie Barrett, Jackie Crossman, Stephen Vercoe (PI) and Rachel Winder |
| Petroc Group Practice | Philippa Haywood, Nicholas Jacobsen (PI), Alison Murton, Rebecca Nicholls, Martin Priest and Kirsty Rogers |
| Richmond House Surgery | Karen Bates (PI), Mary Guest, Sara McNamara, Kathy Polverino and Claire Southgate |
| Rolle Medical Partnership | Merilyn Green, Barbara Welch and William Willcock (PI) |
| Westlake Surgery | Jo Jones, Calli Smith and Lindsay Smith (PI) |
| Wessex primary care | |
| Friarsgate Practice | Tara Clark, Stephen Fowler (PI), Claire Hallett and Elaine Spellerberg |
| Park and St Francis Surgery | Amy Glanville, Natasha Campbell, Samuel Glanville, Jo King, Mark Rickenbach (PI) and Clare Sharland |
| Swanage Medical Centre | Claire Hombersley (PI), Natasha Ritchie and Sara Ward |
Contributions of authors
Graham Devereux contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, interpretation of results and writing/editing the report.
Seonaidh Cotton contributed to the design of the trial, was responsible for the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Shona Fielding contributed to the design of the trial, was responsible for statistical analysis and contributed to the interpretation of results and writing/editing the report.
Nicola McMeekin was responsible for the health economic analysis and contributed to the interpretation of results and writing/editing the report.
Peter J Barnes contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
Andy Briggs contributed to the conception and design of the trial, oversaw the health economic analysis and contributed to the interpretation of results and writing/editing the report.
Graham Burns contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
Rekha Chaudhuri contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Henry Chrystyn contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
Lisa Davies contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Anthony De Soyza contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Simon Gompertz contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
John Haughney contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Karen Innes was responsible for aspects of the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Joanna Kaniewska was responsible for aspects of the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Amanda Lee contributed to the conception and design of the trial, oversaw the statistical analysis and contributed to the interpretation of results and writing/editing the report.
Alyn Morice contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
John Norrie contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of results and writing/editing the report.
Anita Sullivan contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Andrew Wilson contributed to the conception and design of the trial, conduct of the trial, recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
David Price contributed to the conception and design of the trial, the conduct of the trial, the interpretation of the results and writing/editing the report.
Publication
Devereux G, Cotton S, Barnes P, Briggs A, Burns G, Chaudhuri R, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials 2015;16:267.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of COPD 2017. https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd (accessed March 2018).
- British Lung Foundation . Chronic Obstructive Pulmonary Disease (COPD) Statistics n.d. https://statistics.blf.org.uk/copd (accessed March 2018).
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet 2007;370:741-50. https://doi.org/10.1016/S0140-6736(07)61377-4.
- Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J 2001;17:1024-33. https://doi.org/10.1183/09031936.01.17510240.
- Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, et al. American Thoracic Society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003;167:787-97. https://doi.org/10.1164/rccm.167.5.787.
- Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD 2012;9:216-26. https://doi.org/10.3109/15412555.2011.648030.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962-9. https://doi.org/10.1183/09031936.00012408.
- Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005-11. https://doi.org/10.1378/chest.128.4.2005.
- Antonelli Incalzi R, Fuso L, De Rosa M, Forastiere F, Rapiti E, Nardecchia B, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997;10:2794-800. https://doi.org/10.1183/09031936.97.10122794.
- Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005;26:1887-94. https://doi.org/10.1093/eurheartj/ehi291.
- Macchia A, Rodriguez Moncalvo JJ, Kleinert M, Comignani PD, Gimeno G, Arakaki D, et al. Unrecognised ventricular dysfunction in COPD. Eur Respir J 2012;39:51-8. https://doi.org/10.1183/09031936.00044411.
- Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004;27:2478-84. https://doi.org/10.2337/diacare.27.10.2478.
- Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med 2003;114:10-4. https://doi.org/10.1016/S0002-9343(02)01297-4.
- Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med 2006;100:1767-74. https://doi.org/10.1016/j.rmed.2006.01.026.
- Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data from the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003;163:1475-80. https://doi.org/10.1001/archinte.163.12.1475.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. https://doi.org/10.1136/thorax.57.10.847.
- Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:446-52. https://doi.org/10.1164/rccm.200408-1054OC.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. https://doi.org/10.1164/ajrccm.157.5.9709032.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. https://doi.org/10.1136/thx.2005.040527.
- McAllister DA, Maclay JD, Mills NL, Leitch A, Reid P, Carruthers R, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J 2012;39:1097-103. https://doi.org/10.1183/09031936.00124811.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. https://doi.org/10.1056/NEJMoa0909883.
- Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB. Respiratory Effectiveness Group . Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis 2015;10:2439-50. https://doi.org/10.2147/COPD.S94259.
- Department of Health and Social Care (DHSC) . An Outcomes Strategy for COPD and Asthma 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/216531/dh_134001.pdf (accessed March 2018).
- National Institute for Health and Care Excellence (NICE) . Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management 2010. www.nice.org.uk/guidance/cg101 (accessed March 2018).
- Ståhl E, Lindberg A, Jansson SA, Rönmark E, Svensson K, Andersson F, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes 2005;3. https://doi.org/10.1186/1477-7525-3-56.
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar MC, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med 1997;127:1072-9. https://doi.org/10.7326/0003-4819-127-12-199712150-00003.
- Britton M. The burden of COPD in the UK: results from the Confronting COPD survey. Respir Med 2003;97:71-9. https://doi.org/10.1016/S0954-6111(03)80027-6.
- NHS Digital . Hospital Episode Statistics 2016. http://content.digital.nhs.uk/searchcatalogue?topics=2%2fHospital+care%2fAdmissions+and+attendances%2fInpatients&sort=Relevance&size=10&page=1#top (accessed March 2018).
- Price D, Miravitlles M, Pavord I, Thomas M, Wedzicha J, Haughney J, et al. First maintenance therapy for COPD in the UK between 2009 and 2012: a retrospective database analysis. NPJ Prim Care Respir Med 2016;26. https://doi.org/10.1038/npjpcrm.2016.61.
- Gruffydd-Jones K, Brusselle G, Jones R, Miravitlles M, Baldwin M, Stewart R, et al. Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: in UK primary care: a real-world study. NPJ Prim Care Respir Med 2016;26. https://doi.org/10.1038/npjpcrm.2016.2.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. https://doi.org/10.1056/NEJMoa063070.
- Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 2004;363:731-3. https://doi.org/10.1016/S0140-6736(04)15650-X.
- Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J 2013;22:92-100. https://doi.org/10.4104/pcrj.2012.00092.
- Price DB, Russell R, Mares R, Burden A, Skinner D, Mikkelsen H, et al. Metabolic effects associated with ICS in patients with COPD and comorbid type 2 diabetes: a historical matched cohort study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0162903.
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1635-9. https://doi.org/10.1164/ajrccm.160.5.9811058.
- Culpitt SV, Rogers DF, Shah P, De Matos C, Russell RE, Donnelly LE, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:24-31. https://doi.org/10.1164/rccm.200204-298OC.
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med 2002;165:1592-6. https://doi.org/10.1164/rccm.2105025.
- Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 2000;20:6891-903. https://doi.org/10.1128/MCB.20.18.6891-6903.2000.
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967-76. https://doi.org/10.1056/NEJMoa041892.
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 2006;203:7-13. https://doi.org/10.1084/jem.20050466.
- Barnes PJ. Targeting the epigenome in the treatment of asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:693-6. https://doi.org/10.1513/pats.200907-071DP.
- Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, et al. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med 2009;179:542-8. https://doi.org/10.1164/rccm.200810-1570OC.
- Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, et al. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci USA 2002;99:8921-6. https://doi.org/10.1073/pnas.132556899.
- Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med 2004;200:689-95. https://doi.org/10.1084/jem.20040416.
- Sun X, Li Q, Gong Y, Ren L, Wan H, Deng W. Low-dose theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammation. Can J Physiol Pharmacol 2012;90:895-902. https://doi.org/10.1139/y2012-079.
- To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:897-904. https://doi.org/10.1164/rccm.200906-0937OC.
- Perng DW, Su KC, Chou KT, Wu YC, Chen CS, Hsiao YH, et al. Long-acting β2 agonists and corticosteroids restore the reduction of histone deacetylase activity and inhibit H2O2-induced mediator release from alveolar macrophages. Pulm Pharmacol Ther 2012;25:312-18. https://doi.org/10.1016/j.pupt.2012.04.001.
- Cosio BG, Iglesias A, Rios A, Noguera A, Sala E, Ito K, et al. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax 2009;64:424-9. https://doi.org/10.1136/thx.2008.103432.
- Ford PA, Durham AL, Russell RE, Gordon F, Adcock IM, Barnes PJ. Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest 2010;137:1338-44. https://doi.org/10.1378/chest.09-2363.
- Cyr MC, Beauchesne MF, Lemière C, Blais L. Effect of theophylline on the rate of moderate to severe exacerbations among patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol 2008;65:40-5. https://doi.org/10.1111/j.1365-2125.2007.02977.x.
- Fexer J, Donnachie E, Schneider A, Wagenpfeil S, Keller M, Hofmann F, et al. The effects of theophylline on hospital admissions and exacerbations in COPD patients: audit data from the Bavarian disease management program. Dtsch Arztebl Int 2014;111:293-300. https://doi.org/10.3238/arztebl.2014.0293.
- Cosío BG, Shafiek H, Iglesias A, Yanez A, Córdova R, Palou A, et al. Oral low-dose theophylline on top of inhaled fluticasone-salmeterol does not reduce exacerbations in patients with severe COPD: a pilot clinical trial. Chest 2016;150:123-30. https://doi.org/10.1016/j.chest.2016.04.011.
- McKay SE, Howie CA, Thomson AH, Whiting B, Addis GJ. Value of theophylline treatment in patients handicapped by chronic obstructive lung disease. Thorax 1993;48:227-32. https://doi.org/10.1136/thx.48.3.227.
- Napp Pharmaceuticals Ltd . Uniphyllin Continus Tablets 2014. www.medicines.org.uk/emc/medicine/1233 (accessed March 2018).
- Devereux G, Cotton S, Barnes P, Briggs A, Burns G, Chaudhuri R, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0782-2.
- Shakeri-Nejad K, Stahlmann R. Drug interactions during therapy with three major groups of antimicrobial agents. Expert Opin Pharmacother 2006;7:639-51. https://doi.org/10.1517/14656566.7.6.639.
- Hunt SN, Jusko WJ, Yurchak AM. Effect of smoking on theophylline disposition. Clin Pharmacol Ther 1976;19:546-51. https://doi.org/10.1002/cpt1976195part1546.
- Powell JR, Thiercelin JF, Vozeh S, Sansom L, Riegelman S. The influence of cigarette smoking and sex on theophylline disposition. Am Rev Respir Dis 1977;116:17-23. https://doi.org/10.1164/arrd.1977.116.1.17.
- Powell JR, Vozeh S, Hopewell P, Costello J, Sheiner LB, Riegelman S. Theophylline disposition in acutely ill hospitalized patients. The effect of smoking, heart failure, severe airway obstruction, and pneumonia. Am Rev Respir Dis 1978;118:229-38. https://doi.org/10.1164/arrd.1978.118.2.229.
- Jusko WJ, Schentag JJ, Clark JH, Gardner M, Yurchak AM. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther 1978;24:405-10. https://doi.org/10.1002/cpt1978244406.
- Jusko WJ. Role of tobacco smoking in pharmacokinetics. J Pharmacokinet Biopharm 1978;6:7-39. https://doi.org/10.1007/BF01066061.
- Hendeles L, Weinberger M, Bighley L. Disposition of theophylline after a single intravenous infusion of aminophylline. Am Rev Respir Dis 1978;118:97-103. https://doi.org/10.1164/arrd.1978.118.1.97.
- Chrystyn H, Ellis JW, Mulley BA, Peake MD. Bayesian derived predictions for twice daily theophylline under outpatient conditions and an assessment of optimal sampling times. Br J Clin Pharmacol 1989;27:215-21. https://doi.org/10.1111/j.1365-2125.1989.tb05353.x.
- Chrystyn H, Mulley BA, Peake MD. Dose response relation to oral theophylline in severe chronic obstructive airways disease. BMJ 1988;297:1506-10. https://doi.org/10.1136/bmj.297.6662.1506.
- Chrystyn H, Ellis JW, Mulley BA, Peake MD. The accuracy and stability of Bayesian theophylline predictions. Ther Drug Monit 1988;10:299-305. https://doi.org/10.1097/00007691-198803000-00011.
- Chrystyn H, Mulley BA, Peake MD. The accuracy of a pharmacokinetic theophylline predictor using once daily dosing. Br J Clin Pharmacol 1987;24:301-7. https://doi.org/10.1111/j.1365-2125.1987.tb03173.x.
- Shah B, Sucher K, Hollenbeck CB. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract 2006;21:312-19. https://doi.org/10.1177/0115426506021003312.
- Celli BR, MacNee W. ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46. https://doi.org/10.1183/09031936.04.00014304.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modelling valuations for health states: the effect of duration. Health Policy 1996;38:189-203. https://doi.org/10.1016/0168-8510(96)00853-6.
- CAT Governance Board . COPD Assessment Test 2016. www.catestonline.org/ (accessed March 2018).
- Dodd JW, Hogg L, Nolan J, Jefford H, Grant A, Lord VM, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011;66:425-9. https://doi.org/10.1136/thx.2010.156372.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. https://doi.org/10.1183/09031936.00102509.
- Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis. Br Med J 1960;2. https://doi.org/10.1136/bmj.2.5213.1665.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. https://doi.org/10.1136/thx.54.7.581.
- Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73-9. https://doi.org/10.1007/s00408-010-9272-1.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. https://doi.org/10.1183/09031936.05.00034805.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40. https://doi.org/10.1183/09041950.005s1693.
- Barrett B, Byford S. Collecting service use data for economic evaluation in DSPD populations: development of the secure facilities service use schedule. Br J Psychiatry Suppl 2007;49:s75-8. https://doi.org/10.1192/bjp.190.5.s75.
- NHS Health Research Authority . Safety and Progress Reports (CTIMPs) 2007. www.hra.nhs.uk/approvals-amendments/managing-your-approval/safety-reporting/ (accessed March 2018).
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999;21:1074-90. https://doi.org/10.1016/S0149-2918(99)80026-5.
- Ram FS, Jones PW, Castro AA, De Brito JA, Atallah AN, Lacasse Y, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2002;4. https://doi.org/10.1002/14651858.CD003902.
- Chisholm D, Knapp MR, Knudsen HC, Amaddeo F, Gaite L, van Wijngaarden B. Client Socio-Demographic and Service Receipt Inventory – European Version: development of an instrument for international research. EPSILON Study 5. European Psychiatric Services: Inputs Linked to Outcome Domains and Needs. Br J Psychiatry Suppl 2000;39:s28-33. https://doi.org/10.1192/bjp.177.39.s28.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed March 2018).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2018. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed March 2018).
- Information Services Division Scotland . ISD Scotland Costs, Finance 2017. www.isdscotland.org/Health-Topics/Finance/Costs/ (accessed March 2018).
- Oostenbrink JB, Rutten-van Mölken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005;8:32-46. https://doi.org/10.1111/j.1524-4733.2005.03086.x.
- Scott A, Simoens S, Heaney D, O’Donnell CA, Thomson H, Moffat KJ, et al. What does GP out of hours care cost? An analysis of different models of out of hours care in Scotland. Scott Med J 2004;49:61-6. https://doi.org/10.1177/003693300404900208.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed March 2018).
- Baxter K. Stockley’s Drug Interactions. London: Pharmaceutical Press; 2017.
- MedDRA MSSO . Introductory Guide MedDRA Version 13.1 2010. www.meddra.org/sites/default/files/guidance/file/intguide_13_1_english.pdf (accessed March 2018).
- Martinez FJ, Rabe KF, Calverley PMA, Fabbri LM, Sethi S, Pizzichini E, et al. Determinants of response to roflumilast in severe COPD: pooled analysis of two randomized trials. Am J Respir Crit Care Med 2018;198:1268-78. https://doi.org/10.1164/rccm.201712-2493OC.
- Guyatt GH, Townsend M, Pugsley SO, Keller JL, Short HD, Taylor DW, et al. Bronchodilators in chronic air-flow limitation. Effects on airway function, exercise capacity, and quality of life. Am Rev Respir Dis 1987;135:1069-74. https://doi.org/10.1164/arrd.1987.135.5.1069.
- Mehring M, Donnachie E, Fexer J, Hofmann F, Schneider A. Disease management programs for patients with COPD in Germany: a longitudinal evaluation of routinely collected patient records. Respir Care 2014;59:1123-32. https://doi.org/10.4187/respcare.02748.
- Subramanian, Ragulan, Jindal A, Viswambhar V. V AB . The study of efficacy, tolerability and safety of theophylline given along with formoterol plus budesonide in COPD. Journal of Clinical and Diagnostic Research 2015;9:OC10-3.
- Hettle R, Wouters H, Ayres J, Gani R, Kelly S, Lion M, et al. Cost-utility analysis of tiotropium versus usual care in patients with COPD in the UK and Belgium. Respir Med 2012;106:1722-33. https://doi.org/10.1016/j.rmed.2012.09.006.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008;31:416-69. https://doi.org/10.1183/09031936.00099306.
- Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018;391:1076-84. https://doi.org/10.1016/S0140-6736(18)30206-X.
- Martinez FJ, Calverley PMA, Goehring U, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66. https://doi.org/10.1016/S0140-6736(14)62410-7.
- Vestbo J, Leather D, Diar Bakerly N, New J, Gibson JM, McCorkindale S, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med 2016;375:1253-60. https://doi.org/10.1056/NEJMoa1608033.
- Lim S, Tomita K, Caramori G, Jatakanon A, Oliver B, Keller A, et al. Low-dose theophylline reduces eosinophilic inflammation but not exhaled nitric oxide in mild asthma. Am J Respir Crit Care Med 2001;164:273-6. https://doi.org/10.1164/ajrccm.164.2.2006043.
- Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet 1994;343:1006-8. https://doi.org/10.1016/S0140-6736(94)90127-9.
- Ohta K, Fukuchi Y, Grouse L, Mizutani R, Rabe KF, Rennard SI, et al. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med 2004;98:1016-24. https://doi.org/10.1016/j.rmed.2004.02.020.
- Cusack B, Kelly JG, Lavan J, Noel J, O’Malley K. Theophylline kinetics in relation to age: the importance of smoking. Br J Clin Pharmacol 1980;10:109-14. https://doi.org/10.1111/j.1365-2125.1980.tb01726.x.
- Donaldson GC, Wedzicha JA. COPD exacerbations .1: epidemiology. Thorax 2006;61:164-8. https://doi.org/10.1136/thx.2005.041806.
- Vijayasaratha K, Stockley RA. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest 2008;133:34-41. https://doi.org/10.1378/chest.07-1692.
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respir Med 2007;101:1462-9. https://doi.org/10.1016/j.rmed.2007.01.012.
- Quint JK, Donaldson GC, Hurst JR, Goldring JJ, Seemungal TR, Wedzicha JA. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J 2011;37:501-7. https://doi.org/10.1183/09031936.00035909.
- Miravitlles M, Murio C, Tirado-Conde G, Levy G, Muellerova H, Soriano JB, et al. Geographic differences in clinical characteristics and management of COPD: the EPOCA study. Int J Chron Obstruct Pulmon Dis 2008;3:803-14. https://doi.org/10.2147/COPD.S4257.
- Desalu OO, Onyedum CC, Adeoti AO, Gundiri LB, Fadare JO, Adekeye KA, et al. Guideline-based COPD management in a resource-limited setting – physicians’ understanding, adherence and barriers: a cross-sectional survey of internal and family medicine hospital-based physicians in Nigeria. Prim Care Respir J 2013;22:79-85. https://doi.org/10.4104/pcrj.2013.00014.
- Shen N, Yao WZ, Zhu H. Patient’s perspective of chronic obstructive pulmonary disease in Yanqing county of Beijing. Zhonghua Jie He He Hu Xi Za Zhi 2008;31:206-8.
- Tyagi N, Gulati K, Vijayan VK, Ray A. A study to monitor adverse drug reactions in patients of chronic obstructive pulmonary disease: focus on theophylline. Indian J Chest Dis Allied Sci 2008;50:199-202.
- Zhou Y, Wang X, Zeng X, Qiu R, Xie J, Liu S, et al. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology 2006;11:603-10. https://doi.org/10.1111/j.1440-1843.2006.00897.x.
- Berend N, Jenkins CR. Theophylline and Steroids in Chronic Obstructive Pulmonary Disease (COPD) Study (TASCS) 2017. https://clinicaltrials.gov/ct2/show/NCT02261727 (accessed March 2018).
- Jenkins CR, Wen FQ, Barnes PJ, Celli BR, Zhong NS, Zheng JP, et al. Theophylline and systemic corticosteroids in COPD: the TASCS trial. Am J Respir Crit Care Med 2019;199.
- University of Aberdeen . BICS n.d. https://w3.abdn.ac.uk/hsru/BICS/Public/Public/index.cshtml (accessed 24 June 2019).
- García Morales OM, Rojas-Reyes MX, Dennis RJ. Oral xanthine derivatives (theophylline and doxofylline) for patients with stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2017;8. https://doi.org/10.1002/14651858.CD012748.
- Blouin RA, Elgert JF, Bauer LA. Theophylline clearance: effect of marked obesity. Clin Pharmacol Ther 1980;28:619-23.
Appendix 1 Rationale for the low-dose theophylline strategy
Population theophylline pharmacokinetic studies during the 1970s and 1980s demonstrated that disease status, weight and smoking decrease the half-life of theophylline and increase its clearance. 57–62 It has been shown that theophylline clearance is lower in COPD patients who do not smoke than in healthy volunteers. 60 Based on these data and our publications,63–66 an average population clearance value of theophylline in non-smokers is 40 ml/hour/kg, which is reduced to 32 ml/hour/kg in a patient with COPD, and by a further 20% if they have another related disease (e.g. severe congestive heart failure). This corresponds to the fast, normal and slow categories of plasma theophylline pharmacokinetic modelling for COPD patients provided in Table 35. Smoking increases theophylline clearance by ≈60%, and the plasma theophylline level gradually returns to normal levels when a person stops smoking. Another relevant population pharmacokinetic value that is useful for loading doses is a volume of distribution for theophylline of 0.5 l/kg. 57–66
| IBW (kg) | Theophylline 200 mg bd | Theophylline 200 mg od | ||||
|---|---|---|---|---|---|---|
| Steady-state plasma theophylline concentration (Css) (mg/l) | ||||||
| Participant theophylline clearance | ||||||
| Slow | Normal | Fast | Slow | Normal | Fast | |
| Not current smoker | ||||||
| 40.1–50 | 17.4 | 13.0 | 10.4 | 8.7 | 6.5 | 5.2 |
| 50.1–60 | 13.9 | 10.4 | 8.3 | 6.9 | 5.2 | 4.2 |
| 60.1–70 | 11.6 | 8.7 | 6.9 | 5.8 | 4.3 | 3.5 |
| 70.1–80 | 9.9 | 7.4 | 6.0 | 5.0 | 3.7 | 3.0 |
| 80.1–90 | 8.7 | 6.5 | 5.2 | 4.3 | 3.3 | 2.6 |
| 90.1–100 | 7.7 | 5.8 | 4.6 | 3.9 | 2.9 | 2.3 |
| 100.1–110 | 6.9 | 5.2 | 4.2 | 3.5 | 2.6 | 2.1 |
| 110.1–120 | 6.3 | 4.7 | 3.8 | 3.2 | 2.4 | 1.9 |
| > 120 | 5.8 | 4.3 | 3.5 | 2.9 | 2.2 | 1.7 |
| Current smoker | ||||||
| 40.1–50 | 10.9 | 8.1 | 6.5 | 5.4 | 4.1 | 3.3 |
| 50.1–60 | 8.7 | 6.5 | 5.2 | 4.3 | 3.3 | 2.6 |
| 60.1–70 | 7.2 | 5.4 | 4.3 | 3.6 | 2.7 | 2.2 |
| 70.1–80 | 6.2 | 4.7 | 3.7 | 3.1 | 2.3 | 1.9 |
| 80.1–90 | 5.4 | 4.1 | 3.3 | 2.7 | 2.0 | 1.6 |
| 90.1–100 | 4.8 | 3.6 | 2.9 | 2.4 | 1.8 | 1.4 |
| 100.1–110 | 4.3 | 3.3 | 2.6 | 2.2 | 1.5 | 1.2 |
| 110.1–120 | 3.9 | 3.0 | 2.4 | 2.0 | 1.5 | 1.2 |
| > 120 | 3.6 | 2.7 | 2.2 | 1.8 | 1.4 | 1.1 |
The use of actual weight or IBW has been shown to have an effect on the clearance of theophylline in young adults who smoke. 122 If a patient is obese, they may be given a high dose when their actual weight is used. It is good practice to assume that this occurs in all patients and thus use IBW. IBW can be calculated using the following equations:67
The IBW is used unless the actual weight is lower than the IBW.
For oral theophylline dosing, the pharmacokinetic model is:
where Css is the steady-state theophylline concentration, F is the bioavailability of theophylline (F = 1 for theophylline preparations), D is the dose, Cl is the clearance and τ is the dosage interval (either 12 or 24 hours). Using this model and the population theophylline clearance values for COPD patients described above, in smokers and non-smokers, predicted Css values are as shown in Table 35.
Confidence that plasma theophylline in the low-dose range can be achieved using the above dosing strategy is provided from a detailed analysis of a COPD study that measured theophylline concentrations at three different theophylline dosing regimens. 63 In 33 COPD patients [who had a mean weight of 64.6 (SD 14.3) kg and a mean age of 61.2 (SD 5.8) years], we found that the mean plasma theophylline concentration at steady state when they received a mean of 252 (SD 87) mg bd was 6.3 (SD 2.1) mg/l. This represents a clearance value of 51.6 ml/hour/kg. When the theophylline dose was increased to 430 mg bd and then to 597 (SD 153) mg bd, mean steady-state plasma theophylline concentrations rose to 12.1 (SD 1.9) mg/l and 18.3 (SD 3.0) mg/l, respectively. This represents clearance values of 45.8 ml/hour/kg and 42.1 ml/hour/kg, respectively. This will include smokers and non-smokers (numbers of each not recorded) and the latter clearance value is similar to the 40 ml/hour/kg used in the population pharmacokinetics modelling for the ‘fast’ category. Our other publications64,65 (n = 83 patients64 and n = 15 patients65) on plasma theophylline highlight our confidence of using low-dose theophylline in the TWICS trial.
In the clinical situation in which a clinician wished to use intravenous aminophylline to treat a patient participating in the TWICS trial with an acute exacerbation of COPD, the BNF recommends a loading dose of intravenous aminophylline of 5 mg/kg (typically 250 mg); this is usually omitted if the patient is already taking theophylline. This is then followed by an intravenous infusion of aminophylline of 0.5 mg/kg. 86 It is recommended that plasma theophylline be measured after 24 hours to direct the rate of further dosing. The pharmacokinetic model for a loading dose is:
where Co is the concentration immediately after the slow intravenous bolus dose of aminophylline, F is the bioavailability (F = 0.8 for aminophylline) and V is the volume of distribution. A loading dose of 5 mg/kg would provide a Co of:
Because the predicted Css shown in Table 35 ranges from 2.2 to 8.7 mg/l, the maximum theophylline concentration would be 16.7 mg/l. Alternatively, a loading dose of 250 mg of aminophylline could be given, rather than a dose based on weight. A loading dose of 250 mg of aminophylline in COPD patients weighing 40–100 kg would provide a Co ranging from 4 to 10 mg/l. There is a linear relationship between Css and weight. Similarly, if the loading dose was 500 mg of aminophylline, then the predicted Css would be double that for the 250-mg dose.
For an aminophylline infusion of 0.5 mg/kg/hour86 and based on a clearance of 40 ml/hour/kg and the following pharmacokinetic model:
where Css is the steady-state theophylline concentration, F is the bioavailability of theophylline (F = 0.8 for aminophylline preparations), D is the dose, Cl is the clearance (using a clearance of 40 ml/hour/kg) and τ is the dosage interval (1 hour for an intravenous infusion). In this case the predicted Css would be:
Note that the predicted Css is independent of weight (see Equation 9). The predicted Css in non-smokers with COPD with slow, normal or fast theophylline clearance given an infusion of 0.5 mg/hour/kg would be 10.0, 12.5 and 16.7 mg/l, respectively. In smokers, the corresponding predicted Css values would be 6.3, 7.8 and 10.4 mg/l.
Importantly for the TWICS trial, it was safe for participants to receive a 5-mg/kg loading infusion of aminophylline followed by an infusion of 0.5 mg/kg/hour as the plasma concentration would exceed the target 10–20 mg/l range required for conventional theophylline dosing.
Appendix 2 Validation of patient-reported exacerbations
Initially, we planned to validate the total number of COPD exacerbations for ≈20% of participants by examination of general practice records.
At the TSC meeting of 20 March 2017, the validation exercise comparing the number of exacerbations as recorded in general practice records and reported by the participant was discussed. At that time, the focus of this validation had been in two of the largest sites: Aberdeen and Aintree. The validation was done by requesting a care/encounter summary from the GP and comparing this against the patient report. In Aberdeen, 43 records had been checked; in 37 there was complete agreement between patient report and GP report. In Aintree, 24 records had been checked; in 16 there was complete agreement between patient report and GP report. Therefore, 4% of participants had undergone validation, and there was ≈80% concordance. Concerns were raised that there is no ‘gold standard’ for the reporting of exacerbations, and that current general practice records may not be as reliable a source of exacerbation data as in the past, given that patients have rescue packs at home and can access help for their exacerbations through many non-GP sources, for example pharmacies, emergency and walk-in centres, and accident and emergency departments. The published evidence is that patients are able to reliably report the number of exacerbations experienced in the previous year. 112 Furthermore, the patient representatives for the TWICS trial are adamant that it is fairly straightforward to remember the number of exacerbations over this time period. It was also noted that the primary outcome of this trial is patient-reported exacerbations and it is this outcome that drives demand for NHS services. The TSC, therefore, recommended that we completed the validation exercise for the participants for whom we had data, but that the validation exercise did not need to be extended beyond these two sites or to include further participants.
Appendix 3 Breaches
| Site affected (site number) | Description of breach | Assessment |
|---|---|---|
| 36 | The site consented a patient into the trial on 28 April 2014, before the site agreement had been signed by all parties. The trial processes in place prevented the site from randomising the patient on the live randomisation system and also meant that a drug pack could not be dispensed | Non-serious |
| 18 | Participant had rescue medication, including erythromycin (one of the drugs that can increase serum theophylline), and, in response to an exacerbation, the participant started to take the rescue medication without stopping the trial medication. The patient came to no harm, and did not suffer any adverse effects | Non-serious |
| 11 | The chief investigator raised concerns about the monitoring process following a routine trial monitoring visit carried out by R&D monitors at the site. For two patients, the monitors recorded amber findings relating to the recording of comorbities and concomitant medication in the case report form, and for one patient indicated that there was contraindicated medication. However, the data recorded in both case report forms were accurate and both patients were eligible. The breach related to the monitors incorrectly noting amber findings and making the research nurses modify the case report form by entering incorrect information | Non-serious |
| 12 | Participant was admitted to hospital. Prior to the admission, the participant had been prescribed clarithromycin (one of the drugs that can increase serum theophylline). His trial medication was stopped by the hospital pharmacist. The symptoms experienced by the participant (gastro-oesophageal reflux) may have resulted from clarithromycin per se, and/or an interaction between clarithromycin and theophylline. Gastro-oesophageal reflux is a side effect of both clarithromycin and theophylline | Non-serious |
| 11 | The third-party distributor identified that it had despatched a shipment to a participant that contained drug pack numbers 40167 (correctly) and 40166 (in error) on 5 November 2014. The participant was contacted on 29 January 2015 and indicated that he had started using kit number 40167 and that he had not opened kit number 40166. He returned kit number 40166 to the research nurse later that day and it was destroyed. The participant was resupplied with an appropriate box of medication | Non-serious |
| 27 | At the point of randomisation (20 April 2014), the participant had been randomised as a smoker (rather than as an ex-smoker) and was allocated and received a dose of twice-daily trial medication (he should have received a once-daily dose). On 5 November 2014, the patient was diagnosed with atrial fibrillation. No palpitations were noted. Atrial tachycardias are a known side effect of theophylline. The patient was unblinded so he could be managed appropriately. The atrial fibrillation experienced by the patient may have been caused by the theophylline. The participant was seen on 6 January 2015 for a routine appointment, and his pulse was noted to be regular, that is spontaneously reverted to sinus rhythm | Serious |
| 12 | Noted at the 12-month follow-up, the participant had been on diltiazem hydrochloride (Tildiem LA, Sanofi-Aventis) (300 mg od) since recruitment into the study (26 February 2014). Tildiem LA is a form of diltiazem (diltiazem is one of the drugs listed in the trial protocol as known to interact with theophylline). Although this medication had been recorded on the baseline case report form, the patient was assessed as being eligible for the trial. The patient was well throughout the trial. No AEs were noted | Non-serious |
| 11 | During the 12-month follow-up appointment (19 May 2015), the participant mentioned that the community pharmacist had been supplying Uniphyllin 200 mg (theophylline) in his dosette box. After taking the Uniphyllin included in the dosette box for 2 or 3 days, the participant realised that he may be taking theophylline as both the TWICS trial medication and as prescribed medication. He therefore ceased taking the TWICS trial medication. The participant has noted no adverse effects as a result of this | Non-serious |
| 78 | Participant was randomised to twice-daily trial medication – dosing instruction ‘Take ONE tablet every morning and ONE tablet every evening’, but took two tablets each morning and two each evening. After 10 days of taking the trial medication, the participant noted that he was experiencing nausea, tremors and disturbed sleep (which, in part, may have been anxiety related because of a forthcoming bypass operation); his dose was reduced by the trial team to one tablet per day and his symptoms settled. At the 6-month follow-up appointment, the participant noted that he was still taking ‘one tablet’, but as he was feeling well, wished to start taking two tablets again. The trial team agreed that he could increase his dose to two tablets per day (the recommended ‘low dose’ for a smoker of his height and weight). He did this for 3 days and symptoms of nausea/sickness returned. The participant therefore reduced his dose to ‘one tablet’ and the symptoms settled. In subsequent discussion with the participant, it became clear that he had misinterpreted the initial instruction on medication use as two tablets twice a day, and had been taking this dose rather than one tablet twice a day. For approximately 10 days between 3 December 2014 and 17 December 2014, he had therefore been taking a dose in the normal therapeutic range (400 mg twice daily) rather than a low dose (200 mg twice daily). For the period between 17 December 2014 and 10 June 2015 he had been taking one tablet twice a day; this was the appropriate ‘low dose’ used in the trial. For a further 3 days from 10 June 2015, the participant again misinterpreted the instruction on the medication bottle and took two tablets twice a day (i.e. the normal therapeutic range rather than a low dose) | Non-serious |
| 12 | Participant was prescribed Elleste Duet (Piramal Healthcare UK Ltd, Morpeth, UK) (which is an oestrogen; oestrogens may raise theophylline levels to within the normal therapeutic range rather than a low dose) by her GP between being recruited into the study and her 6-month follow-up. At the 6-month follow-up (18 June 2015), she was advised to cease taking trial medication. The interaction between Elleste Duet and theophylline is such that the serum levels of theophylline may be raised into the normal therapeutic range, and not to toxic levels. Thus any interaction does not raise safety concerns | Non-serious |
| Across sites | Following review of emergency hospital admissions captured at follow-up, we identified a number of admissions that should have been captured as SAEs. None was related to trial medication | Non-serious |
| 12 | The participant failed to attend for the 12-month follow-up. Follow-up data were sought from his GP, and during this data-collection exercise, it was noted that the participant had been prescribed Uniphyllin on repeat prescription since 8 May 2012. He had not disclosed this at recruitment or the 6-month follow-up, or during any telephone calls. The prescription he brought to the recruitment appointment did not include the Uniphyllin. No AEs were noted during follow-up (last contact with participant was at the 46-week call) | Non-serious |
| 12 | Late reporting of a SAE in this participant (strangulated small bowel secondary to hernia, not related to trial medication), who had ceased trial medication prior to the event | Non-serious |
| 125 | Participant was randomised on 12 January 2016 (200 mg od); on 26 January it was noted that he was already taking aminophylline (225 mg bd). The participant had taken trial medication as well as routine aminophylline for 8 days. The participant did not experience any ARs. The GP confirmed that the aminophylline (225 mg bd) plus trial medication (if active; 200 mg od) would not have taken the participant over the maximum daily dose | Non-serious |
| 12 | The trial office prepared a waybill for the dispatch of trial medication with the house number transposed (so the trial medication was delivered to house number 35 rather than house number 53). The participant was resupplied, and the incorrect delivery was retrieved from house number 35 | Non-serious |
| 78 | The third-party distributor picked the wrong kit and this was dispatched to the participant. The wrong kit was retrieved from the participant and she was resupplied with the correct kit | Non-serious |
| 14 | Participant was recruited into the TWICS trial while participating in another drug study (in breach of the TWICS trial eligibility criteria). There was no documentation in the medical notes in relation to the other study and the participant did not mention it at recruitment. The participant came to no harm | Non-serious |
| 145 | Participant randomised on 23 June 2016 to once-daily trial medication, and was allocated an appropriate labelled bottle. The participant took the trial medication twice daily for approximately 7 days after commencing medication (this would have brought her into the normal therapeutic range for theophylline rather than a low dose). The participant came to no harm (she noted some initial constipation, which resolved) | Non-serious |
| 122 | Participant was randomised to od study medication. The bottle was correctly labelled, but a dispensing label was added at the time the medication was dispensed that indicated a two-a-day dosing regimen. The error was noted and corrected. The participant took twice daily study medication for approximately 10 days (this would have brought him into the normal therapeutic range for theophylline rather than a low dose) and came to no harm | Non-serious |
| 159 | In an attempt to prevent medication being prescribed that may interact with theophylline, the practice added theophylline to the repeat prescription as a trial drug. The pharmacist dispensed liquid theophylline as part of the repeat prescription; for a period of 7 days, the participant took a dose of liquid theophylline three times per day and also took their trial medication three times per day. This would have brought the participant into the normal therapeutic range for theophylline rather than a low dose. The participant came to no harm | Non-serious |
| 141 | The third-party distributor picked the wrong kit and this was dispatched to the participant. The wrong kit was retrieved from the participant and she was resupplied with the correct kit | Non-serious |
| 115 | Participant was recruited to the trial in October 2015. During data checking in January 2017, it was noted that the participant was already taking Phyllocontin (Napp Pharmaceuticals Ltd). The participant took trial medication for a full 12 months, and no AEs were noted. Subsequent data checking identified five other participants who had been recruited while on a medication that may interact with theophylline:
|
Non-serious |
Appendix 4 Recruitment by site
| Site | Participants recruited (n) |
|---|---|
| Secondary care sites (N = 33) | 1101 |
| Aberdeen Royal Infirmary | 212 |
| Aintree University Hospital NHS Foundation Trust | 127 |
| Belfast City Hospital | 6 |
| Queen Elizabeth Hospital Birmingham | 54 |
| Blackpool Victoria Hospital | 57 |
| Bradford Royal Infirmary | 9 |
| Queen’s Hospital, Burton Hospitals NHS Foundation Trust | 4 |
| Calderdale Royal Hospital, Huddersfield Royal Infirmary, Calderdale & Huddersfield NHS Foundation Trust | 4 |
| University Hospital of North Durham | 9 |
| Lister Hospital, East and North Hertfordshire NHS Trust | 20 |
| Victoria Hospital, Kirkcaldy | 29 |
| Freeman Hospital, Newcastle | 45 |
| Glasgow Hospitals (Gartnavel, Glasgow Royal, Southern General, Victoria Infirmary, Western Infirmary) | 115 |
| Castle Hill Hospital, Hull | 114 |
| Raigmore Hospital, Inverness | 31 |
| University Hospital Wishaw | 12 |
| Royal Lancaster Infirmary | 19 |
| Leighton Hospital, Crewe | 13 |
| Musgrove Park Hospital | 6 |
| Norfolk and Norwich University Hospital | 80 |
| University Hospital of North Tees | 6 |
| City Hospital, Nottingham | 11 |
| Derriford Hospital, Plymouth | 4 |
| South Tyneside District Hospital | 44 |
| Torbay Hospital | 12 |
| New Cross Hospital, Wolverhampton | 33 |
| Worcestershire Royal Hospital | 11 |
| Yeovil District Hospital | 8 |
| York Hospital, York Teaching Hospital NHS Foundation Trust | 6 |
| East of England primary care (N = 48) | 242 |
| Alconbury and Brampton Surgeries | 6 |
| Alexandra and Crestview Surgeries | 5 |
| Andaman Surgery | 7 |
| Attleborough Surgeries | 7 |
| Beccles Medical Centre | 7 |
| Bridge Road Surgery | 4 |
| Bridge Street Medical Centre, Cambridge | 2 |
| Bridge Street Surgery, Downham Market | 8 |
| Campingland Surgery | 2 |
| Castle Partnership | 8 |
| Coltishall Medical Practice | 4 |
| Comberton and Eversden Surgeries | 3 |
| Cutlers Hill Surgery | 2 |
| Davenport House | 3 |
| De Parys Medical Centre | 5 |
| East Norfolk Medical Practice | 1 |
| Elizabeth Courtauld Surgery | 1 |
| Gorleston Medical Centre | 3 |
| Greyfriars Medical Centre | 6 |
| Harvey Group Practice | 6 |
| Holt Medical Practice | 2 |
| Hoveton and Wroxham Medical Centre | 6 |
| Linton Health Centre | 5 |
| Long Stratton Medical Partnership | 4 |
| Ludham & Stalham Green Surgeries | 12 |
| Mount Farm Surgery | 3 |
| Mundesley Medical Centre | 14 |
| Nuffield Road Medical Centre | 4 |
| Orchard Surgery, Dereham | 3 |
| Peninsula Practice | 6 |
| Portmill Surgery | 4 |
| Rosedale Surgery | 3 |
| Roundwell Medical Centre | 5 |
| Salisbury House Surgery | 3 |
| Sheringham Medical Practice | 5 |
| Spinney Surgery | 4 |
| St Stephens Gate Medical Practice | 12 |
| St Johns Surgery, Terrington | 5 |
| Staithe Surgery | 4 |
| The Over Surgery | 1 |
| Trinity and Bowthorpe Medical Practice | 2 |
| Vida Healthcare | 11 |
| Wells Health Centre | 3 |
| Wellside Surgery | 3 |
| Woodhall Farm Medical Centre | 3 |
| Woolpit Health Centre | 19 |
| Wymondham Medical Practice | 1 |
| York Street Medical Practice | 5 |
| North of England primary care (N = 26) | 131 |
| Beacon View Medical Centre | 8 |
| Beaumont Park Medical Group | 4 |
| Belford Medical Practice | 8 |
| Bellingham Practice | 1 |
| Benfield Park Medical Centre | 6 |
| Burn Brae Medical Group | 1 |
| Castlegate & Derwent Surgery | 29 |
| Corbridge Medical Group | 6 |
| Elvaston Road Surgery | 1 |
| Fell Cottage Surgery | 2 |
| Grove Medical Group | 1 |
| Guidepost Medical Group | 7 |
| Haltwhistle Medical Group | 2 |
| Haydon and Allendale Medical Practice | 1 |
| Hetton Group Practice | 4 |
| Humshaugh and Wark Medical Group | 3 |
| Marine Avenue Surgery | 2 |
| Maryport Health Services | 9 |
| Priory Medical Group | 2 |
| Prudhoe Medical Group | 4 |
| Seaton Park Medical Group | 2 |
| Sele Medical Group | 6 |
| Temple Sowerby Medical Group | 5 |
| The Village Surgery | 4 |
| Waterloo Medical Group | 11 |
| West Farm Surgery | 2 |
| South West England primary care (N = 11) | 95 |
| Barton Surgery | 6 |
| Bovey Tracey and Chudleigh Practice | 9 |
| Brunel Medical Practice | 20 |
| Claremont Medical Practice | 4 |
| Coleridge Medical Centre | 3 |
| Helston Medical Centre | 2 |
| Ide Lane Surgery | 2 |
| Petroc Group Practice | 11 |
| Richmond House Surgery | 5 |
| Rolle Medical Partnership | 4 |
| Westlake Surgery | 29 |
| Wessex primary care (N = 3) | 9 |
| Friarsgate Practice | 1 |
| Park and St Francis Surgery | 1 |
| Swanage Medical Centre | 7 |
| Total recruitment | 1578 |
Appendix 5 Supplementary tables
| Characteristic | Primary care (N = 917) | Secondary care (N = 619) | p-value |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 498 (54.3) | 330 (53.3) | 0.701 |
| Female, n (%) | 419 (45.7) | 289 (46.7) | |
| Age (years), mean (SD) | 68.9 (8.2) | 67.7 (8.5) | 0.006 |
| Smoking status | |||
| Current smoker, n (%) | 322 (35.1) | 164 (26.5) | < 0.001 |
| Ex-smoker, n (%) | 595 (64.9) | 455 (73.5) | |
| Pack-years, mean (SD); n | 46.1 (29.5); 910 | 48.4 (27.1); 619 | 0.113 |
| BMI (kg/m2), mean (SD) | 27.4 (6.1) | 27.0 (6.1) | 0.284 |
| BMI group, n (%) | |||
| Underweight | 41 (4.5) | 29 (4.7) | 0.463 |
| Normal | 306 (33.4) | 214 (34.6) | |
| Overweight | 296 (32.3) | 214 (36.6) | |
| Obese | 274 (29.9) | 162 (26.2) | |
| Number of exacerbations in the previous 12 months, mean (SD); n | 3.35 (1.8); 908 | 3.92 (2.5); 619 | < 0.001 |
| Number of exacerbations requiring hospitalisation in the previous 12 months, mean (SD); n | 0.22 (0.6); 907 | 0.61 (1.1); 619 | < 0.001 |
| GOLD 2011 category | |||
| N | 901 | 615 | 0.006 |
| C: two or more exacerbations in the previous year, mMRC dyspnoea score of 0–1 and CAT score of < 10, n (%) | 60 (6.7) | 21 (3.4) | |
| D: two or more exacerbations in the previous year, mMRC dyspnoea score of ≥ 2 and CAT score of ≥ 10, n (%) | 841 (93.3) | 594 (96.6) | |
| FEV1% predicted, mean (SD); n | 54.5 (19.6); 907 | 47.7 (19.9); 619 | < 0.001 |
| FEV1% predicted category | |||
| N | 907 | 619 | < 0.001 |
| ≥ 80% (GOLD mild), n (%) | 100 (11.0) | 40 (6.5) | |
| 50–79.9% (GOLD moderate), n (%) | 399 (44.0) | 207 (33.4) | |
| 30–49.9% (GOLD severe), n (%) | 320 (35.3) | 255 (41.2) | |
| 0–29.9% (GOLD very severe), n (%) | 88 (9.7) | 117 (18.9) | |
| FVC% predicted, mean (SD); n | 86.4 (23.3); 904 | 83.7 (22.1); 619 | 0.022 |
| FEV1/FVC ratio, mean (SD); n | 50.8 (14.2); 904 | 45.8 (20.4); 619 | < 0.001 |
| Current treatment for COPD | |||
| ICS, n (%) | |||
| ICS only | 26 (2.8) | 5 (0.8) | 0.001 |
| ICS/LABA | 176 (19.2) | 84 (13.6) | |
| ICS/LAMA | 12 (1.3) | 10 (1.6) | |
| ICS/LABA/LAMA | 703 (76.7) | 520 (84.0) | |
| Oral mucolytic use, n (%); N | 162 (17.9); 905 | 222 (35.9); 619 | < 0.001 |
| Long-term antibiotic use, n (%); N | 34 (3.8); 905 | 62 (10.0); 619 | < 0.001 |
| Comorbidities | |||
| Asthma, n (%); N | 186 (20.6); 905 | 93 (15.1); 618 | 0.006 |
| Bronchiectasis, n (%); N | 22 (2.4); 905 | 43 (7.0); 617 | < 0.001 |
| Ischaemic heart disease, n (%); N | 107 (11.9); 903 | 97 (15.7); 618 | 0.031 |
| Hypertension, n (%); N | 351 (38.8); 905 | 231 (37.4); 618 | 0.579 |
| Diabetes mellitus, n (%); N | 102 (11.3); 905 | 72 (11.7); 618 | 0.819 |
| Osteoporosis, n (%); N | 99 (10.9); 905 | 96 (15.5); 318 | 0.008 |
| Anxiety/depression treated in the previous 5 years, n (%); N | 231 (25.5); 905 | 195 (31.6); 618 | 0.010 |
| Cerebrovascular event, n (%); N | 58 (6.4); 905 | 45 (7.3); 619 | 0.511 |
| Patient-reported outcome | Primary care | Secondary care | p-value |
|---|---|---|---|
| Degree of breathlessness (mMRC dyspnoea) | |||
| N | 907 | 617 | < 0.001 |
| Not troubled by breathlessness except on strenuous exercise, n (%) | 65 (7.2) | 20 (3.2) | |
| Short of breath when hurrying or walking up a slight hill, n (%) | 286 (31.5) | 143 (23.2) | |
| Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace, n (%) | 267 (29.4) | 216 (35.0) | |
| Stops for breath after walking about 100 m or after a few minutes on level ground, n (%) | 234 (25.8) | 186 (30.2) | |
| Too breathless to leave the house, or breathless when dressing or undressing, n (%) | 55 (6.1) | 52 (8.4) | |
| CAT (N, mean, SD) | 21.6 (7.6); 905 | 23.9 (7.6); 615 | < 0.001 |
| CAT group | |||
| N | 905 | 615 | < 0.001 |
| Low (score of 0–9), n (%) | 60 (6.6) | 21 (3.4) | |
| Medium (score of 10–19), n (%) | 295 (32.6) | 159 (25.9) | |
| High (score of 20–29), n (%) | 391 (43.2) | 285 (46.3) | |
| Very high (score of 30–40), n (%) | 159 (17.6) | 150 (24.4) | |
| EQ-5D-3L utility (N, mean, SD) | 0.66 (0.28); 908 | 0.58 (0.29); 619 | < 0.001 |
| EQ-5D-3L VAS (N, mean, SD) | 61.7 (19.3); 907 | 59.1 (18.9); 617 | < 0.001 |
| Characteristic | HARQ not completed (N = 1134) | HARQ completed (N = 402) | p-value |
|---|---|---|---|
| Sex (male), n (%) | 629 (55.5) | 199 (49.5) | 0.039a |
| Age, mean (SD) | 68.9 (8.3) | 66.8 (8.2) | < 0.001a |
| Smoking status, n (%) | |||
| Current smoker | 352 (31.0) | 134 (33.3) | 0.396a |
| Ex-smoker | 782 (69.0) | 268 (66.7) | |
| Pack-years, mean (SD); N | 46.3 (26.8); 1128 | 49.2 (33.2); 401 | 0.076b |
| BMI, mean (SD) | 27.2 (6.01) | 27.4 (6.4) | 0.689b |
| Exacerbations | Trial arm | Model | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Exacerbations treated with antibiotics only | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number with at least one exacerbation | 230 | 227 | ||||
| Total number of exacerbations | 338 | 368 | ||||
| Mean number of exacerbations | 0.44 | 0.48 | Unadjusted IRR | 0.94 | 0.78 to 1.13 | 0.484 |
| SD (number of exacerbations) | 0.82 | 0.97 | Adjusted IRRa | 0.94 | 0.78 to 1.14 | 0.541 |
| Exacerbations treated with steroids only | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number with at least one exacerbation | 77 | 88 | ||||
| Total number of exacerbations | 117 | 124 | ||||
| Mean number of exacerbations | 0.15 | 0.16 | Unadjusted IRR | 0.93 | 0.66 to 1.32 | 0.697 |
| SD (number of exacerbations) | 0.60 | 0.58 | Adjusted IRRa | 0.88 | 0.62 to 1.25 | 0.476 |
| Exacerbations treated with antibiotics and steroids | ||||||
| Total number included in analysis | 772 | 764 | ||||
| Number with at least one exacerbation | 487 | 479 | ||||
| Total number of exacerbations | 1171 | 1106 | ||||
| Mean number of exacerbations | 1.52 | 1.45 | Unadjusted IRR | 1.05 | 0.93 to 1.17 | 0.446 |
| SD (number of exacerbations) | 1.72 | 1.65 | Adjusted IRRa | 1.02 | 0.92 to 1.14 | 0.725 |
| Category | Trial arm | IRRa | 95% CI | Interaction p-value | |
|---|---|---|---|---|---|
| Theophylline | Placebo | ||||
| All participants | |||||
| n | 772 | 764 | |||
| Mean | 2.24 | 2.23 | |||
| SD | 1.99 | 1.97 | 0.99 | 0.91 to 1.08 | |
| Sex | |||||
| Male | |||||
| n | 418 | 410 | |||
| Mean | 2.23 | 2.18 | 1.01 | 0.87 to 1.17 | |
| SD | 2.04 | 1.92 | |||
| Female | |||||
| n | 354 | 354 | |||
| Mean | 2.25 | 2.28 | 0.97 | 0.83 to 1.14 | 0.609 |
| SD | 1.93 | 2.03 | |||
| Age group (years) | |||||
| < 60 | |||||
| n | 115 | 131 | |||
| Mean | 2.33 | 2.46 | 0.91 | 0.70 to 1.19 | |
| SD | 2.01 | 1.81 | |||
| 60–69 | |||||
| n | 313 | 284 | |||
| Mean | 2.27 | 2.13 | 1.07 | 0.89 to 1.28 | 0.198 |
| SD | 2.06 | 1.81 | |||
| ≥ 70 | |||||
| n | 344 | 349 | |||
| Mean | 2.18 | 2.23 | 0.96 | 0.82 to 1.13 | 0.637 |
| SD | 1.92 | 2.01 | |||
| Smoking status | |||||
| Current | |||||
| n | 241 | 245 | |||
| Mean | 2.40 | 2.47 | 0.96 | 0.80 to 1.16 | |
| SD | 2.01 | 2.07 | |||
| Ex-smoker | |||||
| n | 531 | 519 | |||
| Mean | 2.16 | 2.11 | 1.01 | 0.89 to 1.16 | 0.561 |
| SD | 1.97 | 1.92 | |||
| BMI category | |||||
| Underweight | |||||
| n | 37 | 33 | |||
| Mean | 2.51 | 2.45 | 0.93 | 0.57 to 1.52 | 0.894 |
| SD | 2.34 | 1.72 | |||
| Normal | |||||
| n | 277 | 243 | |||
| Mean | 2.29 | 2.41 | 0.95 | 0.79 to 1.15 | |
| SD | 1.91 | 1.72 | |||
| Overweight/obese | |||||
| n | 458 | 488 | |||
| Mean | 2.18 | 2.13 | 1.02 | 0.88 to 1.17 | 0.478 |
| SD | 2.01 | 1.96 | |||
| COPD treatment at baseline | |||||
| ICS/LAMA/LABA | |||||
| n | 610 | 613 | |||
| Mean | 2.33 | 2.36 | 0.98 | 0.87 to 1.10 | |
| SD | 2.05 | 1.87 | |||
| ICS/LABA or ICS/LAMA | |||||
| n | 148 | 134 | |||
| Mean | 1.89 | 1.78 | 1.00 | 0.76 to 1.32 | 0.832 |
| SD | 1.70 | 1.87 | |||
| ICS only | |||||
| n | 14 | 17 | |||
| Mean | 1.86 | 1.06 | 1.63 | 0.65 to 4.09 | 0.155 |
| SD | 1.92 | 1.43 | |||
| Number of exacerbations in the 12 months prior to baseline | |||||
| 2 | |||||
| n | 286 | 308 | |||
| Mean | 1.61 | 1.53 | 1.05 | 0.86 to 1.28 | |
| SD | 1.66 | 1.85 | |||
| 3 or 4 | |||||
| n | 317 | 298 | |||
| Mean | 2.31 | 2.25 | 1.02 | 0.86 to 1.21 | 0.785 |
| SD | 1.93 | 1.85 | |||
| ≥ 5 | |||||
| n | 169 | 158 | |||
| Mean | 3.16 | 3.55 | 0.89 | 0.73 to 1.09 | 0.139 |
| SD | 2.21 | 2.38 | |||
| GOLD stage | |||||
| I–II | |||||
| n | 370 | 376 | |||
| Mean | 1.93 | 2.03 | 0.97 | 0.82 to 1.14 | |
| SD | 1.89 | 1.99 | |||
| III | |||||
| n | 286 | 289 | |||
| Mean | 2.38 | 2.40 | 1.02 | 0.85 to 1.21 | 0.605 |
| SD | 2.03 | 1.99 | |||
| IV | |||||
| n | 113 | 92 | |||
| Mean | 2.90 | 2.58 | 0.99 | 0.75 to 1.32 | 0.849 |
| SD | 2.03 | 2.04 | |||
| OCSs at baseline | |||||
| No | |||||
| n | 418 | 410 | |||
| Mean | 2.23 | 2.18 | 0.99 | 0.88 to 1.10 | |
| SD | 2.04 | 1.92 | |||
| Yes | |||||
| n | 354 | 354 | |||
| Mean | 2.25 | 2.28 | 1.20 | 0.65 to 2.20 | 0.420 |
| SD | 1.93 | 2.03 | |||
| ICS dose at baseline | |||||
| ≥ 1600 µg per day | |||||
| n | 549 | 547 | |||
| Mean | 2.38 | 2.31 | 0.98 | 0.87 to 1.12 | 0.642 |
| SD | 1.98 | 2.03 | |||
| < 1600 µg per day | |||||
| n | 221 | 215 | |||
| Mean | 1.91 | 2.01 | 1.03 | 0.83 to 1.27 | |
| SD | 1.98 | 1.80 | |||
| Exacerbations and hospital admissions | Trial arm | Model | Estimate | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| Total exacerbations | ||||||
| Total number included in analysis | 753 | 750 | ||||
| Person-years of follow-up | 738.6 | 735.1 | ||||
| Number with at least one exacerbation | 619 | 596 | ||||
| Total number of exacerbations | 1690 | 1678 | ||||
| Mean number of exacerbations | 2.24 | 2.24 | Unadjusted IRR | 1.00 | 0.91 to 1.09 | 0.934 |
| SD (number of exacerbations) | 1.99 | 1.98 | Adjusted IRRa | 0.99 | 0.91 to 1.07 | 0.729 |
| Exacerbations requiring hospital treatment | ||||||
| Total number included in analysis | 753 | 750 | ||||
| Person-years follow-up | 738.6 | 735.1 | ||||
| Number with at least one exacerbation | 99 | 118 | ||||
| Total number of exacerbations | 126 | 172 | ||||
| Mean number of exacerbations | 0.17 | 0.23 | Unadjusted IRR | 0.73 | 0.55 to 0.97 | 0.032 |
| SD (number of exacerbations) | 0.49 | 0.66 | Adjusted IRRa | 0.73 | 0.55 to 0.97 | 0.031 |
| Non-COPD hospital admissions | ||||||
| Total number included in analysis | 744 | 741 | ||||
| Number with at least one admission | 77 | 87 | ||||
| Total number of admissions | 111 | 111 | ||||
| Mean admission rate | 0.15 | 0.15 | Unadjusted IRR | 0.99 | 0.71 to 1.38 | 0.949 |
| SD (admission rate) | 0.54 | 0.45 | Adjusted IRRa | 1.03 | 0.74 to 1.43 | 0.875 |
| Outcome | Trial arm | Model | Overall mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Theophylline | Placebo | |||||
| % predicted FEV1 | ||||||
| Baseline | ||||||
| Total n | 750 | 743 | ||||
| Mean | 51.4 | 52.4 | ||||
| SD | 20.0 | 19.8 | ||||
| 6 months | ||||||
| Total n | 548 | 535 | ||||
| Mean | 52.4 | 53.2 | ||||
| SD | 20.4 | 20.9 | ||||
| 12 months | ||||||
| Total n | 533 | 488 | ||||
| Mean | 51.5 | 52.2 | Unadjusted | –0.59 | –2.54 to 1.36 | 0.551 |
| SD | 20.4 | 21.6 | Adjusteda | –0.58 | –2.46 to 1.29 | 0.543 |
| % predicted FVC | ||||||
| Baseline | ||||||
| Total n | 748 | 742 | ||||
| Mean | 84.5 | 86.5 | ||||
| SD | 22.2 | 23.5 | ||||
| 6 months | ||||||
| Total n | 543 | 531 | ||||
| Mean | 84.0 | 84.6 | ||||
| SD | 22.74 | 24.8 | ||||
| 12 months | ||||||
| Total n | 525 | 485 | ||||
| Mean | 83.1 | 82.5 | Unadjusted | –0.45 | –2.59 to 1.69 | 0.678 |
| SD | 23.8 | 25.1 | Adjusteda | –0.37 | –2.43 to 1.69 | 0.723 |
| CAT score | ||||||
| Baseline | ||||||
| Total n | 745 | 742 | ||||
| Mean | 22.7 | 22.3 | ||||
| SD | 7.6 | 7.9 | ||||
| 6 months | ||||||
| Total n | 668 | 653 | ||||
| Mean | 21.2 | 21.1 | ||||
| SD | 8.1 | 8.3 | ||||
| 12 months | ||||||
| Total n | 633 | 615 | ||||
| Mean | 21.4 | 21.4 | Unadjusted | 0.16 | –0.56 to 0.89 | 0.661 |
| SD | 8.2 | 8.6 | Adjusteda | 0.02 | –0.65 to 0.69 | 0.950 |
| HARQ score | ||||||
| Baseline | ||||||
| Total n | 193 | 197 | ||||
| Mean | 25.2 | 25.8 | ||||
| SD | 16.1 | 14.9 | ||||
| 6 months | ||||||
| Total n | 189 | 187 | ||||
| Mean | 21.9 | 22.8 | ||||
| SD | 15.13 | 15.7 | ||||
| 12 months | ||||||
| Total n | 184 | 172 | ||||
| Mean | 24.1 | 24.2 | Unadjusted | –0.62 | –3.15 to 1.91 | 0.631 |
| SD | 15.70 | 15.94 | Adjusteda | –0.89 | –3.27 to 1.50 | 0.468 |
Appendix 6 Line listings of serious adverse events
| Case ID | Country, sex, age (years) | Event | Outcome | Date of onset | Assessment of relatedness to trial drug | Daily dose route formulation | Dates of treatment | Comments |
|---|---|---|---|---|---|---|---|---|
| System Organ Class: cardiac disorders | ||||||||
| ID 070 | UK, female, 72 | 2 : 1 AV block | Recovered with sequelae | April 2015 | Possible | 200 mg of theophylline od | 4 March–12 April 2015 | PI disputed diagnosis of AV block made by cardiology team. Chief investigator noted normal ECG after discontinuing study drug prior to development of AV block, also noted that theophylline increased heart rate rather than to slow it and therefore unlikely to be related to study drug |
| ID 143 | UK, female, 59 | STEMI, PCI to LCx and OM arteries, cor pulmonale, mild to moderate LVSD on ventriculogram | Recovered | 30 March 2016 | Possible | 200 mg of theophylline od | 21–27 March 2016 | PI suggested that there was possible association with study drug. Chief investigator noted that the participant had ceased study medication 3 days prior to the cardiac event and therefore highly unlikely to be related to study drug |
| Case ID | Country, sex, age (years) | SAE | Outcome | Date of onset | Assessment of relatedness to trial drug | Daily dose route formulation | Dates of treatment | Comments |
|---|---|---|---|---|---|---|---|---|
| System Organ Class: cardiac disorders | ||||||||
| 007 | UK, male, 66 | Atypical atrial flutter/atrial tachycardia | Recovered | 25 August 2014 | Possible | 200 mg theophylline bd | 6–12 August 2014 and 19–25 August 2014 | |
| 018 | UK, male, 79 | Syncopal episode resulting in fracture to right fourth metacarpal | Recovered | 8 November 2014 | Possible | 200 mg theophylline od | 30 March 2014–31 March 2015 | |
| 029 | UK, male, 81 | Atrial fibrillation | Not recovered | 31 October 2014 | Possible | 200 mg theophylline bd | 30 April 2014–26 January 2015 | |
| 072 | UK, male, 82 | Non-sustained ventricular tachycardia | Recovered with sequelae | 14 August 2015 | Possible | 200 mg theophylline od | 25 August 2014–14 August 2015 | |
| 186 | UK, male, 76 | Palpitations | Not recovered | 11 August 2016 | Possible | 200 mg placebo od | 4–11 August 2016 | |
| 189 | UK, female, 73 | Palpitations and tachycardia | Not recovered | 16 August 2016 | Possible | 200 mg theophylline od | 16 February–5 September 2016 | |
| 213 | UK, female, 54 | Palpitations | Unknown | 12 October 2016 | Probable | 200 mg placebo od | 27 May–17 October 2016 | |
| 233 | UK, male, 73 | Sinus tachycardia | Recovered | 18 November 2016 | Possible | 200 mg placebo od | 25 November 2015–ongoing at time of event | |
| System Organ Class: gastrointestinal disorders | ||||||||
| 103 | UK, female, 71 | Dyspeptic pain | Recovered | 22 October 2015 | Possible | 200 mg placebo od | 12–27 October 2015 | |
| System Organ Class: investigations | ||||||||
| 268 | UK, male, 76 | Weight loss, lethargy | Unknown | 19 April 2017 | Possible | 200 mg placebo od | 9 June 2016–ongoing at time of event | |
| Case ID | Country, sex, age (years) | SAE | Outcome | Date of onset | Daily dose route formulation | Dates of treatment | Comments |
|---|---|---|---|---|---|---|---|
| System Organ Class: infections and infestations | |||||||
| 012 | UK, male, 74 | Infected elbow | Recovering | 30 September 2014 | 200 mg of placebo od | 12 September 2014–ongoing | |
| 055 | UK, female, 54 | Right leg cellulitis | Recovered | 10 June 2015 | 200 mg of theophylline od | 9–11 December 2014 | |
| 060b | UK, female, 82 | UTI | Unknown | 9 July 2015 | 200 mg of placebo od | Never commenced trial medication (trial medication not dispensed) | Did not start/initiate study medication |
| 071 | UK, female, 76 | Sepsis | Recovered with sequelae | 19 August 2015 | 200 mg of placebo od | 1 July 2015–ongoing at time of event | |
| 081 | UK, male, 70 | Left arm cellulitis | Recovered | 16 September 2015 | 200 mg of placebo od | 3 April–16 September 2015 | |
| 110 | UK, female, 75 | Cellulitis lower leg secondary to cat bite | Unknown | 13 December 2015 | 200 mg of theophylline od | 2 December 2015–ongoing at time of event | |
| 120 | UK, female, 77 | Cellulitis, delirium | Recovered | 13 December 2015 | 200 mg of theophylline od | 13 February 2015–ongoing at time of event | |
| 127 | UK, female, 82 | UTI | Recovered | 29 December 2015 | 200 mg of theophylline od | 27 February–29 December 2015 | |
| 128 | UK, female, 71 | Sepsis | Unknown | 8 February 2016 | 200 mg of placebo od | 26 January 2016–ongoing at time of event | |
| 134 | UK, male, 79 | UTI and reduced mobility | Recovered | 26 November 2015 | 200 mg of theophylline bd | 16 September 2015–25 January 2016 | |
| 174 | UK, female, 64 | Infection, unknown source | Recovered | 28 May 2016 | 200 mg of theophylline od | 23 June 2015–ongoing at time of event | |
| 181 | UK, female, 76 | Atrial flutter secondary to sepsis from lower-limb cellulitis | Recovering | 4 August 2016 | 200 mg of theophylline od | 19 May 2016–ongoing at time of event | Recorded as infection as this was the primary driver of atrial flutter |
| 201 | UK, male, 57 | Exacerbation of COPD, leading to type 2 respiratory failure, bilateral leg swelling and also became infected Clostridium difficile while in hospital | Unknown | 13 June 2016 | 200 mg of placebo od | 19 April 2016–13 July 2017 | Recorded as infection because of clostridium difficile infection. The exacerbation of COPD captured as primary outcome |
| 203 | UK, female, 78 | Gram-negative bacteraemia | Recovered | 20 August 2016 | 200 mg of placebo od | 15 July–5 September 2016 | |
| 217 | UK, male, 69 | Cellulitis | Recovered | 12 February 2015 | 200 mg of theophylline od | 7 July–24 November 2014 | |
| 220 | UK, female, 77 | Infective gastroenteritis | Recovered | 4 May 2016 | 200 mg of theophylline od | Never commenced trial medication | Did not start/initiate study medication |
| 221 | UK, female, 77 | Cellulitis | Recovered | 10 July 2016 | 200 mg of theophylline od | Never commenced trial medication | Did not start/initiate study medication |
| 222 | UK, female, 77 | Confusion, possible secondary to cellulitis | Recovered | 2 October 2016 | 200 mg of theophylline od | Never commenced trial medication | Did not start/initiate study medicationRecorded as infection as this was the primary driver of confusion |
| 225 | UK, male, 78 | Sepsis | Recovered | 13 November 2016 | 200 mg of placebo od | 14 June 2016–ongoing at time of event | |
| 239 | UK, male, 74 | Fall/possible sepsis | Unknown | 28 December 2016 | 200 mg of theophylline od | 2 August 2016 ongoing at time of event | Recorded as infection as this was the primary driver of falls |
| 244 | UK, male, 66 | Ankle joint infection | Recovering | 7 January 2017 | 200 mg of placebo od | 22 January 2016–ongoing at time of event | |
| 247 | UK, female, 65 | UTI | Recovered | 10 October 2016 | 200 mg of placebo od | 3 August 2016–ongoing at time of event | |
| 249 | UK, male, 77 | UTI | Recovered | 19 January 2017 | 200 mg of theophylline od | 9 February 2016–ongoing at time of event | |
| 251 | UK, female, 71 | Periumbilical abscess | Recovered | 6 October 2016 | 200 mg of theophylline od | 27 November 2015–ongoing at time of event | |
| 253 | UK, female, 60 | UTI and possible viral gastroenteritis | Recovered | 10 December 2016 | 200 mg of theophylline od | 21–29 March 2016 | |
| 281 | UK, male, 71 | Gastroenteritis | Recovered | 6 February 2017 | 200 mg of theophylline od | 9 June–20 July 2016 | |
| System Organ Class: neoplasm (benign, malignant and unspecified) | |||||||
| 010 | UK, female, 74 | Moderately differentiated squamous cell carcinoma of supraglottic submucosal T3 N2c M0 | Recovered | Unknown: reported 29 September 2014 | 200 mg of theophylline od | 14 April 2014–28 February 2015 | |
| 011 | UK, male, 68 | Lung cancer | Not recovered | Unknown: reported 30 September 2014 | 200 mg of placebo od | 19 May–30 September 2014 | |
| 017 | UK, female, 71 | Left lower lobe lesion with pleural effusion | Unknown | 29 October 2014 | 200 mg of theophylline od | 19 May–13 November 2014 | |
| 019 | UK, male, 68 | Metastatic lung cancer stage T2a N3 M1b | Fatal | 18 November 2014 | 200 mg of theophylline od | 7 July–27 August 2014 | |
| 021 | UK, female, 85 | Metastatic bladder cancer | Fatal | 7 October 2014 | 200 mg of placebo od | 2 April–2 June 2014 | |
| 022 | UK, male, 70 | Perforated caecal tumour | Fatal | 24 November 2014 | 200 mg of theophylline od | 24 July–November 2014 | |
| 039 | UK, female, 70 | Intermediate-grade neuroendocrine tumour/atypical carcinoid | Recovering | 16 January 2015 | 200 mg of placebo od | 7 March–3 June 2015 | |
| 040 | UK, female, 77 | Large pelvic mass/sigmoid carcinoma | Not recovered | 12 April 2014 | 200 mg of theophylline od | 21 May 2014–25 April 2015 | |
| 059 | UK, female, 79 | Lung malignancy | Unknown | 6 June 2015 | 200 mg of theophylline od | 17 July 2014–July 2015 | |
| 068 | UK, male, 83 | Lung cancer | Fatal | 12 August 2015 | 200 mg of theophylline od | 18 August 2014–27 May 2015 | |
| 078 | UK, female, 66 | Metastatic colonic malignancy | Fatal | 16 July 2015 | 200 mg of placebo od | 16 December 2014–19 January 2015 | |
| 109 | UK, male, 63 | Left breast cancer | Recovering | 4 November 2015 | 200 mg of theophylline twice daily | 24 June 2015–ongoing at time of event | |
| 112 | UK, male, 61 | Laryngeal cancer | Fatal | 11 September 2015 | 200 mg of placebo od | Never commenced trial medication | Did not start/initiate study medication |
| 117 | UK, female, 64 | Right hilar mass | Not recovered | 15 December 2015 | 200 mg of theophylline od | 5–25 August 2015 | |
| 123 | UK, male, 80 | Metastatic disease in the liver with no obvious primary | Unknown | 11 December 2015 | 200 mg of placebo od | 21 July–31 August 2015 | |
| 132 | UK, female, 67 | Central tumour, mediastinal lymphadenopathy, cerebral metastases | Not recovered | 12 February 2016 | 200 mg of theophylline od | 4–19 February 2016 | |
| 141 | UK, male, 67 | Metastatic cancer | Unknown | 17 March 2016 | 200 mg of placebo od | 14 April 2015–13 April 2016 | |
| 147 | UK, female, 72 | Lung cancer | Not recovered | 1 April 2016 | 200 mg of theophylline od | 15 October 2015–ongoing at time of event | |
| 148 | UK, female, 72 | Haemoptysis secondary to lung cancer | Recovering | 1 April 2016 | 200 mg of theophylline od | 15 October 2015–ongoing at time of event | |
| 160 | UK, female, 76 | Pancreatic malignancy with biliary obstruction | Fatal | 3 May 2016 | 200 mg of theophylline od | 17 March–17 May 2016 | |
| 202 | UK, male, 61 | T2 N2b squamous cell carcinoma of his right pyriform fossa | Unknown | 8 March 2016 | 200 mg of placebo od | 28 August 2015–ongoing at time of event | |
| 219 | UK, female, 68 | Lung neoplasm | Not recovered | Unknown | 200 mg of theophylline od | 19 July 2016–3 January 2017 | |
| 228 | UK, female, 59 | Investigation following CT scan showing nodule – primary lung tumour | Unknown | 15 November 2016 | 200 mg of placebo od | 1 June 2016–ongoing at time of event | |
| 231 | UK, male, 70 | Lung cancer | Not recovered | 4 November 2016 | 200 mg of placebo twice daily | 3 December 2015–ongoing at time of event | |
| 238 | UK, male, 70 | Grade 2 prostate cancer | Unknown | 22 December 2016 | 200 mg of placebo od | 28 July 2016–ongoing at time of event | |
| 241 | UK, female, 58 | Mastectomy for breast cancer | Recovering | 19 December 2016 | 200 mg of theophylline od | 18 February 2016–ongoing at time of event | |
| 254 | UK, female, 67 | Right breast cancer | Unknown | 2 March 2017 | 200 mg of theophylline od | 27 May 2016–ongoing at time of event | |
| 260 | UK, female, 81 | Uterine cancer | Recovering | 27 February 2017 | 200 mg of theophylline od | 3 March 2016–ongoing at time of event | |
| 263 | UK, female, 64 | Chronic lymphocytic leukaemia | Not recovered | 5 April 2017 | 200 mg of placebo od | 25 August 2016–ongoing at time of event | |
| 286 | UK, male, 70 | Hepatic flexure cancer (Dukes’ B) | Recovering | 20 July 2017 | 200 mg of theophylline od | 4 August 2016–ongoing at time of event | |
| 289 | UK, female, 82 | Classification of death: Ia metastatic cholangiocarcinoma, II COPD | Fatal | 8 November 2017 | 200 mg of placebo od | Never commenced trial medication | Did not start/initiate study medication |
| System Organ Class: blood and lymphatic system disorders | |||||||
| 173 | UK, male, 71 | Iron deficiency anaemia | Recovered | 18 February 2016 | 200 mg of placebo od | 29 June 2015–ongoing at time of event | |
| 184 | UK, female, 72 | Abdominal haematoma | Recovering | 3 May 2016 | 200 mg of placebo od | 7 August 2015–ongoing at time of event | |
| System Organ Class: immune system disorders | |||||||
| None | |||||||
| System Organ Class: endocrine disorders | |||||||
| None | |||||||
| System Organ Class: metabolism and nutrition disorders | |||||||
| 245 | UK, male, 79 | Dysphagia | Unknown | 27 January 2017 | 200 mg of theophylline od | 14 July 2016–31 January 2017 | |
| 246 | UK, male, 79 | Refeeding syndrome | Unknown | 28 January 2017 | 200 mg of theophylline od | 14 July 2016–31 January 2017 | |
| System Organ Class: psychiatric disorders | |||||||
| 073 | UK, male, 57 | Overdose of amitriptyline and alcohol | Recovering | 1 September 2015 | 200 mg of placebo od | 4 August–2 September 2015 | |
| 098 | UK, male, 58 | Overdose of amitriptyline and alcohol | Not recovered | 8 November 2015 | 200 mg of placebo od | 4 August–2 September 2015 | |
| 146 | UK, male, 59 | Admission to psychiatric ward with a depressive episode | Recovered | 24 July 2015 | 200 mg of placebo bd | 8 April–30 June 2015 | |
| 183 | UK, male, 73 | Death (suicide) as a result of asphyxiation | Fatal | 13 July 2016 | 200 mg of theophylline od | 9 September 2015–13 July 2016 | |
| System Organ Class: nervous system disorders | |||||||
| 014 | UK, male, 76 | TIA, atrial fibrillation | Recovered with sequelae | 1 September 2014 | 200 mg of theophylline bd | 3 June 2014–9 January 2015 | |
| 025 | UK, male, 76 | Stroke | Recovering | 11 November 2014 | 200 mg of theophylline bd | 3 June 2014–November 2014, restarted briefly at start of January 2015 | |
| 027 | UK, male, 66 | Seizure secondary to intracerebral haemorrhage | Recovered | 13 January 2015 | 200 mg of theophylline od | 31 March 2014–26 March 2015 | |
| 033 | UK, female, 44 | Headache | Recovered | 19 February 2015 | 200 mg of theophylline od | 7 January 2015–ongoing at time of event | |
| 043 | UK, male, 65 | Subdural bleed/haematoma as result of fall prior to trial inclusion | Unknown | 1 April 2015 | 200 mg of theophylline od | 23 April 2015–ongoing at time of event | |
| 050 | UK, female, 78 | Suspected cerebral infarct | Recovering | 23 May 2015 | 200 mg of placebo od | 30 April–21 May 2015 | |
| 064 | UK, male, 78 | Subdural haemorrhage | Fatal | 20 June 2015 | 200 mg of theophylline od | 23 April–23 July 2015 | |
| 077 | UK, male, 73 | Spinal canal stenosis | Recovered | 10 September 2015 | 200 mg of theophylline od | 21 April–9 September 2015 | |
| 080 | UK, female, 82 | Partial anterior circulation infarct | Recovered | 7 September 2015 | 200 mg of theophylline od | 27 February–7 September 2015 | |
| 085 | UK, male, 73 | Chest pain/spinal stenosis | Recovered | 20 July 2015 | 200 mg of theophylline od | 13 October 2014–12 October 2015 | |
| 099 | UK, male, 78 | Lewy body dementia | Recovered | 6 November 2015 | 200 mg of placebo od | 1 December 2014–ongoing at time of event | |
| 107 | UK, female, 82 | Right total anterior circulation stroke syndrome or Todd’s palsy | Recovered | 21 October 2015 | 200 mg of theophylline od | 27 February 2015–ongoing at time of event | |
| 114 | UK, male, 76 | Bilateral thalamic infarct | Not recovered | 31 December 2015 | 200 mg of theophylline od | 15 September 2015–ongoing at time of event | |
| 118 | UK, male, 81 | CVA | Recovering | 31 December 2015 | 200 mg of placebo od | 30 January 2015, then stopped trial drugs as soon as admitted | |
| 131 | UK, female, 58 | Possible TIA | Recovering | 18 January 2016 | 200 mg of placebo od | 24 March–18 August 2015 | |
| 140 | UK, female, 72 | Dizziness and vomiting | Recovered with sequelae | 22 March 2016 | 200 mg of theophylline od | 1 April 2015–ongoing at time of event | |
| 151 | UK, male, 62 | Confusion with worsening headache | Recovered | 16 December 2015 | 200 mg of placebo od | 10 April 2015–15 April 2016 | |
| 172 | UK, male, 72 | Ischaemic stroke, community-acquired pneumonia | Recovering | 1 July 2016 | 200 mg of theophylline od | 2 February 2016–ongoing at time of event | |
| 176 | UK, female, 79 | Subarachnoid haemorrhage | Unknown | 6 June 2016 | 200 mg of placebo od | 15 September 2015–6 June 2016 | |
| 269 | UK, male, 68 | Frontal lobe dementia | Fatal | Unknown | 200 mg of placebo od | 12 April–18 October 2016 | |
| System Organ Class: eye disorders | |||||||
| None | |||||||
| System Organ Class: ear and labyrinth disorders | |||||||
| None | |||||||
| System Organ Class: cardiac disorders | |||||||
| 004 | UK, female, 84 | Pulmonary oedema | Recovered | 24 June 2014 | 200 mg of placebo od | 9 April–20 June 2014 | |
| 013 | UK, male, 72 | Death: cause of death – myocardial infarction, infective exacerbation of COPD, atrial fibrillation | Fatal | 1 October 2014 | 200 mg of placebo od | 30 May–13 August 2014 | |
| 026 | UK, male, 90 | Orthostatic hypotension | Recovered with sequelae | 28 December 2014 | 200 mg of theophylline od | 7 May–6 June 2014 | |
| 031 | UK, male, 75 | Out-of-hospital cardiac arrest; end-stage COPD; mitral valve prolapse | Fatal | 8 January 2015 | 200 mg of theophylline od | 21 June–4 September 2014 | |
| 032 | UK, male, 57 | Chest pain | Recovered | 1 December 2014 | 200 mg of placebo od | 31 March 2014–2 April 2015 | |
| 036 | UK, male, 71 | Anterolateral NSTEMI (myocardial infarction) | Recovered | 26 February 2015 | 200 mg of placebo od | 13 March 2014–26 February 2015 | |
| 042 | UK, male, 75 | Cardiac arrest at home; carcinoma of the right upper lobe and COPD | Fatal | 28 April 2015 | 200 mg of placebo od | 18 August 2014–April 2015 | |
| 052 | UK, female, 76 | Angina | Recovered with sequelae | 11 July 2014 | 200 mg of theophylline od | 3 April 2014–2 April 2015 | |
| 053 | UK, female, 76 | Angina | Recovered with sequelae | 31 July 2014 | 200 mg of theophylline od | 3 April 2014–2 April 2015 | |
| 056 | UK, male, 65 | Atrial fibrillation/flutter | Recovered with sequelae | 16 June 2014 | 200 mg of placebo od | 1–16 June 2015 | Initially reported as possibly related to the study medication; at SAE follow-up, reported as having no relationship to study medication |
| 062 | UK, female, 78 | Carotid vascular disease | Recovered with sequelae | 4 June 2015 | 200 mg of placebo od | 29 April–12 May 2015 | |
| 063 | UK, female, 78 | Angina | Recovered with sequelae | 5 June 2015 | 200 mg of placebo od | 29 April–12 May 2015 | |
| 079 | UK, female, 82 | Missed STEMI versus broken heart syndrome | Recovered | 16 August 2015 | 200 mg of theophylline od | 27 February–16 August 2015 | |
| 082 | UK, female, 70 | Fast atrial fibrillation | Recovering | 2 October 2015 | 200 mg of placebo od | 25 November 2014–28 July 2015 | |
| 086 | UK, male, 59 | Unstable angina | Recovered | 9 September 2015 | 200 mg of placebo od | 8 April–30 June 2015 | |
| 097 | UK, female, 74 | Left ventricular failure | Recovered | 1 November 2015 | 200 mg of placebo od | 6 January 2015–ongoing at time of event | |
| 102 | UK, male, 82 | Collapse not otherwise specified | Unknown | 25 November 2015 | 200 mg of placebo od | 21 May 2015–ongoing at time of event | |
| 119 | UK, female, 77 | Exacerbation of COPD; pulmonary congestion | Recovered | 4 December 2015 | 200 mg of theophylline od | 13 February 2015–ongoing at time of event | |
| 122 | UK, female, 77 | Left ventricular failure, secondary to acute MI, secondary sepsis, secondary to pneumonia | Fatal | 17 January 2016 | 200 mg of theophylline od | 14 February 2015–18 January 2016 | Recorded as cardiac because of pulmonary congestion, the exacerbation of COPD was captured as primary outcome |
| 126 | UK, female, 82 | Congestive cardiac failure | Recovered | 16 December 2015 | 200 mg of theophylline od | 27 February–16 December 2015 | |
| 133 | UK, male, 69 | Cardiac arrest | Fatal | 26 January 2016 | 200 mg of placebo od | 21 October 2015–26 January 2016 | |
| 149 | UK, female, 62 | Heart failure | Recovered | 1 February 2016 | 200 mg of placebo od | 14 April 2015–ongoing at time of event | |
| 164 | UK, male, 79 | Cardiac arrest | Fatal | 12 May 2016 | 200 mg of theophylline od | 31 October–19 December 2015 | |
| 178 | UK, male, 78 | Heart failure, acute kidney injury | Fatal | 21 July 2016 | 200 mg of theophylline od | 1 June–21 July 2016 | |
| 179 | UK, male, 68 | Cardiac arrest | Fatal | 9 July 2016 | 200 mg of theophylline od | 27 November 2015–8 July 2016 | |
| 185 | UK, male, 76 | Possible heart attack | Not recovered | 12 August 2016 | 200 mg of placebo od | 3 June–23 September 2016 | |
| 192 | UK, female, 64 | Narrow complex tachycardia, exacerbation of COPD | Recovering | 29 June 2016 | 200 mg of theophylline od | 26 August 2015–ongoing at time of event | |
| 195 | UK, male, 86 | Chest pain – probably angina | Recovered | 11 February 2015 | 200 mg of placebo od | 5–11 September 2014 | |
| 196 | UK, male, 86 | Unstable angina | Recovered | 9 June 2015 | 200 mg of placebo od | 5–11 September 2014 | |
| 205 | UK, male, 55 | Acute coronary syndrome | Unknown | 14 September 2016 | 200 mg of placebo twice daily | 5 February 2016–ongoing at time of event | |
| 223 | UK, male, 73 | Acute myocardial infarction | Unknown | 24 October 2016 | 200 mg of placebo od | 7 June 2016–ongoing at time of event | |
| 240 | UK, female, 78 | Heart failure, moderate to severe aortic stenosis | Unknown | 29 November 2016 | 200 mg of placebo od | 15 July–5 September 2016 | |
| 242 | UK, male, 73 | NSTEMI | Recovered with sequelae | 15 October 2016 | 200 mg of theophylline od | 1 February 2016–9 January 2017 | |
| 243 | UK, male, 63 | Congestive heart failure | Recovering | 21 December 2016 | 200 mg of placebo twice daily | 26 July 2016–ongoing at time of event | |
| 250 | UK, female, 69 | STEMI | Recovered | 23 February 2016 | 200 mg of placebo od | 27 February–30 July 2015 | |
| 258 | UK, male, 88 | NSTEMI | Recovered | 9 October 2016 | 200 mg of placebo od | 13 November 2015–ongoing at time of event | |
| 265 | UK, male, 64 | End-stage congestive cardiac failure | Fatal | 12 April 2017 | 200 mg of placebo twice daily | 26 July 2016–30 April 2017 | |
| 276 | UK, male, 68 | Acute pulmonary oedema | Fatal | 1 June 2017 | 200 mg of theophylline od | 14 July 2016–1 June 2017 | |
| 282 | UK, female, 65 | Atrial fibrillation and heart failure | Recovered | 10 June 2016 | 200 mg of placebo od | 11 May 2016–ongoing at time of event | |
| 284 | UK, female, 69 | Postural hypotension | Recovered | 31 May 2017 | 200 mg of placebo od | 11 August 2016–ongoing at time of event | |
| System Organ Class: vascular disorder | |||||||
| 001 | UK, male, 57 | Old cerebellar gliosis/stroke | Recovered | 25 April 2014 | 200 mg of placebo od | 31 March 2014–ongoing at time of event | |
| 066 | UK, male, 62 | COPD with lower respiratory tract infection and DVT | Recovered | 20 June 2015 | 200 mg of placebo od | 18 September–October 2014 (18 doses in total) | Recorded as vascular because exacerbation of COPD captured as primary outcome |
| 105 | UK, male, 75 | Ruptured abdominal aortic aneurysm | Fatal | 29 November 2015 | 200 mg of theophylline twice daily | 14 October–29 November 2015 | |
| 168 | UK, female, 76 | Right leg DVT | Recovering | 14 June 2016 | 200 mg of theophylline od | 2 December 2015–ongoing at time of event | |
| 182 | UK, female, 72 | Collapse | Unknown | 5 August 2016 | 200 mg of placebo od | 26 January 2016–ongoing at time of event | |
| 224 | UK, male, 78 | Intracerebral haemorrhage | Recovered | 6 November 2016 | 200 mg of placebo od | 14 June 2016–ongoing at time of event | |
| 252 | UK, male, 69 | Right ICA occlusion | Recovering | 26 December 2016 | 200 mg of placebo od | 1 March 2016–17 February 2017 | |
| 255 | UK, male, 74 | Bilateral subdural haematomas | Recovered with sequelae | 28 December 2015 | 200 mg of theophylline od | 21 April–17 September 2015 | |
| 256 | UK, male, 74 | DVT/pulmonary embolism | Recovered with sequelae | 28 December 2015 | 200 mg of theophylline od | 21 April–17 September 2015 | |
| 267 | UK, female, 73 | Uncontrolled hypertension | Recovered | 13 April 2017 | 200 mg of theophylline od | 13 April 2016–ongoing at time of event | |
| 270 | UK, male, 68 | Collapse, cause unknown | Recovered | 17 June 2016 | 200 mg of placebo od | 12 April–18 October 2016 | |
| 285 | UK, male, 77 | Ruptured abdominal aortic aneurysm | Fatal | 7 August 2017 | 200 mg of theophylline od | 17 August–9 October 2016 | |
| System Organ Class: respiratory, thoracic and mediastinal disorders | |||||||
| 002 | UK, male, 70 | Death: pneumonia, diabetic hypoglycaemia | Fatal | 12 May 2014 | 200 mg of theophylline od | 24 April–11 May 2014 | |
| 003 | UK, female, 74 | Pulmonary embolism | Recovered | 24 June 2014 | 200 mg of placebo od | 16 June 2014–ongoing at time of event | |
| 015 | UK, female, 65 | Death: exacerbation of COPD | Fatal | 30 October 2014 | 200 mg of placebo od | 24 June–30 October 2014 | |
| 023 | UK, female, 69 | Chest infection/chest pain/left upper rib fracture | Recovered | 17 October 2014 | 200 mg of placebo od | 25–29 July 2014 | |
| 024 | UK, female, 68 | Death: type 2 respiratory failure, COPD, multiple sclerosis | Fatal | 20 December 2014 | 200 mg of theophylline od | 22 May–27 August 2014 | |
| 034 | UK, male, 74 | Death: severe COPD | Fatal | 16 February 2015 | 200 mg of placebo od | 16 June 2014–February 2015 | |
| 035 | UK, male, 46 | Hyperventilation | Recovered | 11 November 2014 | 200 mg of placebo bd | 10 September 2014–ongoing at time of event | |
| 044 | UK, male, 73 | Death: exacerbation of COPD | Fatal | 11 April 2015 | 200 mg of theophylline od | 18 February–7 April 2015 | |
| 046 | UK, male, 90 | Symptomatic pleural effusions, hospital-acquired pneumonia | Recovered with sequelae | 11 May 2015 | 200 mg of theophylline od | 7 May 2014–18 May 2015 | |
| 047 | UK, male, 47 | Shortness of breath; most likely exacerbation of COPD | Recovered | 6 December 2015 | 200 mg of placebo bd | 10 September 2014–ongoing at time of event | |
| 057 | UK, female, 73 | Death: end-stage COPD | Fatal | 15 June 2015 | 200 mg of placebo od | 3–31 March 2015 | |
| 058 | UK, female, 74 | Pleuritic chest pain | Recovered | 15 March 2015 | 200 mg of theophylline od | 10 July–11 August 2014 | |
| 061 | UK, male, 72 | Pleuritic chest pain | Recovered | 20 July 2015 | 200 mg of theophylline od | 10 July 2015–ongoing at time of event | |
| 067 | UK, male, 62 | Community-acquired pneumonia, vomited, aspirated and cardiac arrest | Fatal | 17 July 2015 | 200 mg of placebo od | 18 September–October 2014 (18 doses in total) | |
| 069 | UK, female, 67 | Pleuritic chest pain | Recovered | 24 February 2015 | 200 mg of theophylline od | 27 August–9 November 2014 | |
| 083 | UK, male, 71 | Increased breathlessness | Recovered | 1 April 2015 | 200 mg of theophylline od | 23 September 2014–12 October 2015 | |
| 088 | UK, male, 79 | Cor pulmonale secondary to COPD | Fatal | 4 October 2015 | 200 mg of placebo od | 21 April–7 September 2015 | |
| 089 | UK, male, 83 | Infective exacerbation of COPD, pleural effusion | Unknown | 11 July 2015 | 200 mg of theophylline od | 18 August 2014–27 May 2015 | |
| 093 | UK, male, 78 | Shortness of breath | Recovering | 9 October 2015 | 200 mg of placebo od | 1 December 2014–ongoing at time of event | |
| 116 | UK, female, 71 | Pulmonary embolism | Recovering | 19 December 2015 | 200 mg of placebo od | 7 August 2015–ongoing at time of event | |
| 124 | UK, male, 73 | (1a) Acute kidney injury, (1b) septicaemia, (1c) lower respiratory tract infection; (2) COPD, atrial fibrillation, acromegaly | Fatal | 9 April 2015 | 200 mg of placebo od | 1 May 2014–8 April 2015 | Recorded as respiratory because the prime driver was lower respiratory tract infection and acute kidney injury, with septicaemia a secondary driver |
| 125 | UK, male, 80 | Pneumonia | Fatal | 31 December 2015 | 200 mg of placebo od | 24 November–30 December 2015 | |
| 154 | UK, female, 72 | Pleuritic chest pain | Recovered | 12 January 2016 | 200 mg of theophylline od | 14 November–20 December 2015 | |
| 155 | UK, male, 55 | Renal failure, secondary to chest infection | Fatal | 12 April 2016 | 200 mg of placebo bd | 20 October 2015–April 2016 | Recorded as respiratory because prime driver was lower respiratory tract infection, renal failure secondary |
| 165 | UK, male, 71 | Haemoptysis | Recovered | 11 May 2016 | 200 mg of placebo od | 29 June 2015–ongoing at time of event | |
| 166 | UK, male, 76 | Bronchiectasis | Recovered | 7 March 2016 | 200 mg of placebo od | 10 December 2015–ongoing at time of event | |
| 170 | UK, male, 72 | Pneumothorax | Recovered | 1 May 2016 | 200 mg of placebo od | 8 July 2015–ongoing at time of event | |
| 175 | UK, female, 64 | Respiratory failure and CO2 narcosis following exacerbation of COPD and chest infection | Recovering | 12 May 2016 | 200 mg of theophylline od | 14 March–12 May 2016 | |
| 177 | UK, female, 71 | Pulmonary embolism | Recovering | 18 July 2016 | 200 mg of theophylline od | 28 November 2015–ongoing at time of event | |
| 197 | UK, male, 70 | Pneumonia, pulmonary embolism, cavitating lesion on CT scan of chest | Recovered with sequelea | 22 August 2016 | 200 mg of placebo od | 4 May–23 August 2016 | |
| 208 | UK, male, 63 | Right pneumothorax | Recovered | 27 June 2014 | 200 mg of theophylline od | 24 April–23 June 2014 | |
| 209 | UK, male, 63 | Right pneumothorax | Recovered | 31 August 2014 | 200 mg of theophylline od | 24 April–23 June 2014 | |
| 212 | UK, female, 64 | Pleurisy or musculoskeletal pain | Recovered | 10 February 2016 | 200 mg of theophylline od | 9 April 2015–ongoing at time of event | |
| 226 | UK, female, 59 | Bronchiectasis | Unknown | 15 November 2016 | 200 mg of placebo od | 1 June 2016–ongoing at time of event | |
| 230 | UK, female, 55 | Hypoxia | Recovering | 28 November 2016 | 200 mg of placebo od | 20 January 2016–ongoing at time of event | |
| 234 | UK, male, 62 | COPD | Fatal | Unknown | 200 mg of theophylline od | 19 January 2016–ongoing at time of event | |
| 248 | UK, male, 79 | Aspiration pneumonia | Fatal | 2 February 2017 | 200 mg of theophylline od | 14 July 2016–31 January 2017 | |
| 257 | UK, male, 88 | Chest infection | Fatal | 20 February 2017 | 200 mg of theophylline od | 6 March 2016–25 February 2017 | |
| 264 | UK, male, 68 |
|
Fatal | 22 November 2016 | 200 mg of placebo od | 12 April–18 October 2016 | |
| 266 | UK, female, 71 | Pleuritic chest pain | Recovered | 12 July 2015 | 200 mg of placebo od | 15 September 2014–2 February 2015 | |
| 288 | UK, female, 78 | Death, pneumonia, severe COPD, frailty | Fatal | 9 December 2015 | 200 mg of theophylline od | 20 April–17 November 2015 | |
| System Organ Class: gastrointestinal disorders | |||||||
| 009 | UK, male, 55 | Adhesional bowel obstruction | Recovered | 11 September 2014 | 200 mg of theophylline od | 24 May–1 September 2014 | |
| 016 | UK, male, 76 | Blockage in oesophagus | Recovered | 1 November 2014 | 200 mg of theophylline od | 2 October 2014–ongoing at time of event | |
| 020 | UK, male, 72 | Inflammation of oesophagus | Recovered | 16 September 2014 | 200 mg of placebo od | 8 July 2014–24 June 2015 | |
| 030 | UK, female, 82 | Viral gastroenteritis | Recovered | 15 January 2015 | 200 mg of placebo od | 1 April 2014–15 January 2015 | |
| 037 | UK, female, 43 | Abdominal pain and liver steatosis | Unknown | 11 March 2015 | 200 mg of theophylline od | 13 January 2015–ongoing at time of event | |
| 038 | UK, female, 79 | Vomiting, fever, severe abdominal pain | Recovering | 21 March 2015 | 200 mg of placebo od | 4 February–8 August 2015 | |
| 048 | UK, male, 78 | Diverticulitis | Recovered | 8 September 2014 | 200 mg of placebo od | 6 March–15 October 2014 | |
| 049 | UK, male, 78 | Diverticulitis | Recovered | 8 December 2014 | 200 mg of placebo od | 6 March–15 October 2014 | |
| 051 | UK, female, 72 | Severe constipation | Recovered | 7 June 2015 | 200 mg of theophylline od | 2–5 May 2014 | |
| 054 | UK, female, 54 | Gastritis | Recovered | 25 April 2015 | 200 mg of theophylline od | 9–11 December 2014 | |
| 065 | UK, female, 58 | Diverticulitis | Recovered | 26 July 2015 | 200 mg of placebo od | 3 July 2015–ongoing at time of event | |
| 074 | UK, male, 66 | Appendicitis | Recovered | 28 August 2015 | 200 mg of theophylline od | 29 July–27 August 2015 | |
| 087 | UK, male, 71 | Laparoscopic appendectomy | Recovered | 9 November 2014 | 200 mg of theophylline od | 5 September 2014–ongoing at time of event | |
| 090 | UK, female, 82 | Abdominal pain | Recovered | 5 October 2014 | 200 mg of theophylline od | 14–17 May 2014 | |
| 100 | UK, female, 59 | Strangulated small bowel secondary to hernia | Recovered | 1 November 2014 | 200 mg of placebo od | 4 August–1 September 2014 | |
| 101 | UK, female, 60 | Oesophagitis and oesophageal stricture | Recovered | 6 July 2015 | 200 mg of placebo od | 4 August–1 September 2014 | |
| 111 | UK, male, 71 | Haematemesis | Recovered | 22 November 2014 | 200 mg of placebo od | 20 March 2014–ongoing at time of event | |
| 121 | UK, male, 58 | Perforated duodenal ulcer | Recovered | 25 September 2015 | 200 mg of theophylline od | 3 February 2015–15 January 2016 | |
| 129 | UK, male, 49 | Laparotomy and adhesiolysis following severe abdominal pain | Recovering | 15 February 2016 | 200 mg of theophylline bd | 23 April 2015–ongoing at time of event | |
| 136 | UK, male, 70 | Rectal bleed; possible Infective/ischaemic colitis | Recovering | 6 March 2016 | 200 mg of theophylline od | 12 August–31 December 2015 | |
| 150 | UK, male, 71 | Diverticular disease | Recovered | 6 April 2016 | 200 mg of theophylline od | 22 March 2016–ongoing at time of event | |
| 158 | UK, female, 72 | Nausea and vomiting, acute abdominal pain | Recovering | 3 May 2016 | 200 mg of theophylline od | 26 November 2015–ongoing at time of event | |
| 163 | UK, male, 56 | Anal abscess/fistula | Not recovered | 10 January 2016 | 200 mg of theophylline twice daily | 4 July 2015–ongoing at time of event | |
| 171 | UK, male, 71 | Diverticulitis | Unknown | 27 June 2016 | 200 mg of theophylline od | 21 March–29 June 2016 | |
| 193 | UK, male, 59 | Bowel obstruction | Recovered | 5 August 2016 | 200 mg of theophylline od | 29 July 2016–ongoing at time of event | |
| 198 | UK, female, 59 | Gastroenteritis | Recovered | 1 May 2016 | 200 mg of theophylline od | 21–29 March 2016 | |
| 210 | UK, female, 71 | Acute pancreatitis | Recovering | 6 October 2016 | 200 mg of theophylline od | 27 November 2015–ongoing at time of event | |
| 235 | UK, male, 68 | (COPD) and acute upper gastrointestinal haemorrhage due to duodenal ulcer | Fatal | Unknown | 200 mg of theophylline bd | 28 June–19 December 2016 | Recorded as gastrointestinal because exacerbation of COPD captured as primary outcome |
| 262 | UK, male, 80 | Constipation | Recovered with sequelae | 5 March 2017 | 200 mg of placebo od | 11 May 2016–ongoing at time of event | |
| 275 | UK, female, 88 | Constipation | Recovered | 6 December 2016 | 200 mg of placebo od | 1 June 2016–ongoing at time of event | |
| 277 | UK, male, 76 | Constipation | Recovered | 28 December 2016 | 200 mg of theophylline od | 3–22 June 2016 | |
| 279 | UK, female, 73 | Diverticulitis ‘flare-up’ | Recovered | 2 March 2017 | 200 mg of theophylline od | 18 July–2 August 2016 | |
| 287 | UK, male, 77 | Constipation | Unknown | 8 May 2017 | 200 mg of theophylline od | 17 August–9 October 2016 | |
| System Organ Class: hepatobiliary disorders | |||||||
| 008 | UK, male, 66 | Acute hepatitis | Recovered | 25 August 2014 | 200 mg of placebo bd | 23–25 August 2014 | |
| 130 | UK, female, 67 | Obstructive jaundiced and evidence of intraductal calculi | Recovered | 11 January 2016 | 200 mg of placebo od | 24 February 2015–ongoing at time of event | |
| 138 | UK, female, 68 | Vomiting | Recovered | 22 November 2015 | 200 mg of theophylline od | 20–11 March 2016 | |
| 152 | UK, female, 72 | Cholangitis and laparoscopic cholecystectomy | Recovered | 21 December 2015 | 200 mg of theophylline od | 14 November–20 December 2015 | |
| 236 | UK, male, 76 | Groin pain (possible biliary sepsis) | Recovered | 12 August 2016 | 200 mg of placebo od | 7 June 2016–ongoing at time of event | |
| 273 | UK, male, 87 | Gallstones | Recovered with sequelae | 1 November 2016 | 200 mg of placebo od | 24 August 2016–ongoing at time of event | |
| System Organ Class: skin and subcutaneous tissue disorders | |||||||
| 135 | UK, male, 80 | Skin rash | Recovered | 29 December 2015 | 200 mg of theophylline od | 3 February 2015–14 February 2015 | |
| System Organ Class: musculoskeletal and connective tissue disorders | |||||||
| 006 | UK, male, 61 | Suspected fractured ribs | Recovering | 31 May 2014 | 200 mg of placebo od | 4 March–10 May 2014 | |
| 084 | UK, female, 55 | Chest pain | Recovered | 3 July 2015 | 200 mg of placebo od | 24 April 2015–ongoing at time of event | |
| 092 | UK, female, 58 | Atypical chest pain | Recovered | 27 August 2015 | 200 mg of placebo od | 11 May 2015–ongoing at time of event | |
| 139 | UK, female, 69 | Chest tightness | Recovered | 11 January 2016 | 200 mg of theophylline od | 23 September 2015–ongoing at time of event | |
| 144 | UK, male, 82 | Acute stiff neck | Recovering | 25 February 2016 | 200 mg of placebo bd | 21 May 2015–25 February 2016 | |
| 145 | UK, male, 82 | GP referral owing to swallowing problems and neck pain ongoing at time of event for 2 or 3 weeks | Recovered with sequelae | 21 March 2016 | 200 mg of placebo bd | 21 May 2015–25 February 2016 | |
| 156 | UK, female, 72 | Left rib fracture (osteoporotic, not traumatic) | Recovering | 25 April 2016 | 200 mg of placebo od | 7 August 2015–ongoing at time of event | |
| 159 | UK, male, 71 | Musculoskeletal chest pain | Recovering | 10 May 2016 | 200 mg of theophylline od | 17 November 2015–17 May 2016 | |
| 161 | UK, male, 56 | Hyperaesthesia of insulin injection site | Recovered | 11 December 2015 | 200 mg of placebo od | 25 June 2015–ongoing at time of event | |
| 162 | UK, male, 56 | Right ankle pain possibly due to cellulitis | Recovering | 3 May 2016 | 200 mg of placebo od | 25 June 2015–ongoing at time of event | |
| 187 | UK, male, 61 | Musculoskeletal chest pain | Recovered | 3 May 2014 | 200 mg of theophylline od | 24 April–23 June 2014 | |
| 188 | UK, male, 71 | Chest pain | Recovered | 16 August 2016 | 200 mg of placebo od | 17 November 2015–ongoing at time of event | |
| 199 | UK, female, 59 | Musculoskeletal pain | Recovered | 3 July 2016 | 200 mg of theophylline od | 21–29 March 2016 | |
| 211 | UK, female, 76 | Back pain following fall | Recovered | 14 May 2015 | 200 mg of placebo od | 5–9 June 2014 | |
| 259 | UK, female, 72 | Primary diagnosis of gout of her left big toe, with a secondary diagnosis of infection | Unknown | 1 March 2017 | 200 mg of theophylline od | 18 April 2016–ongoing at time of event | |
| 261 | UK, female, 76 | Abdominal pain | Recovered | 1 February 2017 | 200 mg of placebo od | 26 August 2016–ongoing at time of event | Considered to be of musculoskeletal origin |
| System Organ Class: renal and urinary disorders | |||||||
| 076 | UK, male, 60 | Kidney stones | Recovering | 2 September 2015 | 200 mg of placebo od | 6 January 2015–ongoing at time of event | |
| 104 | UK, male, 83 | Urinary retention | Recovering | 18 November 2015 | 200 mg of placebo od | 28 May 2015–ongoing at time of event | |
| 106 | UK, female, 82 | Acute kidney injury | Recovered | 30 November 2015 | 200 mg of theophylline od | 27 February 2015–ongoing at time of event | |
| 157 | UK, male, 63 | Right renal colic | Recovered | 29 Feb 2016 | 200 mg of placebo bd | 4 November 2015–5 January 2016 | |
| 200 | UK, female, 59 | UTI with stage 1 acute kidney injury | Recovered | 16 August 2016 | 200 mg of theophylline od | 21–29 March 2016 | |
| 207 | UK, female, 75 | Multiresistant Escherichia coli UTI | Recovered | 25 November 2014 | 200 mg of theophylline od | 28 August–1 September 2014 | |
| 229 | UK, female, 73 | Deranged renal function, lower respiratory tract infection | Recovered | 20 November 2016 | 200 mg of theophylline od | 11–20 April 2016 | |
| 237 | UK, male, 76 | Haematuria | Recovering | 2 December 2016 | 200 mg of placebo od | 7 June–12 August 2016 | |
| 280 | UK, male, 83 | Shortness of breath due to fluid overload, secondary to renal disease | Recovered | 30 March 2016 | 200 mg of theophylline od | 2 March–12 April 2016 | |
| 283 | UK, female, 78 | Proximal ureteric stone causing obstruction of left kidney | Recovered | 5 December 2016 | 200 mg of theophylline od | 26 July 2016–ongoing at time of event | |
| System Organ Class: pregnancy, puerperium and perinatal conditions | |||||||
| None | |||||||
| System Organ Class: reproductive system and breast disorders | |||||||
| None | |||||||
| System Organ Class: congenital, familial and genetic disorders | |||||||
| None | |||||||
| System Organ Class: general disorders and administration site conditions | |||||||
| None | |||||||
| System Organ Class: investigations | |||||||
| 113 | UK, female, 55 | Asymptomatic raised calcium levels | Recovered | 4 December 2015 | 200 mg of placebo od | 23 June 2015–ongoing at time of event | |
| System Organ Class: injury, poisoning and procedural complications | |||||||
| 005 | UK, female, 68 | Left tibial plateau fracture | Recovering | 1 July 2014 | 200 mg of theophylline od | 8 May 2014–ongoing at time of event | |
| 028 | UK, female, 67 | Fractured pubic ramus and right acetabulum | Recovered | 12 November 2014 | 200 mg of theophylline od | 22 August–9 November 2014 | |
| 041 | UK, male, 55 | Death: head injury | Fatal | 19 April 2015 | 200 mg of theophylline od | 13 March–19 April 2015 | |
| 075 | UK, male, 90 | Fall (mechanical) | Recovered | 12 March 2015 | 200 mg of placebo od | 4 September 2014–3 September 2015 | |
| 091 | UK, female, 58 | Rectus sheath haematoma | Recovered | 23 September 2015 | 200 mg of placebo od | 11 May 2015–ongoing at time of event | Secondary to trauma |
| 094 | UK, male, 85 | Fractured neck of femur | Recovered | 14 August 2015 | 200 mg of placebo od | 23 March 2015–ongoing at time of event | |
| 095 | UK, female, 82 | Fracture left wrist | Recovered | 28 April 2015 | 200 mg of placebo od | 30 October–5 November 2014 | |
| 096 | UK, female, 65 | Fractured distal radius and ulna | Recovered | 4 September 2015 | 200 mg of placebo od | 15 June 2015–ongoing at time of event | |
| 108 | UK, male, 49 | Laceration to left hand | Unknown | 29 September 2015 | 200 mg of theophylline bd | 23 April 2015–ongoing at time of event | |
| 115 | UK, female, 76 | Fall | Recovered | 26 December 2015 | 200 mg of placebo od | 12–31 December 2015 | |
| 137 | UK, male, 60 | Lower back pain lasting 2 hours since fall on floor during the night | Recovered | 7 March 2016 | 200 mg of theophylline od | 7 April 2015–ongoing at time of event | |
| 153 | UK, female, 72 | Post-operative wound infection | Recovered | 9 January 2016 | 200 mg of theophylline od | 13 November–20 December 2015 | |
| 169 | UK, male, 80 | Raised INR of 4.2 and HB 97; possibly due to GI bleed | Recovering | 1 July 2016 | 200 mg of theophylline od | 21 October 2015–ongoing at time of event | Inappropriately high dose of warfarin |
| 180 | UK, male, 83 | Head injury | Recovered | 6 July 2016 | 200 mg of placebo od | 10 February 2016–ongoing at time of event | |
| 190 | UK, male, 69 | Fractured rib | Not recovered | 19 August 2016 | 200 mg of placebo od | 21 March 2016–ongoing at time of event | |
| 204 | UK, female, 81 | Right distal fibula and medial malleolus | Unknown | 3 September 2016 | 200 mg of theophylline od | 19 July–19 December 2016 | |
| 206 | UK, male, 74 | Fell down stairs and fractured clavicle and shoulder, and broke ribs | Recovering | 1 August 2016 | 200 mg of placebo od | 24 August 2015–11 February 2016 | |
| 214 | UK, male, 63 | Fall | Recovered | 15 January 2015 | 200 mg of placebo od | 4 March 2014–ongoing at time of event | |
| 216 | UK, male, 69 | Persistent vomiting | Recovered | 17 January 2015 | 200 mg of theophylline od | 7 July–24 November 2014 | Thought to be related to chemotherapy |
| 218 | UK, male, 69 | Confusion (steroid-induced psychosis) | Recovered | 4 April 2015 | 200 mg of theophylline od | 7 July–24 November 2014 | |
| 271 | UK, female, 76 | Closed fracture neck of femur | Unknown | 14 May 2017 | 200 mg of placebo od | 27 May 2016–ongoing at time of event | |
| 272 | UK, male, 58 | Fall-like syncopal attack | Recovered | 26 March 2015 | 200 mg of theophylline od | 12 March–18 April 2015 | |
| 274 | UK, male, 84 | Fall | Fatal | 14 October 2016 | 200 mg of placebo od | 23 May–6 October 2016 | |
| 278 | UK, female, 61 | Fractured neck of femur | Recovered | 8 May 2017 | 200 mg of placebo od | 17 May 2016–ongoing at time of event | |
| System Organ Class: surgical and medical procedures | |||||||
| 045 | UK, male, 69 | Optical urethrotomy | Recovered | 27 March 2015 | 200 mg of theophylline bd | 30 July 2014 to 29 July 2015 | |
| System Organ Class: social circumstances | |||||||
| None | |||||||
List of abbreviations
- AE
- adverse event
- AR
- adverse reaction
- ASSET
- Low-dose Theophylline as Anti-inflammatory Enhancer in Severe Chronic Obstructive Pulmonary Disease
- ATS
- American Thoracic Society
- bd
- twice a day
- BMI
- body mass index
- BNF
- British National Formulary
- CAT
- COPD Assessment Test
- CHaRT
- Centre for Healthcare Randomised Trials
- CHSS
- Chest Heart & Stroke Scotland
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRN
- Clinical Research Network
- CSRI
- Client Service Receipt Inventory
- Css
- steady-state concentration
- CTIMP
- Clinical Trial of an Investigational Medicinal Product
- DMC
- Data Monitoring Committee
- ECLIPSE
- Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints
- EQ-5D-3L
- EuroQoL-5 Dimensions, three-level version
- ERS
- European Respiratory Society
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- GLM
- generalised linear model
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- GP
- general practitioner
- HARQ
- Hull Airway Reflux Questionnaire
- HDAC
- histone deacetylase
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IBW
- ideal body weight
- ICER
- incremental cost-effectiveness ratio
- ICS
- inhaled corticosteroid
- IL-8
- interleukin 8
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LABA
- long-acting β2 agonist
- LAMA
- long-acting muscarinic antagonist
- mMRC
- modified Medical Research Council
- MR
- modified release
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OCS
- oral corticosteroid
- od
- once daily
- OR
- odds ratio
- PDE
- phosphodiesterase
- PI3K
- phosphoinositide 3-kinase
- PIC
- participant identification centre
- PIL
- participant information leaflet
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SABA
- short-acting β2 agonist
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SVC
- slow vital capacity
- TSC
- Trial Steering Committee
- TWICS
- Theophylline With Inhaled CorticoSteroid
