Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/167/135. The contractual start date was in October 2014. The draft report began editorial review in April 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Hajek received research funding from, and provided consultancy to, manufacturers of stop smoking medications (Pfizer Inc., New York City, NY, USA). Hayden J McRobbie received a grant from the National Institute for Health Research Health Technology Assessment programme; he also received honoraria for speaking at smoking cessation meetings and attended advisory board meetings organised by Pfizer Inc. and Johnson & Johnson (New Brunswick, NJ, USA). Dunja Przulj received a research grant from Pfizer Inc. Maciej Goniewicz provided consultancy to Johnson & Johnson. Lynne Dawkins reports personal fees from attorneys at law outside the submitted work. Jinshuo Li reports grants from the National Coordinating Centre for Health Technology Assessment (NCCHTA) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Hajek et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
E-cigarettes are a popular option for smokers seeking to limit the health risks of smoking. 1 E-cigarettes are unlikely to be harmless, but the risk of their use has been estimated to be < 5% of the risks of smoking. 2,3 No health risks were identified over some 18 months of use,4 and there is some evidence that smokers who successfully switch to vaping reduce their nicotine dependence and often stop vaping as well. For example, in the UK, there are currently some 1.5 million ex-smokers who have stopped smoking with the help of e-cigarettes, of whom some 700,000 have stopped vaping as well. 5
Current smoking cessation treatments provide a combination of behavioural support and medications that target cigarette withdrawal discomfort,6 but sensorimotor factors that accompany smoking and that are likely to be reinforcing for smokers are not well addressed. 7 E-cigarettes pose a promise to fill this gap, and some UK Stop Smoking Services (SSSs) are now including e-cigarettes in their routine work. However, data on e-cigarette efficacy in this context are limited. Information is needed on whether or not e-cigarettes can match or even surpass the efficacy of other evidence-based treatments that are currently used. The question is particularly important because e-cigarettes are much less expensive than stop smoking medications and smokers are purchasing e-cigarettes themselves, which means that their use represents no cost to health-care systems. In addition, they hold a greater appeal to smokers and are used more widely than licensed stop smoking medication,8 which suggests that they could potentially have a bigger population impact.
Only three randomised controlled trials (RCTs) have evaluated the efficacy of e-cigarettes when offered proactively by health professionals as a stop smoking treatment. A Cochrane meta-analysis4 of two trials that provided long-term outcomes9,10 (both trials used early e-cigarette models with poor nicotine delivery) found evidence that e-cigarettes containing nicotine are more likely to help smokers quit than placebo e-cigarettes with one trial showing the same (low) effect for e-cigarettes and for nicotine patches. 9 The third trial11 had only a 2-month outcome and so was not included in the meta-analysis, but it showed a significant effect of a more advanced (second-generation) e-cigarette product. Another trial12 was published in 2018, but its rationale was unclear and is difficult to interpret. Participants had access to cartridge-based e-cigarettes in both trial arms, but one arm was also given stop smoking medications. In addition, treatments were offered to people who did not ask to be treated and, to be classified as an abstainer, participants had to undergo repeated blood sampling. ‘Abstinence rates’ were thus extremely low (1% in the arm allocated e-cigarettes only and 0.5% in the arm allocated medication plus e-cigarettes). The trial12 also evaluated incentives. Paying abstainers US$600 to attend the blood sampling increased ‘abstinence rates’ only to 2.9%. 12
The present trial was set up to evaluate the efficacy of a refillable e-cigarettes compared with the efficacy of nicotine replacement therapy (NRT) products as currently used routinely by SSSs, when accompanied by weekly behavioural support (as provided routinely by the services). In routine use, NRTs are included as a single product of the patient’s choice, selected from among a number of available options, or in combinations, depending on a patient’s preferences.
Chapter 2 Methods
Some parts of this chapter are from The New England Journal of Medicine, Hajek P, et al. 13 A randomized trial of e-cigarettes versus nicotine-replacement therapy, Vol. 380, pp. 629–37. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission.
Overview of trial design
This was a pragmatic RCT conducted between 2015 and 2018 in three sites in England that provide local SSSs. Eligible smokers seeking help to quit were randomised (1 : 1) to receive a NRT of their choice (a single NRT product or product combinations) plus usual care (weekly behavioural support provided by the SSS) or e-cigarettes plus usual care. Participants attended weekly sessions at their SSS, as per standard practice, and were followed up by telephone at 6 and 12 months. Participants reporting abstinence or at least a 50% reduction in smoking at 12 months were invited to attend for carbon monoxide (CO) validation.
Changes to the trial design
There was a change of chief investigator from Professor McRobbie to Professor Hajek (who was a co-investigator) because Professor McRobbie moved to New Zealand. A new advertising strategy (i.e. leaflets distributed to local households) was added. These and other minor changes are shown in Table 32 in Appendix 1.
Participants
Inclusion/exclusion criteria
Participants were included if they were aged ≥ 18 years, were current smokers who wanted to quit smoking and were able to read/write/understand English. Participants were excluded if they were pregnant or breastfeeding, had a strong preference for or against using NRT or e-cigarettes in their quit attempt, were currently enrolled in other interventional research or were currently using NRT or e-cigarettes.
Recruitment
Between May 2015 and January 2017, 886 participants were recruited. SSSs included information about the study in their advertising (typically posters, leaflets, digital media, local papers, through general practices, mail-outs to previous attenders and in local radio/newspaper interviews). Leaflets advertising the trial were also delivered to local households. An example of the advertisements can be seen in the study documentation at www.journalslibrary.nihr.ac.uk/programmes/hta/12167135/#/ (accessed 18 February 2019).
Advertisements directed participants to contact the local trial team, which provided further information about the trial, assessed eligibility and invited potential participants to a screening session at their local SSS.
Setting
Recruitment and delivery of the interventions took place at the Health and Lifestyle Research Unit at Queen Mary University of London (QMUL), which is commissioned to deliver the SSS for the local boroughs of Tower Hamlets and the City of London, and the Leicester and East Sussex SSSs. Follow-up calls were carried out by researchers at the Health and Lifestyle Research Unit.
Trial procedures
Informed consent procedures
Following initial confirmation of eligibility by telephone/e-mail, participants were invited to attend a screening (baseline) session at their local SSS. Potential participants were provided with written information, along with the standard SSS client registration form, prior to their screening session. Participants were given sufficient time to read the written information and to consider whether or not they wanted to participate.
At the screening (baseline) session, trial details were discussed and eligibility was reconfirmed. Those who were interested and eligible gave informed consent obtained by members of staff trained in good clinical practice.
Interventions
Identical multisession behavioural support was provided to both trial arms. The exact procedures differed slightly between trial sites, but they followed the same treatment approach (withdrawal-oriented therapy14) that involves face-to-face support sessions with CO monitoring, which usually begins 1 or 2 weeks prior to the target quit date (TQD). Clients attended sessions weekly, typically for 4 weeks post TQD. Trial data were collected face to face at the baseline session, at TQD and for the first 4 weeks post TQD.
All participants were telephoned at 26 and 52 weeks post TQD to obtain smoking status and assess e-cigarettes/NRT/other product use and adverse reactions (ARs); at 52 weeks, those reporting abstinence or smoking reduction of 50% or more were invited back to the service that they had attended to give a CO reading.
Participants received £20 compensation for their time and travel at the 52-week visit. No other compensation was provided at any time during the trial.
As per usual-care protocol, participants who missed appointments or who were not contactable at follow-up were contacted by other means to check on their progress (i.e. text, telephone, e-mail, letter). When there was no response, up to six attempts at contact were made at 4 weeks, up to five attempts at 6 months and up to eight attempts at 1 year.
Nicotine replacement therapy arm
Participants were advised about the NRT products available at the baseline session. They chose their preferred NRT, as per usual practice, and were also provided with an option to use NRT combinations (normally the patch and one of the oral products) as per usual practice. The supply of NRT differed slightly between the different trial sites. A letter of recommendation (LOR) to supply NRT was issued on a fortnightly basis for up to 12 weeks at the London SSS, which service users exchange at a pharmacy in return for the NRT. The £8.60 prescription charge is paid by service users unless they are exempt (around 50% of SSS users are). East Sussex and Leicester service users receive direct supply of NRT free of charge, for up to 12 weeks.
To avoid a possible bias that could be generated because NRT participants had to visit their local pharmacy and potentially pay a prescription fee whereas participants randomised to e-cigarette did not, the procedure below was followed.
At sites that used LORs, all participants were given a LOR at their baseline session (as per standard practice). They were instructed to collect the NRT and bring it to their TQD session. At the TQD, those randomised to NRT kept their NRT and initiated use at the session. Participants randomised to the e-cigarette condition, swapped their NRT for an e-cigarette starter pack (see E-cigarette arm).
For sites that provided NRT directly, participants were provided with their NRT or e-cigarette during the TQD session following randomisation.
For all sites, instructions on NRT use were provided as per routine clinic support.
At the completion of the trial treatment period, participants could request further supplies of NRT in line with the SSS standard practice.
Participants in the NRT arm were free to switch to other forms of NRT; this was recorded at every contact point.
E-cigarette arm
As noted in the previous section, participants in the e-cigarette group who attended a site using LORs were given a NRT LOR at their baseline session and were asked to collect the NRT and bring it to their TQD session. Participants who were then randomised to e-cigarettes at the TQD swapped their NRT for the e-cigarette starter kit. Participants randomised to the e-cigarette arm who attended a site that did not use LORs were provided with their e-cigarette starter kit at the TQD session.
A starter pack was given to initiate e-cigarette use and demonstrate refillable e-cigarette products. Participants were expected to source their own e-liquid and were also encouraged to purchase a different device if the provided one did not suit their needs. To start the participants on using the e-cigarette, we provided a Conformité Européenne (CE)-marked refillable e-cigarette with 2 or 3 weeks’ supply (1 × 30-ml bottle) of e-liquid. The e-liquid was labelled as 18 mg/ml of nicotine, the most commonly used nicotine content at the time. 15 The e-cigarette used was ‘One Kit’, an Aspire® (Shenzhen Eigate Technology Co. Ltd, Shenzhen, China) device with a 2.1-Ω resistance atomiser coil and a 650-mAh battery, branded by the UK Ecig Store (London, UK). During the trial, the company discontinued the original One Kit, so the new ‘One Kit 2016’ device was used for 42 participants. One Kit 2016 is an Innokin® device (Innokin Technology, Shenzhen, China) with a 1.5-Ω resistance atomiser coil and a 1000-mAh battery, branded by the UK Ecig Store.
The original One Kit was purchased for the wholesale price of £5.99 with the following accessories: an atomiser five pack (£3.49), a UK adapter (£2.99) and a spare battery (£3.89). The total cost was £19.35, including the e-liquid. The One Kit 2016 was purchased for £13 with the following accessories: an atomiser five pack (£3.75), a UK adapter (£2.99) and a spare battery (£7.50). The total cost was £30.23, including the e-liquid. The e-liquid used was 30 ml of Tobacco Royale flavour, purchased from the UK Ecig Store for £2.99.
Verbal and written guidance was given about how to use the e-cigarette. Participants initiated use during the session.
Participants were instructed to obtain further supplies of e-liquid themselves and advised on how to do this via the internet or local vape shops. They were encouraged to try different strengths and flavours of e-liquids if they did not like the supplied one. Participants who did not manage to source their own supplies of e-liquid were provided with one additional supply on request (1 × 10-ml bottle), but this was not proactively offered.
The e-cigarette arm participants had to pay for e-liquid supplies once they had used their trial-allocated e-liquid or if they wanted to try other flavours, but these costs are modest and roughly balanced by the fact that some of the NRT arm participants had to pay prescription charges.
Both trial arms
To help minimise contamination, at the TQD all participants in both trial arms were asked to sign a commitment form stating that they were committed to not using the non-allocated treatment for at least 4 weeks post TQD.
Measurements
Measurements were collected as follows:
-
participant demographics, smoking history and previous/current medical conditions
-
Fagerström Test for Cigarette Dependence (FTCD) score16
-
score on the Mood and Physical Symptoms Scale, which measures severity of urges to smoke and other tobacco withdrawal symptoms17
-
self-reported smoking status
-
end-expired CO reading, collected using a CO monitor [a reading of < 8 parts per million (p.p.m.) was used as a cut-off point for validating self-reported abstinence]
-
an ARs checklist (see Adverse reactions)
-
e-cigarette/NRT use and ratings of helpfulness in refraining from smoking cigarettes (from 1 = not at all helpful to 5 = extremely helpful) and satisfaction and how good it tasted in comparison with usual cigarettes (much less than normal cigarettes = 1, a little less = 2, the same = 3, a little more = 4, much more than normal cigarettes = 5)
-
in the case of participants who stopped using e-cigarettes/NRT or who switched to a different type of e-cigarette/NRT, reasons for doing so
-
additional economic evaluation measures: EuroQoL-5 Dimensions, five-level version (EQ-5D-5L), score at baseline and at 6 and 12 months;18 smoking cessation service and health service use at baseline and during the preceding period at 6 and 12 months.
Adverse reactions
With the agreement of the trial sponsor and Research Ethics Committee (REC), data on adverse events (AEs) were not collected, as the safety profiles of NRT are well established and a number of studies4,19 have now identified the likely ARs to e-cigarettes. Instead, an ARs checklist was used (Table 1 shows the schedule of collection of measurements), which asked whether or not any ARs had been experienced since the last contact. Those reporting ARs were asked whether the AR had stopped them from doing things they would usually do, as an indication of severity. The following were evaluated at each session: nausea, throat/mouth irritation, sleep disturbances, dizziness, headache and four indicators of respiratory health: shortness of breath, cough, wheezing and phlegm.
| Measures/procedures | Trial session | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | TQD | TQD + 1 week | TQD + 2 weeks | TQD + 3 weeks | TQD + 4 weeks | TQD + 26 weeks | TQD + 52 weeks | |
| Informed consent | ✓ | |||||||
| Baseline questionnaire | ✓ | |||||||
| Current illness | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Current medication | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Randomisation | ✓ | |||||||
| Commitment form | ✓ | |||||||
| CO reading | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ (if abstinent or there was a 50% reduction) | |
| MPSS | ✓ | ✓ | ✓ | ✓ | ||||
| Smoking status/CPD | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ARs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| E-cigarette/NRT ratings | ✓ | ✓ | ||||||
| E-cigarette/NRT use and helpfulness | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| E-cigarette dispensed, demonstration on first use | ✓ | ✓ | ||||||
| NRTa dispensed, demonstration on first use | ✓ | ✓ | ✓ | |||||
| EQ-5D-5L questionnaire | ✓ | ✓ | ✓ | |||||
| Smoking cessation service and health service use | ✓ | ✓ | ✓ | |||||
Serious adverse events (SAEs), including death, overnight hospitalisation and permanent disability, were also recorded.
Data management
Data collection and entry
A web-based application, using an Oracle 11g database, was used to collect data. This was set up and hosted by the Barts Clinical Trials Unit (CTU). The electronic data capture forms were web based and built using Java (Oracle Corporation, Redwood Shores, CA, USA) with data validation in JavaScript (Java framework Struts 2) (Netscape Communications Corporation, Dulles, VA, USA; Mozilla Foundation, Mountain View, CA, USA; Ecma International, Geneva, Switzerland). When the web-based application was unavailable, data were collected on paper case report forms (CRFs) and questionnaires and then entered into the database at the earliest opportunity. All data were kept in accordance with good clinical practice and data protection requirements.
Data quality
The co-ordinating site checked electronic CRFs on a weekly basis for anomalies and raised and resolved queries with the researcher/advisor concerned. Once recruitment and follow-up were complete, the trial team cleaned the data.
A sample of paper CRFs/questionnaires were also checked. Ten per cent were randomly selected for comparison between written and database entries. The predetermined quality target of ≤ 2% discrepancies was met.
Sample size
At the time the trial protocol [available at www.journalslibrary.nihr.ac.uk/programmes/hta/12167135/#/ (accessed 23 April 2019)] was drawn up, the 12-month validated abstinence rate (i.e. the primary outcome) associated with usual care in our setting was 14%. 20 The projection of a feasible rate with the e-cigarettes was based on the research team’s work and two published studies. The research team’s work suggested that e-cigarettes deliver nicotine quickly, with time to the maximum concentration occurring within 5 minutes. 21 This is similar to nicotine nasal spray. In a comparative study of nicotine nasal spray plus patch versus patch alone, 1-year abstinence rates were 27% versus 11%, respectively [risk ratio (RR) 2.45]. 22 In a 2014 cohort study23 in Italy, a second-generation e-cigarette achieved 36% CO-validated abstinence at 6 months. Assuming 25% relapse between 6 and 12 months,24 this would translate to a 1-year abstinence rate of 27%. Relative to the assumed usual care rate, this would give a RR of 1.9. However, quit rates in countries with little tradition of stop smoking treatments tend to be much higher than in the UK. The research team wanted to detect a RR of 1.7 (e-cigarette rate = 24%) with 0.95 power, but also have reasonable power (e.g. 0.75) if the RR should be as low as 1.5 (e-cigarette rate = 21%). This figure still represents a clinically significant difference. To achieve these levels of power (two-sided alpha = 0.05, continuity correction), a total of 886 participants (443 in each group) was required.
It is noted in the statistical analysis plan that, since the trial protocol was written, a new evaluation of the UK SSSs has been published. 25 The validated 1-year quit rate has declined to 8%. This figure is derived from quit rates in general practice and pharmacy services of 5% and in specialist support services of 10% for individual and 12% for group support. The decline is probably a result of a ‘hardening’ of treatment population, namely the services see an increasing number of reattenders, people with serious health issues, etc. Using a quit rate of 10% in the usual-care arm, which provides multicontact support, and 17% in the e-cigarette arm (RR 1.70), the trial sample size still provides at least 85% power to detect such a difference with a two-tail test of proportions.
Assuming that the true percentage in both arms is 10%, the 95% confidence interval (CI) for the difference in proportions will have a width of ± 4% around the observed difference.
Randomisation
Randomisation (1 : 1 in permuted blocks of 20) was undertaken using a web-based application, set up by the Barts CTU, and was stratified by trial site. Participants who were eligible and consented to take part were randomly allocated to the NRT arm or the e-cigarette arm on the TQD session by researchers/stop smoking advisors. The TQD was used as the point of randomisation to minimise any differential drop-out. The staff randomising the participant accessed the web-based application when the participant was with them, entering their participant identification number, date of birth and initials into the program. There were no stratification factors. The allocation was immediately provided by the program. In the event of the site having no web access, staff were able to fax/e-mail the relevant CRFs to the CTU for a telephone randomisation to take place during standard working hours.
Treatment blinding
Participants could not be blinded to the intervention they were receiving, and trial staff could not be blinded when providing the interventions and collecting data.
Unblinded data were seen and analysed by the trial statistician for the purposes of the Data Monitoring and Ethics Committee (DMEC) meetings. All other trial staff who had access to outcome data remained blinded until prespecified data analyses were complete. Prespecified data analyses were conducted blind to treatment allocation.
Statistical methods
Changes from planned analysis
There were no changes from the planned analysis, from either the trial protocol or the statistical analysis plan. See the project web page for the statistical analysis plan [www.journalslibrary.nihr.ac.uk/programmes/hta/12167135/#/ (accessed 18 February 2019)].
General analysis principles
The main analysis for each outcome used intention-to-treat principles, meaning that all participants with a recorded outcome were included in the analysis and were analysed according to the treatment group to which they were randomised. More information on which participants were included in each analysis is available in Withdrawn participants.
In all analyses for all outcomes, the following are presented:
-
the number of participants included in the analysis, by treatment group (for the primary outcome data, this will be all randomised participants; see the next section)
-
a summary measure of the outcome by treatment group, for example mean [standard deviation (SD)] for continuous outcomes and number (%) for binary outcomes
-
treatment effect (risk ratio of abstinence for e-cigarettes relative to NRT), with 95% CI
-
two-sided p-values (the significance level was set at 5%).
Missing data
To deal with incomplete data (i.e. when patients had missing data at one of the follow-up time points), the research team:
-
attempted to follow up all randomised patients, even if they had discontinued participation
-
included participants lost to follow-up (i.e. missing cases) or not providing biochemical validation as non-abstainers
-
carried out sensitivity analyses using multiple imputations via chained equation and excluding cases with missing outcomes.
Withdrawn participants
Participants requesting no further follow-up were included in the analysis, as per intention to treat (such participants were counted as smokers for the time points after withdrawal, as per the Russell Standard26).
Participants who died were excluded from the sample, as per the Russell Standard.
Participants who moved to an untraceable address and whose telephone number(s) and e-mail address were no longer in use were excluded from the sample from the point that notification was received that they were no longer living at the address and their telephone number(s)/e-mail address were no longer in use, as per the Russell Standard. They were included in the sample analysis up until this point and coded as relapsed after that point.
Chapter 3 Outcomes
Some parts of this chapter are from The New England Journal of Medicine, Hajek P, et al. 13 A randomized trial of e-cigarettes versus nicotine-replacement therapy, Vol. 380, pp. 629–37. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission.
Primary outcome
Sustained abstinence at 52 weeks was calculated as per the Russell Standard26 (i.e. a self-report of smoking no more than five cigarettes since 2 weeks post TQD), validated by a CO reading of < 8 p.p.m. at the 52-week follow-up. (CO in expired breath is a commonly used biochemical validation tool that is particularly useful when people continue to use nicotine because methods based on the detection of nicotine metabolites are not appropriate in such cases. The CO assay detects only smoking over the previous ≈24 hours, but trial participants do not usually know of the assay’s half-life, and dependent smokers typically smoke daily.)
If outcome data at previous follow-up points were missing but no data were available to contradict claims of sustained abstinence, the participants who reported smoking no more than five cigarettes in total since 2 weeks post TQD at the 52-week follow-up and whose self-report was validated by a CO reading of < 8 p.p.m. were classed as meeting the primary outcome definition.
In the primary analysis, all participants were included in the arm to which they were randomised, but sensitivity analyses were conducted that took into account use of non-allocated products.
Sensitivity analyses for primary outcome
The following sensitivity analyses of the primary outcome were conducted:
-
including only participants who attended at least one treatment session, namely only those who engaged in treatment
-
excluding participants who used the unassigned trial product for 5 consecutive days or more
-
using multiple imputation of smoking status in participants with missing follow-up data by chained equations.
At the request of the chairperson of the Trial Steering Committee (TSC), an analysis limited to participants who completed the 12-month follow-up is also included. (This is normally not done in this field, as it increases quit rates and can obscure treatment effects; treatment failures are more likely to drop out, and so missingness is not random.)
Secondary outcomes
We examined the differences between study arms in the proportions of participants with 6- to 12-month sustained abstinence, abstinence at 4 and 26 weeks, and sustained reduction of 50% or greater in baseline cigarette consumption and CO levels at 52 weeks, using binomial regression.
At each time point, seven-day abstinence rates were also calculated. This measure is less informative because 7 days of not smoking does not convey much health benefit and, compared with sustained abstinence, is a weak predictor of smoking status in future. Seven-day abstinence is also influenced by other, more recently occurring factors, so is likely to diminish the effects of a treatment delivered 1 year ago. However, it is still used in less rigorous studies and so it was included to allow across-trial comparisons.
The time to relapse was examined using a Cox analysis.
Table 2 shows the definitions used for secondary outcomes.
| Outcome | Definition |
|---|---|
| CO-validated sustained abstinence between 26 and 52 weeks post TQD | Reporting no more than five cigarettes smoked between weeks 26 and 52, accompanied by a CO reading of < 8 p.p.m. at week 52 |
| For all outcomes, if data from previous follow-ups were missing but no data contradicted the outcome as measured at the given point, this was accepted | |
| CO-validated sustained abstinence at 4 weeks post TQD | Reporting not a single puff in the previous 2 weeks at Q + 4 follow-up, accompanied by a CO reading of < 8 p.p.m. at Q + 4 |
| Sustained abstinence at 26 weeks post TQD | Reporting no more than five cigarettes smoked since 2 weeks post TQD at Q + 24 |
| 7-day point prevalence at 4 weeks post TQD | Reporting not a single puff in the previous 7 days |
| 7-day point prevalence at 26 weeks post TQD | Reporting not a single puff in the previous 7 days |
| 7-day point prevalence at 52 weeks post TQD | Reporting not a single puff in the previous 7 days |
| Smoking reduction in participants who did not achieve abstinence at 52 weeks | Self-reported daily cigarette consumption at Q + 52 reduced by at least 50% from baseline consumption, accompanied by a CO reading at Q + 52 reduced by at least 50% from that at baseline. Participants with missing data were classified as non-reducers |
Each outcome was adjusted for baseline covariates selected using a stepwise regression approach so that only significant covariates (p = 0.1) were included in the final model.
Tobacco withdrawal symptoms at 1 and 4 weeks
Between-group differences in urges to smoke and changes (from baseline) in tobacco withdrawal symptoms were examined using t-tests in both the whole sample and the abstainers-only sample.
Treatment ratings (satisfaction, taste, helpfulness, reasons for stopping product use)
Differences in mean ratings of satisfaction, taste of the product, product helpfulness and reasons for stopping product use were examined between groups using t-tests at 1 and 4 weeks post TQD. Adjustments for normal distribution were applied when needed and non-parametric tests were used where necessary. When two NRT products were used and rated, the average rating of the two was taken.
Adverse reactions
The chi-squared test was used to compare, between arms, the frequency of participants who reported each AR (sleep disturbance, nausea or throat/mouth irritation) on at least one occasion. The Medical Dictionary for Regulatory Activities (MedDRA) coding system was used.
Changes in respiratory symptoms
Using logistic regression, we compared changes in cough, wheezing, phlegm production and shortness of breath from baseline to 52 weeks in the two arms. Symptoms at 52 weeks were regressed onto trial arm with adjustment for baseline score and study site.
The details of the cost-effectiveness analysis methods are included in the cost-effectiveness section.
Statistical software
All analyses were carried out using Stata® software, version 15 (StataCorp LP, College Station, TX, USA).
Patient and public involvement
Two members of the public served on the TSC. Along with two others, they also contributed to a patient and public involvement (PPI) panel, which convened throughout the trial. Three members of the panel were current e-cigarette users. When PPI members were not available for the meetings/teleconferences, they were contacted by e-mail for their feedback.
Trial committees
The DMEC and the TSC convened every 6–12 months. The Trial Management Group also met regularly throughout the study. Appendix 1, Table 33, shows the members of the trial committees.
The chief investigator, study manager and a minute-taker (who was a member of the trial team) also attended the TSC and DMEC meetings. The trial statistician (originally Mr John Stapleton, followed by Dr Irene Kaimi) also attended the DMEC meetings.
Quality control and quality assurance
A risk assessment was carried out in conjunction with the trial sponsor and Barts CTU, which was used as a basis for the trial monitoring plan. During the recruitment phase, a monitor from the co-ordinating site carried out 6-monthly monitoring visits at each of the sites. The Barts CTU was responsible for oversight of the monitoring process and overall audit of the trial.
Approvals
The trial was sponsored by the QMUL Joint Management Research Office. Ethics approval was obtained from the National Research Ethics Service Committee London – Camden and Islington REC on 19 December 2014 (reference number14/LO/2235).
The results and the discussion of the Trial of E-Cigarettes (TEC) are presented in Chapter 4. The methods and results of the economical evaluation are presented in Chapter 5.
Chapter 4 The TEC trial
Some parts of this chapter are from The New England Journal of Medicine, Hajek P, et al. 13 A randomized trial of e-cigarettes versus nicotine-replacement therapy, Vol. 380, pp. 629–37. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission.
Results
The methods and results of the economical evaluation are presented after the main trial section.
Participant flow
Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
FIGURE 1.
The CONSORT flow diagram. From Hajek P, et al. 13 A randomized trial of e-cigarettes versus nicotine-replacement therapy. The New England Journal of Medicine Vol. 380, pp. 629–37. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission.

The follow-up rates at 12 months were 81% in the e-cigarette arm and 77% in the NRT arm [χ2(1) = 2.1; p = 0.09].
Sample characteristics
Participant characteristics are shown in Table 3.
| Characteristic | Trial arm | Total (N = 884) | |
|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | ||
| Age (years), median (IQR) | 41 (33–53) | 41 (33–51) | 41 (33–52) |
| Female, n (%) | 211 (48.2) | 213 (47.8) | 424 (48.0) |
| Marital status, n (%) | |||
| Single | 224 (51.1) | 249 (55.8) | 473 (53.5) |
| Separated or divorced | 89 (20.1) | 86 (19.3) | 174 (19.6) |
| Married | 116 (26.5) | 105 (23.5) | 221 (24.9) |
| Widowed | 10 (2.3) | 6 (1.4) | 16 (1.8) |
| Ethnicity, n (%) | |||
| White British | 322 (73.5) | 311 (69.7) | 633 (71.6) |
| White other | 35 (8.0) | 41 (9.2) | 76 (8.6) |
| Black | 15 (3.4) | 15 (3.4) | 30 (3.4) |
| Asian | 29 (6.6) | 37 (8.3) | 66 (7.5) |
| Mixed | 22 (5.0) | 28 (6.3) | 50 (5.7) |
| Other | 9 (2.1) | 9 (2.0) | 18 (2.0) |
| Missing | 6 (1.4) | 5 (1.1) | 11 (1.2) |
| Educational qualification, n (%) | |||
| Primary school | 19 (4.3) | 22 (4.9) | 41 (4.6) |
| Secondary school | 141 (32.2) | 130 (29.2) | 271 (30.7) |
| Further education/diploma | 117 (26.7) | 127 (28.5) | 244 (27.6) |
| Higher education | 161 (36.7) | 167 (37.5) | 328 (37.1) |
| Employment status, n (%) | |||
| In paid employment | 299 (68.3) | 316 (70.9) | 615 (69.6) |
| Receives free prescriptions, n (%) | 181 (41.3) | 179 (40.1) | 360 (40.7) |
| Smoking and quitting history | |||
| Cigarettes smoked per day, median (IQR) | 15 (10–20) | 15 (10–20) | 15 (10–20) |
| Baseline CO, median (IQR) | 20 (13–27) | 21 (13–28) | 20 (13–28) |
| FTCD, mean (SD) | 4.5 (2.5) | 4.6 (2.4) | 4.6 (2.4) |
| Previous use of stop smoking products, n (%) | |||
| NRT | 328 (74.9) | 334 (74.9) | 662 (74.9) |
| Varenicline (Champix®; Pfizer Inc., New York City, NY, USA) | 149 (34.1) | 151 (33.8) | 300 (33.9) |
| Bupropion (Zyban®; GlaxoSmithKline plc, Brentford, UK) | 34 (7.8) | 35 (7.9) | 69 (7.8) |
| E-cigarettes | 186 (42.5) | 181 (40.6) | 367 (41.5) |
| Never tried NRT, varenicline or bupropion | 84 (19.2) | 92 (20.6) | 176 (19.9) |
| Age (years) initiated smoking, median (IQR) | 16 (14–18) | 16 (14–18) | 16 (14–18) |
| Spouse/partner smokes, n (%) | 167 (38.1) | 178 (39.1) | 345 (39.1) |
| Study site, n (%) | |||
| London | 289 (66.0) | 295 (66.2) | 584 (66.1) |
| Leicester | 92 (21.0) | 96 (21.5) | 188 (21.3) |
| East Sussex | 57 (13.0) | 55 (12.3) | 112 (12.7) |
The sample comprised mostly middle-aged smokers who started to smoke at a median age of 16 years and tried various smoking cessation aids before joining the trial.
Abstinence rates
Table 4 shows abstinence rates in the two trial arms at different time points. Abstinence rates were consistently higher with e-cigarettes than with NRT for both primary and secondary outcomes. There were no significant differences between quit rates across the trial sites [e.g. χ2(2) = 4.2; p = 0.12 for the primary outcome].
| Outcome | Trial arm, n (%) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | Relative risk (95% CI)a | p-value | Relative risk (95% CI) | p-value | |
| Primary outcome | ||||||
| 52-week abstinence | 79 (18.0) | 44 (9.9) | 1.83 (1.30 to 2.58) | 0.001 | 1.75 (1.24 to 2.46)b | 0.001 |
| Secondary outcomes | ||||||
| Abstinence between 26 and 52 weeks | 93 (21.2) | 53 (11.9) | 1.79 (1.32 to 2.44) | < 0.001 | 1.82 (1.34 to 2.47)c | < 0.001 |
| 4 weeks post TQD | 192 (43.8) | 134 (30.0) | 1.45 (1.22 to 1.74) | < 0.001 | 1.43 (1.20 to 1.71)d | < 0.001 |
| 26 weeks post TQD | 155 (35.4) | 112 (25.1) | 1.40 (1.14 to 1.72) | 0.001 | 1.36 (1.15 to 1.67)b | 0.003 |
| CO-validatede reduction ≥ 50% in non-abstainers at 24–52 weeks; n/N (%) | 44/345 (12.8) | 29/393 (7.4) | 1.75 (1.12 to 2.72) | 0.01 | 1.73 (1.11 to 2.69) | 0.02 |
Sensitivity analyses for the primary outcome
Regarding the primary outcome, sustained 1-year abstinence rates were 18.0% and 9.9% in the e-cigarette and NRT arms, respectively (RR 1.83, 95% CI 1.30 to 2.58; p = 0.001) (see Table 4). The results of the four sensitivity analyses of the primary outcome tallied with the main analysis, indicating greater abstinence in the e-cigarette arm than in the NRT arm (RR 1.75 to RR = 1.85; p < 0.001) (Table 5). The absolute risk difference for the primary outcome was 8.1% (95% CI 3.6% to 12.7%), with a number needed to treat of 12.
| Sensitivity analysesa | RR | p-value | 95% CI |
|---|---|---|---|
| Participants who attended ≥ 1 treatment session (e-cigarette arm, n = 411; NRT arm, n = 418) | 1.79 | 0.001 | 1.27 to 2.52 |
| Participants using non-allocated product for ≥ 5 days excluded (e-cigarette arm, n = 411; NRT arm, n = 345) | 1.84 | 0.001 | 1.27 to 2.66 |
| Participants with missing outcome at 52 weeks excluded (e-cigarette arm, n = 356; NRT arm, n = 342) | 1.75 | 0.001 | 1.25 to 1.45 |
| Multiple imputation of missing information by chained equations (e-cigarette arm, n = 438; NRT arm, n = 446) | 1.85 | < 0.001 | 1.32 to 2.60 |
Among the e-cigarette arm abstainers, two (3%) were using non-allocated NRT at 12 months, whereas, in the NRT arm, nine (20%) were using non-allocated e-cigarettes. An additional sensitivity analysis that was not prespecified, in which the abstainers using non-allocated products were removed from the sample, was carried out. This per-protocol analysis resulted in a 52-week abstinence rate in the e-cigarette and NRT arms of 17.7% and 8.0%, respectively (RR 2.21, 95% CI 1.52 to 3.22; p < .001).
Table 4 also shows the secondary abstinence outcomes. Abstinence rates were consistently higher in the e-cigarette arm, including 7-day point prevalence abstinence rates (Table 6).
| 7-day smoking abstinence at each follow-up | Trial arm, n (%) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| E-cigarette (N = 446) | NRT (N = 438) | Relative risk (95% CI) | p-value | Relative risk (95% CI) | p-value | |
| 4 weeks post TQD | 195 (44.4) | 136 (30.4) | 1.46 (1.23 to 1.74) | < 0.001 | 1.43 (1.20 to 1.70)a | < 0.001 |
| 26 weeks post TQD | 158 (36.0) | 115 (25.7) | 1.39 (1.14 to 1.70) | 0.001 | 1.36 (1.12 to 1.66)b | 0.002 |
| 1 year post TQD | 146 (33.3) | 98 (21.9) | 1.52 (1.23 to 1.90) | < 0.001 | 1.52 (1.22 to 1.89)c | < 0.001 |
Among participants who did not achieve full abstinence, more participants in the e-cigarette arm than in the NRT arm achieved a validated reduction of smoking of ≥ 50% (see Table 6).
Time to relapse was not significantly different in the two trial arms (HR 1.14, 95% CI 0.96 to 1.34; p = 0.12). The relapse rates at 1 year among 4-week abstainers did not differ between the two trial arms (RR 1.27, 95% CI 0.93 to 1.73; p = 0.14).
Figure 2 shows the time to relapse in the two conditions.
FIGURE 2.
Time to relapse.

Attendance and adherence
Table 7 shows session attendance and adherence to each treatment and length of NRT/e-cigarette use. Adherence to e-cigarettes was greater from early on, with many more participants still using e-cigarettes at 6 and 12 months, while only a few still used NRT.
| Measure of adherence | Trial arm | p-value | χ2, Z | |
|---|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | |||
| Number of contacts completed,a median (IQR) | 5 (4–5) | 5 (4–5) | ||
| Maximum sessions completed, n (%) | ||||
| 1 | 8 (1.8) | 10 (2.2) | χ2(4) = 8.8; p = 0.07 | |
| 2 | 25 (5.7) | 40 (9.0) | ||
| 3 | 38 (8.7) | 45 (10.1) | ||
| 4 | 86 (19.6) | 106 (23.8) | ||
| 5 | 281 (64.2) | 245 (54.9) | ||
| Use of allocated products over the initial 4 weeksb | ||||
| On how many days used (0–28), median (IQR) | 28 (25–28) | 24 (19–27) | < 0.001 | Z = 11.6 |
| n (%) using daily over the full 4 weeks | 232 (53.0) | 46 (10.3) | < 0.001 | χ2(1) = 186.5 |
| Days used in past week, median (IQR) (the results were similar for weeks 1–4) | 7 (7–7) | 6.5 (3.5–7) | < 0.001 | Z = 8.1–9.1 |
| Use of allocated products at 26 weeks | ||||
| n (%) using at 26 weeks | 180 (41.1) | 33 (7.4) | < 0.001 | χ2(1) = 137.2 |
| Use of allocated products at 52 weeks | ||||
| n (%) using at 52 weeks | 173 (39.5) | 19 (4.3) | < 0.001 | χ2(1) = 161.4 |
Of the 19 participants in the NRT arm using NRT at 12 months, four were using NRT combinations. Products used were patches (n = 7), chewing gum (n = 6), mouth spray (n = 5), inhalator (n = 2), mouth strips, microtabs and lozenge (n = 1 each). Of the 173 participants in the e-cigarette arm using e-cigarettes at 12 months, 168 (97%) used refillable products.
Table 8 shows reasons for discontinuing product use. Participants in the NRT arm were more likely to dislike the taste, have ARs and find the product not satisfying.
| Reason | Trial arm, n (%) | |
|---|---|---|
| E-cigarette (N = 88) | NRT (N = 166) | |
| Cost | 0 (0) | 0 (0) |
| Did not like the taste | 4 (4.6) | 19 (11.5) |
| AR | 7 (8.0) | 25 (15.1) |
| Not satisfying | 4 (4.6) | 16 (10.0) |
| Difficult to use | 2 (2.3) | 1 (0.6) |
| Embarrassing to use | 0 (0) | 1 (0.6) |
| Difficult to obtain them | 0 (0) | 1 (0.6) |
| Smoking normal cigarettes now | 8 (9.1) | 17 (10.2) |
| To quit nicotine | 0 (0) | 6 (3.6) |
| Other | 63 (71.6) | 80 (48.2) |
Overall, there were more participants assigned to NRT who used e-cigarettes than participants assigned to e-cigarettes who used NRT. The initial cross-contamination levels were low. A 15% level of cross-contamination in the first 4 weeks post TQD was predefined as being acceptable; the reported level was < 3%. Cross-contamination was defined as non-allocated product use on at least 5 consecutive days.
However, later in the trial, more people from the NRT arm were using e-cigarettes than the other way round. Participants who switched from NRT to e-cigarettes also used the non-allocated product for longer than those who switched from e-cigarettes to NRT. Similar very low proportions of participants in both trial arms used other stop smoking medications during the first 4 weeks (Table 9).
| Non-allocated product use | Trial arm | p-value | t-value/χ2 | |
|---|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | |||
| Non-allocated product use within the first 4 weeks | ||||
| Used for ≥ 5 consecutive days, n (%) | 3 (0.7) | 11 (2.5) | 0.06 | Fisher’s exact test |
| Non-allocated product use at 6 months (excludes first 4 weeks) | ||||
| Used for ≥ 5 consecutive days since 4 weeks, n (%) | 16 (3.6) | 57 (12.8) | < 0.001 | χ2(1) = 24.3 |
| Duration (in weeks) of non-allocated product use since previous assessment (0–20), median (IQR) | 3 (1–9) | 8 (1–20) | 0.2 | Z = –1.2 |
| Non-allocated product use at 12 months (excludes first 4 weeks) | ||||
| Used for ≥ 5 consecutive days since 26 weeks, n (%) | 14 (3.2) | 77 (17.3) | < 0.001 | χ2(1) = 47.4 |
| Duration (in weeks) of non-allocated product use since previous assessment (0–24), median (IQR) | 6.5 (0–12) | 20 (6–24) | 0.002 | Z = –3.1 |
| Other non-study stop smoking medication use (including single use) | ||||
| Varenicline, n (%) | 15 (3.4) | 13 (2.9) | 0.7 | |
| Bupropion, n (%) | 0 (0) | 0 (0) | N/A | |
Table 10 shows the NRT products selected initially by the participants in the NRT arm.
| NRT products used | n (%) |
|---|---|
| Type of NRT selected initially | N = 442a,b |
| 24-hour patch (21 mg) | 190 (43) |
| 24-hour patch (14 mg) | 4 (1) |
| 16-hour patch (25 mg) | 149 (34) |
| 16-hour patch (15 mg) | 29 (7) |
| 16-hour patch (10 mg) | 1 (0.2) |
| Microtab (2 mg) | 0 |
| Mouth strips (2.5 mg) | 68 (15) |
| Gum (4 mg) | 25 (6) |
| Gum (2 mg) | 8 (2) |
| Lozenge (4 mg) | 20 (5) |
| Lozenge (2 mg) | 17 (4) |
| Nasal spray | 2 (0.5) |
| Minis (4 mg) | 33 (8) |
| Minis (1.5 mg) | 2 (0.5) |
| Inhalator | 163 (37) |
| Mouth spray | 124 (28) |
| Selecting two NRT products | 393 (88) |
| Switched to different NRT product in first 4 weeks | 260 (59) |
The majority of participants (88.1%) received two NRT products. The nicotine patch was by far the most popular, followed by the nicotine inhalator and nicotine mouth spray. Switching to different NRT products during the first 4 weeks of treatment was fairly common (see Table 10).
Table 11 shows the e-cigarette products used by participants in the e-cigarette arm. Most of those who used e-cigarettes began purchasing e-liquid themselves from early on; very few requested e-liquid supply beyond the first bottle. Hardly any participants (< 1%) switched to a cartridge e-cigarette. The nicotine strength of the e-liquid declined over time. Flavour choices varied with time; fruit and tobacco flavours were most popular, with mint and chocolate/candy flavours following (Table 12).
| E-cigarette products used | |
|---|---|
| E-cigarette arm participants using refillable e-cigarettes, n (%) | |
| 1 week post TQD (N = 384)a | 383 (99.7) |
| 4 weeks post TQD (N = 343)a | 343 (100) |
| 26 weeks post TQD (N = 270)a | 265 (98.2) |
| 52 weeks post TQD (N = 235)a | 227 (96.6) |
| E-liquid nicotine strength in mg/ml, median (IQR) | |
| 4 weeks post TQD (N = 340) | 18 (16–18) |
| 26 weeks post TQD (N = 267) | 12 (6–18) |
| 52 weeks post TQD (N = 232) | 11 (5–18) |
| Requested further e-liquid supply at 2 weeks post TQD, n (% of full sample) | 30 (7) |
| Flavoura | Week, n (%) | |||
|---|---|---|---|---|
| 1 (N = 155) | 4 (N = 156) | 26 (N = 516) | 52 (N = 511) | |
| Tobacco | 15 (10) | 44 (28) | 163 (32) | 127 (25) |
| Fruit | 70 (45) | 51 (33) | 150 (30) | 169 (33) |
| Menthol/mint | 31 (20) | 20 (13) | 75 (15) | 81 (16) |
| Tobacco menthol | 5 (3.2) | 7 (4.5) | 13 (2.5) | 12 (2.3) |
| Vanilla | 5 (3.2) | 1 (0.6) | 11 (2.1) | 14 (2.7) |
| Chocolate, dessert, candy or sweet | 17 (11) | 18 (12) | 62 (12) | 72 (14) |
| No flavour | 0 (0) | 0 (0) | 0 (0) | 2 (0.4) |
| Coffee | 2 (1.3) | 1 (0.6) | 6 (1.2) | 8 (1.6) |
| Alcoholic drink | 2 (1.3) | 2 (1.3) | 7 (1.4) | 3 (0.6) |
| Energy or soft drink | 6 (3.9) | 10 (6.4) | 17 (3.3) | 13 (2.5) |
| Other | 2 (1.3) | 2 (1.3) | 12 (2.3) | 10 (2.0) |
Table 13 compares ratings of NRT and e-cigarettes in terms of perceived helpfulness in stopping smoking, and in terms of taste and satisfaction compared with conventional cigarettes. Only cases with complete data across measures are included. E-cigarettes received significantly better ratings for all three variables at both time points. Both products were perceived as less satisfying compared with cigarettes, but e-cigarettes provided higher satisfaction than NRT (see Table 13).
| Rating | Trial arm, mean (SD) | t-value; p-value | Mean difference (95% CI) | |
|---|---|---|---|---|
| E-cigarette (n = 324)a | NRTb (n = 228)a | |||
| Helpfulnessc | ||||
| 1 week post TQD | 4.3 (0.9) | 3.6 (0.9) | 8.4; < 0.001 | 0.7 (0.5 to 0.9) |
| 4 weeks post TQD | 4.3 (0.9) | 3.7 (0.9) | 7.0; < 0.001 | 0.6 (0.4 to 0.7) |
| Tasted | ||||
| 1 week post TQD | 3.0 (1.4) | 2.7 (1.6) | 2.5; 0.015 | 0.3 (0.1 to.06) |
| 4 weeks post TQD | 3.5 (1.3) | 3.1 (1.5) | 3.3; 0.001 | 0.4 (0.2 to 0.6) |
| Satisfactiond | ||||
| 1 week post TQD | 2.4 (1.0) | 2.0 (1.2) | 4.3; < 0.001 | 0.4 (0.2 to 0.6) |
| 4 weeks post TQD | 2.7 (1.1) | 2.3 (1.2) | 4.7; < 0.001 | 0.5 (0.3 to 0.6) |
Urges to smoke
Table 14 shows urges to smoke in participants abstaining from smoking at 1 and 4 weeks post TQD in the two trial arms. Participants in the e-cigarette arm experienced lower frequency of urges to smoke and reduced strength of urges than participants in the NRT arm.
| Urge to smoke | Trial arm at 1 week, mean (SD) | t-test; p-value | Trial arm at 4 weeks, mean (SD) | t-test; p-value | ||
|---|---|---|---|---|---|---|
| E-cigarette (n = 158) | NRT (n = 131) | E-cigarette (n = 186) | NRT (n = 132) | |||
| Frequency | 2.5 (1.1) | 2.8 (0.9) | –3.1; 0.002 | 1.9 (0.9) | 2.2 (0.8) | –3.3; 0.001 |
| Strength | 2.7 (1.1) | 3.2 (1.0) | –3.6; 0.0004 | 2.1 (1.1) | 2.4 (1.0) | –2.6; 0.001 |
| Composite urge score | 2.6 (1.0) | 3.0 (0.9) | –3.6; 0.0003 | 2.0 (1.0) | 2.3 (0.9) | –3.1; 0.003 |
Among all participants, the level of urges to smoke is higher, but the difference between the two trial arms is similar, with the e-cigarette arm reporting lower frequency of urges to smoke and reduced urge intensity than the NRT arm (Table 15).
| Urge to smoke | Trial arm at 1 week, mean (SD) | t-test; p-value | Trial arm at 4 weeks, mean (SD) | t-test; p-value | ||
|---|---|---|---|---|---|---|
| E-cigarette (n = 389) | NRT (n = 383) | E-cigarette (n = 365) | NRT (n = 334) | |||
| Frequency | 2.8 (0.05) | 3.1 (0.05) | –3.8; 0.0002 | 2.3 (0.06) | 2.7 (0.06) | –4.1; < 0.001 |
| Strength | 3.1 (0.06) | 3.3 (0.05) | –3.8; 0.0002 | 2.6 (0.06) | 2.9 (0.06) | –3.6; 0.0004 |
| Composite urge score | 2.9 (0.05) | 3.2 (0.05) | –4.2; < 0.001 | 2.4 (0.05) | 2.8 (0.06) | –4.1; 0.0001 |
Withdrawal symptoms
Regarding other withdrawal symptoms, abstainers in the e-cigarette arm suffered less restlessness, irritability and inability to concentrate during the first week of abstinence. By week 4, abstainers in both trial arms suffered little discomfort and the differences were no longer significant (Table 16). Among all participants, irritability, restlessness, hunger, poor concentration and the composite withdrawal score were all less severe in the e-cigarette arm than in the NRT arm during the first week of abstinence, with only the difference in hunger and the composite withdrawal score persisting at week 4 (Table 17).
| Withdrawal symptom | Trial arm, mean (SD) | t-value; p-value | |
|---|---|---|---|
| E-cigarette (n = 158) | NRT (n = 131) | ||
| 1 week post TQD | |||
| Depressed | 0.05 (0.7) | 0.08 (.8) | –0.3; 0.77 |
| Irritable | 0.27 (1.2) | 0.78 (0.12) | –3.4; 0.001 |
| Restless | 0.13 (1.1) | 0.43 (1.5) | –2.0; 0.05 |
| Hungry | 0.33 (1.1) | 0.59 (1.3) | –1.9; 0.06 |
| Poor concentration | –0.06 (0.8) | 0.25 (1.2) | –2.6; 0.009 |
| Composite score | 0.14 (0.58) | 0.43 (0.75) | –3.6; 0.001 |
| 4 weeks post TQD | (n = 191) | (n = 134) | |
| Depressed | –0.02 (0.8) | –0.04 (0.9) | –0.2; 0.86 |
| Irritable | –0.01 (0.1) | 0.20 (1.1) | –1.7; 0.09 |
| Restless | –0.13 (1.1) | –0.08 (1.3) | –0.3; 0.74 |
| Hungry | 0.19 (1.2) | 0.31 (1.4) | –0.8; 0.43 |
| Poor concentration | –0.15 (0.9) | –0.04 (1.0) | –1.0; 0.30 |
| Composite score | –0.01 (0.6) | 0.08 (0.8) | –1.3; 0.20 |
| Withdrawal symptom | Trial arm, mean (SD) | t-value; p-value | |
|---|---|---|---|
| E-cigarette (n = 389) | NRT (n = 383) | ||
| 1 week post TQD | |||
| Depressed | 0.1 (0.1) | 0.1 (0.1) | –1.0; 0.30 |
| Irritable | 0.4 (0.1) | 0.6 (0.1) | –2.7; 0.008 |
| Restless | 0.1 (0.1) | 0.4 (0.1) | –2.8; 0.006 |
| Hungry | 0.3 (0.1) | 0.5 (0.1) | –2.6; 0.01 |
| Poor concentration | 0.1 (.01) | 0.2 (0.1) | –2.9; 0.004 |
| Composite score | 0.2 (0.1) | 0.4 (0.1) | –4.1; 0.0001 |
| 4 weeks post TQD | (n = 364) | (n = 334) | |
| Depressed | 0.12 (0.1) | 0.13 (0.1) | –0.2; 0.87 |
| Irritable | 0.11 (0.1) | 0.24 (0.1) | –1.5; 0.13 |
| Restless | –0.02 (0.1) | 0.04 (0.1) | –0.7; 0.51 |
| Hungry | 0.10 (0.1) | 0.39 (0.1) | –2.9; 0.004 |
| Poor concentration | –0.04 (0.1) | 0.06 (0.1) | –1.3; 0.18 |
| Composite score | 0.1 (0.04) | 0.2 (0.04) | –2.2; 0.03 |
Product safety
Safety of the trial products was evaluated in three ways: SAEs were recorded, data were collected on elicited ARs, and changes in respiratory symptoms over the duration of 1 year were monitored.
Two participants died in the follow-up period, one in each trial arm. The cause of death of the NRT participant was traumatic neck injury and that of the e-cigarette participant was ischaemic heart disease. There were 27 SAEs reported in the e-cigarette arm and 22 in the NRT arm (see Appendix 1, Table 34). None of the SAEs were classed as related to study product use. There were six pulmonary events: five in the e-cigarette arm and one in the NRT arm (not counting a hospitalisation concerning a lung mass in the NRT arm). The two participants who were hospitalised with pneumonia were both smoking at the time (one was also vaping). The participant in the e-cigarette arm who was hospitalised with asthma had recently stopped vaping and relapsed to smoking. One of the participants with chronic obstructive pulmonary disease (COPD) exacerbation was smoking and vaping at the time; e-cigarette use was not ascertained in the remaining case.
Adverse reactions
Table 18 shows the incidence of elicited ARs in the two trial arms that were reported at least once. NRT caused more nausea, whereas e-cigarettes caused more throat/mouth irritation.
| AR | Trial arm, n (%) | Relative risk (95% CI)b | |
|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | ||
| Nausea | 137 (31) | 169 (38) | 0.83 (0.69 to 0.99) |
| Sleep disturbances | 279 (64) | 303 (68) | 0.94 (0.986 to 1.04) |
| Throat/mouth irritationa | 286 (65) | 221 (51) | 1.27 (1.13 to 1.43) |
All three reactions (nausea, sleep disturbance and throat/mouth irritation) were mostly mild. Table 19 shows the proportion of participants who rated these reactions as severe. The two trial arms did not differ in this respect.
| AR | Trial arm, n (%) | Relative risk (95% CI)a | |
|---|---|---|---|
| E-cigarette (N = 438) | NRT (N = 446) | ||
| Nausea | 29 (6.6) | 29 (6.5) | 1.02 (0.62 to 1.67) |
| Sleep disturbances | 57 (13) | 58 (13) | 1.0 (0.71 to 1.4) |
| Throat/mouth irritationb | 26 (5.9) | 17 (3.8) | 1.51 (0.84 to 2.74) |
Table 20 compares the two trial arms in terms of self-reported respiratory symptoms among participants who provided relevant data. The p-value and odds ratio relate to the occurrence of respiratory symptoms at 12 months, adjusted for the baseline symptoms occurrence and trial centre. Significantly more participants in the e-cigarette arm than in the NRT arm stopped coughing and producing phlegm.
| Respiratory symptom | Trial arm, n (%) | p-value | Relative riska (95% CI) | |||
|---|---|---|---|---|---|---|
| E-cigarette (N = 315) | NRT (N = 279) | |||||
| Baseline | 12 months | Baseline | 12 months | |||
| Shortness of breath | 120 (38.1) | 66 (21.0) | 92 (33.0) | 64 (22.9) | 0.25 | 0.9 (0.7 to 1.1) |
| Wheezing | 102 (32.4) | 74 (23.5) | 86 (30.8) | 59 (21.2) | 0.73 | 1.1 (0.8 to 1.4) |
| Cough | 173 (54.9) | 97 (30.8) | 144 (51.6) | 111 (39.8) | 0.004 | 0.8 (0.6 to 0.9) |
| Phlegm | 137 (43.5) | 79 (25.1) | 121 (43.4) | 103 (36.9) | 0.002 | 0.7 (0.6 to 0.9) |
To determine whether or not the better cough and phlegm outcomes in the e-cigarette arm participants were a result of their higher quit rate, a non-planned exploratory analysis controlling for 1-year abstinence status was run. This did not alter the results (RR = 0.8, 95% CI 0.6 to 0.9, p = 0.008, for cough; and RR = 0.7, 95% CI 0.6 to 0.9, p = 0.004, for phlegm).
Discussion
Clear differences between the quit rates in the two trial arms emerged early on, with the participants in the e-cigarette arm having significantly higher validated quit rates at all time points. Sustained biochemically validated 1-year quit rate with NRT products of patient choice, including NRT combinations and an opportunity to vary products, was 9.9%, tallying closely with success rates reported previously for the UK SSSs 1-year validated outcome. 25 In the e-cigarette arm, the quit rate was 18.0%. Participants in the e-cigarette arm showed significantly better adherence and experienced fewer urges to smoke, and reduced strength of urges, throughout the initial 4 weeks of their quit attempt than those in the NRT arm. Participants assigned to e-cigarettes reported significantly less coughing and phlegm at 1 year than those assigned to NRT.
In the following sections, key aspects of the trial and of its findings are discussed.
Sample representativeness
The trial sample comprised participants who are typical of clientele of UK SSSs: middle-aged smokers unable to quit smoking unaided, including some 40% in receipt of free prescriptions (a marker of illness or social disadvantage), with high baseline CO readings, who have tried a range of stop smoking aids before. The findings are likely to be valid for clinical samples but may not be generalisable to smokers who are less dependent or who try e-cigarettes for reasons other than quitting smoking.
Representativeness of the two interventions
In this pragmatic trial, e-cigarettes were used as they could be used in routine SSS practice, namely smokers were provided with a starter kit and advised to purchase e-liquids with a flavour and nicotine strength that they found helpful. They were also encouraged to buy a different e-cigarette model if needed. A range of e-cigarette products and e-liquids are available for smokers with different needs and the practise utilises the opportunity this provides. Participants who found e-cigarettes helpful had no problem with purchasing their own supplies. In fact, by the end of the first 2 weeks of treatment, when trial e-liquid was still available, most were already using e-liquids that they had purchased themselves. This approach makes it probable that the results can be achieved in any setting that can replicate the trial conditions, but it also means that the trial results may not be replicated if just one type of e-cigarette is provided to all participants, especially if this is a weak cartridge-based product.
Nicotine replacement therapy was provided under generous conditions that are probably optimal for treatment efficacy. The medication was provided free of charge, apart from the prescription charge applicable at one trial site. Participants were encouraged to select from among the full range of NRT preparations products that best fit their needs; most participants (88%) were provided with two forms of NRT, usually a patch plus a fast-acting NRT formulation, and more than one-third of participants experimented with different NRT products during the initial treatment period. NRT use was also supervised by SSS clinicians trained in guiding clients and optimising NRT use.
Caution was taken to avoid possible bias related to product cost and effort needed to obtain it. At the SSSs that provide NRT on LOR, participants in both groups had to collect NRT from a pharmacy, and the e-cigarette group exchanged this for an e-cigarette at randomisation. Reassuringly, no participant listed cost as the reason for discontinuing product use.
Effect of e-cigarettes on smoking cessation
The only previous trial9 reported a small, non-significant, advantage of e-cigarettes over patches (7.3% vs. 5.8% quit rates at 6 months). However, it used an early unreliable Cig-A-Like e-cigarette (Elusion™, Auckland, New Zealand) with very low nicotine delivery (maximum concentration of 3 ng/ml, compared with 18 ng/ml from cigarettes and 10 ng/ml from refillable e-cigarettes using 20 mg/ml e-liquid)27 and the products were provided with no face-to-face support, which explains the low quit rates. In this trial, e-cigarettes were significantly more effective than NRT at all time points, despite the fact that the number of participants in the NRT arm who also used e-cigarettes was more than three times the number in the e-cigarette arm who used NRT as well. Such ‘contamination’ could not exert much influence on the primary outcome of abstinence from the third week of treatment onwards; at 3 weeks contamination was low, but those who abstained for longer periods included abstainers in the NRT arm who quit with the help of e-cigarettes, making the results conservative.
Product use at 1 year
Most abstainers in the e-cigarette arm (80%) were still vaping at 1 year, whereas only 9% of NRT arm abstainers were still using NRT. Ongoing use of nicotine-containing products could be seen in both a positive and negative light. Product use may assist with maintaining smoking abstinence and preventing relapse, ameliorate the weight gain that typically follows nicotine withdrawal and provide some continuation of the positive subjective effects that smokers used to get from smoking. However, it can be also a cause for concern if overcoming nicotine addiction is seen as an important aim in itself, even if ongoing nicotine use carries negligible health risks, or if ongoing vaping poses clear health risks.
Regarding continuous nicotine use, when NRT was first introduced, the view that combating nicotine use is the main purpose of stop smoking interventions led to claims that someone who stopped smoking successfully but still uses NRT should not be counted as a treatment success. However, the motivation for helping smokers quit is to help them to avoid the health risks of smoking, and, once this rationale became widely accepted, such concerns largely disappeared. The SSS sees its aim as helping smokers avoid smoking-related disease and death, and there are no concerns about long-term use of NRT, especially as such use prevents relapse in ex-smokers with high tobacco dependence and other negative prognostic signs. 28 However, the concerns about ongoing nicotine use are likely to re-emerge as smokers switch to vaping. For those who are concerned about nicotine use per se, some reassurance is provided by data showing that vapers show lower dependence on e-cigarettes than on cigarettes,29,30 and smokers who successfully stop smoking with the help of e-cigarettes tend to eventually stop vaping as well. 10 It is currently estimated that the health risks of long-term vaping are unlikely to exceed 5% of the risks of smoking. 2 Some risks may yet emerge and ongoing monitoring is needed, but no health risks from e-cigarettes used for up to 1.5 years have been identified to date, and biomarkers of risk that have been examined so far show that long-term vapers do not differ from long-term NRT users (and probably also from non-smokers). 31
Product adherence and attractiveness
Among participants who engaged with treatment, adherence was initially high in both trial arms. The difference in adherence to the two products increased markedly over time, with participants being much more likely to continue with e-cigarette use than with NRT use at later follow-ups. This could be related to the fact that NRTs are presented as a medicinal product with an expectation of time-limited use, whereas e-cigarettes are a consumer product that is more easily accessible and not linked to expectations of short-term use only. However, satisfaction with the product may have been the most important factor as suggested by other data on product differences. Unsatisfactory taste, lack of satisfaction and ARs were the main reasons for stopping NRT use and were more frequent in the NRT trial arm than in the e-cigarette trial arm. E-cigarettes were also rated as significantly more helpful, more similar to cigarettes in taste and more satisfying than NRT.
It is possible that, apart from better sensory effects, e-cigarettes were also providing better tailored nicotine replacement than NRT. Allowing participants to select their own e-liquid could have provided better dose tailoring than NRT products allow. It is paradoxical that smokers can select their nicotine levels freely while they smoke, but once they enter NRT treatment this autonomy is removed and nicotine dosing is placed in the hands of those who treat them. In addition, NRT products are formulated to provide very low nicotine doses, far below what smokers normally obtain from their cigarettes. Using NRT combinations could ameliorate this problem, but the short-acting products added to patches are typically used only opportunistically. Future studies should collect cotinine samples to allow comparisons of the actual nicotine intake.
E-cigarette and nicotine replacement therapy products used
The starter pack consisted of a refillable (tank) e-cigarette. Very few users switched to cartridge-based products. This corresponds to data showing that e-cigarette users prefer refillable e-cigarettes5 and that these provide higher nicotine levels,32 better relief from craving and higher satisfaction33 than Cig-A-Like products. Vapers tried a wide range of e-liquid flavours. Fruit flavours were the most popular, with tobacco flavour also popular at 6 months but less so at 1 year. Sweet flavours (chocolate, candy) and menthol increased in popularity over time. This corresponds with data showing that fruit flavours are the most popular, with many smokers also liking candy flavours. 5 NRT product choices were similar to those observed across SSSs. 34 The patch was by far the most popular choice, followed by the inhalator and mouth spray.
Effects of the two treatments on withdrawal symptoms
Participants using e-cigarettes reported significantly lower intensity and frequency of urges to smoke than participants using NRT throughout the initial treatment period. All existing stop smoking medications are assumed to exert their main therapeutic effect via craving reduction, and this is likely to be the case with e-cigarettes too. Most other tobacco withdrawal symptoms were also lower in the e-cigarette arm than in the NRT arm during the first week of abstinence, although this effect subsided by 4 weeks, when most withdrawal symptoms in continued abstainers reduced to almost zero.
Product safety
There was one death in each trial arm. A difference in frequency of acute pulmonary events was noted (five in the e-cigarette arm vs. one in the NRT arm, or two if counting a lung mass in the NRT arm). Two of the e-cigarette-arm participants with pulmonary SAEs were not vaping at the time, two were vaping and smoking, and the vaping status of one is not known. The difference is likely to be due to chance; however, the study design did not set out to examine e-cigarette safety. Future study designs should include more reliable evaluation of any possible pulmonary risks of vaping.
Sleep disturbance was monitored, as this is considered to be an AE that is commonly associated with the use of NRT patches, but sleep disturbance was equally common in both trial arms. As expected, throat/mouth irritation was more frequently noted by e-cigarette users and nausea was reported more frequently by NRT users, but both effects were mostly mild. The two groups did not differ in the incidence of severe reactions.
Contrary to general expectations, e-cigarette use seemed to have a significantly positive impact on elicited respiratory symptoms. Previous findings on this have been contradictory. Studies of cells and animals have suggested that vaping may result in respiratory infections;35,36 however, a large internet survey of vapers found that the switch from smoking to vaping was accompanied by reduction in respiratory infections. 37 It was proposed that this could be because of the antibacterial properties of propylene glycol, which is usually one of the constituents of e-liquid. Respiratory symptoms were monitored to see if vaping has any negative or positive effects. Changes in wheezing and shortness of breath did not differ between the two arms, but there was a significant difference in two other symptoms that are more closely linked to respiratory infections: cough and generating phlegm. There was improvement in both study arms compared to baseline, but the improvement in the e-cigarette arm was significantly larger. Importantly, the effects were not due to the higher quit rate in the e-cigarette arm, and so they support the hypothesis suggested by previous observations that vaping provides protection against respiratory tract infections. Future studies should include objective measures of lung health and respiratory symptoms.
Economic analysis
The full economic analysis is presented in Chapter 5, but, in summary, compared with NRT intervention, e-cigarette intervention was less costly and generated higher quit rates; therefore, it was also more cost-effective. There was an indication that the e-cigarette arm achieved a higher quality-adjusted life-year (QALY) gain than the NRT arm during the 12-month period, but the difference was not significant. The e-cigarette intervention did not appear to lead to an increase in spending on cessation aids on the smokers’ part while reducing the costs to the smoking cessation services.
Trial limitations
One obvious limitation of open-label trials is the possible effect of expectations. There is no evidence that expectations can boost long-term abstinence rates, but it is likely that they can lower the quit rate in the condition that is seen as inferior, for example if therapists communicate low expectations and/or patients think that they have received the short straw they may drop out of treatment or not put as much effort into quitting. This trial was presented as testing whether or not e-cigarettes are as effective as NRT to avoid creating expectations of its superiority. It was also possible to check whether or not a depressed quitting in the NRT condition contributed to the trial results. A study25 of the long-term quit rates among a group of SSSs with above average early outcomes, using the same outcome criteria as this trial, showed an overall validated 1-year quit rate of 8%. Clients receiving identical individual treatment as used in this trial, provided by specialist advisors, had a quit rate of 10%. However, 46% were treated with varenicline, which was associated with significantly higher abstinence than NRT (12% vs. 7%, respectively). A large RCT38 has recently confirmed the superiority of varenicline over other medications. Thus, it is reassuring that the NRT-arm quit rate (10%) was commensurate with or superior to the standard outcome in this treatment setting.
We tried to make treatment access similar in the two trial arms, but all e-cigarette users had to pay for their supplies, whereas only about half of NRT users had to pay prescription charges. However, this is unlikely to play a major role, as cost was not listed among the important reasons for discontinuing product use by either trial arm.
The 79% follow-up rate at 1 year was comparable with the 78% follow-up rate achieved in another recent NIHR-funded trial in the same setting and population,39 79% achieved in SSS service evaluation25 and 75% in an earlier trial that also took place within SSSs. 40 The follow-up rate was somewhat lower in the NRT arm (77% for NRT vs. 81% for e-cigarettes), which probably reflects the fact that unsuccessful quitters are more likely to drop out. Delivering higher rates of follow-up in this population is difficult. Smokers receiving face-to-face support usually feel embarrassed if they do not succeed, and avoid further contact. However, incomplete data are another trial limitation.
Participants’ weight was not monitored, nor were participants asked about weight changes that they experienced. As evidence is emerging that e-cigarette use may ameliorate post-cessation weight gain, future studies should include such monitoring.
As discussed in Sample representativeness, the trial sample comprised dependent smokers seeking treatment, and they received instructions, support and monitoring of their progress. The results may not be generalisable to other types of smokers and other settings. The trial did not evaluate any one particular e-cigarette product, but the choice of e-cigarette devices and e-liquids that smokers can access currently in the UK. Practically all e-cigarette arm participants used refillable e-cigarettes. The results may not apply to the now largely obsolete Cig-A-Like e-cigarette (now produced almost exclusively by the tobacco industry) or to settings and countries that only allow one particular e-cigarette product.
Implications for health care
Stop Smoking Services have become more receptive to e-cigarettes over the past few years and most now condone or encourage e-cigarette use, but only a few provide e-cigarette starter packs. The reluctance to do so is understandable, because evidence of the comparative efficacy of e-cigarettes is limited to one trial9 showing that early e-cigarettes matched NRT patches when used with minimal support. The results of the current trial are likely to change the current practice. The trial provides evidence that e-cigarettes not only match but surpass the efficacy of NRT, even when combination NRT is provided alongside expert advice and supervision.
The adoption of e-cigarettes by SSSs could generate several benefits. It is likely that e-cigarettes as a smoking cessation tool are more effective if accompanied by support and monitoring, as has been shown for NRT. 41 If smokers switching to e-cigarettes do so with SSS support, they are likely to improve their chance of successfully stopping smoking. The offer of e-cigarette starter packs could be particularly attractive to disadvantaged smokers, thus improving the service reach in areas where it is most needed. Offering e-cigarettes as one of the treatment options would also make the services more economical. The full course of a single NRT product is typically > £100 and combinations of two NRT products, widely used by SSSs, cost even more. The more advanced starter pack used in this trial cost £30. If SSSs start to provide e-cigarettes, this may also counteract some of the antivaping reporting in UK tabloids and contribute to dispelling the worrying increase among smokers of a belief that e-cigarettes are as dangerous as cigarettes. 2
Outside clinical settings, the trial results reinforce the general recommendation to smokers to use e-cigarettes to stop smoking as provided by, for example, Stoptober, the Royal College of Physicians and the National Centre for Smoking Cessation and Training. The effects of e-cigarettes without multisession support may well be lower, but there are currently > 1.5 million people in the UK who report that they stopped smoking with the help of e-cigarettes.
The trial is likely to have an international impact, but different countries have different e-cigarette regulations and different attitudes to ‘harm reduction’. We believe that, in this context, if e-cigarettes help smokers who seek treatment to stop smoking, then they should not be seen as a method of harm reduction but as a method of smoking cessation. A small proportion of such smokers are likely to use e-cigarettes in the long term, but the majority will use e-cigarettes only temporarily, over a period of time that is assumed to carry no health risk. The ultimate goal is to remove any health risks altogether and help people who wish to stop all nicotine use to do so, but extended nicotine use can be helpful to smokers who struggle to stop smoking otherwise. Health professionals could now inform dependent smokers that e-cigarettes are superior to NRT as a method of stopping smoking.
Recommendations for research
Longer-term follow-ups of smokers quitting with the help of e-cigarettes and smokers quitting with other methods are needed to establish the frequency of long-term e-cigarette use in this population and any health effects that such use may have. These results also suggest an intriguing possibility that e-cigarette use may reduce respiratory infections, but it may also have some adverse effects. Studies of medical records and lung function in ex-smokers quitting with and without e-cigarettes are needed to clarify this issue.
A combination of varenicline with NRT has not generated clear additional effects,42 but a combination of varenicline and e-cigarettes could hold more promise. Adding a more satisfactory sensorimotor replacement to varenicline could, in theory, improve withdrawal relief and lower the risk of relapse. There may also be smokers who have only limited reaction to one of the treatments but are sensitive to the other; the combination may be of particular benefit to this group.
A replication of this finding is also needed. Most e-cigarette studies that we are aware of plan to compare e-cigarettes with nicotine and e-cigarettes without nicotine. Such studies follow the standard approach for testing drugs, but this does not seem to be a productive paradigm in this field. The objective of these studies is unclear. E-cigarettes without nicotine are rarely used and so there is no practical need to evaluate them. If the objective is to determine whether or not giving smokers nicotine helps with quitting, there are already > 100 RCTs showing that it does. 19 Such studies will provide no information on the main reason that e-cigarettes are much more popular than other forms of NRT, namely the combination of nicotine and sensorimotor input. 43
Population data suggest that e-cigarettes help many smokers quit smoking without any input from health-care professionals. It is not known to what degree an intensive support, such as that provided in this trial, increases e-cigarette efficacy. Studies that compare quit rates of e-cigarettes provided alongside differing degrees of support are needed. This would help to establish if public health messages should focus on encouraging the switch from smoking to vaping within stop smoking services, or on recommending switching to vaping without such need.
Conclusion
Within the context of multisession treatment for smokers seeking help, as provided by the UK specialist SSS, e-cigarettes were significantly more effective than NRT. The provision of e-cigarette starter packs by specialist SSSs may help to improve the treatment efficacy, cost efficacy and reach of SSSs.
Chapter 5 Economic evaluation of the TEC trial
Methods
The economic evaluation was conducted by way of a cost-effectiveness analysis. Following NICE guidance,44 the analysis was performed from the NHS and Personal Social Services (PSS) perspective to reflect the NHS England decision-making framework. The analysis was also conducted from a wider perspective to explore the possible societal impact of the intervention. All costs are presented in 2015/16 Great British pounds. The objectives of the analysis were to:
-
assess the cost-effectiveness of e-cigarettes over and above usual care at the 12-month follow-up (i.e. the primary outcome)
-
estimate the potential long term cost-effectiveness of the intervention.
These objectives were achieved by combining data collected within the trial and existing models.
Intervention training cost
Because e-cigarettes are a relatively new product to the NHS, SSS advisors in their official capacity are provided with additional training on how to use the product before they can confidently provide smokers with advice about the product. The training was estimated to take 1 hour and was delivered once for each site by two of the research team members. A total of 30 advisors or researchers attended the training. Although they provided support for the participants in both arms, this training was specific to the e-cigarette intervention. Each was equipped with one demonstration kit at a cost of £19.35, including liquid and accessories. To reflect routine practice should the intervention be rolled out, all of the attendees were costed as advisors, which was the average of NHS pay band 5 and 6. Trainers were costed at NHS pay band 6. Including salary oncosts, overheads and capital, the cost was estimated at £37 per hour for advisors and £42 per hour for trainers. 45
Intervention delivery cost
The intervention had two components. The first was behavioural support and the second was the provision of a smoking cessation aid. Behavioural support was the same for both arms. It was delivered by the NHS SSS advisors or researchers in a face-to-face setting or by telephone, with one session prior to the TQD followed by one session on the TQD and four weekly sessions after the TQD.
The smoking cessation aid for the e-cigarette arm was the e-cigarette. The research team provided a starter kit (i.e. a 2-week supply) to the participants free of charge. A further 2-week supply of e-liquid could be requested if the participants failed to purchase their own at 2 weeks post TQD. Participants were also provided with a printed information sheet on how to use the e-cigarette.
The smoking cessation aid for the NRT arm was a NRT product. Owing to the different settings in the trial sites, NRT products were provided to the participants either directly or through a LOR. Direct provision of NRT was free of charge. However, LOR requires participants to go to a pharmacy and redeem the prescription. A prescription charge of £8.20 per item might be payable if the participant was not otherwise exempted. 46 These NRT products were provided for 4 weeks on a fortnightly basis. Further supply could be requested on completion of the treatment. The participants were also provided with a printed list of local pharmacies.
The form and dosage of NRT was matched to all the products with the same chemical name, dosage and form in Prescription Cost Analysis – England 201647 to get the weighted average cost per item of each NRT product. Where the information on some aspects was missing (e.g. patches with no dosage recorded), the weighted average cost based on available information was applied. The dispensing fee per item was estimated based on the data published by the NHS Business Services Authorities. 48 Owing to different approaches of dispensing NRT products between the research sites (direct dispense vs. LOR), a conservative assumption was made that once an LOR is provided, the stated NRT products are considered dispensed. This is to avoid underestimating cost to the NHS.
The usage of behavioural support, NRT products and/or e-cigarettes was recorded in the CRF at each support session. The cost of printing the e-cigarette leaflets and a pharmacy list was recorded by the research team.
Smoking cessation service outside the intervention period
Utilisation of smoking cessation services outside the intervention period, including pharmacotherapies and e-cigarettes, was recorded at baseline and at the 6- and 12-month follow-ups by self-report. Each covered the services utilisation in the six-month period before data collection. The quantities reported were then multiplied by the unit costs of corresponding services or net ingredient cost plus dispensing fee of prescribed items using secondary data sources (see Appendix 1, Table 35).
Health-care service use
Health-care and social services use data were collected at baseline and at the 6- and 12-month follow-ups. The quantities of the service use were collected using a self-report questionnaire. The quantities were then multiplied by a set of weighted national average costs following standard health economics methodology (see Appendix 1, Table 35).
Quality of life
Health-related quality of life was measured using the EQ-5D-5L. 49 By applying the UK population tariff to the scores of five domains of EQ-5D-5L, a utility score was to be calculated at different time points. QALYs were then derived by calculating the area under the curve from baseline to 6 months and from 6 months to 12 months. 18 In addition, EuroQol Visual Analogue Scale (EQ VAS) results were also collected at the three time points.
At the time of the analysis, NICE had issued a statement recommending mapping the EQ-5D-5L descriptive system data onto the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), valuation set, despite the valuation set for EQ-5D-5L being made available earlier. 50 Therefore, although the quality-of-life data were collected using the EQ-5D-5L, the valuation of utility scores was conducted using the recommended mapping function. 51
Analysis
Missing data
The primary analysis was conducted on an intention-to-treat basis. For the smoking cessation service use outside the intervention and health-care service use, data were classified as missing when all sections under one question were left blank. For instance, if one question had five subquestions [annotated as a), b), c), etc.], then all five were deemed missing when all five were left blank. If one part was answered, the others were assumed as zero. In the case of the EQ-5D-5L, owing to the structure of the questionnaire, the whole section was considered missing if any of the five questions was not answered. Missing data due to death would result in all costs and QALYs being equal to zero at the time points after the date of death.
Missing data were handled by multiple imputation following Rubin’s rules,52 assuming that any missing data were missing at random. A chained equation model was developed and predictive mean matching by arms was used as the imputation method, using the 10 nearest neighbours to the prediction as a set to draw from.
The imputation model included training costs, intervention delivery costs, smoking cessation help costs at baseline and at 6 and 12 months, pharmacotherapies at baseline and at 6 and 12 months and health-care service use costs, EQ VAS scores and utility scores at baseline and at 6 and 12 months, age, sex, trial site, FTCD at baseline, ethnicity and entitlement to free prescriptions. Although not a cost to the NHS, the costs of NRT products purchased over the counter (OTC), the costs of e-cigarette purchases and prescription charges, when applicable, were also included in the imputation model.
The EQ-5D-5L results were imputed at the utility score level rather than at the individual domain level. Owing to the arrangement of the questionnaire, the costs of service use were imputed at an aggregated level as smoking cessation services, pharmacotherapies and health-care services, rather than at an individual service level. Training costs and intervention delivery costs were imputed as individual variables, as their costing methods were different. As a rule of thumb, the number of imputations was set to approximately the highest percentage of missing data in all variables. 53
Primary analysis
The primary analysis took the form of an incremental cost-effectiveness analysis. The costs included intervention costs, smoking cessation service cost after the intervention period and health-care service use cost that had occurred during the 12-month period. The difference in costs between arms was controlled for smoking cessation service costs and health-care service use at baseline, age, sex, trial site, entitlement to free prescriptions and baseline FTCD. The QALYs were calculated during the same time period. The difference in QALYs between arms was controlled for utility value at baseline, age, sex, trial site, entitlement to free prescriptions and baseline FTCD. Combining both, an ICER of cost per QALY was calculated to assess the cost-effectiveness of e-cigarettes, compared with NRT. The ICER was then compared with the national WTP threshold of £20,000–30,000 per QALY gained. 44
Sensitivity analysis
A non-parametric bootstrap resampling technique was used to assess the uncertainty surrounding the ICER. Bootstrapping has been proposed as an efficient approach for calculating the confidence limits for the ICER, as its validity does not depend on any specific form of underlying distribution. 54–57 A cost-effectiveness plane (CEP) and a CEAC were plotted, based on the outcomes of 5000 bootstrap iterations, to illustrate the uncertainty. 58
To assess the impact of the missing data, an additional set of analyses were carried out using the CCA, analysing results only for those participants for whom both the completed cost and outcome data were available at all time points. 59–62
Secondary analyses
Comparison of weekly nicotine replacement therapy dispense with nicotine replacement therapy use
A separate analysis was carried out to examine the potential impact on the intervention costs of the assumption of NRT dispense equating NRT use. In the primary analysis, the NRT costs were estimated based on the dispensing records in the NRT arm. The dispensing records reflected the NRT dispensed for the week following the session from the TQD. From 1 week post TQD, participants in the NRT arm also reported their use of NRT in the week before the session. The selection of NRT products was the same as for the dispensing records; therefore, the same set of weighted average costs per unit of NRT products as used in the primary analysis was applied. The method employed provided a conservative estimate, as it was possible that not all products used were provided by the trial. In the case of obtaining the NRT products OTC, the actual costs to the participants could be higher than our estimates. Lacking means to distinguish the sources of self-reported NRT products, the net ingredient cost plus dispensing fee was used.
The estimated weekly cost of NRT used in the past week was then compared with the estimated weekly cost of recorded NRT dispensing in the previous session among the participants for whom both were recorded.
Costs of smoking cessation aids to the NHS and participants’ expenses on smoking cessation
As e-cigarettes are not currently provided by the NHS, and NRT products might be purchased OTC outside the intervention period, excluding participants’ expenses on e-cigarettes and OTC NRT products might lead to a conclusion of cost-effectiveness by shifting the costs to the participants. To assess this possibility, the costs of purchasing e-cigarettes (including refills) and NRTs OTC, and prescription charges for redeeming the pharmacotherapies when applicable, were included to compare the distribution of smoking cessation aids costs between the NHS and the participants in both arms. The costs of OTC NRTs were estimated using the same set of net ingredient cost plus dispensing fee (see Appendix 1, Table 35).
In reality, the unit costs of NRT products purchased OTC are different from the ones in Table 35 (see Appendix 1). However, given the complexity of the marketing sizes and prices and the lack of detailed information on the products purchased, the same unit costs were used for the OTC products. It should be noted that the actual expenses could, therefore, be higher than these estimates. The cost of e-cigarettes (including refills) was estimated based on self-report by the participants who answered the question ‘How much money have you spent on e-cigarette products in the last 6 months?’.
Long-term cost and outcome projections
Smoking has been found to present an increased risk of developing many diseases, such as cancer, COPD and cardiovascular disease. 63,64 The relationship between smoking and life expectancy has been proven by the British Doctors’ Study using data collected over 5 decades. 65 The study found that smokers who quit smoking at ages 30, 40, 50 and 60 gained 10, 9, 6 and 3 years of life, respectively. Smoking cessation, therefore, has impacts on both the costs of treating smoking-related diseases and health-related quality of life over people’s lifetime.
The NICE guidance to the methods of technology appraisal44 recommends that the time horizon for estimating cost-effectiveness should be sufficiently long to reflect important differences in costs and outcomes between the interventions being compared. Therefore, in addition to the within-trial cost-effectiveness analysis, a decision-analytic model was used to capture the lifetime cost-effectiveness of the use of e-cigarettes, in comparison with NRT, when used within the UK SSSs.
Model structure
A Markov model was developed to estimate long-term costs and consequences of smoking cessation interventions in this research team’s previous study. 66 The Markov model is considered to be the most appropriate approach to modelling smoking cessation, in which, over time, events such as quitting and relapse recur. 67 Smokers could quit their smoking habit with or without help, and former smokers may relapse for various reasons. 68 In this report, this model was populated with the costs and smoking status during the trial period of the current trial and other updated parameters from a variety of sources.
The Markov model structure and the health-state transitions are demonstrated in Figure 3. This model comprises three Markov states: current smoker, ex-smoker (former smoker/quitter) and death. The model assumes a hypothetical cohort of 1000 individuals who are current smokers when entering the model, and simulates events from there. Trial data were used to populate the first 12 months, for which the costs and QALYs were estimated based on the trial for e-cigarettes and NRT, respectively. At the end of the first year, individuals’ smoking status was assigned in proportion to the 12-month abstinence rates estimated from the trial outcomes in each intervention group. Subsequently, individuals transferred between the three states following the direction of the arrows with a model cycle of 1 year until the survivors completed their 90th year. The assumption was made that the individuals would not take the interventions again after the trial period.
FIGURE 3.
The structure of the Markov model for long-term projection.

The movement of individuals between health states at the end of each cycle was parameterised by transition probabilities. Each health state is associated with a smoking-related health-care cost and health benefit in terms of QALYs gained. The accumulated lifetime smoking-related health-care costs and QALY gains for each intervention were computed by summarising values of all the individuals over all model cycles. Both costs and QALYs were discounted at an annual rate of 3.5%. 44 The model was programmed in Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Model parameter inputs
To populate the model, comprehensive literature reviews were undertaken. The parameter values derived from the literature and the sources are presented in Table 36 (see Appendix 1). Additional parameters estimated from the trial data are presented in Results.
Relapse
It is common for people who achieved abstinence to smoke again. A systematic review and meta-analysis conducted by Hughes et al. 68 reported that the average 1-year relapse rate to smoking after 1 year of abstinence was ≈10%. There is also evidence from retrospective studies that abstinence tends to become stable after 10 years. 69,70 Therefore, in this model we assumed that the risk of relapse for participants reporting successful quit was 10% per year for the first 10 years and 0% thereafter.
Mortality
In the Markov model, all pathways eventually lead to death. The mortality rates for the first 12 months of the model were derived from the number of participants who died during the trial period. From the second cycle onwards, we first obtained mortality rates for people of different age and sex from the deaths registered in England and Wales in 2016. 71 We then took into account the increased risk of death for smokers and ex-smokers compared with never-smokers reported by Doll et al. 65 The estimated mortality rates for both smokers and ex-smokers by age and sex are listed in Appendix 1, Table 36.
Utility
To estimate QALY gains for the two interventions, we need to attach utilities to the different health states in the model. The QALYs estimated from the trial were used as the outcome input for the first cycle of the model. For the period beyond the trial follow-up, the QALY data were derived from a study in England conducted by Vogl et al. 72 using data from the 2006 Health Survey for England. 73 The estimates from this study were considered representative as it had a large sample size of 13,241 and the survey participants were chosen at random from all the addresses in England. The study estimated the utility values, measured by EQ-5D based on age, sex and smoking status. Appendix 1, Table 36, summarises the average yearly QALY gain for both smokers and ex-smokers by age group and sex.
Costs
The within-trial costs of the two interventions, consisting of both intervention costs and wider healthcare resource use costs, were included in the model as a one-off cost for the first 12 months.
The long-term costs for treating smoking-related diseases for smokers and ex-smokers were measured using cost-of-illness methods recommended by the World Health Organization Economics of Tobacco Toolkit. 74 The Hospital Episode Statistics (HES) records all the hospital inpatient care utilisation for patients at NHS hospitals in England by diagnosis, sex and age. 75 By combining the usage of admitted patient care caused by 52 identified smoking-related diseases extracted from the HES data and the national average unit costs extracted from the NHS reference costs, we estimated the annual costs related to smoking in England by sex and age group. 75–77 Because the NHS reference costs report the average unit cost at the level of Healthcare Resource Group, we employed the NHS Code to Group Workbook (HRG4+)78 to link the HES hospital episodes and reference costs. The smoking-attributable proportion was calculated as follows:
where pcur/pex is the proportion of current smokers/ex-smokers and rcur/rex is relative risk for having certain smoking-related diseases for current smokers/ex-smokers compared with people who never smoked.
The estimated annual costs were then multiplied by the smoking-attributable proportion to generate the costs attributable to smoking for smokers and ex-smokers. The results are summarised in Appendix 1, Table 36.
Cost-effectiveness analysis and probabilistic sensitivity analysis
The Markov model was run separately for each intervention and yielded expected costs and effectiveness in terms of QALYs. Similar to the within-trial economic evaluation, an incremental cost-effectiveness analysis was conducted. The ICERs were calculated and the intervention with an ICER of < £20,000 per QALY was considered as cost-effective, following NICE’s guideline. 44
A probabilistic sensitivity analysis was performed to propagate the probabilistic uncertainty surrounding each model parameter and estimate 95% CIs around the cost-effectiveness results. The model was probabilistic in that all parameters were assigned probability distributions to reflect their sample variability. Beta distribution was used for probabilities and a gamma distribution was used for costs and QALYs. 67 Monte Carlo simulation was employed to draw a randomly selected estimate of each model parameter from the assigned distribution, and the expected costs and QALYs for each intervention were calculated using a particular combination of parameter values. 79 This random draw was then repeated 10,000 times, and the results of these simulations were reported in the form of the CEP and CEACs.
Results
In total, 886 participants were randomised: 447 in the NRT arm and 439 in the e-cigarette arm. There was one death before the 6-month follow-up in the NRT arm and one death before the 12-month follow-up in the e-cigarette arm.
Intervention training cost
The cost of trainees’ time for 1 hour of training was calculated by multiplying the number of advisors by the hourly cost. It was recorded as £1110 in total. Additional training was delivered in three sites by two trainers, resulting in £252 of trainer time cost. For each advisor to have a demo kit required the purchase of 30 kits at a cost of £580.50. A training cost of £4.40 per participant was allocated to the e-cigarette arm. There was zero training cost in the NRT arm.
Intervention delivery cost
Behavioural support sessions
The mean number of sessions received was 5.5 (SD 1.0) in the e-cigarette arm and 5.2 (SD 1.2) in the NRT arm, with a range of 2–6 sessions. In the NRT arm (n = 447), 62% of participants had six sessions, 15% had five sessions, 11% had four sessions, 6% had three sessions and 6% had two sessions. In the e-cigarette arm (n = 439), 70% of the participants had six sessions, 15% had five sessions, 7% had four sessions, 5% had three sessions and 3% had two sessions. The proportion of participants who had six sessions was higher in the e-cigarette arm than in the NRT arm (70% vs. 62%).
On average, sessions 1 and 2 each lasted for 30 minutes. The subsequent sessions took approximately 15–25 minutes. For costing purposes, the middle point of the time estimate, 20 minutes per session, was used for sessions 3–6. The cost of behavioural support was, therefore, £80 (SD £12) per participant in the e-cigarette arm and £77 (SD £15) per participant in the NRT arm.
Nicotine replacement therapy/e-cigarette
The e-cigarette starter pack used in the trial was the One Kit, costing £19.35 per kit, including liquid and accessories. During the trial, more had to be purchased because of breakages. The original version of One Kit was no longer manufactured; instead, the newer version, One Kit 2016, was purchased at a cost of £30.54 per kit including liquid and accessories. Overall, 396 participants were provided with the old version and 42 were provided with the new version. One participant in the e-cigarette arm did not accept the pack. The total cost of the e-cigarette starter packs, therefore, was £8945.28. There were 30 participants who were also given an extra bottle (10 ml) at around 2 weeks post TQD. At a cost of £1.34 per bottle, that led to a total of £40.20. Therefore, the mean cost of e-cigarettes per participant in the e-cigarette arm was £20 (SD £4).
Selected NRT products included nicotine patch, gum, microtab, mouth strip, lozenge, nasal spray, inhalator and mouth spray. Table 37 in Appendix 1 presents the details of the products dispensed and their cost per item, extracted from the Prescription Cost Analysis – England 2016,47 plus dispensing fee per item. 48
The participants in the NRT arm were provided with a maximum of two NRT products. At TQD, 49 participants had been provided with one product, 394 participants were provided with two and four did not receive any NRT product. After the TQD, the number of participants who did not receive any product increased to 166 at 1 week post TQD, then dropped slightly to 149 at 2 weeks, remaining unchanged at 3 weeks and 4 weeks post TQD. Post TQD, the number of participants who missed the sessions entirely was 53 at 1 week, 89 at 2 weeks, 120 at 3 weeks and 91 at 4 weeks. The number of participants provided with two products reduced by two-thirds (to 121 at 1 week, 101 at 2 and 3 weeks and 117 at 4 weeks post TQD), whereas the number of participants provided with one product increased (to 107 at 1 week, then 108 at 2 weeks, 77 at 3 weeks and 90 at 4 weeks post TQD).
The 24-hour patch (21 mg) and 16-hour patch (25 mg) were the most popular products from TQD to 4 weeks post TQD, whether used alone or in combination with another product (Figures 4–8).
FIGURE 4.
Proportion of NRT dispensed on TQD in the NRT arm (n = 443).

FIGURE 5.
Proportion of NRT dispensed at 1 week post TQD in the NRT arm (n = 228).

FIGURE 6.
Proportion of NRT dispensed at 2 weeks post TQD in the NRT arm (n = 209).

FIGURE 7.
Proportion of NRT dispensed at 3 weeks post TQD in the NRT arm (n = 178).
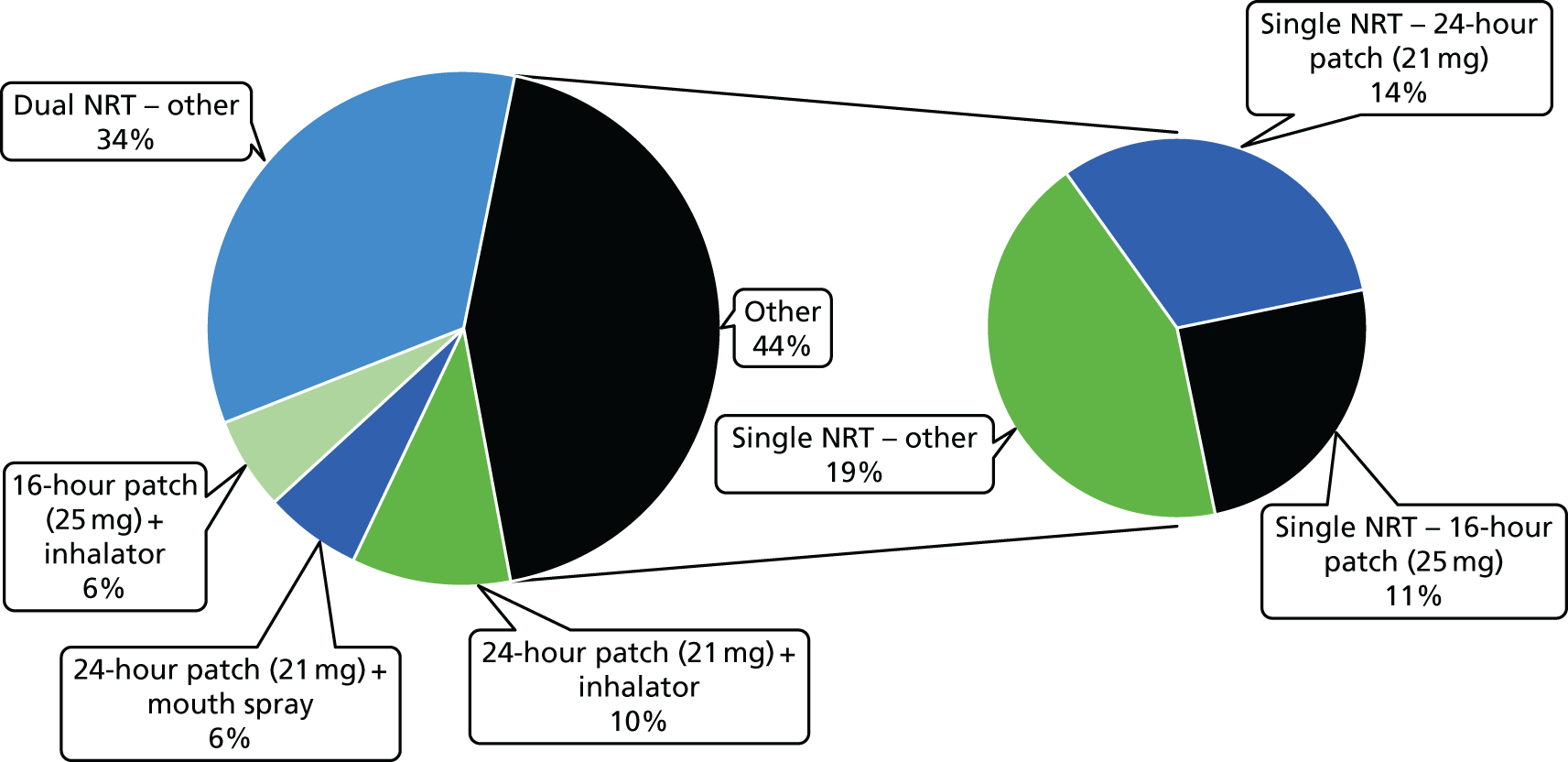
FIGURE 8.
Proportion of NRT dispensed at 4 weeks post TQD in the NRT arm (n = 207).
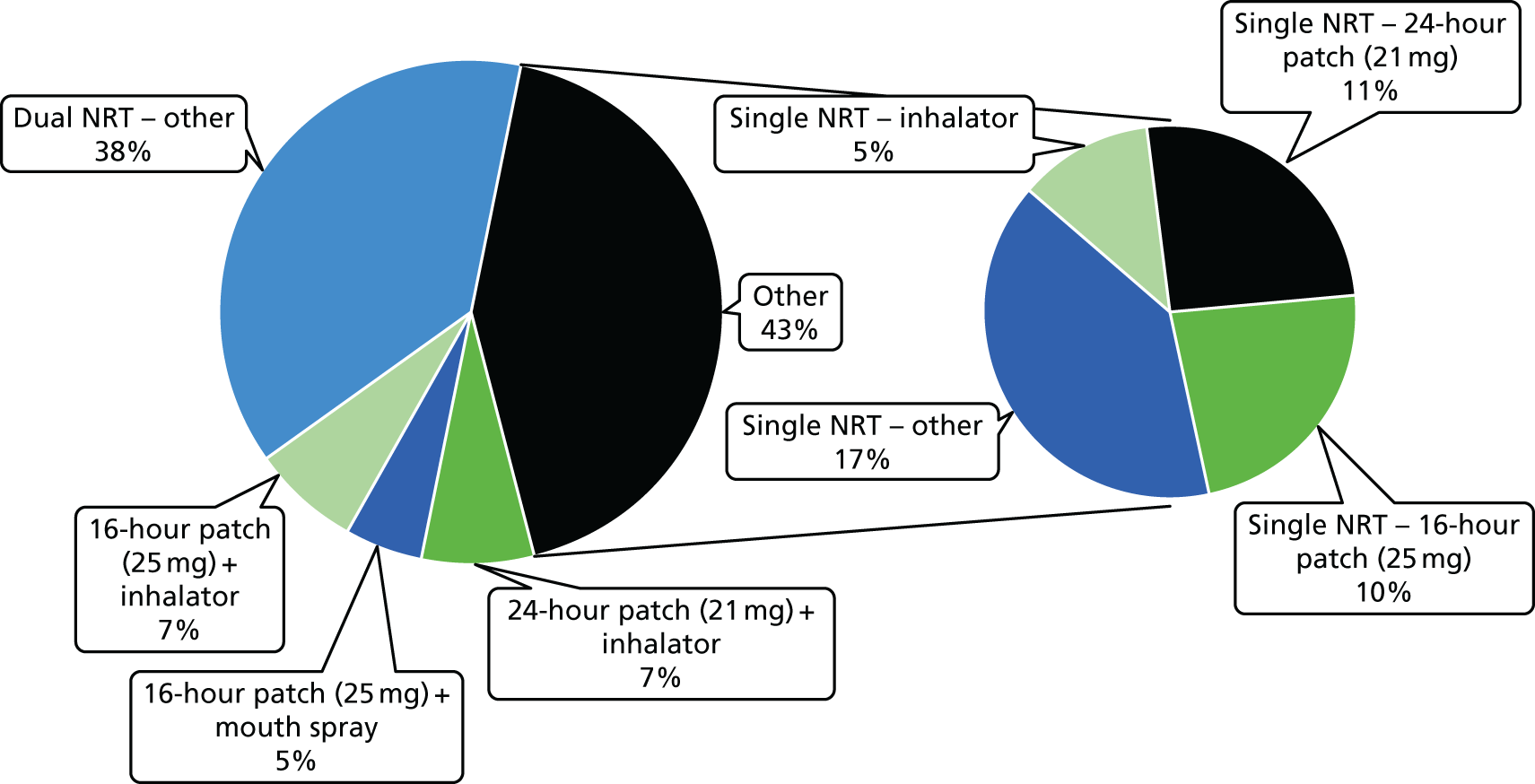
On the TQD, single use of nicotine inhalator and mouth spray was higher than use of the nicotine patch, accounting for 2% and 3% of the participants who were provided with NRT, respectively. However, from 1 week to 4 weeks post TQD the 24-hour patch (21 mg) and 16-hour patch (25 mg) became much more popular for single NRT use. For dual NRT use, the most popular combination among the participants who were provided with NRT was nicotine inhalator in combination with either 24-hour patch (21 mg) or 16-hour patch (25 mg), followed by mouth spray in combination with either patch.
Information sheets for the use of the e-cigarette costed £37.50 in total, which was £0.09 per participant. LOR was used in one of the three sites for NRT. A LOR cost £0.01 each and the total number issued was 732 in the NRT arm from TQD to 4 weeks post TQD. The printing cost of the pharmacy list for the NRT arm was £23.70, which was £0.05 per participant.
In total, the mean intervention delivery cost was £201 (SD £77) per participant in the NRT arm and £100 (£13) in the e-cigarette arm (Table 21). The mean intervention delivery cost in the e-cigarette arm had a bigger SD because of the variation of NRT provided to each participant.
| Cost item | Trial arm | |
|---|---|---|
| NRT (n = 447) | E-cigarette (n = 439) | |
| Behavioural support sessions, mean (SD) | 77 (15) | 80 (12) |
| NRT/e-cigarette supplies (including LORs), mean (SD) | 124 (67) | 20 (4) |
| Pharmacy lists/information sheets | 0.05 | 0.09 |
| Total intervention delivery cost, mean (SD) | 201 (77) | 100 (13) |
Smoking cessation services outside the intervention period
Outside the intervention period, the use of smoking cessation services was recorded at baseline and at 6 and 12 months. It consisted of smoking cessation help from general practitioners (GPs), NHS SSS and NHS Smoking Helpline service. The use of smoking cessation services was low, on average, in both arms (Table 22). In comparison with the mean values, the SDs appear to be bigger, indicating a small group of participants with high usage of services.
| Smoking cessation help | Trial arm, mean (SD) | |||||
|---|---|---|---|---|---|---|
| NRT | E-cigarette | |||||
| Baseline (n = 447) | 6 months (n = 270) | 12 months (n = 285) | Baseline (n = 439) | 6 months (n = 308) | 12 months (n = 314) | |
| GP | 0.17 (0.55) | 0.10 (0.45) | 0.18 (1.01) | 0.12 (0.47) | 0.04 (0.35) | 0.04 (0.36) |
| NHS SSS | 0.16 (0.53) | 0.16 (0.74) | 0.47 (1.99) | 0.18 (1.30) | 0.19 (1.62) | 0.26 (1.47) |
| NHS Smoking Helpline service | 0.02 (0.18) | 0.01 (0.11) | 0.00 (0.06) | 0.03 (0.16) | 0.00 (0.06) | – |
Outside the intervention period, the use of pharmacotherapies/e-cigarettes was also reported at baseline and at 6 and 12 months. All randomised participants reported these uses at baseline. Out of 447 participants in the NRT arm, 60 reported use of NRT products, 57 reported use of e-cigarettes, 19 reported use of varenicline and only one reported use of bupropion. Out of 439 participants in the e-cigarette arm, 47 participants reported use of NRT products, 61 reported use of e-cigarettes, 10 reported use of varenicline and one reported use of bupropion. At 6- and 12-month follow-ups, among the responders, the use of bupropion or varenicline was low in both arms (8/271 and 11/285, respectively, in the NRT arm, and 4/306 and 9/316, respectively, in the e-cigarette arm). The reported use of bupropion at 6 months and 12 months was very low among responders (1/271 and 2/284, respectively, in the NRT arm, none in the e-cigarette arm). The proportion of responders who reported use of e-cigarettes at 6 and 12 months’ follow-up was considerably higher in the e-cigarette arm than in the NRT arm [e-cigarette arm: 272/307 (89%) at 6 months and 232/316 (73%) at 12 months; NRT arm: 96/273 (35%) at 6 months and 98/285 (34%) 12 months]. The use of NRT products in the NRT arm was 48% (131/272) at 6 months and 21% (59/285) at 12 months, higher than in the e-cigarette arm at both follow-ups [12% (37/306) at 6 months and 9% (29/316) at 12 months].
Among those who provided information, e-liquid was purchased more frequently than e-cigarette devices during the period from baseline to 6 months and the period from 7 months to 12 months, especially in the e-cigarette arm. At baseline, although the mean number of purchases of e-cigarette devices was similar between the arms [0.14 (SD 0.51) in the NRT arm and 0.18 (SD 0.64) in the e-cigarette arm], the mean number of purchases of e-liquid was much higher in the e-cigarette arm than in the NRT arm [1.01 (SD 17.44) vs. 0.17 (SD 0.88)]. In the NRT arm, among the 272 of those who provided information at 6 months (on the period from baseline to 6 months), the mean number of purchases of e-cigarette devices was 0.53 (SD 2.01) and of e-liquid was 2.12 (SD 6.27), whereas among the 283 of those who provided information at 12 months (on the period from 7 months to 12 months) it was 0.24 (SD 0.47) for e-cigarette devices and 3.60 (SD 9.44) for e-liquid. In the e-cigarette arm among the 306 of those who provided information at 6 months (on the period from baseline to 6 months), the mean number of purchases of e-cigarette devices was 0.75 (SD 1.61) and of e-liquid was 14.85 (SD 26.68), and among the 315 of those who provided information at 12 months (on the period from 7 months to 12 months) it was 0.86 (SD 2.10) for e-cigarette devices and 14.30 (SD 24.57) for e-liquid. The SDs of the purchase of e-liquid were large, especially in the e-cigarette arm, indicating huge variation in the amount of e-liquid purchased.
Among the responders to the questions, the most-used NRT product was inhalator in both arms at baseline and 6 months (Figure 9). At 12 months, the inhalator was still the most used product in the e-cigarette arm, but use of the nicotine spray and patch outnumbered inhalator use in the NRT arm. The use of NRT products was higher in the NRT arm than in the e-cigarette arm during the 12-month period. In the NRT arm, a larger proportion of these products were purchased OTC than on prescription, whereas most of NRT products used in the e-cigarette arm were available on prescription.
FIGURE 9.
Mean reported usage of NRT products on prescription and purchased OTC among responders at each follow-up point, by arm. (a) Baseline; (b) 6 months; and (c) 12 months.


As few participants reported using varenicline or bupropion, the mean usage of the two medications for smoking cessation was close to zero in both arms. The mean number of packs of varenicline in the NRT arm was 0.12 (SD 0.64) among the 447 participants at baseline, 0.05 (SD 0.34) among the 271 responders at 6 months and 0.12 (SD 0.67) among the 285 responders at 12 months. In the e-cigarette arm, the mean number of packs of varenicline was 0.06 (SD 0.47) among the 439 participants at baseline, 0.04 (SD 0.39) among the 306 responders at 6 months and 0.08 (SD 0.52) among the 316 responders at 12 months. The highest mean number of packs of bupropion was reported among 285 responders in the NRT arm at 12 months, which was 0.01 (SD 0.13).
Combining the unit costs and the usage, the mean costs of smoking cessation help, NRT products, varenicline and bupropion were calculated. Participants’ expenses on purchasing e-cigarettes, including refills, were estimated based on self-report spend. These are presented in Table 23, by time and arm. Large SDs in comparison with the mean values indicated a skewed distribution of costs in either arm.
| Trial arm | ||||||
|---|---|---|---|---|---|---|
| NRT | E-cigarette | |||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Cost of smoking cessation help | ||||||
| Number of responders | 447 | 271 | 286 | 439 | 308 | 315 |
| Mean cost (SD) (£) | 9 (25) | 6 (22) | 14 (55) | 8 (29) | 5 (33) | 6 (28) |
| Cost of NRT products | ||||||
| Number of responders | 447 | 267 | 283 | 439 | 306 | 316 |
| On prescription, mean cost (SD) (£) | 20 (155) | 45 (188) | 17 (132) | 17 (144) | 18 (131) | 22 (185) |
| Over the counter, mean cost (SD) (£) | 22 (221) | 61 (534) | 44 (274) | 9 (88) | 7 (68) | 7 (76) |
| Expenses on e-cigarettes | ||||||
| Number of responders | 447 | 272 | 282 | 439 | 305 | 312 |
| Mean cost (SD) (£) | 3 (12) | 20 (46) | 26 (64) | 4 (27) | 77 (95) | 80 (117) |
| Cost of bupropion | ||||||
| Number of responders | 447 | 272 | 285 | 439 | 306 | 317 |
| Mean cost (SD) (£) | 0 (2) | 0 (2) | 0 (5) | 0 (2) | – | – |
| Cost of varenicline | ||||||
| Number of responders | 447 | 272 | 286 | 439 | 306 | 317 |
| Mean cost (SD) (£) | 4 (24) | 2 (12) | 4 (25) | 2 (17) | 1 (15) | 3 (19) |
Health-care services use
Similar to the use of SSSs, wider utilisation of health-care services showed a skewed distribution among the participants. At baseline, the service with the highest use was hospital outpatient attendance, with one participant in the NRT arm reporting 96 visits. At 6 months, one participant in the e-cigarette arm reported having received 90 prescriptions in the previous 6 months, while one participant in the NRT arm reported 60 prescriptions. One participant in each arm reported 50 hospital outpatient attendances in the 6 months after baseline. At 12 months, one participant in each arm reported having received 50 prescriptions from 6 to 12 months’ follow-up. One participant in the e-cigarette arm reported 49 nights’ inpatient stay, whereas the highest number of hospital inpatient nights in the NRT arm was 42. From 6 to 12 months’ follow-up, one participant in the NRT arm reported 48 home visits from a practice nurse; this service use was rarely seen otherwise.
The mean use of most of the health-care services was less than one occasion during a 6-month period, but the SDs were large (see Appendix 1, Table 38). The most used services were GP visits, followed by hospital outpatient attendance. In correspondence with the use of services, the responders at each time point reported at least two prescriptions on average within a 6-month period.
Combining the usage of the services and their unit costs, the mean health-care services costs were £612 (SD £2294) in the NRT arm at baseline (n = 447), £422 (SD £1388) at 6 months (n = 271) and £519 (SD £3036) at 12 months (n = 284). In the e-cigarette arm, the mean health-care services costs were £512 (SD £1299) at baseline (n = 439), £438 (SD £1489) at 6 months (n = 306) and £575 (SD £2288) at 12 months (n = 317). The large SDs followed the skewed pattern of usage.
Quality of life
The mean EQ-5D-5L scores recorded on the visual analogue scale (VAS) for the NRT arm were 71.1 (SD 17.4) at baseline (n = 447), 73.0 (SD 18.0) at 6 months (n = 272) and 72.1 (SD 18.2) at 12 months (n = 282). In the e-cigarette arm, the mean VAS score was 70.6 (SD 17.8) at baseline (n = 439), 72.9 (SD 17.3) at 6 months (n = 306) and 74.0 (SD 17.9) at 12 months (n = 315).
At all follow-up points, the majority of the responders reported level 1 (no problem in that dimension) across all five dimensions (Table 24). Of the five dimensions, the percentage of responders who reported no problems in mobility, self-care and usual activities remained > 80% at any time point in either arm. The best perceived dimension by the responders was self-care, followed by usual activities and mobility. The proportion of no pain or discomfort was similar in both arms: 70% in the NRT arm and 69% in the e-cigarette arm, at baseline. The proportion who did not feel anxious or depressed was on a similar level (71% in the NRT arm and 69% in the e-cigarette arm). At 6 months, 79% of the responders in both arms reported no pain or discomfort, and 75% of the responders in the NRT arm and 79% of the responders in the e-cigarette arm reported not feeling anxious or depressed. At 12 months, 80% of the responders in both arms reported no pain or discomfort, and 78% of the responders in the NRT arm and 84% of the responders in the e-cigarette arm reported not feeling anxious or depressed. The number reporting being unable to perform tasks, extreme pain/discomfort or anxiety/depression (level 5) did not exceed 10 responders at each follow-up point in either arm.
| Level | EQ-5D-5L dimension, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | ||||||
| NRT | E-cigarette | NRT | E-cigarette | NRT | E-cigarette | NRT | E-cigarette | NRT | E-cigarette | |
| Baseline (NRT arm, n = 447; e-cigarette arm, n = 439) | ||||||||||
| 1 | 389 (87) | 369 (84) | 428 (96) | 415 (95) | 388 (87) | 380 (87) | 313 (70) | 302 (69) | 316 (71) | 305 (69) |
| 2 | 23 (5) | 33 (8) | 10 (2) | 12 (3) | 30 (7) | 32 (7) | 68 (15) | 72 (16) | 74 (17) | 82 (19) |
| 3 | 20 (4) | 26 (6) | 6 (1) | 8 (2) | 21 (5) | 17 (4) | 46 (10) | 46 (10) | 44 (10) | 43 (10) |
| 4 | 15 (3) | 11 (3) | 3 (1) | 4 (1) | 8 (2) | 9 (2) | 19 (4) | 13 (3) | 11 (2) | 5 (1) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 1 (0) | 6 (1) | 2 (0) | 4 (1) |
| 6 months (NRT arm, n = 270; e-cigarette arm, n = 306) | ||||||||||
| 1 | 236 (87) | 264 (86) | 251 (93) | 286 (93) | 241 (89) | 274 (90) | 214 (79) | 241 (79) | 203 (75) | 241 (79) |
| 2 | 15 (6) | 13 (4) | 10 (4) | 6 (2) | 9 (3) | 9 (3) | 16 (6) | 22 (7) | 32 (12) | 29 (9) |
| 3 | 12 (4) | 17 (6) | 6 (2) | 10 (3) | 11 (4) | 18 (6) | 31 (11) | 29 (9) | 26 (10) | 22 (7) |
| 4 | 6 (2) | 11 (4) | 1 (0) | 3 (1) | 6 (2) | 2 (1) | 5 (2) | 12 (4) | 5 (2) | 11 (4) |
| 5 | 1 (0) | 1 (0) | 2 (1) | 1 (0) | 3 (1) | 3 (1) | 4 (1) | 2 (1) | 4 (1) | 3 (1) |
| 12 months (NRT arm, n = 281; e-cigarette arm, n = 314) | ||||||||||
| 1 | 245 (87) | 274 (87) | 267 (95) | 297 (95) | 255 (91) | 289 (92) | 226 (80) | 251 (80) | 220 (78) | 264 (84) |
| 2 | 14 (5) | 15 (5) | 5 (2) | 7 (2) | 8 (3) | 6 (2) | 22 (8) | 27 (9) | 30 (11) | 11 (4) |
| 3 | 7 (2) | 10 (3) | 4 (1) | 3 (1) | 6 (2) | 7 (2) | 21 (7) | 22 (7) | 25 (9) | 31 (10) |
| 4 | 13 (5) | 14 (4) | 3 (1) | 5 (2) | 7 (2) | 9 (3) | 8 (3) | 11 (4) | 4 (1) | 6 (2) |
| 5 | 2 (1) | 1 (0) | 1 (0) | 2 (1) | 4 (1) | 3 (1) | 3 (1) | 3 (1) | 2 (1) | 2 (1) |
Using the mapping technique recommended by NICE and replacing missing values as a result of death with zero, the mean utility scores in the NRT arm were 0.878 (SD 0.180) at baseline (n = 447), 0.881 (SD 0.217) at 6 months (n = 271) and 0.891 (SD 0.206) at 12 months (n = 281). In the e-cigarette arm, the mean utility scores were 0.868 (SD 0.190) at baseline (n = 439), 0.886 (SD 0.200) at 6 months (n = 306) and 0.897 (SD 0.208) at 12 months (n = 315).
Primary analysis
Missing data
Taking into account the costs and quality of life at each time point, and other baseline covariates for adjustment, the level of missing data for individual variables is shown in Table 25. The pattern of missing data showed that in most cases the reason for missing data was loss to follow-up. Missing data on individual sections were rare. The level of the missing data was highest (35%) at 6 months and the number of imputations was set to 35 accordingly.
| Variable | Missing values, n (%) |
|---|---|
| Age | 0 (0) |
| Sex | 0 (0) |
| Trial site | 0 (0) |
| Entitlement to free prescriptions | 0 (0) |
| Ethnicity | 0 (0) |
| Intervention cost | |
| Training | 0 (0) |
| Delivery | 0 (0) |
| Baseline FTCD | 0 (0) |
| Smoking cessation help cost | |
| Baseline | 0 (0) |
| 6 months | 307 (35) |
| 12 months | 285 (32) |
| Pharmacotherapies prescription cost | |
| Baseline | 0 (0) |
| 6 months | 313 (35) |
| 12 months | 288 (33) |
| Expenses on NRT OTC | |
| Baseline | 0 (0) |
| 6 months | 313 (35) |
| 12 months | 287 (33) |
| Health-care services cost | |
| Baseline | 0 (0) |
| 6 months | 309 (35) |
| 12 months | 285 (32) |
| Expenses on e-cigarettes | |
| Baseline | 0 (0) |
| 6 months | 309 (35) |
| 12 months | 292 (33) |
| Prescription charges on pharmacotherapies | |
| Baseline | 0 (0) |
| 6 months | 185 (21) |
| 12 months | 172 (19) |
| Utility score | |
| Baseline | 0 (0) |
| 6 months | 309 (35) |
| 12 months | 290 (33) |
| VAS | |
| Baseline | 0 (0) |
| 6 months | 308 (35) |
| 12 months | 289 (33) |
Incremental cost-effectiveness analysis
The primary analysis was conducted from the NHS and PSS perspectives. Therefore, participants’ spending on purchasing e-cigarettes and OTC NRT products and prescription charges for redeeming the pharmacotherapies were not included in the following costs.
Smoking cessation help costs in the 6 months before baseline were £9 [standard error (SE) £1] per participant in the NRT arm and £8 (SE £1) per participant in the e-cigarette arm. Pharmacotherapy costs (including NRT on prescription, varenicline and bupropion) in the same period were £24 (SE £7) per participant in the NRT arm and £20 (SE £7) per participant in the e-cigarette arm. The mean cost of health-care service use was £612 (SE £108) in the NRT arm and £512 (SE £62) in the e-cigarette arm. In total, the mean cost to the NHS in the 6 months before baseline was £645 (SE £109) in the NRT arm and £539 (SE £62) in the e-cigarette arm.
The mean cost of smoking cessation help was generally low in both arms, in comparison with other costs (Table 26). In the 6 months post TQD, the mean pharmacotherapy cost in the NRT arm was twice that in the e-cigarette arm (£38 vs. £16), whereas the mean cost of health-care service use in the e-cigarette arm was higher than in the NRT arm (£475 vs. £382). From 6 months to 12 months post TQD, the mean cost of pharmacotherapies became similar in both arms (£19 in the NRT arm and £21 in the e-cigarette arm). The mean cost of health-care service use in both arms was higher than in the previous 6-month period, with the e-cigarette arm costs remaining higher than the NRT arm costs. The mean total cost to the NHS increased slightly in both arms from 6 months post TQD to 6–12 months post TQD. In the NRT arm, it increased from £426 (SE £65) per participant in the 6 months post TQD to £490 (SE £124) per participant in the 6 months before the 12-month follow-up. In the e-cigarette arm, costs increased from £496 (SE £100) per participant in the 6 months post TQD to £573 (SE £109) per participant in the 6 months before the 12-month follow-up.
| Resource | Trial arm, mean (SE) (£) | |
|---|---|---|
| NRT (n = 447) | E-cigarette (n = 439) | |
| Mean cost in the 6 months before baseline | ||
| Smoking cessation help cost | 9 (1) | 8 (1) |
| Pharmacotherapy cost | 24 (7) | 20 (7) |
| Health-care service use cost | 612 (108) | 512 (62) |
| Cost to the NHS | 645 (109) | 539 (62) |
| Mean cost in the 6 months post TQD | ||
| Smoking cessation help cost | 6 (1) | 4 (2) |
| Pharmacotherapy cost | 38 (8) | 16 (6) |
| Health-care service use cost | 382 (63) | 475 (100) |
| Cost to the NHS | 426 (65) | 496 (100) |
| Mean cost from 6 months to 12 months post TQD | ||
| Smoking cessation help cost | 14 (3) | 6 (2) |
| Pharmacotherapy cost | 19 (5) | 21 (8) |
| Health-care service use cost | 457 (124) | 546 (109) |
| Cost to the NHS | 490 (124) | 573 (109) |
In the NRT arm, the mean EQ VAS score increased from 71.1 (SE 0.8) at baseline to 73.7 (SE 1.0) at 6 months, then dropped to 72.6 (SE 1.0) at 12 months. In the e-cigarette arm, the mean EQ VAS increased from 70.6 (SE 0.9) at baseline to 73.2 (SE 0.9) at 6 months, then to 74.6 (SE 0.9) at 12 months. The mean EQ-5D-5L utility score in both arms presented an upwards trend. In the NRT arm, the mean utility score was 0.878 (SE 0.008) at baseline, 0.882 (SE 0.011) at 6 months and 0.887 (SE 0.011) at 12 months. In the e-cigarette arm, the mean utility score was 0.868 (SE 0.009) at baseline, 0.888 (SE 0.010) at 6 months and 0.898 (SE 0.011) at 12 months.
The mean EQ VAS score in the e-cigarette arm was lower than that in the NRT arm at baseline and 6 months but became higher at 12 months (Figure 10). Although the increase was maintained in the e-cigarette arm, the mean EQ VAS score in the NRT arm peaked at 6 months and dropped slightly at 12 months. The mean EQ-5D-5L utility score in the e-cigarette arm started lower than that in the NRT arm but became higher from 6 months onwards. The increase in the e-cigarette arm was slightly more drastic than in the NRT arm, especially from baseline to 6 months.
FIGURE 10.
Mean EQ VAS score and EQ-5D-5L utility score at baseline, 6 months and 12 months, by arm.

The mean intervention cost per participant was £201 (SE £4) in the NRT arm and £105 (SE £1) in the e-cigarette arm. The difference was mainly as a result of the provision of NRT products in the NRT arm. Smoking cessation costs outside the intervention were £77 (SE £13) per participant in the NRT arm and £48 (SE £11) in the e-cigarette arm, during the 12 months post TQD. The mean cost of health-care service use was higher in the e-cigarette arm [£1022 (SE £147)] than in the NRT arm [£839 (SE £162)]. In total, the mean cost to the NHS was similar between arms: £1116 (SE £163) in the NRT arm and £1174 (SE £147) in the e-cigarette arm. The unadjusted difference in costs was £58 (95% CI –£339 to £410) per participant, with the e-cigarette arm having higher costs. After adjusting for age, sex, FTCD score at baseline, trial centre, entitlement to free prescription and the costs to the NHS at baseline, the mean cost per participant in the e-cigarette arm was £11 (95% CI –£104 to £147) higher than in the NRT arm (Table 27).
| Costs, QALYs and ICER | Trial arm | |
|---|---|---|
| NRT (n = 447) | E-cigarette (n = 439) | |
| Costs during the trial period (£) | ||
| Intervention cost (SE) | 201 (4) | 105 (1) |
| Smoking cessation costs (SE) | 77 (13) | 48 (11) |
| Health-care costs (SE) | 839 (162) | 1022 (147) |
| Total costs during the trial period (SE) | 1116 (163) | 1174 (147) |
| Difference in total costs during the trial period (95% CI) | ||
| Unadjusted | 58 (–339 to 410) | |
| Adjusted | 11 (–104 to 147) | |
| Quality of life during the trial period | ||
| QALYs (SE) | 0.882 (0.009) | 0.886 (0.008) |
| Difference in QALYs (95% CI) | ||
| Unadjusted | 0.003 (–0.017 to 0.025) | |
| Adjusted | 0.010 (–0.003 to 0.023) | |
| ICER (£) | ||
| ICER at 12 months post TQD (95% CI) | 1100 per QALY gained (–36,947 to 47,414) | |
The mean QALY gain was 0.882 (SE 0.009) per participant in the NRT arm and 0.886 (SE 0.008) per participant in the e-cigarette arm. The unadjusted difference in mean QALYs was 0.003 (95% CI –0.017 to 0.025), with the e-cigarette arm having a greater QALY gain. After adjusting for age, sex, FTCD score at baseline, trial centre, entitlement to free prescription and utility score at baseline, the mean QALY gain per participant was 0.010 (95% CI –0.003 to 0.023) higher in the e-cigarette arm than in the NRT arm.
Dividing the adjusted difference in mean total costs by the adjusted difference in mean QALYs gained, the ICER was, therefore, £1100 per QALY gained, comparing the e-cigarette arm with the NRT arm. This was considered cost-effective, comparing it against the WTP threshold of £20,000 per QALY.
The bootstrapped 95% CI for the ICER was wide, ranging from –£36,947 to £47,414 per QALY gained. The CEP gave a clearer illustration of the uncertainty surrounding the £1100 point estimate (see Figure 11a). It shows that the majority of the bootstrapped ICERs fall under the line marking the £20,000 per QALY threshold indicating that an intervention is likely to be cost-effective. The CEAC presents the probability of e-cigarettes being cost-effective, in comparison with NRT, at different WTP levels (see Figure 11b). The probability reaches 50% at a very low WTP level. By £20,000 per QALY, the probability of an e-cigarette intervention being cost-effective was ≈87%; at £30,000 per QALY, it was 90%. This indicates an intervention that is highly likely to be cost-effective from the NHS and PSS perspectives.
FIGURE 11.
(a) Cost-effectiveness plane and (b) CEAC, comparing the e-cigarette arm with the NRT arm.


Complete-case analysis
Taking into account of all the variables required, the CCA was conducted based on 458 participants (254 in the e-cigarette arm and 204 in the NRT arm). Among complete cases, the mean cost of smoking cessation help in the 6 months before baseline, in the 6 months post TQD and from 6 to 12 months post TQD was relatively low in both arms: £7 (SD £17), £7 (SD £24) and £14 (SD £45), respectively, in the NRT arm and £8 (SD £24), £4 (£29) and £6 (SD £29), respectively, in the e-cigarette arm. The mean pharmacotherapy cost among complete cases in the NRT arm was £15 (SD £67) in the 6 months before baseline; it peaked at £37 (SD £129) in the 6 months post TQD and dropped back to the baseline level from 6 to 12 months post TQD [£14 (SD £54)]. In the e-cigarette arm, the mean pharmacotherapy cost among complete cases reduced from £24 (SD £179) in the 6 months before baseline to £17 (SD £119) in the 6 months post TQD, and remained on the same level from 6 to 12 months post TQD [£19 (SD £125)]. In the NRT arm, the mean health-care service use cost was highest in the 6 months before baseline [£666 (SD £2810)]. It decreased to £462 (SD £1552) in the 6 months post TQD and increased again to £590 (SD £3561) from 6 to 12 months post TQD. In the e-cigarette arm, the mean health-care service use cost went in the opposite direction. It started from £561 (SD £1478) in the 6 months before baseline, reduced to £457 (SD £1565) in the 6 months post TQD and reached the highest value, £653 (SD £2540), from 6 to 12 months post TQD.
In total, the mean costs to the NHS were £688 (SD £2811), £506 (SD £1578) and £617 (SD £3560) in the NRT arm, in the three 6-month periods. In the e-cigarette arm, they were £593 (SD £1490), £478 (£1574) and £678 (£2548), for the same periods. Compared with the primary analysis, except for the e-cigarette arm in the 6 months post TQD, the mean costs to the NHS were all higher among complete cases than in the imputed data set (Figure 12). The biggest differences were in the mean costs from 6 to 12 months post TQD in both arms [£496 (primary analysis) vs. £617 (CCA) in the NRT arm and £573 (primary analysis) vs. £678 (CCA) in the e-cigarette arm]. In both arms, the relative position of the mean costs in the first and last 6-month periods remained the same as in the primary analysis (i.e. the last 6-month period was more costly than the first 6-month period). However, the relative position between arms was reversed in the first 6-month period, with costs higher in the NRT arm than in the e-cigarette arm among complete cases (in contrast to the primary analysis, in which costs in the same period were higher in the e-cigarette arm).
FIGURE 12.
Comparison of mean costs to the NHS between the primary analysis and CCA.
The mean VAS score among the complete cases in the NRT arm (n = 204) was 70.9 (SD 16.6) at baseline, 73.2 (SD 17.0) at 6 months and 72.2 (SD 18.0) at 12 months. In the e-cigarette arm (n = 254), it was 69.9 (SD 17.7) at baseline, 72.7 (SD 17.2) at 6 months and 74.3 (SD 16.9) at 12 months among the complete cases. Compared with the primary analysis, the mean VAS score was lower in the complete cases at all time points and in both arms (Figure 13). The difference in the NRT arm was less pronounced than that in the e-cigarette arm. However, the general pattern of change remained the same: in the NRT arm VAS score rose from baseline to 6 months and dropped slightly at 12 months, whereas in the e-cigarette arm it increased from baseline to 6 months and from 6 to 12 months.
FIGURE 13.
Comparison of mean VAS score between the primary analysis and CCA.

The proportion of the participants in the NRT arm selecting each level of the five dimensions of the EQ-5D-5L was shown in Figure 14. Overall, most participants (> 80%) among the complete cases had no problems in the areas of mobility, self-care and usual activities, with the best scores being in self-care, at any time point (Figures 14 and 15). The proportion of participants reporting no pain/discomfort and not anxious/depressed was relatively lower but increased from ≈70% at baseline to ≈80% at 12 months in both arms. Few participants felt that they were unable to perform usual tasks, felt extreme pain/discomfort or felt extremely anxious/depressed.
FIGURE 14.
Proportion of EQ-5D-5L dimensions among complete cases in the NRT arm.
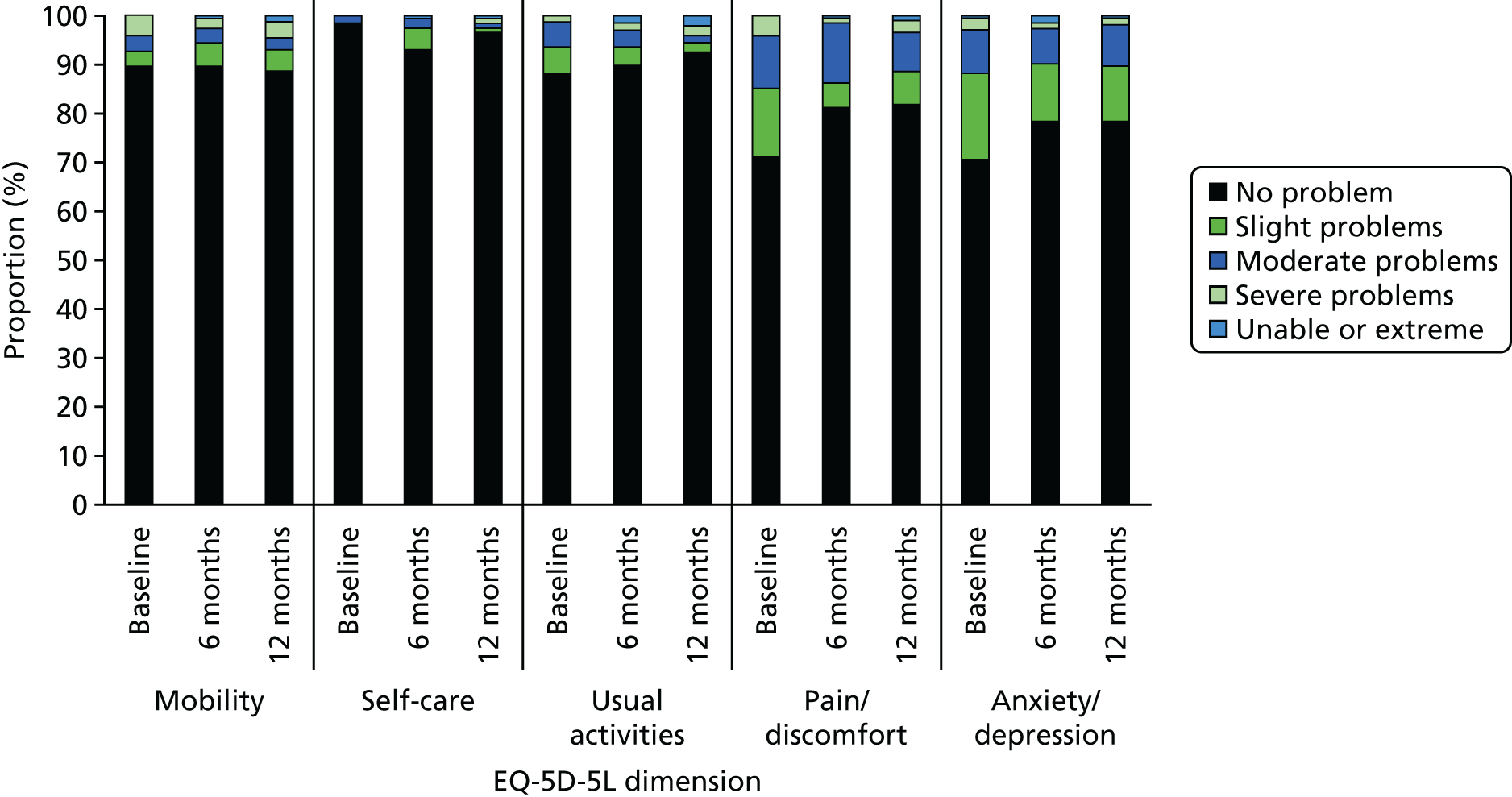
FIGURE 15.
Proportion of EQ-5D-5L dimensions among complete cases in the e-cigarette arm.

Among the complete cases in the NRT arm, the mean utility score was 0.885 (SD 0.162) at baseline, 0.897 (SD 0.198) at 6 months and 0.893 (SD 0.205) at 12 months. In the e-cigarette arm, the mean utility score was 0.868 (SD 0.193) at baseline, 0.882 (SD 0.199) at 6 months and 0.900 (SD 0.202) at 12 months.
In comparison with the primary analysis, the mean utility score among complete cases was higher in the NRT arm at all three time points (Figure 16). Instead of a gradual upwards trend in the primary analysis, the CCA showed a sharp increase from baseline to 6 months and then dropped slightly at 12 months.
FIGURE 16.
Comparison of mean utility scores between the primary analysis and CCA.

In the e-cigarette arm, the mean utility score was the same as in the primary analysis at baseline, became lower at 6 months, and reached a slightly higher level at 12 months. The upwards trend remained but became gentler from baseline to 6 months.
These shifts resulted in a change in relative position at 6 months. Although the mean utility score was still higher in the NRT arm than in the e-cigarette arm at baseline and lower in the NRT arm than in the e-cigarette arm at 12 months, it was much higher in the NRT arm at 6 months than in the e-cigarette arm. This led to an increase in the area under the curve of three data points in the NRT arm and a reduction in the area under the curve in the e-cigarette arm. Therefore, the mean QALY gain was higher than in the primary analysis in the NRT arm (0.893 vs. 0.882) and lower than in the primary analysis in the e-cigarette arm (0.883 vs. 0.886).
The total cost to the NHS during the trial period was £1339 (SD £4616) per participant in the NRT arm, which was higher than that in the e-cigarette arm [£1264 (SD £3031)] (Table 28). After adjusting for age, sex, FTCD score at baseline, trial centre, entitlement to free prescriptions and the costs to the NHS at baseline, the adjusted difference in total costs between arms during the trial period was –£96 (95% CI –£304 to £81) in complete cases, with the e-cigarette arm having lower costs than the NRT arm. The negative cost difference indicated a less costly intervention, compared with the control, at a point estimation.
| Costs, QALYs and ICER | Trial arm | |
|---|---|---|
| NRT (n = 204) | E-cigarette (n = 254) | |
| Costs during the trial period (£) | ||
| Intervention cost (SD) | 216 (73) | 108 (10) |
| Smoking cessation costs (SD) | 71 (165) | 46 (190) |
| Health-care costs (SD) | 1051 (4611) | 1110 (3018) |
| Total costs during the trial period (SD) | 1339 (4616) | 1264 (3031) |
| Difference in total costs during the trial period (95% CI) | ||
| Unadjusted | –75 (–868 to 587) | |
| Adjusted | –96 (–304 to 81) | |
| Quality of life during the trial period | ||
| QALYs (SD) | 0.893 (0.162) | 0.883 (0.170) |
| Difference in QALYs (95% CI) | ||
| Unadjusted | –0.010 (–0.041 to 0.021) | |
| Adjusted | 0.003 (–0.018 to 0.023) | |
| ICER (£) | ||
| ICER at 12 months post TQD (95% CI) | E-cigarette dominant (less costly, more effective) (–194,201 to 171,348) | |
After adjusting for age, sex, FTCD score at baseline, trial centre, entitlement to free prescriptions and utility score at baseline, the adjusted difference in QALYs was 0.003 (95% CI –0.018 to 0.023) in complete cases, which was smaller than in the primary analysis. Combining the difference in costs and in QALY (i.e. looking at the difference in costs and in QALY together), the e-cigarette arm was less costly but more effective than the NRT arm, among complete cases, which made the e-cigarette arm dominant and cost-effective. The bootstrapped replicates produced a wide 95% CI for ICER with the upper bound still lower than £20,000.
The CEP illustrated the 5000 bootstrapped replicates of matching adjusted difference in costs and in QALYs (Figure 17a). The majority of the dots fell under the zero cost difference line, supporting the point estimation of the e-cigarette arm being less costly. The distribution of QALY difference was more balanced between the two sides of the zero QALY difference line, concentrating slightly more on the positive side. The majority of the replicated ICERs fell under the £20,000 per QALY line, indicating a high probability that e-cigarettes are cost-effective. Converting that onto a CEAC, e-cigarettes showed a 75% probability of being cost-effective compared with NRT (Figure 17b). The curve started from close to 90% at the null WTP threshold, and declined gradually to 70% at the £30,000 WTP thresholds. This was because the more monetary value being put on 1 QALY, the less likely an intervention resulting in a negative QALY gain would be considered cost-effective, even if it is less costly.
FIGURE 17.
(a) Cost-effectiveness plane and (b) CEAC comparing the e-cigarette arm with the NRT arm, in the CCA.
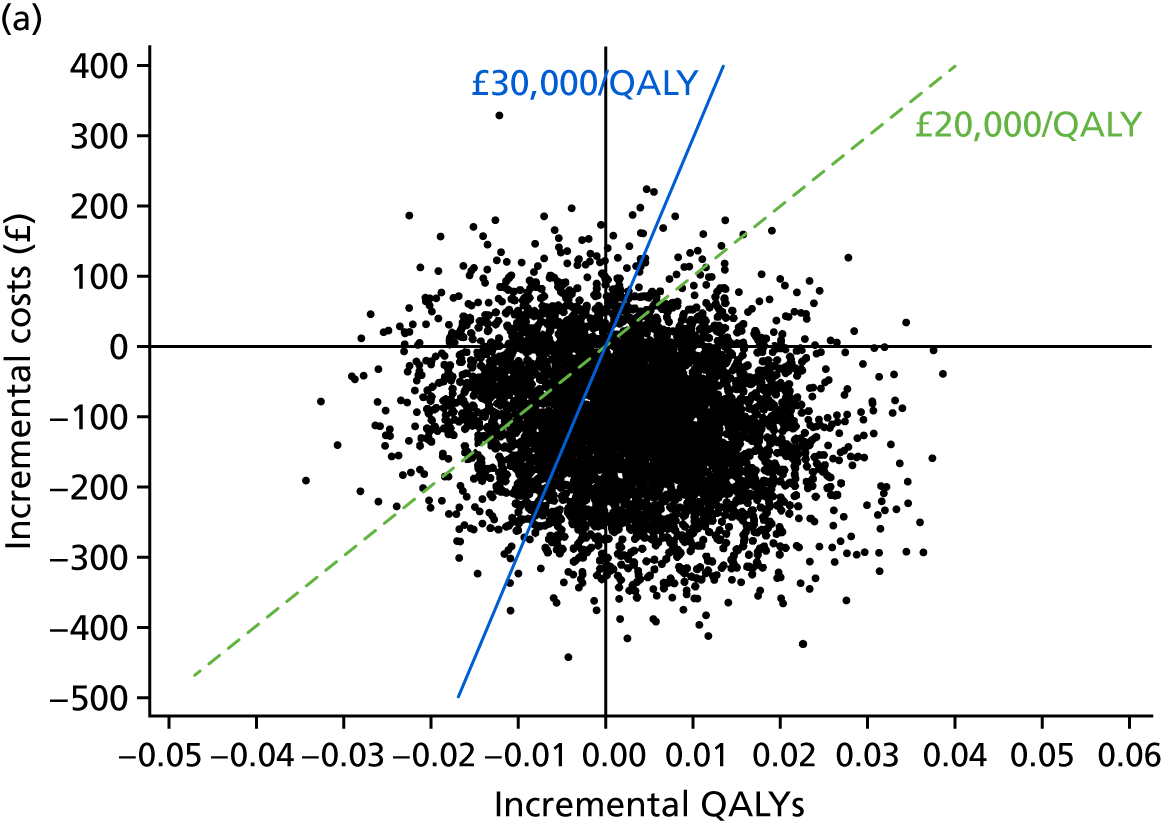
Although the CCA produced a negative point estimate of cost difference, it also gave a less significant improvement in QALYs than did the primary analysis. This suggests that the multiple imputation had the effect of reducing the difference in costs but enlarging the difference in QALYs, though not by much. However, the conclusion did not change.
Secondary analysis
Comparison of weekly nicotine replacement therapy dispensed and nicotine replacement therapy used
Information on NRT use in the first week, both self-reported and from dispensing records, was available for 385 of the 447 participants in the NRT arm. The corresponding numbers at later time points were 346 at 2 weeks post TQD, 321 at 3 weeks post TQD and 335 at 4 weeks post TQD. Figure 18 presents the numbers of each NRT item on record during the same weeks. The numbers do not sum to the number of participants because of the dual use of NRT products.
FIGURE 18.
Comparison of recorded dispense of NRT and self-reported use of NRT in the same week from 1 week to 4 weeks post TQD in the NRT arm. (a) One week post TQD (n = 385); (b) 2 weeks post TQD (n = 346); (c) 3 weeks post TQD (n = 321); and (d) 4 weeks post TQD (n = 335).

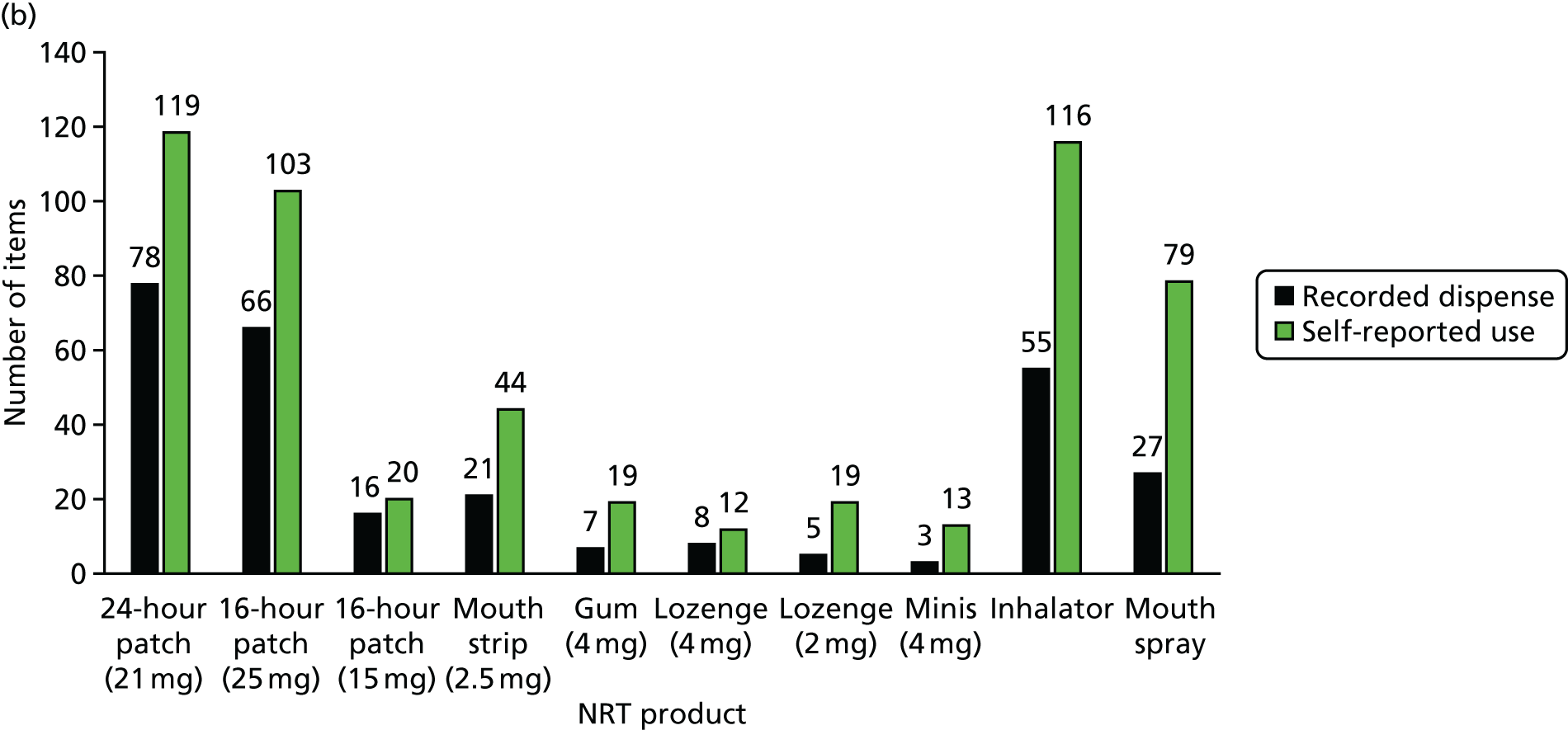

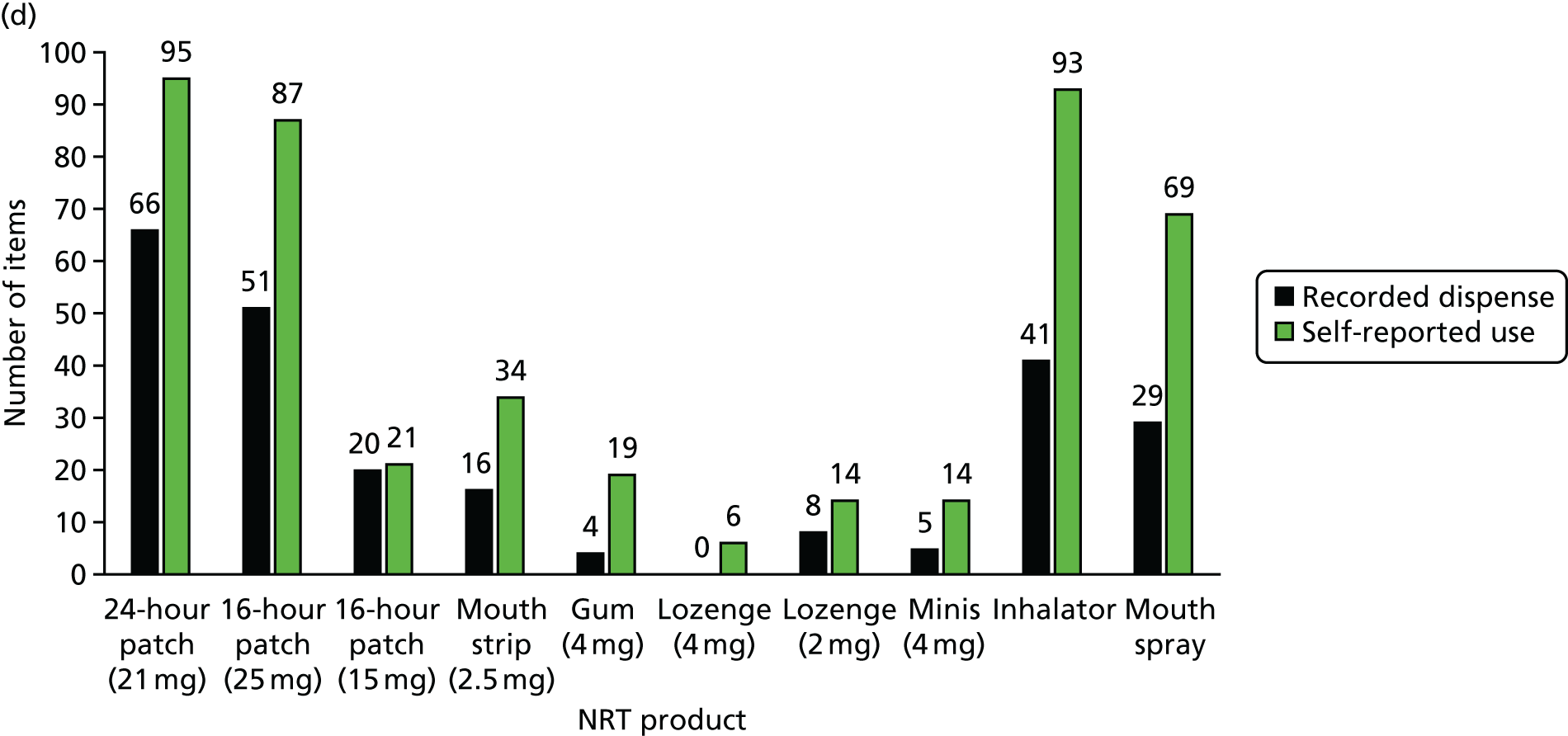
At 1 week post TQD, the self-reported numbers of NRT products were not much different from the numbers recorded as dispensed. The most popular products in both cases were 24-hour patch (21 mg), followed by inhalator, 16-hour patch (25 mg) and mouth spray. At 2 weeks post TQD, the most popular products were still the four products mentioned above, but the self-reported numbers of products used exceeded the numbers recorded as dispensed. Self-reported use of inhalator was similar to self-reported use of 24-hour patch (21 mg) but was twice the number of recorded dispenses of inhalator. The self-reported use of mouth spray was almost three times the number on recorded dispense. These patterns continued, to slightly different degrees, in the third and fourth weeks post TQD.
The mean NRT cost according to the dispensing records on TQD was £50 (SD £12), which is slightly higher than the mean NRT cost according to self-report at 1 week post TQD [£45 (SD £16); n = 385]. The mean NRT cost according to the dispensing record at 1 week post TQD was £23 (SD £23), which is lower than the mean NRT cost according to self-report at 2 weeks post TQD [£43 (SD £18); n = 346]. Similarly, the mean NRT cost according to the dispensing record at 2 weeks post TQD was £23 (SD £22), which is lower than the mean NRT cost according to self-report at 3 weeks post TQD [£42 (SD £19); n = 321]. The mean NRT cost according to the dispensing record at 3 weeks post TQD was £21 (SD £23), which is lower than the mean NRT cost according to self-report at 4 weeks post TQD [£38 (SD £20]); n = 335]. Except for the first week post TQD, the mean self-reported NRT cost was generally higher than the cost of recorded dispense.
A complete record of NRT use, from dispensing on TQD to self-report at 4 weeks post TQD, was available for a total of 266 participants in the NRT arm. The mean cost of NRT according to dispense data was £124 (SD £53), whereas the mean cost of NRT according to self-reported use was £175 (SD £54).
Further exploring the weekly NRT dispense and use, 248 participants were provided with two products in the first week post TQD and used both, and 31 were provided with one product and used it. A total of 82 participants used either one of the two provided products or another product in addition to the one provided product. A total of 10 participants used only products not provided whether or not they already received products provided as part of the trial. A further 14 participants used no NRT products, provided or not (Figure 19).
FIGURE 19.
Participants’ use of dispensed NRT products in the NRT arm, from 1 to 4 weeks post TQD.
In the following weeks, more participants reported using NRT products that were different from their provided products and fewer participants reported using two provided products. These were the causes of discrepancy between two estimated mean NRT costs. Assuming that all products were prescribed or provided by NHS SSSs, the mean intervention cost in the NRT arm would increase by ≈£50 per participant.
Costs of smoking cessation aids to the NHS and participants’ expenses on smoking cessation
The cost to the NHS of pharmacotherapies has been reported in the primary analysis. The following analyses were performed on the same data set as in the primary analysis, that is on the data set comprising 447 participants in the NRT arm and 439 participants in the e-cigarette arm, and missing data were imputed.
Figure 20 showed a comparison of participants’ expenses on smoking cessation aids (pharmacotherapies and e-cigarettes). Pharmacotherapies include various NRT products, bupropion and varenicline. The last two are available only on prescription. Taking into account those who were exempted from prescription charges, the mean cost of prescription products was low in both arms. In the NRT arm, it was £2 (SE £1) in the 6 months before baseline, £18 (SE £2) in the 6 months post TQD and £2 (SE £1) from 6 to 12 months post TQD. In the e-cigarette arm, it was £1 (SE £0) in the 6 months before baseline, £0 (SE £0) in the 6 months post TQD and £3 (SE £2) from 6 to 12 months post TQD.
FIGURE 20.
Participants’ expenses on smoking cessation aids in the 6 months before baseline, after TQD and before the 12-month follow-up, by arm.

The amount spent on OTC NRT products was much higher in the NRT arm than in the e-cigarette arm in all three 6-month periods: £22 (SE £10) versus £9 (SE £4) in the 6 months before baseline, £47 (SE £21) versus £6 (SE £3) in the 6 months post TQD and £42 (SE £15) versus £6 (SE £3) from 6 to 12 months post TQD (see Figure 20).
In contrast, the purchase of e-cigarettes was considerably more common in the e-cigarette arm than in the NRT arm. The mean amount spent on e-cigarettes was similar in both arms in the 6 months before baseline [NRT arm, £3 (SE £1); e-cigarette arm, £4 (SE £1)]. However, the mean amount spent on e-cigarettes in thee-cigarette arm was £73 (SE £5) in the 6 months post TQD and £80 (SE £6) from 6 to 12 months post TQD, whereas participants in the NRT arm spent, on average, £21 (SE £3) on e-cigarettes in the 6 months post TQD and £28 (SE £4) from 6 to 12 months post TQD.
As defined in the primary analysis, SSS costs to the NHS covered smoking cessation help (advice from a GP, Specialist Smoking Cessation Service and Smoking Cessation Helpline) and pharmacotherapies (NRT, bupropion and varenicline) but excluded intervention costs. Participants’ expenses on smoking cessation comprised prescription charges, when applicable, NRT purchased OTC and purchase of e-cigarettes.
In the 6 months before baseline, SSS costs to the NHS were £33 (SE £8) per participant in the NRT arm and £27 (SE £7) per participant in the e-cigarette arm. Participants’ expenses on smoking cessation amounted to £27 (SE £11) per participant in the NRT arm and £14 (SE £4) per participant in the e-cigarette arm (Table 29).
| Time point and costs | Trial arm, mean (SE) (£) | |
|---|---|---|
| NRT (n = 447) | E-cigarette (n = 439) | |
| 6 months before baseline | ||
| SSS costs to the NHS | 33 (8) | 27 (7) |
| Participants’ expenses on smoking cessation | 27 (11) | 14 (4) |
| 6 months post TQD | ||
| SSS costs to the NHS | 44 (8) | 20 (6) |
| Participants’ expenses on smoking cessation | 86 (21) | 80 (6) |
| 6 to 12 months post TQD | ||
| SSS costs to the NHS | 33 (8) | 27 (9) |
| Participants’ expenses on smoking cessation | 72 (15) | 89 (7) |
In the 6 months post TQD, the cost to the NHS of SSSs was £44 (SE £8) per participant in the NRT arm, higher than in the e-cigarette arm [£20 (SE £6)]. Participants’ expenses on smoking cessation averaged £86 (SE £21) per participant in the NRT arm and £80 (SE £6) per participant in the e-cigarette arm.
From 6 to 12 months post TQD, the cost to the NHS of SSSs was £33 (SE £8) per participant in the NRT arm and £27 (SE £9) per participant in the e-cigarette arm. Participants’ expenses on smoking cessation averaged £72 (SE £15) per participant in the NRT arm and £89 (SE £7) per participant in the e-cigarette arm.
In the 12 months post TQD, excluding intervention costs, the mean cost to the NHS of SSSs was £77 (SE £13) per participant in the NRT arm and £48 (SE £11) per participant in the e-cigarette arm. The mean cost of smoking cessation to participants was £158 (SE £27) per participant in the NRT arm and £169 (SE £11) per participant in the e-cigarette arm.
Although participants’ expenses did not appear to be different between arms, the mean cost to the NHS of SSSs was slightly lower in e-cigarette arm than in the NRT arm. Taking into account the intervention costs, the mean smoking cessation cost to the NHS was £278 (SE £14) per participant in the NRT arm and £152 (SE £11) per participant in the e-cigarette arm.
Long-term cost and outcome projection
Parameters estimated from the trial
The abstinence rate at 12 months was 9.9% in the NRT arm and 18.0% in the e-cigarette arm. The intervention costs in both groups are reported in the preceding section. The wider health-care costs and the QALYs gained over the first 12 months were recalculated from the trial results according to a participant’s abstinence status at 12 months. Table 30 presents the model parameters estimated from the trial data. Combining Appendix 1, Table 36, and Table 30, all the model parameters are summarised.
| Parameter | Value (SE) | Distribution |
|---|---|---|
| Probability of quit at 12 months (%) | ||
| NRT arm | 9.9 (1.4) | Beta |
| E-cigarette arm | 18.0 (1.7) | Beta |
| Intervention cost (£) | ||
| NRT arm | 201 (4) | Gamma |
| E-cigarette arm | 105 (1) | Gamma |
| 12-month health-care costs (£) | ||
| NRT arm | ||
| Those who quit | 603 (211) | Gamma |
| Continuing smokers | 950 (180) | Gamma |
| E-cigarette arm | ||
| Those who quit | 1164 (329) | Gamma |
| Continuing smokers | 1049 (165) | Gamma |
| QALY gain at 12 months | ||
| NRT arm | ||
| Those who quit | 0.888 (0.028) | Gamma |
| Continuing smokers | 0.882 (0.009) | Gamma |
| E-cigarette arm | ||
| Those who quit | 0.887 (0.019) | Gamma |
| Continuing smokers | 0.885 (0.009) | Gamma |
Cost-effectiveness analysis
The mean lifetime smoking-related health-care costs (including costs occurred during trial period) were estimated at £3175 (SE £161) per participant who used NRT and £3184 (SE £169) per participant who received e-cigarette. The average QALY gain was estimated at 24.14 (SE 0.31) per person for those in the NRT arm and 24.28 (SE 0.31) per person for those in the e-cigarette arm. The ICER for the base-case cost-effectiveness analysis was £65 (95% CI –£12,537 to £13,423) per QALY gained, with the use of e-cigarette more costly and more effective (see Table 30). This was much lower than the £20,000 per QALY WTP threshold.
| Intervention | Lifetime smoking-related health-care costs, mean (SE) (£) | QALY gain, mean (SE) |
|---|---|---|
| NRT | 3175 (161) | 24.14 (0.31) |
| E-cigarette | 3184 (169) | 24.28 (0.31) |
| ICER (incremental cost per additional QALY), mean (95% CI) (£) | 65 (–12,537 to 13,423) | |
Probabilistic sensitivity analyses
The probabilistic sensitivity analysis incorporated the uncertainty in the parameter estimates to provide estimates of the probability that each intervention would be cost-effective at different WTP thresholds for QALY gains.
Figure 21 illustrates the uncertainty surrounding the ICER estimate at £65 per QALY. Only a small proportion of the dots fell on the left side of the y-axis, indicating that e-cigarettes are highly likely to be more effective, in terms of QALYs gained, than NRT. As for the costs, the dots are scattered almost equally on either side of the x-axis, indicating a similar level of lifetime smoking-related costs between the two arms. The majority of the dots fell under the £20,000 per QALY line, suggesting a robust conclusion.
FIGURE 21.
Lifetime CEP.
The CEAC in Figure 22 shows that e-cigarettes have a higher probability of being cost-effective than NRT at a very low WTP threshold (£65 per QALY). Beyond that, the probability of e-cigarettes being more cost-effective rises sharply, reaching 80% at a WTP threshold of £3000 per QALY. Using the £20,000 to £30,000 per QALY gained WTP thresholds, the CEAC indicates that the probability of e-cigarette being more cost-effective is between 84.6% and 84.8%, in comparison with NRT.
FIGURE 22.
Lifetime CEAC.
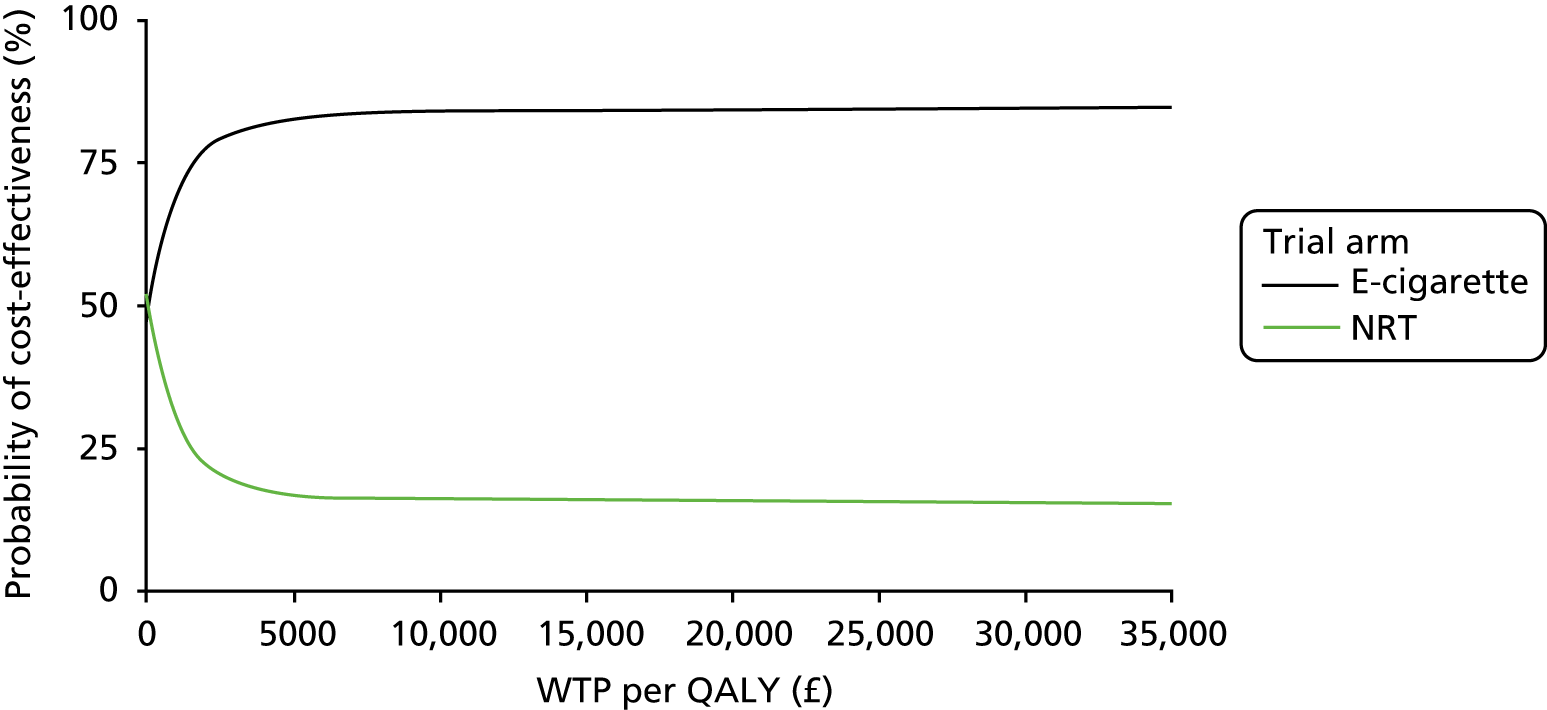
Conclusion
The intervention costs were £201 (SE £4) per participant in the NRT arm and £105 (SE £1) per participant in the e-cigarette arm. In the primary analysis, the total costs to the NHS during the 12-month period were £1116 (SE £163) per participant in the NRT arm and £1174 (SE £146) per participant in the e-cigarette arm. The adjusted difference in mean total costs was £11 (95% CI –£104 to £147), with the e-cigarette arm costs being higher. The mean QALY gain per participant was 0.882 (SE 0.009) in the NRT arm and 0.886 (SE 0.008) in the e-cigarette arm. The adjusted difference in mean QALYs was 0.010 (95% CI –0.003 to 0.023). The ICER was estimated at £1100 per QALY gained (95% CI –£36,947 to £47,414). The CEP and the CEAC showed an 87% probability of cost-effectiveness at a WTP threshold of £20,000 and a 90% probability of cost-effectiveness at a WTP threshold of £30,000, comparing e-cigarettes with NRT. These suggest that e-cigarettes are a cost-effective intervention with relatively low uncertainty.
The CCA reported a higher level of uncertainty surrounding the differences in mean total costs and mean QALYs. It indicated a possible cost-saving intervention with no difference in effectiveness. The conclusion of e-cigarettes being more cost-effective than NRT was therefore still upheld.
Further exploration of participants’ expenditure on smoking cessation outside the intervention found no evidence of cost shift from the NHS to the smokers. On the contrary, although participants’ expenses were similar in each arm, the NHS smoking cessation costs were slightly lower in the e-cigarette arm than in the NRT arm.
The weekly comparison of NRT dispensed and self-reported NRT in the NRT arm suggested an issue in compliance with NRT prescription. Although participants still used some form of NRT products, the NRT products provided by the trial were not always used. If the extra NRT products were obtained through the NHS, the intervention costs of the NRT arm might be underestimated.
The long-term modelling projected a lifetime ICER of £65 per QALY gained (95% CI –£12,537 to £13,423), indicating that e-cigarettes would be a highly cost-effective intervention, compared with NRT. The probability sensitivity analyses proved the conclusion to be robust.
Discussion
The trial collected a large amount of information in a large sample. The total sample size of 886 reduced the possibility of some participants with particularly high health-care services use being randomised to one arm and causing imbalance in the total costs. A wide range of smoking cessation related services/products information and primary and secondary care use information was also collected.
The primary analysis concluded that using e-cigarettes within the UK SSSs is cost-effective compared with pharmacotherapies. A sensitivity analysis and the long-term modelling that was performed and reported after the within-trial analyses (including sensitivity analysis) supported this conclusion. There was no evidence to suggest a difference in costs to the NHS within the 12-month period, although the intervention costs were lower with e-cigarettes. There was an indication that QALY gain was higher in the e-cigarette arm than in the NRT arm during the 12-month period; however, the difference was not significant. The CCA indicated that using e-cigarettes might be cost-saving while having no effect on QALYs in the 12-month period because, among the complete cases, health-care service costs and QALYs, although increased in both arms, were increased to a much greater extent in the NRT arm.
Both EQ VAS and utility scores showed a slight upwards trend in the e-cigarette arm. In the NRT arm, the EQ VAS score increased at 6 months and dropped marginally at 12 months, whereas the utility score showed a more gentle increase than in the e-cigarette arm. Among the five dimensions of EQ-5D-5L, most participants did not report any problems in mobility, self-care or usual activities, which was expected in this population. The proportions of participants reporting problems were highest for the dimensions of pain/discomfort and anxiety/depression. Both dimensions appeared to improve through the trial period, with the greatest improvement occurring in anxiety/depression dimension score in the e-cigarette arm.
It appeared that participants, once introduced to one type of smoking cessation aid, were more likely to continue using the same aid after the intervention period. Participants in the NRT arm reported more NRT use than participants in the e-cigarette arm, whereas the participants in the e-cigarette arm reported more purchase of e-cigarettes than those in the NRT arm. Although some participants switched products or used a mixture of products, they accounted for only a small fraction of the total.
Originally, the research team were concerned that the introduction of e-cigarettes might shift the cost of smoking cessation onto individuals trying to quit, because e-cigarettes are not currently provided by the NHS. However, the results from this analysis showed that participants trying to quit smoking were likely to purchase some form of cessation aid, whether NRT or e-cigarettes. Therefore, there was no evidence to suggest that participants who used e-cigarettes spent more money on smoking cessation aids. As various brands and packages of NRT products are available on the market, we estimated personal expenditure on NRT products based on prescription costs rather than market prices. Participants’ actual expenditure on NRT products could be higher than we estimated. For instance, the net ingredient cost of seven 21 mg/24-hour nicotine patches is £9.87,47 whereas a pack of seven 21 mg/24-hour nicotine patches costs £8.00 to £30.89 on Amazon UK (Amazon.com, Inc., Bellevue, WA, USA) and £11 to £13 on the websites of Boots (Nottingham, UK), Superdrug (London, UK) and LloydsPharmacy (Coventry), without sale discount. Promotional sales and online purchase could reduce the cost to participants but, in general, the price would be higher than the net ingredient cost.
The reasons for a large proportion of participants not using some or all of the NRTs dispensed were unclear. It was possible that between the time of dispense and use, the participants looked into NRT products and decided to use other forms of NRT. The use of a LOR rather than direct product at one of the sites dispensing could also have led participants at that site to change the product used, as a LOR must be redeemed in pharmacies, thereby incurring an extra trip and prescription charge if not exempted. It could also be that, once participants were introduced to the NRT products, they were more likely to be tempted to try other forms of NRT.
The economic evaluation comparing e-cigarettes with NRT products was conducted on the premise that the initial products were provided free of charge to the participants, with some guidance. However, this is not currently the case. To implement this intervention in practice, a systematic shift within various relevant services might be required, the costs of which were not taken into account in this analysis. Furthermore, the price of e-cigarettes is changing rapidly, as reflected in this trial. The One Kit originally used in the trial cost £19.35 per kit, but during the trial period this old version was discontinued and a more expensive version (£30.54 per kit) had to be used as replacement. In contrast to the relatively stable price of NRT products, this makes the cost of the intervention uncertain and likely to increase in the future. With the price rising, participants’ expenditure on e-cigarettes as a smoking cessation aid would also increase. This results in the conclusion regarding SSS costs to the NHS and a cost shift from the NHS to smokers becoming less robust.
Short-term costs and QALYs are not sufficient to reflect the full benefits of smoking cessation. This long-term model showed a result favourable to the use of e-cigarettes mainly because of the higher abstinence rate at 12 months in the e-cigarette arm than in the NRT arm. The model did not take into account the repeated attempt of smoking cessation, in which, in reality, people who failed the first time might try a second or even third time at a later stage. In addition, although the smoking-related diseases have been identified and their costs were estimated, the EQ-5D-5L is not a clinical measurement widely used for these clinical conditions, especially in the acute onset of a condition. The QALYs in the model were derived based on smoking status and were not disease specific; therefore, it is possible that the QALY gain from smoking cessation was underestimated.
Acknowledgements
We would like to thank:
All trial participants and the participating SSSs [Leicester, East Sussex (Quit 51), Tower Hamlets and City of London] that helped to recruit participants and deliver the trial interventions.
Our TSC and DMEC members, in particular the chairpersons, Professor Ian Roberts and Professor Tim Peto, lay members, Benjamin Roberts and Brian Eastwood, and Professor Sarah Lewis, for her independent review of the statistical analysis plan.
Our NIHR research project managers: Simon Bevan, Alexa Cross, Avril Lloyd and Jennifer Cook.
All of the team, current and former, at the Health and Lifestyle Research Unit, QMUL, who assisted with intervention delivery, data collection and data entry.
The Barts CTU, in particular Dr Irene Kaimi (Senior Statistician), Benoit Aigret, Richard Ostler, Samanah Haidary and Alberto Stella, for their support throughout the trial.
Contributions of authors
Professor Peter Hajek (Professor of Clinical Psychology) was the Chief Investigator. He co-wrote the original grant application, co-designed the trial, co-wrote the statistical analysis plan, trained staff, interpreted the trial findings and led the drafting of the report.
Mrs Anna Phillips-Waller (Research Manager) co-designed the trial, managed the trial, co-wrote the statistical analysis plan, trained staff, delivered the interventions, contributed to data collection, interpreted the trial findings and assisted with the drafting of the report.
Dr Dunja Przulj (Postdoctoral Research Assistant) assisted with the management of the trial, trained staff, delivered the interventions, contributed to data collection, co-wrote the statistical analysis plan, interpreted the trial findings and assisted with the drafting of the report.
Dr Francesca Pesola (Study Statistician) co-wrote the statistical analysis plan and analysed the final trial data.
Dr Katie Myers Smith (Research Fellow) was a co-applicant on the grant proposal, trained staff, delivered the interventions, contributed to data collection, interpreted the trial findings and assisted with the drafting of the report.
Ms Natalie Bisal (Research Health Psychologist) delivered the interventions, contributed to data collection and assisted with the drafting of the report.
Ms Jinshuo Li (Research Fellow) co-wrote the statistical analysis plan for health economics analysis, analysed the health economics trial data, interpreted the results and assisted with the report write-up.
Mr Steve Parrott (Reader in Health Economics) co-wrote the statistical analysis plan for health economics analysis, analysed the health economics study data, interpreted the results and assisted with the report write-up.
Professor Peter Sasieni (Professor of Biostatistics and Cancer Epidemiology) co-wrote the statistical analysis plan.
Dr Lynne Dawkins (Associate Professor) assisted with recruitment and was involved in the interpretation of the trial data and write-up of the trial report.
Ms Louise Ross (Stop Smoking Service Manager) advised on trial feasibility, trained staff, supported intervention delivery, contributed to data collection and assisted with the drafting of the report.
Dr Maciej Goniewicz (Associate Professor of Oncology) was involved in the preparation of the grant proposal and the trial report.
Ms Qi Wu (Research Fellow) developed, updated and ran the health economics model.
Professor Hayden J McRobbie (Professor in Public Health Interventions) was a co-investigator. He co-wrote the original grant application, co-designed the trial, co-wrote the statistical analysis plan, trained staff, interpreted the trial findings and assisted with the drafting of the report.
Publication
Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629–37.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Sussan TE, Shahzad FG, Tabassum E, Cohen JE, Wise RA, Blaha MJ, et al. Electronic cigarette use behaviors and motivations among smokers and non-smokers. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4671-3.
- Public Health England . E-Cigarettes and Heated Tobacco Products: Evidence Review 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/684963/Evidence_review_of_e-cigarettes_and_heated_tobacco_products_2018.pdf (accessed 5 January 2018).
- Royal College of Physicians . Nicotine Without Smoke: Tobacco Harm Reduction 2016.
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD010216.pub3.
- Action on Smoking and Health (ASH) . Use of Electronic Cigarettes (Vapourisers) Among Adults in Great Britain 2016.
- McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation: A Guide For Counsellors and Practitioners. Oxford: John Wiley and Sons; 2006.
- Przulj D, McRobbie H, Hajek P. The effect of sensorimotor replacement on smoking cessation and craving. Open Addict J 2012;5:41-50. https://doi.org/10.2174/1874941001205010041.
- West R, Beard E, Brown J. Trends in Electronic Cigarette Use in England. Smoking Toolkit Study 2018. www.smokinginengland.info/latest-statistics/ (accessed 13 May 2019).
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382:1629-37. https://doi.org/10.1016/S0140-6736(13)61842-5.
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0066317.
- Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health 2014;11:11220-48. https://doi.org/10.3390/ijerph111111220.
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med 2018;378:2302-10. https://doi.org/10.1056/NEJMsa1715757.
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629-37. https://doi.org/10.1056/NEJMoa1808779.
- Hajek P. Withdrawal-oriented therapy for smokers. Br J Addict 1989;84:591-8. https://doi.org/10.1111/j.1360-0443.1989.tb03474.x.
- Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014;109:1801-10. https://doi.org/10.1111/add.12659.
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 2012;14:75-8. https://doi.org/10.1093/ntr/ntr137.
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology 2004;177:195-9. https://doi.org/10.1007/s00213-004-1923-6.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD000146.pub4.
- Ferguson J, Bauld L, Chesterman J, Judge K. The English smoking treatment services: one-year outcomes. Addiction 2005;100:59-6. https://doi.org/10.1111/j.1360-0443.2005.01028.x.
- Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res 2015;17:175-9. https://doi.org/10.1093/ntr/ntu153.
- Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ 1999;318:285-8. https://doi.org/10.1136/bmj.318.7179.285.
- Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-1159.
- Stapleton J. Cigarette smoking prevalence, cessation and relapse. Stat Methods Med Res 1998;7:187-203. https://doi.org/10.1177/096228029800700206.
- Bauld L, Hiscock R, Dobbie F, Aveyard P, Coleman T, Leonardi-Bee J, et al. English stop-smoking services: one-year outcomes. Int J Environ Res Public Health 2016;13. https://doi.org/10.3390/ijerph13121175.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. https://doi.org/10.1111/j.1360-0443.2004.00995.x.
- Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology 2017;234:773-9. https://doi.org/10.1007/s00213-016-4512-6.
- Hajek P, Jackson P, Belcher M. Long-term use of nicotine chewing gum. Occurrence, determinants, and effect on weight gain. JAMA 1988;260:1593-6. https://doi.org/10.1001/jama.1988.03410110101035.
- Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction 2013;108:1115-25. https://doi.org/10.1111/add.12150.
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res 2015;17:186-92. https://doi.org/10.1093/ntr/ntu204.
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 2017;166:390-40. https://doi.org/10.7326/M16-1107.
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 2014;4. https://doi.org/10.1038/srep04133.
- Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First- versus second-generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction 2015;110:669-77. https://doi.org/10.1111/add.12807.
- NHS Digital . Statistics on NHS Stop Smoking Services in England April 2017 to September 2017 2018.
- Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0108342.
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0116861.
- Miler J, Mayer B, Hajek P. Changes in the frequency of airway infections in smokers who switched to vaping: results of an online survey. J Addict Res Ther 2016;7. https://doi.org/10.4172/2155-6105.1000290.
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. https://doi.org/10.1016/S0140-6736(16)30272-0.
- The Preloading Investigators . Effects on abstinence of nicotine patch treatment prior to quitting smoking: parallel, two arm, pragmatic randomised trial. BMJ 2018;361. https://doi.org/10.1136/bmj.k2164.
- Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, et al. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 2013;108:2193-201. https://doi.org/10.1111/add.12304.
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD008286.pub3.
- Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-140.
- Hajek P. The development and testing of new nicotine replacement treatments: from ‘nicotine replacement’ to ‘smoking replacement’. Addiction 2015;110:19-22. https://doi.org/10.1111/add.12905.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Department of Health and Social Care (DHSC) . Charges for NHS Prescriptions, Dental Charges, Elastic Stockings and Tights, Wigs and Fabric Supports And Optical Voucher Values: Written Statement – HLWS346 2015. www.parliament.uk/business/publications/written-questions-answers-statements/written-statement/Lords/2015-03-11/HLWS346/ (accessed 1 February 2018).
- NHS Digital . Prescription Cost Analysis – England 2016 2017.
- NHS Business Services Authority . PD1 Reports 2017. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/information-services-pd1-reports (accessed 10 October 2017).
- The EuroQol Group . EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument (Version 2.0) 2013. www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/UserGuide_EQ-5D-5L_v2.0_October_2013.pdf (accessed 4 March 2015).
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 13 September 2017).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Rubin DB. Statistical matching using file concatenation with adjusted weights and multiple imputations. J Bus Econ Stat 1986;4:87-94. https://doi.org/10.1080/07350015.1986.10509497.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. London: Chapman & Hall; 1993.
- Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med 1996;15:1447-58. https://doi.org/10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V.
- Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ 1996;5:297-305. https://doi.org/10.1002/(SICI)1099-1050(199607)5:4<297::AID-HEC216>3.0.CO;2-T.
- Severens JL, De Boo TM, Konst EM. Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999;15:608-14.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Drummond M, Sculpher M, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Coyle D, Davies L, Drummond MF. Trials and tribulations. Emerging issues in designing economic evaluations alongside clinical trials. Int J Technol Assess Health Care 1998;14:135-44. https://doi.org/10.1017/S0266462300010588.
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342. https://doi.org/10.1136/bmj.d1548.
- International Agency for Research on Cancer (IARC) . IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Volume 83: Tobacco Smoke and Involuntary Smoking 2004. http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf (accessed 15 December 2017).
- US Department of Health & Human Services . The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General, 2014 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf (accessed 20 February 2018).
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. https://doi.org/10.1136/bmj.38142.554479.AE.
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost-effectiveness of computer-tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res 2014;16:270-8. https://doi.org/10.1093/ntr/ntt136.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav 2008;33:1516-20. https://doi.org/10.1016/j.addbeh.2008.05.012.
- US Department of Health & Human Services . The Health Benefits of Smoking Cessation: A Report of the US Surgeon General 1990.
- Hawkins J, Hollingworth W, Campbell R. Long-term smoking relapse: a study using the British household panel survey. Nicotine Tob Res 2010;12:1228-35. https://doi.org/10.1093/ntr/ntq175.
- Office for National Statistics (ONS) . Deaths Registered in England and Wales: 2016 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2016 (accessed 23 May 2019).
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-203.
- National Centre for Social Research and University College London . Department of Epidemiology and Public Health, Health Survey for England 2006.
- World Health Organization (WHO) . Economics of Tobacco Toolkit: Assessment of the Economic Costs of Smoking 2011.
- NHS Digital . Hospital Admitted Patient Care Activity, 2016–17 2017.
- Royal College of Physicians . Hiding in Plain Sight: Treating Tobacco Dependency in the NHS 2018.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016.
- Health and Social Care Information Centre (HSCIC) . HRG4+ 2015 16 Reference Costs Code to Group Workbook 2016.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-52.
- National Institute for Health and Care Excellence (NICE) . Smoking Cessation Services Costing Report – Implementing NICE Guidance 2008.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
Appendix 1 Supplementary tables
| Approved versiona | Date | Summary |
|---|---|---|
| 3.0 | 8 April 2015 | Change of site address and principal investigator at QMUL. Clarifications to statistics |
| 4.0 | 29 May 2015 | Change of chief investigator |
| 5.0 | 11 May 2016 | Change to advertising strategies, change to storage of data and clarification regarding modes of contact used for participants who miss appointments/follow-up calls |
| Name | Role | Committee |
|---|---|---|
| Professor Ian Roberts | Chairperson (independent) | TSC |
| Professor Sarah Lewis | Member (independent) | TSC |
| Professor Linda Bauld | Member (independent) | TSC |
| Professor Michael Ussher | Member (independent) | TSC |
| Mr Darush Attar-Zadeh | Member (independent) | TSC |
| Mr Brian Eastwood | Lay member (independent) | TSC |
| Mr Benjamin Roberts | Lay member | TSC |
| Professor Tim Peto | Chairperson (independent) | DMEC |
| Dr Angela Crook | Member (independent) | DMEC |
| Dr Lion Shahab | Member (independent) | DMEC |
| Mrs Anna Phillips-Waller | Study Manager | TMG |
| Professor Peter Hajek | Chief Investigator | TMG |
| Dr Dunja Przulj | Researcher | TMG |
| Mr Benoit Aigret | Head of Barts CTU | TMG |
| Mr Richard Ostler | Barts CTU programmer | TMG |
| E-cigarette arm | NRT arm |
|---|---|
|
|
| Service | Unit cost (£) | Source |
|---|---|---|
| Smoking cessation help | ||
| GP | 36 per session | Curtis and Burns45 |
| NHS SSS | 17 per session | Curtis and Burns45 and NICE80 |
| NHS Smoking Helpline service | 7 per call | Curtis and Burns45 and Wu et al.66 |
| Pharmacotherapies (including dispensing fee) | ||
| Patch | 27 per item | NHS Digital47 and NHS Business Services Authority48 |
| Gum | 15 per item | NHS Digital47 and NHS Business Services Authority48 |
| Microtab | 19 per item | NHS Digital47 and NHS Business Services Authority48 |
| Inhaler | 36 per item | NHS Digital47 and NHS Business Services Authority48 |
| Lozenge | 17 per item | NHS Digital47 and NHS Business Services Authority48 |
| Spray | 30 per item | NHS Digital47 and NHS Business Services Authority48 |
| Mouth strip | 17 per item | NHS Digital47 and NHS Business Services Authority48 |
| Varenicline | 37 per item | NHS Digital47 and NHS Business Services Authority48 |
| Bupropion | 40 per item | NHS Digital47 and NHS Business Services Authority48 |
| Health-care service use | ||
| A&E | 162 per attendance | NHS Reference Costs 2015 to 2016 77 |
| Hospital outpatient | 135 per appointment | NHS Reference Costs 2015 to 2016 77 |
| Hospital inpatient | 606 per night | NHS Reference Costs 2015 to 2016 77 |
| Day case | 733 per episode | NHS Reference Costs 2015 to 2016 77 |
| Emergency ambulance | 96 per use | NHS Reference Costs 2015 to 2016 77 |
| GP (in office) | 31 per consultation | Curtis and Burns45 |
| Practice nurse (in office) | 9 per visit | Curtis and Burns45,81 |
| GP (home visit) | 56 per consultation | Curtis and Burns45,81 |
| Practice nurse (home visit) | 17 per visit | Curtis and Burns45,81 |
| Prescription (NIC + dispensing) | 22 per prescription | NHS Business Services Authority48 |
| Parameters | Value (SE) | Source |
|---|---|---|
| Annual probability of relapse (%) | ||
| ≤ 10 years | 10.00 (3.06) | Hughes et al.,68 US HHS,69 Hawkins et al.70 |
| > 10 years | 0 (0) | Hughes et al.,68 US HHS,69 Hawkins et al.70 |
| Mortality (%) | ||
| Men | ||
| Continuing smokers, age (years) | ||
| 35–44 | 0.24 (0.40) | Doll et al.,65 ONS71 |
| 45–54 | 0.80 (0.40) | Doll et al.,65 ONS71 |
| 55–64 | 1.94 (0.52) | Doll et al.,65 ONS71 |
| 65–74 | 5.15 (0.82) | Doll et al.,65 ONS71 |
| ≥ 75 | 25.36 (2.04) | Doll et al.,65 ONS71 |
| Ex-smokers, age (years) | ||
| 35–44 | 0.18 (0.35) | Doll et al.,65 ONS71 |
| 45–54 | 0.51 (0.73) | Doll et al.,65 ONS71 |
| 55–64 | 1.24 (0.58) | Doll et al.,65 ONS71 |
| 65–74 | 3.08 (0.59) | Doll et al.,65 ONS71 |
| ≥ 75 | 15.12 (1.14) | Doll et al.,65 ONS71 |
| Women | ||
| Continuing smokers, age (years) | ||
| 35–44 | 0.14 (0.31) | Doll et al.,65 ONS71 |
| 45–54 | 0.53 (0.33) | Doll et al.,65 ONS71 |
| 55–64 | 1.30 (0.43) | Doll et al.,65 ONS71 |
| 65–74 | 3.45 (0.68) | Doll et al.,65 ONS71 |
| ≥ 75 | 20.79 (1.90) | Doll et al.,65 ONS71 |
| Ex-smokers, age (years) | ||
| 35–44 | 0.11 (0.27) | Doll et al.,65 ONS71 |
| 45–54 | 0.34 (0.59) | Doll et al.,65 ONS71 |
| 55–64 | 0.83 (0.48) | Doll et al.,65 ONS71 |
| 65–74 | 2.06 (0.49) | Doll et al.,65 ONS71 |
| ≥ 75 | 12.40 (1.05) | Doll et al.,65 ONS71 |
| Annual smoking-related healthcare costs after the first year (£) | ||
| Men | ||
| Continuing smokers, age (years) | ||
| 35–44 | 54.48 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 45–54 | 54.48 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 55–64 | 181.97 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 65–74 | 315.75 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| ≥ 75 | 535.22 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| Ex-smokers, age (years) | ||
| 35–44 | 16.57 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 45–54 | 16.57 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 55–64 | 64.99 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 65–74 | 83.82 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| ≥ 75 | 105.36 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| Women | ||
| Continuing smokers, age (years) | ||
| 35–44 | 41.31 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 45–54 | 41.31 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 55–64 | 119.83 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 65–74 | 249.03 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| ≥ 75 | 470.69 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| Ex-smokers, age (years) | ||
| 35–44 | 10.72 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 45–54 | 10.72 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 55–64 | 40.95 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| 65–74 | 71.25 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| ≥ 75 | 103.18 (0) | NHS Digital,75 DHSC,77 HSCIC78 |
| Annual QALY gain after the first year | ||
| Men | ||
| Continuing smokers, age (years) | ||
| 35–44 | 0.889 (0.007) | Vogl et al.72 |
| 45–54 | 0.841 (0.007) | Vogl et al.72 |
| 55–64 | 0.780 (0.008) | Vogl et al.72 |
| 65–74 | 0.756 (0.008) | Vogl et al.72 |
| ≥ 75 | 0.710 (0.009) | Vogl et al.72 |
| Ex-smokers, age (years) | ||
| 35–44 | 0.889 (0.007) | Vogl et al.72 |
| 45–54 | 0.841 (0.007) | Vogl et al.72 |
| 55–64 | 0.780 (0.008) | Vogl et al.72 |
| 65–74 | 0.756 (0.008) | Vogl et al.72 |
| ≥ 75 | 0.710 (0.009) | Vogl et al.72 |
| Women | ||
| Continuing smokers, age (years) | ||
| 35–44 | 0.870 (0.007) | Vogl et al.72 |
| 45–54 | 0.830 (0.007) | Vogl et al.72 |
| 55–64 | 0.763 (0.008) | Vogl et al.72 |
| 65–74 | 0.751 (0.008) | Vogl et al.72 |
| ≥ 75 | 0.676 (0.009) | Vogl et al.72 |
| Ex-smokers, age (years) | ||
| 35–44 | 0.889 (0.004) | Vogl et al.72 |
| 45–54 | 0.850 (0.005) | Vogl et al.72 |
| 55–64 | 0.784 (0.005) | Vogl et al.72 |
| 65–74 | 0.773 (0.006) | Vogl et al.72 |
| ≥ 75 | 0.700 (0.007) | Vogl et al.72 |
| Annual discount rate (%) | ||
| Cost discount rate | 3.5 | NICE44 |
| Outcome discount rate | 3.5 | NICE44 |
| NRT product | Cost per item + fee (£) |
|---|---|
| 24-hour patch | |
| 21 mg | 25 |
| 14 mg | 25 |
| 7 mg | 25 |
| 16-hour patch | |
| 25 mg | 24 |
| 15 mg | 26 |
| 10 mg | 26 |
| Microtab (2 mg) | 17 |
| Mouth strip (2.5 mg) | 15 |
| Gum | |
| 4 mg | 15 |
| 2 mg | 12 |
| Lozenge | |
| 4 mg | 15 |
| 2 mg | 15 |
| Nasal spray | 41 |
| Minis | |
| 4 mg | 17 |
| 1.5 mg | 15 |
| Inhalator | 33 |
| Mouth spray | 26 |
| Health-care service | Trial arm, mean (SD) | |||||
|---|---|---|---|---|---|---|
| NRT | E-cigarette | |||||
| Baseline (n = 447) | 6 months (n = 270) | 12 months (n = 283) | Baseline (n = 439) | 6 months (n = 306) | 12 months (n = 316) | |
| A&E | 0.19 (0.52) | 0.11 (0.47) | 0.10 (0.32) | 0.24 (0.80) | 0.11 (0.38) | 0.10 (0.34) |
| Outpatient | 1.04 (5.18) | 0.85 (3.92) | 0.68 (2.55) | 0.73 (2.23) | 0.85 (3.71) | 0.68 (3.07) |
| Inpatient (nights) | 0.30 (2.87) | 0.22 (1.57) | 0.32 (2.80) | 0.23 (1.78) | 0.17 (1.61) | 0.50 (3.58) |
| Day case | 0.16 (0.77) | 0.06 (0.24) | 0.14 (1.45) | 0.13 (0.48) | 0.13 (0.97) | 0.08 (0.35) |
| Emergency ambulance | 0.04 (0.22) | 0.03 (0.38) | 0.04 (0.20) | 0.06 (0.31) | 0.01 (0.08) | 0.03 (0.16) |
| GP (in office) | 2.05 (3.17) | 1.40 (2.98) | 1.37 (2.22) | 2.04 (3.69) | 1.41 (2.58) | 1.52 (2.95) |
| Practice nurse (in office) | 0.56 (1.84) | 0.51 (2.02) | 0.31 (0.69) | 0.72 (2.04) | 0.49 (1.88) | 0.37 (0.87) |
| GP (home visit) | 0.01 (0.08) | – | 0.00 (0.06) | 0.00 (0.05) | 0.02 (0.15) | 0.02 (0.23) |
| Practice nurse (home visit) | 0.01 (0.19) | 0.01 (0.18) | 0.17 (2.85) | – | 0.01 (0.08) | 0.01 (0.11) |
| Prescription | 3.13 (6.39) | 2.74 (5.85) | 2.98 (4.78) | 2.92 (5.55) | 2.55 (6.02) | 2.53 (4.43) |
List of abbreviations
- AE
- adverse event
- AR
- adverse reaction
- CCA
- complete-case analysis
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- CTU
- Clinical Trials Unit
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ VAS
- EuroQol Visual Analogue Scale
- FTCD
- Fagerström Test for Cigarette Dependence
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- LOR
- letter of recommendation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OTC
- over the counter
- PPI
- patient and public involvement
- p.p.m.
- parts per million
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QMUL
- Queen Mary University of London
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SSS
- Stop Smoking Service
- TEC
- Trial of E-Cigarettes
- TQD
- target quit date
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WTP
- willingness to pay
