Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/99/01. The contractual start date was in January 2013. The draft report began editorial review in April 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sarah E Lamb reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study and was a member of the following boards: HTA Additional Capacity Funding Board (2012–15), HTA Clinical Trials Board (2010–15), HTA End of Life Care and Add on Studies (2015), HTA Funding Boards Policy Group (previously Commissioning Strategy Group) (2010–15), HTA Maternal, Neonatal and Child Health Methods Group (2013–15), HTA Post-board funding teleconference (Prioritisation Group members to attend) (2010–15), HTA Primary Care Themed Call board (2013–14), HTA Prioritisation Group (2010–15) and NIHR Clinical Trials Unit Standing Advisory Committee (2012–16). Muhammad K Javaid reports personal fees from Optasia Medical Ltd (Cheadle, UK) and Zebra Medical Vision, Inc. (Shefayim, Israel) outside the submitted work. Cyrus Cooper reports personal fees from Alliance for Better Health (Troy, NY, USA), Amgen Inc. (Thousand Oaks, CA, USA), Eli Lilly and Company (Indianapolis, IN, USA), GlaxoSmithKline plc (Middlesex, UK), Medtronic (Watford, UK), Merck & Co. Inc. (Kenilworth, NJ, USA), Novartis Pharmaceuticals UK Ltd (Frimley, UK), Pfizer Inc. (New York, NY, USA), F. Hoffman-La Roche Ltd (Basel, Switzerland), Servier Laboratories Limited (Stoke Poges, UK), Takeda UK Ltd (Wooburn Green, UK) and UCB Pharma (Brussels, Belgium).
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Barker et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The Physiotherapy Rehabilitation for Osteoporotic VErtebral Fracture (PROVE) trial was commissioned by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. It was set up to investigate the effects of physiotherapy on clinical outcomes for people with symptomatic osteoporotic vertebral fractures (OVFs). The PROVE trial compared the effectiveness of two different physiotherapy approaches, exercise therapy and manual therapy, with a single session of physiotherapy (SSPT) for people with at least one previous OVF and back pain. This chapter provides background information about osteoporosis and vertebral fractures. It goes on to provide an overview of the management of OVF and to focus on the background to the interventions examined in the PROVE trial.
Background
In order to fulfil its function, bone changes throughout life and undergoes a constant process of remodelling. 1–3 This involves daily deposition of new bone and removal or resorption of old bone to allow for bone growth, repair and adaptation to load. 1,3 The balance between bone deposition and resorption determines whether there is net bone acquisition, maintenance or loss. Bone mass typically reaches its peak in early adulthood, determined by factors such as genetics, nutrition, levels of physical activity and health during development. 4 After about 40 years of age, bone resorption gradually begins to outpace the formation of new bone and, thus, a gradual loss of bone density takes place as part of the normal ageing process. 3 In women, the rate of bone loss accelerates sharply after the menopause owing to the role that oestrogen plays in the bone renewal cycle. 5 Bone remodelling also results in gradual changes in bone architecture and geometry with gradual loss of spongy, internal trabecular bone, change in cortical bone thickness and widening of the bone cavity seen with ageing. 1,3 These processes are fundamental to the pathophysiology of osteoporosis.
Definition and diagnosis of osteoporosis
The World Health Organization describes osteoporosis as a progressive systemic skeletal disease ‘characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk’. 6 Osteoporosis is defined in terms of bone mass, based on bone mineral density (BMD) assessment. It is diagnosed in postmenopausal women or in men aged ≥ 50 years when BMD lies ≥ 2.5 standard deviations (SDs) below the mean value for a healthy young adult (a T-score of ≤ –2.5 SD) at the same skeletal site. 7 The T-score is calculated from the measurement of BMD using central (hip and/or lumbar spine) dual-energy X-ray absorptiometry (DEXA) scanning. 8 Established or severe osteoporosis is a BMD T-score of < –2.5 SD plus the presence of one or more fragility fractures. 9 Although this operational definition of osteoporosis focuses on BMD, the wider definition of osteoporosis emphasises that skeletal fragility is the product of decreased BMD and poor bone quality (i.e. the deterioration in bone architecture and microstructure associated with osteoporosis). 5
Osteoporosis can be classified as either primary or secondary. Primary or idiopathic osteoporosis includes postmenopausal osteoporosis, juvenile osteoporosis and age-related, senile osteoporosis and is the most common type of osteoporosis. 5 It affects both sexes, but women are at greater risk of developing primary osteoporosis because, on average, they have lower peak bone mass than men, they live longer and the lack of oestrogen after the menopause accelerates the normal rate of bone loss and deterioration in bone structure that occurs with ageing. 5 In contrast, secondary osteoporosis occurs because of other conditions or diseases, such as rheumatoid arthritis, endocrine disorders (diabetes mellitus, hypogonadism, etc.), cancer and gastrointestinal diseases (coeliac disease, inflammatory bowel disease, etc.), or because of medications that affect bone metabolism (e.g. glucocorticoids4). The clinical significance of osteoporosis lies not in low bone mass itself, but in the fractures that may happen as a consequence.
Epidemiology of osteoporosis and osteoporotic fracture
Osteoporosis is a common disorder, affecting hundreds of millions of people worldwide and > 3.2 million people in the UK. 10 Because bone density reduces with age and the global population is ageing, the prevalence of osteoporosis is increasing. 10,11 For example, the number of women living with osteoporosis in the UK has been predicted to increase from 1.8 million in 2010 to 2.1 million by 2020 (+16.5%). 12 Osteoporosis itself is often called a ‘silent disease’ as patients cannot perceive that their bones are getting thinner and this process is not painful or limiting. 13 The clinical importance of osteoporosis arises from its association with bone fractures. 8
In the osteoporotic spine, vertebral fractures often happen without any noticeable stress. 14,15 Rates of osteoporotic fracture are increasing in line with the increase in osteoporosis. 12 In the UK in 2010, the estimated number of osteoporotic fractures was 536,000; this number included approximately 79,000 hip fractures, 66,000 new clinically diagnosed vertebral fractures and 69,000 forearm fractures. 10 This is expected to rise to 682,000 osteoporotic fractures by 2025, an increase of 27% over 15 years. 10
Costs of osteoporosis and osteoporotic fracture
Osteoporosis incurs substantial personal and economic costs. In Europe, the costs associated with osteoporosis are greater than those caused by all cancers (apart from lung cancer) and many other chronic diseases including asthma, stroke, heart disease and rheumatoid arthritis. 3,13 Osteoporotic fractures result in significant excess mortality and morbidity. 8,10,12 The consequences vary by fracture site, with the greatest excess mortality and morbidity attributed to hip and vertebral fractures. 13 Hip fractures are associated with the highest mortality in the year after fracture, with vertebral fractures being the second most important site for fracture-related deaths. 13 Both hip and vertebral fracture result in marked reductions in quality of life (QoL) that persist for ≥ 18 months after fracture. 16
The economic burden of osteoporosis is a combination of direct costs related to fracture care and osteoporosis management (e.g. surgery and hospitalisation, diagnostic scans, therapy and pharmaceuticals), as well as indirect costs (e.g. from lost work time and productivity for the individual and any caregiver) and more intangible costs in terms of the value of the quality-adjusted life-years (QALYs) lost. 13 In 2010, the cost of osteoporosis-related care to the NHS was estimated to be £4.4B, of which acute fracture care accounted for the largest proportion. 10 By 2025, it is estimated that this figure will rise to £5.5B per year. 10 These may be conservative estimates. Burge et al. 17 highlight the difficulties of analysing the total cost of osteoporosis, noting that indirect costs, such as unpaid care time or longer-term, ongoing care provided by Personal Social Services, may be unaccounted for and result in underestimating the total cost of osteoporosis. 17
Osteoporotic vertebral fractures
Vertebral fractures are the most common osteoporotic fracture. 10 They can occur in any part of the spine, but are most frequent in the thoracic and lumbar spine at the transition points between more rigid and more flexible parts of the spine, such as the thoracolumbar junction and at the mid-point of the thoracic kyphosis. 14 The reported prevalence and incidence of OVF vary by country, population observed and method used to define a fracture. 13 Although recognising this, vertebral fractures are estimated to affect ≥ 20% of the older population; thus, around one in five men and women aged ≥ 50 years will have one or more vertebral fractures, with the incidence and prevalence of OVF increasing steadily with age. 10,18,19
Radiological diagnosis of OVF is not always straightforward; vertebral deformity exists on a continuum from barely detectable change through to severe compression fracture and fracture age can be difficult to determine. 14,18,20,21 The Genant method is used to classify deformity and suggests that a vertebral fracture exists where there is loss of > 4 mm (or 20%) of vertebral height (anterior, mid or posterior dimensions) relative to the original or adjacent vertebra. 18 Wedge fractures are the most common type of vertebral fracture and occur when there is a loss of height in the vertebral body anteriorly while the back of the vertebral body maintains its height. 14
Vertebral fractures contribute to spinal deformity (e.g. loss of height at the anterior vertebral body causes the affected segment of the spine to angle forwards, resulting in increased thoracic kyphosis or loss of lumbar lordosis and height loss). 20 This changes spinal biomechanics, increasing load on the vertebral body and adjacent vertebra, back extensor muscles and ligaments and significantly increases the risk of a subsequent vertebral fracture, progressive spinal deformity and hyperkyphotic posture. 22,23 Each OVF is estimated to increase kyphosis by about 3.8 °. 24 The severity of kyphosis is influenced by the number of OVFs but also by the strength of back extensor muscles and the extent of other degenerative changes in intervertebral discs and spinal soft tissues. 15,20,22,23
The experience of vertebral fracture varies markedly. The majority of OVFs are initially asymptomatic or ‘clinically silent’. 18 As a result, despite their high prevalence it is estimated that only 30–40% of all vertebral fractures come to clinical attention and more than two-thirds remain undiagnosed and undertreated. 3,18,21 Asymptomatic OVFs are of less immediate concern than those that cause clinical symptoms, but are important as both symptomatic and asymptomatic OVFs contribute to the progression of disability and both are robust predictors of future vertebral and non-vertebral fractures. 18,21 For instance, a woman who has sustained an OVF has a fivefold increase in the risk of having another OVF within 1 year; sustaining two or more fractures carries up to a 12-fold increase in risk. 4,14 The risk of hip and other non-hip/non-vertebral fractures increases by twofold to threefold, respectively. 4 Appropriate treatment can reduce the risk of subsequent fractures, making identification important. 3
Patients with more severe or multiple fractures are more likely to be symptomatic. The main symptom is back pain: this is typically located at the fracture site or close to it and may be accompanied by muscle spasm. 14 The pain can be acute and can severely restrict function. 14,25 Up to one-fifth of patients require hospitalisation, many fail to return to previous levels of function and some require institutional care. 13,25 Although pain following an acute OVF tends to settle over the first 3 months following the event, around half of those affected go on to develop chronic back pain. 26 The ongoing effects of OVF(s) can be considerable and, together with pain, spinal deformity and height loss, include limitations in physical function, activities of daily living (ADL) and social participation, altered body image, loss of self-esteem, fatigue and low mood, all of which negatively and persistently affect QoL. 16 Vertebral fractures and hyperkyphosis are associated with increased mortality, particularly from falls. 19,27 Thoracic deformity and pain linked to OVF may compromise respiratory function and balance, increasing the risk of death from respiratory disease or from sustaining an injury such as a hip fracture following a fall. 19,28,29 QoL and excess mortality progressively worsen as the number of OVFs increase.
Management
Patients with osteoporosis and vertebral fracture present with varying levels of pain and disability; older patients and those with more severe fractures are likely to have multiple comorbidities and may have significant frailty adding to clinical complexity. 25 Care for people with OVF reflects this complexity and should be multifaceted and individualised; it can include pharmacological treatment together with a range of other interventions, such as education, surgery, spinal orthoses, physiotherapy and falls and pain management. 8,9 Treatment is directed both at the prevention of further fractures and at addressing the effects of OVF on function and QoL.
Lifestyle recommendations
Guidelines recommend that people with osteoporosis and at high risk of osteoporotic fractures are provided with advice about osteoporosis and a range of lifestyle measures to promote bone health and prevent falls. 9,30 These include ensuring adequate calcium and vitamin D intake through diet or with supplementation, stopping smoking and avoiding excessive alcohol consumption. 8,9,30 They detail the value of physical activity, of undertaking regular weight-bearing exercise and muscle strengthening exercise. 4,8,9,30 Over the last 3 years, information accessible to patients (via osteoporosis patient societies etc.) to support exercise recommendations has become more comprehensive. 30,31
Pharmacological treatment
Pharmacological bone-protective medications are central to the management of osteoporosis. Initiated when a high risk of fracture has been identified, the main aim of treatment is to reduce fracture risk. 8 Most work by regulating bone remodelling and may increase BMD [i.e. by inhibiting or slowing down the resorption of old bone (antiresorptive drugs) or by stimulating the osteoclast cells that build new bone (anabolic drugs)]. 32 High-quality evidence exists to support the effectiveness of most major osteoporotic medications in preventing fractures in postmenopausal women, but few data are available about potential benefits or side effects in men. 4,8 Medication is often more effective at preventing OVF than other types of fracture. 2,8
Frequently prescribed, mainly antiresorptive drugs are the bisphosphonates [alendronate (Fosamax®; Merck Sharp & Dohme, Kenilworth, NJ, USA), risedronate (Actonel®; Procter & Gamble Pharmaceuticals, Cincinnati, OH, USA), ibandronate (Boniva®, Genentech Inc., San Francisco, CA, USA, a subsidiary of F. Hoffmann-La Roche AG, Basel, Switzerland) and zolendronate (Reclast® and Zometa®; Novartis International AG, Basel, Switzerland], denosumab (Prolia®; Amgen Inc., Thousand Oaks, CA, USA), which is a monoclonal antibody against the major mediator of bone resorption, and raloxifene (Evista®, Eli Lilly and Company, Indianapolis, IN, USA), which is a selective oestrogen receptor modulator. 32 Common anabolic drugs include parathyroid hormone treatments such as teriparatide (Forteo®, Eli Lilly and Company, Indianapolis, IN, USA). 32 Previously widespread, hormone therapies are also available but are now used only in limited circumstances because of concerns about side effects. 8 The different osteoporotic medications have varying routes of administration, different antifracture efficacy at different sites and different side effects and cost profiles. 4,8,32 Although medications are an essential part of management, they do not address pain or deficits in back extensor muscle strength, balance or posture to improve function or reduce the risk of fracture owing to falls.
Surgical interventions
Surgery is used for a minority of patients after acute OVF, predominantly when conservative treatment is unsuccessful (i.e. when patients have severe, unremitting pain and significant disability). 33,34 Vertebroplasty and balloon kyphoplasty are the two main, minimally invasive surgical techniques employed,33 with newer cement augmentation techniques developing. 34 Vertebroplasty involves the injection of acrylic bone cement into the fractured vertebral body under fluoroscopic imaging guidance. During kyphoplasty, a deflated balloon is inserted into the compressed vertebral body, the balloon is expanded to correct the vertebral deformity and create a cavity, then the void is filled with bone cement. 33 Initial reports about these techniques were controversial, with significant complications such as cement extrusion and fracturing of adjacent vertebrae being documented;33 however, more recent studies report more positive results. 34–36 Current reviews acknowledge the inconsistent messages about the benefits of surgery and highlight the need for robust criteria when selecting patients for these procedures and for further research in the area. 33–35,37
Physiotherapy rehabilitation
Physiotherapy programmes for people with OVF and back pain encompass a wide range of interventions, such as exercise therapies that include varied strengthening, functional or balance training exercises, or ‘hands-on’ manual therapies, such as massage, taping and joint mobilisations. Alongside these, physiotherapists often provide education and substantial reassurance about osteoporosis in general, posture and how to exercise and carry out daily activities safely and efficiently. 38 Preliminary evidence exists to suggest that physiotherapy that addresses pain and physical impairments might have an important role in improving QoL and reducing fracture risk in people with OVF. 39 However, high-quality evidence for efficacy is lacking and which types of physiotherapy interventions might be most helpful is unclear. 39,40
The evidence used to develop the exercise and manual physiotherapy interventions investigated in the trial is summarised in Chapter 3.
The PROVE trial
In summary, there is some evidence that both exercise therapy interventions and manual therapy interventions can be beneficial for people with symptomatic OVF. Whether or not exercise therapy is more effective than manual therapy is unknown and further information is needed about short- and longer-term outcomes of each intervention.
The PROVE trial was commissioned to address these questions.
Research objectives
There are a variety of different packages of physiotherapy that could be used to treat people with OVF. At the start of the trial, the indication for different therapies was not well defined and the effectiveness of different intervention modalities was not known.
The primary objective of the PROVE trial was to:
-
undertake a definitive randomised controlled trial (RCT) to assess the effects of two physiotherapy approaches (exercise therapy and manual therapy), compared with a SSPT, on the clinical outcomes of people with symptomatic OVF.
The secondary objectives were to:
-
compare the clinical outcomes of exercise and manual therapies
-
assess the safety of and identify any significant side effects associated with the intervention programmes
-
investigate the acceptability of and adherence to the interventions
-
conduct a health economic analysis to assess the cost-effectiveness of the different treatment strategies from a NHS and Personal Social Services perspective
-
explore the experiences and views of people with OVF regarding their participation in the PROVE trial, their perceptions regarding the appropriateness and acceptability of the interventions and the factors influencing adherence to the intervention programmes.
Chapter 2 Methods
The protocol for the RCT was published in Trials in 2014. 39 The trial was delivered as published and there were no changes to the protocol.
Trial design
The PROVE trial was a pragmatic, multicentre, three-arm RCT with an adaptive design. The trial assessed the effects of a physiotherapy intervention based on exercise therapy and a physiotherapy intervention based on manual therapy, each intervention consisting of seven sessions of individual physiotherapy and a home programme, and these interventions were compared with a single 1-hour physiotherapy session for people with osteoporosis and a clinically diagnosed vertebral fracture. The trial flow chart is shown in Figure 1.
FIGURE 1.
The PROVE trial flow chart. Adapted from Barker et al. 39 © Barker et al. ;39 licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
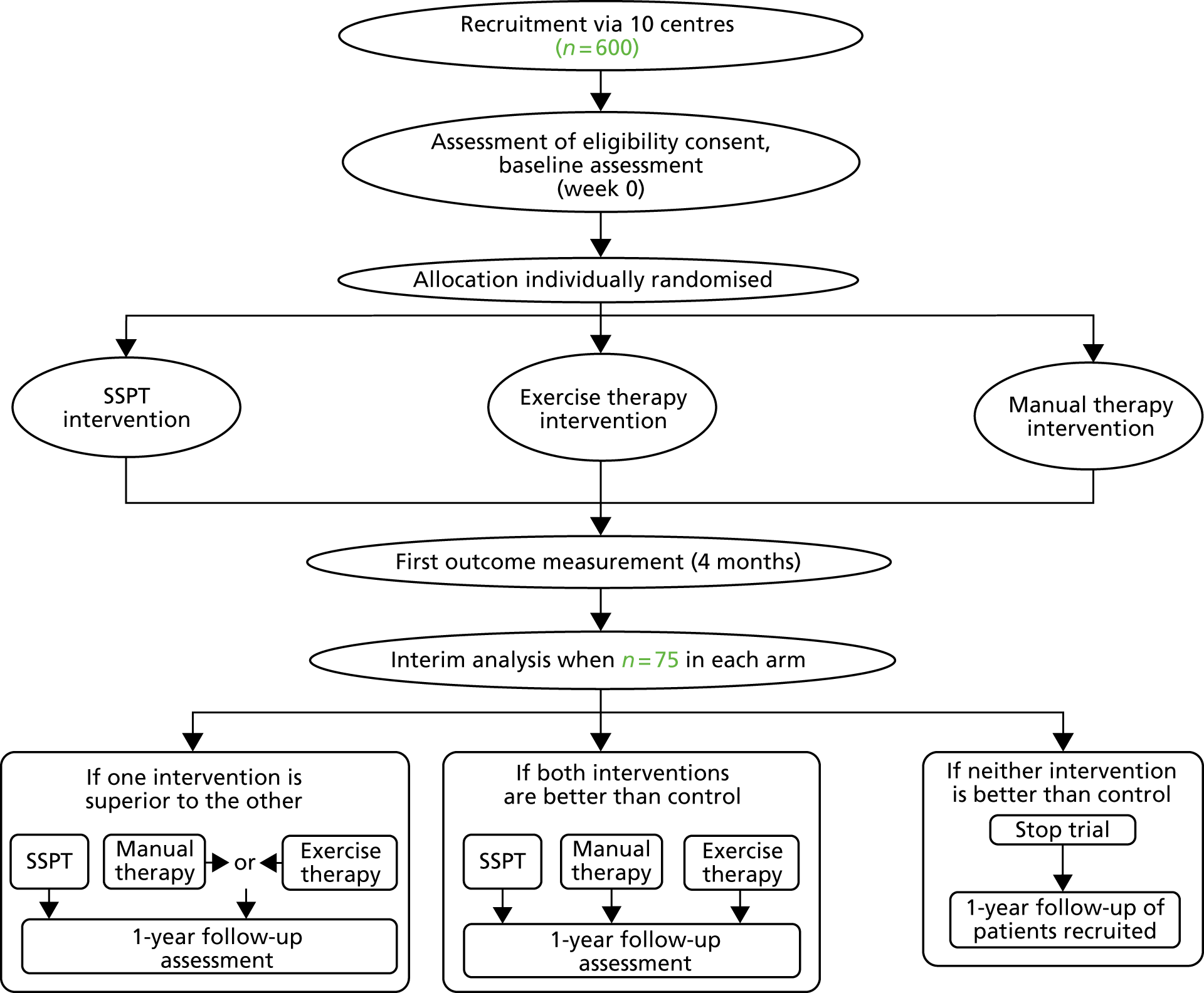
An interim analysis was planned and conducted when 75 participants were recruited to each arm and they had completed their 4-month follow-up. Following this interim analysis, the trial was assessed against prespecified rules to make a decision on whether or not the design should be adapted. These rules stated that if both intervention arms were sufficiently similar and showed advantage over the SSPT then the trial would not be adapted and recruitment would continue into both intervention arms. If one arm, exercise therapy or manual therapy, was not sufficiently promising relative to SSPT or sufficiently similar to the other intervention arm, then this arm would be dropped from the trial and the trial would be adapted to continue as a two-arm RCT with participants randomised between SSPT and the remaining intervention arm. If neither arm was sufficiently superior to the SSPT arm, the trial would be stopped. 39
Participants
Inclusion criteria
-
Patients who had a diagnosis of primary osteoporosis confirmed by a radiograph or DEXA scan (≥ 2.5 SD below the norm) at the lowest lumbar level.
-
Patients who had a history of at least one symptomatic OVF.
-
Patients aged ≥ 18 years.
-
Patients who were postmenopausal (if female).
-
Patients able to walk ≥ 10 m independently with or without an aid.
-
Patients able to understand and participate in the physiotherapy programme.
Exclusion criteria
-
Patients aged < 18 years.
-
Patients who had osteoporosis secondary to other metabolic bone disorders or disease (e.g. rheumatoid arthritis, cancer and osteomalacia), experienced lower-limb joint surgery, or fracture, in the previous 6 months.
-
Patients whose primary problem was back pain with pain radiating into the lower limb.
-
Patients who had undergone vertebroplasty, facet joint injection or any physical therapy (e.g. chiropractic, osteopathic or physiotherapeutic treatment for back pain in the previous 12 weeks).
-
Patients with severe unstable cardiovascular or pulmonary disease or significant psychiatric or neurological conditions that would preclude participation in the physiotherapy treatment arms.
Screening and recruitment
Patients were approached by clinical staff during routine clinic visits. They were provided with a patient information sheet (see Appendix 13) and asked if they would consider participating. A research physiotherapist would then recontact them at a later date to book an appointment at a research clinic. Here, the trial information was discussed, the patient had an opportunity to ask questions, eligibility was rechecked and, if appropriate, patients were consented. Participants were asked to give written informed consent in accordance with the principles of Good Clinical Practice41 and the Declaration of Helsinki. 42 At the time of consent, outcome assessors collected baseline measures.
Settings and locations
The trial was run in 21 NHS trusts (names at time of participation):
-
Cambridge University Hospitals NHS Foundation Trust
-
Countess of Chester NHS Foundation Trust
-
East Sussex Healthcare NHS Foundation Trust
-
King’s College Hospital NHS Foundation Trust
-
Nottingham University Hospitals NHS Foundation Trust
-
Oxford University Hospitals NHS Trust
-
Portsmouth Hospitals NHS Foundation Trust
-
Royal Devon and Exeter NHS Foundation Trust
-
Royal Orthopaedic Hospital NHS Foundation Trust
-
Royal Surrey County Hospital Foundation Trust
-
Royal United Hospitals Bath NHS Foundation Trust
-
Sheffield Teaching Hospitals NHS Foundation Trust
-
Solent NHS Trust
-
South Devon Healthcare NHS Foundation Trust
-
Southend University Hospital NHS Foundation Trust
-
Staffordshire and Stoke-on-Trent Partnership NHS Foundation Trust
-
The Ipswich Hospitals NHS Trust
-
The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust
-
University College London NHS Foundation Trust
-
University Hospitals of Leicester NHS Trust
-
Western Sussex Hospitals NHS Foundation Trust.
Interventions
Full details of the interventions are provided in Chapter 3, and are described briefly here.
All interventions were delivered by physiotherapists experienced in the treatment of spinal pain and/or osteoporosis. The treating therapists were independent of the recruitment and randomisation procedures. They attended a training session delivered by the trial team and received ongoing support and guidance regarding the intervention to ensure that quality standards were met. Physiotherapists were trained to deliver the a manual and exercise therapy interventions and the SSPT assessment and advice intervention and, wherever possible, different therapists within each department delivered each of the treatment interventions. Contamination was minimised through a variety of quality control measures. These included monitoring the treatment logs completed at each session, quality assurance visits to each therapist when they started delivering trial interventions and limiting access to trial materials to therapists and the participants randomised to each of the intervention arms. The rationale and protocol for the interventions were described in training and reference manuals.
Participants randomised to SSPT intervention had a single 1-hour session with a physiotherapist who gave general advice about disease management and lifestyle choices to promote bone health in line with the information available from the Royal Osteoporosis Society (ROS) at the time that the trial started.
Participants randomised to either of the intervention arms received an initial 1-hour assessment visit followed by up to a maximum of six one-to-one sessions in which a package of care based on either exercise therapy or manual therapy was delivered. The treatments were standardised, but it was considered important to allow therapists to personalise treatments as appropriate. For example, therapists were able to omit or adjust the intensity of any technique or exercise to reflect an individual participant’s capabilities and their progress. The treatments were delivered over a 12-week period. Participants in each intervention arm received education about osteoporosis and general advice about exercise, as in the SSPT arm.
Monitoring the intervention delivery
Attendance rates and content of treatment appointments were recorded by the therapist using treatment logs. Logs were completed for each session for all participants and returned to the trial co-ordinating centre. Close contact was maintained between the clinical research team and the therapy departments to address any problems that were highlighted by the treatment logs.
All sites were visited to ensure smooth implementation of the interventions within the trial. This quality control process involved the clinical research team auditing treatment logs and observing treatment sessions.
Outcomes
Follow-up data collection was by face-to-face clinical assessments at 4 and 12 months following randomisation. When face-to-face assessment was not possible, postal and telephone data collection methods were used to obtain self-reported core data.
In addition, a subset of the data were collected by postal questionnaire at 6 and 9 months. The outcome measures are detailed in Table 1.
| Tests | Week 0 | Month 4 | Month 6 | Month 9 | Month 12 |
|---|---|---|---|---|---|
| QUALEFFO-41 | ✓ | ✓ | By post | By post | ✓ |
| EQ-5D-5L | ✓ | ✓ | By post | By post | ✓ |
| Physical Activity Scale for the Elderly | ✓ | ✓ | By post | By post | ✓ |
| TLS test | ✓ | ✓ | ✓ | ||
| Flexicurve ruler | ✓ | ✓ | ✓ | ||
| Short Performance Physical Battery | ✓ | ✓ | ✓ | ||
| Functional reach test | ✓ | ✓ | ✓ | ||
| 6MWT | ✓ | ✓ | ✓ | ||
| 10-point VAS | ✓ | ✓ | ✓ |
There were two primary outcome measures for this trial, one measuring QoL and one measuring physical function:
-
The QUALEFFO-41 (Quality of Life Questionnaire of the European Foundation for Osteoporosis – 41 items) is a disease-specific measure of health-related quality of life (HRQoL) applicable to patients with established vertebral osteoporosis. It is a self-administered questionnaire that provides scores on five domains (pain, physical function, social function, general health perception and mental performance) and a total score. It is validated and reliable and has been shown to be responsive in clinical trials of physiotherapy treatment. 39,43 Both the total score and the scores in the separate domains were used; the lowest QoL score in every domain corresponds to a total of 100 points and the highest is reflected by a total score equal to 0.
-
The timed loaded standing (TLS) test assesses combined shoulder and back extensor muscle endurance in people with osteoporosis and OVF. 44 It records the time in seconds that a person can stand with both arms extended and shoulders flexed to 90° holding a light (0.5-kg or 1-kg) dumbbell in each hand. Initial work has shown it to be well tolerated and easy to administer by a physiotherapist in a clinical setting and to have good concurrent validity and reliability. 44–46 Formal work on the minimally clinically important difference (MCID) in TLS test time has not been conducted.
Secondary outcome measures:
-
Thoracic kyphosis was measured using a flexicurve ruler. This provided a measure of the Flexicurve kyphosis index and of the Flexicurve kyphosis angle and allowed a calculation of an approximate Cobb angle or thoracic kyphosis angle (degrees). 47
-
The Short Physical Performance Battery (SPPB) was used to assess lower-extremity physical function. 48
-
The functional reach test (FRT) was used to specifically evaluate standing balance. 49
-
A 6-minute walk test (6MWT) at self-selected speed (resting as required) was used to measure exercise capacity and walking endurance. 50
-
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L),51 is a short, generic measure of HRQoL and was used to assist comparison with other conditions and assessment of health economics.
-
Participants were also asked to complete the Physical Activity Scale for the Elderly (PASE) to assess activity levels in the previous week. 52
Other outcomes included measures of balance, falls, mobility and physical activity: all areas affected by OVF. Each test is reliable and valid, has been used with older, community-dwelling adults and has been shown to be responsive in previous rehabilitation studies.
Information about relevant physical characteristics, namely age, height, weight, BMD, walking aid use and number and site of osteoporotic vertebral fractures and other fractures, was collected at baseline. Comorbidity data were also collected to provide more complete information about this population.
Randomisation
Randomisation was via a central telephone randomisation service at the Warwick Clinical Trials Unit, University of Warwick.
Sequence generation
The variable block randomisation schedule was prepared by the trial statistician. Randomisation was stratified by centre to control for any confounding factors evident at local recruitment sites.
Allocation concealment
The research clinician telephoned the randomisation service, and only once the participant was registered in the trial was the random allocation generated; hence, allocation was concealed. Allocation was sent directly to the therapist and the participant was informed at the appointment or when their first appointment was arranged.
Blinding
The outcome assessor was blind to the allocation arm of the participant and was independent of intervention delivery. Participants were requested to not disclose their allocation to the outcome assessor. If an outcome assessor was unblinded, this was recorded. If they remained unblinded at each research visit, the assessors were asked to guess which allocation the participant had been given. The participants and therapists could not be blinded to the allocation arm.
Sample size
The initial sample size calculation was based on a traditional approach to a three-arm trial. We proposed to detect a standardised effect of 0.4 in the QUALEFFO-41, at 80% power and an alpha of 0.05, which would require 180–200 participants in each arm, or 540–600 in total.
In the proposed adaptive design, the power of the trial may be defined to be the probability that, given a truly effective intervention, an intervention remains in the trial at the interim analysis, and leads to a significant result compared with the SSPT in the final analysis. The specified sample size was chosen to give 94% power if the better of the two intervention arms had a true standardised treatment effect of 0.4.
The decision rule and sample size for the interim analysis was chosen to ensure that the power was high and the probability of continuing with an ineffective treatment was sufficiently low. If the true (unknown) treatment effect for an intervention is equal to SSPT, the probability of dropping that intervention at the interim analysis was approximately 73%. If neither intervention was truly superior to SSPT, the probability of stopping the entire trial at the interim analysis was approximately 60%. Based on this interim analysis sample size, the standard error (SE) of the estimated difference between the intervention arms and SSPT at the interim analysis would be 0.82.
The assumptions underlying the sample size calculation were monitored by the Data Monitoring and Ethics Committee (DMEC) throughout the trial.
Statistical methods
Participants were analysed in accordance with the intervention arm to which they were randomised, regardless of the intervention that they received [intention-to-treat (ITT) analysis],53 with pairwise comparisons between the SSPT arm and each of the two intervention arms.
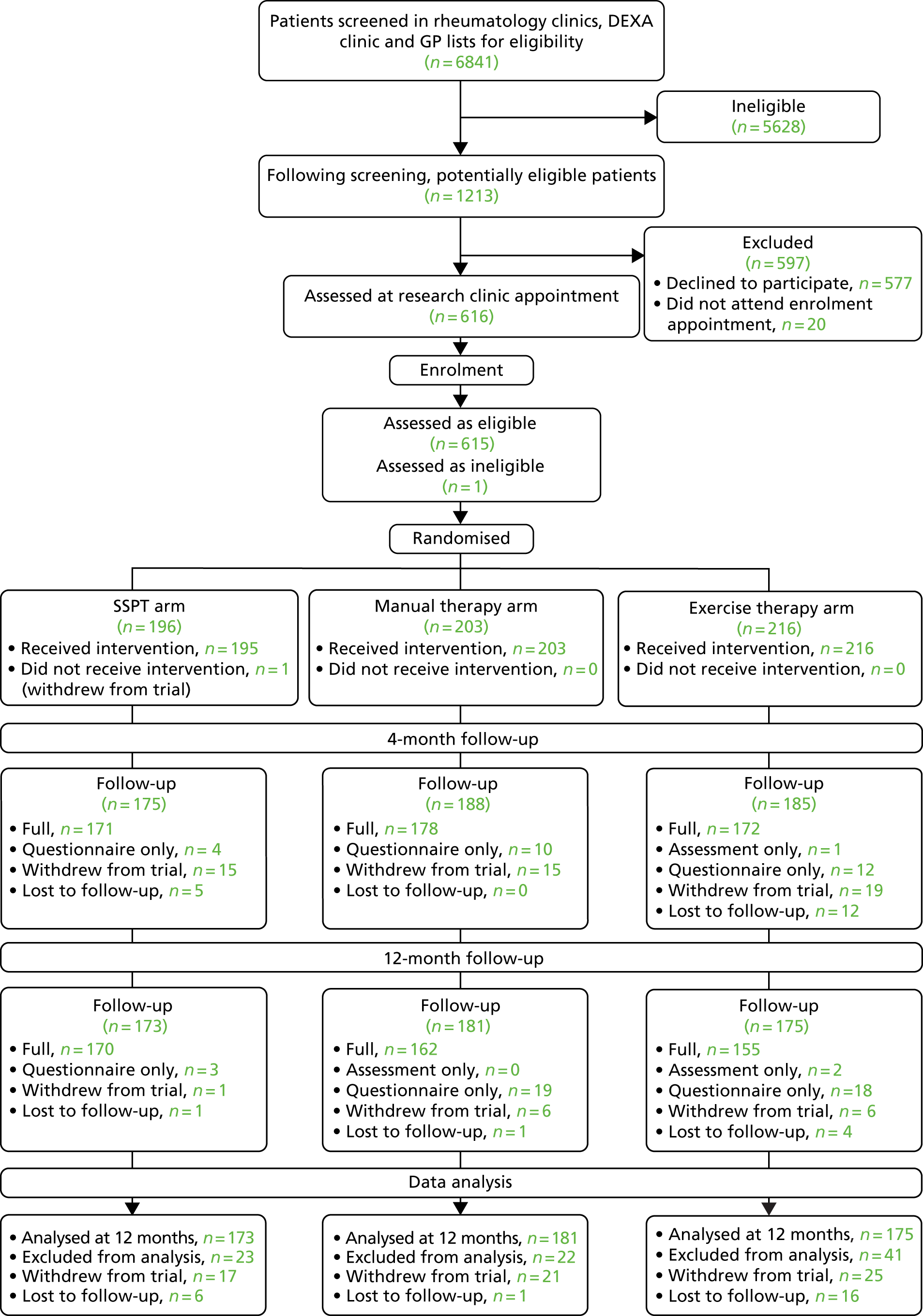
Analyses were guided by an analysis plan prepared before data were available. A Consolidated Standards of Reporting Trials (CONSORT) diagram was produced54 (Figure 2). All analyses were conducted using R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria), with the exception of the complier-average causal effect (CACE) analysis, which was conducted using Stata®/SE version 15.0 (StataCorp LP, College Station, TX, USA).
FIGURE 2.
The PROVE trial CONSORT flow diagram.
Database and data processing
Project data were stored in a Microsoft SQL Server 2012 Enterprise Edition (version: 11.0.6251.0, Microsoft Corporation, Redmond, WA, USA); the database was hosted in the University of Warwick’s data centre and rules were imposed for data entry, which included valid range for responses, linked dates and participant identification numbers.
Data were single entered into the database by trial personnel. The trial statistician carried out checks of the plausibility of values, missing data and form return rates to enable further queries to be resolved prior to freezing data for scheduled DMEC reports and analysis.
For data quality assessment, 10% of all forms at all time points were randomly selected for data checking on a 2-monthly basis. All disagreements found when checking were corrected and any systematic faults found as a result of the checks were also corrected.
Data set access
The final data set was accessible to all trial members after data lock. The chief investigator assumed overall responsibility for the data report and had full access to the trial data set. There were no contractual agreements that limited access for investigators.
Scoring and missing items
The QUALEFFO-41 score and scores for individual domains were calculated in accordance with formulae given by the International Osteoporosis Foundation. 55 The total score was considered missing if all questions were missing, with domain scores considered missing if all questions were missing for that domain.
The EQ-5D-5L was used mainly for economic analyses evaluation, the methods and results of which are described in Chapter 6.
Interim analysis
The interim analysis decision rule was based on a comparison of the estimated mean change from baseline in the QUALEFFO-41 score for each of the three trial arms.
Although the integrity of the trial in terms of type I error rate control does not require prespecification of the decision rule to be used at the interim analysis, the below rule was proposed prior to the start of the trial to decide which intervention arm(s) should continue to be included along with the SSPT in the second stage of the trial. This rule ensured that the interventions would continue only if they were sufficiently promising relative to the SSPT, and that the most promising would be chosen to continue alone along with the SSPT unless both were sufficiently promising and appeared to be of similar efficacy, in which case both would continue:
-
If the mean change from baseline of the QUALEFFO-41 score for an intervention arm was not > 0.5 points greater than that of the SSPT, that intervention arm would be dropped from the trial. Note that under this rule both intervention arms might be dropped, in which case the trial would be stopped because of futility.
-
If the mean change from baseline of the QUALEFFO-41 score for one intervention arm was > 2 points higher than for that of the other arm, the intervention with the lower mean change from baseline would be dropped from the trial.
Demographic and baseline measurements
Demographic characteristics, clinical characteristics and baseline measurements were presented to evaluate the comparability of intervention arms and generalisability to clinical settings. Count data were presented as percentages and continuous data were presented as means and SDs in each arm.
Primary end-point analyses
The analysis of the co-primary end points of change in QUALEFFO-41 score and TLS test time from baseline at 12 months was conducted to allow for the adaptive design used and to allow for the multiple comparisons arising from the two pairwise comparisons between the comparator SSPT arm and the two experimental intervention arms.
Hypothesis tests were conducted using the method proposed by Bretz et al. ,56 modified by Friede et al. ,57 to allow for the use of an early end point, in this case the change in QUALEFFO-41 score from baseline to 4 months, at the interim analysis. Details of the method are given in Appendix 24. The analyses of 12-month change in QUALEFFO-41 and TLS test were based on linear models to test for treatment effects, adjusting for age (dichotomised into ≤ 70 years and > 70 years), number of baseline spinal fractures (dichotomised into two or fewer and more than two), recruitment centre and baseline score for either the QUALEFFO-41 or the TLS test.
Unbiased estimates of differences between each intervention arm and a SSPT, adjusting for the adaptive design used, were based on the method proposed by Stallard and Kimani,58 with corrected confidence intervals (CIs) calculated using the method proposed by Magirr et al. 59 These were presented alongside means from each arm unadjusted for baseline or centre and uncorrected for the adaptive design used.
Secondary end-point analyses
For all secondary end points other than number of falls and falls frequency, changes in secondary end points from baseline to 4 and 12 months (including 4-month changes in the co-primary end points) for the SSPT arm were compared with each intervention arm. Hypothesis tests and estimated treatment differences were obtained using linear models adjusting for baseline value and centre, and these were presented alongside unadjusted means from each arm.
The number of falls in the previous year recorded at 4 and 12 months was analysed using a Poisson regression model, adjusting for baseline number of falls and recruitment centre. The frequency of falls was dichotomised into rarely (one or fewer per year) and sometimes (one or fewer per month) or frequently (one or more per week) and analysed using a logistic regression model, adjusting for baseline frequency and recruitment centre.
Subgroup analyses
Analyses were conducted to investigate subgroup effects specified prior to the data analysis being conducted. The subgroups investigated were sex, age (≤ 70 years vs. > 70 years) and number of spinal fractures at baseline (two or fewer vs. more than two). In each case, linear models adjusting for baseline QUALEFFO-41 or TLS test score and centre were used to test for an intervention*baseline interaction effect and then to estimate and test treatment effects within each subgroup.
Sensitivity analyses
As a sensitivity analysis, we compared demographic and baseline characteristics for participants who had complete baseline and 12-month QUALEFFO-41 or TLS test data and for those for whom this was missing at the 12-month time point. We also compared data from the different recruitment centres.
Data from participants recruited in stage 1 (i.e. with data included in the interim analysis) and data from those recruited in stage 2 were also compared.
Complier-average causal effect analysis
A prespecified CACE analysis was conducted to determine if the level of compliance with the exercise programme affected participant outcomes as measured by the QUALEFFO-41 and TLS test (primary outcome). The CACE estimates were calculated using the instrumental variable method described by Dunn et al. 60 The model adjusted for baseline and recruitment centre, with CIs calculated using a bootstrap approach. No adjustment was made for the adaptive design used.
The analysis was conducted using thresholds of four or more sessions (partial compliance) and the maximum of seven sessions (full compliance). Estimates were obtained when ≥ 50% of participants in each of the intervention arms were classified as compliers.
Monitoring and approval
Formal approvals
The PROVE trial was approved by South Central Research Ethics Committee in June 2012 (Research Ethics Committee reference 12/SC/0411) and by the research and development departments of each participating centre. The final approved trial protocol has been published. 39 A first substantial amendment was granted on 4 January 2013, in which the participant consent and information leaflets were reviewed and approved. A second substantial amendment was approved on 28 March 2014 to update the participant information leaflet, the reconsent form and the letter to participants. A third substantial amendment was granted on 10 September 2014 to update the letter to participants from general practitioners (GPs). A fourth substantial amendment was granted on 22 April 2015 to update the participant reply slip.
Trial Steering Committee
A Trial Steering Committee (TSC) was responsible for monitoring and supervising the progress of the PROVE trial towards the interim and final milestones. The TSC consisted of three independent experts, a lay member and leading members of the Trial Management Group (TMG). Membership of the TSC is detailed in Acknowledgements.
Data Monitoring and Ethics Committee
The DMEC was independent of the trial and was tasked with monitoring ethics, safety and data integrity. The trial statistician provided data and analyses requested by the DMEC at each of the meetings. Membership of the DMEC is detailed in Acknowledgements.
Trial Management Group
A TMG was responsible for the day-to-day management of the trial; it consisted of the chief investigator, research fellow, statistician and trial co-ordinator. They ensured the overall integrity of the trial, compliance with the protocols, the welfare of all participants and that the trial was appropriately reported.
Chapter 3 Intervention description and rationale
Introduction
This chapter describes the multifaceted approach taken to produce the interventions for the experimental and control arms of the PROVE trial and provides details of each intervention. The Medical Research Council (MRC) guidelines for developing and evaluating complex interventions61 and the Template for Intervention Description and Replication (TIDieR) checklist62 highlight the importance of reporting interventions in sufficient depth to enable clinicians and other researchers to implement, replicate and build on research findings, and were considered in the account below.
Overview of the development process
The work of developing, testing and refining interventions took place over an 18-month period. The people involved included specialist physiotherapists and medical consultants working with people with osteoporosis, physiotherapy service managers, physiotherapy and health economic researchers and patient and public members with interest in and experience of osteoporosis and musculoskeletal research and connections to the ROS. We were supported in this development work by collaboration with the Fracture Reduction in South Central Policy (FRiSCy) network: this is a multidisciplinary clinical network of 11 NHS trusts in the south central region of England with an interest in secondary fracture prevention.
Figure 3 displays the information sources and processes involved. The HTA commissioning brief provided initial direction to the intervention development [e.g. it specified a trial of physiotherapy (not tai chi, yoga, osteopathy, etc.) compared with an attention/education control]. As with all NIHR research, the study went through an extensive peer review process before being commissioned and was adapted in response to feedback (e.g. regarding treatment dose).
FIGURE 3.
Intervention design sources and process.
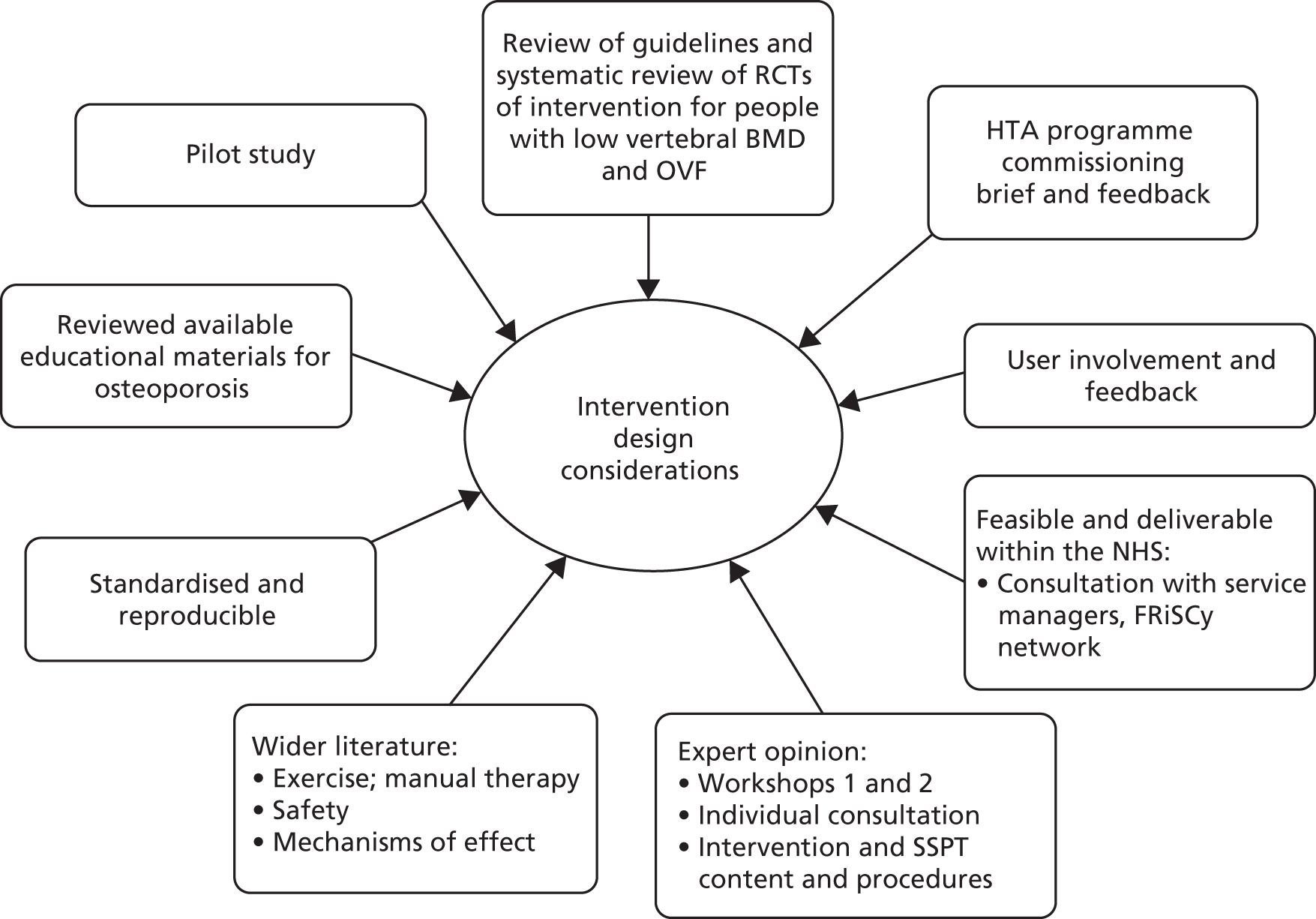
We considered existing reviews and guidelines and undertook a systematic review of RCTs that evaluated the effects of physiotherapy for people with OVF (Table 2). We also examined the wider clinical evidence base regarding manual physiotherapy treatments and exercise interventions for older people and people with back pain. This included examining specific issues such as treatment safety, exercise intensity and progression and adherence. A range of educational materials for people with osteoporosis was reviewed. 3,31,77,78
| Trial (authors, publication year; country; setting) | Study design | Population characteristics | Intervention | Measure timeline and outcomes used | Main findings |
|---|---|---|---|---|---|
| Bautmans et al.63 2010; Brussels, Belgium; outpatient physiotherapy clinics | RCT | Women (n = 48) with vertebral osteoporosis with and without OVF
|
12 weeks (18 sessions plus HEP):
|
Baseline and 12 weeks Thoracic kyphosis C7–T12 Spinal Mouse hand-held inclinometer (degrees), back pain in previous week – VAS (0–100 mm) and QUALEFFO-41 |
Kyphosis improved significantly in IG vs. CG (ITT analysis) and by mean of 7.1 ° (SD 1.9 °) in those who were compliant vs. controls/non-compliant. QUALEFFO-41 mental health was significantly worse in both IG and CG, no difference in other QUALEFFO-41 outcomes of pain |
| Bennell et al.64 2010; Australia; outpatient physiotherapy clinics | Pilot RCT | Adults (n = 20): 3 men, 17 women with osteoporosis and at least one painful OVF
|
10 weeks (10 sessions plus HEP)
|
Baseline and week 11 Back pain on VAS scale (0–10 points), global change in back pain (1–5 scale), AQoL, QUALEFFO-41, TUG, TLS test – back endurance and thoracic kyphosis: Dualer Electric Inclinometer (North American Fork, UT, USA) |
No significant difference in QoL on total QUALEFFO-41 or AQoL. The IG significantly decreased in pain at rest and movement vs. CG (VAS pain, QUALEFFO-41 pain). The IG had significantly longer endurance on TLS test compared with CG and better QUALEFFO-41 physical function. No significant difference in kyphosis, TUG or other QUALEFFO-41 outcomes |
| Bergland et al.65 2011; Norway; hospital physiotherapy gym | RCT | Women (n = 89) with osteoporosis and least one OVF:
|
12 weeks (25 sessions):
|
Baseline and 3 and 12 months MWS of > 20 m, TUG, FRT, QUALEFFO-41 and sum score of the GHQ-20 |
At 3 months, significant improvements for IG vs. CG in MWS, TUG, QUALEFFO-41 mental function and GHQ-20. At 12 months, significant improvements for IG vs. CG in MWS, TUG, QUALEFFO-41 total, QUALEFFO-41 mental function and pain. No change in FRT |
| Olsen and Bergland66 2014; Norway; hospital physiotherapy gym | RCT | See Bergland et al. 2011 | See Bergland et al. 2011 |
Baseline and 3 and 12 months Fear of falling (FES-I Norwegian) and number of falls |
IG: significant reduction in fear of falling at 3 and 12 months. Number of falls reduced but not different between groups |
| Bergström et al.67 2011; Sweden; hospital physiotherapy gym | RCT | Women (n = 36) with osteoporosis, kyphosis and at least one OVF
|
16 weeks (32 sessions):
|
Baseline and 4 months Height (stadiometer), BES in standing (isometeric dynamometer), spinal posture (kyphometer) and thoracic expansion (cm) |
Back strength on ITT analysis showed no significant effects for IG vs. CG. Per-protocol analysis adjusted for BES at baseline: IG was significantly better than CG. Height and thoracic expansion significantly improved in IG vs. CG. No change in kyphosis |
| Gold et al.68 2004; North Carolina, USA; retirement communities |
RCT, modified crossover Phase 1 was cluster randomised |
Women (n = 185) with osteoporosis and at least one OVF:
|
6 months (120 sessions):
|
Baseline and 3, 6, 9 and 12 months BES: peak isometric torque (foot pounds): Isokinetic dynamometer (B-200 Isostation) (Isotechnologies, Inc., Hillsborough, NC, USA), pain with activities (FSI) and psychological symptoms (GSI) |
Phase I: IG vs. CG significantly improved in BES, psychological symptoms but not pain. In phase 2 (self-maintenance), strength declined and psychological symptoms/pain were unchanged |
| Grahn Kronhed et al.69 2009; Sweden; hospital physiotherapy gym | RCT | Women (n = 73) aged 60–81 years with vertebral osteoporosis with and without OVF
|
16 weeks (32 sessions):
|
Baseline, 4 months and 1 year SF-36, QUALEFFO-41, back pain (VAS scale 0–10 cm), number of falls, eyes open and closed SRT, single-leg stand with eyes open and closed, walk forward/back along line, dynamic posturography (Equitest®, NeuroCom, San Carlos, CA, USA), grip strength, kyphosis: C7 to wall (cm) and the Physical Activity Scale |
IG vs. CG significantly improved in worst pain on VAS, mental component of SF-36, SF-36 pain, physical function, general health and social function. Time to first fall later in IG. No difference in QUALEFFO-41 total or subscales, other VAS pain, balance, grip, kyphosis, physical activity scale or falls |
| Hongo et al.70 2007; Akita, Japan; home | RCT | Women (n = 80) with vertebral osteoporosis with and without OVF:
|
16 weeks:
|
Baseline and 16 weeks JOQOL, back extension peak isometric strength (kg) with HHD (digital force gauge) and spinal curvature with digital inclinometer |
QoL on JOQOL was significantly better for the IG vs. CG. The IG also had a significant increase in BES vs. CG. No difference in spinal ROM. Change in JOQOL and BES was significantly correlated |
| Kanemaru et al.71 2010; Tokyo, Japan; home | RCT | Women (n = 63) with vertebral osteoporosis with and without OVF:
|
1 year; one overnight hospital stay plus 3-monthly physiotherapy review:
|
Baseline and 3, 6 and 12 months Strength in right and left legs (Nm/kg), TUG, timed SLS, 10-m walk speed (m/minute), grip strength (kg) – HHD, total daily steps (pedometer), SF-36, lumbar (L2–L4), BMD (DEXA) and fracture rates |
At 1 year, significantly improved grip, walking speed and SF-36 physical function in IG but walking speed, SLS and grip improved in CG. No change in other measures. 86% complied, per-protocol analysis only |
| Madureira et al.72 2007; São Paulo, Brazil; community and home | RCT | Women (n = 60) with vertebral osteoporosis with and without OVF:
|
40 weeks (40 sessions plus HEP):
|
Measures: baseline and 12 months Balance – Berg Balance Scale, CTSIB, TUG, fracture rates and fall rates |
At 1 year, there were significant improvements for the IG vs. CG in balance (BBS and CTSIB) and number of falls. Adherence: 60% classes and 77.7% HEP at least once/week. No change in TUG |
| Madureira et al.73 2010; see Madureira et al.72 2007 | RCT | See Madureira et al.72 2007 | See Madureira et al. 2007 | OPAQ | At 1 year, there were significant improvements for the IG vs. CG in OPAQ in well-being, physical function, psychological status and social function domains |
| Malmros et al.74 1998; Denmark; hospital physiotherapy gym | Assessor-blinded RCT | Women (n = 53) with osteoporosis, at least 1 OVF and back pain, aged 55–75 years:
|
10 weeks (20 sessions plus HEP):
|
Baseline and 5 and 10 weeks QoL questionnaire only – week 22, pain VAS score (0–10), analgesia use, Modified Oswestry, Danish QoL questionnaire, Postural control Sway index (Chattex Balance System), isometric strength back extensors, abdominal flexors and quadriceps (kg) – strain gauge dynamometer |
The IG had a significant reduction in pain and analgesia by week 10. Danish QoL significantly improved by weeks 5, 10 and 22. Significantly improved quadriceps strength for IG at week 5 only. No difference in balance, BES, trunk flexor strength or ODQ in weeks 5 or 10 |
| Papaioannou et al.75 2003; Ontario, Canada; home | Assessor-blinded RCT | Women (n = 74) with osteoporosis and OVF: 6 (8%) with one OVF, 68 (91%) with multiple fractures:
|
26 weeks (seven physiotherapy sessions at home plus ongoing HEP three times a week)
|
Baseline and 6 and 12 months OQLQ, SIP, balance – lateral and anteroposterior displacement, velocity of movement using force plate, TUG and lumbar (L2–L4) BMD (DEXA) |
At 6 months, the IG significantly improved on OQLQ symptoms, emotion and leisure. This did not continue at 12 months. At 12 months only, the IG had significantly improved balance. There was no difference in SIP or TUG at 6 or 12 months or BMD at 12 months. Adherence declined from 62% to 46% at 12 months |
| Yang et al.76 2007; Sichuan, China; West China Hospital inpatients | RCT | Women (n = 30) with vertebral osteoporosis and at least one OVF:
|
4 weeks (20 sessions). Inpatient and outpatient treatment:
|
Baseline, end of weeks 1 and 4 Pain (100 mm on VAS), TUG and TUFB |
No significant difference between groups in pain – reduced in both groups over time. No difference between groups at week 1 in function. At week 4, the IG was significantly faster on the TUG and TUFB then the CG |
Alongside this, we consulted with NHS physiotherapy service managers (n = 12), rheumatologists (n = 10) and GPs (n = 8) to help scope and define usual care for people with osteoporosis and OVF and to gather information about what would make the interventions feasible and deliverable within the NHS. Expert clinicians, managers and users were involved through workshops and were approached individually to help develop and modify the proposed intervention content, assessments and participant- and therapist-facing materials associated with each trial arm. They helped to differentiate and form the treatment interventions into two coherent programmes of outpatient physiotherapy based on either exercise or manual therapy principles and to shape the educational content in the SSPT arm. All trial interventions were standardised and fully manualised to provide clear, documented pathways for assessment, intervention prescription, progression and tailoring to individual patient’s needs. Subsequent testing took place in a pilot study between September 2013 and October 2014 in the outpatient physiotherapy department of the main trial site.
Rationale for the interventions
Rationale for single session of physiotherapy
Guidelines for the management of osteoporosis and fragility fracture focus on areas such as diagnosis and prescription of bone-protective medication, but also recommend that those at high risk of osteoporotic fractures receive education about osteoporosis and lifestyle advice to promote bone health. 8,78,79 Physiotherapy is acknowledged as an important component of rehabilitation after fracture but no specific recommendations about physiotherapy treatment are provided. 78
The commissioning brief from the HTA 10/99 stipulated that the trial should include a control or comparator arm that consisted of simple advice, an attention control and secondary prevention of fractures in line with National Institute for Health and Care Excellence (NICE) guidance (Medication). 80 Prior to the trial, discussions with members of the FRiSCy network confirmed that relatively few people with OVF were referred for physiotherapy; most would be seen in primary care by their GP and started on bone-protective medication, some were given lifestyle advice, some might be provided with written information and some were directed to the ROS for information. Differences in usual care and education provided between clinicians and between areas were apparent. Consequently, to ensure that all participants received recommended education and advice a standardised intervention was developed as a comparator arm.
Rationale for manual therapy
Previous research suggests that there are multiple factors that contribute to the development of back pain and spinal deformity, such as hyperkyphosis in people with osteoporosis. 23,81,82 Physiotherapists use manual techniques, such as spinal mobilisations, soft-tissue mobilisation or taping, to address modifiable elements, such as soft-tissue restrictions and decreased muscle length, with the aim of providing pain relief, improving spinal motion and posture and physical function. 63,64,75,83,84 Manual techniques have been examined in two small RCTs of people with osteoporosis and back pain and some positive effects have been reported on thoracic kyphosis,63 pain and QoL. 64 However, each of these trials was underpowered, and manual therapies were combined with other techniques, making it difficult to determine their effectiveness.
Spinal mobilisations
Physiotherapists routinely use spinal segmental mobilisation to treat back pain and reduced spinal range of movement (ROM). Given the prevalence of osteoporosis and back pain, it is likely that many physiotherapists have used these techniques with patients with compromised bone health. 84 However, specific evidence for the effectiveness and safety of mobilisations in the management of people with osteoporosis and back pain is limited, and physiotherapy guidelines suggest caution. High-velocity spinal manipulation techniques are contraindicated85 and concerns about the use of low-velocity spinal mobilisation techniques have been expressed, but practice surveys, case reports and RCTs suggest that these techniques are used and can be used safely. 63,64,75,86,87
The characteristic deformity seen in vertebral osteoporosis is the anterior wedge or compression fracture. Movements that increase load on the anterior vertebral body, such as flexion and rotation, are thought to have increased fracture risk and actions that reduce load on the anterior vertebral body, such as extension, reduced fracture risk. 88 Manual techniques, such as posterior–anterior accessory mobilisations, promote spinal extension and have been used safely in previous trials. 63,64,85 Restoring thoracic mobility and reducing pain is thought to normalise central sensory input from paraspinal muscle spindles and joints, and, hence, may reduce central inhibition and facilitate gains in back muscle strength in response to loading. 83
Postural training
In addition to other physiotherapy treatments, several studies incorporate postural training. Although detailed descriptions are lacking, common elements include education and feedback about posture, teaching self-correction of posture, practising functional daily life tasks with optimal alignment and postural taping. 63–65,68,89,90
Postural taping over the paraspinal muscles with an extension bias aims to increase proprioceptive feedback about alignment, reduce pain and facilitate back extensor muscle activity, thereby improving thoracic extension and balance. 63,64,91,92 A RCT of 15 osteoporotic women with OVF reported that a single session of postural taping significantly reduced thoracic kyphosis but had no immediate effects on balance or trunk muscle activity. 91 A case study of acute OVF [lumbar vertebral level (L) 1 management] combined thoracolumbar taping with extension bias with other conservative treatments and reported no complications with taping and improved pain and function. 93 Two small RCTs of people with OVF that combine postural taping with other manual interventions report reduced thoracic kyphosis63 and improved pain, QoL and back strength. 64 One trial reported an adverse event of skin irritation. 63
Soft-tissue mobilisation
Soft-tissue mobilisation, or massage, is a widespread treatment for chronic back and musculoskeletal pain that is primarily used to relieve pain but is also used to improve tissue mobility and muscle function. 94,95 It can encompass a range of manual techniques including slow stroking movements (effleurage), kneading, wringing and skin rolling movements (petrissage), penetrating pressure from fingertips with small focal movements (frictions) and similar myofascial trigger-point techniques in which digital pressure is applied to focal, symptomatic areas of soft tissue. 86,94
Soft-tissue mobilisation is recognised as a safe modality with a low risk of adverse events. In 2011, a RCT that compared massage with a SSPT for people with chronic back pain suggested that massage may provide benefits in pain and function that persist for up to 6 months. 96 A systematic review in 2002 also found evidence that massage is beneficial in reducing pain intensity and improving function in people with chronic back pain. 94 However, other reviews highlight the lack of robust studies in the area and suggest that evidence of effectiveness is less conclusive. 95 Few studies have been completed in people with osteoporosis, although surveys, case reports and RCTs show that these techniques are used in combination with other therapies. 64,86,90,97 In 2010, a pilot RCT of 20 patients with OVFs found that a package of physiotherapy that included soft-tissue massage significantly improved QoL, pain and back muscle endurance. 63
Stretches
Restrictions to thoracic and lumbar extension, cervical glide, shoulder flexion and abduction are associated with hyperkyphosis and OVF. 68,86,89 Stretches are a common feature of exercise programmes for people with vertebral osteoporosis that aim to improve flexibility, but unfortunately they are often poorly described and quantified (e.g. ‘stretches’74,75,98–100 and ‘stretch of target muscles’101). They can encompass more ‘static’ passive stretching techniques63 or more active ROM exercises or mobilising movements. 70 Passive stretches to promote thoracic extension are part of the home exercise programme (HEP) in RCTs of individual physiotherapy sessions that include manual therapy. These trials report benefits in terms of spinal posture,63 pain, QoL and back strength. 64
Rationale for exercise therapy
Exercise interventions for people with vertebral osteoporosis or osteopenia range from simple individual back extension exercises to a mix of weight-bearing exercises, balance exercises and progressive strength training interventions. 67,70,72,74,76,99,101 Common elements include exercises to improve cervical glide, scapula retraction, shoulder flexion, trunk extension, hip and knee extension and ankle dorsiflexion. Exercises and activities that increase the load on the vulnerable anterior vertebral body (i.e. exercises that involve excessive or dynamic spinal flexion or rotation), high-impact loading and overhead compression activities are not suitable for people at high risk of recurrent OVF. 38
Exercise interventions have been delivered individually,76 in a class format,65,67–69,99,100,102–106 as a HEP70,101 or as a combination of physiotherapist-led exercise and a HEP. 74,75
Exercise has also been found to positively affect bone mass and low BMD, particularly when intervention durations are prolonged. 107–113 Whether or not exercise can reduce the incidence of OVF remains uncertain. 114 Some studies report improvements in OVF risk and in factors associated with OVF risk but the lack of RCTs with appropriate measures and limited long-term follow-up in most studies make it difficult to determine the impact of exercise on vertebral fracture rates. 114,115
Type of exercise
The optimal type and duration of exercise for this population is uncertain. 109,112 At the inception of the trial, there was some evidence that programmes that combine strength training exercise with weight-bearing aerobic exercise, such as walking and stair climbing, were more effective for preserving bone strength and could positively influence lumbar BMD, physical function and QoL. 69,108,112,113,116
Strength training
Strength training exercises should be targeted to load muscle and bone at affected sites and progressed. 79 Illustrating this, graded trunk extension and lumbar stabilisation exercises are found in several RCTs of people with low vertebral BMD that report improved back extensor strength,68,70,101 pain and QoL. 67,69,70,101,103 Whether or not strengthening back extensor muscles prevents future fracture and whether or not these effects can be sustained is unclear. Few RCTs collect long-term outcomes and none collect information about fractures. 15,40,108
Balance training
Strategies to address falling are an important component of any treatment programme for this patient group. 24,28,117,118 There is evidence that balance training is effective in reducing fall risk. 72,104,106 However, reviews point out that interventions that combine strength training, weight-bearing aerobic exercise and balance exercises may be more effective than single-element interventions for people with low BMD. 108,119
Multimodal interventions
To date, few trials investigate multimodal exercise interventions that combine weight-bearing, strength and balance activities. The RCTs that have been completed report that multicomponent balance and progressive strength training programmes produce better results in terms of maintaining leg strength, balance, BMD and physical function than balance or strength training alone. 99,108,117,120 These results are supported by an earlier trial in healthy elderly women, in which combined balance and strength training produced the best results in terms of leg strength, balance, BMD and physical function compared with balance or strength training alone. 117
There is also some evidence to suggest that exercise programmes that combine upper-body and lower-body exercises load bone and muscle in the spine more effectively than programmes that focus on one area. 110,121 Previous trials of exercise in people with low vertebral BMD that report benefits include a mix of upper-body, trunk and lower-body exercises. Common elements include exercises to improve cervical glide, scapula retraction, shoulder flexion, trunk extension, hip extension, knee extension and ankle dorsiflexion, weight-bearing exercises, such as squats and walking, and balance activities, such as tandem and single-leg standing. 65,67–69,74,75,99,100,102,103,120,122
Previous randomised controlled trials of exercise interventions in vertebral osteoporotic fracture at time of intervention development
There are a limited number of RCTs of exercise interventions in populations in which all participants have OVF, and these are of mixed quality. A RCT in 2011 investigated the effect of a twice-weekly exercise class for 4 months in 36 women with OVF and reported improved back muscle strength but no change in thoracic kyphosis. 67 An earlier RCT evaluated a 3-month course of classes and reported improvements in walking, pain and some aspects of QoL that persisted at 12 months. 65 Their results were comparable to a trial evaluating a 10-week class with HEP, but in this trial, when training stopped, improvements declined. 74 The largest trial of 185 women by Gold et al. 68 found that 6 months of three exercise classes per week improved back strength and some psychological symptoms but not pain or function. Improvements in psychological health were retained but strength changes did not persist at 1 year. Only one RCT of 74 women with OVF has investigated physiotherapist-led home exercise. 75 Here, a 6-month programme of 3 days per week of stretches, strength training and walking improved some aspects of QoL and balance at 6 months and 1 year but did not affect function. The intensity of these interventions is notable. Durations range from 3 to 6 months and the maximum number of sessions range from 24 to 120, raising questions about whether or not effects can be seen at levels of intervention intensity deliverable in line with current NHS resources.
It is also notable that only one study included any male subjects; this point is emphasised in the reviews by Giangregorio et al. 40 and Dusdal et al. 123
Safety
Information about any specific risks of exercise for people with OVFs is scarce. Most studies lack details of how adverse events are monitored or do not report any events. The high incidence of further fractures and falls in this population makes it difficult to quantify if there is any increased risk of fracture due to exercise above the persistently elevated risk. 40 Gold et al. 68 reported that two people in the exercise arm sustained fractures (one in the ribs and one in the costal cartilage) but did not link these to the intervention. Dynamic flexion and rotation exercises were associated with increased risk of further fracture in a small, experimental trial. 88 More minor aggravation of back pain, shoulder or forearm pain following exercise that settles with rest or adapting the exercise is also reported. 64,70,99,101
In addition, hyperkyphosis and vertebral fracture are associated with compromised respiratory function and this may significantly limit maximal aerobic capacity. 29,81 People in this age group are also likely to have other comorbidities such as cardiovascular disease, which can limit exercise capacity. Considering this, moderate intensity exercise is most appropriate for this population and specific screening for comorbidities and use of the Borg Rating of Perceived Exertion (RPE) scale to guide exercise intensity is recommended. 124 All these factors were taken into consideration when setting exercise intensity.
Adherence
Adherence to exercise regimes can present a challenge in this population, and the outcomes may depend on whether or not participants complete their prescribed exercises. A long-term observational study of 33 women with low BMD reported poor compliance (38%) with home exercises and little change in physical outcomes. 125 Exercise class attendance was low, at 58%, in the intervention by Gold et al. ,68 and improvements in muscle strength did not persist. Grahn Kronhed et al. 69 found that pain and physical function improved after a 4-month exercise class but that physical function declined once supervised exercise stopped and benefits did not persist at 1 year. Kanemaru et al. 71 reported improved physical function after a 12-month HEP but eliminated participants who stopped exercising from the analysis. Bautmans et al. 63 reported that participants who complied with rehabilitation including home exercises improved significantly compared with those who were non-compliant.
We recognised that participants might be ambivalent about a HEP; they might have concerns and want to exercise more but might also see barriers, risks or downsides to doing exercise (lack of time, might aggravate pain, etc.). 126 In addition, they might lack knowledge or skills in carrying out exercise or lack confidence in their ability to succeed with an exercise programme. 127 HEPs that offer greater support (e.g. that utilise strategies to promote adherence, such as education, goal setting, diaries and telephone support) achieve higher compliance and more positive outcomes. 64,70,74,101
The PROVE trial interventions
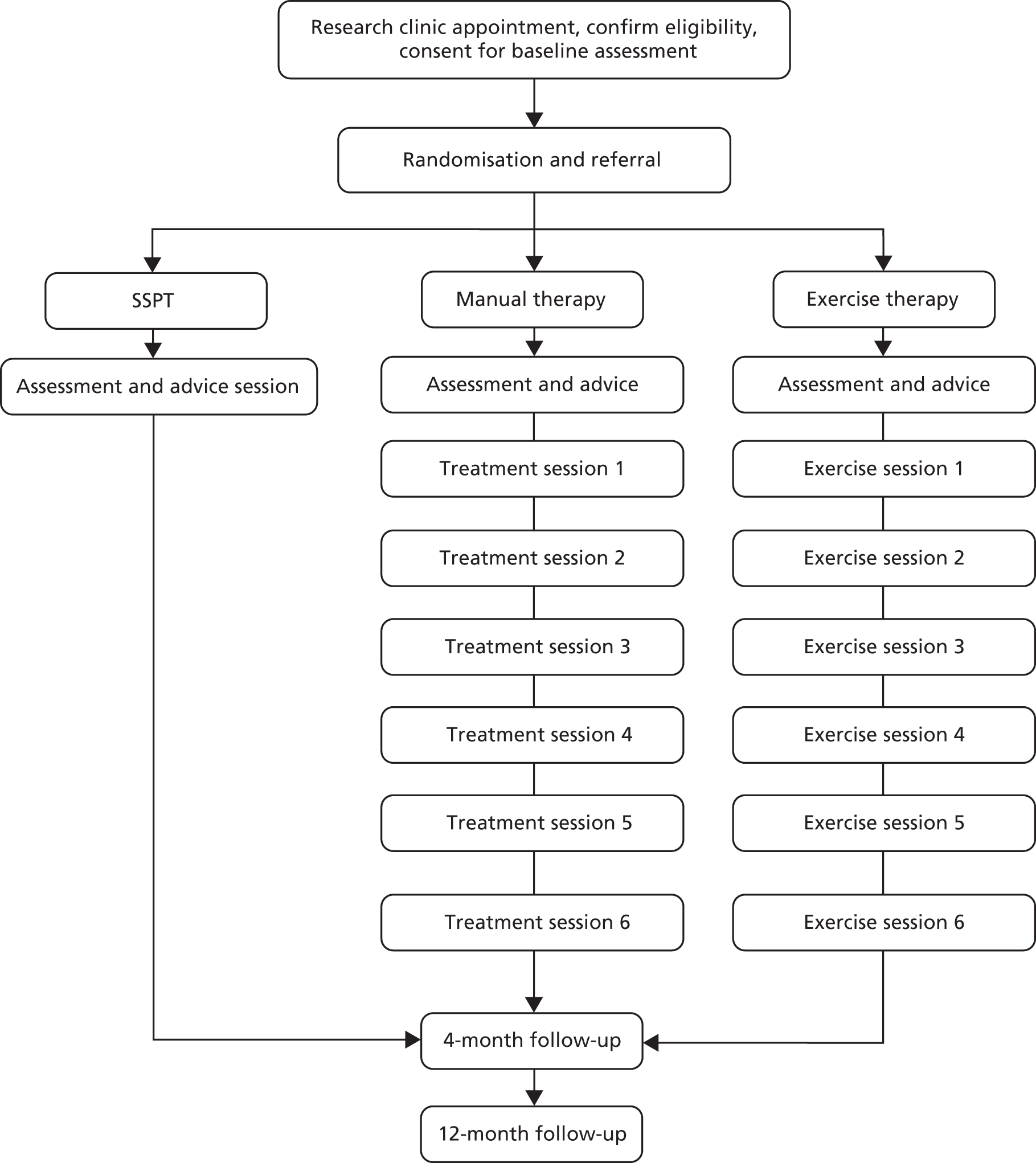
The interventions were delivered by UK physiotherapists with expertise in musculoskeletal rehabilitation. They took place within standard NHS outpatient settings. All physiotherapists were employed by the NHS and treated the PROVE trial participants alongside their standard caseload. In most centres, the delivery of the different treatment arms was carried out by different physiotherapists, matched for level of seniority and experience. All sessions of all arms of the trial were to be delivered within 12 weeks, after which the participant was discharged (Figure 4).
FIGURE 4.
Intervention allocation and arm activities.
Single session of physiotherapy intervention
All trial participants had been prescribed bone-protective medication and all received a single outpatient appointment with a physiotherapist. This started with an assessment that included questions about the person’s current condition, any lifestyle changes that they had made to date to manage their osteoporosis and their educational needs in relation to their osteoporosis. The physiotherapist then provided education and advice following educational materials developed for the trial and considering the individual’s circumstances (e.g. including information about smoking or alcohol consumption and fracture risk). The education did not include any explicit exercise prescription, any manual therapy or any other physiotherapy (e.g. walking aid prescription).
Manual therapy intervention
The manual therapy intervention comprised:
-
An initial assessment.
-
All SSPT education and advice.
-
Low-velocity spinal mobilisations at each treatment session. The start position (prone, prone over pillow or forward lean sitting) was adapted as needed by the treating therapist. Central posterior–anterior accessory mobilisations were applied through the thoracic and lumbar spine at vertebral levels identified by the treating therapist from the assessment. The number of repetitions and grade (grade 2–4) were to be chosen by the treating therapist. Using pain as a guide, the therapist could choose whether or not to mobilise at the level of a known previous vertebral fracture.
-
Postural training comprising:
-
Education – providing a rationale for increasing awareness of posture in daily life, discussing the relationship between posture, pain and function and highlighting that flexed posture is likely to have both fixed and modifiable elements.
-
Teaching self-correction of posture to find patients’ usual ‘best posture(s)’ that promote optimal skeletal alignment and comfort in different positions.
-
Postural taping with extension bias over the thoracic region. After specific screening for precautions and contraindications, tape was applied bilaterally from each anterior aspect of the acromioclavicular joint, over the muscle bulk of the upper trapezius and then diagonally towards the spinous process of thoracic vertebral level (T) 6, intersecting over T6 to promote thoracic extension. 91 Tape was applied for up to 3 days at one time, on up to three occasions, and guidelines for wearing and removing the tape were included in the manual therapy diary (see Appendix 1).
-
-
Soft-tissue mobilisation for a maximum of 5 minutes at each treatment session. Treatment was directed at the soft tissues and muscle groups over the thoracic area. The specific area targeted was identified by the treating physiotherapist on assessment (e.g. over the trapezius, supraspinatus, rhomboid or thoracic erector spinae muscle groups). Physiotherapists might elect to use any of the soft-tissue or trigger-point mobilisation techniques or to combine techniques.
-
A HEP of three passive stretches each day, one stretch each to promote trunk extension, shoulder flexion and abduction, each for a maximum duration of 5 minutes. The three stretches were selected from the graded set of stretches for each muscle group by the physiotherapist to be at an appropriate level for the individual.
-
A manual therapy diary including all stretches, taping records plus strategies to promote adherence, including an agreed personal exercise plan, goal setting, HEP record sheets and participant confidence rating (see Appendices 2 and 3).
-
Documentation of assessments and interventions using standard logs (see Appendix 4).
Exercise therapy intervention
The exercise therapy intervention comprised:
-
An initial assessment.
-
All SSPT education and advice.
-
A multimodal, graded exercise programme with three parts – strength training, balance training and weight-bearing exercise. Each element of the programme progressed in difficulty and the overall aim was for participants to achieve 45 minutes of moderate-intensity exercise three to five times per week by the end of 12 weeks. Specifically, it included:
-
Three balance exercises, one each from a walking balance, tandem standing and single-leg standing balance set of exercises.
-
A community walking exercise programme, using a pedometer to determine baseline walking ability, structure progression and encourage self-monitoring with lower-limb stretches as a cool down.
-
Three strength-training exercises, one each from sets to promote cervical dorsal glide (upper-cervical flexion and lower-cervical extension), scapula retraction, shoulder flexion, trunk extension, lumbar stabilisation and functional lower-limb strength.
-
Advice about exercising safely and effectively (e.g. minimising the risk of falls and considering comfort and posture).
-
Specific selection and progression of exercise intensity at a self-perceived moderate to somewhat hard level of effort (rating 3 or 4) using the Borg RPE scale.
-
-
Provision of an exercise therapy diary including all prescribed exercises and strategies to promote adherence to the HEP, including an agreed personal exercise plan with goal setting, exercise practice record sheets, assessment of the participant’s level of confidence with their HEP and telephone support.
-
Documentation of assessments and interventions using standard logs (see Appendices 5–7).
Intervention materials
Assessment forms were developed for each trial arm to help guide and standardise the initial assessment across sites and formed part of the clinical record. A pamphlet summarising the educational content was created for treating physiotherapists and a set of laminated information cards, illustrated and written in point form in accessible ‘patient-friendly’ language, was produced (see Appendices 8 and 9). The illustrated cards were for use within the education session, to support the discussion between the therapist and the patient and to ensure that relevant areas were covered in a standard way and that no area was forgotten. The content included background information about bone, osteoporosis and OVF, lifestyle factors that affect bone health, ways to protect the spine in daily life and strategies to reduce the risk of falls. The ROS leaflet ‘Healthy living for strong bones’ was provided to all trial participants to reinforce educational messages (see Appendix 10).
All trial stretches and strength and balance training exercises were photographed, described and categorised into sets; each set included exercises that addressed the same area [e.g. shoulder flexion, graded in difficulty (see Appendix 11)]. These were uploaded to the trial website. The website included an area for physiotherapists carrying out trial interventions [https://research.ndorms.ox.ac.uk/prove/therapists.php (accessed 6 February 2019)] and an online HEP prescription tool. It provided access to all trial exercises and participant documentation. Once the physiotherapist had selected the relevant level of exercise from the exercise set, this was recorded on an exercise record sheet, providing details of frequency, number and intensity. The chosen exercises were included in the participant therapy diary along with other documentation including goal setting and a plan to create an individualised HEP. A brief illustrated summary for physiotherapists for setting and progressing strength and balance training exercises was produced for use along with the Borg RPE scale. These were intended to supplement material in the PROVE trial treating therapist manual, to be easily transportable and useable in the clinical setting as needed.
Documents to support the adherence strategies were produced: for example, a goal-setting record, an exercise programme record and a confidence ruler. Each participant in the intervention arms received a therapy diary in accordance with allocation (see Appendices 2 and 5). These contained all prescribed exercises and a range of documents produced for the trial that were designed to monitor and support adherence to the HEP. Each participant in the exercise arm received a pedometer for use in the walking programme. The pedometer was issued with a wear guide, a daily step count record sheet and guide to increasing step count and walking distance. The physiotherapist provided guidance to the participant on the use of the pedometer and agreed the target increases in walking distance with the participant. Participants retained the pedometer at the end of the intervention.
Intervention providers and setting
The intervention was delivered by UK physiotherapists registered with the Health and Care Professions Council and working in musculoskeletal rehabilitation or specifically with people with osteoporosis. The trial was conducted at 22 hospital sites across the UK in physiotherapy outpatient departments. All therapists were NHS employees who treated trial participants alongside their normal caseload. The treatments delivered as part of the PROVE trial did not require enhanced skill sets but were within the normal scope of physiotherapists working within the NHS. In most centres, therapists delivered each of the trial interventions (i.e. SSPT or manual or exercise therapy programmes) as necessary. In other centres, therapists might split these roles (e.g. one therapist provided SSPT, another conducted manual therapy intervention and another conducted exercise therapy). To enhance consistency, physiotherapists delivering the trial interventions received training from the trial research team prior to seeing trial participants and were required to use trial materials and follow procedures. The integrity of the intervention was monitored during site visits (see Intervention monitoring and support).
Intervention structure and dose
Each intervention was delivered via individual, face-to-face sessions of outpatient physiotherapy. The key reason for selecting individual, face-to-face sessions was that, although exercise and educational interventions can be delivered in class formats or individually, manual techniques require individual ‘hands-on’ treatment. In addition, many older adults prefer individual exercise over group exercise and find their home setting most convenient for exercise (e.g. avoids transport issues). 128 We also thought that individual outpatient physiotherapy was feasible in more settings and, thus, could be more easily implemented on a wider scale.
For those allocated to SSPT, the intervention was delivered in a single appointment, of up to 60 minutes in length. To investigate the different content of each intervention arm, it was decided to keep the underlying programme structure and level of attention in each arm as consistent as possible. As a result, both interventions consisted of a maximum of seven sessions of individual outpatient physiotherapy over 12 weeks. The first session was an assessment lasting up to 60 minutes, with subsequent sessions lasting for 30 minutes.
Individual sessions were supplemented with a HEP. Home-based strength and aerobic exercise programmes in older populations report positive outcomes but maximising adherence is a key consideration. 128 Common strategies to promote adherence were used across the intervention arms (e.g. clear illustrated exercise instructions, goal setting, planning practice individually, systematically reviewing confidence with prescription, recording practice and telephone calls). Previous trials of home exercise that include telephone support from a physiotherapist70,101,128 report higher adherence and more positive outcomes than those without telephone support.
Within the NHS, current physiotherapy provision is limited to a ceiling of an average of around six sessions of individual physiotherapy. Thus, a factor considered when deciding the intervention dose was to determine if a positive treatment effect could be seen at levels of intervention deliverable within current NHS resources. It was felt that markedly increasing the duration of treatment and number of sessions would affect the generalisability of trial findings and increase the NHS excess treatment costs. The consultation with physiotherapy service managers had highlighted that increasing session numbers would be likely to reduce centre recruitment. Thus, the overall package of treatment was a maximum of seven physiotherapist contacts over 12 weeks. Exact session timings were agreed individually between the participant and the physiotherapist but with the expectation that there would be more sessions towards the start of the treatment period (as would happen in routine clinical practice).
Intervention prescription, progression or regression
To prompt an increase in physical capacity, sufficient training stimulus (overload) is needed to elicit musculoskeletal system adaptation and achieve motor learning, then, as an individual’s capacity improves, exercises need to progress to promote further improvement. 124 For each strength, balance or stretch exercise set in the home programmes, the physiotherapist estimated the appropriate start level based on their clinical judgement and initial assessment and then assessed this, asking the participant to perform up to three repetitions of the exercise, judging safety, form, comfort and rating intensity (Borg RPE scale). The exercise selected within the group was adjusted, if needed, based on feedback. Throughout the 12-week intervention, the physiotherapist reviewed and updated the HEP. This was to identify and address any problems and to allow for tailored progression as people adapted to the initial exercise prescription.
For the strength programme, the physiotherapist and the participant together selected an exercise from the designated set(s) that could be performed well and without pain for 10 repetitions (at least 8 and up to a maximum of 12) at a moderate intensity (rating 3–4 Borg RPE scale). 129 The number of sets was progressed up to a maximum of three sets of 10 repetitions, then the exercise level was reviewed (e.g. moving from prone trunk extension with arms to assist to prone trunk extension without arm assistance). The same principle applied to exercise levels that used resistance bands to increase the load. For the step-up and sit-to-stand exercises, the physiotherapist used the number of possible repetitions in 30 seconds at baseline to guide the initial number of repetitions per 30-second set, progressing up to a maximum of four sets.
In the walking programme, participants wore a pedometer for 1 week to allow the physiotherapist to evaluate the individual’s baseline walking level by calculating the average daily step count. Pedometers have been successfully used to structure, set intensity and progress self-monitored walking programmes for older people, including people with vertebral osteoporosis. 130 Participants were asked to increase their average daily step count by 15–30% by the end of 12 weeks in a graded fashion. 122 Progress was monitored mid-way, at 6 weeks, using the pedometer.
The balance exercises were made progressively more difficult in a standard way across the three exercise sets. Participants progressed from upper-limb support to no upper-limb support, from eyes open to eyes closed, from bilateral-leg to single-leg activity and from no distractors to mental distraction. 72,100,104,120
If a physiotherapy treatment, home exercise or taping prescription aggravated a pre-existing condition or resulted in new pain or injury, then physiotherapists were required to follow the adverse events protocol. Aside from this, if a participant reported difficulty with an element of the intervention, the physiotherapists would review the intervention and consider whether to adapt or reduce its intensity. If one element of the exercise programme was not tolerated (e.g. exercises in four-point kneeling), physiotherapists were advised to document this and miss out this element from the programme.
Intervention tailoring
We expected trial participants to have a wide range of pain, mobility and activity levels, various comorbidities, different levels of experience and confidence with exercise and a mix of home environments and support systems. To accommodate these factors and prescribe safely and effectively, it was necessary for the treating physiotherapists to tailor the PROVE trial intervention.
In the manual therapy arm, the therapist tailored spinal mobilisation treatments by selecting start position, spinal segment(s) treated, number of repetitions and mobilisation grade. The physiotherapist used their clinical judgement to select the muscle/soft-tissue area and treatment technique from the range of soft-tissue mobilisation techniques. Therapeutic taping was included only following screening regarding safety and the exact number of taping applications (up to three) was determined by the therapist. The specific stretches selected from the stretch sets also individualised the HEP.
In the exercise arm, the baseline assessment was used with the graded list of exercises and Borg RPE scale to allow the physiotherapist to prescribe a HEP tailored to individual participant capacity. Specifically, this involved manipulating the exercise level selected within each set, the number of repetitions and number of sets, the difficulty level in the balance exercises and walking time. Physiotherapists also worked with participants to set up a personal exercise plan that included a goal relevant to that individual and planned how and when to carry out exercises. The way in which the three exercise elements came together was tailored to fit the person’s daily life. For instance, participants might alternate different programme elements across the week (e.g. in weeks 1 to 4, strength training exercise was completed on a Monday and Thursday and the walking programme and balance training were completed on a Wednesday and Saturday). Over the 12 weeks, as the intensity of the programme progressed, elements were likely to overlap to reach the overall goal of 45 minutes of moderate-intensity exercise on 3–5 days per week by 12 weeks.
Intervention training
All physiotherapists delivering the PROVE trial interventions underwent a 3-hour training session prior to seeing the trial participants. The in-person training was supported by Microsoft PowerPoint® presentation manager (Microsoft Corporation, Redmond, WA, USA) slides and training manuals and took place individually or in a group session. The training sessions were structured to provide an overview of the trial, including background to the problem, participant eligibility, data collection, identification and reporting of study-related adverse events as well as to provide specific information about how to deliver each intervention. Training about the interventions could be adapted, depending on whether the physiotherapist would be delivering all intervention arms or a single intervention arm. Training content included the correct use and documentation of the pedometer, demonstration of how to customise and progress the HEP using the trial-specific designed online PROVE exercise tool, demonstration of the manual therapy techniques and delivery of the educational programme as well as using the adherence and self-management strategies. The PROVE trial treating therapist manual also contained detailed instructions and illustrations for physiotherapists treating trial participants. All therapists were required to read the manual prior to seeing trial participants.
Intervention monitoring and support
Routine quality assurance checks were carried out at each site by the research team, which included observation of an intervention assessment and a treatment delivery session. Feedback was provided following monitoring and if training needs were identified these were addressed. Telephone calls and additional visits were made to support the site if required. A structured checklist was used to ensure that all elements of treatment delivery and assessments were carried out as per the protocol (see Appendix 12). Bi-annual teleconference calls were organised for all the sites and this provided an opportunity for physiotherapists and research staff to share any general and site-specific concerns related to the trial. Two research days were organised over the course of the trial to provide additional support and a platform for PROVE trial physiotherapists at different centres to interact with research clinicians and each other.
Chapter 4 Results
This chapter is structured to present the results of the trial, describing the analyses and basis for decisions taken at the interim analysis, which formed the basis for the decisions made regarding adapting the trial. The interim results are followed by the results of the full three-arm randomised trial.
Trial sites
Centre characteristics
Twenty-one NHS trusts participated in the trial (Table 3). The recruitment period at each trust varied from 8 to 36 months; this resulted in variation in the number of participants recruited at each site.
| Centre | Start month | Months of recruitment | Number of therapists |
|---|---|---|---|
| Royal Orthopaedic Hospital NHS Foundation Trust | September 2013 | 28 | 4 |
| Western Sussex Hospitals NHS Foundation Trust | October 2014 | 23 | 2 |
| King’s College Hospital NHS Foundation Trust | February 2015 | 18 | 3 |
| Staffordshire and Stoke-on-Trent Partnership NHS Foundation Trust | April 2014 | 28 | 2 |
| Cambridge University Hospitals NHS Foundation Trust | October 2015 | 11 | 3 |
| The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust | April 2015 | 16 | 1 |
| Royal Surrey County Hospital Foundation Trust | February 2014 | 30 | 6 |
| University Hospitals of Leicester NHS Trust | September 2013 | 36 | 5 |
| Royal Devon and Exeter NHS Foundation Trust | April 2014 | 28 | 4 |
| Nottingham University Hospitals NHS Foundation Trust | May 2014 | 27 | 3 |
| South Devon Healthcare NHS Foundation Trust | March 2014 | 14a | 1 |
| Southend University Hospital NHS Foundation Trust | March 2014 | 29 | 1 |
| The Ipswich Hospitals NHS Trust | March 2014 | 29 | 1 |
| Countess of Chester NHS Foundation Trust | November 2014 | 22 | 2 |
| Oxford University Hospitals NHS Trust | September 2013 | 36 | 6 |
| University College London NHS Foundation Trust | January 2015 | 19 | 2 |
| Sheffield Teaching Hospitals NHS Foundation Trust | January 2016 | 8 | 3 |
| East Sussex Healthcare NHS Foundation Trust | October 2015 | 11 | 3 |
| Royal United Hospitals Bath NHS Foundation Trust | August 2015 | 12 | 4 |
| Portsmouth Hospitals NHS Foundation Trust | October 2014 | 23 | |
| Solent NHS Trust | October 2014 | 23 | 2 |
Participant flow
The overall flow of participants through the trial is described in the CONSORT flow diagram (see Figure 2). Further detail for each stage is provided in the following sections.
Recruitment
Screening
Screening and recruitment took place between September 2013 and September 2016. A total of 6841 patients were screened in rheumatology clinics, DEXA clinics and GP lists (Table 4). Of the screened patients, 1213 (17.7%) were eligible and, of these, 636 (52.4%) were willing to attend a research clinic appointment (Table 5); 616 patients (50.7%) actually attended an appointment. One patient was subsequently found to be ineligible, leaving 615 participants to proceed to the trial. A further withdrawal from the SSPT arm took place following randomisation and prior to assessment and one baseline assessment data set was not obtained from the site, resulting in 613 baseline data sets to be included in the final analysis. The variation in the conversion from screening to recruitment related to the strategy used: some centres screened all clinic attenders, whereas others screened only those participants who had already positively responded to trial invitation information.
| Centre | Number of patients | Percentage of patients recruited | |
|---|---|---|---|
| Screened | Recruited | ||
| Royal Orthopaedic Hospital NHS Foundation Trust | 48 | 10 | 21 |
| Western Sussex Hospitals NHS Foundation Trust | 5 | 3 | 60 |
| King’s College Hospital NHS Foundation Trust | 15 | 7 | 47 |
| Staffordshire and Stoke-on-Trent Partnership NHS Foundation Trust | 538 | 32 | 6 |
| Cambridge University Hospitals NHS Foundation Trust | 44 | 10 | 23 |
| The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust | 37 | 11 | 30 |
| Royal Surrey County Hospital Foundation Trust | 193 | 43 | 22 |
| University Hospitals of Leicester NHS Trust | 41 | 34 | 83 |
| Royal Devon and Exeter NHS Foundation Trust | 44 | 7 | 16 |
| Nottingham University Hospitals NHS Foundation Trust | 110 | 32 | 29 |
| South Devon Healthcare NHS Foundation Trust | 122 | 33 | 27 |
| Southend University Hospital NHS Foundation Trust | 44 | 44 | 100 |
| The Ipswich Hospitals NHS Trust | 664 | 64 | 10 |
| Countess of Chester NHS Foundation Trust | 56 | 29 | 52 |
| Oxford University Hospitals NHS Trust | 4707 | 199 | 4 |
| University College London NHS Foundation Trust | 11 | 1 | 9 |
| Sheffield Teaching Hospitals NHS Foundation Trust | 10 | 8 | 80 |
| East Sussex Healthcare NHS Foundation Trust | 48 | 10 | 21 |
| Royal United Hospitals Bath NHS Foundation Trust | 24 | 13 | 54 |
| Portsmouth Hospitals NHS Trust/Solent Hospitals NHS Foundation Trusta | 80 | 25 | 31 |
| Total | 6841 | 615 | 9 |
| Category | Number of patients | Percentage of patients |
|---|---|---|
| Eligible, willing and research appointment booked | 636 | 9.3 |
| Eligible but not willing | 577 | 8.4 |
| Not eligible (reason known) | 4965 | 72.6 |
| Not eligible (reason unknown) | 663 | 9.7 |
| Total | 6841 |
A total of 5628 screened patients (82.3%) were not eligible; Table 6 provides the reasons for ineligibility when known. Of those eligible but not willing, reasons were specified in the majority of cases (57.2%), with the remaining 42.8% of patients giving no reason (Table 7).
| Reason | Number of patients | Percentage of patients |
|---|---|---|
| No spinal fractures | 2831 | 57.0 |
| Osteopenia not osteoporosis | 315 | 6.3 |
| No back pain | 87 | 1.8 |
| Other reason | 1732 | 34.9 |
| Total | 4965 |
| Reason | Number of patients | Percentage of patients |
|---|---|---|
| Lack of time | 20 | 3.5 |
| Travel difficulties | 37 | 6.4 |
| No pain | 16 | 2.8 |
| Other | 257 | 44.5 |
| No reason | 247 | 42.8 |
| Total | 577 |
Recruitment
A total of 615 participants were enrolled in the PROVE trial between September 2013 and September 2016, with 216 being randomly allocated to the exercise therapy arm, 203 to the manual therapy arm and 196 to the SSPT arm. Although the recruitment target of 600 was achieved in August 2016, a further 15 participants were recruited in September 2016 as appointments made in the later stages before the target was reached were all honoured. The final recruitment total was 615 participants over a 36-month period.
Of the 636 participants initially given appointments for baseline assessments, 20 did not attend or cancelled, resulting in 616 participants being randomised (see Figure 2), one of whom was subsequently found to be ineligible, leaving 615 participants to proceed into the trial. A further withdrawal from the SSPT arm took place following randomisation and prior to assessment and one baseline assessment data set was not obtained from the site, resulting in 613 baseline data sets being included in the final analysis.
Demographic and baseline data
Baseline and demographic data summaries for each of the three intervention arms and for all participants are given in Table 8. The three arms were well matched at baseline.
| Data | All participants | Intervention arm | ||
|---|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | ||
| Female participants, n (%) | 531 (86.6) (N = 613) | 185 (85.6) (N = 216) | 173 (85.6) (N = 202) | 173 (88.7) (N = 195) |
| Age (years), mean (SD) | 72.1 (9.1) (N = 615) | 72.2 (8.4) (N = 216) | 72.4 (9.3) (N = 203) | 71.9 (9.6) (N = 196) |
| Height (cm), mean (SD) | 157.1 (17.8) (N = 612) | 156.4 (20.6) (N = 216) | 159.1 (8.7) (N = 201) | 155.6 (20.8) (N = 195) |
| Weight (kg), mean (SD) | 63.8 (14.2) (N = 613) | 64.3 (16.4) (N = 216) | 64.1 (14.4) (N = 202) | 62.9 (10.9) (N = 195) |
| DEXA lumbar T-score, mean (SD) | –2.7 (1.3) (N = 471) | –2.6 (1.5) (N = 162) | –2.7 (1.2) (N = 162) | –2.8 (1.3) (N = 147) |
| Number of spinal fractures, mean (SD) | 2.6 (1.9) (N = 552) | 2.7 (1.8) (N = 196) | 2.4 (1.8) (N = 187) | 2.5 (2.1) (N = 169) |
| Number of non-spinal fractures, mean (SD) | 0.1 (0.4) (N = 555) | 0.2 (0.4) (N = 194) | 0.2 (0.4) (N = 184) | 0.1 (0.3) (N = 177) |
| Number of fractures, n (%) | ||||
| Upper lumbar (L1 and L2) | 170 (45.9) (N = 370) | 62 (47.3) (N = 131) | 53 (42.7) (N = 124) | 55 (47.8) (N = 115) |
| Lower lumbar (L3–L5) | 216 (52.7) (N = 410) | 75 (49.7) (N = 151) | 78 (55.7) (N = 140) | 63 (52.9) (N = 119) |
| Lower thoracic (T6–T12) | 436 (86.5) (N = 504) | 160 (88.9) (N = 180) | 138 (85.2) (N = 162) | 138 (85.2) (N = 162) |
| Upper thoracic (T1–T5) | 67 (20.5) (N = 327) | 27 (22.9) (N = 118) | 13 (12.6) (N = 103) | 27 (25.5) (N = 106) |
| Walking category, n (%)a | ||||
| 1 | 280 (46.0) (N = 609) | 94 (43.7) (N = 215) | 95 (47.3) (N = 201) | 91 (47.2) (N = 193) |
| 2 | 134 (22.0) (N = 609) | 53 (24.7) (N = 215) | 40 (19.9) (N = 201) | 41 (21.2) (N = 193) |
| 3 | 151 (24.8) (N = 609) | 53 (24.7) (N = 215) | 53 (26.4) (N = 201) | 45 (23.3) (N = 193) |
| 4 | 42 (6.9) (N = 609) | 15 (7.0) (N = 215) | 12 (6.0) (N = 201) | 15 (7.8) (N = 193) |
| 5 | 2 (0.3) (N = 609) | 0 (0.0) (N = 215) | 1 (0.5) (N = 201) | 1 (0.5) (N = 193) |
| 6 | 0 (0.0) (N = 609) | 0 (0.0) (N = 215) | 0 (0.0) (N = 201) | 0 (0.0) (N = 193) |
| Stair use category, n (%)b | ||||
| 1 | 249 (41.5) (N = 600) | 82 (38.9) (N = 211) | 88 (44.2) (N = 199) | 79 (41.6) (N = 190) |
| 2 | 49 (8.2) (N = 600) | 15 (7.1) (N = 211) | 17 (8.5) (N = 199) | 17 (8.9) (N = 190) |
| 3 | 22 (3.7) (N = 600) | 8 (3.8) (N = 211) | 8 (4.0) (N = 199) | 6 (3.2) (N = 190) |
| 4 | 256 (42.7) (N = 600) | 95 (45.0) (N = 211) | 78 (39.2) (N = 199) | 83 (43.7) (N = 190) |
| 5 | 0 (0.0) (N = 600) | 0 (0.0) (N = 211) | 0 (0.0) (N = 199) | 0 (0.0) (N = 190) |
| 6 | 24 (4.0) (N = 600) | 11 (5.2) (N = 211) | 8 (4.0) (N = 199) | 5 (2.6) (N = 190) |
| Aid use category, n (%)c | ||||
| 1 | 405 (66.5) (N = 609) | 136 (63.3) (N = 215) | 139 (68.8) (N = 202) | 130 (67.7) (N = 192) |
| 2 | 130 (21.3) (N = 609) | 49 (22.8) (N = 215) | 37 (18.3) (N = 202) | 44 (22.9) (N = 192) |
| 3 | 33 (5.4) (N = 609) | 13 (6.0) (N = 215) | 8 (4.0) (N = 202) | 10 (5.2) (N = 192) |
| 4 | 4 (0.7) (N = 609) | 2 (0.9) (N = 215) | 1 (0.5) (N = 202) | 1 (0.5) (N = 192) |
| 5 | 4 (0.7) (N = 609) | 0 (0.0) (N = 215) | 2 (1.0) (N = 202) | 2 (1.0) (N = 192) |
| 6 | 33 (5.4) (N = 609) | 15 (7.0) (N = 215) | 13 (6.4) (N = 202) | 5 (2.6) (N = 192) |
| Participants with back pain, n (%) | ||||
| In previous 2 weeks | 591 (96.4) (N = 613) | 209 (96.8) (N = 216) | 194 (96.0) (N = 202) | 188 (96.4) (N = 195) |
| Today | 418 (68.3) (N = 612) | 157 (72.7) (N = 216) | 133 (65.8) (N = 202) | 128 (66.0) (N = 194) |
| Number of falls in previous year, mean (SD) | 0.7 (1.7) (N = 607) | 0.8 (2.3) (N = 214) | 0.7 (1.4) (N = 201) | 0.6 (1.0) (N = 192) |
| Participants with falls, in category, n (%)d | ||||
| 1 | 2 (0.3) (N = 588) | 1 (0.5) (N = 207) | 1 (0.5) (N = 193) | 0 (0.0) (N = 188) |
| 2 | 41 (7.0) (N = 588) | 19 (9.2) (N = 207) | 14 (7.3) (N = 193) | 8 (4.3) (N = 188) |
| 3 | 545 (92.7) (N = 288) | 187 (90.3) (N = 207) | 178 (92.2) (N = 193) | 180 (95.7) (N = 188) |
| Participants with pain, n (%) | ||||
| In previous 2 weeks | ||||
| Lower lumbar (L3–L5) | 340 (72.0) (N = 472) | 121 (70.8) (N = 171) | 115 (74.2) (N = 155) | 104 (71.2) (N = 146) |
| Upper lumbar (L1 and L2) | 239 (56.6) (N = 422) | 83 (55.3) (N = 150) | 77 (56.6) (N = 136) | 79 (58.1) (N = 136) |
| Lower thoracic (T6–T12) | 246 (55.3) (N = 445) | 84 (54.5) (N = 154) | 78 (55.7) (N = 140) | 84 (55.6) (N = 151) |
| Upper thoracic (T1–T5) | 152 (38.2) (N = 398) | 50 (35.2) (N = 142) | 50 (41.3) (N = 121) | 52 (38.5) (N = 135) |
| Today | ||||
| Lower lumbar (L3–L5) | 218 (67.1) (N = 325) | 83 (65.9) (N = 126) | 76 (72.4) (N = 105) | 59 (62.8) (N = 94) |
| Upper lumbar (L1 and L2) | 155 (54.4) (N = 285) | 56 (52.3) (N = 107) | 55 (59.1) (N = 93) | 44 (51.8) (N = 85) |
| Lower thoracic (T6–T12) | 167 (56.0) (N = 298) | 58 (51.8) (N = 112) | 51 (58.0) (N = 88) | 58 (59.2) (N = 98) |
| Upper thoracic (T1–T5) | 89 (34.4) (N = 259) | 28 (28.6) (N = 98) | 28 (36.8) (N = 76) | 33 (38.8) (N = 85) |
| QUALEFFO-41 score (points), mean (SD) | 38.4 (15.6) (N = 609) | 39.9 (16.0) (N = 214) | 37.1 (14.9) (N = 200) | 38.1 (15.9) (N = 195) |
| TLS test (seconds), mean (SD) | 49.6 (54.9) (N = 600) | 48.6 (54.5) (N = 210) | 47.9 (51.7) (N = 197) | 52.4 (58.7) (N = 193) |
| Comorbidities score, mean (SD) | 0.8 (1.4) (N = 606) | 0.75 (1.01) (N = 213) | 0.68 (1.00) (N = 200) | 0.91 (1.89) (N = 193) |
| PASE score, mean (SD) | 110.8 (81.5) (N = 538) | 109.7 (89.7) (N = 191) | 115.6 (81.3) (N = 182) | 106.9 (71.2) (N = 165) |
| Thoracic kyphosis (degrees), mean (SD) | 49.7 (33.4) (N = 606) | 51.2 (34.8) (N = 213) | 48.6 (22.4) (N = 199) | 49.1 (40.6) (N = 194) |
| SPPB score, mean (SD) | 8.4 (2.1) (N = 569) | 8.3 (2.1) (N = 200) | 8.6 (2.1) (N = 185) | 8.4 (2.2) (N = 184) |
| FRT (cm), mean (SD) | 23.6 (9.7) (N = 612) | 23.5 (8.8) (N = 216) | 23.4 (10.5) (N = 201) | 23.8 (9.8) (N = 195) |
| 6MWT (m), mean (SD) | 304.0 (130.9) (N = 611) | 295.4 (128.0) (N = 216) | 304.2 (135.3) (N = 201) | 313.4 (129.4) (N = 194) |
| VAS back pain today, mean (SD) | 4.7 (2.1) (N = 416) | 4.8 (2.2) (N = 156) | 4.5 (2.1) (N = 133) | 4.6 (2.1) (N = 127) |
Follow-up
Across the three arms, 529 out of 615 participants (86%) had a complete QUALEFFO-41 data set at 12 months and 458 out of 615 participants (78%) had completed the TLS test. The follow-up rate for the QUALEFFO-41 was higher than for the TLS test; this is because the QUALEFFO-41 could be collected by post or telephone, unlike the TLS test, which required a physical visit.
Withdrawals
In total, 63 participants (10.2%) withdrew from the trial during follow-up, with a slightly greater proportion of these in the exercise therapy arm. The timing and reasons for withdrawal are shown in Tables 9 and 10. Most withdrawals took place between the baseline and 4-month assessments, with just 13 taking place between the 4- and 12-month assessments.
In addition, 17 participants (2.7%) were unable to be contacted at the 4-month time point, rising to 23 participants (3.7%) at the 12-month time point.
| Time of withdrawal from trial | Number of participants | |||
|---|---|---|---|---|
| Intervention arm | Total | |||
| Exercise programme | Manual therapy | SSPT | ||
| Prior to treatment | 0 | 0 | 1 | 1 |
| After treatment, before the 4-month follow-up | 19 | 15 | 15 | 49 |
| Between the 4- and 12-month follow-upsa | 6 | 6 | 1 | 13 |
| Total | 25 | 21 | 17 | 63 |
| Reason | Intervention arm (number of participants) | Total, n (%) | ||
|---|---|---|---|---|
| Exercise programme | Manual therapy | SSPT | ||
| Travelling/time | 2 | 2 | 2 | 6 (9.5) |
| Poor health | 7 | 7 | 2 | 16 (25.5) |
| Death | 2 | 4 | 0 | 6 (9.5) |
| Unable to attend | 6 | 4 | 4 | 14 (22.2) |
| Does not want to/not committed | 3 | 2 | 1 | 6 (9.5) |
| Not happy with allocated arm | 0 | 1 | 6 | 7 (11.1) |
| Reason not given | 5 | 1 | 2 | 8 (12.7) |
| Total | 25 | 21 | 17 | 63 |
Participants in the SSPT arm were more likely than those in the other two arms to withdraw because they were not happy with their allocated arm. There were more withdrawals due to ill health in the treatment arms. The withdrawal rate met the prespecified 10% allowed for in the sample size calculation.
Interim analysis
Table 11 gives results for the interim analysis for participants recruited in stage 1. As the difference in 4-month change in QUALEFFO-41 scores between each of the intervention arms and the SSPT arm was > 0.5 points and the difference between the two intervention arms was < 2.0 points, all three arms continued to the end of the trial.
| Score change at 4 months | Intervention arm | ||
|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | |
| QUALEFFO-41 (points)a | |||
| Mean (SD) | –3.41 (8.7) (n = 61) | –1.95 (8.4) (n = 73) | –1.21 (6.7) (n = 67) |
| Mean difference from SSPT | –2.20 | –0.74 | |
| TLS test (seconds)b | |||
| Mean (SD) | 6.66 (44.2) (n = 58) | 13.00 (45.4) (n = 68) | –4.30 (48.6) (n = 63) |
| Mean difference from SSPT | 10.96 | 17.30 | |
Primary end-point analyses
The results of the main analysis of the co-primary end points of 12-month change in QUALEFFO-41 and TLS test are given in Table 12. In addition to unadjusted means and SDs for each arm, the table presents p-values adjusted for multiplicity allowing for the adaptive design used and adjusted for baseline, centre, age and baseline fracture history. The pooled SD for the QUALEFFO-41 change at 12 months is 9.94 points, so that the standardised intervention effects are –0.13 and –0.01, respectively. There are no significant differences between interventions on either of the co-primary end points. Unbiased estimates of the intervention differences and CIs, correcting for the adaptive design, are also presented.
| Score change at 12 months | Intervention arm | ||
|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | |
| QUALEFFO-41 (points)a | |||
| Raw mean (SD) | –1.31 (9.97) (n = 176) | –0.15 (10.81) (n = 181) | –1.18 (8.89) (n = 172) |
| Raw mean difference from SSPT | –0.13 | 1.03 | |
| Unbiased estimate mean | –1.55 | 0.03 | –1.32 |
| Unbiased mean difference from SSPT (95% CI) | –0.23 (–3.20 to 1.59) | 1.35 (–1.76 to 2.93) | |
| p-value adjusted for multiplicity and corrected for baseline, centre, fracture history and age | 1.000 | 1.00 | |
| TLS test (seconds)b | |||
| Raw mean (SD) | 9.8 (52.4) (n = 148) | 13.6 (38.9) (n = 153) | 4.2 (55.8) (n = 157) |
| Raw mean difference from SSPT | 5.6 | 9.4 | |
| Unbiased estimate mean | 9.581 | 13.501 | 3.811 |
| Unbiased mean difference from SSPT (95% CI) | 5.77 (–4.85 to 20.46) | 9.69 (0.09 to 24.86) | |
| p-value adjusted for multiplicity and corrected for baseline, centre, fracture history and age | 0.437 | 0.335 | |
Secondary end-point analyses
Tables 13 and 14 summarise the analysis of secondary end points. Mean values and SDs for each intervention arm are given in Table 13 and estimated treatment differences relative to a SSPT are given in Table 14, values in the latter being adjusted for baseline and centre. This adjustment results in the differences reported in Table 14 not being exactly equal to the differences between the mean values given in Table 13. No adjustment for multiplicity or the adaptive design has been made in these analyses. Relative to a SSPT, exercise therapy results in significant improvement at 4 months in the SPPB score, the FRT and the 6MWT, and manual therapy results in significant improvement at 4 months in the TLS test and FRT. The effect of exercise therapy on the QUALEFFO-41 pain and social function subscales at 4 months was also approaching statistical significance.
| Secondary end points | Intervention arm, mean (SD) | ||
|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | |
| QUALEFFO-41 change at 4 months (points)a | –2.16 (8.40) (n = 180) | –0.55 (8.17) (n = 185) | –0.87 (7.05) (n = 173) |
| TLS test change at 4 months (seconds)b | 7.42 (47.90) (n = 162) | 11.80 (40.27) (n = 171) | –0.58 (40.90) (n = 161) |
| QUALEFFO-41 subscale 12-month change (points)a | |||
| Pain | –9.07 (22.88) (n = 176) | –6.75 (22.98) (n = 180) | –6.25 (18.39) (n = 172) |
| Physical function | –0.70 (11.41) (n = 176) | 1.10 (11.56) (n = 181) | –0.90 (10.16) (n = 172) |
| Social function | –1.65 (16.09) (n = 175) | 0.02 (17.26) (n = 181) | –0.45 (16.11) (n = 172) |
| General health perception | –0.17 (18.83) (n = 175) | 1.00 (18.15) (n = 180) | 1.03 (16.09) (n = 170) |
| Mental function | 1.45 (11.69) (n = 174) | 0.22 (13.14) (n = 180) | –0.37 (12.56) (n = 171) |
| QUALEFFO-41 subscale 4-month change (points)a | |||
| Pain | –7.76 (18.51) (n = 180) | –5.58 (21.41) (n = 182) | –3.77 (19.13) (n = 173) |
| Physical function | –2.81 (9.45) (n = 180) | –0.89 (8.54) (n = 185) | –1.66 (8.35) (n = 173) |
| Social function | –3.22 (14.32) (n = 180) | –0.83 (15.75) (n = 185) | –0.10 (13.30) (n = 172) |
| General health perception | –1.99 (15.48) (n = 180) | 0.43 (15.09) (n = 184) | 0.10 (13.45) (n = 173) |
| Mental function | –0.23 (10.32) (n = 180) | –0.84 (10.50) (n = 184) | –2.06 (11.27) (n = 173) |
| PASE changeb | |||
| 12 months | 9.72 (63.64) (n = 116) | 4.22 (67.02) (n = 127) | 6.75 (57.58) (n = 122) |
| 4 months | 10.26 (54.58) (n = 133) | 9.38 (86.20) (n = 136) | 8.02 (66.41) (n = 125) |
| Thoracic kyphosis changea | |||
| 12 months | –6.88 (31.66) (n = 152) | –4.63 (22.28) (n = 156) | –2.73 (49.58) (n = 167) |
| 4 months | –4.21 (40.63) (n = 170) | –2.58 (9.31) (n = 173) | –3.26 (40.33) (n = 167) |
| SPPB changeb | |||
| 12 months | 0.66 (1.78) (n = 134) | 0.15 (1.49) (n = 141) | 0.47 (1.75) (n = 149) |
| 4 months | 0.81 (1.72) (n = 152) | 0.31 (1.51) (n = 157) | 0.39 (1.57) (n = 150) |
| FRT changeb | |||
| 12 months | 0.26 (8.57) (n = 152) | 1.18 (13.00) (n = 155) | –0.21 (12.72) (n = 165) |
| 4 months | 1.28 (9.19) (n = 170) | 1.53 (9.56) (n = 177) | –0.80 (9.85) (n = 168) |
| 6MWT changeb | |||
| 12 months | 19.1 (103.77) (n = 150) | 20.19 (87.45) (n = 157) | 7.16 (88.48) (n = 164) |
| 4 months | 28.09 (102.50) (n = 167) | 16.73 (90.18) (n = 174) | –1.73 (84.71) (n = 166) |
| VAS pain changea | |||
| 12 months | –0.16 (2.17) (n = 73) | –0.12 (2.29) (n = 75) | –0.2 (1.98) (n = 70) |
| 4 months | –0.37 (1.75) (n = 81) | –0.41 (2.33) (n = 80) | –0.15 (1.86) (n = 74) |
| Secondary end points | Change relative to SSPT (95% CI); p-value | |
|---|---|---|
| Exercise therapy arm | Manual therapy arm | |
| QUALEFFO-41 change at 4 months (points)a | –1.23 (–2.84 to 0.37); p = 0.133 | 0.27 (–1.32 to 1.86); p = 0.737 |
| TLS test change at 4 months (seconds)b | 7.46 (–1.43 to 16.35); p = 0.101 | 10.56 (2.22 to 18.90); p = 0.014 |
| QUALEFFO-41 subscale 12-month change (points)a | ||
| Pain | –2.80 (–7.07 to 1.46); p = 0.199 | –1.03 (–5.22 to 3.17); p = 0.632 |
| Physical function | 0.18 (–2.04 to 2.41); p = 0.871 | 1.78 (–0.48 to 4.04); p = 0.124 |
| Social function | –0.66 (–3.68 to 2.37); p = 0.671 | –0.11 (–3.31 to 3.08); p = 0.944 |
| General health perception | –0.06 (–3.61 to 3.50); p = 0.975 | 0.07 (–3.33 to 3.47); p = 0.970 |
| Mental function | 1.65 (–0.81 to 4.10); p = 0.190 | 0.18 (–2.35 to 2.71); p = 0.888 |
| QUALEFFO-41 subscale 4-month change (points)a | ||
| Pain | –3.57 (–7.39 to 0.25); p = 0.068 | –2.54 (–6.56 to 1.48); p = 0.216 |
| Physical function | –1.02 (–2.83 to 0.80); p = 0.273 | 0.73 (–0.99 to 2.45); p = 0.407 |
| Social function | –2.71 (–5.46 to 0.03); p = 0.053 | –0.98 (–3.86 to 1.90); p = 0.507 |
| General health perception | –1.07 (–4.01 to 1.88); p = 0.479 | 0.65 (–2.17 to 3.48); p = 0.651 |
| Mental function | 1.56 (–0.59 to 3.71); p = 0.156 | 0.96 (–1.17 to 3.09); p = 0.376 |
| Number of falls in previous yeara,c | ||
| 12 months | –0.09 (–0.41 to 0.24); p = 0.601 | –0.17 (–0.49 to 0.16); p = 0.319 |
| 4 months | –0.08 (–0.46 to 0.29); p = 0.660 | –0.05 (–0.41 to 0.31); p = 0.776 |
| Falls frequencyb,d | ||
| 12 months | –0.10 (–1.63 to 1.43); p = 0.895 | –1.07 (–2.42 to 0.29); p = 0.123 |
| 4 months | 0.68 (–0.81 to 2.18); p = 0.371 | 0.57 (–0.76 to 1.90); p = 0.402 |
| PASE changeb | ||
| 12 months | 6.33 (–8.40 to 21.06); p = 0.400 | 1.56 (–12.92 to 16.04); p = 0.833 |
| 4 months | 0.95 (–13.58 to 15.48); p = 0.898 | 1.67 (–16.78 to 20.12); p = 0.859 |
| Thoracic kyphosis changea | ||
| 12 months | –2.24 (–6.40 to 1.92); p = 0.291 | –3.02 (–8.14 to 2.10); p = 0.249 |
| 4 months | 0.82 (–3.27 to 4.90); p = 0.696 | –0.71 (–3.96 to 2.55); p = 0.671 |
| SPPB changeb | ||
| 12 months | 0.18 (–0.21 to 0.57); p = 0.361 | –0.19 (–0.54 to 0.16); p = 0.280 |
| 4 months | 0.45 (0.11 to 0.79); p = 0.010 | –0.03 (–0.35 to 0.30); p = 0.878 |
| FRT changeb | ||
| 12 months | 1.02 (–0.96 to 3.01); p = 0.313 | 0.54 (–1.65 to 2.73); p = 0.632 |
| 4 months | 2.43 (0.71 to 4.16); p = 0.006 | 2.11 (0.57 to 3.64); p = 0.007 |
| 6MWT changeb | ||
| 12 months | 6.83 (–13.08 to 26.74); p = 0.502 | 12.02 (–6.46 to 30.50); p = 0.203 |
| 4 months | 26.09 (6.58 to 45.60); p = 0.009 | 16.16 (–1.76 to 34.09); p = 0.078 |
| VAS pain changea | ||
| 12 months | 0.02 (–0.62 to 0.67); p = 0.945 | –0.03 (–0.70 to 0.65); p = 0.941 |
| 4 months | –0.21 (–0.74 to 0.32); p = 0.439 | –0.24 (–0.88 to 0.39); p = 0.454 |
Subgroup analyses
Table 15 presents the results from pre-planned subgroup analyses for QUALEFFO-41 and TLS test change at 4 and 12 months.
| Subgroup analyses | Intervention arm | Number of participants in the exercise therapy arm, manual therapy arm and SSPT arm, respectively | |||
|---|---|---|---|---|---|
| Exercise therapy | Manual therapy | ||||
| Change relative to SSPT (95% CI); p-value | p-value for interaction | Change relative to SSPT (95% CI); p-value | p-value for interaction | ||
| QUALEFFO-41 score 12-month change (points) | |||||
| Sex | |||||
| Female | –0.41 (–2.49 to 1.66); p = 0.695 | 0.357 | 0.87 (–1.27 to 3.00); p = 0.428 | 0.985 | 154, 155, 154 |
| Male | 3.61 (–2.09 to 9.30); p = 0.225 | 0.42 (–7.69 to 8.54); p = 0.919 | 22, 26, 18 | ||
| Age (years) | |||||
| ≤ 70 | –0.00 (–3.22 to 3.22); p = 1 | 0.834 | –0.36 (–3.92 to 3.20); p = 0.842 | 0.937 | 68, 66, 68 |
| > 70 | –0.58 (–3.13 to 1.97); p = 0.656 | 0.83 (–1.73 to 3.38); p = 0.527 | 108, 115, 104 | ||
| Number of spinal fractures | |||||
| ≤ 2 | –0.25 (–2.49 to 2.00); p = 0.829 | 0.975 | 1.39 (–1.17 to 3.95); p = 0.288 | 0.275 | 77, 95, 86 |
| > 2 | –3.45 (–7.65 to 0.74); p = 0.111 | 0.13 (–4.75 to 5.01); p = 0.959 | 52, 49, 49 | ||
| TLS test 12-month change (seconds) | |||||
| Sex | |||||
| Female | 3.94 (–7.63 to 15.52); p = 0.505 | 0.892 | 4.50 (–5.64 to 14.64); p = 0.384 | 0.112 | 130, 130, 141 |
| Male | –5.91 (–51.19 to 39.37); p = 0.801 | 33.77 (–7.27 to 74.82); p = 0.119 | 18, 23, 16 | ||
| Age (years) | |||||
| ≤ 70 | 10.91 (–10.33 to 32.14); p = 0.317 | 0.299 | 8.62 (–10.30 to 27.53); p = 0.374 | 0.294 | 58, 59, 64 |
| > 70 | 2.07 (–10.99 to 15.14); p = 0.756 | 8.34 (–3.36 to 20.04); p = 0.164 | 90, 94, 93 | ||
| Number of spinal fractures | |||||
| ≤ 2 | 8.17 (–9.44 to 25.79); p = 0.365 | 0.254 | 4.41 (–9.43 to 18.25); p = 0.533 | 0.563 | 76, 93, 81 |
| > 2 | –1.10 (–15.95 to 13.75); p = 0.885 | 11.41 (–6.19 to 29.02); p = 0.207 | 62, 53, 66 | ||
| QUALEFFO-41 score 4-month change (points) | |||||
| Sex | |||||
| Female | –1.43 (–3.15 to 0.29); p = 0.103 | 0.531 | –0.18 (–1.79 to 1.43); p = 0.827 | 0.138 | 159, 158, 156 |
| Male | 0.15 (–4.65 to 4.96); p = 0.950 | 4.08 (–3.23 to 11.40); p = 0.283 | 21, 27, 17 | ||
| Age (years) | |||||
| ≤ 70 | –2.01 (–4.67 to 0.65); p = 0.141 | 0.134 | –2.12 (–4.89 to 0.64); p = 0.135 | 0.204 | 69, 70, 67 |
| > 70 | –0.91 (–3.06 to 1.24); p = 0.409 | 1.32 (–0.71 to 3.34); p = 0.204 | 111, 115, 106 | ||
| Number of spinal fractures | |||||
| ≤ 2 | –1.04 (–2.99 to 0.92); p = 0.301 | 0.376 | –0.76 (–3.00 to 1.47); p = 0.505 | 0.595 | 82, 103, 88 |
| > 2 | –1.38 (–4.41 to 1.64); p = 0.373 | 1.39 (–1.76 to 4.55); p = 0.389 | 62, 58, 53 | ||
| TLS test 4-month change (seconds) | |||||
| Sex | |||||
| Female | 7.05 (–2.52 to 16.61); p = 0.150 | 0.702 | 9.29 (0.63 to 17.96); p = 0.036 | 0.345 | 144, 147, 144 |
| Male | 7.43 (–17.87 to 32.74); p = 0.570 | 8.67 (–17.04 to 34.38); p = 0.514 | 18, 24, 17 | ||
| Age (years) | |||||
| ≤ 70 | 20.17 (5.89 to 34.4); p = 0.007 | 0.008 | 21.09 (7.28 to 34.89); p = 0.003 | 0.0006 | 65, 62, 64 |
| > 70 | 0.15 (–11.45 to 11.75); p = 0.980 | 2.28 (–8.26 to 12.82); p = 0.672 | 97, 109, 97 | ||
| Number of spinal fractures | |||||
| ≤ 2 | 9.81 (–4.48 to 24.09); p = 0.181 | 0.195 | 6.97 (–4.78 to 18.73); p = 0.247 | 0.294 | 78, 99, 84 |
| > 2 | 7.41 (–4.31 to 19.12); p = 0.217 | 12.83 (–0.35 to 26.02); p = 0.059 | 76, 67, 71 | ||
The prespecified subgroup analyses were based on age at recruitment (those aged ≤ 70 years vs. those aged > 70 years), sex (male vs. female) and number of spinal fractures (those with fewer than two OVFs vs. those with more than two OVFs).
The table gives p-values for the subgroups by treatment interactions and for tests of treatment effects within each subgroup. There is a highly significant treatment*age group interaction for TLS test change at 4 months, with significant treatment differences between SSPT and both manual and exercise therapy in the ≤ 70 years age group and no effect in the > 70 years age group.
Sensitivity analyses
Comparison of centres
Figure 5 shows the mean change in QUALEFFO-41 and TLS test scores at 12 months for each centre, combining all intervention arms. Plotted circles have radii proportional to the number of observations in each centre, so that larger circles correspond to centres with more observations. Half of the centres randomised < 15 participants and we have not attempted to show means for individual treatment groups as the numbers would be small. As would be expected, because the SE of the mean difference is proportional to the reciprocal of the square root of the sample size for each centre, results for smaller centres show greater variability. Other than this, there is little indication that there is any effect of centre size on either end point nor that there is correlation between centre effects on change in TLS test and change in QUALEFFO-41.
FIGURE 5.
Mean QUALEFFO-41 and TLS test score change at 12 months for each centre.
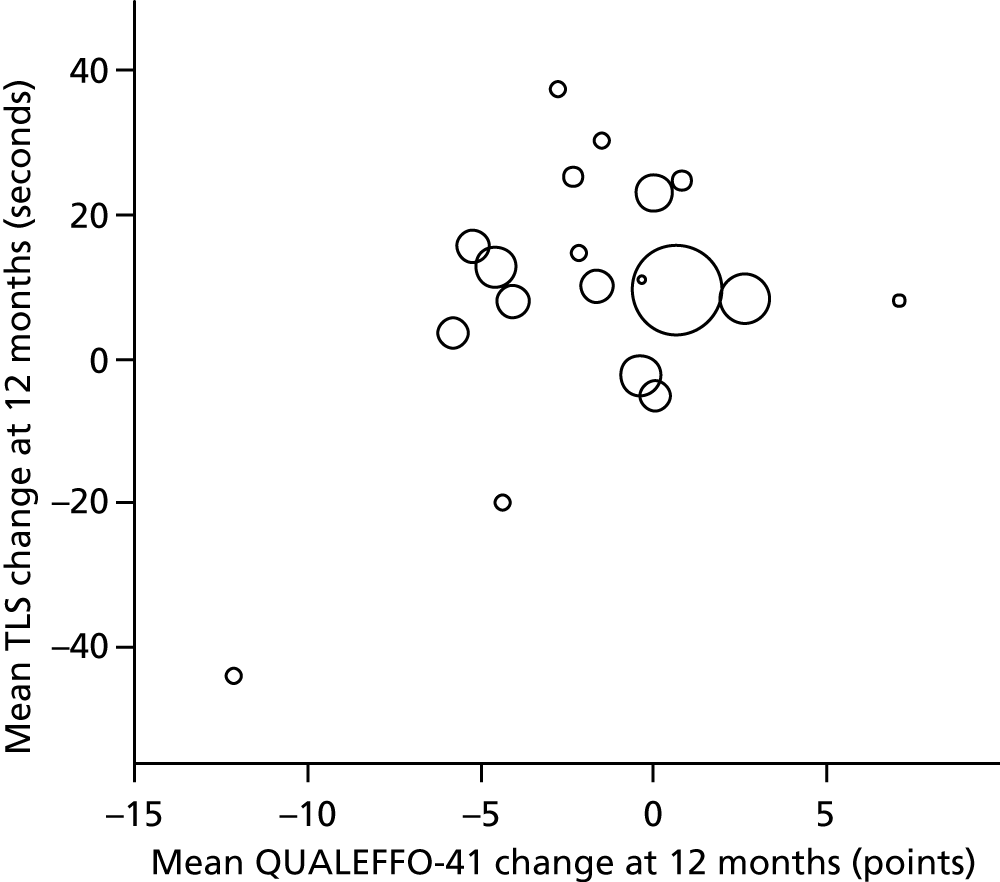
Comparison of baseline characteristics for participants with missing and non-missing primary end-point data
The results of sensitivity analyses comparing baseline and demographic data for participants with and without 12-month QUALEFFO-41 or TLS test data are given in Tables 16 and 17. There is an indication that participants who were missing for the 12-month QUALEFFO-41 or TLS assessment had more restricted walking ability, more back pain on the day of the assessment and in the last 2 weeks, more frequent falls in the previous year, greater thoracic kyphosis and walked shorter distances in 6 minutes at baseline.
| Baseline characteristics | QUALEFFO-41 data | Comparison p-value | |
|---|---|---|---|
| Missing | Non-missing | ||
| Female patients, n (%) | 67 (81.7) (N = 82) | 463 (87.5) (N = 529) | p = 0.1484 |
| Age (years), mean (SD) | 71.15 (10.03) (N = 82) | 72.38 (8.9) (N = 529) | p = 0.2530 |
| Height (cm), mean (SD) | 156.9 (19.71) (N = 82) | 157.1 (17.5) (N = 528) | p = 0.9247 |
| Weight (kg), mean (SD) | 62.0 (15.3) (N = 82) | 64.0 (14.0) (N = 529) | p = 0.2351 |
| DEXA T-score, mean (SD) | –2.7 (1.3) (N = 66) | –2.7 (1.4) (N = 404) | p = 1.0000 |
| Number of spinal fractures, mean (SD) | 2.8 (1.8) (N = 76) | 2.5 (1.9) (N = 474) | p = 0.1987 |
| Number of non-spinal fractures, mean (SD) | 0.14 (0.4) (N = 72) | 0.14 (0.4) (N = 481) | p = 1.0000 |
| Fractures, n (%) | |||
| Upper lumbar (L1 and L2) | 24 (55.8) (N = 43) | 146 (44.8) (N = 326) | p = 0.1727 |
| Lower lumbar (L3–L5) | 29 (59.2) (N = 49) | 186 (51.7) (N = 360) | p = 0.3228 |
| Lower thoracic (T6–T12) | 61 (92.4) (N = 66) | 374 (85.8) (N = 436) | p = 0.1391 |
| Upper thoracic (T1–T5) | 7 (21.2) (N = 33) | 60 (20.5) (N = 293) | p = 0.9212 |
| Walking category, n (%)a | |||
| 1 | 31 (37.8) (N = 82) | 249 (47.3) (N = 526) | p = 0.4590 |
| 2 | 21 (25.6) (N = 82) | 112 (21.3) (N = 526) | |
| 3 | 25 (30.5) (N = 82) | 126 (24.0) (N = 526) | |
| 4 | 5 (6.1) (N = 82) | 37 (7.0) (N = 526) | |
| 5 | 0 (0.0) (N = 82) | 2 (0.4) (N = 526) | |
| 6 | 0 (0.0) (N = 82) | 0 (0.0) (N = 526) | |
| Stair use categoryb | |||
| 1 | 33 (41.3) (N = 80) | 215 (41.4) (N = 519) | p = 0.1934 |
| 2 | 5 (3.8) (N = 80) | 46 (8.9) (N = 519) | |
| 3 | 4 (5.0) (N = 80) | 18 (3.5) (N = 519) | |
| 4 | 33 (41.3) (N = 80) | 223 (43.0) (N = 519) | |
| 5 | 0 (0.0) (N = 80) | 0 (0.0) (N = 519) | |
| 6 | 7 (8.8) (N = 80) | 17 (3.3) (N = 519) | |
| Aid use category, n (%)c | |||
| 1 | 58 (70.7) (N = 82) | 347 (66.0) (N = 526) | p = 0.4573 |
| 2 | 12 (14.6) (N = 82) | 117 (22.2) (N = 526) | |
| 3 | 6 (7.3) (N = 82) | 27 (5.1) (N = 526) | |
| 4 | 0 (0.0) (N = 82) | 4 (0.8) (N = 526) | |
| 5 | 0 (0.0) (N = 82) | 4 (0.8) (N = 526) | |
| 6 | 6 (7.3) (N = 82) | 27 (5.1) (N = 526) | |
| Patients with back pain, n (%) | |||
| In previous 2 weeks | 81 (98.8) (N = 82) | 508 (96.0) (N = 529) | p = 0.2136 |
| Today | 61 (74.4) (N = 82) | 355 (67.2) (N = 528) | p = 0.1955 |
| Falls in previous year, mean (SD) | 0.94 (1.86) (N = 82) | 0.67 (1.64) (N = 523) | p = 0.0961 |
| Falls in category, n (%)d | |||
| 1 | 1 (1.3) (N = 78) | 1 (0.2) (N = 508) | p = 0.0714 |
| 2 | 9 (11.5) (N = 78) | 32 (6.3) (N = 508) | |
| 3 | 68 (87.2) (N = 78) | 475 (93.5) (N = 508) | |
| Patients with pain, n (%) | |||
| In last 2 weeks | |||
| Lower lumbar (L3–L5) | 38 (71.7) (N = 53) | 300 (71.9) (N = 417) | p = 0.9703 |
| Upper lumbar (L1 and L2) | 31 (62.0) (N = 50) | 206 (55.7) (N = 370) | p = 0.3973 |
| Lower thoracic (T6–T12) | 41 (69.5) (N = 59) | 205 (53.2) (N = 385) | p = 0.0194 |
| Upper thoracic (T1–T5) | 24 (47.1) (N = 51) | 128 (37.0) (N = 346) | p = 0.1675 |
| Today | |||
| Lower lumbar (L3–L5) | 27 (67.5) (N = 40) | 189 (66.8) (N = 283) | p = 0.9283 |
| Upper lumbar (L1 and L2) | 21 (56.8) (N = 37) | 132 (53.7) (N = 246) | p = 0.7244 |
| Lower thoracic (T6–T12) | 28 (66.7) (N = 42) | 139 (54.5) (N = 255) | p = 0.1412 |
| Upper thoracic (T1–T5) | 13 (38.2) (N = 34) | 76 (33.9) (N = 224) | p = 0.6225 |
| Functional comorbidities index, mean (SD) | 0.85 (1.06) (N = 80) | 0.76 (1.39) (N = 526) | p = 0.5791 |
| PASE, mean (SD) | 114.9 (99.7) (N = 69) | 110.2 (78.5) (N = 469) | p = 0.6549 |
| Thoracic kyphosis, (degrees) mean (SD) | 55.8 (33.6) (N = 80) | 48.7 (33.4) (N = 524) | p = 0.0773 |
| SPPB, mean (SD) | 8.1 (2.2) (N = 77) | 8.5 (2.1)) (N = 490) | p = 0.1232 |
| FRT (cm), mean (SD) | 22.6 (10.2) (N = 82) | 23.8 (9.6) (N = 528) | p = 0.2968 |
| 6MWT (m), mean (SD) | 286.1 (150.1) (N = 82) | 307.2 (127.6) (N = 527) | p = 0.1748 |
| VAS pain, mean (SD) | 5.3 (2.4) (N = 61) | 4.5 (2.1) (N = 353) | p = 0.0075 |
| Baseline characteristics | TLS test data | Comparison p-value | |
|---|---|---|---|
| Missing | Non-missing | ||
| Female patients, n (%) | 121 (83.4) (N = 145) | 401 (87.6) (N = 458) | p = 0.2063 |
| Age (years), mean (SD) | 72.88 (10.39) (N = 145) | 71.85 (8.51) (N = 458) | p = 0.2300 |
| Height (cm), mean (SD) | 156.3 (15.9) (N = 144) | 157.3 (18.4) (N = 458) | p = 0.5575 |
| Weight (kg), mean (SD) | 62.19 (15.5) (N = 145) | 64.2 (13.6) (N = 458) | p = 0.1346 |
| DEXA T-score, mean (SD) | –2.7 (1.3) (N = 110) | –2.7 (1.33) (N = 356) | p = 1.0000 |
| Number of spinal fractures, mean (SD) | 2.7 (1.8) (N = 128) | 2.6 (1.96) (N = 415) | p = 0.6073 |
| Number of non-spinal fractures, mean (SD) | 0.16 (0.44) (N = 129) | 0.13 (0.39) (N = 420) | p = 0.4591 |
| Fractures, n (%) | |||
| Upper lumbar (L1 and L2) | 38 (47.5) (N = 80) | 129 (45.6) (N = 283) | p = 0.7613 |
| Lower lumbar (L3–L5) | 51 (53.7) (N = 95) | 161 (52.4) (N = 307) | p = 0.8323 |
| Lower thoracic (T6–T12) | 105 (91.3) (N = 115) | 323 (84.8) (N = 381) | p = 0.0745 |
| Upper thoracic (T1–T5) | 10 (14.9) (N = 67) | 57 (22.3) (N = 256) | p = 0.1871 |
| Walking category, n (%)a | |||
| 1 | 48 (33.3) (N = 144) | 231 (50.8) (N = 455) | p = 0.0023 |
| 2 | 41 (28.5) (N = 144) | 93 (20.4) (N = 455) | |
| 3 | 38 (26.4) (N = 144) | 105 (23.1) (N = 455) | |
| 4 | 16 (11.1) (N = 144) | 25 (5.5) (N = 455) | |
| 5 | 1 (0.7) (N = 144) | 1 (0.2) (N = 455) | |
| 6 | 0 (0.0) (N = 144) | 0 (0.0) (N = 455) | |
| Stair use category, n (%)b | |||
| 1 | 50 (35.5) (N = 141) | 197 (43.9) (N = 449) | p = 0.0076 |
| 2 | 7 (5.0) (N = 141) | 42 (9.4) (N = 449) | |
| 3 | 2 (1.4) (N = 141) | 19 (4.2) (N = 449) | |
| 4 | 73 (51.8) (N = 141) | 178 (39.6) (N = 449) | |
| 5 | 0 (0.0) (N = 141) | 0 (0.0) (N = 449) | |
| 6 | 9 (6.4) (N = 141) | 13 (2.9) (N = 449) | |
| Aid use category, n (%)c | |||
| 1 | 87 (60.4) (N = 144) | 313 (68.8) (N = 455) | p = 0.0020 |
| 2 | 28 (19.4) (N = 144) | 102 (22.4) (N = 455) | |
| 3 | 13 (9.0) (N = 144) | 19 (4.2) (N = 455) | |
| 4 | 0 (0.0) (N = 144) | 4 (0.9) (N = 455) | |
| 5 | 1 (0.7) (N = 144) | 2 (0.4) (N = 455) | |
| 6 | 15 (10.4) (N = 144) | 15 (3.3) (N = 455) | |
| Patients with back pain, n (%) | |||
| In previous 2 weeks | 140 (96.6) (N = 145) | 441 (96.3) (N = 458) | p = 0.8827 |
| Today | 113 (78.5) (N = 144) | 298 (65.1) (N = 458) | p = 0.0026 |
| Number of falls in previous year, mean (SD) | 0.88 (1.66) (N = 144) | 0.63 (1.67) (N = 453) | p = 0.3259 |
| Falls in category, n (%)d | |||
| 1 | 1 (0.7) (N = 139) | 1 (0.2) (N = 440) | p = 0.2495 |
| 2 | 13 (9.4) (N = 139) | 26 (5.9) (N = 440) | |
| 3 | 125 (89.9) (N = 139) | 413 (93.9) (N = 440) | |
| Patients with pain, n (%) | |||
| In previous 2 weeks | |||
| Lower lumbar (L3–L5) | 75 (71.4) (N = 105) | 257 (71.8) (N = 358) | p = 0.9427 |
| Upper lumbar (L1 and L2) | 60 (63.2) (N = 95) | 172 (53.9) (N = 319) | p = 0.1112 |
| Lower thoracic (T6–T12) | 66 (63.5) (N = 104) | 180 (53.4) (N = 337) | p = 0.0713 |
| Upper thoracic (T1–T5) | 41 (44.1) (N = 93) | 110 (36.5) (N = 301) | p = 0.1911 |
| Today | |||
| Lower lumbar (L3–L5) | 52 (62.7) (N = 83) | 161 (68.2) (N = 236) | p = 0.3541 |
| Upper lumbar (L1 and L2) | 45 (59.2) (N = 76) | 105 (51.7) (N = 203) | p = 0.2642 |
| Lower thoracic (T6–T12) | 46 (59.0) (N = 78) | 121 (55.8) (N = 217) | p = 0.6233 |
| Upper thoracic (T1–T5) | 25 (36.8) (N = 68) | 63 (33.5) (N = 188) | p = 0.6283 |
| Functional comorbidities index, mean (SD) | 0.89 (1.04) (N = 142) | 0.74 (1.44) (N = 454) | p = 0.2503 |
| PASE, mean (SD) | 102.3 (90.6) (N = 129) | 114.5 (78.7) (N = 400) | p = 0.1411 |
| Flexicurve, mean (SD) | 56.7 (50.4) (N = 142) | 47.6 (25.9) (N = 455) | p = 0.0047 |
| SPPB, mean (SD) | 7.8 (2.2) (N = 129) | 8.7 (2.0) (N = 431) | p = 0.0000 |
| FRT (cm), mean (SD) | 22.0 (11.3) (N = 145) | 24.1 (9.0) (N = 457) | p = 0.0221 |
| 6MWT (m), mean (SD) | 268.1 (143.3) (N = 145) | 318.1 (123.1) (N = 456) | p = 0.0000 |
| VAS pain, mean (SD) | 4.9 (2.2) (N = 112) | 4.6 (2.1) (N = 297) | p = 0.2043 |
Complier-average causal effect analysis
Table 18 presents the results of the planned CACE analysis, estimating the effect of the intervention on QUALEFFO-41 and TLS test score change at 4 and 12 months for compliers; compliance is defined as attending either at least four (partial compliance) or all seven sessions (full compliance). These analyses are adjusted for baseline and centre but are not corrected for the adaptive design used. The effects of the interventions are larger in magnitude for full compliers than for partial compliers at both time points. The small beneficial effects of exercise therapy on QoL and muscle endurance increased for those who attended seven sessions but did not reach significance. The small reduction in QoL and muscle endurance at 12 months in the manual therapy arm was magnified in those who fully complied but, again, this did not reach significance. For both full and partial compliers, a significant treatment effect of manual therapy was observed on the TLS test at 4 months, which is in line with the ITT analysis. Subsequent, unplanned analyses corrected for multiplicity and adaptive design are presented in Appendix 23. These conservative analyses continue to show the beneficial effect of manual therapy on muscle endurance at 4 months and that this increases with increasing compliance (adjusted p = 0.055). They confirm that increasing dose has no significant effect on QoL (both arms) or muscle endurance (exercise therapy arm).
| Analysis | Change relative to SSPT (95% CI); p-value | |
|---|---|---|
| Exercise therapy arm | Manual therapy arm | |
| QUALEFFO-41 change at 12 monthsa | ||
| Compliance = attendance at at least four sessions | –0.11 (–2.63 to 2.40); p = 0.927 | 1.00 (–1.47 to 3.47); p = 0.427 |
| Compliance = attendance at all sessions | –0.21 (–4.61 to 4.20); p = 0.927 | 1.60 (–2.36 to 5.57); p = 0.427 |
| TLS test change at 12 monthsb | ||
| Compliance = attendance at at least four sessions | 5.49 (–8.18 to 19.16); p = 0.431 | 8.96 (–2.05 to 19.98); p = 0.111 |
| Compliance = attendance at all sessions | 9.28 (–13.82 to 32.39); p = 0.431 | 14.37 (–3.29 to 32.03); p = 0.111 |
| QUALEFFO-41 change at 4 monthsa | ||
| Compliance = attendance at at least four sessions | –1.61 (–3.65 to 0.42); p = 0.120 | 0.34 (–1.48 to 2.17); p = 0.714 |
| Compliance = attendance at all sessions | –2.82 (–6.40 to 0.77); p = 0.123 | 0.55 (–2.38 to 3.47); p = 0.714 |
| TLS test change at 4 monthsb | ||
| Compliance = attendance at at least four sessions | 9.70 (–1.49 to 20.90); p = 0.089 | 12.45 (2.87 to 22.03); p = 0.011 |
| Compliance = attendance at all sessions | 16.82 (–2.59 to 36.23); p = 0.089 | 19.93 (4.63 to 35.23); p = 0.011 |
Adverse events
There were no serious adverse events (SAEs) (defined as death, life-threatening adverse experiences or related inpatient hospitalisations) and no reportable adverse events associated with the trial in any of the arms according to the agreed and prespecified criteria.
During the recruitment and follow-up period, 85 SAEs were reported to the trial team. SAEs were classified through discussions with the local principal investigators, the trial lead and one of the co-applicants (Dr Muhammad K Javaid, Consultant in Metabolic Bone Medicine). Full information was requested from the participant when a potential SAE was noted, particularly with regard to the likelihood of trial treatment being the cause. No SAEs were deemed both unexpected and related to the trial involvement. All SAEs were regularly reviewed by the DMEC. The categories of SAE, likelihood of relatedness and reasons are summarised in Table 19 and a comprehensive list and description of each event is reported in Appendix 21. There were numerous non-reportable events in both arms, including falls, further compression fractures and cardiorespiratory problems.
| SAE data | Number of events, by trial arm | Total number of events (%) | ||
|---|---|---|---|---|
| SSPT | Manual therapy | Exercise therapy | ||
| SAEs reported | 22 | 37 | 26 | 85 (100) |
| Falls | 4 | 6 | 5 | 15 (18) |
| Fractures | 8 | 9 | 6 | 23 (27) |
| Related to intervention | 0 | 2 | 0 | 2 (2.3) |
| Unrelated to intervention | 22 | 35 | 26 | 83 (97.7) |
In the exercise therapy arm, 24 participants receiving the intervention disclosed a total of 26 different non-reportable events, including five falls and six fractures (two fractures were sustained during a fall). In the manual therapy arm, 34 participants receiving the intervention disclosed a total of 37 different non-reportable events, with six falls and nine fractures (three fractures were sustained during a fall). In the SSPT arm, 22 participants disclosed 22 different non-reportable events, including four falls and eight fractures (three fractures were sustained during a fall).
No fractures were sustained during delivery of the interventions (a concern raised before the trial for manual therapy), and there was no difference in the number of adverse events between the treatment arms.
Chapter 5 Qualitative study
Introduction and objective of the study
This qualitative study was carried out to explore the experiences and views of people with osteoporosis regarding their participation in the PROVE trial, their treatment interventions, their perceptions regarding the appropriateness of the interventions and to explore factors influencing adherence to the intervention programmes.
Methods
Sampling procedure
Purposive sampling was used to achieve a sample that included:
-
Female and male participants. Because the majority of research with people with osteoporosis fracture has previously been undertaken with women,131 it was considered important to capture the views of male participants within the study.
-
Participants with varying number(s) and location(s) of fracture. It was anticipated that this approach would also include people describing a wide range of levels of physical activity and include middle-aged and older people.
Sample size
We used interpretative phenomenological analysis (IPA), which recommends involving small numbers of participants to gain a rich and in-depth account. 132 We anticipated that approximately five participants from each arm (approximately 15 in total) would provide a rich insight into their experience of the intervention. Eighteen participants were recruited and recruitment halted when a wide range of views had been obtained and no new topic areas were being raised by participants.
Approaching and recruitment of participants
Potential participants to achieve the specified sample were identified by the trial co-ordinator and invitations to participate in the qualitative study were posted out to potential participants. Recruitment took place in two stages: the first stage was early in the trial, to explore the acceptability of the intervention at the outset of the PROVE trial. The PROVE trial was then gradually rolled out across multiple sites. Recruitment to the qualitative research was paused while more sites began recruitment and was then restarted when participants from a wider geographical area and from different treatment centres could be recruited.
Twenty people were approached, of whom 18 were willing to participate in the study. Interviews were held at the homes of participants at a time convenient for them.
Interviews
Participants engaged in in-depth semistructured interviews after they had received their allocated intervention. Written consent was obtained at the start of visits before interviews commenced. An interview guide was developed using data from the PROVE pilot; however, this was not followed rigidly: follow-up questions and probes were used to help the interview flow, ensuring that relevant areas were covered and allowing participants to introduce new areas of relevant discussion. 133 The same experienced physiotherapy researcher (CML) carried out all interviews. Participants were encouraged to discuss any areas they believed to be relevant to their experiences of participating in the PROVE trial. After the interviews, time was spent in everyday conversation with the participants to allow the interviewer to check the well-being of the interviewees. 134 Field notes and memos were recorded in a study diary.
Interviews were digitally audio-recorded and fully transcribed, then all transcripts were checked again against the recordings and transcribing errors were corrected.
Data analyses
Audio-recordings were listened to and transcripts were read until they became familiar. The data were coded in accordance with IPA. 132 Transcript data were broken down into discrete units, making concerted efforts to remain close to the data and continually explore meaning (by CML). The first three transcripts were independently coded by two researchers (CML and KLB). During discussion of these three transcripts, it was agreed that, because similar coding units had been identified by both researchers, it was unnecessary in this instance for all transcripts to be independently coded. All transcripts were coded by one researcher (CML), who then grouped together units found to be conceptually similar into categories. All categories, units and data for each unit were imported into NVivo 11 software (QSR International, Warrington, UK). The units, descriptors of the units and data for each unit were then checked (by Karen L Barker and Dr Francine Toye, who provided independent peer review during analyses) to ensure that the data fully supported the unit and category descriptors. The process of constantly comparing data, codes and categories took place throughout all analyses and the team met regularly to discuss the data. Five randomly chosen transcripts were recoded at least 6 months after first coding. Rigour was therefore promoted by the use of peer review, dependability and confirmability audits, a reflexivity diary and field notes and by using an experienced researcher. 135
Findings
The characteristics of people participating in the qualitative study are presented in Table 20.
| Interview number | Allocated treatment arm | Sex | Age at interview (years) | Number of fractures (location of fractures) | Number of falls | Number of sessions attended/offered) |
|---|---|---|---|---|---|---|
| 1 | Exercise therapy | Female | 70 | 2 (L1–L5) | 1 | 5 |
| 2 | Manual therapy | Female | 72 | 9 (T6–L5) | 0 | 4 |
| 3 | Manual therapy | Female | 90 | 5 (T6–L5) | 0 | 6 |
| 4 | Exercise therapy | Male | 66 | 5 (T1–T12) | 0 | 4 |
| 5 | Manual therapy | Female | 70 | 4 (T6–L5) | 1 | 6 |
| 6 | Manual therapy | Female | 85 | 15 (T6–L5) | 1 | 6 |
| 7 | Manual therapy | Female | 78 | 4 (T10–L3) | 0 | 6 |
| 8 | SSPT | Female | 84 | 1 (T6) | 1 | 1 |
| 9 | SSPT | Male | 70 | 4 (T12–L2) | 1 | 1 |
| 10 | Exercise therapy | Male | 69 | 2 (L3–L5, T6–T12) | 0 | 5 |
| 11 | SSPT | Male | 53 | 5 (T1–L5) | 0 | 1 |
| 12 | SSPT | Male | 99 | Unknown | 1 | 1 |
| 13 | Exercise therapy | Male | 90 | 4 (T6–L5) | 0 | 1 |
| 14 | Manual therapy | Male | 55 | 3 (T6–T12) | 0 | 5 |
| 15 | Exercise therapy | Female | 69 | 1 (T6–T12) | 1 | 6 |
| 16 | Exercise therapy | Male | 52 | 2 (L3–L5) | 0 | 6 |
| 17 | Exercise therapy | Male | 70 | 1 (L1 and L2) | 0 | 0 |
| 18 | SSPT | Female | 54 | 1 (T6–T12) | 0 | 0 |
A wide geographical and urban/rural spread of interviewees was obtained; interviews took place across central and south-eastern England. Interview visit durations ranged from 1 hour and 15 minutes to 3 hours; the average duration of digital recordings was 61 minutes (range 41–81 minutes).
The findings are presented in three main categories:
-
the experiences, perceptions and views of participants regarding the interventions received in the PROVE trial, their physiotherapists and any further treatment received by participants
-
the experiences, perceptions and views of participants regarding participating in the wider PROVE trial, treatment preferences and identified improvements
-
adherence to interventions and being physically active.
The PROVE trial interventions
Manual therapy treatment arm
This intervention was well accepted and appreciated by the participants who were interviewed. It was perceived as a very enjoyable and effective treatment by participants, even if participants described a brief period of symptom reduction (this varied from hours to months):
I was fortunate enough to be in the group whereby there was a hands-on massage of my back as well, I could have laid there forever, that was just wonderful.
Interviewee 3
Participants had no concerns about receiving ‘hands-on’ care because they were confident in the expertise and professionalism of their physiotherapist: ‘I knew they were professionals that knew what they were doing’ (interviewee 7) and ‘I know how good physios [physiotherapists] are’ (interviewee 14).
One participant (interviewee 5) was initially apprehensive (‘a bit ooo to start with’) but then found it ‘very very good’.
A range of benefits from manual therapy were described by participants, including improved mobility, reduced stiffness, soothing, energising and the immediate results of ‘hands-on’ treatment, which were appreciated by participants in this arm:
I think I am more mobile and I am holding up better. I think I would have gone over.
Interviewee 2
Very good. It eased the muscles. It eased the joints. Made me more mobile. Got rid of the niggles in the back, etc., and it did improve, sort of, the quality of life, from the back point of view.
Interviewee 14
I think invigorated, you feel quite mmm yes.
Interviewee 2
However, not all participants’ posture and back pain improved: one person said ‘I think it’s the same’ (interviewee 6). Participants also valued the mental health benefits of the intervention:
Also the fact that I’m doing something towards it, I’m not just sitting there thinking ‘oh well, this is my lot’.
Interviewee 2
No side effects of manual therapy were described, although one participant (interviewee 3) thought that not everyone might like ‘hands-on’ massage.
The use of taping was less positively viewed. Several people spoke of the difficulties of keeping the tape in place and removing it at home or disliked it:
[Taping] was good, but it’s difficult on your own to cope with that tape, you know, it started to come off. So I had to get, I had to get my daughter-in-law to help me get it off.
Interviewee 7
[Tape] which was weird, that drove me mad a bit.
Interviewee 2
The spinal exercises were viewed positively and some participants continued to use them, although one participant valued the manual therapy but felt that they needed (and arranged to have) further treatment to improve their core strength and posture using an exercise programme:
[Manual therapy] did loosen up the muscles and it got rid of the knots and it made me more mobile and it got rid of the pains, but what it didn’t do, it didn’t correct any postural trap problems or the core muscles.
Interviewee 14
The amount/dose of exercises was acceptable, although some participants found it hard to do the exercises. Three participants were unable to exercise in the prone position because it was ‘just too uncomfortable’ (interviewee 3) or was like ‘pinched nerves’ (interviewee 2).
Exercise treatment arm
The exercise treatment intervention was accepted and viewed positively by most, but not all, of the interviewed participants in this arm.
Some participants spoke of the benefits of exercise, including reduced pain and stiffness, improved strength and function/mobility: ‘they got me mobile’ (interviewee 15), ‘loosened up’ (interviewee 10) and ‘my core muscles have really strengthened up’ (interviewee 4).
Participants described treatment being individualised and progressed by their physiotherapist. Some participants (interviewees 1 and 4) initially found some exercises painful/too difficult, which led to the exercise being modified by the physiotherapist or halted by the participant:
At first . . . some of the exercises she gave me were very painful. And when I went back to see her she told me to stop doing those and to do different ones, and she said I’ve got to do exercises that don’t hurt but that help, and then gradually I then, as I built up I went back to the exercises that hurt and found that I could do them. So, it was a very gradual thing, I didn’t notice any benefit at all for quite a few weeks but then, after I had been doing it for a while, I really did see the benefit.
Interviewee 4
You’d start at the beginning and you go through it . . . get a more difficult one.
Interviewee 10
Most participants found the regular contact with, and monitoring by, their physiotherapists a helpful element of their treatment [‘I feel very supported when I’m in a programme’ (interviewee 1)] and useful for keeping them motivated and adhering to the programme [because it ‘focused you on what you were doing’ (interviewee 16), was ‘on my conscience more’ (interviewee 13) and is ‘all supporting . . . the skeleton’ (interviewee 10)]. Some found recording their exercises in the diary a helpful element of their treatment: ‘it gives you a structure . . . you tick tick tick’ (interviewee 10), ‘I have benefited from . . . keeping the diaries . . . gets you in the routine and you don’t forget’ (interviewee 4).
Post treatment, people described being active and continuing doing the exercises they found helpful, ‘good’ (interviewees 1 and 10) and/or easy and were ‘just going to carry on doing them now’ (interviewee 4).
I do things that are easy but make a difference.
Interviewee 1
The participants were more likely to jettison exercises that they perceived provided no benefit or were ‘too hard work’ (interviewee 1), too difficult or were in starting positions (such as on the floor) that participants perceived as potentially unsafe (n = 1).
The dose/amount of exercise was accepted, but some participants (interviewees 13 and 17) admitted that they no longer/had not exercised as much as they should (owing to factors such as time, effort and fatigue):
I’m afraid I haven’t done them as thoroughly, am afraid since I stopped going. I do the stretching exercises now and again. I do lots of stretching ones and pushing up ones nearly every day. Sometimes I haven’t pursued very far.
. . . I’m so tired that I go to the [sic] sleep, I might sleep for an hour and a half in the afternoon and then it’s time to start doing things again but I try and get the walking done, that is good.
Interviewee 13
I have to admit that I didn’t pursue the exercise correctly as well as I should have done because I just didn’t have time and by the time the evening came I was too **** tired to start doing exercises.
Interviewee 17
The exercise intervention was experienced as less valuable/helpful, or of no benefit, by some participants who were already exercising regularly when they entered the trial (e.g. they were attending Pilates groups or were gym members):
Frankly . . . I know about exercise, I belong to a gym, I go walking, not as much as I ought to but who does, so it was a bit of a waste of time.
Interviewee 17
This participant ‘got low’ and fed up during treatment and would have preferred a ‘motivating’ group setting. Another person also stated that they would have preferred a group setting with longer treatment sessions and greater supervision of exercise: ’20-min [minute], 30-min sessions sometimes, that’s not really long enough’ (interviewee 15).
Single session of physiotherapy
The majority of people randomised to SSPT who were interviewed did not expect to receive any physiotherapy treatment and did not consider attending SSPT as having received any physiotherapy treatment in the trial:
Oh well I didn’t have physio [physiotherapy] treatment.
Interviewee 8
I thought there’d be nothing.
Interviewee 16
Some were ‘disappointed’ (interviewee 9) or ‘left out’ (interviewee 18), feeling that they had received lesser care than those in the other treatment arms, and one person acted on this by arranging non-trial treatment:
I’m in the control group, as you know, which in theory nothing is happening to, but I . . . thought I must do something, I am not just going to sit around and do nothing so I have been practising the Alexander Technique with a teacher . . .
Interviewee 9
Some participants (interviewees 9 and 11) felt that they had learnt nothing new, because they knew the information provided prior to entering the trial: ‘I knew because I had read stuff’ (interviewee 11). Others described learning new helpful information (interviewees 8 and 18) and continuing to carry out exercises (interviewees 8 and 12).
Benefits of physiotherapy and clinical physiotherapists
Some participants spoke of their perception of physiotherapy in general, for example the mental health benefits from feeling helped [‘mentally you feel that you are being helped, and I think, makes you feel better’ (interviewee 2)] and being listened to and encouraged [‘she listened to what I was saying about my back . . . and she was encouraging (interviewee 13)]. There were no negative views regarding the physiotherapy clinicians providing treatment in the PROVE trial. Physiotherapists were viewed as pleasant, knowledgeable and effective practitioners:
Whoever does it, needs to have the knowledge, understanding and training, which, I think, is probably, basically a physio [physiotherapist].
Interviewee 14
One participant valued the continuity of care and seeing the same physiotherapist each time:
Obviously they knew what they were doing, I mean it’s quite a busy little department there, isn’t it? And because I tended to see the same person.
Interviewee 16
Additional treatment
In addition to the participant allocated to the SSPT arm who was improving after arranging his own private treatment (interviewee 9), several participants had received further physiotherapy following their participation in the PROVE trial. One participant in the manual therapy arm was referred for further NHS physiotherapy after they noticed that their posture was deteriorating, and they were prescribed an exercise programme (interviewee 14). One participant had been involved in a car accident after the PROVE trial and was receiving physiotherapy as part of their recovery (interviewee 16). Another participant (interviewee 8) arranged for private physiotherapy.
Participating in the PROVE trial
This section includes the perceptions and experiences of participants on the following topics:
-
overall perceptions of participating in the PROVE trial
-
reasons for participating in the PROVE trial
-
trial procedures and processes
-
treatment arm preferences and optimal treatment
-
perceptions of trial personnel
-
identified improvements.
Overall perceptions of participating in the PROVE trial
Some participants expressed how positive and good for them participation in the PROVE trial had been (interviewees 7, 10 and 18). One person appreciated how organised the trial was as a whole, with treatment being superior to usual clinical practice, and was glad that they had taken part (interviewee 14).
Although most participants viewed the trial positively, one participant felt that the trial adversely affected his mood:
I found myself and after I finished the PROVE course, I realised I’d got a bit not clinically depressed but a bit low because it kept bringing to mind the fact that I’d got osteoporosis and again, there wasn’t enough explanation of the purpose of the exercises and so on and question . . . I mean maybe it’s just my individual response but there was a whole psychological area that I thought could usefully be thought about.
Interviewee 17
This person described becoming low in mood because participation in the PROVE trial kept bringing his condition to mind, and he did not believe that he was in the right treatment arm or that it helped him. He believed that this was because he received insufficient explanation of his exercises and received one-to-one treatment rather than exercising with a supportive peer group. He compared this treatment with his previous cardiac rehabilitation care and believed that lifestyle advice and peer support should be included in osteoporosis care. He improved after the trial: ‘I got a lot happier because it was over’ (interviewee 17).
Reasons for participation
The majority of participants took part in the PROVE trial because they wanted to help other people [‘if it helps someone else’ (interviewee 2)] and/or wanted to improve knowledge and treatment for women and men with fractures due to osteoporosis; one participant also thought that it would help them understand their condition (interviewee 4):
Well I feel that this osteoporosis thing ought to be explored to its fullest. I think people ought to get much more information and background knowledge of it because it’s getting more and more relevant. Not only to women but I think perhaps men.
Interviewee 3
The impact of research was understood by participants and there was a confident expectation that the research would be useful and used: ‘people that take part in research really do help, it does count’ (interviewee 1). A few participants were grateful for the NHS treatment they had already received and wanted to support the NHS in return: ‘it’s literally wanting to do anything to support the people that have supported me’ (interviewee 1).
One person participated because they appreciated that not many people of his advanced age would be able to take part in the trial; he was also encouraged by his family (interviewee 12). Another participant (interviewee 16) took part because he was made aware that not many men were in the trial and, as a man, he thought that it would help the trial. One participant (interviewee 5) hoped that the trial would include hydrotherapy, which they had previously ‘loved’. One person (interviewee 15) participated in the trial because they were ‘desperate’ to access treatment and another (interviewee 18) joined because their treatment would be provided sooner in the PROVE trial than in usual clinical care.
Trial procedures and processes
Attending for the PROVE trial treatments
Two participants (interviewees 6 and 7) identified difficulties in attending for physiotherapy treatments because of travel and transport expenses:
I don’t drive and the buses are too unreliable . . . so I used the volunteer car service, which is excellent, they’ve got a really good volunteer service here but it’s £13 every time I go you see, so I spent nearly £200 with the physio [physiotherapist] and the course on just transport.
Interviewee 7
Randomisation and blinding
A minority of participants (n = 6) spoke about blinding and randomisation. One person mentioned that they had to remember to not unblind the research physiotherapist: ‘I had to remember to talk about Christmas, anything! [not to unblind her]’ (interviewee 2).
Importantly, some participants did not appear to understand randomisation. One participant thought that he had been picked to be in a particular arm because he looked as if he could do it:
I think they picked me for that because they probably thought that I was kind of, looked as if I could do it.
Interviewee 10
Another participant’s wife explained randomisation to him after he did not understand the explanation provided by the research physiotherapist and thought that he would have a choice of treatment: ‘I just remember it being a little bit confused, and I wasn’t absolutely sure’ (interviewee 16). Finally, one participant did not know which arm they had been allocated to after randomisation: ‘I don’t quite know really’ (interviewee 13).
Impact of visual scans or spinal curve measures
Two participants described the horror and upset of realising how affected their spine had become by osteoporosis when they viewed scans or graphs of their spine:
When [researcher] measures my spine I am horrified when I see the graph on the paper, I say, I ask her ‘Good ***. Is that my spine?’, and she said ‘Well, yes’, it’s a bit horrifying to come to the realisation that, that is what it looks like.
Interviewee 3
Subjective assessment (questionnaires, diaries and paperwork)
The questionnaires and paperwork for the trial were generally acceptable to most participants. Completion of paperwork, even if lengthy (it varied from ‘not long’ to up to an hour or so), was considered as an expected part of participating in a research study: ‘I felt it was just part of it [the trial]’ (interviewee 1), ‘enjoyed it’ (interviewee 1) and ‘it’s the whole point of the trial’ (interviewee 4). Some participants (interviewees 4 and 13) found the paperwork to be a chore: ‘it a bit of chore to complete . . . I don’t see how you could reduce it’ (interviewee 4). Others ‘did not mind’ (interviewee 1) completing paperwork [‘it’s not too bad’ (interviewee 2)] and, in general, completing it was acceptable to and not considered a problem for most participants, who steadily worked or ’whizzed’ (interviewee 16) their way through questionnaires/paperwork. Participants generally felt that the questionnaire was comprehensive and thorough: ‘it was pretty thorough wasn’t it? It went into everything’ (interviewee 7) and ‘I think you went quite deep there’ (interviewee 15). One person found the questionnaire problematic to answer, appreciating that pain is very subjective to measure and different for different people, but no participants reported difficulties with understanding the questionnaire questions:
Pain, 1 to 10. 1 to 10, for me, is very different to you, my mother, my daughter, etc., and it’s very subjective and it can be a problem to answer those questions, but you’ve got to ask them, then otherwise you haven’t got the information.
Interviewee 14
Objective assessment
The time taken for the assessment was ‘no problem’ (interviewee 26) and ‘fine’ (interviewee 7) for participants, either because they did not feel that the time it took was too long or because they had spare time: ‘that [time] is something I’ve got’ (interviewee 8). There were varied responses to the perceived difficulty of the assessment. One participant found the tests tiring:
Well I find them a bit tiring, like the walking is a bit tiring, I want to give up before I am supposed to. But I’ve never collapsed.
Interviewee 8
One participant found that her step count goal of 10,000 steps per day was unachievable: ‘quite hard work so I didn’t kind of get there’ (interviewee 1). Others explained that, because of their age and/or fitness level, the assessments and/or 10,000 steps per day were too easy. A longer route on the 6MWT, or a more difficult test, would have been preferred:
It would’ve been nicer for a longer route, but you’re doing it indoors and, also, I was lucky. I was young. I was relatively fit when I got identified. So, I was able to do quite a lot of distance.
Interviewee 14
I was doing more than 10,000 steps a day anyway so that wasn’t really a target.
Interviewee 16
Feedback
Several participants (interviewees 4, 15 and 17) wanted feedback on their assessments because they were interested to know if they had improved or deteriorated:
It would be nice to be told, to be given that information so you can see whether you’ve increased/decreased.
Interviewee 4
Treatment arm preferences and optimal treatment
The majority of participants had a preference for a particular trial arm when they entered the PROVE trial. The most popular preference was for the manual therapy arm (seven participants preferred this): ‘Ooh I rather fancied the massage!’ (interviewee 1). This was either because participants had previously enjoyed massages and found them helpful (interviewee 1) or because participants had not yet experienced ‘hands-on’ physiotherapy and were keen to try it (interviewees 4, 7 and 17): ‘I was so pleased when it [manual therapy] came up. I couldn’t believe it!’ (interviewee 7). One participant believed that there would be an ‘immediate response’ (interviewee 14) to manual therapy whereas exercise would take longer to produce results.
A couple of other participants preferred either manual therapy or exercise therapy (they did not mind which) but did not want to be allocated to receive a SSPT (interviewees 4 and 9). According to the trial database, one participant who was randomised to SSPT did not attend for treatment (interviewee 18). One participant reported a preference for a non-PROVE treatment (hydrotherapy), because they had loved previous hydrotherapy treatment: ‘And I was so hoping I was going to be in there again this time actually’ (interviewee 5). Another participant did not want to be allocated to the exercise therapy arm, preferring manual therapy, because they were already active and knowledgeable about exercise; they were disappointed to not be allocated to manual therapy (interviewee 17). According to the trial database, this participant did not attend for treatment.
Some participants ‘did not really mind’ (interviewee 2) which treatment arm they were allocated to (interviewees 2, 10 and 11) and were happy to go with the flow (interviewee 13). One participant stated that receiving any trial treatment would be beneficial for health and/or mental health (interviewee 10). Several participants had reported experiencing fracture(s) during non-physiotherapy manipulations; one was slightly nervous about the thought of manual therapy but also believed physiotherapy to be different to chiropractic care (interviewee 16).
Optimal treatment
Some participants described what they believed constituted the optimal physiotherapy treatment and this varied widely. Some felt that going to an exercise group for/after their physiotherapy treatment would be enjoyable and beneficial [‘you realise you are not on your own’ (interviewee 17)] and would give them ‘motivation’ (interviewee 15) to continue exercising in the longer term:
In an ideal world it would be nice for somebody to have, to see a physiotherapist and do some exercises and then be reinforced by going into a group with other people.
Interviewee 1
Other participants preferred individualised treatment, valuing the opportunity to discuss their condition one to one: ‘the one on one’s best really, yes because then you can talk it through can’t you?’ (interviewee 7).
Other participants believed that early treatment post onset/diagnosis is important to help inform and treat patients (interviewees 7 and 10). Another participant felt that treatment might need to be sequential [i.e. manual therapy first then followed, at some stage, by an exercise programme and with ‘top-ups’ in the future as/if needed (interviewee 14)]. The amount of resources required, and the issues involved in this, were identified by this participant. Hydrotherapy was very positively viewed by people who had experienced this before participating in the PROVE trial (interviewees 7 and 15). One person was unsure whether or not further treatment would be too much for his back:
The amount of damage that is in my spine at the moment, I don’t know whether physio [physiotherapy] would be a good idea because I don’t know if it would cause more fractures.
Interviewee 11
Perceptions of trial personnel
The trial team were viewed positively by trial participants:
Everybody has been brilliant.
Interviewee 2
I couldn’t fault the people in [trial hospital] that carried out this stuff and all of the staff that I met there. They were brilliant. They were very encouraging and they were good fun.
Interviewee 10
Queries raised by participants were promptly addressed (interviewee 2). Staff were described as nice, kind and encouraging people. One participant believed that she received special attention from staff because she was in the trial:
You know, I felt a bit special because I was the one on the trial and all the other people in the waiting room sort of, he’d come out and see me, he was always very, very pleasant, really nice man.
Interviewee 18
Identified improvements
Participants were asked if there was any way that they thought the trial could have been improved. Participants were generally positive about and ‘very impressed’ (interviewee 3) by the trial and its organisation and many were unable to suggest ways in which the trial could be improved (interviewees 2, 6, 7, 9, 11, 12 and 18). The following suggestions were each made by one or two participants (there were no frequently suggested improvements):
-
a further clinical follow-up visit (at 3 or 6 months) to promote adherence to/progress with the exercises (interviewees 1 and 13)
-
include hydrotherapy (interviewee 5) as an intervention
-
offer technologies such as scanning and e-mailing exercises to participants (interviewee 14)
-
‘a reminder [telephone call] of what I had to be doing, every other month perhaps’ (interviewee 13)
-
involving a psychologist in the trial to consider the impact of participation on participants’ mental health [‘I think there is a point there’ (interviewee 17)].
Adherence to interventions and being physically active
Experience of physical activity and exercise: ‘as long as I’m active I’m much better’ (interviewee 4)
Exercise and/or physical activity was viewed as ‘vital’ (interviewee 12) and ‘necessary’ (interviewee 14) and was undertaken by the participants, both by those who had always enjoyed exercise and been active (interviewees 3, 4, 7, 10 and 17) and by those who had never been naturally athletic/sporty (owing to not enjoying it, perceived laziness, busyness of life or having a desk job) but who understood the importance of being/keeping active (interviewees 1 and 14):
No, the programmes have definitely, definitely helped, because I’m not an athletic person. I was never any good at games, I hated gym at school, I’m not naturally athletic at all, but I do know that exercise, exercise and keeping your weight down, which I find really really difficult.
Interviewee 1
I’ve always been active in sport, which I think, stands me in very good stead now, because if I think if I hadn’t, I think I would be in a very much worse way.
Interviewee 3
One participant (interviewee 15) thought that getting a friend to go to an exercise group with them would help their motivation. Several participants (interviewees 10, 13, 14 and 17) also described how, although they did fewer PROVE exercises than they felt they should do, they were going for walks and keeping active: ‘nothing will stop me walking’ (interviewee 10). Several participants who disliked going to the gym enjoyed walking and keeping active that way (interviewees 14 and 15). For those participants who had always enjoyed exercise/being active, it could be depressing/limiting when certain activities become no longer possible/advisable, although one participant (interviewee 16) simply switched to another type of exercise and did not mind doing this. Exercise was seen as being beneficial for mental health and ‘morale’ (interviewee 15) as well as physical health: ‘mentally you feel that you are being helped, and I think, makes you feel better’ (interviewee 2). Exercise/activity was considered beneficial, especially for people with osteoporosis, for whom the need to keep muscles strong and the spine supported is recognised (interviewee 10). Inactivity is considered harmful, resulting in adverse effects of seizing up, deep-vein thrombosis and ever-deteriorating function (interviewees 10 and 12).
Daily or regular routine
All participants described how they had a regular exercise and/or physical activity routine and had built this into their daily/weekly life: by exercising at the same time of day, attending regular gym sessions/classes, going ‘religiously’ for daily walks/walking every other day [‘for the rest of my days’ (interviewee 10)]:
I have a little routine of exercises that I do every morning.
Interviewee 1
One participant described doing one activity a day even though it exhausted them (interviewee 8). Not all participants always managed a daily/regular pattern despite planning to do so; factors such as fatigue could prevent this or reduce motivation, but they did not cease exercising and returned to it. For a couple of participants, keeping an exercise log helped them to monitor/plan their exercises (interviewees 4 and 14).
Perceived difficulty
The extent to which participants perceived exercises to be difficult or easy influenced adherence:
I do things that are quite easy but make a difference.
Interviewee 1
She gave me these exercises and again they seemed very basic, and I said to her at first I didn’t see any benefit in doing them, but over a period of time, you really do feel the benefit, my core muscles have really strengthened up and I’m just going to carry on doing them now.
Interviewee 4
When advised by their physiotherapist to do an exercise that the participants found painful, difficult, tiring or uncomfortable, participants tried and then stopped doing that exercise: ‘she told me to do some things which I just can’t do so I don’t’ (interviewee 1). Exercises in lying positions, especially in the prone position, were perceived as more difficult by some participants (interviewees 1, 3, 5, 13 and 15):
And she wanted me to lay on my front and do something but there was no way I could do that. I just, wasn’t . . . Oh, so uncomfortable, so uncomfortable.
Interviewee 3
A couple of participants also avoided lying because it could be difficult to get up. For one participant, who lived alone and could not get up from the floor, this exercise was perceived as unsafe. Participants were more likely to do exercises they found easy, although one participant (interviewee 17) did not adhere because they felt that the exercises were not adding anything new [‘the rest of them I was already doing’ (interviewee 17)] and preferred going to the gym and doing Pilates.
Perceived or observed benefits promote adherence
The participants (interviewees 1, 2, 4, 5, 6, 7, 8, 14 and 15) adhered to their exercises because of the perceived benefits and observed results of doing them. Some participants (interviewees 3 and 17) who did not see any improvements attributable to exercise either stopped exercising/the exercise or changed to another form of exercise: ‘I don’t think it makes a great deal of difference’ (interviewee 3).
The factors improving or reducing adherence are summarised in Figure 6.
FIGURE 6.
Summary of the factors that reduced or increased adherence to the PROVE trial.
Impact of symptoms and comorbidity
Adherence was adversely affected for participants experiencing symptoms of osteoporosis and comorbid conditions. Some older participants (interviewees 8 and 12) described how tired they were or how tired their back made them (a younger participant, interviewee 14, descried being tired after work) and how they had slowed down with age:
I do try to take a walk every day and I find it exhausting . . . but my whole back it just hurts, aches all the time. I mean, really deeply aches, yeah.
Interviewee 8
Some participants in the exercise arm (interviewees 13 and 17) admitted that they no longer/had not exercised as much as they should (owing to factors such as time, effort and fatigue):
I’m afraid I haven’t done them as thoroughly, am afraid since I stopped going. I do the stretching exercises now and again. I do lots of stretching ones and pushing up ones nearly every day. Sometimes I haven’t pursued very far.
. . . I’m so tired that I go to the [sic] sleep, I might sleep for an hour and a half in the afternoon and then it’s time to start doing things again but I try and get the walking done, that is good.
Interviewee 13
I have to admit that I didn’t pursue the exercise correctly as well as I should have done because I just didn’t have time and by the time the evening came I was too **** tired to start doing exercises.
Interviewee 17
The factors that influenced adherence are described earlier in this section and are summarised in Figure 6.
Visual proof
The impact of participants seeing visual images of their spines from scans is a strong incentive to adhere to exercise and keeping active: ‘it makes me do my exercises more’ (interviewee 1). Strong terms were used to describe how powerful and upsetting seeing such visual evidence of damage is for people with vertebral fractures due to osteoporosis:
Well, I, when [researcher] measures my spine I am horrified when I see the graph on the paper, I say, I ask her ‘Good ***. Is that my spine?’, and she said ‘Well, yes’, it’s a bit horrifying to come to the realisation that, that is what it looks like.
Interviewee 3
She showed me on screen and explained everything and my T12 I have made one hell of a mess of . . . it does upset me, to think what damage I’ve done to my spine . . . it’s quite horrific.
Interviewee 5
The pictures were on the computer so I saw them and I thought, ‘Blimey, hey’. And it’s quite shocking really when you see it in black and white. And you can see that the bones were just broken and really it’s just the fact your muscles are holding it all together.
Interviewee 16
People relate to these images at a deep level: a picture ‘speaks a thousand words’ (interviewee 16).
Visual changes in posture also prompted adherence:
I can remember all the exercises, such that, when I start realising I’m stooping over again, I don’t have to go back to get more physio [physiotherapy] or anything else.
Interviewee 14
I’ve lost my height as well . . . It is upsetting, you don’t want to get wider and shorter and hunched over.
Interviewee 15
Family history
Some, although not all, participants reported a family history of osteoporosis. The effect of this knowledge on adherence varied. Some participants (interviewee 1) feared becoming like their relative and this spurred them on to fully adhere to all of their osteoporosis treatment:
I was livid but it was also kind of quite inevitable because of my mum and I thought ‘Oh **** here we go, I’m going to be just like her’. But I won’t be. One, because of kind of medical science, because I’ve been able to take the Fosamax and because I’ve been really really aware of my diet. And the exercise. So I’m hoping that I’ll be better off than her, I’m sure I will be.
Interviewee 1
This participant felt that, with advances in treatment, the future would/could be different and she hoped that she would be better off than her relative. Not all inherited traits were negative; one participant (interviewee 1) felt that she had inherited her relative’s determination alongside their osteoporosis. Some participants were realistic about their family history, but one was still surprised when they were diagnosed with osteoporosis. Several participants expressed sorrow/awareness that they had/could pass osteoporosis on to their children; they informed their children and encouraged them to be aware and proactively healthy, active and promptly seek health care/checks if/when needed:
Unfortunately my daughter’s now been, she’s 44 [years old], she’s been diagnosed . . . I was sorry really because I felt that, thinking about it, it’s in our family so probably there’s nothing I could do about it. I’m sad, I’m sad she’s got it.
Interviewee 2
I’ve got two sons and we have kept them fully informed haven’t we . . . we’re very conscious, you know, that these things can be passed down and they’re very much aware aren’t they?
Interviewee 4
Fighting osteoporosis
For some participants (interviewees 2, 4, 6, 7, 11 and 12), adherence to exercise and/or keeping active was a way of fighting the progression of their osteoporosis and not letting osteoporosis win or beat them. Participants spoke of exercise being something they could do [‘I have done everything I can’ (interviewee 4)] instead of giving up and thinking that there is nothing that can be done to prevent/stave off deterioration:
I think the exercise, and also the fact that I’m doing something towards it, I’m not just sitting there thinking ‘oh well, this is my lot’.
Interviewee 2
Adhering to treatment can help give a sense of control over the future progression of osteoporosis [‘I will not let it beat me if I can help it’ (interviewee 5)], although one person believed that they had little control over the risk of future fracture (interviewee 9). People want to know that they have done all they possibly can to help themselves and strictly adhering to treatment is seen as part of this approach. One participant tried to not think about the future but still adhered to treatment (interviewee 18).
Potential consequences of osteoporosis
The potential consequences and/or expectation of worsening osteoporosis motivated adherence to both exercise/activity and medical treatment [i.e. all osteoporosis treatment was seen as, in general, ‘preventing’ (interviewee 8) deterioration as much as possible]. Further events and changes in osteoporosis could be depressing and upsetting:
I must say when I hit the ground for the second time I thought ‘Oh ****. I’ve done it again’. That was immensely depressing because I knew I’d got another 6 months of, you know, not being able to [do] very much and having to start all over again.
Interviewee 1
People do not ‘want to get any worse’ (interviewee 5) than they need to be [‘I am hoping I shan’t go over too much’ (interviewee 2), ‘I was kind of worrying about me ending up in a wheelchair’ (interviewee 10)] and do not see a situation in which they will no longer need to exercise or take care of themselves [‘I think most people with that would recognise that you need to do something to help yourself’ (interviewee 9)]. The potential consequences were taken seriously by participants. One participant felt that previous experience of fracture had prepared them for further fractures. Another participant expected his osteoporosis to worsen but not become ‘the be all and end all of life’ (interviewee 14):
I know there is light at the end of the tunnel, you will, you know, as long as it gives up like it did before, yes I could get through it. I think the first time it happens it’s the unknown, and it is quite difficult.
Interviewee 2
Further fracture or events
Further events/fractures influenced the extent to which participants could adhere to the PROVE trial and continued with exercise/activity post the PROVE trial in the long term. One participant was low in mood after appearing to have sustained further fracture(s) during a car accident; they were in pain, off work and receiving further treatment, ‘It does really [get me down], yeah . . . almost 2 years it was sort of fine then now it’s not and you couldn’t make it up could you?’ (interviewee 16). These participants were keen to resume exercise as soon as symptoms permitted because ‘it’s got to help’ (interviewee 5). Fear of further fracture was a concern for participants (interviewee 1). One participant described a light bulb moment: after his first fractures, he was in denial and did not comply with medical advice at all, but after a subsequent fall and fractures, he became fully adherent.
Complying with health-care professionals
Some participants spoke about adherence to the PROVE trial/NHS treatment for their osteoporosis being affected by a motivation to do as they were told by health-care professionals. One participant felt that if the PROVE trial team bothered to prescribe exercises then she should do everything possible to adhere/comply: ‘if somebody is bothering to put me on this, that I should do everything to try’ (interviewee 2). A couple of people felt that you ‘should listen’ (interviewee 11) to health-care professionals and do as they advise [‘everything he told me I just did. I did exactly what he told me to do’ (interviewee 11)], although one participant recognised the role of the individual and that people could not be made to listen or comply unless they wanted to (interviewee 11). Several participants described partial compliance; doing fewer exercises than prescribed when stable, but returning to or increasing exercises when symptoms such as stiffness returned (interviewee 14 and 15).
Monitoring and support
Participants spoke of the support provided during the intervention and follow-up stages of their treatment (interviewees 1, 2, 15 and 16). Most of the participants who spoke about monitoring/supervision believed that this helped their adherence during the trial and that longer follow-up/further monitoring would motivate them to continue adhering post treatment [‘so it really focused you on what you were doing’ (interviewee 16)] and that people would be more likely to continue exercising:
It’s been wonderful to be back in a programme again, because I feel very supported when I’m in a programme . . ., if I were to know that in 5 years’ time I’d be on another programme, and then 5 years after that, I would really keep going . . . Because if you’re actually being seen in 6 months then you really would keep, would be more likely to keep going.
Interviewee 1
Royal Osteoporosis Society
Although not part of adhering to PROVE trial interventions, some participants (interviewees 2, 4, 9, 14, 16 and 17) spoke about the ROS and adhering/following helpful advice provided by the ROS:
I think the information I have read has helped me a lot to understand, mostly it came from the National Osteoporosis Society [former name of the ROS].
Interviewee 4
The ROS support and information was used by participants and included advice on exercise and activity that might have indirectly supported adherence to the PROVE trial.
Letters from PROVE trial participants
Ten PROVE trial participants, and one physiotherapist, wrote letters to the trial team about PROVE trial treatment. Although not part of the qualitative study, we have included excerpts here because they identify additional factors relating to adherence or provide additional valuable information about factors that were already identified. Two participants described how the recent death of their spouse had understandably affected their participation in the PROVE trial and their questionnaire responses (letters 3 and 10): ‘my mind and body are in turmoil . . . extremely difficult’ (letter 3). Bereavement is an important event to capture in studies, especially those involving older people.
One letter spoke of ‘being prone to spontaneous falls’ (letter 1) and another participant felt prone to falling because the exercises drained them (letter 5). This participant also described a breathing and panic attack (letter 5). Another participant described how a new comorbidity (a heart problem) adversely affected their ability to exercise (letter 4).
Discussion
Of the 18 participants who were interviewed, 17 were highly positive about their participation in the PROVE trial. The trial was perceived as important, beneficial, organised and well run by a helpful, pleasant trial team. The recruitment and assessment procedures were generally acceptable, although a few participants were unclear about randomisation and several participants found elements of the objective assessment insufficiently challenging. One participant described their mood being lowered because of participation in the PROVE trial and some participants described the distress and horror of seeing the impact of osteoporosis, especially when they looked at images/scans.
Implications for clinical practice
Participants were keen to receive manual therapy and all participants who were interviewed and had received manual therapy were vocally positive about its effects. Participants perceived manual therapy to be safe, enjoyable and effective. The use of taping was generally well tolerated, but two participants found this difficult to remove or unpleasant. Exercises in lying positions, particularly in the prone position, were painful, difficult, avoided or not possible for some participants. PROVE trial treatments were designed to be individualised by physiotherapists, avoiding uncomfortable, difficult or unsafe exercises; therefore, there may have been a training issue or additional training may have been beneficial. Most participants who were allocated to the exercise arm, with one exception, accepted this intervention as an appropriate and effective treatment approach. Participants allocated to the SSPT arm did not perceive this intervention as an approach that was equal to manual or exercise therapy and some participants believed that they had received no physiotherapy treatment. A few participants found travelling to appointments difficult and expensive; the ability to attend for treatment and pay transport costs may influence a patient’s ability to adhere to a treatment package. The trial database indicated that two participants did not attend for treatment and this seemed to be linked to not receiving their treatment of choice.
Issues identified and recommendations
The findings of this study, as in previous studies, have identified and highlighted issues relevant to the design and implementation of both the PROVE trial and trials more generally.
Qualitative studies allow the findings of trials to be explored and explained, the development of specific recommendations about improving trial design(s) and the generation of questions that contextualise trial findings and help to increase the overall utility of the evidence generated by the trial. 136,137 Table 21 summarises the issues arising from this research and provides recommendations for consideration of further research or to address these issues in future research.
| Issue | Recommendation |
|---|---|
| Clinical | |
| Taping can be problematic/unpleasant for patients | Research is needed to explore the appropriateness, effectiveness and acceptability of taping people with fractures due to osteoporosis |
| Some participants found exercises in the prone position/a lying position painful, difficult or not possible | Research is needed to explore the appropriateness, effectiveness and acceptability of exercising in the prone position/a lying position for older people with fractures due to osteoporosis and to explore the effectiveness of exercising in different starting positions |
| Some participants preferred one-to-one treatment, others preferred group treatment or hydrotherapy | Research is needed to determine which form of treatment delivery is superior and most accepted by people with osteoporosis |
| The value of additional follow-up to promote adherence/progress treatment | Research is needed to determine the effectiveness, cost-effectiveness and acceptability of longer-term clinical follow-up |
| Travel to appointments can be difficult and costly for patients | The ability to attend for treatment and pay transport costs may influence a patient’s ability to adhere to a treatment package |
| Research and clinical | |
| SSPT was perceived by some as no care or not having received a physiotherapy treatment | Research is needed to explore how to improve and ensure understanding of usual care trial arms for participants/potential participants |
| Seeing scans and graphs of their spine can be upsetting and horrifying for patients | Researchers need to consider and address the potential impact on patients during non-clinical research appointments as an ethical issue in future trials. Clinicians need to be aware of the impact that visual images of spinal curve and damage can have on people with osteoporosis |
| Research | |
| Pedometers did not work | Equipment provided in trials needs to be fit for purpose |
| Some participants were unclear about or confused by randomisation | Research is needed to explore how to improve and ensure participants’ understanding of randomisation |
| Assessments did not include taking a detailed history of the condition | Reassure patients when a medical history has been obtained pre assessment by referral/from medical notes |
| Using US questionnaires in UK studies that include US terms/language | Provide a brief explanation to prevent participants believing that the trial team has not bothered to correct/amend tools |
| Insufficient space for comments | Provide space for patients to add comments if they wish |
| Greater explanation and feedback of assessment findings. Being informed of trial findings | Include clear explanations for each test/measure. Provide individual feedback at the end of the final research visit when possible. Provide trial findings to participants |
| One participant experienced low mood attributable to participating in the trial | Consider including a psychologist in future trials. Assessments could include checking if participation is causing any problems for patients. Patient information sheets could state that although many people enjoy taking part in trials, this is not always beneficial for all and encourage people to discuss any problems they experience with the trial team |
| Provide reminder telephone calls to complete diaries/paperwork | Obtain and include patients’ views regarding reminders during the design of future trials |
Strengths and limitations of the study
The sampling strategy successfully recruited a wide range of participants: both men and women with varying numbers of fractures and in a variety of locations. This has provided us with wide-ranging and rich data about participants’ experiences of the PROVE trial, its processes and interventions and adherence to exercise and advice. However, as we interviewed only 3% of all the participants in the PROVE trial, the data cannot be representative of the whole sample but rather provide a snapshot view.
It is difficult to estimate an appropriate sample size for qualitative studies when projects require the estimation of numbers of participants and resources in advance of funding being granted. 138 Previous experience in the team (of carrying out similar studies) suggested that 15 participants would provide an appropriate sample size given the detailed level of analyses involved in IPA. During the study, 18 participants were recruited before the team was satisfied that the range of experiences described by participants was sufficient to ensure that the aim of the study had been achieved. The need to adequately resource the qualitative research in combined qualitative and quantitative research has been previously raised. 136
A further strength of this research is that, rather than being peripheral or an ‘add-on’ to the study, both the qualitative and quantitative research were considered essential elements that provided a better opportunity to maximise the value of the qualitative research. 139 This chapter has detailed the qualitative research with participants post intervention. It builds on the pre-trial collection of qualitative and survey data from the development and piloting of the intervention from people with osteoporosis, clinicians and researchers and for this reason, the qualitative and quantitative strands of the research have intertwined throughout the PROVE trial.
Chapter 6 Health economics
Cost-effectiveness of Physiotherapy Rehabilitation for Osteoporotic VErtebral Fracture
Key points
-
Exercise therapy resulted in more QALYs but higher costs than SSPT, whereas manual therapy was more costly and resulted in fewer QALYs than SSPT.
-
The two physiotherapy interventions were not cost-effective relative to SSPT using the £20,000-per-QALY threshold.
-
Despite the substantial number of missing data, the various sensitivity analyses conducted showed that SSPT remained the most cost-effective option.
-
Overall, exercise therapy was more effective but more costly than usual care, whereas manual therapy was less effective and more costly than SSPT.
Introduction
Osteoporotic vertebral fractures have a significant economic burden in terms of health-care and non-health-care costs140 and a significant impact on patient QoL, ranging from pain to impaired mobility. Current non-invasive management of OVF in the UK involves starting patients on appropriate medication, education about osteoporosis and providing general advice about exercise. There is some evidence that physiotherapy interventions can be beneficial for people with at least one previous OVF and back pain. However, UK-based high-quality evidence on which physiotherapy interventions are more effective and cost-effective in this population is unclear.
This chapter summarises the methods and results of an economic evaluation that was based on evidence from the PROVE trial. Two physiotherapy approaches (exercise therapy and manual therapy) were compared with a single 1-hour session of physiotherapy (SSPT). The cost-effectiveness analysis reports the incremental costs per QALY gained from the alternative options. The target population comprised individuals with at least one previous OVF and back pain identified in a NHS hospital setting.
Existing research
We conducted a literature review of cost-effectiveness studies that were published between 2000 and January 2018 concerning interventions for individuals with vertebral fracture. The MEDLINE and EMBASE electronic databases were searched; the search terms used and results are reported in Appendix 23. We identified eight studies estimating the cost-effectiveness of various treatment approaches in this population but no studies examining physiotherapy interventions. Three studies looked at kyphoplasty compared with standard medical treatment. 140–142 Five studies37,143,144 compared vertebroplasty with standard medical treatment. Of the eight studies identified, two were based on randomised clinical trials,140,143 one was based on a retrospective observational study144 and five used decision models (four used Markov models37,141,142 and one used a treatment state model37).
The three papers comparing kyphoplasty with standard medical treatment studies reported disparate results: one study concluded that kyphoplasty was not cost-effective and the other two studies concluded that it was. The study by Fritzell et al. ,140 a RCT, reported an incremental cost per QALY of Swedish krona (SEK) 884,684, well above what Sweden is willing to pay for a QALY gained (SEK 600,000). In contrast, the two British studies (Svedbom et al. 142 and Ström et al. 141) reported balloon kyphoplasty to be cost-effective compared with standard treatment, with incremental costs per QALY gained of £2706 and £8800, respectively. Fritzell et al. 140 use a Swedish cohort from the Fracture Reduction Evaluation (FREE) trial, Ström et al. 141 use all FREE trial participants to inform the analysis and Svedbom et al. 142 use data from the FREE trial as well as the VERTOS II (Vertebroplasty vs. conservative treatment in acute osteoporotic vertebral compression fractures) trial.
The reasons behind the difference in results are likely to be multifactorial. One possible explanation is that Fritzell et al. 140 used a wider perspective in costs, including more categories of costs (e.g. informal care), which was not the case in the other two studies. Fritzell et al. 140 report the cost-effectiveness analysis over a 2-year period, whereas the British studies used Markov models to estimate the cost-effectiveness of the interventions using a lifetime horizon. Hence, Fritzell et al. 140 report the QALYs gained to be 0.085 for kyphoplasty, whereas Ström et al. 141 and Svedbom et al. 142 reported 0.17 and 0.50 QALYs for kyphoplasty relative to non-surgical management, respectively. The differences in the data inputs, methods and time horizons between the three studies mean that a direct comparison of their findings may not be fully appropriate.
Four out of five studies comparing vertebroplasty with standard medical treatment concluded that it was the most cost-effective option. Masala et al. 144 reported costs as mean costs per point increase in ADL, ambulation and visual analogue scale (VAS) pain, whereas the remaining studies reported incremental costs per QALY gained. Klazen et al. 143 conducted an open-label randomised trial (VERTOS II) comparing vertebroplasty with standard treatment, and found the incremental cost per QALY gained to be cost-effective in the Netherlands. Stevenson et al. 37 reported the results of UK-based cost-effectiveness analyses submitted to NICE by the authors (UK assessment group) and two different manufacturers [Johnson & Johnson (New Brunswick, NJ, USA) and Medtronic (Dublin, Ireland)]. The UK assessment group reported results that were similar to those in the Medtronic and Johnson & Johnson analyses [i.e. vertebroplasty was cost-effective relative to non-invasive treatment; incremental cost-effectiveness ratios (ICERs) were < £5000 per QALY gained].
Methods
A total of 615 participants with at least one OVF and back pain were enrolled in the PROVE trial between September 2013 and September 2016. Of these, 216 participants were randomly allocated to the exercise therapy arm, 203 participants were allocated to the manual therapy arm and 196 were allocated to the SSPT arm. The inclusion and exclusion criteria are described in detail in Chapter 2.
We conducted an incremental cost–utility analysis of three modes of care for participants in the PROVE trial:
-
exercise therapy
-
manual therapy
-
single session of physiotherapy.
Full details of the interventions are provided in Chapter 3. Briefly, all trial participants had been prescribed bone-protective medication and all received individualised education delivered by a musculoskeletal physiotherapist who provided general advice about disease management and lifestyle choices to promote bone health, in line with advice available from the ROS at the time that the trial started. The SSPT arm received only a single 1-hour session of education; this education contained no explicit exercise prescription, no manual therapy or any other physiotherapy.
In addition, participants randomised to either of the two therapy interventions received up to a maximum of six one-to-one treatment sessions over a 12-week period. The manual therapy intervention comprised postural training, low-velocity spinal and soft-tissue mobilisation at each treatment session, a HEP of three passive stretches each day and the provision of a manual therapy diary. Exercise therapy comprised a multimodal, graded exercise programme with three parts (strength training, balance training and weight-bearing exercise) and the provision of an exercise therapy diary. Although care was standardised, the physiotherapists were allowed to personalise treatments as appropriate.
Following NICE recommendations, the base-case cost-effectiveness analysis was conducted from the perspective of the NHS and Personal Social Services. 145 Effectiveness was measured by QALYs, which capture differences in life expectancy and/or QoL. The price year for the costs was 2016 and the time horizon of the analysis was 1 year.
Health economic data collection
All participants were followed up to 12 months. The economic data were collected via two self-reported participant diaries covering the time period from randomisation to 4 months and from 4 months to 12 months. The self-reported economic diaries asked the participants to record primary care contacts (with a GP or nurse, for surgery or home visits), physiotherapy contacts, newly prescribed medication and secondary care contacts (outpatient and inpatient episodes and lengths of stay). The diaries also captured equipment that the participants started using (e.g. walking sticks), over-the-counter (OTC) medication, private health-care visits as well as social care, informal care and impact on work (e.g. sick days). Furthermore, the EQ-5D-5L questionnaire was administered to participants at baseline, 4 months, 6 months (via post), 9 months (via post) and 12 months. This QoL instrument assesses health utilities from five domains: mobility, self-care, usual activity, pain/discomfort and anxiety/depression.
Quality of life
We estimated the QoL in the physiotherapy intervention arms and the SSPT arm using the EQ-5D-5L data collected within the trial. Following NICE recommendations,146,147 to derive utility weights from the five-level version, we applied a UK crosswalk from the EuroQoL group to convert the five-level responses to three-level responses. 148 We then used the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), time trade-off (TTO) values set developed by Dolan149 on a UK sample to map utility values to the three-level responses.
We assumed changes in mean utility values between each time point to be straight-line transitions, and weighted the average change in utility between each time point for each patient by the within-trial survival time to estimate QALYs gained during the study period, equivalent to an area under the curve calculation. We truncated the analysis between the randomisation date and 12 months of follow-up. Differences in the mean numbers of QALYs between the three arms during the 12 months of the trial were adjusted for baseline differences in utilities.
Intervention costs
The interventions delivered did not require enhanced skill sets but were within the normal scope of musculoskeletal physiotherapists working in the NHS. Each trial centre recorded the number of physiotherapy sessions attended by the trial participants. The costs of exercise and manual therapy interventions were estimated by valuing the recorded physiotherapy sessions (£49) attended by the participants using NHS Reference Costs 2015–16. 150 Manual therapy was identified to be £1.50 more expensive per physiotherapy session owing to the additional consumable materials used. Each physiotherapy session amounted to 30 minutes for both interventions, with the exception of the initial assessment session, which lasted for 1 hour and was costed at £98. All participants in the three arms of the trial were offered the initial 1-hour assessment session, which involved clinical assessment of the participant by the therapist and the provision of advice. We also complemented the number of sessions recorded by each trial centre with data from the participant diaries. We assumed that the total number of therapy sessions attended in the first 4 months was the higher of the two independent sources (participant diaries covering the period up to 4 months post randomisation and trial centre data). For example, if a centre reported that a participant attended three physiotherapy sessions but the participant reported five sessions in their diary up to 4 months, we assumed that the total number of sessions in that time period was five. Similarly, if a centre reported five physiotherapy sessions attended but the participant recorded attending only three sessions, we assumed that a total of five sessions were attended. We then added any physiotherapy sessions reported in the participant diaries covering the period between 4 months and 12 months of follow-up. The cost of the intervention per participant was calculated by counting the total number of therapy sessions undertaken by all participants in each arm divided by the number of participants.
Primary and secondary costs
Costs per health-care contact in a primary care setting were calculated using unit costs from a national database,151 including qualification costs and direct care costs when applicable. Hospital inpatient and outpatient contacts were valued using NHS Reference Costs 2015–16. 150 Each hospital admission was assigned into a 2015/16 Healthcare Resource Group using standard software (HRG4+ 2015/16 Reference Costs Grouper; NHS Digital, Leeds, UK) based on what was recorded as the reason for admission (converted into International Classification of Diseases, tenth edition, code) and/or operational procedures [converted into OPCS Classification of Interventions and Procedures (OPCS-4)]. HRGs consist of standard groups of clinically similar treatments that consume a common set of health-care resources. All unit costs are reported in Appendix 23.
Medication costs
Any new medications, both OTC medication and prescribed medication, were recorded in the self-reported participant diaries. This medication use was valued using weighted-average medication unit costs per item prescribed [net ingredient cost (NIC)] from national prescribing volumes and costs in 2016. 152 Each individual medication reported in the participant diaries was reviewed and based on the diary-reported length of treatment, type of medication, indication for the medication and referencing with the British National Formulary; the total number of packs of medication expected over the 12-month period was determined and costed appropriately. For recurring medications, it was assumed that each NIC per item prescribed was incurred once a month for daily use medications. Intravenous medication, injections and medications with frequencies other than once a day were looked at individually to determine the expected number of items used over the period of time when the medication was taken. The cost per NIC was multiplied by the number of prescriptions expected over 12 months and a sum of the costs in each intervention arm was calculated and divided by the number of participants.
Out-of-pocket expenses
Any equipment purchased by participants was recorded in the self-reported participant diaries as well as use of private health-care services. Equipment was valued using prices listed in large UK retailers and a national database. 151 Use of private health-care services was valued using NHS Reference Costs 2015–2016150 and the NHS Choices website. 153 All unit costs are reported in Appendix 24.
Social care costs
The use of social care services (e.g. home care and home nurse) reported in the participant diaries was valued using national databases150,151 by multiplying the unit costs (see Appendix 24) by the number of hours per week and the number of weeks during which Personal Social Services were used.
Informal care and lost earnings attributable to morbidity
Unpaid care provided by friends and relatives of participants was recorded in the participant diaries and valued using the 2016 median weekly gross pay if the carer was employed (£539) or the 2016 minimum hourly wage (£7.20) if the carer was unemployed. These unit costs were multiplied by the number of hours and number of weeks of informal care received (see Appendix 24).
The participants also recorded the number of days taken off work during the trial follow-up in the self-reported diaries. The occupation of each participant was coded using the Office for National Statistics Standard Occupational Classification coding tool154 and matched against the national 2016 full-time and part-time weekly gross pay. 155 The weekly gross pay was converted into daily pay and multiplied by the number of days taken off work to estimate the lost earnings for each participant.
Discounting
As the time horizon of the analysis was 1 year, costs and QALYs were not discounted.
Statistical analysis
We reported descriptive statistics (means, SDs and SEs) for resource use based on the participant diaries at 4 and 12 months. We reported resource use and costs within 12 months of randomisation per diary completed (4 or 12 months) and for participants who had completed both diaries.
We reported descriptive statistics (means, SDs and SEs) for EQ-5D-5L utilities at baseline and 4, 6, 9 and 12 months. We compared EQ-5D-5L utilities between the intervention arms and SSPT using multilevel linear mixed models with no imputation of missing data. We used the scheduled times of EQ-5D-5L questionnaires for this analysis (baseline and 4, 6, 9 and 12 months) and not the dates when the questionnaires were completed (which may have varied from the scheduled times in some cases). The models were run in Stata® version 14.2 (StataCorp LP, College Station, TX, USA) using the mixed command with robust SEs and assuming normal error distributions. These models included participant-level random effects. Fixed effects included baseline EQ-5D-5L weights, treatment assignment and time since baseline. Additional fixed effects representing an interaction between treatment and time since baseline, age at randomisation and sex were also conducted.
In addition to the analyses comparing health utilities described previously, we also estimated QALYs by estimating the area under the curve resulting from the linear interpolation of health utilities between the time points: baseline and 4, 6, 9 and 12 months. We used the dates of completion of the EQ-5D-5L questionnaires to inform the estimation of the area under the curve. We also truncated the analysis between the randomisation date and 12 months of follow-up. Two complete-case analyses (CCAs) were conducted: (1) CCA1, in which all five EQ-5D-5L questionnaires had to be complete, and (2) CCA2, in which only the baseline, 4-month and 12-month EQ-5D-5L questionnaires had to be complete for each participant. Differences in mean QALYs between the three arms during the 12 months of the trial were adjusted for baseline differences in utilities.
We then conducted a within-trial economic analysis, with total health-care costs and QALYs gained per participant calculated for the 12 months of the trial in the exercise and manual therapy arms and SSPT arm. Our base-case analysis used intent-to-treat principles, wherein medical resource use, costs and EQ-5D-based health utilities were analysed in accordance with randomised treatment assignment.
We calculated the ICER by dividing the mean cost difference between the intervention and SSPT arms by the mean QALY difference. Three methods of analysis were used to estimate the ICERs: (1) CCA1, as defined above, and both health economics diaries completed; (2) CCA2, as defined above, and both health economics diaries completed, and (3) MI (see below). The difference in costs and QALYs was estimated with regression analysis, using a system of seemingly unrelated regressions. The mean difference in QALYs was adjusted for baseline imbalances in the utility scores. We report the probability that the intervention is cost-effective at a threshold of £20,000 per QALY gained. The probability of the intervention being cost-effective at the threshold of £20,000 per QALY was determined using the net benefit framework and Fieller’s theorem. 156
To deal with missing QoL and cost data, MIs were conducted using a chained model with 70 iterations, regressed on the baseline complete covariates age at randomisation, sex, recruitment centre, number of falls in previous year, walking distance, number of spine and non-spinal fractures and EQ-5D-5L utility score. Mean imputation was used for missing baseline EQ-5D utility scores. Imputed variables consisted of EQ-5D-5L utility scores at 4 and 12 months and total NHS and social care costs up to 4 months and between 4 and 12 months. The patterns of missing data were evaluated to assess whether or not the missing data were non-monotonic and we examined the association between data missingness and baseline variables and observed EQ-5D-5L scores and costs. 157 Given the non-normality of EQ-5D-5L and cost data, we used predictive mean matching, using the five nearest neighbours, to ensure that only plausible values of the missing variable were imputed.
In sensitivity analysis, we explored the following: (1) using NHS costs only, (2) adjusting the difference in costs and QALYs for baseline imbalances in the utility scores and (3) using different MI models (varying the number of baseline covariates). We also conducted subgroup analysis by estimating the incremental costs, QALYs and ICERs by sex and for participants aged older and younger than 70 years.
Results
Participant sample and health economic data completion
At the end of the 12 months, 90% of individuals were still in the trial (see Appendix 23). The proportion of participants with all EQ-5D-5L questionnaires completed was highest in the SSPT arm (61.73%), followed by the exercise therapy arm (52.78%) and the manual therapy arm (51.23%). The proportion of participants with EQ-5D-5L questionnaires completed at least at baseline, 4 months and 12 months was highest in the SSPT arm (77.55%), followed by the manual therapy arm (74.88%) and the exercise therapy arm (68.06%) (see Appendix 24). In terms of resource use and cost data, 69.39% of participants in the SSPT arm returned both participant diaries, of which 92.65% were returned completed (see Appendix 24); participants in the manual therapy arm had a lower return rate (65.02%) and completion rate (89.39%). Of the three trial arms, participants in the exercise therapy arm reported the lowest return (55.09%) and completion (88.24%) rates for the participant diaries. Combining the health economic data reduced the proportion of participants who were eligible for the health economic analysis to 44% in the exercise therapy arm, 52% in the manual therapy arm and 57% in the SSPT arm. Table 22 reports the baseline demographics and characteristics for participants in the trial and for those with complete and incomplete health economic data. We defined complete data as having EQ-5D-5L data at least at baseline, 4 months and 12 months together with completed self-reported diaries 1 and 2 with NHS and social care resource use. Participant characteristics were found to be similar across participants with complete and incomplete health economic data. In the exercise therapy arm, participants with complete health economic data had a lower body mass index (BMI) (mean difference: –1.82 kg/m2, p = 0.007) and lower rates of back pain (odds ratio 0.48, p = 0.018) than those without complete data. No other significant differences were identified between those with complete and incomplete health economic data.
| Intervention arm | Age at randomisation (years), mean (SD) | Female (%) | BMI (kg/m2), mean (SD) | Lower lumbar OVF (L3–L5) (%) | Upper lumbar OVF (L1 and L2) (%) | Lower thoracic OVF (T6–T12) (%) | Upper thoracic OVF (T1–T5) (%) | Spine fractures, mean (SD) | Non-spinal fractures, mean (SD) | Back pain today (%) | Falls in previous year, mean (SD) | Walking distance (unlimited) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete sample | ||||||||||||
| Exercise therapy | ||||||||||||
| n | 216 | 216 | 213 | 131 | 151 | 180 | 118 | 196 | 194 | 216 | 214 | 215 |
| Result | 72.11 (8.37) | 85.65 | 25.87 (4.95) | 47.33 | 49.67 | 88.89 | 22.88 | 2.64 (1.81) | 0.15 (0.42) | 72.69 | 0.84 (2.29) | 43.72 |
| Manual therapy | ||||||||||||
| n | 203 | 202 | 201 | 124 | 140 | 162 | 103 | 187 | 184 | 202 | 201 | 201 |
| Result | 72.33 (9.33) | 85.64 | 25.16 (5.01) | 42.74 | 55.71 | 85.19 | 12.62 | 2.40 (1.86) | 0.17 (0.44) | 65.84 | 0.70 (1.37) | 47.26 |
| SSPT | ||||||||||||
| n | 196 | 195 | 192 | 115 | 119 | 162 | 106 | 169 | 177 | 194 | 192 | 193 |
| Result | 71.82 (9.61) | 88.72 | 25.18 (4.23) | 47.83 | 52.94 | 85.19 | 25.47 | 2.61 (2.05) | 0.08 (0.32) | 65.98 | 0.56 (1.02) | 47.15 |
| Complete cases for analysis | ||||||||||||
| Exercise therapy | ||||||||||||
| n | 96 | 96 | 94 | 51 | 62 | 79 | 50 | 87 | 88 | 96 | 95 | 96 |
| Result | 72.15 (8.10) | 86.46 | 26.89 (4.85) | 56.86 | 48.39 | 88.61 | 30.00 | 2.57 (1.80) | 0.13 (0.37) | 64.58 | 0.65 (1.65) | 51.04 |
| Manual therapy | ||||||||||||
| n | 105 | 105 | 105 | 60 | 66 | 81 | 48 | 97 | 93 | 105 | 104 | 104 |
| Result | 73.45 (7.87) | 88.57 | 24.95 (4.50) | 41.67 | 57.58 | 86.42 | 14.58 | 2.30 (1.71) | 0.15 (0.42) | 60.00 | 0.77 (1.58) | 50.96 |
| SSPT | ||||||||||||
| n | 112 | 112 | 111 | 65 | 69 | 95 | 60 | 98 | 101 | 111 | 111 | 111 |
| Result | 72.77 (8.63) | 90.18 | 25.15 (4.17) | 46.15 | 55.07 | 89.47 | 28.33 | 2.53 (1.99) | 0.06 (0.28) | 68.47 | 0.44 (0.73) | 48.65 |
| Incomplete cases for analysis | ||||||||||||
| Exercise therapy | ||||||||||||
| n | 120 | 120 | 119 | 80 | 89 | 101 | 68 | 109 | 106 | 120 | 119 | 119 |
| Result | 72.07 (8.61) | 85.00 | 25.07 (4.90) | 41.25 | 50.56 | 89.11 | 17.65 | 2.70 (1.83) | 0.18 (0.45) | 79.17 | 0.99 (2.69) | 37.82 |
| Manual therapy | ||||||||||||
| n | 98 | 97 | 96 | 64 | 74 | 81 | 55 | 90 | 91 | 97 | 97 | 97 |
| Result | 71.14 (10.58) | 82.47 | 25.44 (5.55) | 43.75 | 54.05 | 83.95 | 10.91 | 2.51 (2.01) | 0.19 (0.47) | 72.16 | 0.63 (1.10) | 43.30 |
| SSPT | ||||||||||||
| n | 84 | 83 | 81 | 50 | 50 | 67 | 46 | 71 | 76 | 83 | 81 | 82 |
| Result | 70.55 (10.70) | 86.75 | 25.21 (4.33) | 50.00 | 50.00 | 79.10 | 21.74 | 2.72 (2.15) | 0.12 (0.36) | 62.65 | 0.72 (1.30) | 41.12 |
Utilities
Appendix 23 reports the completion rate of EQ-5D-5L questionnaires at baseline and 4, 6, 9 and 12 months. We also report the timing of the completion of the questionnaires relative to the expected time points. Appendix 23 reports the dimensions and levels of the EQ-5D-5L questionnaires by intervention arm of the trial and time point.
In the mobility and self-care dimensions, there were small changes between baseline and 12 months for all three intervention arms. The pain and discomfort dimension captured improvements across the three interventions. The largest change in the pain and discomfort dimension was seen in the SSPT arm, with a 15.04% increase in the no/slight levels, compared with an 11.16% increase in the exercise arm and a 6.55% increase in the manual therapy arm (see Appendix 24). There was also an improvement in the usual activity dimension for all three intervention arms. The largest change in the usual activity dimension was also seen in the SSPT arm, with an 8.88% increase in no/slight levels, compared with small changes in the no/slight levels in the manual and exercise therapy arms (see Appendix 24). In the anxiety and depression dimension, there was a decrease of 5.50% in the no/slight anxiety levels in the exercise therapy arm (more anxiety and depression reported at 12 months) in contrast to an increase of 4.74% in the SSPT arm (less anxiety and depression reported at 12 months). However, these changes were not statistically significant (see Appendix 24).
Table 23 and Figure 7 report the mean utilities at each time point by intervention arm and their change relative to the baseline value (see also Appendix 24). There was an imbalance at baseline in the reported utilities, with participants in the SSPT arm reporting the highest average utility, followed by those in the manual therapy arm and the exercise therapy arm, albeit these differences were not statistically significant (p = 0.167 and p = 0.159, respectively). The mean changes at each time period and across the three arms were not statistically significant (95% CIs contain 0). Furthermore, the mean changes in EQ-5D-5L index scores in the exercise therapy arm were, overall, positive relative to baseline, with the magnitude of change at 12 months (0.017) being similar to that reported in the SSPT arm (0.016). These changes were more marked at 4 months (0.040 in the exercise arm and 0.021 in the SSPT arm). In the manual therapy arm, the EQ-5D-5L index score was worse at all time points relative to baseline.
| Intervention arm | EQ-5D-5L utility | |||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | All participants (N = 615) | |
| Baseline | ||||
| n (complete data) | 207 | 194 | 189 | 590 |
| Mean (SE) | 0.6177 (0.0154) | 0.6652 (0.0138) | 0.6486 (0.0149) | 0.6432 (0.0086) |
| Not available (%) | 4.17 | 4.43 | 4.59 | 4.07 |
| 4 months | ||||
| n (complete data) | 174 | 177 | 167 | 518 |
| Mean (SE) | 0.6574 (0.0172) | 0.6616 (0.0163) | 0.6692 (0.0157) | 0.6693 (0.0065) |
| Mean change (SE) | 0.0397 (0.0231) | –0.0036 (0.0214) | 0.0206 (0.0217) | 0.0195 (0.0128) |
| Not available (%) | 19.44 | 12.81 | 14.80 | 15.77 |
| 6 months | ||||
| n (complete data) | 166 | 157 | 159 | 482 |
| Mean (SE) | 0.6317 (0.0171) | 0.6541 (0.0172) | 0.6304 (0.0188) | 0.6386 (0.0102) |
| Mean change (SE) | 0.014 (0.0230) | –0.0111 (0.0221) | –0.0182 (0.0240) | –0.0046 (0.0133) |
| Not available (%) | 23.15 | 22.66 | 18.88 | 21.63 |
| 9 months | ||||
| n (complete data) | 153 | 141 | 152 | 446 |
| Mean (SE) | 0.6306 (0.0182) | 0.6424 (0.0194) | 0.6450 (0.0166) | 0.6392 (0.0104) |
| Mean change (SE) | 0.0129 (0.0238) | –0.0228 (0.0238) | –0.0036 (0.0223) | –0.0040 (0.0135) |
| Not available (%) | 29.17 | 30.54 | 22.45 | 27.48 |
| 12 months | ||||
| n (complete data) | 167 | 172 | 164 | 503 |
| Mean (SE) | 0.6345 (0.0175) | 0.6558 (0.0163) | 0.6649 (0.0160) | 0.6517 (0.0096) |
| Mean change (SE) | 0.0168 (0.0233) | –0.0094 (0.0214) | 0.0163 (0.0219) | 0.0085 (0.0129) |
| Not available (%) | 22.69 | 15.27 | 16.33 | 18.21 |
FIGURE 7.
The EQ-5D-5L changes from baseline (95% CI): ITT analysis, observed data.
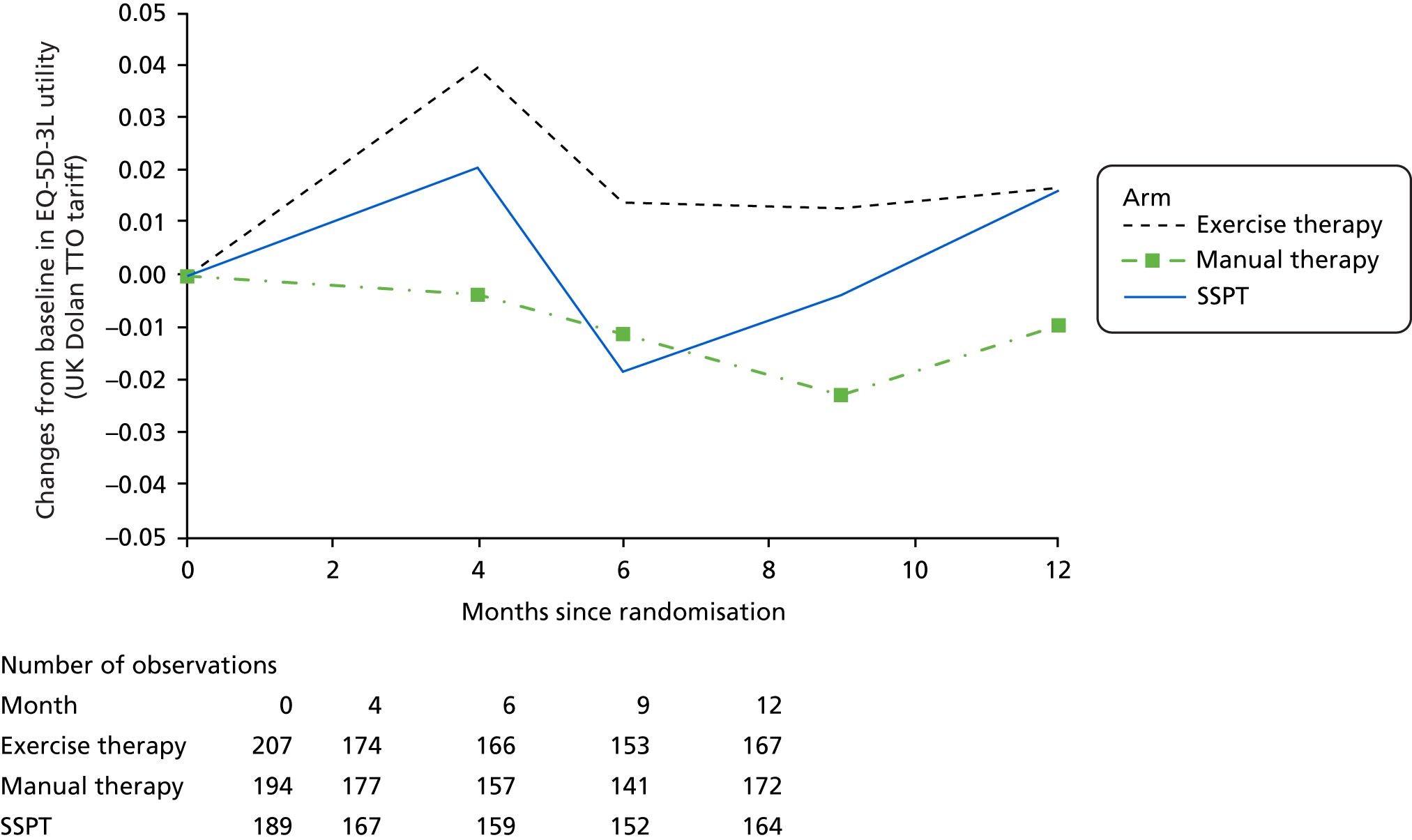
The SSPT and exercise therapy arms reported the highest mean utilities at 4 months, with SSPT reporting a lower mean utility at 6 months (below the baseline value) and increasing again at 12 months (above the baseline value). In contrast, the average utility in the exercise therapy arm was consistently above the mean baseline value at all time points. The variation in utility values in the SSPT arm may be explained by the changes in the pain and discomfort and anxiety and depression dimensions. In the SSPT arm, a greater percentage of participants moved to the more severe levels (moderate/severe/extreme pain and discomfort) between months 4 and 6 relative to the exercise therapy arm. However, in subsequent months, these increases levelled out and moved closer to the baseline values. The manual therapy arm appeared to follow a relatively stable downward trajectory throughout, which was consistently below the mean baseline value.
Table 24 reports the results from the multilevel mixed-effects linear models comparing the utilities and adjusting for several covariates, such as baseline EQ-5D-5L score and number of months since randomisation. There was no significant difference in utilities across the three intervention arms.
| Base case: ITT population, scheduled times of EQ-5D questionnaire (not actual times) | Results, by model | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Total number of observations | 2470 | 2470 | 2470 |
| SSPT | Reference | Reference | Reference |
| Exercise therapy, coefficient (robust SE) | –0.0002 (0.0101) | 0.0041 (0.0069) | 0.0043 (0.0070) |
| Manual therapy, coefficient (robust SE) | –0.0092 (0.0097) | 0.0022 (0.0066) | 0.0023 (0.0067) |
| Exercise therapy × time since randomisation, coefficient (robust SE) | – | –0.0008 (0.0017) | –0.0008 (0.0017) |
| Manual therapy × time since randomisation, coefficient (robust SE) | – | –0.0020 (0.0015) | –0.0020 (0.0015) |
| Time since randomisation (months), coefficient (robust SE) | –0.0010 (0.0007) | –0.0001 (0.0011) | –0.0001 (0.0011) |
| Baseline EQ-5D score, coefficient (robust SE) | 0.8017*** (0.0232) | 0.8017*** (0.0232) | 0.8004*** (0.0232) |
| Age at randomisation, coefficient (robust SE) | –0.0008 (0.0005) | ||
| Female, coefficient (robust SE) | 0.0042 (0.0118) | ||
| Random effects | |||
|
|
|
|
Following MI of the utility scores at 4 and 12 months (Table 25), results similar to those in the CCA were found in terms of direction and magnitude in the SSPT and manual therapy arms. In the exercise therapy arm, the change in utility scores at 4 months became significant (0.031, 95% CI 0.010 to 0.052). The utility score at 12 months was considerably lower than what was observed in the CCA and, conditional on the imputation model used, it could be lower than the mean baseline utility, albeit never statistically significant.
| Intervention arm | EQ-5D utility | |||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | All participants (N = 615) | |
| Baselinea | ||||
| n (complete/imputed data) | 216 | 202 | 195 | 613 |
| Mean (SE) | 0.621 (0.014) | 0.665 (0.01) | 0.649 (0.014) | 0.644 (0.008) |
| Not available (%) | 0.49 | 0.51 | 0.33 | |
| 4 monthsb | ||||
| n (complete/imputed data) | 216 | 202 | 195 | 613 |
| Mean (SE) | 0.652 (0.015) | 0.664 (0.015) | 0.659 (0.015) | 0.658 (0.009) |
| Mean change (SE) | 0.031** (0.011) | –0.002 (0.010) | 0.010 (0.009) | 0.014** (0.006) |
| Not available (%) | 0 | 0.49 | 0.51 | 0.33 |
| 12 monthsb | ||||
| n (complete/imputed data) | 216 | 202 | 195 | 613 |
| Mean (SE) | 0.622 (0.017) | 0.641 (0.016) | 0.650 (0.015) | 0.637 (0.009) |
| Mean change (SE) | 0.0009 (0.015) | –0.024 (0.014) | 0.001 (0.014) | |
| Not available (%) | 0 | 0.49 | 0.51 | –0.007 (0.008) |
Table 26 reports the QALYs for participants with the five EQ-5D-5L questionnaires completed, three questionnaires completed (baseline, 4 months and 12 months) and MI. We found no significant difference in QALYs across the three arms of the trial after adjusting for baseline EQ-5D-5L imbalances in any of the analyses. However, the numbers of QALYs were higher in the exercise therapy arm in the three analyses, followed by the SSPT arm and the manual therapy arm.
| Intervention arm | Exercise | Manual | SSPT |
|---|---|---|---|
| Base case: participants with five EQ-5D data points (actual times of EQ-5D-5L questionnaire, not scheduled times) | |||
| N | 216 | 203 | 196 |
| Participants with complete EQ-5D data, n (%) | 114 (53) | 104 (51) | 121 (62) |
| Baseline ED-5D score, mean (SD) | 0. 635 (0.203) | 0.704 (0.180) | 0.671 (0.186) |
| QALYs, mean (SD) | 0.654 (0.182) | 0.688 (0.183) | 0.667 (0.172) |
| Difference relative to SSPT adjusted for baseline ED-5D score (SE) | 0.015 (0.013) | –0.004 (0.013) | – |
| Participants with baseline, 4-month and 12-month EQ-5D data (actual times of EQ-5D-5L questionnaire, not scheduled times) | |||
| N | 216 | 203 | 196 |
| Participants with complete EQ-5D data, n (%) | 147 (68.06) | 152 (74.88) | 152 (77.55) |
| Baseline ED-5D score, mean (SD) | 0.629 (0.213) | 0.672 (0.198) | 0.658 (0.200) |
| QALYs, mean (SD) | 0.644 (0.195) | 0.659 (0.200) | 0.659 (0.182) |
| Difference relative to SSPT adjusted for baseline ED-5D score (SE) | 0.007 (0.012) | –0.012 (0.012) | – |
| MI of 4-month and 12-month EQ-5D data (actual times of EQ-5D-5L questionnaire, not scheduled times) | |||
| N | 216 | 203 | 196 |
| Participants with complete ED-5D data, n (%) | 216 (100) | 202 (99) | 195 (99) |
| Baseline ED-5D score (SE) | 0.621 (0.014) | 0.665 (0.013) | 0.648 (0.014) |
| QALYs (SE) | 0.628 (0.014) | 0.646 (0.014) | 0.647 (0.013) |
| Difference relative to SSPT adjusted for baseline ED-5D score (SE) | 0.002 (0.011) | –0.014 (0.011) | – |
Resource use and costs
Participants in the manual therapy arm reported the highest number of physiotherapy sessions attended in the first 4 months [mean 5.03 sessions (SD 2.57 sessions)], followed by participants in the exercise therapy arm [mean 4.33 sessions (SD 2.77 sessions)] and the SSPT arm [mean 1.69 sessions (SD 0.59 sessions)] (see Appendix 24).
Table 27 reports the resource utilisation in the three intervention arms during the 12 months of follow-up for participants with complete economic data (n = 349). The most physiotherapy sessions were reported in the manual therapy arm (mean 5.93 sessions), followed by the exercise therapy arm (mean 5.03 sessions) and the SSPT arm (1.69 sessions). The SSPT arm reported the highest number of contacts with a GP or nurse or outpatient visits (7.12 contacts/visits) and this health-care category was the most used across all three intervention arms.
| Intervention arm | Resource utilisation | |||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | All participants (N = 615) | |
| Complete diaries, n/N | 105/216 | 118/203 | 126/196 | 349/615 |
| Newly prescribed medications, mean (SD) | 1.35 (1.80) (n = 105) | 1.46 (1.93) (n = 118) | 1.41 (1.66) (n = 126) | 1.41 (1.79) (n = 349) |
| GP/nurse/outpatient visits, mean (SD) | 6.77 (6.70) (n = 105) | 7.00 (7.10) (n = 118) | 7.12 (5.81) (n = 126) | 6.97 (6.52) (n = 349) |
| Hospitalisations, mean (SD) | 0.30 (0.75) (n = 105) | 0.22 (0.57) (n = 118) | 0.28 (0.68) (n = 126) | 0.27 (0.67) (n = 349) |
| Physiotherapy sessions, mean (SD) | 5.03 (2.44) (n = 105) | 5.93 (1.91) (n = 118) | 1.69 (1.84) (n = 126) | 4.13 (2.78) (n = 349) |
| Home visits, mean (SD) | 0.10 (0.38) (n = 105) | 0.29 (1.05) (n = 118) | 0.42 (1.32) (n = 126) | 0.28 (1.03) (n = 349) |
| Items of equipment purchased, mean (SD) | 0.26 (0.71) (n = 104) | 0.28 (0.80) (n = 117) | 0.39 (1.05) (n = 126) | 0.31 (0.87) (n = 347) |
| Private care contacts, mean (SD) | 0.32 (1.06) (n = 104) | 0.34 (1.25) (n = 117) | 0.71 (2.29) (n = 126) | 0.47 (1.67) (n = 347) |
| New OTC medication, mean (SD) | 0.24 (0.74) (n = 104) | 0.30 (0.67) (n = 117) | 0.29 (0.80) (n = 126) | 0.28 (0.74) (n = 347) |
Converting the resource utilisation into costs, the highest cost category reported was inpatient hospital stays, varying from a mean of £499 (SD £1361) per participant in the SSPT arm to a mean of £767 (SD £2932) in the exercise therapy arm (Table 28). This was followed by GP/nurse/outpatient costs, which varied from a mean of £402 (SD £458) per participant in the exercise therapy arm to a mean of £435 (SD £422) in the SSPT arm. The total NHS costs were found to be higher in the manual therapy arm (mean of £1638 per participant), followed by the exercise therapy arm (mean £1627) and SSPT (mean £1410). The difference in NHS costs was not statistically significant between exercise therapy (mean £217, 95% CI –£446 to £880) or manual therapy (mean difference £228, 95% CI –£340 to £796) and the SSPT arm.
| Intervention arm | Costs | |||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | All participants (N = 615) | |
| Complete diaries, n/N | 105/216 | 118/203 | 126/196 | 349/615 |
| Newly prescribed medications, mean cost (£) (SD) | 141.77 (617.22) (n = 105) | 186.31 (593.06) (n = 118) | 316.92 (719.71) (n = 126) | 220.07 (650.99) (n = 349) |
| Hospitalisations, mean cost (£) (SD) | 766.97 (2932.42) (n = 105) | 620.79 (2524.16) (n = 118) | 499.24 (1361.42) (n = 126) | 620.89 (2321.53) (n = 349) |
| GP/nurse/outpatient visits, mean cost (£) (SD) | 402.14 (458.16) (n = 105) | 414.56 (500.61) (n = 118) | 434.65 (422.40) (n = 126) | 418.08 (459.63) (n = 349) |
| Physiotherapy sessions, mean cost (£) (SD) | 287.47 (134.72) (n = 105) | 344.11 (106.92) (n = 118) | 126.39 (95.49) (n = 126) | 248.46 (146.62) (n = 349) |
| Home visits, mean cost (£) (SD) | 28.97 (253.37) (n = 105) | 72.13 (547.98) (n = 118) | 32.98 (99.86) (n = 126) | 45.01 (352.28) (n = 349) |
| Total cost to NHS (£), mean (SD) | 1627.32 (3109.72) (n = 105) | 1637.89 (2699.15) (n = 118) | 1410.19 (1649.51) (n = 126) | 1552.50 (2515.60) (n = 349) |
| Difference in NHS costs (£), a mean (SD) | 217.13 (337.08) ( n = 105) | 227.70 (288.72) ( n = 118) | – ( n = 126) | |
| Personal Social Services, mean cost (£) (SD) | 92.08 (890.48) (n = 104) | 31.69 (320.16) (n = 117) | 19.56 (123.45) (n = 126) | 45.38 (526.20) (n = 347) |
| Total cost to NHS and Personal Social Services (£), mean (SD) | 1733.52 (3257.71) (n = 104) | 1675.58 (2722.37) (n = 117) | 1429.74 (1652.05) (n = 126) | 1603.68 (2578.33) (n = 347) |
| Difference in costs NHS and Personal Social Services (£), a mean (SD) | 303.77 (351.60) ( n = 104) | 245.84 (291.60) ( n = 117) | – ( n = 126) | – |
| Equipment, mean cost (£) (SD) | 15.58 (94.81) (n = 104) | 9.07 (37.52) (n = 117) | 19.71 (73.89) (n = 126) | 14.89 (71.70) (n = 347) |
| Private care, mean cost (£) (SD) | 19.81 (65.71) (n = 104) | 17.37 (60.25) (n = 117) | 42.24 (134.96) (n = 126) | 27.13 (95.99) (n = 347) |
| OTC medication, mean cost (£) (SD) | 1.61 (6.26) (n = 104) | 1.99 (6.89) (n = 117) | 1.80 (5.01) (n = 126) | 1.81 (6.06) (n = 347) |
| Total cost (£), mean (SD) | 1770.52 (3288.69) (n = 104) | 1704.02 (2740.70) (n = 117) | 1493.49 (1673.94) (n = 126) | 1647.50 (2600.86) (n = 347) |
| Total cost difference a (£), mean (SD) | 277.02 (355.17) ( n = 104) | 210.52 (294.04) ( n = 117) | – ( n = 126) | – |
Combining NHS and social care costs, the exercise therapy arm had the highest mean costs per participant (£1733), followed by manual therapy (£1676) and SSPT (£1430). Although the difference in mean costs between exercise therapy (£304, 95% CI –£388 to £995), manual therapy (£246, 95% CI –£328 to £819) and SSPT increased, relative to using NHS costs only, they remained clinically not significant. Adopting a broader perspective and including participant expenses (e.g. equipment purchased and private care) resulted in the mean costs per participant in the exercise therapy arm and manual therapy arm being £277 (95% CI –£423 to £976) and £211 (95% CI –£368 to £789), respectively, higher than in the SSPT arm. Table 29 reports the costs per individual receiving social care, informal care or reporting lost earnings in the three trial arms.
| Intervention arm | Costs or utilisation | |||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | All participants (N = 615) | |
| Complete diaries, n/N | 105/216 | 118/203 | 126/196 | 426/615 |
| Received Personal Social Services, mean (SD) | 0.04 (n = 105) | 0.03 (n = 118) | 0.05 (n = 126) | 0.04 (n = 349) |
| Personal Social Services costs (£),a mean (SD) | 4788.00 (6058.49) (n = 2) | 1854.00 (2265.57) (n = 2) | 664 (445.42) (n = 4) | 1992.50 (3051.02) (n = 8) |
| Received informal care, mean (SD) | 0.19 (n = 105) | 0.31 (n = 118) | 0.21 (n = 126) | 0.24 (n = 349) |
| Informal care costs (£),a mean (SD) | 6924.29 (12,337.89) (n = 7) | 272.45 (491.59) (n = 5) | 4523.13 (4686.45) (n = 6) | 4276.17 (8238.09) (n = 18) |
| Total employed | 0.18 (n = 105) | 0.12 (n = 118) | 0.20 (n = 126) | 0.17 (n = 349) |
| Employed, taking time off | 0.21 (n = 19) | 0.36 (n = 14) | 0.24 (n = 25) | 0.26 (n = 58) |
| Lost earnings (£),a mean (SD) | 2637.74 (4972.73) (n = 4) | 497.92 (929.77) (n = 5) | 1225.34 (1330.98) (n = 5) | 1369.09 (2704.18) (n = 14) |
Appendix 24 reports resource utilisation and costs by type of health-care category and intervention for participants who completed diary 1 (first 4 months) or diary 2 (4 months to 12 months). Combining the available data from diaries 1 and 2 resulted in lower NHS and social care costs per patient than using only participants with both diaries completed. For example, the total NHS and social care cost at 12 months in the exercise therapy arm was £1472 when adding the available data in the two time periods, compared with £1733 obtained using complete cases only.
Multiple imputation
The pattern of missing data across the three interventions was visually assessed and found to be non-monotonic (e.g. participants completed EQ-5D-5L questionnaires at 12 months but not at 4 months). Lower EQ-5D-5L scores at baseline and allocation to the exercise therapy arm were significantly associated with missing costs, adjusting for sex and age at randomisation. Lower EQ-5D-5L scores at baseline were found to be significantly associated with missing EQ-5D-5L data at 12 months, adjusting for treatment allocation, sex and age at randomisation. However, observed values in the preceding time period were significantly associated with missing EQ-5D-5L data, adjusting for all baseline variables. As a result, the data were assumed to be missing at random. Eight MI models were defined, with the most complex model used as the base case for MI. We checked the fit of the imputation model by comparing the imputed values with the observed ones and found the distributions to be similar for costs and utilities.
Table 30 reports the EQ-5D-5L data and costs in each intervention arm in two CCAs (CCA1, in which all five EQ-5D-5L questionnaires had to be complete, and CCA2, in which only the baseline, 4-month and 12-month EQ-5D-5L questionnaires had to be complete) and the MI data. Overall, participants with incomplete data reported lower mean utility scores than those included in the CCA. Across the three intervention arms, when comparing observed with imputed data in participants with incomplete data, MI resulted in similar EQ-5D-5L scores at baseline, higher EQ-5D-5L scores at 4 months and lower EQ-5D scores at 12 months. There were also considerable differences between CCA1 and CCA2 in terms of costs and EQ-5D-5L scores.
| Intervention arm | CCA | MI of costs and EQ-5Da | |
|---|---|---|---|
| 1 (all five EQ-5D questionnaires) | 2 (EQ-5D at baseline and 4 and 12 months) | ||
| Exercise therapy: complete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.662 (n = 72) | 0.644 (n = 97) | 0.621 (n = 216) |
| 16 weeks | 0.705 (n = 72) | 0.681 (n = 97) | 0.652 (n = 216) |
| 12 months | 0.663 (n = 72) | 0.662 (n = 97) | 0.622 (n = 216) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 504 (n = 72) | 548 (n = 97) | 533 (n = 216) |
| 4–12 months | 1422 (n = 72) | 1215 (n = 97) | 945 (n = 216) |
| Exercise therapy: incomplete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.601 (n = 144) | 0.602 (n = 119) | 0.602 (n = 119) |
| 16 weeks | 0.623 (n = 101) | 0.626 (n = 76) | 0.628 (n = 119) |
| 12 months | 0.612 (n = 94) | 0.595 (n = 69) | 0.590 (n = 119) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 638 (n = 64) | 613 (n = 40) | 525 (n = 119) |
| 4–12 months | 619 (n = 64) | 635 (n = 40) | 733 (n = 119) |
| Manual therapy: complete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.716 (n = 76) | 0.685 (n = 106) | 0.665 (n = 202) |
| 16 weeks | 0.710 (n = 76) | 0.682 (n = 106) | 0.664 (n = 202) |
| 12 months | 0.695 (n = 76) | 0.659 (n = 106) | 0.641 (n = 202) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 581 (n = 76) | 754 (n = 106) | 638 (n = 202) |
| 4–12 months | 737 (n = 76) | 1024 (n = 106) | 868 (n = 202) |
| Manual therapy: incomplete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.635 (n = 127) | 0.644 (n = 97) | 0.643 (n = 96) |
| 16 weeks | 0.625 (n = 101) | 0.631 (n = 71) | 0.643 (n = 96) |
| 12 months | 0.619 (n = 94) | 0.642 (n = 64) | 0.622 (n = 96) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 819 (n = 73) | 565 (n = 44) | 515 (n = 96) |
| 4–12 months | 1085 (n = 59) | 418 (n = 30) | 700 (n = 96) |
| SSPT: complete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.673 (n = 94) | 0.673 (n = 112) | 0.649 (n = 195) |
| 16 weeks | 0.690 (n = 94) | 0.693 (n = 112) | 0.659 (n = 195) |
| 12 months | 0.665 (n = 94) | 0.673 (n = 112) | 0.650 (n = 195) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 511 (n = 94) | 513 (n = 112) | 451 (n = 195) |
| 4–12 months | 879 (n = 94) | 903 (n = 112) | 810 (n = 195) |
| SSPT: incomplete cases | |||
| EQ-5D mean score | |||
| Baseline | 0.626 (n = 102) | 0.617 (n = 84) | 0.616 (n = 83) |
| 16 weeks | 0.626 (n = 73) | 0.599 (n = 55) | 0.613 (n = 83) |
| 12 months | 0.646 (n = 71) | 0.622 (n = 53) | 0.618 (n = 83) |
| NHS and social care mean costs (£) | |||
| Up to 16 weeks | 390 (n = 50) | 315 (n = 32) | 368 (n = 83) |
| 4–12 months | 736 (n = 51) | 579 (n = 33) | 686 (n = 83) |
Cost-effectiveness analysis
Table 31 reports the cost-effectiveness results. Overall, exercise therapy was more effective but more costly than SSPT, whereas manual therapy was less effective and more costly than SSPT. However, the mean incremental costs and QALYs varied across CCA1 and CCA2 and MI. For example, relative to SSPT, the incremental costs of exercise therapy were £536 (95% CI –£183 to £1255) for CCA1, £347 (95% CI –£376 to £1071) for CCA2 and £206 (95% CI –£228 to £641) for MI. The difference in QALYs, adjusted for baseline EQ-5D score, was 0.017 (95% CI –0.013 to 0.048) for CCA1, 0.014 (95% CI –0.013 to 0.041) for CCA2 and 0.002 (95% CI –0.020 to 0.025) for MI. However, using a threshold of £20,000 per QALY gained, the physiotherapy interventions were found to not be cost-effective relative to SSPT across the three methods of analysis. Figure 8 shows the cost-effectiveness acceptability curves for exercise therapy relative to SSPT with the three methods of analysis. CCA2 was the only analysis in which exercise therapy had a probability of > 50% of being the most cost-effective option under a threshold of £30,000 per QALY gained.
| Intervention arm | CCA | MI of costs and EQ-5Da (n = 613; 99%) | |
|---|---|---|---|
| 1 (all five EQ-5D questionnaires) (n = 242; 39%) | 2 (EQ-5D at baseline and 4 and 12 months) (n = 313; 51%) | ||
| Exercise therapy vs. SSPT | |||
| Difference in costs (£) | |||
| Mean | 536 | 347 | 216 |
| SE | 367 | 369 | 224 |
| 95% CI | –183 to 1255 | –376 to 1071 | –223 to 655 |
| Difference in QALYs adjusted for baseline EQ-5D score | |||
| Mean | 0.017 | 0.014 | 0.002 |
| SE | 0.015 | 0.014 | 0.011 |
| 95% CI | –0.013 to 0.048 | –0.013 to 0.041 | –0.020 to 0.025 |
| ICER (£/QALY) | 31,098 | 25,453 | 100,525 |
| Probability of being cost-effective at a threshold of £20,000 per QALY | 36% | 44% | 31% |
| Manual therapy vs. SSPT | |||
| Difference in costs (£) | |||
| Mean | –72 | 361 | 244 |
| SE | 361 | 361 | 224 |
| 95% CI | –780 to 635 | –345 to 1068 | –195 to 683 |
| Difference in QALYs adjusted for baseline EQ-5D score | |||
| Mean | –0.011 | –0.021 | –0.014 |
| SE | 0.015 | 0.013 | 0.012 |
| 95% CI | –0.041 to 0.019 | –0.048 to 0.005 | –0.036 to 0.009 |
| ICER (£/QALY) | 6710 (not cost-effective) | Dominated | Dominated |
| Probability of being cost-effective at a threshold of £20,000 per QALY | 39% | 5% | 7% |
FIGURE 8.
Cost-effectiveness acceptability curve for exercise therapy relative to SSPT, using three methods of analysis: CCA1, CCA2 and MI.

Sensitivity analysis
Table 32 reports the incremental costs, QALYs and ICERs for difference sensitivity analysis scenarios explored using the MI method. Although there was considerable variation in the ICERs across the different MI models used (from £58,828/QALY to £1,490,543/QALY for exercise therapy relative to SSPT), the two physiotherapy interventions were consistently found to not be cost-effective. In all analyses, the manual therapy intervention was dominated by SSPT (i.e. it was more costly and less effective) with a probability of being the most cost-effective option of 5–7% at the £20,000-per-QALY threshold. This is in keeping with our findings in the base-case analysis.
| Intervention arm | Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MI base case | NHS cost perspective | Adjusted for baseline EQ-5D scorea | Adjusted for centre and baseline EQ-5D scorea | MI model | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| Exercise therapy vs. SSPT | |||||||||||
| Difference in costs (£) | |||||||||||
| Mean | 216 | 125 | 182 | 159 | 214 | 218 | 194 | 212 | 197 | 200 | 192 |
| SE | 224 | 213 | 222 | 218 | 220 | 223 | 223 | 226 | 217 | 221 | 220 |
| Difference in QALYs adjusted for baseline EQ-5D score | |||||||||||
| Mean | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 | 0.004 | 0.001 | 0.003 | 0.003 | 0.0001 | 0.001 |
| SE | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.011 | 0.012 | 0.011 | 0.011 | 0.011 |
| ICER (£/QALY) | 100,525 | 55,087 | 75,219 | 54,330 | 110,493 | 58,828 | 160,287 | 76,504 | 66,639 | 1,490,543 | 260,863 |
| Probability of being cost-effective at a threshold of £20,000 per QALY (%) | 31 | 40 | 35 | 38 | 30 | 34 | 31 | 33 | 34 | 28 | 30 |
| Manual therapy vs. SSPT | |||||||||||
| Difference in costs (£) | |||||||||||
| Mean | 245 | 238 | 265 | 246 | 244 | 255 | 247 | 244 | 254 | 242 | 244 |
| SE | 224 | 215 | 223 | 219 | 224 | 225 | 223 | 226 | 224 | 225 | 221 |
| Difference in QALYs adjusted for baseline EQ-5D score | |||||||||||
| Mean | –0.014 | –0.014 | –0.014 | –0.013 | –0.015 | –0.015 | –0.016 | –0.013 | –0.014 | –0.015 | –0.015 |
| SE | 0.012 | 0.011 | 0.012 | 0.011 | 0.011 | 0.012 | 0.011 | 0.012 | 0.012 | 0.011 | 0.011 |
| ICER (£/QALY) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Probability of being cost-effective at a threshold of £20,000 per QALY (%) | 6 | 6 | 6 | 7 | 5 | 5 | 5 | 7 | 6 | 5 | 5 |
Subgroup analysis
Table 33 reports the cost-effectiveness results by participant subgroup using the MI method. There were no statistically significant differences in incremental costs or incremental QALYs across the three arms of the trial. Exercise therapy was more effective than SSPT for females but not for males, albeit these differences were not statistically significant. SSPT was the most cost-effective option only for females and for participants aged > 70 years. Manual therapy was cost-effective only for males, but cheaper and less effective than SSPT. For participants aged ≤ 70 years, exercise therapy was suggested to be the most cost-effective option at the £20,000-per-QALY threshold.
| Intervention arm | Sex | Age (years) | ||
|---|---|---|---|---|
| Females only (n = 531) | Males only (n = 82) | ≤ 70 (n = 237) | > 70 (n = 376) | |
| Exercise therapy vs. SSPT | ||||
| Difference in costs (£) | ||||
| Mean | 235 | 49 | 36 | 332 |
| SE | 244 | 533 | 316 | 301 |
| 95% CI | –244 to 714 | –996 to 1093 | –583 to 656 | –258 to 923 |
| Difference in QALYs adjusted for baseline EQ-5D score | ||||
| Mean | 0.005 | –0.015 | 0.003 | 0.001 |
| SE | 0.012 | 0.032 | 0.019 | 0.015 |
| 95% CI | –0.019 to 0.029 | –0.079 to 0.048 | –0.034 to 0.040 | –0.027 to 0.030 |
| ICER (£/QALY) | 49,923 | Dominated | 12,310 | 244,065 |
| Probability of being cost-effective at a threshold of £20,000 per QALY (%) | 35 | 35 | 52 | 25 |
| Manual therapy vs. SSPT | ||||
| Difference in costs (£) | ||||
| Mean | 325 | –295 | 322 | 196 |
| SE | 245 | 544 | 319 | 303 |
| 95 CI | –155 to 806 | –1361 to 771 | –304 to 948 | –399 to 790 |
| Difference in QALYs adjusted for baseline EQ-5D score | ||||
| Mean | –0.016 | –0.005 | –0.024 | –0.007 |
| SE | 0.012 | 0. 032 | 0.019 | 0.014 |
| 95 CI | –0.039 to 0.009 | –0.067 to 0.058 | –0.062 to 0.014 | –0.035 to 0.021 |
| ICER (£/QALY) | Dominated | 61,192 | Dominated | Dominated |
| Probability of being cost-effective at a threshold of £20,000 per QALY (%) | 4 | 58 | 7 | 23 |
Discussion
We have estimated the cost-effectiveness of programmes of manual and exercise physiotherapy relative to a SSPT education for participants with OVF. We have found that the programmes of physiotherapy, as they were delivered, have a low probability of being cost-effective relative to a SSPT.
To our knowledge, this is the first cost-effectiveness analysis of physiotherapy interventions in this population. Our literature review did not identify other cost-effectiveness studies evaluating such interventions in populations with OVF. Furthermore, the PROVE trial is the first UK RCT of a structured physiotherapy programme designed to be implemented in the NHS in participants with vertebral fractures. The major strengths of this cost-effectiveness analysis include being integrated with a well-designed and relatively large randomised trial to obtain reliable estimates of the interventions’ effectiveness over 1 year of follow-up. We were also able to prospectively measure QoL and health-care use using EQ-5D-5L questionnaires and self-reported participant diaries.
We found that exercise therapy resulted in more QALYs (mean 0.002) than SSPT but was more expensive. Manual therapy was both less effective (mean –0.015 QALYs) and more expensive than SSPT. These differences were not statistically significant. The impact on quality-adjusted life expectancy captured by the EQ-5D-5L questionnaires was consistent with the trial results using the disease-specific QoL instrument (QUALEFFO-41) at 12 months (see Chapter 4). However, we did not find an improvement in QoL, as measured by the EQ-5D-5L questionnaires, in the manual therapy arm relative to baseline. This is at odds with the findings in this arm using the QUALEFFO-41 instrument, which suggested an improvement in QoL at 12 months. Nonetheless, such differences were not statistically significant based on either QoL instrument. In the qualitative study, the participants in the manual therapy arm described only a brief period of symptom reduction (see Chapter 5) and this may help to explain this finding.
Inpatient hospital stays accounted for the highest costs across the three interventions, followed by primary care and outpatient consultations. Physiotherapy sessions were the third most costly resource use category in the two physiotherapy arms, and non-NHS costs were relatively small across the three interventions. However, the number and costs of physiotherapy sessions in the first 16 weeks of follow-up are likely to be an underestimate. To avoid double counting, we did not sum the number of sessions reported in the participant diary and the sessions collected by the recruitment centres.
Using MI, relative to baseline, participants in the exercise therapy arm were significantly better at 4 months (0.031, 95% CI 0.010 to 0.052), but by 12 months they were only marginally better off (0.001, 95% CI –0.030 to 0.031). This was not replicated in the CCAs reported here or in the CCA of the primary outcome of the trial (QUALEFFO-41 change at 12 months; see Chapter 4). However, the findings at 12 months had considerable methodological uncertainty, as seen by their variation across the different imputation models, and they contrast with the results of the CCA. In the case of manual therapy, in the complete-case and MI analyses, there was a decrement in QoL at 4 and 12 months relative to baseline and lower QALYs relative to SSPT. Therefore, the small benefit for or lack of QALYs and the additional costs jointly produced a low probability that both of the physiotherapy interventions will be cost-effective relative to SSPT, albeit in the context of a substantial number of missing data, discussed below.
In terms of subgroup analysis, we found that exercise therapy and manual therapy were the most cost-effective options for individuals aged ≤ 70 years and for males only, respectively. The additional benefit in the younger population was not seen in the CCA of the QUALEFFO-41 outcome at 12 months (see Chapter 4). Although these results are based on MI, we found them to be consistent across the different imputation models; further research is warranted to better understand these findings.
An important limitation of the cost-effectiveness analysis relates to the degree of missing data. Of all participants in the trial, only 54% had complete EQ-5D-5L questionnaires (i.e. at least the baseline, 4-month and 12-month questionnaires) and cost data to allow the complete-case cost-effectiveness analysis to be conducted. Missing data concerning the self-reported participant diaries were more prevalent. Those participants with incomplete EQ-5D-5L data tended to have lower utility scores and lower costs than those with complete data. There were also some issues concerning the timing of the EQ-5D-5L questionnaires at 4 and 12 months, with 23–25% of these being collected > 30 days before or after these dates. Hence, linear interpolation between the observable EQ-5D-5L dates of completion was needed to estimate the QALYs within the 12-month period of analysis. In terms of the pattern of missing data, we found it to be non-monotonic, and the statistical analysis of the missing data seemed to suggest that they were missing at random. Hence, we accounted for missing data using MI, a validated technique157 in the context of RCTs that is superior to CCA when data are missing at random. Furthermore, there was considerable variation in the ICERs of the CCAs and various imputation models to warrant caution regarding the results. However, the sensitivity analysis explored the impact of using different imputation models and we found that the physiotherapy interventions remained not cost-effective relative to SSPT.
Our cost-effectiveness analysis is based on the largest RCT among participants with OVF in the UK. Longer follow-up could reveal whether or not the observed QoL and cost differences are maintained and whether or not any significant differences emerge beyond the first year. However, it seems that the positive impact of the physiotherapy sessions on QoL in the first 4 months does not carry over to 12 months after the sessions have ended.
Conclusion
With the data available in this study, we estimated the QoL and costs associated with physical interventions in participants with OVFs. The results appear robust across a range of assumptions, indicating that a short course of physiotherapy is not cost-effective compared with a single 1-hour physiotherapy session using quality-adjusted survival as the main outcome measure.
Chapter 7 Discussion
This discussion provides an overview of findings from the trial and the issues associated with its internal and external validity and provides interpretation for clinical practice in the NHS.
Overview of trial findings and key messages
Pain and reduced function associated with hyperkyphosis and falls cause significant morbidity for patients with vertebral fractures associated with osteoporosis. Clinical guidelines from NICE recommend targeted pharmacological therapy and structured assessment of fragility fracture risk but make no recommendations about physical therapies. 80,158 The National Osteoporosis Guideline Group guidance states that ‘Physiotherapy is an important component of rehabilitation after fracture. Muscle strengthening and balance training exercise interventions may reduce falls by improving confidence and coordination as well as maintaining bone mass’8 and that ‘Regular weight-bearing exercise should be advised, tailored according to the needs and abilities of the individual patient’. 159 However, these recommendations were informed by a small number of insufficiently sized trials with short follow-up periods and the evidence base for the efficacy of physiotherapy in improving the management of patients with OVF was weak.
The PROVE trial was a rigorous, well-conducted multicentre RCT that was pragmatic in the context of the funding envelope available for physiotherapy within the UK and sought to estimate the effect of treatment strategies based on exercise or manual therapy compared with a single 1-hour session of tailored advice with a physiotherapist. Before the PROVE trial, there was no evidence on the cost-effectiveness of physiotherapy interventions, although the findings of clinical effectiveness from several small studies were promising. Our trial included 615 participants; only one previous trial had more than 100 participants. The PROVE trial used a range of recognised measures with a 12-month follow-up, which is longer than many previous trials.
We used an adaptive trial design and undertook an interim futility analysis after extensive simulation studies to determine the rules about the difference required to make the decision to adapt, stop or continue the trial. At the interim analysis, the differences from the two interventions on the primary outcomes were large enough to warrant further study and met the prespecified rules for continuing as a three-arm trial.
At our specified main end point of 12 months, our primary ITT analysis did not demonstrate any statistically significant difference in favour of either exercise or manual therapy on either of the primary outcomes compared with SSPT, although gains persisted compared with baseline in QUALEFFO-41 pain subscales and muscle endurance. In addition, reductions in thoracic kyphosis in the exercise and manual therapy arms accrued throughout the 12 months to clinically relevant levels, whereas, in the SSPT arm, after an initial small improvement, kyphosis deteriorated between 4 and 12 months. Furthermore, at the outset of the trial, there were considerable reservations about whether or not it was safe to conduct manual therapy with patients with OVF. Among the 202 manual therapy participants, there were no adverse events related to the treatment intervention; this demonstrates its safety.
At 4 months, we examined outcomes immediately after treatment and we found on the ITT analysis that improvements in muscle endurance in the manual therapy arm were statistically significant and likely to be clinically relevant, particularly for those who fully complied. Improvements in muscle endurance were also seen in the exercise therapy arm (again larger for those who fully complied) but these were not statistically significant. The subgroup analysis indicated that benefits were predominantly experienced by those participants aged ≤ 70 years, with significant improvements seen in muscle endurance in both the exercise therapy arm and the manual therapy arm for these participants at 4 months. There were no significant changes in QoL at 4 months in either arm, although improvements seen in QUALEFFO-41 pain and social function subscales approached significance in the exercise therapy arm. There were some statistically and clinically significant results at 4 months in favour of the exercise therapy arm for secondary measures of lower-limb function, walking exercise capacity and balance, and there was also significant improvement in balance for the manual therapy arm. Across the outcomes at 4 months, there was a signal in favour of exercise therapy, although this did not reach statistical significance.
We had prespecified partial compliance of the intervention as being a minimum attendance of four sessions, with full compliance being attendance of seven sessions. However, only 82 participants (38%) in the exercise therapy arm and 99 participants (49%) in the manual therapy arm received seven sessions. The CACE analysis demonstrated that there was a dose-related response, more strongly for manual therapy and larger in magnitude for full compliers than for partial compliers, but these findings did not alter the overall trial results. Analysis of cost-effectiveness demonstrated that exercise therapy resulted in higher QALY gains but higher costs than SSPT, whereas manual therapy was more costly and with fewer QALYs than SSPT. Neither of the two physiotherapy interventions was cost-effective relative to a single physiotherapy session using the £20,000-per-QALY threshold.
The qualitative interviews indicated that most participants perceived that they had received considerable benefit from both exercise and manual physiotherapy treatments, which they enjoyed and valued. However, they found adhering to HEPs difficult and did not maintain them after the formal sessions were completed. No other data were collected, so our understanding of exactly which aspects were difficult is limited. In addition, participants allocated to SSPT did not perceive this as an equal approach to manual or exercise therapy: some felt that they had received no physiotherapy and some were motivated to seek additional treatment. In the SSPT arm, treatment logs and data from the 145 participants who completed health resource use diaries showed that at least 41 out of the 196 SSPT participants (21%) had additional physiotherapy of varied types (NHS or private) within 4 months of randomisation.
The key messages that emerged from the quantitative results are that neither exercise nor manual therapy conferred significant benefits in terms of cost-effectiveness or clinical effectiveness at 12 months compared with a single session of physiotherapy education. However, at 4 months, there was a signal of short-term benefits and participants perceived that they had benefited from the interventions.
Internal validity and methodological limitations
We estimated the sample size based on a traditional three-arm trial, requiring a sample size of 180 participants per arm assuming a moderate mean standardised effect size of 0.4 on the QUALEFFO-41, power of 80% and an alpha of 0.05. To allow for a withdrawal rate of 10%, we allowed for a sample size of 600. This assumption was found to be reasonable; there were 63 withdrawals from the trial (10.2%). We recruited 15 participants in an internal pilot, giving a total sample of 615 participants, which gave sufficient statistical power to detect the difference that was originally specified.
To maximise the efficiency of the trial, we used an adaptive design. Possible adaptations could include stopping the trial early for reasons of safety, futility or efficacy or dropping interventions at an interim analysis. 160 Such approaches potentially enable the research question to be answered in less time with less cost, randomising fewer participants to a less effective treatment. 161 We used a treatment selection design that allowed an intervention to be dropped at an interim analysis if it appeared to be inferior to SSPT or to the other intervention,56 meaning that participants in the second stage of the trial would not be randomised to a less-effective intervention. If neither intervention appeared effective relative to SSPT, the trial would be stopped, resulting in a shorter trial. The trial was not designed to be stopped early for efficacy, as it was considered that an adequate sample size was necessary to provide good evidence of effectiveness for an intervention not dropped from the trial. A key trial decision that was considered was the timing of the interim analysis and which follow-up data would be used to decide this. We were aware of the need to minimise the risk of biased or inefficient adaptions but we were also aware of the need to be pragmatic and collect data from sufficient participants to provide a robust sample to make any decision, but not to leave the point of adaption so late that it would have been futile. Our original aim was to recruit the 600 participants in 15 months. If we had set an interim analysis point based on the final 12-month follow-up point, there would have been a risk that there would not have been enough time to collect and analyse enough data to support an interim trial adaptation with only a small number of participants potentially benefiting from the adaptation and no worthwhile efficiency gains. If we had conducted the interim analysis on earlier outcome data, there was a risk that the adaptation would not correlate strongly enough with the planned primary outcome, resulting in an arm being dropped erroneously, leading to a type II error, or in an ineffective arm being retained in the trial erroneously.
It was disappointing that the positive results in favour of the treatment strategies found at the interim analysis did not carry through to the full trial. In accordance with best practice guidance on adaptive trial design, we had conducted extensive simulations and agreed a clear interim analysis plan approved by the DMEC. 162,163 The interim analysis was based on a comparison of the change in QUALEFFO-41 score at 4 months and indicated that the mean 4-month improvement in QUALEFFO-41 score was greater for both exercise therapy and manual therapy than SSPT (mean differences of –2.20 and –0.74 points for exercise therapy and manual therapy, respectively), although the differences were not statistically significant. On the basis of these mean values, it was decided to continue with both intervention arms and the SSPT arm, following the planned decision rule. The mean 4-month changes in QUALEFFO-41 scores for participants from both stages of the trial combined suggest that the effects of both interventions were overestimated by the interim analysis data set, and in particular that the effect of manual therapy may actually be slightly detrimental. However, the CIs, even from the whole data set, are sufficiently wide to include the mean differences observed at the interim analysis, suggesting that the changes between the interim and full analyses are consistent with random variation. Baseline data for participants included and not included in the interim analysis indicate that the two groups of participants appear to be similar in terms of their baseline characteristics, although there is some suggestion that slightly lower proportions of participants included in the interim analysis have baseline fractures and pain at all sites. Baseline QUALEFFO-41 and TLS test values for the participants from the two trial stages were formally compared, indicating that there was no significant difference between the two stages in either measure (p = 0.729 and p = 0.184 for the comparison of baseline QUALEFFO-41 and TLS test scores, respectively).
Randomisation was conducted by a central facility at the University of Warwick and was stratified by site using variable block lengths, making it unlikely that research staff could anticipate or influence the allocation of treatment for any given participant and resulted in three well-matched arms. Owing to stratification allocating numbers to the arms by recruiting centre, the numbers recruited were not apportioned to exact thirds, but did not differ by any significant amount. Demographic data, self-reported questionnaire data and measures of clinical impairment were all similar between arms.
Owing to the nature of physiotherapy interventions, it is not possible to blind participants to their treatment allocation. Therefore, blind outcome assessment was highly important but is very hard to achieve owing to the continual risk of inadvertent unblinding of research staff by participants. 164 Strategies to reduce the risk of unblinding included explicitly instructing participants to not inform their research physiotherapist of their arm allocation at every visit. We have previously reported that utilising these strategies resulted in successfully maintaining blinding in 81–91% of the assessments, meeting the expected level for a successfully blinded trial as defined by Boutron et al. 165
The qualitative study results showed that both of the intervention arms were well received by participants. Quality assurance checks, observation visits and treatment logs showed that session content was well delivered. The progression of home programmes was delivered as planned, with good evidence that participants had increased the quantity and difficulty of exercises/stretches and incrementally increased their activity levels between the first and last sessions in the exercise therapy arm. However, the specified total number of sessions was not always delivered.
We did not achieve the planned seven sessions per participant in the exercise or manual therapy arms, and attendance was slightly lower in the exercise arm. The reasons for non-attendance varied but included physiotherapists discharging participants early from treatment and participants deciding to stop treatment or not attend. The small percentage of participants who withdrew were spread across the arms, with slightly more in the exercise and manual therapy arms. Exploration of the reasons for withdrawal showed that 35% of withdrawals were attributable to death or developing other serious health problems and 31.5% were related to travel time or inability to attend because of caring commitments rather than the unacceptability of the interventions. More participants in the SSPT arm withdrew because of dissatisfaction with their allocated arm. The difference between the treatments provided and the planned treatments in each arm exposed the trial to the risk that the intervention effects were lost or underestimated.
Despite huge efforts from the trial team, we had more missing data than hoped, with greater completion for data collected by questionnaire (86%) than for the physical outcome tests (78%) that required a visit to the centre. The pragmatic approach to collecting outcome data by post or telephone when participants were unable or unwilling to attend face-to-face assessments resulted in 10% more participants being retained in the trial. Those participants who did not complete their 12-month assessment were significantly frailer at the outset of the trial, with higher levels of pain reported at baseline, greater restriction in walking distance and speed, worse balance and more falls on entering the trial and a greater thoracic kyphosis. There were no significant differences in the missing data based on age, sex, or number of vertebral fractures.
External validity/generalisability of findings
Overall, we believe that the generalisability of the findings from this trial is high. Originally, the trial was designed to recruit from 10 hospitals within the FRiSCy network. However, slow recruitment rates and the late decision by some of the original centres to not participate meant that 21 centres across the UK delivered the trial. This brought the advantage of a much wider geographical spread and variation in settings: from small local district general hospital physiotherapy departments to large facilities within major university teaching hospitals.
At the outset, the physiotherapy departments had variable experience of delivering treatment to patients with OVF and no centres were routinely providing manual therapy to this group. Many areas had no established pathway for referral to physiotherapy on diagnosis of a spinal fragility fracture and these pathways became more established as the trial progressed. The techniques we used within the trial were all commonplace and used within the everyday practice of all the treating therapists, albeit on a different clinical population, so could easily be implemented into the NHS without the need for extensive training or equipment. Nevertheless, the training provided to the treating physiotherapists was thorough and focused on the progression of the intervention and tailoring the appropriate starting point for each intervention.
The recruited population was representative of patients referred to physiotherapy from NHS rheumatology or spinal clinics. The average age of 72.1 (SD 9.1) years matches the known increase in prevalence with advancing age, with a diagnosis in 10% of women aged 60 years, 20% of women aged 70 years and 40% of women aged 80 years. 166,167 It is estimated that one in three women and one in five men aged > 50 years will experience osteoporotic fractures. A white woman aged 50 years has a 16% lifetime risk of OVF compared with a 5% lifetime risk for men, with the incidence of vertebral fractures increasing with age in both sexes. Recent studies indicate that the prevalence of OVF in men is similar to, or even greater than, that seen in women aged 50–60 years but that OVFs are more likely to go undetected in men. 168 Thus, considering this prevalence of OVFs, men were under-represented in our trial; however, the ratio recruited into the trial was representative of those referred to physiotherapy and this is the largest number of men included in any trial of physiotherapy for OVF to date.
As in any trial, the need to define selection criteria shaped the trial population. People were excluded if they had conditions linked to secondary osteoporosis (such as kidney disease, cancer or rheumatoid arthritis) or conditions that might make it unsafe to participate or make them unable to participate (unstable cardiovascular disease) or confound results (Parkinson’s disease and planned joint surgery). The ability to walk 10 m independently with an aid if needed was an inclusion criterion to ensure that individuals were able to participate in the walking programme element of the exercise therapy intervention if randomised to this arm but may have also excluded the most frail individuals with OVF. Screening data highlight that transport problems were a major disincentive to participation and, again, this might have disproportionately affected participants with greater health problems who could not drive or use public transport independently. In addition to a baseline demographic data, data relating to osteoporosis and fracture history were sought to gain an understanding of the underlying health status of our population. Comorbidity data were obtained using a modified version of the comorbidity score by Katz et al. 169 Baseline data suggest that the SSPT arm had slightly more comorbidities than the exercise therapy or manual therapy arms. Furthermore, the relatively low levels of comorbidity reported in each arm suggest that comorbidity has been underestimated in the PROVE trial or that screening prior to the trial resulted in the exclusion of those with higher levels of comorbidity. Key comorbidities, such as depression or musculoskeletal health, are not included in the Functional Comorbidity Index and have not been captured and scored, which may help to explain these findings. However, compared with the descriptions of participants in other trials in this area, the mean TLS test time, kyphosis severity and 6MWT time, in particular, indicate that participants had significant osteoporosis disease severity. Over 60% of adults aged > 65 years have two or more chronic conditions and, as Bell and Saraf170 summarise, comorbidity and multimorbidity then rapidly increases with increasing age, so higher levels of morbidity would be expected in the PROVE trial population.
Critique of methods
We designed this trial to test interventions that could be delivered in routine NHS physiotherapy practice within the current commissioning constraints relating to the number of sessions remunerated by most Clinical Commissioning Groups. The trial showed that it was practicable to deliver the intervention contents but that, for the most part, they were not delivered at the intensity that was originally planned. The results suggest that most of the benefits from the lower-intensity interventions administered were clinically relevant but declined after active treatment ceased; although gains persisted at 12 months in some areas, this was not consistent.
QUALEFFO-41
We chose to use the QUALEFFO-41 as our primary outcome, as it is the recognised disease-specific QoL measure for this population and is targeted towards fracture assessment and deterioration. 73,171 We also chose to use the total QUALEFFO-41 score (sum of all 41 items) as our primary outcome measure rather than specifying one of the five constituent domains: pain, physical function, social function, general health perception and mental performance. There is no published MCID for the QUALEFFO-41, so interpreting the significance of the size of any change would require further research.
At 12 months, a small improvement in the mean QUALEFFO-41 total score was seen in the exercise therapy and SSPT arms and a minimal improvement was seen in the manual therapy arm. Differences between arms were not significant and outcomes in the manual therapy arm compared with SSPT worsened. The QUALEFFO-41 pain subscale score changes at 12 months showed an improvement at a clinically important level: the exercise therapy arm had the largest improvement, with a mean score of –9.07 points (SD 22.88 points) points, and improvements in the manual therapy and SSPT arms were of a similar magnitude, with mean scores of –6.75 and –6.25 points, respectively. Liu-Ambrose et al. 116 conducted a three-arm trial in women with osteoporosis and compared active interventions of resistance training or agility training with a general stretching intervention. As in our trial, with three interventions, they found no statistically significant changes in total QUALEFFO-41 scores between arms but relevant change within the exercise arm for the pain (of a similar magnitude to the PROVE trial) and social activity subscales (in the resistance arm) and for the physical function subscale in the agility arm. They concluded that resistance or agility training provided important QoL benefits to women with osteoporosis.
Several physiotherapy and exercise RCTs that have used the QUALEFFO-41 total score as their outcome measure of QoL have failed to show an improvement. 63,64,69,116,172 Some studies have failed to demonstrate improvements in total QUALEFFO-41 score but show change in QUALEFFO-41 subscale scores. 65,69 Arguably, using subscales might be more sensitive than the total QUALEFFO-41 to detect changes expected from physical interventions. In other trials in which no change has been observed in QUALEFFO-41 score, significant changes have been detected in other QoL measures: Short Form questionnaire-36 items (SF-36) subscales and General Health Questionnaire – 20 items total score and clinical measures that assess similar domains (e.g. pain and physical function at the same time points). 64,65,69 Other disease-specific QoL instruments have been used to measure the effects of physiotherapy interventions. Madureira et al. ,73 Hongo et al. 70 and Chien et al. 101 found improvements in QoL using the Osteoporosis Assessment Questionnaire and its Japanese derivation (Japanese Osteoporosis Quality of Life Questionnaire). These findings need to be treated with caution; Chien et al. 101 did not conduct an ITT analysis, and assessors were not blinded to arm allocation or outcome assessment in the study by Hongo et al. 70 Papaioannou et al. 75 demonstrated QoL benefits on domains of the Osteoporosis Quality of Life Questionnaire (OQLQ) immediately post treatment, but these did not persist at the 12-month follow-up. A comparison of the two measures found the OQLQ to be superior to the QUALEFFO-41, particularly when used with women with more than one OVF. 173 Future studies should consider whether or not the QUALEFFO-41 is the most appropriate measure for assessing the impact of physical interventions on QoL in this population; alternatively, the QUALEFFO-41 could be used in conjunction with other generic measures of QoL, such as the SF-36.
In contrast, the studies by Evstigneeva et al. ,174 Bergland et al. 65 and Angın et al. 107 do report significant between-group changes using the QUALEFFO-41. In their study, Evstigneeva et al. 174 demonstrated an improvement of 5.8 points in the mean QUALEFFO-41 score immediately after their intervention but the comparison with the control group was influenced by the fact that the control group deteriorated by 3.1 points, emphasising the treatment effect of their intervention. Bergland et al. 65 found that changes in QUALEFFO-41 total scores post treatment at 3 months were not significant, but between-group changes at 12 months were significant.
At 4 months, we observed a mean reduction in the total QUALEFFO-41 score in all three arms, the magnitude of the improvement being greatest for exercise therapy, with a mean reduction of 3.41 points, compared with reductions of 1.95 points for manual therapy and 1.21 points for SSPT. The reduction achieved in this trial at 4 months was similar to the 3.3-point reduction demonstrated by Bergland et al. 65 at 3 months, and our 1.31-point reduction at 12 months is comparable to the 2.8-point change they achieved despite our more active comparator intervention. 65 Thus, at 4 months, exercise therapy had a 2.20-point improvement in QoL compared with SSPT. At 4 months, QUALEFFO-41 subscale scores for pain and social function compared with SSPT also showed improvement, with a trend towards statistical significance (p = 0.068 for pain and p = 0.053 for social function). The unadjusted CACE analysis suggested a weak dose–response effect for QoL, but these differences were not significant or evident in the multiplicity-adjusted CACE analysis.
Timed loaded standing
At present, only a small number of studies involving relatively few participants have used the TLS test, resulting in few data to assist clinicians interpreting test times or estimating what a clinically meaningful change is. Average TLS test times in older populations are recorded in the study by Shipp et al. ,44 with a mean of 110.6 seconds (SD 54.4 seconds) in healthy, older women, and in a RCT involving 99 older adults with hyperkyphosis (16% of whom had vertebral fractures) by Katzman et al. ,175 which recorded a mean of 115.7 seconds (SD 60.5 seconds) and 123.0 seconds (SD 53.1 seconds) in the intervention and control groups, respectively. The average TLS test times at baseline in our trial of 49.7 seconds (SD 59.8 seconds), 47.9 seconds (SD 51.5 seconds) and 55.9 seconds (SD 73.7 seconds) for the exercise therapy, manual therapy and SSPT arms, respectively, are considerably shorter and indicate that our population generally had poor back muscle endurance and greater limitations at baseline. Our TLS test times are most comparable to those in (1) the study by Bennell et al. ,64 in which nine adults with at least one painful OVF in the intervention group had a mean TLS test time of 50 seconds (SD 39 seconds), and (2) the study by Shipp et al. ,44 who studied 121 women with osteoporosis and OVF, 45% of whom had back pain, in which average TLS test times were a mean of 67.6 seconds (SD 56.7 seconds).
At 12 months, increases in back muscle endurance were seen in both active intervention arms but not in the SSPT arm. Mean TLS test times in the exercise and manual therapy arms increased from baseline to 4 months. In contrast to the 4-month time point, improvements in TLS test duration were also found in the SSPT arm at 12 months. These changes were smaller and are less likely to be clinically relevant than those seen in either intervention arm (at a mean of 3.8 seconds or 7% relative to baseline), but the gains meant that differences between both the exercise therapy arm and the manual therapy arm compared with SSPT were non-significant at 1 year. Previous studies have also demonstrated improvements in back strength in the control group in RCTs of exercise in people with osteoporosis, attributing this to participants’ increased awareness, confidence or motivation to undertake physical activity. 70 The educational intervention in the SSPT arm in the PROVE trial may have amplified these effects. Certainly, participants in the qualitative study commented on the value of information and link to the ROS (which promotes exercise) highlighted by the educational session. We also know from the qualitative study and participant diaries that 21% of participants allocated to SSPT sought and received additional physiotherapy. This was more likely to happen after 4 months and might help to explain the improvements in TLS test endurance in the SSPT arm between 4 and 12 months. In addition, the lack of supervision and support for the HEP in the active intervention arms from 4 to 12 months may have contributed to the lack of difference, as gains in TLS test times in the active intervention arms were smaller during this period compared with those in the initial 4-month period.
At 4 months, increases in TLS test endurance were seen in both of the active intervention arms but not in the SSPT arm. The mean increase of 10.56 seconds above SSPT in the manual therapy arm was statistically significant in the unadjusted analysis and showed a strong trend on the multiplicity-adjusted analysis (p = 0.055) (see Appendix 24). It was greater than the mean increase of 7.46 seconds above SSPT in the exercise therapy arm, which was not significant. When compliance with treatment was considered greater, increases in holding time were seen in both arms in those who fully complied compared with those who partially complied.
When the low levels of back muscle endurance in the PROVE trial population at baseline are considered together with the fact that deterioration or no improvement in back muscle endurance would be expected in this group, the increases in TLS test times were arguably clinically significant. 64,175 Relative to baseline, TLS test times increased by a mean of 15% and 22% compared with SSPT in the exercise and manual therapy arms, respectively; within these arms, 34% and 42% increases were seen in those participants who fully complied. A longitudinal cohort study has linked increased back muscle strength with lower incidence of OVF at 10 years. Although strength gains persisted at 1 year, the rates of new OVFs were not formally evaluated radiologically and low rates were reported, with no differences between arms emerging at 1 year. 115
Back fatigue and difficulty carrying out functional tasks in standing because of fatigue are commonly reported by people with osteoporosis and chronic back pain and negatively affect QoL. 44,176 Whether or not the improvements in back muscle endurance resulted in improvements in daily life for PROVE trial participants is uncertain. Interestingly, a range of immediate benefits were attributed to manual therapy by participants in the qualitative study (including improved QoL). However, in this group, the EQ-5D-5L index was worse at all time points and minimal change in QUALEFFO-41 scores was seen at 4 months despite significant increases in endurance. Whether or not the impact of increased endurance on daily life could be captured by the measures we used in the PROVE trial needs consideration. Although increased endurance would be expected to improve performance on some of the items in the QUALEFFO-41 (e.g. meal preparation), the weight of the questionnaire is directed towards other areas (e.g. pain, mobility and mental function). In addition, none of the other outcome measures we used (PASE, SPPB and 6MWT) assessed this dimension, instead focusing on lower-limb function, mobility and exercise capacity.
Improvements in back extensor muscle function were likely to underpin the significant, clinically relevant, increases in standing balance at 4 months that were observed in the manual therapy arm, in which participants underwent no specific balance training. These improvements in back extensor muscle strength are also likely to have contributed to improvements in the exercise therapy arm. The FRT requires the participant to lift the extended arm to 90 degrees of shoulder flexion and reach as far forward as possible without taking a step. This prompts activation of the thoracic erector spinae muscles prior to and during the task, creating an extension moment that counteracts and controls movement of the centre of mass to maintain equilibrium in the anterior–posterior direction. 177,178 This is comparable to muscle activity demanded during the TLS test, so it is not surprising to see both of these measures improving in parallel. 46
Overall, the improvements in back muscle endurance at 4 months for those who received manual therapy suggest that these techniques provide at least short-term benefits on back muscle function. Few trials have investigated the effect of manual therapy techniques on muscle strength or endurance in relevant populations, but our findings are consistent with those that have. In a RCT involving 46 younger women with hyperkyphosis, Kamali et al. 86 reported significant improvements in back muscle strength immediately following a manual therapy intervention. This consisted of 15 treatments over 5 weeks, comprising thoracic posterior–anterior spinal mobilisations, stretches and soft-tissue mobilisation techniques. Bennell et al. 64 also found significant improvements in TLS test duration immediately after a multimodal physiotherapy intervention that incorporated manual therapy. This resulted in a mean change of 45.6 seconds (95% CI 16.1 to 77.3 seconds) or a 65% improvement in holding time compared with baseline. The small, pilot nature of this trial and lack of correction for multiple statistical comparisons may partly explain the extent of its changes. In addition, compared with the PROVE trial, participants in this study, on average, were younger and had fewer OVFs. The intervention combined manual techniques with strengthening exercises, was more intensive, involving 10 sessions of physiotherapy over 10 weeks, and had high levels of compliance.
The mechanisms by which manual techniques might improve endurance in the absence of any resistance exercises, endurance exercises or exercises intended to load muscle require consideration. Reduced pain might result in greater endurance. However, although the QUALEFFO-41 pain subscale for the manual therapy arm shows a mean reduction of –2.54 points (95% CI –6.56 to 1.48 points) at 4 months compared with SSPT, these changes are small and not significant. The application of posterior–anterior joint mobilisations promoted extension of local spinal segments; stretches and soft-tissue mobilisation techniques may have also increased tissue flexibility, allowing for increased active thoracic extension. Small reductions were seen in thoracic kyphosis at 4 months in the manual therapy arm, although these were not significant when compared with SSPT. Improvements in scapulothoracic muscle strength have been observed in people with neck pain and in healthy SSPT participants following spinal mobilisations and stretches without strengthening exercises. 83,179 Joint mobilisations are thought to reduce central inhibition due to pain and stiffness and allow for increased recruitment and activation of spinal muscles, allowing for gains in strength. 83,179 The postural retraining element of the manual therapy programme may have been of benefit. Participants were asked to be aware of their posture and were taught to correct their posture and to practise achieving optimal alignment in everyday tasks, which may have provided sufficient load to prompt increased endurance.
The improvement in TLS test duration in the exercise therapy arm at 4 months suggests that the strength training programme was less effective at increasing back muscle endurance. These findings agree with those of Malmros et al. ,74 who found no significant change in back extensor strength after a combined 10-week intervention of exercise classes followed by a HEP. Similarly, Katzman et al. 175 found that three 60-minute classes per week for 6 months of postural education, back strengthening and stretching exercises in participants with hyperkyphosis (some with OVFs) resulted in no improvement in back extensor strength, spinal muscle density or TLS test time. In this study, change in TLS test time in the intervention arm after treatment was minimal [mean 0.3 seconds (95% CI –11 to 11.6 seconds)] and much lower than we observed in the exercise arm in the PROVE trial.
Subgroup analyses highlighted that participants aged ≤ 70 years in both the exercise therapy arm and the manual therapy arm were most likely to have increased TLS test muscle endurance at 4 months, whereas those aged > 70 years were less likely to benefit. Previous studies that report improved back strength64,70,101 were conducted in younger populations with lower disease severity (less pain and fewer OVFs) than the PROVE trial population. Younger participants may find it easier to participate in home interventions, which provide less clinical support and supervision, and may have been more able to undertake exercises such as back extension exercises in the prone position, which have previously been linked to significantly improved back extensor strength. 70,101,115 Increased age is associated with increased osteoporosis disease severity, number of comorbidities and frailty. 25 These factors may have meant that older participants were less able to tolerate treatments, had less capacity to improve, found it more difficult to attend for treatment, might improve at a slower pace or might need greater levels of supervision.
Ageing also results in neuromuscular changes linked to weaker, less fatigue-resistant, muscles,180 and this may combine with deficits due to spinal pathology to exacerbate back muscle weakness and postural deformity in people with osteoporosis. 180,181 Whether or not exercises were sufficiently graded and whether or not physiotherapists found it difficult to individualise the programme to allow those with the lowest capacity to participate is not known. Some participants in the qualitative study described finding initial stretches and exercise prescriptions too difficult or painful at times, and some participants found treatments in the prone lying position difficult or not possible. This may have particularly affected those with the greatest mobility problems, those with more severe pain at the start of treatment and those with fixed or more severe kyphosis, many of whom would have been older participants. A range of starting positions was available to trial physiotherapists, and guidance was given to omit exercises that were painful, unsafe or not possible. Conversely, the qualitative study also noted positive experiences of exercise prescriptions being adjusted and progressed, allowing for a gradual improvement. Further research into the most appropriate exercise prescription for participants with the greatest limitations is needed. A RCT by Yang et al. 76 found that medication review including analgesia prescription combined with a 4-week twice-daily programme of low-intensity, functional exercises that focused on ensuring that exercises were pain free improved mobility and independence in patients with painful OVF. This was a small study with methodological flaws that included both inpatients and outpatients, but a combined intervention of medication review and exercise prescription could be usefully investigated in an outpatient setting.
The CACE analysis suggested that attending more sessions was associated with greater endurance and increasingly significant outcomes. This effect was stronger in the manual therapy arm, in which, on average, participants attended more sessions than those in the exercise therapy arm. These findings could suggest that additional sessions might have resulted in further gains but also highlight the difficulty of achieving full compliance with treatment. This was particularly true of those in the exercise therapy arm. Overall, exercise therapy participants attended a mean of 4.33 sessions (SD 2.7 sessions); although this would be a common number of sessions in the context of a NHS outpatient physiotherapy department, this is a very limited amount of treatment in comparison with other exercise interventions designed for this patient group. Achieving sufficient treatment intensity is a prerequisite for achieving gains in muscle strength or endurance, including in people with OVF. Further studies of intervention dose and alternative ways of delivering physical interventions are needed in people with OVF to determine how this affects compliance.
Functional reach
The FRT is a measure of dynamic standing balance and stability. 49 Performance on the FRT correlates well with performance on a range of other measures of function, dynamic balance and mobility, such as the timed up and go (TUG) test, step climbing, walking speed, tandem walk and single-leg stand. 182–184 A reach distance of 27.2 cm (SD 0.9 cm) is considered normal for older adults,185 and poor performance on the FRT, of ≤ 18.5 cm, is associated with falls and frailty. 186 Baseline measures of the FRT in all arms in the PROVE trial suggested that most participants had mild to moderate levels of balance impairment.
At 12 months, mean changes in FRT distance had declined compared with the 4-month point in both active intervention arms but continued to be greater than baseline. Mean change in the SSPT arm remained lower than baseline at all time points but improved slightly between 4 and 12 months. Consequently, at 12 months, there were no statistically significant differences between arms, although the mean difference between the exercise therapy arm and the SSPT arm of 1.02 cm (CI –0.96 to 3.01 cm) could be considered clinically valuable.
At 4 months, improved balance was seen in both of the active treatments arms, in contrast to a deterioration in balance in the SSPT arm. Changes in both the exercise therapy arm and the manual therapy arm compared with SSPT were statistically and clinically significant at this point. A 1-cm improvement on the FRT is associated with clinical benefits and lowers the risk of having major mobility problems going forward. 182 Mean changes of > 1 cm were seen in both the exercise therapy arm and the manual therapy arm, and, when compared with the SSPT arm, reach distance was a mean of 2.43 cm (95% CI 0.71 to 4.16 cm) longer in the exercise therapy arm and a mean of 2.11 cm (95% CI 0.57 to 3.64 cm) longer in the manual therapy arm. Greater changes in the exercise therapy arm may have been due to the additive effects of balance and strength training.
Similar improvements in balance after physiotherapy treatment have been reported by several RCTs involving women with OVF, all of which involved higher dose and longer duration interventions. Evstigneeva et al. 174 found that standing balance and tandem walk increased after an intensive intervention of two 40-minute classes per week for 1 year (104 sessions). A 12-month (40 sessions) balance training programme by Madureira et al. 72 significantly improved balance measured by the Berg Balance Scale, the TUG test and Clinical Test of Sensory Interaction for Balance, and fewer falls happened in the intervention arm. Long-term follow-up was not conducted in either of these studies, so whether or not benefits persist once interventions cease is unknown. In studies like ours, which include long-term follow-up, there is evidence that treatment effects decline without supervised practice. Bergland et al. 65 saw significant improvements in balance measured by TUG and FRT distance immediately after a 12-week class intervention (32 sessions). Change in FRT distance was of a similar magnitude to that observed in the PROVE trial and, like in our trial, balance declined when active intervention ceased, so at 12 months differences in functional reach were no longer significant.
Short Physical Performance Battery and 6-minute walk test
Findings from the SPPB and 6MWT will be considered together; both evaluate aspects of lower-limb physical function and mobility, and similar patterns of change were seen in these measures in the trial, indicating change in this underlying domain. The SPPB combines the results of tests of walking speed, balance and sit-to-stand function and assesses lower-limb function and mobility in older adults. 48 Performance on the SPPB is predictive of falls, subsequent disability and difficulty carrying out ADL, risk of hospitalisation and death. 187 Summary scores range from 0 to 12 points, with higher scores representing better function. A change of 0.5 points on the SPPB is thought to represent a small clinically meaningful change and a change of 1 point is thought to represent a substantial meaningful change. 188 The SPPB scores across arms at baseline suggested that, on average, participants had mild to moderately impaired lower-limb function at the start of the trial. 187 At 12 months only the exercise therapy arm showed a small clinically meaningful change in SPPB score; at 4 months, this was larger and statistically significant.
The 6MWT is an assessment of submaximal exercise capacity and walking endurance. Proposed values for the 6MWT in healthy adults range from 400 m to 700 m; distances walked reduce with age and 400 m has been proposed as the cut-off point indicative of mobility limitation and sarcopenia. 186,189 In a RCT by Angın et al. 107 involving 41 women with vertebral osteoporosis, the mean 6MWT distance was 407.82 m (SD 95.38 m) and 402.95 m (SD 90 m) at baseline for the intervention and control arms, respectively. In our trial, baseline values of 6MWT distance were considerably lower in all three arms, highlighting our participants’ low exercise capacities and poor walking endurance. 190 Our values are more comparable to groups with pulmonary disease and probably reflect the association between vertebral fractures and impaired lung function. 29,191 For the 6MWT, a change of 20 m has been estimated to be a meaningful change in response to treatment. 188
In both the SPPB and 6MWT, significant increases were seen from baseline to 4 months in the exercise therapy arm compared with the SSPT arm. In the exercise therapy arm, distance walked on the 6MWT increased by 26.09 m (95% CI 6.58 to 45.6 m), suggesting that change in these measures was both statistically and clinically significant. Angın et al. 107 also found a significant improvement in 6MWT distance after a 24-week (73-session) course of clinical Pilates supervised by a physiotherapist. The younger mean age and lower disease severity of their population, unblinded assessment and the more intensive nature of this intervention may explain the larger increase of 46.0 m (SD 22.75 m) between arms. In the manual therapy arm, changes were not statistically significant, and improvements in 6MWT distance were smaller and unlikely to be clinically significant.
The inclusion of simple functional exercises (sit to stand and step-ups) in the strength training programme, combined with balance exercises and a guided walking programme with a pedometer, resulted in short-term gains in functional mobility and exercise capacity. The qualitative study suggested that participants particularly enjoyed the walking component of the programme and were more motivated to undertake this aspect of the exercise therapy rather than other elements, although, for others, fatigue could be a barrier to engagement. Improvements in mobility may have had positive effects on QoL for participants. Outcomes on the 6MWT have been found to correlate well with changes in the SF-36 physical function subscale. 190 A trend for a significant improvement in the QUALEFFO-41 social function (p = 0.053) subscale compared with SSPT was observed in the exercise therapy arm at 4 months. Leisure and social activities, such as gardening or visiting a theatre or friends, asked about in this area of the questionnaire rely on lower-limb function and walking capacity, and positive changes in these areas may underpin results.
By 12 months, treatment effects had declined. Decline in improvement in physical function after supervised practice ceases is familiar in this population. Iwamoto et al. 122 investigated a pedometer-guided walking programme plus strengthening exercises in women with osteoporosis aged 55–77 years, but outcomes focused on lumbar BMD. At 1 year, BMD improved, but, after a subsequent year of detraining, BMD reverted to levels not significantly different from those in the control arm. Maximum walking speed increased significantly following a 12-week (25-session) circuit class in a RCT by Bergland et al. ;65 although speed declined after treatment stopped, it remained significantly better at 1 year than in the control arm, which had no intervention. Overall, exercise capacity and walking ability in people with osteoporosis and OVF is underinvestigated. Few studies to date have designed interventions that address the limited aerobic capacity and walking endurance in this population despite the relevance of these factors to everyday function and QoL. Measures that capture these domains, such as the SPPB and 6MWT, but also those that consider associated fatigue, have not been widely used or formally studied with people with osteoporosis. The PROVE trial suggests that further research in these areas is warranted.
Thoracic kyphosis
Thoracic kyphosis is expected to progress slowly with age, so that reducing kyphosis by even a small amount may be clinically significant, particularly if treatment effects are maintained over time. 175 Each new vertebral fracture in people with osteoporosis is associated with a 3.8 ° increase in thoracic kyphosis. 192 Even mildly increased kyphosis is associated with an elevated risk of further vertebral fractures due to increased spinal loads and is linked to pain, poor physical and respiratory function, impaired balance and falls. 22 Kyphosis of > 40 ° is recognised as hyperkyphosis and thoracic kyphosis of > 50 ° is associated with increased risk of falls and fractures. 175,192 At baseline, most participants in the PROVE trial were hyperkyphotic. Those in the exercise therapy arm had the highest mean kyphosis, of 51.2 ° (SD 34.8 °), and reported more falls in the previous year than those in the manual therapy and SSPT arms.
Improvements in kyphosis were seen within all three arms at 4 months, with no significant difference between arms. Between 4 and 12 months, kyphosis worsened in the SSPT arm, whereas further improvements in kyphosis were seen in both the exercise therapy arm and the manual therapy arm. There were no statistically significant differences between arms at any point; however, at 12 months the mean reduction in kyphosis of –3.02 ° (95% CI –8.14 ° to 2.10 °) in the manual therapy arm compared with SSPT was a clinically relevant difference and of a comparable size to other trials. Bautmans et al. 63 showed significant improvement in thoracic kyphosis of 3.4 ° following 3 months of manual physiotherapy that included up to 18 sessions of spinal mobilisations and taping and a HEP. They also demonstrated a dose–response relationship to treatment and problems with compliance; a per-protocol analysis in this trial showed that those who complied (at least nine sessions) had significantly more benefit, with a mean improvement (reduction in kyphosis) of –7.1 ° compared with the control.
Katzman et al. 175 completed a trial of targeted strengthening exercise and posture training aiming to reduce hyperkyphosis in older adults in the Study of Hyperkyphosis, Exercise and Function (SHEAF). Not all participants had a diagnosis of osteoporosis and vertebral fracture, with 12 out of 51 participants in the intervention arm and 4 out of 48 participants in the control arm having a confirmed vertebral fracture. Final assessment was at 6 months immediately after completing exercise classes. Katzman et al. 175 reported a mean reduction in thoracic kyphosis of 3 ° in their intervention group compared with their control group but no differences in other outcomes of physical function or QoL. They conclude that a targeted spine-strengthening and posture-training intervention over 6 months may be an effective treatment option for older adults with hyperkyphosis. The primary outcome was thoracic kyphosis measured as a Cobb angle from a standing lateral spine radiograph. They also measured kyphosis using the Debrunner kyphometer; using this method, they reported an identical 3 ° reduction in kyphosis angle. In our trial, we measured kyphosis angle using the from flexicurve as a simple and cost-effective method readily adopted in clinical practice, which did not involve any radiation. We found a mean reduction of 6.8 ° in the exercise therapy arm, 4.63 ° in the manual therapy arm and 2.73 ° in the SSPT arm. Although Katzman et al. 175 reported a between-group difference of 3 ° as being statistically significant, the greater difference of 4.15 ° that we found was not statistically significant; however, it may have been clinically important. At trial entry, the exercise therapy arm had the greatest mean kyphosis at 51.2 °, which was the only arm above the 50 ° threshold associated with increased fall risk and which reported the most falls by participant in the previous year. The within-group change brought the mean kyphosis below this threshold; at 12 months, no significant difference in the incidence of falls was seen between arms. This may be evidence of the stability, or of a ‘static success’, of the intervention, helping to maintain and avoid progression of a condition and associated secondary complications.
Our lower-intensity intervention was associated with clinically important changes in kyphosis in both the manual therapy arm and the exercise therapy arm, which increased in size over the 12-month period, in contrast to the SSPT arm, in which effects declined. Most participants did not receive the complete treatment as planned, and this is likely to have resulted in smaller treatment effects and, along with our more active comparator intervention, explains our lack of statistically significant findings. A range of benefits was attributed to manual therapy in the qualitative study, including decreased stiffness, improved mobility and improved well-being. In the qualitative study, the use of taping was highlighted as being difficult to tolerate and a reason for non-compliance for some participants. The qualitative research highlighted that feedback about spinal shape was very powerful and prompted engagement with treatment but orthotic interventions that are burdensome or uncomfortable can also affect well-being. 193 Bautmans et al. 63 asked physiotherapists and patients about their experiences of treatment. Specific reasons for non-compliance varied, as might be expected, but themes emerged that were also raised in the PROVE qualitative study, including barriers due to mobility or health problems and costs related to treatment (e.g. transport and time). Feedback from physiotherapists in this study included that patients needed lots of guidance with home exercises and that motivation for home exercise was low.
Pain
Chronic back pain is a characteristic feature of vertebral fracture. Over 95% of all participants in the PROVE trial reported experiencing back pain in the fortnight before entering the trial and ≥ 65% in each arm reported pain at the baseline assessment. Using a simple VAS (0 to 10), most participants rated their pain at a moderate intensity at this point. We considered the effects of our interventions on pain using the QUALEFFO-41 pain subscale and a VAS (pain today). In a population with chronic musculoskeletal pain, a change of 1 point or a 15% reduction in pain on a 0–10 VAS is thought to represent a MCID. 194
All arms reported that pain improved at 4 months on the QUALEFFO-41 pain subscale and within each arm there was a further reduction in pain intensity on the QUALEFFO-41 at 12 months. The largest improvement was in the exercise therapy arm, and improvements in the manual therapy and SSPT arms were of the same magnitude. Placebo effects or positive effects of attention provided by clinical and research staff are suggested to affect measures of pain more than physical outcome and may have contributed to the similarity of change in these arms. Alternatively, the educational intervention may also have provided support and prompted behaviour change.
At 4 months, we observed a trend towards significance in the QUALEFFO-41 pain subscale in the exercise therapy arm compared with SSPT arm (p = 0.68). However, the size of the improvement, at a mean score of 3.57 points, compared with SSPT was smaller than that seen in the trials by Bergland et al. ,65 Evstigneeva et al. 174 and Bennell et al. 64 This is likely to reflect the low intensity of our intervention and the fact that our control arm received an intervention. Other studies have reported no change in pain on the QUALEFFO-41 pain subscale but significantly reduced pain using measures such as the SF-36;69 the responsiveness of the QUALEFFO-41 pain subscale may be too low to demonstrate early changes after a short intervention.
At 4 months, small improvements on the VAS (pain today) were seen in each arm, none of which could be considered statistically or clinically significant. Relative to baseline levels, the largest changes were following manual therapy (9.1% reduction), followed by exercise therapy (7.5% reduction) then SSPT (3.2% reduction). In contrast to the direction of change on the QUALEFFO-41 pain subscale, these effects had declined at 12 months. Gold et al. 68 also found no significant change in pain in a trial of 185 women with osteoporosis, despite an intensive class intervention (120 sessions) that included education about coping strategies and provided high levels of psychological support. In their trial, participants had lower levels of pain at baseline and the comparator arm received an educational intervention. Other studies have reported changes in VAS pain intensity after exercise interventions. A small RCT by Karakasidou et al. 195 investigated the effectiveness of 12 weeks (36 sessions) of motor control exercise (including graded lumbar and trunk stabilisation exercises) in a group of 20 women with OVFs. They demonstrated a reduction in pain intensity of 33% following treatment; however, outcome assessment was unblinded and conducted by the physiotherapist who delivered the treatment, exposing the trial to significant bias. Angın et al. 107 found that VAS pain and QUALEFFO-41 pain scales significantly improved after a 24-week clinical Pilates intervention, which also involves specific graded lumbar and trunk stabilisation exercises; again, outcome assessment was unblinded. These trials are interesting, as the exercise therapy arm in the PROVE trial included similar lumbar and trunk stabilisation exercises and showed a positive trend towards significance.
Study limitations
There are limitations to our work.
In an attempt to operate within the constraints of standard commissioning in the UK, we designed an intervention that was potentially too low in dose and duration. Compared with other previously reported trials, our intervention was brief and over a relatively short duration. Determining the intervention dose was challenging. Many RCTs of physical interventions for people with low vertebral BMD are delivered in a class format and these types of interventions typically involve more sessions. For instance, in 10 RCTs of group exercise interventions published prior to the trial, the number of sessions ranged from 16 to 72, with a mean of 40 sessions, and intervention periods ranged from 8 to 40 weeks, with a mean of 22 weeks. 65,67–69,72,74,100,102,104,120 In this context, seven sessions over 12 weeks seems limited. However, RCTs of interventions delivered as a HEP or as a combination of individual sessions and home exercise for this population provide fewer sessions and continue to report benefits. 70,71,75,101,122 The PROVE trial interventions more closely resemble these trials. In the two studies that used manual therapy, one offered 10 sessions over 11 weeks and the other offered 18 sessions over 12 weeks. 63,64 Studies of home exercise alone offer even fewer individual sessions. 70,71,101,122 Two high-quality studies lasting 12101 and 16 weeks70 reported short-term benefits from a single session of physiotherapy plus HEP supported by instruction booklets, exercise diaries and telephone calls every 2 weeks, or a maximum of eight contacts. Another RCT found benefits following a total of seven sessions of physiotherapy over 26 weeks: an initial session of physiotherapy supported by regular monthly reviews to foster adherence and progress treatments. 75 One RCT reported benefits from a single session of physiotherapy and HEP supported by checks every 3 months; adherence was high (86%) but these results should be interpreted cautiously as only a per-protocol analysis was completed. 71 It is recognised that achieving positive outcomes from HEPs can be challenging when support for adoption is limited. 125,128
Many of the exercises incorporated within the PROVE trial exercise therapy arm had been used previously in RCTs that reported significant increases in back strength. 67,72,76,89 However, key differences between these trials and the PROVE trial exist. Gold et al. 68 investigated an intensive 6-month (120-session) class intervention that combined exercise classes with classes focused on coping strategies and psychological support. The combination of psychological and exercise interventions, as well as the trial’s setting in retirement communities, which avoided potential barriers to treatment such as transport, may have encouraged adherence and, alongside greater treatment intensity, prompted strength gains. Notably, when supervised exercise ceased at 6 months, improvements in strength declined and by 1 year were no longer significant.
In designing our SSPT arm, we spent considerable effort to design an intervention that would be acceptable to trial participants and was ethically acceptable but that did not mirror the content of either of the intervention arms. We provided a 1-hour tailored assessment and advice session with an experienced physiotherapist who provided individualised advice about lifestyle modifications, falls prevention, posture and physical activity (but no specific exercises). Our results show that, unlike most of the previous trials that have reported deterioration in outcome for the control arm, our SSPT participants demonstrated a mean improvement in the total QUALEFFO-41 score of 1.18 points and a mean improvement in the TLS test score of 4.2 seconds. Similarly, on the secondary outcome measures, the SSPT arm demonstrated small, non-significant, improvements on most outcome measures, with the exception of functional reach. This may be a reflection of a placebo response or participants may have genuinely benefited from the single session with a physiotherapist and acted on the advice given to improve their activity, posture and functional activity. It may also reflect the fact that at least 41 participants (21%) in the SSPT arm had additional non-trial physiotherapy treatments from 0 to 4 months, the effects of which may be being picked up here.
Furthermore, although the changes we found at 12 months were not significant and those at 4 months provided a signal of effect at best, these results should be considered against the expected course of progression for participants with OVF. It is recognised that, following an OVF, patients have a substantial reduction in QoL, which remains impaired for ≥ 18 months. 16 It is also recognised that vertebral fractures are associated with an increased risk of both further vertebral fractures and non-vertebral fractures. 196–198 Women who develop a vertebral fracture are at substantial risk of additional fracture within the next 1–2 years, with the incidence of a new vertebral fracture in the subsequent 12 months being around 20%. 198
In the context of long-term conditions such as osteoporosis, the efficacy of an intervention cannot be judged solely by improved function; physical maintenance and avoidance of associated complications (such as falls) are also important criteria. 199 Considering these factors, the small improvements in pain, QoL and function, together with the low rates of falls within the groups, although not resulting in significant between-group differences, arguably represent a static success and a worthwhile outcome when compared with the deterioration that would be expected with the natural course of the disease in the absence of other changes to the patients’ medical management. It would also have been beneficial to have recorded at baseline assessment the time since latest OVF and to have looked at the variance of time post fracture in relation to the results of the interventions.
Implications for clinical practice and recommendations for future research
Although the trial did not find a significant difference in the two intervention arms at 12 months, there was a signal from one of the primary outcomes and several secondary measures that there was effect at 4 months.
There was a clear problem regarding the willingness of participants to attend all seven sessions or of physiotherapists to deliver the seven sessions in the intervention arms, resulting in an intervention that was arguably underdosed and lacking the power to realise a change. In contrast, at least 41 participants in the SSPT arm received additional (NHS or private) physiotherapy, suggesting underlying demand for treatment. Further work is needed in this population to explore ways of delivering or supporting interventions to maximise engagement (e.g. to look at class vs. individual or hospital vs. community interventions or the effect of additional telephone or online support). The CACE analysis indicated a dose–response effect and strategies that improved adherence to the intervention may have improved effectiveness. Further investigations that consider treatment dose and how this relates to outcomes, including compliance, would also be of benefit. For example, in the context of a long-term condition like osteoporosis, a short ‘burst’ of physiotherapy treatment but with planned review may be more suitable to sustain long-term engagement.
Importantly, it should be noted that our comparator arm was not an absence of treatment but a single 1-hour session with a physiotherapist who provided individualised and tailored advice to participants after an assessment of their presenting problems. There is evidence that this comparator intervention led to improved outcomes from baseline on the majority of the outcomes that we assessed against the expected normal course of the disease. Although there was not strong enough evidence to support the implementation of manual therapy or exercise therapy in the long term, it is arguable that short-term 4-month benefits were worthwhile in terms of patient feedback and that a single session of physiotherapy conferred benefit and was cost-effective.
Patient and public involvement
We benefited from the support of highly engaged patients and members of the public. During the inception of the trial, we were supported by the ROS charity: a representative from this organisation was a co-applicant and another representative was a fully voting member of the TSC. More informally, we were extensively supported by members of our local osteoporosis group and from former and current patients undergoing physiotherapy for osteoporosis-related fractures. Their generous time and comments were hugely influential in shaping the trial, in designing the intervention and in the patient information and documentation that we produced to support the trial. We worked with the ROS in their Strong, Straight, Steady campaign to feed our findings into their new clinical guidelines, which were launched in December 2018.
Further research
It is suggested that further research should focus on the intensity and duration of interventions to determine if the intervention effect could be enhanced by using treatment protocols that exceed current commissioning practice and allow for a greater number of sessions over a longer period.
Research to explore the relative benefits of alternative ways of delivering interventions would also be useful, particularly if this improved adherence.
Acknowledgements
Contributions of authors
Karen L Barker (Professor of Physiotherapy, Chief Investigator) led the funding application, trial conception and design and development of interventions and was responsible for supervision and writing and reviewing the report.
Meredith Newman (Research Fellow, Trial Lead) contributed to the trial design, development of interventions, supervision and writing and reviewing the report.
Nigel Stallard (Statistician) designed and conducted statistical analysis and contributed to writing and reviewing the report.
Jose Leal (Health Economist) designed and conducted economic analysis and contributed to supervision and writing and reviewing the report.
Catherine Minns Lowe (Research Fellow, Qualitative) contributed to the qualitative study design and conduct and writing and reviewing the report.
Muhammad K Javaid (Associate Professor, Consultant in Metabolic Bone Disease) contributed to trial supervision, trial design, the assessment of adverse events and reviewing the report.
Angela Noufaily (Statistician) conducted statistical analysis and contributed to writing and reviewing the report.
Anish Adhikari (Health Economist) contributed to the economic analysis and writing and reviewing the report.
Tamsin Hughes (Research Physiotherapist) contributed to the trial intervention and assessments and writing and reviewing the report.
David J Smith (Trial Co-ordinator) contributed to the trial management and writing and reviewing the report.
Varsha Gandhi (Trial Co-ordinator) contributed to the trial management, quality assurance of interventions and writing and reviewing the report.
Cyrus Cooper (Professor of Rheumatology) contributed to the trial conception and design, trial supervision and writing and reviewing the report.
Sarah E Lamb (Professor of Rehabilitation, Director of Clinical Trials Unit) contributed to the trial conception and design, trial supervision and writing and reviewing the report.
Physiotherapy Rehabilitation for Osteoporotic VErtebral Fracture hospitals
Cambridge University Hospitals NHS Foundation Trust (Addenbrookes Hospital), Countess of Chester NHS Foundation Trust (Countess of Chester Hospital), East Sussex Healthcare NHS Foundation Trust (Eastbourne General Hospital and Conquest Hospital), King’s College Hospital NHS Foundation Trust (King’s College Hospital), Nottingham University Hospitals NHS Foundation Trust (Queen’s Medical Centre), Oxford University Hospitals NHS Trust (Nuffield Orthopaedic Centre), Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital), Royal Devon and Exeter NHS Foundation Trust (Heavitree Hospital), Royal Orthopaedic Hospital NHS Foundation Trust (Royal Orthopaedic Hospital), Royal Surrey County Hospital Foundation Trust (Royal Surrey County Hospital), Royal United Hospitals Bath NHS Foundation Trust (Royal National Hospital for Rheumatic Diseases), Sheffield Teaching Hospitals NHS Foundation Trust (Northern General Hospital and Royal Hallamshire Hospital), Solent Hospitals NHS Foundation Trust (Queen Alexandra Hospital and St Mary’s Hospital), South Devon Healthcare NHS Foundation Trust (Torbay District General Hospital), Southend University Hospital NHS Foundation Trust (Southend Hospital), Staffordshire and Stoke-on-Trent Partnership NHS Foundation Trust (Haywood Hospital), The Ipswich Hospitals NHS Trust (Ipswich Hospital), The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (Royal Bournemouth Hospital and Christchurch Hospital), University College London NHS Foundation Trust (University College Hospital), University Hospitals of Leicester NHS Trust (Leicester General Hospital and Leicester Royal Infirmary) and Western Sussex Hospitals NHS Foundation Trust (Worthing Hospital).
Physiotherapy Rehabilitation for Osteoporotic VErtebral Fracture trial team
-
Chief Investigator: Professor Karen L Barker.
-
Co-investigators: Professor Sarah E Lamb, Professor Cyrus Cooper and Professor Muhammad K Javaid.
-
Trial Lead: Ms Meredith Newman.
-
Trial Co-ordinators/Administration: Ms Varsha Gandhi and Dr David J Smith.
-
Trial Statisticians: Professor Nigel Stallard, Dr Angela Noufaily and Dr Peter Kimani.
-
Health Economists: Dr Helen Campbell, Dr Jose Leal and Dr Anish Adhikari.
Site principal investigators and research clinicians
Charlotte Heywood and Phillip Clarke (Addenbrookes Hospital), Heather Shilliday (Countess of Chester Hospital), Carol McCrum and Kate Weatherly (Eastbourne General Hospital, Conquest Hospital), Nicky Wilson (King’s College Hospital), Ira Pande, Marie-Josephe Pradere and Rachel Nicole (Queen’s Medical Centre), Tamsin Hughes (Nuffield Orthopaedic Centre), Kim Brown, Anna Thornhill and Emma McLoughlin (Queen Alexandra Hospital), Sarah Moore, Liz Jacobs and Stephanie Travers-Griffin (Heavitree Hospital), David Rogers and Gareth Stephens (Royal Orthopaedic Hospital), Rebecca Hull, Joanne Richardson and Michael Dawson (Royal Surrey County Hospital), Julie Russell and Sophie Park (Royal National Hospital for Rheumatic Diseases), Jessica Shipley and Jacqueline Harrison (Northern General Hospital, Royal Hallamshire Hospital), Steven Young Min (Queen Alexandra Hospital, St Mary’s Hospital), Linda Knott, Nicola Donlin and John Clare (Torbay District General Hospital), Hubert van Griensven, Billy Fashanu, Steven Young and Joanne Calver (Southend Hospital), Zoe Paskins and Lucy Huckfield (Haywood Hospital), David Cumming, Sajeed Kaleel, Maggie Dawson, Sheeba Suresh and Mary Knott (Ipswich Hospital), Jonathan Marks and Emily Watson (Royal Bournemouth Hospital, Christchurch Hospital), Sophia Mavrommatis (University College Hospital), Nicola Clague and Ross Dixey (Leicester General Hospital, Leicester Royal Infirmary) and Chris Mercer and Isobel Amey (Worthing Hospital).
Trial Steering Committee
Professor Maria Stokes (Chairperson), Professor Sarah E Lamb, Dr Victoria Allgar, Dr Allison Rushton, Dr Jane Simmonds, Dr Fiona Cramp and Mrs Jane Aldridge (Patient Representative).
Data Monitoring Committee
Professor David Torgerson (Chairperson), Professor Susan Todd and Professor Helen Dawes.
Other acknowledgements
-
Dr Peter Kimani (Statistician).
-
Dr Helen Campbell (Health Economist).
-
Dr Fran Toye for assistance in analysis of the qualitative study.
Publications
Barker KL, Javaid MK, Newman M, Minns Lowe C, Stallard N, Campbell H, et al. Physiotherapy Rehabilitation for Osteoporotic Vertebral Fracture (PROVE): study protocol for a randomised controlled trial. Trials 2014;15:22.
Barker KL, Toye F, Minns Lowe CJ. A qualitative systematic review of patients’ experience of osteoporosis using meta-ethnography. Arch Osteoporos 2016;11:33.
Newman M, Minns Lowe CJ, Barker KL. Spinal orthoses for vertebral osteoporosis and osteoporotic vertebral fracture: a systematic review. Arch Phys Med Rehab 2016;97:1013–25.
Newman M, Newman R, Hughes T, Vadher K, Barker KL. Is the timed loaded standing test a valid measure of back muscle endurance in people with vertebral osteoporosis? Osteoporos Int 2018;29:893–905.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Boskey AL, Coleman R. Aging and bone. J Dent Res 2010;89:1333-48. https://doi.org/10.1177/0022034510377791.
- Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med 2015;277:650-61. https://doi.org/10.1111/joim.12369.
- International Osteoporosis Foundation . Biological Causes of Osteoporosis: International Osteoporosis Foundation n.d. www.iofbonehealth.org/pathophysiology-biological-causes-osteoporosis (accessed 23 July 2018).
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. National Osteoporosis Foundation . Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 2014;25:2359-81. https://doi.org/10.1007/s00198-014-2794-2.
- Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol 2017;18:3-36. https://doi.org/10.1007/s10195-017-0474-7.
- Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-41.
- World Health Organization . Prevention and Management of Osteoporosis 2003.
- Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12. https://doi.org/10.1007/s11657-017-0324-5.
- Scottish Intercollegiate Guidelines Network . SIGN 142 Management of Osteoporosis and the Prevention of Fragility Fractures 2015.
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 2013;8. https://doi.org/10.1007/s11657-013-0137-0.
- Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, et al. Epidemiology of fractures in the United Kingdom 1988–2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone 2016;87:19-26. https://doi.org/10.1016/j.bone.2016.03.006.
- Gauthier A, Kanis JA, Jiang Y, Martin M, Compston JE, Borgström F, et al. Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: estimations from a disease model. Arch Osteoporos 2011;6:179-88. https://doi.org/10.1007/s11657-011-0063-y.
- Sànchez-Riera L, Wilson N. Fragility fractures. Their impact on older people. Best Pract Res Clin Rheumatol 2017;31:169-91. https://doi.org/10.1016/j.berh.2017.10.001.
- Gerdhem P. Osteoporosis and fragility fractures: vertebral fractures. Best Pract Res Clin Rheumatol 2013;27:743-55. https://doi.org/10.1016/j.berh.2014.01.002.
- Mokhtarzadeh H, Anderson DE. The role of trunk musculature in osteoporotic vertebral fractures: implications for prediction, prevention, and management. Curr Osteoporos Rep 2016;14:67-76. https://doi.org/10.1007/s11914-016-0305-4.
- Svedbom A, Borgstom F, Hernlund E, Strom O, Alekna V, Bianchi ML, et al. Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures – results from the ICUROS. Osteoporos Int 2018;29:557-66. https://doi.org/10.1007/s00198-017-4317-4.
- Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: projections for 2000–20. J Med Econ 2001;4:51-62. https://doi.org/10.3111/200104051062.
- Kendler DL, Bauer DC, Davison KS, Dian L, Hanley DA, Harris ST, et al. Vertebral fractures: clinical importance and management. Am J Med 2016;129:221.e1-10. https://doi.org/10.1016/j.amjmed.2015.09.020.
- Puisto V, Rissanen H, Heliövaara M, Impivaara O, Jalanko T, Kröger H, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3210 men and 3730 women with 30 years of follow-up. Eur Spine J 2011;20:2181-6. https://doi.org/10.1007/s00586-011-1852-0.
- Kado DM. The rehabilitation of hyperkyphotic posture in the elderly. Eur J Phys Rehabil Med 2009;45:583-93.
- Tsuda T. Epidemiology of fragility fractures and fall prevention in the elderly: a systematic review of the literature. Curr Orthop Pract 2017;28:580-5. https://doi.org/10.1097/BCO.0000000000000563.
- Broy SB. The vertebral fracture cascade: etiology and clinical implications. J Clin Densitom 2016;19:29-34. https://doi.org/10.1016/j.jocd.2015.08.007.
- Mika A, Unnithan VB, Mika P. Differences in thoracic kyphosis and in back muscle strength in women with bone loss owing to osteoporosis. Spine 2005;30:241-6. https://doi.org/10.1097/01.brs.0000150521.10071.df.
- Kado DM, Miller-Martinez D, Lui LY, Cawthon P, Katzman WB, Hillier TA, et al. Hyperkyphosis, kyphosis progression, and risk of non-spine fractures in older community dwelling women: the study of osteoporotic fractures (SOF). J Bone Miner Res 2014;29:2210-16. https://doi.org/10.1002/jbmr.2251.
- Ong T, Kantachuvesiri P, Sahota O, Gladman JRF. Characteristics and outcomes of hospitalised patients with vertebral fragility fractures: a systematic review. Age Ageing 2018;47:17-25. https://doi.org/10.1093/ageing/afx079.
- Venmans A, Lohle PN, van Rooij WJ. Pain course in conservatively treated patients with back pain and a VCF on the spine radiograph (VERTOS III). Skeletal Radiol 2014;43:13-8. https://doi.org/10.1007/s00256-013-1729-x.
- Eum R, Leveille SG, Kiely DK, Kiel DP, Samelson EJ, Bean JF. Is kyphosis related to mobility, balance, and disability?. Am J Phys Med Rehabil 2013;92:980-9. https://doi.org/10.1097/PHM.0b013e31829233ee.
- van der Jagt-Willems HC, de Groot MH, van Campen JP, Lamoth CJ, Lems WF. Associations between vertebral fractures, increased thoracic kyphosis, a flexed posture and falls in older adults: a prospective cohort study. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0018-z.
- Watanabe R, Shiraki M, Saito M, Okazaki R, Inoue D. Restrictive pulmonary dysfunction is associated with vertebral fractures and bone loss in elderly postmenopausal women. Osteoporos Int 2018;29:625-33. https://doi.org/10.1007/s00198-017-4337-0.
- National Osteoporosis Guideline Group . Assessment of Fracture Risk n.d. www.sheffield.ac.uk/NOGG/mainrecommendations.html (accessed 23 July 2018).
- Royal Osteoporosis Society (ROS) . Bone Building Exercise n.d. https://nos.org.uk/about-osteoporosis/your-bone-strength/bone-building-exercise/ (accessed 8 February 2019).
- Royal Osteoporosis Society (ROS) . Osteoporosis Drugs n.d. https://nos.org.uk/about-osteoporosis/treating-osteoporosis/osteoporosis-drugs/ (accessed 23 July 2018).
- Montagu A, Speirs A, Baldock J, Corbett J, Gosney M. A review of vertebroplasty for osteoporotic and malignant vertebral compression fractures. Age Ageing 2012;41:450-5. https://doi.org/10.1093/ageing/afs024.
- Sebaaly A, Rizkallah M, Bachour F, Atallah F, Moreau PE, Maalouf G. Percutaneous cement augmentation for osteoporotic vertebral fractures. EFORT Open Rev 2017;2:293-9. https://doi.org/10.1302/2058-5241.2.160057.
- Anderson PA, Froyshteter AB, Tontz WL. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J Bone Miner Res 2013;28:372-82. https://doi.org/10.1002/jbmr.1762.
- Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1408-16. https://doi.org/10.1016/S0140-6736(16)31341-1.
- Stevenson M, Gomersall T, Lloyd Jones M, Rawdin A, Hernández M, Dias S, et al. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18170.
- Giangregorio LM, McGill S, Wark JD, Laprade J, Heinonen A, Ashe MC, et al. Too fit to fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int 2015;26:891-910. https://doi.org/10.1007/s00198-014-2881-4.
- Barker KL, Javaid MK, Newman M, Minns Lowe C, Stallard N, Campbell H, et al. Physiotherapy Rehabilitation for Osteoporotic Vertebral Fracture (PROVE): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-22.
- Giangregorio LM, Macintyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD008618.pub2.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Good Clinical Practice Guide 2012.
- World Medical Association . World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Nurs Ethics 2002;9:105-9.
- Mayoux-Benhamou MA, Roux C, Perraud A, Fermanian J, Rahali-Kachlouf H, Revel M. Predictors of compliance with a home-based exercise program added to usual medical care in preventing postmenopausal osteoporosis: an 18-month prospective study. Osteoporos Int 2005;16:325-31. https://doi.org/10.1007/s00198-004-1697-z.
- Shipp KM, Purse JL, Gold DT, Pieper CF, Sloane R, Schenkman M, et al. Timed loaded standing: a measure of combined trunk and arm endurance suitable for people with vertebral osteoporosis. Osteoporos Int 2000;11:914-22. https://doi.org/10.1007/s001980070029.
- Eyskens JB, Nijs J, D’Août K, Sand A, Wouters K, Moorkens G. Timed loaded standing in female chronic fatigue syndrome compared with other populations. J Rehabil Res Dev 2015;52:21-9. https://doi.org/10.1682/JRRD.2014.03.0086.
- Newman M, Newman R, Hughes T, Vadher K, Barker KL. Is the timed loaded standing test a valid measure of back muscle endurance in people with vertebral osteoporosis?. Osteoporos Int 2018;29:893-905. https://doi.org/10.1007/s00198-017-4358-8.
- Greendale GA, Nili NS, Huang MH, Seeger L, Karlamangla AS. The reliability and validity of three non-radiological measures of thoracic kyphosis and their relations to the standing radiological Cobb angle. Osteoporos Int 2011;22:1897-905. https://doi.org/10.1007/s00198-010-1422-z.
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-94. https://doi.org/10.1093/geronj/49.2.M85.
- Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. J Gerontol 1992;47:M93-8. https://doi.org/10.1093/geronj/47.3.M93.
- Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up and Go Test, and gait speeds. Phys Ther 2002;82:128-37. https://doi.org/10.1093/ptj/82.2.128.
- EuroQoL . EQ-5D-5L: About 2009. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 23 July 2018).
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. https://doi.org/10.1016/0895-4356(93)90053-4.
- Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000;21:167-89. https://doi.org/10.1016/S0197-2456(00)00046-5.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- International Osteoporosis Foundation . QUALEFFO-41 Scoring Algorithm 2017 n.d. http://share.iofbonehealth.org/designer/Qualeffo-Scoring-Algorithm_09_06_%2017.pdf (accessed 23 July 2018).
- Bretz F, Schmidli H, König F, Racine A, Maurer W. Confirmatory seamless phase II/III clinical trials with hypotheses selection at interim: general concepts. Biom J 2006;48:623-34. https://doi.org/10.1002/bimj.200510232.
- Friede T, Parsons N, Stallard N, Todd S, Valdes Marquez E, Chataway J, et al. Designing a seamless phase II/III clinical trial using early outcomes for treatment selection: an application in multiple sclerosis. Stat Med 2011;30:1528-40. https://doi.org/10.1002/sim.4202.
- Stallard N, Kimani PK. Uniformly minimum variance conditionally unbiased estimation in multi-arm multi-stage clinical trials. Biometrika 2018;105:495-501. https://doi.org/10.1093/biomet/asy004.
- Magirr D, Jaki T, Posch M, Klinglmueller F. Simultaneous confidence intervals that are compatible with closed testing in adaptive designs. Biometrika 2013;100:985-96. https://doi.org/10.1093/biomet/ast035.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. https://doi.org/10.1191/0962280205sm403oa.
- Medical Research Council . Guidelines for Management of Global Health Trials Involving Clinical of Public Health Interventions 2017 2017. https://mrc.ukri.org/documents/pdf/guidelines-for-management-of-global-health-trials/ (accessed 23 July 2018).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Bautmans I, Van Arken J, Van Mackelenberg M, Mets T. Rehabilitation using manual mobilization for thoracic kyphosis in elderly postmenopausal patients with osteoporosis. J Rehabil Med 2010;42:129-35. https://doi.org/10.2340/16501977-0486.
- Bennell KL, Matthews B, Greig A, Briggs A, Kelly A, Sherburn M, et al. Effects of an exercise and manual therapy program on physical impairments, function and quality-of-life in people with osteoporotic vertebral fracture: a randomised, single-blind controlled pilot trial. BMC Musculoskelet Disord 2010;11. https://doi.org/10.1186/1471-2474-11-36.
- Bergland A, Thorsen H, Kåresen R. Effect of exercise on mobility, balance, and health-related quality of life in osteoporotic women with a history of vertebral fracture: a randomized, controlled trial. Osteoporos Int 2011;22:1863-71. https://doi.org/10.1007/s00198-010-1435-7.
- Olsen CF, Bergland A. The effect of exercise and education on fear of falling in elderly women with osteoporosis and a history of vertebral fracture: results of a randomized controlled trial. Osteoporos Int 2014;25:2017-25. https://doi.org/10.1007/s00198-014-2724-3.
- Bergström I, Bergström K, Kronhed A-CG, Karlsson S, Brinck J. Back extensor training increases muscle strength in postmenopausal women with osteoporosis, kyphosis and vertebral fractures. Adv Physiother 2011;13:110-7. https://doi.org/10.3109/14038196.2011.581696.
- Gold DT, Shipp KM, Pieper CF, Duncan PW, Martinez S, Lyles KW. Group treatment improves trunk strength and psychological status in older women with vertebral fractures: results of a randomized, clinical trial. J Am Geriatr Soc 2004;52:1471-8. https://doi.org/10.1111/j.1532-5415.2004.52409.x.
- Grahn Kronhed A-C, Hallberg I, Ödkvist L, Möller M. Effect of training on health-related quality of life, pain and falls in osteoporotic women. Adv Physiother 2009;11:154-65. https://doi.org/10.1080/14038190902896659.
- Hongo M, Itoi E, Sinaki M, Miyakoshi N, Shimada Y, Maekawa S, et al. Effect of low-intensity back exercise on quality of life and back extensor strength in patients with osteoporosis: a randomized controlled trial. Osteoporos Int 2007;18:1389-95. https://doi.org/10.1007/s00198-007-0398-9.
- Kanemaru A, Arahata K, Ohta T, Katoh T, Tobimatsu H, Horiuchi T. The efficacy of home-based muscle training for the elderly osteoporotic women: the effects of daily muscle training on quality of life (QoL). Arch Gerontol Geriatr 2010;51:169-72. https://doi.org/10.1016/j.archger.2009.10.003.
- Madureira MM, Takayama L, Gallinaro AL, Caparbo VF, Costa RA, Pereira RM. Balance training program is highly effective in improving functional status and reducing the risk of falls in elderly women with osteoporosis: a randomized controlled trial. Osteoporos Int 2007;18:419-25. https://doi.org/10.1007/s00198-006-0252-5.
- Madureira MM, Ciconelli RM, Pereira RM. Quality of life measurements in patients with osteoporosis and fractures. Clinics 2012;67:1315-20. https://doi.org/10.6061/clinics/2012(11)16.
- Malmros B, Mortensen L, Jensen MB, Charles P. Positive effects of physiotherapy on chronic pain and performance in osteoporosis. Osteoporos Int 1998;8:215-21. https://doi.org/10.1007/s001980050057.
- Papaioannou A, Adachi JD, Winegard K, Ferko N, Parkinson W, Cook RJ, et al. Efficacy of home-based exercise for improving quality of life among elderly women with symptomatic osteoporosis-related vertebral fractures. Osteoporos Int 2003;14:677-82. https://doi.org/10.1007/s00198-003-1423-2.
- Yang L, He CQ, Lei W, Xie W, Lan Q. Effect of pain-free exercises on female osteoporosis patients with spinal compressive fractures. J Clin Rehab Tissue Eng Res 2007;11:9108-11.
- Osteoporosis Australia . Men and Osteoporosis n.d. https://osteoporosis.org.au/men (accessed 8 February 2019).
- National Osteoporosis Guideline Group . Osteoporosis Clinical Guideline for Prevention and Treatment Executive Summary 2010. https://sheffield.ac.uk/NOGG/index.html (accessed January 2013).
- Scottish Intercollegiate Guidelines Network . Management of Osteoporosis: A National Clinical Guideline 2003. http://sign.ac.uk (accessed 1 January 2013).
- National Institute for Health and Care Excellence (NICE) . Alendronate, Etidronate, Risedronate, Raloxifene, Strontium Ranelate and Teriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women 2008.
- Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR. Study of Osteoporotic Fractures . Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med 2009;150:681-7. https://doi.org/10.7326/0003-4819-150-10-200905190-00005.
- Katzman WB, Wanek L, Shepherd JA, Sellmeyer DE. Age-related hyperkyphosis: its causes, consequences, and management. J Orthop Sports Phys Ther 2010;40:352-60. https://doi.org/10.2519/jospt.2010.3099.
- Liebler EJ, Tufano-Coors L, Douris P, Makofsky HW, McKenna R, Michels C, et al. The effect of thoracic spine mobilization on lower trapezius strength testing. J Man Manip Ther 2001;9:207-12. https://doi.org/10.1179/106698101790819761.
- Sran MM, Khan KM. Physiotherapy and osteoporosis: practice behaviors and clinicians’ perceptions – a survey. Man Ther 2005;10:21-7. https://doi.org/10.1016/j.math.2004.06.003.
- Maitland GH, Hengeveld E, Banks K, English K. Maitland’s Vertebral Manipulation. Edinburgh: Elsevier Butterworth-Heinemann; 2005.
- Kamali F, Shirazi SA, Ebrahimi S, Mirshamsi M, Ghanbari A. Comparison of manual therapy and exercise therapy for postural hyperkyphosis: a randomized clinical trial. Physiother Theory Pract 2016;32:92-7. https://doi.org/10.3109/09593985.2015.1110739.
- Sran MM, Khan KM. Is spinal mobilization safe in severe secondary osteoporosis? – a case report. Man Ther 2006;11:344-51. https://doi.org/10.1016/j.math.2005.08.008.
- Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil 1984;65:593-6.
- Katzman WB, Sellmeyer DE, Stewart AL, Wanek L, Hamel KA. Changes in flexed posture, musculoskeletal impairments, and physical performance after group exercise in community-dwelling older women. Arch Phys Med Rehabil 2007;88:192-9. https://doi.org/10.1016/j.apmr.2006.10.033.
- Papa L, Mandara A, Bottali M, Gulisano V, Orfei S. A randomized control trial on the effectiveness of osteopathic manipulative treatment in reducing pain and improving the quality of life in elderly patients affected by osteoporosis. Clin Cases Miner Bone Metab 2012;9:179-83.
- Greig AM, Bennell KL, Briggs AM, Hodges PW. Postural taping decreases thoracic kyphosis but does not influence trunk muscle electromyographic activity or balance in women with osteoporosis. Man Ther 2008;13:249-57. https://doi.org/10.1016/j.math.2007.01.011.
- Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program. Mayo Clin Proc 2005;80:849-55. https://doi.org/10.4065/80.7.849.
- Papa JA. Conservative management of a lumbar compression fracture in an osteoporotic patient: a case report. J Can Chiropr Assoc 2012;56:29-3.
- Furlan AD, Brosseau L, Imamura M, Irvin E. Massage for low-back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2002;27:1896-910. https://doi.org/10.1097/00007632-200209010-00017.
- Lewis JS, Wright C, Green A. Subacromial impingement syndrome: the effect of changing posture on shoulder range of movement. J Orthop Sports Phys Ther 2005;35:72-87. https://doi.org/10.2519/jospt.2005.35.2.72.
- Cherkin DC, Sherman KJ, Kahn J, Wellman R, Cook AJ, Johnson E, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Intern Med 2011;155:1-9. https://doi.org/10.7326/0003-4819-155-1-201107050-00002.
- Lange U, Müller-Ladner U, Teichmann J. Physiotherapy in outpatients with osteoporosis. Insufficient evidence for therapy success. Z Rheumatol 2012;71:319-25. https://doi.org/10.1007/s00393-012-0964-1.
- Alp A, Cansever S, Gorgec N, Yurtkuran M, Topsac T. Effects of tai chi exercise on functional and life quality assessments in senile osteoporosis. Turk Klin Tip Bilim 2009;29:687-95.
- Hourigan SR, Nitz JC, Brauer SG, O’Neill S, Wong J, Richardson CA. Positive effects of exercise on falls and fracture risk in osteopenic women. Osteoporos Int 2008;19:1077-86. https://doi.org/10.1007/s00198-007-0541-7.
- Teixeira LE, Silva KN, Imoto AM, Teixeira TJ, Kayo AH, Montenegro-Rodrigues R, et al. Progressive load training for the quadriceps muscle associated with proprioception exercises for the prevention of falls in postmenopausal women with osteoporosis: a randomized controlled trial. Osteoporos Int 2010;21:589-96. https://doi.org/10.1007/s00198-009-1002-2.
- Chien MY, Yang RS, Tsauo JY. Home-based trunk-strengthening exercise for osteoporotic and osteopenic postmenopausal women without fracture – a pilot study. Clin Rehabil 2005;19:28-36. https://doi.org/10.1191/0269215505cr844oa.
- Alp A, Kanat E, Yurtkuran M. Efficacy of a self-management program for osteoporotic subjects. Am J Phys Med Rehabil 2007;86:633-40. https://doi.org/10.1097/PHM.0b013e31806dd428.
- Angın E, Erden Z. Effects of exercise training on pain, spinal mobility, lordosis and kyphosis angle in osteoporosis: a pilot study. Turk J Geriatr 2010;13:117-24.
- Burke TN, França FJ, Meneses SR, Pereira RM, Marques AP. Postural control in elderly women with osteoporosis: comparison of balance, strengthening and stretching exercises. A randomized controlled trial. Clin Rehabil 2012;26:1021-31. https://doi.org/10.1177/0269215512442204.
- Liu-Ambrose TY, Khan KM, Eng JJ, Gillies GL, Lord SR, McKay HA. The beneficial effects of group-based exercises on fall risk profile and physical activity persist 1 year postintervention in older women with low bone mass: follow-up after withdrawal of exercise. J Am Geriatr Soc 2005;53:1767-73. https://doi.org/10.1111/j.1532-5415.2005.53525.x.
- Smulders E, Weerdesteyn V, Groen BE, Duysens J, Eijsbouts A, Laan R, et al. Efficacy of a short multidisciplinary falls prevention program for elderly persons with osteoporosis and a fall history: a randomized controlled trial. Arch Phys Med Rehabil 2010;91:1705-11. https://doi.org/10.1016/j.apmr.2010.08.004.
- Angın E, Erden Z, Can F. The effects of clinical Pilates exercises on bone mineral density, physical performance and quality of life of women with postmenopausal osteoporosis. J Back Musculoskelet Rehabil 2015;28:849-58. https://doi.org/10.3233/BMR-150604.
- de Kam D, Smulders E, Weerdesteyn V, Smits-Engelsman BC. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: a systematic review of randomized controlled trials. Osteoporos Int 2009;20:2111-25. https://doi.org/10.1007/s00198-009-0938-6.
- Hamilton CJ, Swan VJ, Jamal SA. The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women: a systematic review of pQCT studies. Osteoporos Int 2010;21:11-23. https://doi.org/10.1007/s00198-009-0967-1.
- Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD000333.pub2.
- Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-47.
- Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 2000;67:10-8. https://doi.org/10.1007/s00223001089.
- Wen HJ, Huang TH, Li TL, Chong PN, Ang BS. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos Int 2017;28:539-47. https://doi.org/10.1007/s00198-016-3759-4.
- Kemmler W, Häberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int 2013;24:1937-50. https://doi.org/10.1007/s00198-012-2248-7.
- Sinaki M, Itoi E, Wahner HW, Wollan P, Gelzcer R, Mullan BP, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone 2002;30:836-41. https://doi.org/10.1016/S8756-3282(02)00739-1.
- Liu-Ambrose TY, Khan KM, Eng JJ, Lord SR, Lentle B, McKay HA. Both resistance and agility training reduce back pain and improve health-related quality of life in older women with low bone mass. Osteoporos Int 2005;16:1321-9. https://doi.org/10.1007/s00198-005-1842-3.
- Karinkanta S, Heinonen A, Sievänen H, Uusi-Rasi K, Pasanen M, Ojala K, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 2007;18:453-62. https://doi.org/10.1007/s00198-006-0256-1.
- Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int 2005;16:1004-10. https://doi.org/10.1007/s00198-004-1791-2.
- Li WC, Chen YC, Yang RS, Tsauo JY. Effects of exercise programmes on quality of life in osteoporotic and osteopenic postmenopausal women: a systematic review and meta-analysis. Clin Rehabil 2009;23:888-96. https://doi.org/10.1177/0269215509339002.
- Burke TN, França FJ, Ferreira de Meneses SR, Cardoso VI, Marques AP. Postural control in elderly persons with osteoporosis: efficacy of an intervention program to improve balance and muscle strength: a randomized controlled trial. Am J Phys Med Rehabil 2010;89:549-56. https://doi.org/10.1097/PHM.0b013e3181ddccd2.
- Winters-Stone KM, Snow CM. Site-specific response of bone to exercise in premenopausal women. Bone 2006;39:1203-9. https://doi.org/10.1016/j.bone.2006.06.005.
- Iwamoto J, Sato Y, Takeda T, Matsumoto H. Effectiveness of exercise in the treatment of lumbar spinal stenosis, knee osteoarthritis, and osteoporosis. Aging Clin Exp Res 2010;22:116-22. https://doi.org/10.1007/BF03324783.
- Dusdal K, Grundmanis J, Luttin K, Ritchie P, Rompre C, Sidhu R, et al. Effects of therapeutic exercise for persons with osteoporotic vertebral fractures: a systematic review. Osteoporos Int 2011;22:755-69. https://doi.org/10.1007/s00198-010-1497-6.
- Petit MA, Hughes JM, Warpeha JM. Manual for Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
- Kerschan-Shindl K, Uher E, Kainberger F, Kaider A, Ghanem AH, Preisinger E. Long-term home exercise program: effect in women at high risk of fracture. Arch Phys Med Rehabil 2000;81:319-23. https://doi.org/10.1016/S0003-9993(00)90078-9.
- Shannon R, Hillsdon M. Motivational interviewing in musculoskeletal care. Musculoskeletal Care 2007;5:206-15. https://doi.org/10.1002/msc.119.
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305-12.
- Atienza AA. Home-based physical activity programs for middle-aged and older adults: summary of empirical research. J Aging Phys Act 2001;9:S38-58. https://doi.org/10.1123/japa.9.s1.s38.
- Heine PJ, Williams MA, Williamson E, Bridle C, Adams J, O’Brien A, et al. Development and delivery of an exercise intervention for rheumatoid arthritis: strengthening and stretching for rheumatoid arthritis of the hand (SARAH) trial. Physiotherapy 2012;98:121-30. https://doi.org/10.1016/j.physio.2011.03.001.
- Iwamoto J, Takeda T, Ichimura S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci 2001;6:128-32. https://doi.org/10.1007/s0077610060128.
- Barker KL, Toye F, Lowe CJ. A qualitative systematic review of patients’ experience of osteoporosis using meta-ethnography. Arch Osteoporos 2016;11. https://doi.org/10.1007/s11657-016-0286-z.
- Smith JA, Osborn M, Smith JA. Qualitative Psychology: A Practical Guide to Methods. London: Sage Publishing; 2008.
- Rubin HJ, Rubin IS. Qualitative Interviewing. The Art of Hearing Data. Thousand Oaks, CA: Sage Publications; 1995.
- Morse J, Noerager Stern P, Corbin J, Bowers B. Developing Grounded Theory: The Second Generation. Walnut Creek, CA: Left Coast Press; 2009.
- Krefting L. Rigor in qualitative research: the assessment of trustworthiness. Am J Occup Ther 1991;45:214-22. https://doi.org/10.5014/ajot.45.3.214.
- O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-215.
- Toye F, Williamson E, Williams MA, Fairbank J, Lamb SE. What value can qualitative research add to quantitative research design? An example from an adolescent idiopathic scoliosis trial feasibility study. Qual Health Res 2016;26:1838-50. https://doi.org/10.1177/1049732316662446.
- Baker SE, Edwards R. National Centre for Research Methods Review Paper. How Many Qualitative Interviews Is Enough? Expert Voices and Early Career Reflections on Sampling 2018. http://eprints.ncrm.ac.uk/2273/ (accessed 8 December 2017).
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- Fritzell P, Ohlin A, Borgström F. Cost-effectiveness of balloon kyphoplasty versus standard medical treatment in patients with osteoporotic vertebral compression fracture: a Swedish multicenter randomized controlled trial with 2-year follow-up. Spine 2011;36:2243-51. https://doi.org/10.1097/BRS.0b013e3182322d0f.
- Ström O, Leonard C, Marsh D, Cooper C. Cost-effectiveness of balloon kyphoplasty in patients with symptomatic vertebral compression fractures in a UK setting. Osteoporos Int 2010;21:1599-608. https://doi.org/10.1007/s00198-009-1096-6.
- Svedbom A, Alvares L, Cooper C, Marsh D, Ström O. Balloon kyphoplasty compared to vertebroplasty and non-surgical management in patients hospitalised with acute osteoporotic vertebral compression fracture – a UK cost-effectiveness analysis. Osteoporosis Int 2013;24:355-67. https://doi.org/10.1007/s00198-012-2102-y.
- Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (VERTOS II): an open-label randomised trial. Lancet 2010;376:1085-92. https://doi.org/10.1016/S0140-6736(10)60954-3.
- Masala S, Ciarrapico AM, Konda D, Vinicola V, Mammucari M, Simonetti G. Cost-effectiveness of percutaneous vertebroplasty in osteoporotic vertebral fractures. Eur Spine J 2008;17:1242-50. https://doi.org/10.1007/s00586-008-0708-8.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal [PMG9] 2013. https://nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 21 February 2019).
- National Institute for Health and Care Excellence (NICE) . The Guidelines Manual Process And Methods [PMG6] 2012. https://nice.org.uk/process/pmg6/chapter/introduction.
- National Institute for Health and Care Excellence . National Institute for Health and Care Excellence Position Statement on Use of the EQ-5D-5L Valuation Set 2017 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 23 July 2018).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Department of Health and Social Care . Department of Health National Schedule of Reference Costs 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 23 July 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- NHS Digital . Prescription Cost Analysis, England 2016 2017. https://digital.nhs.uk/catalogue/PUB23631 (accessed 23 July 2018).
- NHS . NHS Choices 2018 2018. www.nhs.uk/pages/home.aspx (accessed 23 July 2018).
- Office for National Statistics . Occupation Coding Tool n.d. https://onsdigital.github.io/dp-classification-tools/standard-occupational-classification/ONS_SOC_occupation_coding_tool.html (accessed 23 July 2018).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2017 Provisional and 2016 Revised Results 2017 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2017provisionaland2016revisedresults (accessed 23 July 2018).
- Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ 2000;9:623-30. https://doi.org/10.1002/1099-1050(200010)9:7<623::AID-HEC539>3.0.CO;2-V.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- National Institute for Health and Care Excellence (NICE) . Osteoporosis: Assessing the Risk of Fragility Fracture (CG146) 2012. www.nice.org.uk/guidance/cg146 (accessed 21 February 2019).
- National Osteoporosis Guideline Group (NOGG) . Clinical Guideline for the Prevention and Treatment of Osteoporosis 2017. www.sheffield.ac.uk/NOGG/NOGG%20Guideline%202017.pdf (accessed 4 March 2019).
- Chow SC, Chang M. Adaptive design methods in clinical trials – a review. Orphanet J Rare Dis 2008;3. https://doi.org/10.1186/1750-1172-3-11.
- Thorlund K, Haggstrom J, Park JJ, Mills EJ. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 2018;360. https://doi.org/10.1136/bmj.k698.
- Food and Drug Administration . Adaptive Designs for Clinical Trials of Drugs and Biologics. Draft Guidance for Industry 2018.
- Pallmann P, Bedding AW, Choodari-Oskooei B, Dimairo M, Flight L, Hampson LV. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1017-7.
- Minns Lowe CJ, Wilson MS, Sackley CM, Barker KL. Blind outcome assessment: the development and use of procedures to maintain and describe blinding in a pragmatic physiotherapy rehabilitation trial. Clin Rehabil 2011;25:264-74. https://doi.org/10.1177/0269215510380824.
- Boutron I, Estellat C, Ravaud P. A review of blinding in randomized controlled trials found results inconsistent and questionable. J Clin Epidemiol 2005;58:1220-6. https://doi.org/10.1016/j.jclinepi.2005.04.006.
- Melton LJ. Epidemiology of spinal osteoporosis. Spine 1997;22:2-11. https://doi.org/10.1097/00007632-199712151-00002.
- World Health Organization . Assessment of Osteoporosis at the Primary Health Care Level 2007.
- O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 1996;11:1010-18. https://doi.org/10.1002/jbmr.5650110719.
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review?. Med Care 1996;34:73-84. https://doi.org/10.1097/00005650-199601000-00006.
- Bell SP, Saraf AA. Epidemiology of multimorbidity in older adults with cardiovascular disease. Clin Geriatr Med 2016;32:215-26. https://doi.org/10.1016/j.cger.2016.01.013.
- Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporos Int 2005;16:447-55. https://doi.org/10.1007/s00198-004-1762-7.
- Carter ND, Khan KM, McKay HA, Petit MA, Waterman C, Heinonen A, et al. Community-based exercise program reduces risk factors for falls in 65- to 75-year-old women with osteoporosis: randomized controlled trial. CMAJ 2002;167:997-1004.
- Badia X, Díez-Pérez A, Alvarez-Sanz C, Díaz-López B, Diaz-Curiel M, Guillén F, et al. Spanish GRECO Study Group . Measuring quality of life in women with vertebral fractures owing to osteoporosis: a comparison of the OQLQ and QUALEFFO. Qual Life Res 2001;10:307-17. https://doi.org/10.1023/A:1012200508847.
- Evstigneeva L, Lesnyak O, Bultink IE, Lems WF, Kozhemyakina E, Negodaeva E, et al. Effect of twelve-month physical exercise program on patients with osteoporotic vertebral fractures: a randomized, controlled trial. Osteoporos Int 2016;27:2515-24. https://doi.org/10.1007/s00198-016-3560-4.
- Katzman WB, Vittinghoff E, Lin F, Schafer A, Long RK, Wong S, et al. Targeted spine strengthening exercise and posture training program to reduce hyperkyphosis in older adults: results from the study of hyperkyphosis, exercise, and function (SHEAF) randomized controlled trial. Osteoporos Int 2017;28:2831-41. https://doi.org/10.1007/s00198-017-4109-x.
- Mannion AF, Käser L, Weber E, Rhyner A, Dvorak J, Müntener M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J 2000;9:273-81. https://doi.org/10.1007/s005860000189.
- Aruin AS, Latash ML. Directional specificity of postural muscles in feedforward postural reactions during fast voluntary arm movements. Exp Brain Res 1995;103:323-32. https://doi.org/10.1007/BF00231718.
- Takahashi K, Yamaji T, Wada N, Shirakura K, Watanabe H. Trunk kinematics and muscle activities during arm elevation. J Orthop Sci 2015;20:624-32. https://doi.org/10.1007/s00776-015-0724-6.
- Petersen S, Domino N, Postma C, Wells C, Cook C. Scapulothoracic muscle strength changes following a single session of manual therapy and an exercise programme in subjects with neck pain. Musculoskeletal Care 2016;14:195-20. https://doi.org/10.1002/msc.1132.
- da Silva RA, Vieira ER, Cabrera M, Altimari LR, Aguiar AF, Nowotny AH, et al. Back muscle fatigue of younger and older adults with and without chronic low back pain using two protocols: a case-control study. J Electromyogr Kinesiol 2015;25:928-36. https://doi.org/10.1016/j.jelekin.2015.10.003.
- Kasukawa Y, Miyakoshi N, Hongo M, Ishikawa Y, Noguchi H, Kamo K, et al. Relationships between falls, spinal curvature, spinal mobility and back extensor strength in elderly people. J Bone Miner Metab 2010;28:82-7. https://doi.org/10.1007/s00774-009-0107-1.
- Idland G, Rydwik E, Småstuen MC, Bergland A. Predictors of mobility in community-dwelling women aged 85 and older. Disabil Rehabil 2013;35:881-7. https://doi.org/10.3109/09638288.2012.712195.
- Langley FA, Mackintosh S. Functional balance assessment of older community dwelling adults: a systematic review of the literature. Internet J Allied Health Sci Pract 2007;5.
- Weiner DK, Duncan PW, Chandler J, Studenski SA. Functional reach: a marker of physical frailty. J Am Geriatr Soc 1992;40:203-7. https://doi.org/10.1111/j.1532-5415.1992.tb02068.x.
- Bohannon RW, Wolfson LI, White WB. Functional reach of older adults: normative reference values based on new and published data. Physiotherapy 2017;103:387-91. https://doi.org/10.1016/j.physio.2017.03.006.
- Thomas JI, Lane JV. A pilot study to explore the predictive validity of 4 measures of falls risk in frail elderly patients. Arch Phys Med Rehabil 2005;86:1636-40. https://doi.org/10.1016/j.apmr.2005.03.004.
- Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89-96. https://doi.org/10.1093/gerona/glq167.
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743-9. https://doi.org/10.1111/j.1532-5415.2006.00701.x.
- Enright PL. The six-minute walk test. Respir Care 2003;48:783-5.
- Alison JA, Kenny P, King MT, McKinley S, Aitken LM, Leslie GD, et al. Repeatability of the six-minute walk test and relation to physical function in survivors of a critical illness. Phys Ther 2012;92:1556-63. https://doi.org/10.2522/ptj.20110410.
- Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J 2011;37:150-6. https://doi.org/10.1183/09031936.00194909.
- Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc 2004;52:1662-7. https://doi.org/10.1111/j.1532-5415.2004.52458.x.
- Newman M, Minns Lowe C, Barker K. Spinal orthoses for vertebral osteoporosis and osteoporotic vertebral fracture: a systematic review. Arch Phys Med Rehabil 2016;97:1013-25. https://doi.org/10.1016/j.apmr.2015.10.108.
- Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283-91. https://doi.org/10.1016/j.ejpain.2003.09.004.
- Karakasidou P, Skordilis EK, Dontas I, Lyritis GP. Postural profile and falls of osteoporotic women. J Back Musculoskelet Rehabil 2012;25:55-66. https://doi.org/10.3233/BMR-2012-0310.
- Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O. European Vertebral Osteoporosis Study . Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003;14:61-8. https://doi.org/10.1007/s00198-002-1316-9.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721-39. https://doi.org/10.1359/jbmr.2000.15.4.721.
- Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320-3. https://doi.org/10.1001/jama.285.3.320.
- Pope PM. Severe and Complex Neurological Disability: Management of the Physical Condition. Oxford: Butterworth-Heinneman Elsevier; 2007.
- Bojke C, Castelli A, Grasic K, Howdon D, Street AD. Productivity of the English NHS: 2013 14 Update. Discussion Paper. CHE Research Paper 2016 2016. http://eprints.whiterose.ac.uk/135815/ (accessed 13 February 2019).
- Co-operative Mobility . Co-Operative Mobility n.d. www.co-opmobility.co.uk/ (accessed 23 July 2018).
- Cavendish Chairs & Mobility n.d. https://cavendish-furniture-mobility.co.uk/ (accessed 13 February 2019).
- CareCo . CareCo 2018. www.careco.co.uk/ (accessed 23 July 2018).
- healthandcare.co.uk . Healthandcare.co.Uk 2018. www.healthandcare.co.uk/ (accessed 23 July 2018).
- AposTherapy . AposTherapy Pricing Guide n.d. www.apostherapy.co.uk/ (accessed 13 February 2019).
- In The Wash . How Much Do Cleaners Charge? 2017. www.inthewash.co.uk (accessed 23 July 2018).
Appendix 2 Manual therapy
Appendix 3 Manual therapy assessment form
Appendix 4 Manual therapy treatment log
Appendix 5 Exercise therapy diary
The National Osteoporosis Society (former name of the ROS) and University of Oxford logos have been reproduced with permission.
Appendix 6 Exercise therapy assessment form
Appendix 7 Exercise therapy treatment log
Appendix 8 Therapist advice and education notes
Appendix 9 Participant information laminates
Appendix 10 Royal Osteoporosis Society Healthy Living for Strong Bones leaflet
This leaflet has been reproduced with permission from the ROS (formerly known as the National Osteoporosis Society).
Appendix 12 The PROVE trial monitoring checklist
Appendix 13 Patient information sheet
The University of Oxford logo has been reproduced with permission.
Appendix 14 Consent form
The University of Oxford logo has been reproduced with permission.
Appendix 15 The PROVE trial case report forms
The Qualeffo-41 has been reproduced with permission from the International Osteoporosis Foundation.
The EQ-5D-5L has been reproduced with permission from the EuroQol Group.
The PASE has been reproduced with permission from New England Research Institutes, Inc. A fair-use licensing agreement has been obtained for permission to administer and reproduce the PASE.
Appendix 16 Participant diaries
The University of Oxford logo has been reproduced with permission.
Appendix 17 Qualitative study patient information sheet
The National Osteoporosis Society (former name of the ROS) and the University of Oxford Logos have been reproduced with permission.
Appendix 18 Qualitative study consent form
The University of Oxford logo has been reproduced with permission.
Appendix 19 Trial Steering Committee charter
Appendix 20 Data Monitoring Committee charter
Appendix 21 Serious adverse event details
Safety and adverse events
This section details the reported adverse events. Table 34 presents the adverse events experienced by the participants involved in the trial, the actions taken for safety and the outcomes.
| SAE number | Group | Assessment date | AE | Severity | Action taken | Withdrawn? | AE group | Comment | Related or unrelated to treatment | Expected or unexpected |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Exercise therapy | 1 March 2014 | c2 Peg fracture | Severe | No physical assessment completed for 12-month assessment | No | Fracture |
Occurrence unrelated to exercise intervention Severe |
Unrelated | Unexpected |
| 2 | Manual therapy | 11 April 2015 | Fractured neck of femur | Severe | None | No | Fall and fracture |
Unrelated to manual therapy Severe |
Unrelated | Unexpected |
| 3 | Manual therapy | 21 June 2015 | Fractured and dislocated ankle – not on site | Severe | 12-month assessment not done. Questionnaires sent home | No | Fracture |
Unrelated to manual therapy Severe |
Unrelated | Unexpected |
| 4 | Exercise therapy | 10 November 2015 | Right total knee replacement | Severe | None | No | Arthroplasty | Moderate | Unrelated | Unexpected |
| 5 | Manual therapy | 8 January 2015 | Chesty cough | Mild | None yet – intends to see GP | No | Respiratory | Unrelated | Unexpected | |
| 6 | Manual therapy | 1 April 2015 | Lung fibrosis (breathless) | Mild | CT scan, chest X-ray, medication | No | Respiratory | Moderate | Unrelated | Unexpected |
| 7 | SSPT | 21 May 2015 | Contusion to leg with infection | Mild | Area dressed every 3 days until 13 June 2015; oral antibiotics prescribed | No | Cutaneous | Moderate | Unrelated | Unexpected |
| 8 | Manual therapy | 16 February 2015 | Mild whiplash – RTA | Mild | Sought medical attention | No | MSK other | Unrelated | Unexpected | |
| 9 | Manual therapy | 1 March 2015 | Cracked left rib | Mild | Rest and analgesics (paracetamol and ibuprofen) | No | Fracture |
Unrelated to manual therapy intervention Moderate |
Unrelated | Unexpected |
| 10 | SSPT | 1 April 2015 | Exacerbation of asthma, breathless, chesty due to a chest infection | Mild | Oral antibiotics for 7 days. Oral corticosteroid for 6 days | No | Respiratory | Moderate | Unrelated | Unexpected |
| 11 | Manual therapy | 25 October 2015 | Fall – anterior talofibular ligament sprained right ankle | Mild | Rest and ice, elevation, OTC analgesia – knee injury advice | No | Fall, MSK other | Unrelated to manual therapy | Unrelated | Unexpected |
| 12 | SSPT | 25 October 2014 | Reaction to osteoporosis infusion | Moderate | Hospital stay | No | MSK other | Severe | Unrelated | Unexpected |
| 13 | SSPT | 5 February 2015 | Admitted acute kidney injury | Moderate | Hospital stay | No | Renal | Severe | Unrelated | Unexpected |
| 14 | Exercise therapy | 21 September 2015 | Tachycardia | Mild | Admitted to medical ward for 12-hour monitoring | No | Cardiovascular |
Unrelated to exercise intervention Severe |
Unrelated | Unexpected |
| 15 | Manual therapy | 5 April 2015 | Fall – causing back pain | Moderate | None | No | Fall, no fracture | Unrelated to manual therapy intervention | Unrelated | Expected |
| 16 | Exercise therapy | 1 April 2016 | Fell getting up from sofa | Mild | Got self up from floor, no injuries | No | Fall | Unrelated | Unexpected | |
| 17 | Exercise therapy | 29 December 2015 | Third metacarpal base undisplaced left-hand fracture with second metacarpal base fracture | Moderate | Futuro splint and mobilise as planned | No | Fracture non-major | Unrelated to exercise intervention | Unrelated | Expected |
| 18 | SSPT | 25 February 2015 | Vitamin D insufficiency | Mild | 8-week high dose of vitamin D supplement | No | Abnormal laboratory value | Mild | Unrelated | Unexpected |
| 19 | Manual therapy | 26 November 2015 | Urinary tract infection | Severe | Hospital admission 3 days, intravenous antibiotics for 3 days, oral antibiotics for 5 days | No | GU | Moderate | Unrelated | Unexpected |
| 20 | Manual therapy | 7 December 2015 | Urinary tract infection | Mild | Oral antibiotics for 1 week | No | GU | Moderate | Unrelated | Unexpected |
| 21 | SSPT | 17 February 2015 | Fracture T8 related to osteoporosis | Moderate | Given codeine supplements for 8-week period | No | Fractures | Unrelated | Expected | |
| 22 | Manual therapy | 27 May 2015 | L4/L5 osteoporotic fracture | Moderate | Vertebroplasty done on 27 May 2015 | No | Fracture | Unrelated to manual therapy | Unrelated | Expected |
| 23 | Exercise therapy | 9 February 2016 | Nose bleed | Mild | Bilateral Rapid Rhinos® (Smith & Nephew, Inc., Austin, TX, USA) inserted | No | ENT | Unrelated | Unexpected | |
| 24 | Manual therapy | 17 January 2016 | Caught toe on bean bag and fell onto arm of sofa breaking left ribs | Moderate | Saw GP on same day for medical advice. No X-ray and was told it would take 6 weeks to settle | No | Fracture | Unrelated to exercise intervention | Unrelated | Expected |
| 25 | SSPT | 7 July 2015 | Fell at home and fractured T11 | Moderate | Assessment delayed | No | Fall and fracture | Moderate | Unrelated | Expected |
| 26 | SSPT | 14 July 2015 | Fractured distal radius right wrist | Severe | None | No | Fracture | Moderate | Unrelated | Expected |
| 27 | Manual therapy | 1 November 2015 | Back pain and possible compression fracture | Moderate | Visited the GP. Pain medication prescribed. Waiting for scan | No | MSK other | Unrelated to manual therapy | Unrelated | Expected |
| 28 | Exercise therapy | 1 March 2016 | Acute kidney pain | Severe | Awaiting visit with specialist | No | GU | Moderate | Unrelated | Unexpected |
| 29 | Manual therapy | 1 May 2015 | Worsening of migraine (pre-existing condition) | Mild | HRT started in April 2015, stopped in May 2015. After withdrawal of HRT, started on risedronate. Stopped after one dose because of side effects | No | Neurological | Unrelated | Unexpected | |
| 30 | Manual therapy | 1 July 2016 | Carpal tunnel syndrome | Moderate | Referred for nerve induction study. Seen by orthopaedic surgeon on 28 October 2016 | No | MSK other | Unrelated | Unexpected | |
| 31 | SSPT | 27 September 2015 | Fractured pelvis following a syncope | Moderate | None | No | Fall and fracture | Unrelated | Expected | |
| 32 | Manual therapy | 31 July 2015 | Transient ischaemic attack at home | Mild | Patient is undergoing further heart investigations from 4 August 2015 | No | Cardiac | Moderate | Unrelated | Unexpected |
| 33 | SSPT | 7 February 2016 | Constipation due to pain relief (codeine) | Mild | Enema in A&E | No | GI | Moderate | Unrelated | Unexpected |
| 34 | Manual therapy | 1 September 2015 | New back pain across shoulder blade | Mild | Seen by GP × 2, commenced on NSAIDs | No | MSK other | Moderate | Unrelated | Expected |
| 35 | Manual therapy | 10 May 2016 | Back pain | Mild | Treatment continues with physiotherapy | No | MSK other | Unrelated | Expected | |
| 36 | Exercise therapy | 5 July 2015 | Severe wedge fracture of T12 vertebral body | Moderate | Rest. Seen by surgeon – no further action, discharged from outpatients clinic | No | Fracture |
Unrelated to exercise intervention Moderate |
Unrelated | Expected |
| 37 | SSPT | 27 July 2015 | Painful shoulders | Mild | Saw GP, tested for polymyalgia – tests normal | No | MSK other | Mild | Unrelated | Expected |
| 38 | SSPT | 1 August 2015 | New OVF (T9) | Moderate | Commenced on opioid transdermal patch. Awaiting rheumatologist review | No | Fracture | Unrelated | Expected | |
| 39 | Exercise therapy | 1 November 2015 | Urine infection | Mild | Oral antibiotics – 1 week | No | GU | Moderate | Unrelated | Unexpected |
| 40 | Exercise therapy | 11 June 2016 | Urosepsis | Admitted to hospital. Blood transfusion. Intravenous antibiotics. Discharged on 15 June 2016 | Yes, happy for existing data to be used | GU | Severe | Unrelated | Unexpected | |
| 41 | SSPT | 21 June 2015 | Fractured collarbone – not on site | Severe | Delayed appointments | No | Fracture | Moderate | Unrelated | Expected |
| 42 | Manual therapy | 12 August 2015 | Cerebrovascular accident | Severe | Event occurred at home. Patient was admitted to the hospital | No | CVS | Unrelated | Unexpected | |
| 43 | Exercise therapy | 5 November 2015 | Fall, damage to left knee | Moderate | Investigated by orthopaedics – awaiting MRI scan | No | Fall | Unrelated to exercise intervention | Unrelated | Expected |
| 44 | Manual therapy | 14 August 2015 | Dizziness after treatment | Mild | None. It was resolved with rest | No | Dizzy | Related to treatment | Related | Unexpected |
| 45 | Exercise therapy | 13 September 2015 | Fracture left distal radius (fall out of a taxi) | Moderate | Cast – 6 weeks | No | Fall and fracture | Unrelated | Expected | |
| 46 | Exercise therapy | 15 May 2016 | Fractured right ankle | Moderate | No | Fall and fracture | Unrelated to exercise intervention | Unrelated | Expected | |
| 47 | Manual therapy | 23 September 2015 | Osteoporosis-related flare-up of backpain | Mild | Given advice on telephone on 30 September to manage flare-ups of pain | Yes, happy for existing data to be used | Back pain | Unrelated to manual therapy | Unrelated | Expected |
| 48 | SSPT | 6 October 2015 | Urinary tract infection | Mild | Oral antibiotics – 200 mg of trimethoprim for 5 days | No | GU | Moderate | Unrelated | Unexpected |
| 49 | Exercise therapy | 2 December 2016 | Fractured left patella | Moderate | Completed adverse event form | No | Fracture | Unrelated | Expected | |
| 50 | Manual therapy | 5 October 2015 | Skin laceration – right shin | Mild | Oral antibiotics – commenced on 30 October 2015 (25 mg of oxytetracycline, one a day). Dressing renewed every other day | No | Cutaneous | Unrelated | Unexpected | |
| 51 | SSPT | 4 November 2015 | Impetigo | Moderate | Oral plus topical antibiotics commenced | No | Cutaneous | Unrelated | Unexpected | |
| 52 | Manual therapy | 9 January 2016 | Increase in back pain T6–T12 | Severe | Advised to see GP regarding pain. Analgesia discussed to increase paracetamol. Requested another outpatient appointment to see consultant | No | Back pain | Unrelated | Expected | |
| 53 | Manual therapy | 21 January 2016 | Exacerbation of diverticulitis | Moderate | Had colonoscopy | No | GI | Unrelated | Unexpected | |
| 54 | Exercise therapy | 8 December 2015 | Bronchitis | Mild | Oral steroids commenced, 3-day course. Oral antibiotics commenced 30 October 2015. Dressing renewed every other day | No | Respiratory | Unrelated | Unexpected | |
| 55 | Exercise therapy | 6 May 2016 | Dull ache in chest over sternum (no pain in jaw or arms, no pins and needles) | Mild | None by patient. Patient advised at PROVE visit to make an appointment with GP as soon as possible. Blood pressure and pulse checked. Blood pressure = 148/84 mmHg, pulse 59 b.p.m. | No | CVS | Unrelated | Unexpected | |
| 56 | Exercise therapy | 11 May 2016 | Attended general practice and the hospital A&E with chest pains and shortness of breath | Moderate | Electrocardiogram at general practice was NAD. Patient left A&E prior to being re-evaluated | No | CVS | Unrelated | Unexpected | |
| 57 | Exercise therapy | 1 July 2016 | Cyst on thyroid gland | Moderate | Seen by GP, referred to fast-track ENT appointment with consultant. Lump in throat. Review in 3 months’ time in hospital | No | ENT | Unrelated | Unexpected | |
| 58 | Manual therapy | 18 March 2016 | Fractured hip at home after a fall | Moderate | No further exercises done | Yes, happy for existing data to be used | Fall and fracture | Unrelated | Expected | |
| 59 | Exercise therapy | 16 November 2015 | Right fifth toe ulcer/osteomyelitis | Moderate | Oral antibiotics – 12 weeks. Seen by podiatrist | No | Cutaneous | Unrelated | Unexpected | |
| 60 | Exercise therapy | 8 January 2016 | Glioma | Severe | Patient withdrew under palliative care | No | Tumours | Unrelated | Unexpected | |
| 61 | Manual therapy | 18 January 2016 | Infective exacerbation of chronic obstructive pulmonary disease – admitted to hospital | Discharged on 1 February 2016 | Yes, data destroyed | Respiratory | Severe | Unrelated | Unexpected | |
| 62 | Manual therapy | 1 February 2016 | Chest infection | Mild | GP prescribed 3-week course of antibiotics | No | Respiratory | Moderate | Unrelated | Unexpected |
| 63 | Exercise therapy | 1 March 2016 | Slid down steps and strained right wrist | Mild | Nil medical attention required. All symptoms resolved | No | Fall | Unrelated to exercise intervention | Unrelated | Unexpected |
| 64 | SSPT | 7 March 2016 | Increase in backache (lower thoracic) after gardening, lasted for 3 weeks until 28 March 2016 | Moderate | Took extra painkillers, rested more and used hot water bottle on the affected area – did not consult GP | No | Back pain | Unrelated | Unexpected | |
| 65 | SSPT | 8 December 2016 | Fractured right triquetral following a fall | Moderate | Informed Varsha Gandhi at University of Oxford | No | Fall and fracture | Unrelated | Unexpected | |
| 66 | Manual therapy | 27 December 2016 | Chest infection | Moderate | Oral antibiotics for 7 days | No | Respiratory | Moderate | Unrelated | Unexpected |
| 67 | Manual therapy | 28 April 2016 | Flare of trigeminal nerve – not related to osteoporosis | Mild | None | No | Neurological | Unrelated | Unexpected | |
| 68 | SSPT | 27 March 2016 | Cellulitis both legs | Moderate | Contacted GP, had two lots of antibiotics | No | Cutaneous | Unrelated | Unexpected | |
| 69 | SSPT | 4 April 2016 | Urinary tract infection and thrush | Mild | GP prescribed more antibiotics | No | GU | Moderate | Unrelated | Unexpected |
| 70 | SSPT | 20 October 2016 | New spinal fracture | Severe | Attended A&E, X-ray and MRI – surgery offered; date arranged | No | Fracture | Moderate | Unrelated | Expected |
| 71 | SSPT | 1 April 2017 | Patient thought she had pulled a muscle in January 2017 – worsened on 1 April 2017 | Severe | Consulted spinal doctor, who suspected a further OVF, ordered a MRI on 30 April 2017 | No | Moderate | Unrelated | Expected | |
| 72 | Manual therapy | 3 March 2016 | Extreme back pain due to exercises | Severe | Discontinued exercises | No | Back pain | Moderate withdrawal | Related | Expected |
| 73 | Manual therapy | 17 June 2016 | Painful wrist | Mild | Rest | No | MSK other | Unrelated to manual therapy | Unrelated | Unexpected |
| 74 | Manual therapy | 21 December 2016 | Diagnosed with breast cancer | Severe | Patient happy to complete questionnaires but unable to cope with physical assessments | No | Cancer | Severe | Unrelated | Unexpected |
| 75 | Manual therapy | 27 June 2016 | Gallstones | Moderate | Waiting for operation to remove | No | GI | Moderate | Unrelated | Unexpected |
| 76 | Manual therapy | 24 December 2016 | Fall | Mild | None | No | Fall | Moderate | Unrelated | Unexpected |
| 77 | Manual therapy | 21 December 2016 | Left total hip replacement following a fall | Severe | Hip replaced | No | Fall and fracture | Unrelated to manual therapy | Unrelated | Expected |
| 78 | Exercise therapy | 22 June 2016 | Right groin pain | Severe | Pain management clinic and injection to hip, possibly physiotherapy | No | MSK other |
Unrelated to exercise intervention Moderate |
Unrelated | Unexpected |
| 79 | SSPT | 21 September 2016 | Fell in garden, jarred back and hurt right wrist | Mild | Right wrist is still painful when doing repetitive movements but improving | No | Fall | Unrelated | Unexpected | |
| 80 | Manual therapy | 1 November 2016 | Fractured left femur. Spontaneous fracture occurred at home when walking between rooms | Severe | Was admitted to the hospital. Medical investigation determined diagnosis of multiple myeloma. Surgical intervention done | Yes, happy for existing data to be used | Fracture and cancer | Unrelated | Unexpected | |
| 81 | Exercise therapy | 10 November 2016 | Hiatus hernia | Moderate | Under investigation for a hernia causing a lot of pain. Only questionnaires and diary collected at 16-week assessment | No | GI | Unrelated | Unexpected | |
| 82 | Manual therapy | 3 November 2016 | Increased back pain after all the treatment sessions were completed at L5–S1 | Mild | Participant informed the physiotherapist by e-mail. | No | Back pain | Unrelated to manual therapy | Unrelated | Expected |
| 83 | Exercise therapy | 5 June 2017 | Pulmonary hypertension | Severe | GP – hospital admission, 1 week | No | Cardiovascular | Moderate | Unrelated | Unexpected |
| 84 | Exercise therapy | 4 August 2017 | Chest infection | Moderate | GP visit | No | Respiratory | Moderate | Unrelated | Unexpected |
| 85 | Exercise therapy | 20 September 2016 | Facet joint inflammation and leg symptoms | Mild | Rest – no therapy | Yes, happy for existing data to be used | Back pain | Unrelated | Unexpected |
Appendix 22 Chapter 4 supplementary data
| Baseline data | Intervention arm | ||
|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | |
| Female patients, n (%) | 52 (85.2) (N = 61) | 64 (87.7) (N = 73) | 61 (91.0) (N = 67) |
| Age (years), mean (SD) | 71.6 (8.7) (N = 61) | 72.2 (9.0) (N = 73) | 71.9 (9.1) (N = 67) |
| Height (cm), mean (SD) | 156.3 (21.9) (N = 61) | 159.4 (8.4) (N = 72) | 152.3 (27.7) (N = 67) |
| Weight (kg), mean (SD) | 65.2 (17.2) (N = 61) | 62.4 (15.2) (N = 73) | 62.0 (11.3) (N = 67) |
| DEXA T-score, mean (SD) | –2.5 (1.6) (N = 46) | –2.5 (1.6) (N = 57) | –2.7 (1.4) (N = 48) |
| Spinal fractures, mean (SD) | 2.9 (2.0) (N = 56) | 2.6 (2.3) (N = 68) | 2.4 (2.4) (N = 56) |
| Non-spinal fractures, mean (SD) | 0.1 (0.4) (N = 54) | 0.2 (0.5) (N = 70) | 0.1 (0.4) (N = 62) |
| Fractures, n (%) | |||
| Upper lumbar (L1 and L2) | 21 (39.6) (N = 53) | 26 (40.0) (N = 65) | 21 (42.0) (N = 50) |
| Lower lumbar (L3–L5) | 21 (38.2) (N = 55) | 23 (35.9) (N = 64) | 22 (44.0) (N = 50) |
| Lower thoracic (T6–T12) | 47 (82.5) (N = 57) | 49 (74.2) (N = 66) | 48 (81.4) (N = 59) |
| Upper thoracic (T1–T5) | 8 (15.1) (N = 53) | 6 (10.2) (N = 59) | 9 (18.4) (N = 49) |
| Walking category,a n (%) | |||
| 1 | 27 (44.3) (N = 61) | 32 (44.4) (N = 72) | 25 (38.5) (N = 65) |
| 2 | 13 (21.3) (N = 61) | 14 (19.4) (N = 72) | 15 (23.1) (N = 65) |
| 3 | 15 (24.6) (N = 61) | 22 (30.6) (N = 72) | 18 (27.7) (N = 65) |
| 4 | 6 (9.8) (N = 61) | 4 (5.6) (N = 72) | 6 (9.2) (N = 65) |
| 5 | 0 (0.0) (N = 61) | 0 (0.0) (N = 72) | 1 (1.5) (N = 65) |
| 6 | 0 (0.0) (N = 61) | 0 (0.0) (N = 72) | 0 (0.0) (N = 65) |
| Stair use category,b n (%) | |||
| 1 | 20 (33.9) (N = 59) | 29 (41.4) (N = 70) | 23 (35.9) (N = 64) |
| 2 | 2 (3.4) (N = 59) | 7 (10.0) (N = 70) | 5 (7.8) (N = 64) |
| 3 | 3 (5.1) (N = 59) | 4 (5.7) (N = 70) | 5 (7.8) (N = 64) |
| 4 | 29 (49.2) (N = 59) | 29 (41.4) (N = 70) | 28 (43.8) (N = 64) |
| 5 | 0 (0.0) (N = 59) | 0 (0.0) (N = 70) | 0 (0.0) (N = 64) |
| 6 | 58 (98.3) (N = 59) | 1 (1.4) (N = 70) | 3 (4.7) (N = 64) |
| Aid use category,c n (%) | |||
| 1 | 40 (65.6) (N = 61) | 53 (72.6) (N = 73) | 41 (64.1) (N = 64) |
| 2 | 14 (23.0) (N = 61) | 13 (17.8) (N = 73) | 18 (28.1) (N = 64) |
| 3 | 3 (4.9) (N = 61) | 5 (6.8) (N = 73) | 2 (3.1) (N = 64) |
| 4 | 0 (0.0) (N = 61) | 0 (0.0) (N = 73) | 1 (1.6) (N = 64) |
| 5 | 0 (0.0) (N = 61) | 0 (0.0) (N = 73) | 1 (1.6) (N = 64) |
| 6 | 4 (6.6) (N = 61) | 2 (2.7) (N = 73) | 1 (1.6) (N = 64) |
| Participants with back pain, n (%) | |||
| In previous 2 weeks | 61 (100) (N = 61) | 71 (97.3) (N = 73) | 67 (100) (N = 67) |
| Today | 44 (72.1) (N = 61) | 48 (65.8) (N = 73) | 46 (68.7) (N = 67) |
| Number of falls in previous year, mean (SD) | 1.1 (3.5) (N = 60) | 0.7 (1.4) (N = 72) | 0.6 (1.0) (N = 66) |
| Falls in category,d n (%) | |||
| 1 | 1 (1.7) (N = 58) | 0 (0.0) (N = 67) | 0 (0.0) (N = 66) |
| 2 | 3 (5.2) (N = 58) | 7 (10.4) (N = 67) | 4 (6.1) (N = 66) |
| 3 | 54 (93.1) (N = 58) | 60 (89.6) (N = 67) | 62 (93.9) (N = 66) |
| Participants with pain, n (%) | |||
| In previous 2 weeks | |||
| Lower lumbar (L3–L5) | 36 (62.1) (N = 58) | 47 (70.1) (N = 67) | 43 (69.4) (N = 62) |
| Upper lumbar (L1 and L2) | 32 (57.1) (N = 56) | 29 (43.9) (N = 66) | 33 (53.2) (N = 62) |
| Lower thoracic (T6–T12) | 27 (48.2) (N = 56) | 28 (41.8) (N = 67) | 28 (43.1) (N = 65) |
| Upper thoracic (T1–T5) | 12 (21.1) (N = 57) | 17 (26.6) (N = 64) | 17 (28.3) (N = 60) |
| Today | |||
| Lower lumbar (L3–L5) | 22 (53.7) (N = 41) | 32 (68.1) (N = 47) | 21 (50.0) (N = 42) |
| Upper lumbar (L1 and L2) | 21 (53.8) (N = 39) | 20 (44.4) (N = 45) | 16 (39.0) (N = 41) |
| Lower thoracic (T6–T12) | 15 (40.5) (N = 37) | 20 (45.5) (N = 44) | 18 (42.9) (N = 42) |
| Upper thoracic (T1–T5) | 5 (13.5) (N = 37) | 8 (19.0) (N = 42) | 12 (30.0) (N = 40) |
| QUALEFFO-41 score (points), mean (SD) | 39.7 (15.6) (N = 61) | 35.6 (13.7) (N = 73) | 38.2 (16.5) (N = 67) |
| TLS test score (seconds), mean (SD) | 53.9 (63.2) (N = 61) | 52.7 (60.1) (N = 73) | 66.6 (106.5) (N = 67) |
| Baseline data | Intervention arm | ||
|---|---|---|---|
| Exercise therapy | Manual therapy | SSPT | |
| Female patients, n (%) | 133 (85.8) (N = 155) | 109 (84.5) (N = 129) | 112 (87.5) (N = 128) |
| Age (years), mean (SD) | 72.4 (8.2) (N = 155) | 72.5 (9.6) (N = 130) | 71.8 (9.9) (N = 129) |
| Height (cm), mean (SD) | 156.4 (20.1) (N = 155) | 159.0 (8.8) (N = 129) | 157.3 (16.1) (N = 128) |
| Weight (kg), mean (SD) | 64.0 (16.1) (N = 155) | 65.0 (14.0) (N = 129) | 63.3 (10.7) (N = 128) |
| DEXA T-score, mean (SD) | –2.6 (1.5) (N = 116) | –2.8 (1.0) (N = 105) | –2.8 (1.2) (N = 99) |
| Number of spinal fractures, mean (SD) | 2.6 (1.7) (N = 140) | 2.3 (1.5) (N = 119) | 2.5 (1.9) (N = 113) |
| Number of non-spinal fractures, mean (SD) | 0.2 (0.4) (N = 140) | 0.1 (0.4) (N = 114) | 0.1 (0.3) (N = 115) |
| Fractures, n (%) | |||
| Upper lumbar (L1 and L2) | 41 (52.6) (N = 78) | 27 (45.8) (N = 59) | 34 (52.3) (N = 65) |
| Lower lumbar (L3–L5) | 54 (56.3) (N = 96) | 55 (72.4) (N = 76) | 41 (59.4) (N = 69) |
| Lower thoracic (T6–T12) | 113 (91.9) (N = 123) | 89 (92.7) (N = 96) | 90 (87.4) (N = 103) |
| Upper thoracic (T1–T5) | 19 (29.2) (N = 65) | 7 (15.9) (N = 44) | 18 (31.6) (N = 57) |
| Walking category,a n (%) | |||
| 1 | 67 (43.5) (N = 154) | 63 (48.8) (N = 129) | 66 (51.6) (N = 128) |
| 2 | 40 (26.0) (N = 154) | 26 (20.2) (N = 129) | 26 (20.3) (N = 128) |
| 3 | 38 (24.7) (N = 154) | 31 (24.0) (N = 129) | 27 (21.1) (N = 128) |
| 4 | 9 (5.8) (N = 154) | 8 (6.2) (N = 129) | 9 (7.0) (N = 128) |
| 5 | 0 (0.0) (N = 154) | 1 (0.8) (N = 129) | 0 (0.0) (N = 128) |
| 6 | 0 (0.0) (N = 154) | 0 (0.0) (N = 129) | 0 (0.0) (N = 128) |
| Stair use category,b n (%) | |||
| 1 | 62 (40.8) (N = 152) | 59 (45.7) (N = 129) | 56 (44.4) (N = 126) |
| 2 | 13 (8.6) (N = 152) | 10 (7.8) (N = 129) | 12 (9.5) (N = 126) |
| 3 | 5 (3.3) (N = 152) | 4 (3.1) (N = 129) | 1 (0.8) (N = 126) |
| 4 | 66 (43.4) (N = 152) | 49 (38.0) (N = 129) | 55 (43.7) (N = 126) |
| 5 | 0 (0.0) (N = 152) | 0 (0.0) (N = 129) | 0 (0.0) (N = 126) |
| 6 | 6 (3.9) (N = 152) | 7 (5.4) (N = 129) | 2 (1.6) (N = 126) |
| Aid use category,c n (%) | |||
| 1 | 96 (62.3) (N = 154) | 86 (66.7) (N = 129) | 89 (69.5) (N = 128) |
| 2 | 35 (22.7) (N = 154) | 24 (18.6) (N = 129) | 26 (20.3) (N = 128) |
| 3 | 10 (6.5) (N = 154) | 3 (2.3) (N = 129) | 8 (6.3) (N = 128) |
| 4 | 2 (1.3) (N = 154) | 1 (0.8) (N = 129) | 0 (0.0) (N = 128) |
| 5 | 0 (0.0) (N = 154) | 2 (1.6) (N = 129) | 1 (0.8) (N = 128) |
| 6 | 11 (7.1) (N = 154) | 11 (8.5) (N = 129) | 4 (3.1) (N = 128) |
| Participants with back pain, n (%) | |||
| In previous 2 weeks | 148 (95.5) (N = 155) | 123 (95.3) (N = 129) | 121 (94.5) (N = 128) |
| Today | 113 (72.9) (N = 155) | 85 (65.9) (N = 129) | 82 (64.6) (N = 127) |
| Number of falls in previous year, mean (SD) | 0.8 (1.6) (N = 154) | 0.7 (1.4) (N = 129) | 0.6 (1.0) (N = 126) |
| Falls in category,d n (%) | |||
| 1 | 0 (0.0) (N = 149) | 1 (0.8) (N = 126) | 0 (0.0) (N = 122) |
| 2 | 16 (10.7) (N = 149) | 7 (5.6) (N = 126) | 4 (3.3) (N = 122) |
| 3 | 133 (89.3) (N = 149) | 118 (93.7) (N = 126) | 118 (96.7) (N = 122) |
| Participants in pain, n (%) | |||
| In previous 2 weeks | |||
| Lower lumbar (L3–L5) | 85 (75.2) (N = 113) | 68 (77.3) (N = 88) | 61 (72.6) (N = 84) |
| Upper lumbar (L1 and L2) | 51 (54.3) (N = 94) | 48 (68.6) (N = 70) | 46 (62.2) (N = 74) |
| Lower thoracic (T6–T12) | 57 (58.2) (N = 98) | 50 (68.5) (N = 73) | 56 (65.1) (N = 86) |
| Upper thoracic (T1–T5) | 38 (44.7) (N = 85) | 33 (57.9) (N = 57) | 35 (46.7) (N = 75) |
| Today | |||
| Lower lumbar (L3–L5) | 61 (71.8) (N = 85) | 44 (75.9) (N = 58) | 38 (73.1) (N = 52) |
| Upper lumbar (L1 and L2) | 35 (51.5) (N = 68) | 35 (72.9) (N = 48) | 28 (63.6) (N = 44) |
| Lower thoracic (T6–T12) | 43 (57.3) (N = 75) | 31 (70.5) (N = 44) | 40 (71.4) (N = 56) |
| Upper thoracic (T1–T5) | 23 (37.7) (N = 61) | 20 (58.8) (N = 34) | 21 (46.7) (N = 45) |
| QUALEFFO-41 score (points), mean (SD) | 40.0 (16.2) (N = 153) | 38.0 (15.6) (N = 127) | 38.1 (15.7) (N = 128) |
| TLS test score (seconds), mean (SD) | 48.1 (58.6) (N = 155) | 45.2 (46.2) (N = 129) | 50.3 (49.0) (N = 128) |
| Test | p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global test | Exercise therapy vs. SSPT | Manual therapy vs. SSPT | |||||||||
| Stage 1 | Stage 2 | Combined | Stage 1 | Stage 2 | Combined | Corrected | Stage 1 | Stage 2 | Combined | Corrected | |
| Notationa | |||||||||||
| One-sided (+) test | p + 1,G | p + 2,G | p + G | p + 1,E | p + 2,E | p + C,E | p + E | p + 1,M | p + 2,M | p + C,M | p + M |
| One-sided (–) test | p – 1,G | p – 2,G | p – G | p – 1,E | p – 2,E | p – C,E | p – E | p – 1,M | p – 2,M | p – C,M | p – M |
| Two-sided test | p E | p M | |||||||||
| QUALEFFO-41 12-month change (points) | |||||||||||
| One-sided (+) test | 0.696 | 0.426 | 0.566 | 0.556 | 0.286 | 0.359 | 0.566 | 0.530 | 0.701 | 0.679 | 0.679 |
| One-sided (–) test | 0.609 | 0.441 | 0.521 | 0.444 | 0.714 | 0.641 | 0.641 | 0.470 | 0.299 | 0.321 | 0.521 |
| Two-sided test | 1.000 | 1.000 | |||||||||
| TLS test 12-month change (seconds) | |||||||||||
| One-sided (+) test | 0.014 | 0.684 | 0.168 | 0.092 | 0.517 | 0.218 | 0.218 | 0.008 | 0.530 | 0.077 | 0.168 |
| One-sided (–) test | 0.971 | 0.636 | 0.925 | 0.908 | 0.483 | 0.782 | 0.925 | 0.992 | 0.470 | 0.923 | 0.925 |
| Two-sided test | 0.437 | 0.335 | |||||||||
| QUALEFFO-41 4-month change (points) | |||||||||||
| One-sided (+) test | 0.087 | 0.489 | 0.197 | 0.050 | 0.338 | 0.090 | 0.197 | 0.167 | 0.888 | 0.644 | 0.644 |
| One-sided (–) test | 0.933 | 0.186 | 0.583 | 0.950 | 0.662 | 0.910 | 0.910 | 0.833 | 0.112 | 0.356 | 0.583 |
| Two-sided test | 0.394 | 1.000 | |||||||||
| TLS test 4-month change (seconds) | |||||||||||
| One-sided (+) test | 0.088 | 0.106 | 0.035 | 0.094 | 0.144 | 0.050 | 0.050 | 0.050 | 0.061 | 0.013 | 0.035 |
| One-sided (–) test | 0.970 | 0.946 | 0.992 | 0.906 | 0.856 | 0.950 | 0.992 | 0.950 | 0.939 | 0.987 | 0.992 |
| Two-sided test | 0.100 | 0.070 | |||||||||
| Test | p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global test | Exercise therapy vs. SSPT | Manual therapy vs. SSPT | |||||||||
| Stage 1 | Stage 2 | Combined | Stage 1 | Stage 2 | Combined | Corrected | Stage 1 | Stage 2 | Combined | Corrected | |
| ‘Compliance’ defined as attendance of ≥ 50% of sessions | |||||||||||
| QUALEFFO-41 12-month change (points) | |||||||||||
| One-sided (+) test | 0.748 | 0.417 | 0.596 | 0.723 | 0.279 | 0.46 | 0.596 | 0.586 | 0.646 | 0.666 | 0.666 |
| One-sided (–) test | 0.414 | 0.509 | 0.454 | 0.277 | 0.721 | 0.54 | 0.54 | 0.414 | 0.355 | 0.334 | 0.454 |
| Two-sided test | 1 | 0.908 | |||||||||
| TLS test 12-month change (seconds) | |||||||||||
| One-sided (+) test | 0.017 | 0.614 | 0.142 | 0.161 | 0.449 | 0.239 | 0.239 | 0.009 | 0.509 | 0.076 | 0.142 |
| One-sided (–) test | 0.936 | 0.658 | 0.895 | 0.84 | 0.551 | 0.761 | 0.895 | 0.991 | 0.492 | 0.924 | 0.924 |
| Two-sided test | 0.478 | 0.284 | |||||||||
| QUALEFFO-41 4-month change (points) | |||||||||||
| One-sided (+) test | 0.082 | 0.479 | 0.186 | 0.047 | 0.33 | 0.084 | 0.186 | 0.208 | 0.847 | 0.622 | 0.622 |
| One-sided (–) test | 0.908 | 0.247 | 0.607 | 0.954 | 0.671 | 0.916 | 0.916 | 0.793 | 0.153 | 0.378 | 0.607 |
| Two-sided test | 0.372 | 1 | |||||||||
| TLS test 4-month change (seconds) | |||||||||||
| One-sided (+) test | 0.066 | 0.108 | 0.029 | 0.09 | 0.157 | 0.053 | 0.053 | 0.037 | 0.063 | 0.011 | 0.029 |
| One-sided (–) test | 0.972 | 0.939 | 0.992 | 0.91 | 0.844 | 0.947 | 0.992 | 0.963 | 0.938 | 0.989 | 0.992 |
| Two-sided test | 0.105 | 0.058 | |||||||||
| ‘Compliance’ defined as attendance of all sessions | |||||||||||
| QUALEFFO-41 12-month change (points) | |||||||||||
| One-sided (+) test | 0.748 | 0.418 | 0.597 | 0.724 | 0.28 | 0.461 | 0.597 | 0.586 | 0.646 | 0.666 | 0.666 |
| One-sided (–) test | 0.414 | 0.509 | 0.454 | 0.277 | 0.721 | 0.539 | 0.539 | 0.414 | 0.355 | 0.334 | 0.454 |
| Two-sided test | 1 | 0.908 | |||||||||
| TLS test 12-month change (seconds) | |||||||||||
| One-sided (+) test | 0.019 | 0.614 | 0.148 | 0.164 | 0.449 | 0.241 | 0.241 | 0.01 | 0.509 | 0.08 | 0.148 |
| One-sided (–) test | 0.935 | 0.658 | 0.894 | 0.837 | 0.551 | 0.759 | 0.894 | 0.99 | 0.492 | 0.92 | 0.92 |
| Two-sided test | 0.483 | 0.296 | |||||||||
| QUALEFFO-41 4-month change (points) | |||||||||||
| One-sided (+) test | 0.089 | 0.479 | 0.193 | 0.051 | 0.33 | 0.088 | 0.193 | 0.208 | 0.849 | 0.625 | 0.625 |
| One-sided (–) test | 0.908 | 0.244 | 0.604 | 0.95 | 0.671 | 0.912 | 0.912 | 0.792 | 0.151 | 0.375 | 0.604 |
| Two-sided test | 0.386 | 1 | |||||||||
| TLS test 4-month change (seconds) | |||||||||||
| One-sided (+) test | 0.065 | 0.105 | 0.028 | 0.093 | 0.155 | 0.053 | 0.053 | 0.037 | 0.061 | 0.01 | 0.028 |
| One-sided (–) test | 0.971 | 0.94 | 0.991 | 0.907 | 0.845 | 0.947 | 0.991 | 0.964 | 0.94 | 0.99 | 0.991 |
| Two-sided test | 0.107 | 0.055 | |||||||||
Appendix 23 Hypothesis testing allowing for the adaptive design
Hypothesis tests were conducted using the method proposed by Bretz et al. 56 and modified by Friede et al. 57 to allow for the use of an early end point (in this case, the change in QUALEFFO-41 score from baseline at 4 months) in the interim analysis.
In detail, for each of the co-primary end points (change in the QUALEFFO-41 score and TLS test time from baseline to 12 month), we wish to construct p-values, which we denote using pM and pE, respectively, to test the following two null hypotheses:
-
H0,M (manual therapy and SSPT are equally effective)
-
H0,E (exercise therapy and SSPT are equally effective).
These can be tested by testing the one-sided hypotheses:
-
H+0,M (manual therapy is not better than the SSPT)
-
H+0,E (exercise therapy is not better than the SSPT).
and:
-
H–0,M (manual therapy is not worse than the SSPT)
-
H–0,E (exercise therapy is not worse than the SSPT).
Denoting the p-values for these four one-sided hypotheses (corrected for multiplicity and the adaptive design as described below) by p+M, p+E, p–M and p–E, respectively, we have:
where ‘min’ means minimum.
In order to adjust for multiplicity, we apply the closed testing procedure. Let p+G and p–G be p-values for the global hypotheses:
-
H+0,G (neither manual therapy nor exercise therapy is better than the SSPT)
-
H–0,G (neither manual therapy nor exercise therapy is worse than the SSPT),
and p+C,M, p+C,E, p–C,M and p–C,E be p-values to test H+0,M, H+0,E H–0,M and H–0,E, respectively, unadjusted for multiplicity, then p+M, p+E, p–M and p–E$ are given by:
The unadjusted p-values are based on p-values obtained from the data from patients in the two stages of the trial. Stage 1 data are defined as being all data from patients who were included in the interim analysis and stage 2 data are defined as being all data from all other patients. Note that not all stage 1 data were observed at the time of the interim analysis as these were based on only the 4-month follow-up data.
Let p+j,i and p–j,I be p-values from stage j to test H+0,i and H–0,i G, unadjusted for multiplicity. The p-values p+C,M, p+C,E, p–C,M inverse the normal combination of the stagewise p-values by:
for i = M, E and G, where ɸ denotes the standard normal cumulative density function and w1 and w2 are prespecified weights given by w1=75200 and w2=125200. The value 75200 was the planned fraction of patients in each group to be included in the interim analysis.
The p-values p+1,M, p+1,E, p–1,M, p–1,E, p+2,M, p+2,E, p–2,M and p–2,E, to test each elementary one-sided hypothesis, H+0,M, H+0,E, H–0,M, H–0,E, based on stage 1 and stage 2 data, respectively, can be obtained from a linear model to test the effect of each intervention relative to the SSPT, and p-values, p+1,G, p+1,G, p–2,G and p–2,E, to test the global intersection hypothesis can be obtained using a Dunnett test. Equations 1–5 can then be used to calculate the two-sided p-values to test H0,M and H0,E as required. It is these p-values that are given in Chapter 4.
Appendix 24 Chapter 6 supplementary material
MEDLINE (via Ovid) and EMBASE (via OVID)
Date range searched: January 2000 to Janaury 2018.
Date searched: 1 February 2018.
Search strategy
-
osteoporosis.ti,ab.
-
Osteoporosis, Postmenopausal.ti,ab.
-
bone density.ti,ab.
-
Spinal fractures.ti,ab.
-
((spin* or vertebra*) adj5 (fracture* or broke* or break*)).ti,ab.
-
((compress* or crush or wedge) adj5 fracture*).ti,ab.
-
Back/ and (Fractures, Spontaneous/ or Fractures, Compression/ or Fractures, Stress/)
-
4 or 5 or 6 or 7
-
physical therapy.ti,ab.
-
exp Physical therapy modalities/
-
physiotherapy.ti,ab.
-
exp Exercise/
-
qaly$.ti,ab.
-
(valu$ adj2 quality).ti,ab.
-
utility value$.ti,ab.
-
((disability or quality) adj adjusted).ti,ab.
-
((life adj2 year$) or health year equivalent$).ti,ab.
-
hui$1.ti,ab.
-
(quality adj3 well$).ti,ab.
-
qwb.ti,ab.
-
(qald$ or qale$ or qtime$).ti,ab.
-
(well being or wellbeing).tw.
-
(health adj2 stat$).tw.
-
(daly or qol or hql or hqol or hrqol or hr ql or hrql).tw.
-
cost-utility.ti,ab.
-
cost-effectiveness.ti,ab.
-
cost-benefit.ti,ab.
-
cost-minimisation.ti,ab.
-
cost-minimization.ti,ab.
-
QALY.ti,ab.
-
cost.ti,ab.
-
life year$.ti,ab.
-
incremental cost-effectiveness ratio.ti,ab.
-
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33
-
rehab.ti,ab.
-
rehabilitation.ti,ab.
-
9 or 10 or 11 or 12 or 35 or 36
-
1 or 2 or 3
-
8 and 34 and 37 and 38
Unit costs
| Resource used | Cost (£) | Notes | Source |
|---|---|---|---|
| GP consultation | 36.00 | 9.2 minutes/visit | Curtis and Burns151 |
| General practice nurse consultation | 14.00 | 15.5 minutes/visit | Curtis and Burns151 |
| Physiotherapy | 49.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Manual therapy | 50.50 | Cost/encounter and consumables cost | NHS Reference Costs 2015–16150 and the PROVE trial |
| Outpatient consultations | |||
| Outpatient (OPD) hospital (doctor-weighted average) | 120.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Rheumatology nurse (OPD) | 87.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Rheumatology consultant (OPD) | 137.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Clinical immunology (OPD) | 299.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Emergency medicine (OPD) | 197.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| T&O (OPD) | 109.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Urology (OPD) | 99.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Medical oncology (OPD) | 162.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Cardiology (OPD) | 121.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Dental medicine (OPD) | 102.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Dermatology (OPD) | 99.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Diabetic medicine (OPD) | 158.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Geriatric medicine (OPD) | 187.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| ENT (OPD) | 89.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Ophthalmology (OPD) | 86.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Gastroenterology (OPD) | 132.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| General surgery (OPD) | 123.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Clinical genetics (OPD) | 416.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Clinical haematology (OPD) | 166.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Clinical neurophysiology (OPD) | 285.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Neurology (OPD) | 160.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Orthoptics (OPD) | 65.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Nephrology (OPD) | 153.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Respiratory medicine (OPD) | 145.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Spinal surgery (OPD) | 126.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| Transient ischaemic attack (OPD) | 139.00 | Cost/encounter | NHS Reference Costs 2015–16 150 |
| CT scan | 94.00 | Cost/body part | NHS Reference Costs 2015–16 150 |
| MRI scan | 145.00 | Cost/body part | NHS Reference Costs 2015–16 150 |
| Ultrasound scan | 55.00 | < 20-minute scan | NHS Reference Costs 2015–16 150 |
| X-ray | 30.00 | Cost/body part | NHS Reference Costs 2015–16 150 |
| DEXA scan | 71.00 | Cost/scan | NHS Reference Costs 2015–16 150 |
| Echocardiogram | 72.00 | Cost/scan | NHS Reference Costs 2015–16 150 |
| Home visits | |||
| GP | 118.00 | Cost/visit, 23.4 minutes | NHS Reference Costs 2015–16 150 |
| Nurse | 47.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Physiotherapy | 63.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Occupational therapy | 101.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Podiatrist | 52.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Social worker | 102.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Paramedic 1 | 181.00 | See and treat or refer | NHS Reference Costs 2015–16 150 |
| Paramedic 2 | 236.00 | See and treat and convey | NHS Reference Costs 2015–16 150 |
| Matron | 89.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Respiratory nurse | 84.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Other specialist nurse | 77.00 | Cost/visit | NHS Reference Costs 2015–16 150 |
| Social care worker | 79.00 | Cost/visit | Curtis and Burns151 |
| Bereavement counsellor | 130.00 | Assume cost of clinical psychologist | NHS Reference Costs 2015–16 150 |
| Hospital at home | 108.00 | Cost/visit. Excludes cost of medications | University of York Productivity of the English NHS: 2013/14 Update200 |
| Resource used | Cost (£) | Notes | Source |
|---|---|---|---|
| Walking aids and other aids | 4.00–62.00 | Shoe horn, long-reach nail scissors, long-handled items, grabber, crutch, wheel walker, walking frame, back brace, food trolley | Co-operative Mobility201 |
| Bath/shower modifications | 19.00–4782.00 | Bath board, bath seat, hand-hold and set for shower, walk-in shower | Co-operative Mobility201 and Curtis and Burns151 |
| Stools and other sitting aids | 60.00–450.00 | Recliner, perching stool | Co-operative Mobility201 and the Cavendish Mobility Furniture202 |
| Bedding support | 22.00–48.00 | Bed rail, extra padding, fitting for support, bed raiser | Co-operative Mobility201 |
| Wheelchair | 120.00 | Self-propelled wheelchair | Co-operative Mobility201 |
| Toilet equipment | 19.00–36.00 | Toilet seat, toilet frame, toilet rail | Co-operative Mobility201 |
| Mobility scooter | 949.00 | Rascal 388 S (Electric Mobility Euro Ltd, Ilminster, UK) | CareCo203 |
| Leg support | 120.00 | Hinged leg brace | healthandcare.co.uk204 |
| Stair modifications | 20.00–1917.00 | Rails, stair lift | Co-operative Mobility201 and Curtis and Burns151 |
| Resource used | Cost (£) | Notes | Source |
|---|---|---|---|
| Physiotherapy | 49.00 | Assumed to be the same as NHS | NHS Reference Costs 2015–16 150 |
| Occupational therapist | 79.00 | Assumed to be the same as NHS | NHS Reference Costs 2015–16 150 |
| Osteopath | 42.50 | NHS Choices153 | |
| Chiropractor | 55.00 | www.nhs.uk/conditions/chiropractic (accessed 13 February 2019) | |
| Outpatient consultation | Variable | Assumed to be the same as NHS; refer to Table 34 | NHS Reference Costs 2015–16 150 |
| Acupuncture | 55.00 | www.nhs.uk/conditions/acupuncture/153 (accessed 13 February 2019) | |
| Audiologist | 65.00 | Assumed to be the same as NHS | NHS Reference Costs 2015–16 150 |
| Dentist | 131.00 | NHS Reference Costs 2015–16 150 | |
| Dietitian | 81.00 | Assumed to be the same as NHS | NHS Reference Costs 2015–16 150 |
| Alexander technique | 43.00 | Per session | NHS Choices153 |
| AposTherapy | 2480.00 | Annual cost | AposTherapy205 |
Additional results tables
| Health record diary | Participants who completed/returned diaries, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Returned diary 1 (4 months) | 149/216 (68.98) | 163/203 (80.30) | 151/196 (77.04) | 463/615 (75.28) |
| Completed diary 1 (4 months)a | 135/149 (90.60) | 148/161 (91.92) | 144/150 (96.00) | 427/460 (92.82) |
| Returned diary 2 (12 months) | 144/216 (66.67) | 148/203 (72.91) | 152/196 (77.55) | 444/615 (72.20) |
| Completed diary 2 (12 months)a | 138/144 (95.83) | 140/148 (94.59) | 148/152 (97.37) | 426/444 (95.95) |
| Returned diaries 1 and 2 | 119/216 (55.09) | 132/203 (65.02) | 136/196 (69.39) | 387/615 (62.93) |
| Completed diaries 1 and 2a | 105/119 (88.24) | 118/132 (89.39) | 126/136 (92.65) | 349/387 (90.18) |
| Level by visit | Mobility, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Baseline | ||||
| No problems | 81/207 (39.1) | 73/194 (37.6) | 73/189 (38.6) | 227/590 (38.5) |
| Slight problems | 42/207 (20.3) | 52/194 (26.8) | 55/189 (29.1) | 149/590 (25.3) |
| Moderate problems | 68/207 (32.9) | 55/194 (28.4) | 50/189 (26.5) | 173/590 (29.3) |
| Severe problems | 16/207 (7.7) | 14/194 (7.2) | 11/189 (5.8) | 41/590 (7.0) |
| Unable to walk | 0/207 (0) | 0/194 (0) | 0/189 (0) | 0/590 (0) |
| Not available | 9 | 9 | 9 | 25 |
| 4 months | ||||
| No problems | 71/174 (40.8) | 70/177 (39.55) | 67/167 (40.15) | 208/518 (40.15) |
| Slight problems | 40/174 (22.99) | 55/177 (31.07) | 48/167 (27.61) | 143/518 (27.61) |
| Moderate problems | 47/174 (27.01) | 37/177 (20.9) | 41/167 (24.13) | 125/518 (24.13) |
| Severe problems | 16/174 (9.20) | 15/177 (8.47) | 11/167 (8.11) | 42/518 (8.11) |
| Unable to walk | 0/174 (0.0) | 0/177 (0.0) | 0/167 (0.0) | 0/518 (0.0) |
| Not available | 42 | 26 | 29 | 97 |
| 6 months | ||||
| No problems | 68/166 (40.96) | 65/157 (41.4) | 63/159 (39.62) | 196/482 (40.66) |
| Slight problems | 38/166 (22.89) | 42/157 (26.75) | 43/159 (27.04) | 123/482 (25.52) |
| Moderate problems | 44/166 (26.51) | 31/157 (19.75) | 36/159 (22.64) | 111/482 (23.03) |
| Severe problems | 16/166 (9.64) | 19/157 (12.1) | 15/159 (9.43) | 50/482 (10.37) |
| Unable to walk | 0/166 (0) | 0/157 (0) | 2/159 (1.26) | 2/482 (0.41) |
| Not available | 50 | 46 | 37 | 133 |
| 9 months | ||||
| No problems | 61/153 (39.87) | 55/141 (39.01) | 57/152 (37.5) | 173/446 (38.79) |
| Slight problems | 33/153 (21.57) | 42/141 (29.79) | 45/152 (29.61) | 120/446 (26.91) |
| Moderate problems | 47/153 (30.72) | 33/141 (23.4) | 37/152 (24.34) | 117/446 (26.23) |
| Severe problems | 11/153 (7.19) | 10/141 (7.09) | 13/152 (8.55) | 34/446 (7.62) |
| Unable to walk | 1/153 (0.65) | 1/141 (0.71) | 0/152 (0) | 2/446 (0.45) |
| Not available | 63 | 62 | 44 | 169 |
| 12 months | ||||
| No problems | 62/167 (37.13) | 71/172 (41.28) | 71/164 (43.29) | 204/503 (40.56) |
| Slight problems | 39/167 (23.35) | 41/172 (23.84) | 38/164 (23.17) | 118/503 (23.46) |
| Moderate problems | 48/167 (28.74) | 39/172 (22.67) | 45/164 (27.44) | 132/503 (26.24) |
| Severe problems | 18/167 (10.78) | 21/172 (12.21) | 10/164 (6.1) | 49/503 (9.74) |
| Unable to walk | 0/167 (0.0) | 0/172 (0.0) | 0/164 (0.0) | 0/503 (0.0) |
| Not available | 49 | 31 | 32 | 112 |
| Level by visit | Self-care, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Baseline | ||||
| No problems | 139/207 (67.15) | 145/194 (74.74) | 137/189 (72.49) | 421/590 (71.36) |
| Slight problems | 43/207 (20.77) | 30/194 (15.46) | 30/189 (15.87) | 103/590 (17.46) |
| Moderate problems | 22/207 (10.63) | 16/194 (8.25) | 19/189 (10.05) | 57/590 (9.66) |
| Severe problems | 3/207 (1.45) | 2/194 (1.03) | 3/189 (1.59) | 8/590 (1.36) |
| Unable to wash or dress myself | 0/207 (0) | 1/194 (0.52) | 0/189 (0) | 1/590 (0.17) |
| Not available | 9 | 9 | 9 | 25 |
| 4 months | ||||
| No problems | 123/174 (70.69) | 131/177 (74.01) | 118/167 (70.66) | 372/518 (71.81) |
| Slight problems | 26/174 (14.94) | 29/177 (16.38) | 33/167 (19.76) | 88/518 (16.99) |
| Moderate problems | 24/174 (13.79) | 14/177 (7.91) | 12/167 (7.19) | 50/518 (9.65) |
| Severe problems | 1/174 (0.57) | 2/177 (1.13) | 3/167 (1.80) | 6/518 (1.16) |
| Unable to wash or dress myself | 0/174 (0) | 1/177 (0.56) | 1/167 (0.6) | 2/518 (0.39) |
| Not available | 42 | 26 | 29 | 97 |
| 6 months | ||||
| No problems | 107/166 (64.46) | 108/157 (68.79) | 99/159 (62.26) | 314/482 (65.15) |
| Slight problems | 40/166 (24.1) | 36/157 (22.93) | 34/159 (21.38) | 110/482 (22.82) |
| Moderate problems | 17/166 (10.24) | 10/157 (6.37) | 20/159 (12.58) | 47/482 (9.75) |
| Severe problems | 2/166 (1.2) | 3/157 (1.91) | 6/159 (3.77) | 11/482 (2.28) |
| Unable to wash or dress myself | 0/166 (0.0) | 0/157 (0.0) | 0/159 (0.0) | 0/482 (0.0) |
| Not available | 50 | 46 | 37 | 133 |
| 9 months | ||||
| No problems | 96/153 (62.75) | 99/141 (70.21) | 98/152 (64.47) | 293/446 (65.7) |
| Slight problems | 38/153 (24.84) | 30/141 (21.28) | 39/152 (25.66) | 107/446 (23.99) |
| Moderate problems | 17/153 (11.11) | 8/141 (5.67) | 11/152 (7.24) | 36/446 (8.07) |
| Severe problems | 2/153 (1.31) | 4/141 (2.84) | 4/152 (2.63) | 10/446 (2.24) |
| Unable to wash or dress myself | 0/153 (0.0) | 0/141 (0.0) | 0/152 (0.0) | 0/446 (0.0) |
| Not available | 63 | 62 | 44 | 169 |
| 12 months | ||||
| No problems | 111/167 (66.47) | 124/172 (72.09) | 109/164 (66.46) | 344/503 (68.39) |
| Slight problems | 36/167 (21.56) | 28/172 (16.28) | 35/164 (21.34) | 99/503 (19.68) |
| Moderate problems | 17/167 (10.18) | 18/172 (10.47) | 17/164 (10.37) | 52/503 (10.34) |
| Severe problems | 3/167 (1.8) | 2/172 (1.16) | 3/164 (1.83) | 8/503 (1.59) |
| Unable to wash or dress myself | 0/167 (0.0) | 0/172 (0.0) | 0/164 (0.0) | 0/503 (0.0) |
| Not available | 49 | 31 | 32 | 112 |
| Level by visit | Usual activities, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Baseline | ||||
| No problems | 54/207 (26.09) | 50/194 (25.77) | 55/189 (29.10) | 159/590 (26.95) |
| Slight problems | 66/207 (31.88) | 68/194 (35.05) | 55/189 (29.10) | 189/590 (32.03) |
| Moderate problems | 57/207 (27.54) | 59/194 (30.41) | 57/189 (30.16) | 173/590 (29.32) |
| Severe problems | 23/207 (11.11) | 12/194 (6.19) | 16/189 (8.47) | 51/590 (8.64) |
| Unable to do usual activities | 7/207 (3.38) | 5/194 (2.58) | 6/189 (3.17) | 18/590 (3.05) |
| Not available | 9 | 9 | 9 | 25 |
| 4 months | ||||
| No problems | 62/174 (35.63) | 60/177 (33.9) | 51/167 (30.54) | 173/518 (33.40) |
| Slight problems | 47/174 (27.01) | 57/177 (32.2) | 57/167 (34.13) | 161/518 (31.08) |
| Moderate problems | 41/174 (23.56) | 41/177 (23.16) | 49/167 (29.34) | 131/518 (25.29) |
| Severe problems | 18/174 (10.34) | 14/177 (7.91) | 5/167 (2.99) | 37/518 (7.14) |
| Unable to do usual activities | 6/174 (3.45) | 5/177 (2.82) | 5/167 (2.99) | 16/518 (3.09) |
| Not available | 42 | 26 | 29 | 97 |
| 6 months | ||||
| No problems | 50/166 (30.12) | 51/157 (32.48) | 49/159 (30.82) | 150/482 (31.12) |
| Slight problems | 50/166 (30.12) | 52/157 (33.12) | 49/159 (30.82) | 151/482 (31.33) |
| Moderate problems | 44/166 (26.51) | 39/157 (24.84) | 38/159 (23.9) | 121/482 (25.1) |
| Severe problems | 17/166 (10.24) | 12/157 (7.64) | 16/159 (10.06) | 45/482 (9.34) |
| Unable to do usual activities | 5/166 (3.01) | 3/157 (1.91) | 7/159 (4.4) | 15/482 (3.11) |
| Not available | 50 | 46 | 37 | 133 |
| 9 months | ||||
| No problems | 47/153 (30.72) | 50/141 (35.46) | 41/152 (26.97) | 138/446 (30.94) |
| Slight problems | 46/153 (30.07) | 42/141 (29.79) | 52/152 (34.21) | 140/446 (31.39) |
| Moderate problems | 45/153 (29.41) | 31/141 (21.99) | 42/152 (27.63) | 118/446 (26.46) |
| Severe problems | 10/153 (6.54) | 12/141 (8.51) | 13/152 (8.55) | 35/446 (7.85) |
| Unable to do usual activities | 5/153 (3.27) | 6/141 (4.26) | 4/152 (2.63) | 15/446 (3.63) |
| Not available | 63 | 62 | 44 | 169 |
| 12 months | ||||
| No problems | 49/167 (29.34) | 60/172 (34.88) | 56/164 (34.15) | 165/503 (32.8) |
| Slight problems | 52/167 (31.14) | 46/172 (26.74) | 54/164 (32.93) | 152/503 (30.22) |
| Moderate problems | 50/167 (29.94) | 47/172 (27.33) | 45/164 (27.44) | 142/503 (28.23) |
| Severe problems | 13/167 (7.78) | 14/172 (8.14) | 6/164 (3.66) | 33/503 (6.56) |
| Unable to do usual activities | 3/167 (1.8) | 5/172 (2.91) | 3/164 (1.83) | 11/503 (2.19) |
| Not available | 49 | 31 | 32 | 112 |
| Level by visit | Pain and discomfort, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Baseline | ||||
| No pain or discomfort | 11/207 (5.31) | 18/194 (9.28) | 11/189 (5.82) | 40/590 (6.78) |
| Slight pain or discomfort | 64/207 (30.92) | 70/194 (36.08) | 62/189 (32.80) | 196/590 (33.22) |
| Moderate pain or discomfort | 95/207 (45.89) | 84/194 (43.30) | 94/189 (49.74) | 273/590 (46.27) |
| Severe pain or discomfort | 32/207 (15.46) | 20/194 (10.31) | 20/189 (10.58) | 72/590 (12.20) |
| Extreme pain or discomfort | 5/207 (2.42) | 2/194 (1.03) | 2/189 (1.06) | 9/590 (1.53) |
| Not available | 9 | 9 | 9 | 25 |
| 4 months | ||||
| No pain or discomfort | 23/174 (13.22) | 19/177 (10.73) | 18/167 (10.78) | 60/518 (11.58) |
| Slight pain or discomfort | 68/174 (39.08) | 66/177 (37.29) | 63/167 (37.72) | 197/518 (38.03) |
| Moderate pain or discomfort | 59/174 (33.91) | 68/177 (38.42) | 68/167 (40.72) | 195/518 (37.64) |
| Severe pain or discomfort | 20/174 (11.49) | 20/177 (11.3) | 16/167 (9.58) | 56/518 (10.81) |
| Extreme pain or discomfort | 4/174 (2.3) | 4/177 (2.26) | 2/167 (1.2) | 10/518 (1.93) |
| Not available | 42 | 26 | 29 | 97 |
| 6 months | ||||
| No pain or discomfort | 15/166 (9.04) | 18/157 (11.46) | 16/159 (10.06) | 49/482 (10.17) |
| Slight pain or discomfort | 56/166 (33.73) | 54/157 (34.39) | 44/159 (27.67) | 154/482 (31.95) |
| Moderate pain or discomfort | 66/166 (39.76) | 63/157 (40.13) | 79/159 (49.69) | 208/482 (43.15) |
| Severe pain or discomfort | 25/166 (15.06) | 19/157 (12.1) | 19/159 (11.95) | 63/482 (13.07) |
| Extreme pain or discomfort | 4/166 (2.41) | 3/157 (1.91) | 1/159 (0.63) | 8/482 (1.66) |
| Not available | 50 | 46 | 37 | 133 |
| 9 months | ||||
| No pain or discomfort | 13/153 (8.5) | 13/141 (9.22) | 11/152 (7.24) | 37/446 (8.3) |
| Slight pain or discomfort | 54/153 (35.29) | 57/141 (40.43) | 61/152 (40.13) | 172/446 (38.57) |
| Moderate pain or discomfort | 61/153 (39.87) | 52/141 (36.88) | 61/152 (40.13) | 174/446 (39.01) |
| Severe pain or discomfort | 22/153 (14.38) | 16/141 (11.35) | 19/152 (12.5) | 57/446 (12.78) |
| Extreme pain or discomfort | 3/153 (1.96) | 3/141 (2.13) | 0/152 (0) | 6/446 (1.35) |
| Not available | 63 | 62 | 44 | 169 |
| 12 months | ||||
| No pain or discomfort | 17/167 (10.18) | 20/172 (11.63) | 19/164 (11.59) | 56/503 (11.13) |
| Slight pain or discomfort | 63/167 (37.72) | 71/172 (41.28) | 69/164 (42.07) | 203/503 (40.36) |
| Moderate pain or discomfort | 58/167 (34.73) | 50/172 (29.07) | 53/164 (32.32) | 161/503 (32.01) |
| Severe pain or discomfort | 24/167 (14.37) | 29/172 (16.86) | 20/164 (14.51) | 73/503 (14.51) |
| Extreme pain or discomfort | 5/167 (2.99) | 2/172 (1.16) | 3/164 (1.99) | 10/503 (1.99) |
| Not available | 49 | 31 | 32 | 112 |
| Level by visit | Anxiety and depression, n/N (%) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Baseline | ||||
| Not anxious or depressed | 112/207 (54.11) | 116/194 (59.79) | 110/189 (58.20) | 338/590 (57.29) |
| Slightly anxious or depressed | 63/207 (30.43) | 55/194 (28.35) | 47/189 (24.87) | 165/590 (27.97) |
| Moderately anxious or depressed | 26/207 (12.56) | 21/194 (10.82) | 26/189 (13.76) | 73/590 (12.37) |
| Severely anxious or depressed | 4/207 (1.93) | 1/194 (0.52) | 5/189 (2.65) | 10/590 (1.69) |
| Extremely anxious or depressed | 2/207 (0.97) | 1/194 (0.52) | 1/189 (0.53) | 4/590 (0.68) |
| Not available | 9 | 9 | 9 | 25 |
| 4 months | ||||
| Not anxious or depressed | 95/174 (54.6) | 105/177 (59.32) | 102/167 (61.08) | 302/518 (58.3) |
| Slightly anxious or depressed | 51/174 (29.31) | 50/177 (28.25) | 45/167 (26.95) | 146/518 (28.19) |
| Moderately anxious or depressed | 25/174 (14.37) | 19/177 (10.73) | 17/167 (10.18) | 61/518 (11.78) |
| Severely anxious or depressed | 2/174 (1.15) | 2/177 (1.13) | 1/167 (0.6) | 5/518 (0.97) |
| Extremely anxious or depressed | 1/174 (0.57) | 1/177 (0.56) | 2/167 (1.2) | 4/518 (0.77) |
| Not available | 42 | 26 | 29 | 97 |
| 6 months | ||||
| Not anxious or depressed | 82/166 (49.4) | 85/157 (54.14) | 82/159 (51.57) | 249/482 (51.66) |
| Slightly anxious or depressed | 63/166 (37.95) | 51/157 (32.48) | 45/159 (28.3) | 159/482 (32.99) |
| Moderately anxious or depressed | 18/166 (10.84) | 18/157 (11.46) | 26/159 (16.35) | 62/482 (12.86) |
| Severely anxious or depressed | 3/166 (1.81) | 1/157 (0.64) | 5/159 (3.14) | 9/482 (1.87) |
| Extremely anxious or depressed | 0/166 (0) | 2/157 (1.27) | 1/159 (0.63) | 3/482 (0.62) |
| Not available | 50 | 46 | 37 | 133 |
| 9 months | ||||
| Not anxious or depressed | 73/153 (47.71) | 75/141 (53.19) | 80/152 (52.63) | 228/446 (51.12) |
| Slightly anxious or depressed | 57/153 (37.25) | 45/141 (31.91) | 50/152 (32.89) | 152/446 (34.08) |
| Moderately anxious or depressed | 19/153 (12.42) | 17/141 (12.06) | 18/152 (11.84) | 54/446 (12.11) |
| Severely anxious or depressed | 4/153 (2.61) | 1/141 (0.71) | 2/152 (1.32) | 7/446 (1.57) |
| Extremely anxious or depressed | 0/153 (0) | 3/141 (2.13) | 2/152 (1.32) | 5/446 (1.12) |
| Not available | 63 | 62 | 44 | 169 |
| 12 months | ||||
| Not anxious or depressed | 80/167 (47.9) | 101/172 (58.72) | 101/164 (61.59) | 282/503 (56.06) |
| Slightly anxious or depressed | 52/167 (31.14) | 45/172 (26.16) | 43/164 (26.22) | 140/503 (27.83) |
| Moderately anxious or depressed | 31/167 (18.56) | 23/172 (13.37) | 17/164 (10.37) | 71/503 (14.12) |
| Severely anxious or depressed | 3/167 (1.8) | 3/172 (1.74) | 2/164 (1.22) | 8/503 (1.59) |
| Extremely anxious or depressed | 1/167 (0.6) | 0/172 (0) | 1/164 (0.61) | 2/503 (0.4) |
| Not available | 49 | 31 | 32 | 112 |
| Physiotherapy attendance | Intervention group | All participants (N = 615) | ||
|---|---|---|---|---|
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Completed full course, n/N (%) | 82/216 (37.96) | 99/203 (48.77) | 166/196 (84.69) | 347/615 (56.42) |
| Crossover patients,a n/N (%) | 0/216 (0) | 0/203 (0) | 8/196 (4.08) | 8/615 (1.3) |
| Average (SD) | 4.33 (2.77) (n = 936b); 216 | 5.03 (2.57) (n = 1021b); 203 | 0.98 (0.59) (n = 193b); 196 | 3.50 (2.83) (n = 2150b); 615 |
| Completed full course (data from diaries 1 and 2), n/N (%) | 45/105 (42.86) | 68/118 (57.63) | 112/126 (88.89%) | 225/349 (64.47) |
| Average number of sessions attended (data from diaries 1 and 2) | 4.94 (n = 519b); 105 | 5.72 (n = 675b); 118 | 1.04 (n = 131b); 126 | 3.79 (n = 1318b); 349 |
| Resource use category | Resource utilisation, n/N (mean) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Complete diaries | 137/216 | 149/203 | 145/196 | 428/615 |
| Prescribed medication | 62/137 (0.45) | 82/149 (0.55) | 84/145 (0.58) | 228/431 (0.53) |
| Hospitalisations | 6/137 (0.04) | 7/149 (0.05) | 13/145 (0.09) | 26/431 (0.06) |
| GP/nurse/outpatient | 303/137 (2.21) | 349/149 (2.34) | 352/145 (2.43) | 1004/431 (2.33) |
| Physiotherapya | 664/137 (4.85) | 804/149 (5.40) | 151/145 (1.04) | 1619/431 (3.76) |
| Home visit | 4/137 (0.03) | 11/149 (0.07) | 21/145 (0.14) | 36/431 (0.08) |
| Resource use category | Resource utilisation, n/N (mean) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Complete diaries | 138/216 | 140/203 | 148/196 | 426/615 |
| Prescribed medication | 93/138 (0.67) | 90/140 (0.64) | 89/148 (0.60) | 272/426 (0.64) |
| Hospitalisations | 29/138 (0.21) | 19/140 (0.14) | 21/148 (0.14) | 55/426 (0.13) |
| GP/nurse/outpatient | 713/138 (5.17) | 713/140 (5.09) | 879/148 (5.94) | 2305/426 (5.41) |
| Physiotherapy | 25/138 (0.18) | 25/140 (0.18) | 86/148 (0.58) | 136/426 (0.32) |
| Home visit | 10/138 (0.07) | 20/140 (0.13) | 21/148 (0.14) | 55/426 (0.13) |
| Resource use category | Mean cost (£) (SD) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Completed diaries (n/N) | 137/216 | 149/203 | 145/196 | 428/615 |
| Newly prescribed medication | 11.41 (53.18) (n = 137) | 14.59 (61.86) (n = 149) | 59.28 (212.75) (n = 145) | 28.61 (133.60) (n = 431) |
| Hospitalisations | 47.66 (246.06) (n = 137) | 166.83 (1403.82) (n = 149) | 110.50 (460.32) (n = 145) | 110.00 (877.93) (n = 431) |
| GP/nurse/outpatient | 126.29 (178.67) (n = 137) | 128.32 (183.30) (n = 149) | 139.99 (175.86) (n = 145) | 131.60 (179.04) (n = 431) |
| Physiotherapya | 277.19 (148.37) (n = 137) | 315.25 (131.97) (n = 149) | 93.61 (42.40) (n = 145) | 228.58 (151.85) (n = 431) |
| Home visit | 8.91 (92.62) (n = 137) | 7.01 (34.90) (n = 149) | 11.12 (49.46) (n = 145) | 9.00 (62.88) (n = 431) |
| Total cost to NHS | 471.46 (431.17) (n = 137) | 631.99 (1424.42) (n = 149) | 415.00 (590.54) (n = 145) | 507.79 (939.48) (n = 431) |
| Social care servicesa | 73.42 (603.16) (n = 135) | 40.05 (372.42) (n = 149) | 8.33 (82.33) (n = 144) | 39.91 (406.53) (n = 427) |
| Total cost to NHS and social services | 550.97 (777.04) (n = 135) | 674.08 (1484.17) (n = 148) | 425.03 (597.20) (n = 144) | 551.17 (1039.41) (n = 427) |
| Difference between NHS and social services costa | 125.94 (n = 135) | 249.05 (n = 148) | – | – |
| Equipment | 0.75 (4.00) (n = 135) | 1.95 (11.07) (n = 148) | 3.17 (23.77) (n = 144) | 1.98 (15.43) (n = 427) |
| Private care | 2.70 (16.03) (n = 135) | 7.03 (33.61) (n = 148) | 11.95 (53.55) (n = 144) | 7.32 (38.04) (n = 427) |
| New OTC medication | 0.50 (2.29) (n = 135) | 0.80 (3.92) (n = 148) | 0.68 (2.82) (n = 144) | 0.67 (3.10) (n = 427) |
| Total cost | 554.92 (777.75) (n = 135) | 683.86 (1485.35) (n = 148) | 440.84 (599.62) (n = 144) | 561.14 (1040.39) (n = 427) |
| Cost differenceb | 114.08 (n = 135) | 243.02 (n = 148) | – | – |
| Resource use category | Mean cost (£) (SD) | |||
|---|---|---|---|---|
| Intervention group | All participants (N = 615) | |||
| Exercise therapy (N = 216) | Manual therapy (N = 203) | SSPT (N = 196) | ||
| Completed diaries (n/N) | 138/216 | 140/203 | 148/196 | 426/615 |
| Newly prescribed medication | 77.06 (426.60) (n = 138) | 131.83 (502.50) (n = 140) | 69.86 (273.94) (n = 148) | 92.56 (409.84) (n = 426) |
| Hospitalisation | 560.47 (2561.45) (n = 138) | 381.41 (1870.06) (n = 140) | 288.23 (990.21) (n = 148) | 407.04 (1900.18) (n = 426) |
| GP/nurse/outpatient | 210.81 (291.76) (n = 138) | 212.54 (329.00) (n = 140) | 231.56 (281.59) (n = 148) | 218.59 (300.59) (n = 426) |
| Physiotherapy | 8.88 (39.38) (n = 138) | 8.75 (39.98) (n = 140) | 28.47 (81.84) (n = 148) | 15.64 (58.54) (n = 426) |
| Home visit | 15.47 (130.70) (n = 138) | 52.33 (501.71) (n = 140) | 13.58 (60.40) (n = 148) | 26.93 (299.02) (n = 426) |
| Total cost to NHS | 872.68 (2686.82) (n = 138) | 786.86 (2019.83) (n = 140) | 631.70 (1125.60) (n = 148) | 760.75 (2027.23) (n = 426) |
| Total cost to Personal Social Servicesa | 42.92 (395.59) (n = 137) | 14.35 (148.50) (n = 138) | 11.43 (87.90) (n = 148) | 22.61 (246.19) (n = 422) |
| Total cost to NHS and Personal Social Services | 920.81 (2755.32) (n = 137) | 786.43 (2028.99) (n = 138) | 644.00 (1128.34) (n = 147) | 780.44 (2060.72) (n = 422) |
| Difference between NHS and Personal Social Services costb | 276.81 (n = 137) | 142.42 (n = 137) | – (n = 148) | |
| Equipment | 51.15 (419.01) (n = 138) | 5.96 (23.13) (n = 138) | 13.68 (64.50) (n = 147) | 23.32 (242.31) (n = 422) |
| Private care | 15.10 (55.37) (n = 137) | 9.35 (45.61) (n = 138) | 20.48 (81.06) (n = 147) | 15.10 (62.98) (n = 422) |
| OTC medication | 0.31 (1.95) (n = 137) | 0.72 (3.62) (n = 138) | 0.98 (3.90) (n = 147) | 0.68 (3.29) (n = 422) |
| Total cost | 987.37 (2810.21) (n = 137) | 802.45 (2048.27) (n = 138) | 679.15 (1144.54) (n = 147) | 819.53 (2094.47) (n = 422) |
| Cost differenceb | 308.22 (n = 137) | 123.30 (n = 138) | – (n = 147) | |
| Study | Country | Types of cost included (price year) | Outcome | Study design (follow-up) | Results | Patient population | Intervention | Notes and time horizon |
|---|---|---|---|---|---|---|---|---|
| Fritzell et al. 2011140 | Sweden | Hospital visits plus rehabilitation, primary care visits, pharmaceuticals, support from family or relatives, the use of services from the community including transportation and work absenteeism (2003) | QALYs (EQ-5D instrument) | RCT (24 months) |
Cost/QALY using kyphoplasty vs. standard medical treatment = SEK 884,682 Sweden willing to pay a maximum of SEK 600,000, for 1 QALY gained QALY: 0.085 gained with BPK |
67 patients. Aged > 21 years and suffering from severe thoracic and/or low back pain owing to an acute or subacute OVF | Balloon kyphoplasty vs. standard medical treatment | Swedish patients from FREE trial141 |
| Svedbom et al. 2013142 | UK | Procedure costs, GP visit cost, referral costs, analgesic costs, fracture prevention medication cost and hospitalisation costs (2009) | QALYs (FREE141 and VERTOS II143 trials using the EQ-5D instrument) | Markov simulation model using data from two RCTs (FREE141 and VERTOS II143) (24 months) |
Compared with cost/QALY of kyphoplasty vs. standard medical treatment, incremental cost of £2706 (Great British pounds) (kyphoplasty) Cost/QALY of kyphoplasty vs. vertebroplasty was £15,982 |
Women only. Analysis conducted on hypothetical base patient (70-year-old female). Used data from FREE141 and VERTOS II143 trials |
Balloon kyphoplasty vs. standard medical treatment Kyphoplasty vs. percutaneous vertebroplasty |
Lifetime time horizon The base-case ICERs of balloon kyphoplasty compared with standard medical management and vertebroplasty of £2706 and £15,982 (Great British pounds), respectively, fall below the WTP threshold range of £20,000–30,000 (Great British pounds) per QALY gained |
| Ström et al. 2010141 | UK | Procedure costs, hospitalisation cost, cost of imaging and cost of osteoporosis medication (2007/8) | QALY gains (FREE141 trial using the EQ-5D) and cost/QALY gains | Markov cost-effectiveness model using data from FREE141 trial (12 months) | QALY gains of 0.17 and cost/QALY gains at £8800 | 300 patients. Analysis on base-case patient: 70-year-old male/female with osteoporosis. At least one painful vertebral fracture that had caused oedema assessed by magnetic resonance imaging and ≥ 15% loss of height | Balloon kyphoplasty vs. standard medical treatment |
Assumed that QoL benefits found at 12 months linearly approached zero during another 2 years Willingness to pay for a QALY in the UK lies within the range of £20,000–30,000/QALY Time horizon: not reported |
| Stevenson et al. 201437 | UK (assessment group) | Cost of equipment, hospitalisation and costs of further fractures | EQ-5D | Markov model |
£4480 vertebroplasty vs. non invasive (Johnson & Johnson data) Kyphoplasty vs. non-invasive: dominated £2057 vertebroplasty vs. non-invasive (Medtronic data) £2508 vertebroplasty vs. kyphoplasty (Medtronic data) |
Janssen Pharmaceutica (Beerse, Belgium), Medtronic, and assessment group. 70-year-old, T-score of –3 | Vertebroplasty vs. kyphoplasty vs. standard medical treatment | Time horizon is lifetime; maximum of 101 years |
| Stevenson et al. 201437 | UK (Johnson & Johnson, New Brunswick, NJ, USA) | Hospital and procedure costs only (2009–10) | QALYs gained (EQ-5D) | Treatment state model (1 year) |
£4392 per QALY gained (vertebroplasty vs. non-invasive) £14,643 per QALY gained (kyphoplasty vs. non-invasive) – dominated |
All patients with osteoporotic VCFs refractory to conservative management as the base case. Fractures ≤ 3 months old | Vertebroplasty vs. kyphoplasty vs. standard medical treatment | Time horizon is 1 year |
| Stevenson et al. 201437 | UK (Medtronic, Dublin, Ireland) | Hospital, procedure costs (2009–11) | QALYs (EQ-5D) | Markov model (12 months), data from FREE trial141 and VERTOS II trial143 |
£2053 per QALY gained (non-invasive vs. vertebroplasty) £2510 per QALY gained (kyphoplasty vs. vertebroplasty) |
At the base case, it was assumed that patients were 70-year-olds with a T-score of –3.0 SD, inpatient | Vertebroplasty vs. kyphoplasty vs. standard medical treatment | Lifetime |
| Masala et al. 2008144 | Italy | Cost of drugs, procedure costs, outpatient appointment cost and physical therapy. Price year not reported (study 2004–5) | ADL, ambulation and VAS | Retrospective observational study (12 months) | Average cost per patient per reduction of 1 point on the VAS, ambulation or ADL scale. Standard medical therapy cost at 12 months (VAS, ambulation, ADL):
|
179 patients with single OVF. All patients had 2 weeks of analgesic therapy, those with refractory pain were given the option of PVT or CMT. CMT = 95 and PVT = 58 | Vertebroplasty vs. standard medical treatment |
Authors did not report ICERs but rather average cost-effectiveness No significant differences in cost-effectiveness were found between the two groups at 12 months CMT involved physical therapy, analgesia and back brace |
| Klazen et al. 2010143 | The Netherlands and Belgium | Medication cost, GP costs, hospital costs, physiotherapy cost and imaging cost. Price year not given | QALYs gained | Open-label randomised trial (1 year) | Vertebroplasty vs. conservative treatment was €22,685 per QALY gained | 202 patients aged ≥ 50 years had vertebral compression fractures on spine radiograph (minimum 15% height loss; level of fracture at Th5 or lower; bone oedema on MRI), with back pain for ≤ 6 weeks, and a VAS score of ≥ 5 | Vertebroplasty vs. conservative | 1 year. At baseline, a significantly lower EQ-5D score was recorded in the vertebroplasty group than in the conservative treatment group. This was was adjusted for. Cost-effectiveness only applicable to the Netherlands |
List of abbreviations
- 6MWT
- 6-minute walk test
- ADL
- activities of daily living
- BMD
- bone mineral density
- BMI
- body mass index
- CACE
- complier-average causal effect
- CCA
- complete-case analysis
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DEXA
- dual-energy X-ray absorptiometry
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FRiSCy
- Fracture Reduction in South Central Policy
- FRT
- functional reach test
- GP
- general practitioner
- HEP
- home exercise programme
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IPA
- interpretative phenomenological analysis
- ITT
- intention to treat
- L
- lumbar vertebral level
- MCID
- minimally clinically important difference
- MI
- multiple imputation
- NIC
- net ingredient cost
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OQLQ
- Osteoporosis Quality of Life Questionnaire
- OTC
- over the counter
- OVF
- osteoporotic vertebral fracture
- PASE
- Physical Activity Scale for the Elderly
- PROVE
- Physiotherapy Rehabilitation for Osteoporotic VErtebral Fracture
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUALEFFO-41
- Quality of Life Questionnaire of the European Foundation for Osteoporosis – 41 items
- RCT
- randomised controlled trial
- ROM
- range of movement
- ROS
- Royal Osteoporosis Society
- RPE
- rating of perceived exertion
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SEK
- Swedish krona
- SF-36
- Short Form questionnaire-36 items
- SPPB
- Short Physical Performance Battery
- SSPT
- single session of physiotherapy
- T
- thoracic vertebral level
- TLS
- timed loaded standing
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TTO
- time trade-off
- TUG
- timed up and go
- VAS
- visual analogue scale
- VERTOS II
- Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures
