Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/144/50. The contractual start date was in January 2012. The draft report began editorial review in April 2018 and was accepted for publication in February 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael King is a member of the Clinical Trial Units funded by the National Institute for Health Research and the Rapid Trials and Add-on Studies Board. Rumana Z Omar is a member of the Health Technology Assessment General Board. John Strang reports grants from Camurus (Lund, Sweden), Martindale Pharma (now Ethypharm UK, Woodburn Green, UK), Mundipharma (Mundipharma International Limited, Cambridge, UK) and Braeburn Pharma (Braeburn Inc., Plymouth Meeting, PA, USA), and other from Martindale Pharma and Braeburn Pharma outside the submitted work. In addition, John Strang has a patent Euro-Celtique SA issued and a patent pending with King’s College London.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Johnson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Cannabis is the most commonly used drug among people with psychosis, with rates of current use around the time of the onset of psychosis regularly recorded as between 34% and 50%,1–5 which are well above the use patterns in non-psychotic populations of the same age. Continued use following the onset of psychosis is associated with poorer individual outcomes and greater societal burdens. Hazards include delays in remission, suicidal behaviour, violence and homelessness. 1,5–11 In longitudinal studies in first-episode psychosis, cannabis use is associated with substantially higher relapse rates. 9 An Australian study reported a 51% relapse rate over 15 months’ follow-up among substance users (mostly of cannabis) compared with a 17% relapse rate among non-users;12 this was accompanied by a threefold difference in inpatient admission rates. Similarly, a Dutch study reported a 42% relapse rate among persistent cannabis users compared with a 17% relapse rate among those who had never used cannabis or stopped around the time of first onset. 6 A dose–response relationship between the severity of cannabis misuse and the time to relapse was also reported in this study. 6 The type of cannabis used may also be important. In a study of 410 first-episode psychosis patients in south London, users of skunk, a particularly potent form of cannabis, were approximately three times more likely to experience a future psychotic episode than non-users, with daily skunk use associated with a more than fivefold increase in risk. 13
There are substantial consequences for service use and costs, as well as for clinical course. Studies of comorbid substance misuse among people with established psychosis indicate that people who persist in problematic drug use are heavy users of acute mental health services, are more likely to engage in acts of violence than others with psychotic illness and are less likely to work, sometimes using disability benefits to sustain drug use. 5,12,14–16
Thus, if a reduction in cannabis use can be achieved very early in the course of a psychotic illness, this has the potential to improve the illness course, life chances and social recovery of young people who develop psychosis and to reduce the burden on carers, mental health services, criminal justice and welfare services and wider society over many years. This is the overall aim of the current study. Systematic reviews have found that the evidence on effective interventions for comorbid substance misuse in established psychosis is very limited, with interventions tending to have little effect. 17–19 Despite a promising pilot study,20 a large Medical Research Council-funded trial, the MIDAS (Motivational Interventions for Drugs & Alcohol misuse in Schizophrenia) study, showed no effect on primary or secondary outcomes from a relatively lengthy intervention (29 sessions over 9 months) consisting of motivational interviewing (MI) and cognitive–behavioural therapy (CBT). The difficulties of intervening effectively in established psychosis suggest that it may be fruitful to target an earlier stage of illness when, according to several recent studies, patterns of use are in a state of substantial flux. 21,22 Many people are ambivalent about persisting with cannabis use and have substantial motivation for change, although some who initially abstain soon return to use. 23 This contrasts with the very limited motivation for change found in established psychosis,24 so that early psychosis may well be a stage at which achieving change with a relatively brief intervention is more feasible. However, in a similar study25 to the MIDAS trial, a MI and CBT intervention was trialled for cannabis with Early Intervention in Psychosis (EIP) service users, also over 9 months (24 sessions), and again found no benefit of the intervention compared with treatment as usual (TAU).
The very limited benefits achieved from psychological interventions such as MI and CBT in comorbid substance misuse in psychosis have made us look elsewhere for an effective intervention. Contingency management (CM) is an approach that involves offering rewards contingent on engagement in substance use treatment and on evidence of abstinence. CM has now accumulated a robust evidence base in a variety of contexts, including smoking cessation,26,27 heavy drinking28 and drug misuse29–35 and, in a fairly recent trial of a 12-week CM intervention, it was found to be clinically effective and cost-effective in reducing stimulant misuse in a cohort of patients with severe mental illness. 36 Furthermore, the National Institute for Health and Care Excellence (NICE) has recommended that it be adopted in the UK. 37 However, with the exception of a small number of recent evaluative studies in Europe,38 the evidence base is drawn almost entirely from the USA. There is relatively little UK experience of using CM, with only a few evaluations of CM reported. Recent examples include the CONMAN trial, which provided an evidence base for CM in uptake of hepatitis B vaccines among opiate users,39 and the FIAT trial, which found incentives to be effective in reinforcing adherence to antipsychotic medication. 40 The NICE review of psychosocial interventions37 identified 14 trials of CM (all from the USA) that met the criteria for inclusion, which included cannabis use. A consistent finding of a benefit from CM was reported, with most studies using abstinence at 12 weeks as their outcome measure. Just one North American CM study has so far been reported among people with comorbid substance misuse and psychosis. 41 The substances included were cocaine, heroin and cannabis. This was unusual among treatment studies in this population in finding a positive effect. Bellack et al. 41 reported that CM, combined with a psychological intervention, resulted in more drug-free urines than an enhanced TAU intervention (Supportive Treatment for Addiction Recovery) and resulted in reduced hospitalisation and a better quality of life. However, only a small proportion of participants abused cannabis (7%), with 93% abusing cocaine or heroin. Sigmon et al. 42,43 performed two small feasibility studies using a within-subjects reversal design that also reported a beneficial effect from the intervention. We have found no other evidence of CM studies for cannabis use in a population with psychosis.
In the present study, we investigated the clinical effectiveness and cost-effectiveness of CM for reducing cannabis use among EIP service users. This was evaluated in terms of clinical service use, presence of psychotic symptoms, cannabis use and health economic measures. The primary outcome was whether or not CM improves time to relapse, measured as admission to acute mental health services, compared with recommended standard care. Our overall objectives were as follows:
-
To conduct an internal pilot study of a specific intervention based on CM for cannabis use in early psychosis, acquiring evidence regarding rates of recruitment and follow-up, as well as feasibility and acceptability of the intervention in an Early Intervention Service context.
-
If pilot trial criteria for recruitment and retention were met, to proceed with a full multicentre pragmatic randomised controlled trial (RCT), testing whether or not the intervention results in an increase in time to relapse compared with a control arm. Both the CM arm and the control arm were to receive an optimised form of EIP TAU for cannabis, involving delivery by care co-ordinators of a standardised psychoeducation package.
-
To test whether or not the intervention results in an increase in the time to relapse, a decrease in cannabis use, a decrease in positive psychotic symptoms, and an increase in participation in work or education when compared with the control arm.
-
To assess the cost-effectiveness of the intervention from a NHS perspective.
Chapter 2 Methods
Overall design
The CIRCLE (Contingency Intervention for Reduction of Cannabis in Early Psychosis) trial was a rater-blind, multicentre RCT with two arms. The CM arm received a 12-week CM intervention, as well as a manualised psychoeducation intervention delivered by clinical staff, which represents an optimised version of TAU offered by EIPs in the management of cannabis misuse. The control arm received TAU only. Assessments were performed at the time of consent, at 12 weeks following trial inclusion (at the end of the intervention period) and at 18 months following trial inclusion. The primary outcome was time to relapse, operationalised as an admission to an acute mental health service. A Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) diagram for the trial is shown in Table 1. An initial internal pilot was successfully completed to demonstrate the feasibility of recruitment and of delivering the interventions. Subsequently, the main phase of the trial was approved and received ethics approval in amendment version 4 (approved on 23 July 2013).
| Time point | Enrolment | Allocation | Study period | |||||
|---|---|---|---|---|---|---|---|---|
| Post allocation | Close-out | |||||||
| –t 1 | 0 | Week 1 | Week 2 | Week 3 | Week... | Week 12 | 18 months | |
| Enrolment | ||||||||
| Screened for eligibility | ✗ | |||||||
| Informed consent | ✗ | |||||||
| Allocation | ✗ | |||||||
| Interventions | ||||||||
| CM – CM arm only | ↔ | |||||||
| OTAU – CM and control arms | ✗ | ✗ | ✗ | |||||
| Assessments | ||||||||
| Demographics | ✗ | ✗ | ✗ | |||||
| EQ-5D | ✗ | ✗ | ✗ | |||||
| PANSS | ✗ | ✗ | ✗ | |||||
| SF-12 | ✗ | ✗ | ✗ | |||||
| SCID (Part E) | ✗ | ✗ | ✗ | |||||
| CSRI | ✗ | ✗ | ✗ | |||||
| TLFB | ✗ | ✗ | ✗ | |||||
| Urinalysis for cannabis (not part of CM intervention) | ✗ | ✗ | ✗ | |||||
| Acute admissions data recorded from patient notes | ✗ | |||||||
Setting
Participants were recruited to the trial via EIP services throughout the Midlands and the south-east of England. Sites were added to the trial until the recruitment target (n = 544) was achieved. A total of 70 teams from 23 NHS trusts took part in the trial, representing a range of geographical and demographic characteristics. Willingness to participate and proximity to trial centres drove site selection, with almost all sites that were approached agreeing to participate.
Participants
The following inclusion and exclusion criteria were used to identify participants.
Inclusion criteria
The cohort was EIP service users with recent cannabis use. Recent cannabis use was operationalised as having used cannabis at least once during 12 of the previous 24 weeks. Additional eligibility criteria included (1) being aged 18–36 years, (2) having stable accommodation (i.e. not street homeless or roofless), (3) speaking enough English to be able to understand fully and answer the assessment instruments and (4) being able to give informed consent to participate in the trial.
Early Intervention in Psychosis teams have been set up across England following the 2000 NHS Plan. 45 Standard criteria for EIP include developing symptoms of psychotic illness for the first time, with positive psychotic symptoms persisting for at least 1 week and being accompanied by evidence of significant risk and/or functional decline. Service users are typically discharged after 3 years on the caseload of an EIP team. At the start of the pilot study, EIP service users needed to be recruited within the first 2 years of entry to the EIP service. This was changed with substantial amendment 2 (dated 15 June 2012, approved on 5 July 2012) and in protocol version 3 (dated 15 December 2012) to include all EIP service users. This was very early on in the pilot phase of the trial and was an expansion of the eligibility criteria, and so all participants who were already recruited were still eligible for the trial. Second, following the pilot, the eligibility criteria were changed to explicitly exclude patients who required to receive drug testing as part of a community treatment order or probation. This was because of such conditions potentially biasing the results of the intervention or interfering with the way the intervention was delivered. In fact, we had not recruited such participants during the pilot study and, therefore, this change should not affect the trial results. This change was approved in substantial amendment 4 (dated 23 July 2013) and is contained in protocol version 5, dated 17 June 2013.
Exclusion criteria
Exclusion criteria included (1) those who failed EIP service inclusion criteria, (2) those currently engaged in treatment for cannabis use with another agency, (3) those currently compulsorily detained in hospital or prison and (4) those on probation or a community treatment order requiring drug testing for cannabis.
Measures and research data collection
Trial assessors
The trial research workers performed assessments of outcome. Primary outcome assessors were blinded to randomised allocation. Secondary outcome assessors were blinded at the 18-month assessment interview. Research staff were trained in the use of all measures by members of the CIRCLE trial team. Joint ratings with one another and with senior members of the team supervising them were used to establish reliability.
Obtaining informed consent
In the first instance, an EIP staff member obtained agreement from service users to be contacted by a member of the CIRCLE trial research team. If agreement was obtained, a researcher then met with the service user to provide a participant information sheet that was written in plain English and explained all aspects of the trial. The researcher also explained verbally all benefits of the trial and known risks, and service users’ understanding was checked. If the service user was happy to discuss such details with the researcher, eligibility for the trial was also considered. However, eligibility was confirmed for all service users at the baseline interview assessment following consent. The service user was then given at least 48 hours to consider participation prior to consent being taken. Once consent had been obtained, the baseline interview assessment was performed.
Primary outcome
The primary outcome was time to relapse. Admission to a psychiatric hospital, crisis resolution team or crisis house, or other acute mental health service intended as an equivalent to hospital was used as a proxy marker of relapse. The primary outcome was assessed at 18-month follow-up based on the electronic patient records. The dates of admission were recorded and participants were followed up until the end of the 18-month trial period unless they were lost to follow-up.
Assessment interviews
Participants received three assessment interviews: at the time of consent, at 12 weeks following consent and at 18 months following consent (a time at which a significant proportion of young persons with psychosis will have relapsed if they are going to do so). 46,47 Participants were given a £20 voucher to thank them for their time at the baseline assessment, and at the follow-up assessment participants received an extra £10 for the provision of a urine sample. At 18 months, the primary outcome data and some secondary outcome data were collected from electronic patient records.
Outcome measures at interview
At all assessment interviews, data were collected on the following measures:
-
Demographic and social information –
-
Demographic information included age, sex, ethnicity, education, living arrangements, employment and any state benefits currently being received. Details of the most recent diagnosis recorded in a participant’s patient records were also documented.
-
-
Cannabis use –
-
The timeline followback (TLFB)48 method was used to record self-reported cannabis use over the previous 6 months at baseline and 18 months, and for the previous 3 months at the 3-month follow-up assessment interview. The TLFB method is a retrospective, calendar-based measure of daily substance use, with good test–retest reliability demonstrated for cannabis. 49 This was used to establish eligibility in terms of cannabis use and extent of recent use.
-
The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (SCID), part E, was used to evaluate a participant’s history of alcohol and substance misuse disorders.
-
Specimens for urinalysis were obtained, with the threshold set at a level for detecting cannabis use in the previous 28 days (i.e. 50 ng/ml of cannabis metabolites).
-
-
Psychotic symptoms –
-
Service use and health economic analysis –
-
The Client Service Receipt Inventory (CSRI) developed originally by Beecham and Knapp52 was used to record clinical service use, medication use, receipt of state welfare and use of other state-funded services, including criminal justice services. Data were collected for the previous 6 months at baseline and 18 months, and for the preceding 3 months at the 3-month follow-up assessment interview. At 18 months, to minimise loss to follow-up, data were collected from patient records for a subset of the resources deemed most likely to contribute to higher costs and that could feasibly be collected. This was performed at the same time as data collection for the primary outcome.
-
The Short Form questionnaire-12 items (SF-12)53 and the EuroQol-5 Dimensions (EQ-5D)54 are the widely used measures of health status with good psychometric properties55,56 that were used to derive quality-adjusted life-years (QALYs).
-
Details of a participant’s referral to the EIP service, history of admission to acute mental health services and time spent on a community treatment order were recorded on the demographics form.
-
Secondary outcomes
Secondary outcomes included between-group differences at follow-up for:
-
positive symptom severity, measured by the total score on the PANSS50
-
social functioning, based on self-reports of engagement in work or study
-
number of days of cannabis use in the previous 12 weeks (for 12-week follow-up) or 6 months (for 18-month follow-up) based on the TLFB method
-
proportion of cannabis-free urines
-
number of admissions over 18 months’ follow-up
-
QALYs (SF-12 and EQ-5D)57 and service use (CSRI) were used in the cost-effectiveness analyses, as described in Health economic analyses. Service-utilisation data were augmented when possible from participants’ medical records at 18 months.
Trial processes and interventions
Group allocation
Following pre-trial assessments, consenting clients were randomised to a group with a 1 : 1 ratio, stratified on severity of cannabis use (1–3 uses per week, > 3 uses per week). A remote, impartial randomisation service managed the allocation to groups co-ordinated by Priment Clinical Trials Unit based at University College London.
Interventions
The optimised treatment-as-usual (OTAU) package was the context in which we assessed the impact of the CM intervention. The CM intervention involved the offer of voucher rewards for cannabis-free urines over 12 weeks to recent cannabis users with early psychosis. We first describe OTAU, which was delivered to both the CM arm and the control arm, and then the CM intervention to be received by the CM arm only.
Optimised treatment as usual (psychoeducation package)
Our accounts of the interventions include text reproduced from Johnson et al. 44 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
To be confident that we measured the effects of CM, a psychoeducation intervention was delivered to both the CM arm and the control arm with the aim of achieving currently agreed good practice across both the CM arm and the control arm. Guidelines on EIP care recommends that psychoeducation interventions for cannabis should be an important component of routine care, but consultations with EIP managers and staff suggest that this is not often realised in practice, and we did not find routinely used resources for delivering such interventions. Our aim was to create a standardised version of good-quality psychoeducation to be delivered by staff working in EIP teams who were recruiting to the trial. A training manual for delivering the package, and supporting materials, were provided by the research team to clinicians delivering TAU. The intervention was designed to be sufficiently highly structured for staff without high-level clinical qualifications, such as support workers or assistant psychologists, to be able to deliver it competently following brief training.
The TAU was designed to be an individually tailored psychoeducation approach to cannabis use for generic EIP clinicians, which applies general psychoeducation approaches used in first-episode psychosis. 58 The psychoeducation package was developed by the research team through an iterative process, with frequent input from service users. It drew on the psychoeducation package offered in the control arm of a previous Melbourne pilot study of psychological intervention for cannabis use, the Cannabis and Psychosis trial;59 however, it was a novel package developed for this trial. The package comprised six modules that were delivered via a standard personal computer or laptop. Full delivery of all six modules was intended to take approximately 3 hours, normally offered over six regularly programmed sessions of 30 minutes’ duration. The package included a Portable Document Format (PDF) package for clinicians to work through with their clients, which presents information regarding the effects of cannabis, and motivational materials and strategies for harm reduction or for abstaining from cannabis. The package also included video materials, short quizzes, audio files and further information and written records of the modules for the service user to keep. The primary goal of the materials was to deliver information to meet psychoeducation goals, not to act as a psychological intervention. The clinician’s main aim was harm minimisation, with an acknowledgement that, in a young person with psychosis, cannabis abstinence may be required to ensure that no harm is done. The content was based on MI principles, relapse prevention and harm reduction strategies.
The psychoeducation package presented current information on the potential advantages and disadvantages of cannabis use and of cannabis abstinence. To help the participant to make an informed decision about continued use, EIP staff discussed the positives and negatives of cannabis use by exploring its impact on seven areas: family, finance, activity/engagement in work or education, mental health, physical health, legality and social group/friendships. Finally, staff discussed setting goals regarding the young person’s future use of cannabis in the context of harm minimisation, as well as strategies for achieving their goals and avoiding relapse into patterns of cannabis use that compromise those goals.
Contingency management
The CM intervention offered financial incentives contingent on urinalysis results indicating cannabis abstinence. The intervention voucher schedule and rules were adapted from Budney et al. ,60,61 who offered a voucher-based CM intervention for the treatment of cannabis dependence in the general population. The intervention comprised 12 once-weekly urinalysis sessions and was delivered by clinicians supported by the CIRCLE trial research team. At each session, the participant was required to provide a urine sample.
In week 1 of the intervention, details of the intervention were explained to the participant and they were asked to sign an ‘abstinence contract’ indicating that they understood and accepted its rules, and agreed to abide by the test results. In the first week, participants received a £5 voucher for attending and providing a urine specimen, independent of the drug test results, which provided a ‘baseline’ result. From week 2 to week 12, participants were rewarded if their urinalysis results demonstrated abstinence from cannabis use. In the pilot, the value of vouchers increased by £2 each week, contingent on consecutive negative specimens. A bonus voucher to the value of £10 was earned each time two consecutive specimens suggesting abstinence were provided. However, based on feedback from clinicians and participants gained during the qualitative substudy, changes were made to this reward schedule between the pilot and the main trial to simplify it. In the main trial, from week 2 to week 12, assuming participants provided negative samples, the voucher value rose every 2 weeks by £5. No bonus voucher was offered for passing consecutive weeks. In both the pilot and the main trial, participants who abstained from cannabis use for the full duration of the intervention earned £240 (Table 2). This change to the reward schedule made it easier for clinicians to administer and easier for clinicians and participants to understand. Removing the bonus for two consecutive clean urines made it simpler for participants and clinicians to know what reward a participant should receive for passing. In addition, the new reward schedule meant clinicians needed to stock fewer denominations of vouchers. This change to the intervention was included in the trial protocol version 5 (dated 17 June 2013), approved in substantial amendment 4 (approved 23 July 2013).
| Number of negative urines | Reward (£) |
|---|---|
| 1. Attending session | 5 |
| 2. Negative urine | 10 |
| 3. Negative urine | 10 |
| 4. Negative urine | 15 |
| 5. Negative urine | 15 |
| 6. Negative urine | 20 |
| 7. Negative urine | 20 |
| 8. Negative urine | 25 |
| 9. Negative urine | 25 |
| 10. Negative urine | 30 |
| 11. Negative urine | 30 |
| 12. Negative urine | 35 |
Failure to attend intervention sessions, specimens suggesting cannabis use or failure to submit a scheduled specimen reset the value of vouchers back to the initial £5. If the participant attended the following week and provided a negative sample, they were rewarded with £10. In the subsequent week, if the participant provided a second consecutive negative sample, the voucher values resumed from the highest previous level of reward. Vouchers were for major supermarkets, including Tesco (Welwyn Garden City, UK), Sainsbury’s (London, UK) and Asda (Leeds, UK), depending on a participant’s preference. They could be swapped in store for other shop vouchers, if preferred.
Urinalysis was performed using a small bench-top analyser (Kaiwood CHR-110; Kaiwood Technology Co. Ltd, Hsin-Shi, Taiwan) capable of providing rapid test results of drug misuse via the concentration of the drug in a urine sample. To perform the analysis, the clinician pipetted a fixed amount of urine into a buffer solution tube to give a 7 : 1 serial dilution. This allowed a standard 50 ng/ml marijuana test cassette placed in the analyser to provide a urine cannabis concentration reading of between 0 ng/ml and 350 ng/ml. Guidelines were provided to all staff delivering the intervention to allow interpretation of the test results. The guidelines set out upper and lower thresholds for urinary tetrahydrocannabinol (THC) based on recommendations from the suppliers of the urinalysis equipment, SureScreen Diagnostics (Derby, UK). Results greater than the upper threshold (350 ng/ml) clearly indicated use within the last week, and so participants with these results were not rewarded. Results below the lower threshold indicated urinary THC concentration of < 50 ng/ml, the accepted standard for detecting urinary THC; participants with these results were rewarded. Within these thresholds, urinary THC was expected to fall each week until it reached the lower THC threshold, which should be reached within 1 month. A participant with a drop in urinary THC would receive the reward. A participant with a result similar to, or higher than, the previous week would fail. A temperature strip on the side of the specimen cup allowed staff to check whether or not the sample had been tampered with.
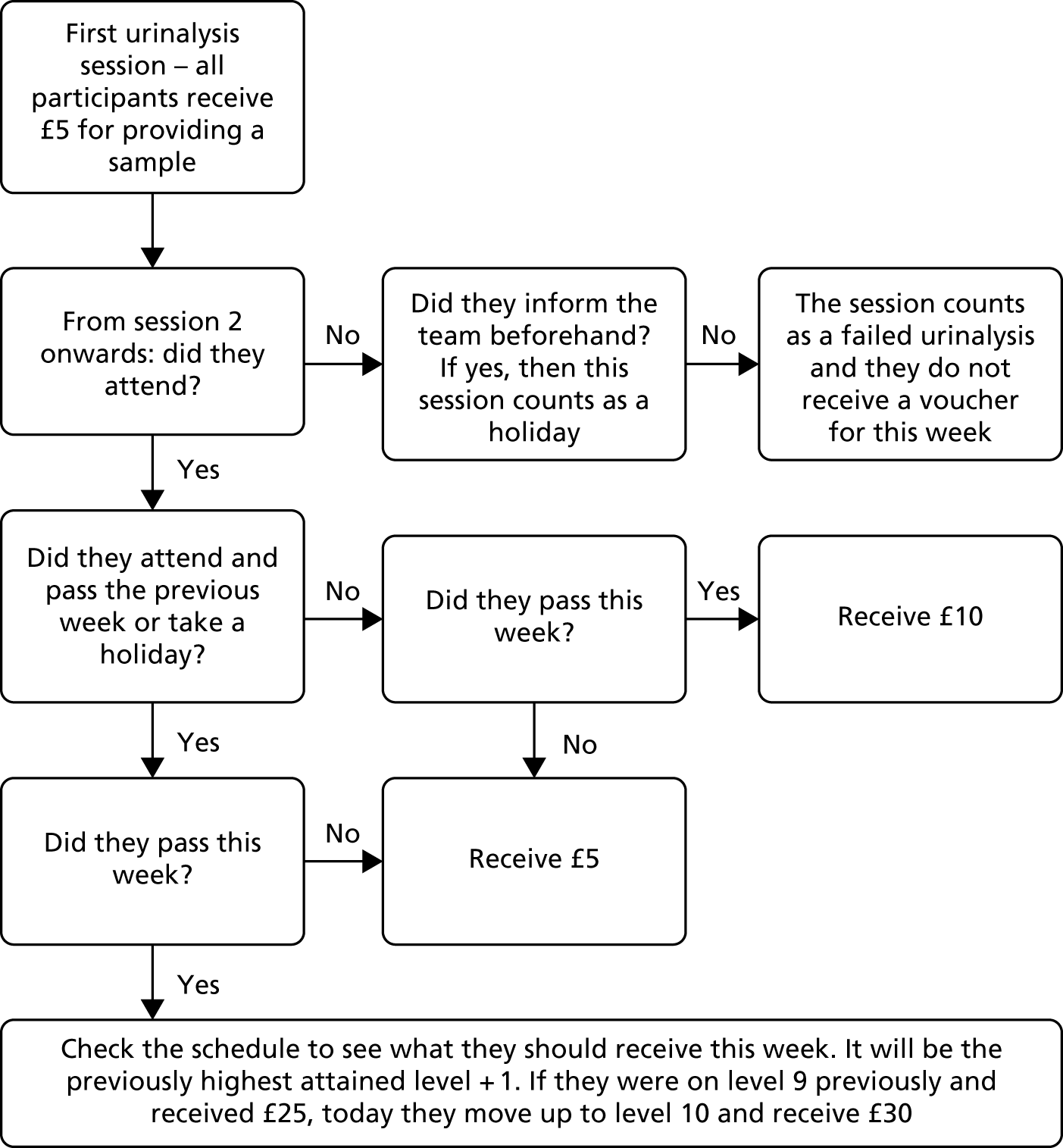
Participants could arrange in advance to miss scheduled sessions (‘holiday week’) and still receive the reward for that week if they had a valid commitment that prevented them from attending. They could do this on a maximum of two occasions for 1 week only each time. They were still expected to show evidence of abstinence at the following session to receive a reward for the holiday week. If a participant missed the following week or provided a positive sample, no financial incentive was received for the holiday week. Holiday weeks normally needed to be arranged with the clinician performing the intervention no later than at the previous scheduled appointment. However, a dispensation of this rule was offered for unforeseeable events, such as illness, transport problems or being offered a work shift. The intervention was suspended for a maximum of 1 month if a participant relapsed or otherwise lost capacity to consent to participate. If capacity was not regained in 1 month, the intervention did not continue. If a participant failed to attend on multiple consecutive weeks or if contact was lost with the participant entirely, each missed week was counted as a failure to attend. Figure 1 is a decision flow chart for the CM intervention.
FIGURE 1.
Decision flow chart for the CM intervention.
Selection and training of staff
Clinicians delivered the CM and TAU interventions, supported by the research team. Training was given to all staff delivering the interventions by members of the research team over a period of half a day face to face, together with the provision of training material and manuals.
Ethics approval
Ethics approval for the trial was received on 16 March 2012 from the National Research Ethics Service Committee London – South East (Research Ethics Committee reference number 11/LO/1939). Written informed consent was obtained from all participants in the trial. The original consent forms are stored at the collaborating universities (University College London, King’s College London, the University of Sussex, and the University of Warwick) and copies were kept in the patients’ clinical notes. Data were also stored at the collaborating universities.
Sample size
The proposed sample size for the trial was 544 participants. This figure was based on data suggesting a usual relapse rate for cannabis users of around 50% over the trial time frame. 6,12 A 15% decrease in this relapse rate as a result of the intervention was agreed to be of substantial clinical benefit. Using a power of 90% and a significance level of 5%, a total sample size of 460 participants was estimated to be required. This sample size was based on an analysis of time to relapse and would allow detection of a 37% decrease in the hazard of relapse [hazard ratio (HR) of 0.63] in the CM arm using a Cox proportional hazards model. This sample size was calculated using Stata® version 11 (StataCorp LP, College Station, TX, USA). The sample size was inflated by a factor of 1.06, assuming that each person delivering the intervention sees an average of four service user participants in the trial, and an intraclass correlation coefficient (ICC) of 0.02 for clinician clustering, which gives a total sample size of 488 participants. Finally, the sample size was inflated by 10% to account for attrition for the primary outcome, giving a total sample size of 544 participants.
Statistical methods
All analyses were carried out by the treatment allocated, using all available data (complete case).
The continuous variables were summarised using mean [standard deviation (SD)] or median [interquartile range (IQR)]. Categorical variables were presented as frequencies and percentages.
Clustering by psychoeducation clinician was accounted for in all modelling. Those participants who declined psychoeducation or for whom the psychoeducation clinician was not known were assigned to a cluster of one (i.e. just that participant). Unadjusted ICCs were calculated for all outcomes except the primary outcome because estimation of the ICC for a survival outcome is not straightforward. 62 We estimated approximate ICCs using log-transformed observed event times and also by using the censoring indicator and treating the outcome as binary. We also obtained an approximate estimate of the correlation by fitting marginal generalised estimating equation models.
Logistic regression was used to determine whether or not those who had no secondary outcome data available at 3 months, 18 months or both had different baseline characteristics to those who remained in the trial. This was repeated for each outcome to determine baseline predictors of missingness.
Kaplan–Meier survival curves by randomised groups were used to examine the primary outcome (time to relapse) descriptively. Cox proportional hazards modelling was used to compare the CM arm and the control arm, adjusting for severity of cannabis use (the stratification variable; 1–3 times per week vs. ≥ 4 times per week) at baseline and whether or not the participant was part of the pilot trial. We carried out a number of supportive analyses for the primary outcome:
-
including significant baseline predictors of missingness
-
excluding those participants who have no secondary outcome data (for 12 weeks and 18 months separately) because those who drop out or die may be different from those who remain in the trial and/or survive
-
including those in the main trial only as some minor changes were made to the protocol at the end of the pilot before the main trial commenced, which might mean that the participants behave differently to those in the pilot trial
-
controlling additionally for the number of psychoeducation sessions attended (which was offered in both arms of the trial)
-
controlling additionally for the number of admissions in the 6 months prior to baseline
-
controlling for the same factors as the primary analysis, but using centre as the clustering variable instead of therapist.
Secondary outcomes were analysed separately at 3 and 18 months. Models were adjusted for severity of cannabis use at baseline and whether or not the participant was part of the pilot trial. For the dichotomous outcomes (cannabis-positive urine, engaged in work or study), logistic regression was used. The continuous outcomes (positive and negative symptoms from PANSS), had non-normal residuals and were, therefore, log-transformed and analysed using linear regression models. For count outcomes (number of cannabis days and number of admissions over follow-up), zero inflated Poisson was applied regression as there were excess zeros in all of these outcomes, except number of cannabis days at 12 weeks which utilised Poisson regression. As with other models, these controlled for severity of cannabis use at baseline and whether or not participants were part of the pilot trial.
All secondary outcomes were also analysed after adjusting for predictors of missingness. An additional supportive analysis compared the number of admissions between trial groups. The analysis included participants who were discharged from psychiatric services within the 18-month follow-up and assumed participants did not have any admissions beyond 18 months.
Robust standard errors63 were used in all regression models to account for clustering of participants by psychoeducation clinician.
Results from all supportive and secondary analyses are presented as estimates and 95% confidence intervals (CIs), as specified in the statistical analysis plan. No p-values are presented to avoid the problem of multiple significance testing. All analyses were carried out using Stata® version 14 (StataCorp LP, College Station, TX, USA).
Health economic analyses
The objective of the cost-effectiveness analysis was to establish the relative cost-effectiveness of CM versus OTAU at 18 months. The primary outcome was QALYs, calculated using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), health profiles combined with health-state preference values from the UK general population. The resultant mean quality-of-life utility scores of the two treatment groups were compared at 18 months using unpaired t-tests. QALYs were calculated using the area under the curve method and compared between groups, adjusting for baseline EQ-5D utility scores.
Costs were calculated based on resource use gathered from an adapted version of the CSRI. The primary analysis will take a NHS and Personal Social Services perspective.
Costs of the CM intervention and OTAU were calculated using the salaries of staff delivering the intervention, plus employer oncosts, overhead costs, the cost of providers of supervision and the cost of any equipment or consumables. A ratio of direct, face-to-face time to indirect, non-face-to-face time was applied.
Total costs at 18 months were calculated by combining resource use with unit costs at 2016 prices. Unit costs were derived from the Personal Social Services Research Unit64 and from NHS reference costs. 65 Medications were costed using NHS prescription cost analysis data. Costs and QALYs were discounted at 3.5% per year.
Costs were compared, adjusting for baseline differences. Patient-level costs and quality-of-life data were bootstrapped with replacement (n = 10,000) to populate incremental cost-effectiveness (ICER) planes, to estimate median cost-effectiveness and (pseudo) 95% CIs. The probabilities of the intervention being cost-effective at different levels of willingness to pay for health benefits were shown by generating cost-effectiveness acceptability curves. Productivity losses were calculated using a human capital approach,66 in which each day off work was valued using information on average salaries from the Office for National Statistics (2018). 67
No subgroup health economics analyses were planned. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement was completed.
Additional health economics analyses:
-
Cost-effectiveness at 3 months was reported.
-
Results from a broader perspective, including costs from criminal justice and cost of unpaid lost productivity because of illness, were reported.
-
Cost-effectiveness using alternative outcomes were reported –
-
QALYs calculated using the SF-12 data
-
time to relapse (to 18 months)
-
number of cannabis-negative urine samples
-
days of reported cannabis abstinence.
-
-
Missing data in baseline resource use, quality of life and other covariates were handled with multiple imputation using chained equations, using guidelines from Gabrio et al. 68 In line with primary outcomes analysis, the base case was a complete-case analysis.
Qualitative substudy
Following the pilot phase of the trial, a qualitative substudy was performed to inform the main trial by exploring the acceptability and feasibility of the trial design and interventions from the perspectives of both participants and clinicians. The full text of the trial design and results can be found in Appendix 3 and was included in version 1 of the trial protocol (24 October 2011). Following the main trial, further qualitative interviews were performed that were included in protocol version 7 (16 August 2016) and approved in substantial amendment 6 (approval received 22 November 2016). The results of that second qualitative data collection are still forthcoming. In the post-pilot qualitative data collection, data were collected from three groups: one-to-one interviews with 11 participants in the intervention arm, five focus groups with clinicians in EIP teams participating in the CIRCLE trial and one carer of a participant in the intervention arm. Results showed that the intervention had good acceptability among all groups, and all three groups viewed it positively. Participants described it as helpful and beneficial, and said that they would recommend it to others. Recommended changes to trial procedures that emerged from the focus groups with EIP staff included (1) simplifying the CM reward schedule; (2) adding a temperature strip to sample cups to prevent participants submitting adulterated urine samples; and (3) having a dedicated EIP team member to deliver the intervention rather than care co-ordinators, such as a support worker/assistant psychologist. As previously mentioned, these recommendations were adopted ahead of the main trial.
Chapter 3 Results
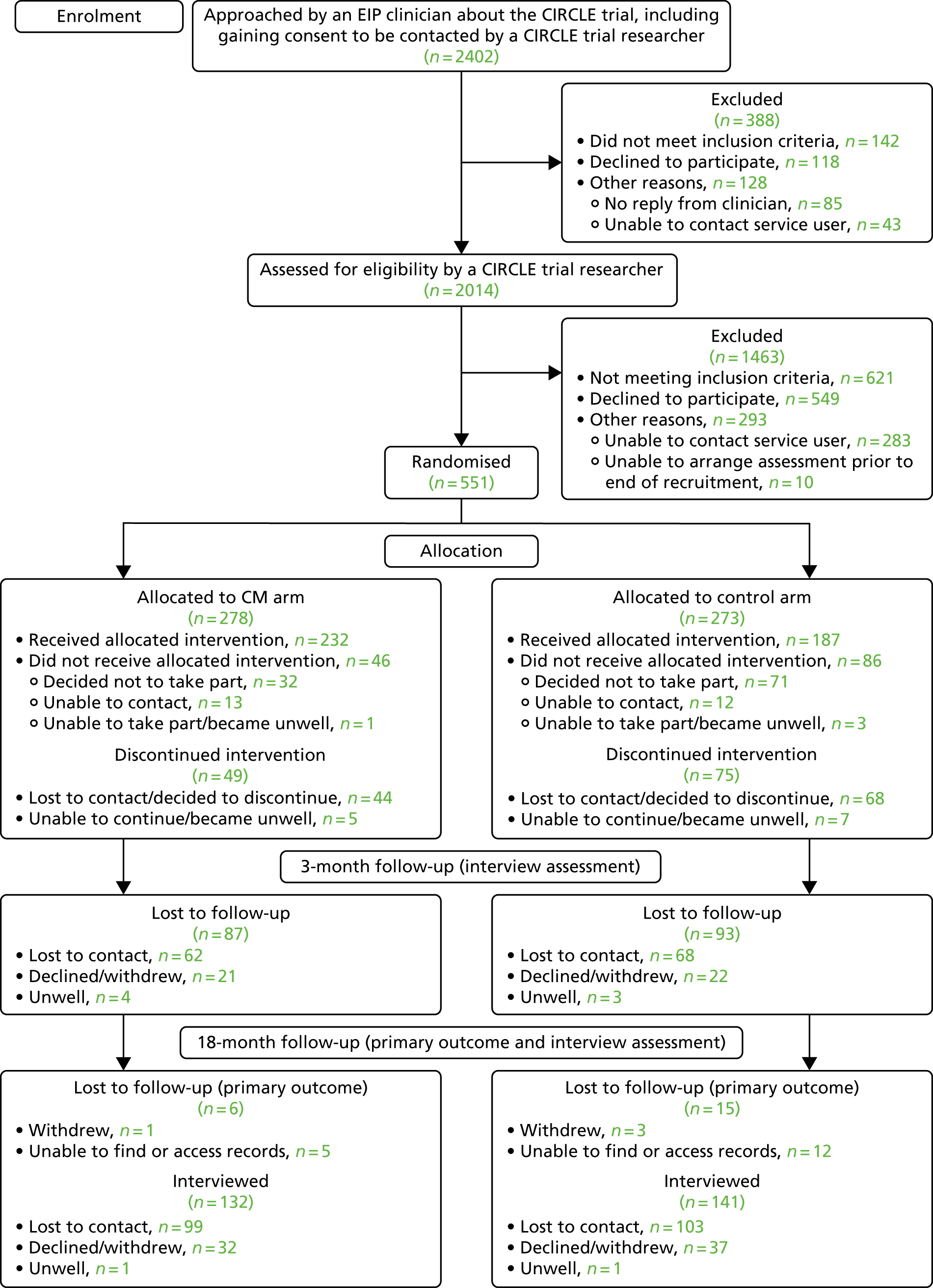
A Consolidated Standards of Reporting Trials (CONSORT)69 flow diagram (Figure 2) shows the number of service users randomised to each arm of the trial and the numbers who have follow-up data available.
FIGURE 2.
The CONSORT flow diagram.
Demographics
The EIP clinicians reported that they had approached 2402 service users to inform them of the trial and seek consent to being contacted by a CIRCLE trial researcher. Following this, initial meetings were held with 2014 service users by the CIRCLE trial researchers, during which the trial was fully explained to potential participants. A total of 551 service users gave informed consent to participate and were then assessed and randomised into the trial.
Table 3 presents baseline characteristics of study participants. More than 85% of participants were male in both randomised arms, with a mean age of aound 25 years (SD 4 years) in both arms. More than half of participants were white and one-quarter were black. A total of 43% of each arm lived with their parents; only 5% of control and 6% of CM arm participants were married or cohabiting. Around one-third of participants were diagnosed with schizophrenia or schizoaffective disorder and half had other types of psychosis. In many participating services, clinicians had significant reservations about making use of schizophrenia as a diagnostic category. One-quarter of participants were engaged in ‘any work or study’ but a large majority had held open-market employment at some point, understood as work that the employee has applied for through an open job market.
| Characteristic | Randomised group | |||
|---|---|---|---|---|
| Control | CM | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Male | 240/273 | 88 | 238/278 | 86 |
| Age (years) | 25 | (4) | 24 | (4) |
| Ethnicity | ||||
| White British, European or other | 144/273 | 53 | 148/277 | 53 |
| Black Caribbean, African or British | 62/273 | 23 | 65/277 | 23 |
| Asian | 30/273 | 11 | 29/277 | 10 |
| Other | 37/273 | 14 | 35/277 | 13 |
| Marital status | ||||
| Single | 253/273 | 93 | 259/278 | 93 |
| Married or cohabiting | 14/273 | 5 | 17/278 | 6 |
| Other | 6/273 | 2 | 2/278 | 1 |
| Educational attainment | ||||
| No qualifications | 48/273 | 18 | 43/277 | 16 |
| GCSE or equivalent | 104/273 | 38 | 133/277 | 48 |
| A level or equivalent | 67/273 | 25 | 58/277 | 21 |
| Post 18 education (including HND, trade, degree) | 54/273 | 20 | 43/277 | 16 |
| Living arrangements | ||||
| Alone | 73/273 | 27 | 73/278 | 26 |
| With parents | 117/273 | 43 | 119/278 | 43 |
| With other adults (only) | 70/273 | 26 | 69/278 | 25 |
| Other | 13/273 | 5 | 17/278 | 6 |
| Housing | ||||
| Independent permanent accommodation | 179/272 | 66 | 184/276 | 67 |
| Independent temporary accommodation | 29/272 | 11 | 27/276 | 10 |
| Supported accommodation | 44/272 | 16 | 44/276 | 16 |
| Other | 20/272 | 7 | 21/276 | 8 |
| Work or study | ||||
| Ever had open-market employment | 223/273 | 82 | 240/278 | 86 |
| Ever kept open-market employment for 1 year | 114/269 | 42 | 128/277 | 46 |
| Had open-market job since first contact with services | 67/272 | 25 | 83/278 | 30 |
| Current paid activity | 39/272 | 14 | 51/278 | 18 |
| Time since last open-market job (years), median (IQR) | 1 | (0.4–3) | 1 | (0.2–3) |
| Open-market full-time employment | 21/273 | 8 | 25/278 | 9 |
| Open-market part-time employment | 17/273 | 6 | 23/278 | 8 |
| Permitted work | 1/273 | 0.4 | 1/278 | 0.4 |
| Voluntary/unpaid work | 6/273 | 2 | 12/278 | 4 |
| Study or training | 38/273 | 14 | 30/278 | 11 |
| Full-time caring | 0/273 | 0 | 2/273 | 1 |
| Unemployed | 136/233 | 58 | 141/227 | 62 |
| Exempt because of disability | 70/271 | 26 | 65/278 | 23 |
| Any work or study | 67/273 | 25 | 73/278 | 26 |
| Benefits | ||||
| Income support | 28/272 | 10 | 30/276 | 11 |
| Incapacity benefit | 36/271 | 13 | 28/276 | 10 |
| DLA care component | 40/271 | 15 | 48/276 | 17 |
| DLA component | 14/271 | 5 | 19/276 | 7 |
| Personal independence payment | 15/270 | 6 | 18/276 | 7 |
| DLA or PIP | 60/272 | 22 | 74/276 | 27 |
| Severe disablement allowance | 3/270 | 1 | 1/275 | 0.4 |
| Council tax benefit | 22/271 | 8 | 26/276 | 9 |
| Housing benefit | 52/271 | 19 | 58/276 | 21 |
| Jobseekers’ allowance | 23/271 | 8 | 17/276 | 6 |
| Working tax credits | 4/271 | 1 | 2/276 | 1 |
| Statutory sick pay | 3/271 | 1 | 0/276 | 0 |
| Employment and support allowance | 102/272 | 38 | 125/275 | 45 |
| Other benefits | 18/271 | 7 | 11/275 | 4 |
| Diagnosis | ||||
| Schizophrenia or schizoaffective disorder | 80/256 | 31 | 90/268 | 34 |
| Bipolar affective disorder | 26/256 | 10 | 19/268 | 7 |
| Depression with psychotic symptoms | 11/256 | 4 | 5/268 | 2 |
| Other psychosis | 139/256 | 54 | 154/268 | 57 |
| Alcohol abuse | 101/217 | 47 | 102/225 | 45 |
| Alcohol dependence | 67/187 | 36 | 60/189 | 32 |
| Cannabis use (previous 6 months) | ||||
| 1–3 times per week | 77/273 | 28 | 78/278 | 28 |
| > 3 times per week | 196/273 | 72 | 200/278 | 72 |
| Drugs taken (lifetime) | ||||
| Sedatives, hypnotics, anxiolytics | 39/273 | 14 | 26/277 | 9 |
| Stimulants | 69/273 | 25 | 72/277 | 26 |
| Opioids | 47/273 | 17 | 29/277 | 10 |
| Cocaine | 128/273 | 47 | 145/277 | 52 |
| Hallucinogens/PCP | 117/273 | 43 | 122/277 | 44 |
| Legal highs | 42/271 | 15 | 32/275 | 12 |
| Other | 56/273 | 21 | 40/277 | 14 |
| PANSS score, median (IQR) | ||||
| Positive symptoms | 12 | (9–17) | 13 | (9, 16) |
| Negative symptoms | 14 | (11, 19) | 14 | (10–19) |
| SF-12 score | ||||
| Physical | 52 | (10) | 52 | (10) |
| Mental | 37 | (16) | 38 | (14) |
| Number of, median (IQR) | ||||
| Days using cannabis in the previous 6 months | 108 | (67–156) | 114 | (70–162) |
| Admissions in the previous 6 months | 0 | (0–1) | 0 | (0–1) |
Most participants were using cannabis more than three times per week. The PANSS positive symptoms median scores were 12 (IQR 9–17) in the psychoeducation group and 13 (IQR 9–16) in the CM group. The PANSS negative symptoms median scores were 14 in both groups (see Table 5). The rates of alcohol misuse or dependence and of reports of using substances other than cannabis were high (e.g. 47% of the control arm and 52% of the CM arm members reported using cocaine; 36% of the control arm members and 32% of the CM arm members met the criteria for alcohol dependence).
At 3 months, demographics and outcome measures were compared alongside delivery of the intervention. At this point, among the sample participating in the interview, almost one-third of participants were engaged in work or study. A total of 72% in the psychoeducation group and 70% in the CM group had cannabis-positive urine, compared with 80% and 79% at baseline, respectively. The median PANSS positive symptom score was 11 (IQR 8–16) in the control arm and 10 (IQR 8–14) in the CM arm. For PANSS negative symptoms, the median scores were 14 (IQR 11–18) and 12 (IQR 9–17) for the psychoeducation and CM groups, respectively. The number of days using cannabis in the previous 3 months was slightly lower in the CM group than in the psychoeducation group. The median number of psychoeducation sessions attended was higher in the CM group than in the psychoeducation group [6 sessions (IQR 1–6) and 4 sessions (IQR 0–6), respectively] (Table 4). Participants in the CM and control arms attended a median of six sessions and four sessions, respectively, of the TAU psychoeducation intervention. However, 86 participants in the control arm declined the intervention or attended no sessions compared with 63 participants in the CM arm. A mean of £64 in voucher rewards was obtained in the CM treatment and the median number of CM sessions attended was 9 (IQR 3–12 sessions). A total of 46 participants declined the CM intervention, despite their initial consent to randomisation, or did not attend any sessions.
| Characteristic | Randomised group | |||
|---|---|---|---|---|
| Control | CM | |||
| n/N | % | n/N | % | |
| Educational attainment | ||||
| No qualifications | 28/182 | 15 | 23/189 | 12 |
| GCSE or equivalent | 63/182 | 35 | 84/189 | 44 |
| A level or equivalent | 40/182 | 22 | 38/189 | 20 |
| Post-18 education (including HND, trade, degree) | 51/182 | 28 | 44/189 | 23 |
| Living arrangements | ||||
| Alone | 60/182 | 33 | 59/189 | 31 |
| With parents | 76/182 | 42 | 79/189 | 42 |
| With other adults (only) | 39/182 | 21 | 39/189 | 21 |
| Other | 7/182 | 4 | 12/189 | 6 |
| Housing | ||||
| Independent permanent accommodation | 125/182 | 69 | 128/189 | 68 |
| Independent temporary accommodation | 15/182 | 8 | 16/189 | 8 |
| Supported accommodation | 29/182 | 16 | 27/189 | 14 |
| Other | 13/182 | 7 | 18/189 | 10 |
| Work or study | ||||
| Ever had open-market employment | 154/183 | 84 | 174/189 | 92 |
| Ever kept open-market employment for 1 year | 84/182 | 46 | 100/187 | 53 |
| Had open-market job since first contact with services | 53/183 | 29 | 73/189 | 39 |
| Current paid activity | 34/183 | 19 | 37/189 | 20 |
| Time since last open-market job (years), median (IQR) | 1.0 | (0.3–3.0) | 1.0 | (0.3–3.0) |
| Open-market full-time employment | 14/183 | 8 | 15/189 | 8 |
| Open-market part-time employment | 20/183 | 11 | 20/189 | 11 |
| Sheltered work | 0/183 | 0 | 1/189 | 0.5 |
| Permitted work | 0/183 | 0 | 2/189 | 1 |
| Voluntary/unpaid work | 5/183 | 3 | 8/189 | 4 |
| Study or training | 31/183 | 17 | 25/189 | 13 |
| Full-time caring | 0/183 | 0 | 1/189 | 0.5 |
| Unemployed | 85/149 | 57 | 90/152 | 59 |
| Exempt because of disability | 52/183 | 28 | 57/189 | 30 |
| Any work or study | 58/183 | 32 | 58/189 | 31 |
| Alcohol abuse | 71/150 | 47 | 69/162 | 43 |
| Alcohol dependence | 51/131 | 39 | 50/148 | 34 |
| Drugs taken | ||||
| Sedatives, hypnotics, anxiolytics | 23/182 | 13 | 18/188 | 10 |
| Cannabis | 181/182 | 100 | 189/189 | 100 |
| Stimulants | 39/182 | 21 | 54/188 | 29 |
| Opioids | 27/182 | 15 | 30/188 | 16 |
| Cocaine | 88/182 | 48 | 98/188 | 52 |
| Hallucinogens/PCP | 79/182 | 43 | 81/188 | 43 |
| Legal highs | 30/181 | 17 | 31/188 | 16 |
| Other | 39/182 | 21 | 42/188 | 22 |
| Cannabis-positive urine | 122/170 | 72 | 128/184 | 70 |
| PANSS score, median (IQR) | ||||
| Positive symptoms | 11 | (8–16) | 10 | (8–14) |
| Negative symptoms | 14 | (10–18) | 12 | (9–17) |
| SF-12 score, median (IQR) | ||||
| Physical | 56 | (48–60) | 55 | (47–60) |
| Mental | 40 | (27–54) | 45 | (30–55) |
| Number of, median (IQR) | ||||
| Days using cannabis in the previous 3 months | 30 | (3–84) | 26 | (1–67) |
| Psychoeducation sessions attended | 4 | (0–6) | 6 | (1–6) |
At 18 months, 33% of the psychoeducation group were engaged in work or study compared with 29% in the CM group. A total of 61% of participants in the control arm and 57% of participants in the CM arm had cannabis-positive urine. The PANSS positive and negative median score and the median number of days using cannabis in the previous 6 months were similar between arms. One-third of participants in both arms were admitted to hospital or a crisis house, were seen by a crisis resolution team or attended an acute day treatment service (Table 5).
| Characteristic | Randomised group | |||
|---|---|---|---|---|
| Control | CM | |||
| n/N | % | n/N | % | |
| Educational attainment | ||||
| No qualifications | 21/134 | 16 | 22/143 | 15 |
| GCSE or equivalent | 41/134 | 31 | 57/143 | 40 |
| A level or equivalent | 33/134 | 25 | 31/143 | 22 |
| Post-18 education (including HND, trade, degree) | 39/134 | 29 | 33/143 | 23 |
| Living arrangements | ||||
| Alone | 49/134 | 37 | 50/143 | 35 |
| With parents | 57/134 | 43 | 55/143 | 38 |
| With other adults (only) | 21/134 | 16 | 25/143 | 17 |
| Other | 7/134 | 5 | 13/143 | 9 |
| Housing | ||||
| Independent permanent accommodation | 87/133 | 65 | 98/144 | 68 |
| Independent temporary accommodation | 13/133 | 10 | 11/144 | 8 |
| Supported accommodation | 21/133 | 16 | 24/144 | 17 |
| Other | 12/133 | 9 | 11/144 | 8 |
| Work or study | ||||
| Ever had open-market employment | 117/135 | 87 | 135/145 | 93 |
| Ever kept open-market employment for 1 year | 69/132 | 52 | 84/145 | 58 |
| Had open-market job since first contact with services | 54/135 | 40 | 63/144 | 44 |
| Current paid activity | 33/135 | 24 | 31/145 | 21 |
| Time since last open-market job (years) | 2.0 | (0.0–4.0) | 2.0 | (0.3–4.0) |
| Open-market full-time employment | 16/135 | 12 | 18/145 | 12 |
| Open-market part-time employment | 16/135 | 12 | 10/145 | 7 |
| Permitted work | 0/135 | 0 | 2/135 | 1 |
| Voluntary/unpaid work | 9/135 | 7 | 9/145 | 6 |
| Study or training | 21/135 | 16 | 16/145 | 11 |
| Full-time caring | 0/135 | 0 | 1/145 | 1 |
| Unemployed | 71/102 | 70 | 68/114 | 60 |
| Exempt because of disability | 33/135 | 24 | 55/145 | 38 |
| Any work or study | 45/135 | 33 | 42/145 | 29 |
| Alcohol abuse | 56/104 | 54 | 62/116 | 53 |
| Alcohol dependence | 41/84 | 49 | 42/81 | 52 |
| Drugs taken (lifetime) | ||||
| Sedatives, hypnotics, anxiolytics | 20/133 | 15 | 13/142 | 9 |
| Cannabis | 133/133 | 100 | 142/142 | 100 |
| Stimulants | 34/133 | 26 | 39/142 | 27 |
| Opioids | 22/133 | 17 | 23/142 | 16 |
| Cocaine | 64/133 | 48 | 77/142 | 54 |
| Hallucinogens/PCP | 59/133 | 44 | 69/142 | 49 |
| Legal highs | 10/130 | 8 | 12/141 | 9 |
| Other | 34/133 | 26 | 30/142 | 21 |
| Cannabis-positive urine | 76/124 | 61 | 77/136 | 57 |
| PANSS score, median (IQR) | ||||
| Positive symptoms | 10 | (8–15) | 11 | (8–13) |
| Negative symptoms | 12 | (8–17) | 12 | (9–17) |
| SF-12 score, median (IQR) | ||||
| Physical | 55 | (47–60) | 55 | (48–59) |
| Mental | 42 | (31–55) | 43 | (30–55) |
| Drug use and admission to acute mental health service | ||||
| Number of days using cannabis in the previous 6 months | 26 | (1–142) | 26 | (0–118) |
| Admitted to a crisis house, seen by crisis resolution team, or attended an acute day treatment service | 85/259 | 33 | 90/272 | 33 |
| Number of admissions during 18-month follow-up, median (IQR) | 0 | (0–1) | 0 | (0–1) |
Associations with missing data
Tables related to missing data are in Appendix 1. The employment status of participants appeared to be associated with missing data on secondary outcomes. For example, participants seemed more likely to be missing data for all 3-month secondary outcomes if they were unemployed (see Table 9) [odds ratio (OR) 1.78, 95% CI 1.06 to 2.99]; however, they seemed less likely to have 3-month data missing if they were exempt from work because of disability (OR 0.51, 95% CI 0.29 to 0.90) (see Table 4). Participants appeared to be less likely to have all 18 secondary outcomes missing if they did some voluntary work at baseline (OR 0.39, 95% CI 0.15 to 0.99) (see Table 10). Participants were more likely to have 3- and 18-month data missing if they had an open-market job since contact with services, had current paid activity at baseline or were employed part time at baseline (see Appendix 1, Table 14).
Outcomes
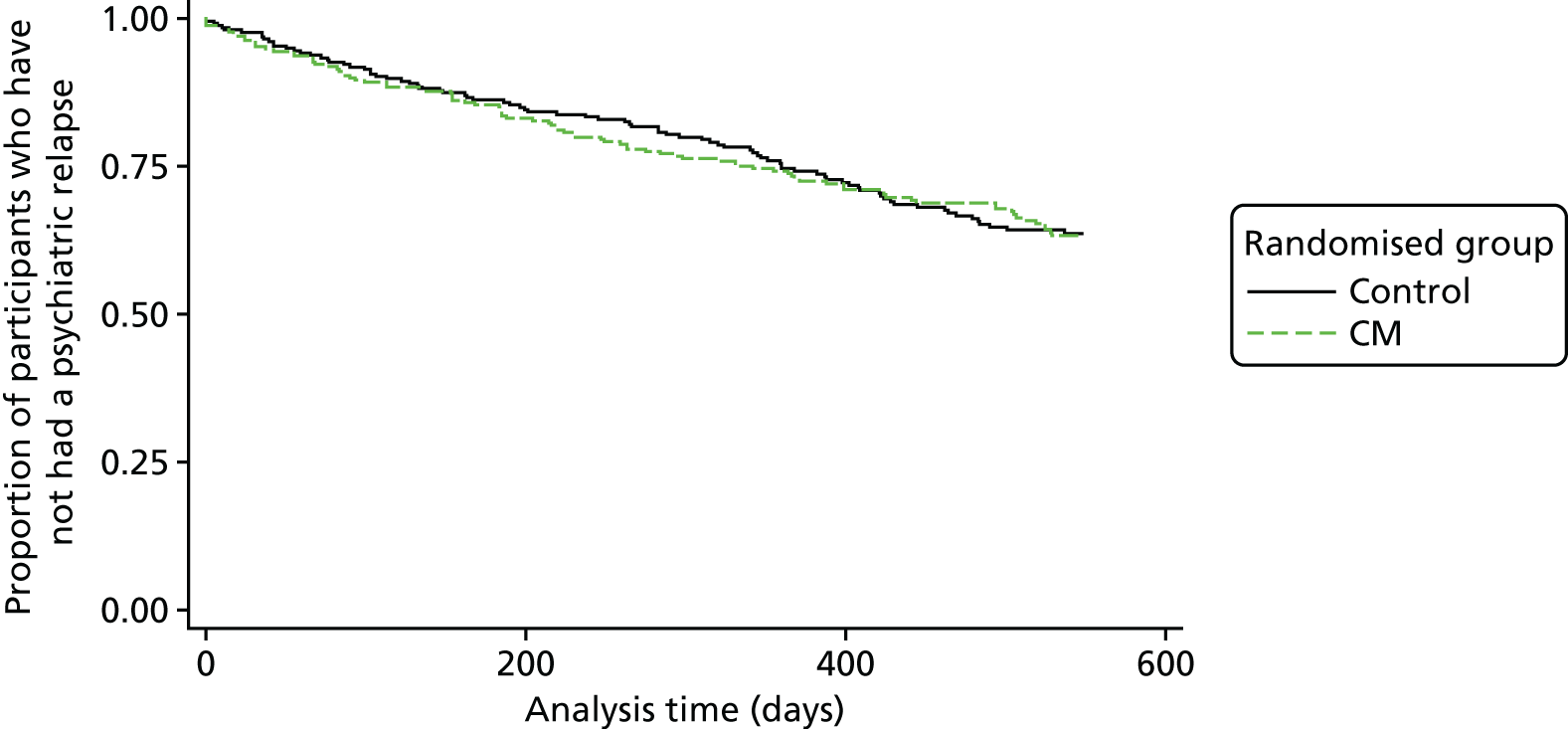
There was no significant difference in time to admission between the randomised groups (HR 1.03, 95% CI 0.76 to 1.40) (Figure 3). Results from the supportive analyses were similar (see Table 4). The odds of at least one admission over 18 months’ follow-up are slightly higher for the CM group than for the psychoeducation group (OR 1.02, 95% CI 0.70 to 1.48), but approximately 33% of participants in both groups were admitted. The ICC estimates obtained from using the log-transformed event times using the analysis-of-variance approach and the marginal models were similar and produced an ICC estimate of 0.01 after adjusting for randomisation group, severity of cannabis use (the stratification variable) at baseline and whether or not the participant was part of the pilot trial. The binary outcome analysis produced a near-zero estimate of the between-cluster variance. In addition, we fitted the primary analysis model without robust standard errors. The estimates of the standard errors and p-values for the covariates were similar to those obtained when robust standard errors were used, suggesting that the ICC is small and consistent with the estimates of 0.01.
FIGURE 3.
Kaplan–Meier survival curve by randomised group for the primary outcome, time to relapse.
Those in the CM arm who had a full 18 months’ follow-up had a slightly higher rate ratio for number of admissions than the controls [incidence rate ratio (IRR) 1.08, 95% CI 0.75 to 1.54]; this changed little when assuming that those who were discharged from services had no admissions during follow-up or when including predictors of missingness. Those randomised to CM had slightly lower odds of cannabis-positive urine at 3 months and 18 months than those randomised to OTAU (OR 0.86, 95% CI 0.56 to 1.34; and OR 0.84, 95% CI 0.49 to 1.41, respectively). However, those in the CM arm also had lower odds of paid work or study at both 3 months and 18 months. For the log-transformed PANSS positive outcome at 3 months, the CM score is, on average, 7% lower than that of the psychoeducation group (95% CI –14% to 0%). Results for the log-transformed PANSS negative score at 3 months are similar. Illicit substance use other than cannabis was very low at both follow-ups [3-month median days of use = 0 (IQR 0–1), in both groups, 18-month median days of use = 0 in both groups (IQR 0–2 in control; IQR 0–1 in experimental)]. On average, the number of alcohol-using days was 4 (control IQR 0–12 days; experimental IQR 0–15 days) in both groups at 3 months, and 6 days (IQR 0–24 days) in both groups at 18 months.
Mean quality-of-life utility scores of the two treatment groups were compared at 18 months using unpaired t-tests. No significant difference was observed between the two groups using EQ-5D utility scores (95% CI –0.02 to –0.078; p = 0.25). The result was the same when using SF-12 utility scores (95% CI –0.022 to –0.037; p = 0.6).
Resource use
Service use is detailed in Table 6. Note that service use for inpatient activity is reported for the full trial period, whereas other types of service use does not cover the period from 3 to 12 months post randomisation.
| Service | Randomised group | |||||
|---|---|---|---|---|---|---|
| Experimental | Control | |||||
| Mean | SD | % | Mean | SD | % | |
| Service use over the trial period (18 months) | ||||||
| Inpatient stays (bed-days) | 89.4 | 92.9 | 24.1 | 90.9 | 97.2 | 24.2 |
| Service use from 0–3 and 12–18 months (contacts) | ||||||
| EIP team member | 11.7 | 9.6 | 68.7 | 11.1 | 8.4 | 68.9 |
| GP | 3.1 | 2.4 | 51.1 | 2.9 | 2.1 | 44.7 |
| Psychiatrist | 3.2 | 2.8 | 54.3 | 3.2 | 3 | 53.1 |
| Psychologist | 5 | 5 | 22.7 | 5.6 | 5.8 | 20.9 |
| Home treatment/crisis team member | 10.7 | 14.5 | 9.0 | 12.8 | 22.6 | 10.3 |
| Mental health nurse | 9.1 | 13.8 | 15.1 | 7 | 7.3 | 13.9 |
| Adult education class | 5.4 | 4.9 | 2.5 | 9 | 8.5 | 3.7 |
| Assertive outreach team member | 8.4 | 11.2 | 1.8 | 9 | 7 | 1.1 |
| Class/group at a leisure centre | 14.6 | 21.9 | 4.0 | 25.2 | 38.7 | 4.4 |
| Community mental health centre | 8.8 | 6.4 | 4.3 | 7.6 | 8.1 | 2.6 |
| Day care centre/day hospital | 5.2 | 3.8 | 1.4 | 27 | 9.9 | 0.7 |
| Drop-in centre | 16.5 | 21.2 | 1.4 | 10.8 | 16 | 4.8 |
| Drug/alcohol service | 16 | 26.9 | 4.0 | 6 | 7.6 | 4.8 |
| Drug and alcohol advisor | 5.5 | 9.2 | 9.4 | 7.9 | 17.2 | 10.6 |
| Occupational therapist | 2.1 | 1.5 | 6.1 | 3.7 | 4.5 | 7.0 |
| Other counsellor/therapist | 7.6 | 7.4 | 4.0 | 7.6 | 7.2 | 5.1 |
| Other doctor | 2.1 | 2.4 | 7.6 | 3.7 | 4 | 8.1 |
| Self-help/support group | 6.8 | 9.3 | 7.2 | 6.5 | 7.7 | 5.1 |
| Social worker | 10.4 | 17.8 | 7.9 | 7.5 | 8.1 | 10.3 |
Use of some services, such as assertive outreach, day care centres/day hospitals, community mental health centres, adult education classes, drop-in centres and classes at leisure centres, was low, being accessed by < 5% of the sample.
Reported use of EIP services was high, with 69% of the sample having a mean of 11 (SD 9) contacts. Use of psychiatrists (54% of the sample) and general practitioners (GPs) (48%) was also high. Over one-fifth of patients reported seeing a psychologist, whereas 15% of patients were seen by a mental health nurse. Around 10% of patients were seen by home treatment or crisis team members. Inpatient use was high among the one-quarter of patients who were admitted, with an average of 90 (SD 95) bed-days over the 18-month period from randomisation.
A small proportion of participants were on a community treatment order at baseline [4.0% (22/551)] and at 18 months [2.7% (14/512)]. Of the 71 people in education at baseline, 50.7% took any days off as a consequence of health problems for a mean of 30 (median 10) days. At 18 months, 29 people were in education, with 31.0% taking any days off as a consequence of health problems for a mean of 6 (median 2) days. A total of 76% of participants claimed at least one benefit in the baseline period, compared with 83% over the follow-up period.
A total of 16% of participants had been in employment in the previous 6 months at baseline, compared with 23% from 12 to 18 months. Of those, the percentage of participants having any days off work as a consequence of health problems fell in both arms, from 55% to 41% (CM) and from 72% to 55% (OTAU).
A total of 10% of participants met with a drug and alcohol advisor at least once, averaging seven contacts (median 14); 4% of participants interacted with drug and alcohol service groups an average of 11 times (median 19).
Total cost
The mean cost of the CM intervention was £295.51 per patient (range £0–602) with a median of five psychoeducation sessions attended and a median of eight urinalysis tests conducted for cannabis. Mean rewards for cannabis abstinence were £68 per patient. The mean cost of the OTAU intervention was £140.33 per patient (range £0–307), with a median of four psychoeducation sessions attended.
Table 7 shows total costs per patient by each period of the trial. Although reported service use notes a gap from 3 to12 months post randomisation, this table imputes those missing costs from the costs of the other period. The imputed portion of the costs for this period represents less than one-quarter of the overall costs in the period.
| Outcome | Estimate | 95% CI | ICC | ICC 95% CI |
|---|---|---|---|---|
| Time to relapse controlling for level of cannabis use and if in the pilot study (HR) – primary outcome, primary analysis | 1.03a | 0.76 to 1.40 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study and predictors of missingness (HR) | 1.02 | 0.75 to 1.40 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study, excluding those who did not have any other data at 12 weeks (HR) | 0.83 | 0.55 to 1.26 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study, excluding those who did not have any other data at 18 months (HR) | 1.04 | 0.66 to 1.63 | ||
| Time to relapse controlling for level of cannabis use, including only those in the main trial (HR) | 0.94 | 0.67 to 1.31 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study and number of psychoeducation sessions attended (HR) | 1.14 | 0.81 to 1.61 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study and number of admissions in the 6 months before baseline as a continuous (HR) | 1.07 | 0.79 to 1.47 | ||
| Time to relapse controlling for level of cannabis use, if in the pilot study and at least one admission in the 6 months before baseline (HR) | 1.04 | 0.77 to 1.41 | ||
| Time to relapse controlling for level of cannabis use and if in the pilot study using the trust as the clustering variable (HR) | 1.03 | 0.79 to 1.35 | ||
| Cannabis-positive urine sample at 12 weeks (OR) | 0.86 | 0.56 to 1.34 | 0.00 | 0.00 to 0.11 |
| Cannabis-positive urine sample at 12 weeks controlling for predictors of missingness (OR) | 0.85 | 0.55 to 1.32 | ||
| Cannabis-positive urine sample at 18 months (OR) | 0.84 | 0.49 to 1.41 | 0.00 | 0.00 to 0.15 |
| Cannabis-positive urine sample at 18 months controlling for predictors of missingness (OR) | 0.85 | 0.50 to 1.43 | ||
| Log-positive symptoms on the PANSS at 12 weeks (coefficient) | –0.07 | –0.14 to –0.00 | 0.06 | 0.00 to 0.17 |
| Log-positive symptoms on the PANSS at 12 weeks controlling for predictors of missingness (coefficient) | –0.07 | –0.14 to –0.00 | ||
| Log-positive symptoms on the PANSS at 18 months (coefficient) | –0.04 | –0.13 to 0.05 | 0.05 | 0.00 to 0.20 |
| Log-positive symptoms on the PANSS at 18 months controlling for predictors of missingness (coefficient) | –0.04 | –0.13 to 0.04 | ||
| Log-negative symptoms on the PANSS at 12 weeks (coefficient) | –0.08 | –0.16 to 0.00 | 0.00 | 0.00 to 0.11 |
| Log-negative symptoms on the PANSS at 12 weeks controlling for predictors of missingness (coefficient) | –0.08 | –0.16 to 0.00 | ||
| Log-negative symptoms on the PANSS at 18 months (coefficient) | 0.01 | –0.08 to 0.11 | 0.19 | 0.03 to 0.34 |
| Log-negative symptoms on the PANSS at 18 months controlling for predictors of missingness (coefficient) | 0.01 | –0.08 to 0.11 | ||
| Paid work or study at 12 weeks (OR) | 0.95 | 0.62 to 1.46 | 0.00 | 0.00 to 0.11 |
| Paid work or study at 12 weeks controlling for predictors of missingness (OR) | 0.94 | 0.60 to 1.47 | ||
| Paid work or study at 18 months (OR) | 0.82 | 0.50 to 1.35 | 0.14 | 0.00 to 0.30 |
| Paid work or study at 18 months controlling for predictors of missingness (OR) | 0.82 | 0.50 to 1.35 | ||
| Number of days that cannabis was used in the previous 12 weeks (12-week follow-up) (IRR) | 0.89 | 0.75 to 1.04 | 0.07 | 0.00 to 0.19 |
| Number of days that cannabis was used in the previous 12 weeks (12-week follow-up) controlling for predictors of missingness (IRR) | 0.88 | 0.75 to 1.04 | ||
| Number of days that cannabis was used in the previous 6 months (18-month follow-up) (IRR) | 1.09 | 0.88 to 1.36 | 0.00 | 0.00 to 0.14 |
| Number of days that cannabis was used in the previous 6 months (18-month follow-up) controlling for predictors of missingness (IRR) | 1.08 | 0.87 to 1.33 | ||
| Number of admissions over 18 months’ follow-up for those with full follow-up or those who died (IRR) | 1.08 | 0.75 to 1.54 | 0.29 | 0.16 to 0.43 |
| Number of admissions over 18 months’ follow-up for those with full follow-up or those who died controlling for predictors of missingness (IRR) | 1.09 | 0.76 to 1.55 | ||
| Number of admissions over 18 months’ follow-up for those with full follow-up, those who died and those who were discharged before the end of follow-up, assuming discharged people did not have any admissions during follow-up | 1.06 | 0.75 to 1.48 | ||
| At least one admission over 18 months’ follow-up (OR) | 1.02 | 0.70 to 1.48 | 0.02 | 0.00 to 0.13 |
| At least one admission over 18 months’ follow-up controlling for predictors of missingness (OR) | 1.01 | 0.69 to 1.48 |
Economic evaluation
In analyses adjusted for baseline cost, the adjusted mean difference between the two arms was –£4277 (95% CI –£12,662 to £4107; p = 0.32). The null hypothesis of no difference in costs between the two treatment groups cannot be rejected. These results remained the same when the period of 3 to 12 months (including some imputed costs) was excluded, or regardless of the time period tested, as Table 8 shows.
| Cost | Randomised group | Adjusted mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Mean | SD | Mean | SD | ||||
| Intervention costs only (n = 533) | 296 | 186 | 140 | 117 | – | – | – |
| Baseline period (6 months) (n = 550) | 2586 | 7345 | 2123 | 4942 | – | – | – |
| Intervention period (3 months) (n = 371) | 2305 | 6826 | 2715 | 8962 | –693 | –2124 to 738 | 0.34 |
| 3–12 months (n = 551) | 8591 | 19,944 | 8902 | 22,282 | –677 | –4140 to 2785 | 0.70 |
| 12–18 months (n = 278) | 4221 | 9325 | 5683 | 11,772 | –1596 | –4103 to 911 | 0.21 |
| Total cost (n = 236) | 14,790 | 33,767 | 17,705 | 35,898 | –4475 | –12,894 to 3945 | 0.30 |
Table 9 shows that costs for inpatient stays were lower for the CM arm (£10,342 vs. £13,247) than the OTAU arm, with large SDs (£34,029 and £35,747, respectively), whereas costs for other items were broadly similar. In the broader perspective, reported benefits received were the largest additional cost.
| Cost item | Randomised group | |||
|---|---|---|---|---|
| Experimental | Control | |||
| Mean | SD | Mean | SD | |
| Costs over the 18-month follow-up period | ||||
| Inpatient stays | 10,342 | 34,029 | 13,247 | 35,747 |
| Costs over the 0- to 3-month and 12- to 18-month follow-up periods | ||||
| EIP team member | 302 | 256 | 303 | 229 |
| GP | 127 | 138 | 134 | 166 |
| Psychiatrist | 404 | 462 | 479 | 522 |
| Psychologist | 416 | 810 | 445 | 894 |
| Home treatment/crisis team member | 47 | 220 | 21 | 60 |
| Mental health nurse | 130 | 438 | 129 | 344 |
| Adult education class | 4 | 39 | 18 | 83 |
| Assertive outreach team member | 4 | 29 | 5 | 33 |
| Class/group at a leisure centre | 10 | 65 | 33 | 225 |
| Community mental health centre | 79 | 469 | 40 | 250 |
| Day care centre/day hospital | 4 | 41 | 3 | 29 |
| Drop-in centre | 12 | 88 | 33 | 246 |
| Drug/alcohol service | 163 | 1221 | 23 | 144 |
| Drug and alcohol advisor | 291 | 2090 | 116 | 423 |
| Medication | 308 | 644 | 288 | 601 |
| Occupational therapist | 37 | 148 | 62 | 250 |
| Other counsellor/therapist | 53 | 347 | 156 | 694 |
| Other doctor | 26 | 90 | 75 | 413 |
| Self-help/support group | 16 | 89 | 9 | 50 |
| Social worker | 44 | 316 | 58 | 193 |
| Broader perspective costs over the 0- to 3-month and 12- to 18-month follow-up periods | ||||
| Court attendance | 81 | 293 | 159 | 581 |
| Police | 48 | 152 | 60 | 235 |
| Police cell | 20 | 75 | 31 | 89 |
| Prison | 53 | 559 | 137 | 977 |
| Probation officer | 10 | 60 | 4 | 20 |
| Solicitor | 4 | 18 | 25 | 92 |
| Benefits | 3609 | 2393 | 3294 | 2298 |
| Productivity losses | 219 | 1054 | 171 | 621 |
Outcomes
The EQ-5D tariff over the 18-month follow-up period is detailed in Table 10 and shown graphically in Figure 4. Utility scores are similar between groups over the follow-up period, with the CM arm having a modestly higher mean raw tariff score at baseline (CM, 0.77; OTAU, 0.76) and all the way through to 18 months (CM, 0.83; OTAU, 0.80). When QALYs are calculated, the mean for the CM arm was higher (CM, 1.21; OTAU, 1.15), but this difference was not significant when adjusting for baseline EQ-5D score (adjusted mean difference 0.053, 95% CI –0.0069 to 0.11; p = 0.083). These differences were smaller when looking at other quality-of-life measures. Figure 4 shows the EQ-5D-3L utility scores over time. Figure 5 shows reported EQ-5D visual analogue scale (VAS) scores over time. The adjusted mean difference for the EQ-5D VAS was 1.2 (95% CI –3.0 to 5.3; p = 0.58). Figure 6 shows SF-6D (Short Form questionnaire-6 Dimensions) utility scores, also showing little difference. Again, the adjusted mean difference had a positive sign (0.015), and again this difference was not significantly different from zero (95% CI –0.018 to 0.048; p = 0.37).
| Quality-of-life measure | Randomised group | Adjusted mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Mean | SD | Mean | SD | ||||
| EQ-5D-3L tariff | |||||||
| Baseline (n = 548) | 0.77 | 0.26 | 0.76 | 0.25 | – | – | – |
| 3 months (n = 372) | 0.82 | 0.24 | 0.78 | 0.26 | – | – | – |
| 18 months (n = 275) | 0.83 | 0.18 | 0.8 | 0.23 | – | – | – |
| QALYs – EQ-5D-3L | |||||||
| 3 months (n = 371) | 0.18 | 0.05 | 0.18 | 0.05 | 0.0046 | –0.01072 to 0.01 | 0.09 |
| 18 months (n = 236) | 1.2 | 0.26 | 1.2 | 0.31 | 0.053 | –0.1169 to 0.11 | 0.083 |
| EQ-5D VAS | |||||||
| Baseline (n = 528) | 59 | 19 | 59 | 21 | – | – | – |
| 3 months (n = 354) | 65 | 20 | 65 | 19 | 0.32 | –3.4 to 4.0 | 0.87 |
| 18 months (n = 277) | 67 | 17 | 66 | 19 | 1.2 | –3.0 to 5.3 | 0.58 |
| SF-12 tariff | |||||||
| Baseline (n = 549) | 0.68 | 0.12 | 0.68 | 0.13 | – | – | – |
| 3 months (n = 370) | 0.71 | 0.14 | 0.71 | 0.14 | – | – | – |
| 18 months (n = 277) | 0.72 | 0.12 | 0.71 | 0.13 | – | – | – |
| QALYs – SF-12 | |||||||
| 3 months (n = 370) | 0.16 | 0.025 | 0.16 | 0.026 | 0.00051 | –0.0023 to 0.0034 | 0.72 |
| 18 months (n = 235) | 1 | 0.15 | 1 | 0.16 | 0.015 | –0.018 to 0.048 | 0.37 |
FIGURE 4.
The EQ-5D-3L utility scores by randomised group over time.
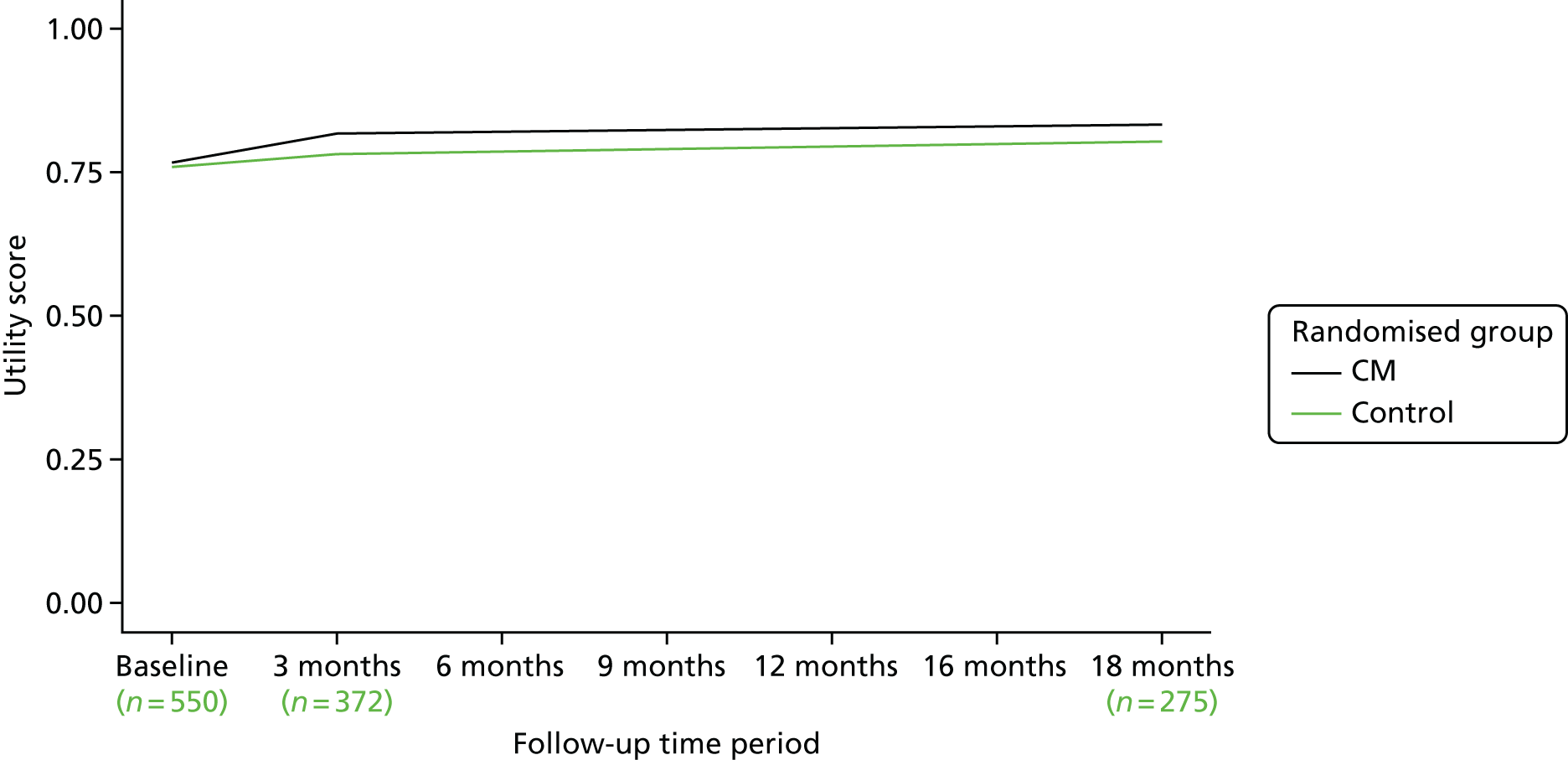
FIGURE 5.
The EQ-5D VAS utility scores over time.
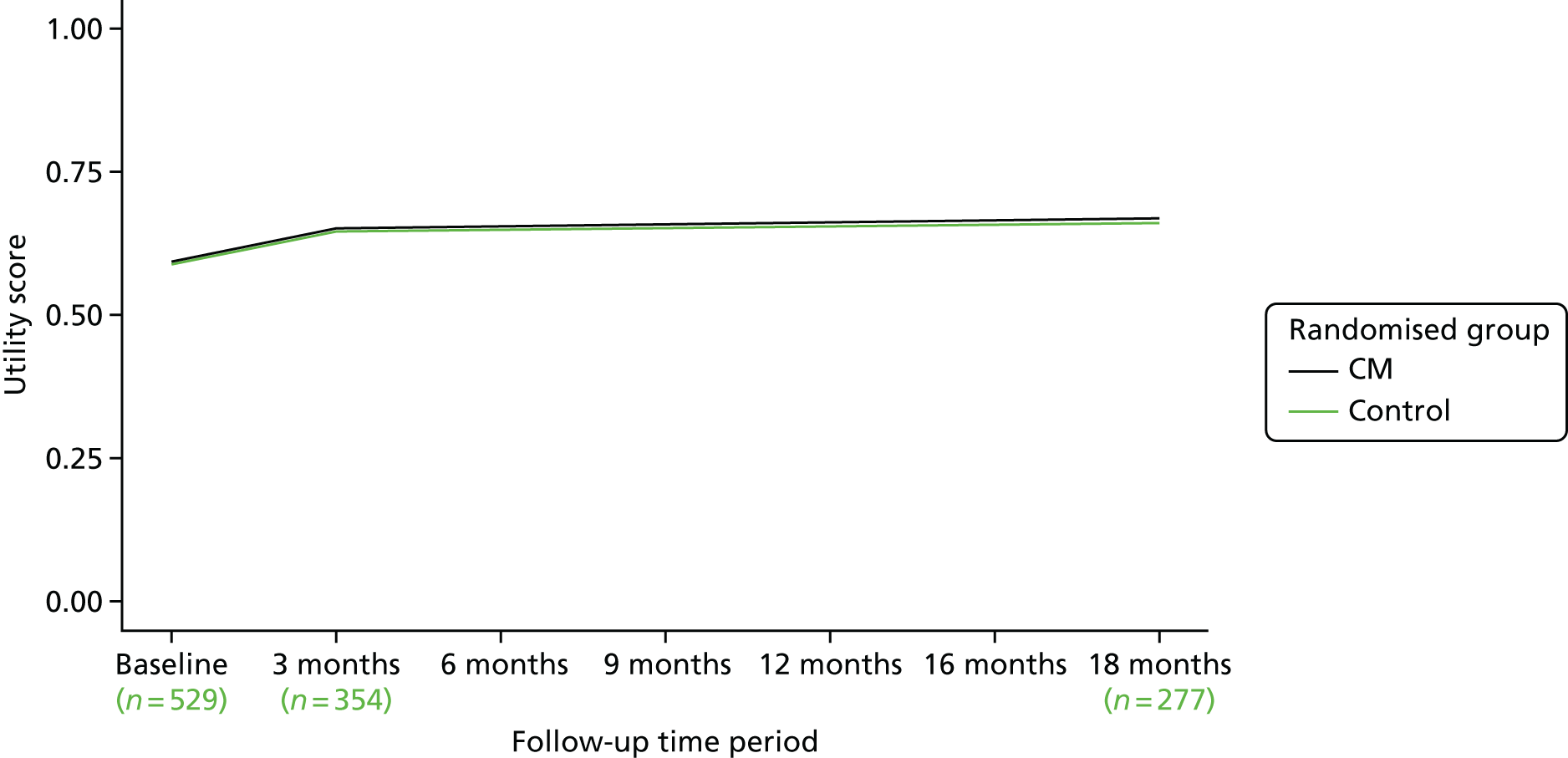
FIGURE 6.
The SF-6D utility scores over time.
Cost-effectiveness analysis
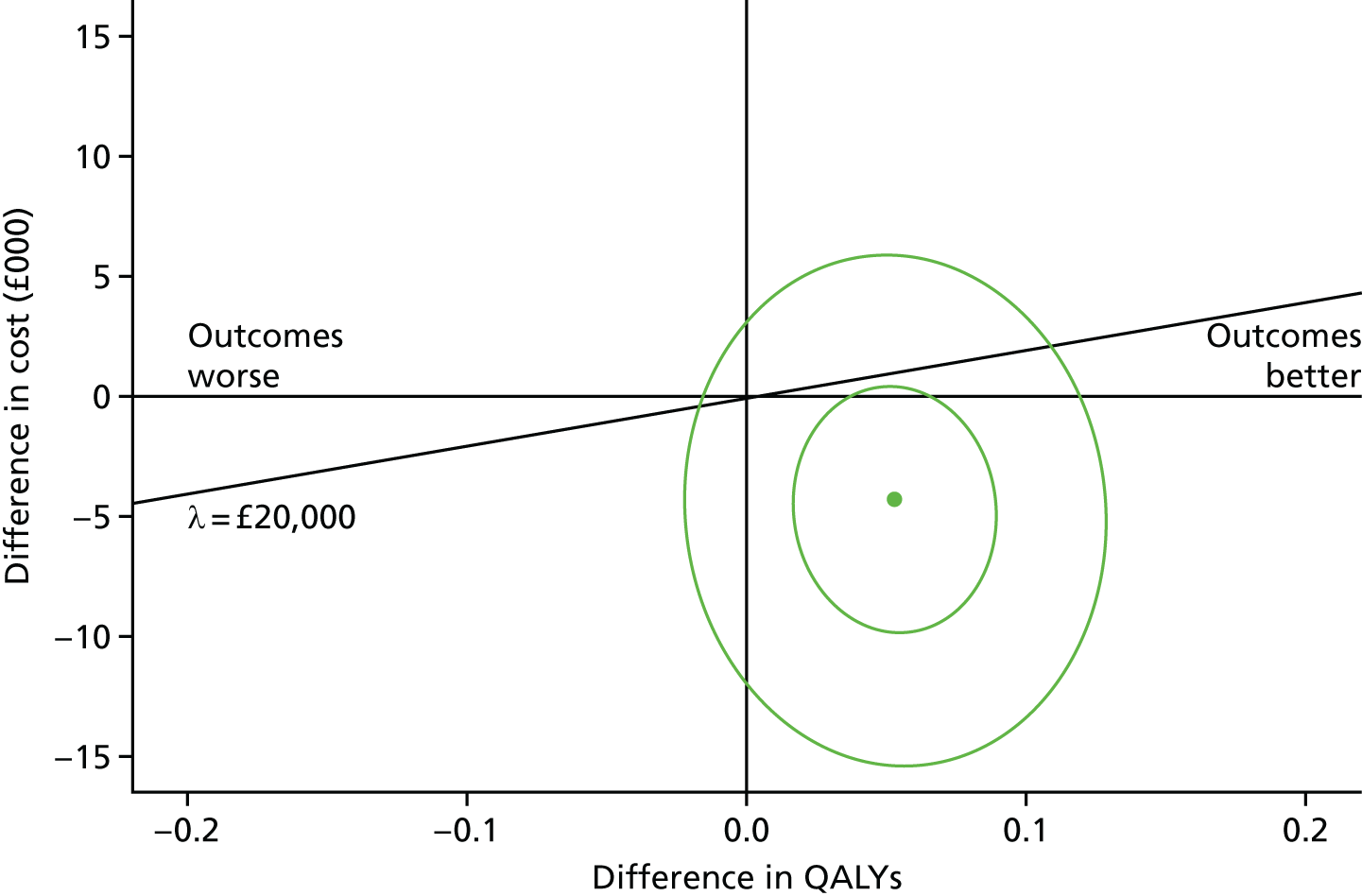
Figure 7 shows the cost-effectiveness plane showing bootstrap cost and QALY (EQ-5D) pairs at the 18-month follow-up. There are 10,000 bootstrapped samples shown, with 50% and 95% confidence ellipses surrounding the bootstrapped pairs. The central dot shows the adjusted mean cost difference of –£4277 and the adjusted mean QALY difference of 0.053. This represents an ICER of –£80,772. The bootstrapped replications of costs and outcomes fall with the majority in the south-east quadrant (81.8%), with very few in the north-west (0.6%), and more in the north-east (14.1%) than in the south-west quadrant (3.5%).
FIGURE 7.
The EQ-5D-3L cost-effectiveness plane.
When this is compared with a cost-effectiveness plane using QALYs generated by the SF-6D (Figure 8), we see less variation in QALY differences, with fewer, but still a majority of, replications to the east of the plot. The relevant mean QALY difference with the SF-6D is 0.015, and the ICER is –£284,937. Both plots suggest that CM may be a cost-effective alternative to OTAU. This is also shown in Figure 9.
FIGURE 8.
The SF-6D cost-effectiveness plane.
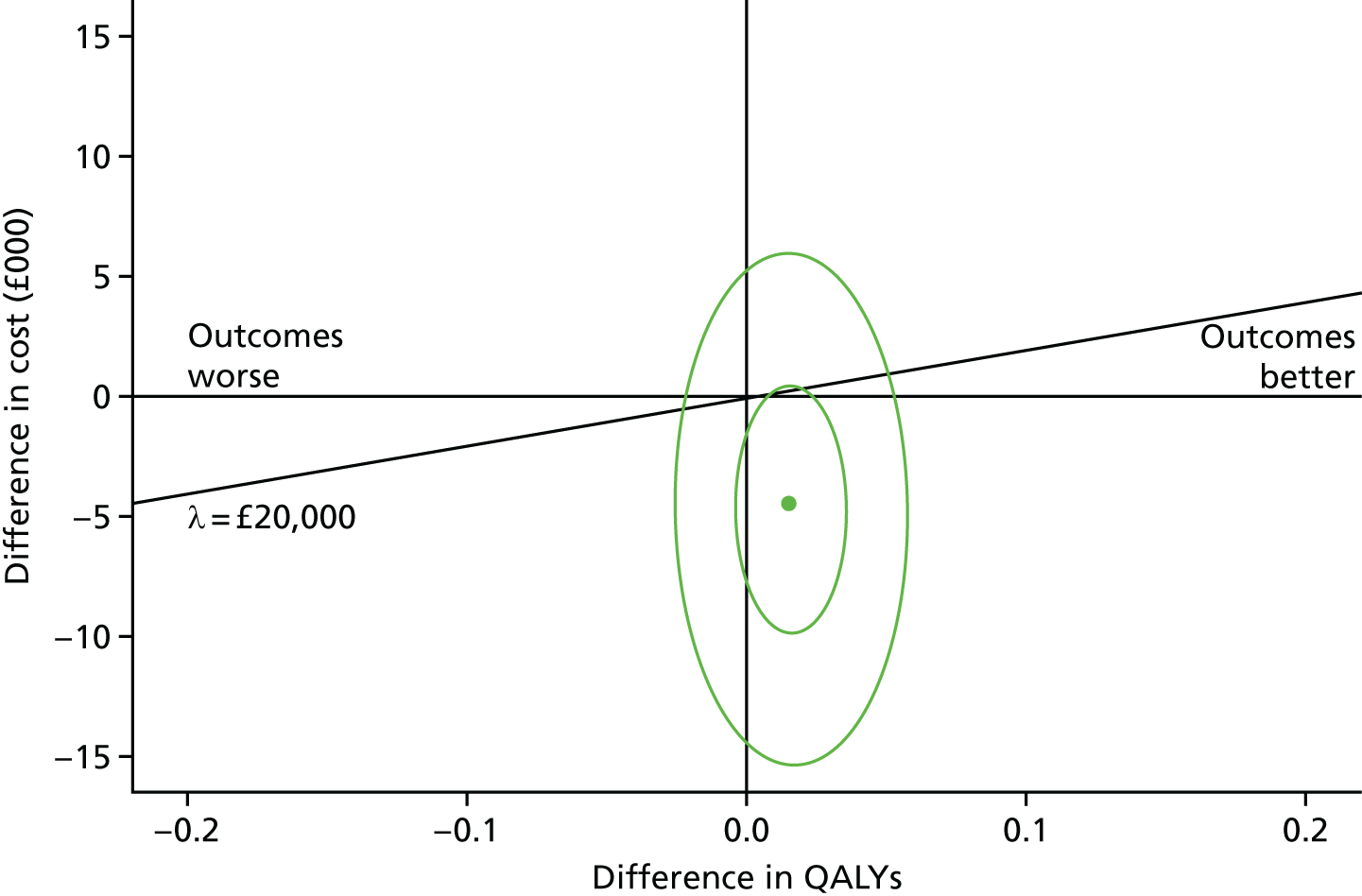
FIGURE 9.
Cost-effectiveness acceptability curves by QALY method.
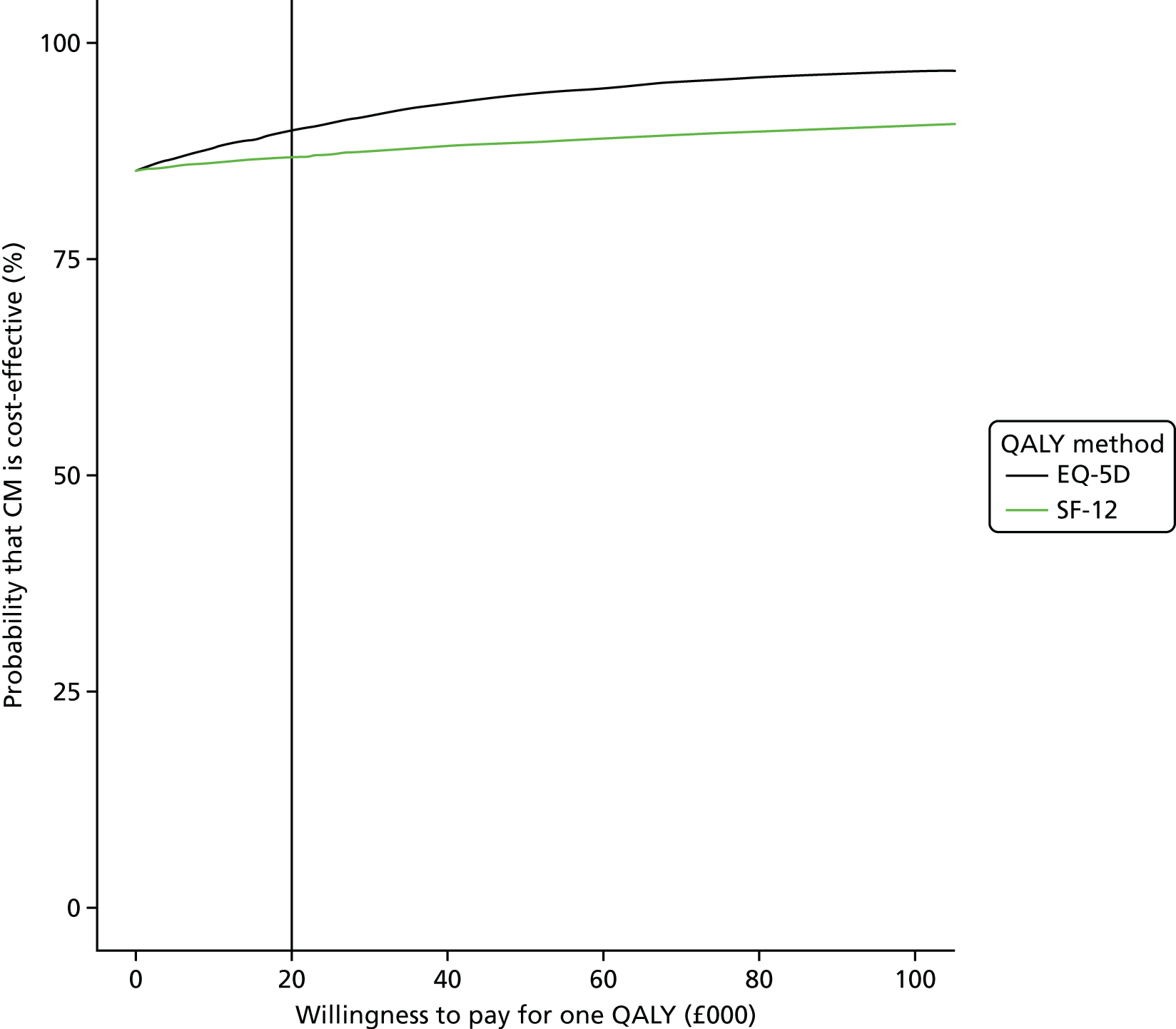
The above analysis shows the probability that CM is more cost-effective than OTAU in terms of QALYs at the 18-month follow-up. The fact that 69.5% of bootstrap replications were in the south-east quadrant suggest that CM is more effective and less costly than OTAU. Again, there were few replications in the north-west quadrant (2.9%), and more replications in the south-west (15.8%) than in the north-east (11.7%). There is a high probability (> 85%) that CM is cost-effective regardless of willingness to pay for one QALY, and irrespective of whether or not QALYs are calculated using EQ-5D or SF-6D. The probability is slightly higher using QALYs generated via the EQ-5D.
An analysis using QALYs at 3 months was conducted, with adjusted mean differences not statistically different from 0 when using EQ-5D or SF-12 tariffs. An analysis of self-reported cannabis-free days was also conducted. From 12 to 18 months, the mean numbers of cannabis-free days in each arm were similar: 107 (SD 66) for CM and 107 (SD 67) for OTAU. The adjusted mean difference was 4.1 days (95% CI –10.7 to 18.9; p = 0.58).
A sensitivity analysis including a broader selection of criminal justice costs, benefits payments and productivity losses did not alter the findings from the base-case analysis. The total mean costs in each arm were £21,793 (SD £30,417) for CM and £24,432 (SD £35,179) for OTAU. The adjusted mean difference was –£4896 (95% CI –£13,000 to £3100; p = 0.23). Another sensitivity analysis using multiple imputation by chained equations of individual cost elements did not alter the findings from the base-case analysis. This analysis assumed that cost elements were missing at random. They did shrink the adjusted mean difference in total costs between the two arms from –£4475 in the base case to –£1075. The CIs remained wide (95% CI –£6191 to £4042, p = 0.68). These results are shown in Table 11.
| Sensitivity analysis | Randomised group | Adjusted mean difference (£) | 95% CI (£) | p-value | |||
|---|---|---|---|---|---|---|---|
| Experimental (£) | Control (£) | ||||||
| Mean | SD | Mean | SD | ||||
| Broader perspective of total costs | 21,793 | 30,417 | 24,432 | 35,179 | –4896 | –13,000 to 3100 | 0.23 |
| Multiple imputation of missing cost elements | 17,928 | 34,076 | 17,845 | 33,584 | –1075 | –6191 to 4042 | 0.68 |
Conclusions from the economic evaluation
The data suggest that the information on cost-effectiveness is complex. Hypotheses of equivalence in costs and QALYs could not be rejected, but bootstrapped replications of cost and QALY pairs largely fell in the south-east quadrant, representing better outcomes for lower costs. This gave a high probability of cost-effectiveness acceptability for any willingness to pay for one QALY.
There are a number of limitations of the economic evaluation. Although some service use information was collected for the whole 18-month trial period, participants at the 18-month follow-up were asked to recall their service use only for the previous 6 months. So, the period from the 3-month follow-up to 12 months has limited service use data. We sought to limit the impact of this by extracting data on the leading service use cost contributor, inpatient activity, from hospital trust databases.
Another limitation was the choice of outcome measure. Although generic measures such as the EQ-5D and SF-6D have been widely used, doubts have been raised about their use for patients with mental health problems (e.g. Brazier et al. 70). We sought to limit the impact of this by using information from other outcomes, but the EQ-5D showed the largest, but not significant, impact.
The sensitivity analysis using a broader, societal perspective also had limitations. Costs from the informal health-care sector were not collected, nor were patient time costs, unpaid caregiver time costs, or transportation costs. Although time missing from education was collected, there is no clear way to calculate a cost for this. Costs from the criminal justice system were calculated; however, the data collected gave no indication of the type of crimes committed and so no attempt was made to calculate the costs of the crimes themselves.
Serious adverse events
There were 58 serious adverse events (SAEs); this included 52 inpatient episodes, five deaths and one arrest. All SAEs were considered by the trial manager and chief investigator, and reported to the Data Monitoring Committee. None was considered to be potentially an adverse reaction to the intervention. All the deaths occurred between treatment cessation and the 18-month follow-up assessment. One was because of a heroin overdose, two were because of road accidents and two were because of health-related issues. The CIRCLE trial research team recorded all SAEs they were aware of, either through contact with the service users directly or via EIP clinicians. However, although all SAEs were recorded during the intervention period, detection of SAEs was harder during the post-treatment follow-up phase as the CIRCLE trial team did not have direct contact with participants and relied on EIP teams to report any information. All deaths were reported by the EIP teams and recorded as SAEs; however, primary data collection shows that not all arrests or inpatient episodes were reported to the CIRCLE trial team once the intervention had ended.
Chapter 4 Discussion
Main findings
For the primary outcome, there was no evidence of a difference in time to relapse when the CM intervention was delivered in addition to OTAU. Most secondary outcomes, including cannabis use and occupation, also showed no substantial evidence of an effect on direct comparison between the CM and control arms. However, positive symptoms were slightly lower in the CM arm than the control arm at 3 months. The cost-effectiveness analysis indicated a > 85% likelihood that the intervention was cost-effective, mainly resulting from higher mean inpatient costs for the control arm than the CM arm. Interpretation of this and the improvement in positive symptoms is, however, complex and there was strikingly little difference between the arms on primary and cannabis-use-related outcomes. Thus, our trial is unfortunately another trial in a growing list of carefully designed intervention trials that have shown no clear effect on cannabis use in the context of psychosis.
Other noteworthy findings included a lower relapse rate than anticipated when reviewing relevant literature and carrying out the power calculation: only around one-third in each group required admission to acute care, possibly indicating that a comparatively stable group were recruited to the trial. Although a majority still had a cannabis-positive urine, the number of reported cannabis-using days in the previous 6 months fell from > 100 days to 26 days in each group by the 6-month follow-up, suggesting that, whether through the natural history of the condition or the effects of the psychoeducation package, cannabis use declined in both groups over this period. Engagement with the psychoeducation package was good in both groups, with six and four sessions attended, respectively. Success in completing the CM programme was limited for most, with a mean of £64 per person obtained in voucher rewards from a potential sum of £240. There was no evidence of participants replacing their cannabis use with use of other illicit substances.
Limitations
A number of important limitations constrain interpretation of the findings.
First, we have demonstrated the likely ineffectiveness of a CM intervention at the threshold set for the trial (although with the caveat that health economic findings suggested that cost-effectiveness was probable), which was intended to be substantial enough to be motivating without being coercive and thus ethically problematic. The threshold set was supported by clinicians, service users who were consulted via the patient and public involvement consultation, and experts in the field. However, there is presumably some threshold at which the incentive would change behaviour, at least in the short term – for example, a reward for abstinence of £1M per week would presumably be a more powerful incentive for abstinence, albeit an unaffordable and ethically unacceptable one. The question thus remains of whether or not there is a level of reward that would shape behaviour more convincingly without appearing ethically and economically unacceptable, and, if so, whether or not effects would be enduring and outcomes would be improved in general.
Second, the inclusion of an active control makes it more difficult to interpret results. The relatively good engagement with the psychoeducation package and fall in cannabis-using days across the sample could be interpreted as suggesting benefit from psychoeducation. Although the OTAU intervention was intended to reduce the impact of variations in TAU, it became apparent that it was a much better-developed and more ambitious psychoeducation intervention than was available in any of the participating teams.
Third, our initial intention preceding the pilot was for the CM package to be delivered by care co-ordinators in teams. It became apparent early in the trial that very few were prepared to do so, partly because of time pressures and partly because some feared disruption to therapeutic relationships. Delivery of the intervention thus tended to become part of the responsibilities of additional workers in the teams such as support workers and assistant psychologists. Protocols were, in general, well adhered to, but it is possible that effectiveness might have been greater with more integration into routine appointments.
Fourth, although the use of routine data meant excellent completion of the primary outcome and a fully powered trial, the drop-out rate was greater than anticipated on the interview measures, potentially introducing bias.
Fifth, using acute service use as a proxy for relapse is a pragmatic choice because of the very high completion rate for this measure, but it is limited in that some relapses are likely to be contained without acute service use, especially in the context of EIP care for which a relatively intensive response is often feasible. The health economic results suggest a missed effect is a possibility.
Sixth, although we approached all EIP patients who were identified to trial researchers as potentially eligible by their clinicians and who agreed to be contacted by a trial researcher, it may be that self-selection by patients could have introduced bias. However, this was a pragmatic RCT and it is likely that the trial sample is a good reflection of the range of characteristics of the EIP service users who would enter treatment if the intervention was offered through EIP or specialist drug treatment services. Some of the more common reasons patients gave for declining participation in the CIRCLE trial included that they were ‘not interested’ or ‘not trying to quit at the moment’ or they denied that they were using cannabis. Similar self-selection seems probable if the intervention was to be introduced as part of routine care.
Seventh, the qualitative substudy found that, although clinicians generally viewed the interventions positively, with most seeing them as acceptable, a small number raised ethical concerns about paying people to stop smoking cannabis, which they should not be doing anyway. Such concerns, if widely held among patients, carers, clinicians and the public, are a potential barrier to adopting CM in routine care. However, most of the clinicians who raised such concerns said that they would still engage their clients in the treatment if it helped. In addition, there was no evidence of these concerns being shared by participants or carers, who viewed the intervention positively, although we were able to interview only one carer. Public acceptability of CM, however, is currently unclear as few studies have been published on the topic. One of the studies that did explore this issue, Promberger et al. ,71 reported that, in the UK, reward-based incentive schemes were viewed favourably for treatment adherence in mental illness but were viewed slightly negatively for drug addiction. These results do not have a straightforward implication for the CIRCLE trial as it trialled CM for drug misuse in severe mental illness; however, it would be interesting to investigate this issue further.
Eighth, we did not systematically record the type of cannabis participants were using. It may be that users of high-potency cannabis would have shown more of an effect in the primary outcome if the intervention had been effective at reducing use.
Finally, we focused only on cannabis, but baseline data indicated that the participants had a considerable history of alcohol and substance use disorders. It may be that an effective intervention would need to address the use of additional substances.
Implications for research and practice
The lack of clear effects in the trial means that immediate implications for practice are limited, although the health economic data may suggest that the intervention should not be discounted entirely and may be effective with a different design or reward level.
Regarding further research, it continues to be very challenging to address the substantial problem of cannabis use in psychosis effectively. Regarding further use of CM, effectiveness at a different threshold or with a different threshold is possible. Investigation of stakeholder perspectives on the threshold tested in our trial is desirable before considering this (a PhD linked to the study will include relevant data of this type).
Beyond this, the persistent failure of a variety of approaches to reduce substance misuse in psychosis requires the research community to take stock and reconsider the theoretical basis underpinning approaches tested and the potential for change. Most approaches tried thus far have been relatively narrowly focused on changing cannabis use, with a variety of psychological approaches taken to do this. Behaviour-change models have been deployed relatively rarely and it is worth considering if these might enhance the approaches taken. A more personalised approach taking account of specific individual triggers might also be of value.
However, a more substantial change in approach is also worth considering. Several studies72,73 have noted that young people who have psychosis and problematic cannabis use are often multiply disadvantaged, and often have restricted social networks in which their main sources of social contact are other cannabis users. This may make cannabis use hard to modify without a broader approach to the social context, for example to support isolated young people with comorbid cannabis use to find new forms of social contact and activity, perhaps alongside psychological approaches to reducing substance misuse. The evidence of multiple substance use should also be noted, suggesting that, although cannabis has been identified as the principal problematic substance in EIP contexts, interventions may also need to address other substance use.
Acknowledgements
We would like to thank all the participants who took part in the trial, as well as all the research assistants who contributed to the trial. This includes Joanne Taylor, Sophie Colman, Catherine Harder, Eleanor Gilbert, Amie Randhawa, Kirsty Labuschagne, Charlotte Jones, Theodora Stefanidou, and Marina Christoforou. We would also like to thank the EIP clinical staff who assisted with recruitment or delivered the interventions. We are also grateful to the members of the Trial Steering Committee (Thomas Barnes, Jonathan Haynes, Sara Brookes, Maurice Arbuthnott, Katherine Barrett, Nick Barber and Beverley Chipp) and the Data and Ethics Committee (David Kingdon, Chris Metcalfe, Rob McPherson and David Li) for providing oversight throughout the trial, and the principal investigators (Charlotte Johnston-Webber, Simon Clark, Nikola Rahaman, Manoj Sukumaran, Peter Carter, Rick Fraser, Nilamadhab Kar, Sajad Yousuf, Jonathan West, Nicholas Chamberlain-Kent, Abu Abraham, Hana Soliman, John Joseph Scanlon, Nandini Chakraborty, Sazgar Hamad, Eileen O’Regan, Chris Harrop, Sanjoo Chengappa, Belinda Lennox and Jesus Perez) for supporting the trial. Finally, we would like to thank Barnaby Major, Kelly Wilson, Leila Hughes, Melanie Whyman, Simon Spencer, Alyssa Milton, Danielle Lamb, Kate Fullarton, Johanna Frerichs, Kirsty Shepherd, Danelle Pettman, Ruth Mintah, Sherene Olusesi, Tayla McCloud and the clinical studies officers who contributed to the trial for all their efforts.
Patient and public involvement
Patient and public involvement groups were consulted during the development of the trial, and were involved in the development of the CM treatment and the psychoeducation package. In the course of designing the trial and applying for funding, we presented our plans and modified them in the light of comments to the London Early Intervention Research Network (by clinicians and a carer representative) and to the SURF (Service User Research Forum) at University College London. The psychoeducation package used in both arms of the trial was based on the Cannabis and Psychosis Trial conducted in Melbourne, Australia by Edwards et al. :59 service users from a lived experience group called the Service User Research Forum had been invited on multiple occasions to comment on this package. In the set-up phase of the trial, we again consulted the London Early Intervention Research Network and the SURF on the content of our intervention and on trial plans. Four service users at Camden and Islington Early Intervention Services were also asked to comment in some detail on the CM and psychoeducation plans during the set-up phase of the trial. Further modifications based on qualitative interviews with patients and on discussions with staff were made to the CM schedule between the pilot and the full trial. There were two service user members on the Trial Steering Committee. Had we been designing the package now, it is probable that service user involvement would be more extensive.
Contributions of authors
Sonia Johnson is Professor of Social and Community Psychiatry in the Division of Psychiatry, University College London, and chief investigator for the CIRCLE trial. She managed the primary research centre, UCL, and designed the study with contributions from Steven Marwaha, David Fowler, Tom Craig, Louise Marston, Rumana Omar, Michael King, Tim Weaver, John Strang and Paul McCrone. She also drafted the final report with Luke Sheridan Rains.
Luke Sheridan Rains has been the trial manager for the CIRCLE trial since the completion of the feasibility study. He also drafted the final report with Sonia Johnson.
Steven Marwaha is a Reader in Psychiatry in the Mental Health and Wellbeing Department in the University of Warwick, and lead investigator for the CIRCLE trial in the Midlands. He contributed to study design and managed the other research centres with David Fowler and Thomas Craig.
John Strang is a Professor in Addictions, Head of the Addictions Department and Director of the National Addiction Centre at the Institute of Psychiatry, Psychology, and Neuroscience, King’s College London. John Strang is an expert in substance misuse and has substantial experience of CM trials in the UK. He provided expertise to the study design and delivery.
Thomas Craig is Professor of Social Psychiatry in the Department of Health Service and Population Research at King’s College London. He is the lead investigator for the CIRCLE trial in south London. He contributed to study design and managed the other research centres with Steven Marwaha and David Fowler.
Tim Weaver is Associate Professor in Mental Health in the department of Mental Health, Social Work and Interprofessional Learning, Middlesex University, London. He has significant experience of CM trials in the UK and is an expert in qualitative research methods. He provided expertise to the study design and delivery.
Paul McCrone is Professor of Health Economics in the Department of Health Services and Population Research, King’s College London. He contributed to study design, authored the cost-effectiveness analysis and, with Jonathan Spencer, performed the analysis.
Michael King is Professor of Primary Care Psychiatry and joint director of PRIMENT Clinical Trials Unit in the Division of Psychiatry, University College London. He contributed to study design, statistical analyses, and the interpretation of the findings through the PRIMENT clinical trial unit.
David Fowler is Professor in Psychology in the University of Sussex and Lead Investigator for the CIRCLE trial in Sussex and the surrounding areas. He contributed to study design and managed the other research centres with Steven Marwaha and Thomas Craig.
Stephen Pilling is Professor of Clinical Psychology and Clinical Effectiveness in the Division of Psychology and Language Sciences in University College London.
Louise Marston is Senior Research Statistician in the Department of Primary Care and Population Health, University College London. She contributed to study design. She also authored and performed the statistical analyses with Rumana Z Omar.
Rumana Z Omar is a Professor of Medical Statistics at the Department of Statistical Science, University College London. She contributed to study design, authored and performed the statistical analyses with Louise Marston.
Meghan Craig was the trial manager for the CIRCLE trial during the feasibility study. She managed the pilot phase of the trial and developed the psychoeducation package with Mark Hinton.
Jonathan Spencer is a Health Economist based in the Department of Health Services and Population Research, King’s College London. He performed the cost-effectiveness analysis with Paul McCrone.
Mark Hinton is a Clinical Psychologist with expertise in substance misuse and CM. He was instrumental in conceiving and developing the CIRCLE trial, and was largely responsible for the development of the contingency management intervention. He developed the psychoeducation package with Meghan Craig.
Publication
Sheridan Rains L, Marston L, Hinton M, Marwaha S, Craig T, Fowler D, et al. Clinical and cost-effectiveness of contingency management for cannabis use in early psychosis: the CIRCLE randomised clinical trial. BMC Med 2019;17:161.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and appropriate agreements being in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Lambert M, Conus P, Lubman DI, Wade D, Yuen H, Moritz S, et al. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand 2005;112:141-8. https://doi.org/10.1111/j.1600-0447.2005.00554.x.
- Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry 2006;188:237-42. https://doi.org/10.1192/bjp.bp.104.007237.
- Stone JM, Fisher HL, Major B, Chisholm B, Woolley J, Lawrence J, et al. Cannabis use and first-episode psychosis: relationship with manic and psychotic symptoms, and with age at presentation. Psychol Med 2014;44:499-506. https://doi.org/10.1017/S0033291713000883.
- Schimmelmann BG, Conus P, Cotton S, Kupferschmid S, McGorry PD, Lambert M. Prevalence and impact of cannabis use disorders in adolescents with early onset first episode psychosis. Eur Psychiatry 2012;27:463-9. https://doi.org/10.1016/j.eurpsy.2011.03.001.
- Patel R, Wilson R, Jackson R, Ball M, Shetty H, Broadbent M, et al. Cannabis use and treatment resistance in first episode psychosis: a natural language processing study. Lancet 2015;385. https://doi.org/10.1016/S0140–6736(15)60394–4.
- Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 1994;51:273-9. https://doi.org/10.1001/archpsyc.1994.03950040017002.
- Verdoux H, Liraud F, Gonzales B, Assens F, Abalan F, van Os J. Predictors and outcome characteristics associated with suicidal behaviour in early psychosis: a two-year follow-up of first-admitted subjects. Acta Psychiatr Scand 2001;103:347-54. https://doi.org/10.1034/j.1600-0447.2001.00202.x.
- Hanna RC, Shalvoy A, Cullum CM, Ivleva EI, Keshavan M, Pearlson G, et al. Cognitive function in individuals with psychosis: moderation by adolescent cannabis use. Schizophr Bull 2016;42:1496-503. https://doi.org/10.1093/schbul/sbw030.
- Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, et al. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry 2016;3:215-25. https://doi.org/10.1016/S2215-0366(15)00363-6.
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014;20:4112-18. https://doi.org/10.2174/13816128113199990620.
- Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry 2014;5. https://doi.org/10.3389/fpsyt.2014.00054.
- Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Substance misuse in first-episode psychosis: 15-month prospective follow-up study. Br J Psychiatry 2006;189:229-34. https://doi.org/10.1192/bjp.bp.105.017236.
- Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case–control study. Lancet Psychiatry 2015;2:233-8. https://doi.org/10.1016/S2215-0366(14)00117-5.
- Walsh E, Buchanan A, Fahy T. Violence and schizophrenia: examining the evidence. Br J Psychiatry 2002;180:490-5. https://doi.org/10.1192/bjp.180.6.490.
- Kooyman I, Dean K, Harvey S, Walsh E. Outcomes of public concern in schizophrenia. Br J Psychiatry Suppl 2007;50:s29-36. https://doi.org/10.1192/bjp.191.50.s29.
- Marwaha S, Johnson S, Bebbington P, Stafford M, Angermeyer MC, Brugha T, et al. Rates and correlates of employment in people with schizophrenia living in the UK, France and Germany: findings from the EuroSC study. Br J Psychiatry 2007;191:30-7. https://doi.org/10.1192/bjp.bp.105.020982.
- Jeffery D, Ley A, McLaren S, Siegfried N. Psychosocial treatment programmes for people with both severe mental illness and substance misuse (review). Cochrane Database Syst Rev 2000;3. https://doi.org/10.1002/14651858.CD001088.
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD001088.pub3.
- Hjorthoj CR, Baker A, Fohlmann A, Nordentoft M. Intervention efficacy in trials targeting cannabis use disorders in patients with comorbid psychosis systematic review and meta-analysis. Curr Pharm Des 2014;20:2205-11. https://doi.org/10.2174/13816128113199990431.
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O’Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. Am J Psychiatry 2001;158:1706-13. https://doi.org/10.1176/appi.ajp.158.10.1706.
- Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr Scand 2007;115:304-9. https://doi.org/10.1111/j.1600-0447.2006.00900.x.
- Archie S, Rush BR, Akhtar-Danesh N, Norman R, Malla A, Roy P, et al. Substance use and abuse in first-episode psychosis: prevalence before and after early intervention. Schizophr Bull 2007;33:1354-63. https://doi.org/10.1093/schbul/sbm011.
- Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospective study. Br J Psychiatry 2006;189:137-43. https://doi.org/10.1192/bjp.bp.105.014308.
- Mueser K, Drake R, Graham H, Copello A, Birchwood M. Substance Misuse in Psychosis: Approaches to Treatment Delivery. Chichester: John Wiley & Sons; 2003.
- Barrowclough C, Marshall M, Gregg L, Fitzsimmons M, Tomenson B, Warburton J, et al. A phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: a randomized controlled trial. Psychol Med 2014;44:2749-61. https://doi.org/10.1017/S0033291714000208.
- Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD004307.pub5.
- Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0090347.
- Godley MD, Godley SH, Dennis ML, Funk RR, Passetti LL, Petry NM. A randomized trial of assertive continuing care and contingency management for adolescents with substance use disorders. J Consult Clin Psychol 2014;82:40-51. https://doi.org/10.1037/a0035264.
- Petry NM. Contingency management treatments. Br J Psychiatry 2006;189:97-8. https://doi.org/10.1192/bjp.bp.106.022293.
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med 2016;92:36-4. https://doi.org/10.1016/j.ypmed.2016.08.008.
- Stanger C, Scherer EA, Babbin SF, Ryan SR, Budney AJ. Abstinence based incentives plus parent training for adolescent alcohol and other substance misuse. Psychol Addict Behav 2017;31:385-92. https://doi.org/10.1037/adb0000279.
- Ainscough TS, McNeill A, Strang J, Calder R, Brose LS. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend 2017;178:318-39. https://doi.org/10.1016/j.drugalcdep.2017.05.028.
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, Zajac K. Contingency management treatment for substance use disorders: how far has it come, and where does it need to go?. Psychol Addict Behav 2017;31:897-906. https://doi.org/10.1037/adb0000287.
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiatry 2013;170:94-101. https://doi.org/10.1176/appi.ajp.2012.11121831.
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction 2014;109:1426-36. https://doi.org/10.1111/add.12589.
- Murphy SM, McDonell MG, McPherson S, Srebnik D, Angelo F, Roll JM, et al. An economic evaluation of a contingency-management intervention for stimulant use among community mental health patients with serious mental illness. Drug Alcohol Depend 2015;153:293-9. https://doi.org/10.1016/j.drugalcdep.2015.05.004.
- National Institute for Health and Care Excellence (NICE) . Clinical Guideline on Drug Misuse in Over 16s: Psychosocial Interventions. Clinical Guideline 51 2007.
- Secades-Villa R, García-Rodríguez O, Higgins ST, Fernández-Hermida JR, Carballo JL. Community reinforcement approach plus vouchers for cocaine dependence in a community setting in Spain: six-month outcomes. J Subst Abuse Treat 2008;34:202-7. https://doi.org/10.1016/j.jsat.2007.03.006.
- Weaver T, Metrebian N, Hellier J, Pilling S, Charles V, Little N, et al. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet 2014;384:153-63. https://doi.org/10.1016/S0140-6736(14)60196-3.
- Priebe S, Yeeles K, Bremner S, Lauber C, Eldridge S, Ashby D, et al. Effectiveness of financial incentives to improve adherence to maintenance treatment with antipsychotics: cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5847.
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry 2006;63:426-32. https://doi.org/10.1001/archpsyc.63.4.426.
- Sigmon SC, Steingard S, Badger GJ, Anthony SL, Higgins ST. Contingent reinforcement of marijuana abstinence among individuals with serious mental illness: a feasibility study. Exp Clin Psychopharmacol 2000;8:509-17. https://doi.org/10.1037/1064-1297.8.4.509.
- Sigmon SC, Higgins ST. Voucher-based contingent reinforcement of marijuana abstinence among individuals with serious mental illness. J Subst Abuse Treat 2006;30:291-5. https://doi.org/10.1016/j.jsat.2006.02.001.
- Johnson S, Sheridan Rains L, Marwaha S, Strang J, Craig T, Weaver T, et al. A randomised controlled trial of the clinical and cost-effectiveness of a contingency management intervention compared to treatment as usual for reduction of cannabis use and of relapse in early psychosis (CIRCLE): a study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1620-x.
- Department of Health and Social Care (DHSC) . The NHS Plan: A Plan for Investment, a Plan for Reform 2000.
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241-7. https://doi.org/10.1001/archpsyc.56.3.241.
- Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001;158:1835-42. https://doi.org/10.1176/appi.ajp.158.11.1835.
- Sobell L, Sobell M, Allen J, Litten R. Measuring Alcohol Consumption: Psychosocial and Biological Methods. New York, NY: Humana Press; 1992.
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 2014;28:154-62. https://doi.org/10.1037/a0030992.
- Kay S, Fiszbein R, Opler L. The positive and negative syndrome scale for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophr Res 2005;79:231-8. https://doi.org/10.1016/j.schres.2005.04.008.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- McCrone P, Patel A, Knapp M, Schene A, Koeter M, Amaddeo F, et al. A comparison of SF-6D and EQ-5D utility scores in a study of patients with schizophrenia. J Ment Health Policy Econ 2009;12:27-31.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and validity of the SF-12 health survey among people with severe mental illness. Med Care 2000;38:1141-50. https://doi.org/10.1097/00005650-200011000-00008.
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health 1999;53:46-50. https://doi.org/10.1136/jech.53.1.46.
- Edwards JE, Cocks J, Bott J, McGorry PD, Jackson HJ. The Recognition and Management of Early Psychosis: a Preventative Approach. Cambridge: Cambridge University Press; 1999.
- Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, et al. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr Scand 2006;114:109-17. https://doi.org/10.1111/j.1600-0447.2006.00783.x.
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051-61. https://doi.org/10.1037/0022-006X.68.6.1051.
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive–behavioral therapy for cannabis dependence. J Consult Clin Psychol 2006;74:307-16. https://doi.org/10.1037/0022-006X.74.2.307.
- Kalia S, Klar N, Donner A. On the estimation of intracluster correlation for time-to-event outcomes in cluster randomized trials. Stat Med 2016;35:5551-60. https://doi.org/10.1002/sim.7145.
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56:645-6. https://doi.org/10.1111/j.0006-341X.2000.00645.x.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017 2006;3:77-101. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2017/ (accessed 16 July 2019).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 16 July 2019).
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. PharmacoEconomics 1996;10:460-6. https://doi.org/10.2165/00019053-199610050-00003.
- Office for National Statistics (ONS) . EARN01 Average Weekly Earnings – January 2018 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/averageweeklyearningsearn01 (accessed February 2018).
- Gabrio A, Mason AJ, Baio G. Handling missing data in within-trial cost-effectiveness analysis: a review with future recommendations. Pharmacoecon Open 2017;1:79-97. https://doi.org/10.1007/s41669-017-0015-6.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Brazier J. Is the EQ-5D fit for purpose in mental health?. Br J Psychiatry 2010;197:348-9. https://doi.org/10.1192/bjp.bp.110.082453.
- Promberger M, Brown R, Ashcroft R, Marteau T. Acceptability of financial incentives to improve health outcomes in UK and US samples. J Med Ethics 2011;37:682-7. https://doi.org/10.1136/jme.2010.039347.
- Carrà G, Battaglia C, Hinton M, Sheridan Rains L, Crocamo C, Johnson S. Social network confidants, duration of untreated psychosis and cannabis use in people with first episode psychosis: an exploratory study. Early Interv Psychiatry 2018;12:942-6. https://doi.org/10.1111/eip.12476.
- Carrà G, Johnson S, Crocamo C, Angermeyer MC, Brugha T, Azorin JM, et al. Psychosocial functioning, quality of life and clinical correlates of comorbid alcohol and drug dependence syndromes in people with schizophrenia across Europe. Psychiatry Res 2016;239:301-7. https://doi.org/10.1016/j.psychres.2016.03.038.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
Appendix 1 Missing data tables
| Characteristic | OR | 95% CI |
|---|---|---|
| Male | 0.85 | 0.48 to 1.49 |
| Age | 0.98 | 0.93 to 1.02 |
| Ethnicity | ||
| White | Ref | |
| Black | 1.34 | 0.80 to 2.25 |
| Asian | 0.71 | 0.36 to 1.38 |
| Other | 1.54 | 0.88 to 2.68 |
| Marital status | ||
| Single | Ref | |
| Married or cohabiting | 1.16 | 0.55 to 2.42 |
| Other | 1.26 | 0.32 to 5.05 |
| Educational attainment | ||
| No qualifications | Ref | |
| GCSE or equivalent | 1.06 | 0.61 to 1.85 |
| A level or equivalent | 1.27 | 0.68 to 2.35 |
| Post-18 education (including HND, trade, degree) | 0.96 | 0.49 to 1.87 |
| Living arrangements | ||
| Alone | Ref | |
| With parents | 0.86 | 0.58 to 1.27 |
| With other adults (only) | 0.95 | 0.59 to 1.55 |
| Other | 0.20 | 0.06 to 0.68 |
| Housing | ||
| Independent permanent accommodation | Ref | |
| Independent temporary accommodation | 1.20 | 0.69 to 2.08 |
| Supported accommodation | 1.29 | 0.81 to 2.07 |
| Other | 0.70 | 0.32 to 1.49 |
| Work or study | ||
| Ever had open-market employment | 0.81 | 0.49 to 1.36 |
| Ever kept open-market employment for 1 year | 0.89 | 0.64 to 1.25 |
| Had open-market job since first contact with services | 1.24 | 0.84 to 1.82 |
| Current paid activity | 1.40 | 0.86 to 2.26 |
| Open-market full-time employment | 1.24 | 0.67 to 2.30 |
| Open-market part-time employment | 1.59 | 0.84 to 3.03 |
| Permitted work | 2.08 | 0.13 to 32.81 |
| Voluntary/unpaid work | 1.04 | 0.36 to 2.97 |
| Study or training | 0.78 | 0.46 to 1.32 |
| Full-time caringa | ||
| Unemployed | 1.78 | 1.06 to 2.99 |
| Exempt because of disability | 0.51 | 0.29 to 0.90 |
| Any work or study | 1.07 | 0.72 to 1.57 |
| Benefits | ||
| Income support | 0.76 | 0.39 to 1.95 |
| Incapacity benefit | 0.93 | 0.44 to 1.95 |
| DLA care component | 0.84 | 0.51 to 1.38 |
| DLA mobility component | 0.89 | 0.36 to 2.18 |
| PIP | 1.36 | 0.69 to 2.69 |
| DLA or PIP | 0.97 | 0.63 to 1.49 |
| Severe disablement allowance | 0.69 | 0.07 to 6.70 |
| Council tax benefit | 0.59 | 0.29 to 1.20 |
| Housing benefit | 1.00 | 0.66 to 1.52 |
| Jobseekers’ allowance | 0.99 | 0.53 to 1.84 |
| Working tax credits | 4.18 | 0.74 to 23.60 |
| Statutory sick pay | 4.15 | 0.38 to 45.38 |
| Employment and support allowance | 1.28 | 0.85 to 1.91 |
| Other benefits | 0.77 | 0.29 to 2.03 |
| Diagnosis | ||
| Schizophrenia or schizoaffective disorder | Ref | |
| Bipolar affective disorder | 1.38 | 0.67 to 2.84 |
| Depression with psychotic symptoms | 1.03 | 0.33 to 3.18 |
| Other psychosis | 1.11 | 0.73 to 1.67 |
| Alcohol abuse | 0.85 | 0.55 to 1.31 |
| Alcohol dependence | 0.83 | 0.51 to 1.37 |
| Cannabis use | ||
| 1–3 times per week | Ref | |
| > 3 times per week | 1.20 | 0.81 to 1.78 |
| Drugs taken | ||
| Sedatives, hypnotics, anxiolytics | 0.84 | 0.46 to 1.54 |
| Stimulants | 0.73 | 0.46 to 1.18 |
| Opioids | 0.60 | 0.32 to 1.14 |
| Cocaine | 0.97 | 0.66 to 1.44 |
| Hallucinogens/PCP | 0.69 | 0.46 to 1.05 |
| Legal highs | 1.00 | 0.58 to 1.73 |
| Other | 0.83 | 0.49 to 1.39 |
| PANSS | ||
| Positive symptoms | 1.03 | 0.99 to 1.07 |
| Negative symptoms | 0.99 | 0.97 to 1.02 |
| SF-12 | ||
| Physical | 1.00 | 0.99 to 1.01 |
| Mental | 1.00 | 0.99 to 1.01 |
| Number of | ||
| Days using cannabis in the previous 6 months | 1.00 | 1.00 to 1.01 |
| Characteristic | OR | 95% CI |
|---|---|---|
| Male | 0.82 | 0.50 to 1.36 |
| Age | 0.99 | 0.96 to 1.03 |
| Ethnicity | ||
| White | Ref | |
| Black | 0.97 | 0.64 to 1.46 |
| Asian | 0.80 | 0.46 to 1.40 |
| Other | 1.07 | 0.63 to 1.81 |
| Marital status | ||
| Single | Ref | |
| Married or cohabiting | 0.86 | 0.43 to 1.70 |
| Other | 3.12 | 0.62 to 15.65 |
| Educational attainment | ||
| No qualifications | Ref | |
| GCSE or equivalent | 1.06 | 0.61 to 1.83 |
| A level or equivalent | 0.82 | 0.44 to 1.54 |
| Post-18 education (including HND, trade, degree) | 0.81 | 0.42 to 1.56 |
| Living arrangements | ||
| Alone | Ref | |
| With parents | 0.86 | 0.55 to 1.32 |
| With other adults (only) | 1.05 | 0.64 to 1.71 |
| Other | 0.55 | 0.25 to 1.18 |
| Housing | ||
| Independent permanent accommodation | Ref | |
| Independent temporary accommodation | 1.17 | 0.67 to 2.07 |
| Supported accommodation | 1.47 | 0.95 to 2.29 |
| Other | 1.83 | 0.95 to 3.55 |
| Work or study | ||
| Ever had open-market employment | 0.77 | 0.48 to 1.26 |
| Ever kept open-market employment for 1 year | 0.90 | 0.64 to 1.25 |
| Had open-market job since first contact with services | 1.21 | 0.83 to 1.75 |
| Current paid activity | 1.28 | 0.83 to 1.97 |
| Open-market full-time employment | 1.38 | 0.76 to 2.51 |
| Open-market part-time employment | 1.29 | 0.71 to 2.33 |
| Permitted work | 1.03 | 0.06 to 16.85 |
| Voluntary/unpaid work | 0.39 | 0.15 to 0.99 |
| Study or training | 0.91 | 0.55 to 1.50 |
| Full-time caringa | ||
| Unemployed | 0.94 | 0.66 to 1.35 |
| Exempt because of disability | 1.01 | 0.70 to 1.46 |
| Any work or study | 1.17 | 0.84 to 1.63 |
| Benefits | ||
| Income support | 0.88 | 0.51 to 1.51 |
| Incapacity benefit | 1.69 | 0.96 to 2.98 |
| DLA care component | 1.08 | 0.69 to 1.68 |
| DLA mobility component | 1.41 | 0.73 to 2.74 |
| PIP | 0.95 | 0.50 to 1.81 |
| DLA or PIP | 1.11 | 0.78 to 1.60 |
| Severe disablement allowance | 0.33 | 0.03 to 3.26 |
| Council tax benefit | 1.02 | 0.59 to 1.78 |
| Housing benefit | 1.23 | 0.78 to 1.93 |
| Jobseekers’ allowance | 1.14 | 0.56 to 2.31 |
| Working tax credits | 1.02 | 0.21 to 5.06 |
| Statutory sick pay | 2.04 | 0.18 to 23.14 |
| Employment and support allowance | 0.86 | 0.59 to 1.25 |
| Other benefits | 0.82 | 0.43 to 1.56 |
| Diagnosis | ||
| Schizophrenia or schizoaffective disorder | Ref | |
| Bipolar affective disorder | 1.06 | 0.60 to 1.90 |
| Depression with psychotic symptoms | 0.22 | 0.06 to 0.79 |
| Other psychosis | 0.83 | 0.55 to 1.25 |
| Alcohol abuse | 0.99 | 0.70 to 1.41 |
| Alcohol dependence | 0.70 | 0.46 to 1.06 |
| Cannabis use | ||
| 1–3 times per week | Ref | |
| > 3 times per week | 1.16 | 0.84 to 1.62 |
| Drugs taken | ||
| Sedatives, hypnotics, anxiolytics | 1.16 | 0.67 to 2.00 |
| Stimulants | 0.92 | 0.61 to 1.39 |
| Opioids | 1.04 | 0.58 to 1.89 |
| Cocaine | 0.89 | 0.63 to 1.26 |
| Hallucinogens/PCP | 0.93 | 0.65 to 1.35 |
| Legal highs | 1.34 | 0.81 to 2.23 |
| Other | 0.70 | 0.43 to 1.12 |
| PANSS | ||
| Positive symptoms | 1.02 | 0.98 to 1.06 |
| Negative symptoms | 1.01 | 0.98 to 1.04 |
| SF-12 | ||
| Physical | 0.99 | 0.97 to 1.00 |
| Mental | 1.00 | 0.99 to 1.01 |
| Number of | ||
| Days using cannabis in the previous 6 months | 1.00 | 1.00 to 1.01 |
| Characteristic | OR | 95% CI |
|---|---|---|
| Male | 0.94 | 0.50 to 1.79 |
| Age | 0.97 | 0.93 to 1.02 |
| Ethnicity | ||
| White | Ref | |
| Black | 1.23 | 0.69 to 2.18 |
| Asian | 0.58 | 0.26 to 1.30 |
| Other | 1.72 | 0.97 to 3.05 |
| Marital status | ||
| Single | Ref | |
| Married or cohabiting | 0.88 | 0.38 to 2.00 |
| Other | 1.80 | 0.45 to 7.21 |
| Educational attainment | ||
| No qualifications | Ref | |
| GCSE or equivalent | 1.11 | 0.61 to 2.01 |
| A level or equivalent | 1.13 | 0.59 to 2.15 |
| Post-18 education (including HND, trade, degree) | 0.81 | 0.39 to 1.69 |
| Living arrangements | ||
| Alone | Ref | |
| With parents | 0.86 | 0.56 to 1.33 |
| With other adults (only) | 0.96 | 0.55 to 1.67 |
| Other | 0.29 | 0.09 to 0.99 |
| Housing | ||
| Independent permanent accommodation | Ref | |
| Independent temporary accommodation | 1.25 | 0.73 to 2.15 |
| Supported accommodation | 1.24 | 0.75 to 2.04 |
| Other | 0.76 | 0.33 to 1.72 |
| Work or study | ||
| Ever had open-market employment | 0.81 | 0.46 to 1.44 |
| Ever kept open-market employment for 1 year | 0.92 | 0.63 to 1.33 |
| Had open-market job since first contact with services | 1.55 | 1.03 to 2.32 |
| Current paid activity | 1.73 | 1.08 to 2.78 |
| Open-market full-time employment | 1.50 | 0.80 to 2.83 |
| Open-market part-time employment | 1.89 | 1.03 to 3.47 |
| Permitted work | 3.01 | 0.19 to 47.81 |
| Voluntary/unpaid work | 0.59 | 0.17 to 2.10 |
| Study or training | 0.75 | 0.41 to 1.37 |
| Full-time caringa | – | – |
| Unemployed | 1.75 | 1.00 to 3.08 |
| Exempt because of disability | 0.57 | 0.30 to 1.09 |
| Any work or study | 1.22 | 0.81 to 1.83 |
| Benefits | ||
| Income support | 0.67 | 0.32 to 1.38 |
| Incapacity benefit | 1.29 | 0.61 to 2.73 |
| DLA care component | 0.99 | 0.59 to 1.64 |
| DLA mobility component | 0.79 | 0.30 to 2.03 |
| PIP | 1.12 | 0.54 to 2.33 |
| DLA or PIP | 0.96 | 0.61 to 1.51 |
| Severe disablement allowancea | – | – |
| Council tax benefit | 0.57 | 0.27 to 1.21 |
| Housing benefit | 1.08 | 0.69 to 1.67 |
| Jobseekers’ allowance | 0.61 | 0.28 to 1.33 |
| Working tax credits | 1.49 | 0.26 to 8.52 |
| Statutory sick pay | 6.00 | 0.54 to 66.68 |
| Employment and support allowance | 1.13 | 0.73 to 1.76 |
| Other benefits | 1.13 | 0.43 to 2.98 |
| Diagnosis | ||
| Schizophrenia or schizoaffective disorder | Ref | |
| Bipolar affective disorder | 1.32 | 0.62 to 2.83 |
| Depression with psychotic symptoms | 0.22 | 0.03 to 1.70 |
| Other psychosis | 1.14 | 0.73 to 1.76 |
| Alcohol abuse | 0.71 | 0.46 to 1.09 |
| Alcohol dependence | 0.70 | 0.42 to 1.17 |
| Cannabis use | ||
| 1–3 times per week | Ref | |
| > 3 times per week | 1.33 | 0.87 to 2.04 |
| Drugs taken | ||
| Sedatives, hypnotics, anxiolytics | 0.97 | 0.52 to 1.80 |
| Stimulants | 0.93 | 0.57 to 1.51 |
| Opioids | 0.77 | 0.39 to 1.50 |
| Cocaine | 0.87 | 0.57 to 1.33 |
| Hallucinogens/PCP | 0.70 | 0.45 to 1.09 |
| Legal highs | 1.05 | 0.59 to 1.87 |
| Other | 0.81 | 0.48 to 1.36 |
| PANSS | ||
| Positive symptoms | 1.03 | 0.99 to 1.08 |
| Negative symptoms | 1.00 | 0.97 to 1.03 |
| SF-12 | ||
| Physical | 1.00 | 0.98 to 1.02 |
| Mental | 1.00 | 0.98 to 1.01 |
| Number of | ||
| Days using cannabis in the previous 6 months | 1.00 | 1.00 to 1.01 |
Appendix 2 Recruitment graph
FIGURE 10.
Recruitment to the trial.
Appendix 3 Qualitative substudy following completion of the pilot phase of the trial
Introduction
The pilot phase of the trial successfully recruited 62 participants and featured 11 EIP teams. Following the pilot study, a qualitative study was performed to explore the usefulness and acceptability of the intervention from the perspective of participants and of clinicians, and potential mechanisms for change.
Methods
Sample
Qualitative data collection was performed with three groups between March and August 2013: one-to-one interviews with participants in the CM arm, focus groups with clinicians in EIP teams participating in the CIRCLE trial, and carers of participants in the CM arm. Interviews were performed by members of the CIRCLE trial research team (Joanne Taylor and LSR).
Focus groups were performed with five EIP teams included in the pilot phase of the trial. Between two and nine clinicians attended each focus group. Service user and carer data collection comprised one-to-one interviews performed by a member of the CIRCLE trial research team using a semistructured interview schedule. Service user qualitative interviews were performed with 11 participants in the CM arm. As a result of significant difficulties in recruiting carers, only one interview was performed with a carer of a participant in the CM arm. The main barrier to recruitment was identifying suitable candidates. For most participants, discussing their cannabis use with family was a sensitive topic and so few participants felt comfortable with researchers interviewing family about the trial.
Interview schedules
Topic guides were refined in collaboration with the service user and carer steering groups. Focus groups with staff explored clinicians’ experiences of implementing CM schedules and any impediments encountered, the impact of the organisational context and culture on CM delivery, their views regarding the ethical and clinical implications of CM and their perceptions of service users’ responses to the intervention. Interviews with service users considered their perception of the effects of being offered incentives, their views regarding ethical aspects of this and suggestions for improvements. The carer interview explored perspectives on the intervention, including their views on the use of voucher rewards and how these should be presented.
Analysis
Interviews and focus groups were digitally recorded and transcribed by a professional transcription service (WayWithWords, London, UK) and imported into the NVivo 9 package (QSR International, Warrington, UK) for data coding and analysis. A thematic approach was taken to analysing the data for trial participants and clinicians. 74 As we were able to conduct only one carer interview, these data were not thematically analysed but are summarised in this report. Clinician and trial participant data were analysed and are discussed here separately, in sections Sevice users and EIP clinicians. The thematic analysis involved the following phases: each transcript was first reviewed by three members of the CIRCLE trial research team (Kate Fullarton, Johanna Frerichs and LSR) independently to familiarise themselves with the data and make initial notes. The following steps were then performed:
-
Kate Fullarton generated initial codes for all the data and collated data relevant to each code. Johanna Frerichs independently analysed a subset of the data (from two focus groups and three participant interviews).
-
Kate Fullarton and JF then independently organised the codes into potential themes, and identified data relevant to those themes.
-
Kate Fullarton, Johanna Frerichs and LSR then reviewed the initial codings and themes each had generated and reviewed whether or not the identified themes accurately represented the data.
Any agreed recoding of data or redefining of themes would then be reviewed further until a consensus between Kate Fullarton and Johanna Frerichs had been achieved. Although no substantial points of difference were identified, some small changes were made following the initial analyses and themes were again reviewed.
-
Following this, themes were defined and organised by Kate Fullarton into a thematic ‘map’, which sorted themes into ‘main’ and ‘sub’ themes. This map was then reviewed with Johanna Frerichs and LSR.
-
Kate Fullarton produced the final report of the results of the analysis.
Relevant conclusions drawn from the qualitative interview informed preparation of materials and changes to the trial protocol ahead of the main trial. Topic guides elicited the service user perspective.
Results
Results from the qualitative interviews are presented by group.
EIP clinicians
Most clinicians did not have a straightforwardly positive or negative view, with some reservations voiced. However, positive comments appeared to predominate with staff reporting that they felt that the scheme was acceptable and potentially useful. Staff reported that the scheme was ‘taken quite well by clients’ and particularly those in the CM arm ‘did enjoy it’. Two staff members reported that they felt that both the psychoeducation and the CM were useful opportunities for their clients, and that they ‘motivated them’ and that ‘they found [them] helpful’. However, some staff felt that there were important limitations to the scheme, particularly with regard to its lasting impact post treatment. We will discuss, in turn, the five main themes emerging from interviews.
1. Opinions about the interventions overall
Initial impressions
Overall, clinicians were generally positive about both the CM and the psychoeducation, but some were concerned that they would not have a long-term impact.
Positive effects
Staff from the range of focus groups reported the positive effects of the scheme for their clients, particularly those assigned to the intervention arm of the trial. These positive outcomes included:
-
Increased engagement with service users. Some clinicians reported that they were seeing their clients more often and found it easier to contact them.
-
Encouraging service users to quit smoking cannabis, at least during the period of the scheme. One staff member said: ‘the people who were on the CM did remain abstinent you know, during the course of the trial’.
-
Even if they did not quit, many service users reduced their use. One clinician said ‘[my client’s cannabis use] is not as bad as it was before, [he only] takes a little bit now’.
-
Encouraging service users to make positive changes due to reducing cannabis use. One clinician said of their client: ‘It kind of helped him . . . he started recognising the changes in himself . . . he became more kind of alert, concentration-wise . . . and he was doing a bit more . . . he was much better off, he was eating better’.
Limitations
There were two main limitations discussed by clinicians:
-
Difficulty recruiting service users to the trial. In addition, some clinicians found that clients did not engage with the intervention, particularly those in the control arm. One clinician said that he was not able to ‘engage any of my clients in the CIRCLE trial’ and felt that that ‘said a lot as they were kind of young 18-year-olds, using quite a lot of cannabis . . . and there was interest but never able to kind of just follow up or see it through’. This staff member said that the people who he felt would most benefit from the scheme were ‘just not interested’ and felt that for participants to be interested they required insight into what happened to them and ‘to kind of make the link to cannabis’, which he felt ‘some people do and some don’t’. Staff felt that it was a difficult area to involve service users in and that service users needed to have their own motivation to be willing and ‘ready’ to engage.
-
Post-intervention relapse. A commonly held concern was that the scheme would not be likely to have a lasting effect on their clients and that participants may go back to using once the scheme ended. One staff member said that the CM was beneficial to her client, but when the trial finished he relapsed. She felt this was because ‘he had been using for a long time in his life and any little stresses he gets, he goes back to it’.
Ethics views
Views on the ethics of CM were generally positive, with few reservations about whether or not it is proper to give financial rewards for abstinence. Many staff members saw no ethical problems and felt that if it worked, then it should continue. However, a few clinicians did raise questions about whether or not people should receive rewards for something ‘they shouldn’t be doing’ and asked ‘what about the clients who don’t smoke in the first place, that is almost incentivising them to smoke, so they can stop’. However, many of those clinicians also felt that these concerns would not stop them from engaging their clients in the scheme if it would help. Important questions were raised about whether or not people would have a problem with public money being given to participants and one staff member felt that people’s ‘own motivation and moral compass, should be enough, perhaps?’. Another staff member felt that other service users could potentially be ‘unhappy about cannabis users getting rewards’ and queried whether or not CM should be used for something like cannabis use at all. Another clinician had been concerned that participants may exchange the vouchers for cash, but his clients had actually used the vouchers appropriately.
2. Voucher scheme
Most staff members thought that the voucher scheme was beneficial for their clients.
Motivation
All staff members reported that the voucher scheme and the monitoring of THC levels motivated their clients to some extent to stop/reduce their cannabis use. One staff member described the scheme as a ‘good incentive’ and reported that his client was ‘looking forward each week . . . in terms of seeing his . . . cannabis result’. Even in cases in which participants did not stop smoking cannabis, staff felt that it had ‘created some kind of level of motivation . . . in terms of making decisions . . . about whether they are ready to stop or not’.
-
Pre-existing motivation. Two staff members reflected that clients who had engaged well with the scheme and had significantly reduced/quit their cannabis use may have quit anyway. One staff member said, ‘people have to be really . . . contemplating giving up smoking to engage in something like that . . . if they are not interested in giving up then – however much money you throw at them – it’s just not going to make a difference’.
-
Not enough incentive. Staff from two teams felt that even the voucher scheme option was not enough of an incentive for their clients to stop using cannabis. One staff member said that the scheme ‘didn’t seem to be enough of a hook’ and felt that this was because ‘they had enough money anyway’.
-
Complicated system. Two staff members reported that a negative aspect of the voucher scheme was the ‘complicated’ reward schedule. One staff member said that it was a ‘confusing mechanism that could possibly be simplified’ as he felt it was ‘confusing to try and explain to people’.
-
Impact on relationship. Three staff members raised concerns about the potentially negative impact the CM intervention could have on their relationship with their client, particularly if the client disagreed with the result. But no clinicians reported any personal experience of this happening. Most clinicians agreed that this could be resolved by a nominated person in the team delivering the intervention, such as a support worker or assistant psychologist. One staff member shared that she had a participant whom she suspected had ‘watered down her sample to attempt to cheat’ and that this had led to the participant stopping coming for a while. She reflected that this could have been because the participant may have been ‘ashamed’ to see her. Another staff member said that he always has very good working relationships with his clients and that delivering the intervention did not have an impact on those relationships at all.
-
Urine testing. When staff were asked for their views on delivering the urinalysis aspect of the intervention, feedback was generally positive with no issues raised; staff found it relatively straightforward. One staff member felt that his client was particularly motivated by seeing his result each week.
3. Psychoeducation
There were mixed views about the psychoeducation package, with some staff reporting that they thought it was less useful than the CM and more difficult to engage their clients with than the CM. Staff who were positive about the psychoeducation package thought that some aspects of the package were particularly useful, but also reported that some improvements that could be made.
Positive aspects
-
Format of material. Staff felt that the way in which the psychoeducation package was presented was very clear and ‘easy to use’. One member of staff commented that they thought it was a ‘good idea’ and felt that it was a ‘different format and a way of helping them think about why they are using’.
-
Knowledge. Four members of staff felt that the knowledge their clients gained from the psychoeducation was useful. Staff felt that it was ‘good for reflection’ and clients would be able to ‘look back and have space to think of their own cannabis use’.
Negative aspects
Staff did have some criticisms of the psychoeducation package.
-
Repetitive. Four staff members felt that the content of the psychoeducation package was quite repetitive and could be improved in this regard.
Support with delivering intervention
Staff felt that they received a good level of support from the CIRCLE trial team, felt ‘adequately informed’ and had a good ‘understanding of the programme’ and recruitment process. One member of staff felt that they had ‘support and teaching sessions done for the whole team, which was quite beneficial‘. Another member of staff said that she ‘always found it easy to ask questions’ of members of staff from the research team and felt ‘well supported’.
However, one staff member felt that different CIRCLE trial team members sometimes gave conflicting answers to the teams’ questions about the urinalysis. The solution he proposed was to have a dedicated member of the team working on the CIRCLE trial.
4. The impact on others
Clinicians delivering intervention
Two staff members highlighted the difficulties of finding time to do the intervention, which they said may have an impact on motivation to engage with the trial. Another staff member said that it would be useful if support workers could help more with delivering the intervention. Some staff from one team spoke of how the perceived lack of support during the pilot meant that staff delivering the intervention felt quite frustrated. Other staff members reported no negative impact.
Families and friends
Two members of staff commented that families were supportive of their relatives taking part in the scheme. One staff member spoke of how one of his clients was using his vouchers to ‘take his mum to Tesco and buy food’, he reported his client’s mum was ‘pleased’ with him talking part in the intervention and ‘they have seen the changes throughout the trial’.
5. Continuing the intervention post scheme
Staff were generally positive about continuing the CM intervention post scheme if it was found to be effective. However, for some, this view tended to focus on psychoeducation rather than the CM, as many staff members felt the CM intervention was not sustainable in a routine EIP setting. The majority of staff felt that the psychoeducation would be ‘helpful’ and a ‘useful tool’ to continue in clients’ normal sessions. A member of staff suggested that she would be keen to suggest ‘doing some kind of group session’ of psychoeducation.
Summary and emerging recommendations for the main trial
Overall, staff impressions of the scheme were positive, believing that the CM and psychoeducation interventions were potentially useful interventions for cannabis use. However, many clinicians expressed mixed opinions about certain aspects of the scheme. Positive views of the CM intervention included that service users liked it and many reported their clients benefiting from it. In addition, the scheme helped to engage service users both in their treatment for cannabis use and with their EIP care team. The main concerns about the CM intervention was its sustainability post treatment cessation, and the potentially negative effect the intervention could have on their relationship with their client. There were also concerns about the details of the CIRCLE trial CM intervention specifically, which included that the reward schedule was too complicated and that the urine testing could be incorrect as a result of adulterated samples. The psychoeducation intervention was also viewed positively. Some clinicians requested to use the psychoeducation package once the trial had ended. The main concern raised about the psychoeducation related to some factual inaccuracies.
When feasible, concerns about the psychoeducation and CM interventions were addressed ahead of the main trial. Specifically, the main recommendations to emerge from the focus groups were to:
-
make the CM reward scheme simpler
-
prevent adulterated urine samples
-
have a dedicated EIP team member to deliver the intervention rather than care co-ordinators, such as a support worker/assistant psychologist; that person should ideally be interested in research
-
ensure that there is the same researcher or group of researchers contacting the EIP team about the CIRCLE trial and the clinician doing the intervention
-
address inaccuracies in the information given in the psychoeducation.
We were able to follow all of these recommendations, but with some limitations. The reward schedule was simplified and a temperature strip was added to the sample cups used in the urinalysis to prevent adulteration. Teams could identify a few dedicated members of staff within the team, such as support workers or assistant psychologists, to deliver the intervention if preferred. Materials were developed and provided to clinicians to make it easier for them to explain the trial to their clients when getting consent to being contacted by a researcher. Significant efforts were made by the research team to ensure that each EIP team were contacted by the same one or two researchers and, when needed, supporting clinicians delivering the intervention. Ahead of the main trial, reference manuals and guidelines were developed for the research team and separate ones were developed for the clinicians delivering the interventions. These aimed to address clinicians’ commonly asked questions and provide researchers with enough information to support the EIP teams effectively. This way, we improved the support EIP teams received and ensured that information they were provided with by the research team was consistent. Changes were made to the psychoeducation programme to make it less repetitive.
Service users
During the analysis, six main themes emerged.
1. Overall impact of the intervention
All 10 participants were generally positive about the scheme, with many saying that they thought it was ‘good’ and ‘beneficial’. Subthemes were as follows:
-
Helpful aspects of the intervention. Participants often felt that having their urinary THC level monitored was very helpful and motivating because they could see what was in their body each week, which was information they would not otherwise have access to. Some also felt that the urine testing meant that they could not cheat the process, and found it motivating to know that the clinician would find out the result because they did not want to let them down. The scheme also made some participants feel proactive and more in control over their use. Finally, there was common agreement that the combination of learning through the psychoeducation sessions and receiving the vouchers was particularly helpful.
-
Improvements recommended for the scheme. Participants often could not think of anything they would have changed to improve the scheme and said that they were perfectly happy with the arrangements. However, some improvements were suggested. One participant felt that there were repeated questions across some of the psychoeducation modules, which he did not find useful. Other suggestions included increasing the voucher amount, more opportunities to meet with someone for support/positive conversation, extending the number of weeks of the intervention, and one participant suggested encouraging socialising between participants and including group outings.
-
Lifestyle changes as a result of the intervention. Several participants talked about changes they had made because of the intervention. These included eating more healthily, saving money, adopting a more active lifestyle by attending the gym and changing the people they spend time with to avoid cannabis users.
-
Recommending the CIRCLE trial. When asked, participants said they would definitely recommend the intervention to friends who would like to give up cannabis.
2. Psychoeducation
-
Helpful. Seven participants found some or all of the psychoeducation helpful.
-
Unhelpful. Two participants did not find the psychoeducation helpful at all; one participant said that he/she would not have chosen to do this part of the scheme by itself. Another participant said that he did not feel that he had learnt anything from it.
-
Improvements. One participant suggested that an improvement to the psychoeducation modules would be to hear more from people in the videos who had had similar experiences.
-
Knowledge. Seven participants felt that the knowledge they gained by doing the psychoeducation modules was valuable. The following sub-subthemes emerged:
-
previously unaware of dangers
-
weighing up pros and cons
-
insight into disadvantages/recognising harm
-
weekly reminder of why they are trying to stop.
-
-
Experiences of peers. Participants felt that the videos they were shown during the psychoeducation modules were especially helpful. Hearing other people’s stories of having smoked cannabis and experiencing similar symptoms was reported to be very beneficial.
3. Quitting cannabis
-
Challenges. Six participants spoke about the challenges of trying to quit cannabis, including cravings, having to stay strong to say ‘no’ to friends who smoke, having to alter social networks and breaking long-term habits. They said that the scheme helped with these challenges.
-
Changes. Positive: six participants explicitly spoke about the positive changes in their lives since reducing/quitting cannabis, including that their minds felt clearer, they felt healthier and their family relationships were better. Negative: three participants spoke of negative changes that occurred since reducing/quitting cannabis, which focused on withdrawal symptoms and cravings/urges for cannabis.
-
Motivation. Eight participants said that they found the scheme motivated them to stop/cut down their cannabis use. A number of participants felt that their THC levels being monitored weekly was the most motivating aspect and others felt that it was receiving the vouchers. Six participants explained that they had already decided to reduce their use/give up cannabis before the scheme commenced, but felt that the scheme made it easier for them to achieve this.
-
Quit. Four participants spoke of managing to cut down and then stop smoking cannabis completely during the scheme.
-
Reduced use. Not all participants quit smoking but all of them felt that they had successfully managed to cut down while taking part in the scheme. Four participants said that they were managing to continue reducing their use after the scheme had ended.
4. Relationships
When participants were asked if they discussed the scheme with staff from the EIP, their care co-ordinator and/or friends or family, the majority of participants stated that they had chosen not to talk about it, but those who had talked about it felt that it had either made no difference to relationships or improved them:
-
EIP staff. Three participants felt that taking part in the scheme had a positive effect on their relationships with their care co-ordinators as they provided them with support/encouragement and someone to talk to about their progress. Six participants felt that it made no difference to their meetings, that is it had no positive or negative impact.
-
Family and friends. Six participants said that taking part in the scheme/discussing the scheme with their family had a positive impact on relationships with certain family members and that the family members they chose to tell were very supportive of them taking part in the scheme. A number of participants did not want to discuss the scheme with their family and/or friends.
5. Testing
-
Failed tests. Five participants shared that they felt disappointment when they failed the test but not about the voucher amount, more about letting themselves down. One participant said that failing the test one week was the motivation they needed to stop completely. One participant felt disappointed that he had failed because he had missed out on the increased voucher amount.
-
Urine test. Most participants did not have a problem with the experience of giving weekly urine samples and thought that the process was fine. One participant mentioned that they found it awkward to carry a urine sample in the EIP.
6. Vouchers
-
Fairness. All participants felt that the voucher system was fair.
-
Feelings about vouchers. Participants’ feelings about receiving the vouchers were positive, with participants saying that they appreciated them, were motivated by them or found them really helpful.
-
Not about the money. Four participants said that the important thing for them was staying focused on the THC levels in their system, and receiving the vouchers was just an added bonus.
-
Uses for vouchers. Participants gave a range of uses for their vouchers, mainly buying food, choosing healthy food, presents for their family and enabling them to save money.
-
Voucher amount. Participants’ views on the voucher amount varied. Two participants felt that the initial voucher amounts and the ‘fail’ £5 voucher amount were too small. One participant linked the motivation with the amount of money and said that if there had been more money they would have been more motivated. Three participants felt that the voucher amount was just right and were satisfied with the process.
Summary and recommendations for the main trial
The CM and psychoeducation interventions were well received. All participants were generally positive about the scheme, describing it as helpful and beneficial, and all participants who were asked said that they would recommend it to friends. Specifically helpful aspects of the intervention included that the intervention gave them motivation to quit and that passing gave them a sense of accomplishment, the urinary THC concentration monitoring provided them with information about how much cannabis was in their system, which many found the most useful aspect of the scheme, and participants said that they felt that the process was fair. The scheme also helped people feel more in control of their cannabis use, and some reported feeling good about proving that they had stopped using cannabis. When asked about the combination of CM and psychoeducation, all agreed that it was important that the CM intervention was accompanied by the psychoeducation, as otherwise the scheme would provide no rationale to explain why quitting was worthwhile. Participants could not think of any areas of improvement, except perhaps that the voucher rewards could be higher or that the duration of the scheme could be longer. The issues raised by participants regarding repetition in the psychoeducation and awkwardness associated with giving urine samples were carefully considered ahead of the main trial. Researchers looked for more discrete options for the urinalysis process and changes were made to the psychoeducation programme to ensure that information was accurate and not repetitive.
Carer
Only one interview was conducted with a carer of a participant in the trial and so thematic analysis was not used. However, LSR summarised the key aspects to emerge from the interview.
Overall
The carer thought that the scheme was ‘brilliant’ as it helped her son come off cannabis, which she believed was the basis of his mental and physical ‘problems’. She said that her son looked forward to the meetings and avoided using cannabis in order to receive the reward:
Being his mother and seeing what he’s been through and the harm that he’s done to himself all because of drugs, you know, it’s been absolutely horrendous and a nightmare . . . Anything that can help these . . . young men and women is definitely, yes, I don’t think enough is being done for it.
Positive aspects of the scheme
The carer reported that she thought that one of the main benefits of the scheme was that it targeted cannabis because there was very little to help people who use cannabis, unlike the use of other substances such as heroin. She said that there was a need for cannabis treatment programmes and that incentives or perhaps cannabis substitutes are ‘very helpful’ and ‘very useful’. She said that she saw a ‘drastic’ change in her son when he took part in the scheme.
Negative aspects of the scheme
The carer said that she had no concerns about the scheme overall. However, she thought that her son would benefit from support over a longer period and suggested that perhaps the scheme should be longer or have ‘follow-up’ sessions.
Views of the urinalysis sessions
She had no concerns about the urinalysis sessions. She said that if her son did not pass a session, he appeared to feel he had let himself down. The more sessions he attended, the more he got from it and the more he felt good about himself and what he had achieved. She knew that her son would occasionally smoke a small amount soon after a urinalysis session, believing that it would not be detectable at the following session. However, she said he would then feel guilty about trying to deceive her and the clinicians delivering the intervention.
Conclusions
Overall, the carer had positive views of the CIRCLE trial. In terms of recommendations, they thought that the scheme could be longer or that there should be more support to help people using cannabis post treatment.
Appendix 4 Outcome measures
1. Demographics
2. Positive and Negative Syndrome Scale
3. SF-12
4. Client Service Receipt Inventory (CSRI)
5. Timeline Follow Back (TLFB)
6. Structured Clinical Interview for the DSM-IV
List of abbreviations
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CIRCLE
- Contingency Intervention for Reduction of Cannabis in Early Psychosis
- CM
- contingency management
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- EIP
- Early Intervention in Psychosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HR
- hazard ratio
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness
- IQR
- interquartile range
- IRR
- incidence rate ratio
- MI
- motivational interviewing
- MIDAS
- Motivational Interventions for Drugs & Alcohol misuse in Schizophrenia
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- OTAU
- optimised treatment as usual
- PANSS
- Positive and Negative Syndrome Scale
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- TAU
- treatment as usual
- THC
- tetrahydrocannabinol
- TLFB
- timeline followback
- VAS
- visual analogue scale
