Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/14/01. The contractual start date was in September 2014. The draft report began editorial review in October 2017 and was accepted for publication in March 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Shirley A Thomas and Avril ER Drummond report grants from the National Institute for Health Research (NIHR) and the Stroke Association outside the submitted work. Rebecca L Palmer is an author of the Consent Support Tool, which was piloted in the work and is discussed in the report. Roshan das Nair is a member of the NIHR Health Services and Delivery Research Board. Nicholas R Latimer is supported by the NIHR (NIHR Post Doctoral Fellowship PDF-2015-08-022). Stephen J Walters is a member of the NIHR Health Technology Assessment (HTA) Clinical Trials Board and the NIHR HTA Funding Boards Policy Group; he also reports grants from the NIHR HTA programme during the conduct of the study, personal fees from royalties, research grants from the NIHR and the Medical Research Council, personal fees from external examining fees and book royalties outside the submitted work. Cindy L Cooper is a member of the NIHR Clinical Trials Unit Standing Advisory Committee.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Post-stroke depression
Stroke
Stroke is a condition in which interruption of the blood supply to the brain causes brain damage. This leads to the impairment of motor, sensory and cognitive abilities. Cognitive impairments include disorders of communication, such as aphasia, and problems with attention, memory, visuospatial abilities and executive function. These cognitive impairments have a negative effect on recovery and long-term outcomes. 1–3
Depression after stroke
Many stroke patients experience emotional consequences, including depression, anxiety, post-traumatic stress, anger, apathy and frustration. Depression is the most commonly investigated emotional consequence of stroke. 4,5
Several recent reviews5–8 have reported that about one-third of stroke patients have depression at any time point. Two of these reviews1,2 included a meta-analysis. Ayerbe et al. 1 analysed data from 43 studies published between 1983 and 2011. They reported an average prevalence of depression among stroke survivors of 29% [95% confidence interval (CI) 25% to 32%] of stroke survivors with depression, with a prevalence of 28% (95% CI 23% to 34%) within 1 month of stroke, 31% (95% CI 24% to 39%) at 1–6 months, 33% (95% CI 23% to 43%) at 6 months to 1 year and 25% (95% CI 19% to 32%) at > 1 year. Hackett and Pickles2 conducted a similar review and identified 61 studies. They obtained a pooled prevalence of depression among stroke survivors 31% (95% CI 28% to 35%) at any time, up to 5 years after stroke. Hackett et al. 3 also highlighted that this figure was not significantly different from the proportion in their earlier review (33%, 95% CI 29% to 36%; difference of 2%, 95% CI < 1% to 3%), suggesting that the management of the problem had not substantially improved over the previous 10 years.
Factors associated with depression after stroke
Effective treatment of depression is important because depression is associated with worse rehabilitation outcomes4–7 and increased disability. 1 Stroke survivors who are depressed may engage less in rehabilitation, which, in turn, can lead to decreased functional recovery. 4 Depression is also associated with increased mortality. 1,8,9 Not only does depression affect stroke survivors themselves but it also has an effect on their carers. 10 It has cost implications for the NHS because it is associated with increased health-care utilisation. 9
Most studies have assessed depression and its potential outcomes at the same time point, making it unclear whether depression is a cause or consequence of the outcome variable. Ayerbe et al. 1 reviewed only those studies in which depression was assessed at an earlier time point than the outcome and found that disability, lower quality of life (QoL) and mortality may be outcomes of depression in stroke survivors. In a more recent review, Towfighi et al. 11 concluded that the most consistent predictors of post-stroke depression are physical disability, stroke severity, history of depression and cognitive impairment. They also reported that post-stroke depression is associated with higher rates of health-care use after stroke. Therefore, in addition to improving mood, effective treatment of post-stroke depression is important as this has the potential to improve functional outcomes and QoL and reduce health-care costs.
Many studies of depression after stroke are based on clinical interviews or questionnaires to assess depression but these may not be appropriate for those with communication problems. About one-third of stroke survivors have aphasia,12,13 which may affect all communication modalities, namely speaking, understanding, reading and writing. Studies that have used measures of depression appropriate for those with communication problems have reported that stroke survivors with aphasia may be particularly susceptible to post-stroke depression. 14,15
Current service provision
Psychological treatments for depression after stroke
Previous research16 indicates that a high proportion of depressed stroke patients are likely to be taking antidepressants and so suggests that antidepressants have not resolved the mood problem. Previous research also suggests that few stroke survivors receive ongoing psychological treatment. 17 The Communication and Low Mood (CALM) trial of behavioural activation (BA) for low mood in people with aphasia17 found that, at 3-month follow-up, only 14% of participants who had been identified as having low mood after stroke had received mental health treatment in the past 3 months (from a mental health nurse, counsellor, psychologist, or psychiatrist). Although Improving Access to Psychological Therapies (IAPT) has extended its remit to include people with physical health problems,18,19 the current uptake by stroke survivors is unknown.
Among the several psychological approaches to the treatment of depression, cognitive–behavioural therapy (CBT) is the most widely used psychological treatment for depression in clinical practice20 and may be appropriate for those with stroke. 21,22 There is evidence from single case design studies that some patients with post-stroke depression improve following CBT. 23,24 However, a randomised controlled trial25 (RCT) of CBT for post-stroke depression found no significant difference between those participants who received CBT, an attention placebo or usual care. One of the possible reasons for the lack of efficacy was that psychological treatments need to be tailored for people with aphasia and cognitive impairment. 22,25 A systematic review26 of the modifications to CBT that were required for people with cognitive impairments caused by acquired brain injury reported promoting an understanding of how specific changes to cognition, affect and behaviour occur as a result of brain injury and the use of memory aids. 27 However, a randomised trial of augmented CBT, in which CBT was adapted to suit those with stroke, also found no evidence of benefit in comparison with a cognitive training control group. In addition, a trial28 of CBT at different time points after stroke found no overall effect of CBT in comparison with usual care, although there was some evidence that CBT improved mood in those who were recruited > 9 months after stroke. Therefore, there is currently little evidence to support the provision of CBT after stroke.
Effectiveness of psychological interventions for depression after stroke
Other psychological interventions that may be appropriate for those with depression after stroke include counselling, motivational interviewing and problem-solving training. Some of these have been provided early after stroke in an attempt to prevent the development of depression,29–35 whereas others have been provided later to those who have developed depression. 36,37
There is currently limited evidence for the clinical effectiveness and cost-effectiveness of these psychological therapies for treating post-stroke depression. 38 Towfighi et al. 11 identified seven trials (n = 775 participants) of psychological interventions for depression after stroke and concluded that these trials suggest that brief psychosocial interventions may be useful and effective in the treatment of post-stroke depression. Two of these trials36,37 evaluated a brief psychosocial behavioural intervention, but details of the content of the intervention are limited. Motivational interviewing31,35 has also been shown to reduce post-stroke depression but studies recruited participants early after stroke and excluded those with severe communication or cognitive problems, so these findings may not be applicable to the broad range of stroke survivors with post-stroke depression.
Behavioural activation
A psychological intervention that may be suitable for stroke survivors is BA therapy. BA is based on the behavioural model of depression, in which depression is believed to result from a lack of response-contingent positive reinforcement. 39 Positive reinforcement is dependent on the person’s actions,40 and reduction in activity can lead to loss of reinforcement. Low positive reinforcement can arise from several sources: a deficiency in the individual’s skills (e.g. lack of social skills), limited availability of potential reinforcers in the environment and a decreased ability to enjoy pleasant events. The individual may engage in few activities that generate reinforcement. These antecedents contribute to a low rate of positive reinforcement, and so there are reduced feelings of mastery and esteem in success, which can lead to feelings of depression. As individuals become depressed they reduce participation in activities and hobbies, decreasing the level of reinforcement further and so leading to a vicious cycle. 41 It is proposed that depression is, therefore, maintained by a cycle of depressed mood, decreased activity and avoidance. 42
A stroke can result in a loss or restriction of rewarding activities and interactions (such as everyday activities, hobbies and social interactions) and this loss may lead to depression. The symptoms of depression (such as reduced motivation and lack of energy), in addition to the consequences of stroke, can mean that some behaviours or activities become more difficult and lose the positive reinforcement that they used to provide. BA aims to increase activity level, particularly the frequency of valued activities, and decrease avoidance behaviours in order to improve mood. In addition to its focus on reduced positive reinforcement leading to depression, BA is also concerned with addressing avoidance behaviours that contribute to depression. Depressed people may use avoidance as a coping strategy. 43 For example, someone who feels low in mood may withdraw from social contacts because they find this activity challenging and causing them discomfort. Avoiding this activity provides short-term relief and is negatively reinforced, thereby increasing the likelihood that they will repeat this avoidance again in the future.
Behavioural activation is effective at treating depression in adults in primary-care settings44–46 and in older adults. 47 It has been found to have comparable effectiveness to CBT in treating depression. 48 In a randomised controlled non-inferiority trial, Richards et al. 49 found that BA had comparable effects to CBT for people with depression in primary care and could be delivered by mental health workers with less intensive and less costly training than that required to deliver CBT and was, thus, also more cost-effective.
Behavioural activation is considered a straightforward approach and, as such, is suitable for those with reduced cognitive or communication ability. Stroke patients have cognitive impairment and some have communication problems, which makes BA an appropriate treatment. A multicentre RCT, the CALM trial,17 evaluated BA delivered by an assistant psychologist (AP) for treating low mood in stroke patients with aphasia. This trial17 found that mood was significantly better at 6-month follow-up in those who received BA than in those who received usual clinical care. In addition, reduced resource use suggested potential cost-effectiveness. 50
The transferability of BA to hard-to-reach populations, such as those with aphasia and severe cognitive problems,51–54 adds to its potential as a psychological intervention for depression after stroke. Given that the CALM trial17 demonstrated that it was possible to deliver BA to stroke survivors with aphasia and that studies of BA in people with dementia indicate that it is suitable for those with cognitive impairment,54 there is significant potential for using BA for treating depression in stroke survivors with mild to moderate communication and cognitive impairment.
Rationale and objectives
The Behavioural Activation Therapy for Depression after Stroke (BEADS) trial was designed and conducted in response to a commissioned call from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme to answer the research question ‘How feasible is a study to investigate the clinical and cost-effectiveness of a psychological intervention for people with post-stroke depression?’.
The BEADS trial was a multicentre trial designed to test the feasibility and clinical outcomes of BA for treating post-stroke depression, as well as its acceptability to patients, carers and therapists. We also collected data on the feasibility of delivering the BA intervention in the NHS as part of routine clinical practice. This feasibility work was essential in informing a proposal for a definitive (Phase III) multicentre RCT evaluating the clinical effectiveness and cost-effectiveness of BA for treating post-stroke depression. However, as a feasibility study, the BEADS trial was not powered to explore any factors that may modify the effects of treatment.
Primary objective
The primary objective was to determine the feasibility of proceeding to a definitive trial.
Secondary objective
The secondary objective was to determine the feasibility of delivering BA to people with post-stroke depression.
Chapter 2 Methods
The feasibility trial
This feasibility trial is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement55 and the pilot and feasibility trials extension. 56 The full protocol has been published. 57
Trial design
This study used a parallel-group, feasibility, multicentre RCT design with nested qualitative research and economic evaluation to compare BA therapy with usual stroke care for patients with post-stroke depression. Participants were allocated to BA or usual stroke care at a ratio of 1 : 1.
Ethics approval
Ethics approval was granted on 29 January 2015 by the National Research Ethics Service (NRES) Committee East Midlands – Leicester (reference number 15/EM/0014).
Important changes to the methods after feasibility trial commencement
Changes made to the essential documentation during the trial and following ethics approval on 29 January 2015 can be found in Appendix 1.
Early in the recruitment phase substantial amendment 2 [Protocol v2.1, Research Ethics Committee (REC) approved 8 July 2015] provided additional options in the recruitment process. This allowed the therapists to contact patients directly by telephone following initial consent to be contacted. This streamlined the recruitment process by providing the opportunity for the therapist to explain more about the research and arrange a home visit to complete screening measures. This amendment also broadened the recruitment routes to include potential participants on acute outpatient caseloads.
In substantial amendment 3 (Protocol v2.2, REC approved 7 September 2015), an additional exclusion criterion were added to exclude patients who were currently receiving psychological intervention. Five out of the 49 participants were recruited prior to this exclusion criterion being added. Furthermore, it was specified that participants in the intervention group could be withdrawn from the intervention if it was subsequently agreed that the patient needed immediate clinical psychology input. This amendment also clarified that participation in the study would not compromise access to other services (i.e. psychological input) that were part of usual care.
Minor amendments were also made including changes in study personnel and contact details. In addition, another secondary end point was added to estimate the sample size for a definitive trial and clarification that two or fewer missing items within the Patient Health Questionnaire-9 (PHQ-9)58 could be imputed if required.
Participants and eligibility criteria
Recruitment of participants
Participants were identified from hospital and community stroke databases at three stroke services, as well as the corresponding acute hospital stroke wards, and from voluntary support groups. Participants were approached by letter or by clinicians in community and acute stroke teams, or by voluntary group leaders. Self-referrals were facilitated by advertising the study in newsletters of relevant charities and societies. Posters were displayed in local voluntary sector groups, libraries and local community centres so that potential participants unknown to local hospital and community stroke teams could self-present to the local research team. The methods of identifying potential participants were kept broad to allow assessment of the optimum recruitment strategy for the definitive study. This also enabled recruitment of a representative cross-section of the population.
Participants were recruited from three centres (see Settings and locations where the data were collected). The process for recruitment varied depending on where the participant was recruited from.
Hospital stroke database and community stroke team database
At each site, the clinical teams sent invitation letters to those on the hospital or community stroke databases of discharged patients. Patients were sent a postal pack containing a covering letter, a participant information sheet, a reply slip, the PHQ-9, the Visual Analogue Mood Scales (VAMS) ‘Sad’ item and a prepaid envelope. Patients who were interested in taking part returned the reply slip and completed PHQ-9 and VAMS ‘Sad’ item to the therapist. Return of completed questionnaires was taken as implied consent to be contacted by the therapist (i.e. for potential recruitment into the trial). Those patients who did not reply were contacted by telephone by the clinical team to remind them about the study. The PHQ-9 and VAMS ‘Sad’ item were used to assess whether or not participants met the inclusion criterion of having depression. Participants who returned the reply slip with the completed PHQ-9 and VAMS ‘Sad’ item were contacted by the therapist if they were identified as depressed (scoring ≥ 10 points on PHQ-9 or ≥ 50 points on the VAMS ‘Sad’ item). Those who were identified as not being depressed (i.e. scores of < 10 points on the PHQ-9 or < 50 points on the VAMS ‘Sad’ item) were thanked for their interest and informed that they were not eligible.
Therapists contacted patients who were classified as depressed to arrange a visit. The purpose of the visit was to check that the participant met the remainder of the inclusion criteria, to explain the study and to formally invite eligible patients to take part, obtain signed consent and complete baseline assessments. If a patient returned the reply slip to express interest in the study but did not return the completed PHQ-9 and VAMS ‘Sad’ item, the therapist offered to visit and support the patient to complete these assessments.
Patients currently on acute hospital stroke wards
At each site, research nurses visited hospital stroke wards to provide information about the research to potential participants and seek their permission to be contacted by the research team and, therefore, permission for their contact details to be passed on to the research team. A screening form was used to collect key demographic and contact information from all consented participants, who were then contacted by the local therapist 3 months from the date of consent to be contacted. Before making contact, the local research nurse or general practitioner (GP) was contacted to check whether or not the patient was still alive.
Patients were then contacted by telephone to tell them more about the research and arrange a home visit, during which they completed the PHQ-9 and VAMS ‘Sad’ item. Those patients who were identified as not being depressed were thanked for their interest and were informed that they were not eligible. For patients who were classified as depressed, the therapist either (1) arranged a subsequent home visit or (2) continued with recruitment as per the steps in Hospital stroke database and community stroke team database. Alternatively, instead of a home visit or telephone call, the patient could be sent a pack containing a covering letter, a participant information sheet, a reply slip, the PHQ-9, the VAMS ‘Sad’ item and a prepaid envelope addressed to the therapist for that site. The same steps outlined in Hospital stroke database and community stroke team database were then followed.
Patients currently on the active caseload of community and acute stroke teams
The BEADS trial was presented to the community and acute stroke teams at each of the study sites. The clinical care teams were asked to explain the study to potential participants at the end of therapy, outpatient appointments or between appointments by telephone. If patients were interested in taking part, the clinician asked permission for their contact details to be passed on to the research therapist. Following this, the therapist then sent a pack to the patients and followed the steps outlined in Hospital stroke database and community stroke team database or arranged a home visit and followed the steps in Patients currently on acute hospital stroke wards.
Voluntary sector (stroke and aphasia groups)
The therapists sought permission to attend stroke and aphasia groups in each site to explain the study to members. Those who were interested in taking part were invited to provide their contact details to the therapist, who then either arranged a home visit and followed the steps in Patients currently on acute hospital stroke wards or sent the patient a pack and followed the steps in Hospital stroke database and community stroke team database.
Potential participants were told that entry into the trial was entirely voluntary and that their treatment and care would not be affected by their decision. It was also explained that they could withdraw at any time, but attempts were made to avoid this happening. Participants were told that, if they withdrew, their data could not be erased and could be used in the final analyses.
Recruitment of carers and therapists
For those participants with carers, the carer was also invited to take part. Carer participants were eligible if they provided informal care to the trial participant. Family members, spouses and friends were all eligible to participate as carers. The study therapists (staff participants) were invited to take part in the qualitative interviews at the end of the study.
The presence of a carer was established by the therapist during the initial telephone call to arrange the first home visit. When the carer was present during the home visit, study therapists provided them with a copy of the participant information sheet to review and gave a verbal explanation of study participation. When appropriate and relevant, written informed consent was taken from carer participants during this first home visit. When the carer was not present during the home visit, a copy of the participant information sheet was provided for the carer and the study therapist followed this up with a telephone call to discuss the study in more depth. When the carer was interested in participating, an additional home visit was undertaken to organise written informed consent from them.
Staff participants were consented by the study therapist; research and staff participants were consented by the interviewer for the qualitative interview and by the trial manager for video recording (fidelity assessment). It was explained that participation was voluntary and that they could withdraw at any time.
At consent for recruitment into the trial, all participants opted in or opted out of receiving invitations for their treatment sessions to be video recorded as part of the fidelity assessment. Participants who declined video recording were offered the option of audio recording instead. Participants were not excluded from the study if they did not want their treatment sessions to be video or audio recorded. We documented the proportion of participants who agreed to be video (or audio) recorded.
Eligibility criteria
Inclusion criteria
The criteria were designed to identify those who would be suitable for the intervention were it to be offered within clinical practice. Participants were included in the study if they:
-
had a diagnosis of stroke
-
were aged ≥ 18 years
-
were living in community settings, including home or nursing home
-
were a minimum of 3 months and a maximum of 5 years post stroke
-
were identified as depressed, defined as –
Exclusion criteria
Participants were excluded if they:
-
had a diagnosis of dementia, based on self-report or carer report, prior to their stroke
-
reported receiving medical or psychological treatment for depression at the time at which they had their stroke
-
were currently receiving a psychological intervention
-
had communication difficulties that would have an impact on their capacity to take part in the intervention, based on assessment with the Consent Support Tool60 (CST) for people with aphasia
-
had visual or hearing impairments that would have an impact on their capacity to take part in the intervention based on their therapist’s opinion at baseline assessment
-
were unable to communicate in English prior to the stroke
-
did not have mental capacity to consent to take part in the trial.
All reasons for patient exclusion were recorded.
Consent process
During training by a speech and language therapist experienced in mental capacity assessment, all recruiting researchers were taught techniques to identify whether or not a potential participant was able to understand key information provided about the project and to retain and weigh this information, as well as methods to assist participants to express their decision, adhering to the four key aspects of mental capacity outlined in the Mental Capacity Act 2005. 61
Written informed consent was obtained from all participants who were are able to give it. Those who lacked the mental capacity to provide consent were excluded from the trial. The therapists explained the details of the trial and provided a participant information sheet, ensuring that the participant had sufficient time to consider participating or not. For patients who were physically unable to sign the form (e.g. weakness in dominant hand attributable to stroke), consent could be given using a mark or line in the presence of an independent witness (who had no involvement in the trial), who would then corroborate this by signing the consent form.
A significant proportion of the stroke population have some degree of cognitive or language impairment (aphasia). The level of support required to enable a person with aphasia to provide informed consent is dependent on the severity and profile of the aphasia. In order to provide information in a format consistent with each individual’s language ability, the CST could be used. The CST provides a means of determining comprehension levels of people with aphasia, or cognitive difficulties, in order to provide information in a format that is likely to be most accessible to the person with aphasia to support their understanding. This tool also helps to identify methods that support the individual to express their decision. The therapist requested verbal consent from the potential participant to carry out part A of the CST (10 minutes). The result indicated how appropriate it was to provide the accessible information sheet. If the CST indicated that the potential participant understood fewer than two key written or spoken words in a sentence, they would be likely to find it difficult to understand all the information required to provide informed consent. These participants were thanked for their time but were not eligible for the study as, despite the intervention using techniques to support the inclusion of those people with reduced language or cognition, the intervention did rely on achieving understanding with support and actively participating in therapeutic communication.
The accessible information sheet was provided to those who understood at least two key written and spoken words. This follows standard aphasia-friendly principles, with one idea presented per page in short simple sentences in large font. Key words are in bold and each idea is represented by a pictorial image to support understanding of what the study is about. The therapists were trained to support understanding further by reading parts of the information aloud and using supportive gestures/actions (as described for information level 3 of the CST and consistent with the types of support offered in the intervention under study).
Once the potential participant was given the information and had sufficient time to ask questions and discuss with family or friends, the therapist checked that the individual had capacity to provide informed consent. This was performed by checking that they understood the information, could remember what the study was about and could clearly express their decision in the way in which they usually communicate (speaking, writing, using a communication aid). The CST provides information on ways people with aphasia might choose to express their intentions.
When the CST was not needed, owing to adequate understanding and verbal expression in conversation, the researchers were still taught to check that the trial information provided had been understood by each individual by asking yes/no questions about the content of the information and the potential consequences of their involvement to confirm the patient’s ability to weigh the information.
Participants with capacity to provide informed consent who used the accessible information provision were provided with an aphasia-friendly consent form and asked to initial all boxes before signing. When stroke symptoms prevented initialling of boxes or providing written consent, the patient could use a mark or line and a relative/friend was asked to witness the fact that the participant was consenting to the study and to sign and date the consent form to confirm this on behalf of the participant.
As participants may become distressed during the study and, therefore, may be advised to consult their GPs, consent to notify the GP was sought from all participants. Participants’ GPs were notified by letter that their patient was taking part in the research and were sent a copy of the participant information sheet for information.
Expected duration of participant participation
Study participants were expected to participate in the study for approximately 6 months.
Settings and locations where the data were collected
The University of Nottingham sponsored the trial. Co-ordination of the trial was undertaken by the Sheffield Clinical Trials Research Unit (CTRU). Participants were identified and recruited from hospital and community stroke services at Sheffield Health & Social Care NHS Foundation Trust, Derby Teaching Hospitals NHS Foundation Trust and Let’s Talk Wellbeing at Nottinghamshire Healthcare NHS Foundation Trust. Screening and identification of potential participants was supported by Clinical Research Network (CRN) staff based at Sheffield Teaching Hospitals NHS Foundation Trust, Derbyshire Community Health Services NHS Foundation Trust and at Sherwood Forest Hospitals NHS Foundation Trust. Baseline data were collected and BA therapy was delivered by the therapists in participants’ own homes. Six-month follow-up data were collected in participants’ own homes, for those with aphasia, by blinded outcome assessors. For those without aphasia, data were collected by post unless help was requested.
Interventions
Intervention: behavioural activation therapy
Behavioural activation therapy is a structured and individualised treatment that aims to increase people’s level of activity, particularly the frequency of valued activities (pleasant or enjoyable events), and decrease avoidance behaviours, in order to improve mood. Participants randomised to receive BA were treated at their place of residence by a research therapist. The research therapists were APs at two sites and a psychological well-being practitioner (PWP) at one site. APs are psychology graduates who support the work of a clinical psychologist and work under the supervision of a clinical psychologist. Many APs aspire to train to become clinical psychologists. The level of expertise and experience of an AP can vary, but they would need to meet the person and job specification for the band at which they would be employed (NHS bands 4 or 5). PWPs have completed an accredited training course to enable them to deliver low-intensity CBT-based interventions to people with mental health conditions.
The two APs in the study were psychology graduates. They had no previous formal training in psychotherapy and had not previously worked in a stroke service. They were newly appointed to working as an AP in the stroke service at their site; their background and level of experience was comparable to the APs who delivered BA to people with aphasia in our previous CALM study. 17
Participants were offered a maximum of 15 sessions of BA over 4 months, with an expected average of 10 sessions. Therapy sessions were delivered face to face on an individual basis and lasted for about 1 hour. The intensity and duration of therapy were based on a study25 of CBT with stroke patients and were informed by the CALM study,17 in which participants received an average of nine 1-hour sessions over 3 months. Experience and criticism of the CBT trial25 was that the 3-month duration of therapy was too short. The trial62 of BA for treating depression in primary care provided 12 sessions over 3 months, but this was not in a stroke sample and patients with communication or cognitive difficulties may require a longer duration of therapy. Therefore, the duration of therapy was 4 months because the CALM study17 showed that it was difficult to complete sessions in 3 months owing to non-availability of the participants and short-term illnesses. Four months also allowed flexibility to provide therapy visits to support maintenance, as might be provided in clinical practice. However, the number of therapy sessions varied depending on the needs of the individual and their progress in therapy. The intensity of treatment was negotiated between the therapist and the participant, based on their progress in achieving their therapy goals, so as to reflect usual clinical practice.
A BA treatment manual was developed for the previous CALM trial17 based on the behavioural component of CBT for depression in stroke patients,23,25 behavioural therapy with older people41 and guidelines on conducting therapy with people who have aphasia. 21,41,51 For the BEADS trial, this therapy manual was further revised to include BA with stroke patients who do not have aphasia, and provided examples and practical guidance relevant to all stroke patients. In revising the manual from the CALM trial,17 we drew on the CBT therapy manuals of Lincoln et al. 23 and Laidlaw (Ken Laidlaw, University of Edinburgh, 2004 personal communication), BA manuals of Martell et al. ,40 Lejuez et al. 63 and Mitchell (Pamela Mitchell, University of Washington School of Nursing, 2002, personal communication), BA strategies used in low-intensity CBT64 and guidelines on adapting CBT for people with stroke. 22,51
The therapy manual contained session content for 10 sessions, using the same behavioural approaches as the CALM study. 17 Participants could receive up to 15 sessions to allow for the fact that, for some people, it may take longer to cover therapy content. Additional guidelines were provided for identifying strategies to support people with aphasia and materials were recommended to enable guidelines on conducting therapy to be followed.
Goals set during treatment to increase enjoyable activities were tailored to the individual. BA also included ‘homework’ tasks to be completed between sessions to practise exercises and increase activity levels. Behavioural treatment strategies focused on maximising mood-elevating activities. The process of BA involved identifying how the person currently spends their time, identifying activities that they enjoyed doing (this included resuming previous activities, increasing current activity levels or introducing new activities) and setting goals to increase the number of enjoyable activities.
Behavioural therapy techniques included:
-
Activity monitoring – therapists identified how participants spent their time to assess current activity level, determined what activities they enjoyed and when activities could be carried out. Participants were given an activity diary or timetable to complete as a homework task. The complexity of the diary varied depending on the cognitive and communication abilities of the patient. The activity diaries were available in a range of formats, including word cards, picture cards and photographs.
-
Activity scheduling – participants were encouraged to plan realistic activities and goals to complete each day. This was intended to increase the likelihood that activities were being carried out. The number of activities was gradually increased in order to increase the amount of positive reinforcement received. Activities were set on the basis of the abilities and goals of the individual.
-
Graded task assignment – tasks were broken down into smaller, manageable, steps to facilitate practising tasks that participants found difficult. This was intended to increase the frequency of self-reward and reduce the chances of failure and avoidance of tasks. For example, someone who wanted to go shopping, were encouraged to start by going to a familiar local shop where they knew people already; this was then extended to going to a larger shop, further away.
-
Problem solving – this included focusing on difficulties a participant may have with completing activities and using behavioural techniques (such as a graded task) for improving success at these tasks. Common problems in carrying out activities or tasks were identified and then a problem-solving approach was used to identify and practise possible solutions.
After each session, therapists completed the therapy recording form. This included the duration and location of the therapy session and whether or not there was another person present. The time taken to travel to the visit was recorded. The therapy recording form also included an estimate of how much time (in 10-minute units) had been spent on each component of therapy. The components of therapy were based on the content of BA approaches in the manual.
The therapist also completed the therapy session log. This included the planned number of treatment sessions, the number of treatment sessions completed and the reasons that sessions were missed.
Ensuring intervention fidelity
The therapists attended a 2-day workshop led by a NHS consultant clinical psychologist and the chief investigator. The workshop covered the rationale of BA therapy for treating depression, application of behavioural techniques for treating post-stroke depression and explanation of the therapy manual. The workshop included fictional case examples and role-play exercises. The workshop also included training from a speech and language therapist on communicating with stroke patients with cognitive and/or communication difficulties. Communication resources were developed during the CALM study17 (such as picture cards and activity schedules) and were provided for each of the therapists. To support between-session activities, worksheets/information-appropriate sheets were developed for varying levels of cognitive difficulties or aphasia.
It was important that the therapists delivered the intervention consistently, in accordance with the therapy manual. Weekly clinical supervision for the therapists was provided by a local clinical psychologist at each site. In addition, therapists delivering the intervention had a monthly teleconference to discuss the content of the intervention, share examples of practice and raise any difficulties with the chief investigator and NHS consultant clinical psychologist.
A sample of therapy sessions were video recorded (see Fidelity assessment for further details).
Control group: usual care
The availability of psychological support in the three sites varied. The content of usual care was decided locally by the clinical team, as per local services. In the three sites, most stroke survivors are admitted to hospital, usually to a stroke unit. On discharge, they may receive input from an early supported discharge team or from a community stroke/rehabilitation team.
Participants in the usual-care group followed the current care pathway. Participants received all other services routinely available to them as local practice but had no contact with the trial therapist. This usual-care control group provided a record of usual care to inform the design of the definitive trial.
Concomitant treatment
Those receiving medical or psychological treatment for depression at the time of stroke onset were excluded as we were interested in those who developed depression following stroke (as per exclusion criteria in Participants and eligibility criteria). Those who were currently receiving antidepressants were included so that we could record how commonly this occurs. Receipt of antidepressant medication or other psychological intervention for depression was recorded in the case report form (CRF).
Compliance
Compliance with BA was regarded as an outcome measure not a covariate and was measured by recording whether or not participants allocated to the BA intervention attended scheduled therapy sessions. The completion rates of follow-up questionnaires were also recorded.
Feasibility criterion
Primary outcome
The primary outcome measures related to the feasibility of (1) proceeding to a definitive trial and (2) delivering the BA therapy intervention with participants with post-stroke depression.
The primary end points were based on:
-
feasibility of recruitment
-
acceptability of the research procedures and measures
-
appropriateness of the baseline and outcome measures for assessing impact
-
retention of participants at outcome
-
potential value of conducting the definitive trial, based on value-of-information analysis.
Other feasibility outcomes
The secondary end points were related to the feasibility of the BA therapy intervention, based on:
-
acceptability of BA therapy to participants, carers and therapists
-
feasibility of delivering the intervention by APs or therapists under supervision of an experienced mental health practitioner
-
documentation of ‘usual care’ using a health-care resource use questionnaire
-
treatment fidelity of the BA therapy
-
feasibility of delivery of BA therapy within current services and within a definitive trial
-
estimating the sample size for a definitive trial.
Clinical outcomes
Primary outcomes
The primary clinical outcome measure at 6 months was the PHQ-9. 58 For participants with moderate to severe language problems who were unable to complete the PHQ-9, the VAMS ‘Sad’ item59 was used – this is a single-item, visual analogue mood measure. The number of participants unable to complete the PHQ-9 was recorded, and the VAMS ‘Sad’ item was completed with all participants so that the relationship between the measures could be explored. This was a pragmatic approach, based on self-completion at baseline.
Secondary outcomes
The secondary clinical outcomes were the questionnaire measures used to assess the potential secondary outcomes at 6 months following BA therapy. These related to the feasibility primary end points (b) and (c). The following measures were used to assess clinical outcomes at 6 months:
-
Stroke Aphasic Depression Questionnaire – Hospital version (SADQ-H) (observer-rated depression)65
-
Nottingham Leisure Questionnaire (NLQ) (leisure activities)66
-
Nottingham Extended Activities of Daily Living (NEADL) (functional outcome)67
-
Carer Strain Index (CSI) (carer-rated level of strain)68
-
EuroQoL-5 Dimensions, five-level version (EQ-5D-5L) [health-related quality of life (HRQoL)] standard version69 and a version for people with cognitive problems70 for participants and carers
-
health-care resource use questionnaire.
Participant withdrawal
Participants had the right to withdraw from the study at any time. The reasons for leaving the study, when given, were recorded on a CRF. Participants who withdrew were still invited to complete the 6-month outcome assessments unless they had specified that they wished to have no further involvement in the trial. Individuals removed from active participation in the intervention were not replaced. Reason for withdrawal from the intervention, if known, was recorded.
Participants were withdrawn from the trial either at their own request or at the discretion of the chief investigator. The investigator could withdraw a participant in the interest of the participant (e.g. if continuation in the trial was considered to be causing undue stress) or because of a deviation from the protocol (e.g. when, following review, it transpired that a participant was incorrectly deemed eligible at the time of consent). Participants could discontinue their allocated intervention or withdraw from the study for the following reasons:
-
withdrawal of consent
-
changes to their health status preventing their continued participation
-
failure to adhere to protocol requirements.
If, during the trial, a patient allocated to the BEADS intervention subsequently required clinical psychology input (as per the protocol of the local service), the BEADS therapist (AP/PWP) discussed this with the clinical psychologist or clinical lead and the patient and all agreed what was best for the participant. If it was agreed that the patient needed immediate clinical psychology input then they were withdrawn from the BEADS intervention and they saw the clinical psychologist, or were referred to alternative provision. Therefore, the patient was withdrawn from the intervention but not the overall trial and, thus, outcome data were still collected from them. We recorded the number of participants who were withdrawn from the BEADS intervention because of a conflict in using clinical services.
Changes to trial outcomes after the trial commenced, with reasons
An additional secondary end point, to estimate the sample size for a definitive trial, was added to the protocol after the trial commenced, as an inconsistency was identified between the statistical analysis plan (SAP) and the protocol.
Sample size
As this was a feasibility study, it was not powered for efficacy and no formal interim analyses of efficacy were conducted; the sample size was adequate to estimate the uncertain critical parameters [standard deviation (SD) for continuous outcomes; consent rates, event rates and attrition rates for binary outcomes] needed to inform the design of the definitive RCT with sufficient precision. The sample size of 60 participants allowed SDs for continuous outcomes, such as the PHQ-9 and VAMS ‘Sad’ item, to be estimated to within precision of approximately ± 19% of its true value (with 95% confidence). Allowing for 15% attrition by 6 months post-randomisation follow-up, 72 participants needed to be recruited. To achieve the target sample size of 72 over the 12-month recruitment period, with three centres, we needed to randomise two participants per centre per month.
In addition to this, we estimated that we would recruit a total of 65 carers and three therapists to the study. The carer estimate was based on the CALM study,17 in which approximately 90% of people with stroke had an informal carer present who completed the study outcome assessments.
Further information on both the quantitative and the health economic analyses is provided in the SAP. This covers both the procedures for missing, unused and spurious data and definitions of populations for which data were analysed.
Explanation of any interim analyses and stopping guidelines
The overall study could have been stopped because of safety concerns or issues with study conduct at the discretion of the sponsor. There were no formal statistical criteria for stopping the trial early. Decisions to stop the trial early on grounds of safety or futility would have been made by the Trial Steering Committee (TSC) on the basis of advice from the Data Monitoring and Ethics Committee (DMEC). No early stopping was planned, but the study could have been terminated early if, in the view of the TSC, no useful information was likely to be obtained by continuing. The criteria for assessing this were primarily the feasibility outcomes listed in Feasibility criterion. The TSC could also have recommended the closure of a centre but that the trial as a whole continued, on the same grounds. Unblinded adverse event (AE) data were reviewed by the DMEC, who could have recommended to the TSC that the trial was stopped if, in their opinion, there was evidence of harm in the intervention group. As this was a feasibility trial, it would not have stopped early for efficacy.
Method used to generate the random allocation sequence
Randomisation was conducted using a computer-generated list with random permuted blocks of varying sizes, created and hosted by the Sheffield CTRU in accordance with their standard operating procedures and was held on a secure server. Once a participant had consented to the study, the therapist logged into the remote, secure, internet-based randomisation system and entered basic demographic information. The allocation for that participant was then revealed to the researcher.
Type of randomisation and details of any restriction (such as blocking and block size)
Block randomisation with randomly varying block sizes of two, four and six was used so that the sequence of allocation could not be predicted. The block sizes were determined by the trial statistician and block size was not revealed to any other member of the study team. Participants were allocated to BA or usual stroke care at a ratio of 1 : 1. Randomisation was stratified by site.
Allocation concealment mechanism
Access to the allocation sequence was restricted to those with authorisation. The sequence of treatment allocations was concealed until interventions had been assigned and recruitment, data collection and analyses were complete.
Neither the participants nor the therapists were blind to which treatment the participants were receiving. The outcome assessors were blind to the treatment received and there was no requirement for them to know the treatment allocation at any stage. As a result, a procedure for breaking the code was not necessary.
Blinding
Participants were randomised at baseline (after consent and baseline assessments) in equal proportions to BA or usual stroke care. It was not pragmatically possible for the participant or therapist to be blind to the group allocation, but the researchers completing the 6-month outcome assessments were blinded and had no involvement in any other aspects of the trial. The researchers were asked to record whether or not they thought they were unblinded and were also asked to guess the group allocation. We followed guidelines71,72 to minimise unblinding during RCTs of rehabilitation.
The trial statisticians remained blind until data freeze, at which point data checks were carried out on unblinded data.
Statistical methods
As the trial was a pragmatic, parallel-group RCT, data were reported and presented in accordance with the CONSORT 2010 Statement. 55 All statistical analyses were performed in R version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria). 73 As a feasibility study, the main analysis was descriptive and focused on CI estimation and not formal hypothesis testing.
Analysis populations
The intention-to-treat (ITT) population includes all participants for whom consent was obtained and who were randomised to treatment, regardless of whether they received the intervention. This is the primary analysis set and end points were summarised for the ITT population unless otherwise stated.
Baseline characteristics
Baseline characteristics were participants’ demographics (age, sex and ethnicity), patient- and carer-reported outcomes [PHQ-9, VAMS ‘Sad’ item, EQ-5D-5L, NEADL, NLQ, Stroke Aphasic Depression Questionnaire (SADQ), CSI and EQ-5D-5L carer], stroke history (time since last stroke, lateralisation of stroke, stroke type, side of weakness, previous stroke and depression treatment) and stroke outcomes [Montreal Cognitive Assessment (MoCA), Frenchay Aphasia Screening Test (FAST) and Modified Rankin Scale].
For continuous variables, the number of observations, mean and SD or median and interquartile range (IQR), and minimum and maximum observations were presented by treatment group and site. For categorical variables, the number and percentage of observations in each category were presented.
Imbalance between treatment arms was not tested statistically but was reported descriptively.
Feasibility outcomes
The numbers of participants screened, eligible and randomised per month per centre and overall were presented with relevant percentages. Attrition was examined by presenting the number of participants who dropped out by treatment arm, site and time since last stroke. The reasons for attrition, where given, were also presented for each participant.
The time of key events including screening, randomisation, baseline and follow-up was plotted by participant to check that these were carried out as planned.
The number and percentage of participants randomised to the BA arm and who received at least two, five, eight and 10 therapy sessions were presented. The mean, SD, median and IQR number of planned sessions that were missed were presented.
A summary of missing patient- and carer-reported outcome measures was also presented. In addition to this, we reported the timing of the post-randomisation follow-up assessment.
As part of the feasibility analysis, the effect size for the 6-month PHQ-9 outcome (the probable primary end point for the definitive study), that is the difference in mean scores between the BA and control groups, was estimated, along with its associated 95% CI estimate,74 using a mixed-effects model; site was included as a random effect and baseline PHQ-9 as a covariate to check that the likely effect was within a clinically relevant range as confirmation that it was worth progressing with the definitive trial.
The following sensitivity analyses were presented alongside the ITT analysis:
-
multiple imputation of missing primary outcome data
-
unadjusted analysis.
Although this was not prespecified, to examine the effect of the treatment, we also examined the change in PHQ-9 depression categories (Table 1).
| PHQ-9 score (points) | Depression category |
|---|---|
| 0–4 | Minimal depression |
| 5–9 | Mild depression |
| 10–14 | Moderate depression |
| 15–19 | Moderately severe depression |
| 20–27 | Severe depression |
Individual PHQ-9 score and depression category at baseline and follow-up were plotted by treatment arm. To assess the level of agreement between PHQ-9 and VAMS ‘Sad’ item, scatterplots were generated using baseline and 6-month follow-up data. Pearson’s correlation coefficient was also calculated using baseline and 6-month follow-up data.
Sample size calculations for a definitive trial
We calculated a sample size for a definitive trial comparing BA with usual care in participants with post-stroke depression. The primary end point used was PHQ-9 at 6 months post randomisation. The sample size was based on a range of differences in PHQ-9 of between 3 and 5 points75 and a range of conservative estimates of SD of 7–11 points, giving a range of standardised effect sizes between 0.27 and 0.71, allowing us to determine the most appropriate option. Feasibility data were used to calculate the intraclass correlation coefficient (ICC) in the intervention arm based on clustering by site. Furthermore, the feasibility trial attrition rate was used to adjust the final sample size calculation.
Clinical outcomes
Primary outcomes
The primary clinical outcome measure at 6 months was the PHQ-9. 58 We planned that those participants with moderate to severe language problems who were unable to complete the PHQ-9 would instead complete the VAMS ‘Sad’ item59 – this was a single-item, visual analogue mood measure. However, we did not have any participants with moderate to severe language problems who did not complete the PHQ-9. A comparison of PHQ-9 to VAMS ‘Sad’ item was carried out as described above to inform a potential definitive trial.
To assess the quality of the primary outcome, the follow-up window, defined as the period between screening and 6-month follow-up assessment, was calculated for each participant. A mean and SD of follow-up time in days were calculated. Timing of key events (screening, consent, randomisation, withdrawal and follow-up) were plotted with number of days on the x-axis and screening number on the y-axis.
The secondary outcomes at 6 months post randomisation were analysed using a multiple linear regression model on the ITT population adjusting for baseline measure and centre to examine the difference between treatment arms. Mean differences and their 95% CIs were presented.
Missing spurious and unused data
The numbers of missing scores for each of the primary and secondary outcomes at baseline and 6 months post randomisation were presented by treatment arm. Furthermore, the number and percentage of missing items were presented for each of these questionnaires.
Multiple imputation was carried out using the Multivariate Imputation by Chained Equations ‘mice’ package in R statistical software. 76 Missing 6-month post-randomisation PHQ-9 scores were imputed using chained equations and 30 multiply imputed data sets. The multiple imputation model included sex, age, treatment group, PHQ-9 score at baseline and/or 6 months, EQ-5D-5L at baseline and 6 months and SADQ at baseline and 6 months as predictors.
Safety outcomes
The number of AEs and serious adverse events (SAEs) was recorded and presented by treatment arm. These events were further categorised by the type of AE (fall, worsening health, etc.) and whether or not they resulted in a hospital stay.
Patient and public involvement
The BEADS trial received input from the patient and public involvement (PPI) group (including one patient with significant aphasia) on aspects of the design and methods development as well as study oversight. Two patients and one carer attended five scheduled meetings to discuss feedback on how the study was being conducted, including ideas about recruitment, study documents, ensuring the well-being of the patients and carers, and supporting the therapists. The meetings were attended by the PPI group members, the trial manager and the chief investigator. Meetings took place prior to receiving ethics approval when study materials were being developed, during recruitment and intervention delivery, and after the study to discuss the study results. The meetings each had an agenda that was agreed by the group. A summary of the discussions was written up by the trial manager and chief investigator and was circulated to the group for them to add any points and to ensure that it was an accurate summary of the meeting. Suggestions were put into practice with the creation of a short study summary card and a spiral-bound version of the aphasia-friendly participant information sheet. At the suggestion of the patient and carer representatives, the therapists were invited to join the PPI meetings. In these meetings, the PPI group members were able to ask the therapists questions and give suggestions. PPI group members asked the therapists if they found their job difficult and whether or not there were any challenges with delivering the intervention. The therapists explained that they were well supported and that they enjoyed their role. The PPI group members felt that this was crucial to the success of the study. Another question asked by the PPI group was whether the therapists felt that having the carer being present during therapy was helpful or not. The therapists explained that the carer provided support to the patient. One of the therapists said that they made sure they addressed any discussion or questions to the participant directly, so that they could choose when they wanted their carer to answer on their behalf.
Fidelity assessment
To ensure the fidelity of the intervention, the content of treatment was described and analysed against the manual.
Therapy sessions were video recorded to ensure that the treatment was being delivered in accordance with the manual and to be potentially used for future training. The plan was to select participants and sessions iteratively, using purposive sampling to represent the range of severity of depression (mild, moderate, severe from baseline scores) and across the phases of therapy (beginning, middle and end). We planned to video record up to 24 therapy sessions (based on recruiting the target sample size). It was anticipated that more sessions would be recorded in the middle phase of therapy because this covered more of the therapy sessions and is when the majority of the BA intervention occurs.
The video recordings were transferred to a secure encrypted device, deleted from the video recorder prior to transportation and stored in a secure area on the University of Nottingham server.
Practices for video recording drew on guidance on minimising intrusiveness of the recording. 77,78 Coding of video recordings was carried out by an independent researcher using a time sampling procedure. Recordings were made on the minute, every minute, throughout the recording. On each observation, the activity of the therapist and participants was given the appropriate activity code.
The assessors analysing the video recordings applied a customised therapy record form designed to capture a variety of key elements spanning all aspects of the intervention. The recordings included activities that were expected in all sessions and those that were session specific. They also included content derived from the treatment manual and other content. The other content included activities that were likely to occur but were not specified in the manual, such as social chat and making travel arrangements. A sample of recordings was checked by another observer and discrepancies were resolved by discussion.
The video-recording categories are shown in Appendix 2.
Data from coding sheets were entered into Statistical Product and Service Solutions (SPSS) version 22 (SPSS Inc., Chicago, IL, USA) for analysis.
Health economic methods
Background
The health economic analysis had two key components that related to the primary end points of the trial:
-
assessing the feasibility of collecting data that may be used in a health economic analysis
-
conducting an economic evaluation and a value-of-information analysis in order to provide information on the potential value of conducting the definitive trial.
Overview
For the feasibility analysis, the number of participants who had complete data for each of the key measures is reported for each time point by treatment group and overall. For patient and carer questionnaires, the item response rate at each visit (baseline and 6 months) is reported. Response rate was measured as a fraction of the total number of items. This provides an analysis of the feasibility of collecting data required to complete a health economic analysis. For the health economics analysis, the data of most relevance are those from the:
-
EQ-5D-5L – standard version (completed by participants who are able)
-
EQ-5D-5L – aphasia-accessible version (completed by all participants)
-
EQ-5D-5L – completed by the carers of participants for themselves
-
EQ-5D-5L – completed by the carers of participants on behalf of the participant
-
resource use questionnaire.
For the economic evaluation, a series of cost-effectiveness analyses were conducted:
-
within-trial analysis from a NHS and Personal Social Services (PSS) perspective
-
within-trial analysis from a societal perspective
-
model-based analysis from a NHS and PSS perspective
-
model-based analysis from a societal perspective.
Owing to the importance of carers for people with post-stroke depression, it was important to include analyses undertaken with a societal perspective to supplement the NHS and PSS analyses.
The within-trial analyses were undertaken both with and without multiple imputation, which was used to estimate values for missing data. Patient-level costs and outcomes were assessed over the full length of the feasibility study and this was supplemented with the construction of a simple economic model to examine the longer-term cost-effectiveness of treatment and priorities for future research. Costs and utilities were estimated for individual patients using data collected at baseline and follow-up, based on responses to EQ-5D-5L and resource use questionnaires, combined with standard cost and valuation sources. 79,80 Differences between costs and quality-adjusted life-years (QALYs) in the two groups were described and the incremental cost-effectiveness ratio (ICER) was calculated.
The main aim of the BEADS trial was to assess the feasibility of conducting a future definitive RCT to investigate the clinical and cost-effectiveness of BA therapy for people with post-stroke depression. Therefore, our analysis cannot provide conclusive cost-effectiveness results. However, early cost-effectiveness modelling remains of value because it provides insight into the likely cost-effectiveness of the intervention and demonstrates the value of pursuing further research, particularly when value-of-information analyses are included. 81,82 The value-of-information framework allows the maximum value of further research to be estimated, taking into account the uncertainty in the parameters included in the economic model. 83,84 We estimated the expected value of perfect information (EVPI), representing the maximum value of further research on all uncertain parameters in the economic model, and also estimated the expected value of perfect partial information (EVPPI), representing the maximum value of obtaining more information on each specific parameter (or group of parameters) included in the model.
Resource use
Costs were estimated for each participant, including intervention costs (based on staff time and number of sessions) and health-care resource use. Questionnaires were tested as a method for collecting resource use data. The resource use questionnaire included questions about a participant’s use of health services over the previous 3 months, representing the final 3 months of the follow-up period. As data were required for the entire 6-month follow-up period, we assumed that costs for the first 3 months were the same as for the final 3 months. In the questionnaire, resources were split by services, such as inpatient, outpatient, primary care and community services, and, where necessary, included average appointment length. Participants were also asked to record dosages of medication relating to depression, and information about carer time and employment. This information was used to calculate total medical costs and societal costs.
Unit costs
Resource use data were combined with unit cost data from the latest versions of the Personal Social Services Research Unit (PSSRU) unit cost publication,85 NHS reference costs79 and the British National Formulary86 in order to calculate costs for inclusion in the economic analysis. When appropriate values were not available from the latest version of the PSSRU unit costs publication, earlier versions were consulted87–89 and prices were inflated using the hospital and community health services index. 85
The unit costs used to estimate the costs associated with the resource use observed in the trial are presented in Appendix 3.
Outcomes
Participants who did not have moderate or severe language problems were asked to complete the standard version of the EQ-5D-5L as well as an amended (and as yet unvalidated) accessible version (based on pictures). 57 Participants who had moderate to severe language problems were asked to complete the accessible version of the EQ-5D-5L. In addition, for participants who had carers, the carer was asked to complete a standard EQ-5D-5L by proxy. This allowed us to test alternative methods for collecting data from which to calculate QALYs.
Analysis
Within-trial analysis
Utility scores, based on EQ-5D-5L responses, were calculated for participants at baseline and follow-up. Differences in costs and QALYs between the two groups were estimated over the 6-month trial period using seemingly unrelated regression (SUR). SUR allows for correlation between costs and utility data. 90–92 The SUR model was specified to adjust for baseline EQ-5D-5L as suggested by Manca et al. 93 and also adjusted for baseline (pre-randomisation) costs. The regression was run for participants with no missing data (complete cases) and also for all participants including imputed values for costs and utilities.
For missing EQ-5D-5L data, multiple imputation was used as described in Analysis populations. Predictive mean matching was used to impute the missing data for costs using a chained regression. 94 Thirty imputations were generated for each missing value and the mean of these was used in the final imputed data set analysis. Differences between costs and QALYs were summarised using the ICER and CIs were algebraically determined by using the variance–covariance matrix.
A supplementary societal perspective analysis involved costing carer time associated with each participant (collected using the resource use questionnaire) using the human capital approach. 95 The resource use questionnaire also collected data on employment changes and private care costs, which were incorporated in the societal analysis.
Model-based analysis
The trial-based analysis was supplemented with an analysis using a simple decision-analytic model, used to estimate the cost-effectiveness of the intervention over the lifetime of participants. This was populated using the trial data combined with unit costs and mortality rates as well as assumptions regarding the maintenance of the treatment effect over time. The base-case analysis was undertaken from a NHS and PSS perspective, but a supplementary societal analysis was also undertaken.
The structure of the model (Figure 1) was based on that used to estimate the cost-effectiveness of computerised aphasia treatment compared with usual stimulation in the CACTUS study. 70 A simple three-state Markov model was used to extrapolate the data from the trial to a simulated cohort over a lifetime horizon. Participants entered the model in the no response state. Each month, they could remain in this state or transition to the good response state or death. Once in the good response state, participants could remain in this state or move back to the no response state or to death.
FIGURE 1.
Markov model.

Transition probabilities were primarily based on the trial data. The primary clinical outcome measure was the PHQ-9; therefore, we based our definition of ‘good response’ on PHQ-9 scores. Specifically, participants moved from the ‘no response’ state to the ‘good response’ state if they achieved a 4.78-point decrease in PHQ-9 score from baseline to follow-up. This definition of a response was chosen based on the minimum important clinical difference of PHQ-9 reported by Löwe et al. 75 In the model, we assumed that the intervention would be given over 4 months, as in the trial, and a response (if achieved) would occur after 3 months. As the trial had only one follow-up time point, it was not possible to estimate a relapse rate for a good response. Hence, in the base case, it was assumed that the relapse rate was zero. A sensitivity analysis was carried out to estimate the effect on cost-effectiveness of changing the relapse rate; in this analysis, it was assumed that participants in the ‘good response’ state could move back to the ‘no response’ state after 6 months.
Transitions from the ‘no response’ and ‘good response’ states to death were based on evidence on long-term survival following stroke,96 combined with background mortality rates from the Office for National Statistics,97 reflecting the approach taken in a previous economic evaluation of an intervention for people with aphasia. 70 The same mortality rate was used for the ‘no response’ and ‘good response’ states.
The HRQoL utility scores applied to each health state were reduced over time on the basis of multipliers estimated by Ara and Brazier. 98 QALYs were estimated for each cycle of the model by combining utility scores with life-years, allowing the total QALYs associated with each treatment strategy to be calculated. Costs and QALYs were discounted at a rate of 3.5% each year, in line with recommendations made by the National Institute for Health and Care Excellence (NICE). 99
Distributions were placed around each of the uncertain parameters included in the model for use in probabilistic sensitivity analysis (PSA), which allowed the estimation of cost-effectiveness acceptability curves (CEACs) and a value-of-information analysis. Gamma distributions were used for costs, log-normal distributions for utilities, and beta distributions for probabilities, with dispersions based on numbers observed in the trial. The PSA was supplemented with deterministic scenario analysis on the relapse rate as this was not observed directly in the trial.
The EVPI and EVPPI analyses were undertaken assuming a cost-effectiveness threshold of £20,000 per QALY gained (based on NICE decision rules99) over a 10-year period (assuming that it might take 10 years before a new treatment for these patients is developed), using a 3.5% discount rate. The Sheffield Accelerated Value of Information tool100 was used to estimate the EVPI and EVPPI.
The qualitative research
A series of qualitative interviews with a sample of participants and carers (from both the intervention and the control arms of the study), as well as all three therapists, were completed by an independent researcher to provide a description of the acceptability of the design and procedures used in the trial and the BA intervention. We interviewed 16 participants and 10 carers. The participant and carer interviews were completed in the interviewees’ homes (or an agreed convenient, private location) and the therapist interviews were completed in private locations, as agreed with the researcher. Participants and carers were interviewed after 6-month outcome assessments had been completed. Therapists were interviewed after they had completed all therapy sessions for the study. The interviews took between 10 and 55 minutes. All participants were provided with information concerning the purpose of the study, issues relating to confidentiality and anonymity of the data, and their rights as a participant. All participants provided informed consent to participate in the interview, which was also audio recorded on an encrypted digital recorder and transferred to a secure area on the University of Nottingham server. The researcher transcribed all of the interviews; the transcripts did not include any personal identifiers and the recordings were deleted on completion of the transcription.
Interviewer characteristics
Three women and one man conducted the interviews with patient and carer participants. They were Doctor of Philosophy (PhD) students and research assistants who were registered allied health professionals (one was a speech and language therapist) or working towards gaining professional registration as an allied health professional (psychologist). They had experience of working with people with neurological conditions and had been trained to conduct interviews with patients and carers, including those with reduced language and cognitive ability. Dr Gogem Topcu, who has expertise in health psychology and research into long-term conditions, conducted the therapist interviews. She is an experienced qualitative researcher who has adopted a realist pragmatic approach to research; she had no previous experience of conducting BA therapy.
Relationship with participants
The interviewers’ role was to guide and facilitate the interview, rather than impose how the interview proceeded. The interviewers had no involvement in the delivery or provision of care for the participants and participants were aware of this. The person who interviewed the therapists did not have any prior relationship with the therapists. The interview analysts did not have a personal view on the benefits or limitations of the intervention or about participants’ experience of taking part in the study.
Theoretical and thematic framework
A framework approach was adopted, which is a hierarchical, matrix-based method for ordering and synthesising qualitative data. 101,102 This approach enables in-depth exploration of the data while simultaneously maintaining an effective and transparent audit trail. 101,103 Adopting this approach consolidated the rigour of the analysis and the credibility of the findings.
The thematic framework was constructed iteratively from the interview objectives and existing literature (e.g. previous trials of BA) as well as the issues the participants raised during the interviews. We followed Ritchie and Spencer’s102 approach in arriving at the final thematic framework, which began with familiarisation of the transcripts and audio recordings of the interviews to gain an overview of the data, noting salient points and recurrent ideas. The thematic framework was further consolidated by amalgamating the notes taken during the familiarisation process and the a priori issues covered in the interview schedule and extant literature. However, we did keep an open mind to incorporate material that did not fit within our predefined structure, and iteratively checked whether or not additional themes were warranted or whether or not some predefined themes needed to be minimised in terms of importance or relevance, based on the interview (see Appendix 4 for a worked example of an audit trail of how a framework was developed and amended).
Participant selection
We used a purposive, maximum-variation sampling strategy to select participants and their carers for the interview phase. We attempted to recruit a heterogeneous sample based on their demographic and stroke characteristics. The selection strategy was designed to balance the sample of participants in the following categories:
-
recruitment site
-
treatment arm
-
sex of participant
-
level of depression (mild, moderate or severe)
-
aphasia status
-
recruitment (early or late to the study).
As there was a large number of stratification factors, we prioritised selecting participants by site and treatment arm, and then by the other factors. The sample size was guided by our previous experiences of soliciting feedback about trials from stroke survivors and patients with neurological conditions (e.g. das Nair and Lincoln104), whereby we felt that we were able to achieve sufficient detail related to each construct under investigation, and the categories had ‘conceptual depth’. 105 We were also guided by the limits placed by the recruitment process of the main trial and our framework analysis method. Our primary criterion for conceptual depth related to the question, ‘Do we have sufficient data for each key question that represents rich, nuanced, wide ranging experiences, that resonate with (agree or depart from) the extant literature?’ (cf. Nelson106). Therefore, we did not seek to achieve ‘data saturation’ per se. The trial therapists from each participating site were invited to take part in interviews about their experiences of working on the trial and delivering the intervention.
Data collection
The interviewers had sufficient knowledge of the interview schedule to loosely follow the questions in line with interests and views offered by the participant. Although a predetermined and structured schedule was used to conduct the interviews, the interviews were flexible, allowing the emergence of issues relevant and important to interviewees that were not in the initial schedule. This allowed participants to say what they felt was important to them, while maintaining a basic framework of inquiry at the same time. Prompts were used throughout interviews to provide cues when participants had difficulties, to clarify questions and encourage responding.
The interview schedule included general questions for all participants and carers concerning their experiences of participating in the study, and specific questions to those who had received the intervention and those who were in the control group (see Appendices 5 and 6). An accessible version of the interview questions was also developed for participants with aphasia. The interview schedule for therapists included questions relating to therapists’ experiences of delivering the therapy, views on the trial procedures and practical aspects of delivering the therapy (see Appendix 7). Interviews were transcribed verbatim. The transcripts did not include any personal identifiers and the recordings were deleted on completion of the transcription.
Data analysis
Data analysis was undertaken by Gogem Topcu using the framework approach,101,102 for which the data were mapped onto the constructed thematic framework. If required, the framework was amended to include new concepts or themes introduced during the interviews. To map the data onto the theoretical framework, we relied on indexing various sections of the data to specific thematic constructs. This, again, was an iterative process, requiring Gogem Topcu to go back to previously analysed transcripts to check whether or not the newly emerged construct was also evident there. After mapping all the data, a matrix was generated in which the data were charted to summarise each main theme. This matrix was then used in the interpretation of the data in addition to the notes made during the coding process. The interpretation process, like the other processes, was iterative and relied on consultation between Gogem Topcu and Roshan das Nair regarding the viability and relevance of a theme, to interrogate theoretical constructs, and to unpack nuances within the data.
To ensure rigour and credibility of the findings, Roshan das Nair reviewed the generated matrix and checked the quotations for their relevance to the themes. Disagreements were resolved by discussion. We provided information regarding the context in which the interviews were conducted, some pertinent descriptions of the interviewers and interviewees, and some verbatim quotations from our participants, to ensure transparency. To ensure quality of study reporting, we used the COnsolidated criteria for REporting Qualitative research (COREQ) checklist (see Appendix 8).
Chapter 3 Results of the feasibility trial
Implementation of the intervention and trial
Implementation summary
The trial was due to start recruitment at the beginning of May 2015 but, owing to delays in set-up, recruitment did not start until 27 May 2015 in site 3, 20 August 2015 in site 1 and 3 September 2015 in site 2. This resulted in a total of 27 recruitment months available rather than the planned 36 months. Recruitment ended as planned on 30 April 2016. Follow-up was completed in November 2016. There were a number of issues in regard to recruitment, data collection and delivery of the interventions, which are discussed below.
In order to maintain anonymity, the sites will be referred to as ‘site 1’, ‘site 2’ and ‘site 3’ rather than by their geographical name.
Recruitment and participant flow
Recruitment to the trial
The study set out to assess feasibility of recruitment by utilising a number of different recruitment routes including hospital database, community database, stroke ward, community caseload, voluntary group, self-referral and outpatients. The CONSORT flow diagram (Figure 2) shows the flow of participants through the trial. See Chapter 2, Feasibility criterion, for the primary end points referred to in the analysis section.
FIGURE 2.
Study CONSORT flow diagram. a, One participant was randomised in error (found to be ineligible within days of being randomised), this participant was excluded from analyses; b, one participant withdrew consent 5 days after randomisation, this participant was included in analyses.

Table 2 displays a summary of recruitment flow by screening route and Table 3 shows the number identified by the different screening routes for each site. The number randomised signifies the success of each route; however, it is necessary to discuss each method in turn to understand its individual efficiency fully. Overall, we randomised 49 participants to the trial in 27 centre-months of recruitment (one participant was randomised in error and was not included in analysis, and one participant withdrew consent 5 days after randomisation and was included in analysis). Recruitment by centre can be seen in Table 4 and numbers recruited by the recruitment route can be seen in Table 5.
| Identified | Screening route, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Hospital database (N = 444) | Community database (N = 8) | Stroke ward (N = 183) | Community caseload (N = 80) | Voluntary group (N = 9) | Self-referral (N = 4) | Outpatients (N = 28) | |
| Pack sent/home visit arranged | 444 (100.0) | 8 (100.0) | 64 (35.0) | 24 (30.0) | 9 (100.0) | 3 (75.0) | 22 (78.6) |
| Pack received/home visit conducted | 156 (35.1) | 2 (25.0) | 12 (6.6) | 22 (27.5) | 9 (100.0) | 3 (75.0) | 21 (75.0) |
| Questionnaires completed | 74 (16.7) | 1 (12.5) | 11 (6.0) | 20 (25.0) | 9 (100.0) | 3 (75.0) | 20 (71.4) |
| Initial eligibility met | 29 (6.5) | 1 (12.5) | 3 (1.6) | 13 (16.3) | 4 (44.4) | 3 (75.0) | 16 (57.1) |
| Eligibility appointment conducted | 25 (5.6) | 1 (12.5) | 2 (1.1) | 10 (12.5) | 3 (33.3) | 3 (75.0) | 13 (46.4) |
| Eligible | 21 (4.7) | 1 (12.5) | 1 (0.5) | 8 (10.0) | 2 (22.2) | 3 (75.0) | 13 (46.4) |
| Consent obtained | 21 (4.7) | 1 (12.5) | 1 (0.5) | 8 (10.0) | 2 (22.2) | 3 (75.0) | 13 (46.4) |
| Randomised | 21 (4.7) | 1 (12.5) | 1 (0.5) | 8 (10.0) | 2 (22.2) | 3 (75.0) | 13 (46.4) |
| Route | Site, n (%) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| Community caseload | 10 (2.6) | 63 (22.7) | 7 (7.9) | 80 (10.6) |
| Community database | 0 (0.0) | 8 (2.9) | 0 (0.0) | 8 (1.1) |
| Hospital database | 198 (50.8) | 193 (69.7) | 53 (59.6) | 444 (58.7) |
| Outpatients | 4 (1.0) | 7 (2.5) | 17 (19.1) | 28 (3.7) |
| Self-referral | 2 (0.5) | 1 (0.4) | 1 (1.1) | 4 (0.5) |
| Stroke ward | 176 (45.1) | 5 (1.8) | 2 (2.2) | 183 (24.2) |
| Voluntary | 0 (0.0) | 0 (0.0) | 9 (10.1) | 9 (1.2) |
| Site | Month (n) | Total (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| July 2015 | August 2015 | September 2015 | October 2015 | November 2015 | December 2015 | January 2016 | February 2016 | March 2016 | April 2016b | ||
| 1 | 0 | 0 | 1 | 5 | 5 | 0 | 4 | 0 | 3 | 6 | 24 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 7 |
| 3 | 1 | 1 | 2 | 1 | 1 | 2 | 3 | 2 | 2 | 3 | 18 |
| Recruitment route | Site, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| 1 | 2a | 3 | ||
| Community caseload | 4 (16.7) | 1 (14.3) | 3 (16.7) | 8 (16.3) |
| Community database | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (2.0) |
| Hospital database | 14 (58.3) | 4 (57.1) | 3 (16.7) | 21 (42.9) |
| Outpatients | 3 (12.5) | 0 (0.0) | 10 (55.6) | 13 (26.5) |
| Self-referral | 2 (8.3) | 1 (14.3) | 0 (0.0) | 3 (6.1) |
| Stroke ward | 1 (4.2) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Voluntary | 0 (0.0) | 0 (0.0) | 2 (11.1) | 2 (4.1) |
Table 6 shows that 28 out of a possible 33 carers were recruited to the study. These carers were evenly spread across treatment arms and centres. Table 7 shows the reasons why a small number of carers (n = 5) refused to consent to the trial.
| Treatment arm | Participants with carers, n (%) | Carers recruited, n (%) |
|---|---|---|
| Overall | 33 (68.8) | 2 (84.8) |
| Treatment arm | ||
| Intervention | 17 (68.0) | 15 (88.2) |
| Control | 16 (73.9) | 13 (81.2) |
| Reason for carer non-consent | n (%) |
|---|---|
| Carer not present at time to give consent | 3 (40) |
| Carer does not speak English | 1 (20) |
| Carer does not have enough time and is not interested in participating in research | 1 (20) |
Hospital database
The highest number of participants was recruited through the hospital database. Therefore, this could be considered the most efficient method as it required only minimal staff time. However, uptake seemed to be even more effective if a potential participant was approached by a clinician and given the opportunity to discuss the study and ask questions. It is worth noting that the success of this route varied between the sites. In site 1, the hospital database was the most effective route of recruitment. However, the therapist in site 1 was based in the hospital setting and worked closely to support the CRN nurse to identify and recruit participants. By comparison, the therapists at the other two sites were frequently based at different hospitals from the CRN nurse and, therefore, worked less closely with the latter.
Stroke ward
Based on the values in Table 3, the stroke ward seemed to be the least effective recruitment route. Although research nurses had the opportunity to approach inpatients on the stroke ward to discuss the study, participants did not become eligible to take part until 3 months after their stroke. The protocol indicated that the therapists should perform a mortality check prior to contacting patients at 3 months post stroke. However, this follow-up might not always have taken place. Furthermore, in this early stage, some stroke patients were focused more on dealing with practical issues, such as walking and talking, rather than with their mood.
Community database
Identifying patients through the community stroke databases was not used consistently across the three sites. Community stroke services varied between trusts, and records were kept less consistently than in secondary care. For a definitive study, it would be important to discuss the composition of community services available to improve understanding of specific recruitment routes at the outset.
Community caseload
This route was used by the study therapists to identify current community patients. Approaching patients by telephone or face to face was more effective than the community database route as it allowed the patients an opportunity to discuss the study, although it was more time-consuming for staff.
Voluntary group
The protocol specified that the therapist could attend stroke and aphasia groups to explain the study and to collect contact details of those interested, although this route was time-consuming and not successful in recruiting many participants. However, if the protocol had been less prescriptive and allowed for the people who run the voluntary groups to discuss the study and collect contact details on behalf of the therapists, it may have been possible to make this route a more efficient method for a definitive study. Site 3 was the only site that recruited through this route. The therapist in site 2 reported that recruitment from voluntary groups was unfruitful as many members were not eligible, few had low mood and many were > 5 years post stroke.
Self-referral
According to Table 3, this route appears very effective; however, few participants were recruited this way. This route could be more efficient if the study had been advertised more widely in the press and through social media.
Outpatients
It could be suggested that this route was successful because patients were screened and identified by clinical care teams in outpatient settings, allowing for the opportunity of face-to-face discussions with patients about the study. This route was particularly successful at one site (site 3), with the principal investigator (PI) and therapist being based in the community team and patients being referred post stroke. This outpatients route was for community-based patients and not hospital outpatients. Outpatients differed from community caseload as this is a longer-term service, identifying participants who were at a longer time point post stroke.
Protocol non-compliances
Table 8 shows protocol non-compliances reported, by site and category. All of the issues identified as non-compliances could be incorporated into site staff training for a future definitive trial.
| Non-compliances | Site (n) | Total (n) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Delegation log | 2 | 0 | 0 | 2 |
| Eligibility | 0 | 1 | 0 | 1 |
| Intervention delivery | 0 | 0 | 1 | 1 |
| Failure to report SAE | 0 | 1 | 0 | 1 |
We also reported a non-compliance relating to the inconsistency in using the CST during recruitment; this is discussed in further detail in Challenges with recruitment and data collection.
In addition to the non-compliance reports, there was one instance of unblinding occurring at a 6-month outcome visit in which a participant revealed their allocation to an outcome assessor.
Losses and exclusions after randomisation
Table 9 shows the number and percentage of participants who dropped out of the trial by treatment arm, site and length of time since stroke. Attrition is defined here as the number of participants who did not complete the primary outcome (PHQ-9) at 6 months post randomisation. (See Appendix 9 for an outline of the reasons, where given, for dropout.) A total of nine participants dropped out of the trial during the 6-month follow-up period. Of these nine participants, seven were in the intervention arm. All of the participants who dropped out of the study had their most recent stroke between 3 and 12 months previously.
| Treatment arm, site and time since stroke | Attrition, n (%) | Overall attrition, n (%) | ||||
|---|---|---|---|---|---|---|
| Number | Withdrew consent | Intervention withdrawal and decision not to follow-up | Investigator decision: patient too ill | Lost to follow-upa | ||
| Overall | 48 | 1 (11.0) | 1 (11.0) | 1 (11.0) | 6 (67.0) | 9 (18.8) |
| Treatment | ||||||
| Intervention | 25 | 1 (11.1) | 1 (11.1) | 0 (0.0) | 5 (55.6) | 7 (28.0) |
| Control | 23 | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (11.1) | 2 (8.7) |
| Site | ||||||
| 1 | 24 | 0 (0.0) | 0 (0.0) | 1 (11.1) | 4 (44.4) | 5 (20.8) |
| 2 | 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 18 | 1 (11.1) | 1 (11.1) | 0 (0.0) | 2 (22.2) | 4 (22.2) |
| Time since strokeb | ||||||
| 3 months to 1 year | 30 | 1 (11.1) | 1 (11.1) | 6 (66.7) | 1 (11.1) | 9 (30.0) |
| 1–2 years | 12 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2–4 years | 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Participants lost to follow-up
Table 10 shows the method for completion of 6-month follow-up data. Reminders involved a telephone call to the participant or carer. We aimed to test the feasibility of collecting 6-month outcome data by post compared with in person using a blinded outcome assessor. For a definitive RCT, it may be necessary to implement additional measures to further minimise loss to follow-up (e.g. reminder telephone calls rather than the initial reminder letter to identify those who need a home visit to complete the questionnaires sooner, sending regular newsletters to participants during the follow-up period to maintain engagement with the study, and offering vouchers to participants to encourage return of postal questionnaires).
| Methods | Follow-up | |
|---|---|---|
| Home visit | Postal pack | |
| Total, n (%) | 17 (37.0) | 29 (63.0) |
| Visit arranged/pack sent, n (%) | 16 (94.1) | 28 (96.6) |
| Visit conducted/pack returned, n (%) | 14 (87.5) | 24 (85.7) |
| At least one reminder contact, n (%) | 1 (6.2) | 11 (39.3) |
| Mean (SD) number of reminders | 0.1 (0.2) | 0.8 (1.5) |
| Reminder resulted in completed questionnaires, n (%) | 1 (100.0) | 8 (72.7) |
| Other method used, n (%) | 0 (0.0) | 2 (6.9) |
Dates defining the periods of recruitment and follow-up
Data collected outside the data collection window
It was agreed that data would be accepted within 4 weeks before or after the 6-month time point. However, data from four participants were collected outside this data collection window as collection was delayed because of illness.
Delay in randomisation following initial eligibility measures
During the analysis, there were some delays between completion of the initial eligibility measures and randomisation. In most cases, there was a delay of between 7 and 30 days between completing the initial eligibility and randomisation, which was probably due to the availability of the therapist and the patient in arranging a home visit (see Appendix 10). However, nine participants had a delay of > 30 days. Each therapist could only deliver the therapy to a limited number of participants at one time and, therefore, some participants had to wait for therapist availability. The PHQ-9 score collected at initial eligibility is considered the baseline PHQ-9 and, therefore, because of these delays the time point between baseline and 6-month PHQ-9 is not always the same. For a definitive trial, we would suggest setting a time frame between collecting initial eligibility and full eligibility at baseline.
Baseline data
Demographic information, patient- and carer-reported outcomes and stroke-related outcome data for randomised participants by treatment arm can be seen in Tables 11–14.
| Demographic characteristics | Treatment arm | Overall | |
|---|---|---|---|
| Intervention | Control | ||
| Age (years) | |||
| n | 25 | 23 | 48 |
| Mean (SD) | 62.6 (14.5) | 68.8 (12.1) | 65.6 (13.6) |
| Median (IQR) | 65 (53–72) | 67 (60–75) | 66 (55–75) |
| Minimum, maximum | 31, 88 | 40, 97 | 31, 97 |
| Sex, n (%) | |||
| Male | 17 (68.0) | 12 (52.2) | 29 (60.4) |
| Female | 8 (32.0) | 11 (47.8) | 19 (39.6) |
| Ethnicity, n (%) | |||
| White | 25 (100.0) | 22 (95.7) | 47 (97.9) |
| Asian | 0 (0.0) | 1 (4.3) | 1 (2.1) |
| Measures | Treatment arm | Overall | |
|---|---|---|---|
| Intervention | Control | ||
| PHQ-9 score (points) | |||
| n | 25 | 23 | 48 |
| Mean (SD) | 16.3 (4.7) | 17.3 (4.8) | 16.8 (4.7) |
| Minimum, maximum | 10, 25 | 10, 27 | 10, 27 |
| PHQ-9 category | |||
| n | 25 | 23 | 48 |
| Moderate (10–14 points), n (%) | 12 (48.0) | 7 (30.4) | 19 (39.6) |
| Moderately severe (15–19 points), n (%) | 6 (24.0) | 9 (39.1) | 15 (31.3) |
| Severe (≥ 20 points), n (%) | 7 (28.0) | 7 (30.4) | 14 (29.2) |
| VAMS ‘Sad’ item score (points) | |||
| n | 25 | 22 | 47 |
| Median (IQR) | 51 (39–74) | 52 (41–83) | 51 (39–82) |
| Minimum, maximum | 3, 100 | 0, 96 | 0, 100 |
| SADQ score (points) | |||
| n | 15 | 12 | 27 |
| Mean (SD) | 25.3 (7.6) | 18.8 (8.1) | 22.4 (8.4) |
| Minimum, maximum | 8, 35 | 5, 29 | 5, 35 |
| NLQ score (points) | |||
| n | 24 | 23 | 47 |
| Mean (SD) | 16.2 (6.0) | 13.1 (5.4) | 14.7 (5.9) |
| Minimum, maximum | 4, 29 | 5, 24 | 4, 29 |
| NEADL score (points) | |||
| n | 24 | 23 | 47 |
| Mean (SD) | 12.3 (7.2) | 11.7 (6.0) | 12 (6.6) |
| Minimum, maximum | 2, 22 | 0, 21 | 0, 22 |
| CSI score (points) | |||
| n | 13 | 11 | 24 |
| Mean (SD) | 7.2 (3.6) | 6.5 (2.7) | 6.9 (3.2) |
| Minimum, maximum | 1, 13 | 3, 11 | 1, 13 |
| EQ-5D-5L score (points) | |||
| n | 24 | 23 | 47 |
| Mean (SD) | 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) |
| Minimum, maximum | 0.045, 0.924 | –0.218, 0.951 | –0.218, 0.951 |
| Stroke characteristics | Treatment arm | Overall | |
|---|---|---|---|
| Intervention | Control | ||
| Modified Rankin Scale score (points) | |||
| n | 24 | 23 | 47 |
| Mean (SD) | 3.2 (0.9) | 3.2 (0.9) | 3.2 (0.9) |
| Minimum, maximum | 2, 4 | 2, 5 | 2, 5 |
| FAST score | |||
| n | 24 | 23 | 47 |
| Median (IQR) | 27 (22–29.5) | 27 (22–29) | 27 (22–29) |
| Minimum, maximum | (18, 30) | (12, 30) | (12, 30) |
| FAST category | |||
| n | 24 | 23 | 47 |
| Aphasia (below cut-off point) | 10 (41.6) | 11 (47.8) | 21 (44.7) |
| MoCA score (points) | |||
| n | 24 | 23 | 47 |
| Median (IQR) | 22 (17.5–25) | 22 (17–26) | 22 (17–25) |
| Minimum, maximum | 11, 30 | 4, 29 | 4, 30 |
| Stroke history | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Time from stroke | |||
| < 3 months | 0 (0) | 0 (0.0) | 0 (0.0) |
| 3 months to 1 year | 16 (64) | 14 (60.9) | 30 (62.5) |
| 1–2 years | 7 (28) | 5 (21.7) | 12 (25.0) |
| 2–4 years | 2 (8) | 4 (17.4) | 6 (12.5) |
| Lateralisation of stroke | |||
| Left | 11 (44) | 9 (39.1) | 20 (41.7) |
| Right | 12 (48) | 10 (43.5) | 22 (45.8) |
| Unknown | 2 (8) | 4 (17.4) | 6 (12.5) |
| Stroke type | |||
| Ischaemic | 19 (76) | 18 (78.3) | 37 (77.1) |
| Haemorrhagic | 6 (24) | 4 (17.4) | 10 (20.8) |
| Side of weakness | |||
| Left | 13 (52) | 10 (43.5) | 23 (47.9) |
| Right | 10 (40) | 7 (30.4) | 17 (35.4) |
| Bilateral | 0 (0) | 2 (8.7) | 2 (4.2) |
| Unknown | 2 (8) | 4 (17.4) | 6 (12.5) |
| Previous stroke | |||
| Yes | 6 (24) | 11 (47.8) | 17 (35.4) |
| No | 19 (76) | 12 (52.2) | 31 (64.6) |
| Depression treatment | |||
| Yes | 10 (40) | 12 (52.2) | 22 (45.8) |
| No | 15 (60) | 11 (47.8) | 26 (54.2) |
The PHQ-9 has a range of 0–27 points, with low scores indicating a low level of depression. VAMS ‘Sad’ item has a range of 0–100 points, with low scores indicating a low mood. EQ-5D-5L standard scores can range from –0.281 to 0.951, with 0, 1 and negative values corresponding to death, full health and health states worse than death, respectively. NLQ scores range from 0 to 60, with a low score indicating a low level of leisure activity. NEADL scores range from 0 to 22, with low scores corresponding to lower independence. SADQ scores range from 0 to 63 on the SADQ-H, with low scores indicating a low level of depression. This questionnaire was completed by carers. CSI scores range from 0 to 13, with low scores corresponding to a low level of strain on the carer.
A total of 25 participants were randomised to the intervention arm and 23 were randomised to the control arm. The mean age of participants was 65.6 years (SD 13.6 years) and most participants were men (60.4%). Site 1 recruited 24 participants, site 3 recruited 18 participants and site 2 recruited seven participants.
Mean scores were consistent across treatment arms. In most participants the time since stroke was 3 months to 1 year (62.5%). The proportion of participants who had had a previous stroke was higher in the control arm (47.8%) than in the intervention arm (24%).
The proportion of men who had had a previous stroke was higher in the intervention arm (72.2%) than in the control arm (52.4%).
Tables 15 and 16 show the baseline demographics and baseline outcome measures for participants included in the primary effectiveness analysis.
| Demographic characteristics | Treatment arm | Overall | |
|---|---|---|---|
| Intervention | Control | ||
| Age (years) | |||
| n | 18 | 21 | 39 |
| Mean (SD) | 63.4 (11.8) | 67.9 (10.7) | 65.8 (11.3) |
| Median (IQR) | 62.5 (54–72) | 67.0 (64–75) | 66.0 (56–75) |
| Minimum, maximum | 47, 85 | 40, 89 | 40, 89 |
| Sex, n (%) | |||
| Male | 13 (72.2) | 11 (52.4) | 24 (61.5) |
| Female | 5 (27.8) | 10 (47.6) | 15 (38.5) |
| Ethnicity/nationality, n (%) | |||
| English/Welsh/Scottish/Northern Irish/British | 18 (100.0) | 20 (95.2) | 38 (97.4) |
| Pakistani | 0 (0.0) | 1 (4.8) | 1 (2.6) |
| Measures | Treatment arm | Overall | |
|---|---|---|---|
| Intervention | Control | ||
| PHQ-9 score (points) | |||
| n | 18 | 21 | 39 |
| Mean (SD) | 16.2 (4.9) | 16.9 (4.6) | 16.6 (4.7) |
| Minimum, maximum | 10, 25 | 10, 27 | 10, 27 |
| VAMS ‘Sad’ item score (points) | |||
| n | 18 | 20 | 38 |
| Median (IQR) | 59.5 (39–82) | 52.0 (35.5–82.5) | 56.0 (39–82) |
| Minimum, maximum | 3, 100 | 0, 96 | 0, 100 |
| SADQ score (points) | |||
| n | 11 | 11 | 22 |
| Median (IQR) | 25 (22–32) | 19 (13–28) | 22 (14–29) |
| Minimum, maximum | 8, 35 | 5, 29 | 5, 35 |
| NLQ score (points) | |||
| n | 18 | 21 | 39 |
| Median (IQR) | 17.5 (10–20) | 14.0 (10–17) | 16.0 (10–19) |
| Minimum, maximum | 7, 29 | 5, 24 | 5, 29 |
| NEADL score (points) | |||
| n | 18 | 21 | 39 |
| Median (IQR) | 12.5 (5–20) | 11.0 (10–18) | 12.0 (8–18) |
| Minimum, maximum | 2, 21 | 2, 21 | 2, 21 |
| CSI score (points) | |||
| n | 9 | 10 | 19 |
| Median (IQR) | 7.0 (5–8) | 5.5 (4–9) | 6.0 (4–9) |
| Minimum, maximum | 1, 13 | 3, 11 | 1, 13 |
| EQ-5D-5L score (points) | |||
| n | 18 | 21 | 39 |
| Median (IQR) | 0.466 (0.3–0.7) | 0.599 (0.4–0.7) | 0.563 (0.4–0.7) |
| Minimum, maximum | 0.045, 0.893 | 0.206, 0.951 | 0.045, 0.951 |
Clinical outcomes and estimation
Primary clinical outcomes
A total of 39 out of the 48 randomised participants had valid PHQ-9 outcomes at follow-up (18 participants in the intervention arm and 21 participants in the control arm). Table 17 shows a summary of the results of the primary effectiveness analysis. Following adjustment for baseline and centre, we observed a mean difference in PHQ-9 of –3.8 points (95% CI –6.9 to –0.58 points) at 6 months post randomisation. This represents a reduction in depression in the BA arm.
| Adjustment | Treatment arm | Mean difference in PHQ-9a (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Median (IQR) | Mean (SD) | n | Median (IQR) | Mean (SD) | ||
| Unadjusted | 18 | 6.5 (5–15) | 10.1 (6.9) | 21 | 14 (10–17) | 14.4 (5.1) | –4.3 (–8.0 to –0.5) |
| Adjusted | –3.8 (–6.9 to –0.58) | ||||||
| MI unadjustedb | 25 | 23 | –3.9 (–7.7 to –0.0041) | ||||
| MI adjustedb | 25 | 23 | –3.4 (–7.0 to 0.094) | ||||
Multiple imputation by chained equation was used to impute this missing outcome data; this was a sensitivity analysis that increased the sample size back up to 48 (25 in the intervention arm, 23 in the control arm). Adjusting for baseline and centre, we observed a mean difference in PHQ-9 score of –3.4 points (95% CI –7 to 0.094 points) at 6 months post randomisation. This is shown in Figure 3. Adjusted models included baseline measure and centre as a random effect (n = 39 in unadjusted and adjusted analysis, n = 48 in MI adjusted and unadjusted analysis).
FIGURE 3.
Results from unadjusted and adjusted primary analyses showing mean difference between intervention and control at 6 months.
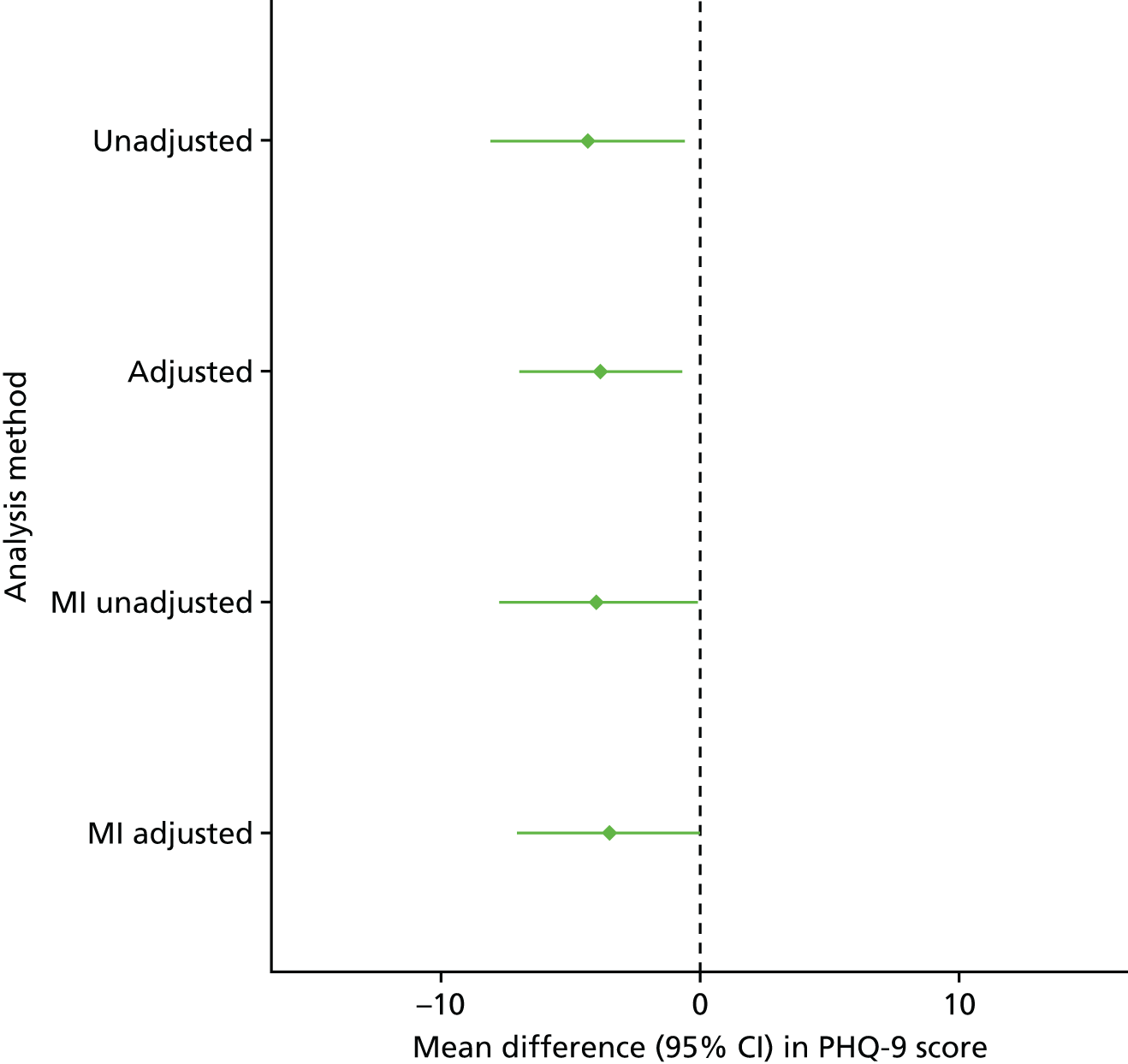
Previous research has suggested that the minimum clinically important difference or change for the PHQ-9 is 4.78 points on the scale. 75 The 95% CI for the mean difference in PHQ-9 scores between the groups includes a difference of ≥ 5 points, suggesting that the likely treatment effect is within a clinically relevant range.
Figure 4 shows that in more participants in the intervention arm than in the control arm the PHQ-9 category was reduced (horizontal dashed lines) from baseline to follow-up. Horizontal dashed lines represent the cut-off scores for depression categories. This information is also presented in Table 18. Most participants in the intervention arm were in the ‘moderate depression’ category at baseline (n = 9), whereas participants in the control arm are distributed across three depression categories: ‘moderate’ (n = 7), ‘moderately severe’ (n = 8) and ‘severe’ (n = 6). Most of the participants in the intervention arm reduced their level of depression by one (n = 7) or two categories (n = 7) at the 6-month follow-up compared with baseline. Most participants in the control arm stayed in the same category (n = 9) or reduced by one category (n = 6).
FIGURE 4.
Line plot showing individual participant change in PHQ-9 score from baseline to follow-up, by treatment arm. (a) Control; and (b) intervention.


| Changes in PHQ-9 category | Treatment arm, n (%) | |
|---|---|---|
| Intervention | Control | |
| Increased by one category | 2 (11.1) | 3 (14.3) |
| No change in category | 2 (11.1) | 9 (42.9) |
| Decreased by one category | 7 (38.9) | 6 (28.6) |
| Decreased by two categories | 7 (38.9) | 2 (9.5) |
| Decreased by three categories | 0 (0.0) | 1 (4.8) |
Table 18 shows that a greater number of participants in the intervention arm than in the control arm decreased by one or two categories.
Figure 5 shows the mean PHQ-9 score in the intervention and control arms at baseline (n = 48) and 6 months (n = 39). Despite the intervention arm having a lower mean score at baseline, the decrease in PHQ-9 score overall was greater in the intervention arm. The dashed lines represent the cut-off points for PHQ-9 depression categories and show that the mean score in the intervention arm has reduced by one depression category.
FIGURE 5.
Mean PHQ-9 score at baseline and 6-month follow-up, by randomised group by treatment arm (n = 48).
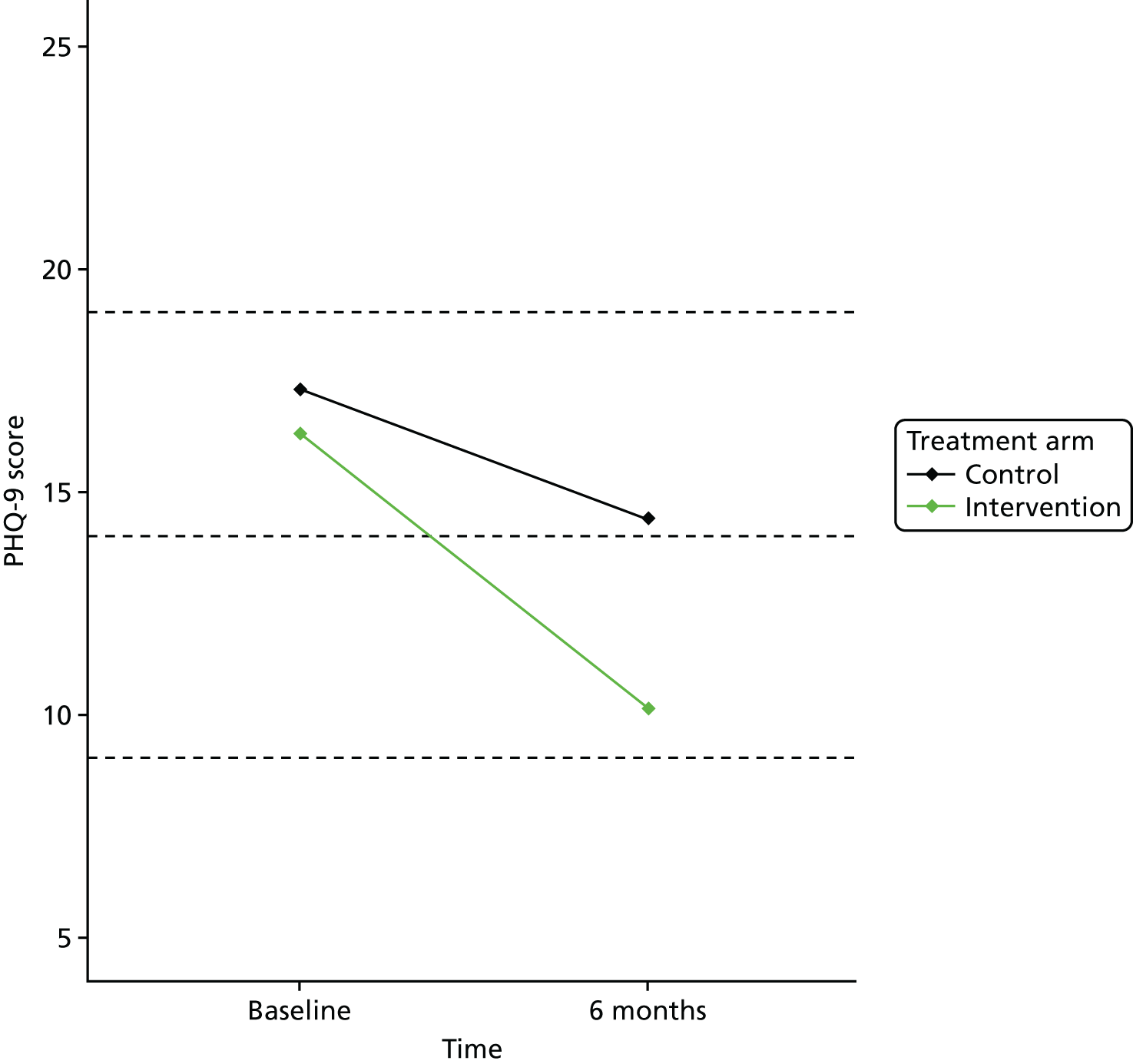
Figure 6 shows the relationship between PHQ-9 and VAMS ‘Sad’ item at baseline and 6 months post randomisation. Correlation was found to be moderate at both baseline and follow-up (r = 0.45 and r = 0.57, respectively). The level of agreement would be considered too low for VAMS ‘Sad’ item to be used to impute PHQ-9. 107
FIGURE 6.
Scatterplot showing correlation between standardised PHQ-9 and VAMS ‘Sad’ item at baseline and 6-month follow-up (n = 48 at baseline and n = 39 at 6 months). (a) Baseline (r = 0.45) and (b) 6 months (r = 0.57).
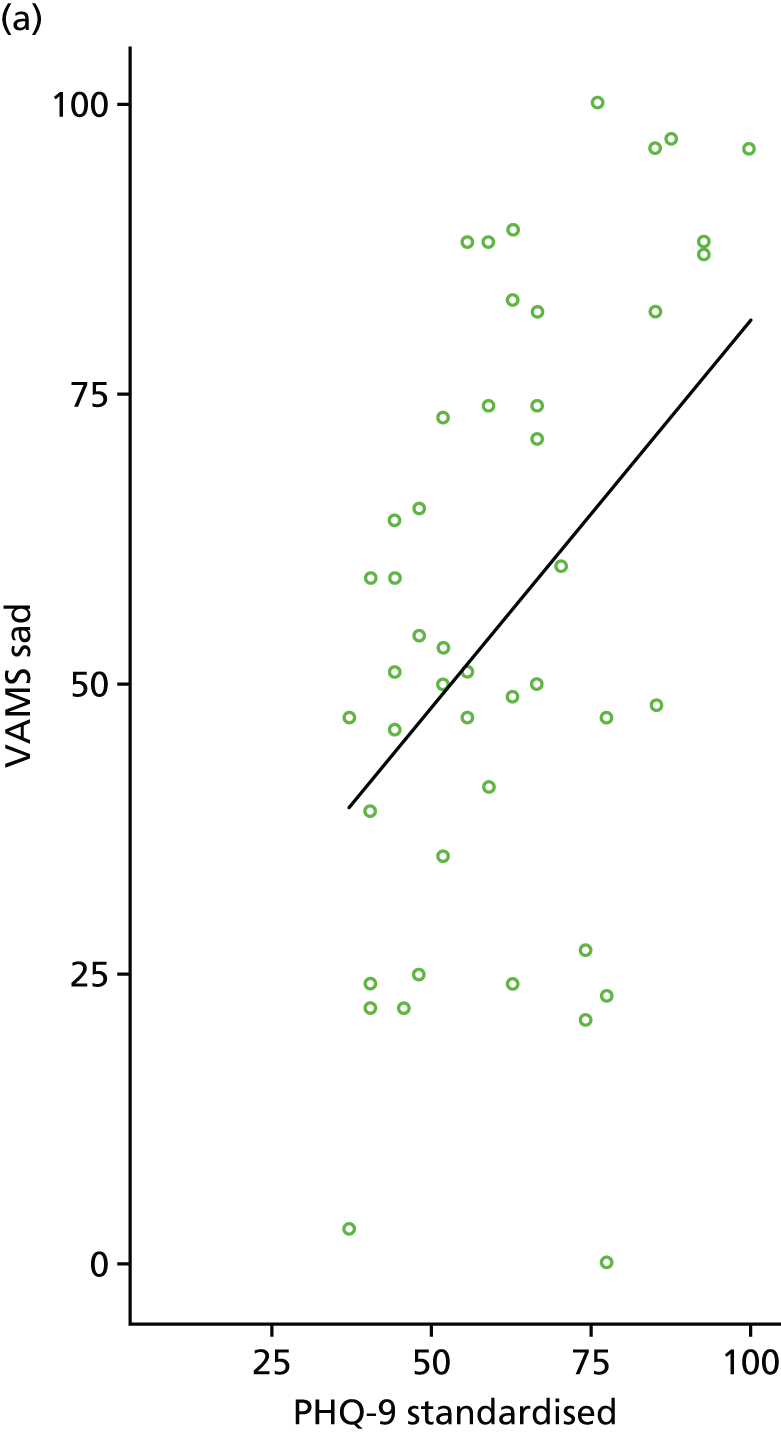
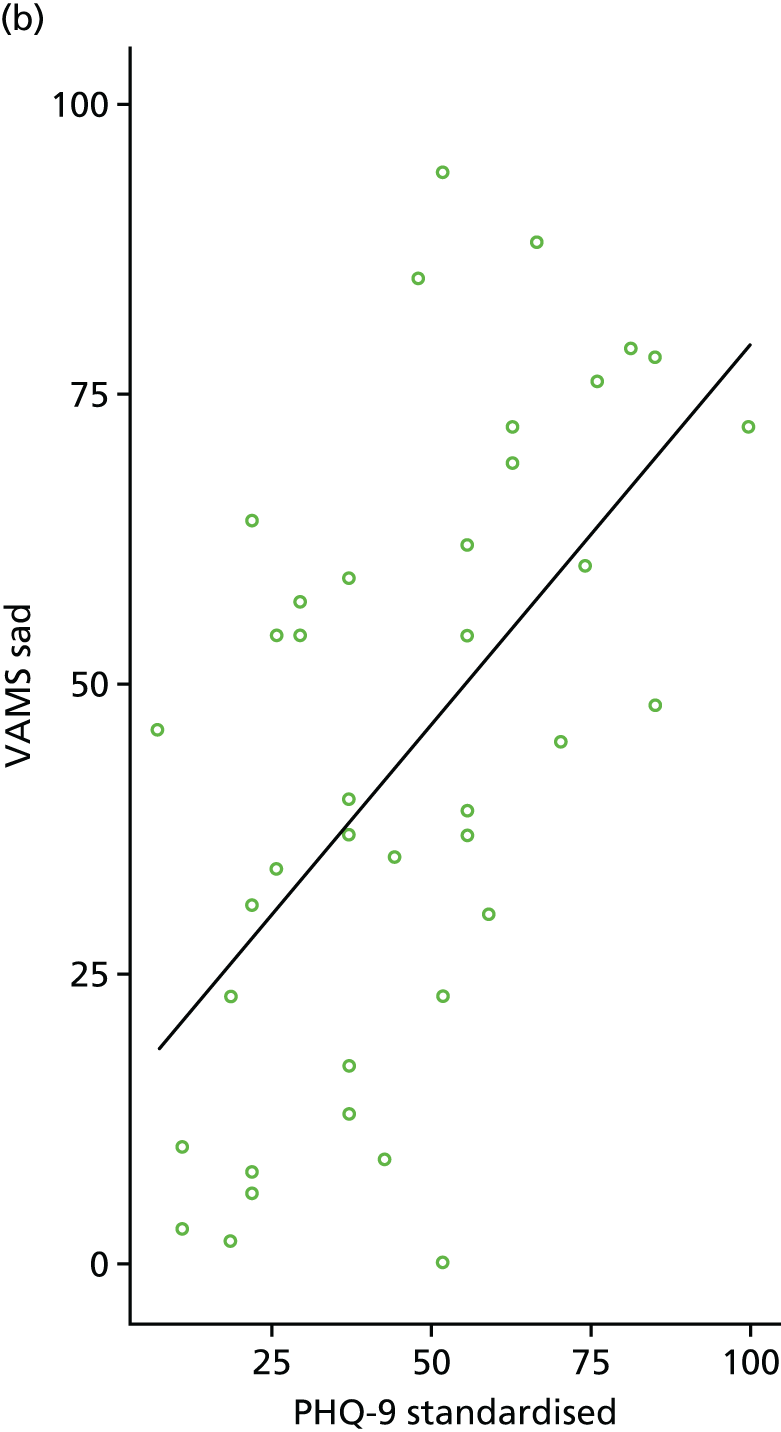
Secondary clinical outcomes
Table 19 shows the analysis of patient-reported secondary outcomes. In terms of the patient-reported secondary outcomes, most of the differences suggest that the intervention has a small positive effect. Table 19 shows the adjusted mean differences and their relative 95% CIs for the patient-reported outcomes including VAMS ‘Sad’ item, NLQ, NEADL and EQ-5D-5L. VAMS ‘Sad’ item and NLQ both suggest that the intervention has a positive effect although the 95% CIs include a difference of zero. The NEADL does not demonstrate any difference between intervention and control arms. EQ-5D-5L score is lower in the intervention arm, which suggests a small negative effect.
| Outcomes | Treatment arm | Mean difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Median (IQR) | Mean (SD) | n | Median (IQR) | Mean (SD) | ||
| VAMS ‘Sad’ item | |||||||
| Unadjusted | 19 | 39.0 (10–64) | 39.8 (28.5) | 21 | 54.0 (34–69) | 48.6 (24.7) | –8.8 (–26.0 to 8.2) |
| Adjusted | –8.6 (–25.0 to 7.7) | ||||||
| NLQ | |||||||
| Unadjusted | 18 | 17.5 (14–26) | 20.5 (9.1) | 21 | 15.0 (12–20) | 15.6 (5.5) | 4.9 (0.087 to 9.7) |
| Adjusted | 3.2 (–0.96 to 7.3) | ||||||
| NEADL | |||||||
| Unadjusted | 18 | 12.0 (5–18) | 12.0 (7.1) | 19 | 11.0 (6–17) | 11.6 (6.1) | 0.37 (–4.0 to 4.8) |
| Adjusted | –0.032 (–2.2 to 2.1) | ||||||
| EQ-5D-5L | |||||||
| Unadjusted | 18 | 0.6 (0.33–0.71) | 0.5 (0.3) | 20 | 0.7 (0.47–0.82) | 0.6 (0.2) | –0.12 (–0.29 to 0.054) |
| Adjusted | –0.04 (–0.17 to 0.092) | ||||||
Table 20 shows the analysis of carer-reported secondary outcomes. CSI score was lower in the intervention group, which represents a positive effect. EQ-5D-5L Carer score was lower in the intervention arm, which suggests a small negative effect of the intervention. SADQ score showed very little difference between groups. CIs are wider because of smaller numbers of carers than participants. CIs around the adjusted mean differences for carer-reported outcomes are very wide because of low numbers of carers who completed the 6-month follow-up (n = 22).
| Outcomes | Treatment arm | Mean difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Median (IQR) | Mean (SD) | n | Median (IQR) | Mean (SD) | ||
| SADQ | |||||||
| Unadjusted | 10 | 23.5 (20.0–27.0) | 23.5 (8.7) | 11 | 17.0 (13.0–25.0) | 17.6 (7.4) | 5.9 (–1.5 to 13.0) |
| Adjusted | –0.55 (–6.5 to 5.4) | ||||||
| CSI | |||||||
| Unadjusted | 9 | 4.0 (1.0–7.0) | 5.1 (4.5) | 10 | 7.5 (2.0–9.0) | 6.0 (3.5) | –0.89 (–4.8 to 3.0) |
| Adjusted | –2.0 (–4.9 to 0.84) | ||||||
| EQ-5D-5L Carer | |||||||
| Unadjusted | 12 | 0.8 (0.67–0.87) | 0.7 (0.2) | 10 | 0.8 (0.75–0.87) | 0.8 (0.2) | –0.062 (–0.23 to 0.11) |
| Adjusted | –0.052 (–0.24 to 0.14) | ||||||
Standardised mean differences and their CIs can be seen in Figure 7, where arrows on the y-axis indicate the direction of a positive intervention effect. Arrows on the y-axis indicate the direction of a desired effect. Outcomes have been standardised onto a 0–100 scale.
FIGURE 7.
Results from secondary analyses showing mean difference between intervention and control at 6 months, adjusted for baseline and centre.

Decision on the primary end point and sample size for a definitive trial
Sample size calculations
We calculated a range of sample sizes for a definitive scale trial comparing BA with usual care in patients with post-stroke depression. The primary end point used was PHQ-9 score at 6 months post randomisation. PHQ-9 has a range of 0–27 points, with low scores meaning a low level of depression. We assumed that a target difference in PHQ-9 scores of approximately 5 points75 would be clinically and practically important, but also used lower estimates of 3 and 4 points so as to produce a range of scenarios. A range of estimates of SD (7, 9 and 11 points) were used, roughly based on the SDs observed in the pilot, which are subject to considerable uncertainty. These scenarios give a range of standardised effect sizes of 0.27–0.71. From the feasibility study, data were used to calculate the ICC of 0.06 in the intervention arm based on clustering by site. We also assumed an average cluster size of around 20 participants per therapist/site. Furthermore, the attrition rate of 18.8% was rounded up to 20% over 6 months and was used to adjust the final sample size calculation.
A sample size of 580 participants would be required to detect a difference of 4 points (SD 9 points) on the PHQ-9 scale with 90% power and 5% significance (see Table 21). It would take approximately 24 months of recruitment in 16 centres, assuming a recruitment rate of 1.5 participants per centre per month, which is similar to the rate of 1.8 participants per month observed in BEADS.
A sample size of 623 participants would be required to detect a difference of 3 points (SD 7 points) on the PHQ-9 scale with 90% power and 5% significance (Table 21).
| Outcome | Significance (%) | Power (%) | Target difference (points) | SD (points) | Standardised effect size | ICC | Average cluster size (n) | Sample size per group (n) | Total sample size (adjusted for 20% attrition) (n) |
|---|---|---|---|---|---|---|---|---|---|
| PHQ-9 | 5 | 90 | 5 | 7 | 0.71 | 0.06 | 20 | 93 | 233 |
| PHQ-9 | 5 | 90 | 4 | 7 | 0.57 | 0.06 | 20 | 142 | 355 |
| PHQ-9 | 5 | 90 | 3 | 7 | 0.43 | 0.06 | 20 | 249 | 623 |
| PHQ-9 | 5 | 90 | 5 | 9 | 0.56 | 0.06 | 20 | 150 | 375 |
| PHQ-9 | 5 | 90 | 4 | 9 | 0.44 | 0.06 | 20 | 232 | 580 |
| PHQ-9 | 5 | 90 | 3 | 9 | 0.33 | 0.06 | 20 | 409 | 1023 |
| PHQ-9 | 5 | 90 | 5 | 11 | 0.46 | 0.06 | 20 | 221 | 553 |
| PHQ-9 | 5 | 90 | 4 | 11 | 0.36 | 0.06 | 20 | 343 | 858 |
| PHQ-9 | 5 | 90 | 3 | 11 | 0.27 | 0.06 | 20 | 608 | 1520 |
Delivery and receipt of the intervention
Table 22 shows the attendance at BA sessions in participants who were randomised to the intervention. Attendance of participants to therapy sessions was high. The mean number of therapy sessions received in the intervention arm was 8.1 (SD 3.4). In total, 92% of participants randomised to the intervention arm received at least two sessions, 88% received at least five sessions, 64% received at least eight sessions and 40% received at least 10 sessions. The mean number of missed sessions was 0.9 (SD 1.3), with 52% of participants missing at least one scheduled therapy session.
| Number of sessions | Site | Overall | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| n | 12 | 3 | 10 | 25 |
| Total number of sessions completed | 110 | 23 | 69 | 202 |
| Mean (SD) number of sessions attended | 9.2 (2.2) | 7.7 (1.2) | 6.9 (4.6) | 8.1 (3.4) |
| Median (IQR) number of sessions attended | 10 (2.25) | 7 (1.00) | 8.5 (7.75) | 9 (3.00) |
| Number (%) of participants attending at least two sessions | 12 (100) | 3 (100) | 8 (80) | 23 (92) |
| Number (%) of participants attending at least five sessions | 12 (100) | 3 (100) | 7 (70) | 22 (88) |
| Number (%) of participants attending at least eight sessions | 9 (75.0) | 1 (33.3) | 6 (60.0) | 16 (64.0) |
| Number (%) of participants attending at least 10 sessions | 7 (58.3) | 0 (0.0) | 3 (30.0) | 10 (40.0) |
| Total number of planned sessions not completed | 6 (5.0) | 1 (4.0) | 16 (19.0) | 23 (10.2) |
| Mean (SD) number of sessions not completed | 0.5 (0.9) | 0.3 (0.6) | 1.6 (1.5) | 0.9 (1.3) |
| Median (IQR) number of sessions not completed | 0 (1.0) | 0 (0.5) | 1 (1.0) | 1 (1.0) |
| Number (%) participants missing at least one session | 4 (33.3) | 1 (33.3) | 8 (80.0) | 13 (52.0) |
Site 3 had a higher rate of missed sessions, probably owing to a higher number of withdrawals. Figure 8 shows that participants who withdrew from the intervention attended a low number of sessions. There were also three participants who attended eight or more sessions and were lost to follow-up.
FIGURE 8.
Number of sessions attended by participants who were randomised to the intervention arm (n = 25).

The timing of therapy sessions was fairly regular, with sessions generally occurring weekly.
Number of missing values/incomplete cases
Missing data in the outcome measures and missing items within these outcomes are presented in Table 23. Baseline completion rate was high in all outcomes. The 6-month follow-up rate was around 80% in most outcomes. Two participants had two or fewer missing items for the PHQ-9 at 6 months post randomisation. Imputation was used on these missing items as specified in the SAP.
| Measures | Time | Total, n (%) | Treatment arm, median (minimum, maximum) | Overall, median (minimum, maximum) | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| PHQ-9,a 9 items | Baseline | 48 (100.0) | 9 (7, 9) | 9 (8, 9) | 9 (7, 9) |
| 6 months | 39 (81.2) | 9 (0, 9) | 9 (0, 9) | 9 (0, 9) | |
| VAMS ‘Sad’ item,b 1 item | Baseline | 47 (97.9) | N/A | N/A | N/A |
| 6 months | 40 (83.3) | N/A | N/A | N/A | |
| EQ-5D Standard, 5 items | Baseline | 47 (97.9) | 5 (5, 5) | 5 (5, 5) | 5 (5, 5) |
| 6 months | 38 (79.2) | 5 (0, 5) | 5 (0, 5) | 5 (0, 5) | |
| NLQ, 30 items | Baseline | 47 (97.9) | 30 (30, 30) | 30 (30, 30) | 30 (30, 30) |
| 6 months | 39 (81.2) | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | |
| NEADL, 22 items | Baseline | 47 (97.9) | 22 (22, 22) | 22 (22, 22) | 22 (22, 22) |
| 6 months | 37 (77.1) | 22 (0, 22) | 22 (0, 22) | 22 (0, 22) | |
| SADQ, 21 items | Baseline | 27 (96.4) | 21 (21, 21) | 21 (20, 21) | 21 (20, 21) |
| 6 months | 21 (75.0) | 21 (0, 21) | 21 (0, 21) | 21 (0, 21) | |
| CSI, 13 items | Baseline | 24 (85.7) | 13 (10, 13) | 13 (12, 13) | 13 (10, 13) |
| 6 months | 19 (67.9) | 13 (0, 13) | 13 (0, 13) | 13 (0, 13) | |
Summary of risks and benefits
This trial was not an investigation of a medicinal product and entailed no invasive procedures. No participants had any existing treatments withdrawn. There was a risk that participants may have experienced some distress from being asked about their mood, but all researchers and therapists were trained to deal with these situations. If, at any point during the baseline assessment, intervention or outcome assessment, the researcher or therapist was concerned about a participant, for example they had severe distress or reported feeling suicidal, then the necessary referrals were made. This process is explained in Adverse events and Challenges with implementation.
Adverse events
For the purposes of this study, AEs were defined as suicidal intentions. Researchers asked participants about any AEs at the 6-month follow-up. This information was collected on outcome questionnaires or recorded in person for those participants who required help at a home visit. Any AEs that were self-reported by participants in the intervention group during the delivery of the therapy sessions were also recorded by the therapist on the CRF and database.
Adverse events have been summarised by treatment arm in Table 24. There were a total of 13 AEs experienced by 10 participants.
| AEs | Treatment arm | All | |
|---|---|---|---|
| Intervention | Control | ||
| Number (%) of participants who experienced more than one AE | 4 (15.4) | 6 (26.1) | 10 (20.4) |
| Number of AEs | 5 | 8 | 13 |
| Type of AE, n (%) | |||
| Fall | 0 (0.0) | 2 (25.0) | 2 (15.4) |
| Health worsened | 1 (20.0) | 3 (37.5) | 4 (30.8) |
| New health condition | 2 (40.0) | 0 (0.0) | 2 (15.4) |
| Suicidal intentions | 2 (40.0) | 3 (37.5) | 5 (38.5) |
| Hospital stay, n (%) | |||
| No | 1 (20.0) | 8 (100.0) | 9 (69.2) |
| Yes | 4 (80.0) | 0 (0.0) | 4 (30.8) |
Serious adverse events (SAEs) are presented in Table 25. There were three SAEs experienced by three separate participants. None of these were related to the intervention.
| BEADS ID | Description | Was the SAE intervention related? | Outcome of SAE |
|---|---|---|---|
| C7/014 | Admitted to A&E following suicide attempt | No | Ongoing |
| C6/004 | Admitted to hospital following a heart attack | No | Improved |
| B4/016 | Repair to hernia | No | Recovered |
Challenges with implementation
Challenges with the delivery of the intervention
Based on the experiences of delivery of the intervention, we have gained some important insights for a definitive trial:
-
The therapists delivering the intervention identified that participants in the early days post stroke had more practical goals, such as walking and speaking, and were less concerned with their mood.
-
It was difficult to find activities for participants with limited mobility.
-
Getting participants to adapt their goals was challenging and one therapist felt that, at this stage, a talking therapy to deal with acceptance would be more beneficial.
-
One of the therapists had experience of delivering CBT in a previous role. Therefore, it may be important to tailor therapist training for those who are inexperienced in delivering psychological therapies and those who are experienced in delivering psychological therapy but not specifically BA therapy. This would ensure that both experienced and inexperienced therapists deliver the therapy in accordance with the manual.
-
This feasibility study recruited only participants who were mildly affected by aphasia and, therefore, did not provide us with knowledge of delivering the intervention to those with aphasia and cognitive impairments.
Challenges with recruitment and data collection
There were a number of issues experienced with recruitment that resulted in lower than planned recruitment rates. However, the final sample recruited was sufficient for this feasibility study, which was powered not for efficacy but to inform the design of the definitive RCT. The original recruitment target of 72 participants was based on three sites recruiting two participants per month over 12 months, giving a total of 36 recruitment months. However, owing to delays in site set-up, none of the sites was able to utilise the full 12-month recruitment period. Instead, a total of 27 recruitment months were available across the three sites.
There were three main challenges for the delays in site set-up: (1) approval of excess treatment costs (ETCs), (2) the knock-on effect on appointment of study therapists in the NHS (as advertisement of the posts was contingent on ETCs) and (3) a change to the IAPT provider close to the time that the study was due to open. Although we under-recruited slightly, this had little impact on determining the feasibility of the trial. Recruitment challenges included the fact that the therapists had a dual role, which meant they were recruiting participants in addition to delivering the intervention. This also meant that they were limited to being able to recruit only as many participants as they could deliver the intervention to at one time.
A second recruitment challenge was the inevitable competition in highly engaged clinical research centres for the resources to recruit participants. The CRN research nurses at sites were able to assist in screening participants from the hospital database and stroke wards. They issued invitation packs to participants they identified as fitting the initial eligibility criteria, but this then required further time to follow up potential participants to discuss the study after issuing the invitation packs.
The third recruitment challenge was the centres being in different settings and, therefore, encountering different problems with recruitment. Recruitment was particularly successful where the therapist was based in the hospital setting and so was able to work closely with the CRN nurse in recruiting from the hospital database and stroke wards. In contrast, the bulk of recruitment in the case of the therapist based in a community setting was gained through the outpatient, community caseload and voluntary group routes as there was limited resourcing to recruit from the hospital database. The therapist based in the IAPT service was heavily reliant on the participants identified from the hospital database and stroke ward by the CRN nurses and so was constrained by the resourcing available. For a definitive RCT, it would be beneficial to fund more research assistant/research nurse time to support recruitment.
Use of the Consent Support Tool
A total of 18 out of the 48 participants recruited had aphasia, which represents one-third of this cohort of stroke survivors, as expected. The intervention was designed to be suitable for people with mild and moderate aphasia, with adaptations being made to support reduced language ability. Only those with aphasia severe enough to make participation in the intervention difficult, even with support, were excluded. The CST was recommended to identify those who were not eligible because they did not have the mental capacity to provide informed consent, and those whose aphasia was too severe to participate in the intervention with support (those with two key written or spoken word comprehension or below). The CST was also recommended to help identify which participants required accessible information and to identify strategies to support the individual’s communication needs during the intervention. The CST was used with only 5 of the 18 participants with aphasia. The FAST scores for these participants show that they had mild/very mild aphasia, confirming that they were all eligible for the study. The therapists reported having a conversation with potential participants and, if they seemed to be communicating well and understanding what was being said to them, then they did not feel the need to use the CST.
Problems with data collection
There was an occasional need to visit some participants twice to collect data outcome because of health and tiredness. Although this was not common, it did have an impact on therapist time.
The main problems experienced were with outcome data collection. The postal method proved to be challenging and it was necessary to chase some participants to return their questionnaires, which resulted in four participants (8%) having their outcome data collected outside the data collection window. This may have been because the patient group required more support to complete the questionnaires. For a definitive trial, the response rate may be more prompt or improved by issuing regular newsletters to participants to keep them engaged or by planning for visits to those who request them.
During the trial, the therapists were funded only until the end of therapy. However, at this stage, 6-month data collection was still ongoing and, therefore, AEs could still be reported and study completion/discontinuation forms required completion. For a definitive trial, it may be beneficial to extend contracts a couple of months beyond the end of treatment completion. Furthermore, organising regular teleconferences with the PIs could improve PI engagement throughout the trial.
Reporting of adverse events
The protocol specified that all AEs were to be assessed for seriousness, expectedness and causality. In addition, the therapists and researchers completed a SAE form for additional events that were classed as serious, including death, suicide, a life-threatening AE, inpatient hospitalisation (or prolongation of existing hospitalisation), disability or incapacity. For other AEs, the researcher completed an AE form. Further stroke-related events were not reported as SAEs because these were expected within this population. However, it was later identified that it was not always possible to assess all AEs for their relationship to the stroke and to the intervention. For example, a score of 3 points ‘nearly every day’ on question 9 of the PHQ-9, ‘Thoughts that you would be better off dead or of hurting yourself in some way’ would trigger the suicide protocol and be reported as an AE. When this occurred, this was referred back to the site for it to follow its local procedures to ensure the safety of the participant. They were also asked to assess if the event was related to the stroke and to the intervention (where applicable). This proved difficult for a number of reasons. First, based on a response to the questionnaire, it is not possible to assess these criteria without asking the participant, which may seem inappropriate and insensitive. Second, as participants were recruited from various routes, they were not always known to the immediate team and, therefore, did not have an established rapport with the therapists. Third, study teams based in community psychology services did not always have access to the patient’s hospital records specifically for admissions to general hospital and, therefore, found it difficult to identify and report details of SAEs.
Summary of usual care
A summary of the provision received by participants in the study by treatment arm can be seen in Table 26. The provision is consistent across treatment arms; however, one participant who was in the intervention arm received significantly more services than other participants.
| Resources used | Treatment arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||
| Total | Number of contacts ≥ 1 (n) | Mean (SD) | Median (IQR) | Mean (SD) duration (minutes) | Total | Number of contacts ≥ 1 (n) | Mean (SD) | Median (IQR) | Mean (SD) duration (minutes) | |
| Inpatient hospital services | 1 | 1 | 0.038 (0.2) | 0.0 (0–0) | – | 0 | 0 | 0.0 (0.0) | 0.0 (0–0) | – |
| Outpatient hospital services | 35 | 3 | 1.3 (4.7) | 0.0 (0–0) | – | 2 | 1 | 0.087 (0.42) | 0.0 (0–0) | – |
| A&E or day hospital attendance | 9 | 4 | 0.35 (1.2) | 0.0 (0–0) | – | 4 | 3 | 0.17 (0.49) | 0.0 (0–0) | – |
| Doctor or nurse contact | 64 | 14 | 2.5 (3.2) | 1.5 (0–5) | 19.0 (26.0) | 96 | 17 | 4.2 (4.9) | 2.0 (0–8) | 27.0 (38) |
| Occupational therapist | 32 | 5 | 6.4 (5.1) | 3.0 (3–12) | 66.0 (33.0) | 12 | 2 | 6.0 (5.7) | 6.0 (2–10) | 42.0 (25) |
| Physiotherapist | 42 | 4 | 10.0 (9.5) | 7.5 (4–17) | 60.0 (0.0) | 29 | 5 | 5.8 (4.9) | 3.0 (3–10) | 44.0 (16) |
| Speech and language therapist | 1 | 1 | 1.0 (–) | 1.0 (1–1) | 60.0 (–) | 13 | 4 | 3.2 (3.3) | 2.0 (1–5.5) | 45.0 (17) |
| Home help/care worker | 522 | 3 | 170.0 (81.0) | 180.0 (90–252) | 68.0 (75.0) | 540 | 3 | 180.0 (160.0) | 90.0 (90–360) | 32.0 (13) |
| NHS counsellor, psychologist or psychotherapist | 8 | 1 | 0.31 (1.6) | 0.0 (0–0) | 3.5 (18.0) | 6 | 1 | 0.26 (1.3) | 0.0 (0–0) | 2.2 (10) |
| Community nurse, social worker, case manager or well-being practitioner | 9 | 1 | 0.35 (1.8) | 0.0 (0–0) | 50.0 (17.0) | 2 | 1 | 0.087 (0.42) | 0.0 (0–0) | – (–) |
| CBT therapist | 13 | 3 | 4.3 (2.9) | 6.0 (1–6) | 1.5 (7.8) | 0 | 0 | – (–) | – (–) | 2.6 (13) |
| Day care centre | 24 | 2 | 12.0 (0.0) | 12.0 (12–12) | 120.0 (85.0) | 13 | 2 | 6.5 (7.8) | 6.5 (1–12) | 240.0 (–) |
| Private counsellor, psychologist, psychotherapist or psychiatrist | 24 | 1 | 0.92 (4.7) | 0.0 (0–0) | – | 0 | 0 | 0.0 (0.0) | 0.0 (0–0) | – |
Chapter 4 Fidelity assessment results
The mean number of sessions attended was 8.5 (SD 4.4), with a range from 0 to 14 sessions. Overall, 90% of scheduled sessions were attended. The main reason that sessions were missed was a change in participants’ availability (n = 14, 61%), illness (n = 4, 17%) and change of therapists’ availability (n = 3, 13%). In addition, two participants (9%) withdrew from treatment.
Table 27 shows the proportion of time (in 10-minute units) spent on individual components of therapy, as reported by therapists.
| Main component | Subcomponent of therapy | Number of 10-minute units | Percentage |
|---|---|---|---|
| Explanation of treatment rationale | 117.8 | 10.2 | |
| Explain research project | 13.0 | 1.1 | |
| Explain BA | 45.2 | 3.9 | |
| Set and agree session agenda | 59.6 | 5.2 | |
| Assessment | 104.5 | 9.0 | |
| Background information | 14.0 | 1.2 | |
| Current problems or difficulties | 46.0 | 4.0 | |
| Depression symptoms, mood | 28.0 | 2.4 | |
| Effects of stroke | 16.5 | 1.4 | |
| Communication and cognitive difficulties | 9.0 | 0.8 | |
| Establish communication skills and difficulties | 1.0 | 0.1 | |
| Developing communication resources | 2.0 | 0.2 | |
| Practice communication skills | 0.0 | 0.0 | |
| Identify strategies for coping with cognitive difficulties | 6.0 | 0.5 | |
| Goals | 117.0 | 10.1 | |
| Set and agree goal | 48.5 | 4.2 | |
| Review progress of goals | 68.5 | 5.9 | |
| Activities | 209.5 | 18.1 | |
| Activity monitoring | 75.0 | 6.5 | |
| Identify enjoyable activities | 69.5 | 6.0 | |
| Activity scheduling | 56.0 | 4.8 | |
| Practise skills or task | 9.0 | 0.8 | |
| Graded task | 240 | 2.1 | |
| Explain graded task principle | 10.5 | 0.9 | |
| Set and agree graded task | 10.0 | 0.9 | |
| Carry out part of the task in therapy | 3.5 | 0.3 | |
| Problem-solving | 44.0 | 3.8 | |
| Identify problems or obstacles arising | 24.0 | 2.1 | |
| Identify and plan solution(s) to a problem | 20.0 | 1.7 | |
| Between-session tasks | 212.4 | 18.3 | |
| Set and agree between-session tasks | 89.0 | 7.7 | |
| Review between-session tasks | 123.4 | 10.6 | |
| Summary and review | 149.1 | 12.9 | |
| Recapping information | 54.3 | 4.7 | |
| Discuss therapy ending | 36.0 | 3.1 | |
| Summarise session | 58.8 | 5.1 | |
| Generalisation | 52.0 | 4.5 | |
| Summary of skills learned during therapy | 31.5 | 2.7 | |
| Plan for future scenarios | 20.5 | 1.8 | |
| Other | 118.5 | 10.2 | |
| Discussion with carer | 29.5 | 2.5 | |
| General conversation | 52.0 | 4.5 | |
| Other (specify) | 37.0 | 3.2 | |
Most time was spent on between-session tasks (18.3%), which included setting and agreeing new between-session tasks (7.7%) and reviewing the previous between-session tasks (10.6%). The second most frequent component was activities (18.1%). This included activity monitoring (6.5%), identifying enjoyable activities (6.0%) and activity scheduling (4.8%), with relatively little time spent on practising skills or tasks (0.8%). The least amount of time was spent on communication and cognitive difficulties (0.8%). In addition, the use of graded tasks (2.1%) and problem-solving (3.8%) was relatively infrequent.
All participants received an explanation of the treatment rationale, assessment, discussion of between-session tasks, summary and review. Two participants (8%) did not receive any goal-setting or activity scheduling. Twelve (50%) did not receive graded task assignments and eight (33%) did not receive training in problem-solving. This indicates that some core components of BA were missed for some participants. The therapy relied heavily on between-session tasks and activities.
The proportion of components of therapy by session number is shown in Table 28. This shows that explanation of the treatment occurred across most sessions and that assessment was mainly concentrated in the first two. Goals, activities, graded task assignments and problem-solving were spread across most sessions. In all sessions, most time was spent discussing between-session tasks and on summary and review. Discussion of generalisation occurred mainly in later sessions.
| Component of therapy | Session number, n (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Explanation of treatment rationale | 60 (29) | 21 (14) | 16 (10) | 17 (10) | 17 (11) | 13 (8) | 15 (10) | 8 (8) | 8 (9) | 4 (7) | 3 (9) | 1 (4) | 0 (0) | 1 (17) |
| Assessment | 84 (40) | 20 (13) | 8 (5) | 2 (1) | 2 (1) | 2 (1) | 3 (2) | 2 (2) | 3 (3) | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Communication and cognitive difficulties | 3 (1) | 0 (0) | 2 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Goals | 0 (0) | 20 (13) | 6 (4) | 8 (5) | 7 (5) | 13 (8) | 19 (13) | 16 (15) | 16 (18) | 9 (15) | 2 (6) | 3 (13) | 1 (17) | 1 (17) |
| Activities | 5 (2) | 9 (6) | 44 (26) | 46 (28) | 34 (22) | 36 (23) | 22 (15) | 11 (10) | 8 (9) | 4 (7) | 2 (6) | 1 (4) | 1 (17) | 0 (0) |
| Graded task | 0 (0) | 2 (1) | 3 (2) | 5 (3) | 6 (4) | 9 (6) | 1 (1) | 2 (2) | 0 (0) | 1 (2) | 2 (6) | 2 (8) | 0 (0) | 0 (0) |
| Problem-solving | 0 (0) | 3 (2) | 11 (7) | 9 (6) | 12 (8) | 4 (3) | 9 (6) | 8 (8) | 2 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Between-session tasks | 22 (11) | 39 (25) | 43 (26) | 42 (26) | 38 (25) | 34 (22) | 31 (21) | 17 (16) | 12 (13) | 5 (8) | 4 (12) | 4 (17) | 0 (0) | 0 (0) |
| Summary and review | 29 (14) | 23 (15) | 25 (15) | 22 (14) | 22 (14) | 30 (19) | 26 (17) | 23 (22) | 23 (26) | 15 (25) | 6 (18) | 4 (17) | 2 (33) | 2 (33) |
| Generalisation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 5 (3) | 13 (9) | 8 (8) | 9 (10) | 16 (26) | 6 (18) | 4 (17) | 0 (0) | 1 (17) |
| Other | 6 (3) | 18 (12) | 10 (6) | 10 (6) | 11 (7) | 9 (6) | 9 (6) | 10 (9) | 8 (9) | 5 (8) | 6 (18) | 5 (21) | 2 (33) | 1 (17) |
| Total | 209 | 155 | 168 | 162 | 152 | 156 | 149 | 106 | 90 | 61 | 33 | 24 | 6 | 6 |
We were unable to video as many therapy sessions as planned. The main difficulty was that there was only one video camera for the study, to be shared between the three therapists who were geographically based in three different sites. The therapists had to take turns using the video camera and had to record available sessions depending on whether or not the participants they were treating at that time had consented to be videoed.
Video recordings of treatment sessions were analysed for eight participants, who were aged 47–76 years (mean 62.6 years, SD 10.8 years); six participants were men. PHQ scores ranged from 11 to 23 (mean 15.8, SD 4.6) and VAMS ‘Sad’ item scores ranged from 22 to 88 (mean 59.3, SD 29.5). Ten treatment sessions were recorded from across the three sites. There were no recordings for sessions 1, 5, 8 and 9.
The frequency of components of therapy was calculated separately for those components of the manual that were applicable on all sessions and those that were session specific (see Appendices 11 and 12 for the results). Most components of the manual that were intended to be delivered on all sessions occurred. However, there were four sessions in which no session summary was observed. In addition, the recording of session 10 comprised almost exclusively social chat and included few of the components of treatment that should apply to all sessions.
The components of treatment that were specific to individual sessions were observed in the appropriate sessions. However, the recording of session 4 did not show any discussion on how enjoyable activities improve mood, nor a list of enjoyable activities being created, and no identification of barriers to engaging in identified activities. These were all components listed in the manual for session 4. In addition, the recording of session 10 showed that the session comprised entirely questions and answers and did not include a review of problems addressed during therapy, a summary of successful strategies and skills used, a discussion of generalisation of skills to future situations or a reminder about the 6-month follow-up.
See Appendix 13 for a summary of the frequency of therapist and participant activities. Most therapist activities occurred on most sessions. The only notable omission was the lack of reference to previous sessions by the therapist. The participants’ activities were similar across sessions. However, there were no observations of participants asking for information or asking questions.
Discussion of fidelity results
Overall, for the assessment of fidelity, the results of the therapy records indicate that most of the components of therapy described in the manual were delivered to participants. Importantly, each session included essential components of therapy and the distribution of time was as expected. This suggests that outcomes reflect the effect of the intervention as described in the manual. However, there were some components of therapy that were not recorded as being delivered to some participants. There was little use of graded task assignments and training in problem-solving, even for those elements that form the core components of BA. This may be a reflection of the coding used, as graded task assignments were often used as between-session tasks. The record form may need to be modified to reflect the content of the between-session tasks, as well as the content of therapy.
The video recordings also indicated that therapy was mainly delivered in accordance with the manual. The main limitations of the video analysis are that recordings were incomplete and some sessions were not covered. The results therefore demonstrate the extent to which video analysis could be reported in a definitive trial. The recordings highlighted that the content of the sessions that were recorded did not cover all aspects of therapy expected from the manual. However, the intervention was designed to be delivered flexibly and it may be that the missed content was delivered in other sessions.
Both methods of checking the fidelity of the intervention were feasible and both highlighted potential ways in which the therapy received deviated from the treatment as described in the manual. The records kept by therapists were simpler to use and more complete. However, the lack of therapeutic content in the video recording of session 10 was not picked up in the therapist records, suggesting that the two methods may complement each other.
Chapter 5 Health economic results
Feasibility outcomes
The percentage of items of complete data for each key outcome measure is presented in Table 29. At baseline, the response rate for the standard and pictorial versions of the EQ-5D-5L and resource use questionnaire was > 90%. There was a lower response rate for the proxy version of the EQ-5D-5L, which was completed by carers, and for the carers’ EQ-5D-5L about their own health (75.8% and 84.8%, respectively, as a proportion of those participants who had carers). At the 6-month follow-up, the response rate was slightly lower for most outcome measures for participants in the intervention group than in the control group. Overall, at follow-up, the response rate for the standard version of EQ-5D-5L and the resource use questionnaire was around 80%. Of the 10 participants who did not have standard version EQ-5D-5L data at 6-month follow-up; eight participants had withdrawn from the study or were lost to follow-up, one participant did not return the questionnaire and one participant returned the questionnaire but had a response for only four out of the five domains, meaning that an EQ-5D-5L score could not be calculated. The resource use questionnaire response rates were analysed by item. At baseline 2% of data were missing and at 6 months 16% of data were missing. The larger proportion of missing data at 6 months was generally attributable to a small number of participants not completing any part of the questionnaire, rather than to missing items within otherwise completed questionnaires.
| Outcome measure | Time point, % (n/N) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | |||||
| Treatment arm | Overall | Treatment arm | Overall | |||
| Control | Intervention | Control | Intervention | |||
| EQ-5D-5L (standard version) | 100.0 (23/23) | 96.0 (24/25) | 97.9 | 87.0 (20/23) | 72.0 (18/25) | 79.2 |
| EQ-5D-5L (aphasia-friendly version) | 95.7 (22/23) | 92.0 (23/25) | 93.8 | 82.6 (19/23) | 72.0 (18/25) | 77.1 |
| EQ-5D-5L (proxy)a | 81.3 (13/16) | 70.6 (12/17) | 75.8 | 68.8 (11/16) | 58.8 (10/17) | 63.6 |
| EQ-5D-5L (carer)a | 87.5 (14/16) | 82.4 (14/17) | 84.8 | 56.3 (9/16) | 70.6 (12/17) | 63.6 |
| Resource use questionnaire | 100.0 (23/23) | 96.0 (24/25) | 97.9 | 91.3 (21/23) | 76.0 (19/25) | 83.3 |
Within-trial analysis
Quality of life
The standard version of the EQ-5D-5L was used to calculate QALYs as no participants had severe aphasia. All participants were able to complete the standard version, including the five participants with moderate aphasia. At the 6-month follow-up, EQ-5D-5L utility scores were slightly higher in the control group than in the intervention group (Table 30).
| EQ-5D-5L by treatment arm | Mean | Standard error | 95% CI |
|---|---|---|---|
| EQ-5D-5L at baseline | |||
| Control | 0.51 | 0.06 | 0.40 to 0.63 |
| Intervention | 0.50 | 0.06 | 0.38 to 0.62 |
| EQ-5D-5L at 6 months | |||
| Control | 0.60 | 0.06 | 0.46 to 0.73 |
| Intervention | 0.51 | 0.07 | 0.35 to 0.67 |
As a supplementary analysis, the pairwise correlation between the different utility measures was calculated. There was a strong correlation (Pearson’s correlation coefficient 0.91) between values of the aphasia accessible version of the EQ-5D-5L and standard version. The correlation between responses to the EQ-5D-5L completed by carers on behalf of the study participant (carer proxy) and the standard version completed by the participants was moderate (Pearson’s correlation coefficient 0.59).
Costs
The cost of the intervention was estimated to be £57 per hour. 85 This was based on the intervention being provided on a one-to-one basis by a grade-5 Agenda for Change (AfC) mental health nurse, using estimates from the PSSRU. PSSRU incorporate training and clinical supervision costs in this estimate and, therefore, the training and supervision received in the BEADS study was not costed separately. Direct intervention costs were calculated by multiplying the hourly intervention cost by the average number of sessions for each individual patient.
There were two participants in the control group and seven in the intervention group with missing cost data at follow-up. One of these participants also had cost data missing at baseline. As there was the same level of missing data across resource use categories, data were imputed on the total cost level. The missing data at 6 months were imputed separately, based on whether or not the participant had achieved a ‘good response’ as measured by the PHQ-9. Analyses that incorporate imputed data are referred to as ‘full data set (with imputation)’ analyses. Analyses that do not use imputed data are referred to as ‘complete-case’ analyses.
Average costs at follow-up for the whole trial period (6 months) are shown in Table 31; these figures contain no imputed data. For all of the categories except societal costs, the average cost per patient in the intervention group was higher than in the control group (although no differences were statistically significant, see Table 32). Inpatient costs were incurred by two participants in the intervention group, compared with no participants in the control group. It is possible that these costs were chance events rather than being related to the intervention. For outpatient hospital services, two participants in the intervention group had costs of > £1000, whereas there were no such outliers in the control group. These costs were associated with psychiatry and psychology outpatient visits. It is unclear whether or not these costs were related to the intervention.
| Treatment arm | Cost (£) | |||||
|---|---|---|---|---|---|---|
| Inpatient hospital services | Outpatient hospital services | Primary and community care | Community day based services | Intervention costs | Societal | |
| Control | 0.0 | 225.12 | 1123.02 | 111.46 | 0.0 | 24,133.84 |
| Intervention | 466.16 | 954.78 | 2175.92 | 217.96 | 460.56 | 19,292.29 |
Home help was the main factor that caused the difference between the control and intervention groups in primary and community care costs. Four participants in the intervention group received home help compared with three participants in the control group. The intervention group received 295.5 hours more home help than the control group over the last 3 months of follow-up. There was one participant in the intervention group who received the equivalent of approximately one visit a day for 3 hours per day, which was more than that received by any other participant in the trial. See Appendix 14 for the distribution of costs relevant to the NHS and PSS perspective for the control and intervention groups.
The human capital approach was used to estimate societal costs. The societal component of costs consisted of work hours lost, carer time, travel expenses, private health-care costs and charity-provided services. These were added to the costs from the NHS and PSS perspective to form costs for the societal perspective. Owing to the nature of the population, there were only two participants in the control group and three participants in the intervention group either working or looking for work. In addition, three carers were reported to have lost work hours as a result of their caring responsibilities. However, data were incomplete, with some carers not recording whether or not they worked, and some carers not recording if they lost any time from work. For this reason, we allocated a cost to all carer time reported for the societal perspective analysis, whether or not it resulted in time being taken off work. Carer time accounted for 93.3% and 78.0% of societal costs for the control and intervention groups, respectively. See Appendix 15 for the distribution of costs relevant for the societal perspective for the control and intervention groups. Aggregated costs for the 6 months of the trial are presented in Table 32.
| Treatment arm | n | Mean (£) | Standard error (£) | 95% CI (£) | Difference (£) | Standard error (£) | 95% CI |
|---|---|---|---|---|---|---|---|
| Full data set (with imputation): health-care costs (including intervention costs) | |||||||
| Control | 23 | 1961.18 | 800.49 | 234.37 to 3688.01 | |||
| Intervention | 25 | 3547.46 | 1072.83 | 1316.47 to 5778.44 | 1586.27 | 1357.62 | –1164.24 to 4336.77 |
| Full data set (with imputation): societal costs (including health-care and intervention costs) | |||||||
| Control | 23 | 26,549.36 | 6078.09 | 13,801.07 to 39,297.65 | |||
| Intervention | 25 | 22,991.18 | 6056.13 | 10,275.64 to 35,706.72 | –3558.18 | 8724.13 | –21,249.47 to 14,133.1 |
| Complete case: health-care costs (including intervention costs) | |||||||
| Control | 21 | 1391.90 | 318.38 | 727.77 to 2056.03 | |||
| Intervention | 19 | 3875.64 | 1307.22 | 1129.27 to 6622.01 | 2483.74 | 1286.12 | –119.88 to 5087.36 |
| Complete case: societal costs (including health-care and intervention costs) | |||||||
| Control | 21 | 25,525.74 | 6039.68 | 12,927.19 to 38,124.29 | |||
| Intervention | 19 | 23,167.92 | 6470.61 | 9573.67 to 36,762.18 | –2357.817 | 8842.79 | –20,259.11 to 15,543.48 |
Results from the within-trial analysis
The within-trial analysis was undertaken on both the complete-case and the imputed data sets. Results are presented in Table 33. Based on the imputed data set, when including all costs relevant to the NHS and PSS [labelled ‘NHS & PSS perspective (1)’] costs were £1316 higher in the intervention group than the control group over the course of the study. To illustrate the influence of inpatient hospital stays we reanalysed the data excluding inpatient costs if the hospital stay was for > 1 night [labelled ‘NHS & PSS perspective (2)’]. In this analysis the incremental cost associated with the intervention reduced to £980. The 95% CI for both these estimates includes negative values, indicating that there was no statistically significant difference in costs between the intervention and control groups.
| Cost perspective | Incremental costs (£) | Standard error (£) | 95% CI (£) | Incremental QALYs | Standard error | 95% CI |
|---|---|---|---|---|---|---|
| Complete case (38 participants) | ||||||
| NHS & PSS perspective (1) | 1634 | 1174.0 | –667.39 to 3934.63 | –0.0107 | 0.015 | –0.04 to 0.02 |
| NHS & PSS perspective (2) | 1228 | 951.9 | –638.13 to 3093.27 | –0.010 | 0.015 | –0.04 to 0.02 |
| Societal perspective | –6479 | 7727.6 | –21,625.08 to 8666.45 | –0.012 | 0.015 | –0.04 to 0.02 |
| Full data set (including imputed data; 48 participants) | ||||||
| NHS & PSS perspective (1) | 1316 | 1275.15 | –1191.66 to 3823.16 | –0.0204 | 0.021 | –0.06 to 0.02 |
| NHS & PSS perspective (2) | 980 | 1245.94 | –1469.93 to 3430.61 | –0.0205 | 0.021 | –0.06 to 0.02 |
| Societal perspective | –8281 | 7945.87 | –23,879.93 to 7318.81 | –0.0207 | 0.021 | –0.06 to 0.02 |
For all analyses, incremental QALYs were negative, meaning that participants in the intervention group accrued fewer QALYs over the 6-month trial period than participants in the control group. Again, differences were not statistically significant. Therefore, based on an NHS and PSS perspective and a within-trial analysis, the intervention is expected to be dominated by the control, that is, it results in higher costs and lower QALYs than usual care.
It was estimated that there would be lower costs (by £8281 per patient) in the intervention group than in the control group, when taking a societal perspective and valuing all carer time regardless of whether it resulted in the carer taking time off work. This results in an ICER of £539,917 saved per QALY lost based on the complete-case analysis, and an ICER of £400,048 saved per QALY lost based on the imputed data analysis. The interpretation of these ICERs is different from the standard interpretation because the intervention is situated in the south-west quadrant of the cost-effectiveness plane (see Figure 12) – it results in cost savings but fewer QALYs.
Figures 9 and 10 show the point estimate of the ICER and CIs around this based on the ‘NHS & PSS perspective (2)’ analysis and the societal analysis, respectively, using the full data set with imputation. Figures 11 and 12 show the corresponding probabilities of the intervention being cost-effective at different cost-effectiveness thresholds for these two analyses.
FIGURE 9.
Confidence ellipses (controlling for baseline utility and costs) for ‘NHS & PSS perspective (2)’ analysis, full data set.

FIGURE 10.
Confidence ellipses (controlling for baseline utility and costs) for the societal perspective, full data set.

FIGURE 11.
Cost-effectiveness acceptability curve (controlling for baseline utility and costs) for ‘NHS & PSS perspective (2)’ analysis, full data set.

FIGURE 12.
Cost-effectiveness acceptability curve (controlling for baseline utility and costs) for the societal perspective, full data set.

Model-based analysis
Model inputs
Transition probabilities
Transitions from the ‘no response’ health state to the ‘good response’ health state were based on the response rates observed in the trial. In the intervention group, 68% of participants achieved a 4.78-point decrease in PHQ-9 from baseline to follow-up and were, therefore, assumed to transit into the ‘good response’ state. In the control group, 22% of participants achieved this response. Model parameter values for all parameters included in the model, their CIs and the distribution used to characterise them in PSA are presented in Appendix 16.
It was not possible to discern relapse rates from the trial and, therefore, these were assumed to be zero in the base case, but a range of possible relapse rates was considered in sensitivity analysis.
Brønnum-Hansen et al. 96 estimated that the annual risk of death from stroke is 18.1% and 10% between 4 weeks to 1 year and after 1 year following stroke, respectively. In the BEADS trial, 60% of participants had a stroke up to 1 year prior to randomisation, with mean time since stroke for this group being approximately 7 months. Hence, for the first 5 months of the model, the mortality rate was weighted between 18.1% and 10%, based on the proportion of participants who had a time since stroke of more or less than 1 year. This mortality rate was applied to both the ‘no response’ and the ‘good response’ health states; hence, we assume that the intervention does not affect survival. From month 6 up to the start of year 6, the transition probability for death was based on the 10% annual risk estimated by Brønnum-Hansen et al. 96 From year 6 onwards, death from other causes was added to the annual mortality rate based on mortality data from the Office for National Statistics97 and the age and sex split observed in the BEADS trial.
Quality of life
We calculated utility scores at baseline and at 6 months separately for participants who achieved a ‘good response’ and those who did not, in order to estimate the increase in utility associated with achieving a good response using a difference-in-differences approach. We found that achieving a good response was more beneficial than not responding, such that achieving a good response was associated with an increase in utility score of 0.066 at 6 months.
It is notable that the increase in utility score in responders was much more substantial in the control group than in the intervention group (see Appendix 17). However, we assumed that this was due to chance and in the economic model simply used the utility benefit estimated using all responders in the study, irrespective of their randomised group.
In the economic model, the utility score for the ‘no response’ health state was 0.52 and the utility score in the ‘good response’ health state was 0.59 (see the table of parameter values in Appendix 16). These scores were based on average utility for each response group at 6 months.
Costs
Health state costs were estimated separately for the ‘good response’ and ‘no response’ states. Outlying costs were observed in the trial analysis; in particular, inpatient hospital costs were observed only in the intervention group, and these were not deemed to be associated with the intervention. These costs were for a 17-night inpatient orthopaedics episode and a 10-night inpatient episode for a myocardial infarction. In order to avoid these costs skewing our model-based analysis, we excluded them from the economic model. Costs associated with the response and non-response health states used in the economic model are presented in the table of parameter values in Appendix 16. Costs were slightly higher in the ‘good response’ health state from the NHS and PSS perspective, but were lower from the societal perspective.
The direct cost of the intervention was estimated to be £460.56, based on an average number of BA sessions completed in the intervention arm of the trial of 8.08. This cost was added to the intervention arm of the model only and included in the PSA (see the table of parameter values in Appendix 16).
Probabilistic sensitivity analysis
For the PSA, we assumed that utility in the response group could not fall below the utility of the non-response group. The difference method was used108 rather than simple (and biased) techniques that involve resampling or adjustment if the desired ordering is not achieved. It seems reasonable to assume that a participant with a lower level of depression (i.e. a ‘good response’) would have a higher utility than a participant with a higher level of depression, all else remaining equal. Without a larger data set of observations of utility values of responders and non-responders, it is impossible to rule out the possibility that this assumption does not hold.
Our base-case analysis incorporated a zero relapse rate. We felt that this was likely to lead to misleading PSA and value-of-information results. Therefore, in a supplementary analysis a hypothetical relapse rate of 10% per month was added to the model. This followed a beta distribution with a standard error of 0.2, and lower and upper bounds of 0.01 and 0.8, respectively. The standard error was chosen based on an inflation of the standard error observed for the response rates for each treatment group (0.09 in the intervention group and 0.08 in the control group).
Results from the model-based analysis
Deterministic results
Based on an NHS and PSS perspective, the economic model predicted that, over a person’s lifetime, costs would be £3852 higher in the intervention group than in the control group. It is notable that this represents a substantial difference compared with the QALY loss estimated using the within-trial analysis. This is because there was a higher response rate in the experimental group, and the utility score was higher in the ‘good response’ health state, and is driven by the fact that we assume that the utility of a responder is not dependent on whether they received the intervention or usual care. This point is revisited in Summary of health economics findings. A QALY gain of 0.2 was estimated for participants in the intervention group. From these results the ICER was estimated to be £19,187. Typically, in the UK, interventions are classed as cost-effective if the ICER is > £20,000 per QALY gained. 99 Under a societal perspective, the intervention was estimated to dominate the control treatment – producing higher QALYs and cost savings. Results are presented in Table 34.
| Cost perspective | Per person treated | ||||
|---|---|---|---|---|---|
| Cost (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| NHS perspective | |||||
| Control | 33,590 | 3.57 | |||
| Intervention | 37,441 | 3.77 | 3852 | 0.2 | 19,186.88 |
| Societal perspective | |||||
| Control | 363,127 | 3.57 | |||
| Intervention | 319,449 | 3.77 | –43,678 | 0.2 | Intervention dominates |
Deterministic sensitivity analysis
Cost-effectiveness analysis results are often more sensitive to the values of certain parameters than others. A one-way sensitivity analysis allows us to see how changing one parameter and keeping other parameters constant would influence the ICER. Owing to the relapse rate not being included in our base-case analyses (as it was not possible to calculate it directly from trial data), the base-case ICERs may be optimistic. Figure 13 demonstrates that when a relapse rate was incorporated within the model, the ICER increases above £30,000 per QALY gained for relapse rates of > 7% per month, under an NHS and PSS perspective.
FIGURE 13.
ICER vs. relapse rate per month, NHS and PSS perspective.
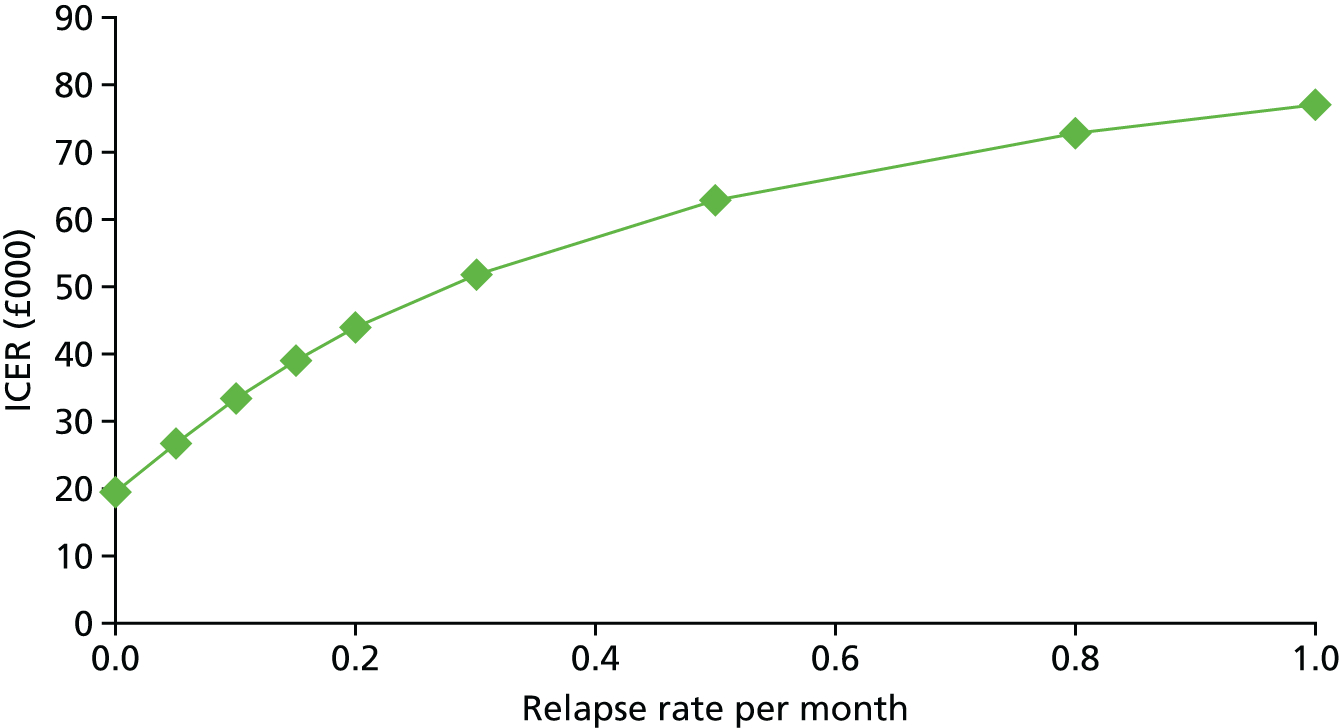
From a societal perspective, the intervention continues to dominate for all possible relapse rates, all else remaining equal.
Probabilistic sensitivity analysis
The results of the deterministic analysis have to be interpreted with caution as they do not account for parameter uncertainty. PSA assigns distributions to parameters and runs the model many times, each time selecting a value from the distribution of each parameter. Estimates from each run of the model are combined and averages are taken to calculate the probabilistic ICER. CEACs are estimated, which represent the proportion of simulations in which the intervention was associated with an ICER below a defined cost-effectiveness threshold. We ran the model a total of 10,000 times in a Monte Carlo simulation. The result of each simulation is presented on the cost-effectiveness plane in Figure 14, for the analysis that took an NHS and PSS perspective and incorporated imputation. The probabilistic ICER was £18,979.82 per QALY gained.
FIGURE 14.
Model-based analysis: probabilistic results on the cost-effectiveness plane, NHS and PSS perspective.
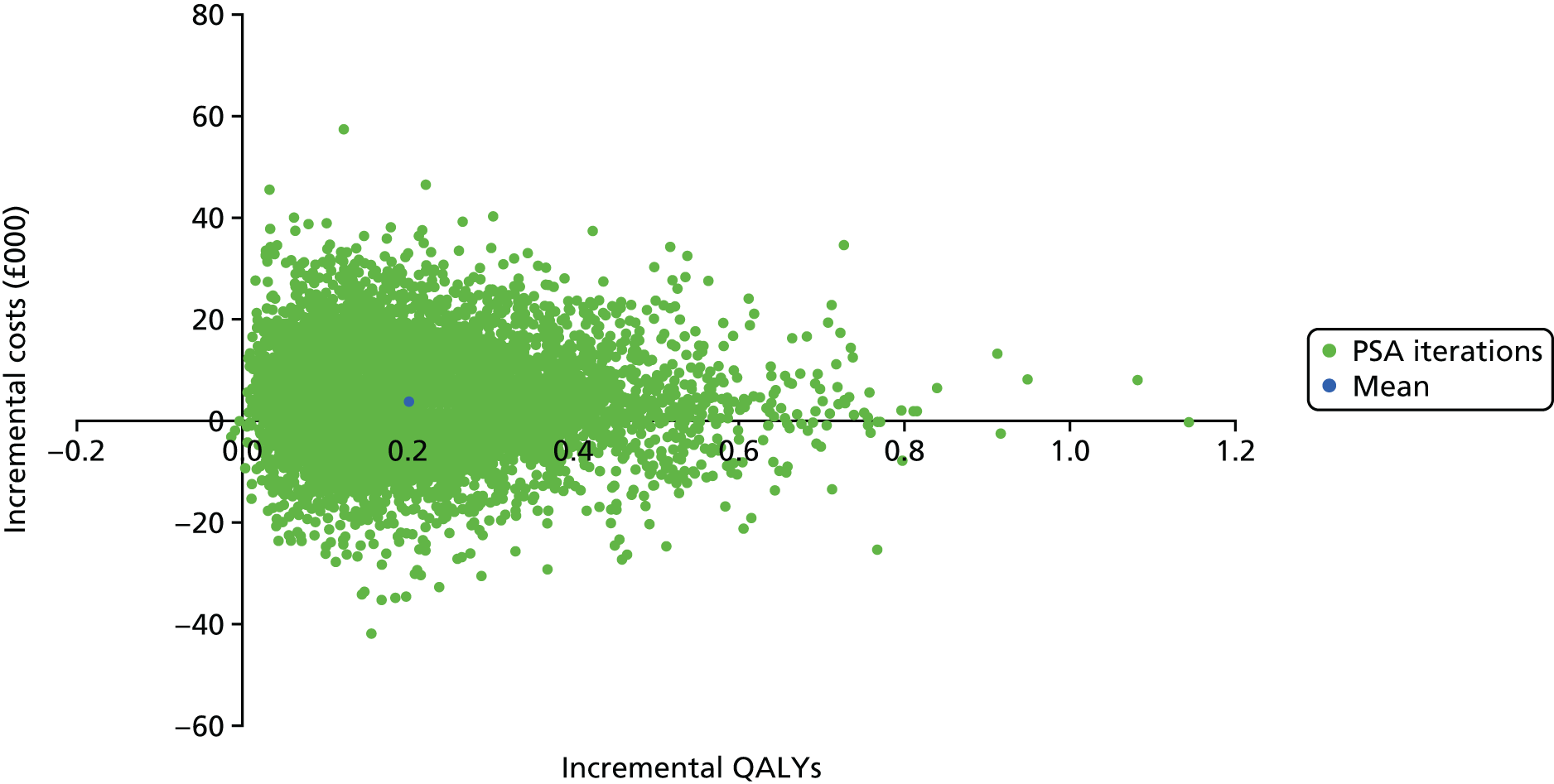
Owing to the substantial uncertainty around monthly costs associated with each health state, incremental costs for each model run varied widely, with approximately 70% of runs estimating that the control group would have lower costs, and approximately 30% estimating that the intervention group would have lower costs. CEACs, showing the probability of the intervention being cost-effective at various thresholds for the NHS and PSS perspective, are plotted in Figure 15. At a threshold of £20,000 per QALY gained, the intervention had a 51% probability of being cost-effective, and at a threshold of £30,000 the probability was 60%. Owing to a small number of model runs producing a QALY decrement for the intervention group, the probability of the intervention being cost-effective does not reach 100%, irrespective of how high the threshold is. For cost-effectiveness thresholds of > £19,000 per QALY gained, it was estimated that the intervention would be most likely to be the cost-effective treatment option.
FIGURE 15.
Cost-effectiveness acceptability curves, NHS and PSS perspective.

From the societal perspective, the probabilistic analysis estimated that the intervention was dominant – the vast majority of model runs resulted in cost-effectiveness estimates that lay in the south-east quadrant of the cost-effectiveness plane (Figure 16). The probability that the intervention was cost-effective at a threshold of £20,000 per QALY gained was 82.8% (Figure 17).
FIGURE 16.
Model-based analysis: probabilistic results on the cost-effectiveness plane, societal perspective.
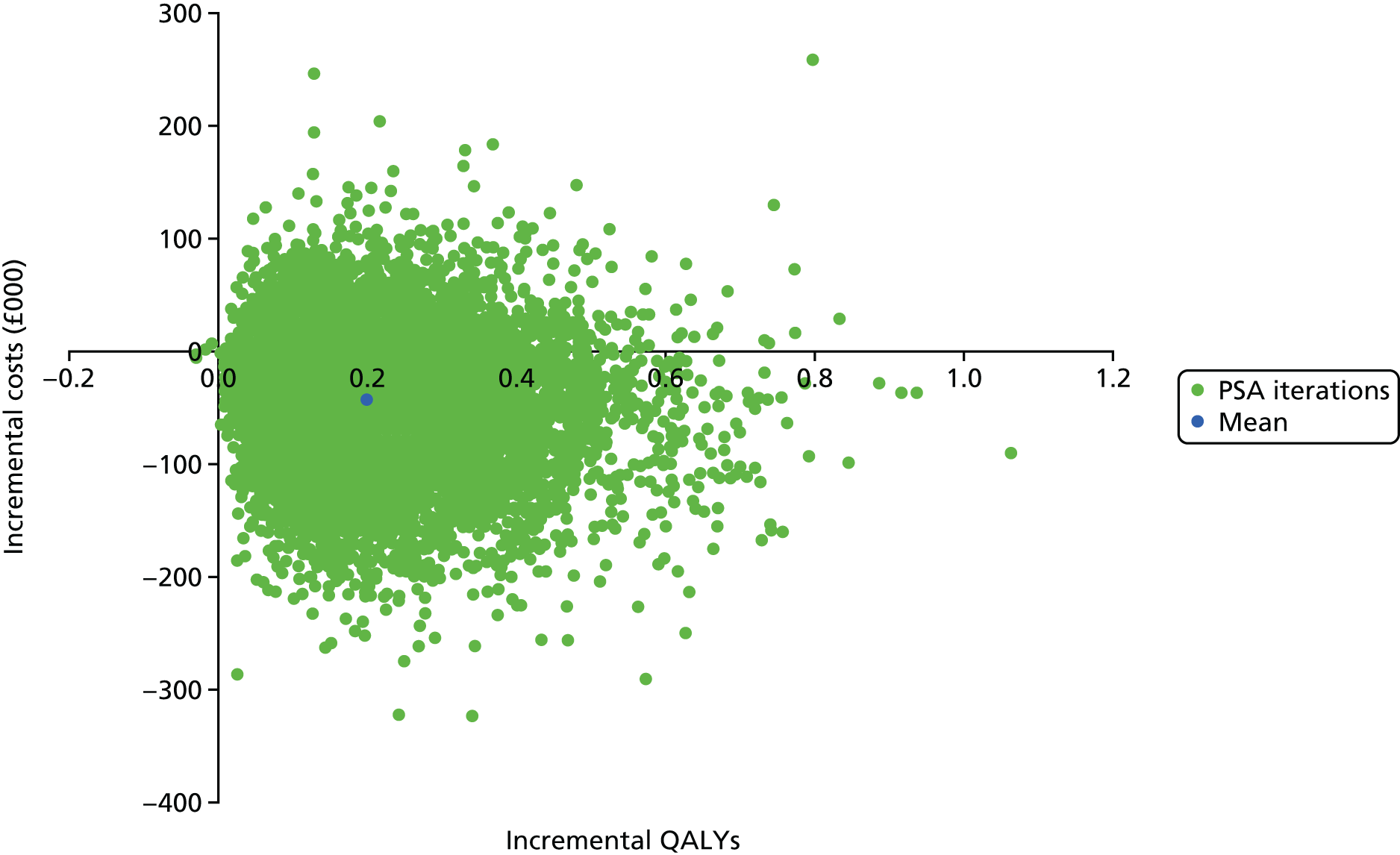
FIGURE 17.
Cost-effectiveness acceptability curves, societal perspective.
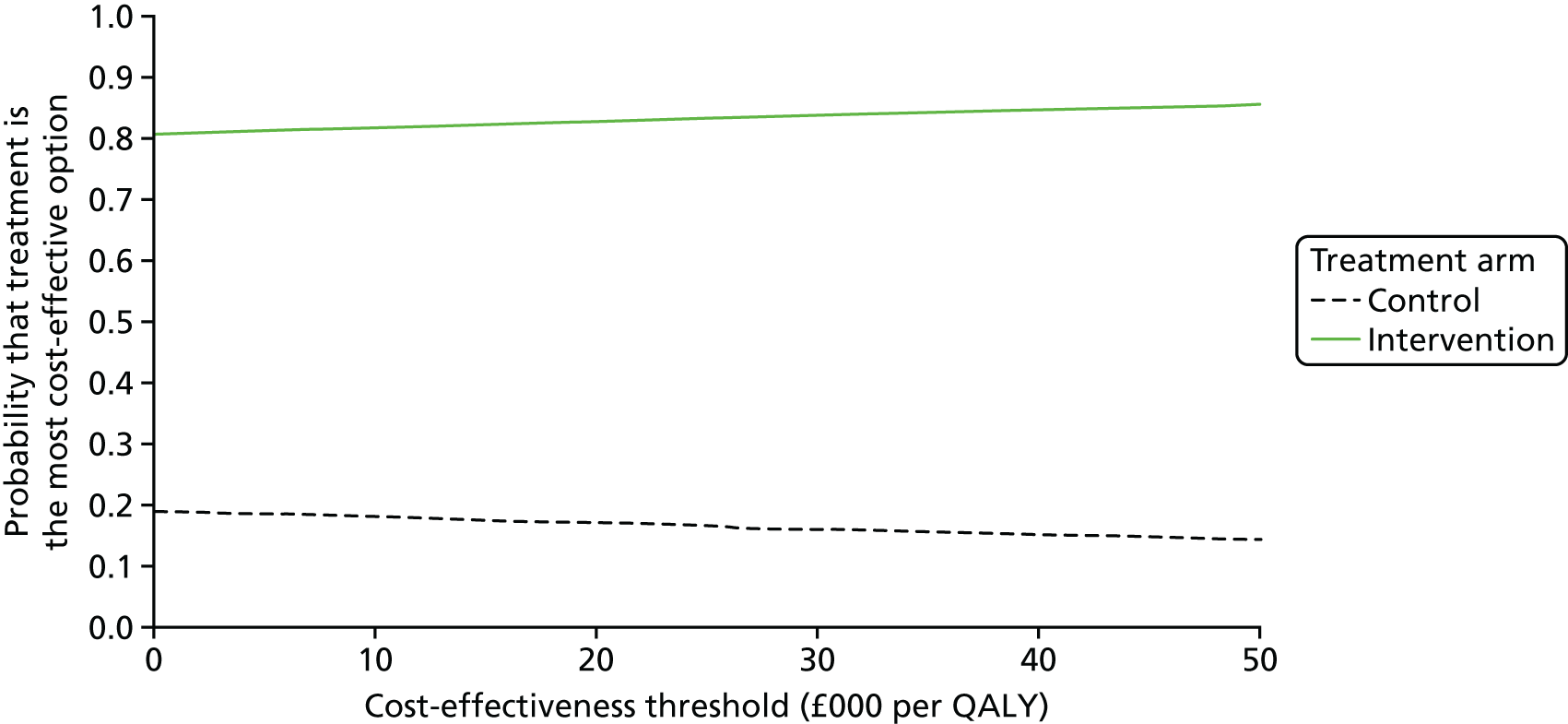
Supplementary analysis
It is important to reiterate that the base-case deterministic and probabilistic results incorporated a relapse rate of zero, with no uncertainty around this. We conducted a supplementary analysis that incorporated a hypothetical relapse rate of 10% per month, with a beta distribution with a standard error of 0.2, and lower and upper bounds of 0.01 and 0.8, respectively. Clearly this is an important assumption. The standard error represents an inflation of the standard errors associated with the response rate in the experimental and control groups, which were 0.09 and 0.08, respectively. This resulted in a probabilistic ICER of £21,626.49 per QALY gained for the NHS and PSS perspective. From the societal perspective the model continued to estimate that the intervention would dominate, leading to cost savings and QALY gains. The cost-effectiveness plane scatters and the CEACs for these analyses are presented in Figures 18–21. At a cost-effectiveness threshold of £20,000 per QALY gained, the probability of the intervention being cost-effective was 42.6% under an NHS and PSS perspective, and 81.0% under a societal perspective.
FIGURE 18.
Model-based analysis: probabilistic results on the cost-effectiveness plane, NHS and PSS perspective with relapse rate.
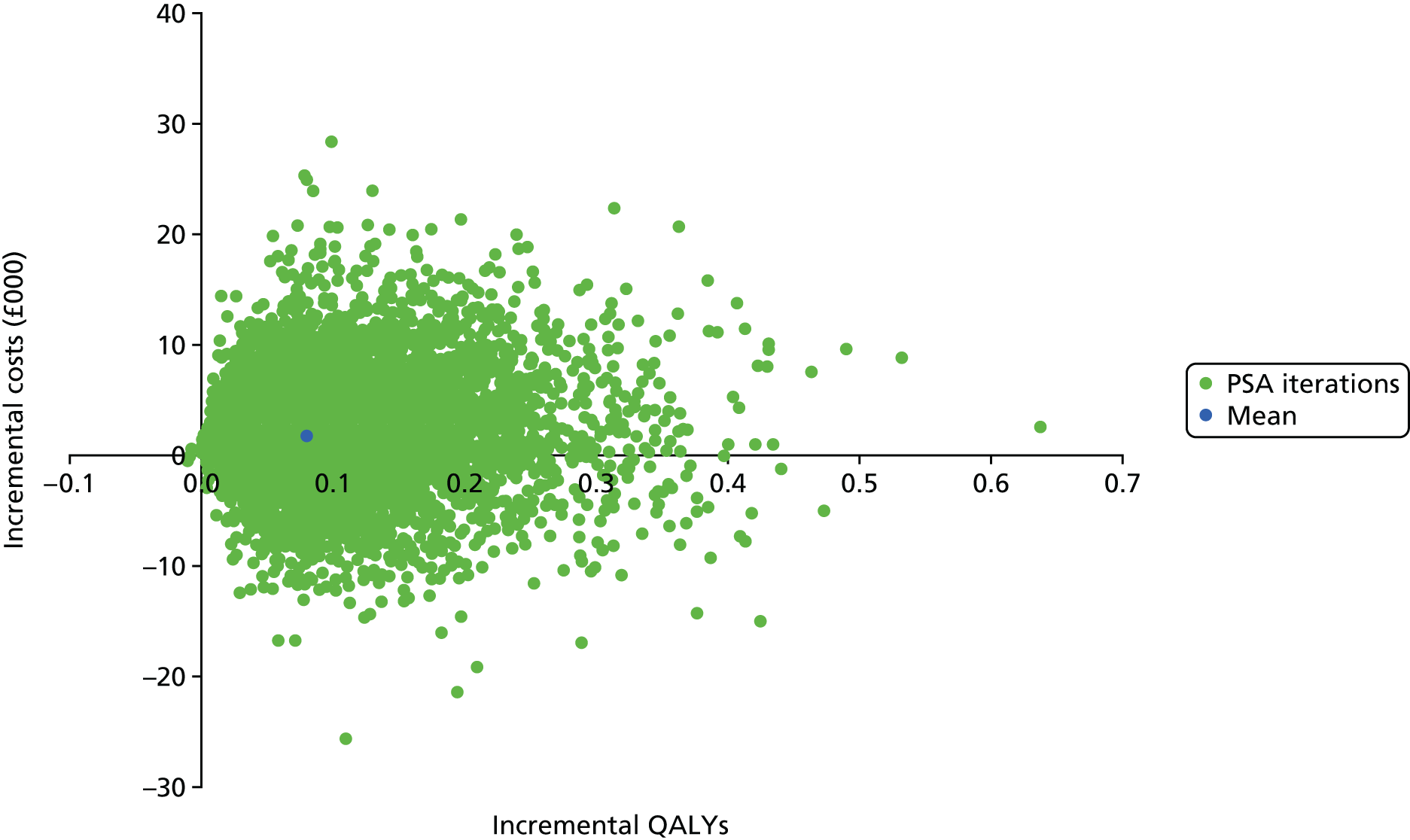
FIGURE 19.
Cost-effectiveness acceptability curves, NHS and PSS perspective with relapse rate.
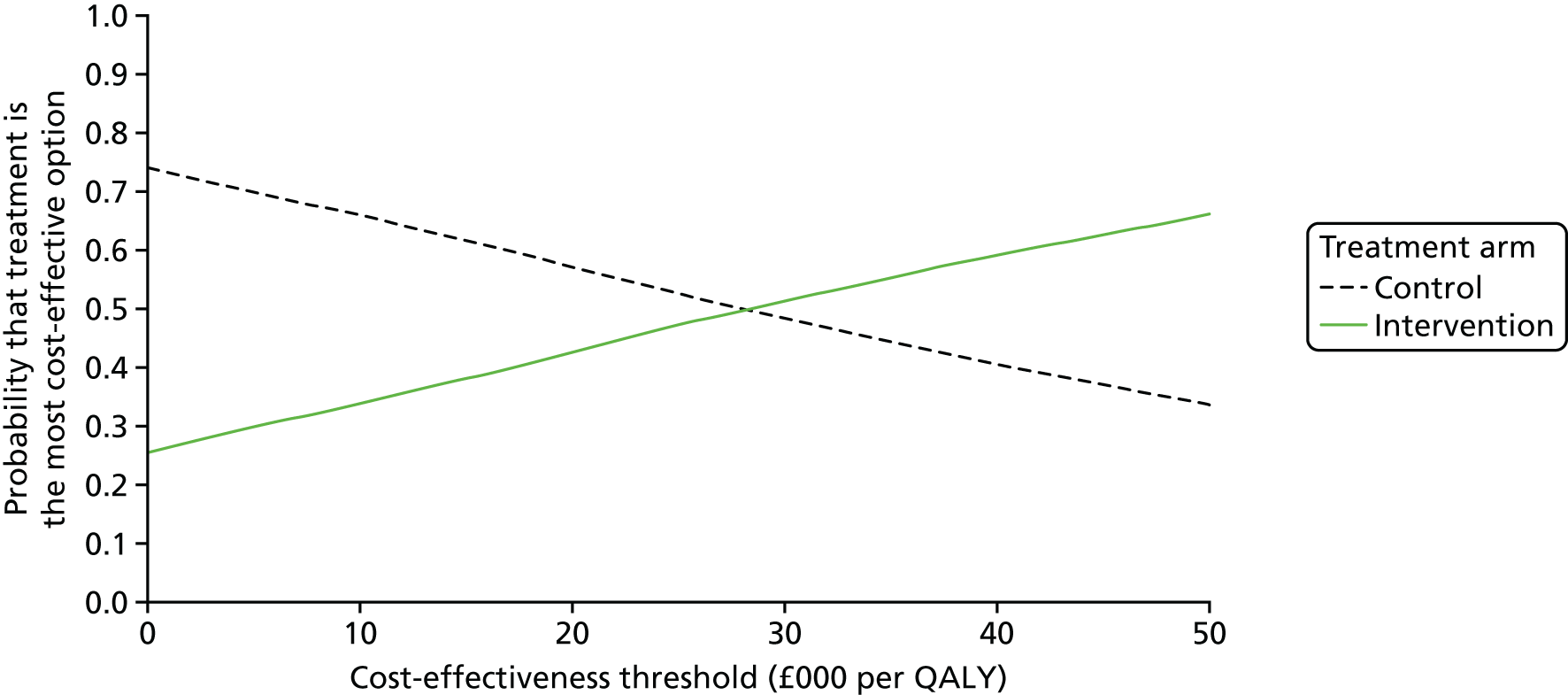
FIGURE 20.
Model-based analysis: probabilistic results on the cost-effectiveness plane, societal perspective with relapse rate.
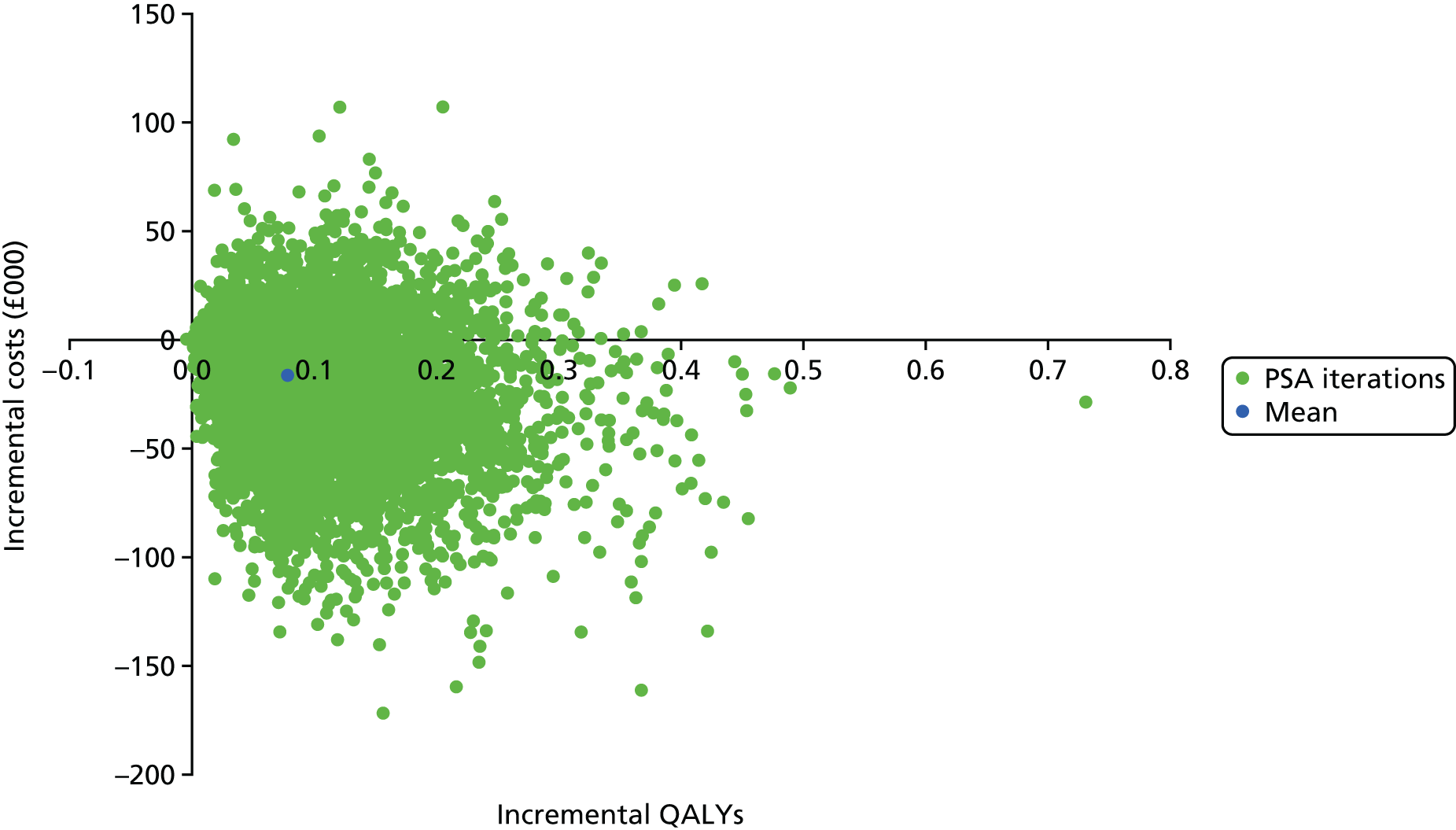
FIGURE 21.
Cost-effectiveness acceptability curves, societal perspective with relapse rate.

Value of information
We conducted our value-of-information analysis based on the supplementary probabilistic analysis that incorporated a non-zero relapse rate, as it was considered to be unrealistic to assume a zero relapse rate with no uncertainty. We estimate that the per-patient value of perfect information is £1348.85, given a cost-effectiveness threshold of £20,000 per QALY gained and taking an NHS and PSS perspective.
We extrapolated the per-patient value-of-information estimates to a population level by estimating the number of participants that would be likely to receive the treatment over a 10-year time period. It is estimated that there are 1,202,053 stroke survivors in the UK. 109 Approximately 29% of these people will also have depression. 1 To be conservative in our estimation of the population size that could benefit from BA therapy, we assumed that the intervention would not be suitable for people with severe depression. No published estimates of the proportion of stroke survivors who had mild, moderate or severe depression were found. Hence, to estimate this we used the proportion of participants with severe depression in the BEADS trial (29.2%); thus, 70.8% of stroke survivors with depression were estimated to have mild or moderate depression and, therefore, to be eligible for the intervention. In total, we estimate that the prevalent population in the UK that could benefit from the BA intervention is 246,922 [1,202,053 × 0.29 × 0.71].
In addition, it is estimated that approximately 152,145 people in the UK have a stroke each year,110 with 67–75% of these estimated to be first (i.e. not recurrent) strokes. A total of 78% of people experiencing a stroke are expected to survive for longer than 6 months. 111 Again, approximately 29% of these people will also have depression and we estimate that 70.8% of these would have mild or moderate depression and be eligible for the intervention. To be conservative, taking the lower bound of the stroke proportion that represents a first stroke, we estimate that the incidence population in the UK that could benefit from the intervention is 16,333 per year (152,145 × 0.67 × 0.78 × 0.29 × 0.71).
In total, we estimate that 410,251 people in the UK could be eligible to receive the intervention over a 10-year time period (246,922 + 10 × 16,333), with, on average, 41,025 treated per year. Based on this, we estimate a population-level EVPI of £552M.
The EVPI was higher when undertaking the analysis from a societal perspective. The per-patient EVPI was £1881 at a cost-effectiveness threshold of £20,000 per QALY gained and the population-level EVPI was £771.8M.
Expected value of perfect parameter information
A further analysis was undertaken to gain an insight into which areas would deliver the most value for future research. The Sheffield Accelerated Value of Information Tool100 was used to estimate the value of obtaining perfect information for one parameter or a group of parameters. The following parameters were included in the analysis:
-
health-care costs in the ‘response’ state
-
health-care costs in the ‘no response’ state
-
utility in the ‘response’ state
-
utility in the ‘non-response’ state
-
probability of good response (control group)
-
probability of good response (intervention group)
-
relapse rate
-
number of BA sessions
-
probability of death.
The results of the analysis taken from the NHS and PSS perspective suggest that the parameters that have the highest value for further research were health costs in both response states (Table 35). For health costs in the response state, the EVPPI was £926 per person, giving a population EVPPI of £379.8M over 10 years, for the NHS and PSS perspective. For health costs in the non-response state, the per-person EVPPI was £891, and for the population was £365.4M over 10 years. The next most important parameter was utility for a non-response, with an estimated per person EVPPI of £108. A zero value for EVPPI suggests that whichever value a parameter takes within its defined distribution would not affect the probability of the intervention being cost-effective at a threshold of £20,000 per QALY gained.
| Parameters | Per-person EVPPI (£) | Standard error (£) | EVPPI for UK over 10 years (£) |
|---|---|---|---|
| Health costs (response) | 925.84 | 21.77 | 379,800,000 |
| Health costs (no response) | 890.61 | 20.3 | 365,400,000 |
| Utility (response) | 0.0 | 2.1 | 0 |
| Utility (no response) | 108.42 | 20.33 | 44,480,000 |
| Probability of good response (intervention) | 13.99 | 11.44 | 5,741,000 |
| Probability of good response (control) | 0.0 | 3.32 | 0 |
| Relapse rate | 21.77 | 22.86 | 8,932,000 |
| Number of BA sessions | 8.24 | 11.72 | 3,381,000 |
| Probability of death | 0.0 | 0.0 | 0 |
Results of the EVPPI analysis from the societal perspective are presented in Table 36. The EVPPI was zero for all parameters except societal costs for both response groups.
| Parameters | Per-person EVPPI (£) | Standard error (£) | EVPPI for UK over 10 years (£) |
|---|---|---|---|
| Health costs (response) | 0.0 | 0.69 | 0 |
| Health costs (no response) | 0.0 | 0.0 | 0 |
| Societal costs (response) | 734.77 | 58.69 | 301,400,000 |
| Societal costs (no response) | 420.6 | 53.15 | 172,600,000 |
| Utility (response) | 0.0 | 0.0 | 0 |
| Utility (no response) | 0.0 | 0.0 | 0 |
| Probability of good response (intervention) | 0.0 | 0.0 | 0 |
| Probability of good response (control) | 0.0 | 0.59 | 0 |
| Relapse rate | 0.0 | 6.25 | 0 |
| Number of BA sessions | 0.0 | 0.0 | 0 |
| Probability of death | 0.0 | 0.0 | 0 |
Given the difficulties associated with collecting accurate data on ‘indirect’ costs (i.e. NHS, PSS and societal resource use is not directly related to the actual receipt of the intervention), details on these are not always collected and included in economic evaluations. For this reason, we reran the NHS and PSS perspective EVPI and EVPPI analyses, including in the economic model only the direct costs associated with the delivery of the intervention. This resulted in a per-patient EVPI of £50.39, a population-level EVPI of £20M, and EVPPI results as presented in Appendix 18.
Summary of health economics findings
The primary objectives of our analysis were to assess the feasibility of collecting data that may be used in a health economic analysis in the context of the BEADS trial, and to conduct an economic evaluation and a value-of-information analysis in order to provide information on the potential value of conducting the definitive trial. Hence, the primary aim of our analysis was not to conclude whether or not the intervention was likely to represent a cost-effective use of NHS resources, but to conclude whether it is feasible to collect the data required to make such conclusions based on a full trial, and whether it appears to represent good value for money to conduct a full trial.
The collection of data required for the economic evaluation was successful in the BEADS trial; it would be feasible to collect similar data in a full trial. The data collection completion rate was 97.9% at baseline for the standard EQ-5D questionnaire and for the resource use questionnaire, and was 79.2% and 83.3% for the EQ-5D and resource use questionnaire, respectively, at the 6-month time point.
Our analyses suggest that there would be a very high value to obtaining further information on key parameters within the economic mode. This value is likely to far exceed the cost of running a full trial whether an NHS and PSS perspective is taken (for which the population-level EVPI was estimated to be £552.6M) or a societal perspective is taken (for which the population-level EVPI was estimated to be £771.8M) in the economic model. The model parameters that are estimated to be the most valuable on which to obtain more information are the NHS and PSS costs associated with ‘no response’ and ‘good response’ health states.
Our aim was not definitively to estimate the cost-effectiveness of the BA therapy. However, our results suggest that the BA therapy is likely to be of borderline cost-effectiveness from an NHS and PSS perspective, given a cost-effectiveness threshold of £20,000 per QALY gained, but may be cost-saving from a societal perspective. However, our analysis is exploratory and the results should be interpreted with care given the small sample size in the BEADS trial.
It is important to note that the results of our model-based analyses differed substantially from those of the within-trial analysis. The within-trial analysis estimated QALY losses for the intervention group, whereas the model-based analyses estimated QALY gains for the intervention. This is because the model was response based; response rates were higher in the intervention group and utility scores were higher in responders and were assumed to be equal irrespective of the treatment that had been received. We feel that this is a reasonable assumption, but we highlight it here as the reason for the difference between the within-trial and model-based analyses of QALY gains/losses. In fact, the average increase in utility reported by responders in the control group was larger than in the experimental group (see Appendix 17). On average, non-responders in the control group also reported a small utility gain, whereas there was a small decrease in utility in non-responders in the intervention group. This explains why the within-trial analysis resulted in a QALY loss for the intervention, whereas the modelled analysis resulted in a QALY gain.
We could have assumed that utility scores for the ‘no response’ and ‘good response’ health states were treatment arm specific, which would have led to modelled QALY losses for the intervention. However, the CIs for the utility scores in the ‘no response’ and ‘good response’ health states overlapped for the control and intervention groups and were based on very low numbers; for example, EQ-5D-5L data at 6 months were available for only four control group participants who achieved a good response, and six intervention group non-responders. We therefore decided to calculate health-state utility scores that were not treatment arm specific, assuming that the utility scores in each response state were not related to treatment received. We feel that this is a reasonable assumption, but we highlight it here as the reason for the difference between the within-trial and model-based analyses of QALY gains/losses. This also highlights that there is considerable uncertainty around the impact of the intervention on QoL, and it is possible that the intervention could lead to reductions in QoL.
Discussion of health economics findings
Costs were collected on all health-care resource use in the BEADS trial, and the ranges of incurred costs varied widely between trial participants. Based on the data collected in BEADS, it is highly uncertain whether a ‘good response’ leads to a reduction or an increase in health-care costs; however, the answer to this is highly influential for the results of the economic evaluation. This is similarly true when the analysis takes a societal perspective – the societal costs associated with ‘no response’ and ‘good response’ health states are highly uncertain and highly influential. Hence, in a definitive trial it would be particularly valuable to obtain more information on costs.
Given the importance of carer costs in the societal analysis, it is relevant to further consider how these were estimated. Resource use questionnaires were used to collect data about informal care, and trial participants were asked the following question at baseline and the end of the trial ‘Over the last 3 months have you been helped and/or cared for by a relative or friend because of your health?’. If the answer was ‘yes’ participants were asked to record how many hours in the last week the person who helped them most spent caring for them, and how many hours resulted in the carer not attending work. However, there were many missing responses for how many hours attending work were lost and, therefore, all carer time was costed equally. The total cost of carer time over the 6-month trial was estimated by extrapolating the data for 1 week over the 6-month period and applying an hourly cost based on median hourly earnings using Office for National Statistics figures (see Appendix 3). Owing to the majority of societal costs comprising carer costs, we believe that any future trial should aim to improve the collection of data on carer time. Carer diaries could be incorporated in the trial, or participants could be asked to complete questionnaires more regularly, to reduce the need to extrapolate information from 1 week over a 6-month time period.
Although societal costs were much higher than NHS and PSS costs, home help costs were a key driver of the analysis undertaken from the NHS and PSS perspective. Therefore, for a definitive trial, care should be taken to collect information on this as accurately as possible. In fact, in our study home help costs were higher in the intervention group, whereas carer costs were lower; in a definitive trial, any potential relationship between these resource uses should be further explored.
Owing to the difficulties associated with obtaining accurate and precise estimates of health state costs when all NHS and PSS (and societal) resource use is included, it may be argued that value-of-information analyses that estimate the value of obtaining perfect information on these costs are likely to vastly overestimate the value of further research because perfect information will never be obtained and high levels of uncertainty are likely to remain even after a full trial has been completed. For this reason, we reran our value-of-information analyses excluding all but direct intervention costs from the economic model. This resulted in a population EVPI over 10 years of £20.67M. This suggests that it would still be worth conducting a full trial to obtain further information on other parameters such as relapse rate, utilities and response rates, even if other resource use costs were ignored. These data are best collected in a RCT.
Although our economic model suggests that the BA therapy is potentially cost-effective, it is important to reiterate that the within-trial analysis resulted in QALY losses for the intervention. These were highly uncertain and were not statistically significant, but it is possible that the intervention could reduce QoL.
Limitations of the health economics analysis
It should be noted that there are limitations with value-of-information analyses. The EVPI represent the value of obtaining perfect information, which is unlikely to occur in reality. It also assumes perfect uptake of the intervention, which may be unrealistic (although by assuming that people with severe depression would not receive the intervention we have attempted to estimate the eligible population size conservatively). Therefore, the EVPI figures may be argued to represent maximum values of obtaining further information. Conversely, our EVPI estimates account for parameter uncertainty only in our economic model – they do not account for structural uncertainty. A very simple model structure was used because it was unlikely to be possible to populate a more elaborate model using data collected in BEADS. For instance, different categories of response could be modelled, as well as potential future neurological events that would be expected to have an impact on the long-term effectiveness of the treatment. We believe that a more elaborate model would not substantially alter the results of our analysis, particularly our supplementary probabilistic analysis that incorporated a relapse rate to account for long-term effectiveness. However, a more complex model structure may be appropriate if and when further data become available. Overall, given that our estimated EVPI values were consistently substantially higher than the likely costs of undertaking a full trial, it seems reasonable to conclude that conducting such a trial would represent good value for money.
It may be considered that our assumption of equal mortality rates in the ‘no response’ and ‘good response’ health states is overly conservative, given the evidence of an association between depression and mortality. 1,8,9 However, the model states are based on response, not on whether or not a patient still has depression – a responder could still be classified as having depression. An alternative model structure could use health states defined around depression instead of response, but this may miss benefits to patients who respond favourably to the treatment but still have depression.
Chapter 6 Qualitative research results
In this chapter we begin by providing an overview of the emerging themes and subthemes. At this stage, we have split the themes based on the data derived from the patient- and carer-participant interviews and the therapist-participant interviews, which are brought together in Chapter 7 to offer a more comprehensive view of similar issues from a patient, carer and therapist perspective.
The major themes covered in this section focus on patient- and carer-participants’ views of the intervention and trial procedures, and therapist-participants’ views of and practical aspects of delivering the therapy, and their views on the trial procedures. Although both groups of participants were asked to comment on some of the same aspects (e.g. trial procedures), both groups also responded to questions that related to their unique experiences. For instance, patient-participants commented on their experience of receiving the intervention, whereas the therapist-participants commented on their experience of the training they received to deliver the intervention.
The findings from these themes are brought together in a brief summary at the end of this chapter.
In this chapter we attribute quotations to ‘patients’, ‘carers’ or ‘therapists’, to distinguish which participant group each individual was from. Patient and carer quotations are labelled as A, B or C to denote the study site anonymously. We had only three therapists in the trial. To protect their identity, we do not attribute quotations to specific therapists. We use the term ‘they’ so as to not gender the therapist.
Interviews were completed with 16 participants and 10 carers as well as the three study therapists. See Appendix 19 for a summary of participant characteristics.
Patients’ views of the interventions and trial
Gains and changes
General changes and gains (not therapy related)
None of the control participants reported that they experienced any changes in their lives as a result of undertaking the study. As might be expected, most patients in the control group explained that they had only completed questionnaires in the study, thus still could not find a way to deal with their problems:
Has being involved in the research study changed anything for [participant] or for yourself at a, at a greater level?
Not really I said because there’s (laughs) all you’re filling it [questionnaires] in isn’t it, nobody’s highlighted the, oh you answered this and you know, can we help you.
Carer C1/009: control group
However, some control participants found being able to talk to someone about their experiences helpful and cathartic. Moreover, they reported that answering questionnaires encouraged them to think about their feelings and experiences. One participant also found the study helpful because it provided useful information about stroke:
It give me [sic] information that I didn’t understand about strokes and that properly ‘til I came into your study and it gave me a lot more information about them that I didn’t know.
Patient A6/001: control group
Therapy-related changes (intervention group only)
Perceived changes in participants’ mood since undertaking therapy
Some intervention participants found it difficult to tell whether or not they felt any changes in their mood since undertaking the therapy, because their mood was still changing, with several ‘ups and downs’. However, some participants reported feeling better and less stressed as a result of having the therapy and engaging in activities, even though they still experienced some ‘bad days’:
Well it was quite uplifting really because [therapist] gave me some good points you know to what to do because I think I was down in the y’know sometimes I was quite nasty, not intentionally, I didn’t know I was doing it y’know but [therapist] gave me some positives, positive ideas what do to do like . . . y’know sometimes I’d be just like I am now and then the next minute me mood’d just gone to the floor so it was ideas how to get over that and it did and then we found this stroke place didn’t we which has been good as well so but the therapy has been really good.
Patient B1/087: intervention group
It’s difficult with [patient] because he changes so much with each . . . even though you’re with him 24 hours a day but he is changing so much. Probably sometimes he sinks a little bit more before he either shouts you to get up in the middle of the night or gets angry, you know.
Carer C7/017: intervention group
Some participants perceived no changes in their moods since undertaking the therapy. One participant provided conflicting accounts on whether or not they perceived any changes in their mood since undertaking the therapy:
It was good, definitely, yeah. Definitely [therapist] made me feel better . . .
Do you think you have changed since, after those sessions?
Ah well, I don’t know really. I can’t think if I have.
Patient A1/084: intervention group
In one case, the participant reported that the therapy had a positive impact on his mood while it lasted. However, when it came to an end, he had not been feeling as good as before:
I think my mood’s all over the place anyway at the minute. Yeah, I think, yeah, ‘cause it makes me feel a bit more positively and plan it has a bit more positive impact on my mood, which it had done, but I think that might be to do with the fact that course came to an end.
So you think it improved your mood but then maybe it’s not carried on or it has carried on?
It has. It’s only recently I’ve been, I’ve not been as good. I’ve been meaning to ring [psychologist], to be honest.
Patient C7/017: intervention group
Other perceived benefits of therapy
Participants described benefits in terms of reassurance, engaging in more activities, looking at the bigger picture and thinking about the future, learning skills to break up tasks into manageable bits, and having more realistic goals and expectations. The therapy was described as a trigger for planning and setting realistic goals, and also provided ideas for engaging in activities to occupy the mind and keep participants’ moods up instead of doing nothing:
I think it was, it was the thought that you weren’t being abandoned altogether, that somebody was there to care for you . . . somebody did come and make various suggestions to do things. So that was quite useful . . . I think the thing that gave her ideas of things to do, to occupy her mind, not just to sit and vegetate but to try and get things done. Which she has done, I mean she’s enjoyed the drawing, for instance, and she very much, and she’s made a good job of it . . . So it’s give her an idea of something to do.
Carer A1/084: intervention group
Moreover, some participants reported that the therapy helped them to think about their needs and reflect on their feelings, and gave them new and positive ideas about things to do. They expressed an understanding of the relationship between thoughts, mood and engaging in activities:
Um, I don’t feel as if I did anything during the trial that I wouldn’t have been doing anyway. Um, but it did help me reflect on how activities were affecting my latest mood, certainly . . . I think it was the reflective process and I think just recording some of the things down to be quite cathartic . . . I think I learned to accentuate the positive maybe. And what I realised was that it would be very very easy to get down and depressed if I concentrated more on the things I can’t do now.
Patient A6/002: intervention group
Some participants reported feeling a sense of achievement for being able to do tasks and engage in activities despite the stroke. They became more aware of their capabilities and realised that they can still perform certain tasks by setting realistic goals:
[Working voluntarily at the museum] helped me realise just what a gap it was filling in my life. It sort of grew and it made me feel useful . . . it’s these weekly sessions discussing what I was doing did help me appreciate what a difference it was starting to make to my life . . . Um, I think it gave me a sense of achievement to actually physically get out. Um, I think generally where I have a day where I haven’t gone anywhere or done anything, then I think I’m feeling a bit down by the end of the day because I feel I’ve wasted the day . . . And I think that’s something that I realised during the sort of weekly reflections, that that’s the life attitude that really fights off downs. You know, my really giving myself a pat on the back for what I have done, rather than concentrating on what I feel I can’t do.
Patient A6/002: intervention group
Some participants in the intervention group found that merely talking to the therapist during the sessions was helpful and cathartic:
I used to get very emotional about it [stroke] and I didn’t like talking about it, I used to bottle it all up. But I’ve found it helps if you do talk about it . . . It’s like a pressure release valve. It lets a bit of steam off.
Patient C7/001: intervention group
Carers also perceived some benefits of the therapy on their own health and well-being as the positive changes in participants helped carers to have a break from their caregiving responsibilities and reduced their burden. Seeing the improvements in participants’ mood also made carers feel happier:
It’s [BA] given me pleasure to think that [patient] enjoys doing things out of it.
Carer A1/084: intervention group
Activities/tasks undertaken (during and after therapy)
Participants talked about a range of tasks and activities undertaken during and after therapy that they found helpful. For example, participants talked about resuming their creative writing, drawing and painting, volunteering in a museum, building kit cars and walking, among other things:
[Therapist] suggested projects I might do which some of them I’ve not got round to changing the box yet and sort of things to do I’ve been doing these paint books you know when I’m sat sort of on me own y’know . . . I’ve done quite a few erm, yeah they help us concentrate.
Patient B1/087: intervention group
Therapy-specific experiences (intervention group only)
Understanding the purpose of therapy
Participants had a broad recognition of what the intervention was for and what it could achieve. There were some initial concerns about what benefit the therapy might offer:
I didn’t know what really to expect. It’s that not knowing bit, isn’t it? But when something like this [stroke] happens to you, everything’s a big question mark, every day of your life is a question mark. Whether I can get up in a morning, be motivated to do things.
Patient C7/017: intervention group
In most cases, however, there was a generally positive assessment of the therapy:
Different tasks, really trying to get me back into realising that you can do things . . . this encourages you to break things down into little bits, rather than the whole thing, which is a better way of doing it.
Patient A1/034: intervention group
Participants felt that therapy was about making them more active to improve their mood by reflecting on self and activities done, and by making them realise that they needed to break tasks into manageable bits in order to complete them:
It’s a big hole out of your life, that. So my days will get pretty boring pretty quickly. I suppose with that, what they were doing with me, was to prevent them things from happening, getting bored and planning things to do and making sure I went through with them. The tasks, which, I suppose, were really important. I suppose they were very good with that. That’s the exercise, I think, it did have a meaning to it. A meaning and an end, if you like. Before that I wasn’t interested in planning nowt, or doing nowt, on a daily basis.
Patient C7/017: intervention group
Reflections or perceptions of therapy
Perceived mediators of change (helpful aspects of therapy)
The identification of different and meaningful activities to perform aligned with goal-setting was frequently described and presented as an important aspect of the therapy. However, in general, participants found it difficult to articulate the mechanisms of change, and they did not explicitly distinguish the effect of BA therapy from the processes involved in it:
Well [therapist] told me how to do drawing and things like that, I hadn’t even thought of it before [therapist] came. And it’s done me good really. Trying to do some knitting and crocheting, drawing. Yeah, fine. I think it’s good the idea, because I hadn’t even thought of it.
Patient A1/084: intervention group
Planning (particularly setting goals that are realistic and manageable) was described as an important aspect of the therapy:
You try to do things and you struggle because you try to do things, you’re not cutting the picture down, you’re just trying to look at the whole thing. But this encourages you to break things down into little bits, rather than the whole thing, which is a better way of doing it . . . I’ve learned how to get round to doing it with the problem, which is what it’s teaching you. There’s always two ways to get somewhere, which I think you’ve got to remember.
Patient A1/034: intervention group
Completing the diary was also found to be one of the most helpful aspects of the therapy because it allowed the participants to reflect on progress, activities and mood on a regular basis. It also functioned as a reminder for some participants to do the tasks and achieve their goals:
I made a note of what I did um every day and how I was feeling and we [with therapist] talked through it. Um, and I did find it useful, interesting, and it sort of um prompted me to reflect on my progress and my activities . . . I came to look forward to the sessions because it was a chance to sit and reflect and um, and it gave um, it became an activity in itself um, which helped me to focus what I was doing over the week. Whereas perhaps if I hadn’t been taking part in this, I would have been taking these activities for granted . . .
Patient A6/002: intervention group
The mere process of having to talk to someone during the sessions about their feelings and experiences was found to be helpful by many participants:
The one thing it does is give you, you know that someone’s gonna come round, someone you can have a friendly chat with as well, which makes a difference.
Patient A1/034: intervention group
The positive characteristics of the therapist (e.g. being ‘friendly’, ‘positive’, trained and encouraging) and having a good relationship with the patient were alluded to on a number of occasions, suggesting that the therapist’s manner might be a mediator for the success or acceptability of the therapy:
In the beginning it needs somebody trained to get your mind-set to change and ask the right questions and get people to think differently.
Carer C7/017: intervention group
There was also a suggestion that continuity (i.e. having the same therapist attend weekly) was important:
Not a stranger because obviously you do get to feel comfortable with the person, with [therapist] and, you know and you kind of, kind of look forward to it.
Carer B1/087: intervention group
Challenging aspects of therapy
Some participants mentioned that the therapy was useful only while it lasted, so they felt the need for follow-up sessions to maintain the benefits. Participants highlighted some challenging aspects of the therapy, even though they found the therapy helpful as a whole:
I’m not 100% certain, I think I did benefit from [therapist’s] weekly visits and going through goal-setting and things. I suppose that, since [therapist] stopped coming, that’s slipped a bit. And I would things, sort of think about doing things. I supposed, [therapist] turning up was more of a reminder for me what I’m doing.
Patient C7/017: intervention group
However, participants understood that the sessions were part of a research project and that it had to end at some point:
Well, we always realised there was a timescale on it. So, I mean, we were prepared that we knew it wouldn’t last forever . . .
Carer A1/084: intervention group
Some participants highlighted that not every stroke patient is affected in the same way and suggested tailoring the therapy based on individual needs:
It’s difficult because just for myself personally, I think it’s been great but I would think it depends on the type of person you’re going to see and how it has affected them y’know whether they would think ‘oh yeah this is fine’ or ‘I can’t be bothered’ y’know that sort of thing but I accepted that everything that was put to me was positive and I did my best to do it.
Patient B1/087: intervention group
A few participants reported having some trouble with filling out the diary. One participant suggested some improvements in the mood diary by breaking it into morning/afternoon/evening, as their mood tended to vary during the day:
His projects that he was supposed to do, I think he found that difficult because [therapist] would give him a weekly chart like a diary to fill in what he’s been doing in the day and I think he just found that; even when he were at work he found it difficult to fill in paperwork so it’s nothing new.
Carer C7/017: intervention group
Two participants found the sessions and the tasks repetitive and boring:
The only thing that I found, you know doing all that work with [therapist], a lot of it repeated itself a lot. It was quite repetitive at times.
Patient C7/017: intervention group
Some of it I thought were [sic] a load of rubbish. On the whole, it was a neutral. Some of it was good, some of it I found boring, some of it I found helpful and it helped me.
Patient C7/001: intervention group
Format of therapy
Number, frequency and duration of sessions
Most participants thought that the number and duration of sessions were ideal. However, some participants suggested having follow-up sessions to ‘keep the pot boiling’ and maintain the positive changes gained in therapy:
Well, it might be worth sometimes to think about some sort of follow-up on a, on a sort of annual basis, sort of thing? See how things were going . . . it may be useful to keep in touch with things, I don’t know. You know, to see whether things are still working or not.
Carer A084 A1/084: intervention group
Role of carer in sessions
The carers’ involvement with the sessions was varied. Although some carers reported assisting the therapist, others stated that they did not get involved with the therapy and supported the patient (if needed) only to do planning. Some carers reported joining the sessions (despite not taking part in the sessions), whereas some stated that they had never been to the sessions. However, carers felt happy with the level of their involvement:
Well, I think it was just assisting whatever [therapist] wanted to do. If [therapist] wanted me to do something then I would, you know, attempt to do it. You know so it was just a sort of, like a, you know, assistant. Assisting [therapist] to do whatever they wanted to do with [patient]. Tried not to interfere more than necessary, really.
Carer A1/084: intervention group
Therapists’ views of delivering the therapy
Experiences of delivering the therapy
General views and experiences
All three therapists found delivering the therapy to be a positive experience and stated that they would recommend the intervention to other therapists and patients because of its ‘simplicity’ and usefulness. They also found implementing the therapy easy and straightforward:
I found it quite easy to implement the therapy . . . I think they [participants] found it really beneficial and they did just you know writing down just the initial activity diary and just getting them to see what they do and what they don’t do and how they could incorporate more things and I think that just really helped so yeah, I’d definitely recommend it.
However, the therapists were also mindful that the therapy would work better for people who had come to terms with having had a stroke and were ready to put in the amount of time and effort required. As for this reason, they suggested that the therapy might be more effective if provided as a supplement to other therapies for adjustment and physiotherapy:
I think it works really well for the people who are kind of at the start following their stroke where they are ready to kind of put in the amount of time and effort required with behavioural interventions . . . some people I work with just seemed to be I guess when, when somebody’s got such a low level of hope and they still kind of haven’t really come to terms with how their stroke has changed them and how its changed what they can do especially if somebody’s experienced a loss of some kind of role . . . those people that were really quite resistive to any kind of introduction of activity or identifying activity they’d just say I can’t do that I can’t do that.
For all three therapists, communicating with participants went well, as they did not have any participants with extreme difficulties (e.g. severe aphasia or cognitive difficulties). There were only a few participants with such difficulties but they were all able to understand, follow the conversation and perform the tasks. One therapist found the communication strategies taught as part of the training to be useful, as most participants had some degree of impairment in communication owing to stroke, even though it was not severe.
Challenges
The therapists also discussed the challenges they encountered while delivering the therapy. Dealing with the individual differences was reported to be one of the most challenging parts of delivering the therapy. Therapists did not rigidly follow the session-by-session plan as not everyone worked at the same speed, and the physical and cognitive capabilities and personalities of people varied. Some participants were more resistant to change or sceptical, whereas others were open to take on board new ideas:
I had a couple of people where I had to take it a little slower, needed a little bit more time or a little bit more time for their writing, again that wasn’t difficult it was just something that I had to be aware of . . . I think some people are a little bit more resistant to change depending on where they are in their journey after stroke. Some people are ready to take on board anything that you want to do others are a little bit more sceptical even though they’ve decided to try it . . . So trying to get people to understand the rationale and why we were doing it, some people I had to repeat that for a lot more and really y’know try and keep them on that frame of mind.
In order to deal with the individual differences, therapists tried to be responsive to individual preferences and tailored the session structure based on individuals’ needs:
I just tried to be responsive to the individuals as I kind of do in my day-to-day therapy and I think again as I mentioned, going back to the rationale of the therapy and the protocol to try and remind them what we were trying to do and why . . . just thinking around the individual needs and exactly sort of what people were frame of mind that they were in at the time.
Two of the therapists also found the process of identifying meaningful and achievable activities difficult in some cases owing to the participants’ levels of disability and unrealistic expectations:
I‘d say the hardest things was just working round practicalities of what people could and couldn’t do now, I think people tended to still get very focussed in their initial goals of ‘I just want to be as I was in the past’ and so the hardest thing was trying to get people to look at perhaps having to adapt that and having a different goal slightly or working with that.
Keeping people engaged with the therapy-related tasks was also challenging. One therapist suggested that this was due to people not being able to come to terms with the stroke and being ready to change. They also argued that, if they were in a clinical situation, they would withdraw the intervention for such people (after a collaborative decision). However, as this was a research study they felt the need to keep pushing to engage people:
Quite a few people that I worked with it seemed like they weren’t quite ready to be increasing their activity and what they needed more at this stage in their recovery was the talking therapy and work on acceptance with kind of the stroke and the changes that have happened in their life so there was some difficulties there in terms of people’s physical capabilities and also engagement with the intervention because they kind of felt like there wasn’t anything that they could do . . . quite a few of the people I worked with were telling me that they didn’t really want to plan things out they didn’t want to plan activity and plan out their lives . . . and I think in a real life clinical situation there would have been a couple of points where I think I would have had quite a frank conversation with somebody about their level of engagement with the intervention and where we’d to go from here and if they really want to carry on.
Improvements needed/suggestions
In order to improve the therapy, two therapists suggested having a standardised interactive therapy notebook or a folder that might be easier for participants to use and might also facilitate their engagement with the tasks, as noted in the following quotation from one of the therapists:
I tended to recommend to people or suggest they consider having like I say a bit of a therapy book because we would obviously have sheets that I could copy for people and things like that and that can become a bit disparate and people end up with piles of paper all over. So one thing I’d say perhaps just to consider, and a couple of people did, was to have a therapy book, erm a notebook that they can make any questions for the session, notes at the end and set the goals in it and kind of almost do that together and then they can sort of slip their diary in it. And one thing for therapy in the future if that wanted to be quite standardised across therapists is that could always be something that came with the therapy so perhaps a bit of a workbook for the clients that BEADS produce so that all the therapists were doing that in the same way. So even if it was just a notebook with like a couple of sections or something like that or even just a folder that sheets could go in or just to standardise it if that’s something to keep quite the same across a large number of people.
Some therapists also argued that delivering the therapy 3 months post stroke was too soon as patients were still in the process of adjusting to stroke, and behavioural change was not a priority for these participants. Therefore, they suggested that delivering the therapy 9 or 12 months post stroke might be more beneficial:
I thought that maybe having the therapy 3 months post stroke was a bit too soon, just because people are still adjusting to what’s happened and having to adapt their homes . . . I felt like it wasn’t so much of a priority for them to do the BA. They were always wanting to walk or improve their speech.
Experiences of participants from therapists’ perspectives
Observed changes in participants
Therapists observed that some participants made considerable progress in terms of increased confidence, engagement in activities and making plans. The level of progress in mood and activity levels varied among participants. However, even in those participants who progressed less, there was an increase in activity levels:
There was definitely a couple of people that I worked with that were hugely different in what they were describing, so anecdotal things like confidence increased, going out more, wanting to speak to other people more.
The people who really engaged with the therapy and kind of did go through this gradual process of introducing a bit more activity, experiencing lift in their moods, a bit more energy, a bit more motivation and then it kind of snowballed from there and people being more motivated to engage the following week because the work they’d carried out had shown benefits for them.
They also observed some secondary benefits of the therapy for participants. Having someone to talk to during the therapy was thought to reduce participants’ feelings of isolation:
I think having someone who was dedicated to talking to them about how their life had changed and what they could do to help them and just that acknowledgement was a huge part of it I know that’s kind of the secondary benefits of any therapy, it’s not the core of BA to be listening to people but that was huge, and most people commented that the stage that they were at once they’d had all their discharge support sort of 6 months down the line from the stroke it was very isolating, so they found that very helpful.
Therapists also observed that those who engaged less with the therapy benefited less than those who were fully engaged:
. . . then there’d seemed to be some people who don’t carry out the work or they do and don’t get, they don’t experience as much of a lift in their mood as they were hoping for or they didn’t quite get from it what they wanted then engagement would drop off and then things kind of snowball in the other direction if you like.
Observed helpful and unhelpful aspects of therapy to participants
Therapists’ observations of the helpful aspects of the therapy for participants echoed the accounts of participants and carers. That is, therapists observed that identifying new and meaningful ideas, undertaking reflective processes during the sessions, having weekly sessions and enabling the participant to talk to the therapist were the most helpful aspects of the therapy:
More than anything it seemed to be just having the chance to talk things through with someone and some, mainly a lot of things that came out, just talking and the ideas that it sort of lead them to think of. So things to do, ways round little problems, getting out the house so all the things that we would do for the BA activities, people said they’d found that really helpful to do that.
Therapists also emphasised that completing the activity diary helped participants to become aware of the link between being active and their mood:
I think looking definitely visualising the mood rating and their activity levels really helped mine and showing them the progress that they were making and how their mood was being marked good when they were doing certain activities over other activities so I think visualising that and getting them to see it on paper rather than just telling them about it actually really helps.
Undertaking the therapy at home and the flexibility of the therapy schedule were also considered helpful:
People really thought it was good that the therapy was available in their own homes, people seemed to respond really well to that especially when I’d go out to and see them and talk about the study and explain that the work’s carried out in their own homes, people spoke positively about that and a couple of people had even kind of before I’d even got to that point indicated they wouldn’t be able to come out and see me so their preference was to be seen at home.
Practical aspects of delivering the therapy from therapists’ perspectives
Support provided to therapists
Views on the manual
All therapists found the manual to be a helpful resource when delivering the therapy as it provided guidance during the intervention, broke down the material and gave examples:
The manual was really helpful, some really good hints in it like at the beginning where it gives you a few more hints about using it with people with difficulties in aphasia again just to remind you, and broke it down into all the different sessions . . . I think all the sheets at the back were quite helpful so having a choice of different appendix and things were helpful yeah.
Although two therapists described the structure of the manual as flexible, one therapist found it somewhat rigid and suggested amending the manual to have a little less structure in how the modules are introduced to increase flexibility, as the second quotation indicates:
So it’s a very good structure and it was always told to me that it was quite flexible so I never felt that I was doing that wrong and obviously discussed it in supervision as well but yeah just for myself and the clients that I had, we didn’t stick very rigidly to that session by session structure . . .
I did find it tricky at times when you kind of reach a road block in one aspect of the therapy like I was saying before if someone doesn’t want to be kind of formally activity scheduling with some kind of planner and you’ve tried everything to come up with a way to modify the strategy in a way that’s acceptable to them but still not getting anywhere and then trying to bring in other strategies like graded tasks or problem-solving it, the manual in that sense felt a little bit rigid it was kind of I think problem-solving got introduced in session six or seven of the manual and graded tasks around the same time but in my head I kind of imagined it being a bit more like a timeline between activity monitoring, identifying enjoyable activity and activity planning so those are kind of the three stages and graded tasks and problem-solving are kind of additional strategies that slot in throughout.
One therapist found the manual ‘a bit dense’, but understood that it needed to be comprehensive to cover people with different needs. They suggested providing a brief overview of each session in the manual to help therapists as a quick reference:
I did find the manual a bit, a bit dense to use as kind of a brief reference but I understand it needs to be as comprehensive as possible and it was useful to have the amount of information that was there but one thing I found really useful was [pause] I flicked through the manual and essentially took out all the headings for each section within each session and made summary cards for each session which fit onto a piece of paper about that big a couple of inches long, which I could use just as a reference . . . As an additional resource I think something like that would have been useful . . .
Views on the training received
The training received was described by all the therapists as useful in providing a good foundation to BA therapy. They found it ‘comprehensive’, supportive and responsive as they were able to ask for help and get answers to their questions:
I’ve found it to be very supportive in all the training and everything that I’ve had . . . the training helped just kind of make sure I was doing everything in line with the protocol . . . we had plenty of time to go through different modules and ask questions and sort of get an idea of how the BEADS wanted us to use the therapy, but without it being . . . and it was nice to meet the other therapists at that stage, doing the training and talk to people, so I think I found that really helpful and a good base definitely.
One therapist also commented that the amount of training provided was just about right and that they had the opportunity to ask for extra support if needed:
I felt very able to ask for help and extra sort of support if I’ve needed it . . . it didn’t feel like it was too much, overkill or anything like that it was helpful.
The speech and language training was found to be particularly useful as it provided therapists with some useful tips on communication with people with aphasia:
[The] Speech and Language Therapist . . . gave us training on communicating with people with aphasia and gave us tools and some resources, that was that was excellent as well that was really useful as I said I didn’t really work with anyone with very severe communication difficulties but just the strategies in general were useful with almost everyone I’ve worked with.
Views on the supervision received
The overall supervision received (including supervision from the BEADS team, direct supervision in service and peer supervision) was also described as useful and supportive. Peer supervision was found to be particularly helpful as it reduced therapists’ feelings of isolation and provided them with an opportunity to share their experiences and tips on how to overcome certain issues they encountered:
The supervision was good I think with us being quite all over the country the supervision that we had with the team, the BEADS team worked well over the phone so it was really, really good to have that sort of peer supervision here we had conference calls because it was, you’re quite isolated in doing this role for what you’re doing for the BEADS so it’s very nice to kind of hear and talk to the others and sort of share experiences and sort of tips and hints of things they’ve overcome so definitely that was a really good way of doing it . . . I always had the direct supervision within my service from the sort the PI as well which was fine . . . I felt very supported.
Views on the monitoring of practice
Therapists did not feel like they were being monitored, and felt that there was a good balance between people keeping an eye on their practice and not making them feel pressured:
It always felt there was a good balance between erm I would have the support where people were keeping an eye on what I was doing so I couldn’t go too wrong but there wasn’t sort of hovering over the shoulder and sort of didn’t feel too pressured and I could ask if I needed to so, that was good.
Therapists also discussed the videotaped sessions and whether they felt that these sessions were different from unrecorded sessions. All three therapists felt that the videotaped sessions were the same as the unrecorded sessions; however, they also admitted being self-conscious at first, but this disappeared after 5–10 minutes. They also reported that they did not observe any differences in participants during the videotaped sessions:
A little bit self-conscious with kind of the videos . . . it wasn’t a huge, huge problem at all and once the session got started you kind of forgot it [camera] was there. So it didn’t bother me . . . I was keeping an eye out for them [participants] maybe not being too different with the camera on from how I’d seen them in other sessions. So I think the biggest thing was yeah, just trying to make sure they were OK with that but everyone seemed fine . . .
It was weird having to video yourself but no it was good, most participants were happy to be videoed, especially when they knew it was just for training purposes and wouldn’t be broadcasted on the news or something.
Experience of working on trial within therapists’ departments
Therapists had varying experiences of working on the trial within their departments. One therapist was autonomous, working completely separately from their clinical team. Apart from receiving supervision and updating the team, they mostly worked independently from home:
I think for me it was completely separate. So I had my supervision from a clinical lead who was the PI for our site, my team do something very different so apart from I kind of let people know what I was doing and sort of gave a bit of information, but generally it was just completely autonomous . . .
One therapist felt integrated within a larger clinical team within the department:
My team . . . were brilliant as well, so yeah I really enjoyed it. I had a lot of help from [names person] . . . everyone was really supportive and always replied to the e-mails and yeah I got a lot of support so it was really good . . . there was always someone there to help and give you any supervision if you needed it and . . . so there was always a wealth of knowledge to ask if ever I needed it so yeah, I couldn’t have asked for better team to be, to be part of.
In contrast, one therapist did not feel integrated within the clinical service and expressed that they were mostly working in isolation:
I was kind of working in isolation a little bit which isn’t ideal . . . so I didn’t really feel integrated into the service in anyway and the way that referrals came to me from the service it seemed I wasn’t really involved in the triage process or really that involved with the other professionals in that team.
Integrating the trial practice into wider service
Barriers
Barriers to integrating the trial practice into wider services included therapists being employed in different hospital trusts and the difficulty of having to keep reminding people in community teams about the trial:
I think because I had the two different [names settings and place], I think it was difficult to keep reminding people of the BEADS trial and that it was running.
A couple of issues in terms of software I guess and the systems that we use for note takings and reports and that was to do with that fact it’s two separate trusts again kind of working across both.
Facilitators
Therapists found sharing information, support from psychological services and regular supervision helpful, and thought that this might facilitate the integration of the trial practice into wider services:
Obviously having the clinical psychologist who was fully supportive and on board . . . so having someone that was, that knew all the different people and could email them and, and tell them about the study was really useful for me so that was good.
Regular supervision was really important and helpful . . . I think with a second larger study just keeping that in mind, the idea of making the therapist feel part of the service by kind of physically locating them in the same place as the service would be, quite an important thing.
Participants’ views on the trial procedures
Rationale of the study
Participants and carers recognised the importance of the study and there was a sense of them having an understanding of its rationale:
I think it’s useful information and the only way you can get it is by talking to people like me, that’s guide through it [sic].
Patient A6/001: control group
You can’t move forward in these situations without these projects and things.
Carer B1/087: intervention group
However, there were a few concerns about what benefit the study and filling out different questionnaires might offer participants on a personal level:
Well, since this [stroke] has happened I’ve had all sorts chucked at me. Different types of questionnaires. Varying different types of questionnaires. I thought it was good, it’s got its good points. Erm, but ultimately what do I achieve from it?
Patient C7/017: intervention group
Motivation and reason to participate
Altruism was one of the most commonly mentioned reasons for participating. Some participants joined the study as they felt grateful to have been invited. Other participants explained that they thought it was better than doing nothing and that there was nothing to lose:
I went into this open-minded and I thought, I’ve got nothing to lose, I’m only sat in the house, I’m not doing anything. If nothing else, it’s somebody to talk to.
Patient C7/001: intervention group
We said yes, you know it’s the least we can do in return for all the care and attention that [patient] had.
Carer C1/047: control group
However, it was evident that some people had specific expectations of the BEADS study. Some wanted to find out about their problem and what could be done to improve their mood. Others chose to participate to see if the treatment would work, and some saw the study as an opportunity to talk to others about their problems:
But err so I’m interested in you know trying to sort out well for ourselves trying to find out what is it frustration or is it depression that [patient]’s got. Is there anything that we can do about it? . . . But yes I am I am in a way desperate or I am keen to try and find a way that we can bring [patient] a little bit more back to what she was prior to certainly the last two strokes, if not all three strokes.
Carer C1/047: control group
Well I thought, well I’ll see what I can do and try and, try and help like, you know. And see if it help me to get me head straight a bit.
Patient A4/002: control group
Some participants reported having no expectations regarding the study when they first got involved:
I don’t think we had a thought about whether it was going to help or not. And I, and I don’t think actually, one way or the other.
Carer A1/124: control group
I didn’t know what to expect, I was just – I’ll do it, I’ll do it, I’m not doing anything else. So yeah, I thought yeah, I’ll give it a go.
Patient A6/001: control group
For others, it was the hospital or the health professionals who suggested the trial to the patient:
It was from [staff name] at [hospital name], and she came out as sort of a stroke counsellor to try and help me adjust with you know the stroke. Because it’s difficult. It’s tough when you’ve had a stroke you know. And she came out and it were [sic] her that suggested it.
Patient A4/002: control group
Understanding the research process
General
From the feedback interviews, we established that, overall, participants and carers thought that the information provided was clear and easy to follow, and that the study itself was a positive experience in general:
Well as I say the interviews aren’t unpleasant in any way.
Patient C4/005: control group
However, some participants had difficulty with remembering details about the study owing to stroke-related memory problems:
I can’t remember anything about the study because, apart from everything else, the stroke’s affecting my memory and I can’t keep them in my mind long, they just disappear after a short while.
Patient A6/001: control group
The follow-ups were highlighted as useful as they provided participants with an opportunity to reflect on the intervention and the trial:
Been very happy, you know I like the fact that you don’t just leave people, you do follow it up. You give it a break before you actually come out again, and so it gives you time to settle down and review, and all that sort of thing.
Carer B1/087: intervention group
Some participants and carers expressed that they would like to know about the findings and receive feedback about the study:
I know the study’s finished and what’s the final outcome when the final study’s done, all its conclusions? What’s that? What happens then?
Patient C7/017: intervention group
For control group participants, the research process was straightforward, involving only the completion of questionnaires. Most control participants (both participants and carers) reported not feeling involved with the study and there was a sense of disappointment as a result:
It’s [study] been quite straightforward as far as I’m concerned. No problems whatsoever . . . I’ve had to do nothing, only attend two visits from people asking me questions. There’s nothing much to say about it really.
Patient A1/124: control group
Recruitment
When prompted to recall the recruitment process, some participants reported that they could not remember the finer details of the process. This could be attributed to (1) stroke-related memory problems, (2) feeling overwhelmed as a result of stroke-related issues happening at the time (e.g. many health professionals getting in touch, having had the stroke recently) and (3) their involvement having been a long time ago:
How did you come to be involved in the study?
I can’t remember haha! It’s that long ago, I can’t, I think we got a phone call or a letter or something. Or even erm I honestly can’t remember . . . I can’t you see this is where the stroke has affected I can’t . . .
Patient C1/047: control group
In general, participants did not mind being asked to participate in the study and some found the process of getting involved in the study easy:
So how did it feel, being asked to do the study?
Ooh I didn’t mind at all. Did I?
Patient A1/084: intervention group
One carer argued that some participants might be discouraged by the use of mental health terms and by the perceived intrusive nature of the study. However, she was happy to get involved and encouraged the patient to take part:
I can see why people wouldn’t be, because people are often a bit put off by the words, you know that are used, the sort of mental health side of it and all that sort of thing. But it was clearly very appropriate in this situation . . . I guess people might think it’s a bit intrusive or you know, quite a bit, not personal stuff I don’t remember there being a lot of personal stuff, but it’s, for the actual patient or you know stroke victim if you like, you know you’re asking how much they can do certain things and all of this. And it’s another stranger that’s coming into the home and all this sort of thing . . . But I mean I’m quite, just for me, I’m quite an open person and I will take any help of any sort given the situation.
Carer B1/087: intervention group
Acceptability of randomisation protocol
In general, intervention group participants felt positive about being allocated to get the therapy and some thought of this as a ‘privilege’:
Well I think it was, we thought it was a privilege to have the chance to do it rather than be sort of abandoned.
Carer A1/084: intervention group
Feelings of disappointment were evident in some control group participants and carers for not having the intervention, as they understood from recruitment processes that they would be receiving treatment:
I was a little bit disappointed, to be fair, but I was happy to go along with it and see what would happen, but I was a little bit disappointed. I wanted to be more involved.
Patient B6/001: control group
However, there was also a sense of understanding about why there was the need to have a control group and a sense of acceptance among participants:
Yeah, I don’t mind that [being in control] because I think it’s useful information and the only way you can get it is by talking to people like me, that’s going through it.
Patient A6/001: control group
Indeed, some intervention group participants would still have taken part even if randomised to the control group:
But yeah I was fine about it really, either way we would have taken part in it I think wouldn’t we?
Yeah, yeah.
Because it’s all, it’s all useful and how can you get, you can’t move forward in these situations without these projects and things. So you can’t lose by it really, we would have gone either way to be honest.
Carer B1/087: intervention group
However, some uncertainty about the randomisation process was evident; some control participants thought that they were not taking part in the therapy sessions because ‘they were already doing activities’ or that they did not ‘qualify’ to get the treatment:
So your research assistant would have called you to let you know that you were part of the control group, so you weren’t going to be taking part in these therapy sessions.
No. Because I already was doing bits myself, you know. So I was like doing a lot of what they were going to do, probably, you know.
So is that why, is that the reason you think that perhaps you weren’t chosen?
I don’t know because I have me books you see, and I sit colouring all day, well most of day!
Patient A4/002: control group
Yeah I didn’t get the treatment, I didn’t qualify for the treatment.
Patient C7/013: control group
There was also a perception among some intervention group participants that the questions they answered were used to ‘qualify’ them to receive the therapy. This demonstrates misunderstanding and perhaps a lack of clarity in how the trial information has been explained to participants:
Um, I was asked, if I remember right, I was asked certain questions which said whether I, to which one [group] I would be going to.
Patient A1/034: intervention group
Participants’ views of the outcome measures
Focus of measures
Overall, participants felt that the focus of the measures was good, ‘pertinent’, ‘pretty thorough’ and ‘interesting’, and they felt that we were asking relevant questions of both the participants and the carers.
Participants and carers were asked to rate the questionnaires on a scale of 1–10 in terms of their content and focus (1 = for not capturing important aspects of their experiences and 10 = fully capturing important aspects of their experiences). On average, participant and carers gave 7.15 (SD = 1.85), indicating that the questionnaires captured important aspects that were relevant to their experiences.
Some participants thought that some of the questions were not relevant to their own personal situation, but they could see the relevance of such questions for other people who experienced different problems, and understood that the questions needed to be broad:
. . . so some might not have been quite so relevant for us, but overall you’re covering it all for people with stroke. So yeah I don’t have a problem with any of it, some more relevant than others, but I wouldn’t say you should leave anything out because you’ve got to cover it for everybody.
Carer B1/087: intervention group
Some participants mentioned that how they answer the questions might depend on how they feel and cope on a daily basis and that their answers might be different if they completed the questions at another time:
Sometimes the ones you know, did he feel weak and stuff, not at all this week . . . they do, are different at the beginning to what they are as you get further on, it depends how they cope with it.
Carer C1/009: control group
Participants felt that the questionnaires were useful as they gave them and the researchers some indication as to how their mental health was affected by their condition and the intervention itself:
Well yes, yeah. I mean obviously this [questionnaire] gave me some indication as what my mental state was at the time.
Patient B1/087: intervention group
One participant thought that there were some missing questions regarding fear and confidence that might be relevant to people affected by stroke:
I know one question which was not on there and I know sometimes I do think it, people who have had a stroke and got off light, or anybody who’s had a stroke, they are frightened they’re going to have another one and that’s at the back of their mind . . . because the confidence goes and in the back of the mind, I might be driving somewhere, have a heart attack because I’ve got AF or could have another stroke.
Carer C1/009: control group
Ease of understanding/completion
General comments
Most participants and carers had positive views of the questionnaires in terms of ease of completion and understanding. A number of people found the questions fairly straightforward to complete:
I think he was fine answering the questions. And he found that a lot of the, the questions that were being asked, or was, well they were asking him when he was in the hospital, I think, we’ve had different people come, different nurses and things come in. So a lot of the questions were the same as that.
Carer A1/124: control group
Some participants felt that space was needed to allow them to express their responses in more detail and they suggested providing more space or asking open-ended questions. In contrast, others mentioned that they preferred ticking boxes, as this was easier:
It was pretty easy because you’d got the options rather than having to think about what you wanted to say. About how easy things were, how difficult things were. You got a, you got an option for each one so, yeah, I would say it was pretty easy.
Patient B6/001: control group
Few participants found completing the questionnaires difficult. One participant found the content of the questionnaires difficult to grasp because of stroke-related problems, whereas another participant found it hard to concentrate when questionnaires were lengthy:
Shattered because I had to uh concentrate. And the problem being is, the questionnaires are that long winded that when your brain’s been affected, you have trouble concentrating, so they could simplify it just a tad.
Patient A1/034: intervention group
For some participants and carers, it was hard to explain their thoughts and feelings, particularly in a continually changing context. However, they also suggested that the questionnaires provided them with a specified amount of time:
You know, even though circumstances can change you can only answer with, you know it’s like, one of them was about me, how was I getting on that particular week. Now that particular week I wasn’t having a good week. If you’d have asked me a week before that or a fortnight it might have been oh I’m fine, so they are pretty good.
But you, you like that it gave you that defining amount of time?
Yeah. Even as though as I said circumstances can change.
Carer C1/009: control group
Did you find that [completing questionnaires] easy or quite hard?
Hard. Hard to explain your thoughts and feelings.
Carer A6/001: control group
Some participants explained that they needed help with filling in the forms in terms of holding a pen, concentrating or remembering. One patient stated that she would not complete the questionnaires alone as she would not have the patience and the ability to concentrate. Thus, participants suggested that, if they complete the questionnaires together with someone (i.e. by having a conversation), it would boost the confidence of the patient and completing the questionnaires would be easier.
The problem with form filling for [patient] she hasn’t got the almost the patience to do it. So if you give her something like that and then she’s absolutely bored by the time she’s got to the bottom of the first page.
Hmm, how did you get around that?
Talked about it, I asked the question . . .
How do you think [patient] would have got on if you hadn’t have been there?
She wouldn’t have done it . . .
I’ve always been frightened of forms, filling a form in, in case, in case I do it wrong. In any case so just looking at it I would have thought I can’t cope with this, and I would have just ignored it, I really wouldn’t know.
Carer and patient C1/047: control group
Specific outcome measures
In general, participants reported positive views on each specific outcome measure. However, some participants and carers found it difficult to answer questions about feelings, as how a participant feels fluctuates all the time depending on the shifting stroke-affected context:
I found it OK. The questionnaire that we had that you know that I filled in for myself at the end, was OK. Some of the questions were a bit erm, I couldn’t say how he was feeling. You know, you have to expect that, you want to know how he was feeling on that day. Well, you know, as far as I’m concerned some days are good days, some days are bad days, so you can’t really make a judgement on each day, because you don’t know what each, you don’t know what each day is going to pan out like.
Carer A1/124: control group
Participants expressed some issues with the mood questions. For instance, they felt that asking about suicidal tendencies was ‘strange’, ‘not appropriate’ and ‘upsetting’, and one participant felt that these questions were ‘not valid’ as people might not answer them truthfully owing to the stigma associated with depression and suicidal thoughts. However, there was also a sense of understanding of the purpose of these questions and that they might be relevant to those who were very depressed:
I found a bit strange I don’t know some people might not, but. It says erm well the question, do you ever think that, do you ever wish you were dead? Do you think you’d be better off like, you know. Well that, to me that’s a question, a strange question. ‘Cause I mean I don’t think anything like that, it’s probably ruddy morbid though . . . I certainly don’t think it’s a relevant question to me . . . I can’t understand the purpose of a question like that quite honestly. I mean whether it erm, whether the answer relates to a person, to an individual state of mind or not I don’t know, it may do. Which I suppose it does in a sense. Because you know people might get all sorts of strange things when they, through frustration and that. Which erm, which is I do feel a lot sometimes.
Patient A1/124: control group
Several participants found the aphasia-friendly questions with pictures and smiley faces helpful as the images made the questions more relatable and they enabled participants to better express their feelings:
I feel they sort of, you could put 0–3 but, yeah. They made it, sort of, I felt that YOU could see exactly how I was feeling . . . I feel that, again, you can see better how I actually feel with the pictures rather than if it was just like before with the questions. I feel this one you can see a lot more.
Patient B6/001: control group
In contrast, one participant was offended by the simplicity of these questions. This was also echoed by another intervention participant who declined to complete the aphasia-friendly questionnaire, as he found these ‘too simplistic’. However, participants recognised that for others more severely affected these questions were needed:
I didn’t really enjoy it. And then there was one with pictures, and she [researcher] said now can you point to what’s on these pictures. And I thought well, course I can! There was a bridge, and a ship in it, and things, and a fence, and things like that! And I thought of course I can! Then you think after, it’s probably for them [sic] people that can’t remember, you know, that’s brain’s got a bit, you see.
Patient A4/002: control group
Most participants had difficulty answering the resource use questionnaire as stroke-related memory problems made it difficult to recall answers:
Quite hard to recall, especially when it’s asking you about the minutes each time. But yeah, if you just round it up, it’s not too hard.
Patient B6/001: control group
Carers found the caregiving questions to be relevant and they appreciated being considered because they often felt forgotten:
I was actually quite pleased because in a lot of these types of situations the carers do get forgotten about, it’s one of those duty things isn’t it. But erm I was pleased to see the carers were at least considered in the equation really, it wasn’t just about the person that was ill.
Carer B1/087: intervention group
Quantity of questionnaires
Most participants and carers were happy with the number of questionnaires that they had to complete and stated that the quantity was ‘just about right’:
There wasn’t too many. It was fine, it was, yeah, I would have liked more (laughs) . . . it wasn’t too short, it wasn’t too long, it seemed to have got all the information.
Patient MB/001: control group
What you thought about the number of times and the number of questions we’ve asked you to complete, whether that’s been OK or whether there’s been too many things?
That’s fine, I mean there’s got to be a reason for it hasn’t there? You know, so that’s fine.
Carer C7/013: control group
Four participants thought that there were too many questions:
It took a long while yeah . . . Well, I did get fed up of answering questions [laughs] to be honest, kept going which is more or which is less, or whatever and things, and I thought not another page you know! [laughs].
Patient A4/003: control group
Some people questioned why we were being repetitive and wondered if this was a way to ‘trick’ them to respond in a certain way or to see if they are being ‘honest’ with their answers. The duplication tempted them to constantly go back and check their answers:
You notice as you filled them in as you’ve gone through them, you notice you’re answering the same question two or three times over . . . Now whether this is just a trick to sort of get you to put yes on the first page and no five pages later I don’t know, but they are a lot of questions duplicated . . . So you can go back and rub all the answers out and make them all agree! Haha! . . . There’s always temptation isn’t there.
Carer C1/047: control group
Therapists’ views on the trial procedures
Recruitment
Each therapist experienced different issues with recruitment depending on the setting in which they were based and their links to recruiting trusts:
I think it was quite difficult in that I couldn’t get hands-on and do things. So we struggled with getting packs out and recruitment to start with . . . so having permission to go in and work with the team and the research nurses to send out packs and then contact patients and follow that up would be really helpful . . . if we could have a few extra staff as it were for the first couple of months to really plough out that invites, information contact people and then even if that dropped off later because we didn’t need that support, to kind of almost do it the other way round that would have helped.
They also found that recruitment from voluntary groups was ‘unfruitful’ as the members were already functioning well or were several years post stroke:
[we] tried a number of approaches for different groups so like the voluntary groups and things like that, which was completely unfruitful. So that didn’t really work because the groups mainly held people who were either functioning quite well because they were active in the groups or they were sort of 15 years post stroke so it was a very long time ago.
One therapist found the process challenging and experienced problems at the start, as the sites had not been set up as planned. They felt stressed when targets were not met as they believed that it was their responsibility to recruit the target number of people:
We had a little bit of an issue with recruitment at the start of the study in [names place] and other sites weren’t quite set up on according to the schedule for the study and that kind of set things back a little bit . . . the impression I had was that recruitment was and the feeling that I got was that recruitment was kind of solely my burden almost it was all my responsibility and kind of it was quite stressful at time when targets weren’t, weren’t being met because of lack of referrals . . . and I think one thing that helped towards the end of that was getting some more support from the admin team that I was kind of working with the [name of] admin team which I guess just didn’t figure out was available at the start.
When the therapist was integrated into the wider stroke research team at the trust, they reported that this went well as they had access to patient data and were able to support all recruitment activity:
I had a lot of help from the stroke co-ordinator . . . she’d given me a database to work from, send out recruitment packs which really helped to kick-start recruitment for [names site] . . . being able to access that data and managing all the recruitment myself and sending out the packs and knowing where was at with everything really helped because I wasn’t having to rely on other people to do it who had their own jobs to do as well so I think that was a real benefit for [names site] because I was able to look at the patient records and send out the invitation packs so it was really good.
Two therapists argued that working part-time limited their ability to recruit more people and slowed down the process, as they did not have sufficient time to exhaust all the available recruitment routes at the same time as delivering the therapy.
Study procedures
As it was their first research experience, two therapists had initial concerns about following the protocol, but they both felt that they were able to ask questions and received support from the trial team, which they found useful:
There was a few things that I had questions on so just to check back on, yeah as I say I’m very clinical based so a lot of the paperwork it was all new to me quite how much paperwork and forms and everything there was. So I was a bit terrified of getting something wrong, but I was, I felt able to ask questions and anytime something was slightly wrong.
Two therapists found the protocol comprehensive, easy to follow and useful as a reference guide. One therapist thought that it needed some amendment to allow for flexibility and streamlining in terms of the processes of screening, baseline assessments, recruitment and consenting:
It [protocol] seemed to work really well, it was nice having that as a reference guide to refer back to, it was very thorough and so yeah I think that worked well, I can’t think of anything to change.
There were a couple of things in terms of recruitment and the recruitment routes themselves I think there’s a couple of amendments there to be made to allow for that flexibility but I think just a little bit more flexibility being inherently more in the protocol would have been useful . . . I think the process for screening, baselines and recruitment and consent could have been streamlined a little bit I think that there were some cases I found that it was a little bit disjointed.
One therapist identified that completing therapy treatment sheets and transferring them to the online system duplicated effort and was time-consuming:
So, for most of the paperwork, like assessment paperwork, you would do it with the client ‘cause you were doing there and then and then you would transpose it onto the system when you got home which was fine because you can understand that, but the therapy treatment write-up sheets just felt like a real duplicate of time because you would write up your notes with the patient, make some notes as you were going along and then you would do a paper copy and then you would do it on the computer. So it was almost like ‘cause that was quite time-consuming.
Measures
Therapists thought that all the measures were relevant and comprehensive and fit with the purpose of the study:
Well I know all the different assessments are capturing different bits of data for various things that they’re going to be looking at. I think they’re all relevant . . . I think the carer questionnaires were particularly good, I think the carers that I worked with really liked being involved in the study and that way being invited to take part as well and have their view . . . They seemed fit for purpose, there wasn’t anywhere I was thinking that it was I didn’t understand why it was being done or that I was thinking it wouldn’t be useful there was just that one where there was a doubling up of the aphasia-friendly and the standard one.
One therapist thought that there were too many questionnaires, but felt that they were not tedious and were relevant for the purpose of the study. Another therapist did not think that there were too many measures to complete and noted that, when needed, the assessment session was split:
There was a lot of them [questionnaires], but I could see why they were there. They didn’t feel too tedious, as I say I never had anyone with great difficulties sort of aphasia or cognitive, they could have been quite overwhelming if there was, it would have taken time.
I didn’t find that it was too much. I just had one lady who did get tired halfway through so we had to split the session up . . . I just thought that that was the information that was needed to collect . . . if participants were struggling I’d read the questions out for them and do and the checking and things like that.
One therapist highlighted the doubling up of the aphasia-friendly version and standard version of the EQ-5D-5L questionnaire at baseline and thought that it would be more efficient and more accurate if people completed them independently at first and got help from the therapist if needed:
There’s one that there was a standard version and an aphasia-friendly version as part of the baseline booklet which I always kind of felt that it was a bit of a confounding variable that some of those baselines were done in person with the therapist as opposed to done by the individual themselves because a lot of the time the baselines were carried out with me going through them, me reading in which case the aphasia-friendly version and standard version it kind of, it’s a bit meaningless if it was me going through them anyway.
Qualitative summary
Most participants’ experiences of being involved in the trial were positive. However, not all participants understood that they would not receive therapy for their mood problems. In addition, the understanding of reasons for being randomised to usual care was variable. These findings suggest that more information should be provided to participants about randomisation before they are consented into the study, possibly with reminders being given during the course of the trial.
The outcome measures were generally felt to be appropriate in terms of content and length. Some participants found questions on death and suicide inappropriate, but there was an understanding of their purpose and their relevance to those who were very depressed. Therefore, outcome assessors may need to introduce these questions or questionnaires with care. Some participants thought that it would be useful to include questions about fear and confidence. Some questions were thought to be duplicated across outcome measures, which is inevitable given that standardised questionnaires were used (with some overlap of questions). Images to support the understanding of questions were found to be useful by many participants, but there is a need to be mindful of participants who may find this childish and insulting. Moreover, some participants had memory issues affecting their ability to complete measures, particularly the service use questionnaire. Therapists felt that the questionnaires could have been overwhelming if participants had more significant language and cognitive difficulties.
The therapists’ views of the trial procedures were generally positive. However, they felt that the process of screening, baseline assessments, recruiting and consenting could be clearer in the protocol. They also felt that it was important for them to be integrated within the existing clinical teams, when possible, for ease of recruitment and understanding site-specific processes. Therapists also found completing therapy forms on paper and on the electronic database a duplication of work that was time-consuming, and they suggested making this process simpler.
Many patient participants reported benefits of having the BA therapy in terms of their improved use of strategies, and increased levels of activities and mood. For many, the follow-up appointment was seen to be useful therapeutically and they suggested that this could be part of the intervention itself. Some therapists felt that the timing of therapy (3 months post stroke) was too early, as people were still in the process of adjusting to stroke, and they suggested providing the therapy later (9 or 12 months post stroke). Therapists also recommended including an interactive therapy notebook to facilitate participants’ engagement with the therapy. Both the training and supervision provided for the therapists were found to be useful. However, therapists found the manual quite large and suggested providing an overview summary to refer to as needed.
Chapter 7 Discussion
Summary of findings
Feasibility was demonstrated across the majority of the selected outcomes and we identified strategies for improvements in a definitive trial. However, the main issue outstanding is whether or not a sufficient number of sites that would be capable of delivering the intervention and maintaining participant numbers over the trial could be recruited.
Feasibility trial
We randomised 49 participants to the trial (one in error) in 27 centre-months of recruitment. Recruitment was lower than the anticipated sample size of 72, although this calculation was based on 36 centre-months of recruitment. The highest number of participants was recruited through hospital databases, whereas recruiting from stroke wards was the least effective method. Nine participants (19%) dropped out of the study during the 6-month follow-up; seven of these were in the BA arm. Delivery of the intervention was good, with high attendance (90%).
Participants received a median of 9 and a mean of 8.1 BA therapy sessions (range 0–14 sessions), with sessions each lasting a mean of 57.3 minutes. Excluding the participants from the BA arm who did not receive the intervention, 75% of participants received at least eight sessions. A positive effect was found in the intervention arm for the primary clinical outcome, the PHQ-9 score. We did not test for statistically significant differences between the groups because this was a feasibility trial but we note that the 95% CI does not include zero. In terms of the secondary outcomes, the intervention had a positive effect for participants on VAMS ‘Sad’ and the NLQ and for carers on the CSI, although these results are not considered statistically significant. There was no difference between intervention and control groups on NEADL. Small negative effects were found for the patient-reported EQ-5D-5L and SADQ.
Fidelity assessment
The therapy record forms, which documented the proportion of time (in 10-minute units) spent on individual components, indicate that the components of BA described in the manual were delivered to participants. The distribution of time on the different components over the course of therapy was as expected. However, there was little use of graded tasks assignments and training in problem-solving documented. This may be because graded tasks were often used as a between-session task and so were coded as such. The video recordings of sessions were an important adjunct to the record forms as they highlighted some aspects not otherwise recorded.
Qualitative
Therapists, participants and carers observed that the most helpful aspects of therapy were identifying new and meaningful activities, reflection during the sessions, having weekly sessions and having the chance to talk with someone. Most participants felt that both the number and the duration of sessions were appropriate, although some participants suggested that follow-up sessions would help to maintain the gains made. Interestingly, some control participants also found participation in the study helpful as it provided opportunities to talk about their experiences. However, others were uncertain as to why they had been randomised to usual care.
The therapists found delivering BA a positive experience and would recommend it to other therapists because of its simplicity and usefulness. The therapists found the manual and training helpful but suggested having a summary of each session and an interactive notebook or workbook for participants. The therapists may also benefit from having a quick reference guide alongside the full treatment manual. The therapists also believed that therapy would be more appropriate for those who had come to terms with their stroke (i.e. those who had a stroke > 3 months ago). The biggest challenge identified was the variation in individual presentations, although the therapy and manual allowed sessions to be tailored for individuals’ needs. However, identifying meaningful and achievable activities was difficult for some owing to their level of disability and unrealistic expectations. The therapists reported different experiences of recruiting participants to the trial, reflecting local site differences.
Health economics
Behavioural Activation Therapy for Depression after Stroke has shown that it is feasible to collect the data to conduct a rigorous economic evaluation. Uncertainty is high and influential, such that very high values of obtaining further information are estimated, irrespective of the perspective of the analysis, but conducting a full trial would appear to represent good value for money.
Evidence of feasibility and implications for a future definitive trial
Population/recruitment
The primary objective of this multicentre feasibility trial was to assess feasibility of recruitment in the study population for a definitive trial. A total of 756 participants were screened across all sites, of whom 49 were recruited, giving a recruitment rate of 1.8 participants per centre per month. Delays in set-up meant that we had a reduced number of months of recruitment as we still closed the study on the planned date. Had recruitment been open for 36 months rather than 27 months, at our recruitment rate of 1.8 participants per centre per month, we would have achieved a sample size of 62. We have identified ways to improve recruitment in future studies. The delays in site set-up have been outlined in Chapter 3 and were due to delays in receiving approval for excess treating costs, with the knock-on effect on the appointment of study therapists in the NHS and the IAPT provider changing close to the time at which the study was due to open. This suggests that a longer set-up time should be scheduled for a definitive trial, particularly given the increased number of sites needed.
The sample size for a definitive trial was calculated with two main options, one based on a difference in PHQ-9 score of 4 points between treatment and control at 6 months and the other based on a difference in PHQ-9 score of 3 points between treatment and control at 6 months. Both options represent moderate standardised effect sizes that are appropriate for a trial of this kind. If a definitive trial were to be powered on the smaller effect size to detect a smaller effect, this would mean that, to have a total sample size of 580 participants, based on a recruitment rate of 1.5 participants per centre per month, 16 centres and 24 months of recruitment would be required. However, recruiting 16 centres capable of participating and delivering the intervention, while maintaining a steady pace of recruitment over 24 months, may be a potentially significant challenge. Given the variability in psychological support services for people with stroke, it is not yet clear if 16 centres would be achievable. Data would need to be collected to explore this aspect further.
The attrition rate, at 19%, was higher than the anticipated 15% and was highest in the BA group. The reasons given for dropping out were varied and included having insufficient time (for those in the BA group) and a deterioration of health. However, people did not have to give a reason or may not have given the real reason for drop-out. The attrition rate was higher than in studies of CBT for post-stroke depression25 and motivational interviewing after stroke. 31,35 The sample size was too small to enable subgroup analysis to explore whether or not particular characteristics were associated with drop-out. One possible factor is that in BEADS we recruited people at a wider range of time points post stroke.
Recruitment varied across the three sites. Recruitment was most successful at site 1, where the therapist was based in the hospital with the CRN research nurse, which enabled close working to facilitate recruitment. This was reflected in the qualitative interviews, in which therapists noted that it was important for them to be integrated within clinical teams to facilitate recruitment. The therapists were generally positive about the trial procedures but suggested that the process of screening, baseline assessments, recruiting and consenting could be clearer in the protocol.
From our observations in the feasibility trial, the use of IAPT as a main site for recruitment in a definitive study may not be ideal, particularly if this is separate to the trust delivering wider stroke services. This is due to the nature of IAPT services, which can be actively sought out by participants, and it was possible that people would be less likely to refer potential participants owing to the risk of an unfavourable randomisation outcome. The IAPT site had lower recruitment, possibly as a result of having fewer stroke patients available to them than the other main sites. This made them heavily reliant on patients identified from the hospital database at the companion site. This adds to the challenge of recruiting the large number of sites required for a full trial.
Recruitment varied across the different routes. The highest number of participants (42.9%) was recruited using hospital databases. Although this requires a large number of potential participants to be contacted, it may be considered an efficient approach in terms of staff time to send out the invitation packs. This approach alone may not be sufficient to sustain recruitment over the duration of a definitive trial depending on the number of patients on the database and how many years previously the details are available (e.g. if recruiting up to 5 years post stroke). The highest uptake from the number of patients screened was via community caseload (13/28 participants; 46.4%), which is possibly attributable to the personal approach by the clinician who had the opportunity to explain the study and answer questions, although this is more time-consuming than using databases. We did not recruit through GP databases, and this could be explored as a future option in order to approach people who are later post stroke (e.g. 2–5 years) to reach the large patient population. We would first need to establish the likely proportion of people on the database who have had a stroke within the past 5 years and the time and resources needed to send out invitation packs. A further suggestion for recruiting more patients who are later post stroke (e.g. after 1 year) and therefore unlikely to be in contact with stroke services would be to increase self-referral. This could be attempted through advertising the study more widely in the press and through social media.
It is possible that equipoise, or lack of individual equipoise, may have affected recruitment when we were reliant on clinicians as the gatekeepers notifying potential participants about the study. Interviewing the PIs and key clinicians from the sites would have provided insight into how they perceived equipoise and to what extent it influenced how they explained the study to participants. We did get an indication, however, that there were issues of equipoise at one site early on. At this site objections were raised about the treatment-as-usual arm not receiving any active intervention, and whether this was ethical. This was resolved with the explanation that it was unknown whether or not the intervention arm would be shown to be beneficial. However, additional guidance for clinicians may help them to explain equipoise to eligible participants. 112
Therapists reported that, as a result of their workloads, they could recruit only the number of participants to whom they could deliver the therapy without undue delay (in the event that participants were randomised to the intervention arm). At one of our sites, the therapist had to delay randomising participants and this meant that there were varied periods of delay between baseline assessments and randomisation. In nine participants this delay was > 30 days. Stratification by site was used but this did not alleviate these delays. To increase recruitment rate and/or reduce this delay, therapists would potentially need to be employed for a greater proportion of the week in order to prevent long delays between consent and intervention delivery.
All of the participants with aphasia had mild aphasia, which may indicate that people with more significant communication support needs may have been missed by the identification and recruitment methods used. It is possible that staff referring to the study simply did not consider people with significant communication difficulties. In addition, the written information sent out to potential participants (covering letter, participant information sheet, reply slip, PHQ-9, VAMS ‘Sad’) may not have provided sufficient support for people with more significant aphasia to understand the study and demonstrate an interest. For a definitive trial, the presence of these difficulties could be one of the factors that help decide whether to send a pack or whether to arrange a home visit to discuss the study.
The therapists reported not using the CST if they felt that the potential participant seemed to be communicating well with them. Yet, how well a person appears to understand can be misleading and is subject to the judgement made by the therapist. If people with more significant communication difficulties are identified for a future trial, it will be important for therapists to use a tool such as the CST routinely with all participants. This would ensure consistency and identify any communication difficulties that need to be supported. It is to be noted that the FAST, which is similar in presentation to the CST, was completed well as it was part of the CRF. However, FAST indicates only the presence and severity of aphasia, whereas the CST identifies the levels of communication ability and thus eligibility for the study and also links to support strategies. These issues could be addressed during staff training in a future study.
Generalisability
The information gained from this feasibility trial was adequate to inform the sample size needed for a definitive trial. Our inclusion criteria were broad to reflect clinical practice and address the issue of the generalisability of the results. In order to recruit a sample representative of the wider stroke population, we identified participants through a range of routes. In most participants (62.5%) the time since stroke was 3 months to 1 year, although one-quarter of participants were between 1 and 2 years post stroke. We included people who had their stroke up to 5 years previously because there is evidence that depression is still common after this time113 and to reflect our original PPI feedback.
The therapists did attend voluntary stroke and aphasia groups in an effort to identify people > 5 years post stroke but found that they were less likely to need support for low mood and were often more than 5 years post stroke. For a future study there is the potential to access hospital and community databases to include more people between 2 and 5 years post stroke.
It is an important point that people with aphasia have higher rates of depression than the rest of the stroke population and it was disappointing that only mildly aphasic participants were recruited. Of the 48 participants recruited, 18 had aphasia, which reflects the incidence rates of aphasia in this population114 (it should be noted that all of these patients were mildly aphasic). This may be a reflection of the recruitment approaches used. We have already shown in a previous study17 that BA can be delivered to a sample of participants with aphasia and can improve mood. At baseline, 78.7% of participants were scored as having mild cognitive impairment on the MoCA, which is in keeping with previous findings that up to 75% of participants have cognitive impairment after stroke. 115
The team discussed the possibility that it is not uncommon for clinical staff to ‘pre-screen’ patients and not identify those who they believe might struggle with the intervention or they feel they want to protect, which is common with patients with communication and cognitive difficulties owing to their increased vulnerability. A possible way to ensure inclusion of a wider range of aphasia severity in a future trial may be to provide training to the clinical teams about ensuring that all patients identified as eligible are given the choice about whether or not to participate in the trial, irrespective of a personal clinical viewpoint. In addition, it may be of benefit to engage more fully and regularly with the speech and language therapists within each clinical team, as they are best placed to identify people with aphasia.
Half of the people (69/138) who completed the PHQ-9 for screening prior to recruitment scored as depressed. This is higher than the prevalence of one-third reported in the literature11 and suggests that our recruitment approaches were able to identify depressed people who were willing to respond to the invitation for the study. The mean PHQ-9 score of 16.8 points (SD 4.7 points) in the recruited sample at baseline falls in the category of major depression, moderately severe. In the sample at baseline, 39.6% of participants scored as having moderate depression, 31.2% were scored as having moderately severe depression and 29.2% were scored as being severely depressed. We excluded people who were receiving medical or psychological treatment for depression when they had their stroke and those currently receiving psychological intervention. Therefore, we would not expect to have many people with severe depression.
We recognise that all but one participant was from a white British ethnic background. We would therefore need to improve the representation of the multicultural, multi-ethnic UK population in a definitive trial. Approaches to improve this representation could involve the selection of a diverse range of study sites as well as the inclusion of people from different ethnic backgrounds in our PPI group.
For the purposes of this trial, only English speakers were offered the intervention as delivering the intervention to non-English speakers is not straightforward. This would need to be addressed if the intervention was shown to be effective with English speakers initially. It is important to explore methods of broadening this intervention out to non-English speakers, for example by using therapists trained in BA who speak the same language as the patient, using translators or using family members (this would need to be explored with caution as translators often do not translate verbatim and, therefore, the content of the therapy delivered may not always be consistent with that received and so careful fidelity measurement would be required).
Intervention
The feasibility of the intervention with regard to delivery was very promising as the results demonstrated that the intervention could be delivered as intended across sites and was acceptable to participants, carers and therapists. Of the 225 intervention sessions scheduled, 202 sessions were completed (i.e. 90% of sessions scheduled were delivered). Reasons for missed sessions included changes to participants’ availability (n = 14, 61%), illness (n = 4, 17%) and change of therapists’ availability (n = 3, 13%). Overall, the therapy seemed to be acceptable to participants, carers and therapists and could be delivered by an AP or IAPT therapist under supervision from an experienced mental health practitioner. The intervention was manualised to support delivery of the intervention by the therapists, to facilitate consistency across sites and therapists and to allow future replication. The therapists did comment in the interviews that the manual was quite large and that an overview summary to refer to would be helpful. They also recommended that an interactive therapy notebook/workbook might facilitate participants’ engagement with therapy. Some participants who received BA suggested that follow-up therapy sessions would be useful to maintain gains. This would be consistent with the booster sessions offered in the study comparing BA and CBT for depression in primary care. 49
The therapy record forms indicate that the components of therapy in the manual were delivered to participants and that the distribution of time was as expected. The therapy record form may need to be modified to reflect the content of between-session tasks, as the infrequent coding of problem-solving and graded tasks on the record form may be because they were covered as between-session tasks. This is supported by the fact that the therapists and participants did refer to problem-solving and graded tasks (i.e. breaking down a bigger goal or task into smaller steps), which suggests that these approaches were used.
In the qualitative interviews, therapists felt that the timing of therapy was too early for some participants as they were still adjusting to stroke, although it is important to note that this was not commented on in participant interviews. Participants had to be a minimum of 3 months post stroke but could be up to 5 years post stroke, so we did not constrain recruitment to the early months post stroke. The sample size is not sufficiently large to allow subgroup analysis of whether time post stroke was related to differences in outcomes. With regard to whether the intervention is sufficiently adaptable for patients who were perceived to come up with unrealistic goals, we could attempt to address this in a definitive trial by including more time in the therapists’ training to provide guidance on goal-setting, using examples from BEADS. We could also seek feedback from our PPI group on how to better explain the purpose of the therapy to patients and provide examples of goals that may be appropriate.
Outcomes
Follow-up for a definite trial could be done via telephone (where appropriate), which may improve follow-up rates. We observed that some participants needed to be reminded by telephone call a few times to complete and send back outcome assessment packs to the central study team; this could be practically challenging in a larger study. Postal outcome assessments supported by telephone reminders with the option of resending questionnaires, completing questionnaires over the telephone or offering a home visit have been used previously31,35 and Parker and Dewey116 highlighted that administration time was important to maximise the quantity and quality of information obtained by postal questionnaires. Follow-up home visits could be offered to all participants; however, this would significantly increase the cost of the study, particularly considering the number of centres and participants needed for a definitive trial. Text message reminders could also be used.
Completion rates of individual questionnaires were good and there are no reasons to change the outcome measures. The outcome measures were generally felt to be appropriate in terms of content and length.
The quality-of-life measures used in the BEADS trial are worthy of further consideration. The standard EQ-5D questionnaire was included, as was a proxy version completed by carers on the behalf of participants, an accessible, unvalidated, aphasia-friendly version of the EQ-5D, and the standard version completed by carers to report their own QoL. Because nearly 98% of participants were able to complete the standard version of the EQ-5D at baseline, this measure was chosen as the basis for the economic evaluation, with neither the proxy version nor the accessible version used any further. It is relevant to note, however, that data completion was slightly worse for proxy measures, and that the agreement between proxy measures and patient-completed questionnaires was only moderate. Agreement between patient-completed standard and accessible measures appeared to be much higher, although this does not represent an appropriate validation of the tool. This reinforces the view that proxy measures should be avoided when possible, hence the recognised need for accessible preference-based HRQoL measures for participants who are unable to complete the standard EQ-5D questionnaire. 117
Carer QoL was collected marginally less successfully than patient QoL in the BEADS trial. Completion rates were 84.8% at baseline and 63.6% at the 6-month follow-up time point. QoL of carers has rarely been taken into account in economic evaluations, but excluding it may underestimate the value of an intervention owing to health spill-over effects not being incorporated, as the health and well-being of a carer can be affected by the health of the person for whom they are caring. 118 Including these spill-over effects can affect the cost-effectiveness of an intervention. 119,120 There is currently no consensus on how spill-over effects should be incorporated, but a conceptual framework has been developed to suggest how they could be. 121 The BEADS trial has shown that it is feasible to collect information on the HRQoL of carers, and this could be incorporated into future analyses to help inform decision-making on the allocation of scarce health-care resources.
Owing to the small mean difference (and 95% CI) found between the intervention and control groups at follow-up on the NEADL, this measure could be omitted from a full trial. Overall, the items on the NEADL may not be sensitive to detect the changes in the types of activities addressed in BA. In the qualitative interviews the therapists commented that some participants showed an increased confidence during the therapy. One participant also suggested that questions about fear and confidence were missing from the measures they completed. A measure of confidence after stroke has recently been published122 and may be suitable as an outcome measure in a full trial, although, as a new measure, its responsiveness to change has not yet been determined.
Adverse event reporting
Trials of non-pharmacological health interventions vary widely in their approach to recording AEs and making attributions in terms of causality. We recommend that the approach taken in any future trial is appropriate to the level of risk and feasible. Given appropriate resource, we would record AEs but an option would be to avoid labelling them as ‘adverse’, specifically to avoid the need to assess causality. For a definitive trial we would recommend that non-serious AEs are not assessed for relatedness to stroke or the intervention as these are difficult to attribute to the intervention. As hospital admission details are not easily accessible for rehabilitation trials and an AE cannot always be assessed for its relatedness to the intervention or condition, we would propose that events are not recorded as AEs but rather as safety outcomes, which are not assessed in the same way and for which no action is taken. These safety outcomes could then be reported regularly to the DMEC for monitoring. We would record and report SAEs.
Strengths and limitations
The feasibility trial
Behavioural Activation Therapy for Depression after Stroke was a multicentre RCT. Allocation concealment was ensured using internet-based randomisation hosted by Sheffield Clinical Trials Unit. The outcomes assessors were blind. It was not possible to blind participants or therapists to group allocation. The SAP was agreed prior to data lock. In addition to the feasibility RCT, we had nested qualitative research, fidelity assessment and a health economics evaluation. As outlined previously, delays in site set-up meant that the start of recruitment was delayed. However, we kept the original recruitment end date and so were able to deliver the trial, intervention and follow-up assessments in the time period originally agreed, albeit with reduced numbers. Issues with recruitment and recommendations for improvements are detailed in Chapter 3, Challenges with recruitment and data collection.
Participants were willing to be randomised, although in the interviews some control group participants seemed to misunderstand why they were randomised to usual care (e.g. they thought that they did not qualify for the intervention). This suggests that the purpose of randomisation and result of the allocation needs to be explained more clearly. We were able to recruit a diverse sample in terms of demographic and clinical characteristics.
The control arm in this trial was treatment as usual; therefore, there was no active control arm or any attempt to control for attention. We decided against including an attention control group, as there is already evidence that the benefit from BA to treat depression in other settings is a specific effect of treatment rather than a non-specific effect of extra attention. In a trial of CBT for post-stroke depression25 it was difficult to deliver an attention placebo intervention that was credible to participants, easy to facilitate and included none of the active intervention strategies. Further consideration should be given to including an attention control third arm, but the benefits of this would have to be weighed up against the difficulties associated with the associated increase in sample size that this would require.
As noted recently by Popp and Schneider,123 there is no ‘gold standard’ for attention placebo controls in trials of psychosocial interventions. Our main argument against including an attention control group is that there is already evidence for the effectiveness of BA for treating depression in other settings. In particular, in a recently updated meta-analysis of RCTs of BA for depression,124 6 of the 25 studies comparing BA to controls used treatment as usual for the control, 15 used a waitlist control, 3 used a psychological placebo intervention and 1 study had a waitlist and a placebo as controls. The meta-analysis found that BA was superior to controls. A smaller effect size was found in studies using a placebo intervention compared with a waitlist or usual care, but these effect sizes were still statistically significant. There is therefore up-to-date evidence that BA is an effective treatment for depression in the general population.
In the qualitative process evaluation from the COBRA trial (non-inferiority trial comparing CBT with BA for depression),49 although common factors, such as being listened to and feeling understood, were valued by participants, the researchers found that specific aspects associated with the CBT and BA were important in addition to these factors. In a definitive trial evaluating BA for treating post-stroke depression, it would therefore be important to use a process evaluation to focus on the mechanisms of change that may be taking place within BA. For example, in an ongoing cluster RCT of BA by mental health nurses for treating late-life depression in primary care, BA is being compared with treatment as usual rather than an attention control condition and one of the research questions is to explore the mechanisms of change that account for the effectiveness of BA compared with treatment as usual. 125
We do not have detailed information on what usual care was at each of the sites. It was intended that treatment-as-usual data would be gathered from the resource use inventory but, as this questionnaire covered all services received, whether treatment was as usual, was sought by participants or paid for, etc., it does not provide the specific usual-care information that would be valuable to collect in a definitive trial.
Regarding the disappointment expressed by some participants who were randomised to the control group, it is worth noting that the attrition rate was lower in the usual-care group than in the BA group. This suggests that allocation to the usual-care group did not deter people from remaining as participants in the study and completing the outcome questionnaires. To alleviate the disappointment for participants allocated to the usual-care group, we would recommend that the training provided to staff who are explaining the study and taking consent should include additional time spent explaining the concept of randomisation in lay terms. In addition, we would recommend that the training includes additional time spent on how to explain the randomisation outcome to participants, in particular to those who have been allocated to the usual-care group (and who will therefore not be receiving the BA therapy). The materials for this training (such as example scripts and role-play tasks) would be developed in collaboration with the PPI group.
Fidelity assessment
We assessed the fidelity of the delivery of the intervention through therapy record forms for every session and video recordings for a sample of sessions. The video analysis was limited as recordings were incomplete and did not include all sites. We had intended to select participants and sessions iteratively using purposive sampling but this was not possible because of practicalities and resources. We had only one video camera for the study; this meant that each therapist had the camera for only a short period of time and they could record available sessions only for those patients who consented to this. Technical issues such as the camera battery running low meant that some sessions were only partially recorded. Therefore, we cannot be sure that all sessions at all sites were delivered in accordance with the manual; in a future study it would be logical to review videos during the trial and ensure sufficient funding for all sites to have a video camera throughout the study. However, in addition to the training and the therapy manual, weekly clinical supervision by a local clinical psychologist and monthly teleconferences with the chief investigator and a NHS consultant clinical psychologist were provided. These meetings did not raise any concerns suggesting that the therapists were not following the manual.
We did not assess the competencies of the therapists in the study (although appropriate clinical supervision was provided). To our knowledge, a validated competency assessment for BA is not currently available. We note that Ekers et al. 62 developed a fidelity checklist for use in their trial evaluating BA (delivered by generic mental health workers) for treating depression and there may be scope for this to inform training and monitoring of therapy in a definitive trial.
Health economics
The BEADS trial has shown that it is feasible to collect the data to conduct a rigorous economic evaluation – including data to help inform an analysis taking a societal perspective, including indirect costs, and potentially including spill-over HRQoL effects. The collection of data required for the economic evaluation was successful and it would be feasible to collect similar data in a full trial. However, it is important to note that participants reported some difficulty answering the resource use questionnaire owing to issues with recall; this should be carefully considered when designing the data collection approach in a definitive trial. In addition, given the importance of carer costs, consideration should be given to collecting information on carer time over a longer time period, to provide more reliable information for a societal analysis. Resource use diaries represent an alternative approach, but also have limitations.
Our analyses suggest that there would be a very high value to obtaining further information on key parameters within the economic model – this value is likely to far exceed the cost of running a full trial. It would be most valuable to obtain more information on the NHS, PSS and societal costs associated with the health states included in the economic model. Often it is difficult to collect such information with precision even in definitive trials, but there remains substantial value to obtaining further information on other parameters such as the relapse rate, utilities and response rates, which would be best achieved through a RCT.
It was not our aim to provide definitive estimates of the cost-effectiveness of the BA treatment. However, our preliminary analysis of the cost-effectiveness of the intervention suggests that it may represent a dominant treatment strategy (i.e. cost saving and QALY gaining) from a societal perspective, but is of borderline cost-effectiveness from a NHS and PSS perspective. However, these results should be interpreted with some caution, particularly because our within-trial analysis resulted in QALY losses for the BA treatment. Owing to the higher response rate observed in the intervention group and the assumed higher utility score associated with good response, our modelled analysis provided an opposite result, but there is clearly considerable uncertainty around this.
The qualitative research
The nested qualitative research has helped our interpretation of the feasibility results. As well as interviewing participants who had received the intervention, we interviewed carers and also participants who were randomised to the control group in order to gain their perspectives. Participants were selected purposively to represent the centres, range of severity of depression, and stroke survivors with and without aphasia.
Results in the context of other studies of psychological interventions for post-stroke depression
As outlined in Chapter 1, Current service provision, there is currently limited evidence for the clinical and cost-effectiveness of psychological therapies for post-stroke depression. 38 Previous studies of psychological interventions have recruited people early after stroke and excluded people with severe communication or cognitive problems. The CALM study17 found that BA improved mood at 6-month follow-up in stroke participants with aphasia and low mood. In BEADS we have shown that BA can be delivered to a broader sample that included people with and without aphasia or cognitive impairment. A reduction in depression was found in the BA arm, although the purpose of BEADS was to evaluate feasibility and so we did not aim for it to be powered for efficacy.
Patient and public involvement
The PPI group (two stroke survivors and a carer) was particularly helpful in improving the patient information materials; this led to us developing a study summary card and a spiral-bound version of the aphasia-friendly information sheet. The therapists were invited to a PPI meeting as the group wanted to ask the therapists about their experience of delivering the therapy and working with people with depression. Both the therapists and the PPI members gave positive feedback about this meeting and we would recommend this approach in a future study. The PPI group gave feedback on the draft of the plain English summary and will be asked to feed back on drafts of the final study newsletter for participants.
As we had a separate PPI group and included people with significant aphasia in this group we did not have a PPI representative on the TSC. With hindsight, it would be beneficial in a definitive trial for a member of the PPI group also to sit on the TSC to ensure that the PPI representation is integrated into the overall monitoring of the study.
Chapter 8 Conclusions
Overall, BEADS has shown that it is feasible for BA to be delivered by an AP or IAPT therapist and that the therapy is acceptable to participants, carers and therapists. We were able to recruit participants but owing to delays in site set-up, the overall recruitment rate was lower than anticipated. We have used the recruitment rate from BEADS to estimate the number of sites and duration of recruitment that would be needed for a definitive trial. Although we have identified approaches for improving recruitment, the issue remains of whether or not 16 study sites could be identified that could deliver the service over 24 months to achieve the sample size estimate required for a definitive trial.
A scoping study is first needed to identify whether or not there are a sufficient number of sites that are willing and able to recruit participants and deliver the BA intervention for a definitive trial. In selecting appropriate sites for the study, it would be necessary for the site to have a PI in the stroke service who is able to commit time to facilitating participant recruitment across the different recruitment routes; this would be essential, given the number of sites in the study. The site would also have to have an appropriately qualified clinician who could provide the local clinical supervision to the study therapist. If a site did not already have a therapist in post to deliver the BA intervention, it would be necessary to secure extra treatment costs to fund this and also to allow sufficient time in the study set-up to appoint therapists.
Implications for health care
-
Half of the people who returned the PHQ-9 at initial screening scored as depressed, which highlights the prevalence of post-stroke depression and the importance of mood screening after stroke. It is particularly important to ensure that people with aphasia and/or cognitive impairments are included within mood screening.
-
As the trial was not powered for efficacy, it is not appropriate to draw conclusions on the efficacy of BA for treating post-stroke depression. However, we have shown that it is feasible to deliver BA to people with no or mild to moderate aphasia or cognitive impairment.
-
We found that, following training, APs and a PWP were able to deliver the BA intervention in this trial. This provides different potential models for the delivery of BA, namely through stroke services or IAPT services. As we found recruitment to be more difficult when using the IAPT service as a main site for recruitment than when using the stroke service sites, this suggests that a referral pathway would need to be established to link stroke and IAPT services at sites where such a pathway does not exist. We would need to explore further the competencies required for delivering BA to stroke patients with depression to identify whether or not the therapy could also be delivered by other clinicians following appropriate training, such as mental health nurses.
Recommendations for a Phase III trial
The first step is to identify whether or not there are sufficient sites willing and able to deliver the services needed that could sustain recruitment over the study time frame. Then, if a definitive trial were to be undertaken, based on our findings, we would recommend the following:
Essential/high priority
-
Recruit through stroke services rather than using IAPT as the main site for recruitment.
-
Provide at-site support or central monitoring of recruitment.
-
Although there were monthly teleconferences between the CI and the therapists, and regular newsletters to update the sites of key changes and phases of the study, for a definitive trial, regular teleconferences could be arranged for the site staff and PIs so that they feel more engaged. This may improve recruitment at sites.
-
Send out regular newsletters to all participants informing them of the study’s progress to help them feel more engaged in the study. This may increase retention as some participants in the control arm commented that they did not think they needed to complete the follow-up assessment that they were sent as they did not realise that they were still involved in the study.
-
Amend the therapy record form so that the content of the between-session task is recorded, as some components of therapy (e.g. graded tasks) may be covered as a between-session task.
-
Ensure that study staff and CRN staff resourcing is allocated accordingly for the most effective recruitment routes (e.g. hospital databases require a large number of letters to be sent out but this is efficient in terms of sending out a mailing to potential participants).
Moderate priority
-
The therapists were in post only until the end of recruitment and so the study manager had to co-ordinate the reporting of AEs and SAEs, including finding information on the causality, during the follow-up period. It would be worth considering funding an administrator at the sites during the follow-up period. This would allow the sites to be more involved in follow-up and in the reporting of AEs and SAEs and would ensure that related activities, such as dealing with database discrepancies, study completion forms, etc., were dealt with. Having at-site support for the full duration of the study would also help with collating information about treatment as usual at the sites and any other issues that arise after the end of the intervention.
-
Explore GP databases and social media as other sources to recruit participants, particularly those no longer receiving rehabilitation.
-
Ensure that data on NHS, PSS and societal costs are captured.
-
Ensure that sufficient data are collected to allow estimation of a relapse rate.
-
Improve the collection of usual-care data. For example, study leads at sites could contact participants regularly (e.g. every 2 to 3 months) to find out whether they have had any other intervention and follow up further details with staff who delivered other interventions.
-
Ensure that therapists have the capacity to start treatment sessions before randomising participants.
-
Consider including a booster session(s) to support the maintenance of therapy gains.
-
Consider providing a quick reference guide for the therapists to use alongside the full treatment manual.
-
Ensure that the training for researchers includes additional time spent on how to explain the randomisation outcome to participants, in particular to those who have been allocated to the usual-care group (and who will therefore not be receiving the BA therapy). The materials for this training (e.g. example scripts and role-play tasks) should be developed in collaboration with the PPI group.
-
Develop a fidelity checklist to be used to inform the training of therapists and the monitoring of the videos of therapy sessions during the trial.
Recommendations for future research
Several of our recommendations for a Phase III trial include approaches that are also relevant to the recruitment of participants and the monitoring of treatment fidelity in multicentre trials in stroke rehabilitation more broadly. In addition to these recommendations, from our experience of BEADS we have identified further areas for future research:
-
identify whether equipoise from clinicians and researchers influences recruitment to RCTs in stroke rehabilitation
-
explore methods for broadening psychological interventions for people who have had a stroke to be accessible for non-English speakers
-
explore the accessibility of psychological interventions for people with severe aphasia and/or cognitive impairments.
Acknowledgements
We thank all those who took part in the trial, and the clinical staff and research nurses at the participating sites for their support.
We gratefully acknowledge the hard work, support and advice from the following: Helen Wakefield and Zoe Furniss (University of Sheffield) for administrative support; Saleema Rex (University of Sheffield) for assistance with data management; Jacqueline Mhizha-Murira (University of Nottingham) for assistance with preparing the final report for submission; Luke Squires and Sara Clarke (University of Nottingham), Mark Jayes, Madeline Harrison and Rosie Duncan (University of Sheffield) for conducting outcome assessments and interviews; and Sara Clarke and Cara Knight (University of Nottingham) for assistance with the treatment fidelity video analysis.
Thank you to the PIs at the sites for their hard work and support of the study: Amanda Farr (Nottinghamshire Healthcare NHS Trust), Martin Cooper (Sherwood Forest Hospitals NHS Foundation Trust), Nigel Schofield (Derby Teaching Hospitals NHS Foundation Trust), Mary Smith (Derbyshire Community Health Services NHS Foundation Trust), Jane Barton (Sheffield Health & Social Care NHS Trust) and Kirsty Harkness (Sheffield Teaching Hospitals NHS Foundation Trust).
We are grateful for the hard work from the study therapists: Michelle Brook, Kara Crossley and Josh Maybin.
We would like to thank the PPI group members (Ian Merriman, Traudel Merriman and Christine Welburn) for their invaluable input throughout the study.
We offer special thanks to the members of our oversight committees, namely the TSC and DMEC: Fergus Gracey (Chairperson of the TSC), Clinical Neuropsychologist and Senior Research Fellow (University of East Anglia); David Ekers, Senior Clinical Lecturer (Durham University); Thompson Robinson, Professor of Stroke Medicine (University of Leicester); Jessica Marsh, Neurospecialist Clinical Psychologist (Royal Derby Hospital); Professor Peter Langhorne (Chairperson of the DMEC), Professor of Stroke Care (University of Glasgow); Professor Anne Forster, Professor of Stroke Rehabilitation (University of Leeds); Professor Helen Rodgers, Professor of Stroke Care (Newcastle University); and Professor Toby Prevost, Chair in Medical Statistics and Clinical Trials (Imperial College London).
Contributions of authors
Shirley A Thomas (Associate Professor in Rehabilitation Psychology) was the chief investigator and co-authored the final report.
Avril ER Drummond (Professor of Healthcare Research, Director of Research and Occupational Therapist) was the acting chief investigator from May 2016 to June 2017, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Nadina B Lincoln (Professor of Clinical Psychology) conducted the treatment fidelity analysis and prepared the results for publication, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Rebecca L Palmer (NIHR-HEFCE Senior Academic Clinical Lecturer) designed and delivered training to the study therapists and researchers on working with people with aphasia, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Roshan das Nair (Professor of Clinical Psychology & Neuropsychology) jointly conducted the qualitative analysis and contributed to the preparation of the qualitative results for publication, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Nicholas R Latimer (Senior Research Fellow) jointly conducted the health economics analysis and contributed to the preparation of the health economics results for publication, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Gemma L Hackney (Research Assistant) was the Trial Manager from June 2016 to January 2017 and contributed to the preparation of the final report.
Laura Mandefield (Medical Statistician) conducted the statistical analysis of the feasibility trial, prepared the statistical results for publication and contributed important intellectual content to the report.
Stephen J Walters (Professor of Medical Statistics and Clinical Trials) oversaw the statistical analysis of the feasibility trial, prepared statistical results for publication, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Rachael D Hatton (Research Assistant) jointly conducted the health economics analysis and contributed to the preparation of the health economics results for publication.
Cindy L Cooper (Director, Clinical Trials Research Unit/Professor of Health Services Research and Clinical Trials) oversaw the clinical trial management, contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Timothy F Chater (Data and Information Systems Manager) was the data manager for the trial and contributed to the preparation of the final report.
Timothy J England (Clinical Associate Professor) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Patrick Callaghan (Professor of Mental Health Nursing) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Elizabeth Coates (Research Associate/Trial Manager) was the Trial Manager from September 2014 to December 2015, contributed to the development of the trial protocol and contributed important intellectual content to the report.
Katie E Sutherland (Research Assistant) was the Research Assistant from January 2017 to April 2017 and contributed to the preparation of the final report.
Sarah Jacob Eshtan (Study Manager) was the Trial Manager from January 2016 to June 2016 and from April 2017 to August 2017 and contributed to the preparation of the final report.
Gogem Topcu (Research Fellow) jointly conducted the qualitative analysis and contributed to the preparation of the qualitative results for publication.
All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publication
Thomas SA, Coates E, das Nair R, Lincoln NB, Cooper C, Palmer R, et al. Behavioural Activation Therapy for Depression after Stroke (BEADS): a study protocol for a feasibility randomised controlled pilot trial of a psychological intervention for post-stroke depression. Pilot Feasibil Stud 2016;2:45.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry 2013;202:14-21. https://doi.org/10.1192/bjp.bp.111.107664.
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014;9:1017-25. https://doi.org/10.1111/ijs.12357.
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005;36:1330-40. https://doi.org/10.1161/01.STR.0000165928.19135.35.
- Gillen R, Tennen H, McKee TE, Gernert-Dott P, Affleck G. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch Phys Med Rehabil 2001;82:1645-9. https://doi.org/10.1053/apmr.2001.26249.
- Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke 1998;29:618-24. https://doi.org/10.1161/01.STR.29.3.618.
- Pohjasvaara T, Vataja R, Leppävuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol 2001;8:315-19. https://doi.org/10.1046/j.1468-1331.2001.00182.x.
- van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil 1999;13:268-72. https://doi.org/10.1191/026921599672495022.
- Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carrà G, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat 2013;2013. https://doi.org/10.1155/2013/862978.
- Ghose SS, Williams LS, Swindle RW. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care 2005;43:1259-64. https://doi.org/10.1097/01.mlr.0000185711.50480.13.
- Cameron JI, Cheung AM, Streiner DL, Coyte PC, Stewart DE. Stroke survivor depressive symptoms are associated with family caregiver depression during the first 2 years poststroke. Stroke 2011;42:302-6. https://doi.org/10.1161/STROKEAHA.110.597963.
- Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke Depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e30-e43. https://doi.org/10.1161/STR.0000000000000113.
- Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging 2005;22:163-82. https://doi.org/10.2165/00002512-200522020-00006.
- Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379-84. https://doi.org/10.1161/01.STR.0000221815.64093.8c.
- Kauhanen ML, Korpelainen JT, Hiltunen P, Määttä R, Mononen H, Brusin E, et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis 2000;10:455-61. https://doi.org/10.1159/000016107.
- Thomas SA, Lincoln NB. Predictors of emotional distress after stroke. Stroke 2008;39:1240-5. https://doi.org/10.1161/STROKEAHA.107.498279.
- Ruddell M, Spencer A, Hill K, House A. Fluoxetine vs placebo for depressive symptoms after stroke: failed randomised controlled trial. Int J Geriatr Psychiatry 2007;22:963-5. https://doi.org/10.1002/gps.1771.
- Thomas SA, Walker MF, Macniven JA, Haworth H, Lincoln NB. Communication and Low Mood (CALM): a randomized controlled trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil 2013;27:398-40. https://doi.org/10.1177/0269215512462227.
- Griffiths H. Investing in Emotional and Psychological Wellbeing for Patients With Long-Term Conditions n.d. www.nhsconfed.org/resources/2012/04/investing-in-emotional-and-psychological-wellbeing-for-patients-with-long-term-conditions (accessed 7 August 2017).
- Department of Health and Social Care . Long-Term Conditions Positive Practice Guide 2008. www.uea.ac.uk/documents/246046/11991919/longterm-conditions-positive-practice-guide.pdf/f9e2b540-2061-4950-a428-b2c9c3ddb35d (accessed 7 August 2017).
- National Institute for Clinical Excellence (NICE) . Depression in Adults: Recognition and Management 2009. www.nice.org.uk/guidance/cg90 (accessed 8 August 2017).
- Broomfield NM, Laidlaw K, Hickabottom E, Murray MF, Pendrey R, Whittick JE, et al. Post-stroke depression: the case for augmented, individually tailored cognitive behavioural therapy. Clin Psychol Psychother 2011;18:202-17. https://doi.org/10.1002/cpp.711.
- Kneebone II. A framework to support cognitive behavior therapy for emotional disorder after stroke. Cogn Behav Pract 2016;23:99-109. https://doi.org/10.1016/j.cbpra.2015.02.001.
- Lincoln NB, Flannaghan T, Sutcliffe L, Rother L. Evaluation of cognitive behavioural treatment for depression after stroke: a pilot study. Clin Rehabil 1997;11:114-22. https://doi.org/10.1177/026921559701100204.
- Rasquin SM, Van De Sande P, Praamstra AJ, Van Heugten CM. Cognitive-behavioural intervention for depression after stroke: five single case studies on effects and feasibility. Neuropsychol Rehabil 2009;19:208-22. https://doi.org/10.1080/09602010802091159.
- Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: a randomized controlled trial. Stroke 2003;34:111-15. https://doi.org/10.1161/01.STR.0000044167.44670.55.
- Gallagher M, McLeod HJ, McMillan TM. A systematic review of recommended modifications of CBT for people with cognitive impairments following brain injury [published online ahead of print 22 November 2016]. Neuropsychol Rehabil 2016. https://doi.org/10.1080/09602011.2016.1258367.
- Kootker JA, Rasquin SM, Lem FC, van Heugten CM, Fasotti L, Geurts AC. Augmented cognitive behavioral therapy for poststroke depressive symptoms: a randomized controlled trial. Arch Phys Med Rehabil 2017;98:687-94. https://doi.org/10.1016/j.apmr.2016.10.013.
- Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clin Rehabil 2017;31:71-8. https://doi.org/10.1177/0269215515626232.
- Hadidi NN, Lindquist R, Buckwalter K, Savik K. Feasibility of a pilot study of problem-solving therapy for stroke survivors. Rehabil Nurs 2015;40:327-37. https://doi.org/10.1002/rnj.148.
- Visser MM, Heijenbrok-Kal MH, Van’t Spijker A, Lannoo E, Busschbach JJ, Ribbers GM. Problem-solving therapy during outpatient stroke rehabilitation improves coping and health-related quality of life: randomized controlled trial. Stroke 2016;47:135-42. https://doi.org/10.1161/STROKEAHA.115.010961.
- Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CI, Lightbody CE, et al. Motivational interviewing early after acute stroke: a randomized, controlled trial. Stroke 2007;38:1004-9. https://doi.org/10.1161/01.STR.0000258114.28006.d7.
- Gurr B. Emotional support for stroke survivors: Share Your Story Group. Int J Ther Rehabil 2009;16:564-72. https://doi.org/10.12968/ijtr.2009.16.10.44566.
- Gurr B. A psychological well-being group for stroke patients. Clin Psychol Forum 2009;202:12-7.
- House A. Problem-Solving Therapy Improves Psychological Outcome After Stroke: A Randomised Controlled Trial 2003. www.cochranelibrary.com/es/cdsr/doi/10.1002/14651858.CD003689.pub3/media/CDSR/CD003689/CD003689/.pdf/es (accessed September 2018).
- Watkins CL, Wathan JV, Leathley MJ, Auton MF, Deans CF, Dickinson HA, et al. The 12-month effects of early motivational interviewing after acute stroke: a randomized controlled trial. Stroke 2011;42:1956-61. https://doi.org/10.1161/STROKEAHA.110.602227.
- Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, et al. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke 2009;40:3073-8. https://doi.org/10.1161/STROKEAHA.109.549808.
- Kirkness CJ, Becker KJ, Cain KC, Kohen R, Tirschwell DL, Teri L, et al. Telephone versus in-person psychosocial behavioral treatment in post-stroke depression. Stroke 2015;46.
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD003437.pub3.
- Lewinsohn PM, Friedman R, Katz M. The Psychology of Depression: Contemporary Theory and Research. Washington, DC: V.H. Winston & Sons; 1974.
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral Activation for Depression: A Clinician’s Guide. New York, NY: Guilford Press; 2010.
- Laidlaw K, Thompson LW, Dick-Siskin L, Gallagher-Thompson D. Cognitive Behaviour Therapy with Older People. Chichester: John Wiley & Sons Ltd; 2003.
- Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract 2001;8:255-70. https://doi.org/10.1093/clipsy.8.3.255.
- Richards DA, Bennett-Levey J, Richards D, Farrand P, Christensen H, Griffiths K, et al. Oxford Guide to Low Intensity CBT Interventions. Oxford: Oxford University Press; 2010.
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol Sci Pract 2009;16:383-411. https://doi.org/10.1111/j.1468-2850.2009.01178.x.
- Soucy Chartier I, Provencher MD. Behavioural activation for depression: efficacy, effectiveness and dissemination. J Affect Disord 2013;145:292-9. https://doi.org/10.1016/j.jad.2012.07.023.
- Chan ATY, Sun GYY, Tam WWS, Tsoi KKF, Wong SYS. The effectiveness of group-based behavioral activation in the treatment of depression: An updated meta-analysis of randomized controlled trial. J Affect Disord 2017;208:345-54. https://doi.org/10.1016/j.jad.2016.08.026.
- Scogin F, Welsh D, Hanson A, Stump J, Coates A. Evidence-based psychotherapies for depression in older adults. J Consult Clin Psychol 2005;12:222-37. https://doi.org/10.1093/clipsy.bpi033.
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013;58:376-85. https://doi.org/10.1177/070674371305800702.
- Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871-80. https://doi.org/10.1016/S0140-6736(16)31140-0.
- Humphreys I, Thomas S, Phillips C, Lincoln N. Cost analysis of the Communication and Low Mood (CALM) randomised trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil 2015;29:30-41. https://doi.org/10.1177/0269215514537656.
- Grober S, Hibbard MR, Gordon WA, Stein PN, Freeman A, Gordon WA. Advances in Stroke Rehabilitation. Andover: Andover Medical Publishers; 1993.
- Kanter JW, Puspitasari AJ, Santos MM, Nagy GA. Behavioural activation: history, evidence and promise. Br J Psychiatry 2012;200:361-3. https://doi.org/10.1192/bjp.bp.111.103390.
- Farrand P, Woodford J, Llewellyn D, Anderson M, Venkatasubramanian S, Ukoumunne OC, et al. Behavioural activation written self-help to improve mood, wellbeing and quality of life in people with dementia supported by informal carers (PROMOTE): a study protocol for a single-arm feasibility study. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0083-x.
- Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci 1997;52:P159-66. https://doi.org/10.1093/geronb/52B.4.P159.
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement. Obstet Gynecol 2010;115:1063-70. https://doi.org/10.1097/AOG.0b013e3181d9d421.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- Thomas SA, Coates E, das Nair R, Lincoln NB, Cooper C, Palmer R, et al. Behavioural Activation Therapy for Depression after Stroke (BEADS): a study protocol for a feasibility randomised controlled pilot trial of a psychological intervention for post-stroke depression. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0072-0.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Stern RA. Visual Analog Mood Scales Professional Manual. Odessa, FL: Psychological Assessment Resources Inc.; 1997.
- Jayes M, Palmer R. Initial evaluation of the Consent Support Tool: a structured procedure to facilitate the inclusion and engagement of people with aphasia in the informed consent process. Int J Speech Lang Pathol 2014;16:159-68. https://doi.org/10.3109/17549507.2013.795999.
- Great Britain . Mental Capacity Act 2005 2005.
- Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry 2011;198:66-72. https://doi.org/10.1192/bjp.bp.110.079111.
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif 2011;35:111-61. https://doi.org/10.1177/0145445510390929.
- Papworth M, Marrinan T, Martin B, Keegan D, Chaddock A. Low Intensity Cognitive-Behaviour Therapy. A Practitioner’s Guide. London: Sage; 2013.
- Lincoln NB, Sutcliffe LM, Unsworth G. Validation of the stroke aphasic depression questionnaire (SADQ) for use with patients in hospital. Clin Neuropsychol Assess 2000;1:88-96.
- Drummond AER, Walker MF. The Nottingham Leisure Questionnaire for stroke patients. Br J Occup Ther 1994;57:414-18. https://doi.org/10.1177/030802269405701102.
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Latimer NR, Dixon S, Palmer R. Cost-utility of self-managed computer therapy for people with aphasia. Int J Technol Assess Health Care 2013;29:402-9. https://doi.org/10.1017/S0266462313000421.
- Minns Lowe CJ, Wilson MS, Sackley CM, Barker KL. Blind outcome assessment: the development and use of procedures to maintain and describe blinding in a pragmatic physiotherapy rehabilitation trial. Clin Rehabil 2011;25:264-74. https://doi.org/10.1177/0269215510380824.
- Siemonsma PC, Walker MF. Practical guidelines for independent assessment in randomized controlled trials (RCTs) of rehabilitation. Clin Rehabil 1997;11:273-9. https://doi.org/10.1177/026921559701100402.
- R Core Team . R: A Language and Environment for Statistical Computing 2008.
- Walters SJ. Quality of Life Outcomes in Clinical Trials and Health-Care Evaluation: a Practical Guide to Analysis and Interpretation. Chichester: John Wiley & Sons Ltd; 2009.
- Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care 2004;42:1194-201. https://doi.org/10.1097/00005650-200412000-00006.
- Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Heath C, Silverman D. Qualitative Research: Theory, Method and Practice. London: Sage; 1997.
- Jordan B, Henderson A. Interaction analysis: foundations and practice. J Learn Sci 1995;4:39-103. https://doi.org/10.1207/s15327809jls0401_2.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015–2016 2016. www.gov.uk/government/collections/nhs-reference-costs (accessed 12 April 2017).
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: an EQ-5D-5L Value Set for England (OHE research paper 16/01. London: Office of Health Economics; 2016.
- Annemans L, Genesté B, Jolain B. Early modelling for assessing health and economic outcomes of drug therapy. Value Health 2000;3:427-34. https://doi.org/10.1046/j.1524-4733.2000.36007.x.
- Hartz S, John J. Contribution of economic evaluation to decision making in early phases of product development: a methodological and empirical review. Int J Technol Assess Health Care 2008;24:465-72. https://doi.org/10.1017/S0266462308080616.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. https://doi.org/10.1016/S0140-6736(02)09832-X.
- Griffin S, Welton NJ, Claxton K. Exploring the research decision space: the expected value of information for sequential research designs. Med Decis Making 2010;30:155-62. https://doi.org/10.1177/0272989X09344746.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Joint Formulary Committee . British National Formulary 2017. www.medicinescomplete.com/mc/bnf/current/ (accessed 1 March 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Willan AR, Briggs AH. Statistical Analysis of Cost-Effectiveness Data. Chichester: John Wiley & Sons Ltd; 2006.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Asso 1962;57:348-68. https://doi.org/10.1080/01621459.1962.10480664.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis 2010;69:i89-91. https://doi.org/10.1136/ard.2009.117150.
- Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Danish MONICA Study Group . Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. https://doi.org/10.1161/hs0901.094253.
- Office for National Statistics . National Life Tables: United Kingdom 2016. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 12 April 2017).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- National Institute for Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013, Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case#framework-for-estimating-clinical-and-cost-effectiveness (accessed 11 April 2017).
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Ritchie J, Spencer L. The Analysis of Qualitative Data: An Approach to Analysis for Applied Social Policy Research. London: Routledge; 1993.
- Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Res 2011;18:52-6. https://doi.org/10.7748/nr2011.01.18.2.52.c8284.
- das Nair R, Lincoln NB. The effectiveness of memory rehabilitation following neurological disabilities: a qualitative inquiry of patient perspectives. Neuropsychol Rehabil 2013;23:528-45. https://doi.org/10.1080/09602011.2013.792290.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage; 2014.
- Nelson J. Using conceptual depth criteria: addressing the challenge of reaching saturation in qualitative research. Qual Res 2016;17:554-70. https://doi.org/10.1177/1468794116679873.
- Campbell MJ, Walters SJ, Machin D. Medical Statistics: A Textbook for the Health Sciences. Chichester: Wiley-Blackwell; 2007.
- Ren S, Minton J, Whyte S, Latimer N, Stevenson M. A new approach for sampling ordered parameters in probabilistic sensitivity analysis. PharmacoEconomics 2018;36:341-7. https://doi.org/10.1007/s40273-017-0584-3.
- Stroke Association . State of the Nation: Stroke Statistics, January 2016 2016. www.stroke.org.uk/sites/default/files/stroke_statistics_2015.pdf (accessed 8 June 2017).
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation; 2012.
- Slot KB, Berge E, Sandercock P, Lewis SC, Dorman P, Dennis M. Oxfordshire Community Stroke Project . Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585-9. https://doi.org/10.1161/STROKEAHA.108.531533.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Lincoln NB, Brinkmann N, Cunningham S, Dejaeger E, De Weerdt W, Jenni W, et al. Anxiety and depression after stroke: a 5 year follow-up. Disabil Rehabil 2013;35:140-5. https://doi.org/10.3109/09638288.2012.691939.
- Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, et al. Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil 2016;97:2188-201.e8. https://doi.org/10.1016/j.apmr.2016.03.006.
- Middleton LE, Lam B, Fahmi H, Black SE, McIlroy WE, Stuss DT, et al. Frequency of domain-specific cognitive impairment in sub-acute and chronic stroke. NeuroRehabilitation 2014;34:305-12. https://doi.org/10.3233/NRE-131030.
- Parker C, Dewey M. Assessing research outcomes by postal questionnaire with telephone follow-up. Int J Epidemiol 2000;29:1065-9. https://doi.org/10.1093/ije/29.6.1065.
- Whitehurst DGT, Latimer NR, Kagan A, Palmer R, Simmons-Mackie N, Hoch JS. Preference-based health-related quality of life in the context of aphasia: a research synthesis. Aphasiology 2015;29:763-80. https://doi.org/10.1080/02687038.2014.985581.
- Christakis NA. Social networks and collateral health effects: have been ignored in medical care and clinical trials, but need to be studied. BMJ 2004;329:184-5. https://doi.org/10.1136/bmj.329.7459.184.
- Dixon S, Walker M, Salek S. Incorporating carer effects into economic evaluation. PharmacoEconomics 2006;24:43-5. https://doi.org/10.2165/00019053-200624010-00004.
- Krol M, Papenburg J, van Exel J. Does including informal care in economic evaluations matter? A systematic review of inclusion and impact of informal care in cost-effectiveness studies. PharmacoEconomics 2015;33:123-35. https://doi.org/10.1007/s40273-014-0218-y.
- Al-Janabi H, van Exel J, Brouwer W, Coast J. A framework for including family health spillovers in economic evaluation. Med Decis Making 2016;36:176-86. https://doi.org/10.1177/0272989X15605094.
- Horne JC, Lincoln NB, Logan PA. Measurement of confidence: the development and psychometric evaluation of a stroke-specific, measure of confidence. Clin Rehabil 2017;31:1529-37. https://doi.org/10.1177/0269215517705424.
- Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials 2015;16. https://doi.org/10.1186/s13063-015-0679-0.
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0100100.
- Janssen N, Huibers MJH, Lucassen P, Voshaar RO, van Marwijk H, Bosmans J, et al. Behavioural activation by mental health nurses for late-life depression in primary care: a randomized controlled trial. BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1388-x.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57.
Appendix 1 Changes to protocol
| Changes to protocol | Date | Approved by |
|---|---|---|
| Detail added on notification of GPs following ethics review by NRES East Midlands – Leicester | Protocol v2.0, 22 Januray 2015 | REC 29 Januray 2015 |
| Additional options added to the recruitment process following early feedback from participating sites, that is, to include the option of a streamlined recruitment process whereby the therapist can contact the patient directly by telephone following consent to contact, and to broaden the recruitment routes to include potential participants on acute outpatient caseloads | Protocol v2.1, 19 July 2015 | REC 8 July 2015 |
| Additional exclusion criteria to the effect that patients are not eligible to be recruited to the BEADS trial if they are currently receiving psychological intervention, that they will be withdrawn from the intervention arm if it is subsequently agreed that the patient needs immediate clinical psychology input and to clarify that usual care can include psychological input post randomisation | Protocol v2.2, 29 July 2015 | REC 7 August 2015 |
|
Change in study personnel and contact details Additional secondary end point of estimating sample size for a definitive trial Clarification that two or fewer missing items within the PHQ-9 questionnaire may be imputed |
Protocol v2.3, 26 February 2016 | Minor amendment therefore REC approval not required. Approval to implement received from CRN on 15 April 2016 |
| Change in study personnel (CI and study manager) and contact details | Protocol v3, 6 May 2016 | REC 23 May 2016 |
Appendix 2 Video-recording categories
| Manual content | Code | Other content | Code |
|---|---|---|---|
| All sessions | |||
| Summarise previous session | P | Social chat | SC |
| Review between-session tasks | R | Information on organisation of sessions (e.g. date, venue, time, etc.) | IO |
| Set agenda and goals for each session | AG | Travel arrangements | T |
| Agree between-session tasks for the next session | AN | Preparing materials, tasks, etc. | PP |
| Session summary | SU | Check whether or not participant needs reminder of the next appointment (telephone call/text message) | AR |
| Breaks | B | ||
| Review events since previous session | RE | ||
| Session 1 | Therapist Activities | ||
| Therapist to introduce themselves | TI | Providing explanation | PE |
| Explain aims of BEADS study | AB | Providing feedback | PF |
| Explain structure of sessions (i.e. frequency, time scales, between-visit tasks etc.) | SS | Providing encouragement/reassurance | PR |
| Assessment of current difficulties and symptoms of depression | DS | Summarising | S |
| Discuss communication abilities/difficulties | CA | Paraphrasing | PH |
| Outline BA treatment rationale | BA | Presenting/discussing BA strategies | PS |
| What else do you want to know? | K | Address problems with task non-completion | AP |
| Checking understanding | U | ||
| Session 2 | Give opportunities for participant to ask questions | OQ | |
| Identification of participants’ problems (problem list) | IP | References to between-session activities | RA |
| Agreeing therapy goals (specific, measurable, time-bound) | TG | Reference to problems list | PL |
| Reference to participant goals | PG | ||
| Session 3 | Addressing appropriateness of activities | A | |
| Discuss relationship between activity level and mood | DR | Ask participant to rate enjoyment of chosen activities | AE |
| Refer to behavioural model of depression | BM | Asking participant questions | |
| Introduce idea of identifying enjoyable activities | IA | Reference to previous sessions | Z |
| Session 4 | Participant Activity | ||
| Review idea of identifying enjoyable activities | ER | Discussing BA strategies | BS |
| Discuss how enjoyable activities improve mood | EM | Discussing activities | D |
| Identify enjoyable activities | E | Asking for information | AI |
| Create list of enjoyable activities | L | Describing problems | DP |
| Identify barriers to engaging in identified activities | EB | Asking questions | Q |
| Check activities are in line with goals | CG | Information about sessions, venue, group, etc. | I |
| Activity scheduling plan | SP | Describing mood | DM |
| Feedback on home activities | F | ||
| Session 5 | Feedback on sessions | FS | |
| Review whether activities were carried out (rate success or problem solve for non-completion) | AO |
Hospital visit discussion Speech and language therapy discussion |
HD ST |
| Identify suitable activities to be completed as between-session tasks (new activities/increased frequency of activities) | SA | ||
| Recap idea of activity scheduling | RS | ||
| Agree which activities to be carried out for following week | AW | Appendices/Worksheets | |
| Graded task principle discussion/explanation | GT | ||
| Session 6 | Use of graded task principle | UG | |
| Review whether activities were carried out as scheduled | CS | Use of/reference to activity diaries/schedules | UD |
| Identify one activity per day to be scheduled | OA | Use of/reference to mood rating scales | UM |
| Identify any potential obstacles to completing activities and address them | PO | Use of/reference to activity lists | AL |
| Use of/reference to activity schedule/activity monitor | AS | ||
| Session 7 | Use of/reference to participant specific communication resources | C | |
| Review relationship between activity schedule and mood | SM | ||
| Identify common problems in planning or carrying out activities | CP | ||
| Problem-solving strategy | PK | ||
| Session 8 | |||
| Review and rate achievements with regard to goals set at start of therapy | GA | ||
| Addressing a new or unachieved goal | N | ||
| Discuss behavioural strategies to achieve goal | DB | ||
| Introduce idea of therapy ending | TE | ||
| Highlight successful behavioural strategies and progress | H | ||
| Session 9 | |||
| Address reasons for non-completion of between-session tasks | NC | ||
| Discuss therapy ending | DE | ||
| Highlight successes and achieved goals and consider continuation of these post therapy | TC | ||
| Identify with participant how behavioural strategies can be used to address goals | BG | ||
| Develop plan to cope with future mood problems | PM | ||
| Session 10 | |||
| Review problems addressed during therapy | TP | ||
| Summarise successful strategies and skills used | US | ||
| Discuss generalisation of skills to future situations (relapse prevention) | GS | ||
| Questions and answers | QA | ||
| Remind participants about 6-month follow-up protocol (indicate date) | RM |
Appendix 3 Resource use costs
| Resource use | Unit cost (£) | Reference |
|---|---|---|
| Inpatient stay per night (general) | 389.10 | NHS Reference Costs 2015–1679 (weighted average of regular day of night admission) |
| Inpatient stay per night (myocardial infarction) | 442.85 | NHS Reference Costs 2015–1679 (weighted average of EL, EL XS, NEL, NEL XS, NES for EB10A-EB10E) |
| Inpatient stay per night (orthopaedics) | 433.54 | NHS Reference Costs 2015–1679 (weighted average of EL, EL XS, NEL, NEL XS, NES for HE11A-HE83C) |
| Outpatient attendance | 116.92 | NHS Reference Costs 2015–1679 (weighted average of total outpatient attendances data) |
| Outpatient attendance: psychiatry | 171.41 | NHS Reference Costs 2015–1679 (total; 715 old age psychiatry) |
| Outpatient attendance: clinical psychology | 144.70 | NHS Reference Costs 2015–1679 (total; 656 clinical psychology) |
| Outpatient attendance: psychotherapy | 199.06 | NHS Reference Costs 2015–1679 (total; 713 psychotherapy) |
| Outpatient attendance: liaison mental health | 105.08 | NHS Reference Costs 2015–1679 (total; 722 liaison psychiatry) |
| Outpatient attendance: accident and emergency | 146.86 | NHS Reference Costs 2015–1679 (total; 180 accident and emergency) |
| Outpatient attendance (other) | 170.60 | NHS Reference Costs 2015–1679 (total; 328 stroke medicine) |
| 127.67 | NHS Reference Costs 2015–1679 (total; 320 cardiology) | |
| 167.05 | NHS Reference Costs 2015–1679 (total; 300 general medicine) | |
| 48.33 | NHS Reference Costs 2015–1679 (total; 650 physiotherapy) | |
| 116.05 | NHS Reference Costs 2015–1679 (total; 652 speech and language therapy) | |
| 79.19 | NHS Reference Costs 2015–1679 (total; 662 optometry) | |
| 117.01 | NHS Reference Costs 2015–1679 (total; 110 trauma and orthopaedics) | |
| 175.60 | NHS Reference Costs 2015–1679 (total; 400 neurology) | |
| 154.77 | NHS Reference Costs 2015–16 (total; 340 respiratory medicine) | |
| 58.33 | NHS Reference Costs 2015–1679 (total; 840 audiology) | |
| 114.60 | NHS Reference Costs 2015–1679 (total; 658 orthotics) | |
| 107.52 | NHS Reference Costs 2015–1679 [diagnostic imaging (outpatient); weighted average RD20 A to RD27Z)] | |
| GP consultation (at surgery) | 3.90 (per minute of patient contact) | PSSRU 201685 (p. 145) |
| GP consultation (home visit) | 4.90 (per minute, including travel) | PSSRU 201387 (p. 191) |
| Practice nurse (at surgery) | 43.00 (per hour) | PSSRU 201685 (p. 143) |
| Community nurse | 67.00 (per hour) | PSSRU 201589 (p. 169) |
| Community occupational therapist | 44.00 (per hour) | PSSRU 201685 (p. 159) |
| Hospital-based physiotherapist | 38.00 (per hour) | PSSRU 201589 (p. 217) |
| Hospital-based speech and language therapist | 38.00 (per hour) | PSSRU 201589 (p. 219) |
| Home care worker | 24.00 (per hour face-to-face, weekday) | PSSRU 201685 (p. 160) |
| NHS counsellor | 50.00 (per hour) | PSSRU 201488 (p. 51) |
| NHS psychologist | 52.00 (per hour, based on AfC band 7) | PSSRU 201685 (p. 137) |
| NHS psychotherapist | 52.00 (per hour, based on AfC band 7) | PSSRU 201685 (p. 137) |
| Community-based mental health nurse | 75.00 (per hour of face-to-face contact) | PSSRU 201589 (p. 170) |
| Social worker/case manager | 79.00 (per hour of client contact) | PSSRU 201685 (p. 156) |
| CBT therapist | 97.00 (per session) | PSSRU 201685 (p. 77) |
| Home care worker | 24.00 (per hour) | PSSRU 201685 (p. 160) |
| Podiatrist | 32.00 (per hour, based on AfC band 5) | PSSRU 201685 (p. 137) |
| Stroke support | 25.00 (per hour, based on higher level clinical support worker; AfC band 3) | PSSRU 201685 (p. 137) |
| Other primary care contact (NHS community mental health services for older people) | 43.00 (per hour) | PSSRU 201685 (p. 167) |
| Day care for people requiring mental health support | 8.20 (per hour) | PSSRU 201685 (p. 38) |
| Reablement service | 43.00 (per hour of client contact) | PSSRU 201679 (p. 179) |
| Private and voluntary day care for people requiring mental health support | 8.00 (per hour) | PSSRU 201679 (p. 39) |
| Lost employment hours/carer time | 12.10 (per hour, based on median hourly earnings) | Annual survey of hours and earnings, ONS 201697 |
| Behaviour activation | 57.00 (per hour of face-to-face contact) | PSSRU 201685 (p. 40; based on Richards et al.49) |
Appendix 4 A worked example of the audit trail of the analysis and framework development
Please note that this is a worked example of the audit trial and some sections/field notes have been removed. The detailed trail can be obtained from the authors.
Step 1 (Framework development): The following categories (thematic constructs) were developed based on a priori issues covered in the interview schedule and extant literature.

Step 2 (Familiarisation and revised framework): Gogem Topcu was immersed in the data by reading and rereading the verbatim transcripts and listening to audio recordings to note salient points, and recurrent ideas noted under each category. Memos were used to provide a visible audit trail of an emerging theme or subtheme.
Patient- and carer-participant interviews

Notes: a, After reading the transcripts, Gogem Topcu and Roshan das Nair decided to consider outcomes as a separate category as they constituted a prominent part of the interview data. This also fit well with the aims of the project (determining the acceptability of the outcome measures). Therefore, category 1 in step 1 was divided into two categories and the titles were changed. b, Category name was changed to better reflect the interview data. c, Another category was created by Gogem Topcu after reading the interview transcripts as the changes occurred in patients’ lives after stroke were frequently discussed by the patients. These changes were stroke related and not study or therapy related. Gogem Topcu and Roshan das Nair agreed to keep this category as it provided context for the interviews. d, This theme was created to incorporate experiences (e.g. family problems) that did not fit with other themes within this category but were considered important by some participants in relation to their stroke experiences.
Therapist-participant interviews
Notes: a, Experiences of participants from the therapists’ perspective was a recurrent theme. Gogem Topcu initially considered this as a separate category, but after discussion with Roshan das Nair, they decided to have this theme under the category ‘Therapist views of delivering the intervention’ as it fit well within this category in terms of providing a context and further explanation/understanding for their experiences of delivering the therapy.
Step 3 (Indexing and mapping): Mapping the data onto the constructed thematic framework by coding and indexing various sections of the data to specific thematic constructs. The indexing process was iteratively completed by Gogem Topcu, with input from Roshan das Nair, and used the constant comparison method to check and compare each item with the rest of the data, requiring Gogem Topcu to go back to previously analysed transcripts as the new themes or subthemes emerged, to check if they were also evident in these transcripts. This ensured that any additional themes/subthemes were added to reflect the nuances within the data. Midway through the coding of the transcripts, Roshan das Nair also examined five transcripts which had been coded by Gogem Topcu, to check the coding of the data independently. Memos were used to provide a visible audit trail.
Patient- and carer-participant interviews
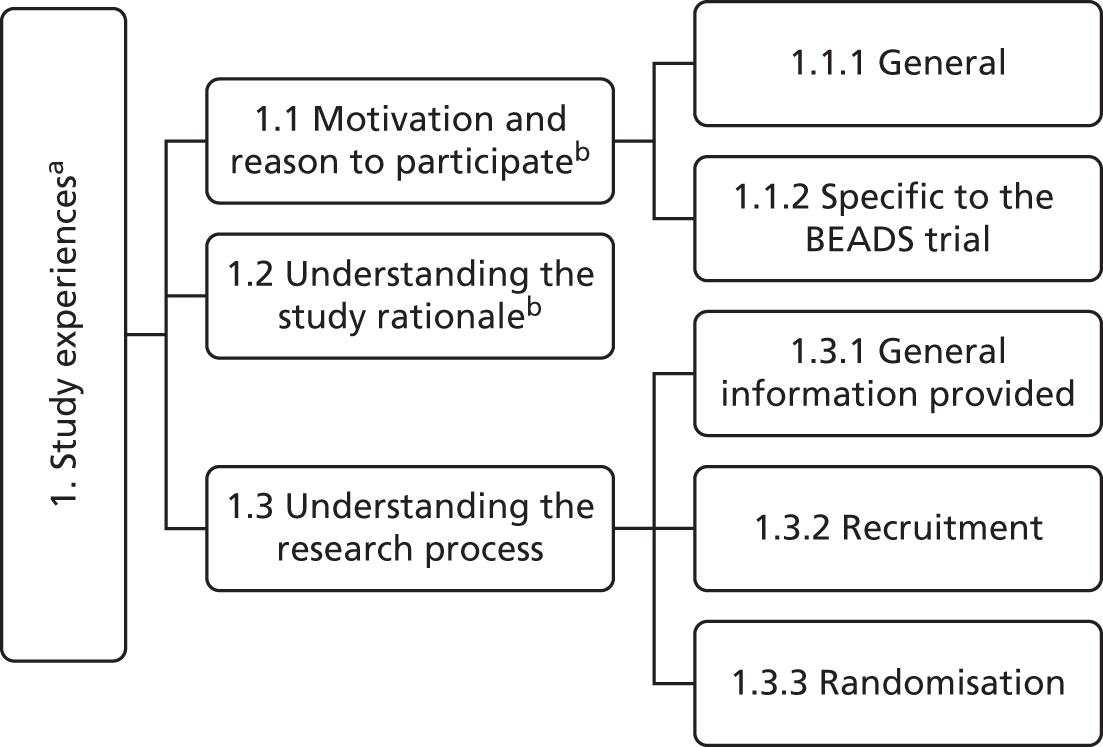
Notes: a, The name of this category was changed from ‘Participant views on trial and the procedures’ to ‘Study experiences’ to cover all the relevant experiences related to the study (e.g. motivation to participate, understanding the rationale and understanding the research process). b, The ’Motivation and understanding’ theme that was developed in step 2 was divided into two separate themes ‘Motivation and reason to participate’ and ‘Understanding the study rationale’ as these represented distinct but inter-related constructs. Theme 1.1 was further developed into two subthemes (i.e. general and specific to the BEADS trial) to reflect participant views better, as the motivation and reason to participate were different for each participant.

Notes: a, Although some participants made general comments about the focus of the measures, some participants commented on specific measures. Therefore, we coded these comments under two subthemes. Another subtheme was created for participants’ ratings. b, Similarly, when talking about the ease of understanding, participants made comments either generally or on specific measures. Therefore, data were coded into two subthemes. c, The subtheme ‘Repetition’ emerged from our reading and interpretation of the data. Therefore, data were coded into two subthemes. Gogem Topcu went back to previously analysed transcripts to check if it was also evident in already analysed transcripts.

Notes: a, As this thematic construct was only evident in intervention group participants, we decided to change the name of the theme to reflect this. b, After discussing this subtheme, Roshan das Nair and Gogem Topcu agreed to rename it as ‘Perceived mediators of change’ to emphasise that certain aspects of therapy were perceived by participants as helpful, mediating a change. c, During coding of carer interviews, a new subtheme, called ‘Role of carer in sessions’, emerged regarding the format of therapy. Gogem Topcu went back to previously analysed transcripts to check whether it was also evident in those transcripts already analysed.

Notes: a, The name of the thematic construct was changed to ‘Gains and changes’ to cover all the themes and subthemes within this category. b, Some participants attributed changes they experienced to the study, whereas others discussed changes they experienced in general and did not attribute these changes to the study. Therefore, we decided to divide this theme into two subthemes.

Notes: a, The name of the thematic construct was changed to make it clearer and also to be more comprehensive of the experiences of the participants. b, The theme ‘Stroke-related changes’ was divided into two subthemes to incorporate carer-specific experiences. c, This theme was also divided into two subthemes to be able to differentiate and compare helpful and unhelpful support/strategies (not therapy-related) used by participants.
Therapist–participant interviews
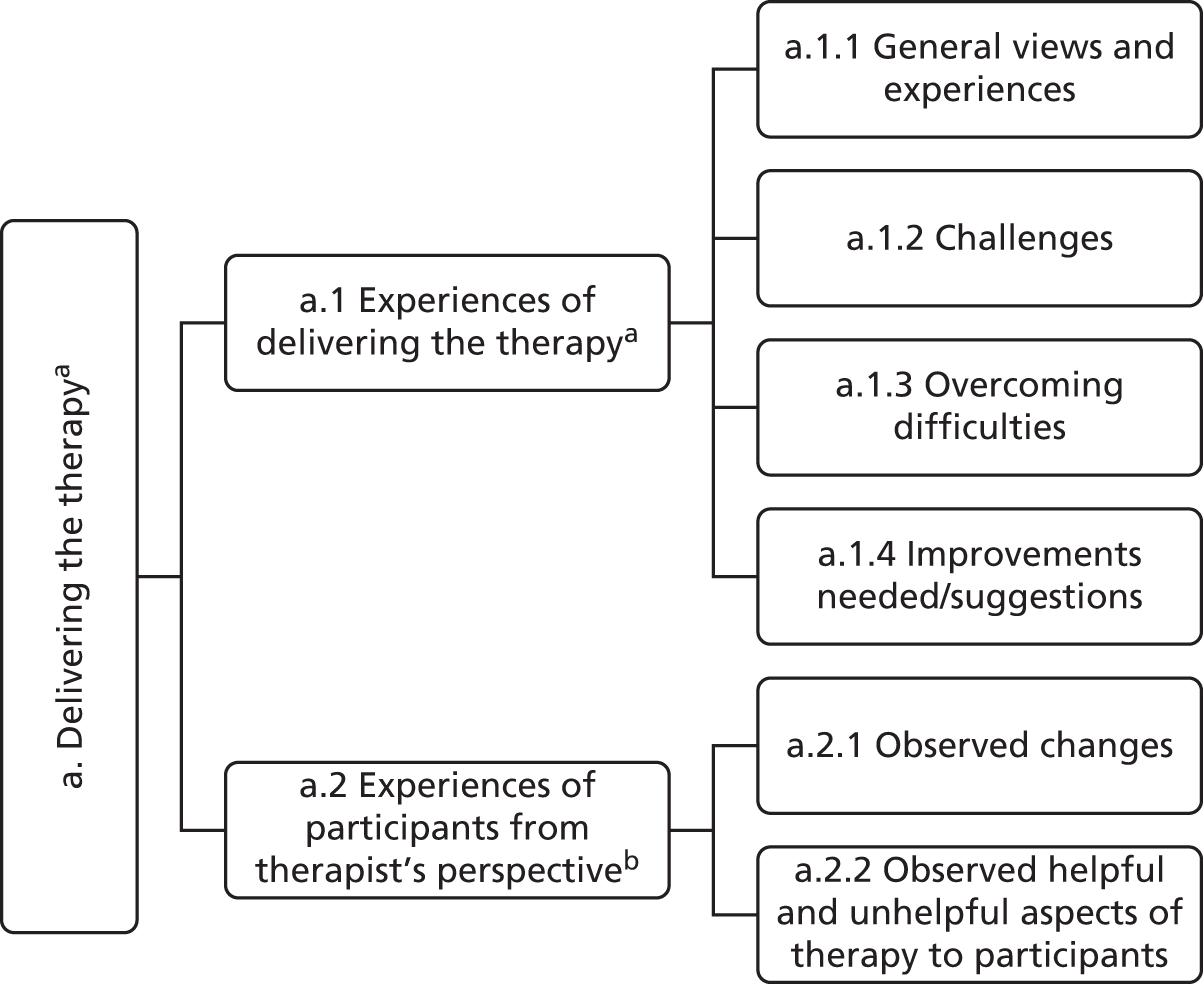
Notes: a, Gogem Topcu revised the names of the thematic construct and the theme a.1, in consultation with Roshan das Nair, for the purpose of clarity. b, This theme was further divided into two subthemes to better differentiate therapists’ observations regarding the changes in participants and the helpful/unhelpful aspects of therapy to participants.
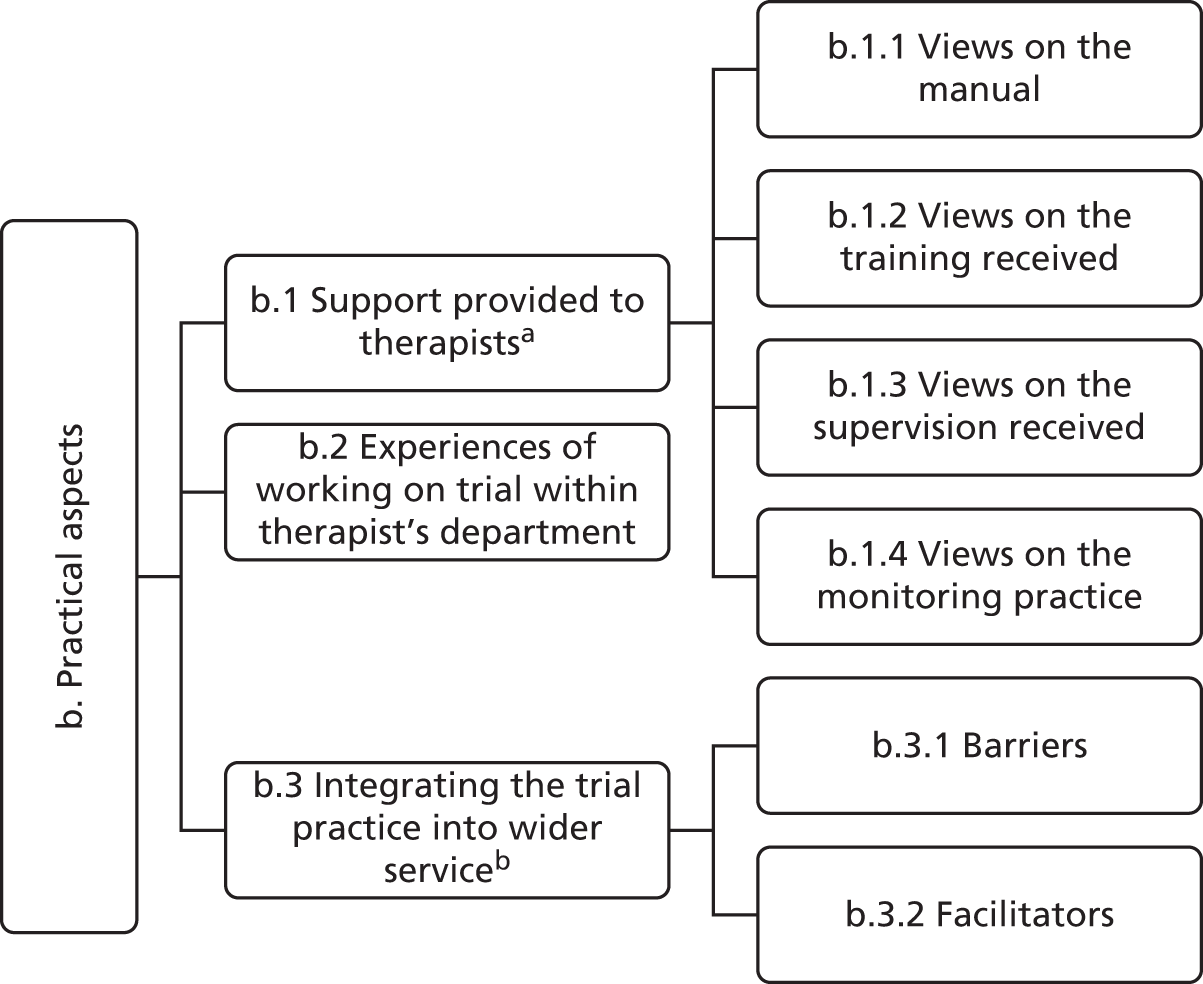
Notes: a, This theme was further divided into four subthemes as the interviews focused on four different types of support provided (i.e. manual, training, supervision, monitoring). b, This theme was further divided into two subthemes as both barriers to and facilitators of integrating the trial into wider services emerged from our reading and interpretation of the data.
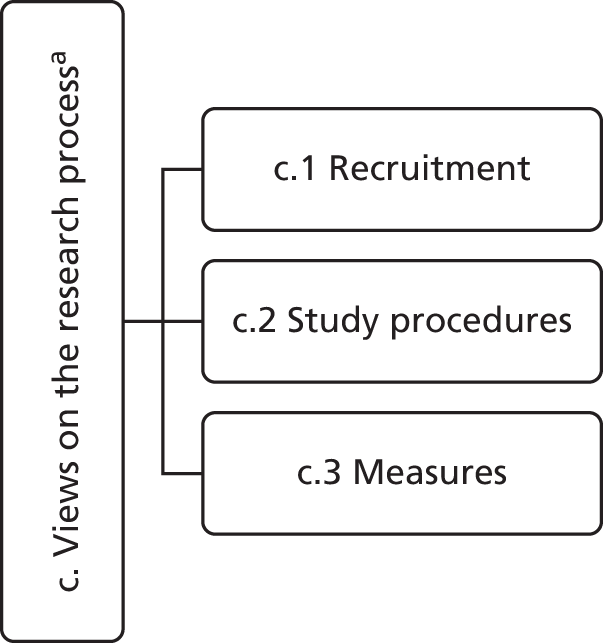
Notes: a, The name of the thematic construct was revised, in consultation with Roshan das Nair, to make it more comprehensive and clear.
Step 4 (Charting): After mapping all the data, a matrix was generated by Gogem Topcu in which the data were charted to summarise each main theme. One matrix per thematic category across participants was developed, containing summaries of the views and experiences of the participants, and references to verbatim quotations in the transcripts. This facilitated the process of comparing and contrasting data from individual participants across themes. Each matrix was reviewed by Roshan das Nair to ensure its rigour and credibility. Disagreements were resolved by discussion. Memos were used to provide a visible audit trail.
(Please note that the matrices and the corresponding field notes have been removed from this worked example of the audit trail. The full audit trail can be obtained from the corresponding author.)
Step 5 (Interpretation): The matrices and the field notes were then used in the interpretation of the data. The interpretation process was iterative and relied on consultation between Gogem Topcu and Roshan das Nair regarding the viability and relevance of a theme, to interrogate theoretical constructs, and to unpack nuances within the data.
(Please note: The field notes regarding the interpretation process have been removed from this worked example of the audit trail. The full audit trail can be obtained from the corresponding author.)
Appendix 5 Semistructured interview guide: participants (version 1)
[Please note: This is a semistructured topic guide that is designed to be used flexibly with each participant. As such, the questions and prompts (presented as subquestions) asked in each interview are likely to vary slightly.]
Opening question
-
Please can you tell me about your experience of being involved in the study?
Recruitment and group allocation
-
How did you come to be involved in the study?
-
What did that feel like?
-
-
How did it feel to be allocated to your study group?
-
How did it feel (not) to be allocated to receive the behavioural activation therapy?
-
Study procedures
-
What did you think about the information we collected from you at the beginning and end of the study?
-
How easy (or not) were the questionnaires to complete?
-
What did you think about how many questionnaires you needed to complete?
-
Did the questionnaires ask about things that were relevant for you, in relation to what the study was about?
-
How would you rate the assessments on a scale of 1–10 (1 did not capture important aspects of my experience to 10 fully captured the important aspects of my experience)
-
For intervention participants only
-
How did you find the therapy?
-
What did you find helpful about the therapy? Any particular aspects?
-
What did you find unhelpful about the therapy? Any particular aspects?
-
Were there any particular aspects which were good or bad?
-
-
How do you think we could improve the therapy in the future?
-
Would you recommend this therapy to other people with low mood after a stroke?
Impact/perceived benefits
-
Have you experienced any changes since taking part in this study?
-
What are these changes?
-
How do you make sense of these changes?
-
Other issues
-
Is there anything else you would like to tell me about?
Appendix 6 Semistructured interview guide: carers (version 1)
[Please note: This is a semistructured topic guide that is designed to be used flexibly with each participant. As such, the questions and prompts (presented as subquestions) asked in each interview are likely to vary slightly.]
Opening questions
-
Please can you tell me about your experience of being involved in the study?
-
Please can you tell me about your (insert appropriate descriptor: spouse/partner/family member/friend)’s experience of being involved in the study?
Recruitment and group allocation
-
How did you come to be involved in the study?
-
What did that feel like?
-
-
How did it feel for your (insert appropriate descriptor) to be allocated to their study group?
-
How did it feel for them (not) to be allocated to receive the behavioural activation therapy?
-
Study procedures
-
What did you think about the information we collected from you at the beginning and end of the study?
-
How easy (or not) were the questionnaires to complete?
-
What did you think about how many questionnaires you needed to complete?
-
Did the questionnaires ask about things that were relevant for you, in relation to what the study was about?
-
How would you rate the assessments on a scale of 1–10? (1: did not capture important aspects of my experience to 10: fully captured the important aspects of my experience)
-
For carers of intervention participants only
-
How much involvement did you have in the therapy?
-
What did you have to do?
-
Were you happy with this level of involvement?
-
What did you find helpful or unhelpful about the therapy? Any particular aspects?
-
-
How do you think we could improve the therapy for people like your (insert appropriate descriptor) in the future?
-
Would you recommend this therapy to other people with low mood after a stroke?
Impact/perceived benefits
-
Have you or your (insert appropriate descriptor) experienced any changes since taking part in this study?
-
What are these changes?
-
How do you make sense of these changes?
-
Other issues
-
Is there anything else you would like to tell me about?
Appendix 7 Semistructured interview guide: staff (version 1)
[Please note: This is a semistructured topic guide that is designed to be used flexibly with each participant. As such, the questions and prompts (presented as subquestions) asked in each interview are likely to vary slightly.]
Opening question
-
Please can you tell me about your experience of being involved in the study?
Intervention delivery
-
Please can you tell me about your experiences of delivering the therapy?
-
How easy/difficult was it to implement the therapy with people who have had a stroke and those with communication difficulties?
-
What went well?
-
What were the difficulties and how did you overcome them?
-
How did you find the training that you received?
-
How did you find using the manual?
-
How did you find the clinical supervision at site/by the trial therapists?
-
How did you find the monitoring of your practice by the research study??
-
-
How did participants find the therapy?
-
Were there any particular aspects which were good or bad?
-
Did participants experience any changes/benefits from the therapy?
-
-
How do you think the therapy could be improved in the future?
-
Would you recommend this therapeutic approach to other psychologists working with people with post-stroke depression?
Study procedures
-
Please can you tell me about the recruitment process?
-
How could this be improved for a future trial?
-
-
How did you find the study procedures, i.e. working to the protocol?
-
How could this be improved for a future trial?
-
-
What did you think about the measures we used at baseline?
-
How would you rate the assessments on a scale of 1–10 (1 did not capture important aspects of the experience to 10 fully captured the important aspects of the experience).
-
Service barriers and facilitators
-
Please can you tell me about your experience of working on the trial within your department?
-
What are the main barriers to integrating the trial practice into the wider service of your department?
-
What are the main facilitators of integrating the trial practice into the wider service of your department?
Other issues
-
Is there anything else you would like to tell me about.
Appendix 8 Completed COnsolidated criteria for REporting Qualitative studies checklist
| Topic | Number item | Guide questions/description | Reported in section |
|---|---|---|---|
| Domain 1: research team and reflexivity | |||
| Personal characteristics | |||
| Interviewer/facilitator | 1 | Which author(s) conducted the interview or focus group? | Chapter 2 , The qualitative research , Interviewer characteristics ; Acknowledgements ; Contributions of authors |
| Credentials | 2 | What were the researcher’s credentials (e.g. PhD, MD)? | Chapter 2 , The qualitative research , Interviewer characteristics Acknowledgements ; Contributions of authors |
| Occupation | 3 | What was their occupation at the time of the study? | Chapter 2 , The qualitative research , Interviewer characteristics ; Acknowledgements ; Contributions of authors |
| Gender | 4 | Was the researcher male or female? | Chapter 2 , The qualitative research , Interviewer characteristics ; Acknowledgements ; Contributions of authors |
| Experience and training | 5 | What experience or training did the researcher have? | Chapter 2 , The qualitative research , Interviewer characteristics |
| Relationship with participants | |||
| Relationship established | 6 | Was a relationship established prior to study commencement? | Chapter 2 , The qualitative research , Interviewer characteristics |
| Participant knowledge of the interviewer | 7 | What did the participants know about the researcher? (e.g. personal goals, reasons for doing the research) | Chapter 2 , The qualitative research ; Chapter 2 , The qualitative research , Relationship with participants |
| Interviewer characteristics | 8 | What characteristics were reported about the inter viewer/facilitator? (e.g. bias, assumptions, reasons and interests in the research topic) | Chapter 2 , The qualitative research , Interviewer characteristics |
| Domain 2: study design | |||
| Theoretical framework | |||
| Methodological orientation and theory | 9 | What methodological orientation was stated to underpin the study? (e.g. grounded theory, discourse analysis, ethnography, phenomenology, content analysis) | Chapter 2 , The qualitative research , Theoretical and thematic framework |
| Participant selection | |||
| Sampling | 10 | How were participants selected? (e.g. purposive, convenience, consecutive, snowball) | Chapter 2 , The qualitative research , Participant selection |
| Method of approach | 11 | How were participants approached? (e.g. face-to-face, telephone, mail, e-mail) | Chapter 2 , The qualitative research , Participant selection ; Chapter 2 , The feasibility trial , Participants and eligibility criteria |
| Sample size | 12 | How many participants were in the study? | Chapter 2 , The qualitative research ; Chapter 6 |
| Non-participation | 13 | How many people refused to participate or dropped out? Reasons? | Appendix 9 |
| Setting | |||
| Setting of data collection | 14 | Where was the data collected? (e.g. home, clinic, workplace) | Chapter 2 , The qualitative research |
| Presence of non-participants | 15 | Was anyone else present besides the participants and researchers? | Chapter 2 , The qualitative research |
| Description of sample | 16 | What are the important characteristics of the sample? (e.g. demographic data, date) | Chapter 6 ; Appendix 19 |
| Data collection | |||
| Interview guide | 17 | Were questions, prompts, guides provided by the authors? Was it pilot tested? | Chapter 2 , The qualitative research , Data collection ; Appendices 5 – 7 |
| Repeat interviews | 18 | Were repeat interviews carried out? If yes, how many? | No |
| Audio/visual recording | 19 | Did the research use audio or visual recording to collect the data? | Chapter 2 , The qualitative research |
| Field notes | 20 | Were field notes made during and/or after the interview or focus group? | Chapter 2 , The qualitative research , Theoretical and thematic framework ; Chapter 2 , The qualitative research , Data analysis ; Appendix 4 |
| Duration | 21 | What was the duration of the interviews or focus group? | Chapter 2 , The qualitative research |
| Data saturation | 22 | Was data saturation discussed? | Chapter 2 , The qualitative research , Participant selection |
| Transcripts returned | 23 | Were transcripts returned to participants for comment and/or correction? | No |
| Domain 3: analysis and findings | |||
| Data analysis | |||
| Number of data coders | 24 | How many data coders coded the data? | Chapter 2 , The qualitative research , Data analysis ; Appendix 4 |
| Description of the coding tree | 25 | Did authors provide a description of the coding tree? | Chapter 6 ; Appendix 4 |
| Derivation of themes | 26 | Were themes identified in advance or derived from the data? | Chapter 2 , The qualitative research , Data analysis ; Appendix 4 |
| Software | 27 | What software, if applicable, was used to manage the data? | N/A |
| Participant checking | 28 | Did participants provide feedback on the findings? | No |
| Reporting | |||
| Quotations presented | 29 | Were participant quotations presented to illustrate the themes/findings? Was each quotation identified? (e.g. participant number) | Chapter 6 |
| Data and findings consistent | 30 | Was there consistency between the data presented and the findings? | Chapter 7 |
| Clarity of major themes | 31 | Were major themes clearly presented in the findings? | Chapter 6 ; Appendix 4 |
| Clarity of minor themes | 32 | Is there a description of diverse cases or discussion of minor themes? | Chapter 6 |
Appendix 9 Reasons given for drop-out
| ID | Type of attrition | Reason given | Treatment arm | Sessions completed |
|---|---|---|---|---|
| A1/048 | Lost to follow-up | Completed other measures but not PHQ-9 | Intervention | 9 |
| C7/006 | Withdrew from intervention and lost to follow-up | Physical health/lack of time | Intervention | 1 |
| C7/009 | Withdrew from intervention and lost to follow-up | Not enough time | Intervention | 2 |
| C1/040 | Withdrew consent | No time and personal/family reasons | Intervention | 0 |
| A1/027 | Lost to follow-up | Unknown | Intervention | 10 |
| B1/155 | Randomised in error | Intervention | 0 | |
| A4/009 | Lost to follow-up | Out of country caring for a relative so could not complete follow-up | Intervention | 8 |
| C7/014 | Withdrew from intervention and did not want to be followed up | Health deteriorated following suicide attempt. Inappropriate to follow-up | Intervention | 5 |
| A1/173 | Lost to follow-up | Too ill on day and could not contact again | Control | N/A |
| A1/123 | Investigator decision not to follow up at end of life | At end of life | Control | N/A |
Appendix 10 Data collected outside collection window and delays in randomisation
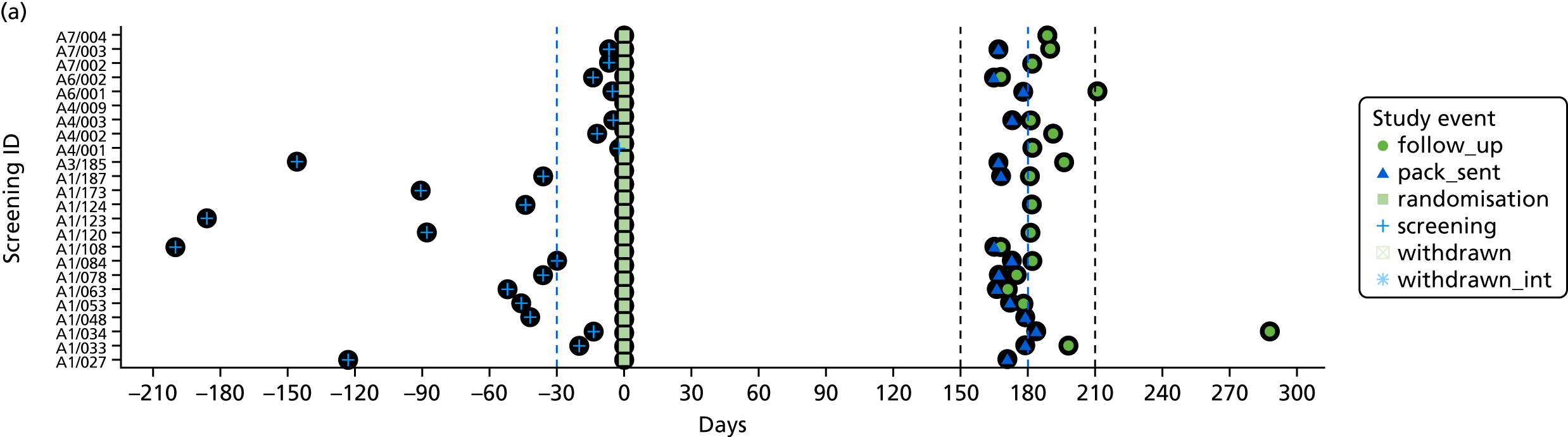
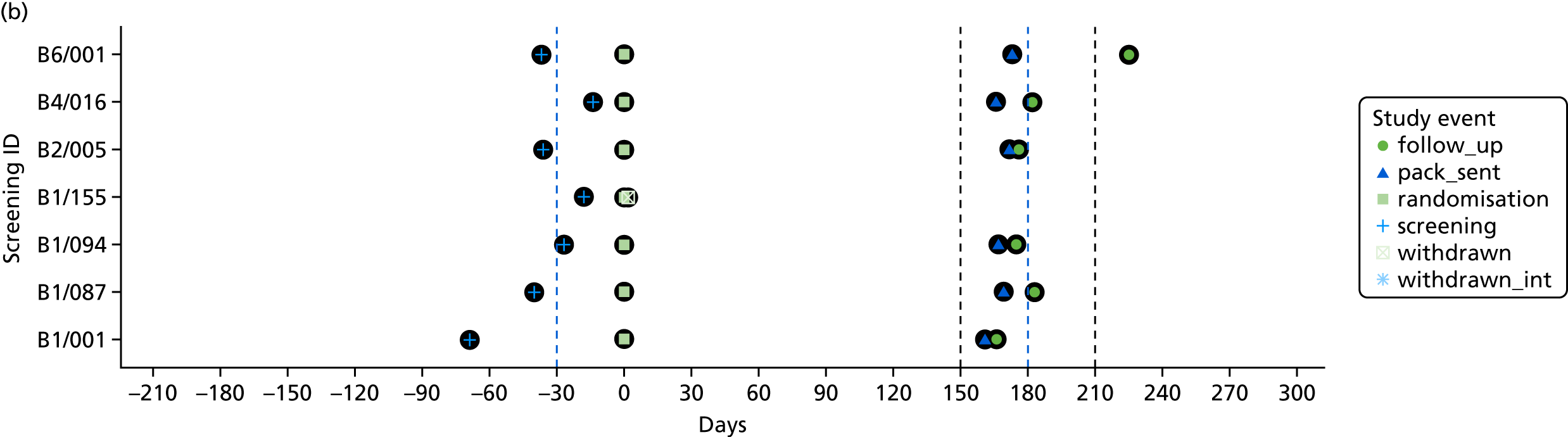
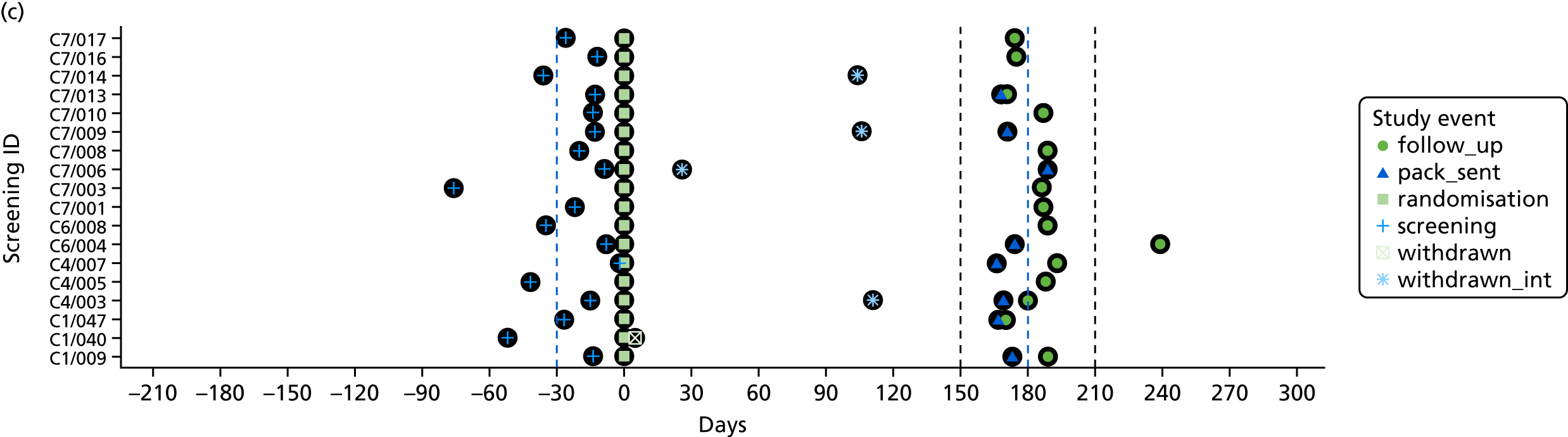
Appendix 11 Frequency of content of manual applicable to all sessions
| Content | Session, frequency of content, n (%) | Total, frequency of content, n (%) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 7 | 10 | ||
| Manual content | |||||||
| Summarise previous session | 4 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 5 (4.6) |
| Review between-session tasks | 2 (5.6) | 6 (22.2) | 1 (12.5) | 1 (7.7) | 1 (5.6) | 0 (0.0) | 8 (7.4) |
| Set agenda and goals for each session | 5 (13.9) | 1 (3.7) | 0 (0.0) | 1 (7.7) | 3 (16.7) | 0 (0.0) | 10 (9.3) |
| Agree between-session tasks for the next session | 3 (8.3) | 3 (11.1) | 0 (0.0) | 1 (7.7) | 5 (27.8) | 0 (0.0) | 12 (11.1) |
| Session summary | 2 (5.6) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.8) |
| Review events since previous session | 3 (8.3) | 6 (22.2) | 6 (75.0) | 7 (53.8) | 6 (33.3) | 1 (11.1) | 29 (26.9) |
| Other content | |||||||
| Social chat | 10 (27.8) | 10 (37.0) | 0 (0.0) | 1 (7.7) | 1 (5.6) | 8 (88.9) | 30 (27.8) |
| Information on organisation of sessions | 3 (8.3) | 1 (3.7) | 0 (0.0) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 6 (5.6) |
| Travel arrangements | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Preparing materials, tasks, etc. | 4 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 5 (4.6) |
| Check whether or not participant needs reminder of the next appointment | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Appendix 12 Frequency of content of manual applicable to specific sessions
| Session number | Session, frequency of content, n (%) | Total, frequency of content, n (%) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 7 | 10 | ||
| Session 2 | |||||||
| Identification of participant’s problems | 14 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 14 (19.2) |
| Agree therapy goals | 20 (58.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (27.4) |
| Session 3 | |||||||
| Discuss relationship between activity level and mood | 0 (0.0) | 6 (60) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (8.2) |
| Refer to behavioural model of depression | 0 (0.0) | 1 (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Introduce idea of identifying enjoyable activities | 0 (0.0) | 3 (30) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (4.1) |
| Session 4 | |||||||
| Review idea of identifying enjoyable activities | 0 (0.0) | 0 (0.0) | 3 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (4.1) |
| Discuss how enjoyable activities improve mood | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Identify enjoyable activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Create list of enjoyable activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Identify barriers to engaging in identified activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Check activities are in line with goals | 0 (0.0) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.7) |
| Activity scheduling plan | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Session 6 | |||||||
| Review whether or not activities were carried out as scheduled | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Identify one activity per day to be scheduled | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 4 (5.5) |
| Identify any potential obstacles to completing activities and address them | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (63.6) | 0 (0.0) | 0 (0.0) | 7 (9.6) |
| Session 7 | |||||||
| Review relationship between activity schedule and mood | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (60) | 0 (0.0) | 6 (8.2) |
| Identify common problems in planning or carrying out activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (40) | 0 (0.0) | 4 (5.5) |
| Session 10 | |||||||
| Review problems addressed during therapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Summarise successful strategies and skills used | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discuss generalisation of skills to future situations | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Questions and answers | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100) | 2 (2.7) |
| Remind participants about the 6-month follow-up protocol | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Appendix 13 Frequency of therapist and participant activities
| Activities | Session, frequency, n (%) | Total, frequency of content, n (%) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 7 | 10 | ||
| Therapist activities | |||||||
| Providing explanation | 9 (13.2) | 2 (3.2) | 2 (7.4) | 1 (5.9) | 6 (9.4) | 0 (0.0) | 20 (7.7) |
| Providing feedback | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Providing encouragement/reassurance | 3 (4.4) | 5 (8.1) | 2 (7.4) | 3 (17.6) | 3 (4.7) | 0 (0.0) | 16 (6.1) |
| Summarising | 5 (7.4) | 1 (1.6) | 2 (7.4) | 2 (11.8) | 3 (4.7) | 1 (4.3) | 14 (5.4) |
| Paraphrasing | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 3 (1.1) |
| Presenting/discussing BA strategies | 0 (0.0) | 9 (14.5) | 2 (7.4) | 0 (0.0) | 6 (9.4) | 0 (0.0) | 17 (6.5) |
| Address problems with task non-completion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Checking understanding | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.3) | 1 (0.4) |
| Giving opportunities for participant to ask questions | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Referring to between-session activities | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (0.4) |
| Referring to problems list | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Referring to participant goals | 2 (1.3) | 6 (9.6) | 1 (3.7) | 0 (0.0) | 2 (3.1) | 0 (0.0) | 11 (4.2) |
| Addressing appropriateness of activities | 1 (1.5) | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 5 (1.9) |
| Asking participant to rate enjoyment of chosen activities | 0 (0.0) | 0 (0.0) | 2 (7.4) | 2 (11.8) | 2 (3.1) | 2 (8.7) | 8 (3.1) |
| Asking participant questions | 4 (5.9) | 3 (4.8) | 1 (3.7) | 0 (0.0) | 5 (7.8) | 3 (13.0) | 16 (6.1) |
| Referring to previous sessions | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Participant activities | |||||||
| Discussing BA strategies | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 2 (0.8) |
| Discussing activities | 14 (20.6) | 12 (19.4) | 6 (22.2) | 0 (0.0) | 8 (12.5) | 8 (34.8) | 48 (18.4) |
| Asking for information | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Describing problems | 20 (12.7) | 18 (29.0) | 3 (11.1) | 5 (29.4) | 19 (29.7) | 1 (4.3) | 66 (25.3) |
| Asking questions | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Being presented with information about sessions, venue, group, etc. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Describing mood | 2 (1.3) | 3 (4.8) | 3 (11.1) | 1 (5.9) | 4 (6.3) | 1 (4.3) | 14 (5.4) |
| Feedback on home activities | 1 (1.5) | 0 (0.0) | 1 (3.7) | 2 (11.8) | 2 (3.1) | 1 (4.3) | 7 (2.7) |
| Feedback on sessions | 1 (1.5) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 4 (1.5) |
| Hospital visit discussion | 2 (2.9) | 0 (0.0) | 1 (3.7) | 1 (5.9) | 1 (1.6) | 1 (4.3) | 6 (2.3) |
| Speech and language therapy discussion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Appendix 14 Distribution of costs relevant to the NHS and Personal Social Services perspective (complete-case analysis)
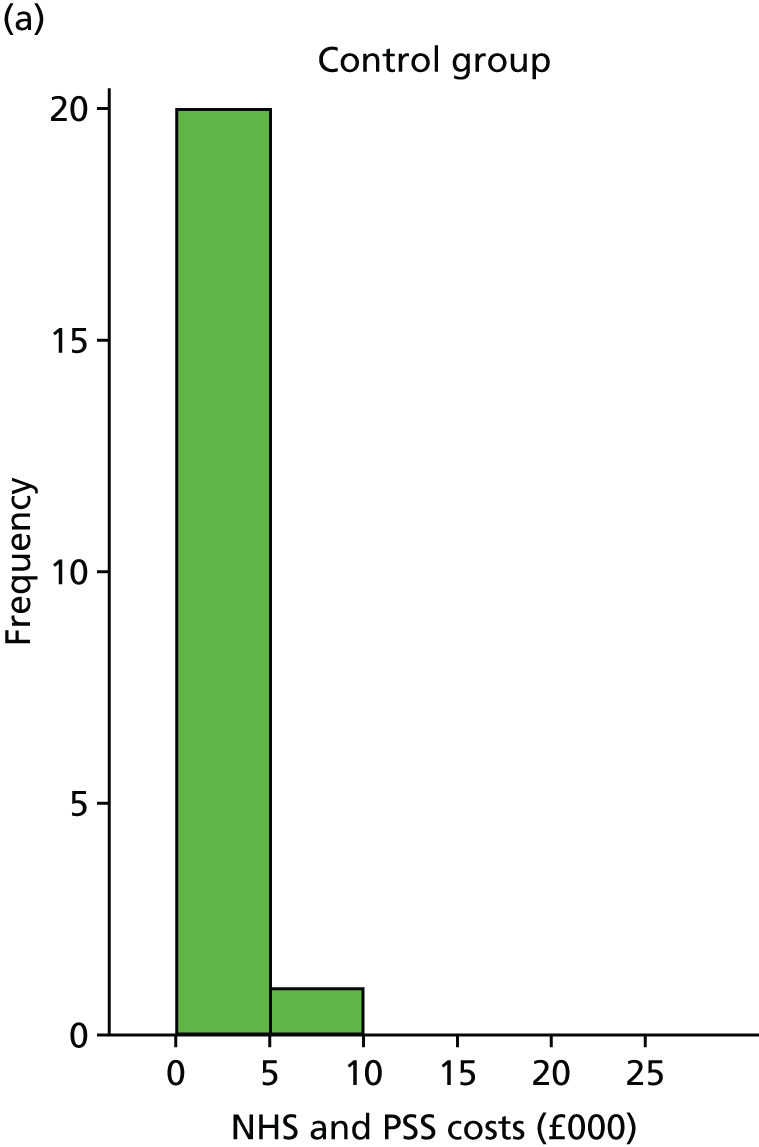

Appendix 15 Distribution of costs relevant to the societal perspective (complete-case analysis)
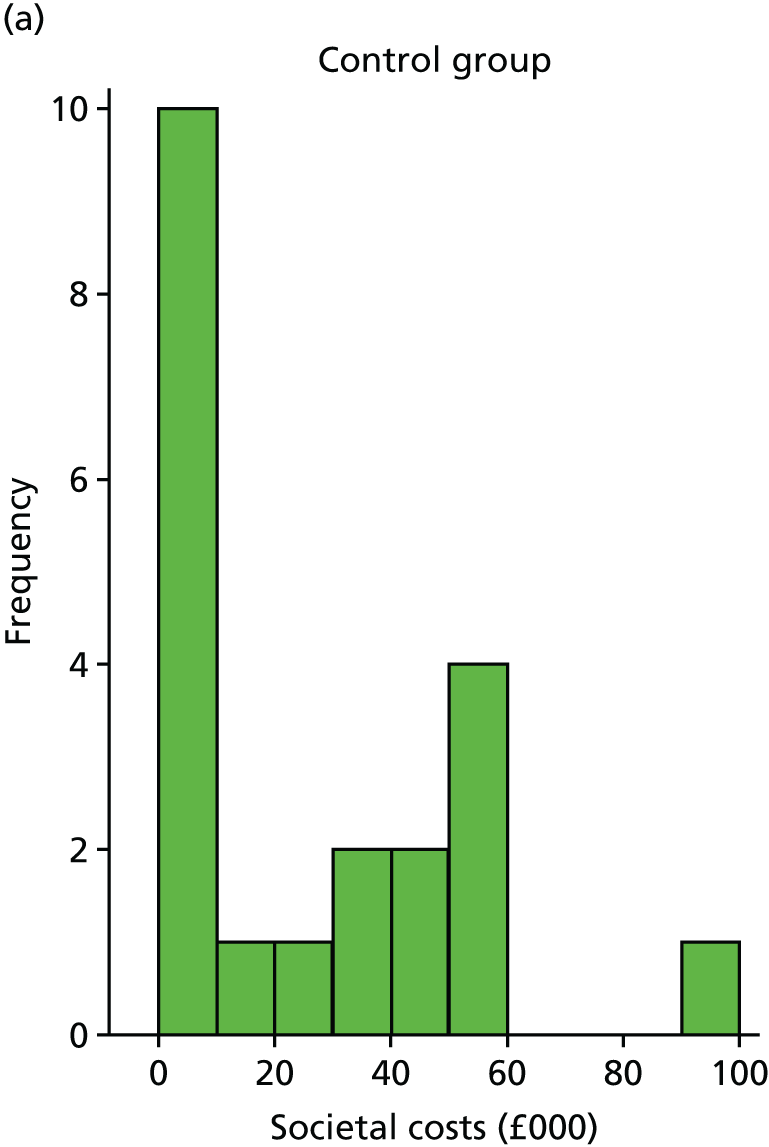
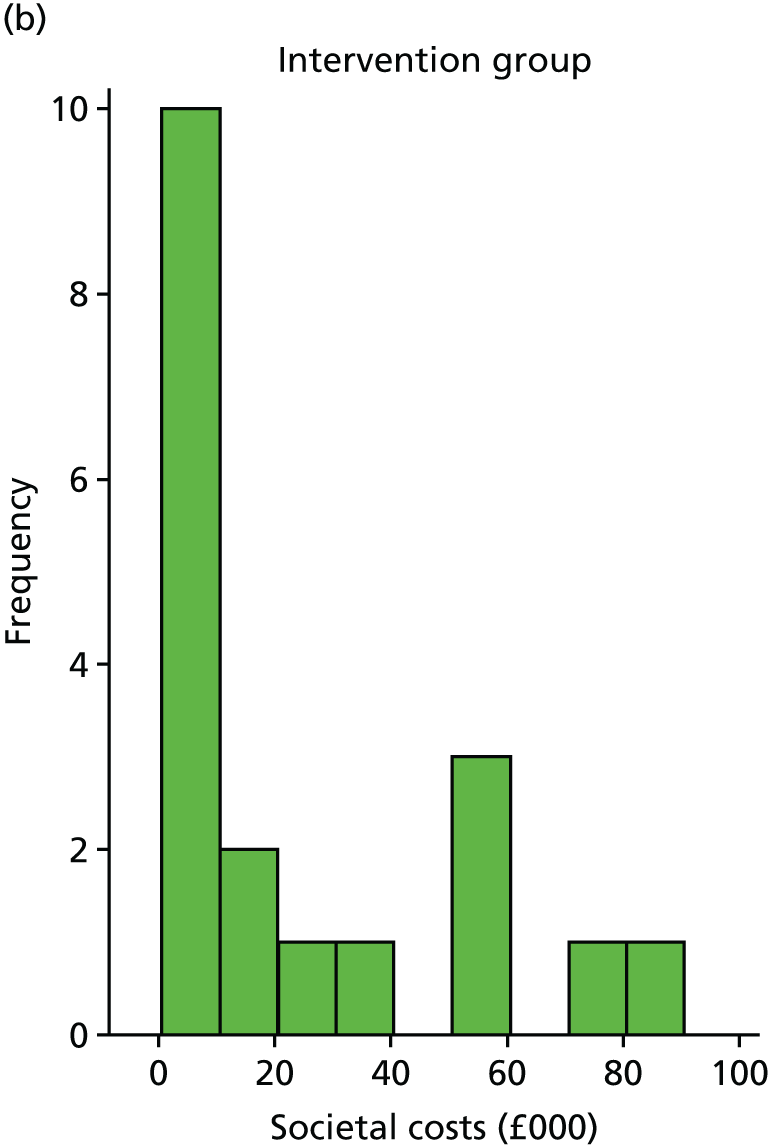
Appendix 16 Model parameters and distributions
| Parameter | Base-case value | Uncertainty distribution | 95% CI |
|---|---|---|---|
| EQ-5D-5L (utility) | |||
| Non-response | 0.52 | Beta(26.22, 24.17) | 0.38 to 0.66 |
| Response | 0.59 | Beta(39.77, 28.08) | 0.47 to 0.70 |
| Monthly costs (NHS and PSS perspective) | |||
| Non-response | £383.61 | Gamma(6.12, 62.68) | 142.52 to 741.94 |
| Response | £473.55 | Gamma(7.77, 60.97) | 201.48 to 860.37 |
| Monthly costs (societal perspective) | |||
| Non-response | £4229.19 | Gamma(18.68, 226.42) | 2534.04 to 6352.24 |
| Response | £2968.70 | Gamma(10.36, 286.67) | 1446.01 to 5032.93 |
| Probability of a good response | |||
| Non-response | 0.22 | Beta(5.00, 18.00) | 0.08 to 0.40 |
| Response | 0.68 | Beta(17.00, 8.00) | 0.49 to 0.84 |
| Other parameters | |||
| Number of BA sessions | 8.08 | Gamma(143.05, 0.06) | 7.34 to 10.05 |
Appendix 17 Change in EuroQoL-5 Dimensions, five-level version split by response (based on imputed data)
| Treatment arm | Number of participants (for whom utility data were missing) | Change in EQ-5D-5L from baseline | Standard error | 95% CI |
|---|---|---|---|---|
| Control (no response) | 18 (2) | 0.04 | 0.05 | –0.07 to 0.16 |
| Intervention (no response) | 8 (2) | –0.04 | 0.12 | –0.29 to 0.22 |
| Control (response) | 5 (1) | 0.23 | 0.18 | –0.16 to 0.62 |
| Intervention (response) | 17 (6) | 0.03 | 0.08 | –0.14 to 0.20 |
Appendix 18 Results from expected value of perfect partial information, excluding all costs except intervention costs
| Parameter | Per-person EVPPI (£) | Standard error | EVPPI for UK over 10 years (£) |
|---|---|---|---|
| Health costs (response) | 0.0 | 0.0 | 0 |
| Health costs (no response) | 0.0 | 0.0 | 0 |
| Societal costs (response) | 0.0 | 0.0 | 0 |
| Societal costs (no response) | 0.0 | 0.0 | 0 |
| Utility (response) | 0.0 | 0.05 | 384 |
| Utility (no response) | 0.0 | 0.0 | 0 |
| Probability of good response (intervention) | 0.02 | 0.03 | 6279 |
| Probability of good response (control) | 0.07 | 0.19 | 29,720 |
| Relapse rate | 34.23 | 4.4 | 14,040,000 |
| Number of BA sessions | 0.0 | 0.0 | 0 |
| Probability of death | 0.0 | 0.0 | 0 |
Appendix 19 Participant characteristics for qualitative feedback interviews
| Participant number | Characteristic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age range (years) | Sex | Lateralisation stroke | Weakness side | FAST score | VAMS ‘Sad’ score (points) | PHQ score (points) | Randomisation | Days since stroke | Carer’s age (years) | Carer’s sex | Carer’s relationship to patient | |
| A1/034 | 50–54 | Male | Right | Left | 26 | 97 | 21 | Intervention | 573 | |||
| A1/053 | 60–64 | Male | Right | Left | 30 | 47 | 10 | Control | 241 | |||
| A1/084 | 66–70 | Female | Left | Right | 21 | 24 | 11 | Intervention | 181 | 80 | Male | Partner |
| A1/124 | 80–84 | Male | Left | Right | 22 | 25 | 13 | Control | 239 | 73 | Female | Partner |
| A4/002 | 80–84 | Female | Left | Left | 27 | 22 | 11 | Control | 297 | 85 | Male | Partner |
| A6/001 | 85–99 | Female | Right | Left | 25 | 23 | 21 | Control | 1192 | 88 | Male | Partner |
| A6/002 | 50–54 | Male | Right | Left | 30 | 50 | 14 | Intervention | 998 | |||
| A7/004 | 70–74 | Female | Left | Right | 24 | 59 | 12 | Control | 489 | 72 | Male | Partner |
| B1/087 | 75–89 | Male | Unknown | Unknown | 27 | 73 | 14 | Intervention | 388 | 58 | Female | Partner |
| B6/001 | 40–44 | Female | Left | Right | 30 | 65 | 13 | Control | 950 | |||
| C1/009 | 65–69 | Male | Right | Left | 30 | 51 | 15 | Control | 1075 | Female | Partner | |
| C1/047 | 70–74 | Female | Right | Left | 26 | 49 | 17 | Control | 370 | 69 | Male | Partner |
| C4/005 | 60–64 | Male | Unknown | Unknown | 30 | 21 | 20 | Control | 199 | |||
| C7/001 | 50–54 | Male | Left | Right | 22 | 3 | 10 | Intervention | 199 | |||
| C7/013 | 65–69 | Male | Right | Left | 27 | 82 | 18 | Control | 207 | 68 | Female | Partner |
| C7/017 | 50–54 | Male | Left | Left | 26 | 47 | 21 | Intervention | 135 | 48 | Female | Partner |
List of abbreviations
- AE
- adverse event
- AfC
- Agenda for Change
- AP
- assistant psychologist
- BA
- behavioural activation
- BEADS
- Behavioural Activation Therapy for Depression after Stroke
- CALM
- Communication and Low Mood
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COREQ
- COnsolidated criteria for REporting Qualitative research
- CRF
- case report form
- CRN
- Clinical Research Network
- CSI
- Carer Strain Index
- CST
- Consent Support Tool
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- ETC
- excess treatment cost
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect partial information
- FAST
- Frenchay Aphasia Screening Test
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MoCA
- Montreal Cognitive Assessment
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NLQ
- Nottingham Leisure Questionnaire
- NRES
- National Research Ethics Service
- PhD
- Doctor of Philosophy
- PHQ-9
- Patient Health Questionnaire-9
- PI
- principal investigator
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SADQ
- Stroke Aphasic Depression Questionnaire
- SADQ-H
- Stroke Aphasic Depression Questionnaire – Hospital version
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SUR
- seemingly unrelated regression
- TSC
- Trial Steering Committee
- VAMS
- Visual Analogue Mood Scales
