Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/136/52. The contractual start date was in March 2015. The draft report began editorial review in October 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Catherine Hewitt is a member of the Health Technology Assessment Commissioning Board. Suzy Ker reports personal fees from the NHS outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Peckham et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Some of the information in this chapter is reported in Peckham et al. 1 This contains information licensed under the Non-Commercial Government Licence v2.0.
Smoking and severe mental ill health
People with severe mental ill health (SMI), such as schizophrenia and bipolar disorder, are more likely to smoke and to smoke more heavily than those without SMI. 2,3 Estimates of the percentage of people with SMI who smoke vary depending on the setting, with up to 70% of inpatients smoking. 4 The presence of mental ill health is associated with an elevated risk of smoking by a factor of odds ratio (OR) 2.2 [95% confidence interval (CI) 1.7 to 2.8]. 2 Smokers with SMI are more nicotine dependent, more likely to become medically ill with smoking-related diseases and less likely to access help in quitting than the general population. 5 People with SMI tend to:
-
begin smoking at a higher rate before diagnosis or treatment for SMI than smokers without SMI6,7
-
smoke each cigarette more intensely, extracting more nicotine per cigarette. 8
People with SMI are also more likely to be unemployed than the general population. Smoking is known to be part of the ‘culture’ of mental health services, among both staff and patients. In addition, people with SMI often lack self-esteem and see the future as ‘bleak’; as a consequence, they may not be motivated to look after their physical health. 9 Many people with SMI are also misinformed about the risks and benefits of smoking versus nicotine dependence treatment. 10 They often fear and overestimate the medical risks of nicotine replacement therapy (NRT). 11 Many believe that smoking relieves depression and anxiety12 (nicotine increases anxiety13).
Smoking contributes to the poor physical health of those with SMI. In the UK, the standardised mortality rate for all causes of death for people with schizophrenia was 289 (95% CI 247 to 337), amounting to a threefold increase in mortality compared with the population of England and Wales. 14 Although people with SMI are more likely to smoke than the general population, there is evidence that general practitioners (GPs) are less likely to intervene with smokers who have a mental disorder than those who do not have a mental disorder. 5 Although the number of people in the general population who smoke has declined over the last 20 years, the number of people with SMI who smoke has not seen a similar decline. 3 The smoking rate among people with SMI in England is 40.5%,15 which is more than double that of the general population. 16 Smoking rates in people with SMI vary across settings, with up to 70% of people in psychiatric units smoking. 4
It is within this context that a number of policy initiatives have emerged that emphasise improving the physical care of those with SMI, including taking initiatives to facilitate smoking cessation and the promotion of smoke-free environments in secondary care services. 2,17,18
Existing knowledge
Smokers most commonly cite ‘stress relief and enjoyment’ as their main ‘reason’ for smoking,19 although the major cause is nicotine dependence. Nicotine acts in the midbrain, creating impulses to smoke in the face of stimuli associated with smoking20 and producing what may be thought of as a kind of ‘nicotine hunger’ (a feeling of needing to smoke) when blood nicotine concentrations are depleted. Smokers also experience nicotine withdrawal symptoms, such as unpleasant mood swings and physical symptoms, that occur on abstinence and are relieved by smoking. 21 Nicotine dependence is the main reason that most unassisted quit attempts fail within a week. 22 Cochrane systematic reviews23–30 and guidance from the National Institute for Health and Care Excellence (NICE)31 highlight the following smoking cessation interventions (including medications used as smoking cessation aids) that help smokers reduce their tobacco intake and quit smoking.
Nicotine replacement therapy
At the time of the trial, there were seven different available forms of administering NRT for use as smoking cessation aids. These were the nicotine patch, gum, lozenge, inhaler, spray (oral or nasal) and sublingual tablet (microtab). These provide a ‘clean’ alternative source of nicotine without the other 4000 toxic chemicals found in cigarette smoke. All deliver a lower dose of nicotine than the dose that would be received through smoking, with the only difference being the differing absorption rates owing to different methods of delivery. Nicotine replacement products can be used singly or in combination, for example a patch combined with a product that delivers nicotine faster, such as the nasal spray. A meta-analysis of > 100 randomised controlled trials (RCTs) shows that any form of NRT is effective in terms of smoking cessation (risk ratio 1.55, 95% CI 1.49 to 1.61). 32 For those who are not ready to stop smoking but are interested in cutting down, NRT prescription has been shown to reduce smoking and to facilitate quit rates later on (reduce to stop, or cut down to quit). 33
Antidepressants and nicotine receptor agonists
Two non-nicotine pharmacotherapies have been licensed as smoking cessation aids. These are varenicline [Chantix® (USA) and Champix® (the European Union and other countries); Pfizer Inc., New York, NY, USA], a nicotinic acetylcholine receptor partial agonist, and bupropion (Zyban®; GlaxoSmithKline plc, Brentford UK), a noradenaline and dopamine reuptake inhibitor, which was first introduced as an atypical antidepressant. Varenicline is almost certainly the most effective treatment to date (OR for 12-month continuous abstinence for varenicline vs. placebo 3.22, 95% CI 2.43 to 4.27). It is more efficacious than bupropion (OR for varenicline vs. bupropion 1.66, 95% CI 1.28 to 2.16). 29 However, its use in people with SMI may be limited by case reports of depression or mental health worsening in populations with a previous history of mental health difficulties. Yet, a recent systematic review found there to be no increase in adverse effects in people with SMI taking varenicline compared with those without SMI,34 and a recent trial among people with a range of mental health problems showed varenicline as being effective and having a good safety profile. 35 Therefore, these fears appear to be unfounded.
Behavioural support
Advice, discussion and encouragement can be delivered via a range of means: individually or in a group, in an open (rolling) or closed group, face to face or over the telephone or internet. Meta-analyses of trials of multisession ‘intensive behavioural support versus brief advice’ found ORs of 1.56 (95% CI 1.32 to 1.84) for individual support and 2.04 (95% CI 1.60 to 2.60) for group support. 25,26 Regular support over the telephone is also effective. A meta-analysis of 10 trials of telephone support for people stopping smoking gave an OR of 1.64 (95% CI 1.41 to 1.92). 28 There is some evidence to suggest that group support may be more effective in general than one-to-one support36 and that it should involve multiple sessions. 36
The accumulated evidence for the use of current smoking cessation interventions has been distilled into clear recommendations for health professionals31 and a manual for those designing and delivering smoking cessation services. 37 In addition, guidance has been issued by NICE to guide the use of smoking cessation interventions for those with SMI. 38
Evidence on the effectiveness of smoking cessation strategies in SMI comes from a systematic review of randomised trials by Banham and Gilbody,39 which was updated in 2017. 40 These reviews draw on the results of 26 RCTs of smoking cessation interventions among those with SMI and show that combinations of behavioural support and pharmacotherapy (NRT and bupropion) are effective in facilitating smoking cessation. The evidence is strongest from varenicline, through the use of which the odds of quitting were improved fourfold (five trials; risk ratio 4.13, 95% CI 1.36 to 12.53).
Rationale for the SCIMITAR+ trial
Despite the higher prevalence of smoking, a substantial proportion of people with SMI express a desire to quit, but they expect to find it harder than those in the general population. 41 The introduction in 2004 of a new General Medical Services (GMS) contract42 created a policy impetus to improve the quality of primary care in priority areas. In terms of mental health, the new GMS contract specified that primary care is responsible for the provision of physical health care. Importantly, for smoking cessation initiatives, it ‘incentivised’ GPs to (1) produce a register of people with severe long-term mental health [Quality and Outcomes Framework (QOF) indicator MH8 – the SMI register] and (2) ensure that at least 90% of SMI patients have had a review that includes smoking status recorded within the previous 15 months (QOF indicator MH9 – SMI health check). This check included patients seen in primary care, secondary care and under shared care arrangements. Though this GMS target has now been withdrawn, this was an incentive at the time of conception of the SCIMITAR+ (smoking cessation intervention for severe mental ill health) trial.
In addition, for those who are admitted to hospital (including inpatient psychiatric units), the introduction of smoke-free policies provides an opportunity to address smoking (Public Health Guidance 4817). The admission of an individual to hospital, while being stressful and occurring at a time of personal crisis, also provides a unique opportunity to provide general health advice and to engage individuals in interventions targeted at smoking reduction and cessation.
Recent guidance issued by NICE17 offers clear statements of purpose to make secondary care services (including mental health services) entirely smoke free and to promote a smoke-free culture among staff and users of the services. Mental health services are highlighted as an area of priority and an unmet need in relation to smoking cessation, and there is clear guidance that services should be developed and implemented as a matter of some priority.
In 2009, the SCIMITAR pilot trial was devised to test whether or not a bespoke smoking cessation (BSC) intervention tailored to the needs of people with SMI would be acceptable to people with SMI. 43 In addition, it tested whether or not it was feasible to recruit and retain participants in a RCT to test this intervention. Recruitment to the pilot trial took place between May 2011 and May 2012. Results from the SCIMITAR pilot trial indicated that the intervention was acceptable to participants and that it was possible to recruit and retain participants in such a trial. 43 Although the trial was not powered to detect a difference between the bespoke intervention and usual care, the results indicated that there was a potential but non-statistically significant benefit of the bespoke intervention over usual care in terms of the proportion of self-reported quitters [12/33 (36%) vs. 8/35 (23%) adjusted OR 2.9, 95% CI 0.8 to 10.5]. Following this, the National Institute for Health Research Health Technology Assessment programme commissioned a fully powered RCT (SCIMITAR+) to explore the clinical effectiveness and cost-effectiveness of the SCIMITAR BSC intervention.
Research objectives44
-
To establish the clinical effectiveness of a BSC intervention compared with usual care for people with SMI.
-
To establish the cost-effectiveness of a BSC intervention for people with SMI.
Chapter 2 Methods
Some of the information in this chapter is reported in Peckham et al. 1 This contains information licensed under the Non-Commercial Government Licence v2.0.
Study design
This study was a pragmatic, two-arm, parallel-group RCT. The setting was in primary care and specialist mental health services within secondary care. Given that patients with SMI are a ‘hard-to-reach population’, a range of complementary strategies were used to try to identify and recruit eligible participants. A two-stage recruitment process was employed to check for eligibility, to check understanding of the study and to obtain consent. Participants were individually randomised to receive usual care or usual care plus a BSC service. Participants were followed up over the course of 12 months, with data collected at 6 and 12 months post randomisation. 44
Approvals obtained
Ethics approval was sought and granted on 15 March 2015 by Leeds East Research Ethic Committee (reference number 15/YH/0051). Approval was also obtained from the relevant research and development departments (see Appendix 1).
Trial sites
The study was conducted in 22 sites in England. Sites recruited throughout the duration of the study.
Participant eligibility
Inclusion criteria
To be eligible for inclusion in this study, participants needed to meet the following inclusion criteria:
-
aged ≥ 18 years
-
have SMI
-
be a smoker who expresses an interest in wanting to cut down on smoking (though not necessarily quitting)
-
smoke at least five cigarettes per day.
There is no agreed definition of SMI, so we adopted a pragmatic definition, that is, a documented diagnosis of schizophrenia or delusional/psychotic illness [International Classification of Diseases, Tenth Edition (ICD-10),45 F20.X and F22.X or Diagnostic and Statistical Manual of Mental Disorders (DSM)46 equivalent] or bipolar disorder (ICD F31.X or DSM equivalent). This SMI-inclusive diagnosis needed to have been made by specialist psychiatric services and to have been documented in either GP notes or psychiatric notes.
Exclusion criteria
People were ineligible if they met the following criteria:
-
currently pregnant or breastfeeding
-
with comorbid drug or alcohol problems (as ascertained by the GP or mental health worker)
-
non-English speakers
-
lacking capacity to participate in the trial (guided by the 2005 Mental Capacity Act 7147)
-
currently receiving advice from a smoking cessation advisor.
People with SMI who smoke while concurrently abusing substances may require additional medication or specialist advice, which was beyond the brief of the mental health smoking cessation practitioner (MH-SCP) and this trial. Similarly, smoking cessation in pregnancy also requires specialist knowledge. It was planned that any participant who became pregnant during the course of the trial would be fully withdrawn from the study and referred to local smoking cessation services specific to pregnancy.
Identifying participants
We used five methods to recruit participants.
Direct general practitioner referral or referral following database screening
General practitioners are encouraged to offer opportunistic advice and information about smoking cessation services to all people who smoke whenever they consult in primary care. GPs taking part in this study were provided with patient study information packs to give to people with SMI who were receptive to participating in the trial. GPs then completed and faxed a referral form and the person’s ‘consent to be contacted’ form to the SCIMITAR+ researchers who approached the person for recruitment.
The GP practices were also asked to consult their patient databases and SMI register, if available, to screen for potentially eligible participants. Information packs were sent from the GP practice, inviting people willing to take part in the study to return a completed ‘consent to be contacted’ form to the SCIMITAR+ researchers, who then approached them to ascertain eligibility and recruitment.
Primary care referral following annual health check
At the time of the trial, the annual primary care health check for people with SMI (MH9) represented an opportunity to address smoking behaviour and to offer enhanced smoking cessation services within the context of a trial. Health checks are generally conducted by practice nurses, and we encouraged all primary care staff to make SMI smokers aware of the trial when they received their annual primary care health check. Information packs were given to interested and potentially eligible people during their health check. Similar to GP referrals, practice nurses were instructed to complete referral forms and to fax the persons’ completed ‘consent to be contacted’ form to the SCIMITAR+ researchers, who then approached them for eligibility and recruitment.
Community mental health teams
Study researchers worked with care co-ordinators and members of the community mental health team (CMHT) to screen their caseloads for potentially eligible participants who matched the inclusion criteria. People identified as potentially suitable for the SCIMITAR+ trial were either provided with a copy of the information pack by their care co-ordinator or other mental health professional or sent an information pack in the post. The information pack contained a ‘consent to be contacted’ form for potential participants to return to the SCIMITAR+ researchers, giving permission for the researcher to contact them by telephone or letter or in person to discuss the trial further.
Service user groups44
Service user groups were provided with information about the study along with copies of a SCIMITAR+ flyer. Interested service users could then contact the mental health professional named on the flyer or their care co-ordinator. Alternatively, the staff member at the service user group could contact the service user’s care co-ordinator on their behalf.
Lifestyle Health and Wellbeing Survey
The Lifestyle Health and Wellbeing Cohort is a prospective study that forms a platform for interventional studies (‘Trials within cohorts’), co-ordinated by the University of York, recruiting adults with SMI aged ≥ 18 years from primary and secondary care. Participants in the Lifestyle Health and Wellbeing Survey complete a series of questions about their health and well-being, including questions about their smoking status. Cohort participants who reported smoking and were potentially interested in cutting down on or quitting smoking were invited to take part in the SCIMITAR+ trial.
Screening for eligibility
After receiving an information pack and once a person gave consent to be contacted (either verbally to the recruiting clinician or in writing to the SCIMTAR+ researchers), a SCIMITAR+ researcher approached them by telephone, face to face or by e-mail, depending on the person’s preference. After briefly explaining the trial, the researcher checked the person’s eligibility by asking about their smoking habits, specifically (1) whether or not they smoke; (2) if so, how much they smoke (they needed to smoke at least five cigarettes per day to be eligible); (3) if they would consider quitting or cutting down, with a view to quitting within the next 6 months; and (4) whether or not they were currently receiving advice from a smoking cessation advisor. These questions ensured that the person currently smoked but was contemplating quitting. The researcher also asked screening questions about pregnancy and breastfeeding, and drug and alcohol usage, which led to exclusion if present. The researcher then arranged a meeting at a mutually convenient time and venue.
Consenting participants
When a potential participant met with the SCIMITAR+ researcher, they were given the opportunity to clarify any points they did not understand and to ask any questions. A full explanation of the trial was given by the SCIMITAR+ researcher. It was emphasised that they may withdraw their consent to participate at any time without loss of treatment options that would otherwise be available to them. They were also informed that, by consenting, they agreed to their GP being informed of their participation in the trial. Written informed consent was then obtained, with both the participant and the researcher signing and dating the consent forms prior to collecting baseline data (see Appendix 2 for participant information sheets and consent forms).
Baseline assessment
Once a participant had consented to take part in the trial, they completed the baseline questionnaires. Height and weight measurements were completed to calculate the participant’s body mass index (BMI) and an exhaled breath carbon monoxide (CO) reading was taken. These made up the baseline data set. The participant was randomised on completion of this data set.
Randomisation
Eligible, consenting individuals were randomised 1 : 1 to either the BSC service or usual care. The researcher contacted a secure telephone randomisation service run by the York Trials Unit. Simple randomisation was used, following a computer-generated number sequence devised by an independent data manager otherwise not involved in the conduct of the trial. Once given the details of the participant’s allocation, the researcher immediately informed the participant of their allocation and what would happen next. If allocated to the BSC arm, researchers at the University of York then contacted the MH-SCP in the participant’s area and organised for them to contact the participant to arrange a mutually convenient time to meet. A letter was sent to the GP and mental health specialist for the participant’s records and to advise them on subsequent smoking cessation management. Owing to the nature of the intervention, it was not possible to blind participants, GPs, researchers or the MH-SCPs to the treatment allocation.
Ineligible and non-consenting individuals
All ineligible and non-consenting persons were referred back to their GP/mental health service.
Sample size
Results from the SCIMITAR pilot trial were used to inform the sample size calculation for this trial. In the pilot trial, a quit rate of 23% was observed at 12 months in the usual-care arm and a quit rate of 36% was observed in the BSC arm. 43 The SCIMITAR+ trial was powered at 80% to detect a relative risk increase in quitting of 1.7, assuming a control quit rate of 20% (an increase to a quit rate of 34% in the intervention group), equal randomisation and a two-sided alpha of 0.05. Allowing for a 20% loss to follow-up at 12 months required a total sample size of at least 393 to be recruited and randomised. We therefore proposed to recruit 400 participants in total to ensure sufficient power according to the preceding statistical assumptions.
Description of interventions
Trial intervention
Participants in the intervention group were allocated to receive a BSC intervention designed specifically to help people with SMI stop smoking. This intervention was delivered by a mental health professional (care co-ordinator, support worker, mental health nurse) trained in smoking cessation interventions. The training took place over 2 days and was provided by trained smoking cessation advisors. The BSC intervention consisted of up to 12 one-to-one face-to-face meetings between the participant and the MH-SCP, who worked in conjunction with the participant and their GP or mental health specialist to ensure that the participant received smoking cessation medication and medication monitoring. The initial meeting generally took 1 hour, with subsequent meetings lasting 30 minutes. Meetings were initially weekly; however, towards the end of the programme the participant had the choice of meeting fortnightly if preferred. Pharmacotherapies were provided for as long as was deemed necessary, in line with NICE guidance,17 and were determined by the GP without the influence of the SCIMITAR+ trial team. In line with NICE recommendations, MH-SCPs offered advice on the range of treatment options available to people in the NHS (including medication, behavioural support and follow-up). 31 It was not the remit of the trial to assess specific smoking cessation pharmacotherapies or treatments per se, although data on frequency of their use were collected. The intervention was based on that used by the National Centre for Smoking Cessation and Training (NCSCT), but the following specific modifications were applied to tailor the intervention to people with SMI: (1) recognising the need for several assessment sessions prior to setting a ‘quit date’; (2) recognising the purpose of smoking in the context of their mental illness, such as the use of smoking to relieve side effects from antipsychotic medication (and how this will be managed during a cessation attempt); (3) recognising the need to involve other members of the multidisciplinary team in planning a successful quit attempt for those with complex care needs and multiagency programmes of care; (4) arranging meetings so that they take place in a mutually agreeable location, often in the participant’s home rather than in the GP surgery or on NHS trust premises; (5) providing additional face-to-face support following an unsuccessful quit attempt or relapse; and (6) informing the GP and psychiatrist of a successful quit attempt so that they can review antipsychotic medication doses in line with changes in metabolism. Participants were encouraged to (1) reduce smoking to quit, (2) set their own quit dates and (3) make several attempts to quit if their initial attempt failed. All participants remained under the care of their GP and continued to receive their usual NHS treatment.
A detailed description of the development and content of the smoking cessation intervention is given in the report of the SCIMITAR pilot trial. 1
Control intervention
This was a ‘usual-care’ control group, whereby participants were encouraged to consult with their GP or local NHS quit smoking services. GPs were given advice to follow current NICE guidelines for smoking cessation, without the additional support of a bespoke MH-SCP. Usual care could include pharmacotherapies to aid smoking cessation (NRTs, bupropion or varenicline either separately or in combination), access to self-help materials and referral to local NHS stop smoking clinics (which would not be specifically tailored to the needs of those with SMI). Participants were encouraged to reduce smoking to quit and set their own quit dates; however, they were managed solely by their own GP or mental health specialist and, crucially, did not receive regular visits from a MH-SCP. Details of NRT that control participants received were gathered by accessing participants’ GP notes, and details of any smoking cessation management were requested from participants in the follow-up questionnaires.
Smoking cessation medication provision
Clear guidance on the prescription of anti-smoking medications in the presence of SMI (including safety considerations) has been published and was made available to all GPs to help inform their prescribing decisions (for both control and intervention participants). 48 A key feature of the SCIMITAR+ trial was to ensure that GPs manage anti-smoking medications within this framework and in accordance with their prior knowledge of the patient and their concomitant use of medication. This was carried out with the aim of replicating ‘real-life practice’ of the use of anti-smoking medications in primary care. The medication profile of the individual participants was reviewed by their GP or mental health specialist to assess any potential safety issues (in line with the latest practice guidance on the provision of smoking cessation interventions in the NHS2). An important aspect of the design of this study was that the SCIMITAR+ team had no direct influence over prescribing decisions by GPs, as this was not a drug trial or an investigation of a medicinal product(s).
Follow-up
Participants were followed up 6 months and 12 months after randomisation.
All assessments were carried out face to face where possible and, where not possible, a systematic approach was used to explore other avenues to collect self-report data; the participant was offered the option of completing the questionnaires over the telephone or having the questionnaires sent by post for them to complete and return. If it was not possible to make contact with the participant, a family member or friend, designated by the participant at their baseline interview, was contacted to verify the participant’s current smoking status.
Outcomes
Primary outcomes
The primary outcome was self-reported abstinence from smoking at 12 months post randomisation, defined as answering ‘not even a puff’ to the question ‘have you smoked in the last week?’, validated by CO breath measure, with abstinence defined as a CO measurement of < 10 parts per million (p.p.m.).
Secondary outcomes
-
Self-reported smoking cessation.
-
Number of cigarettes smoked per day (self-report).
-
Fagerström Test for Nicotine Dependence (FTND). 49
-
Motivation to Quit questionnaire (MTQ). 50
-
Patient Health Questionnaire-9 items (PHQ-9). 51
-
Generalised Anxiety Disorder-7 items (GAD-7). 52
-
Health-related quality of life [Short Form questionnaire-12 items (SF-12) version 1 was used, as this was used in the pilot trial]. 53
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 54
-
BMI.
-
Health service use collected via a bespoke questionnaire.
Table 1 gives details of the measures collected and the time points at which they were collected.
| Assessment | Time point | ||
|---|---|---|---|
| Baseline | 6 months post randomisation | 12 months post randomisation | |
| Eligibility and consent | |||
| Eligibility | ✗ | ||
| Consent | ✗ | ||
| Background and follow-up | |||
| Personal details, general health | ✗ | ||
| BMI | ✗ | ✗ | ✗ |
| Mental health details | |||
| Mental health history | ✗ | ||
| Current mental health status | ✗ | ✗ | ✗ |
| Current medications | ✗ | ✗ | ✗ |
| Referrals to mental health services | ✗ | ✗ | |
| Admissions to hospital related to mental health | ✗ | ✗ | |
| Smoking details | |||
| Smoking history | ✗ | ||
| Current smoking status | ✗ | ✗ | ✗ |
| Use of electronic cigarettes | ✗ | ✗ | ✗ |
| Use of smoking cessation services | ✗ | ✗ | ✗ |
| CO measurement | ✗ | ✗ | ✗ |
| Adverse event reporting | Ongoing collection | ||
| Questionnaires | |||
| FTND | ✗ | ✗ | ✗ |
| MTQ | ✗ | ✗ | ✗ |
| PHQ-9 | ✗ | ✗ | ✗ |
| GAD-7 | ✗ | ✗ | ✗ |
| Health-related quality of life (SF-12) | ✗ | ✗ | ✗ |
| Health-state utility (EQ-5D-5L) | ✗ | ✗ | ✗ |
| Health economics/service utilisation questionnaire | ✗ | ✗ | ✗ |
Withdrawal
A study participant could be withdrawn from the trial by their GP, mental health specialist or smoking cessation practitioner, or may choose to do so themselves, at any time. If the withdrawal was due to an adverse event, procedures followed a trial-specific standard operating procedure (SOP) for adverse events (see Appendix 3).
Where possible, data were collected on the nature of the withdrawal. Reasons for a practitioner to withdraw a participant included pregnancy, admission to hospital for reasons unrelated to the trial and, inability to attend treatment or assessment sessions. Relapse to resuming smoking was not a reason to withdraw a participant, as they could resume treatment and make several attempts to quit smoking.
Participants were given a choice of (1) withdrawal from treatment only (participants were still followed up at 6 and 12 months), (2) withdrawal from follow-up or (3) complete withdrawal from the study, including follow-up and medication data collection. Withdrawal from the study did not affect the participants’ treatment or access to NHS services.
Any data collected from the participant prior to withdrawal were still included in the final analysis of the data.
Participant concordance
Mental health smoking cessation practitioners were asked to complete a treatment log that recorded all participants’ contacts (face-to-face meetings, telephone calls and joint appointments with MH-SCPs and GPs) to judge the degree to which the intervention, as designed on a session-by-session basis, was actually delivered in practice by MH-SCPs.
At each meeting, MH-SCPs would take a CO measurement from the participant. Measurements of ≥ 10 p.p.m. indicated that the participant had not ceased smoking. If the participant claimed to have stopped but their CO readings were ≥ 10 p.p.m., they were probed about when they last smoked and whether or not they had had any minor relapses during their quit attempt.
Statistical analysis
All analyses were conducted following the principles of intention to treat, including all randomised participants in the groups to which they were randomised, where data were available. Analyses were conducted in Stata® version 15 (StataCorp LP, College Station, TX, USA). All statistical tests were two-sided at the 5% significance level.
Recruitment and retention
The flow of participants through the trial is described in Consolidated Standards of Reporting Trials (CONSORT) flow diagrams (see Figures 3–5), two of which describe recruitment up to randomisation (one for primary care and one for secondary care) and one of which presents the flow of participants from randomisation to analysis. The response rates, time to return and mode of completion of the 6- and 12-month participant questionnaires are summarised by treatment group.
Baseline data
Baseline data are summarised by treatment group, using mean standard deviation (SD), median and range for continuous data, and count and percentage for categorical variables. No formal statistical comparisons between the two groups were undertaken on baseline data.
Data manipulations and questionnaire scoring
Number of cigarettes smoked
Participants were asked how many cigarettes they normally smoked per day and how much tobacco they used per day. It was assumed that 0.5 g of tobacco equates to one cigarette. 55
Fagerström Test for Nicotine Dependence
The FTND is a 6-item questionnaire. 49 Item scores are added together to give a total score between 1 and 10: a score of 1–2 indicates low dependence, 3–4 indicates low to moderate dependence, 5–7 indicates moderate dependence and 8–10 indicates high dependence.
Motivation to Quit questionnaire
The MTQ is a 4-item questionnaire scored from 4 to 19, by adding together the responses to each item, with higher scores indicating a greater motivation to stop smoking. 50
Patient Health Questionnaire-9 items
The PHQ-9 is a 9-item instrument. It asks the subject to indicate how often in the last 2 weeks they have been bothered by nine problems, which are each scored on the scale of 0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day. 51 A total score is obtained from adding together the nine item scores. Scores can be categorised as follows: 0–4 indicates minimal depression, 5–9 indicates mild depression, 10–14 indicates moderate depression, 15–19 indicates moderately severe depression and 20–27 indicates severe depression. If one or two values are missing from the score, then they can be substituted with the average score of the non-missing items (scored pro rata and total score rounded to nearest integer). Questionnaires with more than two missing values are disregarded.
Generalised Anxiety Disorder Assessment-7 items
The GAD-7 is a 7-item instrument. 52 For each item, scores of 0, 1, 2 and 3 are given to the possible responses of ‘not at all’, ‘several days’, ‘more than half the days’ and ‘nearly every day’, respectively. The GAD-7 total score ranges from 0 to 21. Scores of 5, 10 and 15 represent cut-off points for mild, moderate and severe anxiety, respectively. If one or two values are missing from the score, then they can be substituted with the average score of the non-missing items (scored pro rata and total score rounded to nearest integer). Questionnaires with more than two missing values are disregarded.
Short Form questionnaire-12 items (version 1)
The SF-12 consists of two subscales – a physical component and a mental component – both of which are scored within the range of 0–100, with 0 indicating the lowest level of health and 100 indicating the highest level of health measured by the scale. Scoring was conducted in accordance with the SF-12 scoring manual. 53
Body mass index
A participant’s weight was measured in kilograms at each follow-up visit and their height was measured in metres at the baseline visit. BMI was calculated by dividing weight by height squared and is expressed in the units kg/m2.
Primary analysis
The primary outcome of CO-verified smoking cessation at 12 months was intended to be analysed via a mixed-effect logistic regression model to compare the BSC service intervention with usual care. The model was to include CO-verified smoking status at 6 and 12 months (repeated measures within participants) and be adjusted for baseline smoking severity (self-reported number of cigarettes smoked per day), time, treatment group and a treatment group-by-time interaction, with site as a random effect. Participants nested within site were also to be treated as a random effect to account for the repeated measures within the subjects. However, this model failed to converge. Therefore, separate multilevel logistic regression models were run to compare the outcomes at 6 and 12 months, that is, two models, instead of one model that incorporates the repeated measurements. The two models were each adjusted for baseline smoking severity, with site as a random effect. The OR, corresponding two-sided 95% CI and p-value for the treatment effect at 6 and 12 months are presented. The treatment effect at 12 months serves as the primary end point, and the effect at 6 months serves as a secondary end point. Participants were analysed according to their original random group allocation and were included in the models if they provided a CO reading and a response to the self-reported question relating to amount smoked in the previous week at that particular time point.
Sensitivity analyses
Unadjusted odds ratio
The unadjusted, marginal OR for smoking status at 6 and 12 months is presented for comparability.
Post hoc generalised estimating equations analysis
Given that the prespecified repeated measures mixed logistic regression model did not converge, post hoc sensitivity analyses were conducted using generalised estimating equation (GEE) techniques. This allowed for the repeated measures within participants to be accounted for, but the multilevel nature of the data (i.e. participants within site) cannot be controlled in the same way that including a random effect for site in the mixed logistic regression can be. Two GEE models were run: (1) one controlling for baseline smoking severity, and including an interaction between allocation and time, and (2) one controlling for both baseline smoking severity and site as covariates, and including an interaction between allocation and time. Both specified a binomial family and logit link, with an exchangeable correlation structure.
Accounting for missing carbon monoxide-verified smoking status
Self-reported smoking status was considered when CO-verified smoking status was missing. Further sensitivity analyses were conducted treating participants who still had missing outcome data in the following two ways: one treated participants with missing data as smokers under a worst case scenario, and the other used multiple imputation techniques. Twenty imputations were generated by multiple imputation using chained equations with a burn-in of 10. To avoid bias, the imputation model used all variables that were included in the analysis models (baseline smoking severity, CO-verified smoking status at 6 and 12 months, site and allocation). 56
Secondary analyses
All data collected at 6 and 12 months are summarised descriptively by treatment group, including an investigation into the number of missing data.
Self-reported smoking cessation
The primary analysis was repeated using self-reported smoking status at 6 and 12 months as the outcome (cessation defined as answering ‘not even a puff’ to the question ‘have you smoked in the last week?’).
Number of cigarettes smoked per day
The number of cigarettes smoked per day, as reported as part of the FTND, at 6 and 12 months was compared between the two groups using a mixed-effect negative binomial regression model, adjusting for the same covariates as the primary analysis and the repeated measures within participants. Incidence rate ratios and their associated 95% CIs and p-values are provided.
Continuous secondary outcomes: Fagerström Test for Nicotine Dependence, Motivation to Quit questionnaire, Patient Health Questionnaire-9 items, Generalised Anxiety Disorder Assessment-7 items, Short Form questionnaire-12 items and body mass index
The FTND, MTQ, PHQ-9, GAD-7, SF-12 physical component and SF-12 mental component scores and BMI were all analysed in the same way. Scores were compared between treatment groups using a covariance pattern linear mixed model. The outcome modelled was the total score at 6 and 12 months. Each model included baseline score, baseline smoking severity, treatment group, time and a treatment group-by-time interaction term as fixed effects, and site as a random effect.
Different covariance structures for the repeated measurements that are available as part of Stata were explored and the most appropriate pattern was used for the final model, based on the one that produced the smallest Akaike information criterion (AIC). Model assumptions were checked via a Q–Q plot to assess the normality of the standardised residuals and a scatterplot of the residuals against fitted values.
Predicted means for each group and the adjusted mean difference (with 95% CI and p-value) between treatment groups at 6 and 12 months are given.
Other outcomes
Self-reported number of attempts to quit, periods of cessation and e-cigarette use will be summarised descriptively by treatment group and time point.
Compliance with the intervention
Treatment session data are summarised for participants in the intervention group, including the number of sessions attended, and the duration and location (e.g. participant’s home) of the sessions. A complier-average causal effect (CACE) analysis for the primary outcome (CO-verified smoking status at 12 months post randomisation) was conducted to obtain an unbiased estimate of the intervention effectiveness in the presence of full compliance. A two-stage least squares instrumental variable approach, with randomised group as the instrumental variable, was used. We defined compliance with the intervention for those allocated to BSC as the number of sessions attended.
Health economic analysis
The health economic analysis for the SCIMITAR+ trial assessed the cost-effectiveness of supplementing usual care with BSC, compared with usual care alone, within the trial. Although the impact of smoking cessation is likely to be observed in the long term, the within-trial evaluation could provide a relatively realistic estimate of intervention costs, necessary parameter inputs for model projection and potentially some insights into the difference in change, in the case of lacking in capacity of a long-term follow-up. The economic evaluation took the form of an incremental cost-effectiveness analysis from a NHS and Personal Social Services perspective, as recommended by NICE guidance. 57
The costs of BSC and usual care were identified, measured and valued. Costs and outcomes were collected at participant level and based on the trial population. Health outcomes were measured using quality-adjusted life-years (QALYs), as per NICE guidance. 57 QALYs were constructed from the EQ-5D-5L,54 which was collected at baseline and during follow-up.
Costs
The total costs encompassed intervention costs and health-care and social services use costs. A wider range of societal costs were collected but analysed separately.
Intervention costs
The intervention costs were identified by two stages corresponding to the timeline of the trial, which were the pre-intervention stage and the intervention stage.
Training and supervision
The pre-intervention stage is the training period for MH-SCPs. To deliver the intervention, four members of the SCIMITAR+ team attended a 2-day training session delivered by the NCSCT, and then acted as trainers for all MH-SCPs. The MH-SCPs received a 2-day training session, in line with that provided by the NCSCT, with specific adaptations for people with SMI. This was delivered by the four trained members of the SCIMITAR+ team, two at a time. The cost of the initial training from the NCSCT was estimated using the invoice. The opportunity cost of time spent on the initial training by our research team and the cost of delivering the training for MH-SCPs were costed at NHS band 6 instead of researchers’ wages to reflect the costs to the NHS. The opportunity costs of time spent on these training sessions by the MH-SCPs were also estimated for 2 working days for NHS band 4 staff. A full day was considered 7.5 working hours. The staff cost included salary oncosts, overheads and capital.
Each practitioner was given a 43-page SCIMITAR+ Standard Treatment Protocol and a 51-page NCSCT Standard Treatment Programme. These were printed in-house at £0.02 per page.
After training, all MH-SCPs received regular supervision, which was delivered by the trainer of the additional training. Supervision time was recorded by the supervisor and allocated to each participant in the BSC group.
Intervention delivery
The intervention stage is the period during which the intervention/control treatment was delivered. Costs were estimated for both the BSC group and the usual-care group accordingly.
For the intervention group, costs included costs of BSC and usual GP care. BSC was costed based on the working time and caseload of MH-SCPs, including travel time to the prearranged location for the appointment. These sessions were recorded in treatment logs by MH-SCPs. Each practitioner was equipped with a CO monitor, which cost £120 and had a life span of 5 years. The depreciation value of the CO monitor in the first year was calculated using double-declining balance to estimate the cost of the CO monitor during the trial period.
Usual GP care included participants’ contact with usual-care practitioners [e.g. GPs, pharmacists, stop smoking services (SSSs), etc.] for smoking cessation and prescriptions of pharmacotherapies for smoking cessation. Usual-care contact was collected through self-reported questionnaires, and prescriptions were extracted from their medical records. The overall cost of contact with practitioners was then calculated by multiplying the number of contact sessions by their national average unit costs (see Table 2). The prescriptions were matched by their generic names, dosage and form to the Prescription Cost Analysis – England,58 and their costs were calculated by multiplying the weighted average net ingredient cost (NIC) per unit by the recorded quantities used within the trial period. In the case of missing values on dosage or form, a weighted average matching available information was used for estimation. Some medications were recorded by prescription instead of quantity; these were consequently estimated based on the weighted average NIC per prescription item.
Health-care and social services costs
Health-care and social service resources utilised by participants outside the trial were measured by an adapted Health Economic/Service Utilisation Questionnaire. These included mainly secondary care and community-based services.
This questionnaire was part of the questionnaire for baseline, 6- and 12-month follow-up and collected the quantity of resources utilised by participants, covering the usage in the 6 months before each time point. After the physical unit of resources was identified by the questionnaire, their quantities were multiplied by their national average unit costs extracted from Unit Costs of Health and Social Care 2017,59 NHS Reference Costs 2016–1760 or inflated from Unit Costs of Health and Social Care 201561 if recent data were unavailable (Table 2).
| Service items/products | Unit cost (2016/17) | Sources |
|---|---|---|
| Usual GP care for smoking cessation | ||
| Cessation consultation with GP | £40/session | 61 |
| Cessation consultation with pharmacist | £7/session | 61 |
| SSS | £19/session | 61,62 |
| SSS helpline | £7/call | 61,63 |
| Health care and social services | ||
| A&E department | £175/attendance | 60 |
| Hospital admission | £604/night; £1938/FCE | 60 |
| Hospital outpatient appointment | £138/attendance | 60 |
| Day case/procedure | £736/episode | 60 |
| Emergency ambulance | £99/use | 60 |
| GP home visit | £56/9.22-minute consultation plus 12 minutes of travel | 59,61 |
| GP surgery | £31/9.22-minute consultation | 59 |
| GP telephone | £15/call | 59 |
| Practice nurse | £9/15.5-minute consultation | 59,61 |
| District nurse | £36/contact | 60 |
| Community psychiatric nurse | £61/contact | 60 |
| Health visitor | £64/contact | 60 |
| Clinical psychologist | £40/45-minute session | 59 |
| NHS counsellor | £39/55-minute session | 59 |
| NHS dentist | £138/contact | 60 |
| Podiatrist | £44/contact | 60 |
| Occupational therapist | £77/contact | 60 |
| Physiotherapist | £53/contact | 60 |
| CBT | £100/session | 59 |
| MBCT | £15/session | 59 |
| Crisis team | £192/team contact | 59 |
| CMHT | £197/contact | 59 |
| Day care service | £92/patient day | 60 |
| Social worker | £30/30-minute visit | 59 |
| Family support worker | £27/30-minute visit | 59 |
| Drug/alcohol support worker | £118/contact | 60 |
| NRT products | ||
| Nasal spray | £23/bottle | Estimated market price |
| Microtab | £16/pack | Estimated market price |
| Patch | £13/7-piece pack | Estimated market price |
| Gum | £13/96-piece pack | Estimated market price |
| Lozenge | £13/96-piece pack | Estimated market price |
| Mouth spray | £19/bottle | Estimated market price |
| Inhalator | £21/20 cartridges | Estimated market price |
All costs were presented in 2016/17 Great British pounds. Discounting was not required in this study, as the evaluation was conducted within a 12-month time frame.
The use of antipsychotics was extracted from participants’ medical notes. As with prescription of pharmacotherapies, they were matched by their generic names, dosage and form to the Prescription Cost Analysis – England. 58
The costs of health-care and social services use in the 6 months before baseline were used as one of the baseline covariates for costs.
Wider societal costs
Costs incurred outside the NHS perspective were collected in the questionnaire by self-report and added to the base-case analysis to present a separate analysis from a societal perspective. These included out-of-pocket purchases of NRT products and e-cigarettes, and travel costs for the participants to attend the smoking cessation appointments. As the market pricing of NRT products is highly complicated because of deals, bundles or packaging, a set of estimated market prices was summarised, based on prices from big supermarkets and pharmacies, such as Sainsbury’s (www.sainsburys.co.uk), Morrison’s (www.groceries.morrisons.com), Boots (www.boots.com) and Lloyds Pharmacy (www.lloydspharmacy.com). It was further simplified by using only one package size for patch, gum, lozenge and inhalator, as they were available in various package sizes (see Table 2).
Health-related quality of life
The EQ-5D-5L was used to measure health-related quality of life in both the BSC group and the usual-care group. The instrument was used at baseline and at 6- and 12-month follow-up. An index score was calculated using the mapping function developed by van Hout et al. 64 The index score ranges from –0.594 to 1, with higher scores indicating better health-related quality of life. QALYs were then derived from EQ-5D-5L index score at three time points by calculating the area under the curve. 65 The accompanied visual analogue scale (VAS) was also reported, ranging from 0 to 100, with higher numbers indicating better health-related quality of life.
After the original plan was finalised, a EQ-5D-5L value set for England was released by the Office of Health Economics. 66 Following that development, NICE released a position statement in August 2017 stating that, for the data gathered using EQ-5D-5L, the mapping function approach is still the preferred method for reference case analysis and the five-level version valuation set is not recommended for use at this time. 67 The updated statement in November 2018 retained this position. 68 Therefore, we maintained the original plan and disregarded the available five-level version valuation set for England.
Missing data
For the baseline covariates, little to no missing data were expected. These missing values were imputed by the mean of the parameter in the whole sample because these were information collected before the intervention began and the randomisation functioned to balance the parameters between two arms.
Methods for dealing with missing data on the primary outcome (CO-verified smoking cessation) followed the approach used in the previous statistical analysis. The missing data on costs and quality-of-life elements at follow-ups were imputed using multiple imputation. A chained equation model was developed, and predictive mean matching was used as the main imputation method for continuous variables, using 10 nearest neighbours to the prediction as a set from which to draw. All missing data were imputed separately by trial group. The imputation was performed on the aggregated level estimate, that is, EQ-5D-5L utility value converted from data provided by participants. As a rule of thumb, the number of imputations was set to the highest percentage of all missing values. 69
Analysis
Primary analysis
The primary health economic analysis was conducted on an intention-to-treat basis, including all randomised participants in the groups to which they were randomised. The CO-verified smoking cessation rate at 12 months was calculated for both groups in the statistical analysis. Intervention cost per CO-verified quitter at 12 months and incremental intervention cost per additional quitter was reported to provide a comparison with the SSS statistics.
Intervention costs and wider health-care and social services costs were used to calculate total cost per participant. The EQ-5D-5L was used to calculate QALYs per participant. An incremental cost-effectiveness ratio (ICER) was calculated and compared with the willingness-to-pay (WTP) threshold recommended by NICE (£20,000–30,000). 57
Sensitivity analysis
Uncertainty around the ICER was quantified using a non-parametric bootstrap technique whereby 5000 samples were generated by resampling. Confidence intervals around the ICER were estimated and plotted on a cost-effectiveness plane to illustrate the overall uncertainty of both costs and QALYs. Cost-effectiveness acceptability curves were constructed from the bootstrapped samples by converting ICER to net monetary benefit. 70
A complete-case analysis was also carried out to assess the uncertainty due to missing data.
Secondary analysis
Several exploratory analyses were also conducted in addition to the reference case analysis recommended by the NICE guidance. 57
Wider societal costs were summarised and presented as a separate analysis to provide an estimate of costs outside the NHS. Using the information on the number of cigarettes smoked per day, an estimate of money spent on cigarettes was calculated to demonstrate the monetary impact on the financial condition of the participants. Previous evidence suggested that smokers with SMI might require a lower dosage of antipsychotics to achieve the same effect if they stop smoking. 2 Therefore, a comparison of the costs of antipsychotics between two halves of the trial period by participants’ smoking status among this sample was conducted.
Adverse events
A trial-specific procedure for detecting and reporting adverse events was implemented.
An adverse event was defined as any unexpected effect or untoward clinical event affecting the participant. Standard criteria for serious adverse events (SAEs) were used.
The participant’s MH-SCP, GP or mental health specialist was requested to inform the research team of any SAEs or non-SAEs. In addition, when participants indicated hospital attendance or use of emergency services either during a visit or in questionnaire responses, this was followed up by the research team as required.
All adverse events and SAEs were independently reviewed by a clinician and were routinely reported to the Data Monitoring and Ethics Committee and the Independent Trial Steering Committee (TSC).
Adverse event data are summarised descriptively by treatment group.
Suicide and self-harm risk protocol
A protocol for identifying and reporting potential suicide risk was implemented (see Appendix 3 for protocol). Item 9 on the PHQ-9, which asks if the participant has had ‘thoughts that you would be better off dead or of hurting yourself in some way’, was used to identify any suicide risk.
If the participant indicated a response of 3 (i.e. nearly every day) for this item at baseline or at any of the follow-up interviews, then the suicide protocol was implemented and the participant was asked if they had talked to their GP, psychiatrist or care co-ordinator/community psychiatric nurse (CPN) about these feelings. If they had not, consent was sought to contact the participant’s GP to inform them of the situation. If the participant refused, the relevant designated psychiatrist/health professional was contacted. If the health professional agreed, the participant’s GP or psychiatrist was contacted immediately. A Suicidal Intent Form was completed and, when applicable, a Suicidal Intent Form: Psychiatrist/Health Professional was also completed. These forms were stored with the participant’s trial records.
Patient and public involvement in research
The SCIMITAR+ trial benefited from the involvement of users of mental health services and carers of people with SMI throughout the research period. Our TSC included representation from carers and service users. Our protocol and study materials were scrutinised and supported by users and carers in the north-west of England.
We invited service users from a patient and public involvement (PPI) group based in the north-west to be involved in the study. One of the collaborators works at the University of Manchester, the institution that organised the PPI group. From this group, service users provided input on the recruitment materials. Service users from this group were also invited to sit on the TSC; we had two service users sit on the TSC and provide input into the running of the trial. We also had the benefit of a carer who sat on the TSC in the pilot trial and continued to sit on the TSC in the main trial.
A service user and carer were invited to attend one of our yearly SCIMITAR+ meetings, which we held for all the organisations taking part in the study. They gave a presentation on smoking and giving up smoking and provided useful input into the study. They continue to remain involved and provide advice on our work. We are seeking advice from service users regarding the dissemination of the SCIMITAR+ trial and have recently formed a PPI group at the University of York for service users with SMI. We will be asking this group to provide input into our dissemination plan.
Chapter 3 Changes to the protocol
The current version of the protocol is version 2.5.
Recruitment from service user groups
After the trial had been recruiting for 3 months, recruitment from service user groups was added as an additional recruitment method. This involved providing service user groups with information about the SCIMITAR+ trial, along with copies of the flyer. Interested service users could then contact the mental health professional named on the flyer or their care co-ordinator, or, if the service user preferred, the staff member at the service user group could contact the service users’ care co-ordinator on their behalf.
This recruitment method was added to broaden the range of recruitment methods and therefore broaden the range of participants invited to take part in the SCIMITAR+ trial. This method also had the potential to offer service users who may not routinely be attending a clinic, and therefore not approached by a clinician, the opportunity to take part in the study.
Recruitment from the Lifestyle Health and Wellbeing Survey
Using the Lifestyle Health and Wellbeing Survey to recruit was added as a recruitment option after the trial had been recruiting for 8 months. The survey is a questionnaire-based prospective study involving adults aged ≥ 18 years with SMI. Participants in the survey complete a series of questions about their health and well-being, including questions about their smoking. Participants in the survey who consented to being contacted by the research team and who smoked and were potentially interested in cutting down on or quitting smoking were invited to take part in the SCIMITAR+ trial.
The use of the Lifestyle Health and Wellbeing Survey was included to broaden the range of recruitment options and to offer people who may not have been invited by their mental health team or GP to take part in the SCIMITAR+ trial the opportunity to do so. During the course of the SCIMITAR+ trial, we noticed that some clinicians were gatekeeping: instead of checking whether or not a person wanted to do something about their smoking, they were stating that the person did not want to stop smoking. In the survey, each person was asked whether or not they wanted to do something about their smoking, thus removing the possibility of an incorrect assumption being made.
Chapter 4 Results
Recruitment
Recruitment started in October 2015 and ended in December 2016. Over the course of the trial, 40 GP surgeries mailed out recruitment packs, and 21 mental health trusts were enlisted to recruit participants.
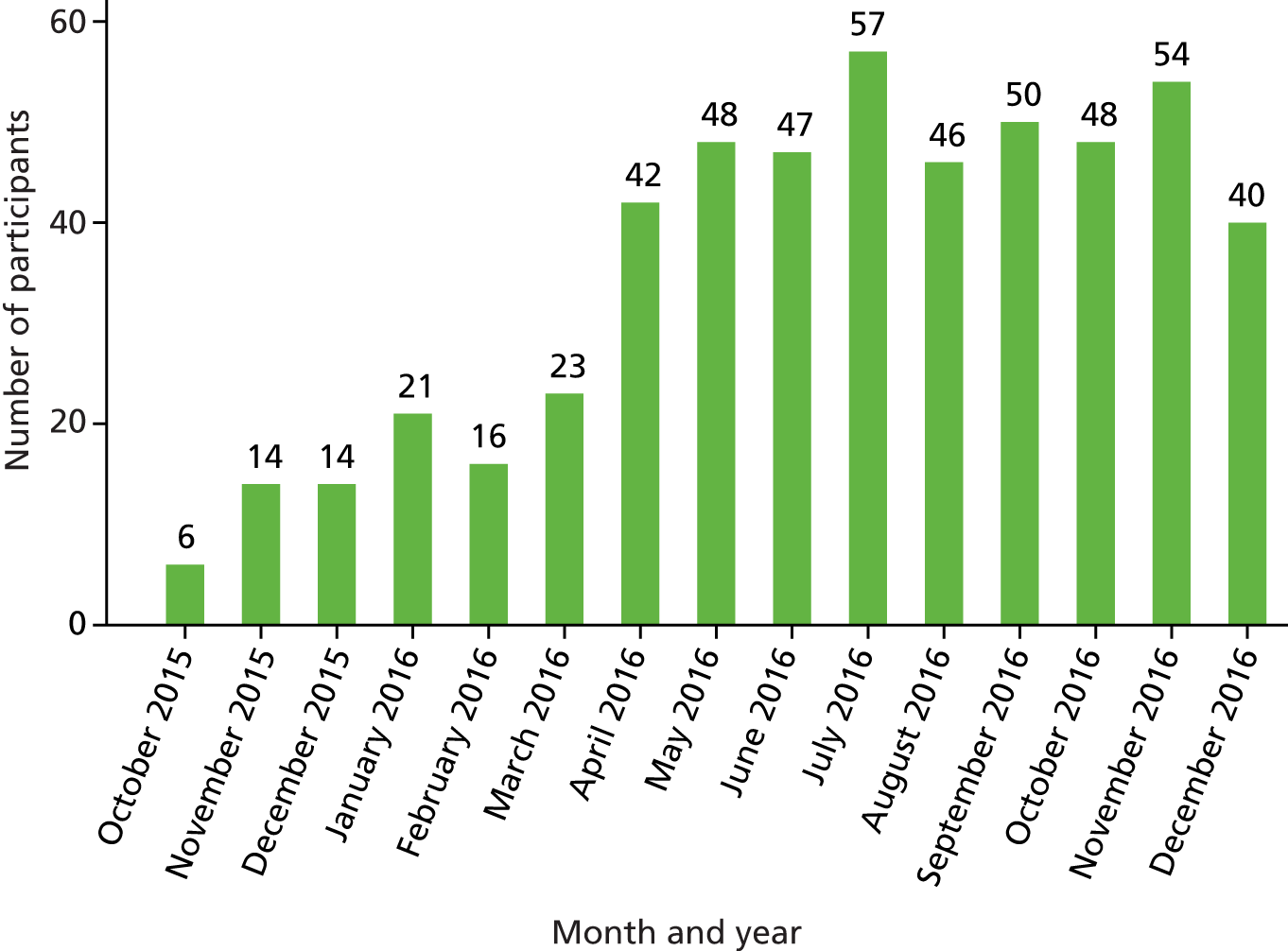
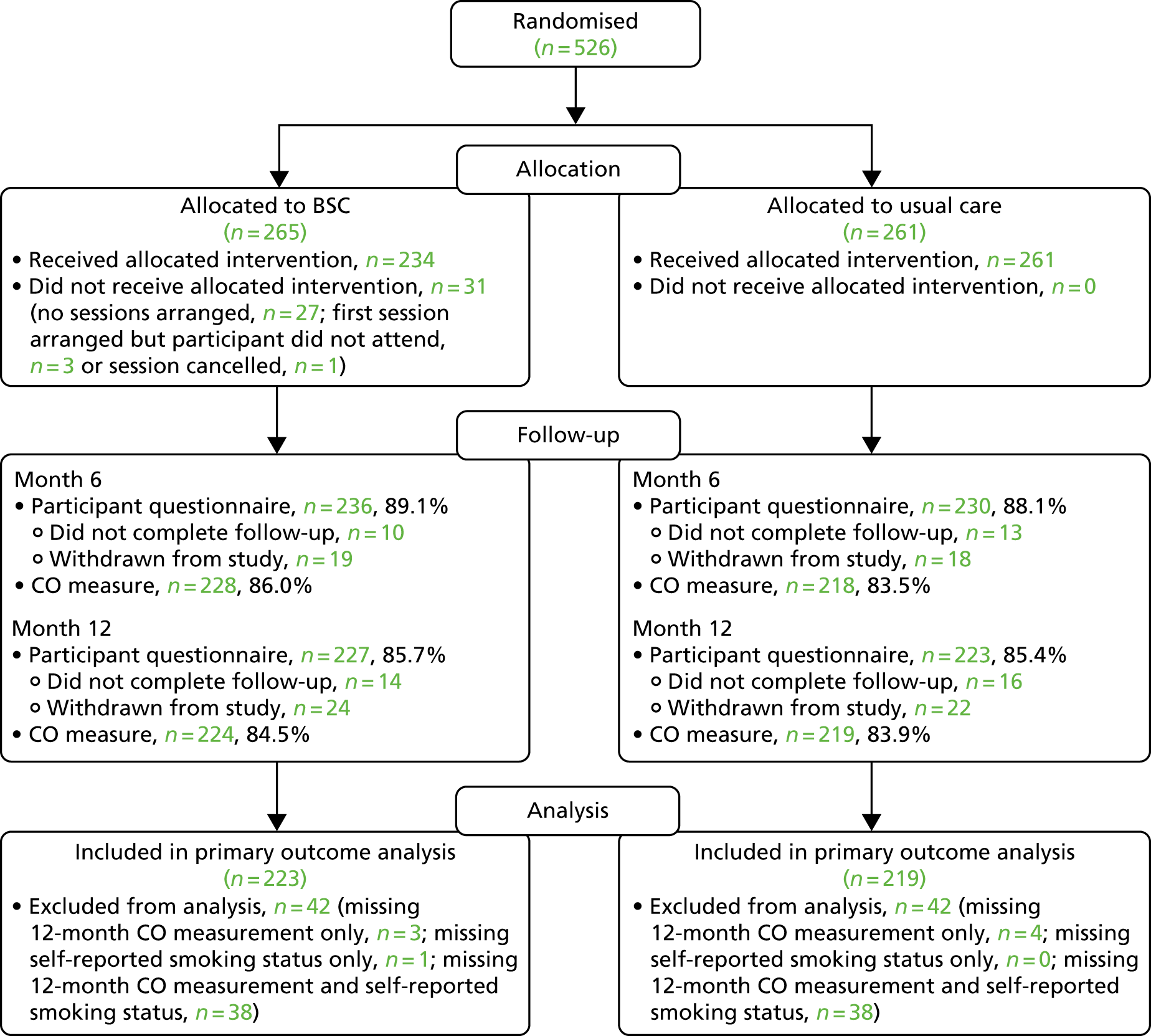
A total of 526 participants were recruited to the trial. The target and actual rates of recruitment are shown in Figure 1, and monthly recruitment figures are shown in Figure 2. Recruitment took place at 21 mental health trusts and 16 GP surgery sites (one GP site was a standalone site and the other GP sites were acting as participant identification centres for the mental health trusts), although eligible and randomised participants were primarily identified from mental health trusts. Table 3 shows the recruitment broken down by method. Mental health trusts recruited between four and 52 participants per trust, and GP surgeries recruited between one and five participants. Participant flow through the trial is shown in CONSORT flow diagrams in Figures 3–5. Of the 526 participants, 265 were randomised to the BSC group and 261 were randomised to the usual-care group.
FIGURE 1.
The SCIMITAR+ target and actual recruitment rates.
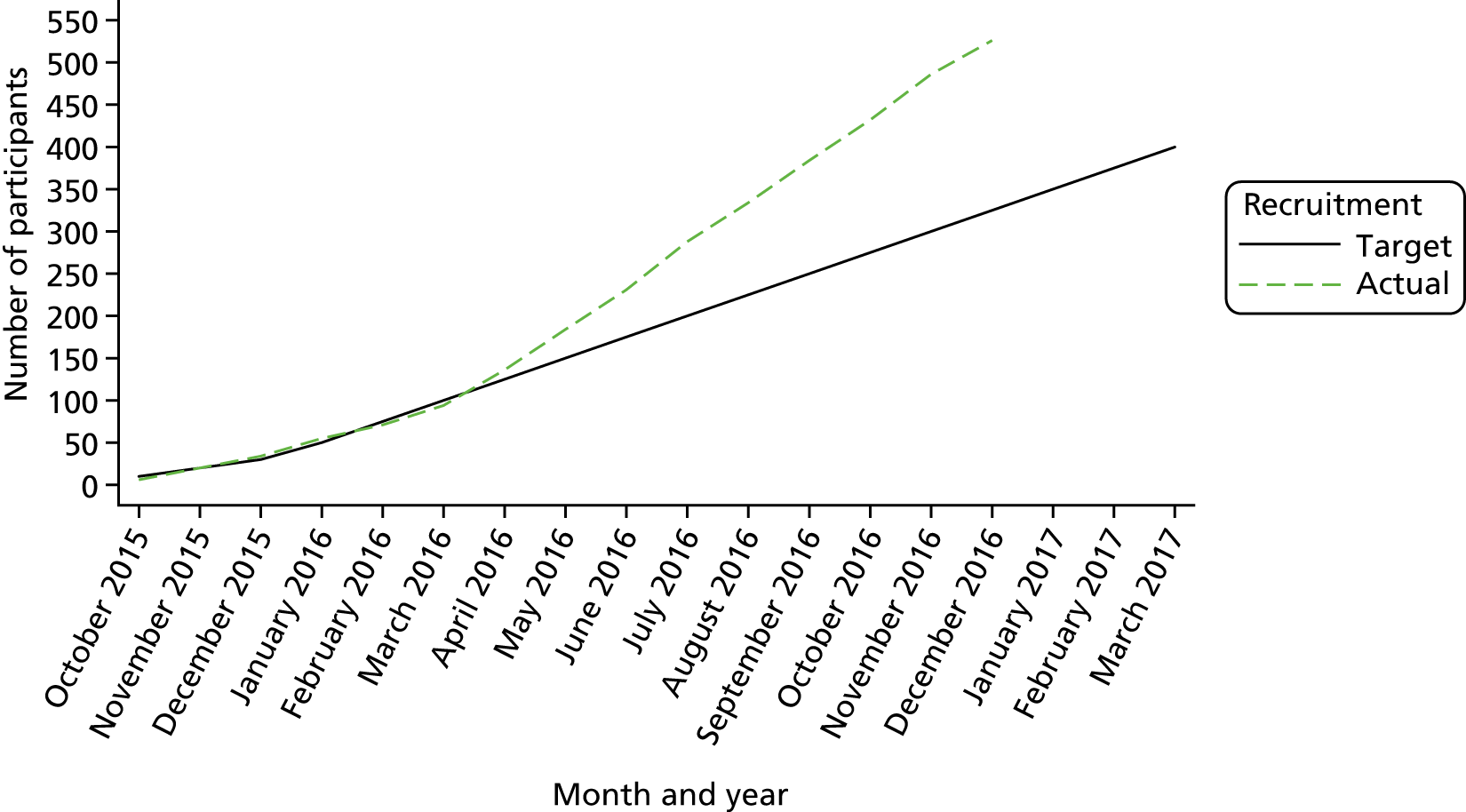
FIGURE 2.
Monthly recruitment into the SCIMITAR+ trial.
| Recruitment method | Number recruited (N = 526), n (%) |
|---|---|
| CMHT direct referral | 377 (72) |
| Psychiatrist direct referral | 40 (8) |
| CMHT database screen | 37 (7) |
| CMHT/psychiatrist unknown | 28 (5) |
| GP database screen | 22 (4) |
| Recruitment from Lifestyle Health and Wellbeing Survey | 13 (2) |
| GP direct referral | 1 (0.2) |
| GP unknown | 1 (0.2) |
| Service user group | 1 (0.2) |
| Unknown | 6 (1) |
FIGURE 3.
Primary care CONSORT flow diagram up to randomisation.

FIGURE 4.
Secondary care CONSORT flow diagram up to randomisation.
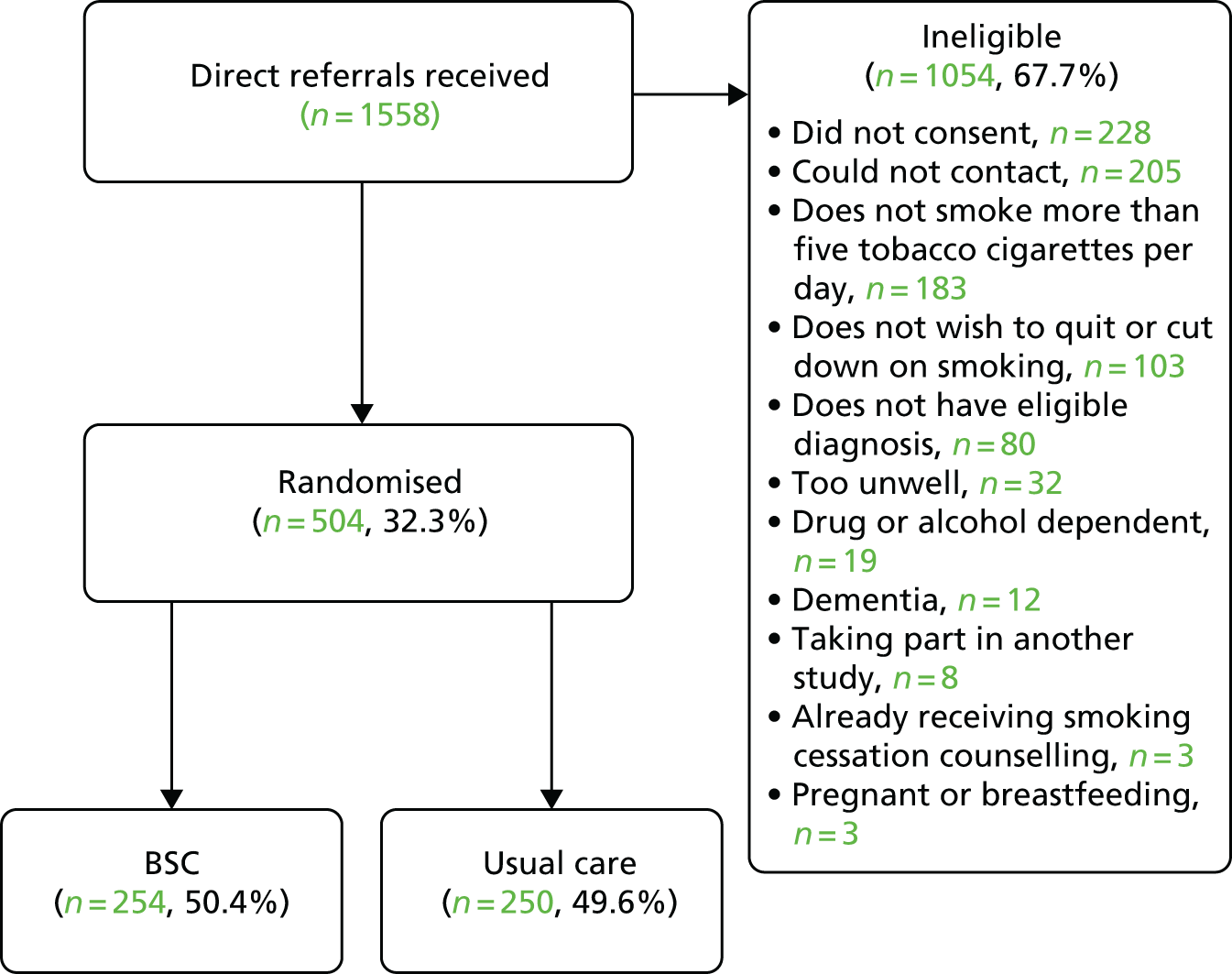
FIGURE 5.
The CONSORT flow diagram showing participant flow through the trial, from randomisation.
Primary care
A total of 1162 participants were identified as potentially eligible from GP practice lists and were sent a recruitment pack, of which 48 (4.1%) returned a ‘consent to contact’ form. Twenty-six of these were ineligible and the remaining 22 were randomised, 11 to each trial arm. The randomisation rate for the GP mailout was 1.9%.
Secondary care
The number of direct referrals received was 1558. Two-thirds of these were ineligible, non-consenting and/or could not be contacted (n = 1054, 67.7%) and the remaining 504 were randomised, 254 to the BSC group and 250 to the usual-care group. The randomisation rate for direct referrals was 32.3%.
Taking part in another study was not one of the exclusion criteria for the SCIMITAR+ trial; however, some people were taking part in a study that precluded participants from taking part in other research studies.
Baseline data
The baseline characteristics of participants are summarised in the following tables, and the treatment groups appear well balanced across baseline data. Table 4 summarises the sociodemographic and employment data for the participants. Overall, 58.7% of the participants were male, 41.1% were female and one participant identified as transgender (0.2%). The mean age of participants was 46 years, with a range from 19 to 72 years. The vast majority of participants were white (89.7%). Over two-thirds of the participants were unemployed but not seeking work owing to ill health (67.7%).
| Characteristic | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| Sex/gender, n (%) | |||
| Male | 159 (60.0) | 150 (57.5) | 309 (58.7) |
| Female | 105 (39.6) | 111 (42.5) | 216 (41.1) |
| Transgender | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Age (years) | |||
| Mean (SD) | 46.5 (12.5) | 45.5 (11.7) | 46.0 (12.1) |
| Median (min., max.) | 47 (19, 72) | 46 (19, 71) | 47 (19, 72) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, n (%) | |||
| White | 241 (90.9) | 231 (88.5) | 472 (89.7) |
| Mixed | 9 (3.4) | 10 (3.8) | 19 (3.6) |
| Asian | 5 (1.9) | 11 (4.2) | 16 (3.0) |
| Black | 9 (3.4) | 7 (2.7) | 16 (3.0) |
| Chinese | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Highest educational qualification, n (%) | |||
| None | 42 (15.8) | 41 (15.7) | 83 (15.8) |
| GCSE/O level | 74 (27.9) | 91 (34.9) | 165 (31.4) |
| GCE A/AS level or Scottish Higher | 20 (7.5) | 23 (8.8) | 43 (8.2) |
| NVQ/SVQ levels 1–3 | 39 (14.7) | 21 (8.0) | 60 (11.4) |
| GNVQ (Advanced) | 2 (0.8) | 7 (2.7) | 9 (1.7) |
| BTEC certificate | 4 (1.5) | 2 (0.8) | 6 (1.1) |
| BTEC diploma | 7 (2.6) | 11 (4.2) | 18 (3.4) |
| National Certificate or Diploma | 7 (2.6) | 5 (1.9) | 12 (2.3) |
| Qualified teacher status | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Higher education diploma | 5 (1.9) | 7 (2.7) | 12 (2.3) |
| Degree (first degree/ordinary degree) | 24 (9.1) | 13 (5.0) | 37 (7.0) |
| Postgraduate certificate | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| Postgraduate diploma | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| Master’s degree | 4 (1.5) | 6 (2.3) | 10 (1.9) |
| PhD | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Other | 30 (11.3) | 25 (9.6) | 55 (10.5) |
| Missing | 2 (0.8) | 5 (1.9) | 7 (1.3) |
| Employment, n (%) | |||
| Employed full time | 3 (1.1) | 16 (6.1) | 19 (3.6) |
| Employed part time | 9 (3.4) | 11 (4.2) | 20 (3.8) |
| Self-employed | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| Retired | 19 (7.2) | 16 (6.1) | 35 (6.7) |
| Looking after family or home | 4 (1.5) | 3 (1.1) | 7 (1.3) |
| Student | 5 (1.9) | 5 (1.9) | 10 (1.9) |
| Voluntary worker | 11 (4.2) | 18 (6.9) | 29 (5.5) |
| Not employed but seeking work | 16 (6.0) | 15 (5.7) | 31 (5.9) |
| Not employed but not seeking work because of ill health | 185 (69.8) | 171 (65.5) | 356 (67.7) |
| Not employed but not seeking work for some other reason | 8 (3.0) | 4 (1.5) | 12 (2.3) |
| Other | 3 (1.1) | 0 (0.0) | 3 (0.6) |
| Marital status, n (%) | |||
| Single | 177 (66.8) | 171 (65.5) | 348 (66.2) |
| Married | 32 (12.1) | 26 (10.0) | 58 (11.0) |
| Living with a partner/co-habiting | 13 (4.9) | 16 (6.1) | 29 (5.5) |
| Divorced/separated | 33 (12.5) | 46 (17.6) | 79 (15.0) |
| Widowed | 10 (3.8) | 2 (0.8) | 12 (2.3) |
| Accommodation, n (%) | |||
| Detached house | 10 (3.8) | 18 (6.9) | 28 (5.3) |
| Semidetached house | 40 (15.1) | 48 (18.4) | 88 (16.7) |
| Terraced house | 45 (17.0) | 32 (12.3) | 77 (14.6) |
| Flat | 123 (46.4) | 113 (43.3) | 236 (44.9) |
| Bedsit/studio | 6 (2.3) | 11 (4.2) | 17 (3.2) |
| Communal establishment | 36 (13.6) | 35 (13.4) | 71 (13.5) |
| Caravan/other mobile shelter | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| No fixed abode | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| Missing | 2 (0.8) | 2 (0.8) | 4 (0.8) |
Table 5 presents data on the general health of the participants at baseline. Most participants reported at least moderate health in the last year (65.4%), despite most participants reporting that they experienced at least one of the listed medical conditions (85.6%). The majority of participants (83.5%) felt that smoking had negatively affected their health, and 70.9% reported that they had been advised to stop smoking by their GP. The mean BMI of participants was 29.9 kg/m2 (range 16.6–59.8 kg/m2), which falls in the overweight range.
| Characteristic | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| Health over the past year, n (%) | |||
| Excellent | 14 (5.3) | 1 (0.4) | 15 (2.9) |
| Good | 50 (18.9) | 62 (23.8) | 112 (21.3) |
| Moderate | 101 (38.1) | 116 (44.4) | 217 (41.3) |
| Poor | 77 (29.1) | 62 (23.8) | 139 (26.4) |
| Very poor | 23 (8.7) | 18 (6.9) | 41 (7.8) |
| Missing | 0 (0.0) | 2 (0.8) | 2 (0.4) |
| Feel smoking has negatively affected health, n (%) | |||
| Yes | 220 (83.0) | 219 (83.9) | 439 (83.5) |
| No | 45 (17.0) | 42 (16.1) | 87 (16.5) |
| GP or doctor gave advice on quitting smoking, n (%) | |||
| Yes | 192 (72.5) | 181 (69.3) | 373 (70.9) |
| No | 73 (27.5) | 80 (30.7) | 153 (29.1) |
| Health problems, n (%) | |||
| Asthma | 89 (33.6) | 82 (31.4) | 171 (32.5) |
| Chronic bronchitis | 29 (10.9) | 34 (13.0) | 63 (12.0) |
| Other chest trouble | 83 (31.3) | 106 (40.6) | 189 (35.9) |
| Diabetes | 39 (14.7) | 38 (14.6) | 77 (14.6) |
| Stomach/digestive disorder | 67 (25.3) | 87 (33.3) | 154 (29.3) |
| Haemorrhoids (piles) | 44 (16.6) | 48 (18.4) | 92 (17.5) |
| Liver trouble | 19 (7.2) | 19 (7.3) | 38 (7.2) |
| Rheumatic disorder or arthritis | 43 (16.2) | 56 (21.5) | 99 (18.8) |
| Lung cancer | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Other cancer | 9 (3.4) | 9 (3.4) | 18 (3.4) |
| Varicose veins | 26 (9.8) | 25 (9.6) | 51 (9.7) |
| Stroke | 12 (4.5) | 11 (4.2) | 23 (4.4) |
| Migraine | 79 (29.8) | 71 (27.2) | 150 (28.5) |
| Back trouble | 116 (43.8) | 122 (46.7) | 238 (45.2) |
| Epilepsy | 17 (6.4) | 11 (4.2) | 28 (5.3) |
| ME or chronic fatigue | 18 (6.8) | 26 (10.0) | 44 (8.4) |
| High blood pressure | 56 (21.1) | 62 (23.8) | 118 (22.4) |
| Healthy life, n (%) | |||
| A very healthy life | 17 (6.4) | 11 (4.2) | 28 (5.3) |
| A fairly healthy life | 118 (44.5) | 114 (43.7) | 232 (44.1) |
| A not very healthy life | 94 (35.5) | 101 (38.7) | 195 (37.1) |
| An unhealthy life | 36 (13.6) | 32 (12.3) | 68 (12.9) |
| Missing | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| BMI (kg/m2) | |||
| Mean (SD) | 30.2 (7.1) | 29.7 (6.3) | 29.9 (6.7) |
| Median (min., max.) | 29.0 (16.6, 59.8) | 29.4 (16.9, 48.4) | 29.3 (16.6, 59.8) |
| Missing, n (%) | 2 (0.8) | 3 (1.1) | 5 (1.0) |
| Eat five portions of fruit and vegetables a day, n (%) | |||
| Yes | 113 (42.6) | 106 (40.6) | 219 (41.6) |
| No | 152 (57.4) | 152 (58.2) | 304 (57.8) |
| Missing | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| Exercise for 20 minutes or more, n (%) | |||
| Daily | 92 (34.7) | 89 (34.1) | 181 (34.4) |
| Weekly | 72 (27.2) | 68 (26.1) | 140 (26.6) |
| Monthly | 9 (3.4) | 7 (2.7) | 16 (3.0) |
| Seldom | 33 (12.5) | 43 (16.5) | 76 (14.4) |
| Never | 59 (22.3) | 51 (19.5) | 110 (20.9) |
| Missing | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| Drink alcohol, n (%) | |||
| Yes | 141 (53.2) | 140 (53.6) | 281 (53.4) |
| No | 122 (46.0) | 121 (46.4) | 243 (46.2) |
| Missing | 2 (0.8) | 0 (0.0) | 2 (0.4) |
| If yes, units in last week | n = 141 | n = 140 | n = 281 |
| Mean (SD) | 10.1 (15.8) | 8.8 (16.0) | 9.5 (15.9) |
| Median (min., max.) | 4 (0, 84) | 4 (0, 140) | 4 (0, 140) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 6 summarises baseline mental health status. The most common severe mental health problems were schizophrenia or other psychotic illness (n = 342, 65.0%), bipolar disorder (n = 115, 21.9%) and schizoaffective disorder (n = 66, 12.5%). Nearly two-thirds of the participants had a care programme approach (CPA) co-ordinator (64.4%) and/or a CPN (64.6%), and 83.3% were under the care of a CMHT. On average, participants were aged 26 years when they were first diagnosed with their mental health problem and had required psychiatric treatment in hospital an average of 2.8 times (range 0–100 times) in the last 10 years. Most (64.3%) participants described their condition as ‘stable’, but 13.7% described their condition as ‘unstable’ (although each participant had been judged to be stable from the point of view of their condition by either their GP or a responsible mental health professional), and 22.0% either were unsure or did not respond to this question.
| Characteristic | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| Age at first diagnosis (years) | |||
| Mean (SD) | 26.1 (10.3) | 26.4 (10.5) | 26.3 (10.4) |
| Median (min., max.) | 24 (2, 60) | 25 (0, 59) | 24 (2, 60) |
| Missing, n (%) | 4 (1.5) | 7 (2.7) | 11 (2.1) |
| Most recent diagnosis, n (%) | |||
| Bipolar disorder | 59 (22.3) | 56 (21.5) | 115 (21.9) |
| Schizoaffective disorder | 25 (9.4) | 41 (15.7) | 66 (12.5) |
| Schizophrenia | 138 (52.1) | 125 (47.9) | 263 (50.0) |
| Other psychotic disorder | 41 (15.5) | 39 (14.9) | 80 (15.2) |
| Missing | 2 (0.8) | 0 (0.0) | 2 (0.4) |
| Type of professional seen by participant, n (%) | |||
| CPA co-ordinator | 166 (62.6) | 173 (66.3) | 339 (64.4) |
| CPN | 171 (64.5) | 169 (64.8) | 340 (64.6) |
| CMHT | 217 (81.9) | 221 (84.7) | 438 (83.3) |
| Time since last health check (months) | |||
| Mean (SD) | 5.4 (11.9) | 4.4 (6.7) | 4.9 (9.6) |
| Median (min., max.) | 1.9 (0.1, 116.5) | 2.1 (0.1, 63.0) | 2.0 (0.1, 116.5) |
| Missing, n (%) | 102 (38.5) | 94 (36.0) | 196 (37.3) |
| Number of times needed psychiatric treatment in hospital in last 10 years | |||
| Mean (SD) | 2.9 (6.9) | 2.7 (4.7) | 2.8 (5.9) |
| Median (min., max.) | 1 (0, 100) | 1 (0, 50) | 1 (0, 100) |
| Missing, n (%) | 4 (1.5) | 6 (2.3) | 10 (1.9) |
| Would you describe your condition as . . . , n (%) | |||
| Stable | 172 (64.9) | 166 (63.6) | 338 (64.3) |
| Unstable | 40 (15.1) | 32 (12.3) | 72 (13.7) |
| Unsure | 53 (20.0) | 62 (23.8) | 115 (21.9) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
Participants reported that they started smoking, on average, at the age of 16 years (range 5–50 years) and had been smoking for a mean of 29.9 (SD 12.9) years (Table 7). The most common form of tobacco used was factory-made cigarettes, reportedly used by 63.3% of participants, followed by hand-rolled cigarettes (58.7%). The mean number of cigarettes smoked per day was 24.9 (range 3–100 cigarettes) (although all participants reported smoking at least five cigarettes per day when the eligibility check was carried out, one participant stated that they smoked three cigarettes per day in the self-complete smoking questionnaire). The median number of attempts to quit ever was 3, with a range of 0–200. The median duration of longest quit attempt reported was 40 days, with a range of 0 days to 188 months. The most common self-reported previous strategies used to stop smoking were ‘cold turkey’ (55.3%) followed by NRTs (20.3%).
| Characteristic | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| Age started smoking (years) | |||
| Mean (SD) | 15.7 (5.3) | 16.5 (6.0) | 16.1 (5.7) |
| Median (min., max.) | 15 (5, 47) | 15 (7, 50) | 15 (5, 50) |
| Missing, n (%) | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Length of time smoking (years) | |||
| Mean (SD) | 30.7 (13.2) | 29.0 (12.5) | 29.9 (12.9) |
| Median (min., max.) | 31.9 (0.1, 61.1) | 29.3 (2.1, 56.2) | 30.6 (0.1, 61.1) |
| Missing, n (%) | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Type of tobacco used, n (%) | |||
| Factory-made cigarettes | 173 (65.3) | 160 (61.3) | 333 (63.3) |
| Hand-rolled cigarettes | 150 (56.6) | 159 (60.9) | 309 (58.7) |
| Cigars | 17 (6.4) | 10 (3.8) | 27 (5.1) |
| Pipe | 4 (1.5) | 2 (0.8) | 6 (1.1) |
| Chewing tobacco | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Water pipe/hookah/shisha pipe | 3 (1.1) | 2 (0.8) | 5 (1.0) |
| Number of cigarettes per day | |||
| Mean (SD) | 24.7 (13.5) | 23.2 (12.8) | 24.0 (13.2) |
| Median (min., max.) | 20 (3, 100) | 20 (0, 80) | 20 (0, 100) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If using roll-ups or pipe, amount of tobacco used per day (g) | n = 151 | n = 159 | n = 310 |
| Mean (SD) | 12.4 (10.7) | 12.8 (10.1) | 12.6 (10.4) |
| Median (min., max.) | 10 (0.25, 75) | 12 (0.5, 80) | 10 (0.25, 80) |
| Missing, n (%) | 18 (11.9) | 13 (8.2) | 31 (10.0) |
| Number of quit attempts in the past 6 months | |||
| Mean (SD) | 1.4 (5.4) | 1.5 (3.4) | 1.5 (4.5) |
| Median (min., max.) | 0 (0, 80) | 0 (0, 30) | 0 (0, 80) |
| Missing, n (%) | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Total number of quit attempts | |||
| Mean (SD) | 6.6 (13.2) | 8.2 (19.0) | 7.4 (16.3) |
| Median (min., max.) | 3 (0, 100) | 4 (0, 200) | 3 (0, 200) |
| Missing, n (%) | 4 (1.5) | 8 (3.1) | 12 (2.3) |
| Length of most recent quit attempt (months) | |||
| Mean (SD) | 1.9 (9.4) | 2.8 (14.5) | 2.4 (12.2) |
| Median (min., max.) | 0.1 (0.0, 105.7) | 0.1 (0.0, 187.9) | 0.1 (0.0, 187.9) |
| Missing, n (%) | 13 (4.9) | 12 (4.6) | 25 (4.8) |
| Longest quit attempt (months) | |||
| Mean (SD) | 8.8 (22.9) | 9.3 (23.8) | 9.0 (23.3) |
| Median (min., max.) | 0.9 (0.0, 152.6) | 1.5 (0.0, 187.9) | 1.3 (0.0, 187.9) |
| Missing, n (%) | 19 (7.2) | 7 (2.7) | 26 (4.9) |
| Previous methods used to stop smoking, n (%) | |||
| Cold turkey | 139 (52.5) | 152 (58.2) | 291 (55.3) |
| NRT | 51 (19.2) | 56 (21.5) | 107 (20.3) |
| Champix (varenicline) | 18 (6.8) | 34 (13.0) | 52 (9.9) |
| Hypnosis | 22 (8.3) | 18 (6.9) | 40 (7.6) |
| E-cigarettes | 15 (5.7) | 14 (5.4) | 29 (5.5) |
| Acupuncture | 15 (5.7) | 12 (4.6) | 27 (5.1) |
| Zyban (bupropion) | 12 (4.5) | 12 (4.6) | 24 (4.6) |
| Other | 2 (0.8) | 9 (3.4) | 11 (2.1) |
The reasons for smoking and their importance are summarised in Table 8. The reasons most commonly reported as very important for smoking were helping to cope with stress (61.2%) and to give them something to do (39.7%).
| Characteristic, n (%) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| I enjoy it | |||
| Very important | 103 (38.9) | 90 (34.5) | 193 (36.7) |
| Quite important | 106 (40.0) | 96 (36.8) | 202 (38.4) |
| Not important | 55 (20.8) | 74 (28.4) | 129 (24.5) |
| Missing | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| It keeps my weight down | |||
| Very important | 36 (13.6) | 45 (17.2) | 81 (15.4) |
| Quite important | 64 (24.2) | 48 (18.4) | 112 (21.3) |
| Not important | 163 (61.5) | 166 (63.6) | 329 (62.5) |
| Missing | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| It helps me to socialise | |||
| Very important | 43 (16.2) | 32 (12.3) | 75 (14.3) |
| Quite important | 68 (25.7) | 72 (27.6) | 140 (26.6) |
| Not important | 153 (57.7) | 154 (59.0) | 307 (58.4) |
| Missing | 1 (0.4) | 3 (1.1) | 4 (0.8) |
| It helps my concentration | |||
| Very important | 44 (16.6) | 38 (14.6) | 82 (15.6) |
| Quite important | 81 (30.6) | 64 (24.5) | 145 (27.6) |
| Not important | 138 (52.1) | 157 (60.2) | 295 (56.1) |
| Missing | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| It gives me something to do | |||
| Very important | 106 (40.0) | 103 (39.5) | 209 (39.7) |
| Quite important | 106 (40.0) | 109 (41.8) | 215 (40.9) |
| Not important | 53 (20.0) | 48 (18.4) | 101 (19.2) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| It helps me to cope with stress | |||
| Very important | 162 (61.1) | 160 (61.3) | 322 (61.2) |
| Quite important | 71 (26.8) | 72 (27.6) | 143 (27.2) |
| Not important | 29 (10.9) | 27 (10.3) | 56 (10.6) |
| Missing | 3 (1.1) | 2 (0.8) | 5 (1.0) |
| It helps combat pain | |||
| Very important | 32 (12.1) | 15 (5.7) | 47 (8.9) |
| Quite important | 37 (14.0) | 31 (11.9) | 68 (12.9) |
| Not important | 194 (73.2) | 211 (80.8) | 405 (77.0) |
| Missing | 2 (0.8) | 4 (1.5) | 6 (1.1) |
| I feel bad when I try to stop | |||
| Very important | 93 (35.1) | 85 (32.6) | 178 (33.8) |
| Quite important | 63 (23.8) | 59 (22.6) | 122 (23.2) |
| Not important | 104 (39.2) | 110 (42.1) | 214 (40.7) |
| Missing | 5 (1.9) | 7 (2.7) | 12 (2.3) |
| I like being a smoker | |||
| Very important | 41 (15.5) | 27 (10.3) | 68 (12.9) |
| Quite important | 60 (22.6) | 59 (22.6) | 119 (22.6) |
| Not important | 162 (61.1) | 172 (65.9) | 334 (63.5) |
| Missing | 2 (0.8) | 3 (1.1) | 5 (1.0) |
| It gives me confidence | |||
| Very important | 38 (14.3) | 22 (8.4) | 60 (11.4) |
| Quite important | 48 (18.1) | 53 (20.3) | 101 (19.2) |
| Not important | 177 (66.8) | 183 (70.1) | 360 (68.4) |
| Missing | 2 (0.8) | 3 (1.1) | 5 (1.0) |
Table 9 summarises the reasons for wanting to try to give up smoking; the reasons most commonly reported as very important were that it is bad for health (87.5%) and that it is expensive (71.9%).
| Characteristic, n (%) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| It is expensive | |||
| Very important | 193 (72.8) | 185 (70.9) | 378 (71.9) |
| Quite important | 49 (18.5) | 49 (18.8) | 98 (18.6) |
| Not important | 23 (8.7) | 26 (10.0) | 49 (9.3) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| It is bad for my health | |||
| Very important | 236 (89.1) | 224 (85.8) | 460 (87.5) |
| Quite important | 20 (7.5) | 31 (11.9) | 51 (9.7) |
| Not important | 9 (3.4) | 5 (1.9) | 14 (2.7) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| I do not like feeling dependent on cigarettes | |||
| Very important |
|
|
|
| Quite important |
|
|
|
| Not important |
|
|
|
| Missing |
|
|
|
| It makes my clothes and breath smell | |||
| Very important | 151 (57.0) | 155 (59.4) | 306 (58.2) |
| Quite important | 74 (27.9) | 64 (24.5) | 138 (26.2) |
| Not important | 40 (15.1) | 40 (15.3) | 80 (15.2) |
| Missing | 0 (0.0) | 2 (0.8) | 2 (0.4) |
| It is a bad example for children | |||
| Very important | 156 (58.9) | 163 (62.5) | 319 (60.6) |
| Quite important | 53 (20.0) | 45 (17.2) | 98 (18.6) |
| Not important | 54 (20.4) | 50 (19.2) | 104 (19.8) |
| Missing | 2 (0.8) | 3 (1.1) | 5 (1.0) |
| It is unpleasant for people near me | |||
| Very important | 140 (52.8) | 139 (53.3) | 279 (53.0) |
| Quite important | 80 (30.2) | 80 (30.7) | 160 (30.4) |
| Not important | 45 (17.0) | 40 (15.3) | 85 (16.2) |
| Missing | 0 (0.0) | 2 (0.8) | 2 (0.4) |
| It makes me less fit | |||
| Very important | 186 (70.2) | 184 (70.5) | 370 (70.3) |
| Quite important | 60 (22.6) | 55 (21.1) | 115 (21.9) |
| Not important | 19 (7.2) | 21 (8.0) | 40 (7.6) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| People around me disapprove of my smoking | |||
| Very important | 107 (40.4) | 101 (38.7) | 208 (39.5) |
| Quite important | 78 (29.4) | 68 (26.1) | 146 (27.8) |
| Not important | 80 (30.2) | 89 (34.1) | 169 (32.1) |
| Missing | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| It is bad for the health of people near me | |||
| Very important | 147 (55.5) | 136 (52.1) | 283 (53.8) |
| Quite important | 72 (27.2) | 72 (27.6) | 144 (27.4) |
| Not important | 45 (17.0) | 51 (19.5) | 96 (18.3) |
| Missing | 1 (0.4) | 2 (0.8) | 3 (0.6) |
Participant use of e-cigarettes is summarised in Table 10. Over two-thirds (70.3%) of the participants reported having ever tried an e-cigarette, of whom 77 (22.0%) had used e-cigarettes at least once per week in the past month. Of the participants who reported ever having tried an e-cigarette, over half stated that their use of tobacco had not changed since they started using e-cigarettes (53.5%), with only 34.1% saying that they now smoke less tobacco. The most commonly cited reason for using e-cigarettes was to try to quit smoking tobacco (51.1%).
| Characteristic, n (%) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| Ever tried an e-cigarette | |||
| Yes | 186 (70.2) | 184 (70.5) | 370 (70.3) |
| No | 79 (29.8) | 77 (29.5) | 156 (29.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If yes: | n = 186 | n = 184 | n = 370 |
| Use of e-cigarette in last 30 days | |||
| Never | 128 (68.8) | 124 (67.4) | 252 (68.1) |
| Less than once per week | 21 (11.3) | 18 (9.8) | 39 (10.5) |
| Less than daily but at least once per week | 22 (11.8) | 20 (10.9) | 42 (11.4) |
| Every day | 14 (7.5) | 21 (11.4) | 35 (9.5) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Length of use of e-cigarettes | |||
| < 1 month | 58 (31.2) | 76 (41.3) | 134 (36.2) |
| 1–6 months | 44 (23.7) | 38 (20.7) | 82 (22.2) |
| 6–12 months | 28 (15.1) | 21 (11.4) | 49 (13.2) |
| > 1 year | 38 (20.4) | 31 (16.8) | 69 (18.6) |
| Missing | 18 (9.7) | 18 (9.8) | 36 (9.7) |
| Nicotine levels in cartridges used in e-cigarette | |||
| None (0 mg) | 10 (5.4) | 10 (5.4) | 20 (5.4) |
| Low (< 8 mg) | 24 (12.9) | 26 (14.1) | 50 (13.5) |
| Medium (8–16 mg) | 56 (30.1) | 60 (32.6) | 116 (31.4) |
| High (> 16 mg) | 51 (27.4) | 44 (23.9) | 95 (25.7) |
| Missing | 45 (24.2) | 44 (23.9) | 89 (24.1) |
| Use of tobacco changed since using e-cigarettes | |||
| Yes, I smoke less tobacco | 62 (33.3) | 64 (34.8) | 126 (34.1) |
| Yes, I smoke more tobacco | 11 (5.9) | 5 (2.7) | 16 (4.3) |
| No, it has not changed | 97 (52.2) | 101 (54.9) | 198 (53.5) |
| Missing | 16 (8.6) | 14 (7.6) | 30 (8.1) |
| Reason started using e-cigarettes | |||
| To quit smoking tobacco | 92 (49.5) | 97 (52.7) | 189 (51.1) |
| To try a safer alternative to tobacco | 25 (13.4) | 23 (12.5) | 48 (13.0) |
| To try something new | 14 (7.5) | 13 (7.1) | 27 (7.3) |
| To smoke less tobacco | 29 (15.6) | 19 (10.3) | 48 (13.0) |
| Other | 14 (7.5) | 20 (10.9) | 34 (9.2) |
| Missing | 12 (6.5) | 12 (6.5) | 24 (6.5) |
Participant questionnaire return rates
Response rates to the participant questionnaires at 6 and 12 months are presented in Table 11. Over 95% of the forms given to participants at 6 months were returned (466/489, 95.3%; 88.6% of 526 randomised participants). The median number of days between the form being due and being returned was 6 (replacing those that were completed early with 0 days), and the majority were completed face to face (95.9%). At the primary time point of 12 months, 450 (93.8%) of the 480 forms sent out were returned (85.6% of 526 randomised participants). The median time to return was 4 days, and only eight (1.8%) were not completed face to face (six over the telephone and two on paper via post).
| Time point | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|
| 6 months | |||
| Sent,a n (%) | 246 (92.8) | 243 (93.1) | 489 (93.0) |
| Completed,b n (%) | 236 (95.9) | 230 (94.7) | 466 (95.3) |
| Days to complete, median (IQR) | 7 (0–20) | 5 (0–20) | 6 (0–20) |
| Mode of completion,c n (%) | |||
| Face to face | 228 (96.6) | 219 (95.2) | 447 (95.9) |
| Telephone | 4 (1.7) | 10 (4.4) | 14 (3.0) |
| Paper | 4 (1.7) | 1 (0.4) | 5 (1.1) |
| 12 months | |||
| Sent,a n (%) | 241 (90.0) | 239 (91.6) | 480 (91.3) |
| Completed,b n (%) | 227 (94.2) | 223 (93.3) | 450 (93.8) |
| Days to complete, median (IQR) | 3 (0–15) | 4 (0–16) | 4 (0–15) |
| Mode of completion,c n (%) | |||
| Face to face | 223 (98.2) | 219 (98.2) | 442 (98.2) |
| Telephone | 3 (1.3) | 3 (1.4) | 6 (1.3) |
| Paper | 1 (0.4) | 1 (0.4) | 2 (0.4) |
Primary analysis
The CO breath measurement readings are summarised by treatment group at baseline, 6 and 12 months in Table 12. The mean reading at baseline was 24.6 (SD 15.2) p.p.m. and was similar between the two groups. Seventy (13.3%) participants had a reading of < 10 p.p.m. at baseline. In both groups, the mean reading decreased from baseline to 6 months and then increased slightly at 12 months.
| Time point | CO measurement | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | Mean (SD) | 24.9 (15.4) | 24.3 (15.1) | 24.6 (15.2) |
| Median (min., max.) | 22.0 (1.0, 95.0) | 21.0 (2.0, 100.0) | 21.0 (1.0, 100.0) | |
| Missing, n (%) | 1 (0.4) | 3 (1.1) | 4 (0.8) | |
| 6 months | Mean (SD) | 18.0 (14.1) | 19.4 (12.9) | 18.7 (13.5) |
| Median (min., max.) | 16.5 (0.0, 89.0) | 17.5 (0.0, 71.0) | 17.0 (0.0, 89.0) | |
| Missing, n (%) | 37 (14.0) | 43 (16.5) | 80 (15.2) | |
| 12 months | Mean (SD) | 18.1 (13.6) | 20.2 (13.7) | 19.2 (13.7) |
| Median (min., max.) | 16.0 (0.0, 71.0) | 20.0 (0.0, 77.0) | 18.0 (0.0, 77.0) | |
| Missing, n (%) | 41 (15.5) | 42 (16.1) | 83 (15.8) |
Self-reported smoking status and quit attempt data reported on the participant questionnaires at 6 and 12 months are presented in Table 13. At 6 months, 463 participants (88.0%) provided a valid response to the self-reported smoking status question, and 51 of these (11.0%) reported that they had not smoked even a puff in the last week [34 (14.5%) in the BSC group and 17 (7.4%) in the usual-care group]. At the primary time point of 12 months, 449 participants (85.4%) provided a valid response to the self-reported smoking status question, and 57 of these (12.7%) reported that they had not smoked even a puff in the last week [35 (15.5%) in the BSC group and 22 (9.9%) in the usual-care group). For those who said that they did still smoke, the most common hour of the day to have the first puff of a cigarette is between 08.00 and 09.00 (20% at both time points). At 12 months, of the participants with valid data for this question, 16.1% of BSC participants and 10.6% of usual-care participants reported that they had stopped smoking completely, and participants reported a median of one quit attempt in the previous 6 months, with the most recent quit attempt lasting an average of 3.5 weeks before the participant returned to smoking. Responses to the items in Table 13 relating to smoking status and smoking in the last week did not always agree, as might be expected. For example, replying ‘not a puff’ in relation to the amount smoked in the last week did not always imply that participants reported that they had ‘stopped smoking completely’, and vice versa. For instance, one participant who reported that they had not smoked a puff in the past week at 12 months in the usual-care group reported that they ‘smoke cigarettes (including hand-rolled) but not every day’, and this could be because they still smoke occasionally but just had not smoked in the previous week.
| Time point | Outcome | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Smoked in last week, n (%) | ||||
| 6 months | Not even a puff | 34 (12.8) | 17 (6.5) | 51 (9.7) |
| Just a few puffs | 5 (1.9) | 0 (0.0) | 5 (1.0) | |
| Between one and five cigarettes | 12 (4.5) | 1 (0.4) | 13 (2.5) | |
| More than five cigarettes | 183 (69.1) | 211 (80.8) | 394 (74.9) | |
| Missing | 31 (11.7) | 32 (12.3) | 63 (12.0) | |
| 12 months | Not even a puff | 35 (13.2) | 22 (8.4) | 57 (10.8) |
| Just a few puffs | 2 (0.8) | 3 (1.1) | 5 (1.0) | |
| Between one and five cigarettes | 11 (4.2) | 10 (3.8) | 21 (4.0) | |
| More than five cigarettes | 178 (67.2) | 188 (72.0) | 366 (69.6) | |
| Missing | 39 (14.7) | 38 (14.6) | 77 (14.6) | |
| Self-reported smoking status, n (%) | ||||
| 6 months | Smoke the same amount of cigarettes (including hand-rolled) every day | 77 (29.1) | 106 (40.6) | 183 (34.8) |
| Cut down on the number of cigarettes (including hand-rolled) I smoke | 100 (37.7) | 96 (36.8) | 196 (37.3) | |
| Smoke cigarettes (including hand-rolled) but not every day | 19 (7.2) | 5 (1.9) | 24 (4.6) | |
| Stopped smoking completely | 37 (14.0) | 18 (6.9) | 55 (10.5) | |
| Missing | 32 (12.1) | 36 (13.8) | 68 (12.9) | |
| At 12 months | Smoke the same amount of cigarettes (including hand-rolled) every day | 91 (34.3) | 101 (38.7) | 192 (36.5) |
| Cut down on the number of cigarettes (including hand-rolled) I smoke | 86 (32.5) | 83 (31.8) | 169 (32.1) | |
| Smoke cigarettes (including hand-rolled) but not every day | 11 (4.2) | 10 (3.8) | 21 (4.0) | |
| Stopped smoking completely | 36 (13.6) | 23 (8.8) | 59 (11.2) | |
| Missing | 41 (15.5) | 44 (16.9) | 85 (16.2) | |
| Quit attempts in last 6 months | ||||
| At 6 months | Mean (SD) | 2.0 (3.7) | 1.6 (2.3) | 1.8 (3.1) |
| Median (min., max.) | 1 (0, 50) | 1 (0, 20) | 1 (0, 50) | |
| Missing, n (%) | 39 (14.7) | 40 (15.3) | 79 (15.0) | |
| At 12 months | Mean (SD) | 1.8 (4.0) | 1.3 (1.7) | 1.6 (3.1) |
| Median (min., max.) | 1 (0, 35) | 1 (0, 12) | 1 (0, 35) | |
| Missing, n (%) | 49 (18.5) | 44 (16.9) | 93 (17.7) | |
| Length of cessation (weeks) | ||||
| At 6 months | Mean (SD) | 5.7 (37.2) | 2.8 (5.6) | 4.3 (26.6) |
| Median (min., max.) | 0.6 (0, 520) | 0.4 (0, 26) | 0.4 (0, 26) | |
| Missing, n (%) | 67 (25.3) | 65 (24.9) | 132 (25.1) | |
| At 12 months | Mean (SD) | 4.0 (10.4) | 3.0 (7.1) | 3.5 (8.9) |
| Median (min., max.) | 0.3 (0, 52) | 0.3 (0, 52) | 0.3 (0, 52) | |
| Missing, n (%) | 87 (32.8) | 71 (27.2) | 158 (30.0) | |
Smoking cessation at 6 and 12 months was defined as a CO measure of < 10 p.p.m. (indicating no smoking in the last 12 hours) and self-reported cessation (indicating no smoking in the last week). At 6 months, 446 participants (84.8%) provided a CO reading, 463 (88.0%) had valid data for self-reported smoking status and 443 (84.2%) had both (Table 14); 32 out of 226 (14.2%; 10.6% of 265 randomised participants) fulfilled the definition for smoking cessation in the BSC group and 14 out of 217 (6.5%; 5.4% of 261 randomised participants) fulfilled the definition in the usual-care group. At 12 months, 443 (84.2%) provided a CO reading, 449 (85.4%) had valid data for self-reported smoking status and 442 (84.0%) had both; 34 out of 223 (15.2%; 12.8% of 265 randomised participants) fulfilled the definition for smoking cessation in the BSC group and 22 out of 219 (10.0%; 8.4% of 261 randomised participants) fulfilled the definition in the usual-care group.
| Time point | Outcome, n (%) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| 6 months | Number with CO measurementa | 228 (86.0) | 218 (83.5) | 446 (84.8) |
| Number with CO < 10 p.p.m.b | 69 (30.3) | 50 (22.9) | 119 (26.7) | |
| Number with self-reporta | 234 (88.3) | 229 (87.7) | 463 (88.0) | |
| Number self-reporting as quitterb | 34 (14.5) | 17 (7.4) | 51 (11.0) | |
| Total number with CO and self-reported measurea | 226 (85.3) | 217 (83.1) | 443 (84.2) | |
| Total number quitb | 32 (14.2) | 14 (6.5) | 46 (10.4) | |
| Total number quita | 32 (12.1) | 14 (5.4) | 46 (8.7) | |
| 12 months | Number with CO measurementa | 224 (84.5) | 219 (83.9) | 443 (84.2) |
| Number with CO < 10 p.p.m.b | 73 (32.6) | 62 (28.3) | 135 (30.5) | |
| Number with self-reporta | 226 (85.3) | 223 (85.4) | 449 (85.4) | |
| Number self-reporting as quitterb | 35 (15.5) | 22 (9.9) | 57 (12.7) | |
| Total number with CO and self-reported measurea | 223 (84.2) | 219 (83.9) | 442 (84.0) | |
| Total number quitb | 34 (15.2) | 22 (10.0) | 56 (12.7) | |
| Total number quita | 34 (12.8) | 22 (8.4) | 56 (10.6) |
The OR for the difference between the two groups from the model adjusting for baseline number of cigarettes smoked, and site as a random effect, at 12 months was 1.6 (95% CI 0.9 to 2.8; p = 0.12). The OR at 6 months was 2.4 (95% CI 1.2 to 4.7; p = 0.01).
Sensitivity analysis
Unadjusted odds ratio
The unadjusted, marginal ORs for CO-verified smoking cessation at 6 and 12 months are 2.4 (95% CI 1.2 to 4.6; p = 0.01) and 1.6 (95% CI 0.9 to 2.9; p = 0.10), respectively.
Post hoc generalised estimating equations analysis
Using GEE to account for the repeated measures, the OR adjusting for baseline number of cigarettes smoked is 2.3 (95% CI 1.2 to 4.5; p = 0.01) at 6 months and 1.6 (95% CI 0.9 to 2.8; p = 0.12) at 12 months. When the GEE model was additionally adjusted for site, the treatment effects were estimated at an OR of 2.5 (95% CI 1.3 to 4.8; p = 0.01) at 6 months and 1.6 (95% CI 0.9 to 2.9; p = 0.12) at 12 months.
Accounting for missing CO-verified smoking status
When imputing self-reported smoking status when CO measures were missing in separate logistic regressions at 6 and 12 months and adjusting for number of cigarettes smoked as a fixed effect and site as a random effect, the following treatment effects were obtained: 6 months, OR 2.6, 95% CI 1.3 to 5.0 (p = 0.005); 12 months, OR 1.7, 95% CI 0.9 to 3.0 (p = 0.08).
Then, assuming that anyone else with missing smoking status data is a smoker yielded the following results: 6 months, OR 2.6, 95% CI 1.4 to 5.0 (p = 0.004); 12 months, OR 1.7, 95% CI 0.9 to 2.9 (p = 0.08).
When multiple imputation was used to impute missing primary outcome data, the treatment effect at 6 months was observed to be an OR of 2.4 (95% CI 1.3 to 4.4; p = 0.007), and at 12 months it was observed to be an OR of 1.7 (95% CI 0.9 to 3.0; p = 0.08).
Secondary analysis
Self-reported smoking cessation
Among those with a valid response to the self-reported smoking status question at 6 months, 34 out of 234 (14.5%) in the BSC group and 17 out of 229 (7.4%) in the usual-care group reported that they had not smoked even a puff in the last week (Table 15) (adjusted OR 2.1, 95% CI 1.1 to 3.9; p = 0.02). At 12 months, 35 out of 226 (15.5%) in the BSC group and 22 out of 223 (9.9%) in the usual-care group reported that they had not smoked even a puff in the last week (see Table 15) (adjusted OR 1.7, 95% CI 0.9 to 3.0; p = 0.08).
| Time point | FTND questionnaire | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | Mean (SD) | 6.5 (2.0) | 6.4 (1.9) | 6.4 (2.0) |
| Median (min., max.) | 7.0 (1.0, 10.0) | 6.0 (1.0, 10.0) | 7.0 (1.0, 10.0) | |
| Missing, n (%) | 7 (2.6) | 7 (2.7) | 14 (2.7) | |
| Month 6 | Mean (SD) | 5.3 (2.1) | 5.4 (2.0) | 5.3 (2.1) |
| Median (min., max.) | 5.0 (1.0, 10.0) | 6.0 (1.0, 10.0) | 5.5 (1.0, 10.0) | |
| Missing, n (%) | 80 (30.2) | 66 (25.3) | 146 (27.8) | |
| Month 12 | Mean (SD) | 5.6 (2.0) | 5.3 (2.3) | 5.5 (2.2) |
| Median (min., max.) | 6.0 (1.0, 10.0) | 6.0 (1.0, 10.0) | 6.0 (1.0, 10.0) | |
| Missing, n (%) | 96 (36.2) | 75 (28.7) | 171 (32.5) |
Number of cigarettes smoked per day
The incidence rate ratio for number of cigarettes smoked per day at 6 months was 0.90 (95% CI 0.80 to 1.01; p = 0.08), marginally favouring the BSC group, and at 12 months it was 1.00 (95% CI 0.89 to 1.13; p = 0.95). Neither of these differences is statistically significant.
Fagerström Test for Nicotine Dependence
The FTND score is summarised by randomised group and time point in Table 15. Adjusted FTND means and group differences are presented in Table 21 and displayed in Figure 6. The adjusted mean difference at 6 months was –0.18 (95% CI –0.53 to 0.17) and at 12 months it was –0.01 (95% CI –0.39 to 0.38), both marginally favouring the BSC group but not statistically significantly (p = 0.32 and 0.97, respectively).
FIGURE 6.
Adjusted means for the FTND by treatment group and time point.

These results were extracted from a mixed-effects model in which a participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline FTND score and baseline smoking severity (fixed effects), and site as a random effect. Different covariance structures were applied to the model. An unstructured pattern that models all variances and covariances separately resulted in the lowest AIC and so was used in the final model. Diagnostics of model fit revealed that the standardised residuals were normally distributed and were uniform against fitted values; therefore, data transformation was not required.
Motivation to Quit questionnaire
The MTQ score is summarised by randomised group and time point in Table 16. Adjusted MTQ means and group differences are presented in Table 21 and displayed in Figure 7. The adjusted mean difference at 6 months was 0.58 (95% CI –0.01 to 1.17) and at 12 months was 0.64 (95% CI 0.04 to 1.24), both marginally favouring the BSC group. The difference is almost statistically significant at the 5% level at 6 months (p = 0.06) and is below this level at 12 months (p = 0.04).
| Time point | MTQ | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | Mean (SD) | 13.9 (2.7) | 13.7 (2.6) | 13.8 (2.7) |
| Median (min., max.) | 14.0 (6.0, 19.0) | 14.0 (5.0, 19.0) | 14.0 (5.0, 19.0) | |
| Missing, n (%) | 5 (1.9) | 2 (0.8) | 7 (1.3) | |
| Month 6 | Mean (SD) | 13.2 (3.4) | 12.4 (3.1) | 12.8 (3.3) |
| Median (min., max.) | 14.0 (4.0, 19.0) | 12.0 (4.0, 19.0) | 13.0 (4.0, 19.0) | |
| Missing, n (%) | 48 (18.1) | 60 (23.0) | 108 (20.5) | |
| Month 12 | Mean (SD) | 13.0 (3.3) | 12.3 (3.4) | 12.6 (3.4) |
| Median (min., max.) | 13.0 (5.0, 19.0) | 13.0 (4.0, 19.0) | 13.0 (4.0, 19.0) | |
| Missing, n (%) | 65 (24.5) | 61 (23.4) | 126 (24.0) |
FIGURE 7.
Adjusted means for the MTQ by treatment group and time point.
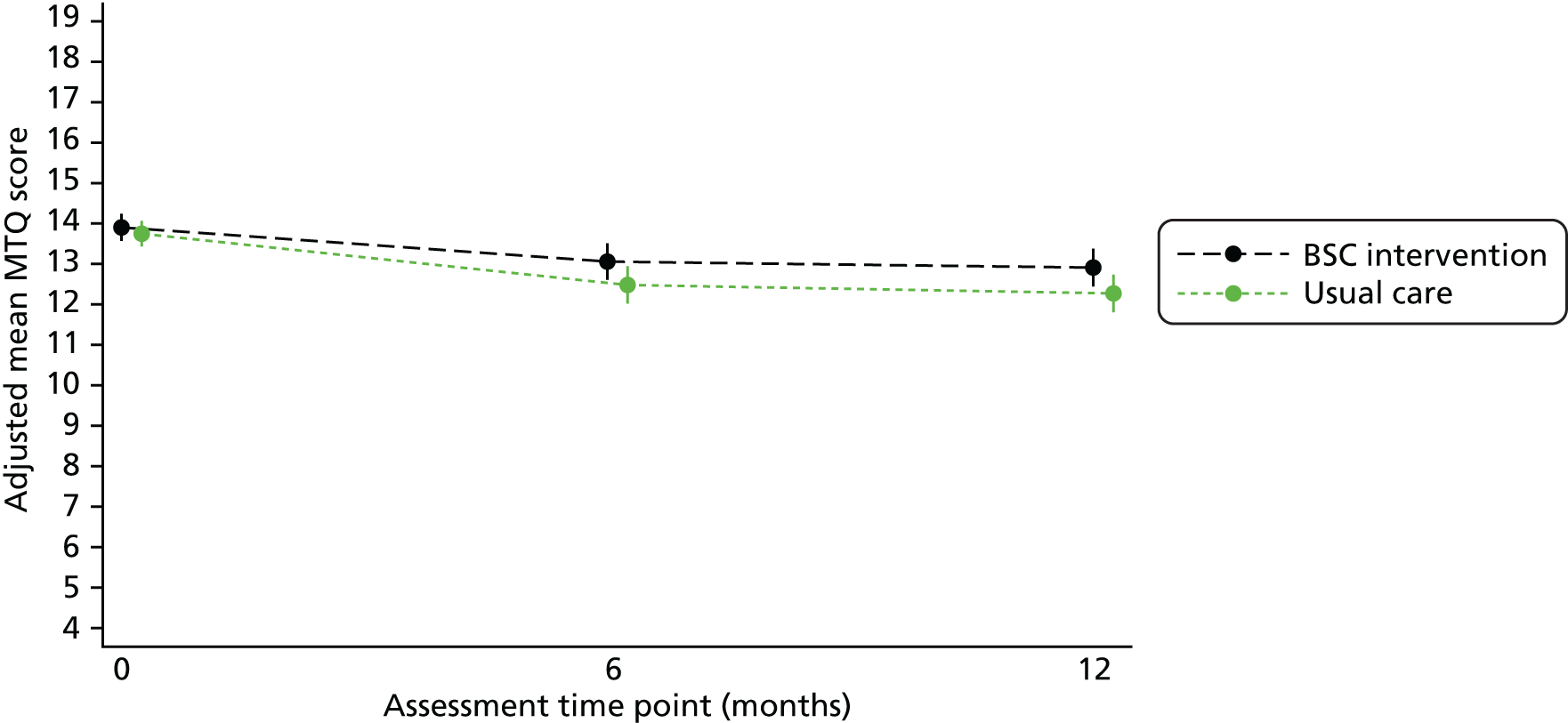
These results were extracted from a mixed-effects model in which participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline MTQ score and baseline smoking severity (fixed effects), and site as a random effect. Different covariance structures were applied to the model. A compound symmetric (or exchangeable) pattern that assumes equal correlation of errors between each pair of time points within participants resulted in the lowest AIC and so was used in the final model. Diagnostics of model fit revealed that the standardised residuals were normally distributed, and were uniform against fitted values; therefore, data transformation was not required.
Patient Health Questionnaire-9 items
The PHQ-9 score is summarised by randomised group and time point in Table 17. Adjusted PHQ-9 means and group differences are presented in Table 21 and displayed in Figure 8. The adjusted mean difference at 6 months was 0.20 (95% CI –0.85 to 1.24), marginally favouring usual care, and at 12 months was –0.12 (95% CI –1.18 to 0.94), marginally favouring BSC, but neither statistically significantly (p = 0.72 and 0.82, respectively).
| Time point | PHQ-9 | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | ||||
| Score | ||||
| Mean (SD) | 10.3 (6.7) | 10.8 (6.6) | 10.5 (6.6) | |
| Median (min., max.) | 10.0 (0.0, 27.0) | 10.0 (0.0, 27.0) | 10.0 (0.0, 27.0) | |
| Missing, n (%) | 1 (0.4) | 1 (0.4) | 2 (0.4) | |
| How difficult,a n (%) | n = 255 | n = 254 | n = 509 | |
| Not at all | 65 (25.5) | 64 (25.2) | 129 (25.3) | |
| Somewhat | 106 (41.6) | 111 (43.7) | 217 (42.6) | |
| Very | 42 (16.5) | 45 (17.7) | 87 (17.1) | |
| Extremely | 42 (16.5) | 34 (13.4) | 76 (14.9) | |
| Month 6 | ||||
| Score | ||||
| Mean (SD) | 9.3 (6.7) | 9.4 (6.4) | 9.3 (6.6) | |
| Median (min., max.) | 8.0 (0.0, 27.0) | 8.0 (0.0, 27.0) | 8.0 (0.0, 27.0) | |
| Missing, n (%) | 42 (15.8) | 47 (18.0) | 89 (16.9) | |
| How difficult,a n (%) | n = 216 | n = 210 | n = 426 | |
| Not at all | 67 (31) | 56 (26.7) | 123 (28.9) | |
| Somewhat | 89 (41.2) | 98 (46.7) | 187 (43.9) | |
| Very | 33 (15.3) | 36 (17.1) | 69 (16.2) | |
| Extremely | 27 (12.5) | 20 (9.5) | 47 (11) | |
| Month 12 | ||||
| Score | ||||
| Mean (SD) | 9.0 (6.7) | 9.7 (6.7) | 9.3 (6.7) | |
| Median (min., max.) | 8.0 (0.0, 27.0) | 9.0 (0.0, 27.0) | 8.0 (0.0, 27.0) | |
| Missing, n (%) | 52 (19.6) | 50 (19.2) | 102 (19.4) | |
| How difficult,a n (%) | n = 206 | n = 206 | n = 412 | |
| Not at all | 70 (34) | 42 (20.4) | 112 (27.2) | |
| Somewhat | 70 (34) | 93 (45.1) | 163 (39.6) | |
| Very | 47 (22.8) | 47 (22.8) | 94 (22.8) | |
| Extremely | 19 (9.2) | 24 (11.7) | 43 (10.4) | |
FIGURE 8.
Adjusted means for the PHQ-9 by treatment group and time point.
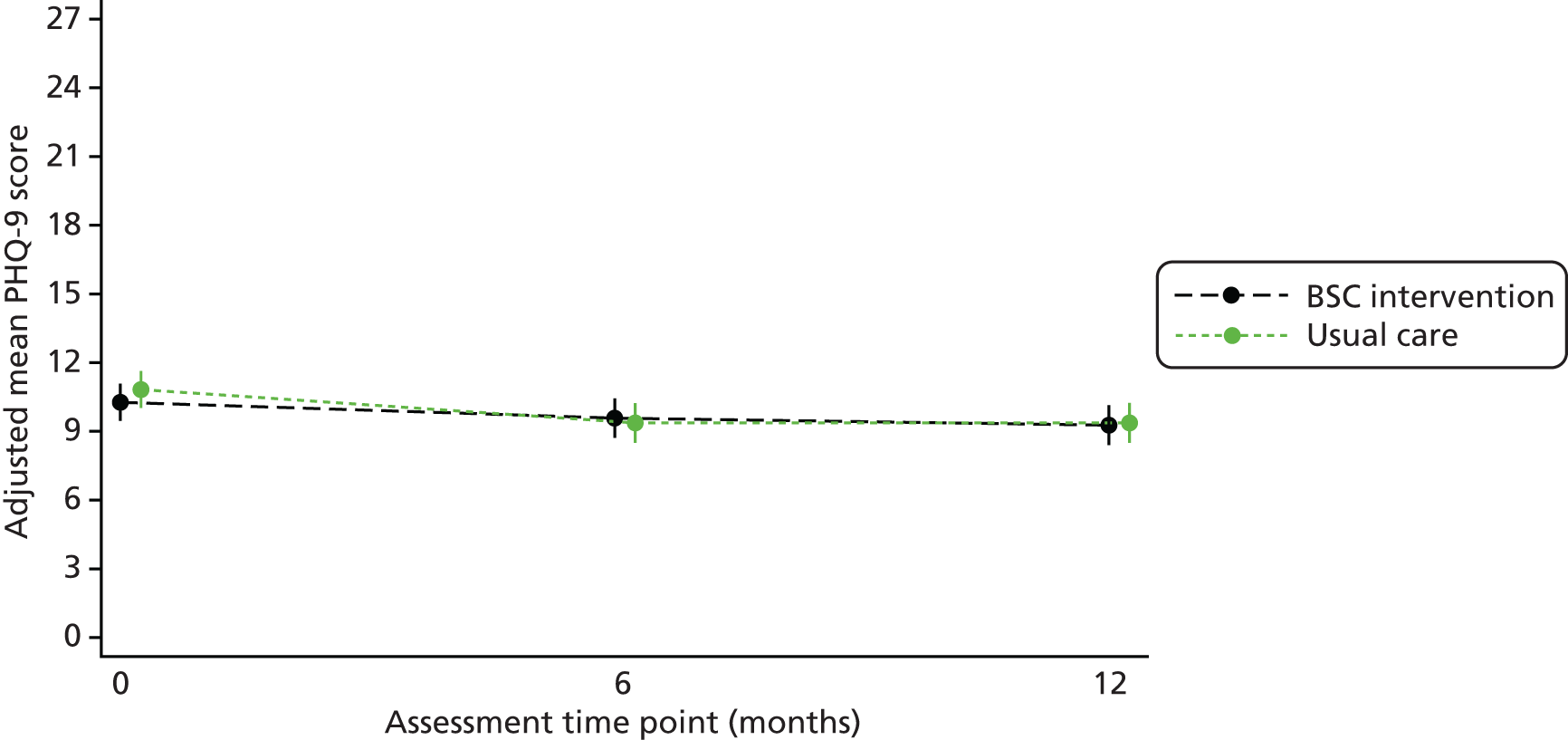
These results were extracted from a mixed-effects model in which participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline PHQ-9 score and baseline smoking severity as fixed effects, and site as a random effect. Different covariance structures were applied to the model. A compound symmetric (or exchangeable) pattern that assumes equal correlation of errors between each pair of time points within participants resulted in the lowest AIC and so was used in the final model. Diagnostics of model fit revealed that the standardised residuals were normally distributed, and were uniform against fitted values; therefore, data transformation was not required.
Generalised Anxiety Disorder Assessment-7 items
The GAD-7 score is summarised by randomised group and time point in Table 18. Adjusted GAD-7 means and group differences are presented in Table 21, and displayed in Figure 9. The adjusted mean difference at 6 months was –0.32 (95% CI –1.26 to 0.62) and at 12 months was –0.10 (95% CI –1.05 to 0.86), both marginally favouring BSC, but neither statistically significantly (p = 0.50 and 0.84, respectively).
| Time point | GAD-7 questionnaire | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | ||||
| Score | ||||
| Mean (SD) | 8.4 (6.2) | 8.4 (6.1) | 8.4 (6.1) | |
| Median (min., max.) | 7.0 (0.0, 21.0) | 8.0 (0.0, 21.0) | 8.0 (0.0, 21.0) | |
| Missing, n (%) | 1 (0.4) | 1 (0.4) | 2 (0.4) | |
| How difficult,a n (%) | n = 249 | n = 249 | n = 498 | |
| Not at all | 72 (28.9) | 63 (25.3) | 135 (27.1) | |
| Somewhat | 93 (37.3) | 113 (45.4) | 206 (41.4) | |
| Very | 53 (21.3) | 41 (16.5) | 94 (18.9) | |
| Extremely | 31 (12.4) | 32 (12.9) | 63 (12.7) | |
| Month 6 | ||||
| Score | ||||
| Mean (SD) | 7.0 (5.9) | 7.3 (5.8) | 7.1 (5.9) | |
| Median (min., max.) | 6.0 (0.0, 21.0) | 7.0 (0.0, 21.0) | 6.0 (0.0, 21.0) | |
| Missing, n (%) | 41 (15.5) | 44 (16.9) | 85 (16.2) | |
| How difficult,a n (%) | n = 202 | n = 197 | n = 399 | |
| Not at all | 70 (34.7) | 52 (26.4) | 122 (30.6) | |
| Somewhat | 73 (36.1) | 86 (43.7) | 159 (39.8) | |
| Very | 35 (17.3) | 45 (22.8) | 80 (20.1) | |
| Extremely | 24 (11.9) | 14 (7.1) | 38 (9.5) | |
| Month 12 | ||||
| Score | ||||
| Mean (SD) | 7.0 (6.3) | 7.4 (6.0) | 7.2 (6.2) | |
| Median (min., max.) | 5.0 (0.0, 21.0) | 7.0 (0.0, 21.0) | 6.0 (0.0, 21.0) | |
| Missing, n (%) | 51 (19.2) | 49 (18.8) | 100 (19.0) | |
| How difficult,a n (%) | n = 196 | n = 202 | n = 398 | |
| Not at all | 62 (31.6) | 51 (25.2) | 113 (28.4) | |
| Somewhat | 74 (37.8) | 88 (43.6) | 162 (40.7) | |
| Very | 38 (19.4) | 41 (20.3) | 79 (19.8) | |
| Extremely | 22 (11.2) | 22 (10.9) | 44 (11.1) | |
FIGURE 9.
Adjusted means for the GAD-7 by treatment group and time point.

These results were extracted from a mixed-effects model in which participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline GAD-7 score and baseline smoking severity as fixed effects, and site as a random effect. Different covariance structures were applied to the model. A compound symmetric (or exchangeable) pattern that assumes equal correlation of errors between each pair of time points within participants resulted in the lowest AIC and so was used in the final model. Diagnostics of model fit revealed that the standardised residuals were normally distributed, and were uniform against fitted values; therefore, data transformation was not required.
Short Form questionnaire-12 items
The SF-12 mental and physical health component subscale scores are summarised by randomised group and time point in Table 19. Adjusted SF-12 means and group differences are presented in Table 21 and displayed in Figure 10. The adjusted mean difference for the mental component score at 6 months was –0.73 (95% CI –2.82 to 1.36) and at 12 months was –0.41 (95% CI –2.35 to 1.53), both marginally favouring usual care, but neither statistically significantly (p = 0.49 and 0.68, respectively). The adjusted mean difference for the physical component score at 6 months was 1.75 (95% CI 0.21 to 3.28), indicating a statistically significant benefit of BSC (p = 0.03); however, this difference reduced at 12 months to 0.59 (95% CI –1.07 to 2.26) and was not statistically significant (p = 0.48).
| Time point | SF-12 | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Mental component score | ||||
| Baseline | Mean (SD) | 38.6 (12.6) | 37.9 (11.7) | 38.2 (12.2) |
| Median (min., max.) | 37.2 (14.8, 63.5) | 37.4 (14.4, 62.6) | 37.4 (14.4, 63.5) | |
| Missing, n (%) | 8 (3.0) | 5 (1.9) | 13 (2.5) | |
| Month 6 | Mean (SD) | 38.4 (13.1) | 38.9 (12.2) | 38.6 (12.6) |
| Median (min., max.) | 37.1 (9.3, 64.4) | 38.3 (14.3, 65.1) | 37.9 (9.3, 65.1) | |
| Missing, n (%) | 51 (19.2) | 53 (20.3) | 104 (19.8) | |
| Month 12 | Mean (SD) | 39.3 (11.9) | 38.9 (11.9) | 39.1 (11.9) |
| Median (min., max.) | 38.7 (12.2, 65.6) | 38.1 (12.4, 63.9) | 38.3 (12.2, 65.6) | |
| Missing, n (%) | 53 (20.0) | 54 (20.7) | 107 (20.3) | |
| Physical component score | ||||
| Baseline | Mean (SD) | 43.7 (10.4) | 42.2 (11.0) | 43.0 (10.7) |
| Median (min., max.) | 45.0 (17.0, 64.1) | 42.0 (19.4, 62.1) | 43.9 (17.0, 64.1) | |
| Missing, n (%) | 8 (3.0) | 5 (1.9) | 13 (2.5) | |
| Month 6 | Mean (SD) | 45.6 (9.8) | 42.9 (11.0) | 44.3 (10.5) |
| Median (min., max.) | 47.0 (22.7, 61.7) | 43.4 (17.4, 63.8) | 45.5 (17.4, 63.8) | |
| Missing, n (%) | 51 (19.2) | 53 (20.3) | 104 (19.8) | |
| Month 12 | Mean (SD) | 44.3 (10.1) | 42.4 (11.4) | 43.3 (10.8) |
| Median (min., max.) | 45.6 (20.2, 63.7) | 44.1 (16.3, 61.3) | 45.3 (16.3, 63.7) | |
| Missing, n (%) | 53 (20.0) | 54 (20.7) | 107 (20.3) | |
FIGURE 10.
Adjusted means for the SF-12 mental and physical health components by treatment group and time point. (a) Mental; and (b) physical. MCS, mental component score; PCS, physical component score.


These results were extracted from mixed-effects models in which participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline SF-12 mental/physical component score and baseline smoking severity as fixed effects, and site as a random effect. Different covariance structures were applied to the model. An unstructured pattern that models all variances and covariances separately resulted in the lowest AIC for both models and so was used. Diagnostics of model fit revealed that the standardised residuals were normally distributed, and were uniform against fitted values; therefore, data transformation was not required.
Body mass index
Body mass index is summarised by randomised group and time point in Table 20. Adjusted BMI means and group differences are presented in Table 21 and displayed in Figure 11. The adjusted mean difference at 6 months was 0.16 (95% CI –0.54 to 0.85) and at 12 months was 0.25 (95% CI –0.62 to 1.13), both marginally favouring usual care, but neither statistically significantly (p = 0.65 and 0.57, respectively).
| Time point | BMI | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) |
|---|---|---|---|---|
| Baseline | Mean (SD) | 30.2 (7.1) | 29.7 (6.3) | 29.9 (6.7) |
| Median (min., max.) | 29.0 (16.6, 59.8) | 29.4 (16.9, 48.4) | 29.3 (16.6, 59.8) | |
| Missing, n (%) | 2 (0.8) | 3 (1.1) | 5 (1.0) | |
| Month 6 | Mean (SD) | 30.5 (7.0) | 29.9 (6.0) | 30.2 (6.5) |
| Median (min., max.) | 30.0 (14.8, 60.7) | 29.1 (16.6, 48.5) | 29.4 (14.8, 60.7) | |
| Missing, n (%) | 49 (18.5) | 56 (21.5) | 105 (20.0) | |
| Month 12 | Mean (SD) | 30.4 (7.2) | 29.7 (6.7) | 30.0 (6.9) |
| Median (min., max.) | 29.7 (0.0, 61.9) | 29.3 (0.0, 45.8) | 29.4 (0.0, 61.9) | |
| Missing, n (%) | 57 (21.5) | 60 (23.0) | 117 (22.2) |
| Time point | BSC, mean (95% CI) | Usual care, mean (95% CI) | Difference (95% CI), SE | p-value |
|---|---|---|---|---|
| FTND | ||||
| Month 6 | 5.25 (5.00 to 5.50) | 5.43 (5.19 to 5.67) | –0.18 (–0.53 to 0.17), 0.18 | 0.32 |
| Month 12 | 5.42 (5.14 to 5.70) | 5.43 (5.16 to 5.69) | –0.01 (–0.39 to 0.38), 0.20 | 0.97 |
| MTQ | ||||
| Month 6 | 13.1 (12.6 to 13.5) | 12.5 (12.0 to 12.9) | 0.58 (–0.01 to 1.17), 0.30 | 0.06 |
| Month 12 | 12.9 (12.4 to 13.4) | 12.3 (11.8 to 12.7) | 0.64 (0.04 to 1.24), 0.31 | 0.04 |
| PHQ-9 | ||||
| Month 6 | 9.6 (8.7 to 10.4) | 9.4 (8.5 to 10.2) | 0.20 (–0.85 to 1.24), 0.53 | 0.72 |
| Month 12 | 9.3 (8.4 to 10.1) | 9.4 (8.5 to 10.2) | –0.12 (–1.18 to 0.94), 0.54 | 0.82 |
| GAD-7 | ||||
| Month 6 | 7.0 (6.3 to 7.7) | 7.4 (6.7 to 8.1) | –0.32 (–1.26 to 0.62), 0.48 | 0.50 |
| Month 12 | 7.1 (6.4 to 7.8) | 7.2 (6.5 to 7.9) | –0.10 (–1.05 to 0.86), 0.49 | 0.84 |
| SF-12 MCS | ||||
| Month 6 | 37.9 (36.2 to 39.5) | 38.6 (36.9 to 40.3) | –0.73 (–2.82 to 1.36), 1.07 | 0.49 |
| Month 12 | 38.6 (37.0 to 40.1) | 39.0 (37.4 to 40.5) | –0.41 (–2.35 to 1.53), 0.99 | 0.68 |
| SF-12 PCS | ||||
| Month 6 | 45.2 (44.1 to 46.3) | 43.5 (42.4 to 44.6) | 1.75 (0.21 to 3.28), 0.78 | 0.03 |
| Month 12 | 43.6 (42.4 to 44.8) | 43.0 (41.8 to 44.2) | 0.59 (–1.07 to 2.26), 0.85 | 0.48 |
| BMI | ||||
| Month 6 | 30.3 (29.8 to 30.8) | 30.1 (29.6 to 30.6) | 0.16 (–0.54 to 0.85), 0.35 | 0.65 |
| Month 12 | 30.2 (29.5 to 30.8) | 29.9 (29.3 to 30.5) | 0.25 (–0.62 to 1.13), 0.45 | 0.57 |
FIGURE 11.
Adjusted means for BMI by treatment group and time point.

These results were extracted from a mixed-effects model in which the participant was treated as a random effect and observations over time (6 and 12 months) were nested within participant. The effect of randomised treatment group was assessed while adjusting for time, group-by-time interaction, baseline BMI and baseline smoking severity as fixed effects, and site as a random effect. Different covariance structures were applied to the model. An unstructured pattern that models all variances and covariances separately resulted in the lowest AIC and so was used in the final model. Diagnostics of model fit revealed a minor violation: the ‘body’ (centre) of the distribution of the standardised residuals was normal, but the distribution was short-tailed; however, the residuals were uniform against fitted values. Data transformations of the outcome (log, square root and square) did not substantially improve the model fit and so analyses were conducted on untransformed data.
Summary of results for secondary outcomes
Use of e-cigarettes and lifestyle data at 6 and 12 months
Participant-reported use of e-cigarettes and lifestyle data collected at 6 and 12 months are summarised in Tables 22 and 23. Fifty-four per cent of randomised participants reported having ever tried an e-cigarette at the 12-month time point (66.7% of those with valid response to this question). Of these, however, most (64.7%) reported that they had not used an e-cigarette in the past month. Approximately half said that their use of tobacco had not changed since they started to use e-cigarettes. Of those with valid data, 59.0% of the BSC group and 56.6% of the usual-care group reported leading a fairly or very healthy life at 6 months. Of those with valid data, 57.1% of the BSC group and 56.8% of the usual-care group reported leading a fairly or very healthy life at 12 months. At month 12, a similar proportion of participants in each group reported that they drank alcohol (48.4% in the BSC group and 47.7% in the usual-care group), with the BSC group consuming an average of 8.7 units in the last week (11.9 units in the usual-care group).
| Characteristic, n (%) | Month 6 | Month 12 | ||||
|---|---|---|---|---|---|---|
| BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) | |
| Ever tried an e-cigarette | ||||||
| Yes | 157 (59.3) | 155 (59.4) | 312 (59.3) | 135 (50.9) | 151 (57.9) | 286 (54.4) |
| No | 69 (26.0) | 59 (22.6) | 128 (24.3) | 79 (29.8) | 64 (24.5) | 143 (27.2) |
| Missing | 39 (14.7) | 47 (18.0) | 86 (16.4) | 51 (19.3) | 46 (17.6) | 97 (18.4) |
| If yes: | n = 157 | n = 155 | n = 312 | n = 135 | n = 151 | n = 286 |
| Use of e-cigarette in last 30 days | ||||||
| Never | 86 (54.8) | 98 (63.2) | 184 (59.0) | 89 (65.9) | 96 (63.6) | 185 (64.7) |
| Less than once per week | 14 (8.9) | 12 (7.7) | 26 (8.3) | 6 (4.4) | 11 (7.3) | 17 (5.9) |
| Less than daily but at least once per week | 21 (13.4) | 13 (8.4) | 34 (10.9) | 11 (8.1) | 17 (11.3) | 28 (9.8) |
| Every day | 36 (22.9) | 32 (20.6) | 68 (21.8) | 28 (20.7) | 27 (17.9) | 55 (19.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.3) |
| Length of use of e-cigarettes | ||||||
| < 1 month | 48 (30.6) | 56 (36.1) | 104 (33.3) | 36 (26.7) | 39 (25.8) | 75 (26.2) |
| 1–6 months | 30 (19.1) | 30 (19.4) | 60 (19.2) | 25 (18.5) | 36 (23.8) | 61 (21.3) |
| 6–12 months | 21 (13.4) | 16 (10.3) | 37 (11.9) | 23 (17.0) | 22 (14.6) | 45 (15.7) |
| > 1 year | 40 (25.5) | 40 (25.8) | 80 (25.6) | 37 (27.4) | 36 (23.8) | 73 (25.5) |
| Missing | 18 (11.5) | 13 (8.4) | 31 (9.9) | 14 (10.4) | 18 (11.9) | 32 (11.2) |
| Nicotine levels in cartridges used in e-cigarette | ||||||
| None (0 mg) | 6 (3.8) | 10 (6.5) | 16 (5.1) | 4 (3.0) | 6 (4.0) | 10 (3.5) |
| Low (< 8 mg) | 22 (14) | 25 (16.1) | 47 (15.1) | 28 (20.7) | 39 (25.8) | 67 (23.4) |
| Medium (8–16 mg) | 39 (24.8) | 41 (26.5) | 80 (25.6) | 39 (28.9) | 32 (21.2) | 71 (24.8) |
| High (> 16 mg) | 34 (21.7) | 37 (23.9) | 71 (22.8) | 28 (20.7) | 40 (26.5) | 68 (23.8) |
| Missing | 56 (35.7) | 42 (27.1) | 98 (31.4) | 36 (26.7) | 34 (22.5) | 70 (24.5) |
| Use of tobacco changed since using e-cigarettes | ||||||
| Yes, I smoke less tobacco | 70 (44.6) | 61 (39.4) | 131 (42.0) | 63 (46.7) | 56 (37.1) | 119 (41.6) |
| Yes, I smoke more tobacco | 4 (2.5) | 9 (5.8) | 13 (4.2) | 4 (3.0) | 3 (2.0) | 7 (2.4) |
| No, it has not changed | 65 (41.4) | 75 (48.4) | 140 (44.9) | 55 (40.7) | 78 (51.7) | 133 (46.5) |
| Missing | 18 (11.5) | 10 (6.5) | 28 (9.0) | 13 (9.6) | 14 (9.3) | 27 (9.4) |
| Reason started using e-cigarettes | ||||||
| To quit smoking tobacco | 67 (42.7) | 71 (45.8) | 138 (44.2) | 74 (54.8) | 84 (55.6) | 158 (55.2) |
| To try a safer alternative to tobacco | 28 (17.8) | 18 (11.6) | 46 (14.7) | 16 (11.9) | 12 (7.9) | 28 (9.8) |
| To try something new | 18 (11.5) | 9 (5.8) | 27 (8.7) | 7 (5.2) | 18 (11.9) | 25 (8.7) |
| To smoke less tobacco | 23 (14.6) | 33 (21.3) | 56 (17.9) | 21 (15.6) | 20 (13.2) | 41 (14.3) |
| Other | 11 (7.0) | 14 (9.0) | 25 (8.0) | 10 (7.4) | 9 (6.0) | 19 (6.6) |
| Missing | 10 (6.4) | 10 (6.5) | 20 (6.4) | 7 (5.2) | 8 (5.3) | 15 (5.2) |
| Characteristic | Month 6 | Month 12 | ||||
|---|---|---|---|---|---|---|
| BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) | BSC (N = 265) | Usual care (N = 261) | Overall (N = 526) | |
| Healthy life, n (%) | ||||||
| A very healthy life | 18 (6.8) | 7 (2.7) | 25 (4.8) | 12 (4.5) | 12 (4.6) | 24 (4.6) |
| A fairly healthy life | 116 (43.8) | 117 (44.8) | 233 (44.3) | 113 (42.6) | 109 (41.8) | 222 (42.2) |
| A not very healthy life | 72 (27.2) | 72 (27.6) | 144 (27.4) | 70 (26.4) | 71 (27.2) | 141 (26.8) |
| An unhealthy life | 21 (7.9) | 23 (8.8) | 44 (8.4) | 24 (9.1) | 21 (8.0) | 45 (8.6) |
| Missing | 38 (14.3) | 42 (16.1) | 80 (15.2) | 46 (17.4) | 48 (18.4) | 94 (17.9) |
| Eat five portions of fruit and vegetables a day, n (%) | ||||||
| Yes | 107 (40.4) | 104 (39.8) | 211 (40.1) | 109 (41.1) | 100 (38.3) | 209 (39.7) |
| No | 121 (45.7) | 114 (43.7) | 235 (44.7) | 110 (41.5) | 115 (44.1) | 225 (42.8) |
| Missing | 37 (14.0) | 43 (16.5) | 80 (15.2) | 46 (17.4) | 46 (17.6) | 92 (17.5) |
| Exercise for ≥ 20 minutes, n (%) | ||||||
| Daily | 94 (35.5) | 94 (36.0) | 188 (35.7) | 98 (37.0) | 77 (29.5) | 175 (33.3) |
| Weekly | 61 (23.0) | 42 (16.1) | 103 (19.6) | 61 (23.0) | 60 (23.0) | 121 (23.0) |
| Monthly | 3 (1.1) | 6 (2.3) | 9 (1.7) | 4 (1.5) | 6 (2.3) | 10 (1.9) |
| Seldom | 30 (11.3) | 25 (9.6) | 55 (10.5) | 21 (7.9) | 36 (13.8) | 57 (10.8) |
| Never | 40 (15.1) | 51 (19.5) | 91 (17.3) | 35 (13.2) | 35 (13.4) | 70 (13.3) |
| Missing | 37 (14.0) | 43 (16.5) | 80 (15.2) | 46 (17.4) | 47 (18.0) | 93 (17.7) |
| Drink alcohol, n (%) | ||||||
| Yes | 119 (44.9) | 106 (40.6) | 225 (42.8) | 106 (40.0) | 103 (39.5) | 209 (39.7) |
| No | 110 (41.5) | 113 (43.3) | 223 (42.4) | 113 (42.6) | 113 (43.3) | 226 (43.0) |
| Missing | 36 (13.6) | 42 (16.1) | 78 (14.8) | 46 (17.4) | 45 (17.2) | 91 (17.3) |
| If yes, units in last week | ||||||
| Mean (SD) | 10.2 (18.5) | 9.9 (18.3) | 10.0 (18.4) | 8.7 (11.5) | 11.9 (27.2) | 10.3 (20.8) |
| Median (min., max.) | 5 (0, 164) | 4 (0, 126) | 4 (0, 164) | 4 (0, 58) | 4 (0, 220) | 4 (0, 220) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Compliance with intervention
Intervention session attendance
A total of 1654 treatment sessions were arranged for 238 of the 265 intervention participants between November 2015 and December 2017, of which 1483 (89.7%) were attended. A total of 48 sessions were cancelled and in 123 cases the participant did not attend the session (Table 24). Overall, 234 out of the 265 intervention participants (88.3%) attended at least one treatment session (the remaining 27 did not partake in any intervention sessions as they withdrew from the trial or were unwell). For these 234 participants, the mean number of sessions attended was 6.4 (SD 3.5, median 6, range 1–14). Sessions lasted an average of 39.4 minutes (SD 17.2 minutes, median 35, range 5–120 minutes). Most (85.0%) sessions took place face to face, 4.1% took place over the telephone and 10.9% took place via another mode. The majority (81.1%) took place at the participant’s home.
| Intervention details | BSC (N = 265) |
|---|---|
| Number of sessions attended, n (%) | |
| 0 | 31 (11.7) |
| 1 | 19 (7.2) |
| 2 | 23 (8.7) |
| 3 | 23 (8.7) |
| 4 | 22 (8.3) |
| 5 | 16 (6.0) |
| 6 | 15 (5.7) |
| 7 | 16 (6.0) |
| 8 | 25 (9.4) |
| 9 | 24 (9.1) |
| 10 | 22 (8.3) |
| 11 | 12 (4.5) |
| 12 | 12 (4.5) |
| 13 | 3 (1.1) |
| 14 | 2 (0.8) |
| Attended at least one session | 234 (88.3) |
| Number of sessions attended | |
| Mean (SD) | 6.3 (3.5) |
| Median (min., max.) | 6 (1, 14) |
| Total number of sessions attended | 1483 |
| Length of sessions (minutes) | |
| Mean (SD) | 39.6 (17.2) |
| Median (min., max.) | 35 (5, 120) |
| Mode, n (%) | |
| Face to face | 1260 (85.0) |
| Telephone | 61 (4.1) |
| Other | 156 (10.5) |
| Missing | 6 (0.4) |
| Location, n (%) | |
| Home | 1202 (81.1) |
| Other | 276 (18.6) |
| Missing | 5 (0.3) |
Complier-average causal effect analysis
The CACE estimate of attending one additional intervention session was a small, non-statistically significant, increase in the likelihood of being a CO-verified quitter at 12 months (OR 1.07, 95% CI 0.97 to 1.18; p = 0.20).
Use of usual-care stop smoking services
The most used usual-care smoking cessation services were consultations with the GP, the pharmacist and the SSS. In the first 6 months of the trial, 100 out of the 212 responders (47.2%) in the usual-care group reported not using any of them, while 144 out of the 218 responders (66.1%) in the intervention group reported having used them. Among those who reported any use, the mean number of consultations was 2.8 (SD 3.5, median 2, range 1–31) in the usual-care group and 3.3 (SD 2.8, median 2, range 1–13) in the intervention group. In the second half of the trial, 142 out of 213 responders (66.7%) in the usual-care group reported not using any of the three services, while 141 out of 213 responders (66.2%) in the intervention group reported so. Among those who reported any use, the mean number of consultations was 3.3 (SD 3.4, median 2, range 1–18) in the usual-care group and 2.7 (SD 3.2, median 2, range 1–24) in the intervention group. Participants also reported consulting with other health-care professionals, such as practice nurse and health-care assistant, but these were rare cases.
Withdrawals
Some of the information reported in this section is given in Peckham et al. 1 This contains information licensed under the Non-Commercial Government Licence v2.0.
There were four categories of participant withdrawal:
-
Full withdrawal – participant withdrawn from the trial with regard to completion of both postal questionnaires and collection of GP data.
-
Withdrawal from follow-up – participant withdrawn from the completion of postal questionnaires but agreed to the continuing collection of GP data.
-
Withdrawal from treatment – participant withdrawn from trial intervention treatment, but agreed to continuing completion of postal questionnaires and collection of GP data.
-
Too unwell to continue – participant was deemed too unwell by medical staff to complete any questionnaires. This generally occurred only when a participant has been hospitalised.
Overall, there were 93 (17.7%) participant withdrawals (all types) during the trial: 23 participants (8.8%) from the usual-care group and 70 participants (26.4%) from the BSC group.
A total of 47 participants (8.9%) withdrew fully from the trial, two participants (0.4%) withdrew from follow-up, two (0.4%) were too unwell to continue and 42 (15.8%) withdrew from the intervention (Table 25).
| Withdrawals type | Usual care | BSC | Total |
|---|---|---|---|
| Full withdrawal | 21 | 26 | 47a |
| Withdrawal from follow-up | 1 | 1 | 2 |
| Withdrawal from treatment | 0 | 42 | 42 |
| Too unwell to continue | 1 | 1 | 2 |
| Total | 23 | 70 | 93 |
Adverse events
There were 365 adverse events among 174 participants during the course of the trial. Of these, 111 were classed as serious. More participants in the BSC group (42.1%) experienced one or more adverse events than those in the usual-care group (24.5%). All adverse events (n = 58) that were deemed to be definitely or probably related to the intervention or trial participation were all non-serious adverse events (Table 26). This includes three events, related to NRT side effects, which were experienced by usual-care participants as part of a routine care quit attempt.
| Adverse event | Relationship | BSC | Usual care | Total |
|---|---|---|---|---|
| Serious adverse event | Definitely related | 0 | 0 | 0 |
| Probably related | 0 | 0 | 0 | |
| Possibly related | 1 | 0 | 1 | |
| Unlikely to be related | 36 | 18 | 54 | |
| Unrelated | 31 | 24 | 55 | |
| Unclassifieda | 1 | 0 | 1 | |
| Non-serious adverse event | Definitely related | 2 | 0 | 2 |
| Probably related | 53 | 3b | 56 | |
| Possibly related | 28 | 3 | 31 | |
| Unlikely to be related | 52 | 33 | 85 | |
| Unrelated | 56 | 24 | 80 | |
| Total | 260 | 105 | 365 |
Of the 111 serious adverse events reported, the majority were related to unplanned hospital admission due to a deterioration in the participant’s mental health condition (n = 98). The remaining serious adverse events were associated with unplanned hospital admissions associated with comorbidities (n = 6) or death (n = 7). The one event deemed to be possibly related was an inpatient hospitalisation due to infective chronic obstructive pulmonary disease.
Of the 254 non-serious adverse events reported, the majority of events were associated with participant comorbidities (n = 124). Sixty-six events were associated with NRT side effects and 57 with the participant’s mental ill health. Of the remaining events, five were associated with accident and emergency or emergency ambulance use, but no information was available with regard to the reasons for this and two were unable to be classified (smoking teabags, n = 1; media coverage of family member, n = 1). Of the 56 events deemed to be probably related, the majority were associated with NRT side effects (n = 50) and of those possibly related, the majority were associated with participant comorbidities (n = 14). The two events deemed to be definitely related were both associated with NRT side effects (nausea following Champix use, n = 1; itching and redness caused by NRT patch, n = 1).
Summary of findings
Attempts were made to contact a total of 1606 people for eligibility and consent to partake in the trial, of which 526 were randomised (a rate of 33%). A primary reason for ineligibility, besides being unable to contact the person and non-consent, was that the person did not smoke or did not smoke at least five tobacco cigarettes a day. Over two-thirds of the participants were recruited from CMHT direct referral (72%). Between October 2015 and December 2016, 265 participants were randomised to BSC and 261 participants were randomised to usual care. This was over our original sample size target of 400 participants. Almost 60% of the participants were male and the average age was 46 years (range 19–72 years). The most common mental health diagnosis was schizophrenia or other psychotic illness (65%), followed by bipolar disorder (22%) and schizoaffective disorder (12%). The mean number of cigarettes smoked per day at baseline was 25, and the most commonly reported reasons for wanting to quit smoking were that it is bad for your health (88%) and is expensive (72%).
Retention rates in the trial were high. At the primary time point of 12 months, 450 (94%) of the 480 participant questionnaires intended to be completed were returned (86% of 526 randomised participants), with most (98%) being completed face to face with the MH-SCP. In total, 442 participants were included in the primary analysis (i.e. provided a CO breath measurement and completed the self-reported smoking status question on the 12-month participant questionnaire). A total of 34 out of 223 participants (15%; 13% of 265 randomised participants) fulfilled the definition for CO-verified smoking cessation in the BSC group, and 22 out of 219 participants (10%; 8% of 261 randomised participants) in the usual-care group. Therefore, participants in the BSC group were more likely to be a CO-verified 1-week quitter at 12 months post randomisation than participants in the usual-care group, but this difference was not statistically significant (OR 1.6, 95% CI 0.9 to 2.8; p = 0.12). A larger difference was, however, observed at the 6-month time point and this was statistically significant (OR 2.4, 95% CI 1.2 to 4.7; p = 0.01), perhaps suggesting an early benefit of the intervention, which waned over time.
The trial was originally powered at 80% to detect a relative increase of 1.7 in quitting from 20% in the usual-care group to 34% in the BSC group, allowing for a 20% loss to follow-up at 12 months. Ultimately, 526 participants were randomised and 442 (84%) were included in the primary analysis, so the loss to follow-up rate was lower than 20%. However, the quit rate was half that expected in the usual-care group (10% as opposed to 20%); therefore, the power needed to detect a relative increase in quit rate of 1.7 (to 17% in the BSC group) was ultimately 58%. Although this was not statistically significant at 12 months, the difference (10% vs. 15%) may still be clinically meaningful.
The primary result was robust to sensitivity checks, both the pre-planned and post hoc analyses, and similar results were seen when considering only self-reported smoking cessation. There was no evidence of a difference between the two groups in secondary outcomes at any time point except for the MTQ, which statistically significantly favoured the BSC group at 12 months, with a borderline p-value of 0.06 at 6 months. The physical component of the SF-12 also statistically significantly favoured the BSC group at 6 months (but not at 12 months).
The proportion of participants reporting that they had ever used e-cigarettes remained reasonably stable throughout the trial, as did the proportion reporting use in the previous month, suggesting that e-cigarettes were not frequently used as a NRT for those attempting to quit in the trial.
The uptake of the BSC intervention was reasonable, with 88% of participants randomised to the BSC group attending at least one session (median 6, range 1–14 sessions). The majority of sessions took place in the participants’ homes.
A total of 111 serious adverse events were reported in the trial and more participants in the BSC group (42%) reported one or more adverse events than in the usual-care group (25%); however, none of the SAEs was deemed to be related to trial participation.
Chapter 5 Health economic analysis
Costs
Intervention costs
Training and supervision
The initial training session delivered by the NCSCT was invoiced at a total of £5325. The opportunity cost for time attended by team members was estimated at £43 per hour59 for 15 hours, costing £2589. The 2-day training sessions for the MH-SCPs were delivered by two trainers at £43 per hour,59 costing £1290. In total, eight 2-day training sessions were held, amounting to £10,320. The time costs spent by the practitioners were costed at £28 per hour,59 resulting in £23,520 for 56 people for 2 days in total. The total printing cost was recorded as £105. Therefore, the total training costs were £41,850. The training costs were then allocated evenly to the participants in the BSC group. Training costs per participant were therefore estimated at £158.
Supervisors recorded the supervision sessions and estimated approximately 30 minutes of supervision per participant. The cost of supervision was estimated at £22 per participant. The first-year depreciation value of 56 CO monitors was £6720. It gave a cost of £10 per participant. In total, training, supervision and material cost per participant was £190 in the BSC group.
The control treatment was usual care that required no special training. The costs of training and supervision were considered zero for the usual-care group.
Intervention delivery
There were two parts of intervention delivery: (1) the BSC session in the BSC group and (2) usual care in both groups.
Bespoke smoking cessation delivery in the bespoke smoking cessation group
For the BSC support sessions, among the 265 participants randomised to the BSC group, two participants attended one session but the session records were missing. There were 27 participants who had withdrawn or been otherwise uncontactable before the treatment began, and four participants who had been contacted but failed to attend the first support session.
Among the 231 participants who had at least one support session, 28% attended one to three support sessions, 22% attended four to six support sessions, 28% attended seven to nine support sessions, and 22% attended ≥ 10 sessions. There were 1481 sessions delivered in total. The duration of the sessions varied hugely, ranging from 5 minutes to 120 minutes. There were 695 (47%) sessions that lasted 5–30 minutes and 715 (48%) sessions that lasted 35–60 minutes. There were 65 (4%) sessions that lasted 65–90 minutes and only six sessions (< 1%) that were > 90 minutes.
For each attended session, an estimated 10 minutes of administration time was added to account for the necessary paperwork for the session. There were 1370 appointments that entailed travel by the practitioners, including some of the did not attend (DNA) or late cancellation. Owing to the difficulty in collecting travel time for all individual appointments, an estimated 40 minutes of travel time for a return journey was added to the appointments that required travelling.
There were 149 participants who recorded no DNA or cancellation during the trial period and 89 participants who recorded 171 occasions of DNA/cancellation, the majority of whom recorded only once (47/89). Once a DNA or cancellation occurred, attempts were often made to re-establish contact or reschedule appointments. The time spent on these attempts ranged from 5 to 30 minutes per DNAs/cancellations but it should be noted that not all DNAs/cancellations entailed this staff time. The majority (87%) of these attempts were within 10 minutes.
In total, among the 263 participants whose session data were not missing, the mean number of attended sessions was 5.6 (SD 3.8) per participant and the duration of total session time per participant was 222 minutes (SD 166 minutes, range 0–775 minutes). The total time on rearrangement in the case of DNA/cancellation was 5 minutes (SD 10 minutes, range 0–65 minutes) per participant, while the mean number of DNAs/cancellations was 1 (SD 1). The total travel time per participant was 208 minutes (SD 155 minutes, range 0–560 minutes). The total administration time per participant was 56 minutes (SD 38 minutes, range 0–140 minutes). Excluding the two participants whose session data were missing, the mean total BSC delivery time was 492 minutes (SD 339 minutes, range 0–1425 minutes) per participant in the BSC group. Applying an hourly cost of £28, the mean cost of BSC delivery was estimated at £229 (SD £158).
Usual-care delivery in both groups
For usual GP care, both groups reported asking for help/advice for smoking cessation from GPs, pharmacists, SSSs and stop smoking helplines. The mean usage was low in both groups, with large standard deviation, indicating a wide variance on a participant level (Table 27). The usage of individual services did not appear to differ between groups, except for SSS. The mean usage of SSSs in the usual-care group was twice as high as that in the BSC group in months 1–6 post randomisation. In months 7–12 post randomisation, the mean usage of SSSs was still higher in the usual-care group than in the BSC group, but not as considerable. The mean cost of these services for smoking cessation was £37 (SD £60) per participant among the 212 responders in the usual-care group and £28 (SD £62) per participant among the 217 responders in the BSC group in months 1–6 post randomisation. In months 7–12 post randomisation, the mean cost of these services for smoking cessation was £25 (SD £59) per participant among the 213 responders in the usual-care group, and £22 (SD £56) per participant among the 212 responders in the BSC group.
| Usual GP care services | Unit cost | BSC | UC | ||
|---|---|---|---|---|---|
| Mean usage (SD) | Mean cost (SD) | Mean usage (SD) | Mean cost (SD) | ||
| Months 1–6 | n = 218 | n = 212 | |||
| Cessation consultation with GP | £40/session | 0.50 (1.38) | £20 (£55) | 0.62 (1.65) | £22 (£46) |
| Cessation consultation with pharmacist | £7/session | 0.35 (1.00) | £2 (£7) | 0.33 (1.78) | £2 (£12) |
| Smoking cessation service | £19/session | 0.27 (1.07) | £5 (£20) | 0.62 (1.65) | £12 (£31) |
| NHS stop smoking helpline | £7/call | 0.07 (0.54) | £1 (£4)a | 0.08 (0.38) | £1 (£3) |
| Total | – | £28 (£62)a | – | £37 (£60) | |
| Months 7–12 | n = 213 | n = 213 | |||
| Cessation consultation with GP | £40/session | 0.35 (1.09) | £14 (£43) | 0.36 (0.96) | £14 (£38) |
| Cessation consultation with pharmacist | £7/session | 0.22 (0.75) | £2 (£5) | 0.28 (0.88) | £2 (£6) |
| Smoking cessation service | £19/session | 0.35 (1.81) | £7 (£34) | 0.45 (1.60) | £8 (£30) |
| NHS stop smoking helpline | £7/call | 0.02 (0.14)b | £0 (£1)b | 0.08 (0.47)b | £1 (£3)b |
| Total | – | £22 (£56)c | – | £25 (£59) | |
In addition to responses to the structured questions, there were individual participants who reported asking for help/advice from community psychiatric nurses, practice nurses, support workers, family therapists, hospital nurses, psychiatrists and dentists. The report of these service uses was rare, with only one or two participants in one group.
Costing was undertaken for a 10-minute brief advice session for stop smoking as equivalent to the length of opportunistic brief advice session by GPs and practice nurses;59 the unit costs of these SSSs are shown in Table 30. Applying the unit, an estimated cost was calculated and added to the costs estimated from the structured questions.
Accounting for both structured and open questions, the mean cost of usual care for SSSs was £37 (SD £60) per participant among the 212 responders in the usual-care group and £28 (SD £62) per participant among the 217 responders in the BSC group, during months 1–6 post randomisation. The mean cost of usual care for SSSs was £26 (SD £59) per participant among the 213 responders in the usual-care group and £23 (SD £56) per participant among the 212 responders in the BSC group, during months 7–12 post randomisation.
Practices were contacted to extract participants’ prescription information on pharmacotherapies for smoking cessation. The prescription information for 160 (61%) participants was returned in the usual-care group and the prescription information for 156 (59%) participants was returned in the BSC group. Of the participants with returned data collection, 45 participants in the usual-care group and 100 participants in the BSC group recorded being prescribed pharmacotherapies at some point during the trial period. There were four participants in the usual-care group and 17 participants in the BSC group who had prescription data insufficient for cost estimation.
Therefore, the mean NIC of pharmacotherapy prescription was £26 (SD £73) per participant among the 156 respondents in the usual-care group and £92 (SD £198) per participant among the 139 respondents in the BSC group.
Health-care and social services costs
Table 28 presents the number of participants who responded to the questions regarding emergency and hospital services, and their mean use of each service. More than 80% of the participants in either group responded to the questions at either follow-up. In months 1–6 post randomisation, the usual-care group reported nearly twice as many hospital admissions as the BSC group among the responders, both in number of times (0.14 vs. 0.08) and number of nights (1.59 vs. 0.88), while the BSC group reported nearly twice as many use of emergency ambulance as the usual-care group (0.28 vs. 0.15). In months 7–12 post randomisation, the usual-care group reported many more hospital admissions than the BSC group (0.80 vs. 0.13). However, the relatively large SD (8.68) of the mean number of admissions in the usual-care group indicated that there was a small group of responders being admitted frequently.
| Services | BSC | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|
| Months 1–6 | Months 7–12 | Months 1–6 | Months 7–12 | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| A&E department | 218 | 0.39 (0.81) | 211 | 0.34 (0.80) | 212 | 0.37 (0.95) | 209 | 0.43 (0.96) |
| Hospital admission | ||||||||
| Times | 221 | 0.08 (0.29) | 211 | 0.13 (0.51) | 214 | 0.14 (0.58) | 209 | 0.80 (6.24) |
| Nights | 219 | 0.88 (6.77) | 212 | 1.00 (4.68) | 210 | 1.59 (10.61) | 208 | 1.58 (8.68) |
| Hospital outpatient appointment | 217 | 1.40 (2.79) | 212 | 1.43 (3.15) | 213 | 1.72 (4.04) | 208 | 1.66 (3.49) |
| Day case/procedure | 219 | 0.13 (0.57) | 211 | 0.18 (0.70) | 213 | 0.14 (0.43) | 209 | 0.12 (0.42) |
| Emergency ambulance | 219 | 0.28 (0.73) | 213 | 0.22 (0.67) | 215 | 0.15 (0.43) | 210 | 0.25 (1.32) |
Over 80% of the participants in either group responded to the questions regarding the primary and community services at follow-ups. The mean use of the services was generally lower than once per responder in both groups with a few exceptions (Table 29). The highest mean usage was reported for community psychiatric nurse, with over five times in the usual-care group in both follow-up periods and over six times in the BSC group. This was followed by CMHT visits. The mean number of visits by CMHT was close to five per responder in the usual-care group throughout the trial period. In the BSC group, the mean number of visits was over five per responder in months 1–6 and over four per responder in months 7–12. The third highest used service was day care. Both groups reported a mean number of use over three times during a 6-month period. Both groups reported over two visits to a GP in surgery during a 6-month period and over one visit to a practice nurse. The large SDs indicated a skewed distribution of service use.
| Services | BSC | Usual care | ||
|---|---|---|---|---|
| Months 1–6 (n = 220) | Months 7–12 (n = 214) | Months 1–6 (n = 214) | Months 7–12 (n = 213) | |
| GP home visit | 0.21 (1.14) | 0.20 (0.87) | 0.26 (1.43) | 0.29 (1.39) |
| GP surgery | 2.34 (2.97) | 2.37 (3.46) | 2.63 (3.69) | 2.69 (3.69) |
| GP telephone | 0.51 (1.39) | 0.61 (1.70) | 0.73 (2.21) | 0.56 (1.26) |
| Practice nurse | 1.16 (2.38) | 1.06 (2.19) | 1.42 (2.93) | 1.15 (2.28) |
| District nurse | 0.20 (1.36) | 0.07 (0.60) | 0.17 (1.07) | 0.20 (1.53) |
| Community psychiatric nurse | 6.25 (10.58) | 6.33 (10.55) | 5.24 (7.13) | 5.48 (10.80) |
| Health visitor | 0.17 (1.79) | 0.15 (1.76) | 0.19 (1.30) | 0.89 (12.33) |
| Clinical psychologist | 1.05 (3.77) | 0.65 (2.97) | 0.81 (3.47) | 0.58 (1.99) |
| NHS counsellor | 0.29 (2.21) | 0.12 (1.64) | 0.18 (1.24) | 0.05 (0.44) |
| NHS dentist | 0.63 (1.20) | 0.78 (1.33) | 0.74 (1.54) | 0.79 (1.42) |
| Podiatrist | 0.26 (0.81) | 0.20 (0.71) | 0.22 (0.76) | 0.15 (0.61) |
| Occupational therapist | 0.64 (3.53) | 0.72 (5.33) | 0.32 (1.48) | 0.49 (2.60) |
| Physiotherapist | 0.22 (1.83) | 0.20 (1.23) | 0.19 (1.00) | 0.23 (1.27) |
| CBT | 0.19 (1.26) | 0.38 (2.27) | 0.50 (3.11) | 0.18 (1.85) |
| MBCT | 0.24 (1.51) | 0.69 (8.26) | 0.19 (1.75) | 0.13 (0.82) |
| Crisis team | 0.47 (2.41) | 1.10 (5.02) | 0.59 (2.09) | 0.62 (2.04) |
| CMHT | 5.67 (10.54) | 4.74 (10.77) | 4.91 (8.17) | 4.98 (8.31) |
| Day care service | 3.72 (19.56) | 4.14 (23.97) | 3.64 (19.46) | 3.54 (20.89) |
| Social worker | 1.66 (12.68) | 0.68 (2.56) | 1.23 (5.96) | 0.73 (2.62) |
| Family support worker | 0.12 (1.02) | 0.76 (7.92) | 1.48 (13.17) | 1.20 (10.44) |
| Drug/alcohol support worker | 0.73 (8.15) | 0.23 (1.68) | 0.37 (2.59) | 0.46 (2.52) |
In addition to the structured service questions, there were a few participants who reported other services used. These included support worker, psychotherapist, specialist nurse, art therapist, forensic community team, health-care assistant, rehabilitation facility, outreach team, phlebotomist and midwife. The most reported service was support worker. There were 11 participants who reported 578 visits in months 1–6 in the usual-care group and 12 participants who reported 1498 visits in the BSC group. In months 7–12, there were nine participants who reported 611 visits in the usual-care group and nine participants in the BSC group reported 994 visits. Some of these participants reported daily visits by a support worker during the entire period. There was one participant in the usual-care group who stayed in a rehabilitation facility for 171 days and another reported 24 visits to a psychotherapist. The other services were reported by only one or two participants for no more than 10 visits.
According to the additional services reported by the participants, the corresponding unit costs were estimated from published resources (Table 30).
| Services | Unit cost (2016/17) | Sources |
|---|---|---|
| Other health-care personnel for stop smoking advice | ||
| Community psychiatric nurse | £6/10-minute brief advice for smoking | 59 |
| Practice nurse | £6/10-minute brief advice for smoking | 59 |
| Support worker | £4/10-minute brief advice for smoking | 59 |
| Nurse at hospital | £15/10-minute brief advice for smoking | 59 |
| Psychiatrist | £18/10-minute brief advice for smoking | 59 |
| Family therapist | £6/10-minute brief advice for smoking | 59 |
| Other health care and social services | ||
| Dentist | £21 | 59 |
| Support worker | £13/30-minute visit | 61 |
| Early intervention team | £184/contact | 61 |
| Psychotherapist | £194/contact | 60 |
| Specialist nurse | £64/contact | 60 |
| Outreach team | £132/contact | 61 |
| Pharmacist | £7/contact | 61 |
| Speech and language therapist | £96/one-to-one contact | 60 |
| Arts therapist | £40/40-minute contact | 61 |
| Forensic community team | £238/contact | 60 |
| Health-care assistant | £12/30-minute contact | 61 |
| Phlebotomist | £3/contact | 60 |
| Midwife | £62/contact | 60 |
Applying the unit costs to the quantities reported, the estimated costs of emergency and hospital services were £1443 (SD £6470) per responder in the usual-care group and £932 (SD £4228) per responder in the BSC group in months 1–6 post randomisation (Table 31). The estimated costs of primary and community care were £2367 (SD £2893) per responder in the usual-care group and £2529 (SD £3178) per responder in the BSC group in months 1–6 post randomisation. In months 7–12 post randomisation, the estimated costs of emergency and hospital services were £1317 (SD £5330) per responder in the usual-care group and £953 (SD £3027) per responder in the BSC group. During the same period, the estimated costs of primary and community care were £2303 (SD £3234) per responder in the usual-care group and £2429 (SD £3458) per responder in the BSC group.
| Services | BSC | Usual care | ||
|---|---|---|---|---|
| n | Mean cost (SD) | n | Mean cost (SD) | |
| Months 1–6 | ||||
| Emergency and hospital services | 211 | £932 (£4228) | 208 | £1443 (£6470) |
| Primary and community care | 220 | £2529 (£3178) | 214 | £2367 (£2893) |
| Months 7–12 | ||||
| Emergency and hospital services | 203 | £953 (£3027) | 202 | £1317 (£5330) |
| Primary and community care | 214 | £2429 (£3458) | 213 | £2303 (£3234) |
Practices were contacted to extract participants’ prescription information on antipsychotic medication. There were 223 (85%) participants for whom prescription information was returned in the usual-care group and 229 (86%) participants for whom prescription information was returned in the BSC group. Of the participants with returned data collection, 207 participants in the usual-care group and 219 participants in the BSC group recorded being prescribed antipsychotics at some point during the trial period. There were 28 participants in the usual-care group and 25 participants in the BSC group who had prescription data that were insufficient for cost estimation. Therefore, the mean NIC of antipsychotic prescription was £736 (SD £1081) per participant among the 195 respondents in the usual-care group and £820 (SD £1296) per participant among the 204 respondents in the BSC group, including those reported for whom no antipsychotics were prescribed.
Wider societal costs
Other sources of smoking cessation advice
In addition to the smoking cessation services provided by the NHS and Personal Social Services, the participants reported their use of other sources of help. These included a smoking cessation helpline run by non-NHS organisations, the internet and books. These were reported by only a small group of participants. The most often used source of advice was the internet. In the 6 months post randomisation, 18 participants reported, in total, 82 times of internet use for advice on smoking cessation in the BSC group, and 22 participants reported a total of 61 times of use in the usual-care group. From 6 months to 12 months post randomisation, 21 participants in the usual-care group reported a total of 86 times of internet use and 16 participants in the BSC group reported 64 times of use. Among them, the highest number of uses appeared in the usual-care group where one participant reported 28 times of use in the 7–12 months post randomisation. It was followed by two participants who reported 25 times of use and 26 times of use in the BSC group in the 6 months post randomisation. The costs of internet use were not estimated because of the lack of details. In the 1–6 months post randomisation, books were used by 18 participants in the BSC group and 10 participants in the usual-care group. In the 7–12 months post randomisation, 10 participants in the BSC group and nine participants in the usual-care group used books. Only two participants in the BSC group and five in the usual-care group called another smoking cessation helpline during the 12-month period.
Participants’ out-of-pocket expenses in relation to smoking cessation
Participants were asked about their expenses for the purchase of NRT products, the purchase of e-cigarettes and travel to GP/SSS at the follow-ups.
There were 157 participants (81% of 195 responders) in the BSC group who reported not purchasing any NRT products and 152 participants (77% of 197 responders) in the usual-care group in the 12 months post randomisation. Among the 38 participants who reported any purchase in the BSC group, 27 purchased a single form of NRT product, eight purchased two forms of NRT products and three purchased three forms of NRT products. Among the 45 participants who reported any purchase in the usual-care group, 27 purchased a single form of NRT product, 13 purchased two forms of NRT products and three purchased three forms of NRT products.
Among all the NRT products asked about the most purchased one was the nicotine patch. There were 27 participants who reported purchasing patches in the usual-care group, compared with 14 in the BSC group (Figure 12). The next most purchased were the lozenge and mouth spray. There were 12 participants who reported purchase of a lozenge in either group and the purchase of mouth spray was almost the same (11 in the usual-care group and 12 in the BSC group). These were followed by nicotine gum, which was reported by nine participants in the usual-care group and 11 participants in the BSC group. There were five participants who reported purchasing an inhalator in the usual-care group and two in the BSC group. Only one participant reported purchasing nasal spray (in the BSC group). None of the participants reported purchasing microtabs.
FIGURE 12.
Number of participants who acquire NRT products on prescription, purchase or both, by group.

Comparing the sources of NRT products in those who had both self-report response and prescription records (132 in the usual-care group and 129 in the BSC group), the majority of the NRT products in the BSC group were acquired on prescription, whereas the usual-care group acquired their NRT products, if any, over the counter. A small number of participants in both groups acquired their products via both prescription and purchase.
Given that the majority of the responders reported no purchase at all during the 12 months post randomisation, the mean amount of purchase of individual forms of NRT products was low and the SD was comparatively large, indicating a skewed distribution of purchase (Table 32).
| NRT products | BSC, mean (SD) | Usual care, mean (SD) |
|---|---|---|
| Months 1–6 | n = 206 | n = 209 |
| Patches (pack) | 0.02 (0.14) | 0.20 (0.90) |
| Gum (pack) | 0.20 (1.79) | 0.09 (0.91) |
| Lozenges (pack) | 0.64 (5.97) | 0.11 (0.80) |
| Microtabs (pack) | – | – |
| Inhaler (bottle) | – | 0.08 (0.75) |
| Nasal spray (bottle) | 0.01 (0.21) | – |
| Mouth spray (bottle) | 0.07 (0.57) | 0.05 (0.33) |
| Months 7–12 | n = 214 | n = 209 |
| Patches (pack) | 0.11 (0.58) | 0.19 (1.01) |
| Gum (pack) | 0.02 (0.17) | 0.03 (0.22) |
| Lozenges (pack) | 0.62 (5.28) | 0.13 (1.16) |
| Microtabs (pack) | – | – |
| Inhaler (bottle) | 0.01 (0.15) | 0.00 (0.07) |
| Nasal spray (bottle) | – | – |
| Mouth spray (bottle) | 0.35 (3.40) | 0.03 (0.21) |
Applying Table 2 to the quantities in Table 32, the mean expense of NRT products was £8 (SD £30) among 209 responders in the usual-care group and £13 (SD £83) among 206 responders in the BSC group in months 1–6 post randomisation. In months 7–12, the mean expense for NRT products was £5 (SD £22) among 209 responders in the usual-care group and £17 (SD £97) among the 214 responders in the BSC group.
There were 136 participants who reported no purchase of e-cigarettes in the usual-care group and 152 participants in the BSC group in months 1–6 post randomisation. In months 7–12, there were 143 participants in the usual-care group and 148 participants in the BSC group who reported no purchase of e-cigarettes. The mean expense of e-cigarettes was £21 (SD £47) among 208 responders in months 1–6 and £21 (SD £59) among 205 responders in months 7–12 in the usual-care group. In the BSC group, the mean expense on e-cigarettes was £17 (SD £64) among 219 responders in months 1–6 and £19 (SD £68) among 210 responders in months 7–12.
The majority of the responders reported walking as their way of travelling to see their GP or SSS, followed by driving, taking a bus or taking a taxi. Fewer than five responders reported taking trains to see their GP or SSS. In the usual-care group, 173 responders in months 1–6 and 171 in months 7–12 reported no expense on travel. In the BSC group, 187 in months 1–6 and 176 in months 7–12 reported no expense on travel. The mean expense on travel to GP/SSS was £6 (SD £30) among 203 responders in months 1–6 and £3 (SD £11) among 199 responders in months 7–12, in the usual-care group. In the BSC group, it was £3 (SD £12) among 213 responders in months 1–6 and £2 (SD £9) among 205 responders in months 7–12. However, this expense might have been underestimated because some of the participants did not take into account when they used cars and others did.
Health-related quality of life
The EQ-5D-5L was returned by 260 participants in the usual-care group at baseline and 265 participants in the BSC group. The mean VAS score was 54.2 (SD 20.5) in the usual-care group and 54.8 (SD 22.9) in the BSC group. Two participants in the usual-care group had missing items on the descriptive system of EQ-5D-5L and, therefore, utility could not be calculated. Among the rest of the participants, the mean utility was 0.640 (SD 0.304) in the usual-care group and 0.638 (SD 0.280) in the BSC group. At 6 months, 217 participants in the usual-care group and 224 participants in the BSC group returned the questionnaire. The mean VAS score was 55.6 (SD 21.2) in the usual-care group and 58.3 (SD 21.8) in the BSC group. The mean utility was 0.659 (SD 0.307) in the usual-care group and 0.682 (SD 0.269) in the BSC group. At 12 months, 213 participants in the usual-care group and 216 participants in the BSC group returned information. The mean VAS score was 55.9 (SD 20.9) in the usual-care group and 55.8 (SD 23.9) in the BSC group. Three participants in the usual-care group and two in the BSC group had missing items on the descriptive system of EQ-5D-5L. Among the remaining participants, the mean utility was 0.662 (SD 0.306) in the usual-care group and 0.688 (SD 0.275) in the BSC group.
Missing data
The percentage of missing data varies from variable to variable (Table 33). As expected, the lowest missing percentage occurs among the variables at baseline. Missing data level ranges between 20% and 30% on participants’ self-reported items at follow-ups, which is consistent with the overall follow-up rate of the trial. The highest percentage of missing values is that of the costs of pharmacotherapy prescriptions in the trial period, which was collected from practices directly. This percentage of missing values is 44%; the number of multiple imputations was therefore set to 45. The missing values in the continuous variables were imputed using the predictive mean matching method, with 10 nearest neighbours to draw from. Binary variables were imputed using the logit method.
| Variables | Number of missing values | Percentage of missing values |
|---|---|---|
| Characteristics | ||
| Age | 1 | 0 |
| Gender | 0 | 0 |
| Study centre | 0 | 0 |
| Pre-existing medical condition | 4 | 1 |
| Duration since first diagnosis of SMI | 10 | 2 |
| Duration since start of smoking | 2 | 0 |
| Costs | ||
| Cost of BSC training and supervision | 0 | 0 |
| Cost of BSC delivery | 2 | 0 |
| Cost of usual smoking cessation in 6 months before baseline | 9 | 2 |
| Cost of usual smoking cessation in months 1–6 | 97 | 18 |
| Cost of usual smoking cessation in months 7–12 | 101 | 19 |
| Costs of pharmacotherapy prescriptions in months 1–12 | 231 | 44 |
| Cost of emergency and hospital services in 6 months before baseline | 14 | 3 |
| Cost of emergency and hospital services in months 1–6 | 107 | 20 |
| Cost of emergency and hospital services in months 7–12 | 121 | 23 |
| Cost of primary and community services in 6 months before baseline | 2 | 0 |
| Cost of primary and community services in months 1–6 | 92 | 17 |
| Cost of primary and community services in months 7–12 | 99 | 19 |
| Costs of antipsychotics in months 1–12 | 127 | 24 |
| Other measures | ||
| Cigarettes per day at baseline | 0 | 0 |
| Cigarettes per day at 6 months | 140 | 27 |
| Cigarettes per day at 12 months | 159 | 30 |
| FTND score at baseline | 14 | 3 |
| FTND score at 6 months | 146 | 28 |
| FTND score at 12 months | 171 | 33 |
| Outcomes | ||
| VAS at baseline | 1 | 0 |
| VAS at 6 months | 85 | 16 |
| VAS at 12 months | 97 | 18 |
| EQ-5D-5L utility at baseline | 3 | 1 |
| EQ-5D-5L utility at 6 months | 85 | 16 |
| EQ-5D-5L utility at 12 months | 102 | 19 |
| Out-of-pocket expenses | ||
| Travel expenses to GP surgery/stop smoking clinic in 6 months before baseline | 21 | 4 |
| Travel expenses to GP surgery/stop smoking clinic in months 1–6 | 110 | 21 |
| Travel expenses to GP surgery/stop smoking clinic in months 7–12 | 122 | 23 |
| Purchase of e-cigarette in 6 months before baseline | 10 | 2 |
| Purchase of e-cigarette in months 1–6 | 99 | 19 |
| Purchase of e-cigarette in months 7–12 | 111 | 21 |
| Purchase of NRT products in 6 months before baseline | 13 | 2 |
| Purchase of NRT products in months 1–6 | 111 | 21 |
| Purchase of NRT products in months 7–12 | 103 | 20 |
There were five participants who died before the 6-month follow-up and another two died before the 12-month follow-up. All of them were unable to be followed up at both follow-up points. For those who died before the 6-month follow-up, the costs and expenses in the first 6 months of the trial were to be imputed as others, while the costs and expenses in months 7–12 were set to zero. For the two who died after the 6-month follow-up but before the 12-month follow-up, their costs and expenses were to be imputed as others. The utility and VAS from EQ-5D-5L were set to zero at 6 months and 12 months for those died before the 6-month follow-up, and those who died after 6 months were to have 6-month values imputed but 12-month values set to zero. Other measures including cigarettes per day, alcohol drinking and FTND score were to be imputed conditioned on the death status at that time point.
Analysis
The BSC intervention cost, consisted of a fixed training cost and delivery cost, was estimated at £418 [standard error (SE) £10] per participant. The cost of usual GP care during the trial period was £65 (SE £6) per participant in the usual-care group and £52 (SE £6) per participant in the BSC group. The BSC group received more pharmacotherapies for smoking cessation on prescription than the usual-care group. The mean cost of pharmacotherapies on prescription was £91 (SE £13) in the BSC group, three times the mean cost in the usual-care group [£29 (SE £6)]. The total smoking cessation costs during the trial period were therefore £93 (SE £9) per participant in the usual-care group and £561 (SE £19) per participant in the BSC group.
In the 6 months before randomisation, both groups had similar levels of cost of smoking cessation from usual GP care [£38 (SE £4) vs. £37 (SE £4)]. The mean costs of emergency and hospital services were higher in the BSC group [£2030 (SE £494), compared with £1507 (SE £406) per participant in the usual-care group]. The costs of primary and community services were the first cost drive in both groups, with £2614 (SE £260) per participant in the usual-care group and £2746 (SE £223) per participant in the BSC group. The total health resource use costs were estimated at £4160 (£512) per participant in the usual-care group and £4813 (£548) per participant in the BSC group in the 6 months before randomisation.
Throughout the trial period, primary and community services remained the first cost driver in both groups, with the mean costs never dropping below £2000 per participant, and the mean costs of emergency and hospital services in the same period never reaching this threshold. The mean costs of emergency and hospital services were £1513 (SE £492) per participant and costs of primary and community services were £2391 (SE £204) in the usual-care group in the first 6 months of the trial. In the same period, the mean costs of emergency and hospital services were £982 (SE £285) per participant and costs of primary and community services were £2597 (SE £218) in the BSC group. In the second 6-month period of the trial, the mean costs of emergency and hospital services were £1404 (SE £361) per participant in the usual-care group and costs of primary and community services were £2320 (SE £232). In the BSC group, the mean costs of the mean costs of emergency and hospital services were £1004 (SE £211) per participant and costs of primary and community services were £2504 (SE £245) in the second 6-month period of the trial. The mean cost of antipsychotics on prescription in the trial period was estimated at £768 (SE £81) per participant in the usual-care group and £799 (SE £84) in the BSC group. The total health resource use costs were therefore £8396 (£774) per participant in the usual-care group and £7886 (SE £594) in the BSC group.
In the usual-care group, the mean VAS score was 54.2 (SE 1.3) at baseline, 54.9 (SE 1.9) at 6 months and 55.3 (SE 1.4) at 12 months. In the BSC group, the mean VAS score was 54.8 (SE 1.4) at baseline, 57.3 (SE 1.5) at 6 months and 55.1 (SE 1.6) at 12 months. In the usual-care group, the mean utility value was 0.640 (SE 0.019) at baseline, 0.647 (SE 0.020) at 6 months and 0.654 (SE 0.020) at 12 months. In the BSC group, the mean utility value was 0.638 (SE 0.017) at baseline, 0.670 (SE 0.018) at 6 months and 0.678 (SE 0.019) at 12 months. The mean QALYs were estimated at 0.647 (SE 0.017) in the usual-care group and 0.664 (SE 0.015) in the BSC group.
Primary analysis
There were 442 participants (219 in the usual-care group and 223 in the BSC group) who had CO-verified smoking status at 12-month follow-up. Without imputing missing data on this outcome, the quit rate at 12 months was 10.0% (SE 2.0%) in the usual-care group and 15.3% (SE 2.4%) in the BSC group. The mean intervention cost among these participants was £93 (SE £9) per participant in the usual-care group and £584 (SE £21) in the BSC group. The mean intervention cost per quitter was £906 (95% CI £612 to £1456) in the usual-care group and £3828 (95% CI £2901 to £5409) in the BSC group. The incremental intervention cost per additional quitter was £9472 (95% CI –£44,016 to £73,503).
Combining costs of smoking cessation and health resource use during the trial period, the total costs were estimated at £8489 (SE £775) per participant in the usual-care group and £8446 (SE £596) per participant in the BSC group (Table 34). The mean total costs were similar between groups during the trial period. After adjusting for age, gender, pre-existing medical conditions, duration since first diagnosis of SMI and health resource use costs in the 6 months before randomisation, the adjusted difference in mean total costs was –£270 (95% CI –£1817 to £1297), with the BSC group lower than the usual-care group. Although the unadjusted difference in QALYs between groups was 0.017, after adjusting for the same set of baseline covariates with the exception of replacing health resource use costs with EQ-3D-5L utility value at baseline, the adjusted difference became 0.026 (95% CI –0.008 to 0.045).
| Results | BSC (n = 265) | Usual care (n = 261) |
|---|---|---|
| Costs | ||
| Smoking cessation, mean (SE) | £561 (£19) | £93 (£9) |
| Health resource use, mean (SE) | £7886 (£594) | £8396 (£774) |
| Total, mean (SE) | £8446 (£596) | £8489 (£775) |
| Adjusted difference,a mean (95% CI) | –£270 (–£1817 to £1297) | |
| QALYs, mean (SE) | 0.664 (0.015) | 0.647 (0.017) |
| Adjusted difference,b mean (95% CI) | 0.026 (–0.008 to 0.045) | |
| Incremental cost-effectiveness ratio | ||
| ICER, mean | BSC dominates (uncertainty: Figure 13) | |
With the incremental costs being negative (BSC was less costly) and the incremental QALYs being positive (BSC was more effective), the BSC group dominated the usual-care group and was cost-effective based on the point estimation. However, both incremental costs and incremental QALYs have wide 95% CIs including zero, indicating the presence of high uncertainty.
Sensitivity analysis
Mapping incremental costs and incremental QALYs estimated from 5000 bootstrapped replicates onto the cost-effectiveness plane, Figure 13a shows the overall majority (92%) of dots that represented ICERs of the replicates, scattered on the right side of the y-axis (zero effect), indicating that the BSC is very likely to achieve higher QALYs. The distribution of these dots in relation to incremental costs is less clear, where 62% are scattered below zero and 38% above. This suggests that the BSC is likely to be cost-saving, but the uncertainty is higher. Taking into account the combination of both, 57% fall in the south-east quadrant, where the BSC group dominates the usual-care group (less costly but more effective). Figure 13b is the cost-effectiveness acceptability curve with WTP thresholds at £20,000/QALY and £30,000/QALY as reference line. For the probability of BSC being cost-effective, compared with usual care, although it starts high (> 60%) and rises with the increase of WTP, the increase in the probability shows a trend of becoming smaller. This leads to a gradual upwards curve and almost flat line close to 90%.
FIGURE 13.
Cost-effectiveness plane and cost-effectiveness acceptability curve of primary analysis results. (a) Cost-effectiveness plane; and (b) cost-effectiveness acceptability curve.


Complete-case analysis
Taking into account the outcome measures and baseline variables for adjustment, there were 80 participants in the usual-care group and 88 participants in the BSC group included in the complete-case analysis. The intervention cost was similar in the usual-care group between primary analysis (£93, SE £9) and complete-case analysis (£92, SD £128) while in the BSC group it was slightly higher in complete-case analysis (£593, SD £338) than in primary analysis (£561, SE £19). In the usual-care group, the mean costs of health services were £4822 (SD £7881) in the 6 months before randomisation, £3440 (SD £3745) in months 1–6 of the trial and £4293 (SD £8490) in months 7–12 of the trial, in complete-case analysis. In the BSC group, the mean costs of health services were £4594 (SD £7449) in the 6 months before randomisation, £3599 (SD £4173) in months 1–6 of the trial and £3555 (SD £5078) in months 7–12 of the trial, in complete-case analysis. The difference between primary analysis and complete-case analysis was more prominent in the usual-care group than in the BSC group.
For the BSC group, the mean costs in complete-case analysis did not vary from those in primary analysis by much. For the usual-care group, however, the mean costs became higher than those in primary analysis in the 6 months before randomisation and months 7–12 of the trial, and were lower than in primary analysis in months 1–6 of the trial. This change led to a reverse of the relative position when comparing between groups and the reduction of the difference in the 6 months before randomisation and months 1–6 of the trial. In the second 6-month period of the trial, although the mean costs of health services were still higher in the usual-care group, the difference between the mean costs in the two groups was widened.
For EQ-5D-5L utility value, the pattern of change was more consistent. In complete-case analysis, the mean utility value was 0.595 (SD 0.319) at baseline, 0.612 (SD 0.336) at 6 months and 0.641 (SD 0.303) at 12 months in the usual-care group, while it was 0.661 (SD 0.257) at baseline, 0.686 (SD 0.254) at 6 months and 0.684 (SD 0.262) at 12 months in the BSC group. Although in the usual-care group the mean utility values were always lower in complete-case analysis than in primary analysis, the BSC group was the opposite.
The mean total cost was £911 (95% CI –£2769 to £2631) higher in the BSC group than in the usual-care group, after adjusting for costs of health resource use in the 6 months before randomisation, age, gender, pre-existing medical conditions and duration since first diagnosis of SMI (Table 35). The mean QALYs were 0.008 (95% CI –0.030 to 0.074) higher in the BSC group than in the usual-care group, after adjusting for utility value at baseline, age, gender, pre-existing medical conditions and duration since first diagnosis of SMI. The ICER ratio was therefore £113,875 (uncertainty: Figure 14) per QALY gained.
| Results | BSC (n = 88) | Usual care (n = 80) |
|---|---|---|
| Costs | ||
| Smoking cessation, mean (SD) | £593 (£338) | £92 (£128) |
| Health resource use, mean (SD) | £7741 (£8649) | £8437 (£11,386) |
| Total, mean (SD) | £8434 (£8642) | £8530 (£11,405) |
| Adjusted difference,a mean (95% CI) | £911 (–£2768 to £2631) | |
| Quality of life | ||
| QALYs, mean (SD) | 0.679 (0.219) | 0.615 (0.283) |
| Adjusted difference,b mean (95% CI) | 0.008 (–0.030 to 0.074) | |
| Incremental cost-effectiveness ratio | ||
| ICER, mean | £113,875 (uncertainty: Figure 14) | |
FIGURE 14.
Cost-effectiveness plane and cost-effectiveness acceptability curve of complete-case analysis results. (a) Cost-effectiveness plane; and (b) cost-effectiveness acceptability curve.


Compared with the primary analysis, Figure 14a shows the dots scattered more widely on the cost-effectiveness plane, indicating a higher level of uncertainty. The BSC is still likely to achieve higher QALYs as 79% of the dots fall to the right side of y-axis. The difference in costs, however, almost disappears as about half (49%) fall below zero. Only 39% of the dots fall in the south-east quadrant, suggesting a less costly but more effective intervention. The cost-effectiveness acceptability curve demonstrates that the probability of the intervention being cost-effective was 61–65% between £20,000 and £30,000 per QALY (see Figure 14b). Similar to the primary analysis, the increase of the probability becomes smaller the higher the WTP gets, and flattens close to 80%.
Secondary analysis
Participants’ expenses
In months 1–6 of the trial, the participants’ expenses on travel were estimated at £5 (SE £2) per participant in the usual-care group and £3 (SE £1) in the BSC group. In months 7–12, it was £3 (SE £1) in the usual-care group and £2 (SE £1) in the BSC group. In months 1–6, the participants in the usual-care group spent on average £22 (SE £3) per person on e-cigarettes, while the participants in the BSC group spent on average £16 (SE £4) per person. In months 7–12, the expense on e-cigarette was £23 (SE £4) per participant in the usual-care group and £20 (SE £5) per participant in the BSC group. The expense on NRT over the counter was £8 (SE £2) per participant in the usual-care group and £12 (SE £5) per participant in the BSC group, in the first 6 months of the trial. In the second half of the trial, this expense was £6 (SE £2) per participant in the usual-care group and £16 (SE £6) per participant in the BSC group. The total expenses during the trial period were £67 (SE £8) per participant in the usual-care group and £70 (SE £11) per participant in the BSC group. In comparison with the huge health resource use costs, the participants’ expenses were almost negligible.
Financial impact of smoking cigarettes
Excluding seven people who died during the trial period, there were 262 participants in the BSC group and 257 in the usual-care group as the basis for estimating the expenses on cigarettes. The average numbers of cigarettes per day were 17.6 (SE 0.9) in the 1–6 months post randomisation and 19.7 (SE 0.8) in the 7–12 months post randomisation in the BSC group. In the usual-care group, average cigarettes per day were 18.5 (SE 0.7) in the 1–6 months post randomisation and 18.5 (SE 0.8) in the 7–12 months post randomisation. Assuming the number of cigarettes smoked per day was the average over the recall period and did not vary day to day, an estimated price of £9.37 per 20 cigarettes was applied to estimate the expenses. 71 The estimated expenses on cigarettes over 6 months were over £1500 per participant in the usual-care group [£1590 (SE £69) and £1596 (SE £70)]. In the BSC group, the estimated expenses were £1516 (SE £76) per participant in the first half of the trial and £1691 (SE £72) per participant in the second. Overall, smoking would add £3186 (SE £111) per person per year to the participants’ cost in the usual-care group and £3207 (SE £128) per person per year in the BSC group.
Antipsychotics among quitters and non-quitters
Without imputing missing smoking status, there were 344 participants in total who had complete data on CO-verified smoking status at both follow-ups and cost of antipsychotics in the trial period. Owing to the incompliance to normality, a Wilcoxon signed-rank test was used to access if the cost of antipsychotics differed from the first half of the trial to the second. Table 36 presents the mean cost of antipsychotics according to participants’ CO-verified quit status. There were 286 participants who did not quit at both follow-ups, 14 quit at 6 months but relapsed at 12 months, 23 did not quit at 6 months but quit at 12 months and 21 quit at both follow-ups. Except for the first category, there was no evidence of significant change between the first and the second half of the trial. However, this might be because limited numbers of participants fell into these categories. The participants who did not quit at any time showed an increased cost on antipsychotics (p = 0.0018).
| CO-verified quit status at 6- and 12-month follow-ups | Cost of antipsychotics [£, mean (SD)] | Wilcoxon signed-rank test | |
|---|---|---|---|
| Months 1–6 | Months 7–12 | ||
| Not quit at both follow-ups (n = 286) | 353 (509) | 369 (511) | z = 3.119; p = 0.0018 |
| Quit at 6 months but not at 12 months (n = 14) | 246 (212) | 243 (216) | z = –0.372; p = 0.7100 |
| Not quit at 6 months but at 12 months (n = 23) | 324 (409) | 248 (418) | z = 1.683; p = 0.0924 |
| Quit at both follow-ups (n = 21) | 582 (929) | 713 (1298) | z = 0.245; p = 0.8061 |
Discussion
This study collected extensive data to provide a comprehensive picture of costs to the NHS and Personal Social Services in this population. The mean intervention cost per quitter in the BSC group was around four times the mean intervention cost per quitter in the usual-care group (£3828 vs. £906). Although the cost per quitter of SSS in the general population was £690 per quitter for CO-validated 4-week quit in 2017/18 in England where data are available,72 this could not be compared directly. Our study offered a more flexible timeline to suit the needs of people with SMI and, therefore, did not measure 4-week smoking status. The participants in the usual-care group showed a more active engagement with usual smoking cessation services, especially in months 1–6 of the trial. This is understandable as the participants in the BSC group would have mostly been engaging with the intervention during that period while the help-seeking behaviour in the usual-care group might have been triggered by participation in the trial. Both groups became less engaged with usual-care services in months 7–12.
The use of various health-care and social services was highly intensive. The costs of primary and community services were the largest component of the overall costs. These costs were relatively stable from before the trial to the end of the trial and did not change significantly during the trial, indicating this population’s constant demand for community support. The lower total costs of the BSC group were mainly the results of the consistently lower costs of emergency and hospital services during the trial and higher costs in the 6 months before the trial, while the costs of emergency and hospital services remained on the same level in the usual-care group. This difference in costs of emergency and hospital services was probably related to the higher frequency and longer stay of hospital admission in the usual-care group than in the BSC group. However, it is unclear if this difference is related to the intervention or smoking status, or if it happened by chance. As the use of services was collected through self-report, the possibility of recall bias should be kept in mind when interpreting the results.
The data on prescriptions of antipsychotics and pharmacotherapies for smoking cessation were extracted from participants’ medical records. The information collected through this approach was more reliable and accurate than participants’ self-report, especially when there were multiple medicines involved. However, the information on prescriptions was completely missing for some participants because of the closure or lack of capacity of their general practices. Although attempts were made to ensure a uniform extraction of data, there were still discrepancies among data returns. As a result, clinic supervision and therefore staff cost necessitated by some antipsychotics [e.g. clozapine (Clozaril®, Sandoz Pharmaceuticals)] were not included in the analysis as individual visits to the clinic could not be identified for all participants. For the same reason, the prescription charge was also not included in the participants’ expenses as individual prescription items were not always identifiable. The cost of pharmacotherapies for smoking cessation might be overestimated. As we extracted data from GPs’ prescription records, it should be noted that not all people redeem their prescriptions.
With the development and increased accessibility of the internet, people become rapidly used to seeking information online. The level and costs of smoking cessation help available online vary. The publicly available information provided by the NHS or charities costs only individuals’ internet charge, whereas the private organisations might offer more intensive online treatment for a fee. It is not clear if this particular population has a preference for internet use or not and to what extent they rely on the internet for smoking cessation help. A brief browse of free information might be negligible in terms of costs but more intensive help could add to participants’ financial burden.
Both groups had an upwards trend of EQ-5D-5L scores, although the BSC group’s increase was more drastic. This resulted in the positive QALY gains of the BSC intervention. This might be related to the lower frequency and shorter stay of hospital admission in the BSC group. Both appeared to reflect a better general health of the participants in this group. However, within such a short period, participants’ smoking status, successfully quit or not, is unlikely to have contributed to their better general health.
A disadvantage of collecting comprehensive service use data is that data are more likely to be missing because of the length of the questionnaire. However, owing to a high follow-up rate, the missing data level was not unacceptably high. When comparing primary analysis (multiple imputation of missing data) and complete-case analysis (remove all incomplete observations), it appears that missing data on costs have a larger impact on costs in the usual-care group than in the BSC group. The impact of missing data on EQ-5D-5L is less straightforward. In the usual-care group, it appears that the participants who managed better dropped out, whereas in the BSC group the participants who did not do well were the source of missing data.
The biggest impact of smoking on the individual’s private costs came from purchase of cigarettes itself. On average, the participants in the in the trial were estimated to spend > £3000 per year on cigarettes and there did not appear to be any difference between groups. Because the amount of money spent on cigarettes was estimated based on the data collected at three time points, the change in smoking patterns in between was not captured. The assumption of unchanging number of cigarettes per day throughout the estimated period might not hold. Another thing to keep in mind is that we estimated the expenses on smoking by converting all tobacco to cigarettes and costing it based on market price of manufactured cigarettes. This might lead to an overestimate as there are cheaper options available for purchasing tobacco. Although there were some participants purchasing e-cigarettes during the trial, the expenses did not appear significant in comparison to those of cigarettes. Previous evidence has recommended that people with SMI could reduce their antipsychotics intake if they stop smoking. Although the results showed that the participants who smoked throughout the trial had a significant increase in their costs of antipsychotics, the current study did not find evidence to support the reverse situation. One of the reasons might be that there were insufficient numbers of quitters in the trial to draw meaningful conclusions. Moreover, the quit status was measured by asking the question ‘have you smoked in the last week’, which is not a measure of abstinence. Clinical guidelines suggest that if a person makes a quit attempt their antipsychotic medication should be closely monitored as smoking affects the way these drugs are metabolised and the person may need to reduce their antipsychotic medication and if they subsequently relapse back to smoking their dose of antipsychotic medication may need to be increased. Finally, this comparison was undertaken based on the participants whose costs of antipsychotics and smoking status were both available. There is a possibility of selection bias.
The primary analysis showed a higher than 50% probability of the BSC intervention being cost-effective, compared with usual care, at a WTP threshold of £0 per QALY gained. This was largely attributable to the lower level of health-care services costs offsetting the intervention costs in the BSC group and a QALY gain with relatively high level of certainty. Although the likely cost-saving effect in the primary analysis disappeared in the complete-case analysis, the offset effect remained. This suggested that in the complete-case analysis, the mean health-care services costs in the BSC group remained lower than those in the usual-care group. Given that the primary outcome of CO-validated quit at 12 months post randomisation did not show a statistical significance, it could raise concerns over the more favourable results of the economic evaluation. As a perfect association between smoking status and health services and quality of life is not expected in 1 year, it is possible that the analysis shows a better outcome in terms of costs and QALYs. However, unless there were some unknown factors in participants’ life affected by the intervention that could be identified later, the result of this study might be hard to generalise.
Conclusion
The BSC intervention for people with SMI is likely (57%) to dominate usual GP care, from a NHS and Personal Social Services perspective. Although neither the difference in costs nor in QALYs was statistically significant, the probability of cost-effectiveness could reach 80% at a threshold of £30,000 based on the primary analysis and 65% based on the complete-case analysis. However, it remains uncertain whether or not this was the result of smoking cessation, as the smoking status at 12 months did not show a significant difference between groups. Furthermore, the impact of smoking cessation on health and wider health services use is unlikely to be observed in a short period of 12 months. A longer-term follow-up would be ideal to observe the sustainability of quit and impact on health.
Chapter 6 Discussion
Some of the information in this section is reported in Peckham et al. 1 This contains information licensed under the Non-Commercial Government Licence v2.0.
This report presents the results of the first UK trial of a BSC intervention designed specifically for people with SMI. The SCIMITAR+ trial was commissioned by the National Institute for Health Research Health Technology Assessment programme in view of the clinical need of this population and the widening health inequalities that exist in relation to smoking and smoking-related illness. We have previously reported the results of the SCIMITAR pilot trial, which demonstrated acceptability but was not sufficiently powered to estimate clinical effectiveness and cost-effectiveness. The SCIMITAR programme has followed a developmental pathway to produce a feasible intervention to the point where we were able to judge the value of an evidence-informed smoking cessation intervention within the context of a definitive trial. We drew on existing evidence (taken from high-quality systematic reviews) of ‘what works’ in helping people to cut down or quit smoking. 23 In linked work, we have also conducted a systematic review of ‘what works’ in relation to people with SMI, and have shown that the same pharmacological and behavioural approaches to smoking cessation are effective among people with SMI as with the rest of the population. 39,40 Despite this evidence, it is clear that people with SMI do not access conventional NHS quit smoking services, and a coherent response is to design a service and intervention that ensures that evidence-supported pharmacotherapies and behaviour change techniques are applied with specific reference to the needs of people with SMI.
The BSC intervention at the centre of the SCIMITAR+ trial was designed to address the unmet needs of and barriers to accessing smoking cessation interventions for this population. We will now review the main findings and address the main objectives of the SCIMITAR+ trial in turn.
Main findings
The main finding of the SCIMITAR+ trial is that smoking cessation can be achieved among people with SMI, and that, in people who express a desire to quit smoking, the use of a BSC intervention increased the chances of sustained quitting as estimated by a gold-standard biologically verified outcome measure (exhaled CO). 73 The observed odds of successful quitting at 12 months was higher among those who received BSC but this was not statistically significant. However, the observed odds of successful quitting at 6 months were higher among those who received BSC and this was statistically significant. The SCIMITAR+ trial used quitting as measured at 12 months post randomisation as its primary end point, and although there was a trend in favour of the BSC intervention, the difference in quit rates was no longer statistically significant at 1 year. This finding is in line with research in the general population, which shows that long-term quit rates are difficult to achieve, and this remains a challenge for the wider evidential basis of treatment for nicotine dependence in any population. 74 The results of the effect of the BSC intervention were seen in secondary outcomes, and we found an improvement in short-term physical health (as measured by the SF-12) and some evidence of reduced numbers of cigarettes smoked per day and increased motivation to quit. We also found that there were no between-group differences on measures of mental health, including depression and anxiety, supporting the notion that offering a smoking cessation intervention is not detrimental to mental health.
To our knowledge, this is the first large-scale UK RCT of a bespoke intervention designed for people with SMI. Trials to date have been small scale and with short periods of follow-up, and have focused on pharmacological treatments, with limited consideration of behavioural approaches. 40 The SCIMITAR+ trial adapted and enhanced an evidence-supported smoking cessation strategy that has been developed and forms the mainstay of successful UK SSSs. This structured intervention was delivered by a mental health professional and a ‘cut down to quit’ approach was also offered. The results of the SCIMITAR+ trial, alongside trials35 and systematic review evidence34,39,40 of the safety and effectiveness of pharmacological treatments for nicotine dependence in people with SMI, represents accumulating evidence of the effectiveness of smoking cessation interventions for people with severe mental ill health. Across both arms of the trial, people smoked a higher number of cigarettes per day and had a higher mean number of years of smoking history than those in the general population (25.5 per day compared with 11.3 per day in the general population). 75 This adds to the justification for the need to intervene and develop new and novel interventions for this population.
Is the treatment acceptable to participants?
The SCIMITAR+ trial found that participants generally engaged with BSC services. The levels of engagement were high, with 88% taking up treatment and with an average of six sessions attended. In an earlier qualitative evaluation conducted in the developmental phase of the SCIMITAR pilot trial, it was found that participants valued the fact that smoking cessation therapists were drawn from staff working within mental health services. 76 Smoking cessation practitioners therefore had a familiarity with SMI and the specific needs of that group, and this was seen as a positive aspect of the intervention by participants. By addressing these factors, the participants felt that their smoking was more readily addressed and they felt less stigmatised than might have been the case in conventional services. Participants were attracted to a service that offered the prospect of cutting down prior to quitting and they appreciated the opportunity to receive NRT prior to setting a quit date. In the control group, there was a lack of engagement with conventional NHS quit smoking services despite control participants being given smoking cessation literature and encouraged to visit their GP or NHS quit smoking service. The intervention was clearly more intensive than what would be offered in conventional NHS services, and the overall cost of BSC was higher than usual care.
Within the SCIMITAR+ trial, participants were encouraged to choose an appropriate form of smoking cessation medication in collaboration with their GP. The mainstay of treatment was NRT and only a small number of participants were prescribed varenicline. None was prescribed bupropion. There were, however, some difficulties observed within the trial with regard to access to NRT, partly because provision (e.g. via SSSs, local councils or GP practices) varied across the trial sites. In some settings, access to provision was restricted, either by a requirement to attend all elements of a SSS provision or by restricting access to specific groups (e.g. pregnant women, those with respiratory disorders). Where this occurred this was often driven by funding being linked with smoking cessation accruals, and through discussions with services it was possible to access NRT provision, without accessing behavioural intervention components provided by the service, once a mechanism was implemented to provide accruals data to the relevant organisation. In some locations it was, however, almost impossible for participants to receive NRT through local provision and in the limited number of cases where this could not be facilitated, NRT was prescribed by the secondary care provider as is recommended by the 2013 NICE guidance on smoking cessation provisions for people with mental ill health,17 which recommends that mental health services make this provision for smokers who use their services. Where NRT was provided, in some instances only one product was prescribed, and in some cases the preferred product identified by the patient in discussion with the smoking cessation practitioner was not prescribed. In both instances this was often attributed to cost implications. There were also some delays in initial prescribing that resulted in participants having to delay their quit attempt. These delays or changes in prescribing had potential to demoralise patients and so reduce their interest in and willingness to make a quit attempt. It is important to note that this was not observed within the trial. NRT was also often not prescribed as a repeat medication or was prescribed only for a fixed duration, which had an impact on longer-term abstinence from smoking. Contact with GPs, councils or SSSs by members of the core SCIMITAR+ trial team helped to identify and to facilitate implementation of methods or routes to remove barriers and so to enable access to NRT provision. Previous qualitative interview data have shown that GPs can be reluctant to prescribe smoking cessation products other than NRT, and, as described, this was evident in the SCIMITAR+ trial. GPs therefore welcomed this direct contact as this enabled the team to provide assurance that GPs would retain oversight for their patient’s care, and to directly tackle concerns or misconceptions with regard to prescribing of pharmacological treatments for patients with SMI.
The advice from the NCSCT regarding e-cigarettes when the SCIMITAR+ trial began in 2015 was that, if people wished to use e-cigarettes in their quit attempt specific advice on their use should not be given, but that behavioural support could still be provided (NCSCT, 2015, personal communication). This was the advice that was followed when the trial began. MH-SCPs in the trial neither encouraged nor discouraged the use of e-cigarettes. A subsequent briefing produced by the NCSCT, Electronic cigarettes: A Briefing for Stop Smoking Services,77 led to a change in the advice MH-SCP gave and although they could not provide advice on which e-cigarette to use, they did give more information about e-cigarettes in line with NCSCT guidance. This updated advice was published towards the end of the recruitment period and, therefore, only the participants recruited towards the end of the trial were given the amended advice. This may have resulted in fewer participants using e-cigarettes than would be the case if the trial was conducted now.
Is bespoke smoking cessation cost-effective?
The results of the SCIMITAR+ trial provide preliminary evidence that a BSC intervention is likely to be cost-effective in terms of total health-care costs and quality of life. However, it is uncertain if this conclusion is the result of the intervention as an approach to nicotine dependence for people with SMI.
The BSC intervention for people with SMI probably dominates usual GP care, from a NHS and Personal Social Services perspective. Depending on the relevant WTP threshold, the probability of cost-effectiveness could be higher (80% at £30,000). Although neither difference in total costs nor difference in QALYs was significant, there is an indication that the reduction of health-care services costs might offset the intervention costs.
Limitations of the SCIMITAR+ trial
There were limitations to the SCIMITAR+ trial. First, we lost 16% of participants to follow-up, but note that this was better than we achieved in our pilot trial43 and that there was non-differential loss to follow-up. Second, a lower 12-month cessation rate was observed in the usual-care group than was hypothesised in the sample size calculation (10% vs. 20%), and the actual percentage increase was lower (5 vs. 14 percentage points). Therefore, although the trial recruited more participants than originally planned and the loss to follow-up rate was lower (16% vs. 20% hypothesised), the trial was ultimately underpowered to detect a difference in quit rates from 10% to 15%. Third, we noted difficulties in ensuring that some participants received pharmacological treatment, as are detailed previously. Given that smoking is the single most modifiable risk factor for early mortality, we recommend that local services have in place robust mechanisms to remove barriers to the provision of medication both when implementing the results of the SCIMITAR+ trial and to support smoking cessation more generally. Last, we were not able to ensure that all follow-up was blind to treatment allocation. However, we also put in place a biochemically verified end point that is less susceptible to observer biases.
Given the trial could not conclude that the intervention is effective in terms of smoking cessation at 12 months, it is uncertain if the result of the economic evaluation was associated with cessation itself. It is possible that the intervention triggered some changes in aspects other than smoking behaviour, which had a positive impact on health, or the differences in health-care costs occurred by chance. In addition, we lacked the capacity to conduct a longer follow-up and a well-correlated relationship between smoking status and health is not expected in the short term.
Conclusions
In the face of significant health inequalities for people with SMI, smoking is the most important modifiable risk factor for poor health and reduced life expectancy. 78 At 12 months we did not find a statistically significant difference between the intervention and usual care; however, we did find that there was an increased quit rate in the intervention at 6 months. Quitting smoking did not appear to have had any impact on mental health and there were no statistically significant differences between PHQ-9 scores in the intervention and control group at 6 or 12 months. Those in the intervention group had a slightly higher number of quit attempts than those in the usual-care arm.
In this trial, we have shown that people with SMI more readily engage with a bespoke intervention that results in increased 6-month quit rates; however, this difference was not sustained at 12 months. Further research is needed to establish how long-term quitting can be supported.
Implications for health care
Clinicians are sometimes reluctant to offer smoking cessation advice to patients under their care and this is in part because of concerns that treatment is ineffective, or that quitting might cause deterioration in mental health. The results of the SCIMITAR+ trial will be helpful in informing clinical practice, as we have shown that quitting can be achieved for people who use mental health services just as it can for all smokers. As per Public Health Guidance 48,17 clinicians should ask all of their patients about smoking status and offer referral to effective smoking cessation services. The SCIMITAR+ intervention could provide a candidate model for such a service. The results of this trial will provide clinicians with increased confidence that smoking cessation is likely to be either beneficial or not harmful to mental health.
The SCIMITAR+ trial experienced a range of difficulties in procuring NRT provision for participants ahead of, or during, their quit attempt. Given that smoking is the single most modifiable risk factor for early mortality, both within the general and SMI populations, it is suggested that policy and practice should be reviewed and revised so as to facilitate ease of access to NRT for any person wishing to make a smoking cessation attempt. Ensuring adequate provision to support smoking cessation is likely to reduce NHS expenditure in the longer term, by virtue of limiting potential for comorbidities attributable to tobacco use. Furthermore, consideration should be given to ensuring sufficient provision to ensure that a quit attempt is sustained; this is particularly imperative for SMI patients where additional support may be required.
The results of the SCIMITAR+ trial will be helpful for service commissioners who seek to implement guidance for smoking cessation among hard-to-reach groups, such as people with SMI. 79
Recommendations for future research
Following on from the SCIMITAR+ trial, further research is needed to establish how quitting can be sustained among people with SMI who engage with an evidence-supported quit smoking intervention.
In addition, although the SCIMITAR+ trial explored the use of e-cigarettes, we suggest that research is needed to evaluate the role of e-cigarettes in helping people with SMI to cut down or to quit smoking.
Additional research is also needed to complement the findings of the SCIMITAR+ trial, specifically to establish the clinical effectiveness and cost-effectiveness of very brief opportunistic interventions for smoking cessation among people with SMI, such as people with SMI being offered advice on giving up smoking in a routine appointment or other opportunistic encounter with a clinician. 80
It may also be useful to explore NRT update and the barriers to this in this population through qualitative research.
In future trials, conducting a process evaluation to analyse aspects of the intervention that did not work, for which groups and in which contexts, may be helpful.
It may be helpful to explore if there are other factors that affect the health of people with SMI and that can be influenced by a BSC intervention.
Finally, in order to capture the whole impact of smoking cessation intervention in this population, a long-term follow-up is needed to establish the cost-effectiveness.
Acknowledgements
We would like to thank the participants for taking part in the trial, the GPs and secondary care staff for recruiting participants to the study and completing trial documentation, the TSC and Data Monitoring and Ethics Committee members for overseeing the study, and Andy McEwan of the NCSCT for proving training for the MH-SCPs.
The SCIMITAR+ trial benefited from the support of the North West Coast, Yorkshire and Humber, East Midlands and South West Peninsula Clinical Research Network.
Trial Steering Committee members
Professor Ann McNeil (Independent Chairperson), Professor of Tobacco Addiction, King’s College London, London, UK.
Dr Andrew Elmslie, Consultant Psychiatrist, Tees, Esk and Wear Valleys NHS Foundation Trust, York, UK.
Dr David Shiers, Retired GP, North Staffordshire, UK.
Mr David Waldram (Service User Representative).
Data Monitoring and Ethics Committee members
Professor Mike Crawford (Chairperson), Professor of Mental Health Research, Imperial College London, London, UK.
Dr Elena Ratschen, Lecturer in Epidemiology/Tobacco Control, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham, UK.
Professor Richard Emsley, Professor of Medical Statistics and Trials Methodology, King’s College London, London, UK.
Hazel Cheeseman, Director of Policy, Action on Smoking and Health, London, UK.
Contributions of authors
Emily Peckham and Simon Gilbody wrote the original protocol.
Catherine Arundel, Della Bailey, Suzanne Crosland, Caroline Fairhurst, Paul Heron, Catherine Hewitt, Jinshuo Li, Steve Parrott, Tim Bradshaw, Michelle Horspool, Elizabeth Hughes, Tom Hughes, Suzy Ker, Moira Leahy, David Osborn, Joseph Reilly, Diane Brennan and Simon Gilbody refined the protocol.
Catherine Arundel, Suzanne Crosland, Tayla McCloud, Paul Heron and Thomas Steare were trial co-ordinators for the study.
Tayla McCloud, Thomas Steare, Emma Ballantyne, Polly Bidwell, Susan Bonner, Diane Brennan, Tracy Callen, Alex Carey, Charlotte Colbeck, Debbie Coton, Emma Donaldson, Kimberley Evans, Hannah Herlihy, Wajid Khan, Lizwi Nyathi, Elizabeth Nyamadzawo, Helen Oldknow, Jamie Rea, Crystal-Bella Romain-Hooper, Kaye Smith, Alison Stribling and Carinna Vickers recruited participants to the study, provided feedback on the recruitment methods and helped refine the study procedures.
Peter Phiri and Shanaya Rathod helped refine the study procedures.
Emily Peckham, Catherine Hewitt, Steve Parrott, Tim Bradshaw, Elizabeth Hughes, Tom Hughes, Suzy Ker, Moira Leahy, David Osborn, Joseph Reilly and Simon Gilbody were co-applicants on the Health Technology Assessment application.
Emily Peckham was the trial manger.
Della Bailey supervised the delivery of the intervention.
Caroline Fairhurst conducted the clinical analysis.
Catherine Hewitt oversaw the conduct of the analysis.
Jinshuo Li designed and undertook the economic analysis in conjunction with Steve Parrott.
Simon Gilbody was the chief investigator and oversaw the study.
Emily Peckham, Catherine Arundel, Della Bailey, Suzanne Crosland, Caroline Fairhurst, Paul Heron, Jinshuo Li and Simon Gilbody were the writing team.
All authors read and reviewed the transcript.
Publication
Gilbody S, Peckham E, Bailey D, Arundel C, Heron P, Crosland S, et al. Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial. Lancet Psychiatry 2019;6:379–90.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following this.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Peckham E, Man MS, Mitchell N, Li J, Becque T, Knowles S, et al. Smoking Cessation Intervention for severe Mental Ill Health Trial (SCIMITAR): a pilot randomised control trial of the clinical effectiveness and cost-effectiveness of a bespoke smoking cessation service. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19250.
- Royal College of Physicians . Smoking and Mental Health: A Report by the Tobacco Advisory Group 2013.
- Szatkowski L, McNeill A. Diverging trends in smoking behaviors according to mental health status. Nicotine Tob Res 2015;17:356-60. https://doi.org/10.1093/ntr/ntu173.
- Jochelson K, Majrowski B. Clearing the Air: Debating Smoke-free Policies in Psychiatric Units. London: The King’s Fund; 2006.
- Szatkowski L, McNeill A. The delivery of smoking cessation interventions to primary care patients with mental health problems. Addiction 2013;108:1487-94. https://doi.org/10.1111/add.12163.
- Weiser M, Reichenberg A, Grotto I, Yasvitzky R, Rabinowitz J, Lubin G, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004;161:1219-23. https://doi.org/10.1176/appi.ajp.161.7.1219.
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2010;6. https://doi.org/10.1002/14651858.CD007253.pub2.
- Williams JM, Ziedonis DM, Abanyie F. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res 2005;79:323-35. https://doi.org/10.1016/j.schres.2005.04.016.
- Phelan M, Stradins L, Morrison S. Physical health of people with severe mental illness – can be improved if primary care and mental health professionals pay attention to it. BMJ 2001;322:443-4. https://doi.org/10.1136/bmj.322.7284.443.
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiatry Res 2007;150:141-52. https://doi.org/10.1016/j.psychres.2006.05.021.
- Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med 2005;61:293-30. https://doi.org/10.1016/j.socscimed.2004.11.057.
- Addington J, el-Guebaly N, Addington D, Hodgins D. Readiness to stop smoking in schizophrenia. Can J Psychiatry 1997;42:49-52. https://doi.org/10.1177/070674379704200107.
- Moylan S, Jacka FN, Pasco JA, Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav 2013;3:302-26. https://doi.org/10.1002/brb3.137.
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 2005;62:649-59. https://doi.org/10.1001/archpsyc.62.6.649.
- Public Health England . PHE Local Tobacco Profiles 2018 n.d. https://fingertips.phe.org.uk/profile/tobacco-control (accessed 23 January 2019).
- Office for National Statistics . Adult Smoking Habits in the UK: 2017 2017.
- National Institute for Care Excellence (NICE) . NICE Public Health Guidance 48, Smoking Cessation in Secondary Care in Acute, Maternity and Mental Health Services 2013.
- Cheeseman H, Harker K. The Stolen Years. London: Action on Smoking and Health; 2016.
- West R. Understanding Nicotine and Tobacco Addiction. London: Wiley; 2006.
- Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens. Nicotine Tob Res 2004;6:899-912. https://doi.org/10.1080/14622200412331324965.
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007;9:315-27. https://doi.org/10.1080/14622200701188919.
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38. https://doi.org/10.1111/j.1360-0443.2004.00540.x.
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD000146.pub2.
- Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev 2004;4. https://doi.org/10.1002/14651858.CD000165.pub2.
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD001292.pub2.
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD001007.pub2.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3. https://doi.org/10.1002/14651858.CD001118.pub2.
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2006;3. https://doi.org/10.1002/14651858.CD002850.pub2.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD006103.pub3.
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD000031.pub3.
- National Institute for Care Excellence (NICE) . Brief Interventions and Referral for Smoking Cessation in Primary Care and Other Settings: NICE Public Health Intervention Guidance 2006.
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD010216.pub3.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12020.
- Wu Q, Gilbody S, Peckham E, Brabyn S, Parrott S. Varenicline for smoking cessation and reduction in people with severe mental illnesses: systematic review and meta-analysis. Addiction 2016;111:1554-67. https://doi.org/10.1111/add.13415.
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. https://doi.org/10.1016/S0140-6736(16)30272-0.
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;31:1650-60. https://doi.org/10.1016/j.addbeh.2005.12.014.
- McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation (Guide for Counsellors and Practitioners). London: Blackwell Publishing Ltd; 2006.
- National Institute for Care Excellence (NICE) . Smoking Cessation in Secondary Care: Acute, Maternity and Mental Health Services 2013.
- Banham L, Gilbody S. Smoking cessation in severe mental illness: what works?. Addiction 2010;105:1176-89. https://doi.org/10.1111/j.1360-0443.2010.02946.x.
- Peckham E, Brabyn S, Cook L, Tew G, Gilbody S. Smoking cessation in severe mental ill health: what works? an updated systematic review and meta-analysis. BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1419-7.
- Office for National Statistics . Opinions and Lifestyle Survey, Smoking Habits Amongst Adults 2013.
- BMA and NHS Employers . Revisions to the GMS Contract, 2006 7. Delivering Investment in General Practice 2006.
- Gilbody S, Peckham E, Man MS, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): a pilot randomised controlled trial. Lancet Psychiatry 2015;2:395-402. https://doi.org/10.1016/S2215-0366(15)00091-7.
- Peckham E, Arundel C, Bailey D, Brownings S, Fairhurst C, Heron P, et al. Smoking Cessation Intervention for Severe Mental Ill Health Trial (SCIMITAR+): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1789-7.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems. 11th Revision 2018.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition 2013. https://doi.org/10.1176/appi.books.9780890425596.
- Great Britain . Mental Capacity Act 2005 2005. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 20 March 2019).
- Royal College of General Practitioners and Royal College of Psychiatrists . Primary Care Guidance on Smoking and Mental Health 2008.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- National Centre for Smoking Cessation and Training . Motivation to Stop Smoking 2015 n.d. www.ncsct.co.uk/publication_motivation-to-stop-smoking.php (accessed 23 January 2019).
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000.
- EuroQol Group . EuroQol – a new facility for the measurement of health related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Laugesen M, Epton M, Frampton CM, Glover M, Lea RA. Hand-rolled cigarette smoking patterns compared with factory-made cigarette smoking in New Zealand men. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-194.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- NHS Digital . Prescription Cost Analysis – England, 2017 2018. https://digital.nhs.uk/catalogue/PUB30246 (accessed 10 April 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Department of Health and Social Care . NHS Improvement. Reference Costs 2016–17 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- National Institute for Health and Care Excellence (NICE) . Smoking Cessation Services Costing Report – Implementing NICE Guidance 2008.
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost-effectiveness of computer-tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res 2014;16:270-8. https://doi.org/10.1093/ntr/ntt136.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Economics 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 26 April 2019).
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) 2018. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 25 January 2019).
- White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an Internet-based alcohol trial. Stat Med 2011;30:3192-207. https://doi.org/10.1002/sim.4360.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Office for National Statistics . RPI: Ave Price – Cigarettes 20 King Size Filter 2018.
- NHS Digital . Statistics on NHS Stop Smoking Services in England – April 2017 to March 2018 2018.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. https://doi.org/10.1111/j.1360-0443.2004.00995.x.
- Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD009670.pub3.
- Office for National Statistics . Adult Smoking Habits in the UK: 2015 2015.
- Knowles S, Planner C, Bradshaw T, Peckham E, Man MS, Gilbody S. Making the journey with me: a qualitative study of experiences of a bespoke mental health smoking cessation intervention for service users with serious mental illness. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0901-y.
- McEwen A, McRobbie H. Electronic Cigarettes: A Briefing for Stop Smoking Services. Dorchester: National Centre for Smoking Cessation and Training; 2016.
- Campion J, Shiers D, Britton J, Gilbody S, Bradshaw T. Primary Care Guidance on Smoking and Mental Disorders – 2014 Update. London: Royal College of General Practitioners and Royal College of Psychiatrists; 2014.
- National Institute for Care Excellence (NICE) . Type 2 Diabetes: Prevention in People at High Risk 2012.
- Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction 2012;107:1066-73. https://doi.org/10.1111/j.1360-0443.2011.03770.x.
Appendix 1 Regulatory approvals
| Trust | Date research and development approval granted |
|---|---|
| 2gether NHS Foundation Trust | 5 February 2016 |
| Berkshire Healthcare NHS Foundation Trust | 14 December 2015 |
| Bradford District Care NHS Foundation Trust | 18 February 2016 |
| Cambridgeshire and Peterborough NHS Foundation Trust | 27 April 2016 |
| Camden and Islington NHS Foundation Trust | 5 February 2016 |
| Greater Manchester West Mental Health NHS Foundation Trust | 25 November 2015 |
| Kent and Medway NHS and Social Care Partnership Trust | 1 October 2015 |
| Lancashire Care NHS Foundation Trust | 29 February 2016 |
| Lincolnshire Partnership NHS Foundation Trust | 28 September 2015 |
| Leeds and York Partnership NHS Foundation Trust | 5 January 2016 |
| Northumberland, Tyne and Wear NHS Foundation Trust | 27 June 2016 |
| Oxford Health NHS Foundation Trust | 30 March 2016 |
| Rotherham, Doncaster and South Humber NHS Foundation Trust | 22 October 2015 |
| Sheffield Health and Social Care NHS Foundation Trust | 12 October 2015 |
| Solent NHS Trust | 28 January 2016 |
| Somerset Partnership NHS Foundation Trust | 8 June 2016 |
| South Essex Partnership University NHS Foundation Trust | 29 March 2016 |
| Southern Health NHS Foundation Trust | 15 October 2015 |
| South West Yorkshire NHS Foundation Trust | 2 November 2015 |
| Sussex Partnership NHS Foundation Trust | 14 September 2015 |
| Tees, Esk and Wear Valleys NHS Foundation Trust | 9 November 2015 |
| Vauxhall Health Centre (formerly Liverpool Primary Care Trust) | 11 April 2016 |
Appendix 2 Patient information sheet and consent form
Appendix 3 Adverse events and risk protocol
Appendix 4 Flow chart for the SCIMITAR+ trial

List of abbreviations
- AIC
- Akaike information criterion
- BMI
- body mass index
- BSC
- bespoke smoking cessation
- CACE
- complier-average causal effect
- CI
- confidence interval
- CMHT
- community mental health team
- CONSORT
- Consolidated Standards of Reporting Trials
- CPA
- care programme approach
- CPN
- community psychiatric nurse
- DNA
- did not attend
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FTND
- Fagerström Test for Nicotine Dependence
- GAD-7
- Generalised Anxiety Disorder Assessment-7 items
- GEE
- generalised estimating equation
- GMS
- General Medical Services
- GP
- general practitioner
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- MH-SCP
- mental health smoking cessation practitioner
- MTQ
- Motivation to Quit questionnaire
- NCSCT
- National Centre for Smoking Cessation and Training
- NIC
- net ingredient cost
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PHQ-9
- Patient Health Questionnaire-9 items
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCIMITAR
- smoking cessation intervention for severe mental ill health
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SMI
- severe mental ill health
- SSS
- stop smoking service
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WTP
- willingness to pay
