Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/36/33. The contractual start date was in March 2013. The draft report began editorial review in December 2017 and was accepted for publication in July 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Christopher McCabe has received grant funding from the University of Alberta and Alberta Innovates Health Solutions (Edmonton, AB, Canada). Julia Brown is Deputy Director of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Clinical Evaluation and Trials Board and has received grant funding for the following studies: the NIHR eRAPID Programme Grants for Applied Research (PGfAR) trial, the NIHR Efficacy and Mechanism Evaluation (EME) GLiSten trial, the NIHR HTA LAVA trial (liver resection surgery versus thermal ablation for colorectal liver metastases), the NIHR EME IntAct (Intraoperative flourescence angiography to prevent Anastomotic leak in rectal cancer surgery), NIHR Research for Patient Benefit LACE (Life After Cancer Epidemiology) trial, the NIHR EME ROLARR (RObotic vs. LAparoscopic Resection for Rectal cancer) trial, the NIHR HTA SaFarI (Sacral nerve stimulation versus the FENIX TM magnetic sphincter augmentation for faecal incontinence: a Randomised Investigation and the NIHR StamINA Programme Development Grant trial. Claire Hulme and E Andrea Nelson have been members of the NIHR HTA Commissioning Board and E Andrea Nelson has received funding for the NIHR HTA CODIFI (Concordance in Diabetic Foot Ulcer Infection) study. Elizabeth McGinnis has received funding for NIHR Health Service and Delivery Research (HSDR) Information Systems, Monitoring and Managing from Ward to Board and the NIHR PGfAR SWHSI (Surgical Wounds Healing by Secondary Intention) trial. Isabelle L Smith and Susanne Coleman have received a NIHR personal fellowship. Benjamin Thorpe has received a NIHR research methods fellowship. Delia Muir has received a Wellcome Trust Engagement Fellowship. Rachael Gilberts has received funding for the NIHR HTA ALPHA (ALitretinoin versus PUVA in severe chronic HAnd eczema) and MIDFUT (Multiple Interventions for Diabetic Foot Ulcer Treatment) trials. Nikki Stubbs has received funding for NIHR PGfAR SWHSI and NIHR HTA AMBER (Abdominal Massage for Bowel Dysfunction Effectiveness Research) trial. Jane Nixon has received funding for NIHR HTA MIDFUT, CODIFI and ALPHA. Sarah Brown has received funding for NIHR HTA ALPHA, MIDFUT, FORVAD (Clinical and cost-effectiveness of posterior cervical FORaminotomy Versus Anterior cervical Discectomy in the treatment of cervical brachialgia: a multicentre, Phase III, randomised controlled trial), the NIHR PGfAR PROMPT (early detection to improve outcome in patients with undiagnosed psoriatic arthritis), ARUK (Arthritis Research UK) SALRISE (SALivary electro-stimulation for the treatment of dry mouth in patients with Sjögren’s syndrome: a multicentRe randomISEd sham-controlled double-blind study).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Nixon et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Pressure ulcers (PUs) are a major burden on patients, carers and the health-care system,1,2 affecting approximately one in seven hospitals and 1 in 20 community patients. 3–6 PUs greatly affect patients and their carers with physical, social and psychological factors. Distressing symptoms including pain, exudate and odour, increased care burden, prolonged rehabilitation, requirement for bed rest, hospitalisation and, for those who work, sickness absences are often reported. 1 They are a cross-specialty problem, a complication of serious acute or chronic illness in patient populations characterised by high levels of comorbidity and mortality. 7
A PU is described as ‘localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear’8 (reproduced with permission from the National Pressure Ulcer Advisory Panel, the European Pressure Ulcer Advisory Panel and the Pan Pacific Pressure Injury Alliance). The primary cause of a PU is mechanical load in the form of pressure, or pressure and shear, applied to soft tissues, generally over a bony prominence. The magnitude or duration of the mechanical load is such that it causes cell deformation leading to cell membrane rupture and/or impairment of the blood supply and tissue ischaemia, resulting in tissue damage. 9,10
Classification
Pressure ulcers range in severity from intact skin with non-blanchable erythema to severe ulcers involving fat, muscle and bone. Various classification systems have been developed8,11,12 using terms such as ‘grade’, ‘stage’ and ‘category’. The most widely used international classifications use a numerical scale of 1–4 to indicate increasing severity of skin and underlying soft tissue/bone involvement, with additional descriptors including ‘unstageable’ and ‘deep tissue injury’ for which a 1–4 classification cannot be determined from clinical examination. 8,12
More recently, Australian and US groups have advocated that the term ‘pressure injury’ be adopted to replace the term ‘pressure ulcer’. 13,14 The European position15 and a NHS Improvement Definitions Task and Finish Group16 both recommend that the term ‘pressure ulcer’ is retained so that the UK is aligned with the most up-to-date International Classification of Diseases, 11th Revision. 17
For the purposes of this report, the term ‘pressure ulcer’ and its abbreviation ‘PU’ will be used.
Risk factors
A systematic review on PU risk factors identified the risk factor domains emerging most frequently in multivariable modelling of independent predictors of PU development as mobility/activity, perfusion (including diabetes) and skin/PU status. 18 Moisture, age, haematological measures, nutrition and general health status are also important, but emerge inconsistently in multivariable modelling. The importance of body temperature and immunity require further confirmatory research and there is no clear evidence that race and gender are important to PU development. 18 The risk factor systematic review informed the development of a PU conceptual framework that maps the potential relationships between the key ‘direct causal factors’ (immobility/inactivity, perfusion and skin status) and the large number of other ‘indirect causal factors’ (e.g. moisture, age, nutrition, acute illness). 9
Importance
Over the past three decades, government health departments, national guideline agencies, commissioners and funders of health care have promoted policies, incentives and guidelines that focus on the prevention of PUs. 5,12,19–22 This reflects the high personal costs incurred by patients1,23 and the high financial costs incurred by health-care funders and providers in the treatment of PUs due to increased length of hospital stay, hospital admission, community nursing, treatments (reconstruction surgery/mattresses/dressings/technical therapies) and complications (serious infection). 24,25 Litigation is also a burden on health-care providers and is predicted to increase because of general societal trends and, in some cases (e.g. the UK), changes in the national policies that have led to investigation by government agencies of severe PUs to detect institutional and professional neglect of vulnerable adults. 21,22
Interventions for pressure ulcer prevention
In all national and international guidelines, there are three main components of PU prevention practice, including:
-
formal risk assessment to identify ‘at risk’ patients and target preventative interventions
-
minimising both the intensity and the duration of exposure to mechanical loads on vulnerable skin sites not adapted to loading8,12 through –
-
minimising exposure to localised pressure through use of pressure-redistributing mattresses/cushions
-
intermittent pressure relief26 through major or minor repositioning of patients and/or mattresses and cushions that alternate temporarily to relieve pressure
-
complete pressure relief through continuous offloading of a specific skin site12 achieved by positioning patients or providing a heel offloading device so that a specific skin site (i.e. a skin site with an existing PU or adverse skin status) is completely offloaded at all times.
-
-
optimising skin condition through cleansing, drying and minimising exposure to moisture. 12
In this randomised controlled trial (RCT), the focus was on the effectiveness of two interventions: one minimising the intensity of pressure and one minimising both the intensity and the duration of pressure.
Pressure ulcer prevention mattresses
Pressure-relieving mattresses either distribute a patient’s weight over a larger contact area, providing ‘constant low pressure’ to reduce pressure intensity, or mechanically distribute a patient’s weight over a large contact area AND vary the pressure beneath the patient in order to reduce both the intensity and the duration of pressure [alternating pressure mattresses (APMs)]. 27 There are a range of ‘constant low pressure’ mattresses that are classified as ‘low technology’ [e.g. high-specification foam, gel, water] and ‘high technology’ [e.g. electrically powered air and air-fluidised (bead) beds]. All the alternating pressure mattresses are electrically powered and are classified as high technology. 27 Unit costs vary considerably; mattresses used frequently in the UK can be as little as £80–35228 for a low-technology high-specification foam mattress or as much as £500–4000 for a high-technology alternating pressure mattress. 28 In this study, these two main mattress types, utilised within the NHS, were compared, namely (1) high-technology APMs and (2) HSFMs, which are low-technology support surfaces. 27
Table 1 illustrates different types of mattresses and their classifications as ‘low technology’ and ‘high technology’, as adapted from the National Institute for Health and Care Excellence (NICE) guidelines12 and Cochrane systematic review. 27 Only HSFMs and APMs were used in this study.
| Mattress type | Technology |
|---|---|
| Static overlay | Low |
| HSFM | Low |
| Static air filled | Low |
| Gel filled | Low |
| Alternating pressure | High |
| Hybrid foam/alternating pressure | High |
| Low air loss | High |
| Hybrid alternating pressure/low air loss | High |
| Specialised | High |
| Hybrid alternating top layer and air-filled static | High |
Mattress intervention effectiveness
Overall in this field, the quality of trials is poor [small underpowered studies without allocation concealment, intention-to-treat (ITT) analysis or a priori sample size estimate]. 27,29 NICE guidelines12 and systematic review evidence27 highlight the fact that NHS resource availability is not based on robust health economic evaluations.
The fifth version of the Cochrane systematic review of support surfaces for PU prevention27 was published in 2015. The review included 59 RCTs, of which 11 compared the effectiveness of APMs with a range of low-technology (including high-specification foam, gel, silicone, water, static air) and high-technology constant low-pressure mattresses (low air loss). Most trials showed no evidence of a difference between treatment groups, although some were too small and underpowered to detect clinically important differences. Pooling of data in a meta-analysis of 10 trials of APMs versus various constant low-pressure mattresses showed no evidence of a difference in effectiveness for PU prevention.
Of the 11 RCTs, only one study compared APMs with a HSFM (viscoelastic)30 as part of a 2 × 2 factorial design incorporating two methods of risk assessment (Braden Scale31 score vs. observation of category 1) and two mattress/repositioning interventions (APM overlay vs. HSFM with turning every 4 hours). The study took place in a single centre and recruited patients from surgery, internal medicine and elderly care. Patients aged ≥ 18 years, with an expected length of stay of > 3 days were recruited and randomised to be ‘risk assessed’ using either the Braden Scale31 or observation of a category 1 skin area. Those identified as being at risk of developing a PU then had a second-level randomisation, with allocation to an APM or HSFM with turning every 4 hours. A total of 447 patients had the second-level randomisation and the incidence of PUs (of category ≥ 2) was 15.3% (34/222) in the APM arm and 15.6% (35/225) in the HSFM plus repositioning arm. Outcome data were recorded by ward staff. There were more heel ulcers reported in the HSFM arm, but more severe ulcers developed in the APM arm. An adjusted analysis incorporating risk assessment was not reported. Results are confounded by the inclusion in the HSFM arm of turning every 4 hours, and the potential for contamination between methods of risk assessment was not explored (including impact on outcome assessment, i.e. staff in the category 1 risk assessment arm were already alert to pressure damage); a major limitation is that outcome data were recorded by ward staff.
The review concluded that the relative merits of APMs and constant low-pressure mattresses are unclear. The review recommended the evaluation of APMs compared with constant low-pressure mattresses (such as HSFMs) because of their widespread use. 27 Similarly, NICE guidelines12 research recommendations acknowledge the limited evidence of effectiveness of pressure-redistributing devices and recommend further research. They particularly note the variation in cost of APMs compared with HSFMs.
The patient perspective
Although NICE guidelines12 are underpinned by the principle that all health-care professionals should deliver patient-centred care, it is exceptional to find a RCT with a patient-centred outcome such as acceptability, comfort or quality of life (QoL). Only four out of 59 RCTs included in the Cochrane review27 report patient comfort or acceptability of the mattress as an outcome and none report a patient’s QoL.
There is evidence from qualitative studies exploring the lived experience of patients with PUs23,32 and secondary end-point data in Pressure RElieving Support SUrfaces: a Randomised Evaluation (PRESSURE) 133 that some patients do not like APMs; a network meta-analysis34 reported that APMs had the lowest probability of being the most comfortable compared with other mattress types. Alternating pressure mattresses comprise large air-filled pockets that inflate and deflate in cycles. The alternating sensation is disliked by some patients and can cause feelings of nausea and affect sleep. In addition, on patient movement, the air-filled pockets are compressed and patients find it difficult to mobilise in bed and also report feeling unstable at the mattress edge, when they are getting in and out of bed, or feeling like they will be ‘rolled out of bed’, creating an unsafe feeling. 23,32,33 Other issues include noise from the pump, technical failure and attendant alarms. This was further supported by members of the Pressure Ulcer Research Service User Network (PURSUN),35 which supports research prioritisation and informs grant development, project delivery and data interpretation. Some members of the group feel strongly that APMs can be uncomfortable and debilitating, restrict movement and independence, exacerbate existing balance/mobility problems and leave patients in need of extra care (i.e. help in repositioning).
Practice guidelines
Previous NICE guidance26 stated that:
. . . decisions about which pressure-relieving device to use should be based on cost considerations and an overall assessment of the individual. Holistic assessment should include all of the following: identified levels of risk, skin assessment, comfort, general health state, lifestyle and abilities, critical care needs and acceptability of the proposed pressure-relieving equipment to the patient and/or carer.
It is not clear what ‘cost considerations’ means, but, in practice, decisions are generally made on unit costs and not cost-effectiveness, this being challenged following publication of PRESSURE 1,33 in which it was demonstrated that, despite no clinical difference between mattresses, there was a 64% probability that the more expensive APM replacement (unit cost ≈£4000) was more cost-effective than the cheaper APM overlay (unit cost ≈£1000).
The current NICE guideline12 for PU prevention utilised the evidence found in the 2011 version of the McInnes et al. 36 Cochrane review and recommended that a HSFM should be used for adults in secondary care settings or those who are assessed as being at a high risk of PUs in community and primary care environments. As this recommendation is based on trials deemed to be of low quality, NICE highlighted the following research priority:
Do pressure redistributing devices reduce the development of pressure ulcers for those who are at risk of developing a pressure ulcer?
The NICE guidelines section for PU treatment, however, do include the following caveat:
Use high-specification foam mattresses for adults with a pressure ulcer. If this is not sufficient to redistribute pressure, consider the use of a dynamic support surface.
The quality of evidence to inform this recommendation was considered to be low and none of the studies followed up patients for a sufficient time. There is no reference to the category of PU in the recommendation; however, the RCTs used to inform this included PUs of all categories.
NHS practice
Traditional hospital foam mattresses (with a marbled cover) have been superseded by HSFMs with both a ‘high-performance’ foam core and a cover designed to minimise ‘hammocking’ and build-up of moisture. There is good evidence of the benefit of HSFMs compared with traditional hospital mattresses in reducing the incidence of PUs in high-risk patient populations27 and HSFMs are in widespread use in the NHS, with many hospitals providing all patients with a HSFM as standard. Following NICE guidance,26 a HSFM is the recommended ‘minimum’ standard care for those assessed as being ‘vulnerable to PUs’.
Despite the lack of evidence of benefit, APMs are also in widespread use in the UK for at-risk patients. In two studies conducted as part of the Pressure UlceR Programme Of reSEarch (PURPOSE),37 their common use in the NHS was identified. First, the multicentre PU prevalence survey,6 involving nine hospitals across three NHS trusts and ≈3000 patients, found that ≈20% of mattresses in the adult care setting were APMs. Second, in a multicentre cohort study of 634 patients, involving high-risk patients with mobility/activity impairment and/or category 1 PUs,38 mattress allocation by ward staff to the study population was 48% HSFM and 52% APM, reflecting a lack of standardised practice.
History of this research
A Health Technology Assessment (HTA) programme commissioning brief in 1998 included multicentre RCTs to compare the clinical effectiveness and cost-effectiveness of APMs with:
-
less costly alternating pressure overlays
-
low-technology constant low-pressure alternatives (such as different types of HSFM).
At that time, there was a reluctance by clinicians to randomise high-risk patients to HSFM, so the trial funded by the HTA programme, PRESSURE 1,33,37,39 dealt with only the first of the two research priorities and compared overlay and replacement APMs.
However, since then, many UK hospitals have replaced traditional hospital mattresses with HSFMs as standard for some or all clinical specialties. In addition, NICE guidance12 and the widespread use of profiling electric beds have increased clinical confidence in the use of HSFMs for high-risk patients. Furthermore, qualitative and quantitative evidence suggests that some patients do not like APMs,23,32,33 and results of the PRESSURE 1 trial33 showed a lack of difference in clinical outcomes between expensive alternating pressure replacement mattresses and cheaper alternating pressure overlay mattresses. These developments in the knowledge base and clinical experiences have challenged previously held views of clinical effectiveness based on non-randomised evaluations, which inferred the superiority of APMs and enabled trial design and delivery to address a key clinical question comparing high-technology APMs and low-technology HSFMs in the prevention of new PUs.
Summary
In the light of the priority being given to PU prevention, the high cost and lack of evidence relating to the effectiveness of mattresses in common use in the NHS, ad hoc practice in mattress allocation and the disadvantages and difficulties reported by patients in the use of APMs, we undertook a RCT to compare APMs with HSFMs in a high-risk inpatient population.
Chapter 2 Trial methods
Aims and objectives
The primary aim was to determine the clinical effectiveness and cost-effectiveness of APMs and HSFMs when used in conjunction with an electric profiling bed frame in secondary and community inpatients with evidence of acute illness, for the prevention of PUs of category ≥ 2.
Secondary aims were to assess the feasibility of using photography to quantify potential bias in the reporting of the PRESSURE 2 trial primary end point (see Chapter 5) and modify and evaluate the Pressure Ulcer Quality-of-Life (PU-QoL) instrument for use in patients at risk of PUs receiving preventative interventions (see Chapter 6).
Primary trial objective
The primary objective was to compare the time taken to develop a new PU of category ≥ 2 in participants using APM with the time taken to develop a new PU of category ≥ 2 in those using HSFM.
Secondary trial objectives (clinical)
-
To compare the time taken to develop a new PU of category ≥ 3 between participants using APM and those using HSFM.
-
To compare the time taken to develop a new PU of category ≥ 1 between participants using APM and those using HSFM.
-
To compare the time to healing of all pre-existing category 2 PUs between participants using APM and those using HSFM.
-
To compare the incidence of mattress change between participants using APM and those using HSFM.
-
To compare safety between participants using APM and those using HSFM.
Secondary trial objectives (health economic)
-
To determine the impact of APM and HSFM on HRQoL.
-
To determine the incremental cost-effectiveness of APM compared with HSFM from the perspective of the health and social care sectors.
Photography substudy objectives
-
To assess over-reporting of category ≥ 2 PUs using photography.
-
To assess under-reporting of category ≥ 2 PUs using photography.
-
To assess consent rates for photography.
-
To assess compliance with photographs (i.e. whether or not the intended number of photographs were actually taken).
-
To assess compliance with secure transfer of photographs between the research centre and the Clinical Trials Research Unit (CTRU).
-
To assess the quality of photographs and confidence in photographic review.
Pressure Ulcer Quality of Life substudy objectives
-
To modify the PU-QoL instrument for use with patients at a high risk of PU development receiving preventative interventions – the Pressure Ulcer Quality of Life – Prevention (PU-QoL-P).
-
To undertake a comprehensive evaluation of the psychometric measurement properties of the PU-QoL-P instrument to ensure that it is acceptable, reliable, valid and responsive, and suitable for use in the UK health-care setting.
Overview of methods
This chapter outlines the main methods for the trial, including all data collection for the trial and substudy work and the analysis methods for the primary and secondary clinical results, which are detailed in Chapter 3. Analysis methods and results for the main trial health economics and QoL comparisons, the photography substudy and the PU-QoL substudy are detailed in Chapters 4, 5 and 6, respectively. The discussion in Chapter 7 draws the work together.
Trial design
The trial was a multicentre, Phase III, open, prospective, randomised, planned as a double-triangular sequential, parallel-group trial, with two planned interim analyses.
High-risk patients from secondary and community inpatient facilities with evidence of acute illness were randomised on a 1 : 1 basis to receive either APM or HSFM in conjunction with an electric profiling bed.
The intervention phase follow-up period was for a maximum of 60 days (referred to as the ‘treatment phase’) and there was a 30-day post-treatment phase follow-up (referred to as the ‘30-day final follow-up’).
The study protocol for this trial has already been published. 40 A study flow diagram/study summary can be found on page 6 of the trial protocol [see Methods on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)]. Summary details of the methods are given in the following sections.
Group sequential trial design
The group sequential design provides a formal framework and efficient design in which clinical trial data can be monitored as they accumulate, with preplanned interim analyses that can inform early stopping for superiority, inferiority or futility. PRESSURE 2 had two preplanned interim analyses and, at each interim analysis, the analysis of the primary end point (time to developing a PU of category ≥ 2) was to be conducted on both the ITT and the per-protocol populations (PPPs). The results were to be presented to the Data Monitoring and Ethics Committee (DMEC), which would advise the Trial Steering Committee (TSC) if the balance of benefits and harms suggested that the trial should be stopped in accordance with planned stopping rules. 40
Ethics approval
Ethics approval for the study was given by Leeds West Research Ethics Committee [Research Ethics Committee (REC) reference number 13/YH/0066; Integrated Research Application System (IRAS) project identification: 122769]. All trial activity took place in accordance with the ethics-approved protocol, principles of Good Clinical Practice41 and the Declaration of Helsinki. 42
Eligibility and informed consent
Acute secondary and community NHS trust inpatient admissions were screened for eligibility by a clinical research nurse/registered health-care professional (CRN/P) in consultation with ward staff. Patients were eligible at any point during their inpatient stay (and irrespective of trust provider) if they fulfilled the following criteria:
-
Had evidence of acute illness through –
-
acute admission to secondary care hospital, community hospital or NHS-funded intermediate care/rehabilitation facility
-
inpatient secondary care, community hospital or NHS-funded intermediate care/rehabilitation facility with an onset of acute illness secondary to elective admission
-
recent secondary care hospital discharge to community hospital or NHS-funded intermediate care/rehabilitation facility.
-
-
Was aged ≥ 18 years.
-
Had an expected total length of stay of ≥ 5 days.
-
Was at a high risk of PU development as a result of one or more of the following:
-
Consented to participate [written, informed consent/witnessed verbal consent/consultee agreement or nearest relative/guardian/welfare attorney (in Scotland)].
-
Was expected to comply with the follow-up schedule.
-
Was on an electric profiling bed frame.
Patients were excluded if:
-
they had previously participated in the PRESSURE 2 trial
-
they had a current or previous PU of category ≥ 3
-
they had a planned admission to an intensive care unit where standard care was APM provision
-
they were unable to receive the intervention (e.g. slept at night in a chair or was unable to transfer to randomised mattress)
-
they weighed less or more than mattress weight limits (< 45 kg or > 180 kg)
-
it was ethically inappropriate to approach them.
A log was completed of all patients who were screened but not randomised, either because they were ineligible or because they declined participation. The following anonymised information was included:
-
age
-
gender
-
ethnicity
-
current mattress type
-
date screened
-
the reason why they were not eligible or declined participation
-
other reason for non-randomisation.
Potentially eligible patients were provided with a verbal explanation of the study and a patient information leaflet [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)]. Assenting patients were formally assessed for eligibility and invited to provide informed consent. Witnessed consent process was used [see Witnessed Consent Form on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)] for those who gave consent but were physically unable to complete the written form.
A large proportion of patients suffering from or at risk of PUs have cognitive impairment affecting their understanding and/or dementia. Cognition affects compliance with repositioning and self-care. To ensure that the study population was representative of the normal NHS clinical population assessed in the course of usual care, recruitment procedures also facilitated consultee or nearest relative/guardian/welfare attorney (in Scotland) agreement [see Consultee Agreement Form on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)].
Interventions
Participants were randomised to either APM or HSFM products used by the participating centre for a maximum of 60 days. All patients were also allocated an electric profiling bed, as per standard care in the participating centres.
As this was a pragmatic trial, operational specifications for both APMs and HSFMs were defined in the PRESSURE 2 mattress specification guide (MSG) (see Appendix 1). Individual products allocated to trial participants were part of the usual-care mattress stock for each participating hospital, maximising generalisability of the trial findings.
The PRESSURE 2 MSG (see Appendix 1) provided details of foam density and cover details for HSFM and cell height (8.5 cm minimum, 29.4 cm maximum) and cycle time and frequency for APMs. Excluded mattresses, for example hybrid foam/air mattresses, were also specified. During feasibility assessment, mattresses in each centre were reviewed against the PRESSURE 2 MSG and only trusts with sufficient access to trial-eligible mattresses and electric profiling beds were able to take part in the trial. As new mattresses were marketed during the trial period, the PRESSURE 2 MSG was updated.
All mattresses had to comply with The Medical Devices Regulations 2002 SI (Système International d’Unités). 43 The allocated randomised mattress was expected to be provided to the trial patient within 24 hours of randomisation.
Randomisation
Participants were randomised once eligibility was confirmed and the baseline assessments and questionnaires were completed. Participants were randomised in a 1 : 1 allocation ratio, to receive either an APM or a HSFM in conjunction with an electric profiling bed. A computer-generated minimisation program, which incorporated a random element of 0.8, was used to ensure that intervention groups were well balanced for the following factors:
-
centre
-
PU status (no PU, category 1 or 2 PU)
-
secondary care hospital, community hospital/intermediate care or rehabilitation facility
-
consent [written, witnessed verbal, or consultee or nearest relative/guardian/welfare attorney (in Scotland) agreement].
The randomisation system included an automated internal check using a patient’s NHS number to confirm that the participant had not been recruited to the trial previously.
Blinding
It was not possible to blind participants, the clinical team or the CRN/P because of the nature of the mattresses (presence/sound of a pump on the APM and different appearance of bed and sheeting on each type of mattress).
A validation substudy, using photography with blinded central review, was therefore included to assess any bias in the reporting of PUs of category ≥ 2, details of which are given in Chapter 5.
Assessments and instruments
Skin assessments
Pressure ulcer status was assessed using the international PU classification11 including categories 1–4, unstageable and suspected deep tissue injury. This classification system was chosen as it was the most frequently used system in the NHS at the start of the trial period. Additional skin status descriptors were also assessed and recorded to characterise other alterations of intact skin and skin site exclusions from the end-point derivation (Box 1), meeting practical data collection requirements for the purpose of research.
Healthy intact skin. No skin changes.
Category AAlterations to intact skin specified with subcategory code:
-
001 = blanching redness which persists
-
002 = bruising – red hue
-
003 = bruising – purple hue
-
004 = scar
-
005 = oedema
-
006 = cellulitis
-
007 = lymphoedema
-
008 = discoloration – ischaemia
-
009 = discoloration – cyanosis
-
010 = dry/flaky
-
011 = papery thin
-
012 = cracks/calloused
-
013 = spongy
-
014 = macerated
-
015 = scratches
-
016 = rash
-
017 = scab
-
018 = induration
-
019 = heat
-
999 = none of the above, please describe.
Non-blanchable erythema of intact skin. Intact skin with non-blanchable erythema of a localised area usually over a bony prominence. Discolouration of the skin, warmth, oedema, hardness or pain may also be present. Darkly pigmented skin may not have visible blanching.
Category 2Partial-thickness skin loss or blister. Partial-thickness loss of dermis presenting as a shallow open ulcer with a red-pink wound bed, without slough. May also present as an intact or open/ruptured serum or serosanguinous-filled blister.
Category 3Full-thickness skin loss. Full-thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Some slough may be present. May include undermining and tunnelling.
Category 4Full-thickness tissue loss. Full-thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present. Often includes undermining or tunnelling.
Category UUnstageable. Full-thickness skin loss in which actual depth of the ulcer is completely obscured by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed.
Category N/ANot applicable. Specify with subcategory code:
-
001 = amputation
-
002 = bandage in situ
-
003 = cast in situ
-
004 = dressing in situ
-
005 = incontinence-associated dermatitis
-
006 = other chronic wound
-
007 = device-related ulcer
-
008 = surgical wound/bruising
-
009 = traumatic wound/bruising
-
010 = dermatological skin condition (e.g. eczema)
-
011 = unable to assess
-
999 = none of the above, please describe.
Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
In addition, photography of all category 2 PUs at first observation was undertaken (subject to consent). At each visit, all major anatomical skin areas at risk of PU development (sacrum, spine and right and left buttocks, ischial tuberosities, hips, heels, ankles and elbows) were assessed.
Risk factors (moderators)
Risk factors were recorded based on the pressure ulcer – minimum data set (PU-MDS)45 derived from a systematic review of the risk-factor literature,18 which was developed using consensus methods. The PU-MDS included descriptors for the key risk factors, namely skin status, mobility status, sensory perception, diabetes, conditions affecting macro- and microcirculatory function, nutrition and skin moisture. Data on skin status, supplementary to the minimum data set, was also recorded (see next section).
In addition, in previous exploratory work, we identified that pain was associated with the development of new PUs of category ≥ 238 and was assessed as a covariate to further explore the prognostic value of pain. Using our established method, the presence of localised skin pain on any pressure area skin site was confirmed if participants responded ‘yes’ to both the following questions:
-
At any time, do you get pain, soreness or discomfort on a pressure area? Prompt – back, bottom, hips, elbows, heels or other sites as applicable?
-
Do you think this is related to your pressure sore/lying in bed for a long time/sitting for a long time (as appropriate)?
Pressure ulcer prevention interventions (mediators) and compliance
In order to monitor selection bias at baseline and mattress compliance at follow-up, mattress type was recorded, including APM or other high-technology mattress and HSFM or other low-technology mattress (see Table 1). In addition, to characterise standard care as provided at the discretion of the attending clinical team and potential mediators, recorded PU prevention interventions included frequency of repositioning in the previous 24 hours, chair type and cushion type (when appropriate), adjuvant dressings for prevention and adjuvant prevention devices, such as heel off-loading devices, including pillows.
Quality-of-life and health resource utilisation data
The following QoL instruments were administered.
Generic quality-of-life instruments
EuroQol-5 Dimensions, five-level version
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L), is a QoL measure consisting of five domains: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. Each of these domains has five levels: (1) no problems, (2) slight problems, (3) moderate problems, (4) severe problems and (5) extreme problems. 46
Short Form questionnaire-12 items acute
The Short Form questionnaire-12 items (SF-12) instrument was used to assess HRQoL, on the basis of evidence from a systematic review of QoL measures for chronic wounds (including PUs)47 and practical issues relating to the patient population.
Use of the Short Form questionnaire-36 items (SF-36) was considered for inclusion; however, it was decided by the project team that it was too long for use with patients with PUs (e.g. these patients are largely elderly, are highly dependent and/or have high levels of comorbidity including acute and chronic illness). Instead, the SF-12, a short version of the SF-36, was selected to reduce respondent burden. The SF-12 is a generic instrument that assesses eight QoL domains: (1) physical functioning, (2) role-physical, (3) body pain, (4) general health, (5) energy/fatigue, (6) social functioning, (7) role-emotional and (8) mental health. A physical component summary score and a mental component summary score are generated. An acute version of the SF-12 is available that incorporates a 1-week recall period, which for this condition has been found to be relevant. 48 It takes 2 minutes to administer and has been validated for researcher administration. Even though the SF-12 has not specifically been validated for use with people with PUs, it is widely used in other chronic wounds and dermatological conditions to assess changes in QoL between groups, has been used with other chronic skin wound conditions to validate their corresponding disease-specific QoL instruments and has been validated for use with elderly people. The acute SF-12 was chosen as the best available QoL instrument for the primary trial analysis at this stage.
Condition-specific instruments
Pressure Ulcer Quality of Life – Prevention
During the National Institute for Health Research (NIHR) PURPOSE,37 PU-QoL, a condition-specific outcome measure, was developed for people with PUs;48 however, there is a need for a similar measure for patients at risk of PU (prevention). The PU-QoL-P was therefore developed and validated during this study. Further details can be found in Chapter 6.
Pressure Ulcer Quality of Life – Utility Index
The Pressure Ulcer Quality of Life – Utility Index (PU-QoL-UI) is a condition-specific utility measure derived from the PU-QoL instrument. 37,49 It is a researcher-administered, participant-completed questionnaire. If enrolment into the study was through consultee agreement or nearest relative/guardian/welfare attorney (in Scotland), a proxy PU-QoL-UI could be completed by the CRN/P, ideally with a carer, on behalf of the participant.
Health-care resource use
Health-care resource utilisation (e.g. diagnostic tests and medical assessments) was abstracted from health-care records (inpatient and outpatient) and a short researcher-administered questionnaire (regarding the use of community health and social care) was completed with participants.
Safety monitoring
The trial population were known to have high levels of comorbidity and mortality. Therefore, it was decided to report only events that were considered ‘related’ to the intervention.
The following adverse events (AEs) and serious adverse events (SAEs) were expected within the patient study population and were reported during the treatment and follow-up phase:
-
death (SAE)
-
hospital re-admission (SAE)
-
institutionalisation (AE)
-
device ulcers that may be considered to be related to the mattress (AE/SAE), such as plaster cast ulcers
-
falls (AE/SAE).
As these events were expected within the study population, they were subject to expedited reporting to the main REC. Further definitions of AEs and SAEs are provided in the protocol. 40
Data collection schedule
All baseline and outcome assessments were undertaken by trained CRN/Ps. Treatment phase follow-up assessments were undertaken from randomisation to the end of the treatment phase (a maximum of 60 days). A final 30-day final follow-up visit was undertaken.
Baseline
The following baseline demographic information was collected: NHS number, date of birth, gender, date of admission, type of admission, category of medical condition (e.g. medical, surgical), ethnicity, confirmation general practitioner (GP) letter sent and confirmation consultant letter sent.
The following clinical assessment information was collected: pre-randomisation mattress, skin assessment, PU category 2 photography (when present), assessment of pain on pressure area skin site, risk factors (mobility status, sensory perception, diabetes, conditions affecting macro- and microcirculatory function, nutrition, skin moisture, friction and shear), height and weight (self-reported or from records), PU prevention mattress and other interventions (mediators), and duration and size of ulcer for participants with a pre-existing category 2 PU.
The following participant questionnaires were administered: health-care resource utilisation and, at the start of the study, EQ-5D-5L, PU-QoL-UI, SF-12 and PU-QoL-P (or a proxy questionnaire pack when necessary).
The following personal data were collected (these were retained in the site file and not returned to the CTRU): name, hospital number, ward location, address and telephone number, GP name and address, and name of the hospital consultant responsible for the care of the patient.
The following randomisation information was collected: mattress allocation and date of mattress provision.
Treatment phase (maximum 60 days)
Mattress compliance and technical faults were recorded for each day (based on retrospective information from staff and participants).
A clinical assessment was conducted twice weekly to day 30, then once weekly to day 60. The clinical assessment involved skin assessment (including pain and photography of PUs of category ≥ 2 when present), PU prevention interventions (mediators), expected AEs and SAEs, unexpected and related SAEs and/or confirmation of the end of the treatment phase (patient discharged or 60 days of treatment or no longer at risk).
Participant questionnaires
The frequency of completion of the full questionnaire pack was reported by the CRN/Ps as a burden for many participants and the schedule was amended during the course of the trial. Initially, the health-care resource utilisation questionnaire, EQ-5D-5L, PU-QoL-UI, SF-12 and PU-QoL-P (or proxy questionnaire pack when necessary) were completed weekly to day 30, then fortnightly to day 60. Between April 2014 and October 2015, a revised schedule was implemented with a randomised allocation to a set of questionnaires (EQ-5D-5L/PU-QoL-UI or the SF-12/PU-QoL-P) at weeks 1 (visit 2) and 3 (visit 6) only. From November 2015 to November 2016, the PU-QoL-P questionnaire was omitted because sufficient data had been collected for conducting the analysis, and the remaining questionnaires (EQ-5D-5L, PU-QoL-UI and SF-12 or proxy questionnaire pack) were reinstated for all participants at weeks 1 and 3.
Thirty-day final follow-up
Following confirmation of the end the treatment phase (maximum 60 days), a final 30-day follow-up visit was scheduled. This was undertaken at the patient’s place of residence (i.e. hospital if an inpatient or home if discharged).
A clinical assessment that comprised skin assessment (including photography if required) was undertaken.
The following participant questionnaires were administered: health-care resource utilisation, EuroQol-5 Dimensions (EQ-5D-5L), PU-QoL-UI, SF-12 and PU-QoL-P (or proxy questionnaire pack when necessary).
Trial completion
Trial completion was defined as the end of the 30-day final follow-up (i.e. 30 days after the end-of-treatment phase), withdrawal or death.
The treatment phase was defined as the time from randomisation to (whichever happened soonest):
-
discharge from an eligible inpatient facility
-
60 days from randomisation
-
no longer at a high risk.
No longer at a high risk was defined as no PU of category ≥ 1 on any skin site AND no localised skin pain on any pressure area skin site AND improved mobility and activity (Braden Scale31 activity score of 3 or 4 AND mobility score of 3 or 4). 50
End points
Primary end point
The primary end point was time to developing a new PU of category ≥ 2 from randomisation, during the (maximum) 60-day treatment phase to the 30-day final follow-up (a maximum of 90 days). The timing of the primary end point was chosen to align with requirements for the health economics analysis. Given that a PU can take between 4 and 22 weeks to completely heal depending on the category,2 and that randomised patients are discharged with an unresolved PU, the 30-day post-treatment phase follow-up assessment was included in the trial to retrieve information on treatment and utility regarding the potential long-term effect of the intervention.
Whether or not a participant developed a new PU of category ≥ 2 was first derived on a skin site basis [see Appendix 2; see also Methods on the project web page for the statistical analysis plan (SAP) (specifically, table 2 in appendix C, and figure 2): www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)]. These data were then used to derive whether or not each participant developed a new PU of category ≥ 2 at any skin site and the time to development of the first new PU, competing event or censoring time [see the project web page for the SAP, section 2.3.2 time to development and censor variables: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)].
For participants who developed a new PU of category ≥ 2, the time to development was calculated as the duration between the date of randomisation and the date that the first new PU of category ≥ 2 was observed. Participants were categorised as a competing risk at the date they stopped the trial if they withdrew from the trial due to clinical condition or loss of capacity, or because the assessment schedule was too burdensome, or if they died; or else participants were censored at the date of their last evaluable skin assessment. Further details of the derivation of the end point and a summary of the derivation of the primary end point is provided in Appendix 2 [see also Methods on the project web page for the SAP (specifically, table 2 in appendix C and figure 2): www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)].
Secondary end points
-
Time to developing a new PU of category ≥ 3.
The time to development of a PU of category ≥ 3 is derived in line with the derivation of the primary end point but adjusted for the development of a more severe PU (i.e. category 3) rather than a category 2.
-
Time to developing a new PU of category ≥ 1.
The time to development of a new PU of category ≥ 1 is derived in line with the derivation of the primary end point but adjusted for the development of a less severe PU (i.e. category 1) rather than a category 2.
-
Time to healing of all pre-existing category 2 PUs.
A category 2 PU was classified as healed if the same skin site was later recorded as healthy or altered skin. Time to healing was derived as the number of days between the date of randomisation and the date all pre-existing category 2 PUs were observed to heal. Participants were categorised as a competing risk at the date they stopped the trial if they withdrew from the trial due to clinical condition or loss of capacity or because the assessment schedule was too burdensome, or they died; or else participants were censored at the date of their last evaluable skin assessment.
Trial organisational structure
The trial sponsor was the University of Leeds and responsibilities were delegated to the CTRU, as detailed in the trial contract.
Trial oversight and management was conducted by the Trial Management Group (TMG), the TSC and the DMEC. All groups met regularly throughout the trial.
Centre-level case report form (CRF) returns, data quality, screening logs, recruitment, patient disposition, protocol deviations and safety were monitored by the CTRU, TMG, TSC and DMEC. In addition, overall mattress compliance was monitored by the TSC and DMEC (with no cause for concern to prompt investigation at a centre level). Compliance with the photography and skin verification substudies and the overall event rate were monitored by the DMEC.
Public involvement methods
The CTRU hosts a network of service users, PURSUN, with personal experience of PUs and/or PU risk. The group consists of patients, carers and family members. PURSUN works to improve public involvement in PU research and raise awareness of the topic. It has an established relationship with the study team and has supported this study from conception. Public involvement activities have included the following:
-
Study design. Meetings were held with PURSUN at both the grant application and the protocol development stages. In particular, PURSUN made an important contribution to the development of the eligibility criteria (at its request, patients with existing PUs of category ≥ 3 were excluded from participation in the trial) and to the consent process for the photography substudy (as PUs commonly develop on sensitive areas of the body, such as buttocks, PURSUN advised whether or not and how the consent process should be conducted).
-
Developing participant materials.
-
Project oversight. A service user co-applicant (KW) was invited to all TMG meetings and two PURSUN members joined the TSC. Update meetings were also held with the wider network throughout the study.
-
Results interpretation. One results interpretation workshop has already been held with a small number of PURSUN members. A larger one is planned for 2019. The aim of the workshops is to discuss what the results mean for service users, and to plan a collaborative approach to dissemination and implementation.
Supporting public involvement
The Pressure Ulcer Research Service User Network is supported by a patient and public involvement (PPI) officer, who acted as the main point of contact for all service users involved in the study. Additional support and facilitation were provided by the chief investigator and trial co-ordinator. PURSUN has an established induction process whereby new members meet with the PPI officer to discuss their specific support and training needs. This same flexible approach was adopted during PRESSURE 2. Support was tailored to the needs of the individuals involved and their role in the project.
All service users involved in the study were invited to attend CTRU’s ‘Introduction to Clinical Trials’ training, a 1-day workshop open to academics, health professionals and public contributors. This training was not mandatory, but was seen as an optional development opportunity. All PURSUN members were reimbursed for their time and expenses in line with PURSUN policy and public involvement good practice guidelines. 51
Public involvement evaluation
An iterative approach to evaluation was adopted to ensure that issues could be identified and dealt with throughout the study. The service user co-applicant and TSC members were offered regular debrief meetings with the PPI officer. These meetings had the dual purpose of providing support and aiding evaluation.
Involvement activities and impact were documented via a public involvement log. The log is a shared document that can be accessed by both the PPI officer and the service user co-applicant. The log contains a series of prompt questions that encourage both documentation and reflection. The co-applicant was encouraged to document any challenges and areas in which she felt that she (or other public contributors) had made a positive impact on the study. She was also asked to think about the impact (positive or negative) that being involved in the study had on her personally.
Statistical methods
Original sample size
A maximum of 588 events (i.e. participants developing a new PU of category ≥ 2), corresponding to 2954 participants, was required for the study to have 90% power for detecting an absolute difference of 5% in the incidence of PU of category ≥ 2 between APM and HSFM, assuming an incidence rate of 18% on APM52 and 23% on HSFM [corresponding to a hazard ratio (HR) of 0.759] and a two-sided significance level of 5% and accounting for 6% loss to follow-up. 52
An incidence rate of 18% for PUs of category ≥ 2 for APMs was estimated on the ITT population for PRESSURE 1,33 the PURPOSE pain cohort study33,38 and the trial reported by Vanderwee et al. ;30 hence, the sample size estimate incorporates the effect of non-compliance. The sample size accounts for multiplicity in the interim analyses using Lan–DeMets α and β spending functions. 53
Pressure ulcer incidence rates could not be estimated accurately for the HSFM group; therefore, the maximum sample size estimate was based on the detection of the smallest relevant difference of 5% (clinical opinion). If the difference was > 5%, then the trial would have sufficient power to stop early having demonstrated superiority (or inferiority) of the APM. If the difference was < 5%, then the trial was likely to stop early for futility.
Original planned recruitment rate
It was planned to involve 10 large and 10 medium NHS trusts (comprising approximately 30 hospital sites). It was estimated that 15,000 patients would need to be screened, of whom ≈40% (6000) would be eligible and ≈50% (3000) of those eligible would consent. Accrual estimates were seven participants per month in three large trusts for 33 months, six participants per month in seven large trusts for 30 months and 3.5 participants per month in 10 medium trusts for 30 months, enabling recruitment of 3003 participants.
Revised sample size and expected accrual
The trial recruited participants at a much slower rate than originally anticipated. A recovery plan and unplanned value of information (VOI) analysis, based on the original trial assumptions, was requested by the funder; this was submitted to the funder in October 2014 and reviewed by the DMEC, which recommended trial continuation.
An unplanned interim analysis and second VOI analysis using confidential data from the trial (see Appendix 3) were requested by the funder and conducted in November/December 2015. A primary end point analysis was conducted on 909 participants. Owing to violation of the proportional hazards assumption, a piecewise Cox model was fitted to the primary end point, splitting the data into two time intervals; a sensitivity analysis was also conducted on the treatment phase (see Appendix 3). The analysis was conducted by the trial statistician, supervising statistician and health economist, and the results of these unblinded analyses were reviewed by the DMEC and remained confidential to these personnel. The hazard ratio (HR) for the treatment phase sensitivity analysis was presented to the DMEC in line with the stopping boundaries calculated for the original trial design. However, as this was an early unplanned analysis, a very low significance value was selected to minimise the impact on the overall significance level of 0.05;54 therefore, no adjustment to the p-value or width of the confidence intervals (CIs) was made at the final analysis. Following the interim analysis, the DMEC asked the team to explore alternative approaches to the piecewise Cox model in order to maximise power in the final analysis. The revised analysis plan [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)] detailed the planned approach to include scenarios when the assumption of proportional hazards and/or independent censoring do not hold.
Following discussion between the DMEC and TSC in January 2016, the independent TSC members were informed of the overall event rate, which was much lower, at 9.9%, than the event rate of 20.5% utilised to estimate the sample size. The DMEC and TSC then asked the TMG, who were blinded to the overall event rate, to consider the minimum clinically relevant differences on varying centred event rates including 15%, 10% and 5% to help inform the DMEC and TSC’s recommendations to the funder on the continuation of the trial. A relative difference of 25% centred on an overall event rate of 15%, corresponding to an absolute difference of 3.75%, was considered to be clinically meaningful and the preferred minimum clinically relevant difference. For an overall event rate of 10%, a relative difference of 33.3%, corresponding to an absolute difference of 3.3%, was considered to be clinically meaningful and the preferred minimum clinically relevant difference. In the third scenario, a relative difference of 50.0% centred on an overall event rate of 5%, corresponding to an absolute difference of 2.5%, was considered to be clinically meaningful and the preferred minimum clinically relevant difference. In all of these cases, a costed extension would have been required to power the study based on these differences. The TMG also noted that relative differences of 33.3%, 40% and 60% centred on event rates of 15%, 10% and 5%, corresponding to absolute differences of 5%, 4% and 3%, respectively, were considered important to detect. In addition, having sufficient precision in the estimate of the treatment effect to conclude futility was also considered important by the TMG.
Following discussion of the minimum clinically relevant differences with the TMG, DMEC and TSC supported a 6-month recruitment extension (requiring no additional HTA funding) request, which was submitted in June 2016, to continue recruitment until the end of November 2016, by which time approximately 1996 participants were expected to be recruited, allowing a difference of 4% to be detected with at least 80% power, assuming an overall event rate of 10%.
A request for additional funding to continue recruitment for 14 months beyond the original planned recruitment end date to achieve a sample size of 2728 participants to detect the minimum clinically relevant difference between mattresses of 3.3% with 80% power, together with a clinically meaningful improvement in precision of a further 3% under the assumption of no difference, was also supported by the TSC and DMEC.
The funder did not support additional funding to achieve the higher sample size, but did approve the 6-month extension request and accruals were maximised by continued recruitment until the end of the extension period, which was 30 November 2016.
Statistical analysis
All analyses were performed using SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA).
A SAP was approved prior to the relevant analysis being conducted [see Methods on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)].
Reflecting the double-triangular sequential design, a maximum of three analyses were originally planned with unequally spaced reviews at event-driven coherent cut-off points, as specified below.
Interim analysis 1 was to be conducted after 300 events, corresponding to the earliest time point for stopping the trial by demonstrating overwhelming evidence of efficacy or futility. This would have also corresponded to the minimum number of events required for conducting the economic evaluation. The futility boundaries were constructed as non-binding in order for the DMEC to over-rule a decision of stopping early for futility in the event that a futility boundary is crossed.
Interim analysis 2 was to be conducted after 445 events, corresponding to the number of expected events required for trial termination under futility (with 434 corresponding to the number of events required for demonstrating superiority or inferiority of APM to HSFM).
The final analysis was to be conducted after 588 events had occurred.
In addition, in the event of an early stopping signal for futility, an assessment of the value of continuing with the trial from the NHS decision-making perspective, via an expected value of sample information (EVSI) analysis, was also planned, to inform the deliberations of the DMEC.
Following the unplanned interim analysis and the extension to recruitment, only the final analysis was conducted.
Patient populations
All participants recruited to the trial were included in the analysis using ITT and analysed in accordance with the randomised allocation and actual stratification factors.
A PPP analysis was also undertaken by allocated mattress; all major protocol violators and participants who did not receive their randomised mattress within 2 days of randomisation or who spent < 60% of their follow-up time on their randomised mattress were excluded (see Methods on the project web page for further details of exclusions: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)].
The safety population included all participants who were recruited to the trial.
The population used to calculate the time to healing of category 2 PUs included all participants with at least one pre-existing category 2 PU at the baseline assessment.
Missing data
Missing categorical data at baseline were included in the analysis models as a specific category. Any missing dates in the end-point derivation were imputed based on visit dates before and after, and in accordance with the visit schedule.
Final analysis
Primary end point
The primary end-point analysis was the time taken to develop a new PU of category ≥ 2 from randomisation to 30-day final follow-up.
A Fine and Gray55 model was fitted to the primary end point, with adjustment for the following minimisation factors: health-care setting, PU status, consent and covariates (i.e. presence of pain on a healthy, altered or category 1 PU skin site and the presence of conditions affecting peripheral circulation). A likelihood ratio test was used to assess the effect of adding mattress group to this model. The HR was calculated for the mattress group effect, and is presented with the corresponding CIs and p-value in Chapter 3. The cumulative incidence of developing a PU of category ≥ 2 by 30-day final follow-up in each group is also presented.
The median time to event is presented for those participants developing a PU of category ≥ 2 by mattress group and overall.
The Fine and Gray55 model was used rather than the Cox proportional hazards model in order to account for competing risks. That is, participants who do not develop a PU of category ≥ 2 during the treatment phase or by 30-day final follow-up were categorised as a competing risk at the date they stopped the trial if they withdrew from the trial due to poorly clinical condition or loss of capacity, or because the assessment schedule was too burdensome. Death was also considered a competing risk at the date of death. Other participants who did not develop a PU of category ≥ 2 during the treatment phase or by 30-day final follow-up were censored at the date of their last evaluable skin assessment. Centre was intended to be fitted as a random effect; however, this was not possible with the Fine and Gray55 model in SAS version 9.4.
Sensitivity analysis
A sensitivity analysis was conducted for the time taken to develop a PU of category ≥ 2 during the treatment phase.
This sensitivity analysis for the end point of time to developing a PU of category ≥ 2 during the treatment phase was specified in version 1.0 of the SAP finalised prior to the unplanned interim analysis being conducted. The end point of time to developing a PU of category ≥ 2 during the treatment phase was analysed using the Fine and Gray55 model, in line with the primary analysis.
Moderator analysis
Potential predictors of response (time to developing a PU of category ≥ 2) were explored using baseline measurements: pre-existing PUs, category A skin status, diabetes, age, mobility, sensory perception, macro- and microcirculatory function, nutritional status, skin moisture, presence of pain at pressure area skin site and consent type by assessing potential predictor by mattress group interactions in the primary model.
Mediator analysis
An analysis using the Baron and Kenny56 method was planned, with the goal of identifying potential mediators such as time on the allocated mattress, patient repositioning, cushions, heel protectors and protective dressings. However, applications of the Baron and Kenny56 method to time-to-event analyses have been strongly criticised and are known to give biased results. 57 Alternative methods have been developed58,59 but do not apply directly to analyses with competing risks. These methods would have required us to assume that competing risks could be treated as uninformative censoring. This assumption was not considered reasonable, and therefore no mediator analyses were performed. However, a descriptive summary of the potential mediator, repositioning, is presented over time by mattress group. Further investigative work is planned for the other mediators, including investigating the quality of the data and how practice changes over time.
Secondary end point analysis
The secondary end points of time to developing a PU of category ≥ 1 and time to developing a PU of category ≥ 3 to 30-day final follow-up were analysed using the Fine and Gray55 model in line with the primary analysis. To note, for the analysis of time to developing a PU of category ≥ 1, the covariate for the presence of pain excluded pre-existing category 1 PUs and was an indicator variable to denote whether or not there was pain at healthy or altered skin sites only.
For time to healing of a pre-existing PU of category 2, a Fine and Gray55 model was fitted to the outcome time to healing of all pre-existing category 2 PUs to 30-day final follow-up. The following covariates were considered for inclusion in the model: health-care setting, consent type, the presence of a condition affecting peripheral circulation and mattress group.
For all models, the adequacy of the proportional hazards assumption was explored through examination of the Schoenfeld residuals and ln (cumulative hazard) over time, and via a formal test for interaction of mattress and ln (time). 60
Mattress compliance
Descriptive statistics on the time to receiving allocated mattress, reasons for not receiving mattress on the day of randomisation, reason for first mattress change from randomised mattress, and time spent on the randomised mattress during the treatment phase are presented by mattress group. In addition, the mattress in use at the time of screening for patients who were ineligible because either the patient or the staff were unwilling to change the mattress is presented in Appendix 4, Table 69.
Safety
Expected AEs and SAEs and related unexpected serious adverse events (RU SAEs) are summarised by allocated mattress and mattress at the time of the AE/SAE.
Summary of main changes to the protocol
Throughout the trial, five substantial amendments to the protocol were submitted and approved by the REC. The important changes from a patient perspective were the collection of the QoL information and questionnaires as described in Data collection schedule. Prior to starting recruitment (August 2013), modifications to the eligibility criteria, collection of the NHS number and collection of PU-QoL-UI at baseline were added. Two additional objectives were added in an amendment approved in September 2013, to improve the analysis. These were to compare the:
-
incidence of mattress change between patients using APM and those using HSFM
-
safety between patients using APM and those using HSFM.
When and who performs the photography substudy data collection were also clarified. In February 2014, the amendment provided details of the EQ-5D-5L, PU-QoL-UI, SF-12, health resource use survey (1-week recall) and PU-QoL-P questionnaires and specified when they would be administered. In April 2014, an urgent safety measure led to the changes in questionnaire administration, Scottish centres were added, which required additional documents to comply with legislation for consenting participants who lacked capacity. The last substantial amendment, implemented in November 2015, made further changes to the frequency of questionnaire administration and incorporated other health-care professionals as well as nurses to collect data.
Chapter 3 Clinical results
This chapter presents the findings of the analysis for the clinical outcomes.
Participant flow
In total, 15,277 patients were assessed for study eligibility and 2030 randomisations took place between August 2013 and 30 November 2016. There were 41 NHS trusts/health boards comprising 47 centres that opened (i.e. met all local regulatory requirements), of which 39 NHS trusts/health boards and 42 centres recruited participants. Of the recruiting trusts/health boards, the numbers of participants randomised by each trust ranged from 1 to 471, with a median of 24 (Figure 1). The mean recruitment per trust was 1.6 participants per month. The recruiting centres/trusts comprised 25 teaching hospitals (21 acute NHS trusts and two health boards), 13 general hospitals (11 acute) and nine community hospitals (in nine community care NHS trusts). Others were combined acute and community trusts.
FIGURE 1.
Recruitment by organisation.
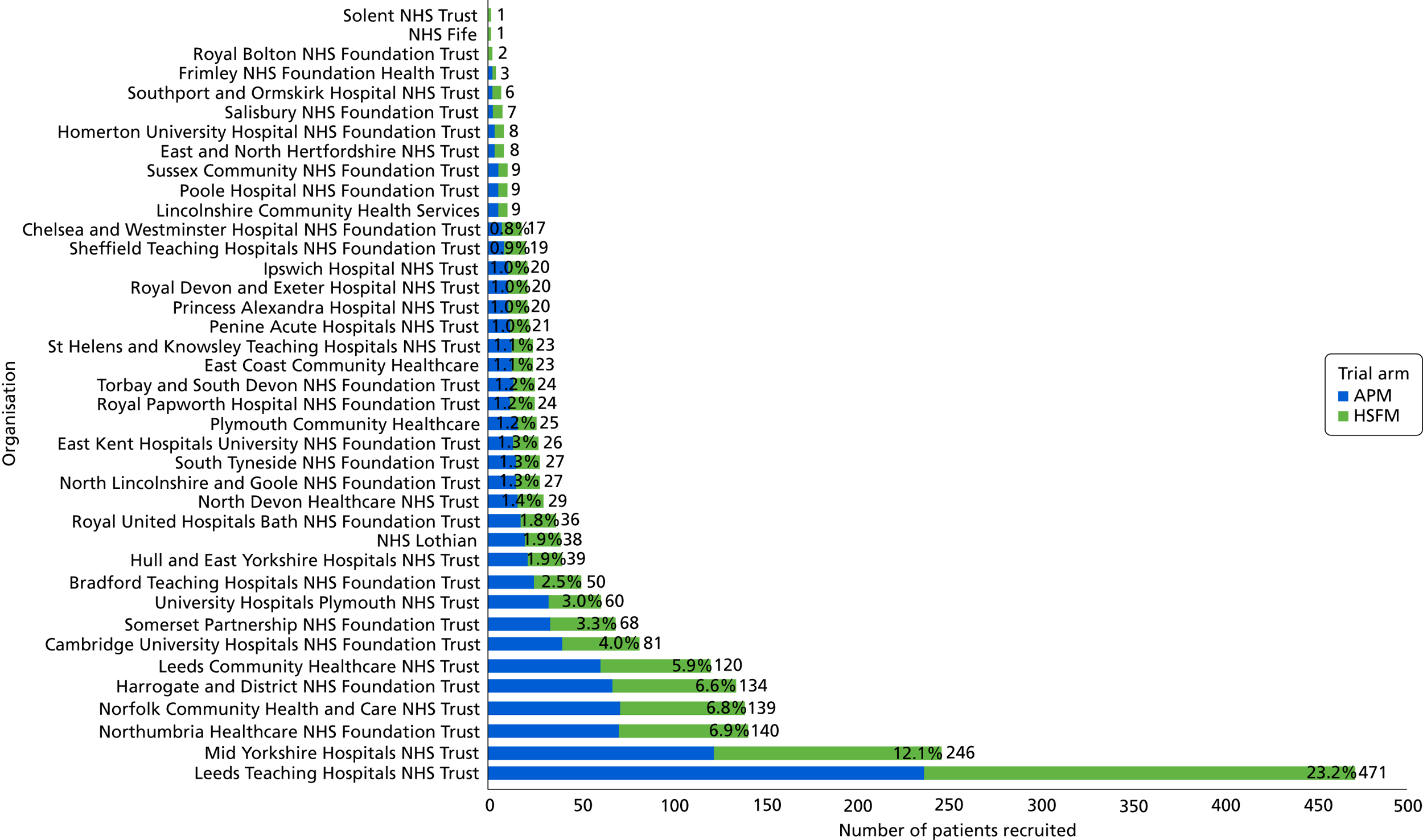
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram of trial progress is presented in Figure 2. Of the 15,277 patients screened, 877 (5.7%) were not assessed for eligibility; the main reasons for this included ethically inappropriate to approach (n = 482, 55.0%), discharged (n = 157, 17.9%) or missed by the research team (n = 68, 7.8%). Of the 14,400 patients assessed for eligibility, 9323 (64.7%) were ineligible, with the main reasons including not at a high risk of PU development (n = 2180, 23.4%), length of stay expected to be < 5 days (n = 1640, 17.6%), patient (n = 938, 10.1%) or staff (n = 1116, 12.0%) unwilling to change mattress and patient too unwell to change mattress (n = 709, 7.6%). Of the 5077 eligible patients, 2068 (40.7%) consented and 2030 (40.0%) were randomised.
FIGURE 2.
The CONSORT flow diagram. ICU, intensive care unit; TVTM, tissue viability team member. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

The screened population and randomised populations were similar in respect of age, gender and ethnicity (see Appendix 4) with the exception of mattress type at screening. In terms of mattress type, in the screened population, 50% (n = 7640) had been allocated an APM or another high-technology device and 48.8% (n = 7462) had been allocated a HSFM or other low-technology mattress by ward staff, whereas in the randomised population a lower proportion of patients (n = 868, 42.2%) were allocated to APM or another high-technology device than to HSFM or another low-technology device by ward staff (n = 1149, 56.6%) (see Appendix 4, Table 68).
In addition, of the 1116 patients for whom staff were unwilling to change mattress, 78.9% (n = 881) were on APMs or other high-technology mattresses and 20.8% (n = 232) were on HSFM or other low-technology mattresses (see Figure 2). This contrasts with the 938 patients who were unwilling to change mattress, of whom 52.0% (n = 488) were on APMs or other high-technology mattress and 47.7% (n = 447) were on HSFM or other low-technology mattress (see Figure 2).
Of those patients randomised, 1017 (50.1%) were allocated to APM and 1013 (49.9%) to HSFM, with 81.5% of participants in each group receiving the allocated mattress within 48 hours. A total of 119 participants were withdrawn [62 (6.1%) of those were allocated to APM and 57 (5.6%) to HSFM] and 166 patients died between randomisation and withdrawal or the 30-day final follow-up period [82 (8.1%) of those were allocated to APM and 84 (8.3%) were allocated to HSFM]. One patient was inadvertently randomised twice and was withdrawn from trial participation as soon as this was identified. Data arising from the second randomisation were excluded from the analysis population; therefore, the analysis population comprises a total of 2029 participants (see Figure 2).
Baseline characteristics
Patient characteristics were balanced across the mattress groups and are detailed in Tables 2–6. In summary, the study population comprised largely elderly patients (median age 81 years, range 21–105 years); 55.2% (n = 1119) were female and 98.2% (n = 1992) were of white ethnicity. Participants were inpatients for a median of 7 days (range 0–388 days) prior to randomisation.
Participants were inpatients on medical (64.6%, n = 1310), orthopaedics and trauma (22.3%, n = 453), surgical (7.6%, n = 155) and other wards (5.2%, n = 106), including oncology, critical care, neurosciences, renal, gastroenterology and spinal injuries unit (Table 2). Participants were inpatients in secondary care hospitals (69.7%, n = 1414), community hospitals (18.7%, n = 379) and intermediate care/rehabilitation facilities (11.5%, n = 234).
| Attribute | Trial arm | Overall (N = 2029) | |
|---|---|---|---|
| APM (N = 1016) | HSFM (N = 1013) | ||
| Gender, n (%) | |||
| Male | 462 (45.5) | 445 (43.9) | 907 (44.7) |
| Female | 553 (54.4) | 566 (55.9) | 1119 (55.2) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Age (years) | |||
| Mean (SD) | 77.8 (13.42) | 78.2 (12.87) | 78.0 (13.1) |
| Median (range) | 81 (21.1–105) | 81 (21.9–101) | 81 (21–105) |
| IQR | (71.3–87.0) | (71.9–87.2) | (71.6–87.1) |
| Missing | 0 | 0 | 0 |
| Ethnicity, n (%) | |||
| White | 1000 (98.4) | 992 (97.9) | 1992 (98.2) |
| Mixed race | 3 (0.3) | 3 (0.3) | 6 (0.3) |
| Non-white | 12 (1.2) | 16 (1.6) | 28 (1.4) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Medical specialty, n (%) | |||
| Medical | 641 (63.1) | 669 (66.1) | 1310 (64.6) |
| Surgical | 83 (8.2) | 72 (7.1) | 155 (7.6) |
| Orthopaedics and trauma | 233 (22.9) | 220 (21.7) | 453 (22.3) |
| Oncology | 21 (2.1) | 16 (1.6) | 37 (1.8) |
| Critical care | 10 (1.0) | 6 (0.6) | 16 (0.8) |
| Neurosciences | 17 (1.7) | 15 (1.5) | 32 (1.6) |
| Spinal injury | 8 (0.8) | 9 (0.9) | 17 (0.9) |
| Other | 2 (0.2) | 2 (0.2) | 4 (0.2) |
| Missing | 1 (0.0) | 4 (0.3) | 5 (0.2) |
| Consent type, n (%) | |||
| Written | 706 (69.5) | 696 (68.7) | 1402 (69.1) |
| Witnessed verbal | 151 (14.9) | 152 (15.0) | 303 (14.9) |
| Consultee agreement | 159 (15.6) | 163 (16.1) | 322 (15.9) |
| Missinga | 0 (0.0) | 2 (0.2) | 2 (0.1) |
| Health-care setting, n (%) | |||
| Secondary care hospital | 710 (69.9) | 704 (69.5) | 1414 (69.7) |
| Community hospital | 191 (18.8) | 188 (18.6) | 379 (18.7) |
| NHS intermediate care/rehabilitation facility | 115 (11.3) | 119 (11.7) | 234 (11.5) |
| Missingb | 0 (0.0) | 2 (0.2) | 2 (0.1) |
| Days between admission to randomising | |||
| Mean (SD) | 12.7 (20.27) | 13.3 (21.23) | 13.0 (20.8) |
| Median (range) | 6 (0.0–306) | 7 (0.0–388) | 7 (0–388) |
| IQR | (3.0–15.0) | (3.0–17.0) | (3.0–16.0) |
| Number of participants with missing data | 1 | 2 | 3 |
In relation to morbidity and risk of PU development, 15.9% (n = 322) of patients lacked capacity, 44.8% (n = 909) had a history of falls in the preceding month (Table 3), a majority of 96.6% (n = 1961) had limitations to independent movement, and 98.7% (n = 2003) and 92.6% (n = 1879) of patients were classified as ‘at risk’ of PU development on the Pressure Ulcer Risk Primary Or Secondary Evaluation Tool (PURPOSE-T) and Braden Scale,31 respectively (see Table 3).
| Risk factor | Trial arm, n (%) | Overall (N = 2029), n (%) | |
|---|---|---|---|
| APM (N = 1016) | HSFM (N = 1013) | ||
| BMI (kg/m2) | |||
| Underweight (< 18.5) | 52 (5.1) | 49 (4.8) | 101 (5.0) |
| Normal weight (18.5 to < 25) | 455 (44.8) | 392 (38.7) | 847 (41.7) |
| Overweight (25 to < 30) | 266 (26.2) | 336 (33.2) | 602 (29.7) |
| Obese (≥ 30) | 235 (23.1) | 217 (21.4) | 452 (22.3) |
| Missing | 8 (0.8) | 19 (1.9) | 27 (1.3) |
| History of falls in the preceding month | |||
| Yes | 458 (45.1) | 451 (44.5) | 909 (44.8) |
| No/not aware of any falls | 554 (54.5) | 559 (55.2) | 1113 (54.9) |
| Missing | 4 (0.4) | 3 (0.3) | 7 (0.3) |
| PURPOSE-T subscales | |||
| Analysis of independent movement | |||
| Moves frequently/major position changes | 28 (2.8) | 32 (3.2) | 60 (3.0) |
| Moves frequently/slight position changes | 141 (13.9) | 139 (13.7) | 280 (13.8) |
| Moves occasionally/major position changes | 110 (10.8) | 110 (10.9) | 220 (10.8) |
| Moves occasionally/slight position changes | 624 (61.4) | 621 (61.3) | 1245 (61.4) |
| Does not move | 109 (10.7) | 107 (10.6) | 216 (10.6) |
| Missing | 4 (0.4) | 4 (0.4) | 8 (0.4) |
| Sensory perception and response | |||
| No problem | 744 (73.2) | 739 (73.0) | 1483 (73.1) |
| Unable to feel and/or respond appropriately to discomfort from pressure | 270 (26.6) | 271 (26.8) | 541 (26.7) |
| Missing | 2 (0.2) | 3 (0.3) | 5 (0.2) |
| Moisture due to perspiration, urine, faeces or exudate | |||
| No problem/occasional | 693 (68.2) | 686 (67.7) | 1379 (68.0) |
| Frequent (2–4 times a day) | 289 (28.4) | 299 (29.5) | 588 (29.0) |
| Constant | 31 (3.1) | 26 (2.6) | 57 (2.8) |
| Missing | 3 (0.3) | 2 (0.2) | 5 (0.2) |
| Perfusion | |||
| No problem | 554 (54.5) | 555 (54.8) | 1109 (54.7) |
| Conditions affecting peripheral circulation | 166 (16.3) | 169 (16.7) | 335 (16.5) |
| Conditions affecting central circulation | 234 (23.0) | 224 (22.1) | 458 (22.6) |
| Conditions affecting central and peripheral circulation | 60 (5.9) | 59 (5.8) | 119 (5.9) |
| Missing | 2 (0.2) | 6 (0.6) | 8 (0.4) |
| Nutrition | |||
| No problem | 544 (53.5) | 553 (54.6) | 1097 (54.1) |
| Problem | 471 (46.4) | 456 (45.0) | 927 (45.7) |
| Missing | 1 (0.1) | 4 (0.4) | 5 (0.2) |
| Previous PU history | |||
| No known PU history | 914 (90.0) | 920 (90.8) | 1834 (90.4) |
| PU history | 101 (9.9) | 90 (8.9) | 191 (9.4) |
| Missing | 1 (0.1) | 3 (0.3) | 4 (0.2) |
| Risk status recorded on PURPOSE-T | |||
| Not at risk | 12 (1.2) | 11 (1.1) | 23 (1.1) |
| No PU but at risk | 820 (80.7) | 816 (80.6) | 1636 (80.6) |
| PU of category ≥ 1, or scarring from previous PU | 183 (18.0) | 184 (18.2) | 367 (18.1) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Braden subscales | |||
| Sensory perception | |||
| No impairment | 657 (64.7) | 678 (66.9) | 1335 (65.8) |
| Slightly limited | 276 (27.2) | 259 (25.6) | 535 (26.4) |
| Very limited | 67 (6.6) | 60 (5.9) | 127 (6.3) |
| Completely limited | 15 (1.5) | 14 (1.4) | 29 (1.4) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Moisture | |||
| Rarely moist | 451 (44.4) | 414 (40.9) | 865 (42.6) |
| Occasionally moist | 360 (35.4) | 419 (41.4) | 779 (38.4) |
| Very moist | 177 (17.4) | 153 (15.1) | 330 (16.3) |
| Constantly moist | 27 (2.7) | 25 (2.5) | 52 (2.6) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Activity | |||
| Walks frequently | 13 (1.3) | 9 (0.9) | 22 (1.1) |
| Walks occasionally | 108 (10.6) | 113 (11.2) | 221 (10.9) |
| Chairfast | 677 (66.6) | 667 (65.8) | 1344 (66.2) |
| Bedfast | 217 (21.4) | 222 (21.9) | 439 (21.6) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Mobility | |||
| No limitation | 22 (2.2) | 20 (2.0) | 42 (2.1) |
| Slightly limited | 125 (12.3) | 115 (11.4) | 240 (11.8) |
| Very limited | 790 (77.8) | 797 (78.7) | 1587 (78.2) |
| Completely immobile | 78 (7.7) | 79 (7.8) | 157 (7.7) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Nutrition | |||
| Excellent | 173 (17.0) | 158 (15.6) | 331 (16.3) |
| Adequate | 528 (52.0) | 539 (53.2) | 1067 (52.6) |
| Probably inadequate | 279 (27.5) | 285 (28.1) | 564 (27.8) |
| Very poor | 35 (3.4) | 29 (2.9) | 64 (3.2) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Friction and shear | |||
| No apparent problem | 89 (8.8) | 84 (8.3) | 173 (8.5) |
| Potential problem | 752 (74.0) | 770 (76.0) | 1522 (75.0) |
| Problem | 174 (17.1) | 157 (15.5) | 331 (16.3) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Overall Braden PU risk | |||
| Not at risk (i.e. a score of > 18) | 78 (7.7) | 69 (6.8) | 147 (7.2) |
| At risk (i.e. a score of ≤ 18) | 937 (92.2) | 942 (93.0) | 1879 (92.6) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
There were high levels of skin morbidity at baseline, which included 53.4% (n = 1084) of patients reporting pressure-related pain on one or more healthy, altered or category 1 PU skin sites (Tables 4 and 5). The ‘worst’ skin classifications recorded were PUs of category 2 in 7.1% (n = 145) of patients, PUs of category 1 in 11.6% (n = 235) of patients and alterations to intact skin in 66.4% (n = 1347) of patients. On a skin site level, of a total number of 28,421 skin sites, there were 177 (0.6%) PUs of category 2, 409 (1.4%) PUs of category 1, 7569 (26.6%) alterations to intact skin, 510 (1.8%) other conditions/wounds (see Box 1), 486 (1.7%) unable to assess (i.e. bandage/cast in situ), 104 (0.4%) amputations and 268 (0.9%) missing.
| Question | Trial arm, n (%) | Overall (N = 2029), n (%) | |
|---|---|---|---|
| APM (N = 1016) | HSFM (N = 1013) | ||
| Worst category of skin reported at baseline (patient level) | |||
| 0 | 147 (14.5) | 152 (15.0) | 299 (14.7) |
| A | 673 (66.2) | 674 (66.5) | 1347 (66.4) |
| 1 | 125 (12.3) | 110 (10.9) | 235 (11.6) |
| 2 | 70 (6.9) | 75 (7.4) | 145 (7.1) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Pressure-related pain on any skin site | |||
| Yes | 577 (56.8) | 584 (57.7) | 1161 (57.2) |
| No | 393 (38.7) | 388 (38.3) | 781 (38.5) |
| Unable to assess | 15 (1.5) | 15 (1.5) | 30 (1.5) |
| Combination of ‘missing’ and ‘no’ | 6 (0.6) | 6 (0.6) | 12 (0.6) |
| Combination of ‘no’ and ‘unable to assess’ | 15 (1.5) | 13 (1.3) | 28 (1.4) |
| Missing | 10 (1.0) | 7 (0.7) | 17 (0.8) |
| Pressure-related pain on a healthy, altered or category 1 skin site | |||
| Yes | 541 (53.2) | 543 (53.6) | 1084 (53.4) |
| No | 440 (43.3) | 439 (43.3) | 879 (43.3) |
| Unable to assess | 15 (1.5) | 15 (1.5) | 30 (1.5) |
| Combination of ‘missing’ and ‘no’a | 2 (0.2) | 1 (0.1) | 3 (0.1) |
| Combination of ‘no’ and ‘unable to assess’a | 5 (0.5) | 3 (0.3) | 8 (0.4) |
| Missing | 9 (0.9) | 5 (0.5) | 14 (0.7) |
| No skin sites reported as healthy, altered or category 1b | 4 (0.4) | 7 (0.7) | 11 (0.5) |
| Skin sites and classifications | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Skin classification all skin areas,a n (%) | |||
| Normal | 9443 (66.4) | 9438 (66.5) | 18,881 (66.4) |
| Category A | 3764 (26.5) | 3805 (26.8) | 7569 (26.6) |
| Category 1 | 212 (1.5) | 197 (1.4) | 409 (1.4) |
| Category 2 | 91 (0.6) | 86 (0.6) | 177 (0.6) |
| Suspected deep tissue injury | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| Not applicable | 596 (4.2) | 520 (3.7) | 1116 (3.9) |
| Missing | 124 (0.9) | 144 (1.0) | 268 (0.9) |
| Totals | 14,230 (100) | 14,191 (100) | 28,421 (100) |
| Skin sites with pre-existing category 2 PU, n (%) | |||
| Spine/back | 3 (3.3) | 1 (1.2) | 4 (2.3) |
| Sacrum | 23 (25.3) | 23 (26.7) | 46 (26.0) |
| Buttock – L | 17 (18.7) | 18 (20.9) | 35 (19.8) |
| Buttock – R | 16 (17.6) | 17 (19.8) | 33 (18.6) |
| Ischial – L | 3 (3.3) | 0 (0.0) | 3 (1.7) |
| Ischial – R | 4 (4.4) | 0 (0.0) | 4 (2.2) |
| Trochanter – L | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Trochanter – R | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Heel – L | 6 (6.6) | 7 (8.1) | 13 (7.3) |
| Heel – R | 10 (11.0) | 6 (7.0) | 16 (9.0) |
| Ankle – L | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Ankle – R | 1 (1.1) | 2 (2.3) | 3 (1.7) |
| Elbow – L | 2 (2.2) | 5 (5.8) | 7 (4.0) |
| Elbow – R | 2 (2.2) | 3 (3.5) | 5 (2.8) |
| Other | 2 (2.2) | 4 (4.7) | 6 (3.4) |
| Total | 91 (100.0) | 86 (100.0) | 177 (100.0) |
| Duration of most severe pre-existing PU (days) | |||
| Mean (SD) | 29.6 (42.4) | 67.6 (179.73) | 48.3 (129.61) |
| Median (range) | 14 (1–182.6) | 7.5 (1.0–730.5) | 10 (1–730.5) |
| < 7 days, n (%) | 10 (14.3) | 13 (17.3) | 23 (15.9) |
| 8–28 days, n (%) | 7 (9.9) | 7 (9.3) | 14 (9.7) |
| > 28 days, n (%) | 10 (14.3) | 6 (8.0) | 16 (11.0) |
| Missing, n (%) | 43 (61.4) | 49 (65.3) | 92 (63.4) |
| Total number (%) of patients with a PU | 70 (100.0) | 75 (00.0) | 145 (100.0) |
| Area of most severe pre-existing PU (mm2) | |||
| Mean (SD) | 462.3 (1357.4) | 245.4 (392.4) | 352.0 (989.2) |
| Median (range) | 68.6 (3.7–5369.8) | 123.7 (3.1–1652.5) | 76.3 (3.1–5369.8) |
| Number of patients with non-missing data | 29 | 30 | 59 |
| Number of patients with missing data | 41 | 45 | 86 |
Pre-randomisation standard pressure ulcer prevention care
Prior to randomisation, preventative care interventions, including mattress, repositioning frequency, sitting, seating and use of adjuvant devices as provided by the attending ward staff, were balanced across the mattress groups and are detailed in Table 6.
| Interventions | Trial arm, n (%) | Overall (N = 2029), n (%) | |
|---|---|---|---|
| APM (N = 1016) | HSFM (N = 1013) | ||
| Current mattress type | |||
| HSFM or other low technology | 575 (56.6) | 574 (56.7) | 1149 (56.6) |
| APM or other high technology | 435 (42.8) | 433 (42.7) | 868 (42.8) |
| Missing | 6 (0.6) | 6 (0.6) | 12 (0.6) |
| Frequency of repositioning in previous 24 hours | |||
| More frequently than every 2 hours | 148 (14.6) | 146 (14.4) | 294 (14.5) |
| Every 2–3 hours | 473 (46.6) | 494 (48.8) | 967 (47.7) |
| Every 4–5 hours | 333 (32.8) | 307 (30.3) | 640 (31.5) |
| Every 6–7 hours | 36 (3.5) | 48 (4.7) | 84 (4.1) |
| Less frequently than every 8 hours | 20 (2.0) | 12 (1.2) | 32 (1.6) |
| Missing | 6 (0.6) | 6 (0.6) | 12 (0.6) |
| Time spent sat out of bed in previous 24 hours | |||
| N/A (i.e. bedfast) | 270 (26.6) | 271 (26.8) | 541 (26.7) |
| < 2 hours | 91 (8.9) | 90 (8.9) | 181 (8.9) |
| 2–3 hours | 134 (13.2) | 134 (13.2) | 268 (13.2) |
| 4–5 hours | 178 (17.5) | 183 (18.1) | 361 (17.8) |
| 6–7 hours | 125 (12.3) | 128 (12.6) | 253 (12.5) |
| > 8 hours | 207 (20.4) | 191 (18.9) | 398 (19.6) |
| Missing | 11 (1.1) | 16 (1.6) | 27 (1.3) |
| Type of cushion | |||
| Standard chair only | 203 (27.3) | 206 (27.9) | 409 (27.6) |
| High-technology specialist cushion | 56 (7.5) | 52 (7.0) | 108 (7.3) |
| Low-technology specialist cushion/chair with integral pressure relief | 434 (58.3) | 429 (58) | 863 (58.2) |
| Pillow | 45 (6.1) | 47 (6.4) | 92 (6.2) |
| Missing | 6 (0.8) | 5 (0.7) | 11 (0.7) |
| Total (number of patients who sat out) | 744 (100) | 739 (100) | 1483 (100) |
| Participant on electronic profiling bedframe | |||
| Yes | 1012 (99.6) | 1008 (99.5) | 2020 (99.6) |
| No | 3 (0.3) | 3 (0.3) | 6 (0.3) |
| Missing | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Adjuvant devices and dressing | |||
| Yes | 143 (14.1) | 141 (13.9) | 284 (14.0) |
| No | 867 (85.3) | 865 (85.4) | 1732 (85.4) |
| Missing | 6 (0.6) | 7 (0.7) | 13 (0.6) |
Prior to randomisation, 868 (42.8%) participants had been allocated an APM or other high-technology mattress and 1149 (56.6%) had been allocated a HSFM or other low-technology mattress by ward staff. Overall, 89.8% (n = 1823) of patients required complete or partial assistance to reposition themselves; the frequency was observed to be every 2 hours and every 2–3 hours in 14.5% (n = 294) and 47.7% (n = 967) of patients, respectively, with a small number of patients (n = 116, 5.7%) repositioned less frequently than every 6 hours. Fewer than one-sixth of patients (14.0%, n = 284) patients received adjuvant devices or dressings, of whom 56.7% (n = 161) were provided devices for heel off-loading. One-quarter of the patients were confined to bed (26.7%, n = 541), and, of those who did sit out (73.1%, n = 1483), only 65.5% (n = 971) had a specialist cushion.
Outcomes: intention-to-treat population
Primary end point: time to development of new category ≥ 2 pressure ulcers to the 30-day final follow-up
A total of 160 (7.9%) patients developed at least one new PU of category ≥ 2 between randomisation and 30-day final follow-up, death or withdrawal (Table 7). An absolute difference between mattress groups of 2.0% was observed, corresponding to incidence rates of 6.9% (n = 70) in the APM arm and 8.9% (n = 90) in the HSFM arm. Of those patients who developed a PU of category ≥ 2, the median time to development was 18 days (range 2–86 days) in the APM group and 12 days (range 2–94 days) in the HSFM group. There was no evidence of a difference between mattress groups in terms of the primary end point in the unadjusted Gray’s test (p = 0.1148, Figure 3a) or the adjusted analysis (Fine and Gray model HR 0.76, 95% CI 0.56 to 1.04; exact p-value = 0.0890) (Table 8). The only factor statistically significantly associated with the primary end point was skin status (Wald p-value = 0.0057); specifically, patients with a pre-existing category 1 PU were more at risk of developing a PU of category ≥ 2 than patients who did not have a pre-existing PU (HR 1.83, 95% CI 1.17 to 2.87). Similarly, patients with a pre-existing category 2 PU had an increased risk of developing a new PU of category ≥ 2 compared with patients with no PU (HR 1.83, 95% CI 1.09 to 3.09). Overall, 11.4% (27/236) of patients with a pre-existing category 1 PU at baseline and 12.4% (18/145) of patients with a pre-existing PU of category ≥ 2 at baseline developed a new PU of category ≥ 2, compared with 7.0% (115/1648) of patients without a pre-existing PU at baseline.
| Attribute | New PU of category ≥ 2 | Not eligiblea | Total | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| No new PU of category ≥ 2 | Died | Withdrawn | ||||
| PU status at baseline | ||||||
| APM | ||||||
| No PU | 49 (6.0) | 682 (83.1) | 59 (7.2) | 30 (3.7) | 1 (0.1) | 821 (100) |
| Category 1 PU | 11 (8.8) | 92 (76.0) | 12 (9.6) | 7 (5.6) | 0 (0.0) | 125 (100) |
| Category 2 PU | 10 (14.3) | 48 (68.6) | 6 (8.6) | 3 (4.3) | 3 (4.3) | 70 (100) |
| Total | 70 (6.9) | 825 (81.2) | 77 (7.6) | 40 (3.9) | 4 (0.4) | 1016 (100) |
| HSFM | ||||||
| No PU | 66 (8.0) | 691 (83.6) | 49 (5.9) | 20 (2.4) | 1 (0.1) | 827 (100) |
| Category 1 PU | 16 (14.4) | 78 (70.3) | 11 (9.9) | 5 (4.5) | 1 (0.9) | 111 (100) |
| Category 2 PU | 8 (10.7) | 43 (57.3) | 12 (16.0) | 6 (8.0) | 6 (8.0) | 75 (100) |
| Total | 90 (8.9) | 812 (80.2) | 72 (7.1) | 31 (3.1) | 8 (0.8) | 1013 (100) |
| Consent type | ||||||
| APM | ||||||
| Written consent | 44 (6.2) | 595 (84.3) | 38 (5.4) | 26 (3.7) | 3 (0.4) | 706 (100) |
| Witnessed verbal consent | 16 (10.6) | 111 (73.5) | 10 (6.6) | 13 (8.6) | 1 (0.7) | 151 (100) |
| Consultee agreement | 10 (6.3) | 119 (74.8) | 29 (18.2) | 1 (0.6) | 0 (0.0) | 159 (100) |
| Total | 70 (6.9) | 825 (81.2) | 77 (7.6) | 40 (3.9) | 4 (0.4) | 1016 (100) |
| HSFM | ||||||
| Written consent | 56 (8.0) | 579 (83.0) | 34 (4.9) | 22 (3.2) | 7 (1.0) | 698 (100) |
| Witnessed verbal consent | 16 (10.5) | 123 (80.9) | 7 (4.6) | 5 (3.3) | 1 (0.7) | 152 (100) |
| Consultee agreement | 18 (11.0) | 110 (67.5) | 31 (19.0) | 4 (2.5) | 0 (0.0) | 163 (100) |
| Total | 90 (8.9) | 812 (80.2) | 72 (7.1) | 31 (3.1) | 8 (0.8) | 1013 (100) |
| Care setting | ||||||
| APM | ||||||
| Secondary care hospital | 43 (6.1) | 569 (80.1) | 64 (9.0) | 30 (4.2) | 4 (0.6) | 710 (100) |
| Community hospital | 16 (8.4) | 161 (84.3) | 9 (4.7) | 5 (2.6) | 0 (0.0) | 191 (100) |
| Intermediate care/rehabilitation | 11 (9.6) | 95 (82.6) | 4 (3.5) | 5 (4.3) | 0 (0.0) | 115 (100) |
| Total | 70 (6.9) | 825 (81.2) | 77 (7.6) | 40 (3.9) | 4 (0.4) | 1016 (100) |
| HSFM | ||||||
| Secondary care hospital | 59 (8.4) | 558 (79.0) | 57 (8.1) | 24 (3.4) | 8 (1.1) | 706 (100) |
| Community hospital | 18 (9.6) | 155 (82.4) | 13 (6.9) | 2 (1.1) | 0 (0.0) | 188 (100) |
| Intermediate care/rehabilitation | 13 (10.9) | 99 (83.2) | 2 (1.7) | 5 (4.2) | 0 (0.0) | 119 (100) |
| Total | 59 (8.4) | 558 (79.0) | 57 (8.1) | 24 (3.4) | 8 (1.1) | 1013 (100) |
| Pain on a healthy/altered/PU of category 1 at baseline | ||||||
| APM | ||||||
| Yes | 35 (6.5) | 453 (83.7) | 30 (5.5) | 23 (4.3) | 0 (0.0) | 541 (100) |
| No | 33 (7.4) | 359 (80.3) | 38 (8.5) | 17 (3.8) | 0 (0.0) | 447 (100) |
| Unable to assess | 0 (0.0) | 7 (46.7) | 8 (53.3) | 0 (0.0) | 0 (0.0) | 15 (100) |
| Missing | 2 (15.4) | 6 (46.2) | 1 (7.7) | 0 (0.0) | 4 (30.8) | 13 (100) |
| Total | 70 (6.9) | 825 (81.2) | 77 (7.6) | 40 (3.9) | 4 (0.4) | 1016 (100) |
| HSFM | ||||||
| Yes | 55 (10.1) | 438 (80.7) | 31 (5.7) | 18 (3.3) | 1 (0.2) | 543 (100) |
| No | 34 (7.7) | 358 (80.8) | 38 (8.6) | 13 (2.9) | 0 (0.0) | 443 (100) |
| Unable to assess | 1 (6.7) | 11 (73.3) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 15 (100) |
| Missing | 0 (0.0) | 5 (41.7) | 0 (0.0) | 0 (0.0) | 7 (58.3) | 12 (100) |
| Total | 90 (8.9) | 812 (80.2) | 72 (7.1) | 31 (3.1) | 8 (0.8) | 1013 (100) |
| Condition affecting peripheral circulation | ||||||
| APM | ||||||
| Yes | 17 (7.5) | 183 (81.0) | 17 (7.5) | 8 (3.5) | 1 (0.4) | 226 (100) |
| No | 53 (6.7) | 641 (81.3) | 60 (7.6) | 32 (4.1) | 2 (0.3) | 788 (100) |
| Missing | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 2 (100) |
| Total | 70 (6.9) | 825 (81.2) | 77 (7.6) | 40 (3.9) | 4 (0.4) | 1016 (100) |
| HSFM | ||||||
| Yes | 22 (9.6) | 180 (78.6) | 19 (8.3) | 7 (3.1) | 1 (0.4) | 229 (100) |
| No | 67 (8.6) | 630 (80.9) | 53 (6.8) | 24 (3.1) | 5 (0.6) | 779 (100) |
| Missing | 1 (20.0) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) | 5 (100) |
| Total | 90 (8.9) | 812 (80.2) | 72 (7.1) | 31 (3.1) | 8 (0.8) | 1013 (100) |
| Overall | 160 (7.9) | 1637 (80.7) | 149 (7.3) | 71 (3.5) | 12 (0.6) | 2029 (100) |
FIGURE 3.
Cumulative incidence functions. (a) Cumulative incidence function for development of a PU of category ≥ 2 by the 30-day final follow-up; (b) cumulative incidence function of development of a PU of category ≥ 2 in treatment phase; (c) cumulative incidence function for development of PU of category ≥ 1 by the 30-day final follow-up; and (d) cumulative incidence function for development of a PU of category ≥ 3 by the 30-day final follow-up. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.


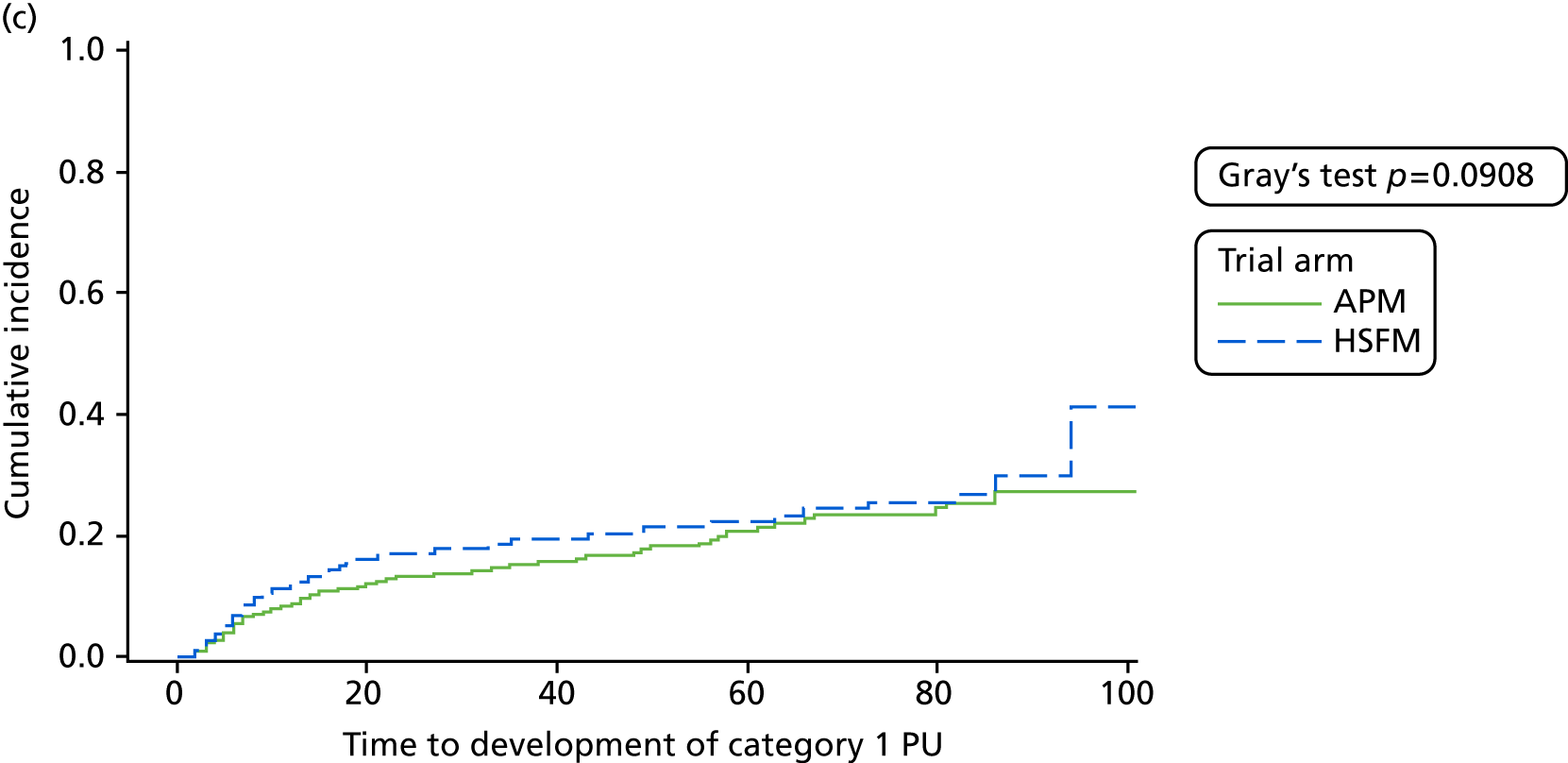
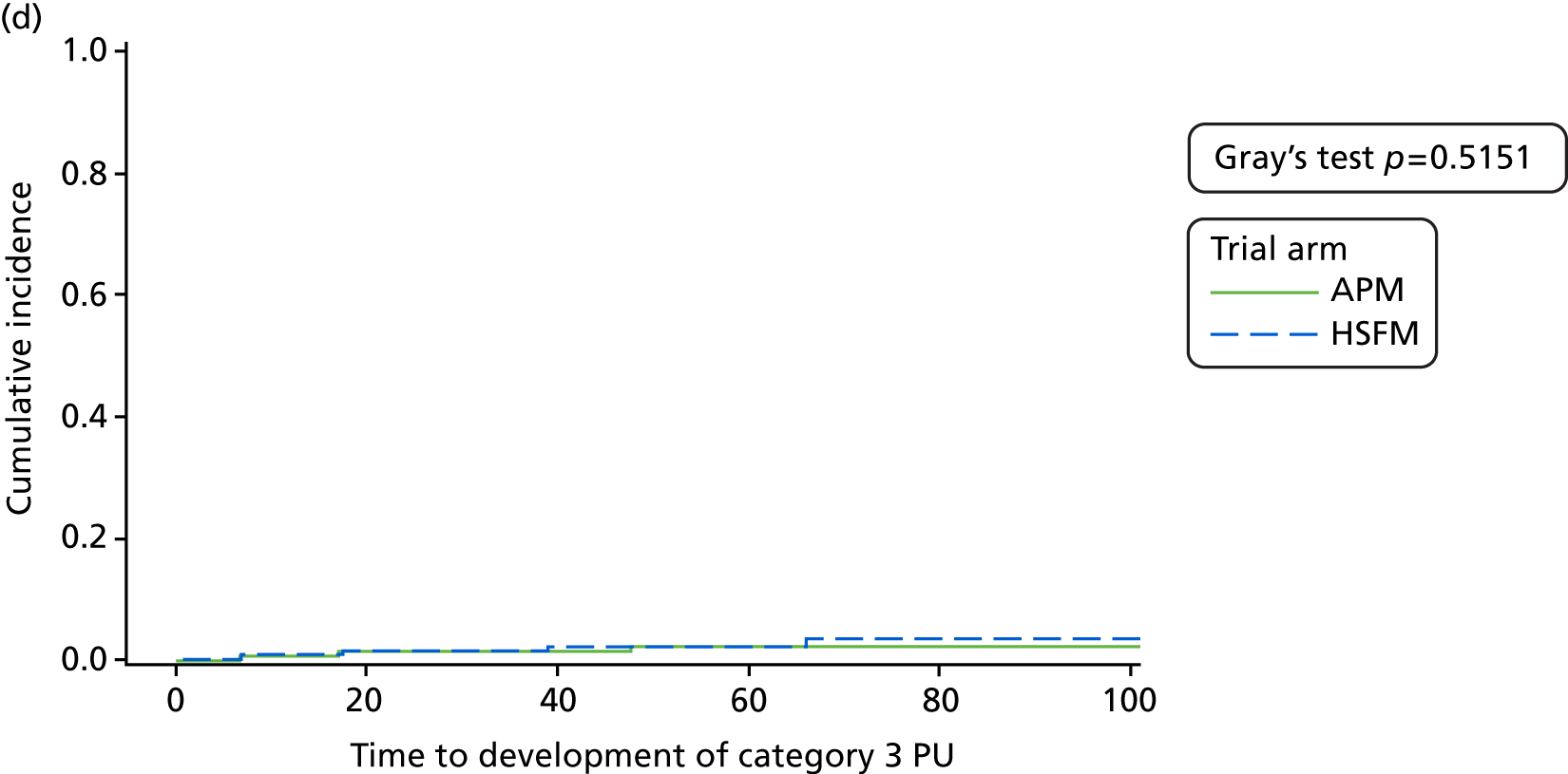
| Covariate | Incidence rate, n/N (%) | Reference level | HR | Wald p-value | |
|---|---|---|---|---|---|
| Point estimate | 95% Wald confidence limits | ||||
| Treatment | |||||
| HSFM | 90/1013 (8.9) | – | – | – | 0.0890a |
| APM | 70/1016 (6.9) | Vs. HSFM | 0.76 | 0.56 to 1.04 | |
| Skin status | |||||
| No PU | 115/1648 (7.0) | – | – | – | 0.0057 |
| Category 1 PU | 27/236 (11.4) | Vs. no PU | 1.83 | 1.16 to 2.87 | |
| Category 2 PU | 18/145 (12.4) | Vs. no PU | 1.83 | 1.09 to 3.09 | |
| Consent type | |||||
| Written | 100/1404 (7.1) | – | – | – | 0.3025 |
| Witnessed verbal | 32/303 (10.6) | Vs. written | 1.34 | 0.90 to 1.99 | |
| Consultee agreement | 28/322 (8.7) | Vs. written | 1.23 | 0.79 to 1.91 | |
| Setting | |||||
| Secondary care hospital | 102/1416 (7.2) | – | – | – | 0.6182 |
| Community hospital | 34/379 (9.0) | Vs. secondary care hospital | 1.06 | 0.71 to 1.58 | |
| NHS intermediate care/rehabilitation facility | 24/234 (10.3) | Vs. secondary care hospital | 1.26 | 0.79 to 1.99 | |
| Pain on a healthy, altered or category 1 PU skin site | |||||
| No | 67/890 (7.5) | – | – | – | 0.5070 |
| Yes | 90/1084 (8.3) | Vs. no | 1.15 | 0.82 to 1.61 | |
| Unable to assess | 1/30 (3.3) | Vs. no | 0.38 | 0.05 to 2.94 | |
| Missing | 2/25 (8.0) | Vs. no | 2.02 | 0.43 to 9.45 | |
| Presence of condition affecting peripheral circulation | |||||
| No | 120/1567 (7.7) | – | – | – | 0.5688 |
| Yes | 39/455 (8.6) | Vs. no | 1.09 | 0.75 to 1.57 | |
| Missing | 1/7 (14.3) | Vs. no | 2.91 | 0.35 to 24.51 | |
A total of 213 new PUs of category ≥ 2 were observed on 160 patients. Fewer PUs developed on patients allocated to APM (n = 89) than on patients allocated to HSFM (n = 124). The most common skin sites to develop new PUs were the sacrum (n = 44, 20.7%), left buttock (n = 42, 19.7%), right buttock (n = 38, 17.8%), left heel (n = 27, 12.7%) and right heel (n = 22, 10.3%). The frequency distribution of skin sites within mattress types were similar (see Appendix 4, Table 70).
Sensitivity analysis: time to development of new pressure ulcers of category ≥ 2 during the treatment phase
A total of 132 (6.5%) patients developed at least one new PU of category ≥ 2 between randomisation and the end of the treatment phase (as a result of no longer being at risk, discharge, death, withdrawal or 60 days) (see Appendix 4, Table 71). An absolute difference between mattress groups of 2.6% was observed corresponding to incidence rates of 5.2% (n = 53) in the APM arm and 7.8% (n = 79) in the HSFM arm. Of those patients who developed a PU of category ≥ 2 during the treatment phase, the median time to development was 12 days (range 2–57 days) in the APM arm and 9 days (range 2–50 days) in the HSFM arm. The difference between mattress groups in terms of the treatment phase end point was statistically significant in the unadjusted Gray’s test (p = 0.0306; see Figure 3b). The adjusted analysis (Fine and Gray model) also demonstrated that there was statistically significant evidence that APM was superior to HSFM (HR 0.66, 95% CI 0.46 to 0.93; exact p-value = 0.0176). The adjusted analysis identified that skin status at baseline was statistically significantly associated with the development of a new PU of category ≥ 2 during the treatment phase (Wald p-value = 0.0089); specifically, patients with a pre-existing category 1 PU were more at risk of developing a PU of category ≥ 2 during the treatment phase than patients who did not have a pre-existing PU (HR 1.83, 95% CI 1.11 to 3.02). Similarly, patients with a pre-existing category 2 PU had an increased risk of developing a new PU of category ≥ 2 during the treatment phase compared with patients with no PU (HR 1.98, 95% CI 1.12 to 3.50). Overall, 9.3% (22/236) of patients with a pre-existing category 1 PU at baseline and 10.3% (15/145) of patients with a pre-existing PU of category ≥ 2 at baseline developed a new PU of category ≥ 2, compared with 5.8% (95/1648) of patients without a pre-existing PU at baseline (Table 9).
| Covariate | Incidence rate, n/N (%) | Reference level | HR | Wald p-value | |
|---|---|---|---|---|---|
| Point estimate | 95% Wald confidence limits | ||||
| Treatment | |||||
| HSFM | 79/1016 (7.8) | – | – | – | 0.0190a |
| APM | 53/1013 (5.2) | Vs. HSFM | 0.66 | 0.46 to 0.93 | |
| Skin status | |||||
| No PU | 95/1648 (5.8) | – | – | – | 0.0089 |
| Category 1 PU | 22/236 (9.3) | Vs. no PU | 1.83 | 1.11 to 3.02 | |
| Category 2 PU | 15/145 (10.3) | Vs. no PU | 1.98 | 1.12 to 3.50 | |
| Consent type | |||||
| Written | 84/1404 (6.0) | – | – | – | 0.7400 |
| Witnessed verbal | 25/303 (8.3) | Vs. written | 1.14 | 0.73 to 1.77 | |
| Consultee agreement | 23/322 (7.1) | Vs. written | 1.18 | 0.72 to 1.92 | |
| Setting | |||||
| Secondary care hospital | 85/1416 (6.0) | – | – | – | 0.8670 |
| Community hospital | 29/379 (7.7) | Vs. secondary care hospital | 1.12 | 0.73 to 1.73 | |
| NHS intermediate care/rehabilitation facility | 18/234 (7.7) | Vs. secondary care hospital | 1.01 | 0.60 to 1.71 | |
| Pain on a healthy, altered or category 1 PU skin site | |||||
| No | 51/890 (5.7) | – | – | – | 0.2081 |
| Yes | 78/1084 (7.2) | Vs. no | 1.37 | 0.94 to 1.99 | |
| Unable to assess | 1/30 (3.3) | Vs. no | 0.44 | 0.06 to 3.48 | |
| Missing | 2/25 (8.0) | Vs. no | 2.57 | 0.56 to 11.73 | |
| Presence of condition affecting peripheral circulation | |||||
| No | 97/1567 (6.2) | – | – | – | 0.5671 |
| Yes | 34/455 (7.5) | Vs. no | 1.13 | 0.76 to 1.67 | |
| Missing | 1/7 (14.3) | Vs. no | 2.65 | 0.32 to 21.80 | |
Secondary end points
Time to development of pressure ulcers of category ≥ 1 to 30-day final follow-up
A total of 350 (17.2%) patients developed at least one new PU of category ≥ 1 between randomisation and 30-day final follow-up, death or withdrawal (see Appendix 4, Table 72). An absolute difference between mattress groups of 3.1% was observed, corresponding to incidence rates of 15.7% (n = 160) in the APM arm and 18.8% (n = 190) in the HSFM arm. Of those patients who developed a PU of category ≥ 1, the median time to development was 12 days (range 1–86 days) in the APM arm and 9 days (range 2–94 days) in the HSFM arm. There was no evidence of a difference between mattress groups in terms of the development of a PU of category ≥ 1 from randomisation to 30-day final follow-up in the unadjusted Gray’s test (p = 0.0908; see Figure 3c) or in the adjusted analysis (Fine and Gray model HR 0.83, 95% CI 0.67 to 1.02; exact p-value = 0.0733). Factors related to the development of a PU of category ≥ 1 included skin status (Wald p-value = 0.0301), consent type (Wald p-value = 0.0140) and pain on a healthy or altered skin site (Wald p-value = 0.0063); HRs and corresponding 95% CIs are provided in Table 10. The incidence rate in patients with no PU at baseline was 16.5% (272/1648), compared with 21.2% (50/236) in patients with a PU of category 1 and 19.3% (28/145) in patients with a pre-existing PU of category 2. In terms of type of consent, 21.4% (69/322) of patients with consultee agreement developed a new PU of category ≥ 1, compared with 19.5% (59/303) of patients with witnessed verbal consent and 15.8% (222/1404) of patients with written consent. The incidence of new PUs of category ≥ 1 was 16.0% (226/1416) in the secondary care setting compared with 17.7% (67/379) in community hospitals and 24.4% (57/234) in NHS intermediate care/rehabilitation facilities. The incidence rate in patients who did not have pain on a healthy or altered skin site at baseline was observed to be 15.6% (147/943), compared with 19.2% (198/1029) in patients who did have pain, 6.7% (2/30) in patients for whom pain could not be assessed and 11.1% (3/27) in patients for whom the pain status was missing.
| Covariate | Incidence rate, n/N (%) | Reference level | HR | Wald p-value | |
|---|---|---|---|---|---|
| Point estimate | 95% Wald confidence limits | ||||
| Treatment | |||||
| HSFM | 190/1013 (18.8) | – | – | – | 0.0741a |
| APM | 160/1016 (15.7) | Vs. HSFM | 0.83 | 0.67 to 1.02 | |
| Skin status | |||||
| No PU | 272/1648 (16.5) | – | – | – | 0.0301 |
| Category 1 PU | 50/236 (21.2) | Vs. no PU | 1.52 | 1.11 to 2.09 | |
| Category 2 PU | 28/145 (19.3) | Vs. no PU | 1.18 | 0.79 to 1.75 | |
| Consent type | |||||
| Written | 222/1404 (15.8) | – | – | – | 0.0140 |
| Witnessed verbal | 59/303 (19.5) | Vs. written | 1.15 | 0.86 to 1.53 | |
| Consultee agreement | 69/322 (21.4) | Vs. written | 1.52 | 1.15 to 2.01 | |
| Setting | |||||
| Secondary care hospital | 226/1416 (16.0) | – | – | – | 0.0970 |
| Community hospital | 67/379 (17.7) | Vs. secondary care hospital | 0.95 | 0.72 to 1.26 | |
| NHS intermediate care/rehabilitation facility | 57/234 (24.4) | Vs. secondary care hospital | 1.35 | 1.01 to 1.82 | |
| Pain on a healthy or altered skin site | |||||
| No | 147/943 (15.6) | – | – | – | 0.0063 |
| Yes | 198/1029 (19.2) | Vs. no | 1.38 | 1.11 to 1.71 | |
| Unable to assess | 2/30 (6.7) | Vs. no | 0.28 | 0.07 to 1.15 | |
| Missing | 3/27 (11.1) | Vs. no | 1.31 | 0.40 to 4.36 | |
| Presence of condition affecting peripheral circulation | |||||
| No | 259/1567 (16.5) | – | – | – | 0.3258 |
| Yes | 90/455 (19.8) | Vs. no | 1.19 | 0.93 to 1.51 | |
| Missing | 1/7 (14.3) | Vs. no | 1.85 | 0.26 to 13.12 | |
Time to development of pressure ulcers of category ≥ 3 to 30-day final follow-up
A total of 32 (1.6%) patients developed at least one new PU of category ≥ 3 between randomisation and 30-day final follow-up, death or withdrawal (see Appendix 4, Table 73). An absolute difference between mattress groups of 0.4% was observed, corresponding to incidence rates of 1.4% (n = 14) in the APM arm and 1.8% (n = 18) in the HSFM arm. Among those patients who developed a PU of category ≥ 3, the median time to development was 16 days (range 2–58 days) in the APM arm and 17 days (range 4–66 days) in the HSFM arm. There was no evidence of a difference between mattress groups in terms of the development of PUs of category ≥ 3 to 30-day final follow-up in the unadjusted Gray’s test (p = 0.5151; see Figure 3d); the adjusted analysis (Fine and Gray model) also showed no evidence of a difference between the APM and HSFM groups (HR 0.81, 95% CI 0.40 to 1.62; exact p-value = 0.5530). Factors related to development of PUs of category ≥ 3 included skin status (Wald p-value = 0.0288), consent type (Wald p-value = 0.0335), pain on a healthy, altered or category 1 PU skin site (Wald p-value < 0.0001) and presence of a condition affecting the peripheral circulation (p < 0.0001); HRs and corresponding 95% CIs are provided in Table 11. Incidence rates within all levels of covariates were low; the incidence rate was 1.3% for patients with no PU (22/1648) or a category 1 PU (3/236) at baseline compared with 4.8% (7/145) of patients with a pre-existing category 2 PU. In terms of consent, the incidence rate was observed to be 1.1% (16/1404) for patients who provided written consent, compared with 2.0% (6/303) for patients who provided witnessed verbal consent and 3.1% (10/322) for patients who were consented via consultee agreement. The presence of pain on a healthy, altered or category 1 PU skin site was statistically significant in the model, with an incidence rate of 0.0% for patients unable to report pain (0/30) and 8.0% for patients for whom the presence of pain was missing (2/25). The incidence rate for patients with pain was marginally higher, at 1.8% (19/1084), than for patients who did not have pain [1.2% (11/890)]. The presence of a condition affecting peripheral circulation was also statistically significant in the model, with an incidence rate of 0.0% (0/7) in patients for whom this factor could not be determined. The incidence rate in patients who had a condition affecting peripheral circulation was 2.2% (10/455), whereas it was 1.4% (22/1567) in patients who did not have such a condition.
| Covariate | Incidence rate, n/N (%) | Reference level | HR | Wald p-value | |
|---|---|---|---|---|---|
| Point estimate | 95% Wald confidence limits | ||||
| Treatment | |||||
| HSFM | 18/1013 (1.8) | – | – | – | 0.5498a |
| APM | 14/1016 (1.4) | Vs. HSFM | 0.81 | 0.40 to 1.62 | |
| Skin status | |||||
| No PU | 22/1648 (1.3) | – | – | – | 0.0288 |
| Category 1 PU | 3/236 (1.3) | Vs. no PU | 0.85 | 0.24 to 2.98 | |
| Category 2 PU | 7/145 (4.8) | Vs. no PU | 3.20 | 1.33 to 7.71 | |
| Consent type | |||||
| Written | 16/1404 (1.1) | – | – | – | 0.0335 |
| Witnessed verbal | 6/303 (2.0) | Vs. written | 1.68 | 0.66 to 4.28 | |
| Consultee agreement | 10/322 (3.1) | Vs. written | 2.97 | 1.31 to 6.74 | |
| Setting | |||||
| Secondary care hospital | 26/1416 (1.8) | – | – | – | 0.3045 |
| Community hospital | 3/379 (0.8) | Vs. secondary care hospital | 0.43 | 0.13 to 1.41 | |
| NHS intermediate care/rehabilitation facility | 3/234 (1.3) | Vs. secondary care hospital | 0.61 | 0.18 to 2.10 | |
| Pain on a healthy, altered or category 1 PU skin site | |||||
| No | 11/890 (1.2) | – | – | – | < 0.0001 |
| Yes | 19/1084 (1.8) | Vs. no | 2.00 | 0.93 to 4.32 | |
| Unable to assess | 0/30 (0.0) | Vs. no | 0.00 | 0.00 to 0.00 | |
| Missing | 2/25 (8.0) | Vs. no | 5.90 | 1.19 to 29.32 | |
| Presence of condition affecting peripheral circulation | |||||
| No | 22/1567 (1.4) | – | – | – | < 0.0001 |
| Yes | 10/455 (2.2) | Vs. no | 1.49 | 0.70 to 3.15 | |
| Missing | 0/7 (0.0) | Vs. no | 0.00 | 0.00 to 0.00 | |
A total of 40 new PUs of category ≥ 3 were observed on 32 patients. The number of category 3 PUs was comparable by trial arm (APM, n = 19, vs. HSFM, n = 21). The most common skin sites to develop the first new PU of category ≥ 3 were the sacrum (n = 8, 20.0%), left heel (n = 10, 25.0%) and right heel (n = 12, 30.0%). In terms of the location of new PUs of category ≥ 3, similar proportions were noted on participants in both the APM and the HSFM groups on the major torso skin sites (sacrum and buttocks) and heels (see Appendix 4, Table 74).
Healing of pre-existing pressure ulcers of category 2 to 30-day final follow-up
Of 145 patients who had a pre-existing PU of category 2, 70 (48.3%) were allocated to APM and 75 (51.7%) were allocated to HSFM. The healing rate was observed to be 62.9% (n = 44) for patients allocated to APM and 60.0% (n = 45) for patients allocated to HSFM, an absolute difference of 2.9%. Of the patients who healed, the median time to healing was 20 days (range 2–85 days) in the APM arm and 14 days (range 2–84 days) in the HSFM arm. There was no evidence of a difference between mattress groups in terms of healing by the 30-day final follow-up in the unadjusted Gray’s test (p = 0.7362; Figure 4), and this was reflected in the adjusted analysis (Fine and Gray model HR 1.12, 95% CI 0.74 to 1.68; exact p-value = 0.6122) for the comparison of APM with HSFM. The only factor related to the time to healing of a pre-existing PU of category 2 was the presence of a condition affecting the peripheral circulation (p = 0.0469); HRs and corresponding 95% CIs for all covariates entered into the model are presented in Table 12. The healing rate for patients who had a condition affecting peripheral circulation was 64.2% (68/106) and was 52.6% (20/38) for patients who did not have such a condition.
FIGURE 4.
Cumulative incidence function for healing end point.
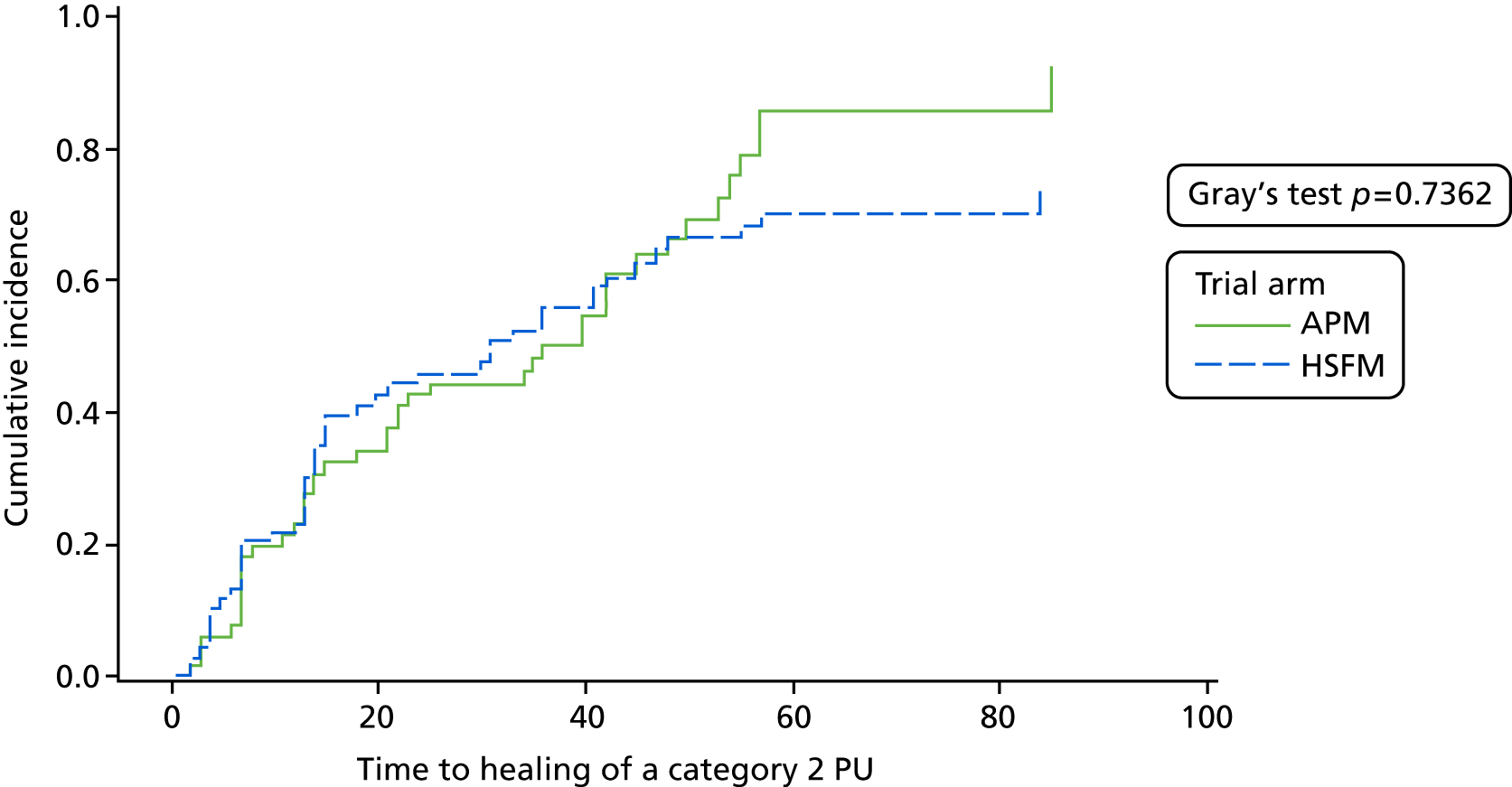
| Covariate | Healing rate, n/N (%) | Reference level | HR | Wald p-value | |
|---|---|---|---|---|---|
| Point estimate | 95% Wald confidence limits | ||||
| Treatment | |||||
| HSFM | 45/75 (60.0) | – | – | – | 0.5990a |
| APM | 44/70 (62.9) | Vs. HSFM | 1.12 | 0.74 to 1.68 | |
| Consent type | |||||
| Written | 63/102 (61.8) | – | – | – | 0.9193 |
| Witnessed verbal | 14/23 (60.9) | Vs. written | 1.08 | 0.65 to 1.81 | |
| Consultee agreement | 12/20 (60.0) | Vs. written | 1.12 | 0.57 to 2.19 | |
| Setting | |||||
| Secondary care hospital | 71/111 (64.0) | – | – | – | 0.3093 |
| Community hospital | 8/20 (40.0) | Vs. secondary care hospital | 0.55 | 0.26 to 1.18 | |
| NHS intermediate care/rehabilitation facility | 10/14 (71.4) | Vs. secondary care hospital | 0.91 | 0.44 to 1.86 | |
| Presence of condition affecting peripheral circulation | |||||
| No | 20/38 (52.6) | – | – | – | 0.0469 |
| Yes | 68/106 (64.2) | Vs. no | 0.59 | 0.36 to 0.97 | |
| Missing | 1/1 (100.0) | Vs. no | 0.56 | 0.31 to 1.04 | |
Moderator analysis (exploratory analysis)
The treatment effects for each level of each covariate (risk factor) for the end points of time to development of a PU of category ≥ 2 to 30-day final follow-up (primary end point) and time to development of a PU of category ≥ 2 during the treatment phase are presented in Figures 5–8. The forest plots present the point estimate of the treatment effect, ln(HR), together with the corresponding 95% CI and, alongside the plot, the corresponding HR and 95% CIs. As this is an exploratory analysis, it is noted where there are observed differences in the estimate of the ln(HR) across the levels of each risk factor together with corresponding 95% CI, ln(HR) and corresponding upper 95% CI to the left of zero indicate a benefit in favour of APM rather than HSFM, whereas ln(HR) and lower 95% CI to the right of zero indicate the reverse.
FIGURE 5.
Forest plot of effect sizes within subgroups for the primary end point, by the following subgroups: skin status, mobility, nutrition, consent type. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

FIGURE 6.
Forest plot of effect sizes within subgroups for the primary end point, by the following subgroups: diabetic status, age group, sensory perception, macro- and microcirculation and moisture.

FIGURE 7.
Forest plot effect sizes within subgroups for the treatment phase end point, by the following subgroups: skin status, mobility, nutrition, consent type.
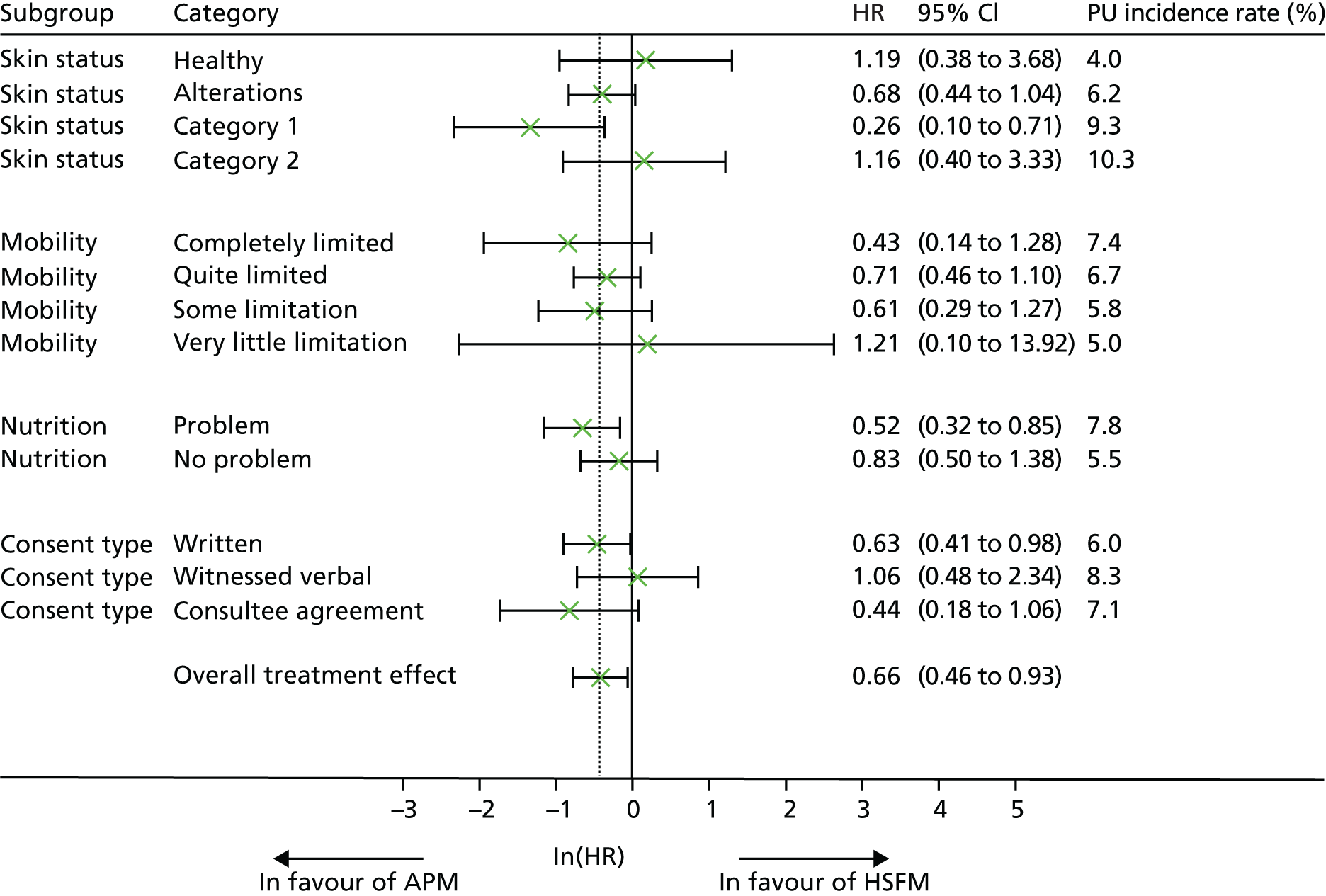
FIGURE 8.
Forest plot of effect sizes within subgroups for the treatment phase end point, by the following subgroups: diabetic status, age group, sensory perception, macro- and microcirculation and moisture.
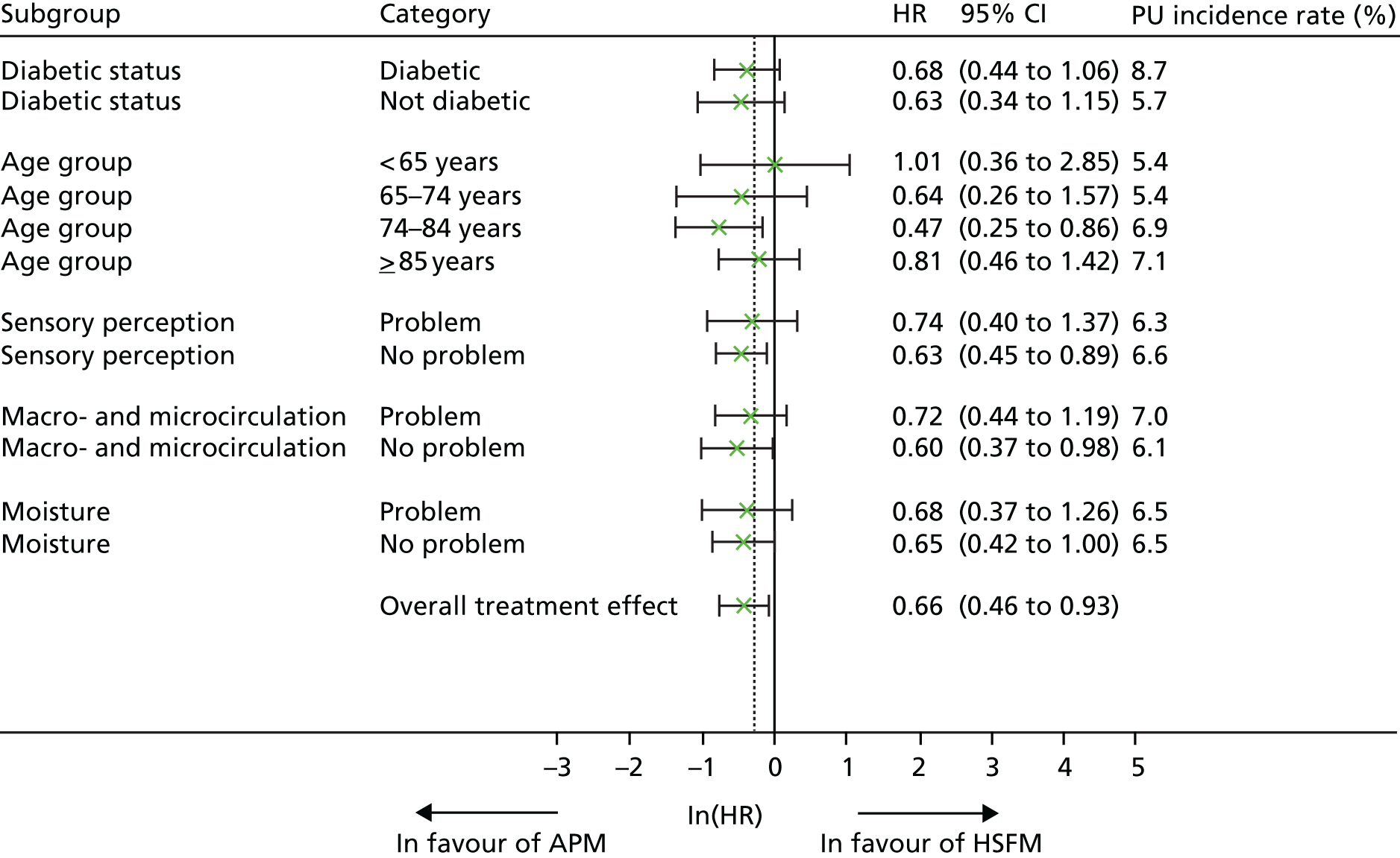
Incidence rates observed within each level of risk factor are aligned with the conceptual framework. Although there is no evidence of differential treatment effects within risk factors at the 5% significance level, treatment effects from these exploratory analyses suggest that there may be a potential benefit of APM compared with HSFM in patients whose worst skin status was assessed as altered or category 1 PU, those with mobility limitations, those with a nutritional problem and patients who participated via consultee agreement. There is a suggestion that patients aged 75–84 years may benefit more from APM, and those aged ≥ 85 years are not observed to benefit from mattress type. The reason for this is not clear; however, these patients have a higher incidence rate and their treatment effect may be confounded by other correlated risk factors. Further work would be required to investigate this.
Mediator analysis (exploratory analysis)
Figure 9 presents repositioning frequencies by mattress allocation during the treatment phase for all patients in the ITT population for whom repositioning data are available. Throughout the treatment phase, ≥ 50% of patients with complete data in both the APM and the HSFM arms at each visit were repositioned every 2–3 hours or more frequently. However, the proportion of patients repositioned every 2–3 hours or more frequently appears to reduce during the treatment phase in both arms. Of those patients repositioned fewer times than every 2–3 hours, similar proportions in the APM and HSFM arms were positioned every 4–5 hours and every 6–7 hours or fewer during the period between visits 1 and 5, but during the period between visits 6 and 10, patients allocated to APM were repositioned less frequently than those allocated to HSFM (i.e. fewer APM patients were positioned every 4–5 hours and more were positioned every 6–7 hours or fewer times than patients in the HSFM arm).
FIGURE 9.
Repositioning during treatment phase by mattress allocation. a, Proportion calculated on a complete-case basis. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
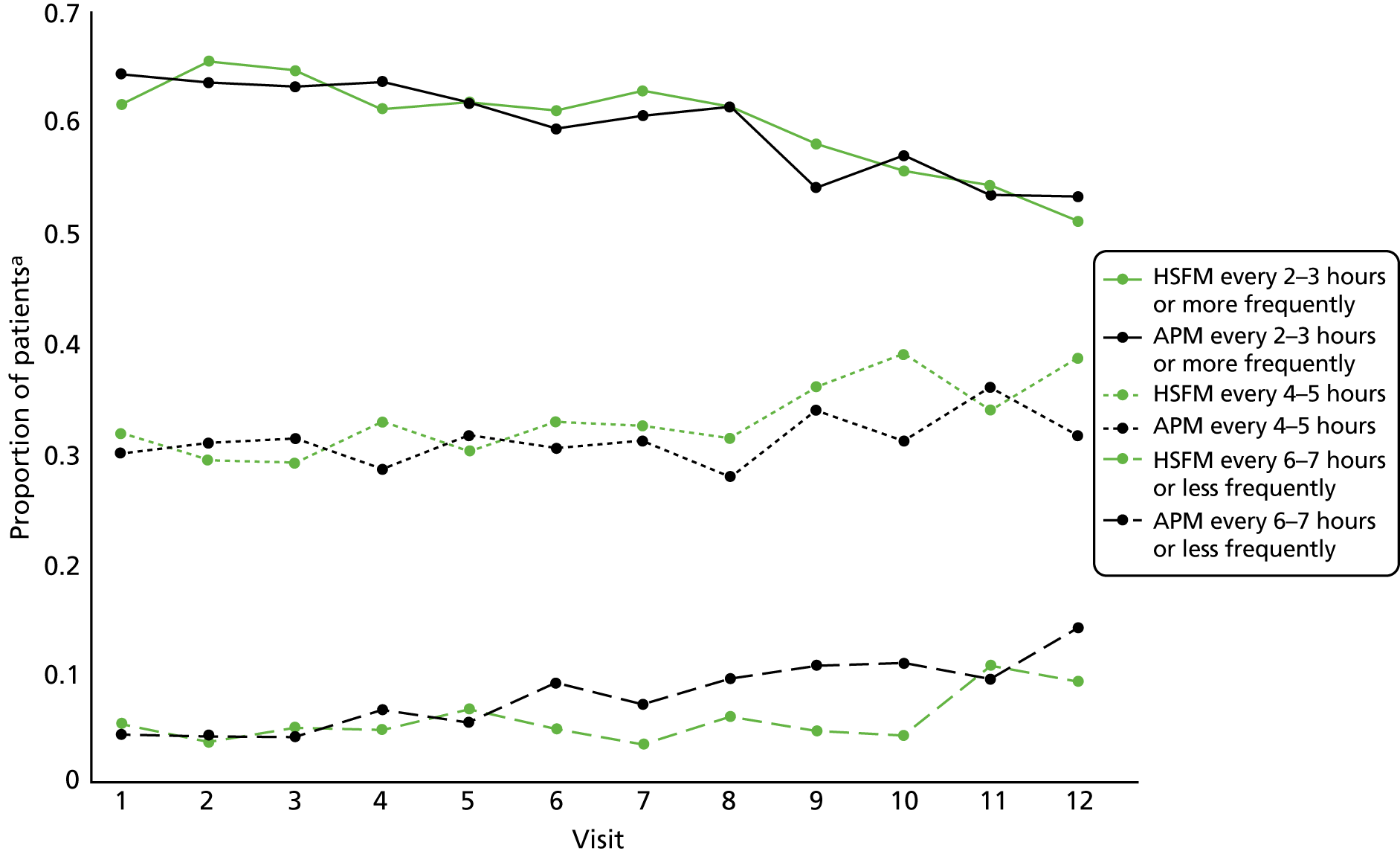
Mattress compliance
The allocated mattress was received by 81.5% of patients within 2 days of randomisation for each mattress group (Table 13). The median proportion of time in the treatment phase that patients spent on the randomised mattress was 92% (range 0–100%) in the APM group and 100% (range 0–100%) in the HSFM group, with 9.3% (n = 94) of patients allocated to APM and 10.9% (n = 110) of patients allocated to HSFM not receiving their allocated mattress at any point during the treatment phase.
| Mattress allocation, compliance and changes | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Allocated mattress received on day 0, n (%) | |||
| Yes | 491 (48.3) | 660 (65.2) | 1151 (56.7) |
| No | 523 (51.5) | 349 (34.5) | 872 (43.0) |
| Mattress log not returned | 2 (0.2) | 4 (0.4) | 6 (0.3) |
| Total | 1016 (100) | 1013 (100) | 2029 (100) |
| If no, reason(s) why, n (%) | |||
| Logistical reasons (e.g. mattress unavailable or awaiting delivery) | 499 (95.1) | 301 (86.2) | 800 (91.7) |
| Clinical decision (e.g. participant’s clinical condition) | 11 (2.1) | 32 (9.2) | 43 (4.9) |
| Patient request | 8 (1.5) | 13 (3.7) | 21 (2.4) |
| Other reason/reason unknown/missing | 5 (1.0) | 3 (0.9) | 8 (0.9) |
| Total | 523 (100) | 349 (100) | 872 (100) |
| If no, mattress the patient is on, n (%) | |||
| APM or other high-technology mattress | 39 (7.5) | 336 (96.3) | 375 (43.0) |
| HSFM or other low-technology mattress | 481 (92.0) | 10 (2.9) | 491 (56.3) |
| Other | 1 (0.2) | 2 (0.6) | 3 (0.3) |
| Missing | 2 (0.4) | 1 (0.3) | 3 (0.3) |
| Total | 523 (100) | 349 (100) | 872 (100) |
| Allocated mattress received within 2 days of randomisation, n (%) | |||
| Yes | 828 (81.5) | 826 (81.5) | 1654 (81.5) |
| No | 186 (18.3) | 183 (18.1) | 369 (18.2) |
| Missing | 2 (0.2) | 4 (0.4) | 6 (0.3) |
| Total | 1016 (100) | 1013 (100) | 2029 (100) |
| Mattress compliance (%) during treatment phase | |||
| Mean (SD) | 72.8 (35.81) | 72.8 (37.81) | 72.8 (36.8) |
| Median (range) | 92 (0–100) | 100 (0–100) | 95 (0–100) |
| IQR | (50.0–100) | (47.1–100) | (50.0–100) |
| Number of patients with missing data | 2 | 4 | 6 |
| Frequency distribution, n (%) | |||
| 0.0% | 94 (9.3) | 110 (10.9) | 204 (10.1) |
| 0.0% to < 20.0% | 74 (7.3) | 78 (7.7) | 152 (7.5) |
| 20.0% to < 40.0% | 51 (5.0) | 50 (4.9) | 101 (5.0) |
| 40.0% to < 60.0% | 59 (5.8) | 51 (5.0) | 110 (5.4) |
| 60.0% to < 80.0% | 80 (7.9) | 57 (5.6) | 137 (6.8) |
| 80.0% to 100.0% | 656 (64.6) | 663 (65.4) | 1319 (65.0) |
| Missing | 2 (0.2) | 4 (0.4) | 6 (0.3) |
| Total | 1016 (100) | 1013 (100) | 2029 (100) |
| Changed from randomised mattress at least once, n (%) | |||
| Yes | 222 (24.1) | 220 (24.4) | 442 (24.2) |
| No | 698 (75.7) | 679 (75.2) | 1377 (75.5) |
| Mattress log not returned | 2 (0.2) | 4 (0.4) | 6 (0.3) |
| Total | 922 (100) | 903 (100) | 1825 (100) |
| Reason for first change from randomised mattress, n (%) | |||
| Participant requested mattress change | |||
| To aid movement | 20 (9.0) | 0 (0.0) | 20 (4.5) |
| Mattress not comfortable | 90 (40.5) | 28 (12.7) | 118 (26.7) |
| Participant no longer at risk | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Ward-led mattress change | |||
| Participant no longer at risk | 4 (1.8) | 1 (0.5) | 5 (1.1) |
| To aid rehabilitation | 29 (13.1) | 5 (2.3) | 34 (7.7) |
| Participant comfort | 5 (2.3) | 17 (7.7) | 22 (5.0) |
| Participant clinical condition | 3 (1.4) | 130 (59.1) | 133 (30.1) |
| Participant safety/health | 4 (1.8) | 2 (0.9) | 6 (1.4) |
| Reason unknown | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| In error | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Ward transfer | 40 (18.0) | 20 (9.1) | 60 (13.6) |
| Technical fault | 11 (5.0) | 0 (0.0) | 11 (2.5) |
| Mattress was required by another patient | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| Home leave | 2 (0.9) | 2 (0.9) | 4 (0.9) |
| Slept in chair | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Hospital transfer | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| Reason unknown | 8 (3.6) | 10 (4.6) | 18 (4.0) |
| Total | 222 (100.0) | 220 (100.0) | 442 (100.0) |
Of 1016 patients allocated to APM, 51.5% (n = 523) did not receive their mattress on the day of randomisation, compared with 34.5% (n = 349) of patients allocated to HSFM. The most common reasons for not receiving the allocated mattress on the day of randomisation were logistical (e.g. mattress unavailable or awaiting delivery), affecting 95.1% (499/523) of those patients in the APM arm and 86.2% (301/349) of those patients in the HSFM arm. Other reasons for non-allocation on the day of randomisation were clinical, affecting 2.1% (11/523) of those in the APM arm and 9.2% (32/349) of those in the HSFM arm. Patients who were not allocated their mattress on the day of randomisation were typically on the alternative mattress type, with 92.0% (481/523) of patients who did not receive their allocated APM being on HSFM or another low-technology mattress pre randomisation and 96.3% (336/349) of patients who did not receive their allocated HSFM being on APM or another high-technology mattress pre randomisation (see Table 13).
In total, there were 1320 mattress changes. The most common reason was that the allocated mattress became available (n = 606, 45.9%).
Of the 922 APM and 903 HSFM patients who received their allocated mattress during the treatment phase, 24.1% (222/922) in the APM arm and 24.4% (220/903) in the HSFM arm were observed to have at least one change from their allocated mattress (see Table 13). These mattress changes included patient- and ward-led reasons. For 40.5% (90/222) of APM participants and 12.7% (28/220) of HSFM participants, the first change from the mattress allocation was a participant request because the mattress was not comfortable. In contrast, changes for 2.3% (5/222) of participants from the APM arm and 7.7% (17/220) from the HSFM arm were ward-led changes to aid participant comfort (see Table 13).
Other reasons were related to clinical condition and movement: 1.4% (3/222) of first mattress changes from APM were ward led as a result of a participant’s clinical condition, compared with 59.1% (130/220) of such changes from HSFM. Of the changes, both patient and ward led, to aid movement/rehabilitation, 22.1% [(20 + 29)/222] were from APM, whereas 2.3% [(0 + 5)/220] were from HSFM (see Table 13).
Ward transfer was the third most common reason for first change from allocated mattress, contributing to 18.0% (40/222) of the first changes from APM and 9.1% (20/220) of the first changes from HSFM.
Per-protocol population
The PPP consisted of 1352 (66.6%) patients after removing 545 (26.9%) patients who did not achieve at least 60% compliance with their allocated mattress prior to developing a PU of category ≥ 2, or the end of the treatment phase, whichever occurred sooner. Patients were also excluded (reasons not mutually exclusive) if they were not at a high risk of PU development (n = 42, 2.1%), had a current or previous PU of category ≥ 3 (n = 8, 0.4%), were outside the permitted weight limits (n = 6, 0.3%), did not receive the mattress within 2 days of randomisation (n = 369, 18.2%) and/or the consent form was not received or the consent date was after randomisation (n = 13, 0.6%). Of the 1352 patients in the PPP, 663 (49.0%) were allocated to APM and 689 (51.0%) were allocated to HSFM. There was no evidence of a difference between mattress groups in the incidents of new PUs of category ≥ 2 (adjusted Fine and Gray model HR 0.79, 95% CI 0.54 to 1.16; p = 0.2249). Incidence rates of new PUs of category ≥ 2 by 30-day final follow-up were observed to be 7.2% (n = 42) in the APM arm and 8.4% (n = 58) in the HSFM arm. Incidents of a new PU of category ≥ 2 in the treatment phase sensitivity analysis were observed to be 5.7% (n = 38) in the APM arm and 7.4% (n = 51) in the HSFM arm, a difference of 1.7%, which was marginally significant, indicating some evidence of a difference between mattress groups (adjusted Fine and Gray model HR 0.76, 95% CI 0.32 to 1.00; p = 0.0508).
Safety data: adverse and serious adverse events
Results are reported for all randomised patients (i.e. including the patient who was randomised twice).
No patient experienced a RU SAE (Table 14); expected (i.e. prespecified) adverse and serious adverse events, including deaths, institutionalisation, falls and device-related ulcers, were balanced across groups.
| Attribute | Trial arm | Total (N = 2030) | |
|---|---|---|---|
| APM (N = 2017)a | HSFM (N = 2013) | ||
| Number of RU SAEs | 0 | 0 | 0 |
| Deaths, n (%) | 82 (8.1) | 84 (8.3) | 166 (8.2) |
| Participants who were readmitted, n (%) | 82 (8.1) | 62 (6.1) | 144 (7.1) |
| Expected AEs/SAEs, n (%) | |||
| At least one AE/SAE reported | 163 (16.0) | 167 (16.5) | 330 (16.3) |
| No AE/SAE reported | 853 (83.9) | 842 (83.1) | 1695 (83.5) |
| CRF not received | 1 (0.1) | 4 (0.4) | 5 (0.2) |
| Total | 1017 (100.0) | 1013 (100.0) | 2030 (100.0) |
| Total number of AEs/SAEs | 259 (100.0) | 25 (100.0) | 511 (100.0) |
| Falls | 246 (95.0) | 240 (95.2) | 486 (95.1) |
| Device-related ulcers | 12 (4.6) | 10 (4.0) | 22 (4.3) |
| Related AEs | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| Falls details | |||
| Patients who experienced a fall, n (%) | 152 (14.9) | 159 (15.7) | 311 (15.3) |
| Total number of falls | 246 | 240 | 486 |
| On allocated mattress at time of fall, n (%) | |||
| Yes | 61 (24.8) | 64 (26.7) | 125 (25.7) |
| No | 15 (6.1) | 18 (7.5) | 33 (6.8) |
| Cannot be determined | 6 (2.4) | 10 (4.2) | 16 (3.3) |
| Missing | 4 (1.6) | 5 (2.1) | 9 (1.9) |
| Fall occurred after treatment phase | 160 (65.0) | 143 (59.6) | 303 (62.3) |
| Injury sustained, n (%) | |||
| Yes | 81 (32.9) | 73 (30.4) | 154 (31.7) |
| No | 163 (66.3) | 166 (69.2) | 329 (67.7) |
| Missing | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| If injury sustained, was the injury serious?, n (%) | |||
| Yesa | 11 (13.6) | 16 (21.9) | 27 (17.5) |
| No | 70 (86.4) | 57 (78.1) | 127 (82.5) |
| If injury was serious, seriousness criteria, n (%) | |||
| Requires prolonged hospitalisation | 7 (63.6) | 9 (56.3) | 16 (59.3) |
| Significantly or permanently disabling or incapacitating | 0 (0.0) | 2 (12.5) | 2 (7.4) |
| Requires surgical intervention | 1 (9.1) | 2 (12.5) | 3 (11.1) |
| Laceration(s) | 1 (9.1) | 3 (18.8) | 4 (14.8) |
| Radiography undertaken but clear | 2 (18.2) | 0 (0.0) | 2 (7.4) |
| If injury was serious, causality of fall, n (%) | |||
| Unlikely to be related | 0 (0.0) | 1 (6.3) | 1 (3.7) |
| Unrelated | 8 (72.7) | 11 (68.8) | 19 (70.4) |
| Missing | 3 (27.3) | 4 (25.0) | 7 (25.9) |
| If injury was serious, mattress type at time of fall, n (%) | |||
| HSFM | 2 (18.2) | 5 (31.3) | 7 (25.9) |
| APM | 0 (0.0) | 1 (6.3) | 1 (3.7) |
| Unknown/participant at home | 1 (9.1) | 2 (12.5) | 3 (11.1) |
| Domestic mattress | 3 (27.3) | 1 (6.3) | 4 (14.8) |
| Missing | 5 (45.5) | 7 (43.8) | 12 (44.4) |
| Device-related ulcer details | |||
| Patients who experienced a device-related ulcer, n (%) | 12 (1.2) | 8 (0.8) | 20 (1.0) |
| Total number of device-related ulcers | 12 | 10 | 22 |
| On allocated mattress at time of device-related ulcer first observed, n (%) | |||
| Yes | 7 (58.3) | 2 (20.0) | 9 (40.9) |
| No | 1 (8.3) | 8 (80.0) | 9 (40.9) |
| Missing | 2 (16.7) | 0 (0.0) | 2 (9.1) |
| Device-related ulcer occurred after treatment phase | 2 (16.7) | 0 (0.0) | 2 (9.1) |
| Was the device-related ulcer serious?, n (%) | |||
| No | 12 (100) | 10 (100) | 22 (100) |
| Related AEs | |||
| Patients who experienced a mattress-related AE, n (%) | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Total number of mattress-related AEs | 1 | 2 | 3 |
| On allocated mattress at time of mattress-related AE, n (%) | |||
| Yes | 1 (100.0) | 1 (50.0) | 2 (66.7) |
| No | 0 (0.0) | 1 (50.0) | 1 (33.3) |
| Was the mattress-related AE serious?, n (%) | |||
| No | 1 (100) | 2 (100) | 3 (100) |
Of the SAES and AEs reported, only three were reported to have an ‘other’ AE related to the mattress: two of these were related to an APM deflating (including one patient randomised to HSFM but allocated to APM by ward staff). The third AE was related to HSFM and the patient reported a burning sensation on their buttocks and sacrum during the night. None of these ‘other’ mattress-related AEs was classified as serious.
In total, there were 82 (8.1%) and 84 (8.3%) deaths, and 82 (8.1%) and 62 (6.1%) re-admissions in the APM and HSFM groups, respectively.
Device-related ulcers were reported in 20 (1.0%) patients: 12 (1.2%) in the APM group and 8 (0.8%) in the HSFM group. Devices resulting in ulcers included catheters, plaster casts, traction, nasal tubing, antiembolic stockings, face mask, bed end, bedpan, hoist, bandage, footstool and wheelchair. None of the device-related ulcers was assessed as serious.
Falls were observed in both mattress groups and affected a total of 311 (15.3%) patients: 152 (14.9%) patients with a total of 246 falls in the APM arm, and 159 (15.7%) patients with a total of 240 falls in the HSFM arm. Only 25.7% (125/486) of falls took place when patients were known to be on their randomised mattress; 166 (34.2%) falls took place inside the home after discharge. An injury was sustained in 154 (31.7%) falls, and 27 (17.5%) of these were classified as SAEs. None of the serious falls was assessed as related to the mattress (although causality data were missing for 25.9% (7/27) incidents) (see Table 14).
Summary of results
From August 2013 to November 2016, 2030 patients were randomised: 1017 were allocated to APM and 1013 were allocated to HSFM.
Primary end point: 30-day final follow-up
Of 2029 patients in the ITT population, 160 (7.9%) developed a new PU of category ≥ 2 with no evidence of a difference between mattress groups: 6.9% (n = 70) in the APM group compared with 8.9% (n = 90) in the HSFM group (Fine and Gray model HR 0.76, 95% CI 0.56 to 1.04; exact p-value = 0.0890).
Treatment phase sensitivity analysis
During the treatment phase, new PUs of category ≥ 2 developed in 5.2% of participants in the APM group compared with 7.8% of HSFM participants, with statistically significant evidence that APM is superior to HSFM (Fine and Gray model HR 0.66, 95% CI 0.46 to 0.93; p = 0.0176).
Secondary end points: 30-day final follow-up
New PUs of category ≥ 1 developed in 350 (17.2%) patients with no evidence of a difference between groups: 15.7% (n = 160) in the APM group compared with 18.8% (n = 190) in the HSFM group (Fine and Gray model HR 0.83, 95% CI 0.67 to 1.02; p-value = 0.0733).
New PUs of category ≥ 3 developed in 32 (1.6%) patients with no evidence of a difference between groups: 1.4% (n = 14) in the APM group compared with 1.8% (n = 18) in the HSFM group (Fine and Gray model HR 0.81, 95% CI 0.40 to 1.62; p = 0.5530).
In terms of time to healing of a pre-existing PU of category 2, there was no evidence of a difference in healing rate between the two groups: 62.9% (n = 44/70) in the APM group compared with 60.0% (n = 45/75) in the HSFM group (Fine and Gray model HR 1.12, 95% CI 0.74 to 1.68; p = 0.6122).
Chapter 4 Health economics
Introduction
Within-trial economic evaluations were carried out, together with a model-based analysis, to assess the cost-effectiveness of APM compared with HSFM in the prevention of PUs of category ≥ 2 in high-risk patients. The model was used to extrapolate the results over the expected lifetime of the trial participants. The methods used are in line with NICE guidance. 61
Measurement of resource use and cost analysis
The analyses take the perspective of the UK NHS and Personal Social Services (PSS), including costs incurred by the NHS in the provision of the treatment and other health and social care resource utilisation together. This includes length of stay in hospital, use of hospital outpatient facilities, contact with community-based health-care services and utilisation of supported living such as care and nursing homes.
Utilisation of health and social care was combined with appropriate unit cost information obtained from national sources such as the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2016. 62 All costs were adjusted by the health-care price index to February 2017. 63 Unit costs and their sources are shown in Appendix 5, Tables 75–77.
Objectives
-
The primary objective was to assess the incremental cost-effectiveness of APM compared with HSFM in the prevention of PUs in high-risk patients at 30-day final follow-up (maximum 90 days post randomisation), from the perspectives of the NHS and PSS.
-
The secondary objective was to assess the long-term incremental cost-effectiveness of APM compared with HSFM in the prevention of PUs of category ≥ 2 in high-risk patients, from the perspectives of the NHS and PSS.
-
A tertiary objective was, in the event of an early stopping signal for futility, we would assess the value of continuing with the trial from the NHS decision-making perspective, via an EVSI analysis, to inform the deliberations of the DMEC. Although a futility boundary was not crossed, this analysis was undertaken twice at the request of the funder. The methods and results are presented in Appendix 3.
Methods
Within-trial analysis
In line with the main trial methods, the within-trial economic analysis adopted an ITT perspective. The analysis used quality-adjusted life-years (QALYs) as the main outcome and adopted the perspectives of the UK NHS and PSS. Utility values were derived from the EQ-5D-5L,64,65 resource use was obtained using a Health Care Resource Utilisation questionnaire comprising abstracted data from health-care records and a researcher-administered questionnaire.
Quality-of-life and resource use data were collected at baseline, at weeks 1 and 3 and at 30-day follow-up after the end of the treatment phase. Given that a PU can take between 4 and 22 weeks to completely heal, depending on the category,2 and that randomised patients are discharged with an unresolved PU, the 30-day post-treatment phase (final) follow-up assessment was included in the trial to retrieve information on the potential long-term effect of the intervention.
Neither costs nor QALYs were discounted, given that the time period was 90 days post randomisation. The results are reported as incremental cost-effectiveness ratios (ICERs).
Quality-adjusted life-years
Quality-adjusted life-years reflect both duration and quality of life. Their estimation requires the production of utility weights for each health state observed in the trial population. HRQoL was assessed using the EQ-5D-5L. 64 The EQ-5D-5L responses were converted to health-state utility values using the UK tariff. 65 In addition to the EQ-5D-5L, sensitivity analyses were undertaken using QALYs estimated from the PU-QoL-UI (a PU condition-specific utility measure) using the appropriate algorithm. 37
Quality-of-life questionnaires were administered by a trained CRN/P at baseline, at weeks 1 and 3 and at 30-day final follow-up.
The values obtained from each of two instruments (EQ-5D-5L and PU-QoL-UI) were multiplied by duration (t) in each health state to generate QALYs. An area-under-the-curve approach was adopted for estimating QALYS. For example, for the EQ-5D-5L:
where EQ5DBaseline, EQ5Dw1, EQ5Dw3 and EQ5DE30 are the EQ-5D-5L scores at baseline, week 1, week 3 and the 30-day final follow-up, respectively.
When an individual died during the trial, it was assumed that their utility value is 0 from the date of death to trial end. A linear transition to this value from their last completed outcome-measure questionnaire was assumed.
Adjusting for baseline imbalance
Despite randomisation, there may be some differences in mean baseline values between groups. This is of particular importance because a patient’s utility value at baseline is likely to be correlated with their utility value over the follow-up period. Therefore, any potential imbalance in baseline utilities has been accounted for. 66,67 Multiple regression analysis was used to estimate differential mean QALYs and to predict adjusted QALYs controlling for utility at baseline, PU status, setting, peripheral circulation and presence of pain. Adjustment of total costs was also performed based on the same baseline characteristics used to adjust the utility (expect utility at baseline).
Missing data
The analysis was conducted under the assumption that missing data were missing at random (MAR). This was based on a descriptive analysis performed on the missing data. Visual and a logistic regression analysis indicated that the data were unlikely to be missing completely at random (MCAR). 68 The visual pattern showed that the proportion of missing data differed by time point, whereas the regression analysis showed that some observed variables predicted missingness. The result of this analysis indicated that MAR may be a plausible assumption. However, it is recognised that missing not at random (MNAR) cannot be completely ruled out, as some variables relevant to the economic data are unknown.
The multiple imputation approach was used to impute data, as it includes randomness to reflect the uncertainty inherent in missing data. This process uses iterative multivariable regression techniques. 69 For the QoL data, missing EQ-5D-5L values were imputed at each of the follow-up periods. The imputed variables were used to estimate the utility values at each time point and then to estimate the QALYs for each treatment arm. For costs, missing data were imputed at the level of total health and social services costs. The imputation was performed in Stata® version 14 (StataCorp LP, College Station, TX, USA) using predictive mean matching to perform multiple imputation by chained equations. This technique ensures that only plausible values of the missing variables are imputed, and the imputed values are estimated from another individual whose predictive value is close to the one of the missing observation. 68
Cost-effectiveness analyses
In line with the clinical analyses, the cost-effectiveness analysis adopted an ITT perspective for analysing and summarising the health economic trial data. The primary analysis consisted of a cost–utility analysis over the 30-day final follow-up (90 days post randomisation). The incremental cost per QALY gain as a result of the use of either APM or HSFM was calculated by dividing the mean difference in cost of the two trial arms by the mean difference in QALYs to produce an ICER, as follows:
where Ci and Ei are the expected cost and effectiveness of the intervention (APM), and Cc and Ec are the expected cost and effectiveness of the comparator strategy (HSFM).
The ICER represents the additional cost per QALY gained for each intervention compared with the next best alternative. 70 NICE consider a cost per QALY within the range of £20,000–30,000 to be acceptable. 61 The lower limit of this threshold (λ = £20,000) was used to determine cost-effectiveness. Interventions with an ICER of < £20,000 per QALY gained will generally be considered cost-effective.
Sensitivity analysis
Alternative scenarios are explored, to test the robustness of the main trial analysis results. The effect of not imputing missing data was considered with an analysis that includes only complete cases. In addition, in the event of an imbalance at baseline, the effect on cost-effectiveness was evaluated. Table 78 in Appendix 5 illustrates the main and secondary analyses carried out.
The level of sampling uncertainty around the ICER was determined using non-parametric bootstrapping to generate 10,000 estimates of incremental costs and benefits. 71,72 The bootstrapped estimates were plotted on the cost-effectiveness plane to illustrate the uncertainty surrounding the cost-effectiveness estimates73 and the cost-effectiveness acceptability curve (CEAC) to show the probability of APM or HSFM being cost-effective as a function of the willingness-to-pay threshold (λ). Mean net benefit is reported in Results (see Table 15). 71
Deterministic and probabilistic analyses for the primary analysis, and probabilistic results for the secondary analysis, are described in Appendix 5, Table 78.
Lifetime decision-analytic model
A decision-analytic model was constructed to estimate the long-term incremental cost-effectiveness of APM compared with HSFM in the prevention of PUs of category ≥ 2 in high-risk patients. Originally, a patient-level simulation model was planned using individual patients defined in terms of age, gender and underlying health condition. However, the economic modelling undertaken as part of the interim trial analysis (see Appendix 3) showed a mean and median age of > 80 years. Given the age of the cohort and the associated likelihood of comorbidities, there was far greater homogeneity within the patient population than anticipated. Following discussion with the trial DMEC, a cohort model was used.
A Markov decision model was constructed using R software version 1.0.136 (The R Foundation for Statistical Computing, Vienna, Austria) (Figure 10). The model structure mirrors that used in the interim VOI analyses (see Appendix 3), but with some differences as the data retrieved from the trial allowed a more detailed patient pathway. The model begins at the point of randomisation and extends for the lifetime of the patients. The model describes patient progression over time through a pathway of health states, with movement between the health states being triggered by development of one or more PUs, healing of PU(s) or death. Compared with the VOI model, the long-term model had two starting points: in hospital PU free or in hospital with a PU. The model also added a stage to account for one PU developed in hospital. The long-term model also used final trial data compared with expectations or partial trial data. Figure 10 shows how patients can move between health states (indicated by the arrows). The model structure was developed in consultations with the clinical expert members of the trial team. It aimed to capture clinical practice according to the retrieved data. Resource use and costs are associated with each health state and patients accumulate costs and health benefits in each state over 3-day cycles. The outcome measure for the model was the QALY. The analysis adopts a lifetime horizon, truncated at 110 years.
FIGURE 10.
Lifetime decision-analytic model to compare APM with HSFM.

The model analysis was conducted from the perspectives of the NHS and PSS. Costs and outcomes were discounted at 3.5%. 61
Model parameters
The model was populated using information from the trial for transition probabilities. Only a few parameters were populated with information from relevant literature. The HR of death when having a PU was obtained from Landi et al. ,74 as this study was based on a similar population as the one in this trial. The percentage reduction in utility for having one PU was assumed based on a previous PU study,37 and the frequency of PU incidence was obtained from Whittington and Briones. 75 In terms of costs, the additional cost of treating a PU was obtained from Dealey et al. 24 Costs of the mattresses were obtained from the NICE PU prevention guideline12 and updated to 2017 costs. The daily cost of treatment of one PU was assumed to be that of the cost per hour of a house visit of a community practice nurse, obtained from the Unit Costs of Health and Social Care 201662 and updated to 2017 prices. Extra costs typical at the end of life were obtained from Halek et al. 76 and Fassbender et al. 77 The parameters are shown in Appendix 5, Table 79.
Cost-effectiveness analysis
As in the within-trial analyses, the incremental cost per QALY gained was estimated. Similarly, the lower threshold value (£20,000 per QALY gained) was used to determine which strategy was the most cost-effective.
Base-case scenario and sensitivity analyses
The base-case scenario included complete cases from the trial (ignoring missing values and based on QALY estimates using the adjusted EQ-5D-5L). Alternative scenarios mirror the within-trial analyses (see Appendix 5, Table 78).
Parameter uncertainty was addressed through probabilistic sensitivity analysis using Monte Carlo simulation. The distributions chosen to characterise the uncertainty were based on the type of variable being investigated. Beta distributions were used to characterise transition probabilities, as this distribution is bounded between 0 and 1. Log-normal distributions were used to characterise HRs as this distribution is bounded at 0 but can sample values > 1. Gamma distributions were assumed for all costs, as the descriptive analysis of trial data suggested that costs follow such a distribution. When standard deviations (SDs) were not available, a relative standard error (RSE) was assumed. The RSE is defined as the ratio of the standard error to the mean. This was assumed at 5% for all required variables.
The outputs of the analysis are presented as the expected ICER, a scatterplot on the cost-effectiveness plane and a CEAC. The expected net monetary benefit (NMB) was also calculated for both interventions using a value of £20,000 for λ.
A deterministic one-way sensitivity analysis was performed to check the results over the most uncertain parameters. Multiway deterministic sensitivity analyses were to be undertaken to test different possible scenarios.
Results
Within-trial analyses
Sample
The total sample size of the ITT analysis was of 2029 participants (APM arm, n = 1016; HSFM arm, n = 1013).
For the complete-case analysis, 267 participants (APM arm, n = 118; HSFM arm, n = 149) had completed the EQ-5D-5L at all four time points, and 233 had completed the PU-QoL-UI at all four time points (APM arm, n = 107; HSFM arm, n = 126). Figure 11 shows a flow diagram showing the number of complete cases by measurement for resource use and EQ-5D-5L.
FIGURE 11.
Flow chart of completed questionnaires by measurement. a, Patients who responded to all five dimensions of the EQ-5D-5L.

Costs
Tables 80–82 in Appendix 5 contain the average per-patient costs from baseline to 30-day final follow-up for those patients who completed the resource use questionnaires at all four time points. (It should be noted that all these patients were included in the ITT analyses, but only those who had also completed the EQ-5D-5L at all four time points were included in the complete-case analysis.)
The cost driver is, as might be anticipated, inpatient care, although the difference between the total mean cost of inpatient stays between the two arms of the trial is not statistically significant (£2810.08 for the APM arm and £2888.68 for the HSFM arm; p = 0.54). Overall, there are few statistically significant differences in costs across the two arms; however, the majority of costs are lower in the APM arm than in the HSFM arm.
Utility values
The utility values (see Appendix 5, Table 83) of both the EQ-5D-5L and the PU-QoL-UI show no statistically significant differences between the arms of the trial across each of the four time periods (baseline, week 1, week 3 and 30-day final follow-up).
Cost-effectiveness
The results of the within-trial analyses using QALYs derived from the EQ-5D-5L are shown in Table 15.
| Strategy | Total | Incremental | ICER (£) | NMB (£) | Probability of being cost-effective (£20,000 per QALY threshold) | Result | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALY | Cost (£) | QALY | |||||
| Deterministic within-trial analysis (adjusted for baseline costs and QALYs) | ||||||||
| APM | 4482 | 0.128 | –1918 | – | Cost-effective | |||
| HSFM | 4621 | 0.127 | 138 | –0.0010 | –136,171 | –2077 | – | Dominated |
| Probabilistic within-trial analysis (adjusted for baseline costs and QALYs) | ||||||||
| APM | 4533 | 0.128 | –1979 | 0.99 | Cost-effective | |||
| HSFM | 4646 | 0.127 | 113 | –0.0011 | –101,699 | –2114 | 0.01 | Dominated |
| Deterministic lifetime analyses | ||||||||
| APM | 17,736 | 7.04 | 123,134 | – | Cost-effective | |||
| HSFM | 18,778 | 6.98 | 1042 | 0.055 | –18,979 | 120,994 | – | Dominated |
| Probabilistic lifetime analyses | ||||||||
| APM | 17,708 | 6.91 | 120,600 | 0.60 | Cost-effective | |||
| HSFM | 18,661 | 6.87 | 953 | –0.046 | –20,465 | 118,715 | 0.40 | Dominated |
Once baseline adjustments were made, the deterministic analysis shows that the mean total costs of APM and HSFM are £4533 and £4646, respectively, with mean QALYs of 0.128 and 0.127, respectively. Thus, APMs dominate HSFM, as APM has lower costs and higher QALY values. Similar results are seen in the probabilistic analysis in which APMs dominate HSFM (mean total costs of APM and HSFM are £4533 and £4646, respectively, and mean QALYs are 0.128 and 0.127, respectively). Within this analysis, APM has a 99% probability of being cost-effective at a threshold of £20,000. Figures 12 and 13 show the cost-effectiveness plane and CEAC.
FIGURE 12.
Cost-effectiveness plane. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
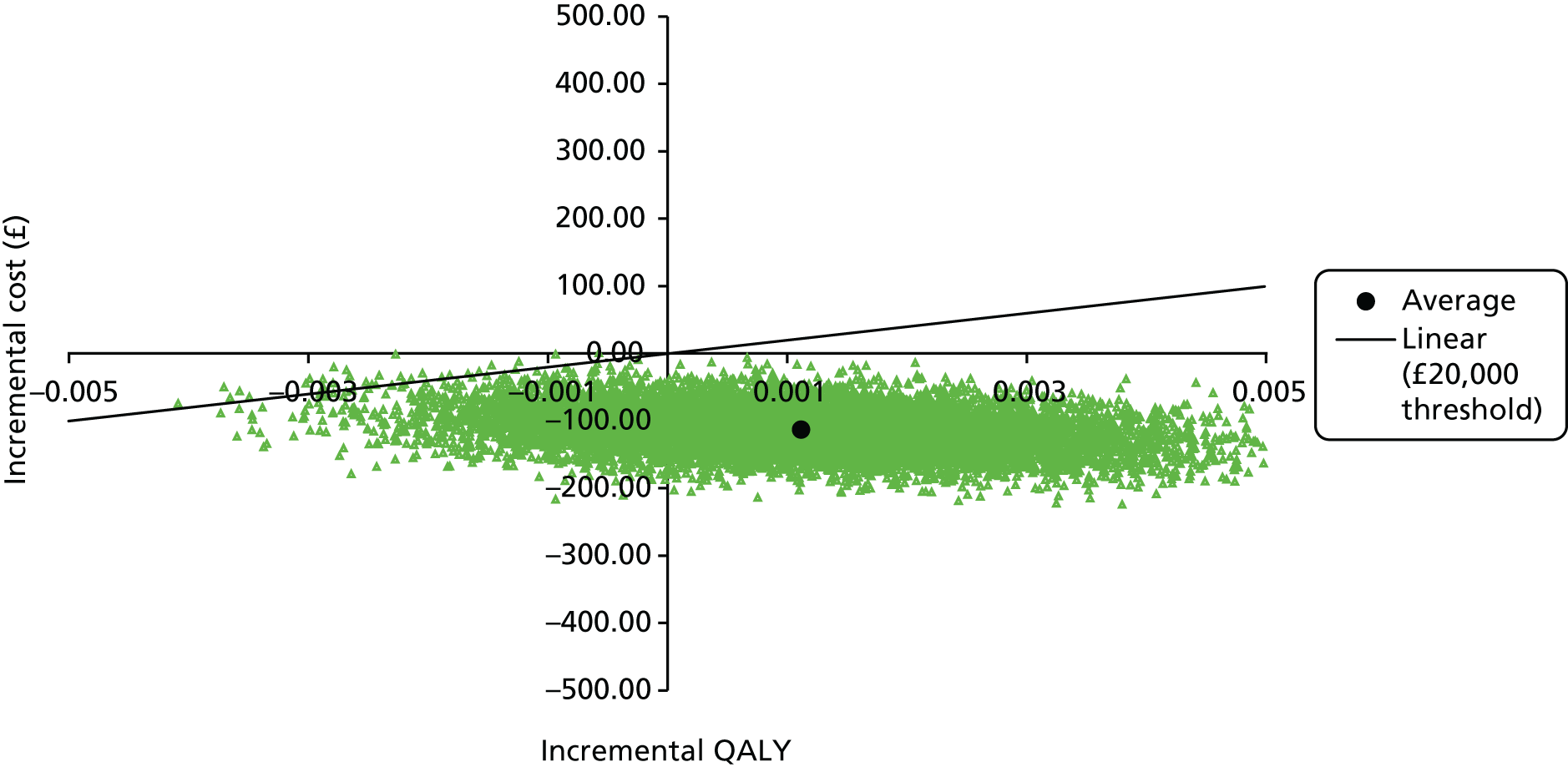
FIGURE 13.
Cost-effectiveness acceptability curve. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

The sensitivity analyses (see Appendix 5, Tables 84–88) show mixed results. The complete-case analysis showed, unlike the previous analyses, that HSFM was cost-effective. An additional analysis was undertaken using only complete cases for the baseline and 30-day final follow-up. This analysis increased the number of complete cases to 934 (APM arm, n = 460; HSFM arm, n = 474). When baseline adjustment was made, the results here showed that HSFM was the most cost-effective; conversely, when no baseline adjustment was made, APM was the cost-effective strategy (see Appendix 5, Tables 89 and 90). ITT analysis without baseline adjustment found that APM was the cost-effective alternative. The results of the sensitivity analyses using QALYs derived from the PU-QoL-UI similarly found differing results (see Appendix 5, Tables 87 and 88).
Decision-analytic cost-effectiveness model
Within the lifetime cost-effectiveness model, APM dominated HSFM with lower costs and higher QALY gains in both the deterministic and the probabilistic analyses (see Table 15). The probabilistic analysis showed a probability of cost-effectiveness of 60%. The cost-effectiveness plane and CEAC are shown in Figures 14 and 15. The sensitivity analyses (see Table 15) show that the results are robust to changes in the main costs and utility variables.
FIGURE 14.
Cost-effectiveness scatterplot (lifetime analyses).
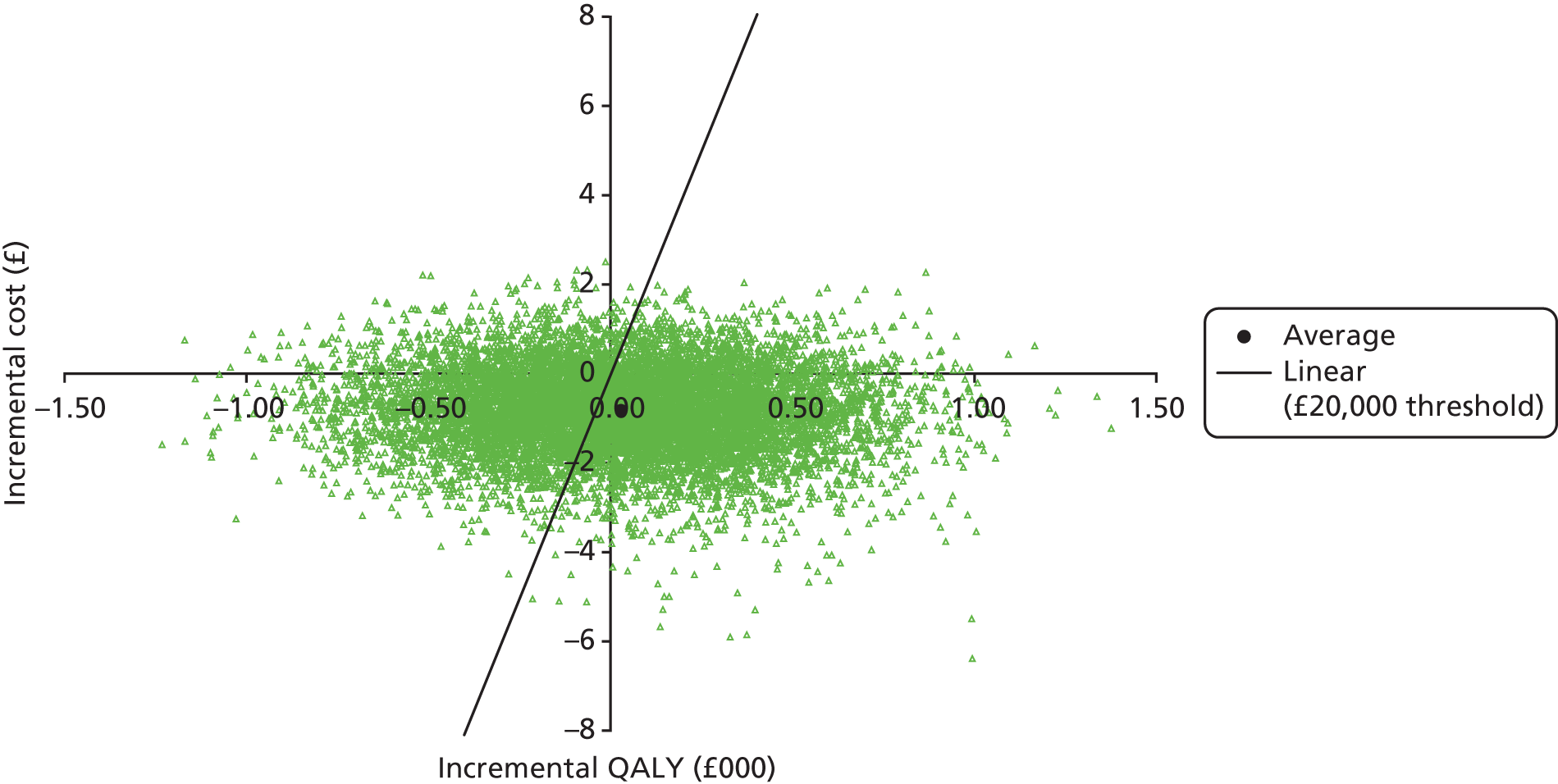
FIGURE 15.
Cost-effectiveness acceptability curve (lifetime analyses).
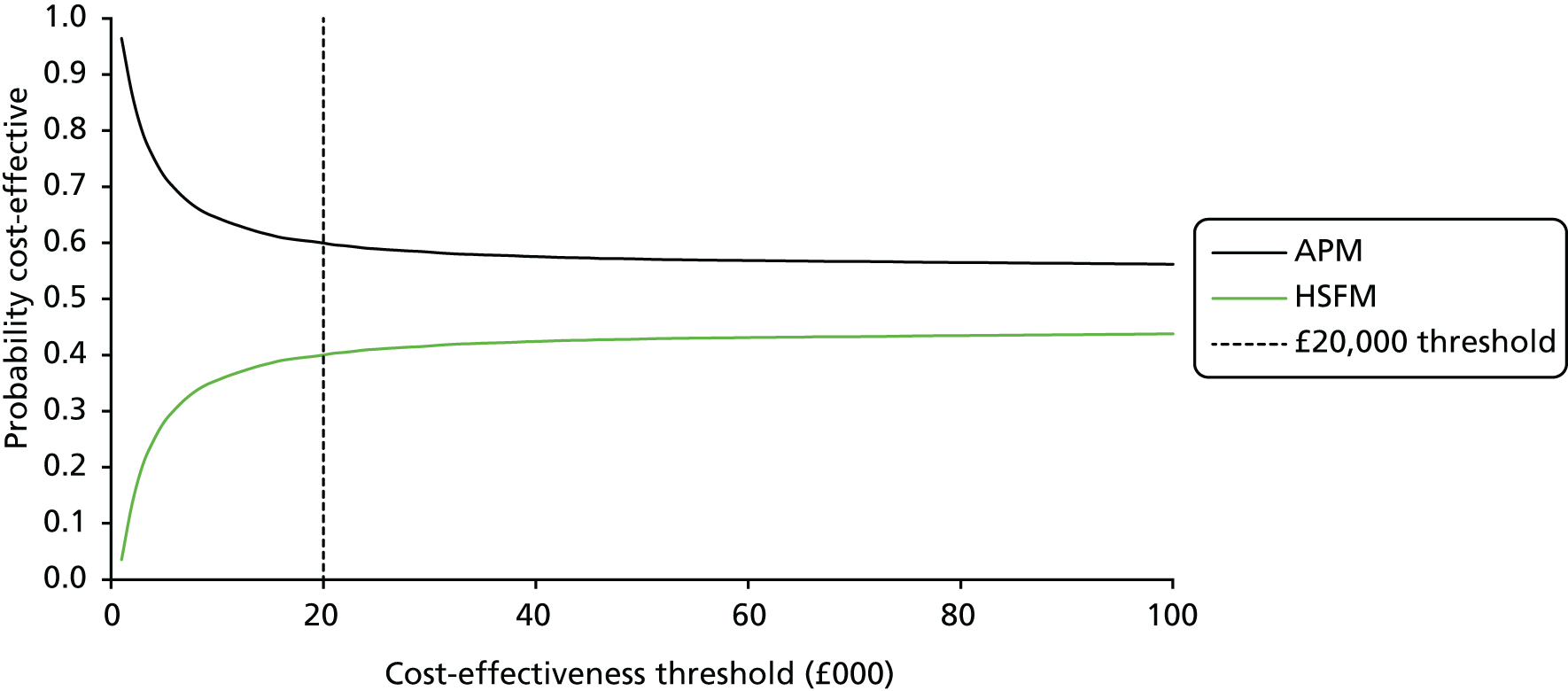
The sensitivity analyses (see Appendix 5, Table 91) all show APM to be cost-effective with lower costs and higher QALY values.
Summary
The value for money of the two mattresses showed APM to be cost-effective over both the short and the long term. However, the results were not robust; differences in costs between the two were modest and the differences in the measure of effectiveness were very small.
Chapter 5 Photography validation substudy
A detailed study protocol for this substudy has been previously published. 78 The following sections give an overview of the background, a summary of the methods and full results and discussion.
Background
Currently, there is no objective laboratory measure for the diagnosis of a PU. The accepted ‘gold standard’ is expert clinical examination of the skin areas at risk of PU development. 8,33 Owing to the visible differences between the two interventions (APM and HSFM) in this study, using the ‘gold standard’ for the end-point assessment meant that blinded assessment of the skin was not achievable. It is possible that the trial primary end point could be misreported, either intentionally or not, by clinical research staff. There is also inherent subjectivity with clinical assessments and misclassification of the skin may also occur. 52,79,80 If there is a risk that estimates of the treatment effect will be biased, then the findings of the trial may not be reliable.
To assess and quantify any potential bias through estimates of over- and under-reporting of PUs, one approach would be to take photographs of all skin sites and assess them blinded. The problem with this would be that taking so many photographs would be a burden to patients and staff and raise concerns about patient consent.
Other issues, such as image quality in ambient lighting conditions and the reliability of the identification of a category 2 PU using photographic evidence,79,80 were of concern.
These issues have been only partially explored previously. At the trial design stage, only two studies were found that report the inter-rater reliability of photography assessed by different clinical experts and photography versus clinical assessment. One study compared experts’ photographic assessments, reporting levels of agreement between two groups of experts assessing photographs to classify PUs. 80 The other study compared the ‘gold standard’ expert clinical nurse assessment against assessment using photographs. 79 Although inter-rater agreement was high in both, the former study reported a high proportion of poor-quality images that could not be assessed80 and, in the latter study, half of the PU photographs were of category ≥ 379 and, therefore, not comparable with the primary end point of ‘early’ category 2 PUs.
Other technological solutions were also considered including laser Doppler, light spectroscopy and multispectral imaging, but these detect erythema and the intensity of skin blood flow and are unable to assess the presence of a category 2 PU. 81
Therefore, recognising the need to establish a method for blinded outcome assessment and respond to the funding body’s request, a validation substudy was undertaken in order to address both scientific and practical questions.
Aims and objectives
The main aim of the photographic substudy was to assess the feasibility of using blinded expert central photographic review to quantify potential bias in the reporting of the PRESSURE 2 trial primary end point.
The primary objectives of the substudy were, therefore, to assess:
-
over-reporting of PUs of category ≥ 2
-
under-reporting of PUs of category ≥ 2.
The secondary objectives of the sub study were to assess:
-
rates of consent/potential impact on trial recruitment
-
acceptability to patients
-
compliance with photographs (i.e. whether or not the intended number of photographs were actually taken)
-
compliance with secure transfer of photographs between the research site and the CTRU
-
the quality of photographs and confidence of photographic review.
Methods
During the recruitment process, participants were asked to consent to photography of their skin sites; this was an optional consent and did not preclude participation in the trial. Participants were able to opt out of photographs at any time.
Photographic data collection
Photographs were taken on the following occasions:
-
All PUs of category ≥ 2 at first observation (baseline or follow-up) by a CRN/P (to enable the assessment of the over-reporting of PUs of category ≥ 2).
-
A random sample of 10% of patients from each centre had a detailed skin assessment and photographs taken by an independent clinical assessor who was a clinical expert in skin assessment and blinded to all CRN/P assessments, to assess the potential for under-reporting of PUs. If the patient had any PU of category ≥ 2, one would be photographed. If there was more than one PU of category ≥ 2, the photographs were to include one skin site with a PU and one skin site without. Compliance was monitored and adjusted to ensure that a 10% proportion of patients were selected.
-
All photographs had blinded, central end-point review by a panel of three clinical experts. Photographic data transfer complied with data protection legislation,82 detailed in a standard operating procedure (SOP). Data collection and transfer was monitored to assess compliance; reasons for non-compliance were recorded.
-
Information from screening logs and consent forms (opt out of consent to photographs) was used to assess the acceptability of photographs and the potential impact on trial recruitment.
Sample size
This study was required to determine the feasibility and reliability of photography against the ‘gold standard’ expert nurse clinical assessment in the assessment of PUs of category ≥ 2. There was no formal sample size calculation because of the exploratory nature of this substudy; however, it was estimated that a maximum of 1653 photographs, corresponding to 1080 PU photographs and 573 PU-free photographs, would be received and reviewed by the central review panel. 78 This was based on the original trial sample size of 2954 patients, 5.7% of whom were expected to have an existing PU33 at baseline and 20.5% of whom were expected to develop a new PU.
At trial completion, as a result of the low trial event rate and reduced trial sample size, a maximum of 918 photos were expected to be received, corresponding to 177 baseline category 2 PUs, 213 new PUs of category ≥ 2 and 528 photos from the sample of patients randomly selected for the clinical assessment (two photos per patient).
Cameras and photographs
The expertise of an independent professional medical photographer was sought for camera selection, testing and standardisation of photographs; full details have been previously published. 78
A practical consideration of the study was the cost of supplying 50 cameras to the centres taking part in the study. With the available budget, potential, affordable camera models were tested using a ColorChecker® Color Rendition Chart (X-Rite, Grand Rapids, MI, USA) to assess colour accuracy.
The same model of camera was used by all centres throughout the study to facilitate more consistent results. Different camera models, even from the same manufacturer, use different sensors, lenses and firmware and could make a difference to the colour and quality of images. The selected camera, the Canon IXUS 510 HS (Canon Incorporated, Tokyo, Japan), was then tested further to assess the best settings and shooting distances to standardise the cameras; details were incorporated into a SOP.
The SOP also included details of the CTRU safe and secure transfer process. 78 Once transferred to CTRU, the photographs were prepared for central, blinded review, which included standardisation of photograph quality using Adobe® Lightroom software (Adobe Systems Incorporated, San Jose, CA, USA) and assignation of a unique random number to the photograph to ensure that the reviewers were blind to patient identifiers. All photographs at centres were destroyed immediately after secure transfer was confirmed.
Central blinded expert review process
During the central photographic review, three clinical expert members of the TMG simultaneously assessed each image with no conferring, using the trial skin assessment classification and coding (see Box 1). Simultaneous assessment was undertaken so that the image conditions were standardised. The three reviewers were blinded to the clinical classification and blinded to each other. The data manager prepared the blinded photographs in batches for review and oversaw each review session, ensuring that blinding was maintained. The category ≥ 2 PU photographs and those from the 10% random sample (the majority of which were assessed clinically as normal, altered or category 1) were combined to maintain the blinding.
It was recognised that (1) the photographs may not have been of high quality because they had not been taken by medical photographers and (2) skin assessment is usually a combination of visual and manual examination and the diagnosis of other skin conditions requires details of the clinical history. Reviewers were therefore asked to rate their confidence in their assessment of the skin from the photograph alone on a scale of 0 (not confident at all) to 10 (very confident). 83–86
Analysis
The SAP [see Methods on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)] included analysis of the photographic data; however, changes were required as a result of the nature of the data collection. These changes were detailed in the analysis amendment log. The analysis conducted is outlined in the following sections.
Primary objectives
Over-reporting of pressure ulcers of category ≥ 2
The blinded expert central review of photographs of PUs of category ≥ 2 sent as part of the main trial (i.e. because a category 2 PU was observed) was compared with the clinical skin assessment recorded by the CRN/P, and the proportion of agreement was summarised.
Under-reporting of pressure ulcers of category ≥ 2
The blinded expert central review of photographs of skin sites sent as part of the 10% random sample was compared with the clinical skin assessment recorded by the independent clinical assessor and the proportion of agreement was summarised. The kappa and prevalence- and bias-adjusted kappa (PABAK) statistics are reported in Results, Primary objective 2: under-reporting of pressure ulcers of category ≥ 2.
In addition, clinical skin assessments undertaken by an independent clinical assessor as part of the 10% random sample were compared with the clinical skin assessments recorded by the CRN/P to assess agreement on classification of PUs of category ≥ 2. Agreement across all skin sites (i.e. on a patient basis) was summarised by mattress allocation and overall, and the kappa and PABAK statistics are reported; guidelines were used to interpret the kappa analysis. 87,88
Secondary objectives
Rates of consent/potential impact on trial recruitment
Consent to photographs at baseline was summarised based on the returned consent forms. Reasons for non-recruitment to the trial were examined to identify whether or not photography was a barrier to recruitment.
Acceptability to patients and compliance with photography
Acceptability of and compliance with photography were assessed in combination. The reasons for not taking the intended number of photographs have been summarised (see Table 21) and incorporate logistics or research team issues and patient-led issues.
Compliance with secure transfer of photographs between the research site and the Clinical Trials Research Unit
The number and details of protocol deviations relating to the transfer of photographs have been summarised (see Secondary objective 4: compliance with secure transfer of photographs between the research site and the Clinical Trial Research Unit), including any remedial actions taken, if required.
Quality of photographs and confidence of photographic review
As a result of the low number of category ≥ 2 PUs observed in the trial, and, therefore, in the random sample of patients identified to assess the under-reporting of PUs of category ≥ 2, kappa and PABAK were calculated for the combined data obtained from photographs taken from the random sample and the photographs of all PUs of category ≥ 2.
Results
A total of 2029 patients were recruited into the PRESSURE 2 trial. A total of 177 PUs of category 2 were observed among 145 patients at baseline, and 213 PUs of category ≥ 2 from 160 patients were observed to develop during the trial; therefore, a total of 390 photographs of PUs of category ≥ 2 were expected. A total of 248 photographs were received.
A total of 264 patients were selected by the 24-hour system to be part of the sample selected for assessment by the independent clinical assessor and a maximum of 528 photographs were expected (two photographs per patient). Only 167 (63.3%) patients actually had a substudy assessment completed, of whom 142 (85.0%) were reported to have had photographs taken. In total, 284 (53.8%) photographs were returned as part of the substudy from 137 (51.9%) patients.
Primary objective 1: over-reporting of pressure ulcers of category ≥ 2
A total of 248 photographs were received as part of the main trial (photography of first presentation of PUs of category ≥ 2): 103 from the APM arm and 145 from the HSFM arm. The expected numbers of photographs of PUs of category ≥ 2 were 180 in the APM arm and 210 in the HSFM arm, indicating compliance with return of photographs of 57% in the APM arm and 69% in the HSFM arm.
Blinded expert central photographic review versus clinical research nurse/registered health-care professional clinical assessment
Of the photographs returned, the overall agreement between the blinded expert central photographic review assessment and the CRN/P clinical assessment was 83.5% (207/248, 95% CI 78.9% to 88.1%), corresponding to agreement of 88.3% (91/103, 95% CI 82.1% to 94.5%) in the APM arm and 80.0% (116/145, 95% CI 73.5% to 86.5%) in the HSFM arm. This does indicate the potential for higher levels of over-reporting in the HSFM arm, although the CIs for the proportion of agreement in each group overlap. Furthermore, the levels of agreement need to be considered in conjunction with the differing return rates of photographs in the two mattress groups, as mentioned in the previous section and in Table 16, and also the diagnostic uncertainty observed in the assessment of the photographs by the three expert reviewers [i.e. in five (2.0%) cases, the assessment could not be determined and, in 226 (91.1%) cases, at least one central review assessment was in agreement with the CRN/P].
| Attribute | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Blinded central expert review assessment | |||
| PU of category ≤ 1 | 6 (10.7) | 25 (17.2) | 36 (14.5) |
| PU of category ≥ 2 | 91 (88.3) | 116 (80.0) | 207 (83.5) |
| Unable to determine | 1 (1.0) | 4 (2.8) | 5 (2.0) |
| Total number of photographs reviewed | 103 (100.0) | 145 (100.0) | 248 (100.0) |
| Did at least one blinded central expert review assessment determine a PU of category ≥ 2? | |||
| Yes | 97 (94.2) | 129 (89.0) | 226 (91.1) |
| No | 6 (5.8) | 16 (11.0) | 22 (8.9) |
| Total number of photographs reviewed | 103 (100.0) | 145 (100.0) | 248 (100.0) |
Primary objective 2: under-reporting of pressure ulcers of category ≥ 2
A total of 264 (13.0%) patients were selected for the skin verification substudy. In terms of compliance with this substudy, a total of 167 (63.3%) patients had a substudy visit completed, equating to 8.2% of the ITT population. Of the patients who had a visit, 142 (85.0%) were reported to have had photographs attempted. A final total of 284 photographs from 137 patients were assessed as part of this substudy. There were two patients with just one photograph returned, 124 patients with two photographs returned, eight patients with three photographs and three patients with four photographs (Table 17).
| Attribute | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Skin verification substudy visit conducted | |||
| Yes | 72 (59.5) | 95 (66.4) | 167 (63.3) |
| No | 49 (40.5) | 48 (33.6) | 97 (36.7) |
| Total | 121 (100.0) | 143 (100.0) | 264 (100.0) |
| Reason skin verification substudy visit not done | |||
| Missed by the independent clinical assessor | 18 (36.7) | 18 (37.5) | 36 (37.1) |
| Participant refused | 1 (2.0) | 1 (2.1) | 2 (2.1) |
| Participant too unwell | 4 (8.2) | 2 (4.2) | 6 (6.2) |
| Participant has been transferred to another eligible inpatient facility | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Participant has been discharged | 9 (18.4) | 15 (31.3) | 24 (24.7) |
| Participant has been transferred to an ineligible inpatient facility | 1 (2.0) | 1 (2.1) | 2 (2.1) |
| Participant has withdrawn from the trial | 2 (4.1) | 2 (4.2) | 4 (4.1) |
| Participant has died | 3 (6.1) | 0 (0.0) | 3 (3.1) |
| Did not receive substudy e-mail | 3 (6.1) | 3 (6.3) | 6 (6.2) |
| Lack of staff capacity | 7 (14.3) | 3 (6.3) | 10 (10.3) |
| Reason unknown | 0 (0.0) | 2 (4.2) | 2 (2.1) |
| Other reason | 0 (0.0) | 1 (2.1) | 1 (1.0) |
| Total | 49 (100.0) | 48 (100.0) | 97 (100.0) |
| If substudy assessment was conducted, were photographs attempted? | |||
| Yes | 63 (87.5) | 79 (83.2) | 142 (85.0) |
| No | 9 (12.5) | 16 (16.8) | 25 (15.0) |
| Total | 72 (100.0) | 95 (100.0) | 167 (100.0) |
| If no photographs were taken, reason | |||
| Consent for photos to be taken not obtained | 9 (100.0) | 9 (56.3) | 18 (72.0) |
| Participant no longer wants photos to be taken | 0 (0.0) | 3 (18.8) | 3 (12.0) |
| Participant does not want photos to be taken at this visit | 0 (0.0) | 1 (6.3) | 1 (4.0) |
| Not appropriate at this time | 0 (0.0) | 2 (12.5) | 2 (8.0) |
| Missing | 0 (0.0) | 1 (6.3) | 1 (4.0) |
| Total | 9 (100.0) | 16 (100.0) | 25 (100.0) |
| Number of photographs received per participant | |||
| 0 | 10 (13.9) | 20 (21.1) | 30 (18.0) |
| 1 | 2 (2.8) | 0 (0.0) | 2 (1.2) |
| 2 | 57 (79.2) | 67 (70.5) | 124 (74.3) |
| 3 | 2 (2.8) | 6 (6.3) | 8 (4.8) |
| 4 | 1 (1.4) | 2 (2.1) | 3 (1.8) |
| Total number of participants who had substudy assessment | 72 (59.5) | 95 (66.4) | 167 (63.3) |
Blinded expert central photographic review versus independent clinical assessment
Overall, there was agreement in 91.5% of cases (260/284) between the blinded expert central review and independent clinical assessor in terms of whether skin sites were healthy, altered or category 1 PU or category ≥ 2 PU: 90.5% (114/126) agreement in the APM arm and 92.4% (146/158) agreement in the HSFM arm. There were 15 PUs of category ≥ 2 assessed on the photographic blinded central expert review; of these, six were also assessed by the independent clinical assessor as a PU of category ≥ 2 and two were assessed as ‘not applicable’, whereas seven were assessed as healthy, altered or category 1 PUs (four out of eight PUs in the APM and five out of seven PUs in the HSFM arm were not classified as a category ≥ 2 PU by the clinical assessor) (Table 18). Conversely, there were 10 PUs of category ≥ 2 assessed by the independent clinical assessor and, of the four disagreements with the photographic blinded expert central review, two were classified as healthy, altered or category 1 PUs and two could not be determined.
| Independent clinical assessor | Blinded expert central photographic review, n (%) | |||||
|---|---|---|---|---|---|---|
| Healthy, altered or category 1 PU | Categories 2–4 or unstageable | Unable to determine | Overall | Kappaa | PABAKa | |
| Overall | ||||||
| Healthy, altered or category 1 PU | 254 (89.4) | 7 (2.5) | 3 (1.1) | 264 (93.0) | 0.53 | 0.93 |
| Categories 2–4 or unstageable | 2 (0.7) | 6 (2.1) | 2 (0.7) | 10 (3.5) | ||
| N/A | 4 (1.4) | 2 (0.7) | 1 (0.4) | 7 (2.5)b | ||
| Missing | 3 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Overall | 263 (92.6) | 15 (5.3) | 6 (2.1) | 284 (100.0) | ||
| APM | ||||||
| Healthy, altered or category 1 PU | 110 (87.3) | 3 (2.4) | 1 (0.8) | 114 (90.5) | 0.59 | 0.92 |
| Categories 2–4 or unstageable | 2 (1.6) | 4 (3.2) | 2 (1.6) | 8 (6.4) | ||
| N/A | 1 (0.8) | 1 (0.8) | 0 (0.0) | 2 (1.6) | ||
| Missing | 2 (1.6) | 0 (0.0) | 0 (0.0) | 2 (1.6) | ||
| Overall | 115 (91.3) | 8 (6.4) | 3 (2.4) | 126 (100.0) | ||
| HSFM | ||||||
| Healthy, altered or category 1 PU | 144 (91.1) | 4 (2.5) | 2 (1.3) | 150 (94.9) | 0.49 | 0.95 |
| Categories 2–4 or unstageable | 0 (0.0) | 2 (1.3) | 0 (0.0) | 2 (1.3) | ||
| N/A | 3 (1.9) | 1 (0.6) | 1 (0.6) | 5 (3.2) | ||
| Missing | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.6) | ||
| Overall | 148 (93.1) | 7 (4.4) | 3 (1.9) | 158 (100.0) | ||
The kappa statistic of 0.53 is in the region of ‘weak agreement’;87,88 however, this is influenced by a small proportion of PUs of category ≥ 2 reported. The PABAK statistic of 0.93 demonstrates ‘very good agreement’ of photograph assessments with clinical assessments.
All photographs: blinded expert central photographic review versus clinical assessment
If the assessment of the 248 photographs of all category 2 PUs observed by the CRN/P is combined with the assessment by the independent clinical assessor of the 284 photographs from the random sample of 10% of patients, then an overall agreement of 87.8% (467/532) between the blinded expert central photographic review and clinical assessment is observed. There were 222 PUs of category ≥ 2 assessed on the blinded expert central photographic review and, of these, 213 were also assessed by the clinical assessors (i.e. CRN/Ps and independent clinical assessors) as a PU of category ≥ 2, two as ‘not applicable’ and seven as healthy, altered or category 1 PUs. Similarly, there were a total of 258 PUs of category ≥ 2 reported by the clinical assessors, of which 38 were classified as healthy, altered or category 1 PUs and seven could not be determined through blinded expert central photographic review. The corresponding kappa statistic is 0.82 (‘very good agreement’) and PABAK is equal to 0.82, indicating that photographic assessment has ‘very good agreement’ when compared with expert clinical assessment.
Independent clinical assessor versus clinical research nurse/registered health-care professional
The skin assessment by the independent clinical assessor was compared with the assessment by the CRN/P that was closest in time. The overall agreement was observed to be 94.6% (157/166): 91.7% (66/72) agreement for patients in the APM arm and 96.8% (91/94) agreement in the HSFM arm. The kappa statistic was observed to be in the region of ‘moderate agreement’; however, this is influenced by the small proportion of PUs of category ≥ 2 that were observed. The corresponding PABAK statistic of 0.89 overall is in the region of ‘very good agreement’.
There were 12 PUs of category ≥ 2 assessed by the independent assessor and, of these, five were reported as healthy, altered or category 1 PUs by the CRN/P (Table 19). All of the PUs of category ≥ 2 reported by the independent clinical assessor, but not by the CRN/P, were in the APM arm. Furthermore, there were four skin sites that were assessed as a PU of category ≥ 2 by the research nurse that the independent assessor categorised as healthy, altered or category 1: one in the APM arm and three in the HSFM arm. These results suggest that there may be some under-reporting by both the CRN/P and the independent assessor; however, the sample size is too small to determine the level of under-reporting and to distinguish whether or not there are any differences between the arms.
| Research nurse | Independent assessor, n (%) | ||||
|---|---|---|---|---|---|
| Overall | Healthy, altered or category 1 PU | Category 2, 3, 4 or unstageable | Overall | Kappa | PABAK |
| Healthy, altered or category 1 PU | 150 (90.4) | 5 (3.0) | 155 (93.4) | 0.58 | 0.89 |
| Categories 2–4 or unstageable | 4 (2.4) | 7 (4.2) | 11 (6.6) | ||
| Overall | 154 (92.8) | 12 (7.2) | 166a (100.0) | ||
| APM | |||||
| Healthy, altered or category 1 PU | 61 (84.7) | 5 (6.9) | 66 (91.7) | 0.58 | 0.83 |
| Categories 2–4 or unstageable | 1 (1.4) | 5 (6.9) | 6 (8.3) | ||
| Overall | 62 (86.1) | 10 (13.9) | 72 (100.0) | ||
| HSFM | |||||
| Healthy, altered or category 1 PU | 89 (94.7) | 0 (0.0) | 89 (94.7) | 0.56 | 0.94 |
| Categories 2–4 or unstageable | 3 (3.2) | 2 (2.1) | 5 (5.3) | ||
| Overall | 92 (97.9) | 2 (2.1) | 94a (100.0) | ||
Secondary objective 1: rates of consent/potential impact on trial recruitment
Overall, 1711 (84.3%) patients in the ITT population consented to photography; this was similar between mattress groups, with 860 (84.6%) patients allocated to APM and 851 (84.0%) patients allocated to HSFM consenting (Table 20). There were no patients who reported the photography element as a barrier to trial participation (see Figure 2).
| Consent for photographs obtained | Trial arm, n (%) | Overall, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Yes | 860 (84.6) | 851 (84.0) | 1711 (84.3) |
| No | 155 (15.3) | 160 (15.8) | 315 (15.5) |
| Original consent form not returned | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Total | 1016 (100.0) | 1013 (100.0) | 2029 (100.0) |
Secondary objectives 2 and 3: acceptability to patients and compliance with photographs
For the photography of PUs of category ≥ 2, on 170 occasions photographs were confirmed by centres as not being attempted. The reasons for these have been summarised in Table 21. The most common reason was because consent for photographs had not been obtained (n = 56, 32.9%). The reasons were reasonably balanced between the two mattress groups, although there was a higher proportion in the HSFM arm for whom photographs were missed in error (n = 14, 14.3%) than in the APM arm (n = 7, 9.7%). In terms of the skin verification substudy, of those patients who had a substudy visit (n = 167), there were 25 (15.0%) patients for whom a photograph was not attempted; the main reason for non-completion of photographs was because consent had not been obtained (n = 18, 72.0%) (see Table 21).
| Reasons for photographs of category ≥ 2 PUs not being taken | Trial arm, n (%) | Overall, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Consent for photographs to be taken not obtained | 23 (31.9) | 33 (33.7) | 56 (32.9) |
| Participant no longer wanted photographs taken as part of this trial | 3 (4.2) | 6 (6.1) | 9 (5.3) |
| Participants did not want photographs taken at this particular visit | 15 (20.8) | 17 (17.3) | 32 (18.8) |
| Not appropriate at this time | 8 (11.1) | 8 (8.2) | 16 (9.4) |
| Pre-photography study set-up | 2 (2.8) | 4 (4.1) | 6 (3.5) |
| Camera technical problem | 3 (4.2) | 0 (0.0) | 3 (1.8) |
| Photograph not taken because of logistical problems (e.g. camera unavailable, not enough time) | 7 (9.7) | 7 (7.1) | 14 (8.2) |
| Dressing/cast in situ | 2 (2.8) | 3 (3.1) | 5 (2.9) |
| Missed in error | 7 (9.7) | 14 (14.3) | 21 (12.4) |
| Unable to reposition the patient | 0 (0.0) | 1 (1.0) | 1 (0.6) |
| Reason unknown | 1 (1.4) | 3 (3.1) | 4 (2.4) |
| Other | 1 (1.4) | 2 (2.0) | 3 (1.8) |
| Total | 72 (100.0) | 98 (100.0) | 170 (100.0) |
Secondary objective 4: compliance with secure transfer of photographs between the research site and the Clinical Trial Research Unit
There were 25 protocol deviations reported relating to photography administration. These were related to the trial general e-mail used for photography transfer (n = 11), greyscale card not being in the photograph (n = 7), incorrect timing (n = 3), photographs received from patients who had not provided initial written consent (n = 2), wrong camera used (n = 1) and camera stolen (n = 1). When transfer was conducted using the incorrect e-mail address, these were deleted from the sender’s and receiver’s e-mail accounts and re-sent using the nhs.net account. When photographs were received from patients who provided verbal agreement at the time of the photography but had refused photography during consent to study participation, these were destroyed by the sender and receiver and not included in the analysis.
Secondary objective 5: quality of photographs and confidence in photographic assessment
In general, each reviewer tended to be more confident when they assessed a photograph as healthy, altered or category 1, with reviewer 1 giving a confidence score of at least 6 in 70.5% of cases compared with 55.5% of the photographs that they assessed as a PU of category ≥ 2. Similarly, reviewer 2 had a confidence of at least 6 in 86.5% of photographs that they assessed as healthy, altered or category 1 compared with 75.6% of those they assessed as PU of category ≥ 2. Reviewer 3 also demonstrated more confidence in photographs they assessed as healthy, altered or category 1, with 79.5% being given a confidence score of at least 6 compared with 68.6% of the photographs they assessed as PU of category ≥ 2. There was a very small number of photographs for which no assessment was made.
Conclusion
Overall, the reliability of photography as a tool for blinded expert central assessment in PU trials is ‘very good’ (according to the PABAK statistics). There is potential imbalance in terms of the over-reporting of PUs between the arms, with an indication that PUs may have been more likely to be over-reported in the HSFM arm; however, the CIs for the level of agreement for each group overlap and this needs to be considered alongside a potential imbalance in terms of the return of photographs of PUs of category ≥ 2 with a lower return rate for the APM arm and the diagnostic uncertainty associated with blinded expert central photographic review.
Overall, there was ‘very good agreement’ (according to the PABAK statistics) between the independent clinical assessor and CRN/P in their clinical assessment.
Similarly, there may have been under-reporting of PUs of category ≥ 2 by the independent clinical assessor compared with the blinded expert central photographic review, but in both of these cases, the sample size was too small to assess under-reporting any further.
There were varying levels of confidence from the blinded expert reviewers of the photographs, with more confidence demonstrated in skin sites assessed as healthy, altered or category 1. In only a very small number of photographs could no assessment be made.
Chapter 6 Evaluation of a patient-reported outcome measure of health-related quality of life for use in pressure ulcer prevention trials
Background
Patient-centred care requires that interventions be evaluated in terms of patient experience, with patient-reported outcomes (PROs), including HRQoL. A widely accepted definition of HRQoL, useful for clinical trials and health services research, states that:
HRQoL is a multidimensional construct encompassing perceptions of both positive and negative aspects of dimensions, such as physical, emotional, social, and cognitive functions, as well as the negative aspects of somatic discomfort and other symptoms produced by a disease or its treatment.
Reproduced from Osoba89
Integral to this definition is that HRQoL is a subjective phenomenon, so the patient’s assessment is preferred to that of a proxy, and it is multidimensional, including core domains plus symptoms that will differ across diseases, treatments and health interventions. 89,90
Another term closely related to HRQoL is PRO. A PRO is ‘any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else’. 91 The term PRO emerged to solve the difficulty of finding a universal definition for HRQoL and related concepts. It does not tell what is being measured, only that the patient is providing the data. PROs can be symptoms (e.g. pain, fatigue), aspects of functioning (e.g. physical, psychological, social) and/or multidimensional constructs, such as HRQoL.
Pressure ulcers and interventions aimed at preventing or treating PUs can affect HRQoL in many ways, including pain,92 exudate and odour, and can compromise all areas of patient functioning. 1,93 The presence of symptoms and impaired functioning can have a distal effect on HRQoL outcomes. 94 Intensive interventions for preventing and treating PUs pose added patient burden and further affect HRQoL. 12 This additional impact on patients is related to increased care burden, prolonged rehabilitation, requirement for bed rest, and hospitalisation. 1,47 In this clinical context, evaluating PROs such as symptoms, functioning and HRQoL is particularly important and relevant, and there is enormous potential for PROs to be integral to patient management and recommendations for PUs.
The need for evaluating health-related quality of life in patients at risk of developing pressure ulcers
The possible burden of symptoms and impacts on HRQoL associated with interventions for preventing and treating PUs provide compelling arguments for incorporating the quality of patients’ lives into decisions about PU prevention and management. The PU field is reliant on health-outcome assessment to provide a strong evidence base, incorporating the patient perspective.
Methods of assessing health-related quality of life in patients with pressure ulcers
A standardised approach to assessment of HRQoL is required to reduce variation in both the way questions are asked and how patients respond. For this reason, questionnaires with standard questions about relevant issues incorporating a standard set of response options are used. These are referred to as PRO instruments or measures.
Patient-reported outcome instruments are increasingly used in clinical studies for measuring outcome variables. In this role, PRO instruments are the central dependent variables on which prevention and treatment decisions are made. They can be useful tools for evaluating health changes following interventions if they are fit for purpose and accord with international standards for rigorous measurement. 12,95 PRO instruments specific to PUs could help improve the evidence base through use in research assessing the clinical effectiveness of PU therapies, facilitate clinician–patient communication and shared decision-making, prioritise patient problems and preferences, monitor changes or outcomes of treatment and measure the performance of health-care providers and services, and could be used in clinical audit. 91,95,96
Previous work by this research team has identified PROs that are important to people with PUs,1,48 established the need for patient-reported measures of outcomes specific to PUs,97 and developed and evaluated a PRO instrument to assess PU-specific symptoms and functioning impacts (the PU-QoL instrument). 98 The PU-QoL instrument is researcher administered and comprises three symptom scales, pain (eight items), exudate (eight items) and odour (six items), and seven function scales: four physical functioning – sleep (six items), movement and mobility (nine items), daily activities (eight items) and vitality (five items); two psychological well-being: emotional well-being (15 items) and self-consciousness and appearance (seven items); and one social participation (nine items), plus a single item for itchiness and a single item for global QoL. It is intended for patients who have any category of PU. Patients rate the amount of ‘bother’ attributed ‘during the past week’ on a three-point response scale (0 = not at all to 2 = a lot). Scale scores are generated by summing items and then transforming to a 0–100 scale. High scores indicate greater patient bother. The PU-QoL instrument has been validated for use with patients with PUs and is most appropriate for people with severe PUs, as demonstrated by a lack of items to represent people with little or no bother due to PUs. Given the heterogeneity of the PU population, further work was required to ensure that the PU-QoL instrument scales met the needs of all people with PUs, including patients with superficial PUs, as well as those at risk of PU development and to test whether the PU-QoL instrument scales were responsive to change. The usefulness of a new PRO instrument is demonstrated by multiple applications in different studies and strengthened by an accumulative body of evidence to support scale measurement properties. 97
Aims
In order to enable assessment of PROs in patients receiving preventative interventions who are at risk of developing PUs, the aims of this study were to:
-
modify the PU-QoL instrument for use with patients receiving preventative interventions who are at a high risk of PU development – the PU-QoL-P
-
undertake a comprehensive evaluation of the psychometric measurement properties of the PU-QoL-P instrument to ensure that it is acceptable, reliable, valid and responsive, and suitable for use in the UK health setting.
This would provide a standardised method for evaluating patients’ self-reports of the impact of PU preventative interventions on HRQoL outcomes.
Methods
Development of the Pressure Ulcer Quality of Life – Prevention instrument
Following international PRO guidelines,91,98 the original PU-QoL instrument was modified to produce a prevention version (the PU-QoL-P instrument). A group of 20 experts, which included specialist tissue viability nurses (recruited from participating sites involved in the PURPOSE programme),37 people with past experience of having PUs (recruited via the Pressure Ulcer Research Service User Network UK)35 and PRO methodologists, were asked to review the original PU-QoL instrument. They completed a questionnaire that asked about content (e.g. how relevant and representative are the issues to people at high-risk of PU development?); if any items were confusing, difficult to understand or needed clarification; if any items were not relevant to people at risk of PUs; and if any important issues were missing.
Expert group review
Feedback from the expert group informed modifications to the PU-QoL instrument.
Item stem
The question stem was changed from ‘During the past week, how much were you bothered by these feelings because of your pressure sore(s)?’ to ‘During the past week, how much were you bothered by these feelings because of any pressure area pain, soreness or discomfort, pressure sores or treatments?’.
Content
Content that was considered missing was added: four items were added to the pain scale [including ‘feeling of altered sensation’, ‘dull ache’, ‘feeling sore’ and ‘loss of feeling (e.g. numbness or paralysis)’], one item was added to the sleep scale (‘being woken during sleep’) and a single item was developed for ‘overall HRQoL’ with response options – improved/got better, the same or worsened. Examples were also added to one item in the daily activities scale, ‘doing things that you enjoy (e.g. reading a book, watching a movie, talking on the telephone, using a computer)’, and to two items in the malaise scale, ‘feeling tired (e.g. in need of sleep or rest)’ and ‘feeling fatigued (extreme tiredness resulting from mental or physical exertion or illness)’.
The two symptom scales (exudate and odour) were considered relevant only for people with PUs; therefore, a skip question was introduced (e.g. only complete if you have a PU). Two items were removed from the daily activities scale, ‘doing shopping’ and ‘doing jobs around the house’, and ‘because of your sore’ was removed from the item ‘feeling like you have no control over your life because of your sore’ in the emotional well-being scale. The participation scale was considered irrelevant to people hospitalised, so it was also excluded. No changes were made to the movement and mobility scale, the appearance and self-consciousness scale or the single item for itchiness.
Evaluation of the Pressure Ulcer Quality of Life – Prevention instrument
To assess the psychometric properties of the PU-QoL-P instrument, a subanalysis was conducted of all participants recruited to the main PRESSURE 2 trial who had completed both the PU-QoL-P and the SF-12 instruments at baseline. This initially included all patients randomised (1 : 1) but, because of data burden, a randomised subset of participants were allocated to complete the PU-QoL-P and SF-12 and finally just the SF-12 instruments (for full details of questionnaire administration, see Chapter 2). Participants completed instruments at baseline, at weeks 1 and 3 and at 30-day final follow-up.
Patient-reported outcome instruments
The PU-QoL-P instrument was administered along with a generic measure of health status, the SF-12. 99 The SF-12 was chosen on the basis of evidence from a systematic review of PRO instruments for chronic wounds (including PUs)48 and practical issues relating to the patient population. Use of the SF-36 was considered; however, it was decided by the project team that it was too long for use with patients at a high risk of PUs (e.g. these patients are mostly elderly, highly dependent and/or with high levels of comorbidity, including acute and chronic illness). Instead, the SF-12, a short version of the SF-36, was selected to reduce respondent burden.
The SF-12 is a generic instrument that assesses health status. It includes eight domains: (1) physical functioning, (2) role-physical, (3) body pain, (4) general health, (5) energy/fatigue, (6) social functioning, (7) role-emotional and (8) mental health. A physical component summary and a mental component summary score are generated. An acute version of the SF-12 is available that incorporates a 1-week recall period, which for this condition has been found to be relevant. 100 The SF-12 takes 2 minutes to administer and has been validated for administration by researchers. Even though the SF-12 has not specifically been validated for use with people with PUs, it has wide-spread use in other chronic wounds and dermatological conditions to assess changes in health status between groups. It has been used with other chronic skin wound conditions to validate their corresponding disease-specific PRO instruments and has been validated for use with elderly people.
Statistical analyses
Detailed analysis methods have been previously reported. 101 Briefly, standard psychometric analyses were used to evaluate the PU-QoL-P scale’s data quality and targeting, scaling assumptions (within-scale validity using multitrait scaling and factor analysis), reliability, construct validity (between-scale validity exploring convergent validity and known-groups validity), and responsiveness against prespecified criteria (see Appendix 6, Table 92). 48,102 Analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA). All analyses were conducted with two-tailed tests at the 95% significance level.
Results
Evaluation of the Pressure Ulcer Quality of Life – Prevention instrument
Baseline demographics
Owing to patient questionnaire burden, and as the PU-QoL-P data were secondary to the main trial data, the frequency of questionnaire administration was reduced for all patients between August 2013 and April 2014, between May 2014 and October 2015 patients were randomised to receive one of two questionnaire packs such that 50% completed the PU-QoL-P; PU-QoL-P data collection then ceased (see Chapter 2, Data collection schedule, Treatment phase (maximum 60 days). Of the 2029 trial participants, 1455 were eligible for the substudy. Of these, 806 completed only the SF-12 at baseline, two completed only the PU-QOL-P at baseline and 30 did not complete PU-QOL-P questionnaire scale items (only descriptive questions). This gave an analysis sample (who completed both PU-QoL-P and SF-12 at baseline) of 617 participants.
The only notable difference observed between the analysis sample and the baseline SF-12 sample was that the analysis sample included more people with category 2 PUs (Table 22). The analysis sample consisted of 617 patients (45.1% male), aged between 21.9 years and 101.3 years (mean 75.7 years), of whom 141 (22.8%) had a category 1 or 2 PU and 61.4% reported the presence of pressure-related pain at baseline. The majority (99.8%) were of white ethnicity. More than half (65.6%) were from a secondary care hospital setting, with a medical condition (58.8%), and 70.7% were considered to have very limited mobility according to the Braden scale (see Table 22). 31
| Characteristic | Instrument completed at baseline | |
|---|---|---|
| PU-QoL-P and SF-12 (N = 617) | Only SF-12 (N = 806) | |
| Age (years), range (mean, SD) | 21.9–101.3 (75.7, 14.458) | 23.4–100.3 (77.6, 12.686) |
| Gender, n (%) | ||
| Male | 278 (45.1) | 368 (45.7) |
| Female | 339 (54.9) | 438 (54.3) |
| Ethnicity, n (%) | ||
| White | 609 (99.8) | 795 (98.6) |
| Non-white | 8 (1.3) | 11 (1.4) |
| Setting, n (%) | ||
| Secondary care hospital | 405 (65.6) | 554 (68.7) |
| Community care hospital | 105 (17.0) | 160 (19.9) |
| Intermediate care/rehabilitation facility | 107 (17.3) | 92 (11.4) |
| Medical condition, n (%) | ||
| Medical | 363 (58.8) | 477 (59.2) |
| Surgical | 56 (9.1) | 56 (6.9) |
| Orthopaedics and trauma | 144 (23.3) | 217 (26.9) |
| Oncology | 13 (2.1) | 10 (1.2) |
| Critical care | 2 (0.3) | 1 (0.1) |
| Rehabilitation | 1 (0.2) | 2 (0.2) |
| Neurological | 11 (1.8) | 8 (1.0) |
| Vascular | 2 (0.3) | 3 (0.4) |
| Stroke | 13 (2.1) | 22 (2.7) |
| Cardiology | 1 (0.2) | 0 (0) |
| Renal | 1 (0.2) | 0 (0) |
| Gastroenterology | 1 (0.2) | 0 (0) |
| Trauma | 0 (0) | 1 (0.1) |
| Other | 9 (1.5) | 8 (1.0) |
| Missing | 0 (0) | 1 (0.1) |
| Mattress allocation, n (%) | ||
| Alternating | 313 (50.7) | 404 (50.1) |
| Foam | 304 (49.3) | 402 (49.9) |
| Mattress type, n (%) | ||
| Static overlay | 0 (0) | 3 (0.4) |
| Foam | 351 (56.9) | 454 (56.3) |
| Static air-filled | 5 (0.8) | 2 (0.2) |
| Gel filled | 0 (0) | 1 (0.1) |
| Hybrid foam/alternating pressure | 14 (2.3) | 25 (3.1) |
| Alternating pressure | 220 (35.7) | 277 (34.4) |
| Low air loss | 15 (2.4) | 25 (3.1) |
| Hybrid alternating pressure/low air loss | 10 (1.6) | 14 (1.7) |
| Other | 1 (0.2) | 1 (0.2) |
| Missing | 1 (0.2) | 3 (0.4) |
| Category 1 PU, n (%) | ||
| Patients with category 1 PU | 80 (13.0) | 100 (12.4) |
| Missing | 8 (1.3) | 2 (0.2) |
| One category 1 PU | 48 (54.6) | 68 (66.7) |
| Two category 1 PUs | 27 (30.7) | 22 (21.6) |
| Three or more category 1 PUs | 6 (6.8) | 11 (10.8) |
| Missing | 7 (7.9) | 1 (0.9) |
| Location | ||
| Elbow | 7 (7.9) | 5 (4.9) |
| Heel/ankle | 17 (19.4) | 24 (23.5) |
| Torso | 51 (58.0) | 65 (63.8) |
| Mixed | 6 (6.8) | 7 (6.9) |
| Missing | 7 (7.9) | 1 (0.9) |
| Category 2 PU, n (%) | ||
| Patients with category 2 PU | 61 (9.9) | 39 (4.8) |
| Missing | 8 (1.3) | 3 (0.4) |
| One category 2 PU | 50 (72.5) | 36 (85.8) |
| Two category 2 PU | 9 (13.0) | 3 (7.1) |
| Three or more category 2 PU | 2 (2.9) | 0 (0) |
| Missing | 8 (11.6) | 3 (7.1) |
| Location | ||
| Elbow | 2 (2.9) | 4 (9.5) |
| Heel/ankle | 7 (10.1) | 9 (21.4) |
| Torso | 49 (71.0) | 26 (61.9) |
| Mixed | 3 (4.4) | 0 (0) |
| Missing | 8 (11.6) | 3 (7.2) |
| Presence of pressure-related pain (yes), n (%) | 379 (61.4) | 499 (61.9) |
| Braden score | ||
| Total score range | 8–23 | 9–22 |
| Completely limited (6–11 points), n (%) | 17 (2.8) | 5 (0.6) |
| Very limited (12–17 points), n (%) | 436 (70.7) | 670 (83.1) |
| No/slight impairment (18–23 points), n (%) | 164 (26.6) | 131 (16.3) |
Scale-to-sample targeting and data quality
Scale scores spanned the entire scale ranges for all scales apart from exudate (0–86) and odour (0–25) scales. However, mean scale scores were < 37, with all scales exceeding the 20% criterion for floor effects (see Appendix 6, Table 93). This was expected given that the sample was predominantly at a high risk of PU development, with few people with PUs at baseline. Scale scores were computable for > 70% of respondents (range 73.8–100.0%) (see Appendix 6, Table 93). Targeting and data quality were also checked for the SF-12 measure and met criteria thresholds (see Appendix 6, Table 94).
Reliability
Internal consistency reliability was high with all scale Cronbach coefficient alphas of > 0.795 (range 0.795–0.970) (Table 23). During the analysis, items ‘putrid smell’ and ‘sickening smell’ were removed from the odour scale because they had zero variance; therefore, the results are based on a four- not six-item scale. No participants in the sample had severe PUs at baseline and these two items assess smell associated with severe PUs.
| PU-QoL-P scale (n items) | Internal consistency | IIC | Scaling assumptions: corrected ITCa | ||
|---|---|---|---|---|---|
| n | Cronbach’s alpha | Mean | Range | ||
| Pain (12) | 617 | 0.864 | 0.355 | 0.128 to 0.713 | 0.364 to 0.642 |
| Exudate (8) | 60 | 0.860 | 0.451 | –0.024 to 0.865 | 0.337 to 0.824 |
| Odour (4) | 61 | 0.795 | 0.492 | –0.017 to 1.000 | –0.017 to 0.893 |
| Sleep (7) | 333 | 0.937 | 0.682 | 0.540 to 0.827 | 0.753 to 0.871 |
| Movement and mobility (9) | 177 | 0.963 | 0.744 | 0.593 to 0.928 | 0.803 to 0.909 |
| Daily activities (6) | 186 | 0.937 | 0.705 | 0.476 to 0.900 | 0.581 to 0.914 |
| Malaise (5) | 215 | 0.915 | 0.680 | 0.559 to 0.844 | 0.669 to 0.846 |
| Emotional well-being (15) | 220 | 0.970 | 0.681 | 0.447 to 0.900 | 0.630 to 0.869 |
| Self-consciousness and appearance (7) | 305 | 0.921 | 0.645 | 0.532 to 0.786 | 0.695 to 0.818 |
Factor analysis
An exploratory factor analysis was performed on items within the six function scales separately from the symptom items because there were fewer than two people, at least one of the variables had zero variance, there was only one variable in the analysis or correlation coefficients could not be computed for all pairs of variables.
The suitability of the data for factor analysis was assessed. The Kaiser–Meyer–Olkin value was 0.530, which is slightly below the recommended value of ≥ 0.6, but the Bartlett’s test of sphericity reached statistical significance (χ2 = 10159.415; p < 0.001). Inspection of the correlation matrix revealed the presence of many correlation coefficients of > 0.30 (all but one item), suggesting that the factor analysis results could be considered. Five factors were revealed with eigenvalues exceeding 1, explaining 26.52, 4.24, 2.31, 1.63 and 1.36 of the variance, respectively (see Appendix 6, Table 95). The five-factor solution explained a total of 73.59% of the variance. The factor analysis mostly supported the six-function scale structure.
Within-scale validity
Scaling assumptions were satisfied (see Table 24). The mean inter-item correlations (IICs) for all scales ranged from 0.355 to 0.744. All item–own-scale correlations were moderate to high [inter-total correlation (ITC), all > 0.45] for the six function scales, but not the three symptom scales. The corrected ITCs were > 0.30 (range 0.337–0.803), satisfying the recommended criterion (i.e. > 0.3), except for the odour scale (corrected ITC range 0.02–0.89). This is not surprising given that symptoms are associated with PUs and few people had severe PUs in the sample.
Between-scale validity
Correlations between PU-QoL-P and SF-12 scales were generally low to moderate (see Appendix 6, Table 96), suggesting that PU-QoL-P scales provide distinction constructs (i.e. disease-specific outcomes) from those measured by the SF-12. Convergent validity was confirmed by significant correlations between hypothesised scales as expected. As predicted, the PU-QoL-P mobility scale correlated significantly with the SF-12 physical function and role-physical scales. In addition, as expected, the PU-QoL-P sleep scale correlated significantly with the SF-12 vitality scale, but, unexpectedly, the malaise scale did not. The PU-QoL-P emotional well-being and self-consciousness/appearance scales correlated significantly with the SF-12 role-emotional and mental health scales. The PU-QoL-P pain scale correlated significantly with the SF-12 pain scale.
Known-groups validity
Presence of category 2 pressure ulcer (no vs. yes category 2 pressure ulcer at baseline)
Known-group comparisons were not found to be statistically significant for the group with no category 2 PUs at baseline compared with the group that had category 2 PUs at baseline. However, small to moderate effect size values were observed for all scales (see Appendix 6, Table 97).
Braden score (completely limited vs. no/slight impairment)
As expected, significant differences were found between completely impaired (M = 49.38, SD = 44.6) and no/slightly impaired (M = 28.72, SD = 33.0) groups for the mobility scale (p = 0.01), and the effect size was moderate (0.6). Significant differences were also found between completely impaired (M = 35.56, SD = 43.2) and no/slightly impaired (M = 14.14, SD = 26.5) groups for the daily activities scale (p = 0.01), and the effect size was moderate (0.7). All mean scores were higher for the completely limited group than for the no/slightly impaired group in all six PU-QoL-P function scales (see Appendix 6, Table 98).
Exploratory known groups included pressure ulcer location (torso vs. limb sites)
Higher mean scores were observed in the three symptom and the mobility scales in people with torso PUs than in those with limb PUs; effect sizes were small (see Appendix 6, Table 99). However, those with limb PUs reported higher (or worse) mean scores in the sleep, daily activities, malaise, emotional well-being and self-consciousness scales than those who had torso PUs.
It is important to note that, for all known groups, the samples were small (range 2–31 participants); therefore, known-groups results are considered preliminary.
Responsiveness to change
In participants who had a category 1 or 2 PU at baseline that healed by visit 30, differences in PU-QoL-P mean scale scores from baseline to visit 30 were statistically significant for the pain, sleep, malaise, emotional well-being and appearance/self-consciousness scales, but not for the two physical function scales (Table 24). All scales showed higher mean scores at baseline in participants who had a category 1 or 2 PU than those who no longer had a PU at visit 30 (mean change range 4.337–18.569).
| Scales (n items) | n | Time points, mean score (SD) | Mean change | Effect size | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Baseline | Visit 30 | ||||||
| Pain (12) | 31 | 27.57 (25.553) | 9.001 (16.927) | 18.569 | 0.86 | 9.637 to 27.493 | < 0.001 |
| Sleep (7) | 29 | 27.82 (32.804) | 14.03 (23.277) | 13.790 | 0.48 | 1.655 to 25.899 | 0.027 |
| Movement and mobility (9) | 21 | 37.86 (36.769) | 21.17 (33.085) | 16.688 | 0.48 | –7.811 to 41.179 | 0.171 |
| Daily activities (6) | 25 | 13.67 (28.956) | 9.333 (24.244) | 4.337 | 0.16 | –10.069 to 18.736 | 0.540 |
| Malaise (5) | 23 | 21.09 (37.049) | 3.913 (13.731) | 17.177 | 0.61 | 3.705 to 30.642 | 0.015 |
| Emotional well-being (15) | 30 | 10.17 (20.837) | 0.476 (2.608) | 9.694 | 0.65 | 1.906 to 17.475 | 0.016 |
| Self-consciousness and appearance (7) | 28 | 8.67 (19.668) | 0.255 (1.350) | 8.415 | 0.60 | 0.803 to 16.034 | 0.032 |
In participants who did not have a category 1 or 2 PU at baseline, but who developed one by visit 30, movement, activities, malaise and self-consciousness scale scores were higher at baseline for people with no PU than for people who developed a PU by visit 30 (Table 25), but these results were not statistically significant. This finding may, in part, be because this sample was acutely ill at baseline (e.g. immobile and unwell), placing them at risk of PUs and, consequently, contributing to the PU developing. All other scale scores were higher at visit 30 than at baseline, suggesting that pain, sleep and emotional well-being is worse in participants with a PU than in those without a PU (see Table 24). These findings are preliminary owing to the small sample sizes.
| Scales (n items) | n | Time points, mean score (SD) | Mean change | Effect size | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Baseline | Visit 30 | ||||||
| Pain (8) | 11 | 30.37 (27.522) | 35.455 (34.676) | –5.085 | –0.16 | –22.166 to 12.000 | 0.522 |
| Sleep (6) | 9 | 17.26 (23.487) | 26.243 (30.829) | –8.983 | –0.33 | –32.791 to 14.828 | 0.410 |
| Movement and mobility (9) | 7 | 61.79 (48.055) | 48.866 (44.792) | 12.924 | 0.28 | –3.955 to 29.806 | 0.110 |
| Daily activities (8) | 7 | 9.64 (16.610) | 3.5714 (9.449) | 6.069 | 0.45 | –4.596 to 16.739 | 0.213 |
| Malaise (5) | 7 | 13.21 (24.440) | 6.548 (12.697) | 6.662 | 0.34 | –8.416 to 21.749 | 0.321 |
| Emotional well-being (15) | 11 | 11.31 (21.949) | 18.375 (31.309) | –7.065 | –0.26 | –24.106 to 9.985 | 0.378 |
| Self-consciousness and appearance (7) | 11 | 4.24 (9.898) | 2.597 (8.615) | 1.643 | 0.18 | –7.771 to 11.061 | 0.705 |
Post hoc analyses
The results from the psychometric analysis suggested modifications that could be made to four of the PU-QoL-P instrument’s scales. As a result, the following four changes were made: (1) ‘appetite’ was removed from the malaise scale, (2) ‘intimacy’ was removed from the daily activities scale, (3) ‘helpless’ was removed from the self-consciousness scale and added to the emotional wellbeing scale and (4) ‘people treat me differently’ was removed from the emotional well-being scale and added to the self-consciousness scale. These changes were considered to make sense conceptually, and reanalysis of the psychometric properties supported these modifications. Specifically, following the modifications, the internal consistency reliability and within-scale construct validity were retained in all four scales, with the daily activities, malaise and emotional well-being scales’ values for Cronbach’s alpha (range 0.914–0.971 for all four modified scales), IICs (all > 0.502) and corrected ITTs increasing (all > 0.681) (see Appendix 6, Table 100). Although, for the self-consciousness scale, the Cronbach alpha decreased marginally from 0.921 to 0.914, mean IICs decreased from 0.645 to 0.628 (range from 0.532–0.786 to 0.502–0.795) and corrected ITCs decreased from 0.695–0.818 to 0.681–0.812; all values remained within acceptable ranges (see Appendix 6, Table 100). Convergent validity results were strengthened following the modifications. The PU-QoL-P daily activity scale was significantly correlated with the SF-12 physical functioning and role-physical scales, both the PU-QoL-P psychological scales were significantly correlated with the SF-12 role-emotional and mental health scales and the PU-QoL-P malaise scale was now significantly correlated with the SF-12 vitality scale (see Appendix 6, Table 101).
The final Pressure Ulcer Quality of Life – Prevention instrument
The final PU-QoL-P instrument is a researcher-administered PRO instrument, comprising three symptom scales [pain (12 items), exudate (8 items) and odour (6 items)]; six function scales: four physical functioning [sleep (7 items), movement and mobility (9 items), daily activities (5 items) and malaise (4 items)] and two psychological well-being [emotional well-being (15 items) and self-consciousness and appearance (7 items)]; and three single items for itchiness, appetite and global QoL. Patients rate the amount of ‘bother’ attributed ‘during the past week’ on a three-point response scale (e.g. 0 = not at all to 2 = a lot). Scale scores are generated by summing items and then transforming to a 0–100 scale. High scores indicate greater patient bother. The PU-QoL-P instrument is intended for interview administration, following a user manual, but could be self-completed by patients, depending on their preference and ability. It is suitable for use with any adults at a high risk of PU development receiving preventative interventions in the UK acute and community health-care settings. Scales can be selected depending on the nature of the research. For example, the exudate and odour scales are not intended for people at risk of PU development or with superficial category 1 PUs. Electronically defined ‘skip’ questions have been added to assist in selecting scales relevant to each individual’s circumstance or the exudate and odour scales could be excluded in future prevention trials.
Discussion
The PU field requires a strong evidence base that incorporates assessment of PROs. To fully capture and quantify patients’ perspectives, appropriately developed and validated PRO instruments are required. The PU-QoL-P instrument mostly satisfies criteria for reliability, validity and responsiveness in line with recommended Food and Drug Administration (FDA) guidelines for PRO instruments for use in clinical trials. 91 The ITCs, alpha coefficient and homogeneity coefficient (IIC mean and range) provide evidence towards the reliability and internal construct validity of the PU-QoL-P scales. The results of the factor analysis mostly supported the use of the items, as hypothesised, into six function scales, as was suggested by the results from the original analysis. Some modifications were made to four scales, which were supported by the retrospective analyses, and the changes made are considered conceptually reasonable. However, as changes were made to the scales, some might argue that a further set of data should be collected for further validation purposes. No one test confirms validity; rather, validation of a PRO instrument is an ongoing process, with the accumulation of clinical validation data building a case for a particular instrument functioning effectively in a particular population for a specific purpose. 103
These findings contribute evidence towards support that people with category 2 PUs experience worse symptoms and functioning outcomes than those without PUs. People who are also physically limited and have PUs experience worse mobility outcomes and ability to participate in daily activities than those who have no or only slight physical impairment. PUs are often a secondary comorbidity and a consequence of the primary condition that a patient may be experiencing (e.g. immobility due to prolonged bed rest following extensive surgery). PUs contribute additional impairment in physical functioning outcomes beyond those experienced from other comorbidities. Furthermore, the exploratory hypothesis testing suggests that patients with torso PUs experience worse symptoms and more mobility problems, whereas patients with limb PUs have more problems with sleep quality, daily activities and malaise, have lower emotional well-being and feel more self-consciousness.
Owing to the small sample sizes in the hypothesised known groups, definitive conclusions cannot be made. However, trends were observed in scores in the right direction and, in some scales, moderate to high effect sizes, even though some were not statistically significant. A small sample size affects the standard error, so large CIs might be expected; however, sample size does not affect means, SDs or effect size. Therefore, effect sizes are still relevant and informative, even if non-significant correlations are observed, which, in this case, may be attributed to sample sizes being too small to detect significant differences.
A limitation of the study was scale-to-sample targeting: mean scores were below scale mid-points and all scales exceeded the 10% criterion for floor effects. However, given that it was intended to recruit an at-risk population (i.e. few people had category 1 or 2 PUs at baseline, and none had a category ≥ 3 PU), this finding is expected. The floor effects indicate more homogeneity in the sample than is representative of the general PU population. However, this study sample is representative of a high-risk PU population, who may be experiencing pressure area-related pain but do not experience symptoms associated with severe PUs such as exudate and odour, and the PU-QoL-P version is intended for prevention trials.
The above limitations do not preclude use of the PU-QoL-P instrument. PU-QoL-P scales can be included as one outcome measure, among others, for group comparisons in future PU prevention research (e.g. clinical trials). Work is under way to develop a short form and to test its clinical utility for use in clinical practice. As the PU-QoL-P was developed and evaluated in the UK, the validity and reliability are characteristics of the instrument for a specific population (i.e. UK nationals). A language translation or cross-cultural adaption may be required to ensure that the PU-QoL-P is appropriate for cultures, languages and ethnic groups outside the UK (see the PU-QoL-P instrument website104 for guidance on language translation and cross-cultural adaptation processes). Further research is also needed to investigate self-complete administration mode, and the development of proxy measures and language translations given the prevalence of cognitively impaired patients with PUs. 33,52 Finally, given the small sample sizes for the known-groups and responsiveness analyses, these analyses could be repeated in larger samples. The process of modifying a newly developed PRO instrument is part of an evolving, ongoing measurement process intended to strengthen the hypothesised conceptual relationships with empiric evidence. 97 The usefulness of new measures is therefore demonstrated by multiple applications in different studies (accumulative body of evidence to support scale measurement properties).
Conclusions
The PU-QoL-P instrument provides a means of comprehensively assessing of PU-specific PROs and for quantifying the benefits and harms of PU preventative interventions from a patient’s perspective, thus far lacking in the area. PRO assessment needs to become more commonplace in the PU field so that the goal of PU prevention and management can be to enhance and maintain the HRQoL of people at risk of or with PUs. The PU-QoL-P is a tool with which to evaluate whether or not PU preventative interventions and the health care given achieve this, outcomes that are ultimately best judged by patients themselves.
Chapter 7 Discussion
Summary of findings
Clinical effectiveness
Overall, only 7.9% of participants recruited to the PRESSURE 2 trial developed one or more new category ≥ 2 PUs and there was no evidence of a difference between mattress groups (absolute difference 2.0%; APM, 6.9% vs. HSFM, 8.9%). Similarly, for all other end points there was no evidence of a difference between mattress groups, with the exception of the development of new category ≥ 2 PUs in the treatment phase sensitivity analysis (absolute difference 2.6%; APM, 5.2% vs. HSFM 7.8% in the ITT population).
It is not clear why the treatment phase benefit seen in the APM arm was not maintained, but it appears that the main benefit of APM over HSFM was a delayed onset of new PUs during the treatment phase (median time to first PU was 18 days in the APM arm vs. 12 days in the HSFM arm), which was not sustained.
The PRESSURE 2 trial is the largest RCT undertaken worldwide and the results are consistent with the study by Vanderwee et al. ,30 who reported new category 2 PU incidence rates of 15.3% for APM and 15.6% for HSFM plus turning. The PRESSURE 2 trial provides ≈80% of the data for the comparison of APMs with HSFMs, with 160 events in 2029 patients, whereas Vanderwee et al. 30 provided data on 69 events in 447 patients. The increased available evidence suggests no difference between mattress types; this needs to be confirmed through meta-analysis.
Cost-effectiveness
Within the health economics analyses, the ITT analyses found APM to be the most cost-effective strategy. However the results were not robust to the sensitivity analyses. In particular, the complete cases found HSFM to be cost-effective. In addition, the difference between the QALY gains of the two arms was very small (≈0.001) equating to around half a quality-adjusted life-day in both the within-trial and the lifetime model analyses. Similarly, the difference between the costs accrued in each arm was relatively modest, with few cost categories showing a statistically significant difference between the arms (see Appendix 5, Tables 80–82). Of interest, the difference in cost between the two mattresses was statistically significant, with the APM attracting a higher mean cost (£28.80) than the HSFM (£1.05) (p < 0.001); however, these costs represented only a small proportion of the total mean costs. In fact, the cost driver for both arms was, as anticipated, inpatient stays and no statistical difference was observed in these costs between the two arms (mean cost £2810.08 for APM vs. £2888.68 for HSFM; p = 0.54) (see Appendix 5, Table 80).
Photography substudy
In relation to the photography substudy, results indicate very good levels of agreement between clinical assessment by CRN/Ps and the expert photographic review and between the independent clinical assessor and expert photographic review. Overall, 84.3% of participants consented at trial recruitment to photography and only small numbers at follow-up subsequently refused.
Pressure Ulcer – Quality of Life – Prevention
The PU-QoL-P instrument substudy suggests that the PU-QoL-P mostly satisfies criteria for reliability, validity and responsiveness, in line with recommended FDA guidelines for PRO instruments for use in clinical trials,91 with modifications recommended. The QoL findings contribute evidence towards support that people with category 2 PUs experience worse symptoms and functioning outcomes, including worse mobility and ability to participate in daily activities, than those without PUs. The exploratory analysis suggested that patients with torso PUs experience worse symptoms and more mobility problems, whereas patients with limb PUs have more problems with sleep quality, daily activities and malaise, have lower emotional well-being and feel more self-conscious.
Key clinical and methodological issues are discussed in the following sections.
Clinical interpretation
Interpretation of the results is complex, in terms of implications for clinical practice, for a number of reasons. First, the event rate was much lower than expected and all differences observed were small. A key question for practitioners is whether or not the overall group differences of 2% in the primary end point and the 2.6% group difference in the treatment phase sensitivity analysis is clinically important when PU incidence rates are low (7.9% and 6.5%, respectively). The difference equates to a number needed to treat of 50 patients for the primary end point and 38 for the treatment phase end point. That means that, in the treatment phase, for every 38 patients allocated an APM, one patient will benefit.
Second, > 10% of participants allocated to APM either refused to change to the APM after randomisation or requested a mattress change because of comfort (118/1017, 11.6%) compared with 3.9% (40/1013) of participants allocated to HSFM. After allocation of mattress, 22.1% (49/222) of participants changed from APM for the first time to aid movement/rehabilitation (patient or ward led) compared with 2.3% (5/220) of participants who changed from HSFM for the first time for the same reason.
Third, data suggest that frequency of repositioning reduced over time and, as the treatment phase progressed, participants allocated to APMs were repositioned less frequently than participants allocated to HSFM (see Figure 9).
A key question by members of PURSUN during a results interpretation event was ‘given the low incidence and the disadvantages of APMs in terms of impact on independent movement and comfort, who will benefit most from APMs?’. The moderator analysis (see Figures 5–8) was included in order to explore the potential benefit of each mattress on patients with known PU risk factors. The analysis suggests that patients who benefit most from APM versus HSFM appear to be those who are completely immobile, have altered skin or PU of category 1 at baseline, have a nutritional problem and those who lack capacity and participate through consultee agreement. In a PU conceptual framework, developed on the basis of epidemiological evidence, immobility, skin condition and tissue perfusion are categorised as direct causal factors, whereas nutritional deficit is classified as an indirect causal factor that impacts on skin condition and tissue perfusion. 9 Lack of capacity is not specified in the conceptual framework but was discussed during its development45 because it was hypothesised that it is a surrogate marker for both direct (immobility) and indirect (sensory perception and response) causal factors affecting repositioning self-care, stimulus to move and ‘compliance’ with repositioning. 45 Direct application of this analysis to practice must be undertaken with caution as it was exploratory, but the results suggest that the impact of altered and category 1 skin status, complete immobility, nutritional deficits and the vulnerability afforded by lack of capacity may be modifiable as risk factors through the use of the APMs, providing marginal gains over HSFMs.
Low event rate
The overall event rate of category ≥ 2 PUs of 7.9% for this participant population was considerably lower than the 2011 grant application sample size estimate, based on a PU incidence rate of 20.5% (APM 18% vs. HSFM 23%), determined by consideration of a range of studies27,105 and two relatively contemporary research studies involving this patient population. 33,38 PRESSURE 133 recruited inpatients between 2001 and 2004 and was a RCT comparing APM overlays with APM replacements (i.e. all participants received an APM) and the incidence of new category 2 PUs was 10.5% overall and 17.7% in acute patients. The pain cohort study38 recruited acutely ill hospital and community patients between 2009 and 2011 and reported that 25.2% of patients developed a new category 2 PU.
A key question regarding the lower than anticipated PU incidence rate is whether this was because the patient population was ‘low risk’ owing to issues around selection bias and equipoise and/or whether it reflects general improvements in clinical practice resulting from national-level PU improvement targets.
In terms of a low-risk population, the screened and randomised populations were similar in respect of age, setting, gender and ethnicity (see Appendix 4, Tables 68 and 69). Of the 15,277 patients screened, the most unwell 1623 (10.6%) were excluded (i.e. it was ethically inappropriate or they felt poorly or unwell); however, the patient population were all acute inpatient admissions, characterised by old age (mean 78.0 years, median 81 years), high levels of pre- and post-randomisation falls (44.8% and 15.3%, respectively) and being at risk on the Braden Scale31 (92.6%) and the PURPOSE-T (98.7%). In addition, adverse skin status at baseline included 11.6% of participants with an existing category 1 PU, 7.1% with a category 2 PU and 53.4% with pain on a healthy, altered or category 1 skin site.
In comparison with the pain cohort population, who had a PU event rate of 25.2%, the study populations were similar in age (pain cohort mean age 77.3 years, median age 80 years). The PRESSURE 2 trial had a higher proportion of participants assessed as being at risk on the Braden Scale31 (pain cohort 72.9%), but a considerably lower proportion of participants with an adverse skin status at baseline (pain cohort 35.5% with an existing category 1 PU, 23.3% with a category 2 PU, 4.1% with a category ≥ 3 PU and 77.1% with pain on a healthy, altered or category 1 skin site). 38
In comparison with the PRESSURE 1 trial combined acute and elective participant population (who had an overall PU incidence of 10.5%), the PRESSURE 2 trial recruited a slightly older population (PRESSURE 1 trial: mean age 75.2 years, median age 76 years), a slightly lower proportion of participants with a category 1 PU at baseline (PRESSURE 1 trial: 15.6%) but a higher proportion of participants with a category 2 PU (PRESSURE 1 trial: 5.7%). 33 A key difference between the two trials is that the PRESSURE 1 trial recruited participants within 24 hours of admission, whereas an unexpected population characteristic in the PRESSURE 2 trial was that the pre-trial duration of hospital stay was a mean of 13 days (median 7 days, range 0–388 days). As the incidence of PU development is highest in the first 2 weeks following admission, the stage at which participants were recruited may have affected PU event rates. However, it is also noteworthy that, in contrast to other prevention trials,27,30 in the PRESSURE 2 trial a small but clinically important minority of 32 (1.6%) participants developed 40 new category 3 PUs.
In terms of mental capacity, screening data from the PRESSURE 1 trial and the pain cohort suggests that the screened population in this study may not be representative of patients at high risk of developing PUs. The PRESSURE 1 trial reported that 39.7% of participants were unable to provide informed consent and in the pain cohort, 24.1% of the screened population were reported to lack capacity, compared with 18.7% of those eligible for the PRESSURE 2 trial. 33,38 However, the actual proportion of participants recruited through consultee agreement was higher in the PRESSURE 2 trial (15.9%) than in the previous study in which consultee agreement was obtained (PRESSURE 1 trial: 4.4%33,52). The inclusion of participants who lack capacity is important, and it was argued that, as there is an element of self-care in repositioning, it was important to include participants who lacked capacity to ensure generalisability. This was evidenced in the results that highlight the poorer outcomes for participants who lack capacity than for participants with capacity in terms of development of PUs of category ≥ 1 (see Table 10) and category ≥ 3 (see Table 11) and for the development of PUs of category ≥ 2 in those allocated to HSFM (see Table 8 and Figures 5–8). It is also noteworthy that participants who provided witnessed verbal consent had a higher incidence of PUs of categories 1, 2 and 3 than those who provided written, informed consent (see Tables 8, 10 and 11); it is suggested that this is also a surrogate indicator of reduced mobility.
From an equipoise perspective, although there were similar numbers of screened patients allocated to APMs and HSFM by ward staff (n = 7640, 50.0%, and n = 7462, 48.8%, respectively), more patients were excluded as the patient or ward staff did not want to change from their existing APM. There was a greater imbalance in the randomised population, with pre-randomisation allocations by ward staff of 42.8% to APM and 56.6% to HSFM (see Appendix 4, Table 68). However, this is only marginally lower than the 48.5% APM allocation by ward staff in the pain cohort participant population.
In terms of impact of general improvements in practice resulting from national targets, these are difficult to elicit from national monitoring such as the Safety Thermometer,106 because of problems of data accuracy4 and difficulties in interpretation of adverse event data. 107,108 However, a key difference between the PRESSURE 2 trial event rate and the pain cohort is that, not only are there fewer participants with an adverse skin status at baseline, but the proportion of participants with a category 1 PU at baseline who subsequently developed a new category 2 PU was much lower in the PRESSURE 2 trial (11.4%) than in the pain cohort study (36.3%).
Overall, the conclusion drawn is that the patient population was indeed characterised by high risk and that the low incidence rate was a result of the prevailing improvements in PU prevention care in the inpatient settings that participated in the trial.
Sample size
A maximum of 588 events (patients developing a new category ≥ 2 PU), corresponding to 2954 participants, were required for the study to have 90% power to detect a difference of 5% in the incidence of category ≥ 2 PUs between APM and HSFM, assuming an incidence rate of 18% on APM and 23% on HSFM, two-sided significance level of 5% and accounting for 6% loss to follow-up. The 5% absolute difference translated to a 24.4% relative difference (absolute difference of 5%/overall event rate of 20.5%) and a hazard ratio of 0.759.
The trial recruited participants more slowly than anticipated originally, leading to a smaller sample size than the planned maximum sample size (2030 compared with 2954), and was therefore underpowered for detecting an absolute difference of 5% at an overall event rate of 20.5%. In addition, the overall event rate was also far lower than originally anticipated (7.9% compared with 20.5%).
Two extension request scenarios were submitted. The first was a fully costed extension request of 14 months to continue recruitment to demonstrate superiority of either mattress for the revised clinically relevant difference of 3.3%, centred on an event rate of 10% with at least 80% power, and to estimate the treatment effect under the assumption of futility with a clinically meaningful improvement in precision. The second was a 6-month extension (requiring no additional HTA programme funding) with the expectation that at least 1996 patients would be recruited and that, although underpowered, it would improve the precision of the estimated treatment effect by at least 3% compared with the trial stopping at the originally planned timescale. The HTA programme approved the 6-month (no additional funding) extension rather than the fully costed extension on the basis that the latter was not seen to be value for money, taking into account the low improvement in precision.
After the interim analysis, the TMG was informed that the event rate was lower than expected and a range of scenarios were considered to inform further discussions with the oversight committees. The TMG noted that the difference relative to the overall event rate was also important when considering the event rates rather than just the absolute difference. Scenarios of the overall event rate centred at 5%, 10% and 15% were considered. The TMG came to a consensus that a relative difference of 33.3% centred on an overall event rate of 10%, corresponding to an absolute difference of 3.3%, was considered to be clinically meaningful and the preferred minimum clinically relevant difference. If the overall event rate was 5%, a relative difference of 50%, corresponding to an absolute difference of 2.5%, was considered to be clinically meaningful and the preferred minimum clinically relevant difference. The TMG also concluded that, with an overall event rate of 5%, a relative difference as small as 40%, corresponding to an absolute difference of 2%, was not considered to be of clinical relevance and that, at these event rates, the decision over which type of mattress to manage a patient on would be based on patient choice, cost and clinical judgement, including factors such as rehabilitation need and self-care considerations.
A HR of 0.76 was observed in the primary analysis of the primary end point, which is in line with the expected HR in the original sample size. However, as the corresponding relative difference was 25.3%, centred on the overall event rate of 7.9%, the a priori discussions with the TMG suggest that this is not considered to be a clinically relevant difference.
In the treatment phase sensitivity analysis, the estimate of the HR was 0.66; the corresponding relative difference of 40%, centred on an overall event rate of 6.5%, may be considered clinically relevant. However in light of the a priori discussion with the TMG, as the overall event rate is low, mattress provision should also be guided by patient choice, clinical opinion and consideration of risk factors for which APMs may have a potential benefit, for example patients with altered or category 1 PU skin, patients who are completely immobile or lack capacity to consent or those with nutritional problems.
Risk factors
The fact that risk factors were found to be predictive of PU development is in line with previous work and adds to the growing body of evidence that a key risk factor in immobile patients is skin status. 8,18 In all adjusted analyses, the presence of a category 1 or 2 PU at baseline was statistically significant in the model (see Tables 8, 10 and 11).
Other factors important in determining outcome in the adjusted analysis for both the category 1 and 3 end points included type of consent and the presence of pain on a healthy, altered (or category 1) skin site. In addition, setting was found to be important in the category 1 model and presence of condition affecting the peripheral circulation in the category 3 model (see Tables 10 and 11).
Usual care
A strength of the study is that data were collected in order that standard care (in terms of efforts made to minimise exposure to mechanical load) in the study population could be characterised. This is the first mattress RCT to provide a detailed description of standard care provision for the patient population27 and will support interpretation and wider application of study findings.
It is noteworthy that 99.6% of participants had an electric profiling bed, as this was a requirement of the trial, and in a population at baseline for which 42.8% of participants were allocated an APM, approximately one in six participants were repositioning more frequently than every 2 hours, whereas 1 in 20 participants were repositioned less frequently than every 6 hours (which is not in line with good practice recommendations). 8,12 Moreover, although one in four participants were confined to bed, one in five of the study population sat out in a chair for > 8 hours a day and 27.6% of those sitting out had a standard chair with no specialist cushion. Adjuvant devices (such as heel off-loading devices and dressings) were used in one in seven participants. A limitation of the data is that the time durations for repositioning and sitting were estimated from a combination of information including patient self-report, staff report, health-care records and observation, and the reliability has not been assessed.
Standard care interventions were also recorded during the treatment phase of the study and a mediator analysis was planned, but could not be undertaken because of the lack of available methods when there are competing risks (see Chapter 2, Final analysis; Mediator analysis). It is noteworthy that participants in the APM arm appeared to be repositioned more frequently than those in the HSFM arm; these data will be explored in more detail in planned work for the future and inform the development of a realist evaluation109 to further inform trial interpretation.
A limitation of the trial is that the discharge date for all trial participants was not collected, as this was recorded on the health resource utilisation questionnaire, and, in line with other inpatient trials,27 post-discharge mattress provision was not recorded. The large proportion of mattress changes during ward transfers and the incidence of PUs from the end of treatment to 30-day final follow-up does indicate, however, that work is required to improve continuity of care as patients transition through services110 and further supports the appropriateness of the treatment phase sensitivity analysis.
Compliance
Overall, mattress compliance was good, with 81.5% of participants on their allocated mattress within 2 days of randomisation for each treatment group. The median time spent on the allocated mattress was high and only 9.3% (n = 94) of those randomised to APM and 10.9% (n = 110) of those randomised to HSFM did not receive their allocated mattress at any point during the treatment phase. As a result, compliance was better than expected compared with the PRESSURE 1 trial. 33 Delays in randomised mattress provision were mainly logistical and, when participants were not allocated their mattress on the day of randomisation, they were, typically, on the alternative mattress pre randomisation.
In total, 158 participants decided, after randomisation, that they did not want to move onto their allocated mattress or requested a mattress change from their allocated mattress. There was an imbalance in this between the two groups: 118 (5.9%) participants randomised to APM and 40 (2.0%) participants randomised to HSFM did not move or requested a change.
Conversely, there were 238 participants whom ward staff decided after randomisation that they did not want to move onto their allocated mattress or who requested a mattress change from their allocated mattress for clinical reasons. This was imbalanced between the two groups: 52 (21.8%) participants allocated to APM and 186 (78.2%) participants allocated to HSFM were not moved onto their allocated mattress or had a change of mattress requested for clinical reasons.
In general, more participants were moved from the APMs to HSFMs for comfort and to aid rehabilitation (reflecting the wider literature)23,32,34,52 and more participants were moved from HSFMs to APMs because of a deteriorating clinical condition.
From a clinical practice perspective, a large number of mattress changes resulted from ward moves. Of the first mattress change after the randomised mattress was received, 40 (18%) changes from APM and 20 (9.1%) changes from HSFM were due to a ward move. Overall, there were 442 (21.8%) participants who had 1320 mattress changes during the course of the treatment phase, although 606 (45.9%) were instigated by the CRN/P to maximise trial compliance. This reflects a lack of continuity during ward transfers, reported in previous research. 110
Safety
Overall, no safety concerns were indicated for either APMs or HSFMs. There were no RU SAEs and only three mattress-related events, none of which was classified as serious.
The proportion of deaths was similar in both trial arms (APM 8.1% vs. HSFM 8.3%) and consistent with a previous trial. 33 There was a difference between re-admission rates (APM 8.1% vs. HSFM 6.1%), but no re-admissions were reported as being mattress related.
The participant population was characterised by high levels of falling in the month preceding randomisation; these levels are in line with those reported in the 2015 national falls audit. 111 Concerns expressed by patients in previous research about feeling unsafe on APMs32,33 were not reflected in falls, with 14.9% (n = 152) of participants in the APM arm and 15.7% (n = 159) of participants in the HSFM arm reported to have fallen, with similar numbers on the allocated mattress at the time of the fall and a high proportion of total falls (n = 486) occurring after the treatment phase (62.3%, n = 303). The fall rate for the number of participants who had a fall is consistent with those reported in acutely ill hospital populations;112 however, the proportion of total falls that resulted in serious injury (5.6%, 27/486) is similar to the 5% rate reported in older people in community-dwelling settings and much lower than the reported 10–25% for institutional falls resulting in fracture, laceration or need for hospital care. 112
Health economic methodological considerations
Small differences in utility values derived from the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), have often been attributed to a lack of sensitivity in the instrument. 113 In an attempt to address this, the EQ-5D-5L was used. The EQ-5D-5L was developed to improve sensitivity. 64 However, recent preliminary evidence suggests that use of the EQ-5D-5L rather than the EQ-5D-3L ‘causes a decrease in the incremental QALY gain from effective health technologies and therefore technologies appear less cost-effective’. 113 Despite the current uncertainty in the choice of EQ-5D-5L measure, a strength of this analysis is the use of data collected using the PU-QoL-UI, a preference-based measure developed to assess the impact of PUs on HRQoL. 37 The sensitivity analyses using this measure produced similar results to the primary analyses, giving the research team confidence in the results and the conclusions drawn.
Although the APM dominates the HSFM in all base-case scenarios (i.e. within-trial and long-term analysis), the difference in terms of QoL between the two types of mattresses is negligible (less than half a day for the within-trial model and 11 days of full health for the long-term model); it could be argued that this could be seen as a zero random effect. Despite the probabilistic sensitivity analysis of both the within-trial and the long-term models suggesting a > 50% probability of APM being cost-effective, the slight difference in the QALYs suggest that the results should be viewed with caution. In terms of costs alone, however, both analyses suggest that using APM would be the less expensive option.
The analyses are limited by a number of factors, not least the under-recruitment, discussed in more detail later in this chapter, and the high proportion of missing data. The base-case analyses mirrored the methods used in the clinical analyses, using ITT. However, primarily because of missing EQ-5D-5L data, the number of complete cases was relatively small (n = 267) compared with the overall sample of the study (n = 2029), which, despite adjustment at baseline and imputation, is a limitation of the study. To address this, the analysis was conducted under the assumption that missing data were MAR, based on a descriptive analysis performed on the missing data. Visual and logistic regression analyses indicated that the data were unlikely to be MCAR. Although MAR was a plausible assumption, it is recognised that MNAR cannot be completely ruled out as some variables relevant to the economic data are unknown, which may affect the results.
When modelling the long-term cost-effectiveness, there is a risk of structural uncertainty – that an excluded clinical pathway could change the results. 114 This is important here as, over the time of the trial, there was a shift from HSFM provision as the minimum standard of provision for all inpatients to substitution of HSFM for hybrid foam and self-adjusting air-cell mattresses to reduce the use of APMs. As noted in Research delivery, Identifying centres prepared to randomise to high-specification foam mattresses, technology shift is an ongoing issue in medical device trials52 in the current regulatory environment, which does not require evidence of effectiveness prior to product marketing. 43
No previous cost-effectiveness analyses were identified that had modelled long-term cost-effectiveness in this area (e.g. Padula et al.,115 model for 1 year from admission to hospital). For both the VOI for the interim analyses and the final, long-term cost-effectiveness modelling, the model structures were validated and discussed with the clinical trial team. The structure of the model was adapted between the two analyses to incorporate additional information from the trial, which allowed a more detailed patient pathway. Specifically, the model also added a stage to account for one PU developed in hospital that was not included in the VOI. In addition, the trial included a 30-day post-treatment phase follow-up assessment. It is well documented that a PU can take up to 22 weeks to heal, with implications for the amount of resources required to treat it and the impact on the QoL of the patient. This additional measurement gave further information regarding the potential long-term implications of the two analysed mattresses.
Trial methodological considerations
The trial was planned as a double-triangular group sequential trial, which is considered an efficient design as it allows for the possibility of early stopping if superiority of either mattress has been demonstrated or if the trial proves to be futile. Therefore, the trial design optimised the potential for producing clinical evidence on the effectiveness of the mattress earlier than in a conventional fixed design. A conclusion of futility was also considered an important outcome for the trial as both mattresses are currently used in clinical practice. Stopping boundaries on a group sequential trial provide statistical guidance to the DMEC on the recommendations for stopping the trial early; however, other factors, including mattress compliance, participant safety, cost-effectiveness and other information external to the trial, were considered in order to make a fully informed decision on the future progression of the trial.
Moreover, the trial utilised an early primary end point, time to developing a PU of category ≥ 2 to a maximum of 90 days post randomisation, thereby allowing stopping of the trial in a timely manner.
The primary end point required close monitoring in order to plan when a prespecified interim analysis needed to be conducted. A benefit of the close monitoring allowed the overall event rate, centred across both mattress arms, to be reviewed against the original trial assumptions. It was considered important to report the estimate of the overall event rate to the DMEC at the scheduled meetings to allow recommendations to be made to the TSC and, subsequently, the funder on future design modifications. The uncertainty of the overall event rate and the impact this has on the accuracy of the power for detecting the prespecified treatment effect led to the funder requesting an unplanned early interim analysis. The recommendations following the unplanned interim analysis were to continue recruiting to the trial; however, the overall event rate observed led to discussions with the TMG on revised minimum clinically relevant differences between mattresses for varying centred event rates. These discussions acknowledged that the event rate was lower than originally assumed but the actual overall event rate observed at the interim analysis was not disclosed, thereby mitigating the risk of any operational bias in the future conduct of the trial. The output from these discussions informed the final target sample size of 1996 participants to be recruited with a 6-month recruitment extension, which allowed a target clinically relevant difference of 4% to be detected with at least 80% power, assuming an overall event rate of 10%.
Observing a lower event rate than originally assumed resulted in not reaching the required number of events to conduct the first planned interim analysis; therefore, the trial was modified to a fixed design with one final analysis.
The unplanned interim analysis did not account for competing risks and also deviated from the assumption of proportional hazards. However, the estimate of the treatment effect observed in the piecewise Cox model and also in the sensitivity analysis conducted on the treatment phase supported the final primary end-point analysis results. The difference was not statistically significant at the point of conducting the interim analysis, suggesting that it was worthwhile to continue the trial following the interim analysis.
A large number of data were reported for 14 skin sites (plus other skin sites identified post hoc) with repeated assessments over a maximum of 90 days post randomisation. All of these data were then reduced into one record per participant, reporting whether or not the participant developed a new PU of category ≥ 2 (i.e. the end point) and the corresponding time to development (or competing risk/censoring). Therefore, although this was a clinically meaningful end point, the multilevel nature of the data arising from skin sites nested within participants and assessed at repeated time points is ignored. This means that potentially important information, such as intermediate changes in skin status, multiple events and other risk factors, fails to be taken into account in the end-point derivation.
The primary end point was defined as time to developing a PU of category ≥ 2 to a maximum of 90 days post randomisation, with a sensitivity analysis conducted on the time to developing a PU of category ≥ 2 during the treatment phase. Interpretation of the trial results is first and foremost based on the analysis of the primary end point, with the sensitivity analysis used to support these findings. Therefore, the results of the sensitivity analysis would always be reported together with those from the primary end-point analysis. The sensitivity analysis was driven by discussions with clinical research staff, who thought that the PU development during the treatment phase was clinically important because factors such as discharge plans could affect PU development at 30 days post treatment.
This is the first study to include a longer-term post-treatment phase follow-up perspective. 27 It could be argued that the primary end point should have been the shorter-term end point of time to developing a PU of category ≥ 2 by the end of the treatment phase, with the 30-day final follow-up as the primary time point for the cost-effectiveness evaluation. This could be considered a more clinically meaningful time frame owing to the majority of participants (132/160, 83%) developing their new category ≥ 2 PUs during the treatment phase, the relevance of the outcome to the institution providing the mattress intervention and the different patient pathways following the treatment phase and associated discharge. In addition, the long interval between the end of the treatment phase and follow-up at 30 days will have resulted in imprecise reporting of the actual date a PU developed during this time period. However, the longer-term outcome provides a realistic estimate of effectiveness within current NHS inpatient and community services. The pros and cons of the primary end-point definition options require further consideration for future trials.
Research delivery
Overall, trial conduct within the NHS research teams was good, with NHS centres returning high-quality data, better than expected intervention compliance and lower than expected losses to follow-up. The centre variation in recruitment and, in particular, recruitment to the top six centres was a reflection of both local CRN/P provision and ‘top-up’ trial-funded CRN/Ps at centres with evidence of available patients and capacity/ability to expand the research team.
There were five main challenges for the delivery of the PRESSURE 2 trial within the NHS clinical service and research infrastructure. This are outlined in the following sections.
Patient population
The PRESSURE 2 trial participants were hard-to-reach inpatient admissions with acute illness, located across multiple hospital wards including acute admission units, medical, elderly, orthopaedics and trauma, vascular surgery and rehabilitation wards. Of those patients screened, 13.3% were recruited (see Figure 2), reflecting a high workload required for ‘walking the wards’ to screen and consent. It is important to note, however, that, where there was dedicated well-managed CRN/P infrastructure, recruitment was delivered at the rate expected and planned in the grant application.
Identifying centres prepared to randomise to high-specification foam mattresses
Although NICE guidelines12 clearly identify the absence of evidence to support the use of high-technology mattresses such as APMs, they are in widespread use. Reporting of PU prevalence and incidence by trusts to commissioners through the Commissioning for Quality and Innovation (CQUIN) framework21 and performance-related payments and penalties have created a risk-averse culture; local policies have been developed advocating APMs for all high-risk patients, despite the lack of evidence or mandated guidance from NICE. 12 Trusts with such policies were unwilling to take part in the trial.
A second issue that limited NHS trust/health board participation was a shift from HSFM provision as the minimum standard of provision for all inpatients to trust-wide substitution of HSFM for hybrid foam and self-adjusting air-cell mattresses to reduce the use of APMs. These mattresses are provided to the NHS without RCT evidence of effectiveness compared with current standard care (HSFM or APM). 12,27 Technology shift is an ongoing issue in medical device trials52 in the current regulatory environment, which does not require evidence of effectiveness prior to product marketing. 43
Working with research-naive local principal investigators
The majority of local principal investigators (PIs) were clinical nurse specialists, many of whom were new to the role of PI. In some centres, the tissue viability nurses were supported by their research and development/network teams in working through feasibility and securing service support costs (SSCs). Intensive support was also provided to promote understanding of basic research principles and practical support was provided through one-to-one sessions to talk through the IRAS registration, feasibility questionnaire completion and site-specific information completion.
For some, however, the challenges of their day-to-day working environment prohibited them from taking forward the trial in a role in which research is not a core function. Barriers include lack of senior nurse support, limited personal computer (PC) access (i.e. sharing desk space and/or PCs) and a lack of support in securing CRN/Ps through SSCs (see following section).
Securing clinical research nurse/registered health-care professional infrastructure
The trial was undertaken during a transition period in the allocation of research funds for CRN/Ps. At the time of the grant application, the NIHR costing model, ARCO,116 was in place and this meant that follow-up activity was classified as a SSC that was funded through NIHR Clinical Research Network allocations to NHS trusts/health boards. It was originally estimated that 15 CRN/Ps were required (80% through SSC and 20% through research grant funding) across 20 centres to accrue 2954 participants in 33 months, but securing the SSCs required negotiation with individual NHS trusts/health boards. This meant that some trusts/health boards were unable to take part in the trial. In addition, although each trust/health board received a research grant, funding per-patient payment, the majority of this funding was not invested directly in the local CRN/P team.
At no point during the recruitment period were the required levels of CRN/Ps allocated by the participating trusts, even when the number of centres was doubled, until the research team were able to use research grant savings to top up the CRN/P allocations in the top recruiting centres. [Please note that under the new AcoRD (attributing the costs of health and social care research and development) guidelines,117 the follow-up activity would be classified as a research cost as the follow-up is not a part of individual patient clinical management and so, in future trials, it is not expected that this barrier would have the same impact.]
An advantage in trial set-up was that some trusts had managed teams of CRN/Ps, for which the CRN/Ps were not ‘tied’ into specific specialty portfolios and were able to support the trial. In trial delivery, performance of both individual CRN/Ps and managed teams was mixed. It was more straightforward when a CRN/P was dedicated to PRESSURE 2 trial performance management by the local PI or manager than when CRN/Ps did not have ring-fenced time and were delivering a large number of competing studies. In the latter centres, it was difficult to establish the true whole-time equivalent (WTE) SSC allocation to the PRESSURE 2 trial and, based on their reported WTE, these sites did not recruit efficiently.
Recruitment difficulties
Participant recruitment per WTE CRN/P was lower than expected, based on previous work. 38,52 In the grant application, it was estimated that 40% of patients screened would be eligible and that, of these, 50% would consent (i.e. overall, 20% of patients screened would be randomised). In September 2014, it was identified that only 29% of patients screened were eligible and, of those, 36% had consented (10% of patients screened were randomised). As this issue was identified early in the recruitment phase, steps were taken to improve the recruitment rate per WTE CRN/P; such steps included workshops with the CRN/Ps focused on efficient screening approaches, relative assent and simplifying trial description, CRN/Ps shadowing experienced PIs, provision of a short-hand patient information leaflet [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/113633/#/ (accessed 20 November 2018)], a reduction in the number of follow-up questionnaires, supplying performance data to each trust/health board and NIHR Clinical Research Network Research Delivery Manager, and use of research grant funding to increase the CRN/P posts in the top six recruiting centres. This improved participant recruitment per WTE CRN/P, increased the proportion of participants recruited using consultee agreement and increased the proportion of those screened and recruited to 13% by the end of the trial period.
Photography substudy
A strict protocol was in place for the photography of skin sites, including camera specification, secure transfer of photographs to CTRU, use of a greyscale card and calibration of photographs for central review, and randomly ordering and naming photographs to maintain blinding during review.
The final sample size for the photography substudy was smaller than expected at the point of designing the trial. This was because the accrual of participants and the event rate was lower than expected and the consent rate was not incorporated in the original sample size estimate.
Overall, the reliability of photography as a tool for blinded assessment in PU trials compared with the current gold-standard expert clinical assessment for the assessment of PUs of category ≥ 2 was found to be ‘very good’. A strength of the work is that agreement from a large number of individual paired assessments was analysed, whereas other studies have utilised multiple assessments of a small number of photographs. 79,80
There were varying levels of confidence in the assessment of photographs and further work is required to explore whether or not the confidence levels varied by skin site.
In line with other inter-rater reliability studies,118–120 which compare clinical assessments undertaken by expert assessors, there was ‘very good agreement’ between the independent assessor and CRN/P in their clinical assessment. A strength of this analysis (compared with the wider literature) is the balance of skin sites assessed as normal/altered/category 1 and PUs of category ≥ 2, as prevalence affects interpretation of agreement.
A sensitivity analysis was planned to assess the impact on the results of conducting a blinded assessment through photographs; however, this was not possible at the stage of the analysis. The primary end point was derived through repeated assessments of the skin; however, only one photograph was taken per PU of category ≥ 2. Therefore, a sensitivity analysis would have been conducted on the primary end point rederived using most of the original data and one photographic assessment of one skin site that would then be collapsed into a single end point. This was not considered an appropriate sensitivity analysis.
The research team set out to assess the potential for over-reporting and under-reporting of PUs of category ≥ 2, but this cannot really be assessed as central photographic review is not the gold standard. Rather, the research team were trying to establish if there were differences between the arms in the agreement between blinded photography central review and unblinded clinical assessment that would suggest systematic bias in under- or over-reporting. Overall, ≈15% of clinically assessed PUs of category ≥ 2 were assessed as normal, altered or category 1 by blind central photographic review and CIs for the proportion of agreement of PUs of category ≥ 2 for each group overlapped. One of the concerns in the utility of central photographic review was the ability to distinguish between non-blanching erythema and a very early category 2 PU characterised by a small area of epidermal loss within a larger area of erythema. 78 Therefore, differences would be expected between the two assessment methods.
The more concerning finding was that photographic compliance was lower in the APM arm than the HSFM arm; in future work, return rates require compliance monitoring by trial arm (without compromising trial conduct). It is not clear why the compliance was lower in the APM arm. Further work is required to understand whether this was related to practical difficulties associated with participant movement on the APM or systematic bias.
Overall, the use of photography as a reliable measure for blinded assessment in PU research is recommended. It is advised that a robust protocol is in place to allow for consistent and accurate photography of skin sites. Repeated photographs should also be taken in order to conduct a robust sensitivity analysis on the primary end point, but should be considered in conjunction with patient acceptability.
The Pressure Ulcer Quality of Life – Prevention substudy
The PU field requires a strong evidence base that incorporates assessment of PROs. To fully capture and quantify the patients’ perspective, appropriately developed and validated PRO instruments are required. The PU-QoL-P instrument mostly satisfies criteria for reliability, validity and responsiveness in line with recommended FDA guidelines for PRO instruments for use in clinical trials. 91 The ITC, alpha coefficient and homogeneity coefficient (IIC mean and range) provide evidence towards the reliability and internal construct validity of the PU-QoL-P scales. The results of the factor analysis mostly supported the use of the items as hypothesised into six function scales, as was suggested by the results from the original analysis. 48 Some modifications were made to four scales, which were supported by the retrospective analyses, and the changes made are considered conceptually reasonable. However, as changes were made to the scales, some might argue that a further set of data should be collected for further validation purposes. No one test confirms validity; rather, validation of a PRO instrument is an ongoing process, with the accumulation of clinical validation data building a case for a particular instrument functioning effectively in a particular population for a specific purpose. 103
These findings contribute evidence towards support that people with category 2 PUs experience worse symptoms and functioning outcomes than those without PUs. People who are also physically limited and have PUs experience worse mobility outcomes and ability to participate in daily activities than those who have no or only slight physical impairment. PUs are often a secondary comorbidity and a consequence of the primary condition a patient may be experiencing (e.g. immobility due to prolonged bed rest following extensive surgery). PUs contribute additional impairment in physical functioning outcomes beyond those experienced from other comorbidities. Furthermore, this exploratory hypothesis testing suggests that patients with torso PUs experience worse symptoms and more mobility problems, whereas patients with limb PUs have more problems with sleep quality, daily activities and malaise, suffer from lower emotional well-being and feel more self-conscious.
Owing to the small sample sizes in the hypothesised known groups, definitive conclusions cannot be made. However, trends in scores in the right direction and, in some scales, moderate to high effect sizes were observed, even though some were not statistically significant. Small sample size affects the standard error so large CIs might be expected; however, sample size does not affect means, SDs or effect size. Therefore, effect sizes are still relevant and informative, even if non-significant correlations are observed, which in this case may be attributed to sample sizes being too small to detect significant differences.
A limitation of the study was scale-to-sample targeting; mean scores were below scale mid-points and all scales exceeded the 10% criterion for floor effects. However, given that it was intended to recruit an at-risk population (i.e. few people had category 1 or 2 PUs at baseline and none had a category ≥ 3 PU), this finding is expected. The floor effects indicate more homogeneity in the sample than is representative of the general PU population. However, the study sample is representative of a high-risk PU population, who may be experiencing pressure area-related pain but do not experience symptoms associated with severe PUs, such as exudate and odour, and the PU-QoL-P is intended for prevention trials.
The above limitations do not preclude use of the PU-QoL-P instrument. PU-QoL-P scales can be included as one outcome measure, among others, for group comparisons in future PU prevention research (e.g. clinical trials). Work is under way to develop a short form and to test its clinical utility for use in clinical practice. As the PU-QoL-P was developed and evaluated in the UK, the validity and reliability are characteristics of the instrument for a specific population (i.e. UK nationals). A language translation or cross-cultural adaption may be required to ensure that the PU-QoL-P is appropriate for cultures, languages and ethnic groups outside the UK (see the PU-QoL-P instrument website104 for guidance on language translation and cross-cultural adaptation processes). Further research is also needed to investigate self-complete administration mode, and the development of proxy measures and language translations, given the prevalence of cognitively impaired patients with PUs. Finally, given the small sample sizes for the known groups and responsiveness analysis, these analyses could be repeated in larger samples. The process of modifying a newly developed PRO instrument is part of an evolving, ongoing measurement process intended to strengthen the hypothesised conceptual relationships with empiric evidence. 97 The usefulness of new measures is therefore demonstrated by multiple applications in different studies (accumulative body of evidence to support scale measurement properties).
Patient and public involvement
Added value of public involvement
Certain areas of the study particularly benefited from a servicer-user perspective. For example, people with a current or previous severe PU were excluded from the study. This was as a direct result of PURSUN discussions. PURSUN members who had experienced particularly severe PUs said they would not be willing to be randomised as they already had a mattress preference. People also highlighted the traumatic nature of having a severe PU and said that fear of recurrence can lead to anxiety during hospital admissions. For this reason, they felt that it was inappropriate to approach or randomise people with experience of a category ≥ 3 PU.
Input from PURSUN was also helpful when developing the photography protocol. There are ethical issues associated with photographing skin sites; therefore, the team worked with PURSUN to develop the photography instructions, with particular input from one member with experience of having wounds photographed. Measures were put in place to maintain patient dignity and limit burden. For example, the photography instructions state that photographs must be taken by a health-care professional, not a medical photographer. They also stress the importance of comfort and dignity when positioning patients or removing dressings. In addition, the consent process allowed people to opt out of photography but remain in the trial.
The photography process was highlighted by both service users and clinical co-applicants as a positive example of public involvement. One clinical co-applicant reflected that she felt reassured by the input of service users and happier about asking her colleagues and patients to take part in this element of the study. The service user co-applicant said that she felt that her personal experience added something to photography discussions that would have otherwise been missing.
The service user co-applicant also reflected on the impact that being a member of the PRESSURE 2 team had on her. She found it to be a rewarding process as she was able to learn and to use her unique experience to aid the research. Being part of the team from the outset aided this as it allowed her to build relationships and seek support if needed.
Reflections on patient and public involvement
The challenges of evaluating public involvement are well documented. 121 It is difficult and, arguably, inappropriate to try and isolate the input of particular individuals within a collaborative research process. As described in the previous section, certain examples naturally stand out, but some aspects of public involvement are more subtle. The PRESSURE 2 PPI officer reflected on the way that research team members spoke when service users were present at meetings. At times, she felt that the presence of a service user caused people to think and speak in a more patient-centred way. However, that is hard to document or measure. The perspective of the wider research team will be explored further after the final PURSUN workshop.
Some practical challenges were encountered when planning and managing public involvement. For example, teleconferences were used as a way of allowing service users to contribute from home, if needed. However, PURSUN members found it hard to stay engaged and contribute by telephone, and they have recommended face-to-face meetings for the future.
It was particularly challenging to integrate the service user perspective into steering committee meetings. Although there are many advantages to having service user input in high-level project oversight, PURSUN members found the meetings challenging, with one member stepping down. Issues such as complicated documents, technical language and the varied number of topics covered within meetings hindered meaningful involvement. After feedback, additional support was implemented for steering committee members, including a glossary of terms, more focused communications before meetings and the option to attend meetings with the PPI officer. Although PURSUN members appreciated these measures, they still felt that more work needs to be done to make steering committees an effective engagement activity.
Chapter 8 Implications for practice and research
Implications for practice
-
Alternating pressure mattresses confer a small treatment-phase benefit on acutely ill inpatients who are bedfast/chairfast and/or have a category 1 PU, which is diminished over time.
-
APM patient compliance, the very low PU incidence rate observed and small group differences indicate the need for improved indicators for targeting of APMs.
-
Individualised decision-making should take into account skin status, patient preferences (e.g. movement ability and rehabilitation needs) and the presence of factors that may be modifiable through APM allocation, including being completely immobile, having nutritional deficits, lacking capacity and/or having altered skin/category 1 PU.
-
Patients with existing category 1 and 2 PUs are most at risk of subsequent PUs of category ≥ 2 and require targeted secondary prevention.
-
Improved communication is required prior to ward transfers to improve continuity of PU prevention care.
-
Improvements are required to ensure continuity of PU prevention post discharge.
Implications for research
-
This study should be incorporated into a meta-analysis of APM versus HSFM RCTs.
-
Objective measurement instruments of key risk factors is required to better inform risk stratification and preventative interventions in practice.
-
Further analysis is required to explore the relationship between mental capacity, levels of independent movement and repositioning, nutritional status and PU development.
-
Further research is required to explore ‘what works for whom and in what circumstances’ to better inform mattress provision for high-risk patients.
-
The health economic analysis was limited by missing data; however, the difference in QoL outcomes between the trial arms was negligible and the difference in cost was small, suggesting no need for further research.
-
Central blinded expert photographic review is a reliable method for assessing PU outcomes in research. A robust method to enable repeated photographic assessments, which minimises patient burden while enabling sensitivity analyses, requires development.
-
Clinical end points should be considered for PU research during the treatment phase because skin changes can occur very quickly and may be influenced by factors such as discharge plans.
-
Skin site-level data collected in PU research should be detailed in order to understand how skin changes over time. Further methodological work is required to be able to fully utilise these data in the analysis of trial outcomes.
-
The PU-QoL-P tool is suitable for capturing patient-reported functioning (core domains of HRQoL) and PU-area pain in patients at risk of PU development and for quantifying the benefits of associated preventative interventions from the patient’s perspective, thus far lacking. It can be used in research with adults at risk of PU development in all UK health-care settings. Further research is needed to investigate self-complete administration mode, and the development of proxy measures and language translations, given the prevalence of cognitively impaired patients with PUs.
Acknowledgements
Participants
We wish to express our gratitude to all patients who participated in this research, for giving up their time and participating in this research study.
Trial Steering Committee members
Chairperson
Professor Julie Brittenden, Professor of Vascular Surgery, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
External/independent members
-
2013–2015: Dr Philip Bell, Service User (PURSUN UK).
-
2013–14: Ms Carole Bennett, Service User (PURSUN UK).
-
Dr Jeannie Donnelly, Teaching Fellow, School of Nursing and Midwifery, Queen’s University Belfast.
-
Ms Andrea McGoverin, Service User (PURSUN UK).
-
Ms Heather Newton, Consultant Nurse Tissue Viability, Tissue Viability Service, Royal Cornwall Hospitals NHS Trust.
-
Ms Yvonne Rawson, Service User (PURSUN UK).
-
Ms Heidi Sandoz, Lead Nurse for Wound Care, Accelerate Health CIC (Community Interest Company) and Honorary Lecturer, University of Hertfordshire.
-
Professor Paula Williamson, Biostatistician, Institute of Translational Medicine, University of Liverpool.
Data Monitoring and Ethics Committee members
Chairperson
Professor Kerry Hood, Director, South East Wales Trials Unit, Cardiff University School of Medicine.
External/independent members
-
Ms Jane James, Tissue Viability Nurse, Hywel Dda University Health Board.
-
Mr Hamish Laing, Consultant, Welsh Centre for Burns and Plastic Surgery, Morriston Hospital, Abertawe Bro Morgannwg University Local Health Board.
-
Dr Lisette Schoonhoven, Professor of Nursing, University of Southampton.
-
Dr Marta Soares, Health Economist, Centre for Health Economics, University of York.
Pressure Ulcer Service User Network UK
We would like to sincerely thank all members of PURSUN UK for their continued hard work and dedication, including those on the TSC and other members involved through participation in general PURSUN UK meetings where the PRESSURE 2 trial was discussed.
Research team members at participating sites
We are grateful to all the clinical research team members and co-authors from the 41 NHS Trusts who have participated in this research and made it a part of their busy daily schedules. Without the commitment and support of the PIs, Tissue Viability Nurse Specialists, CRN/Ps and support staff in gaining local permissions, recruiting patients and collecting data for this trial would not have been possible.
Bolton NHS Foundation Trust
Lisa Murthen [Research Nurse (RN)].
Bradford Teaching Hospitals NHS Foundation Trust
-
Mr Kevin Mercer (PI).
-
Helen Fearnley (TVNS).
-
Fazah Gull (RN).
-
Alan Liu (RN).
Cambridge University Hospitals NHS Foundation Trust
-
Carole Young (PI).
-
Sarah Davies (PI).
-
Joanna Helmy (RN).
-
Sara Jones (Lead RN).
-
Alex Maylon (RN).
-
Petra Polgarova (RN).
-
Alice Robertson (RN).
Chelsea and Westminster Hospital NHS Foundation Trust
-
Valeria Sylvestre (RN).
-
Carina Bautista (RN).
-
Elna Efra (RN).
-
Mini Thankachen (RN).
East and North Hertfordshire NHS Trust
-
Dianne Brett (PI).
-
Hannah Beadle (RN).
-
Angela Cook (RN).
East Coast Community Healthcare
-
Noreen Cushen-Brewster (PI).
-
Amanda Ayres (RN).
-
Sharleen Blundell (RN).
-
Jayne Jode (RN).
-
Dianne Rudge (RN).
East Kent Hospitals University NHS Foundation Trust
-
Judy Elliot (PI).
-
Tracey Cosier (RN).
-
Kylie Freear (RN).
-
Fiona Hammonds (RN).
-
Francesca King (RN).
-
Florin Muscariu (RN).
-
Janine Musselwhite (RN).
-
Eliya Nenkova (CTA).
-
James Rand (CTA).
-
Sarah Stirrup (RN).
Harrogate and District NHS Foundation Trust
-
Alison Layton (PI).
-
Margaret Broome (RN).
-
Agness Bwalya (RN).
-
Rachael Lee (RN).
Homerton University Hospital NHS Foundation Trust
-
Mr Richard Bull (PI).
-
Marites Montemayor (RN).
-
Juancho Salgado (RN).
Hull and East Yorkshire Hospitals NHS Trust
-
Angela Oswald (PI).
-
Emma Clarkson (RN).
-
Sarah Wilson (RN).
Leeds Community Healthcare NHS Trust
-
Nikki Stubbs (PI).
-
Karen Armstrong-Lamb (RN).
-
Rachael Lee (RN).
-
Jane Mayes (RN).
-
Martin Sylvester (RN).
-
Julie Thackray (RN).
-
Lindsey Worstenholme (RN).
Leeds Teaching Hospitals NHS Trust
-
Dr Elizabeth McGinnis (PI).
-
Sally Blundell (RN).
-
Alan Brydon (RN).
-
Jimmy Choo (RN).
-
Clare Greenwood (RN).
-
Fazah Gull (RN).
-
Gary Lamont (RN).
-
Keira Meethan (RN).
-
Martin O’Malley (RN).
-
Ruth Pano (RN).
-
Sally Redfern (RN).
Lincolnshire Community Health Services NHS Trust
-
Colette Longstaffe (PI).
-
Suzanne Archer (RN).
Livewell Southwest (formerly Plymouth Community)
-
Theresa Mitchell (PI).
-
Rena Truscott (RHCP).
Mid Yorkshire Hospitals NHS Trust
-
Lyn Wilson (PI).
-
Rosie Beckitt (Research support).
-
Beverley Hemingway (RN).
-
Sara Holbrook (RN).
-
Tracey Lowry (RN).
-
Toni Rank (RN).
-
Linda Slater (RN).
-
Fiona Stones (RN).
-
Jackie Ward (RN).
NHS Fife
-
Anne Wilson (PI).
-
Polly Buchanan (Lead RN).
NHS Frimley Health Foundation Trust
Wendy Jardine (PI).
NHS Lothian
-
Juliet MacArthur (PI).
-
Lisa Derr (RN).
-
Mary Morrissey (RN).
-
Frank Morrow (RN).
-
Beena Poulose (RN).
Norfolk Community Health and Care NHS Trust
-
Lesley Maloney (PI).
-
Laura Turner (PI).
-
Bianca Bailey (RN).
-
Debby Kelly (RN).
-
Wendy Neale (RN).
Northern Devon Healthcare NHS Trust
-
Melanie Hucker (PI).
-
Tiffany Adams (RN).
-
Geraldine Belcher (RN).
-
Rebecca Holbrook (RN).
-
Jane Hunt (RN).
-
Amanda Skinner (RN).
-
Lucia Stancombe (Research support).
-
Lynne Van Koutrik (RN).
Northern Lincolnshire and Goole Hospitals NHS Foundation Trust
-
Jackie Robinson (PI).
-
Michelle Cheeseman (RN).
-
Lisa Jayne Cottam (RN).
-
Carol Ann Gray (RN).
-
Paula Hardisty (RN).
-
Carole Linfoot (RN).
Northumbria Healthcare NHS Foundation Trust
-
Valerie Henderson (PI).
-
Louise Jones (PI).
-
Deborah Bunn (RN).
-
Christine Hodgson (RN).
-
Sean Phillips (RN).
-
Susan Wharton (RN).
Papworth Hospital NHS Foundation Trust
-
Fiona Downie (PI).
-
Elizabeth Blake Palmer (RN).
-
Philippa Clarke (RN).
-
Katy Rintoul (RN).
-
Victoria Senior (RN).
Pennine Acute Hospitals NHS Trust
Judy Harker (PI).
Plymouth Hospitals NHS Trust
-
Pia Prince (PI).
-
Julie Alderton (RN).
-
Charlotte Eglinton (RHCP).
-
Catherine Harden (RN).
-
Heidi Hollands (RHCP).
-
Madelaine Miles (Research support).
-
Darren Waugh (RN).
-
Natasha Wilmshurt (RN).
Poole Hospital NHS Foundation Trust
-
Andrea Graham (PI).
-
Katie Murphy (RN).
Princess Alexandra Hospital NHS Trust
-
Dr Jane Snook (PI).
-
Amelia Daniel (RN).
-
Ervin Shpuza (RN).
-
Nikki Staines (RN).
-
Shane Walsh (RN).
Royal Devon and Exeter NHS Foundation Trust
-
Judith Potter (PI).
-
Linda Park (RN).
-
Kathy Polverino (RN).
-
Nicola Walker (RN).
Royal United Hospitals Bath NHS Foundation Trust
-
Nikki Heywood (PI).
-
Michaela Arrowsmith (PI).
-
Helen Seymour (RN).
Salisbury NHS Foundation Trust
-
Jenny Brown (PI).
-
Caroline Clarke (RN).
Sandwell and West Birmingham Hospitals NHS Trust
-
Lesley McDonagh (PI).
-
Julie Colley (RN).
Sheffield Teaching Hospitals NHS Foundation Trust
Northern General Hospital
-
Mr Pradeep Thumbikat (PI).
-
Mr Ram Hariharan (Co-PI).
-
Karen Armitage (RN).
-
Lissie Webster (RN).
Royal Hallamshire Hospital
-
Dr Ali Ali (PI).
-
Jo Howe (RN).
-
Emma Richards (RN).
Solent NHS Trust
Marjolein Woodhouse (PI).
Somerset Partnership NHS Foundation Trust
-
Carinna Vickers (PI).
-
Sarah Dunne (RN).
-
Tania Collin (RN).
-
Frances Rockhill (RN).
-
Helen Rowe (RN).
-
Tamlyn Russell (RN).
South Tyneside NHS Foundation Trust
-
Jeanette Milne (PI).
-
Judith Young (PI).
-
Debra Ann Houghton (RN).
-
Seana Kenny (RN).
-
Karen Lowes (RN).
-
Karen Reay (RN).
Southport and Ormskirk Hospital NHS Trust
Dominic Williams (PI).
St Helens and Knowsley Teaching Hospitals NHS Trust
-
Vinod Gowda (PI).
-
Susan Dowling (RN).
-
Caron Jones (RN).
Sussex Community NHS Foundation Trust
-
Neil Hanley (PI).
-
Claire Cox (RN).
-
Kate Weekes (RN).
The Ipswich Hospital NHS Trust
-
Lisa Sutherland (PI).
-
Sue Brixey (RN).
-
Glynis Stansfield (RN).
Torbay and South Devon NHS Foundation Trust
-
Professor Nigel Benjamin (PI).
-
Nicola Donlin (RN).
-
Becky George (RN).
-
Bianca Hulance (RN).
-
Nicola Mann (RN).
-
Natalie Taylor (RN).
York Teaching Hospital NHS Foundation Trust (Scarborough Hospital)
-
Samantha Haigh (RN).
-
Emma Temlett (RN).
We would like to sincerely thank Miss Suzanne Hartley, who was the Head of Trial Management, and Dr Monider Bhogal, who was the Senior Trial Co-ordinator during the set-up and initial recruitment phase of the study.
Contributions of authors
Jane Nixon (Professor of Tissue Viability and Clinical Trials Research) was the Chief Investigator and is responsible for the conception of the PRESSURE 2 trial. She contributed to the design of the trial, protocol development, protocol implementation, interpretation of data, drafting and revising the report for intellectual content and approval of the final version of the report. She also acts as guarantor.
Sarah Brown (Principal Medical Statistician) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation, statistical design and oversight, and interpretation of data. She also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
Isabelle L Smith (Medical Statistician) contributed to the design of the trial, protocol development, protocol implementation, statistical design, statistical analysis of the clinical results and photography analysis and interpretation of data. She also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
Elizabeth McGinnis (Tissue Viability Nurse Consultant and PRESSURE 2 trial Clinical Co-ordinator) was a co-applicant and contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was responsible for protocol implementation and local co-ordination of approvals and data acquisition for Leeds Teaching Hospitals NHS Trust. She was on the blinded expert photographic assessment panel. She also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
Armando Vargas-Palacios (Research Fellow in Health Economics) contributed to protocol implementation, the health economic analysis plan, data analysis and data interpretation. He also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
E Andrea Nelson (Professor of Wound Healing) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Julia Brown (Professor of Clinical Trials Research) was a co-applicant. She contributed to the conception of the trial, design of the PU-QoL-P substudy, protocol development, scientific oversight and interpretation of the PU-QoL-P substudy. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Susanne Coleman (NIHR post-doctoral Research Fellow) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was on the blinded expert photographic assessment panel. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Howard Collier (Senior Data Manager) contributed to the design of the trial, protocol development, protocol implementation and interpretation of data. He also contributed to revising the report for intellectual content and approval of the final version of the report.
Catherine Fernandez (Head of Trial Management) contributed to the design of the trial, protocol development, protocol implementation and interpretation of data. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Rachael Gilberts (Senior Trial Co-ordinator) contributed to the design of the trial, protocol development, protocol implementation and interpretation of data. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Valerie Henderson (Tissue Viability Nurse Specialist) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was responsible for protocol implementation and local co-ordination of approvals and data acquisition for Northumbria Healthcare NHS Foundation Trust. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Christopher McCabe (Professor of Health Economics) was a co-applicant. He was responsible for the conception of the trial and was the study lead for the health economic VOI analysis, including design, data interpretation and drafting the VOI analyses. He also contributed to revising the report for intellectual content and approval of the final version of the report.
Delia Muir (PPI Officer) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was responsible for the co-ordination of PPI. She also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
Claudia Rutherford (Senior Research Fellow) was a co-applicant. She led the conception and design of the PU-QoL-P substudy, including protocol development, analysis plan and data analysis and interpretation. She drafted the PU-QoL-P substudy and contributed to revising the report for intellectual content and approval of the final version of the report.
Nikki Stubbs (Tissue Viability Nurse Specialist) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was responsible for protocol implementation and local co-ordination of approvals and data acquisition for Leeds Community Healthcare NHS Trust. She was on the blinded expert photography assessment panel. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Benjamin Thorpe (Research Methods Fellow) contributed to the design of the mediator and moderator analyses, data analysis and data interpretation. He also contributed to revising the report for intellectual content and approval of the final version of the report.
Klemens Wallner (Research Assistant) was responsible for the health economic VOI analysis, including design, data analysis, data interpretation and drafting the VOI analysis. He also contributed to revising the report for intellectual content and approval of the final version of the report.
Kay Walker (PURSUN representative) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She also contributed to drafting the report and approval of the final version of the report.
Lyn Wilson (Research Manager and PRESSURE 2 trial Clinical Co-ordinator) was a co-applicant. She contributed to the conception and design of the trial, protocol development, protocol implementation and interpretation of data. She was responsible for protocol implementation and local co-ordination of approvals and data acquisition for Mid Yorkshire Hospitals NHS Trust. She was on the blinded expert photography assessment panel. She also contributed to revising the report for intellectual content and approval of the final version of the report.
Claire Hulme (Professor of Health Economics) was a co-applicant. She contributed to the conception and design of the trial, protocol development, oversight of the health economic data analysis and conduct, and data interpretation. She also contributed to drafting and revising the report for intellectual content and approval of the final version of the report.
Publication
Nixon J, Smith IL, Brown S, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Pressure relieving support surfaces for pressure ulcer prevention (PRESSURE 2): clinical and health economic results of a randomised controlled trial [published online ahead of print September 3 2019]. EClinicalMedicine 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57:1175-83. https://doi.org/10.1111/j.1532-5415.2009.02307.x.
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age Ageing 2004;33:230-5. https://doi.org/10.1093/ageing/afh086.
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. J Wound Care 2001;10:530-5. https://doi.org/10.12968/jowc.2001.10.1.26039.
- Smith IL, Nixon J, Brown S, Wilson L, Coleman S. Pressure ulcer and wounds reporting in NHS hospitals in England part 1: audit of monitoring systems. J Tissue Viability 2016;25:3-15. https://doi.org/10.1016/j.jtv.2015.11.001.
- Briggs M, Collinson M, Wilson L, Rivers C, McGinnis E, Dealey C, et al. The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nurs 2013;12. https://doi.org/10.1186/1472-6955-12-19.
- McGinnis E, Briggs M, Collinson M, Wilson L, Dealey C, Brown J, et al. Pressure ulcer related pain in community populations: a prevalence survey. BMC Nurs 2014;13. https://doi.org/10.1186/1472-6955-13-16.
- Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital-acquired pressure ulcers and risk of death. J Am Geriatr Soc 1996;44:1435-40. https://doi.org/10.1111/j.1532-5415.1996.tb04067.x.
- Pan Pacific Pressure Injury Alliance . Prevention and Treatment of Pressure Ulcers: Quick Reference Guide 2014. www.epuap.org/wp-content/uploads/2016/10/quick-reference-guide-digital-npuap-epuap-pppia-jan2016.pdf (accessed 29 June 2017).
- Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222-34. https://doi.org/10.1111/jan.12405.
- Loerakker S, Manders E, Strijkers GJ, Nicolay K, Baaijens FP, Bader DL, et al. The effects of deformation, ischemia, and reperfusion on the development of muscle damage during prolonged loading. J Appl Physiol 2011;111:1168-77. https://doi.org/10.1152/japplphysiol.00389.2011.
- US National Pressure Ulcer Advisory Panel . Prevention and Treatment of Pressure Ulcers: Quick Reference Guides 2009. www.epuap.org/guidelines/guidelines-old/ (accessed 28 June 2017).
- National Institute for Health and Care Excellence (NICE) . Pressure Ulcers: Prevention and Management 2014. www.nice.org.uk/guidance/cg179 (accessed 29 June 2017).
- US National Pressure Ulcer Advisory Panel (NPUAP) . National Pressure Ulcer Advisory Panel (NPUAP) Announces a Change in Terminology from Pressure Ulcer to Pressure Injury and Updates the Stages of Pressure Injury 2016. www.npuap.org/national-pressure-ulcer-advisory-panel-npuap-announces-a-change-in-terminology-from-pressure-ulcer-to-pressure-injury-and-updates-the-stages-of-pressure-injury/ (accessed 25 September 2017).
- Australian Wound Management Association (AWMA) . Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury 2012. www.woundsaustralia.com.au/publications/2012_AWMA_Pan_Pacific_Guidelines.pdf (accessed 12 October 2017).
- European Pressure Ulcer Advisory Panel (EPUAP) . Pressure Ulcer Terminology 2017. www.epuap.org/news/pressure-ulcer-terminology/ (accessed 30 April 2018).
- NHS Improvement . Expert Panels: Do You Want to Represent Your Provider? 2017. https://improvement.nhs.uk/resources/expert-panels-do-you-want-to-represent-your-provider/ (accessed 30 April 2018).
- World Health Organization (WHO) . The 11th Revision of the International Classification of Diseases (ICD-11) Is Due by 2018! 2017. www.who.int/classifications/icd/revision/en/ (accessed 30 April 2018).
- Coleman S, Gorecki C, Nelson EA, Closs SJ, Defloor T, Halfens R, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud 2013;50:974-1003. https://doi.org/10.1016/j.ijnurstu.2012.11.019.
- Department of Health and Social Care . Pressure Sores – A Key Quality Indicator: A Guide For NHS Purchasers And Providers 1993.
- Department of Health and Social Care . Essence of Care: Patient-Focused Benchmarking For Health Care Practitioners 2001.
- Department of Health and Social Care . Using the Commissioning for Quality and Innovation (CQUIN) Payment Framework for the NHS England 2008.
- Department of Health and Social Care . Clinical Governance and Adult Safeguarding 2010.
- Hopkins A, Dealey C, Bale S, Defloor T, Worboys F. Patient stories of living with a pressure ulcer. J Adv Nurs 2006;56:345-53. https://doi.org/10.1111/j.1365-2648.2006.04007.x.
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21:261-2. https://doi.org/10.12968/jowc.2012.21.6.261.
- Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009283.
- National Institute for Health and Care Excellence (NICE) . Pressure Ulcer Prevention: Pressure Ulcer Risk Assessment and Prevention, Including the Use of Pressure-Relieving Devices (Beds, Mattresses and Overlays) for the Prevention of Pressure Ulcers in Primary and Secondary Care 2003. www.nice.org.uk/guidance/cg7 (accessed 22 April 2019).
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD001735.pub5.
- NHS Supply Chain . NHS Supply Chain Online Catalogue 2017. www.supplychain.nhs.uk/catalogue (accessed 5 October 2017).
- Walker A, Nixon J. Improving the quality of reporting in randomised controlled trials. J Wound Care 2004;13:103-6. https://doi.org/10.12968/jowc.2004.13.3.26593.
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age Ageing 2005;34:261-7. https://doi.org/10.1093/ageing/afi057.
- Prevention Plus . Home of the Braden Scale n.d. www.bradenscale.com/index.htm (accessed 20 November 2018).
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57:494-50. https://doi.org/10.1111/j.1365-2648.2006.04140.x.
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332. https://doi.org/10.1136/bmj.38849.478299.7C.
- Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0192707.
- Muir D. The Pressure Ulcer Research Service User Network (PURSUN) for the UK. Eur Wound Manage Asso J 2011;11.
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2011;13. https://doi.org/10.1002/14651858.CD001735.pub4.
- Nixon J, Nelson E A, Rutherford C, Coleman S, Muir D, Keen J, et al. Pressure UlceR Programme Of reSEarch (PURPOSE): using mixed methods (systematic reviews, prospective cohort, case study, consensus and psychometrics) to identify patient and organisational risk, develop a risk assessment tool and patient-reported outcome Quality of Life and Health Utility measures. Programme Grants Appl Res 2015;3.
- Smith IL, Brown S, McGinnis E, Briggs M, Coleman S, Dealey C, et al. Exploring the role of pain as an early predictor of category 2 pressure ulcers: a prospective cohort study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013623.
- Iglesias C, Nixon J, Cranny G, Nelson EA, Hawkins K, Phillips A, et al. Pressure relieving support surfaces (PRESSURE) trial: cost effectiveness analysis. BMJ 2006;332. https://doi.org/10.1136/bmj.38850.711435.7C.
- Brown S, Smith IL, Brown JM, Hulme C, McGinnis E, Stubbs N, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1703-8.
- World Health Organization (WHO) . Handbook for Good Clinical Research Practice (GCP) 2002. www.who.int/medicines/areas/quality_safety/safety_efficacy/gcp1.pdf (accessed 20 November 2018).
- World Health Organization (WHO) . World Medical Association Declaration of Helsinki 2001. www.who.int/bulletin/archives/79(4)373.pdf (accessed 20 November 2018).
- Department of Health and Social Care . The Medical Devices Regulations 2002 2002. www.legislation.gov.uk/uksi/2002/618/contents/made (accessed 7 August 2017).
- Nixon J, Smith IL, Brown S, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Pressure relieving support surfaces for pressure ulcer prevention (PRESSURE 2): clinical and health economic results of a randomised controlled trial [published online ahead of print September 3 2019]. EClinicalMedicine 2019. https://doi.org/10.1016/j.eclinm.2019.07.018.
- Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339-52. https://doi.org/10.1111/jan.12444.
- EuroQol Research Foundation . EQ-5D-5L 2017. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 13 October 2017).
- Gorecki C, Nixon J, Lamping DL, Alavi Y, Brown JM. Patient-reported outcome measures for chronic wounds with particular reference to pressure ulcer research: a systematic review. Int J Nurs Stud 2014;51:157-65. https://doi.org/10.1016/j.ijnurstu.2013.03.004.
- Gorecki C, Brown JM, Cano S, Lamping DL, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-95.
- Czoski MC, Meads DM, McCabe C, Edlin R, Rutherford C, Hulme CT, et al. A utility algorithm for the Pressure Ulcer Quality of Life – Utility Instrument (PUQoL-UI). Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.1582.
- Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs 1987;12:8-12. https://doi.org/10.1002/j.2048-7940.1987.tb00541.x.
- INVOLVE . Budgeting for Involvement 2014. www.invo.org.uk/posttypepublication/budgeting-for-involvement/ (accessed 20 November 2018).
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10220.
- Lan GKK, Demets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659-63. https://doi.org/10.1093/biomet/70.3.659.
- Pocock SJ, Hughes MD. Practical problems in interim analyses, with particular regard to estimation. Control Clin Trials 1989;10:209-21. https://doi.org/10.1016/0197-2456(89)90059-7.
- Fine JP, Gray RL. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. https://doi.org/10.1037/0022-3514.51.6.1173.
- Pratschke J, Haase T, Comber H, Sharp L, de Camargo Cancela M, Johnson H. Mechanisms and mediation in survival analysis: towards an integrated analytical framework. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0130-6.
- Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology 2015;26:e23-4. https://doi.org/10.1097/EDE.0000000000000253.
- Tchetgen Tchetgen EJ. On causal mediation analysis with a survival outcome. Int J Biostat 2011;7. https://doi.org/10.2202/1557-4679.1351.
- Collett D. Modelling Survival Data in Medical Research. London: CRC Press; 2014.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 10 October 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Office for National Statistics (ONS) . Inflation and Price Index CPI INDEX 06: HEALTH 2015=100 2018. www.ons.gov.uk/economy/inflationandpriceindices/timeseries/d7bz/mm23 (accessed 29 April 2018).
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR good research practices task force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171-85. https://doi.org/10.1080/01621459.1987.10478410.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Landi F, Onder G, Russo A, Bernabei R. Pressure ulcer and mortality in frail elderly people living in community. Arch Gerontol Geriatr 2007;44:217-23. https://doi.org/10.1016/j.archger.2007.01.030.
- Whittington KT, Briones R. National prevalence and incidence study: 6-year sequential acute care data. Adv Skin Wound Care 2004;17:490-4. https://doi.org/10.1097/00129334-200411000-00016.
- Halek M, Rosenberg M, Conway J, Valliappan V. Implications of the Cost of End of Life Care: A Review of the Literature. Schaumburg, IL: Society of Actuaries; 2012.
- Fassbender K, Fainsinger RL, Carson M, Finegan BA. Cost trajectories at the end of life: the Canadian experience. J Pain Symptom Manage 2009;38:75-80. https://doi.org/10.1016/j.jpainsymman.2009.04.007.
- McGinnis E, Brown S, Collier H, Faulks P, Gilberts R, Greenwood C, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) photographic validation sub-study: study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1851-5.
- Baumgarten M, Margolis DJ, Selekof JL, Moye N, Jones PS, Shardell M. Validity of pressure ulcer diagnosis using digital photography. Wound Repair Regen 2009;17:287-90. https://doi.org/10.1111/j.1524-475X.2009.00462.x.
- Defloor T, Schoonhoven L. Inter-rater reliability of the EPUAP pressure ulcer classification system using photographs. J Clin Nurs 2004;13:952-9. https://doi.org/10.1111/j.1365-2702.2004.00974.x.
- Sprigle S, Zhang L, Duckworth M. Detection of skin erythema in darkly pigmented skin using multispectral images. Adv Skin Wound Care 2009;22:172-9. https://doi.org/10.1097/01.ASW.0000305465.17553.1c.
- Great Britain . Data Protection Act 1998 1998.
- Keren G. Calibration and probability judgements: Conceptual and methodological issues. Acta Psychologica 1991;77:217-73. https://doi.org/10.1016/0001-6918(91)90036-Y.
- Lichtenstein S, Fischhoff B, Kahneman D, Slovic P, Tversky A. Judgement under Uncertainty: Heuristics and Biases. Cambridge: Cambridge University Press; 1982.
- Petrusic WM, Baranski JV. Context, feedback, and the calibration and resolution of confidence in perceptual judgements. Am J Psychol 1997;110:543-72. https://doi.org/10.2307/1423410.
- Soll JB. Determinants of overconfidence and miscalibration: the roles of random error and ecological structure. Organ Behav Hum Decis Process 1996;65:117-37. https://doi.org/10.1006/obhd.1996.0011.
- Bland JM. Measurement in Health and Disease: Cohen’s Kappa. York: Department of Health Sciences, University of York; 2008.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Osoba D. Lessons learned from measuring health-related quality of life in oncology. J Clin Oncol 1994;12:608-16. https://doi.org/10.1200/JCO.1994.12.3.608.
- Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res 2000;9:887-900. https://doi.org/10.1023/A:1008996223999.
- Food and Drug Administration . Patient Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims 2009.
- Gorecki C, Closs SJ, Nixon J, Briggs M. Patient-reported pressure ulcer pain: a mixed-methods systematic review. J Pain Symptom Manage 2011;42:443-59. https://doi.org/10.1016/j.jpainsymman.2010.11.016.
- Gorecki C, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient-focused approach. Int J Nurs Stud 2010;47:1525-34. https://doi.org/10.1016/j.ijnurstu.2010.05.014.
- Gorecki C, Nixon J, Madill A, Firth J, Brown JM. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. J Tissue Viability 2012;21:3-12. https://doi.org/10.1016/j.jtv.2011.11.001.
- Au HJ, Ringash J, Brundage M, Palmer M, Richardson H, Meyer RM. NCIC CTG Quality of Life Committee . Added value of health-related quality of life measurement in cancer clinical trials: the experience of the NCIC CTG. Expert Rev Pharmacoecon Outcomes Res 2010;10:119-28. https://doi.org/10.1586/erp.10.15.
- Smith SC, Murray J, Banerjee S, Foley B, Cook JC, Lamping DL, et al. What constitutes health-related quality of life in dementia? Development of a conceptual framework for people with dementia and their carers. Int J Geriatr Psychiatry 2005;20:889-95. https://doi.org/10.1002/gps.1374.
- Rothman ML, Beltran P, Cappelleri JC, Lipscomb J, Teschendorf B. Mayo/FDA Patient-Reported Outcomes Consensus Meeting Group . Patient-reported outcomes: conceptual issues. Value Health 2007;10:66-75. https://doi.org/10.1111/j.1524-4733.2007.00269.x.
- Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res 2012;21:1305-14. https://doi.org/10.1007/s11136-011-0054-x.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Rutherford C, Costa D, Mercieca-Bebber R, Rice H, Gabb L, King M. Mode of administration does not cause bias in patient-reported outcome results: a meta-analysis. Qual Life Res 2016;25:559-74. https://doi.org/10.1007/s11136-015-1110-8.
- Rutherford C, Brown JM, Smith I, McGinnis E, Wilson L, Gilberts R, et al. A patient-reported pressure ulcer health-related quality of life instrument for use in prevention trials (PU-QOL-P): psychometric evaluation. Health Qual Life Outcomes 2018;16. https://doi.org/10.1186/s12955-018-1049-x.
- Rutherford C, King MT, Smith DP, Costa DS, Tait MA, Patel MI. NMIBC-SI Working Group . Psychometric evaluation of a patient-reported symptom index for nonmuscle invasive bladder cancer: field testing protocol. JMIR Res Protoc 2017;6. https://doi.org/10.2196/resprot.8761.
- Streiner DL, Norman G, Cairney J. Health Measurement Scales: A Practical Guide to their Development and Use. New York, NY: Oxford University Press; 2015.
- Leeds Institute of Clinical Trials Research (LICTR) . Purpose PUQOL n.d. http://medhealth.leeds.ac.uk/info/423/skin/1738/purpose_puqol/2 (accessed 5 December 2018).
- Santy J. Hospital mattresses and pressure sore prevention. J Wound Care 1995;4:329-32. https://doi.org/10.12968/jowc.1995.4.7.329.
- NHS Improvement . Safety Thermometer 2013. www.safetythermometer.nhs.uk/index.php?option=com_content&view=article&id=2&Itemid=106 (accessed 3 November 2017).
- Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care 2004;13:145-51. https://doi.org/10.1136/qshc.2002.003822.
- Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ 2001;322:517-19. https://doi.org/10.1136/bmj.322.7285.517.
- Pawson R, Tilley N. Realist Evaluation. London: Sage Publications Ltd; 1997.
- Pinkney L, Nixon J, Wilson L, Coleman S, McGinnis E, Stubbs N, et al. Why do patients develop severe pressure ulcers? A retrospective case study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004303.
- Royal College of Physicians . National Audit of Inpatient Falls 2015. www.rcplondon.ac.uk/projects/outputs/naif-audit-report-2015 (accessed 7 April 2019).
- Rubenstein LZ, Powers CM, MacLean CH. Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann Intern Med 2001;135:686-93. https://doi.org/10.7326/0003-4819-135-8_Part_2-200110161-00007.
- Wailoo A, Hernandez Alava M, Grimm S, Pudney S, Gomes M, Sadique Z, et al. Comparing the EQ-5D-3L and 5L Versions: What are the Implications for Cost Effectiveness Estimates?. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2017.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment: A Practical Course. Basel: Adis, Springer International Publishing; 2015.
- Padula WV, Mishra MK, Makic MB, Sullivan PW. Improving the quality of pressure ulcer care with prevention: a cost-effectiveness analysis. Med Care 2011;49:385-92. https://doi.org/10.1097/MLR.0b013e31820292b3.
- Department of Health and Social Care . Attributing Revenue Costs of Externally Funded Non-Commercial Research in the NHS (ARCO) 2005. http://webarchive.nationalarchives.gov.uk/20130104234501/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4125280 (accessed 3 November 2017).
- Department of Health and Social Care . Attributing the Costs of Health and Social Care Research 2015. www.gov.uk/government/publications/guidance-on-attributing-the-costs-of-health-and-social-care-research.
- Bours GJ, Halfens RJ, Lubbers M, Haalboom JR. The development of a national registration form to measure the prevalence of pressure ulcers in the Netherlands. Ostomy Wound Manage 1999;45:28-33.
- Nixon J, Thorpe H, Barrow H, Phillips A, Nelson AE, Mason SA, et al. Reliability of pressure ulcer classification and diagnosis. J Adv Nurs 2005;50:613-23. https://doi.org/10.1111/j.1365-2648.2005.03439.x.
- Vanderwee K, Grypdonck MH, De Bacquer D, Defloor T. The reliability of two observation methods of nonblanchable erythema, Grade 1 pressure ulcer. Appl Nurs Res 2006;19:156-62. https://doi.org/10.1016/j.apnr.2005.06.005.
- Muir D, King N, Brooks J. Applied Qualitative Research in Psychology. Basingstoke: Palgrave Macmillan; 2017.
- Standards Centre . BS 3379:2005 2006. www.standardscentre.co.uk/bs/BS-3379-2005/?s=1 (accessed 7 April 2019).
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. https://doi.org/10.1016/S0140-6736(02)09832-X.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation: Handbooks in Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. https://doi.org/10.1177/0272989X04263162.
- Hall PS, Edlin R, Kharroubi S, Gregory W, McCabe C. Expected net present value of sample information: from burden to investment. Med Decis Making 2012;32:E11-21. https://doi.org/10.1177/0272989X12443010.
- NHS Digital . Hospital Episodic Statistics. Accident and Emergency Attendances in England 2013–14 2015.
- Department of Health and Social Care . Reference Costs 2011–12 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213060/2011-12-reference-costs-publication.pdf (accessed 30 April 2018).
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- R Core Team . A Language and Environment for Statistical Computing 2014. www.r-project.org/ (accessed 30 April 2018).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016: Organisation Level Source Data Part 1: Data 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 1 June 2019).
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- NHS England . NHS National Tariff Payment System. Annex A: 2106 17 National Prices and National Tariff Workbook 2016. www.gov.uk/government/publications/nhs-national-tariff-payment-system-201617 (accessed 1 June 2019).
- National Institute of Health and Care Excellence (NICE) . Costing Statement: PU Implementing the NICE Guidelines on PU (CG179) 2014. www.nice.org.uk/guidance/cg179/resources/costing-statement-248688109 (accessed 1 June 2019).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Office for National Statistics . Statistical Bulletin: National Life Tables, UK: 2014 to 2016 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014to2016 (accessed 10 October 2017).
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht: Springer; 2014.
Appendix 1 Mattress specification guide
Mattress specification guideline: PRESSURE 2 trial
All centres are requested to provide a list of mattresses that are in use in each hospital and to be used in the trial.
-
All participants will have an electric profiling bed frame as an adjunct to the trial mattress.
-
All participants will be randomised to either a HSFM or an APM. Overlay or replacement mattresses may be used.
-
After randomisation, an eligible mattress will be sourced and allocated by the clinical research nurse.
-
All mattresses and their use will comply with the Medical Devices Regulations SI2002/618.
Mattress specifications
Pressure-relieving mattresses can be divided into low-technology devices and high-technology devices:
-
Low-technology devices provide a comfortable surface that redistributes body weight over a large surface area. Examples are standard foam mattresses, HSFMs, visco-elastic mattresses, cubed foam, convoluted foam (these aim to redistribute body weight over a larger contact area).
-
High-technology devices are dynamic systems that include APMs where the patient lies on air-filled sacs that sequentially inflate and deflate and relieve pressure at different anatomical sites for short periods.
Mattresses included in the trial
Alternating pressure mattress
-
All mattresses included in the trial should be currently in use in the recruiting centre and comply fully with local medical devices and infection control standards.
-
All mattresses included should be fully automatic; some may have dual therapy, for example the mattress comprises a combination of alternating pressure or low air loss. The trial will include only those participants nursed on the alternating pressure mode of action.
Alternating pressure mattresses should have the following minimum function, as described in Table 26.
| Specification | APM | |
|---|---|---|
| Replacements | Overlays | |
| Cell height (cm) | 19.6–29.4 | 8.5–12.5 |
| Cycle time (minutes) | 7.5–30 | 7.5–30 |
| Cycle frequency | 1 in 2, 3 or 4 | 1 in 2, 3 or 4 |
High-specification foam mattresses
-
All mattresses included in the trial should be currently in use in the recruiting centre and comply fully with local medical devices and infection control standards.
-
Mattresses can be high-density foam, viscoelastic (memory) foam or a combination of both, and can be castellated (for ventilation and profiling).
-
All mattresses will have a cover with the following characteristics: removable, minimum two-way stretch, vapour permeable and covered zips as defined in BS 3379.36. 122
-
All mattresses should be replacement mattresses with a minimum depth of 150–200 mm.
Excluded mattresses
The following mattresses were excluded from the trial:
-
hybrid mattresses with combination therapy, namely mattresses comprising a static layer with alternating cells/low air loss [e.g. AtmosAir™ (ArjoHuntleigh, Houghton Regis, UK)]
-
alternating mattresses that have collapsible cells [e.g. Nimbus® Professional (ArjoHuntleigh, Houghton Regis, UK)]
-
low air-loss mattresses [e.g. Breeze (ArjoHuntleigh, Houghton Regis, UK)]
-
static foam and gel mattresses
-
continuous static low-pressure mattresses, including fibregel
-
fluid, clay [e.g. Rik® (ArjoHuntleigh, Houghton Regis, UK)]
-
air-filled mattresses. [e.g. Repose (Frontier Medical group, Wales, UK)]
-
beds and mattresses that have a motorised repositioning option. [e.g. Acer (ArjoHuntleigh, Houghton Regis, UK)]
-
Air-fluidised bead beds. [e.g. Clinitron® (Hill-Rom, Chicago, IL, USA)].
Appendix 2 Derivation of primary end point
End points to be analysed
Primary end point
The primary end point was the time taken to develop a new category ≥ 2 PU from randomisation to 30 days post treatment phase or withdrawal/death.
Derivation of primary end point
Each participant had a minimum of 14 prespecified skin sites [spine/back, sacrum, left and right buttocks, ischial tuberosities, trochanters (hips), heels, ankles and elbows] assessed at baseline and at every follow-up assessment thereafter. There was the option at each assessment to add other additional skin sites that were not prespecified on the CRF. The data collection process outlined above led to repeated measures for each skin site. Therefore, before deriving whether or not a participant developed a new category ≥ 2 PU (and the time to development), the derivation of whether or not a new category ≥ 2 PU has developed since baseline needed to be defined on a skin site basis (Figure 16). This led to a data set with one record per skin site. These data were then used to derive the primary end point data set with one record per participant that included a variable denoting whether or not the participant developed a category ≥ 2 PU at any skin site and the time to develop the first new PU or censoring time. A summary of the derivation of the primary end point (time to first new category ≥ 2 PU on a patient level) is provided in Figure 17 and Table 27.
FIGURE 16.
Derivation of PU development on a skin site level (one record per skin site per participant). a, Patients with a category 3/4/U PU should have been excluded from the trial in accordance with the eligibility criteria; however they have been included in this derivation to account for participants who may have been incorrectly recruited to the trial. N/A, not applicable; U, unstageable. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
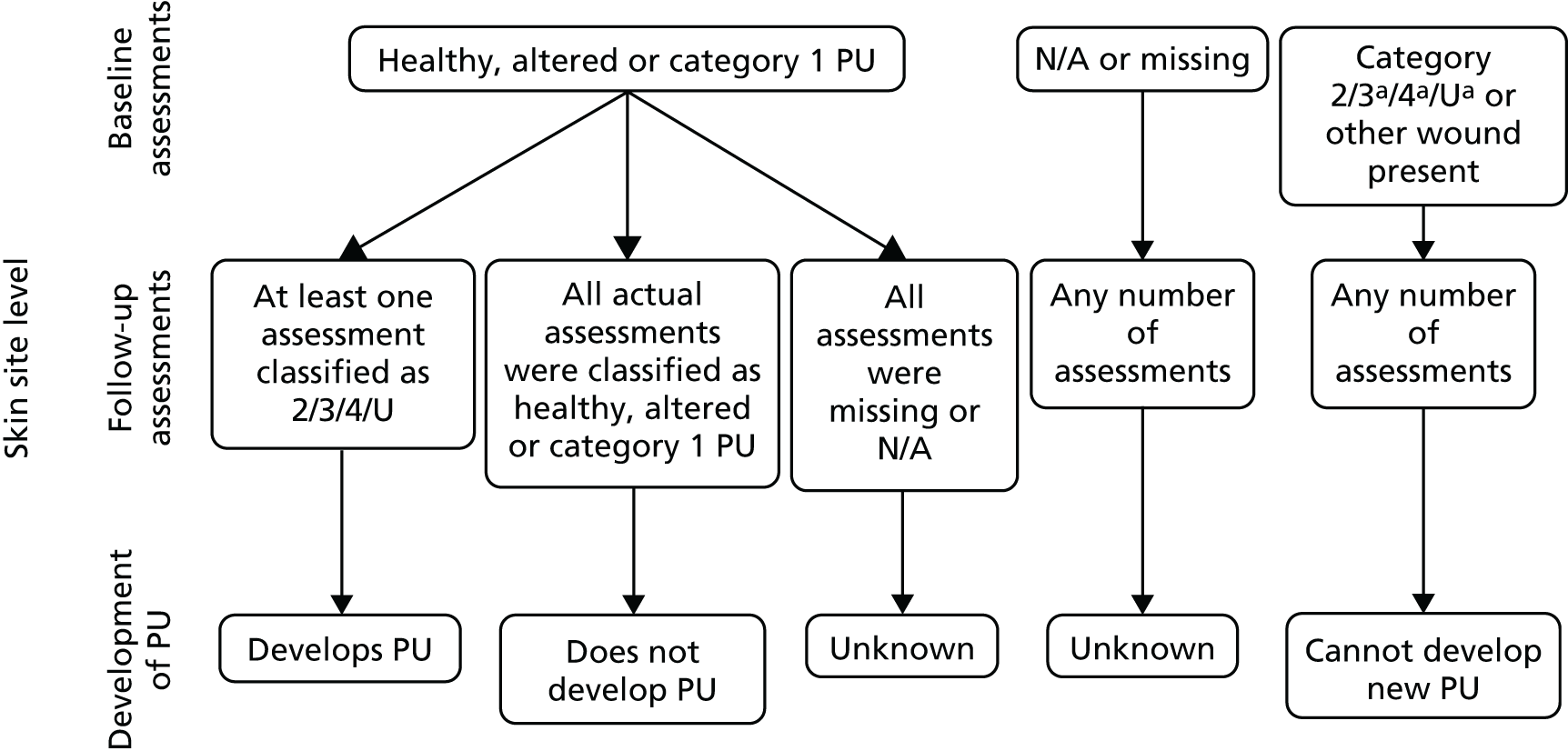
FIGURE 17.
Derivation of PU development at a patient level. a, Evaluable skin sites are such that skin site level outcome can be determined as ‘develops PU’ or ‘does not develop PU’ (see Figure 12). b, The maximum follow-up period was 95 days, to allow for time windows around visits during the treatment phase and around the final visit after the treatment phase. Adapted from Nixon et al. 44 © The Authors, 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

| Scenario | Number of days | Event/censor variable |
|---|---|---|
| Patient is observed to develop a new category ≥ 2 PU before end of follow-up | Number of days between date of randomisation and date first new category ≥ 2 PU was observed (i.e. date first recorded on the CRF) | Event |
| Patient dies before end of follow-up not having developed a category ≥ 2 PU | Number of days between date of randomisation and date of last evaluable skin assessment | Censoreda at date of last evaluable skin assessment |
| Patient withdraws from the trial before end of follow-up not having developed a category ≥ 2 PU | Number of days between date of randomisation and date of last follow-up assessment when an assessment on an evaluable skin site was made | Patient is censoreda at date of last evaluable skin assessmentb |
| Patient is not observed to develop a new category ≥ 2 PU before the end of follow-up (including patients who were lost to follow-up) | Number of days between date of randomisation and date of last follow up assessment where an assessment on an evaluable skin site was made | Patient is censored at date of last evaluable skin site assessment |
The time to development of a new category ≥ 2 PU and the value of the censor variable (a variable denoting whether or not the participant was observed to develop a category ≥ 2 PU) will be derived during the analysis process, as detailed in Table 27.
Other end points
The determination of development of a PU of category 1, 2 or 3 within the treatment phase was derived in line with the above process, adjusted for baseline skin status and relevant time windows.
A category 2 PU was classified as healed if the same skin site was later recorded as healthy or altered skin. The time to healing was calculated as the number of days between the date of randomisation and the date that the last category 2 PU was observed to heal, or patients were censored at the date of last evaluable assessment in line with the rules from above.
Missing data
Attempts were made to retrieve missing data via a thorough data cleaning process. Every effort was made to obtain complete dates for all key data and missing dates were monitored.
Appendix 3 Value-of-information analysis and interim analysis
Reflecting the double-triangular sequential design, a maximum of three interim analyses with unequally spaced reviews at event-driven coherent cut-off points of 300, 445 and 580 PU events were planned originally. As outlined in Chapter 2, Trial design, in the event of an early stopping signal for futility, an assessment of the value of continuing with the trial from the NHS decision-making perspective, via an EVSI analysis, to inform the deliberations of the DMEC was also planned.
As the trial recruited participants at a much slower rate than originally anticipated, a recovery plan and unplanned VOI analysis using parameter estimates from the original trial design were requested by the funder. These were submitted to the funder in January 2015 and reviewed by the DMEC, which recommended trial continuation.
Following a request for a non-costed recruitment extension, an unplanned interim analysis and second VOI analysis, both using confidential data from the trial, were requested by the funder and conducted in November/December 2015. All data were reviewed by the DMEC. Methods and results of the interim analysis and second VOI analysis are detailed later in this appendix.
Part 1: value of information analysis
Background
When deciding whether or not to adopt a new treatment technology, there is always some degree of uncertainty because there is always a chance that the choice turns out to be wrong. VOI analysis tries to measure the expected cost of that uncertainty, which ‘can be interpreted as the expected value of perfect information (EVPI), since perfect information can eliminate the possibility of making the wrong decision’. 123 For a detailed, well-explained description and introduction of how to do a VOI analysis, see Briggs et al. ;124 for the specific methods used in this analysis, see Ades et al. 125 and, especially, Hall et al. 126
First value-of-information analysis
In the first VOI analysis, a computer model was constructed to simulate the differences in costs and effects of two treatment alternatives for prevention of PUs in hospital care. The two treatment alternatives were HSFMs and APMs. The base model was then expanded to simulate clinical trials in that setting, to compare the value of trials with three different patient sample sizes (1508, 2236 and 2954 patients). The value of those trial sample sizes was assessed on generating additional information about the relative effectiveness of the two treatment alternatives compared with the base model results. The model was constructed in the statistical computing software R. Model results and estimates of trial costs were evaluated using the R software and Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Methods
Structure, perspective and inputs of the model were chosen in consultation with, and through input from, the clinical experts of the trial as well as through consulting the relevant published literature.
Model structure
The overall expected net benefit of sampling (ENBS) was calculated by subtracting, for each trial size, the estimated costs of running the trial from the expected net present value of sampling information (ENPVSI). For calculating the ENPVSI, which provides a monetary estimate of the net value of running a trial, the authors followed a three-step approach from the published literature. The first step was a base model run with a cost-effectiveness analysis that, given pre-trial or ‘current information’ knowledge, tried to find the optimal treatment strategy. In this case, it found APMs to be optimal.
The second step was to simulate a trial with half of the trial participants getting one treatment (HSFM) and the other half getting the other treatment (APM), while the remaining participants (all cohort participants minus the in-trial participants) were simulated as being treated with whatever was considered optimal in the first step (i.e. APM in this case).
The third step was then to find the optimal treatment strategy after the trial had finished and implement it for all participants after the trial and for the remaining lifetime of the treatment technology (i.e. 20 years minus trial time in this case). This was done by combining the ‘current information’ before the trial with the new information from the simulated trial results, and then finding the optimal strategy based on this combined ‘updated information’. If the optimal strategy, after the simulated trial, stayed the same, then the trial confirmed the ‘current information’ but had little or negative additional monetary/health value because it did not change the optimal strategy. Alternatively, if the trial did lead to a change in the optimal strategy (compared with step one), then the trial had a high value. This was because switching to the real optimal strategy for all participants after the trial was better (more health improvement per money spent) than sticking with the previous treatment strategy.
Steps 2 and 3 of this approach are repeated many times (i.e. 1000 times in this case) in order to simulate a large number of possible trial outcomes. The resulting mean value of all trial simulations represents the ENPVSI. The ENPVSI was calculated for three different assumptions regarding the possible increase in adoption if the trial finds APM to be more significantly effective (Table 29). The base cost-effectiveness model was a so-called discrete state-transition model, which was used for probabilistic simulation of treatments and disease pathways. The base model ran 2000 simulations initially and afterwards for evaluating each of the simulated trials.
Model inputs and perspective
The model perspective was the NHS; outcomes were measured in QALYs for effectiveness and in 2014 Great British pounds (GBP) for costs. Outcomes were discounted at 3.5% per year. The trial simulation was based on the assumption that information generated in a trial could benefit patients in England within the coming 20 years and that the annual cohort of patients who could benefit has a size of 1,153,535. For the number of patients who could benefit, the current number of patients who receive an APM annually was estimated, and it was estimated that there will be a 50% increase in APM mattress use if the trial demonstrates superior effectiveness. To estimate the number of current patients receiving APMs, annual admissions (excluding paediatrics, obstetrics and day cases) were obtained from two large NHS health trusts (n = 177,000 patients): their mean annual APM mattress usage was 8.5%. These data were extrapolated using figures from NHS England’s accident and emergency (A&E) quarterly activity statistics and quarterly activity return127 and determined the number of NHS admissions as 27,142,004 patients. Based on the previous cohort and prevalence data suggesting that 50% of patients at risk do not currently receive an APM, and in consultation with the clinical co-applicants, it was estimated that mattress use will increase by 50% of 8.5% (4.25 percentage points) and the annual cohort of patients who could benefit was thus determined as n = 1,153,535. Two sensitivity analyses were also ran in which the increase was assumed to be 25% and 75%, to see how that would change the results.
Based on trial enrolment data, the assumed starting age of the hypothetical cohort of patients was, on average, 80 years and their PU status was 89% PU-free and 11% with one PU, whereas only a category ≥ 2 PU was regarded as PU presence. Health status changes could occur every 3 days (cycle length) and the cohort was followed over a model horizon of 3 years. The model used initial result data for mean time in hospital to treat the initial condition and data from the first PRESSURE trial for mean healing time. For relative effectiveness, the authors took the pre-trial expected PU incidence rates of 23% and 18% in the HSFM and APM arms, respectively, and updated them using interim (overall) trial results from November 2014, provided by the trial team.
The cost side of the model assumed, per patient and day, a mean excess treatment cost for the presence of one PU of £158.13 and for the presence of two PUs of £210.79, based on Dealey et al. 24 Mean specialised mattress costs of £0.08 (for HSFM) and £1.95 (for APM) were assumed, both based on the PU prevention guideline12 and durability estimates from the trial team. Furthermore, a standard hospital bed rate of £280.59 was assumed,128 excluding specialised mattresses, and, if discharged with PUs, a daily cost for home care by a visiting nurse of £15.69 for one PU and £20.17 for two PUs was assumed (hourly costs were obtained from Curtis;129 frequency and time requirements were obtained from expert opinion). The rounded estimates for the overall trial costs were, from the smallest to the largest sample size, £2.75M, £3.4M and £4.0M, and were provided by the trial team.
Results
The results of the base-case model indicated that, overall, the APM was slightly more effective and less costly than the HSFM (Table 28). The APM is therefore optimal (i.e. net benefit maximising) at both willingness-to-pay thresholds (£20,000 and £50,000 per QALY), but it is optimal by only a small margin. The higher initial costs of PU prevention in the APM arm of the model were more than offset by cost savings due to the reduced PU incidence and, consequently, the reduced PU treatment costs.
| Treatment | Benefit (QALYs) | Cost (£) | Net benefit (£) | |
|---|---|---|---|---|
| WTP = £20,000 | WTP = £50,000 | |||
| HSFM | 2.2394 | 13,143 | 31,646 | 98,830 |
| APM | 2.2402 | 12,975 | 31,829 | 99,036 |
The results of the trial simulation using different sample sizes showed a positive expected net benefit value for each of the trial sizes at both willingness-to-pay per QALY levels and for all three assumptions concerning the increase in APM usage (Table 29). This means that a trial of any of those sizes is a very useful investment of health budget funds, with NMBs after trial costs of between £92.4M and £290M. However, the exact amount and trends between sample sizes vary depending on what increase-of-usage assumption one deems most realistic. The value of the trial increases together with the increase-of-usage assumption because the more patients who are affected by the adoption decision, the more it pays off to have accurate information on which to base the decision.
| Attribute | Early stopping | Full size | |
|---|---|---|---|
| 1 | 2 | ||
| Completeness of trial (%) | 51.0 | 75.7 | 100.0 |
| Trial size (n participants) | 1766 (1500 + 266a) | 2565 (2236 + 329a) | 2954 |
| Trial costsb (£) | 2,750,000 | 3,400,000 | 4,034,245 |
| Assumed patient population base (% of increase in use of APM or equivalent): 576,768 (25%) | |||
| Willingness-to-pay level = £20,000 (£) | 96,295,281 | 92,421,711 | 96,634,090 |
| Willingness-to-pay level = £50,000 (£) | 109,075,473 | 104,780,678 | 109,613,918 |
| Assumed patient population base (% of increase in use of APM or equivalent): 1,153,535 (50%) | |||
| Willingness-to-pay level = £20,000 (£) | 219,378,516 | 222,483,637 | 211,648,255 |
| Willingness-to-pay level = £50,000 (£) | 248,500,241 | 252,056,859 | 239,896,506 |
| Assumed patient population base (% of increase in use of APM or equivalent): 1,730,303 (75%) | |||
| Willingness-to-pay level = £20,000 (£) | 254,416,174 | 276,231,919 | 290,161,149 |
| Willingness-to-pay level = £50,000 (£) | 287,979,848 | 312,797,604 | 328,484,201 |
The following considerations are based, first, on each and every trial reporting statistically significant results supporting the increase of APM usage and, second, on assuming that the sample variation played only a minor role in the observed outcomes. The latter was deemed to be the case after closer analysis of the results and the former needed to be determined by the trial team.
For a 75% increase, the value of the trial increases with each sample size and therefore suggests continuation of the trial until the full recruitment size is reached. The same suggestion follows from a 25% increase assumption, although going to early stopping 2 only would not be beneficial. On the other hand, for a 50% increase, the simulation indicates that early stopping 2 may be optimal. Again, all those considerations are based on the trial at those sizes reporting statistically significant results, although it can of course turn out that, even for a 50% increase, a larger sample size would be better in order to achieve a higher statistical reliability.
In the trial sampling model runs, HSFM was optimal in < 22% of all trial samples only. However, the positive value of sampling for all three sizes is derived from those cases alone. In the other cases, the simulated trials confirm only that the APM is optimal. Those trial samples for which HSFM was optimal (i.e. < 22% of all trial samples) come from the simulations in which APM practically does not reduce the PU incidence compared with HSFM (no risk reduction).
In the sensitivity analysis, how sensitive the results are to changes in specific model inputs was investigated. During this process, the authors found that there is a strong dependence of the results on the amount of uncertainty associated with the difference in the effectiveness of the two interventions. Depending on this assumption, the expected value of doing a trial can flip from positive to negative when assuming only a small degree of uncertainty. The authors assumed that there was a substantial uncertainty about this model input as this is the primary reason for doing the trial. This uncertainty was incorporated into the model by applying a relative SD of 25% onto the input variable that reflects the difference in the effectiveness of the two mattresses.
Recommendations
The authors’ expectation is that the expected net benefit obtained by carrying on with the trial is positive, taking into account the time it will take for the trial to deliver results and the continued uncertainty with regard to the best practice, while the trial continues. From the VOI perspective, the trial, as originally designed, is well equipped to generate very high-value results, although, when assuming a 50% increase in APM usage, the second largest sample size would be sufficient. However, caution should be exercised around any decision to reduce the target sample size as the acceptability of the trial to its target audience may be significantly damaged by any perception that it is underpowered. In addition, the model assumed an effectiveness difference between the two treatments that is similar to pre-trial expectations. If the interim results show something different, then this needs to be factored into the decision of continuing the trial and the determination of sample size. This observed effectiveness difference and the desired statistical power play an important role in this decision. The authors recommended, fully conditional on the mentioned considerations, that the trial be continued until the full sample size is reached or, when assuming a 50% increase in APM usage, until the second largest sample size is reached.
Second value-of-information analysis
In the second VOI analysis, conducted as part of the unplanned interim analysis, a probabilistic decision-analytic cost-effectiveness model was constructed to simulate the differences in costs and effects of two treatment alternatives for the prevention of PUs in hospital care. The two treatment alternatives were HSFM and APM. This base model was then expanded to simulate clinical trials in that setting, to compare the value of the information provided by trials with two different sample sizes; 902 and 1996 subjects, respectively. The value of the alternative trials was assessed by comparing the impact of the additional information that they would generate on the estimates of the relative clinical effectiveness and cost-effectiveness of the two treatment alternatives, compared with the base model results. The model was constructed in the statistical computing software R. The model130 results and estimates of trial costs were evaluated using the R software and Microsoft Excel.
Methods
The authors chose structure, perspective and inputs of the model in consultation with and through input from the clinical expert members of the trial team, combined with interim trial results and evidence from the published literature.
The overall ENBS was calculated by subtracting, for each trial size, the estimated costs of running the trial from the ENPVSI. For calculating the ENPVSI, which provides a monetary estimate of the net value of running a trial, the authors followed a three-step approach from the published literature. 123,124,126 The first step was a base model run with a cost-effectiveness analysis that, given pre-trial or ‘current information’ knowledge, tried to find the optimal treatment strategy. In this case it found APM to be optimal.
The second step was to simulate a trial with half of the trial participants getting one treatment (HSFM) and the other half getting the other treatment (APM), while the remaining patients (all cohort patients minus the in-trial patients) were simulated as being treated with whatever was considered optimal in the first step (i.e. APM in this case).
The third step was then to find the optimal treatment strategy after the trial had finished and implement it for all patients after the trial and for the remaining lifetime of the treatment technology (which here was 20 years minus trial time). This was done by combining the ‘current information’ before the trial with the new information from the simulated trial results, and then finding the optimal strategy based on this combined ‘updated information’. If the optimal strategy, after the simulated trial, stayed the same, then the trial confirmed the ‘current information’ but had little or negative additional monetary/health value because it did not change the optimal strategy. On the other hand, if the trial did lead to a change in the optimal strategy (compared with step 1), then the trial had a high value. This was because switching to the real optimal strategy for all patients after the trial would be better (more health improvement per money spent) than sticking with the previous treatment strategy.
Steps 2 and 3 of this approach are repeated many times (i.e. 1000 times in this case) to simulate a large number of possible trial outcomes. The resulting mean value of all trial simulations represents the ENPVSI. The ENPVSI was calculated for three different assumptions regarding the possible increase in adoption if the trial finds APM to be more significantly effective (Table 31). The base cost-effectiveness model was a so-called discrete state-transition model, which was used for probabilistic simulation of treatments and disease pathways. The base model ran 2000 simulations initially and afterwards for evaluating each of the simulated trials.
Model inputs and perspective
The model perspective was the NHS and outcomes were measured in QALYs for effectiveness and in 2014 GBP for costs. Outcomes were discounted at 3.5% per year. The trial simulation was based on the assumptions that information generated in a trial could benefit patients in England within the coming 20 years and that the annual cohort of patients who could benefit has a size of 1,153,535. For the cohort size, we estimated the current number of patients who already receive an APM annually and estimated that there would be a 50% increase in APM use if the trial demonstrated superior effectiveness. To estimate the number of current patients receiving APMs, annual admissions (excluding paediatrics, obstetrics and day cases) were obtained from two large NHS health trusts (n = 177,000): their mean annual APM usage was 8.5%. These data were extrapolated using figures from NHS England’s A&E quarterly activity statistics and quarterly activity return and determined that the annual number of NHS admissions was 27,142,004 patients. Based on the previous cohort and prevalence data, suggesting that 50% of patients at risk do not currently receive an APM, and in consultation with the clinical co-applicants, it was estimated that mattress use will increase by 50% of 8.5% (4.25 percentage points); thus, the annual cohort of patients who could benefit was determined as 1,153,535. Two sensitivity analyses were also run in which the increase was assumed to be 25% and 75%, in order to see how that would change the results.
Based on trial enrolment data, the assumed starting age of the hypothetical cohort of patients was, on average, 80 years and their PU status was 89% PU-free and 11% with one PU, whereas only a PU of category ≥ 2 was regarded as PU presence. Health status changes could occur every 3 days (cycle length) and the cohort was followed over a lifetime model horizon. The model used initial result data for mean time in hospital to treat the initial condition and data from the first PRESSURE trial for mean healing time. For relative effectiveness, the authors took the interim results of the trial with the recorded PU incidence rates in the HSFM and APM arms, respectively, using the results from December 2015 provided by the trial team.
The cost side of the model assumed, per patient and day, a mean excess treatment cost for the presence of one PU of £158.13 and for the presence of two PUs of £210.79, based on Dealey et al. 24 Mean specialised mattress costs of £0.08 (for HSFM) and £1.95 (for APM), both based on the PU prevention guideline12 and durability estimates from the trial team. Furthermore, a standard hospital bed rate of £280.59117 was assumed, excluding specialised mattresses, and, if discharged with PUs, a daily cost for home care by a visiting nurse of £15.69 for one PU and £20.17 for two PUs was assumed (hourly costs were obtained from Curtis and Burns,62 frequency and time requirements were obtained from expert opinion). Only costs that were caused by the condition under consideration were modelled; once discharged without a PU, no cost was assumed. The rounded estimates for the overall trial costs were £2.75M for the smaller and £3.40M for the larger trial size, and they were provided by the trial team.
Results
The results of the base-case model indicated that, overall, APMs were slightly more effective and less costly than HSFMs (Table 30). APM is therefore considered optimal, that is net benefit maximising, at both willingness-to-pay thresholds (£20,000 and £50,000 per QALY), but it is optimal by only a small margin. The higher initial costs of PU prevention in the APM arm of the model were more than offset by cost savings due to the reduced PU incidence and, consequently, the reduced PU treatment costs.
| Treatment | Benefit (QALYs) | Cost (£) | Net benefit (£) | |
|---|---|---|---|---|
| WTP = £20,000 | WTP = £50,000 | |||
| HSFM | 63,082 | 9872 | 116,291 | £305,537 |
| APM | 63,091 | 9762 | 116,419 | £305,691 |
The results of the trial simulation using different sample sizes showed a positive expected net benefit value for each of the trial sizes at both willingness-to-pay per QALY levels and for all three assumptions concerning the increase in APM usage (Table 31). This means that a trial of any of those sizes is a very useful investment of health budget funds, with NMBs after trial costs of between £24.3M and £263M. However, the amounts and trends between sample sizes vary depending on the sample sizes themselves and on what increase-of-usage assumption one deems most realistic. The value of the trial increases together with the increase-of-usage assumption because the more patients who are affected by the adoption decision, the more it pays off to have accurate information on which to base the decision.
| Attribute | Early stopping | |
|---|---|---|
| 1 | 2 | |
| Trial size (number of participants) | 902 | 1996 |
| Trial costsa (£) | 2,750,000 | 3,400,000 |
| Assumed patient population base (% increase in use of APM or equivalent): 576,768 (25%) | ||
| Willingness-to-pay level = £20,000 (£) | 24,314,486 | 61,675,336 |
| Willingness-to-pay level = £50,000 (£) | 29,681,444 | 74,688,674 |
| Assumed patient population base (% increase in use of APM or equivalent): 1,153,535 (50%) | ||
| Willingness-to-pay level = £20,000 (£) | 61,774,918 | 144,695,042 |
| Willingness-to-pay level = £50,000 (£) | 74,608,874 | 174,307,316 |
| Assumed patient population base (% increase in use of APM or equivalent): 1,730,303 (75%) | ||
| Willingness-to-pay level = £20,000 (£) | 94,061,129 | 218,794,343 |
| Willingness-to-pay level = £50,000 (£) | 113,316,961 | 263,223,397 |
The following considerations are based, first, on each and every trial reporting statistically significant results supporting the increase of APM usage and, second, on assuming that the sample variation played only a minor role in the observed outcomes. The latter was deemed to be the case after closer analysis of the results. The former needed to be determined by the trial team.
For all three increase-of-usage assumptions, the value of the trial increases with sample size and therefore suggests continuing the trial until the largest recruitment size is reached. Again, all those considerations are based on the trial at those sizes reporting statistically significant results, and a larger sample size would be better in order to achieve a higher statistical reliability.
In the trial sampling model runs, HSFM was optimal in < 16% of all trial samples. However, the positive value of sampling for both possible trials is derived from those cases alone. In the other cases, the simulated trials confirm that the APM treatment only is optimal. Those trial samples for which HSFM was optimal (i.e. < 16%) come from the simulations in which APM treatment does not reduce the PU incidence enough compared with HSFM treatment to offset the higher initial costs of APM treatment.
The sensitivity analysis investigated how sensitive the results are to changes in specific model inputs. During this process, the authors found that there is a strong dependence of the results on the amount of uncertainty associated with the HR of developing a PU in the APM arm. The authors assumed that there was a substantial uncertainty about this model input as this is the primary reason for doing the trial. This uncertainty was incorporated into the model by applying a relative SD of 25% onto this input variable.
Recommendations
The analyses indicate that the net benefit obtained by carrying on with the trial is positive, taking account of the time it will take for the trial to deliver results, and the continued uncertainty with regard to the best practice while the trial continues. From the VOI perspective, the trial, as originally designed, is well equipped to generate very high-value results, especially at the larger sample size. Caution should be exercised around any decision to reduce the target sample size as the acceptability of the trial to its target audience may be significantly damaged by any perception that it is underpowered, possibly even more so in the light of the interim trial results. In addition, the model showed that there is substantial uncertainty around the current cost-effectiveness of both treatment alternatives, with cost-effectiveness being sensitive to changes in the reduction in PU incidence that results from APM use. This needs to be factored into the decision of continuing the trial and the determination sample size. The observed effectiveness difference and the desired statistical power play an important role in this decision. The authors recommend, fully conditional on the mentioned considerations, to continue the trial until the larger sample size is reached.
Part 2: interim analysis
Methods
Primary end-point analysis
Primary analysis
The primary end-point analysis was conducted on both the ITT population and the PPP.
A Cox proportional hazards model was initially fitted to the primary end point with the minimisation factors (i.e. health-care setting, PU status and consent) and the covariates (i.e. presence of pain on a healthy, altered or category 1 PU skin site, conditions affecting peripheral circulation, and mattress group). A competing risks analysis was not conducted.
For the ITT patient population, as there was evidence of non-proportional hazards for mattress group, a piecewise Cox model was fitted, splitting the data into two timeframes corresponding to time to development of PUs of ≤ 60 days and time to development of PUs of > 60 days. A Cox proportional hazards model was then fitted to each of these two timeframes.
For the PPP, the assumption of proportional hazards for mattress group was met.
Sensitivity analysis
A Cox proportional hazards model was fitted to the primary end point (i.e. time to developing a PU of category ≥ 2 during the treatment phase) with the same covariates fitted as for the primary analysis, on both the ITT and the PPPs. For both populations, the assumption of proportional hazards was met for the covariate mattress group.
Monitoring was conducted using East software (East 6, Cytel Software Inc., Cambridge, MA, USA) and the output presented (Figure 18).
FIGURE 18.
The EAST output for the sensitivity analysis of the primary end point for the ITT population. Stopping boundaries.
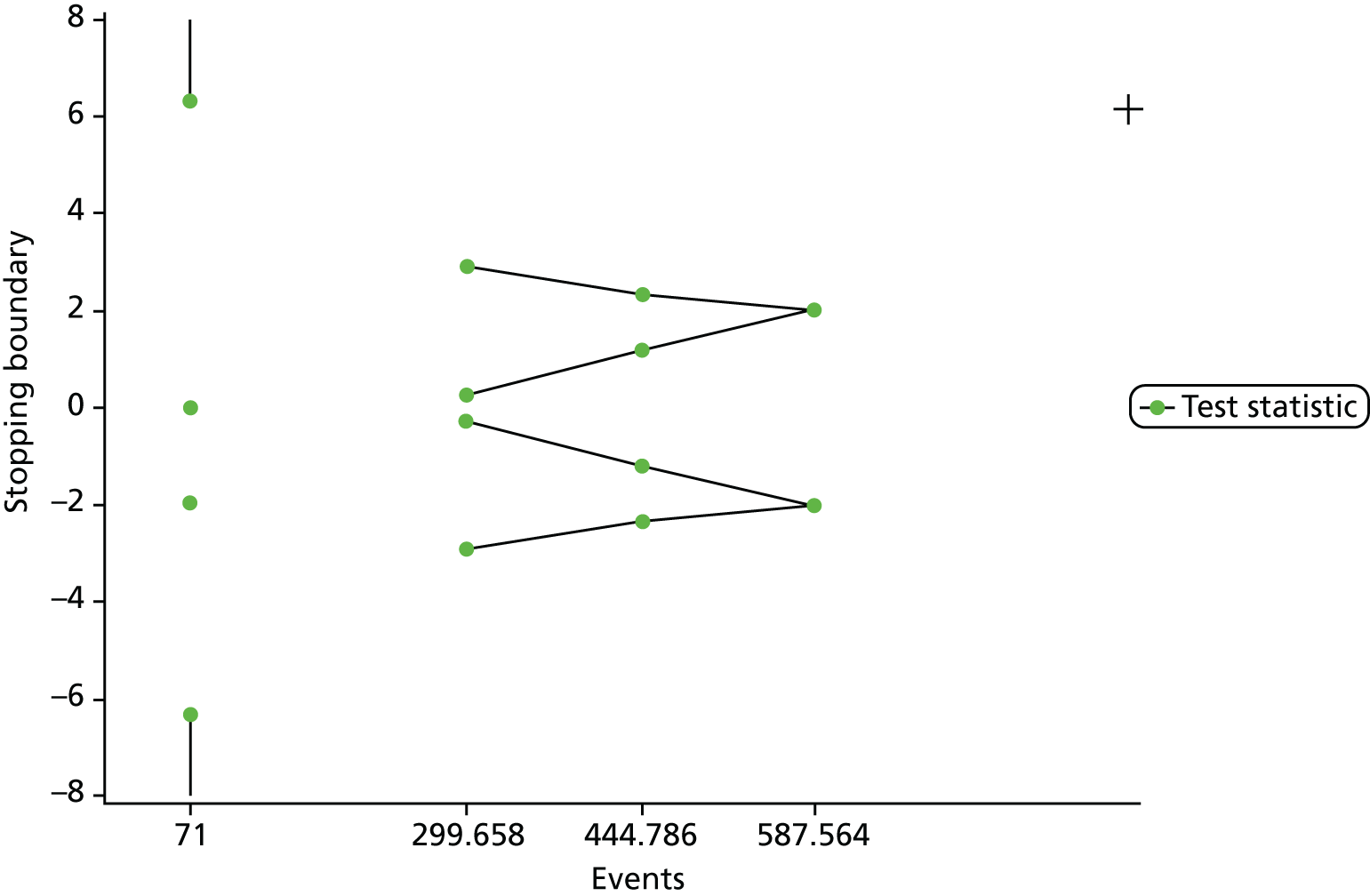
The incidence of new PUs of category ≥ 2 was summarised by mattress group and for each covariate included in the primary end-point analysis models, for both the full 30-day follow-up and the treatment phase.
Mattress compliance
The number of days and proportion of time participants spent on the allocated mattress during the treatment phase were summarised by mattress group. In addition, whether or not participants received the allocated mattress within 2 days of randomisation was summarised.
Safety
Adverse events (i.e. falls, device ulcers and other events) were summarised by mattress group.
Additional Data Monitoring and Ethics Committee report summaries
A CONSORT diagram, recruitment graph and summaries on screening data, minimisation factors and baseline characteristics, participant disposition, data and visit compliance, and protocol deviations and deviations were reported by mattress group. Not all these summaries are provided in this report.
Results
Primary end-point analysis
Intention-to-treat population
A primary end-point analysis was conducted on 909 participants in the ITT population.
Characteristics of the intention-to-treat population
Tables 32–34 give the number of participants randomised to each arm by centre, minimisation factors and participant characteristics.
| Centre | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Royal Devon and Exeter Hospital | 10 (2.2) | 8 (1.8) | 18 (2.0) |
| Addenbrooke’s Hospital | 9 (2.0) | 8 (1.8) | 17 (1.9) |
| Dewsbury District Hospital | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Leeds General Infirmary | 40 (8.7) | 41 (9.1) | 81 (8.9) |
| Pinderfields General Hospital | 49 (10.7) | 46 (10.2) | 95 (10.5) |
| Scunthorpe General Hospital | 14 (3.0) | 13 (2.9) | 27 (3.0) |
| St James’ University Hospital | 67 (14.6) | 60 (13.4) | 127 (14.0) |
| Whiston Hospital | 6 (1.3) | 4 (0.9) | 10 (1.1) |
| South Tyneside District General | 8 (1.7) | 9 (2.0) | 17 (1.9) |
| Harrogate District Hospital | 30 (6.5) | 27 (6.0) | 57 (6.3) |
| Hull Royal Infirmary | 1 (0.2) | 3 (0.7) | 4 (0.4) |
| Poole Hospital | 4 (0.9) | 5 (1.1) | 9 (1.0) |
| The Royal United Hospital | 7 (1.5) | 8 (1.8) | 15 (1.7) |
| University Hospitals Plymouth NHS Trust | 16 (3.5) | 17 (3.8) | 33 (3.6) |
| North Devon District Hospital Trust | 7 (1.5) | 8 (1.8) | 15 (1.7) |
| Torbay District General Hospital | 7 (1.5) | 6 (1.3) | 13 (1.4) |
| Royal Bolton Hospital | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Papworth Hospital | 9 (2.0) | 8 (1.8) | 17 (1.9) |
| Kent and Canterbury Hospital | 5 (1.1) | 6 (1.3) | 11 (1.2) |
| Frimley Park Hospital NHS Foundation Trust | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Princess Alexandra Hospital | 10 (2.2) | 10 (2.2) | 20 (2.2) |
| Chapel Allerton Hospital | 5 (1.1) | 6 (1.3) | 11 (1.2) |
| Royal Oldham Hospital | 11 (2.4) | 10 (2.2) | 21 (2.3) |
| Southport and Ormskirk Hospital | 2 (0.4) | 4 (0.9) | 6 (0.7) |
| Norwich Community Hospital | 21 (4.6) | 22 (4.9) | 43 (4.7) |
| Leeds Community Healthcare Trust | 40 (8.7) | 36 (8.0) | 76 (8.4) |
| St Helens and Knowsley Teaching Hospitals NHS Trust | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| NHS Lothian | 4 (0.9) | 1 (0.2) | 5 (0.6) |
| Northern General Hospital | 3 (0.7) | 3 (0.7) | 6 (0.7) |
| Northumbria Healthcare Trust | 33 (7.2) | 35 (7.8) | 68 (7.5) |
| Beccles and District War Memorial Community Hospital | 3 (0.7) | 3 (0.7) | 6 (0.7) |
| Sussex Community NHS Trust | 5 (1.1) | 4 (0.9) | 9 (1.0) |
| Royal South Hants Hospital | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Somerset Partnership | 16 (3.5) | 16 (3.6) | 32 (3.5) |
| Chelsea and Westminster Hospital | 4 (0.9) | 4 (0.9) | 8 (0.9) |
| Lister Hospital | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Plymouth Community Healthcare | 7 (1.5) | 7 (1.6) | 14 (1.5) |
| Other | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Missing | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| CRF not yet received | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Total | 460 (100) | 449 (100) | 909 (100) |
| Minimisation factor | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Consent type | |||
| Written | 321 (69.8) | 316 (70.4) | 637 (70.1) |
| Witnessed verbal | 65 (14.1) | 60 (13.4) | 125 (13.8) |
| Consultee agreement | 74 (16.1) | 69 (15.4) | 143 (15.7) |
| Missing | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| CRF not yet received | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Total | 460 (100) | 449 (100) | 909 (100) |
| Status of worst PU | |||
| Category 1 | 63 (13.7) | 61 (13.6) | 124 (13.6) |
| Category 2 | 42 (9.1) | 41 (9.1) | 83 (9.1) |
| No PU | 355 (77.2) | 344 (76.6) | 699 (76.9) |
| Missing | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| CRF not yet received | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Total | 460 (100) | 449 (100) | 909 (100) |
| Health-care setting | |||
| Secondary care hospital | 307 (66.7) | 300 (66.8) | 607 (66.8) |
| Community hospital | 73 (15.9) | 78 (17.4) | 151 (16.6) |
| NHS intermediate care/rehabilitation facility | 79 (17.2) | 69 (15.4) | 148 (16.3) |
| Missing | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| CRF not yet received | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Total | 460 (100) | 449 (100) | 909 (100) |
| Baseline characteristics | Trial arm | Total | |
|---|---|---|---|
| APM | HSFM | ||
| Age (years) | |||
| Mean (SD) | 77.2 (13.5) | 77.5 (13.8) | 77.3 (13.7) |
| Median (range) | 81 (21–101) | 80 (21–100) | 81 (21–101) |
| Missing | 0 | 0 | 0 |
| n | 460 | 449 | 909 |
| Macro- and micro-circulatory function, n (%) | |||
| No problem | 251 (54.6) | 243 (54.1) | 494 (54.3) |
| Conditions affecting central circulation | 98 (21.3) | 102 (22.7) | 200 (22.0) |
| Conditions affecting peripheral circulation | 86 (18.7) | 81 (18.0) | 167 (18.4) |
| Conditions affecting central and peripheral circulation | 21 (4.6) | 20 (4.5) | 41 (4.5) |
| Missing | 4 (0.9) | 3 (0.7) | 7 (0.8) |
| Pain on a healthy, altered or category 1 PU skin site, n (%) | |||
| Yes | 245 (53.3) | 241 (53.7) | 486 (53.5) |
| No | 203 (44.1) | 198 (44.1) | 401 (44.1) |
| Unable to assess | 4 (0.9) | 7 (1.6) | 11 (1.2) |
| Missing | 8 (1.7) | 3 (0.7) | 11 (1.2) |
| Total | 460 (100) | 449 (100) | 909 (100) |
Primary analysis
Tables 35–41 provide the data to support the primary analysis.
| Attribute | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Develops new PU, n (%) | |||
| Yes | 39 (8.5) | 51 (11.4) | 90 (9.9) |
| No | 387 (84.1) | 373 (83.1) | 760 (83.6) |
| Last skin assessment same day as randomisation | 32 (7.0) | 20 (4.5) | 52 (5.7) |
| Unknown | 2 (0.4) | 5 (1.1) | 7 (0.8) |
| Time to event/censoring (days) | |||
| Mean (SD) | 35.3 (24.8) | 35.6 (26.0) | 35.5 (25.4) |
| Median (range) | 37 (0–93) | 37 (0–153) | 37 (0–153) |
| Missing | 0 | 0 | 0 |
| n | 458 | 444 | 902 |
| Developed new PU | Type of consent, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Written (N = 638) | Witnessed verbal (N = 127) | Consultee agreement (N = 144) | ||
| Yes | 57 (8.9) | 19 (15.0) | 14 (9.7) | 90 (9.9) |
| No | 541 (84.8) | 96 (75.6) | 123 (85.4) | 760 (83.6) |
| Last skin assessment same day as randomisation | 33 (5.2) | 12 (9.4) | 7 (4.9) | 52 (5.7) |
| Unknown | 7 (1.1) | 0 (0.0) | 0 (0.0) | 7 (0.8) |
| Developed new PU | PU status at randomisation, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| None (N = 701) | Category 1 (N = 125) | Category 2 (N = 83) | ||
| Yes | 60 (8.6) | 18 (14.4) | 12 (14.5) | 90 (9.9) |
| No | 604 (86.2) | 97 (77.6) | 59 (71.1) | 760 (83.6) |
| Last skin assessment same day as randomisation | 37 (5.3) | 10 (8.0) | 5 (6.0) | 52 (5.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 7 (8.4) | 7 (0.8) |
| Developed new PU | Setting, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Secondary care hospital (N = 608) | Community hospital (N = 151) | NHS intermediate care rehabilitation facility (N = 150) | ||
| Yes | 53 (8.7) | 17 (11.3) | 20 (13.3) | 90 (9.9) |
| No | 501 (82.4) | 132 (87.4) | 127 (84.7) | 760 (83.6) |
| Last skin assessment same day as randomisation | 47 (7.7) | 2 (1.3) | 3 (2.0) | 52 (5.7) |
| Unknown | 7 (1.2) | 0 (0.0) | 0 (0.0) | 7 (0.8) |
| Developed new PU | Pain on a healthy, altered or category 1 PU at randomisation, n (%) | Total (N = 909), n (%) | |||
|---|---|---|---|---|---|
| Yes (N = 486) | No (N = 401) | Missing (N = 14) | Unable to assess (N = 8) | ||
| Yes | 49 (10.1) | 39 (9.7) | 2 (14.3) | 0 (0.0) | 90 (9.9) |
| No | 409 (84.2) | 339 (84.5) | 11 (78.6) | 1 (12.5) | 760 (83.6) |
| Last skin assessment same day as randomisation | 28 (5.8) | 23 (5.7) | 1 (7.1) | 0 (0.0) | 52 (5.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (87.5) | 7 (0.8) |
| Developed new PU | Presence of condition affecting peripheral circulation, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Yes (N = 208) | No (N = 694) | Missing (N = 7) | ||
| Yes | 23 (11.1) | 66 (9.5) | 1 (14.3) | 90 (9.9) |
| No | 174 (83.7) | 580 (83.6) | 6 (85.7) | 760 (83.6) |
| Last skin assessment same day as randomisation | 9 (4.3) | 43 (6.2) | 0 (0.0) | 52 (5.7) |
| Unknown | 2 (1.0) | 5 (0.7) | 0 (0.0) | 7 (0.8) |
| Time point | Covariate | n | HR | 99.7% Wald CI | |
|---|---|---|---|---|---|
| ≤ 60 days | Setting (reference = secondary care hospital, n = 522) | Community hospital | 125 | 1.085 | 0.448 to 2.626 |
| NHS intermediate care/rehabilitation facility | 119 | 1.333 | 0.567 to 3.136 | ||
| PU status (reference = no PU, n = 588) | Category 1 | 114 | 1.934 | 0.853 to 4.383 | |
| Category 2 | 64 | 2.752 | 1.045 to 7.244 | ||
| Consent type (reference = written, n = 534) | Consultee agreement | 125 | 1.161 | 0.443 to 3.044 | |
| Witnessed verbal | 107 | 1.990 | 0.871 to 4.547 | ||
| Presence of pain at healthy, altered or category 1 skin site (reference = no, n = 347) | Yes | 419 | 0.871 | 0.433 to 1.753 | |
| Presence of condition affecting peripheral circulation (reference = no, n = 586) | Yes | 180 | 1.010 | 0.479 to 2.129 | |
| Allocated mattress (reference = HSFM, n = 373) | APM | 393 | 0.729 | 0.375 to 1.417 | |
| > 60 days | Setting (reference = secondary care hospital, n = 79) | Community hospital | 26 | 0.483 | 0.017 to 13.669 |
| NHS intermediate care/rehabilitation facility | 31 | 0.841 | 0.061 to 11.505 | ||
| PU status (reference = no PU, n = 113) | Category 1 | 11 | 0.000 | 0.000 to 0.000 | |
| Category 2 | 12 | 0.000 | 0.000 to 0.000 | ||
| Consent type (reference = written, n = 97) | Consultee agreement | 19 | 0.000 | 0.000 to 0.000 | |
| Witnessed verbal | 20 | 0.449 | 0.018 to 11.348 | ||
| Presence of pain at healthy, altered or category 1 skin site (reference = no, n = 69) | Yes | 67 | 1.495 | 0.154 to 14.544 | |
| Presence of condition affecting peripheral circulation (reference = no, n = 110) | Yes | 26 | 0.520 | 0.020 to 13.484 | |
| Allocated mattress (reference = HSFM, n = 71) | APM | 65 | 0.858 | 0.082 to 8.983 | |
Sensitivity analysis: time to development of a category ≥ 2 PU during the treatment phase
Tables 42–48 provide the data to support the sensitivity analysis.
| Attribute | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Developed new PU, n (%) | |||
| Yes | 28 (6.1) | 43 (9.6) | 71 (7.8) |
| No | 388 (84.3) | 369 (82.2) | 757 (83.3) |
| Last skin assessment same day as randomisation | 42 (9.1) | 32 (7.1) | 74 (8.1) |
| Unknown | 2 (0.4) | 5 (1.1) | 7 (0.8) |
| Time to event/censoring (days) | |||
| Mean (SD) | 16.3 (15.2) | 16.8 (15.1) | 16.5 (15.2) |
| Median (range) | 12 (0–58) | 13 (0–65) | 13 (0–65) |
| Missing | 0 | 0 | 0 |
| n | 458 | 444 | 902 |
| Developed new PU | Type of consent, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Written (N = 638) | Witnessed verbal (N = 127) | Consultee agreement (N = 144) | ||
| Yes | 44 (6.9) | 15 (11.8) | 12 (8.3) | 71 (7.8) |
| No | 539 (84.5) | 99 (78.0) | 119 (82.6) | 757 (83.3) |
| Last skin assessment same day as randomisation | 48 (7.5) | 13 (10.2) | 13 (9.0) | 74 (8.1) |
| Unknown | 7 (1.1) | 0 (0.0) | 0 (0.0) | 7 (0.8) |
| Developed new PU | PU status at randomisation, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| None (N = 701) | Category 1 (N = 125) | Category 2 (N = 83) | ||
| Yes | 45 (6.4) | 16 (12.8) | 10 (12.0) | 71 (7.8) |
| No | 600 (85.6) | 99 (79.2) | 58 (69.9) | 757 (83.3) |
| Last skin assessment same day as randomisation | 56 (8.0) | 10 (8.0) | 8 (9.6) | 74 (8.1) |
| Unknown | 0 (0.0) | 0 (0.0) | 7 (8.4) | 7 (0.8) |
| Developed new PU | Setting, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Secondary care hospital (N = 608) | Community hospital (N = 151) | NHS intermediate care rehabilitation facility (N = 150) | ||
| Yes | 43 (7.1) | 12 (7.9) | 16 (10.7) | 71 (7.8) |
| No | 491 (80.8) | 135 (89.4) | 131 (87.3) | 757 (83.3) |
| Last skin assessment same day as randomisation | 67 (11.0) | 4 (2.6) | 3 (2.0) | 74 (8.1) |
| Unknown | 7 (1.2) | 0 (0.0) | 0 (0.0) | 7 (0.8) |
| Developed new PU | Pain on a healthy, altered or category 1 PU at randomisation, n (%) | Total (N = 909), n (%) | |||
|---|---|---|---|---|---|
| Yes (N = 486) | No (N = 401) | Missing (N = 14) | Unable to assess (N = 8) | ||
| Yes | 39 (8.0) | 30 (7.5) | 2 (14.3) | 0 (0.0) | 71 (7.8) |
| No | 407 (83.7) | 338 (84.3) | 11 (78.6) | 1 (12.5) | 757 (83.3) |
| Last skin assessment same day as randomisation | 40 (8.2) | 33 (8.2) | 1 (7.1) | 0 (0.0) | 74 (8.1) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (87.5) | 7 (0.8) |
| Developed new PU | Presence of condition affecting peripheral circulation, n (%) | Total (N = 909), n (%) | ||
|---|---|---|---|---|
| Yes (N = 208) | No (N = 694) | Missing (N = 7) | ||
| Yes | 19 (9.1) | 51 (7.3) | 1 (14.3) | 71 (7.8) |
| No | 173 (83.2) | 578 (83.3) | 6 (85.7) | 757 (83.3) |
| Last skin assessment same day as randomisation | 14 (6.7) | 60 (8.6) | 0 (0.0) | 74 (8.1) |
| Unknown | 2 (1.0) | 5 (0.7) | 0 (0.0) | 7 (0.8) |
| Covariate | n | Pra > χ2 | HR | 99.7% Wald CI |
|---|---|---|---|---|
| Setting (reference = secondary care hospital, n = 601) | ||||
| Community hospital | 151 | 0.9383 | 0.974 | 0.361 to 2.631 |
| NHS intermediate care/rehabilitation facility | 150 | 0.5656 | 1.189 | 0.486 to 2.911 |
| PU status (reference = no PU, n = 701) | ||||
| Category 1 | 125 | 0.0060 | 2.247 | 0.938 to 5.384 |
| Category 2 | 76 | 0.0073 | 2.567 | 0.905 to 7.286 |
| Consent type (reference = written, n = 631) | ||||
| Consultee agreement | 144 | 0.5705 | 1.219 | 0.433 to 3.435 |
| Witnessed verbal | 127 | 0.0899 | 1.674 | 0.679 to 4.127 |
| Presence of pain at healthy, altered or category 1 skin site (reference = no, n = 416) | ||||
| Yes | 486 | 0.7019 | 1.102 | 0.518 to 2.346 |
| Presence of condition affecting peripheral circulation (reference = no, n = 696) | ||||
| Yes | 206 | 0.6755 | 1.120 | 0.501 to 2.506 |
| Allocated mattress (reference = HSFM, n = 444) | ||||
| APM | 458 | 0.0522 | 0.622 | 0.300 to 1.286 |
Primary end-point analysis (per-protocol population)
A total of 570 participants were included in the PPP, corresponding to 62.7% of the ITT population.
Characteristics of the per-protocol population
Tables 49 and 50 describe the characteristics of the PPP.
| Centre | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Royal Devon and Exeter Hospital | 5 (1.8) | 2 (0.7) | 7 (1.2) |
| Addenbrooke’s Hospital | 8 (2.8) | 5 (1.7) | 13 (2.3) |
| Dewsbury District Hospital | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Leeds General Infirmary | 23 (8.2) | 27 (9.4) | 50 (8.8) |
| Pinderfields General Hospital | 24 (8.5) | 26 (9.0) | 50 (8.8) |
| Scunthorpe General Hospital | 11 (3.9) | 12 (4.2) | 23 (4.0) |
| St James’ University Hospital | 41 (14.5) | 30 (10.4) | 71 (12.5) |
| Whiston Hospital | 5 (1.8) | 3 (1.0) | 8 (1.4) |
| South Tyneside District General | 4 (1.4) | 2 (0.7) | 6 (1.1) |
| Harrogate District Hospital | 20 (7.1) | 13 (4.5) | 33 (5.8) |
| Hull Royal Infirmary | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Poole Hospital | 4 (1.4) | 4 (1.4) | 8 (1.4) |
| The Royal United Hospital | 6 (2.1) | 7 (2.4) | 13 (2.3) |
| University Hospitals Plymouth NHS Trust | 11 (3.9) | 10 (3.5) | 21 (3.7) |
| North Devon District Hospital Trust | 6 (2.1) | 6 (2.1) | 12 (2.1) |
| Torbay District General Hospital | 5 (1.8) | 4 (1.4) | 9 (1.6) |
| Royal Bolton Hospital | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Papworth Hospital | 7 (2.5) | 2 (0.7) | 9 (1.6) |
| Kent and Canterbury Hospital | 4 (1.4) | 2 (0.7) | 6 (1.1) |
| Frimley Park Hospital NHS Foundation Trust | 1 (0.4) | 1 (0.3) | 2 (0.4) |
| Princess Alexandra Hospital | 5 (1.8) | 10 (3.5) | 15 (2.6) |
| Chapel Allerton Hospital | 3 (1.1) | 5 (1.7) | 8 (1.4) |
| Royal Oldham Hospital | 9 (3.2) | 8 (2.8) | 17 (3.0) |
| Norwich Community Hospital | 16 (5.7) | 18 (6.3) | 34 (6.0) |
| Leeds Community Healthcare Trust | 19 (6.7) | 33 (11.5) | 52 (9.1) |
| St Helens and Knowsley Teaching Hospitals NHS Trust | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| NHS Lothian | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| Northern General Hospital | 1 (0.4) | 1 (0.3) | 2 (0.4) |
| Northumbria Healthcare Trust | 18 (6.4) | 27 (9.4) | 45 (7.9) |
| Beccles and District War Memorial Community Hospital | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| Sussex Community NHS Trust | 1 (0.4) | 2 (0.7) | 3 (0.5) |
| Somerset Partnership | 11 (3.9) | 11 (3.8) | 22 (3.9) |
| Chelsea and Westminster Hospital | 2 (0.7) | 4 (1.4) | 6 (1.1) |
| Lister Hospital | 1 (0.4) | 1 (0.3) | 2 (0.4) |
| Plymouth Community Healthcare | 4 (1.4) | 3 (1.0) | 7 (1.2) |
| Missing | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| CRF not yet received | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Total | 282 (100) | 288 (100) | 570 (100) |
| Minimisation factor | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Consent type | |||
| Written | 185 (65.6) | 216 (75.0) | 401 (70.4) |
| Witnessed verbal | 37 (13.1) | 39 (13.5) | 76 (13.3) |
| Consultee agreement | 60 (21.3) | 31 (10.8) | 91 (16.0) |
| CRF not yet received | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Total | 282 (100) | 288 (100) | 570 (100) |
| Status of worst PU | |||
| Category 1 | 44 (15.6) | 38 (13.2) | 82 (14.4) |
| Category 2 | 24 (8.5) | 11 (3.8) | 35 (6.1) |
| No PU | 214 (75.9) | 236 (81.9) | 450 (78.9) |
| Missing | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| CRF not yet received | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Total | 282 (100) | 288 (100) | 570 (100) |
| Health-care setting | |||
| Secondary care hospital | 197 (69.9) | 179 (62.2) | 376 (66.0) |
| Community hospital | 48 (17.0) | 57 (19.8) | 105 (18.4) |
| NHS intermediate care/rehabilitation facility | 37 (13.1) | 50 (17.4) | 87 (15.3) |
| CRF not yet received | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Total | 282 (100) | 288 (100) | 570 (100) |
Primary analysis (per-protocol population)
Tables 51–57 provide the data to support the primary end-point analysis.
| Attribute | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Develops new PU, n (%) | |||
| Yes | 28 (9.9) | 18 (6.3) | 46 (8.1) |
| No | 237 (84.0) | 257 (89.2) | 494 (86.7) |
| Last skin assessment same day as randomisation | 17 (6.0) | 13 (4.5) | 30 (5.3) |
| Time to event/censoring (days) | |||
| Mean (SD) | 34.6 (24.9) | 35.9 (24.9) | 35.2 (24.8) |
| Median (range) | 37 (0–93) | 38 (0–153) | 37 (0–153) |
| Missing | 0 | 0 | 0 |
| n | 282 | 288 | 570 |
| Developed new PU | Type of consent, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Written (N = 402) | Witnessed verbal (N = 76) | Consultee agreement (N = 92) | ||
| Yes | 28 (7.0) | 9 (11.8) | 9 (9.8) | 46 (8.1) |
| No | 357 (88.8) | 58 (76.3) | 79 (85.9) | 494 (86.7) |
| Last skin assessment same day as randomisation | 17 (4.2) | 9 (11.8) | 4 (4.3) | 30 (5.3) |
| Developed new PU | PU status at randomisation, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| None (N = 452) | Category 1 (N = 83) | Category 2 (N = 35) | ||
| Yes | 29 (6.4) | 9 (10.8) | 8 (22.9) | 46 (8.1) |
| No | 401 (88.7) | 66 (79.5) | 27 (77.1) | 494 (86.7) |
| Last skin assessment same day as randomisation | 22 (4.9) | 8 (9.6) | 0 (0.0) | 30 (5.3) |
| Developed new PU | Setting, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Secondary care hospital (N = 376) | Community hospital (N = 105) | NHS intermediate care rehabilitation facility (N = 89) | ||
| Yes | 26 (6.9) | 9 (8.6) | 11 (12.4) | 46 (8.1) |
| No | 323 (85.9) | 94 (89.5) | 77 (86.5) | 494 (86.7) |
| Last skin assessment same day as randomisation | 27 (7.2) | 2 (1.9) | 1 (1.1) | 30 (5.3) |
| Developed new PU | Pain on a healthy, altered or category 1 PU at randomisation, (%) | Total (N = 570), n (%) | |||
|---|---|---|---|---|---|
| Yes (N = 313) | No (N = 245) | Missing (N = 11) | Not applicable (N = 1) | ||
| Yes | 23 (7.3) | 21 (8.6) | 2 (18.2) | 0 (0.0) | 46 (8.1) |
| No | 272 (86.9) | 212 (86.5) | 9 (81.8) | 1 (100.0) | 494 (86.7) |
| Last skin assessment same day as randomisation | 18 (5.8) | 12 (4.9) | 0 (0.0) | 0 (0.0) | 30 (5.3) |
| Developed new PU | Presence of condition affecting peripheral circulation, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Yes (N = 132) | No (N = 434) | Missing (N = 4) | ||
| Yes | 13 (9.8) | 33 (7.6) | 0 (0.0) | 46 (8.1) |
| No | 115 (87.1) | 375 (86.4) | 4 (100.0) | 494 (86.7) |
| Last skin assessment same day as randomisation | 4 (3.0) | 26 (6.0) | 0 (0.0) | 30 (5.3) |
| Covariate | n | ln(HR) | Standard error | χ2 | Pra > χ2 | HR | 99.7% Wald CI |
|---|---|---|---|---|---|---|---|
| Setting (reference = secondary care hospital, n = 376) | |||||||
| Community hospital | 105 | 0.08517 | 0.39959 | 0.0454 | 0.8312 | 1.089 | 0.333 to 3.565 |
| NHS intermediate care/rehabilitation facility | 89 | 0.34100 | 0.37990 | 0.8057 | 0.3694 | 1.406 | 0.455 to 4.342 |
| PU status (reference = no PU, n = 452) | |||||||
| Category 1 | 83 | 0.66691 | 0.38693 | 2.9707 | 0.0848 | 1.948 | 0.618 to 6.142 |
| Category 2 | 35 | 1.38660 | 0.41315 | 11.2639 | 0.0008 | 4.001 | 1.174 to 13.636 |
| Consent type (reference = written, n = 402) | |||||||
| Consultee agreement | 92 | 0.32637 | 0.41801 | 0.6096 | 0.4349 | 1.386 | 0.401 to 4.792 |
| Witnessed verbal | 76 | 0.76284 | 0.39301 | 3.7675 | 0.0523 | 2.144 | 0.668 to 6.884 |
| Presence of pain at healthy, altered or category 1 skin site (reference = no, n = 257) | |||||||
| Yes | 313 | –0.27368 | 0.30899 | 0.7845 | 0.3758 | 0.761 | 0.304 to 1.903 |
| Presence of condition affecting peripheral circulation (reference = no, n = 438) | |||||||
| Yes | 132 | 0.06804 | 0.33356 | 0.0416 | 0.8384 | 1.070 | 0.398 to 2.881 |
| Allocated mattress (reference = HSFM, n = 288) | |||||||
| APM | 282 | 0.35728 | 0.31060 | 1.3232 | 0.2500 | 1.429 | 0.569 to 3.593 |
Sensitivity analysis: time to development of a category ≥ 2PU during the treatment phase (per-protocol population)
Tables 58–63 provide the data to support the sensitivity analysis in the PPP.
| Attribute | Trial arm | Overall | |
|---|---|---|---|
| APM | HSFM | ||
| Develops new PU, n (%) | |||
| Yes | 21 (7.4) | 15 (5.2) | 36 (6.3) |
| No | 239 (84.8) | 253 (87.8) | 492 (86.3) |
| Last skin assessment same day as randomisation | 22 (7.8) | 20 (6.9) | 42 (7.4) |
| Time to event/censoring (days) | |||
| Mean (SD) | 16.3 (14.6) | 15.2 (13.9) | 15.7 (14.3) |
| Median (range) | 13 (0–57) | 12 (0–60) | 12 (0–60) |
| Missing | 0 | 0 | 0 |
| n | 282 | 288 | 570 |
| Develops new PU | Type of consent, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Written (N = 402) | Witnessed verbal (N = 76) | Consultee agreement (N = 92) | ||
| Yes | 23 (5.7) | 6 (7.9) | 7 (7.6) | 36 (6.3) |
| No | 355 (88.3) | 60 (78.9) | 77 (83.7) | 492 (86.3) |
| Last skin assessment same day as randomisation | 24 (6.0) | 10 (13.2) | 8 (8.7) | 42 (7.4) |
| Develops new PU | PU status at randomisation, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| None (N = 452) | Category 1 (N = 83) | Category 2 (N = 35) | ||
| Yes | 21 (4.6) | 8 (9.6) | 7 (20.0) | 36 (6.3) |
| No | 397 (87.8) | 67 (80.7) | 28 (80.0) | 492 (86.3) |
| Last skin assessment same day as randomisation | 34 (7.5) | 8 (9.6) | 0 (0.0) | 42 (7.4) |
| Develops new PU | Setting, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Secondary care hospital (N = 376) | Community hospital (N = 105) | NHS intermediate care rehabilitation facility (N = 89) | ||
| Yes | 22 (5.9) | 6 (5.7) | 8 (9.0) | 36 (6.3) |
| No | 317 (84.3) | 95 (90.5) | 80 (89.9) | 492 (86.3) |
| Last skin assessment same day as randomisation | 37 (9.8) | 4 (3.8) | 1 (1.1) | 42 (7.4) |
| Develops new PU | Pain on a healthy, altered or category 1 PU at randomisation, n (%) | Total (N = 570), n (%) | |||
|---|---|---|---|---|---|
| Yes (N = 313) | No (N = 245) | Missing (N = 11) | Unable to assess (N = 1) | ||
| Yes | 18 (5.8) | 16 (6.5) | 2 (18.2) | 0 (0.0) | 36 (6.3) |
| No | 271 (86.6) | 211 (86.1) | 9 (81.8) | 1 (100.0) | 492 (86.3) |
| Last skin assessment same day as randomisation | 24 (7.7) | 18 (7.3) | 0 (0.0) | 0 (0.0) | 42 (7.4) |
| Develops new PU | Presence of condition affecting peripheral circulation, n (%) | Total (N = 570), n (%) | ||
|---|---|---|---|---|
| Yes (N = 132) | No (N = 434) | Missing (N = 4) | ||
| Yes | 11 (8.3) | 25 (5.8) | 0 (0.0) | 36 (6.3) |
| No | 114 (86.4) | 374 (86.2) | 4 (100.0) | 492 (86.3) |
| Last skin assessment same day as randomisation | 7 (5.3) | 35 (8.1) | 0 (0.0) | 42 (7.4) |
Mattress compliance
Table 64 summarises mattress compliance by trial arm.
| Attribute | Trial arm, n (%) | Overall, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Proportion of treatment phase spent on allocated mattress | |||
| Mean (SD) (%) | 75.1 (34.6) | 74.9 (37.1) | 75.0 (35.8) |
| Median (range) (%) | 94 (0–100) | 100 (0–100) | 97 (0–100) |
| Missing, n | 16 | 9 | 25 |
| Non-missing, n | 444 | 440 | 884 |
| Percentage, n (%) | |||
| 0.0 | 35 (7.6) | 40 (8.9) | 75 (8.3) |
| up to 19.9 | 28 (6.1) | 34 (7.6) | 62 (6.8) |
| 20.0–39.9 | 23 (5.0) | 23 (5.1) | 46 (5.1) |
| 40.0–59.9 | 28 (6.1) | 25 (5.6) | 53 (5.8) |
| 60.0–79.9 | 30 (6.5) | 18 (4.0) | 48 (5.3) |
| 80.0–100.0 | 300 (65.2) | 300 (66.8) | 600 (66.0) |
| Missing | 16 (3.5) | 9 (2.0) | 25 (2.8) |
| Total | 460 (100.0) | 449 (100.0) | 909 (100.0) |
| Number of days spent on allocated mattress | |||
| Mean (SD) | 15.4 (15.1) | 14.2 (13.8) | 14.8 (14.5) |
| Median (range) | 11 (0–64) | 10 (0–61) | 10 (0–64) |
| Missing, n | 16 | 9 | 25 |
| n | 444 | 440 | 884 |
| Days | |||
| 0 | 35 (7.6) | 40 (8.9) | 75 (8.3) |
| 1–5 | 112 (24.3) | 95 (21.2) | 207 (22.8) |
| 6–10 | 75 (16.3) | 90 (20.0) | 165 (18.2) |
| 11–20 | 96 (20.9) | 109 (24.3) | 205 (22.6) |
| 21–30 | 57 (12.4) | 56 (12.5) | 113 (12.4) |
| 31–40 | 25 (5.4) | 18 (4.0) | 43 (4.7) |
| 41–50 | 24 (5.2) | 15 (3.3) | 39 (4.3) |
| > 50 | 20 (4.3) | 17 (3.8) | 37 (4.1) |
| Missing | 16 (3.5) | 9 (2.0) | 25 (2.8) |
| Total | 460 (100.0) | 449 (100.0) | 909 (100.0) |
| Received allocated mattress within 2 days of randomisation | |||
| Yes | 370 (80.4) | 371 (82.6) | 741 (81.5) |
| No | 74 (16.1) | 69 (15.4) | 143 (15.7) |
| Missing | 16 (3.5) | 9 (2.0) | 25 (2.8) |
| Total | 460 (100.0) | 449 (100.0) | 909 (100.0) |
Safety
Adverse events
Tables 65–67 provide details of the AEs.
| Attribute | Trial arm | Total | |
|---|---|---|---|
| APM | HSFM | ||
| Participants who experienced an AE, n (%) | |||
| At least one AE reported | 65 (12.1) | 78 (14.6) | 143 (13.4) |
| No AEs reported | 304 (56.8) | 284 (53.1) | 588 (55.0) |
| CRF not yet received | 166 (31.0) | 173 (32.3) | 339 (31.7) |
| Total | 535 (100.0) | 535 (100.0) | 1070 (100.0) |
| Number of device ulcers | 7 (6.3) | 5 (4.0) | 12 (5.0) |
| Number of falls | 104 (92.9) | 117 (92.9) | 221 (92.9) |
| Number of other AEs | 1 (0.9) | 4 (3.2) | 5 (2.1) |
| Total number of adverse events | 112 (100.0) | 126 (100.0) | 238 (100.0) |
| Number of adverse events per participant | |||
| Mean (SD) | 1.7 (1.7) | 1.6 (1.2) | 1.7 (1.4) |
| Median (range) | 1 (1–12) | 1 (1–7) | 1 (1–12) |
| Missing, n | 0 | 1 | 1 |
| Non-missing, n | 65 | 77 | 142 |
| 1, n (%) | 46 (70.8) | 51 (66.2) | 97 (68.3) |
| 2, n (%) | 9 (13.8) | 13 (16.9) | 22 (15.5) |
| 3, n (%) | 3 (4.6) | 8 (10.4) | 11 (7.7) |
| 4, n (%) | 5 (7.7) | 3 (3.9) | 8 (5.6) |
| 6, n (%) | 0 (0.0) | 1 (1.3) | 1 (0.7) |
| 7, n (%) | 1 (1.5) | 1 (1.3) | 2 (1.4) |
| 12, n (%) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| Fall details | Trial arm | Total | |
|---|---|---|---|
| APM | HSFM | ||
| Number of falls | 104 | 117 | 221 |
| Place where fall took place, n (%) | |||
| From the bed | 19 (18.3) | 19 (16.2) | 38 (17.2) |
| By the bed | 25 (24.0) | 32 (27.4) | 57 (25.8) |
| By the chair | 15 (14.4) | 25 (21.4) | 40 (18.1) |
| In the bathroom | 15 (14.4) | 19 (16.2) | 34 (15.4) |
| Away from bed area | 11 (10.6) | 8 (6.8) | 19 (8.6) |
| Other | 19 (18.3) | 14 (12.0) | 33 (14.9) |
| Activity at the time of the fall, n (%) | |||
| Getting into bed | 1 (1.0) | 3 (2.6) | 4 (1.8) |
| Getting out of bed | 22 (21.2) | 28 (23.9) | 50 (22.6) |
| Walking around the bed | 11 (10.6) | 16 (13.7) | 27 (12.2) |
| Standing from chair | 10 (9.6) | 20 (17.1) | 30 (13.6) |
| Going to the bathroom | 20 (19.2) | 24 (20.5) | 44 (19.9) |
| Other | 40 (38.5) | 26 (22.2) | 66 (29.9) |
| Injury sustained, n (%) | |||
| Yes | 40 (38.5) | 42 (35.9) | 82 (37.1) |
| No | 60 (57.7) | 74 (63.2) | 134 (60.6) |
| Missing | 4 (3.8) | 1 (0.9) | 5 (2.3) |
| If injury sustained, was the injury serious?, n (%) | |||
| Yes | 5 (12.5) | 10 (23.8) | 15 (18.3) |
| No | 35 (87.5) | 32 (76.2) | 67 (81.7) |
| If injury was serious, seriousness criteria, n (%) | |||
| Required prolonged hospitalisation | 2 (40.0) | 5 (50.0) | 7 (46.7) |
| Significantly or permanently disabling or incapacitating | 0 (0.0) | 2 (20.0) | 2 (13.3) |
| Required surgical intervention | 0 (0.0) | 1 (10.0) | 1 (6.7) |
| Other | 3 (60.0) | 2 (20.0) | 5 (33.3) |
| Device ulcer details | Trial arm | Total | |
|---|---|---|---|
| APM | HSFM | ||
| Number of device ulcers | 7 | 5 | 12 |
| Type of device, n (%) | |||
| Catheter | 1 (14.3) | 0 (0.0) | 1 (8.3) |
| Plaster of Paris | 2 (28.6) | 1 (20.0) | 3 (25.0) |
| Other | 4 (57.1) | 4 (80.0) | 8 (66.7) |
| Was the device ulcer serious?, n (%) | |||
| No | 7 (100) | 5 (100) | 12 (100) |
Serious adverse events
Fifteen falls were documented as causing a serious injury; no device ulcers or other AEs were documented as serious.
Appendix 4 Clinical results: supplementary tables
| Characteristic | Patients | |
|---|---|---|
| Screened | Randomised | |
| Age (years) | ||
| Mean (SD) | 78.3 (13.2) | 77.5 (13.24) |
| Median (range) | 81 (7–106) | 81 (21.0–104) |
| IQR | (72.0–87.0) | (71.0–87.0) |
| n | 15,138 | 2008 |
| Missing, n | 139 | 22 |
| Gender, n (%) | ||
| Male | 6655 (43.6) | 909 (44.8) |
| Female | 8440 (55.2) | 1111 (54.7) |
| Missing | 182 (1.2) | 10 (0.5) |
| Ethnicity, n (%) | ||
| White | 14,535 (95.1) | 1953 (96.2) |
| Mixed | ||
| White and black Caribbean | 99 (0.6) | 17 (0.8) |
| White and black African | 5 (0.0) | 0 (0.0) |
| White and Asian | 6 (0.0) | 0 (0.0) |
| Other mixed background | 14 (0.1) | 2 (0.1) |
| Asian | ||
| Indian | 71 (0.5) | 9 (0.4) |
| Pakistani | 55 (0.4) | 7 (0.3) |
| Bangladeshi | 15 (0.1) | 1 (0.0) |
| Other Asian background | 15 (0.1) | 0 (0.0) |
| Black | ||
| Caribbean | 45 (0.3) | 10 (0.5) |
| African | 17 (0.1) | 1 (0.0) |
| Other black background | 5 (0.0) | 1 (0.0) |
| Chinese | 14 (0.1) | 1 (0.0) |
| Other | 18 (0.1) | 2 (0.1) |
| Not stated | 31 (0.2) | 0 (0.0) |
| Missing | 332 (2.2) | 26 (1.3) |
| Current mattress type, n (%) | ||
| Static overlay | 153 (1.0) | 13 (0.6) |
| Foam | 7224 (47.3) | 1133 (55.8) |
| Static air filled | 76 (0.5) | 6 (0.3) |
| Gel filled | 9 (0.1) | 2 (0.1) |
| Hybrid foam/alternating pressure | 890 (5.8) | 42 (2.1) |
| Alternating pressure | 6063 (39.7) | 740 (36.5) |
| Low air loss | 315 (2.1) | 47 (2.3) |
| Hybrid alternating pressure/low air loss | 364 (2.4) | 27 (1.3) |
| Specialised | 8 (0.1) | 1 (0.0) |
| Other | 72 (0.5) | 12 (0.6) |
| Missing | 103 (0.7) | 7 (0.3) |
| Current mattress type | Unwilling to change mattress, n (%) | Total (N = 2054), n (%) | |
|---|---|---|---|
| Staff (N = 1116) | Patient (N = 938) | ||
| Static overlay | 11 (1.0) | 3 (0.3) | 14 (0.7) |
| Foam | 219 (19.6) | 438 (46.7) | 657 (32.0) |
| Static air filled | 2 (0.2) | 5 (0.5) | 7 (0.3) |
| Gel filled | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hybrid foam/alternating pressure | 14 (1.3) | 42 (4.5) | 56 (2.7) |
| Alternating pressure | 808 (72.4) | 369 (39.3) | 1177 (57.3) |
| Low air loss | 10 (0.9) | 36 (3.8) | 46 (2.2) |
| Hybrid alternating pressure/low air loss | 46 (4.1) | 40 (4.3) | 86 (4.2) |
| Specialised | 3 (0.3) | 1 (0.1) | 4 (0.2) |
| Other | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Missing | 2 (0.2) | 1 (0.1) | 3 (0.1) |
| Location of PU | Trial arm, n (%) | Overall, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Spine/back | 1 (1.1) | 6 (4.8) | 7 (3.3) |
| Sacrum | 18 (20.2) | 26 (21.0) | 44 (20.7) |
| Buttock – L | 18 (20.2) | 24 (19.4) | 42 (19.7) |
| Buttock – R | 15 (16.9) | 23 (18.5) | 38 (17.8) |
| Ischial tuberosity – L | 1 (1.1) | 3 (2.4) | 4 (1.9) |
| Ischial tuberosity – R | 2 (2.2) | 3 (2.4) | 5 (2.3) |
| Trochanter (hip) – L | 0 (0.0) | 2 (1.6) | 2 (0.9) |
| Trochanter (hip) – R | 1 (1.1) | 0 (0.0) | 1 (0.5) |
| Heel – L | 12 (13.5) | 15 (12.1) | 27 (12.7) |
| Heel – R | 9 (10.1) | 13 (10.5) | 22 (10.3) |
| Ankle – L | 3 (3.4) | 2 (1.6) | 5 (2.3) |
| Ankle – R | 3 (3.4) | 2 (1.6) | 5 (2.3) |
| Elbow – L | 2 (2.2) | 0 (0.0) | 2 (0.9) |
| Elbow – R | 4 (4.5) | 5 (4.0) | 9 (4.2) |
| Total | 89 (100) | 124 (100) | 213 (100) |
| Mattress | New PU of category ≥ 2, n (%) | Baseline assessment not eligible, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| No | Died | Withdrawn | ||||
| APM | 53 (5.2) | 876 (86.2) | 48 (4.7) | 35 (3.4) | 4 (0.4) | 1016 (100) |
| HSFM | 79 (7.8) | 856 (84.5) | 40 (3.9) | 30 (3.0) | 8 (0.8) | 1013 (100) |
| Overall | 132 (6.5) | 1732 (85.4) | 88 (4.3) | 65 (3.2) | 12 (0.6) | 2029 (100) |
| Mattress | New PU of category ≥ 1, n (%) | Baseline assessment not eligible, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| No | Died | Withdrawn | ||||
| APM | 160 (15.7) | 750 (73.8) | 65 (6.4) | 36 (3.5) | 5 (0.5) | 1016 (100) |
| HSFM | 190 (18.8) | 724 (71.5) | 63 (6.2) | 28 (2.8) | 8 (0.8) | 1013 (100) |
| Overall | 350 (17.2) | 1474 (72.6) | 128 (6.3) | 64 (3.2) | 13 (0.6) | 2029 (100) |
| Mattress | New PU of category ≥ 3, n (%) | Baseline assessment not eligible, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| No | Died | Withdrawn | ||||
| APM | 14 (1.4) | 876 (86.2) | 82 (8.1) | 43 (4.2) | 1 (0.1) | 1016 (100) |
| HSFM | 18 (1.8) | 872 (86.1) | 84 (8.3) | 37 (3.7) | 2 (0.2) | 1013 (100) |
| Overall | 32 (1.6) | 1748 (86.2) | 166 (8.2) | 80 (3.9) | 3 (0.1) | 2029 (100) |
| Location of PU | Trial arm, n (%) | Overall, n (%) | |
|---|---|---|---|
| APM | HSFM | ||
| Spine/back | 0 (0.0) | 1 (4.8) | 1 (2.5) |
| Sacrum | 1 (5.3) | 7 (33.3) | 8 (20.0) |
| Buttock – L | 3 (15.8) | 0 (0.0) | 3 (7.5) |
| Buttock – R | 1 (5.3) | 0 (0.0) | 1 (2.5) |
| Ischial tuberosity – R | 1 (5.3) | 0 (0.0) | 1 (2.5) |
| Heel – L | 5 (26.3) | 5 (23.8) | 10 (25.0) |
| Heel – R | 6 (31.6) | 6 (28.6) | 12 (30.0) |
| Ankle – L | 2 (10.5) | 0 (0.0) | 2 (5.0) |
| Ankle – R | 0 (0.0) | 1 (4.8) | 1 (2.5) |
| Elbow – R | 0 (0.0) | 1 (4.8) | 1 (2.5) |
| Total | 19 (100) | 21 (100) | 40 (100) |
Appendix 5 Health economics supplementary tables
| Resource | Unit of measure | Unit cost (£) | Comment/source |
|---|---|---|---|
| Inpatient care | |||
| Medicine | Bed-day | 271 |
aWeighted average per ward of elective inpatient’s excess bed-day NHS Reference Costs 2015 to 2016131 |
| Surgery | 296 | ||
| Orthopaedics | 300 | ||
| Oncology | 397 | ||
| Rehabilitation unit | 241 | ||
| Cardiology | 318 | ||
| Neurology | 347 | ||
| High-dependency unit | 1026 |
Assumed critical care requiring support for 0 or 1 organ NHS Reference Costs 2015 to 2016131 |
|
| Intensive care unit | 1595 |
Assumed critical care requiring support for ≥ 2 organs NHS Reference Costs 2015 to 2016131 |
|
| Medical team | |||
| Consultant | |||
| Medicine | Per consultation | 97 | Time per consultation assumed as 32.9 minutes based on the duration of a surgery consultation reported in Curtis132 |
| Psychiatrist | 99 | ||
| Surgeon | 100 | ||
| Registrar | 51 | ||
| Physiotherapist | 21 |
Annual salary is the same for hospital- or community-based medical professionals Time per consultation assumed as 32.9 minutes based on the duration of a surgery consultation reported in Curtis132 |
|
| Dietitian | 20 | ||
| Occupational therapist | 21 | ||
| Phychologist (counsellor) | 27 | ||
| Speech therapist | 20 | ||
| Social worker | 79 | Assume 1 hour of client-related work (including qualification) reported in Curtis and Burns62 | |
| Tests | |||
| CT | Per test | 108 | NHS Reference Costs 2015 to 2016131 |
| MRI | 148 | NHS Reference Costs 2015 to 2016131 | |
| Radiography | 25 | 2016/17 national prices and national tariff workbook133 | |
| Echocardiography | 67 | NHS Reference Costs 2015 to 2016131 | |
| Ultrasonography | 53 | NHS Reference Costs 2015 to 2016131 | |
| Blood tests | 3 | NHS Reference Costs 2015 to 2016131 | |
| Mattresses type | |||
| HSFM | Per day | 0.08 |
Cost per mattress: £169, with an assumed durability of 6 years Source: NICE134 |
| APM | 2.05 |
Cost per mattress: £3742, with an assumed durability of 5 years Source: NICE134 |
|
| Resource | Unit of measure | Unit cost (£) | Comment/source |
|---|---|---|---|
| Care homes | |||
| Residential care home | Per week | 638.05 | Not-for-profit residential care homes for people aged ≥ 65 years62 |
| Nursing home | 888.22 | Not-for-profit nursing home for people aged ≥ 65 years62 | |
| Rehabilitation unit | |||
| Hospital assessment | Per stay | 331 | Cost per patient stay was estimated by adding the per-stay costs plus the per-day costs and multiplying by the number of days that the patient stayed in the rehabilitation unit135 |
| Discharge | 536 | ||
| Health services provided by CART co-ordinator | 428 | ||
| Cost of typical episode | 2957 | ||
| Wages; overheads; capital costs | Per day | 101 | |
| Resource | Unit of measure | Unit cost (£) | Comment/source |
|---|---|---|---|
| Outpatient care | |||
| GP | |||
| Surgery | Per consultation | 36 | 9.22 minutes per consultation62 at £3.90 per minute62 |
| Clinic | 67 | 17.2 minutes per consultation136 at £3.90 per minute62 | |
| Telephone | 28 | 7.1 minutes per consultation136 at £3.90 per minute62 | |
| Home visit | 92 | 23.4 minutes per consultation including 12 minutes travel time136 at £3.90 per minute62 | |
| Nurse | |||
| Surgery | Per consultation | 14 | |
| Clinic | 14 | ||
| Telephone | 6 |
|
|
| Home visit | 23 | ||
| Physiotherapista | |||
| Surgery | Per consultation | 21 | |
| Clinic | 15 | ||
| Telephone | 8 | ||
| Home visit | 28 |
|
|
| Counsellora | |||
| Surgery | Per consultation | 27 | |
| Clinic | 19 | ||
| Telephone | 11 | ||
| Home visit | 37 |
|
|
| Occupational therapya | |||
| Surgery | Per consultation | 21 |
32.9 minutes per consultation132 £38 per hour with qualifications62 |
| Clinic | 15 |
23.3 minutes per consultation132 £38 per hour with qualifications62 |
|
| Telephone | 8 |
13.1 minutes per consultation132 £38 per hour with qualifications62 |
|
| Home visit | 28 |
Assumed same duration as surgery consultation plus 12 minutes for travel time (as outpatient GP consultation) £38 per hour with qualifications62 |
|
| Outpatient consultations | |||
| Surgery | Per consultation | 131 | NHS Reference Costs 2015 to 2016 (DoH 2016) |
| Orthopaedics | 118 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Oncology | 152 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Cardiology | 128 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Respiratory | 156 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Urology | 106 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Neurology | 177 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Ophthalmology | 91 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Rehabilitation | 126 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Medicine | 168 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| A&E | 138 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Radiology | 85 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Rheumatology | 144 | NHS Reference Costs 2015 to 2016 (DoH 2016) | |
| Social care | |||
| Meal on wheels | |||
| Hot | Per meal | 8.6 | Community meals Leeds |
| Frozen | 4 | Community meals Leeds | |
| Care worker | Per patient visit | 13.50 | Assumes a 30-minute visit (63% of local authority commissioned home-care visits lasted 16–30 minutes;62 the upper value is used here) at cost of £27 per face-to-face visit at the weekend.62 It is assumed that this is paid by the social care sector |
| Social worker | Per hour | 79 | Assume 1 hour of client-related work (including qualification) reported in Curtis and Burns62 |
| Analysis | Time horizon | Outcome measure | Baseline adjustment | Missing data | Cost-effectiveness analysis |
|---|---|---|---|---|---|
| Primary | |||||
| ITT analysis | 30 days after end of treatment | QALYs (EQ-5D-5L) |
|
Imputed | Cost per incremental QALY |
| Secondary | |||||
| 1. Complete case | 30 days after end of treatment | QALYs (EQ-5D-5L) |
|
Excluded | Cost per incremental QALY |
| 2. Baseline adjustment | 30 days after end of treatment | QALYs (EQ-5D-5L) | None | Imputed | Cost per incremental QALY |
| 30 days after end of treatment | QALYs (EQ-5D-5L) | None | Excluded | Cost per incremental QALY | |
| 3. Utility measures | 30 days after end of treatment | PU-QoL-UI |
|
Imputed | Cost per incremental PU-QoL-UI |
| 30 days after end of treatment | PU-QoL-UI |
|
Excluded | Cost per incremental PU-QoL-UI | |
| Parameter description | Name in model | Meana | SE/RSEb | Distribution | Source/comments |
|---|---|---|---|---|---|
| Probabilities | |||||
| Probability of being discharged from hospital | From the trial data: estimated as the daily probability of being discharged using the average days in hospital per treatment arm (APM: 12.87 days; HSFM 12.65 days) and assuming a Poisson distribution | ||||
| HSFM | pDis | 0.0269 | 0.00129 | Beta | |
| APM | pDis_APM | 0.0266 | 0.00132 | Beta | |
| Probability of developing a first PU in hospital | pPU | 0.0620 | 0.0056 | Beta | Overall value during the treatment phase for participants without a PU at randomisation |
| Probability of developing a second PU in hospital | pPU2 | 0.018 | 0.0030 | Beta | Overall value during the treatment phase for participants without a PU at randomisation |
| Probability of developing a PU for participants who already had one at randomisation | pPU_PU2 | 0.103 | 0.025 | Beta | Overall value during the treatment phase for participants with a PU at randomisation |
| HR of developing a PU in the APM arm | HR_PU_APM | 0.68 | 0.08 | Log-normal | HR derived from the mean probability of participants developing a PU during the treatment phase |
| Probability of PU healing (in hospital) | pHeal | 0.44 | 0.041 | Beta | Preliminary data from the trial |
| HR of PU healing when discharged with a PU | HR_heal | 0.95 | RSE: 0.05 | Log-normal | Assumed as 95% of that of healing while in hospital |
| Mortality (probability to die) | mr | Age dependant | Fixed | Fixed | Yearly probability of dying National Life Tables, UK137 |
| HR of death when having a PU | HR_D.PU | 1.92 | RSE: 0.10 | Log-normal | Landi et al.74 |
| Utilities | |||||
| HRQoL utility at starting age of 80 years | u80 | 0.717 | Fixed | – | Szende et al.138 |
| Disutility from being in hospital | uDisutH | 0.02 | RSE: 0.05 | Gamma | Assumed |
| Percentage reduction in utility for having one PU | uDisutPU2 | 65.8% | Fixed | – | Assumed based on data from Nixon et al.37 and frequency of PU from Whittington and Briones75 |
| Disutility from having two PUs | uDisutPU22 | – | – | – | Assumed as 150% of having one PU (uDisutPU2) |
| Disutility typical at the end of life | uTerminal.mean | 0.2000 | RSE: 0.05 | Gamma | Assumed |
| Costs | |||||
| Daily standard hospital bed costs HSFM arm | cPD_SHB_HSF | £271.54 | 2.88 | Gamma | Average cost per day from the trial |
| Daily standard hospital bed costs APM arm | cPD_SHB_APM | £265.12 | 4.09 | Gamma | Average cost per day from the trial |
| Daily costs for HSFM | cPD_HSF | £0.08 | RSE: 0.05 | Gamma | Based on durability of 6 years. Cost of the mattress: £16912 |
| Daily costs for APM mattress | cPD_APM | £2.05 | RSE: 0.05 | Gamma | Based on durability of 5 years. Cost of the mattress: £374212 |
| Daily extra costs for treatment of one PU in hospital | cHPD2 | £166.50 | RSE: 0.05 | Gamma | Dealey et al.24 |
| Daily costs for treatment of one PU when discharged | cDPD2 | £23.54 | RSE: 0.05 | Gamma | Cost per hour of home nursing62 |
| Daily costs for treatment of two or more PU when discharged | cDOD22 | £35.31 | RSE: 0.05 | Gamma | Assumed as 1.5 of the cost of treatment of one PU when discharged |
| Extra costs typical at the end of life (transition cost when entering Dead state) | cTerminal.mean | £20,000 | RSE: 0.05 | Gamma | Halek et al.76 and Fassbender et al.77 |
| Cost category | Trial arm (£) | p-value (Wilcoxon) | |||||
|---|---|---|---|---|---|---|---|
| APM | HSFM | ||||||
| Mean | Median | SD | Mean | Median | SD | ||
| Inpatient care | |||||||
| Medicine | 834.69 | 0.00 | 1806.96 | 814.64 | 0.00 | 1535.37 | 0.51 |
| Surgery | 94.4 | 0.00 | 598.16 | 117.45 | 0.00 | 803.50 | 0.91 |
| Orthopaedics | 211.55 | 0.00 | 903.10 | 226.59 | 0.00 | 855.32 | 0.50 |
| Oncology | 31.78 | 0.00 | 367.01 | 11.99 | 0.00 | 217.26 | 0.16 |
| Rehabilitation unit | 1352.48 | 0.00 | 3421.90 | 1294.56 | 0.00 | 3222.63 | 0.89 |
| Cardiology | 93.11 | 0.00 | 1111.89 | 91.27 | 0.00 | 1067.90 | 0.84 |
| Neurology | 118.83 | 0.00 | 1462.47 | 55.21 | 0.00 | 939.43 | 0.30 |
| High-dependency unit | 42.62 | 0.00 | 743.15 | 58.89 | 0.00 | 1633.24 | 0.51 |
| Intensive care unit | 113.12 | 0.00 | 1460.78 | 276.27 | 0.00 | 3280.45 | 0.07* |
| Total inpatient care costs | 2810.08 | 1897.00 | 4513.13 | 2888.68 | 1897.00 | 5372.53 | 0.54 |
| Medical team | |||||||
| Consultant | 44.06 | 0.00 | 75.08 | 48.06 | 0.00 | 73.69 | 0.22 |
| Registrar | 56.69 | 0.00 | 190.27 | 71.80 | 0.00 | 390.67 | 0.69 |
| Physiotherapist | 104.69 | 62.64 | 123.90 | 108.31 | 83.52 | 131.26 | 0.31 |
| Dietitian | 9.00 | 0.00 | 25.52 | 9.424 | 0.00 | 30.80 | 0.81 |
| Occupational therapist | 40.05 | 0.00 | 80.29 | 39.375 | 20.87 | 79.68 | 0.60 |
| Psychologist (counsellor) | 5.47 | 0.00 | 40.51 | 5.049 | 0.00 | 30.80 | 0.89 |
| Speech therapist | 11.53 | 0.00 | 49.99 | 13.61 | 0.00 | 46.67 | 0.12 |
| Social worker | 17.02 | 0.00 | 58.16 | 11.33 | 0.00 | 40.45 | 0.038** |
| Total medical team costs | 288.55 | 176.00 | 346.28 | 306.97 | 180.52 | 548.59 | 0.71 |
| Tests | |||||||
| CT | 11.885 | 0.00 | 39.26 | 12.88 | 0.00 | 41.90 | 0.68 |
| MRI | 6.191 | 0.00 | 36.79 | 6.702 | 0.00 | 31.84 | 0.37 |
| Radiography | 7.713 | 0.00 | 23.011 | 9.856 | 0.00 | 26.27 | 0.10 |
| Echocardiography | 0.10 | 0.00 | 2.663 | 0.10219 | 0.00 | 2.62 | 0.98 |
| Ultrasonography | 0.74 | 0.00 | 6.22 | 0.720 | 0.00 | 6.143 | 0.95 |
| Bloods | 5.900 | 3.13 | 9.556 | 7.14 | 3.13 | 15.829 | 0.19 |
| Total tests costs | 32.53 | 0.00 | 70.59 | 37.41 | 0.00 | 79.32 | 0.07* |
| Mattress | |||||||
| Mattress type | 28.8 | 14.34 | 26.3 | 1.05 | 0.54 | 0.93 | 0.00 |
| Residential care | |||||||
| Care home | 177.07 | 0.00 | 936.60 | 140.2 | 0.00 | 603.08 | 0.19 |
| Nursing home | 285.02 | 0.00 | 719.53 | 140.25 | 0.00 | 967.151 | 0.63 |
| Rehabilitation unit | 324.62 | 0.00 | 1503.61 | 432.66 | 0.00 | 1722.34 | 0.13 |
| Total residential care cost | 786.72 | 0.00 | 1820.84 | 837.10 | 0.00 | 1959.86 | 0.97 |
| Cost category | Trial arm (£) | p-value (Wilcoxon) | |||||
|---|---|---|---|---|---|---|---|
| APM | HSFM | ||||||
| Mean | Median | SD | Mean | Median | SD | ||
| GP | |||||||
| Surgery | 6.594 | 0.00 | 20.74 | 5.298 | 0.00 | 18.678 | 0.42 |
| Clinic | 2.097 | 0.00 | 21.00 | 0.417 | 0.00 | 5.305 | 0.38 |
| Telephone | 8.713 | 0.00 | 28.128 | 6.494 | 0.00 | 21.02 | 0.018** |
| Home visit | 65.62 | 0.00 | 109.15 | 64.78 | 0.00 | 93.78 | 0.44 |
| Total GP costs | 83.03 | 0.00 | 113.2 | 76.99 | 0.00 | 95.08 | 0.8673 |
| Nurse | |||||||
| Surgery | 0.7585 | 0.00 | 4.093 | 1.452 | 0.00 | 11.87 | 0.87 |
| Clinic | 0.0291 | 0.00 | 0.641 | 0.087 | 0.00 | 1.921 | 0.99 |
| Telephone | 0.070 | 0.00 | 0.7237 | 0.0348 | 0.00 | 0.442 | 0.47 |
| Home visit | 57.19 | 0.00 | 155.47 | 83.26 | 0.00 | 209.5 | 0.089 |
| Total nurse costs | 58.05 | 0.00 | 155.32 | 84.83 | 0.00 | 209.31 | 0.065* |
| Physiotherapist | |||||||
| Surgery | 2.039 | 0.00 | 28.12 | 2.895 | 0.00 | 30.68 | 0.40 |
| Clinic | 1.79 | 0.00 | 13.97 | 0.617 | 0.00 | 6.91 | 0.01** |
| Telephone | 0.3471 | 0.00 | 3.29 | 0.378 | 0.00 | 3.91 | 0.81 |
| Home visit | 39.91 | 0.00 | 107.29 | 58.30 | 0.00 | 146.67 | 0.03 |
| Total physiotherapist costs | 44.10 | 0.00 | 110.72 | 62.19 | 0.00 | 148.62 | 0.060* |
| Counsellor | |||||||
| Surgery | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Clinic | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Telephone | 0.00 | 0.00 | 0.00 | 0.090 | 0.00 | 1.99 | 0.31 |
| Home visit | 1.146 | 0.00 | 18.93 | 0.228 | 0.00 | 3.749 | 0.41 |
| Total counsellor costs | 1.1466 | 0.00 | 18.93 | 0.318 | 0.00 | 4.971 | 0.411 |
| Occupational therapist | |||||||
| Surgery | 0.2169 | 0.00 | 3.161 | 1.944 | 0.00 | 26.50 | 0.30 |
| Clinic | 0.6508 | 0.00 | 9.360 | 0.030 | 0.00 | 0.680 | 0.10 |
| Telephone | 0.165 | 0.00 | 1.775 | 0.263 | 0.00 | 3.39 | 0.60 |
| Home visit | 14.694 | 0.00 | 46.71 | 20.68 | 0.00 | 87.56 | 0.38 |
| Total occupational therapy cost | 15.72 | 0.00 | 47.54 | 22.9 | 0.00 | 91.09 | 0.306 |
| Home care | |||||||
| Frozen meals | 2.11 | 0.00 | 17.03 | 3.28 | 0.00 | 18.69 | 0.23 |
| Hot meals | 4.99 | 0.00 | 43.53 | 3.34 | 0.00 | 28.57 | 0.64 |
| Laundry | 0.61 | 0.00 | 5.58 | 0.698 | 0.00 | 5.51 | 0.64 |
| Care worker | 190.22 | 0.00 | 640.9 | 147.21 | 0.00 | 367.64 | 0.38 |
| Social worker | 7.508 | 0.00 | 31.26 | 13.49 | 0.00 | 106.46 | 0.53 |
| Total home-care cost | 205.45 | 0.00 | 645.92 | 168.03 | 0.00 | 168.03 | 0.56 |
| Cost category | Trial arm (£) | p-value (Wilcoxon) | |||||
|---|---|---|---|---|---|---|---|
| APM | HSFM | ||||||
| Mean | Median | SD | Mean | Median | SD | ||
| Outpatient specialist | |||||||
| Surgery | 6.50 | 0.00 | 55.62 | 6.21 | 0.00 | 45.33 | 0.84 |
| Orthopaedic | 11.21 | 0.00 | 42.16 | 21.37 | 0.00 | 71.92 | 0.003** |
| Oncology | 3.46 | 0.00 | 38.46 | 4.70 | 0.00 | 45.14 | 0.41 |
| Cardiology | 3.45 | 0.00 | 23.91 | 7.42 | 0.00 | 38.96 | 0.07* |
| Respiratory | 2.25 | 0.00 | 21.19 | 1.60 | 0.00 | 15.77 | 0.75 |
| Urology | 4.82 | 0.00 | 50.86 | 5.021 | 0.00 | 60.16 | 0.67 |
| Medicine | 11.49 | 0.00 | 57.76 | 15.25 | 0.00 | 115.54 | 0.76 |
| Neurology | 1.83 | 0.00 | 17.92 | 0.36 | 0.00 | 8.035 | 0.10 |
| Rehabilitation | 1.30 | 0.00 | 12.78 | 1.30 | 0.00 | 15.10 | 0.73 |
| Ophthalmology | 2.07 | 0.00 | 15.97 | 2.25 | 0.00 | 15.37 | 0.66 |
| Rheumatology | 0.29 | 0.00 | 6.54 | 0.59 | 0.00 | 9.22 | 0.56 |
| A&E | 1.72 | 0.00 | 17.79 | 2.00 | 0.00 | 20.82 | 0.99 |
| Radiology | 0.88 | 0.00 | 10.22 | 0.35 | 0.00 | 7.73 | 0.17 |
| Total | 51.33 | 0.00 | 123.41 | 68.45 | 0.00 | 167.57 | 0.05** |
| Tests | |||||||
| CT | 0.00 | 0.00 | 0.00 | 0.44 | 0.00 | 6.92 | 0.15 |
| MRI | 0.61 | 0.00 | 9.50 | 0.00 | 0.00 | 0.00 | 0.15 |
| Radiography | 0.10 | 0.00 | 1.60 | 0.41 | 0.00 | 3.56 | 0.09* |
| Ultrasonography | 0.55 | 0.00 | 6.35 | 0.33 | 0.00 | 5.37 | 0.41 |
| Bloods | 0.00 | 0.00 | 0.00 | 0.013 | 0.00 | 0.20 | 0.15 |
| Total | 1.26 | 0.00 | 11.50 | 1.19 | 0.00 | 9.40 | 0.28 |
| Time point | Mean (SD), median | Trial arm, mean (SD), median | p-value (base case; Wilcoxon) | |
|---|---|---|---|---|
| APM | HSFM | |||
| EQ-5D-5L | ||||
| Baseline | 0.34 (0.23), 0.33 | 0.34 (0.22), 0.32 | 0.34 (0.23), 0.33 | 0.87 |
| Week 1 | 0.41 (0.22), 0.40 | 0.41 (0.21), 0.40 | 0.41 (0.22), 0.40 | 0.50 |
| Week 3 | 0.47 (0.19), 0.47 | 0.47 (0.18), 0.47 | 0.46 (0.20), 0.47 | 0.65 |
| 30 days after the end of the treatment phase | 0.52 (0.22), 0.53 | 0.52 (0.21), 0.54 | 0.52 (0.22), 0.53 | 0.49 |
| PU-QoL-UI | ||||
| Baseline | 0.60 (0.16), 0.60 | 0.60 (0.16), 0.60 | 0.60 (0.16), 0.60 | 0.96 |
| Week 1 | 0.65 (0.14), 0.66 | 0.65 (0.14), 0.66 | 0.65 (0.14), 0.65 | 0.52 |
| Week 3 | 0.69 (0.12), 0.71 | 0.69 (0.11), 0.71 | 0.69 (0.12), 0.70 | 0.65 |
| 30 days after the end of the treatment phase | 0.68 (0.13), 0.69 | 0.69 (0.13), 0.70 | 0.69 (0.13), 0.69 | 0.28 |
Sensitivity analyses for within-trial analyses
| Strategy | Total cost (adjusted) (£) | Total QALY (adjusted) | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| HSFM | 7441 | 0.080 | –5834 | 0.86 | Cost-effective | |||
| APM | 7579 | 0.076 | 138 | –0.0043 | –31,721 | –6059 | 0.14 | Dominated |
| Strategy | Total cost (not adjusted) (£) | Total QALY (not adjusted) | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| APM | 4534 | 0.128 | –1980 | 0.76 | Cost-effective | |||
| HSFM | 4647 | 0.127 | 113 | –0.0011 | –102,406 | –2114 | 0.24 | Dominated |
| Strategy | Total cost (not adjusted) (£) | Total QALY (not adjusted) | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| HSFM | 7454 | 0.080 | –5847 | 0.63 | Cost-effective | |||
| APM | 7555 | 0.076 | 101 | –0.0044 | –22,888 | –6035 | 0.37 | Dominated |
| Strategy | Total cost (£) | Total PU-QoL-UI | Incremental cost (£) | Incremental PU-QoL-UI | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| APM | 4533 | 0.1824 | –855 | 1 | Cost-effective | |||
| HSFM | 4646 | 0.1815 | –113 | –0.0009 | 125,555 | –1016 | 0 | Dominated |
| Strategy | Total cost (£) | Total PU-QoL-UI | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| HSFM | 7542 | 0.128 | 4967 | 0.70 | Cost-effective | |||
| APM | 7571 | 0.124 | 28 | –0.005 | 5627 | 5096 | 0.30 | Dominated |
| Strategy | Total cost (adjusted) (£) | Total QALY (adjusted) | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective(£20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| APM | 5171 | 0.0615 | –3941.00 | 0.09 | Dominated | |||
| HSFM | 5276 | 0.0608 | 105 | –0.0007 | –151,411 | –4059.89 | 0.91 | Cost-effective |
| Strategy | Total cost (not adjusted) (£) | Total QALY (not adjusted) | Incremental cost (£) | Incremental QALY | ICER (£) | NMB (£) | Probability of being cost-effective (at £20,000 threshold) | Result |
|---|---|---|---|---|---|---|---|---|
| APM | 5165 | 0.0612 | –3941.52 | 0.92 | Cost-effective | |||
| HSFM | 5276 | 0.0617 | 111 | 0.0005 | 218,009 | –4042.39 | 0.08 | – |
| Scenario | Baseline result | New ICER (£) | New result |
|---|---|---|---|
| 10% increase in cost of PU treatment (in hospital) | ICER –£18,979 APM dominates | –19,471 | APM dominates |
| 10% increase in cost of home-care PU treatment (once discharged) | –18,506 | APM dominates | |
| 10% decrease in cost of PU treatment (in hospital) | –19,143 | APM dominates | |
| 10% decrease in cost of home-care PU treatment (once discharged) | –18,888 | APM dominates | |
| 20% increase in cost of APM | –18,888 | APM dominates | |
| 50% increase in cost of APM | –18,761 | APM dominates | |
| 10% increase in QALY loss as a result of having a PU | –18,607 | APM dominates | |
| 10% decrease in QALY loss as a result of having a PU | –19,586 | APM dominates |
Appendix 6 Pressure Ulcer Quality of Life – Prevention: supplementary tables
| Psychometric property | Definition/test | Criteria for acceptability |
|---|---|---|
| Item analysis | Identify items for possible elimination as a result of weak psychometric performance, assessed on the basis of:
|
Exploratory factor analysis:
|
| Data quality | The quality of data, assessed by completeness of data and score distributions |
|
| Reliability | ||
| Internal consistency | The extent to which items comprising a scale measure the same construct (e.g. homogeneity of the scale), assessed by Cronbach’s alpha and item-total correlations |
|
| Validity | ||
| Construct validity | ||
| Within-scale analyses | Evidence that a single entity (construct) is being measured and that items can be combined to form a summary score, assessed on the basis of evidence of good internal consistency and correlations between scale scores (which purport to measure related aspects of the construct) |
|
| Analyses against external criteria | ||
| Convergent validity | Evidence that the scale is correlated with other measures of the same or similar constructs, assessed on the basis of correlations between the measure and other similar measures |
|
| Known-groups differences | The ability of a scale to differentiate known groups, assessed by comparing scores for subgroups who are expected to differ on the construct being measured |
|
| Responsiveness | The ability of a scale to detect clinically significant change following treatment of known efficacy, assessed by examining within-person change scores before and after treatment and calculating an effect size statistic (mean change score divided by SD of pre-treatment scores) |
|
| Scale (n items) | Data completeness: computable scale score (%) | Targeting | ||||||
|---|---|---|---|---|---|---|---|---|
| Possible score range | Range mid-point | Observed score range | Mean scorea | SD | Floor/ceiling effectb (%) | Skewness | ||
| Pain (12) | 617 (100) | 0–100 | 50 | 0–100 | 20.90 | 24.594 | 37.5/0.8 | 1.130 |
| Exudate (8) | 62c (100) | 0–100 | 50 | 0–86 | 5.83 | 15.525 | 75.8/1.6 | 3.749 |
| Odour (6) | 62c (100) | 0–100 | 50 | 0–25 | 0.54 | 3.330 | 96.8/1.6 | 6.927 |
| Sleep (7) | 538 (87.2) | 0–100 | 50 | 0–100 | 21.72 | 30.880 | 53.0/4.7 | 1.274 |
| Movement and mobility (9) | 449 (72.8) | 0–100 | 50 | 0–100 | 36.67 | 38.022 | 42.1/12.7 | 0.442 |
| Daily activities (6) | 446 (72.3) | 0–100 | 50 | 0–100 | 20.50 | 32.734 | 64.1/5.7 | 1.346 |
| Malaise (5) | 449 (72.8) | 0–100 | 50 | 0–100 | 18.01 | 30.386 | 67.0/5.0 | 1.537 |
| Emotional well-being (15) | 514 (83.3) | 0–100 | 50 | 0–100 | 15.83 | 27.965 | 63.6/2.7 | 1.744 |
| Self-consciousness and appearance (7 items) | 518 (83.9) | 0–100 | 50 | 0–100 | 8.70 | 20.131 | 75.3/1.1 | 2.730 |
| Itchiness (1) | 549 (89.0) | 0–100 | 50 | 0–100 | 12.28 | 27.496 | 81.3/5.8 | 2.165 |
| Global QoL (1) | 565 (916) | 0–100 | 50 | 0–100 | 47.639 | 38.592 | 32.2/27.5 | 0.081 |
| Scale (n items) | Data completeness: computable scale score (%) | Targeting | ||||||
|---|---|---|---|---|---|---|---|---|
| Possible score range | Range mid-point | Observed score range | Mean scorea | SD | Floor/ceiling effectb (%) | Skewness | ||
| Physical functioning (2) | 1415 (99.4) | 0–100 | 50 | 0–100 | 8.608 | 20.281 | 80.3/1.6 | 2.641 |
| Role-physical (2) | 1399 (98.3) | 0–100 | 50 | 0–100 | 21.497 | 23.653 | 37.7/2.0 | 1.207 |
| Bodily pain (1) | 1359 (95.5) | 0–100 | 50 | 0–100 | 51.51 | 37.040 | 20.0/25.5 | –0.003 |
| General health (1) | 1413 (99.3) | 0–100 | 50 | 0–100 | 34.32 | 30.321 | 29.2/2.8 | 0.488 |
| Vitality (1) | 1369 (96.2) | 0–100 | 50 | 0– 100 | 22.52 | 25.636 | 45.1/2.2 | 1.043 |
| Social functioning (1) | 1345 (94.5) | 0–100 | 50 | 0–100 | 47.73 | 41.620 | 33.1/30.1 | 0.105 |
| Role-emotional (2) | 1382 (97.1) | 0–100 | 50 | 0–100 | 65.41 | 32.008 | 5.3/32.9 | –0.449 |
| Mental health (2) | 1373 (96.5) | 0–100 | 50 | 0–100 | 56.39 | 25.504 | 3.2/5.8 | –0.230 |
| Physical component score | 1356 (95.3) | 0–100 | 50 | 10–59 | 30.55 | 7.700 | 0.1/0.1 | 0.259 |
| Mental component score | 1355 (95.2) | 0–100 | 50 | 13–75 | 45.16 | 12.275 | 0.1/0.1 | –0.176 |
| Item | Factors and loadings (exploratory factor analysis)a | ||||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
| EW: frustrated | 0.816 | ||||
| EW: fed: up | 0.811 | ||||
| EW: miserable | 0.792 | ||||
| EW: concerned/worried | 0.762 | ||||
| EW: annoyed/irritated | 0.750 | ||||
| EW: anxious | 0.737 | ||||
| EW: no control | 0.726 | ||||
| EW: dependent | 0.713 | ||||
| EW: burden on others | 0.687 | ||||
| EW: missing out | 0.668 | ||||
| EW: cut: off/isolated | 0.624 | ||||
| EW: depressed | 0.622 | ||||
| SCA: helpless | 0.616 | ||||
| EW: lonely | 0.596 | ||||
| EW: angry | 0.524 | ||||
| MM: limited in using stairs | 0.946 | ||||
| MM: walking slowed | 0.910 | ||||
| MM: difficulty standing | 0.897 | ||||
| MM: limited in ability to walk | 0.891 | ||||
| MM: difficulty transferring | 0.796 | ||||
| DA: toilet | 0.753 | ||||
| DA: washing self | 0.740 | ||||
| DA: dressing | 0.737 | ||||
| DA: doing regular activities | 0.657 | ||||
| MM: difficulty pushing up | 0.612 | ||||
| MM: difficulty adjusting | 0.522 | 0.377 | |||
| MM: difficulty turning | 0.521 | 0.357 | |||
| DA: doing things you enjoy | 0.507 | ||||
| MM: difficulty sitting | 0.485 | ||||
| M: appetite | 0.332 | 0.319 | |||
| S: kept awake | 0.858 | ||||
| S: not getting sleep | 0.812 | ||||
| S: woken during sleep | 0.797 | ||||
| S: interrupted sleep | 0.746 | ||||
| S: trouble falling asleep | 0.735 | ||||
| S: sleep in one position | 0.613 | ||||
| S: uncomfortable | 0.318 | 0.568 | |||
| M: unwell | 0.493 | ||||
| M: fatigued | 0.310 | 0.487 | |||
| M: reduced energy | –0.377 | 0.425 | |||
| M: tired | 0.389 | 0.422 | |||
| SCA: uneasy being close | 0.831 | ||||
| SCA: lack understanding from others | 0.750 | ||||
| SCA: embarrassed | 0.699 | ||||
| SCA: physically unattractive | 0.612 | ||||
| SCA: self: conscious | 0.549 | ||||
| EW: people treat you differently | 0.492 | ||||
| SCA: lack confidence | 0.357 | 0.483 | |||
| DA: intimacya | |||||
| Scale (n items) | Physical function (2) | Role-physical (2) | Bodily pain (1) | General health (1) | Vitality (1) | Social function (1) | Role-emotional (2) | Mental health (2) |
|---|---|---|---|---|---|---|---|---|
| Pain (12) | 0.072 | 0.109** | 0.320** | 0.220** | 0.168** | 0.046 | 0.201** | 0.261** |
| Exudate (8) | 0.005 | 0.112 | 0.036 | 0.046 | 0.237 | 0.183 | 0.229 | 0.279* |
| Odour (4) | 0.118 | 0.235 | 0.109 | 0.149 | 0.022 | 0.154 | 0.160 | 0.063 |
| Sleep (7) | 0.143** | 0.137** | 0.283** | 0.203** | 0.110* | 0.008 | 0.246** | 0.282** |
| Movement and mobility (9) | 0.151** | 0.133** | 0.350** | 0.214** | 0.171** | 0.036 | 0.299** | 0.300** |
| Daily activities (6) | 0.086 | 0.071 | 0.197** | 0.139** | 0.027 | 0.046 | 0.238** | 0.218** |
| Malaise (5) | 0.030 | 0.017 | 0.229** | 0.205** | 0.024 | 0.076 | 0.293** | 0.274** |
| Emotional well-being (15) | 0.034 | 0.037 | 0.227** | 0.179** | 0.012 | 0.030 | 0.323** | 0.275** |
| Self-consciousness and appearance (7) | 0.034 | 0.053 | 0.219** | 0.119** | 0.053 | 0.045 | 0.249** | 0.206** |
| Itchiness (1) | 0.006 | 0.080 | 0.130** | 0.071 | 0.033 | 0.030 | 0.100* | 0.100* |
| PU-QoL-P scales (n items) | Sample size, n | Category 2 PU at baseline | No PU at baseline | p-value* | Mean difference | CI for difference | Effect size | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Category 2 PU at baseline | No PU at baseline | Mean | SD | Mean | SD | |||||
| Pain (12) | 18 | 151 | 13.342a | 21.520 | 10.908 | 20.679 | 0.639 | 2.434 | –7.79 to 12.66 | 0.12 |
| Exudate (8) | 3 | 2 | 28.274a | 43.671 | 18.750 | 26.517 | 0.805 | 9.524 | –103.21 to 122.26 | 0.25 |
| Odour (6) | 3 | 2 | 33.333a | 57.735 | 25.000 | 35.355 | 0.870 | 8.333 | –140.91 to 157.57 | 0.16 |
| Sleep (7) | 15 | 143 | 13.571a | 31.258 | 8.851 | 19.661 | 0.408 | 4.720 | –6.52 to 15.96 | 0.23 |
| Movement and mobility (9) | 15 | 132 | 17.287a | 34.338 | 13.177 | 28.387 | 0.604 | 4.110 | –11.52 to 19.74 | 0.14 |
| Daily activities (6) | 15 | 134 | 16.111a | 31.255 | 7.152 | 20.055 | 0.126 | 8.959 | –2.54 to 20.46 | 0.42 |
| Malaise (5) | 12 | 124 | 11.667a | 30.101 | 7.950 | 22.254 | 0.594 | 3.717 | –10.04 to 17.47 | 0.16 |
| Emotional well-being (15) | 17 | 145 | 7.010a | 20.179 | 5.733 | 16.825 | 0.772 | 1.277 | –7.43 to 9.98 | 0.07 |
| Self-consciousness and appearance (7) | 18 | 145 | 2.381 | 10.102 | 2.716 | 10.931 | 0.902 | –0.335 | –5.69 to 5.02 | –0.03 |
| PU-QoL-P scales (n items) | Sample size, n | Completely limited | No/slight impairment | p-value | Mean difference | CI for difference | Effect size | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Completely limiteda | No/slight impairmenta | Mean | SD | Mean | SD | |||||
| Pain (12) | 31 | 164 | 16.50 | 27.628 | 24.65 | 24.724 | 0.100 | –8.157 | –17.890 to 1.576 | –0.32 |
| Exudate (8) | 4 | 26 | 0.00 | 0.000 | 2.68 | 8.886 | 0.557 | –2.679 | –11.916 to 6.559 | –0.32 |
| Odour (6) | 4 | 26 | 0.00 | 0.000 | 0.96 | 4.903 | 0.702 | –0.962 | –6.058 to 4.135 | –0.21 |
| Sleep (7) | 28 | 149 | 21.30 | 33.397 | 20.28 | 27.745 | 0.864 | 1.017 | –10.645 to 12.680 | 0.04 |
| Movement and mobility (9) | 20 | 146 | 49.38 | 44.632 | 28.72 | 33.020 | 0.013* | 20.651 | 4.378 to 36.924 | 0.60 |
| Daily activities (6) | 15 | 134 | 35.56 | 43.240 | 14.14 | 26.505 | 0.007* | 21.414 | 6.065 to 36.762 | 0.75 |
| Malaise (5) | 22 | 136 | 24.09 | 31.609 | 15.63 | 30.086 | 0.226 | 8.466 | –5.286 to 22.218 | 0.28 |
| Emotional well-being (15) | 24 | 147 | 16.03 | 26.241 | 13.01 | 26.260 | 0.602 | 3.017 | –8.395 to 14.429 | 0.12 |
| Self-consciousness and appearance (7) | 25 | 145 | 12.29 | 24.530 | 6.69 | 16.014 | 0.141 | 5.596 | –1.880 to 13.072 | 0.32 |
| PU-QoL-P scales (n items) | Sample size, n | Limb | Torso | p-value* | Mean difference | CI for difference | Effect size | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Limb | Torso | Mean | SD | Mean | SD | |||||
| Pain (12) | 29 | 96 | 23.55 | 25.551 | 25.10 | 25.321 | 0.773 | –1.550 | –12.19 to 9.09 | –0.06 |
| Exudate (8) | 8 | 46 | 1.56 | 4.419 | 5.28 | 14.982 | 0.492 | –3.720 | –14.51 to 7.07 | –0.27 |
| Odour (6) | 8 | 46 | 0.00 | 0.00 | 0.72 | 3.859 | 0.601 | –0.720 | –3.48 to 2.04 | –0.20 |
| Sleep (7) | 25 | 89 | 26.90 | 34.537 | 23.53 | 31.505 | 0.644 | 3.370 | –11.06 to 17.80 | 0.10 |
| Movement and mobility (9) | 22 | 74 | 35.32 | 41.949 | 38.57 | 35.930 | 0.721 | –3.250 | –21.26 to 14.76 | –0.09 |
| Daily activities (6) | 21 | 77 | 22.62 | 37.652 | 20.28 | 31.701 | 0.774 | 2.340 | –13.80 to 18.48 | 0.07 |
| Malaise (5) | 19 | 71 | 24.74 | 38.350 | 21.62 | 34.297 | 0.732 | 3.120 | –14.93 to 21.17 | 0.09 |
| Emotional well-being (15) | 24 | 85 | 20.76 | 34.829 | 12.07 | 23.227 | 0.154 | 8.690 | –3.30 to 20.68 | 0.33 |
| Self-consciousness and appearance (7) | 23 | 87 | 18.17 | 30.965 | 6.73 | 16.352 | 0.017 | 11.440 | 2.05 to 20.83 | 0.57 |
Modified scales post hoc analysis
| PU-QoL-P scales (n items) | Internal consistency | Mean IIC | IICa | Scaling assumptions: corrected ITCa | |
|---|---|---|---|---|---|
| n | Cronbach’s alpha | ||||
| Daily activities (5) | 189 | 0.959 | 0.789 | 0.644–0.901 | 0.750–0.927 |
| Malaise (4) | 224 | 0.921 | 0.743 | 0.634–0.849 | 0.715–0.879 |
| Emotional well-being (15) | 215 | 0.971 | 0.689 | 0.513–0.899 | 0.682–0.878 |
| Self-consciousness and appearance (7) | 295 | 0.914 | 0.645 | 0.502–0.795 | 0.681–0.812 |
| Scale (n items) | Physical function (2) | Role-physical (2) | Bodily pain (1) | General health (1) | Vitality (1) | Social function (1) | Role-emotional (2) | Mental health (2) |
|---|---|---|---|---|---|---|---|---|
| Daily activities (5) | 0.120* | 0.130** | 0.224** | 0.184** | 0.085 | 0.047 | 0.278** | 0.266** |
| Malaise (4) | 0.046 | 0.004 | 0.253** | 0.244** | 0.039 | 0.062 | 0.312** | 0.312** |
| Emotional well-being (15) | 0.044 | 0.041 | 0.227** | 0.184** | 0.003 | 0.023 | 0.343** | 0.294** |
| Self-consciousness and appearance (7) | 0.030 | 0.048 | 0.216** | 0.137** | 0.039 | 0.054 | 0.246** | 0.230** |
Glossary
- PU of category ≥ 2
- A pressure ulcer of category 2 or higher.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- APM
- alternating pressure mattress
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN/P
- clinical research nurse/registered health-care professional
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- ENBS
- expected net benefit of sampling
- ENPVSI
- expected net present value of sampling information
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVSI
- expected value of sample information
- FDA
- Food and Drug Administration
- GBP
- Great British pounds
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HSFM
- high-specification foam mattress
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IIC
- inter-item correlation
- IRAS
- Integrated Research Application System
- ITC
- inter-total correlation
- ITT
- intention to treat
- MAR
- missing at random
- MCAR
- missing completely at random
- MNAR
- missing not at random
- MSG
- mattress specification guide
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PABAK
- prevalence- and bias-adjusted kappa
- PC
- personal computer
- PI
- principal investigator
- PPI
- patient and public involvement
- PPP
- per-protocol population
- PRESSURE
- Pressure RElieving Support SUrfaces: a Randomised Evaluation
- PRO
- patient-reported outcome
- PSS
- Personal Social Services
- PU
- pressure ulcer
- PU-MDS
- pressure ulcer – minimum data set
- PU-QoL
- Pressure Ulcer Quality of Life
- PU-QoL-P
- Pressure Ulcer Quality of Life – Prevention
- PU-QoL-UI
- Pressure Ulcer Quality of Life – Utility Index
- PURPOSE
- Pressure UlceR Programme Of reSEarch
- PURPOSE-T
- Pressure Ulcer Risk Primary Or Secondary Evaluation Tool
- PURSUN
- Pressure Ulcer Research Service User Network
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RHCP
- registered health-care professional
- RSE
- relative standard error
- RU SAE
- related unexpected serious adverse event
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SOP
- standard operating procedure
- SSC
- service support costs
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VOI
- value of information
