Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/10/17. The contractual start date was in January 2017. The draft report began editorial review in October 2018 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Irene Higginson reports involvement in the following National Institute for Health Research (NIHR) funding boards: Health Services and Delivery Research Commissioning Board (2009–15), Health Technology Assessment (HTA) Efficient Study Designs (2015–16), HTA End of Life Care and Add on Studies (2015–16) and Service Delivery and Organisation Studies Panel (2009–12). Wei Gao reports involvement in the NIHR HTA End of Life Care and Add on Studies Board (2015–16).
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Koffman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 The management of clinical uncertainty in hospital settings
Parts of this report have been reproduced/adapted from Koffman et al. 1 The Authors 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Summary of the Health Technology Assessment brief
In 2015, the National Institute for Health Research (NIHR) Health Technology Assessment programme published a commissioning brief with a focus on clinical and applied health research into end-of-life care. Interventions for patients in the last 30 days of life were of particular interest. Applicants were asked to consider (1) the use of technologies or interventions that enable and support informed decision-making and choice during the process of end-of-life care, (2) the use of technologies or interventions that enable and support a patient’s ability to die at home if they wish and (3) interventions to support patients, carers and health-care professionals (HCPs) to enable the development of knowledge, skills and confidence in care delivery. This report contains the research conducted in response to this brief (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/151017/#/; accessed 8 February 2019).
Summary of current evidence and policy context
Background rationale
The magnitude of dying in hospital settings
Every year, more than 500,000 people die in the UK,2 and this number is rising. 3 Of these deaths, more than half occur in hospital. 4 However, 69.2% (range 51–84%) of patients and their families would prefer to die at home. 5 Most deaths are anticipated, with up to 75% of all deaths expected, so there is time for discharge to home or more familiar and preferred surroundings. Major reasons for hospital death are poor communication about declining health between patients and HCPs6 and poor identification and management of patients whose situations are ‘clinically uncertain’. 7
Clinical uncertainty in hospital settings
Clinical uncertainty is not a simple concept and a situation of uncertainty usually results from several inter-related factors. Mishel8 was one of the first to develop an overarching theory of uncertainty in an illness, and aimed to explain the underlying processes governing patients’ experiences of uncertainty. Specifically, four concepts contribute to an uncertain state: complexity, unpredictability, ambiguity and lack of information. 8–10 McCormick11 further developed these ideas and described situations of uncertainty in terms of the probability of events occurring, the temporality of events and individuals’ perceptions of their situation.
If clinical uncertainty is not explicitly addressed, there are worse psychological outcomes for patients. 12,13 Evidence suggests that, in the last 30 days of life, the combination of deteriorating health and clinical uncertainty are highly distressing for patients in a hospital and their families. 14,15 This distress is amplified when discussions about their situation and preferences for care and location of death are absent; 67–80% of people want to be informed about poor prognosis. 16 However, research shows that discussions about prognosis rarely occur,17 increasing the likelihood of hospital deaths, but also leading to poor satisfaction, mistrust and loss of confidence in HCPs. 18–21 Indeed, complaints about care at the end of life in hospital settings are frequent. 22
Clinical uncertainty also affects clinicians’ confidence and their practice. Clinicians frequently struggle with uncertainty, which can result in overtreatment or overinvestigation,23 increased costs13 and lack of communication with patients about their future. 24,25 Furthermore, clinicians often feel inappropriately trained to deal with uncertainty; only 4 out of 21 UK postgraduate medical training curricula contain detailed recommendations and curriculum goals relevant to dealing with uncertainty. However, when situations of uncertainty are acknowledged and managed alongside high-quality care, particularly at the end of life, collaborative decision-making is possible. 26 This empowers patients and their carers,27–29 and in turn leads to improved outcomes and increased satisfaction with care. 30,31
The potential for better care
Poor hospital care and inadequate communication, which include patient safety and adverse events, have received increasing attention, particularly regarding the frail elderly and the dying. The Francis Report,32 the independent review of the Liverpool Care Pathway for the Dying Patient33 and the Parliamentary and Health Services Ombudsman’s report34 into complaints about end-of-life care all highlight the devastating effect poor communication and lack of honesty can have on patients and their families towards the end of life. Research has also demonstrated that the costs of patient care in the last year of life are high. 35 Yet when specialist palliative care services are available in hospital and community settings, health service costs can be reduced and patient- and family-centred outcomes improved. 36–38
In 2010, a London hospital identified inconsistencies in the quality of care for patients whose situations were clinically uncertain, for those who were deteriorating and especially for those at the end of life. 34 Issues included inadequate decision-making and poor engagement with patients and carers. A potential solution to caring and supporting patients and their relatives in this situation, developed under the umbrella of a bundle (Box 1 explains the concept of a ‘care bundle’), is referred to as the ‘AMBER care bundle’, where AMBER stands for Assessment; Management; Best practice; Engagement; Recovery uncertain.
The US Institute for Healthcare Improvement describes care bundles as a set of evidence-based interventions for patients that when implemented in combination are aimed at resulting in outcomes that are superior to when delivered alone. 39 They typically consist of a small number of interventions (normally 4–5), which, when implemented together, are associated with improvements in clinical outcomes. 40 The concept of the care bundle approach was originally developed in the USA in intensive care units to achieve the highest levels of reliability in critical care processes that would result in improved outcomes, while at the same time introducing concepts of enhanced teamwork and communication. Since that time, the development and use of care bundles within health care have continued to rapidly evolve both inside and outside the critical care setting.
The AMBER care bundle follows an algorithmic approach to encourage clinical teams to develop and document a clear medical plan, considering anticipated outcomes and resuscitation and escalation status, and to revisit the plan daily. The AMBER care bundle encourages staff, patients, and families to continue with treatment in the hope of a recovery, while talking openly about preferences and priorities should the worst happen. The bundle is modelled on previous empirical work that includes a literature review and examination of clinical records to determine need. 34 However, this bundle was developed to address an identified gap in clinical practice, hence it does not have theoretical underpinnings. Once staff have identified patients who are deteriorating, whose situations are clinically uncertain, with limited reversibility and who are at risk of dying during their current episode of care despite treatment, the multidisciplinary team (MDT) is expected to complete the four tasks within the care bundle. This involves asking:
-
Has a medical plan been documented in the patient records that includes current key issues, anticipated outcomes and their resuscitation status?
-
Has an escalation/de-escalation decision been documented (ward only, high dependency, intensive care)?
-
Has the medical plan been discussed and agreed with nursing staff?
-
Has a patient/carer discussion, or meeting, been held and clearly documented?
With this information, HCPs consider whether or not the patient is still suitable for the intervention, if there had been any medical changes, and if they need to speak with the patient and their relatives daily.
The patient/carer discussion of the AMBER care bundle includes:
-
talking to the patient and family to let them know that the clinical team has concerns about their condition, and to establish their preferences and wishes
-
deciding together how the patient will be cared for should their condition change (i.e. worsen).
The intended benefits of the AMBER care bundle include:
-
increased and improved communication
-
enabling/supporting informed and shared decision-making and choice during end-of-life care
-
improved patient-/family-centred quality of life by reducing anxiety
-
enabling home death if preferred
-
supporting HCPs to develop knowledge, skills and confidence in end-of-life care delivery
-
reducing unnecessary hospital admissions while improving cost-effectiveness (reducing length of hospital stays) and making more efficient use of health services.
The AMBER care bundle is a complex intervention in that it:
-
Comprises multiple components and layers (identification, current and future care planning including escalation and de-escalation decisions, communication delivery, assessment of patient preferences and systematic follow-up, acknowledging dynamic wishes and physical conditions).
-
Aims to change behaviours of health-/social-care professionals delivering the intervention by enhancing recognition of clinical uncertainty in clinical outcomes, patients in the last months of life and management of care expectations.
-
Focuses on staff in primary, hospital and voluntary care, thus including different groups and organisational levels.
-
Includes several complex intended outcomes, including changes in patient involvement in decision-making around situations of clinical uncertainty. The AMBER care bundle is tailored to the individual patient’s and their family’s need and circumstances.
Why research was needed
The AMBER care bundle was identified by NHS England as one of five key enablers of Transforming End of Life Care in Acute Hospitals,41,42 and it is currently being used across a network of approximately 40 hospitals including district general hospitals (DGHs). A further rollout is planned; however, recommendation 7 from the Liverpool Care Pathway for the Dying Patient has suggested that training programmes focused on clinical uncertainty and communication with patients and their relatives must be examined. 33 It was therefore imperative that a clinical trial of the AMBER care bundle took place to accurately quantify patient, clinician and health systems benefits, and that any harms are understood and managed. 43
Now more than ever, with ageing populations and increasing numbers of people dying from cancer and non-malignant conditions,44 health-care systems should provide every patient and their family a dignified death. 45 Facing deteriorating health and uncertain recovery is distressing for patients who may be dying and their families. This is particularly due to the frequent, possibly unnecessary, hospital admissions during the last year of life:46 in England, patients currently spend an average of 29.7 days of their final 12 months in hospital. 35 This is costly for health services and for society. These concerns are endorsed by the NHS Outcomes Framework,47 which includes two outcomes to improve care for people at the end of life: (1) the proportion of patients who die in their preferred place of death (PPD) and (2) bereaved relatives’ experiences of care. Interventional research, including an evaluation of the AMBER care bundle, aimed to understand how these outcomes can be addressed with this intervention.
How the existing literature supports this study
A small but growing body of evidence sheds light on processes and outcomes associated with the AMBER care bundle. When it was introduced at a major London hospital, inpatient deaths declined (from an average of 93 to 81 per month). 34 A recent single-centre study7 identified that rather than being used as a tool to identify patients with an uncertain recovery, the AMBER care bundle was principally used when it became certain that patients would not recover. However, as this was a cross-sectional, observational trial, we were not able to observe changes over time.
We conducted the first comparative observational mixed-methods study of the AMBER care bundle and identified a mixed picture. 48 First, the AMBER care bundle was associated with increased frequency of discussions about prognosis between clinicians and patients, and higher awareness of their prognosis by patients. Second, we observed that those patients who died in locations other than hospitals had shorter lengths of stay than those who received usual care. The mean length of hospital stay for the patients supported by the bundle was 20.3 days (range 1–87 days), compared with 29.3 days (range 6–70 days) in the comparison wards. The mean length of hospital stay for all patients who were discharged and died in a place other than hospital also differed: the mean length of stay for those supported by the AMBER care bundle was 17.6 days (range 1–87 days), compared with 21.4 days (range 6–70 days) in the comparison wards. However, we identified that although the instances of communication were greater, they had lower clarity in relation to the quality of information transmitted about a patient’s condition. Moreover, relatives of patients supported by the AMBER care bundle described more unresolved concerns about caring for them at home. Although this study was informative in evaluating the AMBER care bundle, this was a quasi-experimental study. Non-random selection of comparison sites may have caused bias.
Qualitative data from the same study identified that the intervention was often used as a tool to label or categorise patients, and indirectly served a symbolic purpose in affecting behaviours of individuals and teams. Participants described the importance of training and education alongside the implementation of the intervention. However, adequate exposure to the intervention was essential in order to witness its potential added value or to embed it into practice. This was considered to be very variable. 49
Although the evidence suggests some potential benefits of the care for those supported by the AMBER care bundle, it also identifies downsides, specifically regarding information and communication. Therefore, clinical equipoise in relation to the intervention still exists. These findings pointed to a need for a robust comparative evaluation of the AMBER care bundle compared with standard care. The first step in this direction is to conduct feasibility work to optimise the intervention and to understand the most-appropriate methods to examine its potential benefits.
Feasibility trial aim and objectives
Trial aim
The aim of this exploratory, multicentre, cluster randomised controlled trial (RCT) was to optimise the design of the AMBER care bundle, and to define the outcomes, for a fully powered definitive trial of the AMBER care bundle versus standard care.
Trial objectives
-
To examine recruitment, retention and follow-up rates at both patient and cluster levels.
-
To test trial data collection measures and determine their optimum timing in a larger trial.
-
To assess the degree of contamination at a ward level due to ‘between-ward’ staff and patient movements.
-
To provide a preliminary estimate of the clinical effectiveness of the AMBER care bundle compared with standard care to inform sample size calculation for the full trial.
-
To estimate the intracluster correlation coefficient and likely cluster size.
-
To examine differences in the use of financial resources between the AMBER care bundle and standard care.
-
To examine the extent to which the AMBER care bundle requires further refinement or adaptation (e.g. referral criteria to identify which patients would benefit most) to suit local conditions.
-
To assess the acceptability of the AMBER care bundle to patients, their families and HCPs.
-
To determine the ‘active ingredients’ of the AMBER care bundle that need to be maintained to ensure fidelity of the intervention for a full trial.
-
To assess compliance with and barriers to the delivery of the AMBER care bundle.
Objectives 1 and 6–10 pertain at both individual and cluster levels. Objectives 2 and 4 pertain at the individual level. Objectives 3 and 5 pertain at the cluster level. While assessing acceptability of AMBER, we explored potential benefits and intended harms.
Patient and public involvement
We have sustained patient and public involvement (PPI) throughout all stages of the ImproveCare trial. PPI has been an integral part of all of our research processes, from inception and the development of research ideas, development of the funding application, the application to the NHS Research Ethics Service and the development of all associated documents, through to the delivery of the research project and the interpretation of the trial findings. We have engaged with our PPI members in a number of ways. One of our PPI members (SB) attended the initial Research Ethics Committee (REC) meeting with the chief investigator of the trial, helping to address the REC’s questions and also providing her experiences and highlighting the significance of the trial. We ensured that we met with our PPI members regularly and gave them opportunities to contribute throughout the trial. In close collaboration with our two PPI members (CE and SB), we benefited from their expert views to help us prioritise the research questions and ensure that the trial was undertaken in a way that was meaningful and relevant to patients whose situations are clinically uncertain and their families. We included both our PPI members as part of the Trial Steering and Data Monitoring and Ethics Committee (TS DMEC), which had oversight and responsibility for the conduct of the trial. They reviewed information sheets and contributed to substantial amendments to the trial that aimed to make trial participation easier and more accessible for patients and families. We actively involved both of our PPI members in the analysis and interpretation of data from different components of this trial. For instance, our PPI members were involved in the coding of patient and relative interviews. This provided the trial team with valuable insight in understanding the data and its relevance to addressing the intervention and conduct of the trial.
Chapter 2 Design of the feasibility trial assessing the AMBER care bundle and trial processes
Research design
This trial had a parallel cluster RCT design, with a 1 : 1 allocation ratio. The design of the AMBER care bundle, and the manner in which it was implemented and then operationalised at a ward level, did not strictly follow the Medical Research Council (MRC)’s guidance on the development of complex interventions,50 the MORECare statement51 or the recent guidance on process evaluations. 52 To address this, and to inform the design of a definitive clinical trial (Figure 1), we integrated (concurrent) qualitative components of the trial. Our approach closely followed the guidance (Figure 2). 51,53
FIGURE 1.
Concurrent mixed-methods design for the feasibility cluster RCT. IPOS, Integrated Palliative care Outcome Scale.

FIGURE 2.
Conceptual framework for the evaluation of the AMBER care bundle showing which elements of the MRC’s framework have been completed (top box) and those proposed for testing in this feasibility trial (left box).

Rationale for clustering
The AMBER care bundle is a complex intervention in which ward staff receive training and all patients within the wards are considered for receiving support from the intervention. It would therefore not be possible to randomise patients at the individual level within the same ward. 54 In order to prevent contamination of the control participants, which may have led to biased estimates of the intervention’s potential effect, this trial was designed to be randomised at the cluster level. Clusters, in this case, were identified as being general medical wards, and were selected from different hospitals, as randomised wards within the same hospital might have also been contaminated because of between-ward staff movements.
Components of the trial
-
The clinical trial of the patients supported by the AMBER care bundle compared with those cared for on control wards.
-
A bereavement survey of relatives/close friends of patients who fulfilled the criteria to be supported by the AMBER care bundle on intervention and control wards.
-
Qualitative interviews with patients and their relatives/close friends.
-
Non-participation observation of MDTs at the trial wards.
-
Focus groups with HCPs working on the trial wards.
-
A usual care questionnaire completed by the HCPs working on the trial wards.
-
A review of patients’ case notes and ‘heat maps’ produced at each trial ward.
Consent processes
All participants who provided written informed consent (or assent) to participate in the trial were provided with a copy of the information sheet and their signed consent (or assent) form to keep (see Appendix 2). A copy of the signed consent/assent form was filed in the participant’s medical notes, and a copy was sent to the patient’s general practitioner if they provided their general practitioner’s details. The research team retained the original signed consent form.
Research governance and ethics approval
Favourable ethics opinion was obtained from the National Research Ethics Committee – Camden and King’s Cross (REC reference 16/LO/2010) on 20 December 2016 and from the Health Research Authority (HRA) on 25 January 2017. NHS research governance approvals were obtained from each participating trial hospital. All minor and substantial amendments to trial procedures and material were reviewed and approved by the REC, HRA and local trial hospitals (referred to as trial sites).
Amendments to the protocol
Six substantial amendments were submitted during the course of the feasibility trial. The changes to the protocol and the trial procedures are outlined in this section. All amendments were approved by the REC, HRA and local trial sites (see Appendix 3 for approvals from the REC).
-
The participant information sheets and consent forms for the non-participant observation of MDT meetings were omitted from the original application and a substantial amendment was issued to ensure that these documents were reviewed and approved (REC approval date: 23 January 2017).
-
The participant information sheets for focus groups with HCPs were omitted from the original application and a substantial amendment was issued to ensure that these documents were reviewed and approved (REC approval date: 22 February 2017).
-
Following the enquiries from the Project Advisory Group (PAG) and REC regarding the ambiguity around what ‘standard care’ represented in the control arm of the trial, changes were made to the protocol that aimed to address this issue. A new study measure referred to as the ‘Standard’ or ‘Usual’ Care Questionnaire’ and the accompanying participant information sheet and consent form were developed. To understand how to characterise ‘standard care’ in the control wards, and to examine how well the AMBER care bundle was being used and adapted on the intervention wards, we also added a ‘case note review tool’ and ‘heat maps’ to the trial. Additional questions were included in topic guides for the HCP focus groups to enhance our understanding of the care provided across trial sites (REC approval date: 21 April 2017).
-
An amendment was submitted to temporarily change the chief investigator of the trial to Dr Catherine Evans from Dr Jonathan Koffman. In addition, the trial end date was extended to 31 October 2018. Owing to delays in recruitment of the nurse facilitator, general planned procedures of implementation and data collection were amended. To ensure that data from bereavement surveys were collected in time, changes were made to time points at which bereavement survey packs were sent. Finally, the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) (reference), measure was added at other time points. Owing to these changes, trial documents and the protocol were updated (REC approval date: 24 July 2017).
-
The bereavement survey was amended to include two health economic evaluation measures and to improve the overall layout of the questionnaire (REC approval date: 1 November 2017).
-
After experiencing difficulties in recruitment at the control sites, the inclusion criteria for the trial were amended to remove the ‘Patients who were at risk of dying during their episode of care despite treatment’. Feedback from the local trial sites and the screening logs identified this criterion as challenging to interpret and implement. Furthermore, we improved the language used in the participant information sheets and letters after an incident in which a relative was distressed from a phrase used in the participant information sheet. This amendment also included an addition to conduct qualitative interviews with patients and families over the telephone, as several potential participants who were approached previously mentioned difficulties around arranging a face-to-face interview. Finally, an ‘AMBER readiness criterion’ was included to examine interventional fidelity to the AMBER care bundle at both intervention sites (REC approval date: 14 December 2017).
Data collection
The timeline for data collection for different trial components and trial sites is summarised in Figure 3. Prospective data were collected from the patients at three time points: baseline, 3–5 days and 10–15 days. After obtaining informed consent (or assent), research nurses conducted face-to-face interviews with patients (or relatives) to collect data that captured demographic and clinical circumstances. The questionnaire booklets included candidate patient outcomes using the ‘Patient/family anxiety and communication subscale’ of the Integrated Palliative care Outcome Scale (IPOS) and the ‘howRwe’ measure (see Appendix 4) and health performance status using the Australian-modified Karnofsky Performance Status scale (AKPS). Data on health resource utilisation were collected using the Client Service Receipt Inventory (CSRI). The EQ-5D-5L was used to measure health-related quality of life.
FIGURE 3.
Time frame for training on intervention wards and trial recruitment and data collection points.

Testing the candidate primary outcome measures
The first primary outcome measure we tested was the effect of being supported by the AMBER care bundle on the ‘Patient/family anxiety and communication subscale’ of IPOS. 55,56 This proposed outcome was based on the overall aim of the intervention and findings from our recent comparative observational study48 in which psychosocial issues were shown to be important patient- and family-centred concerns. The Patient/family anxiety and communication subscale incorporates items including being in receipt of information, addressing practical matters, sharing feelings with family, being at peace, and patients’ and families’ levels of anxiety and depression. A general background to the IPOS measure is presented in Box 2.
The POS was developed in the 1990s and includes domains important to patients with advanced progressive illness. 55 It consists of 10 items scored from 0 (best) to 4 (worst). It assesses physical symptoms, psychological and spiritual needs and provision of information and support. Following patient and clinician feedback, a symptom module (POS-S, adapted for specific conditions) was added. 57,58 Staff versions of POS and POS-S – important when the target population is so ill and frequently unable to complete patient-reported versions – are brief, user-friendly clinical outcome measures designed for HCPs to assess an individual’s symptoms and concerns. They typically take less than 10 minutes to complete. Both patient and staff versions of POS and POS-S have undergone extensive psychometric study. POS has validity and internal consistency in a variety of settings, including hospital inpatient care and community and outpatient services, hospice inpatient, day care and home care. 55,59 Moreover, it demonstrates construct validity and re-test reliability, and factor analysis has identified important underlying constructs relating to psychological well-being and quality of care. 56
The IPOS comprises 17 items scored from 0 (best) to 4 (worst) and assesses physical symptoms, psychological and spiritual needs, and provision of information and support. 55 The IPOS has undergone extensive validation testing including cognitive interviewing to assess acceptability and content/face validity and to identify cognitive processing issues following the model of Tourangeau. 60 Face and context validity of IPOS were refined using cognitive interviewing. 61 A recent study provides evidence that the IPOS is a robust, valid and reliable measure and would provide discrimination between relevant groups. 62 We identified among 373 participants that the IPOS can discriminate well between patients with different illness and functional characteristics. For example, in the known-group comparisons (during our testing of construct validity), the total IPOS and subscale scores discriminated well between patients who were in an unstable/deteriorating phase compared with those in a more stable phase of illness (total IPOS: F = 15.0, p < 0.001; Patient/family anxiety and communication subscale: F = 3.6, p < 0.03).
POS, Palliative care Outcome Scale; POS-S, Palliative care Outcome Scale Symptom.
We included another candidate primary outcomes measure to be able to determine the measure best suited to a pragmatic trial. 53 HowRwe, a validated patient-reported experience measure,63 was used to examine changes in patients’ perceptions of their experience of health care, which are highly relevant to those whose situations are clinically uncertain and their families. A general background to the howRwe measure is presented in Box 3. This measure is considered succinct (29 words in length) and highly accessible (Flesch–Kincaid readability score of 2.2). It consists of four items: two relate to the delivery of clinical care (being treated kindly and being listened and explained to). Two further items relate to the organisation of their care, including waiting to see a HCP (time wasted) and how well organised patients perceive the ward to be. The howRwe has been successfully used across inpatient and outpatient general practice, care homes and in domiciliary care.
In England, the NHS undertakes many national surveys of patient experience. However, measures are often unwieldy and lengthy, with many being over 300 words long. The howRwe was developed as the first short generic patient experience measure for use across all health and social care sectors. 63 It comprises just 29 words and includes two items relating to clinical care (treat you kindly and listen and explain) and two items relating to the organisation of care (see you promptly and well organised) as perceived by patients. Each item has four possible responses (excellent, good, fair and poor). The summary howRwe score is calculated for individual respondents by adding the scores for each item, giving a scale with 13 possible values, from the floor, 0 (4 × poor), to the ceiling, 12 (4 × excellent). When reporting the results for a group comprising more than one respondent, mean scores are transformed arithmetically to a 0 to 100 scale, where 100 indicates that all respondents rated all items as excellent and 0 indicates that all rated all items as poor. This allows the mean item scores to be compared with the summary howRwe score on a common scale. The measure was recently trialled among 828 older patients and has undergone extensive psychometric testing64 in a variety of settings, including hospital and care homes in the community. The measure demonstrates good internal consistency, concurrent validity and discriminant and construct validity.
These two measures were reassessed at follow-up points 1 (days 3–5) and 2 (days 10–14). The success of the primary outcomes were examined based on the following criteria:65
-
appropriateness – for patients with an advanced illness (e.g. number of missing data)
-
reliability – the degree of consistency of the measure (i.e. when it gives the same repeated result under the same conditions)
-
feasibility – is the measure easy to administer to patients in an inpatient hospital setting?
Patient recruitment and consent procedures
We aimed to understand how involvement in the trial might influence participants’ situations. We used a successful strategy from a recent study examining the effectiveness of dignity therapy for people living with advanced cancer. 66 Participants in both arms were questioned at all time points to evaluate the extent to which they found participating in the process of obtaining consent and the overall research process ‘helpful’, ‘making life more meaningful’, ‘heightening their sense of purpose’, ‘lessening suffering’ and ‘increasing their will to live’. We have previously observed that self-reports of the benefits of being involved in the research process are generally more positive in the intervention group than in the control group. 67 However, qualitative accounts from participants in the control group from the previous study included statements indicating that being involved in research meant that ‘somebody cared about me’ and represented ‘an opportunity to talk to somebody about problems with a sympathetic and sensitive researcher’. 68
Participant inclusion criteria
-
Patients located on the intervention or control wards.
-
Patients who were aged ≥ 18 years.
-
Patients who were deteriorating (in accordance with the AMBER criteria).
-
Patients whose situations were clinically uncertain, with limited reversibility (in accordance with the AMBER criteria).
-
Patients who were at risk of dying during their episode of care despite treatment (in accordance with the AMBER criteria).
-
Patients who were able to provide written informed consent or for whom a personal consultee could be identified and approached to give an opinion on whether or not the patient would have wished to participate in the trial.
The bereavement survey
The objectives of the bereavement survey within this feasibility trial were to:
-
test feasibility of collecting data retrospectively
-
examine differences in the use of financial resources between the AMBER care bundle and standard care.
Participant inclusion criteria for the bereavement survey
Potential participants for the bereavement survey included the next of kin (NOK) or named relatives of deceased participants who were either (1) supported by the AMBER care bundle on intervention wards or (2) identified as fulfilling the criteria on the control wards. We identified the NOK for participants who died (1) while they were inpatients or (2) on discharge within 100 days.
Recruitment of participants for the bereavement survey
All identified NOKs were sent a letter from the research nurses at each of the participating DGHs 10–12 weeks following bereavement, with an introductory letter, the survey questionnaire and a Royal College of Psychiatrists bereavement support leaflet. Up to two reminders were sent to people who had not responded at 2 and 4 weeks after the initial posting; the second reminder included a second copy of the questionnaire. On receipt of a completed questionnaire (see Data collection for the bereavement survey), the research team recorded the date of receipt into the spreadsheet, checked completion and recorded levels of distress and grief intensity.
Data collection for the bereavement survey
We used a modified version of the QUALYCARE bereavement survey,69 which has previously been shown to be highly acceptable to participants in bereavement research. 48,70 This survey examines the last 1–2 months of the decedent’s life, including quality and consistency of information and communication with clinicians.
The survey comprises four brief and robust measurement tools previously used in cancer and end-of-life care studies. These tools collect information on health and social care services use and informal care (CSRI71,72), patient palliative outcomes in the week prior to death (IPOS55,56), health-related quality of life [EuroQol-5 Dimensions (EQ-5D)73] and respondents’ bereavement outcomes (Texas Revised Inventory of Grief74). Further questions explored preferences for (and actual) place of death, relevant local issues and sociodemographic and clinical data. The format and navigation of the questionnaire have been refined according to cognitive theory literature. 75 Furthermore, the QUALYCARE questionnaire has been piloted and improved to enhance acceptability among 20 bereaved relatives recruited via the palliative medicine department of a London hospital. 70
The qualitative component
The qualitative component of this feasibility trial included interviews with patients, their relatives or close friends, non-participant observation of the MDT meetings and ward-based focus groups with HCPs. The specific objectives of these components of work were:
-
to examine the extent to which the AMBER care bundle requires further refinement or adaptation (e.g. referral criteria to identify which patients would benefit most) to suit local conditions
-
to assess the acceptability of the AMBER care bundle to patients, their families and HCPs
-
to determine the ‘active ingredients’ of the AMBER care bundle that need to be maintained to ensure fidelity of the intervention for a full trial
-
to assess compliance with and barriers to the delivery of the AMBER care bundle.
Participant inclusion criteria for the qualitative interviews
Potential participants included:
-
all patients who were recruited to the trial
-
relatives or close friends of patients who were recruited to the trial
-
patients or relatives who were able to meet face to face or have the interview over the telephone.
Exclusion criteria for the qualitative interviews
Our exclusion criteria for this component of the trial were:
-
patients who were not able to provide informed consent because of capacity-related issues
-
patients, their relatives or close friends considered by HCPs to be too unwell to be interviewed and/or too distressed to approach
-
relatives/close friends, who were not willing to provide informed consent.
Procedure for recruitment of patients and/or relatives for the qualitative interviews
The research nurses were asked to identify up to 20 patients (five per ward), and/or their relatives, who matched the inclusion criteria on intervention and control wards. Identified patients were discussed with the clinical team to see if they were suitable to be approached. Potential participants were also selected according to pre-agreed criteria (range of age groups, gender, disease type and ethnic group). If they were deemed to be appropriate, the research nurse then asked if they would like to be interviewed by the trained researcher.
Relatives were approached while they were visiting the patients and asked if they would be willing to be interviewed by the trained researcher. All participants were provided with a comprehensive participant information sheet explaining the nature of the trial and their potential involvement. This document did not allude to therapeutic promises, nor did it allude to unacceptable inducement or refer to any negative outcome of not participating. If in agreement, the researcher then contacted the potential participants, within 24 hours, to address any questions or concerns that they might have and to establish their decision to take part in the trial or not.
Owing to the challenging nature of the patients’ clinical situations and where the potential participant was not able to meet face to face, a decision, approved by the REC, was made to conduct such interviews over the telephone. A mutually convenient time was arranged to conduct each interview. The researcher explained the trial in full again and the interview commenced after informed consent was obtained (in writing in a face-to-face interview or verbally recorded in a telephone interview). Verbal consent was documented by the researcher on the consent form for telephone interviews and signed and dated. A copy of the signed consent form was sent to the participant after the interview depending on their preference.
Data collection for the qualitative interviews with patients and relatives or close friends
The interview topic guides aimed to explore patients’ and their relatives’/close friends’ insights into the delivery of care, and their perception of involvement in critical decisions regarding their care and treatment while in hospital. Interviews were recorded on an encrypted digital voice recorder. During transcription, all potentially identifiable information was removed or anonymised. Recordings were destroyed following completion of the trial in line with King’s College London’s data-management policy.
Non-participant observation of the multidisciplinary team meetings
Informed consent was obtained prior to the meetings. However, in instances in which HCPs arrived late, informed consent was obtained at the end of the meetings. This was considered the most minimally intrusive option in the group setting, as it did not disrupt natural behaviours of HCPs already participating in the meeting.
On the intervention wards, we recorded who was present at the meetings, the frequency of the meetings, the length of meetings and type of conversations relating to patients identified as fulfilling the criteria to be supported by the AMBER care bundle. We also took note of which professions contributed to conversations, what specific actions were discussed that related to their care and how decision-making processes developed, including the management of end-of-life issues. We conducted similar observations on the two control wards with prior knowledge from the research nurses of patients who fulfilled revised criteria. Observations were written down as field notes during the meeting. All field notes that related to conversations about individual patients and their families were devoid of any identifying characteristics.
Focus groups with health-care professionals
The HCPs were invited to participate in a ward-based multiprofessional focus group to explore their views on caring for patients whose situations were clinically uncertain, views about the AMBER care bundle (if on the intervention wards) and views regarding conduct of the feasibility cluster RCT. Specifically, for the intervention wards, we wanted to understand HCPs’ insights into the ways in which the AMBER care bundle influenced communication with patients and their family members or close friends, improved HCP confidence, competence and empowerment in working with patients with advanced disease and facilitated improved team working, and to explore what changes may be required to enhance its operation. We wanted to explore their views on the acceptability of the care bundle, particularly their views on whether or not the AMBER care bundle required modification or refinement. We purposively recruited a range of HCPs with different levels of experience to share their views on caring for these patients. We worked closely with the research nurses to promote the focus groups and posted information posters in each of the wards in the weeks leading up to the focus group taking place. The focus groups were typically organised during lunch times to optimise participation and were catered with food and refreshments to offset any inconvenience staff might experience. Participants were asked to give informed consent on arrival at the focus group venue.
The ‘standard’ or ‘usual’ care questionnaire
In clinical trials, reference is often made to evaluating the outcomes of an intervention compared with standard care. 76,77 Few studies, however, explain what this type of care comprises or examine the extent to which standard care changes during the trial as a result of involvement in the trial. This methodological issue was highlighted by members of the NHS REC and by our trial statistician. We therefore developed a tool to characterise best standard care applicable for patients, and their families, whose situations are clinically uncertain, across all of the trial sites, at the following time points during the trial:
-
baseline (prior to implementation of the AMBER care bundle for the intervention wards)
-
mid patient recruitment (6 weeks after the recruitment of the first participant)
-
at the end of patient recruitment (we were mindful that some of the procedures in the ward might not change drastically throughout the trial, especially in the control sites, hence why, at the mid patient recruitment and end of patient recruitment time points, we provided respondents with the option of answering only the questions that related to when there had been a change in the procedure since the previous time point).
We collected data from the perspectives of different HCPs (a consultant, a ward manager/sister, a junior doctor/senior house officer, a health-care assistant and a staff nurse), rather than just one representative on each ward, to obtain a broader understanding of this type of care. The format, content and navigation of the ‘standard’ or ‘usual’ care questionnaire were refined based on expert opinions, including members of the PAG and senior clinical colleagues at an inner London teaching hospital. Questions addressed initial care planning and general practices, recognising dying, referral and discharge procedures. The questionnaire took 10–20 minutes to complete depending on the number of free-text data provided.
Case note reviews
The incorporation of the audit tool, which was developed by the bundle developers as part of the quality improvement process, aimed to enhance our understanding of ‘standard’ or ‘usual’ care at ward level and how this might change over time. The nurse facilitator completed the case note reviews. This audit tool is routinely used in over 30 hospitals internationally, including in England, Wales and Australia. The audit tool comprises a case note review of hospital patient records in a standardised format and a ‘heat map’ of patient mortality over a 1-year period.
The case note review was completed retrospectively and involved purposively selected 20 patients per ward, comprising 10 patients who died in the hospital and 10 patients who were discharged and died within 100 days of discharge. It was conducted for all of the trial wards and additionally for the intervention wards after the implementation of the AMBER care bundle. All identifiable patient information was removed and anonymised prior to sharing with the research team.
Trial setting
Selection of the wards
Participant recruitment and implementation of the AMBER care bundle were limited to one or two general medical wards at each hospital site. Wards with the highest number of deaths per year were considered to be suitable for this trial. Selection of the trial wards at each site was informed by ‘heat maps’ that provided contextual information at ward level on the number of deaths during admission and up to 100 days after admission across the hospital wards.
Randomisation and masking
Randomisation was at the level of the NHS trusts via an independent service at King’s Clinical Trials Unit. Four clusters were randomised at once by randomly sequencing the order of randomisation and then randomising the sites in this order into fixed blocks of two, those being the control or intervention arms. All clusters were randomised prior to collection of data at sites but after all sites had agreed to participate. Quantitative analyses masked for the group allocation were conducted. Research nurses collecting the outcome measures were not masked for the group allocation.
Trial sites
Participants were recruited from one (or two) purposefully chosen general medical ward of four DGHs in England. The trial sites were Chesterfield Royal Hospital, East Surrey Hospital, Tunbridge Wells Hospital and Northwick Park Hospital, which are major secondary care facilities typically providing an array of diagnostic and therapeutic services to local populations. There are over 250 DGHs in the UK. 78 They represent abundant settings in which to implement and test the effect of the AMBER care bundle; the findings from this feasibility trial would inform future scalability. However, DGHs are extremely busy environments in which to conduct research, with patients being rapidly assessed and transferred from medical acute admissions units to other wards within the hospital. This makes it challenging to track trial participants and obtain accurate reports of their condition or outcomes. The DGHs selected serve diverse populations including those that comprise ethnic diversity and material deprivation. The hospitals have different strengths and weaknesses in terms of their Care Quality Commission ratings (Table 1).
| Cluster | Specialties | Number of beds | End-of-life care plan | Care Quality Commission rating |
|---|---|---|---|---|
| Control arm | ||||
| One general medical ward |
|
32 |
|
Good |
| One general medical ward |
|
27 | Last days of life care agreement | Requires improvement |
| Intervention arm | ||||
| One general medical ward |
|
30 | Individualised care plan for dying patients | Good |
| Two general medical wards | Care of the elderly | 36 | End-of-life care plan | Requires improvement |
‘Standard’ or ‘usual’ care across trial sites
The standard care provided to patients who might have clinically uncertain recovery was described using the self-reported ‘standard’ or ‘usual’ care survey completed by the HCPs and the case note reviews.
The ‘standard’ or ‘usual’ care surveys were completed at the beginning of the data collection at each trial site by 23 HCPs who represented different seniority levels and professions working on the trial wards (Table 2). The components of care questioned in this survey did not change during the course of the trial. All sites had similar processes for clerking, referring patients to palliative care or intensive care unit (electronic referral and specific triggers) and providing emotional support to the patients and families. However, professionals had various views on who was responsible for producing a medical plan for the patients, varying from all of the MDT and the patients and families to the consultant and medical team only. Delays in recognition of patients’ clinical uncertainty and relaying of information around uncertain recovery from HCPs to patients and families were shown as the main barriers to referrals to palliative care and in escalating care at all trial sites by various HCPs. Three ward sisters [sites Int (intervention) 1, Int2 and Con (control) 2] and one health-care assistant (site Con1) stated that the principal reason for delays in referral to palliative care was the medical team’s decision to continue active treatment: ‘doctors trying to get the patient well’ (Con2001) and ‘medical team wanting to treat for another 24–48 hours’ (Int1005).
| Profession | Number of professions | |||
|---|---|---|---|---|
| Trial arm and site | ||||
| Control | Intervention | |||
| Con1 (n = 5) | Con2 (n = 5) | Int1 (n = 8a) | Int2 (n = 5) | |
| Consultant | 1 | 1 | 2 | 1 |
| Ward sister/manager | 1 | 1 | 2 | 1 |
| Junior doctor | 1 | 1 | 2 | 1 |
| Staff nurse | 1 | 1 | 0 | 1 |
| Health-care assistant | 1 | 1 | 1 | 1 |
| Physician associate | 0 | 0 | 1 | 0 |
The survey also highlighted that advance care plans (ACPs) were devised only once the patient was considered to be at the end of life: ‘when all the care has been given and the patient has not got any better’ (Int2001). After the implementation of the AMBER care bundle, HCPs at site Int1 stated ‘recognising uncertainty’, ‘deteriorating patient’, ‘clinical uncertainty’,7 ‘patient and family wishes’ and ‘frequent hospital admissions’ as reasons for devising an ACP. Within the same teams, there were disagreements around the frequency of revisiting the plans made with the patients and families. Although professionals across all sites stated that they would actively contact families and speak with the rest of the MDT as soon as possible if a patient’s health status deteriorated, HCPs within the same team were in disagreement about how often they would update the patients and families.
Survey findings showed inconsistencies in the manner in which standard care was delivered in relation to shared decision-making among HCPs and the contribution of the MDT to patients’ care and treatment plans. Although all of the survey participants were able to clearly state the systems in place at all sites, the recognition of clinical uncertainty and importance of having conversations with patients and families prior to their last days of life were considered part of the standard care provided.
Case note reviews identified that processes, documentation of plans and discussions at all trial sites were similar (Table 3). The review showed that the uncertain recovery of patients was documented in patients’ notes for the majority (> 60%) of a purposively selected sample of patients across all the trial sites. The majority of patients in the control sites had a cancer diagnosis, unlike the patients in intervention sites. In all trial sites, escalation plans were documented for 60% of participants, and ‘do not resuscitate’ orders were documented for 75% of the sample. However, the majority (> 60%) of the sample did not have an ACP documented. Besides site Con1, medical plans were discussed and agreed with the nursing staff at all other sites. Although the notes identified that discussions with patients and families took place for the majority of the sample, documentation of patients’ preferred places of care (PPCs) and PPDs and their wishes around care and treatment require improvement. With the exception of site Con2 participants, the majority of the sample received daily follow-up. However, it was not possible to examine the quality and the content of the discussions that took place.
| Descriptive variable | Trial arm and site, n (%) | |||
|---|---|---|---|---|
| Control | Intervention | |||
| Con1 (N = 20) | Con2 (N = 20) | Int1 (N = 20) | Int2 (N = 20) | |
| Age (years) | ||||
| 40–60 | 2 (10) | 2 (10) | 0 (0) | 3 (15) |
| 61–70 | 2 (10) | 3 (15) | 0 (0) | 6 (30) |
| 71–80 | 4 (20) | 8 (40) | 2 (10) | 6 (30) |
| 81–90 | 8 (40) | 6 (30) | 11 (55) | 5 (25) |
| ≥ 91 | 4 (20) | 1 (5) | 7 (35) | 0 (0) |
| Primary diagnosis | ||||
| Cardiology | 0 (0) | 0 (0) | 4 (20) | 0 (0) |
| Cancer | 12 (60) | 9 (45) | 2 (10) | 6 (30) |
| Acute respiratory | 5 (25) | 7 (35) | 5 (25) | 2 (10) |
| Chronic respiratory | 0 (0) | 0 (0) | 0 (0) | 10 (50) |
| Stroke | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
| Dementia | 0 (0) | 0 (0) | 2 (10) | 0 (0) |
| Sepsis | 2 (10) | 0 (0) | 1 (5) | 0 (0) |
| Frailty | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Other | 0 (0) | 4 (20) | 5 (25) | 1 (5) |
| Uncertain recovery documented? | ||||
| Yes | 18 (90) | 15 (75) | 18 (90) | 12 (60) |
| No | 2 (10) | 5 (25) | 2 (10) | 8 (40) |
| ACP in place? | ||||
| Yes | 4a (20) | 8b (40) | 7c (35) | 2d (10) |
| No | 16 (80) | 12 (60) | 13 (65) | 18 (90) |
| Escalation plan documented? | ||||
| Yes | 15 (75) | 12 (60) | 18 (90) | 13 (65) |
| No | 5 (25) | 8 (40) | 2 (10) | 7 (35) |
| DNAR/DNACPR status | ||||
| Patient for CPR | 1 (5) | 0 (0) | 0 (0) | 2 (10) |
| Patient not for CPR | 16 (80) | 15 (75) | 20 (100) | 15 (75) |
| No documented decision | 3 (15) | 5 (25) | 0 (0) | 3 (15) |
| Medical plan discussed and agreed with nursing staff? | ||||
| Yes | 9 (45) | 15 (75) | 19 (95) | 16 (80) |
| No | 11 (55) | 5 (25) | 1 (5) | 4 (20) |
| Patient/family discussion? | ||||
| Yes | 19 (95) | 14 (70) | 19 (95) | 13 (65) |
| No | 1 (5) | 6 (30) | 1 (5) | 7 (35) |
| Daily follow-up? | ||||
| Yes | 19 (95) | 12 (60) | 19 (95) | 12 (60) |
| No – should have received | 1 (5) | 7 (35) | 1 (5) | 8 (40) |
| No – not needed | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Assessment of capacity? | ||||
| Yes | 11 (55) | 20 (100) | 10 (50) | 7 (35) |
| No – it was not needed | 7 (35) | 0 (0) | 9 (45) | 12 (60) |
| No – it was needed | 2 (10) | 0 (0) | 1 (5) | 1 (5) |
| PPC | ||||
| Person’s own home | 3 (15) | 7 (35) | 2 (10) | 10 (50) |
| Hospital | 2 (10) | 6 (30) | 2 (10) | 3 (15) |
| Care home | 0 (0) | 3 (15) | 6 (30) | 3 (15) |
| Hospice | 3 (15) | 1 (5) | 1 (5) | 1 (5) |
| Preference not documented | 11 (55) | 1 (5) | 6 (30) | 3 (15) |
| Other (including patients who were undecided) | 1 (5) | 2 (10) | 3 (15) | 0 (0) |
| PPD | ||||
| Person’s own home | 4 (20) | 3 (15) | 1 (5) | 3 (15) |
| Hospital | 2 (10) | 0 (0) | 2 (10) | 0 (0) |
| Care home | 0 (0) | 4 (20) | 1 (5) | 3 (15) |
| Hospice | 3 (15) | 1 (5) | 2 (10) | 0 (0) |
| Preference not documented | 11 (55) | 9 (45) | 3 (15) | 12 (60) |
| Other (including patients who were undecided) | 0 (0) | 3 (15) | 11 (55) | 2 (10) |
| Patient and family wishes documented | ||||
| Wishes documented | 5 (25) | 12 (60) | 16 (80) | 10 (50) |
| DNAR decision only | 4 (20) | 0 (0) | 0 (0) | 5 (25) |
| No wishes documented | 9 (45) | 0 (0) | 4 (20) | 4 (20) |
| Patient offered discussion but refused | 1 (5) | 8 (40) | 0 (0) | 1 (5) |
The case note reviews were also conducted for patients who died within 100 days of discharge from hospital (see Appendix 5) and at the end of the feasibility trial on the intervention wards to identify changes from before to after implementation (see Appendix 6).
Quantitative analysis
A statistical analysis plan was developed to detail the analysis strategy and statistical considerations (missing data checks and model assumption checks). This document was approved by project statisticians and the TS DMEC. We undertook analysis in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines in collaboration with King’s Clinical Trials Unit; two statisticians (WG and RW), the chief investigator (JK) and the health economist (DY) were blind to the randomisation.
All percentages, means, medians, ranges, standard deviations (SDs) and 95% confidence intervals (CIs) were rounded up to one decimal point. No tests of significance were conducted as this was a feasibility trial with no aim to test the effectiveness of AMBER compared with standard care. However, 95% CIs were provided to indicate the precision of the estimates from the preliminary trial.
Data entry
All data were entered into predesigned EpiData (EpiData Association, Odense, Denmark) databases. Ten per cent of the data were double-entered and cross-checks were conducted. No discordance was detected for the primary outcome measures (100% match for IPOS patient/family anxiety and communication subscale and howRwe), with very high accuracy for the rest of the questionnaires.
Sample size for the feasibility cluster randomised controlled trial
A formal power calculation was not appropriate because effectiveness was not being evaluated. Any investigations of changes in trial parameters were exploratory only. Based on the information about number of deaths and prior studies, we aimed to recruit 40 patients per trial arm to meet our feasibility objectives.
Economic evaluation
The data analysis in the economic evaluation examined resource implications from both (1) a health/social care perspective and (2) a societal perspective. We made preliminary cost-effectiveness calculations (e.g. combining CSRI data on costs and EQ-5D score). Economic evaluation is an emergent area in palliative care and uncertainty surrounds best practice. 72 The feasibility trial tested procedures to inform the economic evaluation in the full cluster RCT protocol.
Qualitative data analysis
The qualitative data analysis approach was informed by the Framework approach to inductively code and organise the data and identify emerging themes from the interviews. 79 The Framework approach involves a five-stage matrix-based approach comprising:
-
Familiarisation. We immersed ourselves in the raw data from the interviews by listening in detail to audio-recordings, reading and rereading transcripts and also studying field notes to list key ideas and recurrent themes.
-
Identifying a thematic framework. We developed a thematic framework to identify all of the key issues, concepts and themes so the data could be examined. This was carried out by drawing on a priori issues and questions derived from the aims and objectives of the trial as well as issues raised by the participants themselves. The end product of this stage comprised a detailed ‘index’ of the data so we could ‘label’ the data into manageable chunks for subsequent retrieval and exploration.
-
Indexing. We applied our thematic framework to all the data in textual form by annotating the transcripts with codes from the index, supported by short text descriptors to elaborate the index heading. We made use of NVivo 11 data analysis software (QSR International, Warrington, UK) to facilitate this process.
-
Charting. We rearranged the data according to the part of the thematic framework to which they related, and formed charts. The charting process involved a considerable amount of abstraction and synthesis.
-
Mapping and interpretation. Finally, we made use of the charts to define concepts, map the range and nature of phenomena, create typologies and find associations between themes with a view to providing explanations for the findings.
We addressed issues of rigour and trustworthiness in the analysis. We (JK, EY and HJ – blinded to intervention allocation) randomly selected interview transcripts to review the application of the thematic framework, the coding and the completeness of the framework. When coding differed or areas of the framework were inconsistent, these issues were reconsidered in detail until a consensus was achieved. 80 During this process, we took care to examine what appeared to be more unusual or non-confirmatory views and considered what the data told us about their causes to avoid making unwarranted claims about patterns and regularities in the data. 80 Excerpts from the interview transcripts are presented to illustrate themes representing a range of views rather than being reliant on selected individuals. All quotations from participants (patients, relatives and HCPs, and their specific trial sites) have been anonymised to preserve confidentiality.
Mixed methods: triangulation of data
The aim of the data integration was to examine different aspects of the AMBER care bundle experience and participation in the research trial. Data were integrated using a method of data ‘triangulation’, which combines data sources from more than one source (quantitative and qualitative) to address the same phenomenon. 81,82
The process of triangulating findings from the different methodological approaches took place at the interpretation stage of a feasibility trial after all data sets have been analysed separately. Specifically, we listed the findings from each component of a trial and then considered where findings from each approach agree (convergence), offer complementary information on the same issue (complementarity) or appear to contradict each other (discrepancy or dissonance). We looked for instances of convergence but also for disagreements between findings from different approaches. We believe that disagreement is not a sign that something necessarily went wrong with our feasibility trial. Indeed, instances of ‘intermethod discrepancy’ may lead to a better understanding of how the intervention operates, its effects and where it can be improved. We also looked for instances of silence – where a theme or finding arises from one data set but not from others. Silence might be expected because of the strengths of different methods to examine different aspects of the bundle.
Implementation of the AMBER care bundle
The process of implementing the AMBER care bundle comprised three stages:
-
Findings from the ‘heat maps’ were shared with the hospital staff to inform them about the suitability of the wards for the AMBER care bundle and enabled them to use the data for their own quality assessment. Heat maps did not include any identifiable patient-related information.
-
A baseline review of patients’ clinical case notes was conducted on each of the selected wards prior to the AMBER care bundle implementation. The case notes were scrutinised to –
-
identify patients who would be suitable to be supported by the intervention
-
check if a documented plan for their care was in place
-
identify if there was evidence of an escalation plan and if the medical plan had been agreed with nursing staff
-
identify if there was evidence that a conversation had taken place with the patient and their relatives regarding their uncertain clinical situation and care preferences
-
identify if there was evidence that capacity and or best interests assessments had been appropriately addressed
-
identify, for patients discharged from hospital, any non-elective readmissions in the last 100 days, and the outcome (died or discharged).
-
-
Once a suitable ward had been identified, the nurse facilitator engaged in four steps to introduce and implement the AMBER care bundle on each ward. This involved –
-
familiarisation with the ward
-
introducing the intervention to HCPs and training them on its use
-
supporting HCPs in the practice of using the AMBER care bundle (role modelling and developing relevant communication skills)
-
observing how HCPs used the AMBER care bundle
-
exit plans and completion of a follow-up case note review.
-
The criteria to determine if the ward was perceived to be ready to support suitable patients and their families with the AMBER care bundle included fulfilling the criteria described in Table 4.
| Aspect of training | Baseline criteria |
|---|---|
| Education inputs completed/clinical processes adjusted as planned |
|
| Patients supported by the AMBER care bundle – open communication (observe in practice/notes) – patient/family awareness/feel supported (ad hoc feedback) |
|
| Senior staff (medical, nursing, allied health professionals, critical care outreach) able to identify patients (observe white board rounds without needing to prompt, different HCPs raise the concern) – effective MDT working and ability to work across hierarchies around clinical uncertainty of recovery | At MDT meetings and ward rounds, staff are able to identify patients who meet the AMBER care bundle criteria without prompts from the nurse facilitator. When asked if there are any patients they think may have an uncertain recovery and meet the AMBER care bundle criteria, junior and non-medical members of the team as well as medical colleagues are able to highlight potential patients for discussion with the wider team. They may use different terminology but allude to a clinical uncertainty of recovery – the patient may or may not recover |
| Staff able to describe the AMBER care bundle well (five staff at random) – includes relevant teams working with the ward |
When asked about their understanding of the intervention, staff are aware that it is used to support very unwell patients who may or may not be approaching the end of life and patients who have clinical uncertainty, and that the aim is to have a clear plan (medical/escalation/resuscitation) and involve patients/families in discussions regarding care and preferences NB flexibility is required here – staff may not all use the same terminology depending on their individual experience, but an awareness of the principles is the important factor |
| Awareness of the plan for patients receiving care supported with the AMBER care bundle (MDT aware, observation on handover) – what is important to the patient and escalation plan? | At handover and when asked, senior staff demonstrate an awareness of the patient’s current medical, escalation and resuscitation plan. They are aware of the patient’s/family’s wishes/preferences (e.g. PPC, who they want to care for them) |
| Good documentation and adherence to standards processes. Good communication on discharge and handover to general practitioner and community teams (notes) |
|
Prior to start of the data collection at sites Int1 and Int2, the following were achieved in terms of implementation of the AMBER care bundle:
-
More than 90% of the nursing, medical and therapy staff, the discharge planning teams, ward clerks, the palliative care teams and the respiratory teams were trained; critical care outreach teams were briefed; education inputs were completed; and clinical processes were adjusted to accommodate use of the AMBER care bundle.
-
HCPs were able to discuss ‘clinical uncertainty’ and care preferences with patients and families supported by the AMBER care bundle and provide appropriate support (observed by the nurse facilitator).
-
HCPs were able to identify patients eligible for the intervention without prompting in MDT meetings, including senior medical, junior medical and non-medical staff (observed by the nurse facilitator).
-
Five randomly selected HCPs at each intervention site were able to correctly describe the AMBER care bundle, as per the inclusion criteria, to the nurse facilitator.
-
Senior HCPs discussed what they considered to be important to patients, and their escalation plans when present, at handover meetings (observed by the nurse facilitator).
-
Four main components of the AMBER care bundle were completed in more than 80% of patients’ clinical notes; discharge letters regularly contained information as per the AMBER care bundle inclusion criteria (observed by the nurse facilitator).
Recruitment of the nurse facilitator
The trial successfully recruited a palliative care clinical nurse specialist (CNS) (NHS nursing band 8a) who possessed extensive experience in implementing the AMBER care bundle across all the wards at another NHS trust, with an excellent understanding of the AMBER care bundle, end-of-life care, service and quality improvement, demonstrable knowledge and skills in advanced communication, interpersonal and leadership qualities, an excellent understanding of the NHS infrastructure and a proven ability to think and plan strategically.
Chapter 3 Findings from the feasibility cluster randomised controlled trial of the AMBER care bundle
Findings in relation to each trial objective are presented in this chapter.
Screening processes and initial approach
The screening process varied across the four trial sites. Screening, providing information about the trial, consenting and administration of the questionnaire booklets were completed by the research nurses at all sites. The central research team was updated on a weekly basis.
At site Con1, research nurses screened all of the patients on the trial ward at the beginning of the week and then checked on a daily basis for recently admitted patients who may meet the trial inclusion criteria. They then discussed these patients with clinicians and jointly agreed on each patient’s eligibility status. At site Con2, research nurses screened the patients on a weekly basis. However, the identification of eligible patients and introducing the trial to potential participants were generally completed by the principal investigator and his medical colleagues. At sites Int1 and Int2, research nurses made use of the hospital ward white boards to identify patients who were supported by the AMBER care bundle.
Participant flow
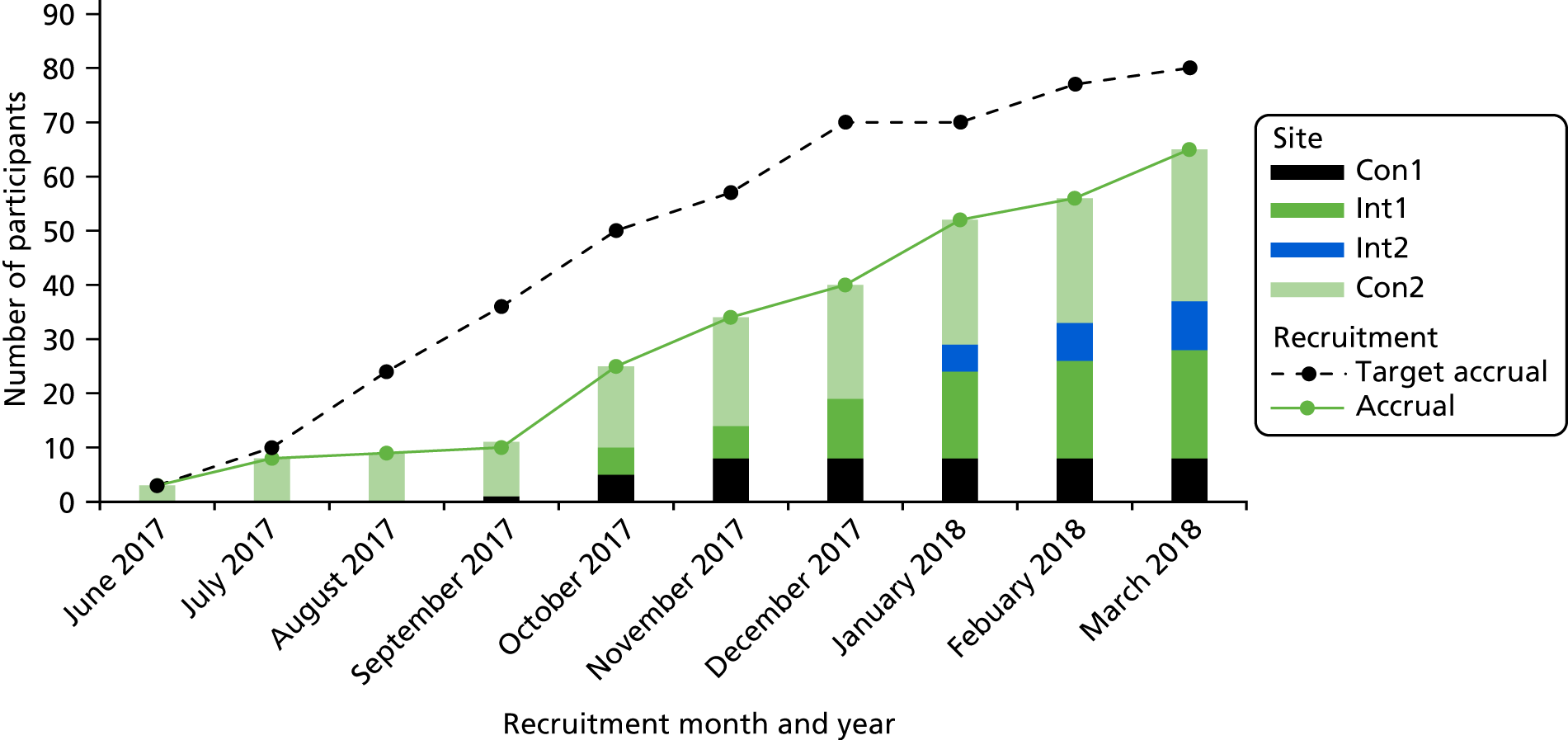
Figure 4 is the CONSORT flow diagram of participant recruitment from screening to baseline to time point 1 (3–5 days), time point 2 (10–15 days) and thenceforth to analysis. In total, 65 patients were recruited to the trial: 29 participants in the intervention arm and 36 participants in the control arm were consented and had completed baseline measures. We aimed to recruit a minimum of 80 participants, and were able to successfully recruit 81.3% of this target. As the AMBER care bundle is an intervention that does not require active participation of the patients or relatives, the intervention completion is not applicable, hence it is not included in the CONSORT flow diagram. We had planned for recruitment to take > 3 months at each of the trial sites, with an average of seven participants consented per month. However, we extended the recruitment period, as the recruitment rate was slower than expected. Figure 5 presents the monthly cumulative recruitment figures for each site (bars), the accrual rate (green line) and target accrual rate (dashed line) over the 9-month data collection period. It should be noted that trial sites opened to recruitment at different time points. Comparing the gradients of the targeted and achieved accrual, the recruitment kept up with the planned rate once the initial delays and issues around understanding the trial were overcome.
FIGURE 4.
The CONSORT flow diagram. Reproduced from Koffman et al. 1 ©The Authors 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. This figure includes minor additions and formatting changes to the original text.

FIGURE 5.
Monthly recruitment rate by site.
Recruitment, retention and follow-up rates
The feasibility of the recruitment strategy was examined by summarising the screening, eligibility, approach and consent processes, reasons for non-participation and the numbers of participants involved at each stage.
Table 5 presents the number of patients screened and recruited at each of the trial sites. Recruitment took place between June 2017 and March 2018. The challenging recruitment of participants in the control arm of the trial is shown: only 1.9% (n = 8) and 8.9% (n = 28) of all those who were screened were recruited at sites Con1 and Con2, respectively. At the intervention sites, recruitment was more successful: 25.0% (n = 20) and 18.0% (n = 9) of those who were screened were recruited at sites Int1 and Int2, respectively. For site Con1, which screened 449 patients and excluded 365 as not eligible, the free-text section of the screening logs stated ‘not at risk of dying’ for 55 (15.1%) patients, and ‘end of life’ or ‘improving’, hence not clinically uncertain, for 21 (5.8%) patients. Other sites did not provide this information in their notes.
| Recruitment | Site (n) | Total (n) | |||
|---|---|---|---|---|---|
| Con1 | Con2 | Int1 | Int2 | ||
| Recruitment period (months) | 4 | 9 | 6 | 3 | |
| Screened | 449 | 315 | 80 | 50 | 894 |
| Not eligible | 365 | 282 | 22 | 5 | 674 |
| Reasons for non-recruitment | |||||
|
4 | 2 | 8 | 6 | 20 |
|
19 | 0 | 2 | 6 | 27 |
|
0 | 0 | 7 | 5 | 12 |
|
13 | 0 | 6 | 2 | 21 |
|
5 | 2 | 6 | 6 | 19 |
|
28 | 1 | 4 | 8 | 41 |
|
7 | 0 | 5 | 3 | 15 |
| Recruited | 8 | 28 | 20 | 9 | 65 |
Participant retention during follow-up, including the number of participants withdrawing or who were lost to follow-up (hereafter referred to as ‘dropouts’), together with the timing and reason for dropouts, is presented by trial arm (see Figure 4).
Data collection timing
Based on the date of admission to the trial ward and date of baseline data completion, in the intervention wards participants completed the baseline measures a median of 10 days (range 0–50 days) after admission to the ward, whereas in the control wards baseline measures were completed a median of 6 days (range 0–64 days) after admission to the ward. In the intervention arm, the mean number of days between the date of being identified as an appropriate recipient of the intervention and the date of baseline questionnaire completion was 6.0 (SD 6.8). In site Int1, the mean number of days between the AMBER care bundle identification and baseline completion was 7.2 (SD 7.6); in site Int2, the mean number of days was 3.3 (SD 3.0).
Participant demographics
The demographic characteristics of participants by trial site (Table 6) and by trial arm (Table 7) are presented. Participants in both trial arms were predominantly white British and married. Moreover, most participants were either ‘living comfortably’ or ‘coping with their present level of income’. Site Con2, located in an urban setting, had a more ethnically diverse sample profile than the other sites.
| Characteristics | Site | |||
|---|---|---|---|---|
| Con1 (N = 8) | Con2 (N = 28) | Int1 (N = 20) | Int2 (N = 9) | |
| Gender, n (%) | ||||
| Male | 15 (53.6) | 15 (53.6) | 8 (40.0) | 3 (33.3) |
| Female | 13 (46.4) | 13 (46.4) | 12 (60.0) | 6 (66.7) |
| Age (years), n (%) | ||||
| 50–64 | 8 (28.6) | 8 (28.6) | 0 (0.0) | 1 (11.1) |
| 65–79 | 13 (46.4) | 13 (46.4) | 2 (10.0) | 5 (55.6) |
| ≥ 80 | 7 (25.0) | 7 (25.0) | 18 (90.0) | 3 (33.3) |
| Age (years), mean (SD) | 71.8 (11.0) | 71.8 (11.0) | 89.0 (5.7) | 77.1 (12.0) |
| Education, n (%) | ||||
| Did not go to school | 2 (7.1) | 2 (7.1) | 0 (0.0) | 0 (0.0) |
| Secondary school (GCSE/O level) | 4 (14.3) | 4 (14.3) | 9 (45.0) | 3 (33.3) |
| Secondary school (A level) | 6 (21.4) | 6 (21.4) | 5 (25.0) | 4 (44.4) |
| Vocational qualification | 1 (3.6) | 1 (3.6) | 1 (5.0) | 1 (11.1) |
| University | 7 (25.0) | 7 (25.0) | 4 (20.0) | 0 (0.0) |
| Prefer not to say | 6 (21.4) | 6 (21.4) | 0 (0.0) | 1 (11.1) |
| Missing | 2 (7.1) | 2 (7.1) | 1 (5.0) | 0 (0.0) |
| Marital status, n (%) | ||||
| Single | 6 (21.4) | 6 (21.4) | 4 (20.0) | 0 (0.0) |
| Widowed | 8 (28.6) | 8 (28.6) | 14 (70.0) | 3 (33.3) |
| Married/civil partnership/long-term relationship | 13 (46.4) | 13 (46.4) | 2 (10.0) | 6 (66.7) |
| Divorced | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, n (%) | ||||
| White British | 10 (35.7) | 10 (35.7) | 19 (95.0) | 9 (100.0) |
| Other white | 1 (3.6) | 1 (3.6) | 1 (5.0) | 0 (0.0) |
| White and black African | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| White and Asian | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Other mixed | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Indian | 7 (25.0) | 7 (25.0) | 0 (0.0) | 0 (0.0) |
| Pakistani | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Other Asian | 4 (14.3) | 4 (14.3) | 0 (0.0) | 0 (0.0) |
| Caribbean | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Other black | 1 (3.6) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Income status, n (%) | ||||
| Living comfortably at present | 12 (42.9) | 12 (42.9) | 8 (40.0) | 4 (44.4) |
| Coping on present income | 10 (35.7) | 10 (35.7) | 5 (25.0) | 4 (44.4) |
| Difficult on present income | 0 (0.0) | 0 (0.0) | 4 (20.0) | 1 (11.1) |
| Very difficult on present income | 2 (7.1) | 2 (7.1) | 0 (0.0) | 0 (0.0) |
| Prefer not to say | 0 (0.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Do not know | 4 (14.3) | 4 (14.3) | 1 (5.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Characteristics | Whole sample (N = 65) | Trial arm | |
|---|---|---|---|
| Control (N = 36) | Intervention (N = 29) | ||
| Gender, n (%) | |||
| Male | 33 (50.8) | 22 (61.1) | 11 (37.9) |
| Female | 32 (49.2) | 14 (38.9) | 18 (62.1) |
| Age (years), n (%) | |||
| 50–64 | 10 (15.4) | 9 (25.0) | 1 (3.5) |
| 65–79 | 25 (38.5) | 18 (50.0) | 7 (24.1) |
| ≥ 80 | 30 (46.2) | 9 (25.0) | 21 (72.4) |
| Age (years), mean (SD) | 77.8 (12.3) | 71.8 (10.8) | 85.3 (9.7) |
| Disease group, n (%) | |||
| Cancer | 30 (46.2) | 23 (63.9) | 7 (24.1) |
| Non-cancer | 35 (53.8) | 13 (36.1) | 22 (75.9) |
| Patient had capacity?, n (%) | |||
| Yes | 23 (35.4) | 17 (47.2) | 6 (20.7) |
| No | 42 (64.6) | 19 (52.8) | 23 (79.3) |
| Education, n (%) | |||
| Did not go to school | 3 (4.6) | 3 (8.3) | 0 (0.0) |
| Secondary school (GCSE/O level) | 21 (32.3) | 9 (25.0) | 12 (41.3) |
| Secondary school (A level) | 15 (23.1) | 6 (16.7) | 9 (31.0) |
| Vocational qualification | 4 (6.2) | 2 (5.6) | 2 (6.9) |
| University | 11 (16.9) | 7 (19.4) | 4 (13.8) |
| Prefer not to say | 7 (10.8) | 6 (16.7) | 1 (3.5) |
| Missing | 4 (6.2) | 3 (8.3) | 1 (3.5) |
| Marital status, n (%) | |||
| Single | 10 (15.4) | 6 (16.7) | 4 (13.8) |
| Widowed | 26 (40.0) | 9 (25.0) | 17 (58.6) |
| Married/civil partnership/long-term relationship | 27 (41.5) | 19 (52.8) | 8 (27.6) |
| Divorced | 1 (1.5) | 1 (2.8) | 0 (0.0) |
| Missing | 1 (1.5) | 1 (2.8) | 0 (0.0) |
| Ethnicity, n (%) | |||
| White British | 45 (69.2) | 17 (47.2) | 28 (96.6) |
| Other white | 2 (3.1) | 1 (2.8) | 1 (3.5) |
| White and black African | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| White and Asian | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Other mixed | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Indian | 7 (10.8) | 7 (19.4) | 0 (0.0) |
| Pakistani | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Other Asian | 4 (6.2) | 4 (11.1) | 0 (0.0) |
| Caribbean | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Other black | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Missing | 1 (2.8) | 1 (2.8) | 0 (0.0) |
| Income status, n (%) | |||
| Living comfortably at present | 26 (40.0) | 14 (38.9) | 12 (41.4) |
| Coping on present income | 21 (32.3) | 12 (33.3) | 9 (31.0) |
| Difficult on present income | 5 (7.7) | 0 (0.0) | 5 (17.2) |
| Very difficult on present income | 2 (3.1) | 2 (5.6) | 0 (0.0) |
| Prefer not to say | 2 (3.1) | 0 (0.0) | 2 (6.9) |
| Do not know | 6 (9.2) | 5 (13.9) | 1 (3.5) |
| Missing | 3 (4.6) | 3 (8.3) | 0 (0.0) |
There were some notable differences between the trial arms that might influence the interpretation of our results. First, the control arm had a higher proportion of men than women, compared with the intervention arm. Second, most patients were aged between 65 and 79 years in the control arm and the majority of patients were aged ≥ 80 years in the intervention arm. Third, the majority of patients had a cancer diagnosis in the control arm, whereas most had non-cancer diagnoses in the intervention arm. The older age and non-cancer diagnoses of the participants in the intervention arm are probably attributable to the inclusion of two care of the elderly wards, which were located at site Int1.
Reasons for admission and morbidities
Reasons for participants’ admissions to hospital varied; the most common reason was shortness of breath, followed by falls and confusion (Table 8). Notably, 62 out of 65 participants (95.4%) had an unplanned admission to hospital through the emergency department. We also collected data on the presence of participants’ morbidities (Table 9). A range of illnesses were present, the most common being circulatory disorders, followed by neoplasms and blood disorder/endocrine disorders. Typically, patients presented with one to four comorbidities, the mean being 2.3 per patient. Participants’ AKPS scores further indicate the poor health status, ranging between ‘almost completely bedfast’ to ‘totally bedfast and requiring extensive nursing care by professionals and/or family’, with mean scores at baseline being 34.2 (range 20–70) in the control arm and 27.3 (range 10–40) in the intervention arm (Table 10).
| Reasons for hospital admission | Site (n) | Trial arm (n) | Total (N = 65) (n) | ||||
|---|---|---|---|---|---|---|---|
| Con1 (N = 8) | Int1 (N = 20) | Int2 (N = 9) | Con2 (N = 28) | Control (N = 36) | Intervention (N = 29) | ||
| Shortness of breath | 0 | 6 | 4 | 5 | 5 | 10 | 15 |
| Fall | 1 | 4 | 0 | 3 | 4 | 4 | 8 |
| Confusion | 0 | 5 | 1 | 1 | 1 | 6 | 7 |
| Infection | 0 | 1 | 1 | 5 | 5 | 2 | 7 |
| Abnormal blood test result | 0 | 1 | 1 | 4 | 4 | 2 | 6 |
| Pain | 3 | 2 | 0 | 0 | 3 | 2 | 5 |
| Nausea/vomiting | 1 | 1 | 0 | 2 | 3 | 1 | 4 |
| Leg swelling | 1 | 0 | 0 | 2 | 3 | 0 | 3 |
| Loss of consciousness | 0 | 0 | 0 | 3 | 3 | 0 | 3 |
| Constipation | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Reduced mobility | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Other | 1 | 0 | 0 | 2 | 3 | 0 | 3 |
| Missing | 1 | 0 | 1 | 0 | 1 | 1 | 2 |
| Morbidities | Site | Trial arm | Total (N = 65) | ||||
|---|---|---|---|---|---|---|---|
| Con1 (N = 8) | Int1 (N = 20) | Int2 (N = 9) | Con2 (N = 28) | Control (N = 36) | Intervention (N = 29) | ||
| Number of morbidities | |||||||
| Mean (SD) morbidities per participant | 2.38 (0.74) | 1.84 (0.96) | 2.13 (1.13) | 2.29 (1.05) | 2.31 (0.98) | 1.93 (1.00) | 2.33 (1.09) |
| Missing (n) | 0 | 1 | 1 | 0 | 0 | 2 | 2 |
| Number (n) | |||||||
|
1 | 9 | 3 | 7 | 8 | 12 | 20 |
|
3 | 5 | 2 | 11 | 14 | 7 | 21 |
|
4 | 4 | 2 | 5 | 9 | 6 | 15 |
|
0 | 1 | 1 | 5 | 5 | 2 | 7 |
| Number of morbidities by International Classification of Diseases, Tenth Revision83 | |||||||
|
7 | 2 | 1 | 17 | 24 | 3 | 27 |
|
0 | 1 | 4 | 7 | 7 | 5 | 12 |
|
1 | 11 | 1 | 3 | 4 | 12 | 16 |
|
4 | 8 | 3 | 14 | 18 | 11 | 29 |
|
0 | 4 | 2 | 4 | 4 | 6 | 10 |
|
4 | 2 | 1 | 11 | 15 | 3 | 18 |
|
3 | 2 | 0 | 3 | 6 | 2 | 8 |
|
0 | 4 | 3 | 4 | 4 | 7 | 11 |
|
0 | 1 | 2 | 1 | 1 | 3 | 4 |
| AKPS score | Trial arm | |
|---|---|---|
| Control | Intervention | |
| Baseline (N = 65) | ||
| Score, n (%) | ||
| 10 | 0 (0.0) | 1 (3.5) |
| 20 | 14 (38.9) | 5 (17.2) |
| 30 | 7 (19.4) | 6 (20.7) |
| 40 | 3 (8.3) | 3 (10.3) |
| 50 | 3 (8.3) | 0 (0.0) |
| 60 | 5 (13.9) | 0 (0.0) |
| 70 | 1 (2.8) | 0 (0.0) |
| Score, mean (SD) | 34.2 (16.2) | 27.3 (8.8) |
| Missing, n (%) | 3 (8.3) | 14 (48.3) |
| 3–5 days (N = 36) | ||
| Score, n (%) | ||
| 20 | 10 (50.0) | 4 (25.0) |
| 30 | 2 (10.0) | 4 (25.0) |
| 40 | 3 (15.0) | 3 (18.8) |
| 50 | 1 (5.0) | 1 (6.3) |
| 60 | 0 (0.0) | 0 (0.0) |
| 70 | 2 (10.0) | 0 (0.0) |
| Score, mean (SD) | 31.7 (16.9) | 30.8 (10.0) |
| Missing, n (%) | 2 (10.0) | 4 (25.0) |
| 10–15 days (N = 12) | ||
| Score, n (%) | ||
| 20 | 1 (20.0) | 1 (20.0) |
| 30 | 1 (20.0) | 0 (0.0) |
| 40 | 1 (20.0) | 0 (0.0) |
| 50 | 1 (20.0) | 1 (20.0) |
| 60 | 0 (0.0) | 0 (0.0) |
| 70 | 0 (0.0) | 0 (0.0) |
| Score, mean (SD) | 35.0 (12.9) | 35 (21.2) |
| Missing, n (%) | 1 (20.0) | 5 (71.4) |
Participant recruitment to qualitative components
Patient and relative interview demographics
In total, 24 interviews with 25 (including one dyad) patients and relatives were conducted (Table 11). Twelve interviews were conducted in each of the intervention and control arms of the trial. The reasons for non-participation are given in Appendix 7. In the control arm, six patients had a cancer diagnosis, compared with just one patient in the intervention arm. In the control group, seven patients and six relatives were interviewed. In the intervention arm, the majority of interviews were conducted with relatives. The relatively small number of patient interviews conducted in the intervention arm of the trial is related to a higher number of older patients, many of whom lacked the mental capacity to consent to be interviewed. The mean interview duration was 41 minutes (range 17–73 minutes). The interviews took place at various locations including the trial hospitals, a nursing home and the participants’ homes, and some were carried out over the telephone.
| Characteristics | Trial arm | |
|---|---|---|
| Control | Intervention | |
| Number of interviews | 12 | 12 |
| Interview participants (n) | ||
| Patient only | 6 | 2 |
| Patient and relative | 1 | 0 |
| Relative only | 5 | 10 |
| Interview participant ethnicity (n) | ||
| White British | 9 | 12 |
| Asian | 4 | 0 |
| Interview participant gender (n) | ||
| Female | 5 | 9 |
| Male | 8 | 3 |
| Relationship with the patient (n) | ||
| Wife | 1 | 1 |
| Husband | 1 | 1 |
| Daughter | 0 | 7 |
| Son | 2 | 1 |
| Daughter-in-law | 1 | 0 |
| Brother-in-law | 1 | 0 |
| Patient disease group (n) | ||
| Cancer | 6 | 1 |
| Non-cancer | 6 | 11 |
| Patient age (years) (n) | ||
| 50–64 | 3 | 1 |
| 65–79 | 3 | 3 |
| 80–94 | 6 | 5 |
| 95–109 | 0 | 3 |
| Mean patient age (years) | 75 | 84 |
| Median patient age (years) | 79 | 87 |
| Patient age (years) (range) | 54–88 | 61–98 |
| Income (n) | ||
| Living comfortably with present income | 4 | 5 |
| Coping on present income | 6 | 3 |
| Difficult on present income | 0 | 1 |
| Do not know | 2 | 2 |
| Pension (n) | ||
| State pension | 8 | 10 |
| Attendance allowance | 1 | 4 |
| Private pension | 4 | 2 |
| Registered disabled | 1 | 3 |
| Blue badge holder | 0 | 2 |
| Other benefits | 1 | 0 |
| Missing | 1 | 0 |
| Interview duration (minutes) | ||
| Mean | 40 | 43 |
| Median | 37 | 44 |
| Range | 20–73 | 17–69 |
Non-participant observation characteristics
Non-participant observations of MDT meetings were conducted at three time points (at the beginning, in the middle and towards the end of the recruitment period) at each trial ward (Table 12). At three trial sites (Int1, Int2 and Con1), daily morning handover meetings were observed; at site Con2, discharge co-ordination meetings, which took place once a week in the afternoon, were observed. All meetings had a major focus on discharge. These meetings were attended by a range of HCPs, and their length varied. The majority of the meetings were led by a senior nursing staff member.
| Characteristics | Trial arm and site | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Int1 | Int2 | Con1 | Con2 | |
| Number of meetings observed | 6 (3 per ward) | 3 | 3 | 3 |
| Specialties involved | Geriatrics | Respiratory |
|
|
| Type of meeting | Morning handover | Board round/morning handover | Morning handover | Discharge co-ordination meeting |
| Professionals involved |
|
|
|
|
| Number of participants per meeting (mean) | 7 | 10 | 8 | 7 |
| Duration (minutes) | 40 | 30–40 | 30 | 60 |
| Gender ratio (women : men) | 5 : 1 | 3 : 1 | 4 : 1 | 4 : 1 |
Focus groups
In total, four focus groups were conducted with HCPs; one was conducted per trial site. The characteristics of the HCPs who participated in the focus groups are presented in Table 13. Focus groups were well attended by a wide range of HCPs.
| Characteristics | Trial arm and site | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Int1 (n = 11) | Int2 (n = 15) | Con1 (n = 9) | Con2 (n = 11) | |
| Specialties involved | Geriatrics | Respiratory |
|
|
| Professionals involved (gender) |
|
|
|
|
| Duration (minutes) | 50 | 49 | 60 | 65 |
Feasibility of conducting trial procedures
During the focus groups, HCPs shared their insights into the conduct of the trial. Their views focused on the following issues: (1) the inclusion criteria for the AMBER care bundle and implications for patient inclusion in the trial, (2) modification of trial inclusion criteria, (3) trial settings, (4) resources required for recruitment and consent, (5) patients’ and relatives’ insights into uncertainty, (6) the participant information sheet and consent forms and (7) the perceived impact of the trial among research nurses and participants.
The inclusion criteria for the AMBER care bundle and implications for patient inclusion in the trial
Focus group participants shared their experiences of disagreement regarding patients they believed to be eligible for the trial. The reasons for this disagreement included confusion about the ‘middle ground’ between patients who were being ‘actively treated’ and those at ‘the end of life’. There was also a sentiment of a power disparity between doctors and nurses, and about how the final decisions were made for patients who could be approached for the trial. Specifically, at site Con1, the research nurse and ward sister explained experiencing difficulty in recruitment because the concept of clinical uncertainty was not fully understood by clinicians as being a legitimate entity in its own right. Often, doctors and nurses had contradictory views about which patients were suitable for the trial. The following two views exemplify this situation:
I think that’s been particularly difficult in this study. I think that is why we struggled to recruit. Because what we perceived to be a patient wasn’t certain (i.e. uncertain), was not necessarily the view of the medical team. I think it’s either ‘We are actively treating’ or ‘End of life’ [approving ‘hmms’ in the background from the other research nurses]. You know there’s no in-between.
Con1021, female, research nurse
Or the medics would say ‘No, they’re not’. But just listening to the handovers, it was like you’d identify everybody on the ward.
Con1018, female, ward sister
It was also evident from the research nurse perspective that despite attempting to explain their reasoning for patient inclusion numerous times, the medical staff were not engaging and had a different understanding of clinical uncertainty. A female research nurse stated the following:
It came to a point where we had explained it, and explained it. I think it got to a point where they said ‘No, no they’re not eligible’.
Con1021, female, research nurse
Other views, which were shared, drew on the more subtle and subjective characteristics of clinical uncertainty, which had implications not only for the inclusion of patients who might benefit from intervention but also for potential recruitment of those individuals to the trial. A consultant from site Int1 believed that the concept of clinical uncertainty should be open to more subjective assessment and was adamant that it was not an objective measurable concept:
It’s quite subjective, but that’s probably good. If you put strict criteria, you might miss some. Like we were saying, it’s almost like a feeling isn’t it, that someone is uncertain. It’s not this metric thing. You know, otherwise like this is a marker of uncertainty. I quite like the fact that the criteria is . . . uncertain.
Int1013, male, consultant
Issues around the inclusion criteria extended further to the risk of dying during their episode of care despite treatment. This criterion relied heavily on the ability to prognosticate, rather than clinical need, which was often challenging.
There was confusion about what represented an ‘episode of care’. In site Con2, a consultant shared his frustration in relation to his understanding of what an ‘episode of care’ implied, wanting to believe that the patients’ and families’ understanding of an episode of care extended far beyond the experience on a particular hospital ward:
My reflection is really, I think it’s just a bad expression; it’s the question of defining what the ‘episode of care’ is. It’s really what’s it came down to, wasn’t it? Initially, I was told that the episode of care finishes as the patient leaves the back door, which really isn’t true, is it? That’s the whole point of whoever is following their care to the community, as far as a patient is concerned. I’m hoping their perception of episode of care isn’t the ‘back door is closed, you’re in the ambulance going home and that’s it’.
Con2020, male, consultant
Concern about the ‘risk of dying’ criterion also opened up a discussion about variability in its perception across trial sites. The same consultant believed that different HCPs might have different interpretations about a patient’s clinical findings, which would have implications for estimating their risk of dying, rather than focusing on their clinically uncertain recovery. He implied that the trial should either focus on all patients where situations of clinical uncertainty exist or be more concrete about what risk of death entailed, and how this could be interpreted with high fidelity across different trial sites:
Yeah, he was very clear; he said probably 50/50 and that’s from his experience of because they’re using the AMBER care bundle in real life. So actually putting something like . . . well actually people will then think, actually there is a 50/50 chance of them surviving this episode of care, actually I would’ve found that much more helpful, because I look at their charts and I look through their organs, and the other things like scores and you can get mortality with the score and 20% chance of dying, is that fitting you or not really. How does that fit against the AMBER care bundle that is to be half and half, so it is difficult? My worry when we set out originally is what happens when that one of your study groups has an 80% death rate and the other has 20% death rate on the other site because they just slightly differently interpreted ‘the risk of death’. So I think being a bit more concrete about risk of death would be good.
Con2020, male, consultant
Modification of trial inclusion criteria
Patient recruitment in the control arm of the trial proved to be highly challenging. At site Con1, 55 patients were logged as ‘not eligible’ in the screening, principally because they were ‘not at risk of dying’ during their episode of care. Without appropriate education and training, research nurses and clinicians experienced enormous difficulty interpreting this criterion. Providing the level of education and support that was available to the intervention sites would have resulted in usual care being altered in the control sites. Consequently, there was considerable variability in the interpretation of this criterion, with the tendency for prognostication rather than consideration of risk. We therefore reviewed this inclusion criterion and how it was working at the control sites across a 4-month recruitment period with our PAG, TS DMEC and the principal investigators of both control sites. Because one of our feasibility objectives was to examine how the trial operated under field conditions and to make changes where necessary, we made a pragmatic decision to remove this criterion.
This change was adopted at the control sites after REC, HRA and local approvals for Substantial Amendment 6 were received (14 December 2017). We planned to monitor the effect of this change on recruitment. However, one of the control sites (site Con1) stopped recruitment owing to challenges around this inclusion criterion and did not have the capacity to reinitiate recruitment by the time REC, HRA and local research governance approvals were obtained. The other control site, site Con2 recruited eight more participants after the change in the inclusion criteria.
Trial settings
The control sites experienced a number of different challenges. It became evident from the principal investigator of site Con1 that consultant oversight of the trial ward was a key factor to take into account when setting up a trial. By the time the consultant on the ward had become familiar with the trial and its requirements, he or she was replaced by a new consultant who similarly needed to be introduced to, or reminded about, the trial:
Because there is a system where the consultant haematologist changes every week and there’s that five or six of them, aren’t there? So, they’re there every fifth week and you know, you happen to tell them every week about the study, remind them that the study is going on. Even the diabetologists change every, is it 2 weeks or month?
Con1023, male, consultant
Because identification of potential participants required clinical input, engagement of HCPs on the ward was crucial to recruitment success. A research nurse highlighted the lack of enthusiasm in relation to the trial from the HCPs:
For the patients, I don’t think there was negative impact . . . I don’t think the staff on the ward were keen.
Con1021, female, research nurse
Contradictorily, a research nurse located at one of the intervention sites witnessed enthusiasm for the intervention, and for her presence in relation to recruitment and data collection. This exceeded her expectations having come from a research background where the focus was not on older people:
So from my perspective, I’m mainly working in paediatric and reproductive health. So coming to care of the elderly, it was completely different and it gives me a unique insight into what’s going on the ward because I’m seeing it completely objectively. So, a few things to say . . . I was really, really impressed about how on board everybody was and everybody knew what AMBER was. On the whole, generally 90% of the time people were very supportive of our presence and what we needed to do.
Int1033, female, research nurse
Resource required for recruitment and consent
Focus group participants discussed the practicalities of identification and recruitment of potential trial participants and the emotive and complex nature of this trial. According to one research nurse at site Con1, they had hoped to access a larger team of research nurses to work on the trial. However, only a few nurses were willing to attend handover meetings and be involved in patient identification and recruitment. Other members of the research team were not comfortable working with a group of very unwell patients. For the research nurses who did collect data, it had been a tiring process:
To start off with as a research team we did it totally different. But then it was just two members of staff who came up every day, looking. A lot of the other staff felt uncomfortable working in this area is if we are honest. The two who did found all the end-of-life care tiring, even though it wasn’t a strictly end-of-life care study. The fact is that we got a paediatric nurse and a stroke nurse and they felt a bit out of their depth.
Con1020, female, research practitioner
Other research nurses drew attention to the challenges of recruiting patients who lacked mental capacity to consent. This was primarily because relatives who might have been able to talk about patients’ intentions regarding their participation were often not immediately available. The ramifications of this were that research nurses had to go back to the ward frequently:
The actual recruitment process was sometimes quite difficult because of what you said, the families not being here. Most of the patients we approached, we had to contact the relatives because the patients were too unwell. So being able to liaise with the families was difficult. We can’t be waiting for the families here, so we were called back about relatives that were here and popping back and asking the ward staff to tell us when they’re here. So the practicalities were difficult.
Int1033, female, research nurse
The non-participant observational work corroborated this concern, whereby a conversation initiated by an occupational therapist informed the rest of the ward staff that she had made numerous attempts to contact a patient’s wife but had yet to successfully speak to her.
Patients’ and relatives’ insight into uncertainty
On several occasions, research nurses spoke of the mismatch between some patients’ inability to comprehend their clinical situation even though they had previously talked in detail to clinicians about the context of the trial and their potential place within it. HCPs who had discussions with patients and relatives would assume that the individual understood that their recovery was uncertain. However, responses that research nurses got in relation to discussions with these patients did not always convince them that this was the case. The following female research nurse based at site Int2 stated the following:
I’m asking someone to do something because they’re on this AMBER care bundle, because they might get worse, or they might get better, and they say ‘Well no, I’m not going to get worse’. It’s not convincing for them to take part in the research project really. If they haven’t got full understanding.
Int2003, female, research nurse
A registrar located at site Con2 shared a similar sentiment, although this time it was focused more on the family members of patients who were very unwell. Although site Con2 had been reasonably successful in the identification and recruitment of patients, there were a few instances where it proved challenging to discuss a patient’s situation with family members as their willingness to accept the situation was critical:
Sometimes it’s like depends on their perception, sometimes you have a patient who’s far more advanced into the illness, whereby unable to give you a ‘Yes’ or ‘No’. So in those cases, you rely on family and it depends on their perception of what they think. So that’s where it . . . It sometimes becomes very tricky. But so far I think the ones who we’ve been trying to recruit, I think most of them have been quite successful. It’s probably one or two perhaps.
Con2019, male, registrar
Participant information sheet and consent forms
Comments here focused on the excessive length and detail of the participant information sheets and the consent forms. Although the development of the participant information sheets and other documentation was reviewed in detail by our PPI members, a research nurse stated that, from her experience, most individuals involved in PPI groups were notably different from the patient population we were aiming to recruit in this trial:
All the PPI groups I’ve ever dealt with are ex-professionals or current professionals, so they are very differently motivated group than actually in a bed, 80 years old, struggling to breathe, still got capacity, you know, you give that to an 80 year old.
You give a four-sided A4 to somebody like. It’s very different.
Int1003, female, research nurse
The nature of the trial documents and the manner in which the trial was sometimes explained to relatives were critical for its success. For instance, while the trial was being introduced to one relative, it became apparent that she interpreted this to mean that the patient was at the end of life. This caused her great distress. Although this was managed sensitively, and the individual was reassured that this was not the case, the principal investigator at the site exemplified the unexpected consequences as follows:
The daughter of the patient told me I was ‘Dr Death’ and ‘the Grim Reaper’! They were very upset about it and I think it was largely because they didn’t understand . . .
Con2020, male, consultant
The research nurse from site Int2 provided comments on the reality of conducting research with an inherently unwell and unstable patient group. Radical modifications to the level of detail of the trial documentation accompanied by a more streamlined process of explaining the trial and less burdensome consent processes should be considered. With these changes, the research nurse believed the trial had a much better chance of success, stating that many potential patients had been put off of the trial:
The consent process also needs to be changed. If you can get away some sort of ‘implied consent’ [would work better]. Because I’ll tell you what, this is a non-CTIMP [Clinical Trial of an Investigational Medicinal Product], so GCP [good clinical practice] is not legal, or the medicines so MHRA [Medicines and Healthcare products Regulatory Agency] doesn’t apply here. The law only applies to CTIMPs. So with this, there is nothing to say, you have to get a written consent form and I think you need to be pushing these boundaries with the ethics committees. This is why research in this specialty is not being done. You would’ve had dozens more questionnaires completed, dozens. Why can’t when I go in to a see a patient ask ‘Mr Smith would you mind answering some questions about your condition and how we’ve been treating you?’. ‘Yes, no problem.’ ‘OK.’ ‘Mr Smith you do understand that you don’t have to do this?’ ‘Yes.’ ‘You do understand that you can stop at any time.’ ‘Yes.’ So I can tick those and start asking questions. You would’ve had an 80% completion rate.
Int2003, female, research nurse
The design of the consent forms needs to be adjusted. There’s lots of improvement to make it easier to get people to consent. Not in a ‘We’re trying to fool people to get them into the study’. But you know reading the patient information leaflet for somebody who is very, very sick. It knackers them out and that’s just day 1. Day 1, talk to them. Day 2, reading the information leaflet. Day 3, I go back to do the consent form and they passed away or gone on to end of life, or they’re just too sick to even contemplate doing it. And it’s quite it’s a long process to trying to read somebody a 3–4 page long document, because they can’t read it themselves. They say read it to me, I get halfway through and they’re falling asleep because they are so, so sick.
Int2003, female, research nurse
The perceived impact of the trial among research nurses and patients
Ward staff who were responsible for potential participants’ care believed that they may have had some influence over patients agreeing to participate in the trial. Although some patients expressed interest in participating in the trial to HCPs who were caring for them, when research nurses followed up, patients declined to participate:
They didn’t want to upset me, because actually, potentially, I’ve done something to improve the quality of their life by giving them symptom control. So they’re thinking, ‘She’s done something for me, I’ll do something for her’. But when the researchers came along, whom they’d never met before, they said ‘no’.
Int2012, female, consultant
Although the research trial had been challenging for many of the research nurses, from consenting potential participants to data collection among an unwell patient population, one research nurse spoke emotively about the relationships she and her colleagues built with patients and/or their relatives during the trial. Unlike some of the studies that they had previously worked on, they felt that the focus of the trial provided them with a privileged position and opportunity to develop relationships with the participants. For example, the following research nurse stated that the perceived impartiality of the trial and her ongoing relationship with certain patients and family participants permitted them to talk frankly about their situation, which they did not feel able to share with the HCPs. When these nurses felt that it was appropriate, they actively encouraged these patients and families to communicate their concerns with the HCPs caring for them so that they could be served to better effect. A research nurse stated:
I think, the same as the other researchers, worked on the study, can’t be here today, he gave us a unique relationship with the relatives. So in a strange way, you’re in a unique position that they talk to you about things that sometimes they feel that they can’t take forward with certain ward staff. So we are able to encourage them into having those conversations making sure that those communications were taking place with the ward staff if they, the family has signed society, or certain query. So, whether because we were seen as external or whether we would able to form a relationship over questionnaires being done at different points, I just don’t know.
Int1033, female, research nurse
Patients’ views of being involved in the trial also highlight the impact of positive relationships being formed between research nurses and the participants as a reason for their continued involvement in the trial.
Further refinements and adaptations required for the AMBER care bundle to suit local conditions
Qualitative trial components were used to understand if, and in what ways, the AMBER care bundle required refinement or modification for it to work successfully in the field, prior to its formal testing in a definitive trial. This included the focus groups held with HCPs at the intervention sites and the non-participant observations of the MDT meetings. Refinements and adaptations required to the intervention focused on the following areas: (1) pragmatic interpretation of the AMBER care bundle criteria, (2) reconsidering the daily reviews, (3) concerns about the ‘labelling’ of the intervention and (4) competences and confidence in advanced communication skills.
Pragmatic interpretation of the AMBER care bundle criteria
A critical issue associated with the delivery of the AMBER care bundle was the inclusion criteria. HCPs shared views on the inherent challenges associated with identifying patients using the criteria. At site Int1, two consultants discussed that their focus was on identifying ‘situations of clinical uncertainty’ in comparison with the criteria of being at ‘risk of dying during their episode of care despite treatment’. This suggested the need for simplified criteria:
There are some people that we’ve been trying to go down . . . sort of get them better but they’re not improving. I think those are the patients I kind of sort of see as uncertain or sort of unpredictable in this recovery. They’re the ones that we identify.
Int1013, male, consultant
I think most of our patients in the elderly ward are already coming in with uncertainty, because generally they’re aged, they’re frail, their history. So for me a lot of my patients would already be on AMBER before they’re even come in to hospital.
Int1018, female, consultant
At site Int2, the issue of who might benefit from being supported by the AMBER care bundle was constricted to the risk of dying. At this site, a female junior doctor explained that although many patients were unwell, with a possibility of dying within the next several months, unless a decision was made that this was likely to happen much sooner, they might not qualify to be supported by the AMBER care bundle. This reiterates the issue that the criteria do not focus exclusively on a patient’s clinical need but on prognostication:
Well sometimes it’s hard predicting whether they’ll die during this admission or when they’re going home. So for example, they might not die in this admission, but they are at the end of life in the next few months.
Int2019, female, senior house officer
Views shared by HCPs during the focus groups were corroborated by the non-participant observations of the MDT meetings that took place at sites Int1 and Int2. We observed a CNS asking her colleagues if they considered a patient as ‘being likely to die during their admission’ as part of their decision-making about whether or not the patient should be supported by the AMBER care bundle. During the same MDT meeting, two patients supported by the intervention were discussed in detail, yet the clinicians mentioned that they possibly had ‘a few more months left to live’. This was inconsistent with the AMBER care bundle inclusion criterion that states that patients should be at ‘risk of dying during their episode of care despite treatment’. We frequently observed that the clinical team made decisions about if a patient was going to be (or continued to be) supported by the AMBER care bundle not based on risk of dying but on their clinical morbidities, their disease progression (e.g. late stage of dementia/Parkinson’s disease), their likelihood of responding to medication or their ceiling of care escalation [e.g. if a patient was documented to be DNAR (do not attempt resuscitation), HCPs mentioned this as a reason].
Incidentally, the non-participant observations on the control wards identified that clinicians were practising ‘AMBER care bundle-like’ rules of care with some of their patients. For example, one doctor was heard to say, ‘If he [the patient] doesn’t respond to antibiotics, we should talk with his wife’. Others were observed as listing a patient’s morbidities and deciding at a MDT meeting that the patient was considered to be frail, with multimorbidities, and they should arrange to speak with the family. However, we did not observe instances in which patients discussed in this manner were also discussed as being at a risk of dying during their episode of care.
This concern about the AMBER care bundle inclusion criterion was also demonstrated in the account of a relative of a patient participant supported by the intervention. We identified a divergence between how this relative viewed their loved one’s situation in comparison to the HCPs who were caring from them, at least in relation to their decline. Despite the intervention’s intention to create awareness among HCPs around the risk of a patient dying during the episode of care, some relatives were surprised that HCPs did not see, or did not want to see, what relatives considered to be obvious. The daughter of a white British, 85-year-old patient participant with acute renal failure illustrated this tension:
I remember having a conversation with the doctor and saying, ‘Do you really actually think he’s going to be discharged out of here? Because he looks like he’s a dying man to me.’. The doctor just said to me ‘You have to be optimistic’, and I just said ‘Optimistic or realistic?’. You know.
Int1017, carer
Reconsidering the daily reviews
The recommendation for the daily monitoring and review of patients supported by the AMBER care bundle was met with a critique. A consultant geriatrician at site Int1 clearly stated that he had accommodated the notion of uncertainty and wanted to instil it into his team. The uncertainty was very likely to be the normal state for many patients from one day to the next. Revisiting daily at the end of each shift to identify whether or not they were still suitable for the intervention, whether or not there had been any medical changes and whether or not a conversation had taken place with the patient and their family was therefore seen as unnecessary:
I think I prefer to have the team feel that there is always an element of uncertainty. It’s unlikely that tomorrow they won’t have any uncertainty. Because even if they recover, our patients they just go back quickly [agreeing chatter in the background]. So I prefer the review to be less often unless there is a drastic change in the person or you know sometimes people make a really good recovery.
Int1013, male, consultant
Building on the concern about the daily review, a junior doctor stated perceiving the overengineering of frequent reviews and sometimes frequent conversations to be distressing for patients and families. This may have been because some patients were frail, elderly and occasionally confused. It may also have been due to the nature of the discussions. Regardless, some individuals were interpreting the discussions differently, which might cause undue distress:
If you discussed it once and explained everything very well, the frequency of further discussions was possible I felt was too high trying to say that to them every day. Again the discussion can go from what we’re doing to make you better and then discussing the uncertainty again every day and it could be quite distressing because a lot of our patients are elderly and going through this with them, they just felt like ‘Oh that means I made progress. I’m well again.’.
Int2022, male, registrar
The concern surrounding the daily reviews also extended to the requirement of placing a sticker on the patient’s clinical notes in addition to recording their ongoing situation in the notes in detail. A male registrar said:
One thing I would dispose of are those stickers everyday about if it has been discussed with them, and there’s a prompt for discussing there.
Int2022, male, registrar
The HCPs’ concerns around the daily review sometimes contrasted with relatives’ expectations of how often they wanted to be kept updated about their loved one’s situation. Relatives suggested that when they came to visit their loved ones, they did not visit just to be with them but also because they wanted to get an up-to-date understanding of their loved one’s clinical situation. The daughter of a white British, 89-year-old patient participant with aspiration pneumonia illustrated this point:
Yeah, well I was there every day, so I spoke to someone every day. Yes, they kept me informed. When they were sending her home, they sat and did the form online with the nurse in charge of the ward. Every single day I was there.
Int1011, carer
For some relatives, visiting daily and seeking out someone to learn about any developments in the patient’s situation was far more challenging and led to frustration. The husband of a white British, 61-year-old patient participant with multiple sclerosis said:
No, it is a little bit in the dark. You got to go and find out yourself . . . The information is not volunteered readily. I think you got to go and find someone to try to explain it to you.
Int2008, carer
Concerns about the ‘labelling’ of the intervention
Some focus group participants raised concerns about the introduction of jargon, such as ‘AMBER’ to patients and families. At instances, family members were confused about the terminology and did not understand the use of the intervention in supporting their loved one. Being informed that their dependant was being supported by the AMBER care bundle became reminiscent of the now-withdrawn Liverpool Care Pathway for the Dying Patient,33 in which families became distressed. A junior doctor said:
We’d keep the family informed, but I think now it’s a bit confusing for the families like ‘Oh what is this AMBER bundle? Is my relative dying?’. And they just get overwhelmed.
Int2017, female, senior house officer
Competence and confidence in advanced communication skills
Focus group participants were aware that the support of patients whose situations were clinically uncertain often involved engaging in difficult conversations that demanded advanced communication skills. Although the nurse facilitator provided training on the rudiments of conducting difficult conversations, there were some HCPs, principally nurses, who were still hesitant about engaging in conversations. It was apparent that advanced training in communication was still required to deliver the AMBER care bundle. Consequently, some suggested that, in future, additional training should be made available to HCPs to enhance their skills and confidence in this area of care. A ward manager said:
So I would have that conversation anyway. I wouldn’t be frightened but I know but I know some of my nurses won’t at all. They wouldn’t talk with them . . .
Int2014, female, ward manager
Some of the HCPs’ hesitancies in having difficult conversations with patients and their families, and their need for more training in advanced communication skills, was also evidenced in the accounts of relatives of patients who had been supported by the AMBER care bundle. For example, during a period of a female patient’s acute deterioration, a doctor spoke with her husband about escalation decisions and tersely brokered information to him about her deteriorating situation. A nurse stepped in, realising how distressed he looked on receiving the news:
But I thought that could’ve been handled a lot better. Especially when he glanced over her and said ‘Well it doesn’t look good, does it?’. And I thought ‘What are you actually saying then? You’re saying that’s it?’ and he said ‘Anyway, I’ll give you a bit of time.’. And then a nurse stepped in and said ‘We’re not saying we’re giving up on her. We’re not at that stage yet. We are still gonna keep treating her.’. But the way it was described to me was that was it. They’ve done as much as they could do and if anything, else happens, that’s it. I didn’t think that’s handled well . . . Like I say, there was a lot of activity around her and I spoke, sort of thrown and get involved to see what was happening and then a doctor sort of said to me ‘We need to know your wishes on resuscitation. If she has a sudden collapse, you know stops breathing or health failure or something, do we resuscitate?’ and I thought well that’s a question. Right, straightaway, literally as soon as I walked in the door really and they said ‘You’re the next of kin. We can’t ask [Int2008]; she’s completely unconscious. What are your views on it?’. Well I said ‘I know what [Int2008’s] views would be’. But I thought that could’ve been handled a lot better. Especially when he glanced over her and said ‘Well it doesn’t look good, does it?’.
Int2008, carer (husband of a white British, 61-year-old patient participant with multiple sclerosis)
Perceived acceptability of the AMBER care bundle to patients, their families and health-care professionals
The acceptability of the AMBER care bundle was explored by analysing data from the qualitative components. In some instances, we were also able to explore the acceptability of being supported by the AMBER care bundle from patients’ and their relatives’ perspectives. We assessed the acceptability of the AMBER care bundle in terms of the resources required, perceived effectiveness and benefits, understanding of the intervention, self-efficacy of HCPs to deliver the intervention and the intervention’s fit within the team’s culture. 84
Prioritisations of patients’ preferences
The HCPs welcomed the departure from solely focusing on patients’ physical symptoms and placing more emphasis on engaging in early and important discussions with patients and their families about their preferences, and the inherent value of documenting these discussions.
Participants stated that they believed that conversations with patients whose situations were clinically uncertain were taking place earlier than they had previously taken place as a result of the intervention. Conversations now included a focus on patients’ and families’ PPC and PPD, whereas previously conversations primarily focused on physical symptoms. Moreover, they believed that the AMBER care bundle discussions not only respected patients’ autonomy but also placed them in a better position to realise their wishes. The following two HCPs, a physician associate and a consultant, shared these views:
It’s actually massively important to the patients, isn’t it? We didn’t appreciate it. Going more than patients want out be out of pain, they want dignity and I think at the end of life, they want control and some of that control is about the environment that they’re in. I think it’s about respecting that patient’s wishes and uhmm empowering them and give them their last wishes.
Int1013, male, consultant
I think it prompted conversations earlier. Because we recognise and we’re looking for these patients, we then initiate the conversations earlier than we may have done previously.
Int1002, female, physician associate
The relatives of patients supported by the AMBER care bundle provided accounts of how they perceived the care. There were instances where holistic care and open and compassionate communication were evident, which chimed with one of the intended benefits of the AMBER care bundle: a reduction in anxiety levels. This was typified by the following accounts from the relatives of three participants:
I think it was, erm, we were very impressed, the doctors worked well, they, yes, they spent a lot of time, in fact, in a way, erm, you could say too much time, because I think they were concerned that my father would be upset, so they had sort of prepared themselves for a very difficult meeting, interview, discussion . . . They were very caring in a sense, they were very concerned that he, you know, that, about how he might react, er, and, it went fine. It was good because the conversation was always involving him too, even though he was in bed or sometimes his hearing isn’t brilliant.
Int1001, carer (son of a white British, 98-year-old patient participant with prostate cancer)
They couldn’t have been more helpful. They really listened to us . . . When we had meeting with the doctors or whatever, they would say to us ‘do you have any questions?’ and they, everyone knows how short of time everyone in the NHS is but they appeared to have all the time in the world for us, which was very comforting. They were happy to, erm, answer as many questions as we had and to give us a full medical explanation . . . They were very open and helpful.
Int1005, carer (daughter of a white British, 84-year-old patient participant who had a heart attack)
Yes, I have to say that the doctors, one doctor in particular, she was very good, she would take us to another room and explain it to us and asked us if we had any questions. And she would stop to explain to dad and to us at the bedside. She was the best doctor there because she could communicate with you and she could come down to like dad’s level of comprehension.
Int1003, carer (daughter of a white British, 96-year-old patient participant who had a heart attack)
However, a number of relatives of participants were disappointed with the lack of appropriate and thoughtful discussions, which they felt were in their dependants’ best interests. The step-daughter of a white British, 92-year-old patient participant who had multiple fractures stated that her family’s views about the patient were never adequately canvassed. Moreover, when they were involved in discussions, the discussions appeared to be conducted in a manner that merely informed them about what was happening, often without consideration of their understandably heightened emotions. This was despite the family appreciating that what was planned was probably appropriate:
No, absolutely nothing . . . We weren’t asked anything about our preferences for her care in the future . . . We were told rather than asked by a consultant a week or ago that they wouldn’t uhm . . . they wouldn’t do anymore to her. Now, I felt I mean I agree with that, but I felt that he said it in such a way that ‘Well, we won’t do it’ [what we wanted]. Never mind what you’re thinking . . .
Int1016, carer
The daughter of a white British, 96-year-old patient participant who had a heart attack stated that although care was provided to her father, she felt that her concerns and questions regarding his care were often dismissed. Moreover, she believed that the HCPs avoided her and were not particularly interested in engaging with the family despite their daily presence on the ward:
You know they sort of ‘poo pooed’ what I was saying . . . The doctors took care of him and but there could’ve been more communication I think, really. We wanted more communication. We were there every day, so there was no reason why they did not stop and speak to us. They did at the end and realised that we were obviously interested in his care and what happened to him uhmm but yeah too many times, they could’ve stopped and spoken to us.
Int1003, carer
Involvement of health-care professionals in the decision-making process and teamwork
At the intervention sites, care and treatment decisions about patients with clinical uncertainty were made with the involvement of the MDT. A consultant stated that although it was the doctors who were perceived as being pivotal in the patient’s care, the views of other professional groups also contributed towards patient-centred decisions. This illustrates the acceptability of the ‘distribution of decision-making process’ among all HCPs, which is a component of the AMBER care bundle:
So it’s an MDT decision, because sometimes you know we as doctors, you know we go in, we see the patient on a ward but we’ve not been caring for the patient. HCAs [health-care assistants], everyone, everyone is involved in the decision.
Int1018, female, consultant
Instances of enthusiastic teamwork were observed during the handover meetings. At site Int2, we witnessed a range of HCPs contributing to discussions about patients’ suitability for the AMBER care bundle. This suggested acceptability of the identification part of the intervention and showed that the team understood its potential in helping patients and their families. This observation was slightly tempered by a number of HCPs, who required the nurse facilitator or the consultant to occasionally prompt them to consider potential patients who might benefit from the AMBER care bundle.
Joint acceptance and teamwork were also observed at site Int1. During a morning handover meeting, when the usual consultant was not available, a colleague from an adjoining ward stepped in to help. During this instance, we observed a number of HCPs informing him about the intervention and carefully explaining the various steps they took when making decisions about which patients were suitable for the intervention. Moreover, talking through the intervention components appeared to be natural and well rehearsed. During this discussion, a number of professionals volunteered information to the other team members about the progress of particular patients they were caring for. Moreover, they also told them they were trying to get hold of close relatives to explain to them the patient’s situation and condition.
Another example of enhanced teamwork was observed when the MDT made clinical decisions together and prioritised understanding the preferences of patients who were supported by the AMBER care bundle. A MDT (a physiotherapist, the ward sister and a consultant) discussed in detail the decision of whether or not to continue to actively treat an elderly patient. They also wanted to discuss the implications of this decision with the patient and learn more about his/her preferences. However, the consultant importantly mentioned that this could not happen before they comprehensively assessed the patient’s mental capacity (which is also highlighted in the AMBER care bundle tool).
Participants’ and their relatives’ perspectives on decision-making processes varied. We identified a number of instances where the views of focus group participants closely corroborated those provided by relatives of patients supported by the AMBER care bundle. For example, they spoke about the various ward staff being closely aligned in getting to know the patients, their thinking about a patient’s situation and their actions regarding care. They spoke of this as being akin to being ‘on the same page’. This is illustrated by the following two comments:
They all seemed to get on very well, yes. All on the same wavelength and saying the same things. Yeah when they sent her home there was an OT [occupational therapist], and he came to the house. The doctor phoned. The nurses made sure she had her cuddly toy with her. They all seemed to group together.
Int1011, carer (daughter of a white British, 89-year-old patient participant with aspiration pneumonia)
They did work well together, I felt, yeah it was a very nice atmosphere on the ward. I mean everybody, for example, the swallowing team and then physio[therapy], and then there was [name of the geriatrician] and [name of the physician associate], and mostly the same nurses who got to know mum, so, I think, there was definitely, erm, a good atmosphere.
Int1002, carer (daughter of a white British, 91-year-old patient participant with Alzheimer’s disease)
For a relative of a patient who had experienced less-satisfactory care in other parts of the hospital prior to their admission to the intervention ward, good teamwork was particularly welcome. The following account illustrates her sentiments:
Ward Y was particularly good from the point of view that they did seem to have some very good support of staff from the point of view of the physiotherapist, the occupational therapist there. It was quite a relief to go on to ward Y and suddenly everybody was planning what they were and how it was going, and um, you know a care plan was actually made for her. And so yeah from that point of view it was they kind of stepped up a notch.
Int1018, carer (daughter of a white British, 95-year-old patient participant with mixed dementia)
Nevertheless, there were times when teamwork was not evident and had negative repercussions for patient care. The daughter of a white British, 84-year-old patient who had a heart attack (Int1005C) mentioned that many of the HCPs had been very considerate and caring, exemplified by the comment ‘They couldn’t have been more helpful . . . very kind’, but she then explained that her father had been in receipt of unnecessary tests by the same HCPs who may not have been aware of the de-escalation for his clinical situation:
Sometimes we’d have people coming to do his blood pressure, erm, and his temperature and when I mentioned to this, to, erm, the consultant geriatrician at ward X, she was notably irritated because people had been instructed not to monitor him and they’d gone back to monitoring, I think, well for whatever reason, lack of communication.
Int1005, carer (daughter of a white British, 84-year-old patient participant who had a heart attack)
Relatives appreciated the challenge of clinical uncertainty but felt that more could be done to keep them in the loop of ‘not knowing’. In the following case, a relative had to take it upon themselves to seek out answers, often from different doctors. The husband of a white British, 61-year-old patient participant with multiple sclerosis stated:
No, it is a little bit in the dark. You got to go and find out yourself. I don’t . . . the information is not volunteered readily; I think you got to go and find someone to try to explain it to you. I suppose it’s a . . . just explanations could’ve been a bit better I suppose but . . . I think once they made up their mind that it was a chest infection, then everyone was heading towards the chest infection for treatment but before that it was a bit inconsistent because there was a lot of doctors with a lot of opinions. Yes, I can. I think that’s the bit that could have been handled better.
Int2008, carer (husband of a white British, 61-year-old patient participant with multiple sclerosis)
Inconsistencies in sharing information were at times frustrating for some relatives. Relatives appreciated that treatment plans may change as necessary; however, these changes in treatment plans were not always shared with them in a timely manner. In the following case, the wife of a white British, 70-year-old patient participant with corticobasal degeneration became confused by the mixed messages from various HCPs about her husband and began to question her own recollection of what had happened. This might have amplified her distress:
One would said one thing, and one would say another, and so it was a little bit like, what’s really happening? What’s going on? Erm, because if they had changed their minds and then I most probably hadn’t been told or heard, do you know what I mean?
Int2012, carer (wife of a white British, 70-year-old patient participant with corticobasal degeneration)
Although our findings from the focus groups and non-participant observations shed light on the acceptability of the AMBER care bundle, we did not observe the MDT meetings prior to implementation of the intervention. Therefore, it is difficult to identify the impact of the AMBER care bundle on teamwork and shared decision-making. Final decisions regarding patients’ care and their treatment are the consultants’ responsibility. Seeking information about patients and taking the MDT members’ views into consideration could depend on the individual consultant. The AMBER care bundle provides a platform for HCPs from various disciplines to share their input about patients whose situations are clinically uncertain. Our findings support the intended benefit of the intervention: enabling the sharing of information for decision-making among HCPs. However, we cannot confidently conclude whether or not the decisions were shared.
The value and simplicity in documenting discussions with patients
Participants expressed views on the value of talking openly with patients about their situation and having a clear plan. The devised plan is beneficial for other HCPs to understand and act on participants’ preferences for care, while acting as an aide-memoire for the person who devised the plan. In a busy ward environment, it could be easy to overlook or to forget important issues without a clear plan. The following core medical trainee voiced this view:
It makes you think about communication and especially when you’re busy and you focus on the medical aspect of it, you [then] remember that we promised the communication with the family.
Int2007, male, specialty registrar–core training 1
The documentation that accompanied the conversations was not perceived as a burden, which is important for HCPs who would be using it:
I think the documented discussion is very important. For the purposes of out of hours, we know the patient during the day and we have the conversations with the family and it’s there documented. At night, they know what the plan is . . . the escalation plan. I think that’s paramount really.
Int1018, female, consultant
This was amplified when HCPs could see that, overall, the document was simple to complete, a departure from many that had been used in the past. A junior doctor illustrates this view:
I think the simplicity of the paperwork itself is good. Because it actually doesn’t . . . we hate paper work. I think keep the simplicity of the paperwork.
Int2017, female, senior house officer
Delivery of care preferences
Where it was possible, the intervention required HCPs to engage in open conversations with patients about their situation and discuss their PPC and in some instances PPD. If HCPs were not honest during conversations about the feasibility of care and treatment options, patients may verbalise preferences that could not be delivered. This highlights the need for allocating adequate time for preparing for these conversations to ensure that the intervention is not causing distress. The following consultant suggested that conversations had the potential to disappoint patients when the system was not able to deliver:
We are . . . giving the patients the impression that we can give them what they want but then not being able to give them what they want. This may disappoint them.
Int1013, male, consultant
Acceptability of inclusion criteria
The HCPs questioned the acceptability of the current inclusion criteria. They felt that modification of the inclusion criteria from ‘patients being at risk of dying in the next 1 to 2 months’ to ‘risk of dying during their episode of care despite treatment’ meant that a reduced number of patients would benefit from the support of the intervention. Although the inclusion criteria are acceptable, alteration of the ‘risk of dying’ criterion might limit their utility. A female consultant stated:
Previously, when I used it before, this was what we talked about before, wasn’t it? [To EY] It was the 2 months or so and obviously they changed it, which meant that now we put less people on AMBER.
Int1018, female, consultant
Long-term acceptability of the AMBER care bundle
In order to operate and sustain the intervention over time, HCPs would need to make critical decisions about (1) who would be the ‘the AMBER care bundle champion’ and (2) what could be de-prioritised to create time for the bundle’s sustainability. HCPs considered the intervention acceptable while they received support from the nurse facilitator; however, they questioned the sustainability of the intervention without dedicated support. A female ward sister said:
The trouble is everyone has so many other responsibilities. Whereas the nurse facilitator’s role was to come in and to facilitate that. So she could focus on that. Whereas everyone else, if you’re asking someone else to take that role on, that’s not going to be their only focus. I feel like it needs someone to drive it.
Int1014, female, ward sister
Active ingredients of the AMBER care bundle to be maintained for intervention fidelity for a definitive trial
To determine the active ingredients of the AMBER care bundle, we explored the perspectives of HCPs from the focus groups, the perspectives of participants and their relatives from interviews and our observations of MDT meetings. The following themes emerged from our analysis: (1) the value in the identification of patients whose situations were clinically uncertain, (2) the routinisation of active engagement and discussion with patients and relatives, (3) the documentation of patient-centred plans, (4) the daily review and re-engagement with patients and (5) the role of the nurse facilitator.
The value in the identification of patients whose situations were clinically uncertain
The AMBER care bundle inclusion criteria were cited as being critical in prompting HCPs to actively consider patients on the ward who might benefit from the intervention. The following physician associate explained that, whereas previously this particular patient group had been absent from the clinical radar, now they had assumed a greater priority in the line-up of daily tasks:
I think for me it [the criteria] bumped it up the priority list, if that makes sense with jobs. Whereas before, it wasn’t highlighted as much . . . I think having conversations with families and updating them. I think what the AMBER care bundle really helped for me was the advance care planning side of doing things.
Int1002, female, physician associate
This finding was corroborated by the observations, where we witnessed discussion among MDTs about patients who fell into three main groupings: those who were acutely unwell, those who were deteriorating and, importantly, those whose recovery was uncertain. Regarding this last group, we often witnessed descriptions of patients as being ‘inbetween-y’ or those who were ‘up and down’.
The routinisation of active engagement and discussion with patients and relatives
Engagements with patients and families became routinised into the daily clinical practice of staff on the intervention wards. One female ward manager stated that although similar conversations with patients like this had previously taken place, the intervention prompted the HCPs to actively seek and prioritise these patients as part of standard practice:
We always recognised them but it became more embedded in our daily board round discussion about highlighting those patients and making a priority that we’re having those conversations but we would think to have those conversations with the relatives when they come in next whereas it’s be more of a we ask them to come in to have the discussions. So we were being more proactive about having those conversations rather than waiting.
Int1022, female, ward manager
We also witnessed instances when HCPs talked about patients who were supported by the AMBER care bundle, their PPCs and their families’ understandings of their situations. For example, we observed a conversation at site Int1 in which a HCP had discussed in detail the situation of a recently admitted patient with the patient’s daughter, who had unrealistic expectations about her mother’s recovery. Discussion moved on to how this conversation had progressed, taking on board the daughter’s views and what she considered to be her PPC for future care. Furthermore, at site Int2, when the nurse facilitator prompted the team to consider a patient to be supported by the AMBER care bundle, she discovered that the team had already identified the patient in question and had spoken in detail with her family.
Documentation of patient-centred plans
The documentation of conversations with patients and families and the development of a clinical plan were perceived to be an active ingredient of the intervention. This ensured transparency among team members. It was particularly useful for staff who worked out of hours, who would have otherwise been less knowledgeable about a patient’s situation. A female consultant and a male trainee doctor shared their perspectives:
I think the documented discussion is very important . . . Oh for the purposes of out of hours, we know the patient during the day and we have the conversations with the family and it’s there documented and this is what we’ll do if they become unwell or whatever. If they get called at night, then they know what the plan is, the escalation plan. I think that’s paramount really.
Int1018, female, consultant
The AMBER care bundle . . . sort of encourages you to have the discussions you wouldn’t have, and then it formalises it in a plan that can be followed by other colleagues. It’s there in writing and we’re requested to engage with that process.
Int2007, male, specialty registrar–core training 1
Documentation of plans and discussions is also intended to provide continuity of the information shared with patients and families by different HCPs. The following example highlights the importance of clear and detailed notes to ensure the correct flow of information to patients and relatives. The instance below highlights the importance of clear and detailed notes to ensure the correct flow of information to patients and relatives. It is here that some study participants suggested that the training that accompanied the AMBER care bundle placed enormous emphasis on understanding their concerns rather than being be delivered in a ‘tick-box’ manner. A white British, 65-year-old patient with motor neurone disease who was supported by the AMBER care bundle at the time of the interview stated the following when asked about the consistency of the information he receives:
OK, so it became clearer over time. So when you first got admitted, they weren’t that clear and then it got clearer?
Ask questions as we go.
So you ask questions as you go?
[He had a notepad that he used to communicate with the staff. He was not able to communicate verbally. He showed the interviewer all the questions he had asked before in his notepad.]
OK, so do you think that you’re getting consistent information from all the doctors and nurses or does it change or differ sometimes?
Occasionally different. Like a drug this morning. The nurse said no I am not for that drug. I questioned that. I was right.
Daily review and re-engagement with patients
Although initial discussion with patients and/or their relatives was considered to be an important ingredient in supporting their care and treatment, the subsequent re-engagement component of the intervention was also considered crucial. This took place daily and required the team to review whether or not a patient’s clinical uncertainty was still ongoing (whether they had deteriorated or improved). Re-engagement with the patients served two main purposes: checking the clinical uncertainty/AMBER care bundle status of the patient, which was fed back to the team for daily review, and following up about their requests, concerns and preferences highlighted during the initial discussion. Whereas follow-up conversations previously occurred on an ad hoc basis, the intervention required the HCPs to review and re-engage in conversations with the patient and their family throughout their admission. This helped the HCPs to understand how the patient’s situation and their preferences changed in the intervening time. The following core medical trainee stated that this drip-feed effect of continuing to address issues and engage with them had the benefit of improving overall communication:
So previously we might have discussed it with the family when they come in initially. Obviously, the admitting doctor is different to the ongoing team and then there may be ‘Oh someone else has talked about it, we won’t need to re-engage with it’, but I think with AMBER care bundle, because they have you have to keep discussing with patient, etc., it does encourage with this process of re-engagement with the family and therefore it probably does improve the communication with the family.
Int2007, male, specialty registrar–core training 1
The HCPs were also observed discussing the clinically uncertain recovery of the patients who were supported by the AMBER care bundle during their daily handover meetings. In the following instance, a patient who was currently supported by the AMBER care bundle had started to recover and the team were reconsidering mobilising him. The ward manager was noted to say ‘He’s no longer AMBER’ and then made sure that that the letter ‘A’ (indicative of being supported by the AMBER care bundle) was removed from the white board next to his name.
The role of the nurse facilitator
Although not considered to be a component of the intervention, a number of focus group participants spoke in positive terms about the critical role the nurse facilitator played in implementing the intervention both on wards and in supporting staff in its delivery to relevant patients and their families. The facilitator should be seen as an ‘active ingredient’ in the delivery of the intervention because achieving the intended benefits would be highly challenging, if not impossible, without her presence. According to the HCPs, she prompted them to think about issues that previously would not have been identified until the last days of life. She also supported the staff at critical moments, for instance when reflecting on the emotive challenges of having difficult conversations with patients and their families. The following male medical consultant and female ward manager explained:
I think the nurse facilitator showed that it works having someone like that. Two phrases that always stick to my mind were ‘preferred place of care’ and ‘preferred place of dying’ which is not something I would think about for a patient probably until we got to the discharge stage. Now, it’s sort of when I’ve seen the patient and identified that they’re AMBER, it’s almost uhm you know I kind of address that sooner so I guess that’s been a good thing.
Int1013, male, consultant
I was just going to say, having a dedicated facilitator helped the staff in having those conversations and supported them with the practical aspects of AMBER.
Int1022, female, ward manager
Our findings from the non-participant observations corroborate the views regarding the importance of the nurse facilitator in the delivery of the intervention. We observed at numerous points that the nurse facilitator encouraged and prompted HCPs to discuss patients’ day-to-day needs and to critically consider their clinical uncertainty. Typically, at handover meetings, the list of patients on the ward was briefly discussed by different HCPs. The following dialogue illustrates the role of the nurse facilitator in directing discussion towards thinking in what ways the AMBER care bundle might be appropriate for a patient. Patient 6 was briefly discussed and a junior doctor stated ‘We made him palliative’. This was followed by a discussion between the palliative care CNS and the junior doctor about whether or not they felt that he might die during that admission. After fielding the discussion between the two HCPs, the nurse facilitator summarised the situation by stating that the patient ‘sounded AMBER’ to her and an escalation plan was required. We also observed the nurse facilitator stating ‘She looks quite fail. She’s 90 years old. She looks AMBER, looking at her notes. COPD [chronic obstructive pulmonary disease] and vascular dementia.’. Other HCPs discussed in what ways the patient could be supported.
At other points during the same meeting, the fast pace of discussion was interrupted to focus in more detail on a patient’s situation. The nurse facilitator’s role in ensuring the intervention’s fidelity was evident: she was heard to say ‘Also still AMBER?’, to which the consultant replied ‘She’s not. She has definitely stabilised.’. A junior doctor stated ‘She came off [AMBER]’. The facilitator then reminded the team that they must now document the patient’s new clinical status regarding the AMBER care bundle in her notes. She was similarly noted to have told the team that when writing a discharge letter about a patient supported by the AMBER care bundle, they should remember to clearly state the patient’s clinical situation and the escalation decisions that had been made previously.
Overall, our findings suggest that a dedicated facilitator for the AMBER care bundle is needed to ensure that HCPs are trained, supported and prompted to consider all components of the intervention regularly.
Compliance with and barriers to the delivery of the AMBER care bundle
First, we examined the extent to which the five key components [(1) documentation of a medical plan (current key issues, anticipated outcomes), (2) documentation of an escalation plan, (3) a medical plan discussion and agreement with nursing staff, (4) discussion with patient/family and documentation of the discussion and (5) daily review of the patient’s clinically uncertain recovery] associated with the delivery of the intervention were fulfilled by looking at the clinical notes of the participants recruited in the intervention arm of the trial. Documentation of these components enabled HCPs to learn about a patient’s situation and act on them. Second, the focus groups with HCPs, held on the intervention wards, explored views on the components of the intervention that they believed were central to its success in supporting patients. Last, we drew on the findings from the non-participant observations of the MDT meetings.
The review of the clinical notes identified acceptable compliance in relation to the delivery of the five components (Table 14). It is possible that there were also instances when tasks may have been carried out but were not recorded, which has implications for the staff caring for and supporting these patients and their families. Moreover, we also identified that in many instances tasks were not recorded within the required 12-hour window stipulated by the AMBER care bundle guidance.
| Site | Compliance with component, n (%) | |||||
|---|---|---|---|---|---|---|
| Discussion with patient/family held and documented | Medical plan (current key issues, anticipated outcomes) | Escalation plan documented | Medical plan discussed and agreed with nursing staff | Daily review | All completed within 12 hours | |
| Int1 (N = 20) | 18 (90) | 18 (90) | 16 (80) | 18 (90) | 8 (40) | 5 (25) |
| Int2 (N = 9) | 6 (67) | 7 (78) | 7 (78) | 6 (67) | 6 (67) | 5 (56) |
| Total (N = 29) | 24 (83) | 25 (86) | 23 (79) | 24 (83) | 14 (48) | 10 (35) |
Availability of relatives and health-care professionals
Participants in the focus groups stated that a large number of patients who had clinical uncertainty were elderly, confused and lacking mental capacity. Engaging in conversations about their situation with them was often challenging and not always possible. It was in the patients’ best interests that timely discussions took place with their relatives to better understand the patients’ preferences. However, relatives were not always available when required, and when they did come in, for example at the weekend, key staff familiar with their circumstances were often not present on the ward. The following ward manager made this clear:
Sometimes the families are not available. So a lot of the time we have some patients who come and their relatives don’t always come or they come in the weekend, or they come in the evening and then there aren’t the people [HCPs] there to have those conversations with them. So, I suppose the challenges on our ward, our patients have confusion or have dementia, and quite often those relatives are not on that same page necessarily . . .
Int1022, female, ward manager
The non-participant observations on the intervention wards corroborate this concern in relation to the unavailability of family members. For example, we witnessed a discussion about a patient’s wife who was receiving chemotherapy, and therefore would not be available to come in that day to have a discussion. In another case, a relative was not fully aware that her mother had dementia, and a significant time would need to be allocated to talk through the dementia diagnosis before it was reasonable to discuss her loved one’s situation of clinical uncertainty.
Communication skills and emotional labour
Focus group participants spoke in detail about communication being central in delivering important information to patients and their families. Communication skills are also critical for understanding patients’ concerns and worries and how they could be resolved. Participants believed that good communication was dependent on the possession of well-honed skills and confidence in having difficult conversations. Some consultants reported that they were experienced in this area, illustrated by the following comment:
It’s our bread and butter and we understand, we are better would you say, not better, better is not the right word but we’re a bit more . . . we can have those conversations.
Int1018, female, consultant
This was reiterated by other medical staff who felt that the intervention was acceptable as they already possessed the skills and knowledge required for the delivery of the AMBER care bundle components, such as the identification of clinical uncertainty and communication:
I think a lot of our . . . at least the medical side of it, a lot of our training has been around medical ethics, communication, breaking bad news, so it’s kind of drilled into us from the get go, I’ll probably say it’s a large part of our training.
Int1013, male, consultant
However, other HCPs, particularly nurses, stated that skills and confidence related to having difficult conversations were not necessarily equally shared. The following ward manager stated:
I think from a nursing point of view, for the junior staff to have some more kind of advanced communication training about you know breaking bad news and having those difficult conversations. Because for a lot of us it comes with experience and I don’t think you were saying in the doctors’ training there’s a lot of focus on it, the nursing training there isn’t.
Int1022, female, ward manager
The emotive context in which discussions regarding clinical uncertainty are required to take place was highlighted as a potential barrier to the delivery of the AMBER care bundle by some junior doctors. A ward manager reiterated this concern, suggesting that there is a need to engage in clinical supervision to support the staff involved in emotive discussions. This concern was challenged by the lack of protected time required to debrief and discuss what had potential to have a negative impact on the staff. The following two quotations illustrate these issues:
Broaching it with the family. It’s quite negative with the patients with uncertainty . . . we have to discuss the end-of-life care, DNACPR [do not attempt cardiopulmonary resuscitation]. So it’s hard to make it a positive thing that we are gonna keep with treatment and then nothing changes with taking care, if that make sense.
Int2023, male, foundation year 1
I mean it is a very emotive subject and you’re at the front of it. You know the relatives can take their frustration out on you uhmm so I think it is important that we all support each other with that and you know, having some kind of clinical supervision would be excellent. Because then as a team, we don’t ever have protected time to have a debrief, or discuss the difficult situations.
Int2022, female, ward manager
Some of the HCPs’ hesitancies around having difficult conversations with patients and their families, and their need for training in advanced communication skills, were also evidenced in the interviews with relatives of patients who had been supported by the AMBER care bundle. For example, during a period of acute deterioration, the husband of a patient recounted how tersely a doctor had spoken to him about his wife’s clinical situation and the consequent escalation decision.
Candidate primary outcome measures
Completion rates for all the patient/proxy responses to the questionnaires, as well as missing item-level data, are summarised at each of the data collection time points. In this section, missing data are classed as a category in their own right, and all percentages are calculated using the total number of participants expected in the relevant population as the denominator (i.e. including participants with missing data for that variable).
Missing outcome measures data
Table 15 presents the level of missing data for the IPOS patient/family anxiety and communication subscale for those participants who continued in the trial from baseline to subsequent time points. The level of missing data for each of the IPOS symptoms, at all three time points, are also presented (see Appendix 9). Notably, the numbers of missing data were relatively small, with very few people choosing ‘I don’t know’, indicating that the participants could report outcomes using the IPOS.
| Trial time point | Item | Completeness, n (%) | |||
|---|---|---|---|---|---|
| Complete | ‘Cannot assess’ | ‘I don’t know’ | Missing | ||
| Baseline (N = 65) | Feeling anxious and worried | 57 (87.7) | 6 (9.2) | 0 (0.0) | 2 (3.1) |
| Friends and family feeling anxious and worried | 65 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Feeling depressed | 60 (92.3) | 5 (7.7) | 0 (0.0) | 0 (0.0) | |
| Feeling at peace | 62 (95.4) | 2 (3.1) | 0 (0.0) | 1 (1.5) | |
| Sharing feelings with family and friends | 61 (93.9) | 3 (4.6) | 0 (0.0) | 1 (1.5) | |
| Being informed | 55 (84.6) | 9 (13.9) | 0 (0.0) | 1 (1.5) | |
| Resolving practical issues | 61 (93.9) | 4 (6.2) | 0 (0.0) | 0 (0.0) | |
| Total | 49 (75.4) | 12 (18.5) | 0 (0.0) | 4 (6.2) | |
| 3–5 days (N = 36) | Feeling anxious and worried | 31 (86.1) | 4 (11.1) | 0 (0.0) | 1 (2.8) |
| Friends and family feeling anxious and worried | 34 (94.4) | 0 (0.0) | 1 (2.8) | 1 (2.8) | |
| Feeling depressed | 32 (88.9) | 2 (5.6) | 0 (0.0) | 2 (5.6) | |
| Feeling at peace | 32 (88.9) | 2 (5.6) | 0 (0.0) | 2 (5.6) | |
| Sharing feelings with family and friends | 32 (88.9) | 2 (5.6) | 1 (2.8) | 1 (2.8) | |
| Being informed | 32 (88.9) | 3 (8.3) | 0 (0.0) | 1 (2.8) | |
| Resolving practical issues | 32 (88.9) | 3 (8.3) | 0 (0.0) | 1 (2.8) | |
| Total | 26 (72.2) | 7 (19.4) | 1 (2.8) | 2 (5.6) | |
| 10–15 days (N = 12) | Feeling anxious and worried | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Friends and family feeling anxious and worried | 11 (91.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) | |
| Feeling depressed | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Feeling at peace | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Sharing feelings with family and friends | 11 (91.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) | |
| Being informed | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Resolving practical issues | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 11 (91.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) | |
Table 16 reports the level of missing data for the howRwe. The howRwe is a patient-only-reported measure of their experience of care and was not supplemented by proxies, such as a relative or close friend. For those who completed this measure, completeness of data could be considered to be very good, with virtually no missing data at all three time points.
| Trial time point | Item | Completeness, n (%) | ||
|---|---|---|---|---|
| Complete | Missing | ‘Not applicable’ | ||
| Baseline (N = 24) | Treating you kindly | 24 (100.0) | 0 (0.0) | 0 (0.0) |
| Listening and explaining | 24 (100.0) | 0 (0.0) | 0 (0.0) | |
| Seeing you promptly | 24 (100.0) | 0 (0.0) | 0 (0.0) | |
| Well organised | 24 (100.0) | 0 (0.0) | 0 (0.0) | |
| Total | 24 (100.0) | 0 (0.0) | 0 (0.0) | |
| 3–5 days (N = 12) | Treating you kindly | 11 (91.7) | 1 (8.3) | 0 (0.0) |
| Listening and explaining | 11 (91.7) | 1 (8.3) | 0 (0.0) | |
| Seeing you promptly | 11 (91.7) | 1 (8.3) | 0 (0.0) | |
| Well organised | 11 (91.7) | 1 (8.3) | 0 (0.0) | |
| Total | 11 (92.7) | 1 (8.3) | 0 (0.0) | |
| 10–15 days (N = 4) | Treating you kindly | 4 (100.0) | 0 (0.0) | 0 (0.0) |
| Listening and explaining | 4 (100.0) | 0 (0.0) | 0 (0.0) | |
| Seeing you promptly | 4 (100.0) | 0 (0.0) | 0 (0.0) | |
| Well organised | 4 (100.0) | 0 (0.0) | 0 (0.0) | |
| Total | 4 (100.0) | 0 (0.0) | 0 (0.0) | |
Missing participant demographic data
Completion rates for all participants’ responses to the outcome measures, as well as missing item-level data, are summarised at each time point. Within this section of the analysis, missing data are classified as a category in their own right and all percentages are calculated using the total number of participants in the relevant population.
Characteristics of participants with missing outcome data
We described the factors with regard to the levels of missing data for the IPOS. In Table 17, we identified that the only meaningful factor associated with missing data was the hospital site. Sites Int1 and Con2 had more missing data at baseline and 3–5 days than the other two sites.
| Characteristics | Time point, missing data | ||
|---|---|---|---|
| Baseline (N = 16) | 3–5 days (N = 10) | 10–15 days (N = 1) | |
| Site, n (%) | |||
| Con1 | 3 (18.8) | 2 (20.0) | 0 (0.0) |
| Int1 | 6 (37.5) | 3 (30.0) | 0 (0.0) |
| Int2 | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Con2 | 6 (37.5) | 5 (50.0) | 1 (100.0) |
| Gender (male), n (%) | 8 (50.0) | 6 (60.0) | 1 (100.0) |
| Age (years), mean (SD) | 78.3 (14.5) | 77.7 (13.4) | 78.0a |
| Disease (non-cancer), n (%) | 10 (62.5) | 8 (80.0) | 1 (100.0) |
| Education, n (%) | |||
| Did not go to school | 2 (12.5) | 1 (10.0) | 0 (0.0) |
| Secondary school (GCSE/O level) | 6 (37.5) | 3 (30.0) | 0 (0.0) |
| Secondary school (A level) | 2 (12.5) | 3 (30.0) | 1 (100.0) |
| Vocational qualification | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| University | 3 (18.8) | 2 (20.0) | 0 (0.0) |
| Prefer not to say | 2 (12.5) | 1 (10.0) | 0 (0.0) |
| Marital status, n (%) | |||
| Single | 2 (12.5) | 2 (20.0) | 0 (0.0) |
| Widowed | 7 (43.8) | 3 (30.0) | 0 (0.0) |
| Married/civil partner/long-term relationship | 7 (43.8) | 4 (40.0) | 0 (0.0) |
| Divorced | 0 (0.0) | 1 (10.0) | 1 (100.0) |
| Ethnicity, n (%) | |||
| White British | 11 (68.8) | 7 (70.0) | 1 (100.0) |
| Other mixed | 1 (6.3) | 1 (10.0) | 0 (0.0) |
| Indian | 2 (12.5) | 2 (20.0) | 0 (0.0) |
| Other Asian | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Other black | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Income, n (%) | |||
| Living comfortably at present | 9 (56.3) | 4 (40.0) | 0 (0.0) |
| Coping on present income | 1 (6.3) | 3 (30.0) | 1 (100.0) |
| Difficult on present income | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Prefer not to say | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Do not know | 2 (12.5) | 2 (20.0) | 0 (0.0) |
| Missing | 2 (12.5) | 1 (10.0) | 0 (0.0) |
Descriptive analyses of primary outcome measures
Exploratory analysis of patient primary outcomes for the patient/family anxiety and communication subscale of the IPOS and the howRwe are reported in Table 18 as descriptive statistics (means and 95% CIs) for each of the measures at each of the three time points. We also provide the descriptive statistics for the patient/family anxiety and communication subscale of the IPOS and the howRwe by trial arm (see Appendix 10). Table 18 presents the descriptive analysis of the two measures for those participants with data at each of the time points.
| Participant-reported outcome | Time point, mean score (95% CI) | ||
|---|---|---|---|
| Baseline | 3–5 days | 10–15 days | |
| IPOS patient/family anxiety and communication subscalea | 13.1 (11.9 to 14.2) (n = 50) | 13.8 (12.3 to 15.4) (n = 26) | 13.2 (10.1 to 16.3) (n = 11) |
| howRwe b | 12.9 (11.8 to 14.0) (n = 23) | 13.5 (11.9 to 15.0) (n = 11) | 13.3 (9.7 to 16.8) (n = 4) |
This feasibility trial was not intended to be powered to detect differences in outcome measure scores, only to understand to what extent the measures were able to identify to what extent the support provided by the AMBER care bundle to patients whose situations were clinically unstable differed to best standard care. In this section, we present tables comparing the IPOS patient/family anxiety and communication subscale and the howRwe scores across the two trial arms. Subsequent tables examine each of the measures in more detail to explore their ability to capture data across their respective domains of interest.
The mean IPOS patient/family anxiety and communication subscale score at baseline was 13.1 (i.e. within the moderate range). This remained fairly consistent across time points (mean score of 13.8 at 3–5 days and 13.2 at 10–15 days). Although the howRwe scores changed across time points, it is not possible to speculate whether or not this change is clinically significant. Furthermore, owing to a high rate of attrition between time points, it is not possible to know if the patients who were lost to follow-up would show a change in their scores based on the communication and care received from the HCPs and their levels of anxiety.
Focusing on participants with data available at baseline and 3–5 days (n = 24), scores on the IPOS patient/family anxiety and communication subscale slightly increased, indicating poorer outcomes for patients (Table 19). Scores on the howRwe (n = 10) suggested a slight improvement in patients’ care. The discrepancy in the direction of change for these outcome measures may be explained by a large number of relatives completing the IPOS patient/family anxiety and communication subscale, in contrast to the patient self-reported howRwe measure.
| Participant-reported outcomes | Time point, mean score (95% CI) | ||
|---|---|---|---|
| Baseline | 3–5 days | 10–15 days | |
| IPOS patient/family anxiety and communication subscale (n = 24) | 13.3 (11.3 to 15.4) | 14.0 (12.3 to 15.6) | 12.8 (9.4 to 16.2)a |
| howRwe (n = 10) | 12.8 (11.1 to 14.5) | 13.5 (11.8 to 15.2) | 13.3 (9.7 to 16.8)b |
Interviews with patients and relatives who were supported by the AMBER care bundle provided contextual information about the overall experience of participants. Although some participants reported receiving excellent communication from the clinical team, others highlighted the overall complexity of the situation, and their anxieties around discharge from hospital, which could explain the slight increase in the IPOS patient/family anxiety and communication subscale during participants’ hospital stays. Below, the quotation from a relative of a patient who received care at site Int1 provides support for how proxy-completed outcomes might have had an influence in the increase in IPOS patient/family anxiety and communication subscale scores:
We were very impressed, the doctors worked well, they, yes, they spent a lot of time, in fact, in a way, erm, you could say too much time, because I think they were concerned that my father would be upset, so they had sort of prepared themselves for a very difficult meeting, interview, discussion, and my father said ‘oh well that’s fine’ he sort of said ‘well I’m not surprised, I’ve had my time, it doesn’t bother me whether it’s 3 months, 6 months’ and the doctor sort of said ‘look we need, we are going to try and make your last few months as comfortable as possible so, you know’ and he was, he was fine, I, for me it is a bit of a shock, when you consider he’s been there for all of my life.
Int1001, carer
In the following quotation, another relative of a patient who received care at site Int1 praised the care provided while expressing her concerns around discharge:
My anxiety levels absolutely rocketed because I just wanted him to stay comfortable in hospital where the care was fantastic, but as they said, and I’d been reassured, well it had been reaffirmed to me from people I know that no the hospital won’t keep you in if you’re stable.
Int1005, carer
The comparison of primary outcome scores at baseline across trial arms showed that we were able to recruit similar patient populations (Table 20). Both groups scored at a relatively moderate range on the IPOS patient/family anxiety and communication subscale. It is not possible to make valid interpretations about the changes in the scores of the howRwe measure because only two participants completed this measure in the intervention arm.
| Primary outcome measures | Time point, mean (95% CI) | ||
|---|---|---|---|
| Baseline | 3–5 days | 10–15 days | |
| IPOS patient/family anxiety and communication subscale (n = 24) | |||
| Control (n = 12) | 13.3 (10.3 to 16.4) | 13.3 (10.9 to 15.8) | 10.3 (7.5 to 13.2)a |
| Intervention (n = 12) | 13.3 (10.1 to 16.6) | 14.6 (12.0 to 17.2) | 13.9 (8.9 to 18.8)b |
| howRwe (n = 10) | |||
| Control (n = 8) | 13.1 (11.1 to 15.2) | 13.9 (11.8 to 16.0) | 14 (9.0 to 19.0)c |
| Intervention (n = 2) | 11.5 (N/A) | 12.0 (N/A) | 11 (N/A)d |
In Table 21, the scores of each howRwe item are presented by trial arm. In both trial arms, participants rated being treated kindly by the HCPs as ‘good’ to ‘excellent’, whereas being organised was perceived as less satisfactory. The small number of patients who had capacity to complete this self-reported outcome measure limits our ability to interpret scores over time.
| Item | Trial arm, mean score (95% CI) | |
|---|---|---|
| Control | Intervention | |
| Baseline (control, n = 17; intervention, n = 7) | ||
| Treating you kindly | 3.7 (3.5 to 3.9) | 3.7 (3.3 to 4.2) |
| Listening and explaining | 3.4 (3.0 to 3.8) | 3.4 (2.9 to 3.9) |
| Seeing you promptly | 2.9 (2.4 to 3.4) | 3.1 (2.3 to 4.0) |
| Well organised | 2.8 (2.3 to 3.4) | 3.1 (2.3 to 4.0) |
| 3–5 days (control, n = 8; intervention, n = 3) | ||
| Treating you kindly | 3.8 (3.4 to 4.1) | 3.3 (1.9 to 4.8) |
| Listening and explaining | 3.5 (2.9 to 4.1) | 3.0 (N/A) |
| Seeing you promptly | 3.3 (2.5 to 4.0) | 3.0 (N/A) |
| Well organised | 3.4 (2.8 to 4.0) | 3.0 (N/A) |
| 10–15 days (control, n = 3; intervention, n = 1) | ||
| Treating you kindly | 3.7 (2.2 to 5.1) | 3.0 (N/A) |
| Listening and explaining | 3.7 (2.2 to 5.1) | 4.0 (N/A) |
| Seeing you promptly | 3.3 (1.9 to 4.8) | 2.0 (N/A) |
| Well organised | 3.3 (1.9 to 4.8) | 2.0 (N/A) |
Although findings from howRwe indicate ‘good’ to ‘excellent’ experience outcomes, and qualitative data corroborating these findings were recorded, contradictory instances were also obtained from the interviews with patients and relatives. On some occasions, patients and relatives from both intervention sites and control sites felt dismissed and not listened to. The following quotations from a patient participant who was cared for at site Con2 and from a relative of a patient who received care at site Int2 illustrate instances of poor hospital experience:
They just didn’t acknowledge me at all and by that time I had had it, I had it from 6 in the morning from these two, I’m thinking I gotta, and I let rip, I was like ‘Would you two effin’ listen to me, I’ve had enough of this since I got here at 6 o’clock you’ve been nothing but rude, you’re ignoring not only me but all the other patients that really need help, that can’t stick up for themselves, that can’t ask for things themselves’ and they still wouldn’t answer.
Con2002
You know, if you’re saying something, I find this very much with the NHS, that they don’t listen. To them you’re a layman, you don’t know what you’re talking about. You know, uhmm they know how to treat, they have the training, they know it but they don’t listen to the relatives. The relatives do know that patient and what they might want or they might not want. What they need.
Int1003, carer
In Table 22, the scores of each IPOS patient/family anxiety and communication subscale item are presented by trial arm. When asked about how the patient participant was feeling over the previous 3 days, scores indicated that the concerns they selected for each item were experienced either ‘occasionally’ or ‘sometimes’, with the exception of friends and family experiencing ‘anxiety and worries’ ‘most of the time’. Baseline scores indicate that participants in both trial arms experienced some issues that had potential to be addressed by the intervention. With the exception of slight differences across time points, scores remained relatively stable for each arm. The trial data do not enable us to draw conclusions regarding the measures’ responsiveness to capture the impact of the intervention. Friends’ and families’ ‘anxiety and worry’ remained relatively high; this was consistent across both arms.
| Item | Trial arm, mean score (95% CI) | |
|---|---|---|
| Control | Intervention | |
| Baseline | ||
| Feeling anxious and worried | 2.2 (1.5 to 2.8) (n = 32) | 1.8 (1.3 to 2.3) (n = 25) |
| Friends and family feeling anxious and worried | 3.3 (3.0 to 3.7) (n = 36) | 3.1 (2.7 to 3.5) (n = 29) |
| Feeling depressed | 1.6 (1.0 to 2.1) (n = 35) | 1.7 (1.2 to 2.2) (n = 25) |
| Feeling at peace | 1.7 (1.3 to 2.2) (n = 33) | 2.3 (1.8 to 2.8) (n = 29) |
| Sharing feelings with family and friends | 1.6 (1.0 to 2.1) (n = 33) | 1.7 (1.2 to 2.2) (n = 28) |
| Being informed | 1.6 (1.0 to 2.1) (n = 32) | 1.9 (1.4 to 2.4) (n = 23) |
| Resolving practical issues | 1.8 (1.2 to 2.3) (n = 33) | 1.9 (1.2 to 2.5) (n = 28) |
| 3–5 days | ||
| Feeling anxious and worried | 2.1 (1.3 to 2.9) (n = 18) | 1.8 (1.07 to 2.6) (n = 13) |
| Friends and family feeling anxious and worried | 3.4 (3.0 to 3.9) (n = 18) | 2.8 (2.1 to 3.4) (n = 16) |
| Feeling depressed | 1.5 (0.8 to 2.2) (n = 17) | 1.7 (0.8 to 2.5) (n = 15) |
| Feeling at peace | 2.4 (1.6 to 3.2) (n = 16) | 2.4 (1.8 to 3.0) (n = 16) |
| Sharing feelings with family and friends | 1.4 (0.6 to 2.2) (n = 17) | 2.3 (1.5 to 3.1) (n = 15) |
| Being informed | 1.4 (0.7 to 2.1) (n = 19) | 2.0 (1.1 to 2.9) (n = 13) |
| Resolving practical issues | 1.8 (0.9 to 2.7) (n = 16) | 1.9 (1.1 to 2.7) (n = 16) |
| 10–15 days | ||
| Feeling anxious and worried | 1.6 (0.2 to 3.0) (n = 5) | 1.9 (0.7 to 3.0) (n = 7) |
| Friends and family feeling anxious and worried | 3.5 (2.6 to 4.4) (n = 4) | 3.1 (2.3 to 4.0) (n = 7) |
| Feeling depressed | 1.0 (–0.2 to 2.2) (n = 5) | 1.7 (0.6 to 2.9) (n = 7) |
| Feeling at peace | 2.2 (–0.1 to 4.4) (n = 5) | 2.1 (0.9 to 3.4) (n = 7) |
| Sharing feelings with family and friends | 1.8 (–1.5 to 5.0) (n = 4) | 2.0 (1.2 to 2.8) (n = 7) |
| Being informed | 1.0 (–0.5 to 2.5) (n = 5) | 1.6 (0.7 to 2.5) (n = 7) |
| Resolving practical issues | 2.4 (–0.3 to 5.1) (n = 5) | 1.4 (0.4 to 2.5) (n = 7) |
Although participants reported scores about patients’ concerns as experiences ‘occasionally’ or ‘sometimes’, several interviews with participants indicate acceptance of their clinical situation. For instance, when asked about how they were feeling, patients from site Con2 who were interviewed during their hospital stay replied with short answers:
I’m alright, I’m in good care. Fine . . . none . . . no complaints.
Con2021
Yeah I’m OK. What can you do? What will happen, will happen.
Con2024
Detailed descriptive analysis of each of the IPOS symptoms identifies that ‘weakness or lack of energy’, ‘pain’ and ‘poor appetite’ were perceived to be concerns for participants in both arms of the trial (see Appendix 8).
Preliminary effectiveness of the AMBER care bundle
Based on data available from our outcome measures, we cannot draw conclusions about differences between arms. Despite this, our outcome measures indicate that patients experienced moderate levels of anxiety and worry, as expected with this population. In comparison, the perceived experiences of anxiety and worry for family members were consistently higher during the hospital stay for both trial arms.
Choosing the primary outcome measure for a definitive trial
The primary aim of a definitive trial would be to observe a clinically important difference in the outcome and the experience of care between (1) those supported by the AMBER care bundle and (2) those supported by standard care. We selected two ‘candidate’ primary outcomes to be evaluated for a future definitive trial: the patient/family anxiety and communication subscale of the IPOS55 and the patient-reported experience measure, the howRwe. 63 The IPOS patient/family anxiety and communication subscale, although not powered to detect differences in this feasibility trial, showed variance and change over time, which indicates that it will probably detect the differences required as the primary outcome measure in a definitive trial. The completeness of the data and the acceptability of the howRwe measure were good; however, this is a patient-only-reported measure, which reduces the utility of the tool. Further exploration is needed to determine whether or not proxy data collection is feasible for this measure. The primary end point of the trial was at time point 1 (3–5 days), at which 44.6% of participants were lost to follow-up.
During the focus groups, HCPs also shared their views on the candidate primary outcome measures. When asked whether or not the proposed measures could capture the intended benefits of the AMBER care bundle on patient outcomes, a research nurse raised an important point about the potential bias due to involvement of hospital staff in data collection. The following discussion illustrates views on the IPOS patient/family anxiety and communication subscale and howRwe:
I think the howRwe will give you better feedback about communication. It’s difficult though. ‘Are we treating you well?’ Well I’m a health-care professional sat in front of them. That’s kind of . . . that’s question is a little bit loaded, do you know what I mean? You know, when somebody stood there, kind of servicing your car ‘Are you happy with our service?’. Well you got my car or do you know what I mean, my dog in your hands. ‘Yeah, yeah I’m happy.’ That question, I don’t know . . . A bit biased because I’m sat there asking this question.
And the IPOS bits? Do you think those might be things that move in a different direction as a result of AMBER?
No. Because of I think the underlying anxiety from their condition. This is just another admission in a series of admissions. This isn’t, you know our communication in one admission isn’t going to change their anxiety and the fact that this is their third admission in a year, they’re struggling to breathe more and more, how’s gonna take care . . . you know several of our patients are carers for their husbands or wives with dementia. Who’s gonna look after them? Who is gonna look after the dog? I’m supposed to be doing . . . I’m gonna miss my daughter’s wedding. These are . . . AMBER care isn’t gonna make to that scoring. It’s overall gonna give a better satisfaction of the care they’re receiving on this ward, but to their overall well-being I would give it a 3% importance. The other 95–97% of their concerns is about everything else that is going on. This is what I got from the patients.
Another research nurse commented on the potential utility of collecting data regarding patients’ symptoms and concerns (referring to IPOS):
They, OK if they completed the patient outcome measures, they are more conscious, like if they’re in pain, they can actually justify that, how much is the pain. It’s more about their consciousness.
OK, so being able to articulate ‘How much pain I’m in?’ because we’re asking them to tick.
Yeah and their concerns when here.
Sample size
The screening and recruitment data, the attrition rate, the completeness of outcome measures and the size and context of each cluster obtained in this trial will provide useful information to guide future sample size calculations. We were not able to calculate the intracluster correlation coefficient because of the insufficient number of clusters.
Acceptability of trial length and time points
The screening to recruitment rate and time needed to inform and consent potential participants showed that a 3-month recruitment period was not adequate. We found out that the majority of the potential participants lacked capacity and required availability of a proxy to participate in the trial. In a future trial, more time should be allocated to familiarising the local research and ward staff about the trial as the number of recruited participants per month increased once the trial was embedded and queries about the trial procedures were addressed. Data collection at 10–15 days (time point 2) was not feasible owing to high attrition (81.5% of participants). A suggestion to overcome loss to follow-up due to discharge may be to recruit participants earlier (i.e. closer to their hospital admission). By recruiting the participants earlier during their hospital stay, data collection at 10–15 days may be feasible and the outcome measures may be able to detect changes over time.
Descriptive analysis of views on being involved in the trial
Participants with sufficient mental capacity were asked to provide their views on being involved in the trial. The findings in Table 23 identify that no patient participant regarded their involvement in the trial as being negative. Only one participant in the control arm stated that they were not happy to complete this questionnaire (no further reason provided). Free-text comments highlighted that participants were happy to take part in the trial. Specifically, some stated that this was due to the positive interaction with the research nurses. This is typified by the following free-text comment: ‘the research coordinator is very polite and explained everything about the study’ (Con2014). Some stated that it was due to their sense of altruism, believing that their involvement would help others and improve services, illustrated by the following free-text comment: ‘If you can help others, then it’s worth doing’ (Int2007). A number of participants also recommended that other patients take part in the trial, reiterating that their involvement would ‘help others’. The views of proxies’ (i.e. personal consultees of patients who lacked capacity) participation in the trial were not within the scope of the trial, as a patient-reported measure was used to capture this data.
| View | Experience of being involved in the trial, n (%) | Would recommended the trial to other patients, n (%) | ||||
|---|---|---|---|---|---|---|
| Control | Intervention | Total | Control | Intervention | Total | |
| Baseline | N = 17 | N = 6 | N = 23 | N = 17 | N = 6 | N = 23 |
| Positive | 7 (41.2) | 3 (50.0) | 10 (43.5) | 9 (52.9) | 4 (66.7) | 13 (56.5) |
| Neutral | 5 (29.4) | 3 (50.0) | 8 (37.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Don’t know | 2 (11.8) | 0 (0.0) | 2 (8.7) | 5 (29.4) | 1 (16.6) | 6 (26.1) |
| Missing | 3 (17.6) | 0 (0.0) | 3 (13.0) | 3 (17.6) | 1 (16.6) | 4 (17.4) |
| 3–5 days | N = 8 | N = 3 | N = 11 | N = 8 | N = 3 | N = 11 |
| Positive | 1 (12.5) | 2 (66.6) | 3 (27.3) | 4 (50.0) | 3 (100.0) | 7 (63.6) |
| Neutral | 3 (37.5) | 1 (33.3) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Don’t know | 2 (25.0) | 0 (0.0) | 2 (18.2) | 2 (25.0) | 0 (0.0) | 2 (18.2) |
| Missing | 2 (25.0) | 0 (0.0) | 2 (18.2) | 2 (25.0) | 0 (0.0) | 2 (18.2) |
| 10–15 days | N = 4 | N = 0 | N = 4 | N = 4 | N = 0 | N = 4 |
| Positive | 2 (50.0) | N/A | 2 (50.0) | 3 (75.0) | N/A | 3 (75.0) |
| Neutral | 1 (25.0) | N/A | 1 (25.0) | 0 (0.0) | N/A | 0 (0.0) |
| Negative | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | N/A | 0 (0.0) |
| Don’t know | 1 (25.0) | N/A | 1 (25.0) | 1 (25.0) | N/A | 1 (25.0) |
| Missing | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | N/A | 0 (0.0) |
Health economics and cost-effectiveness of the intervention
The objective of the health economics component of the trial was to examine differences in the use of financial resources between the intervention and standard care arms. An auxiliary objective was to explore the feasibility of conducting a cost-effectiveness analysis of the AMBER care bundle.
Data on resource use were collected using the CSRI,71,72 which measured the use of health care, social care and informal care 3 months prior to the hospital admission, at the baseline interview and during the inpatient stay in hospital at 10–15 days. The EQ-5D-5L73 was used to measure health-related quality of life both at baseline and at the follow-up interviews.
The descriptive statistics of service use showed that use was within plausible ranges; for example, length of stay in hospital did not exceed 3 months (Table 24). Patients interviewed at 10–15 days reported the use of investigations/tests and the informal care provided, but health services were not used.
| Service use | Trial arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||||
| N | Users | Use | N | Users | Use | |||||
| n | % | Mean | SD | n | % | Mean | SD | |||
| Overnight stay | ||||||||||
| Intensive care unit | 36 | 6 | 17.0 | 4.2 | 5.4 | 29 | 0 | 0.0 | N/A | N/A |
| Inpatient ward | 36 | 22 | 61.0 | 10.8 | 10.2 | 29 | 15 | 52.0 | 22.3 | 25.9 |
| Hospice | 36 | 3 | 8.0 | 60.0 | N/A | 29 | 0 | 0.0 | N/A | N/A |
| Nursing home | 36 | 2 | 6.0 | 3.0 | N/A | 29 | 1 | 3.0 | 76.0 | N/A |
| Residential home | 36 | 2 | 6.0 | 69.5 | 13.4 | 29 | 4 | 14.0 | 55.0 | 29.8 |
| A&E | 36 | 15 | 42.0 | 1.9 | 1.1 | 29 | 12 | 41.0 | 1.8 | 1.7 |
| Emergency ambulance | 36 | 15 | 42.0 | 1.9 | 1.3 | 29 | 13 | 45.0 | 1.5 | 0.7 |
| Outpatient | ||||||||||
| Palliative care | 36 | 4 | 11.0 | 2.0 | 0.8 | 29 | 1 | 3.0 | 1.0 | N/A |
| Radiotherapy | 36 | 7 | 19.0 | 2.0 | 1.2 | 29 | 0 | 0.0 | N/A | N/A |
| Oncology clinic | 36 | 13 | 36.0 | 2.5 | 1.5 | 29 | 1 | 3.0 | 2.0 | N/A |
| Other appointment | 36 | 12 | 33.0 | 2.5 | 1.4 | 29 | 6 | 21.0 | 2.0 | 1.3 |
| Hospital transport ambulance | 36 | 2 | 6.0 | 12.5 | 16.3 | 29 | 4 | 14.0 | 7.3 | 5.6 |
| GP face to face | 36 | 28 | 78.0 | 2.7 | 1.6 | 29 | 25 | 86.0 | 3.4 | 2.6 |
| GP on the telephone | 36 | 24 | 67.0 | 2.9 | 1.2 | 29 | 17 | 59.0 | 2.6 | 2.5 |
| Nurse | ||||||||||
| Marie Curie (London, UK) | 36 | 4 | 11.0 | 1.3 | 0.5 | 29 | 1 | 3.0 | 2.0 | N/A |
| Macmillan Cancer Support (London, UK) or palliative care | 36 | 9 | 25.0 | 3.4 | 2.3 | 29 | 1 | 3.0 | 1.0 | N/A |
| Other | 36 | 4 | 11.0 | 1.5 | 0.7 | 29 | 3 | 10.0 | 1.0 | 0.0 |
| Palliative care or ‘hospice at home’ team | 36 | 7 | 19.0 | 3.0 | 2.6 | 29 | 0 | 0.0 | N/A | N/A |
| Physiotherapist | 36 | 8 | 22.0 | 2.4 | 0.9 | 29 | 7 | 24.0 | 2.5 | 1.9 |
| Occupational therapist | 36 | 6 | 17.0 | 2.0 | 1.1 | 29 | 6 | 21.0 | 2.4 | 1.5 |
| Psychiatrist | 36 | 0 | 0.0 | N/A | N/A | 29 | 0 | 0.0 | N/A | N/A |
| Psychologist or counsellor | 36 | 3 | 8.0 | 1.7 | 1.2 | 29 | 0 | 0.0 | N/A | N/A |
| Spiritual care person | 36 | 0 | 0.0 | N/A | N/A | 29 | 3 | 10.0 | 6.3 | 3.8 |
| Social worker | 36 | 5 | 14.0 | 3.5 | 4.4 | 29 | 0 | 0.0 | N/A | N/A |
| Paid formal carer | 36 | 4 | 11.0 | 90.0 | 0.0 | 29 | 13 | 45.0 | 20.7 | 24.0 |
| Dietitian | 36 | 9 | 25.0 | 1.8 | 0.7 | 29 | 4 | 14.0 | 3.0 | 2.6 |
| Voluntary service | 36 | 1 | 3.0 | 0.0 | N/A | 29 | 0 | 0.0 | N/A | N/A |
| Other professionals | 36 | 4 | 11.0 | 1.0 | 0.0 | 29 | 2 | 7.0 | 35.5 | 48.8 |
| Investigation/diagnostic tests | ||||||||||
| Blood test | 36 | 35 | 97.0 | 13.8 | 8.7 | 29 | 18 | 62.0 | 5.6 | 6.5 |
| X-ray | 36 | 28 | 78.0 | 3.3 | 3.8 | 29 | 13 | 45.0 | 2.7 | 1.1 |
| Echocardiogram | 36 | 9 | 25.0 | 1.5 | 0.5 | 29 | 5 | 17.0 | 1.0 | 0.0 |
| Electrocardiogram | 36 | 20 | 56.0 | 1.9 | 1.0 | 29 | 10 | 34.0 | 1.2 | 0.4 |
| Ultrasound | 36 | 17 | 47.0 | 1.5 | 0.8 | 29 | 3 | 10.0 | 1.5 | 0.7 |
| CT/CAT scan | 36 | 27 | 75.0 | 1.8 | 1.0 | 29 | 7 | 24.0 | 1.2 | 0.4 |
| Magnetic resonance image | 36 | 13 | 36.0 | 1.6 | 0.9 | 29 | 1 | 3.0 | 2.0 | N/A |
| Other | 36 | 17 | 47.0 | 4.3 | 6.0 | 29 | 7 | 24.0 | 1.2 | 0.4 |
| Informal care (hours) | ||||||||||
| Personal care | 36 | 20 | 56.0 | 30.9 | 44.8 | 29 | 15 | 52.0 | 16.3 | 29.6 |
| Help with medical procedures | 36 | 18 | 50.0 | 8.4 | 5.6 | 29 | 12 | 41.0 | 5.5 | 9.2 |
| Help inside the home | 36 | 24 | 67.0 | 6.6 | 4.3 | 29 | 17 | 59.0 | 6.5 | 4.2 |
| Help outside the home | 36 | 25 | 69.0 | 8.3 | 6.9 | 29 | 17 | 59.0 | 2.3 | 1.4 |
| Time spent ‘on call’ | 36 | 13 | 36.0 | 26.5 | 51.7 | 29 | 11 | 38.0 | 48.2 | 68.6 |
| Other | 36 | 4 | 11.0 | 4.7 | 2.5 | 29 | 4 | 14.0 | 7.3 | 9.5 |
| EQ-5D index score | 33 | N/A | N/A | 0.00 | 0.33 | 28 | N/A | N/A | –0.08 | 0.14 |
Responses to the five items in the EQ-5D-5L were used to generate the index score for each patient and each time point. Theoretically, the index score ranges from 0 (death) to 1 (full health), but some EQ-5D-5L profiles are evaluated as below zero, implying that the individual considers her or his current quality of life as worse than death.
Missing data
For health and social care service use variables, < 5.0% of data were missing at baseline and 100% of data were missing at follow-up, except for the investigations/tests, which means that nobody reported health and social service use between baseline and 10–15 days. Less than 9.0% of data for informal care items were missing at baseline and 0% were missing at follow-up. Three out of 33 participants in the control arm and 1 out of 29 participants in the intervention arm did not complete all five items of the EQ-5D-5L at baseline. Three out of five remaining participants in the control arm and all (n = 7) remaining participants in the intervention arm completed all five items at follow-up.
Intervention costs
Implementation costs, which form part of the intervention costs, could be obtained from the diary kept by the nurse facilitator (NHS band 7), who worked for 6 months preparing and implementing the intervention. The nurse facilitator recorded details of her work (e.g. preparation of materials for staff at the intervention site, travel, meetings with staff, communication with the research team and writing case notes). Senior management was provided by an associate with expertise in quality improvement and was focused in the earlier months of the nurse facilitator’s work. This diary also recorded each of the meetings held at the intervention sites, for example the duration of meetings, the types and grades of participants at the meetings and a short description of the discussion at the meeting.
Resource use in participating sites was extracted from the nurse facilitator’s diary (see Appendix 11). The nurse facilitator had meetings with individual HCPs or joined the regular MDT meetings. Individualised meetings were arranged depending on the availability of HCPs and their needs for implementing the AMBER care bundle. At the introduction stage, the AMBER care bundle meetings were devoted to teaching, case review, discussion and evaluation, but as the bundle was embedded, it was discussed at the MDT meetings as part of the routine activities.
Sites Int1 and Int2 had differences in the types of HCPs and time spent on the AMBER care bundle‘s implementation, which may reflect differences in characteristics of each hospital, such as staffing, care provision, types of patients and catchment population. Many activities in site Int1 happened during the MDT meetings, and HCPs other than doctors and nurses (health-care assistants, clinical social workers, physiotherapists and occupational therapists) were involved in care provision. Implementing the intervention in site Int2 necessitated more individualised contacts with HCPs in addition to the MDT meetings.
Summary
It was feasible to collect the data on health and social care service use, informal care provision and quality of life at baseline and at 10–15 days (see Appendix 12). Missing values in the data were not problematic (< 9.0%). However, health service use at follow-up could be replaced by exploring patients’ medical records assuming that all patients stay in wards. Costs associated with care service use would then be obtained using unit costs for each service item and opportunity costs (e.g. minimum salaries).
Implementation costs are only part of the real intervention costs because changes in time and effort from HCPs cannot be accurately captured. Thus, implementation costs can be considered as the minimum of the actual intervention costs that we could measure. A diary recorded by the nurse facilitator in the central research team successfully tracked the resource use in intervention sites. A predetermined format of the diary could be developed with the prior information on the participating sites in future studies. Implementation costs would be calculated by combining HCPs’ time and salaries in addition to the nurse facilitator’s salary and supervision during the trial period.
Adverse or unforeseen events
Throughout the trial, we were mindful to identify and record any adverse and unforeseen events associated with the trial or associated with the delivery of the AMBER care bundle. All research nurses were provided with a distress protocol.
Research-related events
In two instances, individuals were identified as being distressed as a direct result of the trial. In the first case, a relative was distressed about the wording of the participant information sheet because she was not adequately aware of her relative’s current clinical condition. Consequently, the research nurse and the principal investigator of the trial were required to have a conversation to sensitively inform her of the situation, and the issue was subsequently resolved. We also simplified the language on the information sheets following this incident. In the second case, a relative of a bereaved participant informed us that she was distressed about an administrative error in judging the postage of the bereavement survey. The respondent was required to go to the Post Office sorting office to retrieve the letter and pay the cost of the inadequate postage. As a result, conversations took place between the chief investigator and principal investigators at each of the trial sites to remind them, and their research staff, to check the cost of posting bereavement surveys at each trial site. Subsequently, no errors were reported.
Notably, because the inclusion criteria focused on identifying potential participants who had a clinically uncertain recovery with a risk of dying during their episode of care despite treatment, we made a joint decision with our TS DMEC that ‘death of participants’ should not be recorded as an ‘adverse event’ during the course of the trial. Instead, death in this population is a possible outcome. We did not prespecify events such as emergency readmissions as potential adverse events of the trial or intervention. Emergency attendances are increasing at the end of life,85 hence emergency attendances were also expected in our sample. Nevertheless, we extracted information regarding non-elective readmissions within 30 days discharge for patients who were recruited and discharged from the trial ward. Six participants (16.7%) in the control arm (site Con1, n = 3; site Con2, n = 3) and five participants (17.2%) in the intervention arm (site Int1, n = 3; site Int2, n = 2) were readmitted within 30 days of their discharge.
Intervention-related events
We also observed instances in which the AMBER care bundle might have been causing additional distress to patients, relatives and HCPs. Although no one in our sample explicitly stated that they had been distressed as a direct result of being involved in the intervention, our data raised a number of issues.
For patients and relatives, the main concerns involved the use of confusing terminology and communication. Instances when patients and relatives were confused about the term ‘AMBER’ and what it means in the context of their care were identified. During the implementation of the AMBER care bundle, HCPs were advised not to mention the term ‘AMBER’ to patients and families. However, this instruction regarding not mentioning the AMBER care bundle, and keeping this term within the team as jargon, was not implemented at the intervention sites. However, even when HCPs did not mention the term ‘AMBER’ during their interactions with the patients and families, the AMBER care bundle stickers were placed on patient notes, and the letter ‘A’ positioned next to the patients’ names on the white boards could have been seen by the patients and/or their relatives, which would evoke further questions. Use of unfamiliar terminology might not always necessarily lead to distress if it is explained to the patients and families. However, unanswered questions have the potential to lead to confusion and false assumptions about what the intervention might entail.
Patients and relatives also mentioned instances of poor communication, for example not being informed about their relative’s discharge and not being listened to when they volunteered information regarding their relative’s other health conditions. As instances of poor communication were reported in both arms of the trial, it is difficult to ascertain whether or not the AMBER care bundle was directly responsible for these experiences. The quotations from a junior doctor and a relative of a patient who was supported by the AMBER care bundle from the same trial site provide examples of poor communication:
We’d keep the family informed, but I think now it’s a bit confusing for the families like ‘Oh what is this AMBER bundle? Is my relative dying?’. And they just get overwhelmed.
Int2017, female, senior house officer
But I thought that could’ve been handled a lot better. Especially when he [referring to a doctor] glanced over her and said ‘Well it doesn’t look good, does it?’. And I thought ‘What are you actually saying then? You’re saying that’s it?’ and he said ‘Anyway, I’ll give you a bit of time.’.
Int2008, carer
For HCPs, our main concern was regarding inadequate communication skills, which could subsequently affect patients and relatives, and the personal impact of having difficult conversations and managing patient and relative expectations. The AMBER care bundle encourages HCPs to open up conversations about clinical uncertainty and the end of life, and includes training at the implementation phase, with continuous support from the nurse facilitator. However, some team members might have been engaging in the complex conversations that they were not ready for. Having difficult conversations requires advanced communication skills, which might not be guaranteed to be acquired by every HCP through the training provided during the AMBER care bundle implementation. In addition, HCPs, including a consultant, alluded to distress caused to themselves as a result of not being able to match patients’ and relatives’ expectations, especially when talking about future care preferences:
It was a patient that I had that discussion with but then I felt that I wouldn’t, I wanted him to make this important . . . that he aired what his PPD was but uhmm in a way I felt bad because I couldn’t give him what he wanted. I think with some of patients the PPD is home, but in reality, there’s no way we’ll be able to support them at home or the palliative care would be able to support them at home or social services or fast track. So . . . about sort of giving the patients the impression that we can give them what they want but not them not being able to give them what they want. Disappoint them.
Int1013, male, consultant
Although these were not unexpected instances, they highlight the need for a greater emphasis to be placed on acquiring communication skills.
Assessing contamination at the control sites
When designing the trial, we were concerned that there might be a transfer of information about the AMBER care bundle to the ward staff at the control sites as a result of staff moving. A cluster RCT design was specifically chosen to mitigate this. Despite this, we were not able to systematically measure contamination of AMBER-related knowledge.
Although site Con1 experienced frequent turnover of medical staff, to the best of our knowledge no HCPs were familiar with the AMBER care bundle. Moreover, no HCPs were practising similar interventions. At site Con2, we learned that one of the registrars was well informed about the intervention and had been applying some of the principles of the intervention in his practice. Furthermore, during the focus group conducted at site Con2, participants stated that merely thinking about clinical uncertainty had an effect on their clinical practice. HCPs mentioned that the trial provided them with a platform to broach difficult topics such as clinical uncertainty and ACP with the patients and families. The findings from the focus groups provide important information to understand this situation. For example, a registrar explained:
I mean, I’ve learned a lot from my seniors and especially being involved in the ImproveCare study, I think it made it much more comfortable for me to go for these discussions. I think when I was earlier, pretty early in my training days, it was very difficult, when we got asked all these different questions, probably I didn’t have answers for and they kept asking why can’t we do this, why can’t we do that and I didn’t understand but then when you get better understanding of it, if you’re comfortable in touching these subjects, it just makes it easier. It takes some time, some people take it easier within few minutes or few presentation and we have some time to say, some people it just takes a bit longer but the point is if you are confident and you put it in a way that we just understand it and I think the more you do it, the more comfortable you get. I find it more comfortable to discuss these though compared to 2 years ago.
Con2019, male, registrar
A consultant from site Con2 stated in the baseline standard care survey that her team found palliative care not to be appropriate for non-cancer patients. However, at the focus group conducted at the end of the trial, she stated the following, which alludes to an effect of identifying potential patients for the trial:
It helps us I think reflect a bit more on non-cancer patients.
What reflections are they? How did that help you?
It made the team more aware that this may be a group that we previously missed in terms of getting them identified and also support for end of life.
It was important to record the number of patients who died during their admission and those who died within 100 days after discharge to understand if the correct patient population was recruited to the trial (Table 25). Patients with clinically uncertain recovery are expected to have a condition with limited reversibility. By this definition, it was expected that most of the patients recruited to the trial would die in the given time frame. Overall, 60.0% of participants died, either during their hospital admission or within 100 days after discharge. Similar findings were present for the control arm (58.3%) and the intervention arm (62.1%). Although we were not able to record the survival status of the remaining 26 participants, the number of patients who died and the number of days between discharge and death for each trial arm provide some evidence that, from the perspective of ‘being near to end of life’, we were able to identify the correct patient population.
| Characteristics of deceased patients | Trial arm | Totala | |
|---|---|---|---|
| Controla | Intervention | ||
| Number of participants recruited | 36 | 29 | 65 |
| Number of participants who died, n (%) | 21 (58.3) | 18 (62.1) | 39 (60.0) |
| Died within 100 days after discharge, n (%) | 13 (61.9) | 10 (55.6) | 23 (59.0) |
| Died during admission, n (%) | 8 (38.1) | 8 (44.4) | 16 (41.0) |
| Duration between discharge and death (days) | |||
| Mean (95% CI) | 19.2 (2.3 to 36.0) | 26.7 (12.0 to 41.5) | 22.6 (12.1 to 33.1) |
| Median (range) | 8.5 (2–85) | 25 (2–61) | 13.5 (2–85) |
The bereavement survey
The main purpose of the bereavement survey was to retrospectively examine and compare the experiences of care for deceased patients who were recruited to the trial from the perspectives of their close relatives. This approach was included as part of the trial design to assess the feasibility of collecting data retrospectively from NOKs.
In order to identify potential respondents to the survey, research nurses at each trial site checked the death status of the participants on a regular basis. If the patient participant died during their hospital admission or within 100 days after discharge from the trial hospital, research nurses sent out a bereavement pack to the NOK between 10 and 12 weeks after the participant’s death. If there was no response, two subsequent reminder letters were sent in the following month at fortnightly intervals.
As shown in Table 26, out of 65 participants, 39 participants were eligible for the bereavement survey. We were able to successfully identify NOKs and send out surveys for 36 (92.3%) participants. In total, 25 (69.4%) NOKs sent a response to the research team, with 15 of the NOKs completing the survey. Out of 10 relatives who declined to participate, six did not provide a reason, one mentioned ‘not having capacity/time to complete the survey due to other responsibilities related to their relative’s death’ as their reason for declining to complete the bereavement survey and two mentioned ‘completing multiple components of the study already’ as their reason for declining. We did not know what proportion of our participants would be eligible for the bereavement survey. Although the high response rate and the completeness of the data are indicative of a feasible data collection method, consideration must be given to the potential number of patients who would be eligible.
| Eligibility/response | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Control | Intervention | ||
| Eligible for bereavement survey (n) | 21 | 18 | 39 |
| No NOK | 2 (9.5) | 1 (5.6) | 3 (7.7) |
| Mailed to | 19 (90.5) | 17 (94.4) | 36 (92.3) |
| Returned – completed | 7 (36.8) | 8 (47.1) | 15 (41.7) |
| Returned – declined | 4 (21.1) | 6 (35.3) | 10 (27.8) |
| No response | 8 (42.1) | 3 (17.6) | 11 (30.1) |
It became evident that the bereavement survey was not a practical or effective way of collecting meaningful information about care. Identifying participants who died outside the hospital was challenging due to problems in the accuracy of electronic records. Furthermore, this required a great deal of additional research nurse time to prepare.
A total of 36 NOKs were identified and 30 received an initial pack and two reminder packs. Of these 30, only six NOKs provided a response prior to receiving a reminder pack.
Based on the previous comparative study of AMBER conducted by Bristowe et al. 48 that also made use of this bereavement survey, we chose to focus on questions that were related to communication around clinical uncertainty and those questions about care that might be affected by the AMBER care bundle (see Appendix 13). Owing to the inadequate number of responses to the survey questionnaire, a fair comparison between the two arms is not viable.
Chapter 4 Discussion and conclusions
In this chapter, we discuss our findings in relation to the overall aim and objectives of the feasibility trial, the trial limitations and the implications for future research in this area of health care.
Further refinements or adaptations of the AMBER care bundle
Following the MRC’s guidance50,52 and the MORECare statement,51 we identified the refinements and adaptations required for future implementation and sustainability of the intervention in different settings.
Eligibility criteria
Our qualitative findings from the focus groups and non-participant observations of MDT meetings suggest that the AMBER care bundle eligibility criteria require further refinement. This is particularly related to the criterion ‘risk of dying during their current episode of care’. Although the eligibility criteria prompted the HCPs to prioritise a group of patients who previously received less attention, this criterion restricted their ability to identify a wider group of patients who might have potentially benefited from the intervention. In addition, our observations of HCPs’ decision-making process around which patients should be supported by the AMBER care bundle showed that, in many instances, their focus was on patients’ clinical uncertainty, responsiveness to medication, age, multimorbidities and frailty. The ‘risk of dying’ criterion was frequently not considered when making these decisions.
Furthermore, HCPs often felt uncomfortable with the concept of prognostication and attributing ‘risk’ at the identification stage, despite appreciating that in many instances the identified patients were likely to die. This is also evidenced by the deaths of 60% of participants who were recruited in the trial using the AMBER care bundle eligibility criteria. Previous studies have shown that when HCPs are asked to predict life expectancy, their predictions are frequently inaccurate. 86,87 HCPs’ hesitation around prognostication of death observed in our trial is in line with current evidence. The manner in which ‘risk’ is interpreted can often vary among HCPs. The Neuberger review into the use of The Liverpool Care Pathway for the Dying Patient33 therefore recommended that ‘evidence-based education and competency-based training should be promoted to improve prognostic skills’ (contains information licensed uncle the Open Government License v3.0). However, there is currently no clear guidance on how prognostication of death can be better identified by HCPs. 87
Based on the evidence from this trial, to ensure fidelity in the implementation of the AMBER care bundle in different contexts, the eligibility criteria should be simplified to focus solely on the ‘clinical uncertainty’ of the patient and their clinical needs. Criteria that do not require the need for prognostication would enable HCPs to quickly and more efficiently identify patients who would benefit from the intervention.
Daily reviews and follow-up
Based on qualitative findings and clinical notes review of participants who were supported by the AMBER care bundle, we identified inconsistencies in compliance with reviewing ‘the AMBER care bundle status’ of patients and the daily follow-up. HCPs focused their attention on two aspects: (1) reviewing the clinically uncertain recovery status daily for some patients is unnecessary and (2) discussing issues related to clinical uncertainty with the patients and families had the potential to cause additional distress. In our trial, daily review was evidenced in the notes for only 48% of the participants, whereas the other four components of the intervention showed compliance at around 80%.
Our findings from interviews with patients and relatives identified that, although appreciating the workload and time pressures of HCPs, they greatly valued brief daily updates about the current clinical situation from all ward staff, not only doctors. The patient- and proxy-reported outcomes highlighted the high levels of anxiety experienced by family and friends in both trial arms [IPOS patient/family anxiety and communication subscale at baseline: control arm mean 3.3 (95% CI 3.0 to 3.7), intervention arm mean 3.1 (95% CI 2.7 to 3.5)] during the patients’ hospital stay. This finding further supports the need for more attention and time to be devoted to communicating and sharing information with relatives.
We suggest that the frequency of reviewing changes in patients’ clinical uncertainty within the clinical team should be adapted locally. Regardless of patients’ ‘AMBER care bundle status’, daily brief updates are often valued by the patients and families, and should be prioritised by the whole MDT.
Enhancing competences and confidence in advanced communication skills
Issues around communication were identified from all stakeholders’ perspectives. Findings from the HCP focus groups highlighted discrepancies in the communication skills and confidence of different professional groups. Although a number of doctors stated that they already possessed advanced communication skills, there was a strong sentiment that proficiency in this area was unequally distributed among the ward staff, with implications for patient care. This was especially true for nurses, who, despite having received training around having difficult conversations, when conveying important information to patients and their relatives from the nurse facilitator, still felt unprepared.
Findings from patient and relative interviews also reiterated the range in HCPs’ communication skills. Patients and relatives often mentioned that the main source of information regarding patients’ conditions and progress was provided by doctors. When clinical updates were sought from nurses, they were often unable to provide answers, and patients and relatives were signposted to a doctor. Communication with patients and relatives by nursing staff across both control sites and intervention sites was similar, regardless of the communication training received as part of the AMBER care bundle training. Considering that nursing staff are often more present on wards where there are potentially more opportunities to communicate with patients and their families, the lack of confidence in brokering uncertainties around recovery or treatments should be addressed with adequate support and training.
In future, communication skills training that complements supporting patients with the AMBER care bundle must be broadened to serve different levels of proficiency and confidence across all groups of HCPs. Moreover, evidence from other studies88,89 highlights the need to continue to provide this training at future intervals to build on and consolidate the knowledge and skills HCPs already possess.
Active ingredients of the AMBER care bundle to be maintained in a full trial
Although we were not able to measure the direct effect of the specific components of the AMBER care bundle statistically, through our qualitative data collection methods we nevertheless attempted to identify the ‘active ingredients’ of the bundle that should remain in place. The following were identified:
-
Criteria to identify patients. Asking if the patient’s ‘recovery is clinically uncertain’ compelled HCPs to think carefully about the patient’s status, which previously would have received less attention in both trial arms. This initiated a process of discussion and engagement with the patient and their family.
-
Daily review and follow-up with patients and their families. Continuous engagement with the patients and families was critical. Checking the clinical uncertainty/AMBER care bundle status of the patient should be open to local interpretation. Discussing patients’ needs and plans became routinised into the daily clinical practice on the intervention wards, and was considered to be a central feature of the intervention.
-
Simple documentation. The simple documentation associated with the AMBER care bundle was quick to complete and valued by all HCPs. This provided an assured system of conveying important information to all ward staff, particularly those who worked out of hours and at the weekend.
-
The role of the nurse facilitator. The nurse facilitator was pivotal to successful delivery of the intervention, not only in introducing ward staff to the concept of clinical uncertainty and how to respond to it, but also in providing valuable clinical supervision for staff who were now engaging in a style of care that came with emotive consequences. Continuous training and support provided by a dedicated AMBER care bundle facilitator is required for sustainability of the intervention.
Acceptability of the AMBER care bundle
We identified that the AMBER care bundle was broadly acceptable to patients, their families and the HCPs who delivered it. However, there are still some concerns that might have an impact on the intervention’s acceptability. These include potentially negative consequences for patients and relatives from poor communication and delivery of information, and HCPs’ reluctance to take responsibility for sustaining the AMBER care bundle on their ward.
Focusing on the holistic needs of patients and their families
The HCPs welcomed the intervention as a means of not only focusing on patients’ physical symptoms but also placing greater emphasis on engaging in early and important discussions with them and their families about more-holistic concerns and preferences.
Transmission of information
Some HCPs stated that although conversations with patients and their families had previously taken place in a manner commensurate with the AMBER care bundle, the detail of these conversations had not always been adequately recorded. HCPs therefore widely endorsed the documentation associated with the AMBER care bundle, which provided them with a system of conveying important information to all ward staff.
Wider involvement in contributing to patient-centred decisions
The AMBER care bundle promoted wider involvement of HCPs in important decisions about the care of patients and their families. Although this suggests a greater democratisation of involvement, the final decisions about which patients were deemed to fulfil the AMBER care bundle eligibility criteria rested with clinicians. Nonetheless, our findings indicate that teamworking in decision-making aligned with the intended benefits of the AMBER care bundle.
Sustainability of the AMBER care bundle in the absence of a nurse facilitator
Based on feedback received from HCPs, wards were often short-staffed. The departure of the nurse facilitator and absence of a ward champion to support HCPs in delivering the intervention raised concerns that some of the intervention components and the identification of potential patients might not be sustained. HCPs at intervention sites were reluctant to take on this responsibility, which raised questions around the acceptability and value of the intervention, particularly in the longer term.
Inclusion criteria
The HCPs found the inclusion criteria broadly acceptable, but had differing interpretations of ‘risk’. There were concerns around the wider utility of the intervention for their patient population on the ward, which was limited by the ‘risk of dying’ criterion.
Communication and information
Patients and relatives found discussions around clinically uncertain recovery acceptable, and when these conversations did not take place, they mentioned that they would have welcomed HCPs to communicate with them. HCPs, specifically doctors, found it acceptable to have difficult conversations, which is a core component of the intervention. However, HCPs should be mindful about the information recorded on the patients’ notes and should be aware of the feasible options when discussing preferences for care.
Compliance with and barriers to the delivery of the AMBER care bundle
Regarding the completion of core components of the AMBER care bundle, the review of the clinical records of participants identified that, with the exception of the daily review, components associated with the intervention were completed. However, there were some doubts about the completion of these components within the required time frame (12 hours).
Completing initial conversations and follow-ups with the patients, or their family if they lacked capacity, was generally delayed owing to the unavailability of relatives when the clinical team taking care of the patient was on the ward.
Inadequate communication skills and confidence in communication led to issues in compliance across components. Owing to inconsistency in the skills of ward staff, delivery of difficult conversations and subsequently care and treatment planning were reliant on specific HCPs, and therefore were sometimes delayed.
Implementation of the intervention was standardised and delivered by the same nurse facilitator in both intervention sites, and the embeddedness of the intervention was assessed against predetermined criteria to ensure high fidelity. HCPs complied with the components of the intervention for the duration of the trial. However, it is important to state that the fidelity in which the AMBER care bundle is delivered and recorded can only be viewed as a ‘process measure’ in contributing towards positive patient- and family-centred outcomes.
Feasibility of conducting trial procedures
Overall, although we identified that the trial procedures were technically possible, we provide evidence that scaling up to a future trial of the AMBER care bundle compared with standard care would not be practical. Alternative study designs would need to be feasibility tested to ensure that they provide robust evidence to inform a future evaluation of the AMBER care bundle. Without considerable changes and retesting, ‘de-risking’ of a definitive trial of the AMBER care bundle could be not be ensured.
Feasibility of recruitment
We were successful in recruiting 65 participants, many of whom were elderly with multiple morbidities. Prior to the trial, we hypothesised that patients with clinical uncertainty would die either during their current hospital admission or within a limited time frame (100 days); this was informed by previous quality improvement work carried out by the AMBER care bundle developers. Of 65 recruited participants, 60% did indeed die (n = 39). Of those who died, 41% died during their hospital stay and the remainder died within 100 days of hospital discharge. We are aware that some participants died more than 100 days after hospital discharge, but considering this was not within the scope of this current trial.
Of 220 eligible participants, only 19 (8.6%) declined to participate in the trial; this finding supports the ability of the trial to recruit and the willingness of patients to be involved. Furthermore, these findings provide additional evidence that it is possible to recruit acutely unwell patients (our intended target population) via the screening and recruitment processes developed in this trial. Although we were able to recruit, the yield of recruitment from screened numbers was low.
This feasibility trial provides valuable insights regarding adjustments that would be required to improve recruitment of similar patient populations in similar future trials. First, having a recruitment period of 3 months, with an intended target of seven recruits per month, was not possible. To improve this, the eligibility criteria for the identification of potential patient participants should be reconsidered. If the AMBER care bundle criteria are going to serve as inclusion criteria for a future study, they warrant a discussion with the AMBER care bundle developers. Simplification would be needed, and including age and multimorbidities among other factors in the inclusion criteria would potentially be helpful. The current eligibility criteria are variably understood, and in some instances there is resistance to enact them due to lack of confidence and skills. Second, we would suggest increasing the recruitment period, particularly the time taken to familiarise research staff with the eligibility criteria.
Feasibility of data collection
All participants (n = 65) consented into the trial completed baseline measures. The attrition rate for the trial was high, a finding also evident in other trials of this nature. At time point 1, 29 participants were lost to follow-up; of these, 16 (55.2%) were discharged. At time point 2, 24 participants were lost to follow-up; of these, 8 (33.3%) were lost because of discharge from the hospital. Owing to the number of participants discharged throughout the duration of the trial, data collection at time point 2 was not feasible. Interestingly, the median time from ward admission to baseline data collection for participants in the control and intervention arms was 6 (interquartile range 11) and 10 (interquartile range 11) days, respectively. Based on our findings, capturing potential participants at an earlier point of their hospital admission is advisable for studies of this nature. This may minimise the number lost to follow-up because of discharge at time point 2, and would ensure that data collection could take place over a longer time frame.
Overall, levels of missing data for patient participant self-reported outcomes and those provided by proxies were very low for both of the primary outcome measures we used. For the IPOS patient/family anxiety and communication subscale, 6.2% of data were missing at baseline, 5.6% were missing at time point 1 and 0% were missing at time point 2. For howRwe, 0% of data were missing at baseline, 8.3% were missing at time point 1 and 0% were missing at time point 2. We are mindful that data completeness for the howRwe should be understood within the limitation that it is a patient-completed measure and therefore could not be used for those who lacked adequate mental capacity. Nevertheless, the findings indicate that data collection among an older population with multimorbidities was possible through face-to-face research nurse interviews. We therefore suggest that those undertaking future studies that attempt to conduct research among this patient population, and importantly of the interventions designed to benefit them, may wish to consider embedding the measurement of patient-centred concern, as recorded by the IPOS. This could be incorporated into their daily patient care, as demonstrated by the recent Outcome Assessment and Complexity Collaboration project that was designed to support the implementation of patient-centred outcome measures in specialist palliative care settings in the UK. 90 The use of ‘patient-centred’ rather than ‘patient-reported’ outcome measures is particularly useful in this type of research in which often patients have impaired cognition or are too unwell to complete the measures themselves. 91 Proxy-completed measures have previously been found to be a fair substitute for patient response for assessing symptoms and quality of life. 92 Alternative approaches to data collection might also be considered. For example, it may be prudent to make use of population-based, retrospective hospital-based data to examine and compare patients supported by the AMBER care bundle with controls, adjusted for propensity matching. Similar approaches have been successfully employed to examine the quality of care of cancer patients. 93 Specifically, domains of care would have to be specified for patients who died during their hospital stay or within 100 days of discharge and, importantly, for those who survived. Domains of interest might include informing family members when death was imminent, the use of validated tools to assess common symptoms (e.g. pain), prescribing drugs for anxiety, the use of bereavement support (where available), length of hospital stay, preferred and actual place of death, number of hospital readmissions and admissions to emergency departments.
Within the scope of our feasibility trial, we have refuted the legitimate concerns about engaging with vulnerable patient populations at the end of life. 94–96 This feasibility trial demonstrates that when ethical and pragmatic decisions are made in relation to trial design and combined with highly sensitised research nurses and researchers, the voice of patients can be heard.
We have shown that it was possible to collect the data on health and social care service use, informal care provision and quality of life at baseline and at 10–15 days (time point 2). The level of missing data was very low. However, in future, health service use at follow-up should be replaced by exploring patients’ clinical records, assuming that all patients stay in wards. The nurse facilitator’s time working on the wards was recorded in a diary, but the costs associated with this represents only a part of the AMBER care bundle intervention. This is because it was not possible to accurately quantify the time and work of other HCPs. In future studies, a predetermined ‘diary’ format should be developed to record all relevant HCPs’ activities and the time spent delivering the intervention. This could then be combined with HCPs’ salaries, the nurse facilitator’s salary and the cost of supervision to provide a more accurate estimate of cost during the trial period.
Data collection using qualitative methods was also possible. We were able to conduct 24 interviews, representing 36.9% of our participants. Our focus groups were well attended by a wide range of HCPs.
In this trial, it was possible to collect data prospectively using both qualitative research methods and quantitative research methods. Nonetheless, there were challenges associated with the collection of data, and possible refinements to methods are required to overcome these. Based on the feedback we received from participants and the research nurses, the number of sections in the questionnaire booklets and additional trial components, for example the bereavement survey, was perceived as burdensome. Based on this finding, it is recommended that the number of data collected directly from patients and relatives is reduced to the essential components only. Other data sources, such as CSRI and AKPS, should be extracted from the patients’ medical notes.
We are conscious that the acceptability of data collection extends to understanding how participants perceived the experience of being involved in the trial. Their positive motivations, including notions of altruism, build on a small but growing evidence base that studies of this type are possible and indeed permissible. 97,98
Limitations and key learning points from our trial
This feasibility trial has identified important study limitations to the generalisability and validity of findings. The limitations, their implications and how they may be resolved in similar future studies are discussed in this section.
Sample size
We aimed to recruit 80–90 participants throughout the trial. However, we were only able to recruit 65 participants. This limited our ability to identify differences in the candidate primary outcome measures, hence limiting the assessment of the preliminary effectiveness of the intervention. In addition, the small number of clusters included in the trial meant that we were not able to calculate the intracluster correlation coefficient required for a future trial. In future, we would extend the number of clusters included in the trial and implement learning from the screening and recruitment phase.
Inclusion criteria
Using inclusion criteria based on the eligibility criteria for the AMBER care bundle required subjective clinical judgement. This greatly limited the HCPs’ ability to reliably identify and then recruit patients. Consequently, we simplified the inclusion criteria at the control sites. Although we were not able to capture the effect of this change within the time frame of the feasibility trial, any future study examining this clinical issue would be required to adopt simplified inclusion criteria in order to improve the reliability of the identification of potential participants. Alternative recruitment strategies might also include recruiting and consenting all the patients within the clusters. Patients who fulfilled the simplified inclusion criteria in relation to their situation of clinical uncertainty could then be retrospectively identified from their clinical notes, an approach previously used in our comparative study of the AMBER care bundle. 48 However, the quality and level of detail of information contained within patients’ clinical notes can be highly variable.
Participant information sheets
The participant information sheets, although developed in close collaboration with our PPI members, require further improvement, particularly when attempting to engage frail older people. There were strong views from the research nurses that the documentation developed was overly lengthy, too detailed and too complex for our intended target patient population. Consequently, a number of potential participants were discouraged from engaging in the trial, preventing them from sharing their experiences. Little guidance exists in this research area in developing trial information documentation that is acceptable in both level of detail and length to both potential participants and research ethics committees. 99 Future studies working in this area of health care should be permitted to evaluate to what extent briefer participant information sheets convey adequate information about the trial and the potential benefits and risks to potential participants and the influence of this in relation to trial uptake. This could be compared with the current standard typically required by the HRA and research ethics committees.
Consent processes
Closely related to the above issue is the perceived burdensome manner in which advanced consent was obtained from patients, many of whom were frail, elderly and fatigued. According to the Department of Health and Social Care, informed consent can be written, oral or given ‘by implication’. 100 Therefore, it has been suggested that written consent is neither sufficient nor necessary for valid consent to be present. 101 Based on the previous section, we propose that a future trial incorporates a more pragmatic process of obtaining consent in which the patient participant explicitly agrees to what is proposed by the researcher, and the researcher then notes this. Again, this approach should be compared with approaches currently used to examine the relative acceptability of interventions.
Loss to follow-up
In our trial, the main reason for loss to follow-up was discharge from the trial ward. This limited our ability to utilise the data collection at the follow-up points. This finding was evident in both arms of the trial. As our data collection was limited to the time frame of patients located in the trial ward, we were not able to carry on collecting data from the participants. In response to this limitation, any future study examining this area of health care with a similar patient population should consider designing a study that either aims to recruit patients at an earlier point in their hospital admission or allows follow-up after discharge. A potentially more appropriate commencement point for recruitment within the hospitals could be acute medical units. Instances of clinical uncertainty and decisions regarding patients’ further treatment and care within the hospitals often take place in the acute medical units. This would extend the potential of collecting data at time point 2 for a larger number of participants who would otherwise have been discharged at this point.
Selection of trial wards
Guided by the AMBER care bundle development team, we made use of the ‘heat map’ approach to identify wards with the largest numbers of patient deaths. Consequently, we did not identify wards with similar specialties across the trial arms. This resulted in a case mix of patients who were quite different. The effect of this was most pronounced with the inclusion of care of the elderly wards that skewed the age balance between the trial arms. The mean age of the participants in the intervention arm was inevitably higher than that in the control arm. Future studies should not base the selection of trial wards solely on the number of deaths per ward, but also on other factors, importantly including ward specialty, the potential active engagement of ward staff and the presence of the principal investigator on the ward.
Selection bias
Although the opportunity to participate in the qualitative components was offered to all participants (patients or their relatives and HCPs), it is possible that those who chose to participate differed from those who did not. This may limit the transferability of qualitative findings to different contexts and has implications for conclusions made about the acceptability of the intervention. We aimed to purposively sample a range of patients and their relatives and HCPs. However, more time and resources should be allocated to approaching potential participants who might have opposing views about the trial and intervention to overcome these biases.
Conclusions
This feasibility trial was difficult to undertake for many logistical and methodological reasons, yet provided vital evidence to inform the decision about progressing to a full trial. In this trial, the stringent inclusion criteria for trial participation, which were adapted from the intervention, based on prognostication rather than clinical need, were not compatible with trial procedures. HCPs, especially at the control sites, reported that they lacked the confidence and skills to identify, approach and introduce the trial to patients whose situations were clinically uncertain. Introduction of the trial, including the language on the participant information sheets, required an explanation of the potential patient participant’s clinical situation. This ‘gatekeeping’ function prevented many potentially eligible patients from being invited to consider whether or not they wish to participate in this trial. Although we have shown that the feasibility cluster RCT was technically possible, in the light of the aforementioned concerns, a full trial of the AMBER care bundle is not practical. This represents an important trial outcome for a feasibility trial that is specifically designed to address research questions of this nature. 102–104 Alternative study designs that wish to examine interventions focused on this complex patient group are required. Moreover, they too will need to be tested; examples include embedding patient-centred outcome measures, for example the IPOS,55,56 into routine clinical practice, or the analysis of routinely recorded hospital-based data. 93 Both have appeal but would need to be tested to ensure that they address the anticipated benefits of the AMBER care bundle that are relevant to patients, their families and health-care services.
This feasibility cluster RCT of the AMBER care bundle identified a number of active ingredients that are required to deliver potential positive HCP and patient- and family-centred outcomes. The trial also identified a number of areas that require further consideration to benefit a highly complex group of patients with multimorbidities and whose situations are clinically uncertain. The trial highlighted a number of important contextual moderators that need to be taken into account when implementing the intervention across different settings. This demonstrates that early work in identifying and then appraising existing evidence alongside identifying a theoretical framework for an intervention prior to its use is paramount. In future, complex interventions of this nature should strictly follow guidance developed by the MRC50,52 and the MORECare statement. 51
This trial has shown that research in this area is, at times, challenging. Any future study that focuses on this patient group and interventions designed to serve such patients should simplify study procedures. First, consideration is needed with regard to the type of wards that are being compared across each arm of the study to improve the homogeneity of patients. Second, the manner in which patients in receipt of the intervention and comparable patients on control wards are recruited should be reconsidered. Although labour intensive, recruiting all patients admitted to each arm of the study and then retrospectively identifying those who fulfilled the specific study criteria may be more acceptable to principal investigators and research staff, particularly in the control arm of the study. Finally, consent procedures should be simplified and only data that are vital in addressing the study objectives should be collected directly from the patients and relatives.
Acknowledgements
The authors wish to acknowledge funding for this trial from the NIHR Health Technology Assessment programme, as well as support from the research managers at the NIHR Evaluation, Trials and Studies Coordinating Centre.
We would especially like to thank the principal investigators and the clinical teams at each the four hospital sites:for agreeing to be involved in this trial and for participating in the focus groups. We express particular thanks to the research nurses working across each of the four trial sites, who were instrumental in recruiting patients, collecting data and arranging interview appointments. We also wish to thank the non-clinical staff at each of the four trial sites for their input into the administration of the feasibility trial and for facilitating communication between the research team and research nurses.
-
Henry Penn – Northwick Park Hospital, London North West University Healthcare NHS Trust
-
David Brooks – Chesterfield Royal Hospital, Chesterfield Royal Hospital NHS Foundation Trust
-
Natalie Broomhead – East Surrey Hospital, Surrey and Sussex Healthcare NHS Trust
-
Carole Robinson – Tunbridge Wells Hospital, Maidstone and Tunbridge Wells NHS Trust
We are incredibly grateful to all of the patients and their families who contributed to the ImproveCare trial, either by participating in the interviews or by participating in the feasibility trial. The information and findings gathered from patients and their families over the course of the trial were invaluable in informing potential modifications to the intervention and for informing a future study.
We are very grateful to the AMBER care bundle design team: Susanna Shouls (Design Team Co-ordinator), Dr Irene Carey (Consultant in Palliative Medicine, Guy’s and St Thomas’ NHS Foundation Trust), Dr Adrian Hopper (Consultant Physician and Deputy Medical Director, Guy’s and St Thomas’ NHS Foundation Trust), Charlene Davis (Design Team Administrator). This team offered valuable advice throughout the study. We wish to thank Linda Launchbury (Palliative Care Clinical Nurse Specialist), the nurse facilitator who worked on behalf of the AMBER care bundle design team and represented the nurse facilitator for AMBER at each of the two intervention sites.
We are grateful for the time and expertise Dr Natasha Lovell provided in helping to categorise patients, reasons for admission and their morbidities in accordance with the International Classification of Diseases, Tenth Revision. 83
We are particularly grateful for the members of the PPI group for contributing to the development of the trial and for sharing their time in reviewing patient materials, participant information sheets and consent sheets, and for the interpretation of the trial findings. We express particular thanks to Sylvia Bailey and Colleen Ewart, who provided expert PPI input at all stages of the trial from the inception of the research idea through all the stages of the funding application, interpreting the findings of the feasibility trial and drafting this final report.
Finally, we wish to thank Dr Liz Sampson (Chairperson), Dr Joanne Droney, Dr Morag Farquhar and Professor Toby Prevost, who acted as members of the project’s TS DMEC and who offered invaluable advice at all stages of this trial.
Contributions of authors
Jonathan Koffman (Reader in Palliative Care, Cicely Saunders Institute, King’s College London) led the research as chief investigator, led the design of the overall trial, co-ordinated the funding proposal application, led the NHS Research Ethics Service approval application, co-ordinated substantial amendments for the NHS Research Ethics Service, undertook overall supervision of the research, co-ordinated the day-to-day running of the research study, facilitated the PAG meetings, contributed to the interpretation of the findings from the feasibility trial and led in writing the final report.
Emel Yorganci (Research Assistant Economic and Social Research Council PhD training fellow, Cicely Saunders Institute, King’s College London) was a research assistant, co-ordinated the day-to-day running of the research study, liaised closely with trial sites, conducted interviews with patients and relatives, conducted non-participant observations of MDT meetings, facilitated the PPI meetings, developed the content and format of the ‘standard’ or ‘usual’ care survey questionnaire, performed case note review of clinical notes to examine fidelity of intervention and extracted clinical data from participants’ notes, managed data entry, performed the qualitative and quantitative analyses of the feasibility trial data, interpreted the findings from the feasibility trial and contributed to the writing of the final report.
Fliss Murtagh (Professor of Palliative Care, Wolfson Palliative Care Research Centre, Hull York Medical School, and Honorary Consultant Palliative Physician, Hull University Teaching Hospitals NHS Trust) contributed to the design of the overall study, the funding proposal application, the interpretation of the findings from the feasibility trial and the writing of the final report.
Deokhee Yi (Health Economist, Cicely Saunders Institute, King’s College London) contributed to the design of the overall study and the funding application, led on the cost-effectiveness component of the feasibility trial and contributed to the interpretation of the findings and writing of the final report.
Wei Gao (Reader in Medical Statistics, Cicely Saunders Institute, King’s College London) contributed to the overall design of the study and the funding application and led on the analysis of the findings with a focus on the requirements for a future definitive trial.
Stephen Barclay (Senior Lecturer in General Practice and Palliative Care, General Practitioner, and Department of Public Health and Primary Care, University of Cambridge Honorary Consultant Physician in Palliative Care) contributed to the overall design of the study, the funding application, the interpretation of study findings and the writing of the final report.
Andrew Pickles (Professor in Biostatistics, Department of Biostatistics and Informatics, King’s College London) contributed to the design of the overall study and interpretation of study findings with a focus on the requirements for a future definitive trial.
Irene Higginson [Professor of Palliative Care and Policy, Director, Cicely Saunders Institute Vice Dean (Research), Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care, King’s College London] contributed to the interpretation of the findings with a focus on the requirements for a future definitive trial.
Halle Johnson (Research Projects and Co-ordination Assistant, Cicely Saunders Institute, King’s College London) was a research assistant, facilitated the PPI meetings, interpreted the findings from the qualitative components of the feasibility trial and contributed to the writing of the final report.
Rebecca Wilson (Research Associate, Cicely Saunders Institute, King’s College London) contributed towards the analysis and interpretation of the quantitative components of the trial and the writing of the final report.
Sylvia Bailey (PPI representative) contributed to the design of the overall study and helped to interpret the results of the feasibility trial.
Colleen Ewart (PPI representative) contributed to the design of the overall study and helped to interpret the results of the feasibility trial.
Catherine Evans (Health Education England/NIHR Senior Clinical Lecturer in Palliative Care and Nursing Research, Cicely Saunders Institute, King’s College London, and Development and Innovation Director and Honorary Nurse Consultant, Sussex Community NHS Foundation Trust) contributed to the design of the overall study and the funding proposal application, acted as the interim chief investigator, co-ordinated substantial amendments for the NHS Research Ethics Service, contributed to supervision of the research, co-ordinated the day-to-day running of the research study, undertook site visits, facilitated the PAG meetings, contributed to the interpretation of the findings from the feasibility trial and contributed to the writing of the final report.
Publication
Koffman J, Yorganci E, Yi D, Gao W, Murtagh F, Pickles A, et al. Managing uncertain recovery for patients nearing the end of life in hospital: a mixed-methods feasibility cluster randomised controlled trial of the AMBER care bundle. Trials 2019;20:506.
Data-sharing statement
Data collected as part of the ImproveCare trial are held securely at the Cicely Saunders Institute, King’s College London. Trial documents and the protocol can be found on the ISRCTN registry (https://bit.ly/2QH5Ox4; accessed 12 August 2019). Any enquires regarding the data collected should be made to the corresponding author: Dr Jonathan Koffman (jonathan.koffman@kcl.ac.uk).
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Koffman J, Yorganci E, Yi D, Gao W, Murtagh F, Pickles A, et al. Managing uncertain recovery for patients nearing the end of life in hospital; a mixed-methods feasibility cluster randomised controlled trial of the AMBER care bundle. Trials 2019;20.
- Office of National Statistics . Statistical Bulletin: Deaths Registered in England and Wales: 2012 Deaths, Stillbirths and Infant Mortality Including Death Rates, Causes, Age, and Area of Residence 2013. https://tinyurl.com/ya5dvbj2 (accessed 5 March 2019).
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- National End Of Life Care Intelligence Network . What Do We Know That We Didn’t Know a Year Ago? New Intelligence on End of Life Care in England 2012. www.endoflifecare-intelligence.org.uk/resources/publications/what_we_know_now (accessed 30 August 2019).
- Gomes B, Higginson IJ, Calanzani N, Cohen J, Deliens L, Daveson BA, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012;23:2006-15. https://doi.org/10.1093/annonc/mdr602.
- Almack K, Cox K, Moghaddam N, Pollock K, Seymour J. After you: conversations between patients and healthcare professionals in planning for end of life care. BMC Palliat Care 2012;11. https://doi.org/10.1186/1472-684X-11-15.
- Etkind SN, Karno J, Edmonds PM, Carey I, Murtagh FE. Supporting patients with uncertain recovery: the use of the AMBER care bundle in an acute hospital. BMJ Support Palliat Care 2015;5:95-8. https://doi.org/10.1136/bmjspcare-2013-000640.
- Mishel MH. The measurement of uncertainty in illness. Nurs Res 1981;30:258-63. https://doi.org/10.1097/00006199-198109000-00002.
- Mishel MH. Reconceptualization of the uncertainty in illness theory. Image J Nurs Sch 1990;22:256-62. https://doi.org/10.1111/j.1547-5069.1990.tb00225.x.
- Mishel MH. Uncertainty in illness. Image J Nurs Sch 1988;20:225-32. https://doi.org/10.1111/j.1547-5069.1988.tb00082.x.
- McCormick KM. A concept analysis of uncertainty in illness. J Nurs Scholarsh 2002;34:127-31. https://doi.org/10.1111/j.1547-5069.2002.00127.x.
- Johnson Wright L, Afari N, Zautra A. The illness uncertainty concept: a review. Curr Pain Headache Rep 2009;13:133-8. https://doi.org/10.1007/s11916-009-0023-z.
- Thorne SE, Bultz BD, Baile WF. SCRN Communication Team . Is there a cost to poor communication in cancer care?: a critical review of the literature. Psycho-Oncology 2005;14:875-84. https://doi.org/10.1002/pon.947.
- Brashers DE. Communication and uncertainty management. J Commun 2001;51:477-97. https://doi.org/10.1111/j.1460-2466.2001.tb02892.x.
- Etkind SN, Bristowe K, Bailey K, Selman L, Murtagh FE. How does uncertainty shape patient experience in advanced illness? A secondary analysis of qualitative data. Palliat Med 2017;31:171-80. https://doi.org/10.1177/0269216316647610.
- Harding R, Simms V, Calanzani N, Higginson IJ, Hall S, Gysels M, et al. If you had less than a year to live, would you want to know? A seven-country European population survey of public preferences for disclosure of poor prognosis. Psycho-Oncology 2013;22:2298-305. https://doi.org/10.1002/pon.3283.
- Wright AA, Zhang BZ, Mack JW, Trice E, Balboni T, Mitchell SL, et al. Association between end of life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665-73. https://doi.org/10.1001/jama.300.14.1665.
- Johnson CG, Levenkron JC, Suchman AL, Manchester R. Does physician uncertainty affect patient satisfaction?. J Gen Intern Med 1988;3:144-9. https://doi.org/10.1007/BF02596120.
- Fisher M, Ridley S. Uncertainty in end-of-life care and shared decision making. Crit Care Resusc 2012;14:81-7.
- Ogden J, Fuks K, Gardner M, Johnson S, McLean M, Martin P, et al. Doctors expressions of uncertainty and patient confidence. Patient Educ Couns 2002;48:171-6. https://doi.org/10.1016/S0738-3991(02)00020-4.
- Clayton JM, Butow PN, Arnold RM, Tattersall MH. Discussing life expectancy with terminally ill cancer patients and their carers: a qualitative study. Support Care Cancer 2005;13:733-42. https://doi.org/10.1007/s00520-005-0789-4.
- Parliamentary and Health Service Ombudsman . Dying Without Dignity Investigations by the Parliamentary and Health Service Ombudsman into Complaints About End of Life Care 2015. www.ombudsman.org.uk/sites/default/files/Dying_without_dignity.pdf (accessed 30 August 2019).
- Kassirer JP. Our stubborn quest for diagnostic certainty. A cause of excessive testing. N Engl J Med 1989;320:1489-91. https://doi.org/10.1056/NEJM198906013202211.
- Helft PR, Chamness A, Terry C, Uhrich M. Oncology nurses’ attitudes toward prognosis-related communication: a pilot mailed survey of oncology nursing society members. Oncol Nurs Forum 2011;38:468-74. https://doi.org/10.1188/11.ONF.468-474.
- Elkington H, White P, Higgs R, Pettinari CJ. GPs’ views of discussions of prognosis in severe COPD. Fam Pract 2001;18:440-4. https://doi.org/10.1093/fampra/18.4.440.
- Politi MC, Street RL. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract 2011;17:579-84. https://doi.org/10.1111/j.1365-2753.2010.01549.x.
- Katz J. Why doctors don’t disclose uncertainty. Hastings Cent Rep 1984;14:35-44. https://doi.org/10.2307/3560848.
- Braddock CH, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999;282:2313-20. https://doi.org/10.1001/jama.282.24.2313.
- Sorenson J. Biomedical innovation, uncertainty, and doctor-patient interaction. J Health Soc Behav 1974;15:366-74. https://doi.org/10.2307/2137097.
- Epstein R, Hadee T, Carroll J, Meldrum S, Lardner J, Shields C. Reassurance, uncertainty, and empathy in response to patients’ expressions of worry. J Gen Intern Med 2007;22:1731-9. https://doi.org/10.1007/s11606-007-0416-9.
- Gordon GH, Joos SK, Byrne J. Physician expressions of uncertainty during patient encounters. Patient Educ Couns 2000;40:59-65. https://doi.org/10.1016/S0738-3991(99)00069-5.
- The Stationery Office . The Mid Staffordshire NHS Foundation Trust Public Enquiry, Chaired by Robert Francis QC 2013.
- Neuberger J, Guthrie C, Aaronovitch D. More Care, Less Pathway: A Review of the Liverpool Care Pathway. London: Department of Health and Social Care; 2013.
- Carey I, Shouls S, Bristowe K, Morris M, Briant L, Robinson C, et al. Improving care for patients whose recovery is uncertain. The AMBER care bundle: design and implementation. BMJ Support Palliat Care 2015;5:12-8. https://doi.org/10.1136/bmjspcare-2013-000634.
- National End of Life Care Intelligence Network . Variations in Place of Death in England 2010. www.endoflifecare-intelligence.org.uk/resources/publications/variations_in_place_of_death (accessed 30 August 2019).
- Higginson IJ, Finlay IG, Goodwin DM, Hood K, Edwards AG, Cook A, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers?. J Pain Symptom Manage 2003;25:150-68. https://doi.org/10.1016/S0885-3924(02)00599-7.
- Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families?. Cancer J 2010;16:423-35. https://doi.org/10.1097/PPO.0b013e3181f684e5.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. https://doi.org/10.1056/NEJMoa1000678.
- Resar R, Griffin FA, Haraden C, Nolan TW. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper 2012. www.ihi.org/resources/Pages/IHIWhitePapers/UsingCareBundles.aspx (accessed 5 March 2019).
- Robb E, Jarman B, Suntharalingam G, Higgens C, Tennant R, Elcock K. Using care bundles to reduce in-hospital mortality: quantitative survey. BMJ 2010;340. https://doi.org/10.1136/bmj.c1234.
- NHS England . Transforming End of Life Care in Acute Hospitals: The Route to Success ‘How To’ Guide 2015.
- National End of Life Care Programme . NHS Improving Quality 2014.
- Currow DC, Higginson I. Time for a prospective study to evaluate the Amber Care Bundle. BMJ Support Palliat Care 2013;3:376-7. https://doi.org/10.1136/bmjspcare-2013-000608.
- Evans CJ, Ho Y, Daveson BA, Hall S, Higginson IJ, Gao W. GUIDE_Care project . Place and cause of death in centenarians: a population-based observational study in England, 2001 to 2010. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001653.
- Dignity in death: the triumph of politics over evidence. Lancet Oncol 2013;14:1243-356. https://doi.org/10.1016/S1470-2045(13)70559-X.
- Henson LA, Gao W, Higginson IJ, Smith M, Davies JM, Ellis-Smith C, et al. Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta-analysis. J Clin Oncol 2015;33:370-6. https://doi.org/10.1200/JCO.2014.57.3568.
- Department of Health and Social Care . The NHS Outcomes Framework 2011 12 2010.
- Bristowe K, Carey I, Hopper A, Shouls S, Prentice W, Caulkin R, et al. Patient and carer experiences of clinical uncertainty and deterioration, in the face of limited reversibility: a comparative observational study of the AMBER care bundle. Palliat Med 2015;29:797-80. https://doi.org/10.1177/0269216315578990.
- Bristowe K, Carey I, Hopper A, Shouls S, Prentice W, Higginson IJ, et al. Seeing is believing – healthcare professionals’ perceptions of a complex intervention to improve care towards the end of life: a qualitative interview study. Palliat Med 2018;32:525-32. https://doi.org/10.1177/0269216317711336.
- Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance 2008.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG, Donner A. Methods in health service research. Evaluation of health interventions at area and organisation level. BMJ 1999;319:376-9. https://doi.org/10.1136/bmj.319.7206.376.
- Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 1999;8:219-27. https://doi.org/10.1136/qshc.8.4.219.
- Siegert RJ, Gao W, Walkey FH, Higginson IJ. Psychological well-being and quality of care: a factor-analytic examination of the palliative care outcome scale. J Pain Symptom Manage 2010;40:67-74. https://doi.org/10.1016/j.jpainsymman.2009.11.326.
- Sleeman KE, Higginson IJ. A psychometric validation of two brief measures to assess palliative need in patients severely affected by multiple sclerosis. J Pain Symptom Manage 2013;46:406-12. https://doi.org/10.1016/j.jpainsymman.2012.08.007.
- Higginson IJ, Gao W, Saleem TZ, Chaudhuri KR, Burman R, McCrone P, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0046327.
- Antunes B, Murtagh F, Bausewein C, Harding R, Higginson IJ. EURO IMPACT . Screening for depression in advanced disease: psychometric properties, sensitivity, and specificity of two items of the Palliative Care Outcome Scale (POS). J Pain Symptom Manage 2015;49:277-88. https://doi.org/10.1016/j.jpainsymman.2014.06.014.
- Tourangeau R. Cognitive aspects of survey measurement and mismeasurement. Int J Public Opin Res 2003;15:3-7. https://doi.org/10.1093/ijpor/15.1.3.
- Schildmann EK, Groeneveld EI, Denzel J, Brown A, Bernhardt F, Bailey K, et al. Discovering the hidden benefits of cognitive interviewing in two languages: the first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat Med 2016;30:599-610. https://doi.org/10.1177/0269216315608348.
- Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS) [published online ahead of print June 12 2019]. Palliative Medicine 2019. https://doi.org/10.1177/0269216319854264.
- Benson T, Potts HW. A short generic patient experience questionnaire: howRwe development and validation. BMC Health Serv Res 2014;14. https://doi.org/10.1186/s12913-014-0499-z.
- Benson T, Sizmur S, Whatling J, Arikan S, McDonald D, Ingram D. Evaluation of a new short generic measure of health status: howRu. Inform Prim Care 2010;18:89-101. https://doi.org/10.14236/jhi.v18i2.758.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2140.
- Hall S, Edmonds P, Harding R, Chochinov H, Higginson IJ. Assessing the feasibility, acceptability and potential effectiveness of Dignity Therapy for people with advanced cancer referred to a hospital-based palliative care team: Study protocol. BMC Palliat Care 2009;8. https://doi.org/10.1186/1472-684X-8-5.
- Hall S, Goddard C, Opio D, Speck P, Higginson IJ. Feasibility, acceptability and potential effectiveness of Dignity Therapy for older people in care homes: a phase II randomized controlled trial of a brief palliative care psychotherapy. Palliat Med 2012;26:703-12. https://doi.org/10.1177/0269216311418145.
- Hall S, Goddard C, Speck P, Higginson IJ. ‘It makes me feel that I’m still relevant’: a qualitative study of the views of nursing home residents on dignity therapy and taking part in a phase II randomised controlled trial of a palliative care psychotherapy. Palliat Med 2013;27:358-66. https://doi.org/10.1177/0269216312449272.
- Gomes B, McCrone P, Hall S, Koffman J, Higginson IJ. Variations in the quality and costs of end-of-life care, preferences and palliative outcomes for cancer patients by place of death: the QUALYCARE study. BMC Cancer 2010;10. https://doi.org/10.1186/1471-2407-10-400.
- Koffman J, Higginson IJ, Hall S, Riley J, McCrone P, Gomes B. Bereaved relatives’ views about participating in cancer research. Palliat Med 2012;26:379-83. https://doi.org/10.1177/0269216311405091.
- Beecham J, Knapp M, Thornicroft GJ. Measuring Mental Health Needs. London: Gaskell; 2001.
- McCrone P. Capturing the costs of end-of-life care: comparisons of multiple sclerosis, Parkinson’s disease, and dementia. J Pain Symptom Manage 2009;38:62-7. https://doi.org/10.1016/j.jpainsymman.2009.04.006.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Faschingbauer TR, Zisook S, DeVaul R, Zisook S. Biopsychosocial Aspects of Bereavement. Washington, DC: American Psychiatric Press, Inc.; 1987.
- Mullin PA, Lohr KN, Bresnahan BW, McNulty P. Applying cognitive design principles to formatting HRQOL instruments. Qual Life Res 2000;9:13-27. https://doi.org/10.1023/A:1008923301313.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. https://doi.org/10.1001/jama.2009.1198.
- Johnson MJ, McSkimming P, McConnachie A, Geue C, Millerick Y, Briggs A, et al. The feasibility of a randomised controlled trial to compare the cost-effectiveness of palliative cardiology or usual care in people with advanced heart failure: two exploratory prospective cohorts. Palliat Med 2018;32:1133-41. https://doi.org/10.1177/0269216318763225.
- NHS . Authorities and Trusts n.d. www.nhs.uk/servicedirectories/pages/nhstrustlisting.aspx (accessed 8 February 2019).
- Richie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. Abingdon-on-Thomas: Routledge; 2002.
- Spencer L, Ritchie J, Lewis J. Quality in Qualitative Evaluation: A Framework for Assessing Research Evidence. Occasional Series No 2. London: The Cabinet Office; 2003.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- O’Cathain A, Murphy E, Nicholl J. Research methods & reporting three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision n.d. https://icd.who.int/browse10/2016/en (accessed 9 August 2019).
- Sekhon M, Cartwright M, Francis JJ. Acceptability of health care interventions: A theoretical framework and proposed research agenda. Br J Health Psychol 2018;23:519-31. https://doi.org/10.1111/bjhp.12295.
- Bone AE, Evans CJ, Etkind SN, Sleeman KE, Gomes B, Aldridge M, et al. Factors associated with older people’s emergency department attendance towards the end of life: a systematic review. Eur J Public Health 2019;29:67-74. https://doi.org/10.1093/eurpub/cky241.
- Rinck G, van den Bos GA, Kleijnen J, de Haes HJ, Schadé E, Veenhof CH. Methodological issues in effectiveness research on palliative cancer care: a systematic review. J Clin Oncol 1997;15:1697-707. https://doi.org/10.1200/JCO.1997.15.4.1697.
- White N, Reid F, Harris A, Harries P, Stone P. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts?. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0161407.
- Brighton LJ, Selman LE, Gough N, Nadicksbernd JJ, Bristowe K, Millington-Sanders C, et al. Evaluation of multiprofessional training. BMJ Support Palliat Care 2017;8:45-8. https://doi.org/10.1136/bmjspcare-2017-001447.
- Selman LE, Brighton LJ, Hawkins A, McDonald C, O’Brien S, Robinson V, et al. The effect of communication skills training for generalist palliative care providers on patient-reported outcomes and clinician behaviours: a systematic review and meta-analysis. J Pain Symptom Manage 2017;54:404-16. https://doi.org/10.1016/j.jpainsymman.2017.04.007.
- Pinto C, Bristowe K, Witt J, Davies JM, de Wolf-Linder S, Dawkins M, et al. Perspectives of patients, family caregivers and health professionals on the use of outcome measures in palliative care and lessons for implementation: a multi-method qualitative study. Ann Palliat Med 2018;7:S137-50. https://doi.org/10.21037/apm.2018.09.02.
- Etkind SN, Daveson BA, Kwok W, Witt J, Bausewein C, Higginson IJ, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015;49:611-24. https://doi.org/10.1016/j.jpainsymman.2014.07.010.
- Kutner JS, Bryant LL, Beaty BL, Fairclough DL. Symptom distress and quality-of-life assessment at the end of life: the role of proxy response. J Pain Symptom Manage 2006;32:300-10. https://doi.org/10.1016/j.jpainsymman.2006.05.009.
- Elmstedt D, Mogensen H, Hallmans D-E, Tavelin B, Lundström S, Lindskog M. Cancer patients hospitalised in the last week of life risk insufficient care quality – a population-based study from the Swedish Register of Palliative Care. Acta Oncol 2019;58:432-8. https://doi.org/10.1080/0284186X.2018.1556802.
- Benoliel JQ, Davis AJ, Krueger JC. Patients, Nurses, Ethics. New York, NY: American Journal of Nursing; 1980.
- de Raeve L. Ethical issues in palliative care research. Palliat Med 1994;8:298-305. https://doi.org/10.1177/026921639400800405.
- Koffman J, Morgan M, Edmonds P, Speck P, Higginson IJ. Vulnerability in palliative care research: findings from a qualitative study of black Caribbean and white British patients with advanced cancer. J Med Ethics 2009;35:440-4. https://doi.org/10.1136/jme.2008.027839.
- Gysels M, Shipman C, Higginson IJ. ‘I will do it if it will help others:’ motivations among patients taking part in qualitative studies in palliative care. J Pain Symptom Manage 2008;35:347-55. https://doi.org/10.1016/j.jpainsymman.2007.05.012.
- Gysels M, Shipman C, Higginson IJ. Is the qualitative research interview an acceptable medium for research with palliative care patients and carers?. BMC Med Ethics 2008;9. https://doi.org/10.1186/1472-6939-9-7.
- Karbwang J, Koonrungsesomboon N, Torres CE, Jimenez EB, Kaur G, Mathur R, et al. What information and the extent of information research participants need in informed consent forms: a multi-country survey. BMC Med Ethics 2018;19. https://doi.org/10.1186/s12910-018-0318-x.
- Department of Health and Social Care . Good Practice in Consent Implementation Guide 2001.
- Kennedy I, Grubb A. Medical Law: Text and Materials. London: Butterworths; 2000.
- Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-67.
- Lancaster GA. Pilot and feasibility studies come of age!. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/2055-5784-1-1.
- Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0150205.
- Department of Health and Social Care . Mental Capacity Act 2005 2005. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 12 August 2019).
- McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: a retrospective study. Int J Geriatr Psychiatry 1997;12:404-9. https://doi.org/10.1002/(SICI)1099-1166(199703)12:3<404::AID-GPS529>3.0.CO;2-2.
- Sampson EL, Ritchie CW, Lai R, Raven PW, Blanchard MR. A systematic review of the scientific evidence for the efficacy of a palliative care approach in advanced dementia. Int Psychogeriatr 2005;17:31-40. https://doi.org/10.1017/S1041610205001018.
- University of Bristol . Adults Lacking Capacity – On-Line Toolkit 2010.
- Scott S, Jones L, Blanchard MR, Sampson EL. Study protocol: the behaviour and pain in dementia study (BePAID). BMC Geriatr 2011;11. https://doi.org/10.1186/1471-2318-11-61.
- Jones L, Harrington J, Scott S, Davis S, Lord K, Vickerstaff V, et al. CoMPASs: IOn programme (Care Of Memory Problems in Advanced Stages of dementia: Improving Our Knowledge): protocol for a mixed methods study. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-002265.
- Davies K, Collerton JC, Jagger C, Bond J, Barker SA, Edwards J, et al. Engaging the oldest old in research: lessons from the Newcastle 85+ study. BMC Geriatr 2010;10. https://doi.org/10.1186/1471-2318-10-64.
- Dewing J. Participatory research: a method for process consent with persons who have dementia. Dementia 2007;6:11-25. https://doi.org/10.1177/1471301207075625.
- Mathie E, Goodman C, Crang C, Froggatt K, Iliffe S, Manthorpe J, et al. An uncertain future: the unchanging views of care home residents about living and dying. Palliat Med 2012;26:734-43. https://doi.org/10.1177/0269216311412233.
- Rees E, Hardy J. Novel consent process for research in dying patients unable to give consent. BMJ 2003;327. https://doi.org/10.1136/bmj.327.7408.198.
- Department of Health Scientific Development and Bioethics Division . Guidance on Nominating a Consultee for Research Involving Adults Who Lack Capacity to Consent 2008. www.manchester.gov.uk/download/downloads/id/12218/guidance_on_nominating_a_consultee_for_research_involving_adults_who_lack_capacity_to_consent.pdf (accessed 30 August 2019).
Appendix 1 The AMBER care bundle tool
Reproduced with permission from Guy’s and St Thomas’ NHS Foundation Trust.
Reproduced with permission from Guy’s and St Thomas’ NHS Foundation Trust.
Appendix 2 Recruitment of patients
The recruitment of patients at risk of reduced capacity
To comply with the Mental Capacity Act 2005 (MCA),105 research ethics approval was obtained to seek a ‘personal consultee’ or ‘nominated consultee’ agreement for patients with impaired capacity and for participants who lost capacity following the provision of informed consent.
Process of consent and assent for adults lacking capacity
Capacity was likely to be a major issue in this feasibility trial population; the presence of confusion might reduce patients’ capacity to give consent. People with cognitive impairment in the last year of life experience symptoms and care needs comparable to people with cancer,106 but have a high prevalence of poor symptom management, notably pain management, and often experience aggressive treatment at the end of life. 107 The MCA105 requires that those lacking capacity are only included in research that is likely to be of direct benefit to those taking part or to benefit the particular population under study. In this trial, ward patients receiving the AMBER care bundle intervention may benefit directly from improved quality of care.
Another potential direct benefit for those taking part in the feasibility trial was that screening assessments for delirium on admission to the ward might identify people with delirium at an earlier stage, allowing for earlier treatment. Excluding those without capacity from this research would not be an ethical principle of justice, as it would compromise the generalisability of findings by recruitment of an unrepresentative trial sample and would potentially exclude this vulnerable group from the benefits of research evidence in improving practice.
All participants were considered to have capacity unless it was established that they did not, and all practicable steps were taken to enable individuals to decide for themselves if they wished to participate. For example, with the advice and support of PPI members, the participant information sheet was designed using accessible language. A potential participant’s level of capacity was discussed with the referring clinician to identify participants with possible impaired capacity and to anticipate the likely consent procedure. Capacity was established when meeting the individual using the MCA four-step process:105
-
The individual was able to understand the information about the trial.
-
The individual was able to retain the information (even for a short time).
-
The individual was able to use or weigh up that information.
-
The individual was able to communicate his or her decision (by any means).
Potential participants’ mental capacity was anticipated as ranging from ‘able to give informed consent’ to ‘lacking capacity to give informed consent’ (Figure 6). We have previously developed processes of consent and assent that are tailored to an individual’s level of capacity that incorporate varying levels of capacity. We anticipated that some participants might lose capacity during the course of this feasibility trial because many of them might be close to death. Incorporating different processes of consent and assent is used in end-of-life care research studies and also among those participants of advanced age. 109–111 This feasibility trial aimed to explore how it would be possible to enable individuals with varying levels of capacity to decide for themselves if they wished to participate, and to incorporate a process of assent for adults lacking capacity.
FIGURE 6.
Process of seeking consent. CTIMP, Clinical Trial of an Investigational Medicinal Product. Reproduced with permission. 108

Consent in the moment for participants with impaired capacity
For adults with impaired capacity but able to ‘understand, retain and weigh-up information in the moment’, a process of consent in the moment was used with ongoing consent, whereby informed consent to participate was reaffirmed prior to each data collection point. 112 The approach of ‘consent in the moment’ has been developed and used in studies involving adults with dementia and/or cognitive impairment. 112,113 If a participant’s capacity declines to a point whereby they are no longer able to give informed consent in the moment, the researchers follow the procedure for adults lacking capacity detailed in Assent for adults lacking capacity.
Advanced consent and assent for participants who lose capacity
An advanced consent was incorporated in anticipation that some participants might lose capacity and may no longer have capacity to indicate their desire to withdraw from the trial. The process of advanced consent was informed by previous studies with older people111 and in end-of-life care. 114 Participants able to give informed consent were asked to indicate, should they lose capacity in the future, if they would wish to continue to be involved in the trial, and if they indicated ‘yes’ then they were to nominate a ‘personal consultee’ (e.g. NOK), or if not available a ‘nominated or professional consultee’ (e.g. social worker). The named consultee was then approached if in the future the participant lost capacity to an extent that they were no longer able to indicate their desire to withdraw from the trial and to complete patient-reported outcome measures, instead requiring a proxy informant (e.g. informal or formal carer). The procedure for assent for adults lacking capacity was followed to ascertain the named consultee’s opinion on the individual’s continued participation (see the following section).
Assent for adults lacking capacity
When an adult lacks capacity, a ‘personal consultee’ was sought to give an opinion on whether or not, in his/her knowledge of the potential participant, the participant would have wanted to participate in the feasibility trial had they had capacity to indicate this, and that participation would not cause them undue distress. 105,111 A personal consultee could be the NOK, immediate carer or attorney with lasting power of attorney. Identified consultees were given an information leaflet about the trial, a letter detailing why they had been chosen as a consultee and their responsibilities as a consultee. The consultee documents were informed by our previous research with older people,111 the MCA105 and MCA guidance,115 and PPI involvement. If contact could not be made with a personal consultee within 1 week of initial identification, a ‘nominated consultee’ was contacted. 109,110 The ‘nominated consultee’ had a professional relationship with the potential participant but could not be directly connected to the feasibility trial (i.e. not the principal investigator). 109 The nominated consultee was asked, based on their knowledge of the individual, to give an opinion on whether or not it was in the individual’s best interest to participate in the feasibility trial and that they would not be caused undue distress by participating. Participants’ general practitioners were also informed of their involvement in the trial.
Appendix 3 Research Ethics Committee approvals for substantial amendments
Appendix 4 Primary outcome measures
The Integrated Palliative care Outcome Scale
The IPOS is reproduced with the permission of Irene Higginson, Cicely Saunders Institute and King’s College London as the Intellectual Property owners of the IPOS.
‘HowRwe’ scale
Reproduced with permission from R-Outcomes Ltd (Bristol, UK; https://r-outcomes.com).
Appendix 5 Patients who were discharged and died within 100 days, by site and trial arm
| Descriptive variable | Trial arm and site, n (%) | |||
|---|---|---|---|---|
| Control | Intervention | |||
| Con1 (N = 10) | Con2 (N = 10) | Int1 (N = 10) | Int2 (N = 10) | |
| Number of days survived | ||||
| 1–20 | 3 (30) | 4 (40) | 3 (30) | 3 (30) |
| 21–40 | 2 (20) | 1 (10) | 2 (20) | 3 (30) |
| 41–60 | 3 (30) | 1 (10) | 2 (20) | 3 (30) |
| 61–80 | 1 (10) | 3 (30) | 1 (10) | 0 (0) |
| 81–100 | 0 (0) | 1 (10) | 2 (20) | 1 (10) |
| Unknown | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Non-elective readmission within 30 days of discharge? | ||||
| Yes | 4 (40) | 6 (60) | 2 (20) | 4 (40) |
| No | 6 (60) | 4 (40) | 8 (80) | 6 (60) |
| Place of death | ||||
| Person’s own home | 3 (30) | 1 (10) | 0 (0) | 1 (10) |
| Hospital | 2 (20) | 6 (60) | 1 (10) | 2 (20) |
| Care home | 0 (0) | 0 (0) | 2 (20) | 2 (20) |
| Hospice | 2 (20) | 0 (0) | 1 (10) | 0 (0) |
| Unknown | 3 (30) | 3 (30) | 6 (60) | 5 (50) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Place of death matched PPD? | ||||
| Yes | 3 (30) | 1 (10) | 1 (10) | 2 (20) |
| No | 2 (20) | 0 (0) | 1 (10) | 0 (0) |
| Unknown | 5 (50) | 9 (90) | 8 (80) | 8 (80) |
| Quality of handover information | ||||
| Poor | 2 (20) | 4 (40) | 2 (20) | 1 (10) |
| Average | 4 (40) | 2 (20) | 1 (10) | 6 (60) |
| Good | 2 (20) | 0 (0) | 6 (60) | 1 (10) |
| Very good | 2 (20) | 4 (40) | 1 (10) | 2 (20) |
Appendix 6 Pre- and post-implementation case note reviews (intervention wards)
| Descriptive variable | Implementation, n (%) | |
|---|---|---|
| Pre | Post | |
| Age group (years) | ||
| 50–60 | 0 (0) | 0 (0) |
| 61–70 | 0 (0) | 0 (0) |
| 71–80 | 2 (10) | 3 (15) |
| 81–90 | 11 (55) | 8 (40) |
| ≥ 91 | 7 (35) | 9 (45) |
| Primary diagnosis | ||
| Cardiology | 4 (20) | 2 (10) |
| Cancer | 2 (10) | 2 (10) |
| Acute respiratory | 5 (25) | 7 (35) |
| Dementia | 2 (10) | 2 (10) |
| Frailty | 1 (5) | 3 (15) |
| Stroke | 0 (0) | 1 (5) |
| Sepsis | 1 (5) | 3 (15) |
| Other | 5 (32) | 0 (0) |
| Uncertain recovery documented? | ||
| Yes | 18 (90) | 20 (100) |
| No | 2 (10) | 0 (0) |
| ACP in place? | ||
| Yes | 7a (35) | 20 (100) |
| No | 13 (65) | 0 (0) |
| Escalation plan documented? | ||
| Yes | 18 (90) | 20 (100) |
| No | 2 (10) | 0 (0) |
| DNAR/DNACPR status | ||
| Patient for CPR (decision documented) | 0 (0) | 0 (0) |
| Patient not to be resuscitated (decision documented) | 20 (100) | 20 (100) |
| No documented decision on resuscitation | 0 (0) | 0 (0) |
| Medical plan discussed and agreed with nursing staff? | ||
| Yes | 19 (95) | 20 (100) |
| No | 1 (5) | 0 (0) |
| Patient/family discussion? | ||
| Yes | 19 (95) | 20 (100) |
| No | 1 (5) | 0 (0) |
| Daily follow-up? | ||
| Yes | 19 (95) | 20 (100) |
| No – should have received | 1 (5) | 0 (0) |
| No – not needed | 0 (0) | 0 (0) |
| Assessment of capacity? | ||
| Yes | 10 (50) | 17 (85) |
| No – it was not needed | 9 (45) | 3 (15) |
| No – it was needed | 1 (5) | 0 (0) |
| PPC | ||
| Person’s own home | 2 (10) | 2 (10) |
| Hospital | 2 (10) | 7 (35) |
| Care home | 6 (30) | 10 (50) |
| Hospice | 1 (5) | 0 (0) |
| Preference not documented | 6 (30) | 1 (5) |
| Other (include patients who are undecided) | 3 (15) | 0 (0) |
| PPD | ||
| Person’s own home | 1 (5) | 2 (10) |
| Hospital | 2 (10) | 4 (20) |
| Care home | 1 (5) | 7 (35) |
| Hospice | 2 (10) | 1 (5) |
| Preference not documented | 3 (15) | 5 (25) |
| Other (include patients who are undecided) | 11 (55) | 1 (5) |
| Patient and family wishes documented | ||
| Wishes documented | 16 (80) | 20 (100) |
| DNAR decision only | 0 (0) | 0 (0) |
| No wishes documented | 4 (20) | 0 (0) |
| Patient offered discussion but refused | 0 (0) | 0 (0) |
| Length of hospital stay (days) | ||
| Mean (SD) | 12.6 (9.1) | 25.9 (19.4) |
| Range | 1–37 | 3–79 |
| Descriptive variable | Implementation, n (%) | |
|---|---|---|
| Pre | Post | |
| Number of days survived | ||
| 1–20 | 3 (30) | 2 (20) |
| 21–40 | 2 (20) | 5 (50) |
| 41–60 | 2 (20) | 2 (20) |
| 61–80 | 1 (10) | 0 (0) |
| 81–100 | 2 (20) | 1 (10) |
| Unknown | 0 (0) | 0 (0) |
| Non-elective readmission within 30 days of discharge? | ||
| Yes | 2 (20) | 0 (0) |
| No | 8 (80) | 20 (100) |
| Place of death | ||
| Person’s own home | 0 (0) | 1 (10) |
| Hospital | 1 (10) | 0 (0) |
| Care home | 2 (20) | 8 (80) |
| Hospice | 1 (10) | 0 (0) |
| Unknown | 6 (60) | 1 (10) |
| Other | 0 (0) | 0 (0) |
| Place of death matched PPD? | ||
| Yes | 1 (10) | 6 (60) |
| No | 1 (10) | 2 (20) |
| Unknown | 8 (80) | 2 (20) |
| Quality of handover information | ||
| Poor | 2 (20) | 0 (0) |
| Average | 1 (10) | 1 (10) |
| Good | 6 (60) | 2 (20) |
| Very good | 1 (10) | 7 (70) |
| Descriptive variable | Implementation, n (%) | |
|---|---|---|
| Pre (N = 20) | Post (N = 18) | |
| Age group (years) | ||
| 40–60 | 3 (15) | 2 (11) |
| 61–70 | 6 (30) | 4 (22) |
| 71–80 | 6 (30) | 5 (28) |
| 81–90 | 5 (25) | 6 (33) |
| ≥ 91 | 0 (0) | 1 (6) |
| Primary diagnosis | ||
| Cardiology | 0 (0) | 1 (6) |
| Cancer | 6 (30) | 3 (17) |
| Acute respiratory | 2 (10) | 8 (44) |
| Chronic respiratory | 10 (50) | 1 (6) |
| Stroke | 1 (5) | 0 (0) |
| Dementia | 0 (0) | 1 (6) |
| Sepsis | 0 (0) | 1 (6) |
| Other | 1 (5) | 3 (17) |
| Uncertain recovery documented? | ||
| Yes | 12 (60) | 18 (100) |
| No | 8 (40) | 0 (0) |
| ACP in place? | ||
| Yes | 2a (10) | 18 (100) |
| No | 18 (90) | 0 (0) |
| Escalation plan documented? | ||
| Yes | 13 (65) | 18 (100) |
| No | 7 (35) | 0 (0) |
| DNAR/DNACPR status | ||
| Patient for CPR (decision documented) | 2 (10) | 0 (0) |
| Patient not to be resuscitated (decision documented) | 15 (75) | 18 (100) |
| No documented decision on resuscitation | 3 (15) | 0 (0) |
| Medical plan discussed and agreed with nursing staff? | ||
| Yes | 16 (80) | 18 (100) |
| No | 4 (20) | 0 (0) |
| Patient/family discussion? | ||
| Yes | 13 (65) | 18 (100) |
| No | 7 (35) | 0 (0) |
| Daily follow-up? | ||
| Yes | 12 (60) | 18 (100) |
| No – should have received | 8 (40) | 0 (0) |
| No – not needed | 0 (0) | 0 (0) |
| Assessment of capacity? | ||
| Yes | 7 (35) | 11 (61) |
| No – it was not needed | 12 (60) | 7 (39) |
| No – it was needed | 1 (5) | 0 (0) |
| PPC | ||
| Person’s own home | 10 (50) | 5 (28) |
| Hospital | 3 (15) | 9 (50) |
| Care home | 3 (15) | 4 (22) |
| Hospice | 1 (5) | 0 (0) |
| Preference not documented | 3 (15) | 0 (0) |
| Other (include patients who are undecided) | 0 (0) | 0 (0) |
| PPD | ||
| Person’s own home | 3 (15) | 6 (33) |
| Hospital | 0 (0) | 5 (28) |
| Care home | 3 (15) | 4 (22) |
| Hospice | 0 (0) | 0 (0) |
| Preference not documented | 12 (60) | 3 (17) |
| Other (include patients who are undecided) | 2 (10) | 0 (0) |
| Patient and family wishes documented | ||
| Wishes documented | 10 (50) | 15 (83) |
| DNAR decision only | 5 (25) | 2 (11) |
| No wishes documented | 4 (20) | 1 (6) |
| Patient offered discussion but refused | 1 (5) | 0 (0) |
| Length of hospital stay (days) | ||
| Mean (SD) | 16.7 (18.2) | 19.8 (15.1) |
| Range | 2–82 | 2–52 |
| Descriptive variable | Implementation, n (%) | |
|---|---|---|
| Pre (N = 10) | Post (N = 8) | |
| Number of days survived | ||
| 1–20 | 3 (30) | 5 (63) |
| 21–40 | 3 (30) | 2 (25) |
| 41–60 | 3 (30) | 0 (0) |
| 61–80 | 0 (0) | 1 (12) |
| 81–100 | 1 (10) | 0 (0) |
| Unknown | 0 (0) | 0 (0) |
| Non-elective readmission within 30 days of discharge? | ||
| Yes | 4 (40) | 2 (25) |
| No | 6 (60) | 6 (75) |
| Place of death | ||
| Person’s own home | 1 (10) | 2 (25) |
| Hospital | 2 (20) | 2 (25) |
| Care home | 2 (20) | 4 (50) |
| Hospice | 0 (0) | 0 (0) |
| Unknown | 5 (50) | 0 (0) |
| Other | 0 (0) | 0 (0) |
| Place of death matched PPD? | ||
| Yes | 2 (20) | 6 (75) |
| No | 0 (0) | 1 (12.5) |
| Unknown | 8 (80) | 1 (12.5) |
| Quality of handover information | ||
| Poor | 1 (10) | 1 (12.5) |
| Average | 6 (60) | 1 (12.5) |
| Good | 1 (10) | 2 (25) |
| Very good | 2 (20) | 4 (50) |
Appendix 7 Reasons for not participating in qualitative interviews
| Reasons | Trial arm, n (%) | |
|---|---|---|
| Control | Intervention | |
| Number of participants who agreed to be approached by a researcher (N = 35) | 20 (57.1) | 15 (42.9) |
| Number of participants approached (N = 29) | 16 (80.0) | 13 (86.7) |
| Invalid contact details (N = 4) | 2 (10.0) | 2 (13.3) |
| Participant died prior to contact (N = 2) | 2 (10.0) | 0 (0.0) |
| Reasons for declining (N = 6) | ||
| Agreed but was too unwell on the day of the interview (spouse took part) | 1 (16.7) | 0 (0.0) |
| Main carer (will not have time) | 1 (16.7) | 0 (0.0) |
| Patient died a few days after the carer expressed interest | 3 (49.9) | 0 (0.0) |
| Does not want to relive the experiences | 0 (0.0) | 1 (16.7) |
Appendix 8 Descriptive analysis of participant self- or proxy-reported symptoms
| Item | Trial arm, mean (95% CI) | |
|---|---|---|
| Control | Intervention | |
| Baseline | ||
| Pain | 1.8 (1.3 to 2.3) (n = 32) | 1.5 (1.3 to 1.9) (n = 27) |
| Shortness of breath | 1.2 (0.7 to 1.6) (n = 34) | 1.6 (1.2 to 2.0) (n = 28) |
| Weakness or lack of energy | 2.9 (2.6 to 3.2) (n = 34) | 3.2 (2.9 to 3.5) (n = 29) |
| Nausea (feeling like you are going to be sick) | 1.0 (0.5 to 1.4) (n = 32) | 0.6 (0.2 to 0.9) (n = 27) |
| Vomiting (being sick) | 0.3 (< 0.1 to 0.6) (n = 36) | 0.3 (< 0.1 to 0.6) (n = 28) |
| Poor appetite | 2.4 (1.9 to 2.9) (n = 32) | 2.4 (1.9 to 3.0) (n = 28) |
| Constipation | 1.4 (0.9 to 1.8) (n = 34) | 1.2 (0.6 to 1.8) (n = 23) |
| Sore or dry mouth | 2.2 (1.7 to 2.6) (n = 35) | 3.0 (1.6 to 2.4) (n = 27) |
| Drowsiness | 2.0 (1.5 to 2.5) (n = 33) | 2.5 (2.1 to 3.0) (n = 29) |
| Poor mobility | 3.4 (3.1 to 3.7) (n = 33) | 3.5 (3.2 to 3.8) (n = 28) |
| 3–5 days | ||
| Pain | 2.0 (1.2 to 2.8) (n = 17) | 1.1 (0.6 to 1.7) (n = 14) |
| Shortness of breath | 0.9 (0.3 to 1.5) (n = 17) | 1.3 (0.7 to 1.9) (n = 14) |
| Weakness or lack of energy | 2.7 (2.1 to 3.3) (n = 18) | 2.8 (2.2 to 3.4) (n = 15) |
| Nausea (feeling like you are going to be sick) | 1.0 (0.4 to 1.6) (n = 16) | 0.3 (< 0.1 to 0.6) (n = 14) |
| Vomiting (being sick) | 0.4 (< 0.1 to 0.8) (n = 19) | N/A (n = 14) |
| Poor appetite | 2.4 (1.7 to 3.2) (n = 16) | 1.9 (1.0 to 2.8) (n = 14) |
| Constipation | 1.6 (0.9 to 2.3) (n = 18) | 1.2 (0.3 to 2.0) (n = 11) |
| Sore or dry mouth | 2.1 (1.5 to 2.7) (n = 19) | 1.5 (0.7 to 2.3) (n = 14) |
| Drowsiness | 1.7 (1.0 to 2.3) (n = 15) | 2.1 (1.3 to 2.8) (n = 16) |
| Poor mobility | 3.2 (2.6 to 3.7) (n = 18) | 3.1 (2.6 to 3.6) (n = 16) |
| 10–15 days | ||
| Pain | 2.0 (0.2 to 3.8) (n = 5) | 1.9 (0.4 to 3.3) (n = 7) |
| Shortness of breath | 1.0 (< 0.1 to 2.2) (n = 5) | 1.4 (0.3 to 2.6) (n = 7) |
| Weakness or lack of energy | 2.5 (0.9 to 4.1) (n = 4) | 3.7 (3.3 to 4.2) (n = 7) |
| Nausea (feeling like you are going to be sick) | 1.8 (0.4 to 3.2) (n = 5) | 0.2 (< 0.1 to 0.6) (n = 6) |
| Vomiting (being sick) | 0.2 (< 0.1 to 0.8) (n = 5) | N/A (n = 6) |
| Poor appetite | 2.0 (0.7 to 3.3) (n = 4) | 2.3 (1.1 to 3.4) (n = 7) |
| Constipation | 1.4 (0.3 to 2.5) (n = 5) | 1.8 (0.6 to 3.1) (n = 6) |
| Sore or dry mouth | 1.2 (0.2 to 2.2) (n = 5) | 1.4 (0.5 to 2.3) (n = 7) |
| Drowsiness | 0.8 (< 0.1 to 2.2) (n = 5) | 2.0 (0.8 to 3.2) (n = 7) |
| Poor mobility | 3.6 (2.9 to 4.3) (n = 5) | 3.6 (3.1 to 4.1) (n = 7) |
Appendix 9 Level of missing data: Integrated Palliative care Outcome Scale symptoms
| Item | Data, n (%) | |||
|---|---|---|---|---|
| Complete | ‘Cannot assess’ | ‘Don’t know’ | Missing | |
| Baseline (N = 65) | ||||
| Pain | 59 (90.8) | 5 (7.7) | 0 | 1 (1.5) |
| Shortness of breath | 62 (95.4) | 3 (4.6) | 0 | 0 |
| Weakness or lack of energy | 63 (96.9) | 1 (1.5) | 0 | 1 (1.5) |
| Nausea (feeling like you are going to be sick) | 59 (90.8) | 4 (96.9) | 0 | 2 (3.1) |
| Vomiting (being sick) | 64 (98.5) | 0 | 0 | 1 (1.5) |
| Poor appetite | 60 (92.3) | 5 (7.7) | 0 | 0 |
| Constipation | 57 (87.7) | 4 (6.2) | 1 (1.5) | 3 (4.6) |
| Sore or dry mouth | 62 (95.4) | 2 (98.5) | 0 | 1 (1.5) |
| Drowsiness | 62 (95.4) | 3 (4.6) | 0 | 0 |
| Poor mobility | 61 (93.9) | 4 (6.2) | 0 | 0 |
| 3–5 days (N = 36) | ||||
| Pain | 31 (86.1) | 4 (11.1) | 0 | 1 (2.8) |
| Shortness of breath | 31 (86.1) | 2 (91.7) | 0 | 3 (8.3) |
| Weakness or lack of energy | 33 (91.7) | 2 (5.6) | 0 | 1 (2.8) |
| Nausea (feeling like you are going to be sick) | 30 (83.3) | 4 (11.1) | 0 | 2 (5.6) |
| Vomiting (being sick) | 33 (91.7) | 1 (2.8) | 0 | 2 (5.6) |
| Poor appetite | 30 (83.3) | 5 (13.9) | 0 | 1 (2.8) |
| Constipation | 29 (80.6) | 5 (13.9) | 0 | 2 (5.6) |
| Sore or dry mouth | 33 (91.7) | 2 (5.6) | 0 | 1 (2.8) |
| Drowsiness | 31 (86.1) | 5 (13.9) | 0 | 0 |
| Poor mobility | 34 (94.4) | 2 (5.6) | 0 | 0 |
| 10–15 days (N = 12) | ||||
| Pain | 12 (100.0) | 0 | 0 | 0 |
| Shortness of breath | 12 (100.0) | 0 | 0 | 0 |
| Weakness or lack of energy | 11 (91.7) | 0 | 1 (8.3) | 0 |
| Nausea (feeling like you are going to be sick) | 11 (91.7) | 0 | 0 | 1 (8.3) |
| Vomiting (being sick) | 11 (91.7) | 0 | 0 | 1 (8.3) |
| Poor appetite | 11 (91.7) | 1 (8.3) | 0 | 0 |
| Constipation | 11 (91.7) | 0 | 0 | 1 (8.3) |
| Sore or dry mouth | 12 (100.0) | 0 | 0 | 0 |
| Drowsiness | 12 (100.0) | 0 | 0 | 0 |
| Poor mobility | 12 (100.0) | 0 | 0 | 0 |
Appendix 10 Descriptive analysis of participant self-reported outcomes, by trial arm for all time points
| Data collection time point and outcome measure | Trial arm, mean (95% CI) | |
|---|---|---|
| Control | Intervention | |
| Baseline | ||
| IPOS patient/family anxiety and communication subscale | 13.1 (11.2 to 15.1) (n = 27) | 13.4 (11.5 to 15.3) (n = 22) |
| howRwe | 12.8 (11.4 to 14.2) (n = 17) | 13.4 (10.9 to 15.9) (n = 7) |
| 3–5 days | ||
| IPOS patient/family anxiety and communication subscale | 13.4 (11.1 to 15.6) (n = 13) | 14.3 (11.9 to 16.7) (n = 13) |
| howRwe | 13.9 (11.8 to 16.0) (n = 8) | 12.3 (10.9 to 13.8) (n = 3) |
| 10–15 days | ||
| IPOS patient/family anxiety and communication subscale | 12.0 (6.5 to 17.5) (n = 12) | 13.9 (8.9 to 18.8) (n = 7) |
| howRwe | 14.0 (9.0 to 19.0) (n = 3) | 11.0 (n = 1) |
Appendix 11 Time spent to implement the AMBER care bundle in the treatment sites
| Health-care professional | Activities (time in hours) | ||
|---|---|---|---|
| Individual | Teama | ||
| Central research team | |||
| Research nurse (NHS band 7) | 100% FTE for 3 months | ||
| Senior research associate | 10 | ||
| Site Int1 | In situ | Ex situ | 14.5 |
| Senior nurse (NHS bands 7 or 8) | 2 | 4.7 | |
| Nurse (NHS bands 3–6) | 7.7 | 6.4 | |
| Health-care assistant | 7.3 | ||
| Occupational therapist | 0.3 | 1.3 | |
| Occupational therapist assistant | 0.3 | ||
| Physiotherapist | 1 | 1.3 | |
| Physiotherapist assistant | 0.7 | ||
| Ward clerk | 1 | 4.4 | |
| Consultant | 4.3 | 3.8 | |
| Specialty registrar | 4.7 | 3 | |
| Physician associate | 0.7 | 2.3 | |
| Site Int2 | 5.3 | ||
| Senior nurse (NHS bands 7 or 8) | 13.5 | 7.3 | |
| Nurse (NHS bands 3–6) | 8.3 | 18.7 | |
| Health-care assistant | 0.5 | ||
| Consultant | 4.5 | 1.8 | |
| Specialty registrar | 4 | 5 | |
| Foundation doctor | 2.5 | 1.5 | |
| Clinical social worker | 4.1 | ||
| Ward staff or clerk | 2.5 | 0.8 | |
Appendix 12 Health-care and social care utilisation and informal care provision at the follow-up interview
| Type of care | Trial arm | |||||
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| N | User, n (%) | Utilisation, mean (SD) | N | User, n (%) | Utilisation, mean (SD) | |
| Investigation/diagnostic tests | ||||||
| Blood test | 5 | 5 (100) | 16.25 (22.87) | 7 | 7 (100) | 8.00 (2.00) |
| X-ray | 5 | 3 (60) | 3.50 (2.12) | 7 | 2 (29) | 1.00 (0.00) |
| Echocardiogram | 5 | 1 (20) | 1.00 (N/A) | 7 | 2 (29) | 1.00 (0.00) |
| Electrocardiogram | 5 | 0 (0) | N/A (N/A) | 7 | 3 (43) | 2.00 (1.00) |
| Ultrasound | 5 | 3 (60) | 1.00 (0.00) | 7 | 2 (29) | 1.00 (0.00) |
| CT/CAT scan | 5 | 3 (60) | 1.00 (0.00) | 7 | 3 (43) | 1.00 (0.00) |
| Magnetic resonance image | 5 | 0 (0) | N/A (N/A) | 7 | 1 (14) | N/A (N/A) |
| Other | 5 | 4 (80) | 3.00 (N/A) | 7 | 3 (43) | 1.00 (N/A) |
| Informal care (hours) | ||||||
| Personal care | 5 | 4 (80) | 25.75 (10.90) | 7 | 3 (43) | 3.00 (1.41) |
| Help with medical procedures | 5 | 4 (80) | 13.00 (2.65) | 7 | 3 (43) | 2.00 (1.41) |
| Help inside the home | 5 | 3 (60) | 2.00 (1.00) | 7 | 4 (57) | 2.50 (1.29) |
| Help outside the home | 5 | 2 (40) | 5.50 (2.12) | 7 | 4 (57) | 2.50 (1.00) |
| Time spent ‘on call’ | 5 | 3 (60) | 8.50 (9.19) | 7 | 2 (29) | 5.00 (0.00) |
| Other | 5 | 0 (0) | N/A (N/A) | 7 | 2 (29) | 2.50 (0.71) |
| EQ-5D index score | 3 | 3 | –0.01 (0.23) | 7 | 7 | –0.11 (0.36) |
Appendix 13 Bereavement survey findings
| Survey questions | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 8) | Control (N = 7) | |
| Awareness of prognosis | ||
| Was the patient aware they were going to die because of their illness? | ||
| Yes, she/he certainly knew | 5 (62.5) | 5 (71.4) |
| Yes, she/he probably knew | 3 (37.5) | 2 (28.6) |
| No, she/he probably did not know | 0 | 0 |
| No, she/he definitely did not know | 0 | 0 |
| Not sure if she/he knew or not | 0 | 0 |
| Did any health professional discuss with the patient the fact that she/he was likely to die because of the illness? | ||
| Yes | 0 | 2 (28.6) |
| No | 5 (62.5) | 4 (57.1) |
| Don’t know | 3 (37.5) | 1 (14.3) |
| N/A | 0 | 0 |
| Not ticked | 0 | 0 |
| Communication and information sharing | ||
| Did you receive information about her/his condition that was clear and easy to understand? | ||
| Yes, most of the time | 5 (62.5) | 4 (57.1) |
| Sometimes | 3 (37.5) | 2 (28.6) |
| No, not at all | 0 | 1 (14.3) |
| Not applicable | 0 | 0 |
| Don’t know | 0 | 0 |
| No answer | 0 | 0 |
| Do you remember receiving information on a day-to-day basis that helped you understand the reason for the care she/he received? | ||
| Yes, most of the time | 3 (37.5) | 4 (57.1) |
| Sometimes | 5 (62.5) | 1 (14.3) |
| No, not at all | 0 | 2 (28.6) |
| Not applicable | 0 | 0 |
| Don’t know | 0 | 0 |
| No answer | 0 | 0 |
| Did you receive consistent information about her/his condition? | ||
| Yes, most of the time | 4 (50.0) | 4 (57.1) |
| Sometimes | 4 (50.0) | 2 (28.6) |
| No, not at all | 0 | 1 (14.3) |
| Not applicable | 0 | 0 |
| Don’t know | 0 | 0 |
| No answer | 0 | 0 |
| Involvement of palliative care | ||
| Did the patient see a palliative care or Macmillan Cancer Support team at that hospital? | ||
| Yes | 2 (25.0) | 6 (85.7) |
| No, this was not wanted | 1 (12.5) | 0 |
| No, this was not needed | 2 (25.0) | 0 |
| No, this was never offered | 2 (25.0) | 1 (14.3) |
| No, they offered to visit but never came | 0 | 0 |
| Don’t know | 1 (12.5) | 0 |
| No, but not specified | 0 | 0 |
| N/A | 0 | 0 |
| Not ticked | 0 | 0 |
| Place of death | ||
| Patient preference – place where the patient would have preferred to die | ||
| In his/her own home | 6 (75.0) | 4 (57.1) |
| In the home of a relative or friend | 0 | 0 |
| In a hospice | 0 | 0 |
| Hospital | 1 (12.5) | 0 |
| In a nursing home | 1 (12.5) | 0 |
| Residential home | 0 | 0 |
| Elsewhere | 0 | 0 |
| No preference | 0 | 1 (14.3) |
| Don’t know | 0 | 2 (28.6) |
| Own home or hospice | 0 | 0 |
| Own home or home of a relative or friend | 0 | 0 |
| Hospice or nursing home | 0 | 0 |
| Own home or hospital | 0 | 0 |
| No answer | 0 | 0 |
| Place of death – place where the patient died | ||
| Own home | 0 | 1 (14.3) |
| Home of relative or friend | 0 | 0 |
| Hospice | 1 (12.5) | 3 (42.9) |
| Hospital | 5 (62.5) | 3 (42.9) |
| Nursing home | 2 (25.0) | 0 |
| Residential home | 0 | 0 |
| Elsewhere | 0 | 0 |
| Don’t know | 0 | 0 |
| No answer | 0 | 0 |
List of abbreviations
- ACP
- advance care plan
- AKPS
- Australian-modified Karnofsky Performance Status scale
- AMBER
- Assessment; Management; Best practice; Engagement; Recovery uncertain
- CI
- confidence interval
- CNS
- clinical nurse specialist
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- DGH
- district general hospital
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HCP
- health-care professional
- HRA
- Health Research Authority
- IPOS
- Integrated Palliative care Outcome Scale
- MCA
- Mental Capacity Act 2005
- MDT
- multidisciplinary team
- MRC
- Medical Research Council
- NIHR
- National Institute for Health Research
- NOK
- next of kin
- PAG
- Project Advisory Group
- PPC
- preferred place of care
- PPD
- preferred place of death
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- TS DMEC
- Trial Steering and Data Monitoring and Ethics Committee
