Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/69. The contractual start date was in September 2011. The draft report began editorial review in June 2017 and was accepted for publication in March 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mark T Drayson reports personal fees from Abingdon Health (Abingdon Health, York, UK) outside the submitted work. Stella Bowcock reports personal fees from Amgen (Amgen, CA, USA) and Celgene (Celgene, NJ, USA) outside the submitted work, non-financial support to attend educational meetings and has a patent issued for a device broadly related to the work. Tim Planche reports personal fees from Pfizer (Pfizer, CT, USA), Actellion (Actellion, Allschnil, Switzerland) and Astellas (Astellas Pharma, Tokyo, Japan) outside the submitted work. Kwee Yong reports grants from Janssen (Janssen Pharmaceutica, Beerse, Belgium), Celgene and Chugai (Chugai Pharmaceutical Co Tokyo, Japan) outside the submitted work. David Meads is a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) European Economic and Social Committee (EESC) Methods Group and the NIHR HTA EESC Panel. Claire T Hulme is a member of the NIHR HTA Commissioning Board. Janet A Dunn is a member of the NIHR Efficacy and Mechanism Evaluation board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Drayson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Myeloma is a cancer of bone marrow plasma cells that causes anaemia, skeletal fractures, renal failure and profound immunodeficiency. There are approximately 5500 new cases of myeloma in the UK per annum. 1 However, the overall prevalence is increasing, given the improved survival rates over the past four decades. 2 The median age at presentation is approximately 70 years and only 15% of patients are aged < 60 years. Myeloma has a higher incidence in African-Caribbean ethnic groups than in Caucasians, but there are few other distinctive epidemiological features. 3 The majority of cases present de novo, but it is now recognised that this is preceded by an asymptomatic monoclonal gammopathy of undetermined significance phase in virtually all patients. 4
Myeloma causes profound immunodeficiency and recurrent serious infections. One-quarter of patients will have a serious infection within 3 months of diagnosis. Ten per cent of patients die within the first 60 days of diagnosis, with bacterial infection directly causing 45% of these deaths. 5 Recent advances in antimyeloma treatment have improved overall survival significantly, yet this high early-death rate remains little changed, affecting all prognostic groups. Patients who may have survived long term with current antimyeloma treatment are dying soon after diagnosis, with the biggest single cause being bacterial infection. Therefore, newly diagnosed myeloma patients may benefit from antibacterial prophylaxis to prevent infection, hospital admission and early death. Reducing infection may also improve response to antimyeloma treatment by reducing interruptions of antimyeloma treatment and reducing immune responses to infection that promote myeloma cell survival and growth. In patients with other causes of immunodeficiency, such as neutropenia, asplenia, human immunodeficiency virus infection or reflux nephropathy, the importance of prophylactic antibiotics to prevent infection is well established and the administration of prophyactic antibiotics is common practice in the NHS. However, their usefulness in myeloma has not been established. Furthermore, some of the studies that established the use of antibacterial prophylaxis in other conditions predate the current rise in health care-associated infections (HCAIs), such as Clostridium difficile. The data from these older trials may not reflect current risks associated with antibiotic prophylaxis and so there is a need to reassess the effect of antibiotic prophylaxis on HCAI.
Existing research
Large studies in Europe and North America have identified a high mortality rate (8–20%) in the first 3 months following a diagnosis of myeloma, with bacterial infection being the single biggest identifiable cause. 5–8 Analysis of 3107 myeloma patients registered into UK Medical Research Council (MRC) trials from 1980 to 2002 showed that 10% of patients died within 60 days of trial entry and that 45% of these deaths were directly attributable to bacterial infection. 5
In the ‘MRC myeloma 9’ trial,9 which recruited between 2003 and 2008, overall incidence of infection in non-intensively treated patients was 214 out of 692 (30.9%), with a median time to infection from first diagnosis of myeloma of 43 days. Recent advances in antimyeloma treatment have improved survival significantly, yet this high early-death rate has remained unchanged for > 30 years and affects all prognostic groups. This suggests that current supportive care strategies, including the treatment of an infection once established, may be insufficient. Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli are the most frequent types of bacterial infection in myeloma patients. 10–15 The risk of these infections is associated with myeloma disease activity and abates as the disease is brought under control with antimyeloma treatment.
The mechanism by which the risk of infection is increased in the presence of active myeloma disease is not well understood. Over 90% of 3218 MRC myeloma trial patients had reduced levels of normal antibodies,16 and these patients’ susceptibility to bacterial chest infections is characteristic of antibody deficiency. However, a previous MRC trial (MacLennan ICM, Chapman C, Hazelwood M, North J. University of Birmingham, 1993) of immunoglobulin G (IgG) replacement treatment (double-blind, randomised, placebo-controlled trial of 203 patients) did not significantly reduce mortality or morbidity from infection in the first 3 months after diagnosis, despite effectively increasing total serum IgG levels and titres against specific bacterial pathogens. Myeloma patients are not usually neutropenic at presentation, and, in one study, only 11 out of 135 myeloma patients dying of infection within 60 days of diagnosis had a neutrophil count of < 2.0 × 109/l. 5 Other factors associated with active myeloma disease that might increase the risk of infection include low serum complement component 4 (C4) levels, increased transforming growth factor beta and increased interleukin 10. 17
Antibacterial prophylaxis is an obvious strategy to prevent infection, hospital admission and early death in these patients. Of the only two trials18,19 of prophylactic antibiotics in early myeloma, one prospective randomised study18 was with co-trimoxazole in the early 1990s. This showed a reduction in bacterial infections with prophylactic co-trimoxazole (2/28 treated vs. 11/26 control patients) but the sample size was too small to detect reduced mortality. A recent trial19 of 212 patients given ciprofloxacin, co-trimoxazole and placebo found no difference in the rate of infection. This study19 was, again, underpowered to show differences in infection and mortality. The low incidence of all infections (22%) in this study raises the question whether or not the patients were representative of the normal myeloma clinic population. A retrospective analysis20 of infections in 202 patients on new therapies found that 40% of patients had an infection within 6 months, with 80% of severe infections (16% of patients) occurring in the first cycle of treatment. Antibiotic prophylaxis was effective in preventing infections in those patients with surrogate markers of high tumour burden (monoclonal band of > 3 g/dl, platelet count of < 130 × 109/l), but not in those without these parameters.
Antibiotic prophylaxis should be active against the bacteria commonly causing infections in the patients treated, should be given as ideally oral, once-daily medication to maximise adherence and efficacy, and should have few side effects. For all of the above reasons, the quinolones, particularly ciprofloxacin and levofloxacin, are now the most commonly used antibiotics for chemoprophylaxis.
Although less than one-tenth of myeloma patients dying of infection are neutropenic, the immunosuppressed state in both neutropenic and early myeloma patients leads to bacterial infection. 5 The common organisms causing infection in myeloma are E. coli, S. pneumoniae, Klebsiella spp., S. aureus, Pseudomonas spp., Haemophilus spp. and Proteus spp. These are similar to those organisms seen in neutropenic infections, although Gram-negative infections are more common in neutropenia. Thus, studies on the use of prophylactic antibiotics active against the common pathogens that cause infection in neutropenia are pertinent to myeloma patients.
A large meta-analysis21 including 162 studies with 12,599 neutropenic patients found that all antibiotic prophylaxis significantly reduced the risk of death compared with placebo or no treatment [relative risk (RR) 0.66, 95% confidence interval (CI) 0.55 to 0.79]. Fluoroquinolone prophylaxis was the most effective and reduced the risk of all-cause mortality (RR 0.52, 95% CI 0.37 to 0.74), as well as of infection-related mortality, fever, clinically documented infection and microbiologically documented infections. Fluoroquinolone prophylaxis increased the risk of adverse events (AEs) (RR 1.52, 95% CI 0.79 to 2.92), but these were minor events. The benefit of reduction in infection-related mortality (RR 0.49, 95% CI 0.31 to 0.77) far outweighed any mortality from adverse effects because all-cause mortality was still markedly reduced (RR 0.52, 95% CI 0.37 to 0.74). These studies translate into a number needed to treat of 50 (95% CI 34 to 268) in order to prevent one death from all causes in neutropenic patients.
To date, only two studies22,23 have reported differences in costs, and both showed a cost benefit for prophylaxis. These studies focused on individual resource use elements, such as the total cost of antibiotics or hospital inpatient days. None of the trials included a comprehensive cost analysis or a full economic evaluation.
Levofloxacin prophylaxis may, in addition to preventing infection, improve response to antimyeloma treatment. Delivery of antimyeloma treatment is often delayed by infection and so reducing infectious episodes may increase the amount of antimyeloma treatment given. There is epidemiological and laboratory evidence that the cytokines and inflammatory mediators associated with bacterial infection may promote the growth of myeloma cells. 17 By reducing infections, antibiotic prophylaxis may reduce myeloma growth and potentiate response to antimyeloma treatment. This will be the first trial to assess these factors.
However, quinolones, along with other antibiotics, are implicated in increased risk of colonisation with antibiotic-resistant bacteria and invasive infection by those bacteria. These HCAIs have been an ever-increasing problem to the NHS over the past 10 years, accounting for significant morbidity and mortality. Up to one in four people carry S. aureus, and C. difficile may be carried by 1–3% of healthy people. 24 Up to 30% of long-term hospitalised patients may carry C. difficile. There were 36,095 cases of C. difficile-associated diarrhoea in the UK in 2008–9. 24
There is an increasing perception that antibiotic prophylaxis will increase numbers of HCAIs. A Midlands survey (carried out by the TEAMM trial management group) found that 24 haematologists did not use antibiotic prophylaxis alongside conventional myeloma chemotherapy, whereas eight haematologists did so in selected patients (unpublished audit). Half of the haematologists routinely used antibiotic prophylaxis in patients receiving intensive myeloma chemotherapy. Guidelines for the diagnosis and management of multiple myeloma published in 2009 by the UK Myeloma Forum3 on behalf of the British Committee for Standards in Haematology state that:
. . . there is insufficient evidence to recommend the routine use of prophylactic antibiotics (Grade C recommendation; level IV evidence).
Bird et al. 3
There are insufficient data on the relationship between changes in carriage rate of potentially pathogenic organisms during antibiotic treatment and the risk of subsequent infection with the same organism. From meta-analysis25 on antibiotic prophylaxis trials in neutropenia, there was no significant increase in C. difficile infection (7/1250 patients receiving a fluoroquinolone prophylaxis vs. 5/1279 receiving placebo or no treatment). Furthermore, recruitment to these trials predates Tackling Early Morbidity and Mortality in Myeloma (TEAMM) and the current problems with HCAIs by > 7 years. Although recent European guidelines26 recommend fluoroquinolone prophylaxis in severe neutropenia, adherence to this recommendation is not universal. In trials in which resistance data have been reported, patients on fluoroquinolones did not develop more infections with pathogens resistant to the drug than patients on placebo (RR 1.04, 95% CI 0.73 to 1.5). By reducing the number of clinical infections, levofloxacin may reduce the total amount of antibiotics used in these patients and lessen the emergence of resistance. 22 Although the emergence of bacteria resistant to fluoroquinolones can occur in units using fluoroquinolone antibiotic prophylaxis, there are no clear data on whether or not patients are harmed as a result. 27,28
In summary, the above data show that fluoroquinolone prophylaxis in neutropenia is very effective, but there are concerns about inducing fluoroquinolone-resistant organisms and HCAIs. This supports the equipoise position for this trial. No substantial trial of antibiotic prophylaxis in myeloma has been undertaken. The proven efficacy of levofloxacin in neutropenic patients and the sensitivity to levofloxacin of bacteria that cause infection in myeloma indicate that levofloxacin prophylaxis will also be effective in myeloma. The higher absolute risk of early death in myeloma (≈10% in the first 12 weeks from diagnosis in some risk groups) suggests that antibiotic prophylaxis may be even more effective in myeloma than in neutropenia. As there is a need for such an antibiotic trial in myeloma, it provides an excellent opportunity to collect data on HCAIs and quantify absolute risk of colonisation and infection during antibiotic prophylaxis. Data from the proposed trial will help to inform rational decisions about risks and benefits of antibiotic prophylaxis in many areas of medicine.
Research objectives
To assess the risks, benefits and cost-effectiveness of levofloxacin in newly diagnosed symptomatic myeloma by a prospective, multicentre, randomised, double-blind, placebo-controlled trial.
Research hypotheses
Levofloxacin used once daily as antibacterial prophylaxis in newly diagnosed symptomatic myeloma will:
-
reduce the rate of febrile episodes, hospitalisation and death
-
increase response to antimyeloma treatment
-
improve quality of life (QoL) and overall survival.
The trial will also test if levofloxacin affects the carriage of, and invasive infection by, three important groups of bacteria: (1) C. difficile, (2) S. aureus [including meticillin-resistant Staphylococcus aureus (MRSA)] and (3) extended-spectrum beta-lactamase (ESBL) coliforms, and will answer the following research questions:
-
Is the carriage of these organisms increased in patients receiving levofloxacin compared with those receiving placebo?
-
Is the carriage of these organisms associated with later invasive infections?
-
Does levofloxacin increase the rate of invasive infections by these three groups of organisms?
Chapter 2 Methods
Trial design
Tackling Early Morbidity and Mortality in Myeloma was a randomised, double-blind, placebo-controlled, multicentre phase III trial assessing the benefit of antibiotic prophylaxis and its effect on HCAIs in patients with symptomatic multiple myeloma. Target recruitment was originally 800 patients; however, this was extended to up to 1000 patients because of a high rate of recruitment and availability of investigational medicinal product (IMP). Patients were randomised in a 1 : 1 ratio to 500 mg of levofloxacin for 12 weeks or placebo to match. The primary outcome was time to first febrile episode or death. Secondary outcomes included, but were not limited to, response to antimyeloma treatment, carriage and invasive infections with C. difficile, MRSA and ESBL coliforms, QoL and overall survival.
Amendments to the protocol
Listed below are the amendments to the TEAMM protocol that were significant in increasing recruitment potential:
-
The original protocol stated that patients had to begin trial treatment within 7 days of commencing antimyeloma treatment unless they were already on a broad-spectrum antibiotic for treatment of an infection. Before commencing recruitment, the number of days was increased from 7 to 14 days, irrespective of antibiotic treatment status. This allowed patients more time to consider the trial and research staff more time to obtain baseline samples without affecting the scientific integrity of the trial.
-
Earlier versions of the protocol requested that a diagnostic skeletal survey be performed before patients could enter the trial. The protocol was amended prior to recruitment to relax the wording around diagnostic skeletal surveys. As skeletal surveys are part of the national diagnostic standard for multiple myeloma, it was felt that this did not need to be mandated and this was removed as a potential barrier to inclusion if patients had experienced a slightly different diagnostic journey.
-
The protocol was changed to make it clear that patients were able to enter the trial even if they were taking another antibiotic treatment at the time of recruitment. Our lead microbiologist felt that it was probable that a number of patients would present for randomisation suffering from infections for which they were receiving antibiotic treatment. As long as there was no contraindication, patients could begin their trial treatment at the same time as their antibiotic treatment as this is in line with what is suggested when patients experience infections during the trial. It was felt that this would maximise the pool of eligible patients as long as details of this treatment were collected at baseline.
-
In October 2015, the protocol was amended to allow the trial to recruit up to a further 200 patients as recruitment had been so successful and there was a surplus of IMP.
-
Minor clarifications to eligibility criteria and clarifications on central laboratory testing were submitted alongside the above amendments.
-
The primary end point was ‘number of febrile episodes’ analysed by a Kaplan–Meier curve and log-rank test (i.e. time-to-event analysis censoring deaths as an event). The primary end point, ‘number of febrile episodes’, was changed to ‘time to febrile episode’ to make this clear.
These amendments were all approved by the ethics committee.
Ethics and research and development approvals
Favourable opinion was given by National Research Ethics Service Committee West Midlands – Coventry and Warwickshire on 29 July 2011 (Research Ethics Committee reference number 11/WM/0220). Research and development (R&D) approval was obtained from University Hospitals Birmingham NHS Foundation Trust on 4 October 2011. Permissions to conduct the trial at each site were obtained from individual NHS trusts. Participating hospitals are listed in the Acknowledgements. All sites were activated between April 2012 and August 2015.
Sponsorship
TEAMM was co-ordinated by the Warwick Clinical Trials Unit (WCTU) at the University of Warwick. The University of Warwick had a co-sponsorship agreement with the University of Birmingham (acting as lead sponsor). The University of Birmingham was responsible for the provision of the chief investigator, serious adverse event (SAE) review, drafting and issuing clinical study site agreements, acting as custodian for trial samples collected, contracting with third parties and the haematology laboratory analysis. The University of Warwick was responsible for the administration of the trial, ensuring compliance with Good Clinical Practice, the design and approval of trial documents, and pharmacovigilance.
Participants
The trial sought to recruit patients with symptomatic myeloma who had not previously received active antimyeloma treatment for their disease. These patients were recruited from haematology departments in NHS hospital trusts covering England, Northern Ireland and Wales.
Inclusion criteria
Patients were eligible for this trial if:
-
they were aged ≥ 21 years and able to give informed consent
-
they had newly diagnosed symptomatic myeloma based on internationally agreed criteria
-
there was an intention to treat (ITT) their myeloma actively
-
they were within 14 days of starting, and no more than 14 days into, a programme of antimyeloma treatment
-
they were able to provide written informed consent.
Exclusion criteria
Patients were ineligible for this trial if:
-
they had a contraindication to levofloxacin
-
they were known to have sensitivity/allergy to levofloxacin or other quinolones
-
they had a history of tendon disorders related to fluoroquinolone administration
-
they were receiving amiodarone or arsenic trioxide
-
they were on active antiepileptic treatment
-
they were women of childbearing age who were not willing to use appropriate methods of contraception to prevent pregnancy or women who were breastfeeding
-
they were thought to have mandatory requirement for antibacterial prophylaxis (with the exception of Pneumocystis prophylaxis, if regarded as essential)
-
they had received previous treatment for myeloma, except for the following:
-
local radiotherapy to relieve bone pain or spinal cord compression
-
prior bisphosphonate treatment
-
previous (< 5 years since diagnosis) or concurrent active malignancies, except surgically removed basal or squamous cell carcinoma of the skin, treated carcinoma in situ of the breast or cervix or incidental histological finding of prostate cancer [tumour, node, metastasis (TNM) stage of T1a or T1b]. Patients with remote histories (> 5 years) of other cured malignancies could be entered.
-
Settings and locations
A total of 93 NHS hospitals throughout England, Northern Ireland and Wales took part in the trial. All centres were required to provide confirmation of trust R&D approval to conduct the trial at each site. Each site’s principal investigator (PI) and their delegated team underwent training on the trial protocol by the trial co-ordinator prior to the start of recruitment. This occurred via either a face-to-face meeting or a teleconference. Only when this training had been undertaken and all approvals and documentation were in place was the site opened to recruitment. Activation of recruitment was confirmed in writing by the trial co-ordinator.
Recruitment procedure
Participants were identified in new patient haematology clinics and multidisciplinary team meetings. The trial was then discussed with potential patients and the trial research nurse. Patients had to have had a new diagnosis of symptomatic myeloma and be within 14 days of their antimyeloma treatment start date. Once the trial had been discussed in detail and written patient information was provided, informed consent was taken following the process below.
Informed consent
There was no pre-agreed specified time to consent; however, it was recommended that patients were given at least 24 hours to go away, consider and talk to family about taking part in the trial. Consent was required to be informed and voluntary, with time for questions and reflection. However, the patient had the right also to make an immediate decision to consent.
Consent to participate was sought by the clinician involved in the patient’s care, with the involvement of the research nurse in the consent discussion. Consent to participate was confirmed by the patient initialling each of the appropriate boxes on the consent form and signing the form in the presence of the person taking consent. A copy was given to the patient; one copy was also kept in the patient notes, and the original was kept in the local site file.
The TEAMM intervention
Patient randomisation and blinding
Written informed consent was obtained for all patients recruited to the trial. Randomisation of participants occurred through the WCTU Randomisation Service. Treatment allocation was performed using a minimisation algorithm and was stratified by hospital, estimated glomerular filtration rate (eGFR) and intention to give high-dose chemotherapy with autologous stem cell transplant (Figure 1).
FIGURE 1.
Trial schema.
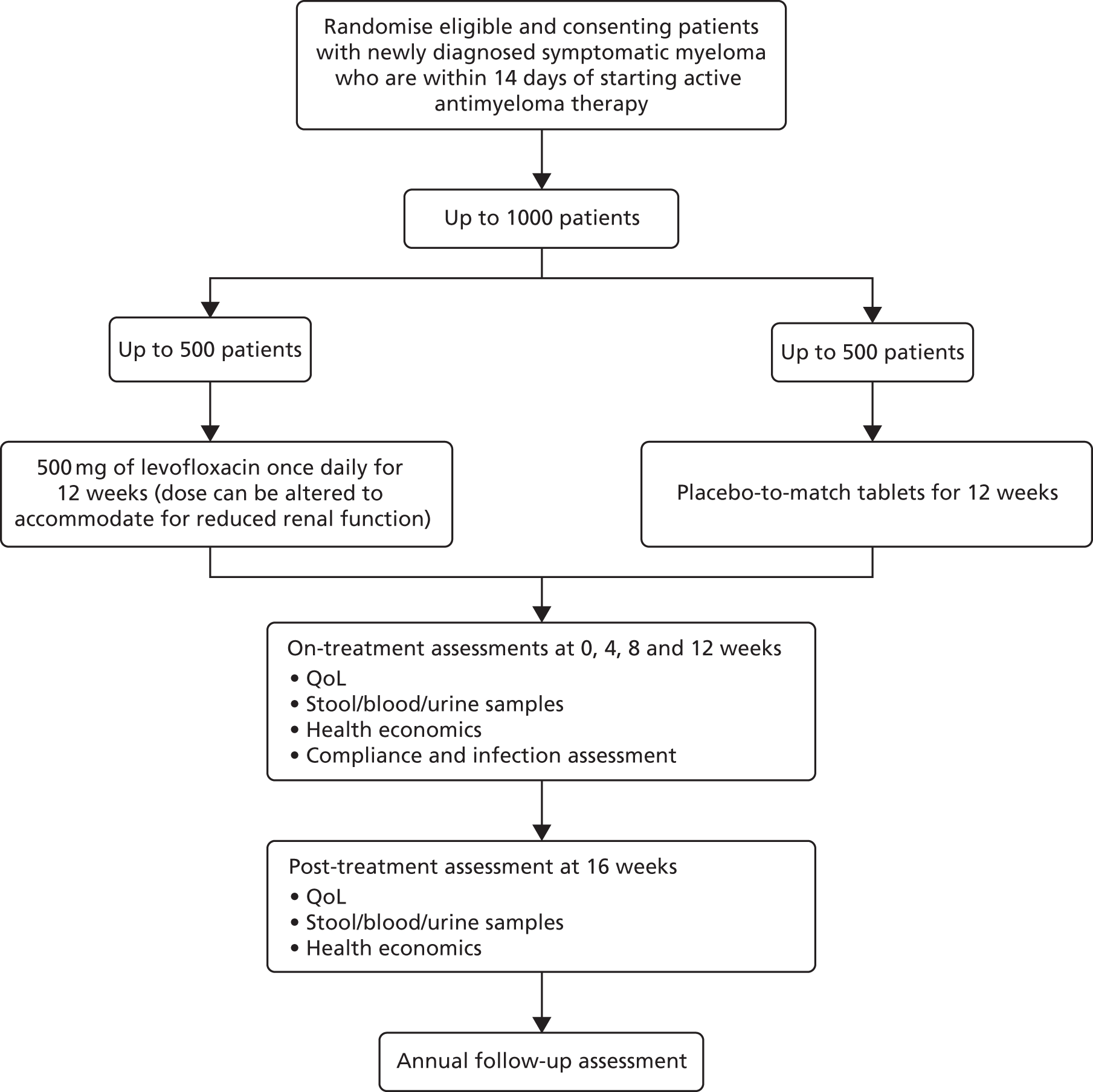
At the point of randomisation, eligibility of the patient was confirmed and a check was performed on the consenting doctor to make sure they had been appropriately trained and delegated by the centre’s PI. Confirmation of the patient’s participation in the trial was sent to their general practitioner (GP) by the research team at the site using an approved template letter provided by the trial team. Trial treatment allocation was blinded to the clinicians, patients and the co-ordinating centre. The trial statistician allocated the term ‘active’ or ‘placebo’ in a 50 : 50 split to a list of 1500 randomly generated drug pack numbers. This list was sent (with password protection) to the drug packaging company that put the active drugs or placebo in the correctly labelled packs. This list was then used to build the bespoke randomisation and drug inventory system after the terms ‘active’ and ‘placebo’ were changed to ‘A’ or ‘B’ for concealment purposes. The trial statistician retained the master list, which revealed the identity of ‘A’ and ‘B’. Ordering of the drug for each site was done via the database that picked at random an even number of ‘A’ pack numbers and ‘B’ pack numbers from those at the storage facility. Once these pack numbers were received by the sites, they were activated and available to be allocated to patients when randomised.
Emergency unblinding could be requested on grounds of safety by any clinician who was involved in the medical care of the patient. Emergency unblinding was performed by telephone contact with the Emergency Scientific and Medical Services team at Guy’s and St Thomas’ Hospital, which held a master unblinding list with the allocated pack numbers. Emergency unblinding was available 24 hours a day, 365 days a year, and was considered an option only when the patient’s future treatment required knowledge of the trial treatment assignment.
Treatment
All patients entering TEAMM received antimyeloma treatment and supportive care including bisphosphonates as per standard practice at their hospital trust. If it was intended that the patient would proceed to high-dose chemotherapy with autologous stem cell transplant, this information was collected at randomisation and taken into account during stratification.
When patients were within 14 days either side of starting a programme of antimyeloma treatment, they received 500 mg of levofloxacin or placebo-to-match tablets daily for 12 weeks (84 days). The start of the antimyeloma treatment was determined as the start of steroids or chemotherapy, whichever came first. In a situation in which patients’ renal function was compromised, it was recommended that patients took a reduced dose of the trial drug, as levofloxacin is eliminated from the body mainly via excretion of unmetabolised drug in the urine by the kidneys. eGFR, provided locally, when possible, was assessed at baseline and reassessed at each scheduled trial visit to identify changes in renal function that would necessitate a change in dose of levofloxacin. It was recommended that eGFR was assessed within the 7- to 14-day period prior to randomisation. Those patients with an eGFR of > 50 ml/minute/1.73 m2 took two tablets once per day (a dose of 500 mg), those patients with an eGFR of 20–50 ml/minute/1.73 m2 took one tablet daily (a dose of 250 mg) and those patients with an eGFR of < 20 ml/minute/1.73 m2 took a half-tablet daily (a dose of 125 mg). The active and placebo tablets were in identical breakable form. Dose reductions were recorded on the front of the patient diary, which was provided at each trial visit in conjunction with a review of eGFR.
Patients were asked to complete diaries during and after the 12-week treatment period, which were used to capture information related to drug compliance, health resource use and febrile episodes. Patients were asked to take their temperature daily (at a similar time each day) using a digital oral thermometer provided by the co-ordinating centre. They were also asked to take and record their temperature at any time they felt unwell. If a temperature of ≥ 38 °C was recorded, they were encouraged to contact the hospital for assessment, whether or not antibiotic treatment was required. In the event of a febrile episode, it was suggested that patients remain on the trial drug and that management of infections should be as for an individual who was taking active levofloxacin. Patients were treated as per standard practice depending on the nature of the infection. On resolution of the infection, it was recommended that patients continue taking the trial drug. Any patient who had stopped taking the trial drug while being treated for an infection was asked to restart promptly on resolution of the infection. Any treatment breaks were recorded on the case report forms (CRFs) and excess tablets remaining as a result were returned to pharmacies for accountability purposes.
Supportive treatment
Supportive treatment practices common to each centre were allowed; this included the use of bisphosphonates, prophylactic antivirals and prophylactic Pneumocystis treatment. The use of prophylactic Pneumocystis treatment was discouraged and the use of nebulised pentamidine over oral co-trimoxazole (Septrin®, Actavis, Barnstaple, UK) was preferred. Other antibacterial prophylaxis was not allowed.
Central laboratory assessments
Microbiology assessments
Microbiological analysis was conducted at the Department for Medical Microbiology at St George’s Hospital, London, and the Birmingham Public Health Microbiology (PMH) laboratory. Nasal swabs and stool samples were requested from patients at baseline (before the first dose of trial medication) and at 4, 8 and 12 weeks when receiving TEAMM treatment and again at 16 weeks. These were used to assess carriage of S. aureus, C. difficile and ESBL coliforms. Any toxigenic strains of C. difficile were identified by culture and ribotyping. Extended multilocus VNTR analysis typing will be performed on all isolates in the Birmingham PMH laboratory. ESBL-positive Gram-negative bacteria from faecal screens and clinical specimens (when available) were identified and sent to the Birmingham PMH laboratory for genotyping of CTX-M beta-lactamase genes using denaturing high-performance liquid chromatography. Nasal swabs were cultured for MRSA, and isolates were typed and stored. Cultures from invasive infections isolated locally were transferred to the central laboratory at St George’s Hospital for typing when possible.
Immunology assessments
Immunology analysis was conducted by the Clinical Immunology Service at the University of Birmingham. Ethylenediaminetetraacetic acid (EDTA) blood, clotted blood and urine were requested from patients at baseline (before the first dose of trial medication) and at 4, 8 and 12 weeks when receiving TEAMM treatment and again at 16 weeks and 1 year. These samples were used to assess paraprotein levels, prognostic factors and markers of immunocompetence.
Measurements at entry and at 8 and 12 weeks included levels of:
-
whole and free light chain (flc) paraprotein in serum and urine
-
β2-microglobulin, albumin, creatinine, calcium and C-reactive protein (CRP)
-
complement components C3 and C4
-
acute-phase response proteins and cytokines
-
serum levels of polyclonal immunoglobulin (specific antibody against panels of both bacterial and viral antigenic targets and type I natural antibody levels)
-
single-platform flow cytometric enumeration of lymphocyte subsets including type I and type 2 B cells, memory B cells; gamma/delta, cluster of differentiation 4 (CD4) and CD8 T cells; naive and memory subsets; Treg cells; and C cells
-
monocyte subsets defined by CD14 and CD16 dendritic cells
-
buffy coat cells, plasma and serum aliquoted and stored at –80 °C (not measured at 8 weeks).
Measurements at 4 and 16 weeks included levels of:
-
whole and flc paraprotein in serum and urine
-
β2-microglobulin, albumin, creatinine, calcium, CRP response, markers of inflammation, and humoral and cellular immunocompetence.
The schedule of assessments is shown in Table 1.
| Event | Start of trial treatment | Trial visits (post start of trial treatment) | ||||
|---|---|---|---|---|---|---|
| 4 weeks (± 2 weeksa) | 8 weeks (± 2 weeksa) | 12 weeks (end of treatment) | 16 weeks | 12 months | ||
| Informed consent taken | ✗ | |||||
| Medical history to include ECOG performance status and weight and comorbidities | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Inclusion criteria satisfied | ✗ | |||||
| Levofloxacin/placebo supplied to patient | ✗ | |||||
| QoL (EQ-5D, EORTC QLQ-C30 and HADS) | ✗ | ✗ | ✗ | |||
| QoL (EQ-5D, EORTC QLQ-C30, EORTC QLQ-MY24 and HADS) | ✗ | ✗ | ||||
| Patient diary supplied to patient (includes questions on health resource use) | ✗ | ✗ | ✗ | |||
| Post-treatment patient diary supplied to patient | ✗ | |||||
| Compliance with trial medication assessed (counting of empty blister packs) | ✗ | ✗ | ✗ | |||
| Details of infections and hospital admissions collected | ✗ | ✗ | ✗ | ✗ | ||
| AEs | ✗ | ✗ | ✗ | ✗ | ||
| Details of supportive care collected | ✗ | ✗ | ✗ | ✗ | ✗ | |
| 12–20 ml of clotted peripheral blood, 8 ml of EDTA blood (at start of treatment), 4 ml of EDTA blood thereafter and 20 ml of urine to the University of Birmingham | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Stool sample and nasal swab to St George’s Hospital | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Bone marrow aspirate ± trephine | ✗ | |||||
| Full blood count | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Biochemistry screen | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| eGFR using modification of diet in renal disease formula | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Patient follow-up
Patients were followed up at approximately 4-week intervals during their 12 weeks of TEAMM treatment. They were seen again at 16 weeks and had a further follow-up at 1 year post TEAMM treatment start date.
Serious adverse events
Investigators were required to inform the WCTU about the occurrence of a SAE within 24 hours of becoming aware of the event. SAEs had to be reported if they occurred between the first dose of trial medication and up to 30 days after the last dose was taken. An AE was considered to be a SAE if one of the following conditions applied:
-
results in death
-
is immediately life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
leads to the development of any grade 4 non-haematological toxicity (excluding alopecia)
-
results in persistent or significant disability or incapacity
-
is otherwise medically significant (e.g. important medical events that may not be immediately life-threatening nor result in death or hospitalisation, but may jeopardise the patient or may require intervention to prevent one of the other outcomes listed above, excluding new cancers or result of overdose).
A serious adverse reaction (SAR) was defined as a SAE that has a definite, probable or possible causal relationship to levofloxacin. Causality was assessed by both a clinician at site and the chief investigator. SARs that were unexpected according to the summary of product characteristics for levofloxacin were considered to be suspected unexpected serious adverse reactions. For every AE symptom, an AE term and grade was applied by the site using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
The following SAEs were not required to be reported as they relate to myeloma and its treatment:
-
disease progression
-
disease-related deaths
-
routine treatment or monitoring if the studied indication is not associated with any deterioration in condition
-
treatment, which was elective or preplanned, for a pre-existing condition, not associated with any deterioration in condition
-
general care not associated with any deterioration in condition
-
treatment on an emergency outpatient basis for an event not fulfilling any of the definitions of ‘serious’ (as provided above) and not resulting in hospital admission
-
hospitalisation for palliative care
-
grade 4 haematological toxicity is an expected consequence of effective treatment and is required to be reported only if it fulfils the criteria of a SAE (as defined above)
-
treatment (including hospitalisation or extension of hospitalisation) for transfusions or pain relief
-
surgical interventions for skeletal-related events (e.g. fixation of fractures or vertebroplasty)
-
skeletal-related events, including bone fractures, spinal cord compression and increased bone pain
-
hypercalcaemia
-
extravasation
-
toxicities that meet serious criteria that developed prior to entry to the trial.
Patients who died on treatment or within 30 days of the last dose of treatment were reviewed separately by the chief investigator and by a consultant haematologist who was independent of the trial.
Patient withdrawal
It was made clear in the patient information sheet (PIS) that patients were free to withdraw at any point after consenting to take part without having to give a reason, and that withdrawal would not affect the standard of care they would receive. When the withdrawal reason was known, it was supplied to the trial office via the withdrawal form. Three different withdrawal options were given to maximise data collection and options were discussed with the patient when possible:
-
Patient withdrew from treatment only – this option meant that the patient continued on the trial with all active assessments other than administration of the trial drug.
-
Patient withdrew from treatment and assessments – this option meant that the patient did not complete any assessments or take an active role in the trial, but was happy for us to collect data about their disease status and standard treatment they were receiving. This will enable long-term follow-up to be conducted on these patients.
-
Patient withdrew consent – for these patients, no further data were collected or used in the analysis after the date of withdrawal. Data collected between the date of consent and the date of consent withdrawal have still been used unless specified by the patient.
Patients could also have been withdrawn from the trial at the discretion of the chief investigator and/or Trial Steering Committee if safety was a concern.
Patients moving out of area
When patients moved area and were no longer attending visits at the same centre, every effort was made to transfer the follow-up of patients to the new centre where their treatment was continuing. This was possible only if the new centre had the relevant approvals to participate in TEAMM.
Outcomes
Primary outcome measure: time to first febrile episode or death from all causes within 12 weeks of starting trial treatment
A febrile episode was identified and counted by a single oral temperature of ≥ 38 °C (recorded either by a health-care professional or by the patient/carer, provided that the patient/carer had been trained and assessed as competent in temperature taking) and the patient was given anti-infectives.
A single febrile episode was defined as the initial febrile event and any subsequent fevers until that course of anti-infectives was stopped.
Capture of febrile episodes was via (1) documentation in hospital and (2) patient diary cards on which patients were asked to self-report temperature on a daily basis and at any time they felt unwell. Information gathered from documentation in hospital or in patient diaries was assessed every 4 weeks and translated onto on-treatment and infection CRFs.
Secondary outcomes
From start of trial treatment to 12 weeks
-
Number of deaths and infection-related deaths: information on deaths was captured via notification of death and SAE forms.
-
Number of days in hospital: information on hospital admissions was collected via SAE forms, on-treatment CRFs and follow-up CRFs.
-
Number of days in hospital on anti-infectives: for each infection, a separate infection CRF was submitted, which collected all anti-infective treatment and any hospital admissions associated with the infection episode.
-
Carriage and invasive infections with S. aureus, C. difficile and ESBL coliforms: nasal swabs and stool samples from patients were sent at 4-weekly intervals when on TEAMM treatment to the central microbiology laboratory. Samples were cultured to detect the presence of the organisms of interest.
-
Patient characteristics, steroid usage and indices of immunocompetence and their relationship to colonisation by, and development of, infection from S. aureus, C. difficile and ESBL coliforms and non-HCAI and Eastern Cooperative Oncology Group (ECOG) performance status: CRFs at all time points collected information on performance status. Steroid information was captured on a treatment summary CRF that requested information about each antimyeloma treatment cycle. This was looked at in conjunction with the indices of immunocompentence, which resulted from the central immunology laboratory analysis of the blood and urine samples collected at all time points.
-
Number of clinically documented total infections, episodes of severe sepsis (CTCAE grade 3 or 4) and suspected infections: febrile episodes (as defined above) and infections in which there was no associated fever (but which were treated with anti-infectives) were recorded on infection CRFs. Episodes of severe sepsis were captured via SAE forms.
-
Incidence of microbiologically proven infections, the pathogens and their susceptibility to antibacterials: the infection CRF captured information about microbiological diagnosis and SAE forms prompted for microbiology reports to be sent to the trials office when there were positive microbiological cultures. Organisms’ sensitivity profiles were usually present on local microbiological reports.
-
Days on anti-infective treatment for treatment of infection: name and duration of treatment with each anti-infective were captured via infection CRFs.
-
Response to antimyeloma treatment and its relationship to infection: response to antimyeloma treatment was looked at centrally using the blood and urine samples collected from patients. This information was looked at in relation to infections reported via infection CRFs. 29,30
From start of trial treatment to beyond 12 weeks
-
Carriage and invasive infections with S. aureus, C. difficile and ESBL coliforms between 12 and 16 weeks to assess for delayed effects from the intervention that was stopped at 12 weeks: organisms of interest were cultured from stool samples and nasal swabs that were taken at the 16-week time point (4 weeks post end-of-trial treatment).
-
Response to antimyeloma treatment at 16 weeks. Because of the half-life of paraproteins, measurement of myeloma response cannot be undertaken until a minimum of 4 weeks after an intervention – blood and urine samples were sent to the central immunology laboratory for analysis at the 16-week time point, approximately 4 weeks after the treatment end date.
-
QoL: the following validated questionnaires were used to assess QoL –
-
EuroQoL-5 Dimensions (EQ-5D)31 – five-point scales and one 100-point summary scale (completed at all time points up to week 16)
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30)32 – five functional scales, three symptom scales and one global scale and six single items for assessment of general QoL (completed at all time points up to week 16)
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-24-item myeloma-specific module (EORTC-QLQ-MY24) – 24 questions covering disease symptoms, treatment side effects, body image and future perspectives of patients with multiple myeloma (completed only at baseline and week 12)
-
Hospital Anxiety and Depression Scale (HADS)33 – a screening tool for anxiety and depression (completed at all time points up to week 16).
-
-
Health economics: daily diary cards completed by patients captured elements of health resource use in combination with information captured on the CRF.
-
Overall survival: captured via notification of death CRFs and flagging with the Office for National Statistics.
Sample size
The primary and first set of secondary outcomes were reached within 12 weeks of trial treatment. The primary outcome measure was time to first febrile episode or death from all causes, using a Kaplan–Meier survival curve. Assuming that the proportion of patients having a febrile episode or death was 30% in the first 3 months and that prophylactic antibacterials would reduce that rate to 20%, recruiting 800 patients into the trial (400 in each arm) would allow differences in excess of 10% to be detected with 90% power using a two-sided test at the 5% level of significance. Recruiting 1000 patients into the trial (500 in each arm) would allow differences in excess of 8% to be detected with 90% power using a two-sided test at the 5% level of significance. Recruiting 1000 patients would also allow detection of a levofloxacin-induced threefold increase in the rate of C. difficile-positive stools (from 5% to 15%) from entry to the trial to 12 weeks, with 95% power and 5% level of significance (two-sided test).
Other analyses include incidence of probable infections with site, severity and treatment; response to antimyeloma treatment and its relationship to infection; patient characteristics and indices of immunocompetence (blood leucocyte subset enumeration and antibacterial antibody titres) as prognostic markers for colonisation and invasive infection by antibiotic-resistant organisms; health economics; and QoL (by daily diary card and 4-weekly EQ-5D up to 16 weeks). With 1000 patients, it would be possible to report reliable estimates for these secondary outcomes.
Statistical methods
The main analysis, comparing time to first febrile episode or death from all causes within the first 12 weeks, was carried out using a log-rank comparison, with the start time being the date on which the patient started trial treatment to the time of a reported event, or to a censor date for those with no events reported after 12 weeks. 34 All randomised patients were included in the analysis of the primary end point as ITT using the date of randomisation as the start date for any patients not starting trial treatment. 35
The secondary end points such as C. difficile-containing stools, MRSA and ESBL coliform carriage rates and number of invasive infections associated with the identical organism previously carried were assessed using chi-squared tests with continuity adjustments. Mantel–Haenszel tests for combining 2 × 2 tables were then used to adjust for stratification variables and various prognostic factors. Patients who were randomised and had started treatment were included in the analyses of the secondary end points.
Overall survival was calculated from the date on which the patient started trial treatment to the date of death or date of censorship, as appropriate. Overall survival analysis was based on all-cause mortality and assessed using Kaplan–Meier curves. 34 The main treatment effect was assessed using the log-rank test. Kaplan–Meier curves for the primary outcome were constructed for each treatment arm and Cox proportional hazards models were used to compare arms after adjustment for stratification variables and imbalances in important baseline factors. 34,36 Covariates were assessed graphically for non-proportionality of hazards. When there was an indication of non-proportionality, a time-dependent variable (its product with time) was introduced to test non-proportionality. If the Wald chi-squared p-value was < 0.05 for this term, then non-proportionality was assumed and a restricted mean survival time approach used with the test of the difference in restricted means presented instead of the hazard ratio (HR). 37,38 These analyses were carried out on an ITT basis.
The analyses of all other secondary end points, incidence of probable infections with site, severity and treatment, response to antimyeloma therapy and its relationship to infection and indices of immunocompetence (blood leucocyte subset enumeration and antibacterial antibody titres) were undertaken using the appropriate statistical analyses tools.
Statistical analyses were performed using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.
Independent Data and Safety Monitoring Committee
An independent Data and Safety Monitoring Committee (DSMC) was established, consisting of an independent statistician, haematologist and microbiologist. Their main objective was to advise the Trial Steering Committee if there is evidence or reason why the study should be amended or terminated based on recruitment rates, compliance, safety or efficacy. The DSMC met after the first 50 patients were recruited, and annually thereafter. Confidential reports containing recruitment, protocol compliance, safety data and interim analyses of outcomes (not formally tested outside the trial statistical analyses plan, which was agreed with the DSMC) were reviewed by the DSMC. Interim analyses of the primary outcome were presented to the DSMC using conservative tests with significance determine by a p-value of 0.001 (to preserve the overall alpha level of 0.05). All analyses were blinded to the trial statistician and the DSMC until agreement to unblind at the end of the trial.
The original power calculations aimed to detect a difference of 10% (i.e. from 30% in the control arm to 20% in the treatment arm) with 90% power at the two-sided 5% level of significance. The first planned look at the primary end point when 150 patients had completed the 12-week assessment indicated that the original assumptions held true (i.e. a 31% rate in the control arm vs. a 21% rate in the treatment arm; documented in the DSMC December 2013 report). The prespecified look at the data before the trial recruitment closed (i.e. 760 patients recruited and 642 recruited patients having completed the 12-week assessment) indicated that the rate in the placebo arm may be reduced to 23% (note that this analysis remained blind to the treatment arm). After much discussion, the DSMC made recommendations to increase the sample size from 800 patients to up to 1000 patients (the maximum that could be accommodated by the available drug supply) within the current funding window. Increasing the sample size from the original 800 patients to up to 1000 patients allowed the power to be retained at 80% with the ability to detect differences in excess of 7% depending on the final rate in the control arm.
Database and data processing
The database was held on WCTU’s Microsoft SQL Server (Microsoft Corporation, Redmond, WA, USA) system and imposed rules for data entry, which included having a valid range for responses, linked dates and patient identification (ID) numbers.
Data were single entered into the database by trial personnel. Checks were carried out on 100% of data entered by new starters and, following full training, 10% of data entered by trial personnel were checked each month. Unacceptable error levels in 10% checks were followed up with further checking and retraining. The trial statistician carried out checks of plausibility of values and missing data to enable further queries to be resolved prior to freezing data for scheduled analyses.
Chapter 3 Results
Screening and recruitment
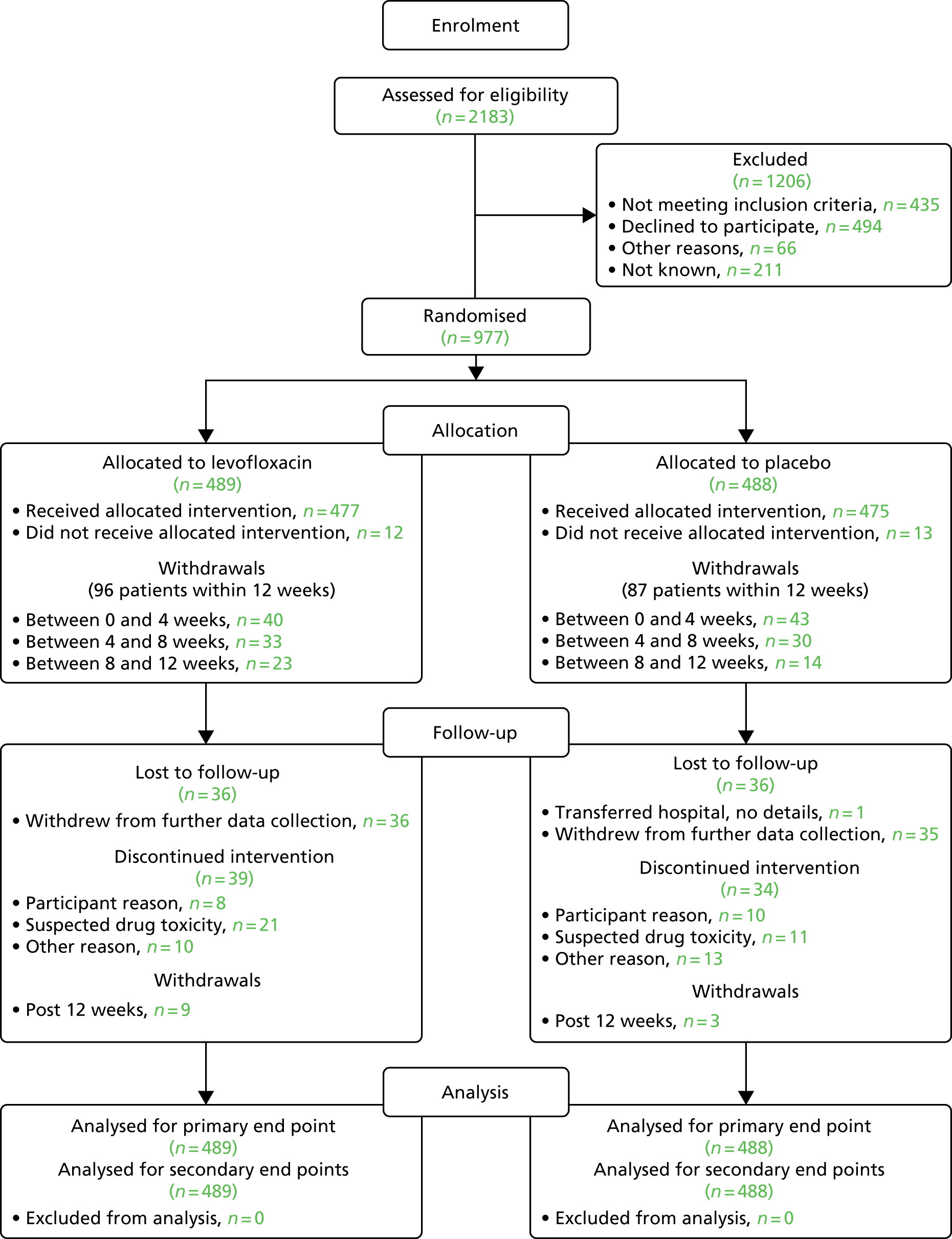
Recruitment
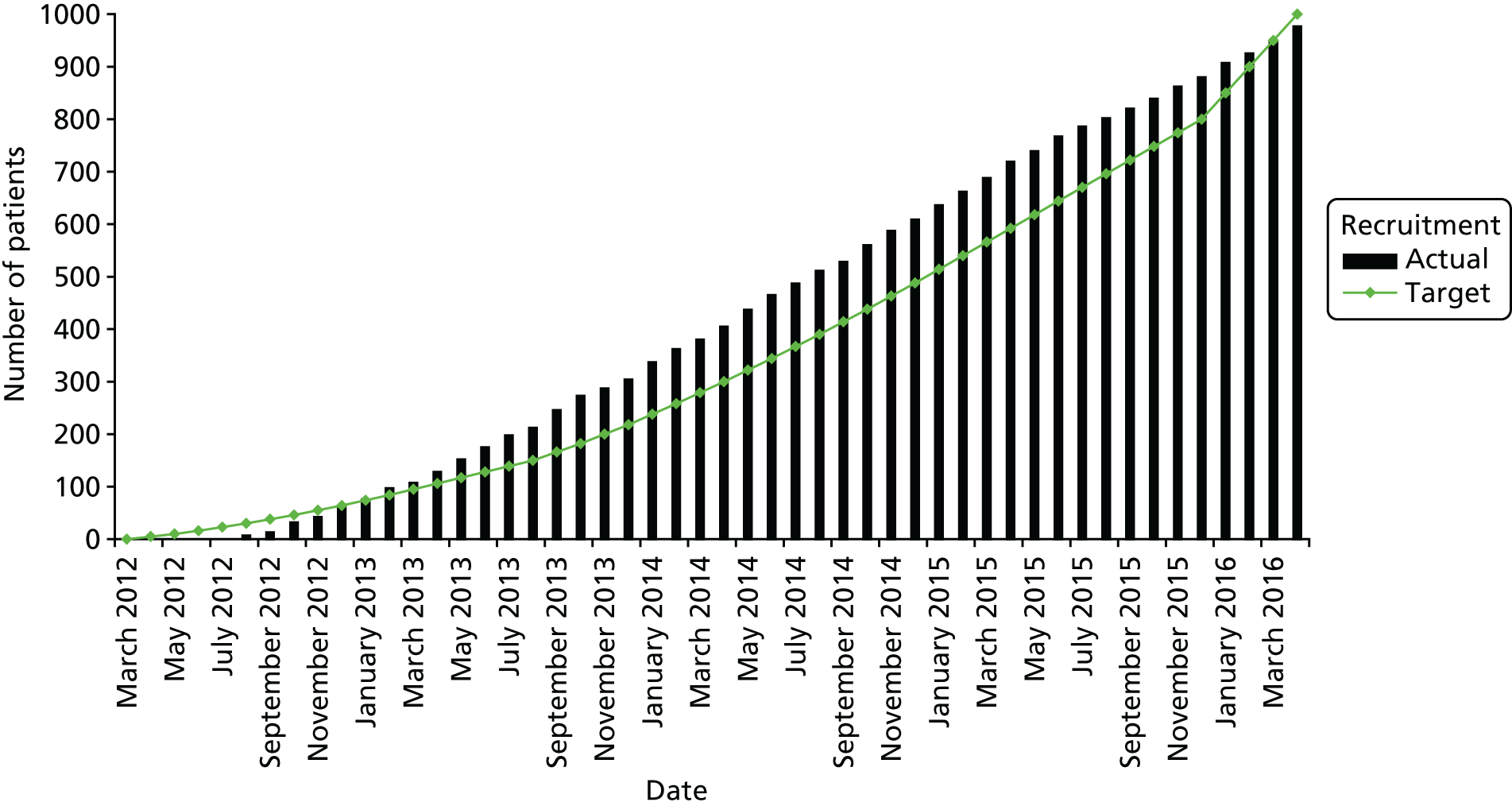
A total of 977 patients were recruited between 3 August 2012 and 29 April 2016 from 93 NHS Hospitals (levofloxacin, n = 489; placebo, n = 488). Figure 2 shows actual compared with target recruitment.
FIGURE 2.
Cumulative recruitment.
Two patients who were randomised withdrew consent on the day of randomisation and were withdrawn from further analyses as no data were collected. Twenty-five patients did not start trial treatment; baseline information was collected for 23 of these patients, and these patients are included in the analysis of the primary end point under ITT.
Screening
Sites were encouraged to screen all patients with newly diagnosed symptomatic myeloma to see if they were eligible. It was requested that all patients with whom the TEAMM trial had been discussed were recorded on the screening log provided. Sites were asked to record patient initials, date of birth, the date screening was initiated, if the patient consented and if they were subsequently randomised; in the case of patients who were not randomised, the sites were asked to provide a reason if possible. Screening logs were requested, on average, once every 6 months. More frequent requests were not necessary because of successful recruitment throughout. A final request was made for screening data after recruitment ended in April 2016. In total, 76 out of the 93 sites returned some screening data. Appendix 1 shows the sites not wishing to participate (see Table 40), the number of patients screened at each site (see Table 41) and the most common reasons for screening failures (see Table 42). A total of 2183 potential patients were screened on the basis of the data available on the returned screening logs; of these, 977 (45%) went on to be randomised.
Of the 1206 patients who were unable to be consented and randomised into the trial, the majority were found to be ineligible as a result of being outside the 14-day window for starting the TEAMM medication after the start of their antimyeloma treatment. This was a problem when patients had started a steroid treatment for their myeloma prior to commencing full cycles of chemotherapy. Of the remaining patients, 278 declined to take part without providing a detailed reason as to why. When explanations were provided, the most common reason for declining to participate was that patients had too much going on in their lives. With a new diagnosis of myeloma, many patients being elderly and having other comorbidities, patients felt that taking tablets and temperatures and completing a diary were too much to cope with. Other common reasons were not wanting to take part in a clinical trial or not wanting to take part in more than one trial as many were also eligible for other complementary myeloma trials.
Recruitment by centre across treatment arms
Table 2 shows the final number of patients recruited from each of the 93 centres, by treatment arm.
| Centre | Treatment arm, n (%) | Total (N = 977), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 489) | Placebo (N = 488) | ||
| Altnagelvin Hospital | 6 (1.2) | 5 (1.0) | 11 (1.1) |
| Antrim Hospital | 3 (0.6) | 1 (0.2) | 4 (0.4) |
| Basildon University Hospital | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Basingstoke and North Hampshire Hospital | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Bradford Royal Infirmary | 4 (0.8) | 4 (0.8) | 8 (0.8) |
| Broomfield Hospital (Chelmsford) | 4 (0.8) | 4 (0.8) | 8 (0.8) |
| Calderdale Royal Hospital (Halifax) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Castle Hill Hospital (Cottingham) | 8 (1.6) | 9 (1.8) | 17 (1.7) |
| Chesterfield Royal Hospital | 7 (1.4) | 6 (1.2) | 13 (1.3) |
| City General Hospital (Stoke-on-Trent) | 19 (3.9) | 20 (4.1) | 39 (4.0) |
| Colchester General Hospital | 15 (3.1) | 16 (3.3) | 31 (3.2) |
| Craigavon Area Hospital | 3 (0.6) | 3 (0.6) | 6 (0.6) |
| Darent Valley Hospital (Dartford) | 12 (2.5) | 11 (2.3) | 23 (2.4) |
| Dewsbury & District Hospital | 2 (0.4) | 0 (0.0) | 2 (0.2) |
| Diana, Princess of Wales Hospital (Grimsby) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Dorset County Hospital (Dorchester) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Ealing Hospital (Southall) | 5 (1.0) | 7 (1.4) | 12 (1.2) |
| Frenchay Hospital (Bristol) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| George Eliot Hospital (Nuneaton) | 3 (0.6) | 3 (0.6) | 6 (0.6) |
| Glan Clwd Hospital (Rhyl) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Gloucestershire Royal Hospital | 3 (0.6) | 2 (0.4) | 5 (0.5) |
| Good Hope Hospital (Sutton Coldfield) | 3 (0.6) | 3 (0.6) | 6 (0.6) |
| Grantham and District Hospital | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Guy’s Hospital (London) | 9 (1.8) | 8 (1.6) | 17 (1.7) |
| Heartlands Hospital (Birmingham) | 10 (2.0) | 11 (2.3) | 21 (2.1) |
| Hereford County Hospital | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Hillingdon Hospital (Uxbridge) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Huddersfield Royal Infirmary | 4 (0.8) | 4 (0.8) | 8 (0.8) |
| Kettering General Hospital | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| King’s College Hospital (Denmark Hill, London) | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Kings Mill Hospital (Sutton-in-Ashfield) | 13 (2.7) | 12 (2.5) | 25 (2.6) |
| Kingston Hospital (Kingston upon Thames) | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Leicester Royal Infirmary | 4 (0.8) | 3 (0.6) | 7 (0.7) |
| Leighton Hospital (Crewe) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Lincoln County Hospital | 6 (1.2) | 5 (1.0) | 11 (1.1) |
| Macclesfield District General Hospital | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Manchester Royal Infirmary | 6 (1.2) | 6 (1.2) | 12 (1.2) |
| Medway Maritime Hospital (Gillingham) | 13 (2.7) | 13 (2.7) | 26 (2.7) |
| Milton Keynes General Hospital | 4 (0.8) | 3 (0.6) | 7 (0.7) |
| New Cross Hospital (Wolverhampton) | 5 (1.0) | 4 (0.8) | 9 (0.9) |
| North Middlesex University Hospital Trust (London) | 5 (1.0) | 5 (1.0) | 10 (1.0) |
| Northampton General Hospital | 9 (1.8) | 10 (2.0) | 19 (1.9) |
| Northwick Park Hospital (Harrow) | 5 (1.0) | 6 (1.2) | 11 (1.1) |
| Pilgrim Hospital (Boston) | 5 (1.0) | 2 (0.4) | 7 (0.7) |
| Pinderfields General Hospital (Wakefield) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Pontefract General Infirmary | 2 (0.4) | 0 (0.0) | 2 (0.2) |
| Poole Hospital | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Princess Royal University Hospital (Orpington) | 16 (3.3) | 15 (3.1) | 31 (3.2) |
| Queen Alexandra Hospital (Portsmouth) | 26 (5.3) | 25 (5.1) | 51 (5.2) |
| Queen Elizabeth Hospital (King’s Lynn) | 8 (1.6) | 8 (1.6) | 16 (1.6) |
| Queen Elizabeth Hospital (London) | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Queen Elizabeth Hospital (Birmingham) | 6 (1.2) | 7 (1.4) | 13 (1.3) |
| Queen’s Hospital (Romford) | 6 (1.2) | 7 (1.4) | 13 (1.3) |
| Queen’s Hospital (Burton upon Trent) | 3 (0.6) | 3 (0.6) | 6 (0.6) |
| Royal Berkshire Hospital (Reading) | 7 (1.4) | 6 (1.2) | 13 (1.3) |
| Royal Bournemouth Hospital | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Royal Devon and Exeter Hospital (Wonford, Exeter) | 5 (1.0) | 5 (1.0) | 10 (1.0) |
| Royal Gwent Hospital (Newport) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Royal Hallamshire Hospital (Sheffield) | 3 (0.6) | 3 (0.6) | 6 (0.6) |
| Royal Hampshire County Hospital (Winchester) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Royal Liverpool University Hospital | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Royal Shrewsbury Hospital | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Royal Surrey County Hospital (Guildford) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Royal United Hospital (Bath) | 5 (1.0) | 7 (1.4) | 12 (1.2) |
| Russells Hall Hospital (Dudley) | 19 (3.9) | 19 (3.9) | 38 (3.9) |
| Salisbury District Hospital | 9 (1.8) | 8 (1.6) | 17 (1.7) |
| Sandwell General Hospital (West Bromwich) | 13 (2.7) | 12 (2.5) | 25 (2.6) |
| South Warwickshire Hospital | 3 (0.6) | 2 (0.4) | 5 (0.5) |
| Southampton General Hospital | 7 (1.4) | 7 (1.4) | 14 (1.4) |
| Southend Hospital (Westcliff-on-Sea) | 5 (1.0) | 6 (1.2) | 11 (1.1) |
| Southmead Hospital (Bristol) | 8 (1.6) | 6 (1.2) | 14 (1.4) |
| St James’s University Hospital (Leeds) | 5 (1.0) | 6 (1.2) | 11 (1.1) |
| St. Helier Hospital (Carshalton) | 14 (2.9) | 14 (2.9) | 28 (2.9) |
| Stafford Hospital | 6 (1.2) | 4 (0.8) | 10 (1.0) |
| Stoke Mandeville Hospital (Aylesbury) | 7 (1.4) | 6 (1.2) | 13 (1.3) |
| Sunderland Royal Hospital | 2 (0.4) | 4 (0.8) | 6 (0.6) |
| The Great Western Hospital (Swindon) | 6 (1.2) | 7 (1.4) | 13 (1.3) |
| Torbay Hospital (Torquay) | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| University Hospital Coventry | 15 (3.1) | 17 (3.5) | 32 (3.3) |
| University Hospital Lewisham (London) | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Ulster Hospital (Belfast) | 9 (1.8) | 9 (1.8) | 18 (1.8) |
| Warrington Hospital | 4 (0.8) | 4 (0.8) | 8 (0.8) |
| West Middlesex University Hospital (Isleworth) | 9 (1.8) | 8 (1.6) | 17 (1.7) |
| West Wales General Hospital (Carmarthen) | 5 (1.0) | 5 (1.0) | 10 (1.0) |
| Wexham Park Hospital (Slough) | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Whipps Cross University Hospital (London) | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Withybush General Hospital (Haverfordwest) | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Wrexham Maelor Hospital | 10 (2.0) | 8 (1.6) | 18 (1.8) |
| Wycombe Hospital (High Wycombe) | 5 (1.0) | 5 (1.0) | 10 (1.0) |
| Wythenshawe Hospital (Manchester) | 6 (1.2) | 5 (1.0) | 11 (1.1) |
| Total | 489 (100) | 488 (100) | 977 (100) |
Withdrawals
Table 3 lists the three levels of withdrawal from the trial and the reasons for withdrawal within those groupings. Of the patients who withdrew, 32% withdrew from treatment but agreed to continue with all trial-related activities with full data collection, 36% were happy for us to collect long-term health and survival data from them but did not want to continue with any trial-related activities and 31% withdrew their consent for us to collect any further information regarding them or their condition. As a minimum, even for withdrawn patients, baseline data were collected for all patients when possible.
| Withdrawal status | Reason for withdrawal | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Levofloxacin | Placebo | |||
| Ceased treatment but remained on follow-up as per protocol (N = 73, 32%) | Suspected trial drug toxicity | 21 (54) | 11 (32) | 32 (44) |
| Participant decision | 8 (20.5) | 10 (29) | 18 (25) | |
| Other reason | 10 (26) | 13 (38) | 23 (31.5) | |
| Withdrawn from treatment and assessments (N = 82, 36%) | Suspected trial drug toxicity | 6 (13) | 9 (24) | 15 (18) |
| Participant decision | 24 (53) | 18 (49) | 42 (51) | |
| Other reason | 15 (33) | 10 (27) | 25 (30) | |
| Withdrawn consent for further data to be collected (N = 71, 31%) | Suspected trial drug toxicity | 5 (14) | 5 (14) | 10 (14) |
| Participant decision | 19 (53) | 23 (66) | 42 (59) | |
| Other reason | 12 (33) | 7 (20) | 19 (27) | |
Unblinding by treatment arm
The unblinding service was contacted regarding TEAMM patients on 29 occasions: 14 of the calls, after further discussion, did not result in the treatment allocation being unblinded, and 15 of the calls resulted in the treatment allocation being unblinded to the caller (two of the calls were redirected to a more appropriate service). Examples of situations in which patients’ treatment allocation was unblinded included tendonitis/tendon rupture, unexpected deterioration in kidney or liver function, confusion, suspected Stevens–Johnson syndrome and unexplained neurological symptoms.
Data return
Data were locked on 24 April 2017. Table 4 reports the number of CRFs returned and expected at the time of analysis.
| Time point | CRF return | Outstanding CRFs being chased (n) | |
|---|---|---|---|
| Received (n) | Expected (n) | ||
| Eligibility | 977 | 977 | – |
| Randomisation | 977 | 977 | – |
| Baseline | 975 | 975 | – |
| Week 4 | 925 | 961 | 36 |
| Week 8 | 856 | 883 | 27 |
| Week 12 | 818 | 832 | 14 |
| Week 16 | 799 | 811 | 12 |
| Treatment summary | 947 | 975 | 28 |
| Annual follow-up | 716 | 758 | 42 |
Consolidated Standards of Reporting Trials flow diagram
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 3.
FIGURE 3.
The CONSORT flow diagram.
Baseline characteristics
Stratification factors of participants by treatment arm
Treatment allocation by minimisation was balanced by site, eGFR on entry into the trial and intention to give high-dose chemotherapy with autologous stem cell transplant, as shown in Table 5.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 977), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 489) | Placebo (N = 488) | |||
| eGFR (ml/minute/1.73 m2) | > 50 | 369 (75) | 369 (76) | 738 (76) |
| 20–50 | 95 (19) | 93 (19) | 188 (19) | |
| < 20 | 25 (5) | 26 (5) | 51 (5) | |
| High-dose chemotherapy with autologous stem cell transplant | No | 223 (46) | 222 (46) | 445 (46) |
| Yes | 266 (54) | 266 (54) | 532 (54) | |
| Sex | Male | 316 (65) | 295 (60) | 611 (63) |
| Female | 173 (35) | 193 (40) | 366 (37) | |
| Age (years) | N | 489 | 488 | 977 |
| Median | 67 | 67 | 67 | |
| IQR | 59–75 | 61–75 | 60–75 | |
Baseline characteristics of participants by treatment arm
Baseline characteristics were well balanced across treatment arms, as shown in Table 6.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 975), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 489) | Placebo (N = 486) | |||
| Performance status at randomisation | 0 | 164 (34) | 173 (36) | 337 (35) |
| 1 | 209 (43) | 188 (39) | 397 (41) | |
| 2 | 80 (16) | 76 (16) | 156 (16) | |
| 3 | 24 (5) | 36 (7) | 60 (6) | |
| 4 | 2 (< 1) | 5 (1) | 7 (1) | |
| Missing | 10 (2) | 8 (2) | 18 (2) | |
| Performance status 6 months prior to randomisation | 0 | 327 (67) | 311 (64) | 638 (65) |
| 1 | 119 (24) | 132 (27) | 251 (26) | |
| 2 | 16 (3) | 22 (5) | 38 (4) | |
| 3 | 5 (1) | 6 (1) | 11 (1) | |
| 4 | 0 (0) | 1 (< 1) | 1 (0) | |
| Missing | 22 (5) | 14 (3) | 36 (4) | |
| Ethnicity | White | 452 (92) | 437 (90) | 889 (91) |
| Mixed | 1 (< 1) | 2 (0.4) | 3 (< 1) | |
| Asian or British Asian | 10 (2) | 17 (4) | 27 (3) | |
| Black or black British | 26 (5) | 28 (6) | 54 (6) | |
| Chinese or other | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Missing | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Prior infection | C. difficile | 2 (< 1) | 1 (< 1) | 3 (< 1) |
| MRSA | 6 (1) | 7 (1) | 13 (1) | |
| ESBL coliforms | 3 (1) | 5 (1) | 8 (1) | |
| Anti-infectives in month prior | No | 332 (68) | 331 (68) | 663 (68) |
| Yes | 75 (15) | 76 (16) | 151 (15) | |
| Missing | 82 (17) | 79 (16) | 161 (17) | |
| Steroids 14 days prior to randomisation | Yes | 248 (51) | 246 (51) | 494 (51) |
| Corticosteroids | Prednisolone | 24 (5) | 18 (4) | 42 (4) |
| Dexamethasone | 226 (46) | 229 (47) | 455 (47) | |
| Other | 0 (0) | 2 (< 1) | 2 (< 1) | |
| Planned antimyeloma treatment | Any bortezomib (Velcade®; Janssen-Cilag Ltd., Beerse, Belgium) regimen | 150 (31) | 144 (30) | 294 (30) |
| Cyclophosphamide, thalidomide, dexamethasone | 116 (24) | 119 (24) | 235 (24) | |
| Cyclophosphamide, thalidomide, dexamethasone (attenuated) | 74 (15) | 83 (17) | 157 (16) | |
| Other | 56 (11) | 56 (12) | 112 (11) | |
| Revlimid, cyclophosphamide, dexamethasone | 33 (7) | 37 (8) | 70 (7) | |
| Revlimid, cyclophosphamide, dexamethasone (attenuated) | 38 (8) | 32 (7) | 70 (7) | |
| Melphalan, prednisolone, thalidomide | 17 (3) | 12 (2) | 29 (3) | |
| Melphalan, prednisolone | 3 (1) | 1 (< 1) | 4 (< 1) | |
| Missing | 2 (< 1) | 2 (< 1) | 4 (< 1) | |
| Bisphosphonate status at randomisation | Not given | 68 (14) | 60 (12) | 128 (13) |
| Given/will be given | 419 (86) | 419 (87) | 838 (86) | |
| Missing | 2 (< 1) | 7 (1) | 9 (1) | |
| Bisphosphonate | Zolendronate | 284 (68) | 280 (67) | 564 (67) |
| Pamidronate | 111 (26) | 105 (25) | 216 (26) | |
| Clodronate | 14 (3) | 23 (5) | 37 (4) | |
| Other | 3 (1) | 7 (2) | 10 (1) | |
| Prophylactic anti-infective status | No | 170 (35) | 166 (34) | 336 (34) |
| Yes | 240 (48) | 237 (50) | 477 (49) | |
| Missing | 82 (17) | 80 (16) | 162 (17) | |
Disease characteristics at presentation were also well balanced, as shown in Table 7.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 975), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 489) | Placebo (N = 486) | |||
| Skeletal disease | ||||
| Corrected calcium (µmol/l) | < 2.5 | 339 (72) | 354 (75) | 693 (74) |
| 2.5–2.75 | 101 (22) | 94 (20) | 94 (21) | |
| > 2.75 | 29 (6) | 20 (5) | 25 (5) | |
| Evidence of bone disease | Yes | 338 (69) | 350 (72) | 688 (71) |
| Site of bone disease | Vertebral fracture/collapse | 118 (24) | 144 (30) | 262 (27) |
| Lytic lesions | 234 (48) | 245 (50) | 479 (49) | |
| Fractured rib | 33 (7) | 24 (5) | 57 (6) | |
| Osteoporosis | 38 (8) | 37 (8) | 75 (8) | |
| Other fracture | 42 (9) | 41 (8) | 83 (9) | |
| Tumour burden | ||||
| Serum β2-microglobulin (mg/l) | < 4 | 189 (46) | 192 (47) | 381 (46) |
| 4–8 | 148 (36) | 152 (37) | 300 (37) | |
| > 8 | 73 (18) | 67 (16) | 140 (17) | |
| Haematopoietic function | ||||
| Anaemia (haemoglobin g/dl) | < 7.5 | 9 (2) | 13 (3) | 22 (2) |
| 7.5–10 | 163 (34) | 166 (34) | 329 (34) | |
| > 10 | 314 (65) | 305 (63) | 619 (64) | |
| Thrombocytopenia (platelets × 109/l) | < 150 | 69 (14) | 79 (16) | 148 (15) |
| > 150 | 417 (86) | 404 (84) | 821 (85) | |
| Neutrophils (× 109/l) | < 1.8 | 35 (7) | 55 (11) | 90 (9) |
| 1.8–3 | 138 (29) | 132 (27) | 270 (28) | |
| > 3 | 311 (64) | 296 (61) | 607 (63) | |
| Lymphocytes | > 0–1 | 118 (24) | 125 (26) | 243 (25) |
| > 1–3.5 | 345 (71) | 339 (70) | 684 (71) | |
| > 3.5 | 21 (4) | 19 (4) | 40 (4) | |
| Renal disease | ||||
| Serum creatinine (µmol/l) | < 130 | 384 (79) | 383 (80) | 767 (80) |
| 130–199 | 57 (12) | 54 (11) | 111 (11) | |
| > 199 | 42 (9) | 43 (9) | 85 (9) | |
| Blood urea (µmol/l) | < 6.5 | 229 (49) | 225 (49) | 454 (48) |
| 6.5–10 | 146 (31) | 148 (32) | 294 (32) | |
| > 10 | 91 (20) | 89 (19) | 180 (19) | |
| Stage | ||||
| International Staging System | Stage I | 100 (24) | 116 (28) | 216 (26) |
| Stage II | 188 (46) | 165 (40) | 353 (43) | |
| Stage III | 121 (30) | 130 (32) | 251 (31) | |
Protocol deviations/non-compliance
One patient was retrospectively found not to be eligible because they were found to have multiple plasmacytoma and not myeloma when further results came to light. A total of 24 patients were, at some point during their treatment, taking the incorrect dose on the basis of their eGFR result. Among this group, clinician decisions not to increase the dose based on patients’ conditions are classed as deviations. The remainder of instances of non-compliance were down to site errors or patient confusion. Details of the type of non-compliances are shown in Table 8.
| Type of protocol non-compliance | Non-compliance | |
|---|---|---|
| Violations (n) | Deviations (n) | |
| Failure to report SAE | 13 | 0 |
| Incorrect dose on basis of eGFR: lower dose than protocol stated | 13 | 6 |
| Incorrect dose on basis of eGFR: higher dose than protocol stated | 5 | 0 |
| Failure to start TEAMM medication within 14 days of antimyeloma treatment | 5 | 8 |
| Continued treatment past the 84-day period stated in the protocol | 11 | 0 |
| Stool sample taken after first dose of TEAMM medication | 0 | 1 |
| Retrospectively found not to be eligible | 1 | 0 |
| Incorrect pack number dispensed | 2 | 0 |
| Prescription and administration of a contraindicated drug | 5 | 1 |
| Patient incorrectly told to stop taking trial drug before 12-week time point | 1 | 0 |
| Total | 56 | 16 |
Protocol deviations are shown in Table 9. A number of patients did not take the TEAMM medication for the correct number of days as stated in the protocol (84 days), some took it for fewer days than expected and some took it for more days than was stated. Those who took TEAMM treatment for fewer days than expected were required to note this either in a withdrawal form or on the on-treatment form as missed treatment. Patients who took the trial medication for more than the 84 days of treatment misunderstood the trial procedure and continued to take remaining tablets as a result of treatment breaks or dose reductions. Patients taking trial treatment for up to 98 days were not classed as non-compliers as it was felt that the data would not be significantly affected by this. The remaining patients who took trial treatment for > 98 days were classed as protocol violations. There were 13 patients who failed to begin their randomised treatment within the 14-day eligibility window stated in the protocol. All of these patients were eligible at the time of randomisation and all violations resulted from a change in the patient’s circumstances that resulted in the anticipated start date changing to one outside the 14-day window. These non-compliances are balanced by treatment arm, as shown in Table 9.
| Non-compliance | Treatment arm, n (%) | Total (N = 925), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 468) | Placebo (N = 457) | ||
| Taken for > 98 days | 7 (1) | 4 (1) | 11 (1) |
| Started > 14 days of starting antimyeloma treatment | 7 (1) | 6 (1) | 13 (1) |
| Lower dose | 10 (2) | 9 (2) | 19 (2) |
| Higher dose | 3 (< 1) | 2 (< 1) | 5 (< 1) |
Treatment data across 12 weeks
Data were captured on participants while on treatment at 4, 8 and 12 weeks. At the time of data lock, a total of 2599 treatment forms had been returned by 925 patients. Table 10 presents on-treatment information by treatment arm.
| Time point | Grouping | Treatment arm, n (%) | Total (N = 925), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 468) | Placebo (N = 457) | |||
| Treatment data at 4 weeks | ||||
| Trial drug dose revised | No | 411 (88) | 420 (92) | 831 (90) |
| Yes | 43 (9) | 26 (6) | 69 (7) | |
| Missing | 14 (3) | 11 (2) | 25 (3) | |
| Revised dose (mg) | 500 | 32 (74) | 18 (69) | 50 (72) |
| 250 | 7 (16) | 6 (23) | 13 (19) | |
| 125 | 4 (9) | 2 (8) | 6 (9) | |
| Randomised treatment missed | No | 298 (64) | 293 (64) | 591 (64) |
| Yes | 74 (16) | 73 (16) | 147 (16) | |
| Missing | 96 (20) | 91 (20) | 187 (20) | |
| Number of days treatment missed, n, median (IQR) | 64, 5 (2–13) | 62, 5 (2–9) | 126, 5 (2–11) | |
| Diarrhoea episodes | No | 409 (87) | 377 (82) | 786 (85) |
| Yesa | 54 (12) | 73a (16) | 127 (14) | |
| Missing | 5 (1) | 7 (1.5) | 12 (1) | |
| Dialysis since last visit | No | 456 (97) | 447 (98) | 903 (98) |
| Yes | 7 (1.5) | 5 (1) | 12 (1) | |
| Missing | 5 (1) | 5 (1) | 10 (1) | |
| Steroids for treatment of myeloma | No | 15 (3) | 9 (2) | 24 (3) |
| Yes | 367 (78) | 365 (80) | 732 (79) | |
| Missing | 86 (18) | 83 (18) | 169 (18) | |
| Participant’s general condition | Improving | 143 (31) | 131 (29) | 274 (30) |
| Stable | 253 (54) | 269 (59) | 522 (56) | |
| Worsening | 61 (13) | 45 (10) | 106 (11) | |
| Missing | 11 (2) | 12 (3) | 23 (2) | |
| Participant’s current bone pain | None | 130 (28) | 116 (25) | 246 (27) |
| Mild | 123 (26) | 120 (26) | 243 (26) | |
| Moderate | 98 (21) | 105 (23) | 203 (22) | |
| Severe | 21 (4) | 15 (3) | 36 (4) | |
| Missing | 96 (20.5) | 101 (22) | 197 (21) | |
| Bone pain compared with last visit | Improving | 138 (29) | 121 (26) | 259 (28) |
| Stable | 204 (44) | 213 (47) | 417 (45) | |
| Worsening | 45 (10) | 37 (8) | 82 (9) | |
| Missing | 81 (17) | 86 (19) | 167 (18) | |
| Time point | Grouping | Treatment arm, n (%) | Total (N = 856), n (%) | |
| Levofloxacin (N = 430) | Placebo (N = 426) | |||
| Treatment data at 8 weeks | ||||
| Trial drug dose revised | No | 389 (90) | 387 (91) | 776 (91) |
| Yes | 27 (6) | 20 (5) | 47 (5) | |
| Missing | 14 (3) | 19 (4) | 33 (4) | |
| Revised dose (mg) | 500 | 15 (56) | 12 (60) | 27 (58) |
| 250 | 11 (41) | 7 (35) | 18 (38) | |
| 125 | 1 (4) | 1 (5) | 2 (4) | |
| Randomised treatment missed | No | 304 (71) | 308 (72) | 612 (72) |
| Yes | 50 (12) | 39 (9) | 89 (10) | |
| Missing | 76 (18) | 79 (18) | 155 (18) | |
| Number of days treatment missed, n, median (IQR) | 47, 3 (2–12) | 29, 10 (3–17) | 76, 6 (2–14.5) | |
| Diarrhoea episodes | No | 378 (88) | 377 (88.5) | 755 (88) |
| Yes | 48 (11) | 41 (10) | 89 (10) | |
| Missing | 4 (1) | 8 (2) | 12 (1) | |
| Dialysis since last visit | No | 423 (98) | 417 (98) | 840 (98) |
| Yes | 5 (1) | 3 (1) | 8 (1) | |
| Missing | 2 (0.47) | 6 (1) | 8 (1) | |
| Steroids for treatment of myeloma | No | 19 (4) | 22 (5) | 41 (5) |
| Yes | 342 (80) | 335 (79) | 677 (79) | |
| Missing | 69 (16) | 69 (16) | 138 (16) | |
| Participant’s general condition | Improving | 154 (36) | 177 (42) | 331 (39) |
| Stable | 219 (51) | 189 (44) | 408 (48) | |
| Worsening | 46 (11) | 47 (11) | 93 (11) | |
| Missing | 11 (3) | 13 (3) | 24 (3) | |
| Participant’s current bone pain | None | 140 (33) | 134 (31) | 274 (32) |
| Mild | 124 (29) | 113 (27) | 237 (28) | |
| Moderate | 80 (19) | 83 (19) | 163 (19) | |
| Severe | 8 (2) | 8 (2) | 16 (2) | |
| Missing | 78 (18) | 88 (21) | 166 (19) | |
| Bone pain compared with last visit | Improving | 127 (30) | 112 (26) | 239 (28) |
| Stable | 192 (45) | 201 (47) | 393 (46) | |
| Worsening | 40 (9) | 31 (7) | 71 (8) | |
| NA/missing | 71 (16.5) | 82 (19) | 153 (18) | |
| Time point | Grouping | Treatment arm, n (%) | Total (N = 856), n (%) | |
| Levofloxacin (N = 430) | Placebo (N = 426) | |||
| Treatment data at 12 weeks | ||||
| Trial drug dose revised | No | 336 (81) | 321 (80) | 657 (80) |
| Yes | 4 (1) | 4 (1) | 8 (1) | |
| Missing | 76 (18) | 77 (19) | 153 (19) | |
| Revised dose (mg) | 500 | 2 (50) | 1 (25) | 3 (38) |
| 250 | 2 (50) | 2 (50) | 4 (50) | |
| Missing | – (–) | 1 (25) | 1 (12) | |
| Randomised treatment missed | No | 293 (70) | 289 (72) | 582 (71) |
| Yes | 46 (11) | 41 (10) | 87 (11) | |
| Missing | 77 (18.5) | 72 (18) | 149 (18) | |
| Number of days treatment missed, n, median (IQR) | 46, 6.5 (2–16) | 35, 6 (2–10) | 81, 6 (2–13) | |
| Diarrhoea episodes | No | 368 (88) | 359 (89) | 272 (89) |
| Yes | 45 (11) | 38 (9) | 83 (10) | |
| Missing | 3 (1) | 5 (1) | 8 (1) | |
| Dialysis since last visit | No | 408 (98) | 393 (98) | 801 (98) |
| Yes | 6 (1) | 5 (1) | 11 (1) | |
| Missing | 2 (0.5) | 4 (1) | 6 (1) | |
| Steroids for treatment of myeloma | No | 23 (5.5) | 25 (6) | 48 (6) |
| Yes | 337 (81) | 321 (80) | 658 (80) | |
| Missing | 56 (13) | 56 (14) | 112 (14) | |
| Participant’s general condition | Improving | 156 (37.5) | 158 (39) | 314 (38) |
| Stable | 203 (49) | 204 (51) | 407 (50) | |
| Worsening | 47 (11) | 35 (9) | 82 (10) | |
| Missing | 10 (2) | 5 (1) | 15 (2) | |
| Participant’s current bone pain | None | 148 (36) | 160 (40) | 308 (38) |
| Mild | 131 (31.5) | 111 (28) | 242 (30) | |
| Moderate | 58 (14) | 58 (14) | 116 (14) | |
| Severe | 8 (2) | 8 (2) | 16 (2) | |
| Missing | 71 (17) | 65 (16) | 136 (17) | |
| Bone pain compared with last visit | Improving | 110 (26) | 114 (28) | 224 (27) |
| Stable | 198 (48) | 194 (48) | 392 (48) | |
| Worsening | 27 (6.5) | 26 (6.5) | 53 (6.5) | |
| NA/missing | 81 (19.5) | 68 (17) | 149 (18) | |
The treatment information for the entire treatment period (from 0 to 12 weeks) is summarised in Table 11.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 2599), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 1314) | Placebo (N = 1285) | |||
| Trial drug dose revised | No | 1136 (86) | 1128 (88) | 2264 (87) |
| Yes | 74 (6) | 50 (4) | 124 (5) | |
| Missing | 104 (8) | 107 (8) | 211 (8) | |
| Revised dose (mg) | 500 | 49 (66) | 31 (62) | 80 (64.5) |
| 250 | 20 (27) | 15 (30) | 35 (28) | |
| 125 | 5 (7) | 3 (6) | 8 (6) | |
| Missing | 0 (0) | 1 (2) | 1 (1) | |
| Randomised treatment missed | No | 895 (68) | 890 (69) | 1785 (69) |
| Yes | 170 (13) | 153 (12) | 323 (12) | |
| Missing | 249 (19) | 242 (19) | 491 (19) | |
| Number of days’ treatment missed, n, median (IQR) | 157, 5 (2–13) | 126, 6 (2–11) | 283, 5 (2–13) | |
| Diarrhoea episodes | No | 1155 (88) | 1113 (87) | 2268 (87) |
| Yesa | 147 (11) | 152 (12) | 299 (11.5) | |
| Missing | 12 (1) | 20 (2) | 32 (1) | |
| Dialysis since last visit | No | 1287 (98) | 1257 (98) | 2544 (98) |
| Yes | 18 (1) | 13 (1) | 31 (1) | |
| Missing | 9 (1) | 15 (1) | 24 (1) | |
| Steroids for treatment of myeloma | No | 57 (4) | 56 (4) | 113 (4) |
| Yes | 1046 (80) | 1021 (80) | 2067 (80) | |
| Missing | 211 (16) | 208 (16) | 419 (16) | |
| Participant’s general condition | Improving | 453 (34) | 466 (36) | 919 (35) |
| Stable | 675 (51) | 662 (51.5) | 1337 (51) | |
| Worsening | 154 (12) | 127 (10) | 281 (10) | |
| Missing | 32 (2) | 30 (2) | 62 (2) | |
| Participant’s current bone pain | None | 418 (32) | 410 (32) | 828 (32) |
| Mild | 378 (29) | 344 (27) | 722 (28) | |
| Moderate | 236 (18) | 246 (19) | 482 (18.5) | |
| Severe | 37 (3) | 31 (2) | 68 (3) | |
| Missing | 245 (19) | 254 (20) | 499 (19) | |
| Bone pain compared with last visit | Improving | 375 (29) | 347 (27) | 722 (28) |
| Stable | 594 (45) | 608 (47) | 1202 (46) | |
| Worsening | 112 (9) | 94 (7) | 206 (8) | |
| NA/missing | 233 (18) | 236 (18) | 469 (18) | |
Infections
Febrile episodes
Table 12 presents the number of febrile episodes experienced by treatment arm. A total number of 264 febrile episodes were reported, with 113 episodes of these being reported by patients in the levofloxacin arm, compared with 151 in the placebo arm. The number of patients reporting febrile episodes was consistently higher in the placebo arm.
| Visit | Treatment arm, n (%) | Total (N = 930), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 470) | Placebo (N = 460) | ||
| 0–4 weeks | |||
| Febrile episodes since last visit | |||
| 0 | 408 (87) | 380 (83) | 788 (85) |
| 1 | 55 (12) | 75 (16) | 130 (14) |
| 2 | 7 (1) | 5 (1) | 12 (1) |
| p-trend = 0.15 | |||
| Number of patients reporting infections | 62 | 80 | 142 |
| Total number of episodes | 69 | 85 | 154 |
| Visit | Treatment arm, n (%) | Total (N = 860), n (%) | |
| Levofloxacin (N = 432) | Placebo (N = 428) | ||
| 4–8 weeks | |||
| Febrile episodes since last visit | |||
| 0 | 410 (95) | 390 (91) | 800 (93) |
| 1 | 21 (5) | 36 (8) | 57 (7) |
| 2 | 1 (< 1) | 2 (< 1) | 3 (< 1) |
| p-trend = 0.03 | |||
| Number of patients reporting infections | 22 | 38 | 60 |
| Total number of episodes | 23 | 40 | 63 |
| Visit | Treatment arm, n (%) | Total (N = 821), n (%) | |
| Levofloxacin (N = 417) | Placebo (N = 404) | ||
| 8-12 weeks | |||
| Febrile episodes since last visit | |||
| 0 | 397 (95) | 379 (94) | 776 (95) |
| 1 | 19 (5) | 24 (6) | 43 (5) |
| 2 | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| p-trend = 0.41 | |||
| Number of patients reporting infections | 20 | 25 | 45 |
| Total number of episodes | 21 | 26 | 47 |
| Visit | Treatment arm, n (%) | Total (N = 934), n (%) | |
| Levofloxacin (N = 471) | Placebo (N = 463) | ||
| 0–12 weeks | |||
| Febrile episodes since last visit | |||
| 0 | 379 (80) | 338 (73) | 717 (77) |
| 1 | 74 (16) | 104 (22) | 178 (19) |
| 2+ | 18 (4) | 21 (5) | 39 (4) |
| p-trend = 0.02 | |||
| Number of patients reporting infections | 92 | 125 | 217 |
| Total number of episodes | 113 | 151 | 264 |
There was a significant trend in the number of febrile episodes reported across treatment arm within the 0- to 12-week summary (Mantel–Haenszel χ = 5.52; p = 0.02), with more febrile episodes reported in the placebo arm. This difference can be explained by the number of febrile episodes reported within the 4- to 8-week period from starting the trial treatment (p = 0.03).
There were 264 febrile episodes reported, for which infection forms were returned. Details on the site, type and confirmation of infections are detailed in Table 13.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 264), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 113) | Placebo (N = 151) | |||
| Randomised treatment stopped during episode | No | 55 (49) | 86 (57) | 141 (53) |
| Yes | 57 (50) | 63 (42) | 120 (46) | |
| Missing | 1 (1) | 2 (1) | 3 (1) | |
| Clinically diagnosed | No | 37 (33) | 58 (38) | 95 (36) |
| Yes | 76 (67) | 93 (62) | 169 (64) | |
| Site of clinically diagnosed infection | Lower respiratory tract | 39 (47) | 49 (51) | 88 (49) |
| Upper respiratory tract | 11 (13) | 11 (11) | 22 (12) | |
| Intravenous catheter associated | 1 (1) | 1 (1) | 2 (1) | |
| Bloodstream | 5 (6) | 8 (8) | 13 (7) | |
| Urinary tract infection | 6 (7) | 7 (7) | 13 (7) | |
| Gastrointestinal tract | 2 (2) | 3 (3) | 5 (3) | |
| Skin soft tissue | 6 (7) | 8 (8) | 14 (8) | |
| Other sitea | 7 (8) | 7 (7) | 14 (8) | |
| Unknown | 6 (7) | 1 (1) | 7 (4) | |
| Type of infection (confirmed or suspected) | Bacterial | 28 (25) | 39 (26) | 67 (25) |
| Fungal | 3 (3) | 1 (1) | 4 (2) | |
| Viral | 12 (11) | 12 (8) | 24 (9) | |
| Bacterial and viral | 0 (0) | 1 (1) | 1 (< 1) | |
| Bacterial and fungal | 1 (1) | 1 (1) | 2 (1) | |
| Not known | 51 (45) | 79 (52) | 130 (49) | |
| Missing | 18 (16) | 18 (12) | 36 (14) | |
| Laboratory specimens taken | No | 13 (11) | 20 (13) | 33 (13) |
| Yes | 88 (78) | 124 (82) | 212 (80) | |
| Missing | 12 (11) | 7 (5) | 19 (7) | |
| Positive cultures (multiple per infection) | Yes | 22 (10) | 38 (16) | 60 (13) |
| Organism suspected or confirmed | No | 76 (67) | 102 (67) | 178 (68) |
| Yes | 18 (16) | 33 (22) | 51 (19) | |
| Missing | 19 (17) | 16 (11) | 35 (13) | |
| Admitted to hospital | No | 24 (21) | 36 (24) | 60 (23) |
| Yes | 88 (78) | 114 (75) | 202 (76) | |
| Missing | 1 (1) | 1 (1) | 2 (1) | |
| Admitted to intensive therapy unit | Yes | 3 (3) | 5 (3) | 8 (3) |
| Infection developed in hospital | No | 101 (89) | 137 (91) | 238 (90) |
| Yes | 10 (9) | 14 (9) | 24 (9) | |
| Missing | 2 (2) | 0 (0) | 2 (1) | |
Other (non-febrile) infections
There were 249 patients who reported to have suffered a non-febrile infectious episode; there were 116 in the levofloxacin arm, compared with 133 patients in placebo arm (Table 14). A total of 323 episodes were reported (144 in the levofloxacin arm vs. 179 in the placebo arm).
| Visit | Treatment arm, n (%) | Total (N = 930), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 469) | Placebo (N = 461) | ||
| 0–4 weeks | |||
| Non-febrile episodes since last visit | |||
| 0 | 400 (85) | 398 (86) | 798 (86) |
| 1 | 66 (14) | 56 (12) | 122 (13) |
| 2 | 3 (1) | 7 (2) | 10 (1) |
| p-trend = 0.95 | |||
| Number of patients reporting infections | 69 | 63 | 132 |
| Total number of episodes | 72 | 70 | 142 |
| Visit | Treatment arm, n (%) | Total (N = 860), n (%) | |
| Levofloxacin (N = 432) | Placebo (N = 428) | ||
| 4–8 weeks | |||
| Non-febrile episodes since last visit | |||
| 0 | 397 (92) | 376 (88) | 773 (90) |
| 1 | 33 (8) | 48 (11) | 81 (9) |
| 2 | 2 (< 1) | 4 (1) | 6 (1) |
| p-trend = 0.05 | |||
| Number of patients reporting infections | 35 | 52 | 87 |
| Total number of episodes | 37 | 56 | 93 |
| Visit | Treatment arm, n (%) | Total (N = 821), n (%) | |
| Levofloxacin (N = 417) | Placebo (N = 404) | ||
| 8–12 weeks | |||
| Non-febrile episodes since last visit | |||
| 0 | 382 (92) | 356 (88) | 738 (90) |
| 1 | 35 (8) | 43 (11) | 78 (9) |
| 2 | 0 | 5 (1) | 5 (1) |
| p-trend = 0.04 | |||
| Number of patients reporting infections | 35 | 48 | 83 |
| Total number of episodes | 35 | 53 | 88 |
| Visit | Treatment arm, n (%) | Total (N = 935), n (%) | |
| Levofloxacin (N = 470) | Placebo (N = 465) | ||
| 0–12 weeks | |||
| Non-febrile episodes since last visit | |||
| 0 | 354 (75) | 332 (71) | 686 (73) |
| 1 | 94 (20) | 95 (20) | 189 (20) |
| 2+ | 22 (5) | 38 (8) | 60 (6) |
| p-trend = 0.06 | |||
| Number of patients reporting infections | 116 | 133 | 249 |
| Total number of episodes | 144 | 179 | 323 |
There was a borderline trend in the number of non-febrile infections reported across treatment arms within the 0- to 12-week summary (Mantel–Haenszel χ = 3.67; p = 0.06), with more episodes reported in the placebo arm.
At the time of data lock, 323 infection forms reporting details of non-febrile episodes had been received: 144 from the levofloxacin arm and 179 from the placebo arm. Details of these non-febrile episodes reported on the infection forms are summarised in Table 15.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 323), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 144) | Placebo (N = 179) | |||
| Randomised treatment stopped during episode | No | 104 (72) | 153 (85) | 257 (80) |
| Yes | 37 (26) | 22 (12) | 59 (18) | |
| Missing | 3 (2) | 4 (2) | 7 (2) | |
| Clinically diagnosed | No | 20 (14) | 17 (10) | 37 (11) |
| Yes | 124 (86) | 161 (90) | 285 (88) | |
| Missing | 0 (0) | 1 (1) | 1 (< 1) | |
| Site of clinically diagnosed infection | Lower respiratory tract | 46 (37) | 48 (30) | 94 (33) |
| Upper respiratory tract | 21 (17) | 22 (14) | 43 (15) | |
| Skin soft tissue | 18 (14) | 24 (15) | 42 (15) | |
| Othera | 17 (14) | 25 (15) | 42 (15) | |
| Urinary tract infection | 12 (10) | 26 (16) | 38 (13) | |
| Dental/abscess | 4 (3) | 6 (4) | 10 (3) | |
| Gastrointestinal tract | 4 (3) | 3 (2) | 7 (2) | |
| Intravenous catheter associated | 1 (1) | 4 (2) | 5 (2) | |
| Bloodstream | 1 (1) | 1 (1) | 2 (1) | |
| Unknown | 1 (1) | 2 (1) | 3 (1) | |
| Bone/joint | 0 (0) | 1 (1) | 1 (< 1) | |
| Type of infection confirmed or suspected | Bacterial | 37 (26) | 60 (34) | 97 (30) |
| Fungal | 12 (8) | 8 (4) | 20 (6) | |
| Viral | 10 (7) | 16 (9) | 26 (8) | |
| Bacterial and viral | 0 (0) | 1 (1) | 1 (< 1) | |
| Bacterial and fungal | 1 (1) | 0 (0) | 1 (< 1) | |
| Fungal and viral | 1 (1) | 0 (0) | 1 (< 1) | |
| Not known | 64 (44) | 77 (43) | 141 (44) | |
| Missing | 19 (13) | 17 (10) | 36 (11) | |
| Laboratory specimens taken | No | 68 (47) | 87 (49) | 155 (48) |
| Yes | 64 (44) | 76 (42) | 140 (43) | |
| Missing | 12 (8) | 16 (9) | 28 (9) | |
| Positive cultures (multiple per infection) | Yes | 27 (27) | 44 (40) | 71 (34) |
| Suspected or confirmed presence of an organism | No | 100 (69) | 113 (63) | 213 (66) |
| Yes | 26 (18) | 48 (27) | 74 (23) | |
| Missing | 18 (13) | 18 (10) | 36 (11) | |
| Admitted to hospital | No | 100 (69) | 142 (79) | 242 (75) |
| Yes | 42 (29) | 37 (21) | 79 (24) | |
| Missing | 2 (1) | 0 (0) | 2 (1) | |
| Admitted to intensive therapy unit | Yes | 1 (1) | 1 (1) | 2 (1) |
| Infection developed in hospital | No | 127 (88) | 155 (87) | 282 (87) |
| Yes | 14 (10) | 16 (9) | 30 (9) | |
| Missing | 3 (2) | 8 (4) | 11 (4) | |
Total infections
A total of 411 patients reported an infection (febrile or non-febrile) on their on-treatment form (190 in the levofloxacin arm and 221 in the placebo arm). A total of 587 infectious episodes were reported (257 in the levofloxacin arm and 330 in the placebo arm), as shown in Table 16.
| Visit | Treatment arm, n (%) | Total (N = 932), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 470) | Placebo (N = 462) | ||
| 0–4 weeks | |||
| Total number of infections since last visit | |||
| 0 | 342 (73) | 329 (71) | 671 (72) |
| 1 | 115 (24) | 111 (24) | 226 (24) |
| 2 | 13 (3) | 22 (5) | 35 (4) |
| p-trend = 0.32 | |||
| Number of patients reporting infections | 128 | 133 | 261 |
| Total number of infections | 141 | 155 | 296 |
| Visit | Treatment arm, n (%) | Total (N = 860), n (%) | |
| Levofloxacin (N = 432) | Placebo (N = 428) | ||
| 4–8 weeks | |||
| Total number of infections since last visit | |||
| 0 | 375 (87) | 341 (80) | 716 (8) |
| 1 | 54 (12) | 78 (18) | 132 (15) |
| 2 | 3 (1) | 9 (2) | 12 (1) |
| p-trend = 0.003 | |||
| Number of patients reporting infections | 57 | 87 | 144 |
| Total number of infections | 60 | 96 | 156 |
| Visit | Treatment arm, n (%) | Total (N = 821), n (%) | |
| Levofloxacin (N = 417) | Placebo (N = 404) | ||
| 8–12 weeks | |||
| Total number of infections since last visit | |||
| 0 | 363 (87) | 331 (82) | 694 (85) |
| 1 | 52 (12) | 67 (17) | 119 (14) |
| 2 | 2 (< 1) | 6 (1) | 8 (1) |
| p-trend = 0.03 | |||
| Number of patients reporting infections | 54 | 73 | 127 |
| Total number of infections | 56 | 79 | 135 |
| Visit | Treatment arm, n (%) | Total (N = 936), n (%) | |
| Levofloxacin (N = 471) | Placebo (N = 465) | ||
| 0–12 weeks | |||
| Total number of infections since last visit | |||
| 0 | 281 (60) | 244 (52) | 525 (56) |
| 1 | 138 (29) | 143 (31) | 281 (30) |
| 2 | 38 (8) | 54 (12) | 92 (10) |
| 3+ | 14 (3) | 24 (5) | 38 (4) |
| p-trend = 0.005 | |||
| Number of patients reporting infections | 190 | 221 | 411 |
| Total number of infections | 257 | 330 | 587 |
There was a significant trend in the total number of infections reported across treatment arms within the 0- to 12-week summary (Mantel-Haenszel χ = 7.89; p = 0.005), with more patients reporting total infections in the placebo arm. This difference can be explained by the number of infections reported per patient between the 4- to 12-week period from starting trial treatment.
At the time of data lock, 587 infection forms reporting details of these episodes had been received: 257 from patients of the levofloxacin arm and 330 from patients of the placebo arm. Infection details are shown in Table 17.
| Factor | Grouping | Treatment arm, n (%) | Total (N = 587), n (%) | |
|---|---|---|---|---|
| Levofloxacin (N = 257) | Placebo (N = 330) | |||
| Randomised treatment stopped during episode | No | 159 (62) | 239 (72) | 398 (68) |
| Yes | 94 (37) | 85 (26) | 179 (30) | |
| Missing | 4 (2) | 6 (2) | 10 (2) | |
| Clinically diagnosed | No | 57 (22) | 75 (23) | 132 (22) |
| Yes | 200 (78) | 254 (77) | 454 (77) | |
| Missing | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Site of clinically diagnosed infection | Lower respiratory tract | 85 (41) | 97 (38) | 182 (39) |
| Upper respiratory tract | 32 (15) | 33 (13) | 65 (14) | |
| Skin soft tissue | 24 (12) | 32 (12) | 56 (12) | |
| Other | 24 (12) | 32 (12) | 56 (12) | |
| Urinary tract infection | 18 (9) | 33 (13) | 51 (11) | |
| Dental/abscess | 4 (2) | 7 (3) | 11 (2) | |
| Gastrointestinal tract | 6 (3) | 6 (2) | 12 (3) | |
| Intravenous catheter associated | 2 (1) | 5 (2) | 7 (2) | |
| Bloodstream | 6 (3) | 9 (3) | 15 (3) | |
| Unknown | 7 (3) | 3 (1) | 10 (2) | |
| Bone/joint | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Type of infection (confirmed or suspected) | Bacterial | 65 (25) | 99 (30) | 164 (28) |
| Fungal | 15 (6) | 9 (3) | 24 (4) | |
| Viral | 22 (9) | 28 (8) | 50 (9) | |
| Bacterial and viral | 0 (0) | 2 (1) | 2 (< 1) | |
| Bacterial and fungal | 2 (1) | 1 (< 1) | 3 (1) | |
| Fungal and viral | 1 (< 1) | 0 (0) | 1 (< 1) | |
| Not known | 115 (45) | 156 (47) | 271 (46) | |
| Missing | 37 (14) | 35 (11) | 72 (12) | |
| Laboratory specimens taken | No | 81 (32) | 107 (32) | 188 (32) |
| Yes | 152 (59) | 200 (61) | 352 (60) | |
| Missing | 24 (9) | 23 (7) | 47 (8) | |
| Positive cultures (multiple per infection) | Yes | 49 (16) | 82 (24) | 131 (20) |
| Suspected or confirmed presence of an organism | No | 176 (68) | 215 (65) | 391 (67) |
| Yes | 44 (17) | 81 (25) | 125 (21) | |
| Missing | 37 (14) | 34 (10) | 71 (12) | |
| Admitted to hospital | No | 124 (48) | 178 (54) | 302 (51) |
| Yes | 130 (51) | 151 (46) | 281 (48) | |
| Missing | 3 (1) | 1 (< 1) | 4 (1) | |
| Admitted to intensive therapy unit | Yes | 4 (2) | 6 (2) | 10 (2) |
| Infection developed in hospital | No | 228 (89) | 292 (88) | 520 (89) |
| Yes | 24 (9) | 30 (9) | 54 (9) | |
| Missing | 5 (2) | 8 (2) | 13 (2) | |
Incidence of microbiologically proven infections, the pathogens and their susceptibility to antibacterials
The identity of species cultured from invasive isolates were extracted from laboratory reports of the 112 specimens. The laboratory reports were obtained for patients in which positive cultures were detected; of the 131 patients reported with positive cultures, 49 were reported in the levofloxacin arm and 82 were reported in the placebo arm (Table 17). A total of 112 organisms were detected: 44 from the levofloxacin arm and 69 from the placebo arm. There were fewer Gram-negative infections reported in the levofloxacin arm than in the placebo arm (Table 18).
| Species | Treatment arm, n (%) | Total (n) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Total number of Gram-negative organisms | 6 (18) | 27 (82) | 33 |
| Enterobacteriaceae | 4 | 14 | 18 |
| Pseudomonas species | 0 | 5 | 5 |
| Other Gram-negative organisms | 2 | 8 | 10 |
| Total number of Gram-positive infections | 16 (44) | 20 (56) | 36 |
| S. aureus | 4 | 6 | 10 |
| Streptococcus pneumoniae | 0 | 3 | 3 |
| Coagulase-negative Staphylococcus | 5 | 5 | 10 |
| Other Gram-positive organisms | 7 | 6 | 13 |
| Total number of other bacterial infections | 4 (50) | 4 (50) | 8 |
| Anaerobic | 1 | 2 | 3 |
| Mixed growth | 3 | 2 | 5 |
| Total number of viral infections | 10 (50) | 10 (50) | 20 |
| Adenovirus | 1 | 0 | 1 |
| Cytomegalovirus | 1 | 0 | 1 |
| Herpes simplex/varicella | 3 | 0 | 3 |
| Influenza | 2 | 5 | 7 |
| Metapneumovirus | 0 | 2 | 2 |
| Parainfluenza | 2 | 3 | 5 |
| Respiratory syncytial virus (RSV) | 1 | 0 | 1 |
| Candida species a | 8 (53) | 7 (47) | 15 |
| Total number of isolates | 44 (39) | 69 (61) | 112 |
Reported sensitivities of invasive organisms from local laboratories
Note that, although the numbers are small, all isolates in the levofloxacin arm of the trial are resistant to quinolones, as shown in Table 19.
| Antibiotic category | Treatment arm | |||
|---|---|---|---|---|
| Levofloxacin | Placebo | |||
| Number sensitive/number tested | % | Number sensitive/number tested | % | |
| Quinolone | 0/3 | 0 | 7/8 | 88 |
| Penicillin | 10/19 | 53 | 16/30 | 53 |
| Aminoglycoside | 5/7 | 71 | 11/13 | 85 |
| Co-amoxiclav | 4/5 | 80 | 9/14 | 64 |
| Piperacillin/tazobactam | 2/2 | 100 | 7/8 | 88 |
| Carbapenem | 2/2 | 100 | 6/6 | 100 |
| Cephalosporin | 2/2 | 100 | 6/8 | 75 |
| Other | 34/44 | 76 | 62/83 | 75 |
Deaths and infection-related deaths
Overall, a total of 116 deaths were reported among the 977 randomised patients (levofloxacin, n = 61; placebo, n = 55). A total of 30 patients (total of 52 causes) died within 12 weeks of starting trial treatment (levofloxacin, n = 8; placebo, n = 22). One patient randomised to placebo died prior to starting trial treatment. A total of 86 patients died post 12 weeks (levofloxacin, n = 53; placebo, n = 33). Table 20 presents causes of death by treatment arm. A total of 220 causes of death were reported for the 116 deaths, as selection of multiple causes is permitted on the death form. Cause of death was not available for one patient on placebo who died in a nursing home.
| Causes of death within 12 weeks | Treatment arm, n (%) | Total (N = 52), n (%) | ||
|---|---|---|---|---|
| Disease related | Cause of death | Levofloxacin (N = 13) | Placebo (N = 39) | |
| Myeloma related | Myeloma/disease progression | 4 (31) | 13 (33) | 17 (33) |
| Overwhelming tumour load | 0 (0) | 0 (0) | 0 (0) | |
| Infection | 2 (15) | 6 (15) | 8 (15) | |
| Renal failure | 2 (15) | 4 (10) | 6 (12) | |
| Skeletal | 0 (0) | 1 (3) | 1 (2) | |
| Subtotal | 8 | 24 | 32 | |
| Non-myeloma related | Cardiac | 2 (15) | 8 (20) | 10 (19) |
| Respiratory | 1 (8) | 2 (5) | 3 (6) | |
| Neurological | 0 (0) | 1 (3) | 1 (2) | |
| Other malignancy | 0 (0) | 0 (0) | 0 (0) | |
| Infection | 1 (8) | 2 (5) | 3 (6) | |
| Other | 1 (8) | 2 (5) | 3 (6) | |
| Subtotal | 5 | 15 | 20 | |
| Total infection related | 3 | 8 | 11 | |
| Causes of death post 12 weeks | Treatment arm, n (%) | Total (N = 168), n (%) | ||
| Disease related | Cause of death | Levofloxacin (N = 106) | Placebo (N = 62) | |
| Myeloma related | Myeloma/disease progression | 44 (42) | 22 (35) | 66 (39) |
| Overwhelming tumour load | 4 (4) | 2 (3) | 6 (4) | |
| Infection | 13 (12) | 10 (16) | 23 (14) | |
| Renal failure | 7 (7) | 6 (10) | 13 (8) | |
| Skeletal | 2 (2) | 1 (2) | 3 (2) | |
| Subtotal | 70 | 41 | 111 | |
| Non-myeloma related | Cardiac | 10 (9) | 6 (10) | 16 (10) |
| Respiratory | 8 (8) | 7 (11) | 15 (9) | |
| Neurological | 2 (2) | 1 (2) | 3 (2) | |
| Other malignancy | 0 (0) | 1 (2) | 1 (1) | |
| Infection | 6 (6) | 2 (3) | 8 (5) | |
| Other | 10 (9) | 4 (6) | 14 (8) | |
| Subtotal | 36 | 21 | 57 | |
| Total infection related | 19 | 12 | 31 | |
| Total causes of death | Treatment arm, n (%) | Total (N = 220), n (%) | ||
| Disease related | Cause of death | Levofloxacin (N = 119) | Placebo (N = 101) | |
| Myeloma related | Myeloma/disease progression | 48 (40) | 35 (35) | 83 (38) |
| Overwhelming tumour load | 4 (3) | 2 (2) | 6 (3) | |
| Infection | 15 (13) | 16 (16) | 31 (14) | |
| Renal failure | 9 (7) | 10 (10) | 19 (8) | |
| Skeletal | 2 (2) | 2 (2) | 4 (2) | |
| Subtotal | 78 (65) | 65 (65) | 143 (65) | |
| Non-myeloma related | Cardiac | 12 (10) | 14 (14) | 26 (12) |
| Respiratory | 9 (8) | 9 (9) | 18 (8) | |
| Neurological | 2 (2) | 2 (2) | 4 (2) | |
| Other malignancy | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Infection | 7 (6) | 4 (4) | 11 (5) | |
| Other | 11 (9) | 6 (6) | 17 (8) | |
| Subtotal | 41 (35) | 36 (35) | 77 (35) | |
The majority of deaths were attributed to myeloma-related causes (n = 143, 65%) as opposed to non-myeloma-related causes (n = 77, 35%). Infection-related cause of death was less common in the levofloxacin arm (three causes) than in the the placebo arm (eight causes) within 12 weeks. However, more infection-related causes of death were reported in the levofloxacin arm (19 causes) than in the placebo arm (12 causes) post 12 weeks.
All deaths within 12 weeks were reviewed by an independent clinician to ascertain causes of death. Deaths post 12 weeks were reviewed by the two clinical chief investigators.
Time to first event by treatment arm across 12 weeks
Table 21 shows the number of febrile episodes, deaths and febrile episodes and deaths combined by treatment arm within the first 12 weeks. A total of 210 patients reported febrile infections (levofloxacin, n = 91; placebo, n = 119) out of the total 977 patients randomised.
| Event category | Treatment arm, n (%) | Total (N = 977), n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Levofloxacin (N = 489) | Placebo (N = 488) | ||||||||
| None | 394 | 354 | 748 | ||||||
| Febrile episode only | 87 | } | 91 | 112 | } | 119 | 199 | } | 210 |
| Death only | 4 | 15 | 19 | ||||||
| Febrile episode and death | 4 | 7 | 11 | ||||||
| Total events | 95 (19) | 134 (27) | 229 (23) | ||||||
Time to first event was calculated from the date of starting trial treatment to the date of febrile episode or date of death for the 229 patients reporting a febrile episode or death within 12 weeks. The remaining patients were censored at date of withdrawal or the last date considered appropriate. Figure 4 shows the 19% of patients randomised to levofloxacin reporting a febrile episode, or death, within 12 weeks from the start of trial treatment compared with the 27% in the placebo arm; log-rank χ2 test = 9.78; HR 0.66, 95% CI 0.51 to 0.86; p = 0.002 in favour of levofloxacin.
FIGURE 4.
Time to first event (febrile or death) within 12 weeks. Log-rank χ2 test = 9.78; HR 0.66, 95% CI 0.51 to 0.86; p = 0.002.
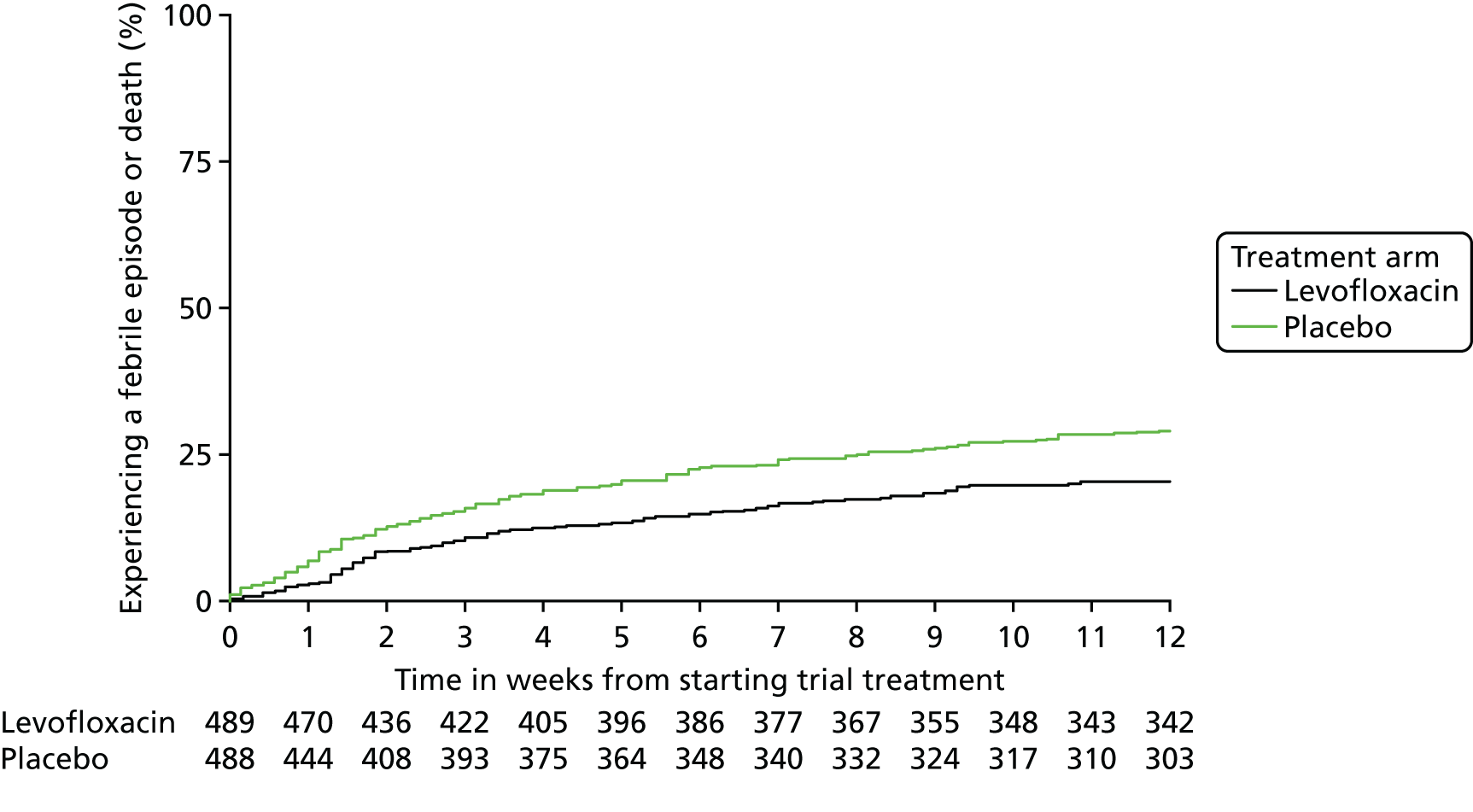
The Cox regression analysis showed that treatment is the most important factor and still retains significance in a multivariate model adjusting for baseline factors. A Cox regression model excluding treatment indicates that no other factors predict time to febrile episode or death. Assessing the individual factors indicates that ECOG performance status is the only factor with a borderline significance. Table 22 shows the treatment effect unadjusted and adjusted by baseline factors. It can be seen that adjustment makes little difference to the overall treatment effect.
| Factor | Grouping | Coefficient | p-value | HR (95% CI) |
|---|---|---|---|---|
| Treatment only (n = 977; 229 events) | ||||
| Treatment | Placebo, levofloxacin | –0.42 | 0.002 | 0.66 (0.51 to 0.86) |
| Treatment adjusted for baseline factors (n = 668; 166 events) | ||||
| Treatment | Placebo, levofloxacin | –0.32 | 0.04 | 0.73 (0.53 to 0.99) |
| ECOG performance status at randomisation | 0–2, 3–4 | 0.46 | 0.11 | 1.58 (0.90 to 2.78) |
| Vertebral fractures | Absent, present | –0.21 | 0.27 | 0.81 (0.56 to 1.17) |
| Neutrophils (× 109/l) | < 1.8, ≥ 1.8 | –0.30 | 0.22 | 0.74 (0.46 to 1.19) |
| ESBL isolated at baseline | No, yes | 0.18 | 0.46 | 1.20 (0.74 to 1.94) |
| International Staging System | Stage I, II, III | –0.05 | 0.61 | 0.95 (0.77 to 1.16) |
| Individual factors – univariate | ||||
| ECOG (n = 957, events = 226) | 0, 1, 2, 3, 4 | 0.13 | 0.07 | 1.14 (0.99 to 1.31) |
| ECOG | 0–2, 3–4 | 0.45 | 0.04 | 1.57 (1.01 to 2.44) |
| Vertebral (n = 977, events = 229) | Absent, present | 0.02 | 0.90 | 1.02 (0.76 to 1.36) |
| ESBL isolated at baseline (n = 785, events = 187) | No, yes | 0.39 | 0.06 | 1.49 (0.98 to 2.27) |
| Neutrophils (n = 967, events = 229) | Continuous | –0.02 | 0.37 | 0.98 (0.94 to 1.02) |
| Prophylactic Septrin (n = 946, events = 224) | No, yes | –0.52 | 0.0008 | 0.59 (0.44 to 0.80) |
| Anti-infective history (treatment of infection month prior to trial treatment) (n = 814, events = 182) | No, yes | 0.36 | 0.04 | 1.43 (1.01 to 2.01) |
| Steroids in 14 days prior to randomisation (n = 970, events = 229) | No, yes | –0.09 | 0.52 | 0.92 (0.71 to 1.19) |
| Treatment and prophylactic Septrin (n = 946, events = 224) | ||||
| Treatment | Placebo, levofloxacin | –0.41 | 0.002 | 0.66 (0.51 to 0.86) |
| Prophylactic Septrin | No, yes | –0.52 | 0.0009 | 0.59 (0.44 to 0.81) |
The most significant individual baseline factor was the use of prophylactic Septrin (HR 0.59, 95% CI 0.44 to 0.80; p = 0.0008), followed by treatment (HR 0.66, 95% CI 0.51 to 0.86; p = 0.002). Adjusting treatment for prophylactic Septrin use made little difference to the treatment effect, indicating that these two variables have independent prognostic value (Figure 5).
FIGURE 5.
Treatment adjusted for Septrin (p = 0.002). (a) Septrin; and (b) no Septrin.

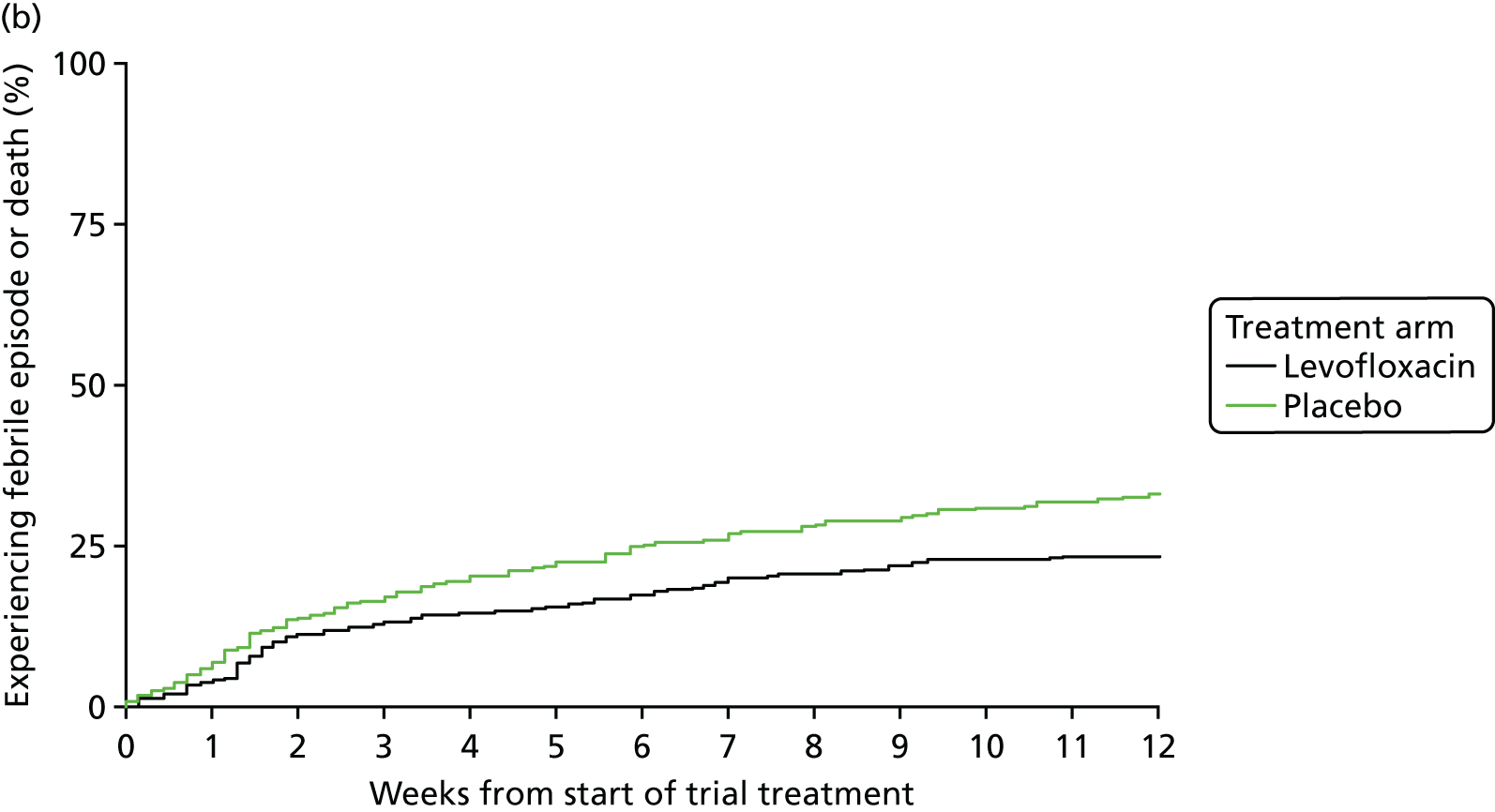
Overall survival
Overall survival was calculated from the date of starting trial treatment to the date of death or being censored at date last alive. Figure 6 shows that 98% of patients on levofloxacin survived for 12 weeks, compared with 95% of patients on placebo (p = 0.94). Twelve-month survival was similar across arms (levofloxacin, 90%; placebo, 91%). Follow-up of surviving patients was the same [median 12 months; interquartile range (IQR) 11–13 months] in both arms.
FIGURE 6.
Overall survival, by treatment. Log-rank χ2 test = 0.004; p = 0.94.
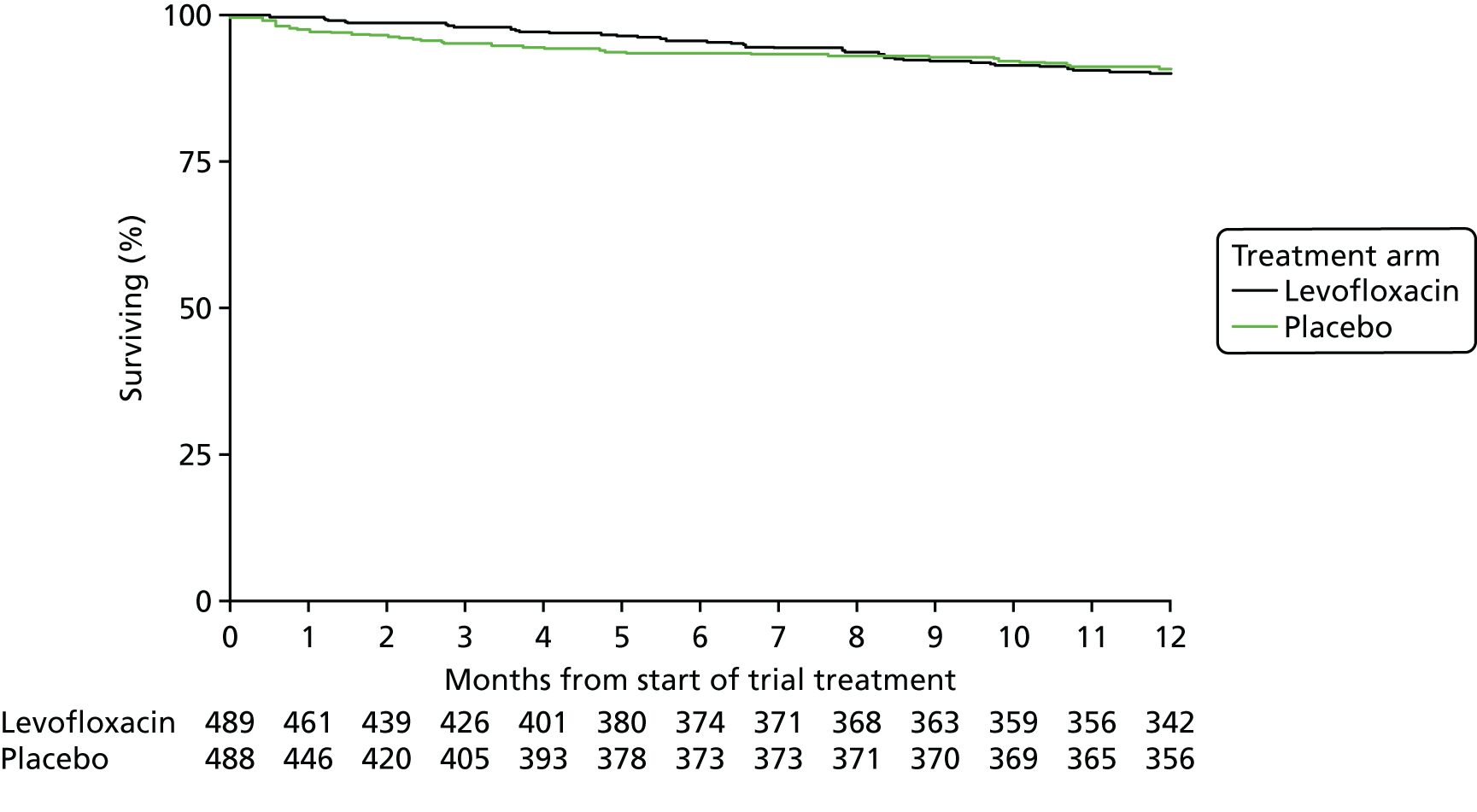
Micro-organism sample return and acquisition
Table 23 shows the numbers of C. difficile, MRSA and ESBL Gram-negative organisms that were present at baseline. It also shows the number of new acquisitions between baseline and 16 weeks. The total number of nasal and stool samples returned between baseline and 16 weeks is indicated in the left-hand column. There were no differences in new ESBL acquisitions between the placebo and levofloxacin arms (30 vs. 25, respectively). There were no differences in acquisitions for C. difficile (8 vs. 11) and MRSA (7 vs. 4) between the placebo and levofloxacin arms.
| Organism | Treatment arm, (n) | Total, (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Levofloxacin | Placebo | ||||||||
| C. difficile | ESBL | MRSA | C. difficile | ESBL | MRSA | C. difficile | ESBL | MRSA | |
| Present at baseline (785 stool and 928 nasal samples) | 1 | 19 | 5 | 5 | 37 | 9 | 6 | 56 | 14 |
| New acquisitions | |||||||||
| Week 4 (706 stool and 805 nasal samples) | 4 | 8 | 0 | 3 | 11 | 4 | 7 | 19 | 4 |
| Week 8 (662 stool and 759 nasal samples) | 0 | 5 | 1 | 2 | 7 | 1 | 2 | 12 | 2 |
| Week 12 (634 stool and 719 nasal samples) | 3 | 3 | 1 | 2 | 7 | 2 | 5 | 10 | 3 |
| Week 16 (593 stool and 650 nasal samples) | 4 | 9 | 2 | 1 | 5 | 0 | 5 | 14 | 2 |
| Total new acquisitions (2595 stool and 2933 nasal samples) | 11 | 25 | 4 | 8 | 30 | 7 | 19 | 55 | 11 |
Days on additional anti-infective treatment and total doses taken for treatment of infection, by treatment arm
A summation of all anti-infective drugs prescribed for the treatment of infections during the trial treatment period by treatment arm, excluding TEAMM trial medication, is shown in Table 24.
| Factor | Treatment arm | Wilcoxon two-sample p-value | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Total number of days on anti-infectives | |||
| n | 186 | 207 | 0.19 |
| Median | 16 | 20 | |
| IQR | 12–30 | 12–32 | |
| Range | 2–129 | 2–235 | |
| Total dose taken (g or l) | |||
| n | 160 | 180 | 0.99 |
| Median | 8 | 9 | |
| IQR | 3–25 | 2–27 | |
| Range | 0.02–65 | 0.02–60 | |
| Total | 99 | 192 | |
Response to antimyeloma treatment
Response to myeloma treatment within the first 12 weeks is an early indication of whether or not patients will reach stable disease. The main reason why patients do not respond is disease progression. Changes between blood samples collected at baseline and the last sample collected within 12 weeks will indicate if patients have had an early response to antimyeloma treatment. This will be presented as part of the TEAMM National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation (EME) grant (reference number 14/24/04).
Serious adverse events
Since the start of the trial, a total of 597 SAEs have been reported to the TEAMM trial office (of these, 308 were from patients on levofloxacin and 289 were from patients on placebo). Tables 25–28 present information relating to the type of event by treatment for all SAEs and summarises the severity and causality assessments, and outcomes of each event by treatment.
| Event type | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Death | 2 (0.6) | 5 (2) | 7 (1) |
| Life-threatening event | 1 (0.3) | 7 (2) | 8 (1) |
| Hospitalisation or prolongation of hospitalisation | 235 (76) | 227 (78) | 462 (77) |
| Persistent or significant disability/incapacity | 5 (2) | 4 (1) | 9 (1) |
| Congenital anomaly/birth defect | – (–) | – (–) | – (–) |
| Other reason | 16 (5) | 19 (7) | 35 (6) |
| Hospitalisation and other reason | 6 (2) | 2 (1) | 8 (1) |
| Life-threatening event, hospitalisation, disability/incapacity | 3 (1) | 3 (1) | 6 (1) |
| Death, life-threatening event, hospitalisation | 0 (0) | 2 (1) | 2 (0.3) |
| Hospitalisation and disability/incapacity | 11 (4) | 2 (1) | 13 (2) |
| Life-threatening event and hospitalisation | 28 (9) | 16 (6) | 44 (7) |
| Disability/congenital anomaly/other reason | 0 (0) | 1 (0.3) | 1 (0.2) |
| Death and hospitalisation | 1 (0.3) | 0 (0) | 1 (0.2) |
| Death and hospitalisation/other reason | 0 (0) | 1 (0.3) | 1 (0.2) |
| Total | 308 (100) | 289 (100) | 597 (100) |
| Severity | Treatment arm, n (%) | Total (N = 597), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 308) | Placebo (N = 289) | ||
| Mild | 38 (12) | 29 (10) | 67 (11) |
| Moderate | 136 (44) | 141 (49) | 277 (46) |
| Severe | 101 (33) | 86 (30) | 187 (31) |
| Fatal/life-threatening | 32 (10) | 33 (11) | 65 (11) |
| Missing | 1 (0.3) | 0 (0) | 1 (0.2) |
| Causality | Treatment arm, n (%) | Total (N = 597), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 308) | Placebo (N = 289) | ||
| Definitely | 1 (0.3) | 0 (0) | 1 (0.2) |
| Probably | 8 (3) | 5 (2) | 13 (2) |
| Possibly | 29 (9) | 17 (6) | 46 (8) |
| Unlikely | 126 (41) | 120 (42) | 246 (41) |
| Unrelated | 144 (47) | 147 (51) | 291 (49) |
| Outcome | Treatment arm, n (%) | Total (N = 597), n (%) | |
|---|---|---|---|
| Levofloxacin (N = 308) | Placebo (N = 289) | ||
| Resolved – no sequelae | 229 (74) | 220 (76) | 449 (75) |
| Resolved – with sequelae | 67 (22) | 44 (15) | 111 (19) |
| Unresolved | 2 (1) | 1 (0.3) | 3 (0.5) |
| Death | 9 (3) | 24 (8) | 33 (6) |
| Consent withdrawn prior to resolution | 1 (0.3) | 0 (0) | 1 (0.2) |
In summary, 597 SAEs were reported for patients (levofloxacin, n = 308; placebo, n = 289), with the majority reported as being unlikely to be related to or unrelated to the study drug (537/597, 90%) but, instead, related to hospitalisation or prolongation of existing hospitalisation (462/597, 77%). Toxicity reported to be related to levofloxacin (38 episodes) compared with placebo (22 episodes) had a trend towards more gastrointestinal disorders (fours events of nausea/vomiting on levofloxacin vs. one event on placebo) but fewer episodes of diarrhoea (five episodes on levofloxacin vs. nine episodes on placebo), rash (six episodes on levofloxacin vs. one episode on placebo), psychiatric disorders (two episodes on levofloxacin vs. no episodes on placebo) and musculoskeletal and connective tissue disorders (seven episodes on levofloxacin and one episode on placebo) (see Table 44). Appendix 2 provides further information as regards the summary of the CTCAE categories of all SAEs reported (see Table 43) together with their terms of SAEs by treatment (see Table 43). In addition, reported SARs are detailed (see Tables 44 and 45).
Quality of life
Within the scope of the TEAMM grant, QoL was measured at baseline and at 4, 8, 12 and 16 weeks for patients who consented to fill in the QoL booklets. These data include the responses to the EQ-5D, which are reported in Chapter 4, as well as to the EORTC QLQ-C30, EORTC QLQ-MY24 and HADS.
Chapter 4 Health economic analysis
Introduction
An economic evaluation was conducted to estimate the cost-effectiveness of prophylactic levofloxacin compared with placebo in newly diagnosed symptomatic myeloma. The economic evaluation was conducted alongside the TEAMM clinical trial so that only the data collected during the trial were analysed. Cost and outcome data were collected from trial participants for 16 weeks. However, if the intervention is successful in reducing mortality, the benefits may extend beyond this period. Consequently, the economic evaluation includes a within-trial cost-effectiveness analysis over 16 weeks but also explores longer-term outcomes using an analysis extrapolated to 12 months from randomisation.
Methods
Aim and end points
The primary aim of this analysis was to assess the cost-effectiveness of antibiotic prophylaxis using levofloxacin alongside antimyeloma therapy compared with using placebo alongside antimyeloma treatment in patients with newly diagnosed symptomatic myeloma. The primary end point was the cost per quality-adjusted life-year (QALY) gained at 16 weeks. The methods used for this within-trial analysis were guided by the recommendations of the National Institute for Health and Care Excellence (NICE) methods guide. 39
Perspective and time frame
The trial adopted a health-care and Personal Social Service provider perspective. Direct costs and outcomes of patients randomised to levofloxacin versus placebo were compared over the 16-week time horizon of the trial. As the time frame of the trial was < 1 year, discounting of the costs and benefits was not required.
Measurement of outcomes
Primary outcome
This analysis used the QALY as the main outcome measure. QALYs are a generic measure of health that take account of both the quality and length of life, such that 1 QALY is equal to 1 year of life lived in a state of full health. 40
Health state utility values were obtained from patient responses to the EQ-5D three-level version questionnaire,31 which was administered at baseline and at weeks 4, 8, 12 and 16 post randomisation. The EQ-5D is a commonly used generic measure of health-related quality of life (HRQoL) and NICE’s39 preferred outcome measure for cost-effectiveness analysis. Patient responses were converted to utility values using the standard UK general population time trade-off tariff values. 41 The utility values represent patients’ QoL and were multiplied by duration (t) in each health state to generate QALYs. An area under the curve approach was adopted for estimating QALYs with a linear transition assumed between adjacent time points:
where EQ-5Dbaseline, EQ-5D4, EQ-5D8, EQ-5D12 and EQ-5D16 are the EQ-5D scores at baseline, week 4, week 8, week 12 and week 16, respectively. If an individual died during the trial, it was assumed that their utility value was 0 from the date of death to trial end and assumed a linear transition to this value from their last completed EQ-5D.
The EORTC QLQ-C30 was also administered alongside the EQ-5D questionnaire at each trial follow-up. QALYs were calculated using the EORTC-8D as a sensitivity analysis in order to examine the effect of using a disease-specific measure of HRQoL compared with a generic measure (EQ-5D). 32
Total QALYs were calculated for each patient over the 16 weeks and summary statistics were generated by intervention group. Differences between groups were compared using independent-sample t-tests.
Secondary outcome
A secondary outcome measure considered in the analysis was number of febrile episodes. This is the primary outcome measure for the clinical analysis and allowed a useful complementary economic analysis of the cost per febrile episode avoided. Details of febrile episodes were collected in clinic visits at the start of trial treatment and then every 4 weeks until the end of treatment in week 12.
Measurement of resource use
All health-care resource use was estimated from the perspective of the health-care and Personal Social Service provider and was collected for the trial period of 16 weeks from randomisation. This included primary care such as GP visits and nurse home visits as well as secondary care such as outpatient visits and other hospital admissions. At 12 months, data were limited to the number of hospital admissions and associated length of stay between 16 weeks and 12 months.
Resource use was captured using three complementary approaches of data collection within the trial: (1) patient diaries, (2) trial CRFs and (3) hospital data. Patients completed diaries of resource use, which they brought with them to follow-up visits. Nurses used the patient diaries to summarise resource use on the CRFs following discussions with patients, at which time corrections to the resource use were made. Hospital data reporting admissions were also collected, which were used alongside patient-reported resource use.
As multiple sources of data were used to capture resource use, there was potential for discrepancies between the sources in the resource use recorded for any given patient. For example, the number of hospital visits recorded on hospital systems may differ from the number recalled by patients. When there were discrepancies between data sources, it was assumed that hospital records were more reliable and these data were given precedence. In order to test the effect of this assumption, sensitivity analyses were conducted using the data from patient recall.
Cost analysis
All resource use data were converted to costs using appropriate UK unit costs estimated at the time of analysis. Unit costs were assigned to health-care resource use from the British National Formulary (BNF),42 Personal Social Services Research Unit’s (PSSRU) Unit Costs of Health and Social Care 201543 and the Department of Health and Social Care’s NHS Reference Costs 2014 to 2015. 44 Costs were assigned on a per-unit basis with unit values taken from the resource use data collected within the trial (i.e. from patient diaries, CRFs and hospital data). All inpatient and outpatient hospital stays or use of community health and social services that occurred within the trial period were costed irrespective of whether or not their use was directly associated with the treatment of myeloma. Unit costs for the main resource use items (those specified on the resource use CRF) are presented in Table 29, together with the costing of the treatment medications. In addition to those resource use items specified in the form, patients were also given a free-text box to enter other items of resource use. These items were costed individually, and full unit costs, including those used to cost additional resource use items recorded, are outlined in Appendix 3 (see Tables 45–47).
| Resource item | Unit cost (£) | Source | Details |
|---|---|---|---|
| Trial medication | |||
| Levofloxacin, 250 mg | 11.57 per 10-tablet pack | BNF 2017 | Normal dose: two tablets per day |
| Moderate renal failure: one tablet per day | |||
| Severe renal failure: half-tablet per day | |||
| Levofloxacin, 500 mg | 11.40 per 10-tablet pack | BNF 2017 | |
| Community health and social services | |||
| GP visit, surgery | 36.00 per visit | PSSRU 2016, p. 14545 | GP, per patient contact lasting 9.22 minutes, including direct care staff costs |
| District nurse visit | 38.00 per visit | NHS Reference Costs 2015 to 2016 46 |
District nurse, adult, face to face CC: N02AF; SC: NURS |
| Home help or care worker visit | 24.00 per visit | PSSRU 2016, p. 16045 | Face-to-face 1-hour weekday session |
| Hospital-based or residential care services | |||
| Hospital inpatient | 298.41 per day | NHS Reference Costs 2015 to 2016 46 | Total Healthcare Resource Groups, average of all non-elective inpatient excess bed-days |
| Hospital critical/intensive care unit | 521.00 per day | NHS Reference Costs 2015 to 2016 46 | Medical adult patients (unspecified specialty), adult critical care, zero organs supported, CC: XC07; SC: CCU03 |
| Hospital outpatient | 135.00 per visit | PSSRU 2016, p. 9545 | Weighted average of all outpatient attendances |
| A&E | 137.74 per visit | NHS Reference Costs 2015 to 2016 46 | Emergency medicine, average unit costs of all emergency medicine attendances |
| Residential home | 90.00 per day | PSSRU 2016, p. 2645 | Private sector residential home cost per permanent resident day |
Patients’ use of health-care resources and total costs were calculated for the ITT population. Total costs for each patient were calculated as the sum of costs assigned for hospital, community health and social services and medication use.
Adjusting for baseline imbalance
Using a randomised controlled study design means that the baseline and socioeconomic characteristics of groups being compared should be well balanced. However, despite randomisation, there will inevitably be some differences in mean baseline values between groups. This is of particular importance because a patient’s utility at baseline is likely to be correlated with their utility over the follow-up period. Therefore, the imbalance in baseline utilities needs to be accounted for when calculating the differential effects between treatment arms. 47,48 Multiple regression analysis was used to estimate differential mean QALYs and to predict adjusted QALYs controlling for utility at baseline.
Missing data
In economic analyses conducted alongside clinical trials, incomplete or missing data are inevitable. Based on descriptive analysis of the missing data, the analysis was conducted under the assumption that the missing data were missing at random (MAR). Analysis of the number of missing data by trial group at each follow-up and visual analysis of missing data patterns indicated that the data were unlikely to be missing completely at random as the proportion of missing data differed by time point (although the proportion of data missing did not differ by treatment allocation). 49 This was explored further using logistic regression to investigate which factors were associated with the probability of missing data. This indicated that MAR may be a plausible assumption under which to conduct the analysis. However, it was noted that it is difficult to rule out missing not at random because the unobserved data are unknown. Consequently, when there were missing data, multiple imputation methods were used to generate estimates of missing values based on the distribution of observed data. The multiple imputation approach is the recommended method of imputation for economic evaluation alongside clinical trials as it includes randomness to reflect the uncertainty inherent in missing data by using iterative multivariable regression techniques. 50
When choosing the level at which to impute missing data (more or less aggregated), a balance needs to be struck between maintaining the data structure and achieving a stable imputation model. 49 Consequently, for QoL data, missing EQ-5D index values were imputed at each follow-up. For costs, missing data were imputed at the level of total health and social services costs, total hospital costs and total treatment costs, not at the unit of resource level. EQ-5D index values were recorded as missing if any EQ-5D items were missing. Missing baseline EQ-5D values were imputed using mean imputation to ensure that imputed values were independent of the treatment allocation. 51 Missing EQ-5D scores at the remaining follow-ups were imputed using multiple imputation methods. Costs were counted as missing if all resource use items on the CRF were missing and missing cost data were imputed at each follow-up using multiple imputation methods.
The imputation was performed in Stata® version 14 (StataCorp LP, College Station, TX, USA) using predictive mean matching to perform multiple imputation by chained equations. Predictive mean matching ensures that only plausible values of the missing variable are imputed as the imputed value is drawn from another individual whose predicted value is close to the predicted value of the individual with the missing observation. 49
Cost-effectiveness analysis
Primary analysis
The cost-effectiveness analysis adopted an ITT perspective for analysing and summarising the health economic trial data. The primary analysis consisted of a cost–utility analysis over the 16-week trial period and included adjustment for baseline variables and imputation of missing data. The incremental cost per QALY gain as a result of the use of levofloxacin compared with placebo was calculated. This was calculated by dividing the mean difference in cost of the two treatment arms by the mean difference in QALYs to produce an incremental cost-effectiveness ratio (ICER), as follows:
The ICER represents the additional cost per QALY gained for each intervention compared with the next best alternative. 52 NICE39 considers a cost per QALY within the range £20,000–30,000 to be acceptable. The lower limit of this threshold (λ = £20,000) was used to determine cost-effectiveness. Interventions with an ICER < £20,000 per QALY gained are generally considered cost-effective.
Secondary analyses
The secondary analyses included a cost-effectiveness analysis, in which the cost per febrile episode avoided attributable to antibiotic prophylaxis compared with placebo was calculated. This analysis was conducted in a similar way to the primary analysis; however, as data on febrile episodes were collected only over the treatment period (baseline to 12 weeks), the time horizon for this analysis was 12 weeks, rather than 16 weeks (as was used in the primary analysis). In addition, a cost–utility analysis was conducted over a 12-month time horizon.
Sensitivity analysis
Alternative scenarios were explored in the sensitivity analysis to test the robustness of the main trial analysis results. The effect of not imputing missing data was considered with an analysis including only complete cases. In addition, the effect of adjusting for baseline imbalance on cost-effectiveness was explored using an analysis with no adjustment for baseline differences between groups. Further sensitivity analyses were conducted to explore the effect of using a disease-specific measure of HRQoL to calculate QALYs rather than EQ-5D. The effect of the decision to prioritise hospital data over patient-recorded data was also explored with an analysis using only resource use data recorded by patients. The effect of costing hospital stays as either long or short stays based on Hospital Episode Statistics (HES) classifications, rather than using a cost per day, was also explored. Finally, the effect of using the same dose (250-mg) tablets to calculate treatment costs for all patients in the levofloxacin arm of the trial was explored in a sensitivity analysis that instead calculated treatment costs using 500-mg levofloxacin tablets for patients who took 250 mg or 500 mg (the same costing method as in the base case was used for patients who took 125 mg of levofloxacin). ICERs from each of the scenarios were compared with the main trial results to identify areas of uncertainty.
For the 12-month analysis, the sensitivity of results to the choice of prediction model employed was also evaluated.
Uncertainty analysis
The level of sampling uncertainty around the ICER was determined using a non-parametric bootstrap to generate 10,000 estimates of incremental costs and benefits. The bootstrapped estimates were plotted on the cost-effectiveness plane to illustrate the uncertainty surrounding the cost-effectiveness estimates. 53 Net monetary benefit (NMB) was also calculated for each of the bootstrapped estimates. NMB combines cost-effectiveness and willingness to pay (WTP) to give an explicit monetary valuation of the health outcome. It is calculated by rearranging the ICER and incorporating the WTP per QALY threshold value, such that NMB is derived for each patient as:
where λ is the value a decision-maker would be willing to pay per incremental QALY gained. For any given threshold value (λ), treatments with an average incremental NMB of > 0 should be adopted. The expected NMB was used to estimate the probability that antibiotic prophylaxis is cost-effective given a range of threshold values (λ = £1000 to λ = £100,000) that were plotted on the cost-effectiveness acceptability curve (CEAC). 54 The CEAC illustrates the probability that antibiotic prophylaxis is cost-effective compared with placebo when used alongside antimyeloma treatment as a function of the WTP threshold (λ). It is constructed using 10,000 bootstrapped samples from the original data and plotting the proportion of times each treatment represents the maximum average net benefit for a range of WTP thresholds (λ). The cost-effectiveness frontier was also plotted to show the intervention that provides the highest net benefit for a given threshold.
Value-of-information analysis
Value-of-information analysis was conducted to estimate the potential gains from the elimination of uncertainty that could result from conducting additional research. 50 As decisions must be made based on the information that is currently available, and as such information may be imperfect, there remains an element of uncertainty and a chance that the wrong decision will be made. In the event of a ‘wrong’ decision, costs will be incurred in the form of health benefit and cost of resources forgone. The expected value of perfect information (EVPI) is derived from the expected costs associated with the uncertainty. The EVPI was calculated using the bootstrapped sample to calculate the average net benefit loss as a result of the ‘wrong’ decision. EVPI was calculated as follows:
where EθmaxjNB(j,θ) is the expected net benefit with perfect information and maxjEθNB(j,θ) is the expected net benefit with current information. The expected net benefit with perfect information is calculated as the mean value of NMB when the intervention with the higher NMB is chosen for each simulation. The expected net benefit with current information is calculated as the mean value of NMB when the intervention with the higher expected net benefit is chosen across all simulations. 55
There were 5540 new cases of myeloma in 2015 in the UK given an age-standardised incidence rate of 9.6 per 100,000 population. 1 It was assumed that the decision is relevant for a period of 10 years, after which time it is reasonable to assume that the treatment pathway may have changed (i.e. alternative therapies becoming available). The EVPI is presented for this population with myeloma in the UK over a range of cost-effectiveness thresholds. A discount rate of 3.5% was used to discount the future value of additional research to the present value. 39 If additional research that removes uncertainty can be conducted at an expected cost lower than the EVPI, then such research may be warranted.
Results
Sample
Of the 977 patients recruited to the trial, complete resource use and EQ-5D results for all follow-ups were available for 511 patients.
Resource use and costs
Table 30 shows the average resource use of all patients in each treatment arm who returned at least one resource use questionnaire over the 16-week duration of the trial (levofloxacin, n = 469; placebo, n = 461). Table 31 also gives a more detailed breakdown of hospital admissions from the available hospital data. Average health-care costs over the trial period are presented in Table 32. The mean total costs of community health and social services were £224.87 [standard deviation (SD) £605.66] for the levofloxacin group and £190.13 (SD £486.41) for the placebo group. The mean total costs for hospital and residential care services were £3620.60 (SD £4579.35) for the levofloxacin group and £3255.17 (SD £4167.70) for the placebo group. For relevant unit costs, see Table 46. For further breakdown of resource use and costs, see Tables 47 and 48.
| Resource use | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Levofloxacin | Placebo | |||||
| Mean (SD) | Min. | Max. | Mean (SD) | Min. | Max. | |
| Community health and social services | ||||||
| GP | 1.02 (1.59) | 0 | 16 | 1.08 (1.78) | 0 | 13 |
| Nurse | 3.0 (10.65) | 0 | 113 | 2.61 (9.77) | 0 | 104 |
| Care worker | 2.01 (11.7) | 0 | 131 | 1.58 (10.00) | 0 | 116 |
| Hospital or residential care services | ||||||
| Hospital inpatient days | 6.23 (12.38) | 0 | 105 | 5.69 (11.24) | 0 | 102 |
| Hospital ICU days | 0.05 (0.65) | 0 | 10 | 0.22 (3.21) | 0 | 66 |
| Hospital outpatient | 10.25 (8.79) | 0 | 44 | 9.76 (9.13) | 0 | 53 |
| Hospital A&E | 0.6 (1.15) | 0 | 14 | 0.51 (0.90) | 0 | 5 |
| Residential care | 0.32 (2.93) | 0 | 42 | 0.3 (4.5) | 0 | 94 |
| Factor | Grouping | Treatment arm | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Number of admissions | 1 | 149 | 140 |
| 2 | 66 | 51 | |
| 3 | 14 | 13 | |
| 4 | 3 | 7 | |
| Total number admissions | 330 | 307 | |
| Length of hospital admission (days) | 1–7 | 180 | 183 |
| 8–14 | 81 | 58 | |
| 15–21 | 28 | 22 | |
| 22–28 | 13 | 19 | |
| > 28 | 27 | 26 | |
| Missing | 6 | 1 | |
| Number of ICU admissions | 3 | 5 | |
| Length of ICU admission (days) | 3 | 2 | 3 |
| 9 | 1 | – | |
| 10 | 1 | 1 | |
| 17 | – | 1 | |
| 66 | – | 1 | |
| Total number of patients admitted | 232 | 211 | |
| Total costs (£) | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Levofloxacin | Placebo | |||||
| Mean (SD) | Min. | Max. | Mean (SD) | Min. | Max. | |
| Community health and social services | 224.87 (605.66) | 0.00 | 5472.00 | 190.13 (486.41) | 0.00 | 4003.76 |
| Hospital and residential care services | 3620.60 (4579.35) | 0.00 | 39,655.81 | 3255.17 (4167.70) | 0.00 | 36,107.61 |
| Trial medication | 164.10 (63.16) | 0.00 | 300.82 | 0.00 (0.00) | 0.00 | 0.00 |
| Total NHS cost | 4009.62 (4669.23) | 34.71 | 40,503.64 | 3445.30 (4228.41) | 0.00 | 36,107.61 |
Independent-sample t-tests were undertaken to explore differences in mean total NHS costs associated with the treatment arms. Although the difference in total NHS costs appears considerable, the difference was not significant at the 5% level (p = 0.053).
Quality-of-life data
Mean (SD) EQ-5D scores for each treatment arm at each time point are presented in Table 33. When patients died during the trial, the EQ-5D score was recorded as 0 from the date of death. EQ-5D scores increase throughout the trial period in both treatment arms. This increase is slightly larger in the levofloxacin group. Baseline EQ-5D scores were very similar and independent-sample t-tests indicated that there was no statistically significant difference in EQ-5D scores at baseline (p = 0.88). The primary analysis uses an adjustment for baseline characteristics; however, the effect of conducting the analysis without this adjustment was explored in a sensitivity analysis.
| Time point | Treatment arm | |
|---|---|---|
| Levofloxacin | Placebo | |
| Baseline | ||
| Mean (SD) | 0.59 (0.33) | 0.59 (0.35) |
| n valid (missing) | 426 (63) | 439 (49) |
| Week 4 | ||
| Mean (SD) | 0.60 (0.3) | 0.58 (0.32) |
| n valid (missing) | 384 (105) | 383 (105) |
| Week 8 | ||
| Mean (SD) | 0.62 (0.28) | 0.60 (0.3) |
| n valid (missing) | 377 (112) | 375 (113) |
| Week 12 | ||
| Mean (SD) | 0.62 (0.28) | 0.62 (0.32) |
| n valid (missing) | 357 (132) | 371 (117) |
| Week 16 | ||
| Mean (SD) | 0.63 (0.29) | 0.62 (0.29) |
| n valid (missing) | 354 (135) | 373 (115) |
Table 34 shows the mean EQ-5D change scores between baseline and each of the follow-up time points. Independent sample t-tests indicated that the variation among groups in the changes in EQ-5D scores was not statistically significant (p = 0.69).
| Time point | Treatment arm | |
|---|---|---|
| Levofloxacin | Placebo | |
| Baseline to week 4 | ||
| Mean (SD) | 0.01 (0.28) | –0.01 (0.28) |
| n valid (missing) | 349 (140) | 360 (128) |
| Baseline to week 8 | ||
| Mean (SD) | 0.01 (0.3) | 0.01 (0.3) |
| n valid (missing) | 340 (149) | 354 (134) |
| Baseline to week 12 | ||
| Mean (SD) | 0.03 (0.31) | 0.03 (0.32) |
| n valid (missing) | 324 (165) | 351 (137) |
| Baseline to week 16 | ||
| Mean (SD) | 0.04 (0.31) | 0.03 (0.33) |
| n valid (missing) | 319 (170) | 347 (141) |
Missing data
A total of 521 patients had complete EQ-5D scores across all time points. The remaining 456 patients had EQ-5D scores missing for at least one of the time periods. There were 112 (11%) missing EQ-5D scores at baseline, which were imputed independently of treatment allocation. A total of 934 (24%) EQ-5D scores were missing across the remaining follow-ups (weeks 4–16) and were imputed for the cost-effectiveness analysis. Of those scores that were missing, 71 (2%) were missing as a result of partially completed EQ-5D forms and the remaining were missing in all elements of the EQ-5D. A total of 704 patients completed resource use questionnaires for all follow-ups. The remaining 273 had missing resource use for at least one of the time periods. At the first follow-up in week 4, 887 (91%) patients completed resource use questionnaires. The completion rate dropped over the duration of the trial to 84% completed at week 8, 80% completed at week 12 and 76% completed at week 16.
Cost-effectiveness results
Cost-effectiveness results are presented in Table 35, which shows the costs and QALYs gained for each treatment arm, the incremental costs and QALYs, and the resulting ICER. The levofloxacin group had the highest QALYs gained over the trial period. The mean total cost was also highest for the levofloxacin group.
| Treatment arm | Cost (£) | QALY | ICER (£/QALY) | ||
|---|---|---|---|---|---|
| Mean (SD) | Incremental | Mean (SD) | Incremental | ||
| Placebo | 3272.19 (4173.23) | 0.1818 (0.006) | |||
| Levofloxacin | 3871.45 (4622.02) | 599.26 | 0.1845 (0.006) | 0.0026 | 231,377.42 |
The results suggest that the use of prophylactic levofloxacin would not be cost-effective compared with placebo. This is as a result of smaller QALY gains and higher costs in the levofloxacin arm than in the placebo arm. An ICER value of £231,377.42 per QALY gain is yielded, which is well above the NICE cost per QALY threshold.
Bootstrapped estimates of the incremental costs and incremental effects are plotted on the cost-effectiveness plane in Figure 7. This shows the joint distribution of incremental costs and incremental effects in the cost-effectiveness plane for levofloxacin compared with placebo. Most of the points in the cloud lie above the cost-effectiveness threshold line, indicating that the use of levofloxacin is unlikely to be a cost-effective use of resources. Although 98.2% of the iterations fall in the north-east quadrant (more costly, more beneficial), only 1.8% fall in the south-east quadrant (less costly, more beneficial).
FIGURE 7.
The cost-effectiveness plane: levofloxacin vs. placebo.
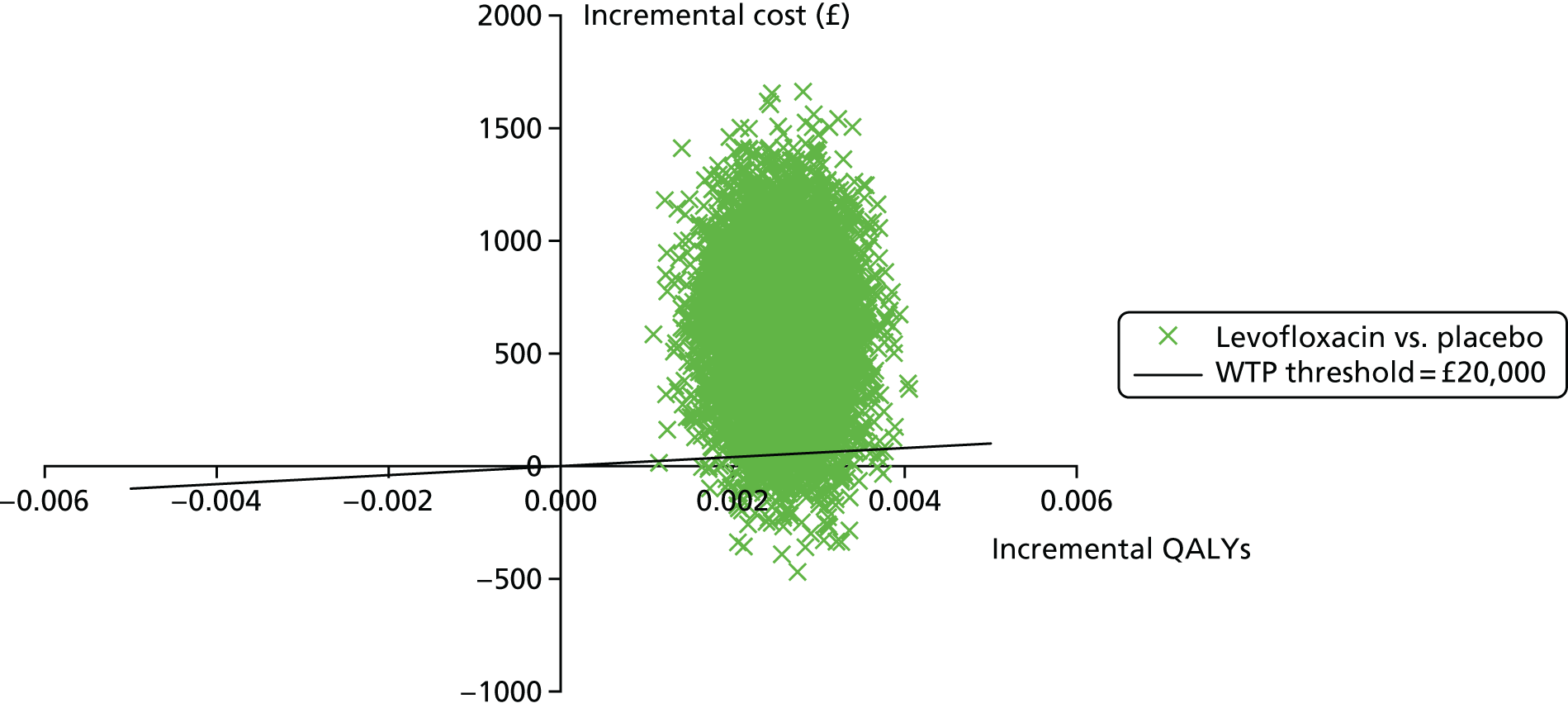
Net monetary benefit
The NMB for each treatment arm, calculated from the bootstrapped estimates of costs and QALYs, is presented in Table 36. Given the decision rule, the NMB results indicate that the use of prophylactic levofloxacin is not a cost-effective use of resources because the expected value of NMB is negative.
| Treatment arm | Expected value NMB (£) | Standard error | 95% CI |
|---|---|---|---|
| Levofloxacin | –184.74 | 2.10 | –188.87 to –180.62 |
| Placebo | 361.43 | 1.89 | 357.72 to 365.14 |
The probability that the treatments are cost-effective is presented on the CEAC shown in Figure 8. This shows that the use of prophylactic levofloxacin is very unlikely to be a cost-effective use of resources even at high cost-effectiveness threshold values.
FIGURE 8.
Cost-effectiveness acceptability curve: levofloxacin vs. placebo.

Sensitivity analysis
The cost-effectiveness results for each scenario explored in the sensitivity analysis are presented in Table 37.
| Treatment arm | Cost (£) | QALY | ICER (£/QALY) | ||
|---|---|---|---|---|---|
| Mean (SD) | Incremental | Mean (SD) | Incremental | ||
| Complete-case analysis | |||||
| Placebo | 3111.76 (3333.24) | 0.195 (0.005) | |||
| Levofloxacin | 4114.17 (4393.45) | 1002.41 | 0.193 (0.005) | –0.002 | Dominated |
| Without baseline adjustment | |||||
| Placebo | 3272.19 (4173.23) | 0.182 (0.080) | |||
| Levofloxacin | 3871.45 (4622.02) | 599.26 | 0.185 (0.071) | 0.003 | 189,648.23 |
| Hospital admissions from patient recall | |||||
| Placebo | 3256.96 (4312.75) | 0.182 (0.006) | |||
| Levofloxacin | 3645.90 (3988.37) | 388.93 | 0.184 (0.006) | 0.003 | 142,490.94 |
| Hospital admissions costed as long/short stays | |||||
| Placebo | 6036.30 (7783.69) | 0.182 (0.006) | |||
| Levofloxacin | 6658.43 (7821.83) | 622.13 | 0.185 (0.006) | 0.003 | 210,385.20 |
| Alternative costing of levofloxacin | |||||
| Placebo | 3272.19 (4173.23) | 0.182 (0.006) | |||
| Levofloxacin | 3796.26 (4624.56) | 524.07 | 0.185 (0.006) | 0.003 | 179,031.58 |
| Condition-specific HRQoL | |||||
| Placebo | 3274.28 (4171.64) | 0.206 (0.007) | |||
| Levofloxacin | 3868.36 (4624.28) | 594.08 | 0.211 (0.006) | 0.006 | 105,386.04 |
Given that the base-case analysis was conducted using imputed data, a sensitivity analysis that used only complete cases was conducted. In addition, sensitivity analyses were conducted to explore the effect of adjusting for baseline differences by repeating the analysis without the baseline adjustment, and to explore the effect of the decision to give precedence to hospital data by using patient-reported hospital admission data instead of the hospital-provided data. The effect of using a condition-specific measure of HRQoL compared with a generic measure was also explored with a sensitivity analysis using the EORTC QLQ-C30 instead of the EQ-5D. A sensitivity analysis was also conducted to explore the effect of costing hospital stays as either long or short stays based on HES classifications, rather than using a cost per day (see Table 46 for relevant unit costs). A final sensitivity analysis was conducted in which treatment costs were calculated using 500-mg levofloxacin tablets for patients who took 250 mg or 500 mg, rather than calculating the cost of treatment using 250-mg tablets for all patients. In each case, the results of the sensitivity analyses support the results of the base-case analysis and indicate that the use of prophylactic levofloxacin is not a cost-effective use of resources.
Secondary analysis
A secondary analysis to explore the cost per febrile episode avoided was also conducted. In line with the primary outcome of the trial, this analysis was conducted over a 12-week time horizon. There were no missing febrile episode data and cost data were imputed using multiple imputation as in the primary health economic analysis. Cost-effectiveness results from the secondary analysis are presented in Table 38. This shows that the additional cost per febrile episode avoided was £4021.26. This amounts to a mean additional cost of £8.15 per patient treated (calculated as the difference in mean cost per patient between the treatment arms/incremental febrile episodes avoided).
| Treatment arm | Total cost (£) | Febrile episodes | ICER (£/febrile episode avoided) | ||
|---|---|---|---|---|---|
| Incurred (12 weeks) | Incremental | Total number | Incremental avoided | ||
| Placebo | 581,503.40 | 149 | |||
| Levofloxacin | 722,247.50 | 140,744.06 | 114 | 35 | 4021.26 |
Explicit predefined reference threshold values to allow interpretation of the cost per febrile episode avoided are not available. However, the cost of treating a febrile episode may be used as a yardstick for interpretive purposes. A generalised linear model (family: gamma; link: identity) was fitted to the trial cost data to estimate the cost of an infection. This produced an estimate of the cost of an infection of £1805.87 (standard error 180.22). As the cost of treating an infection is lower than the cost of preventing a febrile episode, it may be concluded that the use of levofloxacin for prevention is not a cost-effective use of resources.
Secondary analysis: sensitivity analysis
The cost-effectiveness results for each scenario explored as sensitivity analyses are presented in Table 39. Sensitivity analyses were conducted to explore the effect of uncertainty in the estimates of costs. As in the primary analysis, the effect of the decision to give precedence to hospital data was explored in a sensitivity analysis using patient-reported hospital admission data instead. Scenarios considering a reduction in overall costs (–20%) and an increase in overall costs (+20%) were also explored. In each case, the results of the sensitivity analyses support the results of the base-case analysis and indicate that, when compared with an estimated cost per infection of £1806, the use of levofloxacin does not appear to be a cost-effective use of resources.
| Treatment arm | Total cost (£) | Febrile episodes | ICER (£/febrile episode avoided) | ||
|---|---|---|---|---|---|
| Incurred (12 weeks) | Incremental | Total number | Incremental avoided | ||
| Hospital admissions from patient recall | |||||
| Placebo | 581,260.40 | 149 | |||
| Levofloxacin | 722,025.90 | 140,765.50 | 114 | 35 | 4021.87 |
| Increase costs by 20% | |||||
| Placebo | 697,804.10 | 149 | |||
| Levofloxacin | 866,697.00 | 168,892.90 | 114 | 35 | 4825.51 |
| Reduce costs by 20% | |||||
| Placebo | 465,202.80 | 149 | |||
| Levofloxacin | 577,798.00 | 112,595.20 | 114 | 35 | 3217.01 |
Value-of-information analysis
The population EVPI at the NICE cost-effectiveness threshold value of £20,000 per QALY gained is £99,131. The population EVPI for other values of the cost-effectiveness threshold is plotted in Figure 9.
FIGURE 9.
Expected value of perfect information.

Discussion
Principal findings
The trial-based cost-effectiveness analysis indicated that the use of prophylactic levofloxacin alongside antimyeloma treatment was not a cost-effective use of NHS resources compared with placebo.
Both treatment arms showed an increase in EQ-5D score over the trial period but, although levofloxacin was associated with slightly higher total QALY gains, the difference in QALYs was very small. This may, in part, be explained by the fact that benefits were measured only over the trial period of 16 weeks and that any longer-term benefits were not considered. In terms of costs, the levofloxacin arm was found to be the most costly treatment. This was driven by greater resource use in the levofloxacin arm, both in terms of hospitalisations and the use of community health and social services. Consequently, the principal finding that the use of prophylactic levofloxacin would not be a cost-effective use of resources is driven by the (statistically significant) difference in costs.
Base-case cost-effectiveness results were not sensitive to alternative assumptions explored in the sensitivity analyses. The secondary analysis found that the cost per febrile episode avoided in the levofloxacin arm was £8849.31. In the absence of a predefined decision rule for outcomes measures in natural units, the value judgement on whether or not this presents a good use of resources is left to the decision-maker.
An estimate of the value of perfect information suggested that further research to remove uncertainty and enable more robust economic decisions to be made would be worth £99,131. If additional research can be conducted at an expected cost lower than this value, then such research would be warranted to reduce uncertainty.
Strengths and weaknesses of the economic analysis
The main strength of this analysis lies in the randomised controlled design of the trial. This enabled the collection of high-quality data that were subsequently used in this analysis to conduct the 16-week within-trial analysis.
However, the relatively short duration of follow-up has made consideration of longer-term outcomes difficult. This has partly been addressed by conducting an extrapolated analysis over a 12-month time horizon; however, the use of more robust methods to consider a longer follow-up would be beneficial as part of any future research.
Meaning of the trial
Although fewer febrile episodes and deaths were experienced in the levofloxacin arm of the trial, this does not translate into the use of prophylactic levofloxacin being a cost-effective use of resources. This is driven by higher costs in the levofloxacin arm as a result of high levels of resource use in terms of both hospitalisations and the use of community health and social services that are maintained despite the reduction in infections. Consequently, the higher costs in the levofloxacin arm combined with only very small gains in QALYs indicate that the use of prophylactic levofloxacin would not be a cost-effective treatment option alongside antimyeloma treatment.
Unanswered questions and further research
Although the analysis conducted here provides useful insights into the costs and effects over the trial period of 16 weeks, further research is required to provide more robust evidence on the cost-effectiveness over a longer time horizon. This will be particularly relevant if the benefits of prophylactic levofloxacin are maintained or develop in the long term. More research is needed to investigate whether or not the cost for avoided febrile episode is cost-effective. 56
Chapter 5 Discussion
Interpretation
The trial indicated that prophylactic levofloxacin caused a significant reduction in febrile episodes and deaths during the 12-week treatment period (log-rank = 9.78; p = 0.002). The largest benefit was observed within 4–8 weeks. Levofloxacin also reduced the number of other (non-febrile) infectious episodes (p-trend = 0.06). The data gathered on these ‘other infections’ are of particular interest because these data have not, to our knowledge, been previously collected on such a large scale. Our data show that these ‘other infections’ are a substantial burden for both patients and the health-care system. They were more frequent than febrile episodes (323 other infections vs. 264 febrile episodes). Again, the benefit of prophylaxis in these ‘other infections’ became apparent during the 4- to 8-week period and continued up to 12 weeks. The body site experiencing the greatest reduction in infections was the urinary tract, probably because levofloxacin is broadly effective against Gram-negative organisms. Upper respiratory tract infections are frequently caused by viruses and, therefore, would be unaffected by levofloxacin.
Information on infections was collected only up to 12 weeks but longer-term survival data were collected up to 12 months. During months 3–6, patients were not on prophylaxis and yet the survival benefit continued for those who had taken levofloxacin. If levofloxacin had prevented death in those patients destined to respond to antimyeloma treatment and subsequently survive beyond 12 months, the survival benefit might have been expected to continue. A possible interpretation of our finding that the survival curves came together by 6 months is that levofloxacin may reduce or delay the risk of death in patients with poorly responsive or refractory disease. Therefore, the cause of death was analysed in the 116 deaths reported during the 12-month trial period. There were fewer deaths attributed to infection in the levofloxacin arm (three patients) than in the placebo arm (eight patients) during the 12-week treatment period, but more in the levofloxacin arm (19 patients) than in the placebo arm (12 patients) post 12 weeks. These data support our possible interpretation outlined above. Although levofloxacin may have reduced the number of deaths in the first 12 weeks from 22 to 8, there were a further 86 deaths in the next 40 weeks from many causes and the reversal of levofloxacin survival advantage may have been influenced by the volume of the later deaths.
There was little benefit from levofloxacin until after the first 4 weeks, and this time delay in benefit from prophylaxis in reducing febrile episodes and other infections is intriguing. The response to treatment of an established infection with antibiotics is expected within 48 hours. This observed delay in benefit from prophylaxis may suggest that some form of biological process needs to take place before the benefit is realised. One suggestion may be that a change in the patient’s immune response or microbiome may be necessary before the benefit of prophylaxis is established. The theory that a biological process may take place in the patient before the benefit of prophylaxis becomes apparent may also be supported by the observation that the survival curves remained separated for months 3–6. It is possible that the benefit of levofloxacin prophylaxis may continue to reduce infections for a period after withdrawing levofloxacin, but this was not measured in the trial.
There was no significant increase in HCAIs in patients on levofloxacin during the 16 weeks of the trial, disproving the theory that the use of prophylactic levofloxacin may trigger an excess of HCAIs. In addition, despite prospectively looking for colonisation with resistant organisms, there were fewer episodes of colonisation noted in the levofloxacin arm. However, it is possible that a longer period of levofloxacin prophylaxis may induce colonisation or infection with HACIs. There is a need to better understand the reasons for the risks of colonisation with resistant organisms and infections in the first 4 weeks of levofloxacin prophylaxis. There is also a need to explore the effects of a longer duration of levofloxacin prophylaxis.
A total of 597 SAEs were reported (308 in the levofloxacin group and 289 in the placebo group), with most reported as unlikely to be related to or unrelated to the study drug (537/597 events, 90%) and a majority reported to be attributable to hospitalisation or prolongation of existing hospitalisation (462/597 events, 77%). Toxicity reported to be related to levofloxacin (38 episodes) compared with placebo (22 episodes) had a tendency to manifest as gastrointestinal disorders, rash, psychiatric disorders and musculoskeletal/connective tissue disorders, but these were very rare events.
The health economic analysis showed a slight increase in QALYs over 16 weeks for levofloxacin prophylaxis, but this was associated with higher health-care costs. Despite the reduction in febrile episodes and deaths in the patients taking prophylactic levofloxacin, the 16-week cost-effectiveness analysis indicated that prophylactic levofloxacin would not be a cost-effective use of NHS resources. This result was driven by higher use of health-care resources in the levofloxacin arm and only very small gains in QALYs. Consequently, the aforementioned reduction in febrile episodes and deaths did not translate into gains in QALYs that were sufficient to outweigh the additional costs. This result was robust to sensitivity analyses. As the benefit of levofloxacin prophylaxis in reducing infections and death became most apparent after 4 weeks, it would be useful to perform the analysis over the period of 4–16 weeks. This analysis is planned for the future. In addition, the analysis of 12-month data is ongoing. More research is needed to investigate whether or not the cost for avoided febrile episode is cost-effective. 56
Generalisability
Strengths of the study
This was a large randomised, double-blind, placebo-controlled trial for the intervention, with broad entry criteria, and patients with poor performance status were not excluded. Patients could be on any antimyeloma treatment regimen; hence, data were collected prospectively in a real-life situation. All suspected infectious episodes were captured, with the only definition being that patients were given an anti-infective treatment by a health-care professional. These data were the most comprehensive yet recorded for newly diagnosed multiple myeloma patients. A large number of patient and treatment data have been recorded, which allowed the analysis of secondary outcomes as prespecified in the protocol. The longer-term secondary analyses, such as detailed survival analysis and analysis of cost-effectiveness up 12 months and QoL, are ongoing.
Limitations of the study
Despite the study having broad entry criteria, the patient population recruited into the study was healthier than expected, with a median age of 67 years and only 7% of patients of performance status 3 or 4. The 3-month overall survival of patients in our study was in excess of 95%. However, population data show that the median age of myeloma patients at diagnosis is 71 years and the 3-month overall survival is 83%. 57 Therefore, the study was not fully representative of the patient population presenting with multiple myeloma. There was a concern raised by some investigators at the start of the trial that the prophylactic use of antibiotics may trigger HCAIs and, as a consequence, that there may have been a degree of selection bias to randomise fitter patients into the trial. Despite refining the protocol to include patients with impaired renal function, more patients than expected had eGFR values within the > 50 ml/minute/1.73 m2 range, which may be a reflection of over selection of fewer patients with renal impairment.
Patients had to be willing to provide stool samples and nasal swabs throughout the study as well as to complete the daily diary with data on temperature, patient-reported outcomes and QoL. Screening logs were collected and the main reason for not entering the trial was the unwillingness to provide samples and complete daily diaries. There was a protocol amendment early in the study to allow patients to enter the study within 14 days of starting antimyeloma treatments rather than 7 days, as initially stipulated. It may be that recruitment to a supportive care trial needs to accommodate patient emotions around receiving a diagnosis of myeloma and starting antimyeloma treatment before supportive care interventions can be considered.
The recruitment of a relatively healthy patient population to this study has several implications. The benefit of levofloxacin prophylaxis may be even more pronounced in the general myeloma population than the study demonstrated. In addition, if levofloxacin prophylaxis reduces infection and death in some poor performance status patients who have biologically responsive disease but are at risk of early death because of frailty, there could be a prolonged survival benefit for these patients. Our study may not have been able to demonstrate this because it was a selected patient population.
Levofloxacin prophylaxis was given for only 12 weeks. As the benefit in favour of levofloxacin was still evident at this time point, it is possible that there may be a benefit to continuing prophylaxis for longer.
It is important to note that a relatively low C. difficile carriage at baseline was found for patients on the TEAMM trial (0.7% compared with an expected 10% in hospital patients and 1% in the general population), which may be a reflection of the new NHS policies to minimise infections in hospital or the fact that many of these patients are mainly outpatients.
Overall evidence
This study supports the use of levofloxacin as prophylaxis for myeloma patients undergoing antimyeloma treatment. A small study of prophylactic co-trimoxazole in early myeloma showed a reduction in bacterial infections but was too small to detect reduced mortality and 25% of patients were unable to tolerate the drug. 18 Two large studies22–24,58 confirmed the efficacy of prophylactic levofloxacin in preventing bacterial infection in neutropenia, and subsequent meta-analyses showed superiority of fluoroquinolone antibiotics over other classes of antibiotic.
Overall conclusions
In the authors’ opinion, this study supports the use of prophylactic levofloxacin for patients undergoing antimyeloma treatment. This is the largest study in the literature assessing the use of levofloxacin to reduce febrile episodes and other infections for myeloma patients. Most patients in the trial were fitter then expected with a lower carriage rate of C. difficile.
There was no difference for new acquisitions of or invasive infections by C. difficile, MRSA and ESBL Gram-negative organisms when assessed up to 16 weeks.
Despite being clinically effective, levofloxacin in prophylaxis is not a cost-effective health-care programme from the NHS standpoint. However, this result is focused only on incremental QALYs gained, which are usually negligible when measured for such a short period of time (16 weeks). More research is needed to investigate whether or not the cost for avoided febrile episode is cost-effective.
Further research
Laboratory investigation of immunity, inflammation and disease activity on stored samples (funded by the TEAMM NIHR EME programme grant reference number 14/24/04) will provide insights into the triad of (1) myeloma disease activity, (2) immune competence and (3) infections and HCAIs to stratify patients for risk of infection, to guide use of prophylactic antibiotics and to improve response to antimyeloma therapies.
Further research to establish the optimal duration of prophylactic antibiotics for these patients is needed. There may be a benefit to treat for the whole duration of antimyeloma treatment in order to give maximum protection for these patients. In addition, the use of Septrin gave additional benefit to levofloxicin. This needs to be investigated within a future clinical trial.
Acknowledgements
Co-applicants
Mark T Drayson, Stella Bowcock, Janet A Dunn, Tim Planche, Gulnaz Iqbal, Guy Pratt, Kwee Yong, Eric Low, Peter Hawkey, Doug Carroll (QoL advisor) and Claire T Hulme.
Trial Management Group
Mark T Drayson, Stella Bowcock, Tim Planche, Kwee Yong, Guy Pratt, Eric Low, Kerry Raynes, Gulnaz Iqbal, Janet A Dunn, David Meads, Bryony Dawkins, Claire T Hulme and Helen Higgins.
Warwick Clinical Trials Unit staff working on TEAMM trial
Janet A Dunn, Gulnaz Iqbal, Jill Wood, Helen Higgins, Kerry Raynes, Joanne O’Beirne-Elliman, Uzma Manazar, Lauren Betteley, Pankaj Mistry, Catherine Hill, Kiran Bal, David Boss, Bushra Rahman, Sarah Lowe, Kimberly White, Penelope Goode, Claire Daffern, Amy Ismay and Joanne Grummet.
Independent review of early deaths
Graham Jackson provided an independent review of deaths within the first 12 weeks.
Drug supply
Oliver Gupta [Modepharma Limited (Beckenham, UK)] organised the manufacturing and supply of levofloxacin and placebo tablets.
Data Monitoring Committee members
Anthony Child, Tim Boswell and Walter Gregory.
Independent Trial Steering Committee members
Mark Wilcox, Roger Owen and Steven Devereux.
Microbiology
Tim Planche and Irene Monahan at St George’s Hospital, University of London.
Central laboratory Birmingham
Mark T Drayson, Tim Plant, Karen Walker, Nicki Newnham, Alison Adkins, Jennifer Heaney and Zaheer Afzal.
Health economists
David Meads, Bryony Dawkins and Claire T Hulme.
Patient and public involvement
Eric Low was a co-applicant, member of the Trial Management Committee and Trial Steering Committee and, together with colleagues from Myeloma UK, commented on the drafting of the protocol and PIS as well as the main report. We would also like to acknowledge all of the 977 trial participants, especially those who returned their patient diaries and QoL booklets.
The local NHS teams
The TEAMM team would like to thank the NHS trusts and participating PIs and their colleagues for recruiting and monitoring trial participants. These include [in alphabetical order of participating hospitals preceded by the local PI(s)] Feargal McNicholl of Altnagelvin Hospital; Philip Windrum of Antrim Hospital; Joanne Howard and Parag Jasani of Basildon University Hospital; Sylwia Simpson of Basingstoke and North Hampshire Hospital; Paul Kettle and Oonagh Sheehy of Belfast City Hospital; Adrian Williams of Bradford Royal Infirmary; Vijoy Chowdhury of Broomfield Hospital; Sylvia Feyler, Kate Rothwell and Srivasavi Dukka of Calderdale Royal Hospital; Haz Sayala and David Allsup of Castle Hill Hospital; Sally Chown of Cheltenham General Hospital; Robert Cutting, Emma Welch and Peter Toth of Chesterfield Royal Hospital; Michael Hamblin and Gavin Campbell of Colchester General Hospital; Salaheddin Tueger of Countess of Chester Hospital; Aurangzeb Razzak and Kamaraj Karunanithi of County Hospital stafford; Kathryn Boyd of Craigavon Area Hospital; Anil Kamat and Tariq Shafi of Darent Valley Hospital; John Ashcroft of Dewsbury Hospital; Susan Levison-Keating of Diana, Princess of Wales Hospital; Akeel Moosa of Dorset County Hospital; Richard Kaczmarski and Taku Sugai of Ealing Hospital; Alistair Whiteway of Frenchay Hospital; Jagadeesh Gandla and Jhansi Muddana of George Eliot Hospital; Earnest Heartin of Glan Clwyd Hospital; Sally Chown of Gloucestershire Royal Hospital; Bhuvan Kishore of Good Hope Hospital; Sandor Lueff, Kandeepan Saravanamuttu, Caroline Harvey and Charlotte Kallmeyer of Grantham Hospital; Norbert Blesing of Great Western Hospital; Matthew Streetly of Guy’s and St Thomas’ Hospital; Guy Pratt and Bhuvan Kishore of Heartlands Hospital; Lisa Robinson of Hereford County Hospital; Richard Kaczmarski of Hillingdon Hospital; Sylvia Feyler, Kate Rothwell and Srivasavi Dukka of Huddersfield Royal Infirmary; Mark Kwan of Kettering General Hospital; Steve Schey and Stella Bowcock of King’s College Hospital; Rowena Faulkner and Tim Moorby of King’s Mill Hospital; Samir Zebari of Kingston Hospital; Claire Chapman and Mamta Garg of Leicester Royal Infirmary; Kamaraj Karunanithi of Leighton Hospital; Sandor Lueff, Kandeepan Saravanamuttu, Caroline Harvey and Charlotte Kallmeyer of Lincoln County Hospital; John Hudson of Macclesfield District General Hospital; Simon Gibbs, Eleni Tholouli and Alberto Rocci of Manchester Royal Infirmary; Vivienne Andrews, Vijayavalli Dhanapal and Sarah Arnott of Medway Maritime Hospital; Moez Dungarwalla of Milton Keynes Hospital; Nilima Parry-Jones of Nevill Hall Hospital; Abraham Jacobs and Supratik Basu of New Cross Hospital; Claudius Rudin of North Devon District Hospital; Neil Rabin of North Middlesex University Hospital; Jane Parker of Northampton General Hospital; Charalampia Kyriakou of Northwick Park Hospital; Sandor Lueff, Kandeepan Saravanamuttu, Caroline Harvey and Charlotte Kallmeyer of Pilgrim Hospital; John Ashcroft of Pinderfields Hospital; John Ashcroft of Pontefract Hospital; Rebecca Maddams and Fergus Jack of Poole Hospital; Stella Bowcock of Princess Royal University Hospital; Helen Dignum and Edward Belsham of Queen Alexandra Hospital; Mark Cook of Queen Elizabeth Hospital; Ramaprabhahari Sathesh-Kumar and Martin Lewis of Queen Elizabeth Hospital, King’s Lynn; Tullie Yeghen of Queen Elizabeth Hospital, Woolwich; Humayun Ahmad of Queen’s Hospital, Burton upon Trent; Biju Krishnan and Sandra Hassan of Queen’s Hospital, Romford; Karthick Ramasamy and Henri Grech of Royal Berkshire; Rachel Hall of Royal Bournemouth Hospital; Claudius Rudin of Royal Devon & Exeter Hospital; Helen Jackson of Royal Gwent Hospital; Yousef Ezaydi of Royal Hallamshire Hospital; Katharine Lowndes of Royal Hampshire County Hospital; Stephen Hawkins of Royal Liverpool University Hospital; Kamaraj Karunanithi of Royal Stoke University Hospital; Louise Hendry of Royal Surrey County Hospital; Sally Moore of Royal United Hospital Bath; Jeff Neilson of Russells Hall Hospital; Jonathan Cullis of Salisbury District Hospital; Farooq Wandroo of Sandwell General Hospital; Matthew Jenner of Southampton General Hospital; Paul Cervi of Southend University Hospital; Alastair Whiteway of Southmead Hospital; Simon Stern of St Helier Hospital; Gordon Cook of St James University Hospital; Robin Aitchison of Stoke Mandeville Hospital; Rangaiah Madhusadan and Shikha Chattree of Sunderland Royal Hospital; Hussen Baden of Tameside General Hospital; Andreea Corcoz of The Royal Shrewsbury Hospital; Deborah Turner and Heather Eve of Torbay Hospital; Moulod El-Agnaf of Ulster Hospital; Anand Lokare and Beth Harrison of University Hospital Coventry; Tullie Yeghen of University Hospital Lewisham; Simon Watt of University Hospital South Manchester; Chandramouli Nagarajan, Mohamed Kaleel-Rahman and Jayaprakash Ramachandran of Warrington Hospital; Anton Borg and Caroline Arbuthnot of Warwick Hospital; Magda Jabbar Al-Obaidi of West Middlesex University Hospital; Peter Cumber of West Wales General Hospital; Mark Offer of Wexham Park Hospital; Naim Akhtar of Whipps Cross Hospital; Sumant Kundu of Withybush Hospital; Lally Desoya of Wrexham Maleor Hospital; and Robin Aitchison of Wycombe Hospital.
Other acknowledgements
The TEAMM team would like to thank the NIHR programme for funding this research.
The project benefited from facilities funded through Advantage West Midlands Science City Programme. The project received support through the Clinical Research Networks.
Contributions of authors
Mark T Drayson (Chief Investigator) was responsible for identifying the research question and writing the trial protocol, the central laboratory analysis and the review of the blood and urine samples.
Stella Bowcock (Co-Chief Investigator) was responsible for identifying the research question and writing the trial protocol.
Tim Planche (Microbiology Advisor) was responsible for identifying the research question and writing the trial protocol, provided expert microbiology advice and performed the analysis on the stool samples and nasal swabs.
Gulnaz Iqbal (Trial Statistician) was responsible for identifying the research question and writing the trial protocol, and carried out the statistical analysis and interpreted the results.
Guy Pratt (Haematologist) was responsible for identifying the research question and writing the trial protocol.
Kwee Yong (Haematologist) was responsible for identifying the research question and writing the trial protocol.
Jill Wood (Trial Co-ordinator) managed the co-ordination and administration of the trial.
Kerry Raynes (Trial Co-ordinator) managed the co-ordination and administration of the trial.
Helen Higgins (Senior Project Manager) managed the co-ordination and administration of the trial.
Bryony Dawkins (Health Economics) conducted the health economic analysis.
David Meads (Health Economics) conducted the health economic analysis.
Claire T Hulme (Health Economics) oversaw the health economic analysis.
Anna C Whittaker (QoL Advisor) provided expert advice on QoL.
Peter Hawkey (Microbiology) performed additional microbiology tests.
Eric Low (Patient Advocate, Myeloma UK) was responsible for all the patient and public involvement activities throughout the trials.
Janet A Dunn (WCTU Lead) was responsible for identifying the research question and writing the trial protocol, and carried out the statistical analysis and interpreted the results.
All authors provided comments on the report and approved the final version.
Publications
Dunn JA, Harris B, Iqbal G, Wood J, Mistry P, O’Beirne-Elliman J, et al. Use of Patient Diaries in Conjunction with Standard Reporting Methods: Duplication of Data or a Valuable Resource? Paper presented at the National Cancer Research Institute conference, Liverpool, November 2015. Abstract no. 141.
Dunn JA, Harris B, Iqbal G, Wood J, Mistry P, O’Beirne-Elliman J, et al. Use of Patient Diaries in Conjunction with Standard Reporting Methods: Duplication of Data or a Valuable Resource? Paper presented at the Third International Clinical Trials and Methodology conference, Glasgow, 16–17 November 2015. Abstract no. 183.
Bowcock S, Atkin C, Iqbal G, Planche T, Pratt G, Yong K, et al. Diagnostic Pathways of Myeloma Patients Presenting to Hospital Care and Relationship to end organ Damage: An Analysis from the TEAMM (Tackling EArly Morbidity and Mortality in Myeloma) Trial in 977 Patients. Paper presented at the American Society of Haematology conference, Atlanta, GA, December 2017. Abstract no. 2132.
Chicca IJ, Heaney JLJ, Iqbal G, Bowcock S, Planche T, Wood J, et al. Assessment of anti-Bacterial Antibodies in Multiple Myeloma Patients at Disease Presentation and in Response to Therapy: Implication for Patient Management. Paper presented at the American Society of Haematology conference, Atlanta, GA, December 2017. Abstract no. 3036.
Drayson MT, Bowcock S, Planche T, Iqbal G, Wood J, Raynes K, et al. Tackling EArly Morbidity and Mortality in Myeloma: Assessing the Benefit of Antibiotic Prophylaxis and its Effect on Healthcare Associated Infections. Paper presented at the American Society of Haematology conference, Atlanta, GA, December 2017. Abstract no. 903.
Atkin C, Drayson MT, Iqbal G, Yong K, Pratt G, Planche T, et al. Presenting Symptoms of Patients with Multiple Myeloma: Data from the TEAMM (Tackling EArly Morbidity and Mortality in Myeloma) Trial. Paper presented at the British Society of Haematology conference, Liverpool, April 2018. Abstract no. 243.
Bowcock S, Atkin C, Iqbal G, Yong K, Pratt G, Planche T, et al. Does a Long Diagnostic Pathway in Myeloma Lead to More Advanced Disease At Presentation? An Analysis from the TEAMM trial (Tackling EArly Morbidity and Mortality in Myeloma). Paper presented at the British Society of Haematology conference, Liverpool, April 2018. Abstract no. 428.
Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, Wood J, et al. Levofloxacin Prophylaxis in Newly Diagnosed Myeloma Reduces Febrile Episodes and Death without Increasing Healthcare Associated Infections: Results from the TEAMM Trial. Paper presented at the 23rd Congress of the European Hematology Association, Stockholm, June 2018. Abstract no. 2453.
Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, Wood J, et al. Levofloxacin Prophylaxis in Newly Diagnosed Myeloma Reduces Febrile Episodes and Death Without Increasing Healthcare Associated Infections: Results From the TEAMM Trial (Tackling EArly Morbidity and Mortality in Myeloma). Paper presented at the British Society of Haematology conference, Liverpool, April 2018. Abstract no. 234.
Helwick C. For Patients Treated for Myeloma, Antibiotic Prophylaxis May Reduce Infections and Deaths. The ASCO Post. 10 February 2018. URL: www.ascopost.com/issues/february-10-2018/for-patients-treated-for-myeloma-antibiotic-prophylaxis-may-reduce-infections-and-deaths/ (accessed 2 August 2018).
Planche T, Iqbal G, Bowcock S, Wood J, Raynes K, Monahan I, et al. Levofloxacin Prophylaxis – Assessing the Effects and Microbiology of Invasive Infections in a Phase III Study of 977 Patients with Newly Diagnosed Multiple Myeloma. Paper presented at the 28th Annual Congress of the European Congress Of Clinical Microbiology And Infectious Diseases, Madrid, April 2018. Abstract no. 4447.
Planche T, Iqbal G, Bowcock S, Wood J, Raynes K, Monahan I, et al. Levofloxain Prophylaxis does not Increase the Carriage of Resistant Organisms – Tackling EArly Mobidity and Mortality in Myeloma (TEAMM) Phase III Study: Assessing the Benefits of Antibiotic Prophylaxis and its Effect on Carriage of Resistant Organisms in 977 Patients. Paper presented at the 28th Annual Congress of the European Congress Of Clinical Microbiology And Infectious Diseases, Madrid, April 2018. Abstract no. 4479.
Drayson MT, Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial [published online ahead of print October 23 2019]. Lancet Oncol 2019.
Ramasamy K. Reducing infection-related morbidity and mortality in patients with myeloma [comment] [published online ahead of print October 23 2019]. Lancet Oncol 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Myeloma Incidence Statistics 2014. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma (accessed 30 November 2017).
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516-20. https://doi.org/10.1182/blood-2007-10-116129.
- Bird J, Behrens J, Westin J, Turesson I, Drayson M, Beetham R, et al. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2009;147:22-4. https://doi.org/10.1111/j.1365-2141.2009.07807.x.
- Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009;113:5412-17. https://doi.org/10.1182/blood-2008-12-194241.
- Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002 – Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005;23:9219-26. https://doi.org/10.1200/JCO.2005.03.2086.
- Perri RT, Hebbel RP, Oken MM. Influence of treatment and response status on infection risk in multiple myeloma. Am J Med 1981;71:935-40. https://doi.org/10.1016/0002-9343(81)90303-X.
- Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood 2000;95:7-11.
- Blade J, San Miguel JF, Fontanillas M, Esteve J, Maldonado J, Alcala A, et al. Increased conventional chemotherapy does not improve survival in multiple myeloma: long-term results of two PETHEMA trials including 914 patients. Hematol J 2001;2:272-8. https://doi.org/10.1038/sj.thj.6200115.
- Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro Coy N, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica 2012;97:442-50. https://doi.org/10.3324/haematol.2011.043372.
- Cohen HJ, Rundles RW. Managing the complications of plasma cell myeloma. Arch Intern Med 1975;135:177-84. https://doi.org/10.1001/archinte.1975.00330010179023.
- Savage DG, Lindenbaum J, Garrett TJ. Biphasic pattern of bacterial infection in multiple myeloma. Ann Intern Med 1982;96:47-50. https://doi.org/10.7326/0003-4819-96-1-47.
- Espersen F, Birgens HS, Hertz JB, Drivsholm A. Current patterns of bacterial infection in myelomatosis. Scand J Infect Dis 1984;16:169-73. https://doi.org/10.3109/00365548409087137.
- Jacobson DR, Zolla-Pazner S. Immunosuppression and infection in multiple myeloma. Semin Oncol 1986;13:282-90.
- Doughney KB, Williams DM, Penn RL. Multiple myeloma: infectious complications. South Med J 1988;81:855-8. https://doi.org/10.1097/00007611-198807000-00012.
- Rayner HC, Haynes AP, Thompson JR, Russell N, Fletcher J. Perspectives in multiple myeloma: survival, prognostic factors and disease complications in a single centre between 1975 and 1988. Q J Med 1991;79:517-25.
- Heaney JLJ, Campbell JP, Iqbal G, Caims D, Richter A, Child JA. Characterisation of immunoparesis in newly diagnosed myeloma and its impact on progression-free and overall survival in both old and recent myeloma trials [published online ahead of print 20 June 2018]. Leukemia 2018.
- Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 2007;138:563-79. https://doi.org/10.1111/j.1365-2141.2007.06705.x.
- Oken MM, Pomeroy C, Weisdorf D, Bennett JM. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. Am J Med 1996;100:624-8. https://doi.org/10.1016/S0002-9343(95)00043-7.
- Vesole DH, Oken MM, Heckler C, Greipp PR, Katz MS, Jacobus S, et al. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia 2012;26:2517-20. https://doi.org/10.1038/leu.2012.124.
- Offidani M, Corvatta L, Polloni C, Gentili S, Mele A, Rizzi R, et al. Thalidomide, dexamethasone, Doxil and Velcade (ThaDD-V) followed by consolidation/maintenance therapy in patients with relapsed-refractory multiple myeloma. Ann Hematol 2011;90:1449-56. https://doi.org/10.1007/s00277-011-1217-0.
- Vidal L, Gafter-Gvili A, Leibovici L, Dreyling M, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: systematic review and meta-analysis of randomized trials. J Natl Cancer Inst 2009;101:248-55. https://doi.org/10.1093/jnci/djn478.
- Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 2005;353:977-87. https://doi.org/10.1056/NEJMoa044097.
- Cullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med 2005;353:988-98. https://doi.org/10.1056/NEJMoa050078.
- Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 2012;1. https://doi.org/10.1002/14651858.CD004386.pub3.
- Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter-Gvili A, Fraser A, et al. Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer 2006;107:1743-51. https://doi.org/10.1002/cncr.22205.
- Meunier F, Lukan C. The first European conference on infections in leukaemia – ECIL1: a current perspective. Eur J Cancer 2008;44:2112-17. https://doi.org/10.1016/j.ejca.2008.07.008.
- Baum HV, Franz U, Geiss HK. Prevalence of ciprofloxacin-resistant Escherichia coli in hematologic-oncologic patients. Infection 2000;28:278-81. https://doi.org/10.1007/s150100070019.
- Razonable RR, Litzow MR, Khaliq Y, Piper KE, Rouse MS, Patel R. Bacteremia due to viridans group streptococci with diminished susceptibility to levofloxacin among neutropenic patients receiving levofloxacin prophylaxis. Clin Infect Dis 2002;34:1469-74. https://doi.org/10.1086/340352.
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23:3-9. https://doi.org/10.1038/leu.2008.291.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538-48. https://doi.org/10.1016/S1470-2045(14)70442-5.
- EuroQol Group . EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Rowen D, Brazier J, Young T, Gaugris S, Craig BM, King MT, et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 2011;14:721-31. https://doi.org/10.1016/j.jval.2011.01.004.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Kaplan MM P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. https://doi.org/10.1080/01621459.1958.10501452.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977;35:1-39. https://doi.org/10.1038/bjc.1977.1.
- Cox R. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972;34:187-220.
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-152.
- Félix J, Aragão F, Almeida JM, Calado FJ, Ferreira D, Parreira AB, et al. Time-dependent endpoints as predictors of overall survival in multiple myeloma. BMC Cancer 2013;13. https://doi.org/10.1186/1471-2407-13-122.
- National Institute of Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com/mc/bnf/current/ (accessed 30 November 2017).
- Curtis LB. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 30 November 2017).
- Curtis LB. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 30 November 2017).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment. Cham: Adis Springer Nature; 2015.
- Phillips CV. The economics of ‘more research is needed’. Int J Epidemiol 2001;30:771-6. https://doi.org/10.1093/ije/30.4.771.
- National Cancer Registration and Analysis Service . Routes to Diagnosis 2006–2010 Workbook n.d. www.ncin.org.uk/publications/routes_to_diagnosis.
- Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 2005;142:979-95. https://doi.org/10.7326/0003-4819-142-12_Part_1-200506210-00008.
Appendix 1 Additional recruitment details
Table 40 lists the sites that did not want to participate in the trial and their reasons for not participating.
| Site | Reasons for not participating |
|---|---|
| University College London Hospital | C. difficile risk |
| Ysbyty Gwynedd | Withdrew because of a MHRA safety alert for levofloxacin and acute liver injury |
| Peterborough City Hospital | Decided against participating after reading protocol: no reason given |
| Harrogate District Hospital | C. difficile risk |
| Maidstone Hospital | C. difficile risk |
| Scunthorpe General Hospital | No response |
| Luton and Dunstable Hospital | Capacity: may review again |
| East Surrey & Crawley Hospitals | C. difficile risk |
| Frimley Park Hospital | C. difficile risk |
| Ashford and St Peter’s Hospitals | C. difficile risk |
| Chelsea and Westminster Hospital | Postage cost |
Table 41 lists the numbers of patients screened and randomised at each site.
| Site | Patients (n) | |
|---|---|---|
| Screened | Randomised | |
| Altnagelvin Hospital | 24 | 11 |
| Antrim Hospital | 20 | 4 |
| Basildon University Hospital | 28 | 5 |
| Basingstoke and North Hampshire Hospital | 6 | 3 |
| Bradford Royal Infirmary | 44 | 8 |
| Broomfield Hospital (Chelmsford) | 17 | 8 |
| Calderdale Royal and Huddersfield Royal Infirmary | 32 | 9 |
| Castle Hill Hospital (Cottingham) | 34 | 17 |
| Chesterfield Royal Hospital | 33 | 13 |
| Colchester General Hospital | 71 | 31 |
| County Hospital | – | 10 |
| Craigavon Area Hospital | 17 | 6 |
| Darent Valley Hospital (Dartford) | 111 | 23 |
| Diana, Princess Of Wales Hospital (Grimsby) | 5 | 2 |
| Dorset County Hospital (Dorchester) | 10 | 2 |
| Ealing Hospital (Southall) | 25 | 12 |
| Frenchay Hospital (Bristol) | – | 3 |
| George Eliot Hospital (Nuneaton) | 16 | 6 |
| Glan Clwd Hospital (Rhyl) | – | 1 |
| Glangwili General Hospital | 15 | 10 |
| Gloucestershire Royal Hospital | 22 | 5 |
| Good Hope Hospital | – | 6 |
| Grantham and District Hospital | – | 2 |
| Great Western Hospital | 28 | 13 |
| Guy’s and St Thomas’ Hospital | 20 | 17 |
| Heartlands Hospital | 36 | 21 |
| Hereford County Hospital | 16 | 7 |
| Hillingdon Hospital | 4 | 2 |
| Kettering General Hospital | 5 | 2 |
| King’s College Hospital (Denmark Hill, London) | – | 4 |
| King’s Mill Hospital (Sutton-in-Ashfield) | – | 25 |
| Kingston Hospital (Kingston upon Thames) | 17 | 4 |
| Leicester Royal Infirmary | – | 7 |
| Leighton Hospital (Crewe) | 9 | 3 |
| Lincoln County Hospital | 28 | 11 |
| Macclesfield District General Hospital | 10 | 1 |
| Manchester Royal Infirmary | – | 12 |
| Medway Maritime Hospital (Gillingham) | 35 | 26 |
| Mid Yorkshire Hospitals | 21 | 6 |
| Milton Keynes Hospital | 18 | 7 |
| New Cross Hospital (Wolverhampton) | – | 9 |
| North Bristol Hospital | 4 | 0 |
| North Devon District Hospital | 5 | 0 |
| North Middlesex University Hospital Trust (London) | 15 | 10 |
| Northampton General Hospital | 53 | 19 |
| Northwick Park Hospital (Harrow) | 43 | 11 |
| Pilgrim Hospital (Boston) | – | 7 |
| Poole Hospital | – | 2 |
| Princess Royal University Hospital (Orpington) | 42 | 31 |
| Queen Alexandra Hospital (Portsmouth) | 123 | 51 |
| Queen Elizabeth Hospital (Birmingham) | 14 | 13 |
| Queen Elizabeth Hospital (King’s Lynn) | 57 | 16 |
| Queen Elizabeth Hospital (Woolwich) | 10 | 3 |
| Queen’s Hospital (Burton upon Trent) | 18 | 6 |
| Queen’s Hospital (Romford) | 38 | 13 |
| Royal Berkshire Hospital (Reading) | 20 | 13 |
| Royal Bournemouth Hospital | – | 3 |
| Royal Devon and Exeter Hospital (Wonford, Exeter) | 25 | 10 |
| Royal Gwent Hospital (Newport) | – | 1 |
| Royal Hallamshire Hospital (Sheffield) | 29 | 6 |
| Royal Hampshire County Hospital (Winchester) | 17 | 7 |
| Royal Liverpool University Hospital | 6 | 4 |
| Royal Shrewsbury Hospital | 19 | 2 |
| Royal Stoke University Hospital | – | 39 |
| Royal Surrey County Hospital (Guildford) | 11 | 7 |
| Royal United Hospital (Bath) | 33 | 12 |
| Russells Hall Hospital | – | 38 |
| Salisbury District Hospital | 46 | 17 |
| Sandwell General Hospital (West Bromwich) | 58 | 25 |
| Southampton General Hospital | 26 | 14 |
| Southend University Hospital | 16 | 11 |
| Southmead Hospital (Bristol) | 18 | 14 |
| St. Helier Hospital (Carshalton) | 120 | 28 |
| St James’s University Hospital (Leeds) | 16 | 11 |
| Stoke Mandeville Hospital | 36 | 13 |
| Sunderland Royal Hospital | 19 | 6 |
| Tameside General Hospital | 1 | 0 |
| Torbay Hospital | 40 | 4 |
| Ulster Hospital (Belfast) | 37 | 18 |
| University Hospital Coventry | 57 | 32 |
| University Hospital Lewisham (London) | 11 | 5 |
| Warrington Hospital | – | 8 |
| Warwick Hospital | 6 | 5 |
| West Middlesex University Hospital (Isleworth) | 38 | 17 |
| Wexham Park Hospital (Slough) | 11 | 5 |
| Whipps Cross University Hospital (London) | 7 | 4 |
| Withybush General Hospital (Haverfordwest) | 6 | 3 |
| Wrexham Maelor Hospital | 36 | 18 |
| Wycombe Hospital (High Wycombe) | 23 | 10 |
| Wythenshawe Hospital (Manchester) | 19 | 11 |
| Total | 2183 | 977 |
The number of reasons for screening failures are reported in Table 42.
| Reason for screening failure | n | % |
|---|---|---|
| Declined, no reason given | 278 | 23 |
| Declined, too much going on | 88 | 7 |
| Not eligible/out of time window | 290 | 24 |
| Clinical decision | 101 | 8 |
| Location (e.g. moving to a new hospital) | 36 | 3 |
| Medication (e.g. did not want to take any more tablets or did not want to take antibiotics when did not have an infection, etc.) | 49 | 4 |
| Did not want to participate in a clinical trial/already on another clinical trial | 79 | 6.5 |
| Inability to consent (e.g. language barrier/confusion) | 44 | 4 |
| Did not want to/could not take samples | 21 | 2 |
| Died | 9 | 1 |
| Not known | 211 | 17.5 |
| Total | 1206 | 100 |
Appendix 2 Details of serious adverse events reported
| System Organ Class ID/CTCAE category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Missing System Organ Class ID/CTCAE | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Infections and infestations | 96 (18) | 125 (26) | 221 (21) |
| General disorders and administration site conditions | 87 (16) | 75 (15) | 162 (16) |
| Gastrointestinal disorders | 79 (15) | 56 (11) | 135 (13) |
| Respiratory, thoracic and mediastinal disorders | 42 (8) | 34 (7) | 76 (7) |
| Cardiac disorders | 32 (6) | 31 (6) | 63 (6) |
| Vascular disorders | 28 (5) | 24 (5) | 52 (5) |
| Nervous system disorders | 30 (6) | 19 (4) | 49 (5) |
| Musculoskeletal and connective tissue disorders | 30 (6) | 17 (3) | 47 (5) |
| Renal and urinary disorders | 29 (5) | 16 (3) | 45 (4) |
| Skin and subcutaneous tissue disorders | 20 (4) | 17 (3) | 37 (4) |
| Metabolism and nutrition disorders | 17 (3) | 16 (3) | 33 (3) |
| Blood and lymphatic system disorders | 16 (3) | 15 (3) | 31 (3) |
| Investigations | 17 (3) | 13 (3) | 30 (3) |
| Injury, poisoning and procedural complications | 6 (1) | 6 (1) | 12 (1) |
| Psychiatric disorders | 5 (1) | 7 (1) | 12 (1) |
| Immune system disorders | 5 (1) | 5 (1) | 10 (1) |
| Ear and labyrinth disorders | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Eye disorders | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| Hepatobiliary disorders | 0 (0) | 2 (0.4) | 2 (0.2) |
| Endocrine disorders | 0 (0) | 1 (0.2) | 1 (0.1) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 (0) | 1 (0.2) | 1 (0.1) |
| Reproductive system and breast disorders | 0 (0) | 1 (0.2) | 1 (0.1) |
| Social circumstances | 0 (0) | 1 (0.2) | 1 (0.1) |
| Surgical and medical procedures | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Total | 544 (100) | 488 (100) | 1032 (100) |
| System Organ Class ID/CTCAE category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Missing CTCAE | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Infections and infestations | 96 (18) | 125 (26) | 221 (21) |
| Lung infection | 36 | 58 | 94 |
| Infections and infestations – other, specify | 19 | 26 | 45 |
| Sepsis | 19 | 19 | 38 |
| Urinary tract infection | 4 | 8 | 12 |
| Bronchial infection | 2 | 2 | 4 |
| Soft tissue infection | 1 | 3 | 4 |
| Upper respiratory infection | 2 | 2 | 4 |
| Pharyngitis | 2 | 1 | 3 |
| Skin infection | 2 | 1 | 3 |
| Device-related infection | 1 | 1 | 2 |
| Phlebitis infective | 1 | 1 | 2 |
| Bladder infection | 1 | 0 | 1 |
| Endocarditis infective | 0 | 1 | 1 |
| Enterocolitis infectious | 1 | 0 | 1 |
| Joint infection | 1 | 0 | 1 |
| Kidney infection | 1 | 0 | 1 |
| Papulopustular rash | 1 | 0 | 1 |
| Pleural infection | 0 | 1 | 1 |
| Rash pustular | 0 | 1 | 1 |
| Small intestine infection | 1 | 0 | 1 |
| Tooth infection | 1 | 0 | 1 |
| General disorders and administration site conditions | 87 (16) | 75 (15) | 162 (16) |
| Fever | 53 | 56 | 109 |
| Fatigue | 6 | 6 | 12 |
| Chills | 5 | 4 | 9 |
| Pain | 5 | 4 | 9 |
| Oedema limbs | 6 | 1 | 7 |
| Malaise | 3 | 2 | 5 |
| Non-cardiac chest pain | 3 | 1 | 4 |
| Flu-like symptoms | 2 | 0 | 2 |
| Oedema face | 0 | 1 | 1 |
| Oedema trunk | 1 | 0 | 1 |
| Infusion-related reaction | 1 | 0 | 1 |
| Localised oedema | 1 | 0 | 1 |
| Multiorgan failure | 1 | 0 | 1 |
| Gastrointestinal disorders | 79 (15) | 56 (11) | 135 (13) |
| Diarrhoea | 17 | 20 | 37 |
| Vomiting | 14 | 11 | 25 |
| Constipation | 12 | 6 | 18 |
| Nausea | 14 | 3 | 17 |
| Abdominal pain | 3 | 9 | 12 |
| Colonic perforation | 5 | 0 | 5 |
| Abdominal distension | 2 | 2 | 4 |
| Dysphagia | 1 | 2 | 3 |
| Gastrointestinal disorders – other, specify | 1 | 2 | 3 |
| Oral pain | 2 | 0 | 2 |
| Dental caries | 1 | 0 | 1 |
| Duodenal haemorrhage | 1 | 0 | 1 |
| Duodenal perforation | 1 | 0 | 1 |
| Duodenal ulcer | 1 | 0 | 1 |
| Faecal incontinence | 1 | 0 | 1 |
| Mucositis oral | 1 | 0 | 1 |
| Obstruction gastric | 1 | 0 | 1 |
| Rectal haemorrhage | 0 | 1 | 1 |
| Upper gastrointestinal haemorrhage | 1 | 0 | 1 |
| Respiratory, thoracic and mediastinal disorders | 42 (8) | 34 (7) | 76 (7) |
| Dyspnoea | 22 | 12 | 34 |
| Cough | 11 | 9 | 20 |
| Hypoxia | 1 | 3 | 4 |
| Pleural effusion | 3 | 1 | 4 |
| Productive cough | 0 | 4 | 4 |
| Respiratory, thoracic and mediastinal disorders – other, specify | 1 | 2 | 3 |
| Pulmonary oedema | 0 | 2 | 2 |
| Aspiration | 1 | 0 | 1 |
| Bronchospasm | 1 | 0 | 1 |
| Laryngeal inflammation | 1 | 0 | 1 |
| Pneumothorax | 0 | 1 | 1 |
| Respiratory failure | 1 | 0 | 1 |
| Cardiac disorders | 32 (6) | 31 (6) | 63 (6) |
| AF | 11 | 10 | 21 |
| Chest pain – cardiac | 2 | 5 | 7 |
| Cardiac arrest | 2 | 3 | 5 |
| Sinus bradycardia | 3 | 2 | 5 |
| Cardiac disorders – other, specify | 1 | 3 | 4 |
| Heart failure | 4 | 0 | 4 |
| Myocardial infarction | 1 | 3 | 4 |
| Acute coronary syndrome | 1 | 2 | 3 |
| Supraventricular tachycardia | 3 | 0 | 3 |
| Sinus tachycardia | 1 | 1 | 2 |
| Asystole | 0 | 1 | 1 |
| Atrial flutter | 1 | 0 | 1 |
| Atrioventricular block first degree | 1 | 0 | 1 |
| Palpitations | 1 | 0 | 1 |
| Ventricular arrhythmia | 0 | 1 | 1 |
| Vascular disorders | 28 (5) | 24 (5) | 52 (5) |
| Thromboembolic event | 14 | 18 | 32 |
| Hypotension | 13 | 4 | 17 |
| Haematoma | 0 | 1 | 1 |
| Hypertension | 1 | 0 | 1 |
| Vascular disorders – other, specify | 0 | 1 | 1 |
| Nervous system disorders | 30 (6) | 19 (4) | 49 (5) |
| Dizziness | 6 | 6 | 12 |
| Syncope | 5 | 1 | 6 |
| Nervous system disorders – other, specify | 3 | 1 | 4 |
| Vasovagal reaction | 4 | 0 | 4 |
| Depressed level of consciousness | 1 | 2 | 3 |
| Peripheral sensory neuropathy | 3 | 0 | 3 |
| Seizure | 1 | 2 | 3 |
| Headache | 1 | 1 | 2 |
| Lethargy | 2 | 0 | 2 |
| Peripheral motor neuropathy | 1 | 1 | 2 |
| Stroke | 2 | 0 | 2 |
| Ataxia | 0 | 1 | 1 |
| Dysphasia | 0 | 1 | 1 |
| Encephalopathy | 0 | 1 | 1 |
| Ischaemia cerebrovascular | 0 | 1 | 1 |
| Paraesthesia | 0 | 1 | 1 |
| Tremor | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 30 (6) | 17 (3) | 47 (5) |
| Back pain | 9 | 8 | 17 |
| Musculoskeletal and connective tissue disorder – other, specify | 5 | 3 | 8 |
| Bone pain | 3 | 0 | 3 |
| Chest wall pain | 1 | 2 | 3 |
| Generalised muscle weakness | 2 | 1 | 3 |
| Pain in extremity | 2 | 1 | 3 |
| Arthritis | 2 | 0 | 2 |
| Muscle weakness lower limb | 2 | 0 | 2 |
| Myalgia | 2 | 0 | 2 |
| Flank pain | 1 | 0 | 1 |
| Joint range of motion decreased cervical spine | 0 | 1 | 1 |
| Muscle weakness right-sided | 0 | 1 | 1 |
| Neck pain | 1 | 0 | 1 |
| Renal and urinary disorders | 29 (5) | 16 (3) | 45 (4) |
| Acute kidney injury | 20 | 13 | 33 |
| Urinary retention | 4 | 1 | 5 |
| Chronic kidney disease | 1 | 1 | 2 |
| Cystitis non-infective | 1 | 0 | 1 |
| Renal and urinary disorders – other, specify | 0 | 1 | 1 |
| Urinary frequency | 1 | 0 | 1 |
| Urinary incontinence | 1 | 0 | 1 |
| Urinary tract pain | 1 | 0 | 1 |
| Skin and subcutaneous tissue disorders | 20 (4) | 17 (3) | 37 (4) |
| Rash maculopapular | 13 | 10 | 23 |
| Skin and subcutaneous tissue disorders – other, specify | 3 | 1 | 4 |
| Erythroderma | 1 | 1 | 2 |
| Pruritus | 2 | 0 | 2 |
| Stevens–Johnson syndrome | 0 | 2 | 2 |
| Dry skin | 1 | 0 | 1 |
| Palmar–plantar erythrodysaesthesia syndrome | 0 | 1 | 1 |
| Skin ulceration | 0 | 1 | 1 |
| Metabolism and nutrition disorders | 17 (3) | 16 (3) | 33 (3) |
| Dehydration | 1 | 6 | 7 |
| Hyponatraemia | 3 | 3 | 6 |
| Hypocalcaemia | 2 | 3 | 5 |
| Hyperkalaemia | 2 | 1 | 3 |
| Acidosis | 2 | 0 | 2 |
| Anorexia | 1 | 1 | 2 |
| Hyperglyacemia | 2 | 0 | 2 |
| Hypokalaemia | 2 | 0 | 2 |
| Hypercalcaemia | 1 | 0 | 1 |
| Hypernatraemia | 0 | 1 | 1 |
| Hypoglycaemia | 0 | 1 | 1 |
| Hypomagnesaemia | 1 | 0 | 1 |
| Blood and lymphatic system disorders | 16 (3) | 15 (3) | 31 (3) |
| Anaemia | 13 | 8 | 21 |
| Febrile neutropenia | 2 | 6 | 8 |
| Blood and lymphatic system disorders – other, specify | 1 | 1 | 2 |
| Investigations | 17 (3) | 13 (3) | 30 (3) |
| Neutrophil count decreased | 4 | 4 | 8 |
| Creatinine increased | 5 | 2 | 7 |
| Alanine aminotransferase increased | 3 | 2 | 5 |
| Alkaline phosphatase increased | 2 | 1 | 3 |
| Investigations – other, specify | 2 | 1 | 3 |
| Cardiac troponin I increased | 0 | 2 | 2 |
| Electrocardiogram QT corrected interval prolonged | 0 | 1 | 1 |
| Platelet count decreased | 1 | 0 | 1 |
| Injury, poisoning and procedural complications | 6 (1) | 6 (1) | 12 (1) |
| Fall | 2 | 5 | 7 |
| Fracture | 3 | 1 | 4 |
| Vascular access complication | 1 | 0 | 1 |
| Psychiatric disorders | 5 (1) | 7 (1) | 12 (1) |
| Confusion | 2 | 5 | 7 |
| Delirium | 1 | 1 | 2 |
| Hallucinations | 1 | 0 | 1 |
| Restlessness | 0 | 1 | 1 |
| Suicide attempt | 1 | 0 | 1 |
| Immune system disorders | 5 (1) | 5 (1) | 10 (1) |
| Allergic reaction | 5 | 5 | 10 |
| Ear and labyrinth disorders | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Vertigo | 1 | 1 | 2 |
| Ear and labyrinth disorders – other, specify | 0 | 1 | 1 |
| Ear pain | 1 | 0 | 1 |
| Eye disorders | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| Eye disorders – other, specify | 1 | 2 | 3 |
| Retinal vascular disorder | 0 | 1 | 1 |
| Hepatobiliary disorders | 0 (0) | 2 (0.4) | 2 (0.2) |
| Hepatic failure | 0 | 1 | 1 |
| Hepatobiliary disorders – other, specify | 0 | 1 | 1 |
| Endocrine disorders | 0 (0) | 1 (0.2) | 1 (0.1) |
| Endocrine disorders – other, specify | 0 | 1 | 1 |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 (0) | 1 (0.2) | 1 (0.1) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) – other, specify | 0 | 1 | 1 |
| Reproductive system and breast disorders | 0 (0) | 1 (0.2) | 1 (0.1) |
| Testicular pain | 0 | 1 | 1 |
| Social circumstances | 0 (0) | 1 (0.2) | 1 (0.1) |
| Social circumstances – other, specify | 0 | 1 | 1 |
| Surgical and medical procedures | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Surgical and medical procedures – other, specify | 1 | 0 | 1 |
| Total | 544 (100) | 488 (100) | 1032 (100) |
Table 45 shows all SARs that were reported to the trial office. A total of 106 symptoms (69 for patients on levofloxacin and 37 for patients on placebo) were reported for the 60 SARs (38 events on levofloxacin and 22 events on placebo) between the start of treatment and up to 30 days post treatment.
| System Organ Class ID/CTCAE category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Missing System Organ Class ID/CTCAE | 0 (0) | 1 (3) | 1 (1) |
| Gastrointestinal disorders | 15 (22) | 12 (32) | 27 (25) |
| Skin and subcutaneous tissue disorders | 9 (13) | 5 (14) | 14 (13) |
| General disorders and administration site conditions | 6 (9) | 5 (14) | 11 (10) |
| Infections and infestations | 7 (10) | 2 (5) | 9 (8) |
| Musculoskeletal and connective tissue disorders | 7 (10) | 1 (3) | 8 (8) |
| Renal and urinary disorders | 5 (7) | 2 (5) | 7 (7) |
| Blood and lymphatic system disorders | 5 (7) | 0 (0) | 5 (5) |
| Cardiac disorders | 3 (4) | 1 (3) | 4 (4) |
| Investigations | 3 (4) | 1 (3) | 4 (4) |
| Metabolism and nutrition disorders | 1 (1) | 3 (8) | 4 (4) |
| Nervous system disorders | 3 (4) | 0 (0) | 3 (3) |
| Immune system disorders | 1 (1) | 1 (3) | 2 (2) |
| Psychiatric disorders | 2 (3) | 0 (0) | 2 (2) |
| Vascular disorders | 1 (1) | 1 (3) | 2 (2) |
| Ear and labyrinth disorders | 0 (0) | 1 (3) | 1 (1) |
| Injury, poisoning and procedural complications | 1 (1) | 0 (0) | 1 (1) |
| Respiratory, thoracic and mediastinal disorders | 0 (0) | 1 (3) | 1 (1) |
| Total | 69 (100) | 37 (100) | 106 (100) |
| System Organ Class ID/CTCAE category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Levofloxacin | Placebo | ||
| Missing System Organ Class ID/CTCAE | 0 (0) | 1 (3) | 1 (1) |
| Gastrointestinal disorders | 15 (22) | 12 (32) | 27 (25) |
| Diarrhoea | 5 | 9 | 14 |
| Vomiting | 4 | 1 | 5 |
| Nausea | 4 | 0 | 4 |
| Abdominal distension | 1 | 0 | 1 |
| Abdominal pain | 0 | 1 | 1 |
| Gastrointestinal disorders – other, specify | 0 | 1 | 1 |
| Obstruction gastric | 1 | 0 | 1 |
| Skin and subcutaneous tissue disorders | 9 (13) | 5 (14) | 14 (13) |
| Missing CTCAE term | 0 | 1 | 1 |
| Rash maculopapular | 6 | 1 | 7 |
| Skin and subcutaneous tissue disorders – other, specify | 2 | 0 | 2 |
| Erythroderma | 0 | 1 | 1 |
| Pruritus | 1 | 0 | 1 |
| Skin ulceration | 0 | 1 | 1 |
| Stevens–Johnson syndrome | 0 | 1 | 1 |
| General disorders and administration site conditions | 6 (9) | 5 (14) | 11 (10) |
| Fever | 5 | 5 | 10 |
| Chills | 1 | 0 | 1 |
| Infections and infestations | 7 (10) | 2 (5) | 9 (8) |
| Sepsis | 2 | 1 | 3 |
| Lung infection | 2 | 0 | 2 |
| Bladder infection | 1 | 0 | 1 |
| Enterocolitis infectious | 1 | 0 | 1 |
| Rash pustular | 0 | 1 | 1 |
| Small intestine infection | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 7 (10) | 1 (3) | 8 (8) |
| Musculoskeletal and connective tissue disorder – other, specify | 4 | 0 | 4 |
| Chest wall pain | 0 | 1 | 1 |
| Myalgia | 1 | 0 | 1 |
| Neck pain | 1 | 0 | 1 |
| Pain in extremity | 1 | 0 | 1 |
| Renal and urinary disorders | 5 (7) | 2 (5) | 7 (7) |
| Acute kidney injury | 4 | 2 | 6 |
| Chronic kidney disease | 1 | 0 | 1 |
| Blood and lymphatic system disorders | 5 (7) | 0 (0) | 5 (5) |
| Anaemia | 4 | 0 | 4 |
| Febrile neutropenia | 1 | 0 | 1 |
| Cardiac disorders | 3 (4) | 1 (3) | 4 (4) |
| AF | 2 | 0 | 2 |
| Sinus bradycardia | 1 | 1 | 2 |
| Investigations | 3 (4) | 1 (3) | 4 (4) |
| Alanine aminotransferase increased | 1 | 1 | 2 |
| Creatinine increased | 1 | 0 | 1 |
| Neutrophil count decreased | 1 | 0 | 1 |
| Metabolism and nutrition disorders | 1 (1) | 3 (8) | 4 (4) |
| Anorexia | 1 | 0 | 1 |
| Dehydration | 1 | 0 | 1 |
| Hypernatraemia | 1 | 0 | 1 |
| Hyponatraemia | 0 | 1 | 1 |
| Nervous system disorders | 3 (4) | 0 (0) | 3 (3) |
| Dizziness | 1 | 0 | 1 |
| Peripheral sensory neuropathy | 1 | 0 | 1 |
| Vasovagal reaction | 1 | 0 | 1 |
| Immune system disorders | 1 (1) | 1 (3) | 2 (2) |
| Allergic reaction | 1 | 1 | 2 |
| Psychiatric disorders | 2 (3) | 0 (0) | 2 (2) |
| Confusion | 1 | 0 | 1 |
| Delirium | 1 | 0 | 1 |
| Vascular disorders | 1 (1) | 1 (3) | 2 (2) |
| Hypotension | 1 | 1 | 2 |
| Ear and labyrinth disorders | 0 (0) | 1 (3) | 1 (1) |
| Vertigo | 0 | 1 | 1 |
| Injury, poisoning and procedural complications | 1 (1) | 0 (0) | 1 (1) |
| Vascular access complication | 1 | 0 | 1 |
| Respiratory, thoracic and mediastinal disorders | 0 (0) | 1 (3) | 1 (1) |
| Hypoxia | 0 | 1 | 1 |
| Total | 69 (100) | 37 (100) | 106 (100) |
Appendix 3 Health economic tables
| Resource item | Unit cost (£) | Source | Details |
|---|---|---|---|
| Trial medication | |||
| Levofloxacin 250 mg | 11.57 (per 10-tablet pack) | BNF 2017 | Normal dose: two tablets per day |
| Moderate renal failure: one tablet per day | |||
| Severe renal failure: half tablet per day | |||
| Levofloxacin 500 mg | 11.40 (per 10-tablet pack) | BNF 2017 | |
| Community health and social services | |||
| GP visit, surgery | 36.00 (per visit) | PSSRU 2016, p. 14545 | Per patient contact lasting 9.22 minutes, including direct care staff costs |
| GP visit, home | 82.76 (per visit) | PSSRU 2016, p. 14545 | Per patient contact lasting 9.22 minutes (plus an average 12-minute travel time) × £3.90/minute |
| Telephone GP | 14.60 | PSSRU 2016, p. 14745 | Telephone triage, GP led, per clinician consultation lasting 4 minutes |
| District nurse visit | 38.00 (per visit) | NHS Reference Costs 2015 to 2016 46 | District nurse, adult, face to face. CC: N02AF; SC: NURS |
| Practice nurse visit | 12.14 (per visit) |
PSSRU 2015, p. 17443 PSSRU 2016, p. 14345 |
Per 15.5-minute consultation,43 based on £43.00 per hour45 |
| Home help or care worker visit | 24.00 (per visit) | PSSRU 2016, p. 16045 | Face-to-face 1-hour weekday session |
| Acupuncture | 120 | NHS Reference Costs 2015 to 2016 46 | General medicine: acupuncture for pain management CC: AB23Z; SC: 300 |
| Telephone call with research/specialist nurse | 7.90 | PSSRU 2016, p. 14745 | Telephone triage, nurse led, per telephone consultation lasting 6.56 minutes |
| Chiropodist/podiatrist | 32.00 | PSSRU 2016, p. 135–745 | Chiropodist/podiatrist band 5, face to face, 1 hour |
| Specialist nurse | 44.00 | PSSRU 2016, p. 186–845 | Nurse specialist band 6, 1 hour |
| Counsellor | 42.00 | PSSRU 2016, p. 135–745 | Counsellor band 6, face to face, 1 hour |
| Day care | 61.00 | PSSRU 2016, p. 2845 | Local authority provision day care per client attendance |
| Occupational therapist | 32.00 | PSSRU 2016, p. 135–745 | Occupational therapist band 5, face to face, 1 hour |
| Physiotherapy | 32.00 | PSSRU 2016, p. 135–745 | Physiotherapist band 5, face to face, 1 hour |
| Psychologist | 52.00 | PSSRU 2016, p. 135–745 | Clinical psychologist band 7, face to face, 1 hour |
| Phlebotomy | 61.00 (58.00 + 3.00) |
NHS Reference Costs 2015 to 2016 46 PSSRU 2015, p. 16943 |
Phlebotomy DAPS08 and community nurse (1 hour) |
| Palliative care nurse | 136.00 | NHS Reference Costs 2015 to 2016 46 | Medical specialist palliative care SD04A |
| Palliative care (non-medical) | 69.00 | NHS Reference Costs 2015 to 2016 46 | Non-medical specialist palliative care (other) |
| Telephone occupational therapist | 18.00 | NHS Reference Costs 2015 to 2016 46 | Occupational therapy, consultant led, not admitted, non-face to face, first |
| Social worker | 55.00 | PSSRU 2016, p. 15645 | Social worker per hour of client-related work |
| Health visitor – postnatal | 68.55 | NHS Reference Costs 2015 to 2016 46 | Community midwife, post-natal visit N01P |
| Rapid response | 79.00 | PSSRU 2016, p. 10145 | Per hour of service |
| Dentist (band 1) | 51.75 | PSSRU 2015, p. 18143 | Dentist 15 minutes at £207.00 per patient hour |
| Dentist (band 2) | 207.00 | PSSRU 2015, p. 18143 | Per patient hour |
| Hearing centre | 63.00 | NHS Reference Costs 2015 to 2016 46 | Audiometry or hearing assessment, ≥ 19 years CC: CA37A |
| Telephone 111 | 8.00 | www.bbc.co.uk/news/health-22370621 (accessed 22 May 2017) | £7.50–8.50 per call |
| Blood test | 15.14 (12.14 + 3.00) | NHS Reference Costs 2015 to 2016 46 | 15.5-minute consultation with practice nurse (PSSRU 201543) at £43.00 per hour plus blood test at £3.00 |
| Directly accessed pathology services – haematology CC: DAPS05 | |||
| Hospital based or residential care services | |||
| Hospital inpatient | 298.41 (per day) | NHS Reference Costs 2015 to 2016 46 | Total Healthcare Resource Groups, average of all non-elective inpatient excess bed-days |
| Hospital critical/intensive care unit | 521.00 (per day) | NHS Reference Costs 2015 to 2016 46 | Medical adult patients (unspecified speciality), adult critical care, zero organs supported, CC: XC07Z; SC: CCU03 |
| Hospital short stay (hospital stay of 1 day) | 478.00 | NHS Reference Costs 2015 to 2016 46 | Non-elective short stay, malignant disorders of lymphatic or haematological systems |
| Hospital long stay (hospital stay of 2–5 days) | 4850.00 | NHS Reference Costs 2015 to 2016 46 | Non-elective long stay, malignant disorders of lymphatic or haematological systems |
| Hospital excess bed-days (cost per day after first 5 days) | 338.00 (per day) | NHS Reference Costs 2015 to 2016 46 | Non-elective excess bed-days, malignant disorders of lymphatic or haematological systems |
| Hospital outpatient | 135.00 (per visit) | PSSRU 2016, p. 9545 | Weighted average of all outpatient attendances |
| A&E | 137.74 (per visit) | NHS Reference Costs 2015 to 2016 46 | Emergency medicine, average unit costs of all emergency medicine attendances |
| Residential home | 90.00 (per day) | PSSRU 2016, p. 2645 | Private sector residential home cost per permanent resident day |
| Paramedic | 181.00 | NHS Reference Costs 2015 to 2016 46 | Ambulance: see and treat or refer ASS01 |
| Ambulance | 236.00 | NHS Reference Costs 2015 to 2016 46 | Ambulance: see, treat and convey ASS02 |
| Dialysis | 133.36 | NHS Reference Costs 2015 to 2016 46 | Renal dialysis |
| Mammogram | 30.00 | NHS Reference Costs 2015 to 2016 46 | Direct access plain film |
| Chest radiograph | 30.00 | NHS Reference Costs 2015 to 2016 46 | Direct access plain film |
| Resource use, mean (SD) | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Levofloxacin | Placebo | |||||||
| Week 4 | Week 8 | Week 12 | Week 16 | Week 4 | Week 8 | Week 12 | Week 16 | |
| Resources use data from resource use questionnaire only | ||||||||
| n valid (missing) | 450 (39) | 412 (77) | 394 (95) | 374 (115) | 437 (51) | 405 (83) | 385 (103) | 368 (120) |
| GP | 0.38 (0.76) | 0.32 (0.73) | 0.23 (0.59) | 0.22 (0.54) | 0.31 (0.62) | 0.32 (0.9) | 0.32 (0.74) | 0.31 (0.69) |
| Nurse | 0.88 (3.0) | 1.03 (3.72) | 0.81 (3.44) | 0.71 (3.3) | 0.84 (3.26) | 0.78 (3.43) | 0.79 (3.49) | 0.58 (2.62) |
| Care worker | 0.51 (3.24) | 0.71 (5.52) | 0.45 (3.02) | 0.66 (1.20) | 0.49 (3.52) | 0.45 (2.94) | 0.39 (3.04) | 0.5 (4.07) |
| Hospital outpatient | 2.88 (2.70) | 3.14 (2.96) | 2.99 (2.74) | 2.78 (2.66) | 2.79 (2.64) | 2.92 (2.84) | 2.76 (2.80) | 2.82 (3.01) |
| Hospital A&E | 0.29 (0.65) | 0.15 (0.42) | 0.12 (0.43) | 0.12 (0.81) | 0.23 (0.49) | 0.13 (0.39) | 0.12 (0.36) | 0.11 (0.41) |
| Residential care | 0.12 (1.28) | 0.14 (1.91) | 0.03 (0.61) | 0.08 (1.45) | 0.14 (1.97) | 0.02 (0.36) | 0.09 (1.48) | 0.09 (1.58) |
| Resource use data combined from resource use questionnaire and hospital data | ||||||||
| n valid (missing) | 458 (31) | 446 (43) | 440 (49) | 423 (66) | 444 (44) | 428 (60) | 421 (67) | 414 (74) |
| Hospital inpatient days | 3.10 (6.02) | 1.83 (5.5) | 1.59 (5.28) | 0.84 (3.61) | 3.02 (6.54) | 1.40 (5.61) | 0.71 (2.58) | 1.0 (4.26) |
| Resource use data from hospital data onlya | ||||||||
| n | 3 | 1 | 0 | 0 | 4 | 2 | 2 | 0 |
| Hospital ICU days | 5.33 (4.04) | 9 (–) | – | – | 14.75 (15.63) | 19 (12.73) | 2.5 (0.71) | – |
| Total costs (£), mean (SD) | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Levofloxacin | Placebo | |||||||
| Week 4 | Week 8 | Week 12 | Week 16 | Week 4 | Week 8 | Week 12 | Week 16 | |
| Community health and social services | ||||||||
| GP | 13.68 (27.4) | 11.62 (26.22) | 8.41 (21.22) | 7.89 (19.55) | 11.12 (22.43) | 11.38 (28.41) | 11.10 (26.48) | 11.05 (24.67) |
| Nurse | 33.52 (113.91) | 39.2 (141.55) | 30.86 (130.74) | 26.82 (125.43) | 32.09 (123.93) | 29.56 (130.49) | 30.2 (132.5) | 21.89 (99.53) |
| Care worker | 12.16 (77.69) | 17.01 (132.57) | 10.72 (72.37) | 15.72 (100.87) | 11.81 (84.6) | 10.79 (70.58) | 9.29 (73.03) | 12 (97.64) |
| Other community health and social services | 7.76 (75.28) | 6.08 (30.98) | 7.21 (44.75) | 9.11 (67.84) | 6.73 (35.0) | 2.85 (27.39) | 4.48 (30.98) | 1.82 (11.25) |
| Total community health and social services | 67.12 (171.06) | 73.91 (225.25) | 57.20 (171.32) | 59.55 (201.11) | 61.74 (159.69) | 54.57 (160.64) | 55.47 (172.5) | 46.77 (157.83) |
| Hospital and residential care services | ||||||||
| Hospital outpatient | 388.2 (365.05) | 424.0 (399.76) | 404.31 (369.62) | 375.76 (359.66) | 376.58 (355.81) | 394 (383.04) | 372.04 (378.16) | 380.42 (406.21) |
| Hospital A&E | 39.49 (90.08) | 20.06 (57.35) | 16.78 (59.02) | 16.20 (111.14) | 31.20 (66.81) | 17.35 (53.34) | 16.46 (49.95) | 15.35 (55.89) |
| Residential care | 10.4 (115.01) | 12.23 (172.3) | 2.97 (54.59) | 6.98 (130.38) | 12.15 (177.56) | 2.22 (32.21) | 8.42 (133.48) | 8.32 (141.94) |
| Hospital inpatient | 926.50 (1797.03) | 544.63 (1640.47) | 474.07 (1577.02) | 249.73 (1076.89) | 899.93 (1951.76) | 419.03 (1675.11) | 212.64 (770.52) | 296.97 (1271.14) |
| Hospital ICU | 2778.67 (2105.60) | 4689 (–) | – | – | 7684.75 (8142.45) | 9899 (6631.25) | 1302.50 (368.40) | – |
| Total hospital and residential care services | 1376.39 (1831.68) | 980.11 (1776.04) | 853.86 (1598.18) | 602.47 (1155.14) | 1382.89 (2301.84) | 860.36 (1828.71) | 582.30 (864.11) | 660.02 (1327.55) |
| n valid (missing) | 458 (31) | 446 (43) | 440 (49) | 423 (66) | 444 (44) | 428 (60) | 421 (67) | 414 (74) |
| Trial medication | 54.49 (21.46) | 56.25 (28.32) | 53.41 (31.42) | – | 0 (0) | 0 (0) | 0 (0) | – |
| n valid (missing) | 469 (20) | 469 (20) | 469 (20) | – | 461 (27) | 461 (27) | 461 (27) | – |
| Total NHS cost | 1463.01 (1831.86) | 1053.22 (1776.57) | 902.53 (1579.09) | 655.11 (1171.37) | 1390.42 (2279.26) | 846.71 (1789.82) | 578.11 (867.72) | 701.59 (1332.62) |
List of abbreviations
- AE
- adverse event
- AF
- atrial fibrillation
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRP
- C-reactive protein
- CTCAE
- Common Terminology Criteria for Adverse Events
- DSMC
- Data and Safety Monitoring Committee
- ECOG
- Eastern Cooperative Oncology Group
- EDTA
- ethylenediaminetetraacetic acid
- eGFR
- estimated glomerular filtration rate
- EME
- Efficacy and Mechanism
- EORTC QLQ-C30
- European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30
- EORTC QLQ-MY24
- European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-24-item myeloma-specific module
- EQ-5D
- EuroQoL-5 Dimensions
- ESBL
- extended-spectrum beta-lactamase
- EVPI
- expected value of perfect information
- flc
- free light chain
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HCAI
- health-care-associated infection
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IgG
- immunoglobulin G
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ITT
- intention to treat
- MAR
- missing at random
- MRC
- Medical Research Council
- MRSA
- meticillin-resistant Staphylococcus aureus
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PI
- principal investigator
- PIS
- patient information sheet
- PMH
- Public Health Microbiology
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RR
- relative risk
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- TEAMM
- Tackling Early Morbidity and Mortality in Myeloma
- WCTU
- Warwick Clinical Trials Unit
- WTP
- willingness to pay
