Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/140/84. The contractual start date was in December 2015. The draft report began editorial review in August 2018 and was accepted for publication in June 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Timothy Iveson reports honoraria from Amgen Inc. (Thousand Oaks, CA, USA), Bayer AG (Leverkusen, Germany), Bristol-Myers Squibb (New York, NY, USA), Celgene Corporation (Summit, NJ, USA), Pierre-Fabre (Paris, France), Roche (Roche Holding AG, Basel, Switzerland) and Servier (Laboratories Servier, Suresnes, France). Kathleen A Boyd reports grants from the Medical Research Council during the conduct of the study. Mark P Saunders reports personal fees from Servier, Amgen, Merck (Merck and Co., Kenilworth, New Jersey, USA), Eisai (Eisai Co., Ltd., Tokyo, Japan) and Roche outside the submitted work. Jim Cassidy reports grants from the Medical Research Council during the conduct of the study and is currently an employee of Celgene Corporation. Josep Tabernero reports personal fees from Array Biopharma (Boulder, CO, USA), AstraZeneca (Cambridge, UK), Bayer AG (Leverkusen, Germany), BeiGene (Beijing, China), Boehringer Ingelheim (Ingelheim am Rhein, Germany), Chugai (Chugai Pharmaceutical Co., Tokyo, Japan), Genentech, Inc. (South San Francisco, CA, USA), Genmab A/S (Copenhagen, Denmark), Halozyme (Halozyme Therapeutics, San Diego, CA, USA), Imugene Limited (Sydney, NSW, Australia), Inflection Biosciences Limited (Blackrock, Dublin), Ipsen (Paris, France), Kura Oncology (San Diego, CA, USA), Eli Lilly and Company (Indianapolis, IN, USA), Merck, Menarini (The Menarini Group, Florence, Italy), Merck Serono (Rockland, MA, USA), Merrimack Pharmaceuticals (MA, USA), Merus (Utrecht, the Netherlands), Molecular Partners (Molecular Partners AG, Zurich, Switzerland), Novartis (Novartis International AG, Basel, Switzerland), Peptomyc, Pfizer Inc. (New York, NY, USA), Pharmacyclics (Pharmacyclics LLC, Sunnyvale, CA, USA), ProteoDesign SL (Barcelona, Spain), Rafael Pharmaceuticals (Stony Brook, NY, USA), F. Hoffmann-La Roche Ltd, Sanofi (Sanofi S. A., Paris, France), Seattle Genetics (Bothwell, WA, USA), Servier, Symphogen (Symphogen A/S, Ballerup, Denmark), Taiho Pharmaceutical (Tokyo, Japan), VCN Biosciences (Barcelona, Spain), Biocartis (Biocartis Group, Mechelen, Belgium), Foundation Medicine (Cambridge, MA, USA), HalioDX (Marseille, France), SAS Pharmaceuticals (Delhi, India) and Roche Diagnostics outside the submitted work. Bengt Glimelius reports support from PledPharma AB for being on advisory boards. Sherif Raouf reports grants, personal fees and non-financial support from Roche, grants and personal fees from Amgen, and grants and personal fees from Merck outside the submitted work. David Farrugia reports that he received honoraria for speaking in educational events and support for meeting attendance from Bristol-Myers Squibb, Novartis, Ipsen, Amgen, AstraZeneca and Merck. David Cunningham reports grants from 4SC (4SC AG, Planegg, Germany), AstraZeneca, Bayer, Amgen, Celgene, Clovis Oncology (Boulder, CO, USA), Eli Lilly and Company, Janssen Pharmaceuticals (Beerse, Belgium), MedImmune (Gaithersburg, MD, USA), Merck, Merrimack and Sanofi, outside the submitted work. Tamish Hickish reports grants from Pfizer, Roche, Pierre Fabre (Paris, France) and personal fees from Eli Lilly and Company during the conduct of the study. John Bridgewater reports funding from the University College London Hospitals NHS Foundation Trust/University College London Biomedical Research Centre. David Cunningham reports funding from the National Institute for Health Research Biomedical Research Centres at the Royal Marsden.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Iveson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Colorectal cancer is a common malignancy, with 1,360,000 cases annually, leading to 694,000 deaths each year. Colorectal cancer accounts for 12% of all new cancer cases each year in the UK, with approximately 41,265 cases estimated in 2014. 1 The initial treatment for patients presenting with colorectal cancer is usually surgical resection, which is potentially curative; however, 40–50% of patients subsequently relapse and die as a result of the disease becoming metastatic. 2 Postoperative adjuvant fluoropyrimidine-based chemotherapy was first shown to reduce the recurrence of colon cancer in 1990. 3 Initially, adjuvant treatment was given for 12 months, but a randomised study suggested equivalence for 6 months of treatment,4 which is now accepted as the standard duration for adjuvant chemotherapy in patients with high-risk stage II or stage III colorectal cancer. 5
High-risk stage II is defined as having one of the following risk features: T4 disease, tumour obstruction and/or perforation, < 10 lymph nodes harvested, poorly differentiated histology, perineural invasion or extramural venous/lymphatic vascular invasion.
The addition of oxaliplatin to a fluoropyrimidine-based regimen has been shown to improve 3-year disease-free survival (DFS) in patients with colorectal cancer. 6–8 The benefit seen in the MOSAIC6 and NSABP C-077 studies was similar, despite the total oxaliplatin doses being different (1020 mg/m2 and 765 mg/m2, respectively). 6,7 These studies led to the adoption of oxaliplatin and fluoropyrimidine chemotherapy as the adjuvant treatment of choice for most patients with stage III disease who were aged < 70 years. 5,9 However, the administration of oxaliplatin with the fluoropyrimidine backbone results in additional toxicity, with increased neutropenia, thrombocytopenia, diarrhoea, nausea and vomiting. 6,7 There is also increased peripheral neuropathy, which is cumulative, dose-dependent and often irreversible, persisting long term despite the treatment of colorectal cancer having been curative. Neurotoxicity was measured using the National Cancer Institute (NCI) common terminology criteria for adverse events (CTCAE) (version 1) in MOSAIC study,6 and the NCI-Sanofi Neurosensory score in the NSABP C-07 study. 7 In the MOSAIC trial, 12.4% of patients experienced grade 3 sensory neuropathy, with 0.5% having residual problems at 18 months;6 in the NSABP C-07 trial, 8.4% of patients had grade 3 or 4 neuropathy at the end of treatment, with 10% reporting some residual neuropathy beyond 2 years. 10
As the toxicity of oxaliplatin and fluoropyrimidine regimens is cumulative, a reduction in the duration of adjuvant treatment could potentially ameliorate such effects;11 however, whether or not short-duration adjuvant treatment could compromise efficacy is widely debated. Data for one study12 are available in the literature, comparing 3 months with 6 months of adjuvant treatment with a fluoropyrimidine-based chemotherapy regimen; however, the study was conducted before the introduction of oxaliplatin-combination adjuvant treatment. Although the study was somewhat underpowered, reducing treatment duration did not appear to affect patient outcomes and was associated with reduced toxicity and improved health-related quality of life (HRQoL).
The cost of treatment for colorectal cancer in the first year after diagnosis is considerably higher than that of treating other common cancers and was estimated to cost the English health-care system £542M in 2010. 13 Three-month duration adjuvant chemotherapy for patients with colorectal cancer could be anticipated to be more cost-effective than the current standard 6-month duration, provided that efficacy is maintained. Benefits might be associated not only with lower treatment costs but also with reduced expenditure to manage problematic side effects and improvements in HRQoL.
The Short Course Oncology Therapy (SCOT) study was designed to compare 3-month and 6-month oxaliplatin-based adjuvant chemotherapy in patients with colorectal cancer in terms of efficacy, toxicity, HRQoL and economic aspects. At the time of starting this study, no published data were available on the effectiveness of short-duration treatment with adjuvant oxaliplatin and fluoropyrimidine regimens in patients with colorectal cancer. The main objective of the study was to identify whether or not 3-month adjuvant chemotherapy was inferior to 6-month treatment in terms of DFS rate. The trial also aimed to compare overall survival, toxicity and HRQoL in patients between the two treatment groups and to assess the cost-effectiveness of the two regimens. The SCOT study was designed as an international, stand-alone study of adjuvant oxaliplatin and fluoropyrimidine treatment conducted in patients with high-risk stage II or stage III colon or rectal cancers. Although stand-alone, the SCOT study was conducted in parallel with the International Duration Evaluation of Adjuvant chemotherapy (IDEA) collaborative initiative, which aimed to consolidate results from numerous worldwide trials that were attempting to clarify the importance of adjuvant treatment duration for colon cancer patients. The IDEA initiative was restricted to treatment of patients with stage III colon cancer; therefore, it was prospectively planned that patient data from the SCOT study would be pooled with those from six other studies (TOSCA, IDEA France, CALGB/SWOG 80702, ACHIEVE and HORG). 14
Chapter 2 Methods
Study design
The SCOT study was an international, randomised (1 : 1), open-label (non-blinded), non-inferiority, Phase III, parallel-group trial comparing 6 months with 3 months of oxaliplatin plus fluoropyrimidine adjuvant chemotherapy in patients with high-risk stage II or stage III colorectal cancer.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines;15 all aspects of the study received ethics approval from the ethics services in the participating countries. All participants provided written informed consent before enrolment.
Study participants
Parts of this section are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Patients were recruited from 244 oncology clinics from six countries (the UK, Denmark, Spain, Sweden, Australia and New Zealand).
Eligible patients were adults aged ≥ 18 years who had undergone curative resection for high-risk stage II (having one or more of the following risk features: T4 disease, tumour obstruction and/or perforation of the primary tumour, < 10 lymph nodes harvested, poorly differentiated histology, perineural invasion or extramural venous/lymphatic vascular invasion) or stage III adenocarcinoma of the colon or rectum.
Patients were enrolled within 11 weeks of surgery and started treatment in their allocated treatment group within 2 weeks of randomisation. Other eligibility inclusion requirements included having a World Health Organization performance status of 0 or 1, having adequate organ function and having a life expectancy of > 5 years with reference to non-cancer-related disease, accepting that they may die earlier due to colorectal cancer. Patients were to have a normal computed tomography scan of the chest, abdomen and pelvis prior to study enrolment and a carcinoembryonic antigen level of < 1.2 times the local upper limit of normal (ULN) in the week prior to randomisation. Rectal cancer patients were to have undergone total mesorectal excision with negative resection margins (> 1 mm clearance).
Exclusion criteria included undergoing chemotherapy (except chemotherapy administered with curative intent that had been completed > 5 years previously with no residual complications); having undergone previous long-course chemoradiotherapy (preoperative short-course radiotherapy was allowed); having moderate or severe renal impairment (creatinine clearance rate of < 30 ml/minute using the Cockcroft–Gault equation); having a haemoglobin concentration of < 9 g/dl, an absolute neutrophil count of < 1.5 × 109 per litre, a platelet count of < 100 × 109 per litre, and aspartate aminotransferase or alanine aminotransferase levels of > 2.5 × ULN; having clinically significant cardiovascular disease; being pregnant or lactating; being of childbearing potential and not using or being unwilling to use medically approved contraception (postmenopausal women were to have been amenorrhoeic for ≥ 12 months to be considered of non-childbearing potential); having previous malignancy other than adequately treated in situ carcinoma of the uterine cervix or basal or squamous cell carcinoma of the skin (unless there was a disease-free interval of ≥ 5 years); and having known or suspected dihydropyrimidine dehydrogenase deficiency.
Public/patient involvement
The original SCOT study protocol was formally reviewed by consumers as part of the internal review and approval processes at the Glasgow Clinical Trials Unit. Patients were involved informally in the original concept of the trial, and they thought that whether or not shorter chemotherapy could reap the same benefit as longer chemotherapy was an exceptionally important question to define.
Patients at the clinics of the lead investigators were also asked about a proposal to extend study follow-up to increase the number of DFS events for analysis (application to the NIHR programme in 2014). As they felt that the question was exceptionally important, the view was that every effort should be made to extend the study to ensure that a thorough and accurate answer could be obtained for both patients with low-risk and patients with high-risk disease. The proposal to extend the study was also formally discussed and supported both by the main National Cancer Research Institute Colorectal Clinical Studies group and at the meeting of the Adjuvant and Advanced Disease subgroups. The public/patient representative at these meetings was fully supportive.
Study interventions
Parts of this section are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
The adjuvant treatment regimen used was oxaliplatin with 5-fluorouracil (5FU) or oxaliplatin with capecitabine. Participating sites were able to select which treatment combination they wanted to use on an individual-patient basis, reflecting the choice of the patient and/or physician.
Oxaliplatin and 5-fluorouracil
For patients receiving oxaliplatin and 5FU, treatment was given every 2 weeks, the intention being to deliver six cycles to patients assigned 3 months of therapy and 12 cycles to patients assigned 6 months of therapy. On the first day of each cycle, 85 mg/m2 of intravenous (i.v.) oxaliplatin was given over 2 hours, concurrently with 175 mg of L-folinic acid or 350 mg of folinic acid (also known as leucovorin). This was followed by a 400 mg/m2 5FU i.v. bolus injection administered over 5 minutes, and then a continuous i.v. infusion of 2400 mg/m2 of 5FU over 46 hours. At the investigator’s discretion, patients who were aged > 70 years could start both 5FU infusions at 75% of the specified starting dose, if clinically indicated.
If a grade 1 adverse event (AE) occurred as a result of chemotherapy, treatment was to be continued at the full dose. For treatment-related AEs of grade ≥ 2, treatment was to be withheld until recovery to grade 1 and then restarted. If more than one delay or a delay of ≥ 2 weeks occurred, doses of oxaliplatin and infused 5FU were to be kept the same but the bolus 5FU dose was to be omitted; if further delays occurred as a result of myelotoxicity, the oxaliplatin and infusional 5FU doses were to be reduced by 25%. In addition, if after the first cycle the neutrophil count was < 1.0 × 109 cells/l, the bolus 5FU dose was to be omitted and the oxaliplatin and infused 5FU doses were to be reduced by 25%. Wherever possible, the oxaliplatin dose was to be reduced rather than discontinued; if oxaliplatin dosing had to be discontinued, 5FU was to be continued where possible.
Oxaliplatin and capecitabine
For patients receiving oxaliplatin and capecitabine, treatment was given every 3 weeks, the intention being to deliver four cycles to patients assigned 3 months of therapy and eight cycles to patients assigned 6 months of therapy. On the first day of each cycle, 130 mg/m2 of i.v. oxaliplatin was given over 2 hours. Oral capecitabine 1000 mg/m2 was taken twice per day for the first 14 days of each cycle. Patients with a creatinine clearance rate of 30–50 ml/minute were to start capecitabine treatment at 75% of the specified dose. Patients aged > 70 years could be considered for treatment with capecitabine at 75% of the full dose, with the decision to reduce dose being made at the discretion of the investigator depending on the fitness of the individual patient. If the investigator considered that any patient required dose reduction because of any other comorbidity, the patient could receive a minimum starting dose of oral capecitabine of 800 mg/m2 twice per day.
If a grade 1 AE occurred as a result of chemotherapy, treatment was to be continued at the full dose. For treatment-related AEs of grade ≥ 2, treatment was to be withheld until recovery to grade 1 and then restarted. For AEs related to oxaliplatin and capecitabine, if more than one delay or a delay of ≥ 2 weeks occurred, capecitabine and oxaliplatin doses were to be reduced by 25%; if further delays occurred as a result of myelotoxicity, further dose reductions were allowed at the investigator’s discretion.
Objectives
The objective of the SCOT study was to assess the efficacy of 3-month versus 6-month adjuvant chemotherapy for colorectal cancer and to compare the associated toxicity and HRQoL. The study also provided data for an economic analysis of the cost-effectiveness of the two regimens. The primary end point of the study was DFS, the null hypothesis being that 3-month chemotherapy is inferior to 6-month chemotherapy with a hazard ratio (HR) of > 1.13. Secondary end points were overall survival, safety, HRQoL and cost-effectiveness parameters.
The aim of the economic evaluation was to explore the cost-effectiveness of 3-month versus 6-month adjuvant chemotherapy [in terms of incremental cost per quality-adjusted life-year (QALY) gains and net monetary benefit (NMB)], using trial data on treatment and hospitalisations costs, HRQoL and survival outcomes within the timeframe of the SCOT clinical trial.
Outcomes
Parts of this section are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Disease-free survival was defined as the time from randomisation (or from trial registration for those randomised after 3 months of therapy) to relapse, development of a new colorectal cancer, or death from any cause. Overall survival was defined as the time from randomisation (or registration for those randomised at 3 months) to death from any cause. Toxicity was assessed by the investigators after each cycle of chemotherapy with AEs graded using NCI CTCAE version 3.
Patients were followed up for a minimum of 3 years to a maximum of 8 years, with full blood count, urea and electrolyte levels, liver function and carcinoembryonic antigen all being tested at 9, 12, 18, 24 and 36 months, and then annually. Computed tomography of the chest, abdomen and pelvis was conducted at 6, 12, 18, 24 and 36 months.
Health-related quality of life was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires QLQ-C3017 and QLQ-CR29,18 and using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) (with both the visual analogue scale and the health index),19 with UK value sets. 20 Neuropathy was assessed using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT/GOG-Ntx4) questionnaire. 21 Questionnaires were administered at baseline and before each treatment cycle. Additionally, HRQoL was assessed each month in the first 3 months after treatment for the 3-month treatment group. Subsequent assessments were conducted at 9 and 12 months for the EORTC questionnaires; 9, 12, 18 and 24 months and then annually for the EQ-5D-3L; and up to 12 months for the FACT/GOG-Ntx4.
Sample size
Parts of this section are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
In the previous MOSAIC trial, 3-year DFS in the oxaliplatin and 5FU treatment group was 78% compared with 73% for 5FU plus leucovorin. 6 To be able to conclude that the 3-month treatment group in the SCOT study was non-inferior, it was assumed that at least half of this benefit should be retained.
The SCOT study was designed as a randomised (1 : 1) non-inferiority trial aiming to reliably determine whether or not there was < 2.5% decrease in the 3-year DFS for patients in the 3-month treatment group (from 78% in the 6-month treatment group), which corresponds to excluding a HR of > 1.13 with 90% power at the 2.5%, one-sided level of significance. Assuming that the study would recruit over a period of 5 years with a subsequent minimum follow-up of 2 years, this design required 8600 patients to undergo randomisation and 2750 events (relapses, deaths or new colorectal cancers) to be observed; to allow for loss to follow-up, the recruitment target was 9500 patients.
From the outset, it was recognised that detecting meaningful differences based on safety and HRQoL data would not require information from all of the 9500 planned patients. For safety outcomes, 700 patients (350 in each group) were deemed sufficient to detect (80% power and a 2-sided significance level of 5%) a halving in the proportion of patients with grade 3 or 4 toxic effects from 12% to 6% (12% being the rate at which grade 3 or 4 paraesthesia, the most common non-haematological grade 3 or 4 toxic effect, occurred in the oxaliplatin treatment group in the MOSAIC trial). 6 This sample size would allow small changes in global HRQoL to be detected (assuming a difference of magnitude of 7.5322 and a standard deviation of 23.4)17 with 95% power at the 1% significance level. This more stringent level of significance was used to allow for multiple testing across various health-related quality-of-life scales. It should be noted that the power and sample size calculations for safety and health-related quality-of-life outcomes are based on a superiority comparisons, not on non-equivalence.
All sample size calculations were made in EAST 5.3.0.0 (Cytel Corporation, Cambridge, MA, USA).
Information on toxicity and health-related quality-of-life end points was collected from recruited patients until the number required was exceeded and the decision to stop was endorsed by the independent data monitoring committee (DMC) and trial steering committee. An administrative delay in notifying sites about the end of collection of detailed toxicity information resulted in data being collected from 868 patients. The DMC had access to summary plots of EORTC HRQoL data, EuroQol-5 Dimensions (EQ-5D) health status data and FACT/GOG-Ntx4 neuropathy data. In May 2010 (based on interim data from 1047 randomised patients), the committee recommended that the collection of HRQoL data and FACT/GOG-Ntx4 data should be continued because they were concerned that the number of missing data might undermine comparison at later time points. They also recommended that collection of FACT/GOG-Ntx4 data should be extended beyond 12 months for new patients and, where possible, for patients already participating in the study. In November 2010, the DMC recommended that the collection of these data should stop once 1800 patients had been recruited; delays in the amendment of the protocol led to patient recruitment beyond this recommendation. These extensions to data collection were made to compensate for missing data and were not based on formal power calculations.
Randomisation
The adjuvant treatment (oxaliplatin and 5FU or oxaliplatin and capecitabine) that was administered was selected on an individual-patient basis and was not randomised. Patients were randomised (1 : 1) centrally to receive either 3 months or 6 months of treatment using a minimisation algorithm incorporating a random component (80% probability of allocation to the ‘minimum’ group; 1 : 1 randomisation if no preferred group). Minimisation factors were study centre, treatment regimen, sex, disease site (colon or rectum), N stage (X, 0, 1 or 2), T stage (X, 0, 1, 2, 3 or 4) and capecitabine starting dose (from February 2010 for those receiving oxaliplatin and capecitabine). Centralised randomisation was conducted by the Cancer Research UK Clinical Trials Unit (Glasgow, UK). The computerised randomisation system allocated every patient a unique identification number and determined their treatment duration.
Initially, some participating centres were randomly allocated such that patients would be registered in the study prior to starting treatment but then be randomised after completing the first 3 months of treatment (delayed randomisation) to either receive a further 3 months of treatment or stop treatment. The remaining centres randomised patients to 3 months or 6 months of treatment prior to starting treatment. This delayed randomisation approach was discontinued because of a poorer randomisation rate [median 4.09, interquartile range (IQR) 1.29–7.09; n = 41, patients/centre/year] than in centres that randomised patients before the start of treatment (median 5.21, IQR 3.56–11.55; n = 36). 24
The study was open-label for patients, clinicians, and those conducting data analysis.
Statistical analyses
Parts of this section are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Efficacy and safety analyses
The efficacy analyses of DFS and overall survival included, as far as possible, all randomly assigned patients [the intention-to-treat (ITT) population] and were plotted using Kaplan–Meier techniques. Analysis of treatment delivery and safety was based on patients who started the study treatment. The analysis time was prespecified in the study protocol. Statistical analyses used SPSS version 22 (IBM Corporation, Armonk, NY, USA) and R version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria). The data cut-off point for this analysis was 1 December 2016.
Comparison of disease-free and overall survival between treatment groups was based on a Cox regression model incorporating minimisation factors as covariates; this approach was also used to derive the HR and associated 95% confidence intervals (CIs). The p-value for testing the null hypothesis, that the HR comparing 3 months with 6 months of adjuvant treatment was ≥ 1.13, was derived from this model by comparing the log-likelihood of the fitted model with the log-likelihood of a model where the HR between groups was set to 1.13 using a likelihood-ratio test. The proportional hazards assumption implicit in these analyses was examined graphically using a log-minus log plot of survival function against log time and using a test of the interaction between treatment group and time (logged) obtained from a Cox model incorporating an appropriate time-varying covariate.
The components of the forest plot (estimated hazard for the comparison between groups and associated 95% CI) were derived from a Cox model that included separate terms for the effect of duration in each category of the relevant stratification factor or other factors being examined. The p-value for heterogeneity was derived from comparison of the log-likelihoods of a model with separate terms for the effect of duration in each category compared with the model with a single overall term. The aim of this analysis was to establish whether or not the impact of treatment duration varied across important patient subgroups.
Multiple imputation analysis25 was used to fill in missing data for questionnaires in the HRQoL and neuropathy scales. Five multiple imputation sets were produced for each HRQoL or neuropathy scale and the area under the curve (AUC)26 was calculated with imputed data, as prespecified in the statistical analysis plan. The AUC was then adjusted by dividing by the follow-up period and subtracting the baseline value for each patient to produce a standardised-adjusted AUC. The standardised-adjusted AUC was calculated for the five imputed data sets and compared between the randomised treatment groups via a generalised linear model (with treatment group as an independent factor and study minimisation factors as covariates). The test statistics associated with treatment group from each of the five imputations were finally combined to provide an overall p-value that took into account the extent of missing data. To allow for the number of scales being examined, an adjustment for multiple comparisons (separately for the EORTC and the EQ-5D questionnaires) was made using the sharpened Hochberg procedure;27 the p-value threshold for statistical significance was 5% after adjustment. Comparison of these scales at individual time points also made use of multiple imputation and generalised linear models.
The Mann–Whitney U-test was used to compare ordered categorical variables for toxicity grade. Fisher’s exact test was used to compare the incidence of grade 3–5 toxicity and logistic regression was used to estimate the odds ratio and associated CI for the incidence of grade 3–5 toxicity.
Study data were reviewed by the DMC approximately once a year to assess safety and efficacy issues from an ethics viewpoint. Conditional power methods28 were used to aid the committee in reaching decisions about study continuation, but no formal stopping rules were set. The conditional power for DFS was presented at the fifth (June 2012), sixth (January 2013) and seventh (October 2013) meetings of the DMC; the DMC requested the analysis because of apparent differences in DFS curves. The results were discussed by the DMC in the context of the limited follow-up of patients and available survival data. The DMC concluded that no action was required.
Changes to the study protocol
The approach of randomising patients to a treatment group was changed after the trial had been running for 1 year; after this time all patients were randomised as they started adjuvant therapy (rather than some being randomised after 3 months of adjuvant chemotherapy), as this approach proved to have a higher randomisation rate and a lower dropout rate.
From March 2012, the requirement for the neuropathy questionnaire to be completed was extended to follow-up visits at 18 and 24 months; from December 2012 the neuropathy questionnaire was to be completed at follow-up visits to a maximum of 8 years. These extensions to data collection were made to compensate for missing data and to monitor long-term change in neuropathy.
The planned duration of patient follow-up for the primary analysis was extended so that (1) patients with stage III disease were to be followed up until the end November 2014 or for a minimum of 3 years (if they did not have ≥ 3 years of follow-up at the end of November 2014) and (2) all patients with stage II disease were to be followed up until the end of November 2016. Follow-up was extended to increase the number of events for the primary study analysis.
Chapter 3 Results
Participant recruitment and flow
The data cut-off point for the analyses presented here was 1 December 2016, at which time patients in both treatment groups had undergone median follow-up of 37 months (IQR 36–49 months, as calculated using the reverse Kaplan–Meier approach). A total of 88% of patients were followed up for a minimum of 3 years, which allowed for a 2-month window around the follow-up time. A total of 787 patients had died by the time of analysis. Study patients are still undergoing follow-up to support further DFS and overall survival analyses.
A total of 6088 patients were randomised to the trial between 27 March 2008 and 29 November 2013 from 244 centres: 5244 patients were randomised at 164 study centres in the UK, 311 patients at 10 centres in Denmark, 237 patients at 19 centres in Spain, 197 patients at 32 centres in Australia, 83 patients at 14 centres in Sweden, and 16 patients at five centres in New Zealand. The study did not meet its target of 9500 patients as a result of slow recruitment; despite extending the planned enrolment period by 6 months, the target was not reached and recruitment was stopped to allow adequate follow-up of ongoing randomised patients within the budget for the trial. This allowed the minimum follow-up period to be extended to 3 years. A total of 1482 DFS events were observed, which gave the study 66% power rather than the planned 90% power for rejecting the null hypothesis.
A total of 6065 patients were included in the ITT analysis population, 6022 of whom started study treatment. Treatment safety/toxicity was assessed after each chemotherapy cycle for 868 patients. HRQoL was assessed using EORTC QLQ-C30 and CR29 (n = 1829) and EQ-5D-3L (n = 1828) and neuropathy were assessed using FACT/GOG-Ntx4 (n = 2871).
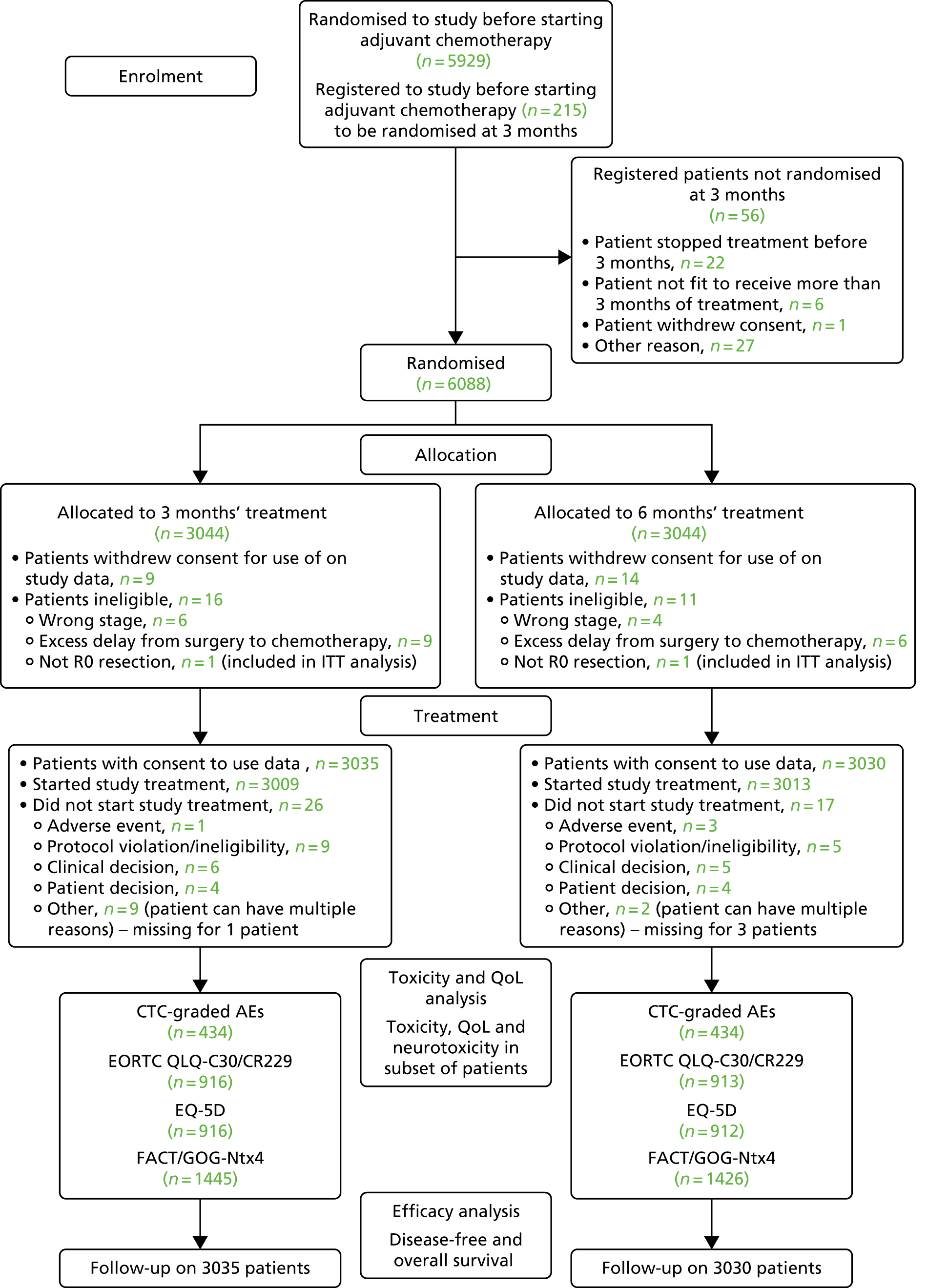
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 1) shows patients who entered and progressed through the trial and provided data for the different assessment parameters. Of the 6088 patients entering the trial, 3044 were randomised into each treatment group (3-month and 6-month duration). Of these 3044 patients, 3035 (99.7% of 3044 randomised to this group) in the 3-month treatment group were included in the ITT analyses, with 3009 patients receiving the study drug, and 3030 (99.5% of 3044 randomised to this group) in the 6-month treatment group were included in the ITT analyses, with 3013 patients receiving the study drug. The reasons patients were not included in the ITT analysis and did not start study treatment are also detailed in Figure 1.
FIGURE 1.
The CONSORT flow diagram of patient progression through the SCOT trial. Reproduced from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Baseline data
Baseline data (recorded at the time of randomisation) identified approximately 60% of patients as male and 40% as female, with the median age being 65 years. Most patients (> 80%) had a diagnosis of colon cancer and approximately 80% had stage III disease. For about 67% of patients, the planned treatment was oxaliplatin and capecitabine, with the remaining 33% planned to receive oxaliplatin and 5FU. Baseline characteristics were comparable for the 3-month and 6-month treatment groups for the overall patient population (Table 1) and for the other analysis sets considering toxicity and HRQoL (see Appendix 1).
| Characteristic | Randomised treatment group | |
|---|---|---|
| 3 months of treatment (N = 3044) | 6 months of treatment (N = 3044) | |
| Gender, n (%) | ||
| Female | 1201 (39.5) | 1200 (39.4) |
| Male | 1843 (60.5) | 1844 (60.6) |
| Total | 3044 (100.0) | 3044 (100.0) |
| Age (years) | ||
| Median | 65 | 65 |
| IQR | 58–70 | 58–70 |
| Range | 23–84 | 20–85 |
| Total | 3044 | 3044 |
| Performance status at randomisation, n (%) | ||
| 0 | 2190 (71.9) | 2144 (70.4) |
| 1 | 854 (28.1) | 900 (29.6) |
| Total | 3044 (100.0) | 3044 (100.0) |
| Disease site, n (%) | ||
| Colon | 2492 (81.9) | 2495 (82.0) |
| Rectum | 552 (18.1) | 549 (18.0) |
| Total | 3044 (100.0) | 3044 (100.0) |
| T stage, n (%) | ||
| 0 | 1 (0.0) | 3 (0.1) |
| 1 | 92 (3.0) | 95 (3.1) |
| 2 | 284 (9.3) | 283 (9.3) |
| 3 | 1749 (57.5) | 1748 (57.4) |
| 4 | 917 (30.1) | 915 (30.1) |
| X | 1(0.0) | 0 (0.0) |
| Total | 3044 (100.0) | 3044 (100.0) |
| N stage, n (%) | ||
| 0 | 559 (18.4) | 557 (18.3) |
| 1 | 1731 (56.9) | 1732 (56.9) |
| 2 | 754 (24.8) | 755 (24.8) |
| Total | 3044 (100.0) | 3044 (100.0) |
| Planned treatment, n (%) | ||
| FOLFOX | 993 (32.6) | 988 (32.5) |
| CAPOX | 2051 (67.4) | 2056 (67.5) |
| Total | 3044 (100.0) | 3044 (100.0) |
| If CAPOX planned, starting dose of capecitabine, n (%) | ||
| 750 mg/m2 | 348 (19.5) | 349 (19.4) |
| 800 mg/m2 | 72 (4.0) | 78 (4.3) |
| 1000 mg/m2 | 1369 (76.5) | 1370 (76.2) |
| Total | 1789 (100.0) | 1797 (100.0) |
| High-risk stage II, n (%) | ||
| No | 2493 (81.9) | 2499 (82.1) |
| Yes | 551 (18.1) | 545 (17.9) |
| Total | 3044 (100.0) | 3044 (100.0) |
| Randomisation time point, n (%) | ||
| Baseline | 2964 (97.4) | 2965 (97.4) |
| 3 months | 80 (2.6) | 79 (2.6) |
| Total | 3044 (100.0) | 3044 (100.0) |
Exposure to study medication
The duration of adjuvant chemotherapy, based on the number of treatment cycles delivered, is presented in Table 2; for the purpose of this analysis, one cycle of oxaliplatin and 5FU equated to 2 weeks of treatment and one cycle of oxaliplatin and capecitabine equated to 3 weeks of treatment. Overall, 83.3% of patients randomised to the 3-month treatment group received 3 months of treatment: the frequency was slightly higher for those receiving oxaliplatin and 5FU (86.2% of patients vs. 81.9% of those receiving oxaliplatin and capecitabine). Overall, 58.8% of those randomised to the 6-month treatment group received 6 months of treatment, with the proportion being similar for those receiving oxaliplatin and 5FU (59.5%) and for those receiving oxaliplatin and capecitabine (58.5%); 6.9% of patients randomised to 6 months of treatment stopped treatment at 3 months. A total of 13.8% of patients stopped treatment before 3 months, with the proportion being similar for those randomised to receive 3 months of treatment (14.1%) and for those randomised to receive 6 months of treatment (13.8%).
| Treatment | Duration of treatment (based on the number of cycles) (weeks) | 3-month treatment (N = 3044), n (%) | 6-month treatment (N = 3044), n (%) |
|---|---|---|---|
| FOLFOX | < 12 | 116 (11.8) | 107 (10.9) |
| 12 | 845 (86.2) | 55 (5.6) | |
| > 12, ≤ 16 | 18 (1.8) | 64 (6.5) | |
| > 16, ≤ 20 | 0 (0.0) | 88 (8.9) | |
| > 20, ≤ 24 | 1 (0.1) | 74 (7.5) | |
| 24 | 0 (0.0) | 586 (59.5) | |
| > 24 | 0 (0.0) | 11 (1.1) | |
| Total | 980 (100.0) | 985 (100.0) | |
| CAPOX | < 12 | 310 (15.2) | 310 (15.3) |
| 12 | 1675 (81.9) | 154 (7.6) | |
| > 12, ≤ 16 | 59 (2.9) | 75 (3.7) | |
| > 16, ≤ 20 | 0 (0.0) | 124 (6.1) | |
| > 20, ≤ 24 | 0 (0.0) | 141 (7.0) | |
| 24 | 0 (0.0) | 1187 (58.5) | |
| > 24 | 0 (0.0) | 37 (1.8) | |
| Total | 2044 (100.0) | 2028 (100.0) | |
| All patients | < 12 | 426 (14.1) | 417 (13.8) |
| 12 | 2520 (83.3) | 209 (6.9) | |
| > 12, ≤ 16 | 77 (2.5) | 139 (4.6) | |
| > 16, ≤ 20 | 0 (0.0) | 212 (7.0) | |
| > 20, ≤ 24 | 1 (0.0) | 215 (7.1) | |
| 24 | 0 (0.0) | 1773 (58.8) | |
| > 24 | 0 (0.0) | 48 (1.6) | |
| Total | 3024a (100.0) | 3013b (100.0) |
The overall median treatment duration based on actual start and end dates was 11.3 weeks (IQR 10.1–12.6 weeks) for the 3-month treatment group and 23.1 weeks (IQR 17.0–25.3 weeks) for the 6-month treatment group. A higher proportion of patients receiving oxaliplatin and 5FU had at least one delayed cycle in both the 3-month (65.0% vs. 45.2% with oxaliplatin and capecitabine) and the 6-month (83.0% vs. 66.3%) treatment groups.
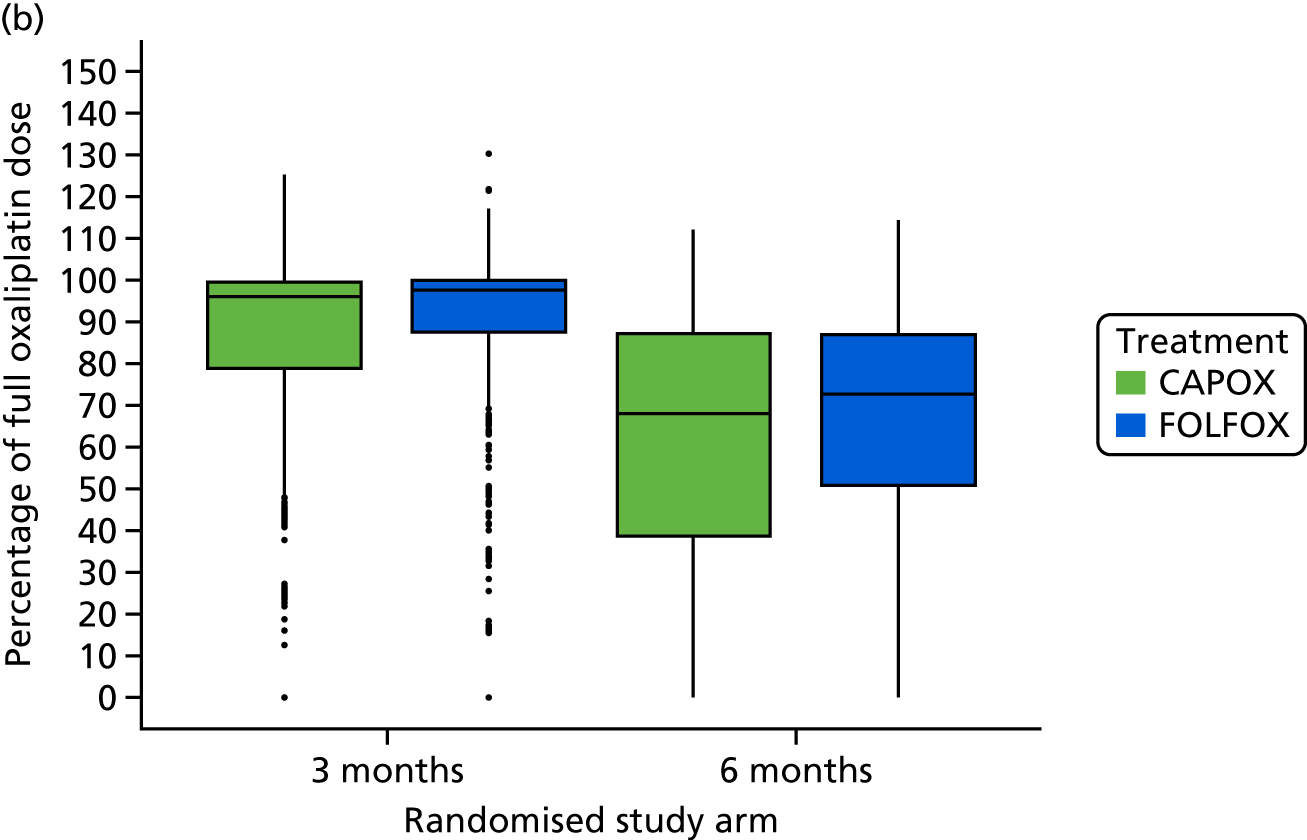
The median percentage of the full fluoropyrimidine dose delivered was 95.3% (IQR 83.1–99.8%) in the 3-month and 83.2% (IQR 56.7–95.7%) in the 6-month treatment groups. The median percentage of the full oxaliplatin dose delivered was 96.6% (IQR 82.3% to 99.7%) in the 3-month and 70.2% (IQR 44.3% to 87.1%) in the 6-month treatment groups. These values were similar irrespective of the fluoropyrimidine backbone (Figure 2).
FIGURE 2.
Treatment delivery by treatment group and adjuvant regimen. (a) Treatment duration; (b) percentage of intended oxaliplatin dose; and (c) percentage of intended 5FU/capecitabine dose. Boxes show median and IQR; whiskers show range; dots represent outliers. FOLFOX, oxaliplatin and 5FU. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).


A total of 788 patients (26.2%) underwent 5FU or capecitabine dose reduction in the 3-month treatment group compared with 1286 patients (42.7%) in the 6-month treatment group; 906 patients (30.1%) underwent oxaliplatin dose reduction in the 3-month treatment group compared with 1869 patients (62.0%) in the 6-month treatment group.
As patients receiving oxaliplatin and 5FU require insertion of a line, this could potentially delay the start of their treatment. However, the difference in mean time from surgery to the start of adjuvant chemotherapy was negligible when comparing patients treated with oxaliplatin and 5FU [mean 58 days, standard deviation (SD) 16 days)] and those treated with oxaliplatin and capecitabine (mean 56 days, SD 14 days).
Comparison of efficacy for 3-month versus 6-month adjuvant chemotherapy
Disease-free survival
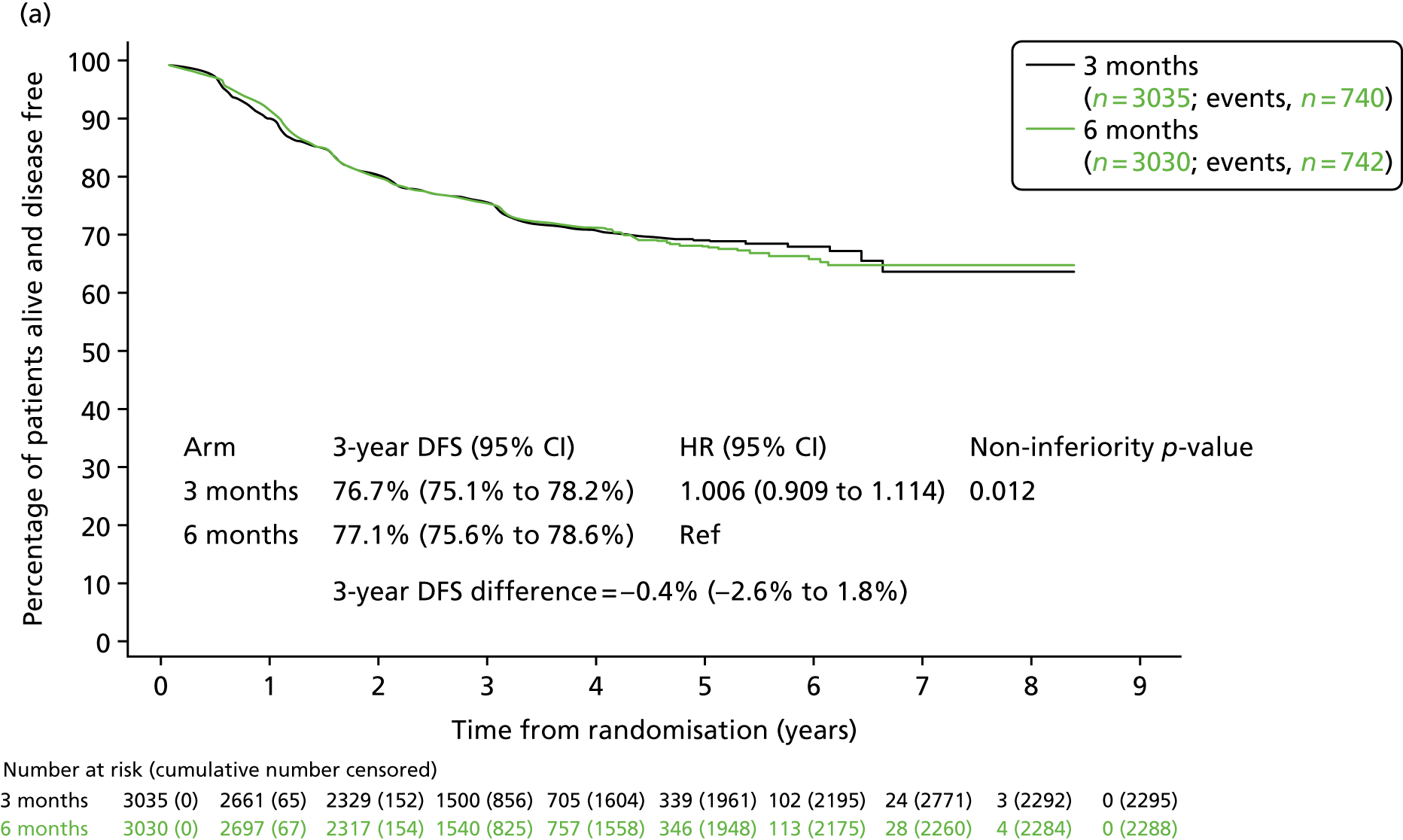
By the time of analysis there had been 1482 DFS events (740 in the 3-month treatment group and 742 in the 6-month treatment group). In the 3-month treatment group, 658 patients (21.7%) experienced disease recurrence, 71 patients (2.1%) died without disease recurrence or a new primary colorectal lesion, and 11 patients (0.4%) had a new primary colorectal cancer lesion; in the 6-month treatment group, 654 patients (21.6%) experienced disease recurrence, 76 patients (2.6%) died without disease recurrence or new primary colorectal lesion, and 12 patients (0.4%) had a new primary colorectal cancer lesion. The 3-year DFS rate in the 3-month treatment group was 76.7% [standard error (SE) 0.8%] and in the 6-month treatment group was 77.1% (SE 0.8%); this equated to a HR of 1.006 [95% CI 0.909 to 1.114; p-value for non-inferiority (pNI) = 0.012] and therefore met the criteria confirming non-inferiority for 3-month compared with 6-month adjuvant chemotherapy (Figure 3).
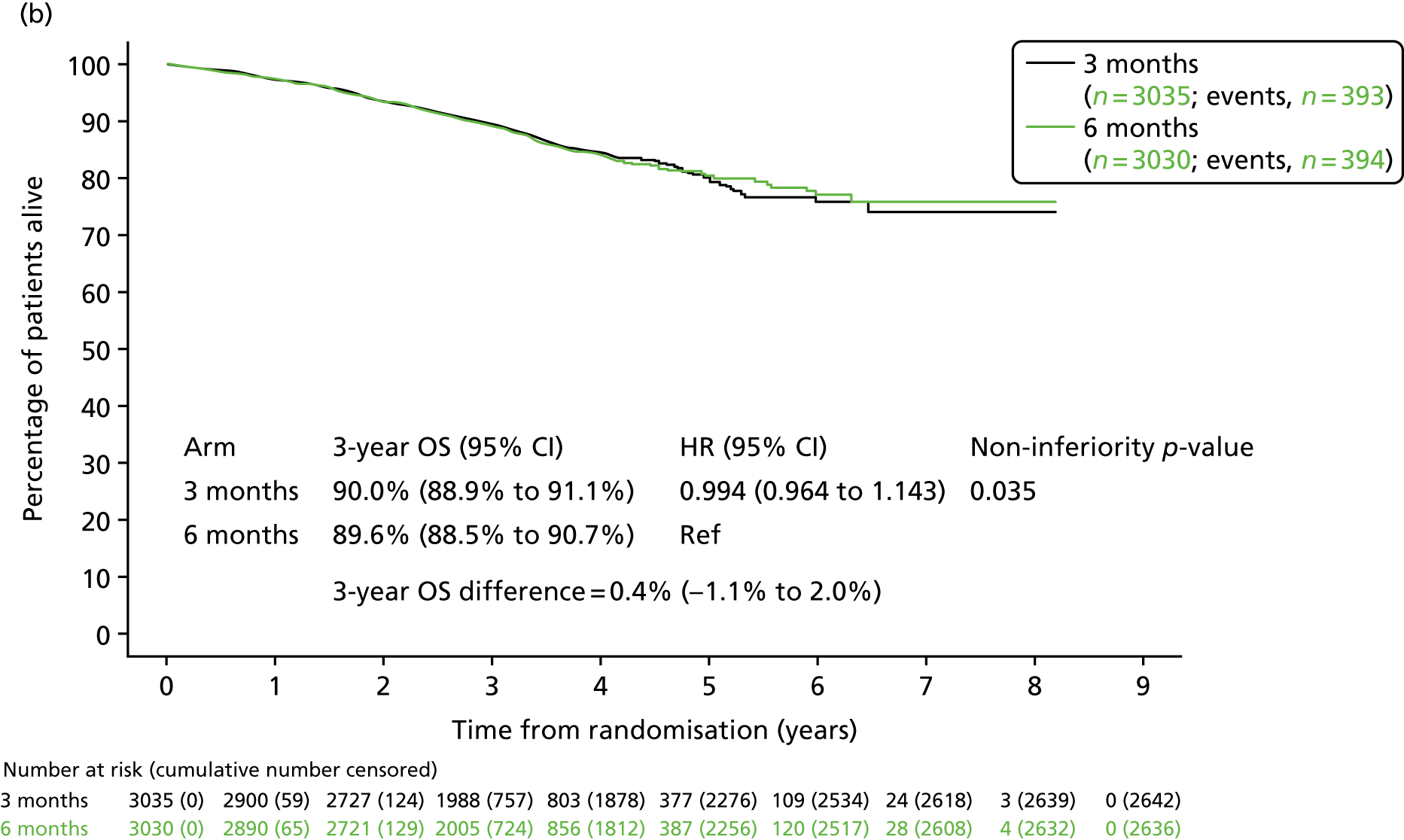
FIGURE 3.
(a) Disease-free survival and (b) overall survival, by treatment group. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Sensitivity analysis considered the difference between treatment groups based on the actual duration of treatment (Figure 4). For eligible patient who received 3 months (2513 patients; see Table 2) or 6 months (1771 patients; see Table 2) of adjuvant chemotherapy, the observed HR was 1.158 (95% CI 1.018, 1.317; p = 0.641) with non-inferiority not confirmed in any of these smaller, non-ITT populations.
FIGURE 4.
Plot of 3-month/6-month HR by actual treatment duration (analysis according to duration restricted to eligible patients who started treatment) with associated non-inferiority p-values. Reproduced from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).

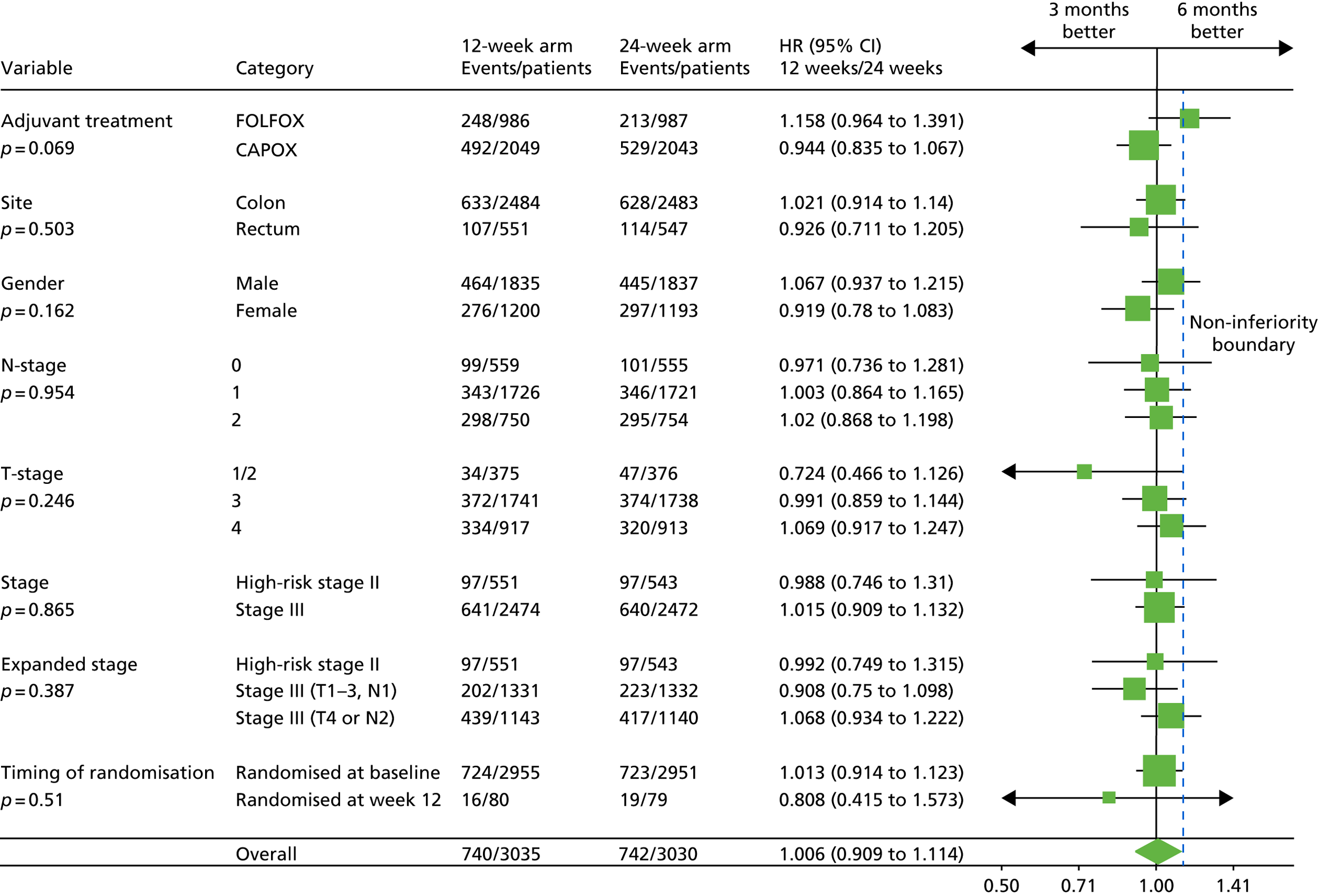
Heterogeneity of the 3-month versus 6-month effect was assessed for the stratification factors used for randomisation and for the randomisation time-point. The resulting HRs, 95% CIs and p-values testing the heterogeneity of the treatment-duration effect for these subgroups are presented in Figure 5. The adjuvant treatment regimen selected at randomisation was associated with a trend towards heterogeneity in effect (p = 0.069).
FIGURE 5.
Disease-free survival and heterogeneity in subgroups by minimisation variables. Categories are listed as recorded at randomisation; 10 patients in the 3-month treatment group and 15 patients in the 6-month treatment group could not be allocated to high-risk stage II or stage III based on T/N data recorded at randomisation. CAPOX, oxaliplatin and capecitabine; FOLFOX, oxaliplatin and 5FU. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
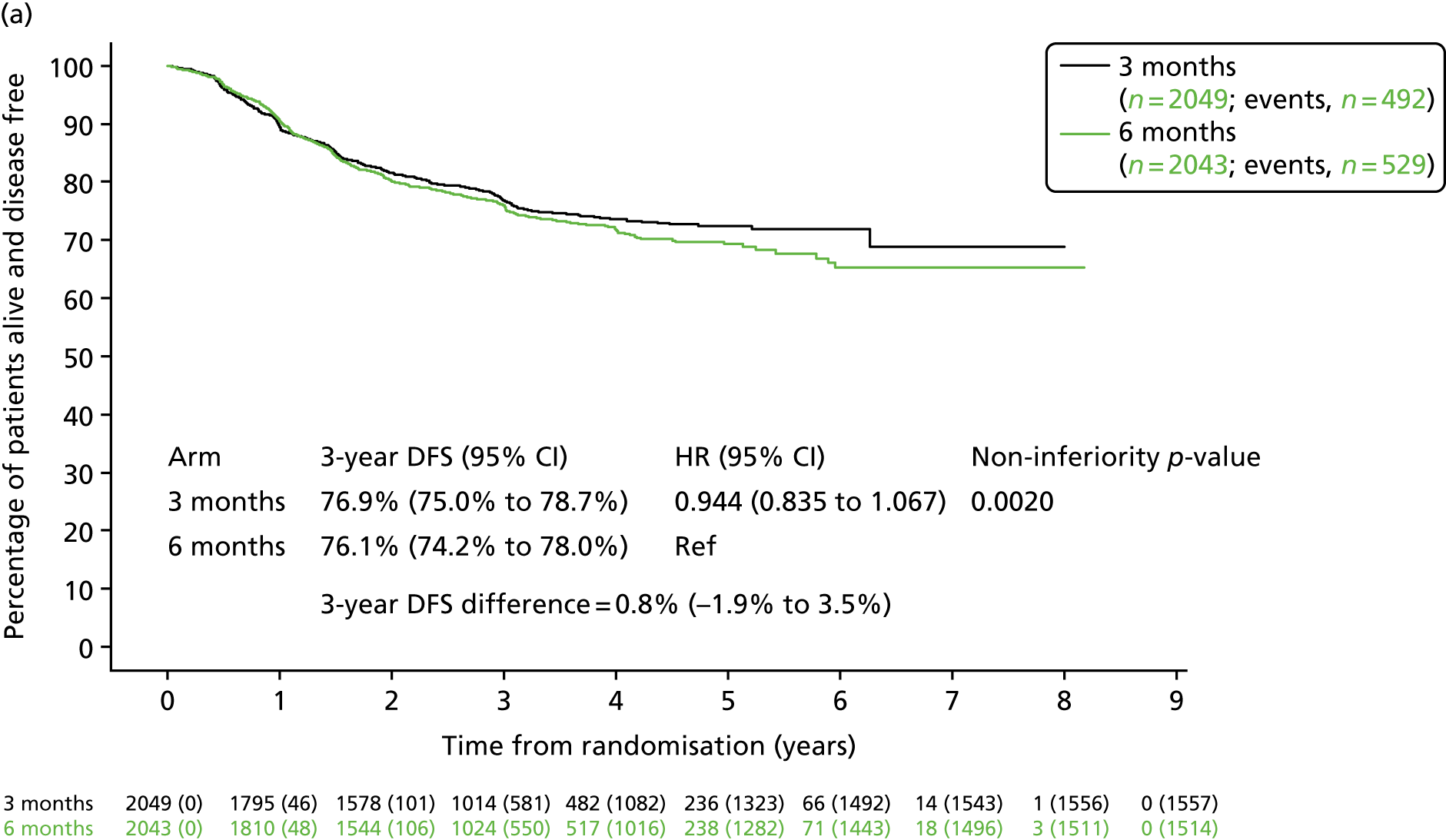
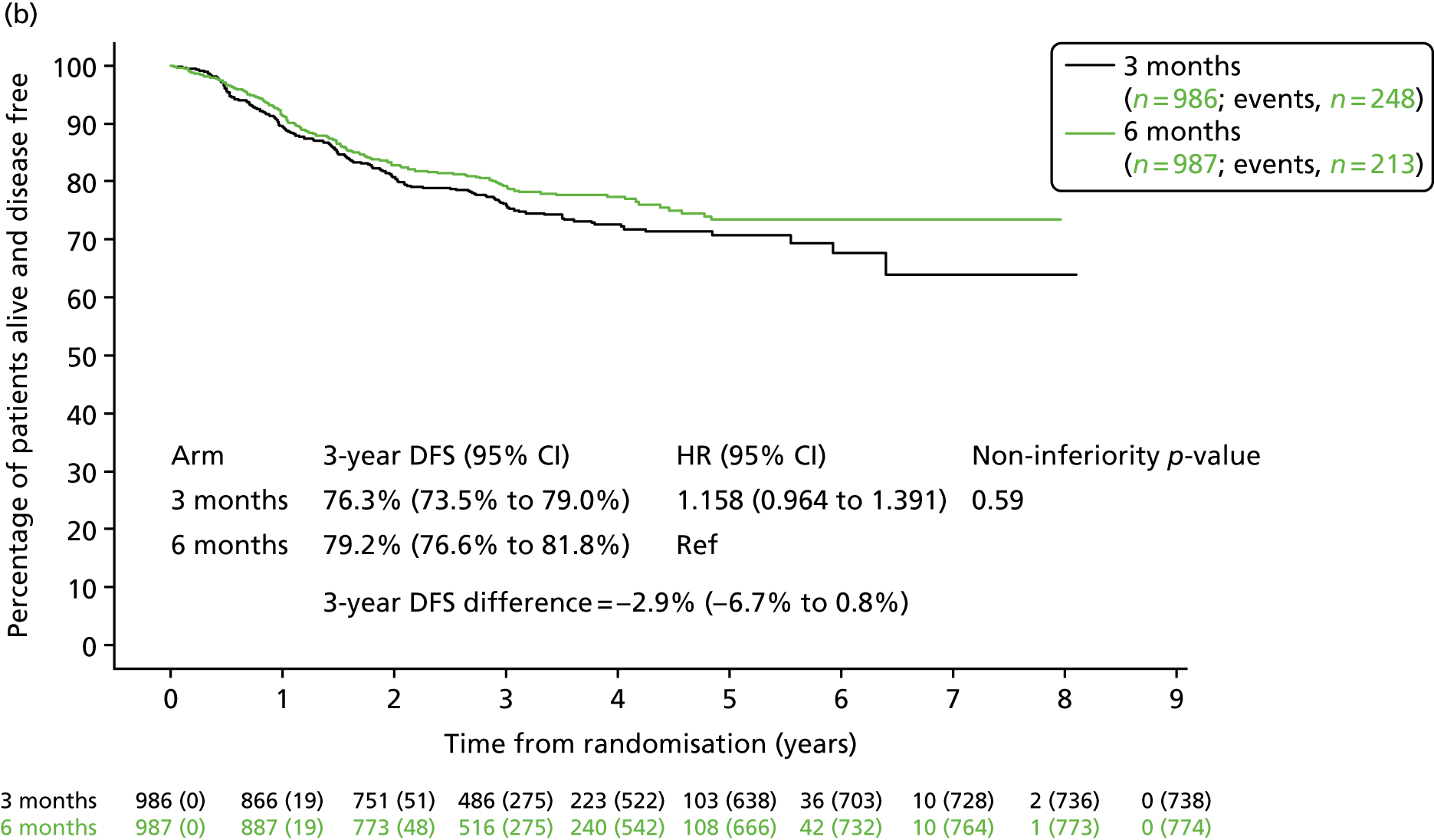
Further (post hoc) analysis of DFS for patients receiving 3-month or 6-month duration adjuvant chemotherapy was conducted separately for patients who received oxaliplatin and 5FU and for patients who received oxaliplatin and capecitabine (Figure 6). For patients receiving oxaliplatin and capecitabine, the 3-year DFS rate for the 3-month treatment group was 76.9% (SE 1.0%) and for the 6-month treatment group was 76.1% (SE 1.0%), resulting in a HR of 0.944 (95% CI 0.835 to 1.067; pNI = 0.002), which met the criteria for non-inferiority. However, for patients receiving oxaliplatin and 5FU, the 3-year DFS for the 3-month treatment group was 76.3% (SE 1.4%) and for the 6-month treatment group was 79.2% (SE 1.3%), resulting in a HR of 1.158 (95% CI 0.964 to 1.391; pNI = 0.591), which did not meet the non-inferiority criteria.
FIGURE 6.
Disease-free survival by treatment group and adjuvant chemotherapy regimen. (a) CAPOX; and (b) FOLFOX. CAPOX, oxaliplatin and capecitabine; FOLFOX, oxaliplatin and 5FU. Adapted with minor corrections from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
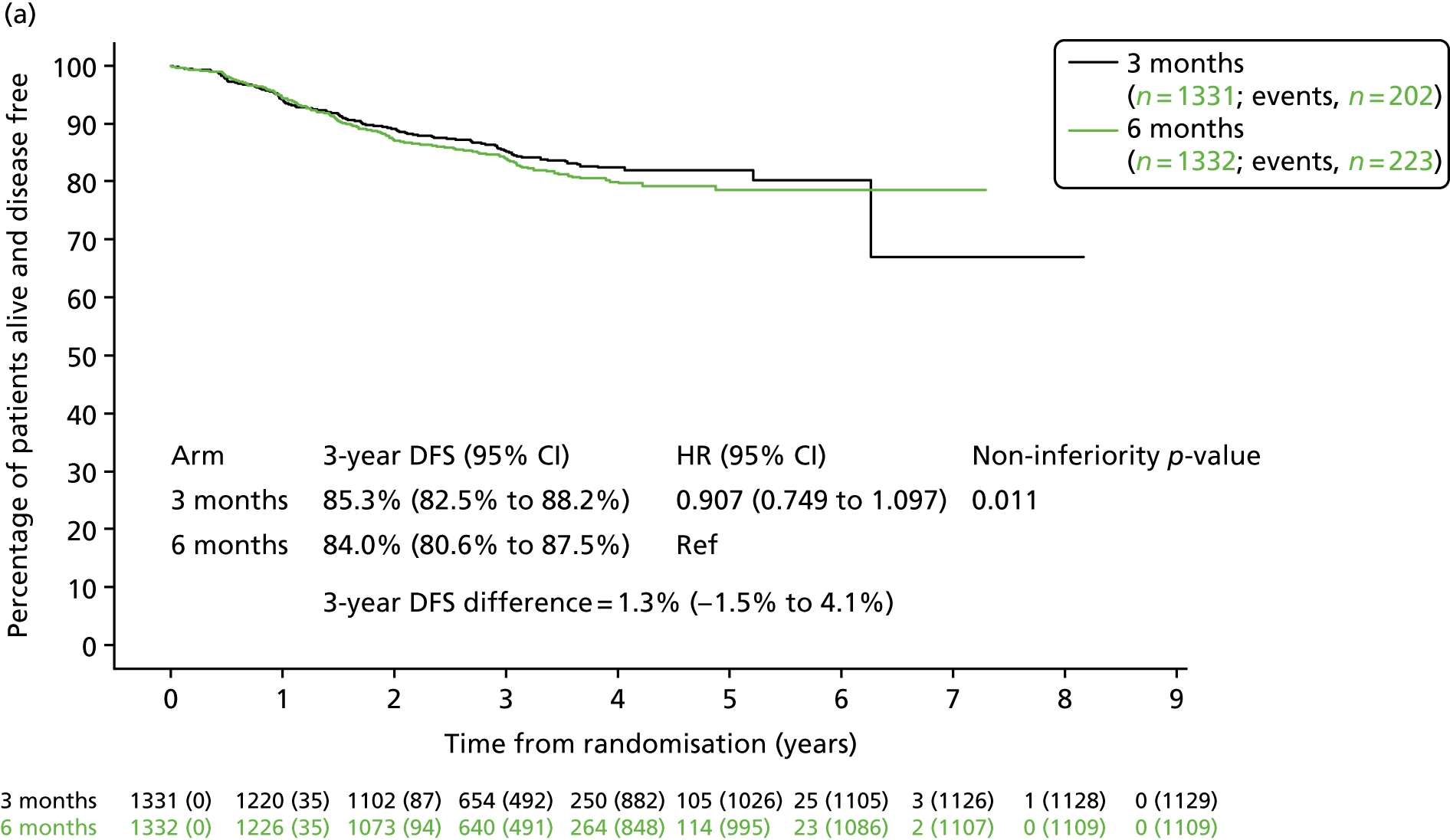
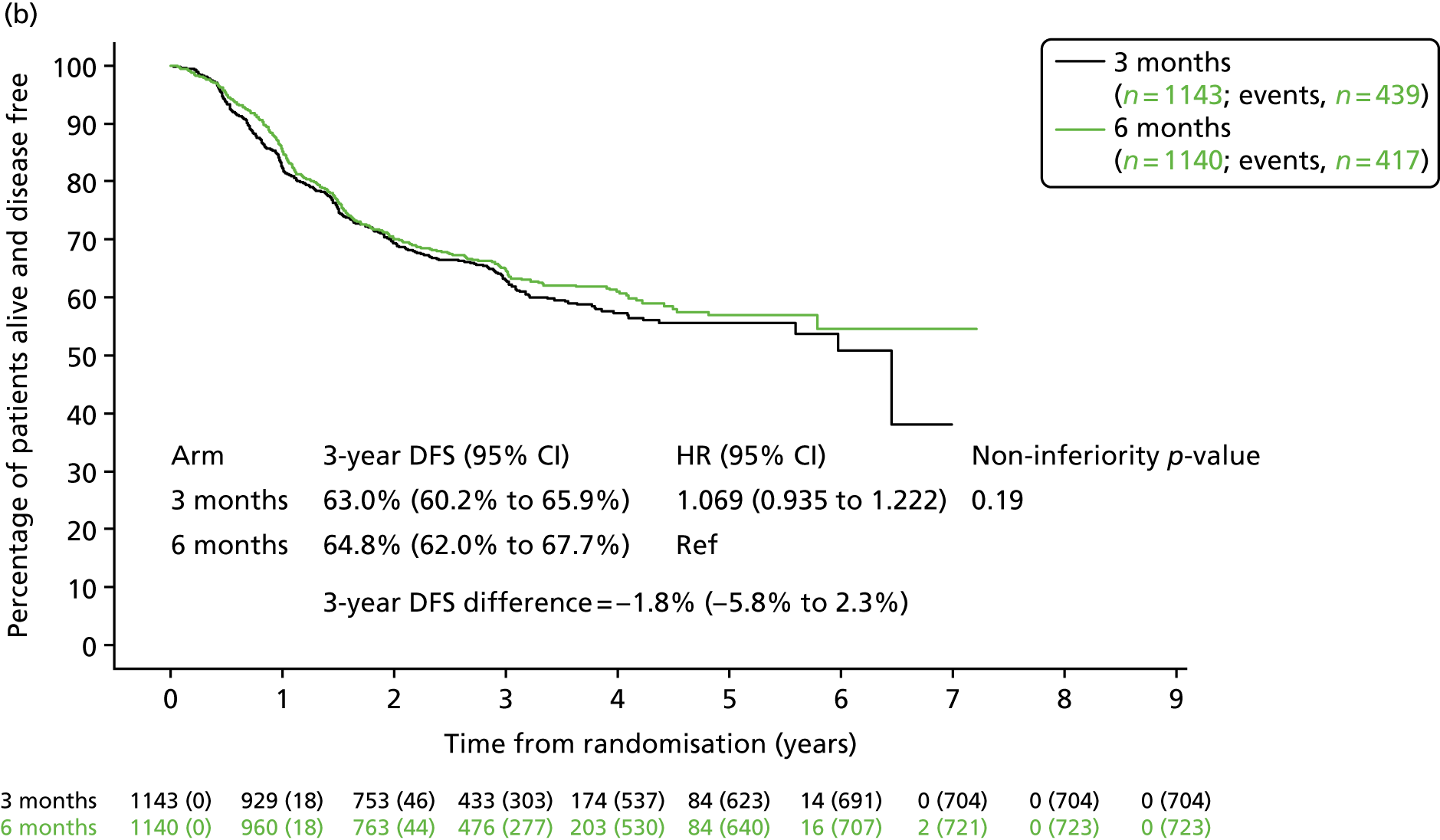
Previous clinical trials in patients with colorectal cancer have shown a marked difference in the risk of relapse between patients with T1–3, N1 disease and those with T4 or N2 disease. 29 Post hoc analyses were therefore conducted to assess whether or not disease stage affected DFS rate for patients with differing adjuvant treatment durations. Kaplan–Meier curves (Figure 7) showed that 3-year DFS for patients with T1–3, N1 primary disease in the 3-month treatment group reached 85.3% (SE 1.0%) and for the 6-month treatment group reached 84.0% (SE 1.0%), giving a HR of 0.907 (95% CI 0.749 to 1.097; pNI = 0.011). For patients with T1–3, N1 disease non-inferiority was, therefore, demonstrated when comparing 3-month with 6-month chemotherapy. For stage III colorectal cancer patients with T4 and/or N2 pathology, 3-year DFS for the 3-month treatment group was 63.0% (SE 1.5%) and for the 6-month treatment group was 64.8% (SE 1.4%), giving a HR of 1.069 (95% CI 0.935 to 1.222; pNI = 0.19), which did not meet the non-inferiority criteria.
FIGURE 7.
Disease-free survival by treatment group and disease stage. (a) T1–3 or N1; and (b) T4 or N2. Adapted with minor corrections from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
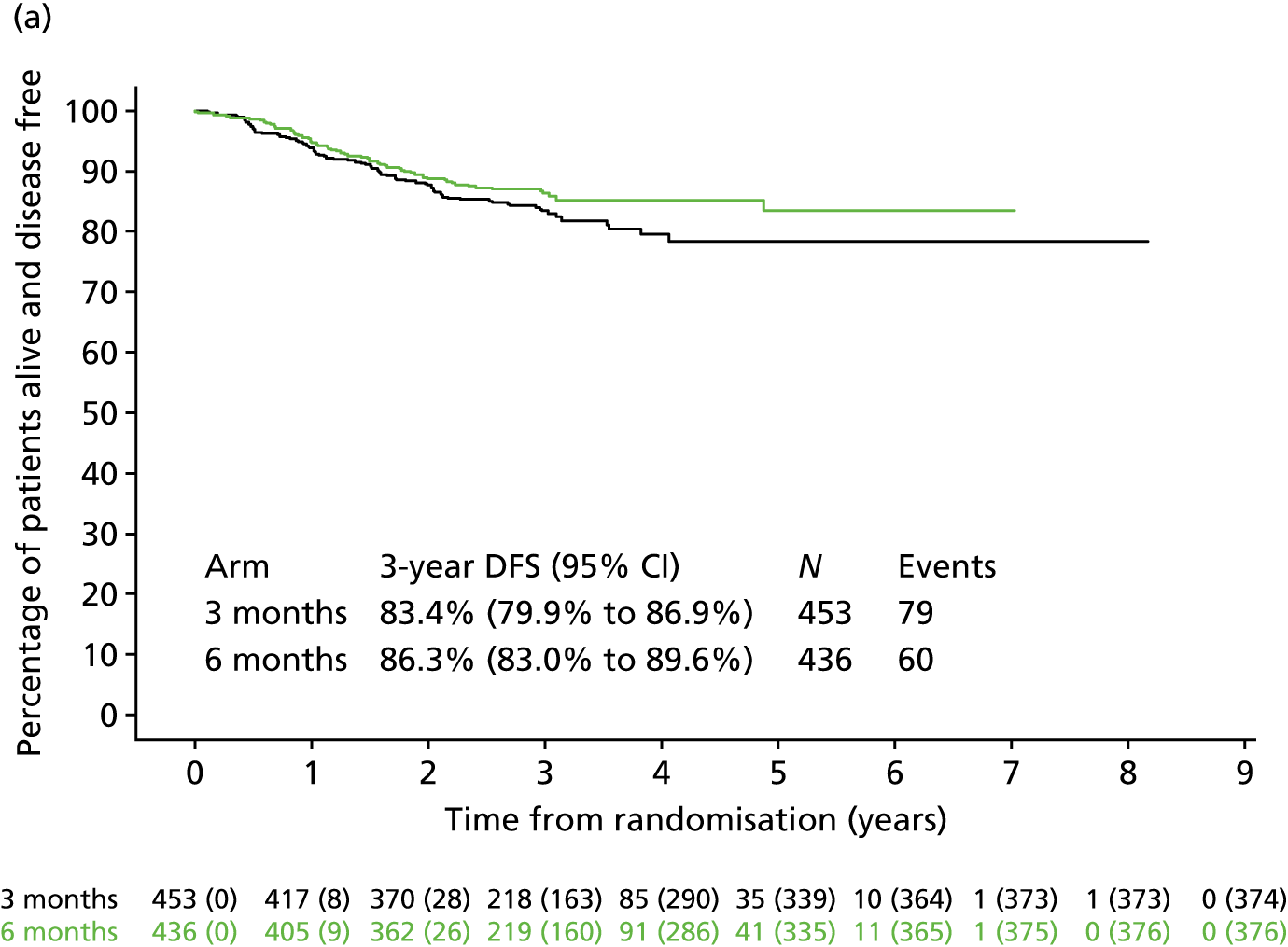
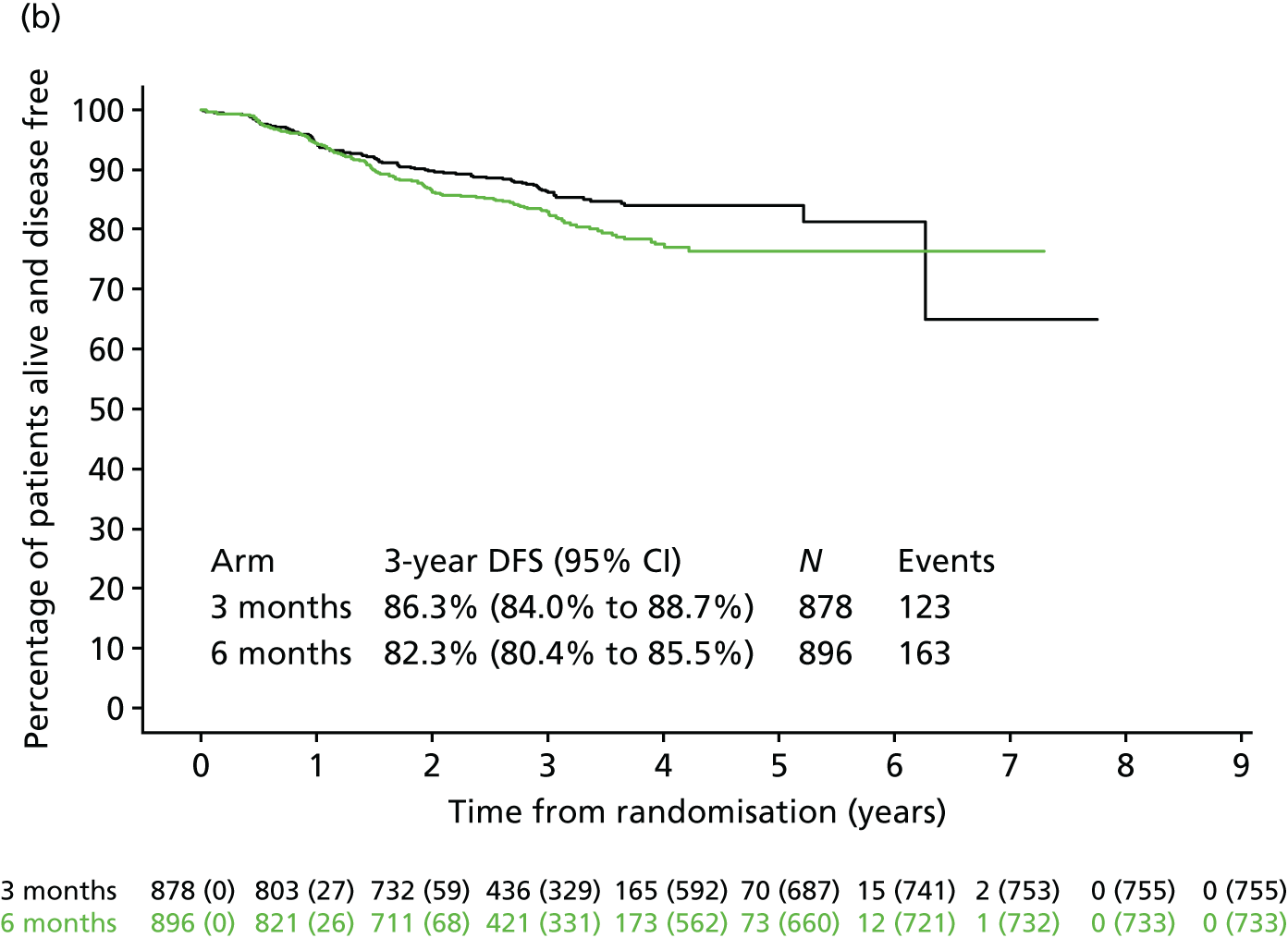
Analysis of these prognostic groups according to the chemotherapy regimen they received (Figure 8) showed that Kaplan–Meier DFS curves suggest that 3 months of treatment with oxaliplatin and oxaliplatin (CAPOX) may be adequate both for T1–3, N1 and for T4 and/or N2 patients (most reliably for T1–3, N1), but this is not as clear for oxaliplatin and 5FU (FOLFOX), particularly for T4 and/or N2.
FIGURE 8.
Disease-free survival by treatment group, disease stage, and adjuvant chemotherapy regimen. (a) Low-risk stage III (T1–3, N1)/FOLFOX; (b) low-risk stage III (T1–3, N1)/CAPOX; (c) high-risk stage III (T4 or N2)/FOLFOX; and (d) high-risk stage III (T4 or N2)/CAPOX. CAPOX, oxaliplatin and capecitabine; FOLFOX, oxaliplatin and 5FU. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).


Note that although curves in some of the subgroups in Figures 7 and 8 cross, there is no suggestion in any case that the proportional-hazards assumption is violated (minimum p-value testing for non-proportionality, p = 0.226).
Overall survival
By the time of analysis, 787 participants in the 3-month treatment group had died [393 (12.9% of 3035 in the efficacy analysis) and 394 participants in the 6-month treatment group had died (13.0% of the 3030 in the efficacy analysis). The 3-year overall survival rate for the 3-month treatment group was 90.0% (SE 0.6%) and for the 6-month treatment group was 89.6% (SE 0.6%), equating to a HR of 0.994 (95% CI 0.964 to 1.143; pNI = 0.035; see Figure 3b). Sixteen deaths in each treatment group were considered related to study medication.
Safety
The occurrence of AEs was analysed for a total of 868 patients, comprising 434 patients in each treatment group. Table 3 shows the maximum NCI CTCAE grade recorded per patient for AEs with an incidence of ≥ 10% in either treatment group. Most of these events (alopecia, anaemia, anorexia, diarrhoea, fatigue, hand–foot syndrome, mucositis, sensory neuropathy, neutropenia, pain, rash, altered taste, thrombocytopenia and watery eye) showed a statistically significant increase in grade for the 6-month treatment group compared with the 3-month treatment group.
| Adverse event | Randomised treatment group, n (%) | p-value (comparison of ordered categories) | p-value (comparison of percentage of grade 3/4/5 adverse events) | Odds ratio for incidence of grade 3/4/5 toxicities (6 months/3 months) from logistic regression (CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months of treatment (N = 434) | 6 months of treatment (N = 434) | |||||||||||||
| 0 | 1–2 | 3 | 4 | Missing | 0 | 1–2 | 3 | 4 | 5 | Missing | ||||
| Alopecia | 345 (83.3) | 69 (16.7) | 0 (0.0) | 0 (0.0) | 20 (–) | 309 (76.1) | 97 (23.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 28 (–) | 0.0094 | – | Not estimable |
| Anaemia | 270 (64.7) | 143 (34.3) | 2 (0.5) | 2 (0.5) | 17 (–) | 212 (52.2) | 190 (46.8) | 3 (0.7) | 1 (0.2) | 0 (0.0) | 28 (–) | 0.00013 | 1.00 | 1.027 (0.255–0.4.136) |
| Anorexia | 312 (75.9) | 92 (22.4) | 7 (1.7) | 0 (0.0) | 23 (–) | 262 (64.7) | 140 (34.6) | 3 (0.7) | 0 (0.0) | 0 (0.0) | 29 (–) | 0.00043 | 0.34 | 0.431 (0.111–1.677) |
| Constipation | 289 (69.8) | 122 (29.5) | 2 (0.5) | 1 (0.2) | 20 (–) | 268 (66.0) | 135 (33.3) | 3 (0.7) | 0 (0.0) | 0 (0.0) | 28 (–) | 0.28 | 1.00 | 1.010 (0.205–5.083) |
| Diarrhoea | 128 (30.7) | 243 (58.3) | 44 (10.6) | 2 (0.5) | 17 (–) | 99 (24.4) | 241 (59.4) | 63 (15.5) | 2 (0.5) | 1 (0.2) | 28 (–) | 0.0079 | 0.033 | 1.566 (1.045–2.345) |
| Fatigue | 58 (14.0) | 320 (77.1) | 35 (8.4) | 2 (0.5) | 19 (–) | 41 (10.1) | 333 (82.0) | 32 (7.9) | 0 (0.0) | 0 (0.0) | 28 (–) | 0.022 | 0.62 | 0.874 (0.533–1.433) |
| Hand–foot syndrome | 277 (66.9) | 129 (31.2) | 8 (1.9) | 0 (0.0) | 20 (–) | 218 (53.7) | 169 (41.6) | 18 (4.4) | 1 (0.2) | 0 (0.0) | 28 (–) | <0.0001 | 0.031 | 2.492 (1.078–5.758) |
| Mucositis (clinical exam) | 355 (86.0) | 56 (13.6) | 2 (0.5) | 0 (0.0) | 21 (–) | 320 (79.0) | 83 (20.5) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 29 (–) | 0.013 | 1.00 | 1.020 (0.143–7.275) |
| Mucositis (functional/symptomatic) | 283 (68.4) | 127 (30.7) | 4 (1.0) | 0 (0.0) | 20 (–) | 242 (59.6) | 159 (39.2) | 4 (1.0) | 0 (0.0) | 1 (0.2) | 28 (–) | 0.0066 | 0.75 | 1.278 (0.341–4.794) |
| Nausea | 147 (35.3) | 249 (59.9) | 20 (4.8) | 0 (0.0) | 18 (–) | 120 (29.6) | 277 (68.2) | 9 (2.2) | 0 (0.0) | 0 (0.0) | 28 (–) | 0.26 | 0.057 | 0.449 (0.202–0.998) |
| Neuropathy: sensory | 37 (8.8) | 365 (86.9) | 18 (4.3) | 0 (0.0) | 14 (–) | 28 (6.8) | 314 (76.8) | 65 (15.9) | 2 (0.5) | 0 (0.0) | 25 (–) | <0.0001 | <0.0001 | 4.375 (2.550–7.508) |
| Neutropenia | 287 (69.0) | 90 (21.6) | 23 (5.5) | 16 (3.8) | 18 (–) | 221 (54.4) | 127 (31.3) | 43 (10.6) | 14 (3.4) | 1 (0.2) | 28 (–) | <0.0001 | 0.031 | 1.611 (1.046–2.480) |
| Pain: other (specify) | 311 (74.0) | 99 (23.6) | 10 (2.4) | 0 (0.0) | 14 (–) | 278 (68.0) | 107 (26.2) | 24 (5.9) | 0 (0.0) | 0 (0.0) | 25 (–) | 0.026 | 0.014 | 2.556 (1.206–5.415) |
| Rash | 359 (86.9) | 52 (12.6) | 2 (0.5) | 0 (0.0) | 21 (–) | 320 (79.0) | 84 (20.7) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 29 (–) | 0.00061 | 1.00 | 0.509 (0.046–5.632) |
| Taste alteration | 231 (56.1) | 180 (43.7) | 1 (0.2) | 0 (0.0) | 22 (–) | 179 (44.4) | 222 (55.1) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 31 (–) | 0.0021 | 0.57 | 2.050 (0.185–22.696) |
| Thrombocytopenia | 290 (69.7) | 117 (28.1) | 5 (1.2) | 4 (1.0) | 18 (–) | 253 (62.3) | 145 (35.7) | 5 (1.2) | 2 (0.5) | 1 (0.2) | 28 (–) | 0.020 | 1.00 | 0.909 (0.347–2.380) |
| Vomiting | 304 (73.1) | 98 (23.6) | 14 (3.4) | 0 (0.0) | 18 (–) | 270 (66.5) | 126 (31.0) | 10 (2.5) | 0 (0.0) | 0 (0.0) | 28 (–) | 0.056 | 0.54 | 0.725 (0.318–1.652) |
| Watery eye | 339 (82.7) | 71 (17.3) | 0 (0.0) | 0 (0.0) | 24 (–) | 310 (76.7) | 92 (22.8) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 30 (–) | 0.028 | 0.25 | Not estimable |
The overall frequency of grade ≥ 3 toxicity was 59.4% for 6-month duration adjuvant chemotherapy and 35.7% for 3-month adjuvant chemotherapy (p < 0.001). Sensory neuropathy, diarrhoea, neutropenia, fatigue, pain, nausea and hand–foot syndrome were the most common grade ≥ 3 AEs reported. The difference in frequency between treatment groups was statistically significant for diarrhoea (p = 0.033), neutropenia (p = 0.023), pain (p = 0.014), hand–foot syndrome (p = 0.031) and sensory neuropathy (p < 0.001). The AE for which there was the most marked increase in the proportion of patients with grade ≥ 3 with 6-month adjuvant chemotherapy was sensory neuropathy (16.4% vs. 4.3% with 3-month treatment).
The proportion of those with febrile neutropenia was 1.6% of patients for the 3-month treatment group and 1.2% for the 6-month treatment group. Figure 9 shows that diarrhoea and hand–foot syndrome were more common in patients receiving oxaliplatin and capecitabine and that neutropenia was more common in those receiving oxaliplatin and 5FU.
FIGURE 9.
Frequency of patients with selected grade ≥ 3 toxicities, by treatment group and treatment regimen. (a) CAPOX 3 months; (b) CAPOX 6 months; (c) FOLFOX 3 months; and (d) FOLFOX 3 months. CAPOX, oxaliplatin and capecitabine; FOLFOX, oxaliplatin and 5FU.


A total of 244 patients in the 3-month treatment group and 576 patients in the 6-month treatment group cited AEs as the reason for stopping treatment early. The most frequently cited AEs leading to treatment discontinuation were diarrhoea (n = 90 patients) for the 3-month treatment group, and diarrhoea (n = 150 patients) and peripheral neuropathy (n = 156 patients) for the 6-month treatment group.
Serious adverse reactions were reported for 421 patients in the 3-month treatment group and for 511 patients in the 6-month treatment group. Gastrointestinal SAEs were most common and occurred in similar proportions of patients in both groups. Thirty-two patients died owing to events attributed to treatment toxicity, with the events distributed equally between the groups (16 patients died in each group); 27 of these deaths occurred during the first 3 months of treatment. A total of 21 patients of the 4108 who received oxaliplatin and capecitabine (0.51%) and 11 patients of the 1980 who received oxaliplatin and 5FU (0.56%) were reported to have died from toxicity.
Peripheral neuropathy was also assessed using a patient-reported outcome (PRO) FACT/GOG-Ntx4 questionnaire. Data were available for 2871 patients who were assessed for up to 7 years. The mean FACT/GOG-Ntx4 questionnaire neuropathy scores for patients receiving 3-month and 6-month adjuvant chemotherapy are shown in Figure 10. The neurotoxicity standardised-adjusted AUC differed markedly between the groups (p < 0.001), with a higher rate of neuropathy for the 6-month treatment group being clearly apparent from 4 months and persisting to ≥ 5 years (p < 0.001). Peak neuropathy occurred at 6 months for the 3-month treatment group and at 9 months for the 6-month treatment group.
FIGURE 10.
Peripheral neuropathy score, by treatment group. Data beyond year 6 were omitted because of small numbers. Error bars show 95% CIs. a, Low completion rate at these time points reflect the fact that neurotoxicity data were initially collected only up to 12 months; b, low return rate because patients were assessed at the start of the last cycle rather than at 6 months. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).

Health-related quality of life
A total of 1829 patients provided data on the EORTC questionnaires. Global health status and all functional and symptom scales of the EORTC QLQ-C30 showed statistically significant differences in terms of standardised-adjusted AUC, indicating better functioning and fewer side effects in patients receiving 3-month adjuvant chemotherapy. Scores for the two groups mirrored each other over the first 3 months of treatment but subsequently improved from months 4 to 6 for patients in the 3-month treatment group, indicative of functional improvement and decreased side effects in those who stopped treatment at 3 months (Figure 11). For the EORTC QLQ-CR29, statistically significant differences were apparent for body image (p = 0.037), dry mouth (p < 0.0001), hair loss (p = 0.035) and taste (p < 0.0001) scales. The magnitudes of the mean differences in functional and global health status scales between treatment groups were indicative of ‘moderate’ differences for global health status, role functioning, and social function and ‘a little’ difference for physical, emotional and cognitive functions. 22
FIGURE 11.
The EORTC global health status, by treatment group. a, Low return rate as patients were assessed at the start of the last cycle rather than at 6 months. Reproduced from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).

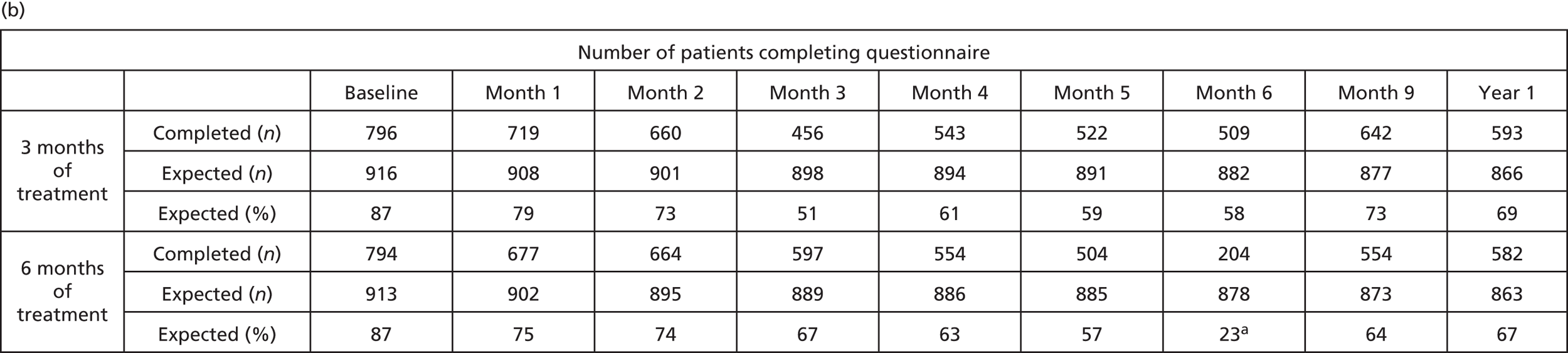
A total of 1828 patients provided data on the EQ-5D. Statistically significant differences in standardised-adjusted AUC were seen for both the EQ-5D self-rated visual analogue scale (p = 0.00081) and the EQ-5D-3L health index (p = 0.00081). Differences between the two treatment groups were apparent from months 4 to 6 (Figure 12), consistent with the time when those in the 6-month treatment group were still receiving treatment but those in the 3-month treatment group had finished. From 9 months to the 7-year follow-up, there were no clinically relevant differences23 between the treatment groups.
FIGURE 12.
The EQ-5D self-rated visual analogue scale, by treatment group. Data beyond year 6 were omitted because of small numbers. a, Low return rate as patients were assessed at the start of the last cycle rather than at 6 months. VAS, visual analogue scale. Reproduced from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
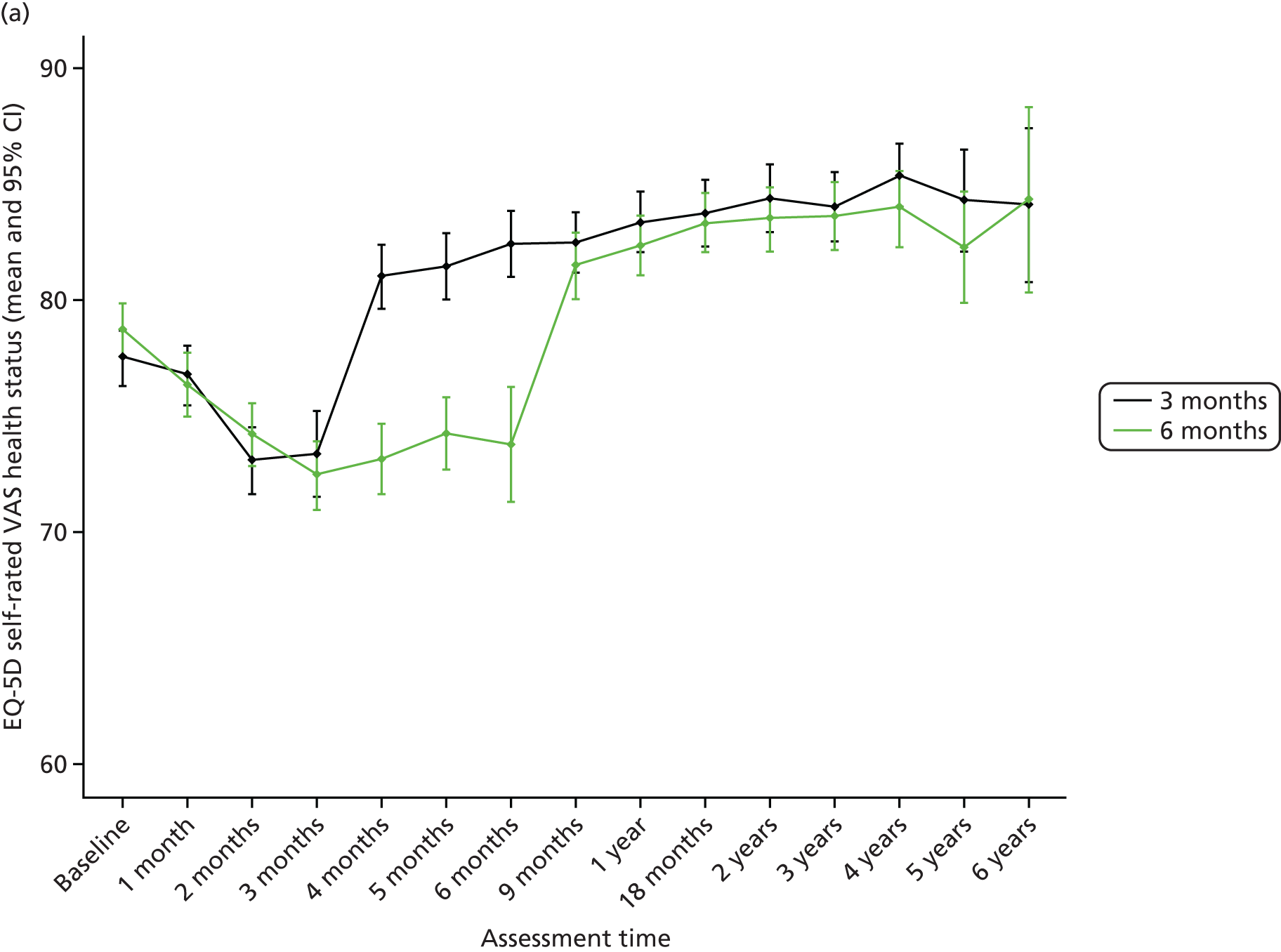
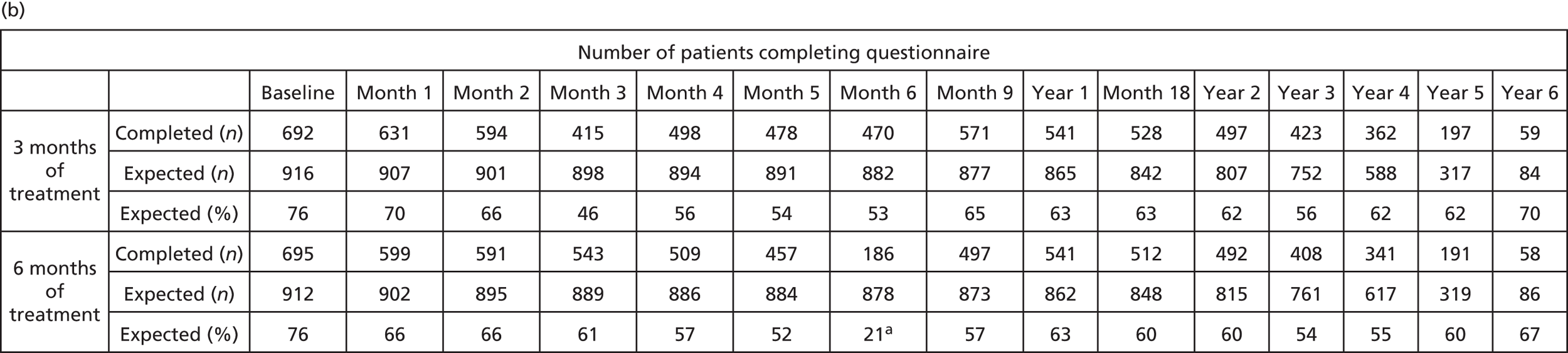
Given the notable number of missing data for HRQoL assessments (FACT/GOG-Ntx4, EORTC and EQ-5D), the reasons for missing questionnaires were analysed and are presented in Appendix 2. Missing questionnaire data were generally related to various errors. Patients who did complete questionnaires were shown to be representative of the overall study population, with no indication that missing data were associated with any particular baseline characteristic. Sensitivity analyses comparing the primary results with those based on just observed data or using data imputed only for patients who completed baseline questionnaires showed similar results.
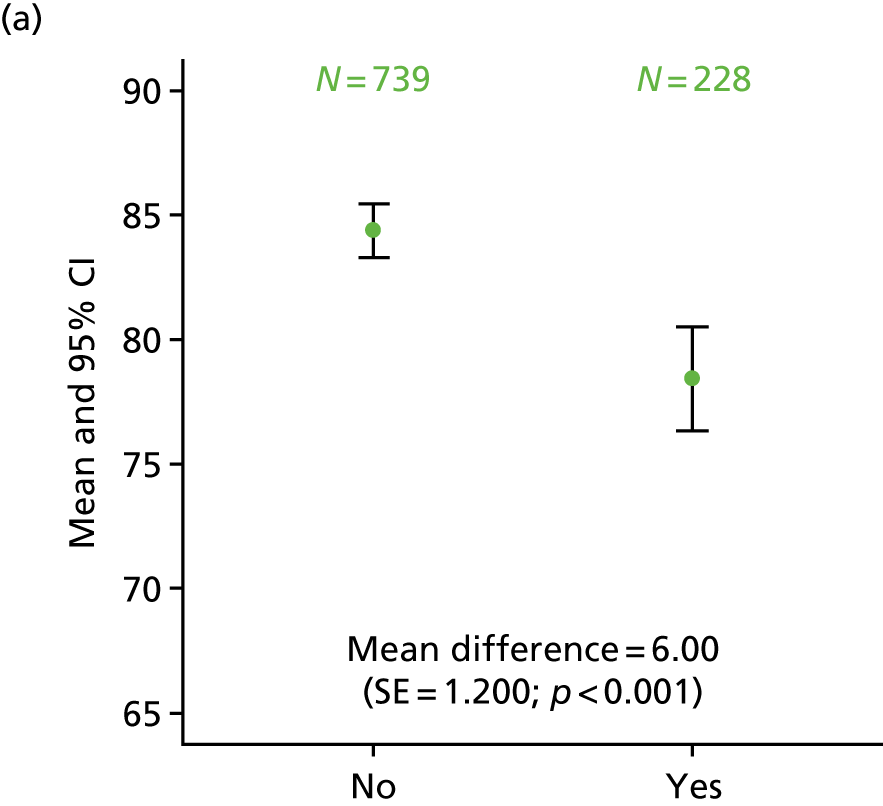
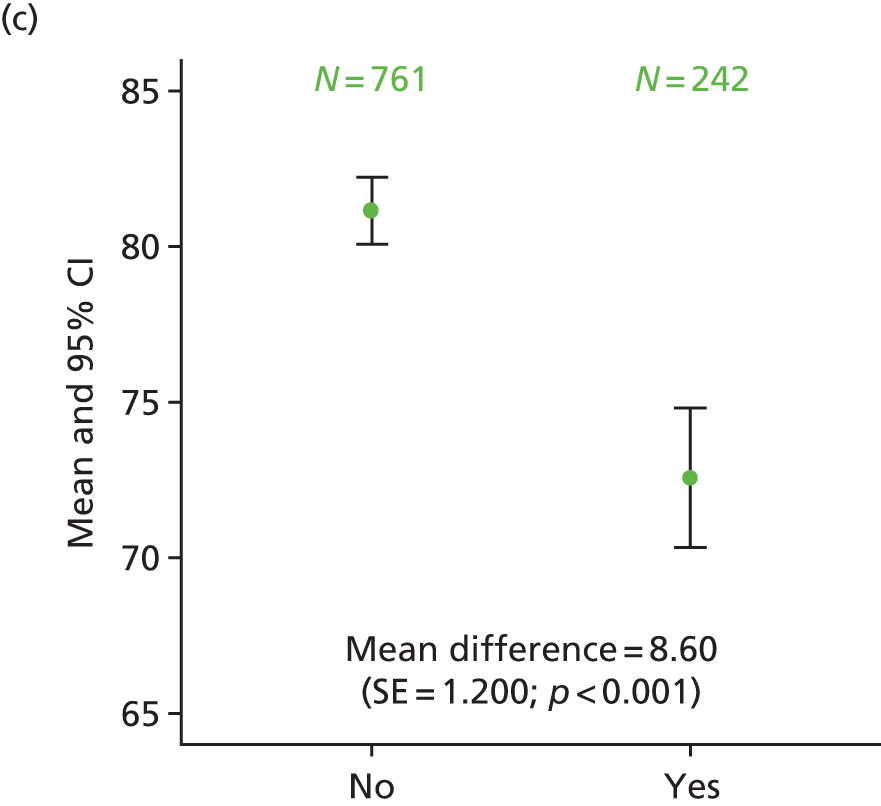
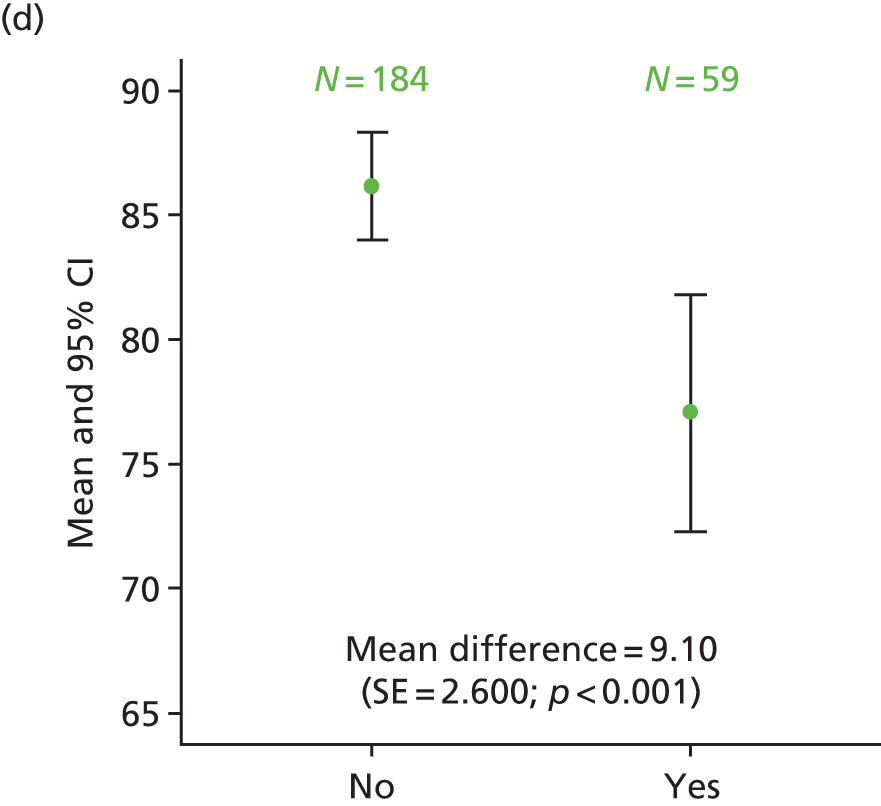
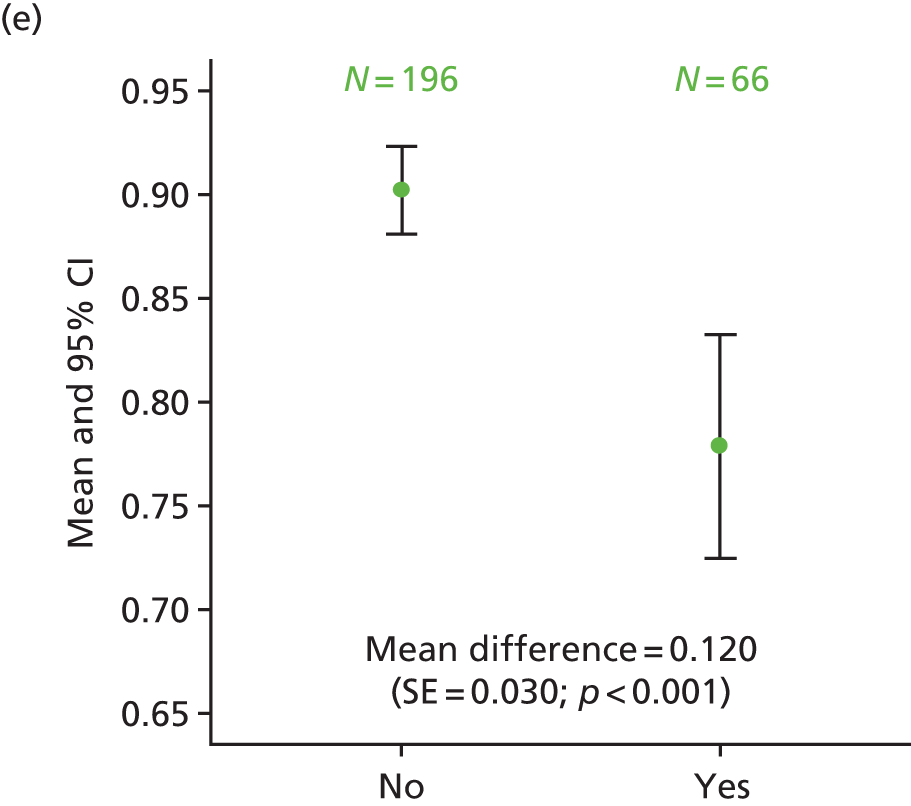
Exploratory analysis considered differences in HRQoL scales when comparing patients with ‘quite a bit’/‘very much’ numbness or tingling or discomfort in hands or feet on the FACT/GOG-Ntx4 toxicity questionnaire with those who rated these symptoms as ‘somewhat’/‘a little bit’/‘not at all’. The proportion of patients recording neuropathy symptoms as being present ‘quite a bit’ or ‘very much’ was higher for patients in the 6-month treatment group at 1 year (34% vs. 14% of patients in the 3-month treatment group), 3 years (32% vs. 17%) and 5 years (29% vs. 16%). This analysis consistently demonstrated statistically significantly poorer HRQoL across the different scales for patients who had ‘quite a bit’ or ‘very much’ recorded for neuropathy symptoms, at all time points (p < 0.001; Figure 13).
FIGURE 13.
Differences in HRQoL assessments based on the degree of numbness/tingling/discomfort in hands or feet. (a) EQ-5D visual analogue scale 1 year; (b) EQ-5D-3L health index 1 year; (c) EORTC global quality of life 1 year; (d) EQ-5D visual analogue scale 3 years; (e) EQ-5D-3L health index 3 years; (f) EQ-5D visual analogue scale 5 years; and (g) EQ-5D-3L health index 5 years. No/yes on all the x-axes refers to whether or not the patient experienced ‘quite a bit/very much numbness or tingling or discomfort in hands or feet’ on the FACT/GOG-Ntx4 toxicity score. Adapted from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).



Chapter 4 Economic analyses
Methodology
The economic analysis was undertaken from the perspective of the UK NHS and Personal Social Services for the 2016 base year, adhering to good practice guidelines. 30,31 A within-trial analysis utilised the individual patient-level data on resource use, HRQoL (EQ-5D-3L) and survival.
Outcome data
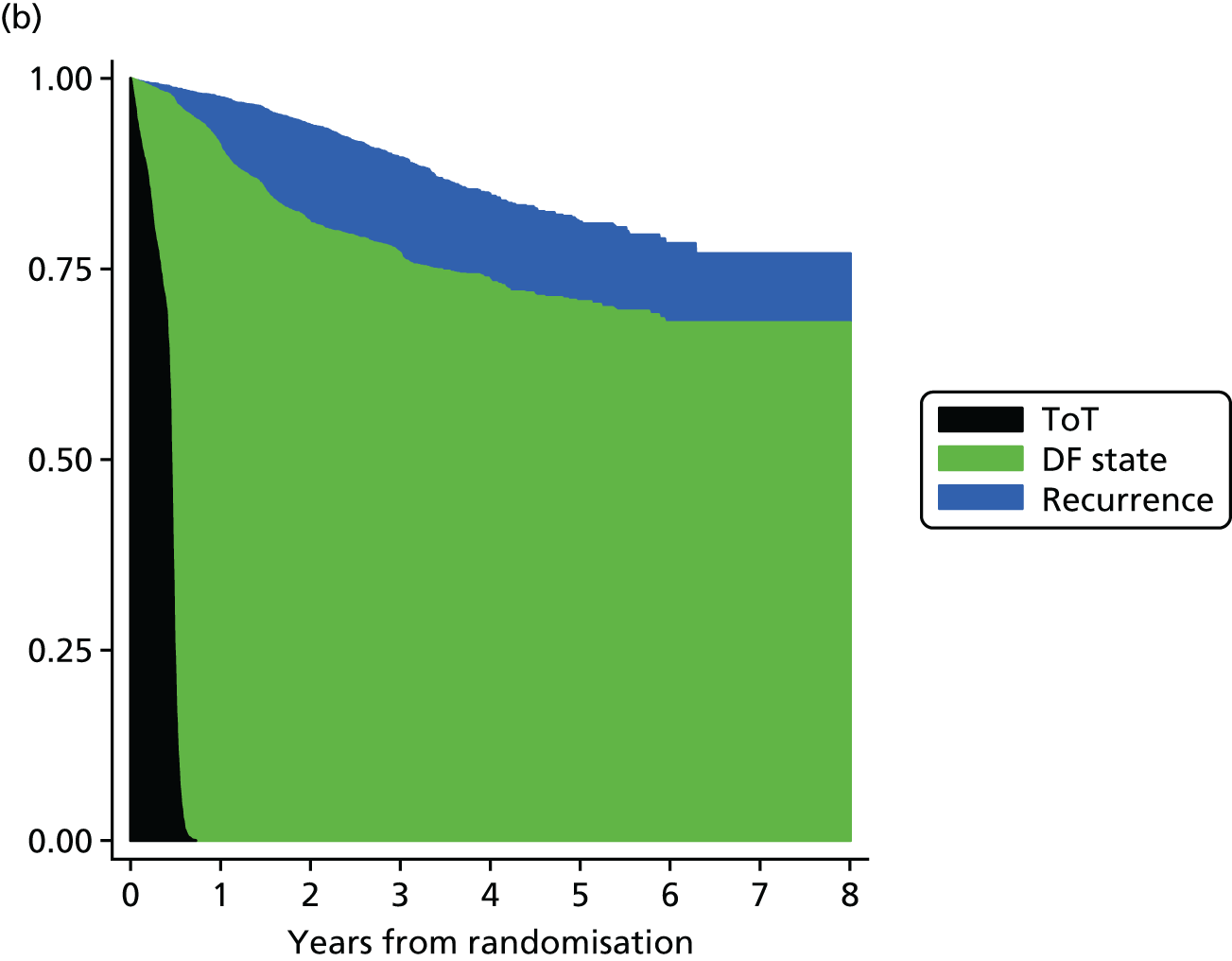
The effectiveness measure for the economic analysis was the discounted QALY gain per patient; QALYs were calculated using quality-adjusted survival analysis. 32 Overall survival data from the SCOT study were partitioned into three health states: time on treatment (ToT), disease-free state post treatment and recurrence (Figure 14). The model begins with patients who have undergone surgery and are now assumed to be disease-free, who begin treatment with chemotherapy (ToT state). After completing treatment, patients move into the disease-free state and remain here until they experience a recurrence or die. Patients who experience a recurrence remain in the recurrence state until they die.
FIGURE 14.
Partitioned survival model.
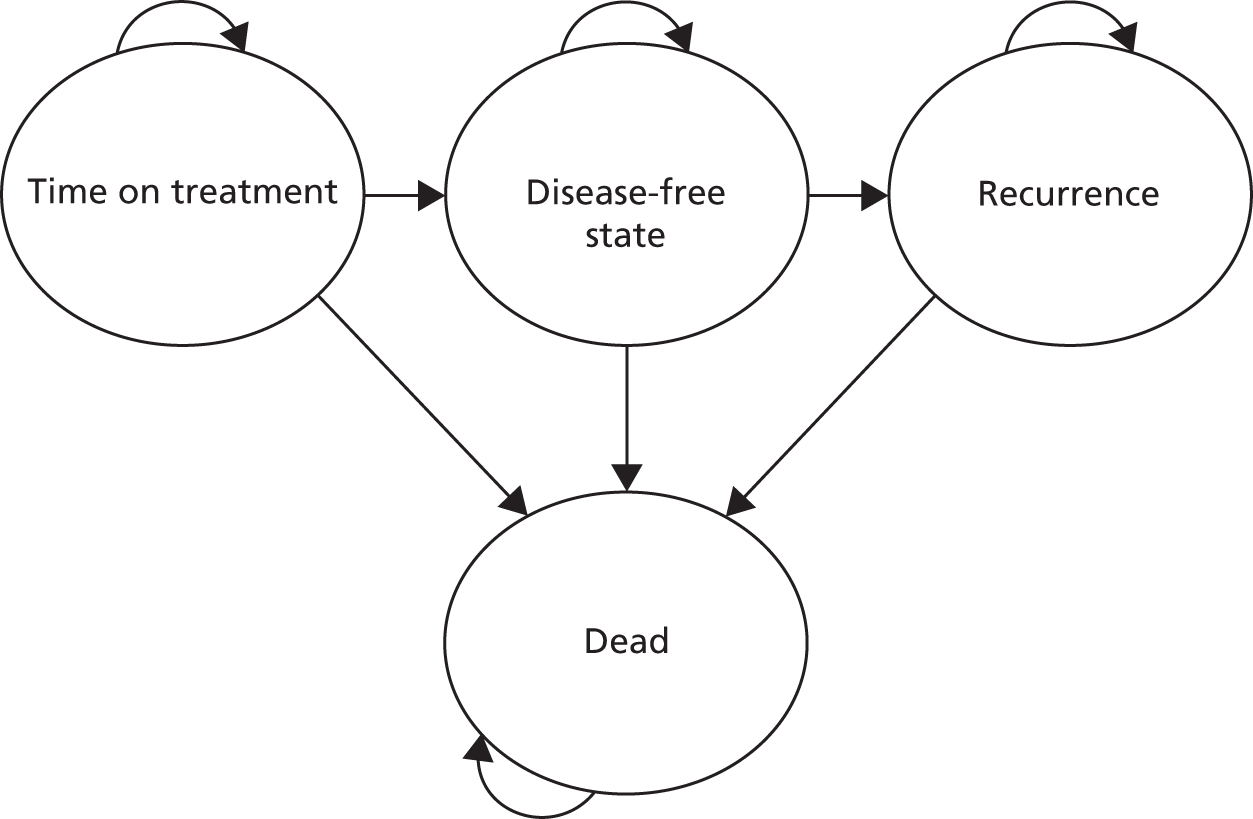
Kaplan–Meier survival estimates were used for computation of quality-adjusted survival time in each health state over the 8-year within-trial period. A separate model estimated HRQoL for each health state.
The EQ-5D-3L data were collected for a subsample of 1832 patients (about 30% of the study sample) at baseline and all follow-up times and combined with the UK EQ-5D-3L health-utility scores20 to calculate utilities. Table 4 details the characteristics of patients by EQ-5D subsample in comparison with the whole study sample. An interim analysis33 of the SCOT study EQ-5D and AE data showed that adequate data were collected from the first 1832 patients in the SCOT study; therefore, discontinuation of the EQ-5D was recommended for new study recruits beyond that point. To control for plausible differences between the EQ-5D and total study populations, the HRQoL model included co-variables such as planned treatment, high-risk disease, gender, age and ethnicity. The model predicts health utilities for the average characteristics of the patients in each health state.
| Characteristic | Total (N = 6065) | Non-EQ-5D (N = 4308) | EQ-5D (N = 1757) | p-value |
|---|---|---|---|---|
| Planned treatment (%) | ||||
| Oxaliplatin and capecitabine | 67.5 | 67.5 | 67.5 | |
| Oxaliplatin and 5FU | 32.5 | 32.5 | 32.5 | 0.99 |
| Gender (%) and age (mean) | ||||
| Female | 39.46 | 39.65 | 38.99 | |
| Male | 60.54 | 60.35 | 61.01 | 0.633 |
| Age | 63.43 | 63.35 | 63.62 | 0.3094 |
| Disease risk (%) | ||||
| High | 53.19 | 54.32 | 50.43 | |
| Low | 46.81 | 45.68 | 49.57 | 0.006 |
| Ethnicity (%) | ||||
| White/Caucasian | 94.02 | 82.61 | 77.95 | |
| African/Caribbean | 1.37 | 1.09 | 0.97 | |
| South Asian | 1.42 | 1.02 | 0.86 | |
| Chinese | 0.4 | 0.25 | 0.19 | |
| Other | 2.79 | 15.04 | 20.03 | < 0.001 |
A linear regression with SEs clustered at the individual level estimated the HRQoL, including the following independent variables: health state, treatment group and individual characteristics. Time in the health states – ToT, disease-free state and recurrence – was computed by integration of the Kaplan–Meier curves and then adjusted by HRQoL using the method of integrated quality-survival product to compute QALYs. This approach to quality-adjusted survival analysis avoids problems with informative censoring in survival analysis based on individual QALYs as an end point. 34
Cost data
The main cost categories were chemotherapy treatment and hospitalisation. Costs associated with patient treatment were calculated by measuring and valuing resources used by trial participants during the treatment and follow-up periods. Patient-level resource-use data were collected for adjuvant chemotherapy dose/duration and hospitalisation during treatment and follow-up for the whole study sample. Costs were valued in 2016 Great British pounds. The oxaliplatin, capecitabine and 5FU doses administered were recorded, and the numbers of each were combined with their unit costs; the cost per mg of each drug was obtained from the British National Formulary 73, where the lowest price per unit available was used. 35 Hospitalisation costs incurred by patients receiving adjuvant chemotherapy and being treated for adverse reactions were recorded. Hospitalisation resource use data included overnight stays in an intensive care unit, a high-dependency unit and general medicine wards,36 and inpatient chemotherapy administration as well as day cases and outpatient cases. Primary care costs (general practitioner visits) were less relevant to this context and were therefore excluded. Direct and non-direct costs for each hospitalisation were obtained from the Information Services Division of NHS Scotland. 37 Direct costs were attributed to medical, nursing, health professional, pharmacy, theatre, laboratory and other costs. For inpatient and day cases occurring within the treatment period, the pharmacy cost was subtracted to avoid double-counting chemotherapy medication. The cost of treating AEs was assumed to be included in hospitalisation costs for patients attending hospital for night or day cases or during outpatient visits.
Given that the follow-up period differed among patients, the total cost per patient was estimated as the Kaplan–Meier survival analysis;38 this allowed the average total cost to be estimated as the sum of the average cost for each period multiplied by the probability of surviving at the beginning of the period.
Cost-effectiveness
The cost and QALY outcomes for each treatment group were estimated and combined with the upper UK decision threshold for cost-effectiveness of £30,000 per QALY30 to report outcomes in terms of NMB according to good reporting practice guidance. 39 The incremental NMB is the difference between the NMB of the two groups.
Analyses were performed using Stata 14.0 (StataCorp LP, College Station, TX, USA) to compare mean cost and mean QALY differences between the treatment groups (3-month vs. 6-month treatment) and the NMB was reported in line with recent reporting guidelines39 and the UK reference case. 30 Discounting of costs and QALY outcomes beyond 1 year was applied at a rate of 3.5%. 30
Bootstrapping (1000 iterations)40 was used to account for uncertainty around the difference in costs and QALYs and to assess how this uncertainty affects the cost-effectiveness outcome. Uncertainty was reported through CIs and the computation of cost-effectiveness acceptability probabilities was estimated as a function of the threshold for the monetary value of a QALY. 41 Subgroup analyses were undertaken, in line with the main trial analyses, to consider cost-effectiveness of the two treatment duration strategies according to planned treatment regimen (oxaliplatin and 5FU or oxaliplatin and capecitabine), disease risk (high or low), gender and age.
Results
Cost-effectiveness analyses were conducted on information provided by the 6065 patients who consented to their data being used (see Figure 1).
Effectiveness
Kaplan–Meier estimates up to 8 years after randomisation were used to determine how overall survival time would be split into the three health states – ToT, disease-free and recurrence – for the two treatment groups, as shown in Table 5. As would be expected, ToT was significantly higher for patients in the 6-month treatment group, whereas disease-free was just outside the 5% statistical-significance level but favoured the 3-month treatment group. No difference was seen between the groups for time in recurrence or overall survival. Figure 15 shows the time of overall survival partitioned into ToT, disease-free state and recurrence state for the two treatment groups, as generated using Kaplan–Meier estimates.
| Survival analysis (restricted mean survival) | 3-month treatment, mean (n = 3035 patients) | 6-month treatment, mean (n = 3030 patients) | Incremental difference | |
|---|---|---|---|---|
| Mean | p-value | |||
| Time-on-treatmenta | 0.21 | 0.39 | –0.18 | 0.000 |
| Disease-free | 5.93 | 5.74 | 0.19 | 0.053 |
| Recurrence | 0.73 | 0.77 | –0.041 | 0.605 |
| Total (overall survival) | 6.87 | 6.90 | –0.032 | 10.695 |
FIGURE 15.
Overall survival partitioned into ToT, disease-free state after treatment, and recurrence, Kaplan–Meier estimates over 8 years, by treatment group at (a) 3 months and (b) 6 months. DF, disease free.

Kaplan–Meier estimates of how overall survival time would be split into the three health states – ToT, disease-free and recurrence – for the two treatment groups, by subgroup, are shown in Table 6.
| Health state | 3-month treatment, mean | 6-month treatment, mean | Incremental difference, mean (p-value) | 3-month treatment, mean | 6-month treatment, mean | Incremental difference, mean (p-value) |
|---|---|---|---|---|---|---|
| By planned treatment | ||||||
| Oxaliplatin and 5FU (N = 1971) | Oxaliplatin and capecitabine (N = 4094) | |||||
| Patients (n) | 984 | 987 | 2051 | 2043 | ||
| Recurrence | 0.78 | 0.67 | 0.11 (0.336) | 0.71 | 0.82 | –0.10 (0.336) |
| Disease-free | 5.81 | 5.96 | –0.14 (0.360) | 5.98 | 5.63 | 0.35 (0.004) |
| ToT | 0.22 | 0.42 | 0.20 (< 0.001) | 0.20 | 0.37 | –0.17 (< 0.001) |
| Total (overall survival) | 6.82 | 7.05 | –0.22 (0.128) | 6.90 | 6.83 | 0.071 (0.508) |
| By risk | ||||||
| High risk (N = 2839) | Low risk (N = 3226) | |||||
| Patients (n) | 1424 | 1415 | 1611 | 1615 | ||
| Recurrence | 0.95 | 0.86 | 0.08 (0.434) | 0.55 | 0.71 | –0.16 (0.158) |
| Disease-free | 5.14 | 5.10 | 0.04 (0.739) | 6.62 | 6.30 | 0.32 (0.016) |
| ToT | 0.21 | 0.40 | –0.18 (< 0.001) | 0.21 | 0.39 | –0.18 (< 0.001) |
| Total (overall survival) | 6.31 | 6.36 | –0.048 (0.736) | 7.39 | 7.42 | –0.023 (0.806) |
| By gender | ||||||
| Female (N = 2393) | Male (N = 3672) | |||||
| Patients (n) | 1199 | 1194 | 1836 | 1836 | ||
| Recurrence | 0.60 | 0.67 | –0.07 (0.576) | 0.82 | 0.84 | –0.02 (0.817) |
| Disease-free | 6.10 | 5.73 | 0.37 (0.006) | 5.80 | 5.74 | 0.053 (0.690) |
| ToT | 0.21 | 0.38 | –0.16 (< 0.001) | 0.21 | 0.40 | –0.19 (< 0.001) |
| Total (overall survival) | 6.92 | 6.78 | 0.14 (0.279) | 6.82 | 6.98 | –0.16 (0.195) |
| By age | ||||||
| ≥ 65 years (N = 3065) | < 65 years (N = 3000) | |||||
| Patients (n) | 1525 | 1540 | 1510 | 1490 | ||
| Recurrence | 0.80 | 0.65 | 0.15 (0.287) | 0.69 | 0.89 | –0.20 (0.042) |
| Disease-free | 5.72 | 5.69 | 0.03 (0.838) | 6.10 | 5.78 | 0.31 (0.013) |
| ToT | 0.21 | 0.38 | –0.16 (< 0.001) | 0.21 | 0.41 | –0.20 (< 0.001) |
| Total (overall survival) | 6.73 | 6.72 | 0.02 (0.900) | 7.01 | 7.09 | –0.077 (0.518) |
Table 7 shows the results of the utility model for non-missing observations. The effect of recurrence and ToT was captured by assigning a value of 1 to the EQ-5D responses occurring in those health states. ToT and recurrence have a significant negative effect on utility, as would be expected; however, the 3-month treatment group was estimated to have higher HRQoL (p < 0.05) even after controlling for recurrence and ToT. These results are consistent with the higher incidence of AEs in the 6-month than in the 3-month treatment group. Patient characteristics were included in the model to adjust health utilities to the average values for the whole SCOT study sample, with and without the EQ-5D data. A statistically significant positive effect on HRQoL was associated with the variables male and age, with a negative effect seen for African/Caribbean and South Asian patients compared with white/Caucasian patients. Planned treatment and disease risk did not have a significant effect.
| Variable | Coefficient | Standard error |
|---|---|---|
| Number of patientsa (n) | 1757 | |
| Number of observations (n) | 16,091 | |
| Health states (reference: disease-free) | ||
| ToT | –0.0394d | 0.00408 |
| Recurrence | –0.0578d | 0.0139 |
| Treatment group: 6 months | –0.0154b | 0.00730 |
| Other characteristics | ||
| Oxaliplatin and capecitabine | 0.00402 | 0.00783 |
| High risk | –0.00911 | 0.00724 |
| Male | 0.0159b | 0.00733 |
| Age | 0.00162d | 0.000429 |
| Ethnicity (reference: white/Caucasian) | ||
| African/Caribbean | –0.0810b | 0.0385 |
| South Asian | –0.145c | 0.0536 |
| Chinese | –0.0447 | 0.0772 |
| Other | 0.0178 | 0.0217 |
| Constant | 0.866d | 0.00944 |
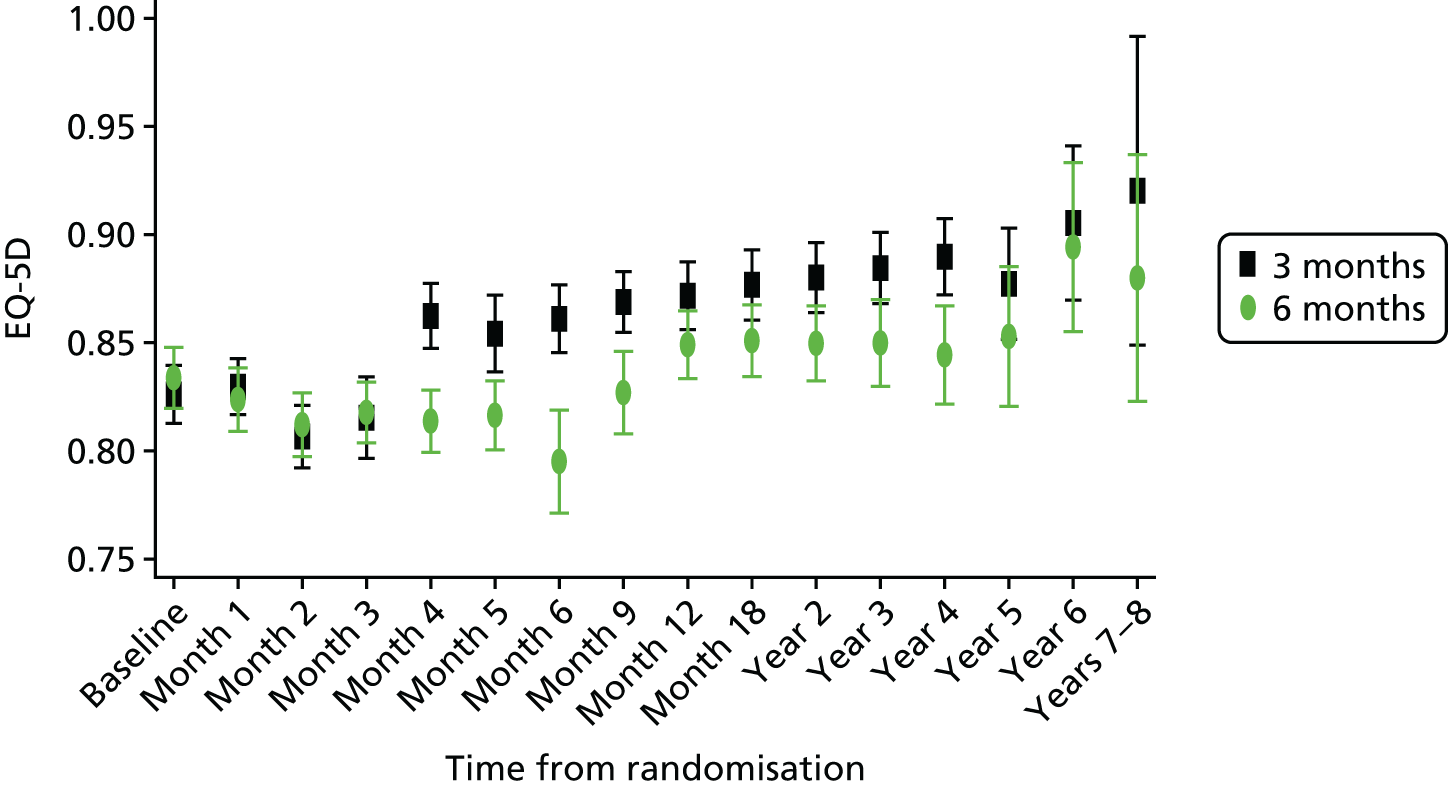
The results of the model follow the pattern of the evolution of EQ-5D scores over time as shown in Figure 16. After baseline, HRQoL decreased for both groups to 3 months; at this point, health utilities for those in the 3-month treatment group increased as they completed treatment, whereas lower HRQoL persisted for patients receiving 6 months of adjuvant chemotherapy. Changes in HRQoL were primarily related to ToT, although Figure 16 illustrates that some difference in HRQoL was evident between the two groups beyond 6 months (completion of adjuvant chemotherapy).
FIGURE 16.
Evolution of EQ-5D utilities over time, by treatment group. Presented as means and 95% CIs.
Costs
The unit costs for treatment and hospitalisation are detailed in Table 8, and Table 9 provides a detailed unit cost breakdown for each of the hospitalisation cost categories. Tables 10 and 11 detail resource use for chemotherapy and hospitalisations, respectively, for each group of the intervention and by planned treatment regimen.
| Adjuvant chemotherapy drug | Description | Cost (£) | Unit cost (£/mg) |
|---|---|---|---|
| Oxaliplatin | 200 mg/40 ml concentrate of oxaliplatin for solution for infusion vials | 595.65 | 2.98 |
| Capecitabine | 500-mg tablets of capecitabine, 120 tablets | 146.00 | 2.43 × 10–3 |
| 5FU (bolus) | 500 mg/20 ml solution of 5FU for injection vials, 10 vials | 64.00 | 1.28 × 10–2 |
| 5FU (infusion) | 2.5 g/100 ml solution of 5FU for infusion vials | 32.00 | 1.28 × 10–2 |
| Hospitalisation type | Description | Unit cost (£/night or case) | |
| Intensive care unit | Night in intensive care unit | 2190.35 | |
| High-dependency unit | Night in intensive care unit | 937.87 | |
| General medicine | Night in general medicine unit | 476.73 | |
| Inpatient (clinical oncology) | Night in clinical oncology unit, as inpatient | 896.88 | |
| Day case (clinical oncology) | Day case at clinical oncology | 813.22 | |
| Outpatient (clinical oncology) | Outpatient attendance for clinical oncology | 251.77 | |
| Hospitalisations | Cost per night or case (£) | Total cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Medical | Nursing | Allied health professional | Pharmacy | Theatre | Laboratory | Other | Non-direct costs | ||
| Intensive care unita | 284.24 | 1029.79 | 36.63 | 263.48 | 9.83 | 76.44 | 22.35 | 467.59 | 2190.35 |
| High-dependency unita | 125.74 | 423.47 | 13.82 | 87.07 | 41.43 | 6.40 | 239.95 | 937.87 | |
| General medicinea | 73.12 | 161.03 | 16.60 | 47.80 | 3.77 | 25.77 | 5.81 | 142.82 | 476.73 |
| Inpatient (clinical oncology)a | 103.47 | 198.66 | 74.87 | 157.15 | 30.71 | 38.25 | 293.77 | 896.88 | |
| Day case (clinical oncology)b | 147.27 | 77.37 | 51.67 | 281.32 | 17.41 | 24.84 | 213.34 | 813.22 | |
| Outpatient (clinical oncology)c | – | – | – | – | – | – | 192.40 | 59.37 | 251.77 |
| Drug | All patients (N = 6065) | Oxaliplatin and 5FU (N = 1971) | Oxaliplatin and capecitabine (N = 4094) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-month treatment, mean | 6-month treatment, mean | Incremental difference: 3-month vs. 6-month; p-value | 3-month treatment | 6-month treatment | Incremental difference: 3-month vs. 6-month; p-value | 3-month treatment | 6-month treatment | Incremental difference: 3-month vs. 6-month; p-value | |
| Patients (n) | 3035 | 3030 | 984 | 987 | 2051 | 2043 | |||
| Oxaliplatin | 842.1 | 1273.0 | –430.8; < 0.001 | 875.8 | 1352.5 | –476.7; < 0.001 | 826.0 | 1234.5 | –408.5; < 0.001 |
| Capecitabine | 114,142.1 | 192,930.5 | –78,788.4; < 0.001 | 3622.8 | 7110.4 | –3487.7; 0.031 | 167,165.6 | 282,702.6 | –115,537.0; < 0.001 |
| 5FU bolus | 1293.9 | 2184.6 | –890.7; < 0.001 | 3871.6 | 6391.7 | –2520.1; < 0.001 | 57.3 | 152.1 | –94.8; < 0.001 |
| 5FU continuous infusion | 8012.8 | 14,196.1 | –6183.3; < 0.001 | 23,991.7 | 41,645.9 | –17,654.3; < 0.001 | 346.6 | 934.7 | –588.1; < 0.001 |
| Resource | All patients (N = 6065) | Oxaliplatin and 5FU (N = 1971) | Oxaliplatin and capecitabine (N = 4094) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-month treatment, mean | 6-month treatment, mean | Incremental difference: 3-month vs. 6-month; p-value | 3-month treatment, mean | 6-month treatment, mean | Incremental difference 3-month vs. 6-month; p-value | 3-month treatment, mean | 6-month treatment, mean | Incremental difference: 3-month vs. 6-month; p-value | |
| Patients (n) | 3035 | 3030 | 984 | 987 | 2051 | 2043 | |||
| 0 to 3 months | |||||||||
| Intensive care unita | 0.013 | 0.022 | –0.010; 0.222 | 0.006 | 0.020 | –0.014; 0.198 | 0.016 | 0.023 | –0.007; 0.475 |
| High-dependency unita | 0.017 | 0.029 | –0.012; 0.391 | 0.008 | 0.014 | –0.006; 0.520 | 0.022 | 0.037 | –0.015; 0.462 |
| General/acute medicinea | 0.846 | 0.753 | 0.093; 0.230 | 0.758 | 0.661 | 0.098; 0.421 | 0.888 | 0.798 | 0.090; 0.361 |
| Inpatient visita | 0.143 | 0.148 | –0.005; 0.859 | 0.186 | 0.209 | –0.023; 0.669 | 0.122 | 0.118 | 0.004; 0.873 |
| Outpatient visitb | 4.588 | 4.633 | –0.045; 0.667 | 6.777 | 6.522 | 0.256; 0.274 | 3.538 | 3.721 | –0.183; 0.045 |
| Day casec | 2.643 | 2.653 | –0.009; 0.919 | 4.254 | 4.193 | 0.062; 0.784 | 1.871 | 1.909 | –0.038; 0.583 |
| 3 to 6 months | |||||||||
| Intensive care unita | 0.042 | 0.012 | 0.030; 0.119 | 0.013 | 0.018 | –0.005; 0.743 | 0.055 | 0.009 | 0.046; 0.088 |
| High-dependency unita | 0.011 | 0.009 | 0.002; 0.785 | 0.006 | 0.017 | –0.011; 0.459 | 0.013 | 0.005 | 0.008; 0.293 |
| General/acute medicinea | 0.524 | 0.203 | 0.321; 0.000 | 0.611 | 0.226 | 0.385; 0.005 | 0.482 | 0.191 | 0.291; 0.001 |
| Inpatient visita | 0.036 | 0.081 | –0.045; 0.005 | 0.029 | 0.128 | –0.098; 0.002 | 0.039 | 0.058 | –0.020; 0.282 |
| Outpatient visitb | 2.123 | 3.585 | –1.462; 0.000 | 2.408 | 5.200 | –2.792; 0.000 | 1.987 | 2.805 | –0.818; 0.000 |
| Day casec | 0.272 | 1.980 | –1.708; 0.000 | 0.398 | 3.275 | –2.876; 0.000 | 0.211 | 1.354 | –1.143; 0.000 |
| 6 to 12 months | |||||||||
| Intensive care unita | 0.027 | 0.059 | –0.032; 0.082 | 0.021 | 0.087 | –0.066; 0.042 | 0.030 | 0.046 | –0.015; 0.489 |
| High-dependency unita | 0.015 | 0.028 | –0.014; 0.200 | 0.024 | 0.030 | –0.006; 0.794 | 0.010 | 0.027 | –0.017; 0.122 |
| General/acute medicinea | 0.842 | 1.353 | –0.511; 0.001 | 1.036 | 1.737 | –0.701; 0.024 | 0.749 | 1.168 | –0.419; 0.011 |
| Inpatient visita | 0.028 | 0.048 | –0.020; 0.147 | 0.048 | 0.048 | 0.000; 0.996 | 0.019 | 0.047 | –0.029; 0.042 |
| Outpatient visitb | 4.071 | 4.566 | –0.495; 0.000 | 4.165 | 4.759 | –0.594; 0.001 | 4.027 | 4.473 | –0.447; 0.000 |
| Day casec | 0.399 | 0.629 | –0.230; 0.000 | 0.477 | 0.888 | –0.411; 0.000 | 0.362 | 0.504 | –0.142; 0.004 |
| > 12 months | |||||||||
| Intensive care unita | 0.050 | 0.053 | –0.003; 0.874 | 0.057 | 0.065 | –0.008; 0.801 | 0.047 | 0.047 | –0.000; 0.993 |
| High-dependency unita | 0.063 | 0.081 | –0.018; 0.520 | 0.053 | 0.062 | –0.009; 0.775 | 0.068 | 0.091 | –0.023; 0.561 |
| General/acute medicinea | 2.317 | 2.474 | –0.158; 0.467 | 2.466 | 2.065 | 0.402; 0.266 | 2.245 | 2.672 | –0.427; 0.113 |
| Inpatient visita | 0.133 | 0.110 | 0.023; 0.537 | 0.147 | 0.094 | 0.053; 0.374 | 0.126 | 0.117 | 0.008; 0.859 |
| Outpatient visitb | 8.623 | 8.755 | –0.132; 0.615 | 8.747 | 8.380 | 0.367; 0.442 | 8.563 | 8.936 | –0.373; 0.236 |
| Day casec | 1.319 | 1.441 | –0.121; 0.330 | 1.311 | 1.498 | –0.188; 0.397 | 1.323 | 1.413 | –0.089; 0.553 |
The mean costs incurred by patients, broken down by hospitalisation period, are presented in Table 12. As expected, adjuvant chemotherapy costs were higher for 6-month treatment duration (p < 0.001). Hospitalisation costs differed between treatment groups for the 4- to 6-month period (p < 0.001); however, the data also showed that 6-month-duration adjuvant chemotherapy was associated with higher hospitalisation costs over the 7- to 12-month period (p = 0.030), possibly reflecting the persistence of treatment-related complications owing to the longer treatment period. Adjuvant chemotherapy and hospitalisation costs for 6-month treatment duration would be expected to be double those for the 3-month regimen; in reality they were 1.67 and 1.45 times higher, respectively, for the 6-month treatment group. This is because only a proportion of patients randomised to the 6-month treatment group were able to complete the full course, owing to tolerability of the adjuvant treatment regimens. Some patients randomised to the 6-month treatment group completed only 4 or 5 months of chemotherapy, and hence treatment and hospitalisation costs are greater, but not doubled, in the 6-month treatment group. Thereby reducing expenditure on chemotherapy agents and time spent in hospital. Whereas 83.3% of patients randomised to 3-month treatment received full-duration therapy, only 58.8% of those randomised to the 6-month regimen received this; this is consistent with the more frequent reporting of AEs of diarrhoea and peripheral neuropathy leading to discontinuation. No difference in cost was seen between treatment groups beyond 12 months. Overall, cost was significantly higher for the 6-month treatment group (p < 0.001), being driven primarily by hospitalisation costs (–£2835) rather than the by cost of the adjuvant chemotherapy agents (–£1829) themselves.
| Time from the start of treatment | 3-month treatment (N = 3035), mean (£/patient) | 6-month treatment (N = 3030), mean (£/patient) | Incremental difference 3-month vs. 6-month | |
|---|---|---|---|---|
| Mean (£/patient) | p-value | |||
| Adjuvant chemotherapy | 2750 | 4579 | –1829 | < 0.001 |
| Hospitalisation (0 to 3 months) | 3576 | 3595 | –19 | 0.816 |
| Hospitalisation (4 to 6 months) | 1790 | 4185 | –2395 | < 0.001 |
| Hospitalisation (7 to 12 months) | 2748 | 3054 | –306 | 0.030 |
| Hospitalisation (> 12 months) | 8473 | 8588 | –115 | 0.876 |
| Total hospitalisation | 16,587 | 19,422 | –2835 | < 0.001 |
| Total | 19,337 | 24,001 | –4663 | < 0.001 |
Cost-effectiveness
The base-case cost-effectiveness analysis showed that the 3-month adjuvant chemotherapy strategy was significantly cheaper than the 6-month strategy, costing £4881 less over the 8-year analysis period (Table 13). The 3-month dosing strategy also resulted in the greatest QALY gain, although this did not reach statistical significance (p = 0.33), with uncertainty over the QALY indicated by wide 95% CIs. QALY gains for the 3-month treatment group are driven by the significantly better HRQoL. The 3-month treatment strategy was dominant, showing cost saving and improvement in QALYs, with an incremental NMB of £7246 per patient.
| Intervention strategy | Cost (£/patient), mean (95% CI) | Life expectancy, mean (95% CI) | QALYs, mean (95% CI) | NMB (Cost/QALY) (£), mean (95% CI) | Probable cost-effectiveness, λ = £30,000 |
|---|---|---|---|---|---|
| 3-month treatment | 18,401 (17,538 to 19,328) | 6.87 (6.73 to 6.99) | 5.30 (5.17 to 5.40) | 140,492 (135,327 to 145,658) | 0.995 |
| 6-month treatment | 23,282 (22,227 to 24,367) | 6.90 (6.78 to 7.02) | 5.22 (5.10 to 5.34) | 133,246 (129,569 to 136,922) | 0.005 |
| Incremental difference, 3-month vs. 6-month | –4881 (–6269 to –3492) | –0.03 (–0.22 to 0.13) | 0.08 (–0.086 to 0.230) | 7246 (3469 to 11,023) | 3-month dominant |
Figure 17 shows the cost-effectiveness acceptability curve. Three-month-duration adjuvant chemotherapy for colorectal cancer shows 99% probability of being cost-effective at both the upper and the lower UK decision thresholds of £30,000 and £20,000 per QALY, respectively. 30 The 3-month regimen also represents the optimal choice across a range of willingness-to-pay (WTP) values.
FIGURE 17.
Cost-effectiveness acceptability curve: base-case analysis.
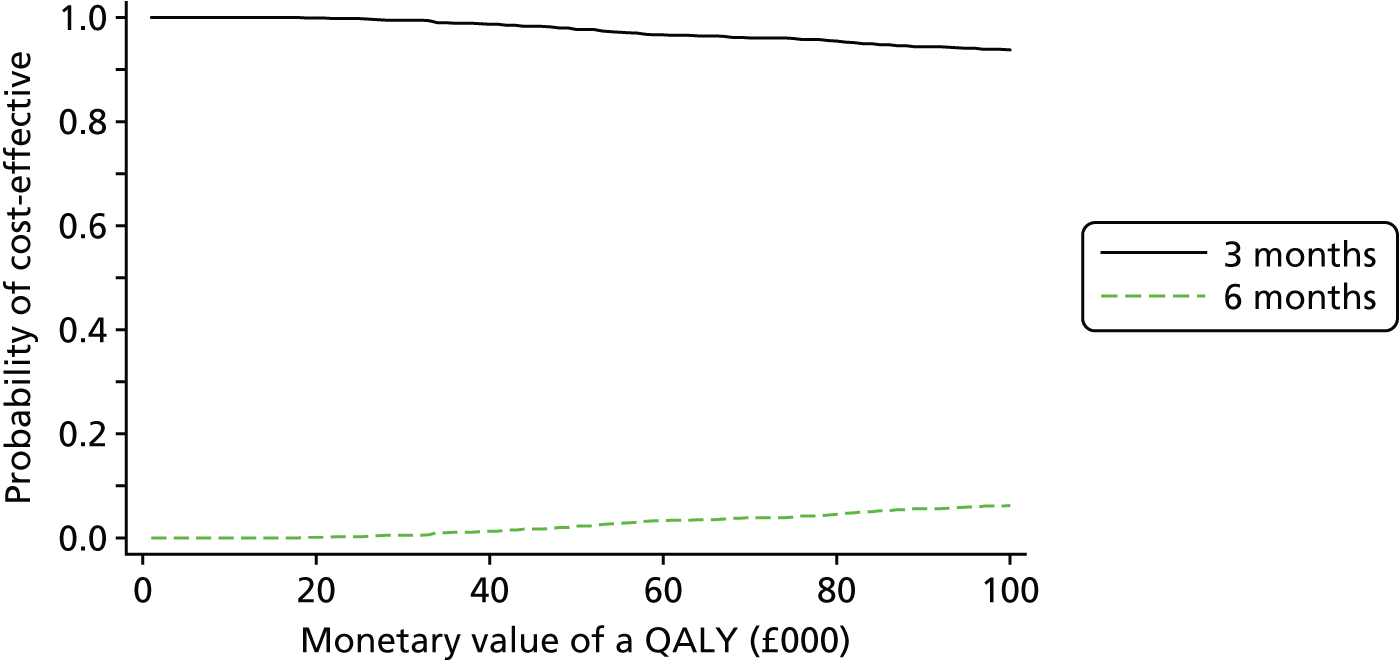
Cost-effectiveness was assessed for various patient subgroups (treatment regimen, level of disease risk, gender and age), as shown in Table 14. Cost-effectiveness results were comparable for the base-case and most subgroups, with the exceptions being oxaliplatin and 5FU treatment and male gender; 3-month treatment was generally dominant and showed a 99% probability of being the cost-effective option. With oxaliplatin and 5FU treatment, the 3-month treatment group showed cost savings but had fewer QALY gains, which was driven by a slightly higher life expectancy in the 6-month treatment group. A relative QALY advantage for 3-month treatment was seen for oxaliplatin and capecitabine chemotherapy, high-risk disease, female patients and older patients. An interaction test of differences in incremental QALYs between subgroups concluded that only in the case of the chemotherapy regimen was there a statistically significant difference in QALYs between the 3-month and 6-month treatment groups, amounting to 0.19 QALY gains for oxaliplatin and capecitabine and –0.12 QALY gains for oxaliplatin and 5FU (p = 0.066). This relative advantage of 6-month treatment duration with oxaliplatin and 5FU was driven by the longer life expectancy gain (0.22 years vs. –0.007 years, interaction test p = 0.106). In the oxaliplatin and 5FU subgroup, 3-month treatment maintained a greater NMB, with an incremental NMB compared with 6-month treatment of £3229 and a 77% probability of being cost-effective. Therefore, although 3-month treatment with oxaliplatin and 5FU is associated with a slightly lower life expectancy, it remains the optimal treatment strategy. An interaction test of differences in incremental QALYs between the disease risk, gender and age subgroups showed no significant differences in incremental QALYs.
| Intervention strategy | Cost per patient (£), mean (95% CI) | Life expectancy, mean (95% CI) | QALYs, mean (95% CI) | NMB (£30,000/QALY), mean (95% CI) | Probability cost-effectiveness λ = £30,000 |
|---|---|---|---|---|---|
| Oxaliplatin and capecitabine | |||||
| 3-month treatment | 17,650 (16,668 to 18,919) | 6.90 (6.75 to 7.04) | 5.34 (5.17 to 5.48) | 142,500 (136,469 to 148,531) | 0.999 |
| 6-month treatment | 21,503 (20,389 to 22,723) | 6.83 (6.67 to 7.99) | 5.16 (5.00 to 5.32) | 133,253 (128,206 to 138,300) | 0.001 |
| Incremental difference | –3,853 (–5487 to –2030) | 0.07 (–0.13 to 0.30) | 0.19 (–0.001 to 0.38) | 9247 (4178 to 14,315) | 3-month dominant |
| Oxaliplatin and 5FU | |||||
| 3-month treatment | 19,641 (18,477 to 20,774) | 6.83 (6.57 to 7.03) | 5.21 (4.99 to 5.39) | 136,679 (128,249 to 145,108) | 0.772 |
| 6-month treatment | 26,483 (24,659 to 28,292) | 7.05 (6.87 to 7.23) | 5.33 (5.15 to 5.51) | 133,449 (127,107 to 139,791) | 0.228 |
| Incremental difference | –6841 (–9040 to –4756) | –0.22 (–0.52 to 0.03) | –0.12 (–0.38 to 0.13) | 3229 (–2494 to 8953) | |
| High risk | |||||
| 3-month treatment | 19,057 (17,888 to 20,339) | 6.31 (6.11 to 6.52) | 4.84 (4.46 to 5.03) | 126,450 (118,973 to 133,927) | 0.997 |
| 6-month treatment | 24,815 (23,481 to 26,362) | 6.36 (6.15 to 6.54) | 4.71 (4.53 to 4.87) | 116,477 (110,700 to 122,254) | 0.003 |
| Incremental difference | –5758 (–7686 to –3924) | –0.05 (–0.32 to 0.24) | 0.13 (–0.08 to 0.37) | 9972 (4664 to 15,281) | 3-month dominant |
| Low risk | |||||
| 3-month treatment | 17,671 (16,596 to 18,925) | 7.39 (7.25 to 7.52) | 5.72 (5.58 to 5.85) | 153,997 (147,785 to 160,208) | 0.922 |
| 6-month treatment | 21,422 (20,305 to 22,573) | 7.41 (7.28 to 7.53) | 5.71 (5.55 to 5.85) | 149,816 (145,425 to 154,206) | 0.078 |
| Incremental difference | –3751 (–5402 to –2153) | –0.02 (–0.20 to 0.17) | 0.01 (–0.17 to 0.21) | 4180 (–429 to 8791) | 3-month dominant |
| Female | |||||
| 3-month treatment | 17,741 (16,408 to 18,934) | 6.92 (6.74 to 7.08) | 5.30 (5.13 to 5.47) | 141,617 (133,821 to 149,413) | 0.997 |
| 6-month treatment | 22,228 (20,716 to 23,936) | 6.78 (6.59 to 6.94) | 5.10 (4.92 to 5.27) | 130,893 (125,233 to 136,553) | 0.003 |
| Incremental difference | –4487 (–6518 to –2637) | 0.14 (–0.11 to 0.39) | 0.20 (–0.03 to 0.44) | 10,723 (4880 to 16,567) | 3-month dominant |
| Male | |||||
| 3-month treatment | 18,857 (17,737 to 20,098) | 6.83 (6.63 to 7.03) | 5.28 (5.11 to 5.43) | 139,377 (132,810.1 to 145,944) | 0.912 |
| 6-month treatment | 23,695 (22,531 to 24,930) | 6.98 (6.82 to 7.13) | 5.29 (5.15 to 5.83) | 135,055 (130,064 to 140,046) | 0.088 |
| Incremental difference | –4837 (–6613 to –3011) | –0.15 (–0.40 to 0.09) | –0.02 (–0.22 to 0.18) | 4321 (–351 to 8994) | |
| ≥ 65 years | |||||
| 3-month treatment | 19,364 (18,101 to 20,831) | 6.73 (6.57 to 6.89) | 5.24 (5.10 to 5.39) | 137,918 (131,146 to 144,689) | 0.985 |
| 6-month treatment | 23,839 (21,981 to 24,917) | 6.72 (6.52 to 6.87) | 5.13 (4.98 to 5.27) | 130,294 (125,355 to 135,234) | 0.015 |
| Incremental difference | –4474 (–5994 to –2090) | 0.01 (–0.22 to 0.27) | 0.11 (–0.09 to 0.32) | 7623 (2789 to 12,457) | 3-month dominant |
| < 65 years | |||||
| 3-month treatment | 17,572 (16,431 to 18,672) | 7.01 (6.83 to 7.17) | 5.35 (5.18 to 5.50) | 142,872 (135,713 to 150,032) | 0.977 |
| 6-month treatment | 23,066 (21,838 to 24,435) | 7.09 (6.94 to 7.24) | 5.29 (5.12 to 5.46) | 135,670 (130,541 to 140,799) | 0.023 |
| Incremental difference | –5493 (–7297 to –3870) | –0.08 (–0.32 to 0.14) | 0.05 (–0.17 to 0.28) | 7202 (1609 to 12,795) | 3-month dominant |
Figure 18 presents the cost-effectiveness acceptability curves for each of the subgroup analyses. For each subgroup, the 3-month strategy had the highest probability of being cost-effective over a wide range of WTP values. Only when a threshold higher than £60,000/QALY was considered did the 6-month regimen become the cost-effective strategy (with highest probability) for the oxaliplatin and 5FU treatment group.
FIGURE 18.
Cost-effectiveness acceptability curves: subgroup analysis. (a) Treatment: CAPOX; (b) treatment: FOLFOX; (c) risk: high; (d) risk: low; (e) gender: female; (f) gender: male; (h) age: ≥ 65 years; and (i) age: < 65 years. CAPOX, oxaliplatin and capecitabine; FOLFOX, oxaliplatin and 5FU.
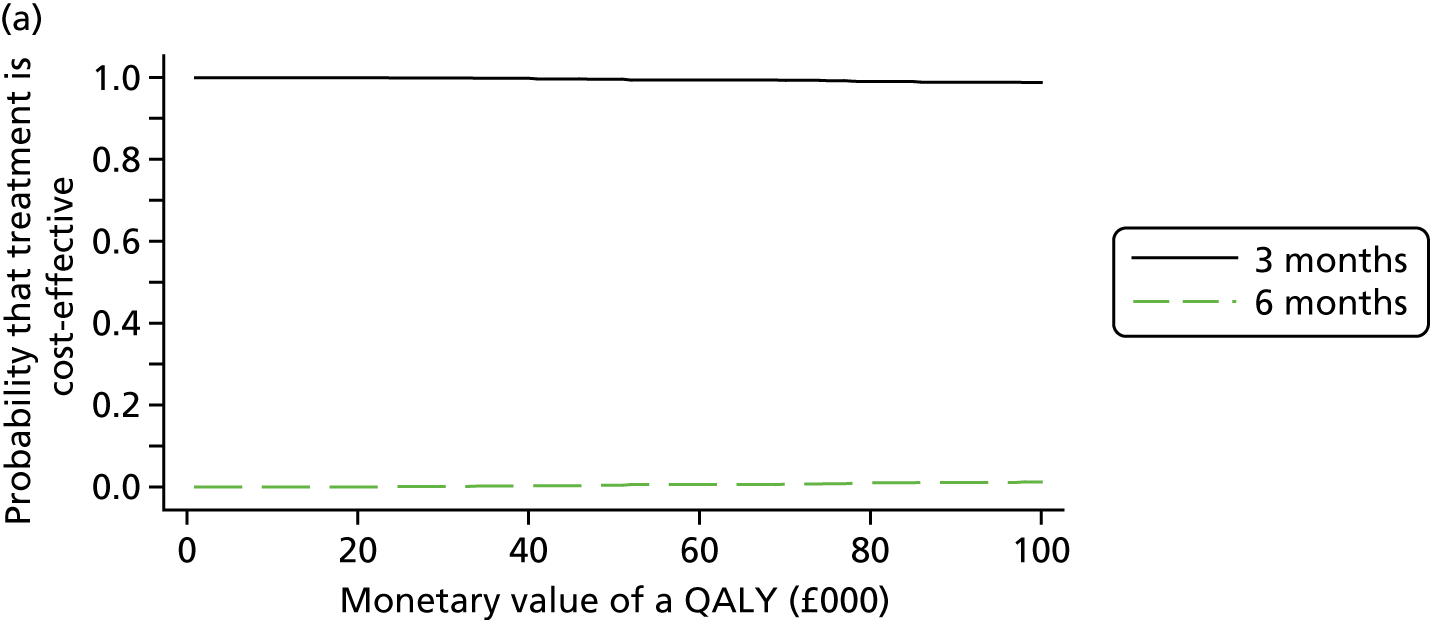
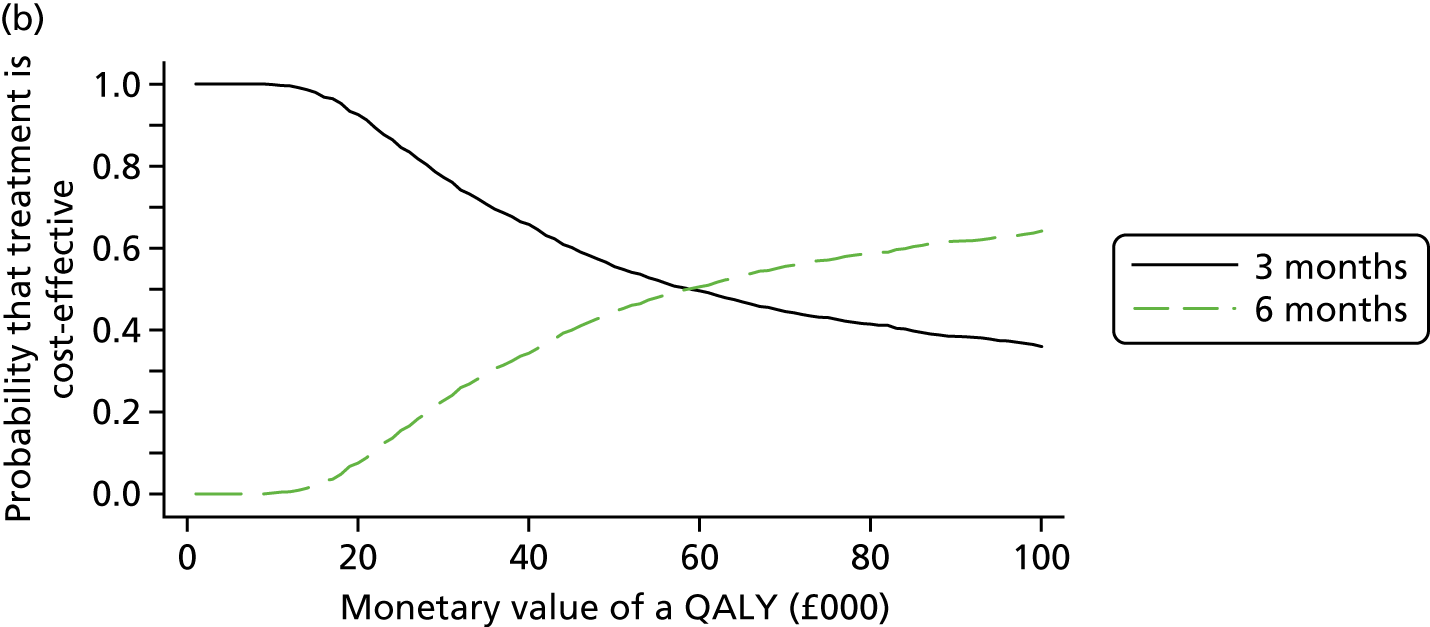




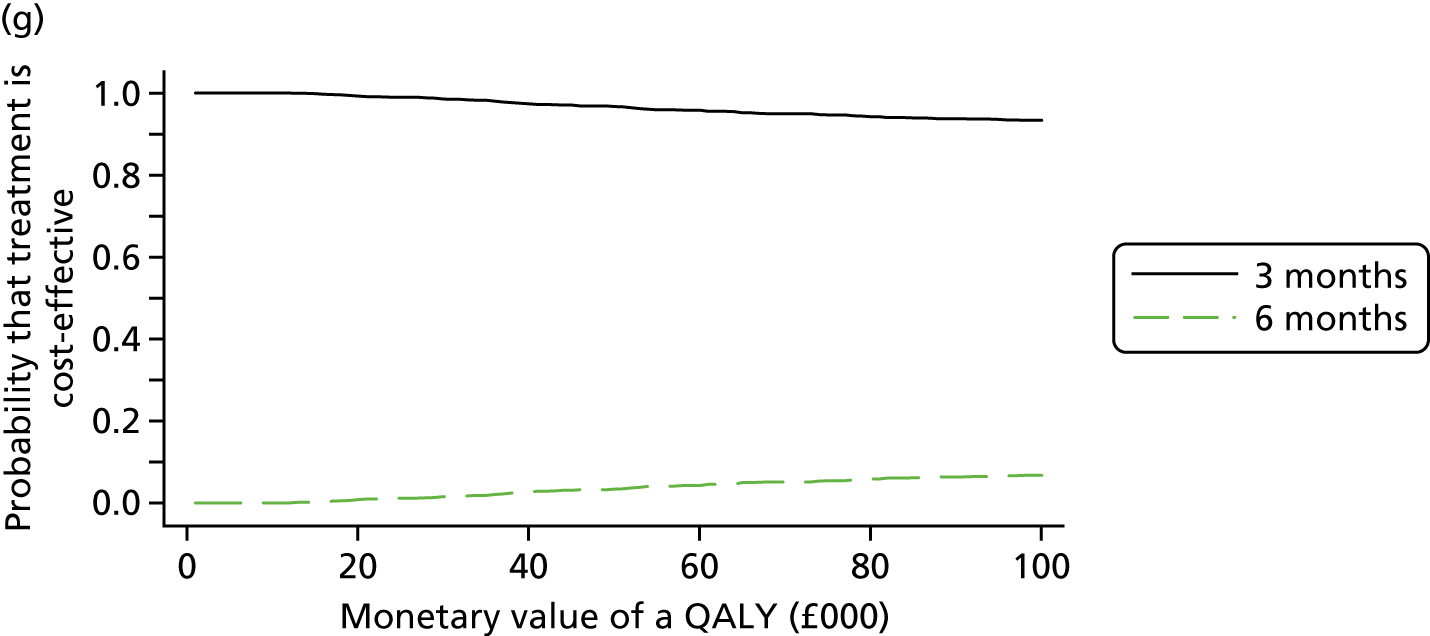
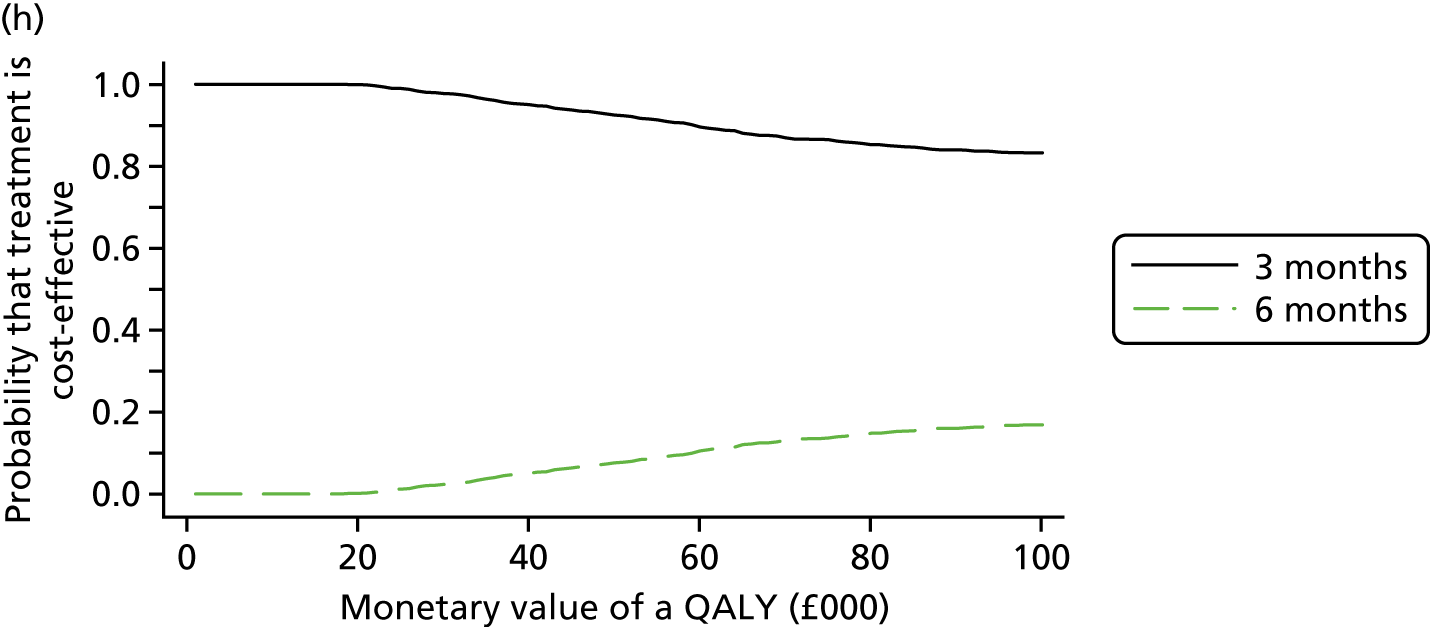
Chapter 5 Discussion
Treatment with oxaliplatin and fluoropyrimidine is the usual adjuvant chemotherapy regimen for patients with high-risk stage II or stage III colorectal cancer,5,9 with 6-month treatment duration being the accepted standard. However, oxaliplatin administration is associated with cumulative toxicity that is often characterised by chronic and often irreversible neuropathy. 6,7 Because the toxicity of oxaliplatin/fluoropyrimidine regimens is cumulative, reducing the duration of adjuvant treatment could potentially ameliorate such effects. 11 The SCOT clinical trial was conducted to determine whether or not reducting the duration of administration of oxaliplatin and fluoropyrimidine adjuvant chemotherapy from 6 months to 3 months is likely to compromise efficacy; the study also considered the effect of reducing treatment duration on toxicity, HRQoL and health economic parameters. It is our understanding that the SCOT study is the largest single randomised study of infusional adjuvant treatment for colorectal cancer to date.
Efficacy assessment
The SCOT study was designed to demonstrate that 3 months of adjuvant oxaliplatin-containing chemotherapy treatment was non-inferior to 6 months of treatment when used to treat patients with high-risk stage II or stage III colorectal cancer. The non-inferiority boundary was set to exclude a maximum 2.5% decrease in 3-year DFS comparing the 3-month and 6-month treatment groups; based on previous trials, this would result in an estimated 3-year DFS rate of 78%. The value of 2.5% was chosen because it was half of the difference in 3-year DFS seen in the MOSAIC study6 when comparing the oxaliplatin-containing treatment and fluoropyrimidine only. 6 Clinicians who routinely treat colorectal cancer considered that this represents a small difference and would be ‘an acceptable pay-off’ for a significant reduction in the sequalae associated with persistent neuropathy and the potential for HRQoL improvements in patients for whom this treatment approach proved curative. This difference corresponds to a HR of 1.13, and it was planned that this would be detected with 90% power at the 2.5% one-sided statistical-significance level.
The study protocol planned for the recruitment of 9500 patients who were expected to experience a total of 2750 DFS events over the course of the trial. However, owing to problems with recruitment, a total of 6088 patients were randomised to study treatment and experienced 1482 events, thereby reducing the statistical power to demonstrate an effect to 66%. Despite this limitation, the trial successfully demonstrated that 3-month oxaliplatin-based adjuvant chemotherapy was non-inferior to 6 months of treatment (pNI = 0.012) across the overall trial population (patients with high-risk stage II or stage III cancer of the colon or rectum); 3-year DFS rates were 77.1% of patients (SE = 0.8%) for 6-month treatment and 76.7% of patients (SE = 0.8%) for 3-month treatment, resulting in a HR of 1.006 (95% CI 0.909 to 1.114). The 3-year DFS rate seen for the 6-month treatment group in this study was similar to that seen with 6 months of oxaliplatin-containing adjuvant chemotherapy in the MOSAIC6 and NSABP C-077 studies (78.2% and 76.1%, respectively), suggesting that the outcome observed in our ‘control’ group was comparable to previous findings. 6,7 The absolute reduction in 3-year DFS rate seen with 3-month treatment was 0.4% (SE = 1.1%).
Sensitivity analyses of DFS rate were conducted based on the actual duration of treatment that patients received. These analyses did not show non-inferiority for 3-month adjuvant chemotherapy but were inherently biased by the differential exclusion of patients not able to receive prolonged therapy because of the different target treatment durations for the treatment groups. In a setting such as this, where differential compliance is intrinsic to the treatments being compared, the ITT analysis is likely to represent a more appropriate reflection of the effect of the intervention.
It is important to note that the proportion of patients randomised with stage II disease (as opposed to stage III) was lower than seen in previous trials (18.3% vs. 40% in the MOSAIC trial and 30% in the NASBP C-07 trial); the SCOT study recruitment, however, was restricted to patients with high-risk stage II disease, whereas the patients in the MOSAIC trial and the NASBP C-07 trial had all-risk stage II disease. The SCOT trial included a lower proportion of patients with stage II disease, which could lead to criticism that the trial populations had different baseline prognoses. However, because the forest plots did not identify dependence of the effect of duration of adjuvant chemotherapy on N-stage or T-stage disease, or between patients with high-risk stage II versus stage III disease, this indicates that effect of duration is not a real concern. It should be noted that, in many parts of the world, clinical practice for patients with high-risk, stage II disease or stage III disease (especially if over 70 years of age) involves single-agent fluoropyrimidine administered over 6 months, as the addition of oxaliplatin to the regimen for these patients has not been shown to improve survival. 42 The results of this trial do not provide information on duration of therapy for patients managed with single-agent fluoropyrimidine.
At the time the SCOT study started few data were available concerning the use of adjuvant chemotherapy for rectal cancer, as many adjuvant-treatment studies exclude these patients. Patients with rectal cancer were eligible to participate in the SCOT study if they had not received preoperative chemotherapy (short-course radiotherapy alone was allowed) and had undergone total mesorectal excision with an R0 resection. Forest plots did not appear to show any differences in the effect of duration of adjuvant chemotherapy when comparing patients with rectal and patients with colon cancer. Although definitive conclusions about the effect of treatment duration could not be drawn for patient subgroups from the SCOT trial, as a result of the small size of many subgroups and fewer numbers of DFS events, the results do raise important questions about how to manage patients with rectal cancer. Data from the SCOT study suggest that 3-month oxaliplatin-containing adjuvant chemotherapy could be considered for rectal cancer patients requiring adjuvant therapy.
This study did not attempt to address the potential impact of various other prognostic factors on the efficacy of standard or short duration oxaliplatin-based adjuvant chemotherapy, such as the sidedness of the cancer; RAS and BRAF status, which are known to have an impact on the prognosis of patients with metastatic disease; and microsatellite instability status. 43 These questions cannot be currently answered by the results of the SCOT study, but the findings of an ongoing translational-research substudy (TransSCOT) will provide more information.
Chemotherapy regimen
The results of both the SCOT study and data pooled for the IDEA collaboration44 indicate that the effectiveness of 3 months of adjuvant chemotherapy may depend on the choice of chemotherapy regimen. When the effect of duration was assessed for the two chemotherapy regimens specified for the trial (oxaliplatin and capecitabine and oxaliplatin and 5FU), clear non-inferiority in terms of DFS was demonstrated for patients receiving oxaliplatin and capecitabine for 3 months compared with 6 months of treatment (HR 0.94, 95% CI 0.84 to 1.07; pNI = 0.002); non-inferiority was not demonstrated when comparing patients receiving oxaliplatin and those receiving 5FU (HR 1.16, 95% CI 0.96 to 1.39; pNI = 0.591). The reasons for this are not clear.
Achieving full-duration adjuvant chemotherapy treatment over 6 months is more difficult than over 3 months. In this study, 83.3% of patients randomised to 3-month adjuvant chemotherapy continued with the regimen for the planned duration; only 58.8% of those randomised to 6-month adjuvant chemotherapy continued for the planned duration. However, compliance data showed that 94–98% of patients in the 3-month treatment group and 82–85% in the 6-month treatment group were administered the full planned fluoropyrimidine dose depending on regimen choice; corresponding figures for oxaliplatin were 96–98% for the 3-month treatment group and 69–73% for the 6-month treatment group. As similar proportions of patients received the full planned doses of the individual chemotherapeutic agents for the two treatment groups, poor treatment compliance is unlikely to explain the difference in the duration effect between the two regimens. It should be noted that capecitabine is an oral drug that is self-administered at home and these assessments are based on the prescribed dose, and so detailed compliance data are not available. This could potentially have led to an underestimation of the dose intensity for patients receiving oxaliplatin and capecitabine. However, this is considered unlikely as compliance with oral chemotherapy treatments is usually high.
The chemotherapy regimen that individual patients received was not randomised but rather was selected by the physician/patient. Thus, the particular chemotherapy combination may have been chosen due to particular patient characteristics; or there could be a real difference between the 3-month and 6-month regimens in the adjuvant setting.
-
There may have been clinical reasons why a particular chemotherapy regimen was selected for an individual patient. For example, clinicians may have selected the oxaliplatin and capecitabine combination for patients who were perceived as frail in order to avoid the risk of neutropenic sepsis inherent with oxaliplatin and 5FU; similarly, the starting dose of capecitabine may have been reduced because of perceived frailty. However, some clinicians specifically avoid giving capecitabine to older patients because of the risks associated with this agent in those with impaired renal function. In this study, the reasons for selecting a particular adjuvant chemotherapy regimen for individual patients are not known.
-
Alternatively, there may be real differences between treatment regimens associated with exposure to the component agents. For the oxaliplatin and capecitabine regimen, the oxaliplatin dose administered in each cycle is higher than that for oxaliplatin and 5FU; therefore, it can be presumed that higher peak exposure may be achieved. Two previous studies suggest that peak oxaliplatin concentration is key to clinical effect, which would be likely to favour the oxaliplatin and capecitabine regimen. 45,46 In addition, peak fluoropyrimidine exposure to is likely to be lower but the continuity of exposure is greater when fluoropyrimidine is given twice daily for 2 out of 3 weeks (compared with 5FU, which is given as bolus and then over 2 days every 2 weeks); if cells are cycling through S-phase sporadically, the greater continuity of fluoropyrimidine exposure with the capecitabine regimen could mean that there is a greater chance that tumour cells will be exposed at a critical phase in the cell cycle. It could be that micrometastatic disease is rendered more sensitive because of one or both of these differences.
Disease stage
Stage III colorectal cancer is a heterogeneous disease group; data from the IDEA collaboration and from multiple adjuvant trials have shown that patients with T1–3, N1 pathology have much better outcomes than those with either T4 or N2 features. 44 This has led to the evolution of the concept of high-risk (with either T4 or N2 disease) and low-risk (with T1–3, N1 disease) stage III patient populations. To optimally treat stage III colorectal cancer, it may be necessary to treat these groups in different ways. The SCOT study has similarly demonstrated that patients with T1–3, N1 disease have better 3-year DFS than those with either T4 or N2 pathology, but it did not show a difference in duration effect between these groups when this was assessed using a test for heterogeneity. Nonetheless, 3-month chemotherapy was clearly non-inferior for patients with T1–3, N1 colorectal cancer (HR 0.91, 95% CI 0.75 to 1.10). For patients with T4 and/or N2 pathology, outcomes were poorer when they received 3 months of treatment; the non-inferiority of this treatment duration in terms of DFS was not seen in this group of patients in the SCOT study (HR 1.07, 95% CI 0.94 to 1.22). The HR was > 1 for the 3-month compared with the 6-month duration, suggesting some loss of efficacy. However, the wide CIs mean that this result could reflect a wide spectrum of possible effects, from a small potential benefit with 3-month treatment to decreased efficacy beyond the non-inferiority boundary. It is important to note that the observed absolute reduction in 3-year DFS between the 3-month and the 6-month treatment groups was only 1.9%. Although non-inferiority was not formally met for high-risk stage III patients, in view of the small absolute difference in 3-year DFS the benefits of longer treatment duration need to be carefully balanced against the increased risk of cumulative chronic toxicity.
Some text in this paragraph is taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Among patients with T4 or N2 disease, the absolute increase in 3-year DFS rate comparing 6-month with 3-month treatment was 2.8% (95% CI –4.1% to 9.7%) for oxaliplatin and 5FU and 1.3% (95% CI –3.7% to 6.2%) for oxaliplatin and capecitabine (see Figure 8). In view of the difference in toxicity seen with longer duration treatment, patients are likely to accept a small reduction in DFS in exchange for reduced toxicity; this is especially true if they are able to receive oxaliplatin and capecitabine. There is less evidence to support use of short-duration adjuvant treatment if it is decided that the patient needs to receive oxaliplatin and 5FU or has T4 disease.
Safety and toxicity
To our knowledge, this is the largest randomised study of adjuvant chemotherapy for colorectal cancer and confirms the findings of previous studies by showing that oxaliplatin-based combination chemotherapy can be safely delivered, with a mortality rate of 0.5%. 6,47 The most common grade 3/4 side effects reported were peripheral neuropathy, diarrhoea, neutropenia, fatigue, pain, nausea and hand–foot syndrome, also consistent with previous studies. Although 12% of patients overall had grade 3/4 neutropenia, only 1.2% had febrile neutropenia; the febrile neutropenia rate in the MOSAIC study was 1.8%. 6
Some text in this paragraph is taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
The SCOT study results show that 6 months of adjuvant chemotherapy is associated with significantly greater toxicity than 3 months of treatment. This difference was most apparent for peripheral neuropathy, with 58% of patients in the 6-month treatment group reporting grade ≥ 2 neuropathy compared with 25% of patients in the 3-month treatment group. Peripheral neuropathy, as reported via a patient questionnaire, was significantly worse in the 6-month treatment group and persisted for ≥ 5 years. Other AEs that are important to patients, such as diarrhoea and hand–foot syndrome, were also significantly more common in patients undergoing 6 months of treatment. Our conclusions regarding long-term neuropathy consequences need to be interpreted in the light of a substantial number of missing data. However, the treatment effect and significance levels are substantial, with no evidence of bias between the treatment groups, and the analysis approach was adapted to make allowance for missing data.
The effects of toxicity early in treatment are important; in the SCOT study, 14% of patients in each group did not complete 3 months of treatment. A similar proportion (13%) of those stopping treatment in the first 3 months in an adjuvant study using oxaliplatin and capecitabine was reported in 2007. 47 Although the choice of chemotherapy was not randomised, in the SCOT study a greater proportion of patients receiving oxaliplatin and capecitabine (15%) appeared to stop treatment before 3 months than estimated for those receiving oxaliplatin and 5FU (11%).
Achieving full-duration adjuvant chemotherapy treatment over 6 months is more difficult than over 3 months. In this study, 83.3% of patients randomised to 3-month adjuvant chemotherapy continued with the regimen for the planned duration, whereas only 58.8% of those randomised to 6-month adjuvant chemotherapy continued for the planned duration. Problems with delivering 6 months of therapy were most pronounced for the oxaliplatin component, where the median percentage of full dose delivered was 96.6% for the 3-month treatment group as compared with 70.2% for the 6-month treatment group. Fluoropyrimidine treatment was not compromised to the same extent, with the median percentage of full doses delivered being 95.3% and 83.2% for the 3-month and 6-month treatment groups, respectively.
As expected, there was a difference in the toxicity profile according to fluoropyrimidine backbone, with more grade 3/4 neutropenia in those receiving oxaliplatin and 5FU (23% vs. 5%) and more grade 3/4 diarrhoea in those receiving oxaliplatin and capecitabine (15% vs. 9%). The frequency of these toxicities is consistent with those reported in other studies of adjuvant treatment. 6,47
Peripheral neuropathy
The only chronic toxicity seen in the SCOT study was sensory peripheral neuropathy, which occurred with significantly lower frequency in the 3-month treatment group than in the 6-month treatment group (overall grade 3/4 toxicity was 4.1% in the 3-month treatment group compared with 16.5% in the 6-month treatment group). The study therefore met its aim of achieving a > 50% reduction in the incidence of grade 3/4 peripheral neuropathy by administering treatment over 3 months. The frequency of peripheral neuropathy reported here is higher than seen for 6-month adjuvant treatment in the MOSAIC, NSABP C-07 and XELOXA trials (12.4%, 8.2% and 11%, respectively). 6,7,47 Peripheral neuropathy in the SCOT study was assessed by the investigator using NCI CTCAE (version 3), which grades the condition on a scale of 0–4; neuropathy in the MOSAIC study was graded using NCI CTCAE (version 1), in the NSABP C-07 study using the NCI-Sanofi Neurosensory score (graded from 0–3) and in the XELOXA study using NCI CTCAE (version 3). As peripheral neuropathy has become recognised as the main dose-limiting toxicity with oxaliplatin, clinicians may be questioning patients more closely about such symptoms in more recent trials. The incidence and course of acute and chronic peripheral neuropathy as a result of oxaliplatin has been well documented using a comprehensive PRO measure (EORTC QLQ-CIPN 20). 11 This study showed that the incidence and severity of sensory peripheral neuropathy increases with the number of cycles of adjuvant chemotherapy received, in broad agreement with the findings of this study.
In the SCOT study, patients were administered either oxaliplatin and capecitabine or oxaliplatin and 5FU in a non-randomised manner. Although the cumulative oxaliplatin dose is similar for the two regimens (1040 mg/m2 and 1020 mg/m2, respectively), the individual doses of oxaliplatin are higher with oxaliplatin and capecitabine (130 mg/m2 every third week compared with 85 mg/m2 every second week with oxaliplatin and 5FU). However, the rates of grade ≥ 2 peripheral neuropathy were similar irrespective of the fluoropyrimidine backbone for both the 3-month (21.3% with oxaliplatin and 5FU and 25.7% with oxaliplatin and capecitabine) and the 6-month (57.7% and 58.1%) groups.
In this study, some of the patients who were randomised to receive either 3- or 6-month duration of adjuvant chemotherapy had persistent neuropathy over ≥ 5 years, with neuropathy frequency being significantly higher (p < 0.001) at 3, 4 and 5 years for those randomised to the 6-month duration treatment. The level of chronic peripheral neuropathy seen in the SCOT study is in line with that previously reported 18 months after treatment using EORTC QLQ-CIPN 20,48 with 50% of patients reporting < 10% reduction in sensory neuropathy scores and 19% reporting > 30% reduction in sensory neuropathy scores compared with baseline. 11
The MOSAIC6 and the NSABP C-077 studies showed rates of grade 3 neuropathy reported at 1 year of 1.1% and 0.6%, respectively. It is now recognised that persistent peripheral neuropathy is a major side effect for patients who have received oxaliplatin, especially in the adjuvant setting. For the majority of these patients neuropathy is a significant problem; because of this, it may be better detected using a more in-depth PRO measure such as the FACT/GOG-Ntx4 questionnaire rather than grading it using the NCI CTCAE.
Health-related quality of life
Health-related quality of life (measured using QLQ-C30 Global Health Status and EQ-5D-3L) declined while patients were receiving adjuvant chemotherapy. HRQoL was, therefore, reduced for longer in patients randomised to receive 6-month treatment compared with 3-month treatment. HRQoL improved after stopping treatment and within 1 year there were no clinically important differences between the two treatment groups. EQ-5D health status, assessed using both the self-rated visual analogue scale and the EQ-5D-3L utility index, did not differ between 9 months and the 7-year follow-up based on the minimal clinically important differences for health utilities in cancer. 23
Health-related quality of life and peripheral neuropathy
It is accepted that clinician assessment of neuropathy using methods such as the CTCAE criteria are less sensitive than PRO tools such as the FACT/GOG-Ntx4 or the EORTC QLQC CIPN 20. 49 In this study, we supplemented the CTCAE analyses with specific assessment of peripheral neuropathy using the FACT/GOG-Ntx4.
Health-related quality of life appeared to recover within 1 year of starting treatment; however, chronic sensory peripheral neuropathy persisted for ≥ 5 years. Patients who recorded a greater degree of neuropathy symptoms had significantly worse HRQoL at 12, 36 and 60 months than those with lesser symptoms. These differences in HRQoL reached at least minimal clinically important levels. Similar findings were reported for a Dutch study, in which there was a correlation between neuropathy symptoms and HRQoL for the 10% of patients with the worst neuropathy symptoms (as measured by the EORTC QLQ-CIPN 20), with a reduction in the HRQoL scores (as measured by the QLQ-C3020) from 2 to 11 years after oxaliplatin treatment for colorectal cancer. 50 It appears that a lack of an overall difference in HRQoL scores does not reflect the meaningful differences experienced by patients with the worst long-term, chronic, neuropathy.
Health economic assessment
The results of the economic evaluation showed that a 3-month duration adjuvant regimen is cheaper and a dominant strategy for chemotherapy treatment for patients with high-risk, stage II or stage III colorectal cancer. The shorter treatment duration significantly reduced costs related to adjuvant chemotherapy administration and hospitalisation. The 3-month treatment group showed significantly better patient HRQoL during the treatment period, with no significant impact on overall survival, leading to an overall QALY gain that did not reach statistical significance. The probabilistic analysis and exploration of cost-effectiveness acceptability showed little uncertainty in the economic results over a wide range of WTP thresholds.
Four subgroup analyses came to similar conclusions, with the 3-month treatment group being the dominant or cost-effective strategy given a £30,000 cost per QALY threshold. The type of chemotherapy administered showed the greatest subgroup affect on cost-effectiveness. The interaction between planned treatment and QALY differences between the two treatment groups was statistically significant. With oxaliplatin and 5FU chemotherapy, the 6-month treatment group generated more QALYs than seen for the 3-month treatment group, which were primarily driven by longer estimates of disease-free and overall survival. HRQoL differences between groups favoured 3-month treatment but the survival gains for the 6-month oxaliplatin and 5FU regimen were relevant over the 8-year follow-up period, resulting in an overall QALY gain. Nonetheless, at a decision threshold of £30,000/QALY the NMB was larger for 3-month treatment (incremental gain of £3229 per patient) and, therefore, the 6-month duration treatment would not be considered cost-effective from a UK perspective. 30
Modelling an 8-year patient follow-up period allowed exploration of cost-effectiveness outcomes over this longer time period. Key cost differences occurred within the first year after randomisation, where 6-month treatment was nearly twice the cost of 3-month chemotherapy when including associated hospitalisations. When considering years 1 to 8 there were minimal differences in hospitalisation costs between groups; however, over the entire 8-year analysis period the total costs per patient were significantly different and favoured 3-month treatment. Differences in costs between the treatment groups over an even longer term would not be expected, with extrapolation beyond 8 years being unlikely to change outcomes unless there were substantial differences in survival rate. The 8-year follow-up also allows thorough consideration of the QALY results, which are driven by event timing. The small differences in life expectancy are subject to uncertainty in both the base-case and subgroup analyses, yet the overall HRQoL is consistently higher for the 3-month treatment group across all analyses. The maximum benefit in terms of HRQoL is seen 3 to 6 months after randomisation, at which time patients receiving 3-month duration chemotherapy have stopped treatment. The negative impact on HRQoL for the 6-month treatment group is accounted for by chemotherapy-related AEs. 33
Study context
The SCOT trial is part of a wider initiative, the IDEA collaboration, which aims to consolidate the results from all trials being conducted worldwide (SCOT, TOSCA,51 IDEA France,52 CALGB/SWOG 80702, ACHIEVE,53 and HORG54) that are attempting to address whether or not the duration of oxaliplatin-based adjuvant chemotherapy can be reduced to 3 months for patients with stage III colon cancer. 14 The results of the SCOT trial are consistent with those from the IDEA collaboration in indicating that the relative effect of shortening the duration of adjuvant chemotherapy may depend on the choice of chemotherapy regimen (oxaliplatin and 5FU vs. oxaliplatin and capecitabine; p = 0.069 heterogeneity in the SCOT study). 55 When the effect of duration was considered separately for each regimen, oxaliplatin and capecitabine showed non-inferiority for 3-month treatment in terms of DFS when compared with 6-month duration adjuvant chemotherapy; for reasons that are not clear, this was not the case for oxaliplatin and 5FU adjuvant chemotherapy. The choice of chemotherapy was not randomised but was chosen by the physician and patient, with the reasons for selection of a particular regimen being unknown.
Limitations of the study
Some text in this paragraph is taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
The SCOT study was underpowered owing to issues with recruitment, which meant that fewer patients than planned participated in the trial. However, the 95% CIs for the HR for the primary analysis lay below the non-inferiority boundary and the results were consistent with those of individual studies conducted within the IDEA group, particularly when considering how the duration effect depended on treatment regimen and disease risk. This consistency with other studies indicates that the results of the SCOT study are unlikely to represent a false-positive, in terms of showing non-inferiority. The concept that underpowered studies are more likely to produce false-positives (ignoring the factor of publication bias, which does not apply to a large scale enterprise such as the SCOT study) is disputed. 56
Despite the size of the SCOT study, there are limitations to the reliability of the conclusions that can be drawn for some patient subgroups. In particular, the SCOT study and the IDEA results indicate that the duration effect differs according to the non-randomised choice of oxaliplatin and 5FU and oxaliplatin and capecitabine chemotherapy; although non-inferiority could be shown for oxaliplatin and capecitabine, the results for oxaliplatin and 5FU are less conclusive. Examination of results within the two stage III risk groups (T1–3, N1 and T4 or N2) are similarly compromised.
Some limitations were also associated with the cost-effectiveness analyses presented, which generally relate to the need for data censoring given the minimum 3-year follow-up and relatively high survival rates (in the region of 75% after 8 years). The cost and quality-adjusted survival methodology used (Kaplan–Meier sample average and partitioned survival analysis, respectively) were chosen to reduce censoring-related bias. Lifetime extrapolation was not undertaken for the base-case within trial analysis given the non-inferiority of overall survival and DFS, and dominance of the 3-month treatment group. However, a lifetime analysis may improve the evidence for subgroup analyses where non-inferiority criterion were not met and the dominance conclusion was subject to uncertainty. Future work will explore lifetime analyses for the subgroups.
It should be noted that, although the SCOT study results are applicable to all patients who might be considered for 6 months of adjuvant combination chemotherapy with oxaliplatin and fluoropyrimidine, there are patients who are currently treated with 6 months of adjuvant fluoropyrimidine only (some patients with high-risk stage II disease or older patients with stage III disease). The SCOT study results are not applicable to patients receiving single agent fluoropyrimidine.
Chapter 6 Conclusions
The SCOT study achieved its primary end point for the overall trial population, showing that 3 months of oxaliplatin-containing adjuvant chemotherapy is non-inferior to 6 months of the same regimen. Three-month oxaliplatin-based chemotherapy should, therefore, be considered as an adjuvant regimen, particularly to oxaliplatin and capecitabine combination therapy. As the study recruited 6088 patients with conventionally defined high-risk stage II and stage III colorectal cancer from a large number of centres and countries and made use of standard chemotherapy regimens, the study findings are applicable to a typical patient with colorectal cancer who needs adjuvant chemotherapy treatment.
Although 3-month treatment with oxaliplatin and capecitabine was statistically non-inferior to 6-month treatment, the study did not demonstrate with statistical certainty non-inferiority for the 3-month oxaliplatin and 5FU regimen. Similarly, for patients with T1–3, N1 disease, the SCOT study demonstrated non-inferiority for 3-month adjuvant treatment; however, for patients with T4 or N2 pathology, a small absolute decrease in 3-year DFS was seen with 3-month duration treatment for both the oxaliplatin and capecitabine and the oxaliplatin and 5FU regimens. In both cases, these absolute differences are small with wide CIs. Therefore, in young, fit patients with very poor prognosis, a more aggressive approach, with longer treatment duration, might be appropriate.
The SCOT results are supported by the IDEA collaboration, which also showed that the effect of duration of adjuvant treatment was influenced by the choice of chemotherapy regimen and was similarly dependent on whether the patient had low- or high-risk stage III disease.
Some text in this paragraph are taken from Iveson et al. 16 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Six-month treatment is associated with a considerable increase in toxicity compared with 3-month treatment. The toxicity profiles of the oxaliplatin and capecitabine and oxaliplatin and 5FU regimens differ, with diarrhoea and hand–foot syndrome more common with capecitabine and neutropenia more common with 5FU. This study confirmed that patients receiving 6-month duration treatment experience significantly greater acute and chronic neurotoxicity, with chronic neurotoxicity persisting to ≥ 5 years. Patients with the worst chronic neuropathy had significantly poorer HRQoL. Neuropathy is, therefore, a significant chronic side effect of oxaliplatin therapy for patients receiving 6 months of adjuvant chemotherapy. In view of the increased toxicity seen with longer therapy and the consequential effects on long-term HRQoL, there should always be a discussion with the individual patient about the most appropriate duration of treatment, balancing the potential for longer treatment to achieve small efficacy benefits with the likelihood of increased toxicity and poorer HRQoL. The final decision on treatment duration and regimen for each individual will depend on a careful discussion between the clinician and patient, taking into account the risk of recurrence, the likely absolute difference in DFS and risk of long-term toxicity, and the strength of evidence for that particular disease context available from both the SCOT study and the wider IDEA analysis.
This study found that, compared with traditional 6-month adjuvant chemotherapy, the 3-month treatment strategy costs significantly less and has no significant detrimental impact on patient outcome (HRQoL and survival). The 3-month regimen was found to dominate 6-month adjuvant chemotherapy for patients with high-risk stage II or stage III colorectal cancer. Cost-effectiveness is affected by the type of chemotherapy regimen used; however, the 3-month strategy was the optimal choice for both the oxaliplatin and capecitabine and the oxaliplatin and 5FU regimens based on relevant WTP thresholds. This economic evaluation adds to the evidence showing that 3-month treatment is not only cost-effective but also dominant over the current standard of 6-month treatment; little uncertainty is associated with this conclusion, which supports the economic case that 3-month treatment is sufficient for most patients.
Implications for practice and implications for research
The findings of this research indicate that 3 months of adjuvant chemotherapy may be sufficient for some patients. For patients with low-risk stage III disease (T1–3, N1) receiving adjuvant oxaliplatin and capecitabine chemotherapy, 3 months of treatment is sufficient. Many low-risk stage III patients receiving adjuvant oxaliplatin and 5FU chemotherapy and high-risk stage III patients receiving adjuvant oxaliplatin and capecitabine chemotherapy may be considered for 3 months of treatment. The optimum duration of adjuvant treatment for high-risk stage III patients is less certain.
Research is needed on the influence of T4 or N2 on the required duration of adjuvant treatment for high-risk stage III patients.
Further research should be conducted to identify any specific patient groups (e.g. patients with specific high-risk pathological features) for whom 6 months of adjuvant chemotherapy might be appropriate and if this is dependent on the regimen selected. The translational tissue samples from the SCOT study (3383 tumour samples and 3100 blood samples) and from other similar studies should be used to build molecular predictors of which patients may benefit from longer treatment. Some of this work is currently under way for the SCOT study.
Acknowledgements
Contributions of authors
Professor Timothy Iveson (https://orcid.org/0000-0002-4681-2712) (Corresponding Author, Medical Oncologist) was involved in the study concept and design, trial supervision, patient recruitment, data analysis and manuscript writing and review.
Dr Kathleen A Boyd (https://orcid.org/0000-0002-9764-0113) (Senior Lecturer in Health Economics) undertook data analysis, manuscript writing and review.
Professor Rachel S Kerr (https://orcid.org/0000-0002-7240-3269) (Medical Oncologist) was involved in study concept and design, trial supervision, data analysis and manuscript writing and review.
Dr Jose Robles-Zurita (https://orcid.org/0000-0002-3862-8798) (Health Economics) undertook data analysis, manuscript writing and review.
Professor Mark P Saunders (Clinical Oncologist) was involved in trial supervision, patient recruitment, data analysis and manuscript writing and review.
Professor Andrew H Briggs (https://orcid.org/0000-0002-0777-1997) (Professor of Health Economics and Health Technology Assessment) was involved in grant submission, study concept and design, manuscript writing and review.
Professor Jim Cassidy (https://orcid.org/0000-0003-3917-561X) (Medical Oncologist) was involved in grant submission, study concept and design, trial supervision, patient recruitment and review of the manuscript.
Dr Niels Henrik Hollander (https://orcid.org/0000-0003-2509-2231) (Clinical Oncologist) was involved in trial supervision, patient recruitment and review of the manuscript.
Dr Josep Tabernero (https://orcid.org/0000-0002-2495-8139) (Medical Oncologist) was involved in trial supervision, patient recruitment and review of the manuscript.
Dr Andrew Haydon (Medical Oncologist) was involved in trial supervision, patient recruitment and review of the manuscript.
Professor Bengt Glimelius (https://orcid.org/0000-0002-5440-791X) (Medical Oncologist): was involved in trial supervision, patient recruitment and review of the manuscript.
Mrs Andrea Harkin (https://orcid.org/0000-0002-8831-7381) (Clinical Trials Unit Operations Director, Glasgow) was involved in grant submission, data interpretation, manuscript writing and review.
Mrs Karen Allan (https://orcid.org/0000-0002-3887-0402) (Clinical Trials Unit Project Manager, Glasgow) was involved in grant submission, data collection, data interpretation, manuscript writing and review.
Mr John McQueen (https://orcid.org/0000-0001-5950-8819) (Clinical Trials Unit Trial Co-ordinator, Glasgow) undertook data collection, data interpretation, manuscript writing and review.
Mrs Sarah Pearson (https://orcid.org/0000-0001-5386-1953) (Trial Co-ordination, Oxford) undertook data interpretation, manuscript writing and review.
Dr Ashita Waterston (https://orcid.org/0000-0002-2080-4749) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Louise Medley (https://orcid.org/0000-0003-0725-3812) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Charles Wilson (https://orcid.org/0000-0002-2597-5694) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Richard Ellis (https://orcid.org/0000-0002-4760-7921) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Sharadah Essapen (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Amandeep S Dhadda (https://orcid.org/0000-0003-3561-3811) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Mark Harrison (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Stephen Falk (https://orcid.org/0000-0001-6106-3062) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Sherif Raouf (https://orcid.org/0000-0002-3870-1828) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Charlotte Rees (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Rene K Olesen (https://orcid.org/0000-0002-3331-6904) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr David Propper (https://orcid.org/0000-0002-4274-5460) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr John Bridgewater (https://orcid.org/0000-0001-9186-1604) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Ashraf Azzabi (https://orcid.org/0000-0003-4801-9109) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr David Farrugia (https://orcid.org/0000-0001-9777-1394) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Andrew Webb (https://orcid.org/0000-0003-1304-0434) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr David Cunningham (https://orcid.org/0000-0001-5158-1069) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Tamas Hickish (Medical Oncology) undertook patient recruitment and review of the manuscript.
Dr Andrew Weaver (https://orcid.org/0000-0001-8919-3598) (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Simon Gollins (Clinical Oncology) undertook patient recruitment and review of the manuscript.
Dr Harpreet Wasan (https://orcid.org/0000-0002-6268-2030) (Medical Oncology) undertook patient recruitment and review of the manuscript.
Mr James Paul (https://orcid.org/0000-0001-7367-5816) (Head of Biostatistics, Clinical Trials Unit, Glasgow) was involved in grant submission, study concept and design, data interpretation, data analysis, manuscript writing and review.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review and appropriate agreements being in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. Lyon: International Agency for Research on Cancer; 2013.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997;40:15-24. https://doi.org/10.1007/BF02055676.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. https://doi.org/10.1056/NEJM199002083220602.
- O’Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ, Erlichman C, Shepherd L, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol 1998;16:295-300. https://doi.org/10.1200/JCO.1998.16.1.295.
- National Comprehensive Cancer Network (NCCN) . Guidelines: Colon Cancer (Version 2.2107) n.d.
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. https://doi.org/10.1056/NEJMoa032709.
- Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. https://doi.org/10.1200/JCO.2006.08.2974.
- Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. https://doi.org/10.1200/JCO.2010.33.6297.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. https://doi.org/10.1093/annonc/mds236.
- Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 2007;25:2205-11. https://doi.org/10.1200/JCO.2006.08.6652.
- Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416-22. https://doi.org/10.1200/JCO.2014.58.8533.
- Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer 2003;88:1859-65. https://doi.org/10.1038/sj.bjc.6600995.
- Laudicella M, Walsh B, Burns E, Smith PC. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer 2016;114:1286-92. https://doi.org/10.1038/bjc.2016.77.
- André T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: trial design and current status. Curr Colorectal Cancer Rep 2013;9:261-9. https://doi.org/10.1007/s11888-013-0181-6.
- World Medical Association . World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ 2001;79:373-4.
- Iveson TJ, Kerr RS, Saunders MP, Cassidy J, Hollander NH, Tabernero J, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol 2018;19:562-78. https://doi.org/10.1016/S1470-2045(18)30093-7.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017-26. https://doi.org/10.1016/j.ejca.2009.08.014.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer 2007;17:387-93. https://doi.org/10.1111/j.1525-1438.2007.00794.x.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139-44. https://doi.org/10.1200/JCO.1998.16.1.139.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-70.
- Paul J, Iveson T, Midgley R, Harkin A, Masterton M, Alexander L, et al. Choice of randomisation time-point in non-inferiority studies of reduced treatment duration: experience from the SCOT study. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-S1-A30.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Qian W, Parmar MK, Sambrook RJ, Fayers PM, Girling DJ, Stephens RJ. Analysis of messy longitudinal data from a randomized clinical trial. MRC Lung Cancer Working Party. Stat Med 2000;19:2657-74. https://doi.org/10.1002/1097-0258(20001015)19:19<2657::AID-SIM557>3.0.CO;2-3.
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75:800-2. https://doi.org/10.1093/biomet/75.4.800.
- Demets DL. Futility approaches to interim monitoring by data monitoring committees. Clin Trials 2006;3:522-9. https://doi.org/10.1177/1740774506073115.
- André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015;33:4176-87. https://doi.org/10.1200/JCO.2015.63.4238.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342. https://doi.org/10.1136/bmj.d1548.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48. https://doi.org/10.1191/0962280202sm269ra.
- Boyd KA, Briggs AH, Paul J, Iveson T, Midgely R, Harkin A, et al. Analysis of adverse events and health-related quality of life data for an economic evaluation of adjuvant chemotherapy incolorectal cancer: when can we stop collecting?. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-S1-A41.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3100.
- Joint Formulary Committee . British National Formulary 2017.
- Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer 2009;115:2081-91. https://doi.org/10.1002/cncr.24246.
- NHS National Services Scotland Information Services Division . Scottish Health Service Costs 2016 2016.
- Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:419-34. https://doi.org/10.2307/2533947.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. https://doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. https://doi.org/10.1200/JCO.2012.42.5645.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. https://doi.org/10.1200/JCO.2009.27.1825.
- Shi Q, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant oxaliplatin-based therapy (3 vs 6 months) for patients with stage III colon cancer: the IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. J Clin Oncol 2017;35. https://doi.org/10.1200/JCO.2017.35.15_suppl.LBA1.
- Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005;16:549-57. https://doi.org/10.1093/annonc/mdi116.
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. https://doi.org/10.1056/NEJMoa043116.
- Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. J Clin Oncol 2007;25:102-9. https://doi.org/10.1200/JCO.2006.08.1075.
- Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 2005;41:1135-9.
- Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL. National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer 2016;24:1439-47. https://doi.org/10.1007/s00520-015-3063-4.
- Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699-707. https://doi.org/10.1200/JCO.2013.49.1514.
- Sobrero A, Lonardi S, Rosati G, Di Bartolomeo M, Ronzoni M, Pella N, et al. FOLFOX or CAPOX in stage II to III colon cancer: Efficacy Results of the Italian Three or Six Colon Adjuvant Trial. J Clin Oncol 2018;36:1478-85. https://doi.org/10.1200/JCO.2017.76.2187.
- André T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol 2018;36:1469-77. https://doi.org/10.1200/JCO.2017.76.0355.
- Yoshino T, Yamanaka T, Oki E, Kotaka M, Manaka D, Eto T, et al. Efficacy and long-term peripheral sensory neuropathy of 3 vs 6 months of oxaliplatin-based adjuvant chemotherapy for colon cancer: The ACHIEVE Phase 3 Randomized Clinical Trial [published online ahead of print September 12 2019]. JAMA Oncol 2019. https://doi.org/10.1001/jamaoncol.2019.2572.
- Souglakos J, Boukovinas I, Kakolyris S, Xynogalos S, Ziras N, Athanasiadis A, et al. Three versus six months adjuvant FOLFOX or CAPOX for high risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant chemotherapy (IDEA) project [published online ahead of print June 22 2019]. Ann Oncol 2019. https://doi.org/10.1093/annonc/mdz193.
- Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177-88. https://doi.org/10.1056/NEJMoa1713709.
- Hooper R. The Bayesian interpretation of a P-value depends only weakly on statistical power in realistic situations. J Clin Epidemiol 2009;62:1242-7. https://doi.org/10.1016/j.jclinepi.2009.02.004.
Appendix 1 Baseline characteristics of analysis subgroups
| Characteristic | Randomised treatment group | |
|---|---|---|
| 3 months of treatment | 6 months of treatment | |
| Gender, n (%) | ||
| Female | 166 (38.2) | 165 (38.0) |
| Male | 268 (61.8) | 269 (62.0) |
| Total | 434 (100) | 434 (100) |
| Age at registration | ||
| Median (years) | 63 | 65 |
| IQR (years) | 59–69 | 58–70 |
| Range (years) | 27–83 | 20–78 |
| Total (n) | 434 | 434 |
| Performance status at randomisation, n (%) | ||
| 0 | 276 (63.6) | 263 (60.6) |
| 1 | 158 (36.4) | 171 (39.4) |
| Total | 434 (100.0) | 434 (100.0) |
| Disease site, n (%) | ||
| Colon | 354 (81.6) | 355 (81.8) |
| Rectum | 80 (18.4) | 79 (18.2) |
| Total | 434 (100.0) | 434 (100.0) |
| T stage, n (%) | ||
| 0 | 0 (0.0) | 1 (0.2) |
| 1 | 11 (2.5) | 10 (2.3) |
| 2 | 43 (9.9) | 39 (9.0) |
| 3 | 246 (56.7) | 250 (57.6) |
| 4 | 134 (30.9) | 134 (30.9) |
| Total | 434 (100.0) | 434 (100.0) |
| N stage, n (%) | ||
| 0 | 58 (13.4) | 56 (12.9) |
| 1 | 250 (57.6) | 255 (58.8) |
| 2 | 126 (29.0) | 123 (28.3) |
| Total | 434 (100.0) | 434 (100.0) |
| Planned treatment, n (%) | ||
| FOLFOX | 155 (35.7) | 158 (36.4) |
| CAPOX | 279 (64.3) | 276 (63.6) |
| Total | 434 (100.0) | 434 (100.0) |
| If CAPOX planned, starting dose of capecitabine, n (%) | ||
| 750 mg/m2 | 5 (27.8) | 5 (22.7) |
| 800 mg/m2 | 0 (0.0) | 0 (0.0) |
| 1000 mg/m2 | 13 (72.2) | 17 (77.3) |
| Total | 18 (100.0) | 22 (100.0) |
| High-risk stage II, n (%) | ||
| No | 377 (86.9) | 378 (87.1) |
| Yes | 57 (13.1) | 56 (12.9) |
| Total | 434 (100.0) | 434 (100.0) |
| Characteristic | Randomised treatment group | |
|---|---|---|
| 3 months of treatment | 6 months of treatment | |
| Gender, n (%) | ||
| Female | 361 (39.4) | 363 (39.8) |
| Male | 555 (60.6) | 549 (60.2) |
| Total | 916 (100.0) | 912 (100.0) |
| Age at registration | ||
| Median (years) | 64 | 65 |
| IQR (years) | 59–70 | 59–70 |
| Range (years) | 24–83 | 20–83 |
| Total (n) | 916 | 912 |
| Performance status at randomisation, n (%) | ||
| 0 | 595 (65.0) | 581 (63.7) |
| 1 | 321 (35.0) | 331 (36.3) |
| Total | 916 (100.0) | 912 (100.0) |
| Disease site, n (%) | ||
| Colon | 755 (82.4) | 750 (82.2) |
| Rectum | 161 (17.6) | 162 (17.8) |
| Total | 916 (100.0) | 912 (100.0) |
| T stage, n (%) | ||
| 0 | 0 (0.0) | 1 (0.1) |
| 1 | 22 (2.4) | 25 (2.7) |
| 2 | 84 (9.2) | 80 (8.8) |
| 3 | 527 (57.5) | 516 (56.6) |
| 4 | 282 (30.8) | 290 (31.8) |
| X | 1 (0.1) | 0 (0.0) |
| Total | 916 (100.0) | 912 (100.0) |
| N stage, n (%) | ||
| 0 | 140 (15.3) | 141 (15.5) |
| 1 | 519 (56.7) | 517 (56.7) |
| 2 | 257 (28.1) | 254 (27.9) |
| Total | 916 (100.0) | 912 (100.0) |
| Planned treatment, n (%) | ||
| FOLFOX | 292 (31.9) | 297 (32.6) |
| CAPOX | 624 (68.1) | 615 (67.4) |
| Total | 916 (100.0) | 912 (100.0) |
| If CAPOX planned, starting dose of capecitabine, n (%) | ||
| 750 mg/m2 | 84 (22.3) | 83 (22.4) |
| 800 mg/m2 | 18 (4.8) | 18 (4.9) |
| 1000 mg/m2 | 274 (72.9) | 270 (72.8) |
| Total | 376 (100.0) | 371 (100.0) |
| High-risk stage II, n (%) | ||
| No | 778 (84.9) | 774 (84.9) |
| Yes | 138 (15.1) | 138 (15.1) |
| Total | 916 (100.0) | 912 (100.0) |
| Characteristic | Randomised treatment group | |
|---|---|---|
| 3 months of treatment | 6 months of treatment | |
| Gender, n (%) | ||
| Female | 361 (39.4) | 363 (39.8) |
| Male | 555 (60.6) | 550 (60.2) |
| Total | 916 (100.0) | 913 (100.0) |
| Age at registration | ||
| Median (years) | 64 | 65 |
| IQR (years) | 59–70 | 59–70 |
| Range (years) | 24–83 | 30–83 |
| Total (n) | 916 | 913 |
| Performance status at randomisation, n (%) | ||
| 0 | 595 (65.0) | 582 (63.7) |
| 1 | 321 (35.0) | 331 (36.3) |
| Total | 916 (100.0) | 913 (100.0) |
| Disease site, n (%) | ||
| Colon | 755 (82.4) | 751 (82.3) |
| Rectum | 161 (17.6) | 162 (17.7) |
| Total | 916 (100.0) | 913 (100.0) |
| T stage, n (%) | ||
| 0 | 0 (0.0) | 1 (0.1) |
| 1 | 22 (2.4) | 25 (2.7) |
| 2 | 84 (9.2) | 80 (8.8) |
| 3 | 527 (57.5) | 517 (56.6) |
| 4 | 282 (30.8) | 290 (31.8) |
| X | 1 (0.1) | 0 (0.0) |
| Total | 916 (100.0) | 913 (100.0) |
| N stage, n (%) | ||
| 0 | 140 (15.3) | 141 (15.4) |
| 1 | 519 (56.7) | 518 (56.7) |
| 2 | 257 (28.1) | 254 (27.8) |
| Total | 916 (100.0) | 913 (100.0) |
| Planned treatment, n (%) | ||
| FOLFOX | 292 (31.9) | 297 (32.5) |
| CAPOX | 624 (68.1) | 616 (67.5) |
| Total | 916 (100.0) | 913 (100.0) |
| If CAPOX planned, starting dose of capecitabine, n (%) | ||
| 750 mg/m2 | 84 (22.3) | 84 (22.6) |
| 800 mg/m2 | 18 (4.8) | 18 (4.8) |
| 1000 mg/m2 | 274 (72.9) | 270 (72.6) |
| Total | 376 (100.0) | 372 (100.0) |
| High-risk stage II, n (%) | ||
| No | 778 (84.9) | 775 (84.9) |
| Yes | 138 (15.1) | 138 (15.1) |
| Total | 916 (100.0) | 913 (100.0) |
| Characteristic | Randomised treatment group | |
|---|---|---|
| 3 months of treatment | 6 months of treatment | |
| Gender, n (%) | ||
| Female | 562 (38.9) | 548 (38.4) |
| Male | 883 (61.1) | 878 (61.6) |
| Total | 1445 (100.0) | 1426 (100.0) |
| Age | ||
| Median (years) | 64 | 65 |
| IQR (years) | 58–70 | 59–70 |
| Range (years) | 24–83 | 20–85 |
| Total (n) | 1445 | 1426 |
| Performance status at randomisation, n (%) | ||
| 0 | 993 (68.7) | 965 (67.7) |
| 1 | 452 (31.3) | 461 (32.3) |
| Total | 1445 (100.0) | 1426 (100.0) |
| Disease site, n (%) | ||
| Colon | 1169 (80.9) | 1155 (81.0) |
| Rectum | 276 (19.1) | 271 (19.0) |
| Total | 1445 (100.0) | 1426 (100.0) |
| T stage, n (%) | ||
| 0 | 0 (0.0) | 1 (0.1) |
| 1 | 35 (2.4) | 42 (2.9) |
| 2 | 133 (9.2) | 126 (8.8) |
| 3 | 831 (57.5) | 806 (56.5) |
| 4 | 445 (30.8) | 451 (31.6) |
| X | 1 (0.1) | 0 (0.0) |
| Total | 1445 (100.0) | 1426 (100.0) |
| N stage, n (%) | ||
| 0 | 232 (16.1) | 230 (16.1) |
| 1 | 830 (57.4) | 816 (57.2) |
| 2 | 383 (26.5) | 380 (26.6) |
| Total | 1445 (100.0) | 1426 (100.0) |
| Planned treatment, n (%) | ||
| FOLFOX | 467 (32.3) | 458 (32.1) |
| CAPOX | 978 (67.7) | 968 (67.9) |
| Total | 1445 (100.0) | 1426 (100.0) |
| If CAPOX planned, starting dose of capecitabine, n (%) | ||
| 750 mg/m2 | 159 (21.8) | 149 (20.6) |
| 800 mg/m2 | 28 (3.8) | 30 (4.1) |
| 1000 mg/m2 | 543 (74.4) | 545 (75.3) |
| Total | 730 (100.0) | 724 (100.0) |
| High-risk stage II | ||
| No | 1215 (84.1) | 1199 (84.1) |
| Yes | 230 (15.9) | 227 (15.9) |
| Total | 1445 (100.0) | 1426 (100.0) |
Appendix 2 Reasons for missing quality of life questionnaires, examination of pattern of missingness and sensitivity analysis
| Missing questionnaires: reason | Treatment group | |
|---|---|---|
| 3 months | 6 months | |
| EORTC questionnaire, n (%) | ||
| Complete | 6629 (81.4) | 7420 (80.2) |
| Patient refused | 175 (2.1) | 168 (1.8) |
| Patient relapsed/evidence of new tumour | 56 (0.7) | 53 (0.6) |
| Patient unfit | 33 (0.4) | 46 (0.5) |
| Other | 1233 (15.1) | 1542 (16.7) |
| Not required | 19 (0.2) | 28 (0.3) |
| EQ-5D questionnaire, n (%) | ||
| Complete | 8924 (76.2) | 9684 (75.3) |
| Patient refused | 256 (2.2) | 273 (2.1) |
| Patient relapsed/evidence of new tumour | 213 (1.8) | 181 (1.4) |
| Patient unfit | 73 (0.6) | 89 (0.7) |
| Other | 2204 (18.8) | 2573 (20.0) |
| Not required | 46 (0.4) | 55 (0.4) |
| GOG-Ntx4 questionnaire, n (%) | ||
| Complete | 9658 (64.4) | 10,858 (65.6) |
| Patient refused | 263 (1.8) | 281 (1.7) |
| Patient relapsed/evidence of new tumour | 190 (1.3) | 187 (1.1) |
| Patient unfit | 74 (0.5) | 101 (0.6) |
| Other | 2890 (19.3) | 3255 (19.7) |
| Not required | 1924 (12.8) | 1871 (11.3) |
It can be seen that the reasons are broadly similar between the treatment groups, the most common reason being ‘other’. Examination of a random sample of the free text on the CRFs when the reason for ‘other’ was entered indicates that in the vast majority of cases this was an error of some sort (e.g. form was not returned, a site error occurred, patient forgot). The high proportion of ‘not required’ for GOG-Ntx4 is a result of administrative delay in implementing the amendment to extend the recording period for the questionnaire.
Table 20 shows the proportion of missing PRO questionnaires by baseline patient characteristics. It can be seen that the proportion is very similar for all baseline characteristics, with the difference in general being very small and never exceeding 5%.
| Characteristic | EORTC missing, n (%) | EQ-5D missing, n (%) | GOG-Ntx4 missing, n (%) | |||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | |
| Gender | ||||||
| Female | 5371 (79.8) | 1362 (20.2) | 7103 (74.9) | 2376 (25.1) | 7753 (64.4) | 4286 (35.6) |
| Male | 8678 (81.3) | 1991 (18.7) | 11,505 (76.2) | 3587 (23.8) | 12,763 (65.4) | 6750 (34.6) |
| Age group (years) | ||||||
| ≤ 50 | 1326 (81.3) | 304 (18.7) | 1715 (75.1) | 570 (24.9) | 1940 (65.4) | 1026 (34.6) |
| ≤ 70 | 9529 (80.9) | 2250 (19.1) | 12,666 (76.2) | 3958 (23.8) | 13,961 (65.0) | 7503 (35.0) |
| > 70 | 3194 (80.0) | 799 (20.0) | 4227 (74.7) | 1435 (25.3) | 4615 (64.8) | 2507 (35.2) |
| Performance status | ||||||
| 0 | 9259 (80.6) | 2222 (19.4) | 12,190 (75.4) | 3980 (24.6) | 14,112 (65.4) | 7451 (34.6) |
| 1 | 4790 (80.9) | 1131 (19.1) | 6418 (76.4) | 1983 (23.6) | 6404 (64.1) | 3585 (35.9) |
| T-stage | ||||||
| X/0/1 | 406 (77.3) | 119 (22.7) | 536 (74.5) | 183 (25.5) | 583 (63.9) | 330 (36.1) |
| 2 | 1278 (81.2) | 296 (18.8) | 1710 (76.8) | 518 (23.2) | 1888 (65.6) | 991 (34.4) |
| 3 | 8082 (81.7) | 1811 (18.3) | 10,783 (76.8) | 3250 (23.2) | 11,866 (65.7) | 6200 (34.3) |
| 4 | 4283 (79.2) | 1127 (20.8) | 5579 (73.5) | 2012 (26.5) | 6179 (63.7) | 3515 (36.3) |
| N-stage | ||||||
| 0 | 2121 (82.5) | 451 (17.5) | 3072 (77.2) | 908 (22.8) | 3566 (66.3) | 1810 (33.7) |
| 1 | 8004 (81.1) | 1863 (18.9) | 10,514 (76.2) | 3287 (23.8) | 11,724 (65.5) | 6172 (34.5) |
| 2 | 3924 (79.1) | 1039 (20.9) | 5022 (74.0) | 1768 (26.0) | 5226 (63.1) | 3054 (36.9) |
| Treatment | ||||||
| FOLFOX | 5479 (82.1) | 1197 (17.9) | 6935 (76.8) | 2090 (23.2) | 7897 (67.7) | 3765 (32.3) |
| CAPOX | 8570 (79.9) | 2156 (20.1) | 11,673 (75.1) | 3873 (24.9) | 12,619 (63.4) | 7271 (36.6) |
Table 21 summarises the comparison of the three most important PRO end points at key time points. The first column is taken from the imputation analysis as presented in the paper, the second column is based on observed data and the last column is based on an imputation restricted to patients who completed a baseline questionnaire. This last analysis was carried out to ensure that the analysis set could not be influenced by a patient's subsequent experience on the study (e.g. patients who should have completed at baseline but were missed and subsequently completed a follow-up form because they developed neurotoxicity are omitted from this analysis).
| Difference | Imputed (as in manuscript), mean (SE) | Observed data only, mean (SE) | Imputed (restricted to patients who completed baseline questionnaire), mean (SE) |
|---|---|---|---|
| GOG-Ntx4 questionnaire | |||
| Differencea at 6 months | –1.99 (0.17)b | –2.31 (0.35)b | –2.16 (0.28)b |
| Difference at 12 months | –2.48 (0.15)b | –2.54 (0.23)b | –2.30 (0.20)b |
| Difference at 5 years | –2.07 (0.33)b | –1.90 (0.42)b | –1.90 (0.34)b |
| EORTC-QLQ-C30 global quality of life | |||
| Difference at 6 months | 11.58 (1.00)b | 12.00 (1.41)b | 12.72 (1.82)b |
| Difference at 12 months | 1.48 (0.85)c | 1.57 (0.99)c | 1.22 (0.86)c |
| EQ-5D Visual Analogue Scale | |||
| Difference at 6 months | 9.80 (1.04)b | 7.05 (1.52)b | 7.36 (0.90)b |
| Difference at 12 months | 1.45 (0.88)c | 1.68 (0.96)c | 1.31 (1.00)c |
At all time points the mean differences are similar and consistent in terms of statistical significance.
Appendix 3 Short Course Oncology Therapy site recruitment and principal investigators
| Site name | Principal investigator(s) | Total recruitment (n) |
|---|---|---|
| Beatson West of Scotland Cancer Centre | Professor Jim Cassidy | 141 |
| Dr Ashita Waterson | ||
| Royal United Hospital Bath | Dr Gareth Rees | 111 |
| Dr Louise Medley | ||
| Dr Emma De Winton | ||
| Addenbrookes Hospital | Dr Charles Wilson | 97 |
| Royal Cornwall Hospital | Dr Richard Ellis | 94 |
| Royal Surrey County Hospital | Dr Gary Middleton | 93 |
| Dr Sharadah Essapen | ||
| Castle Hill Hospital | Dr Amandeep Dhadda | 91 |
| Mount Vernon Hospital | Dr Rob Hughes | 89 |
| Bristol Oncology Centre | Dr Stephen Falk | 85 |
| Queen’s Hospital | Dr Sherif Raouf | 83 |
| Southampton General Hospital | Dr Timothy Iveson | 81 |
| Western General Hospital | Dr Lesley Dawson | 80 |
| Christie Hospital NHS Trust | Dr Mark Saunders | 77 |
| Salisbury District Hospital | Dr Timothy Iveson | 77 |
| Dr Alaaeldin Shablak | ||
| Velindre NHS Trust Hospital | Dr Alison Brewster | 76 |
| St Bartholomews Hospital | Dr David Propper | 74 |
| Queen Alexandra Hospital | Dr Ann O’Callaghan | 73 |
| Sjællands Universites Hospital, Næstved | Dr Neils Henrik Holländer | 70 |
| Princess Alexandra Hospital | Dr John Bridgewater | 69 |
| Royal Marsden Hospital (Sutton) | Professor David Cunningham | 67 |
| Leicester Royal Infirmary | Professor Will Steward | 59 |
| Professor Anne Thomas | ||
| Worcester Royal Hospital | Dr David Farrugia | 59 |
| Dr Mark Churn | ||
| Singleton Hospital | Professor John Wagstaff | 57 |
| Birmingham Heartlands Hospital | Dr Ian Geh | 55 |
| Dr Shobhit Baijal | ||
| Maidstone and Tunbridge Wells NHS Trust | Dr Mark Hill | 55 |
| Royal Shrewsbury Hospitals NHS Trust | Dr Saif Awwad | 54 |
| Dr Abel Zachariah | ||
| Northampton General Hospital NHS Trust | Dr Kinnari Patel | 53 |
| Dr Craig Macmillan | ||
| Dr Roshan Agarwal | ||
| Russells Hall Hospital | Professor David Ferry | 51 |
| Dr Simon Grumett | ||
| Lincoln County Hospital | Dr Tom Sheehan | 50 |
| Dr Zuzana Stokes | ||
| Fife Health Board | Dr Catriona Mclean | 48 |
| King Edward VII Hospital | Dr Marcia Hall | 48 |
| Dr Maher Hadaki | ||
| Royal Hampshire County Hospital | Dr Virginia Hall | 48 |
| Dr Sanjay Raj | ||
| Dr David Nolan | ||
| Sunderland Royal Hospital | Dr Ashraf Azzabi | 48 |
| West Suffolk Hospital | Dr Anne Margaret Moody | 48 |
| Hammersmith Hospital | Dr Charles Lowdell | 47 |
| Churchill Hospital | Dr Andrew Weaver | 46 |
| Aarhus University Hospital | Dr René Krøjgaard Olesen | 45 |
| Yeovil District Hospital | Dr Stephen Falk | 43 |
| Dr Julie Walther | ||
| Dr Erica Beaumont | ||
| Dr Matthew Sephton | ||
| Kettering General Hospital | Dr Craig Macmillan | 42 |
| Dr Guy Faust | ||
| Dr Roshan Agarwal | ||
| Crosshouse Hospital | Dr Jeffery White | 41 |
| Musgrove Park Hospital | Dr Julie Walther | 41 |
| Dr Clare Barlow | ||
| Worthing Hospital | Dr Andrew Webb | 41 |
| Hinchingbrooke Hospital | Dr Cheryl Palmer | 40 |
| Great Western Hospital | Dr Claire Hobbs | 39 |
| Dr Sarah Lowndes | ||
| Hospital Universitari Vall D Hebron | Dr Elena Elez Meelez | 39 |
| Monklands District General Hospital | Dr Ashita Waterston | 39 |
| Dr Clinton Ali | ||
| Dr Anne Mckillop | ||
| Wycombe Hospital | Dr Andrew Weaver | 39 |
| Aalborg Hospital | Dr Jorgen Hansen | 38 |
| Dr Mette Yilmaz | ||
| Ipswich Hospital | Dr Rubin Soomal | 38 |
| Dr Liz Sherwin | ||
| Pilgrim Hospital | Dr Tom Sheehan | 38 |
| Dr Zuzana Stokes | ||
| Raigmore Hospital | Dr David Whillis | 38 |
| Dr Neil Mcphail | ||
| St James’s University Hospital | Dr Daniel Swinson | 38 |
| Stepping Hill Hospital | Dr Jurjees Hasan | 38 |
| Weston Park Hospital NHS Trust | Dr Simon Pledge | 38 |
| Withybush General Hospital | Dr Vallipuram Vigneswaran | 38 |
| Belfast City Hospital | Dr Richard Wilson | 37 |
| Glan Clwyd Hospital | Dr Simon Gollins | 37 |
| Guy’s and St Thomas’ NHS Trust | Dr Nicholas Maisey | 37 |
| Poole Hospital NHS Trust | Dr Tamas Hickish | 37 |
| Dr Amelie Harle | ||
| Royal Bournemouth Hospital | Dr Tamas Hickish | 37 |
| Wrexham Maelor Hospital | Dr Simon Gollins | 37 |
| Hillerød Hospital | Dr Svend Erik Nielsen | 36 |
| Queen Elizabeth Hospital – King’s Lynn | Dr Athar Ahmad | 36 |
| Dr Gail Horan | ||
| St George’s Hospital | Dr Fiona Lofts | 36 |
| University College London Hospital | Dr John Bridgewater | 36 |
| York Hospital | Dr Kim Last | 36 |
| Nottingham University Hospital NHS Trust | Dr Eric Bessell | 35 |
| Dr Vanessa Potter | ||
| Dr Georgina Walker | ||
| Royal Berkshire Hospital | Dr James Gildersleve | 35 |
| Wexham Park Hospital | Dr Marcia Hall | 35 |
| Dr Maher Hadaki | ||
| South Tyneside District Hospital | Dr Shraf Azzabi | 34 |
| Broomfield Hospital | Professor Saad Tahir | 33 |
| Dr Gopalakrishnan Srinivasan | ||
| Eastbourne District General | Dr Fiona Mckinna | 33 |
| ICO L’Hospitalet | Dr Ramon Salazar | 33 |
| Dr Gemma Soler | ||
| Torbay Hospital | Dr Nangi Lo | 33 |
| Conquest Hospital | Dr Timothy Sevitt | 32 |
| Luton and Dunstable Hospital | Dr Suzannah Mawdsley | 32 |
| Dr Faye Lim | ||
| Professor Peter Hoskin | ||
| Aberdeen Royal Infirmary | Dr Leslie Samuel | 31 |
| Basingstoke and North Hampshire Hospital | Dr Charlotte Rees | 31 |
| North Middlesex University Hospital | Dr John Bridgewater | 31 |
| Queen’s Hospital Burton | Dr Prabir Chakraborti | 31 |
| Dr Pugazhenthi Pattu | ||
| Dr Majusha Keni | ||
| Blackpool Victoria Hospital | Dr Shabbir Susnerwala | 30 |
| Dr Sin Chong Lau | ||
| Cheltenham General Hospital | Dr David Farrugia | 30 |
| Regionshospitalet Herning | Dr Nina Keldsen | 30 |
| Diana Princess of Wales Hospital | Dr Rajarshi Roy | 29 |
| Dorset County Hospital | Dr Richard Osborne | 29 |
| Dr Maxine Flubacher | ||
| Cumberland Infirmary | Dr Jonathan J Nicoll | 28 |
| Gloucestershire Royal Hospital | Dr David Farrugia | 28 |
| Hereford County Hospital | Dr Nick Reed | 28 |
| Rigshospitalet | Dr Lone Nørgaard | 28 |
| Wansbeck Hospital | Dr Werner Dobrowsky | 28 |
| Dr Sandeep Singhal | ||
| Charing Cross Hospital | Dr Charles Lowdell | 27 |
| Dr Susan Cleator | ||
| Peterborough Hospital | Dr Karen Mcadam | 27 |
| Dr Abigail Hollingdale | ||
| Queen Elizabeth Hospital (Gateshead) | Dr Werner Dobrowsky | 27 |
| Dr Fiona McDonald | ||
| Dr Mark Katory | ||
| Queen Elizabeth Hospital (Birmingham) | Dr Neil Steven | 27 |
| Scarborough Hospital | Dr Mohan Hingorani | 27 |
| Dr Pugazhenthi Pattu | ||
| Southend Hospital | Dr David Tsang | 27 |
| James Cook University Hospital | Dr Nicholas Wadd | 27 |
| Altnagelvin Hospital | Dr Russell Houston | 26 |
| Dr Claire Harrison | ||
| Dr Sonali Dasgupta | ||
| Scunthorpe General Hospital | Dr Abdel Hamid | 26 |
| Dr Lorcan O’Toole | ||
| Good Hope Hospital | Dr John Glaholm | 25 |
| Dr Shobhit Baijal | ||
| Royal Sussex County Hospital | Dr Andrew Webb | 25 |
| Hospital Donostia | Dr Adelaida-Lacasta Munoa | 24 |
| New Cross Hospital | Dr Mark Churn | 24 |
| Dr Simon Grummet | ||
| University Hospital of North Staffordshire | Dr Fawzi Adab | 24 |
| Countess of Chester Hospital | Dr Adrian Moss | 23 |
| Dr Shaker Abdallah | ||
| Dr Dale Vimalachandran | ||
| University Hospital of Lewisham | Professor George Mikhael | 23 |
| Dr Jessica Brady | ||
| Royal Derby Hospital | Dr Rajendra Kulkarni | 23 |
| Medway Maritime Hospital | Dr Jeff Summers | 22 |
| Dr Christos Mikropoulos | ||
| Royal Preston Hospital | Dr Shabbir Susnerwala | 22 |
| Dr Deborah Williamson | ||
| University Hospital of North Durham | Dr Fareeda Coxon | 22 |
| Complejo Hospitalario Universitario de Santiago | Dr Rafael Lopez | 21 |
| Forth Valley Royal Hospital | Dr Ghazia Shaikh | 21 |
| Dr Saranya Kakumanu | ||
| Dr Stephen Harrow | ||
| Dr Adnan Shaukat | ||
| Dr Aisha Tufail | ||
| Dr Dawn Storey | ||
| Kent and Canterbury Hospital | Professor Roger James | 21 |
| Dr Julia Hall | ||
| Dr Rakesh Raman | ||
| Basildon Hospital | Dr David Tsang | 20 |
| Clatterbridge Centre for Oncology | Dr David Smith | 20 |
| Dr Julie O’Hagan | ||
| Freeman Hospital | Dr Fareeda Coxon | 20 |
| Stoke Mandeville Hospital | Dr Andrew Weaver | 20 |
| Darlington Memorial Hospital | Dr Fareeda Coxon | 19 |
| Harrogate District Hospital | Dr Kim Last | 19 |
| Hospital Del Mar | Dr Joaquim Bellmunt | 19 |
| Queen Elizabeth Hospital (London) | Dr Nicholas Maisey | 19 |
| Dr Asad Qureshi | ||
| Royal Marsden Hospital (London) | Professor David Cunningham | 19 |
| Sjællands Universites Hospital, Roskilde | Dr Jim Stenfatt Larsen | 19 |
| St Helens and Knowsley NHS Trust | Dr Ernest Marshall | 19 |
| Karolinska University Hospital | Dr Gisela Naucler | 18 |
| Dr Jan-Erik Frödin | ||
| Lister Hospital | Dr Rob Hughes | 18 |
| St Mary’s Hospital (Low) | Dr Christopher Baughan | 18 |
| Dr Harish Reddy | ||
| Glangwili General Hospital | Dr Margaret Wilkins | 18 |
| Dr Thi Mau-Don Phan | ||
| William Harvey Hospital | Professor Roger James | 18 |
| Dr Julia Hall | ||
| Bronglais General Hospital | Dr Alan Axford | 17 |
| Dr Sajid Durrani | ||
| Dr Elin Jones | ||
| Darent Valley Hospital | Dr Andrew Gaya | 17 |
| Dr Hazif Al Gurafi | ||
| Dr Riyaz Nazeerul Haq Shah | ||
| Hairmyres Hospital | Dr Grainne Dunn | 17 |
| Kidderminster Hospital | Dr Mark Churn | 17 |
| The Royal Oldham Hospital | Dr Saifee Mullamitha | 17 |
| Ayr Hospital | Dr Jeffery White | 16 |
| Craigavon Area Hospital | Dr Richard Park | 16 |
| Esbjerg Hospital | Dr Brita Bjerregaard | 16 |
| King’s Mill Hospital | Dr Ivo Hennig | 16 |
| Dr Eleanor James | ||
| Dr Eliot Chadwick | ||
| Dr Christina Lopezesccola | ||
| Warrington and Halton Hospitals NHS Trust | Dr Adrian Moss | 16 |
| Dr Amy Ford | ||
| Herlev Hospital | Dr Kirsten Vistisen | 15 |
| Hospital Arnau De Villanova Lleida | Dr Antonia Salud | 15 |
| Macclesfield District General Hospital | Dr Catherine Mcbain | 15 |
| Dr Ganesh Radhakrishna | ||
| Bradford Royal Infirmary | Dr Chris Bradley | 14 |
| Dr Andrew Conn | ||
| North West London Hospitals NHS Trust | Dr Nicola Anyamene | 14 |
| Royal Free Hospital | Dr Astrid Mayer | 14 |
| Sønderborg Hospital | Dr Jurij Bogovic | 14 |
| University Hospital of North Tees | Dr David Wilson | 14 |
| Bendigo Hospital | Dr Robert Blum | 13 |
| Bishop Auckland General Hospital | Dr Nick Wadd | 13 |
| Dr Fareeda Coxon | ||
| Central Hospital Vasteras | Dr Henry Letocha | 13 |
| Dr Andrzej Piwowar | ||
| City Hospital Trust | Dr Daniel Rea | 13 |
| Dr Pankaj Punia | ||
| Manor Hospital | Dr Andrew Hartley | 13 |
| Dr Victoria Kunene | ||
| Royal Hobart Hospital | Dr Ray Lowenthal | 13 |
| Dr David Boadle | ||
| Royal Albert Edward Infirmary | Dr Gregory Wilson | 13 |
| Dr Elena Takeuchi | ||
| Dr Francisca Elena Marti | ||
| Weston General Hospital | Dr Marjorie Tomlinson | 13 |
| Dr Reuben West | ||
| Austin Hospital | Dr Niall Tebbutt | 12 |
| Bankstown – Lidcombe Hospital | Dr Ray Asghari | 12 |
| Nambour General Hospital | Dr Michelle Cronk | 12 |
| Queen Elizabeth The Queen Mother Hospital | Professor Roger James | 12 |
| Dr Julia Hall | ||
| Bedford Hospital NHS Trust | Professor Robert Thomas | 11 |
| Hospital Universitario De Canarias | Dr Marta Llanos | 11 |
| Hospital Clinico Universitario De Valencia | Dr Andres Cervantes | 11 |
| Royal Marsden Hospital (Kingston) | Dr David Cunningham | 11 |
| Uppsala University Hospital | Dr Bengt Glimelius | 11 |
| West Cumberland Hospital | Dr Jonathan J Nicoll | 11 |
| West Middlesex University Hospitals | Dr Pippa Riddle | 11 |
| Hospital Clinic I Provincial De Barcelona | Dr Cristina Nadal | 10 |
| Dr Estela Pineda | ||
| North Devon District Hospital | Dr Mark Napier | 10 |
| Sandwell General Hospital | Dr Daniel Rea | 10 |
| Dr Pankaj Punia | ||
| Southport and Formby District General Hospital | Dr Arthur Sun Myint | 10 |
| Dr Nasim Ali | ||
| St Mary’s Hospital London | Dr Susan Cleator | 10 |
| The Tweed Hospital | Professor Ehtesham Abdi | 10 |
| Ysbyty Gwynedd Hospital | Dr Nick Stuart | 10 |
| Dr Catherine Bale | ||
| Ballarat Hospital | Dr Geoff Chong | 9 |
| Canberra Hospital | Professor Desmond Yip | 9 |
| Centrallasarettet Vaxjo | Dr Eva Fernebro | 9 |
| Dr Ulrika Palenius | ||
| Complejo Hospitalario Universitario De Albacete | Dr Carmen Alonso Lopez | 9 |
| Hospital Universitario Marques De Valdecilla | Dr Fernando Rivera-Herrero | 9 |
| St John of God Hospital Bunbury | Dr Martin Buck | 9 |
| Airedale General Hospital | Dr Andrew Conn | 8 |
| Aintree University Hospitals | Dr David Smith | 8 |
| Dr Julie Hagan | ||
| Alfred Hospital | Dr Andrew Haydon | 8 |
| Auckland Hospital | Dr George Laking | 8 |
| Concord Repatriation General Hospital | Professor Philip Beale | 8 |
| Flinders Medical Centre | Dr Chris Karapetis | 8 |
| Hospital Universitario Madrid Norte Sanchinarro | Dr Antonio Cubillo | 8 |
| North Tyneside General Hospital | Dr Philip Atherton | 8 |
| Royal Devon and Exeter Hospital | Dr Melanie Osborne | 8 |
| Dr Mark Napier | ||
| County Hospital Ryhov | Dr Helga Hagman | 7 |
| Hospital La Fe | Dr Jorge Aparicio | 7 |
| Hospital Virgen De La Arrixaca | Dr Miguel Marin Vera | 7 |
| Inverclyde Royal Hospital | Dr Stephen Harrow | 7 |
| Dr Dawn Storey | ||
| Royal Lancaster Infirmary | Dr David Fyfe | 7 |
| Dr David Eaton | ||
| Royal Brisbane and Women’s Hospital | Dr Matthew Burge | 7 |
| Whittington Hospital | Dr Daniel Hochhauser | 7 |
| Dr Pauline Leonard | ||
| University Hospital of Hartlepool | Dr David Wilson | 7 |
| University Hospital Linköping | Dr Maria Albertsson | 7 |
| Cabrini Hospital | Dr Andrew Haydon | 6 |
| Calderdale and Huddersfield NHS Foundation Trust | Dr Jo Dent | 6 |
| Dumfries and Galloway Royal Infirmary | Dr Lesley Dawson | 6 |
| Hospital Miguel Servet | Dr Antonio Anton | 6 |
| Hospital De Txagorritxu | Dr Severina Dominguez | 6 |
| Leighton Hospital | Dr Chan Ton | 6 |
| Dr Michael Braun | ||
| Milton Keynes General Hospital | Dr Jill Stewart | 6 |
| Dr Gerard Andrade | ||
| Dr Somnath Mukherjee | ||
| Prince of Wales Hospital | Professor David Goldstein | 6 |
| Queen Elizabeth Hospital (Australia) | Professor Tim Price | 6 |
| Royal North Shore Hospital | Dr Alex Guminski | 6 |
| Associate Professor Nick Pavlakis | ||
| St Vincent’s Hospital | Dr Eva Segelov | 6 |
| Essex County Hospital | Dr Bruce Sizer | 5 |
| Falu Hospital | Dr Ake Berglund | 5 |
| Pinderfields General Hospital | Dr Gireesh Kumaran | 5 |
| Dr Konstantinos Kamposioras | ||
| Campbelltown Hospital | Dr Lorraine Chantrill | 4 |
| Christchurch Hospital | Dr Mark Jeffery | 4 |
| Furness General Hospital | Dr Alison Birtle | 4 |
| Dr David Eaton | ||
| Illawarra Cancer Centre (Wollongong Hospital) | Dr Morteza Aghmesheh | 4 |
| Kalmar County Hospital | Dr Charlotte Bratthall | 4 |
| Nevil Hall Hospital | Dr Nayyer Iqbal | 4 |
| Dr Mohammed Harb | ||
| Dr Keith Yinn | ||
| Nepean Cancer Care Centre | Dr Jenny Shannon | 4 |
| Norrlands University Hospital | Dr Birgitta Lindh | 4 |
| Princess Alexandra Hospital (Australia) | Dr Warren Joubert | 4 |
| Royal Darwin Hospital | Dr Narayan Karanth | 4 |
| St John of God Hospital Subiaco | Dr Siobhan Ng | 4 |
| Dr Tom Van Hagen | ||
| Tamworth Base Hospital | Dr Mathew George | 4 |
| Antrim Area Hospital | Dr Colin Purcell | 3 |
| St Richard’s Hospital | Dr Suhail Baluch | 3 |
| Dr Ann O’Callaghan | ||
| Dr Yasser Haba | ||
| Wellington Hospital | Dr Anne O’Donnell | 3 |
| Ballarat Oncology and Haematology Services | Dr George Kannourakis | 2 |
| Coffs Harbour Health Campus | Dr Karen Briscoe | 2 |
| Gosford Hospital | Dr Susan Tiley | 2 |
| Hallands Hospital | Dr Lotta Lundgren | 2 |
| Royal Perth Hospital | Dr David Ransom | 2 |
| University Hospital of Skåne | Dr Margareta Heby | 2 |
| Armidale Hospital | Associate Professor Nick Pavlakis | 1 |
| Dunedin Hospital | Dr Chris Jackson | 1 |
| Hospital De Cruces | Dr Guillermo Lopez Vivanco | 1 |
| ICO Del Hospital Josep Trueta | Dr Bernardo Queralt | 1 |
| Royal Liverpool University Hospital | Dr David Smith | 1 |
| Sundsvall Härnösand County Hospital | Dr Petra Flygare | 1 |
List of abbreviations
- 5FU
- 5-fluorouracil
- AE
- adverse event
- AUC
- area under the curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTCAE
- common terminology criteria for adverse event
- DFS
- disease-free survival
- DMC
- Data Monitoring Committee
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FACT/GOG-Ntx4
- Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- IDEA
- International Duration Evaluation of Adjuvant chemotherapy
- IQR
- interquartile range
- ITT
- intention to treat
- i.v.
- intravenous
- NCI
- National Cancer Institute
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- pNI
- p-value for non-inferiority
- PRO
- patient-reported outcome
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SCOT
- Short Course Oncology Therapy
- SD
- standard deviation
- SE
- standard error
- ToT
- time on treatment
- ULN
- upper limit of normal
- WTP
- willingness to pay
