Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/62/03. The contractual start date was in January 2013. The draft report began editorial review in April 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Khalida Ismail has received honoraria for educational lectures from Sanofi SA (Paris, France), Novo Nordisk (Bagsværd, Denmark), Janssen Pharmaceutica (Beerse, Belgium) and Eli Lilly and Company (Indianapolis, IN, USA). Kirsty Winkley received consultancy fees from Merck Sharp & Dohme (Kenilworth, NJ, USA).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Ismail et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Epidemiology of cardiovascular disease and its risk factors
Cardiovascular disease (CVD) is the leading cause of mortality, morbidity and disability in the UK and in other developed countries. 1 However, many of the major determinants of CVD are modifiable, including tobacco smoking, a diet high in saturated fat, high low-density lipoprotein (LDL) cholesterol, obesity, a sedentary lifestyle, hypertension and diabetes mellitus. 2–6 Lower educational attainment and lower socioeconomic status are associated with a greater risk of CVD, and this association is strongest for females. 7 The risk of CVD varies markedly between ethnic groups, with a higher rate of ischaemic heart disease in those of South Asian ethnicity and a higher rate of cerebrovascular disease in those of African ethnicity among those living in England and Wales. 8
Although CVD remains the most common cause of death in developed nations, mortality rates have been falling. Between 1981 and 2000, CVD mortality in the UK fell by 62% in men and 45% in women. 9 Cohort studies and prediction models suggested that a fall in the prevalence of tobacco smoking, a decline in population blood pressure levels and changes in cholesterol levels were important contributors. 9,10 Population-wide changes in modifiable risk factors, such as dietary intake, can bring about substantial benefits and further changes in blood lipids, particularly reductions in levels of non-high-density lipoprotein (HDL) cholesterol. 10 However, limited changes in physical activity (PA) and rising levels of obesity have limited the decline in CVD mortality. 10 Further efforts are therefore needed to bring about positive changes, particularly in diet, obesity and PA.
Cardiovascular risk identification
The NHS in England introduced the Health Check programme in 2009 as part of the Department of Health and Social Care (DHSC)’s long-term vision for the future of public health in England. 11 In offering Health Checks to all those aged 40–74 years without a known diagnosis of CVD, the aim is to prevent heart disease, stroke, diabetes mellitus and kidney disease and to reduce health inequalities. The risk assessment includes collection of demographic data, smoking status, cholesterol level, blood pressure and diabetes mellitus status. An individualised risk management plan is given in accordance with the assessment to support lifestyle changes, such as referral to smoking cessation sessions, exercise prescriptions, lifestyle advice and signposting to local resources.
A lower-than-anticipated coverage of NHS Health Checks has been reported,12 with regional variations in attendance ranging from 27% to 52%,12 with greater uptake in patients of older age and in regions of lower deprivation. 13 Reductions in CVD risk have been reported for those attending Health Checks;14 however, a systematic review of the implementation of Health Checks found no reduction in mortality or morbidity. 15 The programme has attracted criticism owing to a lack of an up-to-date evidence base and Clinical Commissioning Groups (CCGs) lacking the resources to implement them. 16
Several risk algorithms have been developed and validated to estimate the risk of developing CVD based on known risk factors; these risk algorithms include the Framingham Risk Score,17 the ASSIGN score,18 QRISK®19 and QRISK®220 (both QResearch, Nottingham, UK). The latter is now recommended by the National Institute for Health and Care Excellence (NICE) for the identification of people at risk of CVD up to the age of 84 years in England. 21 The QRISK2 algorithm takes into account self-report ethnicity, deprivation and other relevant clinical conditions, and is updated annually to reflect changes in clinical evidence, data recording and population demographics. Once a high CVD risk is ascertained, the primary prevention of CVD is recommended via lifestyle advice and interventions.
The evidence for increasing physical activity
Physical inactivity increases mortality and the risk of diseases such as CVD and diabetes mellitus. 22 The DHSC advises adults to perform ≥ 30 minutes of at least moderate-intensity PA on ≥ 5 days per week, in ≥ 10-minute bouts, for optimum health benefits. 23 Walking is the most common form of PA in adults and is associated with reductions in CVD and all-cause mortality, and walking pace is a stronger predictor of improved outcomes than walking duration. 24 Walking is promoted as a near-perfect exercise as it has the lowest risk of harm and is now included in UK public health policy. 25,26
However, the proportion of those achieving PA recommendations is low, particularly when objective measures are used to assess PA. In England, 39% of men and 29% of women self-report achieving the recommended PA levels, but objective assessment of PA using accelerometers in a subsample of the Health Survey for England found that only 5% of men and 4% of women aged 35–64 years achieved the recommended levels. 27
Systematic reviews and meta-analyses report moderate positive short-term increases in PA following lifestyle interventions, in either a group or an individual format, but findings are limited because most studies used self-report measures. 28,29 Evidence for brief PA interventions suggests improvement in PA in the short term, but there is limited evidence for the long-term impact, cost-effectiveness and deliverability in a primary care setting. 30,31 A review of 32 trials reported that walking interventions led to improvement in a number of cardiovascular risk factors, including blood pressure and weight, but not in lipids. 32 The majority of the reviewed intervention trials recruit motivated volunteers and report low response rates, which may limit the representativeness of observed findings.
The contents of assessed interventions to promote PA differ significantly, but social support and cognitive–behavioural therapy (CBT) strategies, rather than health education alone, are recommended in older adults. 33 The use of pedometers as a method of self-monitoring can increase PA and improve health in the short term. 34 Compared with usual care (UC), the use of pedometers and a brief walking intervention in primary care led to improvements in 12-month PA in a sample of adults not achieving the recommended activity levels at baseline. 35 The intervention was as effective when delivered by post as when delivered by nurses in primary care, suggesting that PA can be improved in physically inactive patients with minimal resources. Details of the components used in trials of interventions to promote PA, including information on the behaviour change techniques (BCTs) used, are recommended to improve implementation and evidence syntheses. 29,36
The evidence for dietary interventions
Modest beneficial changes to dietary intake, specifically changes to fat, fibre, fruit and vegetable intake, are found following healthy diet interventions in primary care, but there is variability in intervention design and delivery as well as the methodological quality of previous studies. 37 Based on the limited evidence available, estimates of the cost-effectiveness of dietary interventions in primary care suggest that interventions need to be targeted towards the older population at greatest risk of disease to be cost-effective. 38
A systematic review of randomised controlled trials (RCTs) assessing generic dietary advice for reducing CVD risk found modest beneficial effects on mean total and LDL cholesterol levels and small reductions in blood pressure up to 12 months later, suggesting that dietary advice may contribute to an improved CVD risk profile. 39 However, the longer-term effects of dietary interventions are unknown owing to limited follow-up periods of reviewed studies. Compared with UC, dietary interventions produce modest weight losses that diminish over time. 40 Further work is needed to understand how the modest benefits of dietary interventions may be maintained.
The evidence for motivational interviewing
Motivational interviewing (MI) is a common approach to behaviour change in health care, defined as a collaborative, goal-oriented style of communication with particular emphasis on the language of change. 41 It is designed to strengthen motivation for and commitment to a specific behavioural goal by eliciting and exploring personal reasons for change within an atmosphere of acceptance and compassion. 42 The appeal of MI is that it is brief, can be delivered by a range of health-care providers, has a competency framework and can be applied to a range of health-care settings, with evidence of benefits to health outcomes when compared with other interventions. 43
Systematic reviews and meta-analyses have demonstrated significant, moderate effects of MI on diet and exercise behaviours,44 as well as on health outcomes such as reduced weight, cholesterol and blood pressure, although the number of trials is small. 45,46 However, a systematic review of MI used in lifestyle interventions for people at risk or with a diagnosis of CVD found little evidence of the benefits of MI, noting the considerable variability between interventions and the outcomes measured and that few have included a long-term follow-up to assess whether or not any observed benefits are sustained. 47 A 14-month follow-up of diabetic patients who had received a MI lifestyle intervention found no benefits in lifestyle, biomedical or psychological outcomes compared with UC. 48 A RCT with a 12-month postintervention follow-up period found that the benefits of up to five MI sessions delivered over a period of 6 months on walking and cholesterol levels were maintained in a sample of overweight or obese patients, but other CVD risk factor outcomes were not maintained. 49 Effects were stronger for those found to be at higher risk at baseline, suggesting that interventions should target high-risk patients to achieve the best results.
A taxonomy of behaviour change techniques
The limitations of current models of lifestyle interventions, particularly their short-term effects, have led to a search for more sophisticated and targeted behavioural interventions. 50 A Cochrane Database Systematic Review of multiple risk factor interventions for the primary prevention of coronary heart disease observed that techniques based on instruction and information were associated with small improvements in lipid levels and blood pressure, especially when embedded in a theoretical framework related to behaviour change. 51 A systematic review of behavioural interventions found that the techniques most effective in improving diet and PA were based on self-regulatory behaviours, such as goal-setting, self-monitoring, giving feedback, utilising social support and MI. 52 Interventions based on a psychological theory, such as the theory of planned behaviour,53 were more effective, as were those for high-risk populations. There is less evidence to support the case for any minimum threshold of intensity, mode of delivery, intervention provider and setting;50–52 therefore, further study is required to understand how the benefits of behavioural interventions on lifestyle and health outcomes can be maintained. Evaluating interventions in the context of a taxonomy of BCTs and an intervention map offers a framework that is easier to teach, test, replicate and translate. 52–54
In a pilot RCT, a group intervention developed in line with evidence of the most effective BCTs52 led to reductions in weight, when co-interventions and comorbidities were controlled for, but did not increase PA at the 12-month follow-up in people at high risk of CVD, compared with UC. 55 As this was a pilot RCT, the authors note that the intervention was acceptable to participants and that outcomes could be improved by adapting the intervention accordingly. The effects of the group-based setting were not compared with a one-to-one setting, and there is little evidence in the literature on the differences between group and individual approaches. It has been found that the benefits of group sessions outweigh individual sessions even when it is not preferred by participants,56 and that group sessions are highly valued by participants. 57 However, it is also reported that at least one individual session is critical to the success of interventions and in ameliorating disengagement in some participants. 57,58 The variability in patient samples involved in these studies, the intervention delivered and the methodology of each study limit the generalisability of the findings.
By enhancing a MI approach with effective BCTs, the modest effect sizes of each may lead to improvement in outcomes. In people with type 1 diabetes mellitus, four sessions of MI alone was not associated with improved glycaemic control, but four sessions of MI followed by eight sessions of CBT was associated with improved glycaemic control, compared with UC. 59 However, the effects were not maintained after 12 months,60 and participants stated that MI helped them to become more ready to change their behaviour but that further support was needed to implement the change. 61 The evidence for enhancing MI with CBT is not consistent, as the landmark COMBINE (Combined Pharmacotherapies and Behavioural Interventions for Alcohol Dependence) study did not demonstrate increased abstinence from alcohol in those receiving the psychological intervention. 62
The role of health trainers
The DHSC recommended the deployment of health trainers into the most deprived areas of the UK in a White Paper published in 2004. 63 A health trainer is employed by the NHS from the local community in which they serve to provide lifestyle advice to those at risk of disease, with the overall aim of the programme being to address health inequalities, an important issue within multi-ethnic and variably deprived settings. 64 The role involves identifying clients from hard-to-reach and disadvantaged groups, providing one-to-one support in identifying potential problems in lifestyles, setting goals, supporting behaviour change and reviewing client progress. 65 The health trainer programme has been found to increase uptake of NHS Health Checks, particularly in men, younger age groups and those from less affluent areas. 66
A comprehensive review of interventions delivered by lifestyle advisors found little evidence of the benefits of interventions promoting healthy diet and/or increased PA in North American trials; there was no effect on weight and little evidence of improvement in PA levels, but the intervention contents, delivery and goals varied considerably. 67 A review of the available evidence on the NHS health trainer programme thus far indicates positive outcomes and acceptability, but critics argue that the models of service provision are varied, evaluations have included no comparator group and there is a lack of evidence for the maintenance of behaviour change. 68 A health trainer programme for CVD risk reduction in patients with at least one CVD risk factor found significant reductions in CVD risk after 12 months for only those who were identified as being at high risk of CVD (a Framingham Risk Score for CVD of ≥ 20.0%) at baseline. 69 The service also led to high levels of behavioural goal achievement and small, but significant, increases in quality of life. However, as this was a service evaluation there was no comparator group and achievement of behavioural goals was self-reported and unrelated to changes in CVD risk. Further work is needed to assess the potential for health trainers to deliver complex behavioural interventions, including the employment of objective outcome measures, the assessment of maintenance of behavioural change and clinical benefits, and comparison of outcomes with a control group.
The case for an enhanced motivational interviewing intervention
At the population level, the potential benefits of reducing weight and increasing PA in those who are at risk of CVD are considerable. Modest, short-term beneficial effects of various behaviour change interventions for the primary prevention of CVD emphasise the need for more complex, targeted interventions and RCTs that assess long-term benefits. MI provides broad appeal for its collaborative patient-centred style, evidence base and deliverability, and by enhancing a MI approach with the specific BCTs that have the strongest evidence, and incorporating techniques designed to enhance maintenance, observed outcomes may be improved. The relative effectiveness of a group intervention versus an individual intervention remains uncertain, but the former offers social support and may be more cost-effective, if it is acceptable to participants. Health trainers may improve the acceptability of interventions to harder-to-reach populations and positive outcomes have been reported in health trainer programmes thus far. The investigation of the potential for a health trainer to deliver more sophisticated interventions, and for the effects to be compared with UC in a RCT setting, is yet to be undertaken.
The overall aim was to compare the effectiveness of MOVE IT (enhanced MOtiVational intErviewing InTervention), which integrates MI with CBT BCTs, in reducing weight and increasing PA in those at high risk of CVD over 24 months across three arms: (1) enhanced MI in a group format versus (2) in an individual format versus (3) UC. The primary interest was whether or not MOVE IT in a group format was more effective and cost-effective than the individual format or UC because of its potential for social support.
Chapter 2 Research objectives
The following material has been primarily reproduced from our published study protocol, Bayley et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided he original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The purpose of this study was to design and evaluate MOVE IT for people at high risk of CVD, to be delivered by a healthy lifestyle facilitator (HLF) employed from the local community and trained in the intervention. We opted for the job title of HLF, rather than health trainer or lifestyle advisor, as this was thought to better reflect the principles of collaborative work underpinning MI.
Primary objective
To assess whether or not MOVE IT delivered in either a (1) group or (2) individual format is more effective than UC in reducing weight (kg) and increasing PA (average number of steps per day assessed via accelerometry) 24 months later.
Secondary objectives
-
To assess whether or not group MOVE IT is more effective than the individual format in reducing weight and increasing PA 24 months later.
-
To assess whether or not MOVE IT, delivered in either (1) a group format or (2) an individual format, is more effective than UC in reducing LDL cholesterol and in reducing CVD risk score 24 months later.
-
To compare the number of fatal or non-fatal cardiovascular events, and other recorded adverse events (AEs), per treatment allocation.
-
Cost-effectiveness: to assess whether or not MOVE IT delivered in either (1) a group format or (2) an individual format is more cost-effective than UC, in terms of quality-adjusted life-years (QALYs) gained over the 24-month follow-up period.
-
Process evaluation: using mixed methods, we conducted the following –
-
Mediational analysis: to examine whether or not changes in behavioural and psychological factors, such as dietary intake, health beliefs, depressive symptoms and self-efficacy, mediate the association between the intervention and outcomes.
-
Participation bias: to assess whether or not the RCT recruited those it intended, we assessed the participation bias by comparing the sociodemographic characteristics and QRISK2 scores of those who responded to the invitation to participate and those who did not.
-
Fidelity analysis: to assess whether or not MOVE IT was delivered in accordance with the manual and to compare whether or not the levels of competencies among the HLFs were associated with variations in outcomes using thematic contents analysis of sessions.
-
Participant and therapist experience: using qualitative methods, we describe the perceived expectations, benefits, strengths and limitations of the intervention from the patient and intervention provider perspective.
-
Chapter 3 Methods
The following material has been primarily reproduced from our published study protocol, Bayley et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided he original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text. The revised protocol was published before recruitment ended. 70
Trial design
This was a three-arm parallel RCT for individuals at high risk of CVD. The three arms were (1) UC and enhanced MI in a group format, (2) UC and enhanced MI in an individual format and (3) UC. As participants in the group intervention arm, but not in the other two arms, were clustered within groups, this trial has a partially clustered (or nested) design. Interventions were delivered in 10 sessions across a period of 12 months. Participants were followed up at 12 and 24 months from baseline.
Ethics approval and research governance
The Dulwich Research Ethics Committee (REC) (reference number 12/LO/0917) granted ethics approval. The trial was registered with an International Standard Randomised Controlled Trial Number (ISRCTN84864870).
Setting
The study was set in 12 South London CCGs (Bexley, Bromley, Croydon, Greenwich, Kingston, Lambeth, Lewisham, Merton, Richmond, Southwark, Sutton and Wandsworth) that are linked to each other by the South London Health Innovation and Education Cluster (SLHIEC) and inherent in this infrastructure is an efficient method for recruitment. South London has additional advantages: the population is approximately 3 million;71 nearly one-quarter of the population is African, Caribbean or South Asian; it spans the range of population densities, urbanisation and socioeconomic profiling; the development of a Health Innovation Network in South London can allow for rapid dissemination of research findings; and research resources can be shared across adjacent CCGs during periods of varying workload. General practices with list sizes of > 5000 patients were invited to take part, representing approximately 60% of all practices within the SLHIEC.
Eligibility criteria
The following eligibility criteria were used by general practitioners (GPs) during the initial search for eligible patients. In addition, exclusions were made following patient response to the invitation when the research assistant found that the patient did not meet the following criteria. When in any doubt, the opinion and approval of the patient’s GP was sought.
The inclusion criteria were:
-
being aged ≥ 40 years and ≤ 74 years
-
having a CVD risk score of ≥ 20.0%, calculated using QRISK2, a validated predictive tool for identifying the percentage risk of having a fatal or non-fatal cardiovascular event in the next 10 years72
-
being fluent in conversational English
-
being permanently resident and planning to stay in the UK at least three-quarters of a year.
The exclusion criteria were:
-
having established CVD (including congenital heart disease, angina, myocardial infarction, coronary revascularisation procedures, peripheral artery disease, coronary artery bypass graft or angioplasty)
-
having a pacemaker
-
being on a register for diabetes mellitus, kidney disease, atrial fibrillation or stroke (either ischaemic or haemorrhagic, including transient ischaemic attacks)
-
having chronic obstructive pulmonary disease
-
having a disabling neurological disorder
-
having a severe mental illness such as psychosis, a learning disability, dementia or cognitive impairment
-
being registered blind
-
being housebound or resident in a nursing home
-
being unable to move about independently or not being ambulatory
-
having more than three falls in the previous year
-
being pregnant
-
having advanced cancer
-
being morbidly obese [body mass index (BMI) of > 50 kg/m2]
-
currently participating in a weight-loss programme
-
living in the same household as another participant who was already randomised.
Sample size
The power calculation of our main outcome variables is based on the findings of previous research. 34,73 We selected a very conservative effect size of 0.25, expressed as the difference in units of pooled standard deviations (SDs), which translates to an ability to detect differences between two arms of 675 steps per day, 1.25 kg of weight and total cholesterol of 0.25 mmol/l. Our study was powered to detect changes that may be modest at the individual level but that would have an important impact if occurring at the population level. 74
We took into account clustering effect within the group intervention [intraclass correlation coefficient (ICC) of 0.05] by calculating the optimal sample size in presence of differential clustering effects. 75 A sample size of 1420 participants in total was needed to detect these differences in our primary hypotheses, and a two-tailed alpha of 0.025 was used to take account of multiple comparisons for ‘individual versus UC’, ‘group versus UC’ and ‘group versus individual’. Assuming an approximate 17% loss to follow-up, a total sample size of 1704 was calculated.
Recruitment
Invitation procedure
Participating general practices screened primary care databases for eligible patients using either EMIS (EMIS Health, Leeds, UK) or Vision (In Practice Systems, London, UK) medical records systems, two of the clinical software programmes most commonly used in UK primary care. Patients who met the eligibility criteria were invited to participate via a standardised letter from their general practice, which was posted along with a participant information sheet. A prepaid return envelope was included for the return of a reply slip, for the patient to express interest to take part, to the main study centre. After the patient had given permission to be contacted, a research assistant made telephone contact with the patient to confirm that they met all eligibility criteria and to schedule a first appointment.
Consenting participants
As participants attended a first appointment with the research assistant, they were asked to read and complete a consent form. Participants were given the opportunity to ask any questions regarding the study or the consent statements.
Calculation of cardiovascular disease risk score
Once consented, data collection began with CVD risk screening; a summary is given in Table 1. High CVD risk was calculated using QRISK2, a validated predictive tool for identifying those at a ≥ 20.0% chance of having a fatal or non-fatal cardiovascular event over the next 10 years. 72 The measures required for the calculation of QRISK2 score are:
-
Age (years).
-
Sex.
-
Self-report ethnicity.
-
Postcode.
-
Smoking status – current (if current, how many cigarettes or equivalent per day), ex-smoker or non-smoker. We also collected the number of years smoking for current and ex-smokers.
-
Diabetes mellitus status (none, type 1 or type 2).
-
Rheumatoid arthritis status.
-
Chronic kidney disease status.
-
Atrial fibrillation status.
-
Hypertensive treatment status.
-
Family history of CVD (a first-degree relative diagnosed with angina or heart attack when < 60 years of age).
-
Height (cm) and weight (kg) were taken for a calculation of BMI (kg/m2).
-
Systolic blood pressure (mmHg) – the third of three measurements taken.
-
The ratio of HDL cholesterol (mmol/l) to total cholesterol (mmol/l).
| Data collection | Time point | ||||||
|---|---|---|---|---|---|---|---|
| CVD risk screening | Baseline | Post baseline and pre randomisation | 12 months | Post 12 monthsa | 24 months | Post 24 monthsa | |
| Eligibility form | ✓ | ||||||
| Consent form | ✓ | ||||||
| Sociodemographic characteristics | ✓ | ✓ | |||||
| Changes to sociodemographic characteristics | ✓ | ✓ | |||||
| 7-day accelerometer data returned | ✓ | ✓ | ✓ | ||||
| Biomedical data | ✓ | ✓ | ✓ | ||||
| Blood results analysed | ✓ | ✓ | ✓ | ||||
| Record of past interventions | ✓ | ✓ | ✓ | ||||
| AUDIT | ✓ | ✓ | ✓ | ||||
| Smoking status | ✓ | ✓ | ✓ | ||||
| PHQ-9 | ✓ | ✓ | ✓ | ||||
| GPPAQ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| IPAQ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| BIPQ | ✓ | ✓ | ✓ | ||||
| Self-efficacy scale | ✓ | ✓ | ✓ | ||||
| EQ-5D | ✓ | ✓ | ✓ | ||||
| 24-hour dietary recall | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| CSRI | ✓ | ✓ | ✓ | ||||
| Medication data | ✓ | ✓ | ✓ | ||||
| AE questionnaire | ✓ | ||||||
| Participant feedback questionnaire | ✓ | ||||||
Postcode data were collected for the calculation of Index of Multiple Deprivation (IMD) 2010 scores76 (based on lower-layer super output area). 77 The IMD incorporates seven domains: income deprivation, employment deprivation, health deprivation and disability, education deprivation, crime, barriers to housing and services and living environment.
Levels of LDL cholesterol (mmol/l), triglycerides (mmol/l), plasma glucose (mmol/l) and glycated haemoglobin (HbA1c) (mmol/mol) were also taken at this time point, and patients with a HbA1c level of > 47 mmol/mol were subsequently excluded.
Baseline measures
If QRISK2 score was calculated as ≥ 20.0%, participants were subsequently invited to a second appointment for the collection of the following data prior to randomisation.
Sociodemographic data
These were age, sex, self-report ethnicity and family history of CVD collected for the QRISK2 calculation, occupational status, educational attainment, marital status, literacy [using the Rapid Estimate of Adult Literacy in Medicine (REALM)78] and place of residence.
Biomedical data
These were weight, height, BMI, blood pressure, plasma glucose level, lipids and HbA1c level, which were collected for the QRISK2 calculation. Data collection included a full-body composition analysis and measurement of waist, hip and arm circumferences.
Weight and body composition were measured in light clothing without shoes on the Class 3 Tanita® SC-240 digital scale (Tanita, Amsterdam, the Netherlands) to 0.1 units for weight (kg), fat range (%), fat mass (kg), fat-free mass (kg), body water (kg), muscle mass (kg), bone mass (kg) and to the nearest 1 unit for visceral fat (level) and impedance (ohm) and basal metabolic rate (kcal). Height was measured to 0.1 cm using stadiometers with the supported stretch stature method and weight and height measurements were used to calculate BMI (kg/m2).
Waist circumference (cm) was measured horizontally halfway between the lowest rib and the upper prominence of the pelvis using a non-extensible steel tape against the bare abdomen. Hip circumference (cm) was measured at the widest part of the hip. Arm circumference (cm) was taken from the mid-upper arm, at the midpoint between the top of the shoulder and the point of the elbow. Two measurements were taken for each circumference, with a third taken if the first two measurements differed by > 0.5 cm.
Blood pressure (mmHg/mmHg) and resting heart rate (beats per minute) were measured with digital Omron blood pressure monitors [Omron Healthcare (UK) Ltd, Milton Keynes, UK) using standardised procedures of three readings taken 1 minute apart while seated.
Lifestyle data
These were smoking status collected for QRISK2 calculation, PA, alcohol intake and dietary intake.
Physical activity was measured objectively using the ActiGraph GT3X accelerometer (ActiGraph, Pensacola, FL, USA), a validated tri-axial movement sensor that also records step count. 79 The research assistant explained to the participant how to wear the accelerometer: on a belt over the hip for 7 days, from waking in the morning until going to bed at night and removing only for bathing. Participants were asked to keep a log of activities, including sedentary activity, to assist with the qualitative interpretation of the data. The output from the accelerometer includes number of steps taken and time spent doing PA using standard cut-off points for sedentary activity and light, moderate, vigorous and very vigorous PA. The research assistant ensured that, on the participant returning the accelerometer, it had been worn for ≥ 540 minutes on each of ≥ 5 days, and, if not, the participant was asked to wear the accelerometer for another 7 days. Step count per day and a measurement of PA at an at least moderate level [moderate to vigorous PA (MVPA)] in > 10-minute bouts were extracted from the collected data. Standardised vertical axis cut-off points for PA were used to classify sedentary (0–99 counts per minute), light (100–1951 counts per minute), moderate (1952–5724 counts per minute), vigorous (5725–9498 counts per minute) and very vigorous (≥ 9499 counts per minute) activity, with MVPA equating to ≥ 1952 counts per minute. 80 Self-report PA was also measured using the General Practice Physical Activity Questionnaire and the International Physical Activity Questionnaire81 at both the baseline appointment and after 1 week of accelerometer wear.
Alcohol intake was measured using the Alcohol Use Disorders Identification Test (AUDIT),82 which has a range of scores values from 0 to 40. Participants were categorised as abstainers (score 0), low-risk drinkers (score 1–7) or possibly harmful drinkers (score ≥ 8).
Dietary intake was assessed using a standardised multiple-pass 24-hour dietary recall, which provides a more objective and reliable measure of change in intervention studies, at both the baseline appointment with the research assistant and independently after 1 week of accelerometer wear. Research assistants were trained to follow a standardised protocol and ask neutral probing questions to encourage recall of food items, and were taught about different methods of food preparations and brands in different cultures. Portion size was assessed using food photographs to estimate daily calorie intake. 83,84 Dietplan7 software (Forestfield Software Ltd, Horsham, UK) was used for coding the 24-hour recall diaries and deriving nutrient intake. For food not listed in the software’s database, we created new food codes and inputted the available nutritional information. If nutritional information was unavailable for an unlisted type of food, we selected the most similar type of food already in the database.
Psychological data
These included depressive symptoms, health beliefs and health state. Depressive symptoms were collected using the Patient Health Questionnaire-9 items (PHQ-9),85 as depression is associated with worse outcomes in CVD. 86 Each of the nine items are scored from 0 to 3 depending on frequency of occurrence of symptoms, for a score in the range of 0–27. One or two missing items can be substituted with the average score of non-missing items. When there are more than two missing values, the questionnaire is not scored. The questionnaire is followed by a functioning question, and the questionnaire results are provided to the patient’s GP.
The Brief Illness Perception Questionnaire was adapted to be used for ‘high risk of CVD’ rather than for an illness. 87 The questionnaire asks the participant to rate their beliefs of the causes of their high risk of CVD and how being at high risk affects them. A total score is calculated by summing together all items, with items 3, 4 and 7 reverse-scored, a higher score reflecting a more serious view of the high risk of CVD.
We also used a validated self-efficacy questionnaire for exercise,88 and adapted this to assess self-efficacy in keeping to a healthy diet as well as psychological processes we were seeking to change through the intervention. Average scores for both exercise and diet self-efficacy were calculated by summing together the score of each answered item and dividing by the number of items answered.
The EuroQol-5 Dimensions (EQ-5D) was used to measure the health state of the participant on the dimensions of mobility, self-care, usual activities, pain or discomfort and anxiety or depression using three levels: no problems, some problems or extreme problems. 89 Participants are asked to rate their current health state within the 0–100 range.
Health-care usage data
Health-care usage was assessed using the Client Service Receipt Inventory (CSRI),90 which included usage of hospital services, community-based services and support received from friends or relatives for any health reasons for the 12-month period leading up to the appointment. Data on current prescription medication were also collected from medical records.
Randomisation and allocation concealment
Simple randomisation was used, with general practice included as a random factor in the model and emphasis being on more practices and fewer patients per practice. Randomisation of participants was conducted by the data manager from an independent clinical trials unit (King’s College London) using computer-generated randomisation blocks with block sizes of 10. In each block, 10 participants were randomised to the group intervention arm, the individual intervention arm or the UC arm in a 4 : 3 : 3 ratio. The unequal allocation ratio ensured that the group intervention arm had approximately 33% more participants, allowing the group sessions to run with enough participants.
As this is a complex psychological intervention, it was not possible to conceal the allocation to the participants or the HLFs post randomisation. Research assistants, statisticians and technicians were blind to the allocation. Participants were reminded in advance of and during the follow-up appointments not to reveal their allocation.
Outcome measures
At the 12- and 24-month follow-up assessments, participants were asked if there had been any change to their sociodemographic status, such as accommodation, relationship status and educational attainment. AE and participant feedback questionnaires were administered. All other baseline measures were repeated at the 12- and 24-month follow-ups (see Table 1).
Trial arms and intervention details
Arm 1: usual care and enhanced motivational interviewing in a group format
Theoretical framework
The intervention is based on the theory of planned behaviour for initiation of behaviour change: to change behaviour, people need to form an intention. 53 Intention formation is influenced by three constructs: (1) expected value or positive attitude (people see the value in making the change), (2) subjective norm (significant others and peers also value the change) and (3) self-efficacy (people believe that they are capable of making the change).
Our intervention taps into all three constructs using principles and techniques from MI,41 CBT91 and social cognitive theory92 (Figure 1). MI is used to support participants in forming healthy intentions. MI is a collaborative conversation style for strengthening a person’s own motivation for, belief in and commitment to change. Hobbis and Sutton91 highlight the gap between translating intention into action and illustrate how CBT can be applied to bridge this gap. For this intervention, techniques from CBT are used to support the transition from intention to action, and action to maintenance. 93 Identifying and challenging unhelpful thoughts or thinking styles can promote more positive emotions and behaviours, for example ‘When I get breathless after some exercise (bodily sensation), this means that I am going to damage my heart (incorrect cognition)’ or ‘I have eaten one doughnut (behaviour), I might as well eat the whole bag (all or nothing cognition)’.
FIGURE 1.
Theoretical framework and intervention map. White background indicates psychological process; green background indicates behaviour change technique.
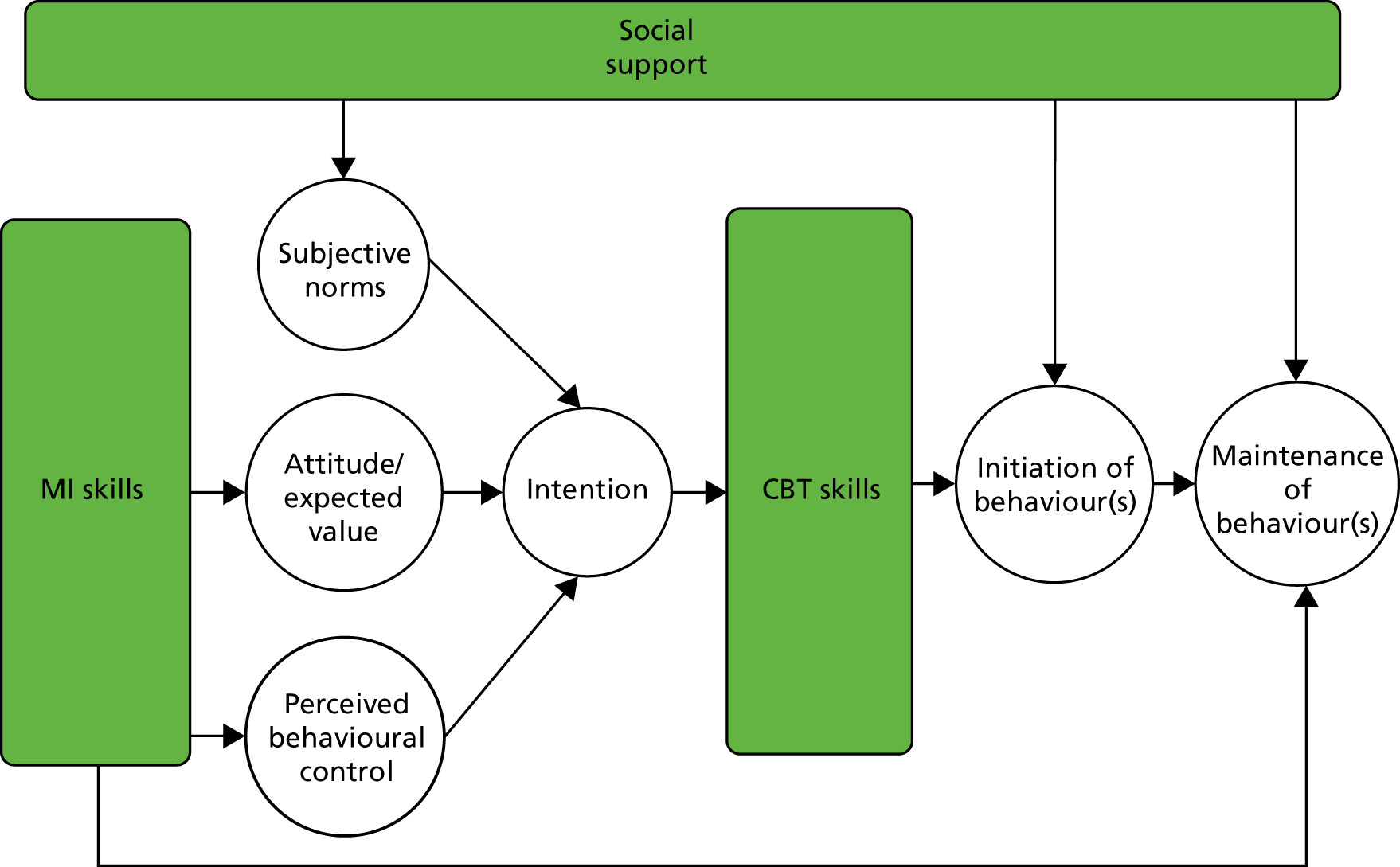
Social cognitive theory emphasises the importance of significant others in shaping people’s behaviours. The theory of planned behaviour also highlights this aspect through the ‘subjective norm’ construct. In our intervention, social networks from the participants’ own lives and/or group members (in the group intervention arm) were actively utilised to provide practical and emotional support and opportunities for modelling health behaviours during all phases of the intervention.
Intervention development
We conducted a scoping study to identify manuals published in English in the previous 5 years to improve diet and/or PA in the peer-reviewed and grey literature. The aim was to map the quality, contents and cultural diversity of these manuals to inform the content of our intervention. The clinical psychologist (CP) devised the intervention based on this synthesis and on our expertise in developing lifestyle interventions. We used an iterative process to draft the manual and refine it over 2–3 cycles. There are two manualised outputs from the trial:
-
An intervention curriculum. This provides an outline of each intervention session, including key learning points, interactive activities and action planning.
-
A participant workbook. This includes key learning points from each session, action planning worksheets, case studies and a self-monitoring diary for each participant.
The participants also received a pedometer, with guidance on how to use it effectively, and access to online, DVD (digital versatile disc) and paper resources on CVD risk.
The programme consisted of 10 sessions, plus an introductory telephone session, spread over 12 months. The outline of each session is given in Table 2. Each participant allocated to a treatment arm had a ‘session 0’ telephone call as an introduction to the intervention, to receive their intervention packs and to become familiar with the HLF. The intensive phase consisted of six weekly sessions at the beginning of the first quarter. The first three sessions focused on PA and the second three sessions focused on diet. The maintenance phase consisted of four sessions delivered at 3, 6, 9 and 12 months after intervention commencement.
| Session (time from intervention commencement) | Content |
|---|---|
| Intensive phase | |
| Session 0 |
Focus: introduce the intervention Examples of delivery: structure of the programme, ice breaker, rapport building with HLF, give out pedometers and baseline measures |
| Session 1: PA (week 1) |
Focus: increasing routine activity Examples of delivery: elicit views regarding walking more and sitting less and instruction on use of pedometer. Support individual goal-setting |
| Session 2: PA (week 2) |
Focus: increasing non-routine activity Examples of delivery: elicit views on recommended activity levels and reflect on previously enjoyed exercise and its benefits. Provide information/demonstration/leaflets regarding local exercise options. Support individual goal-setting |
| Session 3: PA (week 3) |
Focus: to maintain PA changes Examples of delivery: elicit views regarding lapse vs. relapse using case studies. Discuss lapse triggers and strategies to manage them. Support individual relapse-prevention plans (including implementation intentions) |
| Session 4: diet (week 4) |
Focus: increasing health food choices Examples of delivery: elicit views on healthy eating principles. Interactive games regarding healthy snacks. Support individual goal-setting |
| Session 5: diet (week 5) |
Focus: decreasing unhealthy food choices Examples of delivery: elicit views on foods to avoid in excess. Interactive games regarding food labelling and high-fat and high-salt foods. Support individual goal-setting |
| Session 6: diet (week 6) |
Focus: to maintain dietary changes Examples of delivery: elicit views regarding lapse vs. relapse using case studies. Discuss lapse triggers and strategies to manage them. Support individual relapse-prevention plans (including implementation intentions) |
| Maintenance phase | |
| Session 7 (3 months) |
Focus: review progress and problem-solve setbacks Examples of delivery: highlight positive changes in review session, discuss setbacks and potential ways forward. Support individual relapse-prevention plans |
| Session 8 (6 months) |
Focus: review progress and problem-solve setbacks Examples of delivery: highlight positive changes in review session, discuss setbacks and potential ways forward. Support individual relapse-prevention plans |
| Session 9 (9 months) |
Focus: review progress and problem-solve setbacks Examples of delivery: highlight positive changes in review session, discuss setbacks and potential ways forward. Support individual relapse-prevention plans |
| Session 10 (12 months) |
Focus: review progress and problem-solve setbacks Examples of delivery: highlight positive changes in review session, discuss setbacks and potential ways forward. Support individual relapse-prevention plans |
Those randomised to the group intervention arm were encouraged to use peer learning and the peer support environment to facilitate change during both the intensive phase and the maintenance phase. Each group had a maximum of 11 participants and sessions lasted for 120 minutes. The intervention was delivered in local venues, such as community halls and health centres. Between sessions and during follow-up, participants were encouraged to communicate with each other and the HLF. Novel methods and teaching aids were used to supplement the delivery of BCTs, such as visual aids (food labels)/cue cards, exercise demonstrations, video/audio material of patient testimonials, activity-based learning around meal planning, and text/e-mail reminders. HLFs were expected to offer sessions between 08.00 and 21.00, enabling flexibility for participants in full-time work or with carer roles. Cultural and religious awareness was built in to the intervention.
For ease of translation, the key components of the programme have been defined in accordance with Abraham and Michie’s BCT taxonomy:54
-
provide information on consequences
-
prompt intention formation
-
prompt barrier identification
-
prompt specific goal-setting
-
prompt review of behavioural goals
-
prompt self-monitoring of behaviour
-
teach to use prompts or cues
-
agree on behavioural contract
-
use follow-up prompts
-
plan social support or social change
-
relapse prevention
-
MI.
Training the healthy lifestyle facilitators
The HLFs were employed at NHS band 3 level by King’s College Hospital and seconded as appropriate to the CCG. The training programme lasted for 8 weeks and involved a standardised package of training materials. The teaching was a combination of didactic learning, role playing and feedback, group exercises, reading and case study discussion. The HLFs used rating scales for self-monitoring of skill progression during role playing.
Each HLF’s competency was assessed at the end of training via a knowledge test and through observing delivery of two sessions (one intensive, one maintenance). Four domains were assessed: MI, BCTs, group skills and time management, utilising relevant coding frameworks (see Appendix 1). The competency thresholds were adapted for the study,94–96 requiring each HLF to demonstrate ‘moderate proficiency’ in each of the domains before delivering the intervention, meaning that they delivered 70% of BCTs, were 90% MI-adherent and scored in the moderate range in at least two of the other Motivational Interviewing Treatment Integrity (MITI)97 categories, in at least three of the group skills categories and in time management. HLFs that were not competent on initial assessment received further training and reassessment to reach the required competency level.
Healthy lifestyle facilitator competency was monitored throughout the intervention by the CP, who facilitated fortnightly group supervision and weekly e-mail supervision (based on the HLFs completing reflective practice logs). The CP also conducted quality assurance of the intervention administration by regularly reviewing audiotaped sessions (one individual session per month and two group sessions every 8 months) and providing feedback, as needed.
Arm 2: usual care and enhanced motivational interviewing in an individual format
This had the same components as the group intervention arm but was delivered individually (i.e. one to one). There was no opportunity/expectation/guidance for participants to form groups with each other between sessions. The number, content and spread of sessions was the same and sessions lasted for 40 minutes; we reduced the duration of each session to approximately control for attention in the two treatment arms.
Arm 3: usual care only
General practitioners participating in the study were expected to follow their local Health Check pathway for those patients who had a CVD risk score of ≥ 20.0%.
Clinical effectiveness
Primary outcomes
The primary outcomes were change in weight (kg) and PA (average number of steps per day) between arms.
Secondary outcomes
Changes in LDL cholesterol and CVD risk score were assessed. The QRISK2 measurement of CVD risk is sensitive to changes in weight, cholesterol level, blood pressure, HbA1c level and smoking status. The number of fatal and non-fatal CVD events and hospital admissions were recorded using an AE questionnaire and by recording hospital services usage using the CSRI. Changes in dietary habits were measured via analysis of dietary recall data. Health beliefs and depression at 12 and 24 months were assessed as measures of mediating processes.
Cost-effectiveness
The main perspective for the economic evaluation is that of the health-care system. The EuroQol-5 Dimensions, three-level version (EQ-5D-3L) is used to generate QALYs for use in economic analyses. 98 Intervention costs were calculated taking into account staff time for delivering the sessions and the unit costs include elements for overheads and oncosts and account for the ratio of direct-to-indirect contact time. We assumed that the clinician would be grade 3 and that the unit cost per hour was £32.40. For the group intervention, the costs are apportioned over the number of attendees, which averaged 5.5. Other service use was measured at baseline, 12 months and 24 months using the CSRI. EQ-5D instruments were developed in the 1980s and have been subsequently adapted for use in around 500 studies. Service costs were calculated by combining service use data with information on unit costs. 99,100 We had originally planned to explore the cost of lost employment. However, these data were not adequately recorded and so this was omitted.
Process evaluation
The overall aim of the qualitative analysis is to identify and describe factors and processes that affect the delivery, receipt and outcome of the study to aid interpretation and translation of the observed findings. Process data are analysed before outcome data wherever possible to reduce bias in interpretation. The main themes are described in the following sections.
Participation bias
The extent to which the intervention reached out to eligible participants is assessed by (1) the makeup of general practices that agreed and declined to participate, (2) participation biases and (3) attrition biases. We also invited participants who did not complete the intervention to attend a focus group to give feedback on the programme.
Fidelity analysis
We measured adherence and competence of the trained HLF team in delivering the manualised intervention. See Chapter 8 for a full description.
Processes of change
The HLFs would record if targets were met (not at all, partially or fully) at the beginning of each session as a measure of participant adherence to the intervention. Supervision and interviews with the HLFs were used to assess which BCTs were popular, why and for which lifestyle behaviour. We administered a process questionnaire at the end of the study that required all randomised participants to discuss, in open-ended and standardised structured questions, which techniques they had found most useful, their appraisal of the techniques and their level of satisfaction with the interventions of their allocated arms. We included the UC arm to assess the similarity and differences with the intervention arms as there may have been some overlap. In addition to the questionnaire, we conducted focus groups with a small number of participants to elicit more detailed feedback (see Chapter 4).
Statistical analysis
Analysis and reporting is aligned with Consolidated Standards of Reporting Trials (CONSORT) guidelines,101 with primary analyses being on an intention-to-treat (ITT) basis. A description of the sample will be presented using mean (SD) or count (percentage). The baseline characteristics of those who withdrew and those who did not complete the intervention are compared with those of participants who completed follow-up. Descriptive data also include maximum values, minimum values, medians and interquartile ranges (IQRs) when appropriate. Significance is reported to a two-tailed alpha level of 0.05 as appropriate for comparisons.
The differences in treatment effect between the three arms at 12 and 24 months of this partially nested design are analysed using mixed-effects models, with pre-randomisation values as a covariate. 102 In the linear mixed model, ‘treatment arm’, ‘time’ (as a categorical variable with two levels: 12 and 24 months), the ‘interaction between treatment group and time’, ‘borough’, ‘ethnicity’ and the ‘baseline values’ of the outcome variable are the fixed part of the model. The random parts of the models are ‘general practice’ (participants are nested in practices) and ‘therapy group’. The design of the study is complex because we have a partially clustered cross-classified design. In the previous version of the analysis plan, we planned to account for the partially nested design of ‘therapy group’ in an approach that matches the non-parallel data structure. 103 However, preliminary analyses with blinded data revealed that this complex model of a partially nested, cross-classified design did not converge. Thus, in agreement with the Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) (on 6 July 2017), we decided to drop the random effect for therapist from the primary analyses. We are aware that we may underestimate standard errors of treatment effects; however, health intervention therapist effects in similar studies [e.g. the National Institute for Health Research (NIHR)-funded IMPACT104 and Diabetes-6105 RCTs] ranged between negligible and small (ICC range 0 to 0.02).
The dependency of the repeated observations of the same participants at 12 and 24 months and the covariance between the residuals within the lowest-level group ‘patients’ are correlated by using an unstructured covariance pattern model. For the final model, the group difference estimates and associated confidence intervals (CIs) are reported for 12 (for secondary analyses) and 24 months after randomisation.
In a sensitivity analysis, the random effect of borough was replaced by a partially nested random effect for therapist group, as described previously, and results compared.
The described analysis approach provides valid inferences under the assumption that the missing data mechanism can be ignored (missing at random). Sensitivity of results to missing data was further assessed by including covariates predictive of missingness in the analyses model.
The significance level was 2.5% (two-sided) for the two main comparisons of (1) group format versus UC and (2) individual format versus UC. The (secondary) comparison (group format vs. individual format) was assessed on a 5% significance level as our research hypotheses is a null hypothesis of no difference.
The large sample size should have ensured that all possible confounding variables are equally distributed between treatment arms. However, in the sensitivity analysis, we extended the model of the primary analysis by including baseline variables with substantial imbalance, thought to be important in determining outcome. The potential baseline variables were age, sex, IMD, education, marital status and smoking status.
The following further sensitivity analyses were conducted and, for all analyses, changes in predicted treatment outcome differences are presented in the analysis plan – available on the project webpage [see www.journalslibrary.nihr.ac.uk/programmes/hta/106203/#/ (accessed 29 April 2019)].
Sensitivity analysis adjusting for delay in intervention start
Participants allocated to the intervention arms began intervention sessions at different time points relative to randomisation. Therefore, we conducted a sensitivity analysis to include time between randomisation and session 0 of the intervention in the model.
Sensitivity analysis adjusting for unblinding of research assistants at follow-up
Every effort was made to avoid participants revealing their treatment allocation to the research assistant at follow-up. Research assistants recorded whether or not they were unblinded to treatment allocation at either the 12- or the 24-month follow-up. We conducted a sensitivity analysis to include unblinding (yes/no) at follow-up time into the model.
Sensitivity analysis adjusting for insufficient accelerometer wear at baseline
We conducted a sensitivity analysis to include the number of valid days (≥ 540 minutes) of accelerometer wear at baseline in the model.
Sensitivity analysis adjusting for insufficient accelerometer wear at follow-up
We conducted a sensitivity analysis to include the number of valid days (≥ 540 minutes) of accelerometer wear at 12 and 24 months in the model.
Sensitivity analysis adjusting for the recruitment of participants with a body mass index of < 25 kg/m2
Although BMI was not included in the eligibility criteria, an aim of the study was to assess reduction in weight, which would be inappropriate for participants who were of healthy weight or underweight at baseline. We conducted a sensitivity analysis to include a BMI of < 25 kg/m2 (yes/no) at baseline in the model.
Sensitivity analysis adjusting for the recruitment of participants with a QRISK2 score of < 20.0%
We conducted a sensitivity analysis to include a QRISK2 score of ≥ 20.0% (yes/no) at baseline in the model.
Dietary intake analysis
When available, the dietary recall diary from the appointment (or, if missing, the later diary) was used. Owing to resource constraints, we a priori elected to analyse a random sample of 602 participants who had a recall diary available for each time point (baseline and 12- and 24-month follow-ups). The random selection of the 602 participants approximately followed the treatment allocation ratio used for the study (4 : 3 : 3). Thus, the arm sizes for the group, individual and UC arms were 240, 180 and 182, respectively.
Food quantities and nutrient intake were checked for outliers, indicating possible data entry errors. Eight nutrients (water, protein, fat, carbohydrates, total sugar, fibre, saturated fat and sodium) and total energy intake (kcal) were selected as variables of interest. These variables were selected based on their relevance to the intervention (i.e. its emphasis on reducing fat/sugar/salt intake and increasing water/fibre intake).
Dietary intake data are summarised at each time point using means (SDs). A linear mixed-effects model, accounting for the partially nested design, tested if there were any effects of treatment arm and time or their interaction on nutrient intake. The model controlled for participant age, sex, ethnicity, IMD 2010 baseline score76 and if the day recalled was Saturday/Sunday (binary yes/no variable).
A series of mediation analyses were conducted to test if dietary intake measured at the 12-month follow-up mediated the effect of the intervention on the primary outcomes (weight and PA) or QRISK2 score at the 24-month follow-up. Owing to the limitations of the software used – R version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria) and the mediation package [URL: https://cran.r-project.org/web/packages/mediation/mediation.pdf (accessed 29 April 2019)] – each mediation analysis was constrained to testing differences between two of the treatment arms. Thus, a total of 81 mediation analyses were run [three outcomes × nine diet variables × three treatment arm comparisons (UC vs. group, UC vs. individual, group vs. individual)]. The mediation models controlled for participant age, sex, ethnicity, IMD 2010 baseline score,76 if the day recalled was Saturday/Sunday (binary yes/no variable), baseline diet and baseline value for the respective outcome.
Although many statistical tests were carried out for the dietary intake analysis, these tests were largely exploratory. Therefore, a two-tailed alpha level of 0.01 was used to determine statistical significance. For the linear mixed-effects models, the fixed-effect estimates for the effects of time and treatment arm and their interaction were bootstrapped with 1000 replicates and their 95% CIs calculated.
Cost-effectiveness analysis
Health-care costs are compared between the two treatment arms and UC. Given that the data are likely to be skewed, we use bootstrapping methods to estimate 95% CIs around the mean cost differences. QALYs are calculated from the EQ-5D-3L administered at baseline, 12 months and 24 months. Area under the curve methods allow us to calculate the QALY gain over the entire follow-up period and QALY differences are analysed controlling for baseline EQ-5D-3L score in a regression model. If costs were higher for one arm than for another and QALY gains are greater, we then constructed an incremental cost-effectiveness ratio (ICER) to show the cost per extra QALY gained. There will be uncertainty around cost and QALY estimates and this is explored using cost-effectiveness planes generated from 1000 bootstrapped resamples of the data for each of the comparisons. Bootstrapping was carried out using the bsample routine in Stata® Version 15 (StataCorp LP, College Station, TX, USA), with the strata option used so resampling was by arm. Separate regressions of costs and QALYs were performed on each bootstrapped sample and results retrieved and plotted on the plane. Finally, we generated cost-effectiveness acceptability curves, using the net-benefit approach and bootstrapping, to indicate the probability that any of the three approaches is the most cost-effective for different values placed on a QALY gain. The range of values used is £0 to £100,000. This includes the threshold that is used by the NICE: £20,000–30,000. Sensitivity analyses are carried out around key costs, particularly those for the interventions themselves. We did not impute for missing values and a complete-case analysis was instead conducted.
Ethics issues
The trial was reviewed by the Dulwich REC and has been approved (reference 12/LO/0917). The TSC provided overall trial supervision, supported by the DMEC. The main ethics consideration was to ensure that the risk of harm to participants was minimised and that they were fully informed of any risks. We considered literacy and cultural sensitivities in obtaining informed consent. Other ethics considerations were in ensuring that recruitment and informed consent were handled in such a way that potential participants were not put under pressure to take part and that confidentiality was preserved. All participant data are stored using a unique study identifier and electronic data are password protected.
In general, regular PA is associated with improved health outcomes and this outweighs the risk of sedentary lifestyles. However, sudden increases in vigorous PA for otherwise sedentary individuals is associated with a higher risk of myocardial infarction and of musculoskeletal injuries, which may be pertinent as we were intervening in a group that is at high risk of CVD. However, one of the components of BCTs is to deliver the message that PA should be increased in a graded manner rather than suddenly. The intervention discouraged excessive and/or sudden changes to lifestyles. Weight loss could worsen frailty by accelerating the usual age-related loss of muscle that leads to sarcopenia, but combining weight loss with increased PA can actually ameliorate frailty. 106 Importantly, our intervention is based on healthier diets and gradual and sustainable weight loss as opposed to commercial weight-loss programmes. We considered risks to be small and minimal owing to the exclusion of patients with existing CVD and the use of GP advice when CVD events occurred during participation.
Adverse events
An AE, which may be classed as serious, was defined as any untoward occurrence during the study that should be reported to the REC and TSC within an agreed time frame. A suspected unexpected serious AE was defined as an untoward occurrence that is related to the intervention and is unexpected. Participants had the opportunity to report AEs at 12-month and 24-month study appointments with the research assistant as an AE questionnaire was administered, and participants receiving the intervention were able to report at any time during the intervention period to the HLF. All serious AEs and laboratory values were reviewed by the principal investigator (PI) and a co-investigator, and the PI was responsible for determining causality and reporting any AE related to the study to the REC using the National Research Ethics Service guidance.
The AE questionnaire included asking specifically about physical injuries, which were coded as one of the following types of injury: (1) dislocation, (2) fracture, (3) sprain/strain, (4) other injury to a muscle/tendon or (5) other. Details were also collected of how the injury occurred (context, e.g. if the participant fell) and whether or not any treatment was administered. Cardiovascular events were similarly coded, with the following categories used: (1) angina, (2) atrial fibrillation, (3) coronary heart disease, (4) coronary bypass, (5) myocardial infarction, (6) stroke or transient ischaemic attack, or (7) other. Details were also added and verified by checking the participant’s medical records, as necessary.
Obtaining informed consent
General practice staff conducted the searches using our guidance and invited potential participants to give permission for research assistants to contact them. Research assistants invited potential participants to meet them in the general practice, and they were given verbal and written information about the study and at least 1 week to think about participating. We invited patients who were eligible but declined participation to give informed consent for the collection of baseline data to assess the generalisability of our findings.
Withdrawal and stopping rules
Participants were free to withdraw from the study at any time. If the participant withdrew from the intervention, they were asked if they were happy to attend follow-up study appointments and for further data to be collected. If they withdrew from the study without consent to follow-up, no further data were collected.
There were no formal stopping rules. This is because the intervention did not ask participants to do anything more than follow usual GP advice regarding diet and PA. However, the PI evaluated the causality of any AE and advised withdrawal if necessary.
Time period for retention of trial documentation
Copies of patient consent forms were kept for 12 months after the study ended. Personal data that are identifiable by patient name or address will be destroyed 3 years after the study ended. Other trial records will be archived for 7 years after the trial ended before being destroyed.
Patient and public involvement
Before the trial began, we held a focus group with 10 patients from a general practice in Peckham, London. All patients were of African, Caribbean or South Asian ethnicity, first generation migrants and at high risk of CVD, and were distributed equally between sexes. The group understood the rationale of the MOVE IT trial. They recognised that daily stressors affect lifestyle choices and welcomed the chance to talk about this and think differently. The preference was for interventions in an individual format, but they could see the potential benefits of learning from others in a group. Flexible appointment times were stressed as being important, so that the intervention could fit around other activities. The patients felt that it was necessary to provide more information about individual health status rather than just telling patients that they are at high risk of heart disease. They also gave tips on how to recruit; they thought that by getting some patients on board, these patients could network in the local community. These comments were incorporated into the intervention.
We invited this group of patients to form a patient and public involvement (PPI) group to help with the development of study documentation, including the patient information sheet and consent form. A PPI group of five patients was formed, and two members remained involved until the end of the trial. Their role during recruitment involved advertising the trial to both general practices and potential participants, advising on the development of poster and leaflet campaigns to increase public awareness and actively recruiting general practice sites across four of the CCGs. As part of the process evaluation, we sought PPI in the development of topic guides to be used in focus groups to gain feedback from participants who attended the intervention.
Throughout the trial, the trial team updated PPI members with progress and welcomed feedback. The PPI members attended all TSC meetings throughout the trial to gain more detailed feedback and discuss points of interest with the trial manager, PI and co-investigators.
Chapter 4 Protocol changes
It was necessary to make amendments to the original protocol for a number of unforeseen reasons and to enable improvement in our study methodology and the collected data.
Low uptake of NHS Health Checks
In parallel to setting up MOVE IT, there were increasing numbers of reports and concerns that there was a lower-than-anticipated uptake of NHS Health Checks,12 with regional variations in attendance ranging from 27% to 52% and greater uptake among older patients and in regions of lower deprivation. 13 We also observed this when we started recruiting and that this was delaying the project.
It was necessary to amend the protocol to detail that the researcher would repeat all CVD risk algorithm measures at screening. General practice searches for eligible participants could therefore not rely on recent Health Check data but instead on estimates of CVD risk using outdated data or inaccurate substitutions for missing data.
This change to the protocol led to the exclusion of a large number of patients whose medical record data suggested that they were at a ≥ 20.0% QRISK2 score but on screening were found to have a score of < 20.0% (see Chapter 5). Consequently, more resources were required to complete recruitment to target (substantial amendment; approved in July 2013).
Change to recruitment and study time frame
As research assistants were screening a larger proportion of ineligible patients who could not be randomised, the recruitment period had to be extended from 12 months to 21 months to allow the target sample size to be reached. This required an increase in funding to support a greater number of research assistants and for a longer period of time to support the recruitment procedures at a greater number of research sites. As a result, the end-of-study follow-ups were also extended by a further 9 months (minor amendment; approved on 16 March 2015).
Research sites
We initially invited general practice sites with list sizes of > 8000 patients. We extended invitations to smaller practices with > 5000 patients, as we required an increase in the number of sites owing to the high number of responses from ineligible patients (substantial amendment; approved in July 2013).
Randomisation
Randomisation was not stratified by general practice and ethnicity as planned, as the numbers of patients in many practices are not large enough to stratify by both factors. Simple randomisation was agreed to be the best method, with surgery included as a random factor in the model, and emphasis being on more practices and fewer patients per practice (substantial amendment; approved in July 2013).
Research measures
Three blood pressure measures were taken. The third, rather than the mean, systolic blood pressure is used in the QRISK2 algorithm and will be reported in this final report (substantial amendment; approved on 9 June 2015).
Intervention details
The proposed health trainer job title was changed to HLF to better incorporate the MI aspect of the intervention whereby the participant was ‘facilitated’ rather than ‘trained’ to make healthy lifestyle changes.
We revised the definition and description of MI to that used in the third edition of the MI textbook. 42 The intervention was updated to include a session 0, which was an introductory session via telephone. The length of group intervention sessions was increased to 120 minutes, rather than 90 minutes, to better match for attention with the individual intervention sessions (substantial amendment; approved in July 2013).
Further to the above changes, we changed the protocol to state that participants would be seen for intervention sessions within 6 months from randomisation, as this time period was not initially stated and the start of intervention sessions was presumed to be immediate. The time period varied greatly owing to (1) large numbers of participants randomised and waiting to be seen, as a result of the accelerated recruitment rate to meet the target, and (2) HLF staff turnover and the subsequent recruitment and training of new staff members, which created delays. When this time period was exceeded, we made every effort to still engage participants in the intervention and continued to follow-up for the ITT analysis (substantial amendment; approved on 9 June 2015).
Additional consent procedures
Participants were given the opportunity to consent to the following additional items post randomisation:
-
Once participants had completed all research measures, they were asked if we could link their data to other collaborating research studies in which they also participated (substantial amendment; approved on 21 September 2015).
-
We requested access to Hospital Episodes Statistics data,107 information on all hospital appointments for up to a 6-year period beginning 12 months before participating in the study. However, as this was requested after participants completed the study, we did not gain consent from all participants for this and thus did not seek the Hospital Episodes Statistics data and used only CSRI information on hospital services usage (substantial amendment; approved on 21 September 2015). We requested that participants provided consent to these points remotely, via a letter to and a telephone call with the participant, to avoid unnecessary appointment scheduling (minor amendment; approved on 13 January 2016).
-
A small number of participants who received the intervention (group or individual) were randomly selected to be invited to a focus group to provide feedback. They were asked to provide written informed consent to take part in an audiotaped focus group (substantial amendment; approved on 7 March 2016).
-
A small number of participants were asked to consent to a video recording or photograph of an intervention session to be used to promote the study for educational and training purposes only (substantial amendment; approved on 7 March 2016).
Elaboration of process evaluation: participation bias
We gained ethics approval to collect anonymised demographic data on all patients who were identified as potentially eligible and invited to participate when screening the GP medical records. These data enabled an estimation of participation bias through examining differences in age, sex, ethnicity, deprivation and QRISK2 score between responders and non-responders to the study invitation (substantial amendment; approved on 8 January 2016).
King’s College London network outage and impact on the fidelity analysis
On 18 October 2016, a university-wide information technology (IT) network outage occurred as a result of an infrastructure fault, restricting us from accessing the shared network drives in which various trial data were stored (as per university protocol) and resulting in some data being lost. Although King’s College London IT services made an extensive effort to forensically retrieve data from backup sources, all audiotaped intervention session data were lost from the university-managed shared network drive. We estimated this to be almost 2000 hours of intervention delivery. In addition, we lost data from approximately 200 dietary recall diaries (which could be re-inputted from their paper copies) and 95 accelerometry data files from the 24-month follow-up. For the accelerometry data, we asked the 95 participants to rewear the accelerometer; of those, eight declined and consequently had missing data for the PA outcome. We conducted a sensitivity analysis adjusting for participant rewear of the accelerometer.
The loss of the intervention session data was reported to the TSC. At the subsequent TSC meeting (on 6 July 2017), its members recommended that we attempt to recover the session recordings from other sources and conduct the fidelity analysis in accordance with the original plan as closely as possible. Of the data lost, we were able to recover approximately 400 hours from local backups and physical audiotapes, with the co-operation of university IT services and previous HLF staff. This precluded us from conducting the a priori fidelity analysis and randomly selecting coding data from a larger sample of tapes in the database. Instead, we adapted our analysis plan to code and analyse all of the limited, non-random data that were available. Furthermore, we had planned to use the data from the fidelity assessment to determine whether or not the levels of competencies between the HLFs were associated with variations in patient outcomes or mediated the treatment effect. However, the quality of the data recovered precluded us from conducting this analysis as well. See Chapter 8 for further details on our adapted fidelity analysis.
Chapter 5 Main clinical results of the trial
Practice recruitment
Of the 310 general practices with a list size of > 5000 in the 12 South London CCGs that were invited, 126 (40.6%) agreed to participate. A further nine (out of 145) general practice sites with a list size of < 5000 were recruited. Therefore, a total of 135 (29.7%) of the 455 general practices within the 12 South London CCGs participated. 108 The list of participating general practices is provided in Appendix 2. The distribution of practices participating in each CCG and the differences in IMD 2010 scores of participating and non-participating practices are given in Table 3. There were no differences in general practices’ participation by IMD 2010 score.
| CCG | General practices | |||
|---|---|---|---|---|
| Participating in MOVE IT | Not participating in MOVE IT | |||
| n (% of all CCG practices) | IMD 201076 score, mean (SD) | n (% of all CCG practices) | IMD 201076 score, mean (SD) | |
| Bromley | 22 (48.9) | 16.65 (8.93) | 23 (51.1) | 15.95 (8.42) |
| Merton | 10 (40.0) | 16.40 (5.16) | 15 (60.0) | 15.62 (7.09) |
| Lewisham | 16 (39.0) | 30.32 (4.26) | 25 (61.0) | 31.28 (3.62) |
| Southwark | 16 (36.4) | 31.36 (6.45) | 28 (63.6) | 30.72 (5.71) |
| Greenwich | 14 (33.3) | 30.40 (6.89) | 28 (66.7) | 30.80 (6.59) |
| Bexley | 9 (33.3) | 18.19 (9.98) | 18 (66.7) | 16.94 (4.68) |
| Sutton | 8 (29.6) | 13.00 (4.52) | 19 (70.4) | 17.44 (6.34) |
| Croydon | 14 (24.1) | 22.02 (6.52) | 44 (75.9) | 25.02 (7.97) |
| Kingston | 6 (23.1) | 10.42 (1.09) | 20 (76.9) | 11.39 (1.35) |
| Lambeth | 9 (18.8) | 32.19 (4.58) | 39 (81.3) | 30.84 (3.72) |
| Richmond | 5 (17.2) | 9.69 (1.21) | 24 (82.8) | 11.28 (3.12) |
| Wandsworth | 6 (14.0) | 20.67 (6.24) | 37 (86.0) | 22.60 (5.38) |
| Total | 135 (29.7) | 22.42 (9.85) | 320 (70.3) | 23.14 (9.20) |
There was greater initial interest from practices in outer-London CCGs, such as Bromley, Bexley and Merton, and feedback from inner-London CCGs, such as Lambeth and Wandsworth, was that practices are overexposed to research studies and may not have the resources to participate. No formal process of collecting reasons for non-participation of general practices was conducted.
Participant recruitment and flow through the trial
An electronic search for eligible patients was conducted by each general practice using inclusion and exclusion criteria as Read codes109 and including a calculated estimate of QRISK2 value based on values for risk factors already recorded. Participants were recruited between June 2013 and February 2015; recruitment per CCG is given in Table 4. Figure 2 is the CONSORT flow diagram showing participant progression through the trial. Screening eligible participants at general practices led to invitations being sent to 17,775 patients. An expression of interest to take part was received from 3515 patients (a response rate of 19.8%). Participation biases (the differences between those who responded to the invitation and those who did not) are explored in Chapter 7 as part of the process evaluation. Following a brief telephone screening questionnaire, 3183 patients attended for a first appointment with a researcher and provided consent.
| CCG | Date first participant recruited to trial | Participants, n (%) | |
|---|---|---|---|
| Consented (N = 3183) | Randomised (N = 1742) | ||
| Sutton | 7 June 2013 | 265 (8.3) | 151 (8.7) |
| Merton | 10 June 2013 | 149 (4.7) | 79 (4.5) |
| Kingston | 20 June 2013 | 107 (3.4) | 59 (3.4) |
| Croydon | 21 June 2013 | 472 (14.8) | 259 (14.9) |
| Bexley | 3 July 2013 | 177 (5.6) | 103 (5.9) |
| Richmond | 5 July 2013 | 98 (3.1) | 54 (3.1) |
| Bromley | 9 July 2013 | 885 (27.8) | 510 (29.3) |
| Lewisham | 11 September 2013 | 350 (11.0) | 182 (10.4) |
| Southwark | 10 October 2013 | 134 (4.2) | 82 (4.7) |
| Wandsworth | 22 October 2013 | 139 (4.4) | 61 (3.5) |
| Lambeth | 11 November 2013 | 219 (6.9) | 97 (5.6) |
| Greenwich | 6 March 2014 | 188 (5.9) | 105 (6.0) |
FIGURE 2.
The CONSORT flow diagram. COPD, chronic obstructive pulmonary disease. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
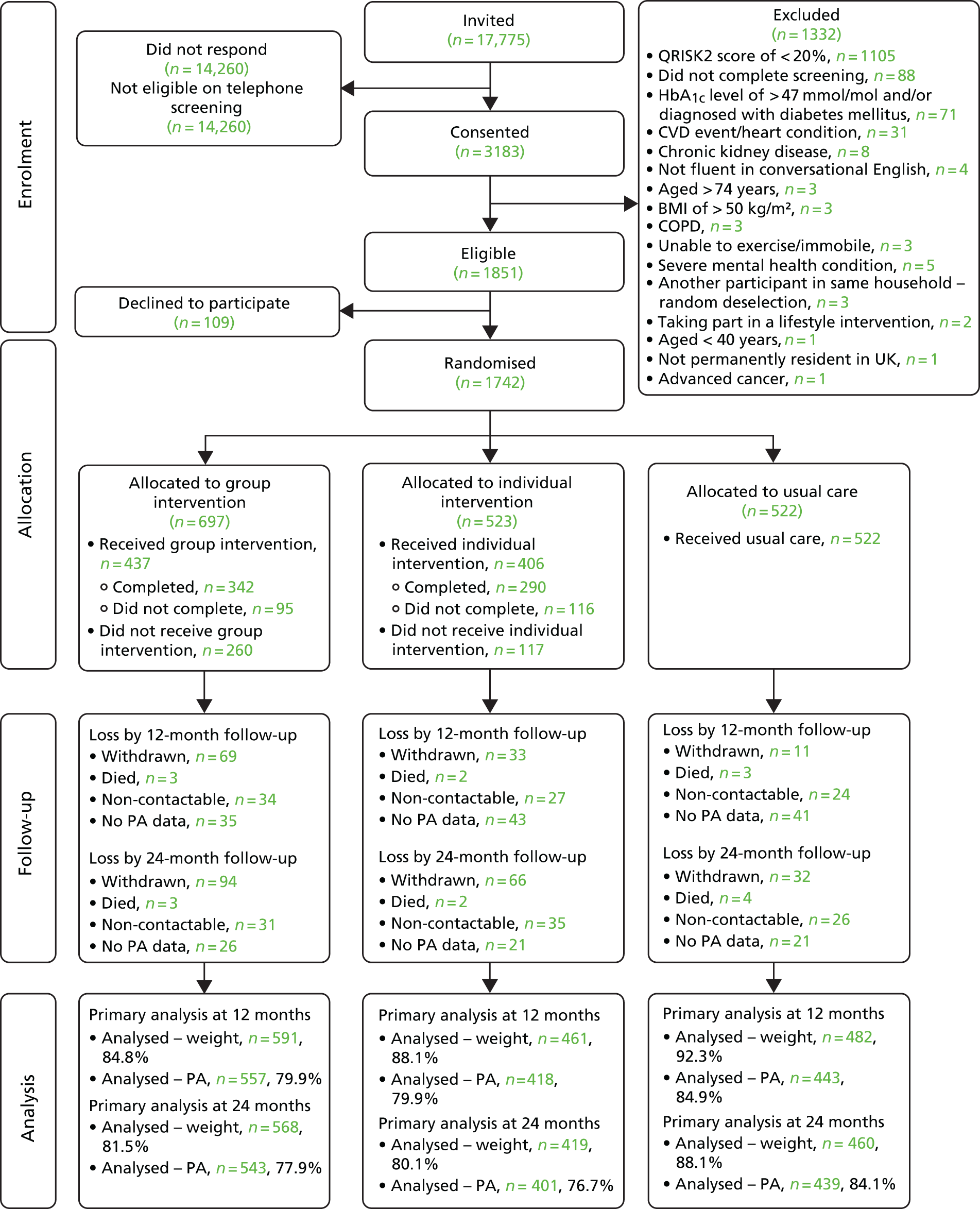
In the first appointment, eligibility was assessed using all measures required for QRISK2 score calculation. This led to the exclusion of 1332 participants as they did not meet the eligibility criteria. The main reason for this (1105 participants; 83.0% of all exclusions) was that the QRISK2 score was < 20.0% on recalculation at face-to-face screening. For 71 participants, blood test results indicated a raised HbA1c level (> 47 mmol/mol) and they were directed to their GP for further advice and informed that they were not eligible to participate. Eighty-eight participants did not complete all screening measures and, therefore, QRISK2 could not be calculated (see Figure 2 for further details of exclusions).
Of the 1851 participants eligible to be randomised, 1742 were randomised and the remaining 109 declined to participate further. Of the randomised sample, 697 were allocated to the group intervention arm, 523 to the individual intervention arm and 522 to the UC arm.
Intervention delivery
Sessions received
A summary of the intervention received by participants randomised to either of the intervention arms is presented in Table 5. Overall, 665 participants (54.5%) completed the intervention, whereas 344 participants (28.2%) did not start the intervention sessions and 211 participants (17.3%) started but did not complete the intervention sessions. Differences in intervention receipt were significantly different between the intervention arms [χ2(2) = 38.30; p < 0.001].
| Intervention received | Participants randomised to intervention arms, n (%) | ||
|---|---|---|---|
| Group (N = 697) | Individual (N = 523) | Total (N = 1220) | |
| Did not start intervention | 260 (37.3) | 117 (22.4) | 377 (30.9) |
| Completed intervention | 342 (49.1) | 290 (55.4) | 632 (51.8) |
| Did not complete intervention | |||
| Attended 1/11 sessions | 34 (4.9) | 33 (6.3) | 67 (5.5) |
| Attended 2/11 sessions | 11 (1.6) | 14 (2.7) | 25 (2.0) |
| Attended 3/11 sessions | 9 (1.3) | 13 (2.5) | 22 (1.8) |
| Attended 4/11 sessions | 5 (0.7) | 5 (1.0) | 10 (0.8) |
| Attended 5/11 sessions | 6 (0.9) | 4 (0.8) | 10 (0.8) |
| Attended 6/11 sessions | 10 (1.4) | 12 (2.3) | 22 (1.8) |
| Attended 7/11 sessions | 11 (1.6) | 15 (2.9) | 26 (2.1) |
| Attended 8/11 sessions | 5 (0.7) | 11 (2.1) | 16 (1.3) |
| Attended 9/11 sessions | 3 (0.4) | 7 (1.3) | 10 (0.8) |
| Attended 10/11 sessions | 1 (0.1) | 2 (0.4) | 3 (0.2) |
For all participants who started the intervention (n = 843), a mean of 8.2 (SD 3.2) sessions (median 9 sessions, IQR 7–11 sessions), including the introductory session 0, were received. The differences in attendance between treatment arms is given in Table 6, with the individual intervention arm attending significantly more sessions (U = 55,388; p < 0.001).
| Sessions | Intervention arm | |||
|---|---|---|---|---|
| Group (n = 437) | Individual (n = 406) | |||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| All session types | ||||
| Including session 0 | 7.62 (2.96) | 8 (6–10) | 8.82 (3.38) | 11 (8–11) |
| Excluding session 0 | 6.72 (2.93) | 7 (5–9) | 7.83 (3.39) | 10 (7–10) |
| Intensive sessions (n = 6) | 4.58 (1.80) | 5 (4–6) | 5.07 (1.93) | 6 (6–6) |
| Maintenance sessions (n = 4) | 2.14 (1.56) | 3 (0–4) | 2.76 (1.70) | 4 (1–4) |
Participants attended a mean of 4.8 sessions (SD 1.9, median 6, IQR 4–6 sessions) out of the six intensive sessions and a mean of 2.4 sessions (SD 1.7, median 3, IQR 0.5–4 sessions) out of the four maintenance sessions. Participants in the individual intervention arm attended significantly more intensive sessions (U = 62,806; p < 0.001) and maintenance sessions (U = 65,424; p < 0.001) than participants in the group intervention arm. A comparison of attendance between the arms and session types is presented in Table 6.
Delays to intervention commencement
There were unexpected delays to intervention commencement, due to factors mentioned in Chapter 4. The date of intervention commencement was missing for 35 participants (2.9%). We endeavoured to start the intervention within 6 months of randomisation, and this was possible for 986 out of 1185 participants (83.2%): 561 out of 682 (82.3%) in the group intervention arm and 425 out of 503 (84.5%) in the individual intervention arm. The time between randomisation and the start of the intervention for participants randomised to each intervention arm is illustrated in Figure 3.
FIGURE 3.
Time between randomisation and intervention start date (session 0), by intervention arm. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

Participants who were randomised to receive the intervention (in either the group intervention arm or the individual intervention arm) waited a mean of 3.12 ± 2.70 months (minimum 0.16, maximum 15.21, median 2.07, IQR 0.92–4.99 months) from randomisation to their scheduled session 0. The wait time did not differ between the group (3.17 ± 2.78 months; n = 682) and individual (3.04 ± 2.59 months; n = 503) arms [t(1120.5) = 0.83; p = 0.41].
Intervention duration
For those participants attending at least one of sessions 1–10 (n = 782), we calculated the time between session 0 and the final session attended. Across the intervention arms, the mean time between session 0 and the final session was 330.6 ± 173.8 days [minimum 6, maximum 808, median 366, IQR 231–440 days; dates were unavailable for one participant (0.1%)]. The time between session 0 and the final session was significantly longer in the individual intervention arm (355.6 ± 173.1 days; n = 373) than in the group intervention arm (307.8 ± 171.4 days; n = 408) [t(771.4) = 3.87; p < 0.001].
Reasons for not starting or not completing the intervention
When participants decided not to start the intervention that they had been randomly allocated to, they were invited to provide a reason for this. When participants decided to stop attending intervention sessions before the course finished, they were also asked for a reason. Table 7 presents the reasons given for not starting or not completing the intervention by participants in each intervention arm.
| Reason | Intervention arm, n (%) | |||
|---|---|---|---|---|
| Group | Individual | |||
| Did not start intervention (N = 103) | Did not complete intervention (N = 116) | Did not start intervention (N = 241) | Did not complete intervention (N = 95) | |
| Delay to start of intervention | 3 (1.2) | 1 (1.1) | 2 (1.7) | 4 (3.4) |
| Ill health | 12 (4.6) | 7 (7.4) | 6 (5.1) | 10 (8.6) |
| Location of sessions | 24 (9.2) | 3 (3.2) | 2 (1.7) | 1 (0.9) |
| Multiple reasons | 9 (3.5) | 3 (3.2) | 3 (2.6) | 2 (1.7) |
| No benefit | 6 (2.3) | 11 (11.6) | 5 (4.3) | 4 (3.4) |
| No longer interested | 41 (15.8) | 13 (13.7) | 14 (12.0) | 18 (15.5) |
| Other | 7 (2.7) | 6 (6.3) | 2 (1.7) | 6 (5.2) |
| Too busy | 68 (26.2) | 20 (21.1) | 26 (22.2) | 38 (32.8) |
| Unable to contact | 31 (11.9) | 13 (13.7) | 25 (21.4) | 20 (17.2) |
| Unhappy with intervention design | 7 (2.7) | 9 (9.5) | 6 (5.1) | 10 (8.6) |
| Unknown | 52 (20.0) | 9 (9.5) | 26 (22.2) | 3 (2.6) |
We qualitatively explore the reasons for non-completers withdrawing from treatment, as part of the process evaluation, in Chapter 9.
Participant adherence to the intervention
After the completion of each intervention session, the HLF was responsible for recording (1) if the participant set a target for that session (yes/no) and (2) if that target was achieved (coded as no/partially/fully). Session 0 was not included as participants were not asked to set goals for that session.
Of the 1220 participants randomised to the intervention arms, 774 (63.44%) had available adherence data. Of those, 407 (58.39% of those randomised) were in the group intervention arm and 367 (70.17% of those randomised) were in the individual intervention arm.
The 774 participants attended a total of 5932 sessions. Table 8 shows the rate of targets set overall and by intervention arm and Figure 4 shows the number of targets set at each session. The overall rate of targets set differed significantly between arms [χ2(1) = 6.12; p = 0.01] but the distribution of targets set at each session did not differ significantly between arms [χ2(9) = 16.18; p = 0.06].
| Target set? | Participants randomised to intervention arms with available data, n (%) | ||
|---|---|---|---|
| Group (N = 407) | Individual (N = 367) | Total (N = 774) | |
| Target set at session | 2582 (90.66) | 2852 (92.48) | 5434 (91.60) |
| No target set | 266 (9.34) | 232 (7.52) | 498 (8.40) |
| Total | 2848 | 3084 | 5932 |
FIGURE 4.
Number of targets set, by each session and intervention arm.

For each target set, the HLF also recorded if the participant achieved it; achievement was rated as ‘not at all’, ‘partially’ or ‘fully’. Table 9 shows the number of targets achieved by intervention arm and in total; the distribution of targets achieved (excluding missing data) differed significantly between arms [χ2(2) = 7.25; p = 0.03]. Figure 5 shows the distribution of target achievement at each session number. The rate of target achievement differed by session number [χ2(18) = 116.99; p < 0.0001]. When looking within each session number, the rate of target achievement differed significantly between the intervention arms at sessions 1 [χ2(2) = 6.40; p = 0.04] and 6 [χ2(2) = 6.75; p = 0.03] only.
| Target achieved? | Targets set by trial arm, n (%) | ||
|---|---|---|---|
| Group intervention | Individual intervention | Total | |
| Not at all | 143 (5.54) | 158 (5.54) | 301 (5.54) |
| Partially | 664 (25.72) | 679 (23.81) | 1343 (24.71) |
| Fully | 1376 (53.29) | 1676 (58.77) | 3052 (56.16) |
| Missing rating | 399 (15.45) | 339 (11.89) | 738 (13.58) |
| Total | 2582 | 2852 | 5434 |
FIGURE 5.
Distribution of target achievement, by intervention arm and session number.
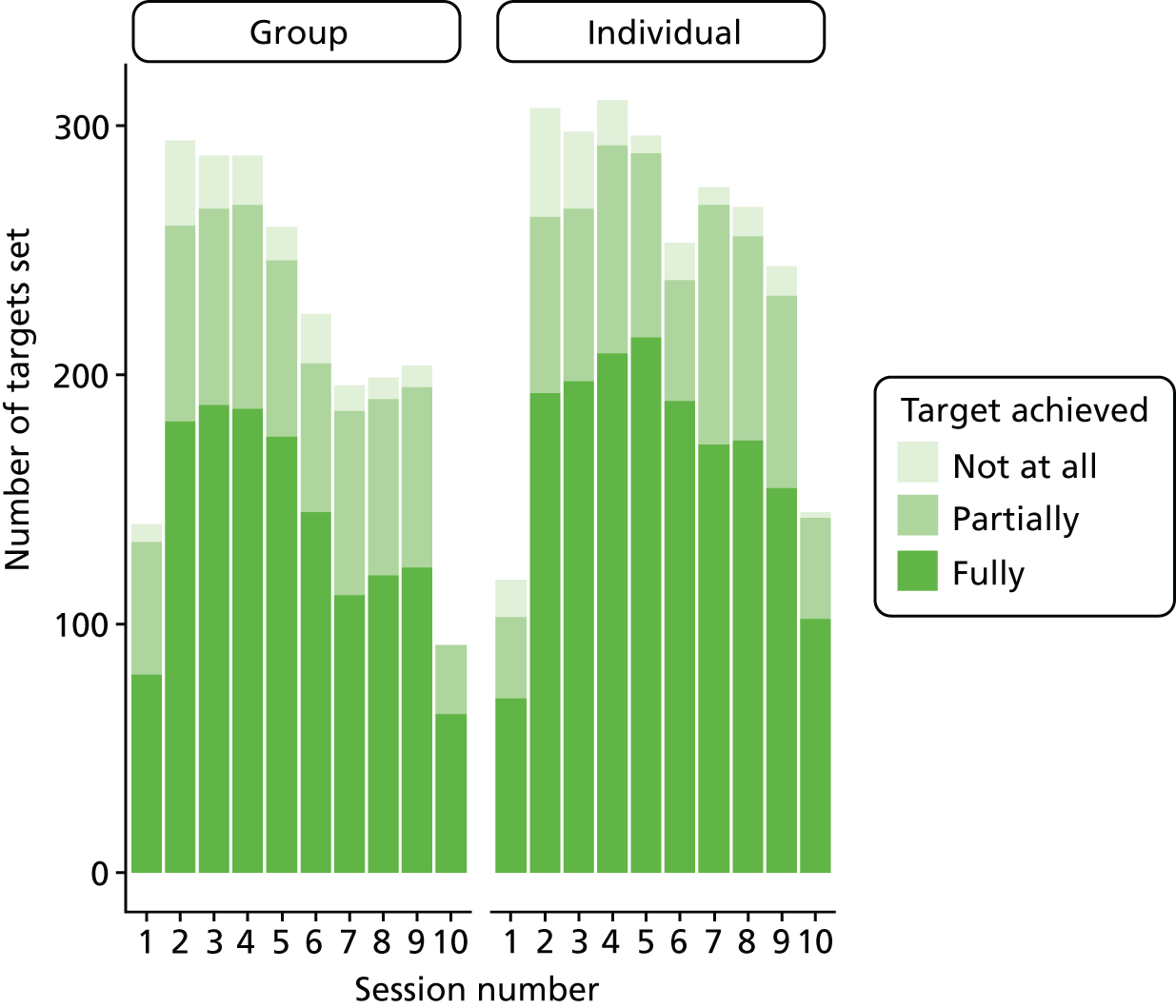
Loss to follow-up
Participants were not seen at follow-up for one of three reasons: (1) they had withdrawn from the study, (2) they had died or (3) they were non-contactable at the follow-up due date (attempts were made to contact the participant up to 6 months after the due date). Participants who were randomised to either of the intervention arms but who did not take part in the intervention did not necessarily withdraw from the study, and their data are included at follow-up to carry out the ITT analysis. Table 10 shows loss to follow-up at both the 12-month follow-up and the 24-month follow-up, with the reasons for withdrawal given. Those who were permanently lost to follow-up at 12 months (withdrawn/died) were not contacted again at 24 months, whereas those who were non-contactable at 12 months were contacted again at 24 months.
| Reason | Trial arm, n (%) | ||
|---|---|---|---|
| Group intervention | Individual intervention | UC | |
| By 12-month follow-up | |||
| Death | 3 (2.8) | 2 (3.2) | 3 (7.9) |
| Withdrawal | |||
| Ill health | 11 (10.4) | 5 (8.1) | 3 (7.9) |
| Too busy | 9 (8.5) | 10 (16.1) | 1 (2.6) |
| Unhappy with study | 6 (5.7) | 5 (8.1) | 1 (2.6) |
| No perceived benefit | 6 (5.7) | 6 (9.7) | 1 (2.6) |
| No longer interested | 11 (10.5) | 2 (3.2) | 3 (7.9) |
| Relocated | 8 (7.5) | 2 (3.2) | 2 (5.3) |
| Unknown | 18 (17.0) | 3 (4.8) | 0 (0.0) |
| Non-contactable | 34 (32.1) | 27 (43.5) | 24 (63.2) |
| Total | 106 (100.0) | 62 (100.0) | 38 (100.0) |
| By 24-month follow-up | |||
| Death | 3 (2.3) | 2 (1.9) | 4 (6.5) |
| Withdrawal | |||
| Ill health | 22 (17.2) | 13 (12.6) | 15 (24.2) |
| Too busy | 12 (9.4) | 15 (14.6) | 2 (3.2) |
| Unhappy with study | 8 (6.3) | 9 (8.7) | 5 (8.1) |
| No perceived benefit | 8 (6.3) | 8 (7.8) | 1 (1.6) |
| No longer interested | 14 (10.9) | 10 (9.7) | 6 (9.7) |
| Relocated | 12 (9.4) | 3 (2.9) | 3 (4.8) |
| Unknown | 18 (14.1) | 8 (7.8) | 0 (0.0) |
| Non-contactable | 31 (24.2) | 35 (34.0) | 26 (41.9) |
| Total | 128 (100.0) | 103 (100.0) | 62 (100.0) |
We exceeded the target follow-up of 83% of participants required by the original sample size calculation. The total loss to follow-up across all arms of the study at 12 months was 11.8%; it was 15.2% in the group intervention arm, 11.9% in the individual intervention arm and 7.3% in the UC arm. The total loss to follow-up across all arms of the study at 24 months was 16.8%; it was 18.4% in the group intervention arm, 19.7% in the individual intervention arm and 11.9% in the UC arm. Data were collected for 91.6% of all participants for at least one of the 12- and 24-month follow-ups, and for 79.7% of participants at both 12 and 24 months. The differences in loss to follow-up between the treatment arms were significant at the 12-month follow-up [χ2(2) = 17.99; p < 0.001] and the 24-month follow-up [χ2(2) = 13.39; p = 0.001]. Withdrawal rates differed significantly between treatment arms at both the 12-month follow-up [χ2(2) = 27.08; p < 0.001] and the 24-month follow-up [χ2(2) = 15.98; p < 0.001].
Accelerometer data completeness
The remaining participants did attend follow-up appointments but did not necessarily complete accelerometer wear for the collection of PA outcomes. Table 11 provides details of the number of accelerometer data collected by trial arm at each time point. The required PA data at baseline was ≥ 5 days of ≥ 540 minutes of accelerometer wear. A number of participants did not wear the accelerometer for the sufficient amount of time at baseline and, therefore, were randomised in error. In the original analysis plan, we stated that we included those with ≥ 4 days of data in the primary analysis to limit exclusions; however, after discussions with the statistician of the TSC (James Carpenter) we included all participants to minimise loss of power and to avoid inducing a potential bias. In agreement with the TSC, for all time points we used the wearable data points if participants wore the accelerometer for at least 1 full day (≥ 540 minutes). Otherwise, the data were considered missing.
| Accelerometer data collection | Time point (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month follow-up | 24-month follow-upa | |||||||
| Trial arm | |||||||||
| Group intervention | Individual intervention | UC | Group intervention | Individual intervention | UC | Group intervention | Individual intervention | UC | |
| No accelerometer issued | |||||||||
| Withdrawn | – | – | – | 69 | 33 | 11 | 94 | 66 | 32 |
| Died | – | – | – | 3 | 2 | 3 | 3 | 2 | 4 |
| Non-contactable | – | – | – | 34 | 27 | 24 | 31 | 35 | 26 |
| Number of valid days (≥ 540 minutes) that the accelerometer was worn | |||||||||
| 0 | 9 | 7 | 5 | 35 | 43 | 41 | 26 | 21 | 21 |
| 1 | 4 | 3 | 4 | 2 | 4 | 3 | 2 | 3 | 5 |
| 2 | 6 | 7 | 8 | 8 | 5 | 7 | 10 | 8 | 8 |
| 3 | 12 | 3 | 8 | 12 | 4 | 9 | 11 | 8 | 9 |
| 4 | 21 | 16 | 25 | 20 | 13 | 17 | 20 | 10 | 18 |
| 5 | 63 | 46 | 28 | 47 | 31 | 40 | 44 | 40 | 43 |
| 6 | 122 | 87 | 94 | 115 | 65 | 84 | 98 | 70 | 77 |
| 7 | 460 | 354 | 350 | 353 | 296 | 283 | 358 | 262 | 279 |
The total percentage of missing activity data across all arms of the study at baseline was 0.5% (n = 8 participants) and at 12 months it was 18.6%: 20.1% in the group intervention arm, 20.1% in the individual intervention arm and 15.1% in the UC arm [χ2(2) = 5.9; p = 0.052]. The total percentage of missing activity data across all arms of the study at 24 months was 20.6%: 22.1% in the group intervention arm, 23.3% in the individual intervention arm and 15.9% in the UC arm [χ2(2) = 10.4; p = 0.006]. At least one activity follow-up measurement was available for 88.1% of the study participants: 86.4% in the group intervention arm, 86.6% in the individual intervention arm and 91.8% in the UC arm [χ2(2) = 9.7; p = 0.008].
Baseline characteristics
Tables 12 and 13 present the baseline characteristics and secondary outcome measures, respectively, of participants by treatment arm for baseline comparability. [For baseline values of the primary outcomes, see Tables 16 (PA) and 18 (weight).] A comparison of baseline characteristics between arms did not reveal any major imbalance, including all prespecified variables that may affect primary outcome (age, sex, ethnicity, IMD 2015 score, education status, marital status and smoking status).
| Characteristic | Trial arm | Total (N = 1742) | ||
|---|---|---|---|---|
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | ||
| Age (years) | ||||
| Mean (SD) | 69.59 (4.16) | 69.76 (4.11) | 69.96 (4.05) | 69.75 (4.11) |
| Median (minimum, maximum) | 70.34 (48.94, 75.83) | 70.77 (48.27, 75.83) | 70.61 (54.02, 75.51) | 70.56 (48.27, 75.83) |
| IQR (lower quartile–upper quartile) | 5.24 (67.44–72.68) | 5.20 (67.66–72.86) | 5.54 (67.6–73.14) | 5.33 (67.55–72.88) |
| Sex, n (%) | ||||
| Male | 593 (85.1) | 457 (87.4) | 440 (84.3) | 1490 (85.5) |
| Female | 104 (14.9) | 66 (12.6) | 82 (15.7) | 252 (14.5) |
| Ethnicity,a n (%) | ||||
| White | 614 (88.1) | 471 (90.1) | 473 (90.6) | 1558 (89.4) |
| Asian or Asian mixed | 75 (10.8) | 45 (8.6) | 41 (7.9) | 161 (9.2) |
| Black or black mixed | 8 (1.1) | 7 (1.3) | 8 (1.5) | 23 (1.3) |
| Current employment?, n (%) | ||||
| Yes | 166 (23.8) | 114 (21.8) | 99 (19.0) | 379 (21.8) |
| No | 531 (76.2) | 409 (78.2) | 423 (81.0) | 1363 (78.2) |
| Qualifications, n (%) | ||||
| No formal qualifications | 186 (27.2) | 126 (24.4) | 122 (23.8) | 434 (25.3) |
| O level/GCSE/CSE/NVQ | 188 (27.4) | 141 (27.3) | 143 (27.9) | 472 (27.6) |
| A level or higher | 311 (45.4) | 249 (48.3) | 247 (48.2) | 807 (47.1) |
| Relationship status, n (%) | ||||
| Married/cohabiting | 521 (74.7) | 412 (78.8) | 371 (71.1) | 1304 (74.9) |
| Divorced/separated/widowed | 100 (14.3) | 62 (11.9) | 82 (15.7) | 244 (14.0) |
| Single | 76 (10.9) | 49 (9.4) | 69 (13.2) | 194 (11.1) |
| IMD 2015 score111 | ||||
| n | 695 | 522 | 522 | 1739 |
| Mean (SD) | 15.52 (10.75) | 16.26 (10.82) | 16.43 (11.17) | 16.02 (10.90) |
| IMD 2015 quintile, n (%) | ||||
| First (least affluent) | 63 (9.1) | 46 (8.8) | 52 (10.0) | 161 (9.3) |
| Second | 122 (17.6) | 125 (23.9) | 108 (20.7) | 355 (20.4) |
| Third | 136 (19.6) | 88 (16.9) | 93 (17.8) | 317 (18.2) |
| Fourth | 166 (23.9) | 116 (22.2) | 124 (23.8) | 406 (23.3) |
| Fifth (most affluent) | 208 (29.9) | 147 (28.2) | 145 (27.8) | 500 (28.8) |
| Smoking status, n (%) | ||||
| Current smoker | 112 (16.1) | 75 (14.3) | 81 (15.5) | 268 (15.4) |
| Ex-smoker | 380 (54.5) | 315 (60.2) | 290 (55.6) | 985 (56.5) |
| Non-smoker | 205 (29.4) | 133 (25.4) | 151 (28.9) | 489 (28.1) |
| Number of cigarettes per day if current smoker | ||||
| Mean (SD) | 11.60 (8.36) | 11.04 (8.12) | 11.15 (9.15) | 12.70 (10.88) |
| Median (minimum, maximum) | 10 (0, 50) | 10 (1, 45) | 9 (1, 40) | 10 (0, 100) |
| IQR (lower quartile–upper quartile) | 13 (5–18) | 10 (5–15) | 14 (4–18) | 13 (5–8) |
| Alcohol intake (AUDIT score), n (%) | ||||
| Abstainer (0) | 73 (10.5) | 54 (10.3) | 55 (10.5) | 182 (10.4) |
| Low risk (1–7) | 506 (72.6) | 397 (75.9) | 383 (73.4) | 1286 (73.8) |
| Possibly harmful (≥ 8) | 118 (16.9) | 72 (13.8) | 84 (16.1) | 274 (15.7) |
| Depressive symptoms (PHQ-9 score) | ||||
| Mean (SD) | 2.07 (3.38) | 1.98 (3.05) | 1.88 (3.13) | 1.99 (3.21) |
| Median (minimum, maximum) | 0 (0, 26) | 0 (0, 18) | 0 (0, 21) | 0 (0, 26) |
| IQR (lower quartile–upper quartile) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) |
| Severity of depressive symptoms (PHQ-9 score), n (%) | ||||
| None (0–4) | 583 (83.6) | 436 (83.4) | 448 (85.8) | 1467 (84.2) |
| Mild (5–9) | 82 (11.8) | 72 (13.8) | 51 (9.8) | 205 (11.8) |
| Moderate (10–14) | 23 (3.3) | 10 (1.9) | 18 (3.4) | 51 (2.9) |
| Moderately severe (15–19) | 7 (1.0) | 5 (1.0) | 4 (0.8) | 16 (0.9) |
| Severe (20–27) | 2 (0.3) | 0 (0.0) | 1 (0.2) | 3 (0.2) |
| Rheumatoid arthritis?, n (%) | ||||
| Yes | 17 (2.9) | 18 (3.9) | 17 (3.5) | 52 (3.4) |
| No | 574 (97.1) | 443 (96.1) | 465 (96.5) | 1482 (96.6) |
| Prescribed blood pressure medication?, n (%) | ||||
| Yes | 385 (55.2) | 280 (53.5) | 276 (53.0) | 941 (54.0) |
| No | 312 (44.8) | 243 (46.5) | 245 (47.0) | 800 (46.0) |
| Family history of angina or myocardial infarction?, n (%) | ||||
| Yes | 192 (27.5) | 140 (26.8) | 148 (28.4) | 480 (27.6) |
| No | 505 (72.5) | 383 (73.2) | 374 (71.6) | 1262 (72.4) |
| Measure | Trial arm | Total | ||
|---|---|---|---|---|
| Group intervention | Individual intervention | UC | ||
| QRISK2 score (%) | ||||
| n | 697 | 523 | 522 | 1742 |
| Mean (SD) | 24.95 (4.79) | 25.26 (5.27) | 24.93 (4.81) | 25.04 (4.94) |
| BMI (kg/m2) | ||||
| n | 697 | 523 | 522 | 1742 |
| Mean (SD) | 28.17 (4.11) | 28.31 (4.28) | 28.37 (4.59) | 28.27 (4.31) |
| Total cholesterol (mmol/l) | ||||
| n | 697 | 522 | 522 | 1741 |
| Mean (SD) | 5.09 (1) | 5.10 (1.01) | 5.05 (1.00) | 5.08 (1.00) |
| LDL cholesterol (mmol/l) | ||||
| n | 694 | 515 | 517 | 1726 |
| Mean (SD) | 3.11 (0.85) | 3.14 (0.89) | 3.07 (0.88) | 3.11 (0.87) |
| Total cholesterol/HDL ratio | ||||
| n | 697 | 523 | 522 | 1742 |
| Mean (SD) | 4.17 (1.13) | 4.20 (1.16) | 4.11 (1.13) | 4.16 (1.14) |
| HbA1c level (mmol/mol) | ||||
| n | 684 | 515 | 515 | 1714 |
| Mean (SD) | 37.58 (3.39) | 37.55 (3.48) | 37.56 (3.45) | 37.56 (3.43) |
| Waist circumference (cm) | ||||
| n | 692 | 520 | 520 | 1732 |
| Mean (SD) | 102.37 (11.64) | 103.24 (11.83) | 102.54 (12.29) | 102.68 (11.89) |
Missing data
Patterns of missing follow-up data for the primary outcome were investigated by comparing the baseline data of those with missing primary outcome data at follow-up with the data of those who provided follow-up data. Participants missing 12-month follow-up assessment data walked significantly less at baseline (6509.0 vs. 6802.6 steps per day; t = 1.99; p = 0.048) and were significantly more likely to be current smokers [23.1% vs. 14.3%; χ2(2) = 13.6; p = 0.001]. Participants with missing 24-month follow-up assessment data walked significantly less at baseline (6376.9 vs. 6833.1 steps per day; t = –2.78; p = 0.006), were significantly more likely to be current smokers [22.0% vs. 14.4%; χ2(2) = 6.4; p = 0.041], were significantly more likely to have no formal qualifications [23.8% vs. 33.2%; χ2(2) = 12.3; p = 0.002] and were significantly more likely to be more depressed (PHQ-9 score of 1.89 vs. 2.47; t = 244; p = 0.015). There were no differences between numbers of participants missing at either follow-up point compared with participants who were present in age, sex, weight, BMI, QRISK2 score, relationship status, ethnicity, IMD 2015 score111 or alcohol consumption (AUDIT score).
Predictors of missing outcome data at 12 and 24 months
In addition to participants missing the follow-up assessment, activity data were also missing because accelerometers were not worn for sufficiently long enough. Two participants did not provide weight data at 24 months. An analysis of missing outcome data was therefore carried out in addition to an analysis of missing attendance data. A stepwise logistic regression with baseline variables, treatment arm, borough at baseline and potential predictors of missing outcome data at 12 and 24 months for weight and PA separately revealed that education, smoking status, PHQ-9 depression score and treatment arm were the most important predictors. However, only little variation was explained by the models (< 2.5% pseudo-R2). Education, smoking status and PHQ-9 depression score were included as predictors of missingness in a sensitivity analysis to assess potential bias due to missing outcome data.
Table 14 presents a summary of baseline comparisons between participants with missing data at the 12-month follow-up and those who attended the 12-month follow-up. Table 15 presents a summary of the baseline comparison between participants with missing data at the 24-month follow-up and those who attended the 24-month follow-up.
| Missing/present data | Characteristic | |||||
|---|---|---|---|---|---|---|
| Weight (kg) | BMI (kg/m2) | PA (number of steps) | Age (years) | QRISK2 score (%) | PHQ-9 total score | |
| Missing | ||||||
| Mean | 84.07 | 28.89 | 6509.043 | 68.94 | 24.82 | 2.63 |
| SD | 18.45 | 5.47 | 2024.38 | 4.10 | 5.67 | 3.79 |
| n | 208 | 208 | 202 | 208 | 208 | 208 |
| Present | ||||||
| Mean | 83.61 | 28.32 | 6802.64 | 70.04 | 24.94 | 1.82 |
| SD | 14.91 | 4.51 | 2755.58 | 4.04 | 4.74 | 3.07 |
| n | 1534 | 1534 | 1532 | 1534 | 1534 | 1534 |
| Missing/present data | Characteristic | |||||
|---|---|---|---|---|---|---|
| Weight (kg) | BMI (kg/m2) | PA (number of steps) | Age (years) | QRISK2 score (%) | PHQ-9 total score | |
| Missing | ||||||
| Mean | 84.43 | 28.63 | 6376.94 | 69.71 | 25.32 | 2.47 |
| SD | 15.35 | 4.44 | 2497.19 | 4.32 | 5.12 | 3.87 |
| n | 293 | 293 | 287 | 293 | 293 | 293 |
| Present | ||||||
| Mean | 83.43 | 28.20 | 6833.13 | 69.76 | 24.98 | 1.89 |
| SD | 15.00 | 4.28 | 2752.55 | 4.07 | 4.91 | 3.05 |
| n | 1449 | 1449 | 1447 | 1449 | 1449 | 1449 |
Primary outcomes
Physical activity
Table 16 presents a descriptive summary of the PA (number of steps) outcome by trial arm and time point. These are the imputed data (methods are described in Chapter 3) and are used for the analyses. Report Supplementary Material 1 presents a descriptive summary of the original (non-imputed) data, which are simply the means of all wearable data points per participant. We observed a near-perfect correlation (r’s > 0.98) between the imputed and original data at each time point. Figure 6 shows the mean PA level with 95% CIs for each arm over time.
| Time point | Trial arm | Total (N = 1742) | ||
|---|---|---|---|---|
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | ||
| Baseline | ||||
| n (%) | 695 (99.71) | 519 (99.24) | 520 (99.62) | 1734 (99.54) |
| Mean (SD) | 6692.78 (2702.08) | 6820.87 (2747.26) | 6781.18 (2708.37) | 6757.63 (2716.55) |
| 12-month follow-up | ||||
| n (%) | 557 (79.91) | 418 (79.92) | 443 (84.87) | 1418 (81.40) |
| Mean (SD) | 6847.70 (2921.44) | 6918.56 (2867.54) | 6738.29 (2872.85) | 6834.41 (2889.32) |
| 24-month follow-up | ||||
| n (%) | 543 (77.91) | 401 (76.67) | 439 (84.10) | 1383 (79.39) |
| Mean (SD) | 6412.51 (2716.39) | 6584.61 (2729.06) | 6520.21 (2861.38) | 6496.60 (2765.79) |
FIGURE 6.
Mean PA (number of steps) with 95% CIs for each arm over time. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

Assessment of assumptions
Distribution of data
Box plots, Q–Q plots and histograms reveal unimodal distribution with some positive skew for PA (see Report Supplementary Material 1); however, the skew is similar in all groups at all time measurements. Furthermore, there are no serious outliers. This suggests that the distribution of the outcome measures does not seriously violate the assumption of being normally distributed and that there is no need for transformations.
Assessment of standard deviations between arms and over time
Assessments of SDs reveal only small differences in variances between groups or over time for PA (see Table 16), which further justifies the use of a mixed-effects model with continuous outcome data assuming a multivariate normal distribution for the analysis of PA.
The primary analysis (mixed-effects regression) of the treatment effect on PA was conducted on 2801 observations. There were 133 unique general practices, with a mean of 21.1 observations for each (range 1–99 observations). There were 1534 unique participant identifiers, with a mean of 1.8 observations for each (range 1–2 observations). Table 17 presents the pairwise comparisons. The fixed and random-effects outputs can be found in Report Supplementary Material 1.
| Comparisons | Outcome | |||
|---|---|---|---|---|
| PA (number of steps) | Weight (kg) | |||
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| 12-month follow-up | ||||
| Individual and UC | 210.22 (–19.46 to 439.91) | 0.073 | –0.55 (–0.95 to –0.14) | 0.008 |
| Group and UC | 131.1 (–85.28 to 347.48) | 0.24 | –0.52 (–0.90 to –0.13) | 0.009 |
| Individual and group | 79.12 (–157.73 to 315.98) | 0.51 | –0.03 (–0.43 to 0.37) | 0.89 |
| 24-month follow-up | ||||
| Individual and UC | 7.24 (–224.01 to 238.5) | 0.95 | –0.42 (–0.93 to 0.09) | 0.104 |
| Group and UC | 70.05 (–288 to 147.9) | 0.53 | –0.03 (–0.49 to 0.44) | 0.91 |
| Individual and group | 77.29 (–153.37 to 307.95) | 0.51 | –0.40 (–0.86 to 0.07) | 0.096 |
There were only minor and non-significant differences between treatment arms in PA levels at 12 or 24 months. At 12 months, compared with participants in the UC arm, participants in the individual intervention arm walked, on average, 210 steps (95% CI –19.5 to 439.9 steps) more and those in the group intervention arm walked, on average, 131 steps (95% CI –85.3 to 347.5 steps) more. All differences (including limits of the 95% CIs) were less than the minimum clinically significant difference (MCD) of 675 steps as defined in the study protocol. Similarly, at the 12-month follow-up and using 97.5% CIs, we observed minor and non-significant differences between the individual and UC arms (mean difference 210.22 steps, 97.5% CI –52.44 to 472.89 steps) and the group and UC arms (mean difference 131.10 steps, 97.5% CI –116.35 to 378.55 steps).
Weight
Table 18 presents a descriptive summary of weight by trial arm and at each time point. Figure 7 shows the mean weight with 95% CIs for each arm over time.
| Time point | Trial arm | Total (N = 1742) | ||
|---|---|---|---|---|
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | ||
| Baseline | ||||
| n (%) | 697 (100) | 523 (100) | 522 (100) | 1742 (100) |
| Weight (kg), mean (SD) | 83.15 (14.75) | 84.15 (15.35) | 83.65 (15.19) | 83.60 (15.06) |
| 12-month follow-up | ||||
| n (%) | 591 (84.79) | 461 (88.15) | 482 (92.34) | 1534 (88.06) |
| Weight (kg), mean (SD) | 82.59 (15.19) | 83.31 (15.13) | 83.43 (15.07) | 83.07 (15.13) |
| 24-month follow-up | ||||
| n (%) | 568 (81.49) | 419 (80.11) | 460 (88.12) | 1447 (83.07) |
| Weight (kg), mean (SD) | 82.72 (15.29) | 82.98 (15.40) | 83.10 (15.13) | 82.92 (15.26) |
FIGURE 7.
Mean weight with 95% CIs for each arm over time. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

Assessment of assumptions
Distribution of data
Box plots, Q–Q plots and histograms reveal unimodal distribution with only minor positive skew for weight (see Report Supplementary Material 1). The skew is similar in all groups at all time points. Furthermore, there are no serious outliers. This suggests that the distribution of the outcome measures could be treated as normally distributed without the need for transformations.
Assessment of standard deviations between arms and over time
Assessments of SDs reveal little differences in variances between groups or over time for each scale (see Table 18), which further justifies the use of a mixed-effects model with continuous outcome data, assuming a multivariate normal distribution for the analysis of weight.
The primary analysis (mixed-effects regression) of the treatment effect on weight was conducted on 2981 observations. There were 133 unique general practices, with a mean of 22.4 observations for each (range 2–104 observations). There were 1595 unique participant identifiers, with a mean of 1.9 observations for each (range 1–2 observations). Table 17 presents the pairwise comparisons. The fixed and random-effects outputs can be found in Report Supplementary Material 1.
There was a small but significant mean difference between the individual and UC arms of –0.55 kg (95% CI –0.95 to –0.14 kg) and between the group and UC arms of –0.52 kg (95% CI –0.90 to –0.13 kg). However, the differences (including the 95% CI limits) are below the MCD of 1.25 kg. There was no difference between the group and individual intervention arms (mean –0.03 kg, 95% CI –0.43 to 0.37 kg). At 24 months, no significant differences were observed. Similarly, at the 12-month follow-up and using 97.5% CIs, we observed minor differences between the individual and UC arms (mean difference –0.55 kg, 97.5% CI –1.01 to –0.08 kg) and the group and UC arms (mean difference –0.52 kg, 97.5% CI –0.96 to –0.08 kg).
Sensitivity analyses
Sensitivity analysis adjusting for imbalances in baseline characteristics
No imbalances were observed in any of the prespecified baseline characteristics; therefore, a sensitivity analysis adjusting for these confounders was not carried out for either of the primary outcomes.
A total of 15 sensitivity analyses were conducted for each (except for two instances; the weight outcome was not completed) of the primary outcomes:
-
adjusting for partially nested random effect for therapist
-
adding therapist and general practice as random factors and exchangeable residual covariance matrix
-
removing potential outliers (removed 12 for weight and 6 for PA)
-
only including patients with a BMI of > 25 kg/m2 (analysed 2298 for weight and 2159 for PA)
-
adjusting for treatment compliance, including participants in the group or individual intervention arms who attended at least one intervention session (analysed 2427 for weight and 2305 for PA)
-
adjusting for the delay in intervention start (continuous variable)
-
adjusting for the unblinding of the research assistant at each follow-up appointment (binary variable)
-
adjusting for the number of days of accelerometer wear at baseline (> 3 days, binary variable)
-
adjusting for the number of days of accelerometer wear at baseline (> 5 days, binary variable)
-
adjusting for the number of valid days of accelerometer wear at baseline (≥ 540 minutes, continuous variable); not completed for weight
-
adjusting for the number of days of accelerometer wear at each follow-up appointment (continuous variable); not completed for weight
-
adjusting for a BMI score of < 25 kg/m2 at baseline (binary variable)
-
adjusting for a QRISK2 score ≥ 20.0% at baseline (binary variable)
-
adjusting for predictors (PHQ-9, smoking status and education) of missing outcome data
-
adjusting for accelerometer rewear at the 24-month follow-up (binary variable); not completed for weight.
Report Supplementary Material 1 presents the output of the sensitivity analyses for each of the primary outcomes, following the numbering above. None of the above sensitivity analyses altered our conclusions for either of the primary outcomes.
Complier-average causal effect analysis
Because there is no evidence of any clinically important treatment effect using a per-protocol analysis (analysing only compliers in the treatment arms), we did not conduct a complier-average causal effect (CACE) analysis. A lack of a clear binary adherence variable for all participants also prevented us from undertaking a reliable CACE analysis.
Secondary outcomes
Table 19 presents the pairwise comparison output for each of the secondary outcomes. We did not observe any treatment effects for any of the secondary outcomes at 12 or 24 months.
| Comparison | Secondary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QRISK2 score (%) | HbA1c level (%) | LDL cholesterol level (mmol/l) | Diastolic blood pressure (mmHg) | Systolic blood pressure (mmHg) | ||||||
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| 12-month follow-up | ||||||||||
| Individual and UC | –0.14 (–0.68 to 0.40) | 0.62 | 0 (–0.37 to 0.38) | 0.99 | 0 (–0.07 to 0.08) | 0.94 | –1.12 (–2.25 to 0.01) | 0.051 | –1.83 (–3.96 to 0.29) | 0.09 |
| Group and UC | –0.28 (–0.79 to 0.23) | 0.29 | 0.08 (–0.27 to 0.42) | 0.66 | 0 (–0.07 to 0.07) | 0.96 | –0.25 (–1.29 to 0.80) | 0.64 | –0.61 (–2.58 to 1.37) | 0.55 |
| Individual and group | 0.14 (–0.36 to 0.64) | 0.59 | –0.08 (–0.41 to 0.25) | 0.65 | 0 (–0.07 to 0.08) | 0.90 | –0.88 (–1.94 to 0.19) | 0.107 | –1.23 (–3.23 to 0.77) | 0.23 |
| 24-month follow-up | ||||||||||
| Individual and UC | 0.05 (–0.63 to 0.72) | 0.89 | 0.02 (–0.39 to 0.43) | 0.91 | 0.05 (–0.04 to 0.14) | 0.26 | –1.04 (–2.20 to 0.11) | 0.077 | –0.29 (–2.37 to 1.79) | 0.79 |
| Group and UC | 0.01 (–0.68 to 0.71) | 0.97 | 0.19 (–0.20 to 0.59) | 0.33 | 0.07 (–0.01 to 0.15) | 0.108 | –0.07 (–1.21 to 1.07) | 0.90 | 1.74 (–0.21 to 3.68) | 0.08 |
| Individual and group | 0.03 (–0.64 to 0.71) | 0.92 | –0.17 (–0.50 to 0.16) | 0.31 | –0.02 (–0.10 to 0.07) | 0.69 | –0.97 (–2.09 to 0.14) | 0.088 | –2.03 (–4.01 to –0.04) | 0.045 |
Dietary intake analysis
Table 20 shows nutrient intake at each time point and by treatment arm. The linear mixed-effects models showed four significant effects for three of the nutrients:
-
fat: a time effect (b = –3.19, 95% CI –6.10 to –0.13)
-
fibre: a treatment-arm-by-time interaction effect for the group intervention arm versus the UC arm (b = 1.20, 95% CI 0.15 to 2.29)
-
saturated fat: a treatment arm effect for the group intervention arm versus the UC arm (b = –3.32, 95% CI –6.37 to –0.11)
-
saturated fat: a time effect (b = –1.53, 95% CI –2.89 to –0.20).
| Nutrient | Trial arm, mean (SD) | |||
|---|---|---|---|---|
| Group (n = 240) | Individual (n = 180) | UC (n = 182) | Total (N = 602) | |
| Baseline | ||||
| Water (g) | 1685.90 (954.28) | 1704.70 (1052.29) | 1557.10 (745.33) | 1652.58 (929.12) |
| Protein (g) | 73.41 (32.03) | 75.00 (31.06) | 73.07 (34.89) | 73.78 (32.60) |
| Fat (g) | 66.79 (34.40) | 70.51 (38.66) | 70.01 (35.34) | 68.87 (35.98) |
| Carbohydrates (g) | 205.78 (80.57) | 211.91 (86.02) | 207.46 (79.44) | 208.12 (81.81) |
| Energy (kcal) | 1769.25 (619.96) | 1828.19 (719.03) | 1817.39 (647.54) | 1801.43 (658.65) |
| Total sugar (g) | 91.63 (49.53) | 94.89 (56.35) | 93.04 (50.68) | 93.03 (51.93) |
| Fibre (g) | 19.54 (8.54) | 19.98 (9.33) | 19.90 (8.77) | 19.78 (8.84) |
| Saturated fat (g) | 23.84 (14.51) | 26.21 (16.99) | 25.90 (14.93) | 25.17 (15.43) |
| Sodium (mg) | 2116.47 (1273.13) | 2386.72 (1393.46) | 2195.32 (1347.91) | 2221.11 (1335.23) |
| 12-month follow-up | ||||
| Water (g) | 1583.16 (808.22) | 1570.28 (823.08) | 1564.05 (756.09) | 1573.53 (796.10) |
| Protein (g) | 66.04 (28.50) | 68.45 (28.44) | 70.27 (26.28) | 68.04 (27.84) |
| Fat (g) | 58.55 (29.98) | 61.82 (33.61) | 66.90 (32.30) | 62.05 (31.94) |
| Carbohydrates (g) | 194.66 (76.52) | 200.70 (75.91) | 211.24 (77.02) | 201.48 (76.67) |
| Energy (kcal) | 1607.90 (588.22) | 1673.23 (616.31) | 1771.27 (567.89) | 1676.83 (593.68) |
| Total sugar (g) | 80.70 (46.11) | 83.33 (41.69) | 88.90 (42.59) | 83.96 (43.83) |
| Fibre (g) | 19.95 (9.42) | 19.42 (8.83) | 20.20 (8.30) | 19.87 (8.91) |
| Saturated fat (g) | 20.69 (13.12) | 22.81 (15.20) | 24.72 (14.23) | 22.54 (14.18) |
| Sodium (mg) | 1902.25 (1194.57) | 1971.90 (1258.57) | 2022.70 (1098.10) | 1959.49 (1185.31) |
| 24-month follow-up | ||||
| Water (g) | 1674.34 (814.50) | 1628.88 (867.23) | 1640.77 (802.37) | 1650.60 (825.89) |
| Protein (g) | 72.35 (31.53) | 70.52 (28.10) | 71.58 (27.00) | 71.57 (29.16) |
| Fat (g) | 66.47 (35.00) | 61.53 (29.60) | 63.60 (26.40) | 64.13 (31.02) |
| Carbohydrates (g) | 206.11 (74.14) | 208.88 (80.67) | 206.72 (73.29) | 207.12 (75.78) |
| Energy (kcal) | 1752.88 (637.69) | 1706.12 (562.62) | 1726.69 (528.81) | 1730.98 (583.60) |
| Total sugar (g) | 84.60 (42.88) | 88.93 (47.73) | 86.92 (41.14) | 86.60 (43.85) |
| Fibre (g) | 21.31 (10.11) | 19.59 (9.73) | 19.50 (7.73) | 20.25 (9.36) |
| Saturated fat (g) | 22.89 (14.54) | 23.24 (13.61) | 22.84 (11.42) | 22.98 (13.36) |
| Sodium (mg) | 1988.68 (1283.79) | 2099.04 (1316.58) | 2087.66 (1150.50) | 2051.61 (1254.18) |
The mediation analyses revealed that none of the nutrients of interest at the 12-month follow-up mediated the effect of the intervention on the 24-month outcomes (p > 0.24 for all analyses).
Adverse events
A total of 523 AEs were reported between baseline and the 24-month follow-up. Seventy-four participants reported experiencing multiple AEs: nine reported experiencing three AEs and 65 reported experiencing two AEs (Table 21). The mean number of AEs experienced by each participant was 0.35 ± 0.58 and the means did not differ across trial arms [F(2) = 0.68; p = 0.51]. The proportions of AEs by subcategories were largely similar across the trial arms (Table 22).
| AE reporting | Trial arm | Chi-squared test outputa | ||
|---|---|---|---|---|
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | ||
| Any AE reported, n (%) | 212 (30.4) | 151 (28.9) | 160 (30.7) | 0.48 |
| Multiple AEs, n (%) | 34 (4.9) | 17 (3.3) | 23 (4.4) | 4.20 |
| 2 | 27 (3.9) | 16 (3.1) | 22 (4.2) | |
| 3 | 7 (1.0) | 1 (0.2) | 1 (0.2) | |
| Number of AEs per participant, mean (SD) | 0.37 (0.61) | 0.33 (0.54) | 0.35 (0.57) | |
| AE type | Trial arm, n (%) | Chi-squared test outputa | ||
|---|---|---|---|---|
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | ||
| Death | 3 (0.4) | 2 (0.4) | 4 (0.8) | 0.92 |
| Any physical injury | 109 (15.6) | 65 (12.4) | 85 (16.3) | 3.61 |
| Dislocation | 5 (0.7) | 1 (0.2) | 2 (0.4) | 1.96 |
| Fracture | 16 (2.3) | 8 (1.5) | 7 (1.3) | 1.82 |
| Sprain/strain | 10 (1.4) | 17 (3.3) | 12 (2.3) | 3.90 |
| Muscle/tendon | 28 (4.0) | 16 (3.1) | 25 (4.8) | 2.33 |
| Other | 48 (6.9) | 22 (4.2) | 39 (7.5) | 5.72 |
| Fall | 37 (5.3) | 17 (3.3) | 23 (4.4) | 3.00 |
| Any cardiovascular event | 42 (6.0) | 22 (4.2) | 22 (4.2) | 2.94b |
| Angina | 2 (0.3) | 2 (0.4) | 1 (0.2) | 0.33 |
| Atrial fibrillation | 7 (1.0) | 2 (0.4) | 4 (0.8) | 1.56 |
| CHD | 0 (0.0) | 0 (0.0) | 1 (0.2) | 2.34 |
| Coronary bypass | 4 (0.6) | 0 (0.0) | 0 (0.0) | 6.01c |
| Myocardial infarction | 6 (0.9) | 3 (0.6) | 3 (0.6) | 0.50 |
| Stroke/TIA | 9 (1.3) | 3 (0.6) | 6 (1.1) | 1.60 |
| Other | 14 (2.0) | 15 (2.9) | 7 (1.3) | 3.03 |
The PI and a clinical co-investigator evaluated whether or not any reported AE could have been related to trial participation. It was determined that no AE was the direct consequence of participating in the study or receiving the intervention. There were nine deaths reported during the trial: three participants in the group intervention arm, two participants in the individual intervention arm and four participants in the UC arm. The causes of death, when provided, were not deemed to be related to the intervention.
We asked specifically about CVD events at follow-up, as a number of these would be expected in a sample of participants at high risk of CVD. However, there were no significant differences in numbers of CVD events reported between trial arms [χ2(2) = 2.94; p > 0.05]. We also asked specifically about physical injuries, as this was the only AE with the potential to be linked to an increase in PA as a result of participating. There were also no significant differences in numbers of physical injuries reported between trial arms [χ2(2) = 3.61; p > 0.05].
Chapter 6 Cost-effectiveness
The number and percentage of participants using specific services or groups of services are shown in Table 23. In the 12-month period prior to baseline assessment, the use of all services was very similar between the three treatment arms. There were relatively few people attending accident and emergency (A&E) or day hospitals or being admitted as inpatients. About half of the participants in each arm had outpatient contacts and nearly every participant had community contacts. This was largely due to GP visits.
| Service | Time point, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month follow-up | 24-month follow-up | |||||||
| Trial arm | Trial arm | Trial arm | |||||||
| Group intervention (N = 697) | Individual intervention (N = 523) | UC (N = 522) | Group intervention (N = 597) | Individual intervention (N = 466) | UC (N = 493) | Group intervention (N = 570) | Individual intervention (N = 422) | UC (N = 459) | |
| Intervention | 0 (0) | 0 (0) | 0 (0) | 437 (63) | 406 (78) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| A&E | 59 (8) | 43 (8) | 40 (8) | 82 (14) | 54 (12) | 58 (12) | 80 (14) | 67 (16) | 64 (14) |
| Day hospital | 55 (8) | 42 (8) | 42 (8) | 40 (7) | 36 (8) | 30 (6) | 26 (5) | 18 (4) | 12 (3) |
| Inpatient | 43 (6) | 31 (6) | 20 (4) | 47 (8) | 27 (6) | 31 (6) | 37 (6) | 24 (6) | 29 (6) |
| Outpatient | 355 (51) | 268 (51) | 266 (51) | 346 (58) | 280 (60) | 301 (61) | 383 (67) | 272 (65) | 302 (66) |
| Community | 667 (96) | 502 (96) | 503 (96) | 588 (98) | 461 (99) | 489 (99) | 564 (99) | 412 (98) | 449 (98) |
In the 12 months prior to the 12-month follow-up, around two-thirds of the group intervention arm and three-quarters of the individual intervention arm received the relevant intervention. Numbers of visits to A&E and outpatient attendances had increased slightly from baseline but there were no clear differences between treatment arms. These patterns were again observed in the 12-month period prior to the second follow-up, although there was again a slight increase in the proportions having outpatient attendances.
For those with specific service contacts, the average number of contacts is shown in Table 24. At baseline, community services were used more intensely than other services. The inpatient contacts refer to the number of days in hospital. It can be seen that there are no major differences between arms. The data for the 1-year follow-up show that the individual intervention arm had slightly more intervention contacts than the group intervention arm. Those admitted to hospital from the individual intervention arm had more days in hospital than the other two arms. There were no clear differences between arms in the period up to the 24-month follow-up.
| Service | Time point, mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month follow-up | 24-month follow-up | |||||||
| Trial arm | Trial arm | Trial arm | |||||||
| Group intervention (n = 697) | Individual intervention (n = 523) | UC (n = 522) | Group intervention (n = 597) | Individual intervention (n = 466) | UC (n = 493) | Group intervention (n = 570) | Individual intervention (n = 422) | UC (n = 459) | |
| Intervention | – | – | – | 7.6 (3.0) | 8.8 (3.4) | – | – | – | – |
| A&E | 1.5 (1.6) | 1.3 (0.6) | 1.3 (0.7) | 1.5 (1.4) | 1.3 (1.1) | 1.5 (1.0) | 1.8 (3.3) | 1.3 (0.8) | 1.6 (1.7) |
| Day hospital | 3.4 (4.1) | 1.8 (2.3) | 2.4 (2.0) | 1.8 (1.4) | 1.7 (1.9) | 1.6 (1.2) | 1.5 (1.0) | 1.7 (1.5) | 1.0 (0.0) |
| Inpatient | 4.7 (10.1) | 4.7 (5.0) | 3.8 (5.9) | 7.4 (12.4) | 14.7 (47.9) | 6.9 (21.1) | 5.8 (9.3) | 3.0 (3.1) | 4.0 (3.3) |
| Outpatient | 3.2 (4.2) | 3.0 (4.1) | 3.2 (4.5) | 3.6 (4.2) | 4.1 (4.5) | 4.0 (5.4) | 3.9 (4.2) | 4.2 (4.6) | 4.1 (4.0) |
| Community | 7.1 (5.4) | 7.5 (8.0) | 7.0 (6.9) | 8.8 (6.5) | 9.2 (7.1) | 9.1 (6.5) | 9.4 (8.1) | 8.9 (7.3) | 9.2 (6.8) |
Service costs (including zero costs for non-users) were similar for inpatient care, outpatient attendances and community contacts (Table 25). Costs of services did not differ markedly between arms, although inpatient costs were somewhat higher for the individual intervention arm at the 12-month follow-up and lower at the 24-month follow-up. The intervention cost was highest for those in the individual intervention arm.
| Service | Time point, mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month follow-up | 24-month follow-up | |||||||
| Trial arm | Trial arm | Trial arm | |||||||
| Group intervention (n = 697) | Individual intervention (n = 523) | UC (n = 522) | Group intervention (n = 697) | Individual intervention (n = 523) | UC (n = 522) | Group intervention (n = 697) | Individual intervention (n = 523) | UC (n = 522) | |
| Intervention | 0 (0) | 0 (0) | 0 (0) | 55 (50) | 136 (98) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| A&E | 19 (94) | 16 (58) | 15 (59) | 30 (107) | 23 (83) | 27 (90) | 36 (204) | 32 (88) | 33 (123) |
| Day hospital | 193 (1049) | 105 (573) | 137 (618) | 85 (405) | 95 (497) | 68 (341) | 49 (271) | 53 (328) | 19 (114) |
| Inpatient | 172 (1605) | 164 (955) | 85 (788) | 340 (2333) | 499 (6940) | 256 (3207) | 219 (1609) | 99 (590) | 149 (745) |
| Outpatient | 222 (460) | 210 (442) | 221 (485) | 284 (492) | 327 (542) | 329 (630) | 355 (524) | 366 (568) | 361 (505) |
| Community | 225 (260) | 241 (331) | 222 (187) | 306 (253) | 317 (233) | 306 (220) | 324 (253) | 306 (232) | 321 (279) |
| Total | 831 (2127) | 736 (1299) | 680 (1132) | 1107 (2520) | 1407 (7067) | 985 (3312) | 984 (1915) | 855 (1079) | 884 (1114) |
Compared with the UC arm, the total costs at baseline were, on average, £151 more for the group intervention arm (95% CI –£27 to £328) and £55 more for the individual intervention arm (95% CI –£96 to £203). The group intervention arm costs were, on average, £95 (95% CI –£93 to £299) more than those for the individual intervention arm. After controlling for baseline in a regression model, the total costs in the 12-month period prior to the first follow-up were, on average, £89 (95% CI –£274 to £390) more for the group intervention arm than for the UC arm and £409 (95% CI –£171 to £1133) more for the individual intervention arm than the UC arm. The individual intervention arm costs were, on average, £320 (95% CI –£170 to £1133) more than those for the UC arm. By the second follow-up, and again controlling for baseline, the mean costs for the group intervention arm were £82 (95% CI –£93 to £263) more than those for UC and £39 (95% CI –£108 to £188) more for UC than for the individual intervention arm. Costs were £121 (95% CI –£37 to £309) more for the group intervention arm than for the individual intervention arm.
Mean total costs over the whole 24-month follow-up period with year 2 costs discounted by 3.5% were £2071 (SD £3363) for the group intervention arm, £2230 (SD £7645) for the individual intervention arm and £1852 (SD £3726) for the UC arm. Compared with UC and controlling for baseline, the group intervention arm had costs that were, on average, £172 higher (95% CI –£237 to £599) and the individual intervention arm had costs that were £352 higher (95% CI –£309 to £1271). For cases in which both cost and QALY data were available, the group intervention arm had incremental costs of £173 compared with the UC arm and the individual intervention arm had incremental costs of £356 compared with the UC arm. These are the incremental costs subsequently used in the ICERs.
Mean EQ-5D tariff scores were similar for each arm and did not change markedly over time (Table 26). Controlling for baseline utility and compared with the UC arm, the group intervention arm had 0.0150 fewer QALYs (95% CI –0.0388 to 0.0088 QALYs) and the individual intervention arm had 0.0039 more QALYs (95% CI –0.0217 to 0.0294 QALYs). For cases in which both cost and QALYs were available, the group intervention arm produced 0.0149 fewer QALYs than the UC arm and the individual intervention arm produced 0.0064 more QALYs than the UC arm. These figures are used as the denominators in the ICERs.
| Time point | Trial arm, mean (SD) | ||
|---|---|---|---|
| Group intervention | Individual intervention | UC | |
| EQ-5D tariff scores | |||
| Baseline | 0.8738 (0.1973) | 0.8831 (0.1870) | 0.8828 (0.1630) |
| 12-month follow-up | 0.8869 (0.1958) | 0.9085 (0.1632) | 0.8947 (0.1688) |
| 24-month follow-up | 0.8994 (0.1639) | 0.9064 (0.1475) | 0.9144 (0.1459) |
| QALYs | 1.7717 (0.3073) | 1.8123 (0.2306) | 1.8014 (0.2462) |
The group intervention arm was less effective and more expensive than the UC arm. For this reason, it was dominated. The individual intervention was more expensive and more effective than UC. The ICER was £55,625 per QALY (£356 divided by 0.0064 QALYs). Uncertainty around the estimates are shown in the cost-effectiveness planes (Figures 8 and 9). In Figure 8, comparing the group intervention arm with the UC arm, it can be clearly seen that the majority (69%) of bootstrapped replications fall in the north-west quadrant, in which the group intervention arm has higher costs and produces fewer QALYs. In the north-east quadrant, in which costs are still higher but more QALYs are produced, 8.5% of replications fall. In the south-east quadrant, in which costs are lower and more QALYs are produced, 4% of replications fall. Finally, 18.5% of replications fall in the south-west quadrant, in which the group intervention arm is less expensive but produces fewer QALYs.
FIGURE 8.
Cost-effectiveness plane comparing the group intervention arm and UC. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

FIGURE 9.
Cost-effectiveness plane comparing the individual intervention arm and UC. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

In Figure 9, comparing the individual intervention arm with the UC arm, 52.3% of replications are in the north-east quadrant, in which the individual intervention arm has higher costs and produces more QALYs; 15.7% are in the south-east quadrant, in which costs are lower and there are more QALYs produced; 5.8% are in the south-west quadrant, in which costs are lower and there are fewer QALYs produced; and, finally, 26.2% of replications are in the north-west quadrant, in which the individual intervention arm has higher costs and produces fewer QALYs.
Figure 10 shows the cost-effectiveness acceptability curves in which all three arms are compared for different values placed on a QALY gain. When a zero value is placed on a QALY, UC has by far the highest probability of being the most cost-effective option (in this situation, only costs are relevant and UC is less expensive). As the value placed on a QALY is increased, the probability that the individual intervention arm is the most cost-effective option increases steadily and the other two arms see a fall in the probability that they are the most cost-effective. This is to be expected as the individual intervention arm is more expensive and more effective than the other options, and as the effect (i.e. increased QALYs) is valued more, it increasingly offsets the cost. However, at a value of £30,000 (above which NICE is likely to decide an intervention is not cost-effective) the individual intervention arm has a 37.4% likelihood of being the most cost-effective option, compared with 58.1% for UC. At this value, the group intervention arm has a likelihood of 4.5% of being the most cost-effective option.
FIGURE 10.
Cost-effectiveness acceptability curves. Reproduced from Ismail et al. 110 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

In sensitivity analyses, the intervention costs were increased and decreased by 25% and 50%, respectively, to reflect different grades of staff delivering the interventions. When intervention costs were reduced by 25%, the group intervention arm had costs that were, on average, £188 more than for the UC arm, whereas costs for the individual intervention arm were £395 more than for the UC arm; the differences when intervention costs were increased by 50% were £204 and £434, respectively. Not surprisingly, these increased differences mean that the interventions are even less likely to be cost-effective compared with UC.
When intervention costs are reduced by 25%, the group intervention arm has costs that are, on average, £157 more than for UC. With fewer QALYs, UC is still dominant. The individual intervention now costs £317 more than the cost of UC, resulting in a cost per QALY of £49,531. With a reduction of 50%, the group intervention arm still has higher costs than UC (by £141) and so continues to be dominated, whereas the individual intervention arm has costs that are £278 higher, resulting in a cost per QALY of £43,438.
Summary
The health economic analyses show that the group intervention is not a cost-effective alternative to UC as it produces fewer QALYs and results in higher health-care costs. The individual intervention is more effective than UC in terms of QALYs produced but the higher costs result in an ICER that is in excess of the £20,000- to £30,000-per-QALY threshold usually used in England to determine cost-effectiveness and so is also not cost-effective. The ICER for the individual intervention arm compared with the group intervention arm is below this threshold, but UC is still the preferred option. Sensitivity analyses did not alter the results in a substantial way.
Chapter 7 Participation biases
Background
Lifestyle intervention trials have previously reported low response rates and do not always recruit those most at risk, which will limit the generalisability of observed associations. 112,113 Failure to recruit those with the highest risk of CVD to the MOVE IT trial could have led to limited representativeness and underestimated effect sizes, and could have contributed to increasing rather than reducing health inequalities. It is important, therefore, that RCTs such as MOVE IT report on participation bias.
The factors found to increase likelihood of participation in walking and lifestyle intervention trials are mid-life, female sex, living in more affluent areas, white ethnicity and university education. 112,114 Ethnic minorities are at higher risk of CVD and related disorders, such as type 2 diabetes mellitus. 115 Regarding the health of participants versus non-participants, some trials report that participants are more likely to be of poorest health,112,116–118 whereas other studies report that participants are healthier or more active. 113,117,119,120
We compared the anonymised sociodemographic data and CVD risk of responders and non-responders to the MOVE IT invitation to participate. We aim to test the hypothesis that people who are older, female, living in more affluent areas, at lower risk of CVD and of white ethnicity are more likely to respond.
Methods
Using a cross-sectional design, we compared anonymous data from medical records of patients who responded to the invitation to participate in MOVE IT and those of patients who did not. We gained REC approval to extract anonymised data for all patients invited to participate unless an informed dissent code was present on medical records.
The measures collected anonymously for all patients invited to participate included age (at time of screening), sex, postcode data to calculate IMD 2010 score,76 QRISK2 score and ethnicity.
QRISK2 score was estimated on medical records via a batch calculator, using age- and sex-based national averages for missing data values.
Ethnicity data from medical records were grouped into white, black African or Caribbean, South Asian, other Asian, other/mixed or missing for this analysis. South Asian and other Asian ethnicities are coded separately because South Asian ethnicity is associated with a higher CVD risk. 72 The category of other/mixed incorporates any ethnicity that is reported and does not fit into the previous categories, as well as including mixed ethnic backgrounds.
Data are summarised as mean (SD) or percentages. The adjusted odds ratios (AORs) for response to invitation by the sociodemographic and CVD risk data collected were calculated using logistic regression in Stata version 14. General practice was included as a random effect in the model to allow for clustering by practice. AORs for age are presented per 5-year increase, for IMD 2010 scores per 10-point increase and for QRISK2 scores per 5-percentage-point increase to provide a better comparison of the strength of the relationships.
Results
Lists of patients invited to participate were not saved by general practices and we returned to practices later to extract anonymous data. Sixty practices provided the original anonymised data. Sociodemographic data and QRISK2 scores were extracted for 8902 patients, representing 50.5% of the 17,618 patients originally invited to participate. Table 27 shows the CCGs from which anonymised data were collected and the deprivation and ethnicity census data of each local authority. 71 General practice deprivation scores (IMD 2010)76 of practices from which anonymised data were extracted did not differ significantly from that of all other practices in South London [t(440) = 0.57; p = 0.57). 108
| Characteristics | CCG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bexley | Bromley | Croydon | Greenwich | Kingston | Lambeth | Lewisham | Merton | Richmond | Southwark | Sutton | Wandsworth | |
| Borough deprivation (IMD 2010 rank76)a (n) | 180 | 217 | 99 | 19 | 252 | 14 | 16 | 208 | 286 | 25 | 193 | 102 |
| Borough ethnicity (%)b | ||||||||||||
| White | 81.9 | 84.3 | 55.2 | 62.5 | 74.5 | 57.1 | 53.6 | 64.9 | 85.9 | 54.3 | 78.6 | 71.4 |
| Black African or Caribbean | 8.5 | 6.0 | 20.2 | 19.1 | 2.4 | 25.9 | 27.2 | 10.4 | 1.5 | 26.8 | 4.8 | 10.6 |
| South Asian | 3.6 | 2.7 | 10.5 | 4.7 | 6.5 | 3.3 | 2.8 | 8.9 | 3.9 | 4.0 | 5.4 | 6.5 |
| Other Asian | 2.9 | 2.5 | 5.9 | 7.0 | 9.9 | 3.5 | 6.5 | 9.2 | 3.4 | 5.5 | 6.2 | 4.4 |
| Other/mixed | 3.0 | 4.5 | 8.4 | 6.7 | 6.6 | 10.1 | 10.0 | 6.5 | 5.2 | 9.4 | 5.0 | 7.1 |
| Anonymous data collected (n) | ||||||||||||
| General practices | 1 | 11 | 11 | 1 | 1 | 6 | 11 | 2 | 0 | 9 | 3 | 4 |
| Patients | 328 | 2463 | 1663 | 255 | 27 | 845 | 1281 | 41 | 0 | 666 | 786 | 646 |
The sociodemographic and QRISK2 data of responders (n = 1489) and non-responders (n = 7413) are presented in Table 28. The mean age of all patients at the time of invitation was 67.3 ± 5.7 years, and 20.7% were female. The mean IMD 201076 score of patients invited was 21.7, a score that falls within the second-most-deprived quintile, and the mean QRISK2 score of invitees was 25.2%. Of all patients invited, 69.9% had a white ethnic background, 13.9% had ethnic minority backgrounds and 16.2% had no ethnicity data recorded in their medical records.
| Characteristics | Patients | Response rate (%) | OR for response to mailout (95% CI) | Test for trend (p-value) | |||
|---|---|---|---|---|---|---|---|
| All invited (N = 8902)a | Responded to invitation (N = 1489) | Did not respond to invitation (N = 7413) | Unadjusted | Adjusted | |||
| Age at invitation (years), mean (SD) | 67.3 (5.7) | 68.1 (5.1) | 67.2 (5.8) | 1.14 (1.08 to 1.21)b | 1.19 (1.12 to 1.26)b | < 0.001 | |
| Age group at invitation (years), n (%) | |||||||
| 40–59 | 976 (11.0) | 124 (8.4) | 852 (11.6) | 12.7 | |||
| 60–64 | 1462 (16.4) | 200 (13.5) | 1262 (17.0) | 13.7 | |||
| 65–69 | 2989 (33.6) | 536 (36.0) | 2453 (33.1) | 17.9 | |||
| 70–75 | 3475 (39.0) | 629 (42.2) | 2846 (38.4) | 18.1 | |||
| Sex, n (%) | |||||||
| Female | 1847 (20.7) | 291 (19.5) | 1556 (21.0) | 15.8 | 1.00 | 1.00 | |
| Male | 7055 (79.3) | 1198 (80.5) | 5857 (79.0) | 17.0 | 1.07 (0.93 to 1.23) | 1.24 (1.07 to 1.44) | 0.004 |
| Ethnicity, n (%) | < 0.001c | ||||||
| White | 6223 (69.9) | 1128 (75.8) | 5095 (68.7) | 18.1 | 1.00 | 1.00 | |
| Black African or Caribbean | 272 (3.1) | 34 (2.3) | 238 (3.2) | 12.5 | 0.74 (0.50 to 1.07) | 0.66 (0.45 to 0.96) | 0.032 |
| South Asian | 578 (6.5) | 98 (6.6) | 480 (6.5) | 17.0 | 0.90 (0.70 to 1.14) | 1.07 (0.84 to 1.37) | 0.593 |
| Other Asian | 240 (2.7) | 31 (2.1) | 209 (2.8) | 12.9 | 0.69 (0.47 to 1.03) | 0.71 (0.48 to 1.05) | 0.086 |
| Other/Mixed | 147 (1.7) | 16 (1.1) | 131 (1.8) | 10.9 | 0.62 (0.36 to 1.05) | 0.61 (0.36 to 1.05) | 0.072 |
| Missing | 1442 (16.2) | 182 (12.2) | 1260 (17.0) | 12.6 | 0.58 (0.48 to 0.69) | 0.55 (0.46 to 0.66) | < 0.001 |
| IMD 2010 score,d mean (SD) | 21.7 (12.0) | 19.4 (11.3) | 22.2 (12.1) | 0.82 (0.76 to 0.87)e | 0.84 (0.79 to 0.90)e | < 0.001 | |
| IMD 2010 quintile, n (%) | |||||||
| 1 (least deprived) | 1407 (15.8) | 293 (19.7) | 1114 (15.0) | 20.8 | |||
| 2 | 1531 (17.2) | 285 (19.1) | 1246 (16.8) | 18.6 | |||
| 3 | 1616 (18.2) | 315 (21.2) | 1301 (17.6) | 19.5 | |||
| 4 | 2725 (30.6) | 420 (28.2) | 2305 (31.1) | 15.4 | |||
| 5 (most deprived) | 1604 (18.0) | 171 (11.5) | 1433 (19.3) | 10.7 | |||
| Unknown | 19 (0.2) | 5 (0.3) | 14 (0.2) | 26.3 | |||
| QRISK2 score, mean (SD) | 25.2 (5.0) | 24.6 (4.5) | 25.3 (5.1) | 0.86 (0.81 to 0.92)f | 0.83 (0.77 to 0.88)f | < 0.001 | |
| QRISK2 score category, n (%) | |||||||
| 20–24.9% | 5357 (60.2) | 968 (65.0) | 4389 (59.2) | 18.1 | |||
| 25–29.9% | 2245 (25.2) | 347 (23.3) | 1898 (25.6) | 15.5 | |||
| ≥ 30% | 1300 (14.6) | 174 (11.7) | 1126 (15.2) | 13.4 | |||
The logistic regression analyses that were conducted to estimate the odds of response are also presented in Table 28. Response rates were higher with increasing age (AOR 1.19, 95% CI 1.12 to 1.26); the odds of response increased by 19% for each 5-year increase in age. The response rate was greater in male patients (AOR 1.24, 95% CI 1.07 to 1.44) and decreased with greater levels of deprivation (AOR 0.84, 95% CI 0.79 to 0.90); for every 10-point increase in IMD 2010 score, the odds of responding decreased by 16%. Increased CVD risk also lowered the response rate (AOR 0.83, 95% CI 0.77 to 0.88); for every 5-point increase in QRISK2 score, the odds of responding decreased by 17%. The response rate was lower for patients of black African or Caribbean ethnicity (AOR 0.66, 95% CI 0.45 to 0.96) and those with missing ethnicity data (AOR 0.55, 95% CI 0.46 to 0.66) than for patients of white ethnicity. The response rates of Asian and other ethnic backgrounds were not significantly different from those of patients of white ethnicity.
Pairwise comparisons were conducted to explore differences in response between different ethnic minority groups, but there were no significant differences. Response rates of patients with missing ethnicity data were significantly lower than those of patients of South Asian ethnicity (p = 0.002). Owing to small numbers of invitees from non-white ethnic backgrounds, a sensitivity analysis was undertaken to investigate predictors of response to invitation with ethnicity removed from the model. Older age (p < 0.001), male sex (p = 0.017), being more affluent (p < 0.001) and having a lower CVD risk (p < 0.001) remained significant predictors of response.
Summary
In Chapter 5, we demonstrated that the trial successfully recruited general practices that did not differ significantly from all other practices in South London in practice deprivation, and this was also true for the smaller group of practices involved in this analysis. Therefore, the MOVE IT trial successfully recruited from socioeconomically varied areas. For this analysis, we found evidence of participation bias towards patients who were older, male, residing in more affluent areas and at a lower CVD risk and we found that patients of black African or Caribbean ethnicity were less likely to respond than patients of white ethnicity. South Asian patients were as likely to respond as patients of white ethnicity but the QRISK2 score identified a smaller sample size of people of African Caribbean and Asian ethnicities. This could be partly because those CCGs with the highest IMD 2010 scores and greater ethnic diversity were less likely to participate in MOVE IT. It is noteworthy that these CCGs were the slowest to respond to participate although all CCGs were invited at the same time. We also observed high rates of missing ethnicity data on medical records and this group of patients with missing ethnicity data was less likely to respond than patients of white or South Asian ethnicity. These findings suggest that there may have been a participation bias towards those with lower CVD risk compared with those most at risk.
Chapter 8 Fidelity analysis
Introduction
As part of the process evaluation of the study, we assessed fidelity, here defined as delivery of the enhanced MI intervention by the HLFs in accordance with the manual. Furthermore, we were interested in determining if the level of HLF competency in delivering the intervention differed between the individual and group intervention arms.
As described in Chapter 3, HLFs received extensive training and supervision before and during the intervention to ensure that they were competent in delivering MOVE IT. HLFs were asked to audiotape each intervention session, and these data were stored on King’s College London’s shared network drives, as per the university protocol. Chapter 4 describes how a university-wide network outage caused the recorded session data to be completely lost, with only a limited sample being subsequently recovered from other sources. In this chapter, we will describe our original aims and methods and how these were adapted following the network outage.
Aims
For the analysis, we were originally interested in answering five questions:
-
What was the degree of adherence by the HLFs to the MI and BCT elements in the group and individual intervention arms?
-
What was the level of HLF competency in delivering the MI aspects?
-
What was the level of HLF competency in delivering the BCTs?
-
Were there any differences in competency in delivering the MI and BCT elements between the group and individual treatment arms?
-
Were the HLF competency levels in delivering the intervention associated with patient outcomes or mediators of any treatment effects?
Owing to the network outage and the number and quality of data that were subsequently recovered, we were unable to address question 5. In addition, although the HLFs were trained to a certain level of competency in two other domains (group skills and time management; see Chapter 3), we were not interested in assessing their competency in these domains for this fidelity analysis.
Original methods
Identifying sessions to rate
Ideally, if every patient randomised to the individual or group intervention attended each of the 10 sessions, we would have obtained 6200 hours of recordings (Table 29). In addition, by assuming a 20% dropout rate halfway through the intervention, we would have obtained 5201 hours of recordings. We originally had 2756 tapes of the intervention sessions, comprising 1989 hours of content.
| Scenario | Number of participants/groups | Average length (hours) | Total number of hours |
|---|---|---|---|
| Ideal | |||
| Individual | 523 (10 sessions) | 0.70 | 3671 |
| Group | 139 (10 sessions) | 1.81 | 2529 |
| Total | 6200 | ||
| Anticipated | |||
| Individual | 418 (105) (10 sessions, 5 dropouts) | 0.70 | 3304 |
| Group | 125 (14) (10 sessions, 5 dropouts) | 1.81 | 1897 |
| Total | 5201 | ||
| Original (pre outage)a | |||
| Individual | 2251 | 0.57 | 1289 |
| Group | 400 | 1.55 | 619 |
| Total | 1908b | ||
| Recovered (post outage)b | |||
| Individuals | 324 | 0.70 | 227 |
| Groups | 71 | 1.81 | 129 |
| Total | 356 | ||
We planned to randomly select and rate 25% of the available recordings, stratified by intervention arm (group vs. individual), ensuring that every participant in the individual intervention arm and every group in the group intervention arm had at least one recording assessed for fidelity. Sessions lasting ≥ 20 minutes would be selected. We planned that a 20-minute segment (randomly selected from the beginning, middle or end) of each tape would be rated for individual sessions, and we selected a 50-minute segment of the group sessions, as this would allow a fairer opportunity for MI skills to be demonstrated in a group context. During our preparation for the fidelity coding, we found that a 20-minute segment was not suitable for group sessions, as the structure and format of group sessions meant that significant amounts of time were devoted to group exercises that could not be coded.
Measures of competency
We assessed the MI and BCT aspects of the intervention. To assess HLF fidelity to the MI aspects of the intervention, we used the MITI manual (version 3.1.1),97 which counts the number of MI techniques used. The MITI tool has been validated and is viewed as the gold standard of MI fidelity, has been translated into multiple languages121 and is used widely as a coding system, including in other large-scale studies. 122,123
The MITI rating tool comprises several variables. ‘Global’ ratings are given for each of ‘spirit’ and ‘empathy’, scored on a scale from 1 to 5 (with the coder assuming a beginning score of 3 and moving up or down based on the HLF’s observed skill). Behaviour count scores are provided for ‘giving information’, ‘MI adherent’ behaviours, ‘MI non-adherent’ behaviours, ‘closed questions’, ‘open questions’, ‘simple reflections’ and ‘complex reflections’. The MITI guidance provides minimum scores that a practitioner must achieve to reach proficiency and competency (Table 30).
| Measure | Beginning proficiency | Competency |
|---|---|---|
| Global spirit rating, mean score | 3.5 | 4 |
| Reflection-to-question ratio | 1 | 2 |
| Percentage with open questions | 50 | 70 |
| Percentage with complex reflections | 40 | 50 |
| Percentage who were MI adherent | 90 | 100 |
To assess HLF adherence to administering the 11 BCTs, each was coded as present (at least once) or absent during a session. The 11 BCTs were:
-
provide information on consequences
-
prompt intention formation
-
prompt barrier identification
-
prompt specific goal-setting
-
prompt review of behavioural goals
-
prompt self-monitoring of behaviour
-
teach to use prompts or cues
-
agree on behavioural contract
-
plan social support or social change
-
self-talk
-
relapse prevention.
Raters were provided with a BCT coding framework, which listed each BCT label alongside a description and an example quotation (see Appendix 3). MI, which is considered the 12th BCT in the taxonomy,54 was removed and considered separately, as the CPs developing the intervention deemed this to be the communication process by which the BCTs were used with participants, rather than as a BCT itself. The BCT coding framework was developed by the authors and raters specifically for this trial, as our review of the extant literature did not reveal an existing coding framework that would be appropriate. The coding framework was developed through an iterative process and piloted internally; however, it was not validated before being utilised in the study.
Interrater reliability
Two independent psychologists who had completed formal training in using the MITI tool and were experienced in this method (having worked together on previous studies) coded the recordings. To determine interrater reliability, the two raters initially coded the same selection of tapes. First, 40 tapes (20 individual and 20 group sessions) were randomly selected. Then, for each tape, a segment that was 20–50 minutes in length was randomly selected, reflecting the variability in quality and length of the tapes.
The raters worked together extensively in adapting the MITI tool to suit the group format. They met on several occasions to discuss discrepancies between the coding of individual and group transcripts. Individual issues were also discussed as they arose until agreement was reached on coding principles.
To ensure that rater drifting did not happen, the raters initially met weekly. As the coding proceeded and any raised issues were resolved, the raters and one of the CPs met regularly. The raters also engaged in peer supervision, in which they would discuss with each other any coding issues that were raised for a tape and how to resolve them. The raters closely followed the principles detailed in the MITI tool and referred to this document on a regular basis. For example, it was necessary to identify the nature of facilitative and structural statements compared with the utterances that constituted MITI behaviour counts, such as complex and simple reflections or giving information.
Intraclass correlation coefficients using a two-way consistency model were calculated for global spirit and empathy scores and absolute agreement percentages for each BCT. For the individual sessions, the two raters achieved ICCs of 0.89 (95% CI 0.75 to 0.96) and 0.84 (95% CI 0.65 to 0.94) for the global ratings of empathy and spirit, respectively; both ICCs are considered good to excellent. 124 The mean absolute agreement was 94.09% (range 85–100%) for the 11 BCTs.
For the group sessions, the two raters achieved ICCs of 0.69 (95% CI 0.37 to 0.87) and 0.71 (95% CI 0.41 to 0.88) for the global ratings of empathy and spirit, respectively; both ICCs are considered good. The mean absolute agreement was 86.82% (range 70–100%) for the 11 BCTs.
Revised methods
Identifying sessions to rate
The network outage at King’s College London resulted in all tapes being lost, with 84.22% of them irretrievably lost. We were able to recover 435 tapes that could be coded. Table 31 summarises the characteristics of the recovered tapes, excluding the 40 used for determining interrater reliability (described in the previous section). When comparing the characteristics of the original and recovered tapes, we found that there were significant differences with regards to the distribution of session number and which HLF administered the session, but no difference in session type (see Table 31). Furthermore one HLF was missing from the recovered tapes (HLF13).
| Characteristics | Recordings, n (%) | Statistical test output | |
|---|---|---|---|
| Original (N = 2651)a | Recovered (N = 395) | ||
| Session number | |||
| 0 | 40 (1.87) | 17 (4.30) | χ2(10) = 86.31; p < 0.0001 |
| 1 | 255 (11.93) | 49 (12.41) | |
| 2 | 237 (11.09) | 54 (13.67) | |
| 3 | 233 (10.90) | 46 (11.65) | |
| 4 | 232 (10.86) | 57 (14.43) | |
| 5 | 197 (9.22) | 61 (15.44) | |
| 6 | 190 (8.89) | 50 (12.66) | |
| 7 | 199 (9.31) | 33 (8.35) | |
| 8 | 180 (8.42) | 9 (2.28) | |
| 9 | 189 (8.84) | 4 (1.01) | |
| 10 | 185 (8.66) | 15 (3.80) | |
| Session type | |||
| Individual | 2251 (84.91) | 324 (82.03) | χ2(1) = 1.98; p = 0.16 |
| Group | 400 (15.09) | 71 (17.97) | |
| HLF | |||
| 1 | 571 (20.72) | 200 (50.63) | χ2(12) = 398.05; p < 0.0001 |
| 2 | 472 (17.13) | 64 (16.20) | |
| 3 | 140 (5.08) | 55 (13.92) | |
| 4 | 30 (1.09) | 30 (7.59) | |
| 5 | 97 (3.52) | 15 (3.80) | |
| 6 | 109 (3.96) | 7 (1.77) | |
| 7 | 194 (7.04) | 5 (1.27) | |
| 8 | 86 (3.12) | 5 (1.27) | |
| 9 | 455 (16.51) | 4 (1.01) | |
| 10 | 155 (5.62) | 4 (1.01) | |
| 11 | 66 (2.39) | 3 (0.76) | |
| 12 | 341 (12.37) | 3 (0.76) | |
| 13 | 40 (1.45) | 0 (0.00) | |
We also adapted the protocol and decided that the entirety of each recovered tape, as opposed to a 20- or 50-minute segment, would be coded and its data used in the fidelity analysis. This protocol change was discussed with and approved by the TSC. The initial 5 minutes of each tape were not coded, as we found this time to be devoted to content other than delivering the intervention (e.g. introductions and housekeeping from previous sessions). Approximately 50% of available tapes were randomly allocated to each rater using minimisation to ensure balance with respect to session type, session number and HLF.
Association between healthy lifestyle facilitator competencies and patient outcomes
We were unable to conduct this analysis (question 5) because the competency data had unacceptable levels of measurement error. Namely, the quality of the recovered data prevented us from matching session-level HLF competencies to patient-level outcomes.
Results
Fidelity to the intervention
A total of 395 sessions were coded for fidelity (324 individual sessions, 82.03%). We observed treatment arm differences in several of the MITI domains, generally favouring the group sessions (Table 32). None of the HLFs achieved proficiency across the five necessary MITI domains, based on the MITI guidance (Table 33). Two HLFs were at least proficient in four domains and two were at least proficient in three domains. Using our adapted competency framework, three HLFs met the competency criteria. Table 34 shows the MITI scores for each HLF by session type.
| MITI domains | Intervention arm, mean (SD) | Total (n = 395), mean (SD) | Statistical test output | |
|---|---|---|---|---|
| Group (n = 71) | Individual (n = 324) | |||
| Global ratings | ||||
| Empathy | 3.07 (0.82) | 3.03 (0.92) | 3.04 (0.90) | t(112.67) = 0.39; p = 0.70 |
| Spirita | 3.44 (0.63) | 3.52 (0.74) | 3.51 (0.72) | t(115.48) = –1.00; p = 0.32 |
| Behaviour counts | ||||
| Giving information | 25.54 (18.45) | 12.54 (10.04) | 14.88 (12.97) | t(79.32) = 5.75; p < 0.0001 |
| MI adherent | 6.83 (4.49) | 6.35 (4.27) | 6.44 (4.31) | t(99.72) = 0.83; p = 0.41 |
| MI non-adherent | 1.30 (2.19) | 1.15 (1.88) | 1.17 (1.94) | t(94.01) = 0.54; p = 0.59 |
| Percentage who were MI adherenta | 83.74 (27.30) | 84.68 (21.61) | 84.51 (22.71) | t(88.91) = –0.27; p = 0.79 |
| Closed questions | 22.48 (13.29) | 14.44 (8.76) | 15.89 (10.19) | t(83.8) = 4.87; p < 0.0001 |
| Open questions | 21.21 (10.65) | 10.81 (6.63) | 12.68 (8.50) | t(82.27) = 7.90; p < 0.0001 |
| Total questions | 43.69 (20.80) | 25.26 (13.63) | 28.57 (16.72) | t(83.63) = 7.14; p < 0.0001 |
| Percentage with open questionsa | 49.35 (14.01) | 43.32 (14.74) | 44.40 (14.78) | t(106.74) = 3.26; p < 0.0001 |
| Simple reflections | 30.73 (18.86) | 18.35 (12.20) | 20.57 (14.42) | t(83.28) = 5.30; p < 0.0001 |
| Complex reflections | 15.31 (6.88) | 10.31 (6.01) | 11.21 (6.46) | t(94.84) = 5.66; p < 0.0001 |
| Total reflections | 46.04 (22.07) | 28.71 (15.12) | 31.83 (17.85) | t(85) = 6.30; p < 0.0001 |
| Percentage with complex reflectionsa | 36.28 (14.17) | 37.26 (16.98) | 37.08 (16.50) | t(118.63) = –0.51; p = 0.61 |
| Reflection-to-question ratioa | 1.17 (0.57) | 1.37 (0.95) | 1.33 (0.90) | t(168.66) = –2.28; p = 0.024 |
| HLF | Number of tapes coded | MITI domains, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Global spirit | Percentage with complex reflections | Percentage with open questions | Reflection-to-question ratio | Percentage who were MI adherent | ||
| 1 | 200 | 3.71 (0.61) | 39.80 (16.42) | 43.28 (13.15) | 1.58 (0.80) | 90.34 (18.74) |
| 2 | 64 | 3.25 (0.66) | 37.55 (15.68) | 41.74 (16.10) | 1.15 (0.73) | 83.33 (23.58) |
| 3 | 55 | 3.20 (0.77) | 30.27 (15.78) | 54.51 (15.32) | 0.82 (0.75) | 68.13 (25.57) |
| 4 | 30 | 3.36 (1.11) | 32.39 (18.10) | 41.48 (11.67) | 0.68 (0.30) | 81.05 (23.59) |
| 5 | 15 | 3.33 (0.50) | 30.70 (12.45) | 47.66 (15.27) | 2.39 (2.05) | 83.60 (20.09) |
| 6 | 7 | 4.00 (0.00) | 48.12 (13.11) | 51.31 (15.63) | 1.10 (0.32) | 87.43 (18.66) |
| 7 | 5 | 3.93 (0.49) | 40.02 (6.91) | 45.85 (6.24) | 0.82 (0.12) | 94.18 (8.85) |
| 8 | 5 | 3.33 (0.47) | 48.81 (18.31) | 24.43 (19.31) | 0.99 (0.77) | 80.00 (44.72) |
| 9 | 4 | 3.42 (0.42) | 34.38 (8.90) | 39.59 (16.27) | 1.33 (0.41) | 78.98 (21.91) |
| 10 | 4 | 2.92 (0.68) | 22.65 (22.06) | 23.28 (5.69) | 1.00 (0.29) | 71.39 (23.10) |
| 11 | 3 | 3.67 (0.58) | 23.46 (13.10) | 50.46 (10.48) | 1.12 (0.77) | 94.44 (9.62) |
| 12 | 3 | 2.77 (0.84) | 33.58 (3.49) | 46.76 (7.58) | 1.51 (0.19) | 54.26 (26.85) |
| HLF | Number of tapes coded | MITI domains, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Global spirit | Percentage with complex reflections | Percentage with open questions | Reflection-to-question ratio | Percentage who were MI adherent | ||
| Individual sessions | ||||||
| 1 | 152 | 3.76 (0.61) | 41.00 (17.36) | 42.11 (13.41) | 1.64 (0.87) | 91.88 (13.97) |
| 2 | 50 | 3.25 (0.67) | 37.32 (15.58) | 39.14 (16.08) | 1.28 (0.76) | 82.76 (24.58) |
| 3 | 47 | 3.22 (0.80) | 29.69 (14.94) | 53.47 (13.54) | 0.85 (0.80) | 67.98 (25.41) |
| 4 | 30 | 3.36 (1.11) | 32.39 (18.10) | 41.48 (11.67) | 0.68 (0.30) | 81.05 (23.59) |
| 5 | 15 | 3.33 (0.50) | 30.70 (12.45) | 47.66 (15.27) | 2.39 (2.05) | 83.60 (20.09) |
| 6 | 7 | 4.00 (0.00) | 48.12 (13.11) | 51.31 (15.63) | 1.10 (0.32) | 87.43 (18.66) |
| 7 | 4 | 4.00 (0.54) | 39.31 (7.77) | 45.08 (6.92) | 0.83 (0.14) | 92.73 (9.51) |
| 8 | 5 | 3.33 (0.47) | 48.81 (18.31) | 24.43 (19.31) | 0.99 (0.77) | 80.00 (44.72) |
| 9 | 4 | 2.92 (0.68) | 22.65 (22.06) | 23.28 (5.69) | 1.00 (0.29) | 71.39 (23.10) |
| 10 | 4 | 3.42 (0.42) | 34.38 (8.90) | 39.59 (16.27) | 1.33 (0.41) | 78.98 (21.91) |
| 11 | 3 | 2.77 (0.84) | 33.58 (3.49) | 46.76 (7.58) | 1.51 (0.19) | 54.26 (26.85) |
| 12 | 3 | 3.67 (0.58) | 23.46 (13.10) | 50.46 (10.48) | 1.12 (0.77) | 94.44 (9.62) |
| Group sessions | ||||||
| 1 | 48 | 3.55 (0.62) | 35.97 (12.42) | 46.98 (11.67) | 1.41 (0.52) | 85.44 (28.85) |
| 2 | 14 | 3.26 (0.63) | 38.38 (16.59) | 51.05 (12.76) | 0.68 (0.28) | 85.34 (20.39) |
| 3 | 8 | 3.04 (0.60) | 33.59 (20.89) | 60.65 (23.58) | 0.66 (0.31) | 68.93 (28.20) |
| 7 | 1 | 3.66 (–) | 42.86 (–) | 48.94 (–) | 0.74 (–) | 100 (–) |
The 11 BCTs were generally coded as present in most of the group (median 45/71, range 20–58) and individual (median 179/324, range 69–267) sessions (Table 35). The distributions of each BCT being coded present were similar between session types, except for BCT 1 (‘provide information on consequences’).
| BCT | Intervention arm, n (%) | Total (N = 395), n (%) | Statistical test output | |
|---|---|---|---|---|
| Group (N = 71) | Individual (N = 324) | |||
| 1 | 48 (67.61) | 169 (52.16) | 217 (54.94) | χ 2 (1) = 5.01; p = 0.025 |
| 2 | 57 (80.28) | 267 (82.41) | 324 (82.03) | χ2(1) = 0.09; p = 0.76 |
| 3 | 45 (63.38) | 224 (69.14) | 269 (68.10) | χ2(1) = 0.70; p = 0.40 |
| 4 | 53 (74.65) | 239 (73.77) | 292 (73.92) | χ2(1) = 0.00; p = 1.00 |
| 5 | 54 (76.06) | 216 (66.67) | 270 (68.35) | χ2(1) = 1.87; p = 0.17 |
| 6 | 40 (56.34) | 147 (45.37) | 187 (47.34) | χ2(1) = 2.32; p = 0.13 |
| 7 | 35 (49.30) | 179 (55.25) | 214 (54.18) | χ2(1) = 0.65; p = 0.42 |
| 8 | 58 (81.69) | 241 (74.38) | 299 (75.70) | χ2(1) = 1.23; p = 0.27 |
| 9 | 33 (46.48) | 160 (49.38) | 193 (48.86) | χ2(1) = 0.11; p = 0.74 |
| 10 | 20 (28.17) | 69 (21.30) | 89 (22.53) | χ2(1) = 1.18; p = 0.28 |
| 11 | 21 (29.58) | 99 (30.56) | 120 (30.38) | χ2(1) = 0.00; p = 0.97 |
| Overall | 464 (59.41) | 2010 (56.40) | 2474 (56.94) | χ2(1) = 2.02; p = 0.16 |
Table 36 shows mean BCT adherence by HLF and session type, aggregated over the 11 BCTs, with ≥ 70% indicating competency.
| HLF | Session type, % (n) | |
|---|---|---|
| Group | Individual | |
| 1 | 62.12 (48) | 66.75 (152) |
| 2 | 62.99 (14) | 37.64 (50) |
| 3 | 35.23 (8) | 47.97 (47) |
| 4 | – | 50.61 (30) |
| 5 | – | 32.73 (15) |
| 6 | – | 66.23 (7) |
| 7 | 72.73 (1) | 81.82 (4) |
| 8 | – | 56.36 (5) |
| 9 | – | 68.18 (4) |
| 10 | – | 54.55 (4) |
| 11 | – | 66.67 (3) |
| 12 | – | 72.73 (3) |
Discussion
We coded 395 recorded intervention sessions for fidelity. We observed that nearly all (11/13) of the HLFs achieved high levels of competency on at least one dimension of MI but fewer did so with the BCT, in which only 2 out of 13 HLFs achieved competency. The HLFs received extensive training in delivering the MI and BCT elements of MOVE IT and were supervised throughout the trial; our analysis of a non-random and probably underpowered sample of tapes indicates that the intervention was administered with acceptable levels of competencies in MI but the competency of HLFs in delivering the BCTs is not known.
When combining MITI scores across HLFs, we found that the MI aspects of the intervention were not delivered at the desired competency level. At the HLF level, none of the HLFs reached the minimum MITI-based proficiency level and only three met the competency criteria adapted for the study. By further stratifying by session type, however, we found that two HLFs delivered the MI elements in individual sessions at a level deemed competent for our study.
We observed treatment arm differences in several of the MITI domains. Most of these differences were in domains with count scores, hence these were expected given the longer length of the group session format (i.e. HLFs had more opportunity to use the technique repeatedly). Of particular interest, HLFs in group sessions demonstrated a greater percentage of open questions, whereas in individual sessions they made more reflections relative to questions. This may be because in group sessions a large proportion of time was spent facilitating group discussion, which lends to repeated use of open questions. In addition, in group sessions there may have been less opportunity for HLFs to ask closed questions, which naturally follow open questions in individual sessions (e.g. to clarify what the individual has just said or encourage further conversation). In terms of the reflection-to-question ratio, this could be explained by the fact that reflections require a deeper level of listening and understanding, which the group session format does not lend itself to as easily as individual sessions do.
Most HLFs were ‘partially proficient’ in delivering BCTs. Only three BCTs (‘prompt intention formation’, ‘prompt specific goal-setting’ and ‘agree on behavioural contract’) were administered in > 70% of sessions. Furthermore, two BCTs (‘self-talk’ and ‘relapse prevention’) were delivered in approximately < 30% of sessions. Across the BCTs defined for the trial, we observed treatment arm differences in only one (‘provide information on consequences’); however, this is possibly a false positive owing to the number of tests conducted. Finally, only two HLFs delivered, on average, ≥ 70% of the BCTs across the sessions assessed (however, this was only five sessions for one HLF and three individual sessions for the other). These results should be interpreted cautiously as we used an unvalidated BCT coding framework developed for this study; thus, we may have underestimated HLFs’ competency in delivering the BCTs.
Limitations
Owing to the network outage and loss of recorded sessions, the characteristics of the recovered tapes are based on a non-random subsample of the original tapes. Thus, it is highly likely that our findings are biased and probably an underestimation of the HLFs’ competency. We are uncertain when these sessions were recorded. For instance, if the sessions were recorded earlier in the study, the HLFs may have had less competency than later in the study. We do not know why these specific tapes were saved. As part of supervision, the HLFs were required to identify sessions in which they had had difficulties and, therefore, these may be tapes representing lower competencies.
As the data loss occurred while we were preparing our database of recorded sessions, some session details were left incomplete. When comparing the characteristics of the sessions in our original (pre-IT outage) and recovered databases, we found that distributions of session numbers and HLFs were different. In particular, the recovered sessions contained fewer maintenance sessions. The majority were retained by one HLF and another HLF had no sessions recovered at all. Thus, it is likely that this fidelity analysis of the recovered sessions is biased towards an underestimation.
We did not observe any pattern concerning the number of sessions delivered by each HLF and their MI skills. The HLF who also supervised and trained other HLFs had the largest number of sessions recovered.
Furthermore, the fidelity analysis that we conducted was possibly unsuited to the content of the intervention. For instance, although the MITI tool was the optimum coding system for assessing fidelity in the individual sessions, it has not been previously validated for MI delivered in a group format. Thus, the raters faced challenges in using this to code the group sessions. For example, as the structure of the group sessions contained more group exercises and more didactic interactions, it was difficult to detect sufficient utterances that demonstrated empathy. Instead, it may have been better to use a different coding system for MI in the group sessions.
Similarly, the nature of MOVE IT may have prevented the BCTs from being consistently captured by the fidelity raters. For example, six of the BCTs (ii–iv and vii–ix) were intended to be delivered by the HLFs during one-to-one portions of the group sessions when participants completed their action plans, which were probably not captured by the recordings. Furthermore, three of the BCTs (ix–xi) were intended to be delivered in the maintenance sessions only; hence, it is not surprising that these were among the least captured in the fidelity analysis. And this issue is compounded by the fact that fewer maintenance sessions were recovered after the network outage. Thus, we felt that the data loss from the outage barred us from conducting a more robust and detailed analysis of BCT delivery by the HLFs.
Chapter 9 Qualitative findings: the views of participants
Background
Randomised controlled trials provide a reliable and rigorous method of assessing the effectiveness of an intervention. 125 However, owing to their complex nature there are a number of additional challenges that may arise when reproducing the intervention. Therefore, it is important to evaluate the delivery and processes of the intervention from the patient’s perspective. 126
Focus groups give participants the opportunity to provide in-depth reflections of their experiences and views of the intervention. 127,128 Understanding the experiences of participants is vital to provide an insight into potential barriers and improvements that often occur in lifestyle interventions. 129 Therefore, the use of focus groups can enable researchers to review the different behavioural or social processes that may not be evident from the use of quantitative analysis only, leading to the successful implementation of an intervention in the future. 126
A recent systematic review and meta-analysis revealed the effectiveness of structured lifestyle interventions that target both diet and PA as being successful in reducing CVD risk factors. 130 Studies have found that the delivery of MI for health-related behaviour change in lifestyle interventions to be beneficial, particularly in the short term. 49,131,132 However, few have investigated the maintenance of behaviour change in the long term with the use of qualitative methodology.
The aim of this qualitative analysis was to explore the views of participants of MOVE IT in terms of reasons for engagement, factors enabling behaviour change and the potential barriers to engagement.
Methods
Two groups of participants were approached for process evaluation: (1) those who attended the intensive phase of the intervention, that is, six or more sessions (completers), and (2) those who withdrew before the intensive phase was completed or did not attend at all (non-completers). Participants were invited, based on a purposive sampling method to form heterogeneous groups. Only those who had completed all three follow-up appointments were approached.
Participants were separated into six focus groups: four groups of completers (labelled as 1A–1D) and two groups of non-completers (2A and 2B). Each group had two facilitators, who were research assistants from the MOVE IT programme and had not met the patients before, to avoid response bias. For each group, we aimed to include at least one female participant, an individual with an ethnic minority background and a mix of those who attended individual and group sessions.
Focus groups using a semistructured interview schedule were carried out with the use of a topic guide (see Appendix 4). The topic guide was informed by preliminary analysis from the HLF contribution, input from the research team and investigators, and feedback from PPI members. It consisted of open-ended questions relating to (1) the invitation to take part in the study, (2) lifestyle changes made since joining the MOVE IT programme, (3) most and least helpful aspects of the sessions, (4) participant diversity and (5) potential online interventions.
Informed consent was obtained and each group was audiotaped. Data were anonymised and transcribed verbatim. The transcripts were analysed using thematic analysis. 133 The analysis was descriptive and interpretative and facilitated by the use of NVivo version 10 (QSR International, Melbourne, VIC, Australia) to code emerging themes and subthemes.
Results
A total of 74 participants were invited to attend a focus group, with 26 ultimately participating. There were four groups of completers and two groups of non-completers. The characteristics of those who took part are outlined in Appendix 5.
A thematic analysis of all six focus groups resulted in three generic themes. A fourth theme was generated from only the non-completers’ responses, as shown in Table 37.
| Themes | Subthemes |
|---|---|
| 1. Perceived benefits of the study |
|
| 2. Factors enhancing behaviour change |
|
| 3. Perceived risk of CVD |
|
| 4. Potential barriers to change and overcoming these barriers |
|
Theme 1: perceived benefits of the study
Personal behaviour changes
Participants described positive lifestyle changes they had made with regards to their diet and PA during the study:
I found it beneficial. I took on the pedometer stuff, I took on the five a day, I took on the number of glasses of water a day, the oily fish, all of those, you know, don’t just live a sedentary lifestyle of a night, get up and move around, all of those things, were lessons that I took on board . . . they have been beneficial to me.
Participant 9
I am in a habit of doing daily exercises in the morning. Although I do it at home at least half an hour stretching and dancing. So that makes up for the lack of walking. All in all it has made quite a positive impact.
Participant 5
Lifestyle changes were sustained following the completion of the sessions:
They’re in there. They’re programmed in. So on an evening, I try not to sit there plonked in front of the television, I get up and do stuff. Make the tea, go and get some fruit. You know, just keep getting up and doing stuff rather than just have a sedentary evening every evening.
Participant 9
I found I am counting the number of different fruits and veg that I’m having each day . . . and then I was like ‘Ohh . . . five today,’ ‘Uh fine gosh, seven yesterday oh well’. You know, I am still doing it every single day . . . what am I going to eat . . . when am I having it. Oh yeah . . . every day.
Participant 4
Increased health awareness
The main benefits described by the participants are the increased knowledge and health awareness from taking part in the intervention. Primarily, individuals reported that the sessions highlighted the importance of keeping active and making healthy lifestyle choices regarding their food intake:
I think I’ve got much more of an awareness of how . . . Oh perhaps I can leave the car behind for a short journey. Do I need it? It’s that permanent awareness. Do I need to? Are we having enough fruit and veg? Oh gosh. We haven’t had fish for quite a . . . quite a long time. You know . . . Just. It’s there all the time.
Participant 2
But what MOVE IT did for me certainly did highlight that I wasn’t doing it. So it’s made me more aware, and that’s why I ate oily fish before I came out today, not particularly because I was coming to this focus group, but it’s ingrained in my subconscious now that I ought to eat a certain amount. I . . . so just to reinforce . . . what this has done is benefited me in that it’s now in my consciousness. I don’t adhere to it religiously but overall I feel it’s been beneficial.
Participant 9
More specifically, there was an increase of awareness and understanding of the use of the labels on packaging (i.e. the traffic light system):
It wasn’t until I noted how much sugar and how much saturated fat was in food that we eat. So when we buy things we’re more aware of what we’re buying and now and then we’ve got food that’s got a bit of red in it [laughs] on the traffic lights . . . But as long as . . . it’s not really frequently we don’t get too upset about it.
Participant 16
Theme 2: factors enhancing behaviour change
Social support and learning from others
Participants who had attended group sessions rather than individual sessions felt that being part of a group had a positive impact on their ability to engage with the study. The opportunity to discuss their achievements or setbacks and be in a positive social environment was something they appreciated and enjoyed:
It was nice to, have other people there were going through the same experiences if you like. And you would talk about it and bring back the same sort of problems or any success. So it’s nice to have experience with it.
Participant 16
I think it’s quite good really, because you know, you. People in our group you know got on quite well, quite nice to talk to other people. Yeah . . . how you been doing, how many steps they done that week. No . . . I think it’s quite a good social atmosphere.
Participant 13
In addition, those who had individual sessions felt that they would have benefited from having support from others:
Whether it’s an individual session or a group session, maybe a group session more, you have to develop a kind of . . . a group dynamic, that compels people to want to come back, because they’re a part of that group. I think the first three groups, the first three meetings or so, if you can really get people hooked in, they’ll come back because they’re a part of that group, or something like that. More will come back, because they’re kind of part of that group.
Participant 21
Social support had a beneficial effect for people close to the participants. Participants had the opportunity to share their experiences with family members and see positive effects on them too:
Yeah. I suppose so. For me the family as well. I did tell my wife about it . . . and it did have an effect on her. You know, what she ate and stuff.
Participant 13
What was helpful – because of all the things about the diet etcetera, my son went to for blood test and was told he was potentially diabetic, put me in the panic. So I cooked everything for him . . . I did all the cooking and sent it round. And he lost about 3 stone in very short time and then they said he wasn’t gonna be a diabetic at all . . . So it did for him as well as for me . . . because of what I’ve learnt. Yeah. So that was a bonus.
Participant 15
Ease of programme structure and content
An important factor reported to enhance behaviour change was the simplicity of the techniques that participants developed throughout the intervention. For example, setting small goals, awareness-raising and the preparation of setbacks enabled them to achieve sustainable lifestyle changes:
And they told me the procedure was very simple once I started. I found that if I avoid snacking, then my weight goes down. So I lost quite a bit of weight.
Participant 9
I thought personally, the pedometer was a very very good thing and it was so simple.
Participant 3
That training was excellent. You know, everybody has got a sometime or the other or you can have a setback. So, they told us how to keep going, not to stop. So that was a very good training. There will be step backs but those techniques help keep going.
Participant 7
You are now conditioned to be a bit more aware, and that is very important. It doesn’t mean that I do 10,000 (steps) a day, but it is that awareness, that keeps my mind going.
Participant 7
Continuity and strength of therapeutic alliance
To sustain healthy behaviour changes, some participants felt that it was important that there was continuity of sessions. It was reported that participants felt less motivated to continue during the later stages of the programme because the sessions were less frequent:
I found the first six sessions really easy, because it was close together and it was just like we knew that every Thursday you would go. It’s, when they were spread out then I felt like oh! You know, for the last one I really did not want to go. But you know, I said it to the nice lady.
Participant 2
Participants highlighted the importance of the therapeutic relationship built with the HLFs and how this encouraged them to return to the later sessions. During the process, there were changes to the HLFs, which some participants found to be quite disruptive:
I found when they spread out was, so in the first six it was one and the same person and you got almost on the one-to-one thing. So you got this rapport with someone then you hear . . . ‘Hello, she’s left I am taking over’.
Participant 4
‘Well, she’s left, I’ve taken over.’ Well it was complete stranger, there was no rapport, and well they thought, ‘How are you doing?’ And well . . . I don’t know . . . you don’t know . . . And it was a complete stranger and you have no idea.
Participant 4
Maintaining continuity of staff to sustain engagement with participants was highlighted by participant 2:
I was definitely one of the lucky ones ‘cause I had the same lady all the way through . . . so I mean we had usually an hour and a half. You know, ‘cause you know, when you to get to know somebody you talk a lot. I mean, that was very useful.
Participant 2
Theme 3: perceived risk of cardiovascular disease
Participants’ perceptions of their own cardiovascular disease risk
Participants dismissed themselves from being at risk of CVD owing to a lack of family history or an absence of current health problems. Some associated their risk of CVD solely with their age and discredited the contribution of other risk factors (e.g. poor diet and lack of PA):
When I got a letter . . . from the King’s College. I was a bit . . . I was a bit surprised. I don’t have a problem with the heart, so why they did . . . wrote letter like that, join. So I thought it might have some benefit . . . so I joined.
Participant 14
You know . . . go to the doctor and the doctor had never said . . . oh . . . you know . . . you’re a particularly high risk of heart disease. So it comes in the post . . . and it says, you’re in this high-risk group. So . . . you know . . . you do a bit of a double take. I don’t know whether. But anyway . . . you’ve got nothing to lose and do the . . . come on and do the study and find out. But I think, as [participant 12] said, on the things they use to work out the risk factor, as soon as you put in your age and put in you’re 70, you’re immediately in the 70% category.
Participant 13
Perceptions of the cardiovascular disease risk to others
The participants distanced themselves from having a risk of developing CVD and projected this onto others. Some felt that those who were selected were not appropriate for the study as they were not at risk or had no history of being at risk of CVD:
I said the thing is, I’m old anyway, surely it’s people who are in their 30s and 40s to preclude them from maybe getting a risk of or having an inherent risk of heart trouble or whatever. Surely, it’s the people who drive a hundred yards, or a hundred metres to the fish and chip shop, load themselves up – mind you I love fish and chips – every day of the week, and they are the people, surely at risk.
Participant 8
Well, I think all the people in our group. You know . . . they seemed to be reasonably fit and you know. You know . . . you harp on about diet really. You know . . . these things . . . are kind of self-selective aren’t they?
Participant 13
Participant 13 suggests that those who agreed to take part may not actually be the individuals who need help:
So you wonder . . . the people that really . . . I’m not saying we didn’t benefit from it, but you know, there weren’t really obese people there. And I think I . . . so you wonder whether the people . . . the people who would really benefit from it. You know . . . the people who are possibly volunteering, are the ones that need it the least.
Participant 13
Theme 4: potential barriers to change and overcoming these barriers
Lack of feedback
A recurrent subtheme for participants in both the individual intervention arm and the group intervention arm was the lack of feedback received during the study. Particularly, participants felt that feedback seemed to be predominantly negative throughout, without the encouragement of positive results to engage them further with the study.
One suggestion was to provide positive feedback regarding weight loss and participants’ progress as a means of potentially increasing motivation:
The person doing the testing didn’t want us to give feedback about the group. I understand. But we didn’t seem to get any feedback, like ‘Oh, your weight’s gone up’ or you know . . . because that’s a motivation in itself. You know . . . there was never that kind of discussion. Like ‘this has changed, your cholesterol’s . . . whatever . . . or’. ‘Cause the things they test, there didn’t seem to be any feedback. Now, I thought it would have been important to say to people ‘well, this is OK . . . but this is not good’ you know . . . and whatever. ‘Cause that’s a motivation in itself.
Participant 21
So you’d get the negative, we would get it if it was a negative result but if you wanna keep people more motivated they need to know about, the positive stuff as well, because that’s why they’re going. They want to know, they’re getting better. If you’re not gonna tell me you’re getting better . . . well why?
Participant 23
Lack of engagement
A further barrier that was described was the lack of engagement or a relationship being developed on a personal level during the study:
There you well ‘mm . . . you’ve been doing this for a year, it’s been bought to your mind. What are the consequences? What’s changed in your life at all . . . that’s not enabled you to do it’ whatever . . . you know it’s just. I think that relates to my earlier point. I think there is a lack of engagement on a personal level. If you want to motivate people, you need to get people hooked in partly, as an individual as well as the group . . . I think I became this name and this number without any particular relationship being developed.
Participant 21
The role of authority
There were varying suggestions surrounding possible reasons why some individuals might not have decided to take part and changes that may improve this in the future. A recurrent theme was the importance of the role of authority from a health-care professional, such as a GP.
One participant suggested that their involvement may therefore be helpful to encourage those who are more reluctant to take part:
Now . . . people tend to listen to their doctor . . . or respond to the doctor, better than the MOVE IT study from, King’s Univ- well, who the hell are they? You know, you got to . . . there’s no engagement there. Whereas, if the doctor sends the letter, or a covering letter . . . then . . . as a as an intro, that’s a more powerful intro than just a letter from you know because clearly . . . well . . . if a doctor said, we are collaborating or co-operating whatever word you choose, with this study from ah, and from your records it would appear that you are at risk of . . . whatever. Perhaps you would be, you’d, perhaps it would be ah, they could suggest that the study might be beneficial to you. Now, now that would be, that would be a fairly powerful introduction I think.
Participant 21
This was also highlighted by the completers, who mentioned that their GPs were unaware of their risk:
. . . and I thought . . . oh . . . all right . . . I’ll go along with it. Then when I next saw my doctor, I said, like you know, what’s this about high risk of heart attack and he looked at me vacantly and he says ‘I dunno’. He said probably your age. He said, that’s all I can think of.
Participant 12
Summary
This qualitative analysis explored the views of participants allocated to the intervention arms of MOVE IT. The main themes that emerged were (1) perceived benefits of the study, (2) factors enhancing behaviour change and (3) perceived risk of CVD. One further theme that emerged solely for the non-completers was (4) potential barriers to change and overcoming these barriers.
We found that participants described having positive experiences during the intervention sessions, citing the benefits of increased health awareness, positive lifestyle changes and the opportunity to learn from others. We identified several factors that might have affected the maintenance of participant behaviour change. Primarily, participants noted that the continuity of sessions and having the staff involved throughout were very important. The changes to the HLFs who carried out the sessions were disruptive for some and led to reduced engagement with the intervention. The continuity and frequency of sessions also had an impact on participants’ motivation to take part and make healthy lifestyle changes. Owing to the pragmatic design, duration of the intervention and short-term contracts of the HLFs, there was a natural staff turnover that could not be prevented, except with a vast increase in funding to potentially hire more HLFs or offer longer contracts.
Non-completers had expressed that there was a lack of feedback and engagement throughout the intervention. To improve this, it would be beneficial to provide more positive feedback from HLFs or to have an alternative method of self-reflection during the sessions. This would increase motivation for patients to continue participation and for behaviour change to be sustained. Participants’ perceptions of their own CVD risk may be viewed as a barrier to engagement.
Most participants did not believe CVD risk to be an issue and often attributed the risk to their age rather than their own lifestyle choices. In addition, some GPs were not aware of their patients’ risks or the study itself, which may have altered individuals’ perceptions of CVD risks. Therefore, for future interventions, it is important to work more closely with GPs to emphasise the benefits for patients’ health and potential to ameliorate CVD risk. There may be other reasons why the non-completers felt a lack of engagement, as they may have higher levels of psychological distress or social problems that were not articulated during the focus group.
MOVE IT emphasised the importance of the use of qualitative methodology in providing detailed experiences of participants for assessing the effectiveness of a RCT. As previously reported, studies have supported the benefits of lifestyle interventions in the short term but few have supported them in the long term. 49,131,132
The detailed reflections from participants involved in a long-term intervention support the use of qualitative methods for gaining greater understanding of the factors facilitating engagement and barriers to engagement. The responses from participants highlight the benefits of lifestyle interventions and the maintenance of behaviour changes for diet and PA levels. Finally, this study reveals areas of improvement for the future delivery and implementation of the study, including continuity of care and duration of the intervention. Although attendance at group sessions was lower than attendance at individual sessions, those who attended group sessions appeared to value the peer support aspect.
Chapter 10 Qualitative findings: the views of healthy lifestyle facilitators and clinical psychologists
Background
The HLF team were trained by a CP to deliver MOVE IT. Training in MI involves achieving a number of core competencies,134 and HLFs were also trained to incorporate BCTs into intervention delivery. 52 There is no fixed length for the training of MI; however, a review of 28 studies found that most training schedules required 9–16 hours of training. 135 The training methods for MI involve didactic supervision and learning activities, such as role play, group exercises, discussions and feedback. 42
The application of MI is considered helpful in working with patients towards lifestyle changes in primary care by nurses who incorporate it into their practice. 136,137 Previous studies of the application of MI in practice have suggested that it can be demanding but rewarding and that the spirit of MI and simple competencies are frequently achieved, but that the application of MI skills reduces over time and further support is required for more complicated competencies. 136,138 The ability to reflect on practice, continuing to develop skills and ongoing supervision are thought to be crucial to enhancing the long-term benefits of a MI approach. 139 There is evidence that enhancing MI with CBT techniques is achievable for nurses supporting diabetes mellitus control140 or alcohol cessation,62 and that intervention providers perceive both positive and negative impacts of the intervention on patient care depending on the patient’s ability to engage. 141 Evaluating any novel intervention, from the perspective of stakeholders such as the intervention providers, is important for translation to policy. 142
The views and experiences of intervention providers are vital to assessing the feasibility of widespread implementation into practice, by exploring the challenges in training and delivery. The HLF role was newly developed for the MOVE IT trial, drawing on the promising preliminary findings of the NHS health trainer programme, and adapted to be in line with MI, which underpins the intervention. Therefore, as the intervention and job role are both novel, we sought to gain detailed feedback on HLF training and intervention delivery from both the CPs who were responsible for training and supervision and the HLF team.
Methods
A qualitative approach was adopted to study HLFs’ and CPs’ views and experiences of developing and delivering the interventions. All 13 HLFs and two CPs were invited to interviews. Semistructured interviews were carried out, consisting of open-ended questions relating to the training, supervision, intervention, participants and values in psychological therapies. The topic guides used for HLFs and CPs are provided in Appendix 6. Interviewees were invited to give their feedback and the discussion was audiotaped. To gather meaning from the data, inductive thematic analysis explored any underlying concepts that were mentioned regularly.
The interviews were conducted by an independent research assistant to avoid response bias, which could exist if the interviewer was part of the MOVE IT team. Audiotapes were transcribed verbatim and the data collected were anonymised. NVivo software was used to analyse the data. Emerging themes from the interview transcripts were coded and categorised into themes and subthemes.
Results
A total of nine female health-care professionals (seven HLFs and the two CPs) were interviewed. All HLFs except one were working on the MOVE IT trial at the time of their interviews. The HLFs who no longer worked on the trial were unavailable to participate. The characteristics of those interviewed are given in Table 38.
| Identifier | Number of months in position at time of interview | In post at time of interview? |
|---|---|---|
| HLF1 | 23 | Yes |
| HLF2 | 9 | No |
| HLF3 | 18 | Yes |
| HLF4 | 8 | Yes |
| HLF5 | 8 | Yes |
| HLF6 | 8 | Yes |
| HLF7 | 23 | Yes |
| CP1 | 10 | No |
| CP2 | 12 | Yes |
A thematic analysis resulted in three generic themes:
-
challenges and suggested improvement for the training
-
supervision issues and peer support
-
challenges with delivering the intervention.
Theme 1: challenges and suggested improvement for the training
The healthy lifestyle facilitator perspective
All HLFs were required to attend training during a 6-week period, with most agreeing that all subjects were adequately covered in this time. The HLFs appreciated the MI and CBT skills and the knowledge of how to apply these skills gained in the sessions. Although the information was thorough and prepared the HLFs for various situations that could occur during their sessions, the HLFs identified the 6-week training period as intensive and time consuming:
I think towards the end people felt like ‘OK I am ready to go now’ so it could have been maybe a little bit shorter.
HLF4
We, I say we because we have actually discussed this already, would have preferred it if it would have actually been pushed out quicker so that we experience it rather than just continuing to do scenarios which we kept doing every day.
HLF6
At the beginning was kind of a lot of practising, it was a bit too much and it became mechanic because we did it with each other so much.
HLF7
There was particular reference to the role-playing exercises during the training, as reflected by comments such as:
. . . since we were role playing between ourselves we were taking it easy on each other.
HLF6
So we had training for 2 months, September and October. It was intense and it got quite repetitive and in my opinion it got quite tedious to the point where we started overthinking everything we did.
HLF7
The HLFs reported that sessions varied considerably in terms of the participant and their motivations to participate in the MOVE IT trial. This differed from the preparation they had undertaken in the training period:
The patients that we had were totally opposite of what we were trained to do and that’s what threw everyone off. Everyone was trained to talk a certain way, got to their patients but then everyone was the total opposite.
HLF2
The clinical psychologist perspective
Both CPs were interviewed regarding their views on the training that had been developed by CP1. CP1 trained and handed over responsibilities to CP2 over an 8-week period. Within the first year, five out of the eight HLFs that CP2 had trained had left the study, leaving three HLFs to split the workload. When CP2 was employed, three new HLFs joined the study, bringing the total number of HLFs to six. The curriculum was adapted as the training was designed for larger groups. Despite this, CP2 felt that the HLFs managed the situation very well despite the intensity of the curriculum and amount of material covered:
I think it was kind of split into sort of face-to-face interactive sessions . . . They were given resources to go and self-study and some ideas of what to practice and how to practice the sessions . . . None of them really had previous experience of actually delivering psychology-based interventions . . . I suppose the tricky thing with something like MI, which I suppose is the key approach that is underpinning everything in MOVE IT, is that actually there is only so much you can teach directly because a lot of it is actually through practice and getting things wrong and reflecting on it . . . I guess that was also another challenge, just to know which were the crucial elements to kind of teach and how to get those across in a simplified and manageable way . . . It is quite a challenge because the concept of MI is still quite undefined in some ways as an intervention.
CP2
They came more from a health promotion background so their thing is being much more directive, telling people what to do, getting information, giving education, so they love all that . . . what’s difficult is to try to pick them up into it and to try not to destroy their confidence too much so that they do not want to come back to training . . . what we can teach people and what we can actually take on board in my experience is quite different, so you can probably cut it [the curriculum] down by half and they might get some of that. Maybe the content, maybe it’s more about doing less, better.
CP1
Along with MI and CBT skills and the process of being assessed, requiring knowledge of CVD and its risk factors contributed to the length of the training, as CP1 explains:
. . . we had been working on another trial which was training diabetes nurses also in MI and some CBT techniques, so we were looking for a length of time which would allow them enough time to understand the theory, enough time to get to know each other and role play with each other, and understand a whole new curriculum and how to deliver it, plus everyone needed to be assessed which is a really lengthy process . . . I think the additional thing is that it is also the third strand is having to provide education about CVD.
CP1
Theme 2: supervision issues and peer support
Issues with supervision
A recurrent theme regarded the supervision received and lack of support provided, as seen in the following excerpts:
If we had a problem it was hard to know who we should go to and sort it out quickly because they tended to palm it off on each other quite a lot, not to be disrespectful or anything.
HLF1
But in terms of managerial supervision, we had that, but it was kind of formalities as opposed to kind of a necessity, I don’t think they wanted to be there and we didn’t really want to be there either and I think towards the end there was some tension between the HLFs and the supervisor.
HLF2
The project manager didn’t really come to our supervision that much, I thought they were going to be there more to like help out with any other problems. I suppose the project manager could have been a bit more hands on, in supervision I mean.
HLF3
In addition, the HLFs felt that there were positive and negative consequences of some of them being at different stages of their sessions. On the one hand, a HLF further along in the training provided useful information to listen to and learn from. However, for those HLFs who were ahead, they felt that this was time consuming and that the time could be used to complete other tasks:
After the training, which was an intense period, you go out so it was good to constantly have that reminder of techniques and things, and then also that other girls are at different stages in their delivery so you can kind of hear about what’s upcoming if someone is a session or two ahead of you . . . I think timing was probably a thing with supervision because they do seem to drag and it seemed like talking about the same thing a lot.
HLF4
Peer support
In relation to the lack of support experienced from management, the HLFs recognised the importance of supporting each other through difficult situations:
We, I say we because we have actually spoken about it. It’s very useful in the sense that you get to hear what other HLFs are experiencing as well, so you might pick up on something someone else brings up and because we are talking about it in the supervision you get to help each other. Therefore, if that kind of situation presents itself again you know what to do.
HLF6
If there are similar problems and successes then it’s nice to hear it but we talk about them anyway. And it’s not nice to brag about it if someone else has an issue so I tend not to say too much, I didn’t really participate too much.
HLF2
Once you are away from that sort of life it can be hard to come back to it, but they all encouraged me and the clinical psychologist is really very supportive . . . They are so supportive. At the beginning I was really slow with the IT and that sort of thing. Each other they helped with something, all of them. So they helped me a lot.
HLF5
We were a tight family; there were eight of us and all around similar ages and personalities. We got on so well that we were like one big team and family, we supported each other more than anyone else did in the study.
HLF2
Healthy lifestyle facilitators 4 and 5 also expressed a lack of guidance for the administrative work that was required. However, the situation improved after the MOVE IT administrator assisted in liaising with booking rooms for sessions, scheduling sessions with participants and contacting potential volunteer group facilitators:
. . . I know probably everyone is saying it, but I feel like I wasted a lot of time trying to sort that out [administrative work] when I could have actually been doing what I am supposed to be doing.
HLF4
The administrator is helping us quite a lot and that way is fine. Even the last group invitations the administrator helped send it and is looking for volunteers, there is help for us from there and we are lucky in that aspect.
HLF5
One HLF was promoted to a senior position and organised meetings to discuss practicalities and problem-solve non-clinical issues that arose. The senior HLF (HLF1) could then mediate between the group and the CPs:
I think that’s really helped [promoting HLF] because it means that supervision with me is a bit more protective for perhaps other more therapeutic issues or issues with the curriculum itself.
CP2
Reflective logs
It was noted that the communication and structure of fortnightly supervision meetings improved after a senior HLF was appointed. Both CP1 and CP2 highlighted the benefits of the weekly reflective log that each HLF completed:
Sometimes when we would start, maybe because it’s a little anxiety-provoking as well, they don’t necessarily have issues that they would come out with then the way around that is that they have these reflective practice logs . . . They fill in these weekly practice logs and then supervision is every fortnight so often I would make a few notes of things that they brought up in their practice logs and then we would talk about it in more depth.
CP2
The supervision was in two parts, one was a face-to-face [meeting] every fortnight and the other was weekly e-mail exchange where they would fill in a reflective practice log about what had gone well and less well and what we needed to focus on for the following week. I would read everyone’s entry and reply by e-mail, so they would get that support as well which worked pretty well because everyone could read everyone else’s logs so they were all learning from each other continuously. Everyone had to log at the end of the week.
CP1
The CPs felt that adequate support was provided, consisting of face-to-face supervision and reflective practice logs. CP1 provided an example of the importance of a weekly log when a HLF was unable to attend face-to-face supervision:
. . . there was one person who had a string of supervision that clashed with her running groups, that was kind of bad because I felt like she really needed to come to supervision but again you have this comfort with getting numbers through for the trial so we couldn’t have her abandon the group, but thankfully she was active with the logs.
CP1
Theme 3: challenges with delivering the intervention
Challenges with patients
All HLFs mentioned enjoying interactions with patients and building a bond with them; they saw the patients benefiting from the interventions and received positive feedback:
What I love is when they come back with the results, when they say they used to be size 20 and now they are size 18, that’s really nice. I had someone who came off one of their medications, so they were on medication before to control their blood pressure and cholesterol but now they are not anymore because they have managed their diet and changed their lifestyle.
HLF6
However, challenges with delivering the intervention emerged as a common theme. Resistant patients disputing any advice proved challenging for the HLFs, as did those who did not engage:
. . . one person walked out of the session as soon as we started with introductions the person just said ‘I’m out I’m out!’ and that scared me.
HLF7
Two of the HLFs mentioned the age of the participants as a barrier to delivering the intervention:
The participants are of an age range that aren’t used to ‘collaborative’ care and often just want to be told/prescribed advice. This can make session delivery difficult as participants get frustrated at not being given a straight answer.
HLF4
The patients that we had were totally opposite of what we were trained to do and that’s what threw everyone off. Everyone was trained to talk a certain way, got to their patients but then everyone was the total opposite.
HLF2
Another challenge that the majority of the HLFs encountered was that participants often thought that the HLFs were medical health experts; CP1 also noted this as a potential issue when delivering the intervention:
There are other issues that they has like their health problems, we are only trained to do the intervention but if they come to you with other problems you are not skilled in it you don’t really know what to do . . . I had to keep telling them that I am not in the medical profession.
HLF2
If you say that five a day is good for you then he would immediately disagree and ask where I researched that from and how do I know if the fruit is actually good for you and not full of chemicals.
HLF6
I think other things that HLFs found challenging, especially with an older population with realistic medical problems was when participants would say that they couldn’t exercise due to back pains, angina or other various reasons. Then the HLFs are a bit stumped because they weren’t sure how to respond to that because they think that it could be dangerous for them to make the participants do X, Y.
CP1
The HLFs also mentioned that the initial information sheet sent out to the participating patients was not sufficient to prepare them for what the intervention would involve. HLF7 felt that a lot of patients would be less reluctant to participate in the trial if they knew what each session would include:
I feel like people aren’t aware of what they are signing up to at the researcher stage, so many people are not sure what we are talking about and I just wonder what they are told in the beginning. I wonder if it’s been made clear that once they’ve done this then they are going to be given something, some people are really hot on it and some have no clue what you’re talking about . . . That’s one big thing that I feel that people aren’t aware of what they are signing up to.
HLF4
From my perspective people do not want to join the group because some expected to be weighed, or do some physical activity because of our name like healthy lifestyle facilitators – facilitate what, is it exercise? The name is good for using and the letter didn’t explain anything. I would expect less dropout if you explain yourself better in the letter, because people become less nervous.
HLF7
Issues with the activity booklet
The lengths of the sessions (group and individual) were praised by the HLFs as there was adequate time to cover the material and manage digressive conversations. A common issue referred to the provided activity booklet being inappropriate for the age group. Participants had found the activity booklet childish or patronising:
It’s probably the actual booklet; some people like it and some people think it’s referred to a sort of working class. Some of the examples and case studies in there don’t particularly relate to them . . . I guess they’ve had to write it for it to cater to different sort of educations, so some people are obviously very well educated and it’s pretty much very basic. Some people find that a little offensive almost so that builds up some resistance and then you are battling against that almost, so myself and loads of the other girls have found that kind of tricky in terms of sort of putting the workbook a bit to the side and chatting with them about things . . . There are bits in the actual goal-setting that people don’t particularly like, which makes us feel like you almost don’t want to mention the question and just want to put your hand over it and pretend it isn’t there.
HLF1
Aspects of the actions plan such as rewards and reminders can be difficult to have this ‘older’ generation understand. Often they will say ‘why do I need a reward/reminder, if I’m going to do it . . . I’ll do it’.
HLF4
People often say that this is not us but then I refer them and couple of individuals have mentioned that this is quite patronising, the curriculum examples could be better and more age-fitting because the patients do not relate.
HLF5
CP1 observed the struggles that the HLFs had with the activity booklet:
I think the HLFs found that difficult because they were trying to do a manualised structured worksheet that they must fill in and the participants were just not on board, and they struggled with that.
CP1
Despite the issues with the activity booklet, CP2 highlighted the competence of the HLFs in guiding the participants towards the booklet during an observation of a session:
They are also very good at, I suppose this is where their comfort zone is, giving the information in a very clear way and making use of the resources in the workbook and pointing and steering people towards those.
CP2
Summary
This qualitative analysis explored the views of HLFs and CPs regarding the process of implementing MOVE IT. Of all 13 HLFs invited to interviews, we gained feedback from seven; we also gained feedback from both of the CPs who trained and supervised the HLF team. The main themes that emerged were (1) challenges faced during training and suggested improvements, (2) issues with supervision and peer support and (3) difficulties in delivering the intervention.
The HLFs enjoyed learning how to implement MOVE IT in addition to working with the patients. The training was described as time consuming and arduous, and HLFs felt that it could have been completed in a shorter time allowing more time for hands-on learning. Although there was a lot of information to cover, none of the HLFs mentioned a lack of confidence in the delivery of the intervention. With regards to the relationship with their supervisors, many of the HLFs felt that the fortnightly sessions were not utilised efficiently or that they could become repetitive.
The HLFs developed a strong support network between one another, and the relationship with their superiors improved once a HLF was promoted and could mediate between them, making better use of their time. It was noted that the activity booklets were age-inappropriate for the participants involved, which became a barrier during some sessions. In addition, some participants assumed that the HLFs were medical professionals who were able to discuss other health complaints and HLFs would have to reiterate that they were not medically trained while ensuring that the participants did not lose confidence in the help that they could provide. Both CPs noted the proficiency of the HLFs in working around these difficulties.
Enhanced MI was reported as an enriching and effective approach. The training undertaken by the HLFs in this study could have been adapted to allow the HLFs to learn via experience as opposed to continual rehearsal. This analysis also demonstrated the importance of effective and useful communication and support between HLFs and those managing them. It is therefore important to consider a practical and ongoing support structure. Furthermore, it may be useful to adapt the tools used in such an intervention to the age of the target group to remove potential barriers to engagement.
Chapter 11 Discussion
Summary of the clinical effectiveness of MOVE IT
This three-arm parallel RCT study tested the effectiveness of an enhanced MI intervention, delivered by specially trained health trainers (HLFs), in a group versus individual format, versus UC for reducing weight and increasing PA in adults at high risk (≥ 20.0%) of developing CVD in the next 10 years.
For the primary objective, the main findings were that there were no significant changes in weight or PA 24 months later between group enhanced MI or individual enhanced MI compared with UC.
For the secondary objectives, we found that MOVE IT delivered in a group format was not more effective than when delivered in the individual format in reducing weight and increasing PA or in reducing LDL cholesterol or CVD risk score 24 months later. There were no differences in the number of fatal or non-fatal cardiovascular events and other recorded AEs between the three treatment arms.
The health economic results revealed that there was little difference in terms of service use and costs between the three arms other than that resulting from the interventions themselves. Total service costs over the follow-up period were highest for the individual intervention arm, followed by the group intervention arm, followed by the UC arm. Differences were not statistically significant. QALYs were very similar for each arm. The group intervention was dominated by UC in that the latter had lower costs and produced more QALYs. The individual intervention did produce more QALYs than UC but the ICER indicated a cost per QALY far in excess of the threshold commonly used by NICE. For this reason, neither form of the intervention was cost-effective. There was much uncertainty around the cost and outcome differences but the conclusions of a lack of cost-effectiveness hold.
The findings of the process evaluation were as follows.
-
Mediators: we found that dietary changes did not mediate any treatment effects on the primary outcomes. We did not conduct any further mediational analyses, as there was no change in the primary or secondary outcomes.
-
Participation bias: we found that there was significant evidence of reduced reach in that those with a higher CVD risk, higher level of deprivation status and of African-Caribbean ethnicity were less likely to reply to invitations to participate from their general practice.
-
Fidelity analysis: there were significant methodological limitations of conducting a fidelity analysis but, based on the much-reduced highly selected sample, there was evidence that nearly all of the HLFs had sufficient competencies in at least one MI skillset.
-
Patient experience: the main themes that emerged were (1) perceived benefits of the study (benefits of increased health awareness, positive lifestyle changes and the opportunity to learn from others), (2) factors enhancing behaviour change (continuity of sessions over a longer period and having continuity of the same HLF) and (3) perceived risk of CVD (this was lower than was expected). One further theme that emerged for the non-completers only was (4) potential barriers to change and overcoming these barriers, such as lack of feedback and lack of engagement by their GPs.
-
Therapist experience: the overall view was that the formal training period could have been shortened, more exposure to training cases could have been provided and the HLFs had not been prepared for the real-world challenges once in the clinical setting. They perceived themselves as competent in the MI approach and BCTs. They observed the importance of working with patients towards their goals but there were some common challenges, such as patients not engaging and some of the intervention materials not being deemed age-appropriate. The HLFs felt that support from supervisors, and administrative support, was insufficient but that they could problem-solve by supporting each other.
Strengths and limitations
This RCT was powered to detect small effect sizes and, therefore, we can be confident that our (negative) findings are statistically valid. We used accelerometers as the gold standard for measuring PA. However, there may still have been a Hawthorne effect because the participants were aware of equipment and were not blind to allocation as this was a talking therapy. If there was a Hawthorne effect of wearing the accelerometer then it was negligible as the overall effect of the intervention was minimal on PA and weight loss. We had greater attrition for complete PA follow-up data. Previous research has set a standard for the amount of accelerometer wear time required per wear-day owing to variability between days of the week. 143 We found that those not providing sufficient data at follow-up were less active and more likely to smoke, had no formal educational qualifications and had more depressive symptoms. However, in the Health Survey for England, participants who provided sufficient accelerometer wear time did not differ from those who did not wear their accelerometer for the requested time. 144 As participants in our recruited sample were older than the target population, the use of fewer days of accelerometer wear data may be acceptable, as previous work has detailed that 3 consecutive wear-days accurately predicts PA levels in older adults. 145 Analysis of sedentary behaviour can require more wear-days, but this was not a main outcome of our study.
We recruited from all 12 CCGs covering the South London region, which has > 3 million residents representing a diverse socioeconomic and ethnically diverse community, including some inner-city London boroughs with high rates of CVD mortality. This was a positive aspect of the study. On the other hand, we did not include residents from the rest of the UK where there are high rates of CVD mortality, such as the north of England, south Wales and central Scotland, and therefore this sample may not have been representative of all people at high CVD risk. 146 Another limitation is that the South London sample may not be representative of the rest of the UK (i.e. owing to the health inequalities between the north and south of the UK). In addition, those London CCGs with more deprivation were slower to respond to the invitation and therefore when we were already halfway through recruitment and by the time we had completed recruitment, less time had been spent recruiting from these CCGs. This may also have led to under-recruitment of those most at risk, namely from deprived areas and non-white ethnic groups.
A pragmatic feature and strength of this study included using the GP QRISK2 tool, which is routinely available on the electronic records software for calculating CVD risk. Although this was a strength because it is an objective measure of risk, which could potentially be easily used by GPs, the limitation was that it had a high false-positive rate, generating a very high denominator number of patients, all of whom were invited for consent for screening for eligibility. The accuracy, completeness and age of data required for the QRISK2 algorithm based on the data stored in medical records were variable. This resulted in inviting and consenting many patients who turned out to have lower and therefore ineligible QRISK2 scores when screened. We had to assess an additional 1332 patients who turned out to be ineligible to participate, which was costly and time consuming. However, there were few other options. The NHS Health Check programme was not appropriate as it achieved a lower-than-expected uptake across England. 12
A limitation of the study was that there were delays in recruitment that had repercussions in that the intervention delivery was delayed for some participants. The unexpected inefficiency in using the NHS Health Check led to delaying recruitment. Switching recruitment strategy to using the QRISK2 tool introduced another delay, which had repercussions for the delivery of the intervention. This is because the switch from Health Checks to QRISK2 required a protocol change, ethics approval and application of a cost extension from NIHR. The delays in recruitment and uncertainty of funding extension approval resulted in most of the HLFs seeking jobs elsewhere as their contracts started coming to an end. At around the same time, the co-applicant’s clinical health psychologist also resigned. We had to employ and complete the induction of a second clinical health psychologist and train a new cohort of HLFs. This meant that when recruitment returned once additional funding and ethics approval had been secured there was a waiting list for delivery of the intervention. This may have contributed to some of the dropout from the intervention.
A strength was that our intervention was embedded and delivered in the heart of the community to match the real-world setting and we were able to be flexible with timing of the sessions (including late afternoons and early evenings). Our intervention focused on supporting maintenance once changes had been made in the initial phases. However, a limitation was that we could not offer sessions immediately and there was a waiting list for patients while HLFs were trained to competency. Approximately 50% of participants randomised to the individual and group interventions dropped out of the intervention after the introductory session. As most of our participants were retired, providing flexibility in the timing of sessions around work schedules was less important. We observed that participants in both the group and individual intervention arms generally adhered well to the intervention, based on the HLFs asking participants to set targets at each session and record if these were achieved or not.
We developed a standardised health trainer manual that could be readily replicated and used for training and supervision. A limitation was that the HLFs preferred practical training rather than didactic learning and classroom-based role play. We would have preferred to have the HLFs undertake more practical training with patients and this might have improved competencies, but this was impossible in terms of resources and the inherent nature of delivering a complex intervention as a research study. The HLFs were employed as NHS staff on short-term contracts; thus, we would have had to set up a CVD risk clinic very quickly, screen patients and then offer the intervention. As this was a 12-month intervention, this was not feasible.
We conducted a comprehensive process evaluation to understand the underlying mechanisms of action. We ensured a long follow-up to capture both short-term and long-term, or maintenance, outcomes. As a result of the process evaluation, we found that we did not reach those at the highest risk of CVD, participants who did not complete the intervention expressed lack of feedback and engagement as a reason, and participants attributed their high CVD risk to their age rather than their own lifestyle choices. Although we did observe several participation biases (see Chapter 7), it is unclear if more specific targeting of the intervention would have had a clinical effect, given that the absolute differences observed were small and a large sample was recruited. In addition, participants noted a lack of engagement from their GP concerning their high CVD risk, which may have negatively influenced any treatment effects. However, given the large number of GPs recruited to the study, it would not have been feasible to incorporate a larger amount of GP engagement in the study design.
Our PPI group was interactive and involved throughout. There are a number of areas in which the methodology of the trial may be improved. For instance, the importance of the invitation process and materials were highlighted by both PPI members and the intervention providers, and appropriate targeting of those who may benefit most from participation in the trial may improve the reach of the study.
There were limitations in the cost-effectiveness analyses. We relied on self-report of service use information and this may have led to recall inaccuracy. This was largely unavoidable given the range of services we wished to include in the measure of cost. Other studies have found this approach to be reasonable and there is no reason to suppose that any inaccuracy biased the findings. 147,148 A further limitation was that the EQ-5D-3L may not have been sensitive to change in this patient group. It is the recommended measure for such analyses but further work on assessing its appropriateness is warranted. The lack of differences in QALYs does support the main clinical findings of the study. Finally, we had hoped to include lost employment costs in a societal perspective; however, data on this were not available.
Interpretation
There are several possible explanations for the results of this trial: (1) although the clinical population was at high risk of CVD, the risk factors were not modifiable by lifestyle interventions, or (2) the psychological theories were not the most appropriate and/or psychotherapeutic approaches are not sufficiently potent alone.
One explanation for the negative findings is that we may not have recruited those with high CVD risk who had modifiable risk factors. The use of a QRISK2 score of ≥ 20.0% ensured that our study recruited those at the highest risk of CVD in accordance with an objective risk algorithm that takes into account numerous modifiable risk factors. However, the risk factors with the greatest weighting in the algorithm are the non-modifiable risk factors: age, sex and ethnicity. 20 It has previously been shown that risk calculators, including the QRISK2, may not have the specificity to identify CVD risk at the individual, as opposed to the population, level. 149 Those patients of older age, male sex and South Asian ethnicity are at the highest risk, but these individuals may be otherwise healthy in terms of their modifiable risk factors that are inputted into the algorithm, and therefore the aims and contents of the intervention may not be applicable to them. The use of a QRISK2 score of ≥ 20.0% may have meant that the recruitment strategy led to a sample skewed against those with high BMIs, blood pressure and LDL cholesterol levels (exactly those participants who were most likely to benefit as these risk factors are modifiable).
Indeed, we found that older people with a normal or underweight BMI were recruited, raising challenges for the intervention team considering the primary aim of a reduction in weight. Previous research has demonstrated that participants at the highest risk benefit most from preventative interventions. In Gidlow et al. ,69 an observational study based in primary care in Stoke-on-Trent, followed three groups of patients: those with at least one modifiable CVD risk factor using the Framingham risk engine score, those with diabetes mellitus or coronary heart disease (secondary prevention) and those who were obese and had a CVD risk of > 15%. They received a patient-tailored, health-trainer-delivered, lifestyle intervention over 6–12 months with face-to-face and telephone/SMS (short message service) support. They observed modest improvements in risk factors and health-related quality of life, but not in CVD risk score, except in the subsample of patients who were at highest risk (> 20%) at baseline. In the study by Hardcastle et al. ,49 the investigators focused on CVD risk factors, such as excess weight (BMI of ≥ 28 kg/m2, based on a value used in the recruiting general practice), hypertension (systolic/diastolic blood pressure of ≥ 150/90 mmHg) or hypercholesterolemia (≥ 5.2 mmol/l), rather than CVD risk score. They observed that the MI intervention was particularly effective for patients with elevated numbers of CVD risk factors at baseline.
The participants in this trial had average step counts at baseline that were in line with that previously reported for healthy older adults who were participating in exercise classes. 150 Therefore, on average, the participants recruited to MOVE IT may at baseline have been at their ceiling in terms of PA achieved, leaving little room for improvement. Previous studies of PA interventions for older adults, using accelerometer measurement as an outcome measure, tend to report significant increases up to a 12-month follow-up but there have been few psychological interventions to help maintain the increased PA. 151 Another explanation for the negative findings is that patients in our sample were not obese. The association between increasing PA and decreasing HbA1c level following behavioural intervention is stronger in those who are obese (BMI of ≥ 30 kg/m2),152 and it may be that our sample was not at a high enough risk (the mean BMI was 28.3 kg/m2) to see significance in outcomes.
The objective accelerometer measurement of PA provides a more reliable measure than self-report as it is less prone to bias. However, the days for which the PA measure is collected may not represent normal PA levels of participants; they may, for example, be elevated above the usual level owing to social desirability. 153 A review of the impact of interventions combining BCTs that target diet and PA found no differences in PA outcomes with UC when self-report PA outcome measures were used. 154 These studies did find a significant impact on BMI, suggesting that beneficial effects of the intervention had taken place and that perhaps a change in PA levels could not be successfully captured by the self-report measure or at the long-term follow-up. It may be that participants in our study made some initial increases in PA levels that were not captured at the 12- and 24-month follow-ups through accelerometry or at baseline; in addition, outcome PA measures of all participants may have been artificially raised. To further investigate the impact of interventions on PA, studies would need to undertake continuous objective measurement throughout participation, as opposed to the norm of 4–7 consecutive days’ wear. 143 Low-cost and unobtrusive means to monitor PA throughout participation in a RCT, such as wearable and smartphone technology, provide this opportunity, although the reliability and validity of these methods are yet to be assessed. 155,156
A second possible explanation for the negative findings is that the intervention potency or overall dose was suboptimal. The psychological skills were based on sound well-established psychological theories: (1) the theory of planned behaviour for initiation of behaviour change, which states that in order to change behaviour, people need to form an intention,53 (2) principles underpinning MI that emphasise that the patient’s motivation to change can be used as a therapeutic tool,41 (3) CBT91 and (4) social cognitive theory,92 which emphasises the importance of peer support in shaping behaviour, which was applied to the group intervention arm of MOVE IT.
We used well-established and evidence-based psychotherapeutic techniques to apply the theoretical framework for MOVE IT. MI was used to support participants in forming healthy intentions. MI is a collaborative conversation style for strengthening a person’s own motivation, belief and commitment to change. We used principles from CBT to support the transition from intention to action and from action to maintenance93 by identifying and challenging unhelpful thoughts or thinking styles and promoting more positive emotions and behaviours. Using an iterative process, we devised the intervention curriculum and a participant workbook to support training, supervision, collaboration and replication. We had an intensive phase and the maintenance phase consisted of four sessions delivered at 3, 6, 9 and 12 months from intervention commencement. Those randomised to the group intervention arm were encouraged to use peer learning and the peer-support environment to facilitate change during both the intensive and maintenance phases. Novel methods and teaching aids were used to supplement the delivery of BCTs, such as visual aids (food labels)/cue cards, exercise demonstrations, video/audio material of patient testimonials, activity-based learning around meal planning and SMS/e-mail reminders. Cultural and religious awareness is built in to the intervention.
We employed and trained health trainers with an intensive package of didactic learning, role playing and feedback, group exercises, reading and case study discussion over 3 months. Competency in administering the intervention was assessed through a knowledge quiz and delivering several sessions to volunteers. 94–96 For HLFs that were not competent, they underwent further training until competent. HLF competency was monitored throughout the intervention by the CP regular supervision and quality assurance by reviewing audiotaped sessions.
This process is a standard approach to developing and evaluating a set of psychotherapeutic skills. It may be that for this clinical setting this approach is, or components of this approach are, insufficient. The theoretical frameworks were originally developed for mental health conditions that can remit, such as alcohol dependence, anxiety and depressive disorders. It would seem that a clinical state such as risk of CVD as a construct that could remit if one could develop and change one’s thinking patterns about lifestyles has face validity for applying similar psychotherapeutic techniques. However, perhaps a psychological model is not appropriate and intensive instruction is more apt.
Landmark studies have repeatedly shown that intensive lifestyle instruction that focuses primarily on behaviour change, such as the diabetes prevention studies,157–159 weight reduction programmes160 and, recently, studies on reversal of type 2 diabetes mellitus,161 does lead to significantly improved outcomes. Even the simple behavioural act of prescribing a pedometer leads to improved PA. 34 In these interventions, the clinically active ingredients were access to intensive, highly-structured and prescribed dietary and/or PA instruction following a counselling approach with minimal psychotherapeutic techniques. Our approach was to use talking therapies to instigate behaviour change rather than instruction, with the philosophy that intentions need to change before behaviours do and that changing people’s way of thinking (as opposed to instructing them) would also be cheaper in the long term and more likely to be maintained. It could be that with lifestyles interventions the active ingredients are intensive instruction and advice. This suggests that the theoretical models of theory of planned behaviour and the theory that everyone has a degree of motivation and intent may not be appropriate for lifestyle change.
A related, but perhaps separate, explanation is that the techniques we used to apply these theories were too weak. Secondary to factors totally outside our control, we could not assess competencies fully, but the extremely limited evidence we had was that the competencies, although acceptable, were not highly proficient. Assuming that this was indeed the case, then despite the intensive MI training of the HLFs this in itself was insufficient and one needs to establish very high levels of competencies in these skills. The health trainers did refer to this in the process evaluation; they thought that there was too much didactic and classroom-based teaching when they would have preferred more clinical cases and more supervision during the intervention. There have been very few studies that measure the competencies of low-intensity psychotherapy in lifestyle-based interventions. In the field of supporting self-management in type 2 diabetes mellitus, there have been only three studies that have formally tested competencies in MI. 105,162,163 The original landmark studies of MI were delivered by those who had developed the models, were leaders in the field and specialised wholly in MI. 41 As champions of MI and developers of the competency materials, it is likely that the earlier studies of MI would have shown high competencies and that this led to improved outcomes. It is unfortunate that although these data were collected we are not able to measure the competencies that would allow us to examine the level of skill. It may be reasonable to assume that the competencies delivered were probably adequate even if not excellent, in keeping with what would be expected for a large-scale delivery of MI by NHS band 3 equivalent health trainers. The reasons are that the health trainers were self-selected, most already had experience in this field and were given excellent references, they received high-intensity training and supervision, and their own feedback suggested that they were very motivated to improve their skills and seek more practical support and had insight into their clinical needs.
Although we observed that the HLFs generally had lower than expected competency in delivering the BCTs, it is important to note that the employed BCT coding framework was developed by us and was not validated, potentially leading us to underestimate HLFs’ competencies in this domain. If we had the full data set of recorded intervention sessions, we believe that we would have observed much higher levels of competency. Anecdotes from the HLFs suggested that they were highly motivated, professional, committed, ambitious and knew that they were being observed; these are all the key generic therapist elements often needed to be competent in delivering talking therapies.
Finally, it is worth considering that participants in the UC arm may have received a high level of standard care over the course of the study, which could have positively affected their PA and weight, thus obscuring any treatment effects from MOVE IT. However, given that the UC arm did not have a significant increase in PA or reduction in weight, this alternative is unlikely.
Clinical implications
There are a number of clinical implications to consider. These findings suggest that low-intensity psychological intervention in the form of MI to the general population at high risk of CVD risk is not effective. The acquisition of a workforce of health trainers who have received extensive training in MI to a level of low-to-moderate skills in MI is probably also not indicated. If this kind of training was to be offered, perhaps the focus should use the manual we have developed as a basis but to focus on practical learning through case work (i.e. practical experience and case studies). There is also some consideration to be given as to whether these findings apply to other clinical settings that require lifestyle change, such as obesity, prediabetes, diabetes mellitus and people with CVD. Some older, male patients have a perception that they are not at risk of CVD or that this risk is to be expected at this stage in their lives. This view may need to be studied further to understand if it is associated with worse health outcomes.
The implication of the cost-effectiveness analyses is that introducing a generic enhanced MI-based health trainer programme for people at high risk of CVD is not a cost-effective use of resources.
Research implications and future directions
There are number of potential directions for the future. The first is to test whether or not this intervention would be more successful with people at much higher risk of CVD who have modifiable risk factors. This would involve selecting patients from primary care or community settings, and perhaps younger patients, with higher levels of obesity, lipids and other CVD risk factors that are potentially modifiable (unlike risk factors such as age, ethnicity and sex, which are not modifiable). One potential next step could be to model the intervention in different populations, as we are not aware of intensive MI interventions to support lifestyle change in which there was improved targeting.
An alternative approach would have been to increase the actual CVD risk for eligibility and to restrict the study to a younger population, those living in deprived areas and those of non-white ethnicity. Another improvement could be to change the format of the intervention or its delivery (i.e. being led by a practice nurse). 164 Setting BMI thresholds for recruitment would be another improvement to our methodology, which may mean that we recruit patients at higher risk and who may have benefited more from the intervention. However, our sensitivity analyses adjusting for different BMI thresholds did not indicate that this factor influenced the effect of intervention.
Second, there may need to be a paradigm shift in the psychological constructs underpinning lifestyle change in chronic disease self-management. Brief interventions for medical conditions that are chronic are increasingly showing to be not effective and outdated. New concepts are needed that take into account the burden, or the long haul, of living with a chronic condition. Our intervention was for 12 months but perhaps interventions that consist of bursts of support over years need to be evaluated; the dilemma is that these are expensive to fund and the results take a long time to be reported, and there may be a cohort effect as medical science might move forward in that time. MOVE IT emphasised the importance of the use of qualitative methodology in providing detailed experiences of participants for assessing the effectiveness of a RCT. As previously reported, studies have supported the benefits of lifestyle interventions in the short term but few have supported their benefits in the long term. 49,131,132 In MOVE IT, continuity of care and duration of the intervention was of value to those who attended. Although attendance to group sessions was less than attendance to individual sessions, those who attended group sessions appear to value the peer support. Future research should include more qualitative work on why those at the highest risk of CVD or those with modifiable risk factors do not attend lifestyle interventions. It was impossible to do this in MOVE IT as we could not gain ethics approval to contact patients who had not replied to our invitation sent via their GPs.
Summary
An intensive lifestyle intervention using enhanced MI skills was not associated with reduced weight or increased PA in a sample of people at risk of CVD. The reasons for this are probably attributable to the sample having predominantly non-modifiable risk factors. Future interventions should focus on those at high CVD risk and with modifiable risk factors and should be delivered at high levels of measured competency.
Acknowledgements
First, we would like to thank the 1742 participants who took part in this trial, as well as the 1441 who were screened but did not participate. We are grateful for the support of the 135 participating general practices across South London, including the collaboration of GP partners, health-care professionals and practice staff. We also wish to thank the TSC and DMEC members for their oversight and support of the study (details are provided in Trial Steering Committee and Data Monitoring and Ethics Committee). We would like to thank the research team, the HLF team, CPs, PPI members and the South London Clinical Research Network, who have supported the study, and Waad Al-Fawaz (PhD student) who led the dietary assessments.
Contributions of authors
Khalida Ismail (Professor of Psychiatry and Medicine and Consultant Liaison Psychiatrist) led the grant application and conduct of all aspects of the trial as PI.
Daniel Stahl (Reader in Biostatistics and Head of the Statistics Learning Group) contributed to the grant application, protocol writing and ongoing study monitoring, developed the statistical analysis plan, advised on the collection of outcome measures and data management and conducted the primary and secondary analyses.
Adam Bayley (Clinical Trial Manager) provided oversight of the research team, contributed to the day-to-day conduct of the trial and was involved in writing and collating contributions to the report.
Katherine Twist (Clinical Trial Manager) provided oversight of the research team and contributed to recruitment of research sites and the day-to-day conduct of the trial.
Kurtis Stewart (Trial Administrator) provided administrative support to the trial and contributed to sections of the report, including intervention details and fidelity analysis.
Katie Ridge (Assistant Trial Manager) contributed to the recruitment of research sites, data collection schedule development and ongoing day-to-day running of the trial.
Emma Britneff (Assistant Trial Manager) provided oversight of the research team and contributed to the day-to-day conduct of the trial.
Mark Ashworth (Senior Lecturer in General Practice) contributed to the grant application, protocol writing and ongoing study monitoring. He networked with, advised and facilitated the study for the new CCGs in South London and advised on primary-care-related issues.
Nicole de Zoysa (Senior Clinical Psychologist) contributed to the grant application, protocol writing and intervention development. She developed the manual, curriculum and competency framework and trained and supervised the HLF team.
Jennifer Rundle (Clinical Psychologist) provided ongoing training and supervision of the HLF team and contributed to the development of the process evaluation, including preliminary fidelity analyses.
Derek Cook (Professor of Epidemiology) contributed to the grant application, protocol writing and ongoing study monitoring. He supervised setup and collection of PA data and the interpretation of outcome measures.
Peter Whincup (Professor of Cardiovascular Epidemiology) contributed to the grant application, protocol writing and ongoing study monitoring. He was responsible for senior supervision of all aspects of CVD epidemiology, Health Checks and health service aspects of the trial.
Janet Treasure (Professor of Psychiatry) contributed to the grant application, intervention development (MI approach, training and manual development) and ongoing study monitoring.
Paul McCrone (Professor of Health Economics) contributed to the grant application, protocol writing and ongoing study monitoring. He conducted the cost-effectiveness analysis.
Anne Greenough (Professor of Neonatology and Clinical Respiratory Physiology) contributed to the grant application, protocol writing and ongoing study monitoring.
Kirsty Winkley (Postdoctoral Fellow and Lecturer in Diabetes and Psychology) contributed to the grant application, protocol writing and ongoing study monitoring. As the senior project manager, she provided oversight of both the research team and the HLF team during recruitment and intervention delivery. She supervised the recruitment of staff members, the quality assurance of data collection and the process evaluation.
The MOVE IT trial team
We set up a multidisciplinary team to ensure that all scientific and practical considerations were addressed for the successful conduct of this study. This represented international reputations and expertise in health psychology, clinical psychology, nursing, clinical epidemiology of long-term conditions (obesity, depression, CVD and diabetes mellitus), clinical trials, health economics of complex interventions, statistics in behavioural sciences, academic and commissioning primary care and project management. Collectively, we have a wealth of expertise in running large cohort clinical trials of complex interventions and in the dissemination and translation into local services and training programmes, especially in the local multicultural setting. Our strength also lies in the collaboration between two teaching hospitals via the health innovation and education cluster.
Members
-
Principal investigator: Khalida Ismail.
-
Co-investigators: Mark Ashworth, Derek Cook, Anne Greenough, Paul McCrone, Daniel Stahl, Janet Treasure, Peter Whincup and Kirsty Winkley.
-
Trial managers: Adam Bayley, Emma Britneff, Katie Ridge and Katherine Twist.
-
Trial administrators: Kurtis Stewart and Clare Tucker.
-
Research assistants: Hina Akram, Jasmine Anilmis, Jennifer Bales, Naomi Croft, Stephanie Fincham-Campbell, Lucinda Gledhill, Ana Jovovic, Alexandra Keyes, Paulina Kuczynska, Lisa Kuriakose, Leona McGarrigle, Louise Mercer, Anna Rokakis, Arann Rowe, Katherine Sparrow and Emily Staite.
-
Clinical psychologists: Nicole de Zoysa and Jennifer Rundle.
-
HLFs: Mwaita Bonga, Anna-Marie Bowtle, Nurcan Cahill, Emily Costelloe, Elizabeth Cruz, Salwa Dafalla, Charlotte Irving, Jessica Mark, Kashmir Mangat, Arbaktun Mohammed, Ekta Patel and Raquel Ramos-Fraga.
-
PPI: Jennifer Bostock and Carole Haynes.
-
Volunteers and students: Gita Ahluwalia, Emily Bralee, Christina Deller, Aurelia Harjanto, Vera Ludwig, Tracy Tang, Patrick Timpel, Alexander Warrilow-Wilson, Farah Yakub and Natalie Zaremba.
Trial Steering Committee
We would like to thank the TSC members: Professor Steve Iliffe (Chairperson), University College London; Professor Tom Marshall (member of the Health Technology Assessment and Efficacy and Mechanism Evaluation boards, 2009–11), University of Birmingham; Professor James Carpenter, London School of Hygiene and Tropical Medicine, Medical Research Council Clinical Trials Unit; and Dr Tim Anstiss, independent medical doctor and consultant. We would also like to thank the PPI members for attending all TSC meetings throughout the trial.
Data Monitoring and Ethics Committee
We would like to thank the DMEC members: Professor Betty Kirkwood (previous Chairperson), London School of Hygiene and Tropical Medicine; Professor Helen Weiss (Chairperson), London School of Hygiene and Tropical Medicine; Professor Stephanie Taylor, Queen Mary University of London, Barts and The London School of Medicine and Dentistry, Blizard Institute; and Dr David Blane, Imperial College London.
Publications
Bayley A, de Zoysa N, Cook DG, Whincup PH, Stahl D, Twist K, et al. Comparing the effectiveness of an enhanced MOtiVational intErviewing InTervention (MOVE IT) with usual care for reducing cardiovascular risk in high risk subjects: study protocol for a randomised controlled trial. Trials 2015;16:112.
Bayley A , Stahl D, Ashworth M, Cook D, Whincup P, Treasure J, et al. Response bias to a randomised controlled trial of a lifestyle intervention in people at high risk of cardiovascular disease: a cross-sectional analysis. BMC Public Health 2018;18:1092.
Ismail K, Bayley A, Twist K, Stewart K, Ridge K, Britneff E, et al. Reducing weight and increasing physical activity in people at high risk of cardiovascular disease: a randomised controlled trial comparing the effectiveness of enhanced motivational interviewing intervention with usual care [published online ahead of print December 12 2019]. Heart 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- World Health Organization . Global Status Report on Noncommunicable Diseases 2010 2011.
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. https://doi.org/10.1016/S0140-6736(09)60318-4.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. https://doi.org/10.1016/S0140-6736(02)11911-8.
- Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829-39. https://doi.org/10.1016/S0140-6736(07)61778-4.
- Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med 2010;170:711-18. https://doi.org/10.1001/archinternmed.2010.76.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. https://doi.org/10.1016/S0140-6736(04)17018-9.
- Backholer K, Peters SAE, Bots SH, Peeters A, Huxley RR, Woodward M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health 2017;71:550-7. https://doi.org/10.1136/jech-2016-207890.
- Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001-2003. J Public Health 2007;29:191-8. https://doi.org/10.1093/pubmed/fdm010.
- Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 2004;109:1101-7. https://doi.org/10.1161/01.CIR.0000118498.35499.B2.
- Hardoon SL, Whincup PH, Lennon LT, Wannamethee SG, Capewell S, Morris RW. How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based study. Circulation 2008;117:598-604. https://doi.org/10.1161/CIRCULATIONAHA.107.705947.
- Department of Health and Social Care (DHSC) . Vascular Checks: Risk Assessment and Management 2008.
- Robson J, Dostal I, Sheikh A, Eldridge S, Madurasinghe V, Griffiths C, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-008840.
- Usher-Smith JA, Mant J, Martin A, Harte E, MacLure C, Meads C, et al. NHS Health Check Programme Rapid Evidence Synthesis 2017. www.rand.org/content/dam/rand/pubs/external_publications/EP60000/EP67129/RAND_EP67129.pdf (accessed 1 November 2017).
- Cochrane T, Davey R, Iqbal Z, Gidlow C, Kumar J, Chambers R, et al. NHS health checks through general practice: randomised trial of population cardiovascular risk reduction. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-944.
- Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, Gøtzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e7191.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. https://doi.org/10.1093/pubmed/fdr034.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8. https://doi.org/10.1016/0002-8703(91)90861-B.
- Woodward M, Brindle P, Tunstall-Pedoe H. SIGN group on risk estimation . Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172-6. https://doi.org/10.1136/hrt.2006.108167.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335. https://doi.org/10.1136/bmj.39261.471806.55.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82. https://doi.org/10.1136/bmj.39609.449676.25.
- National Institute for Health and Care Excellence (NICE) . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification 2016.
- Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 2016;5. https://doi.org/10.1161/JAHA.115.002495.
- Department of Health and Social Care (DHSC) . UK Physical Activity Guidelines 2011.
- Hamer M, Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. Br J Sports Med 2008;42:238-43. https://doi.org/10.1136/bjsm.2007.039974.
- Morris JN, Hardman AE. Walking to health. Sports Med 1997;23:306-32. https://doi.org/10.2165/00007256-199723050-00004.
- Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, et al. Interventions to promote walking: systematic review. BMJ 2007;334. https://doi.org/10.1136/bmj.39198.722720.BE.
- Craig R, Mindell J, Hirani V. Health Survey for England 2008. Volume 1: Physical Activity and Fitness 2009. https://files.digital.nhs.uk/publicationimport/pub00xxx/pub00430/heal-surv-phys-acti-fitn-eng-2008-rep-v1.pdf (accessed 1 November 2017).
- Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health 2011;101:751-8. https://doi.org/10.2105/AJPH.2010.194381.
- Foster C, Hillsdon M, Thorogood M, Kaur A, Wedatilake T. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005;1. https://doi.org/10.1002/14651858.CD003180.pub2.
- Lamming L, Pears S, Mason D, Morton K, Bijker M, Sutton S, et al. What do we know about brief interventions for physical activity that could be delivered in primary care consultations? A systematic review of reviews. Prev Med 2017;99:152-63. https://doi.org/10.1016/j.ypmed.2017.02.017.
- GC V, Wilson EC, Suhrcke M, Hardeman W, Sutton S. VBI Programme Team . Are brief interventions to increase physical activity cost-effective? A systematic review. Br J Sports Med 2016;50:408-17. https://doi.org/10.1136/bjsports-2015-094655.
- Murtagh EM, Nichols L, Mohammed MA, Holder R, Nevill AM, Murphy MH. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta-analysis of randomised control trials. Prev Med 2015;72:34-43. https://doi.org/10.1016/j.ypmed.2014.12.041.
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, et al. Best practices for physical activity programs and behavior counseling in older adult populations. J Aging Phys Act 2005;13:61-74. https://doi.org/10.1123/japa.13.1.61.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. https://doi.org/10.1001/jama.298.19.2296.
- Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, et al. Effect of a primary care walking intervention with and without nurse support on physical activity levels in 45- to 75-year-olds: the Pedometer And Consultation Evaluation (PACE-UP) cluster randomised clinical trial. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002210.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- Bhattarai N, Prevost AT, Wright AJ, Charlton J, Rudisill C, Gulliford MC. Effectiveness of interventions to promote healthy diet in primary care: systematic review and meta-analysis of randomised controlled trials. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-1203.
- Gulliford MC, Bhattarai N, Charlton J, Rudisill C. Cost-effectiveness of a universal strategy of brief dietary intervention for primary prevention in primary care: population-based cohort study and Markov model. Cost Eff Resour Alloc 2014;12. https://doi.org/10.1186/1478-7547-12-4.
- Rees K, Dyakova M, Ward K, Thorogood M, Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.CD002128.pub4.
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med 2007;147:41-50. https://doi.org/10.7326/0003-4819-147-1-200707030-00007.
- Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 2002.
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York, NY: Guilford Press; 2012.
- Lundahl B, Moleni T, Burke BL, Butters R, Tollefson D, Butler C, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93:157-68. https://doi.org/10.1016/j.pec.2013.07.012.
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71:843-61. https://doi.org/10.1037/0022-006X.71.5.843.
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305-12.
- VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med 2014;37:768-80. https://doi.org/10.1007/s10865-013-9527-4.
- Lee WW, Choi KC, Yum RW, Yu DS, Chair SY. Effectiveness of motivational interviewing on lifestyle modification and health outcomes of clients at risk or diagnosed with cardiovascular diseases: a systematic review. Int J Nurs Stud 2016;53:331-41. https://doi.org/10.1016/j.ijnurstu.2015.09.010.
- Jansink R, Braspenning J, Keizer E, van der Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scand J Prim Health Care 2013;31:119-27. https://doi.org/10.3109/02813432.2013.797178.
- Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act 2013;10. https://doi.org/10.1186/1479-5868-10-40.
- Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract 2011;91:1-12. https://doi.org/10.1016/j.diabres.2010.06.030.
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011;1. https://doi.org/10.1002/14651858.CD001561.pub3.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-119.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. https://doi.org/10.1016/0749-5978(91)90020-T.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. https://doi.org/10.1037/0278-6133.27.3.379.
- Greaves C, Gillison F, Stathi A, Bennett P, Reddy P, Dunbar J, et al. Waste the waist: a pilot randomised controlled trial of a primary care based intervention to support lifestyle change in people with high cardiovascular risk. Int J Behav Nutr Phys Act 2015;12. https://doi.org/10.1186/s12966-014-0159-z.
- Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol 2001;69:717-21. https://doi.org/10.1037/0022-006X.69.4.717.
- Kozica SL, Lombard CB, Ilic D, Ng S, Harrison CL, Teede HJ. Acceptability of delivery modes for lifestyle advice in a large scale randomised controlled obesity prevention trial. BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-1995-8.
- Després JP, Poirier P. Diabetes: Looking back at Look AHEAD – giving lifestyle a chance. Nat Rev Cardiol 2013;10:184-6. https://doi.org/10.1038/nrcardio.2013.16.
- Ismail K, Lawrence-Smith G, Cheah Y, Winkley K, Yadav R, Thomas S, et al. Motivational enhancement therapy with and without cognitive-behavioural therapy to treat type 1 diabetes: the long-term outcomes of a randomised controlled trial. Diabetologia 2009;52.
- Ridge K, Bartlett J, Cheah Y, Thomas S, Lawrence-Smith G, Winkley K, et al. Do the effects of psychological treatments on improving glycemic control in type 1 diabetes persist over time? A long-term follow-up of a randomized controlled trial. Psychosom Med 2012;74:319-23. https://doi.org/10.1097/PSY.0b013e31824c181b.
- Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al. A randomised controlled trial of cognitive–behavioural therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: a Diabetes and Psychological Therapies (ADaPT) study. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14220.
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006;295:2003-17. https://doi.org/10.1001/jama.295.17.2003.
- Department of Health and Social Care (DHSC) . Choosing Health: Making Healthy Choices Easier 2004.
- South J, Woodward J, Lowcock D. New beginnings: stakeholder perspectives on the role of health trainers. J R Soc Promot Health 2007;127:224-30. https://doi.org/10.1177/1466424007081791.
- Lhussier M, Carr SM. Health-related lifestyle advice: critical insights. Crit Public Health 2008;18:299-30. https://doi.org/10.1080/09581590802225738.
- Visram S, Carr SM, Geddes L. Can lay health trainers increase uptake of NHS Health Checks in hard-to-reach populations? A mixed-method pilot evaluation. J Public Health 2015;37:226-33. https://doi.org/10.1093/pubmed/fdu041.
- Carr SM, Lhussier M, Forster N, Geddes L, Deane K, Pennington M, et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost-effectiveness, equity and acceptability of different versions of health-related lifestyle advisor role in improving health. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15090.
- Attree P, Clayton S, Karunanithi S, Nayak S, Popay J, Read D. NHS health trainers: a review of emerging evaluation evidence. Crit Public Health 2012;22:25-38. https://doi.org/10.1080/09581596.2010.549207.
- Gidlow CJ, Cochrane T, Davey R, Beloe M, Chambers R, Kumar J, et al. One-year cardiovascular risk and quality of life changes in participants of a health trainer service. Perspect Public Health 2014;134:135-44. https://doi.org/10.1177/1757913913484419.
- Bayley A, de Zoysa N, Cook DG, Whincup PH, Stahl D, Twist K, et al. Comparing the effectiveness of an enhanced MOtiVational intErviewing InTervention (MOVE IT) with usual care for reducing cardiovascular risk in high risk subjects: study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0593-5.
- Office for National Statistics (ONS) . Census 2011 2011. www.ons.gov.uk/census/2011censuspages/1b.asp (accessed 5 May 2017).
- Collins GS, Altman DG. Predicting the 10 year risk of cardiovascular disease in the United Kingdom: independent and external validation of an updated version of QRISK2. BMJ 2012;344. https://doi.org/10.1136/bmj.e4181.
- Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. The SHED-IT randomized controlled trial: evaluation of an internet-based weight-loss program for men. Obesity 2009;17:2025-32. https://doi.org/10.1038/oby.2009.85.
- Rose G. Sick individuals and sick populations. Int J Epidemiol 2001;30:427-32. https://doi.org/10.1093/ije/30.3.427.
- Batistatou E, Roberts C, Roberts S. Sample size and power calculations for trials and quasi-experimental studies with clustering. Stata J 2014;14:159-75. https://doi.org/10.1177/1536867X1401400111.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2010 2011. www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 1 November 2017).
- Noble M, Wright G, Smith G, Dibben C. Measuring multiple deprivation at the small-area level. Environ Plann A 2006;38:169-85. https://doi.org/10.1068/a37168.
- Davis TC, Crouch MA, Long SW, Jackson RH, Bates P, George RB, et al. Rapid assessment of literacy levels of adult primary care patients. Fam Med 1991;23:433-5.
- Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity 2007;15:2371-9. https://doi.org/10.1038/oby.2007.281.
- Mâsse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 2005;37:544-54. https://doi.org/10.1249/01.mss.0000185674.09066.8a.
- Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health-UK 2006;14:66-70. https://doi.org/10.1007/s10389-006-0024-x.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Nelson M, Haraldsdóttir J. Food photographs: practical guidelines I. Design and analysis of studies to validate portion size estimates. Public Health Nutr 1998;1:219-30. https://doi.org/10.1079/PHN19980038.
- Welch AA, Luben R, Khaw KT, Bingham SA. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J Hum Nutr Diet 2005;18:99-116. https://doi.org/10.1111/j.1365-277X.2005.00593.x.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 1999;282:1737-44. https://doi.org/10.1001/jama.282.18.1737.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25. https://doi.org/10.1001/jama.1993.03510150053029.
- Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res 2000;49:154-9. https://doi.org/10.1097/00006199-200005000-00007.
- Rabin R, Gudex C, Selai C, Herdman M. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health 2014;17:70-6. https://doi.org/10.1016/j.jval.2013.10.006.
- Chisholm D, Knapp MRJ, Knudsen HC, Amaddeo F, Gaite L, Van Wijngaarden B. Client Socio-Demographic and Service Receipt Inventory-European Version: development of an instrument for international research. Br J Psychiatry 2000;177:s28-33. https://doi.org/10.1192/bjp.177.39.s28.
- Hobbis ICA, Sutton S. Are techniques used in cognitive–behavioural therapy applicable to behaviour change interventions based on the theory of planned behaviour?. J Health Psychol 2005;10:7-18. https://doi.org/10.1177/1359105305048549.
- Bandura A. Social Foundation of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1986.
- Varley R, Webb TL, Sheeran P. Making self-help more helpful: a randomized controlled trial of the impact of augmenting self-help materials with implementation intentions on promoting the effective self-management of anxiety symptoms. J Consult Clin Psychol 2011;79:123-8. https://doi.org/10.1037/a0021889.
- Miller WR, Moyers TB, Arciniega L, Ernst D, Forcehimes A. Training, supervision and quality monitoring of the COMBINE Study behavioral interventions. J Stud Alcohol Suppl 2005;15:188-95. https://doi.org/10.15288/jsas.2005.s15.188.
- Michie S, Churchill S, West R. Indentifying evidence-based competencies required to deliver behavioural support for smoking cessation. Ann Behav Med 2011;41:59-70. https://doi.org/10.1007/s12160-010-9235-z.
- Dixon D, Johnston M. Health Behaviour Change Competency Framework: Competences to Deliver Interventions to Change Lifestyle Behaviours that Affect Health. NHS Health Scotland; 2010.
- Moyers TB, Martin T, Manuel JK, Miller W, Ernst D. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1.1 (MITI 3.1.1) 2010. https://casaa.unm.edu/download/miti3_1.pdf (accessed 1 November 2017).
- Williams A. The Role of the EuroQol Instrument in QALY Calculations. York: University of York Centre for Health Economics; 1995.
- Beecham J, Knapp M. Costing Psychiatric Interventions. London: Gaskell; 2001.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Campbell MK, Elbourne DR, Altman DG. CONSORT group . CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. https://doi.org/10.1136/bmj.328.7441.702.
- Bauer DJ, Sterba SK, Hallfors DD. Evaluating group-based interventions when control participants are ungrouped. Multivariate Behav Res 2008;43:210-36. https://doi.org/10.1080/00273170802034810.
- Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton, FL: CRC Press; 1997.
- Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1571-0.
- Ismail K, Winkley K, De Zoysa N, Patel A, Heslin M, Graves H, et al. Nurse-led psychological intervention for type 2 diabetes: a cluster randomised controlled trial (Diabetes-6 study) in primary care. Br J Gen Pract 2018;68:e531-e540. https://doi.org/10.3399/bjgp18X696185.
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. Obstet Gynecol Surv 2011;66:488-9. https://doi.org/10.1097/OGX.0b013e3182352181.
- Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017;46:1093-1093i. https://doi.org/10.1093/ije/dyx015.
- Public Health England . National General Practice Profiles 2016. https://fingertips.phe.org.uk/profile/general-practice (accessed 21 July 2017).
- Stuart-Buttle C, Read J, Sanderson H, Sutton Y. A language of health in action: Read codes, classifications and groupings. Proc AMIA Annu Fall Symp 1996:75-9.
- Ismail K, Bayley A, Twist K, Stewart K, Ridge K, Britneff E, et al. Reducing weight and increasing physical activity in people at high risk of cardiovascular disease: a randomised controlled trial comparing the effectiveness of enhanced motivational interviewing intervention with usual care [published online ahead of print December 12 2019]. Heart 2019. https://doi.org/10.1136/heartjnl-2019-315656.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2015 2015. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 1 November 2017).
- Rogers A, Harris T, Victor C, Woodcock A, Limb E, Kerry S, et al. Which older people decline participation in a primary care trial of physical activity and why: insights from a mixed methods approach. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-46.
- Tully MA, Cupples ME, Chan WS, McGlade K, Young IS. Brisk walking, fitness, and cardiovascular risk: a randomized controlled trial in primary care. Prev Med 2005;41:622-8. https://doi.org/10.1016/j.ypmed.2004.11.030.
- Foster CE, Brennan G, Matthews A, McAdam C, Fitzsimons C, Mutrie N. Recruiting participants to walking intervention studies: a systematic review. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-137.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014;129:399-410. https://doi.org/10.1161/01.cir.0000442015.53336.12.
- Ives DG, Traven ND, Kuller LH, Schulz R. Selection bias and nonresponse to health promotion in older adults. Epidemiology 1994;5:456-61. https://doi.org/10.1097/00001648-199407000-00013.
- Harris TJ, Victor CR, Carey IM, Adams R, Cook DG. Less healthy, but more active: opposing selection biases when recruiting older people to a physical activity study through primary care. BMC Public Health 2008;8. https://doi.org/10.1186/1471-2458-8-182.
- Groeneveld IF, Proper KI, van der Beek AJ, Hildebrandt VH, van Mechelen W. Factors associated with non-participation and drop-out in a lifestyle intervention for workers with an elevated risk of cardiovascular disease. Int J Behav Nutr Phys Act 2009;6. https://doi.org/10.1186/1479-5868-6-80.
- van Heuvelen MJ, Hochstenbach JB, Brouwer WH, de Greef MH, Zijlstra GA, van Jaarsveld E, et al. Differences between participants and non-participants in an RCT on physical activity and psychological interventions for older persons. Aging Clin Exp Res 2005;17:236-45. https://doi.org/10.1007/BF03324603.
- Golomb BA, Chan VT, Evans MA, Koperski S, White HL, Criqui MH. The older the better: are elderly study participants more non-representative? A cross-sectional analysis of clinical trial and observational study samples. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-000833.
- Forsberg L, Berman AH, Kallmén H, Hermansson U, Helgason AR. A test of the validity of the Motivational Interviewing Treatment Integrity code. Cogn Behav Ther 2008;37:183-91. https://doi.org/10.1080/16506070802091171.
- Martin T, Christopher PJ, Houck JM, Moyers TB. The structure of client language and drinking outcomes in project match. Psychol Addict Behav 2011;25:439-45. https://doi.org/10.1037/a0023129.
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat 2005;28:19-26. https://doi.org/10.1016/j.jsat.2004.11.001.
- Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 1981;86:127-37.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Hotham ED, Atkinson ER, Gilbert AL. Focus groups with pregnant smokers: barriers to cessation, attitudes to nicotine patch use and perceptions of cessation counselling by care providers. Drug Alcohol Rev 2002;21:163-8. https://doi.org/10.1080/09595230220139064.
- Paterson C, Zheng Z, Xue C, Wang Y. ‘Playing their parts’: the experiences of participants in a randomized sham-controlled acupuncture trial. J Altern Complement Med 2008;14:199-208. https://doi.org/10.1089/acm.2007.0682.
- Tonnon SC, Proper KI, van der Ploeg HP, Westerman MJ, Sijbesma E, van der Beek AJ. A qualitative study of the anticipated barriers and facilitators to the implementation of a lifestyle intervention in the Dutch construction industry. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-1317.
- Zhang X, Devlin HM, Smith B, Imperatore G, Thomas W, Lobelo F, et al. Effect of lifestyle interventions on cardiovascular risk factors among adults without impaired glucose tolerance or diabetes: a systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0176436.
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns 2004;53:147-55. https://doi.org/10.1016/S0738-3991(03)00141-1.
- Burke BL, Dunn CW, Atkins DC, Phelps JS. The emerging evidence base for motivational interviewing: a meta-analytic and qualitative inquiry. J Cogn Psychother 2004;18:309-22. https://doi.org/10.1891/jcop.18.4.309.64002.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Miller WR, Moyers TB. Eight stages in learning motivational interviewing. J Teaching Addict 2006;5:3-17. https://doi.org/10.1300/J188v05n01_02.
- Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat 2009;36:101-9. https://doi.org/10.1016/j.jsat.2008.05.005.
- Brobeck E, Bergh H, Odencrants S, Hildingh C. Primary healthcare nurses’ experiences with motivational interviewing in health promotion practice. J Clin Nurs 2011;20:3322-30. https://doi.org/10.1111/j.1365-2702.2011.03874.x.
- Östlund AS, Wadensten B, Kristofferzon ML, Häggström E. Motivational interviewing: experiences of primary care nurses trained in the method. Nurse Educ Pract 2015;15:111-18. https://doi.org/10.1016/j.nepr.2014.11.005.
- van Eijk-Hustings YJ, Daemen L, Schaper NC, Vrijhoef HJ. Implementation of Motivational Interviewing in a diabetes care management initiative in the Netherlands. Patient Educ Couns 2011;84:10-5. https://doi.org/10.1016/j.pec.2010.06.016.
- Hirdle J, Vaughan T. Exploring the impact of motivational interviewing training for qualified health visitors. Community Pract 2016;89:38-42.
- Maissi E, Ridge K, Treasure J, Chalder T, Roche S, Bartlett J, et al. Nurse-led psychological interventions to improve diabetes control: assessing competencies. Patient Educ Couns 2011;84:e37-43. https://doi.org/10.1016/j.pec.2010.07.036.
- Graves H, Garrett C, Amiel SA, Ismail K, Winkley K. Psychological skills training to support diabetes self-management: qualitative assessment of nurses’ experiences. Prim Care Diabetes 2016;10:376-82. https://doi.org/10.1016/j.pcd.2016.03.001.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Schrack JA, Cooper R, Koster A, Shiroma EJ, Murabito JM, Rejeski WJ, et al. Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. J Gerontol A Biol Sci Med Sci 2016;71:1039-48. https://doi.org/10.1093/gerona/glw026.
- Roth MA, Mindell JS. Who provides accelerometry data? Correlates of adherence to wearing an accelerometry motion sensor: the 2008 Health Survey for England. J Phys Act Health 2013;10:70-8. https://doi.org/10.1123/jpah.10.1.70.
- Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults?. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-62.
- British Heart Foundation . CVD Statistics – BHF UK Factsheet 2018. www.bhf.org.uk/what-we-do/our-research/heart-statistics (accessed 1 November 2017).
- Goldberg RW, Seybolt DC, Lehman A. Reliable self-report of health service use by individuals with serious mental illness. Psychiatr Serv 2002;53:879-81. https://doi.org/10.1176/appi.ps.53.7.879.
- Calsyn RJ, Allen G, Morse GA, Smith R, Tempelhoff B. Can you trust self-report data provided by homeless mentally ill individuals?. Eval Rev 1993;17:353-66. https://doi.org/10.1177/0193841X9301700306.
- van Staa TP, Gulliford M, Ng ES, Goldacre B, Smeeth L. Prediction of cardiovascular risk using Framingham, ASSIGN and QRISK2: how well do they predict individual rather than population risk?. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0106455.
- Tudor-Locke C, Bassett DR. How many steps/day are enough?. Sports Med 2004;34:1-8. https://doi.org/10.2165/00007256-200434010-00001.
- Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-75.
- Bakrania K, Yates T, Edwardson CL, Bodicoat DH, Esliger DW, Gill JM, et al. Associations of moderate-to-vigorous-intensity physical activity and body mass index with glycated haemoglobin within the general population: a cross-sectional analysis of the 2008 Health Survey for England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014456.
- Pedišić Ž, Bauman A. Accelerometer-based measures in physical activity surveillance: current practices and issues. Br J Sports Med 2015;49:219-23. https://doi.org/10.1136/bjsports-2013-093407.
- Hankonen N, Sutton S, Prevost AT, Simmons RK, Griffin SJ, Kinmonth AL, et al. Which behavior change techniques are associated with changes in physical activity, diet and body mass index in people with recently diagnosed diabetes?. Ann Behav Med 2015;49:7-17. https://doi.org/10.1007/s12160-014-9624-9.
- Bauman A, Pedišić Ž, Bragg K, Shephard RJ, Tudor-Locke C. The Objective Monitoring of Physical Activity: Contributions of Accelerometry to Epidemiology, Exercise Science and Rehabilitation. Berlin: Springer; 2016.
- Paul L, Brewster S, Wyke S, McFadyen AK, Sattar N, Gill JM, et al. Increasing physical activity in older adults using STARFISH, an interactive smartphone application (app); a pilot study. J Rehabil Assist Technol Eng 2017;4. https://doi.org/10.1177/2055668317696236.
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-44. https://doi.org/10.2337/diacare.20.4.537.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. https://doi.org/10.1056/NEJM200105033441801.
- Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346.
- Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JCG, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet 2017;389:2214-25. https://doi.org/10.1016/S0140-6736(17)30647-5.
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541-51. https://doi.org/10.1016/S0140-6736(17)33102-1.
- Welch G, Zagarins SE, Feinberg RG, Garb JL. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients?. Diabetes Res Clin Pr 2011;91:54-60. https://doi.org/10.1016/j.diabres.2010.09.036.
- Waker CL. The Effects of Motivational Interviewing on Diabetes Self-Management Behaviors and Glycemic Control in Type 2 Diabetes: A Translational Study. Cincinnati, OH: University of Cincinnati; 2012.
- Harris T, Kerry SM, Limb ES, Furness C, Wahlich C, Victor CR, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: long-term follow-up of participants from two randomised controlled trials in UK primary care. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002526.
- Ingersoll K. Rating the Fidelity of MI Groups Sessions: The AMIGOS Coding System, Bridging the Gap Between MI Practice, Evaluation and Research n.d.
Appendix 1 Proficiency levels adapted for healthy lifestyle facilitator training
| Technique/skill | Proficiency levela | Notes | |||
|---|---|---|---|---|---|
| None | Partial | Moderate | Full | ||
| BCTs (× 11) (% of participants) | 0 | 50 | 70 | 100 | |
| MI (with MITI) | |||||
| Global (n) | < 3 | 3 | 3.5 | 4 | |
| Reflection-to-question ratio (n) | 0 | 0.5 | 1 | 2 | |
| Percentage with open questions (% of participants) | 10 | 30 | 50 | 70 | |
| Percentage with complex reflections (% of participants) | 10 | 30 | 40 | 50 | |
| Percentage who were MI adherent (% of participants) | 50 | 70 | 90 | 100 | |
| Group skills (adapted from AMIGOS)165 (n) | |||||
| Activities | 0 | 1 | 2 | 3 | 0 = none, 1 = a little, 2 = somewhat, 3 = extensively |
| Dynamics | 0 | 1 | 2 | 3 | |
| Rapport | 0 | 1 | 2 | 3 | |
| Boundaries | 0 | 1 | 2 | 3 | |
| Time management (n) | 0 | 1 | 2 | 3 | 0 = none, 1 = a little, 2 = somewhat, 3 = extensively |
Appendix 2 List of participating general practices
| CCG and general practice name | General practice code | Date when the first participant was recruited | List size on 1 April 2013 | Number of patients aged 40–74 years | General practice IMD 2010 score76 | Anonymised search saveda | Number of potentially eligible patients invited | Number of patients | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Who respondedb | Who consented | Randomised | ||||||||
| Bexley | ||||||||||
| Good Health | G83630 | 4 December 2013 | 5807 | 1836 | 30.20 | No | 63 | 12 | 11 | 4 |
| Ingleton Avenue Surgery | G83024 | 20 September 2013 | 5038 | 2110 | 11.00 | No | 87 | 23 | 21 | 18 |
| Lakeside Medical Practice | G83018 | 8 July 2013 | 15,320 | 5253 | 33.50 | Yes | 335 | 38 | 34 | 15 |
| Lyndhurst Road Medical Centre | G83049 | 3 April 2014 | 7943 | 3600 | 14.30 | No | 70 | – | 13 | 5 |
| Plas Meddyg Surgery | G83029 | 7 October 2013 | 7220 | 3188 | 7.77 | No | 111 | 36 | 34 | 28 |
| Slade Green Medical Centre | G83062 | 2 August 2013 | 6521 | 2315 | 29.70 | No | 107 | 14 | 4 | 2 |
| The Westwood Surgery | G83002 | 5 August 2013 | 7770 | 3191 | 10.90 | No | 157 | 40 | 22 | 10 |
| Welling Medical Practice | G83025 | 5 August 2013 | 13,368 | 5984 | 15.00 | No | 85 | 35 | 17 | 7 |
| Woodlands Surgery | G83057 | 14 August 2013 | 10,424 | 4749 | 11.30 | No | 158 | 47 | 21 | 14 |
| Bromley | ||||||||||
| Ballater Surgery | G84040 | 21 August 2014 | 6685 | 2890 | 11.50 | Yes | 138 | – | 31 | 21 |
| Bromley Common Practice | G84024 | 2 December 2013 | 7814 | 3031 | 14.50 | No | 29 | 5 | 4 | 2 |
| Broomwood Road Surgery | G84019 | 27 August 2013 | 9978 | 3923 | 32.90 | No | 107 | – | 42 | 17 |
| Charterhouse Surgery | G84021 | 9 July 2013 | 9127 | 4374 | 8.37 | Yes | 150 | – | 49 | 30 |
| Chelsfield Surgery | G84020 | 18 July 2013 | 7552 | 3591 | 8.91 | No | 211 | 51 | 61 | 27 |
| Cornerways Surgery | G84018 | 24 June 2014 | 8531 | 3810 | 9.61 | No | 179 | 46 | 42 | 30 |
| Derry Downs Surgery | G84005 | 21 January 2014 | 5630 | 2440 | 26.50 | No | 110 | 35 | 27 | 15 |
| Eden Park Surgery | G84011 | 9 October 2013 | 8180 | 3609 | 11.80 | No | 171 | 31 | 45 | 24 |
| Forge Close Surgery | G84030 | 2 April 2014 | 6563 | 3002 | 6.87 | Yes | 113 | 27 | 32 | 19 |
| Links Medical Practice | G84003 | 27 August 2013 | 10,638 | 3974 | 30.60 | Yes | 221 | 23 | 33 | 14 |
| London Lane Clinic | G84016 | 7 August 2013 | 15,558 | 6699 | 15.00 | No | 304 | 20 | 72 | 47 |
| Park Group Practice | G84025 | 1 August 2013 | 8147 | 2983 | 30.10 | No | 126 | 16 | 15 | 8 |
| Pickhurst Surgery | G84033 | 12 December 2013 | 6786 | 3106 | 6.82 | Yes | 180 | 44 | 56 | 28 |
| Poverest Medical Centre | G84007 | 25 September 2013 | 9141 | 3791 | 23.40 | Yes | 199 | 34 | 53 | 32 |
| Robin Hood Surgery | G84029 | 27 February 2014 | 5725 | 2541 | 27.20 | Yes | 93 | 14 | 14 | 6 |
| South View Partnership | G84001 | 20 March 2014 | 6114 | 2647 | 11.70 | No | 195 | 51 | 69 | 45 |
| Station Road Surgery | G84015 | 23 July 2013 | 12,572 | 5873 | 7.80 | Yes | 100 | – | 79 | 47 |
| Stock Hill Surgery | G84004 | 31 July 2013 | 11,241 | 5386 | 10.40 | Yes | 323 | – | 92 | 55 |
| Sundridge Medical Centre | G84629 | 16 December 2013 | 5066 | 1844 | 16.80 | Yes | 112 | 18 | 15 | 10 |
| Trinity Medical Centre | G84022 | 28 July 2014 | 6006 | 2346 | 27.80 | No | 120 | – | 13 | 6 |
| Tudor Way Surgery | G84035 | 20 August 2014 | 7108 | 3076 | 7.76 | No | 63 | – | 10 | 8 |
| Woodlands Practice | Y00542 | 31 July 2014 | 9161 | 3812 | 19.80 | Yes | 202 | – | 31 | 19 |
| Croydon | ||||||||||
| Brigstock Family Practice | H83608 | 21 June 2013 | 4211 | 1474 | 25.00 | Yes | 172 | – | 30 | 13 |
| East Croydon Medical Centre | H83044 | 18 November 2013 | 12,308 | 4189 | 17.90 | Yes | 287 | – | 63 | 30 |
| Greenside Medical Practice | H83631 | 16 August 2013 | 7833 | 2347 | 33.60 | No | 36 | – | 2 | 1 |
| Keston Medical Practice | H83016 | 30 January 2014 | 9406 | 3968 | 15.80 | Yes | 216 | – | 58 | 34 |
| Leander Road Surgery | H83042 | 25 June 2014 | 7035 | 2620 | 21.20 | Yes | 127 | – | 25 | 14 |
| London Road Medical Practice (Cavendish House) | H83021 | 30 September 2014 | 5866 | 2240 | 28.10 | Yes | 114 | – | 12 | 6 |
| Morland Road Surgery | H83023 | 10 July 2013 | 6942 | 2638 | 23.00 | Yes | 193 | – | 24 | 15 |
| Old Coulsdon Medical Practice | H83013 | 27 January 2014 | 12,527 | 5526 | 13.20 | Yes | 237 | – | 58 | 32 |
| Parchmore Medical Centre | H83053 | 16 June 2014 | 13,878 | 5173 | 27.70 | Yes | 186 | – | 29 | 17 |
| Parkside Group Practice | H83015 | 2 April 2014 | 12,505 | 5020 | 15.40 | Yes | 206 | – | 43 | 31 |
| Portland Medical Centre | H83001 | 3 July 2013 | 12,829 | 4987 | 28.40 | No | 164 | – | 15 | 7 |
| Selsdon Park Medical Practice | H83018 | 22 July 2013 | 10,679 | 4759 | 13.10 | Yes | 258 | – | 42 | 23 |
| Stovell House Surgery | H83039 | 25 September 2013 | 7116 | 2973 | 18.50 | Yes | 250 | – | 61 | 31 |
| Upper Norwood Group Practice | H83005 | 12 September 2013 | 11,077 | 3981 | 27.40 | No | 35 | – | 10 | 5 |
| Greenwich | ||||||||||
| All Saints Medical Centre | G83030 | 27 May 2014 | 5006 | 1945 | 36.70 | No | 93 | 22 | 18 | 11 |
| Burney Street | G83065 | 6 January 2015 | 13,146 | 3594 | 28.50 | No | 55 | 8 | 10 | 5 |
| Coldharbour Hill | G83003 | 14 November 2014 | 4098 | 1706 | 25.10 | No | 141 | 31 | 23 | 13 |
| New Eltham | G83628 | 27 June 2014 | 5788 | 2188 | 13.40 | No | 125 | 20 | 15 | 9 |
| Glyndon | G83060 | 13 November 2014 | 7014 | 2466 | 40.80 | No | 66 | 8 | 4 | 2 |
| Manor Brook | G83001 | 18 June 2014 | 12,590 | 4831 | 27.20 | Yes | 276 | 54 | 47 | 27 |
| Mostafa | G83647 | 17 June 2014 | 5701 | 2050 | 32.50 | No | 96 | 20 | 12 | 6 |
| Plumstead Health Centre | G83019 | 18 December 2014 | 5445 | 2400 | 30.50 | No | 89 | 10 | 7 | 4 |
| Royal Arsenal Medical Centre | G83016 | 3 June 2014 | 5823 | 2207 | 36.20 | No | 60 | 9 | 6 | 2 |
| Sherard Road Medical Centre | G83027 | 19 December 2014 | 9790 | 3828 | 31.30 | No | 171 | 6 | 5 | 3 |
| St Mark’s | G83039 | 26 June 2014 | 7325 | 2662 | 39.00 | No | 55 | 11 | 8 | 6 |
| Tewson Road Practice | G83007 | 25 June 2014 | 4972 | 2073 | 30.60 | No | 102 | 11 | 10 | 3 |
| Vanbrugh Group Practice | G83021 | 6 March 2014 | 9520 | 3349 | 26.20 | No | 174 | 24 | 16 | 10 |
| Waverley | G83635 | 6 November 2014 | 5253 | 2103 | 27.60 | No | 77 | 11 | 7 | 4 |
| Kingston | ||||||||||
| Brunswick Surgery | H84015 | 22 April 2014 | 6397 | 2594 | 10.40 | No | 73 | 14 | 13 | 5 |
| Central Surgery | H84030 | 5 August 2013 | 12,203 | 5130 | 10.10 | No | 163 | 51 | 47 | 26 |
| Claremont Medical Centre | H84619 | 20 June 2013 | 9823 | 3245 | 10.70 | No | 52 | 12 | 12 | 6 |
| Hook Surgery | H84025 | 16 April 2014 | 5957 | 2259 | 12.30 | Yes | 137 | 24 | 21 | 15 |
| The Groves Medical Centre | H84016 | 11 November 2013 | 11,513 | 4884 | 8.99 | No | 45 | 12 | 9 | 2 |
| Kingston Health Centre | H84061 | 17 December 2014 | 6569 | 2457 | 10.00 | No | 62 | – | 5 | 5 |
| Lambeth | ||||||||||
| Brockwell Park Surgery | G85137 | 27 November 2013 | 5841 | 1895 | 26.60 | Yes | 91 | – | 27 | 11 |
| Crown Dale Medical Centre | G85022 | 29 July 2014 | 10,813 | 4245 | 29.50 | Yes | 160 | 23 | 30 | 22 |
| Hetherington Group Practice | G85045 | 19 November 2013 | 10,094 | 3344 | 29.80 | No | 136 | – | 23 | 11 |
| The Hurley Clinic | G85053 | 27 November 2013 | 14,255 | 5235 | 31.90 | Yes | 206 | – | 41 | 19 |
| Lambeth Walk Group Practice | G85054 | 10 February 2014 | 7543 | 2988 | 34.10 | Yes | 177 | – | 26 | 12 |
| Minet Green Health Practice (Iveagh House) | G85135 | 14 January 2014 | 7711 | 2666 | 41.10 | No | 136 | 18 | 11 | 1 |
| Stockwell Group Practice | G85028 | 13 November 2013 | 13,802 | 5201 | 35.90 | Yes | 155 | 24 | 20 | 7 |
| Streatham Common Group Practice | G85014 | 11 November 2013 | 7496 | 2796 | 27.20 | Yes | 230 | – | 38 | 14 |
| Streatham Place Surgery | G85118 | 10 December 2013 | 4656 | 1710 | 33.60 | No | 54 | – | 4 | 1 |
| Lewisham | ||||||||||
| Bellingham Green Surgery | G85124 | 9 June 2014 | 7113 | 2431 | 38.90 | No | 97 | – | 12 | 6 |
| Brockley Road Surgery | G85048 | 30 October 2013 | 4561 | 1634 | 23.20 | No | 90 | – | 12 | 6 |
| Grove Medical Centre | G85085 | 26 September 2013 | 8101 | 2321 | 35.10 | Yes | 90 | 11 | 9 | 5 |
| Hilly Fields Medical Centre | G85055 | 17 October 2013 | 12,856 | 4696 | 24.90 | Yes | 225 | – | 60 | 32 |
| Honour Oak Group Practice | G85089 | 1 November 2013 | 9290 | 3093 | 30.00 | No | 143 | 10 | 7 | 2 |
| Jenner Practice | G85004 | 5 June 2014 | 15,045 | 5901 | 28.70 | No | 135 | – | 23 | 7 |
| Morden Hill Surgery | G85035 | 8 May 2014 | 8654 | 3079 | 32.10 | Yes | 112 | 20 | 19 | 11 |
| Nightingale Surgery | G85727 | 18 August 2014 | 5099 | 1628 | 26.60 | Yes | 90 | – | 16 | 11 |
| Queens Road Partnership | G85015 | 23 June 2014 | 11,163 | 4264 | 34.00 | Yes | 156 | – | 22 | 14 |
| Rushey Green GP | G85633 | 29 October 2013 | 11,332 | 3699 | 33.90 | Yes | 208 | 32 | 31 | 16 |
| South Lewisham Group Practice | G85005 | 9 October 2013 | 14,094 | 5592 | 33.30 | Yes | 250 | – | 44 | 23 |
| St John’s Medical Centre | G85038 | 29 September 2014 | 12,553 | 4621 | 29.30 | Yes | 178 | – | 27 | 16 |
| Torridon Road Medical Practice | G85032 | 11 September 2013 | 10,021 | 3982 | 24.30 | Yes | 113 | – | 30 | 11 |
| Triangle Group Practice | G85120 | 25 February 2014 | 7159 | 2665 | 31.80 | Yes | 86 | – | 12 | 8 |
| Woodlands Health Centre | G85722 | 11 July 2014 | 7091 | 2099 | 29.40 | No | 45 | – | 6 | 2 |
| Woolstone Medical Centre | G85061 | 11 June 2014 | 7133 | 2992 | 29.60 | Yes | 112 | 20 | 20 | 12 |
| Merton | ||||||||||
| Alexandra Surgery | H85656 | 3 July 2014 | 5566 | 1928 | 12.10 | No | 106 | 8 | 13 | 5 |
| Central Medical Centre | H85070 | 6 September 2013 | 8271 | 2935 | 20.90 | No | 174 | – | 5 | 2 |
| Grand Drive Surgery | H85101 | 1 October 2013 | 9065 | 3204 | 8.39 | No | 170 | – | 33 | 16 |
| Lambton Road Medical Practice | H85051 | 17 September 2013 | 6113 | 2058 | 9.11 | No | 117 | – | 18 | 8 |
| Merton Medical Practice | H85634 | 5 August 2013 | 6740 | 1687 | 16.60 | Yes | 30 | – | 9 | 6 |
| Mitcham Family Practice (Graham Road) | H85078 | 27 May 2014 | 3074 | 1202 | 21.50 | No | 79 | 10 | 5 | 2 |
| Morden Hall Medical Centre | H85037 | 10 June 2013 | 13,788 | 5123 | 17.10 | No | 138 | – | 34 | 19 |
| Riverhouse Medical Practice | H85092 | 15 May 2014 | 5621 | 1820 | 15.00 | No | 55 | 11 | 10 | 10 |
| Rowans Surgery | H85035 | 14 June 2013 | 9147 | 3468 | 21.70 | No | 43 | – | 10 | 4 |
| Wide Way Medical Centre | H85029 | 5 November 2013 | 7208 | 2541 | 21.60 | Yes | 64 | – | 12 | 7 |
| Richmond | ||||||||||
| Brockbank (Park Road) | H84002 | 5 July 2013 | 12,339 | 5553 | 8.02 | No | 94 | 26 | 20 | 11 |
| Flood (Essex House) | H84023 | 5 November 2013 | 8919 | 3682 | 11.10 | No | 118 | 28 | 24 | 12 |
| Jackson (Acorn) | H84007 | 3 July 2014 | 7950 | 3488 | 8.94 | No | 83 | – | 21 | 12 |
| O’Flynn (Hampton Wick) | H84032 | 25 July 2013 | 8835 | 3885 | 10.30 | No | 103 | 21 | 17 | 11 |
| Pennycook (Hampton Hill) | H84623 | 22 August 2013 | 8819 | 3570 | 10.10 | No | 117 | 20 | 16 | 8 |
| Southwark | ||||||||||
| Albion Street Group Practice | G85138 | 13 February 2014 | 11,970 | 3536 | 24.30 | Yes | 81 | 5 | 5 | 5 |
| Bermondsey and Lansdowne Medical Mission | G85094 | 8 May 2014 | 15,673 | 4527 | 28.80 | No | 112 | – | 17 | 9 |
| Concordia Parkside | G85030 | 7 July 2014 | 6634 | 2338 | 34.30 | No | 53 | 3 | 1 | 1 |
| Lister Primary Care Centre | G85134 | 11 December 2013 | 5824 | 1975 | 35.90 | No | 51 | 4 | 3 | 0 |
| East Street | G85721 | 17 March 2014 | 7080 | 2497 | 37.70 | Yes | 53 | 4 | 4 | 3 |
| Acorn and Gaumont | G85006 | 10 October 2013 | 11,879 | 3964 | 38.80 | Yes | 196 | 25 | 10 | 5 |
| Camberwell Green | G85013 | 28 March 2014 | 10,881 | 4042 | 33.10 | Yes | 61 | – | 11 | 6 |
| Elm Lodge Surgery | G85051 | 1 May 2014 | 7373 | 3155 | 16.40 | Yes | 96 | – | 30 | 24 |
| Hambleden Clinic | G85112 | 24 February 2014 | 3318 | 1093 | 25.00 | No | 34 | 4 | 3 | 0 |
| Lordship Lane Surgery | G85681 | 28 November 2013 | 3924 | 1383 | 26.90 | Yes | 69 | 10 | 8 | 5 |
| Melbourne Grove | G85132 | 27 June 2014 | 7158 | 2498 | 24.20 | No | 45 | 3 | 2 | 2 |
| Nexus Health Group (Manor Place) | G85034 | 18 July 2014 | 12,021 | 4003 | 34.70 | Yes | 102 | 6 | 6 | 4 |
| Nunhead Surgery | G85685 | 23 April 2014 | 7640 | 2777 | 33.30 | Yes | 130 | – | 24 | 13 |
| Old Kent Road Surgery | G85052 | 9 January 2014 | 5920 | 1869 | 36.90 | No | 57 | 6 | 5 | 1 |
| Queens Road Surgery | G85040 | 12 February 2014 | 3672 | 1519 | 38.90 | No | 56 | 3 | 2 | 1 |
| Sir John Kirk Close Surgery | G85050 | 11 July 2014 | 3894 | 1343 | 32.60 | Yes | 60 | 2 | 2 | 2 |
| Sutton | ||||||||||
| The Beeches Surgery | H85662 | 24 June 2014 | 5593 | 2671 | 9.89 | No | 46 | – | 19 | 15 |
| The GP Centre | H85019 | 25 July 2013 | 5066 | 2270 | 8.76 | No | 273 | – | 34 | 16 |
| Cheam GP Centre | H85054 | 9 June 2014 | 5065 | 2196 | 9.02 | No | 116 | 30 | 28 | 20 |
| Dr Scott and partners | H85063 | 26 July 2013 | 5088 | 2236 | 8.61 | No | 73 | – | 17 | 8 |
| The Health Centre (Robin Hood Lane) | H85095 | 21 June 2013 | 10,017 | 3528 | 15.90 | Yes | 180 | – | 26 | 12 |
| Manor Practice | H85116 | 7 June 2013 | 8583 | 3159 | 20.60 | No | 114 | – | 16 | 8 |
| Shotfield Medical Practice | H85115 | 16 July 2014 | 10,370 | 4466 | 15.20 | Yes | 209 | 52 | 50 | 28 |
| Wallington Family Practice | H85653 | 11 September 2014 | 11,081 | 4772 | 16.00 | Yes | 264 | – | 75 | 44 |
| Wandsworth | ||||||||||
| Brocklebank Group Practice | H85048 | 9 January 2014 | 16,781 | 5046 | 19.90 | Yes | 248 | 35 | 29 | 17 |
| Chartfield Surgery | Y01132 | 17 December 2013 | 11,662 | 3946 | 18.90 | Yes | 212 | 33 | 31 | 12 |
| Elborough Street Surgery | H85057 | 5 March 2014 | 6008 | 1973 | 11.30 | Yes | 120 | 31 | 31 | 10 |
| Mayfield Surgery | H85006 | 28 November 2013 | 5521 | 1874 | 29.90 | No | 139 | 20 | 16 | 4 |
| Open Door Surgery | H85087 | 17 April 2014 | 9312 | 3823 | 24.70 | No | 179 | 10 | 8 | 6 |
| Wandsworth Medical Centre | H85001 | 22 October 2013 | 14,145 | 2860 | 19.30 | Yes | 168 | 30 | 24 | 12 |
| Total | 1,154,963 | 439,100 | 17,775 | 3515 | 3183 | 1742 | ||||
Appendix 3 Behaviour change technique coding framework for the fidelity analysis
| BCT code | BCT label | BCT description | Score (0 = not delivered, 1 = delivered) | Line/page numbers (transcript) | Sample quotations | Total number and frequency |
|---|---|---|---|---|---|---|
| 1 | Provide information on the consequences (elicit-provide-elicit) | Give, or make salient, information about the risk factors of CVD and how a healthier diet and/or more PA could lower risk | There are a number of risk factors associated with CVD, some are non-modifiable which include . . . and some are modifiable which include . . .By following national guidelines of healthy eating and physical activity, you can reduce your risk of CVD and therefore your overall health | |||
| 2 | Prompt intention formation | Support patient to form a specific statement about what they intend to do this week (i.e. ‘I will . . . for X times per day/week’) | So you have identified that you would like to . . . What would you like to focus on in the week ahead?What do you intend to do this week? | |||
| 3 | Prompt barrier identification | Support patient to think in advance of the barriers to achieving their goal and how they can be managed | What barriers might occur to you achieving this goal? | |||
| 4 | Prompt specific goal-setting | Support patient to list specific behavioural actions that will support their goal. Ensures goal and action steps are realistic and meaningful. Offer ‘other’ option if prompts are not relevant | How will you go about achieving this goal?How many times a week will you do that?How realistic do you think this goal is? | |||
| 5 | Prompt review of behavioural goals | Use neutral, open questions to review past progress with goals set in previous sessions | I am interested to hear how you got on with your goals set in our last sessionPlease tell me how you got on . . . | |||
| 6 | Prompt self-monitoring of behaviour | Support patient to use diary sheet and e-monitoring to record progress | I would encourage you to use this diary to monitor how you get on with your goal by ticking each day that you achieve itI would encourage you to use the pedometer to keep track of your number of steps | |||
| 7 | Teach to use prompts or cues | Support patient to use reminder prompts | What might help to remind you? | |||
| 8 | Agree on behavioural contract | Support patient to complete action plan and talk through with HLF or another group member | Let’s complete this action plan togetherWould you like to complete this action plan? | |||
| 9 | Plan social support or social change | Support patient to consider who can support them at home. If no one, suggest buddying | Who might be able to support you with this goal, e.g. someone at home or a friend or another group member? | |||
| 10 | Prompt self-talk | Explain/elicit CBT lapse–relapse model. Support patient to find their own cheerleading statements when they have a setback | One way of helping to prevent a relapse is to think of some cheerleading statements you can say to yourself when you have a setback, what might these be for you? | |||
| 11 | Relapse prevention | Elicit common barriers to diet/exercise changes and ways to manage/minimise. Support patient to identify lapse triggers and ‘if . . . then’ statements | What situations would you be most likely to relapse?If that occurs, let’s think about what you could do now to avoid a relapse | |||
| Comments | Please list any necessary comments or points of clarification | |||||
Appendix 4 Participant focus group topic guides
Focus group 1: participants who received six or more intervention sessions
Invitation/information received
Our first set of questions are about the invitation you received from your GP informing you of the study and inviting you to take part. This letter would have informed you of your high risk of heart disease, who was running the study and the motivational interviewing that would be used. In order to participate you would have had to send back a reply slip expressing your interest.
Q1) What made you volunteer to take part?
How did the following influence your decision to take part?
-
Finding out that you were at high risk of heart disease (according to the NHS screening)
-
Potential health benefits of a healthy lifestyle intervention
-
Potential for helping others prevent heart disease in the future
-
The researcher appointment, which included clinical tests and questionnaires about your lifestyle.
Sessions
You would have received sessions with a healthy lifestyle facilitator. You may have had different experiences of this – different HLFs, different number of sessions, different types of session (one-to-one or in a group). We want to hear about all of your thoughts and experiences. The weekly sessions consisted of an introduction to the concept of heart disease and its risk factors, followed by two sessions on exercise, two on diet and two on maintaining healthy habits. After which there were follow-up meetings once every 3 months.
Q2) Did you change any aspects of your lifestyle as a result of the treatment?
-
If there were changes, could you give some examples?
-
If there were no changes, why do you think that was?
Q3) How did the treatment impact on your lifestyle?
-
Could you tell us more about how effective you thought the following aspects of the intervention were in helping you change your lifestyle?
-
Content of sessions (information given, tools, workbook)
-
Format/structure (length, number, spacing of sessions)
-
Behaviour change techniques (action plans, reminders, talking about setbacks)
-
Conversation style (including relationship with HLF)
-
Social support (other group members, family and friends).
-
Q4) The study aimed to invite a diverse range of people from different cultural, social and ethnic minority backgrounds. Based on the treatment you received, what aspects of the treatment made it suitable or unsuitable for a wide range of populations to participate?
Were any of the following aspects of the treatment a help or a hindrance?
-
The materials used.
-
What was it about the HLF that helped/did not help?
-
Did you feel you had something in common with the HLF?
-
Or the opposite, having nothing in common (e.g. did not come from your world/culture/age group).
-
-
The other members of your group (if applicable).
-
Did you feel you had something in common with the other group members?
-
Or the opposite, having nothing in common (e.g. did not come from your world/culture/age group).
-
Q5) Is there anything I have not mentioned that you would like to add?
-
Is there anything you would change about MOVE IT?
Focus group 2: participants who received fewer than two intervention sessions
Invitation/information received
Our first set of questions are about the invitation you received from your GP informing you of the study and inviting you to take part. This letter would have informed you of your high risk of heart disease, who was running the study and the motivational interviewing that would be used. In order to participate you would have had to send back a reply slip expressing your interest.
Q1) What made you volunteer to take part?
How did the following influence your decision to take part?
-
Finding out that you were at high risk of heart disease (according to the NHS screening)
-
Potential health benefits of a healthy lifestyle intervention
-
Potential for helping others prevent heart disease in the future
-
The researcher appointment, which included clinical tests and questionnaires about your lifestyle.
Sessions
You were allocated to receive either one-to-one or individual sessions with a healthy lifestyle facilitator. However, you either did not attend any sessions or attended only the first session. We think that it is important for us to find out why this may be.
Q2) What were the key reasons that made you drop out?
Examples of reasons we thought some people might drop out are:
-
Lack of time to take part. If time was an issue for you, can you tell us more specifically about what resulted in time being a problem?
-
The MOVE IT workbook/documents you may have received from the HLF in session 0; if this was a reason for dropping out, can you tell us what things you didn’t like?
-
Participants not necessarily feeling that the treatment relates to them or would not be useful. If this influenced your reason for dropping out, can you tell us what aspects of the treatment you felt did not relate to you?
-
The allocation (group or individual) you were assigned to. Would you have been more likely to take part if allocated to the other condition?
-
The duration of each session, the number of sessions and the intervention duration as a whole (12-month period)? If this relates to you, can you tell us more about how this affected your participation?
-
Ill health or illness within your family. If this relates to you, can you tell us more about how this affected your participation?
-
Not wanting to share personal information, with either the HLF or other group members. If this relates to you, can you tell us more about how this affected your participation?
-
Can you tell us more about what influenced your decision to drop out?
Q3) The study aimed to invite a diverse range of people from different cultural, social and ethnic minority backgrounds. Based on the treatment you received, what aspects of the treatment made it suitable or unsuitable for a wide range of populations to participate?
Were any of the following aspects of the treatment a help or a hindrance?
-
The materials used.
-
What was it about the HLF that helped/did not help?
-
Did you feel you had something in common with the HLF?
-
-
Or the opposite, having nothing in common (e.g. did not come from your world/culture/age group).
-
The other members of your group (if applicable).
-
Did you feel you had something in common with the other group members?
-
Or the opposite, having nothing in common (e.g. did not come from your world/culture/age group).
Q4) What would have helped you to continue with the treatment?
Q5) Is there anything I have not mentioned that you would like to add?
Appendix 5 Characteristics of focus group attendees
| Group and participant | Borough | Trial arm | Number of sessions attendeda | Age (years) | Sex | Ethnicity | Deprivation quintileb | Educational attainment | Relationship status | Occupational status | QRISK2 score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | |||||||||||
| P1 | Bexley | Group | 10 | 74 | Male | White | 3 | No formal qualifications | Married | Retired | 19.9 |
| P2 | Bromley | Individual | 9 | 75 | Female | White | 2 | A level or above | Divorced | Retired | 22.7 |
| P3 | Lewisham | Individual | 10 | 70 | Male | White | 2 | A level or above | Married | Retired | 20.0 |
| P4 | Croydon | Individual | 10 | 72 | Female | White | 5 | A level or above | Married | Retired | 21.3 |
| P5 | Croydon | Group | 6 | 69 | Male | Asian | 2 | A level or above | Married | Employed | 22.3 |
| P6 | Sutton | Individual | 10 | 69 | Male | Asian | 4 | A level or above | Married | Employed | 20.4 |
| P7 | Merton | Individual | 10 | 73 | Male | White | 5 | A level or above | Married | Retired | 20.4 |
| 1B | |||||||||||
| P8 | Bromley | Individual | 7 | 76 | Male | White | 4 | No formal qualifications | Married | Retired | 20.5 |
| P9 | Croydon | Individual | 8 | 71 | Male | Mixed | 4 | O level/GCSE | Married | Retired | 19.4 |
| P10 | Kingston | Group | 7 | 68 | Female | Asian | 5 | A level or above | Married | Retired | 22.1 |
| 1C | |||||||||||
| P11 | Bromley | Group | 7 | 73 | Female | Asian | 4 | O level/GCSE | Married | Retired | 22.9 |
| P12 | Merton | Group | 8 | 76 | Male | White | 4 | No formal qualifications | Widowed | Retired | 21.4 |
| P13 | Lambeth | Group | 8 | 73 | Male | White | 3 | A level or above | Married | Retired | 18.7 |
| P14 | Lambeth | Individual | 10 | 76 | Male | Asian | 3 | A level or above | Married | Retired | 39.9 |
| 1D | |||||||||||
| P15 | Bexley | Individual | 10 | 74 | Female | White | 2 | A level or above | Married | Retired | 28.9 |
| P16 | Bexley | Group | 10 | 69 | Male | White | 4 | O level/GCSE | Married | Retired | 20.3 |
| P17 | Bromley | Individual | 10 | 75 | Female | White | 5 | O level/GCSE | Divorced | Retired | 21.3 |
| P18 | Southwark | Individual | 10 | 67 | Male | White | 1 | No formal qualifications | Married | Retired | 22.0 |
| P19 | Croydon | Individual | 6 | 73 | Female | White | 2 | O level/GCSE | Single | Retired | 26.4 |
| P20 | Croydon | Individual | 10 | 71 | Male | White | 5 | A level or above | Married | Employed | 25.1 |
| 2A | |||||||||||
| P21 | Richmond | Group | 1 | 71 | Male | White | 5 | A level or above | Married | Retired | 21.6 |
| P22 | Sutton | Individual | 2 | 71 | Male | Asian | 5 | A level or above | Married | Retired | 33.4 |
| P23 | Kingston | Group | 0 | 66 | Male | White | 5 | A level or above | Married | Employed | 36.6 |
| 2B | |||||||||||
| P24 | Southwark | Individual | 0 | 74 | Male | White | 3 | A level or above | Cohabiting | Employed | 21.2 |
| P25 | Merton | Group | 1 | 71 | Male | Asian | 4 | A level or above | Married | Retired | 32.6 |
| P26 | Sutton | Group | 0 | 63 | Male | White | 5 | A level or above | Divorced | Employed | 22.8 |
Appendix 6 Health-care professional feedback topic guides
Healthy lifestyle facilitator interview topic guides
**Some content has not been reproduced here**
Training
You attended the training and supervision at King’s.
-
What were your views on the supervision?
-
Can you tell me any ways in which you think the supervision could have been improved?
-
Have you had any previous supervision in facilitating psychological therapies?
Supervision
-
What are your views about the supervision you received throughout the study?
-
What are your views about the support you received from the research team in general?
-
How did other members of staff at your practice feel about you taking part in the study?
-
Were they supportive/not supportive? How so?
-
-
What would have made it easier for you to participate?
Intervention
Now I’d like to ask you about delivering the intervention.
-
What went well, could you give me some examples?
-
What went less well?
-
How did you find the sessions with patients?
-
How did you find the length of the sessions?
-
-
What are your views about the admin time involved in the study?
Self-awareness
-
How confident did you feel about delivering the group intervention?
-
How confident did you feel delivering the individual intervention?
-
How confident did you feel about managing the caseload?
Patients
-
Can you tell me about any difficulties you experienced in getting patients to attend the sessions?
-
Was there any difference in response between the individual and group sessions?
-
Which method (group or individual) do you feel is more effective?
-
Did you book the patients in yourself?
-
-
What were the challenges that you faced in using CBT skills with these patients?
-
What other challenges were there when delivering the intervention?
Clinical psychologist interview topic guides
Training
You delivered the training and supervision to the HLFs.
-
What were your views on the supervision?
-
HLF engagement?
-
Frequency?
-
Length?
-
-
Can you tell me about any problems you experienced with the supervision?
-
What would have made it easier for you?
-
What were the challenges that you faced when teaching CBT skills to the HLFs?
-
-
Can you tell me any ways in which you think the supervision could have been improved?
-
Have you had any previous experience in facilitating psychological therapies?
-
What are your views about the support you received from the trial manager and your team (NDZ) in general?
-
Were they supportive/not supportive? How so?
-
What would have made it easier for you to participate more?
-
Supervision (fortnightly)
What are your views about the supervision you have delivered throughout the study?
-
Can you tell me about any difficulties you experienced in getting HLFs to attend the supervision?
-
Do the HLFs find it useful/helpful?
-
Have you had any negative feedback about the supervision from the HLFs, if so could you give me some examples?
-
Have you had any positive feedback about the supervision from the HLFs, if so could you give me some examples?
Intervention
Now I’d like to ask you about your views on delivery of the intervention. You sometimes shadow the HLFs at their surgeries, from your perspective:
-
What goes well, could you give me some examples?
-
What goes less well, could you give me some examples?
-
What are your views about the HLF admin time involved in the study?
-
Which method (group or individual) do you feel is more effective?
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AOR
- adjusted odds ratio
- AUDIT
- Alcohol Use Disorders Identification Test
- BCT
- behaviour change technique
- BMI
- body mass index
- CACE
- complier average causal effect
- CBT
- cognitive–behavioural therapy
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CP
- clinical psychologist
- CSRI
- Client Service Receipt Inventory
- CVD
- cardiovascular disease
- DHSC
- Department of Health and Social Care
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HDL
- high-density lipoprotein
- HLF
- healthy lifestyle facilitator
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IT
- information technology
- ITT
- intention to treat
- LDL
- low-density lipoprotein
- MCD
- minimum clinically significant difference
- MI
- motivational interviewing
- MITI
- Motivational Interviewing Treatment Integrity
- MOVE IT
- enhanced MOtiVational intErviewing InTervention
- MVPA
- moderate to vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PA
- physical activity
- PHQ-9
- Patient Health Questionnaire-9 items
- PI
- principal investigator
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- SLHIEC
- South London Health Innovation and Education Cluster
- SMS
- short message service
- TSC
- Trial Steering Committee
- UC
- usual care
Notes
-
Additional data and results for Chapter 5 (main clinical results of the trial)
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hta/106203/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
