Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/44/03. The contractual start date was in April 2010. The draft report began editorial review in October 2018 and was accepted for publication in April 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Elaine McColl was a member of the National Institute for Health Research (NIHR) Journals Library Editorial Group from 2013 to 2016; her employer was remunerated for her work in this regard. Luke Vale was a member of the NIHR Health Technology Assessment (HTA) Clinical Trials Board for a period of time between the years 2014 and 2018. Nicola PT Innes received a grant from The 3M Company (Maplewood, MN, USA)/ESPE to support a Clinical Fellowship in 2000; the work investigated the Hall Technique, which forms an aspect of one arm of the randomised controlled trial reported in this work. The results of the trial were reported independently.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Maguire et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Scientific background
Dental caries (decay) is the most common disease of childhood, and carries a large health and economic impact for the UK. 1–4 Treating oral disease is expensive, costing NHS England £3.4B annually. 5 Extraction of decayed primary teeth alone, carried out under general anaesthesia, costs an estimated £36M annually.
In the UK, the majority of dental care for children takes place in primary care and is carried out by general dental practitioners (GDPs) rather than by specialists. GDPs’ remuneration under the general dental service (GDS) funding system varies depending on the nation. In Scotland, a capitation and fee per item of service system operates, whereas in England and Wales, GDPs claim ‘units of dental activity’ (UDAs) to treat dental patients. Against this background, there is a long-standing debate about the best way of managing decay in children’s primary teeth.
In 2002, the results of two studies6,7 questioned the success of conventional restorative interventions [placing local anaesthesia (injections), removal of carious lesions (decay) and placement of a restoration (filling)] carried out in UK primary dental care practices in preventing pain and infection in children with decayed primary teeth. This apparent lack of effective management has caused considerable uncertainty for the dental profession and for patients and parents. There is universal agreement that guidance for the effective practice-based prevention and management of dental caries is needed, with that evidence drawn from primary dental care. This prompted the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) panel to commission the research that resulted in the Filling Children's Teeth: Indicated Or Not? (FiCTION) trial, a three-arm participant-randomised controlled trial (RCT) investigating three approaches to dental care for children with carious lesions in their primary teeth:
-
Conventional treatment with best-practice prevention (C+P) arm. Based on what has been considered standard practice for the management of carious lesions for > 50 years, this comprises the use of local anaesthetic, complete removal of carious tissue using rotary instruments and placing a restoration.
-
Biological treatment with best-practice prevention (B+P) arm. Sealing-in carious lesions is a minimally invasive approach, based on recent understanding that carious tooth tissue does not need to be completely removed to stop disease progression, and uses a variety of sealing techniques, including restorative materials or crowns, to seal the carious lesion and prevent progression.
-
Best-practice prevention alone (PA) arm. Avoiding restorative intervention, the four ‘pillars’ or components of prevention are promoted to arrest existing carious lesions and prevent any more from developing: (1) reducing frequency of sugars intake, (2) effective twice-daily brushing with fluoridated toothpaste (steps 1 and 2 involve behaviour change by the parents/guardians and children to reduce the cariogenic challenge), (3) application of topical fluoride varnishes and (4) placement of fissure sealants on first permanent molar (FPM) teeth.
Dental caries (decay) experiences of children (globally and in the UK)
Dental caries remains an important public health problem globally,8 recognised by the World Health Organization as a disease that affects 60–90% of school children and the vast majority of adults, contributing to extensive loss of natural teeth in older people. 9,10
The social gradient in health inequity means that the poorest have the worst health,11 and there is strong evidence relating oral health (especially dental caries) and socioeconomic status; children in lower socioeconomic groups are disproportionately affected by the disease, which has a linear relationship with poverty. 12 Disease levels vary starkly in the UK, with 56% of 5-year-old children in Blackburn and Darwen but only 4% of children in less deprived South Gloucester showing visible carious lesions. 13 In England, dental caries remains the primary reason for children’s admissions to hospital. 1 In 2013/14, a total of 62,747 children and young people were admitted to hospital in England, Scotland and Wales with a diagnosis of dental caries; the most common age group was 5–9 years. 1–3
Dental caries and its consequences are not limited to young children or the primary dentition. There is evidence surrounding the trajectory of disease, showing that children who experience carious lesions in their primary dentition carry a much greater burden of disease into adolescence and beyond. 14 Dental caries also affects one in three UK 12-year-olds, is positively associated with deprivation15 and is experienced by almost half of those aged 12–15 years in deprived areas. In 2013 in England, 32% of 12-year-olds experienced dental caries and required treatment, ranging from 46% of those eligible for free school meals to 30% of those ineligible. 16
Changing strategies for management of carious lesions in primary teeth
Until recently, dental caries was considered an infectious disease. As a result, treatment required removal of all bacteria, biofilm and affected tooth tissue before restoration. However, the disease is now understood to result from an ecological shift in the varying proportions of different commensal bacteria, resulting in a dysbiotic microflora in the dental biofilm. 17 Acidogenic, aciduric and cariogenic bacteria are highly competitive within the biofilm and, where there is a frequent supply of fermentable (dietary) carbohydrates, conditions develop that favour them, supporting their domination of the biofilm ecology. This ecological shift in biofilm composition leads to a concurrent shift in bacterial activity, with long periods of acidity and net mineral loss from the dental enamel and dentine resulting in the carious lesion. 18
This more recent view of the disease has allowed a rationale for the development of alternative treatments, rather than the traditional complete surgical excision (drill-and-fill) model. These alternative treatments involve controlling precipitating factors for the disease, through carbohydrate restriction (diet change to reduce sugars intake), biofilm removal (through tooth-brushing), promoting tooth tissue remineralisation (through professional- and home-applied fluoride) and biofilm sealing from substrate (using restorative techniques). 19 A resultant shift away from cariogenic- to non-cariogenic biofilm promotes remineralisation, inactivates the disease process and preserves tooth tissue. This minimal intervention dentistry approach20 follows the contemporary tenet of quaternary prevention. In addition, it avoids, or slows down, the cycle of re-restoration21 and is less invasive for patients.
The evidence for effective management of carious lesions in primary teeth
Teaching in UK dental schools has been based on guidance from the British Society of Paediatric Dentistry (BSPD). Until recently, the BSPD recommended that the optimum treatment of carious lesions in primary teeth is complete removal of the carious tissue, followed by the placement of a conventional restoration (filling) to repair lost tooth tissue. 22,23 However, these recommendations were largely based on evidence from studies conducted in either a secondary care or a specialist paediatric dental practice setting,24 rather than the primary dental care environment, where the vast majority of children are treated. More recently, the Scottish Dental Clinical Effectiveness Programme (SDCEP) has recognised the growing evidence for minimally invasive, biologically based approaches to carious lesion management and has developed national guidance for the management of caries in children,25 which is based on this approach.
Despite long-standing teaching in paediatric dentistry and high-quality guidance for primary dental care in the UK, the proportion of primary teeth with visible carious lesions that are restored (the Care Index) remains low. In Scotland it is 13%,26 whereas in England it is 14%. 27 The perceived ineffectiveness of the traditional ‘drill-and-fill’ methods of managing decayed primary teeth has been hypothesised as one reason why this approach is unpopular with GDPs. 28
Evidence from conventional/traditional approaches to managing carious lesions
Traditional approaches to carious lesion management involve complete carious tissue removal and placement of a restoration. However, a myriad of treatment options are available to dentists when they are deciding how to restore carious primary molars. Clinical decisions are required with respect to how much carious tooth tissue to remove, given the changing face of cariology, as well as which material to use to restore the tooth, in the light of the ongoing advances in dental materials. Restorations provided in specialist clinical environments can be effective, with studies conducted in secondary care showing high success rates for restorations in primary teeth;29,30 subsequent guidance has largely been based on this research. However, both the research quality and its generalisability to a primary dental setting is limited7,31 and a closer look at the evidence shows less clarity, because a limitation in its interpretation is the lack of an agreed core outcome set. 32,33 Cariology studies use a variety of different outcomes and different outcomes measures, making it often impossible to synthesise or directly compare materials and techniques. Few studies have measured caries-related pain and infection as an outcome when comparing different treatment approaches and fewer still have compared conventional (complete) carious lesion removal and restoration approaches with other approaches to managing carious lesions. Two RCTs have compared conventional restorative techniques with other approaches and recorded pain and/or infection as an outcome. One trial,34 a split-mouth RCT set in general dental practice, involved 18 GDPs in Scotland and compared the Hall Technique35 (a biological approach in which carious lesions are sealed in) with conventional restorations. Children, parents and dentists preferred the Hall Technique, and it outperformed the conventional restorations, with the 2-year follow-up showing that participants experienced pain and/or infection in 2% of teeth treated with the Hall Technique and in 15% of teeth treated with conventional restorations. 34 The relatively high failure rates for conventional restorations (as defined by occurrence of pain and/or infection) continued at the 5-year follow-up, with 3% failure for the Hall Technique and 17% for conventional restorations. 36 In terms of restoration longevity, the Hall Technique showed a 5% failure rate at 5 years, compared with 42% for conventional restorations. The lack of effectiveness for conventional restorations in multisurface lesions in primary teeth was replicated in a second study in Germany, in which specialists and experienced postgraduate trainees carried out treatment in a secondary care environment. 37 Conventional restorations were compared with the Hall Technique and a non-restorative cavity control (NRCC) approach (using a prevention alone approach with brushing of carious lesions to arrest them) for management of multisurface cavities [International Caries Detection and Assessment System (ICDAS) codes 3 to 5]. After 2.5 years, participants in the conventional and NRCC arms experienced pain and infection in 9% of teeth whereas participants treated with the Hall Technique experienced pain and infection in 2.5% of teeth.
Evidence from non-destructive/minimally invasive approaches to managing carious lesions
Managing carious lesions using a biological approach is based on evidence that began to emerge in the 1990s, which increasingly supports the idea that carious tissue does not need to be completely removed38 to achieve success in the management of decay. There are advantages to this minimally invasive approach: less tooth tissue is lost, there is less likelihood of irreversible damage to the dental pulp and children tolerate it more easily as there is less use of injections and rotary instruments. These biological approaches include sealing-in a carious lesion with preformed metal crowns (PMCs) using the Hall Technique and sealing in carious lesions under a fissure sealant material.
These minimally invasive strategies and their evidence are summarised in the following sections.
Selective carious lesion removal
Selective removal of carious dentine is carried out to avoid damage to the dental pulp resulting in ingress of oral bacteria and pulpal death. A Cochrane review29 compared complete with selective caries removal strategies and found that selective excavation of carious lesions (sealing some of the decay in the tooth rather than drilling it all out) was effective; in symptomless, vital, primary or permanent teeth this approach reduced the incidence of pulp exposure. Selective carious tissue removal is therefore advocated for primary teeth with the definitive restoration placed at the first visit.
Atraumatic restorative treatment
Atraumatic restorative treatment (ART) comprises removal of softened carious tooth structure with dental excavators and then placement of adhesive materials, usually glass ionomer cement (GIC), to restore cavities, pits and fissures. 39 ART is generally accepted as being less anxiety-provoking than conventional restorative techniques. 20,40,41 However, a recent Cochrane systematic review42 has cast some doubt on earlier conclusions regarding restoration longevity. The review included 11 trials on primary teeth (children aged 3–13 years) comparing ART with conventional restorations and found an odds ratio for restoration failure of 1.60 [95% confidence interval (CI) 1.13 to 2.27] for ART compared with conventional restorations over a follow-up period of 12–24 months in 643 participants. No evidence was found of a difference in cavity types, whether single or multiple surface. Only one study,43 with 40 participants, reported on pain (children aged 4–7 years) and found that children whose teeth were treated with ART reported less pain (on the Wong–Baker FACES® Pain Rating Scale44) than those treated with conventional restorations, with a mean difference of –0.65 (95% CI –1.38 to 0.07).
No carious tissue removal and no restoration (non-restorative cavity control)
This option was initially considered for lesions with no viable repair option using sealing-in methods, either because the lesion or cavity is too extensive to repair or because the child has limited ability to tolerate treatment. In these cases, tooth-brushing and remineralisation strategies remove the biofilm and arrest the decay, maintaining the tooth and avoiding an extraction. NRCC relies on frequent, effective tooth-brushing to physically debride tooth tissue and disrupt the ecology and composition of the cariogenic plaque biofilm, causing it to shift to a more balanced, non-cariogenic and healthy state. 45 The aim is to minimise the time that any substrate and cariogenic microorganisms are in contact with a susceptible tooth surface. NRCC as a carious lesion management strategy relies heavily on a child’s parents/guardians to change behaviour and adopt effective preventative strategies. Moreover, although biofilm removal with a toothbrush is theoretically possible, gaining adequate access to a lesion to scrub it effectively enough to remove the biofilm is challenging. This problem is compounded when trying to remove biofilm from dentine when it is embedded in the exposed collagen matrix. The RCT37 that found a 29% failure rate for NRCC after 2.5 years in the hands of specialists in secondary care and a prospective observational study in a community dental setting46 seem to confirm the purported difficulties found with NRCC.
No carious tissue removal and restoration with a crown using the Hall Technique
Several observational studies47,48 and RCTs with short-term follow-up49,50 support this technique but the strongest evidence comes from two RCTs with long-term follow-up (see Evidence from conventional/traditional approaches to managing carious lesions). The first RCT, by Innes et al. ,34,36 supported the use of the Hall Technique in primary dental care; however, the practitioners involved were from a single geographical area, limiting the generalisability of the findings. The second hospital-based RCT51 in Germany found lower failure rates (based on longevity of restoration and pain/infection) after 1 year with the Hall Technique (0%) than with conventional restorations (9%) or prevention alone with NRCC (8%); after 2.5 years, the respective failure rates were 7%, 33% and 29%. 37
The high success rate of the Hall Technique compared with conventional restorations is also evident in secondary care and private practice. A recent systematic review and meta-analysis52 of adhesive restorations in primary molars found that the mean survival times of restorations range from 20 to 42 months, with composite resin, compomer and resin-modified glass ionomer (RMGI) performing similarly. A Cochrane review30 that included the Hall Technique supported this, concluding that crowns placed on primary molar teeth, regardless of technique, were likely to reduce pain and infection compared with fillings and that crowns placed using the Hall Technique reduced discomfort at the time of crown fitting.
Fissure sealants applied over non-cavitated carious lesions
Fissure sealants (low viscosity, unfilled resins) successfully prevent carious lesions occurring on the occlusal (biting) surfaces of teeth. 53 They are also effective at stopping the progression of carious lesions54,55 in permanent teeth, as they prevent lesion access to the carbohydrate that is necessary for the biofilm to thrive and stay actively cariogenic. 56,57 Although the pathophysiological evidence supports the efficacy of sealing-in to slow or stop progression of carious lesions, the clinical evidence for fissure sealants shows mixed success in permanent teeth. Fissure sealant materials wear and fracture and regularly need to be replaced and, therefore, require continual observation to ensure that the seal is maintained. Similarly, for primary teeth, there is little direct evidence to inform recommendations for the size of lesion that can be sealed or the best sealant material to use. 58
Evidence from Good Practice Prevention approaches to managing caries
The four main approaches to preventing dental caries are well established and represent the four ‘pillars of prevention’. These are:
-
dietary investigation, analysis and intervention to reduce fermentable carbohydrates in the diet
-
tooth-brushing twice daily with a fluoridated toothpaste
-
fissure sealants for permanent teeth
-
topical fluoride varnish applied to primary and permanent teeth by a dental professional.
There is substantial and high-quality evidence from Cochrane reviews on the effectiveness of tooth-brushing,59 fissure sealants60 and fluoride varnish59 in preventing dental caries. However, the evidence base supporting the effectiveness of behaviour change to enact fluoride use at home (tooth-brushing with fluoride toothpaste) and of dietary change to reduce the intake of fermentable carbohydrates (primarily sugars) is more tenuous. 61
Pain and dental infection (sepsis)
Although a highly prevalent condition, the impact of childhood dental caries is often under-appreciated, as the disease itself is rarely life-threatening or overtly limiting on daily activities. However, the consequences of the disease, including pain62, interference with sleep and reduced school attendance,63 can have significant effects on children’s daily lives. In 2013, 6% of 12-year-olds and 3% of 15-year-olds reported difficulty with schoolwork because of the condition of their teeth and mouth over the previous 3 months. 16 Dental caries can also affect the general health and quality of life (QoL) of children, impairing growth and cognitive development,64 as well as interfering with nutritional status,63 and may even have an effect on attainment. 65
Estimates of pain and infection from dental caries in children are difficult to establish. However, the 2016 National Dental Inspection Programme26 epidemiological study in Scotland, in which 86% of Primary 1 children were inspected (at approximately 5 years of age), reported that 7.5% of the children examined had a dental status that required them to be issued with a letter indicating that they ‘should seek immediate dental care on account of severe decay or abscess’.
One of the difficulties with measuring pain and infection in young children is their limited ability to communicate. Young children do not find it easy to describe and report pain. 66 Cognitively, the ability to understand pain and differentiate a chronic pain from the absence of pain is a complex phenomenon. Young children who grow up with pain from an early age may not realise that this is not normal. When they do realise they have pain, it can be difficult to have them describe this precisely to help with reaching an accurate diagnosis.
Dental caries incidence
Since the turn of the millennium, it has been widely acknowledged that the commonly used threshold for the measurement of dental caries in clinical trials is no longer appropriate, because this threshold represents disease that is already well advanced (into dentine). 67,68 The National Institutes of Health (US) (NIH) Consensus Development Conference in Bethesda, MD, USA, in 200167 identified the benefits of including the recording of early dental caries in clinical trials, stating ‘There was a paradigm shift in the management of dental caries toward improved diagnosis of early non-cavitated lesions and treatment for prevention and arrest of such lesions’. In addition, the panel recommended that ‘clinical trials of established and new treatment methods . . . should conform to contemporary standards of design implementation, analysis and reporting’. Many technical adjuncts to carious lesion detection and diagnosis have become available over the years,69 but their use is not widespread in clinical dentistry and they remain, primarily, a clinical research tool.
The most widely used adjunct for carious lesion detection, which is in general use in clinical dentistry, is radiographs. The mandatory use of radiographs as part of a clinical dental research trial is universally agreed as unethical. However, they are recommended at risk-based intervals in UK guidelines. 70 Nevertheless, it is clear from the literature,71 and was highlighted in the FiCTION pilot trial and feasibility study,72,73 that radiograph use in children does not follow the expected frequency.
Child- and parent-reported outcomes: oral health-related quality of life
The value of patient-reported observations on dental treatment experiences is becoming widely accepted and patients’ perspectives are seen as valuable. Patient-reported outcome measures (PROMs) are now included in the vast majority of trials to evaluate changes in the impact of the condition following treatment from the patient’s perspective.
Many PROMs have been produced to measure oral health-related quality of life (OHRQoL), which was defined by Locker and Allen74 as ‘the impact of oral diseases and disorders on aspects of everyday life that a patient or person values, that are of sufficient magnitude, in terms of frequency, severity or duration to affect their experience and perception of their life overall’. Most existing measures of OHRQoL in children, including the Child Perceptions Questionnaire (CPQ), the Child – Oral Impacts on Daily Performances (C-OIDP) index and the Child – Oral Health Impact Profile (C-OHIP), are designed to cover a variety of oral conditions, such as dental caries, malocclusion and craniofacial anomalies. Although these measures have been developed for children to self-report, for young children (< 8 years of age) most studies rely on parental reports of the impact of their child’s health on their daily lives. 75
Child- and parent-reported outcomes: dental anxiety and worry
The prevalence of child dental anxiety, across Europe, ranges from 3% to 21%,76–78 with 14% of children experiencing extreme dental anxiety. 78 These findings suggest a continuum of child dental fear across populations of children, including those who attend for dental treatment. 79
The literature suggests that younger preschool children may find any form of dental intervention frightening; consequently, they may be disruptive during treatment. 80 For the younger, preschool child, it is not the actual dental intervention or seriousness of the treatment that is important, but the imaginings or fantasies stirred up by it. 81 These imaginings include feeling helpless, having to submit passively to treatment, fear of pain and being separated from their parent. Similar observations are made for children who experience dental pain and for those who suffer from high dental anxiety. 79
With psychological development, the once fearful and disruptive preschool child usually becomes able to manage dental treatment. 80 Therefore, it is important when investigating different dental treatment modalities, for preschool and primary school-aged children, to assess child dental anxiety and to examine how dental anxiety status changes with chronological age. Different dental treatment modalities may also influence children’s dental fear, and some may be more appropriate for children with dental anxiety. Therefore, it is important to assess not only dental trait anxiety (anxiety that is more stable) but also dental state anxiety (anxiety that is associated with specific situations) to assess the effect of the treatment on the dental anxiety experienced by the child patient during and after treatment.
In summary, to assess child dental anxiety in the FiCTION trial, to demonstrate how this affect changed with the experience of pain and chronological age and to identify the most appropriate treatment modality to treat carious lesions in the dentally anxious child, state measures of dental fear were chosen. The justification for choice of measures to assess child dental anxiety is described in Chapter 2, Child- and parent-reported outcomes: participant dental anxiety, worry and discomfort during dental treatment.
Economic analysis to determine the cost-effectiveness of different treatment approaches
(See Chapter 2, Cost-effectiveness of managing dental decay in primary teeth, and Chapter 4). Dental care for dental caries has high direct costs for the NHS;82 therefore, we need to ensure that health benefits are being maximised within the budget. An economic evaluation was conducted alongside the FiCTION trial to determine the cost-effectiveness of the different treatment approaches. These results can be used to facilitate efficient resource allocation for managing carious lesions in primary teeth.
Acceptability and associated experiences of the FiCTION trial arms for children, parents/guardians and dental professionals
Dental treatments vary in their degree of acceptability to patients. The more acceptable a treatment is to the patient, parent or care-deliverer, the more likely it is to be delivered and received. The value of children’s opinions on their dental treatment experiences has become more widely accepted and their perspectives are seen as credible. Although a vast body of research has compared different types of treatment to manage carious lesions, most of the research has focused on outcomes related to choice and characteristics of dental materials;33 very little research has included the preferences or perspectives of participants as outcomes.
Rationale for the FiCTION trial
As a pragmatic, parallel-group, patient-RCT in general dental practice, the aim of the FiCTION trial was to provide evidence for the most clinically effective and cost-effective approach to managing caries in children’s primary teeth in primary dental care. Such evidence would remove persistent uncertainty among dental practitioners when treating and managing carious lesions in children’s primary teeth and support dental practitioners in treatment decision-making for child patients to minimise pain and infection in primary teeth.
The implication of this research is likely to be a change in policy for service and education in the NHS and beyond.
As described earlier (see Scientific background), there is growing evidence in favour of less invasive management of dental decay, informed by the principles of minimal intervention dentistry83 and based around sealing-in carious lesions or removing biofilms and supporting remineralisation strategies with a NRCC approach. The natural separation of these interventions, therefore, into conventional management, less invasive sealing management options and non-restorative management options provided the basis for the three arms of the FiCTION RCT.
Design and key findings of the FiCTION trial pilot phase
The FiCTION trial was delivered in two phases: Phase I comprised a pilot trial, qualitative study72 and feasibility survey84 and Phase II was the FiCTION main trial. Phase II was dependent on the success and recommendations of Phase I.
As planned, purposively selected primary dental care practices in Dundee, Newcastle and Sheffield were recruited to the pilot trial. The target patient participant recruitment figure was 200 children, aged 3–7 years, with carious lesions into dentine in primary molar teeth. Once recruited, participants were randomised to a three-arm pilot trial with allocation to the caries treatment strategies described in Scientific background. Trial procedures mirrored those planned for employment in the FiCTION main trial, with the exception that follow-up was for 6 months only.
Appendix 1 presents the 26 recommendations from the pilot phase of the FiCTION trial, made to inform Phase II, the main FiCTION trial.
In summary, the experience of undertaking the FiCTION pilot trial and the feasibility study [HTA number 07/44/03, Newcastle Clinical Trials Unit (NCTU) number FS77044005] resulted in minor refinements to the design and conduct of the main trial, including changes to the presentation of parent and child information and streamlining of the recruiting process. This preparatory work enhanced our confidence in being able to recruit the target number of dental practices for the main trial, and confirmed our expectations that the time scale for recruiting the required number of participants aged 3–7 years would be challenging.
Dentists in the pilot trial were reimbursed for their time, following a detailed costing framework developed by the Primary Care Research Network (PCRN) Northern and Yorkshire; participating practices’ feedback about the process and value of the reimbursement was positive. Primary care trusts (PCTs) were also supportive of the trial and agreed that the FiCTION trial work and ‘accrual’ reimbursement would not interfere with the practice contract or UDAs (current payment system). The opportunity cost of treatment sessions forgone as a result of trial participation was not an issue for the dentists.
Chapter 2 Randomised controlled trial methods
Aims and objectives of the trial
The aim of the FiCTION trial was to compare the clinical effectiveness and cost-effectiveness of three treatment strategies for the management of dental caries in primary teeth over a period of up to 3 years (minimum 23 months) in children aged 3–7 years at the time of initiation of treatment. The three strategies compared were as follows:
-
conventional management of decay, with best-practice prevention
-
biological management of decay, with best-practice prevention
-
best-practice prevention alone.
These strategies are described in detail in Interventions.
Objectives
The objectives were to assess:
-
the clinical effectiveness and cost-effectiveness of three treatment strategies for managing dental caries in primary teeth
-
the three treatment strategies with respect to –
-
child- and parent-reported outcomes, including child OHRQoL, dental anxiety, worry and discomfort during treatment
-
acceptability and associated experiences for children, parents and members of the dental team
-
caries development and/or progression in primary and permanent teeth.
-
Summary/overview of trial design
The FiCTION trial was a multicentre, three-arm, parallel-group, participant-randomised controlled trial, with an integrated economic evaluation (see Chapter 4) and qualitative evaluation (see Chapter 5).
Trial registration and protocol availability
The FiCTION pilot trial was initially registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry on 27 January 2009; it was extended to include the main trial on 8 May 2013. The protocol was published in BioMed Central Oral Health in 201385 and is also available on the NIHR HTA project web page [see www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Over the course of the trial, five protocol amendments were made (see Appendix 2).
Ethics and governance
The University of Dundee acted as sponsor for this trial. Favourable ethics opinion was obtained from the East of Scotland Research Ethics Committee (REC) 1 on 30 July 2012. All subsequent substantial amendments were also approved by the same REC.
All members of the trial team and practice staff had training in Good Clinical Practice (GCP), in keeping with their role in the trial. Practice staff directly involved with recruitment and consent received further training in taking informed consent in a paediatric setting.
Dental practices were required to maintain an investigator site file containing details of staff involved in the FiCTION trial, their training and their delegated roles. Dentists were named as practice leads once they satisfied the GCP/Research Governance Framework requirements.
Trial setting
The trial was conducted in primary dental care, reflecting the setting within which the vast majority of children’s dentistry is carried out. General dental practices across three of the four UK nations (Scotland, England and Wales) were grouped into five clinical centres (CCs) for management purposes:
-
Scotland, with one CC covering Tayside, Glasgow, Edinburgh and the Borders (hereafter referred to as Scotland)
-
England, with three CCs covering –
-
North East England/Cumbria (hereafter referred to as Newcastle)
-
Leeds, Sheffield, Derbyshire, Manchester and Liverpool (hereafter referred to as Leeds/Sheffield)
-
London
-
-
Wales, with one CC covering Cardiff (hereafter referred to as Wales).
To be eligible for participation in the trial, practices had to:
-
see and treat children aged 3–7 years under NHS contracts
-
see children with carious lesions in primary teeth (around one child per week was considered an appropriate frequency)
-
have the infrastructure to support the trial, preferably to include electronic patient management systems and internet access.
Practice recruitment
The initial target was to recruit 50 practices, 10 from each of the five CCs (see Sample size calculation). The selection of practices was designed to reflect the sociodemographic mix of the catchment communities. A range of strategies were used to identify and invite practices to participate. Practices from which an expression of interest in trial participation was received were visited by the research team to assess their eligibility before their inclusion was confirmed.
General strategy
The 11 practices that had participated in the FiCTION pilot trial,86 carried out in Scotland, Newcastle and Leeds/Sheffield, were invited to participate in the main trial. A further 44 practices that indicated interest in main trial participation when surveyed as part of the FiCTION feasibility study and in which at least one GDP had expressed willingness to randomise patients in that survey exercise were re-contacted and formally invited to participate by letters sent to the senior partner as well as to the GDPs working in these practices.
Practices that formed the overall sampling frame for the feasibility study but that had not been contacted as part of that study were also invited to express an interest in the FiCTION trial (total: 632 practices across the five CCs).
Any practice responding to general advertising in the national and local dental press and expressing an interest in participating in the trial was considered in accordance with practice eligibility criteria and proximity to the CCs [Scotland (Dundee), Newcastle, Leeds/Sheffield, Wales (Cardiff) and London].
Local strategy
In addition to the general practice recruitment strategy described above, a local recruitment strategy was developed by the clinical leads at each CC in liaison with the research networks in England and Wales and the Scottish Primary Care Research Network in Scotland. This comprised e-mail and postal mailing of FiCTION trial flyers to practices and practitioners by PCRNs in England and their equivalents in Wales and Scotland. Practices wanting to express an interest in the trial were asked to contact the Dundee FiCTION trial office. Expressions of interest were followed up locally by the clinical leads with the support of their local PCRNs. Local practice recruitment meetings were held in the CCs to inform interested GDPs about the FiCTION trial and to answer any questions they may have had.
By July 2013, 56 practices had been recruited and it was recognised that additional practices would be needed to reach the target number of participants. Following consultation with the Trial Steering Committee (TSC), Independent Data Monitoring Committee (IDMC) and HTA, the target number of practices was increased to 70 and catchment areas for two of the CCs (Leeds/Sheffield and Newcastle) expanded geographically into Derbyshire/Manchester/Liverpool and Cumbria, respectively.
Practice retention
The trial manager, clinical leads and their secretaries, and the clinical researcher actively maintained regular contact with all participating practices throughout the trial. They identified practice retention or associated problems early, using the formally established communication strategy and informally through their ongoing engagement with the practice, working closely with the practitioners to troubleshoot any problems. Regular e-mail and telephone updates from clinical leads’ secretaries and the trial manager, plus quarterly newsletters from the trial core team, were issued during the trial. Active support was sought from the PCRNs, research networks and local research champions for recruitment and retention of practices.
Continuing professional development (CPD) credit for all members of each FiCTION trial practice dental team was made available for attendance at any trial training events.
The practices were remunerated with service support costs for participant screening and recruitment activities, as well as with research costs based on the additional time spent on trial-related data collection. Dental treatment was remunerated in the normal way, depending on the nation where the practice was located.
Trial participants
Child participant inclusion criteria
Children (aged 3–7 years) who:
-
were willing to be dentally examined
-
had at least one primary molar tooth with decay into dentine (i.e. carious lesion) on clinical examination
-
were known regular attendees or, if new to the practice, considered likely to return for follow-up.
Child participant exclusion criteria
-
Children aged < 3 or > 7 years.
-
Children aged 3–7 years who:
-
at the recruitment appointment, were accompanied by an adult who lacked the legal or mental capacity to give informed consent
-
at the recruitment appointment presented with either dental pain and/or dental sepsis due to caries (as diagnosed by the GDP from patient history, examination, radiographs). These participants were not enrolled into the trial at that point, but, after treatment, could be reassessed for eligibility. Discomfort or pain associated with erupting or exfoliating teeth or an incident of trauma or oral ulceration was not an exclusion criterion
-
had a medical condition requiring special considerations with their dental management, for example cardiac defects or blood dyscrasias
-
were currently involved in any other research that might have affected this trial
-
were part of a family that knew they would be moving out of the area during the 3 years following recruitment.
-
Interventions
Three multicomponent treatment strategies for managing carious lesions in the primary dentition were tested. Each patient was randomly allocated to one strategy, with the expectation that they would be managed in that arm of the trial for up to 3 years. These strategies were documented in detail in the clinical protocols used in the training of practices and were available to practices for reference thereafter.
Conventional management of carious lesions, with best-practice prevention
[This intervention will hereafter be referred to as conventional with prevention (C+P).] (See Chapter 1, Evidence from conventional/traditional approaches to managing carious lesions.) Conventional management is commonly known as the ‘drill-and-fill’ method and is the traditional approach to managing dental caries that has been taught and practised for many years. It is based on active management of carious lesions by complete removal of the carious tissue. The teeth are numbed with local anaesthesia (a dental injection), carious tissue is mechanically removed using rotary instruments (drill) or by hand excavation (using hand tools), and a restoration (filling) is placed in the tooth to fill the cavity. If the dental pulp (nerve) is exposed during carious tissue removal or there are symptoms of pulpitis, a pulpotomy (removal of some of the nerve) may be carried out, followed by provision of a metal crown (usually) or a filling. Retained roots, and teeth for which the crowns are unrestorable or the pulp chamber is open, are managed by extraction (removal) of the tooth/root following local anaesthesia.
Best-practice prevention was carried out in line with current guidelines and as per the prevention alone (PA) arm.
Biological management of carious lesions, with best-practice prevention
[This intervention will hereafter be referred to as biological with prevention (B+P).] (See Chapter 1, Evidence from non-destructive/minimally invasive approaches to managing carious lesions.) This minimally invasive approach to managing carious lesions involves sealing decay into the tooth and separating it from the oral cavity; this is achieved by application of an adhesive filling material over the caries or by covering with a metal crown. It may be clinically necessary, on occasion, to partially remove superficial carious tissue prior to the tooth being sealed. Local anaesthetic injections are rarely needed. Retained roots and teeth for which the crowns are unrestorable, or dental pulp is exposed, are managed on a tooth-by-tooth basis. In situations when a tooth has active carious lesions (decay still progressing) or when the clinician decides that the tooth is likely to give the patient pain or sepsis before it exfoliates (falls out), the caries is managed by extraction following local anaesthesia.
Best-practice prevention was carried out in line with current guidelines and as per the PA arm.
Best-practice prevention alone
[This intervention will hereafter be referred to as prevention alone (PA).] (See Chapter 1, Evidence from Good Practice Prevention approaches to managing caries.) For the PA arm, no drilling, filling or sealing of primary teeth occurred. Treatment plans for participants were based on best-practice preventative care for teeth and oral health. This involved the four component pillars of prevention, carried out according to current guidelines:25,87
-
dietary investigation, analysis and intervention to reduce fermentable carbohydrates in the diet
-
tooth-brushing twice daily with a fluoridated toothpaste, plus fluoride mouth-rinsing in children > 7 years of age
-
topical fluoride varnish applied to primary and permanent teeth by a dental professional
-
fissure sealants for permanent teeth.
Training of dentists and practice staff
Between July and October 2012, each CC hosted a practice training day to deliver clinical and trial process training (which included GCP and informed consent training) to all enrolled dentists. Whenever possible, dental therapists/hygienists/nurses and practice receptionists/managers were also trained at the practice training day. For dental team staff who could not attend a practice training day, training was delivered as part of a site initiation visit by the trial manager and clinical researcher.
Training was provided for the individual clinical procedures with which dentists were unfamiliar and was tailored as far as possible to each group of dentists. Topics included, but were not limited to, recording dental caries using ICDAS, taking radiographs in children, the Hall Technique and conventional crown provision. Additional training materials were developed for taking radiographs and for the Hall Technique;35 these materials were made available to participating dentists in their practice. Although the detection of dental sepsis (infection) is a standard part of a dental clinical examination, given its importance as one of the primary outcomes, specifically directed training was included.
Training was given during appropriate treatment planning and delivery to children according to the randomised trial arm. This comprised a didactic teaching session from members of the core team followed by practical treatment planning with cases and discussion with the local clinical lead and co-chief investigators.
Outcomes
Primary outcomes
When the trial was originally designed it was powered only for a single primary outcome of incidence of dental pain and/or dental sepsis. However, as the trial progressed, it became clear that the number of episodes of dental pain and/or dental sepsis experienced by a child is a more clinically relevant outcome and, statistically, a more sensitive measure (compared with dichotomising the number of episodes into zero episodes vs. one or more episodes). Therefore, it was decided that the number of episodes of dental pain and/or dental sepsis should be a co-primary outcome.
Originally, it was planned that participants would be followed up in the study for a fixed 3-year period. However, the extension to the trial recruitment period resulted in the maximum potential follow-up ranging from 23 to 36 months; hence, the co-primary outcomes were assessed across a variable follow-up period.
Definition of the clinical effectiveness outcomes
The co-primary outcomes for clinical effectiveness were the:
-
proportion of children with at least one episode of dental pain or dental sepsis or both during the follow-up period (incidence)
-
number of episodes of dental pain or dental sepsis or both for each child during the follow-up period.
The primary outcome measure of incidence was a binary indicator of dental pain and/or dental sepsis at each treatment visit during the follow-up period (minimum of 23 months to a maximum of 36 months). Treatment visits included scheduled appointments and unscheduled/emergency appointments.
Dental pain was defined on the case report form (CRF) by a ‘yes’ response to question 7 and a ‘yes’ response to question 7a (dental caries).
Dental sepsis was defined as confirmed infection on the CRF by a ‘yes’ response to question 8.
Schedule of assessment of primary outcomes
Data for the primary outcomes of dental pain and/or dental sepsis due to caries were recorded on the CRF during or following all visits to the GDP (Table 1) [see also the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
| Event | Type of outcome | Completed by | Scale | Where recorded | Screening visit | Baseline examination visit | Treatment visits (scheduled treatment or recall and unscheduled/emergency) | Non-attendance postal questionnaire | Final visit |
|---|---|---|---|---|---|---|---|---|---|
| Assessment of eligibility (presence of caries, absence of dental pain and dental sepsis) | GDP | Screening log and participant’s dental record | ✗ | ||||||
| Consent/assent | GDP | Consent form | ✗ | ||||||
| Bitewing radiographs | GDP | Risk-based, in line with standard guidance – NOT a trial specific procedure | |||||||
| Caries assessment | Secondary | GDP | ICDAS | ICDAS chart | ✗ | ✗ | |||
| Pain: pre-treatment questions to GDP | Primary | GDP | CRF | ✗ | ✗ | ||||
| Sepsis: pre-treatment questions to GDP | Primary | GDP | CRF | ✗ | ✗ | ||||
| Child’s co-operation with treatment | Secondary | GDP | CRF | ✗ | ✗ | ||||
| Treatment cost data | Economic | GDP | CRF | ✗ | ✗ | ||||
| Parent-reported child dental discomfort (toothache) outside dental visits | Secondary | Parent | DDQ-8 | Parent questionnaire | ✗ | ✗ | ✗ | ✗ | |
| Parent/carer proxy report of child’s OHRQoL | Secondary | Parent | P-CPQ-16 | Parent questionnaire | ✗ | ✗ | ✗ | ||
| Parental perception of child’s pain related to treatment | Secondary | Parent | Global rating | Parent questionnaire | ✗ | ✗ | ✗ | ||
| Parental perception of child’s worry pre/post treatment | Secondary | Parent | Global rating | Parent questionnaire | ✗ | ✗ | ✗ | ||
| Use of health services | Economic | Parent | Parent questionnaire | ✗ | ✗ | ✗ | |||
| Trait dental anxiety | Secondary | Child | MCDASf | Child questionnaire | ✗ | ✗ | ✗ | ✗ | |
| Child’s perception of pain related to treatment | Secondary | Child | Pictorial rating scale | Child questionnaire | ✗ | ✗ | ✗ | ✗ | |
| Child’s perception of worry pre/post treatment | Secondary | Child | Pictorial rating scale | Child questionnaire | ✗ | ✗ | ✗ | ✗ | |
| Treatment referrals | Economic | GDP | CRF | ||||||
| Treatment deviations | Contextual information | GDP | CRF | ||||||
Dental pain due to caries (toothache)
Assessments for dental pain were made at each visit (scheduled or emergency treatment) throughout a child’s participation in the trial using the CRF completed by dentists. To differentiate between pain originating from a decayed tooth and pain from other causes (e.g. erupting or exfoliating teeth, mouth ulcers), the dentist formed a judgement based on the patient/parent history and the clinical evidence available from examination, which was recorded on the CRF completed at each appointment.
Dental sepsis (dental infection)
Clinical visual examination for dental sepsis was specifically undertaken at every visit by the GDPs and recorded on the CRF. The clinical detection criterion for the positive recording of dental sepsis was the presence of a swelling, dental abscess or draining sinus. Clinical examination was expected to be supplemented with examination of any radiographs taken [in line with Faculty of General Dental Practice (FGDP) guidelines],70 to record radiographic signs of inter-radicular pathology. Our statistical analysis plan (SAP) indicated that, if it was found that < 80% of children had radiographs within 1 year of entry to the trial, this source of data would be considered unrepresentative and would be disregarded in the definition of dental sepsis.
Definition of an episode
Reproduced from Innes NP, Clarkson JE, Douglas GVA, Ryan V, Wilson N, Homer T, et al. , Journal of Dental Research, 99(1), pp. 36–43, copyright © 2020 by International & American Associations for Dental Research 2019. Reprinted by Permission of SAGE Publications, Inc. 88
Several treatment visits (i.e. a course of treatment) can be associated with the same ‘episode’ of dental pain and/or dental sepsis. Therefore, we needed a definition of an ‘episode’ of dental pain and/or dental sepsis due to caries; this definition was operationalised on a tooth-by-tooth basis using CRF data, according to the following algorithm:
-
Let Y (yes) = the presence of dental pain and/or dental sepsis at a single treatment visit (as defined above); N (no) otherwise.
-
Let YY = the presence of dental pain and/or dental sepsis at consecutive treatment visits (i.e. on consecutive CRFs).
-
Y on one or more teeth at a single treatment visit = an episode.
-
Any number of consecutive ‘yeses’ on the same tooth, regardless of timeframe = a single episode (e.g. YYYYY over 5 months).
-
YY on different teeth (regardless of timeframe) = two separate episodes.
-
YNY on the same tooth = two separate episodes (regardless of the timeframe).
Although episodes were defined on a tooth-by-tooth basis, for a given child, if there were two (or more) teeth with dental pain and/or dental sepsis at the same visit, this was recorded as one episode at that visit for that child. For example, if a particular tooth had dental pain and/or dental sepsis at two consecutive visits and at the second visit a different tooth also had dental pain and/or dental sepsis this would be counted as one episode.
It was assumed that those who did not return for regular appointments during their follow-up did not experience dental pain and/or dental sepsis.
Secondary outcomes
See the project web page [www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)] for the SAP and study documentation, patient information sheet and questionnaires, for additional detail on the secondary outcome measures used.
Incidence of dental caries in primary and permanent teeth
A systematic review of clinical caries detection systems revealed a vast selection of scales with many inconsistencies in how the caries process was measured. 89 This review and the NIH consensus statement67 led to the development of an evidence-based and histologically validated system for staging dental caries, known as the ICDAS system. 90 This system is widely used in clinical research as a reference standard for measuring caries prevalence and incidence that all clinicians can use ethically, without harm and irrespective of the equipment they have. These features, along with the reported acceptable validity and reliability of ICDAS in primary teeth91 and the permanent dentition,92 make it an appropriate tool for dental caries measurement. Because, at a population level, nearly all caries experience in the permanent teeth of children in this age group are accounted for in the FPM teeth, the analysis of caries in permanent teeth focuses on these teeth alone.
Detailed measurements of caries experience were recorded at baseline and final assessment by the GDPs using the CRF and ICDAS charting. The dentists measured both early and more advanced stages of dental caries. The primary requirement for the examination was clean, dry teeth. All surfaces of all teeth were examined and the status of each was recorded in terms of caries and restorations. In the event of enough data (at least 80% of children with a radiograph taken within 1 year of entry to the trial) being available to provide a valid measure in this population, it was planned that bitewing radiographs, taken in line with FGDP guidelines (with blinded, independent assessment), would be used as an independent measure of dental caries. However, as the guidance for frequency of bitewing radiographs is based on caries risk assessment, and as some children may have moved out of the high-risk group during the course of the trial, it was anticipated that the frequency of bitewing radiographs taken for some children might reduce over the period of the trial. In addition, some children might have been unco-operative with this type of assessment, in view of their young age.
As the SAP [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)] describes, an incident of caries was defined in terms of the observations made on the ICDAS charts scored at baseline and at the final visit. As observations on surfaces of the same tooth and between adjacent surfaces of different teeth were likely to be correlated, a single whole-mouth Caries Assessment Score for each child was derived and a computer program written to define for each child whether or not there had been disease development/progression from baseline to the final visit in teeth that were ‘sound/reversible’ at baseline, that is caries-free or with non-cavitated enamel caries [see appendix 1 in the SAP, as found on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. Primary and permanent teeth were analysed separately.
Child- and parent-reported outcomes: participant oral health-related quality of life
Our chosen measure of OHRQoL was the Parental–Caregivers Perceptions Questionnaire (P-CPQ). The P-CPQ, in its original 31-item version,93 was found to be reliable and valid for use in the UK. 94 More recently, a modified 16-item short form, Parental–Caregivers Perceptions Questionnaire-16 items (P-CPQ-16), has been developed and validated;95,96 to minimise respondent burden, this shortened version was selected for use in the trial [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 22 February 2019)]. The questionnaire includes four domains: oral symptoms, functional limitations, emotional well-being and social well-being. Parents are asked to indicate, using a six-point Likert scale (‘never’ = 0, ‘once or twice’ = 1, ‘sometimes’ = 2, ‘often’ = 3, ‘every day or almost every day’ = 4, and ‘do not know’ = 7), the frequency with which the events affected their children in the previous 3 months. The P-CPQ-16 also includes two global ratings: the parent’s ratings of the child’s oral health and their rating of the extent to which the oral/orofacial condition affects his/her life overall;97,98 these are rated on five-point response scales ranging from ‘excellent’ to ‘poor’ for the former and from ‘not at all’ to ‘very much’ for the latter.
Parents were asked to complete the P-CPQ-16 at the baseline or the final visit; if the final visit was not attended, parents were sent a non-attendance questionnaire [see Table 1 and the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. A score for each visit was calculated, ranging from 0 to 64 (a lower score represents better OHRQoL). The global ratings are not included in the calculation of the scores.
Child- and parent-reported outcomes: participant dental anxiety, worry and discomfort during dental treatment
In measuring participant dental anxiety, we distinguished between underlying trait anxiety and treatment-related state anxiety. As with the assessment of OHRQoL, the challenges of child self-report and the need for parental proxy report for some constructs was recognised. The choice of measures was informed by a published systematic review of measures of child dental anxiety by Porritt et al. 99
The chosen measure of trait anxiety was a six-item version of the Modified Child Dental Anxiety Scale (faces) (MCDASf). As the last two questions of the standard MCDASf ask about conscious sedation and dental general anaesthesia, neither of which was relevant to FiCTION trial patients, these were omitted, as recommended by SDCEP. 100 The Modified Child Dental Anxiety Scale (MCDAS)101 aims to assess dental anxiety in children and has been shown to be an acceptable measure of child dental anxiety in children aged 8–15-years, exhibiting good internal consistency and validity. 101,102 The MCDASf is a modified version for younger children and has also been tested for criterion and construct validity and test–retest assessment reliability. 103 The response format for each of the six items is in the form of five faces showing different expressions, from ‘relaxed/not worried’ to ‘very worried’. Howard and Freeman103 concluded that the MCDASf can be used with confidence to assess dental anxiety in children, although they indicated that it has not been formally validated in those aged < 8 years. The MCDASf was completed by the child before treatment at baseline and at every visit to provide information on his or her anxiety at each dental encounter throughout the trial. The total score for each assessment was calculated, ranging from 6 to 30, with lower scores indicating less dental trait anxiety.
The measurement of dental state anxiety, to assess how the child responded to the particular treatment provided, was an important dimension of this trial. As with the MCDASf, it was necessary to choose an instrument appropriate to the age and cognitive abilities of the child participants. Visual analogue and pictorial scales are known to be useful in assessing single item constructs such as pre- and post-operative child dental anxiety (‘worry’). Although difficulties are noted with regard to the consistency of overall test–retest reliability, the reliability is noted to improve in the middle and the extremes of the scale. 104 Therefore, we judged that a pictorial scale using faces as the descriptors105 would be an appropriate means of assessing child dental state anxiety. Accordingly, at the start of each treatment visit the child completed a faces-based pictorial scale, scored 1 to 3, where 1 was ‘not at all worried’ and 3 was ‘very worried’, to report on their level of worry prior to arriving at the dentist’s for their visit (anticipatory anxiety). The child subsequently completed a second pictorial scale with the response options and scoring system at the end of each treatment visit to report on treatment-related dental state anxiety.
Although accounts of the use and psychometric properties of parent-proxy measures of children’s state anxiety are equivocal,104,106,107 such assessments have been shown to be valuable when assessing anxiety in younger children. 104,108 Therefore, in parallel with the child self-reports of anticipatory and treatment-related anxiety, parents were asked to make assessments of their child’s worry levels prior to arrival at the dentist’s for their visit and following treatment; these assessments were recorded using a single categorical global worry question for each time point, scored 1 to 5, where 1 was ‘not at all worried’ and 5 was ‘very worried’.
Discomfort during dental treatment was assessed by the child in relation to their treatment experience at the end of each visit using a global question on hurt, with a face format pictorial scale, scored 1 to 3, where 1 was ‘not at all hurt’ and 3 was ‘hurt a lot’. In addition, parents were asked to report on their perceptions of their child’s levels of pain regarding that particular visit to the dentist using a global question on pain due to treatment, scored 1 to 5, where 1 was ‘not at all painful’ and 5 was ‘very painful’.
Dentists also estimated child discomfort at every visit using a global discomfort question scored 1 to 5, where 1 was ‘no apparent discomfort’ and 5 was ‘significant and unacceptable’ discomfort, with these ratings being reported via the CRF.
There was no specific validation of any of the above pictorial or global rating scales.
Cost-effectiveness of managing dental decay in primary teeth
(See Chapter 4.) The objective of the economics analysis in the FiCTION trial was to determine the relative cost-effectiveness of alternative ways of managing dental decay in primary teeth. The primary (base-case) analysis focused on estimating the cost of the three treatment strategies based on the resources (staff time, consumable materials and reusable materials) used to provide care at each visit. The differences in costs between different treatments were equated to the differences in effectiveness measured in terms of dental pain and/or dental sepsis. Sensitivity analyses determined the effect on results when costs were based on current charges to the NHS. Finally, a wider societal perspective was adopted to account for parental costs. The inclusion of a thorough economic evaluation as part of the FiCTION trial was crucial to help address uncertainties surrounding the effectiveness and efficiency of each treatment strategy.
Acceptability and associated experiences of treatment strategy for children and parents/carers and dental professionals
(See Chapter 5.) Given the difficulty in measuring children’s attitudes towards treatment strategies, identified in the pilot trial, the acceptability of the three treatment strategies was explored using a child-centred approach with qualitative methods, and specifically with child participatory activities, to allow children rather than adults to shape the data collection process. 109 Separate ethics approval was sought for this study.
Dentist-reported child behaviour and compliance was measured at each appointment using a behaviour score of 1 to 4, where 1 was ‘The child refused the treatment: . . . It was very difficult to make any progress’ and 4 was ‘Child was completely co-operative’, recorded on the CRF.
The dental professionals’ experiences of the three treatment strategies was explored qualitatively through interviews and focus groups. Topic guides were derived from qualitative information collected during the FiCTION pilot trial.
Safety (harms)
The three interventions tested in this trial are all in common use in general dental practice and were considered to be of relatively low risk. Expected adverse events (AEs) are summarised in Appendix 3.
Because of the type of trial, non-serious AEs were not captured. However, the practice lead was required to report all serious adverse events (SAEs) to NCTU via a secure fax line within 24 hours of the practice learning of an occurrence.
A SAE is defined as any untoward and unexpected medical occurrence or effect that:
-
results in death
-
is life-threatening (refers to an event in which the subject was at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death if it were more severe)
-
requires hospitalisation (for > 24 hours), or prolongation of the participant’s existing hospitalisation
-
results in persistent or significant disability or incapacity
-
is a congenital anomaly or birth defect.
Dental practice staff were reminded that clinical judgement should be exercised in deciding whether or not an AE was serious in other situations. Important AEs that were not immediately life-threatening or did not result in death or hospitalisation but might jeopardise the subject or require intervention to prevent one of the other outcomes listed in the definition above were also considered to be serious.
Hospitalisations for elective treatment of a pre-existing condition did not require reporting as SAEs. Unrelated hospitalisations were to be elicited at the post-treatment follow-up appointment, scheduled subsequent appointments and all unscheduled/emergency appointments.
All SAEs that, in the opinion of a co-chief investigator, were ‘related’ (i.e. resulted from the administration of any of the research procedures) and ‘unexpected’ (see Appendix 3) were reported to the REC.
Sample size calculation
At the planning stage, the proposed primary outcome was the proportion of children reporting either dental pain and/or dental sepsis during 3 years of follow-up. Based on evidence from previous studies on similar populations receiving no fillings,6,7 conventional fillings and the Hall Technique,34 sepsis rates of 20%, 10% and 3% were expected in the PA, C+P and B+P arms, respectively. Using the ‘sampsi’ procedure (a sample size calculation based on a two-sample test of proportions assuming a normal approximation and incorporating a continuity correction) in Stata® version 9 (StataCorp LP, College Station, TX, USA), and assuming a significance level of 2.5% (to allow for the multiple testing involved in a three-arm trial), the required sample size was calculated as:
-
two groups of 334 children to detect a difference in rates of between 20% and 10%, with 90% power, for PA versus C+P
-
two groups of 334 children to detect a difference in rates of between 3% and 10%, with 90% power, for B+P versus C+P.
The sample size was then increased by an inflation factor of 1.09 (giving 365 children per arm at end of follow-up) to allow for adjustment of estimates of effect size, taking into account variation between randomisation strata (dental practices).
Based on previous experience (Jan E Clarkson, University of Dundee, 1 July 2010, personal communication) of conducting RCTs in primary dental care, the sample size was further inflated to allow for a loss to follow-up of 25% over 3 years, requiring 487 children to be consented and randomised to each intervention arm (a target sample size of 1460 in total). In the pilot trial, participants were followed up for only 6 months, rather than the 3 years proposed for the main trial. Therefore, although follow-up in the pilot was complete, no adjustment was made to the assumed rate of loss to follow-up (25%) for the full trial.
The original aim, therefore, was to invite 18,717 children to attend for screening, of whom 12,166 (65%) were expected to actually attend and agree to be screened for the trial. Based on findings from the pilot trial, it was initially assumed that 1825 (15%) of those screened would be eligible and 1460 (80%) of those eligible would consent to be randomised.
However, as the main trial progressed, the rates of participant identification, recruitment and consent proved lower than anticipated from experience in the pilot trial, amounting to 994 accruals by the end of June 2014. Therefore, a contract variation request was submitted to the HTA programme in August 2014 explaining that, based on the recruitment trajectory at the time, and with recruitment anticipated to continue until 31 December 2014 and follow-up until 30 June 2016, the trial would recruit only 1113 children. This would correspond to an effective sample size (after allowing for 25% loss to follow-up) of three groups of 278 children with a mean length of follow-up of 24.6 months. Assuming a linear incidence of dental pain and/or dental sepsis over the follow-up period, this would result in only 61% power to detect a difference between the arms for the primary outcome, dental pain and/or dental sepsis, assuming a type 1 error rate of 2.5%. In August 2014, it was therefore agreed by the NIHR HTA programme that there should be a 12-month extension to the trial, with the end of recruitment, end of follow-up and end of trial set at 31 December 2014, 30 June 2017 and 31 December 2017, respectively. No new practices would be recruited and those children recruited after June 2014 would not have the full 3 years of follow-up.
Allowing for 25% loss to follow-up, the effective sample size under this scenario would be three groups of 278 children followed up for, on average, 35.5 months. Assuming a linear incidence of dental pain and/or dental sepsis over the period of follow-up, this would result in 82.0% power (77.4% power if an adjustment for strata was necessary) to detect the hypothesised effect sizes (19.72% vs. 9.86% for PA vs. C+P and 2.96% vs. 9.86% for B+P vs. C+P), assuming a type 1 error rate of 2.5%.
It was subsequently (November 2014) agreed with the NIHR HTA programme that, owing to the already variable follow-up and in order to maximise the chances of reaching the desired power, recruitment could continue until 30 June 2015 (with follow-up still finishing on 30 June 2017) and that new sites could be added to facilitate this recruitment on the understanding that any additional costs would be absorbed by the existing budget.
Participant timeline: screening, recruitment, consent, retention, withdrawal and visit schedule
The flow diagram of the FiCTION trial is shown in Figure 1. The participant identification and recruitment strategy were informed by the experiences in the pilot trial. 86
FIGURE 1.
Flow diagram of the FiCTION trial.

Identification and screening of participants
Potential trial participants were children aged 3–7 years who were identified from participating dental practices, which invited the potential participants to participate through two routes:
-
Simple searches of practice databases to identify potentially eligible children, using a date of birth query. Potentially eligible children due for a recall visit were invited to participate by letter of invitation from the child’s GDP. This letter, together with information sheets for parents and the child (the child’s version was pictorial and age-appropriate), was sent with their dental appointment card at least 1 week ahead of the scheduled recall visit.
-
Parents of children presenting opportunistically, and identified as being potentially eligible for participation, were invited to participate at the time of presentation. Unless they declined immediately these parents were given the invitation letter and the parent and child information sheets, and time (a minimum of 24 hours) was allowed to consider participation in the trial before consent was sought.
Potential participants identified through both routes had a routine screening examination (‘check-up’) to confirm eligibility. This screening consisted of standard dental recall clinical investigations (including medical and dental history taking, questioning regarding oral pain since last visit and current oral pain, clinical examination of the soft tissues and teeth, and radiographs in line with national guidance). This allowed the identification of children with dental caries and the exclusion of children with current dental pain or dental sepsis due to caries, as well as medically compromised children.
For those children without evidence of caries into dentine or where dental pain due to caries and/or dental sepsis were present at the screening visit, the GDP explained why it was not possible to take part in the FiCTION trial at that time and, if relevant, treated the carious lesion(s) and related pain and/or sepsis. The family was informed that if the child re-presented with dental caries but without pain or sepsis, he or she could then be considered for the FiCTION trial. If a child was caries-free at screening but subsequently presented to the practice with caries during the recruitment phase of the trial, he or she could be invited to join the trial.
Recruitment and consent
Post screening, if there was evidence of dental caries and absence of both dental pain due to caries and dental sepsis, a FiCTION trial-trained dentist in the practice discussed the trial with the parent and child, supplementing the written trial information, and answered any questions they may have had. If the parent and child were willing to participate, and once eligibility had been confirmed, parental written informed consent, and oral or written assent from the child, were obtained by the FiCTION trial-trained dentist prior to any trial-specific procedures being carried out.
The child was then given a subsequent treatment appointment. It was intended that randomisation to a treatment arm should be carried out via the NCTU secure web-based randomisation service before the child returned for the subsequent visit, at which time the child and parent would be informed of the treatment arm to which the child had been allocated. A detailed ICDAS dental chart was completed at the initial treatment visit. Treatment was commenced as per the child’s randomised arm and clinical protocol. Families were also presented with a letter to give to their general medical practitioner to inform them of the child’s involvement in the trial.
For those children for whom consent was not given for participation in the trial, the dentist carried out the child’s normal dental care.
Participant management and visit schedule
The timing and frequency of subsequent visits was at the discretion of the GDP and the family. The original trial design assumed a fixed follow-up period of 3 years, but, as indicated in Sample size calculation, an extension to the trial recruitment period resulted in the maximum potential follow-up, post randomisation, ranging from 23 to 36 months, with those recruited after May 2014 having a truncated follow-up.
Any recurrence of caries in the teeth that were originally treated and any subsequent incidence of caries in other primary teeth were expected to be managed according to the randomly allocated arm and its associated clinical protocol. At any visit during which there was deviation from the allocated clinical protocol, either within or between arms, treatment deviation forms (TDFs) were completed by the GDP.
Data on all treatment provided over the trial period were collected at each FiCTION trial visit from the dental team (usually the GDP), and parents and children completed outcome questionnaires at all visits [see Table 1 and the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
A practice used its usual contact procedures to reschedule any missed visits until the child had not been seen for > 540 days. After this point had been reached the practice sent a did-not-attend (DNA) questionnaire, with a covering letter and pre-paid envelope, to the parent for them to complete and return to the NCTU.
All participants had a final trial visit date, with an associated defined final visit window, for collection of ‘final-visit’ data (see Table 1). A similar approach to that described above was used for children failing to attend their final visit: in this case, a DNA final visit questionnaire was posted by the practice to the parent for them to complete and return to the NCTU.
Participant retention
The concept of ‘marketing’ of clinical trials informed participant recruitment and retention strategies. 110 A distinctive FiCTION trial logo and branding was developed and used on all trial-related materials. On enrolment, parents and children were given FiCTION trial membership cards; this card carried details of their dentists and whom to contact should they change dentist/practice or require out-of-hours or emergency dental care. The card also included FiCTION trial website details, which gave the children access to colouring-in and other activities as well as providing trial updates and contact details of the trial team. Leaflets and posters were distributed for display in practice waiting rooms to convey the research team’s thanks to the FiCTION trial families for their participation, while enhancing the trial’s prominence in practices. Children were sent FiCTION trial birthday cards via the practice and practice feedback was used to guide the development of additional suitable FiCTION trial-branded merchandise promoting the trial.
Participant withdrawal
Participants had the right to withdraw from the trial at any time without having to give a reason. Practices were asked to try to ascertain the reason for withdrawal and document this on a Withdrawal Form [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. Participants who failed to return for follow-up visits were not considered to have withdrawn, unless an explicit request to withdraw was received or the practice confirmed that the child had moved to a practice that was not participating in the FiCTION trial. When practices withdrew from the trial, all of their FiCTION trial participants were considered to have been withdrawn at the time of the practice leaving.
Trial dates
The trial opened to recruitment on 1 October 2012. The first child was recruited on 12 October 2012 and the last child was recruited on 18 June 2015. The end of the trial was defined as last patient, last visit; follow-up of the last child was completed on 29 June 2017.
Randomisation
The unit of randomisation was the child, with randomisation into the three carious lesion treatment strategies (C+P, B+P and PA) in a 1 : 1 : 1 ratio. Randomisation was stratified by site (dental practice).
Sequence generation
The allocation sequence was generated by a statistician not otherwise involved in the trial, using variable length random-permuted blocks of size 6 and 9 (based on the ‘ralloc’ function in Stata).
Allocation concealment
Allocation concealment was achieved by use of a centralised web-based randomisation facility hosted by NCTU. Randomisation was intended to be carried out once the consent process was completed and the child had left the dental practice. The variable length random-permuted blocking ensured concealment of allocation.
Implementation
The intention was that participants were managed throughout their time in the trial according to the arm to which they had been randomly allocated, that is any subsequent dental caries would be managed in the same way (as per random allocation) as the initial occurrence. Any deviation from the allocated treatment strategy (i.e. when treatment of carious lesions involved elements of a treatment strategy other than that to which the child had been randomised, because of decisions or behaviours of the dental practitioner, parent or child) was recorded on a TDF at each follow-up visit at which it occurred.
Blinding (masking)
This was an open randomised trial; the different treatment strategies being used meant that it was not possible to blind the parents, children or dentists as to which arm the child was participating in. The statisticians were not blind; however, there was no analysis conducted by arm until the final database lock.
Statistical methods
A complete SAP, which provides full details of all statistical analyses, variables and outcomes, was finalised and signed before the final database lock and analysis [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Primary analysis of the co-primary outcomes
At the start of the trial, the single proposed primary outcome was the proportion of children with at least one episode of dental pain and/or dental sepsis during the planned 3-year follow-up period. However, the extension to the trial recruitment period resulted in the maximum potential follow-up ranging from 23 to 36 months; hence, the analysis strategy was adjusted to incorporate variable follow-up. As indicated in Primary outcomes, it was agreed that the incidence of dental pain and/or dental sepsis during the follow-up period and the total number of episodes of dental pain and/or dental sepsis for each child during the follow-up period would become co-primary outcomes. The original power calculation for the trial was based on a comparison of proportions and, as a result, was the only powered analysis; therefore, an exploratory hypothesis test for the unpowered comparison of the mean number of episodes is reported.
The analyses of the co-primary outcomes were conducted on the basis of intention to treat (ITT), defined as all randomised children with at least one CRF completed and in MACRO (Elsevier, Amsterdam, the Netherlands), including protocol violators and ineligible randomised participants, retaining participants in their randomised treatment groups. The results for the co-primary outcomes are reported as unadjusted and adjusted models. Models were adjusted for age in years at randomisation and time in the trial in years (defined as the time from randomisation to the date of last CRF in MACRO). Differences between dental practices were included as a random intercept; therefore, all adjusted models were mixed-effects models. Randomised treatment arm was included in the models as a factor with three levels, with the C+P arm as the reference group. The adjusted analyses were defined as the primary analyses and, therefore, the models to use for reporting purposes.
The primary analyses of the co-primary outcomes were:
-
The analysis of the incidence of dental pain and/or dental sepsis during the follow-up period, using logistic regression.
The dependent variable was a binary indicator of whether or not there was a reported incidence of dental pain and/or dental sepsis during the follow-up period. We generated 97.5% CIs for the difference between treatment arms (PA vs. C+P and B+P vs. C+P), expressed as risk differences [by integrating over the covariates in the model (time in study and age) and the random effects]; p-values were reported.
-
The numbers of episodes of dental pain and/or dental sepsis were analysed using negative binomial regression.
The dependent variable was the total number of episodes reported by a child during the follow-up period. Both negative binomial and zero-inflated negative binomial models were considered; the Vuong test was used to determine the best-fitting model. We generated 97.5% CIs for the difference between treatment arms (PA vs. C+P and B+P vs. C+P), expressed as incidence rate ratios (IRRs); exploratory p-values were reported.
Sensitivity analysis specific to the primary analysis of co-primary outcomes
As a planned sensitivity analysis, the models were also re-fitted including only participants with at least 23 months’ follow-up, to assess the influence of shorter lengths of follow-up on the treatment effect estimates (the rationale being that all participants had the opportunity to have at least 23 months of follow-up).
Secondary analysis of the co-primary outcomes
The time to the first episode of dental pain and/or dental sepsis has been identified as particularly important in relation to the age of the child, as the impact of dental pain and/or dental sepsis is greater on younger children and they have a reduced capacity to tolerate dental treatment.
Therefore, an analysis of time to first episode of dental pain and/or dental sepsis was undertaken using a Cox proportional hazards model, including age in years at randomisation as a continuous covariate and dental practices as a random effect. We generated 97.5% CIs for the difference between treatment arms (PA vs. C+P and B+P vs. C+P), expressed as hazard ratios (HRs); exploratory p-values were reported.
Analysis of the secondary outcomes
As described in Secondary outcomes, a number of secondary outcomes were measured during the period of follow-up. The timing of measurements is described earlier (see Table 1), with further description of the outcome measure index or scale given in the SAP [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Quantitative secondary outcome data (P-CPQ-16 and MCDASf) were analysed using the same approach as for the co-primary outcomes. Models were adjusted for age in years at randomisation and time in the trial in years. When a baseline measure was taken into consideration, the baseline measurement was also included as a covariate. Differences between dental practices were included as a random effect. If outcomes were measured at each visit (MCDASf), an additional random effect was added to account for repeated measures nested within child. Randomised treatment arm was included in the models as a factor with three levels, with the C+P arm as the reference group. Within this framework, the mean difference between randomised treatment arms at final visit or across the whole of the follow-up period was estimated.
Ordinal categorical outcomes (anticipatory and treatment-related anxiety and worry) measured at each visit were modelled using multilevel mixed-effects ordinal logistic regression. The assumption of proportional odds was assessed graphically and using the approximate likelihood ratio test. When this assumption was not satisfied, multilevel mixed-effects multinomial regression was considered. The frequencies of observations in each outcome category at each visit were also taken into consideration. When necessary, outcome categories were collapsed to create a binary variable and multilevel mixed-effects logistic regression was used.
Scoring of questionnaires and handling of missing data
For the parent questionnaire (P-CPQ-16) measuring perceived child OHRQoL, missing and ‘do not know’ responses were treated as ‘missing data’ and the same method of imputation applied to both, without distinction or prioritisation. The method adopted was the imputation of the ‘subject–subscale mean’ [i.e. the respondent-specific mean across a minimum of two valid (i.e. 0–4) responses in the item’s own P-CPQ-16 subscale] for missing and ‘do not know’ responses in the subscale [only if two or more of the constituent subscale items had originally valid (0–4) responses]. There was no imputation for participants with missing or ‘do not know’ responses for all 16 items of the P-CPQ-16.
A similar approach was used for MCDASf, although, as there are no subscales in this instrument, this was effectively a ‘subject–overall mean’ imputation, if three or more of the six items had originally valid (1 to 5) responses.
These methods assume that the data are missing completely at random.
Adherence to randomised treatment arm’s clinical treatment protocol
Deviations from the randomised treatment arm’s clinical treatment protocol were recorded on the TDF. These forms were entered into an electronic clinical data management system, MACRO, and then verified by the clinical researcher and classified as a ‘major’ deviation from the randomised treatment arm clinical treatment protocol (if there had been a from-arm tooth treatment change) or otherwise. For the purposes of assessing adherence to the clinical treatment protocol, only ‘major’ deviations were considered.
Adherence to the clinical treatment protocol for each randomised treatment arm could not be evaluated at an individual tooth level because TDFs did not collect tooth numbers. Because multiple teeth could have been treated at any particular visit, a TDF could not be linked to a specific tooth or number of teeth. In addition, tooth numbers were not collected for prevention activities. Therefore, adherence to the clinical treatment protocol was evaluated using child-level summaries.
The prespecified per-protocol analysis set
The prespecified per-protocol (PP) analysis set excluded participants from the ITT analysis set who:
-
were deemed likely to have had dental pain and/or dental sepsis at consent and/or
-
were ‘non-compliant’ with the clinical treatment protocol corresponding to their randomised treatment arm (i.e. defined as having a TDF involving a ‘major’ deviation from the randomised treatment arm’s clinical treatment protocol at > 20% of their visits). A ‘major’ deviation was defined as a cross-arm tooth treatment change.
Planned exploratory multivariable analyses
The variables listed below were included in prespecified exploratory multivariable regression models for both the co-primary outcomes of incidence and the number of episodes of dental pain and/or dental sepsis, regardless of their univariate association with the outcome:
-
Age of participant, in years, at randomisation as a continuous covariate.
-
Number of decayed teeth (level 5/6 cavitation) at baseline from ICDAS charting for each participant.
-
Participant ethnicity. Six ethnicities were listed in the parent baseline questionnaire: (1) white, (2) black, (3) Indian, Pakistani or Bangladeshi, (4) Chinese, (5) mixed race and (6) other. Ethnicity was explored as far as possible within the limitations imposed as a result of the distribution of participants across the categories and incomplete data.
-
Fluoride concentration in tap drinking water. Tap-water fluoride concentration [in parts per million (fluoride ion) (p.p.m.F–)] at the dental practice was used as a proxy for a child’s fluoridation status, in terms of the tap-water supply they received at home.
-
Index of deprivation. The dental practice index of deprivation, based on the dental practice postcode, was used as a proxy for a child’s index of deprivation. The index was extracted from census data collected prior to the recruitment phase of the trial. Index of deprivation can change significantly both spatially and temporally (dependent on the neighbourhood), and so was expected to be a very approximate measure of the individual children’s level of household deprivation. In addition, each country of the UK uses different domains, indicators and weighting of domains to calculate the index of deprivation [England: see www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 22 February 2019); Scotland: see www.gov.scot/Topics/Statistics/SIMD (accessed 22 February 2019); and Wales: see http://wimd.wales.gov.uk/ (accessed 22 February 2019)]. Methods have been proposed111,112 for cross-country comparisons, but were not employed in this trial.
Data archiving
Original questionnaires, CRFs and consent forms have been securely archived at the University of Dundee and are to be held for 7 years following publication of the last paper or report from the trial; patient identifiers have been separated from clinical and outcome data. Archiving of trial data was carried out according to the relevant standard operating procedure, as detailed below.
A full set of source data, including trial definition data and audit trail data were exported from the clinical data management system database (MACRO) for archiving. Archived data were saved on a read-only compact disc (CD) or digital versatile disc (DVD) for archiving in the trial master file, stored with the sponsor. For future longevity, all data were saved to CD/DVD using a comma-separated values (CSV) format; other files were saved in portable document format (PDF) or hypertext markup language (HTML). The archived MACRO data set included the full audit trails of data changes, including who made the change and when. Additional data such as questionnaires and ICDAS data were also included on the archive CDs in CSV format, ensuring that all files/data could be linked by the participant trial identifier. In addition to this, the statistics analysis folder with Stata data files and associated Stata analyses programmes was archived.
Patient and public involvement
Extensive consultation with all stakeholders – dental practitioners, parents and children – informed this trial from the outset.
Prior to submission of the original funding application, GDPs attending a CPD-accredited meeting were asked about their willingness to take part in a trial of traditional fillings versus no active treatment. They fed back that they would not take part in any trial in which their child patients might be randomised to no care at all, likening it to ‘supervised neglect’. Therefore, an alternative approach, with the ‘no active treatment’ arm comprising prevention alone, was posed to them. With this qualification, they said they would then be willing to take part in a trial. This caveat was pivotal to subsequent trial design, in which best-practice prevention was put at the heart of all treatment, as it also formed a key component of intervention in the C+P and B+P arms.
Prior to submission for REC approval for the pilot trial, informal discussions were held with parents and children in Sheffield who had experience of receiving treatment of carious lesions. The feedback was to review the format and content of the participant information sheets and consent/assent forms and to make changes to these documents to improve their acceptability to participants and their families. The focus on preparing materials that allowed children to be part of the consent/assent process113 was commended by the REC. Two participant representatives were recruited and actively engaged in the TSC throughout the project. These representatives were the mothers of young children who had experienced premature loss of primary teeth as a result of dental decay.
Initial involvement of the participant representatives included working with the research team on the development of parental and child questionnaires for use at each visit in the trial. The front cover of the FiCTION trial child questionnaire was a drawing by one of the participant representative’s 6-year-old daughter, illustrating a visit to the dentist.
Following the pilot trial, focus groups were held with child and parent participants in Sheffield to gain insight into their pilot trial experiences. This information, although an element of planned qualitative data collection rather than a patient and public involvement activity, also informed a number of changes to reduce the burden on participants in the main trial. The participant representatives were also engaged in the design and provision of material presented on the FiCTION trial website. An example of one of the initiatives taken was the inclusion of two competitions: one for colouring and a subsequent one for drawing, as the children in the trial became older.
The active engagement of children, their parents and the participant representatives, in the development of, and feedback on, the trial, was very beneficial to its smooth running, particularly in ensuring continued commitment of the child and parent participants throughout the 3 years of engagement for each family.
Chapter 3 Trial results and clinical effectiveness
Introduction
This chapter presents the findings relating to clinical effectiveness. It starts by presenting the characteristics of the practices recruited. Next, participant flow is described, followed by the baseline characteristics of the participants in the three trial arms. This is followed by a description of the treatment provided, including treatment deviations. Next we report trial findings for the co-primary outcomes of dental pain and/or dental sepsis: the ITT analyses, the pre-planned sensitivity and exploratory analyses, and the PP analyses. The chapter concludes with the ITT analyses of the secondary outcomes of caries incidence and child- and parent-reported outcomes.
Dental practices
Dental practice recruitment
The original practice (site) recruitment target was 50 dental practices, comprising approximately 10 practices from each of the five CCs (see Chapter 2, Practice recruitment): Scotland, Newcastle, Leeds/Sheffield, Wales and London. However, patient recruitment rate was lower than predicted within practices and some practices that originally initiated later withdrew. Following consultation with the TSC, the IDMC and the NIHR HTA programme, the target number of practices was increased to 70. The CCs were also expanded geographically, as described in Chapter 2, Practice recruitment.
Of the 93 practices that agreed to participate and received a site initiation visit, 21 did not randomise any participants, leaving 72 sites across the five CCs that recruited at least one participant. Ten of these 72 practices subsequently withdrew.
Dental practice characteristics
Practice characteristics collected were size (number of registered patients), deprivation index in quintiles (see Chapter 2, Planned exploratory multivariable analyses) and tap-water fluoridation status (p.p.m.F–). These data are presented descriptively (Table 2) for the 72 practices that recruited at least one participant.
| Characteristic | Practices, n (%) (N = 72) |
|---|---|
| Region | |
| Scotland | 25 (35) |
| Newcastle | 19 (26) |
| Leeds/Sheffield | 13 (18) |
| Wales | 4 (6) |
| London | 11 (15) |
| Practice size (number of registered patients) | |
| 1–4999 | 19 (26) |
| 5000–9999 | 15 (21) |
| 10,000–14,999 | 1 (1) |
| ≥ 15,000 | 1 (1) |
| No information | 36 (50) |
| Deprivation index (quintile) | |
| 1 (most deprived) | 23 (32) |
| 2 | 21 (29) |
| 3 | 10 (14) |
| 4 | 12 (17) |
| 5 (least deprived) | 6 (8) |
| Tap water fluoridation status (p.p.m.F-)a | |
| < 0.3 | 63 (88) |
| 0.3–0.7 | 5 (7) |
| > 0.7 | 4 (6) |
Participant journey
Screening at the sites
Patient/participant journey through invitation, screening, eligibility assessment, randomisation and subsequent progress through the trial is shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 2.
FIGURE 2.
The CONSORT flow diagram of participant journey through the trial. a, Prior to the start of the study, it was estimated that 18,717 children would be invited; b, prior to the start of the study, it was estimated that 65% of children invited would attend a screening appointment; 64% attended; c, prior to the start of the study, it was estimated that 85% of children screened would be ineligible – 76% were ineligible and 1% declined screening; d, prior to the start of the study, it was estimated that 20% of children screened and found eligible would decline to take part in the trial – 19% of those eligible declined; e, prior to the start of the study, it was estimated that 12% of children screened would be randomised – 15% of those screened were randomised. Reproduced from Innes NP, Clarkson JE, Douglas GVA, Ryan V, Wilson N, Homer T, et al. , Journal of Dental Research, 99(1), pp. 36–43, copyright © 2020 by International & American Associations for Dental Research 2019. Reprinted by Permission of SAGE Publications, Inc. 88

Recruitment rate
Recruitment opened on 1 October 2012; the first child was randomised on 12 October 2012. The trial closed to recruitment on 30 June 2015; the last child to enter the trial was randomised on 18 June 2015.
Figure 3 shows the recruitment rates originally anticipated, the revised target (October 2013), the further revised target (August 2014) and actual recruitment.
FIGURE 3.
Cumulative number of child participants randomised by month (all randomised analysis set, n = 1144).
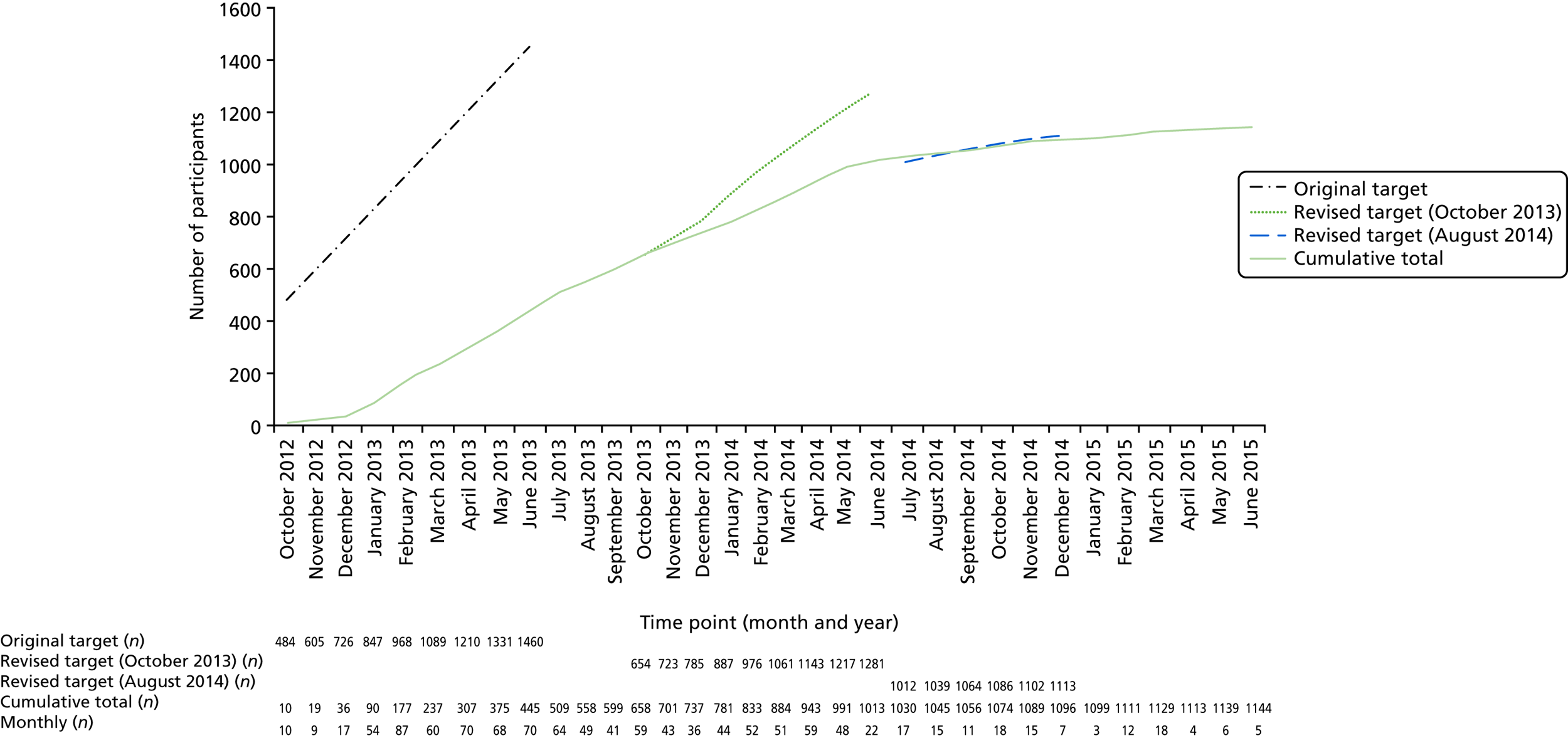
Recruitment by arm
Seventy-two sites had randomised at least one child by the trial recruitment closure date. There was variation across centres in the numbers of participants recruited and randomised: Scotland, 307; Newcastle, 315; Leeds/Sheffield, 171; Wales, 125; and London, 226 participants. The number of randomised participants per practice ranged from 1 to 55, with a median of 14 [interquartile range (IQR) 5–23.5] (see Appendix 4, Table 22).
Numbers analysed: intention-to-treat analysis (1058 participants)
The ITT analysis set was defined as all randomised children with at least one CRF completed and in MACRO, including protocol violators and ineligible randomised participants, retaining participants in their randomised treatment groups. Of the 1144 randomised individuals, 86 (7.5%) did not return for any treatment and therefore had no CRF data collected: C+P, 34 out of 386 (8.8%); B+P, 29 out of 381 (7.6%); and PA, 23 out of 377 (6.1%). This left 1058 participants (from 68 of the 72 randomising practices) who constituted the ITT analysis data set on which subsequent analyses were based.
Ineligible participants
Ineligible participants in the ITT analysis set were classed as those randomised but subsequently found to not meet the eligibility criteria of the trial (see Chapter 2, Trial participants). Of the 22 participants who were considered thus ineligible, five were outside the age range of 3–7 years at consent date and 17 had dental pain and/or dental sepsis associated with carious teeth at the consent date. The 22 ineligible participants were distributed across the three arms (C+P, n = 6; B+P, n = 5; and PA, n = 11). As the proportion of ineligible participants was small [22 of 1058 in the ITT analysis set (2.1%)], a sensitivity analysis without them was not conducted.
First trial visits for intention-to-treat analysis set (1058 participants)
For the ITT analysis set, time from randomisation to first trial visit was similar across arms, with most participants (n = 736, 69.6%) having their first trial visit within 1 month of randomisation. For the remaining participants, the timing of their first trial visit post randomisation was as follows:
-
more than 1 and up to and including 4 months, n = 241 (22.8%)
-
more than 4 and up to and including 12 months, n = 63 (6.0%)
-
more than 12 months, n = 18 (1.7%).
The distribution of time to first trial visit was balanced across arms.
Follow-up and final trial visits
Summary data for the time in trial and number of visits, by arm, are shown in Table 3. Participants spent a median of just less than 34 months in the trial, with a median of 2.5 visits per year. These parameters were well balanced across arms. A total of 711 participants attended a final trial visit (67% of the ITT participants); this was well balanced across the three arms.
| Time in trial and number of visits | C+P (n = 352) | B+P (n = 352) | PA (n = 354) | Total (n = 1058) |
|---|---|---|---|---|
| Time in trial (months) | ||||
| Minimum | 0.03 | 0.03 | 0.03 | 0.03 |
| Median (IQR) | 34.0 (24.7, 36.6) | 33.6 (24.4, 36.8) | 33.5 (22.2, 36.8) | 33.8 (23.8, 36.7) |
| Maximum | 51.4 | 53.7 | 46.7 | 53.7 |
| Total number of trial visits | ||||
| Minimum | 1 | 1 | 1 | 1 |
| Median (IQR) | 7 (5–10) | 7 (5–10) | 6 (4–9) | 7 (5–9) |
| Maximum | 30a | 22 | 20 | 30b |
| Median number of trial visits over median time (years) in trial | 2.5 | 2.5 | 2.1 | 2.5 |
| Number (%) of the ITT participants who attended a final trial visit | 244 (69.3) | 237 (67.3) | 230 (65.0) | 711 (67.2) |
| In or after their scheduled final visit window | 234 (66.5) | 219 (62.2) | 212 (59.9) | 665 (62.9) |
| Before their scheduled final visit window, but identified as a final visit | 10 (2.8) | 18 (5.1) | 18 (5.1) | 46 (4.3) |
| Number (%) of the ITT participants who did not attend a final trial visit | 108 (30.7) | 115 (32.7) | 124 (35.0) | 347 (32.8) |
| Withdrawn | 40 (11.4) | 35 (9.9) | 43 (12.1) | 118 (11.2) |
| Lost to follow-up | 68 (19.3) | 80 (22.7) | 81 (22.9) | 229 (21.6) |
Withdrawals
There were 118 documented withdrawals from the ITT analysis set. All withdrawals were complete; that is, there was consent to use participants’ data up to the point of withdrawal but with no further follow-up or data collection after that point. Reasons for withdrawal were ascertained for 108 recruited participants.
Seven randomising practices withdrew (n = 38 participants). The median time from first randomisation to date of practice withdrawal was 561 days (IQR 482–822 days).
Other reasons for withdrawal were the participant moved away (n = 34); trial fatigue (n = 4); dental reason, for example a traumatic event (n = 8); and personal or another reason (n = 24). The numbers of participants who withdrew were evenly distributed across the three trial arms with no differences in the length of time in trial (time from randomisation to time of last known trial visit) or in the number of trial visits (see Appendix 4, Table 23).
Baseline data
Baseline comparability
Demographic and clinical baseline characteristics were compared across treatment arms descriptively (Table 4). No significance testing was carried out because of the randomised nature of the trial. Characteristics were well balanced across arms.
| Participant characteristic | Non-missing data points (n) | C+P (N = 352) | Non-missing data points (n) | B+P (N = 352) | Non-missing data points (n) | PA (N = 354) | Non-missing data points (n) | Total (N = 1058) |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 352 | 351 | 354 | 1057 | ||||
| Mean (SD) | 5.97 (1.3) | 6.01 (1.3) | 5.91 (1.2) | 5.96 (1.3) | ||||
| Female | 349 | 349 | 349 | 1047 | ||||
| n (%) | 175 (50.1) | 181 (51.9) | 180 (51.6) | 536 (51.2) | ||||
| Ethnicity | 313 | 322 | 320 | 955 | ||||
| White, n (%) | 236 (75.4) | 248 (77.0) | 243 (75.9) | 727 (76.1) | ||||
| Black, n (%) | 9 (2.9) | 11 (3.4) | 10 (3.1) | 30 (3.1) | ||||
| Indian, Pakistani or Bangladeshi, n (%) | 37 (11.8) | 38 (11.8) | 36 (11.3) | 111 (11.6) | ||||
| Chinese, n (%) | 5 (1.6) | 3 (0.9) | 3 (0.9) | 11 (1.2) | ||||
| Mixed race, n (%) | 11 (3.5) | 13 (4.0) | 13 (4.1) | 37 (3.9) | ||||
| Other, n (%) | 15 (4.8) | 9 (2.8) | 15 (4.7) | 39 (4.1) | ||||
| d3mft | 339 | 333 | 334 | 1006 | ||||
| Mean (SD) | 2.8 (2.7) | 2.8 (2.7) | 2.6 (2.6) | 2.7 (2.7) | ||||
| Median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | ||||
| P-CPQ-16 | 300 | 314 | 309 | 923 | ||||
| Mean (SD) | 8.9 (6.7) | 8.0 (6.3) | 8.3 (6.2) | 8.4 (6.4) | ||||
| MCDASf | 336 | 324 | 329 | 989 | ||||
| Mean (SD) | 13.8 (4.9) | 14.2 (5.3) | 14.3 (5.3) | 14.1 (5.1) |
As Table 4 and Appendix 4, Table 24, show, children’s caries experience at entry to the trial (baseline) was balanced between the three trial arms for the number of primary teeth affected and the amount and severity of the lesions. The mean number of primary teeth affected by caries (d3mft) was 2.72 [standard deviation (SD) 2.66] [the national mean d3mft values for 5-year-olds who are not free of obvious carious lesions (i.e. d3mft of > 0) are England (2015) 3.4, Scotland (2016) 3.93 and Wales (2015–16) 3.58]. 13,26,115 It should be noted, however, that the national surveys are of randomly selected children recruited in the school setting and not from those attending general dental practices, whose oral health may be better than non-attenders.
As the mean age of participants was 6 years, they had few FPMs present on entry to the trial; the mean number of FPMs recorded as at least partially erupted was 1.74 (SD 1.88). The decay experience in these teeth was very low, again as might be expected for relatively newly erupting permanent teeth, with a mean D3MFT for FPMs of 0.05 (SD 0.35), and this was balanced between the trial arms (see Appendix 4, Table 25).
Baseline questionnaires were returned for almost all children [n = 1045 (99%)] and had a high level of completeness.
Child OHRQoL at baseline also showed balance between arms, with an overall mean score of 8.4 (SD 6.4), reflecting a group with an OHRQoL score similar to that found in other child populations. 95 The results of the MCDASf, with an overall mean score of 14.1 (SD 5.1), were also balanced across arms at baseline and similar to scores reported in other UK children. 103
Treatment provision and adherence to protocol
Treatment received (intention-to-treat analysis set, n = 1058)
A detailed breakdown of the treatments received is provided as part of the economic evaluation (see Chapter 4). Appendix 5, Table 74, summarises the average total treatment provided at the first visit and at follow-up visits by arm. This table provides insight into how the children were initially treated, how the treatment progressed over the follow-up period and the differences in treatment by arm.
By visit
The interventions were multicomponent and were designed with preventative strategies (also consisting of more than one component) being common to all three arms. Prevention (or at least one prevention component or ‘pillar’) was delivered, on average, at 81% (0.81) of all visits [B+P, mean 0.79 (SD 0.22); C+P, mean 0.79 (SD 0.22); and PA, mean 0.85 (SD 0.19)]. All four prevention pillars were delivered in each of the three arms, in line with the clinical protocols for each arm, but rates of delivery were higher in the PA arm and similar between the C+P and B+P arms. The most commonly provided prevention component was tooth-brushing/plaque control advice (at 75% of all visits) and diet investigation/advice (69%), followed by fluoride varnish applications (57%) and fissure of permanent teeth (15%). The vast majority of preventative care was provided by GDPs (71% of all visits) and the average length of time spent providing preventative treatment was 7.5 minutes [B+P, mean 7.14 (SD 4.8); C+P, mean 7.0 (mean 4.5); and PA, mean 8.5 (SD 4.3) minutes].
Operative care occurred at 34% (0.34) of all visits [B+P, mean 0.42 (SD 0.26); C+P, mean 0.42 (SD 0.26); and PA, mean 0.19 (SD 0.23)] and was also primarily undertaken by GDPs (91% of operative treatment visits were undertaken by a GDP). The provision of operative care decreased as the trial progressed, as highlighted in Appendix 5, Table 74. On average, those children who had operative treatment had four primary teeth treated during their time in the trial.
The rate of restoration placement was estimated as the average of the total number of primary teeth treated with a restoration, divided by the total number of primary teeth treated at each visit per child. The main non-prevention-based care provided was placement of a restoration (at 28% of all visits) or extractions (3% of teeth managed by non-prevention-based care at a visit were extracted). Extractions of primary teeth were balanced across the three arms [B+P, mean 0.03 (SD 0.08); C+P, mean 0.04 (SD 0.07); and PA, mean 0.03 (SD 0.09)]; this can be interpreted as, on average, each child randomised to B+P having 3% of their operatively treated primary teeth extracted at each visit. However, this did not account for all tooth extractions as, additionally, extractions were carried out following referral to secondary/tertiary care centres.
Referral rates differed slightly between arms (C+P, 9%; B+P, 10%; and PA, 11%), with the majority (C+P, 69%; B+P, 61%; and PA, 69%) of those referrals being for day case dental extractions under general anaesthetic (group B) or inhalational sedation for extractions and/or restorations (group D) (see Appendix 5, Table 74). For explanations of groups B and D, see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019).
In line with the intervention arms, and as specified in the clinical protocols, the provision of restorations varied between the three arms; the percentages of visits with a restoration provided over the course of the trial were as follows: C+P, 36%; B+P, 36%; and PA, 12%. In the C+P arm, the majority of routine restorations performed were GIC restorations (on average, each child randomised to the C+P arm had 11% of their operatively treated primary teeth treated with a GIC restoration at each visit), followed by RMGI restorations (8%) and composite restorations (7%). In the B+P arm, the majority of restorations were GIC restorations (11%), followed by PMCs placed using the Hall Technique (9%) and RMGIs (7%). As expected, the PA arm involved little operative care, being largely prevention and extraction based, with 6% of operatively treated teeth receiving a GIC restoration.
By course of treatment
It was expected that, for every course of treatment [for a definition, see Chapter 4 and the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)], a participant in any arm would receive fluoride varnish plus at least one of the remaining three prevention components, when relevant: (1) brushing/plaque control advice, (2) diet investigation/advice or (3) fissure sealing of permanent teeth. Slightly more fluoride varnish was provided in the PA arm as part of a course of treatment in the C+P arm, 62% of courses of treatment had fluoride varnish; in the B+P arm this was 64%, and in the PA arm 73%.
Over the follow-up period, fluoride varnish was provided at 67% of all courses of treatment. Similarly for the components of prevention by visit, the order in which they were provided per course of treatment remained the same (brushing/plaque control advice, 84%; diet investigation/advice, 78%; and fissure sealants for permanent teeth, 18%) (see Appendix 5, Table 75, for the course of treatment summaries by arm).
Adherence to protocol
‘Major’ deviations (i.e. clinical treatment provided not according to the randomised arm) reflected non-adherence to the clinical treatment protocol. At 6% (429/7713) of visits, we observed a ‘major’ deviation from the clinical protocol for a participant’s randomised treatment arm, that is a cross-arm deviation. These major deviations occurred in 263 participants, with 46% among participants randomised to the C+P arm, 15% in the B+P arm and 39% in the PA arm. The most frequently reported direction of treatment deviation for the C+P arm was to B+P (69%); for the B+P arm it was to C+P (80%); and for the PA arm it was to C+P (53%). The main reason for major deviations were parent factors (28%) and dentists’ clinical judgements (29%). The participants with major deviations were not equally distributed across trial arms: C+P, 99 out of 352 (28%); B+P, 51 out of 352 (14%) and PA, 113 out of 354 (32%). Further details on the reason for and direction of all major deviations are shown in Appendix 4, Tables 26 and 27.
For 104 participants (10%), > 20% of their treatment visits involved a major treatment deviation from their randomised treatment arm, and they accounted for 182 of the 429 major treatment deviations recorded on TDFs. Across the trial arms, these participants were distributed as follows:
-
C+P arm, 37 out of 352 participants (11%)
-
B+P arm, 19 out of 352 (5%)
-
PA arm, 48 out of 354 (14%).
These 104 participants remained in the ITT analyses but were removed from the PP analyses.
Use of radiographs
For the ITT analysis set, radiographs were taken at 11% (886/7713) of visits over the duration of the trial. This was less than originally anticipated [see Chapter 2, Schedule of assessment of primary outcomes, and the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. The 886 radiographs were taken in 48% (511/1058) of children and distributed evenly across all three treatment arms: C+P, 49% (174/352); B+P, 47% (165/352); and PA, 49% (172/354). Sixty per cent (308/511) of children who had a radiograph taken had at least one taken during their first year in the trial; this accounted for 39% (349/886) of all radiographs taken.
The reasons for not using radiographs were collected via the CRF at 94% of visits. The justifications given were similar across treatment arms:
-
not due to be taken, according to FGDP guidelines (42%)
-
radiograph(s) not attempted (child compliance issues) (18%)
-
already taken during this course of treatment (18%)
-
other (12%)
-
attempted but failed (5%)
-
parental wish (4%).
When radiographs were taken, the presence or absence of periradicular infection was recorded in 73% of cases. The rate of periradicular infection was 11% and was balanced across arms; however, 17% of radiographs assessed for periradicular infection were considered to be of limited diagnostic use.
Given that the percentage of children with radiographs taken was less than our pre-defined threshold of 80% [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)], this source of data was excluded from contributing to the analysis of outcomes related to dental sepsis.
Co-primary outcomes: dental pain and/or dental sepsis
The primary outcome analysis was a comparison of children’s experience of dental pain and/or dental sepsis, during the follow-up period, across the three treatment arms. This was analysed in two ways: (1) as the proportion of children with at least one episode of dental pain and/or dental sepsis during the follow-up period (incidence) and (2) as the total number of episodes of dental pain and/or dental sepsis for each child during the follow-up period.
Proportion of children with at least one episode of dental pain and/or dental sepsis during the follow-up period (incidence)
Descriptives: incidence of dental pain and/or dental sepsis
For the ITT analysis set, 42.5% of participants experienced at least one episode of dental pain and/or dental sepsis during the follow-up period (Table 5). Although the absolute differences in the incidence of dental pain and/or dental sepsis observed between the arms were smaller than anticipated at the design stage, the rank order was as expected, with the lowest rate in the B+P arm and the highest in the PA arm.
| Outcome | Treatment arm, n (%) | Total (N = 1058), n (%) | ||
|---|---|---|---|---|
| C+P (N = 352) | B+P (N = 352) | PA (N = 354) | ||
| Dental pain ever | 126 (35.8) | 113 (32.1) | 140 (39.5) | 379 (35.8) |
| Dental sepsis ever | 90 (25.6) | 87 (24.7) | 91 (25.7) | 268 (25.3) |
| Dental pain and/or dental sepsis ever | 148 (42.0) | 141 (40.1) | 161 (45.5) | 450 (42.5) |
When the descriptive analysis was restricted to participants with at least 23 months’ follow-up as a planned sensitivity analysis, the overall incidence of dental pain and/or dental sepsis increased from 42.5% to 47% (see Appendix 4, Table 28). For the PP analysis set, the overall incidence reduced to 40% (see Appendix 4, Table 29).
Statistical modelling: incidence of dental pain and/or dental sepsis
The results of fitting logistic regression models to the incidence of dental pain and/or dental sepsis during the follow-up period for the ITT analysis set (Table 6) are presented as unadjusted and adjusted risk differences, comparing the B+P arm and the PA arm with the C+P arm. A negative risk difference indicates a reduced risk of dental pain and/or dental sepsis during the follow-up period, in comparison with the C+P arm. The adjusted risk difference in the incidence of dental pain and/or dental sepsis over the follow-up period in the B+P arm compared with the C+P arm was –0.02 (97.5% CI –0.10 to 0.06), which indicates, on average, a 2% reduced risk of dental pain and/or dental sepsis in the B+P arm compared with the C+P arm. For the PA arm compared with the C+P arm, the adjusted risk difference was 0.04 (97.5% CI –0.04 to 0.12), which indicates, on average, a 4% increased risk of dental pain and/or dental sepsis in the PA arm compared with the C+P arm. There was no evidence of a statistically significant difference between the arms when compared with the C+P arm. The comparative results remained almost unchanged when the adjusted analysis was restricted to participants with at least 23 months’ follow-up and when the PP analysis set was used (see Appendix 4, Table 30).
| Analysis set | Model | Arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|---|---|
| ITT | Unadjusted (n = 1058) | C+P | 0.00 | ||
| B+P | –0.02 | –0.10 to 0.06 | 0.6 | ||
| PA | 0.04 | –0.05 to 0.12 | 0.3 | ||
| Adjusted (n = 1057) | C+P | 0.00 | |||
| B+P | –0.02 | –0.10 to 0.06 | 0.6 | ||
| PA | 0.04 | –0.04 to 0.12 | 0.2 |
Total number of episodes of dental pain and/or dental sepsis during the follow-up period
Descriptives: number of episodes of dental pain and/or dental sepsis
For the ITT analysis set, a total of 676 episodes of dental pain and/or dental sepsis were recorded over the follow-up period, which is an average of 0.64 episodes per participant. The number of episodes ranged from 0 to 7, but the majority of participants (86%) had 0 or 1 episodes (Table 7). The distribution of the number of episodes was similar across the arms, although the unadjusted rate of episodes in the PA arm was slightly higher, at 0.72 episodes per participant, than in the C+P arm (0.62 episodes per participant) or the B+P arm (0.58 episodes per participant). The PA arm had more participants experiencing ≥ 2 episodes of dental pain and/or dental sepsis than the C+P or B+P arm (17.5% vs. 12% vs. 12.5%, respectively) (Figure 4).
| Episodes of dental pain and/or dental sepsis | C+P (N = 352) | B+P (N = 352) | PA (N = 354) | Total (N = 1058) |
|---|---|---|---|---|
| Minimum | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Mean (SD) | 0.62 (0.95) | 0.58 (0.87) | 0.72 (0.98) | 0.64 (0.94) |
| Maximum | 7 | 6 | 5 | 7 |
| Number, n (%) | ||||
| 0 | 204 (58.0) | 211 (59.9) | 193 (54.5) | 608 (57.5) |
| 1 | 106 (30.1) | 97 (27.6) | 99 (28.0) | 302 (28.5) |
| 2 | 23 (6.5) | 29 (8.2) | 40 (11.3) | 92 (8.7) |
| 3 | 15 (4.3) | 13 (3.7) | 15 (4.2) | 43 (4.1) |
| 4 | 2 (0.6) | 1 (0.3) | 5 (1.4) | 8 (0.76) |
| 5 | 0 (0.0) | 0 (0.0) | 2 (0.6) | 2 (0.2) |
| 6 | 1 (0.3) | 1 (0.3) | 0 (0.0) | 2 (0.2) |
| 7 | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
FIGURE 4.
Number of episodes of pain and/or sepsis by randomised treatment arm (ITT analysis set). (a) C+P (n = 352); (b) B+P (n = 352); (c) PA (n = 354); and (d) total (n = 1058).
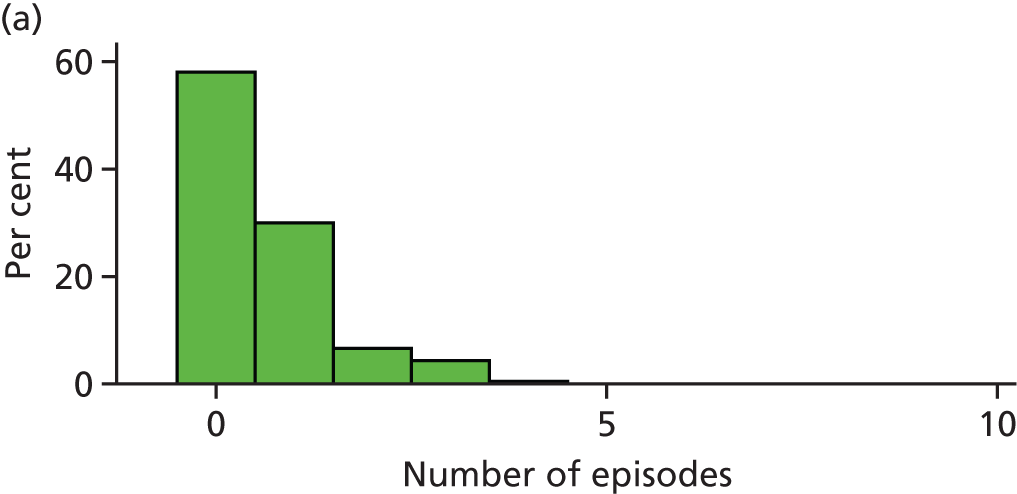
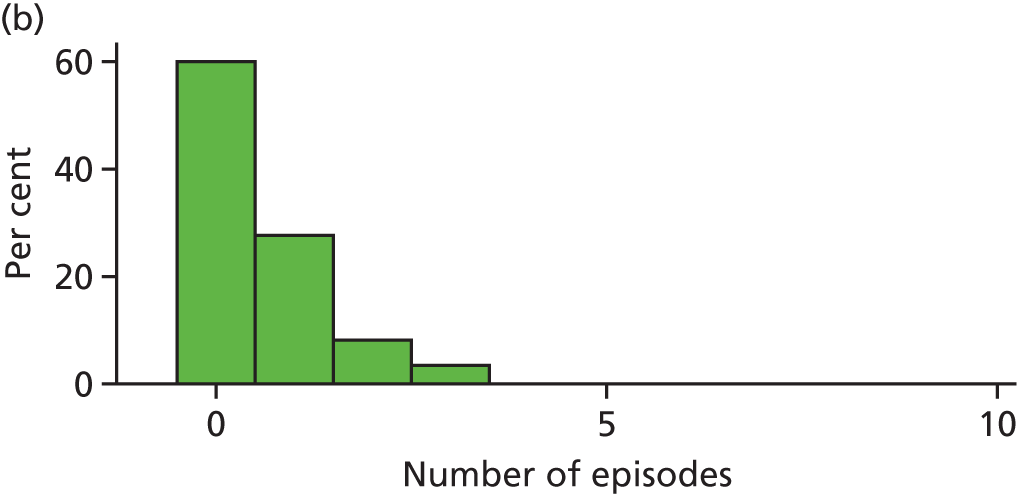

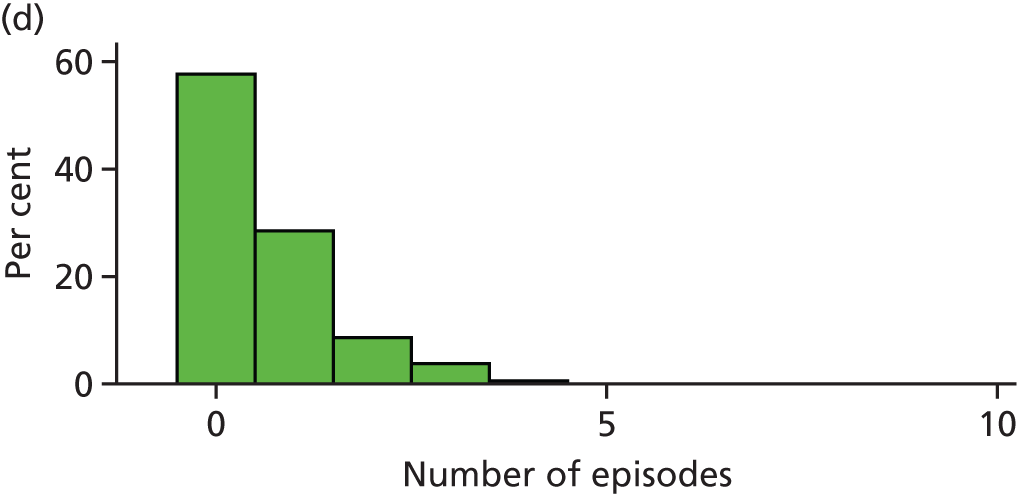
When the descriptive analysis of episodes was restricted to participants with at least 23 months’ follow-up (n = 797), the overall mean number of episodes per participant increased from 0.64 to 0.72 (see Appendix 4, Table 31). For the PP analysis set (n = 940), the overall rate of episodes reduced to 0.59 (see Appendix 4, Table 32).
Statistical modelling: number of episodes of dental pain and/or sepsis
The results of fitting negative binomial regression models to the number of episodes of dental pain and/or dental sepsis during the follow-up period are presented in Table 8 as unadjusted and adjusted IRRs, comparing the B+P arm and the PA arm with the C+P arm. An IRR of < 1 indicates a reduction in the rate of episodes of dental pain and/or dental sepsis during the follow-up period in comparison with the C+P arm. The adjusted IRR of 0.95 (97.5% CI 0.75 to 1.21) for the B+P arm relative to the C+P arm indicates that there were 5% fewer episodes, on average, in the B+P arm than in the C+P arm; for the PA arm relative to the C+P arm, the adjusted IRR of 1.18 (0.94 to 1.48) indicates that, on average, there were 18% more episodes in the PA arm than in the C+P arm. There was no evidence of a statistically significant difference between the arms when comparing the B+P arm with the C+P arm or the PA arm with the C+P arm. The comparative results of the adjusted analysis remained almost unchanged when the PP analysis set was used (see Appendix 4, Table 33). However, when the adjusted analysis was restricted to participants with at least 23 months’ follow-up, the IRR for the comparison of the PA arm with the C+P arm increased from 1.18 to 1.26 (97.5% CI 0.98 to 1.63), indicating that there were 26% more episodes, on average, in participants with at least 23 months’ follow-up in the PA arm when compared with the C+P arm, although this was not statistically significant at the 2.5% level. This was because a higher proportion of children had more than one episode of dental pain and/or dental sepsis (i.e. rather than just more children with one episode) (see Appendix 4, Table 33).
| Analysis set | Model | Arm | IRR | 97.5% CI | p-value |
|---|---|---|---|---|---|
| ITT | Unadjusted (n = 1058) | C+P | 1.00 | ||
| B+P | 0.94 | 0.74 to 1.21 | 0.6 | ||
| PA | 1.17 | 0.93 to 1.48 | 0.1 | ||
| Adjusted (n = 1057) | C+P | 1.00 | |||
| B+P | 0.95 | 0.75 to 1.21 | 0.6 | ||
| PA | 1.18 | 0.94 to 1.48 | 0.1 |
Secondary analysis of the primary outcome: time to first episode of dental pain and/or dental sepsis
Descriptives: time to first episode of dental pain and/or dental sepsis
The Kaplan–Meier survival curves for the time to first episode of dental pain and/or dental sepsis for the ITT analysis set are shown in Figure 5. From these curves, the estimated probability of having no dental pain and/or dental sepsis at 2 years post randomisation in the C+P arm is 0.64 (97.5% CI 0.58 to 0.69), in the B+P arm it is 0.65 (97.5% CI 0.59 to 0.70) and in the PA arm it is 0.56 (97.5% CI 0.50 to 0.61). Further Kaplan–Meier probabilities by length of time in the trial in years and treatment arm are given in Appendix 4, Table 34. The median time to first episode and associated quartiles by treatment arm are given in Appendix 4, Table 35.
FIGURE 5.
Kaplan–Meier estimates of survivor functions by randomised treatment arm, generated using Stata version 14 (StataCorp LP, College Station, TX, USA) (ITT analysis set, n = 1058). (a) Total; (b) C+P arm; (c) B+P arm; and (d) PA arm.


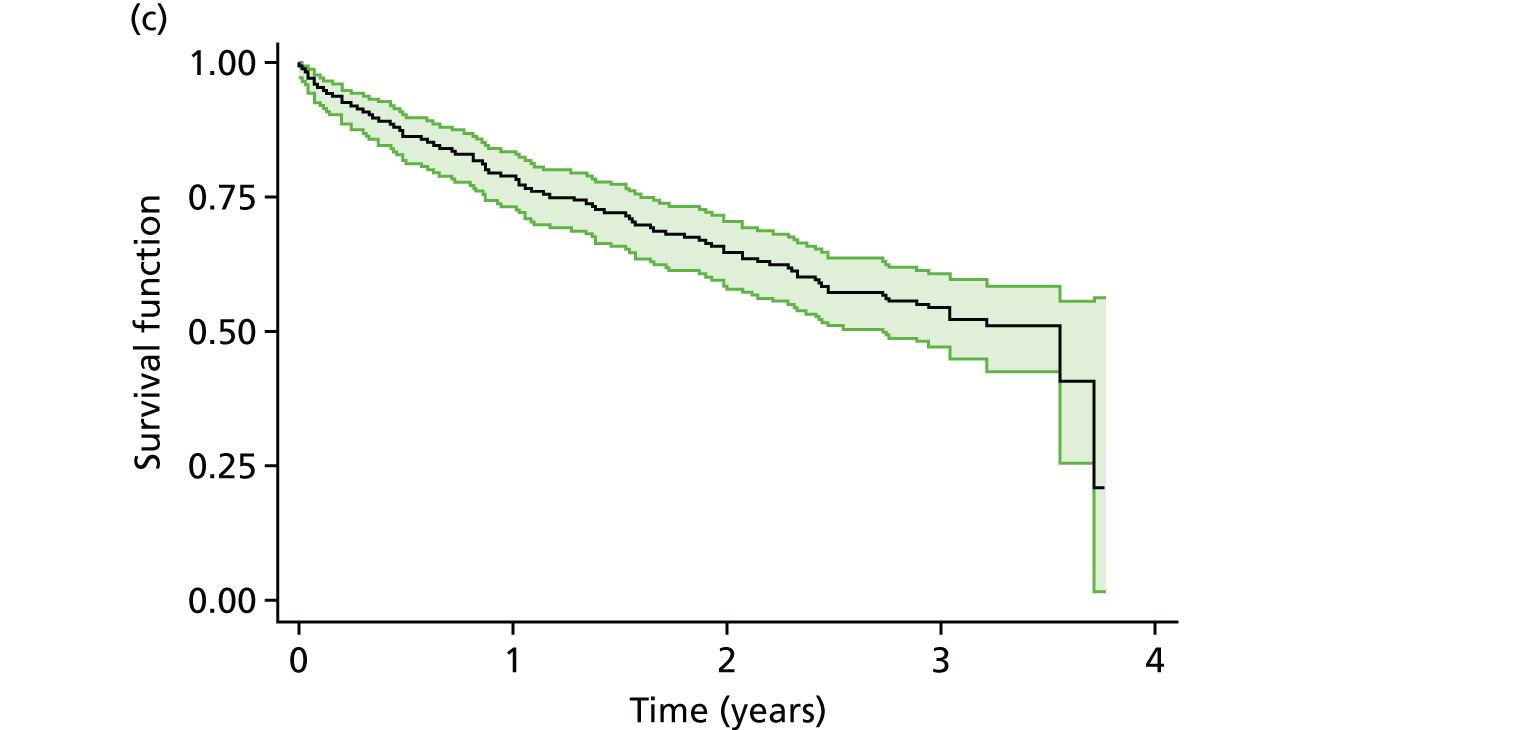

Statistical modelling: time to first episode of dental pain and/or dental sepsis
The results of fitting a Cox proportional hazards model to the time to first episode of dental pain and/or dental sepsis data are presented in Table 9, as adjusted HRs comparing the B+P and PA arms with the C+P arm. A HR of < 1 indicates a reduction in the risk of a first episode of dental pain and/or dental sepsis in comparison with the C+P arm. The HR of 0.95 (97.5% CI 0.73 to 1.24) for the B+P arm relative to the C+P arm indicates that there was a 5% reduced risk of a first episode of dental pain and/or dental sepsis in the B+P arm when compared with the C+P arm. The HR of 1.19 (97.5% CI 0.92 to 1.53) in the PA arm relative to the C+P arm indicates a 19% increased risk of a first episode of dental pain and/or dental sepsis in the PA arm when compared with the C+P arm. There was no evidence of a statistically significant difference between the arms in the risk of a first episode of dental pain and/or dental sepsis.
| Trial arm | HR | 97.5% CI | p-value |
|---|---|---|---|
| C+P | 1.00 | ||
| B+P | 0.95 | 0.73 to 1.24 | 0.7 |
| PA | 1.19 | 0.92 to 1.53 | 0.1 |
Pre-planned exploratory analyses: dental pain and/or dental sepsis ever (ITT analysis set)
The relationship between the incidence of dental pain and/or dental sepsis during the follow-up period and the explanatory variables of age, ethnicity, practice tap water fluoride level, index of deprivation and number of decayed teeth at baseline from ICDAS charting (level 5/6 cavitation), which are described in Chapter 2, Planned exploratory multivariable analyses, were explored descriptively (Table 10) and in univariate and multivariable logistic regression models (see Appendix 4, Tables 36 and 37). From the summary statistics presented in Table 10, those participants who had dental pain and/or dental sepsis had, on average, more teeth with level 5/6 cavitation at baseline than those who did not. All other variables had very similar distributions across the two groups. This is also shown in the exploratory univariate analysis in which the only variable significantly associated with the incidence of dental pain and/or dental sepsis was the number of teeth with level 5/6 cavitation (see Appendix 4, Table 36). The exploratory multivariable model for incidence of dental pain and/or dental sepsis that was adjusted for all exploratory variables did not provide evidence at the 2.5% level of a difference between the arms (see Appendix 4, Table 37).
| Variable | Dental pain and/or sepsis ever | |||
|---|---|---|---|---|
| Non-missing data points (n) | Yes (N = 450) | Non-missing data points (n) | No (N = 608) | |
| Age (years), mean (SD) | 450 | 5.9 (1.2) | 607 | 6.0 (1.3) |
| Ethnicity (white), n (%) | 402 | 312 (77.6) | 553 | 415 (75.1) |
| Fluoride level (p.p.m.)a | 450 | 608 | ||
| Minimum | 0.003 | 0.003 | ||
| Median (IQR) | 0.093 (0.039–0.181) | 0.096 (0.049–0.231) | ||
| Maximum | 1.024 | 1.024 | ||
| Index of deprivation (deciles)a | 450 | 608 | ||
| Minimum | 1 | 1 | ||
| Median (IQR) | 3 (2–5) | 3 (1–5) | ||
| Maximum | 10 | 10 | ||
| Decayed teeth at baseline from ICDAS charting (level 5/6 cavitation) | 433 | 0 | 573 | |
| Minimum | 0 | 0 | ||
| Median (IQR) | 2 (1–3) | 1 (0–2) | ||
| Mean (SD) | 2.1 (2.1) | 1.2 (1.6) | ||
| Maximum | 14 | 9 | ||
Secondary outcomes, part 1: incidence of carious lesions in primary and permanent teeth
The results presented in this section are from the visual assessment of carious lesions, as recorded by each dentist who had been trained to use the ICDAS method of recording carious lesions. Although radiographs could have informed on some additional carious lesions not visible during a clinical visual examination, markedly < 80% of the children had radiographs on entry to the trial (or within 1 year of entry); only 308 out of 1058 (29%) participants had at least one radiograph taken in the first year of the trial. Because the threshold for use of radiographs described in Chapter 2, Schedule of assessment of primary outcomes, was not met, ICDAS data alone are presented.
As previously described [see Chapter 2, Incidence of dental caries in primary and permanent teeth, and see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)], an ICDAS examination was scheduled at baseline and at the final visit. A total of 1006 children had an ICDAS examination completed at the baseline visit and 667 had an ICDAS examination completed at the final visit [see Appendix 4, Tables 24 and 25 (baseline) and Tables 38 and 39 (final visit)]. The following measures of incidence of new carious lesions were analysed for both the primary dentition and the FPMs for those children (n = 653 of the ITT analysis data set) who had both a baseline and final visit ICDAS chart completed (see Appendix 4, Table 40):
-
incidence of carious lesions (i.e. has there been new lesion development in at least one tooth surface for a given child, as a binary variable)
-
severity of carious lesion development (i.e. number of teeth with new lesions).
New dental carious lesions
As children entered the trial with caries experience in some of their primary teeth, the assessment of carious lesion incidence in the primary dentition over the trial period considered only teeth that were neither filled nor had dentinal lesions at baseline. We have used the term ‘sound/reversible’ for these teeth, that is those that were caries free or had non-cavitated enamel lesions only. Tables 24 and 25 in Appendix 4 show that the numbers of such teeth were balanced at baseline between the three trial arms. Teeth that were ‘sound/reversible’ at baseline but that experienced dentinal decay during the trial were taken as having caries progression and included in the analysis of caries incidence.
As children exhibited little decay experience in their FPM teeth on entry to the trial (mean age 6 years, around the time when the FPMs are erupting), and both the number of FPMs and their limited caries experience was balanced between the groups, caries incidence in the permanent teeth is based on only the assessment of the FPMs at the end of the trial.
Carious lesion incidence was analysed in this way at both the child (progression in any primary tooth or FPM) and the tooth level (number of primary teeth or FPMs that exhibited progression).
For all participants in the ITT data set with at least one ‘sound/reversible’ primary tooth or FPM at baseline and ICDAS data at follow-up (n = 653), the baseline characteristics were balanced across the three arms (see Appendix 4, Table 40).
Overall, 399 out of 653 (61.1%) participants had caries development/progression in one or more ‘sound/reversible’ primary teeth over the follow-up period [C+P, 57.9%; B+P, 61.6%; and PA, 64.1% (see Appendix 4, Table 41)]. The adjusted risk difference in the incidence of caries development/progression in primary teeth over the follow-up period (Table 11) in the B+P compared with the C+P arm was 0.03 (97.5% CI –0.06 to 0.11), indicating, on average, a 3% increased risk of caries development/progression in the B+P arm compared with the C+P arm. For the PA arm compared with the C+P arm, the adjusted risk difference was 0.05 (97.5% CI –0.03 to 0.14), indicating, on average, a 5% increased risk of lesion development/progression in the PA arm compared with the C+P arm. However, it must be noted that this analysis is on a subset (n = 653) of the ITT analysis set (61.7% of participants are included), and so must be treated with caution (see Table 11).
| Analysis set | Model | Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|---|---|
| Primary teeth: ITT | Unadjusted (n = 653) | C+P | 0.00 | ||
| B+P | 0.03 | –0.06 to 0.11 | 0.5 | ||
| PA | 0.06 | –0.03 to 0.14 | 0.1 | ||
| Adjusted (n = 652) | C+P | 0.00 | |||
| B+P | 0.03 | –0.06 to 0.11 | 0.5 | ||
| PA | 0.05 | –0.03 to 0.14 | 0.2 | ||
| FPMs: ITT | Unadjusted (n = 653) | C+P | 0.00 | ||
| B+P | 0.03 | –0.04 to 0.09 | 0.4 | ||
| PA | 0.02 | –0.04 to 0.09 | 0.5 | ||
| Adjusted (n = 652) | C+P | 0.00 | |||
| B+P | 0.03 | –0.04 to 0.09 | 0.3 | ||
| PA | 0.03 | –0.04 to 0.09 | 0.4 |
On average, this subset (n = 653) had 1.3 progressed primary teeth that had been ‘sound/reversible’ (i.e. were sound or had non-cavitated enamel lesions at baseline) (see Appendix 4, Table 41). The range of progressed primary teeth per participant was 0–6, but the majority of participants were at the lower end of this range; 81.3% of the 653 participants had between 0 and 2 teeth that progressed (0 teeth progressed, 38.9%; 1 tooth progressed, 25.6%; 2 teeth progressed, 16.8%) and the distribution between arms was similar (Figure 6). The unadjusted mean number of progressed primary teeth was 1.2 in the C+P arm, 1.2 in the B+P arm and 1.4 in the PA arm.
FIGURE 6.
Number of progressed teeth (primary teeth and FPMs) by randomised treatment arm (n = 653). (a) C+P arm, primary teeth; (b) C+P arm, FPMs; (c) B+P arm, primary teeth; (d) B+P arm, FPMs; (e) PA arm, primary teeth; and (f) PA arm, FPMs.





With regard to the number of primary teeth in which dental caries developed/progressed from ‘sound/reversible’ at baseline, as Table 12 shows, the IRR for the B+P arm relative to the C+P arm, adjusted for time in trial, age and including a random effect for practice, was 1.01 (97.5% CI 0.83 to 1.24), and for the PA arm to relative to the C+P arm it was 1.15 (97.5% CI 0.95 to 1.41). There was no evidence of a statistically significant difference at the 2.5% level between the arms when comparing the B+P with the C+P arm or the PA with the C+P arm.
| Analysis set | Model | Trial arm | IRR | 97.5% CI | p-value |
|---|---|---|---|---|---|
| Primary teeth, ITT | Unadjusted (n = 653) | C+P | 1.00 | ||
| B+P | 1.01 | 0.83 to 1.24 | 0.8 | ||
| PA | 1.16 | 0.95 to 1.41 | 0.09 | ||
| Adjusted (n = 652) | C+P | 1.00 | |||
| B+P | 1.01 | 0.83 to 1.24 | 0.8 | ||
| PA | 1.15 | 0.95 to 1.41 | 0.1 | ||
| FPMs, ITT | Unadjusted (n = 653) | C+P | 1.00 | ||
| B+P | 1.29 | 0.59 to 2.82 | 0.5 | ||
| PA | 1.29 | 0.59 to 2.79 | 0.5 | ||
| Adjusted (n = 652) | C+P | 1.00 | |||
| B+P | 1.12 | 0.52 to 2.42 | 0.7 | ||
| PA | 1.09 | 0.51 to 2.34 | 0.8 |
There was no evidence of a difference in carious lesion development/progression in FPMs between the three arms (see Tables 11 and 12, Figure 6 and Appendix 4, Table 41).
Secondary outcomes, part 2: child- and parent-reported outcomes
Children’s oral health-related quality of life
The P-CPQ-16 was administered at baseline and at the final visit. At baseline, 93% (984/1058) of parents returned at least partially completed questionnaires. Baseline and final visit parent questionnaires were received from 59% (627/1058) of parents. The P-CPQ-16 was well completed at both baseline and the final visit, with P-CPQ-16 scores being calculable, following imputation, for 94% and 95% of respondents at baseline and the final visit, respectively. Just over half of the children in the trial [560/1058 (53%)] had a P-CPQ-16 score following imputation at both baseline and the final visit (see Appendix 4, Tables 42–46). Box plots of the P-CPQ-16 scores at baseline and the final visit, and the change in score from baseline to the final visit, are shown in Figure 7.
FIGURE 7.
Box plots of P-CPQ-16 scores for the participants with both baseline and final data (n = 560). (a) Baseline scores; (b) final visit scores; and (c) change in score from baseline to final visit.

By the end of the trial scores had changed, on average, very little. In the analysis adjusted for baseline P-CPQ-16 score, age at randomisation and time in trial, the estimated mean P-CPQ-16 at the final visit was, on average, 0.27 points higher (97.5% CI –1.08 to 1.62) in the B+P arm than in the C+P arm, and 0.17 points higher, on average, (97.5% CI –1.20 to 1.53) in the PA arm than in the C+P arm. These estimated positive mean differences represent a very slightly poorer OHRQoL for children in the B+P and PA arms than for children in the C+P arm, but the differences were very small, not statistically significant and would not be considered clinically meaningful (see Appendix 4, Tables 42–46).
Children’s general anxiety and worry about dentistry (see Appendix 4, Tables 47–50)
The MCDASf was used to assess whether or not the way in which children’s teeth were managed influenced how they felt about dentistry (trait dental anxiety); lower scores represent lower anxiety. Child questionnaires were completed at every visit and were returned for 88% (6793/7713) of visits.
After adjusting for baseline MCDASf, age at randomisation and time in the trial, there was no evidence of any statistically or clinically significant difference between arms in levels of trait dental anxiety, averaged over all follow-up visits. The estimated post-baseline mean MCDASf score was 0.07 points lower for children in the B+P arm than for those in the C+P arm (97.5% CI –0.74 to 0.59), and was 0.22 points higher in the PA arm than in the C+P (97.5% CI –0.44 to 0.89 (see Appendix 4, Table 50).
Anticipatory and treatment-related anxiety and worry (state anxiety) (see Appendix 4, Tables 51–59)
Using the child and adult questionnaires, anticipatory anxiety was recorded at baseline; at every subsequent treatment visit, both anticipatory and treatment-related anxiety were recorded in the same way, with one question posed before treatment and one after treatment. A small proportion of questionnaires had missing data for these pre- and post-treatment questions (child questionnaires: < 5% missing; parent questionnaires: 6% missing). See Appendix 4, Table 51. The percentages of children and parents answering ‘not worried’ at baseline and over all subsequent visits were compared between trial groups.
Anticipatory anxiety and worry
At the baseline visit, 71% (1021/1045) of children who completed this question responded that they were ‘not worried’ before they saw the dentist. This was balanced across all three arms. During the follow-up period (i.e. all visits excluding baseline), the proportion of visits for which a child indicated that they were ‘not worried’ before treatment decreased slightly, to 67% (3729/5605) (see Appendix 4, Table 52). After adjusting for baseline anticipatory anxiety, age at randomisation and time in trial, there was no evidence of any statistically or clinically significant difference between arms in levels of anticipatory anxiety, averaged over all follow-up visits. The adjusted risk difference for the B+P arm compared with the C+P arm was 0.02 (97.5% CI –0.04 to 0.07), which indicates, on average, a 2% increased risk of anticipatory anxiety in the B+P arm compared with the C+P arm post baseline. For the PA arm compared with the C+P arm, the adjusted risk difference was –0.03 (97.5% CI –0.09 to 0.02), which indicates, on average, a 3% reduced risk of anticipatory anxiety in the PA arm compared with the C+P arm post baseline (see Appendix 4, Table 53).
At baseline, 67% (620/929) of parents thought that their child was ‘not worried’ before treatment. Prior to treatment visits during the follow-up period, parents indicated that at 69% (3757/5459) of visits their child was ‘not worried’ (see Appendix 4, Table 54). When considering the risk difference for anticipatory anxiety between treatment arms, adjusted as noted in the previous paragraph, there was no evidence of a statistically significant difference between the B+P and C+P arms (risk difference –0.04, 97.5% CI –0.09 to 0.02). However, parental-reported child anticipatory anxiety was, on average, 6% lower (risk difference –0.06, 97.5% CI –0.11 to –0.003) in the PA arm than in the C+P arm (see Appendix 4, Table 55).
Treatment-related anxiety and worry
The proportion of visits for which children reported not being worried after treatment was 70% (4553/6487). Averaged over all visits, and after adjusting for time in the trial, age and including a random effect for practice, there was no evidence of any statistically or clinically significant difference between arms in post-treatment worry [risk difference for B+P arm vs. C+P arm –0.002 (97.5% CI –0.05 to 0.04); risk difference for PA arm vs. C+P arm –0.04 (97.5% CI –0.08 to 0.01)]. See Appendix 4, Tables 56 and 57.
The proportion of visits for which parents reported that, after treatment, their child had not been worried was 67% (4331/6498). See Appendix 4, Table 58.
When considering the risk difference between treatment arms, adjusted as noted earlier in this section, there was no evidence of a statistically significant difference between the B+P and C+P arms (risk difference –0.04, 97.5% CI –0.09 to 0.02) or between the PA and C+P arms (risk difference –0.04, 97.5% CI –0.10 to 0.01). See Appendix 4, Table 59.
Discomfort during treatment
Discomfort during treatment was recorded independently from the child’s, parent’s and dentist’s perspectives at each treatment visit. Out of the 7713 visits, the question on treatment discomfort was answered 6468 (84%) times by children, 6498 (84%) times by parents and 7301 (95%) times by dentists. The data are summarised in Appendix 4, Tables 60–63.
Children reported having experienced no discomfort at the majority of their appointments; 71% (4571/6468) of the time they reported that their treatment did ‘not hurt at all’. The proportion of visits eliciting this response was higher in the PA arm, 76% (1528/2018), than in either the C+P arm [68% (1551/2278)] or B+P arm [69% (1492/2172)].
At 77% (4713/6093) of visits, parents thought that their child’s treatment was ‘not at all painful’. This was reported more often in the PA arm, 83% (1596/1915), than in the C+P arm [73% (1536/2116)] or B+P arm [77% (1581/2062)].
Dentists perceived ‘no apparent discomfort’ on behalf of the children in 68% (4990/7301) of treatment visits. They reported this more often for the PA arm, 76% (1736/2292), than for the C+P arm [63% (1614/2578)] or B+P arm [68% (1640/2431)].
Secondary outcomes, part 3: dentist-reported child behaviour and co-operation
As another measure of the children’s experience in each of the treatment arms, the dentists were asked to rate children’s behaviour during treatment and difficulties in providing treatment, as well as the reasons for the difficulties. Appendix 4, Tables 64–67, gives details of the findings relating to this aspect of the children’s experience of treatment.
Dentists reported full co-operation without any reservations or caution being expressed by the children at 61% (4426/7289) of all treatment visits. This was higher for PA arms, 66% (1508/2290), than for the B+P arm [62% (1492/2425)] or C+P arm [55% (1426/2574)].
In terms of difficulties providing treatment, dentists reported having no difficulty in 80% (5808/7294) of the visits. This varied little between arms. Concerning the visits for which difficulty was reported, the most common reason given was the child’s behaviour [852/1486 (57%)], followed by moisture control [327/1486 (22%)]. Inability to give local anaesthetic was reported as a difficulty in 9% (54/594) of C+P arm visits for which a treatment difficulty was reported. However, this was also reported as a problem at a small number of visits in the B+P [13/493 (3%)] and PA [13/399 (3%)] arms.
When asked about the use of inhalation sedation, dentists reported using this infrequently, at < 1% of all visits (see Appendix 4, Table 67, and Appendix 5, Table 74).
Secondary outcomes, part 4: other reported outcomes
Child dental discomfort (caused by toothache rather than treatment discomfort)
The Dental Discomfort Questionnaire (DDQ-8) was well completed, with few missing items (< 8%) (see Appendix 4, Table 68). This descriptive additional analysis of parent-reported child dental discomfort suggested that the scores between treatment arms were very similar and at the low end of the scale, indicating that parents’ perception was of little child discomfort experienced from toothache (see Appendix 4, Table 69).
Harms: safety analysis
Serious adverse events
No SAEs were reported.
Chapter 4 Health economics
Introduction
This chapter reports the economic evaluation that was performed as part of the FiCTION trial to determine whether or not any clinical benefit found from the three treatment strategies under evaluation was worthwhile for the UK NHS. This economic evaluation included a within-trial cost-effectiveness analysis and a number of sensitivity analyses that explored different reimbursement methods found in the UK NHS. The primary aim of the economic evaluation was to determine the relative efficiency of the treatment strategies in reducing the number of episodes of dental pain and/or dental sepsis in children with carious primary teeth. In these analyses, the treatment strategies were ordered in terms of increasing average total cost. Relative efficiency in the cost-effectiveness analyses was estimated by taking the difference in average total costs between the trial arms and dividing this by the difference in effects. All analyses were based on an ITT principle.
To allow a full understanding of cost-effectiveness and add value to the analysis, two ways of measuring incremental costs were compared: (1) time/material-based costs (microcosting) and (2) the costs associated with current charges to the NHS for treating children with dental decay in primary teeth in Scotland, England and Wales. The second analysis is important because the payment systems differ for areas with a fee-for-service (FFS) arrangement, which is used in Scotland, and the agreed UDA, which is used in England and Wales. Both costing measures are explained in further detail in Costing methods. The primary (base-case) analysis estimated costs using microcosting; costs based on FFS and UDA were used in the sensitivity analyses.
The health economic evaluation took the perspective of the health service provider (NHS) and Personal Social Services. The main costs collected related to health service utilisation, which, for the FiCTION trial, was the average total cost to manage children with dental decay in primary teeth during the time in trial of at least 23 months (maximum 36 months). A wider perspective was also taken by including the costs borne by parents of these children. These included direct (e.g. out-of-pocket purchase of pain relief medication) and indirect costs (e.g. time away from usual activities, such as work, to care for a child).
The results of the economic analyses are reported as the following outcomes:
-
health-care costs to manage dental decay in primary teeth over the child’s time in the trial of at least 23 months
-
direct and indirect costs to parents of managing dental decay in their children’s primary teeth
-
incidence of dental pain and/or dental sepsis
-
average total number of episodes of dental pain and/or dental sepsis
-
regression models estimating the key predicators of costs and effects (e.g. age, time in trial).
Methods
This analysis was designed and conducted in accordance with best practice, conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Cost data collection
NHS costs and frequency of use of health-care services
The costs for treating dental decay in primary teeth depended on the quantity of dental care resources used for each child during their time in the trial, for up to 3 years post randomisation. The use of dental services was recorded in the CRFs for visits to a GDP and for patient referrals. The frequency of use of health-care resources was calculated for each child and combined with the cost of each resource to generate the average total cost to manage dental decay in primary teeth in each arm over at least 23 months.
Treatment costs
The cost of each treatment was based on information provided in the CRF and was estimated on an individual child level (microcosting) and, in sensitivity analyses, on an aggregate level using FFS and UDA data.
The CRF captured any information on treatments that occurred during the first and subsequent visits to a practice. The CRF collected detailed information on prevention and operative treatments and who performed such treatments.
Information on the visit start time and end time on the CRF allowed us to estimate the duration of each visit for every child. The length of time for each visit was used to estimate the cost of dental personnel providing treatment, taking into account that the same personnel may not provide prevention and operative treatment. We assumed that a dental nurse would be present for the full duration of each visit. Information on the total length of visit and the time providing prevention was used to estimate length of time providing operative treatment.
Operative treatment refers to conventional management and biological management of decay, and includes (when indicated) carious tissue removal, restorations, anaesthesia, extractions and opening up lesions. GDPs or dental therapists were the dental personnel options provided on the CRF for operative treatment. Additional information on the number of surfaces treated per tooth was also collected, because if more than one surface of a tooth was treated this could incur additional costs, such as the use of additional restorative material or a matrix band [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Best-practice preventative care was expected to have been provided at every visit regardless of randomised allocation. Information on prevention collected by the CRF included what prevention activities were performed, how much time was spent on those activities and which personnel performed them. We categorised the prevention activities into the four generally recognised components (or pillars): (1) diet, (2) oral hygiene, (3) fissure sealing of FPMs and (4) fluoride applications. 87,116
Patient referral costs
A patient referral was classified as a child referred to a dental hospital/clinic for a consultation and/or operative treatment. The team were notified of patient referrals through the CRF and further data collection on referrals was made through contact with the referring clinician [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. Additional data collected included the modality of adjunctive behavioural management used (behaviour management alone and type of sedation), specific information relating to the tooth or teeth requiring treatment, and the outcome of the referral (treatment complete, referral ongoing or failure to attend).
To estimate costs associated with referrals, ‘packages of care’, categorised A to F [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)], were generated that defined where the treatment took place, who provided the treatment and how many visits were involved. The ‘packages of care’ were then costed using routine sources and the total cost of each treatment package was estimated based on advice from staff at Newcastle Dental Hospital (Ben Cole, July 2017, personal communication). It was originally anticipated that we would source this information from one site in both Newcastle and Scotland; however, after preliminary research, it was agreed that no additional information would be provided from the Scottish site because both countries use the same NHS reference costs for secondary/tertiary care.
Parent costs
These costs were included in a sensitivity analysis to explore the impact of widening the cost perspective. The parent questionnaire containing questions on parent costs was completed at all scheduled and unscheduled visits, excluding the baseline visit. Parents were asked whether or not their child had experienced any toothache since their previous visit. If they had, they were asked to complete the parent cost questions; however, if the child had not experienced toothache, they could skip this section. The parent questionnaire provided information on any absence from school owing to toothache, parental/carer time off paid work to care for a child with toothache, any additional paid child care arising as a result of toothache, non-prescribed (over-the-counter) medication and any time spent away from usual activities because of toothache (for the child and parent).
In a further sensitivity analysis, children’s time away from school and usual activities because of toothache were incorporated. Children are not economically active; however, children’s time has an opportunity cost, so we costed their time as leisure time117 and determined the effect, if any, that this had on overall results.
To summarise, data collection on resource use and cost can be split into three areas (see Appendix 5, Tables 70 and 74):
-
Treatment costs (first and all subsequent visits, including scheduled and unscheduled visits) were collected via the CRF.
-
Patient referrals were collected via the patient referral form, completed once a referral for a FiCTION trial child was identified. Each referral was categorised into a prespecified ‘care package’ (see NHS costs and frequency of use of health-care services).
-
Parent costs were collected via the ‘about your child’s teeth’ questionnaire completed at scheduled and unscheduled visits.
Costing methods
As previously mentioned, two costing techniques were undertaken as part of the FiCTION trial economic evaluation: microcosting and the current charges to the NHS. A sensitivity analysis explored FFS and UDAs individually and the impact the two different fee structures had on the overall cost-effectiveness of the treatment strategies being explored.
Microcosting
A time/materials-based costing was used to estimate the treatment costs for each child at every visit. Capital costs were not included in our analysis. A spreadsheet was developed using information collected in the CRF and clinical advice obtained to determine what consumable and reusable resources were used to perform each dental treatment. These resources were collated to design an itemised data collection tool and unit costs were assigned to each resource.
We used existing data sources, when possible, to estimate the unit costs. 118–121 The unit costs used in the microcosting analysis are detailed in Appendix 5, Table 70. Further information on how these costs were derived is provided in the health economic analysis plan [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. In brief, staff costs were inflated to account for national insurance and pension contributions to estimate the total gross annual salary. From this, we were able to estimate the cost per minute for each member of dental personnel who could have provided treatment.
Consumable resources were inflated by 20%, when appropriate, to account for value-added tax (VAT) and costs of individual items were estimated by dividing a total ‘pack’ cost by the number of items used.
Reusable resources were also inflated to account for VAT, and the total cost of a pack (e.g. of mirror heads) was divided by the total number of items in that pack. The total cost per item was then converted to the equivalent annual cost, using a 3.5% discount rate. Based on clinical advice, we assumed that all dental equipment would have a 3-year lifespan. In addition, we applied an autoclave cost to every use of a reusable item, when applicable. All assumptions (e.g. 3-year lifespan for reusables) were explored in sensitivity analyses. The costs estimated using microcosting were used in the base-case analysis.
Charges to the NHS
Fee for service
In Scotland, GDPs are reimbursed for every unit of activity they perform. As it has been suggested that this fee-per-item system may create an incentive to overtreat participants,122 this was explored in a sensitivity analysis. Unit costs were collected from the Information Services Division Scotland. 123 The cost of treating each child for every visit was used to estimate the average total cost in Scotland for treating dental decay in primary teeth over the time in the trial of at least 23 months. The unit costs used in the FFS analysis are detailed in Appendix 5, Table 71.
Units of dental activity
In England and Wales, a reimbursement similar to the current Scottish FFS-based system was in play until 2006, when UDAs were introduced. 124 UDAs are a fixed reimbursement that GDPs receive for dental work and vary between practices (between £16 and £40 per UDA). 119 Under the UDA system, dental procedures can be classified into three bands:124
-
Band 1 (1 UDA) – diagnosis, treatment planning and maintenance. It includes examination, radiography, scale and polish and preventative care.
-
Band 2 (3 UDAs) – simple treatments such as fillings, extractions and periodontal treatment.
-
Band 3 (12 UDAs) – complex treatment that includes a laboratory element. Examples include bridges, crowns and dentures.
In addition, GDPs can receive 1.2 UDAs for a band 1 urgent treatment. To incorporate urgent treatment into the UDA analysis, we defined urgent treatment as an unscheduled visit that occurred at the start of a course of treatment. We assumed that needing urgent treatment would begin a new course of treatment, as it was unlikely that a child would require urgent care during an existing course of treatment. This assumption underestimated UDA reimbursement by 0.2 UDAs if an unscheduled visit occurred during a course of treatment. However, the number of visits affected by this assumption was minimal [n = 15 (< 1%)] and, in these cases, the number of days between the unscheduled visit and the previous visit was < 28 (mean 18.8); hence, it was assumed that the unscheduled visit was related to the previous visit.
It is important to note that UDA(s) are reimbursed for only one course of treatment. A course of treatment definition is provided in the health economic analysis plan [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)] and Figure 8 is an illustrative presentation of the course of treatment pathway.
FIGURE 8.
Course of treatment pathway [see HEAP at project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
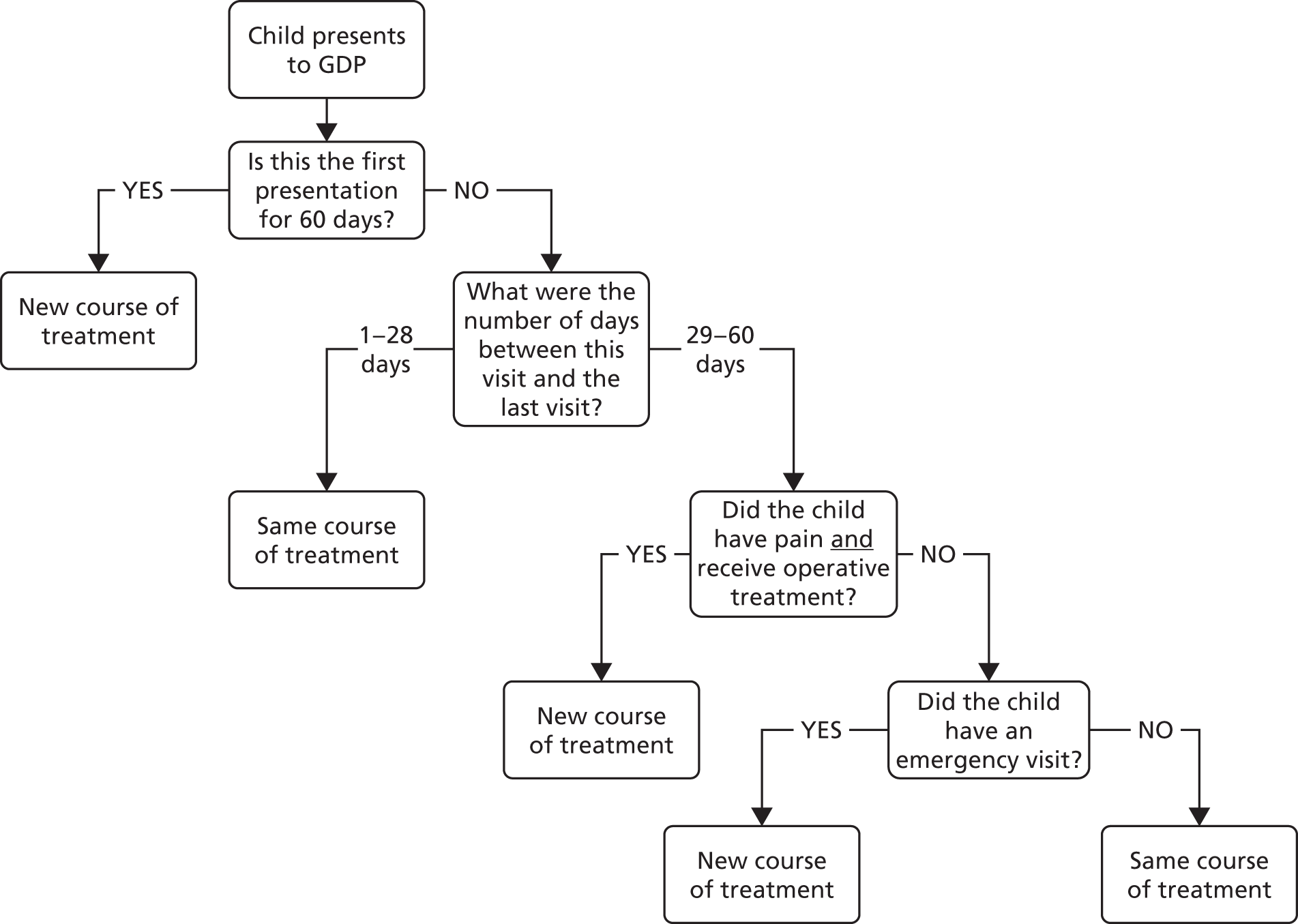
We collected UDA values for FiCTION trial practices in England (n = 43) from the NHS Business Services Authority website,119 which has information on dental processing and payments. The UDA value was estimated to be the value of the total contractual payments divided by the number of contracted UDAs based on the value of GDS contracts only. The average health board UDA value was adopted for the Welsh practices (n = 4). This information was obtained through personal communication (Research and Development Division, Welsh Government, October 2016, personal communication). Practice-level UDA values used in the UDA analysis are summarised in Appendix 5, Table 72. UDAs were discounted based on when a course of treatment started.
The UDAs incurred for each course of treatment were combined into a total number of UDAs received for each child over their time in the trial (see Appendix 5, Table 75). These UDAs were combined with practice UDA values to produce a total cost per child and, hence, the average total costs in England and Wales for treating dental decay in primary teeth.
Parent costs
Information on child and parent costs was collected via the parent questionnaire, excluding baseline visits. The unit costs for time were based on data obtained from the Department for Transport117 and the Office for National Statistics. 125 All medication costs were estimated based on the over-the-counter costs of medications from high-street pharmacies. 126 Child-care costs were sourced from recent UK online reports of current child-care costs. 127 Parent and children costs are detailed in Appendix 5, Table 70.
Effectiveness measure
Health outcomes were measured in natural units; incidence of dental pain and/or dental sepsis and episodes of dental pain and/or dental sepsis. The methods for deriving these outcomes measured are described in detail in Chapter 2. It was assumed that those who did not return for regular appointments during their follow-up did not experience dental pain and/or dental sepsis.
Cost-effectiveness analysis
Adjusted analyses were performed to estimate cost-effectiveness. All results were presented as point estimates of the mean incremental differences in costs and effects, the incremental cost per incidence of dental pain and/or sepsis avoided, and the incremental cost per episode of dental pain and/or dental sepsis avoided.
Adjusted analysis
Costs
Using the data on total costs per child (estimated using microcosting, FFS and UDAs), regression techniques were applied to identify whether or not there was a difference between the treatment strategies when compared with C+P, in the first instance, while controlling for potentially modifying factors, such as age and length of follow-up. An ordinary least squares regression technique was applied:
In Equation 1, a dummy variable for the randomised arm estimates the difference in costs between the arms when compared with C+P, controlling for all other factors in the model. Estimated beta values describe the direction and magnitude of the relationship between each variable and the dependent variable. For example, if the beta estimate of B+P is +50 and the beta estimate of PA is –50, this indicates that B+P is more costly on average, and PA is less costly on average, than C+P, controlling for all other factors.
Benefits
As with costs, regression techniques were carried out to derive the drivers of the difference in effects between the three treatment strategies after controlling the key predictors of dental pain and/or dental sepsis. Equation 2 is the equation applied to estimate effects:
The two equations differed in terms of their dependent variables, but the same independent variables were adopted and used in the statistical analysis.
The variables considered were:
-
Dependent variable(s) –
-
Costs: estimated using microcosting, FFS and UDAs. This equation was replicated to incorporate parent costs with total treatment costs.
-
Dental pain and/or dental sepsis – estimated as the average incidence of dental pain and/or sepsis and replicated for the average total number of episodes of dental pain and/or dental sepsis.
-
-
Independent variables –
-
Randomised arm (i.R): this is an indicator variable that allows us to compare two of the treatment strategies with our comparison strategy (C+P) independently.
-
Time in trial (T) in months (minimum 23 months, maximum 36 months).
-
Age (A) of the child in years.
-
Individual practice variations (P).
-
Seemingly unrelated regression
The cost and outcome regressions (see Equations 1 and 2) were run simultaneously using seemingly unrelated regression (SUR). 128 SUR permits the simultaneous estimation of costs and effects, calculated at the individual level, while accounting for unobserved individual characteristics that could affect both costs and effects and lead to potential correlation between these two dependent variables. 129 In addition, the SUR allowed us to control for additional covariates that may affect costs, effects or both. This analysis was conducted in Stata using the SUR command ‘sureg’.
Sensitivity analyses
Sensitivity analyses were conducted to assess the robustness of the results to realistic variations in the levels of underlying data. Deterministic sensitivity analyses were used to address any uncertainty in the assumptions used in our analysis. A stochastic sensitivity analysis using the bootstrapping technique130 explored the impact of the statistical imprecision surrounding estimates of costs, effects and cost-effectiveness. The bootstrapped results from the three-arm comparison were presented as a cost-effectiveness frontier. The cost-effectiveness frontier131 allowed us to determine the treatment strategy that maximised net benefits at each willingness-to-pay (WTP) value for an additional unit of health effect (i.e. dental pain and/or dental sepsis avoided).
The base-case analysis was replicated to include children who were treated in accordance with the clinical protocol for at least 80% of visits (see Chapter 2, The prespecified per-protocol analysis set, and Chapter 3, Adherence to protocol) to determine what effect, if any, this had on the overall results. The results of the PP analysis are presented in Appendix 5, Table 79.
All costs and effects were discounted at the recommended rate of 3.5%. 132 Discounting for incidence was based on when the child incurred the dental pain and/or dental sepsis during their follow-up period. Discounting for episodes was based on when the episode began. Similar to the co-primary outcome analysis, 97.5% CIs were generated for point estimates of costs and effects and for the differences between the management strategies.
Complete-case analysis
A complete-case analysis was performed on all children who attended their final visit, regardless of the number of visits they had over their period of follow-up. The inclusion of only these children allowed us to estimate the cost-effectiveness of the three treatment strategies with at least 23 months’ follow-up data and overcome some of the issues relating to unscheduled visits. It is important to note that this analysis was underpowered (as the sample size was based on the primary outcomes) and so was less likely to detect a difference should one exist. The results of the complete-case analysis are presented in Appendix 5, Table 80.
Results
A total of 1114 children were randomised to the FiCTION trial. Children included in the economic analysis were those who were deemed eligible to be included in the primary ITT analysis [see Chapter 2, Statistical methods, and Chapter 3, Numbers analysed: intention to treat analysis (1058 participants)]. Data on these 1058 children were used in the health economics base-case analysis and data on 1057 children were used in the adjusted analyses, as one child had missing information on age (age was one of the independent variables in the regression analysis described in Cost-effectiveness analysis, Adjusted analysis).
The children included in the health economic analyses were evenly distributed across the three treatment arms; 352 were randomised to B+P, 352 to C+P and 354 to PA. On average, children had seven visits during their time in the trial.
Data validity and completeness
This section reports on the completeness of the data used to estimate costs and effects.
Case report form
The percentage of missing data was relatively low (< 5%) for all of the questions used in the economic analysis collected via the CRF. A detailed description of missing CRF data, data validity checks and any assumptions made in the base-case analysis (n = 1058) is provided in Appendix 5, Table 73. All decisions made were verified with clinical advice. For operative treatment, we made no assumptions for missing treatments as there was no way to identify whether this was just a check-up visit or, if operative treatment was provided, what potential treatment could have been provided. This assumption therefore slightly underestimates the average total cost in each of the three treatment strategies, but the numbers of missing data are similar across the three treatment strategies (missing data on whether or not restoration materials were used was 36% in the B+P arm, 33% in the C+P arm and 31% in the PA arm).
Information on prescribed medications (painkillers and antibiotics) was missing for 2% of all visits. We assumed that no medications were prescribed in these visits. Again, this assumption leads to a slight underestimate of the average total cost. It also affects the B+P arm more than the other two treatment strategies, as there were more missing medications data in this group than in the other two groups (40% vs. 30% vs. 30% of the missing data for the B+P, C+P and PA arms, respectively). When an analgesic [n = 2 (< 1%)] or antibiotic [n = 2 (< 1%)] was prescribed but no information on the name, dose or frequency was provided, we did not assume a cost for this prescription.
Unit of dental activity values
Information on UDAs was available for 41 out of 43 English practices in the FiCTION trial. The average UDA value for practices in the local area, based on the first half of their postcode, was used to estimate a UDA value for practices with no information on the Business Services Authority website (n = 2). 119 There was information from a minimum of three practices in the same area for this imputation. The following assumptions were adopted for duplicate practices (n = 12): a duplicate may exist if more than one GDP at that practice had a GDS contract, duplicates were removed if there was no UDA information available (n = 2) or the UDA information was the same (n = 5). For the remaining duplicates (n = 5), we estimated the weighted average UDA based on the baseline contract size.
Parent questionnaire
Data on 968 children from 5632 visits were used to inform parental costs, as outlined in Parent costs. Questionnaire data on 145 visits were excluded from the economics analysis (108 visits had no dates and, hence, could not be discounted; 32 visits had a date of questionnaire that was before the first CRF; five were duplicates). We analysed the responses to the parent questionnaire at an aggregate level and used this to assign ‘unit costs’ at a participant level. We estimated the average total time parents had to take off work and the total out-of-pocket expenses associated with the child’s dental treatment. The response rates of the parent questionnaire for the health economic questions were high (≈95%). On average, parents reported experiencing few direct and indirect costs associated with their child’s toothache over their follow-up (< 10% responded ‘yes’ to any single question). As a result, any missing data (< 5%) were assumed to be ‘no’.
On average, nearly all parents who completed the parent questionnaire reported that their child had experienced toothache once during their follow-up [mean 0.98 (SD 1.46)]. All summary statistics on estimating the parent costs are provided in Appendix 5, Tables 70 and 74.
Resource use and costs
The average total resource use based on staff time spent providing treatment and type of treatment was estimated for all three treatment strategies and is presented in Appendix 5, Table 74. A summary of types of treatment provided according to arm is also included [see Chapter 3, Treatment received (intention-to-treat analysis set, n = 1058)]. On average, all three treatment strategies were similar in terms of average total frequency and duration of visits. The three strategies differed in terms of operative treatment, with < 20% of all PA visits receiving operative treatment, compared with > 40% of B+P and C+P visits. The type of operative treatment also differed across the arms, which is reflective of the different treatment strategies (e.g. complete carious tissue removal was more routinely performed during C+P visits, whereas B+P visits with operative treatment were expected to involve partial or no carious tissue removal). GDPs provided the majority of prevention and operative treatment.
Costs were estimated for the first visit and for all follow-up visits during the time in the trial. The average total cost per randomised arm was estimated based on the three costing strategies.
Table 13 illustrates the difference in the total cost per child of treating dental decay in primary teeth, based on costs estimated using microcosting over a minimum of 23 months (maximum 36 months) in the trial. As would be expected, the total cost is lower for PA than for the other two treatment strategies, as children being managed by the PA arm had less operative treatment and shorter appointment times.
| Resource | Total cost per child (£) | |||||
|---|---|---|---|---|---|---|
| C+P arm (n = 352) | B+P arm (n = 352) | PA arm (n = 354) | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Staff costs per visit | 18.78 (6.07) | 17.80 (14.44–22.47) | 18.28 (6.27) | 17.12 (14.00–21.64) | 17.36 (5.95) | 16.20 (13.35–20.77) |
| Prevention costs per visit | 0.66 (0.76) | 0.36 (0.14–0.90) | 0.78 (0.88) | 0.53 (0.16–1.13) | 0.81 (0.88) | 0.58 (0.20–1.07) |
| Operative treatment costsb per visit | 8.18 (6.72) | 6.75 (4.09–10.88) | 7.84 (5.96)) | 6.02 (4.09–9.48) | 4.09 (4.05) | 2.66 (1.89–4.56) |
| Other treatments costs per visit | 0.66 (2.56) | 0.10 (0.00–0.30) | 0.47 (1.84) | 0.00 (0.00–0.30) | 0.52 (1.90) | 0.11 (0.00–0.32) |
| Referral costs per visit | 5.22 (23.35) | 0.00 (0.00–0.00) | 4.96 (23.65) | 0.00 (0.00–0.00) | 10.23 (43.81) | 0.00 (0.00–0.00) |
| Prescription costs per visit | 0.07 (0.29) | 0.00 (0.00–0.00) | 0.04 (0.14) | 0.00 (0.00–0.00) | 0.08 (0.32) | 0.00 (0.00–0.00) |
| Total practice-level treatment cost (excluding referrals) per child per visit | 28.36 (11.08) | 27.02 (20.88–33.08) | 27.40 (10.81) | 25.16 (20.17–31.56) | 22.86 (8.11) | 20.74 (17.43–26.71) |
| Total cost of first visit per child | 37.75c (19.71) | 34.20 (25.50–46.56) | 37.47 (19.95) | 3.36 (24.44–44.48) | 31.07 (60.96) | 24.52 (19.81–32.70) |
| Total cost per follow-up visit per child | 32.47 (30.10) | 25.68 (18.71–33.52) | 30.52 (33.13) | 23.52 (17.46–32.05) | 36.39 (75.18) | 20.61 (16.32–28.65) |
| Total treatment cost per child over the follow-up period | 250.48d (221.70) | 197.42 (122.13–291.38) | 231.27 (214.47) | 172.78 (118.85–283.04) | 211.32 (257.28) | 147.00 (89.44–215.50) |
If we assumed a cost for the missing medications, mentioned in Data validity and completeness, it would have a trivial effect on the overall average total cost estimates for each treatment strategy and, given that B+P had the lowest average prescription costs, it would not affect the order of the three treatment strategies in terms of mean (or median) total cost.
The mean costs have a direct relationship to the total cost of adopting a given strategy. However, the median costs presented in Table 13 are arguably more representative of the typical total cost associated with managing dental decay in primary teeth over a minimum of 23 months and up to a maximum of 36 months. Typically, PA had more referrals and more referrals involving a general anaesthetic, which has inflated the average total cost of this treatment strategy. The assumptions we made previously to exclude costs when information was missing do not affect our results and would only strengthen the difference in costs between PA and the other treatment strategies if they were included.
Fee for service
The unit costs used in the FFS analysis are detailed in Appendix 5, Table 71. Costs were taken to be the fees provided under ‘treatment under capitation’ and specific to minors when possible. For every child, a basic fee for their care and treatment was adopted and multiplied by the number of months they were in the trial. This fee includes examinations, oral hygiene advice, tooth-brushing advice, dietary advice, radiographs and all clinical prevention. Additional costs incorporated were reimbursed based on the number of teeth treated, number of visits or course of treatment. A new course of treatment for the FFS analysis was defined as a visit at least 5 calendar months after the previous visit, as outlined in the Statement of Dental Remuneration Amendment No. 135. 123
Unit of dental activity
The median number of courses of treatment per child during their time in trial was six, with the average number of UDAs reimbursed per course of treatment being 1.75 [C+P arm, mean 1.88 (SD 0.55); B+P arm, mean 1.92 (SD 0.53); and PA arm, mean 1.45 (SD 0.53)]. The unit costs used in the UDA analysis are detailed in Appendix 5, Table 72.
Table 14 illustrates the difference in the total cost per child to treat dental decay in primary teeth based on the different reimbursement strategies over a minimum of 23 months (maximum 36 months). Costs based on UDAs were generally lower than those based on FSS. There is very little difference in the total costs between C+P and B+P in the UDA analysis, which is expected given that both treatment strategies are in the same band category (3 UDAs). In the FFS analysis, B+P is the most costly treatment strategy. Again, this is not surprising as fillings are reimbursed at a lower rate than preformed crowns under the FFS reimbursement system. In all three costing methods, PA is the least costly treatment strategy.
| Reimbursement system | C+P arm | B+P arm | PA arm | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| FFS (Scotland) (N = 287) | n = 90 | n = 100 | n = 97 | |||
| Total NHS reimbursement cost for treating dental decay in primary teeth | 359.28 (203.45) | 335.43 (217–460) | 374.54 (222.76) | 357.45 (207–479) | 321.46 (167.13) | 336.68 (191–415) |
| UDA (England and Wales) (N = 771) | n = 262 | n = 252 | n = 257 | |||
| Total NHS reimbursement cost for treating dental decay in primary teeth | 286.71 (145.77) | 269.55 (186–371) | 291.56 (155.89) | 273.97 (191–357) | 219.19 (127.68) | 195.87 (125–302) |
| FFS and UDA (all areas) (N = 1058) | n = 352 | n = 352 | n = 354 | |||
| Total NHS reimbursement cost for treating dental decay in primary teeth | 305.26 (165.23) | 291.85 (189–399) | 315.14 (181.05) | 284.59 (194–395) | 247.21 (146.65) | 227.68 (136–342) |
Effectiveness
Summaries of incidence of dental pain and/or dental sepsis and episodes of dental pain and/or dental sepsis are provided in Table 15. The effectiveness outcomes were discounted at 3.5%, as per the National Institute for Health and Care Excellence guidelines. 132 The effectiveness point estimates used in the economic analysis are presented in Table 16, alongside the comparative results, which are described in the following section.
| Outcome | C+P arm | B+P arm | PA arm | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Incidence of dental pain and/or dental sepsis | 0.421 (0.494) | 0.00 (0.00–1.00) | 0.401 (0.491) | 0.00 (0.00–1.00) | 0.455 (0.499) | 0.00 (0.00–1.00) |
| Episodes of pain dental pain and/or dental sepsis | 0.618 (0.947) | 0.00 (0.00–1.00) | 0.580 (0.867) | 0.00 (0.00–1.00) | 0.718 (0.984) | 0.00 (0.00–1.00) |
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects(97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| C+P vs. B+P | ||||||||||
| C+P (n = 352) | 244.65 (219 to 271) | 0.410 (0.35 to 0.47) | Dominated | 0.16 | 0.16 | 0.17 | 0.18 | 0.20 | ||
| B+P (n = 352) | 226.76 (201 to 252) | –15.60 (–52 to 21) | 0.390 (0.33 to 0.45) | –0.016 (–0.10 to 0.06) | 0.84 | 0.84 | 0.83 | 0.82 | 0.80 | |
| C+P vs. PA | ||||||||||
| C+P (n = 352) | 244.65 (219 to 271) | 0.410 (0.35 to 0.47) | 799.30 | 0.03 | 0.04 | 0.08 | 0.14 | 0.30 | ||
| PA (n = 354) | 206.42 (176 to 237) | –34.37 (–71 to 2) | 0.445 (0.39 to 0.50) | 0.043 (–0.04 to 0.12) | 0.97 | 0.96 | 0.94 | 0.86 | 0.70 | |
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| C+P vs. B+P | ||||||||||
| C+P (n = 352) | 244.65 (219 to 271) | 0.603 (0.49 to 0.71) | Dominated | 0.16 | 0.17 | 0.18 | 0.18 | 0.23 | ||
| B+P (n = 352) | 226.76 (201 to 252) | –15.60 (–52 to 21) | 0.565 (0.46 to 0.67) | –0.031 (–0.18 to 0.12) | 0.84 | 0.83 | 0.82 | 0.82 | 0.77 | |
| C+P vs. PA | ||||||||||
| C+P (n = 352) | 244.65 (219 to 271) | 0.603 (0.49 to 0.71) | 309.64 | 0.03 | 0.07 | 0.14 | 0.39 | 0.68 | ||
| PA (n = 354) | 206.42 (176 to 237) | –34.37 (–71 to 2) | 0.701 (0.58 to 0.82) | 0.111 (–0.04 to 0.26) | 0.97 | 0.93 | 0.86 | 0.61 | 0.32 | |
Cost-effectiveness results
Biological arm versus conventional arm and prevention alone arm versus conventional arm
In the first instance, we compared both B+P and PA with C+P as if they were two independent comparisons. In this analysis, we assumed that C+P was equivalent to usual care. We replicated these analyses for both of our primary outcome measures: incidence of dental pain and/or dental sepsis and number of episodes of dental pain and/or dental sepsis.
Table 16 illustrates the incremental difference in average total costs and average total effects between the treatment strategies. On average, B+P dominates C+P in both analyses, as it is, on average, less costly and more effective. The comparison between PA and C+P is less clear. On average, PA is less costly than C+P, but it is also less effective in terms of both incidence of dental pain and/or dental sepsis and episodes of dental pain and/or dental sepsis. In this circumstance, an incremental cost-effectiveness ratio (ICER) was estimated to judge whether or not the extra costs of the more effective C+P are worthwhile. If society were willing to pay up to £310 per episode of dental pain and/or dental sepsis avoided, then we would choose C+P. If society were not willing to pay this amount, then we would choose PA. Considering incidence of dental pain and/or dental sepsis as our measure of effect, the threshold for choosing C+P for this decision increases to approximately £800.
The deterministic results alone are not sufficient to support decision-making: we need to consider the imprecision surrounding estimates of costs, effects and cost-effectiveness. For this, stochastic sensitivity analysis was used. The probabilities of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis are based on the bootstrapped results of the cost-effectiveness analysis. We can clearly see in Table 16 that B+P always has a higher probability of being considered cost-effective than C+P at all presented WTP values for both measures of effectiveness. A similar pattern can be seen for PA compared with C+P, with PA having the higher probability of being considered cost-effective at most WTP thresholds presented. However, PA’s probability of being considered cost-effective decreases as our WTP value increases because we progressively put more value on avoiding dental pain and/or dental sepsis, which C+P is more likely to avoid. The more we value avoiding dental pain and/or dental sepsis, the more probable it is that the higher costs of C+P will be offset.
Incremental analysis
Arguably, an incremental analysis is more appropriate from an economics perspective as it allows us to compare the three treatment strategies simultaneously. In this analysis, we first ordered the treatment strategies in terms of increasing average total cost and then compared a more costly strategy with a less costly strategy in terms of incremental cost-effectiveness. As PA is the least costly option,6 we compared this to B+P, the next least costly option, and estimated an ICER as B+P is, on average, more costly but more effective than PA. We then incorporated the most costly treatment strategy, C+P, into the analysis and compared this with B+P. As B+P was, on average, less costly and more effective than C+P, it dominated the comparison; hence, we would not consider C+P to be cost-effective compared with the other two treatment strategies. The ICER for B+P is approximately £300 to avoid one case of incidence and £130 to avoid one episode of dental pain and/or dental sepsis, compared with PA.
Again, the bootstrapped results present the uncertainty surrounding our results; Table 17 shows the probabilities of the three treatment strategies, compared with each other, being considered cost-effective at the different WTP thresholds. Figures 9 and 10 illustrate which treatment strategy has the highest probability of being considered cost-effective at each WTP threshold when the measure of effectiveness is, respectively, incidence of dental pain and/or sepsis avoided or episode of dental pain and/or sepsis avoided.
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 354) | 206.42 (176 to 237) | 0.445 (0.39 to 0.50) | 0.87 | 0.81 | 0.75 | 0.54 | 0.31 | |||
| B+P (n = 352) | 226.76 (201 to 252) | 18.77 (–18 to 55) | 0.390 (0.33 to 0.45) | –0.058 (–0.14 to 0.02) | 323.62 | 0.12 | 0.17 | 0.22 | 0.36 | 0.58 |
| C+P (n = 352) | 244.65 (219 to 271) | 0.410 (0.35 to 0.47) | Dominated by B+P | 0.01 | 0.02 | 0.03 | 0.05 | 0.11 | ||
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 354) | 206.42 (176 to 237) | 0.701 (0.58 to 0.82) | 0.87 | 0.72 | 0.54 | 0.23 | 0.19 | |||
| B+P (n = 352) | 226.76 (201 to 252) | 18.77 (–18 to 55) | 0.565 (0.46 to 0.67) | –0.143 (–0.29 to 0.01) | 131.26 | 0.12 | 0.25 | 0.41 | 0.64 | 0.72 |
| C+P (n = 352) | 244.65 (219 to 271) | 0.603 (0.49 to 0.71) | Dominated by B+P | 0.01 | 0.03 | 0.05 | 0.13 | 0.09 | ||
FIGURE 9.
Probability of being cost-effective at different WTP thresholds to avoid an incidence of dental pain and/or dental sepsis: PA vs. B+P vs. C+P. C+P does not appear in the figure because C+P did not have the highest probability of being considered cost-effective at the WTP values presented.

FIGURE 10.
Probability of being cost-effective at different WTP thresholds to avoid an episode of dental pain and/or dental sepsis: PA vs. B+P vs. C+P. C+P does not appear in the figure because C+P did not have the highest probability of being considered cost-effective at the WTP values presented.
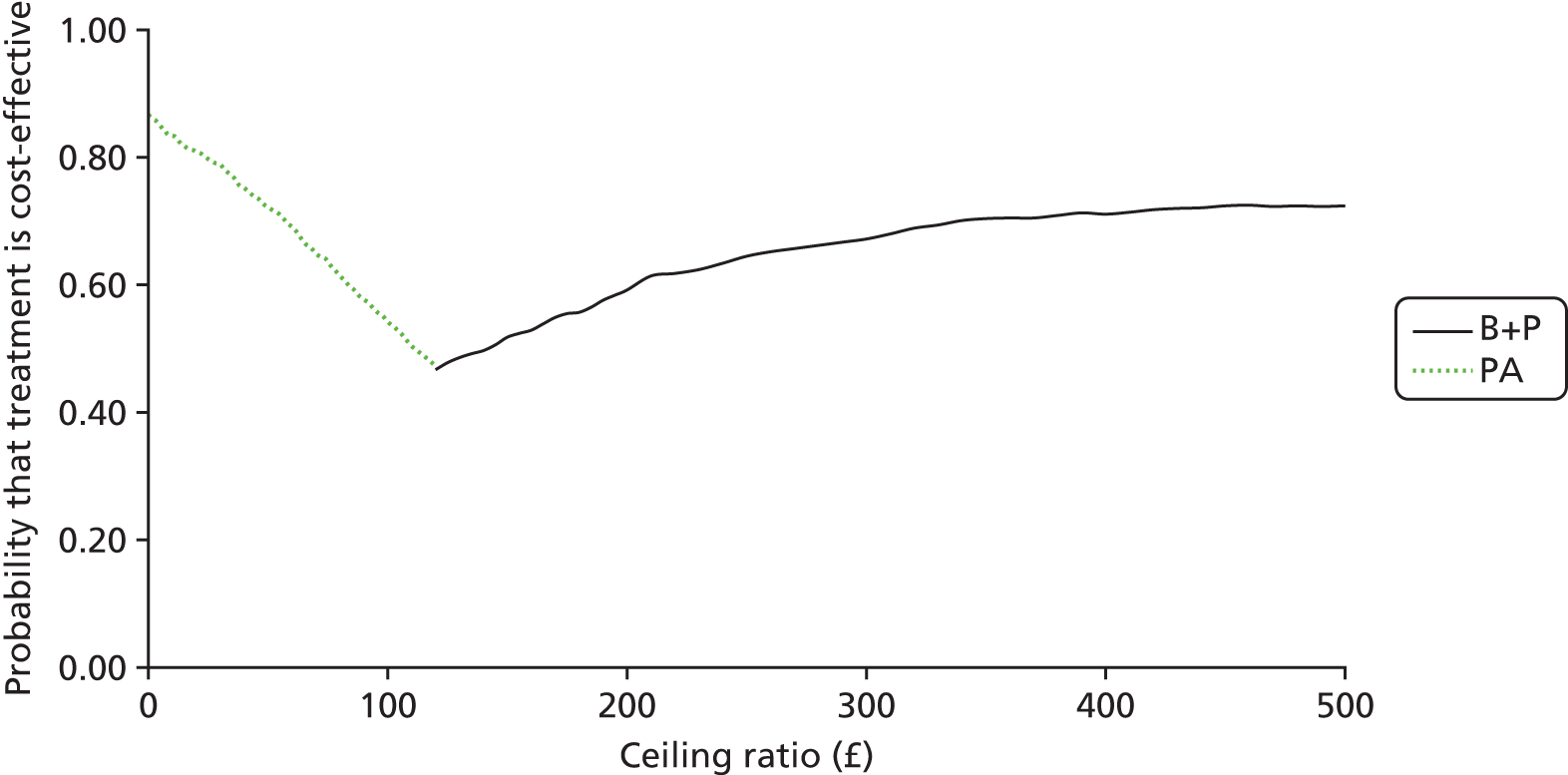
The stochastic analysis shows that PA would be the most cost-effective treatment if a decision were to be based on cost alone. As society’s WTP for avoidance of dental pain and/or dental sepsis increases, it becomes more probable that B+P would be considered cost-effective (see Figures 9 and 10, respectively). C+P would not be considered cost-effective compared with PA and B+P; hence, it is not presented in Figures 9 and 10.
The complete-case analysis, as described in Complete-case analysis, has similar results, in that B+P would be considered cost-effective at reducing the number of episodes of dental pain and/or dental sepsis at a WTP threshold of £130 per episode avoided (see Appendix 5, Table 80).
Sensitivity analysis
Fee for service analysis
Data on 287 children treated in Scotland were used in the FFS analyses (C+P = 90, B+P = 100 and PA = 97). The average total cost per treatment arm was higher in the FFS analysis than in the analysis in which costs were based on microcosting (see Table 16). Estimates of mean effect remain similar to the microcosting-based estimates, except that now PA has fewer incidences of dental pain and/or dental sepsis than C+P (see Appendix 5, Table 76). This change in direction of effects can be explained by the smaller number of children included in the FFS analysis than in the base-case analysis (257 vs. 1058) and suggests that children in the PA arm based in Scotland are less likely to experience dental pain and/or dental sepsis.
When comparing the full incremental analysis based on FFS costs (Table 18) with that based on the microcosting (see Table 17), the conclusions drawn are similar. C+P is unlikely to be considered cost-effective regardless of the basis of costs. Regarding incremental cost per episode of dental pain and/or dental sepsis avoided, C+P was extendedly dominated by B+P. Extended dominance occurs when the ICER for the management strategy is higher than that of the next, more effective, alternative. 134 B+P would probably not be considered cost-effective at reducing incidence of dental pain and/or dental sepsis compared with PA with an ICER of > £1000. However, the incremental cost to avoid an episode of dental pain and/or dental sepsis was £235.
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 97) | 321.46 (282 to 360) | 0.423 (0.31 to 0.54) | 0.98 | 0.98 | 0.97 | 0.91 | 0.69 | |||
| C+P (n = 90) | 359.28 (310 to 408) | 34.89 (–6 to 76) | 0.442 (0.33 to 0.56) | 0.016 (–0.14 to 0.17) | Dominated by PA | 0.02 | 0.02 | 0.03 | 0.04 | 0.22 |
| B+P (n = 100) | 374.54 (324 to 425) | 52.84 (12.79 to 93) | 0.37 (0.26 to 0.48) | –0.052 (–0.20 to 0.10) | 1016.15c | 0.00 | 0.00 | 0.00 | 0.05 | 0.09 |
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 97) | 321.46 (282 to 360) | 0.706 (0.47 to 0.94) | 0.98 | 0.94 | 0.81 | 0.38 | 0.13 | |||
| C+P (n = 90) | 359.28 (310 to 408) | 34.89 (–6 to 76) | 0.586 (0.40 to 0.77) | –0.126 (–0.39 to 0.14) | 276.90 | 0.02 | 0.05 | 0.13 | 0.26 | 0.25 |
| B+P (n = 100) | 374.54 (324 to 425) | 17.95 (–59 to 23) | 0.478 (0.31 to 0.64) | –0.097 (–0.36 to 0.17) | 185.05 | 0.00 | 0.01 | 0.06 | 0.36 | 0.62 |
| B+P vs. PA | 52.84 (12.79 to 93) | –0.223 (–0.49 to 0.04) | 236.95 | 0.00 | 0.01 | 0.08 | 0.50 | 0.82 | ||
Unit of dental activity analysis
Data on 771 children treated in England and Wales were used in the UDA analyses (C+P = 262, B+P = 252 and PA = 257). When the total average UDA-based costs of each treatment strategy are compared with the microcosting-based data (see Table 16), B+P is more costly than C+P but remains more effective on average (see Appendix 5, Table 77).
In terms of the incremental cost per episode of dental pain and/or dental sepsis avoided, the UDA-based results (Table 19) are substantially different from those based on microcosting (see Table 17) and suggest that PA would be most likely to be considered cost-effective over the range of WTP values presented (in Table 17, B+P was most likely to be considered cost-effective when society was willing to pay £130 per episode of dental pain and/or dental sepsis avoided). A broadly similar pattern was found for incremental cost per incidence of dental pain and/or dental sepsis avoided.
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 257) | 219.19 (201 to 237) | 0.452 (0.38 to 0.52) | 1.00 | 1.00 | 1.00 | 1.00 | 0.79 | |||
| C+P (n = 262) | 286.71 (266 to 307) | 59.67 (35 to 84) | 0.398 (0.33 to 0.46) | –0.063 (–0.16 to 0.03) | 947.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 |
| B+P (n = 252) | 291.56 (269 to 314) | 0.398 (0.33 to 0.47) | Dominated by C+P | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | ||
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 257) | 219.19 (201 to 237) | 0.700 (0.47 to 0.75) | 1.00 | 1.00 | 1.00 | 0.83 | 0.39 | |||
| C+P (n = 262) | 286.71 (266 to 307) | 59.67 (35 to 84) | 0.610 (0.47 to 0.75) | –0.106 (–0.29 to 0.08) | 562.92 | 0.00 | 0.00 | 0.00 | 0.09 | 0.33 |
| B+P (n = 252) | 291.56 (269 to 314) | 5.85 (–19 to 31) | 0.599 (0.47 to 0.73) | –0.006 (–0.19 to 0.18) | 975 | 0.00 | 0.00 | 0.00 | 0.08 | 0.28 |
Sensitivity analysis on course of treatment definition
The duration of 60 days, used as an assumption to define a course of treatment in the primary UDA analysis, was explored in a further sensitivity analysis. When an alternative duration of 90 days was incorporated, the average number of courses of treatment per child per treatment strategy was similar to the original UDA analysis [C+P, mean 5.13 (SD 1.9); B+P, mean 5.00 (SD 2.04); and PA, mean 5.08 (2.04)].
Looking at the UDA-based analysis, and when 90 days represented a course of treatment, there was a negligible difference in costs. The difference in average total cost between B+P and C+P was £8.80 and between PA and C+P was £49.97 (results not shown). The difference in effects was, as would be expected, the same as the previous analyses. As a result, the ICER between C+P and PA decreased to £471 to avoid an episode of dental pain and/or dental sepsis, and PA would be considered the most cost-effective treatment strategy owing to the associated cost savings (data not shown). As there was very little difference in our conclusions regardless of whether a course of treatment was defined as 60 days or 90 days, we did not consider the 90-day assumption when looking at the combined FFS and UDA analysis, presented in Fee for service and unit of dental activity analysis.
Fee for service and unit of dental activity analysis
Given the comparatively greater number of children in the England and Wales practices than in the Scotland practices (771 vs. 287), the combined analysis reflects UDA costs more than FFS costs. As a consequence, the average total costs reported are higher than for the microcosting (see Table 16), although the effectiveness data remain the same, as all practices contributed to the analysis. The comparison between PA and C+P is similar to the microcosting-based analysis (see Table 16 and Appendix 5, Table 78).
For the full incremental analysis (Table 20), B+P is the most costly management strategy and it no longer dominates C+P (as it did for the analysis based on microcosting; see Table 17). However, a combination of PA and B+P has extended dominance over C+P, meaning that the most appropriate incremental analysis is for B+P against PA. As Table 20 shows, PA is considerably more likely to be considered cost-effective for both measures of effectiveness owing to the cost savings associated with PA.
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effect (97.5% CI) | Incremental effect (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA | 247.21 (230 to 265) | 0.701 (0.58 to 0.82) | 1.00 | 1.00 | 1.00 | 0.80 | 0.29 | |||
| C+P | 305.26 (285 to 325) | 50.91 (28 to 73) | 0.603 (0.49 to 0.71) | –0.111 (–0.26 to 0.04) | 458.65 | 0.00 | 0.00 | 0.00 | 0.11 | 0.32 |
| B+P | 315.26 (293 to 337) | 10.71 (–11 to 33) | 0.565 (0.46 to 0.67) | –0.031 (–0.18 to 0.12) | 357 | 0.00 | 0.00 | 0.00 | 0.09 | 0.39 |
| B+P vs. PA | 61.62 (39 to 84) | –0.143 (–0.26 to 0.01) | 430.91 | 0.00 | 0.00 | 0.00 | 0.10 | 0.57 | ||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA | 247.21 (230 to 265) | 0.410 (0.35 to 0.47) | 1.00 | 1.00 | 1.00 | 1.00 | 0.88 | |||
| C+P | 305.26 (285 to 325) | 50.91 (28 to 73) | 0.410 (0.35 to 0.47) | –0.043 (–0.12 to 0.04) | 1183.95 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 |
| B+P | 315.26 (293 to 337) | 10.71 (–11 to 33) | 0.390 (0.33 to 0.45) | –0.016 (–0.10 to 0.06) | 669.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 |
| B+P vs. PA | 61.62 (39 to 84) | –0.058 (–0.14 to 0.02) | 1062.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | ||
Parent costs
Table 21 is a summary of the direct and indirect costs incurred by parents because of their child’s toothache over their follow-up. On average, parents incurred direct and indirect costs of £14 because of their child’s toothache over the trial follow-up period [mean £13.71 (SD £50.00)]. This average total cost increased by £10 when we incorporated the opportunity cost of children’s time away from usual activities owing to toothache in a sensitivity analysis [mean £24.13 (SD £77.85)].
| Resource | Total cost per child (£) | |||||
|---|---|---|---|---|---|---|
| C+P (n = 323) | B+P (n = 322) | PA (n = 323) | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Parent questionnaire (excluding baseline) | ||||||
| Missed paid work costs | 4.65 (28.53) | 0.00 (0.00–0.00) | 6.22 (37.00) | 0.00 (0.00–0.00) | 3.19 (14.14) | 0.00 (0.00–0.00) |
| Missed usual activities costs | 6.23 (26.73) | 0.00 (0.00–0.00) | 7.94 (28.60) | 0.00 (0.00–0.00) | 5.40 (17.71) | 0.00 (0.00–0.00) |
| Additional paid child-care costs | 0.92 (5.45) | 0.00 (0.00–0.00) | 1.48 (7.82) | 0.00 (0.00–0.00) | 1.05 (5.20) | 0.00 (0.00–0.00) |
| Painkiller costs | 1.32 (2.65) | 0.00 (0.00–2.89) | 1.27 (2.60) | 0.00 (0.00–2.79) | 1.48 (2.89) | 0.00 (0.00–2.89) |
| Child usual activities cost | 10.25 (40.90) | 0.00 (0.00–0.00) | 12.81 (40.86) | 0.00 (0.00–0.00) | 8.21 (25.79) | 0.00 (0.00–0.00) |
| Total parent costs (excluding child costs) | 13.12 (49.96) | 0.00 (0.00–2.99) | 16.90 (63.66) | 0.00 (0.00–2.99) | 11.13 (30.92) | 0.00 (0.00–2.99) |
| Total parent costs (including child costs) | 23.37 (80.15) | 0.00 (0.00–2.99) | 29.71 (96.34) | 0.00 (0.00–6.14) | 19.34 (49.86) | 0.00 (0.00–5.78) |
On average, the inclusion of parental costs is negligible and would not change our overall conclusions.
Chapter 5 Acceptability and associated experiences of the FiCTION trial arms for children, parents/guardians and dental professionals: a qualitative evaluation
Introduction
Qualitative research can add value to RCTs by exploring deliverers’ and recipients’ responses to an intervention, and provide insight into how the intervention was delivered in practice. 135–137
The purpose of the qualitative component of the FiCTION trial was to address two of the secondary objectives of the trial: (1) to compare the three treatment strategies with respect to acceptability and associated experiences for child participants and parents/guardians and (2) to compare the three treatment strategies (C+P, B+P and PA) with respect to dentists’ and dental practice team members’ experiences.
In this chapter, the acceptability for children and parents/guardians and the experiences of dental professionals will be described separately.
Child participants and their parents/guardians
Aims and objectives
To explore the acceptability of the three treatment strategies to manage carious lesions from the perspective of the child participants and their parents/guardians.
The objectives were to:
-
explore child participants’ and parents’/guardians’ experiences of the three treatment strategies and compare and contrast the impact of the treatment strategies on their daily lives
-
identify factors of importance when considering the acceptability of the three treatment strategies.
Methods
Participants and sampling
Participants were children participating in the FiCTION trial and their parents/guardians. The children were selected from all FiCTION trial participants who had not explicitly withdrawn, and whose dental practice had not explicitly withdrawn from the FiCTION trial in two of the five FiCTION trial clinical centres: Scotland and Yorkshire. Parents/guardians of the children selected were also invited to participate. Children were identified from FiCTION trial records by means of purposive maximum variation sampling using the variables of sex, age, regional location and the setting (general practice or community dental service) where the treatment had been delivered. The sample included children from each of the three treatment arms, including some whose treatment deviated from the clinical protocol for the arm to which they had been randomised as these were cases of particular interest.
Recruitment and consent
Potential child/parent participants were initially contacted by dental practices. Prior to this, the research team had identified potential child participants by their participant identification numbers and passed this information to their dental practice. The practices then sent a letter of invitation along with information sheets about the study to the identified child–parent/guardian dyads, which invited them to take part in this component of the FiCTION trial [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. Each potential dyad was also given an expression of interest form [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]) with a stamped addressed envelope to be returned to the research team if they wanted to take part in the study, or if they wanted more information before making a decision. If they expressed an interest in being interviewed, they were contacted by telephone by the researcher to arrange a suitable time and location for interview. Before the start of each interview, the researcher obtained written informed consent from the parent/guardian for their child and themselves to be interviewed; oral or written assent was also obtained from the child [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)].
Procedure
Each child–parent/guardian dyad took part in one interview together. The interviews were conducted in person and took place at each participant’s home or another convenient location. The interviews were audio-recorded. To facilitate communication, the interactions with the children during the interviews adopted participatory approaches [e.g. drawings, playing with Play-Doh dentist (Hasbro, Inc., Pawtucket, RI, USA), Dentist Barbie (Mattel, Inc., Segundo, CA, USA) or other dental toys]. 109 A topic guide [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)] was developed from literature on the concept of acceptability and its operationalisation138 and through discussions with the trial management group. The specific wording of questions was tailored to the participants and the guides were deliberately kept flexible, allowing for the discussion of unanticipated issues.
Each participating child and each parent/guardian received a £10 Love2Shop gift voucher to thank them for taking part. Children received an additional FiCTION trial-branded gift.
Data analysis
Audio-recordings of the interviews were transcribed verbatim by an external company. The data were anonymised by the use of participant identifiers. The participant identifiers related to their geographical location, the nature of the participant (C = child, P = parent) and their study numbers.
Framework analysis139 was employed, which is a matrix-based method for the analysis of cross-sectional qualitative data. The analysis involved the following stages:
-
identifying initial themes
-
labelling the data
-
sorting the data by theme
-
synthesising the data.
To ensure the coherence of the analysis and interpretation of the data, several members of the research team from different disciplines (paediatric dentistry, dental public health, trial methodology, psychology and sociology) were involved in the analysis process.
Results
Data saturation was reached after 13 interviews had been conducted across Scotland and Yorkshire between January 2017 and November 2017. The 13 child participants were aged from 5 to 11 years and comprised eight girls and five boys; four of the participants were being managed in the C+P arm, five in the B+P arm and four in the PA arm. These children had been treated by six dental teams and treatment deviation forms had been completed for four of them. Three of the 13 parents were male (see Appendix 6, Table 81).
Summary
The two key themes of the acceptability of carious lesion management in primary teeth for children and parents were, first, their experiences of specific procedures and, second, associated factors such as anticipatory anxiety, perceptions of effectiveness (particularly whether or not the child was in pain) and the impact on home or work life from attending dental appointments. These themes were underpinned by the notion of trust in the dental professionals; this notion was pervasive, regardless of which treatment approach was experienced.
Experiences of specific procedures
Overall, although there was some variation between dyads and between specific dental procedures delivered by different dentists, the procedures delivered within all three arms were generally acceptable to both children and parents.
Conventional arm
Children reported their dislike for specific dental procedures: a local anaesthetic injection, the placement of PMCs and extractions. Parents echoed their children’s concern and raised an additional concern about PMCs in a child’s mouth being a visible sign of inadequacies in their parenting practices. However, overall, children found the procedures to be tolerable and they would be willing to undergo them again. Their apparent acceptability appeared to stem from children’s underlying trust in the dentist.
For example, a 6-year-old boy reported his experiences of having an injection and compared the current with the previously seen dentist (see Appendix 6, Table 81, for participant codes):
Not nice . . . Well, it was kind of nice . . . Because it was my third time coming. And then, I kept really, really still. It didn’t hurt. It feels like I . . . my tooth, when I was brushing my tooth.
LC03: child, Yorkshire
It’s a bit painful when they do that, injection. That’s why he was a little bit scared. But he was fine. I was very happy with everything.
LP03, parent, Yorkshire
She was, like doing it really roughly. Yeah, the first one. But this one, like, does it carefully.
LP03, parent, Yorkshire
She didn’t say anything about brushing my teeth.
LC03: child, Yorkshire
She didn’t have time. She’s only got, like, about 10, 15 minutes, just quick . . . more time here.
LP03, parent, Yorkshire
And she said, ‘Bye.’ She does that.
LC03: child, Yorkshire
The conventional treatment was described by some parents as the ‘traditional’ or ‘expected’ treatment based on their own experience; they were not aware of other options:
I think it was the filling, the traditional filling . . . I didn’t realise there were other treatment options, to be fair. I had to get a filling, it was a needle . . . and I expected it before I was told about any other options.
SP05: parent, Scotland
Parents had concerns regarding the aesthetic aspect of PMCs but felt that, if it was the best option, it was justified:
Obviously, I’m to blame as the mum for the overall hygiene of his teeth. But you just felt like OK, everyone would see how bad mum I am . . . But then I thought, you know, what– whatever is best for you. That’s what I was going to do.
LP02: parent, Yorkshire
On the other hand, children were not concerned with the aesthetics of PMCs and were excited to show them to others:
Like the whole class. And my next-door neighbour. And she’s got three . . . They said, ‘Oh, he’s got a silver tooth. Did they pull it out and put it in?’ . . . I like it.
LC03: child, Yorkshire
Children also disliked having injections:
. . . OK until I found out I had to get a needle.
SC05: child, Scotland
Definitely when he numbs your teeth . . . Because it feels like your lips are about three-and-a-half miles long.
LC08: child, Yorkshire
The more invasive nature of the conventional approach for managing carious primary teeth was viewed negatively by some parents:
I wondered why they were gonna put her through that when this thing was going to fall out anyway . . . why are you filling a tooth that’s gonna just fall out?
SP05: parent, Scotland
Parents’ past experiences affected their preferences and expectations of fillings and injections:
. . . when I was little, I was petrified because I had a bad experience. And obviously because my dentist weren’t as good as what they got now, I ended up losing my teeth on bottom . . . once you have a bad experience, it puts you off it a little bit.
LP08: parent, Yorkshire
Biological arm
Children and parents found the B+P arm acceptable and expressed the value they placed on avoiding fillings and injections. As in the C+P arm, children reported their dislike of specific procedures, such as the actual fitting of a PMC and removal of carious tissue, because of their potential for pain or discomfort. Parents were concerned with the ‘unnatural’ aesthetics of a PMC and felt that they may make the child feel self-conscious, although children were not concerned. The apparent acceptability of procedures was linked with their trust in the dental professional.
For example, when an 8-year-old boy was asked if he was worried about having treatment in the future, he replied:
No, because she doesn’t really hurt your teeth. She uses the tools pretty gently.
SC02: child, Scotland
A dentist’s gentle manner towards the child resulted in the child trusting the dentist and limited any concerns regarding future visits. Parents also spoke of the welcoming manner of the dental team:
She’s not frightened of the dentist at all. You go in and they’re lovely . . . They make you feel like you’re at home.
LP06: parent, Yorkshire
Parents found B+P acceptable, particularly because it did not involve any drilling or injections:
Well, I felt good with this one.
LP04: parent, Yorkshire
I’m very glad she got this one . . . if someone came near her mouth with the drill, she wouldn’t be happy at all. And I think we’d have had a lot more problems in getting her to sit down and keep coming back.
LP05: parent, Yorkshire
It’s better that than a filling and drilling and injections.
SP01: parent, Scotland
Some children disliked the process of fitting the PMC because of the pressure applied when placing it or its discomfort if it was the wrong size:
Quite sore . . . He made sure it fit your tooth and then he put it on . . . It’s just stinging when he tries to fit it on.
The first one, as I say, he did say this was the wrong size. She wouldn’t cry in front him . . . she cried when she got out . . . She said it was sore.
But I didn’t cry the second time.
No, the second time was fine.
Conversely, other children described the removal of carious tissue as uncomfortable:
They’re, like, trying to clean it out . . . It hurt a bit and I also felt a bit weird . . .
LC06: child, Yorkshire
. . . she weren’t too keen on when they were cleaning her teeth, you know, the little metal thing that they’re putting in. I think it was just the tugging and the noises she could hear and feel that put her off a bit. But other than that, no she, we, were fine with everything.
LP06: parent, Yorkshire
The aesthetics of PMCs was a concern for some parents, whereas for others it was not:
It seemed sensible . . . don’t really care what the look of it is.
SP01: parent, Scotland
Slightly worried that with . . . all of her back teeth capped now . . . that, like, she’d notice, that other children didn’t . . . But she’s been absolutely fine. She’s not bothered by it.
LP05: parent, Yorkshire
A parent explained their preference for a more restorative approach over prevention alone, but had reservations about the ‘unnatural’ appearance of PMCs:
I’d have been a bit iffy about probably leaving it and waiting and seeing, but I’m not quite sure [how] I feel about the stainless steel thing, to be quite honest with you. I think I’d have preferred them to try and fill it rather than . . . you say it looks more natural.
LP06: parent, Yorkshire
Prevention alone arm
The PA approach was viewed as preferable to having restorations and injections, and some parents also preferred it to having PMCs for the previously mentioned aesthetic reasons. Parents, however, voiced concerns about the potential for further deterioration resulting in pain and/or affecting the permanent successor tooth. Trusting the dentist to make the right decision was apparently an important factor in parents’ acceptability of this arm:
I’m all for that, provided it doesn’t cause any more damage . . . My two concerns were (a) . . . the decay was going to cause more damage and, therefore, she’s going to get some pain from it. And the second thing is whether it’s going to damage the adult teeth underneath . . . the fact I trust . . . She’s very clear, she explains things very . . . and really takes the time both with [name] and me . . . And that helps, I think, to make a decision.
LP01: parent, Yorkshire
Parents preferred to avoid fillings and found other aspects of this arm a positive experience:
Obviously, if he doesn’t have to get treatment then, we would rather it wasn’t . . . if he doesn’t need, then I don’t want them doing it.
SP02: parent, Scotland
I’d say a lot of positive things has come out of it. There’s nothing negative, definitely something positive. And it makes the children aware . . . what they’re eating and what they’re doing . . . I think it’s been really helpful.
SP04: parent, Scotland
Parents’ preferences also appeared to be affected by their experiences:
I prefer the preventative, because I’ve had fillings and I didn’t like getting them at all. And that kind of puts the fear in. So, if we can stop getting fillings, then we can stop the fear.
SP03: parent, Scotland
Parents in the PA arm preferred this approach as they wanted to avoid PMCs for aesthetic reasons, although some children wanted one:
A silver cap on that tooth but we’ve, at the moment, decided not to.
I want one.
She wants one, I’m not sure. I think it’s me that’s saying no. I just . . . well, partly the aesthetics. I think having a piece of lump of silver in her mouth is not ideal at this age.
It is.
A PA route was acceptable as long as the carious teeth were pain free; PA was seen as the less ‘radical’ or ‘significant’ method of treatment:
But none of the three are causing her any pain. I think that’s the key thing for me. So we’re trying to do this thing with diet and brushing, with full-strength toothpaste and all that kind of things . . . I think that it’s all down to pain. So that would obviously influence that decision.
LP01: parent, Yorkshire
If she’d have been having a lot of pain, I’d have thought differently and I think that have been more something more radical to either get rid of it, take the tooth out or to have a filling or whatever, so do something more significant rather than just the painting.
LP01: parent, Yorkshire
Parents also reported that, in addition to their child’s experience, they found the PA approach to be beneficial to them as a parent in terms of ways to reduce sugar consumption and improve tooth-brushing.
In summary, all three of the trial arms were generally acceptable to children and parents and their views about some of the arm-specific procedures involved were captured. A procedure that was common to the three arms was radiographs (X-rays) and the following section describes the acceptability of radiographs.
Radiographs
Having radiographs (X-rays) taken was found to be acceptable to most children and parents; however, some children and parents described it as uncomfortable:
. . . he seems nice and still and she finds no problem when taking them. It’s like one, count one and that’s it.
LP02: parent, Yorkshire
Cool and I really liked it.
LC04: child, Yorkshire
It did not fit.
LC05: child, Yorkshire
Well, it’s . . . there’s this plastic bit that they put in and it kind of makes me gag a little and then I feel like I won’t breath. The first time I gagged. The second time . . . and then the third time I was crying.
SC04: child, Scotland
A mother explained that the radiograph procedure was what made her daughter anxious about attending the dentist:
She’s petrified because she went for that . . . an X-ray and they couldn’t get it because she was just gagging on the thing. So now every time we go she’s petrified they’re going to ask for these X-rays again and she’s . . . gets herself in a bit of a state . . .
SP04: parent, Scotland
Factors of importance when considering acceptability of treatment options
Anticipatory anxiety
Children and parents reported being anxious at the thought of certain procedures, such as drilling, injections and extractions. Even in children with positive experiences, the thought of certain procedures made them dislike attending.
One 6-year-old girl had mixed feelings about attending the dentist:
I don’t know. Sometimes I’m a bit grumpy and sometimes I’m really happy.
What makes you grumpy? What is it about coming to the dentist that makes you grumpy?
I don’t know.
No? Is there something that the dentist does that makes you a bit grumpy?
Pulls my teeth out.
She’s never pulled your teeth out.
The reason for being ‘grumpy’ about visiting the dentist was the thought of the dentist extracting her teeth, not any actual prior extraction experience. In general, however, children who had experienced tooth extraction reported being worried about it.
Parents also reported anxiousness at the thought of certain procedures because of potential pain and concern that the child would not co-operate:
I think I were more nervous at first then she were . . . It was just the thought of her having an injection I thought ‘oh no it’s going to hurt’ . . . She’s not going to let them do it. But no, she were fine.
LP06: parent, Yorkshire
Despite anticipating their child’s non-co-operation, parents found that the dental team was able to facilitate the child’s acceptance of the procedure. Parents also reported being concerned about their child’s willingness to return for certain procedures:
And she’s playing with the drill but, like, if someone came near her mouth with the drill, she wouldn’t be happy at all. And I think we’d have had a lot more problems in getting her to sit down and keep coming back.
LP05: parent, Yorkshire
Impact on schooling, home life and work life
Attending regular appointments generally appeared to be manageable and did not significantly interfere with a family’s routine:
Not really. We choose the earliest, quarter to nine. He’s only maybe half an hour late for school or so. And work, I take half an hour off as well.
LP03: parent, Yorkshire
No, we’re trying to make it, like, on times where she’s off school so she won’t miss any school terms. But she likes to miss it, but we’re trying to keep it . . .
LP04: parent, Yorkshire
Parents also mentioned the efforts of the dental practice in being flexible:
We were fine. They were ever so flexible. They sort of put on . . . so she never had to have no time off school, it were always after school and that.
LP06: parent, Yorkshire
Perceptions of effectiveness
Children and parents across all arms reported a positive impact on their children in terms of less pain and improved oral health. Parents felt that it was important that, when their child was in pain, it was quickly relieved and efforts were made to prevent repeat episodes:
Yes, it seems fine. And we have no complaints, no problems, no toothaches with it . . . she doesn’t complain when she’s eating or anything anymore.
LP05: parent, Yorkshire
She was about 3 [years of age] when we came first to the dentist; I was very worried because, like, up to 6 or 5 or 7 [years of age], they won’t fall out as well and I didn’t want her to be ill with these holes. But nothing worries me now because it did improve and like it’s been repaired.
LP04: parent, Yorkshire
Parents reported an overall improvement in their children’s oral health, with changes in their oral health behaviours including improved tooth-brushing and sugar reduction:
There’s a massive change in his teeth.
LP02: parent, Yorkshire
When the dentist says to her that she has to let us brush . . . she was saying ‘OK, you’re allowed to do it’, just because of the dentist.
LP04: parent, Yorkshire
And about fizzy drinks, they’re all quite conscious about, they’ll go ‘Oh, [name] says but only as a treat’. So they’re all . . . [name] does it in a nicest possible way, but in a way that they remember so. And it’s good for them, especially as they’re getting older.
SP04: parent, Scotland
Some children also reported changes in their oral health behaviour and gaining oral health knowledge:
He’s taught me how to brush my teeth . . . I didn’t know.
LC07: child, Yorkshire
Less unhealthy food . . . fat . . . sugar . . . and fizzy juice . . . I’ve started to cut down on it.
SC05: child, Scotland
Trust
Trust was a significant factor in the acceptability of all treatment options. Continuity of care with the same dentist appeared to be particularly important to allow trust to be built up. Child participants were co-operative and less anxious when they trusted the dentist and child–parent dyads spoke about the importance of their relationship with the dentist and how being able to trust the dentist resulted in a more acceptable experience. Both children and parents spoke of listening, explaining procedures and being gentle, caring and patient as being important characteristics in a dentist and described their positive experience with the dentist:
Yeah, and he’s fantastic with him.
He’s pretty good. He’s just good at giving advice and everything like that.
He’s caring isn’t he, as well?
Parents appeared to go beyond viewing the acceptability of the treatment just in terms of effectiveness but also in terms of the empathy the dentist had when caring for their child. The following parent described a ‘good’ dentist and a disliked previous dentist:
Shows concern . . . it helps if you’ve got a bit of time, ask them what they’re doing at school. It’s just that he was a bit short with them . . . and they hadn’t a ton of patience.
LP07: parent, Yorkshire
Parents reported their child’s co-operation when receiving treatment as a result of the dentist’s approach; the dentists’ ability to make children feel comfortable and less anxious was important for parents:
We’re very lucky because she really, really likes ‘the dentist’, don’t you? . . . She’s made you feel really, really comfortable. Sometimes, even if when you’re feeling a bit nervous, she’ll still get in the chair and at least let her look and things.
LP05: parent, Yorkshire
She’s very patient with [name], particularly with the children in helping her to understand. When she first came she was very reluctant to even open her mouth.
LP01: parent, Yorkshire
Parents also appreciated a dentist’s engagement with them, allowing them the opportunity to support their child while undergoing treatment:
[Name] explains everything really well. And, like, she’s very big on pull a chair up, hold her hand, have a look at what we’re doing.
LP05: parent, Yorkshire
Continuity of care with the same dentist and regular visits was an important factor in allowing the dentist to gain the trust of the child participant and parent:
It kind of made me feel a bit braver going there, because I used to be terrified going there and now I feel a bit, like, more braver.
SC03: child, Scotland
It has made it a lot easier. He was quite well and happy going to the dentist before. But now he sort of gets quite excited.
SP02: parent, Scotland
Parents who trusted the dentist were confident that their child was being well cared for and spoke of their trust of their own personal dentists and related this to acceptability and trust of a child’s FiCTION trial dentist:
Basically, I left everything to the dentist. He knows what he’s doing and he’s brilliant; he’d do whatever he could and do his best in his power. So I trusted his decision and choices.
LP06: parent, Yorkshire
She knows what she’s doing and she takes care of my teeth . . . I mean, my teeth were a disgrace when I went to her. And she’s fixed them all up and I’ll just go and say, ‘What do you think?’ And, kind of, whatever she says, I’ll go with it.
SP02: parent, Scotland
Dental professionals
Aim and objectives
The aim was to explore the three treatment strategies with respect to the experiences of dentists and dental practice team members (hereafter collectively referred to as ‘dental professionals’).
The objectives were to explore:
-
the experiences of dental professionals providing the three treatment strategies and whether or not their past use had an impact
-
dental professionals’ perspectives on children’s and parent/guardians’ preferences between the three treatment strategies
-
how dental professionals’ experiences of the trial might shape future management of children with carious lesions in primary teeth and identify any associated training needs.
Methods
Participants and sampling
Participants were dental professionals (comprising dentists, dental hygienists and therapists, practice managers and dental nurses) selected from the list of all practices participating in the trial. A purposive sample was drawn from across the trial sites, including Scotland, north-east England, Yorkshire and London. Participants were identified by means of purposive maximum variation sampling using the variables of sex, the dental professional’s role in the dental team, time since qualifying, the number of FiCTION trial child participants in their practice, research experience, dental setting (community/public dental service or general dental practices) and regional location. The sample included those who had recorded instances of having deviated from the FiCTION trial clinical protocol for a variety of reasons, as these were cases of particular interest.
Recruitment and consent
Lead dentists in practices participating in the FiCTION trial were sent a letter inviting them to take part in the qualitative component, which also included a detailed information sheet [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. If they expressed an interest in being interviewed, they were asked whether or not other practice team members might also be willing to take part. A suitable time and location to hold the interview or focus group was organised, prior to which written informed consent was obtained from all participants [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)]. Each participant received a £30 Love2Shop gift voucher to thank him/her for taking part.
Interviews and focus groups
Participants were offered a choice of an individual interview or a focus group with other practice team members. Participants completed a brief demographic questionnaire, which recorded time since qualifying and research experience. Individual interviews were carried out either in person or by telephone whereas focus group interviewing took place in dental practices. Interviews and focus groups were audio-recorded, transcribed verbatim by an external company and the transcripts checked. Data were anonymised by the use of study numbers.
The interviews and focus groups followed a topic guide [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019)], derived from the literature on behaviour change,140 process evaluation,141,142 the implementation of research findings in clinical practice143,144 and discussions with the Trial Management Group. This enabled thorough exploration of the dentists’ and dental team members’ perspectives on carrying out the three treatment strategies, their delivery in practice and the contextual factors that influenced this. Finally, it aided identification of the barriers to and facilitators of implementing the findings of the trial in clinical practice, as well as appropriate strategies for disseminating and encouraging adoption of the findings. Specific wording of questions was tailored to participants and the guide was flexible, allowing for the discussion of unanticipated issues.
Data analysis
Interview transcripts were imported into the qualitative data analysis software NVivo (QSR International, Warrington, UK) for coding and management. The data were analysed, using thematic framework analysis139 guided by the theoretical framework described in the following section, by two members of the research team, then discussed with the Trial Management Group as the analysis progressed.
Theoretical framework
To understand how dental professionals engaged with the treatment arms used in the FiCTION trial, the theoretical framework of practice theory was used. Individual behaviour is routinely shaped by our understanding of ‘knowing how to do’ a particular activity or ‘practice’. Practice theory145–147 seeks to explain how human activities are organised across individuals, how the standards of such activities are set and recognised and how these activities develop and change over time.
This report draws on the ‘deliberately slim-line version of practice theory’. 148 This model consolidates what are seen as the key aspects of social practices. A social practice is composed of three elements: (1) materials, (2) competences and (3) meanings. These elements are described as:
-
materials – including things, technologies, tangible physical entities and the stuff of which objects are made
-
competences – which encompasses skill, know-how and technique
-
meanings – in which we include symbolic meanings, ideas and aspirations. 148
The focus of practice theory is not on the individual but on changes over time in (1) how particular activities are done, (2) the meanings that these activities have and (3) the non-human agents (such as technologies and policies) that shape those activities. Practice theory has value to the FiCTION trial as it can engage with the real-world ‘messiness of the clinical encounter’. 149 Rather than focusing on individual choice, practice theory pays attention to the ‘larger socio-cultural, economic and technical transitions that re-define “normal” social patterns of activity’. 150
Results
The FiCTION trial dental practice staff from the Scotland, Newcastle, Leeds/Sheffield and London CCs were interviewed, either face to face or by telephone. Overall, 31 dental professionals were interviewed between August 2016 and March 2017: 22 dentists (four community-based and 18 GDPs), two dental therapists, four nurses and three practice managers (see Appendix 6, Table 82).
Exploring experiences and their impact
Differences between trial arms are outlined by illustrating the themes that emerged from the interviews and their comprising elements of materials, competences and meanings.
Managing carious lesions
As well as the activities involved in each treatment arm (removing carious tissue and filling teeth, sealing-in decay and offering preventative treatment), participants also referred to an overarching activity of managing carious lesions, referred to throughout this chapter. Applying the definition of an activity as ‘a recognisable entity of interacting elements that can be spoken about’,148 this activity was referred to across interviews and focus groups. The activity of managing carious lesions involved determining what was best for a particular patient and selecting a suitable treatment option (see Appendix 6, Table 82, for participant codes):
The child dictates what you do. You are going to . . . there’s no point in having a fancy plan about what you’re going to do with a child who can’t keep their mouth open or a child who’s frightened of local [anaesthetic]. So . . . or child with ADHD [attention deficit hyperactivity disorder]. So, very much the child will dictate what your treatment plan is.
D01: dentist, Scotland
To perform the activity of managing carious lesions, dental professionals were willing to deviate from the FiCTION trial clinical protocol at times:
It was the best . . . it was in the best interests of the child, needed a change. And I changed the plan and that reverted back to being more of a prevention at that stage.
N09: dental therapist, North East
This often involved referring to interpersonal knowledge about the individual child, developed through the dental professional–patient relationship over time, in particular regarding their ability to cope with the treatment provided. On reflection, other dental professionals demonstrated some discomfort that they were not able to do what they felt was ‘best’ for a patient:
It wasn’t so much about doing the treatment, but I’m just wondering whether or not we’re definitely doing the right thing for that child.
D03: dentist, Scotland
As performing the activity of managing carious lesions involved competence in selecting the best treatment option, being told what to do in each case could result in a dental professional feeling ‘guilty’ or finding it ‘difficult’ not to intervene, as a particular treatment option might not be the ‘right thing’ for an individual child. For example, the C+P arm might not be the ‘right thing’ for a child who may have a problem accepting local anaesthetic.
However, as this dentist acknowledges, the lack of definitive evidence on the best way to treat carious lesions can also make it difficult to engage in the activity of selecting the ‘best’ approach for an individual patient:
Well, you don’t know what’s best. And I think dentists are scared of saying that because then they think that ‘Well, I’m looking as though I’m a bit thick and I don’t know what the treatment is,’ you know? Or, ‘I’ve been providing you with this, and now I’m telling you I don’t know’, you know, ‘I don’t know what’s best’.
S07: dentist, Yorkshire
Competence in selecting the ‘best’ treatment for each patient requires practical knowledgeability in the sense of which activity is best under which circumstances for particular children. Conflicting evidence in this area can make it difficult for dental professionals to confidently engage in this activity.
Conventional arm
Participants provided little detail about what was involved in delivering the C+P treatment, although it was described as what they had ‘always’ or ‘generally’ done, or what they were doing ‘before’:
That conventional arm, that’s how we generally treat people anyway. So that’s no different to us.
S02: dental nurse, Yorkshire
They felt that this approach had a meaning in terms of what they were ‘supposed to do’ (i.e. was an ‘element’ referred to in practice theory), as opposed to the other arms:
So, that is a little bit of a difference to get your head around when you maybe see a cavity and you’re not doing anything per se, you’re not picking up a drill, which kind of goes against all of the teaching that you’ve had before.
N08: dental associate, North East
The actions of delivering local anaesthetic, removing carious tissue and filling the tooth cavity are labelled as ‘conventional’ in the trial, but are also regarded as ‘conventional’ by dental professionals (i.e. they have a meaning of being ‘routine’). However, there were examples of participants questioning the effectiveness of this approach:
Because I think parents already know, and perhaps dentists already know as well, it’s very, very difficult to predict what’s going to happen to these baby teeth. And whether actually putting an amalgam filling makes any difference whatsoever.
D02: dentist, Scotland
Removing carious tooth tissue and filling teeth are competences involved in this particular activity. This dentist highlights that, as she is confident in this approach, it has the meaning to her of being ‘effective’:
I think, for me, because I’m accustomed to doing conventional fillings and I’m good at them, they’re . . . to me it often still feels like a cop-out putting on a crown.
D01: dentist, Scotland
This is an example of the importance of considering the performance of a particular activity (e.g. the activity of filling teeth). When a dental professional does not demonstrate the competences required, this affects the meaning of the activity to patients and parents, and, thus, its perceived effectiveness.
Using local anaesthetic was another competence involved in this treatment arm (although some dental professionals reported removing carious tooth tissue without using local anaesthetic):
I mean, if you can get away with doing local without the kids realising, which you can, if you got a good parent. But if they [the parents] are saying, ‘Oh, they don’t want the needle. You’re not going to give them the needle’. That’s when you get a problem.
S06: dentist, Yorkshire
The competence here is not just physically delivering local anaesthetic, but also persuading a child’s parent that this is an appropriate treatment and gaining their co-operation.
Biological arm
Whereas removing decay and filling teeth was labelled and understood as ‘conventional’ (albeit seen as ‘ineffective’ in some cases), sealing decay into teeth had different meanings. First, the PMCs or Hall crowns were viewed as a particularly effective option by some dental professionals:
So, the biological arm, you’ve done something. And you feel as well that you’ve probably treated it the best way, so you have that at the back of your mind, you know.
N03: dentist, North East
In terms of the Hall crowns that we have placed in practice, they have lasted surprisingly well. And the patients have been symptom-free.
N01: dentist, North East
The use of ‘surprisingly’ here indicates that the meaning of sealing decay into the tooth could be contentious; importantly, there is scope for the meaning to change as activities evolve through repeated performance. When participants had prior or routine experience of using B+P arm techniques, they were generally positive about this minimally invasive approach. They understood that it required particular competences that could be developed through training and that it was effective.
Participants identified the importance of finding the right size of crown; this could be difficult and time-consuming if they were not used to using PMCs and were unfamiliar with the PMC kit:
You have to get the right size. That’s the most important one, to get the right size. It’s hard because children’s teeth, they are different shapes and they’re sometimes very strange shaped. So, it’s difficult to find the right one. But once you got the right one, it’s easy.
LDN01: dentist, London
You’ve got to get the right crown for the right tooth and the box is set up quite awkwardly actually, because it’s not right and left.
D04: dentist, Scotland
The materials involved here could be understood as ‘strange’ or ‘awkwardly’ set up, reinforcing the view that this activity is not necessarily familiar to all the participants and supports ‘conventional’ activity as a more established approach:
Historically, there weren’t that many people doing Hall crowns regularly in this area.
N06: dentist, North East
Nevertheless, the dental professionals demonstrated that increasing knowledge of different treatment options affected their understanding, particularly in terms of effectiveness, and illustrated a potential for their meanings to change over time. Ease of performance is central to the adoption of an activity.
Prevention alone arm
The dental professionals spoke about prevention activities as part of what they ‘normally’ did:
If they had a carious lesion, then we would apply fluoride varnish. We’d stress more and more about the oral hygiene and about diet. We would get them in and out more often to see if they were following our advice about fluoride and helping to arrest the caries early before it leads to any more complicated treatment. So nothing really new.
D01: dentist, Scotland
As with performing the activity of removing carious tissue and filling teeth, preventative treatment also has a meaning of being ‘conventional’, in the sense of being routine. However, being in the FiCTION trial involved giving more preventative advice than usual:
I do find that outside of FiCTION, we try to deliver some of that advice. But there’s a lot of advice to deliver there . . . Certainly, at the moment, I feel that, sort of, I can give that time to my FiCTION patients. But outside of FiCTION, they get some of that advice, but perhaps not as much as I’d like.
N01: dentist, North East
This may indicate that, outside the FiCTION trial, providing preventative advice is affected by the financial context of NHS remuneration. Alternatively, the additional element of specifically recording the prevention given, required in the FiCTION trial processes, may have impacted the performance of this activity.
Nevertheless, although prevention is something ‘we do all the time’ (D04: dentist, Scotland), several participants suggested that the PA arm was insufficient:
I think the prevention alone has been quite difficult. Especially when you know there’s a cavity and the parent knows there’s a cavity and they’ve sometimes found it quite difficult leaving that cavity and so have I. On several occasions that there’s been a few where I’ve had to just say, ‘This is getting worse, we need to intervene’.
D05: dentist, Scotland
I think we’d get accused of leaving cavities to progress if we went on the prevention arm.
S06: dentist, Yorkshire
As this second quotation shows, this idea of using prevention on its own as ‘doing nothing’ was a shared meaning, and dental professionals explained that parents could be unsatisfied and that other health professionals, such as hospital doctors, could accuse them of ‘supervised neglect’ (S05: dentist, Yorkshire). Participants questioned the effectiveness of not intervening, as preventative treatments, such as applying fluoride varnish or fissure sealant, were not seen as ‘interventions’.
Not having a preference
Dental professionals did not necessarily express ‘preferences’ for certain treatments, although some favoured treatments that they were familiar with prior to the FiCTION trial. Participants also referred explicitly to not having a ‘favourite’ treatment option or explained how all three options were useful in different circumstances. This may be because the dental professionals knew the importance of maintaining clinical equipoise, and therefore recognised that the language of ‘preferences’ would be inappropriate in a clinical trial context.
Returning to the activity of ‘managing carious lesions’, the data showed that dental professionals would appreciate having a choice, with the options seen as part of a toolbox at their disposal depending on a patient’s needs:
I would prefer to have the range . . . the whole range at my fingertips.
D01: dentist, Scotland
Dental professionals’ perspectives on children’s and parents’/guardians’ preferences
Conventional arm
Some dental professionals reported that parents and children expected fillings; as well as being ‘conventional’ for the dental professional, this approach was ‘conventional’ or ‘normal’ for patients:
The parents that I’m thinking of are expecting, on a whole, that they’re going to come in and they are going to have a filling if there was a problem.
N07: former practice owner, north-east
In relation to this, dental professionals reported that some parents were concerned about the PA arm because it went against these expectations, whereas other parents did not want any interventions:
Well, the parents don’t really want their children to have any interventive [sic] treatment. They don’t mind maybe a Hall crown being fitted and so on, they don’t like the look of them, but they don’t really want the children to get a jag [sic] and get an amalgam filling. The parents are a bit against that.
D02: dentist, Scotland
This is similar to the meaning of offering only preventative treatment as ‘not intervening’. As with the example of D02, a key issue here was with local anaesthetic:
I do remember deviating once from conventional to biological just because the mother didn’t want her to be numbed up.
N02: dentist, North East
And a lot of parents have come in and said ‘can you take this tooth out or, you know, do a filling but I don’t want my little boy getting freezed [sic] because I had it and I had a bad experience when I was 15’.
D05: dentist, Scotland
These negative associations linked to local anaesthetic reflect a meaning of ‘unpleasant experience for the child’, also indicated by dental professionals, who reported discomfort with performing this activity. The appearance of fillings was also a consideration:
You know you’ll get . . . a mother that comes in with a child with a gaping hole in the lower sets. And they don’t want you to put amalgam in it. ‘Can he not get a white filling?’, you know? And it’s just that’s the way people are. I suppose function’s not necessarily their first option, you know, their first priority.
G01: dentist, Scotland
As with the materials, local anaesthetic, needles and amalgam fillings had negative connotations, being understood as a visible sign of previous decay that could also be associated with poor oral health in a family.
Biological arm
The B+P arm was seen by dental professionals as generally popular with parents:
I’d say 99% of the patients would normally . . . do choose a biological . . . with their choice and me explaining the advantages and disadvantages.
N03: dentist, North East
However, the meaning of the B+P arm seemed to differ between dental professionals and they highlighted parents’ objections to the PMCs on aesthetic grounds:
They didn’t want a metal tooth. And that was the only reason. It had nothing to do with it that it was better for them or not or whatever, they didn’t want their child to have that.
S02: dental nurse, Yorkshire
In one case, a dental professional explicitly associated this with parents’ economic situation:
Actually, demographic side, the area that I work in is a more deprived area; there’s more children with the crowns on and the parents are less concerned about it. I don’t know if you were to go to a more affluent area of the city and say to a parent there, maybe where the children had one hole or two holes, like ‘you’re going to need this’ . . . I think you may find a lot more resistance to their use.
D03: dentist, Scotland
In other situations, familiarity with crowns among a child’s peer group [where a crown is referred to as a ‘silver hat’, for example (N09: dental therapist, north-east)] could make them a more acceptable option:
They’re pretty acceptant of the crowns and they’re aware of them and could end up . . . that could be . . . it doesn’t come as a big surprise.
N11: dental therapist, north-east
The meanings associated with the material involved (the PMCs itself) affected how sealing-in decay was interpreted; nevertheless, parents were thought to recognise the benefits of the biological approach:
I think if you try and explain the benefits of the crown and . . . I guess it’s how you spin it a little bit, how you sell it to them, how the uptake is going to be. And I guess if you try to sell it that this is going to be the best for their child, most people have been fine in the end.
N01: dentist, north-east
Here, what is important is that the minimally invasive biological approach of sealing-in decay has the meaning of being effective. The selection of the PMC as a treatment option involves the dental professional determining an appropriate treatment for an individual child, and thus managing carious lesions. The language used here, around ‘explaining’ and ‘selling’ an option, points to the influence dental professionals have in shaping how parents interpret a particular treatment through conveying meaning.
The use of GIC was also mentioned as a parental preference by dental professionals:
I’d say 90% of the people would choose just to place GIC in the cavity and see what happens and review it. I think that, for them, they’re looking for the easiest option, and sort of most painless option for the child to get a dental check, get some sort of treatment done. And then, feel that something’s been done.
N03: dentist, north-east
He identifies that, to a parent, sealing-in decay means ‘lack of pain’ and ‘doing something’ (as opposed to providing preventative treatment alone, which was understood by some parents and dental professionals as ‘doing nothing’).
Although the B+P arm did not have the meaning of being ‘conventional’ in the sense of being ‘routine’ (apart from in a few dental practices), if this approach was understood as ‘effective’ then this meaning could be conveyed to patients:
This is a misconception with patients who have metal crowns. We talk to the parent, say it’s OK and this is recognised that it’s actually better for your child, we think they wouldn’t have any issues.
S07: dentist, Yorkshire
Parents were less familiar with the activity of sealing-in caries than with other treatment options. Nevertheless, when considering the interaction between elements discussed in practice theory, these interviews indicated that the material of PMCs could become associated with the meaning of being ‘effective’ as familiarity increases.
Prevention alone arm
Dental professionals found that parents questioned the effectiveness of using prevention on its own:
When they have this prevention only, they’re a bit, like, cautious and a bit, like, not sure whether it would work.
LDN01: dentist, London
I think the ones that we’re saying that we won’t be filling the teeth, we’ll just be keeping them clean, I think they do have a number of questions, saying, ‘Is this safe? Are they going to get worse?’ They ask those questions.
N06: dentist, north-east
This reflected an expectation that the dental professional should ‘do something’:
There’s probably an expectation on the parents that some sort of active treatment is provided for the patient, some sort of restorative treatment.
N01: dentist, north-east
Furthermore, the prospect that a dental professional could ‘do nothing’ could put parents off participating in the FiCTION trial:
I did have some parents decline going on the trial because they didn’t like the idea that I wasn’t doing anything if they went on the prevention arm, even though we were doing something, they just perceived it as I wasn’t doing anything.
D04: dentist, Scotland
In these accounts, the materials involved in the C+P and B+P arms are recognised by parents as acceptable restorative treatments, whereas ‘just keeping teeth clean’ is viewed as insufficient. Nevertheless, another recognised meaning associated with the provision of prevention was positively framed as ‘not intervening’ and mentioned as particularly preferable for the children themselves:
The patients like it and the parents like it because basically the way you put it to them is, ‘You know we’re really trying to hang on to these teeth for as long as possible without you having to get fillings for teeth’. So, they love it.
G01: dentist, Scotland
A key issue here was that preventative treatment was seen as the preferred option ‘when it works’. However, dental professionals questioned whether or not the PA arm was sufficient; therefore, it is important to recognise that the perceived effectiveness of this activity was conditional.
Trusting the dental professionals
Participants highlighted the importance of parents trusting dental professionals to decide what was best for their child:
But the parents tend to be . . . if they’re prepared to take part they tend to be pretty trusting and they just . . . they just know we’ll do the best for their children and go with the flow.
D01: dentist, Scotland
Getting to know the staff at a particular practice location was also important to build up trust; participants spoke about ‘familiarity’ and ‘continuity’. As already shown by the parents, the dental professionals recognised the importance of being seen to be engaged in an activity of managing carious lesions, which involved displaying the competence of being able to select an appropriate treatment, and which meant that they were acting in the best interests of the patient. Nevertheless, as described earlier, parents were prepared to question dental professionals’ decisions.
Dental professionals’ views on how experiences of the trial may shape their future management of children with carious lesions and any associated training needs
Conventional arm
Some dental professionals spoke about preferring to avoid conventional methods in the future and other dental professionals described how they would be ‘surprised’ or ‘astounded’ if the FiCTION trial was to recommend the C+P arm. Other dental professionals had a less extreme response, although they did want to reduce local anaesthetic use in future. Nevertheless, conventional treatments were seen to have their place, for example for small lesions or to satisfy parents who dislike the appearance of stainless steel crowns.
Although participants spoke about moving away from the ‘conventional’ activities of delivering local anaesthetic, removing carious tissue and filling teeth, it was recognised that the meaning of this approach as ‘conventional’ made this difficult:
I’m still filling children’s teeth. That’s what I was trained to do and it’s very hard to get out of a rut, isn’t it?
D04: dentist, Scotland
Biological arm
Participants seemed willing to move towards minimally invasive biological treatments, rather than necessarily providing fillings and described how the meaning of sealing-in decay had changed:
It might be a bit more towards using a biological approach. Because it does take time when you have to talk a patient through having LA [local anaesthetic] or, you know. I think probably it’s a lot quicker to be able to manage things biologically.
S04: community dental officer, Yorkshire
I don’t feel I’m cheating now if I put on a PMC. You know, before I would have tended to think ‘well, in most cases you should either be doing an extraction or you should be doing a conventional filling’. So I think . . . I think I probably will feel less guilty about doing a PMC.
D01: dentist, Scotland
The idea that performing a particular activity is ‘cheating’ reflects the idea of not doing what one is ‘supposed to’. However, dental professionals were generally positive about non-conventional options if they had been trained in these, understood them to be ‘effective’ and displayed the relevant competences.
Nevertheless, when dental professionals did not feel confident about the techniques involved, their responses suggested that the ‘unfamiliarity’ of the activity of sealing decay into crowns could put them off engaging in this carious lesion management option.
The following dentist had previously said that she would probably use crowns more often, being more ‘aware’ about available options, but the second comment shows that, although this was a choice she wanted to make, the competence required made this less likely than continuing with a ‘conventional’ approach. Furthermore, the expense involved was also a relevant factor:
Because I’m still not confident so much in Hall crowns. I would like to think I would do them. But I know that it just takes so much longer, and the expense of them. There’s a huge difference between doing a filling on a child.
N02: dentist, north-east
In the B+P arm, it was recognised that knowing when to use a PMC and when to use GIC was also a competence. Furthermore, it was important for practices to invest in the required materials and to seek training.
Dental professionals suggested a need to display confidence in the competences required in the B+P arm. Providing training to develop the selection and fitting skills for PMCs would allow more dental professionals to engage in this activity.
Prevention alone arm
Planning to engage in the activity of providing prevention on its own in the future was highlighted:
I mean, I’m more swayed by the preventive arm. Personally, I think it’s all about prevention, oral hygiene and dietary instruction.
N01: dentist, north-east
Nevertheless, prevention alone was still seen as being potentially ‘insufficient’, and some dental professionals spoke about planning to combine prevention alone with B+P treatment options:
And I would’ve said that, in terms of cost efficiency, the preventive way is probably the best. [. . .] And then if you have to do a glass ionomer to prevent food trapping or a Hall crown or something like that.
G01: dentist, Scotland
Prevention alone was also seen as a last resort option when a patient rejected other treatments.
The trial had encouraged several dental professionals to reflect on their approach to managing carious lesions. They commented that taking part in the FiCTION trial had resulted in increased knowledge and altered the meanings associated with particular treatment options, referring to having to ‘think’ about what they are doing, and avoiding acting in a routinised way.
When asked a hypothetical question about what the results of the trial might show, dental professionals spoke about being willing to accept the results, but also recognised that changing one’s approach might be a lengthy process. Participants spoke about the value of ‘evidence’, and their responses indicate that a trial such as FiCTION can alter the meanings of particular activities and, thus, how these are performed. Nevertheless, it is important to recognise that experiential knowledge, both one’s own and that of colleagues, was also valued:
But that’s when people start to accept things, because it’s not just the . . . it is important to get evidence, but it’s the word of mouth of people’s experiences.
N11: dental therapist, north-east
Several dental professionals emphasised their intention to carry on acting in the best interests of the patient when managing carious lesions in routine dental practice (i.e. selecting a suitable treatment for the individual patient):
The whole purpose of evidence-based, in my understanding, is that it’s not just based on scientific research, it’s in consultation with the patient and clinical expertise as well. So, I think, you know, combining more together, you’ll get an individual plan for the patient provided you can justify what you’re doing.
S07: dentist, Yorkshire
Similarly, other dental professionals suggested that the results of the trial would not be definitive; therefore, it would be important to continue to draw on experiential and interpersonal knowledge to manage carious lesions and work in the best interests of the patient:
But I don’t think we’ll get anything as definitive as that so we will carry on, I would think, looking at each child individually [. . .] And giving treatment that best works for them.
D01: dentist, Scotland
Financial context
When considering how they would treat carious lesions in the future, several dental professionals commented on the financial context in which they operated and raised concerns about the UDA-based contract that operates in England and Wales, with UDAs allocated per course of treatment rather than per item of treatment provided. The value of a UDA varies between dental practices. The time involved in fitting a PMC, particularly when dentists were unfamiliar with the technique, meant this was not the best choice financially:
I feel if stainless steel crowns were . . . if dentists were given more money, paid higher UDAs, I think that would maybe change the decision, maybe. If dentists are struggling with something, trying to put stainless steel crowns, and they will revert some of that in what they’re more comfortable doing. And then, if you’re getting paid the same amount of money, then, I think the dentist . . . that will sway the decision that you’re going to make.
N03: dentist, north-east
The requirement to purchase PMCs made this a more expensive option, and a problem if dental practices were paid the same for sealing-in decay using crowns as for filling teeth in the ‘conventional’ approach. Receiving a fee per item in Scotland made PMCs a better option financially for dental practices.
It was also suggested that providing preventative treatment alone could look like ‘profiteering’ under the UDA system, as parents might question a treatment option that involves applying a small amount of fluoride every 3 months. However, other dental professionals suggested they were not paid sufficiently for all preventative interventions when paid a fee per item:
I mean, I don’t think you make any, I can’t think of how you would make any money doing [prevention].
E01: dentist, Scotland
Nevertheless, some dental professionals emphasised that they would provide preventative treatments anyway:
. . . wanting to do the right thing for your patient.
D02: dentist, Scotland
The co-existence of different activities that may lead to conflict for individual dental professionals and the dental practice was clear. Overall, these comments illustrate how material aspects of different activities (including the objects used and the underlying infrastructure) can sometimes undermine dental professionals’ ability to perform the activity of selecting the most appropriate treatment.
Chapter 6 Discussion and conclusions
Summary of findings
Trial findings
The FiCTION RCT compared three treatment approaches to the management of carious lesions involving dentine in the primary teeth of children aged 3–7 years and found that over a median follow-up period of 33.8 (IQR 23.8–36.7) months, there was no evidence of a difference between the arms for the co-primary outcomes of (1) the proportion of children with at least one episode (incidence) and (2) the number of episodes of dental pain or dental sepsis or both.
Overall, 43% of the 1058 children in the ITT analysis set experienced at least one episode of dental pain and/or dental sepsis over a 3-year period (dental pain ever, 36%; dental sepsis ever, 25%).
For incidence, there was no evidence of a difference between arms. Comparing B+P arm children with those in the C+P arm, the risk difference was –0.02 (97.5% CI –0.10 to 0.06; 2% less pain/sepsis for B+P arm); comparing PA arm children with those in the C+P arm, the risk difference was 0.04 (97.5% CI –0.04 to 0.12; 4% less pain/sepsis for C+P arm).
For number of episodes, there was no evidence of a difference between arms. Comparing the B+P and C+P arms, the IRR was 0.95 (97.5% CI 0.75 to 1.21) (slightly fewer for the B+P arm); comparing the PA and C+P arms, the IRR was 1.18 (97.5% CI 0.94 to 1.48) (slightly more for the PA arm).
A similar pattern, but with less marked observed differences, was noted in the pre-planned PP analysis, which excluded from the analysis children with treatment deviations on > 20% of visits.
Child oral health-related quality of life remained high (median P-CPQ-16 score was 5–7) throughout the trial and MCDASf had a median score of 14–15, representing low to moderate dental anxiety. There was no evidence of a difference in the child OHRQoL or dental anxiety measures between treatment arms at the end of the trial and no evidence that they changed over the period of the trial. However, parent-reported child anticipatory anxiety was, on average, 6% lower in the PA arm than in the C+P arm (risk difference –0.06, 97.5% CI –0.11 to –0.003).
Caries development or progression throughout the trial was considered for teeth that were entirely sound or had non-cavitated carious lesions restricted to enamel at baseline, hereafter referred to as ‘sound/reversible’. This analysis was on a subset of the ITT analysis set (61.7% of the 1058 ITT participants included) and so must be treated with caution. Overall, 399 out of 653 (61.1%) of the participants with complete ICDAS data exhibited development or progression of a carious lesion in one or more primary teeth (C+P 57.9%, B+P 61.6% and PA 64.1%). There was no statistical evidence of a difference in carious lesion development/progression in primary teeth. Comparing the B+P arm with the C+P arm, the risk difference was 0.03 (97.5% CI –0.06 to 0.11). Comparing the PA arm with the C+P arm, the risk difference was, on average, 5% higher in the PA arm: risk difference 0.05 (97.5% CI –0.03 to 0.14). Of the 399 participants with development/progression of caries, 69% had one (42%) or two (28%) teeth that were ‘sound/reversible’ at baseline, but in which dental caries developed or progressed [mean 1.3 (SD 1.4) teeth per child], and the distribution between arms was similar. There was no statistical evidence of a difference in carious lesion development/progression in FPMs between the three arms.
Findings from the economic evaluation
In terms of cost-effectiveness, the PA arm was, on average, the least costly treatment but the least effective for both of the co-primary outcomes. This means that if society is not willing to pay to avoid dental pain and/or dental sepsis, then PA would have the highest probability of being considered cost-effective compared with B+P and C+P for both of the co-primary outcomes. The other two treatment strategies would, on average, provide more benefits, albeit at higher cost. A judgement is required as to what value the NHS places on avoiding dental pain and/or dental sepsis, as B+P is, on average, more costly and more effective than PA and dominates C+P. Should the WTP to avoid an episode of dental pain and/or dental sepsis be ≥ £130, then B+P would have the highest probability (49%) of being considered cost-effective compared with PA (45%) and C+P (6%). As this WTP threshold increases, so does the probability of B+P being considered cost-effective. A WTP threshold of approximately £330 per incidence of dental pain and/or dental sepsis avoided would be needed for B+P to be considered the most cost-effective treatment strategy to avoid an incidence of dental pain and/or dental sepsis.
Fee-for-service and UDA analyses represent the current methods of reimbursement for NHS treatments in Scotland, England and Wales. Three variants of the analysis were completed: one using FFS data only, one using UDA data only and a combined analysis in which FFS and UDA data were both used (an individual participant would have either a FFS cost or a UDA cost but not both).
For these analyses, the average total cost per arm was higher than the corresponding average total costs that were estimated in the microcosting analysis. This was to be expected, as the FFS and UDA analyses account for additional overheads and capital costs that were excluded from the microcosting analysis. The impact of this is that the difference in average total costs between B+P and C+P was smaller in the FFS and UDA analyses than in the base-case analysis (which was based on the microcosting). This was expected for the UDA analysis, given that the operative treatments provided in both the B+P and the C+P arms are reimbursed at a rate of 3 UDAs per treatment episode. In the FFS analyses C+P was, on average, less costly than B+P because the reimbursement of a filling (used in C+P) is less than the reimbursement of a crown (used in B+P). Overall, analyses based on FFS and UDAs result in similar conclusions being drawn: PA has the highest probability of being considered cost-effective if we are not willing to pay to avoid an episode or an incidence of dental pain and/or dental sepsis. However, unlike the base-case analyses (which used the microcosting data), PA continues to have the highest probability of being considered cost-effective compared with B+P and C+P at higher WTP thresholds to avoid an episode or incidence of dental pain and/or dental sepsis. For example, at a WTP threshold of £130 to avoid an episode of dental pain and/or dental sepsis, PA has the highest probability of being considered cost-effective compared with B+P and C+P in the FFS (72% vs. 17% vs. 11%, respectively), UDA (99% vs. < 1% vs. < 1%, respectively) and FFS and UDA (99% vs. < 1% vs. < 1%, respectively) analyses. At a WTP threshold of £330 to avoid an incidence of dental pain and/or dental sepsis, PA has the highest probability of being considered cost-effective compared with B+P and C+P in the FFS (84% vs. 6% vs. 10%, respectively), UDA (97% vs. 2% vs. 1%, respectively) and FFS and UDA (99% vs. < 1% vs. < 1%, respectively) analyses. This could lead to incentive incompatibility in that practices could be driven to provide a treatment (i.e. PA) that, when costs are considered rather than fees, might be viewed as inefficient. Further work is needed to understand the implications of this and how dental practices might change practice as reimbursement policies change.
Findings from the qualitative study
The qualitative interviews with child–parent dyads indicated that each treatment arm was felt to be generally acceptable to children and parents, but trust in the dental professional played a significant role. Certain procedures, including local anaesthetic and extractions, were more likely to be viewed negatively. Other factors identified by children and parents were anticipatory anxiety, perceptions of effectiveness and the impact on home or work life from attending dental appointments. Children and parents had similar perspectives on most aspects of the management of carious tooth tissues, except with respect to PMCs: some parents were concerned about the aesthetics of PMCs, but children did not share this view.
The qualitative interviews with dental professionals illustrated how managing carious lesions was a recognised activity, involving the competence of selecting the most appropriate treatment option to act in the best interests of the child patient. Being a dental professional involves drawing on experiential and interpersonal knowledge, as well as research-based evidence. Participants indicated that parents/guardians shared this understanding, trusting dental professionals to treat each child in the ‘best’ way possible. This understanding of dentistry and the activity of managing carious lesions meant that some dental professionals deviated from the allocated trial arm to treat a particular patient in a way other than that to which the child had been randomised. Such treatment deviations indicate the importance to dental professionals of being able to select the most appropriate treatment option; when they continued with a treatment that they felt was less than ideal, they described this as a ‘difficult’ and an ‘uncomfortable’ experience. The interviews with dental professionals also revealed that treatment options were assessed in terms of perceived effectiveness. Although delivering local anaesthetic, removing carious tissue and filling tooth cavities were understood as elements constituting the ‘conventional’ activity of treating carious lesions, dental professionals familiar with minimally invasive biological methods of sealing-in decay spoke about this as more effective, particularly in terms of the negative connotations of local anaesthetic with the traditional ‘drill-and-fill’ approach. Nonetheless, it was recognised that, as an unfamiliar activity, the materials involved in the biological method could be ‘strange’ and dental professionals needed to develop the competencies of selecting the right crown and judging when to use a crown and when to use adhesive restorative material. Providing preventative treatment was viewed positively (and was also ‘routine’), but this was often seen as an activity that needed to be combined with other treatments, otherwise it could be ‘insufficient’.
Dental professionals reflected on how parents and children viewed different treatment options: fillings were ‘expected’, but local anaesthetic could be problematic for many children; crowns were initially viewed with suspicion but understood as beneficial when the process was fully explained; and providing only preventative treatment was often questioned and seen as ‘doing nothing’. Nevertheless, dental professionals reported that parents had other concerns, particularly around the aesthetic aspects of different treatment options. Children were also seen to prefer no intervention if possible; therefore, providing preventative treatment could have positive meanings if this involved avoiding unpleasant experiences. Participants also commented on how they planned to engage in different activities of treating carious lesions in the future. The data revealed some movement towards minimally invasive biological treatments and away from conventional methods; this was also reflected in the numbers and directions of the reported treatment deviations. Nevertheless, it was recognised that it might be difficult to change one’s habitual way of treating carious lesions. It was also clear that dental professionals expected to continue to use a range of different treatment options to manage carious lesions. Although some dental professionals spoke about accepting the results of the trial (should one management strategy be proven to be the best), it was also suggested that dental professionals should continue to have a choice in how they treat patients. The qualitative data indicate that, although the results of the FiCTION trial will form part of their knowledge base, dental professionals will also continue to draw on what they know about an individual patient and their own clinical experience to manage carious lesions.
Strengths and limitations
Overview
The size of the FiCTION trial is a huge strength, with 1144 children recruited across 72 general dental practices in three countries; therefore, the trial is thoroughly embedded in the environment where this research question is most pertinent. The trial has collected a vast number of important data from different perspectives (dental professionals and their teams, children and parents) and using different methods (quantitative and qualitative). To our knowledge, no other RCT of this size has been undertaken in this area of dental care and no RCTs have taken the pragmatic approach adopted in the FiCTION trial, with a child (as opposed to a tooth) forming the basis of the assessment level. Efforts were made to recruit a random and representative sample of practices and to also recruit dentists in clinical equipoise in each of our chosen areas. 86
The pragmatic approach taken, observing what dental professionals did for patients in each of the arms when requested to follow carious lesion management protocols, is akin to establishing what might happen if guidance or policy were put in place to direct clinical practice towards managing carious lesions in primary teeth using one particular approach.
Strengths
The sample of children who participated in the RCT was balanced across groups. At baseline, there was balance between arms in terms of age [mean age 5.96 (SD 1.3) years], sex (51% female), ethnicity (76% white) and d3mft [2.72 (SD 2.66)], including the amount of untreated decay (d3) [2.04 (SD 2.15)]. There was also balance across the arms in respect of fluoridation status and index of multiple deprivation, although these variables were captured only at dental practice level. Overall, 67% of the ITT participants attended a final visit and the median follow-up period was 33.8 (IQR 23.8–36.7) months, also balanced across the three arms, with no evidence of differential attrition across the arms. This balance confirms our confidence in the randomisation process, and the baseline participant characteristics demonstrate the representativeness of the participants as typical high-risk child dental patients attending dental practices with untreated decay. This aspect of the trial is discussed further in Generalisability.
Although the rate of recruitment was relatively slow and a time-only extension was required, the FiCTION trial exceeded its revised target sample size of 1113 participants, and 1058 out of 1144 (92%) children randomised attended at least one trial visit and were included in the ITT analysis set. Allowing for 25% attrition, the revised effective sample size was to retain 834 children who were followed up to their maximum potential of between 23 and 36 months. The FiCTION trial was able to follow up 797 out of 1144 (70%) participants for over 23 months and maximised the utility of the data collected by including in the analyses all data available for each of the 1058 children in the ITT analysis set, regardless of time in the study. The trial was adequately powered to detect the clinically meaningful differences between the arms in the incidence of dental pain and/or dental sepsis, hypothesised at the design stage. However, the estimated level of dental pain and/or dental sepsis was higher than had been anticipated at the design stage; the consequence of this was that the associated CIs were also wider.
A further strength of the trial was the simultaneous evaluation of clinical effectiveness and cost-effectiveness of the three treatment approaches. The economic evaluation was based on extensive data collection undertaken in the RCT. In particular, the different costing approaches adopted allowed us to estimate the cost-effectiveness using both the ‘true’ cost and the NHS reimbursement rates for each of the treatments. The economic results allow us to draw out the implications of the trial findings on policy and practice.
The inclusion of the qualitative study component also added much value to the trial, in addition to providing data for two secondary objectives: first, an understanding of the acceptability and associated experiences of the three treatment strategies for child participants and parents/guardians; and, second, an understanding of dentists’ and dental practice team members’ preferences between the three treatment strategies. The latter, combined with information from the CRFs and TDFs, provided a detailed insight into GDPs’ ability and willingness to deliver the three treatment strategies. By explaining patients’ and health-care professionals’ perceptions of the interventions, the qualitative study also helped the interpretation of findings,151 while also providing some insight into how the three treatment strategies were delivered in practice.
In the qualitative component, we aimed to follow best practice. In research with children, efforts are required to minimise the power imbalance that exists between the researcher and the child. During the interviews with children, efforts were made to reduce the power imbalance by ensuring that interviews took place where children were comfortable, using participatory activities and emphasising that children could stop the interview at any time. 109 The study was designed with input from the multidisciplinary Trial Management Group; the interviews were conducted by experienced qualitative interviewers, including two research associates (non-dental) and one clinical academic. The interviewers were experienced at assessing signs of distress or boredom in participants, but none was observed. Most of the interviews were conducted in children’s own homes, with steps taken to ensure interviewer safety. The analysis was conducted by several members of the team from different disciplines, with two researchers (one dentally qualified) independently identifying initial themes before discussing these with the wider team. The qualitative work with dental professionals was designed by the Trial Management Group with further support from a sociologist experienced in the use of practice theory. The decision to use practice theory to guide the analysis was driven by the data themselves. The interviews were conducted by three experienced qualitative researchers, two of whom were clinical dental academics, to enable the more technical aspects of the dental treatment to be explored while also ensuring that the broader sociocultural and economic aspects were not ignored. The analysis was initially conducted independently by two members of the team (one dentally qualified), with ongoing discussions with the Trial Management Group and undertaken before the results of the primary and secondary outcomes were revealed to the lead for the qualitative component.
Limitations
The trial experienced challenges throughout its course, some of which were anticipated and others that could not have been predicted. These resulted in some limitations, as discussed below and in Methodological rigour.
Recruitment and retention of participants in RCTs is recognised as a significant challenge,152,153 especially among young children and in a relatively research-naive environment such as NHS primary dental care services. The slower than predicted recruitment rate affected the length of time a practice was involved in the trial; some practices were involved for up to 5 years in the RCT if they were practices that had recruited participants at the beginning of the recruitment period and had continued to recruit participants throughout the whole duration of the trial. The trial length also had an impact on the FiCTION trial practice training, which sometimes required repetition or supplementation, in view of staff turnover, particularly in long-serving and vocational training practices. In addition, some secondary outcomes were measured only at baseline and the scheduled final visit and, as 33% of ITT participants did not attend their scheduled final visit, the opportunity to capture these data for those individuals was missed. From some of the qualitative interviews, as well as anecdotally, it was also clear that increasing numbers of practices and participants experienced study fatigue as the trial approached the final visits period, requiring greater levels of motivational input from the research support staff and clinical leads.
As noted above, fluoridation status and deprivation were based on practice address, not patient address, and may well have masked some intrapractice variation (especially in deprivation). Given that the study was patient randomised, it would have been preferable, although more resource intensive, to have been able to calculate these variables at the participant level.
There were several limitations in the economic evaluation. First, there were a number of challenges associated with implementing the different costing methods. In particular, the FFS is a more complex system with reimbursements based on different criteria; for example, an extraction rate was based on the number of teeth extracted in one course of treatment and a per-visit extraction rate was also available. However, the detailed costing undertaken allowed us to cope with the complexity of both reimbursement systems and provided us with robust estimates of the current charges to the NHS to manage dental decay in primary teeth.
Second, an inflated UDA value was incorporated for urgent band 1 visits, which may have led to an overestimation of UDAs for that course of treatment. In the UDA analysis, 3% of courses of treatment (n = 133) were classified as urgent visits, although such visits were evenly distributed across the three treatment strategies. In addition, for UDAs, discounting was applied to costs that occurred after the first year of follow-up based on when a course of treatment started, not when it ended. This assumption may not be reflective of current practice, given that practices are reimbursed retrospectively; however, given that resources used are incurred at the start of the course of treatment, we deemed this to be a sensible assumption. Both of these assumptions potentially give an overinflation of costs that may not be true. However, the effect is small overall and is balanced across the three treatment strategies.
Third, the SUR model used in the adjusted analysis may not be an appropriate fit for the co-primary outcomes. In the primary adjusted analyses, a logit model was adopted for incidence and a negative binomial model was fitted for the number of episodes. Although these models are arguably more appropriate for estimating these outcomes, there is a trade-off between fitting the most appropriate model and applying a model that allows for the correlation of costs and outcomes, which is arguably more appropriate for the economic analysis.
Finally, capital costs were excluded from the analysis as all three management strategies are being provided as part of current care; therefore, these costs would have been incurred regardless of which strategy was considered. Excluding capital costs reduces the total cost of each arm equally; hence the incremental costs and ICER would remain unchanged.
There were several limitations of the qualitative study. First, it was not possible to embed the qualitative component throughout the main trial, with interviews conducted only once recruitment had been completed. Second, only the perspectives of those parents and children who were retained in the trial were gained; it was not possible to interview children or parents who had withdrawn from the trial. In addition, during the interviews with children and parents, it was difficult to distinguish between procedures children had received during the trial from those before or since, especially if the child had subsequent treatment to their permanent teeth, although this is unlikely to alter the findings about acceptability. Finally, although a range of dental professionals were interviewed, it was not possible to interview those who had withdrawn from the study.
Methodological rigour
Generalisability
To our knowledge, the FiCTION trial is the first multicentre three-arm, parallel-group participant-RCT to evaluate three clinical management approaches for carious primary teeth at high risk of decay, in young children, in NHS primary dental care services, the environment where the vast majority of dental care for children takes place. As a pragmatic trial in NHS primary dental care it reflects, as closely as possible, the real clinical practice and issues encountered in the provision of dental care services for children in this environment, including the fact that the inclusion criteria were broad, thereby minimising exclusion, to try to ensure full representation of this group of children. However, the restrictions that were imposed for inclusion meant that children did not join the trial from a very early stage before they had developed a carious lesion. The age criterion for the trial was 3–7 years but the median age of the recruited children was 6 years, at the older end of the planned age range, and, therefore, arguably more compliant with treatment. However, the requirement for children to have at least one primary molar with dental caries into dentine and knowing that most children were screened for carious lesions on a visual basis alone meant that these FiCTION trial participants had advanced caries, which meant that they may or may not have been amenable to avoiding dental pain and/or sepsis. In terms of generalisability of the trial to the reality of general dental practice, management of carious lesions in a trial encourages continuity of care, which is not always observed in primary dental care.
The demographic and clinical characteristics of the practices and participants demonstrate a wide representation of the characteristics of UK primary care dentistry, including the different contractual and funding systems currently operating. Hence, we believe that this study provides generalisable findings for regularly attending child patients at high risk of decay and attending with decay in their primary teeth.
A total of 72 practices participated in the study, which were linked to five centres in Scotland, England and Wales. The large number of practices and diversity of location, including urban and rural areas, strengthened the trial not only in terms of being able to achieve the required sample size but also in terms of its generalisability to UK dental practice.
The selection of the area of north-east England around Newcastle upon Tyne as one of the five study centres was made as the city sits in a water fluoridation area. Based on the fluoridation status of the practice, the proportion of randomised participants [114/1144 (10%)] associated with a practice receiving optimally fluoridated water across the trial was similar to the proportion of the UK population receiving a fluoridated water supply (12%). 154
The RCT was also designed to include regions of the UK where children from ethnic minority populations could be recruited, as it was considered important to understand any cross-cultural differences between treatment approaches. The potential for such differences was anticipated to relate to cultural, behavioural and acceptability aspects of dental treatment, rather than to the actual clinical management of dental decay, which would be expected to be similar for all children in a given practice. The non-white population of the UK is 8.17 million (12.9% of the overall UK population),155 and 24% of the FiCTION trial children were non-white. Major efforts were made to engage with and recruit from these populations as there is evidence to suggest that families from ethnic minority groups in populations researched internationally are less likely to express an interest in or be recruited to RCTs. This is an ongoing problem in terms of research evidence, in view of the disparity in caries experience seen between the larger ethnic minorities and majorities in the UK;13,156 further research is needed to ascertain the main reasons for the difficulties seen in recruiting ethnic minority participants, including children, to RCTs. Achieving 24% of children in the FiCTION trial who were identified as non-white was an additional strength of the trial.
The overall mean caries experience in the primary dentition for all FiCTION trial participants at baseline was 2.72 (SD 2.66) d3mft. This is lower than the caries experience found in 5-year-old children with experience of dental decay (i.e. d3mft > 0) in Scotland, for whom the mean d3mft in 2016 was 3.93;156 in England, for whom the mean d3mft in 2015 was 3.4;13 and in Wales, for whom the mean d3mft in 2015/16 was 3.58. 115 It should be noted, however, that the national surveys are of randomly selected children recruited in the school setting and not from those attending general dental practices and are therefore likely to include those with the poorest oral health, who are not engaged with regular dental care.
In summary, we believe that the practices recruited to the FiCTION trial represented practices delivering primary dental care services in the NHS in Scotland, England and Wales in terms of their size, location (in terms of social deprivation), skill mix and patient mix and were therefore generalisable to the UK. With regard to participants recruited to the FiCTION trial, we believe that they were representative of UK 3- to 7-year-old regular attenders who are at high risk of dental caries, in terms of their age, caries experience, water fluoridation status and ethnicity.
Choice of primary and secondary outcomes
Failure to prevent or treat dental decay ultimately results in dental pain and/or dental sepsis with a substantial impact on individuals, families and NHS resources. Pain and sepsis are therefore appropriate outcomes to measure when comparing the impact of decay management approaches, and were the two primary outcome measures requested in the HTA commissioning brief. The use of co-primary outcomes of the incidence of dental pain and/or dental sepsis in the child (yes/no) and the number of episodes of dental pain and/or dental sepsis in the child (an interval measure) was the optimal way of measuring these most important outcomes, although challenging in terms of the data collection and its analysis.
At the trial design stage, these child- or mouth-based measures were deliberately chosen to focus on the primary mouth-based consequences arising from failure to prevent, arrest or manage decay. The addition of the co-primary outcome of number of episodes of dental pain and/or dental sepsis to the originally planned measure of incidence alone provided a refined combination of measures that strengthened the analysis and also provided further insight into the child participant experiences. Although 43% of children experienced dental pain and or dental sepsis, 67% of these children experienced it only once during the FiCTION trial. We believe that the wording of the pain question may have contributed to the high levels of ‘dental pain ever’ recorded (an overall incidence of 36%, with no evidence of differences between arms), as it asked about ‘a history of pain at this visit’ rather than pain since the last visit, leading to a probable inflation of the instances of positive recordings.
The use of dental sepsis as an outcome measure provided the necessary objectivity, which was less evident with the measure of dental pain. It was the prevalence of dental sepsis seen in other studies that provided the basis for the sample size and power calculation for the trial. However, although pain was a less objective outcome measure, being participant/parent observed (and reported to the dentist), it was the measure that is important to patients.
The secondary outcomes included in the trial were diverse, but all were important contributors to the overall assessment of the clinical, economic, behavioural and psychological impact of dental management approaches to managing decay in children’s primary teeth. At baseline and at the end of follow-up, the use of the ICDAS caries index – an objective measure suitable for recording carious lesion prevalence and incidence – allowed the derivation of a binary indicator variable at a child level (progression of disease in one or more teeth: yes/no) and a measure of disease severity (number of teeth with caries development/progression). From the behavioural and psychological perspective, the child- and parent-reported outcomes measuring OHRQoL, anxiety/worry and dental discomfort were important to capture, particularly as the three treatment approaches differ substantially from one another in that one (C+P) requires the routine use of local anaesthetic when removing decay involving dentine. Parents tend not to recognise dental anxiety in their children very well: they usually under-report it, which is why child self-reported measures are preferred if possible;106 however, it was difficult to identify instruments appropriate and/or valid for the target age group. For example, the use of anticipatory and treatment-related anxiety and worry questions (MCDASf scales) at every visit aimed to distinguish between state and trait dental anxiety. However, in 2007, Howard and Freeman103 concluded that, although the MCDASf scale was a reliable measure of dental anxiety, demonstrating good reliability and validity, this had been demonstrated only with children aged 8–12 years. At baseline scoring, FiCTION trial participants were aged 3–7 years. Notwithstanding this, the pilot study gave some reassurance that the tools used were valid.
With regard to parental and caregiver perceptions, the P-CPQ-16 scale was used. In 2014, Thomson et al. 96 compared the responsiveness and functionality of the Early Childhood Oral Health Impact Scale (ECOHIS) and P-CPQ scales and found them similar and of good quality for internal consistency, reliability, validity and responsiveness. However, when used for health services research, when the child participants had significant amounts of decay as in the FiCTION trial, the P-CPQ did not have the shortcomings that ECOHIS157 demonstrated (it focuses minimally on any ‘oral symptoms’ domain), instead centring on general function and social and emotional well-being.
Financial costs for managing decay in the primary teeth of children in the UK are a substantial call on NHS resources. 5 It was important, therefore, that the FiCTION trial included appropriate clinical effectiveness and cost-effectiveness comparisons using valid, reliable measures. The costs of the interventions were assessed in terms of incremental cost, using data collected at an individual participant level using microcosting, as well as being based on charges to the NHS on an aggregate level using UDA and FFS values. These robust estimates of cost-effectiveness can be used in conjunction with the clinical effectiveness results to help inform policy decisions regarding the management of dental decay in primary teeth.
Blinding and other methodological issues
For ethics reasons, the trial design did not allow for an arm receiving no treatment; therefore, all three arms included the same active prevention element.
Dental professionals, parents and children could not be blinded to the intervention, as major features of the C+P and B+P arms were the use of local anaesthetic and the Hall Technique, respectively. However, they were all responsible for recording a number of the outcome variables, leaving the study open to an element of (detection) bias. The measurement of caries would have been less open to bias if undertaken by independent examiners. However, this would have been another very large resource implication in terms of time and cost given the number of participants, their locations and the 3-month final visit time period.
Although blinding of treatment arm was not possible, we tried to minimise other forms of bias. Of the 12,078 people sent a screening invitation, 4379 (36%) never attended, and, of the 1756 screened and known to be eligible, 612 (35%) did not participate. It is almost certain that biases were introduced at both of these stages, especially in respect of the 331 children who were eligible but declined the trial, but the impact of this cannot be assessed. Selection bias was minimised by using a central randomisation process operated by the NCTU, while detection bias was limited by including a simple observable reporting outcome (dental sepsis) as part of the composite primary outcome alongside the more subjective dentist- and participant-/parent-reported pain measures. We also tried to validate the reported pain by recording the tooth involved.
Dentists were asked to recruit eligible children when they attended their routine dental check-up appointments and, in view of the pragmatic nature of the trial, subsequent treatments and recall intervals varied as they do in real life. As a result, the number of data collection points varied not only with length of follow-up but also between participants and between arms. Overall, the median rate of visits was 2.5 per year in the trial, ranging from 2.1 for the PA arm to 2.5 for the other two arms. This is a lower rate of attendance than would be anticipated as, on the basis of the inclusion criteria for the trial, all children had at least one primary molar with dental caries involving dentine and, therefore, all were at high caries risk. This further indicates that, in the trial, GDPs tended to stick to a 6-monthly ‘check-up’ regime, rather than providing more frequent preventative interventions (e.g. application of fluoride varnish three or four times per year). The reason for this is unclear, but may relate to administrative or financial factors tending to dictate the frequency of recall intervals for children, or may be parent determined (e.g. not attending or arranging visits).
In addition, primarily for ethics reasons, the radiographs taken were only those that would be taken during normal clinical practice, that is those taken in line with FGDP guidelines;70 therefore, they were not a mandatory requirement for data collection. We had originally planned to use any available radiographs to assess the primary outcome of dental pain and/or dental sepsis as well as the caries incidence and progression outcomes. However, the level of adherence to guidelines recommending clinical radiography in children is known to be low. 158 The children entering the trial fell into the ‘high-risk’ category for dental caries because having a carious primary tooth was an inclusion criterion. Therefore, all children in the trial should, in principle, have had a radiograph taken within 1 year of entry to the trial (as they may have had one taken prior to entry) and then annually or more frequently until they moved out of the ‘high-risk’ category. However, in practice, we found that only 308 (29%) of the children had radiographs taken on entry to the trial (or within 1 year of entry). We decided that this rate of radiography was too low (and not representative enough of the children across the trial) to be able to use the radiograph data to supplement the clinical data. We therefore relied on assessment of the clinical data for the primary outcome measure of sepsis and/or pain due to caries. Approximately half of the carious lesions that exist in a child’s mouth, especially affecting proximal surfaces, are not detected by clinical visual examination alone;159 instead, detection relies on the use of further investigation through the use of bitewing radiographs.
Adherence to protocol
As reported in Chapter 3, Treatment provision and adherence to protocol, and Appendix 5, Table 74, we are confident that dentists and dental teams provided treatment for the children in line with the clinical protocols. Prevention was common to all arms and at least one item of prevention was delivered, on average, at 81% of all visits, balanced across arms. According to the trial protocol, recording of treatment deviations was required at every visit at which they occurred. Of the 7713 CRFs completed, a major treatment deviation recorded on a TDF occurred at 429 (6%) visits affecting 263 children, which indicated good fidelity with the protocol. The majority (46%) of deviations were away from the C+P arm and most of these were because of issues associated with local anaesthetic. The reported major deviations from clinical protocol resulted in 10% of the children from the ITT analysis set having > 20% of their treatment visits deviating from the clinical protocol. There was some evidence from the data collected via the CRF that additional protocol deviations had occurred around the failure to use local anaesthetic in the C+P arm, but, as the CRF had not been designed to specifically collect this information, the data around additional deviations lacked sufficient clarity for any meaningful analysis.
As discussed earlier, the clinical protocol for the FiCTION trial was developed in 2007 and was based on evidence-based guidelines available at that time. 25 These provided a degree of flexibility between adhesive intracoronal restorations and Hall Technique use and it is clear that some dentists chose to avoid the Hall Technique use in many situations where the protocol provided a legitimate alternative (ie. intracoronal adhesive restoration); in the B+P arm, the majority of restorations used GIC (11%), followed by PMCs placed using the Hall Technique (9%), and RMGIs (7%).
Patient and public involvement and engagement
Children and parents were involved throughout the FiCTION trial from the outset, including during trial design and the development of recruitment and communication strategies, as well as later during the planning of the qualitative study.
An important aspect of the trial was the inclusion of a child-centred approach to collecting data. Informal discussions were held in Sheffield with parents and children who had experience of the treatment of carious lesions, prior to submission for REC approval for the pilot trial when the format and content of written and web-based participant literature and data collection materials were reviewed, to optimise their acceptability to participants and their families. These discussions informed a number of changes to reduce the burden on participants during the main trial and maximise the enjoyment that children had experienced by being able to participate by answering their own version of a questionnaire when their parents completed the parental questionnaire. Some participating dentists noted that children particularly enjoyed the experience of being part of a trial and being engaged in trial discussions, helped in part by initiatives aimed at keeping them engaged, including provision of small gifts carrying the FiCTION trial logo, personalised birthday and thank-you cards, FiCTION trial medals and a FiCTION trial passport to bring to dental visits.
High retention rates were achieved for practices and participants. The positivity of many of the practices that remained with the trial throughout its prolonged course (up to 7 years for some practices also involved in the pilot trial), which was still evident at close-down of the trial, is a strength and testament to the motivation and dedication of parents, practices and the research team.
Interpretation of results
The evidence for a minimally invasive biological approach for the management of primary teeth as well as permanent teeth was increasing when the trial was set up, which is why, when the original call from the HTA programme was for a two-arm trial comparing a restorative and non-restorative approach, a third (biological) arm was justified. This evidence has strengthened over the past 10 years since the FiCTION trial interventions were designed in 2008. Indeed, for increasing numbers of dentists across the UK, a biological approach has become the ‘conventional’ with a small ‘c’ approach and is now taught widely across UK dental schools. It is against this backdrop that the results of the FiCTION trial are now interpreted and compared.
Previous research findings, including RCTs and systematic reviews, have been reviewed in Chapter 1, and have consistently shown that, at the individual tooth level, when compared with a conventional approach, a minimally invasive biological approach to managing dental carious lesions results in less dental pain and dental sepsis. However, although at least some of the evidence for a biological approach (notably the Hall Technique) has come from primary care and field settings,34,36,50 the evidence for the effectiveness of the conventional approach has been limited to specialist centres. Favourable findings from meta-analyses must be interpreted cautiously, given the variations across trials in patient selection, the actual treatments studied, the definitions of outcomes and other differences in trial design and conduct. 160 The FiCTION RCT’s findings cannot be directly compared with published evidence on the most clinically effective approach to managing carious lesions in individual primary teeth. There are several fundamental differences between the FiCTION trial and the other published evidence: FiCTION was a child-level intervention for the whole primary dentition, whereas the vast majority of the literature around interventions for carious lesion management is based on individual tooth management and outcomes. In addition, the FiCTION trial was designed to be a pragmatic trial and to use the most clinically appropriate outcome measure, the child’s experience of dental pain and/or dental sepsis, rather than the traditional outcome measure used in dental research, that of restorative material performance.
Based on evidence that has emerged since the start of the trial,30,36,37,49,51,161 current guidelines for use of PMCs and the Hall Technique in a biological treatment approach are now less stringent25 with a lower threshold for use, that is they are indicated at an earlier stage. The Hall Technique is now recommended as the treatment of choice for proximal and multisurface lesions on primary molar teeth (cavitated or non-cavitated), whereas the FiCTION trial protocol allowed GDPs to choose between the Hall Technique and an intracoronal adhesive restoration for such lesions, presenting them with a hierarchy in which the Hall Technique was not necessarily the primary choice. Evidence from the qualitative component of the study suggests that the reason for dentists tending to prefer using intracoronal restorations rather than PMCs in the B+P arm may have been related to their past clinical experience. The B+P arm was new to many of the dentists taking part; although they were provided with training in the technique and supplied with additional learning support materials, they recognised a number of challenges to their use. These included judging when to use a PMC, selecting the correct one and when to use adhesive restorative materials, especially when the intracoronal approach was more familiar to them: this represented (only) cautious acceptance of the Hall Technique.
The pre-planned PP analysis compared arms for those 89% of children in the ITT analysis set in whom < 20% of visits had a reported between-arm treatment deviation or where the child was not deemed likely to have had dental pain and/or dental sepsis at consent and still found no evidence of a difference between arms.
We are confident about the power of the trial to detect any true differences between arms, particularly with regard to the incidence of sepsis events, as they formed the basis of the original power calculation. A possible explanation for finding no evidence of clinical benefit is the combination of (1) the inevitability of the co-primary outcomes being observed in these children as they already had dentinal carious lesions and possibly asymptomatic but unhealthy pulps on entry to the trial; (2) not all carious lesions were detected and diagnosed at baseline (especially the less clinically obvious approximal lesions) because bitewing radiographs were used infrequently and not in line with the trial or national guidelines;70 (3) the co-primary outcomes being measured at child rather than tooth level, and therefore including outcomes for teeth treated prior to FiCTION; and (4) dentists using materials and techniques that were accessible and familiar to them rather than newer materials and less familiar techniques meant that optimal clinical outcomes were not possible.
A child’s experience of dental pain and/or dental sepsis in the FiCTION trial was a result of one or more of the following scenarios: (1) a lesion treated using one of the three approaches described in the FiCTION trial clinical protocol, (2) a pre-existing restoration and/or (3) a lesion that was undetected and undiagnosed at baseline. It would be important to try to interrogate the FiCTION trial database further to determine a tooth’s journey through the FiCTION trial to distinguish between these three scenarios, as opposed to a child’s journey, and this is an area for further research. However, it is important to note that the results from this tooth analysis would not be comparable with those from studies that randomised a tooth instead of a child.
Experience of dental pain ever (overall 36%) during the follow-up period was higher than dental sepsis ever (overall 25%) over the same period. As these ‘management strategy failure’ rates are at a mouth level, they might be expected to be higher than those reported in previous studies that have compared different treatment strategies at an individual primary tooth level. Previous tooth-level trials in children have shown that median survival times for conventional adhesive restorations in primary molars range from 20 to 42 months,52 and a Cochrane systematic review by Innes et al. 30 found moderate-quality evidence that risk of major failure in the long term (> 12 months) was lower in crowns than in fillings [risk ratio 0.18, 95% CI 0.06 to 0.56; 346 teeth in three studies, one using conventional (tooth reduction) technique and two using Hall Technique]. The suspected underdetection of carious lesions through clinical examination alone, due to the low use of radiographs, may have increased the potential for unmanaged carious lesions, as well as pre-existing restorations, to contribute to the primary outcome of dental pain and/or dental sepsis. A B+P approach, when treated with the Hall Technique, is less vulnerable to lower carious lesion detection rates as all surfaces are sealed. In terms of ‘dental sepsis ever’ as an outcome, the incidence at a child level across all arms at 25% for the FiCTION trial was slightly higher than the dental sepsis rates predicted for a tooth-level preventative approach based on the work by Levine et al. 7 and Tickle et al. 6
The FiCTION trial found no evidence that one management approach performed better than another when used by dental professionals (non-specialist) working in primary care. This differs from secondary care where there is more resource with respect to time and expertise, leading to more favourable outcomes. 24,162 However, it was the delivery of care in general dental practice that was under test in the FiCTION trial and not the secondary care environment.
The trial has shown that, in primary care, the B+P approach would have the highest probability of being considered cost-effective in terms of avoidance of dental pain and/or dental sepsis if society is willing to pay to avoid an episode (≥ £130) or an incidence (≥ £330) of dental pain and/or dental sepsis.
In a cost-effectiveness analysis, the ICER can be difficult to interpret, given that there is no threshold to determine what society’s WTP for a unit of effect is, that is WTP to avoid an incidence of dental pain and/or dental sepsis in a primary tooth. Recent (2015) research conducted by Lord et al. 163 estimated the WTP to avoid dental decay with pain in a primary tooth. If we adopted this WTP threshold of £153 (95% CI £93 to £213, inflated to 2017 prices),120 the PA arm would have a 68% probability of being considered cost-effective compared with B+P (29%) and C+P (3%) in terms of an incidence of dental pain and/or dental sepsis avoided. A WTP threshold to avoid an episode of dental pain and/or dental sepsis needs to be determined but, based on the Lord et al. 163 threshold, B+P would have the highest probability (53%) of being considered cost-effective compared with PA (40%) and C+P (7%).
A further strength of the trial is that it gives a significant insight to how children with carious lesions are managed in general practice; however, the results of the trial also raise the issue of where a child’s dental care is best managed. The levels of sepsis experienced by participants in the FiCTION trial (re-) open the debate about whether or not children should have their own paediatric dental service and be cared for by specialist teams with an appropriate skill mix.
The C+P arm was designed to encompass the clinical procedures needed to achieve complete carious tissue removal prior to provision of a direct intracoronal plastic restoration (filling) or a direct extracoronal restoration, that is a preformed crown placed following endodontic treatment and some tooth reduction. As a result, it was heavily reliant on the properties of materials used at the dentine: restorative interface within a much reduced tooth structure. In contrast, the B+P arm was based around a minimally invasive approach with no, or partial, carious tissue removal, in which both infected and affected dentinal carious tissue remains. The clinical effectiveness of this approach, which is designed to retain tooth structure, relies on the reduction in further decay potential in the remaining carious dentine and underlying pulp as a result of the reduced viability of the bacteria, which are now sealed-in and starved of nutrients.
The finding that the clinical outcome was dependent on the level of dental experience at baseline, with those children with more decay more likely to experience dental pain and/or dental sepsis, emphasises the need to prevent this disease.
Conclusions
There was no evidence of an overall difference between the three treatment approaches for incidence, or number of episodes, of dental pain and/or dental sepsis experienced by the children. It is important to understand that ‘no evidence of a difference’ does not mean that there was no difference, or that the three treatment approaches were equivalent. Nor was there evidence of a difference in quality of life or dental anxiety. All three strategies were acceptable to children, parents and dental professionals and did not provoke anxiety or discomfort.
The PA arm was, on average, the least costly and least effective treatment with respect to co-primary outcomes. A judgement is required as to what value the NHS places on avoiding dental pain and/or dental sepsis. Over the WTP values considered, the probability of B+P being considered cost-effective was approximately no higher than 60% to avoid an incidence and no higher than 70% to avoid an episode of pain and/or sepsis.
The level of dental pain and/or dental sepsis observed emphasises the importance of early prevention for young children. The outcomes of the FiCTION trial were for the individual child and we suspect the small number of radiographs taken may have led to undetected and misdiagnosed dental caries. This, together with dental professionals’ preferences for familiar materials and techniques, will have contributed to the overall findings.
As there was no evidence of a difference between the three treatment arms in the dental pain or dental sepsis experienced, the choice of treatment should continue to be based on shared decision-making with a conversation between child, parent and clinician to agree the best option for the individual child.
Implications for health care
-
The results of the FiCTION trial emphasise the importance of preventing disease before it occurs to avoid dental pain and/or dental sepsis. Prevention must start early to avoid the need for operative management of disease.
-
The FiCTION trial results indicate that, for successful management of carious lesions in young children, the health-care system may require adjustment to ensure effective detection and diagnosis of dental caries and the use of effective materials and techniques.
-
The FiCTION trial’s findings open the debate around (1) where dental care for children at high risk of dental caries is best provided, (2) the most appropriate way of identifying children at high risk of dental caries and (3) the optimal clinical and funding environment for children at high risk of dental caries.
Implications for parents and practice
-
Parental and dental professional roles are key to ensure that prevention starts early at home, and early attendance at a dental practice is an opportunity for reinforcement of prevention, monitoring of oral health, provision of effective advice and early intervention, if necessary.
-
The FiCTION trial findings indicate that treatment of carious lesions in young children can be tailored to meet their behavioural and clinical needs and, therefore, the role of shared decision-making is important. Although there is no evidence of a difference in clinical outcome between arms, the biological approach may offer an advantage when taking into account the disease process, the child’s needs and the technical requirements, and is the strategy most likely to provide value for money if preventing dental pain/sepsis is valued.
Implications for dental education/teaching and training
-
There appears to be a need for knowledge tools, key evidence-based guidance and additional clinical training and support of dental professionals to address their clinical skills with regard to use of radiographs and choice of restorative materials and techniques.
-
The FiCTION trial has evidence of facilitators of and barriers to the investigation, detection, diagnosis and management of carious lesions, learnt through the qualitative study, which could inform implementation strategies for Public Health England and SDCEP guidance. Messages and techniques need to be clear, evidence based and tailored for delivery and uptake in the primary care environment.
The undergraduate and postgraduate curricula in all dental schools may need to be reviewed to ensure that all caries management strategies evidenced as suitable for use in young children are included, along with competencies in managing the dental care of young children and shared decision-making.
Recommendations for research
-
Explore barriers to use of conventional techniques for the detection and diagnosis of carious lesions (e.g. radiographs) and develop and evaluate suitable tools for use in young children in primary dental care.
-
Explore individual tooth outcomes descriptively by treatment provided, including dental materials and techniques, through further analysis of the FiCTION trial data set.
-
Identify the appropriate service structure necessary to provide cost-effective and acceptable prevention for young children in a primary care setting.
-
Explore clinicians’ decision-making around the use of minimally invasive techniques for caries management in young children treated in primary care.
Acknowledgements
Trial Steering Committee
We would like to thank the external members of the TSC for their advice and support during the project: Professor June Nunn (Trinity College Dublin, chairperson), Professor Lorna McPherson (Glasgow), Dr Ronnie Levine (Leeds), Ms Tina McGuff (patient representative) and Mrs Nicole Douglas (patient representative).
Independent Data Monitoring and Ethics Committee
Our thanks go also to the Independent Data Monitoring and Ethics Committee, comprising Professor Helen Worthington (Manchester University, chairperson), Professor Marie-Therese Hosey (London) and Professor Gerry Humphris (St Andrews).
Recruitment sites
We are grateful to the participants, their parents and the GDPs and their clinical and administrative teams who supported the study, giving so generously of their time and also sharing their experiences with us. The practices were:
Alderman Road Dental Practice, Amble Dental Practice, Anita Belbin Dental Surgery, Archway, Ash Dental, Atlas Road Dental Surgery, B Davidoff Dental Surgery, Barnhill Dental Practice, BG Easton, Bridge of Don Dental Practice, Bridge Street Dental Care, Broxden Dental Centre, Brundholme Dental Practice, Burnett Dental Group, Church Road Dental Practice, Colchester Dental Surgery, DCO Dental, Dean Road Dental Practice, Dental Care Perth, Devonshire, E2 Dental Practice, Eastside Dental Practice, Eston Dental Practice, Family Dental Care, Family Dental Practice, Forth Valley Smile Design, Framwellgate Dental Surgery, Hafren House, Hampden Dental Care, High Green, Hillcrest Dental Practice, Horizon (Blyth) Dental Clinic, Horizon (Whitley Bay) Dental Clinic, Jedburgh Dental Clinic, JEM, Kilbirnie Dental Centre, King’s Cross Health and Community Care Centre, Kingsmeadows Dental Practice, Kingsway Dental Practice, Leeds Community Dental Service, Llantarnam Dental Practice, Lomond Dental Centre, Louise Hunter & Associates, Montgomery Street Dental Care, Montrose Dental Care, Park View Family Dental (formerly Mr A I Robson & Associates), Nanodent, Orgreave Dental Surgery, Parkhead Public Dental Service, Pearl Dental, Perfect Smile, Pollock Dental Care, Port Talbot Resource Centre (Dental Teaching Unit), Possilpark Dental Practice, Queensway Dental Clinic, Roseberry Dental Practice, Salmon Lane Dental Care, Shiremoor Dental Practice, Shotley Bridge Dental Care, Springburn Public Dental Service, Springfield Public Dental Service, Stanley Dental Practice, Stoke Newington Dental Practice, Sunderland Road Dental Practice, The Square Dental Practice, The Whitley Bay Dental Clinic, Thompson & Thomas Dental Care, Triangle Dental Practice, Wanstead Village Dental & Health Centre, Westbury Dental Practice and Whickham Dental Practice.
Non-author contributors
We would like to thank a number of people who helped towards the successful completion of the study (note that ‘former’ means that the person no longer worked on the FiCTION trial at the time of writing):
Paul Averley (Collaborator), Jennifer Ball (Project Secretary, NCTU, former), Hazel Braid (Trial Administrator), Elspeth Barker, (Clinical Lead Secretary, Scotland CC, former), Paul Blaylock (Research Champion for South Tyneside), Tam Bekele (Research Champion for London CC), Amy Caldwell-Nichols, (Trial Administrator, former), Ivor Chestnut (Collaborator), Ben Cole (Collaborator, NHS Consultant in Paediatric Dentistry), Michelle Corsi (Clinical Lead Secretary, Wales CC), Kathryn Cunningham, (Collaborator, Qualitative Clinical Researcher, former), Mark Deverill (Co-Applicant, Health Economics, former), Pina Donaldson, (Trial Administrator), Mojtaba Dorri, (Collaborator, Clinical Researcher, former), Monty Duggal (Co-Applicant), Dafydd Evans (Co-Applicant), Stephen Fayle (Collaborator, NHS Consultant in Paediatric Dentistry), Andrea Henderson-Burton (Clinical Lead Secretary, London CC), Nicola Howe (Collaborator, Database Manager, NCTU, former), Bev Howell (Clinical Lead Secretary, Wales CC, former), Alex Keightley (Collaborator, Clinical Researcher, former), Marilyn Laird (Senior Trial Administrator, former), Shahana Lais (Clinical Lead Secretary, London CC, former), Chris Longbottom, (Collaborator, Trialist, Primary Dental Care), Claire MacDonald (Collaborator, Senior TM, NCTU, former), Bill Montelpare (Collaborator, Biostatistician), Valeria Morenio (Collaborator), Shelley O’Rourke (Project Secretary, NCTU, former), Mark Palmer (Collaborator, TM, NCTU, former), Julia Phillipson (Clinical Trial Administrator, NCTU), Beverly Philpott (Clinical Lead Secretary, Yorkshire CC), Victoria Pickering (Clinical Lead Secretary, Scotland CC), Nigel Pitts (Co-Applicant), Katherine Rennie (TM, NCTU), David Ricketts (Collaborator), Helen Rodd (Collaborator), Chris Speed (Collaborator, Senior TM, NCTU, former), the late Jimmy Steele (Co-Applicant), Vidya Srinivasan (Clinical Lead, Manchester), Nick Steen (Co-Applicant, Statistician, former), Mathew Stewart (Collaborator, Clinical Researcher, former), Fiona Szeller (Trial Administrator, former), Laura Ternent (Collaborator, Health Economist, former), Lynn Thompson (Project Secretary, NCTU), Sue Thompson (Clinical Lead Secretary, North East England CC), Jared Thornton (Senior TM, NCTU), Elizabeth Treasure (Collaborator), Richard Watt (Collaborator) and Richard Welbury (Collaborator).
Ethics approval
Ethics approval for the study was given by the East of Scotland Research Ethics Service in July 2012 (REC reference number 12/ES/0047); Integrated Research Application System project identifier: 103239.
The appropriate local research and development approvals and site-specific assessments were obtained from the PCTs covering practices recruited from the CCs in England and Wales and the Health Boards covering the practices recruited from the Scotland CC.
The trial was registered with the International Standard Randomised Controlled Trial Number registry (reference number ISRCTN77044005).
Sponsorship and service support
The University of Dundee agreed to act as sponsor for the research and the study was adopted by the Scottish Dental Practice Based Research Network and the relevant local research networks in England and Wales.
Service support was provided by a central subvention from NIHR and the local PCTs and health boards.
Contributions of authors
Anne Maguire (https://orcid.org/0000-0003-2930-7412) (Professor, Co-Chief Investigator and Clinical Lead for north-east England and Cumbria CC) contributed to the design and conduct of the trial, was responsible for the conduct of the trial in north-east England and Cumbria, contributed to recruitment of dentists and the interpretation of data and made significant contributions to the drafting and editing of the monograph.
Jan E Clarkson (https://orcid.org/0000-0001-5940-2926) (Professor and Co-Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of the results and the editing of the monograph.
Gail VA Douglas (https://orcid.org/0000-0002-0531-3909) (Professor, Co-Chief Investigator and Joint Clinical Lead for Leeds/Sheffield CC) contributed to the conception and design of the trial, was responsible for the conduct of the trial in Leeds, and contributed to recruitment of dentists, the interpretation of data and the drafting of the monograph.
Vicky Ryan (https://orcid.org/0000-0002-7008-3193) (Senior Statistician) developed the SAP, led the statistical analyses, contributed to the interpretation of the data, made a significant contribution to the drafting of Chapters 2 and 3 and reviewed and approved the monograph.
Tara Homer (https://orcid.org/0000-0002-6664-0671) (Health Economist) developed the health economic analysis plan, conducted the economic analysis, contributed to the interpretation of the data, drafted Chapter 4 and reviewed and approved the monograph.
Zoe Marshman (https://orcid.org/0000-0003-0943-9637) (Professor, Lead for the Qualitative Study) was responsible for the conduct and analysis of the qualitative study, drafted Chapter 5 and reviewed and approved the monograph.
Elaine McColl (https://orcid.org/0000-0001-8300-3204) (Professor, Trialist) contributed to the conception and design of the trial and the interpretation of the data, made a significant contribution to the drafting of Chapter 2 and reviewed and approved the monograph.
Nina Wilson (https://orcid.org/0000-0001-5908-1720) (Statistician) contributed to the development of the SAP, conducted the statistical analyses, contributed to the interpretation of the data, contributed to the drafting of Chapter 3 and reviewed and approved the monograph.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Health Economist) supervised the conduct of the economic analysis, contributed to the interpretation of the data and the drafting of Chapter 4 and reviewed and approved the monograph.
Mark Robertson ((https://orcid.org/0000-0002-0005-3044) (Clinical Research Fellow) contributed to the day-to-day running of the trial, contributed to the interpretation of data, made a significant contribution to the drafting of Chapter 1 and reviewed and approved the monograph.
Alaa Abouhajar (https://orcid.org/0000-0001-7220-6380) (Database Manager) was responsible for the day-to-day management of the data and database, contributed to the drafting of Chapter 2 and reviewed and approved the monograph.
Richard D Holmes (https://orcid.org/0000-0002-1713-7690) (Senior Lecturer, Honorary Consultant in Dental Public Health) made a significant contribution to the design, data collection and interpretation of data in the qualitative study, contributed to the drafting of Chapter 5 and reviewed and approved the monograph.
Ruth Freeman (https://orcid.org/0000-0002-8733-1253) (Professor and Trialist) contributed to the conception and design of the trial, contributed to the interpretation of data and the drafting of Chapter 2 and reviewed and approved the monograph.
Barbara Chadwick (https://orcid.org/0000-0003-4827-3473) (Professor and Clinical Lead for Wales CC) contributed to the design of the trial and the recruitment of practices, was responsible for the conduct of the trial in Wales and reviewed and approved the monograph.
Christopher Deery (https://orcid.org/0000-0001-7526-7736) (Professor and Joint Clinical Lead for Leeds/Sheffield CC) contributed to the design of the trial and the recruitment of practices, was responsible for the conduct of the trial in Sheffield and reviewed and approved the monograph.
Ferranti Wong (https://orcid.org/0000-0001-7681-3647) (Professor and Clinical Lead for London CC) contributed to the design of the trial and the recruitment of dentists, was responsible for the conduct of the trial in London and reviewed and approved the monograph.
Nicola PT Innes (https://orcid.org/0000-0002-9984-0012) (Professor, Co-Chief Investigator and Clinical Lead for Scotland CC) contributed to the concept and design of the trial and the recruitment of practices, was responsible for the conduct of the trial in Scotland, contributed to the interpretation of data and the drafting of the monograph and reviewed and approved the monograph.
Publications
Marshman Z, Innes N, Deery C, Hall M, Speed C, Douglas G, et al. The management of dental caries in primary teeth – involving service providers and users in the design of a trial. Trials 2012;13:143.
Innes NPT, Clarkson JE, Speed C, Douglas GVA, Maguire A. The FiCTION dental trial protocol – filling children’s teeth: indicated or not? BMC Oral Health 2013;13:25.
Allen N. Minimum Intervention – A Nurse’s Perspective. Premium Practice Dentistry. Shenley: FMC; 2014.
Deery C, Doherty R. ‘The Hall technique will revolutionise children’s dentistry’. Br Dent J 2014;216:156–7.
Keightley A, Clarkson J, Maguire A, Speed C, Innes N. Participant recruitment to FiCTION, a primary dental care trial – survey of facilitators and barriers. Br Dent J 2014;217:E22.
Martin-Kerry JM, Lamont TJ, Keightley A, Calache H, Martin R, Floate R, et al. Practical considerations for conducting dental clinical trials in primary care. Br Dent J 2015;218:629–34.
Stewart M, Keightley A, Maguire A, Chadwick B, Vale L, Homer T, et al. Investigating the management of carious primary teeth in general dental practice: an overview of the development and conduct of the FiCTION trial. Prim Dent J 2015;4:67–73.
Innes NP, Clarkson JE, Douglas GVA, Ryan V, Wilson N, Homer T, et al. Child caries management: a randomized controlled trial in dental practice. J Dent Res 2020;99:36–43.
Data-sharing statement
Anonymised data from this trial may be available to the scientific community subject to regulatory and ethics approval. Requests for data should be directed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Faculty of Dental Surgery . The State of Children’s Oral Health in England 2015.
- Public Health Wales . Child Dental General Anaesthetics in Wales 2014.
- Information Services Division (ISD) Scotland . Dental Statistics: NHS Registration and Participation 2014.
- Vernazza CR, Rolland SL, Chadwick B, Pitts N. Caries experience, the caries burden and associated factors in children in England, Wales and Northern Ireland 2013. Br Dent J 2016;221:315-20. https://doi.org/10.1038/sj.bdj.2016.682.
- NHS England . Improving Dental Care and Oral Health: A Call to Action 2014.
- Tickle M, Milsom K, King D, Kearney-Mitchell P, Blinkhorn A. The fate of the carious primary teeth of children who regularly attend the general dental service. Br Dent J 2002;192:219-23. https://doi.org/10.1038/sj.bdj.4801338.
- Levine RS, Pitts NB, Nugent ZJ. The fate of 1,587 unrestored carious deciduous teeth: a retrospective general dental practice based study from northern England. Br Dent J 2002;193:99-103. https://doi.org/10.1038/sj.bdj.4801495a.
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res 2015;94:650-8. https://doi.org/10.1177/0022034515573272.
- Petersen PE, Quah SR, Heggenhougen K. International Encyclopedia of Public Health. Oxford: Elsevier; 2008.
- World Health Organization (WHO) . WHO Expert Consultation on Public Health Intervention Against Early Childhood Caries. 2016.
- Marmot M. Status syndrome. Significance 2004;1:150-4. https://doi.org/10.1111/j.1740-9713.2004.00058.x.
- Broadbent JM, Zeng J, Foster Page LA, Baker SR, Ramrakha S, Thomson WM. Oral health-related beliefs, behaviors, and outcomes through the life course. J Dent Res 2016;95:808-13. https://doi.org/10.1177/0022034516634663.
- Public Health England . National Dental Epidemiology Programme for England: Oral Health Survey of Five-Year-Old Children 2015: A Report on the Prevalence and Severity of Dental Decay 2016.
- Hall-Scullin E, Whitehead H, Milsom K, Tickle M, Su TL, Walsh T. Longitudinal study of caries development from childhood to adolescence. J Dent Res 2017;96:762-7. https://doi.org/10.1177/0022034517696457.
- Pine CM, Adair PM, Nicoll AD, Burnside G, Petersen PE, Beighton D, et al. International comparisons of health inequalities in childhood dental caries. Community Dent Health 2004;21:121-30.
- Pitts NB, Chadwick B, Anderson T. Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland 2015.
- Marsh PD. Contemporary perspective on plaque control. Br Dent J 2012;212:601-6. https://doi.org/10.1038/sj.bdj.2012.524.
- Fejerskov O, Nyvad B, Fejerskov O, Nyvad B, Kidd EAM. Dental Caries: The Disease and Its Clinical Management. Chichester: John Wiley & Sons; 2015.
- Kidd E, Fejerskov O. Changing concepts in cariology: forty years on. Dent Update 2013;40:277-8. https://doi.org/10.12968/denu.2013.40.4.277.
- Frencken JE, Peters MC, Manton DJ, Leal SC, Gordan VV, Eden E. Minimal intervention dentistry for managing dental caries: a review – report of a FDI task group. Int Dent J 2012;62:223-43. https://doi.org/10.1111/idj.12007.
- Elderton RJ. Clinical studies concerning re-restoration of teeth. Adv Dent Res 1990;4:4-9. https://doi.org/10.1177/08959374900040010701.
- Fayle SA, Welbury RR, Roberts JF. British Society of Paediatric Dentistry (BSPD) . British Society of Paediatric Dentistry: a policy document on management of caries in the primary dentition. Int J Paediatr Dent 2001;11:153-7. https://doi.org/10.1046/j.1365-263x.2001.011002153.x.
- Kindelan SA, Day P, Nichol R, Willmott N, Fayle SA. British Society of Paediatric Dentistry . UK National Clinical Guidelines in Paediatric Dentistry: stainless steel preformed crowns for primary molars. Int J Paediatr Dent 2008;18:20-8. https://doi.org/10.1111/j.1365-263X.2008.00935.x.
- Chadwick B, Treasure E, Dummer P, Dunstan F, Gilmour A, Jones R, et al. Challenges with studies investigating longevity of dental restorations: a critique of a systematic review. J Dent 2001;29:155-61. https://doi.org/10.1016/S0300-5712(01)00003-3.
- Scottish Dental Clinical Effectiveness Programme . Prevention and Management of Dental Caries in Children: Dental Clinical Guidance 2018.
- Macpherson LMD, Ball G, Carson S, Ireland J, McStravick M, Conway DI, et al. Report of the 2015 Detailed National Dental Inspection Programme of Primary 7 Children and the Basic Inspection of Primary 1 and Primary 7 Children 2015.
- Davies G, Neville J, Rooney E, Robinson P, Jones A, Perkins C. National Dental Epidemiology Programme for England: Oral health Survey of Five-Year-Old-Children 2012. London: Public Health England; 2012.
- Threlfall AG, Pilkington L, Milsom KM, Blinkhorn AS, Tickle M. General dental practitioners’ views on the use of stainless steel crowns to restore primary molars. Br Dent J 2005;199:453-5. https://doi.org/10.1038/sj.bdj.4812746.
- Ricketts D, Lamont T, Innes NPT, Kidd E, Clarkson JE. Operative caries management in adults and children. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.CD003808.pub3.
- Innes NPT, Ricketts D, Chong LY, Keightley AJ, Lamont T, Santamaria RM. Preformed crowns for decayed primary molar teeth. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.CD005512.pub3.
- Innes NP, Evans DJ, Clarkson JE, Foley JI. Obtaining an evidence-base for clinical dentistry through clinical trials. Prim Dent Care 2005;12:91-6. https://doi.org/10.1308/1355761054348459.
- Innes NP, Schwendicke F, Lamont T. How do we create, and improve, the evidence base?. Br Dent J 2016;220:651-5. https://doi.org/10.1038/sj.bdj.2016.451.
- Levey C, Innes N, Schwendicke F, Lamont T, Göstemeyer G. Outcomes in randomised controlled trials in prevention and management of carious lesions: a systematic review. Trials 2017;18. https://doi.org/10.1186/s13063-017-2256-1.
- Innes NP, Evans DJ, Stirrups DR. The Hall Technique; a randomized controlled clinical trial of a novel method of managing carious primary molars in general dental practice: acceptability of the technique and outcomes at 23 months. BMC Oral Health 2007;7. https://doi.org/10.1186/1472-6831-7-18.
- Innes N, Evans D. The Hall Technique: A Minimal Intervention, Child Centred Approach to Managing the Carious Primary Molar. A Users Manual. Dundee: University of Dundee; 2015.
- Innes NP, Evans DJ, Stirrups DR. Sealing caries in primary molars: randomized control trial, 5-year results. J Dent Res 2011;90:1405-10. https://doi.org/10.1177/0022034511422064.
- Santamaría RM, Innes NPT, Machiulskiene V, Schmoeckel J, Alkilzy M, Splieth CH. Alternative caries management options for primary molars: 2.5-year outcomes of a randomised clinical trial. Caries Res 2018;51:605-14. https://doi.org/10.1159/000477855.
- Kidd EA. How ‘clean’ must a cavity be before restoration?. Caries Res 2004;38:305-13. https://doi.org/10.1159/000077770.
- Frencken JE. Atraumatic restorative treatment and minimal intervention dentistry. Br Dent J 2017;223:183-9. https://doi.org/10.1038/sj.bdj.2017.664.
- Mickenautsch S, Yengopal V. Failure rate of high-viscosity GIC based ART compared with that of conventional amalgam restorations: evidence from an update of a systematic review. SADJ 2012;67:329-31.
- Mickenautsch S, Yengopal V, Banerjee A. Atraumatic restorative treatment versus amalgam restoration longevity: a systematic review. Clin Oral Investig 2010;14:233-40. https://doi.org/10.1007/s00784-009-0335-8.
- Dorri M, Martinez-Zapata MJ, Walsh T, Marinho VC, Sheiham Deceased A, Zaror C. Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries. Cochrane Database Syst Rev 2017;12. https://doi.org/10.1002/14651858.CD008072.pub2.
- de Menezes Abreu DM, Leal SC, Frencken JE. Self-report of pain in children treated according to the atraumatic restorative treatment and the conventional restorative treatment: a pilot study. J Clin Pediatr Dent 2009;34:151-5. https://doi.org/10.17796/jcpd.34.2.9k67p786l7126263.
- Wong DL, Baker CM. Smiling face as anchor for pain intensity scales. Pain 2001;89:295-7. https://doi.org/10.1016/S0304-3959(00)00375-4.
- Innes NP, Manton DJ. Minimum intervention children’s dentistry: the starting point for a lifetime of oral health. Br Dent J 2017;223:205-13. https://doi.org/10.1038/sj.bdj.2017.671.
- Hansen NV, Nyvad B. Non-operative control of cavitated approximal caries lesions in primary molars: a prospective evaluation of cases. J Oral Rehabil 2017;44:537-44. https://doi.org/10.1111/joor.12508.
- Page LA, Boyd DH, Davidson SE, McKay SK, Thomson WM, Innes NP. Acceptability of the Hall Technique to parents and children. N Z Dent J 2014;110:12-7.
- Ludwig KH, Fontana M, Vinson LA, Platt JA, Dean JA. The success of stainless steel crowns placed with the Hall Technique: a retrospective study. J Am Dent Assoc 2014;145:1248-53. https://doi.org/10.14219/jada.2014.89.
- Narbutaite J, Innes NPT, Santamaria RM, Splieth CH, Machiulskiene V. One-year outcomes of dental caries management using different approaches in primary molars of Lithuanian children. Caries Res 2017;51:290-385.
- Araujo MP, Olegario IC, Hesse D, Innes NP, Bonifacio CC, Raggio DP. ART versus Hall Technique in primary molars: 1-year survival cost and analysis of a RCT. Caries Res 2017;51:290-385.
- Santamaria RM, Innes NP, Machiulskiene V, Evans DJ, Splieth CH. Caries management strategies for primary molars: 1-yr randomized control trial results. J Dent Res 2014;93:1062-9. https://doi.org/10.1177/0022034514550717.
- Santos AP, Moreira IK, Scarpelli AC, Pordeus IA, Paiva SM, Martins CC. Survival of adhesive restorations for primary molars: a systematic review and metaanalysis of clinical trials. Pediatr Dent 2016;38:370-8.
- Wright JT, Crall JJ, Fontana M, Gillette J, Nový B, Dhar V, et al. Evidence-based clinical practice guideline for the use of pit-and-fissure sealants. Pediatr Dent 2016;38:120-36. https://doi.org/10.1016/j.adaj.2016.06.001.
- Dorri M, Dunne SM, Walsh T, Schwendicke F. Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. Cochrane Database Syst Rev 2015;11. https://doi.org/10.1002/14651858.CD010431.pub2.
- Fontana M, Platt JA, Eckert GJ, González-Cabezas C, Yoder K, Zero DT, et al. Monitoring of sound and carious surfaces under sealants over 44 months. J Dent Res 2014;93:1070-5. https://doi.org/10.1177/0022034514551753.
- Tellez M, Gomez J, Kaur S, Pretty IA, Ellwood R, Ismail AI. Non-surgical management methods of noncavitated carious lesions. Community Dent Oral Epidemiol 2013;41:79-96. https://doi.org/10.1111/cdoe.12028.
- Wright JT, Tampi MP, Graham L, Estrich C, Crall JJ, Fontana M, et al. Sealants for preventing and arresting pit-and-fissure occlusal caries in primary and permanent molars. Pediatr Dent 2016;38:282-308. https://doi.org/10.1016/j.adaj.2016.06.003.
- Hesse D, Bonifácio CC, Mendes FM, Braga MM, Imparato JC, Raggio DP. Sealing versus partial caries removal in primary molars: a randomized clinical trial. BMC Oral Health 2014;14. https://doi.org/10.1186/1472-6831-14-58.
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;3. https://doi.org/10.1002/14651858.CD002284.
- Ahovuo-Saloranta A, Hiiri A, Nordblad A, Worthington H, Mäkelä M. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD001830.pub2.
- Harris R, Gamboa A, Dailey Y, Ashcroft A. One-to-one dietary interventions undertaken in a dental setting to change dietary behaviour. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD006540.pub2.
- Shepherd MA, Nadanovsky P, Sheiham A. The prevalence and impact of dental pain in 8-year-old school children in Harrow, England. Br Dent J 1999;187:38-41. https://doi.org/10.1038/sj.bdj.4800197.
- Jackson SL, Vann WF, Kotch JB, Pahel BT, Lee JY. Impact of poor oral health on children’s school attendance and performance. Am J Public Health 2011;101:1900-6. https://doi.org/10.2105/AJPH.2010.200915.
- American Academy of Pediatric Dentistry . Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pediatr Dent 2014;37:15-6.
- Blumenshine SL, Vann WF, Gizlice Z, Lee JY. Children’s school performance: impact of general and oral health. J Public Health Dent 2008;68:82-7. https://doi.org/10.1111/j.1752-7325.2007.00062.x.
- Gilchrist F, Marshman Z, Deery C, Rodd HD. The impact of dental caries on children and young people: what they have to say?. Int J Paediatr Dent 2015;25:327-38. https://doi.org/10.1111/ipd.12186.
- National Institute of Health Consensus Development Panel . NIH Consensus Development Conference on Diagnosis and management of dental caries throughout life. Bethesda, MD, 26–28 March 2001. J Am Dent Assoc 2001;65:935-1179.
- Pitts NB, Stamm JW. International Consensus Workshop on Caries Clinical Trials (ICW-CCT): final consensus statements – agreeing where the evidence leads. J Dent Res 2004;83:C125-8. https://doi.org/10.1177/154405910408301s27.
- Pretty IA. Caries detection and diagnosis: novel technologies. J Dent 2006;34:727-39. https://doi.org/10.1016/j.jdent.2006.06.001.
- Horner K, Eaton KA. Selection Criteria for Dental Radiography. London: Faculty of General Dental Practice; 2013.
- Heasman PA, Macpherson LE, Haining SA, Breckons M. Clinical research in primary dental care. Br Dent J 2015;219:159-63. https://doi.org/10.1038/sj.bdj.2015.645.
- Marshman Z, Innes N, Deery C, Hall M, Speed C, Douglas G, et al. The management of dental caries in primary teeth: involving service providers and users in the design of a trial. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-143.
- Stewart M, Keightley A, Maguire A, Chadwick B, Vale L, Homer T, et al. Investigating the management of carious primary teeth in general dental practice: an overview of the development and conduct of the fiction trial. Prim Dent J 2015;4:67-73. https://doi.org/10.1308/205016815816682146.
- Locker D, Allen F. What do measures of ‘oral health-related quality of life’ measure?. Community Dent Oral Epidemiol 2007;35:401-11. https://doi.org/10.1111/j.1600-0528.2007.00418.x.
- Pantell RH, Lewis CC. Measuring the impact of medical care on children. J Chronic Dis 1987;40:99-115. https://doi.org/10.1016/S0021-9681(87)80039-5.
- Klinberg G. Dental anxiety and behaviour management problems in paediatric dentistry: a review of background factors and diagnostics. Eur Arch Paediatr Dent 2008;9:11-5. https://doi.org/10.1007/BF03262650.
- Nuttall NM, Gilbert A, Morris J. Children’s dental anxiety in the United Kingdom in 2003. J Dent 2008;36:857-60. https://doi.org/10.1016/j.jdent.2008.05.014.
- Tsakos G, Hill K, Chadwick B, Anderson T. Children’s Dental Health Survey 2013: Report 1 Attitudes, Behaviours and Children’s Dental Health England, Wales and Northern Ireland 2015.
- Townend E, Dimigen G, Fung D. A clinical study of child dental anxiety. Behav Res Ther 2000;38:31-46. https://doi.org/10.1016/S0005-7967(98)00205-8.
- Klaassen MA, Veerkamp JS, Hoogstraten J. Changes in children’s dental fear: a longitudinal study. Eur Arch Paediatr Dent 2008;9:29-35. https://doi.org/10.1007/BF03262653.
- Freeman R. A fearful child attends: a psychoanalytic explanation of children’s responses to dental treatment. International Journal of Paediatric Dentistry 2007;17:407-18. https://doi.org/10.1111/j.1365-263X.2007.00871.x.
- NHS Digital . NHS Dental Statistics. England, 2013 14 2014. https://files.digital.nhs.uk/publicationimport/pub14xxx/pub14738/nhs-dent-stat-eng-13-14-rep.pdf (accessed 5 September 2019).
- Schwendicke F, Doméjean S, Ricketts D, Peters M. Managing caries: the need to close the gap between the evidence base and current practice. Br Dent J 2015;219:433-8. https://doi.org/10.1038/sj.bdj.2015.842.
- Keightley A, Clarkson J, Maguire A, Speed C, Innes N. Participant recruitment to FiCTION, a primary dental care trial: survey of facilitators and barriers. Br Dent J 2014;217. https://doi.org/10.1038/sj.bdj.2014.1009.
- Innes NPT, Clarkson JE, Speed C, Douglas GVA, Maguire A. The FiCTION dental trial protocol: filling children’s teeth – indicated or not?. BMC Oral Health 2013;13. https://doi.org/10.1186/1472-6831-13-25.
- National Institute for Health Research . NETSCC, HTA: 9th May 2011. FiCTION Feasibility Study 2011.
- Public Health England . Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention 2014.
- Innes NP, Clarkson JE, Douglas GVA, Ryan V, Wilson N, Homer T, et al. Child caries management: a randomized controlled trial in dental practice. J Dent Res 2020;99:36-43.
- Ismail AI. Visual and visuo-tactile detection of dental caries. J Dent Res 2004;83:C56-66. https://doi.org/10.1177/154405910408301s12.
- International Caries Detection and Assessment System (ICDAS) Coordinating Committee . Rationale and Evidence for the International Caries Detection and Assessment System (ICDAS II) 2011.
- Shoaib L, Deery C, Ricketts DN, Nugent ZJ. Validity and reproducibility of ICDAS II in primary teeth. Caries Res 2009;43:442-8. https://doi.org/10.1159/000258551.
- Braun A, Guiraud LM, Frankenberger R. Histological validation of ICDAS II and radiological assessment of occlusal carious lesions in permanent teeth. Odontology 2017;105:46-53. https://doi.org/10.1007/s10266-016-0245-6.
- Jokovic A, Locker D, Stephens M, Kenny D, Tompson B, Guyatt G. Validity and reliability of a questionnaire for measuring child oral-health-related quality of life. J Dent Res 2002;81:459-63. https://doi.org/10.1177/154405910208100705.
- Marshman Z, Rodd H, Stem M, Mitchell C, Robinson PG. Evaluation of the Parental Perceptions Questionnaire, a component of the COHQoL, for use in the UK. Community Dent Health 2007;24:198-204.
- Thomson WM, Foster Page LA, Gaynor WN, Malden PE. Short-form versions of the Parental-Caregivers Perceptions Questionnaire and the Family Impact Scale. Community Dent Oral Epidemiol 2013;41:441-50. https://doi.org/10.1111/cdoe.12036.
- Thomson WM, Foster Page LA, Malden PE, Gaynor WN, Nordin N. Comparison of the ECOHIS and short-form P-CPQ and FIS scales. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/1477-7525-12-36.
- Atchison KA, Gift HC. Perceived oral health in a diverse sample. Adv Dent Res 1997;11:272-80. https://doi.org/10.1177/08959374970110021001.
- Brook PH, Shaw WC. The development of an index of orthodontic treatment priority. Eur J Orthod 1989;11:309-20. https://doi.org/10.1093/oxfordjournals.ejo.a035999.
- Porritt J, Buchanan H, Hall M, Gilchrist F, Marshman Z. Assessing children’s dental anxiety: a systematic review of current measures. Community Dent Oral Epidemiol 2013;41:130-42. https://doi.org/10.1111/j.1600-0528.2012.00740.x.
- Scottish Dental Clinical Effectiveness Programme . Oral Health Assessment and Review Guidance in Brief 2011.
- Humphris GM, Wong HM, Lee GTR. Preliminary validation and reliability of the Modified Child Dental Anxiety Scale. Psychol Rep 1998;83:1179-86. https://doi.org/10.2466/pr0.1998.83.3f.1179.
- Christophorou S, Lee GTR, Humphris GH. The reliability and validity of the Modified Child Dental Anxiety Scale: a study of Greek-Cypriot school children. Eur Arch Paediatr Dent 2000;1:75-81.
- Howard KE, Freeman R. Reliability and validity of a faces version of the Modified Child Dental Anxiety Scale. Int J Paediatr Dent 2007;17:281-8. https://doi.org/10.1111/j.1365-263X.2007.00830.x.
- Nelson S, Slusar MB, Albert JM, Liu Y, Riedy CA. Psychometric properties of a caregiver illness perception measure for caries in children under 6 years old. J Psychosom Res 2016;81:46-53. https://doi.org/10.1016/j.jpsychores.2016.01.002.
- Porritt J, Marshman Z, Rodd HD. Understanding children’s dental anxiety and psychological approaches to its reduction. Int J Paediatr Dent 2012;22:397-405. https://doi.org/10.1111/j.1365-263X.2011.01208.x.
- Patel H, Reid C, Wilson K, Girdler NM. Inter-rater agreement between children’s self-reported and parents’ proxy-reported dental anxiety. Br Dent J 2015;218. https://doi.org/10.1038/sj.bdj.2015.98.
- Krikken JB, van Wijk AJ, ten Cate JM, Veerkamp JS. Measuring dental fear using the CFSS-DS: do children and parents agree?. Int J Paediatr Dent 2013;23:94-100. https://doi.org/10.1111/j.1365-263X.2012.01228.x.
- Ersig AL, Kleiber C, McCarthy AM, Hanrahan K. Validation of a clinically useful measure of children’s state anxiety before medical procedures. J Spec Pediatr Nurs 2013;18:311-9. https://doi.org/10.1111/jspn.12042.
- Marshman Z, Hall MJ. Oral health research with children. Int J Paediatr Dent 2008;18:235-42. https://doi.org/10.1111/j.1365-263X.2008.00922.x.
- Francis D, Roberts I, Elbourne DR, Shakur H, Knight RC, Garcia J, et al. Marketing and clinical trials: a case study. Trials 2007;8. https://doi.org/10.1186/1745-6215-8-37.
- Payne R, Abel G. UK indices of multiple deprivation: a way to make comparisons across constituent countries easier. Health Stat Q 2012;53:1-16.
- Abel GA, Barclay ME, Payne RA. Adjusted indices of multiple deprivation to enable comparisons within and between constituent countries of the UK including an illustration using mortality rates. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012750.
- Shilling V, Williamson P, Hickey H, Sowden E, Beresford M, Smyth R, et al. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLOS One 2011;6. https://doi.org/10.1371/journal.pone.0021604.
- O’Mullane DM, Baez RJ, Jones S, Lennon MA, Petersen PE, Rugg-Gunn AJ, et al. Fluoride and oral health. Community Dent Health 2016;33:69-9.
- Morgan M, Monaghan N. Picture of Oral Health 2017: Dental Caries in 5 Year Olds (2015/2016). Cardiff: Cardiff University; 2017.
- Scottish Intercollegiate Guidelines Network (SIGN) . Dental Interventions to Prevent Caries in Children 2014.
- Department for Transport . Transport Analysis Guidance 2015.
- Dental Sky . Dental Supplies UK 2018. www.dentalsky.com (accessed 21 May 2018).
- NHS Business Services Authority (NHSBSA) . NHS Payments to Dentists: Data for 2016 to 17 2017. www.nhsbsa.nhs.uk/fp17-processing-and-payments/nhs-payments-dentists-201617 (accessed 16 October 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2018.
- NHS Health Education England . Agenda for Change: Pay Rates 2018. www.healthcareers.nhs.uk/working-health/working-nhs/nhs-pay-and-benefits/agenda-change-pay-rates (accessed 21 May 2018).
- Stolls N. Now and then: NHS dentistry – pre- and post-2006. Br Dent J 2016;220. https://doi.org/10.1038/sj.bdj.2016.214.
- Scottish Dental . Statement of Dental Remuneration Amendment No. 135 2017. www.scottishdental.org/wp-content/uploads/2017/09/Amendment-No.-135-SDR.pdf (accessed 16 October 2017).
- Watson M. What is a UDA?. Vital 2010;7. https://doi.org/10.1038/vital1131.
- Office for National Statistics (ONS) . Time Series: Average Actual Weekly Hours of Work for Full-Time Workers (Seasonally Adjusted) 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/timeseries/ybuy/lms (accessed 8 December 2017).
- Lloyds Pharmacy . Online Pharmacy, Prescriptions, Chemist and Health 2017. www.lloydspharmacy.com/ (accessed 8 December 2017).
- Harding C, Cottell J. Childcare Survey 2018. Family and Childcare Trust; 2018.
- Fiebig DG, Baltagi BH. A Companion to Theoretical Econometrics. Maldon, MA: Blackwell Publishing; 2007.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis: principles of good practice – report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5-14. https://doi.org/10.1016/j.jval.2013.08.2291.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Drabble SJ, O’Cathain A, Thomas KJ, Rudolph A, Hewison J. Describing qualitative research undertaken with randomised controlled trials in grant proposals: a documentary analysis. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-24.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Spencer L, Ritchie J, O’Connor W, Lewis J, Ritchie J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Saunders RP, Evans MH, Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract 2005;6:134-47. https://doi.org/10.1177/1524839904273387.
- Green LA, Seifert CM. Translation of research into practice: why we can’t ‘just do it’. J Am Board Fam Pract 2005;18:541-5. https://doi.org/10.3122/jabfm.18.6.541.
- Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-50.
- Schatzki TR. Social Practices: A Wittgensteinian Approach to Human Activity and the Social. Cambridge: Cambridge University Press; 1996.
- Giddens A. The Constitution of Society Outline of the Theory of Structuration. Oxford: Polity Press; 1984.
- Bourdieu P. The Logic of Practice. Cambridge: Polity Press; 1990.
- Shove E, Pantzar M, Watson M. The Dynamics of Social Practice: Everyday Life and How it Changes. London: SAGE Publication; 2012.
- Wieringa S, Engebretsen E, Heggen K, Greenhalgh T. Has evidence-based medicine ever been modern? A Latour-inspired understanding of a changing EBM. J Eval Clin Pract 2017;23:964-70. https://doi.org/10.1111/jep.12752.
- Vihalemm T, Keller M, Kiisel M. From Intervention to Social Change: A Guide to Reshaping Everyday Practices. Surrey: Ashgate; 2015.
- Flemming K, Adamson J, Atkin K. Improving the effectiveness of interventions in palliative care: the potential role of qualitative research in enhancing evidence from randomized controlled trials. Palliat Med 2008;22:123-31. https://doi.org/10.1177/0269216307087319.
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrolment and participation study: the STEPS study. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11480.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Landes DP, Jardine C. Targeting dental resources to reduce inequalities in oral health in the North East of England: a health equity audit methodology to evaluate the effects of practice location, practice population and deprivation. Br Dent J 2010;209. https://doi.org/10.1038/sj.bdj.2010.676.
- Northern Ireland Statistics Research Agency . 2011 Census Aggregate Data: UK Data Service 2017. https://doi.org/10.5257/census/aggregate-2011-2 (accessed 26 November 2019).
- National Dental Inspection Programme (NDIP) . Report of the 2016 Detailed National Dental Inspection Programme of Primary 1 Children and the Basic Inspection of Primary 1 and Primary 7 Children 2016.
- Pahel BT, Rozier RG, Slade GD. Parental perceptions of children’s oral health: the Early Childhood Oral Health Impact Scale (ECOHIS). Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-6.
- Taylor GK, Macpherson LM. An investigation into the use of bitewing radiography in children in Greater Glasgow. Br Dent J 2004;196:563-8. https://doi.org/10.1038/sj.bdj.4811228.
- Newman B, Seow WK, Kazoullis S, Ford D, Holcombe T. Clinical detection of caries in the primary dentition with and without bitewing radiography. Aust Dent J 2009;54:23-30. https://doi.org/10.1111/j.1834-7819.2008.01084.x.
- Pocock SJ, Stone GW. The primary outcome fails – what next?. N Engl J Med 2016;375:861-70. https://doi.org/10.1056/NEJMra1510064.
- Hesse D, de Araujo MP, Olegário IC, Innes N, Raggio DP, Bonifácio CC. Atraumatic restorative treatment compared to the Hall Technique for occluso-proximal cavities in primary molars: study protocol for a randomized controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1270-z.
- BaniHani A, Deery C, Toumba J, Duggal M. Effectiveness, costs and patient acceptance of a conventional and a biological treatment approach for carious primary teeth in children. Caries Res 2019;53:65-7. https://doi.org/10.1159/000487201.
- Lord J, Longworth L, Singh J, Onyimadu O, Fricke J, Bayliss S, et al. Economic Analysis of Oral Health Promotion Approaches for Dental Teams: NICE Public Health Guideline on Oral Health Promotion Approaches for Dental Teams (GID-PHG60). London: National Institute for Health and Care Excellence; 2015.
- National Institute for Health and Care Excellence (NICE) . Sedation in Children and Young People 2010.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 8 December 2017).
- NHS Business Services Authority, NHS Dental Services . Pay Statements and Schedules n.d. www.nhsbsa.nhs.uk/activity-processing-and-payment-services/pay-statements-and-schedules (accessed 8 December 2017).
Appendix 1 Recommendations from the pilot phase
-
The structures and processes for trial management deployed in the pilot trial were effective and will be mirrored in the main trial.
-
Practices with previous experience of participating in research will be considered for the FiCTION main trial.
-
Vocational dental practitioners working in FiCTION trial practices will be invited to participate by one of the FiCTION trial dentists in their practice, to participate in the screening of patients for recruitment.
-
The practice training material is being developed to include role-play scenarios and video footage explaining the trial process, including consent, randomisation and participant responsibilities. Clinical protocol documents are being enhanced to explain the treatment planning options and treatment variation between arms more clearly. Tools are being developed to help practice staff explain these to parents and child participants. These will form a part of the study set-up, initiation and training session for the FiCTION main trial.
-
Training of the GDPs and other members of the team for the FiCTION main trial will be carried out as close to the start of patient recruitment as possible. ‘Top-up’ training will be provided for the dentists and other members of the practice team when necessary during the 12-month recruitment phase.
-
Feedback from the service providers emphasised the need for involvement of the whole dental team. Receptionists were viewed as key members of staff to deal with patient queries and administration. Dentists preferred to have other colleagues in their practice involved to share the workload. Approval from the practice principal, as the business owner, was felt to be required. Practice recruitment for the main trial will focus on larger practices and/or those with an interest in research.
-
Clinical leads should be supported by a dedicated secretary to be the main point of contact and to ensure that paperwork is accurately completed and returned from practices to the trial office.
-
The responsibility for printing information packs for patients will rest with the clinical leads’ offices and not the participating practice, although individual practice letterheads will need to be used. Ensuring the appropriate trial documentation and printing will form part of the remit for the clinical leads’ secretaries.
-
Effective communication strategies that were developed and adopted in the pilot trial will be replicated for the main trial.
-
Flexibility will be offered over the process of patient identification and invitation to participate, allowing the approaches to be tailored for individual practices. This will include the option of opportunistic recruitment of children who present to the practice with caries into dentine.
-
The timing of written information will be changed, and a case made to the REC to simplify the information packs and reduce the amount of initial written information sent to patients/parents.
-
Feedback from the GDPs indicated that the approach taken to screening patients for recruitment in the pilot trial was convenient as they did not have to discuss the trial with those who were ineligible, and there was little difference from their regular recall appointment. This approach will be adopted in the main trial.
-
Regularly attending patients will continue to be the main source of patients for screening as they are more likely to re-attend for treatment and future follow-up. However, to keep the patient sample representative, opportunistic identification of patients should continue.
-
Despite differences between the expected and observed rates of eligibility in the pilot trial, the recruitment period in the main trial of 12 months (compared with 6 months in the pilot trial) will not require adjustment. There will be sufficient time for the dentists to invite (234), screen (152) and recruit (18) the required number of children.
-
The trial team is exploring the possibility of using the Community Dental Service to help with increasing the number of patients with decay who are screened and, thus, the rate of eligibility. An options appraisal will be carried out for this method of recruitment.
-
To generate interest and encourage retention, the trial materials will be made more appealing and child-friendly. In addition, newsletters will be sent to the parents and children updating them on the progress of the trial.
-
For the FiCTION main trial, the importance of the explanations and reassurances provided by the dentists to parents and children will be given more prominence and the projected time for this part of the process will be increased.
-
Training on dental charting using the ICDAS will be strengthened, with both dentists and their dental nurses trained in the completion of the dental chart correctly. The supplementary training resources [an annotated Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation and laminated summary sheet for the dental surgery, identifying how the dental charts should be completed] will be adopted as part of the FiCTION main trial training.
-
Because caries incidence in the primary and permanent dentition is one of the secondary outcome measures for the FiCTION main trial, consideration will be given to utilising the clinical researcher to complete dental chartings on a random sample of patients at the end of the trial period if the quality of baseline dental charts is poor despite improved training.
-
Given the generally small numbers of missing data, the CRF tool appears to be a feasible and acceptable method of collecting economic data relating to resource use and it will be used in the main trial. However, some refinement is required to allow accurate detail on the treatments and any reasons for diverging from the clinical protocol.
-
The instructions and format of the child questionnaire will be amended. In addition, the child measure of OHRQoL will be removed to simplify the questionnaire. In the absence of a more appropriate child measure, data from parents will be used as a proxy, and more child-centred approaches will be incorporated to explore the impact of dental caries and its treatment on the young children participating.
-
Changes to the parental questionnaire will include clarifying the wording of the instructions and re-ordering questions. The measure of parental OHRQoL will be changed to the short-form P-CPQ. The section on dental discomfort will remain the same.
-
As well as shortening the child questionnaires by removing the QoL elements, the burden on both the dental staff in administering and the participants in completing questionnaires will be reduced by decreasing the frequency of measuring OHRQoL. It is proposed that this measure be recorded at baseline and annually, rather than at each appointment.
-
It is proposed that improvements could be made in the explanation of the three treatment strategies by including reassurance that have all been shown to be effective; this should be conveyed through face-to-face discussions with dentists.
-
Dentists who have expressed an interest in participating in the main trial will be considered for recruitment.
-
The processes for the reimbursement of expenses for the pilot trial dentists were acceptable and will be followed in the main trial.
Appendix 2 List of trial protocol amendments
| Amendment number | Protocol version number | Date of REC favourable opinion | Details of changes made |
|---|---|---|---|
| AM06 | 2.0 | 6 November 2014 | Updated to allow appropriate time point questionnaires, accompanied by a covering letter and prepaid return envelope, to be sent out to participants who have failed to attend a scheduled or final appointment |
| AM12 | 3.0 | 8 May 2015 | Updated to reflect new study sites. The total number UK sites was increased from 50 to 70. The distribution of sites per region was also changed:
|
|
|||
|
|||
|
|||
| Section 4.1 primary outcomes and section 4.2 secondary outcomes sections of the trial protocol: updated with more detail regarding the statistical analysis and study-specific estimates of unit costs added as a secondary outcome measure to Section 4.2 | |||
| Development and distribution of promotional material to improve participant retention: the protocol was updated to state that FiCTION trial promotional material (posters and leaflets) would be distributed to sites for display in practice waiting rooms. Birthday cards were also sent to participating children via the practice. Feedback would be actively sought from practices in order to guide development of suitable FiCTION trial-branded merchandise promoting the study | |||
Minor administrative changes:
|
|||
| AM14 | 4.0 | 25 November 2015 | Updated to give patients the opportunity to be contacted with regard to future studies |
| AM15 | 5.0 | 7 June 2016 | Updated to further detail the qualitative aspects of the study. Change of senior trial manager |
| AM16 | 6.0 | 13 June 2017 |
Section 3.2 updated under incidence of caries in primary and secondary teeth, adding paragraph about radiographs Sections 4.1 and 4.2: re-defined primary outcome to co-primary outcomes and expanded statistical analysis methods |
Appendix 3 Expected adverse events (see Chapter 2)
| Procedure | Adverse event | ||
|---|---|---|---|
| Common and well-understood consequences of treatment | Less common and unpleasant side effects | Rare events | |
| Fillings in teeth and crowns on teeth (C+P) |
|
|
Trauma to soft tissues |
| Crowns on teeth (Hall Technique) |
|
|
Localised reaction to crowns |
| Inhalational sedation | Dizziness and nausea | Under- or over-sedation | |
| Local anaesthetic | Pain at site of injection (during or immediately following injection) | Self-inflicted trauma to soft tissues |
|
| Extraction of tooth |
|
|
Temporomandibular joint pain |
| Fluoride varnish |
|
||
| Fissure sealants | Caries progression | ||
| Acid etch on teeth prior to restoration or fissure sealant | Discomfort and minor irritation of oral tissues | ||
Appendix 4 Trial results and clinical effectiveness (see Chapter 3)
Recruitment and baseline characteristics
| Centre | Practice code | Number of children randomised | Children as % of clinical centre’s total participants | Total number of children per clinical centre (% of the 1144 randomised) |
|---|---|---|---|---|
| Scotland | 1 03 | 23 | 7.5 | 307 (26.84) |
| 1 04 | 37 | 12.1 | ||
| 1 06 | 18 | 5.9 | ||
| 1 07 | 18 | 5.9 | ||
| 1 09 | 9 | 2.9 | ||
| 1 11 | 2 | 0.7 | ||
| 1 13 | 15 | 4.9 | ||
| 1 14 | 17 | 5.5 | ||
| 1 15 | 2 | 0.7 | ||
| 1 16 | 4 | 1.3 | ||
| 1 17 | 31 | 10.1 | ||
| 1 18 | 9 | 2.9 | ||
| 1 19 | 6 | 2.0 | ||
| 1 20 | 1 | 0.3 | ||
| 1 21 | 17 | 5.5 | ||
| 1 22 | 9 | 2.9 | ||
| 1 23 | 24 | 7.8 | ||
| 1 24 | 1 | 0.3 | ||
| 1 27 | 1 | 0.3 | ||
| 1 30 | 2 | 0.7 | ||
| 1 31 | 21 | 6.8 | ||
| 1 32 | 2 | 0.7 | ||
| 1 36 | 9 | 2.9 | ||
| 1 37 | 19 | 6.2 | ||
| 1 38 | 10 | 3.3 | ||
| North-east | 2 01 | 4 | 1.3 | 315 (27.53) |
| 2 02 | 38 | 12.1 | ||
| 2 03 | 31 | 9.8 | ||
| 2 04 | 9 | 2.9 | ||
| 2 06 | 26 | 8.3 | ||
| 2 07 | 2 | 0.6 | ||
| 2 08 | 6 | 1.9 | ||
| 2 09 | 7 | 2.2 | ||
| 2 11 | 22 | 7.0 | ||
| 2 12 | 21 | 6.7 | ||
| 2 13 | 33 | 10.5 | ||
| 2 14 | 15 | 4.8 | ||
| 2 15 | 14 | 4.4 | ||
| 2 16 | 27 | 8.6 | ||
| 2 17 | 17 | 5.4 | ||
| 2 18 | 5 | 1.6 | ||
| 2 19 | 16 | 5.1 | ||
| 2 20 | 8 | 2.5 | ||
| 2 22 | 14 | 4.4 | ||
| Yorkshire | 3 01 | 20 | 11.7 | 171 (14.95) |
| 3 03 | 4 | 2.3 | ||
| 3 06 | 21 | 12.3 | ||
| 3 07 | 2 | 1.2 | ||
| 3 08 | 2 | 1.2 | ||
| 3 11 | 5 | 2.9 | ||
| 3 12 | 24 | 14.0 | ||
| 3 13 | 32 | 18.7 | ||
| 3 16 | 21 | 12.3 | ||
| 3 17 | 3 | 1.8 | ||
| 3 18 | 22 | 12.9 | ||
| 3 21 | 4 | 2.3 | ||
| 3 22 | 11 | 6.4 | ||
| Wales | 4 01 | 13 | 10.4 | 125 (10.93) |
| 4 03 | 55 | 44.0 | ||
| 4 04 | 29 | 23.2 | ||
| 4 05 | 28 | 22.4 | ||
| London | 5 02 | 32 | 14.2 | 226 (19.76) |
| 5 03 | 33 | 14.6 | ||
| 5 06 | 7 | 3.1 | ||
| 5 09 | 39 | 17.3 | ||
| 5 10 | 35 | 15.5 | ||
| 5 11 | 13 | 5.8 | ||
| 5 14 | 5 | 2.2 | ||
| 5 15 | 40 | 17.7 | ||
| 5 16 | 13 | 5.8 | ||
| 5 18 | 3 | 1.3 | ||
| 5 24 | 6 | 2.7 |
| Time in study (months) | Treatment arm | Total (n = 118) | ||
|---|---|---|---|---|
| C+P (n = 40) | B+P (n = 35) | PA (n = 43) | ||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (lower quartile, upper quartile) | 9.8 (4.1, 14.9) | 11.1 (1.0, 12.3) | 8.6 (1.4, 18.0) | 9.4 (1.4, 14.7) |
| Maximum | 33.8 | 42.0 | 32.5 | 42.0 |
| Primary dentition (maximum: 20 teeth) | Trial group | All (n = 1006) | ||
|---|---|---|---|---|
| C+P (n = 339) | B+P (n = 333) | PA (n = 334) | ||
| Total number of primary teeth per child | ||||
| Minimum | 4.0 | 5.0 | 4.0 | 4.0 |
| Median (IQR) | 19.0 (14.0–20.0) | 19.0 (15.0–20.0) | 20.0 (14.0–20.0) | 19.0 (15.0–20.0) |
| Mean (SD) | 17.23 (3.40) | 17.41 (3.35) | 17.30 (3.39) | 17.31 (3.38) |
| Maximum | 20.0 | 20.0 | 20.0 | 20.0 |
| Number of caries-free primary teeth (ICDAS summary code 00) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 14.0 (9.0–17.0) | 14.0 (10.0–17.0) | 13.0 (10.0–16.0) | 14.0 (10.0–17.0) |
| Mean (SD) | 12.87 (4.63) | 13.22 (4.48) | 13.00 (4.34) | 13.03 (4.49) |
| Maximum | 20.0 | 20.0 | 20.0 | 20.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 0–2 | ||||
| d ICDAS 0–2 | ||||
| Minimum | 1.0 | 1.0 | 0.0 | 0.0 |
| Median (IQR) | 15.0 (11.0–18.0) | 16.0 (12.0–18.0) | 16.0 (12.0–18.0) | 16.0 (12.0–18.0) |
| Mean (SD) | 14.53 (4.06) | 14.83 (3.96) | 14.75 (4.06) | 14.70 (4.03) |
| Maximum | 20.0 | 20.0 | 20.0 | 20.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 3–4 | ||||
| d ICDAS 3–4 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 1.0 (0.0–2.0) | 0.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| Mean (SD) | 1.04 (1.45) | 1.01 (1.43) | 1.03 (1.29) | 1.03 (1.39) |
| Maximum | 9.0 | 8.0 | 6.0 | 9.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 5–6 | ||||
| d ICDAS 5–6 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| Mean (SD) | 1.65 (2.02) | 1.57 (1.83) | 1.48 (1.72) | 1.57 (1.86) |
| Maximum | 14.0 | 12.0 | 8.0 | 14.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 4–6 | ||||
| d ICDAS 4–6 [equivalent to d3] | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 1.0 (1.0–3.0) | 1.0 (1.0–3.0) | 1.0 (0.0–3.0) | 1.0 (1.0–3.0) |
| Mean (SD) | 2.20 (2.40) | 2.00 (2.02) | 1.91 (2.00) | 2.04 (2.15) |
| Maximum | 14.0 | 12.0 | 11.0 | 14.0 |
| Missing primary teeth due to caries (ICDAS summary code 97) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.22 (0.86) | 0.31 (1.25) | 0.21 (1.25) | 0.24 (1.13) |
| Maximum | 7.0 | 12.0 | 16.0 | 16.0 |
| Filled (but not decayed) primary teeth (number of teeth with at least one surface with ICDAS restoration code 3–7 and caries severity code 0–3) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.37 (0.89) | 0.44 (0.98) | 0.49 (1.12) | 0.44 (1.00) |
| Maximum | 7.0 | 5.0 | 6.0 | 7.0 |
| d3mft | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) |
| Mean (SD) | 2.79 (2.69) | 2.75 (2.71) | 2.61 (2.57) | 2.72 (2.66) |
| Maximum | 14.0 | 19.0 | 19.0 | 19.0 |
| Permanent dentition: first permanent molars – teeth 16/26/36/46 | Trial group | All (n = 1006) | ||
|---|---|---|---|---|
| C+P (n = 339) | B+P (n = 333) | PA (n = 334) | ||
| Total number of first permanent molars per child | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–4.0) | 1.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) |
| Mean (SD) | 1.68 (1.89) | 1.82 (1.87) | 1.72 (1.89) | 1.74 (1.88) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of caries-free first permanent molars (ICDAS summary code 00) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–4.0) | 1.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) |
| Mean (SD) | 1.39 (1.74) | 1.62 (1.77) | 1.49 (1.78) | 1.50 (1.76) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 0–2 | ||||
| D ICDAS 0–2 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–4.0) | 1.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) |
| Mean (SD) | 1.63 (1.85) | 1.78 (1.84) | 1.63 (1.85) | 1.68 (1.84) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 3–4 | ||||
| D ICDAS 3–4 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.05 (0.30) | 0.03 (0.16) | 0.06 (0.37) | 0.04 (0.29) |
| Maximum | 4.0 | 1.0 | 4.0 | 4.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 5–6 | ||||
| D ICDAS 5–6 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.01 (0.09) | 0.01 (0.11) | 0.02 (0.22) | 0.01 (0.15) |
| Maximum | 1.0 | 1.0 | 3.0 | 3.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 4–6 | ||||
| D ICDAS 4–6 (equivalent to D3) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.02 (0.13) | 0.02 (0.13) | 0.06 (0.35) | 0.03 (0.23) |
| Maximum | 1.0 | 1.0 | 4.0 | 4.0 |
| Missing first permanent molars due to caries (ICDAS summary code 97) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.000 (0.000) | 0.003 (0.055) | 0.000 (0.000) | 0.001 (0.032) |
| Maximum | 0.0 | 1.0 | 0.0 | 1.0 |
| Filled (but not decayed) first permanent molars (number with at least one surface with ICDAS restoration code 3–7 and caries severity code 0–3) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.01 (0.18) | 0.03 (0.24) | 0.02 (0.18) | 0.02 (0.20) |
| Maximum | 3.0 | 3.0 | 2.0 | 3.0 |
| D3MFT | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.03 (0.22) | 0.05 (0.36) | 0.08 (0.45) | 0.05 (0.35) |
| Maximum | 3.0 | 4.0 | 4.0 | 4.0 |
Primary outcome-related analyses
| Reason for ‘major’ deviation | Trial arm, n (% of non-missing) | Total (n = 429), n (% of non-missing) | ||
|---|---|---|---|---|
| C+P (n = 195) | B+P (n = 65) | PA (n = 169) | ||
| Total (non-missing), n | 188 | 65 | 164 | 417 |
| Parent factors | 33 (17.6) | 29 (44.6) | 55 (33.5) | 117 (28.1) |
| Child pre co-operative for local anaesthetic | 82 (43.6) | 3 (4.6) | 1 (0.6) | 86 (20.6) |
| Dentist’s clinical judgement | 23 (12.2) | 19 (29.2) | 78 (47.6) | 120 (28.8) |
| Child anxiety | 41 (21.8) | 6 (9.2) | 0 (0.0) | 47 (11.3) |
| Food packing (PA arm only) | 0 (0.0) | 1 (1.5) | 16 (9.8) | 17 (4.1) |
| Child factors (not anxiety/co-operation) | 5 (2.7) | 5 (7.7) | 6 (3.7) | 16 (3.8) |
| Other | 4 (2.1) | 2 (3.1) | 8 (4.9) | 14 (3.4) |
| Arm randomised to | Arm(s) treatment deviated toa | Number (%) of ‘major’ deviations by arm (n = 429) | Randomised arm deviated from – group total, n (%) |
|---|---|---|---|
| C+P | B+P | 135 (69.2) | 195 (45.5) |
| PAb | 52 (26.7) | ||
| B+P and PAc | 3 (1.5) | ||
| C+P and B+Pc | 3 (1.5) | ||
| C+P and PAc | 2 (1.0) | ||
| B+P | C+P | 52 (80.0) | 65 (15.2) |
| PAc | 10 (15.4) | ||
| C+P and B+Pc | 1 (1.5) | ||
| C+P, B+P and PAb,c | 1 (1.5) | ||
| C+P and PAb,c | 1 (1.5) | ||
| PA | C+P | 90 (53.3) | 169 (39.4) |
| B+P | 71 (42.0) | ||
| B+P and PAb,c | 4 (2.4) | ||
| C+P and PAc | 3 (1.8) | ||
| C+P and B+Pc | 1 (0.6) |
| Outcome | Trial arm, n (%) | Total (n = 797), n (%) | ||
|---|---|---|---|---|
| C+P (n = 269) | B+P (n = 267) | PA (n = 261) | ||
| Dental pain ever | 102 (37.9) | 97 (36.3) | 116 (44.4) | 315 (39.5) |
| Dental sepsis ever | 73 (27.1) | 74 (27.7) | 76 (29.1) | 223 (28.0) |
| Dental pain and/or dental sepsis ever | 121 (45.0) | 122 (45.7) | 130 (49.8) | 374 (46.9)a |
| Outcome | Trial arm, n (%) | Total (n = 940), n (%) | ||
|---|---|---|---|---|
| C+P (n = 311) | B+P (n = 329) | PA (n = 300) | ||
| Dental pain ever | 106 (34.1) | 103 (31.3) | 109 (36.3) | 318 (33.8) |
| Dental sepsis ever | 77 (24.8) | 78 (23.7) | 76 (25.3) | 231 (24.6) |
| Dental pain and/or dental sepsis ever | 124 (39.9) | 127 (38.6) | 126 (42.0) | 377 (40.1) |
| Analysis set | Model | Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|---|---|
| ITT | Unadjusted (n = 1058) | C+P | 0.00 | ||
| B+P | –0.02 | –0.10 to 0.06 | 0.6 | ||
| PA | 0.04 | –0.05 to 0.12 | 0.3 | ||
| Adjusted (n = 1057) | C+P | 0.00 | |||
| B+P | –0.02 | –0.10 to 0.06 | 0.6 | ||
| PA | 0.04 | –0.04 to 0.12 | 0.2 | ||
| ITT, at least 23 months in study | Unadjusted (n = 797) | C+P | 0.00 | ||
| B+P | 0.005 | –0.09 to 0.10 | 0.9 | ||
| PA | 0.05 | –0.04 to 0.14 | 0.2 | ||
| Adjusted (n = 797) | C+P | 0.00 | |||
| B+P | 0.01 | –0.09 to 0.10 | 0.9 | ||
| PA | 0.05 | –0.04 to 0.14 | 0.2 | ||
| PP | Unadjusted (n = 940) | C+P | 0.00 | ||
| B+P | –0.01 | –0.10 to 0.07 | 0.7 | ||
| PA | 0.02 | –0.07 to 0.10 | 0.7 | ||
| Adjusted (n = 939) | C+P | 0.00 | |||
| B+P | –0.01 | –0.09 to 0.08 | 0.9 | ||
| PA | 0.02 | –0.06 to 0.11 | 0.5 |
| Outcome | Trial arm | Total (n = 797) | ||
|---|---|---|---|---|
| C+P (n = 269) | B+P (n = 267) | PA (n = 261) | ||
| Number of episodes of dental pain and/or dental sepsis | ||||
| Minimum | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 1 (0–1) | 0 (0–1) |
| Mean (SD) | 0.66 (0.97) | 0.67 (0.92) | 0.84 (1.06) | 0.72 (0.99) |
| Maximum | 7 | 6 | 5 | 7 |
| Number, n (%) | ||||
| 0 | 148 (55.0) | 145 (54.3) | 130 (49.8) | 423 (53.1) |
| 1 | 88 (32.7) | 85 (31.8) | 74 (28.4) | 247 (31.0) |
| 2 | 18 (6.7) | 22 (8.2) | 36 (13.8) | 76 (9.5) |
| 3 | 12 (4.5) | 13 (4.9) | 14 (5.4) | 39 (4.9) |
| 4 | 1 (0.4) | 1 (0.4) | 5 (1.9) | 7 (0.9) |
| 5 | 0 (0.0) | 0 (0.0) | 2 (0.8) | 2 (0.3) |
| 6 | 1 (0.4) | 1 (0.4) | 0 (0.0) | 2 (0.3) |
| 7 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Outcome | Trial arm | Total (n = 940) | ||
|---|---|---|---|---|
| C+P (n = 311) | B+P (n = 329) | PA (n = 300) | ||
| Number of episodes of dental pain and/or dental sepsis | ||||
| Minimum | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Mean (SD) | 0.57 (0.89) | 0.57 (0.87) | 0.66 (0.94) | 0.59 (0.90) |
| Maximum | 7 | 6 | 5 | 7 |
| Number, n (%) | ||||
| 0 | 187 (60.1) | 202 (61.4) | 174 (58.0) | 563 (59.9) |
| 1 | 92 (29.6) | 87 (26.4) | 76 (25.3) | 255 (27.1) |
| 2 | 18 (5.8) | 25 (7.6) | 34 (11.3) | 77 (8.2) |
| 3 | 11 (3.5) | 13 (4.0) | 12 (4.0) | 36 (3.8) |
| 4 | 2 (0.6) | 1 (0.3) | 3 (1.0) | 6 (0.6) |
| 5 | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| 6 | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| 7 | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Analysis set | Model | Trial arm | IRR | 97.5% CI | p-value |
|---|---|---|---|---|---|
| ITT | Unadjusted (n = 1058) | C+P | 1.00 | ||
| B+P | 0.94 | 0.74 to 1.21 | 0.6 | ||
| PA | 1.17 | 0.93 to 1.48 | 0.1 | ||
| Adjusted (n = 1057) | C+P | 1.00 | |||
| B+P | 0.95 | 0.75 to 1.21 | 0.6 | ||
| PA | 1.18 | 0.94 to 1.48 | 0.1 | ||
| ITT, at least 23 months in study | Unadjusted (n = 797) | C+P | 1.00 | ||
| B+P | 1.02 | 0.78 to 1.32 | 0.9 | ||
| PA | 1.27 | 0.98 to 1.64 | 0.04 | ||
| Adjusted (n = 797) | C+P | 1.00 | |||
| B+P | 1.02 | 0.78 to 1.33 | 0.9 | ||
| PA | 1.26 | 0.98 to 1.63 | 0.04 | ||
| PP | Unadjusted (n = 940) | C+P | 1.00 | ||
| B+P | 1.01 | 0.78 to 1.32 | > 0.9 | ||
| PA | 1.15 | 0.89 to 1.50 | 0.2 | ||
| Adjusted (n = 939) | C+P | 1.00 | |||
| B+P | 1.03 | 0.80 to 1.34 | 0.8 | ||
| PA | 1.17 | 0.90 to 1.51 | 0.2 |
| Trial arm | Interval in years | Number at risk | Number of episodes of first dental pain and/or dental sepsis | Censored (last CRF, no episode), n | Probability of remaining pain/sepsis free beyond the end of the interval | 97.5% CI |
|---|---|---|---|---|---|---|
| B+P | 0 ≤ t < 1 | 352 | 70 | 41 | 0.79 | 0.74 to 0.83 |
| 1 ≤ t < 2 | 241 | 40 | 26 | 0.65 | 0.59 to 0.70 | |
| 2 ≤ t < 3 | 175 | 25 | 86 | 0.53 | 0.46 to 0.59 | |
| 3 ≤ t < 4 | 64 | 6 | 58 | 0.44 | 0.35 to 0.52 | |
| C+P | 0 ≤ t < 1 | 352 | 79 | 30 | 0.77 | 0.72 to 0.81 |
| 1 ≤ t < 2 | 243 | 38 | 27 | 0.64 | 0.58 to 0.69 | |
| 2 ≤ t < 3 | 178 | 26 | 92 | 0.51 | 0.45 to 0.57 | |
| 3 ≤ t < 4 | 60 | 5 | 54 | 0.44 | 0.35 to 0.51 | |
| 4 ≤ t < 5 | 1 | 0 | 1 | 0.44 | 0.35 to 0.51 | |
| PA | 0 ≤ t < 1 | 354 | 95 | 40 | 0.72 | 0.66 to 0.76 |
| 1 ≤ t < 2 | 219 | 45 | 24 | 0.56 | 0.50 to 0.61 | |
| 2 ≤ t < 3 | 150 | 18 | 77 | 0.47 | 0.41 to 0.53 | |
| 3 ≤ t < 4 | 55 | 3 | 52 | 0.42 | 0.35 to 0.49 |
| Dental pain and/or dental sepsis free survival (years) | Trial arm | Total (n = 1058) | ||
|---|---|---|---|---|
| C+P (n = 352) | B+P (n = 352) | PA (n = 354) | ||
| n | 352 | 352 | 354 | 1058 |
| Median (97.5% CI) | 3.1 (2.8 to a) | 3.6 (2.7 to a) | 2.8 (1.8 to a) | 3.1 (2.8 to 3.6) |
| Lower quartile (97.5% CI) | 1.1 (0.7 to 1.5) | 1.3 (0.9 to 1.6) | 0.9 (0.6 to 1.1) | 1.0 (0.9 to 1.2) |
| Upper quartile (97.5% CI) | a | 3.7 (3.7 to a) | 3.6 (3.6 to a) | a (3.7 to a) |
| Variable | n | Risk ratio | 97.5% CI | p-value |
|---|---|---|---|---|
| Age (years) | 1057 | 0.99 | 0.92 to 1.06 | 0.6 |
| Ethnicity (white) | 955 | 1.08 | 0.85 to 1.37 | 0.6 |
| Water fluoridation (p.p.m.)a | 1058 | 0.75 | 0.49 to 1.15 | 0.4 |
| Index of deprivation (deciles)a | 1058 | 1.03 | 0.98 to 1.07 | 0.3 |
| Number of decayed teeth at baseline from ICDAS charting (level 5/6 cavitation) | 1006 | 1.12 | 1.09 to 1.16 | < 0.001 |
| Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|
| C+P | 0.00 | ||
| B+P | –0.0006 | –0.08 to 0.08 | > 0.9 |
| PA | 0.07 | –0.01 to 0.16 | 0.06 |
Secondary outcomes: part 1 – caries progression
| Primary dentition (maximum: 20 teeth) | Trial arm | All (n = 653) | ||
|---|---|---|---|---|
| C+P (n = 228) | B+P (n = 216) | PA (n = 209) | ||
| Total number of primary teeth per child | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 11.0 (6.0–12.0) | 10.5 (8.0–12.0) | 10.0 (6.0–12.0) | 11.0 (7.0–12.0) |
| Mean (SD) | 9.60 (4.51) | 10.15 (4.86) | 9.36 (4.76) | 9.71 (4.71) |
| Maximum | 20.0 | 20.0 | 20.0 | 20.0 |
| Number of caries-free primary teeth (ICDAS summary code 00) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 7.0 (1.5–11.0) | 7.0 (2.0–10.5) | 7.0 (2.0–10.0) | 7.0 (2.0–10.0) |
| Mean (SD) | 6.48 (4.96) | 6.98 (5.38) | 6.39 (4.69) | 6.62 (5.02) |
| Maximum | 18.0 | 20.0 | 19.0 | 20.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 0–2 | ||||
| d ICDAS 0–2 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 9.0 (3.0–12.0) | 9.0 (4.0–11.0) | 8.0 (4.0–11.0) | 9.0 (4.0–11.0) |
| Mean (SD) | 7.69 (5.16) | 8.12 (5.33) | 7.47 (4.91) | 7.76 (5.14) |
| Maximum | 20.0 | 20.0 | 19.0 | 20.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 3–4 | ||||
| d ICDAS 3–4 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Mean (SD) | 0.49 (1.00) | 0.54 (1.08) | 0.55 (1.08) | 0.52 (1.05) |
| Maximum | 6.0 | 9.0 | 8.0 | 9.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 5–6 | ||||
| d ICDAS 5–6 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Mean (SD) | 0.61 (1.36) | 0.67 (1.40) | 0.87 (1.70) | 0.71 (1.49) |
| Maximum | 10.0 | 9.0 | 9.0 | 10.0 |
| Number of primary teeth for which the highest surface caries severity code is ICDAS 4–6 | ||||
| d ICDAS 4–6 [equivalent to d3] | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Mean (SD) | 0.79 (1.52) | 0.90 (1.72) | 1.06 (1.89) | 0.91 (1.71) |
| Maximum | 10.0 | 9.0 | 9.0 | 10.0 |
| Missing primary teeth due to caries (ICDAS summary code 97) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.28 (1.07) | 0.19 (0.84) | 0.28 (1.26) | 0.25 (1.07) |
| Maximum | 8.0 | 8.0 | 10.0 | 10.0 |
| Filled (but not decayed) primary teeth (number of teeth with at least one surface with ICDAS restoration code of 3–7 and caries severity code of 0–3) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| Mean (SD) | 1.12 (1.15) | 1.19 (1.14) | 1.20 (1.19) | 1.17 (1.16) |
| Maximum | 5.0 | 5.0 | 5.0 | 5.0 |
| d3mft | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (0.0–4.0) | 2.0 (1.0–3.0) |
| Mean (SD) | 2.19 (2.18) | 2.28 (2.21) | 2.54 (2.70) | 2.33 (2.37) |
| Maximum | 11.0 | 12.0 | 16.0 | 16.0 |
| Permanent dentition: first permanent molars – teeth 16/26/36/46 | Trial arm | All (n = 653) | ||
|---|---|---|---|---|
| C+P (n = 228) | B+P (n = 216) | PA (n = 209) | ||
| Total number of first permanent molars per child | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) |
| Mean (SD) | 3.74 (0.87) | 3.63 (1.00) | 3.73 (0.95) | 3.70 (0.94) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of caries-free first permanent molars (ICDAS summary code 00) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 4.0 (2.0–4.0) | 4.0 (2.0–4.0) | 4.0 (2.0–4.0) | 4.0 (2.0–4.0) |
| Mean (SD) | 3.05 (1.41) | 3.05 (1.36) | 3.08 (1.37) | 3.06 (1.38) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 0–2 | ||||
| D ICDAS 0–2 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 4.0 (4.0–4.0) | 4.0 (3.0–4.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) |
| Mean (SD) | 3.65 (0.92) | 3.50 (1.03) | 3.63 (0.99) | 3.59 (0.98) |
| Maximum | 4.0 | 4.0 | 4.0 | 4.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 3–4 | ||||
| D ICDAS 3–4 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.08 (0.33) | 0.09 (0.32) | 0.03 (0.21) | 0.07 (0.30) |
| Maximum | 3.0 | 2.0 | 2.0 | 3.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 5–6 | ||||
| D ICDAS 5–6 | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.01 (0.11) | 0.04 (0.21) | 0.07 (0.30) | 0.04 (0.22) |
| Maximum | 1.0 | 2.0 | 2.0 | 2.0 |
| Number of first permanent molars for which the highest surface caries severity code is ICDAS 4–6 | ||||
| D ICDAS 4–6 [equivalent to D3] | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.02 (0.13) | 0.06 (0.30) | 0.07 (0.30) | 0.05 (0.26) |
| Maximum | 1.0 | 2.0 | 2.0 | 2.0 |
| Missing first permanent molars due to caries (ICDAS summary code 97) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.004 (0.066) | 0.000 (0.000) | 0.000 (0.000) | 0.002 (0.039) |
| Maximum | 1.0 | 0.0 | 0.0 | 1.0 |
| Filled (but not decayed) first permanent molars (number with at least one surface with ICDAS restoration code of 3–7 and caries severity code of 0–3) | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.12 (0.47) | 0.13 (0.50) | 0.12 (0.50) | 0.13 (0.49) |
| Maximum | 4.0 | 4.0 | 3.0 | 4.0 |
| D3MFT | ||||
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Mean (SD) | 0.14 (0.52) | 0.20 (0.64) | 0.19 (0.60) | 0.18 (0.59) |
| Maximum | 4.0 | 4.0 | 3.0 | 4.0 |
| Participant characteristic | Non-missing data points, n | C+P (n = 228) | Non-missing data points, n | B+P (n = 216) | Non-missing data points, n | PA (n = 209) | Non-missing data points, n | Total (n = 653) |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 228 | 215 | 209 | 652 | ||||
| Mean (SD) | 6.0 (1.3) | 6.0 (1.3) | 6.0 (1.2) | 6.0 (1.3) | ||||
| Sex | 228 | 215 | 209 | 652 | ||||
| Female, n (%) | 124 (54.4) | 105 (48.8) | 106 (50.7) | 335 (51.4) | ||||
| Ethnicity | 208 | 203 | 192 | 603 | ||||
| White, n (%) | 153 (73.6) | 150 (73.9) | 147 (76.6) | 450 (74.6) | ||||
| Black, n (%) | 6 (2.9) | 8 (3.9) | 6 (3.1) | 20 (3.3) | ||||
| Indian, Pakistani or Bangladeshi, n (%) | 27 (13.0) | 28 (13.8) | 20 (10.4) | 75 (12.4) | ||||
| Chinese, n (%) | 4 (1.9) | 3 (1.5) | 1 (0.5) | 8 (1.3) | ||||
| Mixed race, n (%) | 7 (3.4) | 9 (4.4) | 8 (4.2) | 24 (4.0) | ||||
| Other, n (%) | 11 (5.3) | 5 (2.5) | 10 (5.2) | 26 (4.3) | ||||
| Number of decayed teeth at baseline from ICDAS charting (level 5/6 cavitation) | 228 | 216 | 209 | 653 | ||||
| Minimum | 0 | 0 | 0 | 0 | ||||
| Median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | ||||
| Mean (SD) | 1.6 (2.1) | 1.6 (1.9) | 1.5 (1.8) | 1.6 (1.9) | ||||
| Maximum | 14 | 12 | 8 | 14 |
| Outcome | Trial arm | Total (n = 653) | ||
|---|---|---|---|---|
| C+P (n = 228) | B+P (n = 216) | PA (n = 209) | ||
| Primary dentition | ||||
| Progression, n (%) | ||||
| Yes | 132 (57.9) | 133 (61.6) | 134 (64.1) | 399 (61.1) |
| Number of progressed primary teeth that were sound or had reversible caries at baseline | ||||
| Minimum | 0 | 0 | 0 | 0 |
| Median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Mean (SD) | 1.2 (1.3) | 1.2 (1.3) | 1.4 (1.4) | 1.3 (1.4) |
| Maximum | 6 | 6 | 6 | 6 |
| Number, n (%) | ||||
| 0 | 96 (42.1) | 83 (38.4) | 75 (35.9) | 254 (38.9) |
| 1 | 56 (24.6) | 59 (27.3) | 52 (24.9) | 167 (25.6) |
| 2 | 36 (15.8) | 38 (17.6) | 36 (17.2) | 110 (16.8) |
| 3 | 25 (11.0) | 21 (9.7) | 24 (11.5) | 70 (10.7) |
| 4 | 10 (4.4) | 10 (4.6) | 17 (8.1) | 37 (5.7) |
| 5 | 3 (1.3) | 3 (1.4) | 2 (1.0) | 8 (1.2) |
| 6 | 2 (0.9) | 2 (0.9) | 3 (1.4) | 7 (1.1) |
| First permanent molars | ||||
| Progression, n (%) | ||||
| Yes | 21 (9.2) | 26 (12.0) | 24 (11.5) | 71 (10.9) |
| Number of progressed first permanent molars | ||||
| Minimum | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Mean (SD) | 0.1 (0.5) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) |
| Maximum | 4 | 4 | 3 | 4 |
| Number, n (%) | ||||
| 0 | 207 (90.8) | 190 (88.0) | 185 (88.5) | 582 (89.1) |
| 1 | 14 (6.1) | 17 (7.9) | 13 (6.2) | 44 (6.7) |
| 2 | 5 (2.2) | 3 (1.4) | 6 (2.9) | 14 (2.1) |
| 3 | 0 (0.0) | 4 (1.9) | 5 (2.4) | 9 (1.4) |
| 4 | 2 (0.9) | 2 (0.9) | 0 (0.0) | 4 (0.6) |
Secondary outcomes: part 2 (child- and parent-reported outcomes) – oral health-related quality of life
| ITT analysis set participants (n = 1058) | CRFs (N) | Parent questionnaires (N) | Both baseline and final parent questionnaires (n) | Non-missing P-CPQ-16 score after imputation,a n/N (%) | Both baseline and final P-CPQ-16 score, n/N (%) |
|---|---|---|---|---|---|
| Baseline | 1058 | 984 | 627 | 923/1058 (87.2) | 560/1058 (52.9) |
| Final | 711 (final visit attended) | 664 | 630b/711 (88.6) |
| Variable | Mean difference | 97.5% CI | p-value |
|---|---|---|---|
| Baseline P-CPQ-16 score | 0.39 | 0.30 to 0.48 | < 0.001 |
| Arm (compared with C+P arm) | |||
| B+P | 0.27b | –1.08 to 1.62 | 0.7 |
| PA | 0.17b | –1.20 to 1.53 | 0.8 |
| Age (years) at randomisation | –0.08 | –0.52 to 0.36 | 0.7 |
| Time in trial (years) | 0.10 | –1.42 to 1.62 | 0.9 |
| Questionnaire | C+P | B+P | PA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-CPQ-16a domain at baseline | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range |
| Oral | 200 | 2.9 (2.4) | 2 (1–4) | 0–12 | 199 | 2.8 (2.3) | 2 (1–4) | 0–10 | 192 | 2.8 (2.4) | 2 (1–4) | 0–12 |
| Functional | 201 | 2.6 (2.5) | 2 (0–4) | 0–12 | 196 | 2.4 (2.5) | 2 (0–4) | 0–13 | 191 | 2.3 (2.3) | 2 (0–4) | 0–12 |
| Emotional | 201 | 2.2 (2.1) | 2 (0–3) | 0–10 | 198 | 2.0 (2.0) | 2 (0–3) | 0–12 | 185 | 2.1 (2.1) | 2 (0–3) | 0–9.3b |
| Social | 195 | 1.0 (1.9) | 0 (0–1.3b) | 0–12 | 196 | 1.0 (1.9) | 0 (0–1) | 0–12 | 184 | 1.1 (2.0) | 0 (0–1.3b) | 0–10.7b |
| Overall P-CPQ-16 score | 189 | 8.7 (6.5) | 7 (4–12) | 0–38 | 189 | 7.9 (6.2) | 7 (3–11) | 0–44 | 182 | 8.2 (6.4) | 7 (4–11) | 0–34 |
| Questionnaire | C+P | B+P | PA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-CPQ-16a domain at final visit | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range |
| Oral | 189 | 2.8 (2.4) | 2 (1–4) | 0–13 | 189 | 2.5 (2.3) | 2 (1–4) | 0–12 | 182 | 2.6 (2.5) | 2 (1–3) | 0–13.3b |
| Functional | 189 | 2.1 (2.5) | 2 (0–3) | 0–16 | 189 | 2.2 (2.4) | 2 (0–4) | 0–15 | 182 | 2.0 (2.3) | 1.3 (0–3) | 0–11 |
| Emotional | 189 | 1.5 (2.0) | 1 (0–2) | 0–9 | 189 | 1.6 (2.3) | 1 (0–2) | 0–11 | 182 | 1.7 (2.2) | 1 (0–3) | 0–14 |
| Social | 189 | 0.7 (1.7) | 0 (0–1) | 0–14 | 189 | 0.8 (1.8) | 0 (0–1) | 0–16 | 182 | 0.9 (1.7) | 0 (0–1) | 0–8 |
| Overall P-CPQ-16 score | 189 | 7.1 (6.7) | 5 (2–9) | 0–38.7b | 189 | 7.1 (6.5) | 6 (3–9.7b) | 0–46 | 182 | 7.1 (6.1) | 6 (3–9) | 0–36 |
| Questionnaire | C+P | B+P | PA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Meana (SD) | Median (IQR) | Range | n | Meana (SD) | Median (IQR) | Range | n | Meana (SD) | Median (IQR) | Range | |
| Change in overall P-CPQ-16 score (final minus baseline) | 189 | –1.6 (7.6) | –1 (–5 to 2) | –28 to 29 | 189 | –0.8 (6.2) | –1 (–4 to 3) | –24 to 30.3b | 182 | –1.1 (7.2) | –1 (–5 to 2) | –34 to 26 |
Dental anxiety
Child questionnaires: returns, Modified Child Dental Anxiety Scale (faces) completeness and reliability
| ITT analysis set participants (n = 1058) | CRFs (N) | Child questionnaires, n/N (%) |
|---|---|---|
| Baselinea | 1058 | 1045/1058 (98.8) |
| Total | 7713 | 6793/7713 (88.1) |
| Number of missing items | Baseline, n (% of 1045) | All visits, n (% of 6793) |
|---|---|---|
| 0 | 865 (82.8) | 6032 (88.8) |
| 1 | 72 (6.9) | 277 (4.1) |
| 2 | 25 (2.4) | 108 (1.6) |
| 3 | 27 (2.6) | 115 (1.7) |
| 4 | 35 (3.4) | 112 (1.7) |
| 5 | 2 (0.2) | 23 (0.3) |
| 6 | 19 (1.8) | 126 (1.9) |
| MCDASf scorea | C+P | B+P | PA | Total |
|---|---|---|---|---|
| Baseline (n = 1045) | ||||
| n | 336 | 324 | 329 | 989 |
| Mean (SD) | 13.8 (4.9) | 14.2 (5.3) | 14.3 (5.3) | 14.1 (5.1) |
| Median (IQR) | 14 (10–17) | 14 (10.8–18) | 14 (10–18) | 14 (10–18) |
| Range | 6–28 | 6–28 | 6–30 | 6–30 |
| All visits except baseline (n = 5748) | ||||
| n | 1986 | 1850 | 1707 | 5543 |
| Mean (SD) | 15.1 (5.3) | 15.1 (5.5) | 15.3 (5.5) | 15.2 (5.5) |
| Median (IQR) | 15 (11–19) | 15 (11–19) | 15 (11–19) | 15 (11–19) |
| Range | 6–30 | 6–30 | 6–30 | 6–30 |
| Variable | Mean difference | 97.5% CI | p-value |
|---|---|---|---|
| Baseline MCDASf | 0.40 | 0.35 to 0.46 | < 0.001 |
| Arm | |||
| C+P (reference category) | 0.00 | ||
| B+P | –0.07 | –0.74 to 0.59 | 0.8 |
| PA | 0.22 | –0.44 to 0.89 | 0.5 |
| Time in study (years) | 0.17 | –0.22 to 0.56 | 0.5 |
| Age (years) | –0.12 | –0.35 to 0.10 | 0.2 |
Child-reported anticipatory anxiety and worry: Q4 and Q6 (child questionnaire)
| Question | n (% of 6793) |
|---|---|
| Q4. Before you saw the dentist today, were you? Not worried, a little worried, very worried | 167 (2.5) |
| Q6. Thinking about your visit to the dentist today, were you? Not worried, a little worried, very worried | 306 (4.5) |
| Q4: Before you saw the dentist today, were you? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| Baseline (N = 1045) | ||||
| n | 344 | 337 | 340 | 1021 |
| Not worried, n (%) | 243 (70.6) | 237 (70.3) | 240 (70.6) | 720 (70.5) |
| A little worried, n (%) | 82 (23.6) | 83 (24.6) | 69 (20.3) | 233 (22.8) |
| Very worried, n (%) | 20 (5.8) | 17 (5.0) | 31 (9.1) | 68 (6.7) |
| All visits excluding baseline (N = 5748) | ||||
| n | 1990 | 1888 | 1727 | 5605 |
| Not worried, n (%) | 1303 (65.5) | 1232 (65.3) | 1194 (69.1) | 3729 (66.5) |
| A little worried, n (%) | 586 (29.5) | 550 (29.1) | 444 (25.7) | 1580 (28.2) |
| Very worried, n (%) | 101 (5.1) | 106 (5.6) | 89 (5.2) | 296 (5.3) |
| Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|
| C+P (reference category) | 0.00 | ||
| B+P | 0.02 | –0.04 to 0.07 | 0.5 |
| PA | –0.03 | –0.09 to 0.02 | 0.1 |
Parent-reported child anticipatory anxiety and worry
| Before seeing the dentist today, do you think your child was? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| Baseline (N = 1044) | ||||
| n | 306 | 314 | 309 | 929 |
| Not at all worried, n (%) | 215 (70.3) | 206 (65.6) | 199 (64.4) | 620 (66.7) |
| Very slightly worried, n (%) | 57 (18.6) | 75 (23.9) | 65 (21.0) | 197 (21.2) |
| Fairly worried, n (%) | 16 (5.2) | 19 (6.1) | 22 (7.1) | 57 (6.1) |
| Quite worried, n (%) | 13 (4.3) | 8 (2.6) | 14 (4.5) | 35 (3.8) |
| Very worried, n (%) | 5 (1.6) | 6 (1.9) | 9 (2.9) | 20 (2.2) |
| All visits excluding baseline (n = 5703) | ||||
| n | 1918 | 1848 | 1693 | 5459 |
| Not at all worried, n (%) | 1269 (66.2) | 1277 (69.1) | 1211 (71.5) | 3757 (68.8) |
| Very slightly worried, n (%) | 458 (23.9) | 369 (20.0) | 349 (20.6) | 1176 (21.5) |
| Fairly worried, n (%) | 114 (5.9) | 124 (6.7) | 74 (4.4) | 312 (5.7) |
| Quite worried, n (%) | 52 (2.7) | 49 (2.7) | 32 (1.9) | 133 (2.4) |
| Very worried, n (%) | 25 (1.3) | 29 (1.6) | 27 (1.6) | 81 (1.5) |
| Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|
| C+P (reference category) | 0.00 | ||
| B+P | –0.04 | –0.09 to 0.02 | > 0.9 |
| PA | –0.06 | –0.11 to –0.003 | 0.02 |
Child-reported treatment-related anxiety and worry
| Q6: Thinking about your visit to the dentist today, were you? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| All visits (N = 6793) | ||||
| n | 2277 | 2188 | 2022 | 6487 |
| Not worried, n (%) | 1572 (69.0) | 1514 (69.2) | 1467 (72.6) | 4553 (70.2) |
| A little worried, n (%) | 571 (25.1) | 540 (24.7) | 439 (21.7) | 1550 (23.9) |
| Very worried, n (%) | 134 (5.9) | 134 (6.1) | 116 (5.7) | 384 (5.9) |
| Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|
| C+P (reference category) | 0.00 | ||
| B+P | –0.002 | –0.05 to 0.04 | > 0.9 |
| PA | –0.04 | –0.08 to 0.01 | 0.06 |
Parent-reported child treatment-related anxiety and worry
| Thinking about being at the dentist today, do you think your child was? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| All visits (N = 6747) | ||||
| n | 2262 | 2188 | 2048 | 6498 |
| Not at all worried, n (%) | 1451 (64.2) | 1474 (67.4) | 1406 (68.7) | 4331 (66.7) |
| Very slightly worried, n (%) | 569 (25.2) | 485 (22.2) | 443 (21.6) | 1497 (23.0) |
| Fairly worried, n (%) | 117 (5.2) | 133 (6.1) | 108 (5.3) | 358 (5.5) |
| Quite worried, n (%) | 76 (3.4) | 55 (2.5) | 44 (2.2) | 175 (2.7) |
| Very worried, n (%) | 49 (2.2) | 41 (1.9) | 47 (2.3) | 137 (2.1) |
| Trial arm | Risk difference | 97.5% CI | p-value |
|---|---|---|---|
| C+P (reference category) | 0.00 | ||
| B+P | –0.04 | –0.09 to 0.02 | 0.1 |
| PA | –0.04 | –0.10 to 0.01 | 0.06 |
Child-, parent- and dentist-reported child discomfort during treatment
| Experience of discomfort during treatment | C+P | B+P | PA | Total |
|---|---|---|---|---|
| Child reported (n) | 2278 | 2172 | 2018 | 6468 |
| ‘Not hurt at all’, n (%) | 1551 (68.1) | 1492 (68.7) | 1528 (75.7) | 4571 (70.7) |
| Parent reported (n) | 2116 | 2062 | 1915 | 6093 |
| ‘Not at all painful’, n (%) | 1536 (72.6) | 1581 (76.7) | 1596 (83.3) | 4713 (77.4) |
| Dentist reported (n) | 2578 | 2431 | 2292 | 7301 |
| ‘No apparent discomfort’, n (%) | 1614 (62.6) | 1640 (67.5) | 1736 (75.7) | 4990 (68.4) |
| Q7: Thinking about being at the dentist today, did it? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| All visits (N = 6793) | ||||
| n | 2278 | 2172 | 2018 | 6468 |
| Not hurt at all, n (%) | 1551 (68.1) | 1492 (68.7) | 1528 (75.7) | 4571 (70.7) |
| Hurt a little, n (%) | 586 (25.7) | 559 (25.7) | 397 (19.7) | 1542 (23.8) |
| Hurt a lot, n (%) | 141 (6.2) | 121 (5.6) | 93 (4.6) | 355 (5.5) |
| Thinking about being at the dentist today how do you think your child found the treatment? | C+P | B+P | PA | Total |
|---|---|---|---|---|
| All visits (N = 6747) | ||||
| n | 2116 | 2062 | 1915 | 6093 |
| Not at all painful | 1536 (72.6) | 1581 (76.7) | 1596 (83.3) | 4713 (77.4) |
| A little painful | 476 (22.5) | 394 (19.1) | 259 (13.5) | 1129 (18.5) |
| Somewhat painful | 61 (2.9) | 50 (2.4) | 25 (1.3) | 136 (2.2) |
| Painful | 25 (1.2) | 21 (1.0) | 23 (1.2) | 69 (1.1) |
| Very painful | 18 (0.9) | 16 (0.8) | 12 (0.6) | 46 (0.8) |
| Discomfort | Trial arm, n (% of non-missing) | Total (N = 7713), n (% of non-missing) | ||
|---|---|---|---|---|
| C+P (N = 2706) | B+P (N = 2593) | PA (N = 2414) | ||
| No apparent discomfort | 1614 (62.6) | 1640 (67.5) | 1736 (75.7) | 4990 (68.4) |
| Very mild, almost trivial | 453 (17.6) | 429 (17.7) | 313 (13.7) | 1195 (16.4) |
| Mild, not significant | 257 (10.0) | 192 (7.9) | 110 (4.8) | 559 (7.7) |
| Moderate, but child coped | 219 (8.5) | 139 (5.7) | 105 (4.6) | 463 (6.3) |
| Significant and unacceptable | 35 (1.4) | 31 (1.3) | 28 (1.2) | 94 (1.3) |
| Total | 2578 | 2431 | 2292 | 7301 |
Dentist-reported child-behaviour and co-operation
| Please rate the child’s behaviour during the treatment session | C+P (N = 2706) | B+P (N = 2593) | PA (N = 2414) | Total (N = 7713) |
|---|---|---|---|---|
| 1. The child refused treatment: cried forcefully, fearful, evidence of extreme negativism. It was very difficult to make any progress, n (% of non-missing) | 92 (3.6) | 56 (2.3) | 54 (2.4) | 202 (2.8) |
| 2. The child appeared reluctant to listen, respond or to accept the treatment and had some evidence of negative attitude. Some progress was possible, n (% of non-missing) | 181 (7.0) | 137 (5.7) | 105 (4.6) | 423 (5.8) |
| 3. The child was accepting the treatment but was cautious. The child was willing to comply with the dentist, but appeared to have some reservations, n (% of non-missing) | 875 (34.0) | 740 (30.5) | 623 (27.2) | 2238 (30.7) |
| 4. Child was completely co-operative; he/she was interested in the dental procedure and even enjoyed the experience, n (% of non-missing) | 1426 (55.4) | 1492 (61.5) | 1508 (65.9) | 4426 (60.7) |
| Total non-missing (N) | 2574 | 2425 | 2290 | 7289 |
| Were there any difficulties providing treatment? | C+P (N = 2706) | B+P (N = 2593) | PA (N = 2414) | Total (N = 7713) |
|---|---|---|---|---|
| Yes, n (% of non-missing) | 594 (23.0) | 493 (20.3) | 399 (17.5) | 1486 (20.4) |
| No, n (% of non-missing) | 1987 (77.0) | 1933 (79.7) | 1888 (82.6) | 5808 (79.6) |
| Total non-missing (N) | 2581 | 2426 | 2287 | 7294 |
| If yes please explain | C+P (N = 2706) | B+P (N = 2593) | PA (N = 2414) | Total (N = 7713) |
|---|---|---|---|---|
| Compliance of the child, n (% of non-missing) | 363 (61.1) | 259 (52.5) | 230 (57.6) | 852 (57.3) |
| Compliance of the parent, n (% of non-missing) | 26 (4.4) | 18 (3.7) | 19 (4.8) | 63 (4.2) |
| Small mouth, n (% of non-missing) | 79 (13.3) | 92 (18.7) | 72 (18.0) | 243 (16.4) |
| Moisture control, n (% of non-missing) | 107 (18.0) | 127 (25.8) | 93 (23.3) | 327 (22.0) |
| Unable to deliver local anaesthetic, n (% of non-missing) | 54 (9.1) | 13 (2.6) | 13 (3.3) | 80 (5.4) |
| Other clinical (please list), n (% of non-missing) | 82 (13.8) | 98 (19.9) | 44 (11.0) | 224 (15.1) |
| Total answered ‘yes’ to difficulties (N) | 594 | 493 | 399 | 1486 |
| Did you use inhalation sedation/relative analgesia during treatment? | C+P (N = 2706) | B+P (N = 2593) | PA (N = 2414) | Total (N = 7713) |
|---|---|---|---|---|
| Yes, n (% of non-missing) | 23 (0.9) | 16 (0.6) | 14 (0.6) | 53 (0.7) |
| No, n (% of non-missing) | 2657 (99.1) | 2545 (99.4) | 2366 (99.4) | 7568 (99.3) |
| Total non-missing (N) | 2680 | 2561 | 2380 | 7621 |
| Number of missing items | All visits, n (% of 6747) |
|---|---|
| 0 | 6229 (92.3) |
| 1 | 194 (2.9) |
| 2 | 36 (0.5) |
| 3 | 12 (0.2) |
| 4 | 5 (0.1) |
| 5 | 6 (0.1) |
| 6 | 11 (0.2) |
| 7 | 41 (0.6) |
| 8 | 213 (3.2) |
| Overall DDQ-8a score | n | C+P (n = 2347) | n | B+P (n = 2276) | n | PA (n = 2124) | n | Total (n = 6747) |
|---|---|---|---|---|---|---|---|---|
| Minimum | 2254 | 0 | 2177 | 0 | 2040 | 0 | 6471 | 0 |
| Median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | ||||
| Mean (SD) | 1.9 (2.4) | 2.1 (2.4) | 2.1 (2.5) | 2.0 (2.4) | ||||
| Maximum | 16 | 16 | 15 | 16 |
Appendix 5 Health economics (see Chapter 4)
Cost data collection (see Chapter 4, Cost data collection, and Costing methods)
| Resource | Unit | Cost (£) | Source |
|---|---|---|---|
| Treatment provider | |||
| GDP | Cost per minute | 0.68 | CRF (Q10a/11a)/PSSRU 2017120 |
| Dental therapist | Cost per minute | 0.28 | CRF (Q10a/11a)/Agenda for Change band 5 point 20121 |
| Dental hygienist | Cost per minute | 0.28 | CRF (Q10a)/Agenda for Change band 5 point 20121 |
| Oral health educator | Cost per minute | 0.28 | CRF (Q10a)/Agenda for Change band 5 point 20121 |
| Childsmile/extended duty dental nurse | Cost per minute | 0.23 | CRF (Q10a)/Agenda for Change band 4 point 14121 |
| Vocational therapist | Cost per minute | 0.23 | CRF (Q10a)/Agenda for Change band 4 mid-point121 |
| Dental nurse | Cost per minute | 0.21 | CRF (Q10a)/Agenda for Change band 4 point 1121 |
| CT1a | Cost per minute | 0.35 | CRF (Q10a)/Agenda for Change band 6 point 6/band 7 point 1121 |
| Dental nurse trainee | Cost per minute | 0.16 | CRF (Q10a)/Agenda for Change 75% of band 4 point 1121 |
| Resources used at every visit | |||
| Resources at every visit | Cost per visit | 2.19 | CRF completed/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Resources at every visit: fluoride varnish | Cost per visit | 1.74 | CRF (Q10b)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Prevention | |||
| Fissure sealants of permanent teeth (resin) | Cost per visit | 3.99 | CRF (Q10b)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Fluoride varnish | Cost per visit | 0.27 | CRF (Q10b)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Operative treatment | |||
| Topical anaesthetic gel | Cost per visit | 0.05 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Local anaesthetic | Cost per visit | 1.08 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Fissure sealant: glass ionomer | Cost per tooth | 4.54 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Preformed metal crown | Cost per tooth | 14.48 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Hall Technique crown | Cost per tooth | 6.92 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Pulpotomy | Cost per tooth | 25.02 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Extraction | Cost per tooth | 2.58 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Opening a lesion | Cost per tooth | 2.55 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling | |||
| Amalgam | Cost per tooth | 4.11 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Glass ionomer | Cost per tooth | 4.54 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Composite | Cost per tooth | 3.48 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Compomer | Cost per tooth | 3.48 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| RMGI | Cost per tooth | 4.82 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Resources used to administer a filling | Cost per tooth | 5.10 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| More than one surface used | |||
| Filling: amalgam | Cost per tooth | 4.27 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling: glass ionomer | Cost per tooth | 5.09 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling: composite | Cost per tooth | 4.04 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling: compomer | Cost per tooth | 4.04 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling: RMGI | Cost per tooth | 5.38 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Filling: resources used to administer a filling | Cost per tooth | 5.38 | CRF (Q12)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Miscellaneous | |||
| Radiographs | Cost per image | 1.51 | CRF (Q9)/see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/ (accessed 27 November 2019) |
| Inhalation sedation (excluding staff costs) | Cost per visit | 59.76 | CRF (Q17)/NICE 2010164 (inflated to current prices) |
| Medications | |||
| Paracetamol | Cost per dose | 0.04 | CRF (Q20)/BNF165 |
| Ibuprofen | Cost per dose | 0.09 | CRF (Q20)/BNF165 |
| Mouth rinse | Cost per bottle | 7.14 | CRF (Q20)/BNF165 |
| Mouth spray | Cost per spray | 4.64 | CRF (Q20)/BNF165 |
| Mouth gel | Cost per tube | 1.56 | CRF (Q20)/BNF165 |
| Bonjela® (Reckitt Benckiser Group plc, Slough, UK) | Cost per tube | 3.55 | CRF (Q20)/BNF165 |
| Amoxicillin | Cost per dose | 0.05 | CRF (Q21)/BNF165 |
| Penicillin | Cost per dose | 0.74 | CRF (Q21)/BNF165 |
| Metronidazole | Cost per dose | 1.03 | CRF (Q21)/BNF165 |
| Erythromycin | Cost per dose | 0.22 | CRF (Q21)/BNF165 |
| Grouping A | Cost per referral | 118.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Grouping B | Cost per referral | 793.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Grouping C | Cost per referral | 793.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Grouping D | Cost per referral | 418.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Grouping E | Cost per referral | 418.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Grouping F | Cost per referral | 118.00 | Patient referral form (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/personal communication (Ben Cole) |
| Time off paid work | Cost per day | 67.73 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/ONS 2017125 |
| Time off leisure activities | Cost per day | 39.84 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/Department for Transport 2017117 |
| Time off school | Cost per day | 39.84 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/Department for Transport 2017117 |
| Additional child care | Cost per day | 24.90 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/Family and Childcare Trust 2017127 |
| Pain medication (over the counter) | |||
| Calprofen® (ibuprofen) (Johnson & Johnson, New Brunswick, NJ, USA)b | Cost per bottle | 3.59 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/calprofen-174-ibuprofen-3-months-100 ml (accessed 8 December 2017) |
| Calpol® (paracetamol) (Johnson & Johnson)b | Cost per bottle | 2.99 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/calpol-sixplus-suspension-sugar-free-strawberry-flavour-6-years-80ml(accessed 8 December 2017) |
| Bonjelab | Cost per tube | 2.99 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/bonjela-teething-gel-15 g (accessed 8 December 2017) |
| Anbesol® (Alliance Pharmaceuticals, Chippenham, UK)b | Cost per tube | 2.39 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/anbesol-teething-gel-6007901–44 (accessed 8 December 2017) |
| Clove oilb | Cost per bottle | 1.00 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/care-clove-oil-10 ml (accessed 8 December 2017) |
| Child’s soluble dispirinb | Cost per tablet | 0.11 | Parent questionnaire (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/074403/#/; accessed 27 November 2019)/www.lloydspharmacy.com/en/disprin-aspirin-32-soluble-tablets (accessed 8 December 2017) |
| Resource | Unit | Cost (£) | Source |
|---|---|---|---|
| Examination costs (6–12 years)a | Cost per month | 4.26 | Statement of dental remuneration (page 41)123 |
| Prevention | |||
| Fissure sealant | Cost per tooth | 8.75 | CRF (Q10b)/statement of dental remuneration (page 43)123 |
| Fluoride varnish | Cost per course of treatmentb | 41.45 | CRF (Q10b)/statement of dental remuneration (page 10)123 |
| Operative treatment | |||
| Filling | Cost per tooth | 15.45 | CRF (Q12)/statement of dental remuneration (page 43)123 |
| Crown | Cost per tooth | 22.85 | CRF (Q12)/statement of dental remuneration (page 43)123 |
| Extraction (one tooth) | Cost per course of treatmentb | 8.75 | CRF (Q12)/statement of dental remuneration (page 50)123 |
| Extraction (two teeth) | Cost per course of treatmentb | 15.75 | CRF (Q12)/statement of dental remuneration (page 50)123 |
| Extraction | Cost per visit | 7.10 | CRF (Q12)/statement of dental remuneration (page 51)123 |
| Pulpotomy | Cost per tooth | 17.40 | CRF (Q12)/statement of dental remuneration (page 43)123 |
| Sedation | Cost per visit | 13.30 | CRF (Q12)/statement of dental remuneration (page 53)123 |
| Resource | Cost (£), mean (SD) | Cost (£), median (IQR) | Minimum (£) | Maximum (£) | Source |
|---|---|---|---|---|---|
| Total UDA value | 29.68 (11.31) | 28.01 (24.31–31.83) | 21.45 | 98.74 | British Dental Association website166 |
Missing data (see Chapter 4, Data validity and completeness)
| n (%)a | |
|---|---|
| PA | |
| PA missing | |
| Assumed PA was not provided at that visit and would have been already provided as part of the child’s course of treatment | 14 (< 1) |
| PA pillars missing | |
| Assumed they had pillars brushing and diet advice | 9 (< 1) |
| PA staff | |
| Student provided PA – assumed a GDP was present | 7 (< 1) |
| Dental nurse provided PA – assumed no cost as dental nurse was assumed to be present for the duration of the visit | 151 (2) |
| Operative treatment | |
| Operative treatment missing | |
| Assumed no operative treatment provided | 33 (< 1) |
| Operative treatment staff missing | |
| Assumed to be the same as PA staff, if it was a GDP or therapist providing PA. This assumption was verified by the data. In 90% of visits when both PA and operative treatment were provided, the PA staff were the same staff providing operative treatment | 68 (< 1) |
| Assumed to be a GDP | 41 (< 1) |
| Restoration materials missingb | |
| No assumptions could be made for materials used | 125 (< 1) |
| Caries removal missingb | |
| No assumptions were made to imply whether or not caries removal had been undertaken | 417 (< 1) |
| Local anaesthetic missingb | |
| No assumptions were made to imply whether or not local anaesthetic had been provided | 221 (< 1) |
| Topical anaesthetic missingb | |
| No assumptions were made to imply whether or not topical anaesthetic had been provided | 236 (< 1) |
| Number of surfaces missingb | |
| No assumptions were made to imply the number of surfaces treated | 424 (< 1) |
| Number of extractions missingb | |
| No assumptions were made to imply whether or not an extraction took place | 245 (< 1) |
| Number of lesions opened missingb | |
| No assumptions were made to imply whether or not lesions were opened | 270 (< 1) |
| Other treatments | |
| Number of radiographs missing | |
| Assumed no radiographs were taken | 18 (< 1) |
| Number of inhalation sedation missing | |
| Assumed no inhalation sedation took place | 92 (1) |
| Time | |
| Negative total visit time (start time > end time) | |
| Issues with the 24-hour clock (e.g. 14.40–13.10 should be 14.40–15.10) | 13 (< 1) |
| Outstanding negative times – if no operative treatment provided, we took the shortest treatment time | 2 (< 1) |
| Outstanding negative times – if operative treatment provided, we took the longest treatment time | 1 (< 1) |
| Outstanding negative times: total treatment time was – 2 minutes – assumed total treatment time = PA time, as no operative treatment was provided | 2 (< 1) |
| PA time > total visit time | |
| No operative treatment provided: assumed total visit time = PA time | 71 (1) |
| Operative treatment provided: total treatment time = total visit time and new total visit time = treatment time (original visit time) + PA time | 17 (< 1) |
| Operative treatment provided but estimated total treatment time was not reflective of the treatment provided (e.g. lesion opened to make cleansable): assumed PA time captured this | 2 (< 1) |
| Missing PA time | |
| Additional information in PA other details | 9 (< 1) |
| Estimated time based on the pillars of PA provided and the PA time observed in the data for these pillars | 384 (5) |
| PA was missing: assumed no PA provided, so time = 0 minutes | 14 (< 1) |
| Missing total visit time | |
| PA provided, PA time not missing and no operative treatment: assumed visit time = PA time | 28 (< 1) |
| Assumed 10-minute appointments for one practice that said that was their policy and never recorded visit times | 24 (< 1) |
| Outstanding missing times: estimated based on treatments provided using clinical advice | 75 (1) |
| Medications | |
| Missing painkiller information | |
| Assumed no painkillers were prescribed | 90 (1) |
| Missing antibiotics information | |
| Assumed no antibiotics were prescribed | 102 (1) |
Resource use [see Chapter 3, Treatment received (intention-to-treat analysis set, n = 1058) and Chapter 4, Resource use and costs]
| Resource use (per visit) | C+P, mean (SD) | n | B+P, mean (SD) | n | PA, mean (SD) | n |
|---|---|---|---|---|---|---|
| Number of visits | ||||||
| Number of visits (all) (n = 1058) | 7.69 (4.21) | 352 | 7.37 (4.08) | 352 | 6.82 (3.65) | 354 |
| Number of first visits (n = 1058) | 1 (–) | 352 | 1 (–) | 352 | 1 (–) | 354 |
| Number of follow-up visits (n = 1006)a | 6.96 (4.06) | 338 | 6.73 (3.89) | 333 | 6.15 (3.47) | 335 |
| Length of visits (minutes) | ||||||
| Length of visits (all) | 21.76 (6.91) | 352 | 21.24 (7.18) | 352 | 20.11 (6.65) | 354 |
| Length of first visit | 28.80 (11.93) | 347 | 28.14 (11.14) | 350 | 25.56 (10.20) | 354 |
| Length of follow-up visit | 20.54 (6.99)b | 338 | 19.38 (6.90) | 333 | 18.64 (6.85) | 335 |
| Prevention | ||||||
| Prevention | 0.79 (0.22) | 352 | 0.79 (0.22) | 352 | 0.85 (0.19) | 354 |
| Prevention at first visit | 0.81 (0.39)c | 350 | 0.83 (0.37) | 351 | 0.91 (0.29) | 353 |
| Prevention at follow-up visits | 0.79 (0.23)d | 338 | 0.78 (0.23) | 333 | 0.85 (0.21) | 335 |
| Prevention staff | ||||||
| GDP providing prevention at first visit | 0.71 (0.46)e | 349 | 0.72 (0.45) | 349 | 0.77 (0.42) | 344 |
| Dental therapist providing prevention at first visit | 0.07 (0.25) | 349 | 0.07 (0.25) | 349 | 0.08 (0.26) | 344 |
| Dental hygienist providing prevention at first visit | 0.02 (0.13) | 349 | 0.02 (0.14) | 349 | 0.03 (0.17) | 344 |
| Oral health educator providing prevention at first visit | 0.01 (0.11) | 349 | 0.02 (0.15) | 349 | 0.04 (0.19) | 344 |
| Childsmile/extended duty dental nurse providing prevention at first visit | 0.03 (0.16) | 349 | 0.02 (0.13) | 349 | 0.03 (0.16) | 344 |
| Other staff (dental nurse) providing prevention at first visit | 0.01 (0.11) | 350 | 0.01 (0.09) | 351 | 0.01 (0.12) | 353 |
| Other staff (dental nurse trainee) providing prevention at first visit | 0 (–) | 350 | 0 (–) | 351 | 0 (–) | 353 |
| Other staff member (CT1) providing prevention at first visit | 0 (–) | 350 | 0 (–) | 351 | 0 (–) | 353 |
| Other staff member (dental student) providing prevention at first visit | 0 (–) | 350 | 0 (–) | 351 | 0 (–) | 353 |
| GDP providing prevention at follow-up visits | 0.69 (0.27)f | 338 | 0.68 (0.27) | 333 | 0.76 (0.26) | 335 |
| Dental therapist providing prevention at follow-up visits | 0.07 (0.14) | 338 | 0.06 (0.13) | 333 | 0.05 (0.12) | 335 |
| Dental hygienist providing prevention at follow-up visits | 0.01 (0.04) | 338 | 0.01 (0.04) | 333 | 0.01 (0.07) | 335 |
| Oral health educator providing prevention at follow-up visits | 0.01 (0.07) | 338 | 0.01 (0.06) | 333 | 0.01 (0.05) | 335 |
| Childsmile/extended duty dental nurse providing prevention at follow-up visits | 0.02 (0.08) | 338 | 0.01 (0.04) | 333 | 0.02 (0.06) | 335 |
| Other staff member (dental nurse) providing prevention at follow-up visits | 0.03 (0.15) | 338 | 0.02 (0.13) | 333 | 0.03 (0.15) | 335 |
| Other staff member (dental nurse trainee) providing prevention at follow-up visits | 0 (–) | 338 | 0 (–) | 333 | < 0.01 (0.01) | 335 |
| Other staff member (CT1) providing prevention at follow-up visits | 0 (–) | 338 | < 0.01 (< 0.01) | 333 | 0 (–) | 335 |
| Other staff member (dental student) providing prevention at follow-up visits | < 0.01 (0.01) | 338 | < 0.01 (0.01) | 333 | < 0.01 (0.01) | 335 |
| Prevention ‘pillars’ (components) | ||||||
| Brushing/plaque control advice provided at first visit | 0.76 (0.43)g | 350 | 0.79 (0.41) | 351 | 0.88 (0.32) | 353 |
| Fissure sealants provided at first visit | 0.12 (0.33) | 350 | 0.15 (0.35) | 351 | 0.15 (0.36) | 353 |
| Fluoride varnish provided at first visit | 0.53 (0.50) | 350 | 0.56 (0.50) | 351 | 0.74 (0.44) | 353 |
| Diet investigation/advice provided at first visit | 0.70 (0.46) | 350 | 0.75 (0.43) | 351 | 0.84 (0.37) | 353 |
| Brushing/plaque control advice provided at follow-up visits | 0.73 (0.26)h | 338 | 0.71 (0.26) | 333 | 0.78 (0.24) | 335 |
| Fissure sealants provided at follow-up visits | 0.13 (0.20) | 338 | 0.15 (0.22) | 333 | 0.16 (0.23) | 335 |
| Fluoride varnish provided at follow-up visits | 0.51 (0.31) | 338 | 0.54 (0.31) | 333 | 0.62 (0.31) | 335 |
| Diet investigation/advice provided at follow-up visits | 0.66 (0.29) | 338 | 0.64 (0.30) | 333 | 0.71 (0.29) | 335 |
| Prevention time (minutes) | ||||||
| Length of time providing prevention at first visit) | 10.18 (10.44)i | 331 | 10.08 (8.75) | 335 | 12.82 (8.03) | 336 |
| Length of time providing prevention at follow-up visits | 6.58 (4.21)j | 338 | 6.40 (3.96) | 333 | 7.58 (4.16) | 335 |
| Operative treatment | ||||||
| Operative treatment at first visit | 0.62 (0.49)k | 349 | 0.63 (0.48) | 351 | 0.16 (0.37) | 353 |
| Operative treatment at follow-up visits | 0.36 (0.28)l | 338 | 0.34 (0.26) | 333 | 0.19 (0.24) | 335 |
| Operative treatment time (minutes) | ||||||
| Length of time providing operative treatment at first visit | 18.31 (11.21) | 336 | 17.94 (11.27) | 337 | 12.42 (10.48) | 350 |
| Length of time providing operative treatment at follow-up visits | 12.86 (7.08) | 338 | 12.08 (6.47) | 333 | 10.16 (6.55) | 335 |
| Operative treatment staff | ||||||
| GDP providing operative treatment at first visit | 0.58 (0.49)m | 342 | 0.60 (0.49) | 343 | 0.14 (0.34) | 349 |
| Dental therapist providing operative treatment at first visit | 0.04 (0.18) | 342 | 0.03 (0.18) | 343 | 0.02 (0.15) | 349 |
| GDP providing operative treatment at follow-up visits | 0.32 (0.29)n | 338 | 0.29 (0.25) | 333 | 0.17 (0.23) | 335 |
| Dental therapist providing operative tx at follow-up visits | 0.03 (0.09) | 338 | 0.03 (0.08) | 333 | 0.01 (0.04) | 335 |
| Primary teeth treated | ||||||
| Number of primary teeth treated operatively at first visit | 0.98 (1.12)o | 349 | 1.16 (1.32) | 351 | 0.26 (0.70) | 353 |
| Number of surfaces treated at first visit | 0.98 (1.05) | 349 | 1.29 (1.50) | 351 | 0.28 (0.80) | 353 |
| Number of primary teeth treated operatively at follow-up visits | 0.55 (0.59)p | 338 | 0.50 (0.46) | 333 | 0.29 (0.48) | 335 |
| Number of surfaces at follow-up visits | 0.67 (0.71) | 338 | 0.74 (0.77) | 333 | 0.35 (0.53) | 335 |
| Operative treatment: caries removal | ||||||
| Average total complete caries removal per treated primary tooth at first visit | 0.46 (0.49)q | 349 | 0.06 (0.22) | 351 | 0.04 (0.19) | 353 |
| Average total partial caries removal per treated primary tooth at first visit | 0.08 (0.25) | 349 | 0.31 (0.44) | 351 | 0.05 (0.21) | 353 |
| Average total ‘none’ caries removal per treated primary tooth at first visit | 0.06 (0.24) | 349 | 0.24 (0.41) | 351 | 0.05 (0.21) | 353 |
| Average total complete caries removal per treated primary tooth at follow-up visits | 0.21 (0.23)r | 338 | 0.05 (0.12) | 333 | 0.06 (0.16) | 335 |
| Average total partial caries removal per treated primary tooth at follow-up visits | 0.05 (0.11) | 338 | 0.11 (0.16) | 333 | 0.04 (0.11) | 335 |
| Average total ‘none’ caries removal per treated primary tooth at follow-up visits | 0.06 (0.13) | 338 | 0.12 (0.18) | 333 | 0.05 (0.11) | 335 |
| Restorations | ||||||
| Restorations at first visit | 0.58 (0.49)s | 352 | 0.59 (0.49) | 352 | 0.10 (0.30) | 354 |
| Average total amalgam restorations per treated primary tooth at first visit | 0.08 (0.26)t | 349 | 0.01 (0.11) | 351 | 0.01 (0.08) | 353 |
| Average total glass ionomer restorations per treated primary tooth at first visit | 0.13 (0.33) | 349 | 0.15 (0.35) | 351 | 0.05 (0.21) | 353 |
| Average total composite restorations per treated primary tooth at first visit | 0.17 (0.37) | 349 | 0.07 (0.25) | 351 | 0.01 (0.08) | 353 |
| Average total conventional preformed metal crown restorations per treated primary tooth at first visit | 0.01 (0.10) | 349 | < 0.01 (0.05) | 351 | 0 (–) | 353 |
| Average total Hall Technique preformed metal crown restorations per treated primary tooth at first visit | 0.02 (0.12) | 349 | 0.12 (0.32) | 351 | 0.01 (0.10) | 353 |
| Average total compomer restorations per treated primary tooth at first visit | 0.04 (0.19) | 349 | 0.03 (0.15) | 351 | 0.01 (0.08) | 353 |
| Average total RMGI restorations per treated primary tooth at first visit | 0.13 (0.33) | 349 | 0.12 (0.32) | 351 | 0.01 (0.08) | 353 |
| Average total sealant only restorations per treated primary tooth at first visit | 0.02 (0.12) | 349 | 0.08 (0.26) | 351 | 0.01 (0.11) | 353 |
| Average total sealant over restoration per treated primary tooth at first visit | 0.01 (0.09) | 349 | 0.04 (0.18) | 351 | 0 (–) | 353 |
| Average total pulpotomy restorations per treated primary tooth at first visit | 0.01 (0.08) | 349 | < 0.01 (0.05) | 351 | 0 (–) | 353 |
| Average total restorations per treated primary tooth at follow-up visits | 0.30 (0.27)u | 338 | 0.27 (0.24) | 333 | 0.12 (0.21) | 335 |
| Average total amalgam restorations per treated primary tooth at follow-up visits | 0.03 (0.09)v | 338 | < 0.01 (0.03) | 333 | < 0.01 (0.04) | 335 |
| Average total glass ionomer restorations per treated primary tooth at follow-up visits | 0.10 (0.19) | 338 | 0.09 (0.17) | 333 | 0.06 (0.15) | 335 |
| Average total composite restorations per treated primary tooth at follow-up visits | 0.05 (0.12) | 338 | 0.03 (0.09) | 333 | 0.01 (0.08) | 335 |
| Average total conventional preformed metal crown restorations per treated primary tooth at follow-up visits | 0.01 (0.05) | 338 | < 0.01 (0.02) | 333 | < 0.01 (0.02) | 335 |
| Average total Hall Technique preformed metal crown restorations per treated primary tooth at follow-up visits | 0.01 (0.06) | 338 | 0.07 (0.14) | 333 | 0.01 (0.07) | 335 |
| Average total compomer restorations per treated primary tooth at follow-up visits | 0.01 (0.06) | 338 | 0.01 (0.03) | 333 | < 0.01 (0.03) | 335 |
| Average total RMGI restorations per treated primary tooth at follow-up visits | 0.07 (0.15) | 338 | 0.06 (0.15) | 333 | 0.03 (0.10) | 335 |
| Average total sealant only restorations per treated primary tooth at follow-up visits | 0.01 (0.06) | 338 | 0.01 (0.05) | 333 | 0.01 (0.03) | 335 |
| Average total sealant over restoration per treated primary tooth at follow-up visits | < 0.01 (0.03) | 338 | 0.01 (0.05) | 333 | < 0.01 (0.01) | 335 |
| Average total pulpotomy restorations per treated primary tooth at follow-up visits | 0.01 (0.04) | 338 | 0.01 (0.06) | 333 | 0.01 (0.04) | 335 |
| Local anaesthetic | ||||||
| Average total local anaesthetics attempted per treated primary tooth at first visit | 0.26 (0.43)w | 349 | 0.02 (0.12) | 351 | 0.02 (0.11) | 353 |
| Average total local anaesthetics achieved per treated primary tooth at first visit (successful) | 0.22 (0.41)x | 349 | 0.01 (0.10) | 351 | 0.01 (0.11) | 353 |
| Average total local anaesthetics not achieved per treated primary tooth at first visit (unsuccessful) | 0.03 (0.17) | 349 | < 0.01 (0.06) | 351 | < 0.01 (0.03) | 353 |
| Average total local anaesthetics not attempted per treated primary tooth at first visit | 0.22 (0.41) | 349 | 0.37 (0.48) | 351 | 0.05 (0.22) | 353 |
| Average total local anaesthetics attempted per treated primary tooth at follow-up visits | 0.13 (0.19)y | 338 | 0.05 (0.10) | 333 | 0.04 (0.11) | 335 |
| Average total local anaesthetics achieved per treated primary tooth at follow-up visits (successful) | 0.12 (0.18)z | 338 | 0.04 (0.10) | 333 | 0.04 (0.10) | 335 |
| Average total local anaesthetics not achieved per treated primary tooth at follow-up visits (unsuccessful) | 0.01 (0.07) | 338 | < 0.01 (0.03) | 333 | < 0.01 (0.02) | 335 |
| Average total local anaesthetics not attempted per treated primary tooth at follow-up visits | 0.15 (0.21) | 338 | 0.18 (0.21) | 333 | 0.07 (0.15) | 335 |
| Other procedures | ||||||
| Average total extractions per treated primary tooth at first visit | 0.01 (0.09)aa | 349 | 0.01 (0.08) | 351 | 0.01 (0.10) | 353 |
| Average total lesions opened per treated primary tooth at first visit | 0.01 (0.08) | 349 | 0.02 (0.12) | 351 | 0.04 (0.19) | 353 |
| Average total extractions per treated primary tooth at follow-up visits | 0.04 (0.11)bb | 338 | 0.04 (0.10) | 333 | 0.04 (0.11) | 335 |
| Average total lesions opened per treated primary tooth at follow-up visits | 0.01 (0.03) | 338 | 0.01 (0.03) | 333 | 0.02 (0.08) | 335 |
| Radiographs | ||||||
| Radiographs at first visit | 0.18 (0.39)cc | 350 | 0.18 (0.38) | 351 | 0.19 (0.39) | 353 |
| Radiographs at follow-up visits | 0.10 (0.15)dd | 338 | 0.08 (0.14) | 333 | 0.11 (0.17) | 335 |
| Inhalation sedation/relative analgesia | ||||||
| Inhalation sedation/relative analgesia at first visit | 0.01 (0.08) | 345 | 0 (–) | 347 | < 0.01 (0.05) | 348 |
| Inhalation sedation/relative analgesia at follow-up visits | 0.01 (0.07) | 338 | 0.01 (0.04) | 333 | 0.01 (0.03) | 335 |
| Painkillers | ||||||
| Painkillers prescribed at first visit | 0 (–)ee | 344 | 0 (–) | 346 | 0 (–) | 349 |
| Paracetamol prescribed at first visit | 0 (–) | 352 | 0 (–) | 352 | 0 (–) | 354 |
| Ibuprofen prescribed at first visit | 0 (–) | 352 | 0 (–) | 352 | 0 (–) | 354 |
| Painkillers prescribed at follow-up visits | < 0.01 (0.03)ff | 338 | < 0.01 (0.03) | 333 | < 0.01 (0.01) | 335 |
| Paracetamol prescribed at follow-up visits | < 0.01 (0.01) | 338 | < 0.01 (0.02) | 333 | < 0.01 (0.01) | 335 |
| Ibuprofen prescribed at follow-up visits | < 0.01 (0.02) | 338 | < 0.01 (0.02) | 333 | < 0.01 (0.01) | 335 |
| Antibiotics | ||||||
| Antibiotics prescribed at first visit | 0.01 (0.08)gg | 343 | 0.01 (0.09) | 344 | 0.01 (0.12) | 349 |
| Amoxicillin prescribed at first visit | 0.01 (0.08) | 352 | < 0.01 (0.05) | 352 | 0.01 (0.12) | 354 |
| Penicillin prescribed at first visit | 0 (–) | 352 | 0 (–) | 352 | 0 (–) | 354 |
| Metronidazole prescribed at first visit | 0 (–) | 352 | 0 (–) | 352 | 0 (–) | 354 |
| Erythromycin prescribed at first visit | 0 (–) | 352 | < 0.01 (0.05) | 352 | 0 (–) | 354 |
| Antibiotics prescribed at follow-up visits | 0.02 (0.08)hh | 338 | 0.02 (0.07) | 333 | 0.03 (0.10) | 335 |
| Amoxicillin prescribed at follow-up visits | 0.02 (0.08) | 338 | 0.02 (0.07) | 333 | 0.03 (0.09) | 335 |
| Penicillin prescribed at follow-up visits | < 0.01 (0.01) | 338 | 0 (–) | 333 | < 0.01 (0.02) | 335 |
| Metronidazole prescribed at follow-up visits | < 0.01 (0.01) | 338 | 0 (–) | 333 | 0 (–) | 335 |
| Erythromycin prescribed at follow-up visits | 0 (–) | 338 | < 0.01 (0.01) | 333 | < 0.01 (0.02) | 335 |
| Referrals | ||||||
| Referrals | 0.09 (0.30)ii | 352 | 0.10 (0.35) | 352 | 0.11 (0.36) | 354 |
| Resource use | ||||||
| Referrals grouping – A | 0.09 (0)jj | 32 | 0.20 (0) | 36 | 0.02 (0) | 39 |
| Referrals grouping – B | 0.44 (0) | 32 | 0.33 (0) | 36 | 0.54 (0) | 39 |
| Referrals grouping – C | 0.03 (0) | 32 | 0 (–) | 36 | 0.10 (0) | 39 |
| Referrals grouping – D | 0.25 (0) | 32 | 0.28 (0) | 36 | 0.15 (0) | 39 |
| Referrals grouping – E | 0.03 (0) | 32 | 0.11 (0) | 36 | 0.08 (0) | 39 |
| Referrals grouping – F | 0.13 (0) | 32 | 0.08 (0) | 36 | 0.03 (0) | 39 |
| Referrals grouping – DNA | 0.03 (0) | 32 | 0 (–) | 36 | 0.08 (0) | 39 |
| Parent questionnaire (excluding baseline) | ||||||
| Completed questionnaires | 6.06 (3.90)kk | 323 | 5.94 (3.87) | 322 | 5.46 (3.41) | 323 |
| Self-reported toothaches | 0.93 (1.48)ll | 323 | 0.92 (1.45) | 322 | 1.10 (1.43) | 323 |
| Children missing school because of child’s toothache | 0.17 (0.47)mm | 323 | 0.22 (0.63) | 322 | 0.22 (0.58) | 323 |
| Days missed from school because of child’s toothache | 1.27 (1.58)nn | 45 | 1.29 (1.31) | 49 | 1.23 (1.38) | 53 |
| Carers missing paid work because of child’s toothache | 0.07 (0.31) | 323 | 0.08 (0.39) | 322 | 0.07 (0.27) | 323 |
| Days missed from paid work because of child’s toothache | 1.25 (1.37) | 18 | 1.50 (1.72) | 20 | 0.70 (0.45) | 22 |
| Paid child care because of child’s toothache | 0.05 (0.26) | 323 | 0.08 (0.32) | 322 | 0.07 (0.29) | 323 |
| Days’ paid child care because of child’s toothache | 0.08 (0.68) | 15 | 0.85 (0.87) | 23 | 0.70 (0.57) | 20 |
| Self-reported painkillers taken because of child’s toothache | 0.50 (1.05) | 323 | 0.52 (1.12) | 322 | 0.52 (0.95) | 323 |
| Days taking painkillers because of child’s toothache | 3.33 (3.55) | 92 | 3.10 (3.31) | 88 | 2.40 (2.28) | 111 |
| Parents missing usual activities because of child’s toothache | 0.13 (0.48) | 323 | 0.17 (0.51) | 322 | 0.15 (0.45) | 323 |
| Days parents missed usual activities because of child’s toothache | 1.78 (1.55) | 29 | 1.68 (1.41) | 39 | 1.06 (0.77) | 42 |
| Resource use | C+P, mean (SD) | n | B+P, mean (SD) | n | PA, mean (SD) | n |
|---|---|---|---|---|---|---|
| Courses of treatment | 5.44 (2.44)a | 352 | 5.32 (2.41) | 352 | 5.38 (2.62) | 354 |
| UDAs per course of treatment | 1.93 (0.58)b | 352 | 1.92 (0.56) | 352 | 1.45 (0.53) | 354 |
| Prevention provided by course of treatment | ||||||
| Prevention provided per course of treatment | 0.88 (0.19)c | 352 | 0.87 (0.19) | 352 | 0.91 (0.17) | 354 |
| Brushing/plaque control advice provided per course of treatment | 0.83 (0.22) | 352 | 0.82 (0.22) | 352 | 0.86 (0.20) | 354 |
| Fissure sealants provided per course of treatment | 0.17 (0.23) | 352 | 0.19 (0.24) | 352 | 0.19 (0.24) | 354 |
| Fluoride varnish provided per course of treatment | 0.62 (0.32) | 352 | 0.64 (0.31) | 352 | 0.73 (0.29) | 354 |
| Diet investigation/advice provided per course of treatment | 0.77 (0.27) | 352 | 0.76 (0.27) | 352 | 0.81 (0.25) | 354 |
Cost-effectiveness results (see Chapter 4, Cost-effectiveness results)
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| C+P (n = 90) | 359.28 (310 to 408) | 0.442 (0.33 to 0.56) | 0.80 | 0.75 | 0.67 | 0.47 | 0.34 | |||
| B+P (n = 100) | 374.54 (324 to 425) | 17.95 (–23 to 59) | 0.37 (0.26 to 0.48) | –0.068 (–0.22 to 0.09) | 263.97 | 0.20 | 0.24 | 0.33 | 0.53 | 0.66 |
| C+P (n = 90) | 359.28 (310 to 408) | 0.442 (0.33 to 0.56) | Dominated | 0.02 | 0.02 | 0.02 | 0.05 | 0.13 | ||
| PA (n = 97) | 321.46 (282 to 360) | –34.89 (–76 to 6) | 0.423 (0.31 to 0.54) | –0.015 (–0.17 to 0.14) | 0.98 | 0.98 | 0.98 | 0.95 | 0.87 | |
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| C+P (n = 90) | 359.28 (310 to 408) | 0.586 (0.40 to 0.77) | 0.80 | 0.78 | 0.61 | 0.40 | 0.30 | |||
| B+P (n = 100) | 374.54 (324 to 425) | 17.95 (23 to 26) | 0.478 (0.31 to 0.64) | –0.097 (–0.36 to 0.17) | 185.05 | 0.20 | 0.22 | 0.39 | 0.60 | 0.70 |
| C+P (n = 90) | 359.28 (310 to 408) | 0.586 (0.40 to 0.77) | 276.90 | 0.02 | 0.06 | 0.13 | 0.44 | 0.64 | ||
| PA (n = 97) | 321.46 (282 to 360) | –34.89 (–76 to 6) | 0.706 (0.47 to 0.94) | 0.126 (–0.14 to 0.39) | 0.98 | 0.94 | 0.87 | 0.56s | 0.36 | |
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| C+P (n = 262) | 286.71 (266 to 307) | 0.398 (0.33 to 0.46) | 0.68 | 0.68 | 0.67 | 0.64 | 0.61 | |||
| B+P (n = 252) | 291.56 (269 to 314) | 5.85 (–19 to 31) | 0.398 (0.33 to 0.47) | 0.002 (–0.09 to 0.10) | Dominated | 0.32 | 0.32 | 0.33 | 0.36 | 0.39 |
| C+P (n = 262) | 286.71 (266 to 307) | 0.398 (0.33 to 0.46) | 947.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | ||
| PA (n = 257) | 219.19 (201 to 237) | –59.67 (–84 to –35) | 0.452 (0.38 to 0.52) | 0.063 (–0.03 to 0.16) | 1.00 | 1.00 | 1.00 | 1.00 | 0.87 | |
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| C+P (n = 262) | 286.71 (266 to 307) | 0.610 (0.47 to 0.75) | 0.68 | 0.66 | 0.64 | 0.58 | 0.53 | |||
| B+P (n = 252) | 291.56 (269 to 314) | 5.85 (–19 to 31) | 0.599 (0.47 to 0.73) | –0.006 (–0.19 to 0.18) | 975 | 0.32 | 0.34 | 0.36 | 0.42 | 0.47 |
| C+P (n = 262) | 286.71 (266 to 307) | 0.610 (0.47 to 0.75) | 562.92 | 0.00 | 0.00 | 0.00 | 0.11 | 0.43 | ||
| PA (n = 257) | 219.19 (201 to 237) | –59.67 (–84 to –35) | 0.700 (0.47 to 0.75) | 0.106 (–0.08 to 0.29) | 1.00 | 1.00 | 1.00 | 0.89 | 0.57 | |
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| C+P | 305.26 (285 to 325) | 0.603 (0.49 to 0.71) | 0.85 | 0.77 | 0.72 | 0.55 | 0.47 | |||
| B+P | 315.26 (293 to 337) | 10.71 (–11 to 33) | 0.565 (0.46 to 0.67) | –0.031 (–0.18 to 0.12) | 357 | 0.15 | 0.23 | 0.28 | 0.45 | 0.53 |
| C+P | 305.26 (285 to 325) | 0.603 (0.49 to 0.71) | 458.65 | 0.00 | 0.00 | 0.00 | 0.15 | 0.56 | ||
| PA | 247.21 (230 to 265) | –50.91 (–73 to –28) | 0.701 (0.58 to 0.82) | 0.111 (–0.14 to 0.26) | 1.00 | 1.00 | 1.00 | 0.85 | 0.44 | |
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| C+P | 305.26 (285 to 325) | 0.410 (0.35 to 0.47) | 0.85 | 0.82 | 0.78 | 0.69 | 0.57 | |||
| B+P | 315.26 (293 to 337) | 10.71 (–11 to 33) | 0.390 (0.33 to 0.45) | –0.016 (–0.10 to 0.06) | 669.38 | 0.15 | 0.18 | 0.22 | 0.31 | 0.43 |
| C+P | 305.26 (285 to 325) | 0.410 (0.35 to 0.47) | 1183.95 | 0.00 | 0.00 | 0.00 | 0.01 | 0.10 | ||
| PA | 247.21 (230 to 265) | –50.91 (–73 to –28) | 0.445 (0.39 to 0.50) | 0.043 (–0.04 to 0.12) | 1.00 | 1.00 | 1.00 | 0.99 | 0.90 | |
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)b | Effects (97.5% CI) | Incremental effects (97.5% CI)b | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 300) | 206.31 (174 to 239) | 0.410 (0.35 to 0.47) | 0.86 | 0.83 | 0.78 | 0.64 | 0.46 | |||
| B+P (n = 329) | 230.23 (203 to 257) | 24.82 (–13 to 63) | 0.376 (0.32 to 0.43) | –0.033 (0.12 to 0.05) | 752.12 | 0.06 | 0.08 | 0.10 | 0.16 | 0.26 |
| C+P (n = 311) | 230.39 (206 to 255) | –3.95 (–42 to 34) | 0.388 (0.33 to 0.45) | 0.003 (–0.08 to 0.09) | 1316.67 | 0.08 | 0.09 | 0.12 | 0.20 | 0.28 |
| C+P vs. PA | 20.87 (–18 to 59) | –0.030 (–0.12 to 0.05) | 695.66 | 0.10 | 0.12 | 0.15 | 0.25 | 0.36 | ||
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 300) | 206.31 (174 to 239) | 0.641 (0.52 to 0.76) | 0.86 | 0.76 | 0.62 | 0.32 | 0.16 | |||
| B+P (n = 329) | 230.23 (203 to 257) | 24.82 (–13 to 63) | 0.551 (0.44 to 0.66) | –0.087 (–0.24 to 0.07) | 258.29 | 0.06 | 0.10 | 0.15 | 0.27 | 0.33 |
| C+P (n = 311) | 230.39 (206 to 255) | –3.95 (–42 to 34) | 0.550 (0.44 to 0.66) | –0.019 (–0.17 to 0.13) | Dominates B | 0.08 | 0.14 | 0.23 | 0.41 | 0.51 |
| C+P vs. PA | 20.87 (–18 to 59) | –0.106 (–0.26 to 0.050) | 196.89 | 0.10 | 0.18 | 0.29 | 0.56 | 0.75 | ||
| Investigation strategy | Cost (£) (97.5% CI) | Incremental cost (£) (97.5% CI)c | Effects (97.5% CI) | Incremental effects (97.5% CI)c | ICER (£) | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid dental pain and/or dental sepsis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £50 | £100 | £250 | £500 | ||||||
| Incremental cost per incidence of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 261) | 238.35 (201 to 276) | 0.488 (0.42 to 0.56) | 0.85 | 0.81 | 0.77 | 0.62 | 0.43 | |||
| B+P (n = 267) | 258.03 (228 to 288) | 22.01 (–24 to 68) | 0.444 (0.38 to 0.51) | –0.044 (–0.14 to 0.05) | 500.23 | 0.14 | 0.17 | 0.21 | 0.33 | 0.42 |
| C+P (n = 269) | 282.05 (251 to 313) | 22.68 (–23 to 68) | 0.436 (0.37 to 0.50) | –0.008 (–0.10 to 0.09) | 2835 | 0.01 | 0.02 | 0.02 | 0.05 | 0.15 |
| Incremental cost per episode of dental pain and/or dental sepsis avoided | ||||||||||
| PA (n = 261) | 238.35 (201 to 276) | 0.814 (0.67 to 0.96) | 0.85 | 0.71 | 0.54 | 0.22 | 0.06 | |||
| B+P (n = 267) | 258.03 (228 to 288) | 22.01 (–24 to 68) | 0.648 (0.52 to 0.77) | –0.166 (–0.35 to 0.02) | 132.59 | 0.14 | 0.26 | 0.40 | 0.59 | 0.61 |
| C+P (n = 269) | 282.05 (251 to 313) | 22.68 (–23 to 68) | 0.638 (0.51 to 0.77) | –0.011 (–0.20 to 0.17) | 2061.82 | 0.01 | 0.03 | 0.06 | 0.19 | 0.33 |
Appendix 6 Qualitative study (see Chapter 5)
| Code | Participant | Age of the child (years) | Sex | Research arm | FiCTION trial clinical centre | Treatment deviation |
|---|---|---|---|---|---|---|
| LC01 | Child | 5 | Female | PA | Yorkshire | Y |
| LP01 | Parent | Female | ||||
| LC02 | Child | 6 | Male | C+P | Yorkshire | N |
| LP02 | Parent | Female | ||||
| LC03 | Child | 6 | Male | C+P | Yorkshire | Y |
| LP03 | Parent | Male | ||||
| LC04 | Child | 6 | Female | B+P | Yorkshire | N |
| LP04 | Parent | Female | ||||
| LC05 | Child | 6 | Female | B+P | Yorkshire | N |
| LP05 | Parent | Female | ||||
| LC06 | Child | 7 | Female | B+P | Yorkshire | Y |
| LP06 | Parent | Female | ||||
| LC07 | Child | 8 | Male | B+P | Yorkshire | Y |
| LP07 | Parent | Male | ||||
| LC08 | Child | 11 | Male | C+P | Yorkshire | N |
| LP08 | Parent | Female | ||||
| SC01 | Child | 9 | Female | B+P | Scotland | N |
| SP01 | Parent | Female | ||||
| SC02 | Child | 9 | Female | PA | Scotland | N |
| SP02 | Parent | Female | ||||
| SC03 | Child | 9 | Female | PA | Scotland | N |
| SP03 | Parent | Male | ||||
| SC04 | Child | 8 | Male | PA | Scotland | N |
| SP04 | Parent | Female | ||||
| SC05 | Child | 10 | Female | C+P | Scotland | N |
| SP05 | Parent | Female |
| Code | Professional role | Public or general dental service | Sex | Patients recruited (n) | Research experience | Time (years) since qualifying | FiCTION trial region |
|---|---|---|---|---|---|---|---|
| D01 | Dentist | Community | Female | 17 | Yes | 37 | Scotland |
| D02 | Dentist | General | Female | 24 | Yes | 30 | Scotland |
| D03 | Dentist | Community | Male | 4 | No | 17 | Scotland |
| D04 | Dentist | General | Female | 23 | No | 20 | Scotland |
| D05 | Dentist | General | Female | 36 | Yes | 18 | Scotland |
| E01 | Dentist | General | Male | 2 | No | 10 | Scotland |
| G01 | Dentist | General | Female | 24 | Yes | 20 | Scotland |
| L01 | Dentist | Community | Female | 22 | Yes | 14 | Yorkshire |
| L02 | Dental nurse | Community | Female | 22 | No | 25+ | Yorkshire |
| LDN01 | Dentist | General | Male | 37 | No | 27 | South-east |
| N01 | Dentist | General | Male | 20 | No | 17 | North-east |
| N02 | Dentist | General | Female | 14 | Yes | 16 | North-east |
| N03 | Dentist | General | Male | 14 | No | 2 | North-east |
| N04 | Senior dental nurse | General | Female | 30 | No | 15 | North-east |
| N05 | Practice manager | General | Female | 24 | No | 13 | North-east |
| N06 | Dentist | General | Male | 30 | No | 36 | North-east |
| N07 | Dentist | General | Male | 30 | Yes | 16 | North-east |
| N08 | Dentist | General | Female | 15 | No | 7 | North-east |
| N09 | Dental therapist | General | Female | 17 | No | 4 | North-east |
| N10 | Dentist | General | Male | 17 | No | 17 | North-east |
| N11 | Dental therapist | General | Male | 17 | No | 2 | North-east |
| N12 | Dentist | General | Female | 17 | No | 10 | North-east |
| N13 | Dental nurse | General | Female | 17 | Yes | 8 | North-east |
| S01 | Dentist | General | Female | 4 | No | 16 | Yorkshire |
| S02 | Dental nurse | General | Female | 4 | No | Not given | Yorkshire |
| S03 | Practice manager | General | Male | 4 | No | N/A | Yorkshire |
| S04 | Dentist | Community | Female | 22 | Yes | 14 | Yorkshire |
| S05 | Dentist | General | Female | 21 | No | 11 | Yorkshire |
| S06 | Dentist | General | Female | 21 | No | 8 | Yorkshire |
| S07 | Dentist | General | Male | 32 | Yes | 15 | Yorkshire |
| S08 | Practice manager | General | Female | 32 | No | N/A | Yorkshire |
Glossary
- B+P
- Biological management of carious lesions with best-practice prevention.
- C+P
- Conventional management of carious lesions with best-practice prevention.
- MACRO
- An electronic clinical data management system (Elsevier, Amsterdam, the Netherlands).
- PA
- Best-practice prevention alone.
List of abbreviations
- AE
- adverse event
- ART
- atraumatic restorative treatment
- B+P
- biological with prevention
- BSPD
- British Society of Paediatric Dentistry
- CC
- clinical centre
- CD
- compact disc
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- C+P
- conventional with prevention
- CPD
- continuing professional development
- CRF
- case report form
- CSV
- comma-separated values
- DDQ-8
- Dental Discomfort Questionnaire
- DNA
- did not attend
- DVD
- digital versatile disc
- ECOHIS
- Early Childhood Oral Health Impact Scale
- FFS
- fee for service
- FGDP
- Faculty of General Dental Practice
- FiCTION
- Filling Children’s Teeth: Indicated Or Not?
- FPM
- first permanent molar
- GCP
- Good Clinical Practice
- GDP
- general dental practitioner
- GDS
- general dental service
- GIC
- glass ionomer cement
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICDAS
- International Caries Detection and Assessment System
- ICER
- incremental cost-effectiveness ratio
- IDMC
- Independent Data Monitoring Committee
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ISRCTN
- International Standard Randomised Controlled Trials Number
- ITT
- intention to treat
- MCDASf
- Modified Child Dental Anxiety Scale (faces)
- NCTU
- Newcastle Clinical Trials Unit
- NIH
- National Institutes of Health (US)
- NIHR
- National Institute for Health Research
- NRCC
- non-restorative cavity control
- OHRQoL
- oral health-related quality of life
- PA
- prevention alone
- P-CPQ
- Parental–Caregivers Perceptions Questionnaire
- P-CPQ-16
- Parental–Caregivers Perceptions Questionnaire-16 items
- PCRN
- Primary Care Research Network
- PCT
- primary care trust
- PMC
- preformed metal crown
- PP
- per protocol
- p.p.m.F–
- parts per million (fluoride ion)
- PROM
- patient-reported outcome measure
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RMGI
- resin-modified glass ionomer
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SDCEP
- Scottish Dental Clinical Effectiveness Programme
- SUR
- seemingly unrelated regression
- TDF
- treatment deviation form
- TSC
- Trial Steering Committee
- UDA
- unit of dental activity
- VAT
- value-added tax
- WTP
- willingness to pay
