Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 16/51/20. The protocol was agreed in January 2017. The assessment report began editorial review in August 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Fleeman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Thyroid cancer: overview
Thyroid cancer is a rare cancer, representing only 1% of all malignancies in England and Wales. 1 It is caused by the growth of abnormal cells in the thyroid gland. This is a small gland at the base of the neck that secretes three hormones: triiodothyronine (T3), thyroxine (T4) and calcitonin. T3 and T4 control the rate of metabolism in the body, and calcitonin works with the parathyroid hormone to control the amount of calcium in the blood. 2 Thyroid cancer is usually asymptomatic and is often discovered incidentally via imaging studies [e.g. sonograms, computerised tomography (CT) scans and magnetic resonance imaging (MRI)] that are carried out for another reason, or when patients present with a large palpable nodule in the neck. 3 The actual diagnosis of thyroid cancer is usually made using ultrasonography and biopsy (typically, a fine-needle aspiration). 4
The incidence of thyroid cancer is increasing worldwide. 4–10 In the UK, between the period 2003–5 and the period 2012–14, thyroid cancer incidence rates increased by 74% (Figure 1). 1 In 2014, there were 3404 patients diagnosed with thyroid cancer in the UK, 2941 in England and 123 in Wales. 1 The reasons for the increase in incidence are unknown, but are thought to be, at least in part, attributable to improved diagnostic and detection techniques. 11
FIGURE 1.
Average number of new cases per year per 100,000 people in the UK. Based on a graphic created by Cancer Research UK. 1
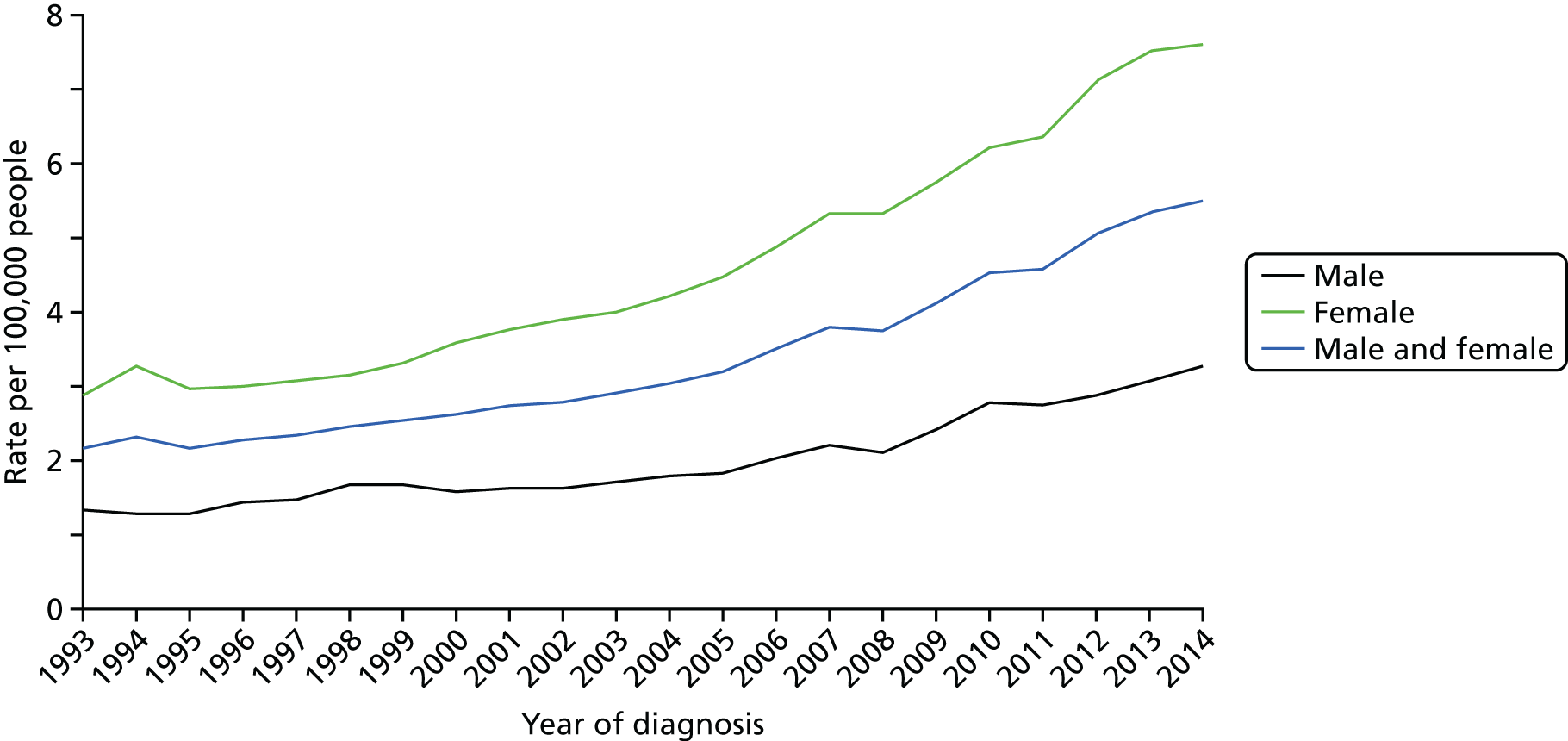
The incidence of thyroid cancer is 2.5 times greater in women than in men. 1 The reasons for this disparity are unclear. 12 Thyroid cancer incidence is strongly related to age, with the highest incidence rates being in older males, and the highest incidence rates among females being in younger and middle-aged women (Figure 2).
FIGURE 2.
Age-specific incidence rates per 100,000 people in the UK. Based on a graphic created by Cancer Research UK. 1

In the UK, thyroid cancer accounts for < 1% of male cancer deaths and < 1% of female cancer deaths. 13 The mortality rate in the UK is reported to be < 1 death per 100,000 people. 13 In 2014, there were 376 thyroid cancer deaths in the UK, 154 (41%) in males and 222 (59%) in females, giving a male-to-female ratio of around 7 : 10. In England and Wales, there were 331 thyroid cancer deaths: 137 in males and 194 in females. 13
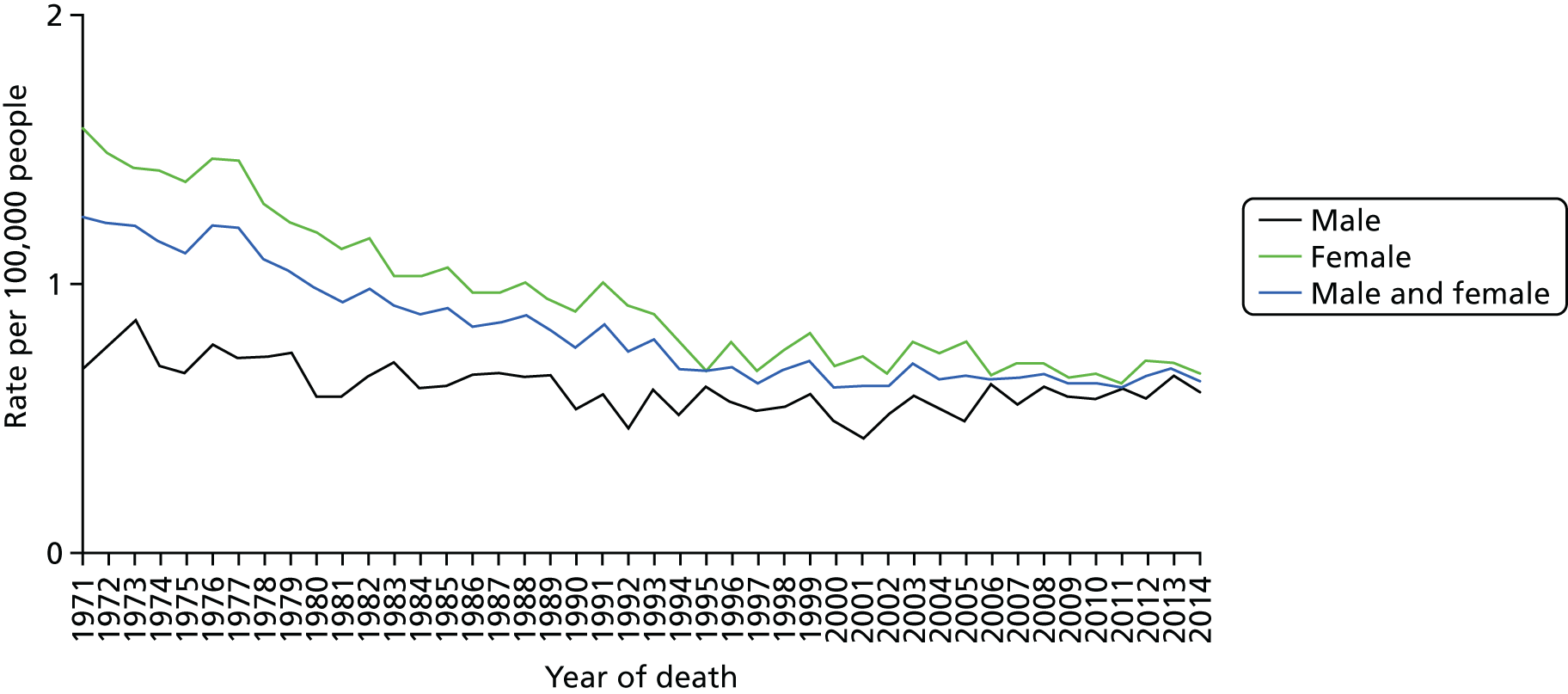
Although the incidence of thyroid cancer in the UK increased between the period 2003–5 and the period 2012–14, overall mortality rates remained stable during this time (Figure 3);13 however, between 1970 and 2014, thyroid cancer mortality rates decreased by 46% in the UK, the decrease being more marked in females (54%) than in males (24%). 13 Mortality rates for thyroid cancer are projected to rise in the future: in the UK, it is expected that between 2014 and 2035 mortality will increase by 7%; however, the overall rate is expected to remain relatively low at 1 death per 100,000 people. 13
FIGURE 3.
European age-standardised thyroid cancer mortality rates in the UK: 1971–2014. Based on a graphic created by Cancer Research UK. 13
Differentiated thyroid cancer
The most common form of thyroid cancer is differentiated thyroid cancer (DTC); DTC is reported to account for approximately 94% of thyroid carcinomas. 14,15 Less common types of thyroid cancer include medullary carcinoma and anaplastic carcinoma; these have been reported to account for approximately 4% and approximately 2% of all thyroid carcinomas, respectively. 15
Differentiated thyroid cancer is a specific type of thyroid cancer made up of different subtypes including papillary carcinoma (PTC), follicular carcinoma (FTC) and Hürthle cell carcinoma. PTC is the most common type of DTC, accounting for approximately 83%15 to 86%16 of all cases; FTC accounts for approximately 10%16 to 13%,15 and Hürthle cell carcinoma accounts for approximately 3%15 to 4%. 16 Hürthle cell carcinomas are usually grouped with FTCs because they present and behave similarly. 17
The median age for all patients with DTC is reported to be 45 years;18 however, estimates of the median age at onset for the subtypes of DTC have been reported to vary:
-
Papillary carcinoma often affects people aged < 40 years,17 but it is also reported that the median age of patients with PTC is 45 years. 19
-
The peak age for the onset of FTC has been stated to be between 40 and 60 years,20 but, again, the median age has been reported to be approximately 45 years. 21
-
The median age of patients with Hürthle cell carcinoma has been reported to be 55 years. 21
In general, the prognosis for patients with DTC is relatively good. The overall 10-year survival rate for middle-aged adults is reported to be 80–90%. 4 It has also been reported that > 85% of patients with DTC have a ‘normal’ life expectancy;22 however, the prognosis generally gets worse with increasing age at the time of diagnosis, particularly for patients aged ≥ 45 years. 4 In addition, young children (aged < 10 years) are at higher risk of recurrence than older children. 4 Prognosis may also be affected by DTC subtype (histology). An analysis of US National Cancer Database data on 41,375 patients with DTC who were treated between 1985 and 199515 has shown that the 10-year relative survival for patients with PTC is 93%, whereas for patients with FTC it is 85%, and for patients with Hürthle cell carcinoma it is 76%.
The size and spread of the tumour affect prognosis. Studies cited by the British Thyroid Association (BTA)4 are reported to show that the risk of recurrence and mortality correlates with the size of the primary tumour. Extrathyroidal invasion, lymph node metastases and distant metastases are also reported to be important prognostic factors. 4
First-line treatment options for patients with differentiated thyroid cancer
There are currently no National Institute for Health and Care Excellence (NICE) guidelines and no NICE guidance for treating patients with DTC or any other type of thyroid cancer. Other clinical guidelines do present some recommendations. In chronological order from date of publication, relevant clinical guidelines include the European Society for Medical Oncology (ESMO) guidelines (2012),23 BTA guidelines (2014),4 American Thyroid Association guidelines (2015)24 and National Comprehensive Cancer Network (NCCN) guidelines (2017). 25
Owing to the indolent course of the disease, many patients with DTC, even if they have metastatic disease, do not require therapy for several years after diagnosis. 26 Treatments for DTC depend on factors including age, extent of disease, and histology, but usually involve surgery to remove all or part of the thyroid gland (thyroidectomy) followed by lifelong thyroxine for thyroid-stimulating hormone (TSH) suppression from the low–normal to fully suppressed range dependent on risk factors. 4,23–25
Treatment options for patients with differentiated thyroid cancer that has progressed following surgery
Following initial surgery, it is estimated that between 5% and 20% of patients with DTC develop local or regional recurrences (approximately two-thirds of these involve cervical lymph nodes27) and between 10% and 15% of patients with DTC develop distant metastases. 4,24 The most common sites for metastases are reported to be the lungs (50%), bones (25%), lungs and bones (20%) or other (5%). 24 It has been noted that the presence of bone metastases has been associated with a worse prognosis than metastases in other sites. 23
The sites that DTC is most likely to spread to vary by histology. For patients aged > 40 years, it has been reported that 10% of patients with PTC, 25% of patients with FTC and 35% of patients with Hürthle cell carcinoma develop distant metastases. 28,29 PTC tends to spread to lymph nodes in the neck, whereas FTC usually spreads to the bones or lungs. 17 Hürthle cell carcinoma is more likely than FTC to spread to lymph nodes in the neck. 30
A radioactive iodine uptake test is commonly used to determine whether or not DTC has spread. The test involves a patient being given a liquid or capsule containing radioactive iodine (iodine-123) to swallow. Two separate uptake measurements are then commonly obtained at different time points within a 24-hour period. The patient is then scanned to see how much of this radioactive iodine has been absorbed by the thyroid (radioactive uptake). Positive results (evidence of iodine-123 uptake) denote the presence of disease, whereas negative results (no radioactive uptake) denote the absence of disease.
It is recommended in clinical guidelines4,23–25 that patients with DTC and evidence of radioactive iodine uptake should undergo treatment with radioactive iodine (also known as radioactive iodine ablation) to treat residual, recurrent or metastatic disease. Patients are typically tested 1–2 months after surgery. Radioactive iodine treatment has been used for > 60 years. It is administered in hospital (during an inpatient stay) and can be given to patients on more than one occasion, as necessary. 4
Like the radioactive iodine uptake test used to diagnose DTC, radioactive iodine treatment involves swallowing radioactive iodine in either liquid or capsule form; however, the radioactive iodine is a different form (iodine-131) to that used for scans (iodine-123): the purpose of radioactive iodine treatment is to destroy cancerous cells. Thus, patients with iodine-131 uptake are responsive to treatment, which can be confirmed by imaging studies.
Approximately 33% of patients with advanced disease can be cured and many others achieve long-term disease stabilisation. 31 From published French registry data,32 the 10-year survival rate for patients with distant metastases who successfully responded to treatment with radioactive iodine is 92%. 32
Radioactive iodine-refractory differentiated thyroid cancer
Although for many patients radioactive iodine is an effective treatment, some patients become resistant to the treatment (decreased or no radioactive iodine uptake) or are unable to safely tolerate additional doses. These patients are considered to have radioactive iodine-refractory DTC (RR-DTC) and are the focus of this multiple technology appraisal (MTA).
Although clinical criteria and algorithms have been developed and reported in clinical guidelines,4,23–25 there is no agreed precise definition of RR-DTC;33 however, a review of the literature published in February 201731 highlights key features that can be considered in defining RR-DTC:
-
Metastatic disease that does not take up radioactive iodine at the time of the first radioactive iodine treatment.
-
Ability to take up radioactive iodine has been lost after previous evidence of uptake of radioactive iodine.
-
Radioactive iodine uptake is retained in some lesions but not in others.
-
Metastatic disease that progresses despite substantial uptake of radioactive iodine.
-
Absence of complete response to treatment after > 600 mCi of cumulative activity of radioactive iodine.
-
Evidence of high uptake of fludeoxyglucose (FDG) 18F on positron emission tomography or CT scan; however, importantly, the authors of this review31 state that this reason alone should not be used to abandon radioactive iodine treatment.
Before deciding whether or not a patient’s disease can be described as being RR-DTC, it is important to determine that decreased radioactive iodine uptake is not due to iodine contamination or insufficient TSH. 34
Radioactive iodine-refractory DTC is a life-threatening form of thyroid cancer with a tendency to progress and metastasise. 14 From published French registry data,32 the 10-year survival rate and median overall survival (OS) for patients with distant metastases who failed to respond to treatment (no iodine-131 uptake) was 10% and 3 years, respectively. For those who appear to respond to radioactive iodine treatment (iodine-131 uptake) but who did not then attain negative imaging studies, the 10-year survival and median OS was 29% and 6 years, respectively. A separate analysis of patients with lung and/or bone metastases35 found that 10-year survival and median OS for those who did not have a complete response to treatment with radioactive iodine was 14% and 5 years, respectively. Data from Canada5 have suggested that the median OS for patients with RR-DTC may be between 2.5 and 3.5 years.
The proportion of patients whose disease becomes refractory to treatment with radioactive iodine is relatively small, and so RR-DTC is described as an ultra-orphan condition. 7,8 Estimates of the proportion of patients who become refractory vary but commonly lie within the range of 5–15%. 7,8,14,16,32,35–37
As with early-stage DTC, many patients with RR-DTC are initially asymptomatic. As highlighted in a literature review published by Schmidt et al.,31 even patients with distant metastases may have a disease that does not progress for many years; however, as noted by Thyroid Cancer Canada,5 the cancer continues to progress with no obvious symptoms.
For patients with rapidly progressing disease, which is characterised by symptomatic disease, the symptoms of RR-DTC can be severe, profoundly debilitating and result in patients becoming increasingly dependent on carers. 8 Clinical advice to the Assessment Group (AG) is that the percentage of patients with RR-DTC with rapidly progressing disease is likely to be approximately 25% to 30%. As a result of their symptoms, patients with clinically significant progressive RR-DTC may suffer a poor quality of life and the psychological impact of the disease can also be substantial, resulting in low mood and fatigue. 38 It has also been stated that patients with RR-DTC often experience multiple complications. 39
Treatment options for patients with radioactive iodine-refractory differentiated thyroid cancer
Radioactive iodine-refractory DTC is typically asymptomatic, but symptoms start to occur as the disease progresses. Symptoms associated with lymph nodes of the neck include difficulty swallowing and/or breathing, pain or sensitivity in the front of the neck or throat, hoarseness or other voice changes, and swelling of the lymph nodes in the neck. 4 Symptoms associated with lung metastases also include swallowing and breathing difficulties. 26 Pain often presents as the principal symptom of metastatic bone involvement. 29,40 Fractures and spinal cord compression are also associated with bone metastases.
Because many treatments, particularly systemic treatments, can have severe side effects and impact significantly on health-related quality of life (HRQoL), clinical advice to the AG is that best supportive care (BSC) tends to be the preferred treatment option, at least until symptoms occur. BSC typically entails TSH suppression therapy and imaging every 3 to 12 months. Palliative radiotherapy and symptom relief are also offered when necessary.
Patients experiencing RR-DTC symptoms and/or patients with rapidly progressing disease are those in need of systemic treatment,31 as reflected in clinical guidelines. 4,23–25 The aim of systemic treatment for patients with rapidly progressing and/or symptomatic RR-DTC is to gain local disease control in the neck and manage systemic disease. 41 Another important objective of treatment is to prolong survival;27 however, treatment options for patients with RR-DTC are limited. Within the ESMO guidelines published in 2012,23 it is stated that chemotherapy should not be given to patients with RR-DTC as it is associated with significant toxicity with no proven evidence of effectiveness. The authors of these guidelines stated that surgical resection and external beam radiotherapy represented the only therapeutic options and they strongly encouraged enrolment of patients in experimental trials with targeted therapy. Similarly, the authors of the guidelines published by the BTA in 20144 only recommended chemotherapy for patients with rapidly progressive, symptomatic RR-DTC who have good performance status (PS), and only when access to targeted therapies in clinical trials is unavailable or when targeted therapies have proved unsuccessful. The authors of the more recent US guidelines published by the American Thyroid Association24 and NCCN25 recommend that patients with RR-DTC should usually avoid treatment with chemotherapy. Clinical advice received by the AG is that chemotherapy is rarely used to treat RR-DTC in UK NHS practice.
Targeted therapies were not widely available and were only the subject of clinical trials between 2012 and 2014, when the ESMO guidelines23 and the BTA guidelines4 were published. The authors of the BTA guidelines4 considered the most promising targeted therapies at that time to be lenvatinib and sorafenib. 4 By 2017, the authors of the NCCN guidelines25 recommended lenvatinib or sorafenib as the treatment of choice for patients with progressive and/or symptomatic disease; lenvatinib is stated to be the ‘preferred’ option based on a response rate of 65% for lenvatinib, compared with 12% for sorafenib, although these agents have not been directly compared. However, the authors state that the decision should be based on the individual patient, taking into account the likelihood of response and comorbidities. 25 In cases in which lenvatinib or sorafenib are not available or not appropriate, drugs not regulated by the US Food and Drug Administration (FDA) but used in the context of clinical trials are also recommended by the authors of the NCCN guidelines. 25
Description of technology under assessment
The two interventions under consideration in this MTA are lenvatinib (Lenvima), manufactured by Eisai Ltd, and sorafenib (Nexavar), manufactured by Bayer HealthCare. Both are a type of tyrosine kinase inhibitor (TKI) known as a multikinase inhibitor (MKI).
A brief comparison of the key features of the two interventions is given in Table 1. The AG notes that lenvatinib and sorafenib appear to have slightly different mechanisms of action. 42 Both drugs have been approved for treating RR-DTC in the USA43,44 and Europe,49,50 with sorafenib being the first of the two agents to be approved in both jurisdictions. In the USA and Europe, the marketing indications for both lenvatinib and sorafenib are for identical patient populations. Approval in the USA and Europe was based largely on evidence from two Phase III randomised controlled trials (RCTs): SELECT,51 in which lenvatinib was compared with placebo, and DECISION (StuDy of sorafEnib in loCally advanced or metastatIc patientS with radioactive Iodine-refractory thyrOid caNcer),52 in which sorafenib was compared with placebo.
| Feature | Lenvatinib | Sorafenib |
|---|---|---|
| Brand name | Lenvima | Nexavar |
| Manufacturer | Eisai Ltd | Bayer HealthCare |
| Class of drug | Oral MKI | Oral MKI |
| Mechanism of action | Targets VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, FGFR3, FGFR4, PDGFR alpha, PDGFR beta, RET and KIT42 | Targets BRAF, RET, VEGFR2 and VEGFR342 |
| US marketing indication | For the treatment of locally recurrent or metastatic, progressive, RR-DTC (15 February 2015)43 | For the treatment of locally recurrent or metastatic, progressive, differentiated thyroid carcinoma refractory to radioactive iodine treatment (22 November 2013)44 |
| European Union marketing indication | For the treatment of adult patients with progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hürthle cell) thyroid carcinoma, refractory to radioactive iodine (28 May 2015)45 |
For the treatment of patients with progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hürthle cell) thyroid carcinoma, refractory to radioactive iodine (25 January 2015)46 In addition to RR-DTC, sorafenib is also indicated for treatment of hepatocellular carcinoma and the treatment of advanced renal cell carcinoma46 |
| Dose information for treating RR-DTC |
24 mg (two 10-mg capsules and one 4-mg capsule) once daily AEs can be managed through dose reduction and treatment is continued until disease progression or unacceptable toxicity45 |
400 mg (two 200-mg tablets) twice daily, taken without food or with a low-fat meal AEs can be managed through dose reduction and treatment is continued until disease progression or unacceptable toxicity46 |
| Important identified risks |
Important risks highlighted by the EMA27 include hypertension, proteinuria, renal failure or impairment, hypokalaemia, cardiac failure, posterior reversible encephalopathy syndrome, hepatotoxicity, haemorrhagic events, arterial thromboembolic events, QTc prolongation and hypocalcaemia Further information, including how to manage some of the risks (e.g. the use of hypertensives for hypertension) is provided in the SmPC46 |
Important risks highlighted by the EMA26 include severe skin AEs; hand–foot syndrome; hypertension; posterior reversible encephalopathy syndrome; haemorrhage including lung haemorrhage, gastrointestinal haemorrhage and cerebral haemorrhage; arterial thrombosis (myocardial infarction); congestive heart failure; squamous cell cancer of the skin; gastrointestinal perforation; symptomatic pancreatitis and increases in lipase and amylase; hypophosphataemia; renal dysfunction; interstitial lung disease-like events; and drug-induced hepatitis Further information, including how to manage some of the risks (e.g. the use of topical therapies, temporary treatment interruption and/or dose modification or treatment discontinuation for hand–foot syndrome) is provided in the SmPC46 |
| List price per pack | £1437.00 for a pack of 30 4-mg capsules and £1437.00 for a pack of 30 10-mg capsules8 | £3576.56 for a pack of 112 200-mg tablets47 |
| Cost per yeara | £52,30738 | £38,74648 |
Approval for use in NHS Scotland was granted to sorafenib in June 201548 and to lenvatinib in September 2016. 38 Both approvals are for the treatment of patients with progressive, locally advanced or metastatic RR-DTC. In NHS Scotland, the use of both lenvatinib and sorafenib is contingent on the continuing availability of Patient Access Scheme (PAS) prices that have been assessed by the PAS Assessment Group.
Sorafenib has been available in England, since July 2016, via the Cancer Drugs Fund (CDF). It is currently funded for all patients with RR-DTC for whom the treating specialist has established that treatment with sorafenib may be beneficial. According to Bayer HealthCare, based on its analysis of notification data from July 2013 to June 2016, sorafenib has become the standard of care for patients for whom systemic treatment is appropriate. 7 Lenvatinib is not currently available to patients treated in the English or Welsh NHS.
Eisai Ltd8 has estimated the incidence of patients in England and Wales with RR-DTC who are potentially eligible for treatment with lenvatinib or sorafenib to be approximately 280 each year. Bayer HealthCare7 has estimated the incidence to be approximately 225 patients per year. The AG notes that the estimates given by the companies differ in how they are calculated. The estimates provided by the companies are reflective of the population defined by the agreed final scope of this appraisal; however, neither estimate appears to account for the fact that lenvatinib and sorafenib are likely to only be preferred for patients with symptomatic and/or rapidly progressing disease. Clinical advice to the AG is that there are no generally agreed definitions of ‘symptomatic’ or ‘rapidly progressive disease’ and that, in clinical practice, definition of a patient’s disease status depends on individual patient characteristics. Therefore, it is difficult to further segment the population.
Chapter 2 Definition of the decision problem
Decision problem addressed by the Assessment Group
The decision problem for this appraisal, as described in the final scope issued by NICE,53 is summarised in Table 2.
| Parameter | In the NICE scope53 | Addressed by the AG |
|---|---|---|
| Interventions |
Lenvatinib Sorafenib |
As per scope |
| Population | Adults with progressive, locally advanced or metastatic, differentiated thyroid carcinoma refractory to radioactive iodine | As per scope |
| Comparators |
The interventions listed above will be compared with each other BSC |
Explore the feasibility of comparing lenvatinib with sorafenib Comparisons of interventions with BSC |
| Outcomes | The outcome measures to be considered include:
|
As per scope |
| Economic analysis |
The reference case stipulates that the cost-effectiveness of treatments should be expressed in terms of incremental cost per quality-adjusted life-year The reference case stipulates that the time horizon for estimating clinical effectiveness and cost-effectiveness should be sufficiently long to reflect any differences in costs or outcomes between the technologies being compared Costs will be considered from a NHS and Personal Social Services perspective |
As per scope |
| Other considerations |
If the evidence allows, consideration will be given to subgroups based on previous treatment with TKIs Guidance will only be issued in accordance with the marketing authorisation. When the wording of the therapeutic indication does not include specific treatment combinations, guidance will be issued only in the context of the evidence that has underpinned the marketing authorisation granted by the regulator |
As per scope |
The overall aims and objectives of the assessment
The aim of this research was to assess the clinical effectiveness and cost-effectiveness of lenvatinib versus sorafenib, within their respective EU marketing authorisations,45,46 for the treatment of patients with RR-DTC. The research objectives are given below:
-
To carry out systematic reviews to compare the clinical effectiveness and cost-effectiveness of –
-
treatment with lenvatinib with treatment with sorafenib for RR-DTC
-
treatment with lenvatinib with BSC for RR-DTC
-
treatment with sorafenib with BSC for RR-DTC.
-
-
To develop an economic model to compare the cost-effectiveness of –
-
treatment with lenvatinib with treatment with sorafenib for RR-DTC
-
treatment with lenvatinib with BSC for RR-DTC
-
treatment with sorafenib with BSC for RR-DTC.
-
Chapter 3 Methods for reviewing clinical effectiveness literature
Search strategy
The AG identified clinical studies and systematic reviews by searching EMBASE, MEDLINE, PubMed and The Cochrane Library from 1999 onwards. All databases were searched on 10 January 2017. Based on the fact that the FDA approved sorafenib for its first indication in 2005, and lenvatinib in 2015, the AG considered that this date span would allow all relevant clinical evidence to be identified. Searches were restricted to publications in English language. The AG did not use any other search filters. The search strategies used by the AG are provided in Appendix 1. In addition to the electronic database searches, information on studies in progress was sought (on 16 May 2017) by searching the ClinicalTrials.gov website, the International Clinical Trials Registry Platform and the European Union Clinical Trials Register. The references in the systematic reviews included in the AG’s review of systematic reviews, and those listed in the submissions from professional stakeholders that were submitted to NICE, as part of the NICE MTA process, were cross-checked to identify any relevant studies not retrieved from the electronic database searches. Literature search results were uploaded to and managed using EndNote X7.4 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] software.
Study selection
The eligibility criteria listed in Table 3 were used to identify studies for inclusion in the AG’s literature review.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Patient population | Adults with progressive, locally advanced or metastatic, differentiated thyroid carcinoma refractory to radioactive iodine | Patients with other types of thyroid cancer or diseases |
| Interventions | Lenvatinib or sorafenib monotherapy (or in combination with BSC) | Lenvatinib or sorafenib in combination with other agents |
| Comparators | Lenvatinib or sorafenib monotherapy (or in combination with BSC), BSC and placebo | A comparator other than lenvatinib, sorafenib, BSC and placebo |
| Outcomes | The outcome measures to be considered include OS, progression-free survival, response rate, adverse effects of treatment and HRQoL | No study was excluded based on outcomes |
| Study design | RCTs, systematic reviews and prospective observational studies | Retrospective cohort studies, case series, case reports, comments, letters, editorials, in vitro, animal and genetic or histochemical studies |
| Restrictions | English language only | Non-English-language studies |
Two reviewers (JH and RH) independently screened all titles and abstracts that were identified in the initial searches (screening stage 1). Based on the titles and abstracts, full-text papers that appeared to be relevant were obtained and assessed for inclusion by the same two reviewers in accordance with the AG’s eligibility criteria (screening stage 2). When necessary, discrepancies were resolved by consultation with a third reviewer (NF). At both stages of screening, studies that did not meet the inclusion criteria were excluded, and, at screening stage 2, the reasons for excluding studies were noted.
The eligibility criteria in Table 3 differ slightly from those specified in the AG’s systematic review protocol. 55 The AG, responding to a suggestion from NICE in relation to the final protocol,55 agreed to include evidence from prospective observational studies that had been submitted to the European Medicines Agency (EMA); however, as only reviewing studies included in the EMA submissions26,27 would have introduced selection bias, the AG included all prospective observational studies of patients with RR-DTC identified by its searches.
Data extraction and quality assessment strategy
Data relating to RCT study characteristics and outcomes were extracted by one reviewer (NF) and independently checked for accuracy by a second reviewer (YD). Data relating to study characteristics and outcomes of systematic reviews and observational studies were extracted by one reviewer (JH or NF) and independently checked for accuracy by a second reviewer (JG). In all cases, a consensus was reached. Study data reported in multiple publications were extracted and reported as a single study. Data were extracted into tables in Microsoft Word (Microsoft Corporation, Redmond, WA, USA).
As specified in the AG’s systematic review protocol,55 the quality of included RCTs and systematic reviews was assessed according to the criteria set out in the Centre for Review and Dissemination’s guidance56 for undertaking reviews in health care. The quality of the included RCTs was assessed by one reviewer (YD) and independently checked for agreement by a second reviewer (NF). In all cases, a consensus was reached. The quality of the included systematic reviews was assessed by one reviewer (JG) and independently checked for agreement by a second reviewer (YD). When necessary, discrepancies were resolved by consultation with a third reviewer (MR).
Methods of analysis/synthesis
The AG’s data extraction and quality assessment results are presented in structured tables and as a narrative summary. Data from RCTs are considered to provide primary clinical effectiveness evidence, with data from systematic reviews and observational studies considered to provide supporting evidence.
As the available evidence did not include two or more RCTs comparing the same intervention, the AG was not able to conduct a meta-analysis of RCT data.
The AG assessed the feasibility of conducting an indirect comparison of effectiveness data (including a comparison to assess effectiveness according to previous treatment with TKIs) by evaluating the clinical and methodological heterogeneity of the included RCTs. Heterogeneity was assessed by comparing (1) trial characteristics, (2) participant characteristics, (3) outcome data and (4) study quality.
Chapter 4 Findings from the systematic review of clinical effectiveness literature
Quantity and quality of research available
Included studies
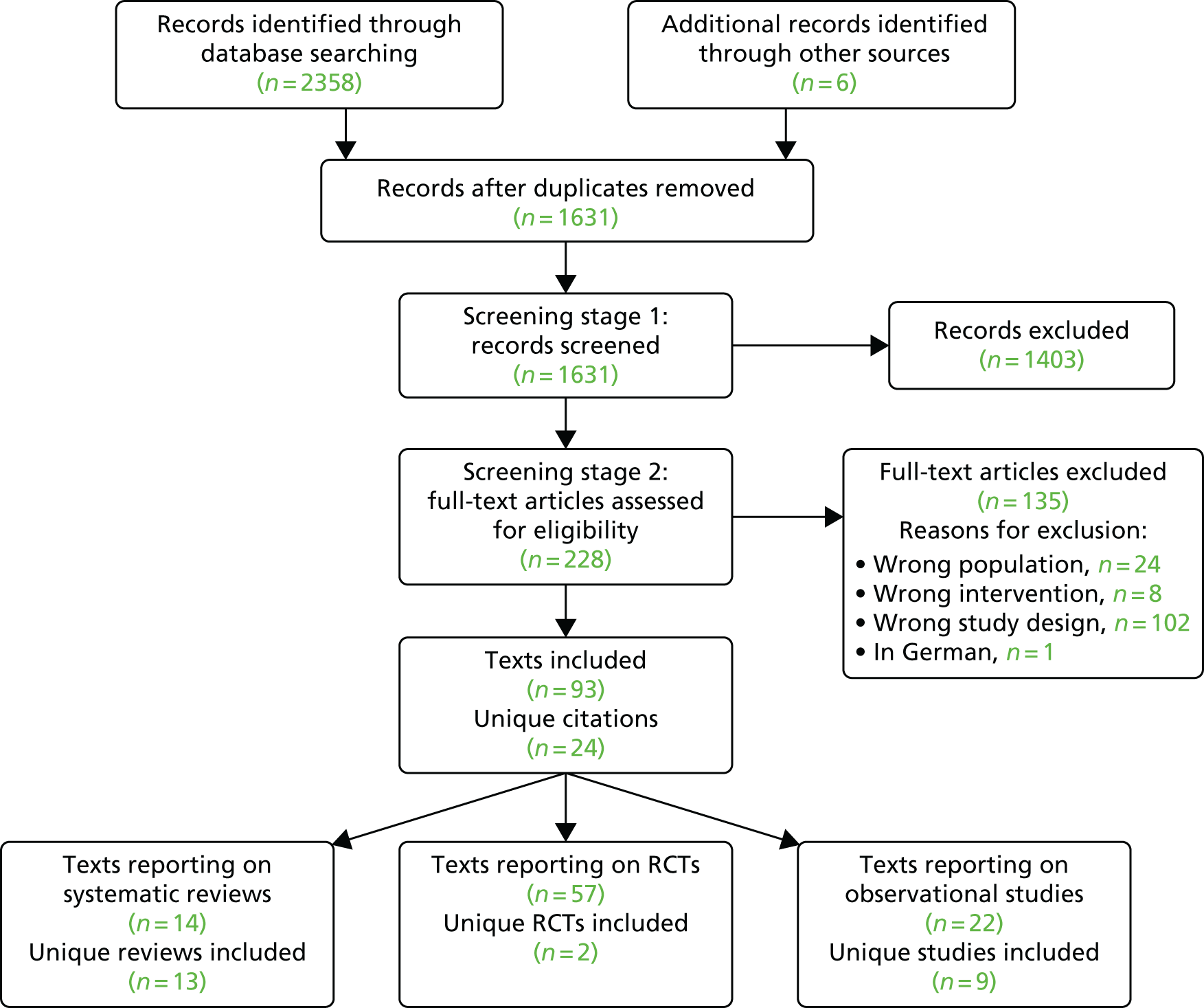
The process of study selection is shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram in Figure 4. The electronic searches yielded 2358 papers, and six additional references5–8,57,58 were identified through other sources. In total, the AG included 93 papers5–8,33,51,52,57–142 reporting on 24 separate studies and reviews: two unique RCTs,51,52 13 unique systematic reviews5–8,33,57,61,93,97,104,127,138,141 and nine unique prospective observational studies. 59,77,78,81,88,101,103,126,135
FIGURE 4.
The PRISMA flow diagram: studies included in AG’s systematic review. Reproduced with permission from Fleeman et al. 54 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
Excluded studies
A full list of studies excluded at stage 2 with reasons for exclusion is presented in Appendix 2, Table 32.
Evidence from randomised controlled trials
Only two RCTs were identified as relevant for inclusion in the AG’s systematic review: SELECT and DECISION. Except when stated otherwise, all information about these two trials has been extracted from the two key trial publications. 51,52
Trial characteristics
A summary of the characteristics of the two included trials is provided in Table 4. Both trials were Phase III, multicentre, double-blind RCTs designed to compare the intervention of interest (lenvatinib or sorafenib) with placebo. Subjects were randomised in a ratio of 2 : 1 to the intervention and comparator arms of SELECT, whereas they were randomised in a ratio of 1 : 1 in DECISION. In both trials, the primary outcome was progression-free survival (PFS) assessed by blinded independent review. Both trials also reported investigator-assessed PFS. Unless otherwise specified, in the remainder of this AG report on clinical effectiveness, PFS refers to PFS assessed by blinded independent review.
| Parameter | Study | |
|---|---|---|
| SELECT | DECISION | |
| Primary reference | Schlumberger et al. 201551 | Brose et al. 201452 |
| Number of centres | 117 | 81 |
| Stratification factors | Subjects were stratified by age (≤ 65 years or > 65 years), geographical region (Europe, North America or other) and receipt or non-receipt of prior VEGFR-targeted therapy (0 or 1) | Subjects were stratified by age (< 60 years or ≥ 60 years) and geographical region (North America, Europe or Asia) |
| Country | Centres were distributed as follows: Europe, 60 (51.3%); North America, 31 (26.5%); Asia Pacific, 13 (11.1%); Japan, 6 (5.1%); and Latin America, 7 (6.0%) | 18 countries from Europe (59.7%) (Austria, Belgium, Bulgaria, Denmark, France, Germany, Italy, Poland, Russia, Spain, Sweden, the Netherlands and the UK), the USA (17.3%) and Asia (23%) (China, Japan, South Korea and Saudi Arabia) |
| Recruitment period | 5 August 2011 to 4 October 2012 | 5 November 2009 to 29 August 2012 |
| Participants (n) | 612 assessed, 392 randomised | 556 enrolled, 419 randomised |
| Intervention dose and schedule | Lenvatinib 24 mg (two 10-mg capsules and one 4-mg capsule) continuous once daily (n = 261) | Sorafenib 400 mg (two 200-mg tablets) twice daily for a total daily dose of 800 mg (n = 207) |
| Comparator arm (n) | Placebo: 131 | Placebo: 210 |
| Primary outcome | PFS, assessed every 8 weeksa and determined by blinded independent imaging review conducted by the imaging core laboratory using RECIST 1.1 | PFS, assessed every 8 weeks by central independent blinded review using RECIST 1.0 |
| Relevant secondary outcomes |
|
|
| Primary analysis | ≥ 214 progression events or deaths | ≈267 progression events |
| Data cut-off points | November 2013, June 2014 and August 2015 | August 2012, May 2013 and July 2015 |
Analysis of clinical efficacy
All efficacy outcomes from both trials, including tumour response evaluations in SELECT, were undertaken using data from the intention-to-treat (ITT) population. Tumour response evaluations in DECISION were undertaken using data from the per-protocol population (i.e. randomised patients who were evaluable for tumour response with imaging data, had received the intervention or placebo as allocated and had no major protocol deviations).
Analysis of safety
Safety analyses for both trials were undertaken using data from the population of patients who were randomised and received at least one dose of study drug and had at least one post-baseline safety evaluation. In SELECT, the numbers of patients included in the ITT and safety populations were identical.
Patients eligible for inclusion
A summary of the criteria describing patient eligibility for entry into SELECT and DECISION is presented in Appendix 3, Table 33. Both trials only included patients with RR-DTC and who had an Eastern Cooperative Oncology Group (ECOG) PS of 0–2. As highlighted in Chapter 1, Radioactive iodine-refractory differentiated thyroid cancer, there is no universally agreed definition of RR-DTC. The definitions used to define RR-DTC in the two trials were broadly similar (see Appendix 3, Table 34, for definitions employed by the trials for RR-DTC).
The main difference in trial eligibility was that SELECT permitted the enrolment of patients who had been previously treated with a vascular endothelial growth factor receptor (VEGFR)-targeted therapy (including sorafenib) and DECISION did not. Age, region and VEGFR-targeted therapy were stratification factors in SELECT, whereas age and region were stratification factors in DECISION.
Dose modifications/interruptions and concomitant therapy
In both trials, the starting dose for treatment with lenvatinib or sorafenib was the licensed dose (24 mg and 800 mg, respectively). Both trials permitted dose modifications or interruptions. The criteria were not stated in the protocol for SELECT but the summary of product characteristics (SmPC)45 includes a dose/toxicity management plan for lenvatinib. For DECISION, Brose et al. 72 stated that dose modifications or interruptions were allowed, based on specific criteria, for grade 2 to grade 3 hand–foot syndrome and other adverse events (AEs).
A summary of the concomitant therapies permitted and prohibited in each trial is presented in Appendix 3, Table 35. Although neither trial describes BSC for patients in either arm, permitted concomitant therapies could be considered to be BSC and were available to patients in both arms of both trials. The main difference between the two trials is that palliative radiotherapy, which is commonly available as part of BSC in UK NHS clinical practice, was not permitted in either arm of SELECT.
Subgroup analyses
In SELECT, subgroup analyses were prespecified for patients previously treated with a VEGFR-targeted therapy and for those who were not. Both trials also included prespecified subgroup analyses for age, region, sex and histology. Subgroup analyses were prespecified for PFS, OS and objective tumour response rate (ORR) in SELECT but only for PFS in DECISION. Other prespecified subgroup analyses in SELECT were for race and for patients whose TSH level was highest prior to progression. Other prespecified subgroup analyses in DECISION included site of metastasis, FDG take-up, prior radioactive iodine cumulative dosing, tumour burden as measured by number of target or non-target lesions and as measured by the sum of target diameters. Many other post hoc subgroup analyses were also conducted for both trials (see Appendix 3, Table 37).
Follow-up, dose intensity and treatment crossover and other subsequent therapy received
At the time of the primary data cut-off points for both trials, OS data were immature. Therefore, for both trials, OS was updated at two subsequent data cut-off points. The median duration of follow-up at each data cut-off point was approximately 17 months at the first data cut-off point in both trials and there were approximately 20 months of additional follow-up in both trials by the final data cut-off point (see Appendix 3, Table 36).
Patients were eligible to receive treatment (intervention or placebo) in both trials until disease progression. An important feature of both trials is that, on disease progression, patients were unblinded and permitted to cross over from the placebo arm to the active treatment arm. In both trials, patients who crossed over were entered into an open-label extension phase of the same trial. In DECISION, patients who had progressed on sorafenib were also eligible to enter the open-label extension phase of the trial and receive further sorafenib until further disease progression. However, patients who progressed on lenvatinib in SELECT were not permitted to receive additional lenvatinib in the open-label extension phase. Information on treatment crossover and subsequent treatment received is reported in Table 5; it is evident that the majority of patients in both placebo arms, and particularly in the placebo arm of SELECT, crossed over to receive lenvatinib or sorafenib.
| Characteristic | Study, n (%) | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| Patients who crossed over: first data cut-off point | N/A | 109 (83.2) | 55 (26.6)a | 150 (71.4) |
| Patients who crossed over: second data cut-off point | N/A | 115 (87.8) | NR | 157 (74.8) |
| Patients who crossed over: third data cut-off point | N/A | 115 (87.8) | NR | 158 (75.0) |
In addition, some patients received subsequent anti-cancer treatments, not part of the trial protocols, on disease progression (see Appendix 3, Table 39). In SELECT, at the first data cut-off point (November 2013), 15.7% of patients randomised to lenvatinib and 12.2% of patients randomised to placebo received subsequent treatment. In DECISION, at the first data cut-off point (August 2012), 20.3% of patients randomised to sorafenib and 8.6% of patients randomised to placebo received subsequent treatments. For the most part, subsequent treatment in both trials comprised antineoplastic and immunomodulating agents. The specific antineoplastic and immunomodulating agents were reported only for SELECT, as data were not collected on the specific agents used during the trial follow-up for DECISION. Most commonly, patients received pazopanib (Votrient®, Novartis) (17.1% and 18.8% of patients who received subsequent therapy in the lenvatinib and placebo arms, respectively) and/or sorafenib (14.6% and 12.5% of patients who received subsequent therapy in the lenvatinib and placebo arms, respectively).
Methods used for adjusting for treatment crossover
As patients in both trials were permitted to cross over to receive the intervention drug on disease progression, the OS results are likely to be confounded. The authors of the SELECT publication51 employed the rank-preserving structural failure time model (RPSFTM) to adjust the OS results for patient crossover. The OS results from DECISION have been adjusted using both the RPSFTM and the iterative parameter estimation (IPE). The unadjusted and adjusted OS analyses have been reported in conference abstracts for SELECT,87 DECISION58,68,110 and in the company submissions. 7,8
As patients were not censored when they received postprogression treatments, the RPSFTM and IPE methods implicitly included all subsequent therapies as an inherent part of the intervention/control treatment effect. In other words, it is assumed that the subsequent therapy administered to patients in each arm of the trial is reflective of the subsequent therapy that would have been offered to patients receiving the same treatment in clinical practice.
The RPSFTM and IPE methods also both rely critically on the ‘common treatment effect’ assumption, that is, the effect of receiving the experimental treatment is the same when received on diagnosis (i.e. in patients initially randomised to the experimental arm) as it is in treatment switchers (i.e. patients from the control arm who switch to receive the experimental treatment). In practice, it is unlikely that the ‘common treatment effect’ assumption will ever be completely true; however, it is appropriate to use RPSFTM/IPE methods if the assumption is likely to be approximately true. 143 Clinical advice to the AG was that for both SELECT and DECISION it is reasonable to assume that patients who switched from the placebo arm to receive the experimental treatment (i.e. lenvatinib/sorafenib) would experience the same treatment effect as patients who were originally randomised to the experimental arm.
In addition to the assumptions that are common to both the RPSFTM and the IPE methods, the IPE method also assumes that survival times follow a parametric distribution. To implement this method, a suitable parametric model must be identified, which can be problematic. The AG has been unable to identify information on how the IPE analysis was carried out using data from DECISION, including details of the parametric model chosen, and so is not able to comment on the suitability of this method.
Generally, the key assumption of a ‘common treatment effect’ that underpins RPSFTM appears to be valid, and because a large number of placebo patients crossed over to active treatment in both trials, the AG is of the opinion that RPSFTM is the most suitable method for adjusting for treatment switching in SELECT and DECISION. However, a caveat to the use of the RPSFTM-adjusted OS results for both trials is that differences in poststudy (postprogression) anti-cancer treatments administered to patients in each treatment arm are not accounted for in this analysis.
Participant characteristics
Overall, the baseline characteristics of patients included in SELECT and in DECISION were balanced between treatment arms (Table 6). Nevertheless, there are a few notable differences between the treatment arms and also across the trials.
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| Age (years), median (minimum to maximum) | 64 (27 to 89) | 61 (21 to 81) | 63 (24 to 82) | 63 (30 to 87) |
| Male, n (%) | 125 (47.9) | 75 (57.3) | 104 (50.2) | 95 (45.2) |
| Race, n (%) | ||||
| White | 208 (79.7) | 103 (78.6) | 123 (59.4) | 128 (61.0) |
| Black of African American | 4 (1.5) | 4 (3.1) | 6 (2.9) | 5 (2.4) |
| Asian | 46 (17.6) | 24 (18.1) | 47 (22.7) | 52 (24.8) |
| Other | 3 (1.2) | 0 | 2 (1.0) | 2 (1.0) |
| Missing or uncodeable | 29 (14.0) | 23 (11.0) | ||
| Region, n (%) | ||||
| Europe | 131 (50.2) | 64 (48.9) | 124 (59.9) | 125 (59.5) |
| North America | 77 (29.5) | 39 (29.8) | 36 (17.4) | 36 (17.1) |
| Other | 53 (20.3) | 28 (21.4) | 47 (22.7) | 49 (23.3) |
| Time from diagnosis of DTC to randomisation (months), median (range) | 66 (0.4–573.6) | 73.9 (6.0–484.8) | 66.2 (3.9–362.4) | 66.9 (6.6–401.8) |
| ECOG PS, n (%) | ||||
| 0 | 144 (55.2) | 68 (51.9) | 130 (62.8) | 129 (61.4) |
| 1 | 104 (39.8) | 61 (46.6) | 69 (33.3) | 74 (35.2) |
| 2 | 12 (4.6) | 2 (1.5) | 7 (3.4) | 6 (2.9) |
| 3 | 1 (0.4) | 0 | 0 | 0 |
| Not available | 0 | 0 | 1 (0.5) | 1 (0.5) |
| Histology, n (%) | ||||
| Papillary | 132 (50.6) | 68 (51.9) | 118 (57.0) | 119 (56.7) |
| Poorly differentiated | 28 (10.7) | 19 (14.5) | 24 (11.6) | 16 (7.6) |
| Follicular, not Hürthle cell | 53 (20.3) | 22 (16.8) | 13 (6.3) | 19 (9.0) |
| Hürthle cell | 48 (18.4) | 22 (16.8) | 37 (17.9) | 37 (17.6) |
| Other | 0 | 0 | 2 (1.0) | 5 (2.4) |
| Missing or non-diagnosed | 0 | 0 | 13 (6.3) | 14 (6.7) |
| Metastases, n (%) | ||||
| Locally advanced | 4 (1.5) | 0 | 7 (3.4) | 8 (3.8) |
| Distant | 257 (98.5) | 131 (100) | 200 (96.6) | 202 (96.2) |
| Metastases site, n (%) | ||||
| Lung | 226 (86.6) | 124 (94.7) | 178 (86.0) | 181 (86.2) |
| Lymph node | 138 (52.9) | 64 (48.9) | 113 (54.6) | 101 (48.1) |
| Bone | 104 (39.8) | 48 (36.6) | 57 (27.5) | 56 (26.7) |
| Pleura | 46 (17.0) | 18 (13.7) | 40 (19.3) | 24 (11.4) |
| Head and neck | NR | NR | 33 (15.9) | 34 (16.2) |
| Liver | 43 (16.5) | 28 (21.4) | 28 (13.5) | 30 (14.3) |
| Thyroid surgery, n (%) | 261 (100) | 131 (100) | 207 (100) | 208 (99.0) |
| Median cumulative radioiodine activity (mCi) | 350 | 400 | 376 | |
| Target tumour size, n (%) | ||||
| < 35 | 65 (24.9) | 28 (21.4) | 44 (21.3) | 51 (24.3) |
| 36–60 | 72 (27.6) | 32 (24.4) | 34 (16.4) | 48 (22.9) |
| 61–92 | 63 (24.1) | 34 (26.0) | 51 (24.6) | 34 (16.2) |
| > 92 | 61 (23.4) | 37 (28.2) | 78 (37.7) | 77 (36.7) |
| Prior VEGFR-targeted therapy, n (%) | 66 (25.3) | 27 (20.6) | 0 | 0 |
In SELECT, there was a lower proportion of males in the lenvatinib arm (47.9%) than in the placebo arm (57.3%). The median time from diagnosis of DTC to randomisation was shorter in the lenvatinib arm than in the placebo arm (66.0 vs. 73.9 months). Compared with the placebo arm, a smaller proportion of patients in the lenvatinib arm had metastases in the lung [86.6% (lenvatinib) vs. 94.7% (placebo)] or liver [16.5% (lenvatinib) vs. 21.4% (placebo)].
In DECISION, a higher proportion of patients in the sorafenib arm had metastases in the lymph node (54.6%) or pleura (19.3%) than in the placebo arm (48.1% and 11.4%, respectively). There was a higher proportion of males in the sorafenib arm (50.2%) than in the placebo arm (45.2%).
As previously highlighted, patients in SELECT could have been previously treated with a VEGFR-targeted therapy (including sorafenib) prior to trial entry whereas patients in DECISION could not. Approximately one-quarter (23.7%) of patients in SELECT had received prior treatment with a VEGFR-targeted therapy. In the lenvatinib arm, of 66 patients previously treated with a VEGFR-targeted therapy, 51 patients (77.2%) were treated with sorafenib. In the placebo arm, of 27 patients previously treated with a VEGFR-targeted therapy, 21 patients (77.8%) were treated with sorafenib. Other VEGFR-targeted therapies used prior to trial entry in SELECT included sunitinib (Sutent®, Pfizer) and pazopanib. The median duration of any prior therapy was ≈11 months in both arms.
A higher proportion of enrolled patients were from North America in SELECT than in DECISION (29.6% vs. 17.3%, respectively) and a lower proportion of patients were from Europe in SELECT than in DECISION (49.7% vs. 59.7%, respectively). A greater proportion of patients were white in SELECT (79.3%) than in DECISION (60.2%). A higher proportion of patients had bone metastases in SELECT than in DECISION (38.8% vs. 27.1%, respectively).
Comparison of assessments of risk of bias
A summary of the risk-of-bias assessments for both trials is reproduced in Appendix 3, Table 48. Overall, the AG considered the risk of bias to be low in both trials.
Consideration of proportional hazards assumption
Cox proportional hazards (PHs) modelling was used to generate PFS, unadjusted OS and adjusted OS hazard ratios (HRs) from data collected during SELECT and DECISION. The validity of this method relies on the event hazards associated with the intervention and comparator data being proportional over time within each trial. The AG assessed the validity of the PH assumption for all analyses, when possible, provided in the submissions from Eisai Ltd8 and Bayer HealthCare7 that included a HR result (see Appendix 6 for methods and results). The AG concluded that the PH assumption was not valid for PFS, unadjusted OS or RPSFTM-adjusted OS in SELECT or for PFS or RPSFTM-adjusted OS in DECISION. This means that the majority of the survival HRs generated using data from SELECT and DECISION and, consequently, statements about the statistical significance of results should be interpreted with caution.
Overall survival
A summary of the unadjusted and adjusted OS findings from the most recent data cut-off points from both trials is presented in Table 7. The findings for all data cut-off points are summarised in Appendix 3, Table 38.
| Outcome | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| Data cut-off pointa | Third data cut-off point (August 2015) | Third data cut-off point (July 2015) | ||
| Deaths, n (%) | 121 (46.4) | 70 (53.4) | 103 (49.8) | 109 (51.9) |
| OS (months), median (95% CI) | 41.6 (31.2 to NE) | 34.5 (21.7 to NE) | 39.4 (32.7 to 51.4) | 42.8 (34.7 to 52.6) |
| Unadjusted HR (95% CI); p-value | 0.84 (0.62 to 1.13); nominal p = 0.2475 | 0.92 (0.71 to 1.21); one-sided p = 0.28 | ||
| RPSFTM-adjusted HR (95% CI); p-value, cox method | NR | 0.77 (0.58 to 1.02); NR | ||
| RPSFTM-adjusted HR (95% CI); p-value, bootstrapping method | 0.54; nominal p = 0.0025 (0.36 to 0.80) | 0.77 (0.42 to 1.79); NR | ||
| IPE-adjusted HR (95% CI); p-value, cox method | N/A | 0.80 (0.61 to 1.05); NR | ||
| IPE-adjusted HR (95% CI); p-value, bootstrapping method | N/A | 0.80 (0.48 to 1.71); NR | ||
In both trials, there was no statistically significant difference in unadjusted OS between trial arms. However, when RPSFTM was used, patients in the lenvatinib arm had a statistically significant improvement in OS when compared with patients in the placebo arm in SELECT. The difference in OS between sorafenib and placebo was not reported to be statistically significant when using either the RPSFTM or IPE method in DECISION.
Progression-free survival
In both trials, the primary outcome was PFS by blinded independent review. The findings for PFS reported in SELECT and DECISION are summarised for the first data cut-off points (November 2013 and August 2012, respectively) in Table 8 because this was the only data cut-off point for which PFS results had been published for both trials.
| Outcome | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| First data cut-off point (November 2013) | First data cut-off point (August 2012) | |||
| PFS by blinded independent review | ||||
| Events, n (%) | 93 (35.6) | 109 (83.2) | 113 (54.6) | 137 (65.2) |
| Died before progression, n (%) | 14 (5.4) | 4 (3.1) | NR | NR |
| PFS (months), median (95% CI) | 18.3 (15.1 to NE) | 3.6 (2.2 to 3.7) | 10.8 (NR) | 5.8 (NR) |
| Stratified HR (95% CI);a p-value | 0.21 (0.14 to 0.31); < 0.001 | 0.59 (0.45 to 0.76); < 0.0001 | ||
| Investigator-assessed PFS | ||||
| Events, n (%) | 91 (34.9) | 104 (79.4) | 140 (67.6) | 184 (87.6) |
| Died before progression, n (%) | 16 (6.1) | 6 (4.6) | NR | NR |
| PFS (months), median (95% CI) | 16.6 (4.8 to NE) | 3.7 (3.5 to NE) | 10.8 (NR) | 5.4 (NR) |
| Stratified HR (95% CI);a p-value | 0.24 (0.16 to 0.35); < 0.001 | 0.49 (0.39 to 0.61); < 0.0001 | ||
In SELECT, there was a median improvement in PFS (blinded independent review) of 14.7 months with lenvatinib when compared with placebo. In DECISION, there was a 5-month median improvement in PFS (blinded independent review) with sorafenib when compared with placebo. The differences in median PFS assessed by investigators were marginally decreased in SELECT (12.9 months) and marginally increased in DECISION (5.4 months). However, the HRs in both trials were similar to those from the assessments by blinded independent review.
The SELECT trial is the only trial that also reports PFS for another data cut-off point. 85,86 This was available for investigator-assessed PFS at the third data cut-off point (August 2015). Compared with the first data cut-off point, median PFS was reported to be slightly higher in the lenvatinib arm at the third data cut-off point (19.4 months), but the median PFS remained the same in the placebo arm (3.7 months), a difference of 15.7 months. However, for both data cut-off points, the HR between arms was identical (0.24) and reported to be statistically significant (p < 0.001).
The findings for all data cut-off points are summarised in Appendix 3, Table 41.
Objective tumour response
The findings for objective tumour response are reported in Appendix 3, Table 42. In both trials, the tumour response assessment was conducted by blinded independent review at the first data cut-off point and favoured patients in the intervention arms compared with patients in the placebo arms. It is noticeable that the difference in ORR between the intervention and placebo arms was much greater for patients treated with lenvatinib in SELECT (63.2%) than for those treated with sorafenib in DECISION (11.7%). This is attributable to the much higher proportion of patients who were treated with lenvatinib and had a partial response in SELECT than the proportion of patients treated with sorafenib in DECISION. Complete responses were only reported for patients treated with lenvatinib, albeit there were very few patients (1.5%). ORR was statistically significantly improved in both trials for patients treated with either lenvatinib or sorafenib when compared with placebo.
The objective tumour response evaluations for SELECT were conducted using an ITT analysis. In DECISION, patients for whom it was not possible to evaluate a tumour response were excluded from the analysis (as per the requirements of a per-protocol analysis). If all patients are included in the evaluations using ORR data from DECISION, the ORR is marginally decreased in both arms: 11.6% for sorafenib and 0.5% for placebo.
Time to response was reported only for SELECT. The median time to response was 2.0 months for patients treated with lenvatinib compared with 5.6 months for patients in the placebo arm. The median duration of response was not estimable for patients in SELECT; however, for those treated with lenvatinib, the restricted mean was 17.34 months. Time to response was not reported in DECISION, but the median duration of response was 10.2 months for patients treated with sorafenib.
Both trials also assessed disease control rates (complete response + partial response + stable disease), and SELECT reported clinical benefit rate (complete response + partial response + durable stable disease). In each trial, the findings were statistically significantly in favour of lenvatinib or sorafenib compared with placebo. However, comparisons between trials cannot be easily made as the definition of disease control rate differed across trials because of differences in the length of stable disease required for control. SELECT required a stable disease of ≥ 7 weeks whereas DECISION required a length of ≥ 4 weeks; however, both trials did report the proportion of patients with stable disease of ≥ 6 months. This was similar in the placebo arms of both trials (SELECT, 29.8%; DECISION, 33.2%), whereas in the intervention arms, it was 15.3% for patients treated with lenvatinib and 41.8% for patients treated with sorafenib. Therefore, a clinical benefit at 6 months was reported by 79.5% of patients treated with lenvatinib compared with 31.3% of patients who received the placebo in SELECT, and 54.0% patients treated with sorafenib compared with 33.7% who received the placebo in DECISION. In the submission from Bayer HealthCare,7 it is noted that most sorafenib-treated patients (77%) experienced target lesion tumour shrinkage (compared with 28% of patients in the placebo arm).
Safety findings
Safety data from SELECT and DECISION were reported for the first data cut-off point (November 2013 and August 2012, respectively). For the individual types of AEs experienced by patients, the published paper for SELECT presented data for treatment-related AEs whereas the published paper for DECISION presented data for any treatment-emergent AEs. Therefore, data for specific types of treatment-emergent AEs were extracted from the pharmaceutical company submission (Eisai Ltd8) for SELECT.
All-grade and grade ≥ 3 adverse events
Nearly all of the patients who received lenvatinib or sorafenib reported an AE, and ≈90% of patients who received placebo reported an AE. AEs that were reported by ≥ 30% of patients and grade ≥ 3 AEs that were reported by ≥ 1.5% of patients in any of the arms are summarised in Appendix 3, Tables 44 and 45. All types of AEs were more common in patients treated with lenvatinib or sorafenib than in patients in the placebo arms of both trials. Hand–foot syndrome was reported by approximately three-quarters of patients in DECISION. Approximately two-thirds of patients reported all-grade hypertension or diarrhoea when treated with lenvatinib in SELECT, similar to the proportion treated with sorafenib reporting all-grade diarrhoea or alopecia in DECISION. Weight loss was reported by approximately half of all patients treated with either lenvatinib or sorafenib. By far the most common grade ≥ 3 AEs for patients treated with lenvatinib and sorafenib were hypertension (> 40%) and hand–foot syndrome (> 20%), respectively.
Serious adverse events (including fatal adverse events)
Serious adverse events (SAEs) reported in SELECT and DECISION are summarised in Appendix 3, Table 45. In SELECT, approximately half of the patients in the lenvatinib arm reported a SAE. Just over one-third of patients reported a SAE in the sorafenib arm of DECISION. Approximately one-quarter of patients in the placebo arms of both trials reported a SAE. The only SAE reported by ≥ 2% of patients in both trials was dyspnoea, which was at least as common for patients who received placebo as for those who received lenvatinib or sorafenib. The most common SAEs (reported by ≥ 3% of patients) reported for patients treated with lenvatinib in SELECT were pneumonia and hypertension. The most common SAEs (reported by ≥ 3% of patients) reported by patients treated with sorafenib in DECISION were secondary malignancy and pleural effusion.
Deaths from AEs were reported for 7.7% of patients treated with lenvatinib and 4.6% of patients in the placebo arm in SELECT. Fatal AEs in DECISION were reported for 5.8% of patients treated with sorafenib and 2.9% of patients in the placebo arm.
Treatment-related adverse events
A summary of treatment-related AEs is presented in Appendix 3, Table 46. A very high proportion of all-grade AEs (≥ 96%) were considered to be related to treatment with lenvatinib or sorafenib. The proportion of all-grade AEs considered to be treatment related was high (> 50%) in the placebo arms of both trials.
In SELECT, the causes of death considered to be treatment related in the lenvatinib arm were one case each of pulmonary embolism, haemorrhagic stroke and general deterioration of physical health; three cases were reported as deaths or sudden deaths (not otherwise specified). DECISION was the only trial in which a patient in the placebo arm was considered to have died because of a treatment-related AE. The cause of death for this patient was subdural haematoma. The cause of death for a patient in the sorafenib arm that was considered to be treatment related was myocardial infarction.
Timing of adverse events
In both trials, there have been subsequent analyses of the timing of AE occurrences in the treatment cycle reported. For SELECT, Haddad et al. 91 reported the incidence and timing of five AEs: proteinuria, diarrhoea, fatigue/asthenia/malaise, rash and hand–foot syndrome. Hypertension was a notable AE omitted from the analysis. For DECISION, detailed analysis of the AE occurrence patterns in patients is published in a peer-reviewed paper by Worden et al. 139 Findings from the two trials cannot be easily compared because Haddad et al. 91 reported their findings as median time to first onset and median time to last resolution, whereas Worden et al. 139 reported the proportion of AEs occurring during each cycle. The AEs reported included hand–foot syndrome, rash/desquamation, diarrhoea, fatigue, hypertension, weight loss, increased TSH levels and hypocalcaemia. Increased TSH levels were described as a ‘study-specific’ AE, with a maximum severity of grade 1; this AE was reported by 69 patients (33.3%) treated with sorafenib. 139
In SELECT, Haddad et al. 91 found that generally AEs for patients treated with lenvatinib occurred early in the treatment process and were resolved. Median time to onset for patients treated with lenvatinib ranged from 3.0 weeks with fatigue/asthenia/malaise to 12.1 weeks with diarrhoea. With regard to resolution, this ranged from a median of 5.9 weeks with rash to a median of 20.0 weeks with hand–foot syndrome.
In DECISION, Worden et al. 139 found that in patients treated with sorafenib, the incidence of AEs was usually highest in the first cycle or the first two cycles. Severity tended to diminish with each cycle (over the first nine cycles). The prevalence of AEs (defined as the number of patients with a new or continuing AE during a treatment cycle) tended to remain stable. Diarrhoea and TSH were notable exceptions in that prevalence steadily increased over the first five or six cycles, at which point the prevalence peaked. Only weight loss, which was primarily grade 1 or grade 2 and highest in the first four cycles, tended to increase in severity over time (from grade 1 to grade 2: a greater proportion of patients experienced grade 2 toxicity in cycle 9 compared with cycles 1 and 2). The authors noted that in general, AEs with sorafenib were manageable over time following dose modification and/or concomitant medications, such as antidiarrhoeals, antihypertensives or dermatological preparations.
Dose modifications
Dose modifications as a result of AEs were more common for patients treated with lenvatinib and sorafenib than for those who received placebo (Table 9). It is of note that the incidence of dose interruptions with lenvatinib in SELECT was higher than with sorafenib in DECISION. The incidence of dose interruptions and dose reductions were lower in the placebo arm of SELECT than in the placebo arm of DECISION.
| Outcome | Study, n (%) | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 209) | |
| Dose interruptions because of an AE | 215 (82.4) | 24 (18.3) | 137 (66.2) | 54 (25.8) |
| Dose reductions because of an AE | 177 (67.8) | 6 (4.6) | 133 (64.3) | 19 (9.1) |
| Discontinued treatment because of an AE | 43 (16.5) | 6 (4.6) | 39 (18.8) | 8 (3.8) |
It is reported that, in SELECT, the most common AEs developing during treatment that led to a dose interruption or reduction among patients receiving lenvatinib were diarrhoea (22.6%), hypertension (19.9%), proteinuria (18.8%) and decreased appetite (18.0%). It is also noted that four patients in the lenvatinib arm (1.5%) required dose adjustments owing to hypocalcaemia. In the submission from Eisai Ltd,8 it is further noted that 1.1% of patients discontinued treatment because of hypertension and 1.1% of patients discontinued because of asthenia. In DECISION, it is reported that hand–foot syndrome was the most common reason for sorafenib dose interruptions (26.6%), reductions (33.8%) and withdrawals (5.3%).
Health-related quality-of-life findings
It was reported in the European Public Assessment Report (EPAR)27 that, although HRQoL data were not collected in the randomised part of SELECT,51 HRQoL would be assessed in 30 patients who participated in the open-label extension phase of the trial. The AG is unaware of whether or not these findings have been published.
For DECISION, HRQoL was reported in a conference abstract by Schlumberger et al. 120 More detailed HRQoL results were also reported in the submission from Bayer HealthCare. 7 Cancer-specific HRQoL was measured using the Functional Assessment of Cancer Therapy – General (FACT-G) questionnaire144 and general health status was measured using the generic EuroQol-5 Dimensions, three-level version (EQ-5D-3L) and the EuroQol-5 Dimensions (EQ-5D) visual analogue scale (VAS). 145 The FACT-G questionnaire is a validated 27-item questionnaire designed to assess the following dimensions in cancer patients: physical well-being, social/family well-being, emotional well-being and functional well-being. The FACT-G total score ranges from 0 to 108 points, with higher scores representing a better HRQoL. Similarly, the EQ-5D is a validated instrument in which higher scores represent better health status.
All questionnaires were self-administered at baseline and day 1 of every 28-day cycle. The overall questionnaire completion rate during the trial was reported by the authors to be 96%. 120 However, the actual number of patients completing the questionnaires reduces with each cycle because only patients with progression-free disease were asked to complete the questionnaires. Thus, for example, as shown in the submission from Bayer HealthCare7 by the response to one of the physical well-being questions, by cycle 13 the number of patients who responded was 87: 40.1% of all patients enrolled into the trial.
Functional Assessment of Cancer Therapy – General
Minimally important differences in the FACT-G total score (i.e. a difference considered to be clinically meaningful) have been reported to range between 3 and 7 points. 144 At baseline, it was reported7,120 that FACT-G scores were comparable to a normative adult cancer population, the mean scores being 81 points [standard deviation (SD) 15 points] in the sorafenib arm and 82 points (SD 14 points) in the placebo arm. However, at the first assessment (cycle 2, day 1), the score for the sorafenib arm had fallen to 76 points (SD 15 points), whereas the score in the placebo arm remained very similar to baseline. The authors of the conference abstract120 reported that the scores in the sorafenib arm thereafter remained similar to the scores at the first assessment, whereas the scores remained similar to the baseline scores in the placebo arm. A mixed linear model estimated that, compared with placebo, the FACT-G score was 3.45 points lower in the sorafenib arm (p = 0.0006), representing a clinically meaningful difference between arms in favour of the placebo arm. The authors attributed the diminished HRQoL score to AEs. Indeed, the submission from Bayer HealthCare7 noted that in response to the FACT-G physical well-being domain question ‘I am bothered by side effects’, the proportion of patients in the sorafenib arm who replied ‘quite a bit’ or ‘very much’ increased from 1.5% at cycle 1 to 29.6% at cycle 2. However, this proportion gradually diminished over time: it was 16.8% by cycle 6 and 8.0% by cycle 13.
EuroQol-5 Dimensions index and visual analogue scale
For UK utility scores, a change of 0.10 points on the EQ-5D index has been reported by Pickard et al. 146 to be clinically meaningful for all cancers (using ECOG PS as the anchor). Similarly, the same study reported a change of at ≥ 7 points on the VAS to be clinically meaningful. 146 It was reported in DECISION7,120 that the patterns for EQ-5D index and VAS were similar to that of the FACT-G; after the first assessment, the scores in the sorafenib arm were lower than the scores in the placebo arm. Although the between-arm differences were statistically significant (p < 0.0001 for both EQ-5D index and VAS), the treatment effects (–0.07 and –6.75, respectively) were of a small magnitude and did not reach the threshold for a clinically meaningful difference. It is reported in the submission from Bayer HealthCare7 that dimensions in the EQ-5D index that are sensitive to AEs include mobility, usual activities and pain/discomfort.
Subgroup analyses from randomised controlled trials
Only subgroup analyses considered by the AG to be of direct relevance to the decision problem have been reported in the remainder of this report. The AG considered the following subgroup analyses to be relevant (with rationale given):
-
patients previously treated and not previously treated with TKIs (prespecified subgroup in the NICE scope53 and AG decision problem)
-
patients with and without symptomatic disease at baseline (as highlighted in Chapter 1, Treatment options for patients with radioactive iodine-refractory differentiated thyroid cancer, systemic treatment is recommended for patients who have symptomatic disease)
-
analyses of subgroups that were prespecified in the trials and in which there appeared to be differences in baseline characteristics within or across trials (as differences in baseline characteristics may influence results).
As previously highlighted, the AG concluded that the assumption of PH does not hold in any of the analyses that it was able to check other than unadjusted OS in DECISION. This means that the majority of the survival HRs generated using data from SELECT and DECISION and, consequently, statements about the statistical significance of results should be interpreted with caution.
Patients previously treated and patients not previously treated with tyrosine kinase inhibitors
Subgroup analyses have been reported for patients previously treated with a TKI (e.g. VEGFR-targeted therapy) in SELECT but only for PFS and ORR. 51,105,106 No patients in DECISION had received prior treatment with a TKI.
Results from subgroup analyses using data from SELECT51,105,106 showed that PFS was statistically significantly longer for patients treated with lenvatinib compared with placebo for patients previously treated with VEGFR-targeted therapy (including sorafenib) (Table 10). For patients who were VEGFR-targeted therapy naive, PFS was also statistically significantly longer for patients treated with lenvatinib compared with placebo.
| Outcome | Treatment with VEGFR-targeted therapy | |||
|---|---|---|---|---|
| Prior treatment | No prior treatment | |||
| Lenvatinib (N = 66) | Placebo (N = 27) | Lenvatinib (N = 195) | Placebo (N = 104) | |
| Events, n (%) | 31 (47.0) | 25 (92.6) | 76 (39.0) | 88 (84.6) |
| Median PFS (months) | 15.1 | 3.6 | 18.7 | 3.6 |
| HR (95% CI) | 0.22 (0.12 to 0.41) | 0.20 (0.14 to 0.27) | ||
Compared with patients in the placebo arm, ORR was statistically significantly improved for patients treated with lenvatinib whether or not they had been previously treated with a VEGFR-targeted therapy (see Appendix 3, Table 47). 51,105,106 In both subgroups, ORRs were similar to the ORRs observed in the overall trial population (lenvatinib 64.8% and placebo 1.5%).
Newbold et al. 105,106 reported that any all-grade and grade ≥ 3 AEs were similar in the two subgroups of patients receiving lenvatinib (prior VEGFR-targeted therapy 100.0% and 87.9%, respectively; no prior VEGFR-targeted therapy 99.5% and 86.7%, respectively). However, SAEs were more common in the lenvatinib arm among patients who had received prior VEGFR-targeted therapy (60.6%) than among those who had not (50.8%). For patients in the placebo arm, the opposite was the case, with SAEs being less common among patients who had received prior VEGFR-targeted therapy (18.5%) than among those who had not (25.0%).
Patients who had not received prior VEGFR-targeted therapy were treated with more cycles of lenvatinib (median 16 cycles) than those who had received prior VEGFR-targeted therapy (median 12.5 cycles). The proportion of patients who had at least one lenvatinib dose reduction was also similar between subgroups (prior VEGFR-targeted therapy 81.8% and no VEGFR-targeted therapy 86.7%). Patients with no prior VEGFR-targeted therapy had an earlier median time to first dose reduction (8.9 weeks) than patients with prior VEGFR-targeted therapy (14.8 weeks). Patients with no prior VEGFR-targeted therapy also had a lower median daily dose of lenvatinib than those with prior VEGFR-targeted therapy (16.1 vs. 20.1 mg, respectively).
Patients with and without symptomatic disease at baseline
Subgroup analyses were not conducted for patients with symptomatic or asymptomatic disease at baseline in SELECT. In DECISION, the median PFS for patients who were retrospectively categorised as being symptomatic at baseline was longer for patients who were asymptomatic than those who were symptomatic in the placebo arm but was similar in the intervention arm (Table 11). Patients were classified as being symptomatic if they had symptoms/findings that were consistent with RR-DTC reported in the medical history or pre-treatment AE data set at trial entry. 113,119 It is noted in the EPAR26 for sorafenib that ≈20% of patients had symptoms that were likely to be related to thyroid cancer at baseline.
| Outcome | Symptom status | |||
|---|---|---|---|---|
| Symptomatic (≈20%) | Asymptomatic (≈80%) | |||
| Sorafenib | Placebo | Sorafenib | Placebo | |
| Events, n (%) | NR | NR | NR | NR |
| Median PFS (months)a | 10.7 | 3.6 | 10.8 | 7.2 |
| HR (95% CI) | 0.386 (0.207 to 0.720) | 0.602 (0.448 to 0.807) | ||
Bayer HealthCare7 has stated that although tumour shrinkage was not always sufficient to be confirmed as an objective response for some patients, it was often sufficient to alleviate symptoms. Further evidence has not been presented to support this statement.
Safety analyses for patients with symptomatic or asymptomatic disease at baseline have not been reported in SELECT or DECISION.
Other subgroup analyses of interest
Some OS subgroup analyses in SELECT have been reported in conference abstracts. 67,73,82,89 No OS subgroup analyses have been reported using data from DECISION. For OS (first data cut-off point in November 2013) in SELECT, it has been reported that:
-
There was no statistically significant difference in OS between older and younger lenvatinib-treated patients [HR 0.78, 95% confidence interval (CI) 0.49 to 1.26; p = 0.304] but there was a statistically significant difference in the placebo arm, favouring younger patients (HR 0.48, 95% CI 0.27 to 0.85; p = 0.010). 67,73
-
Median OS was not reached in either arm in patients treated in North America. 89
-
A statistically significant OS advantage was observed in patients with FTC treated with lenvatinib compared with placebo (HR 0.41, 95% CI 0.18 to 0.97). 82
In addition to the subgroup analyses, Haddad et al. 91 found from a post hoc exploratory multivariate analysis of SELECT (first data cut-off point) that ECOG PS and histology (favouring FTC vs. PTC) were statistically significantly associated with OS.
For PFS, all prespecified and some post hoc subgroup analyses (first data cut-off points) have also been reported in the appendix to the primary published paper for SELECT51 and in the published paper for DECISION. 52 The results for both trials showed that for all subgroups, PFS favoured lenvatinib or sorafenib versus placebo. In the majority of instances, the differences were statistically significant. Regarding PFS for prespecified subgroup analyses, the following results are noted:
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) for patients aged ≤ 65 or > 65 years in SELECT; the effect was statistically significantly in favour of sorafenib (compared with placebo) for patients aged < 60 or ≥ 60 years in DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) and for sorafenib (compared with placebo) for males and females in SELECT and DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) for patients with PTC, poorly differentiated carcinoma, FTC or Hürthle cell carcinoma in SELECT; the effect was statistically significantly in favour of sorafenib (compared with placebo) for patients with PTC or Hürthle cell carcinoma but not for those with FTC or poorly differentiated carcinoma in DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) for patients classified as white or Asian in SELECT; no subgroup analyses have been presented for race in DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) for patients treated in Europe and North America or other regions in SELECT; the effect was statistically significantly in favour of sorafenib (compared with placebo) for patients treated in Europe or Asia but not for patients treated in North America in DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) for those with and without lung metastases in SELECT and the effect was statistically significantly in favour of sorafenib (compared with placebo) for those with lung metastases only and for those without lung metastases only in DECISION.
-
The effect was statistically significantly in favour of lenvatinib (compared with placebo) and for sorafenib (compared with placebo) for patients with and without bone metastases in SELECT and DECISION.
It is recommended by the EMA26 that before initiating treatment, physicians should carefully evaluate the prognosis in the individual patient, considering the maximum lesion size, symptoms related to the disease and the progression rate.
As reported in the appendices to the submission from Bayer HealthCare,7 a post hoc analysis of investigator-assessed PFS by number of target lesions in DECISION found statistically significant improvements with sorafenib compared with placebo for patients with at least three lesions. For patients with fewer than three lesions, PFS was numerically improved with sorafenib compared with placebo. It is also reported that another post hoc subgroup analysis of investigator-assessed PFS showed a treatment effect in favour of sorafenib compared with placebo for patients with maximum tumour size of ≥ 1.5 cm (HR 0.54, 95% CI 0.41 to 0.71). A numerically lower effect was reported for patients with a maximum tumour size of < 1.5 cm (HR 0.87, 95% CI 0.40 to 1.89).
Aside from the caveat surrounding the use of HRs to determine statistical significance as a result of PH assumption being violated, it is important to note that subgroup analyses are not powered to detect statistical significance. Therefore, when no statistically significant differences are reported, it could be that the numbers of patients in the subgroups were not large enough to detect a difference.
Extended open-label phases of SELECT and DECISION
In the extended open-label phase of SELECT, the starting daily dose of lenvatinib was originally 24 mg. This was later modified to 20 mg and then reverted to 24 mg. It is important to note that this phase of the trial only included 115 patients who crossed over from the placebo arm to lenvatinib and therefore does not present evidence from a randomised or controlled patient population. Furthermore, only placebo-treated patients who had confirmed disease progression (independent blinded review) during the randomisation phase and who met protocol-specified eligibility criteria were treated with lenvatinib. Consequently, it is noted in the EPAR27 for lenvatinib that these patients had very advanced disease, because they had experienced two sequential, confirmed disease progressions: the first before randomisation at the time of study entry and the second during treatment with the study drug in the randomisation phase.
The extended open-label phase of DECISION differed to that of SELECT in that as well as including patients who crossed over from the placebo arm to receive sorafenib, it also included patients who remained on sorafenib. In total, 150 patients in the placebo arm crossed over to receive sorafenib at progression and of these, data from 137 patients were evaluable for efficacy. In addition, 55 patients randomised to the sorafenib arm continued on sorafenib treatment in the open-label extension phase, of which 46 patients were evaluable for efficacy. It is reported by Schlumberger et al. 122 and Paschke et al. 114 that patients evaluable for efficacy had poorer risk features at enrolment compared with patients who were not evaluable. Like the extended open-label phase of SELECT, evidence from this patient population does not comprise evidence from a randomised or controlled patient population.
Findings from the extended open-label phase of SELECT for only ‘. . . the more mature dataset of patients who started treatment at the 24mg lenvatinib dose’ were reported in a conference abstract118 describing the first data cut-off point (November 2013). Findings reported at the second data cut-off point (June 2014) were presented for patients who started treatment at the 20-mg dose of lenvatinib and for patients who started the 24-mg dose of lenvatinib in the EPAR for lenvatinib. 27 In the EPAR,27 it is reported that patient characteristics, previous treatments, geographical allocation, on-study placebo exposure, lenvatinib exposure in the extended open-label phase and median follow-up times vary considerably for these two dose regimens. Thus, patients receiving the different dose regimens are considered by the EMA to represent different populations of patients.
In addition to conference abstracts,114,122 the findings from the extended open-label phase of DECISION have been reported in the EPAR46 for sorafenib. Safety data for the extended open-label phase of DECISION are reported in the submission from Bayer HealthCare. 7
The efficacy and safety findings from the open-label phases of both trials are summarised in Appendix 7, Tables 56 and 57. OS data have not been reported. With the exception of median PFS for patients receiving sorafenib for a second time, which was 6.7 months, the efficacy findings for PFS from the extended phase of SELECT and DECISION were similar to the findings reported in the randomised phase of the trials. The incidence of AEs for patients treated with lenvatinib and sorafenib in the open-label phases of the two trials tended to be slightly lower than reported during the double-blind phase.
In addition, Kappeler et al. 94 and Fassnacht et al. 83 have reported exploratory analyses of tumour growth rate in the randomised double-blind and extended open-label phases of DECISION. The authors found that the tumour growth rate (mean changes per month of sum of target lesion diameters) from baseline to nadir was –3.9% then +2.6% from nadir to progression for patients treated with sorafenib in the randomised phase; for those continuing with additional sorafenib in the open-label phase, from second baseline to progression the tumour growth rate was +1.7%. For patients randomised to the placebo arm, the tumour growth rate was +5.0% for all placebo patients and it was +6.1% for placebo patients subsequent to crossing over to receive sorafenib. Patients in the placebo arm who crossed over to sorafenib in the open-label phase then experienced a tumour growth rate pattern similar to that of patients who started on sorafenib in the randomised phase: –4.4% from second baseline to nadir and then +1.8% from nadir to progression.
Associations between tumour response, progression-free survival, overall survival, safety and health-related quality of life
Gianoukakis et al. 86 examined the association between ORR and PFS for patients treated with lenvatinib in SELECT. The analysis is based on the third data cut-off point (August 2015) using investigator-assessed ORR [60.2% (the proportion of patients who achieved an objective tumour response)] and investigator-assessed mean PFS (19.4 months). The authors found that the median PFS in patients who received lenvatinib and who demonstrated a tumour response was 33.1 months (95% CI 27.8 months to not estimable). In lenvatinib-treated patients who did not show tumour response, the median PFS was 7.9 months (95% CI 5.8 to 10.7 months). Robinson et al. 117 reported that an exploratory multivariate analysis found that percentage change in tumour size at the first assessment was a marginally statistically significant positive predictor for PFS (p = 0.06).
Using data from the first data cut-off point of SELECT, Newbold et al. 108 analysed PFS by patients who had responded to treatment with lenvatinib at the first tumour assessment (median time to response 1.9 months) and by those who responded later (median time to response 3.8 months). The authors found that there was no difference in PFS between patients who achieved objective response at the time of first tumour assessment compared with thereafter.
Haddad et al. 91 found from a multivariate analysis (first data cut-off point) that in SELECT, all-grade diarrhoea was statistically significantly associated with OS (median OS for lenvatinib-treated patients with diarrhoea was not reached and median OS for lenvatinib-treated patients without diarrhoea was 17.1 months). Choi et al. 79 reported that the results of a post hoc analysis showed that lenvatinib-treated patients with hypertension had higher median PFS than those without hypertension (18.8 vs. 12.9 months; p = 0.009). Haddad et al. 91 also reported results from multivariate analyses of associations between five other AEs (proteinuria, diarrhoea, fatigue/asthenia/malaise, rash and hand–foot syndrome) and PFS in SELECT. No statistically significant associations between any of the AEs and PFS were found.
Using data from DECISION, Kappeler et al. 95 carried out an exploratory analysis to explore the association between tumour growth rate and PFS and OS. It is reported that the data cut-off points used for PFS and OS were the first data cut-off point (August 2012) and third data cut-off point (July 2015), respectively. Values of early tumour growth rate were split into quartiles [by median times derived from Kaplan–Meier (K–M) curves and from modelling with a Weibull distribution] separately by treatment arm. Better prognosis for PFS and OS with sorafenib was associated with the second and third tumour growth rate quartiles.
No other analyses have been conducted for patients treated with either lenvatinib or sorafenib in SELECT or DECISION examining the relationships between any of the efficacy or safety outcomes and HRQoL. As reported earlier (see Health-related quality-of-life findings), it has been speculated that AEs did affect HRQoL based on data from FACT-G and EQ-5D questionnaires, but no formal analyses have been conducted in an attempt to correlate the findings.
Indirect comparison feasibility assessment
In the absence of direct clinical evidence comparing treatment with lenvatinib versus sorafenib, the AG considered whether or not it was appropriate to carry out an indirect comparison to obtain estimates of the relative efficacy and safety of these two treatments.
The first step was to determine whether or not SELECT and DECISION shared a common comparator. The comparator arm of both trials was placebo. From the limited information on the placebos reported by Eisai Ltd,8 Bayer HealthCare7 and in the published papers,51,52 the AG considered that the comparator arms were likely to be similar and that a network could be constructed (Figure 5).
FIGURE 5.
Indirect comparison network.
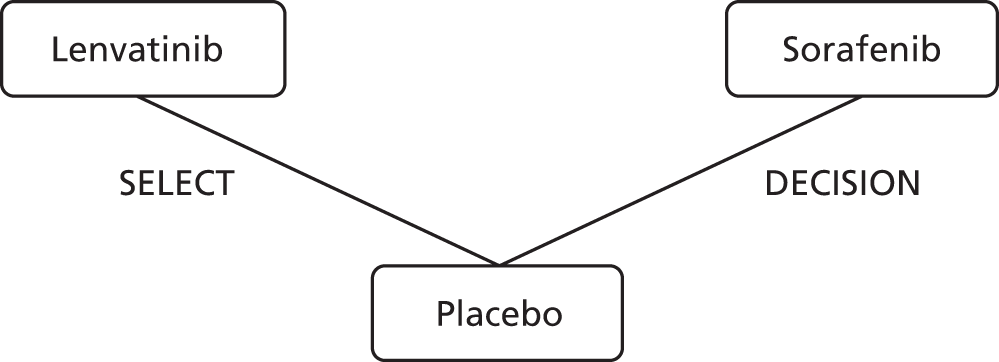
The second step was to check the comparability of the participant and trial characteristics between the two trials. As described in Trial characteristics and Participant characteristics, the AG has noted that there are several trial design and participant differences, both within and between SELECT and DECISION. These differences raised concerns about whether or not data from these trials should be included in the same network of evidence.
The final step undertaken by the AG was to examine the PFS K–M data from the placebo arms of SELECT and DECISION to determine the extent to which the risk profiles of the populations in these arms of the two trials were comparable. The AG concluded that the risks were not sufficiently comparable and that these two trials should, therefore, not be included in the same network of evidence.
The Assessment Group’s detailed commentary on progression-free survival Kaplan–Meier data from the placebo arms
An indirect comparison implicitly assumes that the randomised patients are drawn from similar populations with reference to their risk profile for the time-to-event outcomes (PFS and OS). Because PFS is the primary outcome specified in both clinical trials, it is important that the equivalence of the placebo arms of the two trials can be confirmed by comparison of PFS outcomes: any significant discrepancy in progression risk would invalidate an indirect comparison between lenvatinib and sorafenib.
Figure 6 compares the K–M PFS trial results for the placebo arms of the two trials. After similar trends over the first 2 months, the curves separate markedly for more than a year before crossing over in the long term. Visual examination is sufficient to establish that these data are not amenable to either a simple HR adjustment or a time ratio adjustment.
FIGURE 6.
Comparison of PFS in the placebo arms of DECISION and SELECT. Reproduced with permission from Fleeman et al. 54 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
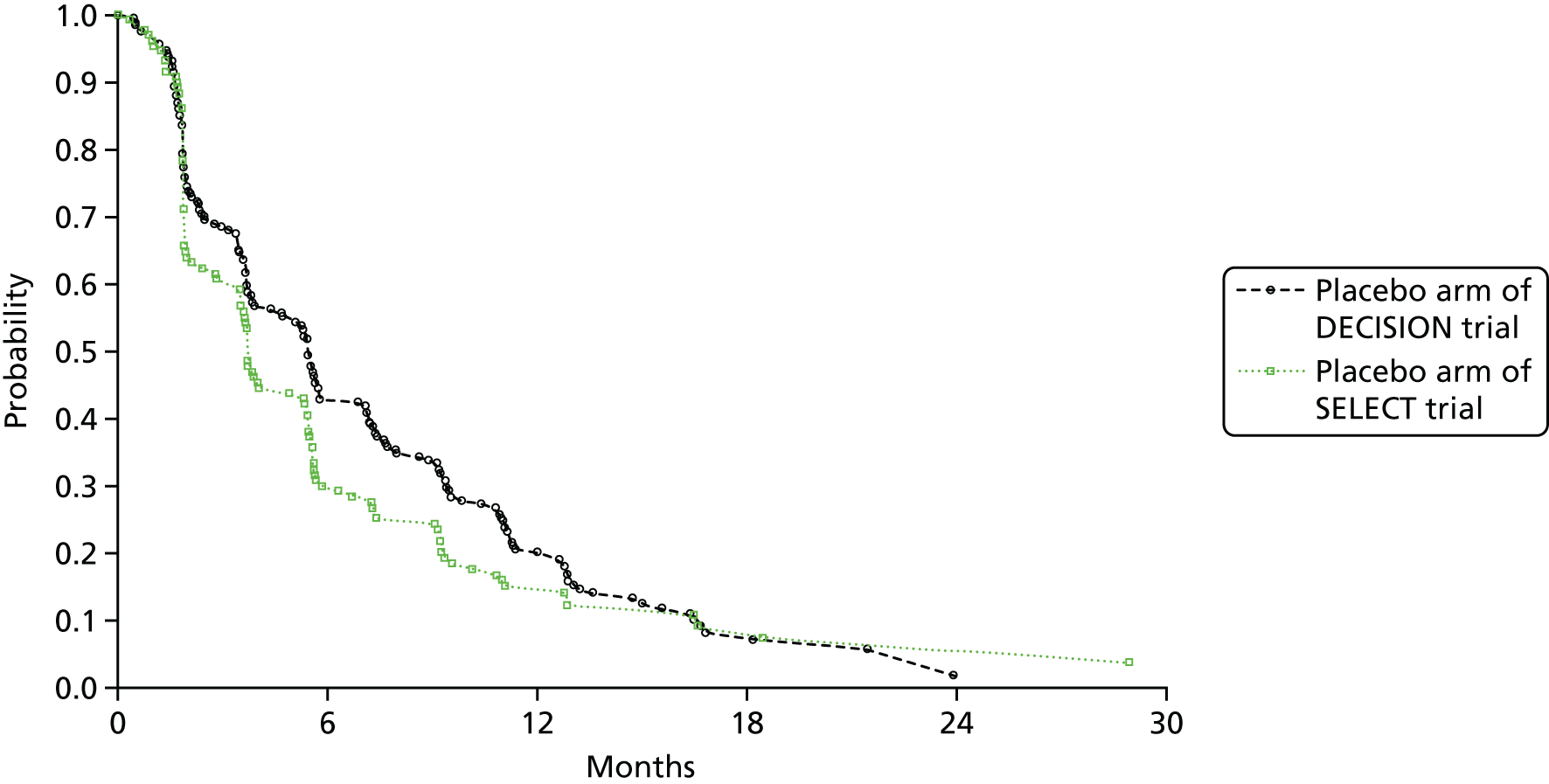
Further exploration of these data trends through a plot of cumulative hazards in the two trial arms at common time points reveals a clear divergence from a simple linear (PH) relationship (Figure 7). The trial data indicate a higher initial risk of disease progression in SELECT in the first 10 months, followed by a sharp reversal in which the risk in the SELECT placebo arm reduces by > 50%.
FIGURE 7.
Comparison of PFS hazard trends in the placebo arms of DECISION and SELECT.
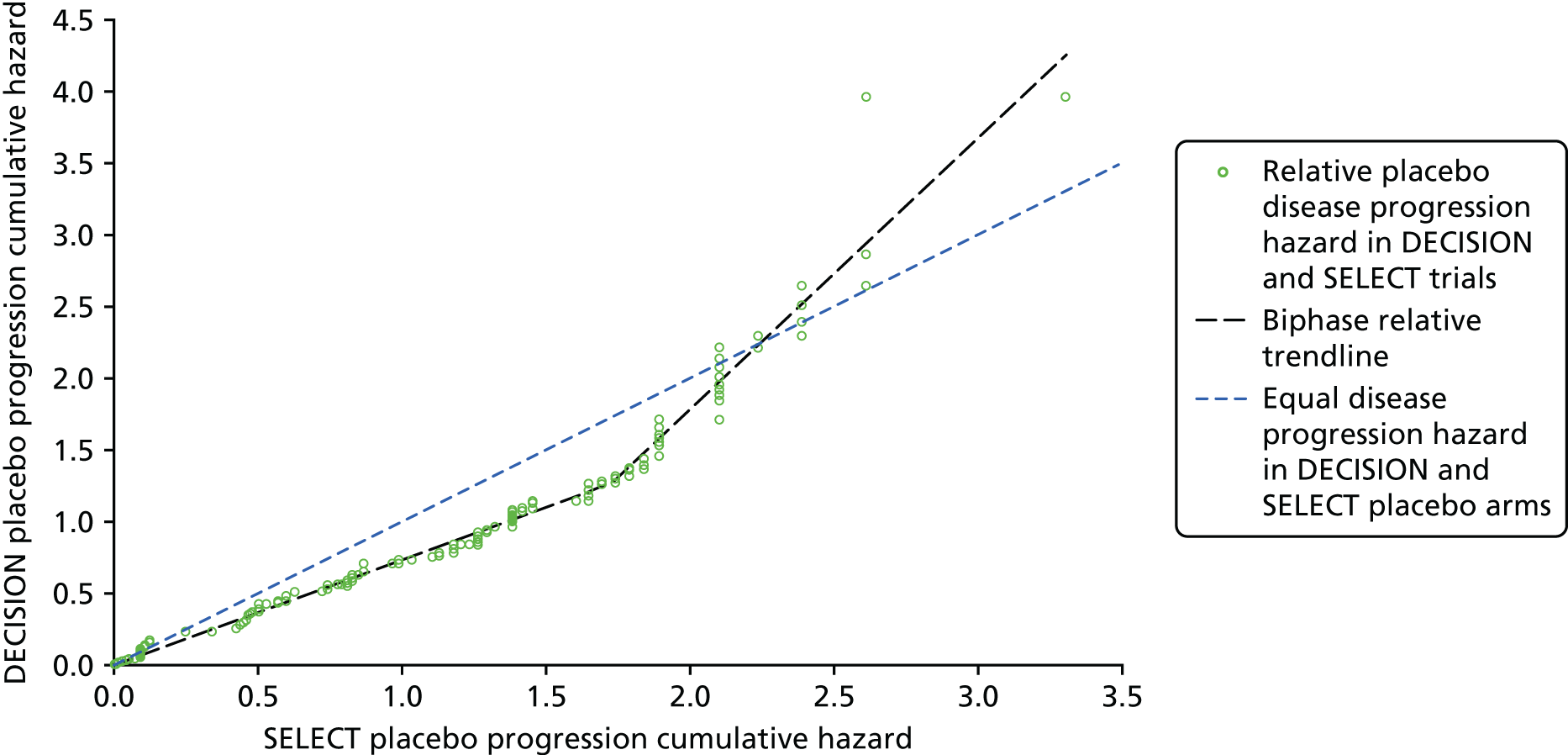
The AG considers that the placebo arms of SELECT and DECISION exhibit unexpectedly inconsistent patterns of temporal change that are not compatible with the assumption that these are similar patient groups. Consequently, patients enrolled in the two trials cannot be considered to be derived from a common population and, therefore, conducting an indirect comparison to obtain estimates of relative efficacy for lenvatinib and sorafenib is not appropriate.
Differences in trial and participant characteristics in the placebo arms of the trials
As reported earlier (see Trial characteristics and Participant characteristics), a number of differences in trial and participant characteristics were observed between arms within trials and across trials. Given the apparent differences in the placebo arms of the two trials, as demonstrated by differing hazard trends, the AG highlights the following differences in characteristics between the two placebo arms:
-
The SELECT trial permitted the enrolment of patients who had been previously treated with a VEGFR-targeted therapy (including sorafenib), whereas DECISION did not: 20.6% had received prior therapy in the placebo arm of SELECT compared with no patients in the placebo arm of DECISION.
-
Palliative radiotherapy, which is commonly available as part of BSC in UK NHS clinical practice, was not permitted for patients in the placebo arm of SELECT.
-
The proportion of patients who crossed over from the placebo arm of SELECT was 87.8% at the third data cut-off point, compared with 75.0% in DECISION.
-
There were proportionately more males in the placebo arm of SELECT than in the placebo arm of DECISION (57.3% and 45.2%, respectively).
-
A higher proportion of patients in the placebo arm of SELECT were classified as being white than were similarly classified in the placebo arm of DECISION (78.6% and 61.0%, respectively), whereas the opposite was the case for patients classified as Asian (18.1% and 24.8%, respectively).
-
Proportionately fewer patients in the placebo arm of SELECT were from Europe (48.9%) and proportionately more were from North America (29.8%) compared with the patients in the placebo arm of DECISION (59.5% and 17.1%, respectively).
-
A greater proportion of patients in the placebo arm of SELECT had an ECOG PS of ≥ 1 (48.1%) than in the placebo arm of DECISION (38.6%).
-
A greater proportion of placebo patients had FTC and poorly differentiated thyroid cancer in the placebo arm of SELECT (16.8% and 14.5%, respectively) than in the placebo arm of DECISION (9.0% and 7.6%, respectively).
-
The time from diagnosis to randomisation was greater in the placebo arm of SELECT (73.9 months) than in the placebo arm of DECISION (66.9 months).
-
A greater proportion of patients in the placebo arm of SELECT had lung, bone and liver metastases (94.7%, 36.6% and 21.4%, respectively) than in DECISION (86.2%, 26.7% and 14.3%, respectively).
Proportional hazards assumption
As discussed in Consideration of proportional hazards assumption, the AG concluded that the PH assumption was not valid for PFS, unadjusted OS or adjusted OS in SELECT or for PFS or adjusted OS in DECISION. The violation of the PH assumption, for all but unadjusted OS in DECISION, means that the network of evidence is compromised for all outcomes.
Assessment Group summary statement
The AG considers that is not appropriate to conduct an indirect comparison to obtain HRs for lenvatinib versus sorafenib for the outcomes of PFS, unadjusted OS and adjusted OS. This is because the risk profiles of the patients in the placebo arms of the trials are not comparable and any indirect comparison would produce results that could not be considered to be robust. This also precluded indirect comparison for subgroups of patients according to previous treatment with TKIs.
As described in Chapter 3, Methods of analysis/synthesis, in addition to trial characteristics, participant characteristics and outcome data, the AG stated that it would consider the quality of the included trials when conducting its feasibility assessment. The results of the AG’s risk-of-bias assessment are reported in Comparison of assessments of risk of bias. However, given the issues already highlighted, the quality of the trials was not a factor in the AG’s decision to not conduct an indirect comparison.
Systematic review evidence
The AG included 13 systematic reviews5–8,33,57,61,93,97,104,127,138,141 in its review; these reviews included the evidence submissions reporting systematic reviews and indirect comparisons for this MTA from Eisai Ltd8 and Bayer HealthCare,7 and also the evidence reported in a paper by Tremblay et al. 57 Although Tremblay et al. 57 did not report the conduct of a systematic review, this paper was included as it did report results from an indirect comparison and a matching-adjusted indirect comparison (MAIC) using data from SELECT and DECISION.
A summary of the characteristics of the included systematic reviews is presented in Appendix 5, Table 49. Most of the evidence was derived from observational studies of treatment with sorafenib. However, four of the reviews,7,8,57,97 including the submissions from Eisai Ltd8 and Bayer HealthCare,7 included evidence from SELECT and DECISION and results from indirect comparisons, including MAICs.
The AG’s assessment of the quality of the included reviews is presented in Appendix 5, Table 50. Overall, the AG considered that the quality of nine5–8,61,97,104,127,138 of the identified systematic reviews was good. However, only 45–8 of the 13 reviews included a quality assessment of the included primary studies. Four33,57,93,141 of the reviews were considered to be of poorer quality than the rest. Of these, only one33 reported the use of an adequate search strategy. In addition, methods of cross-checking during either the study selection process or the data extraction process were not reported by the authors of three reviews. 33,57,93 No quality assessment of the primary studies was reported in any of these four reviews. 33,57,93,141
The conclusions reached by the authors of the systematic reviews are presented in Appendix 5, Table 51. The earliest of the reviews was carried out by Anderson et al. 61 and was published in 2013. The authors concluded that certain treatments, notably TKIs, showed promise in Phase II trials. Gruber and Colevas33 concluded that the most likely outcome of treatment with a TKI was stable disease. McFarland and Misiukiewicz104 concluded that sorafenib slowed the progression of disease in the majority of cases. For treating thyroid cancer, Ye et al. 141 reported that the clinical effects of sorafenib and lenvatinib outweigh the toxicities [relative risk (RR) 1.27, 95% CI 1.05 to 1.53] and deaths (RR 15.24, 95% CI 6.99 to 33.21). Ye et al. 141 concluded that lenvatinib and sorafenib were more useful for thyroid cancer than for RR-DTC, based on the results of the subgroup analyses that were conducted. However, the AG considers that all of the studies that included patients with DTC also included patients with RR-DTC and so the validity of this subgroup analysis and the conclusions reached based on these subgroup analyses are questionable.
Jean et al. 93 found AEs reported for sorafenib for treating RR-DTC to be higher than for AEs reported for treating renal cell carcinoma (RCC) or hepatocellular carcinoma (HCC). In two reviews127,138 ORR data and AE data were pooled for sorafenib from seven observational studies59,78,88,101,126,147,148 (five prospective and two retrospective). In the review by Shen et al. ,127 all of the studies59,78,88,101,126,147,148 included patients with RR-DTC, whereas the review by Thomas et al. 138 included five prospective observational studies of RR-DTC,59,88,101,126,147 a retrospective study of RR-DTC149 and a Phase II study150 of patients with medullary thyroid cancer. Although the incidences of hand–foot syndrome (≥ 73%), diarrhoea (≥ 68%) and weight loss (≥ 50%) included in both meta-analyses were broadly similar to the incidence of the same AEs in DECISION, it was noticeable that the incidences of rash (≥ 66%) and fatigue (≥ 60%) were higher than reported in DECISION. Similarly, the pooled ORR (20.9% to 22%) from the two reviews127,138 was higher than the ORR reported in DECISION. The pooled median PFS (17.9 months) from the review by Thomas et al. 138 was also higher than median PFS reported in DECISION, but the pooled analysis for PFS also included patients with medullary thyroid cancer. The key results from these three reviews93,127,138 are summarised in Appendix 5, Table 52.
In addition, Shen et al. 127 noted that rare but severe AEs were observed mainly due to intracranial haemorrhage, cardiac arrest, angiooedema, small cell lung cancer, carcinoma of the tongue, and grade 5 event of sudden death. Because of the limited data, the authors did not pool these high-grade AEs. Thomas et al. 138 also reported that bleeding at any site occurred in 13.6% of patients, 3.8% of patients reported acute myocardial infarctions and 2.2% experienced congestive heart failure. Severe hypocalcaemia (grade ≥ 3) occurred in 2.5% of patients and 8.7% of patients developed cutaneous squamous cell carcinoma. However, it should be cautioned that in the meta-analyses conducted by Shen et al. 127 and Thomas et al. ,138 the authors did not investigate the heterogeneity of the studies included in the meta-analyses.
For RR-DTC, all of the indirect comparison results (including results from MAICs7,57) showed that lenvatinib was statistically significantly superior to sorafenib in terms of PFS but not OS. 6–8,57,97 Kawalec et al. 97 also reported lenvatinib to result in statistically significantly fewer cases of alopecia but statistically significantly more cases of hypertension and treatment-related SAEs, when compared with sorafenib. Bayer HealthCare7 reported sorafenib to result in fewer grade ≥ 3 AEs, SAEs and withdrawals owing to AEs when compared with lenvatinib. However, caveats about the generalisability of the results of the indirect comparisons have been raised,6 and Kawalec et al. 97 stated that indirect comparison results should be interpreted with caution because of differences in trial characteristics. Furthermore, during the current appraisal, Bayer HealthCare confirmed that it considered that the data from its indirect treatment comparison (ITCs)7 did not enable a robust comparison of sorafenib and lenvatinib given important differences between SELECT and DECISION. 151 Of the indirect comparisons conducted, only the indirect comparison by Kawalec et al. 97 was not sponsored by Eisai Ltd or Bayer HealthCare. A summary of the findings from the indirect comparisons is presented in Appendix 5, Tables 53–55.
Evidence from prospective observational studies
The AG included nine prospective observational studies in its review. 59,77,78,81,88,101,103,126,135 Five of these studies59,78,88,101,126 were included in the meta-analyses conducted by Shen et al. 127 and by Thomas et al. 138 Seven of the studies were included in the EPARs26,27 for lenvatinib77,135 and sorafenib. 59,78,88,101,126 The study and participant characteristics and efficacy and safety findings are summarised in Appendix 8, Tables 58–68.
All studies included patients whose disease was described as being radioactive iodine-refractory59,77,78,101,126,135 or resistant to radioactive iodine,81,88 or who may have received multiple treatments of radioactive iodine. 103 Two studies77,135 investigated the efficacy and safety of lenvatinib, six studies59,78,88,101,103,126 assessed the efficacy and safety of sorafenib and one study81 considered the efficacy of sorafenib. Some patients included in four of the studies59,88,101,135 had anaplastic or medullary carcinoma. Safety data from these four studies59,88,101,135 are, therefore, not reported for RR-DTC only. However, all nine studies59,77,78,81,88,101,103,126,135 reported efficacy findings for patients with RR-DTC only and all efficacy data reported in this section related to patients with RR-DTC only.
The E7080-G000-201 study (hereafter referred to as Study 20177) of lenvatinib was conducted in the UK, France, Italy, Poland, the USA and Australia. The E7080-J081-208 study of lenvatinib (hereafter referred to as Study 208135) was conducted in Japan. Studies of sorafenib were carried out in the UK,59 the Netherlands,126 Italy,103 Greece,81 the USA101 and China. 78 The earliest study was conducted between 2004 and 2005,101 and the most recent study was conducted between 2012 and 2015. 135 The length of study follow-up varied from a minimum of 3 months78 to a median of 51.6 months. 27
The number of patients included in the studies varied from 978 to 58. 77 In total, 109 patients were treated with lenvatinib, of whom 83 had RR-DTC; 213 patients were treated with sorafenib, of whom 186 had RR-DTC. In most studies, the majority of patients with RR-DTC had a histology of PTC,59,77,78,88,101,126 the exception being the study by Marotta et al. 103 in which the ratio of patients with FTC to patients with PTC was 2 : 1. The average age of participants ranged from 55 years59 to 64 years. 101 Four studies59,77,88,101 included a majority of males and three studies81,103,126 had a majority of females. Two studies78,135 did not report information on sex. The authors of only two studies77,101 reported information on race and these included a majority of white participants. Only two studies that reported ECOG status included patients with an ECOG PS of ≥ 2 (6.9%77 and 35.3%103). The same two studies were the only studies to explicitly state that patients could have received a prior TKI (11.8%103 to 29.3%77). There was scant and inconsistent reporting of the sites of metastases.
Median OS was reported in five studies. 77,88,101,126,135 Median OS ranged from 31.8 months135 to 32.3 months77 for lenvatinib and from 23 months101 to 34.5 months126 for sorafenib. Median PFS was reported in six studies77,88,101,103,126,135 and ranged from 12.6 months77 to 25.8 months135 for lenvatinib and from 12 months103 to 22.1 months for sorafenib88 (this last finding was reported in a subsequent conference abstract137). Chen et al. 78 (sorafenib) reported mean PFS (9.7 months). The ORRs ranged from 50.0%77 to 68.0%135 for patients treated with lenvatinib, and from 15% (histology of PTC)101 to 38.3%88 for those treated with sorafenib (this latter finding was reported in a subsequent conference abstract137). Median time to response and median duration of response were only reported in two studies. 77,126 For lenvatinib,77 median time to response was 3.6 months and, for sorafenib,126 all responses were reported to have happened within 6 months. The median duration of response was 12.7 months for lenvatinib77 and 29.6 months for sorafenib. 126
Key AEs are summarised in Appendix 8, Tables 61–66. Two studies88,101 (sorafenib) only reported treatment-related AEs. Two of the sorafenib studies,78,81 presented only as abstracts, reported very little information about AEs.
Incidences of the same types of AEs varied across the studies: for lenvatinib, hypertension and proteinuria were very commonly reported; for sorafenib, hand–foot syndrome, rash and alopecia were common; and diarrhoea and fatigue were common with both drugs. Data on SAEs were available only from Study 20177 (lenvatinib). Information on fatal AEs were reported only in two studies77,135 of lenvatinib and in one study101 of sorafenib. For patients treated with lenvatinib, 48% reported a SAE77 and up to 8%135 died from an AE. Only one death from AEs has been reported in one of the studies of sorafenib;101 it is unclear if the lack of reporting of fatal AEs in the other sorafenib studies59,78,81,88,103,126 means that there were no deaths from AEs in these studies. None of the deaths from AEs in any of the three studies77,101,135 reporting fatal AEs were described as being treatment related.
Ongoing studies and studies for which there are no results
The AG identified four ongoing studies,152–155 as summarised in Appendix 9 (see Table 69). None of the study results has been published or reported as a conference abstract. Only the two studies of lenvatinib154,155 are RCTs: E7080-G000-211 (Study 211)154 is a Phase II postauthorisation study that includes a randomised controlled phase, comparing two different starting doses of lenvatinib (24 vs. 18 mg) with placebo; E7080-C086-308 (Study 308)155 is a Phase III RCT being conducted in China comparing lenvatinib at its licensed dose of 24 mg with placebo. Eisai Ltd sponsors both of these trials. The other two studies are prospective observational Phase II studies of sorafenib:152,153 a pilot study sponsored by the Royal Marsden NHS Foundation Trust152 and a postauthorisation study sponsored by Bayer HealthCare. 153
In addition, although not strictly meeting the inclusion criteria for the current MTA, the AG is aware of an ongoing global prospective non-interventional study [Radioactive Iodine reFractory asymptomatic patients (RIFTOS), NCT02303444]156 of asymptomatic patients with RR-DTC treated with any type of MKI. The primary objective is to compare the time to symptomatic progression from study entry. Bayer HealthCare sponsors this study. The planned enrolment is approximately 700 patients and the expected study end date is 1 July 2020.
Discussion of clinical effectiveness: interpretation of results
The AG’s assessment of lenvatinib and sorafenib for the treatment of patients with RR-DTC focused on evidence from two RCTs: SELECT (lenvatinib vs. placebo) and DECISION (sorafenib vs. placebo). Supporting evidence was derived from 13 systematic reviews5–8,33,57,61,93,97,104,127,138,141 (including two systematic reviews described in the submissions from Eisai Ltd8 and Bayer HealthCare7) and nine prospective observational studies. 59,77,78,81,88,101,103,126,135
Clinical efficacy
Summary and interpretation of evidence: lenvatinib versus sorafenib
The primary objective of the AG’s systematic review was to compare the clinical effectiveness of lenvatinib with that of sorafenib. Results from the AG’s literature search revealed that there have been no head-to-head trials comparing the effectiveness of treatment with lenvatinib with the effectiveness of treatment with sorafenib. However, five studies6–8,57,97 have reported results from indirect comparisons and/or MAICs. Results from all of these analyses show that, compared with sorafenib, treatment with lenvatinib improves PFS but not OS.
The AG explored whether or not it was appropriate to conduct an indirect comparison. Although it was possible to construct a network, the AG identified issues that raised concerns about whether or not evidence from SELECT and DECISION could be included in the same network. First, there were differences between trial characteristics (prior treatment with TKIs, concurrent use of palliative radiotherapy and differences in subsequent treatment received on disease progression). Second, there were differences in participant characteristics (sex, race, geographic region, ECOG PS, time from diagnosis, histology and site of metastases) both within and between the trials. Third, the analysis of the PFS K–M data from the placebo arms of SELECT and DECISION showed that the risk profiles of the two trial populations were not comparable. The reasons for the differences in risk are currently unknown. Fourth, the AG considered that, for the majority of patient survival hazards assessed in the two trials, PHs were violated, the exception being unadjusted OS in DECISION.
The AG is unable to conclude whether or not treatment with lenvatinib is more effective than treatment with sorafenib for patients with RR-DTC. The AG considers that the results from the four published indirect comparisons7,8,57,97 should be interpreted with caution. This warning also extends to the results from the MAICs. 7,57 It is unknown whether or not the MAIC adjustments would fully account for all of the differences in the trial populations because the AG was unable to compare the adjusted risk profiles of patients included in the MAIC.
The AG highlights that Kawalec et al. 97 stated that their indirect comparison results should be interpreted with caution because of differences in the characteristics of the included trials. In addition, the EMA,27 Scottish Medicines Consortium (SMC)38 and Canadian Agency for Drugs and Technologies in Health (CADTH)6 all highlighted that differences in populations might have contributed to differences in results observed between the two trials. The SMC38 also highlighted that the validity of the results from the MAIC submitted by Eisai Ltd may be limited by weaknesses including heterogeneity across the studies in inclusion criteria, assessment of disease progression and analysis of PFS. The CADTH6 highlighted that the MAIC approach does not have the ability to control for the potential for unobserved or unrecorded differences between trials such as differences in standards of care or baseline characteristics. Furthermore, during the current appraisal, Bayer HealthCare confirmed that it considered that the data from its ITCs7 did not enable a robust comparison of sorafenib and lenvatinib given important differences between SELECT and DECISION (Bayer HealthCare response to AG report to the NICE Appraisal Committee, 6 September 2017). 157
Summary and interpretation of evidence: lenvatinib and sorafenib versus best supportive care
The secondary objective of the AG’s systematic review was to compare treatment with lenvatinib and sorafenib with BSC. The AG has assumed that, in both trials, treatment with lenvatinib plus BSC or sorafenib plus BSC is compared with placebo plus BSC. The unadjusted OS results from SELECT and DECISION demonstrated that there was no statistically significant difference in OS between treatment with lenvatinib and treatment with sorafenib versus placebo. After adjusting OS data for treatment crossover using RPSFTM, there was a statistically significant improvement in OS from treatment with lenvatinib compared with placebo; however, the difference in effect of sorafenib versus placebo was not statistically significant. The AG highlights that the unadjusted median OS estimates for patients treated with lenvatinib and sorafenib in SELECT and DECISION are higher than those reported for patients treated with lenvatinib and sorafenib in prospective observational studies.
For PFS and ORR, the results from SELECT and DECISION demonstrated that treatment with both lenvatinib and sorafenib were statistically significantly better than treatment with placebo for patients with RR-DTC. For all of the prespecified subgroups, the results from SELECT and DECISION favoured treatment with the intervention (lenvatinib or sorafenib) when compared with placebo. Median PFS and ORR for patients treated with lenvatinib in SELECT were higher than the prospective observational results from Study 20177 and lower than the results from Study 208. 135 In contrast, median PFS and ORR results reported for patients treated with sorafenib (DECISION) were lower than findings from any of the prospective observational studies or the two meta-analyses. 127,138
Patients in DECISION were permitted to receive concomitant palliative radiotherapy, a common component of BSC in NHS clinical practice, whereas patients in SELECT were not; full details of the BSC provided in the two trials are not available. Whether or not patients in the trials received BSC similar to that provided by the NHS is unknown and this raises uncertainty about whether or not the trial results are generalisable to NHS patients. If the BSC delivered in the two trials is not comparable, then using the placebo arms to connect the two trials in an indirect comparison becomes even more challenging. However, as the rates of palliative radiotherapy administered to patients in DECISION are low (10.6% of patients treated with sorafenib and 21.4% of patients treated with placebo), then perhaps this issue is not important.
There are two important issues to consider when interpreting the RCT evidence. First, a caveat to the use of the RPSFTM-adjusted OS results from both trials is that the method requires the assumption that postprogression anti-cancer treatments, other than those permitted by treatment crossover, represent routine clinical practice. For patients with RR-DTC, there is currently no standard of care for patients with progressive disease. Therefore, it is unknown whether or not the poststudy anti-cancer treatments administered to patients in SELECT and DECISION reflect the treatments that would be offered to patients in the NHS. Second, the AG’s examination of the PH assumption for OS (unadjusted and adjusted) and PFS in SELECT and DECISION showed that the PH assumption does not hold for any of these outcomes other than unadjusted OS in DECISION. This means that the majority of the HRs reported in the company submissions should be interpreted with caution. However, clinical advice to the AG is that the PFS results for the overall populations of SELECT and DECISION are clinically meaningful.
Safety
Summary and interpretation of evidence: lenvatinib versus sorafenib
The AG did not conduct its own indirect comparison to facilitate a comparison of the effect of treatment with lenvatinib with the effect of treatment with sorafenib for AEs. However, two other reviews7,97 reported results from indirect comparisons of AEs. Kawalec et al. 97 reported that treatment with lenvatinib resulted in statistically significantly less alopecia but statistically significantly more hypertension and treatment-related SAEs than sorafenib. Bayer HealthCare7 reported sorafenib to result in fewer grade ≥ 3 AEs, SAEs and withdrawals owing to AEs when compared with lenvatinib.
Summary and interpretation of evidence: lenvatinib and sorafenib versus best supportive care
When compared with placebo, treatment with both lenvatinib and sorafenib resulted in increased AEs. However, although diarrhoea was experienced by just over two-thirds of patients treated with both drugs in SELECT and DECISION, there were some notable differences in the safety profiles. Hypertension and decreased appetite were reported by over half of patients in SELECT, whereas the most common AEs reported by half or more of patients in DECISION were hand–foot syndrome, alopecia and rash. Grade ≥ 3 hypertension was very common in patients treated with lenvatinib (> 40%), and grade ≥ 3 hand–foot syndrome was very common in patients treated with sorafenib (> 20%). Hypertension was also reported to be one of the most common SAEs in SELECT (3.4%). Data on the median time to onset of AEs91,139 from SELECT and DECISION suggest that AEs typically occur early, with a decrease in incidence, prevalence and severity over time. In DECISION, exceptions were diarrhoea that increased in prevalence over the first six cycles and weight loss that increased in severity (from grade 1 to grade 2) over the first nine cycles.
Overall, the safety findings from the RCTs were consistent with the findings from prospective observational studies of lenvatinib77,135 and sorafenib,59,78,81,88,101,126 although it is noticeable that the incidence of some AEs varied quite widely in observational studies for patients treated with sorafenib. However, meta-analyses127,138 of data from observational studies for hand–foot syndrome and diarrhoea reported incidences of all-grade and grade ≥ 3 AEs to be similar to those reported in DECISION. It has, however, been found in a systematic review by Jean et al. 93 that the incidence of common all-grade AEs tends to be higher for patients with RR-DTC than for patients with RCC or HCC and also for some patients with grade ≥ 3 hand–foot syndrome and rash.
After diarrhoea, hypertension was the most common reason for dose modifications, as well as being the most common reason (alongside asthenia) for discontinuations in SELECT. In DECISION, the most common reason for dose modifications and discontinuations was hand–foot syndrome. Dose reductions were frequent (> 60%) for patients treated with both lenvatinib and sorafenib. Life-threatening AEs from treatment with lenvatinib and sorafenib were rare. The AG considers that the AEs associated with treatment with lenvatinib and sorafenib can be managed with usual medical care and dose modifications, including treatment withdrawal. Clear guidance for managing AEs is set out in the SmPCs for lenvatinib45 and sorafenib. 46
Health-related quality-of-life findings
The HRQoL data were not collected as part of SELECT, and HRQoL data from the 30 patients who participated in the open-label extension phase of SELECT are not yet available. This is disappointing given that the investigators in the earlier DECISION had measured and reported HRQoL outcomes and highlighted that HRQoL may be negatively impacted by treatment with TKIs. 7,120 AE rates were high in SELECT and it would have been informative if HRQoL data had been collected. HRQoL research is much needed as HRQoL is one of the most important outcomes to consider, both from the perspective of patients and for assessing comparative cost-effectiveness.
The HRQoL data collected during DECISION demonstrated that the FACT-G scores were higher for patients in the placebo arm than for patients in the sorafenib arm, indicating a higher HRQoL for patients receiving placebo. The negative impact of treatment with sorafenib on HRQoL may be linked to the high rates of AEs. 7,120 Indeed, it has been noted by Bayer HealthCare7 that in response to the question on the FACT-G questionnaire ‘I am bothered by side effects’, the proportion of patients in the sorafenib arm who replied ‘quite a bit’ or ‘very much’ increased from 1.5% in cycle 1 to 29.6% in cycle 2 but then gradually diminished over time.
There are, however, limitations to the results from the HRQoL analyses. Although the overall questionnaire completion rate during DECISION was reported to be 96%,120 the number of patients eligible to complete the questionnaires diminished with every cycle because only those who had not experienced progression were asked to complete the questionnaire. This also means that there are no HRQoL data available from patients whose disease has progressed. It is also unknown whether or not there is a direct correlation between HRQoL and AEs and how the different types of AEs experienced by patients treated with lenvatinib (e.g. hypertension) and sorafenib (e.g. hand–foot syndrome) affect HRQoL. Finally, to what extent a patient’s HRQoL is affected by their symptom status (symptomatic vs. asymptomatic) is unknown.
Generalisability of findings
The AG considers that the generalisability of the findings from SELECT and DECISION to NHS clinical practice is questionable. This concern is driven by the fact that clinical advice to the AG is that in clinical practice there are concerns about the toxicity of TKI therapy in patients and effects on the quality of life of patients with asymptomatic disease and so treatment is more commonly given when symptomatic or clinically significant progressive disease develops. Hence, BSC is a common treatment option for this group. The authors of two of the meta-analyses of sorafenib127,138 concluded that the high incidence of AEs associated with sorafenib may affect the quality of patients’ lives and most patients with metastatic disease do not require systemic therapy. This view is supported by several clinical guidelines4,24,25 as patients experiencing RR-DTC symptoms and/or those with rapidly progressing disease are considered to be in greatest need of systemic treatment. 31 In addition, the EMA concluded that maximum lesion size, symptoms related to the disease and progression rate should be carefully considered for each individual patient before initiating treatment. 26
Although all of the patients in SELECT and DECISION had RR-DTC, it is unclear how many had symptomatic and/or rapidly progressing disease; however, it is reported in the EPAR26 for sorafenib that results from a post hoc subgroup analysis of data from DECISION suggest that 20% of patients were likely to be symptomatic. Clinical advice to the AG is that this is probably typical of the proportion seen in clinical practice. It is unclear how many patients in SELECT were symptomatic and/or had progressive disease.
The post hoc retrospective analysis of data from patients participating in DECISION113,119 categorised patients as having symptomatic disease if they had symptoms/findings that were consistent with RR-DTC reported in the medical history or pre-treatment AE data set at baseline. Clinical advice to the AG is that there are no generally agreed definitions of ‘symptomatic’ or ‘rapidly progressive disease’ and that, in clinical practice, the definition of a patient’s disease status depends on individual patient characteristics.
Results from the post hoc analysis show that median PFS was similar for all patients treated with sorafenib, irrespective of whether or not they were symptomatic or asymptomatic (10.7 months and 10.8 months, respectively, compared with 10.8 months for all patients in the sorafenib arm of the trial). 7 However, for patients in the placebo arm, median PFS was much lower for symptomatic patients (3.6 months) than for asymptomatic patients (7.2 months), and it was also lower than for all patients in the placebo arm of the trial (5.8 months).
No analyses have been undertaken to compare the effectiveness of treatment with lenvatinib for symptomatic patients with that for asymptomatic patients. In the absence of such analyses, no assumptions can be made about relative effectiveness. However, clinical advice to the AG is that, like sorafenib, only patients with symptomatic and/or progressive disease are likely to be treated with lenvatinib in the NHS.
The most recent published guidelines for treating RR-DTC, by the NCCN,25 recommend lenvatinib or sorafenib as the treatment of choice for patients with progressive and/or symptomatic disease. However, the choice between lenvatinib and sorafenib should be based on the individual patient, taking into account the likelihood of response and comorbidities. 25
There are further important caveats regarding the generalisability of the findings from SELECT and DECISION to NHS clinical practice.
The first caveat is that, although most patients participating in the trials had a diagnosis of PTC, as would be expected in clinical practice, there were proportionately more patients with other types of DTC than would be expected in NHS clinical practice. Patients with these other types of DTC are reported to have a worse prognosis than patients with PTC. 15 However, subgroup and exploratory analyses of SELECT data showed that for unadjusted OS, there was a statistically significant OS gain for patients with FTC treated with lenvatinib compared with those treated with placebo,82 and that histology (favouring FTC vs. PTC) was statistically significantly associated with increased OS. 91 These exploratory results warrant further investigation.
The second caveat relates to the age of patients. Thyroid cancer incidence is strongly related to age, with the highest incidence rates being in older males (aged > 60 years) and the highest incidence rates in females being in younger and middle-aged women (aged 40–60 years). 1 The median age of patients was 61 years in the lenvatinib arm and 64 years in the placebo arm of SELECT and 63 years in both arms of DECISION, and approximately half of the patients in both trials were male. Given that the median time from diagnosis in the trials varied from between 5.5 and 6 years, it appears that, in general, patients were older than may be seen in clinical practice. Moreover, the prognosis of patients tends to differ for patients aged < 45 years and for those aged ≥ 45 years, as reflected in the staging criteria used for DTC. 4 Detailed data on the age range of included patients were not reported for either trial.
Other issues of relevance to clinical practice
The relative importance of ORR also warrants some discussion, particularly given the marked reported differences in effect between treatment with lenvatinib and sorafenib indicated by results from SELECT and DECISION and the prospective observational studies. 59,77,78,81,88,101,103,126,135 Although studies of lenvatinib51,77,135 suggest that at least half of all patients achieve a response, meta-analyses of data from observational studies of sorafenib127,138 suggest that no more than 22% of patients receiving this treatment respond. This finding reflects the finding from a systematic review of TKIs33 that shows that the most likely outcome of treatment with a TKI is stable disease. Indeed, in DECISION, 42% of patients in the sorafenib arm had stable disease for ≥ 6 months (and 12.2% had an objective tumour response) compared with 33% in the placebo arm (and 0.5% had an objective tumour response). However, given that lenvatinib and sorafenib are likely to be preferred treatment options for patients with clinically significant progressive disease, reducing the rate of disease progression may be a more relevant outcome. The AG notes that in the submission from Bayer HealthCare,7 it is reported that most patients (77%) in the sorafenib arm of DECISION experienced target lesion tumour shrinkage, compared with 28% of patients in the placebo arm. The authors of a systematic review of sorafenib104 for treating RR-DTC concluded that, although the data in the review came primarily from non-randomised Phase II trials (but also included DECISION), the results suggest that treatment with sorafenib slows the progression of disease in the majority of cases.
The findings from the extended open-label phases of SELECT and DECISION should also be considered. These findings show that the median PFS and ORR outcome results for patients previously randomised to the placebo arms but who crossed over to receive lenvatinib or sorafenib at the licensed doses were similar to the median PFS and ORR reported for patients treated with lenvatinib and sorafenib in the double-blind phases of the trials. Given that patients in the placebo arm received no active systemic therapies during the double-blinded phase, these results appear to support the view that patients with progressive disease do not need to be treated immediately and can be treated when showing symptoms and/or rapidly progressing. However, the AG cautions that data on symptoms and/or whether or not patients were rapidly progressing are lacking, although patients were progressing to the extent that, on the basis of RECIST (Response Evaluation Criteria in Solid Tumours) criteria, they were considered to have progressive disease. The AG also cautions that no OS data were available for these specific cohorts of patients.
The results from the open-label phase of SELECT, which included patients who crossed over from placebo to receive treatment with two different doses of lenvatinib, suggest that PFS may be improved for those starting at the 20-mg dose (median PFS not reached) as opposed to the licensed dose of 24 mg (median PFS of 17.5 months). However, the numbers of patients in each group, particularly in the 20-mg dose cohort, were small, and definitive conclusions could not be reached. Study 211,154 an ongoing Phase II RCT, compares two different starting doses of lenvatinib (24 vs. 18 mg) with placebo. This trial is expected to end in October 2020.
Although patients treated with lenvatinib in SELECT were not permitted to receive additional lenvatinib in the extended open-label phase of that trial, around one-quarter of patients had received treatment with a VEGFR-targeted therapy, including sorafenib, prior to enrolment. SELECT subgroup PFS and ORR findings suggest that patients benefited from treatment with lenvatinib, regardless of whether or not they had received prior treatment with a VEGFR-targeted therapy. This result suggests that lenvatinib could be used as a first- or second-line treatment for patients with RR-DTC. Further research is required to identify the effect on OS of treating patients with lenvatinib followed by sorafenib. Furthermore, it has also been reported that SAEs were more common in the lenvatinib arm among patients who had received a prior VEGFR-targeted treatment (60.6%) than among those who had not (50.8%). 105,106
Some patients in DECISION who had experienced disease progression while receiving sorafenib were also eligible to receive sorafenib for a second time in the extended open-label phase of DECISION. Clinical advice to the AG was that, in NHS practice, patients could be prescribed sorafenib post progression as there is a view that continued treatment with sorafenib will slow the progression of disease. This expectation is supported, to some extent, by exploratory post hoc findings. 83,94 These findings suggest that despite evidence of tumour growth or prior RECIST progression, treatment with sorafenib continued to slow tumour growth for patients who had also been treated with sorafenib during the randomised phase, when compared with tumour growth for patients treated with placebo during the randomised phase. 83,94 However, as concluded by authors of other abstracts114,122 reporting results from the open-label extension phase of DECISION, the effect of continued treatment with sorafenib after progression needs to be explored further.
Finally, there are no data for patients treated with sorafenib followed by lenvatinib. Further research is needed to identify the effect on OS and other efficacy and safety outcomes of treating patients with lenvatinib followed by sorafenib, and sorafenib followed by lenvatinib.
Chapter 5 Assessment of cost-effectiveness
The AG conducted a systematic review of the economic literature to identify the existing evidence assessing the cost-effectiveness of treatment with lenvatinib and sorafenib (vs. each other and vs. BSC) for people with progressive, locally advanced or metastatic RR-DTC. The review focused on the decision problem outlined in the final scope issued by NICE. 53 The economic evaluations presented in the submissions by Eisai Ltd8 and Bayer HealthCare7 are discussed and critiqued separately in Summary of the key features of the companies’ economic models.
Search strategy
The AG identified cost-effectiveness studies by searching EMBASE, MEDLINE, the NHS Economic Evaluation Database via The Cochrane Library and EconLit from 1999 onwards. The starting date for all of the searches was 1999 and all databases were searched on 10 January 2017. Based on the fact that the FDA approved sorafenib for its first indication in 2005, and lenvatinib in 2015, the AG considered that this date span would allow all relevant economic evidence to be identified. The reference lists of included publications, in addition to the NICE, SMC and CADTH websites, were hand-searched. The results of the searches were entered into an EndNote X7.4 library and de-duplicated.
Study selection and inclusion criteria
Publications were selected for inclusion in the review based on their relevance to the decision problem and the specific economic criteria presented in Table 12. In addition to costs, quality-adjusted life-years (QALYs), cost–benefit and cost-effectiveness outcomes, such as cost per PFS year, were also extracted from relevant publications.
| Criteria | Inclusion |
|---|---|
| Population | Adults with progressive, locally advanced or metastatic RR-DTC |
| Intervention |
|
| Comparators |
|
| Costs | Direct health-care costs |
| Outcomes | Incremental cost per life-year gained and/or incremental cost per QALY gained |
| Study design | Full economic evaluations that consider both costs and consequences (cost-effectiveness analysis, cost–utility analysis, cost-minimisation analysis and cost–benefit analysis) |
| Date span | 1999 to 10 January 2017 |
| Language | English language only |
Two reviewers (RH and NF) independently screened the titles and abstracts of all publications identified by the searches. The same two reviewers then independently retrieved and assessed (for inclusion) the full texts of the publications that had been identified as being potentially relevant to the review. Disagreements about inclusion in the review were resolved through discussion and, in all cases, a consensus was reached; it was, therefore, not necessary to consult a third reviewer during the screening and selection process.
Quantity of evidence
The searches for economic evidence identified 19 citations in total: 14 were obtained from the database searches and five were identified from other sources. Once duplicates were removed, 18 publications remained and, after assessment of the titles and abstracts, 10 publications5,38,48,158–164 were retrieved and a detailed assessment of their eligibility was undertaken.
Nine of these 10 publications were included in the review. The AG included four publications158–160,163 that clearly met the inclusion criteria. The AG considered that the economic evidence for lenvatinib and sorafenib that had been submitted to the SMC38,48 and CADTH5,162 was also relevant to this review and so these four records,5,38,48,162 one for each drug’s individual submission to each regulatory agency, were included in the review. One further relevant publication161 was identified during the citation search of the included publications; this publication became available online only after the AG’s database searches had been completed.
One publication164 was a budget impact analysis and was, therefore, excluded from the review.
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the process of study selection is shown in Figure 8.
FIGURE 8.
The PRISMA flow diagram: the AG economic literature review.

A summary of the characteristics of the nine included publications5,38,48,158–163 is presented in Table 13.
| Study | Characteristic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Country; perspective | Intervention | Study design/purpose | Comparators | Reported measures | Cost/outcome source | Time horizon/cycle length/discount rate | Cost year | Further information on publication type | |
| Erdal et al. 2015163 | Turkey; Turkish health-care system | Sorafenib | Cost-effectiveness/utility analysis | BSC | QALYs and LYs; costs calculated in Turkish lira and converted (2.2) to US$ |
Clinical inputs from DECISION Resource use via expert panel |
Time horizon: lifetime (maximum 30 years) Cycle length: 28-days Discount rate: NR |
Mid-2014 | Abstract only |
| Huang et al. 2016158 | USA; US health-care system | Lenvatinib, sorafenib | Cost–utility analysis | Placebo and each other | QALYs; costs in US$ |
Effectiveness estimates taken from DECISION and SELECT Costs and utilities from RED BOOK Online® (Truven Health Analytics, IBM Micromedex®, Ann Arbor, MI, USA, Chicago, IL, USA and Denver, CO, USA), Healthcare Cost and Utilization Project (Agency for Healthcare Research and Quality, Rockville, MD, USA), Medicare Fee Schedule (US Department of Health and Human Services, Centers for Medicare and Medicaid Services, Baltimore, MD, USA) and published literature (additional references NR) |
Time horizon: lifetime Cycle length: bimonthly Discount rate: 3% |
2015 | Abstract only |
| Huang et al. 2016159 | USA; US health-care system | Lenvatinib, sorafenib | Expected value of perfect information analysis | Placebo and each other | ICER per QALY and EVPI per person; costs in US$ |
Effectiveness estimates taken from DECISION and SELECT Costs and utilities from RED BOOK Online®, Healthcare Cost and Utilization Project, Medicare Fee Schedule and published literature (additional references NR) |
Time horizon: lifetime Cycle length: bimonthly Discount rate: 3% |
2015 | Abstract only |
| Tremblay et al. 2016160 | USA; US health-care system |
Lenvatinib sorafenib |
Cost-effectiveness/utility analysis | Each other | Costs and QALYs, cost per PFS year, cost per LY, cost per QALY, cost per responder; costs in US$ |
IHS global pricing database,165 CMS database166 and published sources Kerr et al. 167 is the source of EQ-5D utilities |
Time horizon: 10 years (5-year horizon outcomes also reported) Cycle length: 1 month Discount rate: 5% [via correspondence with author: Dr Gabriel Tremblay, Purple Squirrel Economics (previously at Eisai Ltd), June 2017] |
Not fully reported but states that the costs used to estimate BSC are from 2014 2014 used as cost year for currency conversion estimate |
Abstract and accompanying poster and correspondence with author |
| Wilson et al. 2017161 | USA; US health-care system | Lenvatinib, sorafenib | Cost–utility analysis | Placebo and each other | QALYs; costs in US$ |
Effectiveness estimates taken from DECISION and SELECT Costs and utilities from RED BOOK Online®, Healthcare Cost and Utilization Project, Medicare Fee Schedule and published literature, including Fordham et al. 168 for utilities |
Time horizon: lifetime Cycle length: bimonthly Discount rate: 3% |
2015 | Peer-reviewed journal article |
| SMC 201548 | Scotland; Scottish NHS | Sorafenib | Cost–utility analysis | BSC | ICER per QALY; costs in Great British pounds | Rates of effectiveness and resource use from DECISION |
Time horizon: not explicitly stated, but text implies that it is > 15 years Cycle length: NR Discount rates: NR |
NR – 2015 used as cost year for currency conversion estimate |
Summary of model and submission to the SMC |
| SMC 201638 | Scotland; Scottish NHS | Lenvatinib | Cost–utility analysis | BSC and sorafenib | ICER per QALY, incremental LYs; costs in Great British pounds | Effectiveness and resource use evidence from SELECT and DECISION |
Time horizon: lifetime Cycle length: NR Discount rates: NR |
NR – 2016 used as cost year for currency conversion estimate |
Summary of model and submission to the SMC |
| CADTH 20155 | Canada; Canadian health-care system | Sorafenib | Cost–utility analysis | BSC | ICER per QALY; incremental costs, QALYs and LYs; costs in CA$ | NR |
Time horizon: 10-year base-case horizon (re-estimated at 7 years for main results) Cycle length: NR Discount rates: NR |
NR – 2015 used as cost year for currency conversion estimate | Summary of model and submission to CADTH |
| CADTH 2016162 | Canada; Canadian health-care system | Lenvatinib | Cost–utility analysis | BSC and sorafenib (results reported for BSC comparison only) | ICER per QALY; incremental costs, QALYs and LYs; costs in CA$ | Effectiveness data from SELECT and DECISION |
Time horizon: 10-year base-case horizon (re-estimated at 7 years for main results) Cycle length: 30.4 days Discount rate: NR |
2016 | Summary of model and submission to CADTH |
Quality of the included evidence
The quality of the included evidence was assessed using the NICE reference case checklist169 and the Drummond checklist. 170 Summary tables of the AG’s quality assessments are presented in Appendices 11 (see Table 72) and 12 (see Table 73). Full details of the completed checklists are presented in Appendices 13 (see Tables 74–81) and 14 (see Tables 82–89). The publications by Huang et al. 158,159 have been evaluated together as the same economic model was used to generate results for both publications.
Only the Wilson et al. 161 publication was available as a full-text paper published in a peer-reviewed journal. Three of the included publications158,159,163 were available only as abstracts, and one publication160 was available as a poster. The submissions to the regulatory bodies in Scotland38,48 and Canada5,162 were available only as summary reports. As a result, only limited information was available from most of the included publications and this hindered the quality assessment of some of the methodologies described in the publications.
The authors of all of the included publications produced incremental cost-effectiveness estimates enabling a single metric [an incremental cost-effectiveness ratio (ICER) per QALY gained] to be used for comparative purposes. All of the publications included a discussion of the certainty associated with study results; however, full details of the sensitivity analyses and parameter values were not always available in the text.
Generally, the text describing the assumptions and data sources used to generate resource use, costs and HRQoL estimates within the economic models was not clear. In addition, it was unclear whether or not the costs and benefits described in the publications were discounted appropriately. Results from analyses of the cost-effectiveness of all the relevant comparators (lenvatinib, sorafenib and BSC) were available from only four of the reviewed publications. 158–161
None of the publications considered the decision problem from the perspective of the NHS in England. However, as the Scottish NHS provides a sufficiently similar environment to the NHS in England, the AG considered that, for the purposes of this appraisal, the results from the SMC submissions38,48 are broadly generalisable to patients in England. The characteristics of the health-care systems, in terms of the way treatments are procured and used in the USA,158,159,161 Canada5,162 and Turkey,163 make the results from analyses based on these perspectives less useful when considering treatment options for patients in the NHS in England. However, including these studies5,158,159,161–163 in this review allows a broad range of cost-effectiveness estimates to be considered and provides some indication of the effect of varying assumptions, such as the model time frame and estimates of HRQoL.
Assessment Group economic review: overview of included publications
The AG identified nine relevant publications5,38,48,158–163 describing the cost-effectiveness of treatment with lenvatinib and sorafenib in a population of patients with RR-DTC. When necessary, authors were contacted and asked to provide further information on methodological aspects that lacked clarity in the publications; only one lead author160 replied and provided the discount rate used in the model.
One publication163 considered the cost-effectiveness of treatment with sorafenib compared with usual care in the Turkish setting. Four publications158–161 compared treatment with lenvatinib with treatment with sorafenib from a US perspective. The SMC submissions38,48 considered resource use in the Scottish NHS, and the CADTH submissions5,162 included analyses that were undertaken from the perspective of the Canadian health-care system. The results reported in the publications5,38,48,158–162 comparing the cost-effectiveness of lenvatinib with the cost-effectiveness of sorafenib are based on the results of indirect comparisons. This means that the authors considered that the trial and patient characteristics of SELECT and DECISION were sufficiently comparable for their data to be compared using this methodology. The AG discusses the limitations of using data from SELECT and DECISION in an indirect comparison in Chapter 4, Indirect comparison feasibility assessment.
The costs, benefits and incremental results from each of the publications are presented in Table 14. All costs from 2014 have been inflated to 2015/16 prices using the hospital and community health services index. 171 Analyses conducted using 2015 and 2016 prices have not been inflated as the 2016/17 inflation indices were not available. When the year that costs used within the model is not reported, the year of publication is used as a proxy. When necessary, all cost data have been converted to Great British pounds using the Bank of England exchange rate as of 25 May 2017. 172
| Study | Interventions | Costs (£) | LYs | QALYs | Incremental | ICER (£) | |||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£)a | LYs | QALYs | Per LY gained | Per QALY gained | |||||
| Erdal et al. 2015163 | BSC | NR | NR | NR | |||||
| Sorafenib | NR | NR | NR | 19,084 | 1.29 | 0.80 | 14,754 | 23,859 | |
| Huang et al. 2016158 | Placebo | 657,493 | NR | NR | |||||
| Lenvatinib | 152,448 | NR | NR |
–505,045 (vs. BSC) 25,491 (vs. sorafenib) |
NR | NR | NR | 61,109 (vs. sorafenib) | |
| Sorafenib | 126,957 | NR | NR | –530,536 (vs. BSC) | NR | NR | NR | ||
| Huang et al. 2016159 | Lenvatinib vs. sorafenib | NR | NR | NR | NR | NR | NR | 73,913 | |
| bTremblay et al. 2016160 | Lenvatinib | 217,527 | 2.71 | 1.77 | 40,697 | 0.33 | 0.42 | 124,843 | 96,671 |
| Sorafenib | 176,830 | 2.38 | 1.35 | ||||||
| cTremblay et al. 2016160 | Lenvatinib | 228,637 | 3.38 | 2.10 | 44,626 | 0.58 | 0.54 | 76,835 | 81,338 |
| Sorafenib | 184,010 | 2.80 | 1.56 | ||||||
| Wilson et al. 2017161 | Placebo | 107,898 | NR | 0.71 | |||||
| Lenvatinib | 127,819 | NR | 1.34 | 7368 (vs. sorafenib) 19,921 (vs. placebo) | NR |
0.37 (vs. SOR) 0.63 (vs. placebo) |
NR |
19,522 (vs. sorafenib) 31,566 (vs. placebo) |
|
| Sorafenib | 120,451 | NR | 0.96 | 12,553 (vs. placebo) | NR | 0.25 (vs. placebo) | NR | 49,484 (vs. placebo) | |
| SMC 201548 | Sorafenib vs. BSC | NR | NR | NR | NR | NR | NR | NR | 32,083 |
| SMC 201638 | Lenvatinib vs. sorafenib | NR | NR | NR | NR | NR | NR | NR | 49,525 |
| dCADTH 20155 | Sorafenib vs. BSC | NR | NR | NR | 42,824 | 0.86 | 0.52 | 49,795 | 82,080 |
| eCADTH 20155 | Sorafenib vs. BSC | NR | NR | NR | 45,744 to 46,054 | NR | 0.38–0.42 | NR | 108,974 to 118,913 |
| dCADTH 2016162 | Lenvatinib vs. BSC | NR | NR | NR | 60,784 | 1.01 | 0.84 | 60,182 | 72,536 |
| eCADTH 2016162 | Lenvatinib vs. BSC | NR | NR | NR | 84,687 | 1.03 | 0.84 | 98,343 | 101,293 |
Erdal et al.163
The authors described a partition survival model that used clinical evidence from DECISION, supplemented with Turkey-specific resource use and cost information, to generate estimates of the cost-effectiveness of treatment with sorafenib versus BSC in a population of people with locally advanced or metastatic RR-DTC. Deterministic results were presented and the ICER per QALY gained for the comparison of treatment with sorafenib with treatment with BSC was £23,859. The authors concluded that the results of the one-way deterministic analyses and probabilistic sensitivity analysis (PSA) were similar to the main set of deterministic results. However, as few details of the parameters and values that were used to estimate the level of uncertainty around results were reported in the publication, the AG was not able to ascertain the reliability of results generated by the sensitivity analyses. Despite not reporting a willingness-to-pay threshold, the authors considered sorafenib to be a cost-effective treatment compared with BSC.
Huang et al.158
The Markov model described by the authors used effectiveness evidence from the Phase III trials SELECT and DECISION. Results from one-way sensitivity analyses showed that the base-case model results were sensitive to changes to the costs of lenvatinib and sorafenib and the utility benefit of continuing with lenvatinib. The AG notes that the value and duration of the utility benefits were not reported. The base-case ICER for the comparison of treatment with lenvatinib with treatment with sorafenib was £61,109 per QALY gained.
Huang et al.159
The authors reported the methods and results of an expected value of perfect information (EVPI) analysis using the same model described in the abstract by Huang et al. 158 An ICER of £73,913 per QALY gain was reported, indicating that treatment with lenvatinib offers an increase in benefit over sorafenib, but at an additional cost. At a willingness-to-pay threshold of approximately £77,000 per QALY gained, the probabilities of lenvatinib and sorafenib being cost-effective were low (37% and 33%, respectively). Owing to uncertainty around the reliability of model results, the authors were not certain that treatment with lenvatinib was cost-effective when compared with sorafenib and placebo.
Tremblay et al.160
The poster included results from a cost-effectiveness analysis from a partition survival model designed to compare treatment with lenvatinib and treatment with sorafenib using clinical evidence from the Phase III SELECT and DECISION. The base-case ICER for the comparison of treatment with lenvatinib with treatment with sorafenib was £81,338 per QALY gained when a 10-year time horizon was modelled, and £96,671 per QALY gained when a 5-year time horizon was modelled.
Costs per PFS year (£58,833 with a 5-year time horizon and £62,318 with a 10-year time horizon), costs per responder (£77,372 with a 5-year time horizon and £84,841 with a 10-year time horizon) and life-year saved were also reported in the publication. The authors did not set a willingness-to-pay threshold to determine at what level the cost per responder, for example, would offer good value for money. The authors refer to PSA in the publication but do not report the methods or the results of the analysis.
Wilson et al.161
The same set of authors who produced the abstracts by Huang et al. 158,159 authored a full-text paper comparing the cost-effectiveness of treatment with lenvatinib with that of sorafenib, in which they described a Markov model that used effectiveness data from the Phase III trials SELECT and DECISION. ITCs to compare the effectiveness of lenvatinib with the effectiveness of sorafenib were made following adjustments to the placebo arms of the trials as the authors considered that the placebo arm of SELECT included patients who appeared to be healthier than those in the comparator arm of DECISION. However, the AG does not consider that the adjustments are sufficient to generate reliable estimates of the comparative effectiveness of lenvatinib and sorafenib. In addition, as discussed in Chapter 4, Indirect comparison feasibility assessment, the AG does not consider that it is appropriate to undertake an indirect comparison of the effectiveness of lenvatinib and the effectiveness of sorafenib using data from SELECT and DECISION.
The results of the authors’ cost–utility analysis differ from those reported in the abstracts. 158,159 In the base-case analysis, treatment with lenvatinib generated more benefits (+ 1.34 QALYs) than treatment with sorafenib (+ 0.96 QALYs), as well as more benefits than placebo (+ 0.71 QALYs), but at an increased cost of £7368 versus sorafenib and £19,921 versus placebo. The base-case ICER for the comparison of treatment with lenvatinib with treatment with sorafenib was £19,522 per QALY gained. The base-case ICERs for the comparison of treatment with lenvatinib with placebo and treatment with sorafenib with placebo were £31,566 and £49,484 per QALY gained, respectively.
Sorafenib Scottish Medicines Consortium submission48
For the comparison of treatment with sorafenib with BSC, the ICER was £32,083 per QALY gained; the Scottish PAS price of sorafenib was used in the analysis. 48 These results were sensitive to the time horizon of the model and the approach used to estimate OS, with the ICER increasing with a shortened time horizon and with a change to the OS extrapolation method employed.
Lenvatinib Scottish Medicines Consortium submission38
For the comparison of treatment with lenvatinib with treatment with sorafenib, the base-case ICER was £49,525 per QALY gained; this analysis used the Scottish PAS price for lenvatinib and Eisai Ltd’s estimate of the Scottish PAS discount currently in place for sorafenib. 38 The ICERs per QALY gained were sensitive to the estimates of OS for lenvatinib (ranged from £29,000 to £96,000 per QALY gained with PAS prices) and to changing the utility rates used in the model by 20% (ranged from £41,000 to £62,000 per QALY gained with PAS prices).
Sorafenib Canadian Agency for Drugs and Technologies in Health submission5
The company’s base-case cost-effectiveness estimate was that treatment with sorafenib versus BSC resulted in an ICER of £82,080 per QALY gained. Several other ICERs per QALY gained were also presented as a result of reanalyses suggested by the Economic Guidance Panel. The reanalyses included amendments to the time horizon, the duration of treatment and estimates of OS. The results from the reanalyses ranged from £108,974 to £118,913 per QALY gained.
Lenvatinib Canadian Agency for Drugs and Technologies in Health submission162
The base-case analysis for the comparison of lenvatinib with BSC, submitted by the company, generated an ICER of £72,536 per QALY gained. This increased to £101,293 per QALY gained when the amendments suggested by the Economic Guidance Panel were implemented. The reanalysis included amendments to OS estimates, time horizon, use of the intervention drug in terms of both wastage and the appropriate pack size to reach the required dosage, and the utility values used within the model.
Although the company submitted results from additional analyses comparing the cost-effectiveness of lenvatinib with the cost-effectiveness of sorafenib to CADTH, these results were not presented in the available CADTH guidance report. 162
The AG notes that the SMC38,48 and CADTH5,162 reports highlight concerns about the clinical effectiveness data derived from SELECT and DECISION. Key issues of concern related to median OS not being reached and the high rates of treatment crossover from the placebo (BSC) arms to the intervention arms (lenvatinib or sorafenib) that took place during the trials.
The Assessment Group’s review of economic evidence: summary and conclusions
The published economic evidence163 shows that the ICER of £23,859 per QALY gained for the comparison of sorafenib with BSC (after conversion from Turkish lira) is within the willingness-to-pay threshold that is considered to reflect a cost-effective use of NHS resources. However, without further details of the economic model inputs, in particular the resource use and costs, the relevance of this finding to the NHS setting is unclear.
In the US setting, when compared with placebo, both treatment with lenvatinib and treatment with sorafenib appear to provide additional health benefits while either saving resources158 or yielding ICERs per QALY gained of < £50,000 after conversion from US$ (£31,566 per QALY gained161 for lenvatinib versus placebo and £49,484 per QALY gained161 for sorafenib vs. placebo). When treatment with lenvatinib is compared with sorafenib in the US setting, lenvatinib offers a health benefit over sorafenib but at an increased cost. Cost-effectiveness results ranged from £19,522 per QALY gained161 (lenvatinib vs. sorafenib) to £96,671 per QALY gained160 (lenvatinib vs. sorafenib), at current UK prices. Again, it is unclear whether or not these results are relevant to the NHS setting.
In 2015, sorafenib became the standard of care for patients in Scotland with locally advanced or metastatic RR-DTC, provided that the company supplied the drug to the NHS at the Scottish PAS price agreed by the company with NHS Scotland. 48 The SMC sorafenib report48 states that sorafenib generated more benefit than BSC but at an increased cost. The ICER for this comparison was £32,083 per QALY gained. In 2016, an appraisal of treatment with lenvatinib38 versus sorafenib was submitted to the SMC; lenvatinib was considered by the SMC to be both an orphan drug and an end-of-life treatment. For the comparison of treatment with lenvatinib with treatment with sorafenib, based on survival outcome results generated using indirect comparison methods, and using the Scottish PAS price for lenvatinib, the ICER per QALY gained was estimated to be £49,525 and lenvatinib was accepted for use in NHS Scotland.
The AG notes that any discount to the list prices of the drugs agreed with the NHS in Scotland does not equate to an equivalent agreement with the NHS in England. All PAS prices are confidential and thus the applicability of the results presented within the Scottish submissions to the appraisal of lenvatinib and sorafenib for use in the NHS in England is unclear as it is not known whether or not the discounts agreed with the NHS in Scotland are the same as those agreed with the NHS in England.
In 2015, sorafenib was appraised by CADTH5 and, after reanalyses suggested by the Economic Guidance Panel, estimates of the most plausible ICERs for the cost-effectiveness of treatment with sorafenib versus BSC ranged from £108,974 to £118,913 per QALY gained (after conversion from CA$). Lenvatinib was considered for use by the Canadian health-care system in 2016. Estimates of the cost-effectiveness of treatment with lenvatinib versus both BSC and sorafenib were generated but only the comparisons with BSC are reported in the CADTH report. 162 After the Economic Guidance Panel’s suggested amendments were carried out, the best estimate for the comparison of treatment with lenvatinib versus BSC was £101,293 per QALY gained. Both lenvatinib and sorafenib have been recommended for use in Canada. The relevance of these results to patients in the NHS is unknown.
What is lacking from the current evidence base are any cost-effectiveness analyses of direct relevance to the NHS in England. The SMC submissions38,48 provide an insight into the costs and consequences associated with treatment with lenvatinib, sorafenib and BSC, and these are likely to be similar for patients treated in England. However, the PAS prices agreed with the NHS in Scotland are confidential and this prevents the reported cost-effectiveness estimates being directly applicable to the NHS in England.
Head-to-head comparisons of the effectiveness of treatment with lenvatinib with the effectiveness of treatment with sorafenib depend on results from indirect comparisons, whether conducted in a formal statistical framework5,38,48,160,162 or with adjustments made to the placebo arms of the Phase III trials,161 which provide estimates based on the pooling of the comparator arms within the SELECT and DECISION Phase III trials. The AG considers that, because of the issues discussed in Chapter 4, Indirect comparison feasibility assessment, it is not appropriate to employ indirect comparisons of the effectiveness of lenvatinib and the effectiveness of sorafenib using data from SELECT and DECISION.
Summary of the companies’ systematic reviews of economic evidence
Both of the companies carried out systematic reviews to identify published cost-effectiveness studies that included lenvatinib and/or sorafenib. Both companies concluded that there are no cost-effectiveness studies conducted in the UK from the perspective of the NHS that were relevant to decision-making in England. Therefore, both companies produced their own de novo economic evaluations.
Summary of the key features of the companies’ economic models
This section includes summary details of the key features of the economic models submitted to NICE, from Eisai Ltd and Bayer HealthCare, as part of the MTA process. All of the company data presented in this section are drawn from the company submissions7,8 and models.
Population
Both companies state that their economic evaluations focus on patients with progressive RR-DTC. However, in the submission from Eisai Ltd,8 it is highlighted that the SELECT definition of progressive RR-DTC was locally advanced or metastatic DTC confirmed by radiographic evidence of disease progression within the prior 13 months and that some patients participating in this trial had received prior vascular endothelial growth factor (VEGF) therapy. Eisai Ltd8 points out that, in contrast, no patients recruited to DECISION had received prior VEGF therapy and that, to be eligible for recruitment, evidence of disease progression within the 14 months prior to commencing the trial was required. The AG describes other differences in the two trial populations in Chapter 4, Trial characteristics, Participant characteristics and Indirect comparison feasibility assessment.
Model structure
Key elements of the structure of the economic models submitted by Eisai Ltd and Bayer HealthCare are included in Table 15. The structure of the two company models is similar and is in line with the structure of models that have previously been submitted to NICE to inform appraisals of interventions used to treat patients with cancer. The structure of both models conforms to specifications detailed in the final scope issued by NICE. 53
| Parameter | Eisai Ltd’s model (lenvatinib) | Bayer HealthCare’s model (sorafenib) |
|---|---|---|
| Intervention | Lenvatinib | Sorafenib |
| Comparators |
|
|
| Model structure | A four-state (stable disease, response, progressive and death) partitioned survival cost–utility model developed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) | A three-state (progression-free, progressed and death) partitioned survival cost–utility model developed in Microsoft Excel® |
| Cycle length | 1 month (30.43 days) | 28 days |
| Model time horizon | 33.35 years (5 years and 10 years are considered as scenario analyses) | 30 years |
| Discounting | Costs and benefits were discounted at a rate of 3.5% annually in line with the NICE reference case169 | |
| Perspective | The perspective is stated to be that of the NHS and PSS. However, no specific PSS elements are considered to be relevant to the RR-DTC population and none is included in either model | |
Therapies
Details about the intervention and comparators included in the company models are provided in Table 16. Both models included the therapies listed in the final scope issued by NICE. 53 The AG highlights that the lenvatinib and sorafenib doses in the models are based on average levels of use in SELECT and DECISION and are lower (approximately 17 mg for lenvatinib and 651 mg for sorafenib) than the licensed doses (24 mg for lenvatinib and 800 mg for sorafenib). Possible reasons include dose interruptions/reductions as a result of AEs; in some cases, intolerance may lead to a treatment being stopped.
| Parameter | Eisai Ltd’s model (lenvatinib) | Bayer HealthCare’s model (sorafenib) |
|---|---|---|
| Lenvatinib |
Price: list price used in the CS; however, a completed PAS submission template was made available to the ERG during the review period Daily dose: 17.4 mg (based on SELECT data, Eisai Ltd8). Treatment duration: SELECT TTD data |
Price: list price Daily dose: 17.4 mg (based on published data;8 estimate does not account for dose interruption) Treatment duration: the sorafenib TTD K–M data were adjusted to fit the SELECT median duration of treatment |
| Sorafenib |
Price: MiMS price Daily dose: 651 mg (based on data from DECISION) Treatment duration: assumed until disease progression |
Price: CMU price Daily dose: 651 mg (based on data from DECISION) Treatment duration: DECISION TTD K–M data (these data are complete and, therefore, no extrapolation was required) |
| Placebo/BSC | Assumption: no additional costs | BSC is defined as concurrent use of radiotherapy (10.6% in the sorafenib arm and 21.4% in the placebo arm of DECISION) |
| Administration cost | Deliver oral chemotherapy (SB11Z): £183.50 | None |
| Subsequent therapies | None (assumption based on expert advice) | |
Survival modelling
Summary details of the general approach the companies used to model patient survival (OS and PFS) are provided in Tables 17 and 18, respectively.
| Model | Lenvatinib | Sorafenib | Placebo/BSC |
|---|---|---|---|
| Eisai Ltd | SELECT data from third data cut-off point (August 2015) extrapolated using piecewise exponential curve | The curve, generated to represent OS for patients receiving sorafenib, was adjusted using the HR generated by the company’s ITC using data from the third data cut-off points of DECISION and SELECT (July 2015 and August 2015, respectively) | SELECT data from third data cut-off point (August 2015), recensored and RPSFTM-adjusted, and extrapolated using piecewise exponential curve |
| Bayer HealthCare | The curve, generated to represent OS for patients receiving sorafenib, was adjusted using the HR generated by the company’s ITC using data from the second data cut-off points of SELECT and DECISION (June 2014 and May 2013, respectively) | DECISION data from second data cut-off point (May 2013) allowed a direct comparison. The data were extrapolated using an exponential distribution | DECISION-adjusted ITT data from second data cut-off point (May 2013) allowed a direct comparison. The data were extrapolated using an exponential distribution |
| Model | Lenvatinib | Sorafenib | Placebo/BSC |
|---|---|---|---|
| Eisai Ltd | SELECT data from first data cut-off point (November 2013) extrapolated using piecewise gamma curve | The curve, generated to represent PFS for patients receiving sorafenib, was adjusted using the HR generated by the company’s ITC using data from the third data cut-off points of DECISION and SELECT (July 2015 and August 2015, respectively) | Not affected by crossover – SELECT data from first data cut-off point (November 2013) extrapolated using piecewise gamma curve |
| Bayer HealthCare | The curve, generated to represent PFS for patients receiving sorafenib, was adjusted using the HR generated by the company’s ITC using data from SELECT and DECISION | DECISION data from second data cut-off point (May 2013) allowed a direct comparison. The data were extrapolated using an exponential distribution | DECISION data (May 2013 data cut-off point) allowed a direct comparison. The data from each arm were extrapolated using exponential distributions |
Measurement and valuation of health effects
Sources of utility values
The base-case utility values used in the Eisai Ltd model were stated to be taken from EQ-5D values for sorafenib from DECISION. Disutilities were then applied as a weighted proportion, based on values obtained from a vignette study carried out by Fordham et al. 168 The AG notes that only the utility values used in the progressive state were the same as the utility values derived from DECISION.
The source of the utility values used in the Bayer HealthCare model7 was the EQ-5D data collected during DECISION. No additional utility decrements associated with AEs were included in the model.
The use of utility values derived from EQ-5D data collected during clinical trials is in line with the approach set out in the NICE Guide to the Methods of Technology Appraisal 2013. 169
Utility values
The utility values used in the companies’ models are provided in Table 19.
| Health state | Lenvatinib | Sorafenib | Placebo/BSC |
|---|---|---|---|
| Eisai Ltd’s model | |||
| Stable disease | 0.76 | 0.68 | 0.77 |
| Response | 0.82 | 0.74 | 0.83 |
| Progressive | 0.64 | 0.64 | 0.64 |
| Bayer HealthCare’s model | |||
| Progression-free | 0.72 (SE 0.08) | 0.72 (SE 0.08) | 0.8 (SE 0.07) |
| Post progression | 0.64 (SE 0.06) | 0.64 (SE 0.06) | 0.64 (SE 0.06) |
Health-care costs
Levels of resource use
Eisai Ltd obtained estimates of the level of health-care utilisation inputs for the pre-progression and progressive disease states from physician surveys conducted in Europe; these estimates were then validated by four practising clinical experts employed by NHS England. Mortality-related costs were obtained from the Nuffield Trust173 and adjusted for inflation to 2016 values based on PSSRU171 inflation rates for 2016.
Expert advice from oncologists was the basis for Bayer HealthCare’s resource use estimates. Unit costs were obtained from the NHS Reference Costs 2015 to 2016174 and the Unit Costs of Health and Social Care 2016. 171 In the model, it is assumed that resource use associated with treatment with lenvatinib is the same as the resource use associated with treatment with sorafenib.
The monthly routine care costs used in both company models are provided in Appendix 10 (see Table 70).
Eisai Ltd’s routine costs included physician visits and disease-associated hospitalisation days. Bayer HealthCare’s routine costs included inpatient stay, outpatient appointments and pharmaceutical costs.
Eisai Ltd’s end-of-life costs (£7450) included secondary care, local-authority-funded social care, district nursing and GP contacts.
Adverse event costs
The Eisai Ltd model includes the following AEs:
-
lenvatinib – grade 3 and 4 treatment-emergent AEs and AEs that required hospitalisation in SELECT
-
sorafenib – grade 3 and 4 treatment-emergent AEs in DECISION and AEs that required hospitalisation based on proportions from SELECT.
The Bayer HealthCare model7 only includes grade 3 and 4 AEs occurring in > 5% of patients in the lenvatinib arm of SELECT or in the sorafenib arm of DECISION.
Bayer HealthCare also included AE management costs (per 28 days), see table 29 in the company submission for details. 7
Frequencies/rates and costs associated with AEs included in the company models are presented in Appendix 10 (see Table 71). Eisai Ltd’s cost sources are a mix of NHS Reference Costs 2015 to 2016174 and Unit Costs of Health and Social Care 2016. 171 Bayer HealthCare’s cost sources are a mix of NHS Reference Costs 2014 to 2015,175 Unit Costs of Health and Social Care 2015176 and British National Formulary (BNF) costs. 47
Cost-effectiveness results
Base-case cost-effectiveness results
The base-case cost-effectiveness results from the Eisai Ltd8 and Bayer HealthCare7 submitted economic models are shown in Table 20.
| Technology | Total | Incremental | ICER per QALY gained | ||||
|---|---|---|---|---|---|---|---|
| Costs (£) | LYG | QALYs | Costs (£) | LYG | QALYs | Deterministic | |
| Eisai Ltd’s model results | |||||||
| Lenvatinib | 107,182 | 4.34 | 3.18 | ||||
| Sorafenib | 82,839 | 3.18 | 2.10 | 24,342 | 1.16 | 1.08 | £22,491 |
| Placebo/BSC | 42,115 | 2.80 | 1.84 | 65,067 | 1.54 | 1.34 | £48,569 |
| Bayer HealthCare’s model results | |||||||
| Placebo/BSC | CiC | 3.49 | 2.35 | ||||
| Sorafenib | CiC | 4.79 | 3.16 | CiC | 1.30 | 0.81 | CiC |
| Lenvatinib | CiC | 5.92 | 4.04 | CiC | 1.12 | 0.88 | CiC |
Bayer HealthCare also carried out cost-effectiveness analyses using the adjusted MAIC HRs. The effect on the company’s ICERs was small. The resultant base-case ICERs per QALY gained for the comparison of treatment with sorafenib with BSC and the comparison of treatment with lenvatinib with BSC are commercial in confidence and cannot be reported.
Probability of being the most cost-effective
For the Eisai Ltd model, the PSA results suggest that, at a willingness-to-pay threshold of £50,000 per QALY gained, the probability of lenvatinib being more cost-effective than sorafenib or BSC is 60%.
For the Bayer HealthCare model,7 the PSA results suggest that, at a willingness-to-pay threshold of £30,000 per QALY gained, the probability of sorafenib being cost-effective is 30%, the probability of BSC being cost-effective is 54% and the probability of lenvatinib being cost-effective is 16%.
The PSA results from the Eisai Ltd and Bayer HealthCare submitted economic models are shown in Table 21.
| Technology | Total, mean (95% CI) | Incremental | ICER/QALY gained (vs. BSC) | ICER/QALY gained | ||
|---|---|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | |||
| Eisai Ltd’s model | ||||||
| Lenvatinib vs. sorafenib | NS | NS | NS | NS | NS | £21,578 |
| Lenvatinib vs. placebo/BSC | NS | NS | NS | NS | £48,683 | |
| Bayer HealthCare’s model (all based on results of indirect comparison) | ||||||
| BSC | CiC | 2.41 (1.00 to 5.19) | ||||
| Sorafenib | CiC | 3.25 (1.81 to 5.30) | CiC | 0.84 | CiC | CiC |
| Lenvatinib | CiC | 4.11 (2.02 to 6.67) | CiC | 0.86 | CiC | CiC |
Sensitivity and scenario analyses
Both companies carried out a range of deterministic sensitivity analyses and scenario analyses.
In the Eisai Ltd model, for the comparison of lenvatinib and sorafenib, the two most influential parameters in the deterministic sensitivity analysis were OS HR versus sorafenib (lenvatinib dominates) and PFS HR versus sorafenib (£5000 to £35,000 per QALY gained). In the scenario analyses, the most influential parameters were the treatment duration for lenvatinib (treatment to progression rather than clinical trial duration; £71,978 per QALY gained) and the cut-off point for OS and PFS extrapolation (20 weeks for OS and PFS; £29,874 per QALY gained).
In the Bayer HealthCare model, for the comparison of sorafenib and lenvatinib, the largest deviations from the base-case ICER per QALY gained were attributable to variation in the OS HR for lenvatinib and lower lenvatinib progression-free utility. The scenario analyses that had the biggest effects on the companies’ cost-effectiveness results were the time horizon (reduction to 10 years) and lower lenvatinib progression-free utility. The ICERs per QALY gained for these analyses are commercial in confidence and cannot be reported here.
The Assessment Group’s independent cost-effectiveness assessment
Model design
In common with the two companies, the AG has used a standard partitioned survival model structure, applied to the patient population specified in the final scope issued by NICE,53 to consider the cost-effectiveness of treatment with lenvatinib and sorafenib compared with BSC (as represented by data from the placebo arms of SELECT and DECISION).
Two particular differences should be noted:
-
The AG has not included a separate health state for patients who respond to treatment. On clinical advice, the AG considers that there is little merit in this addition to the standard three-state structure (in which patients begin in the progression-free health state and, following assessed disease progression, transfer to the postprogression state in which they receive only BSC prior to death). For responding patients, who are mostly symptom-free, response alone is unlikely to have a measurable effect on patient-perceived quality of life/utility and has no effect on resource use.
-
The AG has designed a model that allows each intervention (lenvatinib and sorafenib) to be represented in its natural time metric: 30-day cycles for lenvatinib and 28-day cycles for sorafenib. This involved creating two parallel models using the same assumptions and model parameters, but each with its own placebo arm calibrated from its respective clinical trial data. Although not ideal, the AG has provided an illustrative structural sensitivity analysis (Figure 9) based on applying data from the counterfactual placebo arm of both trials to illustrate the extent of uncertainty involved in comparisons between the active treatments with the currently available clinical evidence. The reason for this unusual approach is to demonstrate non-equivalence of the placebo arms of the two clinical trials, which renders indirect comparison of the two treatments via a common comparator invalid (as discussed in Chapter 4, Indirect comparison feasibility assessment, and illustrated graphically in Figure 9).
FIGURE 9.
Model structure featuring two simple trial-based comparisons, with additional cross-trial comparisons as a structural sensitivity analysis to illustrate the uncertainty associated with choice of comparator.

Resource use estimation, the sources for unit costs and selection of health-related utility values used in the AG’s model are presented in this section. Standard discount rates of 3.5% per annum are used for discounting both costs and benefits (measured as QALYs), but not for life-years (survival). The AG model is structured with a maximum time horizon of 40 years.
Effectiveness data
Modelling long-term outcomes from trial data
Both companies have followed a conventional approach to the general problem of identifying an appropriate method by which to extrapolate time-limited follow-up trial data for PFS, OS and time to treatment discontinuation. This involves attempting to fit a range of prespecified statistical functions to the available evidence, and selecting one that appears to be optimal according to particular ‘measures of fit’ (principally the Akaike information criterion and the Bayesian information criterion).
This paradigm is wholly dependent on the limited data available and the restricted armoury of ‘standard’ models. In particular, it fails to take into account a wider evidence base specifically related to the natural history of the disease, and the influence of particular characteristics of both the recruited patient group and of the trial design.
The AG has investigated long-term survival trends in patients diagnosed with Stage 3 or 4 (locally advanced or metastatic) thyroid cancer in the USA and recorded on the Surveillance Epidemiology and End Results (SEER) database. 177 A total of 32,818 patients (male and female) followed for 15 years yielded a persistent trend from 18 months after diagnosis. Figure 10 demonstrates the very close match between these data and a simple linear model, indicating that the risk of death remained unchanged throughout this period, which is indicative of a simple exponential survival process.
FIGURE 10.
Cumulative hazard data from follow-up of patients diagnosed with stage III/IV thyroid cancer for 15 years.

This evidence is sufficiently compelling to give the AG confidence to employ exponential extrapolation as the default method of modelling incomplete trial data in this appraisal. The nature of clinical trials (selecting patients who have suffered a recent disease progression, and administering a novel treatment that takes time to reach full effectiveness) means that the initial period post randomisation will give rise to temporary distortions to the underlying disease process. However, thereafter, it is likely that the natural history of the condition will be re-established, so that a long-term exponential function will reappear. The mean time since diagnosis of patients randomised in DECISION was 7.24 years, suggesting that the trial cohort lies in the middle of the follow-up range shown in Figure 10. The AG is therefore confident that outcome data extrapolation should be focused on fitting exponential models to estimate lifetime survival expectation.
Data issues
Following the initial stakeholders’ meeting for this appraisal (17 February 2017), the AG submitted identical requests to the two companies, asking for a set of detailed analyses of the latest data available from the two clinical trials, based on common analytical methods to allow comparative analyses to be carried out by the AG; thus, minimising the risk of methodological bias. Eisai Ltd provided the requested data relating to SELECT as an appendix to the submission. 8 Unfortunately, Bayer HealthCare chose not to address the AG’s request. As a consequence, the AG was unable to conduct some comparative analyses based on common assumptions, and the potential for bias and uncertainty in the data available to the AG remains.
The two clinical trials that provide the effectiveness evidence for this appraisal share common features, which result in interpretive complexity and uncertainty. In particular, in both trials patients were permitted to cross over from the placebo control to the active treatment (lenvatinib or sorafenib) following disease progression. As a consequence, randomisation was broken in both trials and some outcome variables may not be mutually compatible, even after attempts to adjust for crossover effects.
Both companies assume that, in addition to the active treatments, a third comparator (BSC) may be represented by the placebo arms of the two trials. Moreover, it is implicitly assumed that the randomised patients are drawn from similar populations with reference to their risk profile for the various time-to-event outcomes measured [PFS, OS, postprogression survival (PPS) and time-to-treatment discontinuation]. In Chapter 4, Indirect comparison feasibility assessment, the non-equivalence of PFS data from the placebo arms of the two clinical trials has been clearly demonstrated. This is of crucial importance to attempts to employ relative effectiveness measures reliant on the PHs assumption in relation to PFS, which is the only standard outcome variable reported in these trials that is free from any contamination by crossover effects (both trial protocols required confirmation of disease progression before patients were allowed to enter the open-label phase in which patients in the placebo arm were offered crossover treatment).
The problem of devising a credible approach to indirect comparison between lenvatinib and sorafenib for PFS cannot be resolved by appeal to technical argument alone. The pattern of hazard over time for disease progression in the two active arms is sufficiently similar to justify a simple HR approach. However, the placebo arms exhibit unexpectedly inconsistent patterns of temporal change, not compatible with the assumption of similarity between the patient groups not receiving active treatment. The AG, therefore, considers that the patients enrolled in the two trials cannot be considered to derive from a common population. This degree of difference precludes the use of either placebo arm as being representative of untreated patients across both trials.
The data for both placebo arms exhibit an unexpected improvement in long-term survival (reducing progression hazard) for which there is no obvious explanation. The effect of this phenomenon is to produce a varying differential in performance when comparing survival components across the two trials without any clear confirmatory evidence. Therefore, the AG is unable to support the use of a conventional ITC in this appraisal. The AG considers that it is preferable to model the relative effectiveness and cost-effectiveness of each active treatment against its own placebo comparator, and then generate results for each drug relative to the placebo of the other clinical trial as a sensitivity analysis, in order to allow assessment of the uncertainty associated with the choice of comparator.
Progression-free survival
The AG chose to use data for locally assessed PFS rather than centrally assessed PFS, as local assessment is generally more closely related to normal clinical practice.
Lifetime mean PFS for patients in DECISION who received placebo may be readily estimated from trial data (for the period available) and a simple exponential curve that conforms closely to the reported trial data (Figure 11). The AG estimated lifetime mean PFS from the area under the K–M data to 16.5 months of elapsed time followed by the area under the exponential function thereafter, giving a lifetime mean PFS estimate of 7.56 months. The sorafenib PFS arm of DECISION exhibits a simple constant hazard (exponential) relationship (see Figure 11), allowing the lifetime mean PFS to be estimated in a similar fashion, using the area under the curve (AUC) of the K–M data until 25 months, and the exponential extrapolation thereafter. This shows a lifetime mean PFS estimate of 47.18 months for patients receiving sorafenib, and a mean gain in PFS of 39.62 months compared with receiving placebo.
FIGURE 11.
Progression-free survival K–M data from DECISION modelled by an exponential function.

The SELECT data for PFS exhibit a more complex pattern in each arm. The cumulative hazard plots (Figure 12) reveal two distinct phases, both of which follow a constant hazard. Patients in the placebo arm who remain progression-free after 312 days experience a reduction in hazard of about 53%, which is sustained thereafter. Similarly, patients in the lenvatinib arm experience a reduction of progression hazard of about 47% at 529 days. As before, the estimated mean lifetime PFS for these patient groups was estimated as the sum of the AUC in each trial arm, followed by lifetime extrapolation using the long-term exponential hazard of progression or death. This approach yields estimates of mean lifetime PFS of 41.00 months for patients receiving lenvatinib and 6.92 months for patients in the placebo arm of SELECT. Thus, the estimated net lifetime gain in PFS for patients receiving lenvatinib is estimated to be 34.08 months.
FIGURE 12.
Cumulative hazard for disease progression for SELECT, with two-phase fitted exponential models.
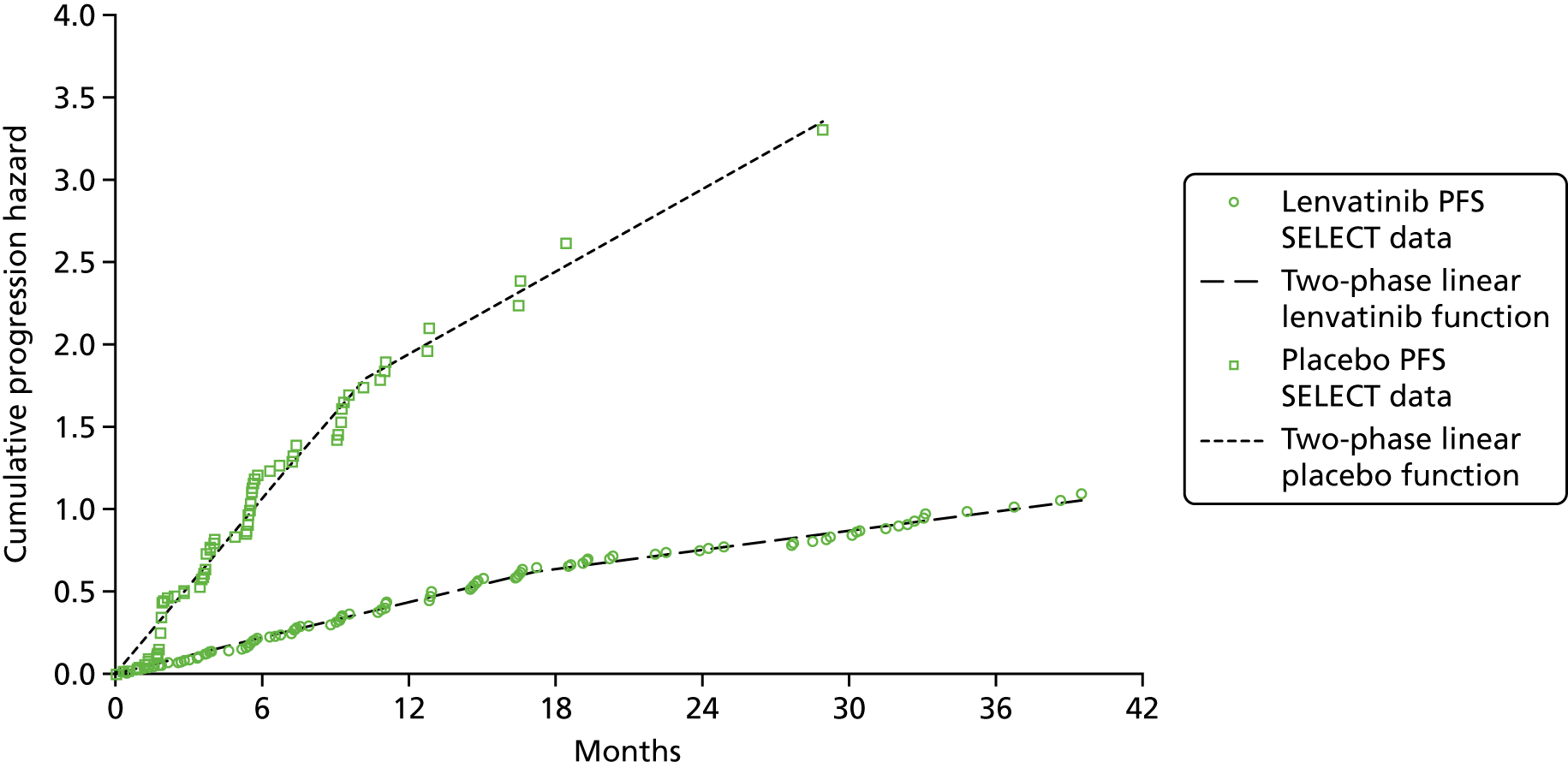
Time to treatment discontinuation
As illustrated in Figure 13, the SELECT data are virtually complete for the cycles of lenvatinib dispensed during the trial. The AG estimates mean usage of lenvatinib as 12.61 30-day cycles per patient.
FIGURE 13.
The 30-day cycles of lenvatinib dispensed in SELECT.
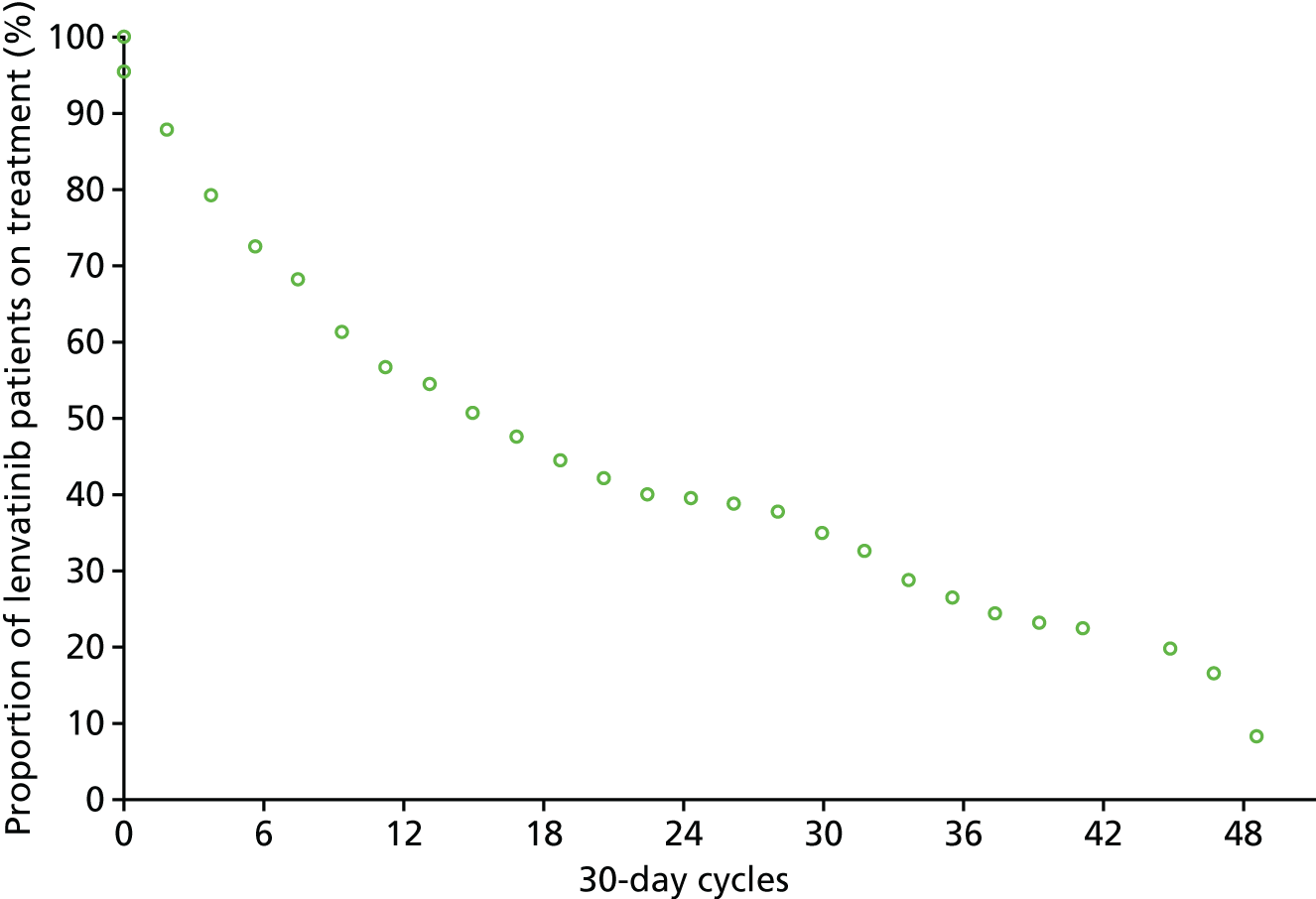
The DECISION trial data are also complete for the cycles of sorafenib dispensed during the trial, as illustrated in Figure 14. The AG estimates mean usage of sorafenib as 14.36 28-day cycles per patient.
FIGURE 14.
The 28-day cycles of sorafenib dispensed in DECISION.

Overall survival
Data provided by the company for lenvatinib-treated patients in SELECT (Figure 15) show a simple long-term exponential trend indicating a constant mortality risk throughout the trial period (19.6% per year). This allows the mean lifetime OS for patients treated with lenvatinib to be estimated using the AUC of the trial K–M curve until 34.7 months plus a simple exponential extrapolation thereafter, giving a total mean OS of 55.1 months.
FIGURE 15.
Overall survival: lenvatinib-treated patients in SELECT with a fitted exponential model, and RPSFTM adjusted for placebo patient crossover with a long-term exponential-fitted model.

Both companies have employed RPSFTM adjustments to data from the placebo arms of their clinical trials to correct for patients crossing over to the active treatment following disease progression. Adjusted OS placebo arm data from SELECT are also displayed in Figure 15 and indicate that, after RPSFTM adjustment, a similar long-term exponential (constant risk) trend also applies to the placebo arm beyond 6 months. Using the AUC of the adjusted K–M curve until 19.1 months plus the exponential extrapolation thereafter yields a lifetime estimated mean OS for the corrected placebo arm of 29.9 months and a net estimated OS gain attributable to treatment with lenvatinib of 25.3 months.
An examination of the OS data from DECISION (Figure 16) indicates that both patients in both treatment arms were subject to a period of relatively low mortality hazard, followed by transition to a higher constant risk of death. This transition took place after 11.2 months for sorafenib patients and 6.4 months for placebo patients.
FIGURE 16.
Cumulative mortality hazard for sorafenib-treated patients in DECISION with a fitted two-phase exponential model, and for RPSFTM-adjusted placebo patients with a fitted two-phase exponential model.

Using the AUC of the RPSFTM-adjusted K–M curve for the placebo arm until 6.4 months plus the AUC of the exponential extrapolation thereafter yields a lifetime estimated mean OS for the placebo arm of 47.18 months. Similarly, combining the AUC of the sorafenib arm up to 11.96 months with the exponential trend thereafter yields an estimated lifetime mean OS of 56.66 months. Thus, the net mean OS gain attributable to sorafenib is 9.48 months.
Postprogression survival
Assessment of PPS may be carried out at an aggregate level by calculating the difference between model estimates of OS and PFS. However, it can also be informative to consider this outcome at the level of individual patients, at which it may provide useful insight into possible post-treatment long-term effects of treatments even after active treatment has ceased. The AG asked both companies to provide PPS data from their respective primary clinical trials. Unfortunately, only data from SELECT have been received. As with OS, it is important to allow for the effects of crossover on PPS by using RPSFTM-adjusted data.
In Figure 17, the beneficial effect of crossover to lenvatinib for patients initially randomised to the placebo arm is clearly apparent. Both trial arms exhibit a similar early pattern, albeit at different absolute levels of survival, and thereafter show similar long-term exponential trends after 15 to 18 months from the time of disease progression. When the RPSFTM adjustment is applied, the corrected placebo arm very closely follows the trajectory of the lenvatinib arm (although the effect of RPSFTM revised censoring does not allow direct comparison beyond 16 months). Nonetheless, these data suggest that, after crossover adjustment, there is probably no additional benefit to individual patients crossing from placebo to lenvatinib beyond what would have been gained by treatment prior to disease progression.
FIGURE 17.
Postprogression survival: lenvatinib in SELECT with a fitted exponential model, and RPSFTM adjusted for placebo patient crossover with a long-term exponential-fitted model.

Summary of time-to-event outcome data analysis
Estimates of PFS, OS and PPS and mean cycles of active treatment received in the two clinical trials are displayed in Table 22. The main difference occurs in the PFS results in which lenvatinib provides substantially greater benefit than sorafenib (34.1 additional months before progression compared with only 6.3 months, respectively). However, the estimated OS results are very similar (55.1 for lenvatinib vs. 56.8 months for sorafenib), and, consequently, estimated PPS is reduced with lenvatinib treatment but increased for sorafenib treatment). Thus, it appears that lenvatinib shows effect more strongly in initially delaying progression, but does not offer additional benefit over sorafenib in terms of long-term survival. The duration of active treatment in the two trials is very similar when measured in days rather than cycles, with a difference of < 7%.
| Study, treatment arm | PFS (months) | OS (months) | PPS (months) | TTD (cycles) |
|---|---|---|---|---|
| SELECT | ||||
| Lenvatinib | 41.0 | 55.1 | 14.1 | 12.6 (30-day cycle) |
| Placebo | 6.9 | 30.2a | 23.3 | N/A |
| Change attributable to lenvatinib | + 34.1 | + 24.9 | –9.2 | N/A |
| DECISION | ||||
| Sorafenib | 13.8 | 56.8 | 42.9 | 14.4 (28-day cycle) |
| Placebo | 7.6 | 43.8a | 36.2 | N/A |
| Change attributable to sorafenib | + 6.3 | + 13.0 | + 6.7 | N/A |
Health-related utility data
The AG has carefully considered the opposing approaches used by the two companies to estimate appropriate health-related utility values to assign to health states and to AEs. The Eisai Ltd model relies heavily on the Fordham et al. 168 vignette study (which it sponsored), whereas the Bayer HealthCare model draws on EQ-5D-3L data collected during DECISION.
On theoretical grounds, directly collected evidence from patients with the condition (as used in the Bayer HealthCare model7) should always be preferred to the results of an artificial study without recourse to the views of patients either in design or calibration (as used in the Eisai Ltd model). Of particular concern is the serious overestimation of baseline utility values in the Fordham et al. 168 study when compared with UK general population values for people of a similar age. The contrary position argues that DECISION data include the disutility of AEs in estimates of health-state utilities, and, therefore, are biased without any objective means of adjusting the health-state estimates.
On balance, the AG considers that the data from DECISION should be used in the base case (Table 23) with a sensitivity analysis using the Eisai Ltd model values.
| Health state | Treatment arm | Base-case utility value | SE | Sensitivity analysis utility value | SE |
|---|---|---|---|---|---|
| PFS | Lenvatinib/sorafenib | 0.72 | 0.08 | 0.76/0.68 | 0.08 |
| PFS | BSC | 0.80 | 0.07 | 0.80 | 0.019 |
| PPS | All | 0.64 | 0.06 | 0.50 | 0.028 |
Resource use and cost data used in the Assessment Group’s model
Active treatments (lenvatinib and sorafenib)
The lenvatinib full acquisition cost is £4311.00 per 30-day treatment (NHS Indicative Price, BNF June 2017). 47 This is reduced by the SELECT dose intensity factor (72.5%) so the true cost per cycle is £3089.55.
The sorafenib full acquisition cost is £3576.56 per 28-day treatment (NHS Indicative Price, BNF June 2017). 47 This is reduced by the DECISION dose intensity factor (81.40%) so the true cost per cycle is £2911.32.
There is no administration cost associated with either drug, both of which can be safely taken unsupervised. The NHS Reference Costs174,175 figures quoted by both companies for administration of oral treatment relate to particular drugs that may cause serious rapid-onset reactions, and so the patient must be monitored following administration. Thus, it is not appropriate to use these costs when estimating the cost of either sorafenib or lenvatinib.
Routine care costs
Table 24 summarises the schedule of itemised routine care tests, treatments and specialist visits identified by the AG’s clinical advisor, in terms of use per quarter (3 months), per 28-day cycle and per 30-day cycle. These items are considered to be applicable to all patients.
| Resource item | Number per quarter | Unit cost (£) | SE (£) | Reference cost (source: NHS Reference Costs 2015 to 2016174) |
|---|---|---|---|---|
| Blood test | 1 | 3.10 | 0.07 | NHS Reference Cost DAPS05 |
| Coagulation test | 1 | 3.10 | 0.07 | NHS Reference Cost DAPS05 |
| Urine test | 1 | 7.63 | 0.22 | NHS Reference Cost DAPS07 |
| Liver function test | 7 | 1.18 | 0.03 | NHS Reference Cost DAPS04 |
| Thyroid function test | 3 | 1.18 | 0.03 | NHS Reference Cost DAPS04 |
| Protein test | 1 | 1.18 | 0.03 | NHS Reference Cost DAPS04 |
| Bone scan | 1 | 242.39 | 7.56 | NHS Reference Cost NMOP/RN15A |
| MRI scan | 1 | 204.67 | 5.07 | NHS Reference Cost IMAGOP/RD03Z |
| CT scan | 1 | 118.53 | 2.92 | NHS Reference Cost IMAGOP/RD22Z |
| Thyroxine (4-weekly) | 3.26 | 4.04 | NS | BNF NHS indicative prices |
| Calcium and vitamin D | 3 | 7.13 | NS | BNF NHS indicative prices |
| Specialist oncology visit | 1 | 162.84 | 4.37 | NHS Reference Cost 370/WF01A |
| Total per 3 months | 789.81 | |||
| Total per 28-day cycle | 242.19 | |||
| Total per 30-day cycle | 259.48 |
Adverse events
Three common AEs feature in the two company models for which treatment types and resource use were estimated by the AG’s clinical advisor. The cost estimates shown in Table 25 are for only a single cycle (28 days or 30 days) and take no account of AE episodes that do not resolve within that time or that subsequently recur.
| AE | Resource item | Unit cost (£) | Incidence rate (%) | |||
|---|---|---|---|---|---|---|
| Sorafenib | Lenvatinib | Placebo vs. sorafenib | Placebo vs. lenvatinib | |||
| Hand–foot syndrome | Diprobase 500-g pump pack | 10.00 (typical retail price) | 20.29 | 3.45 | 0.00 | 0.00 |
| Proteinuria | 2.5 mg of Ramipril × 28 | 0.27 (eMIT, April 2016179) | 0.00 | 3.45 | 0.00 | 0.00 |
| Hypertension | 10 mg of Amlodipine × 28 | 0.19 (eMIT, April 2016179) | 0.00 | 42.91 | 1.91 | 3.82 |
| 10 mg of Ramipril × 28 | 0.41 (eMIT, April 2016179) | 0.00 | 42.91 | 1.91 | 3.82 | |
| Two extra oncology consultations | 162.84 per visit (NHS Reference Costs 2015 to 2016174) | 0.00 | 42.91 | 1.91 | 3.82 | |
| Total cost (£) | ||||||
| Per 28 days | 33.55 | 140.37 | 6.24 | 12.45 | ||
| Per 30 days | 35.95 | 150.40 | 6.69 | 13.34 | ||
End-of-life care
Health-care costs during the last 90 days of life were estimated using the results presented in Table 9 of the paper by Georghiou and Bardsley;173 costs were uplifted from 2010/11 to 2015/16 using the Hospital and Community Heath Services inflation index178 as shown in Table 26.
| Care item | Mean cost per patient (£) | SE (£) |
|---|---|---|
| GP consultation | 391.78 | 4.98 |
| District nursing | 631.14 | 53.77 |
| Local authority social care | 476.57 | 11.28 |
| Emergency inpatient episode | 4369.67 | 6.28 |
| Non-emergency inpatient episode | 1459.78 | 5.06 |
| Outpatient attendance | 405.73 | 1.10 |
| Accident and emergency visit | 85.87 | 0.15 |
| Total | 7820.54 |
Cost-effectiveness results
Deterministic cost–utility results from the AG model using public list prices are compared with submitted results from the two companies in Tables 27 (vs. the Eisai Ltd model) and 28 (vs. the Bayer HealthCare model). Overall, the estimates of incremental costs from the three models are not very different, but estimates of outcomes (life-years and QALYs) show larger discrepancies across the models, reflecting the different assumptions and estimation methods employed. The ICERs per QALY gained reported from the AG model are substantially greater than those obtained from the Bayer HealthCare model, but the Eisai Ltd’s model results show a much larger ICER per QALY gained for sorafenib versus BSC than that obtained from either of the other models.
| Results component | AG’s model preferred scenario | Eisai Ltd’s model estimates | |||||
|---|---|---|---|---|---|---|---|
| Lenvatinib vs. BSC | Sorafenib vs. BSC | ||||||
| Lenvatinib | BSC | Sorafenib | BSC | Lenvatinib | Sorafenib | BSC | |
| Costs (£) | |||||||
| Drug acquisition | 68,217 | 0 | 41,281 | 0 | 68,061b | 37,267 | 0 |
| Drug administration | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Routine care | 12,742 | 7495 | 13,227 | 10,523 | 31,022 | 38,937 | 35,582 |
| AEs | 7385 | 385 | 1833 | 274 | 107 | 21 | 0 |
| End-of-life care | 6758 | 7314 | 6848 | 7157 | 6316 | 6615 | 6532 |
| Total | 95,102 | 15,195 | 63,188 | 17,954 | 107,182 | 82,839 | 42,115 |
| Life-years | |||||||
| Response (in PFS) yearsa | N/A | N/A | N/A | N/A | 0.533 | 0.325 | 0.017 |
| Progression-free yearsa | 3.413 | 0.565 | 1.064 | 0.635 | 3.062 | 0.922 | 0.640 |
| Postprogression yearsa | 1.171 | 1.967 | 3.661 | 3.014 | 1.277 | 2.258 | 2.159 |
| Totala | 4.584 | 2.532 | 4.725 | 3.649 | 4.339 | 3.180 | 2.800 |
| QALYs | |||||||
| PFS | 2.182 | 0.446 | 0.755 | 0.504 | 2.380 | 0.746 | 0.447 |
| PPS | 0.633 | 1.156 | 1.997 | 1.720 | 0.800 | 1.351 | 1.393 |
| Total | 2.815 | 1.602 | 2.752 | 2.224 | 3.179 | 2.097 | 1.840 |
| Incremental cost (£) | 79,907 | 45,234 | 65,067 | 40,724 | N/A | ||
| Incremental life-years | 2.052 | 1.076 | 1.539 | 0.380 | N/A | ||
| Incremental QALYs | 1.213 | 0.528 | 1.339 | 0.257 | N/A | ||
| ICER per QALY vs. BSC (£) | 65,872 | 85,644 | 48,569 | 158,232 | N/A | ||
| Results component | AG-preferred scenario | Bayer HealthCare model estimates | |||||
|---|---|---|---|---|---|---|---|
| Lenvatinib vs. BSC | Sorafenib vs. BSC | ||||||
| Lenvatinib | BSC | Sorafenib | BSC | Lenvatinib | Sorafenib | BSC | |
| Costs (£) | |||||||
| Drug acquisition | 68,217 | 0 | 41,281 | 0 | 41,641 | 33,187 | 0 |
| Drug administration | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Routine care | 12,742 | 7495 | 13,227 | 10,523 | 46,018 | 37,886 | 25,695 |
| AEs | 7385 | 385 | 1833 | 274 | 141 | 81 | 17 |
| End-of-life care | 6758 | 7314 | 6848 | 7157 | 0 | 0 | 0 |
| Total | 95,102 | 15,195 | 63,188 | 17,954 | 87,800 | 71,154 | 25,712 |
| Life-years | |||||||
| Response years | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Progression-free years | 3.413 | 0.565 | 1.064 | 0.635 | 3.767 | 1.342 | 0.808 |
| Postprogression years | 1.171 | 1.967 | 3.661 | 3.014 | 3.589 | 4.381 | 3.161 |
| Total life-yearsa | 4.584 | 2.532 | 4.725 | 3.649 | 7.356 | 5.723 | 3.969 |
| QALYs | |||||||
| PFS | 2.182 | 0.446 | 0.755 | 0.504 | 2.394 | 0.920 | 0.628 |
| PPS | 0.633 | 1.156 | 1.997 | 1.720 | 1.645 | 2.237 | 1.724 |
| Total | 2.815 | 1.602 | 2.752 | 2.224 | 4.039 | 3.158 | 2.352 |
| Incremental cost (£) | 79,907 | 45,234 | 62,088 | 45,441 | 62,088 | ||
| Incremental life-yearsa | 2.052 | 1.076 | 3.487 | 1.754 | 3.487 | ||
| Incremental QALYs | 1.213 | 0.528 | 1.687 | 0.805 | 1.687 | ||
| ICER (per QALY) (£) | 65,872 | 85,644 | 36,802 | 56,417 | 36,802 | ||
Inevitably, the relative economic performance of the treatments in all three models will change significantly when final discounted acquisition prices are applied.
Structural sensitivity analysis
The AG cross-trial ICERs per QALY gained can be readily calculated by interchanging the results shown in the two AG BSC columns of Tables 27 and 28. For sorafenib, this results in an incremental cost per patient of £47,993 and incremental QALYs per patient of 1.150, leading to an exploratory ICER of £41,716 per QALY gained. However, for lenvatinib, the incremental cost per patient is £77,148 and the incremental QALYs per patient are 0.591, leading to an amended ICER of £130,592 per QALY gained.
These very large changes (an increase of 105% in the lenvatinib ICER per QALY gained, and a decrease of 54% in the sorafenib ICER per QALY gained) serve to illustrate that the choice of BSC comparator is of major importance in this appraisal, and that the absence of credible indirect comparison results precludes any simple resolution of this difficulty.
Deterministic sensitivity analyses
Sensitivity analyses have been conducted on the cost-effectiveness results obtained using the AG model and the results from these analyses are shown in Tables 29–31.
| Treatment | Source of uncertainty | AG-preferred scenario: cost per QALY gained (£) | Option A | Option B | ||
|---|---|---|---|---|---|---|
| Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | |||
| Lenvatinib vs. BSC | Discount rate – costs: A = 0%, B = 5% | 65,872 | 70,033 | 4161 | 64,368 | –1504 |
| Discount rate – outcomes: A = 0%, B = 5% | 65,872 | 53,592 | –12,280 | 71,274 | +5402 | |
| Drug use data source: A = PFS, B = least of TTD and PFS | 65,872 | 106,178 | +40,306 | 65,872 | 0 | |
| Drug dose intensity ratio: A = not used | 65,872 | 87,203 | +21,331 | N/A | N/A | |
| Utility value set: A = Eisai Ltd | 65,872 | 54,981 | –10,891 | N/A | N/A | |
| Sorafenib vs. BSC | Discount rate – costs: A = 0%, B = 5% | 85,644 | 88,747 | +3104 | 84,561 | –1082 |
| Discount rate – outcomes: A = 0%, B = 5% | 85,644 | 67,645 | –17,999 | 93,751 | +8108 | |
| Drug use data source: A = PFS, B least of TTD and PFS | 85,644 | 85,814 | +170 | 83,076 | –2568 | |
| Drug dose intensity ratio: A = not used | 85,644 | 103,503 | +17,859 | N/A | N/A | |
| Utility value set: A = Eisai Ltd | 85,644 | 105,666 | +20,023 | |||
| Source of uncertainty | AG-preferred scenario: cost per QALY gained (£) | LCL | UCL | ||
|---|---|---|---|---|---|
| Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | ||
| Dose intensity ratio | 65,872 | 63,892 | –1980 | 67,852 | +1980 |
| Blood/coagulation test cost | 65,872 | 65,871 | –2 | 65,874 | +2 |
| Urine test cost | 65,872 | 65,871 | –1 | 65,876 | +4 |
| Liver/thyroid/protein test cost | 65,872 | 65,870 | –2 | 65,877 | +5 |
| Bone scan cost | 65,872 | 65,792 | –80 | 65,955 | +83 |
| CT scan cost | 65,872 | 65,842 | –30 | 65,905 | +33 |
| MRI scan cost | 65,872 | 65,819 | –53 | 65,928 | +56 |
| Oncology visit cost | 65,872 | 65,524 | –348 | 66,223 | +351 |
| Hand–foot syndrome incidence: lenvatinib | 65,872 | 65,866 | –6 | 65,888 | +15 |
| Proteinuria incidence: lenvatinib | 65,872 | 65,873 | +1 | 65,874 | +2 |
| Hypertension incidence: lenvatinib | 65,872 | 65,018 | –854 | 66,759 | +887 |
| Hypertension incidence: BSC (vs. lenvatinib) | 65,872 | 66,074 | +202 | 65,431 | –441 |
| End-of-life care costs | 65,872 | 65,883 | +11 | 65,864 | –8 |
| PFS utility values | 65,872 | 77,475 | +11,603 | 42,352 | –23,520 |
| PPS utility values | 65,872 | 60,739 | –5133 | 71,956 | +6084 |
| PFS lenvatinib hazard rate | 65,872 | 63,127 | –2745 | 63,853 | –2019 |
| PFS BSC hazard rate (SELECT) | 65,872 | 63,672 | –2200 | 63,389 | –2483 |
| OS lenvatinib hazard rate | 65,872 | 63,231 | –2641 | 63,791 | –2081 |
| OS BSC hazard rate (SELECT) | 65,872 | 68,374 | +2502 | 65,455 | –417 |
| TTD lenvatinib hazard rate | 65,872 | 65,006 | –866 | 63,201 | –2671 |
| Source of uncertainty | AG-preferred scenario: cost per QALY gained (£) | LCL | UCL | ||
|---|---|---|---|---|---|
| Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | Cost per QALY gained (£) | Effect on ICER per QALY gained (£) | ||
| Dose intensity ratio | 85,644 | 83,009 | –2635 | 88,278 | +2635 |
| Blood/coagulation test cost | 85,644 | 85,642 | –2 | 85,645 | +2 |
| Urine test cost | 85,644 | 85,643 | –1 | 85,648 | +5 |
| Liver/thyroid/protein test cost | 85,644 | 85,641 | –2 | 85,649 | +6 |
| Bone scan cost | 85,644 | 85,549 | –94 | 85,741 | +98 |
| CT scan cost | 85,644 | 85,608 | –35 | 85,682 | +39 |
| MRI scan cost | 85,644 | 85,581 | –63 | 85,710 | +66 |
| Oncology visit cost | 85,644 | 85,446 | –198 | 85,845 | +201 |
| Hand–foot syndrome incidence: sorafenib | 85,644 | 85,592 | –51 | 85,710 | +66 |
| Hypertension incidence: sorafenib | 85,644 | 84,460 | –1184 | 87,356 | +1712 |
| Hypertension incidence: BSC (vs. sorafenib) | 85,644 | 85,999 | +355 | 84,782 | –862 |
| End-of-life care costs | 85,644 | 85,657 | +14 | 85,633 | –10 |
| PFS utility values | 85,644 | 97,212 | +11,568 | 59,422 | –26,221 |
| PPS utility values | 85,644 | 95,450 | +9806 | 77,668 | –7976 |
| PFS sorafenib hazard rate | 85,644 | 85,294 | –349 | 85,367 | –277 |
| PFS BSC hazard rate (DECISION) | 85,644 | 85,298 | –346 | 85,383 | –261 |
| OS sorafenib hazard rate | 85,644 | 78,853 | –6790 | 92,528 | +6884 |
| OS BSC hazard rate (DECISION) | 85,644 | 89,074 | +3430 | 82,063 | –3581 |
The AG identified five modelling issues, which do not involve stochastic uncertainty, and the implications, in terms of changes to the size of the estimated ICER per QALY gained in the AG model, that result from changes to these parameter values are shown in Table 29. Assuming that a change in the estimated ICER per QALY gained of < £5000 is not considered substantial, all but one of the five issues generated important changes in the ICER per QALY gained estimates for either sorafenib or lenvatinib (the exception being the discount rate applied to costs).
The AG identified 18 parameter values for which stochastic uncertainty could be quantified in the AG model, and the findings from adjusting these values are summarised in Tables 30 and 31. Only three parameters (the utility values for the PFS and PPS health states estimated from EQ-5D-3L patient data in DECISION, and the sorafenib OS AG extrapolation hazard) were found to lead to substantial effects on the size of the estimated ICER per QALY gained when varied between the lower and upper 95% confidence limits. In particular, the AG considers that uncertainty in specific unit costs (other than drug acquisition costs) is not an important factor when generating uncertainty in ICER per QALY gained estimates.
Probabilistic sensitivity analyses
The AG carried out PSA varying model parameters subject to quantifiable stochastic sampling uncertainty:
-
nine routine care cost variables
-
seven AE incidence rates
-
seven health-related utility values
-
seven end-of-life health and social care costs.
In most cases, probabilistic values were drawn from normal distributions around the standard error of the mean, except for incidence rates, for which beta distributions were employed.
Using list prices, the in-trial comparisons of lenvatinib and BSC (Figure 18) and of sorafenib and BSC (Figure 19) yielded similar deterministic and probabilistic ICERs per QALY gained.
FIGURE 18.
Probabilistic sensitivity analysis: lenvatinib vs. BSC in SELECT.

FIGURE 19.
Probabilistic sensitivity analysis: sorafenib vs. BSC in DECISION.
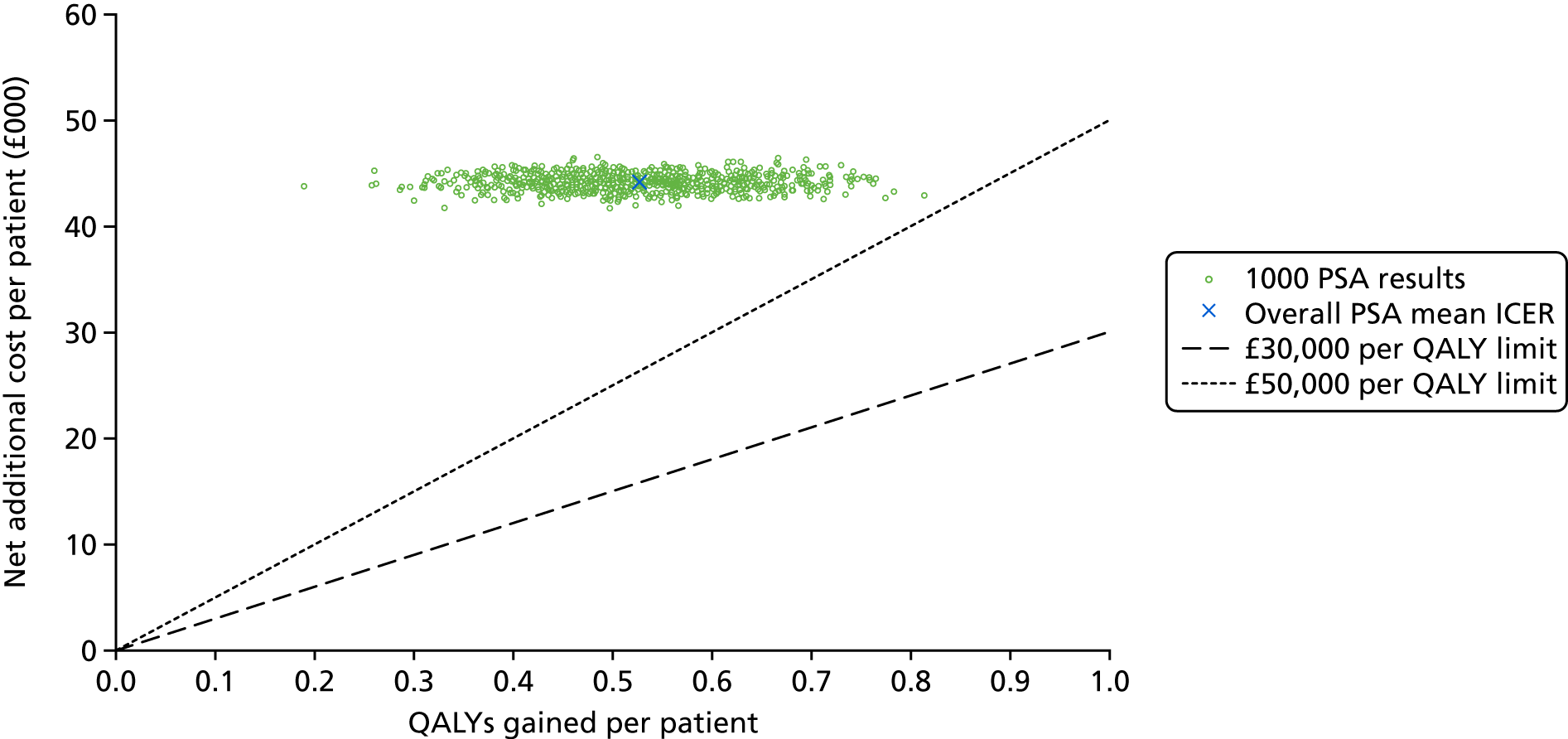
Unfortunately, information relating to the key outcome variables (PFS, OS and time to treatment discontinuation) provided to the AG by one of the companies was not in the form requested, and information on uncertainty in the estimated treatment dose intensity was not included by the other company in its submission or their model. Without these key data items, it was not possible to incorporate these important components of the normal PSA on this occasion. Therefore, the results presented below should be treated with caution.
For the comparison of lenvatinib with BSC, the deterministic ICER is £65,872 per QALY gained and the probabilistic ICER is £66,038 per QALY gained.
For the comparison of sorafenib with BSC, the deterministic ICER is £85,644 per QALY gained and the probabilistic ICER is £83,547 per QALY gained.
The variation in additional cost per patient is much smaller relative to the uncertainty in outcomes (QALYs) gained because of the dominance of drug acquisition costs, which constitute 85–90% of the incremental cost per patient when full list prices are assumed to apply.
Clearly, both treatments exhibit estimated ICERs well above £50,000 per QALY gained if list prices are applied. This is confirmed by the cost-effectiveness acceptability curves (CEACs) presented in Figures 20 and 21. An examination of the CEACs shows that, compared with BSC, the probability of sorafenib being cost-effective at a threshold of £50,000 per QALY gained is < 0.05% and the probability of lenvatinib being cost-effective is 5.4%.
FIGURE 20.
Cost-effectiveness acceptability curves for sorafenib vs. BSC (DECISION).

FIGURE 21.
Cost-effectiveness acceptability curves for lenvatinib vs. BSC (SELECT).
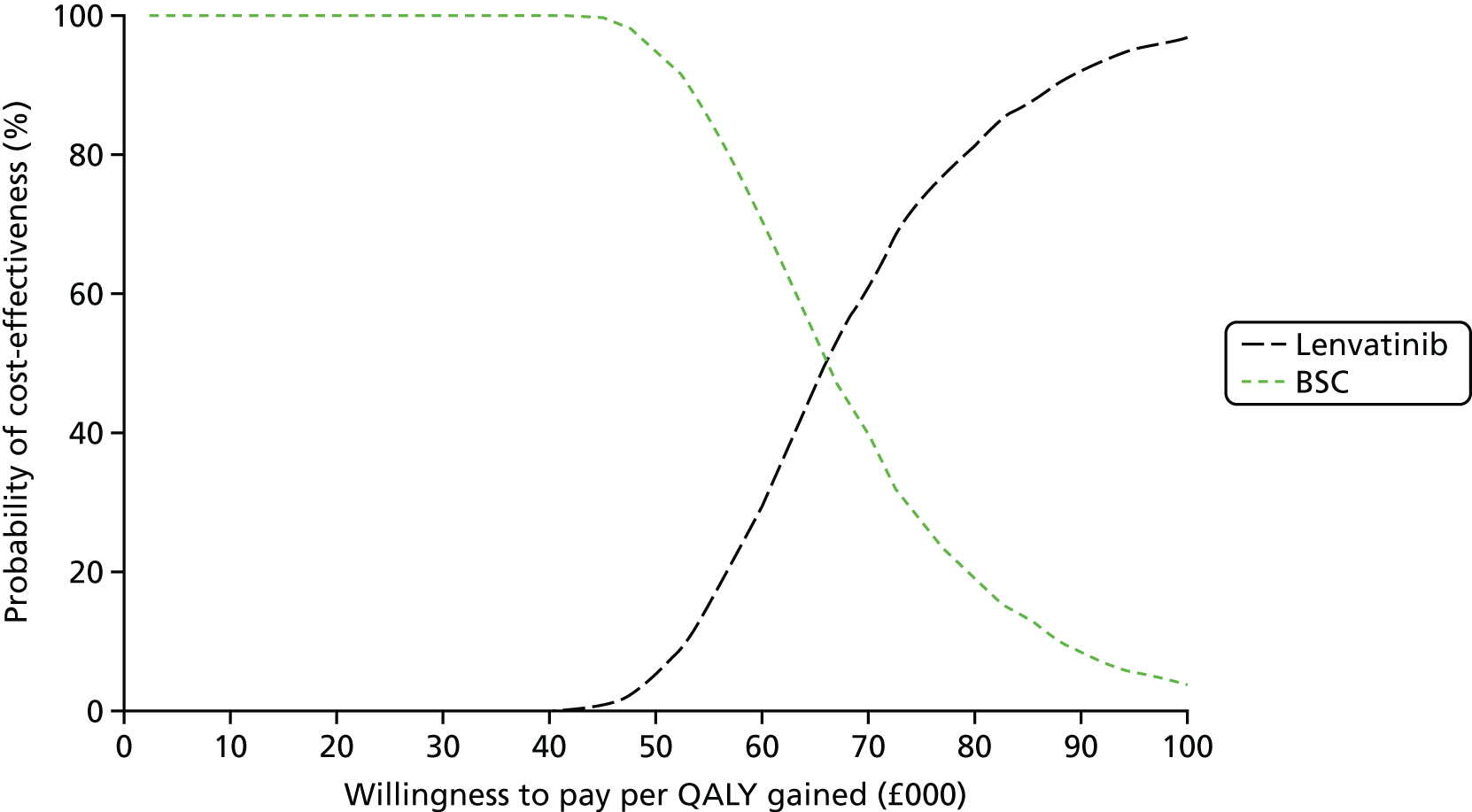
Discussion and summary of cost-effectiveness results
The comparison of data from the placebo arms of SELECT and DECISION indicated that the experience of patients differed markedly for PFS, the principal outcome of both trials, to the extent that the PHs assumption is violated. This invalidates the derivation and application of HRs in order to model an indirect comparison to compare the effectiveness of lenvatinib with that of sorafenib. As a consequence, the AG was only able to carry out separate economic assessments of each active treatment against its trial comparator, using common methods and shared parameter values.
In order to assess the importance of the available placebo data (used to represent long-term BSC), a structural sensitivity analysis was carried out substituting the placebo arm data from each trial as the comparator for the intervention treatment. These analyses resulted in very large changes to the AG’s estimated base-case ICERs per QALY gained, and confirmed the suspicion that the two trial populations are not equivalent.
Using published list prices47 in the AG model, neither treatment was found to be cost-effective at a willingness-to-pay threshold of £50,000 per QALY gained. Moreover, neither treatment meets the NICE end-of-life criteria for special consideration [the AG analyses show that both are indicated to have a lifetime mean estimated OS of 55 to 57 months, and a survival gain versus standard of care (BSC/placebo) of > 9 months].
A comparison of the patterns of clinical effectiveness of the two treatments suggests that the proportion of the average gain in PFS, which is subsequently translated to a gain in OS, is very different between the treatments (73% for lenvatinib vs. 24% for sorafenib). This suggests quite different modes of action, which may have important consequences for patients’ long-term prognoses.
The estimated mean time spent in the PFS and OS health states in the AG model show little difference between the two active treatments, so that apparently different net outcome gains are mainly attributable to large differences in the experience of patients in the comparator arms of the two trials. This consistency of outcomes for the active treatments, and the apparently different modes of action, may suggest that these treatments could be used sequentially to generate additional long-term benefit.
Assessment of factors relevant to the NHS and other parties
Lenvatinib and sorafenib are both MKIs and have been approved for use for treating RR-DTC in NHS Scotland (contingent on the continuing availability of PAS prices). Sorafenib is currently available in NHS England via the CDF. Therefore, it is not anticipated that, if recommended by NICE, the use of lenvatinib and sorafenib would have major implications for NHS service provision, particularly as the administration and AEs from both therapies are broadly in line with those of other TKIs already used to treat patients with cancer in the NHS.
Chapter 6 Discussion
Statement of principal findings
Clinical effectiveness results
The main sources of clinical effectiveness evidence were two good-quality RCTs (SELECT51 and DECISION52). Results from these trials show that treatment with either lenvatinib or sorafenib statistically significantly improves median PFS and ORR when compared with placebo. Median OS results demonstrate that there is no statistically significant difference in effect when treatment with lenvatinib or sorafenib are compared with placebo. Treatment crossover confounds the OS results from both trials and, to adjust for this effect, OS data were modified using RPSFTM. The results from the adjusted analyses show that, when compared with placebo, treatment with lenvatinib statistically significantly improves OS but there is still no statistically significant improvement in OS from treatment with sorafenib. However, the AG considers that the assumption of PH for unadjusted OS, adjusted OS and PFS is violated in SELECT and is violated for adjusted OS and PFS in DECISION; therefore, these results should be interpreted with caution. Nonetheless, clinical advice to the AG is that the improvements in PFS and the benefits from active treatment do appear to be clinically meaningful.
The AG considers that the improvements in OS and PFS for patients treated with lenvatinib and sorafenib when compared with placebo are likely to reflect improvements in OS and PFS when compared with BSC, notwithstanding the possible differences in the BSC received by the patients in the two trials.
The AG highlights that differences exist between the median OS and PFS results from the observational studies,59,77,78,81,88,101,103,126,135,137 and those from SELECT and DECISION: OS for patients treated with lenvatinib and sorafenib in SELECT and DECISION was longer than the OS reported in the observational studies. In contrast, in DECISION, PFS for patients treated with sorafenib was shorter than for in any of the prospective observational studies and the two meta-analyses. 127,138 Median PFS for patients treated with lenvatinib in SELECT was longer than suggested by the prospective, observational results from Study 20177 and shorter than in Study 208. 135
Results from indirect comparisons and MAICs7,8,57,97 show that treatment with lenvatinib leads to better PFS (but not OS) than treatment with sorafenib. The AG did not conduct an indirect comparison as preliminary analyses suggested that using data from SELECT and DECISION in the same network would generate unreliable results. The AG’s preliminary analyses showed that the PFS risk profiles (as demonstrated by a comparison of K–M data) of the SELECT and DECISION populations receiving placebo were not comparable. In addition, results from the AG’s analyses showed that, within SELECT and DECISION, the PH assumption did not hold for the majority of survival outcomes. For data to be included in a network, the assumption of PH should hold both across trials and within trials. The AG’s analyses have demonstrated that this assumption is often violated. As a consequence of this violation, the AG has been unable to compare lenvatinib with sorafenib. The AG considers that the relative clinical effectiveness of these two drugs cannot currently be reliably determined.
As expected, both treatment with lenvatinib and with sorafenib resulted in more AEs than treatment with placebo. Both all-grade and grade ≥ 3 diarrhoea were common for patients treated with lenvatinib and those treated with sorafenib. However, the most common AE experienced by patients treated with lenvatinib was hypertension and the most common AE experienced by patients treated with sorafenib was hand–foot syndrome. Dose reductions were frequent for patients treated with lenvatinib (67.8%) and for patients treated with sorafenib (64.3%). The results of published indirect comparisons97 suggest that when treatment with sorafenib is compared with lenvatinib, the incidence of alopecia is higher but the incidence of hypertension is reduced, and those treated with sorafenib experience fewer grade ≥ 3 AEs, SAEs and withdrawals owing to AEs.
The impact of treatment with lenvatinib on HRQoL was not assessed in SELECT and is, therefore, unknown; this is a limitation of the trial given the difference in the safety profiles for some of the AEs associated with lenvatinib and sorafenib. Sorafenib is reported7,120 to have a ‘mild’ negative impact on patients’ HRQoL, possibly attributable to the high rates of AEs experienced by patients in DECISION.
Cost-effectiveness evidence
The two submitting companies and the AG agree that there are no published cost-effectiveness studies relevant to the decision problem set out in the final scope issued by NICE. 53 The AG considered that none of the cost-effectiveness studies identified via the AG’s literature review were carried out from a NHS England perspective and that, when treatment with lenvatinib and sorafenib were compared, the results were based on the results of flawed indirect comparisons. In addition, the prices of the drugs reported in the studies were generally not consistent with the discounted prices that will likely be charged in the NHS in England. As a result of the absence of relevant published evidence, the AG developed a de novo cost-effectiveness model for the specific purpose of this appraisal and carried out several cost-effectiveness comparisons.
As the AG did not consider that it was appropriate to carry out an indirect comparison, the AG compared the cost-effectiveness of treatment with lenvatinib with the cost-effectiveness of BSC (using data from SELECT) and the cost-effectiveness of treatment with sorafenib with the cost-effectiveness of BSC (using data from DECISION). The AG also compared the cost-effectiveness of each of the SELECT and DECISION intervention drugs with BSC data from the other trial as a sensitivity analysis.
In the AG’s base-case analysis, using list prices only, the comparison of treatment with lenvatinib versus BSC yields an ICER per QALY gained of £65,872, and the comparison of treatment with sorafenib versus BSC yields an ICER per QALY gained of £85,644. The base-case deterministic and probabilistic results were similar for both comparisons. The AG’s deterministic sensitivity analysis involved varying 18 parameters; the results showed that none of the variations lowered the AG’s base-case ICERs to < £50,000 per QALY gained.
When the AG compared the cost-effectiveness of treatment with lenvatinib with the cost-effectiveness of BSC (using placebo data from SELECT) and the cost-effectiveness of treatment with sorafenib with the cost-effectiveness of BSC (placebo data from DECISION), the ICERs per QALY gained were approximately doubled (£130,592) and halved (£41,716), respectively. These results confirm that the choice of BSC comparator is hugely influential in this appraisal.
Strengths and limitations of the assessment
Strengths
A key strength of this review is that it has brought together all of the available relevant evidence (RCTs, observational studies, systematic reviews, indirect comparisons and cost-effectiveness studies) for assessing the clinical and cost-effectiveness of treatment with lenvatinib versus sorafenib in patients with RR-DTC.
The wide array of clinical results available demonstrate that treatment with lenvatinib is more effective when compared with placebo/BSC for all patients and that prior VEGFR-targeted therapy (or even a treatment delay) does not influence the potential for a patient to benefit from treatment.
Another strength of the research is the AG’s detailed investigation of the PFS (and OS) risk profiles of the patients in the two main trials. The AG’s analytical critique shows that the assumptions of PH underpinning the indirect comparison calculations are violated and explains why data from these two trials should not be compared in an indirect comparison. The AG’s critique challenges the validity of published indirect comparison results7,8,57,97 as well as those from published economic evaluations7,8,38,160,162 that have used indirect comparison results in their analyses.
The results from the AG’s economic analyses demonstrate that the choice of BSC comparator has a big influence on the size of the estimated ICERs per QALY gained.
Limitations
The main limitation of this review is that the AG was unable to compare the clinical effectiveness and cost-effectiveness of lenvatinib with those of sorafenib. The AG did not consider that it was appropriate to conduct an indirect comparison because of key differences in the intervention and placebo arms of SELECT and DECISION (both within and across the trials) and because the results of AG analyses demonstrated that the risk profiles of the patients in the placebo arms were different. Therefore, the AG concluded that it was not possible to determine the comparative clinical effectiveness and cost-effectiveness of lenvatinib versus sorafenib; this is problematic as lenvatinib and sorafenib are two relatively new treatments that appear to work well compared with placebo/BSC for patients with RR-DTC who have limited treatment options.
Uncertainties
Although it is recommended4,23–25 that only patients who are symptomatic and/or have rapidly progressing disease are treated with lenvatinib or sorafenib, it is unclear how many patients in SELECT and DECISION met these criteria. As there are no universally accepted objective criteria for describing patients who are symptomatic and/or rapidly progressing, it is difficult to retrospectively identify these groups of patients with any confidence.
Therefore, it is unclear whether or not the efficacy findings from SELECT and DECISION differ in patients who are symptomatic and/or rapidly progressing compared with those who are not. It is also unknown whether or not the frequency and type of AEs differ between these groups of patients and/or whether patient HRQoL is also influenced by symptom status.
There is considerable uncertainty around the HRQoL of patients with RR-DTC in general. Although it appears that treatment with sorafenib may have a ‘mild’ negative impact on HRQoL, the HRQoL data collected during DECISION were limited. As HRQoL data were not collected as part of SELECT, the impact of treatment with lenvatinib on HRQoL, whether positive or negative, is unknown. To what extent a patient’s HRQoL is affected by their symptom status (symptomatic vs. asymptomatic) is also unknown.
Although, for patients with RR-DTC, RCT evidence has shown clinically meaningful improvements in PFS for those treated with lenvatinib and sorafenib compared with placebo, the question remains whether treatment with lenvatinib or sorafenib can deliver a true OS benefit to patients. The adjusted RPSFTM OS estimates suggest that this may be the case for patients treated with lenvatinib but not for patients treated with sorafenib.
Other relevant factors
The AG considers that it is important to reiterate that the cost–utility analyses presented in this MTA report are based on list prices only. As lenvatinib has a confidential PAS price and sorafenib has a confidential Commercial Unit Access price, the cost-effectiveness comparisons presented in this AG report cannot be used as the basis for decision-making. The AG provided cost-effectiveness results generated using the discounted prices for lenvatinib and sorafenib in a confidential appendix presented to NICE.
Chapter 7 Conclusions
Compared with placebo, treatment with lenvatinib or sorafenib results in an improvement in PFS, ORR and, possibly, OS. However, compared with placebo, both drugs also increase the incidence of AEs, in particular hypertension, hand–foot syndrome and diarrhoea. Dose reductions with both drugs are, therefore, frequently required.
The AG considers that it is not possible to compare the clinical effectiveness or cost-effectiveness of lenvatinib with those of sorafenib. Primarily, this is because the risk profiles of the patients in the placebo arms of SELECT and DECISION do not appear to be comparable.
Using list prices, compared with BSC, both treatments exhibit estimated ICERs of > £50,000 per QALY gained. Compared with BSC, the probability of sorafenib being cost-effective at a threshold of £50,000 per QALY gained is < 0.05% and the probability of lenvatinib being cost-effective is 5.4%.
Suggested research priorities
In order of priority, the AG suggests the following further research priorities:
-
Head-to-head RCT evidence.
-
Clinical advice to the AG is that only RR-DTC patients experiencing symptoms, or those who have clinically significant progressive disease, are likely to be treated in routine clinical practice. Subgroup analyses suggest that the effects on PFS are similar for patients treated with sorafenib regardless of whether they are symptomatic or asymptomatic. However, these findings are post hoc and include only a minority of symptomatic patients. It is unclear if other outcomes, such as OS, ORR, AEs and HRQoL, differ by symptomatic or asymptomatic disease. Future studies of patients should aim to include a greater proportion of patients with symptomatic disease and investigate possible differences. Consideration should be given to using the classification of patients as symptomatic or asymptomatic as a randomisation stratification factor.
-
It would be useful to record, and report, HRQoL outcomes from any future clinical study of lenvatinib and sorafenib. In particular, data should be collected, using the EQ-5D questionnaire, throughout the whole trial period, not only from patients whose disease has not progressed. Further research on HRQoL from treating patients who have symptomatic disease compared with those who do not is also required.
-
Currently, evidence does not allow a comparison of the effectiveness of treatment with lenvatinib with the effectiveness of treatment with sorafenib. A head-to-head trial considering these treatments and placebo would generate results that would be valuable to decision-makers.
-
It would be useful to explore how lenvatinib, sorafenib and BSC should be positioned in the treatment pathway.
-
-
Statistical research.
-
The AG considers that it is important to explore more than just standard differences in participant and trial characteristics when considering the heterogeneity of studies that may be included in an indirect comparison. The AG suggests that, before undertaking an indirect comparison, the risk profiles of patient populations for the relevant outcome should be checked to confirm that they are proportional both within and across all trials that are being considered for inclusion in the network. This assessment would avoid generating indirect comparison results that are of unknown reliability.
-
Acknowledgements
The authors would like to thank to Gareth Jones (Liverpool Reviews and Implementation Group) for administrative support and Eleanor Kotas (Liverpool Reviews and Implementation Group) for conducting the searches. The AG would also like to thank Dr Caroline Brammer (Consultant in Clinical Oncology, The Clatterbridge Cancer Centre) for reading and commenting on a draft of the report.
Contributions of authors
Nigel Fleeman (Research Fellow, University of Liverpool) reviewed the evidence for clinical effectiveness, including study selection, data extraction, synthesis and interpretation. He contributed to the study selection for inclusion into the cost-effectiveness review and provided editorial input.
Rachel Houten [Research Associate (Health Economics Modelling), University of Liverpool] reviewed the evidence for cost-effectiveness, including study selection, data extraction, synthesis and interpretation. She contributed to the study selection for inclusion into the clinical effectiveness review and developed cost models for routine treatment and AEs.
Adrian Bagust (Professor of Health Modelling, University of Liverpool) developed the de novo economic model.
Marty Richardson [Research Associate (Medical Statistician), University of Liverpool] provided statistical advice and quality assessment of the systematic reviews included in the clinical effectiveness review.
Sophie Beale [Research Associate (Decision Analyst), University of Liverpool] interpreted the clinical effectiveness and cost-effectiveness evidence, summarised the company economic models and provided editorial input.
Angela Boland (Associate Director, University of Liverpool) interpreted the clinical effectiveness and cost-effectiveness evidence and provided editorial input.
Yenal Dundar (Research Fellow, University of Liverpool) contributed to data extraction and the quality assessment of studies included in the clinical effectiveness review.
Janette Greenhalgh [Senior Research Fellow (Clinical Effectiveness), University of Liverpool] contributed to data extraction and the quality assessment of studies included in the clinical effectiveness review.
Juliet Hounsome (Research Associate, University of Liverpool) contributed to the protocol development and the selection of studies for inclusion into the clinical effectiveness review.
Rui Duarte (Health Technology Assessment Lead, University of Liverpool) provided editorial input.
Aditya Shenoy (Consultant in Clinical Oncology, The Clatterbridge Cancer Centre NHS Foundation Trust) provided clinical advice on all aspects of the review.
All authors contributed to the writing of the report.
Publication
Fleeman N, Houten R, Chaplin M, Beale S, Boland A, Dundar Y, et al. A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer 2019;19;1209.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Thyroid Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/thyroid-cancer/incidence (accessed 5 May 2017).
- Cancer Research UK . About Thyroid Cancer n.d. www.cancerresearchuk.org/about-cancer/type/thyroid-cancer/about/the-thyroid-gland (accessed 5 May 2017).
- Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am 2010;43:229-38. https://doi.org/10.1016/j.otc.2010.01.002.
- Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol 2014;81:1-122. https://doi.org/10.1111/cen.12515.
- Canadian Agency for Drugs and Technologies in Health (CADTH) . Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Sorafenib (Nexavar) for Differentiated Thyroid Cancer 2015. www.cadth.ca/sites/default/files/pcodr/pcodr_sorafenib_nexavar_dtc_fn_cgr.pdf (accessed 16 May 2017).
- Canadian Agency for Drugs and Technologies in Health (CADTH) . Pan-Canadian Oncology Drug Review Final Clinical Guidance Report: Lenvatinib (Lenvima) for Differentiated Thyroid Cancer 2016. www.cadth.ca/sites/default/files/pcodr/pcodr_lenvatinib_lenvima_dtc_fn_cgr.pdf (accessed 16 May 2017).
- Bayer HealthCare . Multiple Technology Appraisal. Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine. Company Submission to NICE 2017. www.nice.org.uk/guidance/gid-ta10101/documents/committee-papers (accessed 30 July 2018).
- Eisai Ltd . Lenvatinib for Treating Differentiated Thyroid Cancer After Radioactive Iodine. Multiple Technology Appraisal [ID1059]. Company Submission to NICE 2017. www.nice.org.uk/guidance/gid-ta10101/documents/committee-papers (accessed 30 July 2018).
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017;317:1338-48. https://doi.org/10.1001/jama.2017.2719.
- Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 2016;23:313-22. https://doi.org/10.1530/ERC-15-0445.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164-7. https://doi.org/10.1001/jama.295.18.2164.
- Yao Y, Chiu CG, Strugnell SS, Gill S, Wiseman SM. Gender differences in thyroid cancer. Expert Rev Endocrinol Metab 2011;6:215-43. https://doi.org/10.1586/eem.11.9.
- Cancer Research UK . Thyroid Cancer Mortality Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/thyroid-cancer/mortality (accessed 5 May 2017).
- Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res 2012;2012. https://doi.org/10.1155/2012/618985.
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 1998;83:2638-48. https://doi.org/10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1.
- Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229-42. https://doi.org/10.1089/thy.2006.16.1229.
- Cancer Research UK . Types of Thyroid Cancer n.d. www.cancerresearchuk.org/about-cancer/type/thyroid-cancer/about/types-of-thyroid-cancer (accessed 5 May 2017).
- Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11. https://doi.org/10.1016/S0140-6736(03)12488-9.
- Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc 2002;113:241-60.
- Sajid-Crokett S, Hershman J, De Groot LJ, Chrousos G, Dungan K, Feingold KR, et al. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
- Clayman G. Hürthle Cell Thyroid Cancer: A Type of Thyroid Tumor and Thyroid Cancer n.d. www.endocrineweb.com/conditions/thyroid-cancer/hurthle-cell-thyroid-tumor (accessed 19 June 2017).
- Verburg FA, Mäder U, Tanase K, Thies ED, Diessl S, Buck AK, et al. Life expectancy is reduced in differentiated thyroid cancer patients ≥ 45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab 2013;98:172-80. https://doi.org/10.1210/jc.2012-2458.
- Pacini F, Castagna MG, Brilli L, Pentheroudakis G. ESMO Guidelines Working Group . Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii110-9. https://doi.org/10.1093/annonc/mds230.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. https://doi.org/10.1089/thy.2015.0020.
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma 2017. www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed 10 May 2017).
- European Medicines Agency . CHMP Extension of Indication Variation Assessment Report: Nexavar 2014. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000690/WC500168976.pdf (accessed 10 May 2017).
- European Medicines Agency . Assessment Report. Lenvima 2015. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003727/WC500188676.pdf (accessed 10 May 2017).
- Lopez-Penabad L, Chiu AC, Hoff AO, Schultz P, Gaztambide S, Ordoñez NG, et al. Prognostic factors in patients with Hürthle cell neoplasms of the thyroid. Cancer 2003;97:1186-94. https://doi.org/10.1002/cncr.11176.
- Muresan MM, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer 2008;15:37-49. https://doi.org/10.1677/ERC-07-0229.
- Vachani C, Hoff K. All About Hürthle Cell Carcinoma n.d. www.oncolink.org/cancers/thyroid/all-about-huerthle-cell-carcinoma (accessed 19 June 2017).
- Schmidt A, Iglesias L, Klain M, Pitoia F, Schlumberger MJ. Radioactive iodine-refractory differentiated thyroid cancer: an uncommon but challenging situation. Arch Endocrinol Metab 2017;61:81-9. https://doi.org/10.1590/2359-3997000000245.
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892-9. https://doi.org/10.1210/jc.2005-2838.
- Gruber JJ, Colevas AD. Differentiated thyroid cancer: focus on emerging treatments for radioactive iodine-refractory patients. Oncologist 2015;20:113-26. https://doi.org/10.1634/theoncologist.2014-0313.
- Sacks W, Braunstein GD. Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr Pract 2014;20:263-75. https://doi.org/10.4158/EP13305.RA.
- Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med 1996;37:598-605.
- Brose MS. In search of a real ‘targeted’ therapy for thyroid cancer. Clin Cancer Res 2012;18:1827-9. https://doi.org/10.1158/1078-0432.CCR-12-0153.
- Newbold KL, Flux G, Wadsley J. Radioiodine for high risk and radioiodine refractory thyroid cancer: current concepts in management. Clin Oncol 2017;29:307-9. https://doi.org/10.1016/j.clon.2016.12.008.
- Scottish Medicines Consortium . Lenvatinib 4 mg and 10 mg Hard Capsules (Lenvima®) SMC No. (1179 16) 2016. www.scottishmedicines.org.uk/files/advice/lenvatinib_Lenvima_FINAL_Sept_2016_amended_30.09.16_for_website.pdf (accessed 17 November 2016).
- Schlumberger M, Sherman SI. Clinical trials for progressive differentiated thyroid cancer: patient selection, study design, and recent advances. Thyroid 2009;19:1393-400. https://doi.org/10.1089/thy.2009.1603.
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. https://doi.org/10.1158/1078-0432.CCR-06-0931.
- Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer 2011;2:193-9. https://doi.org/10.7150/jca.2.193.
- Covell LL, Ganti AK. Treatment of advanced thyroid cancer: role of molecularly targeted therapies. Target Oncol 2015;10:311-24. https://doi.org/10.1007/s11523-014-0331-z.
- Food and Drug Administration . Lenvatinib (Lenvima) 2015. https://wayback.archive-it.org/7993/20170111231641/http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm434347.htm (accessed 16 May 2017).
- Food and Drug Administration . Sorafenib (Nexavar) 2015. https://wayback.archive-it.org/7993/20170111231704/http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm376547.htm (accessed 16 May 2017).
- European Medicines Agency . Product Information: 23 03 2017 Lenvima-EMEA H C 003727-WS 1123. Annex I – Summary of Product Characteristics n.d. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003727/WC500188674.pdf (accessed 10 May 2017).
- European Medicines Agency . Product Information: 02 09 2016 Nexavar-EMEA H C 000690-N 38. Annex I – Summary of Product Characteristics n.d. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000690/WC500027704.pdf (accessed 10 May 2017).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- Scottish Medicines Consortium . Sorafenib 200mg Film-Coated Tablets (Nexavar®) SMC No. (1055 15) 2015. www.scottishmedicines.org.uk/files/advice/sorafenib_Nexavar_FINAL_June_2015_for_website.pdf (accessed 10 May 2017).
- European Medicines Agency . Nexavar (Sorafenib) n.d. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000690/human_med_000929.jsp&mid=WC0b01ac058001d124 (accessed 10 May 2017).
- European Medicines Agency . Lenvima (Lenvatinib) n.d. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003727/human_med_001864.jsp&mid=WC0b01ac058001d124 (accessed 10 May 2017).
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. https://doi.org/10.1056/NEJMoa1406470.
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet 2014;384:319-28. https://doi.org/10.1016/S0140-6736(14)60421-9.
- National Institute for Health and Care Excellence (NICE) . Multiple Technology Appraisal. Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine 2016. www.nice.org.uk/guidance/indevelopment/gid-ta10101 (accessed 20 July 2017).
- Fleeman N, Houten R, Chaplin M, Beale S, Boland A, Dundar Y, et al. A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-6369-7.
- Liverpool Reviews and Implementation Group . Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine. Draft Protocol 2016. www.nice.org.uk/guidance/gid-ta10101/documents/final-protocol (accessed 21 May 2017).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare 2008. www.york.ac.uk/crd/SysRev/!SSL!/WebHelp/SysRev3.htm#5_5_QUALITY_ASSESSMENT.htm (accessed 24 January 2017).
- Tremblay G, Holbrook T, Milligan G, Pelletier C, Rietscheli P. Matching-adjusted indirect treatment comparison in patients with radioiodine-refractory differentiated thyroid cancer. Comp Eff Res 2016;6:13-21.
- Brose MS, Jarzab B, Elisei R, Siena S, Bastholt L, De La Fouchardiere C, et al. Updated overall survival analysis of patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) treated with sorafenib on the Phase 3 DECISION trial. Ann Oncol 2014;27.
- Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold KL, Viros A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a Phase II study in a UK based population. Eur J Endocrinol 2011;165:315-22. https://doi.org/10.1530/EJE-11-0129.
- Ahmed M, Barbachano Y, Riddell AM, Whittaker S, Newbold K, Harrington K, et al. An open labelled Phase 2 study evaluating the safety and efficacy of sorafenib in metastatic advanced thyroid cancer. Ann Oncol 2008;19:viii-218. https://doi.org/10.1200/jco.2008.26.15_suppl.6060.
- Anderson RT, Linnehan JE, Tongbram V, Keating K, Wirth LJ. Clinical, safety, and economic evidence in radioactive iodine-refractory differentiated thyroid cancer: a systematic literature review. Thyroid 2013;23:392-407. https://doi.org/10.1089/thy.2012.0520.
- Ball D, Sherman S, Jarzab B, Cabanillas M, Licitra L, Pacini F, et al. A Phase II trial of the multi-targeted kinase inhibitor lenvatinib (E7080) in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC): Correlation of treatment outcomes with tumor genetic analysis, serum biomarkers and pharmacokinetics. Thyroid 2011;21:A6-7.
- Ball DW, Sherman SI, Jarzab B, Cabanillas ME, Martins R, Shah MH, et al. Lenvatinib treatment of advanced RAI-refractory differentiated thyroid cancer (DTC): Cytokine and angiogenic factor (CAF) profiling in combination with tumor genetic analysis to identify markers associated with response. J Clin Oncol 2012;30.
- Bastholt L, Brose MS, Jarzab B, Schlumberger M, Siena S, De La Fouchardiere C, et al. Population PK modeling and exposure-response analyses of sorafenib in patients with radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) in the Phase III DECISION trial. J Clin Oncol 2014;32.
- Bockisch A, Brose MS, Nutting C, Jarzab B, Elisei R, Siena S, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer (DTC): The Phase III DECISION trial. Exp Clin Endocrinol Diabetes 2014;122. https://doi.org/10.1055/s-0034-1372011.
- Brose M, Jarzab B, Elisei R, Giannetta L, Bastholt L, De La Fouchardiere C, et al. Final overall survival analysis of patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) treated with sorafenib in the Phase 3 DECISION trial: an exploratory crossover adjustment analyses. Ann Oncol 2016;27. https://doi.org/10.1093/annonc/mdw376.06.
- Brose M, Schlumberger M, Tahara M, Wirth L, Robinson B, Elisei R, et al. Effect of age and lenvatinib treatment on overall survival for patients with 131I-refractory differentiated thyroid cancer in SELECT. Asia Pac J Clin Oncol 2015;11.
- Brose MS, Jarzab B, Elisei R, Siena S, Bastholt L, De La Fouchardiere C, et al. Updated overall survival analysis of patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) treated with sorafenib on the Phase 3 DECISION trial. J Clin Oncol 2014;32.
- Brose MS, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: the Phase III DECISION trial. J Clin Oncol 2013;31.
- Brose MS, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: The Phase 3 decision trial. Oncol Res Treat 2014;37:130-1.
- Brose MS, Nutting C, Shong YK, Sherman SI, Smit JWA, Chung J, et al. Association between tumor BRAF and RAS mutation status and clinical outcomes in patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) randomized to sorafenib or placebo: sub-analysis of the Phase III DECISION trial. Eur J Cancer 2013;49.
- Brose MS, Nutting CM, Sherman SI, Shong YK, Smit JW, Reike G, et al. Rationale and design of decision: a double-blind, randomized, placebo-controlled Phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 2011;11. https://doi.org/10.1186/1471-2407-11-349.
- Brose MS, Schlumberger M, Tahara M, Wirth LJ, Robinson B, Elisei R, et al. Effect of age and lenvatinib treatment on overall survival for patients with 131I-refractory differentiated thyroid cancer in SELECT. J Clin Oncol 2015;33.
- Brose MS, Teng A, Rietschel P, Habra MA. Lenvatinib and the effect of age on overall survival for patients with radioiodine-refractory differentiated thyroid cancer. Thyroid 2015;25.
- Brose MS, Troxel AB, Harlacker K, Redlinger M, Chalian AA, Loevner LA, et al. Completion of a Phase II study of sorafenib for advanced thyroid cancer. Eur J Cancer 2009;7. https://doi.org/10.1016/S1359-6349(09)72086-5.
- Brose MS, Troxel AB, Redlinger M, Harlacker K, Redlinger C, Chalian AA, et al. Effect of BRAFV600E on response to sorafenib in advanced thyroid cancer patients. J Clin Oncol 2009;27.
- Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B, et al. A Phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015;121:2749-56. https://doi.org/10.1002/cncr.29395.
- Chen L. Sorafenib at a low dose against radioiodine refractory metastatic papillary thyroid carcinoma in lung. Thyroid 2011;21. https://doi.org/10.1089/thy.2010.0199.
- Choi J, Abouzaid S, Li X, Rietschel P. Characteristics of patients on lenvatinib with treatment-emergent hypertension in the SELECT trial. Thyroid 2015;25.
- Cohen AB, Yarchoan M, Troxel AB, Puttaswamy K, Harlacker K, Loevner LA, et al. Phase II trial of sorafenib in advanced thyroid cancer: a disease site analysis. J Clin Oncol 2014;32.
- Duntas LH, Vlassopoulou V, Boutsiadis A, Mantzou E, Anapliotou M, Tsatsoulis A. Sorafenib in the treatment of radioiodine refractory thyroid cancer. A multicenter Phase II study. Eur Thyroid J 2011:102-3.
- Elisei R, Schlumberger M, Tahara M, Robinson B, Brose M, Dutcus C, et al. Subgroup analysis according to differentiated thyroid cancer histology in Phase 3 (SELECT) trial of lenvatinib. Oncol Res Treat 2015;38:25-6.
- Fassnacht M, Kappeler C, Healy DP, Baumer C, Meinhardt G, Elisei R, et al. Analysis of tumor growth rate for radioiodine (RAI)-refractory differentiated thyroid cancer patients receiving placebo and/ or sorafenib in the Phase III DECISION study. Oncol Res Treat 2016;39.
- Fassnacht M, Smit JW, Schlumberger M, Kappeler C, Meinhardt G, Brose MS. Management of thyroid stimulating hormone (TSH) and possible impact on outcomes for patients with radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) receiving sorafenib or placebo on the Phase III DECISION trial. Oncol Res Treat 2016;39:8-9.
- Gianoukakis AG, Mathias E, Dutcus CE, Kalantari P, Yoon S. Response to lenvatinib treatment in patients with radioiodine-refractory differentiated thyroid cancer (RRDTC). Oncol Res Treat 2016;39.
- Gianoukakis AG, Mathias EG, Dutcus C, Kalantari P, Yoon S. Response to lenvatinib treatment in patients with radioiodine refractory differentiated thyroid cancer (RR-DTC): updated results from SELECT. J Clin Oncol 2016;34.
- Guo M, Sherman S, Wirth L, Schlumberger M, Dutcus C, Robinson B, et al. Overall survival gain with lenvatinib vs. placebo in radioactive iodine refractory differentiated thyroid cancer (RR-DTC): an updated analysis. Eur J Cancer 2015;51. https://doi.org/10.1016/S0959-8049(16)31549-0.
- Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714-19. https://doi.org/10.1200/JCO.2008.16.3279.
- Habra MA, Schlumberger M, Wirth L, Robinson B, Brose MS, Taylor MH, et al. Phase 3 study of (e7080) lenvatinib in differentiated cancer of the thyroid (SELECT): Results and subgroup analysis of patients from North America. Thyroid 2014;24:A100-A1.
- Habra MA, Song J, Rietschel P. Outcomes by site of metastasis for patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib versus placebo: Results from a Phase 3, randomized trial. Thyroid 2015;25:A23-A4.
- Haddad R, Schlumberger M, Wirth L, Sherman E, Shah MH, Robinson B, et al. Incidence and timing of common adverse events in lenvatinib-treated patients with radioiodine-refractory thyroid cancer from the SELECT trial. Thyroid 2015;25.
- Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 2009;161:923-31. https://doi.org/10.1530/EJE-09-0702.
- Jean GW, Mani RM, Jaffry A, Khan SA. Toxic Effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol 2016;2:529-34. https://doi.org/10.1001/jamaoncol.2015.5927.
- Kappeler C, Healy DP, Baumer C, Meinhardt G, Elisei R, Schlumberger M, et al. Analysis of tumor growth rate for radioiodine (RAI)-refractory differentiated thyroid cancer patients receiving placebo and/or sorafenib in the Phase III DECISION study. J Clin Oncol 2015;33.
- Kappeler C, Meinhardt G, Elisei R, Brose M, Schlumberger M. Tumor growth rate analysis of progression-free survival (PFS) and overall survival (OS) for thyroid cancer patients receiving placebo or sorafenib in the Phase 3 DECISION trial. Ann Oncol 2016;27.
- Kawalec P. Efficacy and safety profile of lenvatinib and sorafenib in the treatment of adult patients with advanced radioactive-iodine-refractory differentiated thyroid cancer (RR-DTC). Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.09.2075.
- Kawalec P, Malinowska-Lipień I, Brzostek T, Kózka M. Lenvatinib for the treatment of radioiodine-refractory differentiated thyroid carcinoma: a systematic review and indirect comparison with sorafenib. Expert Rev Anticancer Ther 2016;16:1303-9. https://doi.org/10.1080/14737140.2016.1247697.
- Keefe SM, Troxel AB, Rhee S, Puttaswamy K, O’Dwyer PJ, Loevner LA, et al. Phase II trial of sorafenib in patients with advanced thyroid cancer. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.5562.
- Kiyota N, Robinson B, Shah M, Hoff AO, Taylor M, Li D, et al. Defining 131I-refractory differentiated thyroid cancer: efficacy and safety of lenvatinib by 131I-refractory criteria in the SELECT trial. Eur J Cancer 2015;51. https://doi.org/10.1016/S0959-8049(16)31602-1.
- Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, et al. Subgroup analysis of Japanese patients in a Phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 2015;106:1714-21. https://doi.org/10.1111/cas.12826.
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675-84. https://doi.org/10.1200/JCO.2008.18.2717.
- Kroiss M, Worden F, Shi J, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib for treatment of locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC): Detailed analyses from the Phase III DECISION trial. Oncol Res Treat 2014;37.
- Marotta V, Sciammarella C, Capasso M, Testori A, Pivonello C, Chiofalo MG, et al. Preliminary data of VEGF-A and VEGFR-2 polymorphisms as predictive factors of radiological response and clinical outcome in iodine-refractory differentiated thyroid cancer treated with sorafenib. Endocrine 2016;16.
- McFarland DC, Misiukiewicz KJ. Sorafenib in radioactive iodine-refractory well-differentiated metastatic thyroid cancer. Onco Targets Ther 2014;7:1291-9. https://doi.org/10.2147/OTT.S49430.
- Newbold K, Elisei R, Taylor MH, Krzyzanowska M, Shah MH, Hoff AO, et al. Efficacy and safety of lenvatinib for the treatment of patients with 131I-refractory differentiated thyroid cancer with and without prior VEGF-targeted therapy. Asia Pac J Clin Oncol 2015;11.
- Newbold K, Elisei R, Taylor MH, Krzyzanowska MK, Shah MH, Hoff AO, et al. Efficacy and safety of lenvatinib for the treatment of patients with 131I-refractory differentiated thyroid cancer with and without prior VEGF-targeted therapy. J Clin Oncol 2015;33.
- Newbold K, Robinson B, Schlumberger M, Tahara M, Brose MF, Elisei R, et al. Phase 3 study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT): results and subgroup analysis of patients from Europe. Eur Thyroid J 2014;3.
- Newbold K, Sherman S, Wirth LJ, Habra MA, Rietschel P, Song J, et al. The influence of time to objective response on lenvatinib clinical outcomes in the Phase 3 SELECT trial. Eur J Cancer 2015;51. https://doi.org/10.1016/S0959-8049(16)31600-8.
- Paschke R, Bastholt L, Brose MS, Jarzab B, Schlumberger M, Siena S, et al. Population PK modeling and exposure-response analyses of sorafenib in patients with radioactive iodinerefractory differentiated thyroid cancer (RAI-rDTC) in the Phase III DECISION trial. Oncol Res Treat 2014;37:268-9.
- Paschke R, Brose MS, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Updated overall survival analysis of patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) treated with sorafenib on the Phase 3 DECISION trial. Oncol Res Treat 2014;37.
- Paschke R, Brose MS, Nutting C, Jarzab BJ, Elisei R, Siena S, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: the Phase 3 DECISION trial. Onkologie 2013;36.
- Paschke R, Brose MS, Nutting C, Shong YK, Sherman SI, Smit JWA, et al. Association between tumor BRAF and RAS mutation status and clinical outcomes in patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) randomized to sorafenib or placebo: sub-analysis of the Phase III DECISION trial. Exp Clin Endocrinol Diabetes 2014;122. https://doi.org/10.1055/s-0034-1372012.
- Paschke R, Schlumberger M, Elisei R, Pacini F, Jarzab B, Giannetta L, et al. Prognostic and predictive factors correlated with treatment outcomes for radioactive Iodine-refractory differentiated thyroid cancer (RAI-rDTC) patients receiving sorafenib or placebo on the Phase III DECISION trial. Oncol Res Treat 2015;38. https://doi.org/10.1055/s-0035-1547604.
- Paschke R, Schlumberger M, Nutting C, Jarzab B, Elisei R, Siena S, et al. Exploratory analysis of outcomes for patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) receiving open-label sorafenib post-progression on the Phase III DECISION trial. Oncol Res Treat 2015;38. https://doi.org/10.1055/s-0035-1547632.
- Pena CE, Wilhelm S, Meinhardt G, Schlumberger M, Brose MS. Biomarkers of prognosis in patients with differentiated thyroid cancer: results from the DECISION trial. J Clin Oncol 2016;34.
- Robinson B, Schlumberger M, Wirth LJ, Dutcus CE, Binder TA, Guo M, et al. Responses in specific metastases following treatment with lenvatinib (LN): results from the Phase 3 SELECT trial. Ann Oncol 2016;27. https://doi.org/10.1093/annonc/mdw376.14.
- Robinson B, Schlumberger M, Wirth LJ, Dutcus CE, Song J, Taylor MH, et al. Characterization of tumor size changes over time from the Phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab 2016;101:4103-9. https://doi.org/10.1210/jc.2015-3989.
- Robinson BG, Schlumberger M, Sherman SI, Tahara M, Wirth L, Brose MS, et al. Open-label extension phase outcomes of the Phase 3 SELECT trial of lenvatinib in patients with 131I-refractory differentiated thyroid cancer. Endocr Rev 2015;36.
- Schlumberger M, Elisei R, Pacini F, Jarzab B, Giannetta L, Bastholt L, et al. Prognostic and predictive factors correlated with treatment outcomes for radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) patients receiving sorafenib or placebo on the Phase III decision trial. Thyroid 2014;24:A6-7.
- Schlumberger M, Jarzab B, Elisei R, Siena S, Bastholt L, De La Fouchardiere C, et al. Phase III randomized, double-blinded, placebocontrolled trial of sorafenib in locally advanced or metastatic patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC)-exploratory analyses of patient-reported outcomes. Thyroid 2013;23:A49-50.
- Schlumberger M, Jarzab B, Elisei R, Siena S, Bastholt L, De La Fouchardiere C, et al. A randomized, double-blind, placebo controlled Phase III trial (decision) of sorafenib in locally advanced or metastatic patients with progressive radioactive iodine-refractory differentiated thyroid cancer. Eur Thyroid J 2013;2.
- Schlumberger M, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Exploratory analysis of outcomes for patients with locally advanced or metastatic radioactive iodinerefractory differentiated thyroid cancer (RAI-rDTC) receiving open-label sorafenib post-progression on the Phase III decision trial. Eur Thyroid J 2014;3.
- Schlumberger M, Tahara M, Wirth L, Robinson B, Brose M, Elisei R, et al. A Phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with I-refractory differentiated thyroid cancer (SELECT). Oncol Res Treat 2014;37. https://doi.org/10.1200/jco.2014.32.18_suppl.lba6008.
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. A Phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (e7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT). J Clin Oncol 2014;32.
- Schneider T, Abdulrahman R, Kapiteijn E, Smit JW. Long term efficacy and tolerability of sorafenib in differentiated thyroid carcinoma: final results of a Phase II trial. Eur Thyroid J 2012;1.
- Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a Phase II trial. Eur J Endocrinol 2012;167:643-50. https://doi.org/10.1530/EJE-12-0405.
- Shen CT, Qiu ZL, Luo QY. Sorafenib in the treatment of radioiodine-refractory differentiated thyroid cancer: a meta-analysis. Endocr Relat Cancer 2014;21:253-61. https://doi.org/10.1530/ERC-13-0438.
- Sherman SI, Jarzab B, Cabanillas ME, Licitra L, Pacini F, Martins RG, et al. E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC); results of a multi-center Phase II trial. Eur Thyroid J 2011.
- Sherman SI, Jarzab B, Cabanillas ME, Licitra LF, Pacini F, Martins R, et al. A Phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC). J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.5503.
- Sherman SI, Schlumberger M, Tahara M, Robinson BG, Newbold K, Gianoukakis AG, et al. Relationship between thyroid-stimulating hormone levels and outcomes from the randomized, double-blind, Phase 3 study of (e7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Endocr Rev 2015;36.
- Sherman SI, Tahara M, Wirth L, Robinson B, Brose MF, Elisei R, et al. Analyses of a Phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT). Eur Thyroid J 2014;3:74-5.
- Smit JW, Schlumberger M, Kappeler C, Meinhardt G, Brose MS. Management of thyroid stimulating hormone (TSH) and possible impact on outcomes for patients with radioactive iodine-refractory differentiated thyroid cancer (RAI-RDTC) receiving sorafenib or placebo on the Phase III decision trial. Thyroid 2015;25.
- Tahara M, Schlumberger M, Elisei R, Habra MA, Kiyota N, Dutcus C, et al. Pharmacodynamic biomarkers of outcomes in the Phase III study of lenvatinib in 131I-refractory differentiated thyroid cancer (SELECT). J Clin Oncol 2015;33.
- Tahara M, Wirth L, Brose MS, Newbold K, Schlumberger M, Ductus C, et al. Efficacy and safety of lenvatinib by body mass index in patients with 131I-refractory differentiated thyroid cancer from the Phase 3 SELECT study. Thyroid 2015;25:A275-6.
- Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. Phase II study of lenvatinib in patients with differentiated, medullary, and anaplastic thyroid cancer: final analysis results. J Clin Oncol 2016;34.
- Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. Phase 2 study of lenvatinib in patients with differentiated, medullary and anaplastic thyroid cancer: Final analysis results. Asia Pac J Clin Oncol 2016;12.
- Terry RD, Keefe SM, Grande CM, Zifchak L, Brose MS. Timing and severity of skin-related adverse events in a Phase II trial of sorafenib (BAY43-9006) in patients with advanced thyroid cancer. J Clin Oncol 2013;31.
- Thomas L, Lai SY, Dong W, Feng L, Dadu R, Regone RM, et al. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist 2014;19:251-8. https://doi.org/10.1634/theoncologist.2013-0362.
- Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer 2015;22:877-87. https://doi.org/10.1530/ERC-15-0252.
- Worden FP, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib for treatment of locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC): detailed analyses from the Phase III DECISION trial. J Clin Oncol 2014;32.
- Ye X, Zhu Y, Cai J. Relationship between toxicities and clinical benefits of newly approved tyrosine kinase inhibitors in thyroid cancer: a meta-analysis of literature. J Cancer Res Ther 2015;11:C185-90. https://doi.org/10.4103/0973-1482.168182.
- Young L, Habra M. Outcomes by site of metastasis for patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib versus placebo: results from a Phase 3, randomized trial. Asia Pac J Clin Oncol 2016;12.
- Latimer NR, Abrams KR. Adjusting Survival Time Estimates in the Presence of Treatment Switching. Sheffield: School of Health and Related Research, University of Sheffield; 2014.
- Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-79.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-70.
- Capdevila J, Iglesias L, Halperin I, Segura A, Martínez-Trufero J, Vaz MÁ, et al. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer 2012;19:209-16. https://doi.org/10.1530/ERC-11-0351.
- Marotta V, Ramundo V, Camera L, Del Prete M, Fonti R, Esposito R, et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin Endocrinol 2013;78:760-7. https://doi.org/10.1111/cen.12057.
- Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab 2010;95:2588-95. https://doi.org/10.1210/jc.2009-1923.
- Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010;28:2323-30. https://doi.org/10.1200/JCO.2009.25.0068.
- National Institute for Health and Care Excellence (NICE) . Multiple Technology Appraisal. Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine [ID1059]. Committee Papers 2017. www.nice.org.uk/guidance/ta535/documents/committee-papers (accessed 2 August 2018).
- MATiSSe . A Pilot Study in Metastatic Advanced Thyroid Cancer Evaluating the Safety and Efficacy of Sorafenib n.d. www.clinicaltrialsregister.eu/ctr-search/trial/2006-006615-80/GB#A (accessed 7 July 2017).
- NEXAVAR-TC-01 (Study 17391) . Prospective, Non-Interventional, Post-Authorization Safety Study That Includes All Patients Diagnosed As Unresectable Differentiated Thyroid Carcinoma and Treated With Sorafenib (JPMS-DTC) n.d. https://clinicaltrials.gov/ct2/show/NCT02185560 (accessed 7 July 2017).
- E7080-G000-211 . A Phase 2 Trial of Lenvatinib (E7080) in Subjects With Iodine-131 Refractory Differentiated Thyroid Cancer to Evaluate Whether an Oral Starting Dose of 18 mg Daily Will Provide Comparable Efficacy to a 24 mg Starting Dose, But Have a Better Safety Profile n.d. https://clinicaltrials.gov/ct2/show/NCT02702388 (accessed 7 July 2017).
- E7080-C086-308 . A Trial of Lenvatinib (E7080) in Radioiodine (131I)-Refractory Differentiated Thyroid Cancer in China n.d. https://clinicaltrials.gov/ct2/show/record/NCT02966093.
- Brose MS, Smit JW, Lin C, Pitoia F, Fellous M, Schlumberger M, et al. Optimal timing of multikinase inhibitor initiation in radioactive iodine refractory asymptomatic patients with differentiated thyroid cancer – a global non-interventional study (RIFTOS MKI). Thyroid 2015;25.
- NICE . Bayer HealthCare Response to AG Report to the NICE Appraisal Committee 2017. www.nice.org.uk/guidance/ta535/documents/committee-papers (accessed 7 September 2017).
- Huang W, Chen L, Cao V, Sung H, Yokokura M, Ting J, et al. Cost effectiveness of lenvatinib, sorafenib, and placebo in treatment of radioiodine-refractory differentiated thyroid cancer. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.03.1307.
- Huang W, Chen L, Ting J, Cao V, Sung H, Yokokura M, et al. The EVPI of treatment strategies for radioiodine-refractory differentiated thyroid cancer. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.08.258.
- Tremblay G, Pelletier C, Copher R, Forsythe A, Majethia U. Cost-effectiveness analysis of lenvatinib as a treatment for radioactive iodine refractory differentiated thyroid cancer in the United States. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.03.1595.
- Wilson L, Huang W, Chen L, Ting J, Cao V. Cost effectiveness of lenvatinib, sorafenib and placebo in treatment of radioiodine-refractory differentiated thyroid cancer. Thyroid 2017;27:1043-52. https://doi.org/10.1089/thy.2016.0572.
- Canadian Agency for Drugs and Technologies in Health . Pan-Canadian Oncology Drug Review Final Economic Guidance Report: Lenvatinib (Lenvima) for Differentiated Thyroid Cancer 2016. www.cadth.ca/sites/default/files/pcodr/pcodr lenvatinib lenvima dtc fn egr.pdf (accessed 16 May 2017).
- Erdal E, Sayman H, Turkmen C, Aral F, Yildiz O, Okutur K, et al. Cost-effectiveness of sorafenib for treatment of radioactive iodine (RAI)-refractory locally advanced/metastatic differentiated thyroid cancer (DTC) in Turkey. Value Health 2015;18:A203-4. https://doi.org/10.1016/j.jval.2015.03.1178.
- Sussman M, Munsell M, Valderrama A, Seal BS, Wen L. Estimating the economic impact of sorafenib in treatment of locally recurrent or metastatic, progressive, differentiated thyroid carcinoma (DTC) that is refractory to radioactive iodine (RAI) treatment. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.2200.
- I.H.S. Global Pricing Database n.d. http://myinsight.ihsglobalinsight.com/ (accessed 18 April 2016).
- American Academy of Professional Coders (AAPC) . 2014 OPPS Collapses Clinic Visit E M Levels for G0463 n.d. www.aapc.com/blog/26975-2014-opps-collapses-clinic-visit-em-levels-for-g0463/ (accessed 19 April 2016).
- Kerr C, Fordham B, de Freitas HM, Pelletier CL, Lloyd A. Health state utility valuation in radio-iodine refractory differentiated thyroid cancer (RR-DTC). Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.2339.
- Fordham BA, Kerr C, de Freitas HM, Lloyd AJ, Johnston K, Pelletier CL, et al. Health state utility valuation in radioactive iodine-refractory differentiated thyroid cancer. Patient Prefer Adherence 2015;9:1561-72. https://doi.org/10.2147/PPA.S90425.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 7 February 2017).
- Drummond M, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Bank of England . Statistical Interactive Database 2016. www.bankofengland.co.uk/boeapps/iadb/Rates.asp (accessed 26 May 2017).
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life. London: Nuffield Trust; 2014.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 28 February 2017).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2014 to 2015 n.d. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 16 November 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Surveillance Epidemiology and End Results (SEER) Program . SEER*Stat Database Research Data 1973–2013 n.d. www.seer.cancer.gov (accessed 20 July 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Department of Health and Social Care (DHSC) . Drugs and Pharmaceutical Electronic Market Information (eMIT) 2016. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 16 November 2016).
- Abbadessa G, Santoro A, Claudio PP. 42nd Annual Meeting of the American Society of Clinical Oncology (ASCO) (June 2–6, Atlanta) 2006. Drugs Future 2006;31:617-23.
- Alonso-Gordoa T, Díez JJ, Durán M, Grande E. Advances in thyroid cancer treatment: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7:22-38. https://doi.org/10.1177/1758834014551936.
- Andrews A. Sorafenib effective in metastatic differentiated thyroid cancer. Am Health Drug Benefits 2013;6.
- Anonymous . Sorafenib makes headway on metastatic thyroid cancer. Cancer Discov 2013;3.
- Anonymous . From ASCO-targeted therapies: Sorafenib shows efficacy in thyroid cancer. Nat Rev Clin Oncol 2013;11.
- Anonymous . Lenvatinib slows progression of thyroid cancer. Cancer Discov 2014;4. https://doi.org/10.1158/2159-8290.CD-NB2014-088.
- Anonymous . Lenvatinib receives approval in DTC. J Nucl Med 2015;56.
- Anonymous . Lenvatinib approved for certain thyroid cancers. Cancer Discov 2015;5. https://doi.org/10.1158/2159-8290.CD-NB2015-029.
- Anonymous . Sorafenib (NEXAVAR) and differentiated thyroid cancer. Toxic, and no proof of improved survival. Prescrire Int 2016;25.
- Anonymous . New drugs, new indications in 2015: little progress, and threats to access to quality healthcare for all. Prescrire Int 2016;25:136-9.
- Antonelli A. ‘Molecular profiling and ways towards personalized medicine in advanced differentiated thyroid cancer’. Curr Genomics 2014;15. https://doi.org/10.2174/138920291503140609094751.
- Baudin E, Soria JC, Schlumberger MJ. Towards effective treatment for papillary and follicular metastatic thyroid cancer. EJC Suppl 2005;3:449-51. https://doi.org/10.1016/S1359-6349(05)80317-9.
- Belum VR, Marulanda K, Ensslin C, Gorcey L, Parikh T, Wu S, et al. Alopecia in patients treated with molecularly targeted anticancer therapies. Ann Oncol 2015;26:2496-502. https://doi.org/10.1093/annonc/mdv390.
- Ho AL, Sherman E. Clinical development of kinase inhibitors for the treatment of differentiated thyroid cancer. Clin Adv Hematol Oncol 2011;9:32-41.
- Benvenga S. Emerging therapies in sight for the fight against dedifferentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2011;96:347-50. https://doi.org/10.1210/jc.2010-2799.
- Bernet V, Smallridge R. New therapeutic options for advanced forms of thyroid cancer. Expert Opin Emerg Drugs 2014;19:225-41. https://doi.org/10.1517/14728214.2014.894017.
- Bible KC. Treating advanced radioresistant differentiated thyroid cancer. Lancet Oncol 2012;13:854-5. https://doi.org/10.1016/S1470-2045(12)70342-X.
- Bikas A, Vachhani S, Jensen K, Vasko V, Burman KD. Targeted therapies in thyroid cancer: an extensive review of the literature. Expert Rev Clin Pharmacol 2016;9:1299-313. https://doi.org/10.1080/17512433.2016.1204230.
- Blair HA, Plosker GL. Sorafenib: a review of its use in patients with radioactive iodine-refractory, metastatic differentiated thyroid carcinoma. Target Oncol 2015;10:171-8. https://doi.org/10.1007/s11523-015-0363-z.
- Boudou-Rouquette P, Thomas-Schoemann A, Bellesoeur A, Goldwasser F. Sorafenib for patients with differentiated thyroid cancer. Lancet 2015;385:227-8. https://doi.org/10.1016/S0140-6736(15)60054-X.
- Bradford Carter W, Tourtelot JB, Savell JG, Lilienfeld H. New treatments and shifting paradigms in differentiated thyroid cancer management. Cancer Control 2011;18:96-103. https://doi.org/10.1177/107327481101800204.
- Brose MS. Thyroid cancer update: dramatic changes in the treatment of a rare disease. Oncology 2009;23:778-81.
- Butler T, Maravent S, Boisselle J, Valdes J, Fellner C. A review of 2014 cancer drug approvals, with a look at 2015 and beyond. P T 2015;40:191-205.
- Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev 2016;42:47-55. https://doi.org/10.1016/j.ctrv.2015.11.003.
- Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res 2011;2011. https://doi.org/10.4061/2011/985780.
- Capdevila J, Iglesias L, Halperin I, Segura A, Vaz M, Corral J, et al. Sorafenib in patients (pts) with advanced thyroid carcinoma (TC): a compassionate use program. J Clin Oncol 2010;28.
- Cappagli V, Bottici V, Molinaro E, Agate L, Viola D, Valerio L, et al. Metastatic thyroid cancer unresponsive to conventional therapy treated with sorafenib ‘off-label’: an update of our experience. Eur Thyroid J 2011;107.
- Clayman GL. Local treatment of differentiated thyroid carcinoma. Clin Adv Hematol Oncol 2015;13:6-9.
- Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. https://doi.org/10.1089/thy.2009.0110.
- Corrado A, Ferrari SM, Politti U, Mazzi V, Miccoli M, Materazzi G, et al. Aggressive thyroid cancer: targeted therapy with sorafenib. Minerva Endocrinol 2017;42:64-76. https://doi.org/10.23736/S0391-1977.16.02229-X.
- Costa R, Carneiro BA, Chandra S, Pai SG, Chae YK, Kaplan JB, et al. Spotlight on lenvatinib in the treatment of thyroid cancer: patient selection and perspectives. Drug Des Devel Ther 2016;10:873-84. https://doi.org/10.2147/DDDT.S93459.
- Cully M. Trial watch: Multikinase-targeting therapy finds potential niche in thyroid cancer. Nat Rev Drug Discov 2015;14. https://doi.org/10.1038/nrd4599.
- De La Fouchardiere C, Massicotte MH, Borget I, Brassard M, Claude-Desroches M, Giraudet AL, et al. Sequential TKI treatments for iodine-refractory differentiated thyroid carcinomas. J Clin Oncol 2013;31.
- De Lartigue J. Lenvatinib for advanced differentiated thyroid cancer. J Community Support Oncol 2015;13:237-9. https://doi.org/10.12788/jcso.0145.
- Deshpande HA, Gettinger SN, Sosa JA. Novel chemotherapy options for advanced thyroid tumors: small molecules offer great hope. Curr Opin Oncol 2008;20:19-24. https://doi.org/10.1097/CCO.0b013e3282f28373.
- Dezso Z, Ino M, Minoshima Y, Tohyama O, Sugi NH, Agoulnik S, et al. Systems Biology Analysis to Identify Biomarkers for Lenvatinib in the Preclinical Cancer Cell Line Panels n.d.;75.
- Droz J, De La Fouchardiere C, Bournaud C, Borson-Chazot F. Molecularly-targeted therapies in thyroid cancer. Ann Oncol 2010;21.
- Duntas LH, Bernardini R. Sorafenib: rays of hope in thyroid cancer. Thyroid 2010;20:1351-8. https://doi.org/10.1089/thy.2010.0056.
- Fala L. Lenvima (Lenvatinib), a multireceptor tyrosine kinase inhibitor, approved by the FDA for the treatment of patients with differentiated thyroid cancer. Am Health Drug Benefits 2015;8:176-9.
- Fallahi P, Ferrari SM, Santini F, Corrado A, Materazzi G, Ulisse S, et al. Sorafenib and thyroid cancer. BioDrugs 2013;27:615-28. https://doi.org/10.1007/s40259-013-0049-y.
- Féliz LR, Tsimberidou AM. Anti-vascular endothelial growth factor therapy in the era of personalized medicine. Cancer Chemother Pharmacol 2013;72:1-12. https://doi.org/10.1007/s00280-013-2124-y.
- Funakoshi T, Latif A, Galsky MD. Risk of hypertension in cancer patients treated with sorafenib: an updated systematic review and meta-analysis. J Hum Hypertens 2013;27:601-11. https://doi.org/10.1038/jhh.2013.30.
- Gadaleta-Caldarola G, Infusino S, Divella R, Ferraro E, Mazzocca A, De Rose F, et al. Sorafenib: 10 years after the first pivotal trial. Future Oncol 2015;11:1863-80. https://doi.org/10.2217/fon.15.85.
- Ghatalia P, Morgan CJ, Je Y, Nguyen PL, Trinh QD, Choueiri TK, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228-37. https://doi.org/10.1016/j.critrevonc.2014.12.008.
- Ghatalia P, Je Y, Mouallem NE, Nguyen PL, Trinh QD, Sonpavde G, et al. Hepatotoxicity with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 2015;93:257-76. https://doi.org/10.1016/j.critrevonc.2014.11.006.
- Giuffrida D, Prestifilippo A, Scarfia A, Martino D, Marchisotta S. New treatment in advanced thyroid cancer. J Oncol 2012;2012. https://doi.org/10.1155/2012/391629.
- Gyawali B, Shimokata T, Honda K, Ando Y. Risk of serious adverse events (SAEs) and fatal adverse events (FAEs) with sorafenib use in patients with solid cancers: a meta-analysis of Phase 3 RCTs. Ann Oncol 2016;27. https://doi.org/10.1093/annonc/mdw390.04.
- Haddad R. Role of multi-targeted tyrosine kinase inhibitors in RAI-refractory differentiated thyroid cancer. Clin Adv Hematol Oncol 2014;12:13-8.
- Hannallah J, Rose J, Guerrero MA. Comprehensive literature review: recent advances in diagnosing and managing patients with poorly differentiated thyroid carcinoma. Int J Endocrinol 2013;2013. https://doi.org/10.1155/2013/317487.
- Haraldsdottir S, Shah MH. New era for treatment in differentiated thyroid cancer. Lancet 2014;384:286-8. https://doi.org/10.1016/S0140-6736(14)60663-2.
- Hasskarl J. Sorafenib: targeting multiple tyrosine kinases in cancer. Recent Results Cancer Res 2014;201:145-64. https://doi.org/10.1007/978-3-642-54490-3_8.
- Hesselink EK, Steenvoorden D, Kapiteijn E, Corssmit E, Van Der Horst-Schrivers A, Lefrandt J, et al. Effect and toxicity of small molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: systematic review and meta-analysis. Eur Thyroid J 2014;3.
- Hewett Y, Ghimire S, Farooqi B, Shah BK. Lenvatinib – a multikinase inhibitor for radioiodine-refractory differentiated thyroid cancer. J Oncol Pharm Pract 2018;24:28-32.
- Hodak SP, Carty SE. Radioiodine-resistant differentiated thyroid cancer: hope for the future. Oncology 2009;23:775-6.
- Hoftijzer HC, Kapiteijn E, Schneider T, Hovens GC, Morreau H, Gelderblom H, et al. Tyrosine kinase inhibitors in differentiated thyroid carcinoma: a review of the clinical evidence. Clin Investig (Lond) 2011;1:241-53. https://doi.org/10.4155/cli.10.29.
- Hong DS, Koetz BS, Kurzrock R, Senzer NN, Hanekom W, Naing A, et al. Phase I dose-escalation study of E7080, a selective tyrosine kinase inhibitor, administered orally to patients with solid tumors. J Clin Oncol 2010;28.
- Hong S, Fang W, Liang W, Yan Y, Zhou T, Qin T, et al. Risk of treatment-related deaths with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analysis of 41 randomized controlled trials. Onco Targets Ther 2014;7:1851-67. https://doi.org/10.2147/OTT.S68386.
- Ibrahim N, Yu Y, Walsh WR, Yang JL. Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies. Oncol Rep 2012;27:1303-11. https://doi.org/10.3892/or.2012.1675.
- Ito Y, Suzuki S, Ito K, Imai T, Okamoto T, Kitano H, et al. Tyrosine-kinase inhibitors to treat radioiodine-refracted, metastatic, or recurred and progressive differentiated thyroid carcinoma [Review.]. Endocr J 2016;63:597-602. https://doi.org/10.1507/endocrj.EJ16-0064.
- Iwasaki H, Nakayama H, Suganuma N, Yoshida T, Okamoto H, Yamanaka T, et al. Tentative treatment outcomes for radioactive iodine-refractory differentiated thyroid cancer (RAI-RDTC) patients receiving sorafenib or lenvatinib. Thyroid 2015;25.
- Iwasaki H, Nakayama H, Suganuma N, Yoshida T, Yamanaka T, Hatori S, et al. Treatment outcomes of sorafenib and lenvatinib for advanced thyroid cancers and anaplastic thyroid cancers. Eur Thyroid J 2016;5.
- Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother 2010;11:1943-55. https://doi.org/10.1517/14656566.2010.496453.
- Kapiteijn E, Schneider TC, Morreau H, Gelderblom H, Nortier JW, Smit JW. New treatment modalities in advanced thyroid cancer. Ann Oncol 2012;23:10-8. https://doi.org/10.1093/annonc/mdr117.
- Killock D. Neuroendocrine cancer: SELECT—lenvatinib in thyroid cancer. Nat Rev Clin Oncol 2015;12. https://doi.org/10.1038/nrclinonc.2015.30.
- Klein Hesselink EN, Steenvoorden D, Kapiteijn E, Corssmit EP, van der Horst-Schrivers AN, Lefrandt JD, et al. Therapy of endocrine disease: response and toxicity of small-molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol 2015;172:R215-25. https://doi.org/10.1530/EJE-14-0788.
- Kojic KL, Kojic SL, Wiseman SM. Differentiated thyroid cancers: a comprehensive review of novel targeted therapies. Expert Rev Anticancer Ther 2012;12:345-57. https://doi.org/10.1586/era.12.8.
- Krajewska J, Jarzab B. Lenvatinib for the treatment of radioiodine-refractory follicular and papillary thyroid cancer. Expert Opin Orphan Drugs 2014;2:1331-40. https://doi.org/10.1517/21678707.2014.962514.
- Krajewska J, Handkiewicz-Junak D, Jarzab B. Sorafenib for the treatment of thyroid cancer: an updated review. Expert Opin Pharmacother 2015;16:573-83. https://doi.org/10.1517/14656566.2015.1005601.
- Krajewska J, Kukulska A, Jarzab B. Efficacy of lenvatinib in treating thyroid cancer. Expert Opin Pharmacother 2016;17:1683-91. https://doi.org/10.1080/14656566.2016.1206078.
- Krajewska J, Kukulska A, Jarzab B. Drug safety evaluation of lenvatinib for thyroid cancer. Expert Opin Drug Saf 2015;14:1935-43. https://doi.org/10.1517/14740338.2015.1102883.
- Launay-Vacher V, Aapro M, De Castro G, Cohen E, Deray G, Dooley M, et al. Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN). Ann Oncol 2015;26:1677-84. https://doi.org/10.1093/annonc/mdv136.
- Lerch C, Richter B. Pharmacotherapy options for advanced thyroid cancer: a systematic review. Drugs 2012;72:67-85. https://doi.org/10.2165/11594890-000000000-00000.
- Liu B, Ding F, Liu Y, Xiong G, Lin T, He D, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget 2016;7:67661-73. https://doi.org/10.18632/oncotarget.11813.
- Liu M, Ruan M, Chen L. Update on the molecular diagnosis and targeted therapy of thyroid cancer. Med Oncol 2014;31. https://doi.org/10.1007/s12032-014-0973-9.
- Lorusso L, Newbold K. Lenvatinib: a new option for the treatment of advanced iodine refractory differentiated thyroid cancer?. Fut Oncol 2015;11:1719-27. https://doi.org/10.2217/fon.15.68.
- Lorusso L, Pieruzzi L, Biagini A, Sabini E, Valerio L, Giani C, et al. Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. Onco Targets Ther 2016;9:6467-77. https://doi.org/10.2147/OTT.S84625.
- Ma Q, Gu LY, Ren YY, Zeng LL, Gong T, Zhong DS. Increased risk of severe infections in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analysis. Onco Targets Ther 2015;8:2361-74. https://doi.org/10.2147/OTT.S87298.
- Majethia U, Frolov M, Podvyaznikov S, Rumyantsev P, Bukharova E, Tremblay G, et al. Budget impact and incremental survival benefit of using lenvatinib as a treatment for radioactive iodine refractory differentiated thyroid cancer in Russia. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.03.1552.
- Mayor S. Lenvatinib improves survival in refractory thyroid cancer. Lancet Oncol 2015;16. https://doi.org/10.1016/S1470-2045(15)70066-5.
- Moreo A, Vallerio P, Ricotta R, Stucchi M, Pozzi M, Musca F, et al. Effects of cancer therapy targeting vascular endothelial growth factor receptor on central blood pressure and cardiovascular system. Am J Hypertens 2016;29:158-62. https://doi.org/10.1093/ajh/hpv077.
- Nair A, Lemery SJ, Yang J, Marathe A, Zhao L, Zhao H, et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin Cancer Res 2015;21:5205-8. https://doi.org/10.1158/1078-0432.CCR-15-1377.
- Nixon IJ, Shaha AR, Tuttle MR. Targeted therapy in thyroid cancer. Curr Opin Otolaryngol Head Neck Surg 2013;21:130-4. https://doi.org/10.1097/MOO.0b013e32835aa2c2.
- Okamoto K, Kawada MI, Jestel A, Von Konig K, Funahashi Y, Matsushima T, et al. Distinct binding mode of lenvatinib to VEGFR2 revealed by biochemical characterization. Cancer Res 2015;75.
- Pacini F, Brilli L, Marchisotta S. Targeted therapy in radioiodine refractory thyroid cancer. Q J Nucl Med Mol Imaging 2009;53:520-5.
- Pall G. ASCO annual meeting 2013: head and neck cancer. Memo 2013;6:240-3. https://doi.org/10.1007/s12254-013-0107-7.
- Pall G. Post ASCO update 2014: head and neck cancer. Memo 2014;7:231-6. https://doi.org/10.1007/s12254-014-0183-3.
- Pfister DG, Fagin JA. Refractory thyroid cancer: a paradigm shift in treatment is not far off. J Clin Oncol 2008;26:4701-4. https://doi.org/10.1200/JCO.2008.17.3682.
- Puxeddu E, Romagnoli S, Dottorini ME. Targeted therapies for advanced thyroid cancer. Curr Opin Oncol 2011;23:13-21. https://doi.org/10.1097/CCO.0b013e328340cf94.
- Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol 2013;76:348-57. https://doi.org/10.1111/bcp.12149.
- Qi WX, Tang LN, Sun YJ, He AN, Lin F, Shen Z, et al. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Ann Oncol 2013;24:2943-52. https://doi.org/10.1093/annonc/mdt292.
- Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y. Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2014;89:394-403. https://doi.org/10.1016/j.critrevonc.2013.10.002.
- Ramadan S, Ugas MA, Berwick RJ, Notay M, Cho H, Jerjes W, et al. Spinal metastasis in thyroid cancer. Head Neck Oncol 2012;4. https://doi.org/10.1186/1758-3284-4-39.
- Safavi A, Vijayasekaran A, Guerrero MA. New insight into the treatment of advanced differentiated thyroid cancer. J Thyroid Res 2012;2012. https://doi.org/10.1155/2012/437569.
- Saiyed MM, Ong PS, Chew L. Extent and outcomes of off-label drug use in oncology practice: a systematic review of literature. Ann Oncol 2015;26. https://doi.org/10.1093/annonc/mdv535.07.
- Schlumberger M. Kinase inhibitors for refractory thyroid cancers. Lancet Oncol 2010;11:912-13. https://doi.org/10.1016/S1470-2045(10)70226-6.
- Schlumberger M. French TUTHYREF Network . Targeted therapy in refractory thyroid cancer. Eur J Cancer 2011;47:328-9. https://doi.org/10.1016/S0959-8049(11)70190-3.
- Schutt P, Eberhardt W. Targeted therapy for patients with thyroid cancer. Onkologie 2010;33.
- Sherman SI. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am 2008;37:511-24. https://doi.org/10.1016/j.ecl.2008.02.005.
- Sherman SI. Molecularly targeted therapies for thyroid cancers. Endocr Pract 2009;15:605-11. https://doi.org/10.4158/EP09131.RAR.
- Sherman EJ, Ho AL, Fury MG, Baxi SS, Haque S, Korte SH, et al. A Phase II study of temsirolimus/sorafenib in patients with radioactive iodine (RAI)-refractory thyroid carcinoma. J Clin Oncol 2012;30.
- Sherman EJ, Ho AL, Fury MG, Baxi SS, Haque S, Lipson BL, et al. Phase II study of everolimus and sorafenib for the treatment of metastatic thyroid cancer. J Clin Oncol 2013;31.
- Sherman EJ, Ho AL, Fury MG, Baxi SS, Dunn L, Lee JS, et al. Combination of everolimus and sorafenib in the treatment of thyroid cancer: update on Phase II study. J Clin Oncol 2015;33.
- Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 2012;320:130-7. https://doi.org/10.1016/j.canlet.2012.03.008.
- Smit J, Brose M, Lin CC, Fellous M, Pitoia F, Sugitani I, et al. Baseline patient characteristics from RIFTOS: a global noninterventional study evaluating the use of multikinase inhibitors for treatment of asymptomatic differentiated thyroid cancer refractory to radioactive iodine (RIFTOS MKI). Eur Thyroid J 2016;5.
- Takahashi S. Molecular targeting therapy for thyroid cancer: problems in clinical practice. Ann Oncol 2014;25. https://doi.org/10.1093/annonc/mdu431.3.
- Terada T, Noda S, Inui K. Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors. Pharmacol Ther 2015;152:125-34. https://doi.org/10.1016/j.pharmthera.2015.05.009.
- Thanigaimani S, Kichenadasse G, Mangoni AA. The emerging role of vascular endothelial growth factor (VEGF) in vascular homeostasis: lessons from recent trials with anti-VEGF drugs. Curr Vasc Pharmacol 2011;9:358-80. https://doi.org/10.2174/157016111795495503.
- Tracy ET, Roman SA. Current management of pediatric thyroid disease and differentiated thyroid cancer. Curr Opin Oncol 2016;28:37-42. https://doi.org/10.1097/CCO.0000000000000250.
- Tremblay G, Li X, Abouzaid S, Pelletier C. Number-needed-to-treat (NNT) analysis of therapies in radioiodine-refractory differentiated thyroid cancer (RR-DTC) using indirect comparison. Thyroid 2015;25:A266-A7.
- Tremblay G, Pelletier C, Forsythe A, Majethia U. Matching-adjusted indirect treatment comparison and survival extrapolation in radioiodine-refractory differentiated thyroid cancer (RAI-refractory DTC): updated analysis. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.09.1048.
- Tremblay G, Holbrook T, Milligan G, Pelletier C. Matching-adjusted indirect treatment comparison and survival extrapolation in radioiodine-refractory differentiated thyroid cancer (Rai-Refractory DTC). Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.03.072.
- Tremblay G, Livings C, Crowe L, Kapetanakis V, Briggs A. Determination of the most appropriate method for extrapolating overall survival data from a placebo-controlled clinical trial of lenvatinib for progressive, radioiodine-refractory differentiated thyroid cancer. ClinicoEcon 2016;8:323-33. https://doi.org/10.2147/CEOR.S107498.
- Tsimberidou AM, Vaklavas C, Wen S, Hong D, Wheler J, Ng C, et al. Phase I clinical trials in 56 patients with thyroid cancer: the M. D. Anderson Cancer Center experience. J Clin Endocrinol Metab 2009;94:4423-32. https://doi.org/10.1210/jc.2009-0743.
- Tu SM, Bilen MA, Tannir NM. Personalised cancer care: promises and challenges of targeted therapy. J R Soc Med 2016;109:98-105. https://doi.org/10.1177/0141076816631154.
- Tuttle RM, Leboeuf R. Investigational therapies for metastatic thyroid carcinoma. J Natl Compr Canc Netw 2007;5:641-6. https://doi.org/10.6004/jnccn.2007.0055.
- Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, et al. Thyroid carcinoma, version 2.2014. J Natl Compr Canc Netw 2014;12:1671-80. https://doi.org/10.6004/jnccn.2014.0169.
- Vetter C. First new treatment option after 40 years: sorafenib closes therapeutic gaps in the radioiodine-refractory thyroid cancer. Oncol Res Treat 2014;37:2892-9.
- Wagner M, Khoury H, Bennetts L, Willet J, Lister J, Berto P, et al. Appraising the value of lenvatinib for radio-iodine refractory differentiated thyroid cancer (RR-DTC): a multi-country study applying holistic multicriteria decision analysis (MCDA). Value Health 2015;18:A477-8. https://doi.org/10.1016/j.jval.2015.09.1287.
- Warpakowski A. Radioiodine-resistant differentiated thyroid cancer: sorafenib extends progression-free survival. Arzneimitteltherapie 2014;32:341-2.
- Wendling P. Sorafenib slows progression of advanced thyroid cancer. Oncol Rep 2013;30:11-2.
- Wirth LJ. Targeted therapy for advanced or metastatic differentiated thyroid carcinoma. Clin Adv Hematol Oncol 2015;13:9-16.
- Wong KP, Lang BH. New molecular targeted therapy and redifferentiation therapy for radioiodine-refractory advanced papillary thyroid carcinoma: literature review. J Thyroid Res 2012;2012. https://doi.org/10.1155/2012/818204.
- Worcester S. SELECT trial: lenvatinib effects similar regardless of site, number of metastases. Oncol Rep 2015;33.
- Yang X, Pan X, Cheng X, Cheng Y, Kuang Y. Risk of treatment-related mortality with sorafenib in cancer patients: a meta-analysis of 20 randomly controlled trials: Risk of sorafenib-associated death. Int J Clin Pharm 2015;37:1047-56. https://doi.org/10.1007/s11096-015-0151-y.
- Yang X, Pan X, Cheng X, Kuang Y, Cheng Y. Risk of hypertension with sorafenib use in patients with cancer: a meta-analysis from 20,494 patients. Am J Ther 2017;24:e81-e101. https://doi.org/10.1097/MJT.0000000000000331.
- Yeung KT, Cohen EE. Lenvatinib in advanced, radioactive iodine-refractory, differentiated thyroid carcinoma. Clin Cancer Res 2015;21:5420-6. https://doi.org/10.1158/1078-0432.CCR-15-0923.
- Yimaer W, Abudouyimu A, Tian Y, Magaoweiya S, Bagedati D, Wen H. Efficacy and safety of vascular endothelial growth factor receptor tyrosine kinase inhibitors in the treatment of advanced thyroid cancer: a meta-analysis of randomized controlled trials. Onco Targets Ther 2016;9:1167-73. https://doi.org/10.2147/OTT.S102265.
- Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K, et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget 2016;7:44545-57. https://doi.org/10.18632/oncotarget.10019.
- Zygulska AL, Krzemieniecki K, Sowa-Staszczak A. The use of sorafenib in the thyroid cancer. Euro Endocrinol 2013;9:28-31. https://doi.org/10.17925/EE.2013.09.01.28.
- National Cancer Institute . Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed 12 July 2017).
- Bayer HealthCare . A Double-Blind, Randomized Phase III Study Evaluating the Efficacy and Safety of Sorafenib Compared to Placebo in Locally Advanced Metastatic RAI-Refractory Differentiated Thyroid Cancer 2015.
- Eisai Ltd . Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine [ID1059]. Eisai Response to the Assessment Report n.d. www.nice.org.uk/guidance/gid-ta10101/documents/committee-papers (accessed 9 October 2018).
- Al-Qahtani KH, Tunio MA, Al Asiri M, Fatani H, Bayoumi Y. Calvarium and dura mater as delayed sites of distant metastasis from papillary thyroid carcinoma. Int Med Case Rep J 2015;8:251-4. https://doi.org/10.2147/IMCRJ.S86183.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. https://doi.org/10.1016/S0895-4356(97)00049-8.
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. https://doi.org/10.1056/NEJMoa060655.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. https://doi.org/10.1056/NEJMoa0708857.
- Eisai Ltd . Phase II, Multicenter, Open-Label, Single Arm Trial to Evaluate the Safety and Efficacy of Oral E7080 in Medullary and Iodine-131 Refractory, Unresectable Differentiated Thyroid Cancers, Stratified by Histology. Protocol # E7080-G000-201. CSR Synopsis n.d. http://eisaiclinicaltrials.com/study/test-study-1/ (accessed 30 October 2018).
Appendix 1 Literature search strategies
This appendix is reproduced with permission from Fleeman et al. 54 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Search strategies for evidence of clinical effectiveness
EMBASE
Date searched: 10 January 2017.
Search from 1980 to 2017 week 2.
| 1 | exp Thyroid Neoplasms/ |
| 2 | ((thyroid* or papillar* or follicular*) adj4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)).tw. |
| 3 | (DTC or FTC or PTC).tw. |
| 4 | adenocarcinoma, follicular/ or carcinoma, papillary, follicular/ or adenocarcinoma, papillary/ |
| 5 | 1 or 2 or 3 or 4 |
| 6 | (Lenvatinib or Lenvima or E7080).tw. |
| 7 | (Nexavar or Sorafenib or bay439006).tw. |
| 8 | lenvatinib/ |
| 9 | sorafenib/ |
| 10 | 6 or 7 or 8 or 9 |
| 11 | 5 and 10 |
| 12 | limit 11 to yr=‘1999 -Current’ |
MEDLINE
Date searched: 10 January 2017.
| 1 | exp Thyroid Neoplasms/ |
| 2 | ((thyroid* or papillar* or follicular*) adj4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)).tw. |
| 3 | (DTC or FTC or PTC).tw. |
| 4 | adenocarcinoma, follicular/ or carcinoma, papillary, follicular/ or adenocarcinoma, papillary/ |
| 5 | 1 or 2 or 3 or 4 |
| 6 | (Lenvatinib or Lenvima or E7080).tw. |
| 7 | (Nexavar or Sorafenib or bay439006).tw. |
| 8 | 6 or 7 |
| 9 | 5 and 8 |
| 10 | limit 9 to yr=‘1999 -Current’ |
PubMed
Date searched: 10 January 2017.
| #1 | Search (((thyroid* or papillar* or follicular*))) AND ((Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)) |
| #2 | Search (DTC or FTC or PTC) |
| #3 | Search (#1 or #2) |
| #4 | Search (Lenvatinib or Lenvima or E7080 or Nexavar or Sorafenib or bay439006) |
| #5 | Search (#3 and #4) |
| #6 | Search (‘2016/07/01’[Date - Entrez] : ‘3000’[Date - Entrez]) |
| #7 | Search (#5 and #6) |
The Cochrane Library (Cochrane Database of Systematic Review/Cochrane Central Register of Controlled Trials/Database of Abstracts of Reviews of Effects/Health Technology Assessment Database)
Date searched: 10 January 2017.
| #1 | MeSH descriptor: [Thyroid Neoplasms] explode all trees |
| #2 | ((thyroid* or papillar* or follicular*) near/4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)) |
| #3 | (DTC or FTC or PTC) |
| #4 | MeSH descriptor: [Adenocarcinoma, Follicular] explode all trees |
| #5 | MeSH descriptor: [Carcinoma, Papillary, Follicular] explode all trees |
| #6 | MeSH descriptor: [Adenocarcinoma, Papillary] explode all trees |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 |
| #8 | (Lenvatinib or Lenvima or E7080) |
| #9 | (Nexavar or Sorafenib or bay439006) |
| #10 | #8 or #9 |
| #11 | #7 and #10 Publication Year from 1999 to 2017 |
Economic filter for database search
EMBASE
Date searched: 10 January 2017.
| 1 | exp Thyroid Neoplasms/ |
| 2 | ((thyroid* or papillar* or follicular*) adj4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)).tw. |
| 3 | (DTC or FTC or PTC).tw. |
| 4 | adenocarcinoma, follicular/ or carcinoma, papillary, follicular/ or adenocarcinoma, papillary/ |
| 5 | 1 or 2 or 3 or 4 |
| 6 | (Lenvatinib or Lenvima or E7080).tw. |
| 7 | (Nexavar or Sorafenib or bay439006).tw. |
| 8 | lenvatinib/ |
| 9 | sorafenib/ |
| 10 | 6 or 7 or 8 or 9 |
| 11 | 5 and 10 |
| 12 | limit 11 to yr=‘1999 -Current’ |
| 13 | Socioeconomics/ |
| 14 | Cost benefit analysis/ |
| 15 | Cost effectiveness analysis/ |
| 16 | Cost of illness/ |
| 17 | Cost control/ |
| 18 | Economic aspect/ |
| 19 | Financial management/ |
| 20 | Health care cost/ |
| 21 | Health care financing/ |
| 22 | Health economics/ |
| 23 | Hospital cost/ |
| 24 | (fiscal or financial or finance or funding).tw. |
| 25 | Cost minimization analysis/ |
| 26 | (cost adj estimate$).mp. |
| 27 | (cost adj variable$).mp. |
| 28 | (unit adj cost$).mp. |
| 29 | or/13-28 |
| 30 | 12 and 29 |
MEDLINE
Date searched: 10 January 2017.
| 1 | exp Thyroid Neoplasms/ |
| 2 | ((thyroid* or papillar* or follicular*) adj4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)).tw. |
| 3 | (DTC or FTC or PTC).tw. |
| 4 | adenocarcinoma, follicular/ or carcinoma, papillary, follicular/ or adenocarcinoma, papillary/ |
| 5 | 1 or 2 or 3 or 4 |
| 6 | (Lenvatinib or Lenvima or E7080).tw. |
| 7 | (Nexavar or Sorafenib or bay439006).tw. |
| 8 | 6 or 7 |
| 9 | 5 and 8 |
| 10 | Economics/ |
| 11 | ‘costs and cost analysis’/ |
| 12 | Cost allocation/ |
| 13 | Cost-benefit analysis/ |
| 14 | Cost control/ |
| 15 | Cost savings/ |
| 16 | Cost of illness/ |
| 17 | Cost sharing/ |
| 18 | ‘deductibles and coinsurance’/ |
| 19 | Medical savings accounts/ |
| 20 | Health care costs/ |
| 21 | Direct service costs/ |
| 22 | Drug costs/ |
| 23 | Employer health costs/ |
| 24 | Hospital costs/ |
| 25 | Health expenditures/ |
| 26 | Capital expenditures/ |
| 27 | Value of life/ |
| 28 | exp economics, hospital/ |
| 29 | exp economics, medical/ |
| 30 | Economics, nursing/ |
| 31 | Economics, pharmaceutical/ |
| 32 | exp ‘fees and charges’/ |
| 33 | exp budgets/ |
| 34 | (low adj cost).mp. |
| 35 | (high adj cost).mp. |
| 36 | (health?care adj cost$).mp. |
| 37 | (fiscal or funding or financial or finance).tw. |
| 38 | (cost adj estimate$).mp. |
| 39 | (cost adj variable).mp. |
| 40 | (unit adj cost$).mp. |
| 41 | (economic$ or pharmacoeconomic$ or price$ or pricing).tw. |
| 42 | or/10-41 |
| 43 | 9 and 42 |
The Cochrane Library (NHS Economic Evaluation Database)
Date searched: 10 January 2017.
| #1 | MeSH descriptor: [Thyroid Neoplasms] explode all trees |
| #2 | (thyroid* near/4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*)) |
| #3 | DTC or FTC or PTC |
| #4 | #1 or #2 or #3 |
| #5 | (Lenvatinib or Lenvima or E7080 or Nexavar or Sorafenib or bay439006) |
| #6 | #4 and #5 |
EconLit
Date searched: 10 January 2017.
(thyroid* N4 (Neoplasm* or Cancer* or Carcinoma* or Adenocarcinom* or Tumour* or Tumor* or Malignan* or Lump* or adenoma*))
Appendix 2 Table of excluded studies withrationale
The studies excluded by the AG at screening stage 2 are summarised in Table 32.
| Study and year of publication | Reason for exclusion |
|---|---|
| Abbadessa et al. 2006180 | Wrong study design |
| Alonso-Gordoa et al. 2015181 | Wrong study design |
| Andrews 2013182 | Wrong study design |
| Anonymous 2013183 | Wrong study design |
| Anonymous 2013184 | Wrong study design |
| Anonymous 2014185 | Wrong study design |
| Anonymous 2015186 | Wrong study design |
| Anonymous 2015187 | Wrong study design |
| Anonymous 2016188 | Wrong study design |
| Anonymous 2016189 | Wrong study design |
| Antonelli 2014190 | Wrong study design |
| Baudin et al. 2005191 | Wrong study design |
| Belum et al. 2015192 | Wrong population |
| Benvenga 2011194 | Wrong study design |
| Bernet and Smallridge 2014195 | Wrong study design |
| Bible 2012196 | Wrong study design |
| Bikas et al. 2016197 | Wrong study design |
| Blair and Plosker 2015198 | Wrong study design |
| Boudou-Rouquette 2015199 | Wrong study design |
| Bradford Carter et al. 2011200 | Wrong study design |
| Brose 2009201 | Wrong study design |
| Brose et al. 2015156 | Wrong study design |
| Butler 2015202 | Wrong study design |
| Cabanillas and Habra 2016203 | Wrong study design |
| Cabanillas et al. 2011204 | Wrong study design |
| Capdevila et al. 2010205 | Wrong study design |
| Cappagli et al. 2011206 | Wrong study design |
| Clayman 2015207 | Wrong study design |
| Cooper et al. 2009208 | Wrong study design |
| Corrado et al. 2017209 | Wrong study design |
| Costa et al. 2016210 | Wrong study design |
| Covell and Ganti 201542 | Wrong study design |
| Cully 2015211 | Wrong study design |
| De La Fouchardiere et al. 2013212 | Wrong study design |
| De Lartigue 2015213 | Wrong study design |
| Deshpande et al. 2008214 | Wrong study design |
| Dezso 2015215 | Wrong study design |
| Droz et al. 2010216 | Wrong study design |
| Duntas and Bernardini 2010217 | Wrong study design |
| Fala 2015218 | Wrong study design |
| Fallahi et al. 2013219 | Wrong study design |
| Féliz and Tsimberidou 2013220 | Wrong population |
| Funakoshi 2013221 | Wrong population |
| Gadaleta-Caldarola et al. 2015222 | Wrong study design |
| Ghatalia et al. 2015223 | Wrong population |
| Ghatalia et al. 2015 224 | Wrong population |
| Giuffrida et al. 2012225 | Wrong population |
| Gyawali et al. 2016226 | Wrong population |
| Haddad 2014227 | Wrong study design |
| Hannallah et al. 2013228 | Wrong study design |
| Haraldsdottir and Shah 2014229 | Wrong study design |
| Hasskarl 2014230 | Wrong study design |
| Haugen et al. 201624 | Wrong study design |
| Hesselink 2014231 | Wrong population |
| Hewett et al. 2018232 | Wrong study design |
| Ho and Sherman 2011193 | Wrong study design |
| Hodak and Carty 2009233 | Wrong study design |
| Hoftijzer et al. 2011234 | Wrong study design |
| Hong et al. 2010235 | Wrong population |
| Hong et al. 2014236 | Wrong population |
| Ibrahim et al. 2012237 | Wrong study design |
| Ito et al. 2016238 | Wrong study design |
| Iwasaki et al. 2015239 | Wrong study design |
| Iwasaki et al. 2016240 | Wrong intervention (no data for lenvatinib or sorafenib alone) |
| Iyer et al. 2010241 | Wrong study design |
| Kapiteijn et al. 2012242 | Wrong population (too broad) |
| Killock 2015243 | Wrong study design |
| Klein Hesselink et al. 2015244 | Wrong population (too broad) |
| Kojic et al. 2012245 | Wrong study design |
| Krajewska and Jarzab 2014246 | Wrong study design |
| Krajewska et al. 2015247 | Wrong study design |
| Krajewska et al. 2016248 | Wrong study design |
| Krajewska et al. 2015249 | Wrong study design |
| Launay-Vacher et al. 2015250 | Wrong study design |
| Lerch and Richter 2012251 | Wrong population (too broad) |
| Liu et al. 2016252 | Wrong population (too broad) |
| Liu et al. 2014253 | Wrong study design |
| Lorusso and Newbold 2015254 | Wrong study design |
| Lorusso et al. 2016255 | Wrong study design |
| Ma et al. 2015256 | Wrong population |
| Majethia et al. 2016257 | Wrong study design |
| Marotta et al. 2013148 | Wrong study design |
| Mayor 2015258 | Wrong study design |
| Moreo et al. 2016259 | Wrong population |
| Nair et al. 2015260 | Wrong study design |
| Nixon et al. 2013261 | Wrong study design |
| Okamoto et al. 2015262 | Wrong study design |
| Pacini et al. 2009263 | Wrong study design |
| Pall 2013264 | Wrong study design |
| Pall 2014265 | Wrong study design |
| Pfister and Fagin 2008266 | Wrong study design |
| Puxeddu et al. 2011267 | Wrong study design |
| Qi et al. 2013268 | Wrong intervention (no data for lenvatinib or sorafenib alone) |
| Qi et al. 2013269 | Wrong intervention (no data for lenvatinib or sorafenib alone) |
| Qi et al. 2014270 | Wrong intervention (no data for lenvatinib or sorafenib alone) |
| Ramadan et al. 2012271 | Wrong study design |
| Sacks and Braunstein 201434 | Wrong study design |
| Safavi 2012272 | Wrong population |
| Saiyed et al. 2015273 | Wrong population |
| Schlumberger 2010274 | Wrong study design |
| Schlumberger 2011275 | Wrong study design |
| Schutt and Eberhardt 2010276 | Wrong population |
| Sherman 2008277 | Wrong study design |
| Sherman 2009278 | Wrong study design |
| Sherman et al. 2012279 | Wrong intervention (not sorafenib monotherapy) |
| Sherman et al. 2013280 | Wrong intervention (not sorafenib monotherapy) |
| Sherman et al. 2015281 | Wrong intervention (not sorafenib monotherapy) |
| Shojaei 2012282 | Wrong study design |
| Smit et al. 2016283 | Wrong study design |
| Takahashi 2014284 | Wrong study design |
| Terada et al. 2015285 | Wrong study design |
| Thanigaimani et al. 2011286 | Wrong study design |
| Tracy and Roman 2016287 | Wrong study design |
| Tremblay et al. 2015288 | Wrong study design (reports the findings from a matched ITC but no reporting of a systematic review) |
| Tremblay et al. 2015289 | Wrong study design [reports the findings (number needed to treat) from an ITC but no reporting of a systematic review] |
| Tremblay et al. 2015290 | Wrong study design (reports the findings from a matched ITC but no reporting of a systematic review) |
| Tremblay et al. 2016291 | Wrong study design (cost-effectiveness methods paper) |
| Tsimberidou et al. 2009292 | Wrong interventions |
| Tu et al. 2016293 | Wrong study design |
| Tuttle and Leboeuf 2007294 | Wrong study design |
| Tuttle et al. 2014295 | Wrong study design |
| Vetter 2014296 | Wrong study design |
| Wagner et al. 2015297 | Wrong study design |
| Warpakowski 2014298 | In German |
| Wendling 2013299 | Wrong study design |
| Wirth 2015300 | Wrong study design |
| Wong and Lang 2012301 | Wrong study design |
| Worcester 2015302 | Wrong study design |
| Yang et al. 2015303 | Wrong population |
| Yang et al. 2017304 | Wrong population |
| Yeung and Cohen 2015305 | Wrong study design |
| Yimaer et al. 2016306 | Wrong population |
| Zhu et al. 2016307 | Wrong population |
| Zygulska et al. 2013308 | Wrong study design |
Appendix 3 Data extraction tables from randomised controlled trials not presented in the main body of the report
| Criteria | Study | |
|---|---|---|
| SELECT | DECISION | |
| Inclusion |
|
|
| Exclusion |
|
|
| Criteria | Study | |
|---|---|---|
| SELECT | DECISION | |
| To be classified as having DTC refractory to radioactive iodine, patients were required to meet at least one of the criteria specified |
|
|
| Concomitant treatment allowed and disallowed | Study | |
|---|---|---|
| SELECT | DECISION | |
| Permitted |
|
|
| Prohibited |
|
|
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (n = 261) | Placebo (n = 131) | Sorafenib (n = 207) | Placebo (n = 210) | |
| First data cut-off point | November 2013 | August 2012 | ||
| Length of follow-up (months), median (95% CI) | 17.1 (16.0 to 17.6) | 17.4 (15.9 to 19.0) | 17.4 (NR) | NR |
| Average dose (mg) | 17.2 | NR | 651 | 793 |
| Dose intensity (% of maximum dose) | 71.7 | NR | 81.4 | 99.1 |
| Second data cut-off point | June 2014 | May 2013 | ||
| Length of follow-up (months), median (95% CI) | 23.6 (22.7 to 24.5) | 24.1 (22.1 to 26.1) | 24.1 (NR) | NR |
| Average dose (mg) | NR | NR | NR | NR |
| Dose intensity (% of maximum dose) | NR | NR | NR | NR |
| Third data cut-off point | August 2015 | July 2015 | ||
| Length of follow-up (months), median (95% CI) | 37.8 (NR) | 37.9 (NR) | 36.0 (NR) | NR |
| Average dose (mg) | 16.5a | NR | 651.2 | 793.6 |
| Dose intensity (% of maximum dose) | 68.8a | NR | 81.4 | 99.2 |
| Study | |
|---|---|
| SELECT | DECISION |
| Prespecified subgroup analyses | |
|
Age (≤ 65 years or > 65 years) Geographic region (Europe, North America or other) Prior VEGF-targeted therapy (0 or 1) Sex (male or female) Race (white or non-white) Histology (PTC or FTC) TSH (≤ 0.5, > 0.5 to 2.0, > 2.0 to 5.5 or > 5.5 ml/UL) |
Age (< 60 years or ≥ 60 years) Geographical region (North America, Europe or Asia) Sex (male or female) Histology (PTC, FTC: Hürthle cell, FTC: other subtypes, or poorly differentiated) Site of metastasis [bone (yes or no) and lung only (yes or no)] 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) uptake (negative or positive) Prior radioactive iodine cumulative dosing [< 600 mCi (22.2 GBq) or ≥ 600 mCi (22.2 GBq)] Tumour burden as measured by number of target or non-target lesions (fewer than median or at least median) Tumour burden as measured by sum of target diameters (less than median or at least median) |
| Post hoc subgroup analyses | |
|
Number of sites of metastasis (1, 2, 3 or ≥ 4)a Site of metastasis (brain, bone, liver, lung or lymph node)a Site of metastasis [bone (yes or no) and lung (yes or no)] Target tumour size (≤ 35 mm, 36–60 mm, 91–92 mm or ≥ 92 mm) BRAF status (wild type or mutant) RAS status (wild type or mutant) TSH levels (≤ 0.5, 0.5 to 2.0 or > 2.0 ml/UL) Pharmacodynamic biomarkers [TG and CAF levels (Ang2, VEGF, sTie2, and FGF23)]a Body mass index [underweight and normal weight (< 25 kg/m2), overweight (25 kg/m2 to 29.99 kg/m2) and obese (≥ 30 kg/m2)]a With or without treatment-emergent hypertensiona |
BRAF status (wild type or mutant)a RAS status (wild type or mutant)a TSH levels [less than median (449.4 ng/mL) or at least median (449.4 ng/m)]a Maximum tumour size (< 1.5 cm or ≥ 1.5 cm) Category of lesion size (< 1.5 cm, ≥ 1.5 cm, < 2 cm, ≥ 2 cm, < 3 cm, ≥ 3 cm, < 4 cm or ≥ 4 cm) Lesion category: number of target lesions (< 3, ≥ 3, < 4, ≥ 4, < 5 or ≥ 5)b Symptomatic or asymptomatic at baselineb,c Subgroup analyses on safety parameters by region, body mass index, sex and age (full details not reported)d Subgroup analyses of baseline factors predictive of HRQoL (full details not reported)d |
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| Received anti-cancer treatment following progression, n (%) | 41 (15.7) | 16 (12.2) | 42 (20.3) | 18 (8.6) |
| OS: first data cut-off point | November 2013 | August 2012 | ||
| Patients who crossed over, n (%) | N/A | 109 (83.2) | 55 (26.6) | 150 (71.4) |
| Deaths, n (%) | 71 (27.2) | 47 (35.9) | 45 (21.7) | 54 (25.7) |
| Median OS (months) (95% CI) | NE (22.0 to NE) | NE (14.3 to NE) | NE | NE |
| Unadjusted HR (95% CI); p-value | 0.73 (0.50 to 1.07); 0.1032 | 0.80 (0.54 to 1.19); 0.14 | ||
| RPSFTM-adjusted HR (95% CI); p-value, cox method | NR | 0.61 (0.40 to 0.94); 0.0125 | ||
| RPSFTM-adjusted HR (95% CI); p-value, bootstrapping method | 0.62 (0.40 to 1.00); 0.0510 | 0.61 (0.18 to 2.16); NR | ||
| IPE-adjusted HR (95% CI); p-value, cox method | N/A | 0.70 (0.47 to 1.04); 0.0388 | ||
| IPE-adjusted HR (95% CI); p-value, bootstrapping method | N/A | 0.70 (0.40 to 1.38); NR | ||
| OS: second data cut-off point | June 2014 | May 2013 | ||
| Patients who crossed over, n (%) | N/A | 115 (87.8) | NR | 157 (74.8) |
| Deaths, n (%) | 93 (35.6) | 55 (42.0) | 66 (31.9) | 72 (34.3) |
| Median OS (months) (95% CI) | NE (30.9 to NE) | 19.1 (21.7 to NE) | NE | 36.5 (32.2 to NE) |
| Unadjusted HR (95% CI); p-value | 0.80 (0.57 to 1.12) nominal p = 0.1993 | 0.88 (0.63 to 1.24); 0.24 | ||
| RPSFTM-adjusted HR (95% CI); p-value, cox method | NR | 0.69 (0.49 to 0.99); NR | ||
| RPSFTM-adjusted HR (95% CI); p-value, bootstrapping method | 0.53; nominal p = 0.0051 (0.34 to 0.82) | 0.69 (0.33 to 1.65); NR | ||
| IPE-adjusted HR (95% CI); p-value, cox method | N/A | 0.79 (0.57 to 1.11); NR | ||
| IPE-adjusted HR (95% CI); p-value, bootstrapping method | N/A | 0.79 (0.46 to 1.61); NR | ||
| OS: third data cut-off point | August 2015 | July 2015 | ||
| Patients who crossed over, n (%) | N/A | 115 (87.8) | NR | 158 (75.0) |
| Deaths, n (%) | 121 (46.4) | 70 (53.4) | 103 (49.8) | 109 (51.9) |
| Median OS (months) (95% CI) | 41.6 (31.2 to NE) | 34.5 (21.7 to NE) | 39.4 (32.7 to 51.4) | 42.8 (34.7 to 52.6) |
| Unadjusted HR (95% CI); p-value | 0.84 (0.62 to 1.13) nominal p = 0.2475 | 0.92 (0.71 to 1.21) one-sided p = 0.28 | ||
| RPSFTM-adjusted HR (95% CI); p-value, cox method | NR | 0.77 (0.58 to 1.02); NR | ||
| RPSFTM-adjusted HR (95% CI); p-value, bootstrapping method | 0.54; nominal p = 0.0025 (0.36 to 0.80) | 0.77 (0.42 to 1.79); NR | ||
| IPE-adjusted HR (95% CI); p-value, cox method | N/A | 0.80 (0.61 to 1.05); NR | ||
| IPE-adjusted HR (95% CI); p-value, bootstrapping method | N/A | 0.80 (0.48 to 1.71); NR | ||
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT, n (%) | DECISION, n (%) | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| Any anti-cancer treatment | 41 (15.7) | 16 (12.2) | 42 (20.3) | 18 (8.6) |
| Antineoplastic and immunomodulating agents | 29 (11.1) | 13 (9.9) | 38 (18.4) | 17 (8.1) |
| Variousa | 17 (6.5) | 5 (3.8) | 4 (1.9) | 2 (1.0) |
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| PFS by blinded review: first data cut-off point | November 2013 | August 2012 | ||
| Events, n (%) | 93 (35.6) | 109 (83.2) | 113 (54.6) | 137 (65.2) |
| Died before progression, n (%) | 14 (5.4) | 4 (3.1) | NR | NR |
| Median PFS (months) (95% CI) | 18.3 (15.1 to NE) | 3.6 (2.2 to 3.7) | 10.8 | 5.8 |
| Stratified HR (95% CI); p-value | 0.21 (0.14 to 0.31); < 0.001 | 0.59 (0.45 to 0.76); < 0.0001 | ||
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 210) | |
| PFS by investigator: first data cut-off point | November 2013 | August 2012 | ||
| Events, n (%) | 91 (34.9) | 104 (79.4) | 140 (67.6) | 184 (87.6) |
| Died before progression, n (%) | 16 (6.1) | 6 (4.6) | NR | NR |
| Median PFS (months) (95% CI) | 16.6 (4.8 to NE) | 3.7 (3.5 to NE) | 10.8 | 5.4 |
| Stratified HR (95% CI); p-value | 0.24 (0.16 to 0.35); < 0.001 | 0.49 (0.39 to 0.61); < 0.0001 | ||
| PFS by investigator: second data cut-off point | June 2014 | May 2013 | ||
| Events, n (%) | N/A | N/A | N/A | N/A |
| Died before progression, n (%) | N/A | N/A | N/A | N/A |
| Median PFS (months) (95% CI) | N/A | N/A | N/A | N/A |
| Stratified HR (95% CI); p-value | N/A | N/A | ||
| PFS by investigator: third data cut-off point | August 2015 | July 2015 | ||
| Events, n (%) | 121 (46.4) | 107 (81.7) | N/A | N/A |
| Died before progression, n (%) | 19 (7.3) | 6 (4.6) | N/A | N/A |
| Median PFS (months) (95% CI) | 19.4 (14.8 to 29.3) | 3.7 (3.5 to 5.4) | N/A | N/A |
| Stratified HR (95% CI); p-value | 0.24 (0.17 to 0.35); < 0.001 | N/A | ||
| Characteristic | Study | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 196) | Placebo (N = 201) | |
| ORR (%) (95% CI) | 64.8 (59.0 to 70.5) | 1.5 (0.0 to 3.6) | 12.2 (8.0 to 17.7) | 0.5 (0.0 to 2.7) |
| Difference (%) (95% CI) | 63.2 (57.1 to 69.4) | 11.7 (7.0 to 16.5) | ||
| Odds ratio (95% CI); p-value | 28.87 (12.46 to 66.86); < 0.0001 | NR; < 0.0001 | ||
| Complete response, n (%) | 4 (1.5) | 0 | 0 | 0 |
| Partial response, n (%) | 165 (63.2) | 2 (1.5) | 24 (12.2) | 1 (0.5) |
| Stable disease for ≥ 4 weeks | ≥ 7 weeks: 60 (23.0) | ≥ 7 weeks: 71 (54.2) | 145 (74.0) | 149 (74.1) |
| Durable stable disease (stable disease for ≥ 23 weeks or 6 months) | 40 (15.3) | 39 (29.8) | 82 (41.8) | 67 (33.2) |
| Progressive disease, n (%) | 18 (6.9) | 52 (39.7) | 20 (10.2) | 46 (22.9) |
| Patients unevaluable for response/not known, n (%) | 1 (0.4)/13 (5.0) | 2 (1.5)/4 (3.1) | N/A per-protocol analysisa | N/A per-protocol analysisa |
| Time to response (months) | ||||
| Median (95% CI) | 2.0 (1.9 to 3.5) | 5.6 (1.8 to 9.4) | NR | NR |
| Restricted mean (SD) | 3.38 (0.18) | 5.63 (3.79) | NR | NR |
| Duration of response (months) | ||||
| Median (95% CI) | NE (16.8 to NE) | NE | 10.2 (7.4 to 16.6) | NR |
| Restricted mean (SD) | 17.34 (0.76) | NE | NR | NR |
| Outcome | Study, n (%) | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 209) | |
| Any AE | 260 (99.6) | 118 (90.1) | 204 (98.6) | 183 (87.6) |
| Hypertension | 181 (69.3) | 19 (14.5) | 84 (40.6) | 26 (12.4) |
| Diarrhoea | 173 (66.3) | 22 (16.8) | 142 (68.6) | 32 (15.3) |
| Decreased appetite/anorexia | 139 (53.3) | 24 (18.3) | 66 (31.9) | 10 (4.8) |
| Weight loss | 132 (50.6) | 19 (14.5) | 97 (46.9) | 29 (13.9) |
| Nausea | 121 (46.4) | 33 (25.2) | 43 (20.8) | 24 (11.5) |
| Fatigue | 110 (42.1) | 32 (24.4) | 103 (49.8) | 53 (25.4) |
| Headache | 100 (38.3) | 15 (11.5) | 37 (17.9) | 15 (7.2) |
| Stomatitis (oral mucositis) | 93 (35.6) | 9 (6.9) | 48 (23.2) | 7 (3.3) |
| Vomiting | 92 (35.2) | 19 (14.5) | 23 (11.1) | 12 (5.7) |
| Proteinuria | 84 (32.2) | 4 (3.1) | 2 (1.0) | 0 (0.0) |
| Hand–foot syndrome | 84 (32.2) | 1 (0.8) | 158 (76.3) | 20 (9.6) |
| Dysphonia | 82 (31.4) | 7 (5.3) | 25 (12.1) | 6 (2.9) |
| Rash or desquamation | 48 (18.4) | 2 (1.5) | 104 (50.2) | 24 (11.5) |
| Alopecia | 32 (12.3) | 7 (5.3) | 139 (67.1) | 16 (7.7) |
| Outcome | Study, n (%) | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 209) | |
| Any grade ≥ 3 AE | 223 (85.4) | 39 (29.8) | 133 (64.3) | 63 (30.1) |
| Hypertension | 112 (42.9) | 5 (3.8) | 20 (9.7) | 5 (2.4) |
| Weight loss | 31 (11.9) | 1 (0.8) | 12 (5.8) | 2 (1.0) |
| Proteinuria | 26 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhoea | 22 (8.4) | 0 (0.0) | 12 (5.8) | 2 (1.0) |
| Decreased appetite/anorexia | 15 (5.7) | 1 (0.8) | 5 (2.4) | 0 (0.0) |
| Asthenia | 15 (5.7) | 3 (2.3) | 0 (0.0) | 0 (0.0) |
| Fatigue | 12 (4.6) | 2 (1.5) | 12 (5.8) | 3 (1.4) |
| Stomatitis (oral mucositis) | 11 (4.2) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Hand–foot syndrome | 9 (3.4) | 0 (0.0) | 42 (20.3) | 0 (0.0) |
| Headache | 8 (3.1) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| Nausea | 6 (2.3) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| Hypocalcaemia | 14 (5.4) | 0 (0.0) | 19 (9.2) | 3 (1.4) |
| Dyspnoea | 4 (1.5) | 4 (3.1) | 10 (4.8) | 6 (2.9) |
| Dysphagia | 4 (1.5) | 4 (3.1) | 3 (1.4) | 2 (1.0) |
| Rash/desquamation | 1 (0.4) | 0 (0.0) | 10 (4.8) | 0 (0.0) |
| Outcome | Study, n (%) | |||
|---|---|---|---|---|
| SELECTa | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 209) | |
| SAEs | 133 (51.0) | 31 (23.7) | 77 (37.2) | 55 (26.3) |
| Pneumonia | 10 (3.8) | 3 (2.3) | 1 (0.5) | 0 (0.0) |
| Hypertension | 9 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dehydration | 7 (2.7) | 0 (0.0) | 0 (0.0) | 2 (1.3) |
| General physical health deterioration | 6 (2.3) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Dysphagia | 3 (1.1) | 3 (2.3) | 2 (1.0) | 1 (0.7) |
| Dyspnoea | 3 (1.1) | 5 (3.8) | 7 (3.4) | 6 (2.9) |
| Haemoptysis | 0 (0.0) | 3 (2.3) | 0 (0.0) | 2 (1.3) |
| Secondary malignancy | NR | NR | 9 (4.3) | 4 (1.9) |
| Pleural effusion | 3 (1.1) | 1 (0.8) | 6 (2.9) | 4 (1.9) |
| Outcome | Study, n (%) | |||
|---|---|---|---|---|
| SELECT | DECISION | |||
| Lenvatinib (N = 261) | Placebo (N = 131) | Sorafenib (N = 207) | Placebo (N = 209) | |
| Treatment-related all-grade AEs | 254 (97.3) | 78 (59.5) | 200 (96.6) | 112 (53.6) |
| Treatment-related grade ≥ 3 AEs | 198 (75.9) | 13 (9.9) | 113 (54.6) | 15 (7.2) |
| Treatment-related SAEs | 79 (30.3) | 8 (6.1) | 26 (12.6) | 8 (3.8) |
| Treatment-related fatal AEs | 6 (2.3) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Outcome | Treatment | |||
|---|---|---|---|---|
| Prior | No prior | |||
| Lenvatinib (n = 66) | Placebo (n = 27) | Sorafenib (n = 195) | Placebo (n = 104) | |
| ORR (%) (95% CI) | 62.1 (50.4 to 73.8) | 3.7 (0.0 to 10.8) | 65.6 (59.0 to 72.3) | 1.0 (0.0 to 2.8) |
| HR (95% CI) | 15.57 (4.06 to 59.72) | 58.88 (18.95 to 182.91) | ||
Appendix 4 Risk-of-bias assessment of included trials
| Parameter | Study | |
|---|---|---|
| SELECT | DECISION | |
| Was the method used to assign participants to the treatment groups really random? | ✓ | ✓ |
| Was the allocation of treatment concealed? | ✓ | ✓ |
| Was the number of participants who were randomised stated? | ✓ | ✓ |
| Were details of baseline comparability presented in terms of prognostic factors? | ✓ | ✓ |
| Was baseline comparability achieved in terms of prognostic factors? | ✓/✗a | ✓/✗a |
| Were the eligibility criteria for study entry specified? | ✓ | ✓ |
| Were any co-interventions identified that may influence the outcomes for each group? | ✓ | ✓ |
| Were the outcome assessors blinded to the treatment allocation? | ✓ | ✓ |
| Were the individuals who administered the intervention blinded to the treatment allocation? | ✓b | ✓ |
| Were the participants who received the intervention blinded to the treatment allocation? | ✓c | ✓d |
| Was the success of the blinding procedure assessed? | ✗ | ✗ |
| Were ≥ 80% of the participants originally included in the randomisation process followed up in the final analysis? | ✓ | ✓ |
| Were the reasons for withdrawals stated? | ✓ | ✓ |
| Is there any evidence to suggest that the authors measured more outcomes than they reported? | ✓ | ✓ |
| Was an ITT analysis included? | ✓ | ✓ |
Appendix 5 Evidence from systematic reviews
| Study and year of publication | Cancer type | Intervention | Number of studies | Note | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | RR-DTC | Lenvatinib | Sorafenib | RCT | Non-RCT (prospective) | Non-RCT (retrospective) | ||||
| Anderson et al. 201361 | RR-DTC | Potential treatment options for RR-DTC | 45 | 45 | 1 | 3 | 1 | 44 | 0 | SLR |
| Gruber and Colevas 201533 | RR-DTC | TKIs | 18 | 18 | 2 | 6 | 2 | 16 | 0 | SLR |
| Jean et al. 201693 | DTC vs. other cancer | Sorafenib | 9 | 4 | 0 | 4 | 4 | 5 | 0 | SLR (PubMed only) |
| Kawalec et al. 201697 | RR-DTC | Lenvatinib and sorafenib | 2 | 2 | 1 | 1 | 2 | 0 | 0 | SR and ITC |
| McFarland and Misiukiewicz 2014104 | RR-DTC | Sorafenib (single or in combination) | 18 | 18 | 0 | 18 | 1 | 12 | 5 | SLR |
| Shen et al. 2014127 | RR-DTC | Sorafenib | 7 | 7 | 0 | 7 | 0 | 5 | 2 | SLR |
| Thomas et al. 2014138 | Metastatic thyroid cancer | Sorafenib | 7 | 6 | 0 | 7 | 0 | 6 | 1 | SLR |
| Tremblay et al. 201657 | RR-DTC | Lenvatinib vs. sorafenib | 2 | 2 | 1 | 1 | 2 | 0 | 0 | Does not report SLR or SR methodology, but reports ITC and MAIC results |
| Ye et al. 2015141 | Thyroid cancer | Lenvatinib and sorafenib | 10 | 9 | 2 | 8 | 2 | 8 | 0 | SR and meta-analysis |
| CADTH (lenvatinib) 20166 | RR-DTC | Lenvatinib | 2 | 2 | 1 | 1 | 2 | 0 | 0 | Includes only SELECT but reports on ITC from Eisai Ltd8 |
| CADTH (sorafenib) 20155 | RR-DTC | Sorafenib | 1 | 1 | 0 | 1 | 1 | 0 | 0 | Includes only DECISION |
| Eisai Ltd 20178 | RR-DTC | Lenvatinib | 2 | 2 | 1 | 1 | 2 | 0 | 0 | Includes ITC |
| Bayer HealthCare 20177 | RR-DTC | Sorafenib | 2 | 2 | 1 | 1 | 2 | 0 | 0 | Includes ITC |
| Assessment criterion | Study | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al. 201361 | Gruber and Colevas 201533 | Jean et al. 201693 | Kawalec et al. 201697 | McFarland and Misiukiewicz 2014104 | Shen et al. 2014127 | Thomas et al. 2014138 | Trembaly et al. 201657 | Ye et al. 2015141 | CADTH (lenvatinib) 20166 | CADTH (sorafenib) 20155 | Eisai Ltd 20178 | Bayer HealthCare 20177 | |
| Was the review question clearly defined in terms of population, interventions, comparators, outcomes and study designs? | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was the search strategy adequate and appropriate? | ✓ | ✓ | ✗a | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were preventative steps taken to minimise bias and errors in the study selection process? | ✓ | NR | NR | ✓ | ✓ | ✓ | NR | NR | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were appropriate criteria used to assess the quality of the primary studies, and were preventative steps taken to minimise bias and errors in the quality assessment process | NR | NR | NR | ✗b | NR | ✗ | ✗ | NR | NR | ✓c | ✓c | ✓ | ✓d |
| Were preventative steps taken to minimise bias and errors in the data extraction process? | ✓ | NR | NR | ✓ | ✓ | ✓ | ✓ | NR | ✓ | NR | NR | NR | ✓ |
| Were adequate details presented for each of the primary studies? | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were appropriate methods used for data synthesis? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗e | ✓/✗e | ✓ | ✓/✗f | ✓ | ✓ | ✓ | ✓ |
| Do the authors’ conclusions accurately reflect the evidence that was reviewed? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗f | ✓ | ✓ | ✓ | ✓ |
| Was the review published in a peer-reviewed journal? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
| Was the review sponsored by a pharmaceutical company? | ✓g | ✗ | ✗ | ✗ | ✗ | ✗ | ✓/✗g | ✓/✗h | ✗ | ✗ | ✗ | ✓h | ✓g |
| Study and year of publication | Analysis | Overall findings/conclusions |
|---|---|---|
| Anderson et al. 201361 | Descriptive analysis | Certain treatments, notably TKIs, have shown promise in Phase II trials, and two Phase III randomised placebo controlled trials (SELECT and DECISION) are ongoing |
| Gruber and Colevas 201533 | Descriptive analysis | The most likely outcome of treatment with a TKI is stable disease. Lenvatinib appears to be the most active agent but is not yet available, with a PFS vs. placebo triple that of sorafenib and a RECIST response rate five times that of sorafenib in the Phase III setting |
| Jean et al. 201693 | Descriptive analysis | There is a distinct increase in the rate of occurrence of AEs of sorafenib when used in DTC compared with RCC and HCC. Although many theoretical explanations have been proposed, the exact mechanism for this differential in toxic effects remains unclear |
| Kawalec et al. 201697 | Indirect comparison (conducted using the Bucher et al.313 method) | Lenvatinib and sorafenib are drugs with strong evidence on efficacy in treatment of RR-DTC. Based on the currently available clinical data, lenvatinib appeared to be more efficacious then sorafenib in RR-DTC therapy. The safety profiles of the drugs were acceptable and quite comparable. Indirect comparison results should be interpreted with caution because of the differences in trial characteristics |
| McFarland and Misiukiewicz 2014104 | Descriptive analysis | Although the data are based primarily on non-randomised Phase II trials and on only one randomised Phase III trial, it has been convincingly shown that sorafenib slows the progression of disease in the majority of cases |
| Shen et al. 2014127 | Descriptive analysis and meta-analysis | As far as PR and AEs are concerned, the results of this meta-analysis indicate that sorafenib has a modest effect in patients with RR-DTC and the high incidence of AEs associated with this agent may affect the quality of patients’ lives |
| Thomas et al. 2014138 | Descriptive analysis and meta-analysis | ORR from meta-analysis is higher than recently reported in DECISION. The difference could be explained by the study design of DECISION and the challenges that arise from using RECIST criteria. The targeted therapy agents are associated with significant incidence of AEs and a small risk of death. Although there is evidence of efficacy with TKIs, these drugs may diminish quality of life because of significant toxicities; therefore, it is important to assess the need for treatment. Most patients with metastatic disease do not require systemic therapy |
| Tremblay et al. 201657 | Indirect comparison (conducted using the Bucher et al.313 method) and MAIC | Based on a MAIC of individual patient data from SELECT and aggregate data from DECISION, lenvatinib was associated with statistically significantly longer PFS compared with sorafenib. However, only patient characteristics common to both trials that were reported in DECISION could be matched. The results may therefore have been influenced by other unobserved factors. The conclusions are limited to patients not previously treated with a VEGFR-targeted therapy as these were excluded from the analysis |
| Ye et al. 2015141 | Descriptive analysis and meta-analysis | Lenvatinib and sorafenib are useful in the treatment of TC. Although their toxicities remain high (57.4%) in the patients, the death rate is controlled (4.1%). Lenvatinib and sorafenib are more useful for thyroid cancer compared with RR-DTC |
| CADTH (lenvatinib) 20166 | Descriptive analysisa | The Endocrine Clinical Guidance Panel concluded that there is a net overall clinical benefit of lenvatinib in the treatment of RR-DTC. In making this conclusion, the Clinical Guidance Panel also noted that OS was a secondary end point and confounded by crossover; HRQoL was not studied but AE profiles were similar to AEs seen with sorafenib in DECISION. Hypertension was more common with lenvatinib but hand-foot syndrome and drug discontinuation owing to AEs was more common with sorafenib |
| CADTH (sorafenib) 20155 | Descriptive analysis | The Endocrine Clinical Guidance Panel concluded that there is a net overall clinical benefit of sorafenib compared with placebo in patients with clinically progressive RR-DTC. Toxicity was increased with sorafenib compared both with placebo and with other trials studying sorafenib in cancer, and there may be an increased risk of squamous cell cancers of the skin during sorafenib use. As HRQoL was reduced by sorafenib, the decision to initiate treatment and the monitoring of treatment should be by a clinician experienced in the use of targeted agents and in the treatment of thyroid cancer |
| Eisai Ltd 20178 | Descriptive analysis and indirect comparison (conducted using the Bucher et al.313 method) | Lenvatinib was shown to be of superior efficacy to placebo in SELECT (crossover-adjusted OS, PFS and ORR) and to sorafenib (PFS) from an ITC. Comparative safety information with sorafenib has shown that sorafenib and lenvatinib share many of their AEs, although their safety profiles are not identical and lenvatinib is associated with lower rates of some AEs that have been shown to impact patients’ daily lives |
| Bayer HealthCare 20177 | Descriptive analysis and indirect comparison (conducted using the Bucher et al.313 method) and MAIC | Crossover makes it difficult to detect and attribute improvements in OS in DECISION. Although there were no statistically significant differences between arms, analyses of OS, at 9 months and 36 months after the original data cut-off point, showed a consistent separation of the K–M curves in favour of sorafenib. Results from the indirect comparison show the safety profile of sorafenib to be statistically superior to that of lenvatinib with respect to AEs. Overall, AEs in DECISION were consistent with the known safety profile of sorafenib in other indications, and effectively managed by supportive care, pharmacological treatment, dose interruption or dose reduction. In addition, sorafenib was shown to be associated with a lower risk of treatment discontinuation attributable to AEs. Sorafenib is an efficacious treatment option, especially for patients presenting with comorbidities or in circumstances in which managing and maintaining quality of life is a primary treatment objective. The results of DECISION are directly relevant to the progressive RR-DTC patients within routine clinical practice in England. The safety results from the indirect comparison support sorafenib as a tolerable treatment option. This may be important in patients with comorbidities in whom managing and maintaining quality of life is a primary treatment objective. Please note that Bayer HealthCare has since clarified that it considers indirect comparison results to be unreliable owing to important differences between SELECT and DECISION (communication with the AG via NICE, 2017, personal communication) |
| Outcome | Systematic review | ||||
|---|---|---|---|---|---|
| Jean et al. 201693 | Shen et al. 2014127 (95% CI) | Thomas et al. 2014138 (95% CI) | |||
| TARGET trial (RCC)314 | SHARP trial (HCC)315 | DECISION | Meta-analysisa | Meta-analysisa | |
| Efficacy | |||||
| PFS (months) | 5.5b | 5.5b,c | 10.8 | – | 17.9 (17.9 to 18.0)d |
| ORR (%) | 1.6b | 0.7b | 12.2b | 22 (15 to 28) | 20.9 (14.3 to 27.5)d |
| All-grade AEs (%) | |||||
| Hand–foot syndrome | 30b | 21b | 76b | 80 (68 to 91) | 73.5 (64 to 83) |
| Rash | 40b | 16b | 50b | 66 (50 to 82) | 66.7 (51.7 to 81.7) |
| Diarrhoea | 43b | 39b | 69b | 68 (59 to 77) | 70.3 (62.3 to 78.3) |
| Hypertension | 17b | 5b | 41b | 52 (33 to 72) | 36.1 (26.6 to 45.6) |
| Fatigue | 37b | 22b | 50b | 67 (57 to 78) | 60.6 (44.8 to 76.4) |
| Weight loss | 10b | 9b | 51b | 52 (33 to 72) | 56.8 (38.8 to 74.8) |
| Mucositis | NR | NR | 36b | – | 35.4 (23.1 to 47.7) |
| Grade ≥ 3 AEs (%) | |||||
| Hand–foot syndrome | 6 | 8 | 20 | – | 19.4 (8.3 to 30.5) |
| Rash | 1 | 1 | 5 | – | 6.8 (2.7 to 10.9) |
| Diarrhoea | 2 | 8 | 6 | – | 6.8 (3.3 to 10.3) |
| Hypertension | 4 | 2 | 10 | – | 7.3 (2.5 to 12.1) |
| Fatigue | 5 | 4 | 6 | – | 10.3 (4.4 to 16.2) |
| Weight loss | < 1b | 2b | 12b | – | 5.2 (1.2 to 90.2) |
| Mucositis | NR | NR | 4b | – | 3.9 (0.6 to 7.2) |
| Dose modifications owing to AEs (%) | |||||
| Dose reductions | 13b | 26b | 64b | 62 (36 to 89) | 56 (43.4 to 69.3) |
| Discontinued | 10b | 38b | 19b | – | 16 (8.6 to 23.4) |
| Outcome | Relative effectiveness | Source |
|---|---|---|
| OS (RPSFTM adjusted) | HR 0.78 (95% CI 0.42 to 1.42) | Kawalec et al. 201697 |
| OS (RPSFTM adjusted) | HR 0.77 (95% CI 0.44 to 1.35) | Tremblay et al. 2015289 |
| OS (RPSFTM adjusted) | Academic in confidencea | Eisai Ltd 20178 |
| OS (MAIC and RPSFTM adjusted) | HR 0.73 (95% CI 0.40 to 1.35) | Tremblay et al. 2015289 |
| PFS | HR 0.36 (95% CI 0.22 to 0.57) | Kawalec et al. 201697 |
| PFS | HR 0.36 (95% CI 0.22 to 0.57) | Tremblay et al. 2015289 |
| PFS | Academic in confidencea | Eisai Ltd 20178 |
| PFS (MAIC-adjusted) | HR 0.33 (95% CI 0.22 to 0.57) | Tremblay et al. 2015289 |
| Outcome | Relative effectiveness | Source |
|---|---|---|
| OS (MAIC and RPSFTM adjusted) | Academic in confidencea | bTremblay et al. 2015289 |
| OS (MAIC and RPSFTM adjusted) | Academic in confidencea | Bayer HealthCare 20177 |
| PFS (MAIC adjusted) | Academic in confidencea | bTremblay et al. 2015289 |
| PFS (MAIC adjusted) | Academic in confidencea | Bayer HealthCare 20177 |
| Outcome | Comparison | |
|---|---|---|
| Lenvatinib vs. sorafenib (Kawalec et al. 201697), HR (95% CI) | Sorafenib vs. lenvatinib (Bayer HealthCare 20177), HR (95% CI) | |
| Grade ≥ 3 AE | NR | Academic in confidencea |
| SAE | 1.54 (0.99 to 2.40) | Academic in confidencea |
| Treatment-related SAE | 4.02 (1.69 to 9.60) | Academic in confidencea |
| Discontinuation owing to AE | 1.26 (0.32 to 4.96) | Academic in confidencea |
Appendix 6 Proportional hazards assumption
The AG assessed the validity of the PH assumptions in DECISION and SELECT.
The H–H (cumulative hazard vs. cumulative hazard) plot for PFS by investigator assessment from SELECT (final data cut-off point) is provided in Figure 22. The estimated constant for a linear relationship is statistically significantly different from zero (–0.0589, 95% CI –0.075 to –0.043; p = 6.73 E-12). Comparison by analysis of variance (ANOVA) of the linear trend with a quadratic trend shows an improved fit [F(146,1) = 252.3; p = 1.25 E-33], indicating that the assumption of PH does not hold for investigator-assessed PFS data from SELECT.
FIGURE 22.
The H–H plot for PFS data from SELECT.

The H–H plot for OS unadjusted for treatment crossover from SELECT (final data cut-off point) is provided in Figure 23. The estimated constant for a linear relationship is statistically significantly different from zero (–0.0103, 95% CI –0.0200 to –0.00005; p = 0.039). Comparison by ANOVA of the linear trend with a quadratic trend shows a significantly improved fit for the quadratic relationship [F(146,1) = 63.6; p = 1.86 E-13), indicating that the assumption of PH does not hold for unadjusted OS data from SELECT.
FIGURE 23.
The H–H plot for unadjusted OS data from SELECT.

The H–H plot for OS adjusted by RPSFTM for treatment crossover using data from SELECT (final data cut-off point) is provided in Figure 24. In this case, the estimated constant for the fitted linear trend does not show a significant deviation from zero (–0.0041, 95% CI –0.0166 to 0.0084; p = 0.52). However, a comparison by ANOVA of the linear trend with a fitted quadratic trend shows an improved fit for the quadratic relationship [F(166,1) = 12.03; p = 0.000665], indicating that the assumption of PH is questionable on the basis of evidence of non-linearity in the relationship between the two arms of the trial following adjustment for crossover.
FIGURE 24.
The H–H plot for OS data adjusted by RPFST for treatment crossover from SELECT.
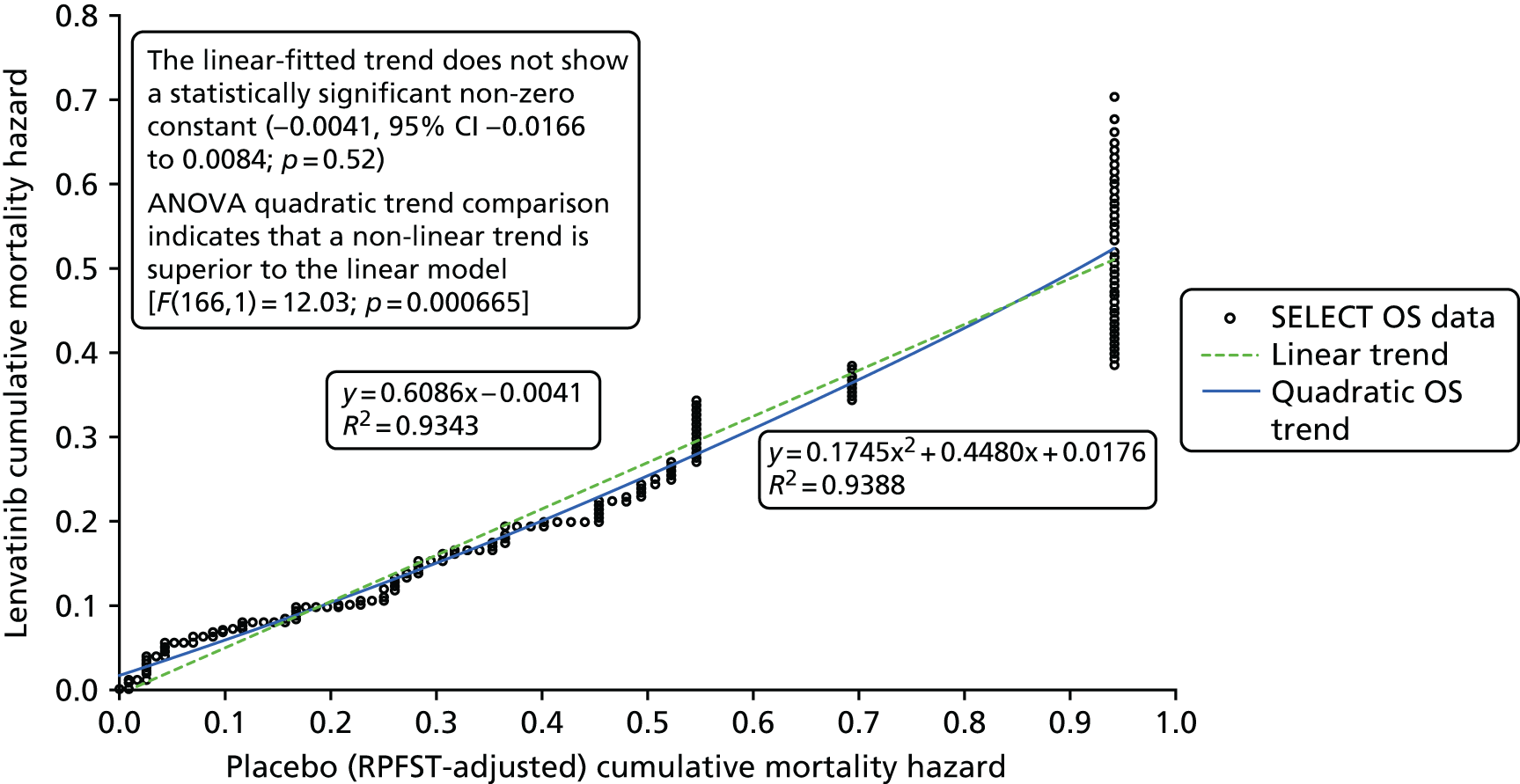
The linear trend fitted to the PFS DECISION data (final data cut-off point) in Figure 25 shows a statistically significant non-zero constant of –0.1263 (95% CI –0.1635 to –0.0892; p = 2.59 E-10). In addition, the ANOVA test for non-linearity indicates a statistically significant deviation from linearity [F(177,1) = 6.722; p = 0.0103]. On both criteria, the PH assumption is called into question.
FIGURE 25.
The H–H plot for PFS from DECISION.

The linear trend fitted to the unadjusted OS data from the DECISION trial (final data cut), shown in Figure 26, shows a very small constant of 0.0018 (95% CI –0.0036 to 0.0073; p = 0.505), consistent with the PH requirement for a zero constant. In addition, the ANOVA test for non-linearity indicates no statistically significant deviation from linearity [F(89,1) = 0.0675; p = 0.796]. On both criteria, the PH assumption is supported for unadjusted OS trial data.
FIGURE 26.
The H–H plot for unadjusted OS data from DECISION.

Figure 27 shows the linear trend fitted to the RPFST-adjusted OS DECISION data (final data cut-off point), which shows a statistically significant non-zero constant of 0.0115 (95% CI 0.0026 to 0.0204; p = 0.0117). In addition, the ANOVA test for non-linearity indicates a statistically significant deviation from linearity [F(122,1) = 56.915; p = 9.03 E-12]. On both criteria, the PH assumption is questionable.
FIGURE 27.
The H–H plot for RPFST-adjusted OS from DECISION.

Appendix 7 Data extraction tables from extended open-label phases of the trials not presented in the main body of the report
| Outcome | Study | ||||
|---|---|---|---|---|---|
| SELECT | DECISION | ||||
| Lenvatinib: 24-mg dose (n = 85) | Lenvatinib: 20-mg dose (n = 30) | Lenvatinib: either dose (n = 115) | Sorafenib after sorafenib (n = 46) | Sorafenib after placebo (n = 137) | |
| Data cut-off point | Second data cut-off point: June 2014 | First data cut-off point: August 2012 | |||
| OS | NR | NR | NR | NR | NR |
| Median PFS (months) (95% CI) | 17.5 (8.3 to NE) | NE (10.9 to NE) | 22.1 (9.4 to NE) | 6.7 | 9.6 |
| ORR (%) (95% CI) | 52.9 (41.8 to 63.9) | 60.0 (40.6 to 77.3) | 54.8 (45.2 to 64.1) | 12.2 | 9.5 |
| Parameter | Study | |
|---|---|---|
| SELECT | DECISION | |
| Lenvatinib: 24-mg dose (n = 82) | Sorafenib after placebo (n = 150) | |
| Data cut-off point | First data cut-off point: November 2013 | First data cut-off point: August 2012 |
| Median duration of treatment (months) (range) | 8.9 (0–25) | 13.1a |
| Median dose intensity (mg) (range) | 19.4 (7–24) | NR |
| Dose reductions owing to AEs (%) | 43.9 | NR |
| Dose interruptions owing to AEs (%) | 70.7 | NR |
| Treatment-related AEs (%) | 85.4 | NR |
| Common AEs (%)b | ||
| Hypertension | 54 | 28.7 |
| Diarrhoea | 52 | 56.0 |
| Decreased appetite | 43 | 25.3 |
| Weight loss | 39 | 41.3 |
| Fatigue | 38 | 24.7 |
| Hand–foot syndrome | NR | 56.7 |
| Alopecia | NR | 56.7 |
| Rash | NR | 29.3 |
| Common grade ≥ 3 AEs (%)b | ||
| Hypertension | 24 | NR |
| Weight loss | 9 | NR |
| Proteinuria | 7 | NR |
| Asthenia | 6 | NR |
| Fatigue | 6 | NR |
| Treatment-related fatal AEs (%) | 4.9 | NR |
Appendix 8 Evidence from observational studies
| Parameter | Observational study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305 | Chen et al. | Duntas et al. | Kloos et al. | Study 12791 | Marotta et al. | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 | RR-DTC: 17 |
| Primary source | Cabanillas et al. 201577 | Takahashi et al. 2016135 (abstract) | Ahmed et al. 201159 | Gupta-Abramson et al. 200888 | Chen 201178 (abstract) | Duntas et al. 201181 (abstract) | Kloos et al. 2009101 | Schneider et al. 2012126 | Marotta et al. 2016103 |
| Other sources | Two abstracts128,129 and lenvatinib EPAR27 | One other abstract136 and lenvatinib EPAR27 | One other abstract60 and lenvatinib EPAR27 | Five abstracts75,76,80,98,137 | None | None | Lenvatinib EPAR27 | One abstract125 and one other study92 | None |
| Country | USA, Italy, UK, Australia, Poland and France | Japan | UK | USA | China | Greece | USA | The Netherlands | Italy |
| Recruitment period | October 2008 to February 2010 | 3 September 2012 to 9 July 2015, latest cut-off datea | Patient accrual commenced in May 2007 | February 2006 to August 2009 | NR | NR | October 2004 and August 2005 | October 2007 and February 2011 | NR |
| Length of follow-up |
September 2013: median 16.1 months (range 15.0–16.6 months) June 2014: median 51.6 months |
Safety: 2 years Secondary outcomes: 40 monthsa |
Median 19 months | b,cMedian 9 months75 | Minimum 3 monthsb | 4 to 9 months | NR | Median 25 months (range 3.5–39 months) | Median 17 months |
| Parameter | Observational study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305 | Chen et al. | Duntas et al. | Kloos et al. | Study 12791 | Marotta et al. | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 | RR-DTC: 17 |
| Median age (years) (range) | 63 (34–77) | NR | All: 55 (21–78) | Initial 30 patients: 63 (31–89) | NR | NR |
PTC/no prior chemotherapy (n = 19): 67 (33–90) PTC/prior chemotherapy (n = 22): 56 (27–75) |
Median 64 (53–82) | 58 |
| % male | 59 | NR | All: 55.9 | All: 49.075 | NR | 36.4 |
All: 55.4 PTC (n = 41): 51.2 |
61.2 | 23.5 |
| Race (%) | White: 86 | NR | NR | NR | NR | NR | White
|
NR | NR |
| ECOG PS ≥ 2 (%) | 6.9 | NR | All: 0 | Initial 30 patients: 0 | NR | NR | NR | NR | 35.3 |
| PTC (%) | 74.1 | NR | All: 23.5 | All: 52.7 | 100 | NR | 73.2 | 41.9 | 35.3 |
| FTC (%) | 25.9a | NR | All: 14.7 | 32.7a | 0 | NR | 19.6a | 48.4 | 64.7 |
| Lung metastases (%) | 93 | NR | NR | NR | NR | NR | NR | Lung only: 25.8 | NR |
| Bone metastases (%) | 45 | NR | NR | NR | NR | NR | NR | Lung and bone only: 25.8 | 23.5 |
| Prior TKI | 29.3 | NR | NR | NR | NR | NR | NR | 0 | 11.8 |
| Parameter | Observational study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305a | Chen et al. | Duntas et al. | Kloos et al. | Study 12791 | Marotta et al. | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 | RR-DTC: 17 |
| Median OS (months) (95% CI) |
September 2013: 27.7 (27.7 to NE)b June 2014: 32.3 (23.3 to 35.8)b |
RR-DTC only: 31.8 (31.8 to NE)b | RR-DTC only: median not met | RR-DTC: 32.4 (21.6 to NE)c | NR | NR | 23 (18 to 43)d | 34.5 (19 to 50) (n = 26) | No patient died during follow-up |
| Median PFS (months) (95% CI) | 12.6 (9.9 to 16.1) | RR-DTC only: 25.8 (18.4 to NE) | RR-DTC only: median not met | RR-DTC only: 22.1 (17.3 to 31.1)c | Mean: 9.7 (6.8 to 12.4)c | NR | All PTC (n = 41): 15 (10 to 27.5) | 18 (7 to 29) (n = 26) | 12 |
| ORR (%) (95% CI) | 50.0 (36.6 to 63.4) | RR-DTC only: 68.0 | 21d | RR-DTC only: 38.3 | 33.3 | 27.3 | All PTC (n = 41): 15d | 30.8 (n = 26) | 35.3 |
| Median time to response (months) | 3.6 (95% CI 1.8 to 3.7) | NR | NR | NR | NR | NR | NR | All responses achieved in the first 6 months of treatment (n = 26) | NR |
| Duration of response (months) | 12.7 (8.8 to NE) (n = 29) | NR | NR | NR | NR | NR | NR for all PTC patients | 29.6 (range 3–33) (n = 26) | NR |
| Event | Prospective observational study | ||
|---|---|---|---|
| Lenvatinib, two studies,77,135 treatment emergent (%) | Sorafenib, four studies,59,78,81,126 treatment emergent (%) | Sorafenib, two studies,88,101 treatment related (%) | |
| All-grade AEs | 100a | NR | NR |
| Hypertension | 76 to 90a | 21 to 42c | 43a |
| Diarrhoea | 55 to 67a | 52 to 77c | 75 to 80a |
| Decreased appetite | 52 to 78a | 29b | 20 to 82a |
| Weight loss | 69b | 29 to 58a | 60 to 82a |
| Nausea | 50b | 10 to 27a | 30 to 55a |
| Fatigue | 60 to 73a | 59b | 63 to 66a |
| Headache | 43b | 15b | 16b |
| Stomatitis/mucositis | 31 to 57a | 27 to 48c | 16 to 47a |
| Vomiting | 38a | 18b | 18b |
| Proteinuria | 61 to 64a | NR | NR |
| Hand–foot syndrome | 22 to 77a | 71 to 79c | 63 to 93a/63 to 91a,d |
| Dysphonia | 43b | NR | NR |
| Rash | 24b | 55 to 88a | 79 to 80a/79 to 85a,d |
| Alopecia | 9b | 52 to 74a | 43 to 79a |
| Other types of all-grade AEs | Other AEs experienced by ≥ 25% of patients in Study 20177 (Study 208135 only reported AEs that were experienced by ≥ 55% of patients):
|
Other AEs experienced by ≥ 25% of patients in any one study:59,126
|
Other treatment-related AEs experienced by ≥ 25% of patients in Kloos et al. 2009:101
|
| Parameter | Observational study | |||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | ||||||||
| Study 201 | Study 208 | Study 12636 | UPCC-03305a,b | Chen et al. | Duntas et al. | Kloos et al.a | Study 12791 | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 |
| All-grade AEs | 58 (100) | 51 (100) | NR | NR | NR | NR | NR | NR |
| Hypertension | 44 (76) | 46 (90) | 7 (21) | 13 (43) | NR | 3 (27) | 24 (43) | 13 (42) |
| Diarrhoea | 39 (67) | 28 (55) | 26 (77) | 24 (80) | NR | One of the most frequent AEsc | 42 (75) | 16 (52) |
| Decreased appetite/anorexia | 30 (52) | 40 (78) | 10 (29) | 6 (20) | NR | NR | 46 (82) | NR |
| Weight loss | 40 (69) | NR | 10 (29) | 18 (60) | NR | NR | 46 (82) | 18 (58) |
| Nausea | 29 (50) | NR | 9 (27) | 9 (30) | NR | NR | 31 (55) | 3 (10) |
| Fatigue | 35 (60) | 37 (73) | 20 (59) | 19 (63) | NR | One of the most frequent AEsc | 37 (66) | NR |
| Headache | 25 (43) | NR | 5 (15) | NR | NR | NR | 9 (16) | NR |
| Stomatitis/mucositis | 18 (31) | 29 (57) | 9 (27) | 14 (47) | NR | NR | 9 (16) | 15 (48) |
| Vomiting | 22 (38) | NR | 6 (18) | Included with nausea | NR | NR | 10 (18) | NR |
| Proteinuria | 37 (64) | 31 (61) | NR | NR | NR | NR | NR | NR |
| Hand–foot syndrome | 13 (22) | 39 (77) | 27 (79) | 28 (93) | NR | One of the most frequent AEsc | 35 (63) | 22 (71) |
| Dysphonia | 25 (43) | NR | NR | NR | NR | NR | NR | NR |
| Rash | 14 (24) | NR | Dermatology (other): 30 (88) | 24 (80) | NR | NR | 44 (79) | 17 (55) |
| Alopecia | 5 (9) | NR | 25 (74) | 13 (43) | NR | NR | 44 (79) | 16 (52) |
| Other types of all-grade AEs | Other AEs experienced by ≥ 25% of patients:
|
None; note that abstract only reports AEs reported by ≥ 55% of patients | Other AEs experienced by ≥ 25% of patients:
|
Terry et al. 2013137 later examined treatment-related hand–foot syndrome and rash. AE data for all 55 patients not RR-DTC only (n = 47):
|
NR | NR | Other AEs experienced by ≥ 25% of patients:
|
|
| Event | Prospective observational study (%) | ||
|---|---|---|---|
| Lenvatinib, two studies,77,135 treatment emergent | Sorafenib, four studies,59,78,81,126 treatment emergent | Sorafenib, two studies,88,101 treatment related | |
| Grade ≥ 3 AEs | 72a | NR | NR |
| Hypertension | 10b | 6 to 16a | 4 to 13a |
| Diarrhoea | 10b | 3 to 7a | 4 to 7a |
| Decreased appetite | 2b | 0b | 3b |
| Weight loss | 12b | 0 to 10a | 5 to 10a |
| Nausea | 0b | 0a | 0a |
| Fatigue | 9b | 9a | 3 to 16a |
| Headache | 2b | 3b | 0b |
| Stomatitis/mucositis | 2b | 9 to 10a | 0 to 2 |
| Hand–foot syndrome | 2b | 23 to 44a | 7 to 10a/7a,c |
| Proteinuria | 10b | NR | NR |
| Asthenia | NR | NR | NR |
| Dyspnoea | 0b | NR | 0b |
| Dysphagia | NR | 0b | NR |
| Rash | 0b | 6 to 16a | 4 to 10a/4 to 18a,c |
| Other types of grade ≥ 3 AEs | Other grade ≥ 3 AEs experienced by ≥ 5% of patients in Study 201:
|
Other grade ≥ 3 AEs experienced by ≥ 5% of patients in any one of the studies:
|
Other grade ≥ 3 treatment-related AEs experienced by ≥ 5% of patients in either study:
|
| SAEs | 48b | NR | NR |
| Fatal AEs | 5 to 8a | 1b | NR |
| Type of SAEs | SAEs that occurred in ≥ 3.5% of patients in Study 201:
|
NR | NR |
| Parameter | Observational study, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305a | Chen et al. | Duntas et al. | Kloos et al.a | Study 12791 | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 |
| Grade ≥ 3 AEs | 42 (72) | RR-DTC: 12 (72) | NR | NR | NR – see ‘other’ | NR | NR | NR |
| Hypertension | 6 (10) | NR | 2 (6) | 4 (13) | NR | NR | 2 (4) | 5 (16) |
| Diarrhoea | 6 (10) | NR | 1 (3) | 2 (7) | NR | NR | 2 (4) | 2 (7) |
| Decreased appetite | 1 (2) | NR | 0 | 1 (3) | NR | NR | 0 | NR |
| Weight loss | 7 (12) | NR | 0 | 3 (10) | NR | NR | 3 (5) | 3 (10) |
| Nausea | 0 | NR | 0 | 0 | NR | NR | 0 | 0 |
| Fatigue | 5 (9) | NR | 3 (9) | 1 (3) | NR | NR | 9 (16) | NR |
| Headache | 1 (2) | NR | 1 (3) | NR | NR | NR | 0 | NR |
| Stomatitis/mucositis | 1 (2) | NR | 3 (9) | 0 | NR | NR | 1 (2) | 3 (10) |
| Hand–foot syndrome | 1 (2) | NR | 14 (44) | 3 (10) | NR | NR | 4 (7) | 7 (23) |
| Proteinuria | 6 (10) | NR | NR | NR | NR | NR | NR | NR |
| Asthenia | NR | NR | NR | NR | NR | NR | NR | NR |
| Dyspnoea | 0 | NR | NR | NR | NR | NR | 0 | NR |
| Dysphagia | NR | NR | 0 | NR | NR | NR | NR | NR |
| Rash | 0 | NR | Dermatology (other): 2 (6) | 3 (10) | NR | NR | 2 (4) | 5 (16) |
| Other types of grade ≥ 3 AEs | Other grade ≥ 3 AEs in ≥ 5% of patients:
|
NR | Other grade ≥ 3 AEs reported:
|
|
‘Although the types of toxicities were consistent with other sorafenib trials, their severity was relatively mild’78 | NR |
Grade ≥ 3 AEs reported, in text Most common (≥ 5% frequency) grade 3 AEs included: |
Grade 3 AEs:
|
| Parameter | Observational study, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305 | Chen et al. | Duntas et al. | Kloos et al. | Study 12791 | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 |
| SAEs | 28 (48) | NR | NR | NR | NR | NR | NR | NR |
| Types of SAEs | SAEs that occurred in at least two patients:
|
NR | NR | NR | NR | NR | NR | NR |
| Fatal AEs | Deaths attributable to AEs, 3 (5%):
|
Four deaths, all unrelated to study drug | NR | NR | NR | NR | 1 (not considered treatment related) | NR |
| Event | Prospective observational study | ||
|---|---|---|---|
| Lenvatinib, two studies,77,135 treatment emergent (%) | Sorafenib, four studies,59,78,81,126 treatment emergent (%) | Sorafenib, two studies,88,101 treatment related (%) | |
| AE dose interruptions | 74a | 82a | NR |
| AE dose reductions | 66a | 42 to 100a | 47 to 52b/47 to 55b,c |
| AE discontinued | 2 to 26b | 23a | 20a |
| Other | AEs that led to lenvatinib withdrawal and occurred in ≥ 3.5% patients in Study 201:
|
Two out of three patients with a PR withdrew from the study after 5–7 months of treatment in one study 79% of patients required a dose reduction by one dose level to 400 mg daily and one-third of these patients underwent a further reduction to the lowest dose level of 400 mg on alternate days in one study |
|
| Parameter | Observational study, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305a | Chen et al. | Duntas et al. | Kloos et al.a | Study 12791 | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 |
| AE dose interruptions | 43 (74) | NR | 28 (82) | NR | NR | NR | NR | NR |
| AE dose reductions | 38 (66) | NR | NR | 14 (47) | 0 | 11 (100) | 29 (52) |
3 months: 13 (42) 6 months: 15 (52) 12 months: 18 (58) |
| AE discontinued | 15 (26) | 1 | NR | 6 (20) | NR | NR | NR | 7 (23) |
| Other | AEs that led to lenvatinib withdrawal and occurred in at least two patients were:
|
79% of patients required a dose reduction by one dose level to 400 mg daily and one-third of these patients underwent a further reduction to the lowest dose level of 400 mg on alternate days | Terry et al. 2013137 later reported 30 (55) dose reductions (n = 55) | 2/3 with a PR withdrew from the study after 5 to 7 months of treatment | ||||
| Parameter | Observational study, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 201 | Study 208 | Study 12636 | UPCC-03305 | Chen et al. | Duntas et al. | Kloos et al. | Study 12791 | |
| Intervention | Lenvatinib | Lenvatinib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib |
| Number of patients | RR-DTC: 58 |
All: 51 RR-DTC: 25 |
All: 34 RR-DTC: 19 |
All: 55 RR-DTC: 47 |
RR-DTC: 9 | RR-DTC: 11 |
All: 56 RR-DTC: 52 |
RR-DTC: 31 |
| Laboratory AEs | Clinically important changes in mean vital signs from baseline to the end points at various visits were observed. Blood pressure changes occurred and were reported as AEs if deemed clinically important by the investigator. Lenvatinib treatment was correlated with an increase in blood pressure |
Liver abnormalities were common (32% of patients experiencing a grade 1/2 transaminitis; 15% of patients developed grade 3 amylasaemia) but no patients developed acute pancreatitis Lipase levels were found to be raised in 22% of patients, half of which were grade ≥ 3 12% of patients developed an elevated TSH. As all patients were on thyroxine (T4) replacement therapy and asymptomatic, this was interpreted as subclinical hypothyroidism corrected by increasing the T4 dose |
There was a marked and rapid change in the serum thyroglobulin level after start of treatment, with a mean decrease of 60% within 12 weeks, consistent with radiographic findings | Tg level was variably decreased by up to 85% | Although dramatic sustained decreases in serum Tg levels were observed in some patients with PRs and stable disease, neither baseline Tg nor Tg response consistently correlated with degree or duration of objective response | Tg response reflected the radiological response; patients with a PR had a median decrease in their serum Tg levels. Patients with stable or progressive disease showed an increase in their serum Tg levels | ||
| Timing of AEs | Most of the increases in blood pressure were observed in the first cycle. Downwards trends in both systolic and diastolic blood pressure were observed after an increase, mainly owing to treatment with antihypertensive medications and/or dose interruption or reduction316 |
From Terry et al. 2013137 (n = 55): The severity of skin toxicity peaked by cycle 1 for rash and cycle 2 for HFSR. The severity improved dramatically for rash by cycle 3 and for HFSR by cycle 6. Our data support the close supervision of skin-related AEs in the first six cycles of treatment with sorafenib. However, the sustained high prevalence of rash and HFSR requires that all patients receive ongoing skin care for the duration of therapy |
The majority of AEs were seen in the first year of treatment and were controllable with dose reduction, medication, or supporting measures (i.e. dietary consultation and additional feeding)126 | |||||
| Other | The authors316 concluded that lenvatinib had an acceptable safety profile for patients with refractory thyroid cancer and no new safety concerns were observed | The authors207 state:Toxicities were manageable with dose modifications | The authors59 state:This study demonstrates that sorafenib is tolerable at reduced doses over prolonged periods of time in patients with thyroid cancer. Sorafenib leads to radiological and biochemical stabilisation of disease in the majority of these patients despite dose reductions | The authors137 state:Our data support the close supervision of skin-related AEs in the first six cycles of treatment with sorafenib. However, the sustained high prevalence of rash and hand-foot syndrome requires that all patients receive ongoing skin care for the duration of therapy | The authors78 state:Prospective controlled randomised studies with more patients and longer observation times are greatly needed | The authors81 state:However, the aggressiveness of disease in some patients implies that targeted therapy should take into account biomarkers and consider combinations with other TKIs or with mTOR inhibitors, adapting the dose, to enhance tolerability and response | The authors101 state:Sorafenib is a reasonably well-tolerated therapy with clinical and biologic antitumour activity in metastatic PTC | The authors126 concluded:Toxicity was consistent with other sorafenib trials |
Appendix 9 Ongoing studies (summary)
| Parameter | Study | |||
|---|---|---|---|---|
| E7080–G000–21 (Study 211) | E7080–C086–308 (Study 308) | MATiSSe | NEXAVAR-TC-01 (Study 17391) | |
| Description | Postmarketing safety study of lenvatinib (Study 211) | Lenvatinib for RR-DTC in China | A pilot study evaluating the safety and efficacy of sorafenib | Postmarketing safety study of sorafenib |
| Sponsor | Eisai Ltd | Eisai Ltd | Royal Marsden NHS Foundation Trust | Bayer HealthCare |
| Commencement date | 28 March 2016 | 7 February 2017 | Ethics approval, 8 January 2007 | 27 June 2014 |
| Expected end date | 30 October 2020 | April 2020 | NR | 30 June 2021 |
| Participants | 161 patients with RR-DTC | 150 patients with RR-DTC | 33 patients with RR-DTC or MTC | 443 patients with RR-DTC |
| Outcomes |
|
|
|
|
Appendix 10 Additional tables summarising key features of the companies’ economic models
| Parameter | Model costs (£) | |
|---|---|---|
| Eisai Ltd | Bayer HealthCare | |
| Pre-progression | ||
| Response | 280.61 | – |
| Stable disease | 297.98 | – |
| Sorafenib and lenvatinib | – | Commercial in confidence |
| Placebo/BSC | – | Commercial in confidence |
| Progressive disease/post progression | 1315.56 | Commercial in confidence |
| Parameter | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Eisai Ltd (lenvatinib) | Bayer HealthCare (sorafenib) | |||||||
| Frequency of grade 3 to 4 AE hospitalisations (%) | Hospitalisation costs (£) | Rate of grade 3 and 4 AEs (per 28 days) (%) | Cost per patient per 28 days (£) | |||||
| Lenvatinib | Sorafenib | Lenvatinib | Sorafenib | Placebo/BSC | Grade 3 | Grade 4 | ||
| Hypertension | 3.5 | 0.79 | 850.67 | 3.55 | 0.76 | 0.43 | 158 | 65.06 |
| Weight decrease | 0.40 | 0.19 | 639.83 | 0.67 | 0.58 | 0.19 | 345 | – |
| Diarrhoea | 0.40 | 0.28 | 571.30 | 0.55 | 0.55 | 0.13 | 223 | 102 |
| Decreased appetite | 0.40 | 0.00 | 639.83 | – | – | – | – | – |
| Hypocalcaemia | 0.40 | 0.69 | 615.83 | 0.18 | 0.72 | 0.30 | 9 | 9 |
| Hypokalaemia | 0.00 | 0.00 | 615.83 | – | – | – | – | – |
| Asthenia | 0.00 | 0.00 | 658.83 | – | – | – | – | – |
| Fatigue | 0.00 | 0.00 | 658.83 | 0.64 | 0.48 | 0.18 | 61 | 74 |
| Hand–foot syndrome | 0.00 | 1.40 | 450.35 | 0.23 | 1.64 | – | 155 | – |
| Proteinuria | 0.40 | 0.19 | 778.67 | – | – | – | – | – |
Appendix 11 The NICE reference case checklist (summary)
| Attribute | Reference case | Erdal et al. 2015163 | Huang et al. 2016158,159 | Tremblay et al. 2016160 | Wilson et al. 2017161 | SMC 201548 | SMC 201638 | CADTH 20155 | CADTH 2016162 |
|---|---|---|---|---|---|---|---|---|---|
| Decision problem | The scope developed by NICE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Comparator(s) | As listed in the scope developed by NICE | ✓/✗ | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓/✗ | ✓/✗ |
| Perspective costs | NHS and PSS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Perspective benefits | All direct health effects, whether for patients or carers | ✓/✗ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Time horizon | Long enough to reflect all important differences in costs or outcomes | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Synthesis of evidence on outcomes | Based on systematic review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Outcome measure | Health effects should be expressed in QALYs (EQ-5D preferred) | ✓ | NR | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Health states for QALY | Reported directly by patients and/or carers | ✓ | NR | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Benefit valuation | Time trade-off or standard gamble | ✓ | NR | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | NR | NR | ✓ | ✗ | NR | ✗ | NR | ✗ |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | NR | ✗ | ✓ | ✓ | NR | NR | NR | NR |
Appendix 12 The Drummond checklist (summary)
| Question | Publication | |||||||
|---|---|---|---|---|---|---|---|---|
| Erdal et al. 2015163 | Huang et al. 2016158,159 | Tremblay et al. 2016160 | Wilson et al. 2017161 | SMC 201548 | SMC 201638 | CADTH 20155 | CADTH 2016162 | |
| Was a well-defined question posed in answerable form? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was a comprehensive description of the competing alternatives given? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was the effectiveness of the programme or services established? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were all the important and relevant costs and consequences for each alternative identified? | ✓ | Unclear | ✓ | ✓ | ✓ | ✓ | Unclear | ✓ |
| Were costs and consequences measured accurately in appropriate physical units? | Unclear | Unclear | ✓ | ✓ | Unclear | ✓ | Unclear | ✓ |
| Were the cost and consequences valued credibly? | Unclear | Unclear | ✓ | ✓/✗ | Unclear | ✓ | Unclear | ✓/✗ |
| Were costs and consequences adjusted for differential timing? | Unclear | ✓ | ✓ | ✓ | Unclear | Unclear | Unclear | Unclear |
| Was an incremental analysis of costs and consequences of alternatives performed? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was allowance made for uncertainty in the estimates of costs and consequences? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Did the presentation and discussion of study results include all issues of concern to users? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Appendix 13 The NICE reference case checklists in full
| Attribute | Reference case | Does the economic evaluation match the reference case? (Erdal et al. 2015163) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Partial – sorafenib is compared with BSC but not to lenvatinib |
| Perspective: costs | NHS and PSS | Turkish payer’s perspective taken |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Partial – patient related direct health effects were considered |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – lifetime horizon |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily taken from DECISION |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs and based on EQ-5D data collected in DECISION |
| Health states for QALY | Reported directly by patients and/or carers | Yes – reported directly by patients in DECISION |
| Benefit valuation | Time trade-off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Does not state in abstract which valuation set is used for the EQ-5D estimates of utility |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Not stated |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | Sensitivity analysis was conducted, but no details of the methods used were reported |
| Attribute | Reference case | Does the economic evaluation match the reference case? (Huang et al. 2016158,159) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Yes – lenvatinib vs. sorafenib and both drugs vs. placebo. The placebo evidence is derived from the Phase III trials; the AG assumes placebo and BSC are equivalent comparators |
| Perspective: costs | NHS and PSS | US perspective. The authors states that direct medical costs were used, but some costs were sourced from Medicare Fee Schedule, which reflects tariffs rather than direct costs |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Partial – patient-related direct health effects were considered, although source and values were not reported in the abstract |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – lifetime horizon |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from DECISION and SELECT |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Authors state the utility values were taken from published sources, but it is unclear which measurement tools were used as the published sources were not referenced |
| Health states for QALY | Reported directly by patients and/or carers | Unclear |
| Benefit valuation | Time trade-off or standard gamble | Unclear |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Unclear but unlikely to be representative of UK population as the study is set in the USA |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Yes – 3% used |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | Sensitivity analysis was conducted, but no details of the methods used were reported |
| Attribute | Reference case | Does the economic evaluation match the reference case? (Tremblay et al. 2016160) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Yes – lenvatinib vs. sorafenib |
| Perspective: costs | NHS and PSS | US perspective |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – 5-year and 10-year results reported |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from DECISION and SELECT |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | QALYs – not EQ-5D |
| Health states for QALY | Reported directly by patients and/or carers | UK general population |
| Benefit valuation | Time trade-off or standard gamble | Neither |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Yes |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Yes – 5% (details provided by lead author) |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | Sensitivity analysis was conducted, but no details of the methods used were reported |
| Attribute | Reference case | Does the economic evaluation match the reference case? (Wilson 2017161) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Yes |
| Perspective: costs | NHS and PSS | US health-care perspective |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – lifetime |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from SELECT and DECISION |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs |
| Health states for QALY | Reported directly by patients and/or carers | No – utility is estimated from a vignette study generated from a sample of the general UK population in which participants were asked to value health-state scenarios they were presented with |
| Benefit valuation | Time trade-off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Yes – 3% |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | Yes |
| Attribute | Reference case | Does the economic evaluation match the reference case? (SMC 201548) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Partial – sorafenib is compared with BSC but not with lenvatinib |
| Perspective: costs | NHS and PSS | NHS Scotland |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – time horizon up to 10 years |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily taken from DECISION |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs and taken from EQ-5D data collected in DECISION |
| Health states for QALY | Reported directly by patients and/or carers | Yes – reported directly by patients in DECISION |
| Benefit valuation | Time-trade off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Does not state which valuation set is used for the EQ-5D estimates of utility |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Not stated |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | One-way parameter sensitivity analysis conducted, but no mention of PSA |
| Attribute | Reference case | Does the de novo economic evaluation match the reference case? (SMC 201638) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Yes |
| Perspective: costs | NHS and PSS | NHS Scotland |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – time horizon up to lifetime |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from DECISION and SELECT |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs |
| Health states for QALY | Reported directly by patients and/or carers | No – utility is estimated from a vignette study generated from a sample of the general UK population in which participants were asked to value health-state scenarios they were presented with |
| Benefit valuation | Time trade-off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Not applicable |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Not stated |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | One-way parameter sensitivity analysis was conducted, but there was no mention of PSA in the publication |
| Attribute | Reference case | Does the economic evaluation match the reference case? (CADTH 20155) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Partial – sorafenib is compared with BSC but not with lenvatinib |
| Perspective: costs | NHS and PSS | Canadian health-care perspective |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – up to 10 years |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from DECISION |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs and based on the EQ-5D data collected in DECISION |
| Health states for QALY | Reported directly by patients and/or carers | Yes – reported directly by patients in DECISION |
| Benefit valuation | Time trade-off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Does not state in the abstract which valuation set is used for the EQ-5D estimates of utility |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Not stated |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | One-way parameter sensitivity analysis was conducted, but there is no mention of PSA in the publication |
| Attribute | Reference case | Does the de novo economic evaluation match the reference case? (CADTH 2016162) |
|---|---|---|
| Decision problem | The scope developed by NICE | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Partial – lenvatinib is compared with BSC but not with sorafenib |
| Perspective: costs | NHS and PSS | Canadian health-care perspective |
| Perspective: benefits | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Form of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes – up to 10 years |
| Synthesis of evidence on outcomes | Based on systematic review | Data have been primarily derived from SELECT |
| Outcome measure | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes – health effects were expressed in QALYs |
| Health states for QALY | Reported directly by patients and/or carers | No – utility is estimated from a vignette study generated from a sample of the general UK population in which participants were asked to value health-state scenarios they were presented with |
| Benefit valuation | Time trade-off or standard gamble | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No |
| Discount rate | The same annual rate for both costs and effects (currently 3.5%) | Not stated |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes – all QALYs estimated by the economic model have the same weight |
| Sensitivity analysis | The scope developed by NICE | One-way parameter sensitivity analysis was conducted, but there is no mention of PSA in the publication |
Appendix 14 Drummond checklists in full
| Erdal et al. 2015:163 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from DECISION |
| Were all the important and relevant costs and consequences for each alternative identified? | Yes | Resource use estimates generated from an expert panel |
| Were costs and consequences measured accurately in appropriate physical units? | Unclear | Sources of cost evidence described, but no details of what was measured were reported |
| Were the cost and consequences valued credibly? | Unclear | Not reported |
| Were costs and consequences adjusted for differential timing? | Unclear | Not reported |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were calculated accurately |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | One-way and probabilistic sensitivity analysis were undertaken, but details of the methods and parameters varied were not reported |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| Huang et al. 2016:158,159 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from DECISION and SELECT |
| Were all the important and relevant costs and consequences for each alternative identified? | Unclear | Based on the Phase III trials, but does not report resource use or costs used within the model |
| Were costs and consequences measured accurately in appropriate physical units? | Unclear | Sources of cost evidence described, but no details of what was measured were reported |
| Were the cost and consequences valued credibly? | Unclear | Details of resource use estimates were not reported |
| Were costs and consequences adjusted for differential timing? | Yes | 3% discount rate used |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | One-way and probabilistic sensitivity analyses were undertaken, but details of the methods and parameters that were varied were not reported |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| Tremblay et al. 2016:160 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from DECISION and SELECT |
| Were all the important and relevant costs and consequences for each alternative identified? | Partially unclear | Based on data from the Phase III trials, time trade-off utility values that were taken from the Kerr et al.167 abstract (details provided via correspondence by lead author of paper). Details of resource use and costs were presented in the abstract. Details of discount rates were provided via correspondence with lead author (5%) [Dr Gabriel Tremblay, Purple Squirel Economics (previously at Eisai), June 2017] |
| Were costs and consequences measured accurately in appropriate physical units? | Yes | |
| Were the cost and consequences valued credibly? | Yes | |
| Were costs and consequences adjusted for differential timing? | Yes | % discount rate used for both costs and outcomes obtained through correspondence with lead author |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | PSA was mentioned in the conclusion, but no results or methods were reported |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| Wilson 2017:161 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from data collected in DECISION and SELECT |
| Were all the important and relevant costs and consequences for each alternative identified? | Yes | |
| Were costs and consequences measured accurately in appropriate physical units? | Yes | |
| Were the cost and consequences valued credibly? | Partially | Utility estimates were from a published study rather than directly from the trial population |
| Were costs and consequences adjusted for differential timing? | Yes | |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Incremental cost, QALYs, LYs and ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Parameter and probabilistic sensitivity analyses were conducted |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| SMC 2015:48 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from DECISION |
| Were all the important and relevant costs and consequences for each alternative identified? | Yes | |
| Were costs and consequences measured accurately in appropriate physical units? | Unclear | |
| Were the cost and consequences valued credibly? | Unclear | |
| Were costs and consequences adjusted for differential timing? | Unclear | |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Results of multiple-parameter sensitivity analysis were reported |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| SMC 2016:38 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from DECISION and SELECT |
| Were all the important and relevant costs and consequences for each alternative identified? | Yes | |
| Were costs and consequences measured accurately in appropriate physical units? | Yes | |
| Were the cost and consequences valued credibly? | Yes | |
| Were costs and consequences adjusted for differential timing? | Unclear | |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Scenario and sensitivity analysis was completed |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| CADTH 2015:5 question | Critical appraisal | AG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Not detailed in the report but effectiveness data were derived from DECISION |
| Were all the important and relevant costs and consequences for each alternative identified? | Unclear | Not reported |
| Were costs and consequences measured accurately in appropriate physical units? | Unclear | |
| Were the cost and consequences valued credibly? | Unclear | |
| Were costs and consequences adjusted for differential timing? | Unclear | |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Results of several sensitivity analyses were presented |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
| CADTH 2016:162 question | Critical appraisal | ERG comment |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | |
| Was a comprehensive description of the competing alternatives given? | Yes | |
| Was the effectiveness of the programme or services established? | Yes | Outcomes from data collected in DECISION and SELECT |
| Were all the important and relevant costs and consequences for each alternative identified? | Yes | |
| Were costs and consequences measured accurately in appropriate physical units? | Yes | |
| Were the cost and consequences valued credibly? | Partially | From a published study168 rather than directly from the trial population |
| Were costs and consequences adjusted for differential timing? | Unclear | |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Incremental cost, QALYs, LYs and ICERs were reported |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Parameter sensitivity analysis was conducted |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes |
List of abbreviations
- AE
- adverse event
- AG
- Assessment Group
- ANOVA
- analysis of variance
- AUC
- area under the curve
- BNF
- British National Formulary
- BSC
- best supportive care
- BTA
- British Thyroid Association
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CDF
- Cancer Drugs Fund
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CT
- computerised tomography
- DECISION
- StuDy of sorafEnib in loCally advanced or metastatIc patientS with radioactive Iodine-refractory thyrOid caNcer
- DTC
- differentiated thyroid cancer
- ECOG
- Eastern Cooperative Oncology Group
- EMA
- European Medicines Agency
- EPAR
- European Public Assessment Report
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ESMO
- European Society for Medical Oncology
- FACT-G
- Functional Assessment of Cancer Therapy – General
- FDA
- US Food and Drug Administration
- FDG
- fludeoxyglucose
- FTC
- follicular carcinoma
- HCC
- hepatocellular carcinoma
- H–H
- cumulative hazard versus cumulative hazard
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IPE
- iterative parameter estimation
- ITC
- indirect treatment comparison
- ITT
- intention to treat
- K–M
- Kaplan–Meier
- MAIC
- matching-adjusted indirect comparison
- MKI
- multikinase inhibitor
- MRI
- magnetic resonance imaging
- MTA
- multiple technology appraisal
- NCCN
- National Comprehensive Cancer Network
- NICE
- National Institute for Health and Care Excellence
- ORR
- objective tumour response rate
- OS
- overall survival
- PAS
- Patient Access Scheme
- PFS
- progression-free survival
- PH
- proportional hazard
- PPS
- postprogression survival
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PS
- performance status
- PSA
- probabilistic sensitivity analysis
- PTC
- papillary carcinoma
- QALY
- quality-adjusted life-year
- RCC
- renal cell carcinoma
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumours
- RPSFTM
- rank-preserving structural failure time model
- RR
- relative risk
- RR-DTC
- radioactive iodine-refractory differentiated thyroid cancer
- SAE
- serious adverse event
- SELECT
- Study of [E7080] LEnvatinib in 131I-refractory differentiated Cancer of the Thyroid
- SMC
- Scottish Medicines Consortium
- SmPC
- summary of product characteristics
- T3
- triiodothyronine
- T4
- thyroxine
- TKI
- tyrosine kinase inhibitor
- TSH
- thyroid-stimulating hormone
- VAS
- visual analogue scale
- VEGF
- vascular endothelial growth factor
- VEGFR
- vascular endothelial growth factor receptor
