Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 18/13/01. The protocol was agreed in August 2018. The assessment report began editorial review in March 2019 and was accepted for publication in July 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Disclaimer
The British Medical Journal (BMJ) Technology Assessment Group (BMJ-TAG) and the editorial team of the BMJ work independently of one another. The views and opinions expressed in this report are those of the BMJ-TAG.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Edwards et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background and definition of the decision problem
The scope of this Diagnostic Assessment Report (DAR) is to assess the cost-effectiveness of implantable cardiac monitors (ICMs) to detect suspected paroxysmal atrial fibrillation (AF) in people who have had a cryptogenic stroke (CS). 1 The review compares the diagnostic accuracy, clinical outcomes and costs of three types of ICM with no further testing after at least 24 hours of outpatient external ambulatory electrocardiography, the alternative AF monitoring strategy in UK clinical practice.
Description of the health condition and aetiology
Population: cryptogenic stroke or transient ischaemic attack
Stroke is the third most common cause of premature death in the UK2 and a major cause of preventable disability. 3 Improvements in care have greatly improved mortality and morbidity over the last two decades, but there are still around 30,0002 stroke-related deaths each year in England, and around one-quarter of patients leave hospital with moderate to severe disability. 4
Strokes and transient ischaemic attacks (TIAs) are caused by the interruption of the blood supply to part of the brain, either due to the narrowing or blockage of a blood vessel by a blood clot (ischaemic stroke), or due to a bleed from a blood vessel in the brain (haemorrhagic stroke). The main difference between a stroke and a TIA is that the symptoms caused by damage to brain tissue from a TIA resolve within 24 hours, whereas in an untreated stroke, the symptoms last for longer. Common symptoms of stroke include numbness, weakness or paralysis, slurred speech, blurred vision, confusion and severe headache. 5
The causes of stroke are manifold and include the build-up of plaque in the artery supplying the ischaemic region of the brain (atherosclerosis), occlusion of small arteries deep in the brain (lacunar) and a clot (embolism), which often originates in the heart and travels to a blood vessel in the brain (e.g. as a result of AF). Other less common causes of stroke are tumours in the heart, heart abnormalities, recent myocardial infarction (MI), migraine, malignancy and drug misuse. 6 However, up to one-third of first-time strokes are cryptogenic, meaning no known cause can be identified, which is most common in younger patients. 6 The Evidence Assessment Group’s (EAG’s) clinical experts reported that patients in the UK who have had a stroke will generally undergo a series of tests to identify a cause before the event is classed as cryptogenic, although some definitions include insufficient testing or identification of more than one cause. 6,7 Diagnostic tests to identify the cause of stroke generally include blood tests, inpatient electrocardiography, echocardiography and Doppler ultrasonography of the carotid arteries.
In 1994, the Oxfordshire community stroke project reported a stroke recurrence rate of ≈30% by 5 years and that people are at highest risk of a subsequent stroke in the first year, when mortality rates are also at their highest. 8 However, a systematic review9 from 2011 suggests that there is a temporal reduction in the 5-year risk of stroke recurrence from 32% to 16.2% across its included studies and that risk of stroke recurrence increases over time, with higher rates at 10 years than at 30 days post stroke. It should also be noted that the review authors reported substantial heterogeneity in the included studies and recurrence rates varied depending on the definition of stroke applied. 9 Establishing the cause of a stroke is paramount to decrease the risk of recurrence by selecting appropriate preventative care. 10
Target condition: atrial fibrillation
Atrial fibrillation is an irregular, rapid heart rhythm that can be intermittent or continuous. People with AF may experience heart palpitations, fatigue, dizziness and shortness of breath, but many people do not experience symptoms. 11 An estimated 1.4 million people in England have AF (approximately 2.5% of the population), and it is estimated that 425,000 people are undiagnosed, making it the most common arrhythmia. 12 The prevalence of AF is higher in men than in women (2.9% vs. 2.0%) and increases with age, with 80.5% of cases in people aged > 65 years. 12,13
The intermittent nature of paroxysmal AF can make diagnosis with short-term electrographic monitoring problematic because patients having infrequent episodes may not experience one during the monitoring. Asymptomatic AF can also remain undiagnosed unless a patient develops symptoms or is monitored incidentally for another reason or during a hospital stay. If AF is suspected, the likelihood of detecting asymptomatic paroxysmal AF increases with duration of monitoring or with repeated monitoring strategies. 14,15
People with AF have a fivefold higher risk of having a stroke or TIA than people without AF. 13,16 The irregular heart rhythm means the heart can fail to empty properly and the remaining blood can form a clot. Stroke or TIA can occur if the clot moves and narrows or blocks the arteries supplying blood flow to the brain (embolic stroke). Although the relationship between AF and stroke is established, there has been some debate regarding the temporal relationship between them, with some studies suggesting that AF acts as a marker of atrial dysfunction, rather than as a direct cause of stroke. 6,17,18 The EAG’s clinical experts advised that AF detected > 2 years post stroke may not be related to the index event, although its management is still likely to be the same and the patient would be considered for treatment with a long-term oral anticoagulant (OAC). Clinical experts also reported that it is thought that up to half of all recurrent strokes may be due to an unrelated mechanism to that of the index event. Clinical experts also reported that there is no consensus on the duration of AF required prior to the commencement of an OAC and that the ICM devices have varying programmable thresholds for the detection of AF, for example 30 seconds, 2 minutes, 6 minutes. Clinical experts suggested that commencement of an OAC for AF of any duration in a CS patient should be considered because of the risk of recurrent stroke, although how beneficial anticoagulation is for AF detected at varying time points after a CS is unknown and is beyond the scope of this review.
Current pathway of care
The EAG’s clinical experts reported that there is no standard guideline on the diagnostic tests required in the UK to further investigate patients who have had a CS or TIA for underlying AF and there is no consensus on the duration or mode of monitoring for AF. The National Institute for Health and Care Excellence (NICE)’s guideline on stroke and TIA in those aged > 16 years (NICE guideline 128)5 was updated in May 2019 and provides no specific recommendations on the diagnosis of AF in people who have had an acute stroke.
The NICE guideline on AF19 recommends that people with asymptomatic suspected paroxysmal AF undetected by standard electrocardiography recording have a 24-hour ambulatory electrocardiographic monitor, although this recommendation is not specific for patients with CS or TIA. The European Society of Cardiology Guidelines20 for the management of AF recommend that patients with ischaemic stroke (IS) or TIA are investigated for AF using a short-term electrocardiography recording and then continuous electrocardiographic monitoring for a minimum of 72 hours.
The EAG’s clinical experts reported that patients with a CS or TIA diagnosis will typically have short-term electrocardiography as an inpatient to detect cardiac arrhythmias, such as AF, as part of the standard suite of diagnostic tests to identify the cause of stroke or TIA. Patients with no AF during inpatient monitoring will often receive outpatient external ambulatory electrocardiographic monitoring for 24–48 hours (e.g. using a Holter monitor). Clinical experts reported that, in some areas, this may be extended to 2 weeks or even 30 days of monitoring depending on local practices and patient–clinician preferences. Clinical experts reported that ICMs are not routinely used in UK clinical practice for AF detection after CS or TIA and that they are likely to be used in the NHS only after patients have received an initial period of at least 24 hours’ external ambulatory monitoring.
Patients with AF detected after stroke or TIA can be treated to reduce the risk of a further stroke. NICE recommendations19 for stroke prevention therapy include rate or rhythm control and anticoagulation based on bleeding risk and CHA2DS2-VASc score {Congestive heart failure (or left ventricular systolic dysfunction), Hypertension [blood pressure consistently > 140/90 mmHg (or treated hypertension on medication)], Age ≥ 75 years (doubled), Diabetes mellitus, prior Stroke or transient ischaemic attack or thromboembolism (doubled), Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque), Age 65–74 years, Sex category (e.g. female)}. CHA2DS2-VASc is measure of stroke risk in patients with AF based on age; sex; and history of congestive heart failure, stroke or TIA, vascular disease and diabetes. 21 Patients with prior stroke or TIA have a minimum CHA2DS2-VASc score of 2 and automatically qualify for anticoagulation according to current NICE guidance,19 regardless of the presence of other stroke risk factors. The NICE pathway for preventing stroke in people with AF22 recommends anticoagulation with apixaban (Eliquis®, Bristol-Myers Squibb Company, New York, NY, USA), dabigatran etexilate (Pradaxa®, Boehringer Ingelheim, Ingelheim am Rhein, Germany), edoxaban (Lixiana®, Daiichi Sankyo Company Ltd, Tokyo, Japan), rivaroxaban (Xarelto®, Bayer AG, Leverkusen, Germany) or a vitamin K antagonist, and the NICE guideline for AF management19 recommends review at least annually, and recommends against aspirin monotherapy. If anticoagulation is contraindicated because of bleeding risk, NICE recommends rate or rhythm control measures, annual review to assess stroke and bleeding risk, and consideration for left atrial appendage occlusion. 19 Clinical experts reported that patients who have had a CS and are diagnosed with AF during follow-up with an ICM are most likely to have paroxysmal AF, for which the management would usually be anticoagulation. Clinical experts also reported that patients identified in advance as being unsuitable for anticoagulation, for example because of their risk of bleeding, may not receive an ICM. However, clinical experts also reported that some patients diagnosed with AF may receive a left atrial appendage occlusion device as an alternative to OAC therapy.
Description of the technologies under assessment
Implantable cardiac monitors, also known as insertable cardiac monitors or implantable loop recorders, are small devices inserted beneath the skin of the chest. The devices allow extended monitoring and automatic recording of heart rhythm. The devices are inserted under local anaesthetic via a small incision and capture continuous electrocardiograms (ECGs) to detect various arrhythmias, including AF. ICMs are currently used in the NHS primarily as a method of monitoring patients experiencing syncope (fainting) to detect and treat underlying arrhythmias. The devices offer the possibility of continuous rhythm monitoring of people who have had a CS or TIA to increase the detection of intermittent or paroxysmal AF to help guide appropriate treatment for secondary stroke prevention.
The devices are usually inserted by cardiologists, cardiac physiologists and nursing staff in a sterile environment such as a catheterisation laboratory (hereafter referred to as cath lab), but clinical experts report that there is variation across devices and with the ICM experience of the service in which the patient is being treated. Devices can be explanted once an arrhythmia has been detected or at the end of the battery life, but can also be left in situ. Adverse events (AEs) are rare, but can include infection or reaction at the insertion site, bleeding, excessive fibrotic tissue growth, extrusion, hematomas or cysts, keloid formation, and erosion or migration of the device.
Once implanted, the devices automatically capture continuous ECGs, and record and transmit detected arrhythmia episodes for clinical review. Recording of episodes can also be activated manually by the patient if symptoms occur using optional external handheld patient devices or smartphone applications (hereafter referred to as ‘apps’), depending on the ICM. Detection parameters, data storage, method of data transmission and notification settings vary by device (Table 1), but all have capabilities to recognise a range of arrhythmias and alert clinicians when an episode is detected. Data are transmitted via internet or cellular networks and encrypted for online storage. Clinical experts reported that programming of ICMs in relation to use of inbuilt automatic programmes varies depending on the patient characteristics and clinician preference. The clinical experts reported that, often, the ICMs’ standard setting for arrhythmia detection in CS patients is used to start with and this is then adjusted as necessary. The clinical experts also reported that the patient activator device is generally of little benefit if used in CS patients, as they are generally asymptomatic in terms of AF and other cardiac arrhythmias.
| Device features | In scope1 | Not in scope | ||
|---|---|---|---|---|
| BioMonitor 2-AF™ (Biotronik SE & Co. KG, Berlin, Germany) | Confirm Rx™ (Abbott Laboratories, Lake Bluff, IL, USA) | Reveal LINQ™ (Medtronic plc, Minneapolis, MN, USA) | Reveal XT | |
| Standard components |
|
|
|
|
| Cost of device (£) | 1030 | 1600 | 1800 | N/A |
| ICM dimensions (mm) and weight (g) |
|
|
|
|
| Insertion procedure | Commonly by cardiologist (± assistant) in cath lab; nurse- or physician-led insertion increasing | Commonly by cardiologists, cardiac physiologists and nursing staff in a cath lab | By cardiologists, cardiac physiologists and nursing staff in a cath lab, although company submission reported that ‘out-of-laboratory’ procedures are possible | By cardiologists, cardiac physiologists and nursing staff in cath lab |
| Patient activation | Optional hand-held patient assistant available | Integrated™ in myMerlin app | Patient assistant device as standard | Patient assistant device – 1- and 2-button models available |
| Detection and sensing parameters | Adjustable or pre-set functions to detect various AF characteristics, high ventricular rate, bradycardia, sudden rate drop and asystole | AF (regularity, R–R variance and sudden onset), brady arrhythmias, tachy arrhythmias, pauses, TLoC conditions, epilepsy exclusion | Atrial tachyarrhythmia (including atrial flutter/AF) (exclusive algorithm) P-wave morphology discriminator algorithm, bradyarrhythmia, ventricular tachyarrhythmia, pause episodes | Atrial tachyarrhythmia/AF (exclusive algorithm), bradyarrhythmia, asystole, ventricular tachyarrhythmia |
| Device storage | 55 automatically detected episodes and four patient-activated episodes (total duration of 60 minutes) | Up to 250 AF episodes plus 250 auto-activated and patient-activated episodes (total duration of 60 minutes) | 14 months of daily time spend in AF (AF burden), 27 minutes of automatically detected episodes, 2 minutes of the longest AF episode, 30 minutes of symptomatic patient-activated episodes | 27 minutes of automatic detections and 22.5 minutes of patient activation |
| Telemetry | Daily message to Home Monitoring Service Centre via cellular phone network | Via app to Merlin.net PCN, accessed by clinicians | Via myCareLink Patient Monitor to a CareLink server using a cellular telephone connection network | Via CareLink programmer to CareLink server |
| Clinician notification | Alerts via e-mail, SMS or fax | E-mail/SMS alerts through website. Auto follow-up via app monthly | Alerts via cellular telephone connection network | Follow-up via the Programmer at pre-set intervals or programmable notification on detection |
| Estimated battery life (years) | 4 | 2 | 3 | 3 |
| Additional features | – |
|
|
– |
Characteristics of the three ICMs included in the NICE scope1 – BioMonitor 2-AF23 Confirm Rx24 and Reveal LINQ25 – are summarised in Table 1. The EAG has also included information about the Reveal XT device, which is an earlier Medtronic model, because it was the device used in the only randomised controlled trial (RCT) identified in the clinical evidence search. Earlier Biotronik and Abbott ICM models are also available, but have not been included because no relevant evidence in the CS or TIA population was submitted by the companies, and the capabilities of these earlier models were not considered relevant to the decision problem. However, it should be noted that some data on the Confirm DM202 in a non-CS population is discussed in Chapter 3, in the absence of data on the Confirm Rx in a CS or non-CS population.
BioMonitor 2-AF
The BioMonitor 2-AF ICM is supplied with programmer and software specific to the device, together with a tool designed to facilitate insertion of the ICM. 23 An optional extra accessory is the Remote Assistant, which enables the patient to trigger recording of heart rhythm. The BioMonitor 2-AF comprises a solid housing section and a flexible component, which is the lead body and carries the antenna for Home Monitoring. Only the BioMonitor 2-AF is included in the scope of this review because information provided by the company indicate that other models, such as the BioMonitor 2-S, do not have functionality for AF detection.
During implantation, the standard program is activated in the BioMonitor 2-AF via the programmer, which is used to set parameter combinations, and for interrogation and saving of data from the device. The parameters in the sensing settings, such as high-pass filter, target sensing threshold or noise window, can be adjusted to individual patients. Alternatively, standard and preconfigured settings are available, all contained in the SensingConsult program. The signals are automatically recorded and stored once a detection type is activated and the detection occurs; multiple detection types can be activated simultaneously.
With Home Monitoring, diagnostic information, as well as technical data of the ICM, are automatically and wirelessly sent to a stationary or mobile transmitter via the antenna in the lead body. The data are encrypted and sent from the transmitter to the Biotronik Home Monitoring Service Centre via the cellular phone network. The received data are deciphered and evaluated. Clinicians can set the criteria for evaluation to be used for each patient and can configure the time of notification via e-mail, short message service (SMS) or fax. An overview of the results of the analysis is displayed on the protected internet platform Home Monitoring Service Centre. Data are transmitted with a daily device message. Messages that indicate an arrhythmia episode or a problem with the device are forwarded to the patient’s clinician at a pre-set time, and a test message can be initiated by the programmer at any time to check the Home Monitoring function.
A total of 55 individual episodes with a length of at least 40 seconds each can be stored automatically. The device can store four recordings triggered by the patient (using the optional patient Remote Assistant device), each with a duration of at least 7.5 minutes. The recording includes 7 minutes of pre-episode history and 0.5 minutes of post-episode history relative to the time of triggering. The maximum recording duration for an individual episode is 10 minutes. The BioMonitor 2-AF can store multiple subcutaneous ECGs, up to a total duration of ≥ 60 minutes. It is reported by Biotronik that the BioMonitor 2-AF has a battery life of 4 years, which is the longest battery life of the three ICMs under review in this DAR.
Confirm RX
The Confirm Rx (developed by St Jude Medical, which was acquired by Abbott) is designed to detect arrhythmias and wirelessly transmit data to the Merlin.net Patient Care Network. 24 The Confirm Rx ICM comprises internal and external components. The physical ICM unit constitutes the internal portion of the ICM system. The Merlin Patient Care System with software version 23.0 (or later), magnet, myMerlin mobile app and Merlin.net Patient Care Network constitute the external components of the system. The Merlin Patient Care System and magnet are used to interrogate and program the device in the clinic, and remote transmissions are performed using the associated smartphone app. The app also allows patients to record and send ECGs of symptomatic events to the clinic without the need for an additional patient activator device, which is required with some other ICM devices (e.g. Reveal LINQ and BioMonitor 2-AF).
The ICM has a CS programmable setting in which certain device parameters are automatically programmed to detect and record arrhythmias in CS patients. The detection algorithms combine regulatory, variance and sudden-onset measures to recognise and trigger an alert for AF. Clinicians can choose fixed settings or program parameters, including episode duration threshold, AF burden alerts and storage of pre- and post-AF recordings. All remotely transmitted data are made available on Merlin.net, where clinicians can log in, review data and make a diagnosis. Additional accessories include specialised tools for incision and insertion of the device. The company reports that the battery life of the Confirm Rx is 2 years, although this is based on the assumption of an average of one auto-detected episode per day, one patient-activated symptom episode per month and up to 6 months’ shelf storage time prior to implantation. 26
Information provided by the company included physical specifications and a list of warnings and precautions, including physician training and insertion procedures. Additional information about the detection capabilities were provided by the company on request (Abbott Laboratories, 2018, personal communication) (see Table 1).
The EAG notes, from literature available on the company website,27 that there were two earlier models of ICM released by St Jude Medical: (1) the SJM Confirm™ DM2100 and (2) the SJM Confirm™ DM2102. The model under review in this DAR is the Confirm RX™ DM3500; the EAG is unclear how this differs to the earlier models. The EAG requested clarification from the company, which reported that the DM2102 is a pacemaker-sized device that requires a larger incision and cath lab or pacing suite facilities for insertion by a cardiologist. The company also reported that the DM3500 is the Confirm Rx, and that this is a much smaller device that is injectable; requires only clean facilities, such as a side room; and can be inserted by a specialist nurse or cardiac physiologist. Owing to the absence of clinical data on the Confirm Rx DM3500, the EAG reports some data in Chapter 3 from a clinical study relating to the SJM Confirm DM2102.
Reveal LINQ
The Reveal LINQ™ Insertable Cardiac Monitoring System consists of a Reveal LINQ ICM, Patient Assistant, MyCareLink Programmer and remote monitoring system (MyCareLink Patient Monitor and MyCareLink network). The Reveal LINQ ICM kit also includes tools tailored to facilitate insertion of the device. The Reveal XT is an earlier and larger Medtronic ICM model that has AF detection functionality for patients who have had a CS. Clinical expert opinion and evidence from a mixed population suggest that the Reveal LINQ has better specificity than the XT (see Chapter 3), is easier to implant and leads to fewer complications due to its size, and that AF detection accuracy between the devices is similar. 28
Medtronic highlighted that the size of Reveal LINQ differentiates it from other devices and means that a smaller incision in the skin is required (< 1 cm). Clinical experts at the NICE scoping workshop reported that the procedure can be done by health-care professionals other than cardiologists (e.g. cardiac physiologists, nurses, neurologists or stroke physicians) and in a procedure room rather than a cath lab. Training in inserting the device is provided by the Medtronic field team and is also available online. Medtronic also offer a monitoring service (FocusOn) to interpret and triage electrocardiographic recordings made by the device before a patient’s clinician is notified.
The device can be programmed by placing the Medtronic CareLink™ programmer head over the device and there are pre-programmed settings that the EAG’s clinical experts reported are generally used for patients with CS. Electrocardiographic recordings for episodes of AF are stored, although the device uses a detection window of 2 minutes in its algorithm for AF detection; therefore, the ICM cannot reliably detect AF episodes of < 2 minutes. The ICM can be programmed to store only episodes of AF exceeding a set threshold (all episodes, 6, 10, 20, 30 or 60 minutes), although the default setting in CS would be to store all detected episodes of AF. Total AF burden can be calculated, and tachyarrhythmia, bradyarrhythmia and pause episodes can also be detected. The battery-operated and hand-held Patient Assistant device allows the patient to press a button to trigger a recording in the event of symptoms (e.g. onset of loss of consciousness or palpitations).
The battery life of the device is estimated by the company to be 3 years with average use assumptions (one auto-detected episode per day and one patient-activated episode per month). As for the other devices, it is for single-patient use and, although it does not need to be removed, the company recommend doing so if it is no longer needed. The ICM can store up to 27 minutes of ECGs from arrhythmias detected automatically and up to 30 minutes from patient-activated episodes. The device also contains an accelerometer to allow changes in patient activity over time to be monitored.
Rhythm abnormalities recorded by the Reveal LINQ ICM are wirelessly transmitted to the MyCareLink Patient Monitor and then sent to a CareLink server in the Netherlands using a cellular telephone connection network. Transmitted and stored data are encrypted. A care alert is sent to clinicians when the device detects a rhythm abnormality, and clinicians can access the data through the CareLink website using a password protected log-in. Alternatively, daily notifications of cardiac activity can be sent. The device will also send alerts if the battery charge is low, and the device will register as ‘disconnected’ if it is unable to communicate with CareLink.
Comparators and the reference standard
The diagnostic accuracy and clinical outcomes of ICMs are considered for patients who have had a CS or TIA in whom no AF has been detected following a minimum of 24 hours of external electrocardiographic cardiac monitoring. The clinical outcomes for ICMs (after a minimum of 24 hours of external electrocardiographic monitoring) will be compared with no further monitoring (also after a minimum period of 24 hours of external electrocardiographic monitoring). The diagnostic test accuracy (DTA) of the ICMs will be compared with 24-hour external ambulatory electrocardiographic monitoring or other commonly used electrocardiographic monitoring regimens, such as 7-day Holter monitoring, which is the reference standard. External electrocardiographic monitoring is most commonly conducted with a Holter monitor, a portable battery-operated device that records continuous ECGs, usually for 24–48 hours, via electrodes that attach to the skin.
Chapter 2 Methods for assessing clinical effectiveness
A systematic literature review was conducted to evaluate the clinical effectiveness of the Reveal LINQ insertable cardiac monitor,25 the BioMonitor 2-AF ICM23 and the Confirm Rx ICM24 for detecting suspected asymptomatic AF after CS, and the diagnostic accuracy of these three ICMs for the diagnosis of AF.
The systematic review methods follow the general principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for conducting reviews in health care,29 the NICE Diagnostics Assessment Programme manual30 and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 31 The protocol32 for this review is registered on PROSPERO as CRD42018109216.
Eligibility criteria
Study populations eligible for inclusion in the review of clinical effectiveness were those comprising people who had had a cryptogenic embolic stroke or cryptogenic TIA for whom there was a suspicion of paroxysmal AF. In the protocol32 it was specified that, if possible, patients were to have had at least 24 hours of outpatient external ambulatory electrocardiographic monitoring that had not detected AF, although this was not applied as an inclusion criterion in the final review because of the small number of eligible studies identified. Based on the available evidence, and in line with the protocol, study-defined CS or TIA was permitted. The study definitions and inclusion criteria are discussed alongside the results in Chapter 3.
Study setting (as planned in the protocol32) was not used to determine study eligibility. However, in the protocol, it was anticipated that the relevant study setting would be secondary or tertiary care, which was consistent with the studies included.
The interventions under investigation in this diagnostic assessment report are:
Data from earlier versions of each of the devices were included as deemed necessary; in particular, data from an earlier model of the Reveal LINQ, known as the Reveal XT, were included. The comparators for included studies were each of the interventions versus each other or versus no further testing after outpatient external ambulatory electrocardiographic monitoring.
The anticipated comparator for the assessment of diagnostic accuracy was 24-hour external ambulatory electrocardiographic monitoring, with the reference standard being clinical validation of ICM-detected AF or ECG validation. In addition, papers that included other commonly used electrocardiographic monitoring methods as the comparator, such as 7-day external electrocardiographic monitoring, were considered, although no diagnostic accuracy studies were identified that met the population inclusion criteria (CS), irrespective of the comparator selected.
The following outcomes were considered in the review:
-
diagnostic accuracy (sensitivity, specificity and the numbers of true positive, true negative, false positive and false negative test results)
-
diagnostic yield in terms of the number of AF diagnoses
-
diagnostic yield in terms of the detection of other cardiac pathologies or incidental findings (i.e. non-AF)
-
time to diagnosis of AF
-
time to initiation of anticoagulants
-
uptake of anticoagulants
-
incidences of device failure (e.g. inability to transmit data or unexpectedly short battery life) and device removal because of failure or AEs
-
hospitalisations caused by AF
-
number of outpatient visits related to monitoring for AF
-
ease of use of devices for clinicians (including insertion)
-
mortality
-
morbidity (including further strokes or TIAs; other thromboembolisms and heart failure; any complications arising from preventative treatment, such as AEs due to anticoagulation treatment; and any AE related to implanting or removing the devices, such as infection or inflammation)
-
health-related quality of life (HRQoL)
-
acceptability of the devices to patients.
It was planned to include the following types of studies:
-
Randomised controlled trials or observational studies, in which participants are assigned to a minimum of 24 hours’ external electrocardiographic monitoring plus an ICM or a minimum of 24 hours’ external electrocardiographic monitoring for diagnosis of AF, and in which outcomes are compared at follow-up.
-
Test accuracy studies assessing the test accuracy of Reveal LINQ/BioMonitor 2-AF/Confirm Rx and/or 24 hours’ external electrocardiographic monitoring with 24 hours’ external electrocardiographic monitoring as the reference standard. In addition, papers that included a reference standard of other commonly used electrocardiographic monitoring, such as 7-day external electrocardiographic monitoring, were considered.
As insufficient studies were identified for the ICMs following a minimum of 24 hours’ external electrocardiographic monitoring, studies of ICMs following shorter durations or no external electrocardiographic monitoring were also considered for inclusion. However, there were still insufficient data for the Reveal LINQ and no suitable comparative studies identified for the Confirm Rx or BioMonitor 2-AF in the CS population. The study design inclusion criteria were therefore relaxed to also allow inclusion of single-arm observational studies for any of the three ICM devices and their earlier models, and the review protocol was amended. 33 The rationale for choosing to amend the study design inclusion criteria rather than another part of the population, intervention, comparator and outcome inclusion criteria was that the current searches limited studies by their population and interventions only. The interventions are already unrestricted in terms of the model of the devices specified in the NICE final scope for the review1 and so no further changes could be made to broaden the included interventions. The population inclusion criteria were also considered unsuitable for extending further, as the definition of CS was unrestricted and the AF detection rates in ICM devices are dependent on the patient population, as is the incidence of the other clinical outcomes of interest in this DAR. 34 Therefore, allowing the inclusion of studies in non-CS patients was deemed to be unsuitable as they are likely to have different incidence rates of AF and of the other clinical outcomes of relevance to this DAR. 34 It was therefore considered that data from non-CS populations would not be representative of ICM device performance in CS or TIA (hereafter referred to together as CS) patients.
The following study/publication types were excluded:
-
pre-clinical and animal studies
-
reviews, editorials and opinion pieces
-
case reports or studies of fewer than 10 patients
-
non-English language studies.
Search strategy
The electronic database searches combined terms for the condition (AF) and terms for the technology being assessed. For the technology, generic terms (e.g. ICM) and terms for the specific product (e.g. Reveal LINQ) were used. There were no study design filters applied, although animal and non-English language articles were excluded using search syntax. The search strategy was refined by scanning key papers identified during the review and through discussion with the review team, clinical experts and information specialists.
The following electronic sources were searched: MEDLINE (via Ovid), EMBASE (via Ovid), The Cochrane Library [including the Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL)] and the CRD database for the Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment (HTA) database.
The electronic databases were all searched from inception until the latest available version. The searches were conducted on 13 September 2018. A copy of the final search strategies is provided in Appendix 1, Tables 34–37.
Ongoing and unpublished studies were also searched and identified using:
-
clinicaltrials.gov (accessed 9 September 2019)
-
controlled-trials.com (accessed 9 September 2019)
-
clinicaltrialsregister.eu (accessed 9 September 2019)
-
company submissions from Abbott, Biotronik and Medtronic
-
the clinical effectiveness electronic database search results.
Relevant reviews and guidelines were identified through electronic database searches, consultation with clinical experts and searching the NICE website, and the reviews were used to identify additional potentially relevant studies.
Reference lists of included papers were also assessed for additional relevant studies. It was planned to hand-search the European Stroke Organisation Conference, International Stroke Conference and UK stroke forum conference proceedings for the previous 2 years, but this was deemed unnecessary as abstracts from those conferences were identified in the literature searches and supplemented by the submissions from companies.
Handling information from the companies
Data submitted by companies were originally going to be considered only if received by the EAG no later than 30 September 2018. However, all data submitted by companies during the writing of the report has been considered for inclusion and additional information has also been requested and provided by each of the three companies involved. Data that met the inclusion criteria for the review have been extracted and assessed for quality, as stated in the methods section of the protocol. 32
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Study selection and data extraction
The titles and abstracts of all identified studies from the electronic database searches were independently assessed for inclusion by two reviewers to identify the potentially relevant full-text articles to be retrieved. Full-text copies of the selected studies agreed for inclusion after title and abstract screening were obtained and all full-text articles were again assessed independently by two reviewers for inclusion using the eligibility criteria outlined in Eligibility criteria. Any disagreements were resolved by discussion; it was not necessary to consult with the third reviewer.
Data for the comparative studies were extracted independently by two reviewers using a standardised data extraction form. Data for five of the single-arm and observational studies were extracted independently by two reviewers to pilot the data extraction form. After agreeing the final data extraction form, one reviewer completed the data extraction for the remaining studies and the second reviewer validated 25% of the included studies. Information extracted included details of the study’s design and methodology, intervention and comparator tests, reference standard, baseline characteristics of participants, and outcome measures, including clinical outcome efficacy and any AEs (see Appendix 3). If there was incomplete information, attempts were made to contact authors with a request for further details. Discrepancies in the data extraction were resolved by discussion, and a third reviewer was available if necessary, although they were not required.
Quality assessment
The quality of included comparative studies has been independently assessed by two reviewers and any differences were resolved by consensus with a third reviewer who was consulted if necessary. The included RCT was assessed according to recommendations by the CRD14 and the Cochrane Handbook for Systematic Reviews of Interventions,18 and recorded using the Cochrane Risk of Bias 2.0 tool. 35 The observational studies were not quality assessed as the majority of them were single-arm studies and there is no standardised quality assessment tool suitable for assessing single-arm clinical effectiveness studies. It should also be noted that their results are reported only narratively or in tables (no evidence synthesis was conducted using them). There were no diagnostic accuracy studies in CS patients included; therefore, quality assessment with the quality assessment of diagnostic accuracy studies–2 (QUADAS-2) tool36 was not required.
Methods of analysis and evidence synthesis
Details of results on clinical effectiveness and quality assessment for each included study are presented in structured tables and as a narrative summary. There were insufficient clinically and methodologically homogenous data available to enable data to be pooled and meta-analysed. Clinical and methodological heterogeneity were investigated and are discussed narratively.
For test accuracy data, positive predictive values (PPVs), negative predictive values (NPVs), sensitivity values and specificity values, with 95% confidence intervals (CIs) are presented for each study, when available.
Potential subgroup analyses
The subgroups that were investigated, when evidence allowed, were as follows:
-
people with varying durations of previous outpatient external ambulatory electrocardiographic monitoring that had not detected AF (for example 1, 2, 7, 14 or 30 days)
-
people who had a cryptogenic TIA (excluding stroke)
-
people who had a CS (excluding TIA).
Sensitivity analyses
The planned sensitivity analyses were to include studies deemed to be at a high risk of bias that were excluded from the primary analyses. Sensitivity analyses were not conducted as there were insufficient data for any data synthesis to be conducted.
Chapter 3 Results of clinical effectiveness review
Quantity and quality of the available evidence
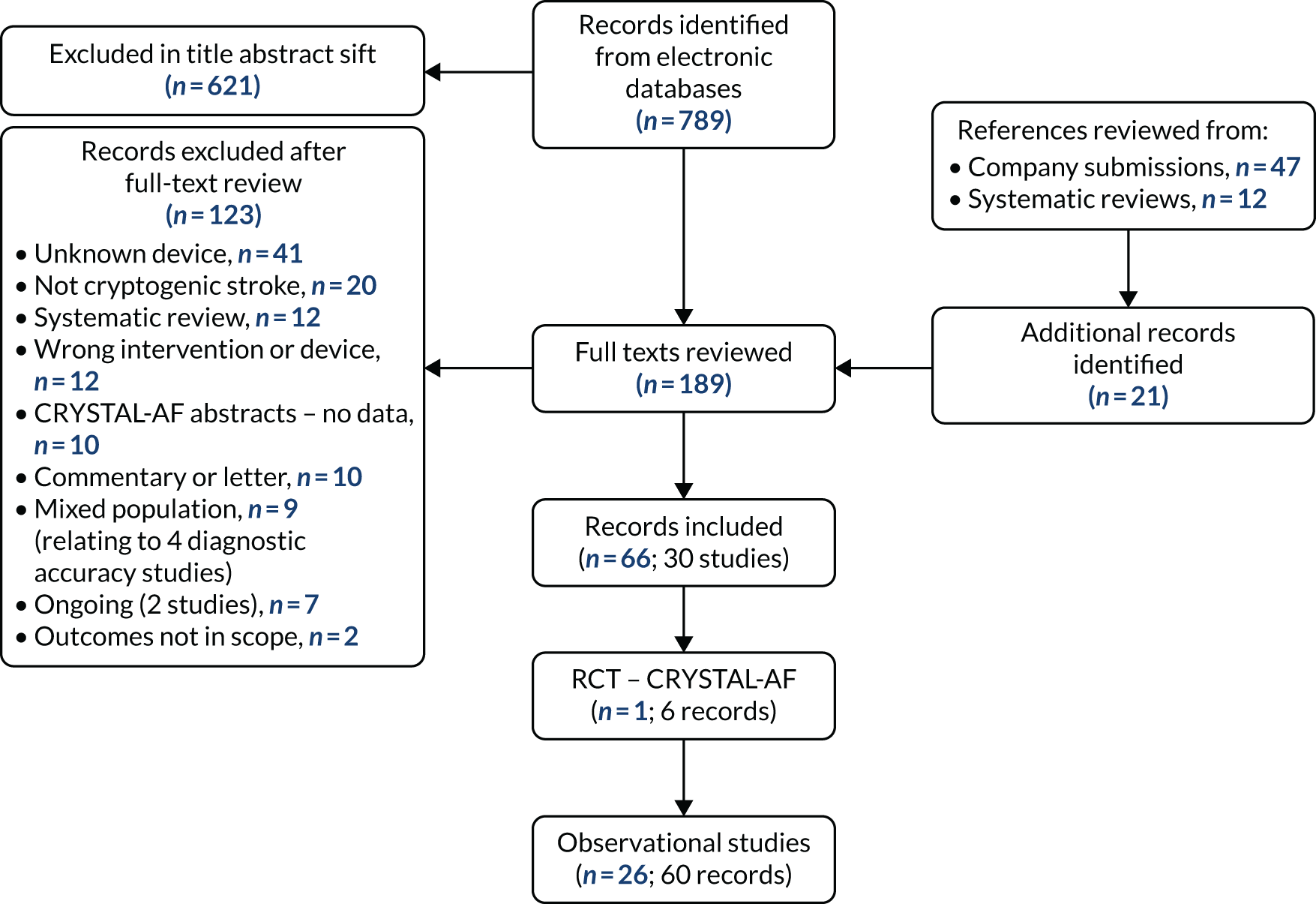
The electronic database searches were run on 13 September 2018. The results of the electronic database searches are summarised in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram in Figure 1. There were 72 references identified in the Cochrane database searches (CDSR and CENTRAL), one reference from resources searched through the CRD (DARE and the HTA database), 758 references from EMBASE (via Ovid) and 123 references from MEDLINE (via Ovid). The 954 results from the electronic database searches were all imported into EndNote X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and de-duplicated. Following de-duplication, 789 articles from electronic database searches were assessed for eligibility in the review through title and abstract screening. The reference lists of 12 systematic reviews identified in the database searches were also screened for potentially relevant studies, along with 47 documents supplied by the companies of the three ICM devices (Confirm Rx, Abbott; BioMonitor 2-AF, Biotronik; and Reveal LINQ, Medtronic).
FIGURE 1.
The PRISMA flow diagram for the review of clinical effectiveness. CRYSTAL-AF, Cryptogenic Stroke and underlying Atrial Fibrillation
As discussed in Chapter 2, initially the results were screened for comparative studies, but comparative data were available for only one device, albeit for a different model (Reveal LINQ, rather than XT). As comparative studies proved to be unavailable for two of the devices (Confirm Rx and BioMonitor 2-AF), single-arm observational studies were also reviewed for the following:
-
to identify any useful information that could be obtained for Confirm Rx, BioMonitor 2-AF and Reveal LINQ
-
in addition, to –
-
find confirmatory evidence for the outcome data identified for the Reveal XT
-
inform any outcomes in the NICE final scope1 not covered by the comparative study identified for Reveal XT.
-
This protocol amendment affected only the screening of the results and was implemented following the first sift of the title and abstracts. The results are, therefore, presented for the revised inclusion criteria to avoid double-counting of articles that met the original and the revised inclusion criteria. In total, 189 full-text articles were screened and 66 of these (relating to 27 studies) were included in the DAR. A list of excluded studies along with the reasons for exclusion is provided in Appendix 2.
The 66 included articles relate to one RCT (six publications) and 26 observational studies (60 publications). The RCT relates to the Cryptogenic Stroke and underlying Atrial Fibrillation (CRYSTAL-AF) trial,37 which compared the Reveal XT ICM with conventional follow-up for AF in patients who had had a CS. The results of the CRYSTAL-AF trial37 are discussed separately to the observational studies. The rationale for discussing the CRYSTAL-AF RCT data separately is that they were deemed to be the most robust clinical evidence for the Reveal LINQ ICM, despite the fact that they relate to an earlier model, the Reveal XT. In addition, the EAG noted that all the included observational studies related to the Reveal LINQ or its earlier model, the Reveal XT, with one study also including a small proportion of patients with the BioMonitor (an earlier model of the BioMonitor 2-AF), but reporting no data by device. The observational studies, therefore, do not provide clinical data for the other ICM devices under review in this DAR (BioMonitor 2-AF or Confirm Rx), but they do supplement the evidence from the CRYSTAL-AF trial37 by providing data for an additional outcome from the NICE scope and providing a larger data set to reflect the generalisability of the results from the RCT. The observational studies provide additional outcome data for all of the outcomes for which data were obtained from the CRYSTAL-AF trial,37 with the exception of HRQoL. In addition, the observational studies provided data for the outcome of diagnostic yield of cardiac pathologies other than AF.
Company submission data on non-CS populations were therefore included to enable some discussion on the clinical effectiveness of BioMonitor 2-AF and Confirm Rx.
Eight ongoing studies were identified from the registry searches (n = 4), the electronic database searches (n = 1, plus 1 duplicate) and from material submitted by the companies (n = 3). Seven records were excluded from the registry searches for having populations that were not of interest and two were already included in the review (Pedersen et al. 38 and the LINQ registry reported in Ziegler et al. 15). In addition to studies already reviewed in the registry searches, the Stroke of known cause and underlying Atrial Fibrillation (STROKE-AF) study (NCT02700945)39 was excluded from the company submission lists because it recruited people with stroke of known origin.
The Silent Atrial Fibrillation aFter Ischaemic StrOke (SAFFO) trial (NCT02684825)40 is a prospective, multicentre, open-label RCT based in Italy. The trial aimed to randomise 424 patients with thrombotic or lacunar stroke to receive a Reveal LINQ ICM or standard monitoring for AF detection. The primary outcome is AF or flutter within 12 months, to be assessed by blinded reviewers. The trial began in October 2015 and planned to recruit 424 patients. The estimated primary completion listed on clinicaltrials.gov is June 2018 but no results have yet been reported.
The Nordic atrial Fibrillation and stroke trial (NOR-FIB) (NCT02937077)41 is a multicentre prospective observational trial of the Reveal LINQ ICM, based in Norway. The trial is designed to evaluate AF detection and identify biomarkers over 12 months in 500 patients who have had a CS and is due to report in 2019. Another study, NCT03720639,42 plans to recruit a mixed diagnosis cohort of 500 patients to compare the transmission capabilities of the Confirm Rx with those of the Reveal LINQ, and is due to be completed in 2020. Two further ongoing studies identified in the registry searches have no status, results or associated publications: (1) the Cryptogenic stroke and atrial fibrillation detection through implantable loop recorder (CRYPTONITE) study (NCT01025947)43 is listed as an Italian observational study of the Reveal XT with a planned enrolment of 100 patients who have had a CS, but there has been no update since 2013, and (2) NCT0221637044 is a Slovakian case–control study with planned enrolment of 125 patients who have had CS.
Relevant ongoing studies outlined in the company submissions were the SMART registry (NCT03505801)45 (Confirm RX); the extended rhythm SCreening for AtRial Fibrillation in cryptogenic stroke patients (SCARF)46 active non-comparative observational study of 50 CS patients with unspecified ICMs, which was due to be completed in April 2017 (NCT01550042); and a Canadian RCT [Post-Embolic Rhythm Detection with Implantable versus External Monitoring (PERDIEM)] comparing the clinical effectiveness and cost-effectiveness of the Reveal LINQ ICM with external loop recording in 300 CS patients, which is due to be completed in December 2019 (NCT02428140). 47 Abbott outlined that the SMART registry is a post-approval study planning to recruit at least 2000 patients with Confirm Rx45 across multiple indications, but with a planned subgroup analysis for CS; completion is expected in December 2020.
As discussed previously in this section, there were no published or ongoing studies identified that assess the diagnostic accuracy of any of the three ICM devices exclusively in a CS population. However, this is not altogether unsurprising given that the incidence of AF is very low in the CS patient population; therefore, a very large study with long-term follow-up consistent with the battery life of the ICM device would be required to have enough patients detected with AF on a short-term Holter monitor in order to assess the DTA of an ICM. As a result, it is unsurprising that DTA data were not identified for any of the three ICMs under review in the CS population. As discussed in Chapter 2, it was decided not to widen the population inclusion criteria for the review, despite the small number of relevant studies in the CS population; this is because the performance (e.g. PPV and NPV) of AF detection in ICM devices is dependent on the patient population, incidence rate of AF, the duration of monitoring and the type of AF. 34 However, the EAG noted that the companies of the three ICMs under review also submitted evidence from non-CS populations for their devices; in the absence of data in the CS population, the EAG decided to narratively review these data. Test accuracy data from the applicable ICM models of each of the three devices under review are discussed later in this chapter, but it should be noted that the populations from which these data are generated are likely to be heterogenous, and the devices and software to which these test accuracy data relate are not necessarily the most up to date. These results should be interpreted with caution as the performance (e.g. PPV and NPV) of AF detection in ICM devices is dependent on the patient population, incidence rate of AF, the duration of monitoring and the type of AF. 34 Moreover, these results are not necessarily representative of the ICM device performance in CS patients and they are not directly comparable between the devices.
The CRYSTAL-AF trial
The CRYSTAL-AF trial details
The CRYSTAL-AF37 trial was an open-label, parallel-group RCT sponsored by the company, Medtronic. There were various conflicts of interest relating to the authors of the different publications of the trial, including employment, grants and personal fees from Medtronic. The EAG also noted that the CRYSTAL-AF trial formed the basis of the clinical data in the company submission from Medtronic for this DAR, despite CRYSTAL-AF being a trial of the Reveal XT, a predecessor model of the Reveal LINQ, the model under review in this DAR. The differences between the two models are discussed in Chapter 1 and data provided by the company on the DTA of the two devices (albeit not from an exclusively CS population) is discussed in Chapter 3, Medtronic.
In the CRYSTAL-AF trial, patients were randomised 1 : 1 to receive the Reveal XT ICM or conventional follow-up care. Details of the follow-up received by both groups is reported in Table 2. Randomisation was stratified in the trial groups according to the type of index event (stroke or TIA) and the presence or absence of a patent foramen ovale (PFO). The EAG’s clinical experts reported that the rationale for stratification by PFO is likely to be because its presence is associated with CS. There is no known difference in the incidence of AF in patients with TIA compared with patients with stroke as their index event, although clinical experts considered it reasonable for it to also be applied as a stratification factor.
| Treatment | ICM: continuous monitoring | Conventional follow-up |
|---|---|---|
| Randomised (n) | 221 (208 received device) | 220 |
| Withdrawals, n (%) at 6 months |
|
|
| Details of follow-up for AF detection | Patients assigned to the ICM group were scheduled to have the REVEAL XT ICM device inserted within 10 days after randomisation. The ICM was to automatically detect and record AF, irrespective of symptoms. The Medtronic CareLink Network was used to remotely transmit the device data | Patients assigned to the control group underwent assessment at scheduled and unscheduled visits, with electrocardiographic monitoring performed at the discretion of the site investigator. Monitoring type, duration and all results were recorded |
| Mean days from index event | To randomisation: 38.1 (SD 27.6) | |
| To insertion of device: 184 participants out of 208 (88.5%) had the device inserted within 10 days. Scheduling delays (22 patients) or medical justification (two patients) accounted for delayed insertions (median delay, 6 days, IQR 1–32) | N/A | |
| Mean duration/length of follow-up for AF detection | 20.3 ± 9.4 months (407.4 patient-years) | 19.2 ± 9.9 months (patient-years not reported) |
| Number of patients completing follow-up at | ||
| 6 months | 205 | 208 |
| 12 months | 194 | 185 |
| 24 months | 88 | 89 |
| 36 months | 24 | 24 |
Patients were enrolled to the CRYSTAL-AF trial between June 2009 and April 2012 from 55 centres in 14 countries across Europe, Canada and the USA. The study closure was planned to be at 12 months after the last patient was randomised, with the primary study follow-ups scheduled at 6 and 12 months. The study inclusion criteria were as follows:
-
A recent episode of cryptogenic symptomatic TIA or a recent episode of cryptogenic IS; recent was defined in a protocol amendment as from 60 to 90 days prior to enrolment. TIAs were required to have a visible lesion on a magnetic resonance imaging (MRI) or computerised tomography (CT) scan that fitted the symptoms of the TIA, and associated speech problems, or weakness of arm or leg, or hemianopia.
-
The patient or their legally authorised representative had to be willing to sign a patient consent form.
-
The patient had to be aged ≥ 40 years.
The definition of a CS in the CRYSTAL-AF trial was that no possible cause could be determined despite extensive work-up according to the standard protocol of the participating study centre. Before randomisation, the following clinical tests were required to establish the diagnosis of CS:
-
A MRI or CT scan.
-
12-lead electrocardiography for AF detection.
-
24-hour electrocardiographic monitoring for AF detection and premature atrial contraction analysis (e.g. Holter monitoring).
-
Transoesophageal echocardiography (TOE).
-
Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) of the head and neck to rule out other causes of stroke pathologies. A later protocol amendment allowed ultrasonography of cervical arteries and transcranial Doppler ultrasonography of intracranial vessels, in place of MRA or CTA of the head and neck in patients aged > 55 years.
The EAG’s clinical experts reported that the tests required in the CRYSTAL-AF trial to define CS were broadly consistent with the tests expected to be conducted in England. The clinical experts also reported that there are standard blood tests that would be required as part of the diagnostic work-up, and that all patients should receive transthoracic echocardiography prior to TOE; a small minority of patients may not receive TOE because of its invasive nature, but they may still be classified as having had a CS and go on to have an ICM.
The actual pre-enrolment screening for AF in the CRYSTAL-AF trial consisted of Holter monitoring, with a median duration of 23 hours [interquartile range (IQR) 21–24 hours] in 71.2% of patients {n = 314, mean 31.0 ± 66.7 hours [assumed to be the standard deviation (SD), although this was not specified in the paper]}, and inpatient telemetry monitoring, with a median duration of 68 hours (IQR 40–96 hours) in 29.7% of patients [n = 131, mean 74.6 ± 51.4 hours (assumed to be the SD, although this was not specified in the paper)]. The EAG considers it important to highlight that, in the DAR protocol, it was specified that patients were required to have a minimum of 24 hours of outpatient external electrocardiographic monitoring to be diagnosed as having had a CS. The EAG notes that 29.7% of patients in the CRYSTAL-AF trial did not receive outpatient electrocardiographic monitoring and that even the patients who did receive the outpatient Holter monitoring did not necessarily receive it for a full 24 hours (median 23 hours).
The main exclusion criteria for the CRYSTAL-AF trial were a history of AF or atrial flutter, an indication or contraindication for permanent OAC therapy at enrolment, or an indication for a pacemaker or implantable cardioverter defibrillator (full exclusion criteria are presented in Sinha et al. 48). The EAG’s clinical experts reported that these exclusion criteria are as expected for a clinical trial and in keeping with what would be expected in clinical practice in England and Wales, with the exception of a recent history of MI; if left ventricular function remained good, then MI would not necessarily be a reason for not implanting an ICM device in CS patients in clinical practice in England and Wales.
In total, 447 patients were enrolled to the CRYSTAL-AF trial, although only 441 underwent randomisation, with 221 randomised to the ICM trial arm and 220 to the conventional follow-up arm. Only 208 randomised participants (94.1%) in the ICM arm received the ICM device; 5.4% of these had withdrawn from the trial by the 6-month follow-up assessment. Reasons for withdrawals are presented in Table 2; with the exception of cross-over, there were similar numbers of withdrawals between the two trial arms. In relation to cross-over, 2.7% of participants in the conventional follow-up arm received an ICM, whereas 5.4% of participants in the ICM arm received conventional follow-up. In addition, there was an issue relating to delayed implantation of the ICM device in 11.5% of participants, which may have affected the AF-detection results of Reveal XT in the CRYSTAL-AF trial. The possible impact of the withdrawals and delayed ICM implantation on the results is discussed further in The CRYSTAL-AF trial: quality assessment.
The standard scheduled follow-up for patients in both of the arms of the CRYSTAL-AF trial was follow-up visits at 1, 6 and 12 months, and every 6 months thereafter until trial closure, with unscheduled visits in the event of symptom occurrence or after the transmission of ICM data, if advised by the investigator. If patients reported AF, then source documentation was acquired for adjudication, when possible. As reported in Table 2, the number of patients who reached 36 months’ follow-up was low in both trial arms, although the numbers were balanced across the two arms (24 patients in each trial arm).
The primary efficacy outcome in the CRYSTAL-AF trial was the time to first detection of AF (lasting > 30 seconds) at 6 months’ follow-up and the secondary outcome was AF detection at 12 months’ follow-up. The rate of AF detection was estimated with the use of the Kaplan–Meier (KM) method and compared between groups on an intention-to-treat (ITT) basis with the use of a log-rank test. Participants were censored in the primary analysis at the time of death, trial exit or completion of 6 months of follow-up. Pre-planned subgroup analyses were age, sex, race or ethnic group, type of index event, presence or absence of PFO, and CHADS2 score. As only the type of index event was relevant to the NICE final scope,1 the results for the other subgroups are not discussed in detail in this report; however, they are summarised in Diagnostic yield: atrial fibrillation detection rate.
The baseline characteristics of the randomised participants in the CRYSTAL-AF trial are presented in Table 3. The EAG notes that, although there were no significant differences between the trial arms at baseline (p < 0.05), there were some small baseline differences, for example in the distribution of participants with PFO and history of prior stroke. These differences were small and unlikely to be a result of any systematic issues with randomisation.
| Baseline participant characteristics | ICM – continuous monitoring (N = 221) | Conventional follow-up (N = 220) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 61.6 (11.4) | 61.4 (11.3) | 0.84 |
| Sex, n (%) | |||
| Male | 142 (64.3) | 138 (62.7) | 0.77 |
| Female | 79 (35.7) | 82 (37.3) | |
| Ethnicity, n (%) | |||
| Asian | 1 (1.4) | 2 (0.9) | 0.60 |
| Black | 7 (3.2) | 10 (4.5) | |
| Hispanic or Latino | 2 (0.9) | 2 (0.9) | |
| White | 194 (87.8) | 191 (86.8) | |
| Other | 0 (0) | 1 (1.4) | |
| Not available | 15 (6.8) | 12 (5.5) | |
| Geographic region, n (%) | |||
| North America | 83 (37.6) | 72 (32.7) | 0.32 |
| Europe | 138 (62.4) | 148 (67.3) | |
| PFO, n (%) | 52 (23.5) | 46 (20.9) | 0.57 |
| Index event, n (%) | |||
| Stroke | 200 (90.5) | 201 (91.4) | 0.87 |
| TIA | 21 (9.5) | 19 (8.6) | |
| Prior stroke/TIA, n (%) | |||
| Stroke | 37 (16.7) | 28 (12.7) | 0.28 |
| TIA | 22 (10.0) | 27 (12.3) | 0.45 |
| Score on mRS (scale: 0 to 6; lower = better), n (%) | |||
| 0–2 | 184 (83.3) | 186 (84.5) | 0.85 |
| > 2 | 36 (16.3) | 34 (15.5) | |
| NIH Stroke Scale (scale: 0 to 42; lower = better), mean score (SD) | 1.6 (2.7) | 1.9 (3.8) | 0.37 |
| Hypertension, n (%) | 144 (65.2) | 127 (57.7) | 0.12 |
| Diabetes, n (%) | 34 (15.4) | 38 (17.3) | 0.61 |
| CHADS2 score, n (%) | |||
| 2 | 69 (31.2) | 81 (36.8) | 0.17 |
| 3 | 92 (41.6) | 91 (41.4) | |
| 4 | 50 (22.6) | 34 (15.5) | |
| 5 | 9 (4.1) | 14 (6.4) | |
| 6 | 1 (0.5) | 0 (0) | |
| Hypercholesterolaemia, n (%) | 125 (56.6) | 128 (58.2) | 0.77 |
| Current smoker, n (%) | 43 (19.5) | 44 (20.0) | 0.91 |
| Coronary artery disease, n (%) | 16 (7.2) | 9 (4.1) | 0.22 |
| Use of antiplatelet agent, n (%) | 212 (95.9) | 212 (96.4) | 1.00 |
In terms of applicability of the patients in the CRYSTAL-AF trial to the equivalent patients in the UK who may be eligible for an ICM for AF detection following a CS, the EAG’s clinical experts reported that, as expected in a clinical trial, the patients in the CRYSTAL-AF trial were slightly younger than those likely to be eligible for an ICM after CS in the UK. In addition, clinical experts reported that if the CRYSTAL-AF criteria for cryptogenic TIA are used, then, possibly, a higher proportion of people who had a TIA would be expected to be eligible for an ICM in clinical practice, and estimated the proportion of people who had a TIA to be closer to 20% of the total ICM-eligible CS population. In addition, all patients would be expected to be on an antiplatelet agent. If patients are contraindicated to antiplatelets, they are likely to also be unsuitable for OAC (the treatment likely to be provided if AF is detected).
The CRYSTAL-AF trial: quality assessment
As discussed in Chapter 2, it was decided to conduct the quality assessment for the CRYSTAL-AF trial using the Cochrane Risk of Bias 2.0 tool; the only outcomes assessed were AF detection at 6, 12 and > 12 months. The results of the risk-of-bias assessment are presented in Appendix 4 and summarised in Table 4.
| Risk-of-bias domain | Time point | ||
|---|---|---|---|
| 6 months | 12 months | > 12 months | |
| 1. Risk of bias arising from the randomisation process | Low | Low | Low |
| 2. Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | Some concerns: lack of blinding unlikely to affect relative AF detection rates between groups. Only small numbers of patients received the alternative interventions [12 (5.4%) patients assigned to the ICM arm and 6 (2.7%) patients in the standard care arm]. Results were analysed for the ITT population (see Sanna et al.37); therefore, by including patients who did not receive an ICM, received one late or crossed over to standard care, the estimated benefit of receiving an ICM may be conservative. Delays in ICM insertion were mostly short and unlikely to affect this outcome | ||
| 3. Missing outcome data | Low | Low | Some concerns: the reasons for loss to follow-up beyond 6 months were not reported and a large number of patients were censored in the 24-month and 36-month analyses (only 88 patients in the ICM arm completed 24 months’ follow-up and 89 in standard care arm; this dropped to only 24 patients in each trial arm by 36 months’ follow-up) |
| 4. Risk of bias in measurement of the outcome | Low | Low | Low |
| 5. Risk of bias in selection of the reported results | Low | Low | Low |
| Overall risk of bias | Some concerns | Some concerns | Some concerns |
| Optional: what is the predicted direction of bias due to selection of the reported result? | Including patients who did not receive an ICM, received one late or crossed over to standard care in the ITT analysis may give a conservative estimate of the true benefit of ICM, although these issues may reflect clinical practice | ||
| Incomplete follow-up at later than 24 months is likely to make these results less reliable than those at 6 and 12 months, although the direction of this bias is unpredictable | |||
The overall risk-of-bias rating for all three time points of AF detection was that there were ‘some concerns’. For the 6- and 12-month follow-up results, this was mostly related to the open-label trial design and patients not receiving the randomised intervention as per the trial protocol [12 (5.4%) patients assigned to the ICM arm received conventional follow-up and 6 (2.7%) patients in conventional follow-up arm received an ICM]; in the ICM arm, device implantation was delayed in 24 (11.5%) of the patients who actually received the ICM (median delay 6 days; IQR 1–32 days). Results were analysed for ITT population; therefore, by including patients who did not receive an ICM, received one late or crossed over to standard care, the estimated benefit of receiving an ICM may be conservative. In addition to these issues around the open-label nature of the trial and the intervention not being received as per the trial protocol, the small number of patients achieving follow-up beyond 12 months is likely to make the 24- and 36-month results less reliable than those at 6 and 12 months, although the direction of this bias is unpredictable.
The CRYSTAL-AF trial: diagnostic test accuracy results
Device sensitivity and specificity
There were no data on the sensitivity or specificity of the Reveal XT reported in the identified CRYSTAL-AF trial publications. Information from the CRYSTAL-AF trial and advice from the EAG’s clinical experts indicate that alerts generated by an ICM will need to be reviewed by a clinician to confirm AF before the initiation of anticoagulation treatment, and so there are essentially no false positives with the ICM.
One study (Choe et al. )14 conducted simulations using the the CRYSTAL-AF trial data to establish the relative sensitivity of the Reveal XT compared with various simulated external monitoring strategies, including one-off 24-hour Holter monitoring and 30 days’ continuous Holter monitoring, assuming that the Reveal XT had a sensitivity of 100%. This study, along with its results, is discussed further alongside the observational studies, as it was not a RCT.
Diagnostic yield: atrial fibrillation detection rate
The AF detection rate at 6 months was the primary outcome of the CRYSTAL-AF trial. The definition of AF in the CRYSTAL-AF trial was an episode of irregular heart rhythm, without detectable P-waves, lasting > 30 seconds. However, AF episodes are detected by an ICM using an automatic algorithm that is based on R-wave interval variability detected within 2-minute analysis windows. 49,50 It is therefore possible that some AF episodes of between 30 seconds’ and 2 minutes’ duration may have been missed in the ICM arm because of the 2-minute analysis window of the ICM. 49,51 As a result, there was a potential discrepancy in the duration of episodes of AF between the ICM and conventional follow-up arms in the CRYSTAL-AF trial that potentially bias the results in favour of conventional follow-up. In addition, as discussed in The CRYSTAL-AF trial: quality assessment, the open-label nature of the CRYSTAL-AF trial may have resulted in bias in the conventional follow-up arm as the outcome assessor was aware of the intervention assignment and was able to influence the electrocardiography or other assessment of AF. The ICM arm was unlikely to be affected by bias relating to the outcome assessor as all episodes of AF that qualified for analysis were adjudicated by an independent committee. These factors should therefore be taken into consideration when interpreting the results for AF detection, along with the risk-of-bias assessment findings. However, it is unclear what the resulting direction of the potential biases would be on the results. For the 6-month and 12-month results, it is most probable that the bias would favour AF detection with conventional follow-up, although beyond 12 months it is much less certain what direction the bias would be because of the large number of people censored in the analyses.
The results for AF detection demonstrated a trend in favour of the ICM across all time points (Table 5). At 6 months, 8.6% of patients were diagnosed with AF in the ICM arm compared with only 1.4% of patients in the conventional follow-up arm. The number of patients with AF diagnosed had risen to 19.0% in the ICM arm at 36 months, compared to only 2.3% in the conventional follow-up arm; this is despite small numbers of patients followed up at 36 months. The estimated AF detection rates are therefore higher in the 36-month KM analysis because of the non-informative censoring of patients lost to follow-up (the AF detection rate was estimated as 30% in the ICM arm and 3% in the conventional follow-up arm).
| Diagnostic yield | Months | ICM | Conventional follow-up | Notes | ||
|---|---|---|---|---|---|---|
| Events, n (% ITT) | Participants (n) | Events, n (% ITT) | Participants (n) | |||
| AF detection | 0–1 | 8 (3.6) | 221 | 1 (0.5) | 220 | |
| 0–6 | 19 (8.6) | 221 (208 with ICM) | 3 (1.4) | 220 | Control group AF from 88 ECGs (65 patients), 20 24-hour Holters (17 patients) and event recording in one patient | |
| 6–12 | 10 (4.5) | 221 (189 with ICM and no AF before 6 months) | 1 (0.5) | 220 | Control group AF from 34 ECGs (33 patients) and 12 Holters (10 patients) | |
| 0–12 | 29 (13.1) | 221 (208 with ICM) | 4 (1.8) | 220 | Control group AF from 122 ECGs, 32 Holters and 1 event recorder | |
| 12–24 | 9 (4.1) | 221 (208 with ICM) | 1 (0.5) | 220 | Control group AF from 62 ECGs and 14 Holters | |
| 0–24 | 38 (17.2) | 221 | 5 (2.3) | 220 | ||
| 24–36 | 4 (1.8) | 221 (208 with ICM) | 0 | 220 | Control group AF from 19 ECGs and 6 Holters | |
| 0–36 | 42 (19) | 221 | (2.3) | 220 | Control group AF from 256 AF monitoring tests | |
| Asymptomatic AF detection (of all detected AF) | 0–6 | 14 (73.3) | 19 | 1 (33.3) | 3 | |
| 0–12 | 23 (79.3) | 29 | 2 (50.0) | 4 | ||
| 0–36 | 34 (76.2) | 42 | 2 (40.0) | 5 | ||
| AF detection by index event | ||||||
| Stroke | 0–6 | 17 (8.3) | NR | 4 (1.6) | NR | Index event numbers from baseline table. p-value for interaction: 0.99 |
| TIA | 3 (15) | NR | 0 | NR | ||
| Stroke | 0–12 | 23 (11.6) | NR | (2.2%) | NR | |
| TIA | 4 (20.0) | NR | 0 | NR | ||
| Stroke | 0–36 | (31.2%) | NR | (3.3%) | NR | |
| TIA | NR | NR | (0.0%) | NR | ||
| Time to event | Median (IQR) | Median (IQR) | HR for detection of AF: ICM versus conventional follow-up, HR, 95% CI; p-value | |||
| First AF detection, unadjusted | 6 | 41 days (4–84 days) | 19 detected | 32 days (2–73 days) | 3 detected | 6.4, 1.9 to 21.7; < 0.001 |
| 12 | 84 days (18–265 days) | 29 detected | 53 days (17–212 days) | 4 detected | 7.3, 2.6 to 20.8; < 0.001 | |
| 36 | 8.4 months (NR) | 42 detected | 2.4 months (NR) | 5 detected | 8.8, 3.5 to 22.2; < 0.001 | |
| First AF detection, adjusted for PFO, hypertension and coronary artery disease | 6 | – | – | – | – | 5.9, 1.7 to 19.8; 0.009 |
| First AF detection, censoring data at the time of crossover | 6 | – | – | – | – | 6.1, 1.8 to 20.8; 0.009 |
Only one patient was diagnosed with AF beyond 12 months’ follow-up in the conventional follow-up arm, whereas in the ICM arm, a further 13 patients were diagnosed with AF (nine patients between 12 and 24 months and four patients between 24 and 36 months; see Table 5). These results would suggest that long-term monitoring with an ICM, such as the Reveal XT, is beneficial in detecting more cases of AF; thus, enabling the treatment of AF to help reduce the risk of a further stroke or TIA.
Atrial fibrillation detection with the ICM compared with conventional follow-up was reported to be consistent across all the prespecified subgroups in the CRYSTAL-AF trial (age, sex, race or ethnic group, index event, presence or absence of PFO and CHADS2 score), with no significant interactions. In addition, it was reported that the subgroup analysis results at 12 months were consistent with those at 6 months. The EAG notes that the subgroup results by index event (i.e. stroke or TIA) suggest a higher incidence of AF in the ICM arm of the TIA subgroup than in the stroke subgroup, although it is also noted that the number of patients in the TIA subgroup was very small (21 patients in the ICM arm). The trend favouring ICM over conventional follow-up seen in the primary study results was consistent in both the TIA and stroke subgroups.
Diagnostic yield: detection of other cardiac pathologies
There were no results reported for the detection of other cardiac pathologies in the CRYSTAL-AF trial.
The CRYSTAL-AF trial: clinical outcome results
Atrial fibrillation
Time to diagnosis
Only five cases of AF were detected in the conventional follow-up arm of the CRYSTAL-AF trial during the 36 months’ follow-up (compared with 42 cases in the ICM arm); owing to the low incidence of AF in the conventional follow-up trial arm, it is difficult to draw any conclusions on the median time to AF detection data. Nevertheless, the data show that the number of patients for whom AF was detected increased with longer follow-up; therefore, the median time to AF detection also increased. However, there was a greater increase in the median time to AF detection with the ICM than with conventional follow-up across all three time points (see Table 5). The timing of trial follow-up visits may have caused interval censoring in the conventional follow-up arm (and thereby influenced the estimated median time to AF detection), whereas, in the ICM arm, trial follow-up is less influential as the device is constantly monitoring for episodes of AF. However, the low detection rate of AF in the conventional follow-up arm is likely to be the main reason for the discrepancy in median time to AF detection between the ICM and conventional follow-up arms.
Hospitalisations
There were no results reported for AF-related hospitalisations in the CRYSTAL-AF trial.
Outpatient monitoring
There were no results reported for outpatient monitoring in the CRYSTAL-AF trial.
Anticoagulant use
Uptake of anticoagulants
The data reporting the use of OACs in the CRYSTAL-AF trial suggest that some patients not diagnosed with AF were commenced on OACs and a small proportion of patients diagnosed with AF did not receive an OAC (Table 6). The rationale for patients having an ICM for AF detection following a CS, and being diagnosed with AF but not started on OACs, is unclear. However, the results suggest that the majority of patients diagnosed with AF in the ICM arm were commenced on OACs (> 90% of patients). Results were not reported for OAC uptake after AF detection in the conventional follow-up trial arm.
| Outcome | Time (months) | ICM | Conventional follow-up | Difference (ICM – control) (%), 95% CI; p-value | ||
|---|---|---|---|---|---|---|
| Events, n (%) | Participants (n) | Events, n (%) | Participants (n) | |||
| Use of OACs | 6 | 21 (10.1) | 208 | 9 (4.6) | 197 | 5.5, 0.5 to 10.6; 0.0375 |
| 12 | 29 (14.7) | 197 | 11 (6.0) | 185 | 8.8, 2.8 to 14.8; 0.0069 | |
| 24 | 23 (26.1) | 88 | 5 (5.6) | 89 | 20.5, 10.2 to 30.9; 0.0002 | |
| 36 | 10 (38.5) | 26 | 2 (8.3) | 24 | 30.1, 8.4 to 51.8; 0.0195 | |
| Use of OACs in patients diagnosed with AF | 6 | 18 (94.7) | 19 | NR | NR | NR |
| 12 | 28 (96.6) | 29 | NR | NR | NR | |
| 24 | 36 (92.3) | 39 | NR | NR | NR | |
| 36 | 38 (90.5) | 42 | NR | NR | NR | |
Time to initiation of anticoagulants
There were no results reported for the time to initiation of anticoagulants in the CRYSTAL-AF trial.
Incidences of device failure and removal
No data were reported to suggest any incidences of device failure in the CRYSTAL-AF trial, although premature removal of the device, by 36 months, due to infection or pocket erosion was reported in 5 out of the 208 (2.4%) participants in the ICM trial arm who received the Reveal XT device (see Appendix 3). Data were also reported on the number of participants who no longer had their ICM device in situ at the 6-month (1.9% participants) and 12-month (3.4% participants) follow-ups; although the numbers of ICMs that had been removed was low, it was unclear why the devices were removed if it was not related to infection or pocket erosion. The EAG also note that the number of ICMs removed was much lower than the number of patients with AF detected at 6 or 12 months, suggesting that many patients kept the ICM in situ after AF was diagnosed.
Ease of use of devices for clinicians
There were no results reported for the ease of use of devices for clinicians in the CRYSTAL-AF trial.
Mortality
There were no results reported for mortality in the CRYSTAL-AF trial.
Further strokes or transient ischaemic attacks
Outcome data on recurrent stroke or TIAs during the CRYSTAL-AF trial follow-up were presented for the composite of recurrent stroke or TIA and demonstrated a non-significant trend in favour of fewer recurrent events in the ICM arm than in the conventional follow-up arm (p > 0.05) (Table 7). It should also be noted that, in the ICM arm, there were fewer recurrent strokes or TIAs than the number of patients with AF detected at each of the time points, whereas, in the conventional follow-up arm, there were higher numbers of recurrent stroke and TIA events than the number of patients diagnosed with AF at each time point. However, outcome data were not reported by intervention and diagnosis of AF, and so it is unclear whether the recurrent stroke or TIA events occurred in patients diagnosed with AF or in the undiagnosed subgroup.
| Time (months) | ICM (N = 221) | Conventional follow-up (N = 220) | HR, 95% CI; p-value | ||
|---|---|---|---|---|---|
| Events (n) | % | Events (n) | % | ||
| 6 | 11 | 4.98 | 18 | 8.18 | NR |
| 12 | 15 | 6.79 | 19 | 8.64 | 0.63, 0.22 to 1.80; 0.39 |
| 36 | 20 | 9.05 | 24 | 10.91 | 0.77, 0.30 to 1.97; 0.59 |
Other thromboembolisms
There were no results reported for other non-stroke- or TIA-related thromboembolisms in the CRYSTAL-AF trial.
Heart failure
There were no results reported for the diagnosis of heart failure in the CRYSTAL-AF trial.
Adverse events
Device-related adverse events
All AE data identified from the CRYSTAL-AF trial were extracted and are presented in Table 8. The data suggest that the incidence of device-related AEs, such as pain and infection, was relatively low with the ICM, although AEs did lead to device removal in 2.4% (n = 5) of participants (see Table 10). In addition, it was reported that > 25% of participants in both the ICM and conventional follow-up trial arms suffered from a serious adverse event (SAE), although it is unclear what the SAEs were. The proportion of SAEs was slightly higher in the ICM arm than in the conventional follow-up arm (30.8% vs. 27.9%, respectively) and there was also a much higher proportion of non-serious AEs in the ICM arm than in the conventional follow-up arm (18.6% vs. 4.1%, respectively). No details were reported on what the non-serious AEs were for either trial arm, so it is unclear why there was such a large difference in AEs between the trial arms.
| Adverse events | Months | ICM | Conventional follow-up | ||
|---|---|---|---|---|---|
| Events, n (%) | Participants (N) | Events, n (%) | Participants (N) | ||
| ICM removal following infection or pocket erosion | 36 | 5 (2.4) | 208 | NA | NA |
| AE: infection | Unclear | 3 (1.4) | 208 | NA | NA |
| AE: pain | Unclear | 3 (1.4) | 208 | NA | NA |
| AE: irritation or inflammation | Unclear | 4 (1.9) | 208 | NA | NA |
| CV or stroke/TIA-related hospital admissions | 12 | 23 (10.5) | 221 | 16 (7.2) | 220 |
| Patients with SAE | Uncleara | 68 (30.8) | 221 | 58 (27.9) | 220 |
| Total patients with non-serious AE | Uncleara | 41 (18.6) | 221 | 9 (4.1) | 220 |
Anticoagulant-related adverse events
There were no results reported for anticoagulant-related AEs in the CRYSTAL-AF trial.
The CRYSTAL-AF trial: participant-reported outcome results
Health-related quality of life
The EuroQol 5-Dimensions (EQ-5D) tool was used to collect HRQoL data during the CRYSTAL-AF trial. However, these results are confidential and therefore cannot be reproduced by the EAG.
Acceptability of the devices to patients
There were no results reported, beyond the HRQoL data mentioned in the previous section, for the acceptability of the devices to participants in the CRYSTAL-AF trial.
Observational studies
As outlined at the beginning of Chapter 3, the eligibility criteria of the systematic literature search were broadened to identify observational studies of ICM use in CS populations. The EAG’s searches were cross-checked with study lists provided by the company, which identified 26 relevant observational studies. The studies are primarily single-arm prospective observational studies and therefore subject to internal biases associated with this study design, but the EAG considered them useful to supplement the evidence from the CRYSTAL-AF trial37 by providing data for an additional outcome from the NICE scope and providing a larger data set to reflect the generalisability of the results from the RCT. The EAG did not consider data synthesis appropriate owing to the clinical heterogeneity between studies across a range of variables that are likely to affect AF detection and clinical outcomes. Key sources of heterogeneity between studies include patient characteristics, rigour of stroke assessment, stroke risk score, definition and adjudication of AF, and length of follow-up (Tables 9 and 10).
| First author, year | Device | Country (n sites) | Participants (n) | Design | Enrolment | Eligibility and diagnostic work-up | Baseline characteristics |
|---|---|---|---|---|---|---|---|
| Asaithambi, 201852 | Reveal LINQ | USA (1) | 234 | Retrospective single arm | April 2014–October 2017 (implanted) | CS (TOAST); no other details | Median age 72 (IQR 61–78) years; 55% male; median CHADS2VASC score of 5 (IQR 4–6) |
| Chalfoun, 201653 | Reveal LINQ | USA (NR) | 192 | Retrospective single arm | May 2014–October 2015 (implanted) | CS and no prior AF after 48 hours of inpatient telemetry | NR |
| Ferrara, 201754 | Reveal LINQ | USA (NR) | 68 | Prospective single arm | NR | CS; no other details | Mean age 71 years; 63% male; mean CHADS2VASC score of 4.1 (SD 2) |
| Heckle, 201855 | Reveal LINQ | USA (2) | 133 | Retrospective single arm | September 2014–November 2017 (implanted) | CS; no other details | Mean age 65.2 years; 73.7% white |
| Kotlarz-Bottcher, 201856 | Reveal LINQ | Germany (1) | 100 | Prospective single arm | Implanted in 2016 | ‘ESUS criteria’; no other details | None reported |
| Li, 201857 | Reveal LINQ | USA (1) | 19a | Retrospective single arm | April 2014–April 2017 (implanted) | CIS or TIA not attributed to large-vessel atherosclerosis, apparent cardioembolism source or small-vessel disease. Extensive cardiac, vascular, haematological and serological evaluation; life expectancy > 18 months | Median (assumed) age 67 years; 92% male (not CS subgroup) |
| Seow, 201858 | Reveal LINQ | Singapore (1) | 71 | Prospective single arm | August 2014–February 2017 (referred) | CS or TIA after MRI or CT, TOE, duplex carotid artery ultrasonography, transcranial Doppler, ≥ 24 hours’ inpatient continuous electrocardiography, 24-hour Holter, eligible for OAC, no prior AF | Mean age 61.9 years; 77.5% male; 0% white; mean CHADS2VASC score of 4.2 (SD 1.3), median 4 (range 2–7) |
| Ziegler, 201715 | Reveal LINQ | International (NR) | 1247 | ICM registry vs. simulated intermittent monitoring | February 2014–July 2014 (implanted) | CS designated by implanting physicians | Mean age 65.3 years; 53% male |
| Pallesen, 201759 | Reveal LINQ (NeuroLINQ) | Germany (NR) | 75 | Prospective single arm | January 2014–June 2015 (implanted) | ESUS, 95% of patients complied with the CRYSTAL-AF trial eligibility criteria | Median age 61 years; 64% male |
| Carrazco, 201860 |
|
USA (1) | 100 | Prospective and retrospective single arm | September 2013–September 2015 (admitted) | CIS, eligible for implant after brain MRI/CT, magnetic resonance/computed tomographic angiography, TTE or TOE, ≥ 24 hours’ cardiac telemetry, electrocardiography, blood work. Excluded patients with severe disabling stroke | Mean age 65.8 years; 48.5% male; 57% white; mean NIHSS score of 5.6 (SD 6.2) with AF, 5.3 (SD 5.8) without AF |
| Abichandani, 201661 |
|
USA (1) | 74 | Prospective single arm | October 2009–September 2015 | CS; no other details | Mean age 66 years; 49% male |
| Poli, 201662 |
|
Germany (1) | 75b | Prospective single arm | NR | CIS (89%) or TIA (TOAST), ≥ 1 AF risk factor (CHADS2VASC score of ≥ 4, atrial runs, left atrium size > 45 mm, LAA flow ≤ 0.2 m/s or spontaneous echo contrast), CT or MRI (with angiography), neurosonology, TOE, ≥ 72 hours’ electrocardiography, ≥ 1 24-hour Holter electrocardiography, thrombophilia screening if aged < 55 years | Mean age 66.4 years; 47% male; median CHADS2VASC score of 5 (IQR 4–6) |
| Joseph, 201563 | Reveal LINQ or XT | USA (NR) | 64 | Prospective single arm | Ongoing registry enrolment | CS with embolic-appearing infarct, ≥ 48 hours’ inpatient telemetry, brain MRI ± angiography, no prior AF, TOE | Mean age 66.9 years; 58.4% male; median NIHSS score of 5.2 |
| Salahuddin, 201564 | Reveal LINQ or XT | USA (1) | 31 | Retrospective single arm | May 2012–September 2014 (implanted) | CS (96.8%) or TIA (3.2%) diagnosed by board-certified vascular neurologists | Mean age 66.1 years; 45.2% male; 38.7% had a prior stroke (other than the index event); 16.1% had a PFO |
| Pedersen, 201838 |
|
Denmark (1) | 105 | Prospective single arm | November 2013–October 2015 (diagnosed) | TIA (neurological deficit episode, presumed ischaemia, symptoms remission within 24 hours regardless of evidence of brain infarction), standard electrocardiography, 72-hour Holter, 12-lead electrocardiography, carotid ultrasonography, brain CT or MRIs, aged 18–81 years, eligible for OAC | Median age 65.4 years; 46% male; median CHADS2VASC score of 4 (range 2–7) |
| Choe, 201514 | Reveal XT | The CRYSTAL-AF trial population: International (55) | 168 | Simulated intermittent monitoringc using the CRYSTAL-AF trial ICM arm | June 2009–April 2012 | CS as defined for the CRYSTAL-AF trial | Mean age 61.3 years; 68% male; mean CHADS2VASC score of 2.9 (SD 0.8) |
| Christensen, 201465 (SURPRISE) | Reveal XT | Denmark (1) | 85 | Prospective single arm | NR | CS after 12–24 hours’ telemetric monitoring and standard work-up, CT- or MRI-verified acute ischaemic lesion, mRS score of ≤ 2, no prior AF | Mean age 56.7 years (pooled); 55.1% male; median CHADS2VASC score of 4 for those with AF and 3 for those without AF |
| Cotter, 201366 | Reveal XT | UK (1) | 51 | Unclear | August 2010–October 2011 (implanted) | CIS (TOAST), ASCO-defined brain infarct, no prior AF, no high-risk cardiac embolic source, structural cardiac imaging, standard electrocardiography, ≥ 24 hours’ Holter. Excluded TIA and prior AF | Mean age 51.5 years; 54.9% male; median CHADS2VASC score of 3 (IQR 2–4); 22/30 had a known PFO |
| Etgen, 201367 | Reveal XT | Germany (1) | 22 | Prospective single arm | Admitted in 2011 | CS (TOAST) after MRI, 12-lead electrocardiography, 24–72-hours’ continuous electrocardiography, ≥ 1 additional 24-hour Holter electrocardiography, TTE, TOE, computed tomographic/magnetic resonance angiography, aged < 55 years prothrombotic screening, eligible for OAC. Exclusion as for the CRYSTAL-AF trial | Mean age 61.6 years (pooled); 50% male |
| Holtzman, 201368 | Reveal XT | USA (NR) | 22 | Prospective single arm | NR | CS with embolic-appearing infarct, TOE, no AF on cardiac telemetry, MRI or computed tomographic angiography, carotid Doppler of < 50% ipsilateral stenosis | None reported |
| Mercé, 201369 | Reveal XT | Spain (1) | 14 | Prospective single arm | August 2009–February 2011 (referred) | CS, daily electrocardiography, laboratory tests, brain CT, Holter, TOE and TTE, Doppler, brain MRA, no prior AF | Mean age 65.4 years; 71.4% male |
| Müller, 201770 | Reveal XT | Germany (4) | 90 | Prospective single arm | March 2013–April 2015 (recruited) | Acute CS (TOAST), aged ≥ 18 years, 12-lead electrocardiography, 72-hour electrocardiography, additional 24-hour electrocardiography and TOE. Brain and vascular imaging (MRI scan with DWI and CTA), eligible for OAC. No prior AF or pacemaker | Mean age 57.7 years; 52% male; mean CHADS2VASC score of 3.4 (SD 1.7) |
| Reinke, 201871 | Reveal XT | Germany (1) | 105 | Prospective single arm | March 2013–December 2014 (admitted) | CS (TOAST) or TIA (18.1%) after accurate work-up: MRI or cardiovascular CT, standard 12-lead electrocardiography on admission, 24-hour Holter electrocardiography, ultrasonography of the brain supplying arteries and TOE | Mean age 64.4 years; 56.2% male; median CHADS2VASC score of 4 (IQR 3–6); median NIHSS score of 2 (IQR 1–5) |
| Ritter, 201372 | Reveal XT | Germany (1) | 60 | Within-patient comparison of 7-day electrocardiography vs. ICM | November 2010–May 2012 | CS (TOAST), embolic patterns on brain MRI or CT; duplex ultrasonography, CTA or MRA, routine electrocardiography, 72-hour continuous electrocardiography, 24-hour Holter electrocardiography, TOE with PFO testing. Excluded lacunar strokes, prior AF | Median age 63 years; 56.7% male; median CHADS2VASC 4 (IQR 3–5) |
| Rojo-Martinez, 201373 | Reveal XT | Spain (1) | 86 | Prospective single arm | NR | CS patients with high suspicion of embolic cerebral ischaemia. Full diagnostic work-up including brain MRI with diffusion and FLAIR during admission | Mean age 67 years; 47.7% male |
| Israel, 201774 |
|
Germany (1) | 123 | Prospective single arm | June 2013–January 2015 (admitted) | Acute ESUS, embolic pattern on cranial CT or MRI, serial 12-lead electrocardiography, 24-hour Holter, 72-hour telemetry, TTE, TOE, cervical duplex, transcranial Doppler, blood tests. Excluded: known AF, stroke mimics, TIA, lacunar strokes | Mean age 65 years; 60.2% male; mean CHADS2VASC score of 4.5 (SD 1.3) |
| First author, year | Device | Follow-up (months)a | Diagnostic accuracy |
|---|---|---|---|
| Ziegler, 201715 | Reveal LINQ | 19 | Assuming 100% sensitivity of Reveal LINQ in a registry cohort, modelled sensitivities of other strategies:
|
| Li, 201857 | Reveal LINQ | 13.4 (median) | 79.7% (98/123) of algorithm-detected AF episodes were not confirmed in the clinician review (i.e. false positives); 20.3% (25/123) were true positives |
| Choe, 201514 | Reveal XT (the CRYSTAL-AF trial) | 11.3 (minimum) | Assuming 100% sensitivity of Reveal LINQ in the CRYSTAL-AF trial, modelled sensitivities of other strategies:a
|
| Mercé, 201369 | Reveal XT | 11.5 (median) | The devices in 10 patients (71%) recorded 24 episodes of AF that were not confirmed after manual review |
| Israel, 201774 |
|
12.7 | > 90% of algorithm-detected AF episodes were not confirmed in the clinician review (i.e. false positives) |
The EAG emphasises that the CRYSTAL-AF trial37 is the only study that met the original eligibility criteria and, representing the most robust evidence for ICMs in the population of interest, is the primary source of clinical data to answer the NICE final scope. 1 Formal quality assessment was not possible owing to the single-arm designs, but the EAG considers the 26 studies discussed hereafter to be at high risk of bias. The observational evidence base is presented to illustrate the existing evidence outside RCTs and to provide clinical data on the Reveal LINQ in the absence of data from RCTs.
Observational studies: study details
Study design and brief population characteristics of the 26 non-RCTs are presented in Table 9. Details of time to ICM insertion, AF threshold (e.g. 30 seconds), method and frequency of data transmission, and how episodes were adjudicated are presented with AF detection rates for each study in Table 11. AF detection rate was the main outcome in all studies; other outcomes of relevance to the NICE scope1 were time to AF detection, uptake of anticoagulants, device failure, subsequent stroke and AEs.
| First author, year | Device | Time from index event to implant | AF threshold, data transmission, and adjudication | Follow-up (months)a | AF detection rate (%) |
|---|---|---|---|---|---|
| Asaithambi, 201852 | Reveal LINQ | Median 4 (IQR 2–9) days | Threshold, programming and data transmission not reported. AF episodes adjudicated by group of cardiac electrophysiologists | 1 | 9 |
| 6 | 20.1 | ||||
| Median 18 | 29.1 | ||||
| Chalfoun, 201653 | Reveal LINQ | At discharge vs. 30 days later | NR | 0.5 | 7.3 |
| 0.5 to 1 | 2.1 | ||||
| 1 to 6 | 7.8 | ||||
| 6 | 17.2 | ||||
| Ferrara, 201754 | Reveal LINQ | NR | Threshold not reported; AF detection settings, daily automatic data transmission, episodes adjudicated | 11 | 14.7 |
| Heckle, 201855 | Reveal LINQ | NR | Threshold and programming not reported. Episodes interrogated remotely and at clinic visits | 10 | 27.1 |
| Kotlarz-Bottcher, 201856 | Reveal LINQ | NR | NR | 12 | 17.0 |
| Li, 201857 | Reveal LINQ | NR | AF of ≥ 2 minutes; AF high sensitivity settings, episodes stored and transmitted daily to CareLink. Home monitoring device for patient-triggered events. Reviewed by physicians and adjudicated independently if disagreement. Seen in clinic after 2–4 weeks; routine follow-ups at physician discretion | 14 | 31.6 |
| Seow, 201858 | Reveal LINQ | 66 days (median) | AF of ≥ 2 minutes, autodetected, patient-activated recordings and daily ECGs transmitted via CareLink and adjudicated by cardiac electrophysiologists. Patients with AF counselled for OACs. No scheduled clinic visits until the battery expired | 6 | 12.7 |
| 12 | 15.5 | ||||
| Ziegler, 201715 | Reveal LINQ | NR | Patient registry data used to simulate comparison with intermittent monitoring strategies (see Table 10). Threshold of ≥ 2 minutes; daily autotransmission or patient-initiated via CareLink. Adjudicated by a single, blinded reviewer | 1 | 4.6 |
| 6 | 12.2 | ||||
| 12 | 16.3 | ||||
| 24b | 21.5 | ||||
| Pallesen, 201759 | Reveal LINQc | Within 1 month | NR | 12 | 19.2 |
| Carrazco, 201860 |
|
Mean 4.2 (± 2.6) days from admission | AF of ≥ 2 minutes, and shorter flutter. AF adjudicated by study cardiac electrophysiologist | Minimum 8 | 25.0 (31 including flutter) |
| Abichandani, 201661 |
|
NR | NR | 12 | 20.3 |
| Poli, 201662 |
|
NR | AF of ≥ 2 minutes; XT patients instructed to do daily readings and present to clinic if alarm activated. All patients included in CareLink Network with automatic daily transmission; telephoned if AF detected. Episodes reviewed by cardiologists blinded to AF risk factors. Clinic visit after 1 month and every 3 months thereafter | 6 | 28.0 |
| 12 | 33.3 | ||||
| Joseph, 201563 | Reveal LINQ or XT | NR | AF of ≥ 10 seconds. No other details | 7 | 17.2 |
| Salahuddin, 201564 | Reveal LINQ or XT | NR | AF of ≥ 15 seconds. Significant PAF was defined as an episode of irregular heart rhythm, without detectable P-waves | NR | 32.3 |
| Pedersen, 201838 |
|
Median 113 (range 30–294) days | AF of ≥ 2 minutes; AF = irregularly irregular heart rhythm without P-waves. Monitored via CareLink. XT data transmitted at 1, 3, 6, 9 and 12 months; LINQ: daily transmissions. Other arrhythmias stored. Adjudicated by two experienced senior electrophysiologists | 12 | 6.7 |
| Choe, 201514 | Reveal XT | NR | Subset of the CRYSTAL-AF trial used to simulate comparison with intermittent monitoring strategies (see Table 10). AF of ≥ 30 seconds, standard programming, automatic detection and recording of AF, remote data transmission via CareLink. AF episodes adjudicated by independent committee | Minimum 11 | 17.9 |
| Christensen, 201465 (SURPRISE) | Reveal XT | Median 69, mean 107 days (usually within 1 week of work-up) | AF of ≥ 2 minutes; AF = irregular R–R intervals and no visible P-waves; minimum biweekly patient data transmission. Programmed to detect and store one-lead ECG of all arrhythmia episodes. Adjudicated by two independent cardiologists | 19 | 16.1 (20.7 including those not detected by an ICM) |
| Cotter, 201366 | Reveal XT | 174 (mean) | AF of ≥ 2 minutes or by patient activation; 0.05 mV threshold, standard detection limits; AF = irregularly irregular R–R interval and no distinct P-waves. Independent verification by a second cardiologist. Follow-up recommended at 1-month intervals by hospital or CareLink. Daily CareLink assessment recommended | 8d | 25.5 |
| Etgen, 201367 | Reveal XT | 9 days (mean) | AF of ≥ 6 minutes; AF detection algorithm. No other details | 12 | 27.3 |
| Holtzman, 201368 | Reveal XT | NR | No details | NR | 40.9 |
| Mercé, 201369 | Reveal XT | ≤ 1 month | Follow-up at 1 month and every 3 months thereafter, or additional if symptoms or recorder’s alarm was activated | Median 6 | 35.7 |
| Müller, 201770 | Reveal XT | NR | AF of ≥ 30 seconds. 0.05 mV sensitivity. Adjudicated by a cardiologist blinded to TTE results | 1 | 8.9 |
| 11 | 17.8 | ||||
| Reinke, 201871 | Reveal XT | ≤ 4 weeks | AF of ≥ 30 seconds; standard AF algorithm and hand-held Patient Assistant. Monitored for 20 months and analysed by experienced cardiologists | 20 | 18.1 |
| Ritter, 201372 | Reveal XT | Median 13 (IQR 10–65) days | AF of ≥ 30 seconds; daily patient transmission of 7-minute ECG, reviewed independently by two cardiologists. All patients received platelet aggregation inhibitors at study start and were seen in clinic every 3 months. Immediately phoned if AF detected; OAC recommended if confirmed | 0.25 | 5.0 |
| 3 | 11.7 | ||||
| Median 13 | 16.7 | ||||
| Rojo-Martinez, 201373 | Reveal XT | No details | 10 | 30.2 | |
| Israel, 201774 |
|
20 days; mostly before discharge | AF of ≥ 2 minutes; automatic AF detection algorithms and ECG storage. Manually analysed and adjudicated. Daily transmission by patient via CareLink or HomeMonitoring® (Biotronik). In-hospital follow-up at 1 month and every 6 months thereafter | 3 | 12.2 |
| 9 | 22.8 | ||||
| 13 | 23.6 |
Nine studies tested the Reveal LINQ device,15,52–59 six included a mix of Reveal LINQ and XT38,60–64 and 10 studies tested only the XT device. 14,65–73 One study included a mix of Reveal XT and Biotronik BioMonitor (an earlier model of the BioMonitor 2-AF), although only 13% were inserted with a BioMonitor (n = 16);74 no studies reported using the Confirm Rx. None of the identified studies provides comparative data between groups of patients receiving an ICM versus those who were monitored with alternative strategies. Three studies conducted within-patient comparisons of ICM versus other monitoring strategies,14,15,72 two of which used ICM detection data to simulate outcomes for intermittent monitoring (discussed in Observational studies: diagnostic test accuracy results). 14,15
All studies included CS populations, although the terms and definitions used varied [e.g. embolic stroke of undetermined source, cryptogenic ischaemic stroke (CIS)], as did the range of exploratory tests performed before patients were considered to have had a CS (see Table 9). Mean or median age was between 60 and 70 years in most studies (range 51.5 to 72 years), the percentage of males ranged from 45% to 92% (median 55%) and median CHADS2VASC score was between 3 and 5, indicating moderate to high risk of AF-related stroke (see Table 9). Two studies exclusively recruited patients who had had a TIA or minor stroke. 38,65
Most studies recruited patients at a single centre and sample sizes ranged from 1469 to 124715 (median 80, mean 131) participants. The most common countries in which studies were conducted were the USA (n = 10) and Germany (n = 8); only one was conducted in the UK. 66 Devices were implanted from 2011 in line with the emergence of each model. Seventeen studies were prospective single-arm observational studies that followed patients who met predefined inclusion criteria and were implanted with an ICM after a CS during a set time frame. 38,54,56,58–63,65,67–71,73,74 Five studies collected data retrospectively from CS patients who had received an ICM52,53,55,57,64 and one study did not report a clear methodology. 66 The EAG reiterates the inherent biases within the observational evidence due to the single-arm designs and the clinical heterogeneity identified, and encourages caution in drawing conclusions from naive comparisons between studies.
Observational studies: diagnostic test accuracy results
Device sensitivity and specificity
None of the observational studies provided comparative DTA between a group of patients who were monitored with an ICM and a group that received standard monitoring. However, two studies14,15 used AF detection data for a group of patients who had had a CS who were monitored for AF with an ICM to estimate the sensitivity of intermittent monitoring strategies if the ICM is assumed to have a sensitivity of 100%. Choe et al. 14 used data from 168 patients who received the Reveal XT in the CRYSTAL-AF trial (those with adequate follow-up from the 221 randomised to the ICM group), and Ziegler et al. 15 used data from a large registry of patients with a Reveal LINQ device15 (n = 1247). Choe et al. 14 used a 30-second episode threshold and Ziegler et al. 15 used a 2-minute threshold, but both studies used the same technique of modelling episodes of AF detected by the ICM; repeated iterations (10,000) were run to estimate the number of patients whose AF would not have been detected had alternative intermittent monitoring strategies been used.
Based on the assumption that the ICMs had 100% sensitivity for AF after a CS, Table 10 shows the estimated sensitivity of other monitoring strategies from the model simulations. Ziegler et al. 15 found sensitivities of between 2.9%, from a single 24-hour Holter monitor, and 29.9%, from quarterly 7-day Holter monitoring, and results were similar in Choe et al. 14 based on the CRYSTAL-AF trial cohort. As a result, even the best-performing intermittent monitoring strategy detected less than one-third of the AF detected by the ICM.
Two other studies reported false-positive rates as the proportion of episodes detected by ICM algorithm that were not subsequently verified as AF by a clinician. Li et al. 57 reported a 79.7% false-positive rate from the Reveal LINQ and Israel et al. 74 reported that > 90% of detected episodes were not confirmed by manual review (Reveal XT and BioMonitor). In their response to queries about individual studies identified by the EAG, Medtronic emphasised that false positive rates vary considerably depending on the model of device, sensitivity configuration and episode detection threshold.
Diagnostic yield: atrial fibrillation detection rate
All 26 included observational studies reported AF detection rates during follow-up, although information about time from stroke to insertion, AF threshold, data transmission and adjudication were inconsistently reported (see Table 11). Nine studies used an AF episode threshold of 2 minutes,15,38,57,58,60,62,65,66,74 four studies used a 30-second threshold in line with the CRYSTAL-AF trial (including Choe et al. ,14 which is based on the CRYSTAL-AF trial ICM population),14,70–72 two studies used shorter thresholds of 10–15 seconds63,64 and nine studies did not state a threshold. 52–56,59,61,68,73 When reported, studies generally stated that standard AF detection settings were used and recordings were automatically transmitted daily. Sixteen studies described episode verification and adjudication,14,15,38,52,54,55,57,58,60,62,65,66,70–72,74 although to varying levels (e.g. by a study clinician or by two independent cardiologists). Patient-activated recording was outlined in seven studies of Reveal LINQ and XT. 14,15,57,58,62,71,74
Atrial fibrillation detection rates at the main follow-up (ranging from 6 to 24 months) were highly variable, ranging from 6.7% (Pedersen et al. ,38 Reveal LINQ and XT, 12-month follow-up) to 40.9% (Holtzman et al. ,68 Reveal XT, unknown follow-up). The EAG reiterates that data synthesis was considered inappropriate because of the clinical heterogeneity between studies across a range of variables that are likely to affect AF detection and clinical outcomes, including but not limited to device model and detection settings, patient characteristics, rigour of stroke assessment, stroke risk score, definition and adjudication of AF, and length of follow-up (see Tables 9 and 10).
Seven studies15,52,53,58,70,72,74 reported AF detection after different lengths of follow-up, which gives an indication of the rate of AF detection over time. In general, the studies indicate that a minority of patients are diagnosed within the first month (mostly in the region of 10% detected by 1 year), around 70–80% by 6 months and a small number beyond 1 year of monitoring. Clinical experts advised the EAG that, for patients detected with AF after > 2 years of cardiac monitoring, the AF may not be related to the index event, although its management is likely to still be the same and the patient would be considered for long-term treatment with an OAC. In the large registry population reported by Ziegler et al. ,15 around 20% of those with AF detected by 2 years were picked up in the first month, 60% by 6 months and 80% by the end of the first year. In Seow et al. ,58 80% of patients with AF detected by 12 months had been diagnosed by 6 months, and in Ritter et al. ,72 around 70% of detected AF by 13 months had been picked up at 3 months. Very few patients reported in Asaithambi et al. 52 had AF detected in the first month and around 70% of those detected by 18 months had been diagnosed by 6 months; the AF detection rate in the first month of Chalfoun et al. 53 had roughly doubled by 6 months. AF detection in the first month was much higher in Müller et al. ,70 with just under half of detected AF (by 11 months) picked up in the first month. Around half of those detected by 13 months in Israel et al. 74 had been detected at 3 months, and nearly all by 9 months.
When described, all or most AF detected was asymptomatic and so would probably not have been picked up without continuous ICM monitoring. 58,62,69 All patients with detected AF in Poli et al. 62 (by 6 months), Mercé et al. 69 and Seow et al. 58 (at 6 and 12 months) were asymptomatic. Two additional patients who had AF detected between 6 and 12 months in Poli et al. 62 experienced symptoms of AF.
Diagnostic yield: detection of other cardiac pathologies
The primary aim of the observational studies was to detect AF in patients who had had a CS, but five studies also reported incidental detection of other arrhythmias by the ICM. Three studies of the Reveal LINQ (or primarily LINQ in a mix of LINQ and XT) suggest that the proportion of patients who had other arrhythmias detected is in the region of 10%, consisting mainly of bigeminy, pause and bradycardia. Two, primarily Reveal XT, studies that reported the breakdown of arrhythmias gave rates of 1% (atrial flutter, cardiac arrest, sick sinus node, bigeminy, ventricular tachycardia) to 7–8% (atrioventricular block and ventricular extra systole). No information was presented about whether or not, or how, the detected arrhythmias were treated, and whether or not outcomes were improved for patients by having the arrhythmias identified. Table 12 summarises the incidental detection of other arrhythmias by the ICM reported in those observational studies.
| First author, year | Device | Follow-up (months) | Other arrhythmias |
|---|---|---|---|
| Asaithambi, 201852 | Reveal LINQ | 17.6 (median) | 12% any arrhythmia (28/234) |
| Li, 201857 | Reveal LINQ | 13.4 (median) | True positive episodes detected by ICM:
|
| Carrazco, 201860 |
|
8 (minimum) |
|
| Pedersen, 201838 |
|
12.5 (mean) |
|
| Christensen, 201465 (SURPRISE) | Reveal XT | 18.7 (mean) |
|
Observational studies: clinical outcome results
Time to atrial fibrillation diagnosis
Eighteen observational studies reported time from device insertion to AF detection: five with the Reveal LINQ, seven with Reveal LINQ or XT, five with the Reveal XT and one with Reveal XT or BioMonitor (Table 13). Overall, average follow-up ranged from 7 to 20 months and median time to first AF detection was highly variable, ranging from 21 to 217 days. Where reported, IQRs also indicate a high degree of variability within studies.
| First author, year | Device | Follow-upa (months) | Days to AF detection | Anticoagulant use, of those with detected AF, % (n/N) | |
|---|---|---|---|---|---|
| Median | IQR unless otherwise stated | ||||
| Asaithambi, 201852 | Reveal LINQ | 17.6 (median) | 94.5 | 16–239 | 91.2 (62/68) |
| Heckle, 201855 | Reveal LINQ | 10.2 | 42 | NR | NR |
| Seow, 201858 | Reveal LINQ | NR | 50 | NR | 90.9 (10/11) |
| Pallesen, 201759 | Reveal LINQb | 12 | 57 | NR | NR |
| Ziegler, 201715 | Reveal LINQ | 24 | 112 | 35–293 | NR |
| Carrazco, 201860 |
|
8 (minimum) |
34 (mean 108) |
0–514 (range) | 96.8 (30/31) |
| Abichandani, 201661 |
|
12 | 243.3 | NR | NR |
| Poli, 201662 |
|
12 |
105 (mean) |
0–361 (range) | NR |
| Joseph, 201863 | Reveal LINQ or XT | 7.3 | 35 | NR | NR |
| Salahuddin, 201564 | Reveal LINQ or XT | 10.4 |
52 (mean 57.1) |
21–57 | NR |
| Pedersen, 201838 |
|
12.5 | 21–146 (range) | NR | |
| Reinke, 201871 | Reveal XT or LINQ | 20 | 217 | 72.5 to 338 | NR |
| Cotter, 201366 | Reveal XT | 7.5c | 48 | 34–118 (range 0–54) | NR |
| Etgen, 201367 | Reveal XT | 12 |
152.8 (mean) |
61.6 to 244.1 (95% CI) | 100 (6/6) |
| Mercé, 201369 | Reveal XT | 11.5 (median) | 176.4 | NR | 100 (5/5) |
| Müller, 201770 | Reveal XT | 10.9 |
30 (mean 40.7) |
SD 42.2 | NR |
| Ritter, 201372 | Reveal XT | 12.5 | 64 | 1–556 (range) | NR |
| Israel, 201774 |
|
12.7 | 109.5 | SD 103.4 | NR |
| Li, 201857 | Reveal LINQ | 13.4 (median) | 83.3 (5/6) | ||
| Christensen, 201465 (SURPRISE) | Reveal XT | 18.7 | 14 patients diagnosed with AF; 19 in total ICM cohort started OAC | ||
Anticoagulant use
In seven studies of Reveal LINQ and/or XT, uptake of OACs in patients detected with AF was consistently high (see Table 13). Most of the studies had small populations, but the evidence suggests that uptake of anticoagulation is in the region of 90% to 100% once AF is detected. Christensen et al. 65 (SURPRISE) reported the overall uptake of OACs regardless of whether AF was detected and did not report whether or not the 19 patients starting OACs included all 14 patients with AF.
Incidences of device failure and removal
Three studies of Reveal LINQ and/or XT52,65,72 reported the number of device removals during follow-up. Ritter et al. 72 (Reveal XT) offered removal to patients once AF was detected, but, for 18 out of 60 removals (30%), it was not clear if the removal was for this reason or other reasons such as tolerability or battery life. Christensen et al. 65 (Reveal XT) reported that the device was prematurely explanted in 5 of 87 (5.7%) patients (three owing to skin reactions and two owing to discomfort) and that the median time to removal was 45 days; a further three patients (3.4%) chose to have the device removed after > 1 year of monitoring without AF being detected. Asaithambi et al. 52 reported that, of the 234 patients implanted with Reveal LINQ, 5.1% of the ICMs were removed from patients who died or required palliative care, 2.6% were removed electively, 1.3% of patients were lost to follow-up and 0.9% of ICMs migrated or fell out.
Further strokes or transient ischaemic attacks
Six studies reporting recurrent stroke indicate that a minority of patients have recurrence in the first year after device implantation, although the data were variable (0–14.6%). The data for recurrent stroke or TIA in patients with AF suggest higher rates than in those without AF detected, but it is unclear how many of these strokes and TIAs in the AF patients occurred prior to the detection of AF. Table 14 summarises the recurrent strokes or TIAs reported in those observational studies.
| First author, year | Device | Mean follow-up (months) | Recurrent stroke/TIA, % (n/N) | Notes |
|---|---|---|---|---|
| Poli, 201662 |
|
12 | Recurrent stroke: 1.4 (1/74) | No AF detected in the patient with recurrent stroke, and the stroke occurred 14 months after index event |
| Pedersen, 201838 |
|
12.5 |
|
In patients with new-onset AF, only one patient experienced a new TIA and none had a stroke. The difference in TIA recurrence in patients with and without AF was not statistically significant (log-rank test, p = 0.98) |
| Christensen, 201465 (SURPRISE) | Reveal XT | 18.7 |
|
Ischaemic event rate, defined as either stroke or TIA (independent of imaging confirmation), was higher in the AF group [6 (33.3%)] than in the non-AF group [7 (10.1%)], p = 0.024 |
| Etgen, 201367 | Reveal XT | 12 | Recurrent stroke: 0 (0/22) | |
| Ritter, 201372 | Reveal XT | 12.5 | Recurrent stroke: 0 (0/60) | |
| Israel, 201774 |
|
12.7 | Recurrent stroke: 14.6 (18/123) |
|
Adverse events
In addition to the device removal data summarised in Incidences of device failure and removal, some of which related to tolerability, five studies reported AEs. Three studies of Reveal XT,69,71,72 one of Reveal LINQ and XT,62 and one of Reveal XT and BioMonitor74 reported that no complications of the procedure or insertion site were noted during follow-up.
Evidence on implantable cardiac monitors in non-cryptogenic stroke populations
All the studies discussed in this section are a different population to that specified in the NICE final scope as they are not patients who had a prior CS (or the studies do not report subgroup data for the included CS patients and > 50% of the study population are not CS patients). As discussed at the beginning of Chapter 3, the performance (e.g. PPV and NPV) of AF detection in ICM devices, is dependent on the patient population, incidence rate of AF, the duration of monitoring and the type of AF. 34 Therefore, the data reported here are not necessarily representative of the performance of the different ICM devices in CS patients. In addition, none of the results is directly comparable between the devices. However, the decision was made to consider these data from non-CS populations as no data have been identified for the Confirm Rx or BioMonitor 2-AF in the CS population and only limited outcome data were identified in the CS population for the Reveal LINQ. It should be noted that the studies discussed in Abbott, Biotronik and Medtronic were obtained directly from company recommendations and a full systematic literature search was not conducted to validate their inclusion owing to time constraints and concerns regarding the applicability of their results to the CS population. 34 The data presented in the following subsections may be subject to study selection bias as well as clinical heterogeneity owing to the variation in the patient populations of each of the studies.
The eligibility criteria applied when selecting studies to report from non-CS populations were as follows:
-
sources searched – references supplied in the individual company submissions
-
study type – RCT or observational studies with or without a comparator arm
-
population – no restrictions applied
-
intervention – any one of the following ICMs: SJM Confirm DM2102, Confirm Rx DM3500, BioMonitor 2-AF, Reveal XT or Reveal LINQ
-
comparator – no restrictions applied. DTA data required Holter monitoring (of any duration) as the reference standard
-
outcomes – all outcomes listed in the protocol and as listed in Chapter 2.
The results of the searches are presented in the following subsections for each of the three ICM companies.
Abbott
The information provided in the company submission by Abbott regarding the Confirm Rx was that the only relevant study was the Diabetes Cardiovascular Risk Evaluation: Targets and Essential Data for Commitment of Treatment (DETECT) AF study (Nölker et al. ). 75 The EAG notes that the patient group in the DETECT AF study was not restricted to CS patients; therefore, the EAG did not consider this study to meet the review inclusion criteria. In addition, the ICM device used in the DETECT AF study was the Confirm ICM, Model DM2102, whereas supporting documents for the company submission included a user guide for the Confirm Rx Model DM3500. The company clarified that Confirm DM2102 was an older and larger model of the Confirm Rx, which is the model specified in the NICE final scope. 1 The EAG is unsure how the firmware in the models differs, but, in the absence of any suitable clinical data for the Confirm Rx DM3500, the data for the Confirm DM2102 are summarised below.
In addition, the company reported that Healey et al. 76 may provide some useful clinical data for the assessment of the Confirm Rx. The EAG notes that this was an observational cohort study that used the Confirm DM2100, the predecessor to the DM2102. The study population in Healey et al. 76 comprised patients at risk of AF who were aged ≥ 65 years and attending outpatient cardiology and neurology clinics. Healey et al. 76 do not specifically report whether or not the study population included any CS or cryptogenic TIA patients, although 48.0% had a history of stroke, TIA or systemic embolism. The EAG considers the data from the DETECT AF study75 to be more appropriate given that they are based on a more recent model of the Confirm ICM, and so the results from Healey et al. 76 are not discussed further.
The DETECT AF study75 was a prospective observational study conducted to assess the diagnostic accuracy of the Confirm ICM in detecting AF compared with Holter monitoring. The intervention comprised 4 days of simultaneous monitoring for AF using the Confirm ICM and a Holter monitor, and was required to take place at least 2 weeks after ICM implantation. A total of 90 patients were enrolled from 12 centres in Germany and the Netherlands between September 2012 and December 2013, although only 79 patients were deemed eligible for inclusion in the analyses. Reasons for exclusion from the analysis included clock synchronisation issues due to batteries running low in the Holter or patient ICM external symptom activator (for a total of five patients) and insufficient duration of analysable Holter recordings (four patients). Patients were required to have been diagnosed with paroxysmal AF or to have a clinical suspicion of paroxysmal AF. In total, eight of the enrolled patients had a history of prior stroke or TIA, although it is unclear whether any of these were CS or cryptogenic TIA patients.
The ICM monitored for AF episodes lasting at least 2 minutes and the Holter monitor data were analysed by a blinded, independent core laboratory. Patient and episode sensitivity, specificity, PPV and NPV were calculated.
At least one AF episode was detected in 16 of the 79 patients analysed, and all 16 patients had episodes of AF recorded by both the ICM and the Holter monitor. There were no incidences in which the Holter monitor detected additional episodes of AF compared with the ICM, but nine patients had at least one 2-minute AF detection by the ICM, without any corresponding AF episode detected on the Holter recording. However, most of these false positives were due to irregular sinus rhythms and not noise (44/58 episodes; number of patients was not reported and the clinical consequence of detecting these was not reported). In a per-patient analysis, the sensitivity was 100% (95% CI 79.4% to 100%), PPV was 64.0% (95% CI 42.5% to 82.0%), specificity was 85.7% (95% CI 74.6% to 93.3%) and NPV was 100% (95% CI 93.4% to 100%) for the Confirm ICM using Holter monitoring (minimum of 45 hours’ analysable data) as the reference standard. The results of the per-patient analysis, therefore, suggest that the Confirm DM2102 ICM can detect AF with a high sensitivity and a reasonably high specificity.
The DETECT AF study also reported no AEs during the follow-up time for any of the 90 enrolled patients who received the ICM device.
Biotronik
Biotronik provided 12 publications in support of their company submission with clinical data that they deemed to be of relevance to the assessment of the BioMonitor 2-AF in patients who had a CS. However, (confidential information has been removed) were deemed to meet the inclusion criteria for a discussion of non-CS or mixed population data. The key characteristics of the five included studies (two publications and six personal communications)77–84 are summarised in Table 15 and their results are discussed in the paragraphs below. The EAG notes that only (confidential information has been removed) and that the primary indication for the ICM is CS (confidential information has been removed) of the study participants for each of the included studies. As discussed in Quantity and quality of the available evidence, AF detection in ICM devices is dependent on the patient population, incidence rate of AF, duration of monitoring and type of AF, and so these results may not be a true reflection of the ICM performance in CS patients. 34 It is also unclear in (confidential information has been removed) what proportion of the study participants received the BioMonitor 2-AF model of the BioMonitor 2 as specified in the NICE final scope and it should be noted that (confidential information has been removed).
| First author/company, year | Study name | BioMonitor 2-AF model (%) | Country (n sites) | Enrolment | Total study population (n) | CS patients, n (%) | Last planned follow-up |
|---|---|---|---|---|---|---|---|
| Ooi, 201878 | BioMonitor 2 pilot study | NR | Australia (5) | Patients with an accepted indication for long-term cardiac monitoring. The most common indications for ICM were syncope (42%) and symptomatic or asymptomatic AF or flutter at baseline (42%) | 31 | 1 (3.2) | 1 month |
| Biotronik, 201877,81,82 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Biotronik, 201883,84 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Biotronik, 201879 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Reinsch, 201880 | N/A | 36 | Germany (1) | Consecutive patients with an indication for long-term cardiac monitoring. Most common indication was unexplained syncope (80%) | 30 | 1 (3) | 3 months |
(Confidential information has been removed.)
(Confidential information has been removed.)
Clinician time taken to insert the BioMonitor 2 was reported in five studies. 77–84 Reinsch et al. 80 reported that all devices were successfully implanted in a cath lab with a median time from skin cut to last suture of 8 minutes (IQR 7–10 minutes) and Ooi et al. 78 reported that all implantations were successfully performed in cath labs on the first attempt, with a median time from incision to last suture of 9 minutes (IQR 5–14 minutes). (Confidential information has been removed.)
(Confidential information has been removed.)
Four studies77,78,80,83 reported data on AEs, with one study77 also reporting subgroup data for CS patients. Ooi et al. 78 reported that there was one pocket infection observed and successfully treated with oral antibiotics. Reinsch et al. 80 reported that no devices had migrated by the 3-month follow-up, but two patients experienced AEs: one patient (3%) who was immunosuppressed developed a device-related pocket infection requiring implantable loop recorder explantation and oral antibiotic treatment; the second patient (3%) complained of slight discomfort in the area of the flexible ICM antenna. (Confidential information has been removed.)
(Confidential information has been removed.)
One study80 also provided data on additional patient-related outcomes of interest to the NICE final scope. Reinsch et al. 80 reported that at least one therapeutic intervention was performed in 23% of patients following the recording of arrhythmias during follow-up, and this included initiation of OAC in one patient (3%). Reinsch et al. 80 also reported results from patient satisfaction surveys at 1 day and 3 months. The results were generally good, with only 7% reporting moderate to severe pain and 20% reporting mild pain within 24 hours post intervention at the implantation site. Sustained paraesthesia was moderate in 7% and mild in 17% of patients and moderate impairment in daily life was reported by one (3%) patient. The cosmetic result was mostly reported to be very satisfying (63%) or satisfying (30%).
In summary, the studies of the BioMonitor 2 suggest that it is clinically effective in detecting AF and that it is associated with low levels of AEs and reasonably good levels of patient satisfaction. However, it should be noted that these results are not exclusively for the BioMonitor 2-AF or for a CS population; therefore, they should be interpreted with caution.
Medtronic
The documents supplied by Medtronic were reviewed for data and all relevant studies relating to the CS population have been included and discussed in the CRYSTAL-AF and observational studies sections; however, owing to time constraints and the large volume of citations of potential relevance for data in a non-CS or mixed population provided by the company, the EAG took the pragmatic decision to limit the inclusion of non-CS studies to those studies directly referred to and reporting clinical outcome data that were reported in the request for information document received on 28 July 2017. Five studies34,50,85–87 in non-CS or mixed populations were identified from the company submission, of which four34,50,86,87 reported data on the DTA of the Reveal ICMs and two85,87 provided AE data for the Reveal LINQ. These five studies are discussed briefly in the following paragraphs, along with their relevant results. As discussed earlier, it is important to remember when interpreting these results that the performance (e.g. PPV and NPV) of AF detection in ICM devices is dependent on the patient population, incidence rate of AF, duration of monitoring and type of AF, and so these results may not be a true reflection of the ICM performance in CS patients. 34
The Reveal XT Performance Trial (XPECT) (Hindricks et al. )50 was a single-arm prospective observational study of 247 patients conducted to assess the performance of the Reveal XT in detecting AF (of at least 2 minutes). Patients were enrolled between September 2007 and July 2008 from 24 medical centres, mainly in Europe and Canada. Eligible patients were those who fulfilled at least one of the following criteria:
-
scheduled for pulmonary vein ablation or surgical rhythm control intervention
-
had documented frequent AF or frequent symptoms attributable to AF
-
had undergone pulmonary vein ablation in the previous 6 months and still had symptoms attributable to AF.
The study protocol required the enrolled patients to be implanted with the Reveal XT; 4 to 6 weeks after the ICM implantation, they were to receive 46 hours of Holter monitoring (with a minimum of 18 hours’ Holter recording required for inclusion in the analyses). A total of 206 patients had analysable Holter recordings, of which 76 (37%) had at least one episode of AF, although only 73 (96.1% of the Holter-detected AF patients) of these patients were also identified as having AF by the ICM. The XPECT study results demonstrate that the dedicated AF detection algorithm in the Reveal XT identified the presence or absence of AF with an accuracy of 98.5%, compared with the Holter monitor (Table 16). In addition, statistical analyses demonstrated that the AF burden measured with the ICM was well correlated with the reference value derived from the Holter monitor (Pearson coefficient = 0.97).
| ICM outcome | Study: ICM (%) | |||
|---|---|---|---|---|
| Hindricks et al.50 (XPECT study): Reveal XT | Pürerfellner et al.86 (XPECT data set): Reveal XT with P-sense enhancement | Sanders et al.87 (LINQ usability study): Reveal LINQ | Pürerfellner et al.34 (LINQ usability data set): Reveal LINQ with adaptive P-sense (TruRhythm™; Medtronic plc) | |
| Sensitivity | 96.1 | 96.1 | 97.4 | 100 |
| Specificity | 85.4 | 90.0 | 97.0 | 99.0b,c |
| PPV | 79.3 | 84.9a | 92.5 | 97.4b,d |
| NPV | 97.4 | 97.5a | 99.0 | 100d |
| Accuracy | 89.3 | 92.2a | 97.1 | 99.3e |
Pürerfellner et al. 86 used the XPECT trial data set and applied a change to the ICM AF detection algorithm so that it also incorporated data on P-waves when classifying patients with AF (this algorithm change was applied in the Reveal LINQ). The revised data set was compared with the original Holter monitor data and the results are presented in Table 16.
The Reveal LINQ usability study (Sanders et al. 87) was a non-randomised, single-arm prospective multicentre observational study to assess the diagnostic accuracy of the new Reveal LINQ ICM for AF detection using 24 hours of Holter monitoring as the reference standard. The Holter monitoring was scheduled to occur at the 1-month follow-up visit, 1 month following ICM insertion, and AF had to be of ≥ 2 minutes’ duration. The patients enrolled in the Reveal LINQ usability study comprised 30 patients with any indication for an ICM and 121 patients with a documented history of AF (including patients awaiting AF ablation). The reference standard and patient population are, therefore, different in this study to those in the XPECT study. The results of the Reveal LINQ usability study are summarised in Table 16. A total of 138 patients had Holter monitor recordings that were suitable for inclusion in the analyses and the ICM correctly identified 37 of the 38 patients with Holter-detected AF (diagnostic sensitivity of 97.4%). The results of the new AF detection algorithm in the Reveal LINQ ICM demonstrate an improvement in terms of AF detection compared with the Reveal XT.
The Pürerfellner et al. 34 study was similar to Pürerfellner et al. 86 study in that it was applying a further P-wave-related algorithm enhancement for the Reveal ICMs AF detecting capability to existing data sets to see what impact it had on the diagnostic accuracy of the ICMs. The Pürerfellner et al. 34 study used both the XPECT and Reveal LINQ usability study data sets. The first 56 patients in the XPECT study with suitable data were used as the development data set for testing the algorithm enhancement and then data from 176 patients were used as the validation data set. In addition, the algorithm enhancement [adaptive P-sense (TruRhythm)] was applied to the Reveal LINQ. The per-patient results were reported in the paper only for the LINQ usability study data set (see Table 16), although the EAG notes that no explanation was provided for the discrepancy in the number of patients with AF diagnosed on Holter monitor in the TruRhythm analysis reported in Pürerfellner et al. 34 compared with in the Sanders et al. 87 publication for the LINQ usability study (37 vs. 38 patients, respectively). Nonetheless, assuming the results of the Pürerfellner et al. 34 study are accurate, they suggest that the new adaptive P-sense (TruRhythm) enhancement results in an improvement in sensitivity, specificity and accuracy in the Reveal LINQ in detecting AF. Medtronic reported in their company submission that the Reveal LINQ with TruRhythm detection was rolled out in 2017.
The results of the DTA studies in the non-CS population suggest that the enhancements over time to the AF diagnosis algorithm in the Reveal XT and Reveal LINQ ICMs have improved the DTA of the ICMs (sensitivity and specificity; see Table 16). However, it should be noted that these data are not in the CS population and the data in the XPECT and Reveal LINQ usability studies used to make some of these comparisons are heterogeneous owing to differences in the way in which the reference standard was applied (Holter monitoring 48 hours vs. 24 hours for the XPECT and Reveal LINQ usability studies, respectively) and differences in the patient populations (e.g. reasons for ICM insertion). Nonetheless, these data suggest that the Reveal LINQ is likely to be as effective at, if not better than, detecting AF as the Reveal XT (as the Reveal LINQ has fewer false positives and fewer false negatives); therefore, the AF detection rate from the CRYSTAL-AF trial is potentially a conservative estimate for the Reveal LINQ, given that it was the Reveal XT that was used in the CRYSTAL-AF trial.
Mittal et al. 85 reported AE data for two observational studies of the Reveal LINQ; one was the LINQ usability study87 already discussed and the second was the Reveal LINQ registry. The registry is a post-market surveillance study of patients with a Reveal LINQ ICM for any indication (the proportion of people with a CS indication is not reported) and the AE data discussed in Mittal et al. 85 for this study were limited to 122 patients from seven centres who were enrolled pre device insertion. The combined cohort of 273 patients from the two studies had an infection rate of 1.5% (n = 4), an AE rate of 4.0% (n = 11) and a SAE rate of 1.1% (n = 3). The company highlighted that the definition of an AE varies across studies and the EAG notes that the analysis in Mittal et al. 85 does not take into account the differences between the two study populations; thus, the analysis is subject to clinical heterogeneity. The results do, however, suggest that the Reveal LINQ is associated with a low rate of AEs and SAEs.
Summary of clinical effectiveness results
Quantity and quality of evidence
-
The clinical evidence searches sought to identify RCTs and comparative observational studies that compared any of the three devices [Confirm Rx (Abbott), BioMonitor 2-AF (Biotronik) and Reveal LINQ (Medtronic)] with at least 24 hours of Holter monitoring to detect AF in people with CS. Electronic database searches were run on 13 September 2018 and results were assessed together with reference lists of systematic reviews and evidence submitted by the companies.
-
A single RCT37 assessing an earlier Medtronic Reveal model (XT rather than LINQ) met the original eligibility criteria (the CRYSTAL-AF trial), so the criteria were widened to find evidence for the BioMonitor 2-AF, Confirm Rx and Reveal LINQ. First, non-comparative observational studies were sought within the correct CS population, and then evidence was considered from studies of mixed populations submitted by each company. Only the CRYSTAL-AF trial37 falls within the eligibility criteria outlined in the original published protocol for this diagnostics assessment, so the additional evidence should be interpreted with caution.
-
The CRYSTAL-AF trial37 (n = 441 participants) represents the most robust clinical evidence to inform the decision problem, despite assessing the Reveal XT. The study was open-label and compared the ICM with conventional follow-up in a population of people who had a CS or TIA and no history of AF after extensive diagnostic work-up. The study was conducted in North America and Europe and the population was considered generally applicable to patients who would be eligible for an ICM in UK clinical practice.
-
Twenty-six single-arm observational studies were identified after widening the eligibility criteria to include non-comparative studies. The studies were conducted in North America and western Europe (one in the UK) and all assessed the Reveal XT and Reveal LINQ in populations of people who had had a CS or TIA; none provided evidence to assess the efficacy of the BioMonitor 2-AF (other than a mixed device study that did not provide separate results) or Confirm Rx for patients who had had a CS. The observational studies represent a wide sample of patients who have received an ICM in practice (n = 3414) and provide evidence for the Reveal LINQ and for additional outcomes that were not available from the CRYSTAL-AF trial.
-
In total, one study65 for Confirm Rx (of an older model, the Confirm DM2102), five studies of the BioMonitor 2-AF77–84 (all BioMonitor 2 but only one80 of which we can be certain was of the ‘-AF’ model) and five studies34,50,85–87 of the Reveal LINQ [three studies34,85,87 of the Reveal LINQ and three50,86 studies of the Reveal XT (one study34 included both devices)] in mixed populations were included based on the recommendations of the companies. All of these mixed population studies are either single-arm observational studies or they provide DTA data for the ICM using Holter monitoring as the reference standard.
-
All the observational studies were single-arm and therefore were deemed to be at a high risk of bias. Three conducted within-patient comparisons of ICM versus other monitoring strategies. 14,15,72 Key sources of heterogeneity between the observational studies include patient demographics (mean or median age 52–72 years), rigour of stroke assessment, stroke risk score (CHA2DS2VASC score of 3–5), definition and adjudication of AF and length of follow-up; all are likely to affect AF detection and other clinical outcomes.
-
Eight ongoing studies40–46 of potential relevance were identified, although only five (three RCTs40,42,47 and two observational studies41,45) reported details of their current status and the ICM being studied and none relates to the BioMonitor 2-AF. The three ongoing RCTs all involve the Reveal LINQ, with only one RCT solely in a CS population: a Canadian randomised trial comparing the clinical effectiveness and cost-effectiveness of the Reveal LINQ ICM with external loop recording in 300 CS patients, which is estimated to complete in December 2019 (PERDIEM). 47 There was only one ongoing study identified relating to the Confirm RX: the SMART registry,45 a post-approval study planning to recruit at least 2000 patients with Confirm RX across multiple indications, but with a planned subgroup analysis for CS; completion is expected in December 2020.
Overview of effectiveness results
-
Atrial fibrillation detection rate was the primary outcome in the CRYSTAL-AF trial (at 6 months), and all 26 observational studies. Other outcomes reported by the CRYSTAL-AF trial and the observational studies were AF at longer follow-ups (up to 36 months), time to AF detection, uptake of anticoagulants, device removals, subsequent stroke and AEs. Quality-of-life data are available only from the CRYSTAL-AF trial, and diagnostic accuracy and detection of other arrhythmias were available only from the observational or mixed population studies.
-
Diagnostic accuracy (CRYSTAL-AF and observational studies): the CRYSTAL-AF trial was designed to measure diagnostic yield rather than accuracy, and none of the observational studies provided comparative DTA between an ICM and standard monitoring. Two studies modelled patient AF detection data from the CRYSTAL-AF trial (Choe et al. 14) and a large patient registry (Ziegler et al. 15) with repeated iterations (10,000) to estimate the number of patients whose AF would not have been detected should an intermittent monitoring strategy have been used (based on assumption that the ICM has 100% sensitivity). The studies found that the best-performing intermittent monitoring strategy detected less than one-third of AF detected by the ICM (ranging from around 3% for a single 24-hour Holter monitor to 30% with a quarterly 7-day Holter monitor). Studies reporting false positive rates as the proportion of episodes detected by ICM algorithm that were not subsequently verified by a clinician were highly dependent on model and sensitivity configuration.
-
Diagnostic accuracy (mixed population studies): the results of the mixed population DTA studies suggest that the enhancements over time to the AF diagnosis algorithm in the Reveal XT and Reveal LINQ ICMs has improved the DTA (sensitivity and specificity) of the ICMs. A naive comparison of the sensitivity and specificity data from non-CS or mixed populations in the studies flagged of relevance by the respective companies of the Confirm DM2102 (older model of Confirm Rx) and Reveal LINQ suggests that they both have 100% sensitivity for AF detection, although specificity varies (85.7% and 99.0%, respectively); the BioMonitor 2 (confidential information has been removed). However, it should be noted that this analysis is subject to clinical heterogeneity in terms of the patient populations, interventions and study designs. In addition, as discussed earlier, the device-related performance of ICMs is dependent on the patient population and the incidence rate of AF. These data are thus not necessarily reflective of the respective ICM performance in CS patients and, also, they do not necessarily reflect the performance of the current device model firmware; for example, the Confirm Rx data are based on an earlier model.
-
Diagnostic yield: AF detection in the CRYSTAL-AF trial was higher with the Reveal XT than with conventional follow-up at all time points. At the primary 6-month analysis, AF had been detected in 19 (8.6%) patients with an ICM and in 3 (1.4%) patients in the conventional follow-up group. By 36 months, the numbers of patients detected were 42 (19%) with an ICM and 5 (2.3%) with conventional follow-up, demonstrating the continued and increasing benefit of ICM monitoring. AF detection rates reported at the primary follow-up (6–24 months) across the 26 observational studies were highly variable, ranging from 6.7%38 (Reveal LINQ and XT at 12 months) to 40.9%68 (Reveal XT, unknown follow-up). These data demonstrate that, even within a CS population, AF detection rates are highly variable, and it is impossible to make any meaningful comparison between the observational studies and the CRYSTAL-AF trial. Observational studies reporting AF detection at different lengths of follow-up indicate that a minority of patients are diagnosed within the first month (mostly in the region of 10% of those detected by 1 year), around 70–80% by 6 months and a small number beyond 1 year of monitoring. 15,52,58,72,74 In comparison, the 36-month data from the ICM arm of the CRYSTAL-AF trial show higher proportions of AF diagnosed at 1 month (19.0%) and beyond 12 months (31.0%) and a lower proportion at 6 months (45.2%) than the observational studies. The EAG reiterates that synthesis of the observational studies was considered inappropriate because of clinical heterogeneity (see Limitations of the evidence). When described, all or most AF detected was asymptomatic and so would probably not have been picked up without continuous ICM monitoring.
-
Time to diagnosis of AF: the median time to AF detection was longer for patients with the Reveal XT in the CRYSTAL-AF trial than for patients receiving conventional follow-up at 6, 12 and 36 months [36 months: hazard ratio (HR) 8.8, 95% CI 3.5 to 22.2; p < 0.001], which is partly because of the significantly higher AF detection rates with the ICM. The benefit of the ICM increased with length of follow-up because very few patients in the conventional follow-up arm were diagnosed, whereas detection continued steadily in the group with an ICM. Eighteen observational studies (five Reveal LINQ, seven Reveal LINQ or XT, five with Reveal XT, and one with Reveal XT or BioMonitor), at average follow-up of between 7 and 20 months, showed highly variable median time to first AF detection, ranging from 21 to 217 days. These results are, however, broadly consistent with the results from the CRYSTAL-AF trial, in which median time to AF diagnosis was 41 (IQR 4–84) days at 6 months’ follow-up, 84 (IQR 18–265) days at 12 months’ follow-up and 8.4 months (IQR not reported) at 36 months’ follow-up.
-
Detection of other arrhythmias: three of the observational studies, primarily of the Reveal LINQ, suggest that the proportion of patients for whom the ICM detected other arrhythmias is in the region of 10%, consisting mainly of bigeminy, pause and bradycardia. No information was presented about whether or not and how the detected arrhythmias were treated to prevent related complications, and other arrhythmias were not available from the CRYSTAL-AF trial.
-
Uptake of anticoagulation: in the CRYSTAL-AF trial, > 90% of patients diagnosed with AF in the ICM arm started an OAC. Data were available only for the conventional follow-up group irrespective of AF diagnosis, indicating that 8.3% were on an anticoagulant by 36 months (24 patients, whereas five had been diagnosed with AF by that time point). In seven observational studies of Reveal LINQ and/or XT, uptake of anticoagulants in patients detected with AF was in the region of 90–100%. Time to anticoagulation and AEs related to anticoagulant use were not reported in any of the identified evidence.
-
Device failures (battery, transmission, removal): after 36 months, five devices had been removed because of infection or pocket erosion in the CRYSTAL-AF trial (2.4%). In the observational evidence, three studies of Reveal LINQ and/or XT52,65,72 reported removals, but it was often not clear if they were for tolerability, battery life or after AF detection. Two observational studies reported a small number of premature device removals for reasons such as skin reactions, migration or discomfort (0.9–5.7%) in line with the CRYSTAL-AF trial (2.4%). At 12 months’ follow-up, 3.4% of ICMs had been removed in the CRYSTAL-AF trial; in contrast, in the Ritter et al. 72 study (Reveal XT), in which removal after AF detection was offered in addition to removal for other reasons, 30% of patients had their ICM device removed during the study (median follow-up time in the study for all patients was 13 months).
-
Subsequent stroke and TIA: in the CRYSTAL-AF trial, recurrent stroke or TIA rates were 5.0% in the ICM arm versus 8.2% in the conventional follow-up arm at 6 months (p > 0.05). At 12 months, rates were 6.8% and 8.6% for the ICM and conventional follow-up arms, respectively, and at 36 months, rates were 9.0% and 10.9%, respectively; none suggests statistically significant stroke prevention benefits of the Reveal XT compared with conventional monitoring. Six of the observational studies, primarily assessing the Reveal XT, also observed relatively low stroke recurrence rates in the first year after device implantation (most were < 7% in line with the CRYSTAL-AF trial, range 0–14.6%). It was unclear how many recurrent strokes occurred in patients diagnosed with AF, and no studies reported other thromboembolisms or related morbidities.
-
Adverse events: Device-related AEs, such as pain and infection, were low in the CRYSTAL-AF trial, the single-arm observational studies and the mixed population studies. In the CRYSTAL-AF trial, the rate of SAEs was similar between groups (around 25–30%), but more patients in the ICM group than in the conventional follow-up group had non-serious AEs (18.6% vs. 4.1%, respectively). No procedure or insertion site complications were reported in the Reveal LINQ and XT observational studies, and none of the studies reported AEs relating to anticoagulation.
-
Health-related quality of life: EQ-5D data collected throughout the CRYSTAL-AF trial were (confidential information has been removed).
-
Ease of use for clinicians and acceptability to patients: the CRYSTAL-AF trial did not collect any ease-of-use or acceptability data, and information from the observational studies was anecdotal. However, company submissions and the EAG’s clinical experts reported that the newer models of the ICMs (e.g. Reveal LINQ and Confirm Rx) were easier to insert and were suitable for insertion by trained nurses and cardiac physiologists, which could help to free up clinician time.
Limitations of the evidence
-
Despite extensive evidence searches, the clinical evidence for this DAR is based primarily on a single RCT for the older Medtronic Reveal XT device. Clinical expert opinion and evidence from a mixed population suggest that the Reveal LINQ has better sensitivity and specificity than the XT and leads to fewer complications because of its size, but there are no head-to-head clinical trials to confirm these findings in a CS population. 28
-
Despite widening the eligibility criteria to include low-quality non-comparative observational studies, no data were found for the BioMonitor 2-AF or Confirm Rx devices; evidence for these devices is limited to mixed population diagnostic accuracy and single-arm observational studies submitted by the companies.
-
No evidence was found for any device for several outcomes (mortality, hospital and outpatient care for AF, related morbidities, AEs related to anticoagulation), and information about the ease of using each device for clinicians and their acceptability to patients was anecdotal or limited to data supplied in observational studies flagged by the companies.
-
The EAG’s clinical experts considered the CRYSTAL-AF trial generally reflective of UK clinical practice, although all UK patients undergo transthoracic echocardiography and some patients who were excluded from the trial might be considered for an ICM (i.e. those with a history of MI). Patients in the CRYSTAL-AF trial were slightly younger than would be expected and all patients would be expected to be on an antiplatelet agent in UK clinical practice.
-
Most patients in the CRYSTAL-AF trial had received a median of 23 hours of Holter monitoring (71.2%), but the remainder received a median of 68 hours of inpatient telemetry monitoring (29.7%), which is not in line with the scope of this DAR, which required a minimum of 24 hours’ outpatient monitoring. Other issues noted with the CRYSTAL-AF trial, such as baseline differences (e.g. in the proportion of patients with PFO and history of prior stroke), crossover between groups, insertion delays (11.5%) and withdrawals, are unlikely to have an important impact on the results of the CRYSTAL-AF trial.
-
Atrial fibrillation detection rates vary considerably between and within the types of evidence considered by the EAG, (the CRYSTAL-AF RCT, uncontrolled observational studies, mixed population studies). The EAG recommends caution in drawing conclusions from naive comparisons between the additional studies owing to the number of uncontrolled variables and inherent biases of their single-arm design. Sources of heterogeneity that probably contribute to the differences in AF detection include the episode threshold used (varying from 10 seconds to 2 minutes), population characteristics (such as stroke risk score), time from stroke to ICM insertion, duration of follow-up and method of AF adjudication. The CRYSTAL-AF trial is the most robust evidence on which to base conclusions of ICM efficacy.
-
There is evidence from the observational studies that the ICMs also detected some non-AF cardiac arrhythmias, although no data on this additional potential benefit of ICMs were available from the CRYSTAL-AF trial, and there were no data comparing ICMs with electrocardiography monitoring in terms of the detection of non-AF cardiac arrhythmias. It is also unclear whether or not detecting these additional arrhythmias led to any change in the management of the patients in whom they were identified.
-
The open-label design of the CRYSTAL-AF trial introduces potential bias because the outcome assessor was aware of the intervention assignment and was able to influence the ECG or other assessment of AF. The ICM arm was unlikely to be affected by bias relating to the outcome assessor as all episodes of AF that qualified for analysis were adjudicated by an independent committee.
-
The results of the mixed population DTA studies suggest that the Reveal LINQ is likely to be as effective as, if not better than, the Reveal XT at detecting AF (as the Reveal LINQ has fewer false positives and false negatives); therefore, the AF detection rate data from the CRYSTAL-AF trial are, potentially, a conservative estimate for the Reveal LINQ, given that it was the Reveal XT that was used in the CRYSTAL-AF trial.
Chapter 4 Methods for assessing cost-effectiveness
The EAG’s economic evaluation assessed the cost-effectiveness of ICMs compared with no further monitoring, to detect AF in people who have had a CS, including TIAs, and have received at least 24 hours of non-invasive external cardiac monitoring. A systematic literature review (SLR) of existing economic evaluations was undertaken to inform the conceptualisation and development of a de novo economic model.
Systematic literature review for cost-effectiveness studies
Methods
A systematic review of the literature was undertaken in September 2018 to identify published economic evaluations of ICMs to detect AF in people who have had a CS. The sources identified in those searches were also used to identify resource use and cost data that could be utilised in the economic model. In addition, one further systematic review was conducted, in September 2018, aiming to identify studies providing utility (generic, preference-based) data on the HRQoL of people with AF and stroke, that could be used for the estimation of quality-adjusted life-years (QALYs) in the economic model.
The following databases were searched for relevant studies:
-
MEDLINE and Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Daily and Versions (via Ovid)
-
EMBASE (via Ovid)
-
EconLit (via Ovid)
-
NHS Economic Evaluation Database (via the CRD)
-
CDSR (via The Cochrane Library)
-
CENTRAL (via The Cochrane Library)
-
DARE (via the CRD)
-
HTA database (via the CRD).
Further to the database searches, experts in the field were contacted with a request for details of relevant published and unpublished studies of which they may have knowledge; reference lists of key identified studies were also reviewed for any potentially relevant studies.
The search strategy for existing economic evaluations and studies reporting resource use or cost data combined terms capturing the interventions of interest (ICM, i.e. Reveal LINQ, BioMonitor 2-AF and Confirm Rx) and the target population (patients who have had a CS) with economic or health-care resource use terms, applied to all electronic databases. The search strategy for HRQoL data was not restricted by intervention, and combined terms capturing the target population with HRQoL terms.
The search for resource use and cost data was limited to the UK NHS setting, as the aim of this search was to identify data directly relevant to the NHS context that could inform the economic model; however, no country restrictions were applied to searches for existing economic evaluations.
Owing to the high volume of hits in the searches for HRQoL evidence, searches were restricted by date, starting from 1997; the year 1997 was selected as this was the year the utility index for the EQ-5D was published. Studies were then restricted to those collecting data in the Organisation for Economic Co-operation and Development countries, as HRQoL data collected in low income countries were unlikely to be generalisable to the UK.
Initially, the EAG considered studies reporting utility data elicited using a generic, preference-based measure [EQ-5D, Health Utilities Index, Short Form questionnaire-36 items (SF-36), Short Form questionnaire-6 Dimensions (SF-6D)] or self-reported validated, choice-based technique for valuation (i.e. time trade-off or standard gamble). However, given the availability of relevant EQ-5D data in this population (made apparent to the EAG during the first sift) and NICE’s preference for EQ-5D data, the EAG decided to restrict studies to primary sources of EQ-5D data.
Limits were applied to all searches to remove animal studies, letters, editorials, comments or case studies. Only conference abstracts published in the previous 2 years were considered for inclusion; it was assumed that any high-quality studies reported in abstract form before that date would have been published in a peer-reviewed journal. Full details of the search strategies are presented in Tables 38–46 in Appendix 5.
The titles and abstracts of papers identified through the searches were independently assessed for inclusion by two health economists using predefined eligibility criteria. Owing to the high volume of studies retrieved by the HRQoL search, one health economist reviewed all identified citations and a second health economist reviewed 20% of citations to confirm that the same studies were included for second pass.
The inclusion and exclusion criteria for each of the three systematic reviews described above are outlined in Box 1. The methodological quality of the full economic evaluations identified in the review was assessed using the Drummond checklist. 88
-
Intervention or comparators according to the scope of the assessment (ICMs).
-
Study population according to the scope of the assessment (people with AF or who have had a cryptogenic embolic stroke or cryptogenic TIA).
-
Full economic evaluations (cost–utility, cost-effectiveness, cost–benefit or cost–consequence analyses) that assess both costs and outcomes associated with the interventions of interest.
-
Economic evaluations that utilise clinical effectiveness data from randomised or non-randomised clinical trials, prospective cohort studies or systematic reviews and meta-analyses of clinical studies; economic analyses that utilise clinical data from studies with a mirror-image or other retrospective design will not be considered.
-
Study population according to the scope of the assessment (people with AF or who have had a cryptogenic embolic stroke or cryptogenic TIA).
-
UK resource use or costing studies.
-
Any setting (to be as inclusive as possible).
-
Study population according to the scope of the assessment (people with AF or who have had a cryptogenic embolic stroke or cryptogenic TIA).
-
UK resource use or costing studies.
-
Any setting (to be as inclusive as possible).
-
Abstracts with insufficient methodological details.
-
Conference papers published 2 years before the search was performed (September 2018).
Results
Economic evaluations
The SLR identified a total of 41 papers after de-duplication and, based on title and abstract, a total of nine papers (including one unpublished report supplied by Biotronik) were identified as potentially relevant and were obtained for full-text review based on the criteria listed in Box 1. Of the nine papers identified for full-text review, five papers were included for data extraction (see Appendix 7, Table 50). 89–93 The results of the searches are summarised in Figure 2. Reasons for exclusion of the ordered papers are provided in Appendix 6 (see Tables 47 and 48).
FIGURE 2.
The PRISMA flow diagram of the economic evaluation SLR. NHS EED, NHS Economic Evaluation Database.
All economic evaluations meeting the inclusion criteria in the SLR were based on a Markov model structure with model cycles ranging from 1 to 3 months. 89–93 Two studies assessed the cost-effectiveness of Medtronic’s Reveal XT device with standard of care monitoring (SoC). 89,90 One study was based on Biotronik’s BioMonitor 2-AF device compared with SoC. 91 (Confidential information has been removed.) Two studies did not indicate which model or brand of ICM was being assessed in the economic evaluations. 92,93
Of the five studies, only one was based on the UK (NHS) payer perspective; therefore, it will be the focus of a more in-depth analysis of model structure and parameter estimation. 90 In addition to the search, the manufacturer of BioMonitor 2 made an unpublished report and economic model available to the EAG, which assessed the cost-effectiveness of BioMonitor 2-AF in patients who had had a CS. (Confidential information has been removed.)
The study by Diamantopoulos et al. 90 is a cost–utility analysis assessing the use of an ICM (Reveal XT) to detect AF in patients who have had a stroke or TIA that is considered cryptogenic after an initial 24-hour period of non-invasive external Holter monitoring. The comparator in the study was no further monitoring after the initial 24-hour period. The perspective of the analysis was the UK NHS, and the time horizon of the model was lifetime.
The model was developed using a Markov structure with three main health states for AF status: AF free, AF detected and AF undetected. Figure 3 presents the model schematic.
FIGURE 3.
Model schematic of the CRYSTAL-AF trial cost-effectiveness analysis. CRNMB, clinically relevant non-major bleeding; DOAC, direct oral anticoagulant; ECH, extracranial haemorrhage; HS, haemorrhagic stroke; IS, ischaemic stroke. Reproduced from Diamantopoulos et al. 90 International Journal of Stroke (volume 11, issue 3), pp. 302–12, copyright © 2016 by SAGE Publications. Reprinted by Permission of SAGE Publications, Ltd.

Patients start in the AF-free state, from which they can move to the AF-undetected or AF-detected states at any given model cycle. From the AF-undetected state, patients can either remain or move to the AF-detected state, and a patient remains in the AF-detected state unless she/he experiences a subsequent cerebrovascular event or bleeding event as follows.
The consequences of these subsequent events were modelled in two separate categories for either temporary or permanent effects. Events with temporary consequences were non-fatal extracranial haemorrhage (ECH) or intracranial haemorrhage (ICH), or a clinically relevant non-major bleed. Events with permanent consequences were non-fatal IS, non-fatal haemorrhagic stroke (HS), or fatal ECH, ICH, IS or HS events. Deaths of any cause could also occur from any heath state in the model.
Following a temporary event, patients return to their previous AF status health state and can continue to move between these health states as described previously. For patients who moved to a post-stroke health state following a permanent event, patients were assumed to remain there and face no further risk of stroke or bleeding events, with the only possible remaining transition being to the death state.
Treatment in the AF-free and AF-undetected states was assumed to be aspirin. In the AF-detected state, treatment was assumed to change to a directly acting oral anticoagulant (DOAC) until a bleeding event (HS, other ICH or ECH) occurs, at which point patients were assumed to revert to aspirin.
The risk of subsequent IS was determined by AF status, virtual CHADS2 score, age and treatment received. Evidence was synthesised from six studies, which included systematic reviews, RCTs and registry data. The severity of IS was considered to measure the expected impact on quality of life and resource use. The distribution of severity (mild, moderate, severe and fatal) was taken from two published cost-effectiveness analyses,94,95 comparing anticoagulant treatments for stroke prevention in patients with AF. The distribution of severity was assumed to be independent of treatment, so the average across all treatments was used.
Bleeding consequences were also included in the model and the risks were assumed to be treatment and age related. Data for these risks were derived from five studies96–100 including a systematic review plus various trials comparing anticoagulants for patients with AF. The same cost-effectiveness analyses used to inform the distribution of IS severity were used to inform the distribution of type and severity of bleeding events, which were also assumed to be independent of treatment.
Age-dependent mortality was applied in the model and based on interim UK life tables. 101 It was adjusted, when applicable, to exclude deaths caused by cerebrovascular events, as these were modelled separately. Following a non-fatal stroke, the mortality risk was increased depending on the severity of stroke and the treatment received for it.
Health-related quality-of-life data for patients experiencing stroke events were collected in the Oxford Vascular Study (OX-VASC). 102 Disutilities associated with bleeding events were also included and informed by two published models. 94,95,103 Utilities were adjusted to account for age and sex using previously published methods.
The price year of the model was 2013. Costs for the insertion of the ICM (£1836) were included in the economic analysis, as were per-cycle costs to account for follow-up visits and monitoring, as well as drug treatments. The resource use required was determined by an unpublished post hoc analysis of the CRYSTAL-AF trial data. The lifetime of the ICM was assumed to be 3 years, at which point the device was removed. The cost of removal (£491) was also included. These costs were sourced from NHS Reference Costs 2012 to 2013. 104 Costs associated with events such as stroke were included, as well as estimated long-term costs associated with living in a post-stroke health state.
Results of the deterministic base-case analysis showed that an ICM was £2587 more expensive than SoC and provided a benefit of 0.151 QALYs, resulting in an incremental cost-effectiveness ratio (ICER) of £17,175 per QALY gained. A probabilistic sensitivity analysis (PSA) was performed, which reduced the incremental cost to £2574 and increased the QALY gain to 0.161, thereby reducing the ICER.
The EAG considers that the results produced by the model are potentially unreliable as there is significant uncertainty around the estimation of the clinical parameters in the model, particularly around the estimation of treatment effects by indirect comparison, AF incidence and detection rates used in Medtronic’s analysis. The authors conducted an indirect comparison to estimate HRs for IS, bleeding events, ICHs, ECHs and mortality that are conditional on treatment received. The EAG attempted to validate the HRs used in the model but could not verify the source data used by the authors: details of how the indirect comparison was conducted, as well as how the publications informing the analysis were identified, were not sufficiently described. Furthermore, the EAG considers that estimation of some of the HRs could be flawed; for example, the authors estimate a HR to adjust mortality in the model, but the source data used is based on standardised mortality ratios.
Finally, patients who are not detected as having AF are assumed to be given aspirin as their treatment option. However, the EAG’s clinical experts stated that patients would be given clopidogrel (75 mg) as their antiplatelet treatment.
However, the EAG considers that the initial health states of the Diamantopoulos et al. 90 model to determine AF status is useful to inform a short-term model, in which the time horizon is linked to the battery life of an ICM device. From the short-term model, patients with AF (whether detected or undetected) will then feed into a long-term (lifetime) model, assessing the costs and benefits of anticoagulation therapy.
Models assessing the long-term impact of anticoagulation therapy for patients with atrial fibrillation
In addition to the SLR, the EAG were notified by NICE of an ongoing DAR for lead-I ECG devices for detecting AF using single-time point testing in primary care [Diagnostic Assessment Programme 39 (DAP39)]. 105 The population considered in DAP39105 is adults presenting to primary care with signs and symptoms of AF who have an irregular pulse. Lead-I ECG devices are handheld instruments that can be used in primary care to detect AF at a single time point in people who present with relevant signs and symptoms (i.e. palpitations, dizziness, shortness of breath and tiredness). 105 If a lead-I device detects AF, the patient initiates anticoagulation and rate control therapy (unless contraindicated) and a 12-lead ECG is conducted to provide more diagnostic information and inform treatment. The EAG assumed anticoagulation therapy would be with apixaban, which is a simplifying assumption.
The comparator in this study was no further immediate testing after manual pulse palpation (MPP), with patients referred for a 12-lead ECG if the general practitioner (GP) was suspicious of AF after MPP (standard care pathway). In the standard care pathway, no AF treatment is initiated if the GP is suspicious of AF until after the results from the 12-lead ECG are available, confirming diagnosis.
Although the technology and population under assessment are not relevant to the decision problem of the current report, the EAG was interested in the approach taken to estimate long-term costs and benefits of anticoagulation therapy once patients have been identified as having AF using lead-I devices. For DAP39,105 once patients had a diagnosis of AF confirmed, they entered a post-diagnostic Markov model with either no history of cardiovascular events (CVEs), one CVE or two CVEs. In each cycle, patients can remain in their current health state, have a CVE and move to a worse health state, or die. Patients with two CVEs can remain in their current health state only until death.
The model parameters used to estimate the transition probabilities for the post-diagnostic Markov model were derived mainly from a cost-effectiveness study by Sterne et al. ,106 which assessed the long-term costs and benefits of anticoagulation therapy for prevention of stroke in patients with AF (hereafter referred to as the DOAC model). The EAG reviewed the publication for the DOAC model and deemed it relevant for the current decision problem, and contacted the authors to obtain a copy of the model for assessment. After reviewing the DOAC model and discussing it with the model developer, the EAG was made aware of an adapted version of the DOAC model, which was used for another publication,107 that would be appropriate to review and potentially use for development of the ICM model.
The adapted DOAC model was developed to assess the cost-effectiveness of screening strategies for AF. 107 The model structure employed by the authors was a hybrid model, with a short-term decision tree that used sensitivity and specificity estimates of different screening strategies to detect AF (confirmed by a 12-lead ECG) and initiate anticoagulation therapy and a long-term adapted version of the DOAC Markov model. In the analysis, it was assumed that 75% of patients not contraindicated to anticoagulation therapy and who are prescribed anticoagulants use DOACs, with the remaining 25% prescribed warfarin. Patients who are diagnosed with AF, but who are contraindicated to anticoagulation therapy, not prescribed or choose not to take OACs, would receive aspirin.
The results of the screening decision-tree model feed directly into the adapted DOAC model (Figure 4). The discrete-time Markov multistate model implemented a cycle length of 3 months and employed a lifetime horizon with a cut-off point at 100 years. Patients who are prescribed an OAC enter the model either on first-line apixaban or warfarin [international normalised ratio (INR) range 2–3], with the remainder on aspirin. The authors assumed the use of apixaban, as it was determined to be the most cost-effective DOAC in the anticoagulation therapy cost-effectiveness analysis, but state that the results are similar when considering other available DOACs.
FIGURE 4.
Prevention of stroke in AF model structure. MB, major bleed; S, stroke. Reproduced from Sterne et al. 106 Contains information licensed under the Non-Commercial Government Licence v2.0.

Depending on the occurrence of IS or SAEs (such as ICH), treatment switching can occur (Figure 5). For patients on first-line apixaban, second-line treatment may be either warfarin or no treatment. No treatment is the only third-line treatment available. For those who fail on warfarin, no further treatment would be given. 106
FIGURE 5.
Treatment strategies and switching/discontinuation rules for the prevention of stroke in the AF model. MB, major bleed; SE, systemic embolism. Reproduced from Sterne et al. 106 Contains information licensed under the Non-Commercial Government Licence v2.0.

The same model structure is used for each treatment option (see Figure 4), but is adjusted for the different costs, utilities and transition probabilities relevant to treatment. Patients start the model in the AF well health state (no event). From any health state in the Markov model, patients can have an IS, a MI, a clinically relevant (extracranial) bleed (CRB), an ICH, a systemic embolism, a TIA or die. The authors of the DOAC model assumed that systemic embolism and TIA have only short-term impacts on future risks, costs and utilities, but IS, ICH, CRB and MI have long-term impacts that will change future risks, costs and utilities. For example, a patient who experiences a MI and ICH will have different risks, costs and utilities compared with a patient who experiences only a MI or ICH. In addition, the model does not distinguish between minor and major ISs because of limited published evidence on the relative rates of these events from the RCTs.
As with all Markov models, patient history through the model is not recorded; therefore, future health state transitions depend only on the current health state the patient occupies. An assumption is made in the model that transition probabilities do not change with time but that, as the cohort ages, mortality risk increases in line with general population life tables.
The authors of the screening model adapted the long-term DOAC model by including HRs for events (stroke, systemic embolism, TIA) affected by AF type (paroxysmal relative to permanent or persistent). Furthermore, the DOAC model depends on age, sex, previous history of IS or TIA and previous history of MI.
Treatment effects implemented in the model were based on a competing risks network meta-analysis (NMA) to jointly estimate log HRs of each treatment relative to warfarin for the different possible health states in the model.
The costs included in the analysis comprised pharmacotherapy costs and costs of acute and chronic AF and anticoagulant-related events. Sources of cost data included the British National Formulary (BNF) for drug costs (March 2015 update),108 NHS reference costs109 and other published sources. When necessary, cost data were inflated to 2015 prices using the Office for National Statistics (ONS) Consumer Price Inflation Index for Medical Services (DKC3). 110
Quality-adjusted life-years were estimated by applying health-state utility values to the proportion of patients occupying each health state per model cycle. Utilities were identified from a previous NICE technology appraisal submission on rivaroxaban (TA256),111 which included a systematic literature search for evidence on EQ-5D utility index scores in health states related to AF. For acute health states (such as CRB, systemic embolism, TIA, ICH, acute IS and acute MI), disutilities were applied for one model cycle. For patients who have multiple chronic health conditions, utilities for the health states were assumed to be multiplicative. All utilities were adjusted for age.
Total costs and QALYs estimated for each first-line anticoagulation therapy were generated as well as incremental results compared with warfarin. The authors did not calculate ICERs, but instead calculated the incremental net benefit (INB) of each DOAC compared with warfarin, when a QALY is valued at either £20,000 or £30,000. Compared with warfarin, all DOACs had a positive INB, with apixaban (5 mg twice daily) estimated to have the highest expected INB (£7533), followed by dabigatran (150 mg twice daily; £6365), rivaroxaban (20 mg once daily; £5279) and edoxaban (60 mg once daily; £5212). The 95% CI around the INB for apixaban was positive, suggesting that apixaban is cost-effective compared with warfarin.
The EAG considers that the adapted DOAC model is suitable to inform the long-term costs and benefits of anticoagulation treatment versus antiplatelet treatment in the CS population, who have suspected AF; therefore, the adopted DOAC model will be incorporated into the model structure assessing ICMs in this population. More detail on the integration of the DOAC model into the EAG’s Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) model is given later in the subsection that describes the development of the economic model (see Model structure and Chapter 5, Probabilistic sensitivity analysis).
Health-related quality-of-life evidence
The systematic literature search identified a total of 7641 papers after deduplication. Based on a review of titles and abstracts, 112 papers were identified as potentially relevant and were obtained for full-text review based on the criteria listed in Box 1. An additional two papers were identified from the reference lists of identified papers. Of the 114 papers identified for full-text review, 25 papers were included for data extraction (see Appendix 7, Table 51). Reasons for exclusion of the 89 papers are provided in Appendix 6, Table 49. The results of the process to identify HRQoL evidence is summarised in Figure 6.
FIGURE 6.
The PRISMA flow diagram of the HRQoL SLR. NHS EED, NHS Economic Evaluation Database.

Data from patients with cerebral infarction, IS, haemorrhagic stroke (intracranial, intracerebral or subarachnoid) or TIA were collected by 21 studies,102,103,112–130 and data on patients specifically with AF (with or without stroke) were collected in six studies. 112,113,121,131–133 Four of the included studies also provided data for patients with MI103,113,121,131 and two assessed the impact of additional bleeding events. 121,133 The studies differed in how stroke was defined, with some having much broader definitions than others, which hindered comparisons by type of stroke.
All studies reported EuroQol-5 Dimensions, three-level version, (EQ-5D-3L) data and two118,119 also collected EuroQol-5 Dimensions, five-level version, (EQ-5D-5L) data. EQ-5D responses were converted into utilities using UK population tariffs developed by Dolan134 in nine studies. 102,103,117,122–124,127,128,131 Two of those studies were undertaken in the UK. 102,132 The remaining studies were undertaken in Canada, Finland, Germany, Korea, Norway, Poland, Spain, Sweden, the USA, the Netherlands or in multiple countries.
The EAG considers that the most relevant utilities for the model are those from OX-VASC,102 which were also utilised in the CRYSTAL-AF trial economic evaluation90 and Berg et al. 131
The OX-VASC study consisted of stroke and TIA patients whose quality of life was assessed using the EQ-5D-3L questionnaire at regular follow-ups of 1, 6, 12, 24 and 60 months after their stroke or TIA. The baseline population consisted of 748 patients with stroke and 440 TIA patients. EQ-5D-3L responses were available for 759 patients at 1 month, 723 patients at 12 months and 479 at 60 months. EQ-5D-3L responses were converted into utilities using UK population tariffs. 102 The mean age of the population was 75 years and 44% were female. Utilities were estimated for different events including, TIA, all stroke, IS, ICH and subarachnoid haemorrhage, as well as different severities of stroke.
The study by Berg et al. 131 assessed HRQoL for patients with AF, based on data from the Euro heart survey. 135 The mean age of the population was 66 years and 41.9% were female. HRQoL was measured at baseline and at 1-year follow-up using the EQ-5D-3L questionnaire. At baseline, 5050 EQ-5D-3L responses were recorded, with 3045 responses recorded at 1-year follow-up. EQ-5D-3L responses were then converted into utilities using UK population tariffs. Baseline utility for patients with AF were estimated, as well as utility decrements for AEs during follow-up, including MI, stroke, congestive heart failure and other major AEs.
The utility values for events that have been included in the economic model are given in the subsection that describes the development of the economic model (see Utility values).
Development of a health economic model
Population
The population considered in the model are patients who have had a CS, including TIAs, for whom there is a suspicion of paroxysmal AF, and who have received at least 24 hours of outpatient external ambulatory electrocardiography monitoring that has not detected AF. The diagnostic data included in the model are based on the results of the SLR that identified the CRYSTAL-AF RCT37 assessing the Reveal XT ICM compared with SoC in the patient population of interest. The mean age (61 years) and sex split (≈65% male) of patients in the model is based on data from the CRYSTAL-AF trial. The EAG’s clinical experts considered that, in general, the population in the CRYSTAL-AF trial is reflective of UK patients; some inconsistencies were noted but were not deemed significant. See Chapter 3 for further detail.
Intervention and comparator
As per the NICE final scope, the interventions included in the model are as follows:
-
BioMonitor 2-AF
-
Confirm Rx
-
Reveal LINQ.
The comparator for the analysis listed in the NICE final scope was no further monitoring after at least 24 hours of outpatient external ambulatory electrocardiography monitoring that has not detected AF. Data for the comparator arm are taken from the CRYSTAL-AF trial, in which patients in the control arm underwent assessment at scheduled visits (every 3 months) and unscheduled visits, if patients were experiencing symptoms of AF. 90 To match the monitoring period of the ICM devices (3 years), the SoC period was also 3 years. Tests for the control arm included ECGs and Holter monitoring (for 24 hours, 48 hours or 7 days). Table 17 presents the tests performed per person per year in the control arm of the CRYSTAL-AF trial.
| Period (months) | No test | ECG | Holter at | ||
|---|---|---|---|---|---|
| 24 hours | 48 hours | 7 days | |||
| 0–12 | 0.31 | 0.55 | 0.06 | 0.02 | 0.06 |
| 12–24 | 0.51 | 0.40 | 0.04 | 0.01 | 0.05 |
| 24–36 | 0.58 | 0.31 | 0.02 | 0.00 | 0.08 |
Model structure
The EAG developed a two-stage economic model to assess the cost-effectiveness of using ICMs to detect AF in patients who have had a CS. The comparator in the analysis was 24 hours of external ambulatory electrocardiography monitoring.
The development of the model was informed by published models identified in the SLR. The first stage of the model (short-term model) outlines the initial patient-flow over a 3-year period (i.e. the battery life of the Reveal XT). The second stage of the model (long-term DOAC model) estimates the lifetime risks, costs and benefits for patients on either long-term anticoagulation or antiplatelet therapy. Figure 7 presents the schematic for the short-term model. The long-term DOAC model is presented in Figure 4. Each stage of the model is described in more detail below.
FIGURE 7.
Short-term patient flow model.
All patients enter the model as CS patients who have received at least 24 hours of outpatient external ambulatory electrocardiography monitoring that has not detected AF. The initial cohort is a mixture of patients with and without pre-existing paroxysmal AF who are given antiplatelet therapy for stroke prevention. Over the time horizon of the short-term model (3 years), patients who have an episode of AF may have that AF detected and thus will move to the AF-detected health state, where they enter the anticoagulation arm of the long-term DOAC model. However, episodes of AF may not be detected; thus, patients will then move to the AF-undetected health state where they enter the antiplatelet arm of the long-term DOAC model. Patients who do not have an episode of AF over the 3-year time horizon of the model remain in the CS patient health state (see Figure 7) and are not considered in the long-term model, as treatment, costs and benefits would be the same with or without an ICM. Therefore, the incremental analysis would equal to zero. However, in the short-term, ICM patients incur the costs of having an ICM and the associated costs of follow-up appointments and SoC patients incur the costs of monitoring and associated follow-up appointments.
The proportion of patients who are identified as having AF in each of the 12 3-month cycles is informed by data from the CRYSTAL-AF trial. It is assumed that patients in the SoC arm are detected either during follow-up appointments or owing to developing symptoms of AF. Explicit transitions from the AF-undetected to the AF-detected states are not modelled, as the data from the CRYSTAL-AF trial present cumulative detection rates. However, as a simplifying assumption, patients who receive SoC and have undetected AF by the end of the short-term model (3 years) will remain undetected and on antiplatelet treatment for the remainder of the modelled time horizon.
Sensitivity data for the Reveal LINQ device, in a broader AF population, indicate a sensitivity of 100%, enabling the calculation of the AF-undetected health state occupancy for the SoC arm of the model (see the evidence from Medtronic on ICMs in non-CS populations given in Chapter 3, Medtronic, for more detail). 34 Furthermore, based on the sensitivity and on data on AF detection rates for the ICM arm, patients from the initial cohort who do not have AF are excluded from the long-term analysis of outcomes as the proportion is assumed to be the same in each arm of the model (i.e. no false positives), and thus incremental costs and QALYs are zero. The assumption of no false positives in the model is based on information from the CRYSTAL-AF trial and advice from the EAG’s clinical experts that state alerts generated by an ICM will need to be reviewed by a clinician to confirm AF and initiate anticoagulation treatment. It should be noted that all patients in the ICM cohort incur the cost of the device, implantation, removal of device and follow-up. All SoC patients incur the cost of monitoring. Patients who do not have AF detected are assumed to incur the cost of follow-up appointments with a consultant cardiologist at 1, 3, 6 and 12 months as per the advice of the EAG’s clinical experts.
The second-stage long-term model uses the adapted version of the DOAC model [previously described in the subsection that reports the results of the SLR (see Economic evaluations)]. The DOAC model is a probabilistic model that outputs total costs and QALYs for DOAC treatments (apixaban, rivaroxaban, edoxaban and dabigatran etexilate), warfarin and antiplatelet treatment. The cycle length of the DOAC model is 3 months. The EAG adapted the model code to allow the output to be given as per-cycle costs and QALYs over a lifetime time horizon. This enabled the application of costs and QALYs specific to each cycle (i.e. time dependent per cycle costs and QALYs) in the ICM Excel model from the point when patients have AF detected and start anticoagulation treatment or are in the AF-undetected state and continue with antiplatelet treatment.
Data inputs were updated to reflect the CRYSTAL-AF trial population, for example the starting age was set at 62 years and the ratio of males-to-females was set at 65 : 35 to weight the general mortality death rates. The model was adapted to include all DOACs plus warfarin. Costs in the DOAC model were updated or inflated to 2018 prices, where appropriate, to reflect current values. Based on the HRQoL SLR, utility inputs were also updated. Life tables were also updated to the most recent year available (2015–17). 101
Mean costs and benefits per cycle (based on 10,000 samples run in the long-term model) related to AF patients treated with either anticoagulation or antiplatelet medication and are estimated for each individual DOAC treatment in the model. Figure 4 outlines the model schematic for the adapted DOAC model. The same structure is used for each treatment included in the model (i.e. DOACs for patients with detected AF and antiplatelet treatments for patients with undetected AF) and is adjusted for treatment-specific transition probabilities, costs and utilities. It should be noted that, for the current model, the adapted DOAC model population was prespecified for previous history of IS and paroxysmal AF (e.g. the risks of events in the model were adjusted to reflect a secondary stroke population with paroxysmal AF).
The mean, time-dependent per-cycle costs and benefits of anticoagulation treatment are then applied to the proportion of patients in each cycle of the AF-detected health state and the mean, time-dependent per-cycle costs and benefits of antiplatelet therapy are applied to the proportion of patients in each cycle of the AF-undetected health state. The economic assessment is taken from the perspective of the NHS and Personal Social Services and both costs and benefits are discounted at 3.5% per annum.
Clinical input parameters
Diagnostic efficacy of implantable cardiac monitors
The clinical effectiveness SLR identified diagnostic yield data only for the Reveal XT device from the CRYSTAL-AF RCT. AF detection rates for the comparator arm of the model are also derived from the CRYSTAL-AF trial. In the CRYSTAL-AF trial, an episode of AF was defined as irregular heart rhythm lasting > 30 seconds. 37 Table 18 presents the cumulative AF detection rate per model cycle (3 months) for both Reveal XT and SoC implemented in the short-term Excel model. These data are based on a KM analysis and include non-informative censoring of patients lost to follow-up. See Chapter 3, Diagnostic yield: atrial fibrillation detection rate, for further details. As no data were identified for BioMonitor 2-AF and Confirm Rx, the EAG sought advice from clinical experts as to whether or not there would be any differences in the detection rates between the devices. The EAG’s clinical experts acknowledged that the main source of efficacy for ICMs is the CRYSTAL-AF trial, but that there would not be any substantial differences in detection rates for the devices. As a result, the EAG has assumed equal efficacy for all devices.
| Month | Cycle | Detection rate (%) | |
|---|---|---|---|
| Reveal XT | SoC | ||
| 0 | 0 | 0 | 0 |
| 3 | 1 | 8 | 1 |
| 6 | 2 | 9 | 1 |
| 9 | 3 | 10 | 1 |
| 12 | 4 | 12 | 2 |
| 15 | 5 | 16 | 2 |
| 18 | 6 | 18 | 2 |
| 21 | 7 | 19 | 3 |
| 24 | 8 | 21 | 3 |
| 27 | 9 | 24 | 3 |
| 30 | 10 | 26 | 3 |
| 33 | 11 | 30 | 3 |
| 36 | 12 | 30 | 3 |
The BioMonitor 2-AF has the longest battery life of all the devices, at 4 years. The battery life of the Reveal LINQ is 3 years and Confirm Rx has the shortest battery life, at 2 years. The EAG’s clinical experts advised that it is improbable that a device will be replaced once the battery has expired. This means that those implanted with the Reveal LINQ or BioMonitor 2-AF who have AF detected between 24 and 36 months would not have AF detected with the Confirm Rx. Thus, the EAG adjusted the detection rates of the Confirm Rx to reflect the number of AF cases that would be missed because of the relatively shorter battery life of the device.
The battery life of BioMonitor 2-AF is 4 years; however, data for AF detection are available for only 3 years. Therefore, in the absence of additional data, the EAG has capped the BioMonitor 2-AF detection rate to 3 years. It is difficult to predict what impact this assumption has for the cost-effectiveness of the BioMonitor 2-AF, as the additional year of monitoring with the device could mean that there is potential (if limited) for additional cases of AF to be picked up compared with the SoC arm.
Diagnostic accuracy data for the Reveal LINQ device indicates a sensitivity and specificity of 100% and 98.1%, respectively. 34 However, the sensitivity and specificity values for the Reveal LINQ are based on an update to the Reveal XT algorithm that incorporates P-waves and is applied to the data set of the XPECT trial, which assessed the performance of the Reveal XT device in a population with known AF. 34 Although the sensitivity estimated in a population with known AF may not be a reliable measure for a population with paroxysmal AF, the EAG’s clinical experts advised that the ICM will probably pick up all cases of paroxysmal AF. As a result, an assumption has been made in the model that the detection rate of the device estimates the true prevalence of AF in the CS population. Please see the evidence from Medtronic on ICMs in non-CS populations given in Chapter 3, Medtronic, for further detail.
Therefore, the detection rate in each cycle of the ICM arm provides an estimate of the proportion of patients in the cohort who have AF at any given cycle. The proportion of AF-undetected per cycle in the SoC arm can then be calculated as the difference between the detection rate of the ICM and the SoC arm per cycle.
It should be noted that, based on the CRYSTAL-AF trial data, the proportion of patients at the end of the 3-year follow-up period who have AF is estimated to be 30%. Theoretically, the subset of CS patients who have AF is known at the start of the model; therefore, all patients could enter the model in the AF-undetected state and over time this would reduce as patients are detected in each arm. However, the EAG chose to start all patients in the model without AF status known and to use the per-cycle incidence of AF, based on the detection rate and sensitivity of the ICM, to calculate the number of patients with undetected AF (calculated as per-cycle incidence of AF minus the per-cycle AF-detection rate). If the overall AF prevalence is used, then the calculation of AF-undetected patients in the ICM arm infers that there is a large proportion of AF patients whom the ICM devices miss, which contradicts the 100% sensitivity. In fact, because of the nature of paroxysmal AF, it may not be true that all patients have AF at the start of the model, particularly those that are detected by the ICM late in the model time horizon, as they may have developed AF as they age in the model. However, using either method to define the starting population of the model, based on the detection rates of the CRYSTAL-AF trial has no impact on the results.
Long-term clinical outcomes
Long-term outcomes for patients with AF (whether detected or undetected) are modelled using the adapted DOAC model. 106,107 Outcomes assessed in the model include IS, MI, TIA, systemic embolism, CRB, ICH and death (all causes). The long-term model is structured so that, as well as experiencing single events, patients can have multiple events (up to a maximum of three).
As discussed earlier in Model structure, each treatment considered in the model (OACs and antiplatelets) has the same long-term model structure, but adjusted for treatment-specific risks, costs and benefits. The authors of the model estimated treatment effects by performing a competing-risks NMA, based on the clinical effectiveness SLR conducted for the study, to estimate HRs for the different events considered. 106
The clinical effectiveness SLR conducted by Sterne et al. 106 identified 23 completed RCTs for inclusion in the review. Seventeen types of events were included in the NMA to account for correlation and competing risks. However, as mentioned previously, the events of interest for the economic model are IS, MI, TIA, systemic embolism, CRB, ICH and death (all causes). Three types of outcome data were incorporated into the model to estimate the HRs: number of first events, number of individuals experiencing at least one event of a given type and total number of events.
Baseline treatment in the adapted DOAC model is warfarin (INR 2–3); therefore, the authors developed a competing-risks model for warfarin separately to estimate the baseline hazard for the outcomes of interest in the model. Further detail on the methodology and estimates used in the long-term model can be found in the publication by Sterne et al. 106
The treatment effects for antiplatelet therapy used in the model have been estimated in the competing-risks NMA using outcomes for aspirin treatment. The EAG consulted with clinical experts to confirm that patients would be given aspirin after stroke and if, in lieu of any diagnosis of AF, they would remain on lifetime treatment with aspirin. The clinical experts advised that, in current clinical practice, treatment for CS patients is, in fact, with clopidogrel (75 mg, once daily) and would be the long-term treatment if patients are not diagnosed with AF.
Consequently, the EAG performed targeted searches to identify evidence on the relative efficacy of clopidogrel and aspirin in AF patients at risk of ischaemic events, as this population reflects the cohort that occupies the AF-undetected health state and would therefore be receiving antiplatelet treatment. The EAG found that much of the literature assesses clopidogrel in combination with aspirin. 136,137 The EAG identified a systematic review and NMA by Cameron et al. 138 comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with AF. The review included aspirin and aspirin plus clopidogrel (dual antiplatelet therapy). Compared with standard dose vitamin K antagonist (e.g. warfarin), aspirin and dual antiplatelet therapy produced similar odds ratios (ORs) for an increase in risk of all-cause stroke or systemic embolism (aspirin OR 1.87, 95% CI 1.26 to 2.8; dual antiplatelet therapy OR 1.93, 95% CI 1.42 to 2.64). Similar results were seen for major bleeding (aspirin OR 1.05, 95% CI 0.60 to 1.87; dual antiplatelet therapy OR 1.1, 95% CI 0.83 to 1.47).
Although the review did not assess clopidogrel on its own, the NMA demonstrated a non-significant increase in risk with dual antiplatelet therapy. Thus, in the economic analysis, the EAG has used this evidence to form the assumption that, in the long-term model, clopidogrel is as effective as aspirin for patients with undetected AF; therefore, the effectiveness estimates used for antiplatelet therapy in the adapted DOAC model remain unchanged. The EAG acknowledges that this is a simplifying, conservative assumption, and a limitation of the analysis.
Mortality
Mortality risk implemented in the long-term DOAC model was estimated using the competing-risks NMA, as described previously for the age and sex split obtained from the CRYSTAL-AF trial. The mortality risk is then adjusted for general population mortality, using life tables for England and Wales101 for each age group beyond the baseline age and weighted by the proportion of males and females in the CRYSTAL-AF trial.
Anticoagulation treatment
For patients diagnosed with AF, anticoagulation treatment with either a DOAC or warfarin would be prescribed. However, analysis of prescribing trends, based on data from the openprescribing.net database139 published by the University of Oxford, has shown that prescriptions of warfarin have been declining, with prescriptions of DOACs overtaking warfarin in April 2018. It should be noted that the prescribing data are not broken down by indication, but an assumption can be made that DOACs are becoming the preferred treatment for patients requiring anticoagulation. Therefore, for the base-case analysis, the EAG assumed that all patients with detected AF will start treatment on a DOAC (i.e. apixaban, dabigatran etexilate, rivaroxaban or edoxaban).
The results from the DOAC model indicate that DOACs are more cost-effective than warfarin and that apixaban is the most cost-effective DOAC treatment (see the subsection that reports the results of the SLR: Models assessing the long-term impact of anticoagulation therapy for patients with atrial fibrillation). However, prescribing data show that apixaban accounts for only 48% of all DOAC prescriptions, with the remainder distributed between rivaroxaban (44%), dabigatran etexilate (6%) and edoxaban (3%). 139 In the base-case analysis, these proportions are used in the short-term Excel model. The proportion of patients on each of the treatments is based on the proportion of prescriptions of each drug between September 2017 and September 2018 from the openprescribing.net database. 139 In the short-term Excel model, the proportion of patients on each DOAC treatment is used to weight long-term costs and benefits. The EAG performed a scenario analysis including a proportion of patients on warfarin, as there may be clinicians who would prescribe this treatment to newly diagnosed AF patients. See Chapter 5 for further details.
Treatment switching probabilities
In the long-term DOAC model, depending on the occurrence of IS or SAEs (such as ICH), treatment switching can occur (see Figure 5). For patients on first-line DOAC treatment, second-line treatment may be either warfarin or no treatment. For patients who fail on warfarin, no further treatment is given. 106 The probability of a patient switching treatment after experiencing an event was based on clinical expert opinion obtained by the authors of the DOAC model.
Utility values
As described in Chapter 4, the EAG conducted a HRQoL SLR to identify relevant utility values to be used, when possible, to update the DOAC model. Two papers were identified as providing relevant utility values for IS, ICH, MI and TIA events (both acute and chronic) that were used to update the long-term DOAC model. 102,131 The papers estimate utilities using EQ-5D-3L data converted into UK population tariffs. The SLR did not identify any relevant studies that published utility values for clinically relevant bleeds (acute and chronic) and acute MI. As a result, the EAG used the values already populated in the DOAC model. 106
Table 19 presents the utility values applied for acute events and Table 20 presents the values used for each health state of the model. The utility value used for the AF-well health state is 0.78, based on data from Berg et al. 131 As per the assumption made in the DOAC model, the duration for an acute event is assumed to be 3 months (1 model cycle).
| Utilities by event | Acute event | Duration of event (months) | Reference or assumption |
|---|---|---|---|
| TIA utility decrement | –0.07 | 3 |
Control value for TIA from study was 0.85, which is higher than the baseline value of 0.78 used in this analysis. Furthermore, TIA utility from the study was estimated as 0.78. As a result, the EAG implemented a utility decrement to account for the impact of TIA |
| IS | 0.64 | 3 | aLuengo-Fernandez et al.102 |
| ICH | 0.56 | 3 | aLuengo-Fernandez et al.102 |
| MI | 0.68 | 3 | Same as DOAC model106 |
| Major bleed utility decrement | –0.03 | 3 | Same as DOAC model106 |
| Systemic embolism | –0.07 | 3 | Assumed to be equal to TIA (same as DOAC model106) |
| Health state | Utility value | Reference |
|---|---|---|
| IS | 0.70 | aLuengo-Fernandez et al.102 |
| ICH | 0.67 | aLuengo-Fernandez et al.102 |
| MI | 0.72 | Same as DOAC model106 |
| Major bleed | 0.70 | Assumed to be equal to stroke (same as DOAC model106) |
In the original DOAC model, utilities were adjusted for age and weighted by sex. Furthermore, as patients can experience more than one chronic health condition in the model, utilities for chronic health states are assumed to be multiplicative. 106
Costs
The following costs are considered in the model:
-
device and standard monitoring costs
-
cost of implantation and removal of devices
-
follow-up costs
-
pharmacotherapy costs
-
acute and chronic care costs of AF and anticoagulant related events.
All costs considered in the model are valued in 2018 Great British pounds (£). When unit costs were obtained from the published literature before 2018, costs were uplifted using the ONS Consumer Price Inflation Index for Medical Services (DKC3). 110
Device costs
The device costs of the Reveal LINQ, BioMonitor 2-AF and Confirm Rx ICMs used in the model are £1800, £1030 and £1600, respectively. These costs were supplied by the manufacturers of each device. The manufacturer of the Reveal LINQ also provides an optional triage service, FOCUSON. The company provided two cost options for FOCUSON: the first option is £187 per patient per year and the second option is £374 per patient per device. Both options are explored in scenario analyses, presented in Chapter 5.
The manufacturers of each of the devices have indicated that no additional training is required to perform the insertion procedure, as it is expected that staff will already have the necessary skills, competencies and experience in performing sterile device insertion procedures. However, the manufacturer of the Reveal LINQ device stated that training for staff is included in the cost of the Reveal LINQ ICM system. The manufacturer of BioMonitor 2-AF also stated that training is offered by the company but did not indicate whether the cost of this is covered by the device cost. Therefore, the EAG has assumed no additional costs of training for the base-case analysis.
Furthermore, the costs of reviewing alerts generated by the ICM have not been included in the base-case analysis as no data were available regarding the average number of alerts generated per day/month that require review. However, based on discussions during the scoping phase of the topic, clinical experts advised that reviewing alerts is relatively quick and would form part of the clinician’s normal workload, although it is dependent on volume.
Implantation and device removal costs
Table 21 presents the ICM implantation costs per patient implemented in the short-term Excel model. The costs of implantation for the base-case analysis are based on resource use assumptions provided by the EAG’s clinical experts for this report. The clinical experts also provided alternative resource use assumptions that are explored in a scenario analysis. The company for the Reveal LINQ device also provided a costing study comparing the costs of the Reveal XT implanted in a cath lab setting versus the Reveal LINQ implanted in a sterile procedure room setting. 141 The resource use assumptions for this study were costed by the EAG and used in a scenario analysis. The cost for removal of an ICM device implemented in the Excel model is £238, taken from the NHS reference costs schedule 2017–18 (EY13Z – removal of electrocardiography loop recorder, outpatient setting, treatment function code 320). 109
| Resource | Role for procedure | Unit cost (£) per hour | Time taken for procedure (minutes) | Cost (£) per procedure | Source |
|---|---|---|---|---|---|
| Clinical expert assumptions (base case) | |||||
| Cardiologist | Implanter | 108 | 10 | 18.00 | PSSRU 2018140 |
| Nurse (band 5) | Assistant | 37 | 10 | 6.17 | PSSRU 2018140 |
| Total | 24.17 | ||||
| Clinical expert assumptions (scenario) | |||||
| Cardiac physiologist (band 7) | Implanter | 57 | 10 | 9.50 | PSSRU 2018140 |
| Nurse (band 5) | Assistant | 37 | 10 | 6.17 | PSSRU 2018140 |
| Total | 15.67 | ||||
| Kanters et al.141 (scenario) | |||||
| Cardiac physiologist (band 7) | Implanter | 57 | 25.6 | 24.32 | PSSRU 2018140 |
| Nurse (band 5) | Assistant | 37 | 43.1 | 26.58 | PSSRU 2018140 |
| Total | 50. 90 | ||||
The most common AEs associated with implantation of an ICM, based on data from the CRYSTAL-AF trial, include infection (1.4%), pain (1.4%) and irritation or inflammation at the insertion site (1.9%). 37 However, there was no further detail on how severe the AEs were in the CRYSTAL-AF trial; as a result, the EAG has not included AE costs in the base-case analysis. However, it is anticipated that, given the relatively small proportions of patients experiencing each AE, it is not expected to have a substantial impact on the cost-effectiveness of the ICM devices.
Comparator arm costs
Costs of the comparator are based on the standard of care arm from the CRYSTAL-AF trial. 90 As presented in Table 17, the standard of care arm comprises various monitoring tests that are performed at 3-monthly intervals for the duration of the trial (3 years). The unit cost of monitoring is estimated to be £141, based on the NHS reference costs schedule 2017–18 [Healthcare Resource Group (HRG) code EY51Z – ECG monitoring or stress testing (outpatient procedures, service code 320)]. 109 Table 22 presents the weighted cost of monitoring per cycle based on number of tests per patient recorded in the CRYSTAL-AF trial (see Table 17).
| Period (months) | Cost (£) | ||||||
|---|---|---|---|---|---|---|---|
| No test | ECG | Holter 24 hours | Holter 48 hours | Holter 7 days | Total | Per cycle | |
| 0–12 | 0.00 | 77.22 | 8.86 | 3.16 | 8.23 | 97.48 | 24.37 |
| 12–24 | 0.00 | 55.94 | 5.09 | 1.02 | 7.12 | 69.17 | 17.29 |
| 24–36 | 0.00 | 44.10 | 2.94 | 0.00 | 11.76 | 58.80 | 14.70 |
In UK clinical practice, it is probable that monitoring tests will be performed only if a patient presents with symptoms. Therefore, the EAG explored a conservative scenario in which the cost of SoC is zero. See Chapter 5 for more detail.
Follow-up costs
In the CRYSTAL-AF trial, follow-up visits were scheduled at 1, 6 and 12 months and then every 6 months until trial closure, for both arms of the trial. However, the EAG’s clinical experts advised that patients with an ICM are likely to have a follow-up visit 1 month post surgery only, and then after that will be remotely monitored, unless patients request a face-to-face appointment. The clinical experts’ advice aligns with information provided in the company submissions. As a result, because of the nature of virtual continuous follow-up with the ICM device, there is a reduction in the need for physical follow-up visits. However, once AF is detected, patients will need to be seen by a clinician to start anticoagulation treatment.
Therefore, the EAG assumed, for the base case, that all patients with an ICM will have one face-to-face follow-up appointment after 1 month and then a subsequent follow-up appointment when AF has been detected. For the SoC arm, follow-up is at 1, 3, 6 and 12 months, as per advice from the EAG’s clinical experts, and the costs of these follow-up appointments are applied to all patients who do not have detected AF. However, after 12 months, any newly AF-detected patients in the SoC arm will have the cost of a subsequent follow-up appointment applied to account for being identified. Table 23 presents the unit cost of follow-up appointments implemented in the short-term Excel model.
Pharmacotherapy costs
As mentioned previously, the DOACs considered in the model are apixaban, dabigatran etexilate, edoxaban and rivaroxaban. Based on clinical expert opinion, antiplatelet treatment in the model is clopidogrel. Warfarin (INR 2–3) was considered only in a scenario analysis. Drug costs used in the DOAC model are presented in Table 24. The costs of DOACs and clopidogrel used in the DOAC model were updated using prices obtained from the BNF September 2018–March 2019 edition. 142 The original cost of warfarin used in the DOAC model (which including monitoring costs) was uplifted to 2018 prices for the current analysis. 106 All drugs considered in the model are taken orally; therefore, it has been assumed that there are no administration or monitoring costs.
| Drug | Dose | Pack size (n tablets) | Cost per pack (£) | Cost per day (£) | Cost per 3-month cycle (£) |
|---|---|---|---|---|---|
| Apixaban | 5 mg, twice daily | 56 | 53.20 | 1.90 | 173.85 |
| Dabigatran etexilate | 110–150 mg twice daily (depending on age) | 60 | 51.00 | 1.70 | 155.55 |
| Rivaroxaban | 20 mg, once daily | 28 | 50.40 | 1.80 | 167.40 |
| Edoxaban | 30–60 mg once daily (depending on weight) | 28 | 49.00 | 1.75 | 162.75 |
| Clopidogrel | 75 mg, once daily | 30 | 1.52 | 0.05 | 4.71 |
| Warfarin (INR 2–3) | 112.07a |
Acute and chronic care costs of atrial fibrillation and anticoagulant-related events
In the long-term adapted DOAC model, acute management costs for IS, ICH, systemic embolism, TIA, MI, deep-vein thrombosis (DVT), pulmonary embolism (PE) and CRB are considered. 106 The acute costs of IS and ICH in the DOAC model are derived from a UK-based population study, which estimated the acute and long-term costs of stroke in AF patients. 143 For the current analysis, costs were uplifted to 2017 prices using the ONS Consumer Price Inflation Index for Medical Services (DKC3). 2 All other event costs were derived from NHS reference costs and updated using the latest schedule (2017–18). 109 Acute event costs are presented in Table 25.
| Event | Mean event cost (£) | Source and assumptions |
|---|---|---|
| IS | 14,522 (SD 21,070) | Luengo-Fernandez et al.143 Based on data for all strokes, IS |
| ICH | 14,307 (SD 17,256) | Luengo-Fernandez et al.143 Based on data for all strokes, haemorrhagic stroke |
| Systemic embolism (non-fatal) | 1666 | NHS Reference Costs.109 Weighted average of cost codes YQ50A-F |
| TIA | 988 | NHS Reference Costs.109 Weighted average of cost codes AA29C-F |
| CRB | 1397 | NHS Reference Costs.109 Weighted average cost of FD03A-H and VB07Z |
| MI | 5804 | NHS Reference Costs.109 Weighted average cost of EB10A-E for non-elective long and short stay. Sterne et al.106 assumed that costs doubled to included follow-up costs |
To ensure consistency, cost assumptions from the original model have been maintained. The authors of the original model assumed that the cost of MI obtained from NHS reference costs accounts for only direct hospitalisation, and, therefore, doubled the total costs to account for follow-up costs. Furthermore, the cost of sudden fatal PE is assumed to be zero, and patients who have a non-fatal PE are assumed to accrue the full cost of PE.
The costs of chronic IS and ICH management in the DOAC model are also derived from the study by Luengo-Fernandez et al. 143 The study estimated the annual cost of stroke, stratified by severity, in the post-acute phase (3 months post index event). The mean cost was calculated by weighting the cost of stroke by severity by the number of events, excluding deaths within 90 days, uplifted to 2018 prices (Table 26) for the current analysis. As per the original model, it is assumed that the cost for ICH is the same as stroke.
| Stroke severity | Number of events (N = 136), n (%) | Mean (SD) annual cost (£) |
|---|---|---|
| Non-disabling | 66 (49) | 2135 (3675) |
| Moderately disabling | 58 (43) | 4165 (7768) |
| Totally disabling | 12 (9) | 6324 (14,898) |
| Total weighted cost (uplifted to 2018 prices) | 4514 (8585) |
Summary of base-case assumptions
Table 27 presents an overview of the parameter assumptions used in the base-case model.
| Parameter | Assumption or source | Justification |
|---|---|---|
| Mean age (years) | 62 | Mean age reported in the CRYSTAL-AF trial was 61.5 years. Age rounded up as a simplifying assumption37 |
| Females (%) | 36.5 | Proportion obtained from the CRYSTAL-AF trial37 |
| Prevalence of AF | Based on the detection rate of Reveal XT in the CRYSTAL-AF trial37 | A 100% sensitivity was assumed for the ICM arm of the model, based on data for the Reveal LINQ.34 Based on the sensitivity and the detection rates of the ICM in the CRYSTAL-AF trial, it is assumed that the detection rate of the device picks up all AF events and, as a result, estimates the true prevalence of the disease in the population |
| AF detection rates for Reveal LINQ | The CRYSTAL-AF trial37 | Efficacy data were available only for the Reveal XT ICM; therefore, it was assumed that the efficacy would be at least as good for the Reveal LINQ, which is a later version of the device. This is a conservative assumption |
| AF detection rates for BioMonitor 2-AF and Confirm Rx | Assumed the same effectiveness as Reveal LINQ | No data were available for the devices; on the advice of the EAG’s clinical experts, it was assumed that all devices are likely to perform as well as each other. However, this is considered an optimistic assumption |
| Percentage uptake of anticoagulation treatment for patients with AF detected | 100 | Simplifying assumption. Data from the CRYSTAL-AF trial suggest that only a small proportion of patients diagnosed with AF did not receive anticoagulation. See Chapter 3 for more detail37 |
| Percentage of patients receiving anticoagulation who receive DOACs | 100% | Prescribing trends that show prescriptions for DOACs overtook prescriptions for warfarin in 2018. Thus, the EAG interpreted the data to show that DOACs are becoming the treatment of choice for clinicians. Therefore, the EAG assumed that all newly diagnosed patients with AF will be prescribed a DOAC |
| Distribution of DOACs | Openprescribing.net139 | Data are available on the proportion of prescriptions for each DOAC, allowing the total costs and benefits of anticoagulation to be appropriately weighted |
| Efficacy of clopidogrel | Assumed to be the same as aspirin, which is the modelled treatment in the DOAC model | Simplifying assumption based on evidence from a NMA that demonstrated a non-significant increase in risk of dual antiplatelet (aspirin + clopidogrel) versus aspirin alone138 |
| Detection rates for BioMonitor 2-AF | Detection rates capped at 3 years, even though battery life of device is 4 years | AF detection data are available for only 3 years; as a result, it is unknown how many more cases of AF will be detected by an ICM in year 4. Therefore, the analysis for the BioMonitor 2-AF device is capped at 3 years |
| Detection rates for Confirm Rx | After 2 years, no further cases of AF are detected | The battery life of the Confirm Rx is 2 years and clinical experts have indicated that the device is unlikely to be replaced when the battery expires |
| Detection rates post 3 years | Assumed no differential detection between ICMs and SoC post 3 years | When an ICM battery expires, it is no longer able to detect AF episodes; therefore, detection rates would reflect those seen in SoC |
| Implantation resource use | Assumed device would be implanted by a cardiologist, with a band 5 nurse assisting | Assumption based on advice provided by the EAG’s clinical experts |
| Time taken to implant an ICM device | 10 minutes | Assumption based on advice provided by the EAG’s clinical experts |
| Costs of reviewing ICM alerts | Not included | Data on average volume of alerts were not available; therefore, this cost was not included in the model. However, based on clinical expert opinion, reviewing alerts is relatively quick and forms part of a clinician’s daily workload. However, if the volume of alerts is high, then this could become burdensome. The direction of bias for this assumption is in favour of ICMs, but it is anticipated that this would not be a key driver of cost-effectiveness |
| Administration and monitoring costs of oral medicines | Nil | All drugs considered in the model are taken orally; therefore, it has been assumed that there are no administration or monitoring costs |
| Cost of MI | Double the total costs estimated from NHS reference costs109 | In the original DOAC model, it was assumed that the cost of MI obtained from NHS reference costs accounts for only direct hospitalisation; therefore, the total costs were doubled to account for follow-up costs106 |
| Cost of sudden fatal PE | Nil | Original assumption from the DOAC model106 |
| Cost of ICH | Assumed to be the same as the cost of stroke | Original assumption from the DOAC model106 |
| Follow-up costs: ICM | Assumed one follow-up appointment, 1 month after device implantation | Advice from EAG’s clinical experts and company submissions to NICE |
| Follow-up costs: SoC | Assumed follow-up appointments would occur at 1, 3, 6 and 12 months | Based on advice from EAG’s clinical experts |
| Duration of disutility for acute events | 3 months (one model cycle) | Original assumption from the DOAC model106 |
| Utility decrement for TIA | –0.07 |
Based on data from Luengo-Fernandez et al. 102 Control value for TIA from study was 0.85, which is higher than the baseline value of 0.78 used in this analysis. Furthermore, TIA utility from the study was estimated as 0.78. Therefore, the EAG implemented a utility decrement to account for the impact of TIA |
| Utility for systemic embolism | Assumed to be the same as TIA | Original assumption from the DOAC model.106 |
| Detection of non-AF arrhythmias | Not included | Data on detection of non-AF arrhythmias were not available and therefore have not been included in the modelling. It is anticipated that the direction of bias for the cost-effectiveness analysis is in favour of SoC |
Uncertainty
Parametric uncertainty in the economic model is explored through deterministic sensitivity analysis and PSA, as well as running various scenarios around the base-case results (see Chapter 5). PSA considers the uncertainty characterising the input parameter estimates by assigning probability distributions to them to reflect their imprecision. Probability distributions were determined by the available data or, when data were lacking, by plausible assumptions. Monte Carlo simulation was then employed to reflect this uncertainty in the model’s results: 10,000 iterations were performed, each drawing random values out of the distributions fitted onto the model input parameters. Results of the PSA were averaged across the 10,000 iterations to provide a mean estimate of costs and QALYs for each intervention. PSA results have been presented as cost-effectiveness acceptability curves (CEACs), for which different willingness-to-pay (WTP) thresholds for a QALY are used to show which strategy is likely to have the largest net benefit for that threshold.
Interpretation of results
The results of the cost-effectiveness analysis are presented as ICERs. ICERs are can be interpreted as cost per QALY gained when comparing two interventions and are calculated as follows:
To compare several interventions with one another, incremental analyses are performed to calculate the ICERs. The incremental analyses involves ranking the interventions by cost, from least to most expensive, and then excluding interventions that are more expensive and less effective than the preceding strategy (i.e. ‘dominated’) and interventions that have ICERs higher than that of the next most effective strategy (i.e. extended dominance). 144 ICERs for the remaining interventions are recalculated to form an ‘efficiency frontier’ of interventions that are cost-effective and can be judged against the NICE cost-effectiveness threshold of £20,000–30,000 per QALY gained.
Chapter 5 Cost-effectiveness results
Base-case deterministic and probabilistic results
Table 28 presents the pairwise, deterministic base-case ICERs for Reveal LINQ, BioMonitor 2-AF and Confirm Rx compared with SoC. The results show that ICMs could be considered cost-effective against the £20,000–30,000 ICER threshold used by NICE. 145 The results are also plotted on the cost-effectiveness plane in Figure 8.
| Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| SoC | 7600 | 1.74 | – | – | – |
| Reveal LINQ | 9092 | 1.89 | 1492 | 0.14 | 10,340 |
| BioMonitor 2-AF | 8322 | 1.89 | 722 | 0.14 | 5005 |
| Confirm Rx | 8866 | 1.84 | 1267 | 0.10 | 12,875 |
FIGURE 8.
Cost-effectiveness plane showing the ICERs for each ICM vs. SoC in relation to the £20,000 and £30,000 per QALY thresholds.
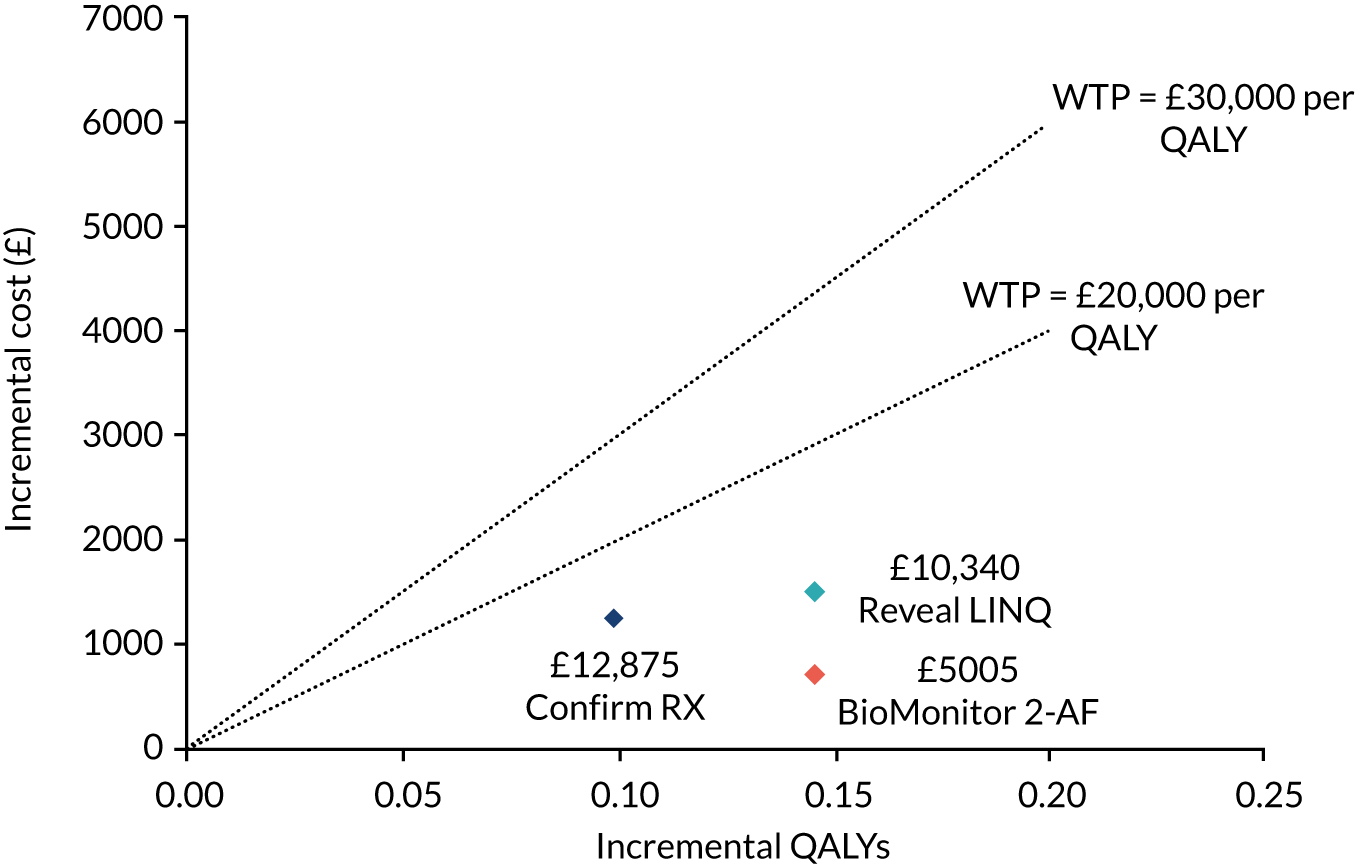
Table 29 presents the fully incremental analysis of cost-effectiveness results and demonstrates that, of the ICMs under consideration, Reveal LINQ and Confirm Rx are dominated by BioMonitor 2-AF.
| Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| SoC | 7600 | 1.74 | – | – | – |
| BioMonitor 2-AF | 8322 | 1.89 | 722 | 0.14 | 5005 |
| Confirm Rx | 8866 | 1.84 | 544 | -0.05 | Dominated |
| Reveal LINQ | 9092 | 1.89 | 770 | 0.00 | Dominated |
It should be noted that the differences in QALYs for Confirm Rx compared with the other two devices are driven by the assumption that, after 2 years, no further episodes of AF are detected for Confirm Rx, as the battery would have expired and the device would not be replaced. In addition, detection rates for BioMonitor 2-AF were capped at 3 years, even though the battery life of the device is 4 years. The impact of this assumption is that the BioMonitor 2-AF may potentially pick up more episodes of AF. However, the results for BioMonitor 2-AF and Confirm Rx should be viewed with caution, as no data were available for any version of these devices in the CS population; therefore, they are based on a strong assumption of equivalence with Reveal LINQ, which is not proven.
Scenario analyses
The EAG conducted the following scenario analyses to assess the potential impact of the uncertainty around some of the assumptions made in the model.
Addition of optional FocusOn triage costs
For the Reveal LINQ device only, the company provides a triage service, which can be provided in two ways. Option 1 provides the service at a cost of £187 per patient per year, whereas option 2 provides the same service but at a one-off fee of £374 per patient per device. Each option was considered as a separate scenario.
Addition of optional BioMonitor 2-AF devices
The BioMonitor 2-AF has the option to include a remote assistant device and CardioMessenger transmitter, at a cost of £230 and £400, respectively. These costs were included as part of the intervention cost and considered as separate scenarios.
Different time horizons (1 year, 2 year)
This scenario assumed that the ICM devices detect for a period of 1 year and 2 years only. This means that any detections that were identified in the CRYSTAL-AF trial beyond these time points were assumed to be missed by the devices, thereby reducing the benefits of the ICMs in comparison with SoC.
Constant detection rate
As an alternative to using the detection data directly from the CRYSTAL-AF trial, this scenario uses the 36-month detection proportion to calculate a constant monthly detection rate using the following formula:
where rm is the monthly rate and p36 is the proportion who are detected at 36 months. The monthly proportions, pm, are then calculated as:
where t is the time in months.
Using each directly acting oral anticoagulant separately to determine the long-term outcomes following atrial fibrillation detection
Instead of taking the weighted long-term DOAC outcomes based on the usage data, this applied the outcomes for each DOAC alone as separate scenarios.
Inclusion of warfarin as a treatment option for patients diagnosed with atrial fibrillation
Currently, warfarin is still in use for the treatment of AF, although, based on clinical expert opinion, the current primary treatments for newly diagnosed AF patients are DOACs. However, given that data suggest that ≈50% of anticoagulation usage comprises warfarin, the EAG conducted a scenario to test the impact on the ICER of this usage. 139 This scenario applied the same approach to weight the costs and QALYs for DOAC treatment from the DOAC model, but also included warfarin as an option in this weighting. Therefore, this applied 50% of the warfarin outcomes and reduced the weighted DOAC outcomes used in the base case by 50%.
No removal of devices
The base-case analysis assumes that all devices are removed at the end of their battery life. This scenario assumes that the devices will not need to be removed at all, as clinical expert advice suggests that they are safe to remain in place indefinitely.
Implanter and implanter assistant assumptions
Two separate scenarios were conducted, which assumed that the implantation was performed by a cardiac physiologist (band 7) and assisted by a cardiac physiologist (band 5), respectively.
Implantation assumptions based on Kanters et al.141
This scenario assumes that a cardiac physiologist (band 7) performs the implantation, assisted by a nurse (band 5). The assumed time required for the cardiac physiologist (band 7) is 25.6 minutes, and for the nurse (band 5) is 43.1 minutes, based on data from Kanters et al. 141
No monitoring for standard of care
This scenario removes all monitoring costs from the SoC group and assumes that no incidences of AF are detected, that is assuming a greater benefit for the ICM groups but also an increased total cost relative to the SoC group.
A discounted ICER for each scenario analysis is given in Table 30.
| Scenario | ICERs vs. SoC (£) | ||
|---|---|---|---|
| Reveal LINQ | BioMonitor-2 | Confirm Rx | |
| Base case | 10,340 | 5005 | 12,875 |
| Addition of FocusOn triage costs (option 1) | 14,097 | 5005 | 12,875 |
| Addition of FocusOn triage costs (option 2) | 12,931 | 5005 | 12,875 |
| Addition of BioMonitor 2-AF remote assistant device | 10,340 | 6598 | 12,875 |
| Addition of BioMonitor 2-AF CardioMessenger | 10,340 | 7776 | 12,875 |
| Time horizon for ICM monitoring (1 year) | 24,955 | 11,497 | 21,460 |
| Time horizon for ICM monitoring (2 year) | 14,908 | 7081 | 12,875 |
| Constant detection rates (exponential) | 10,283 | 4935 | 12,752 |
| Long-term DOAC outcomes based on apixaban | 8386 | 3358 | 10,753 |
| Long-term DOAC outcomes based on dabigatran etexilate | 9989 | 3578 | 12,993 |
| Long-term DOAC outcomes based on edoxaban | 11,664 | 5206 | 14,722 |
| Long-term DOAC outcomes based on rivaroxaban | 12,668 | 7143 | 15,333 |
| Inclusion of warfarin as a treatment option for patients diagnosed with AF | 18,227 | 8600 | 22,612 |
| No explantation of devices | 8850 | 3515 | 10,613 |
| Implantation by cardiac physiologist (band 7) | 10,281 | 4946 | 12,789 |
| Implantation assisted by cardiac physiologist (band 5) | 10,339 | 5004 | 12,874 |
| Implantation assumptions based on Kanters et al.141 | 10,525 | 5190 | 13,147 |
| No SoC or AF detections | 11,615 | 6821 | 14,301 |
Sensitivity analyses
One-way and two-way sensitivity analyses
The EAG conducted a number of sensitivity analyses around the cost inputs that were based on estimates (e.g. NHS reference costs), the outcomes applied from the long-term DOAC model, that is total costs and QALYs per cycle obtained from the long-term DOAC model, and the discount rate applied.
The most recent publication of NHS reference costs (2017–18) no longer gives an IQR for the costs associated with each HRG. Given the lack of data to inform the variation around the mean estimate, the EAG assumed a standard error of 20% of the mean value for each parameter. For DOAC outcomes (costs and QALYs), two-way sensitivity analyses around the 2.5th and 97.5th percentiles of the 10,000 samples for each cycle were used as the lower and upper limits, respectively, and the discount rate was lowered to 1.5% (as per the NICE Guide to the Methods of Technology Appraisal 2013145), as well as being increased to 6%. The summary of the inputs along with the results is given in Table 31.
| Parameter | Base case | Lower value | Upper value | Reveal LINQ (£) | BioMonitor 2-AF (£) | Confirm Rx (£) | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower ICER | Upper ICER | Lower ICER | Upper ICER | Lower ICER | Upper ICER | ||||
| Initial follow-up cost | £163 | £99 | £227 | 10,340 | 10,340 | 5005 | 5005 | 12,875 | 12,875 |
| Device implantation cost | £24 | £15 | £34 | 10,274 | 10,405 | 4939 | 5070 | 12,779 | 12,972 |
| Cost of SoC | £141 | £85 | £196 | 10,722 | 9958 | 5387 | 4623 | 13,435 | 12,315 |
| Device removal cost | £238 | £145 | £332 | 9756 | 10,924 | 4421 | 5589 | 11,989 | 13,762 |
| Subsequent follow-up cost | £128 | £78 | £178 | 11,257 | 9423 | 5922 | 4088 | 14,262 | 11,488 |
| Discount rate | 3.5% | 1.5% | 6% | 8417 | 13,112 | 4091 | 6313 | 10,477 | 16,322 |
| DOAC outcomesa | Mean | 2.5th percentile | 97.5th percentile | 12,835 | 7934 | 7785 | 1997 | 15,064 | 10,747 |
Probabilistic sensitivity analysis
The EAG conducted a PSA to assess the impact of the combined uncertainty from all parameters in the model. This was performed by sampling from distributions of the uncertain parameters 10,000 times, to generate the equivalent number of sampled ICERs. The methods for the inclusion of parameter uncertainty are discussed for each parameter type in turn.
The key uncertainties in the model are captured in the long-term DOAC model [coded using R statistical software (The R Foundation for Statistical Computing, Vienna, Austria)]. This model is probabilistic and produced 10,000 per-cycle samples of costs and QALYs for each DOAC, warfarin and aspirin. These outcomes were pasted into separate tabs of the short-term Excel model, with each of 10,000 columns representing a single sample of per-cycle costs and QALYs over the lifetime horizon. The columns were sampled in the PSA one by one, from 1 to 1000, to avoid sampling from the same column more than once. This sampling is performed for each DOAC treatment (plus warfarin). The samples are then weighted according to the treatments that are included in the analysis and the usage proportions applied to weight them.
The usage proportions were sampled using the data from openprescribing.net,139 from which the mean estimates were derived. The total monthly usage values for each treatment between September 2017 and September 2018 (inclusive) were used to estimate correlated samples using the mvrnorm and cov functions from the MASS and stats packages in R, respectively. 146,147 The cov function generates a covariance matrix (using Pearson’s product moment correlation coefficient as the default) for the monthly usage totals of each treatment, which was inputted into the function, along with the mean monthly usage, to generate 10,000 sampled estimates of the monthly usage totals. These values were used to sample the weights applied to the DOAC treatment (plus warfarin) outcomes.
For cost estimates, gamma distributions were applied using 20% of the mean value to estimate standard errors. The cost estimates that were varied in the PSA were:
-
SoC
-
initial follow-up
-
subsequent follow-up
-
device implantation
-
device removal.
The parameters used for the distribution of each variable are given in Table 32.
| Variable | Mean cost (£) | SEa (£) | Distribution | Alphab | Betac |
|---|---|---|---|---|---|
| SoC | 141 | 28 | Gamma | 25.00 | 5.62 |
| Initial follow-up | 163 | 33 | Gamma | 25.00 | 6.53 |
| Subsequent follow-up | 128 | 26 | Gamma | 25.00 | 5.12 |
| Device implantation | 24 | 5 | Gamma | 25.00 | 0.97 |
| Device removal | 238 | 48 | Gamma | 25.00 | 9.53 |
The results of the PSA for each ICM and SoC are given in Table 33, and scatterplots showing the spread of results from the individual samples and the cost-effectiveness planes are presented in Figures 9–11 for the Reveal LINQ, BioMonitor 2-AF and Confirm Rx, respectively; each is compared with SoC. In addition to these, CEACs, showing the probability of each ICM being cost-effective compared with SoC over a range of WTP thresholds, are presented in Figures 12–14 for the Reveal LINQ, BioMonitor 2-AF and Confirm Rx, respectively.
| Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| SoC | 7599 | 1.74 | – | – | – |
| Reveal LINQ | 9092 | 1.89 | 1493 | 0.14 | 10,347 |
| BioMonitor 2-AF | 8322 | 1.89 | 723 | 0.14 | 5011 |
| Confirm Rx | 8867 | 1.84 | 1267 | 0.10 | 12,883 |
FIGURE 9.
Cost-effectiveness plane for the Reveal LINQ vs. SoC.

FIGURE 10.
Cost-effectiveness plane for the BioMonitor 2-AF vs. SoC.

FIGURE 11.
Cost-effectiveness plane for the Confirm Rx vs. SoC.
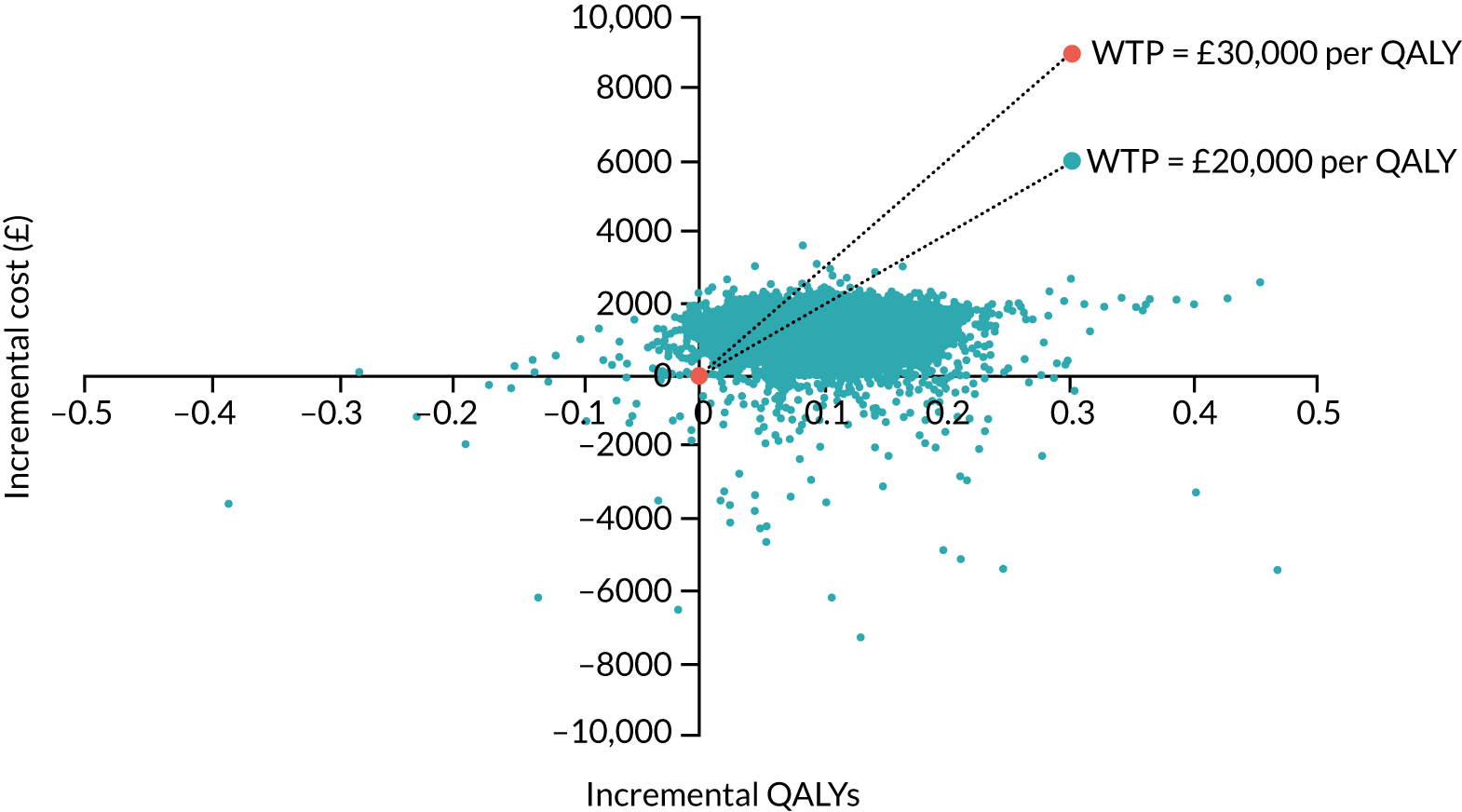
FIGURE 12.
Cost-effectiveness acceptability curve for the Reveal LINQ vs. SoC.

FIGURE 13.
Cost-effectiveness acceptability curve for the BioMonitor 2-AF vs. SoC.

FIGURE 14.
Cost-effectiveness acceptability curve for the Confirm Rx vs. SoC.

Chapter 6 Discussion
Statement of principal findings
Clinical
The clinical evidence systematic review sought to identify RCTs and comparative observational studies that compared any of the three devices (Confirm Rx, BioMonitor 2-AF and Reveal LINQ) with at least 24 hours of Holter (external electrocardiography monitoring to detect AF in people who have had a CS). Only a single RCT assessing an earlier Medtronic Reveal model (XT rather than LINQ) met the original review eligibility criteria (the CRYSTAL-AF trial),37 and so the criteria were widened in an attempt to find evidence for the BioMonitor 2-AF, Confirm Rx and Reveal LINQ. First, non-comparative observational studies were sought within the correct CS population, and then evidence was considered from studies of mixed populations submitted by each company. Only the CRYSTAL-AF trial37 falls within the eligibility criteria outlined in the original published protocol for this DAR, and so the additional evidence should be interpreted with caution. Therefore, the CRYSTAL-AF trial37 (n = 441) represents the most robust clinical evidence available to inform the decision problem, albeit assessing the Reveal XT.
The CRYSTAL-AF trial was an open-label study that compared the Reveal XT ICM with conventional follow-up in a population that had had CS and no history of AF after extensive diagnostic work-up. The study was conducted in North America and Europe and the population was considered by the EAG’s clinical experts to be generally applicable to patients who would be eligible for an ICM in UK clinical practice.
Twenty-six single-arm observational studies were identified after widening the eligibility criteria to include non-comparative studies. The studies were conducted in North America and western Europe (one in the UK) and all assessed the Reveal XT and Reveal LINQ in populations that had had a CS; none provided evidence to assess the efficacy of the BioMonitor 2-AF (other than a mixed-device study that did not provide results by individual device) or the Confirm Rx for patients who have had a CS. The observational studies represent a wide sample of patients who have received an ICM in practice (n = 3414) and provide evidence for the Reveal LINQ and for additional outcomes specified in the NICE final scope that were not available from the CRYSTAL-AF trial.
All of the observational studies were single-arm and therefore were at a high risk of bias, although three conducted within-patient comparisons of ICM versus other monitoring strategies. 14,15,72 Key sources of heterogeneity between the observational studies include patient demographics (mean or median age of 52–72 years), rigour of stroke assessment, stroke risk score (CHA2DS2VASC score of 3–5), definition and adjudication of AF, and length of follow-up; published data and the EAG’s clinical experts suggest that these are all likely to affect AF detection and other clinical outcomes. 34
Mixed population studies recommended by the companies as having potential data for inclusion in the review have also been included, as no data were identified for the Confirm Rx or BioMonitor 2-AF in the CS population and only limited outcome data were identified in the CS population for the Reveal LINQ. A full systematic literature search was not conducted to validate their inclusion owing to time constraints and concerns regarding the applicability of their results to the CS population. 34 The data presented from these studies may therefore be subject to study selection bias as well as clinical heterogeneity due to the variation in the patient populations of each of the studies. In total, there was one study of the Confirm DM2102 (an older model of the Confirm Rx), five studies of the BioMonitor 2-AF (all used BioMonitor 2 but only one specified it as the ‘-AF’ model) and five studies of the Reveal LINQ or XT [three studies used the Reveal LINQ and three studies used the Reveal XT (note that one study included both devices)]. All of these mixed population studies are either single-arm observational studies or they provide DTA data for the ICM using Holter monitoring as the reference standard.
The CRYSTAL-AF trial was designed to measure diagnostic yield rather than accuracy, and none of the observational studies provided comparative DTA between an ICM and standard monitoring. Two studies modelled patient AF detection data from the CRYSTAL-AF trial (Choe et al. 14) and a large patient registry (Ziegler et al. 15) with repeated iterations (10,000) to estimate the number of patients whose AF would not have been detected should an intermittent monitoring strategy have been used (based on the assumption that an ICM has 100% sensitivity). The studies found that the best-performing intermittent monitoring strategy detected less than one-third of the AF detected by the ICM (ranging from around 3% for a single 24-hour Holter monitor to 30% with a quarterly 7-day Holter monitor). Studies reporting false positive rates as the proportion of episodes detected by an ICM algorithm that were not subsequently verified by a clinician were highly dependent on the ICM model and device programme settings.
The results of the mixed population DTA studies suggested that the enhancements over time to the AF diagnosis algorithm in the Reveal XT and Reveal LINQ ICMs has improved the DTA (sensitivity and specificity) of the ICMs. However, it should be noted that these data are not exclusively in the CS population and the data in the XPECT50 and Reveal LINQ Usability87 studies used to make some of these comparisons are heterogeneous owing to differences in the way in which the reference standard was applied (Holter monitoring for 48 hours vs. 24 hours, respectively) and differences in the patient populations (e.g. reasons for ICM insertion). Nonetheless, these data suggest that the Reveal LINQ is likely to be as effective as, if not better than, the Reveal XT at detecting AF (as the Reveal LINQ has fewer false positives and false negatives). Therefore, the AF detection rate from the CRYSTAL-AF trial is potentially a conservative estimate for the Reveal LINQ, given that it was the Reveal XT that was used in the CRYSTAL-AF trial.
A naive comparison of the sensitivity and specificity data from non-CS or mixed populations in the studies flagged of relevance by the respective companies of the Confirm DM2102 (an older model of the Confirm Rx) and Reveal LINQ suggests that they both have 100% sensitivity for AF detection, whereas specificity varies (85.7% and 99.0%, respectively); the BioMonitor 2 (confidential information has been removed). However, it should be noted that the studies are subject to clinical heterogeneity in terms of the patient populations, interventions and study designs, as well as the reference standards. The device-related performance of ICMs is known to be dependent on the patient population and the incidence rate of AF, as well as the reference standard; therefore, this naive comparison should be interpreted with caution as these data are not necessarily reflective of the respective ICMs performance in CS patients. In addition, they do not necessarily reflect the performance of the current device model firmware; for example, the Confirm Rx data are based on an earlier model.
Atrial fibrillation detection rate was the primary outcome in the CRYSTAL-AF trial (at 6 months) and in all 26 observational studies. In the CRYSTAL-AF trial, AF detection was higher with the Reveal XT than with conventional follow-up at all time points. At the primary 6-month analysis, AF had been detected in 19 (8.6%) patients with an ICM and in 3 (1.4%) patients in the conventional follow-up group. By 36 months, the number of patients detected were 42 (19%) with an ICM and 5 (2.3%) receiving conventional follow-up, demonstrating the continued and increasing benefit of ICM monitoring. AF detection rates reported at the primary follow-up (6–24 months) across the 26 observational studies were highly variable, ranging from 6.7%38 (Reveal LINQ and XT at 12 months) to 40.9%68 (Reveal XT, unknown follow-up). These data demonstrate that, even within a CS population, AF detection rates are highly variable, and it is impossible to make any meaningful comparison between the observational studies and the CRYSTAL-AF trial. Observational studies reporting AF detection at different lengths of follow-up indicate that a minority of patients are diagnosed within the first month (mostly in the region of 10% of those detected by 1 year), around 70–80% by 6 months, and a small number beyond 1 year of monitoring. 15,52,58,72,74 In comparison, the 36-month data from the ICM arm of the CRYSTAL-AF trial show higher proportions of AF diagnosed at 1 month (19.0%) and beyond 12 months (31.0%), and a lower proportion at 6 months (45.2%), than with the observational studies. When described, all or most AF detected was asymptomatic and so would probably not have been picked up without continuous ICM monitoring.
Median time to AF detection was longer for patients with the Reveal XT in the CRYSTAL-AF trial than for those receiving conventional follow-up at 6, 12 and 36 months (p-value not reported). Nevertheless, the benefit of the ICM increased with length of follow-up because very few patients in the conventional follow-up arm were diagnosed, whereas detection increased steadily in the group with an ICM (36 months: HR 8.8, 95% CI 3.5 to 22.2; p < 0.001). The observational studies showed highly variable median time to first AF detection, ranging from 21 to 217 days (average follow-up of between 7 and 20 months); nevertheless, the results are still broadly consistent with the results from the CRYSTAL-AF trial, in which median time to AF diagnosis was 41 (IQR 4–84) days at 6 months’ follow-up, 84 (IQR 18–265) days at 12 months’ follow-up and 8.4 months (IQR not reported) at 36 months’ follow-up.
Three of the observational studies, primarily of the Reveal LINQ, suggest that the proportion of patients for whom the ICM detected other non-AF cardiac arrhythmias is in the region of 10%; these arrhythmias consisted mainly of bigeminy, pause and bradycardia. No information was presented about whether or not and how the detected arrhythmias were treated to prevent related complications, and data on detection of other arrhythmias were not available from the CRYSTAL-AF trial. The value of this additional potential benefit of the ICMs is, therefore, unclear.
In the CRYSTAL-AF trial, > 90% of patients diagnosed with AF in the ICM arm started an OAC. Data were available for the conventional follow-up group irrespective of AF diagnosis, indicating that 8.3% were on an anticoagulant by 36 months (24 patients, whereas five had been diagnosed with AF by that time point). These data, along with the data from the observational studies, suggest that most patients with ICMs diagnosed with AF go on to receive long-term OACs. Time to anticoagulation and AEs related to anticoagulant use were not reported in any of the identified evidence. Subsequent stroke or TIA rates in the CRYSTAL-AF trial were reported to be 5.0% in the ICM arm versus 8.2% in the conventional follow-up arm at 6 months, 6.8% versus 8.6% at 12 months and 9.0% versus 10.9% at 36 months (p > 0.05). None of these data suggests statistically significant stroke prevention benefits of the Reveal XT versus conventional monitoring, although there is a trend to fewer events in the ICM arm. It was unclear how many recurrent strokes occurred in patients diagnosed with AF or on OACs, and no studies reported other thromboembolisms or related morbidities.
In the CRYSTAL-AF trial, the overall rate of serious AEs was similar between groups (≈25–30%), but more patients in the ICM group than in conventional follow-up group had non-serious AEs (18.6% vs. 4.1%, respectively). The CRYSTAL-AF trial reported that five devices (2.4%) were removed because of infection or pocket erosion, which was in line with the premature removal rates seen in the observational studies (0.9–5.7%). At 12 months’ follow-up, 3.4% of ICMs had been removed in the CRYSTAL-AF trial; this contrasts with results reported in the Ritter et al. 72 (Reveal XT) study, in which removal after AF detection was offered in addition to removal for other reasons; 30% of patients had their ICM device removed during the study (median follow-up time in the study for all patients was 13 months). In the absence of further data, it is unclear why the removal rate was so high in Ritter et al. 72 However, device-related AEs, such as pain and infection, were consistently low in the CRYSTAL-AF trial, the single-arm observational studies and mixed population studies, suggesting that ICMs are generally well tolerated.
The EQ-5D data collected throughout the CRYSTAL-AF trial were (confidential information has been removed). The CRYSTAL-AF trial did not collect any other ease of use or patient acceptability data, and information from the observational studies was anecdotal. However, company submissions and the EAG’s clinical experts reported that the newer models of the ICMs (e.g. Reveal LINQ and Confirm Rx) were easier to insert and were suitable for insertion by trained nurses and cardiac physiologists.
Eight ongoing studies of potential relevance were identified, although only five (three RCTs and two observational studies) reported details of their status and the ICM being studied. None of the ongoing studies include the BioMonitor 2-AF. The three ongoing RCTs all include the Reveal LINQ but only one RCT is in a discrete CS population; this is a Canadian trial comparing the clinical effectiveness and cost-effectiveness of the Reveal LINQ ICM with external loop recording in 300 CS patients, which is estimated to complete in December 2019 (PERDIEM). 47 There was only one ongoing study identified relating to the Confirm Rx: the SMART registry,45 a post-approval study planning to recruit at least 2000 patients with Confirm Rx across multiple indications, but with a planned subgroup analysis for CS; completion is expected in December 2020. These studies may help to provide further clinical data for these two ICMs, although they will not address the lack of comparative data between the ICMs and do not provide any comparative data for the Confirm Rx or BioMonitor 2-AF against either Holter monitoring or other ICMs.
Economic
As mentioned previously, only one RCT (CRYSTAL-AF) was identified in the clinical effectiveness SLR, which assessed the impact of using an ICM compared with SoC, in a CS population in which there was a suspicion of paroxysmal AF. The CRYSTAL-AF trial reported data on AF detection rates for SoC and the Reveal XT device, which is an earlier model of the Reveal LINQ device. No data were obtained for BioMonitor 2-AF or Confirm Rx. As a result, a strong assumption was made in the economic analysis, based on clinical expert opinion, that the effectiveness of different ICMs is similar; thus, the detection rates obtained from the CRYSTAL-AF trial were used for all the ICM devices under assessment.
The results from the de novo economic model were ICERs, also known as cost per QALY gained. The results of the pairwise analysis, that is each ICM device compared with SoC, demonstrate that ICMs could be considered cost-effective at a £20,000–30,000 threshold compared with SoC. When each device is compared incrementally, the BioMonitor 2-AF dominates the Reveal LINQ and Confirm Rx. However, the results for the BioMonitor 2-AF and Confirm Rx should be viewed with caution, as no data were available for any version of these devices in the CS population and, as a result, there is substantial uncertainty in the results.
The EAG conducted various scenario and sensitivity analyses and found that the scenario that caused the most substantial change in the ICER for all three devices was the inclusion of warfarin. From the one-way sensitivity analysis, the key driver of the cost-effectiveness results relates to outcomes (i.e. total costs and QALYs) obtained from the long-term DOAC model, which for Reveal LINQ and Confirm Rx exceeded the £30,000 cost-effectiveness threshold.
The EAG conducted a SLR to identify any published economic evaluations of ICM devices for the detection of AF in a CS population that could be used to inform the current analysis. One study was identified that assessed the cost-effectiveness of the Reveal XT ICM (a predecessor of the Reveal LINQ) compared with SoC in a CS population from the UK perspective.
The model was developed using a Markov structure with three main health states for AF status: AF free, AF detected and AF undetected. Patients start in the AF-free state, from which they can move to AF-undetected or AF-detected states at any given model cycle. From the AF-undetected state, patients can either remain or move to the AF-detected state, and patients remain in the AF-detected state unless the patient experiences a subsequent cerebrovascular event or bleeding event. Detection rates of AF were based on data from the CRYSTAL-AF trial. 37
Results of the deterministic base-case analysis showed that the ICM was £2587 more expensive than SoC and provided a benefit of 0.151 QALYs, resulting in an ICER of £17,175 per QALY gained. This ICER is lower than the EAG’s results for the Reveal LINQ, which estimated that the ICM was £1687 more expensive than SoC and provided a benefit of 0.07 QALYs, resulting in an ICER of £24,875. The EAG’s short-term model was informed by the model structure used by Diamantopoulos et al. ,90 as it includes the health states of AF detected and AF undetected, with data informing the proportions in each health state per model cycle based on the results from the CRYSTAL-AF trial. 90 However, the approach to modelling long-term outcomes for patients with AF who are either detected and on anticoagulation treatment or undetected and on antiplatelet treatment, is based on a published DOAC cost-effectiveness model. 106
The EAG’s model produces incremental costs that are lower than those of Diamantopoulos et al. 90 and this can be attributed to a lower baseline hazard of IS used in the long-term DOAC model and, therefore, lower health-state costs. Furthermore, there were differences between the two models in the way in which monitoring costs were estimated. The EAG used data on the monitoring tests performed per person per year in the control arm of the CRYSTAL-AF trial, obtained from Diamantopoulos et al. ,90 to estimate costs for SoC in the current analysis. Minor differences in SoC costs between the two models are attributed to a change in the NHS reference cost used in the analysis (£137 in 2016, increased to £141 in 2018). 90,109 In addition, the EAG used a different methodology of calculating the per-cycle cost of SoC, by calculating the cost per year of the monitoring tests and dividing the costs by the number of model cycles per year. In the Diamantopoulos et al. 90 model, the per-cycle probability of each test was estimated and used to weight the unit cost per cycle.
In addition, the incremental QALY gained for the EAG model is lower than that of the Diamantopoulos et al. 90 model. The EAG considers that the difference in QALYs can also be attributed to a lower baseline hazard of IS used in the long-term DOAC model.
It should be noted that, in the model by Diamantopoulos et al. ,90 the entire cohort (no AF, AF detected and AF undetected) is modelled for clinical outcomes. However, the EAG considered that, clinically, outcomes for the no-AF cohort would be the same in each arm of the model (ICM and SoC), and so essentially cancel out, hence a focus on the overall incremental costs and QALYs between the two models.
Clinical expert opinion suggests that an additional benefit of ICMs devices is the ability to detect non-AF arrhythmias, potentially preventing other events. However, data on incidental findings from ICMs were found only in single-arm observational studies, as previously mentioned, and are of poor quality. As a result, it is unclear how detection of other non-AF arrhythmias differs between standard care and ICMs and, furthermore, how a patient’s treatment pathway changes. Therefore, understanding the differences in costs and benefits for incidental findings for ICMs is problematic. However, the EAG considers that, if without an ICM, some of these arrhythmias remain undetected, then the impact on the cost-effectiveness estimates would be favourable towards ICMs, but the extent of the impact is difficult to determine.
Strengths and limitations
Clinical
Despite extensive evidence searches, the clinical evidence for this DAR is based primarily on a single RCT for the older Medtronic Reveal XT device. Clinical expert opinion and evidence from a mixed population suggest that the Reveal LINQ may have better sensitivity and specificity for detecting AF than the Reveal XT and is likely to lead to fewer complications owing to its size, but there are no head-to-head clinical trials to confirm these findings in a CS population. 28 In addition, no clinical or DTA data suitable for inclusion were identified for the BioMonitor 2-AF or Confirm Rx devices, despite widening the eligibility criteria to include low-quality non-comparative observational studies. Data for the BioMonitor 2-AF or Confirm Rx devices were limited to mixed population diagnostic accuracy and single-arm observational studies submitted by the companies. The EAG considers it important to highlight that there are data to suggest that the performance (e.g. PPV and NPV) of AF detection with ICM devices is dependent on the patient population, incidence rate of AF, the duration of monitoring and the type of AF. 34 The mixed population studies were also not obtained through the robust and comprehensive searches that would ideally be used in a systematic review owing to time constraints and concerns about the applicability of their findings in a CS population. The mixed population studies may, therefore, be subject to study selection bias as well as clinical heterogeneity due to the variation in the patient populations of each of the studies, and so the results of any comparison between them should be interpreted with caution.
A further limitation of the review of the clinical effectiveness of the ICMs was that no evidence was found for any of the devices for the outcomes of mortality, hospital and outpatient care for AF, related morbidities, AEs related to anticoagulation or information about the ease of using each device for clinicians. Their acceptability to patients was anecdotal or from mixed population studies. There were also no DTA data identified for the latest versions of any of the ICMs in CS patients and the DTA data that were identified did not use a consistent reference standard across the ICMs, which limits the ability to compare the accuracy of any model of the ICMs, even in non-CS populations.
Nonetheless, clinical data were available for one of the ICMs (Reveal LINQ) from a RCT of an earlier model (Reveal XT) compared with conventional follow-up; the EAG’s clinical experts considered the CRYSTAL-AF37 trial generally reflective of UK clinical practice. The minor differences highlighted by the EAG’s clinical experts between UK clinical practice and the CRYSTAL-AF trial were that all UK patients undergo transthoracic echocardiography and then a minority may not go on to have TOE before receiving an ICM. They also considered that some patients who were excluded from the trial owing to a recent MI might still be considered for an ICM. Patients in the CRYSTAL-AF trial were also slightly younger than would be expected and all patients would be expected to be on an antiplatelet agent in UK clinical practice.
The overall risk-of-bias rating for the CRYSTAL-AF trial for the outcome of AF detection was that there were ‘some concerns’. These concerns included issues around the open-label nature of the study, the intervention not being received as per the study protocol and the small number of patients achieving follow-up beyond 12 months. The open-label design of the CRYSTAL-AF trial introduces potential bias because the outcome assessor was aware of the intervention assignment and so would be able to influence the electrocardiography or other assessment of AF. However, the ICM arm was unlikely to be affected by bias relating to the outcome assessor, as all episodes of AF that qualified for analysis were adjudicated by an independent committee. Despite this, the open-label design potentially biases the results in favour of the ICM over conventional follow-up, compared with a double-blind design. However, there may also have been bias in the detection of AF because of the 2-minute analysis window used by the ICM. This is because the threshold for AF diagnosis in the CRYSTAL-AF trial was defined as being at least 30 seconds, but the ICM uses an automatic algorithm for AF detection that is based on R-wave interval variability within 2-minute analysis windows. 49,50 It is therefore possible that some AF episodes of between 30 seconds and 2 minutes in duration may have been missed in the ICM arm,49,51 and this may bias the results of the CRYSTAL-AF trial in favour of conventional follow-up.
Most patients in the CRYSTAL-AF trial had received a median of 23 hours of Holter monitoring (71.2%), but the remainder received a median of 68 hours of inpatient telemetry monitoring (29.7%), which is not in line with the NICE final scope, which requested outpatient monitoring for a minimum of 24 hours. In addition, it means that, in the CRYSTAL-AF trial,37 the baseline monitoring was not consistent and there were no subgroup data reported to demonstrate whether or not the split between inpatient and outpatient electrocardiography monitoring in establishing the diagnosis of a CS was consistent between the ICM and comparator arm. The EAG is also unable to comment on whether or not subsequent AF detection or other long-term clinical outcomes are influenced by whether or not patients received inpatient or outpatient electrocardiography monitoring in the work-up to receiving their diagnosis of CS, as this was beyond the scope of this review. There were also other issues with the CRYSTAL-AF trial noted by the EAG and its clinical experts, such as baseline differences (e.g. in the proportion of patients with PFO and history of prior stroke), crossover between groups, insertion delays (11.5%) and withdrawals, although they are unlikely to have had an important impact on the results of the CRYSTAL-AF trial.
The use of ICMs in CS patients is for the detection of AF in CS patients who may otherwise have undetected AF or have AF detected much later in standard follow-up, so that treatment can be started to help reduce the risk of subsequent stroke or TIA. However, the AF detection rate in the ICM studies varies considerably between and within the types of evidence considered by the EAG, (i.e. the CRYSTAL-AF trial, uncontrolled observational studies, mixed population studies) The EAG recommends caution in drawing conclusions from naive comparisons between the additional studies owing to the number of uncontrolled variables and inherent biases of their single-arm design. Sources of heterogeneity that probably contribute to the differences in AF detection include the episode threshold used (varying from 10 seconds to 2 minutes), population characteristics (e.g. stroke risk score), time from stroke to ICM insertion, duration of follow-up and method of AF adjudication. As a result, the EAG considers the CRYSTAL-AF trial to provide the most robust evidence available on which to base conclusions of ICM efficacy and safety in this review.
Finally, it should be noted that there is evidence from the observational studies that the ICMs also detected some non-AF cardiac arrhythmias, although no data on this additional potential benefit of ICMs were available from the CRYSTAL-AF trial, nor were data available comparing ICMs with external electrocardiography monitoring for the detection of non-AF cardiac arrhythmias. It is also unclear whether or not detecting these additional arrhythmias led to any change in the management of the patients in whom they were identified. The actual benefit to patients of detecting non-AF cardiac arrhythmias is, therefore, unclear and requires further research to establish if there is a true benefit.
Economic
One of the main strengths of the economic analysis is that outcome data were available from a RCT on the effectiveness of an ICM compared with SoC in the CS population. Therefore, reliable estimates of AF detection rates for an ICM (Reveal XT in this case) and SoC were used to estimate the long-term outcomes, costs and benefits of anticoagulation therapy versus antiplatelet therapy, using a previously developed and established economic model.
Even though the strength of the economic analysis is data being available for an ICM device in the correct target population, data were not available for each of the devices in the NICE final scope of this DAR (i.e. Reveal LINQ, BioMonitor 2-AF and Confirm Rx). For the Reveal LINQ, this is less of a limitation, as the data used in the analysis are based on an earlier model: the Reveal XT. However, for the BioMonitor 2-AF and Confirm Rx, the assumption of clinical equivalence with the Reveal XT is a strong assumption. The manufacturer of the Reveal devices advised that, with each iteration of the device, improvements are made to the algorithm to improve sensitivity and specificity, such that the Reveal LINQ has been estimated to have 100% sensitivity. Furthermore, the EAG’s clinical experts advised that the detection rates for each of the devices will be at least as good as the rates seen in the CRYSTAL-AF trial. With this in mind, caution should be applied when interpreting the cost-effectiveness results for the BioMonitor 2-AF and Confirm Rx, as the strong assumption of equivalence with the Reveal LINQ is not based on evidence of the performance of any version of these devices in the CS population, resulting in substantial uncertainty around the ICER.
Chapter 7 Conclusions
Clinical effectiveness
There is extremely limited DTA or comparative clinical effectiveness evidence for the use of ICMs in the detection of AF, particularly in the CS population. There is also evidence to suggest that the performance (e.g. PPV and NPV) of AF detection in ICM devices is dependent on the patient population, incidence rate of AF, the duration of monitoring and the type of AF,34 thus limiting the use of data in non-CS populations to draw meaningful conclusions.
Only the Reveal LINQ device has good-quality clinical evidence from which it is possible to draw conclusions for the CS population, albeit using RCT data from an older model, the Reveal XT. The clinical data for the Reveal XT suggest that it is significantly more effective at detecting AF than conventional follow-up, although it is also associated with a low risk of device-related AEs. Clinical expert opinion and evidence from a mixed population suggest that the Reveal LINQ, the newer device that is under investigation in this review, is likely to have better sensitivity and specificity for detecting AF than the Reveal XT and that it is also likely to be associated with fewer complications because of its smaller size. Nonetheless, there are no clinical studies to confirm these findings in a CS population.
The limited clinical data available for the Confirm Rx and BioMonitor 2-AF suggest that they both have good sensitivity and specificity for detecting AF, although it is not possible to draw conclusions about how they perform in CS patients or how any of the devices compare with each other. (Confidential information has been removed.) As a rapidly evolving clinical diagnostic field, it makes it extremely difficult to enable any direct comparison between the diagnostic accuracy of the three devices (Reveal LINQ, BioMonitor 2-AF and Confirm Rx). The absence of comparative clinical effectiveness data also limits the ability to draw any meaningful conclusions on the potential patient benefit of the ICMs, but the CRYSTAL-AF trial37 and the other clinical data for the Reveal devices suggest that cases of AF in CS patients who may otherwise go undetected are more likely to be identified using the Reveal LINQ than with no further monitoring. However, the benefit of detecting AF in CS patients is unclear, as no data were identified to demonstrate whether or not anticoagulation of CS patients with AF resulted in fewer strokes.
Cost-effectiveness
The EAG’s economic evaluation assessed the cost-effectiveness of ICMs compared with no further monitoring, to detect AF in people who have had a CS and have received at least 24 hours of non-invasive external cardiac monitoring. The devices included in the scope of this assessment were the Reveal LINQ, BioMonitor 2-AF and Confirm Rx. As mentioned previously, clinical effectiveness data, in the form of AF detection rates, were available only for the Reveal XT device from the CRYSTAL-AF RCT. 37 As a result, the entire economic analysis is based on the detection rates obtained from the CRYSTAL-AF trial, under the assumption that all the devices are likely to have similar efficacy. For the Reveal LINQ, this assumption is deemed reasonable by the EAG, as the manufacturer of both devices is the same; therefore, the data from the CRYSTAL-AF trial are more closely related to Reveal LINQ. For BioMonitor 2-AF and Confirm Rx, this is a strong assumption. Based on the assumption of clinical equivalence of all devices, the economic analysis found that ICMs could be considered cost-effective at a £20,000–30,000 threshold compared with SoC. When each device is compared incrementally, the BioMonitor 2-AF dominates the Reveal LINQ and Confirm Rx. However, the results for the BioMonitor 2-AF and Confirm Rx should be viewed with caution, as no data were available for any version of these devices in the CS population; as a result, there is substantial uncertainty in the results.
Suggested research priorities
High-quality head-to-head clinical trials of the Reveal LINQ, BioMonitor 2-AF and Confirm Rx in CS patients are required to enable a direct comparison between the ICMs in terms of clinical effectiveness. In addition, DTA studies for each of the three ICMs (Reveal LINQ, BioMonitor 2-AF and Confirm Rx) using a consistent reference standard (which would ideally be a minimum of 24 hours of external electrocardiography monitoring) are required in a CS population to both confirm the diagnostic accuracy of the ICM devices in detecting AF in CS patients and to enable a robust comparison of diagnostic accuracy between the ICMs. The key important factor in any clinical or diagnostic studies of the ICMs will be to ensure that they use the latest model and version of the device software to ensure that they provide the most clinically relevant data, (confidential information has been removed). In addition, research is required into the long-term benefits and harms of anticoagulation for CS patients with AF to confirm any clinical benefit of detecting additional cases of AF in CS patients, particularly in relation to secondary stroke prevention.
Acknowledgements
The EAG would like to thank Dr Alastair Sandilands (Consultant Cardiac Electrophysiologist, Glenfield Hospital, Leicester), Dr Klaus Witte (Senior Lecturer in Cardiology, University of Leeds, Leeds), Miss Cara Mercer (Chief Cardiac Physiologist, United Lincolnshire Hospitals NHS Trust), Dr David Fox (Consultant Cardiologist/Cardiac Electrophysiologist, Manchester University Hospitals NHS Foundation Trust), Dr Justin Lee (Consultant Cardiac Electrophysiologist, Sheffield Teaching Hospitals NHS Trust), Dr Sreeman Andole (Consultant in Stroke, King’s College Hospital NHS Foundation Trust), Mrs Joanne Denman (Lead Cardiology Advanced Clinical Practitioner, United Lincolnshire Hospitals NHS Trust) and Dr Anand Dixit (Consultant Stroke Physician, Newcastle upon Tyne NHS Foundation Trust) for providing clinical advice throughout the project, and Dr Howard Thom (Research Fellow in statistical modelling, University of Bristol) for providing statistical modelling input for the economic analysis. The EAG would also like to thank Dr Samantha Barton for her contributions to the protocol, background section and proofreading, and Ms Mariana Bacelar for her contribution to the quality assurance of the economic model.
Contributions of authors
Steven J Edwards (https://orcid.org/0000-0002-9049-3421) was the project lead and supervised the production of the final report, report writing, critical appraisal of the clinical evidence and critical appraisal of the economic evidence.
Victoria Wakefield (https://orcid.org/0000-0002-2058-6411) devised and carried out the clinical literature searches, study selection, data extraction, critical appraisal of the clinical evidence and report writing.
Tracey Jhita (https://orcid.org/0000-0002-8041-0346) contributed to the study selection, development of the conceptual economic model and report writing.
Kayleigh Kew (https://orcid.org/0000-0002-0623-6087) contributed to the study selection, data extraction, critical appraisal of the clinical evidence and report writing.
Peter Cain (https://orcid.org/0000-0001-8580-9391) contributed to the development of the economic model and carried out the economic analysis and report writing.
Gemma Marceniuk (https://orcid.org/0000-0001-9365-0384) devised and carried out the economic literature searches, study selection, data extraction, critical appraisal of the economic evidence and report writing.
All authors read and commented on draft versions of the report.
Publication
Wakefield V, Edwards S, Kew K, Jhita T, Cain P, Marceniuk G. The Benefits of Including Non-comparative Observational Studies in a Systematic Review of Implantable Cardiac Monitors in Cryptogenic Stroke. Cochrane Colloquium, Santiago, Chile, 22–25 October 2019. Poster P2-078.
Data-sharing statement
Further information and requests for access to the data used in this report can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence . Implantable Cardiac Monitors (BioMonitor 2-AF, Confirm Rx Insertable Cardiac Monitor and Reveal LINQ Insertable Cardiac Monitoring System) to Detect Atrial Fibrillation After Cryptogenic Stroke [GID-DG10023]. Final Scope: September 2018 n.d. www.nice.org.uk/guidance/gid-dg10023/documents/final-scope (accessed 21 January 2019).
- Office for National Statistics . Deaths Registered in England and Wales (Series DR): 2017. Registered Deaths by Age, Sex, Selected Underlying Causes of Death and the Leading Causes of Death for Both Males and Females 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredinenglandandwalesseriesdr/2017 (accessed 2 January 2019).
- Newton JN, Briggs AD, Murray CJ, Dicker D, Foreman KJ, Wang H, et al. Changes in health in England, with analysis by English regions and areas of deprivation, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2257-74. https://doi.org/10.1016/S0140-6736(15)00195-6.
- Sentinel Stroke National Audit Programme (SSNAP) . Rising to the Challenge: The Fourth SSNAP Annual Report 2015. www.strokeaudit.org/Documents/AnnualReport/2016-17-SSNAP-Annual-Report.aspx (accessed 2 January 2019).
- National Institute for Health and Care Excellence . Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management 2019. www.nice.org.uk/guidance/ng128 (accessed 9 September 2019).
- Nouh A, Hussain M, Mehta T, Yaghi S. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol 2016;7. https://doi.org/10.3389/fneur.2016.00037.
- Zhang C, Kasner S. Diagnosis, prognosis, and management of cryptogenic stroke. F1000Res 2016;5. https://doi.org/10.12688/f1000research.7384.1.
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 1994;25:333-7. https://doi.org/10.1161/01.STR.25.2.333.
- Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489-94. https://doi.org/10.1161/STROKEAHA.110.602615.
- National Stroke Association . Cryptogenic Stroke: Etiology, Diagnosis &Amp; Recurrent Preventive Management 2014. www.stroke.org/we-can-help/healthcare-professionals/improve-your-skills/pre-hospital-acute-stroke-programs-8 (accessed 31 July 2018).
- NHS . Overview: Atrial Fibrillation 2018. www.nhs.uk/conditions/atrial-fibrillation/ (accessed 2 January 2018).
- Public Health England . Atrial Fibrillation Prevalence Estimates in England: Application of Recent Population Estimates of AF in Sweden 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/644869/atrial_fibrillation_AF_briefing.pdf (accessed 31 July 2018).
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. https://doi.org/10.1161/01.STR.22.8.983.
- Choe WC, Passman RS, Brachmann J, Morillo CA, Sanna T, Bernstein RA, et al. A comparison of atrial fibrillation monitoring strategies after cryptogenic stroke (from the Cryptogenic Stroke and Underlying AF Trial). Am J Cardiol 2015;116:889-93. https://doi.org/10.1016/j.amjcard.2015.06.012.
- Ziegler PD, Rogers JD, Ferreira SW, Nichols AJ, Richards M, Koehler JL, et al. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol 2017;244:175-9. https://doi.org/10.1016/j.ijcard.2017.06.039.
- National Stroke Association . Atrial Fibrillation n.d. www.stroke.org.uk/what-is-stroke/are-you-at-risk-of-stroke/atrial-fibrillation (accessed 31 July 2018).
- Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke 2015;46:936-41. https://doi.org/10.1161/STROKEAHA.115.008714.
- Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474-80. https://doi.org/10.1161/CIRCEP.109.849638.
- National Institute for Health and Care Excellence . Atrial Fibrillation: Management 2014. www.nice.org.uk/guidance/cg180/chapter/1-Recommendations#diagnosis-and-assessment (accessed 31 July 2018).
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. https://doi.org/10.1093/eurheartj/ehw210.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. https://doi.org/10.1378/chest.09-1584.
- National Institute for Health and Care Excellence . NICE Pathway: Preventing Stroke in People With Atrial Fibrillation 2018. http://pathways.nice.org.uk/pathways/atrial-fibrillation (accessed 21 January 2019).
- Biotronik SE & Co. KG . BioMonitor 2-AF -S 2018. https://manuals.biotronik.com/wps/portal/emanuals/emanual/!ut/p/z1/04_Sj9CPykssy0xPLMnMz0vMAfIjo8ziPY0MDQy9DYy8LcI8TQwcPd2cvXyCTQ2DvQ31w8EKDHAARwP9KEL6o_ArMYAqwGNFQW6EQaajoiIA3yATlQ!!/dz/d5/L2dBISEvZ0FBIS9nQSEh/?country=GB&product=ImplCardMon/BioMonitor2/BioMon2_AF_S (accessed 31 July 2018).
- St. Jude Medical . Confirm Rx™ Model DM3500 Insertable Cardiac Monitor: User’s Manual 2019.
- Medtronic plc . Reveal LINQ ICM System 2018. www.medtronic.com/us-en/patients/treatments-therapies/heart-monitors/our-monitors/reveal-linq-icm.html (accessed 31 July 2018).
- St. Jude Medical . Help Manual for the Following Devices: SJM Confirm™ Implantable Cardiac Monitor; Confirm Rx™ Insertable Cardiac Monitor 2016. https://manuals.sjm.com/∼/media/manuals/product-manual-pdfs/d/7/d7408b10-0c87-4066-ae6d-e4ec9716f4fa.pdf (accessed 21 January 2019).
- Abbott Laboratories . Cardiac Arrhythmia and Heart Failure: Product Performance Report 2018 First Edition 2018. www.cardiovascular.abbott/content/dam/bss/divisionalsites/cv/pdf/reports/1.3.7.3/2018_1stEd_05022018.pdf (accessed 21 January 2019).
- Sethi A BM, Garberich R, Hoffman E, Langeberg T, Abdelhadi R, Katsiyiannis W, et al. Evolution of implantable cardiac monitors: a comparison of Reveal XT and LINQ patient selection, arrhythmia detection, and clinical outcomes. J Minneapolis Heart Institute Foundation 2017;1:130-5. https://doi.org/10.21925/mplsheartjournal-D-17-00012.
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Healthcare 2011. www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed 8 November 2018).
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 16 April 2019).
- Cochrane Methods . Screening and Diagnostic Tests. Handbook for DTA Reviews 2018. https://methods.cochrane.org/sdt/handbook-dta-reviews (accessed 16 April 2019).
- National Institute for Health and Care Excellence . Implantable Cardiac Monitors (BioMonitor 2-AF, Confirm Rx Insertable Cardiac Monitor and Reveal LINQ Insertable Cardiac Monitoring System) to Detect Atrial Fibrillation After Cryptogenic Stroke. DAR Protocol n.d. www.nice.org.uk/guidance/gid-dg10023/documents/final-protocol (accessed 9 September 2019).
- Wakefield V, Edwards S, Jhita T, Kew K, Cain P. Protocol: Implantable Cardiac Monitors (BioMonitor 2-AF, Confirm Rx Insertable Cardiac Monitor and Reveal LINQ Insertable Cardiac Monitoring System) to Detect Atrial Fibrillation After Cryptogenic Stroke: A Diagnostic Assessment Review n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018109216 (accessed 21 January 2018).
- Pürerfellner H, Sanders P, Sarkar S, Reisfeld E, Reiland J, Koehler J, et al. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. Europace 2018;20:f321-f328. https://doi.org/10.1093/europace/eux272.
- Higgins JPT, Savović J, Page MJ, Sterne JAC. on behalf of the ROB2 Development Group . Revised Cochrane Risk-Of-Bias Tool for Randomized Trials (RoB 2) 2018. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 21 January 2018).
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478-86. https://doi.org/10.1056/NEJMoa1313600.
- Pedersen KB, Madsen C, Sandgaard NCF, Diederichsen ACP, Bak S, Brandes A. Subclinical atrial fibrillation in patients with recent transient ischemic attack. J Cardiovasc Electrophysiol 2018;29:707-14. https://doi.org/10.1111/jce.13470.
- ClinicalTrials.gov . Rate of Atrial Fibrillation Through 12 Months in Patients With Recent Ischemic Stroke of Presumed Known Origin n.d. https://clinicaltrials.gov/ct2/show/NCT02700945 (accessed 2 October 2019).
- ClinicalTrials.gov . Detection of Silent Atrial Fibrillation AFter Ischemic StrOke (SAFFO) n.d. https://clinicaltrials.gov/ct2/show/NCT02684825 (accessed 2 October 2019).
- ClinicalTrials.gov . Atrial Fibrillation in Cryptogenic Stroke and TIA (NOR-FIB) n.d. https://clinicaltrials.gov/ct2/show/NCT02937077 (accessed 2 October 2019).
- ClinicalTrials.gov . Confirm Rx™ Versus Reveal LINQ™ - Which Is More Reliable in Data Transmission? A Randomized Clinical Study n.d. https://clinicaltrials.gov/ct2/show/NCT03720639 (accessed 2 October 2019).
- ClinicalTrials.gov . “Cryptogenic Stroke and Atrial Fibrillation Detection Through Implantable Loop Recorder (ILR)” (CRYPTONITE) n.d. https://clinicaltrials.gov/ct2/show/NCT01025947 (accessed 2 October 2019).
- ClinicalTrials.gov . Detection of Occult Paroxysmal AF in Cryptogenic Stroke or TIA Patients Using an Implantable Loop Recorder and Correlation With Genetic Markers n.d. https://clinicaltrials.gov/ct2/show/NCT02216370 (accessed 2 October 2019).
- ClinicalTrials.gov . Confirm Rx SMART Registry n.d. https://clinicaltrials.gov/ct2/show/NCT03505801 (accessed 2 October 2019).
- ClinicalTrials.gov . Extended Rhythm SCreening for AtRial Fibrillation in Cryptogenic Stroke Patients (SCARF) n.d. https://clinicaltrials.gov/ct2/show/NCT01550042 (accessed 2 October 2019).
- ClinicalTrials.gov . Post-Embolic Rhythm Detection With Implantable Versus External Monitoring (PERDIEM) n.d. https://clinicaltrials.gov/ct2/show/NCT02428140 (accessed 2 October 2019).
- Sinha AM, Diener HC, Morillo CA, Sanna T, Bernstein RA, Di Lazzaro V, et al. Cryptogenic Stroke and underlying Atrial Fibrillation (CRYSTAL AF): design and rationale. Am Heart J 2010;160:36-41.e1. https://doi.org/10.1016/j.ahj.2010.03.032.
- Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the Cryptogenic Stroke and underlying Atrial Fibrillation trial. Circ Arrhythm Electrophysiol 2016;9. https://doi.org/10.1161/CIRCEP.115.003333.
- Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck KH, Lebedev D, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3:141-7. https://doi.org/10.1161/CIRCEP.109.877852.
- Thijs V, Brachmann J, Morillo C, Passman R, Sanna T, Bernstein R. Predictors for detection of atrial fibrillation in cryptogenic stroke patients: insights from insertable cardiac monitor data in the CRYSTAL AF study. Int J Stroke 2014;9.
- Asaithambi G, Monita JE, Annamalai MR, Ho BM, Marino EH, Hanson SK. Prevalence of atrial fibrillation with insertable cardiac monitors in cryptogenic stroke: a single-center experience. J Electrocardiol 2018;51:973-6. https://doi.org/10.1016/j.jelectrocard.2018.08.033.
- Chalfoun NT, Sagorski R, Fox J, Pieroban J, Rosema S, Khan M, et al. Detection of atrial fibrillation following acute cryptogenic stroke using the insertable cardiac monitor Reveal LINQ. Heart Rhythm 2016;1:S438-S9.
- Ferrara M, Milstein N, Varghese M, Bhatt A, Preminger MW, Sichrovsky T, et al. Pattern of atrial fibrillation detected during long-term ECG monitoring by implantable cardiac monitors in patients with cryptogenic stroke. Heart Rhythm 2017;14.
- Heckle M, Shahan E, Longserre SE, Hernandez HC, Barry N, Gopinathannair R, et al. Racial differences in the incidence of atrial fibrillation in patients with cryptogenic stroke – a two-center study. Heart Rhythm 2018;15.
- Kotlarz-Bottcher MJ, Busch M, Frenzel M, Hummel A, Schminke U, Von Sarnowski B. Risk factors for atrial fibrillation in patients with embolic stroke of undetermined source (ESUS). Eur Stroke J 2018;3.
- Li Y, Nantsupawat T, Olson M, Tholakanahalli V, Adabag S, Wang Z, et al. A single center experience on the clinical utility evaluation of an insertable cardiac monitor. J Electrocardiol 2018;51:583-7. https://doi.org/10.1016/j.jelectrocard.2018.05.002.
- Seow SC, How AK, Chan SP, Teoh HL, Lim TW, Singh D, et al. High incidence of occult atrial fibrillation in Asian patients with cryptogenic stroke. J Stroke Cerebrovasc Dis 2018;27:2182-6. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.03.019.
- Pallesen LPM, Mayer J, Barlinn K, Barlinn J, Prakapenia A, Siepmann T, et al. Real-life detection rate of insertable cardiac monitors in patients with ESUS. Stroke 2017;48.
- Carrazco C, Golyan D, Kahen M, Black K, Libman RB, Katz JM. Prevalence and risk factors for paroxysmal atrial fibrillation and flutter detection after cryptogenic ischemic stroke. J Stroke Cerebrovasc Dis 2018;27:203-9. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.022.
- Abichandani A, Signarovitz D, Branch K, Prutzman D, Agrawal S, Sadowski L, et al. Impact of CHA2DS2VASc score on atrial fibrillation detection in patients with cryptogenic stroke. Cardiology 2016;134.
- Poli S, Diedler J, Härtig F, Götz N, Bauer A, Sachse T, et al. Insertable cardiac monitors after cryptogenic stroke – a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol 2016;23:375-81. https://doi.org/10.1111/ene.12843.
- Joseph K, Wanjiku S, Jumaa M, Richards M. Improved efficacy of ICM detection of atrial fibrillation in cryptogenic stroke patients with MRI-defined infarcts: preliminary results from the SPIDER registry. Stroke 2015;46.
- Salahuddin H, Zaidi S, Tietjen G, Cummings J, Jumaa M. Paroxysmal atrial fibrillation detection rates in cryptogenic stroke patients selected by stroke specialists for prolonged cardiac monitoring (P1.053). Neurology 2015;84.
- Christensen LM, Krieger DW, Højberg S, Pedersen OD, Karlsen FM, Jacobsen MD, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014;21:884-9. https://doi.org/10.1111/ene.12400.
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013;80:1546-50. https://doi.org/10.1212/WNL.0b013e31828f1828.
- Etgen T, Hochreiter M, Mundel M, Freudenberger T. Insertable cardiac event recorder in detection of atrial fibrillation after cryptogenic stroke: an audit report. Stroke 2013;44:2007-9. https://doi.org/10.1161/STROKEAHA.113.001340.
- Holtzman MC, Foster N, Shah D. Implantation with Reveal XT after cryptogenic stroke is a useful tool to detect previously undiagnosed atrial fibrillation. Stroke 2013;44.
- Mercé J, Garcia M, Ustrell X, Pellisé A, de Castro R, Bardají A. Implantable loop recorder: a new tool in the diagnosis of cryptogenic stroke. Rev Esp Cardiol 2013;66:665-6. https://doi.org/10.1016/j.rec.2013.02.001.
- Müller P, Ivanov V, Kara K, Klein-Wiele O, Forkmann M, Piorkowski C, et al. Total atrial conduction time to predict occult atrial fibrillation after cryptogenic stroke. Clin Res Cardiol 2017;106:113-19. https://doi.org/10.1007/s00392-016-1029-2.
- Reinke F, Bettin M, Ross LS, Kochhäuser S, Kleffner I, Ritter M, et al. Refinement of detecting atrial fibrillation in stroke patients: results from the TRACK-AF Study. Eur J Neurol 2018;25:631-6. https://doi.org/10.1111/ene.13538.
- Ritter MA, Kochhäuser S, Duning T, Reinke F, Pott C, Dechering DG, et al. Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013;44:1449-52. https://doi.org/10.1161/STROKEAHA.111.676189.
- Rojo-Martinez E, Sandín-Fuentes M, Calleja-Sanz AI, Cortijo-García E, García-Bermejo P, Ruiz-Piñero M, et al. High performance of an implantable Holter monitor in the detection of concealed paroxysmal atrial fibrillation in patients with cryptogenic stroke and a suspected embolic mechanism. Rev Neurol 2013;57:251-7. https://doi.org/10.33588/rn.5706.2013187.
- Israel C, Kitsiou A, Kalyani M, Deelawar S, Ejangue LE, Rogalewski A, et al. Detection of atrial fibrillation in patients with embolic stroke of undetermined source by prolonged monitoring with implantable loop recorders. Thromb Haemost 2017;117:1962-9. https://doi.org/10.1160/TH17-02-0072.
- Nölker G, Mayer J, Boldt LH, Seidl K, VAN Driel V, Massa T, et al. Performance of an implantable cardiac monitor to detect atrial fibrillation: results of the DETECT AF study. J Cardiovasc Electrophysiol 2016;27:1403-10. https://doi.org/10.1111/jce.13089.
- Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, et al. Subclinical atrial fibrillation in older patients. Circulation 2017;136:1276-83. https://doi.org/10.1161/CIRCULATIONAHA.117.028845.
- Biotronik SE & Co. KG . BioMonitor 2 Study Report I 2018.
- Ooi SY, Ng B, Singarayar S, Hellestrand K, Illes P, Mohamed U, et al. BioMonitor 2 pilot study: early experience with implantation of the Biotronik BioMonitor 2 implantable cardiac monitor. Heart Lung Circ 2018;27:1462-6. https://doi.org/10.1016/j.hlc.2017.09.005.
- Biotronik SE & Co. KG . Results of the Post Market Observation (PMO)-FIT (fast Insertion Tool) Update for BioMonitor 2 2018.
- Reinsch N, Ruprecht U, Buchholz J, Diehl RR, Kälsch H, Neven K. The BioMonitor 2 insertable cardiac monitor: clinical experience with a novel implantable cardiac monitor. J Electrocardiol 2018;51:751-5. https://doi.org/10.1016/j.jelectrocard.2018.05.017.
- Piorkowski C, Busch M, Nölker G, Schmitt J, Roithinger FX, Young G, et al. Clinical evaluation of a small insertable cardiac monitor with a long sensing vector. National Institute for Health and Care Excellence; 2018.
- Biotronik SE & Co. KG . BioMonitor 2 Study Report II 2018.
- Biotronik SE & Co. KG . BioInsight Study Final Report 2018.
- Awad K, Weiss R, Yunus A, Bittrick JM, Nekkanti R, Houmsse M, et al. BioMonitor 2 In-office setting insertion safety and feasibility evaluation with device functionality assessment: results from the BioInsight study. National Institute for Health and Care Excellence; 2018.
- Mittal S, Sanders P, Pokushalov E, Dekker L, Kereiakes D, Schloss EJ, et al. Safety profile of a miniaturized insertable cardiac monitor: results from two prospective trials. Pacing Clin Electrophysiol 2015;38:1464-9. https://doi.org/10.1111/pace.12752.
- Pürerfellner H, Sanders P, Pokushalov E, Di Bacco M, Bergemann T, Dekker LR. Reveal LINQ Usability Study Investigators . Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm 2015;12:1113-19. https://doi.org/10.1016/j.hrthm.2015.02.030.
- Sanders P, Pürerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B, et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm 2016;13:1425-30. https://doi.org/10.1016/j.hrthm.2016.03.005.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997.
- De Angelis G, Cimon K, Sinclair A, Farrah K, Cairns J, Baranchuk A, et al. Monitoring for Atrial Fibrillation in Discharged Stroke and Transient Ischemic Attack Patients: A Clinical and Cost-Effectiveness Analysis and Review of Patient Preferences. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2016.
- Diamantopoulos A, Sawyer LM, Lip GY, Witte KK, Reynolds MR, Fauchier L, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke 2016;11:302-12. https://doi.org/10.1177/1747493015620803.
- Maervoet J, Bossers N, Borge RP, Schollbauer V, Van Engen A, Smala A. Clinical and economic value of device-based detection of atrial fibrillation in patients with cryptogenic stroke. Value Health 2017;20. https://doi.org/10.1016/j.jval.2017.08.1053.
- Quiroz M, Wolff C, Eggington S. Insertable cardiac monitor versus standard of care for detection of atrial fibrillation in patients following cryptogenic stroke: a Dutch cost-effectiveness analysis. Value Health 2017;20. https://doi.org/10.1016/j.jval.2017.08.1075.
- Thijs V, Guarnieri C, Makino K, Tilden D, Huynh M. Cost-effectiveness of long-term continuous monitoring with an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke: an Australian payer perspective. J Neurol Neurosurg Psychiatry 2018;89. https://doi.org/10.1136/jnnp-2018-ANZAN.12.
- Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J 2014;35:1897-906. https://doi.org/10.1093/eurheartj/ehu006.
- Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210.e20. https://doi.org/10.1016/j.clinthera.2013.12.011.
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003;34:2060-5. https://doi.org/10.1161/01.STR.0000080678.09344.8D.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. https://doi.org/10.1056/NEJMoa0905561.
- Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503-11. https://doi.org/10.1016/S1474-4422(12)70092-3.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. https://doi.org/10.1056/NEJMoa1107039.
- Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012;11:315-22. https://doi.org/10.1016/S1474-4422(12)70042-X.
- Office for National Statistics . National Life Tables: England 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables (accessed 16 January 2019).
- Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Oxford Vascular Study . Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology 2013;81:1588-95. https://doi.org/10.1212/WNL.0b013e3182a9f45f.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Department of Health and Social Care . NHS Reference Costs 2012 to 2013 n.d. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013.
- Duarte R, Stainthorpe A, Greenhalgh J, Richardson M, Nevitt S, Mahon J, et al. Lead-I ECG for detecting atrial fibrillation in patients with an irregular pulse using single time point testing: a systematic review and economic evaluation. Health Technol Assess 2020;24.
- Sterne JA, Bodalia PN, Bryden PA, Davies PA, López-López JA, Okoli GN, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21090.
- Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21290.
- Joint Formulary Committee . British National Formulary 2015. www.medicinescomplete.com (accessed 30 November 2018).
- Department of Health and Social Care . NHS Reference Costs 2018. www.gov.uk/government/collections/nhs-reference-costs (accessed 15 February 2019).
- Office for National Statistics . Consumer Price Inflation Index for Medical Services (DKC3) 2018. www.ons.gov.uk/economy/inflationandpriceindices/timeseries/dkc3/mm23 (accessed 12 December 2018).
- National institute for Health and Care Excellence . Rivaroxaban for the Prevention of Stroke and Systemic Embolism in People With Atrial Fibrillation 2012. www.nice.org.uk/guidance/ta256 (accessed 19 February 2018).
- Alvarez-Sabín J, Santamarina E, Maisterra O, Jacas C, Molina C, Quintana M. Long-term treatment with citicoline prevents cognitive decline and predicts a better quality of life after a first ischemic stroke. Int J Mol Sci 2016;17. https://doi.org/10.3390/ijms17030390.
- Bach JP, Riedel O, Pieper L, Klotsche J, Dodel R, Wittchen HU. Health-related quality of life in patients with a history of myocardial infarction and stroke. Cerebrovasc Dis 2011;31:68-76. https://doi.org/10.1159/000319027.
- Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, et al. Sex differences in quality of life after ischemic stroke. Neurology 2014;82:922-31. https://doi.org/10.1212/WNL.0000000000000208.
- Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, et al. Predictors of functional level and quality of life at 6 months after a first-ever stroke: the KOSCO study. J Neurol 2016;263:1166-77. https://doi.org/10.1007/s00415-016-8119-y.
- Christensen MC, Mayer S, Ferran JM. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 2009;40:1677-82. https://doi.org/10.1161/STROKEAHA.108.538967.
- Ghatnekar O, Eriksson M, Glader EL. Mapping health outcome measures from a stroke registry to EQ-5D weights. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-34.
- Golicki D, Niewada M, Buczek J, Karlińska A, Kobayashi A, Janssen MF, et al. Validity of EQ-5D-5L in stroke. Qual Life Res 2015;24:845-50. https://doi.org/10.1007/s11136-014-0834-1.
- Golicki D, Niewada M, Karlińska A, Buczek J, Kobayashi A, Janssen MF, et al. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual Life Res 2015;24:1555-63. https://doi.org/10.1007/s11136-014-0873-7.
- Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193-8. https://doi.org/10.1161/01.STR.0000196990.69412.fb.
- Hallinen T, Soini EJ, Linna M, Saarni SI. Cost-effectiveness of apixaban and warfarin in the prevention of thromboembolic complications among atrial fibrillation patients. Springerplus 2016;5. https://doi.org/10.1186/s40064-016-3024-5.
- Lindgren P, Glader EL, Jönsson B. Utility loss and indirect costs after stroke in Sweden. Eur J Cardiovasc Prev Rehabil 2008;15:230-3. https://doi.org/10.1097/HJR.0b013e3282f37a22.
- Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, Perestelo Perez L, Serrano-Aguilar P, Montón-Álvarez F. Social and economic costs and health-related quality of life in stroke survivors in the Canary Islands, Spain. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-315.
- Lunde L. Can EQ-5D and 15D be used interchangeably in economic evaluations? Assessing quality of life in post-stroke patients. Eur J Health Econ 2013;14:539-50. https://doi.org/10.1007/s10198-012-0402-y.
- Mar J, Begiristain JM, Arrazola A. Cost-effectiveness analysis of thrombolytic treatment for stroke. Cerebrovasc Dis 2005;20:193-200. https://doi.org/10.1159/000087204.
- Mar J, Masjuan J, Oliva-Moreno J, Gonzalez-Rojas N, Becerra V, Casado MÁ, et al. Outcomes measured by mortality rates, quality of life and degree of autonomy in the first year in stroke units in Spain. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0230-8.
- Pickard AS, Johnson JA, Feeny DH. Responsiveness of generic health-related quality of life measures in stroke. Qual Life Res 2005;14:207-19. https://doi.org/10.1007/s11136-004-3928-3.
- Pickard AS, Johnson JA, Feeny DH, Shuaib A, Carriere KC, Nasser AM. Agreement between patient and proxy assessments of health-related quality of life after stroke using the EQ-5D and Health Utilities Index. Stroke 2004;35:607-12. https://doi.org/10.1161/01.STR.0000110984.91157.BD.
- van Eeden M, van Heugten C, van Mastrigt GA, van Mierlo M, Visser-Meily JM, Evers SM. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008220.
- Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, et al. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke 2006;37:2567-72. https://doi.org/10.1161/01.STR.0000240506.34616.10.
- Berg J, Lindgren P, Nieuwlaat R, Bouin O, Crijns H. Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res 2010;19:381-90. https://doi.org/10.1007/s11136-010-9591-y.
- Roalfe AK, Bryant TL, Davies MH, Hackett TG, Saba S, Fletcher K, et al. A cross-sectional study of quality of life in an elderly population (75 years and over) with atrial fibrillation: secondary analysis of data from the Birmingham Atrial Fibrillation Treatment of the Aged study. Europace 2012;14:1420-7. https://doi.org/10.1093/europace/eus102.
- Wang K, Li H, Kwong WJ, Antman EM, Ruff CT, Giugliano RP, et al. Impact of spontaneous extracranial bleeding events on health state utility in patients with atrial fibrillation: results from the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.117.006703.
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2005;26:2422-34. https://doi.org/10.1093/eurheartj/ehi505.
- Creager MA. Results of the CAPRIE trial: efficacy and safety of clopidogrel. Clopidogrel versus aspirin in patients at risk of ischaemic events. Vasc Med 1998;3:257-60. https://doi.org/10.1177/1358836X9800300314.
- Hao Q, Tampi M, O’Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ 2018;363. https://doi.org/10.1136/bmj.k5108.
- Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, et al. Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004301.
- EBM DataLab University of Oxford . Explore England’s Prescribing Data n.d. https://openprescribing.net (accessed 11 December 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 15 February 2019).
- Kanters TA, Wolff C, Boyson D, Kouakam C, Dinh T, Hakkaart L, et al. Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace 2016;18:919-24. https://doi.org/10.1093/europace/euv217.
- Joint Formulary Committee . British National Formulary 2018. www.medicinescomplete.com (accessed 30 November 2018).
- Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke 2013;8:308-14. https://doi.org/10.1111/j.1747-4949.2012.00812.x.
- Caro JJ, Nord E, Siebert U, McGuire A, McGregor M, Henry D, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ 2010;19:1117-27. https://doi.org/10.1002/hec.1629.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 18 December 2018).
- Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016.
- Venables WN, Ripley BD. Modern Applied Statistics with S. New York, NY: Springer; 2002.
- Medtronic Cardiac Rhythm and Heart Failure . Study of Continuous Cardiac Monitoring to Assess Atrial Fibrillation After Cryptogenic Stroke (CRYSTAL-AF) 2014. https://clinicaltrials.gov/ct2/show/results/NCT00924638 (accessed 19 November 2019).
- Passman RS, Morillo CA, Brachmann J, Sanna T, Di Lazzaro V, Bernstein R, et al. A comparison of monitoring strategies for the detection of atrial fibrillation after cryptogenic stroke: results from the CRYSTAL AF study. Heart Rhythm 2014;1.
- Kammersgaard LP, Olsen TS. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis 2006;21:187-93. https://doi.org/10.1159/000090531.
- Saposnik G, Fang J, O’Donnell M, Hachinski V, Kapral MK, Hill MD, et al. Escalating levels of access to in-hospital care and stroke mortality. Stroke 2008;39:2522-30. https://doi.org/10.1161/STROKEAHA.107.507145.
- Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012;43:1795-9. https://doi.org/10.1161/STROKEAHA.111.630731.
- Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107-14. https://doi.org/10.1001/jamainternmed.2013.11912.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. https://doi.org/10.7326/0003-4819-146-12-200706190-00007.
- Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology 2007;69:546-54. https://doi.org/10.1212/01.wnl.0000267275.68538.8d.
- Xian Y, Wu J, O’Brien EC, Fonarow GC, Olson DM, Schwamm LH, et al. Real world effectiveness of warfarin among ischemic stroke patients with atrial fibrillation: observational analysis from Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. BMJ 2015;351. https://doi.org/10.1136/bmj.h3786.
- Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-50. https://doi.org/10.1016/S0140-6736(07)61233-1.
- An J, Niu F, Lang DT, Jazdzewski KP, Le PT, Rashid N, et al. Stroke and bleeding risk associated with antithrombotic therapy for patients with nonvalvular atrial fibrillation in clinical practice. J Am Heart Assoc 2015;4. https://doi.org/10.1161/JAHA.115.001921.
- Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice?. JAMA 2003;290:2685-92. https://doi.org/10.1001/jama.290.20.2685.
- The Stroke Prevention in Atrial Fibrillation Investigators . Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med 1996;156:409-16. https://doi.org/10.1001/archinte.156.4.409.
- Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010;9:1157-63. https://doi.org/10.1016/S1474-4422(10)70274-X.
- Krueger H, Lindsay P, Cote R, Kapral MK, Kaczorowski J, Hill MD. Cost avoidance associated with optimal stroke care in Canada. Stroke 2012;43:2198-206. https://doi.org/10.1161/STROKEAHA.111.646091.
- Bjorck F, Renlund H, Svensson PJ, Sjalander A. Warfarin persistence among stroke patients with atrial fibrillation. Thromb Res 2015;136:744-8. https://doi.org/10.1016/j.thromres.2015.07.028.
- Singh SM, Micieli A, Wijeysundera HC. Economic evaluation of percutaneous left atrial appendage occlusion, dabigatran, and warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Circulation 2013;127:2414-23. https://doi.org/10.1161/CIRCULATIONAHA.112.000920.
- Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Cote R, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci 2012;39:793-800. https://doi.org/10.1017/S0317167100015638.
- Cohen D, Manuel DG, Tugwell P, Sanmartin C, Ramsay T. Direct healthcare costs of acute myocardial infarction in Canada’s elderly across the continuum of care. J Econ Ageing 2014;3:44-9. https://doi.org/10.1016/j.jeoa.2014.05.002.
- Drugs Funded by Ontario Drug Benefit (ODB) Program: e-formulary. Toronto, ON: Ontario Ministry of Health and Long-Term Care; 2015.
- Ontario Drug Benefit Program: Dispensing Fees 2015. http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx.
- Canadian Institute for Health Information (CIHI) . National Health Expenditure Trends, 1975 to 2014 2014.
- Fassbender K, Fainsinger RL, Carson M, Finegan BA. Cost trajectories at the end of life: the Canadian experience. J Pain Symptom Manage 2009;38:75-80. https://doi.org/10.1016/j.jpainsymman.2009.04.007.
- Tanuseputro P, Wodchis WP, Fowler R, Walker P, Bai YQ, Bronskill SE, et al. The health care cost of dying: a population-based retrospective cohort study of the last year of life in Ontario, Canada. PLoS ONE 2015;10. https://doi.org/10.1371/journal.pone.0121759.
- Dorman P, Dennis M, Sandercock P. Are the modified “simple questions” a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry 2000;69:487-93. https://doi.org/10.1136/jnnp.69.4.487.
- Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 1996;156:1829-36. https://doi.org/10.1001/archinte.1996.00440150083009.
- Smith DW, Davies EW, Wissinger E, Huelin R, Matza LS, Chung K. A systematic literature review of cardiovascular event utilities. Expert Rev Pharmacoecon Outcomes Res 2013;13:767-90. https://doi.org/10.1586/14737167.2013.841545.
- Bohmer E, Kristiansen IS, Arnesen H, Halvorsen S. Health-related quality of life after myocardial infarction, does choice of method make a difference?. Scand Cardiovasc J 2014;48:216-22. https://doi.org/10.3109/14017431.2014.923581.
- Bager P, Dahlerup JF. Patient-reported outcomes after acute nonvariceal upper gastrointestinal hemorrhage. Scand J Gastroenterol 2014;49:909-16. https://doi.org/10.3109/00365521.2014.910544.
- Office for National Statistics . National Life Tables, 2011–2013 2014. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014-09-25.
- Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens Stroke Registry. Cerebrovasc Dis 2008;26:381-7. https://doi.org/10.1159/000151678.
- Brønnum-Hansen H, Davidsen M, Thorvaldsen P, Danish MSG. Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. https://doi.org/10.1161/hs0901.094253.
- Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 2012;11:225-31. https://doi.org/10.1016/S1474-4422(12)70017-0.
- Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke 2012;43:3298-304. https://doi.org/10.1161/STROKEAHA.112.673558.
- European Atrial Fibrillation Trial (EAFT) Study Group . Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255-62. https://doi.org/10.1016/0140-6736(93)92358-Z.
- Pisters R, Lane DA, Marin F, Camm AJ, Lip GY. Stroke and thromboembolism in atrial fibrillation. Circ J 2012;76:2289-304. https://doi.org/10.1253/circj.CJ-12-1036.
- Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-92. https://doi.org/10.1161/01.CIR.0000145172.55640.93.
- Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012-18. https://doi.org/10.1136/jnnp.2008.170456.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Greiner W, Weijnen T, Neuwenhuizen M, Oppe S, Badia X, Busschbach J, et al. A single European currency for EQ-5D health states. Eur J Health Econom 2003;4:222-31. https://doi.org/10.1007/s10198-003-0182-5.
- Agency for Healthcare Research and Quality. Calculating the U.S. Population-Based EQ-5D Index Score 2005. http://www.ahrq.gov/rice/EQ5Dscore.htm.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D2 valuation model. Med Care 2005;43:203-20. https://doi.org/10.1097/00005650-200503000-00003.
- Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult US population as assessed by the EQ-5D and Health Utilities Index. Med Care 2005;43:1078-86. https://doi.org/10.1097/01.mlr.0000182493.57090.c1.
- Golicki D, Jakubczyk M, Niewada M, Wrona W, Busschbach JJ. Valuation of EQ-5D health states in Poland: First TTO-based social value set in Central and Eastern Europe. Value Health 2010;13:289-97. https://doi.org/10.1111/j.1524-4733.2009.00596.x.
- Golicki D, Niewada M, van Hout B, Janssen MF, Pickard AS. Interim EQ-5D-5L value set for Poland: First crosswalk value set in Central and Eastern Europe. Value Health Reg Issues 2014;4:19-23. https://doi.org/10.1016/j.vhri.2014.06.001.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Heistaro S. Methodology report. Health 2000 survey. Publications of the National Public Health Institute B26. Helsinki; 2008.
- Badía X, Roset M, Montserrat S, Herdman M, Segura A. La versión española del EuroQol: descripción y aplicaciones. Med Clin 1999;112:S79-86.
- Lamers LM, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Ned Tijdschr Geneeskd 2005;149:1574-8.
- The EuroQol Group . Euro-Qol: a new facility for measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
Appendix 1 Clinical search strategies
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal adj2 LINQ$).tw. | 35 |
| 2 | (Reveal adj2 XT$).tw. | 45 |
| 3 | BioMonitor$.tw. | 6297 |
| 4 | (Confirm adj2 RX$).tw. | 2 |
| 5 | (SJM adj2 Confirm$).tw. | 1 |
| 6 | (insertable adj3 cardiac adj3 monitor$).tw. | 80 |
| 7 | (implantable adj3 cardiac adj3 monitor$).tw. | 131 |
| 8 | (insertable adj3 loop adj3 recorder$).tw. | 35 |
| 9 | (implantable adj3 loop adj3 recorder$).tw. | 458 |
| 10 | (ICM or ICMs).tw. | 3782 |
| 11 | or/1-10 | 10,693 |
| 12 | exp STROKE/ | 116,275 |
| 13 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 218,619 |
| 14 | Ischaemic Attack, Transient/ | 19,490 |
| 15 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 12,728 |
| 16 | (TIA or TIAs or mini-stroke or ministroke or mini-strokes or ministrokes).tw. | 7998 |
| 17 | or/12-16 | 265,082 |
| 18 | 11 and 17 | 137 |
| 19 | animals/not humans/ | 4,461,144 |
| 20 | 18 not 19 | 128 |
| 21 | limit 20 to english language | 123 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal adj2 LINQ$).tw. | 96 |
| 2 | (Reveal adj2 XT$).tw. | 185 |
| 3 | BioMonitor$.tw. | 7678 |
| 4 | (Confirm adj2 RX$).tw. | 4 |
| 5 | (SJM adj2 Confirm$).tw. | 2 |
| 6 | (insertable adj3 cardiac adj3 monitor$).tw. | 187 |
| 7 | (implantable adj3 cardiac adj3 monitor$).tw. | 272 |
| 8 | (insertable adj3 loop adj3 recorder$).tw. | 51 |
| 9 | (implantable adj3 loop adj3 recorder$).tw. | 977 |
| 10 | (ICM or ICMs).tw. | 5864 |
| 11 | implantable cardiac monitor/ | 11,841 |
| 12 | reveal.dv. | 362 |
| 13 | or/1-12 | 26,150 |
| 14 | exp cerebrovascular accident/ | 172,589 |
| 15 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 341,320 |
| 16 | transient ischaemic attack/ | 33,423 |
| 17 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 19,087 |
| 18 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes).tw. | 17,067 |
| 19 | or/14-18 | 404,456 |
| 20 | 13 and 19 | 791 |
| 21 | nonhuman/not human/ | 4,201,609 |
| 22 | 20 not 21 | 771 |
| 23 | limit 22 to english language | 758 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal near/2 LINQ*):ti,ab,kw | 14 |
| 2 | (Reveal near/2 XT*):ti,ab,kw | 27 |
| 3 | BioMonitor*:ti,ab,kw | 40 |
| 4 | (Confirm near/2 RX*):ti,ab,kw | 0 |
| 5 | (SJM near/2 Confirm*):ti,ab,kw | 1 |
| 6 | (insertable near/3 cardiac near/3 monitor*):ti,ab,kw | 31 |
| 7 | (implantable near/3 cardiac near/3 monitor*):ti,ab,kw | 254 |
| 8 | (insertable near/3 loop near/3 recorder*):ti,ab,kw | 1 |
| 9 | (implantable near/3 loop near/3 recorder*):ti,ab,kw | 101 |
| 10 | ICM:ti,ab,kw | 228 |
| 11 | (OR #1-#10) | 565 |
| 12 | MeSH descriptor: [Stroke] explode all trees | 7713 |
| 13 | (stroke* or apoplexy* or CVA or CVAS):ti,ab,kw | 42,050 |
| 14 | MeSH descriptor: [Ischaemic Attack, Transient] explode all trees | 645 |
| 15 | (transient near/3 (ischaemi* or ischaemi*) near/3 attack*):ti,ab,kw | 2397 |
| 16 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes):ti,ab,kw | 1185 |
| 17 | (OR #12-#16) | 43,095 |
| 18 | #11 and #17 | 72 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal NEAR2 LINQ*) | 0 |
| 2 | (Reveal NEAR2 XT*) | 0 |
| 3 | (BioMonitor*) | 0 |
| 4 | (Confirm NEAR2 RX*) | 0 |
| 5 | (SJM NEAR2 Confirm*) | 0 |
| 6 | (insertable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 7 | (implantable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 8 | (insertable NEAR3 loop NEAR3 recorder*) | 5 |
| 9 | (implantable NEAR3 loop NEAR3 recorder*) | 9 |
| 10 | (ICM*) | 12 |
| 11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 25 |
| 12 | MeSH DESCRIPTOR Stroke EXPLODE ALL TREES | 1354 |
| 13 | (stroke* or apoplexy* or CVA or CVAS) | 3165 |
| 14 | MeSH DESCRIPTOR Ischaemic Attack, Transient EXPLODE ALL TREES | 89 |
| 15 | (transient NEAR3 (ischaemi* or ischaemi*) NEAR3 attack*) | 243 |
| 16 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes) | 86 |
| 17 | #12 OR #13 OR #14 OR #15 OR #16 | 3202 |
| 18 | #11 AND #17 | 3 |
| 19 | (#18) IN DARE, HTA | 1 |
Appendix 2 Clinical excluded studies
| Study/reference | Reason for exclusion |
|---|---|
| Assar M, Thijs V, Brachmann J, Morillo C, Passman R, Sanna T, et al. Predictors for detection of atrial fibrillation in cryptogenic stroke patients: insights from insertable cardiac monitor data in the CRYSTAL AF study. Eur Heart J 2014;1:1109 | No outcome data: CRYSTAL-AF, no unique data |
| Bernstein RA, Di Lazzaro V, Rymer MM, Passman RS, Brachmann J, Morillo CA, et al. Infarct topography and detection of atrial fibrillation in cryptogenic stroke: the CRYSTAL-AF study. Stroke Conf 2015;46:A207 | No outcome data: CRYSTAL-AF, no unique data |
| Bernstein RA, Di Lazzaro V, Rymer MM, Passman RS, Brachmann J, Morillo CA, et al. Infarct topography and detection of atrial fibrillation in cryptogenic stroke: results from CRYSTAL AF. Cerebrovasc Dis 2015;40:91–6 | No outcome data: CRYSTAL-AF, no unique data |
| Diamantopoulos A, Sawyer LM, Lip GY, Witte KK, Reynolds MR, Fauchier L, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke 2016;11:302–12 | No outcome data: CRYSTAL-AF, no unique data |
| Passman RS, Koehler JL, Ziegler PD. Atrial fibrillation begets atrial fibrillation in cryptogenic stroke patients: results from the CRYSTAL-AF trial. Circulation 2014;130:A13915 | No outcome data: CRYSTAL-AF, no unique data |
| Passman RS, Rymer MM, Liu S, Ziegler PD. Incidence of atrial fibrillation among patients with an embolic stroke of undetermined source. Stroke Conf 2017;48:A78 | No outcome data: CRYSTAL-AF, no unique data |
| Passman RS, Ziegler PD, Kwong C, Crawford MH, Koehler JL, Zhao SX. Validation of a clinical risk score for predicting atrial fibrillation in cryptogenic stroke patients with insertable cardiac monitors: insights from the CRYSTAL-AF study. Stroke Conf 2018;49:A125 | No outcome data: CRYSTAL-AF, no unique data |
| Sanna T, Bernstein R, Brachmann J, Diener HC, Di Lazzaro V, Morillo C, et al. Detection rates in patients with cryptogenic stroke: a comparison of the CRYSTAL-AF and EMBRACE trials. Eur Stroke J 2016; Conference: 2nd European Stroke Organisation Conference, 10–12 May 2016, Barcelona, Spain, abstract no. 651 | No outcome data: CRYSTAL-AF, no unique data |
| Thijs V, Brachmann J, Morillo C, Passman R, Sanna T, Bernstein R. Predictors for detection of atrial fibrillation in cryptogenic stroke patients: insights from insertable cardiac monitor data in the CRYSTAL AF study. Int J Stroke 2014;9:25 | No outcome data: CRYSTAL-AF, no unique data |
| Verma N, Ziegler PD, Liu S, Passman RS. Incidence of atrial fibrillation among patients with an embolic stroke of undetermined source: insights from insertable cardiac monitors. Int J Stroke 2019;14:146–53 | No outcome data: CRYSTAL-AF, no unique data |
| Lambert AT, Ratajczek-Tretel B, Russell D, Halvorsen B, Sandset EC, Naess H, et al. Atrial fibrillation in cryptogenic stroke – the Nordic atrial fibrillation and stroke study (NOR-FIB). Eur Stroke J 2017;2(Suppl. 1):336 | Ongoing study |
| Tancin Lambert A, Kong XY, Ratajczak-Tretel B, Bente Evy H, Skjelland M, Russell D, et al. Atrial fibrillation in cryptogenic stroke the Nordic atrial fibrillation and stroke study (NOR-FIB) Eur Stroke J 2018;3(Suppl. 1):613–4 | Ongoing study |
| Tancin Lambert A, Kong XY, Ratajczak-Tretel B, Halvorsen B, Russell D, Bjerkeli V, et al. Atrial fibrillation in cryptogenic stroke: The Nordic Atrial Fibrillation and Stroke Study (NOR-FIB). Eur J Neurol 2018;25(Suppl. 2):60 | Ongoing study |
| Ratajczak-Tretel B, Aamodt AH, Johansen H, Atar D, Halvorsen B, Sandset EC, et al. Cryptogenic stroke in – The Nordic and stroke study (NOR-FIB). Eur Stroke J 2016;1(Suppl. 1):778 | Ongoing study |
| Johansen H, Aamodt AH, Ratajczak-Tretel B, Atar D, Halvorsen B, Naess H, et al. Cryptogenic stroke in atrial fibrillation – the Nordic atrial fibrillation and stroke study (NOR-FIB). Eur J Neurol 2016;23:356–7 | Ongoing study |
| Toni D, Lorenzano S, Strano S. Detection of silent atrial fibrillation after ischaemic stroke (SAFFO) guided by implantable loop recorder. A multicentre Italian trial based on neurocardiology unit network. Int J Stroke 2015;10:274 | Ongoing study |
| Toni D, Lorenzano S, Strano S. Detection of Silent Atrial Fibrillation aFter Ischaemic StrOke (SAFFO) guided by implantable loop recorder: multicentre Italian trial based on stroke unit network with paired cardio-arrhythmology units (Italian Neurocardiology Unit Network). Int J Stroke 2016;11:361–7 | Ongoing study |
| Ghrooda EM, Dobrowolski P, Basir G, Yaseen I, Khan N, Ahmad A, et al. Paroxysmal atrial fibrillation is common in patients with defined etiology for stroke: prolonged monitoring of cardiac rhythm for detection of atrial fibrillation after a cerebral ischaemic event (PEAACE) study. Stroke Conf 2014;45:A25 | Wrong intervention: prolonged monitoring but not ICM |
| Dion F, Bonnaud I, Fauchier L, Friocourt P, Bonneau A, Poret P, et al. Unexpected low prevalence of atrial fibrillation in cryptogenic ischaemic stroke: a prospective continuous monitoring study. Arch Cardiovasc Dis Suppl 2010;2:73 | Wrong intervention: Reveal Plus 9526 with no AF detection algorithm |
| Dion F, Saudeau D, Bonnaud I, Friocourt P, Bonneau A, Poret P, et al. Unexpected low prevalence of atrial fibrillation in cryptogenic ischemic stroke: a prospective study. J Interv Card Electrophysiol 2010;28:101–7 | Wrong intervention: Reveal Plus 9526 with no AF detection algorithm |
| De Lera M, Bulnes LR, Cortijo E, Bombin S, Calleja AI, Garcia-Moran E, et al. Predictors of arrhythmic load in patients with cryptogenic stroke and covertatrial fibrillation detected by implantable loop recorders. Eur Stroke J 2017;2(Suppl. 1):115 | Wrong intervention: unknown device – abstract only |
| De Lera M, Largaespada G, Cortijo E, Sandin M, Calleja A, Garcia E, et al. Predictors of higher arrhythmic load in patients with cryptogenic stroke and covert detected by implantable loop recorders. Eur Stroke J 2016;1(Suppl. 1):641–2 | Wrong intervention: unknown device – abstract only |
| Evans N, Sobala C, Belham M, Pugh P, Warburton E. Age is an independent predictor of paroxysmal atrial fibrillation in embolic strokes of undetermined source. Int J Stroke 2016;11(Suppl. 1):9 | Wrong intervention: unknown device – abstract only |
| Giobbe D, Balducci A, Paglia G, Giobbe ML, Budano C, Alunni GL, et al. Detection of paroxysmal atrial fibrillation by implantable loop recorder in cryptogenic stroke. Eur J Neurol 2016;2:138 | Wrong intervention: unknown device – abstract only |
| Goetz N, Poli S, Haertig F, Bauer A, Duckheim M, Eick C, et al. Insertable cardiac monitors for detection of atrial fibrillation in patients with embolic stroke of undetermined source (ESUS) selected by risk factors. Eur Heart J 2015;1:988 | Wrong intervention: unknown device – abstract only |
| Heinrich J, Feil K, Kupper C, Wollenweber F, Sinner M, Kaab S, et al. Follow up in embolic stroke of undetermined source: new clinical treatment algorithm including longterm cardial monitoring and PFO closure in a prospective open-label observational study. Eur Stroke J 2018;3(Suppl. 1):317–8 | Wrong intervention: unknown device – abstract only |
| Jurjans K, Skarsta L, Miglane E, Millers A, Priede Z. Our experience of using implantable loop recorder devices to specify stroke etiology in Pauls Stradins clinical university hospital, Riga, Latvia from 2014 to 2017. Eur J Neurol 2018;25(Suppl. 2):219 | Wrong intervention: unknown device – abstract only |
| Kalejs O, Jurjans K, Jubele K, Kamzola G, Nikrus N, Nesterovics N, et al. Results of monitoring large artery cryptogenic embolic stroke survivors using implantable loop recorder device. Europace 2016;18(Suppl. 1):i111 | Wrong intervention: unknown device – abstract only |
| Llerena Butron SI, San Roman Calvar JA, Sandin Fuentes M, Bulnes Garcia LR, Largaespada Perez G, Bombin Gonzalez S, et al. Implantable loop recorders and short episodes of rapid atrial rate: relevant in the medical work-up of patients with embolic strokes of unknown source. Europace 2017;19(Suppl. 3):iii143–iii4 | Wrong intervention: unknown device – abstract only |
| Llerena Butron SI, Sandin Fuentes M, Bombin Gonzalez S, Bulnes Garcia LR, Largaespada Perez G, Gomez Salvador I, et al. Rapid atrial rate and implantable loop recorders for embolic strokes of unknown source: report of more than 2-years follow up in a single centre. Eur Heart J 2017;38(Suppl. 1):165 | Wrong intervention: unknown device – abstract only |
| Makimoto H, Kurt M, Gliem M, Jander S, Schmidt J, Blockhaus C, et al. High incidence of occult atrial fibrillation by insertable cardiac monitor in patients with cryptogenic stroke in the territory of vertebrobasilar system. Eur Heart J 2016;37(Suppl. 1):275 | Wrong intervention: unknown device – abstract only |
| Makimoto H, Kurt M, Gliem M, Lee JI, Schmidt J, Müller P, et al. High incidence of atrial fibrillation after embolic stroke of undetermined source in posterior cerebral artery territory. J Am Heart Assoc 2017;6:e007448 | Wrong intervention: unknown device – abstract only |
| Marzella F, Salek F, Calkins L, Welton M, Abou-Eid M, Choi M. Evaluation of medication therapy in cryptogenic stroke patients diagnosed with atrial fibrillation following loop recorder implantation. J Am Pharm Assoc 2018;58:e15 | Wrong intervention: unknown device – abstract only |
| Navarro Perez MP, Perez Lazaro C, Pelegrin Diaz J, Rodrigo Trallero G, Sanchez Val A, Garces Anton E, et al. Paroxysmal atrial fibrillation detection after cryptogenic stroke: a single center experience. Eur Stroke J 2018;3(Suppl. 1):460 | Wrong intervention: unknown device – abstract only |
| Noone I, Meagher MK, Mc Creery C, Cassidy T. The role of loop recorders in Embolic Stroke of Uncertain Source (ESUS). Eur Stroke J 2016;1(Suppl. 1):16 | Wrong intervention: unknown device – abstract only |
| Rafanelli M, Ceccofiglio A, Tesi F, Toffanello G, Chisciotti VM, Rivasi G, et al. Implantable loop recorder: a syncope unit experience. Eur Geriatr Med 2015;1:S73 | Wrong intervention: unknown device – abstract only |
| Rafanelli M, Ceccofiglio A, Tesi F, Toffanello G, Chisciotti VM, Rivasi G, et al. Implantable loop recorder: a syncope unit experience. Europace 2015;3:iii50 | Wrong intervention: unknown device – abstract only |
| Ramaiah G, Salahuddin H, Espinosa A, Zaidi S, Jumaa M, Tietjen G. Left atrial size is not a predictor for detection of atrial fibrillation in cryptogenic stroke. Stroke Conf 2018;49:AWP205 | Wrong intervention: unknown device – abstract only |
| Ricci B, Chang AD, Hemendinger M, Dakay K, Cutting S, Burton T, et al. A simple score that predicts paroxysmal atrial fibrillation on outpatient cardiac monitoring after embolic stroke of unknown source. Stroke 2018;49:AWP202 | Wrong intervention: unknown device – abstract only |
| Scacciatella P, Jorfida M, Omede P, Budano C, Castagno D, Zema D, et al. Insertable cardiac monitor in older patients candidates to percutaneous PFO closure. Preliminary results of a perspective registry study. Eur Heart J 2017;38(Suppl. 1):1024–5 | Wrong intervention: unknown device – abstract only |
| Seiler A, Allred J, Biby S, Sethi P. Surveillance for atrial fibrillation in patients with cryptogenic stroke using an implantable loop recorder in a community hospital setting: real world validation of CRYSTAL AF. Eur J Neurol 2015;1:98 | Wrong intervention: unknown device – abstract only |
| Seiler A, Biby S, Sethi P, Allred J. Use of dedicated protocol and implantable loop recorder to evaluate for atrial fibrillation in cryptogenic stroke patients: real world validation of CRYSTAL AF. Circulation Conf 2015;132:A14805 | Wrong intervention: unknown device – abstract only |
| Sethi P, Allred J, Seiler A, Biby S. Surveillance for atrial fibrillation in patients with cryptogenic stroke using an implantable loop recorder in a community hospital setting: real world validation of CRYSTAL atrial fibrillation trial. Cerebrovasc Dis 2015;2:30 | Wrong intervention: unknown device – abstract only |
| Sethi P, Biby S, Allred J, Seiler A, Xu J. Surveillance for atrial fibrillation in patients with cryptogenic stroke using an implantable loop recorder during an inpatient hospitalization in a community hospital setting. Eur Stroke J 2017;2(Suppl. 1):115–6 | Wrong intervention: unknown device – abstract only |
| Sethi P, Biby S, Xu J, Seiler A, Allred J. Lowincidence of atrial fibrillation in recurrent strokes in a cohort of cryptogenic stroke patients on long term cardiac monitoring. Eur Stroke J 2018;3(Suppl. 1):459 | Wrong intervention: unknown device – abstract only |
| Summo CS, Riesinger L, Mehr M, Siebermair J, Fichtner S, Schuhmann C, et al. Potential role of PFO in patients after an ESUS event and with implantable cardiac monitor. J Interv Card Electrophysiol 2018;51(Suppl. 1):S41–S2 | Wrong intervention: unknown device – abstract only |
| Ungar A, Rieger G, De Melis M, Mangoni L, Reinke F, Bucx J, et al. Incidence of atrial fibrillation and medication changes in cryptogenic stroke patients with an implantable loop recorder. Cerebrovasc Dis 2014;37(Suppl. 1):517 | Wrong intervention: unknown device – abstract only |
| Ungar A, Rieger G, Puererfellner H, Duru F, De Melis M, Bonizzi T, et al. Incidence of atrial fibrillation and subsequent medication changes in cryptogenic stroke patients with an implantable loop recorder. Eur Heart J 2014;1:292 | Wrong intervention: unknown device – abstract only |
| Ungar A, Rieger G, West T, Purerfellner H, Bucx J, Topper RF, et al. Atrial fibrillation and treatment changes in cryptogenic stroke patients with an implantable loop recorder for continuous cardiac rhythm monitoring. Eur Heart J 2013;1:62 | Wrong intervention: unknown device – abstract only |
| Venzo S, Rafanelli M, Schipani E, Ceccofiglio A, Tesi F, Rivasi G, et al. Implantable loop recorder in cryptogenic stroke: a cardio-geriatric experience. Eur Geriatr Med 2016;7(Suppl. 1):S169–S70 | Wrong intervention: unknown device – abstract only |
| Verbeet T, Castro J, Morissens M, Arbraud C, Knecht S. The belgian implantable loop recorder database: analysis of three years of activity. Acta Cardiol 2012;67:115 | Wrong intervention: unknown device – abstract only |
| Weinstock J, Marks D, Andriulli JA, Collins J, Ortman ML, Russo AM. Subclinical atrial fibrillation identified in the setting of cryptogenic stroke: a real-world experience. Heart Rhythm 2018;15(Suppl. 1):S71–S2 | Wrong intervention: unknown device – abstract only |
| Yasmeh B, Liu Z, Verdick C, DeMazumder D, Rajsheker S, Costea A. Clinical utility of implantable loop recorders for the diagnosis of paroxysmal atrial fibrillation in patients with cryptogenic stroke-a single large center retrospective analysis. Heart Rhythm 2018;15(5 Suppl. 1):S72 | Wrong intervention: unknown device – abstract only |
| Yeneneh B, Munro J, Wilansky S, Behai J, Scott L. Predictors of atrial fibrillation detection in patients with implantable loop recorders for cryptogenic stroke. Europace 2017;19(Suppl. 3):iii234 | Wrong intervention: unknown device – abstract only |
| Zeitzen T, Chan W. Loop recorder implantation: keeping up with the guidelines with a single-centre experience. Heart Lung Circ 2017;26(Suppl. 2):S185 | Wrong intervention: unknown device – abstract only |
| Rodriguez-Campello A, Cuadrado-Godia E, Ois A, Giralt-Steinhauer E, Jimenez-Conde J, Puig-Pijoan A, et al. Early detection of atrial fibrillation in embolic stroke of unknown origin (ESUS). Int J Stroke 2015;2:268–9 | Wrong intervention: unknown device – abstract only |
| Rodríguez-Campello A, Giralt-Steinhauer E, Ois A, Jiménez-Conde J, Avellaneda-Gómez C, Serra-Martínez M, et al. Atrial fibrillation detection and stroke recurrence in patients with early insertable cardiac monitor. A case-control study. Eur Stroke J 2018;3(1 Suppl. 1):459 | Wrong intervention: unknown device – abstract only |
| Benito B, Valles E, Cuadrado E, Cabrera S, Ramos P, Ois A, et al. Improving AF detection in patients with cryptogenic stroke. Insights from a prospective cohort with insertable cardiac monitor. Eur Heart J 2015;1:164–5 | Wrong intervention: unknown device – Medtronic not involved in study |
| Benito B, Valles E, Cuadrado E, Ramos P, Cabrera S, Ois A, et al. Improving AF detection in patients with cryptogenic stroke. Learning concepts from a prospective cohort with insertable cardiac monitor. Europace 2015;3:iii205 | Wrong intervention: unknown device – Medtronic not involved in study |
| Maddox S, Hoskins M, Lloyd M, Mengistu A, Rangaraju S, Henriquez L, et al. High false positive rates of atrial fibrillation detection among stroke patients who receive Medtronic implantable loop recorders. Stroke Conf 2016;47:A206 | Wrong intervention: unknown device – Medtronic suggest Reveal XT and Non-TruRhythm Reveal LINQ based on timing/location of study |
| Giralt-Steinhauer E, Cuadrado-Godia E, Soriano-Tarraga C, Ois A, Jimenez-Conde J, Rodriguez-Campello A, et al. New-onset paroxysmal atrial fibrillation diagnosis in ischaemic stroke patients. Eur Neurol 2015;74:211–7 | Wrong intervention: unknown device – not identified by companies and no reply from study authors |
| Miller DJ. Randomised controlled trial: prolonged cardiac monitoring after cryptogenic stroke superior to 24 h ECG in detection of occult paroxysmal atrial fibrillation. Evid Based Med 2014;19:235 | Wrong intervention/not comparison of interest/wrong publication type |
| Kanters, TA, Wolff C, Boyson D, Kouakam C, Dinh T, Hakkaart L, Rutten-Van Mölken MP. Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace 2016;18:919–24 | Wrong outcome: cost comparison, no clinical outcomes |
| Gianatasio RM, Shams T, Aashish A, Abrol R, Thambidorai S, Janardhan V. Implanting longterm cardiac monitors by stroke interventionalists in a collaborative cryptogenic stroke program. Interv Neurol 2016;5(Suppl. 1):54 | Wrong outcome: measuring increase in implants after stroke network initiated. No clinical outcomes |
| Healy C, Burleson HD, Rivner H, Goyal V, Grevious S, Lambrakos LK, et al. Indications and utility of traditional, insertable, and smartphone-based ambulatory ECG monitoring systems. Heart Rhythm 2016;1:S516–S7 | Wrong population |
| Katz JM, Eng MS, Carrazco C, Patel AV, Jadonath R, Gribko M, et al. Occult paroxysmal atrial fibrillation in non-cryptogenic ischaemic stroke. J Neurol 2018;24:24 | Wrong population: described as non-CS and limited information in abstract to check population characteristics |
| Katz JM, Gribko M, Jadonath R, Arora R, Salamon E, Garlitzki A, et al. Prevalence of occult paroxysmal atrial fibrillation in non-cryptogenic ischaemic stroke patients. Stroke Conf 2017;48:AWMP63 | Wrong population: described as non-CS and limited information in abstract to check population characteristics |
| De Ruvo E, Panuccio M, Sette A, Martino A, Fagagnini A, Grieco D, et al. Effectiveness of remote monitoring in patients implanted with a new miniaturized injectable cardiac monitor. Europace 2016;18(Suppl. 1):i138 | Wrong population: mixed diagnoses and not disaggregated |
| Iskandar S, Reddy M, Lavu M, Atoui M, Vodapally M, Neerumalla R, et al. Real world experience with Medtronic Reveal LINQ. Circulation Conf 2016;134:A16029 | Wrong population: mixed diagnoses, including only nine who had had a CS |
| Brahmbhatt DH, Chari A, Cotter PE, Martin P, Belham MRD, Pugh PJ. Atrial fibrillation detection algorithms alone are inadequate for identifying atrial arrhythmia by implantable loop recorder after ischaemic stroke. Eur Heart J 2013;1:738–9 | Wrong population: not CS, related to Cotter et al.,66 which reports CS |
| Drak-Hernández Y, Toquero-Ramos J, Fernández JM, Pérez-Pereira E, Castro-Urda V, Fernández-Lozano I. Effectiveness and safety of remote monitoring of patients with an implantable loop recorder. Rev Esp Cardiol 2013;66:943–8 | Wrong population: not limited to stroke |
| Kipp R, Young N, Barnett A, Kopp D, Leal MA, Eckhardt LL, et al. Injectable loop recorder implantation in an ambulatory setting by advanced practice providers: analysis of outcomes. Pacing Clin Electrophysiol 2017;40:982–5 | Wrong population: not limited to stroke |
| Maines M, Zorzi A, Tomasi G, Angheben C, Catanzariti D, Piffer L, Del Greco M. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQ™. Europace 2018;20:1050–7 | Wrong population: not limited to stroke |
| Musat DL, Deihl S, Preminger MW, Bhatt A, Sichrovsky TC, Ferrara M, et al. Understanding automatic connectivity limitations in patients undergoing long-term ECG monitoring with an implantable cardiac monitor. Heart Rhythm 2017;14(Suppl. 1):S245 | Wrong population: not limited to stroke |
| Ching M, Lail N, Tirunagri D, Magun R, Kandel A, Deline C, et al. Predictors of paroxsymal atrial fibrillation in embolic stroke patients with insertable cardiac monitor – a comprehensive stroke center experience. Cerebrovasc Dis 2017;43(Suppl. 1):110 | Wrong population: stroke not cryptogenic |
| Ching MI, Zhang C, Vaughn C, Lail N, Leahy T, Kandel A, et al. Left atrial volume index and PR interval are independent predictors of atrial fibrillation in embolic stroke patients with insertable cardiac monitor. Stroke Conf 2018;49:AWP199 | Wrong population: stroke not cryptogenic |
| Kamel H, Yaghi S, Passman R, Allred J, Sarkar S, Kohler J, et al. Comparison of atrial fibrillation diagnosis and oral anticoagulation utilization among ischaemic stroke patients with vs. without insertable cardiac monitors. Eur Stroke J 2018;3(Suppl. 1):452–3 | Wrong population: stroke not cryptogenic, and unknown device model |
| Prakapenia A, Pallesen LP, Mayer J, Barlinn J, Barlinn K, Siepmann T, et al. Interactions detection rate of insertable cardiac monitors is not influenced by embolic pattern in neuroradiological imaging. Eur Stroke J 2017;2(Suppl. 1):113–4 | Wrong population: stroke population, not cryptogenic. Abstract only |
| Diederichsen SZ, Haugan KJ, Højberg S, Holst AG, Køber L, Pedersen KB, et al. Complications after implantation of a new-generation insertable cardiac monitor: Results from the LOOP study. Int J Cardiol 2017;241:229–34 | Wrong population: those at high risk of stroke, not limited to CS. Large ongoing RCT (LOOP) |
| Diederichsen SZ, Haugan KJ, Køber L, Højberg S, Brandes A, Kronborg C, et al. Atrial fibrillation detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (the LOOP study): Rationale and design of a large randomized controlled trial. Am Heart J 2017;187:122–32 | Wrong population: those at high risk of stroke, not limited to CS. Large ongoing RCT (LOOP) |
| Velu S, Head L, Upright J, Bradley L, Petkar S. Remote monitoring of implantable loop recorders significantly improves diagnostic outcomes. Europace 2012;14(Suppl. 4):iv22–iv27 | Wrong population: unexplained syncope |
| Sutton B, Zigler JD, Gopinathannair R, Deam AG, Graver R. Improved health outcomes and cost-savings with remote monitoring of cardiac implantable electronic devices. Heart Rhythm 2013;1:S455 | Wrong population/not comparison of interest |
| Sethi A, Buescher M, Garberich R, Hoffman E, Sengupta J. An investigation of the evolution of implantable cardiac monitors: a comparison of Reveal XT™ and Reveal LINQ™ based on accuracy measurements and patient outcomes post-device implant. J Am Coll Cardiol 2017;69(Suppl. 1):518 | Wrong population/wrong outcome/not comparison of interest |
| Healey JS. What do implanted cardiac monitors reveal about atrial fibrillation? JAMA Cardiol 2017;2:1128–9 | Wrong population/wrong study design |
| Miller DJ. Increasing the yield of atrial fibrillation detection in cryptogenic stroke using risk factor stratification: a more satisfying approach. Eur J Neurol 2016;23:239–40 | Wrong publication type: comment on Poli et al.62 |
| Wachter R, Weber-Krüger M, Gröschel K. Letter by Wachter et al regarding article, ‘occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors’. Stroke 2013;44:e111 | Wrong publication type: letter |
| Abdul-Rahim AH, Lees KR. Paroxysmal atrial fibrillation after ischaemic stroke: how should we hunt for it? Expert Rev Cardiovasc Ther 2013;11:4:485–94 | Wrong publication type: narrative paper |
| Kamel H, Smith WS. Detection of atrial fibrillation and secondary stroke prevention using telemetry and ambulatory cardiac monitoring. Curr Atheroscler Rep 2011;13:4:338–43 | Wrong publication type: narrative paper |
| Sundararajan K, Strbian D, Sundararajan S. Evaluation of patients for paroxysmal atrial fibrillation after ischemic stroke. Stroke 2013;44:e168–70 | Wrong publication type: narrative paper |
| Di Odoardo LAF, Ambrosini F, Giavarini A, Vicenzi M, Venturini F, Lombardi F. Reveal LINQ™ experience out of the electrophysiology lab. J Cardiovasc Med 2017;18:550–2 | Wrong intervention/not comparison of interest |
| Ellis D, Rangaraju S, Duncan A, Hoskins MH, Raza SA, Rahman H, et al. Measures of coagulation and hemostatic activation outperform left atrial structural parameters in identifying Embolic Stroke of Undetermined Source (ESUS) patients who may benefit from early anticoagulation. Stroke Conf 2018;49:A120 | Wrong intervention/not comparison of interest |
| McCarthy L. ACP Journal Club: ambulatory ECG monitoring for 30 d increased AF detection more than 24 h of ECG monitoring after cryptogenic stroke. Ann Intern Med 2014;161:JC2 | Wrong intervention/not comparison of interest |
| Beinart SC, Natale A, Verma A, Amin AN, Kasner S, Diener HC, et al. Real-world comparison of in-hospital Reveal LINQ insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Heart Rhythm 2016;13(Suppl. 1):S15, abstract number AB07-01 | Wrong intervention/not comparison of interest |
| Roebuck A, Mercer C, Denman J, Houghton AR, Andrews A. Experiences from a non-medical, non-catheter laboratory implantable loop recorder (ILR) service. Br J Cardiol 2015;22:36 | Wrong intervention/not comparison of interest |
| Wong GR, Lau DH, Middeldorp ME, Harrington JA, Stolcman S, Wilson L, et al. Feasibility and safety of Reveal LINQ insertion in a sterile procedure room versus electrophysiology laboratory. Int J Cardiol 2016;223:13–17 | Wrong intervention/not comparison of interest |
| Muller P, Ivanov V, Kara K, Klein-Wiele O, Forkmann M, Piorkowski C, et al. PA-TDI interval to predict occult atrial fibrillation after cryptogenic stroke. Heart Rhythm 2016;1:S263–S4 | Wrong intervention/not comparison of interest |
| Afzal M, Kanmanthareddy A, Gunda S, Atkins D, Reddy M, Atoui M, et al. Cryptogenic stroke and underlying atrial fibrillation: a systematic review and meta-analysis of randomized control trials. J Am Coll Cardiol 2015;1:A360 | Wrong study design: systematic review |
| Afzal MR, Gunda S, Waheed S, Sehar N, Maybrook RJ, Dawn B, Lakkireddy D. Role of outpatient cardiac rhythm monitoring in cryptogenic stroke: a systematic review and meta-analysis. Heart Rhythm 2015;1:S528 | Wrong study design: systematic review |
| Afzal MR, Gunda S, Waheed S, Sehar N, Maybrook RJ, Dawn B, Lakkireddy D. Role of outpatient cardiac rhythm monitoring in cryptogenic stroke: a systematic review and meta-analysis. Pacing Clin Electrophysiol 2015;38:1236–45 | Wrong study design: systematic review |
| Bhatnagar UB, Sethi P, Gedela M, Thompson PA, Pham R, Pham S. Predictors of diagnostic yield of implanted loop recorder in patients with cryptogenic stroke: a systemic review and meta-analysis. Stroke Conf 2018;49:AWMP61 | Wrong study design: systematic review |
| Burkowitz J, Merzenich C, Grassme K, Brüggenjürgen B. Insertable cardiac monitors in the diagnosis of syncope and the detection of atrial fibrillation: A systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:1261–72 | Wrong study design: systematic review |
| Dahal K, Chapagain B, Maharjan R, Farah HW, Nazeer A, Lootens RJ, Rosenfeld A. Prolonged cardiac monitoring to detect atrial fibrillation after cryptogenic stroke or transient ischemic attack: a meta-analysis of randomized controlled trials. Ann Noninvasive Electrocardiol 2016;21:382–8 | Wrong study design: systematic review |
| Glotzer TV, Ziegler PD. Cryptogenic stroke: Is silent atrial fibrillation the culprit? Heart Rhythm 2015;12:234–41 | Wrong study design: systematic review |
| Korompoki E, Del Giudice A, Hillmann S, Malzahn U, Gladstone DJ, Heuschmann P, Veltkamp R. Cardiac monitoring for detection of atrial fibrillation after TIA: a systematic review and meta-analysis. Int J Stroke 2017;12:33–45 | Wrong study design: systematic review |
| Maylin E, Johnson D, Patel R, Hair C, Kraemer T, Lau M, et al. Predicting atrial fibrillation in ischaemic stroke: a systematic review. Eur Stroke J 2018;3(1 Suppl. 1):457 | Wrong study design: systematic review |
| Musat DL, Milstein N, Mittal S. Implantable loop recorders for cryptogenic stroke (plus real-world atrial fibrillation detection rate with implantable loop recorders). Card Electrophysiol Clin 2018;10:111–18 | Wrong study design: systematic review |
| Thijs V, Bernstein RA, Morillo C, Diener HC, Rymer M, Di Lazzaro V, et al. Does neurological symptom duration affect the incidence of atrial fibrillation in patients monitored continuously following cryptogenic stroke? Int J Stroke 2016;11(Suppl. 3):226 | Wrong study design: systematic review |
| De Angelis G, Cimon K, Sinclair A, Farrah K, Cairns J, Baranchuk A, et al. Monitoring for Atrial Fibrillation in Discharged Stroke and Transient Ischemic Attack Patients: A Clinical and Cost-Effectiveness Analysis and Review of Patient Preferences. CADTH Optimal Use Report, No. 5.2b. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2016 | Wrong study design: systematic review/HTA |
| Rabinstein AA. Prolonged cardiac monitoring for detection of paroxysmal atrial fibrillation after cerebral ischemia. Stroke 2014;45:1208–14 | Wrong publication type: narrative paper |
| Raviele A. Asymptomatic atrial fibrillation after cryptogenetic stroke. Circ Arrhythm Electrophysiol 2015;8:249–51 | Wrong publication type: narrative paper |
| Kim Y, Lee SH. The optimal approach to detect atrial fibrillation in potential cardioembolic stroke. Eur J Neurol 2016;23:e35 | Wrong publication type: narrative paper |
| Lau YC, Lane DA, Lip GY. Atrial fibrillation in cryptogenic stroke: look harder, look longer, but just keep looking. Stroke 2014;45:3184–5 | Wrong publication type: narrative paper |
| Jorfida M, Antolini M, Cerrato E, Caprioli MG, Castagno D, Garrone P, et al. Cryptogenic ischaemic stroke and prevalence of asymptomatic atrial fibrillation: a prospective study. J Cardiovasc Med 2016;17:863–9 | Wrong intervention: Reveal PlusXT 9526 with no AF detection algorithm |
Appendix 3 Clinical data extraction tables
| Item | Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Section 1: reviewer and study information | |||||||||
| Study | CRYSTAL-AF | ||||||||
| Publications |
|
||||||||
| Type of report (full paper/conference abstract) | Multiple full papers and abstracts | ||||||||
| Section 2: study information | |||||||||
| Location and number of sites | 55 centres in 14 countries across Europe, Canada and the USA | ||||||||
| Trial sponsor | Medtronic | ||||||||
| Conflicts of interest |
|
||||||||
| Patient enrolment (method and dates of enrolment) | Enrolled between June 2009 and April 2012 | ||||||||
| Trial design | Open-label, parallel-group RCT | ||||||||
| Trial duration (including any period of follow-up) | 6- and 12-month primary follow-ups. Study closure was planned at 12 months after the last patient was randomised, but long-term follow-up of 36 months was reported for some patients | ||||||||
| Inclusion criteria |
1. Recent episode (protocol amendment from < 60 days to < 90 days) of cryptogenic symptomatic TIA or recent episode of cryptogenic ischaemic stroke. Only TIAs with the following documented characteristics could be included: visible lesion on MRI or CT scan that fits the symptoms of the TIA and at least one of the following symptoms:The minimum standard tests required for diagnosis of CS prior to randomisation being allowed were:2. Patient or legal representative is willing to sign patient consent form 3. Aged ≥ 40 years (Protocol amendment) ultrasonography of cervical arteries and transcranial Doppler ultrasonography of intracranial vessels, in place of MRA or CTA of the head and neck, were allowed for patients aged > 55 years |
||||||||
| Exclusion criteria |
The main exclusion criteria were a history of AF or atrial flutter, an indication or contraindication for permanent oral anticoagulant therapy at enrolment and an indication for a pacemaker or implantable cardioverter–defibrillator Full criteria from Sinha et al.:48 |
||||||||
| Subgroups evaluated |
Age, sex, race or ethnic group; type of index event; presence or absence of PFO; and CHADS2 score at baseline Note that only type of index event relevant to the NICE scope1 |
||||||||
| Stratification | Within the study groups according to the type of index event (stroke or TIA) and the presence or absence of a PFO | ||||||||
| Definition of cryptogenic stroke or TIA |
A stroke/TIA is considered to be cryptogenic if no possible cause can be determined despite extensive work-up according to the standard protocol of the participating centre Before randomisation, the following tests are minimally required as standard tests to establish the diagnosis of CS:Pre-enrolment screening for AF consisted of Holter monitoring with a median duration of 23 hours (IQR 21–24 hours) in 71.2% of patients (n = 314, mean 31.0 ± 66.7 hours) and inpatient telemetry monitoring with a median duration of 68 hours (IQR 40–96 hours) in 29.7% of patients (n = 131, mean 74.6 ± 51.4 hours) |
||||||||
| Definition of AF | AF was defined as an episode of irregular heart rhythm, without detectable P-waves, lasting > 30 seconds. Episodes of AF that qualified for analysis were adjudicated by an independent committee | ||||||||
| Treatment | ICM – continuous monitoring | Conventional follow-up | |||||||
| Randomised or number in study (n) | 447 enrolled – 441 randomly allocated | ||||||||
| 221 (208 received device) | 220 | ||||||||
| Withdrawals (including reasons for withdrawal) (n) | At 6 months:
|
At 6 months:
|
|||||||
| Details of follow-up for AF detection | Both groups: follow-up visits scheduled at 1, 6 and 12 months, and then every 6 months until study closure, with additional visits in the event of AF symptoms or after the transmission of ICM data, if recommended by the investigator. If patients reported AF, source documentation was acquired for adjudication | ||||||||
| Patients assigned to the ICM group were scheduled to have the device inserted within 10 days after randomisation. ICM settings were programmed in a standardised fashion. The ICM that was used (REVEAL XT, Medtronic) automatically detects and records AF, irrespective of heart rate or symptoms. The Medtronic CareLink Network was used to remotely transmit the device data | Patients assigned to the control group underwent assessment at scheduled and unscheduled visits, with electrocardiography monitoring performed at the discretion of the site investigator. Monitoring type, duration, and all results were recorded | ||||||||
| Mean days from index event | To randomisation: 38.1 (SD 27.6) | ||||||||
| To insertion of device: 184 out of 208 (88.5%) within 10 days. Scheduling delays (22 patients) or medical justification (2 patients) accounted for delayed insertions [median delay 6 days (IQR 1–32 days)] | |||||||||
| Mean duration/length of follow-up for AF detection | 20.3 ± 9.4 months (407.4 patient-years) | 19.2 ± –9.9 months (patient-years not reported) | |||||||
| Number completing the | |||||||||
| 6-month follow-up | 205 | 208 | |||||||
| 12-month follow-up | 194 | 185 | |||||||
| 24-month follow-up | 88 | 89 | |||||||
| 36-month follow-up | 24 | 24 | |||||||
| Baseline patient characteristics | ICM – continuous monitoring (n = 221) | Conventional follow-up (n = 220) | p-value | ||||||
| Mean age, years (SD) | 61.6 (11.4) | 61.4 (11.3) | 0.84 | ||||||
| Sex, n (%) |
|
|
0.77 | ||||||
| Ethnicity, n (%) | |||||||||
| Asian | 3 (1.4) | 2 (0.9) | 0.60 | ||||||
| Black | 7 (3.2) | 10 (4.5) | |||||||
| Hispanic or Latino | 2 (0.9) | 2 (0.9) | |||||||
| White | 194 (87.8) | 191 (86.8) | |||||||
| Other | 0 (0) | 3 (1.4) | |||||||
| Not available | 15 (6.8) | 12 (5.5) | |||||||
| Geographic region, n (%) | |||||||||
| North America | 83 (37.6) | 72 (32.7) | 0.32 | ||||||
| Europe | 138 (62.4) | 148 (67.3) | |||||||
| PFO, n (%) | 52 (23.5) | 46 (20.9) | 0.57 | ||||||
| Index event, n (%) | |||||||||
| Stroke | 200 (90.5) | 201 (91.4) | 0.87 | ||||||
| TIA | 21 (9.5) | 19 (8.6) | |||||||
| Prior stroke/TIA, n (%) | |||||||||
| Stroke | 37 (16.7) | 28 (12.7) | 0.28 | ||||||
| TIA | 22 (10.0) | 27 (12.3) | 0.45 | ||||||
| Score on modified Rankin Scale (0 to 6, lower = better), n (%) | |||||||||
| 0–2 | 184 (83.3) | 186 (84.5) | 0.85 | ||||||
| > 2 | 36 (16.3) | 34 (15.5) | |||||||
| Mean (SD) NIH Stroke Scale (0 to 42, lower = better) | 1.6 (2.7) | 1.9 (3.8) | 0.37 | ||||||
| Hypertension, n (%) | 144 (65.2) | 127 (57.7) | 0.12 | ||||||
| Diabetes, n (%) | 34 (15.4) | 38 (17.3) | 0.61 | ||||||
| CHADS2 score, n (%) | |||||||||
| 2 | 69 (31.2) | 81 (36.8) | 0.17 | ||||||
| 3 | 92 (41.6) | 91 (41.4) | |||||||
| 4 | 50 (22.6) | 34 (15.5) | |||||||
| 5 | 9 (4.1) | 14 (6.4) | |||||||
| 6 | 1 (0.5) | 0 (0) | |||||||
| Hypercholesterolaemia, n (%) | 125 (56.6) | 128 (58.2) | 0.77 | ||||||
| Current smoker, n (%) | 43 (19.5) | 44 (20.0) | 0.91 | ||||||
| Coronary artery disease, n (%) | 16 (7.2) | 9 (4.1) | 0.22 | ||||||
| Use of antiplatelet agent, n (%) | 212 (95.9) | 212 (96.4) | 1.00 | ||||||
| Section 3: outcomes | |||||||||
| Outcome | Definition | ||||||||
| Diagnostic accuracy (sensitivity and specificity, and/or TP, TN, FP and FN) | Not defined/reported | ||||||||
| Diagnostic yield (number of AF diagnoses) |
AF detected at 1, 6, 12, 24 and 36 months Duration of AF, including median, maximum and mean time in AF per day (with IQR) was reported but not extracted as not part of the NICE scope1 |
||||||||
| Detection of other cardiac pathologies or incidental findings (non-AF) | Not defined/reported | ||||||||
| Time to diagnosis of AF | Time to first detection of AF at 6 months (primary) and 12 months of follow-up (secondary). The rate of detection of AF was estimated with the use of the KM method and groups were compared on an ITT basis with the use of a log-rank test. Patients were censored at the time of death, study exit or completion of 6 months of follow-up | ||||||||
| Time to initiation of anticoagulants | The time-to-event analytic methods used to analyse the primary end point were also used to analyse other time-to-event end points | ||||||||
| Uptake of anticoagulants | Change in use of OACs. The between-group difference in the proportion of participants taking OACs at follow-up visits was compared with the use of Fisher’s exact test | ||||||||
| Incidences of device failure (such as inability to transmit data or battery life) and removal owing to failure or AE | Not defined/reported | ||||||||
| Hospitalisations for AF | Not defined/reported | ||||||||
| Number of outpatient visits related to monitoring for AF | Not defined/reported | ||||||||
| Ease of device use for clinicians (including insertion) | Not defined/reported | ||||||||
| Mortality | Not defined/reported | ||||||||
| Further strokes or TIAs, other thromboembolisms and heart failure | Recurrent stroke or TIA | ||||||||
| Complications arising from preventative treatment, such as AE from anticoagulation | Not defined/reported | ||||||||
| AE related to implanting or removing the device, such as infection or inflammation | AEs relating to ICM | ||||||||
| HRQoL | EQ-5D and VAS | ||||||||
| Acceptability of the device to patients | Not defined/reported | ||||||||
| Section 4: data extraction form | |||||||||
| Outcome | Intervention | Comparator | |||||||
| Dichotomous outcomes | |||||||||
| Diagnostic yield | Months | Patients with the outcome ( n ) | Patients assessed ( N ) | Patients with the outcome ( n ) | Patients assessed ( N ) | Notes | |||
| AF detection | 1 | 8 | 221 | 1 | 220 | ||||
| 6 | 19 (8.6%) | 221 (208 with ICM) | 3 (1.4%) | 220 | Control group AF from 88 ECGs (65 patients), 20 24-hour Holters (17 patients) and 1 event recording | ||||
| 6–12 | 10 | 221 (189 with ICM and no AF before 6 m) | 1 | 220 | Control group AF from 34 ECGs (33 patients) and 12 Holters (10 patients) | ||||
| 12 | 29 | 221 (208 with ICM) | 4 | 220 | Control group AF from 122 ECGs, 32 Holters and 1 event recorder | ||||
| 12–24 | 9 | 221 (208 with ICM) | 1 | 220 | Control group AF from 62 ECGs and 14 Holters | ||||
| 24 | 38 | 221 | 5 | 220 | |||||
| 24–36 | 4 | 221 (208 with ICM) | 0 | 220 | Control group AF from 19 ECGs and 6 Holters | ||||
| 36 | 42 | 221 | 5 | 220 | Control group AF from 256 AF monitoring tests | ||||
| Asymptomatic AF detection (of all detected AF) | 6 | 14 | 19 | 1 | 3 | ||||
| 12 | 23 | 29 | 2 | 4 | |||||
| 36 | 34 | 42 | 2 | 5 | |||||
| AF detection by index event | Stroke | 6 | 17 (8.3%) | 200 | 3 (1.6%) | 201 | Index event numbers from baseline table. p-value for interaction, 0.99 | ||
| TIA | 3 (15%) | 21 | 0 | 19 | |||||
| Stroke | 12 | 23 (11.6%) | 200 | 4 (2.2%) | 201 | ||||
| TIA | 4 (20.0%) | 21 | 0 | 19 | |||||
| Stroke | 36 | (31.2%) | 200 | (3.3%) | 201 | ||||
| TIA | NR | 21 | 0.0% | 19 | |||||
| Time-to-event outcomes | |||||||||
| Time to event | Months | Median (IQR) | Patients ( n ) | Median (IQR) | Patients ( n ) | HR for detection of AF (95% CI; p- value) | |||
| First AF detection, unadjusted | 6 | 41 days (4–84) | 19 detected | 32 days (2–73) | 3 detected | 6.4 (1.9 to 21.7; < 0.001) | |||
| 12 | 84 days (18–265) | 29 detected | 53 days (17–212) | 4 detected | 7.3 (2.6 to 20.8; < 0.001) | ||||
| 36 | 8.4 months (NR) | 42 detected | 2.4 months (NR) | 5 detected | 8.8 (3.5 to 22.2; < 0.001) | ||||
| First AF detection, adjusted for PFO, hypertension and coronary artery disease | 6 | – | – | – | – | 5.9 (1.7 to 19.8; 0.009) | |||
| First AF detection, censoring data at the time of crossover | 6 | – | – | – | – | 6.1 (1.8 to 20.8; 0.009) | |||
| Other clinical outcomes | Months | Patients with the outcome ( n ) | Patients assessed ( N ) | Patients with the outcome ( n ) | Patients assessed ( N ) | HR (95% CI; p- value) | |||
| IS or TIA | 6 | 11 | 221 | 18 | 220 | NR | |||
| 12 | 15 | 221 | 19 | 220 | 0.63 (0.22 to 1.80; 0.39) | ||||
| 36 | 20 | 221 | 24 | 220 | 0.77 (0.30 to 1.97; 0.59) | ||||
| Use of OACs | 6 | 21 (10.1%) | 208 | 9 (4.6%) | 197 | Difference 5.5% (0.5% to 10.6%; 0.0375) | |||
| 12 | 29 (14.7%) | 197 | 11 (6.0%) | 185 | Difference 8.8% (2.8% to 14.8%; 0.0069) | ||||
| 24 | 23 (26.1%) | 88 | 5 (5.6%) | 89 | Difference 20.5% (10.2% to 30.9%; 0.0002) | ||||
| 36 | 10 (38.5%) | 26 | 2 (8.3%) | 24 | Difference 30.1% (8.4% to 51.8%; 0.0195) | ||||
| Use of OACs in patients diagnosed with AF | 6 | 94.7% | 19 | NR | NR | ||||
| 12 | 96.6% | 29 | NR | NR | |||||
| 24 | 92.3% | 39 | NR | NR | |||||
| 36 | 90.5% | 42 | NR | NR | |||||
| AEs | Months | Patients with the outcome (n) | Patients assessed (N) | Patients with the outcome (n) | Patients assessed (N) | Notes | |||
| ICM removal due to infection or pocket erosion | 36 | 5 | 208 | NA | NA | ||||
| ICM no longer in situ | 6 | 4 | 208 | NA | NA | ||||
| 12 | 7 | 208 | NA | NA | |||||
| AE: infection | Unclear | 3 | 208 | NA | NA | ||||
| AE: pain | Unclear | 3 | 208 | NA | NA | ||||
| AE: irritation or inflammation | Unclear | 4 | 208 | NA | NA | ||||
| ICM still inserted | 6 | 204 (98.1%) | 208 | NA | NA | ||||
| ICM still inserted | 12 | 201 (96.6%) | 208 | NA | NA | ||||
| Cardiovascular- or stroke-/TIA-related hospital admissions | 12 | 10.5% 23 | 221 | 16 (7.2%) | 220 | From ct.gov148 | |||
| Patients with SAE | See note | 68 | 221 | 58 | 220 | From ct.gov148: average follow-up was 19.7 ± 9.7 (range 0–42.7) months | |||
| Total patients with non-serious AE | 41 | 221 | 9 | 220 | |||||
| Health-related quality of life (summary of EQ-5D domain responses provided by Medtronic) | |||||||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Continuous outcomes | |||||||||
| Mean | SD | n | Mean | SD | n | Notes | |||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Section 5: additional comments | |||||||||
| Additional comments |
|
||||||||
| Further information that could be requested | HRQoL data, as reported as an outcome in the study protocol | ||||||||
Appendix 4 The CRYSTAL-AF trial quality assessment
Appendix 5 Economic search strategies
Economic evaluations and cost and resource use evidence
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal adj2 LINQ$).tw. | 96 |
| 2 | (Reveal adj2 XT$).tw. | 184 |
| 3 | BioMonitor$.tw. | 7669 |
| 4 | (Confirm adj2 RX$).tw. | 4 |
| 5 | (SJM adj2 Confirm$).tw. | 2 |
| 6 | (insertable adj3 cardiac adj3 monitor$).tw. | 186 |
| 7 | (implantable adj3 cardiac adj3 monitor$).tw. | 271 |
| 8 | (insertable adj3 loop adj3 recorder$).tw. | 51 |
| 9 | (implantable adj3 loop adj3 recorder$).tw. | 976 |
| 10 | (ICM or ICMs).tw. | 5846 |
| 11 | implantable cardiac monitor/ | 11,836 |
| 12 | reveal.dv. | 362 |
| 13 | or/1-12 | 26,118 |
| 14 | exp cerebrovascular accident/ | 172,215 |
| 15 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 340,745 |
| 16 | transient ischaemic attack/ | 33,353 |
| 17 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 19,057 |
| 18 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes).tw. | 17,043 |
| 19 | or/14-18 | 403,705 |
| 20 | exp “cost utility analysis”/ | 8302 |
| 21 | exp “cost benefit analysis”/ | 78,398 |
| 22 | exp “cost effectiveness analysis”/ | 134,340 |
| 23 | exp “cost minimization analysis”/ | 3169 |
| 24 | health economics.mp. | 34,419 |
| 25 | economic evaluation.mp. | 20,232 |
| 26 | statistical model/ | 150,051 |
| 27 | exp fee/ | 37,762 |
| 28 | exp budget/ | 25,710 |
| 29 | (“unit cost” or unit-cost or unit-costs or “unit costs” or “drug cost” or “drug costs” or “hospital costs” or “health-care costs” or “health care cost” or “medical cost” or “medical costs”).tw. | 46,693 |
| 30 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 197,333 |
| 31 | (decision adj1 (tree$ or analys$ or model$)).tw. | 18,116 |
| 32 | (econom$ or price$ or pricing or financ$ or fee$ or pharmacoeconomic$ or pharmaeconomic$ or pharmaco-economic$).tw. | 1,043,164 |
| 33 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 8185 |
| 34 | Markov.tw. | 23,325 |
| 35 | or/20-34 | 1,514,111 |
| 36 | 13 and 19 and 35 | 53 |
| 37 | (letter or editorial or comment or case reports or review).pt. | 3,946,733 |
| 38 | nonhuman/ not human/ | 4,197,535 |
| 39 | or/37-38 | 7,967,184 |
| 40 | 36 not 39 | 37 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal adj2 LINQ$).tw. | 35 |
| 2 | (Reveal adj2 XT$).tw. | 45 |
| 3 | BioMonitor$.tw. | 6300 |
| 4 | (Confirm adj2 RX$).tw. | 2 |
| 5 | (SJM adj2 Confirm$).tw. | 1 |
| 6 | (insertable adj3 cardiac adj3 monitor$).tw. | 79 |
| 7 | (implantable adj3 cardiac adj3 monitor$).tw. | 131 |
| 8 | (insertable adj3 loop adj3 recorder$).tw. | 35 |
| 9 | (implantable adj3 loop adj3 recorder$).tw. | 459 |
| 10 | (ICM or ICMs).tw. | 3783 |
| 11 | or/1-10 | 10,697 |
| 12 | exp STROKE/ | 116,324 |
| 13 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 218,734 |
| 14 | Ischaemic Attack, Transient/ | 19,490 |
| 15 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 12,733 |
| 16 | (TIA or TIAs or mini-stroke or ministroke or mini-strokes or ministrokes).tw. | 8006 |
| 17 | or/12-16 | 265,195 |
| 18 | Health economics.mp. | 4003 |
| 19 | Economic evaluation.mp. | 8421 |
| 20 | exp “Costs and Cost Analysis”/ | 218,208 |
| 21 | exp Cost-Benefit Analysis/ | 74,027 |
| 22 | exp Models, economic/ | 13,515 |
| 23 | exp “Fees and Charges”/ | 29,393 |
| 24 | exp Budgets/ | 13,358 |
| 25 | Cost Effectiveness Analysis.mp. | 8807 |
| 26 | Cost Minimi?ation Analysis.mp. | 623 |
| 27 | Cost Utility Analysis.mp. | 2120 |
| 28 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 145,093 |
| 29 | (“unit cost” or “unit-cost” or “unit-costs” or “unit costs” or “drug cost” or “drug costs” or “hospital costs” or “health-care costs” or “health care cost” or “medical cost” or “medical costs”).tw. | 30,594 |
| 30 | (decision adj1 (tree$ or analys$ or model$)).tw. | 12,890 |
| 31 | (econom$ or price$ or pricing or financ$ or fee$ or pharmacoeconomic$ or pharmaeconomic$ or pharmaco-economic$).tw. | 835,398 |
| 32 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 6030 |
| 33 | Markov.tw. | 18,760 |
| 34 | or/18-33 | 1,125,771 |
| 35 | 11 and 17 and 34 | 10 |
| 36 | (letter or editorial or comment or case reports or review).pt. | 5,630,094 |
| 37 | animals/ not humans/ | 4,462,509 |
| 38 | or/36-37 | 8,310,735 |
| 39 | 35 not 38 | 7 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal NEAR2 LINQ*) | 0 |
| 2 | (Reveal NEAR2 XT*) | 0 |
| 3 | (BioMonitor*) | 0 |
| 4 | (Confirm NEAR2 RX*) | 0 |
| 5 | (SJM NEAR2 Confirm*) | 0 |
| 6 | (insertable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 7 | (implantable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 8 | (insertable NEAR3 loop NEAR3 recorder*) | 5 |
| 9 | (implantable NEAR3 loop NEAR3 recorder*) | 9 |
| 10 | (ICM*) | 12 |
| 11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 25 |
| 12 | MeSH DESCRIPTOR Stroke EXPLODE ALL TREES | 1354 |
| 13 | (stroke* or apoplexy* or CVA or CVAS) | 3165 |
| 14 | MeSH DESCRIPTOR Ischaemic Attack, Transient EXPLODE ALL TREES | 89 |
| 15 | (transient NEAR3 (ischaemi* or ischaemi*) NEAR3 attack*) | 243 |
| 16 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes) | 86 |
| 17 | #12 OR #13 OR #14 OR #15 OR #16 | 3202 |
| 18 | #11 AND #17 | 3 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | MeSH descriptor: [Stroke] explode all trees | 7713 |
| 2 | MeSH descriptor: [Ischaemic Attack, Transient] explode all trees | 645 |
| 3 | (stroke* or apoplexy* or CVA or CVAS):ti,ab,kw | 42,050 |
| 4 | (transient near/3 (ischaemi* or ischaemi*) near/3 attack):ti,ab,kw | 2268 |
| 5 | (TIA or TIAs or mini-stroke or mini-strokes or ministroke or ministrokes):ti,ab,kw | 1185 |
| 6 | (OR) #1-#5} | 43,047 |
| 7 | (Reveal near/2 LINQ*):ti,ab,kw | 14 |
| 8 | (Reveal near/2 XT*):ti,ab,kw | 27 |
| 9 | BioMonitor*:ti,ab,kw | 40 |
| 10 | (Confirm near/2 RX*):ti,ab,kw | 0 |
| 11 | (SJM near/2 Confirm*):ti,ab,kw | 1 |
| 12 | (insertable near/3 cardiac near/3 monitor*):ti,ab,kw | 31 |
| 13 | (implantable near/3 cardiac near/3 monitor*):ti,ab,kw | 254 |
| 14 | (insertable near/3 loop near/3 recorder*):ti,ab,kw | 1 |
| 15 | (implantable near/3 loop near/3 recorder*):ti,ab,kw | 101 |
| 16 | ICM:ti,ab,kw | 228 |
| 17 | OR/ #7-#16 | 565 |
| 18 | #6 and #17 | 72 |
| 19 | MeSH descriptor: [Costs and Cost Analysis] explode all trees | 9518 |
| 20 | MeSH descriptor: [Cost-Benefit Analysis] explode all trees | 6179 |
| 21 | MeSH descriptor: [Fees and Charges] explode all trees | 251 |
| 22 | MeSH descriptor: [Budgets] explode all rees | 33 |
| 23 | MeSH descriptor: [Models, Economic] explode all trees | 298 |
| 24 | (“unit cost” or “unit-cost” or “unit-costs” or “unit costs” or “drug cost” or “drug costs” or “hospital costs” or “health-care costs” or “health care cost” or “medical cost” or “medical costs”):ti,ab | 4117 |
| 25 | (cost near/2 (util* or effective* or efficac* or benefit* or consequence* or analys* or minimi* or allocation* or control* or illness* or affordabl* or fee* or charge*)):ti,ab | 20,485 |
| 26 | (decision near/1 (tree* or analys* or model*)):ti,ab | 682 |
| 27 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*):ti,ab | 50,415 |
| 28 | ((value or values or valuation) near/2 (money or monetary or life or lives or costs or cost)):ti,ab | 578 |
| 29 | Markov:ti,ab | 903 |
| 30 | OR/ #19-#29 | 69,869 |
| 31 | #18 and #30 | 5 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal adj2 LINQ$).tw. | 0 |
| 2 | (Reveal adj2 XT$).tw. | 0 |
| 3 | BioMonitor$.tw. | 3 |
| 4 | (Confirm adj2 RX$).tw. | 0 |
| 5 | (SJM adj2 Confirm$).tw. | 0 |
| 6 | (insertable adj3 cardiac adj3 monitor$).tw. | 0 |
| 7 | (implantable adj3 cardiac adj3 monitor$).tw. | 0 |
| 8 | (insertable adj3 loop adj3 recorder$).tw. | 0 |
| 9 | (implantable adj3 loop adj3 recorder$).tw. | 0 |
| 10 | (ICM or ICMs).tw. | 91 |
| 11 | or/1-10 | 94 |
| 12 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 365 |
| 13 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 6 |
| 14 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes).tw. | 13 |
| 15 | or/12-14 | 376 |
| 16 | 11 and 15 | 0 |
Health-related quality-of-life evidence
| # | Terms | Hits (n) |
|---|---|---|
| 1 | exp cerebrovascular accident/ | 172,439 |
| 2 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 341,008 |
| 3 | transient ischaemic attack/ | 33,402 |
| 4 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 19,067 |
| 5 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes).tw. | 17,054 |
| 6 | or/1-5 | 404,125 |
| 7 | ((quality adj2 life) or QOL).ti,ab. | 373,791 |
| 8 | (HRQL or HRQOL).ti,ab. | 26,321 |
| 9 | (“quality-adjusted life year$” or QALY or QALYs or “quality adjusted life year$”).ti,ab. | 19,666 |
| 10 | exp quality adjusted life year/ | 21,653 |
| 11 | (“disability-adjusted life year$” or DALY or DALYs or “disability adjusted life year$”).ti,ab. | 38,892 |
| 12 | (sf36 or sf-36 or “sf 36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”).ti,ab. | 36,096 |
| 13 | (sf6 or “sf 6” or sf-6 or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six”).ti,ab. | 2001 |
| 14 | (sf6d or “sf 6d” or sf-6d or “short form 6d” or “shortform 6d” or “sf six dimension” or “short form six dimension”).ti,ab. | 1281 |
| 15 | (sf12 or “sf 12” or sf-12 or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve”).ti,ab. | 7811 |
| 16 | (sf16 or “sf 16” or sf-16 or “short form 16” or “shortform 16” or “sf sixteen” or sfsixteen or “shortform sixteen” or “short form sixteen”).ti,ab. | 50 |
| 17 | (sf20 or “sf 20” or sf-20 or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty”).ti,ab. | 407 |
| 18 | (euroqol or “euro qol” or eq5d or “eq 5d” or eq-5d).tw. | 15,700 |
| 19 | (hye or hyes or “healthy year$ equivalent$”).ti,ab. | 133 |
| 20 | (“standard gamble” or SG).ti,ab. | 12,967 |
| 21 | (“time trade off” or “time tradeoff” or TTO or “time trade-off”).ti,ab. | 2356 |
| 22 | (utility adj3 value).ti,ab. | 1308 |
| 23 | disutil$.ti,ab. | 726 |
| 24 | ((quality adj3 wellbeing index) or QWB).ti,ab. | 230 |
| 25 | (“health utilities index” or HUI).ti,ab. | 2051 |
| 26 | or/7-25 | 420,375 |
| 27 | 6 and 26 | 12,159 |
| 28 | (letter or editorial or comment or case reports or review).pt. | 3,949,526 |
| 29 | nonhuman/ not human/ | 4,199,086 |
| 30 | or/28-29 | 7,971,522 |
| 31 | 27 not 30 | 10,215 |
| 32 | limit 31 to english language | 9614 |
| 33 | limit 32 to yr=“1997 -Current” | 9414 |
| 34 | limit 33 to (conference abstract and last 2 years) | 1321 |
| 35 | limit 33 to conference abstract | 4401 |
| 36 | 33 not 35 | 5013 |
| 37 | 36 or 34 | 6334 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | exp STROKE/ | 116,324 |
| 2 | (stroke$ or apoplexy$ or CVA or CVAS).tw. | 218,734 |
| 3 | Ischaemic Attack, Transient/ | 19,490 |
| 4 | (transient adj3 (ischaemi$ or ischaemi$) adj3 attack$).tw. | 12,733 |
| 5 | (TIA or TIAs or mini-stroke or mini-strokes or ministroke or ministrokes).tw. | 8006 |
| 6 | or/1-5 | 265,195 |
| 7 | ((quality adj2 life) or QOL).ti,ab. | 237,735 |
| 8 | (HRQL or HRQOL).ti,ab. | 16,383 |
| 9 | (“quality-adjusted life year$” or QALY or QALYs or “quality adjusted life year$”).ti,ab. | 11,707 |
| 10 | exp Quality-Adjusted Life Years/ | 10,391 |
| 11 | (“disability-adjusted life year$” or DALY or DALYs or “disability adjusted life year$”).ti,ab. | 3033 |
| 12 | (sf36 or sf-36 or “sf 36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”).ti,ab. | 22,797 |
| 13 | (sf6 or “sf 6” or “sf-6” or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six”).ti,ab. | 1887 |
| 14 | (sf6d or “sf 6d” or sf-6d or “short form 6d” or “shortform 6d” or “sf six dimension” or “short form six dimension”).ti,ab. | 717 |
| 15 | (sf12 or “sf 12” or sf-12 or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve”).ti,ab. | 4852 |
| 16 | (sf16 or “sf 16” or sf-16 or “short form 16” or “shortform 16” or “sf sixteen” or sfsixteen or “shortform sixteen” or “short form sixteen”).ti,ab. | 30 |
| 17 | (sf20 or “sf 20” or sf-20 or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty”).ti,ab. | 383 |
| 18 | (euroqol or “euro qol” or eq5d or “eq 5d” or eq-5d).tw. | 8555 |
| 19 | (hye or hyes or “healthy year$ equivalent$”).ti,ab. | 70 |
| 20 | (“standard gamble” or SG).ti,ab. | 9065 |
| 21 | ((quality adj3 wellbeing index) or QWB).ti,ab. | 194 |
| 22 | (“time trade off” or “time tradeoff” or TTO or “time trade-off”).ti,ab. | 1646 |
| 23 | (utility adj3 value).ti,ab. | 863 |
| 24 | disutil$.ti,ab. | 382 |
| 25 | (“health utilities index” or HUI).ti,ab. | 1447 |
| 26 | or/7-25 | 267,789 |
| 27 | 6 and 26 | 6061 |
| 28 | (letter or editorial or comment or case reports or review).pt. | 5,630,094 |
| 29 | animals/ not humans/ | 4,462,509 |
| 30 | or/28-29 | 9,858,961 |
| 31 | 27 not 30 | 4450 |
| 32 | limit 31 to english language | 4069 |
| 33 | limit 32 to yr=“1997 -Current” | 3933 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | (Reveal NEAR2 LINQ*) | 0 |
| 2 | (Reveal NEAR2 XT*) | 0 |
| 3 | (BioMonitor*) | 0 |
| 4 | (Confirm NEAR2 RX*) | 0 |
| 5 | (SJM NEAR2 Confirm*) | 0 |
| 6 | (insertable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 7 | (implantable NEAR3 cardiac NEAR3 monitor*) | 0 |
| 8 | (insertable NEAR3 loop NEAR3 recorder*) | 5 |
| 9 | (implantable NEAR3 loop NEAR3 recorder*) | 9 |
| 10 | (ICM*) | 12 |
| 11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 25 |
| 12 | MeSH DESCRIPTOR Stroke EXPLODE ALL TREES | 1354 |
| 13 | (stroke* or apoplexy* or CVA or CVAS) | 3165 |
| 14 | MeSH DESCRIPTOR Ischaemic Attack, Transient EXPLODE ALL TREES | 89 |
| 15 | (transient NEAR3 (ischaemi* or ischaemi*) NEAR3 attack*) | 243 |
| 16 | (TIA or TIAs or ministroke or mini-stroke or ministrokes or mini-strokes) | 86 |
| 17 | #12 OR #13 OR #14 OR #15 OR #16 | 3202 |
| 18 | #11 AND #17 | 3 |
| 19 | (quality NEAR2 life) OR (QOL) | 11,586 |
| 20 | (HRQL) OR (HRQOL) | 198 |
| 21 | (QALY) OR (QALYs) | 3263 |
| 22 | (quality-adjusted life year*) OR (quality adjusted life year*) | 5265 |
| 23 | MeSH DESCRIPTOR Quality-Adjusted Life Years EXPLODE ALL TREES | 3547 |
| 24 | (disability-adjusted life year*) OR (disability adjusted life year*) | 174 |
| 25 | (DALY) OR (DALYs) | 210 |
| 26 | (euroqol) OR (euro qol) | 263 |
| 27 | (eq5d) OR (eq 5d) OR (eq-5d) | 661 |
| 28 | (hye) OR (hyes) OR (healthy year* equivalent*) | 10 |
| 29 | (standard gamble) OR (SG) | 455 |
| 30 | (TTO) | 18 |
| 31 | (time trade off) OR (time tradeoff) OR (time trade-off) | 372 |
| 32 | (utility NEAR3 value) | 151 |
| 33 | (disutil*) | 184 |
| 34 | (health utilities index) OR (HUI) | 201 |
| 35 | (sf36 or sf-36 or “sf 36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”) OR (sf6 or “sf 6” or “sf-6” or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six”) OR (sf6d or “sf 6d” or sf-6d or “short form 6d” or “shortform 6d” or “sf six dimension” or “short form six dimension”) | 439 |
| 36 | (sf12 or “sf 12” or sf-12 or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve”) OR (sf16 or “sf 16” or sf-16 or “short form 16” or “shortform 16” or “sf sixteen” or sfsixteen or “shortform sixteen” or “short form sixteen”) OR (sf20 or “sf 20” or sf-20 or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty”) | 65 |
| 37 | #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 | 12,312 |
| 38 | #17 AND #37 | 805 |
| 39 | * IN DARE | 45,418 |
| 40 | #38 AND #39 | 231 |
| 41 | * IN NHSEED | 17,613 |
| 42 | #38 AND #41 | 516 |
| 43 | * IN HTA | 17,351 |
| 44 | #38 AND #43 | 58 |
| # | Terms | Hits (n) |
|---|---|---|
| 1 | MeSH descriptor: [Stroke] explode all trees | 7713 |
| 2 | MeSH descriptor: [Ischaemic Attack, Transient] explode all trees | 645 |
| 3 | (stroke* or apoplexy* or CVA or CVAS):ti,ab,kw | 42,050 |
| 4 | (transient near/3 (ischaemi* or ischaemi*) near/3 attack):ti,ab,kw | 2268 |
| 5 | (TIA or TIAs or mini-stroke or mini-strokes or ministroke or ministrokes):ti,ab,kw | 1185 |
| 6 | OR/ #1-#5} | 43,047 |
| 7 | (Reveal near/2 LINQ*):ti,ab,kw | 14 |
| 8 | (Reveal near/2 XT*):ti,ab,kw | 27 |
| 9 | BioMonitor*:ti,ab,kw | 40 |
| 10 | (Confirm near/2 RX*):ti,ab,kw | 0 |
| 11 | (SJM near/2 Confirm*):ti,ab,kw | 1 |
| 12 | (insertable near/3 cardiac near/3 monitor*):ti,ab,kw | 31 |
| 13 | (implantable near/3 cardiac near/3 monitor*):ti,ab,kw | 254 |
| 14 | (insertable near/3 loop near/3 recorder*):ti,ab,kw | 1 |
| 15 | (implantable near/3 loop near/3 recorder*):ti,ab,kw | 101 |
| 16 | ICM:ti,ab,kw | 228 |
| 17 | OR/ #7-#16 | 565 |
| 18 | #6 and #17 | 72 |
| 19 | MeSH descriptor: [Costs and Cost Analysis] explode all trees | 9518 |
| 20 | MeSH descriptor: [Cost-Benefit Analysis] explode all trees | 6179 |
| 21 | MeSH descriptor: [Fees and Charges] explode all trees | 251 |
| 22 | MeSH descriptor: [Budgets] explode all trees | 33 |
| 23 | MeSH descriptor: [Models, Economic] explode all trees | 298 |
| 24 | (“unit cost” or “unit-cost” or “unit-costs” or “unit costs” or “drug cost” or “drug costs” or “hospital costs” or “health-care costs” or “health care cost” or “medical cost” or “medical costs”):ti,ab | 4117 |
| 25 | (cost near/2 (util* or effective* or efficac* or benefit* or consequence* or analys* or minimi* or allocation* or control* or illness* or affordabl* or fee* or charge*)):ti,ab | 20,485 |
| 26 | (decision near/1 (tree* or analys* or model*)):ti,ab | 682 |
| 27 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*):ti,ab | 50,415 |
| 28 | ((value or values or valuation) near/2 (money or monetary or life or lives or costs or cost)):ti,ab | 578 |
| 29 | Markov:ti,ab | 903 |
| 30 | OR/ #19-#29 | 69,869 |
| 31 | #18 and #30 | 5 |
| 32 | MeSH descriptor: [Quality-Adjusted Life Years] explode all trees | 1029 |
| 33 | (“quality-adjusted life year*” or QALY or QALYs or “quality adjusted life year*”):ti,ab | 2647 |
| 34 | (“quality near/2 life” or QOL):ti,ab | 11,493 |
| 35 | (“disability-adjusted life year*” or DALY or DALYs or “disability adjusted life years*”):ti,ab | 148 |
| 36 | (HRQL or HRQOL):ti,ab | 4251 |
| 37 | (sf36 or sf-36 or “sf 36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”):ti,ab | 7486 |
| 38 | (sf6 or “sf 6” or “sf-6” or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six”):ti,ab | 145 |
| 39 | (sf6d or “sf 6d” or “sf-6d” or “short form 6d” or “shortform 6d” or “sf six dimension” or “short form six dimension”):ti,ab | 219 |
| 40 | (sf12 or “sf 12” or sf-12 or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve”):ti,ab | 1395 |
| 41 | (sf16 or “sf 16” or “sf-16” or “short form 16” or “shortform 16” or “sf sixteen” or sfsixteen or “shortform sixteen” or “short form sixteen”):ti,ab | 4 |
| 42 | (sf20 or “sf 20” or sf-20 or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty”):ti,ab | 66 |
| 43 | (euroqol or “euro qol” or eq5d or “eq 5d” or eq-5d):ti,ab,kw | 4069 |
| 44 | (hye or hyes or “health* year* equivalent*”):ti,ab | 9 |
| 45 | (“standard gamble” or SG):ti,ab | 1039 |
| 46 | ((quality near/3 wellbeing index) or QWB):ti,ab | 107 |
| 47 | (“time trade off” or “time tradeoff” or TTO or “time trade-off”):ti,ab | 207 |
| 48 | (utility near/3 value):ti,ab | 106 |
| 49 | disutil*:ti,ab | 46 |
| 50 | (“health utilities index” or HUI):ti,ab | 190 |
| 51 | OR/ #32-#50 | 26,945 |
| 52 | #6 and #51 | 909 |
Appendix 6 Studies excluded from the economic evaluation
| Reference | Reason for exclusion |
|---|---|
| Bravo Y, Marti B, Grifols MA. Cost analysis of an implantable loop recorder, Reveal© XT, for the diagnosis of atrial fibrillation in patients who underwent cryptogenic stroke from the perspective of a tertiary Spanish Hospital. Value Health 2012;15:A351 |
|
| Merino JL, Rodriguez-Barrios JM, Brosa M, Tsintzos S. Cost-effectiveness model of Implantable Cardiac Monitors (ICM) for patients treated with radiofrequency catheter ablation for atrial fibrillation (PAAF). Eur Heart J 2009;1:118 |
|
| Sadri H, Tsintzos S, Yee R, Skanes A, Gula L. Cost-effectiveness analysis of the insertable cardiac monitor for detecting recurrent atrial fibrillation (AF) following radiofrequency catheter ablation (RCA): a Canadian perspective. Value Health 2009;12:A148 |
|
| Steinhaus DA, Zimetbaum PJ, Passman RS, Leong-Sit P, Reynolds MR. Cost effectiveness of implantable cardiac monitor-guided intermittent anticoagulation for atrial fibrillation: an analysis of the react.com pilot study. J Cardiovasc Electrophysiol 2016;27:1304–11 |
|
| Reference | Reason for exclusion |
|---|---|
| Bravo Y, Marti B, Grifols MA. Cost analysis of an implantable loop recorder, Reveal© XT, for the diagnosis of atrial fibrillation in patients who underwent cryptogenic stroke from the perspective of a tertiary Spanish Hospital. Value Health 2012;15:A351 | Non-UK |
| Maervoet J, Bossers N, Borge RP, Schollbauer V, Van Engen A, Smala A. Clinical and economic value of device-based detection of atrial fibrillation in patients with cryptogenic stroke. Value Health 2017;20:A584 | Non-UK |
| Merino JL, Rodriguez-Barrios JM, Brosa M, Tsintzos S. Cost-effectiveness model of Implantable Cardiac Monitors (ICM) for patients treated with radiofrequency catheter ablation for atrial fibrillation (PAAF). Eur Heart J 2009;1:118 | Non-UK |
| Quiroz M, Wolff C, Eggington S. Insertable cardiac monitor versus standard of care for detection of atrial fibrillation in patients following cryptogenic stroke: a Dutch cost effectiveness analysis. Value Health 2017;20:A588 | Non-UK |
| Sadri H, Tsintzos S, Yee R, Skanes A, Gula L. Cost-effectiveness analysis of the insertable cardiac monitor for detecting recurrent atrial fibrillation (AF) following radiofrequency catheter ablation (RCA): a Canadian perspective. Value Health 2009;12:A148 | Non-UK |
| Steinhaus DA, Zimetbaum PJ, Passman RS, Leong-Sit P, Reynolds MR. Cost effectiveness of implantable cardiac monitor-guided intermittent anticoagulation for atrial fibrillation: an analysis of the react.com pilot study. J Cardiovasc Electrophysiol 2016;27:1304–11 | Non-UK |
| Thijs V, Kaffenberger T, Bernhardt J, Koehler J, Ziegler P. Early assessment of patient activity predicts functional outcome and quality of life at 6 months following cryptogenic stroke. Eur Stroke J 2017;2(Suppl. 1):168 | Non-UK |
| Reference | Reason for exclusion |
|---|---|
| Ali M, MacIsaac R, Quinn TJ, Bath PM, Veenstra DL, Xu Y, et al. Dependency and health utilities in stroke: data to inform cost-effectiveness analyses. Eur Stroke J 2017;2:70–6 | Utility values not relevant to the pathway in the model |
| Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–9 | Not primary source |
| Ayis S, Wellwood I, Rudd AG, McKevitt C, Parkin D, Wolfe CDA. Variations in health-related quality of life (HRQoL) and survival 1 year after stroke: five European population-based registers. BMJ Open 2015;5:e007101 | SF-12 mapped to EQ-5D |
| Barclay-Goddard R, Lix LM, Tate R, Weinberg L, Mayo NE. Health-related quality of life after stroke: does response shift occur in self-perceived physical function? Arch Phys Med Rehabil 2011;92:1762–9 | Utility values not reported |
| Barreto AD, Ford GA, Shen L, Pedroza C, Tyson JE, Cai C, et al. Patient-centered quality of life utility values are superior to modified rankin scale outcomes in stroke – experience from the artss-2 trial (randomized, multi-center trial of argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke Conference: American Heart Association/American Stroke Association 2018;49 | Conference abstract with insufficient detail |
| Bennaghmouch N, de Veer AJWM, Mahmoodi BK, Jofre-Bonet M, Lip GYH, Bode K, et al. Economic evaluation of the use of non-vitamin K oral anticoagulants in patients with atrial fibrillation on anti-platelet therapy: a modelling analysis using the healthcare system in the Netherlands. Eur Heart J Qual Care Clin Outcomes 2018;16:16 | Not available |
| Boehme C, Toell T, Mayer L, Prantl B, Knoflach M, Willeit J, et al. Gender differences in quality of life after a 1-year follow-up in stroke and high-risk TIA patients. Eur Stroke J 2017;2(Suppl. 1):470–2 | Conference abstract with insufficient detail |
| Bulkova V, Fiala M, Wichterle D, Haman L, Chovancik J, Havranek S, et al. Quality of life and costs of conventional therapy in patients treated by catheter ablation for atrial fibrillation. Cor et Vasa 2012;54:e421–7 | Irrelevant population. Utility values not reported |
| Cadilhac DA, Andrew NE, Lannin NA, Middleton S, Levi CR, Dewey HM, et al. Quality of acute care and long-term quality of life and survival: the Australian Stroke Clinical Registry. Stroke 2017;48:1026–32 | Utility values not reported |
| Canestaro WJ, Patrick AR, Avorn J, Ito K, Matlin OS, Brennan TA, et al. Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2013;6:724–731 | Not primary source |
| Choi JG, Ali A, Hur C, Lubitz SA. Population screening for atrial fibrillation: results of a cost-effectiveness modeling analysis. Heart Rhythm 2017;14(Suppl. 1):S222 | Conference abstract with insufficient detail |
| Chun H-YY, Whiteley WN, Dennis MS, Mead GE, Carson AJ. Anxiety after stroke: the importance of subtyping. Stroke 2018;49:556–64 | Utility values not reported |
| Chun Y, Carson A, Mead GE, Dennis M, Whiteley W. Anxiety after stroke and TIA: subtypes, predictors, and patient outcomes at 3 months. Int J Stroke 2017;12(Suppl. 2):16 | Conference abstract with insufficient detail |
| Contreras Muruaga MDM, Vivancos J, Reig G, Gonzalez A, Cardona P, Ramirez-Moreno JM, et al. Satisfaction, quality of life and perception of patients regarding burdens and benefits of vitamin K antagonists compared with direct oral anticoagulants in patients with nonvalvular atrial fibrillation. J Comp Eff Res 2017;6:303–12 | Text in Spanish |
| Costa J, Fiorentino F, Caldeira D, Ines M, Lopes Pereira C, Pinheiro L, et al. Cost-effectiveness of non-vitamin K antagonist oral anticoagulants for atrial fibrillation in Portugal. Revista Portuguesa de Cardiologia 2015;34:723–37 | Not available |
| Davidson T, Husberg M, Janzon M, Oldgren J, Levin L-A. Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur Heart J 2013;34:177–83 | Not primary source |
| De Caterina R, Bruggenjurgen B, Darius H, Kohler S, Lucerna M, Pecen L, et al. Quality of life and patient satisfaction in patients with atrial fibrillation on stable vitamin K antagonist treatment or switched to a non-vitamin K antagonist oral anticoagulant during a 1-year follow-up: a PREFER in AF Registry substudy. Arch Cardiovasc Dis 2018;111:74–84 | Utility values not relevant to the pathway in the model |
| De Caterina R, Kirchhof P, Le Heuzey JY, Brueggenjuergen B, Laeis P, Schmitt J. Patients’ convenience and satisfaction as important factors related to switching from vitamin K antagonists to NOACs-a PREFER in AF Registry analysis. Eur Heart J 2016;37(Suppl. 1):502 | Conference abstract with insufficient detail |
| Demel SL, Khoury J, Moomaw CJ, Sucharew H, Alwell K, Kissela BM, et al. Degree of functional independence after an ischemic stroke affects quality of life similarly in men and women. Stroke Conference: American Heart Association/American Stroke Association 2016;47 | Conference abstract with insufficient detail |
| Dewilde S, Thijs V, Annemans L, Peeters A, Belgian Stroke Council NP. Quality of life decrements after stroke. Value Health 2014;17:A331 | Conference abstract with insufficient detail |
| Dorman P, Dennis M, Sandercock P. Are the modified "simple questions" a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry 2000;69:487–93 | Utility values not reported |
| Dorman P, Slattery J, Farrell B, Dennis M, Sandercock P. Qualitative comparison of the reliability of health status assessments with the EuroQol and SF-36 questionnaires after stroke. Stroke 1998;29:63–8 | Utility values not reported |
| Dorman PJ, Dennis M, Sandercock P. How do scores on the EuroQol relate to scores on the SF-36 after stroke? Stroke 1999;30:2146–51 | Utility values not reported |
| Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke 1997;28:1876–82 | Utility values not reported |
| Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Are proxy assessments of health status after stroke with the EuroQol questionnaire feasible, accurate, and unbiased? Stroke 1997;28:1883–7 | Utility values not relevant to the pathway in the model |
| Dudink EAMP, Erkuner O, Berg J, Nieuwlaat R, de Vos CB, Weijs B, et al. The influence of progression of atrial fibrillation on quality of life: a report from the Euro Heart Survey. Europace 2018;20:929–34 | Utility values not relevant to the pathway in the model |
| Duncan PW, Samsa GP, Weinberger M, Goldstein LB, Bonito A, Witter DM, et al. Health status of individuals with mild stroke. Stroke 1997;28:740–5 | EQ-5D not used to measure HRQoL |
| Eckman MH, Wise RE, Speer B, Sullivan M, Walker N, Lip GYH, et al. Integrating real-time clinical information to provide estimates of net clinical benefit of antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014;7:680–6 | Utility values not reported |
| Escolar-Albaladejo G, Baron-Esquivias G, Zamorano JL, Betegon-Nicolas L, Canal-Fontcuberta C, de Salas-Cansado M, et al. Cost-effectiveness analysis of apixaban versus acetylsalicylic acid in the prevention of stroke in patients with non-valvular atrial fibrillation in Spain. Atencion Primaria 2016;48:394–405 | Text in Spanish |
| Fadrna T, Skoloudik D. Quality of life in self-sufficient patients after stroke. Eur Stroke J 2017;2(Suppl. 1):318 | Conference abstract with insufficient detail |
| Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1–11 | Not primary source |
| Freriks RD, Luijckx GJ, Van Der Zee DJ, Pizzo E, Mierau JO, Lahr MMH. Comparing cost-effectiveness of a centralised versus decentralised stroke care system in northern Netherlands-using patient-level data to estimate real-world effects. Cerebrovascular Diseases 2018;45(Suppl. 1):30 | Conference abstract with insufficient detail |
| Gage B, Cardinalli A, Albers G, Owens D. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 1995;274:1839–45 | Pre-1997. EQ-5D utility values not reported |
| Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 1996;156:1829–36 | Pre-1997. EQ-5D utility values not reported |
| Gage BF, Cardinalli AB, Owens DK. Cost-effectiveness of preference-based antithrombotic therapy for patients with nonvalvular atrial fibrillation. Stroke 1998;29:1083–91 | Not primary source |
| Gall S, Phan H, Blizzard L, Thrift A, Anderson C, Kim J, et al. Women and stroke poorer quality of life at 3-6 months after stroke in women compared to men is due to age and severity but not clinical care. Eur Stroke J 2017;2(Suppl. 1):66 | Conference abstract with insufficient detail |
| Ganesh A, Luengo-Fernandez R, Wharton RM, Gutnikov SA, Silver LE, Mehta Z, et al. One-month modified Rankin scale (mRS) score predicts five-year disability, death, quality-of-life, and healthcare costs in ischaemic stroke: a prospective cohort study. Stroke Conference: American Heart Association/American Stroke Association 2017;48 | Conference abstract with insufficient detail |
| Gupta D, Mildred M, Mattam SR, Linker NJ. The cost-effectiveness of dabigatran versus warfarin in patients undergoing ablation for atrial fibrillation: analysis based on data from the re-circuit trial. Heart Rhythm 2018;15(Suppl. 1):S434 | Conference abstract with insufficient detail |
| Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. the SAFE study. Health Technol Assess 2005;9(40) | Not primary source |
| Jacobs M, Kaasenbrood F, Postma M, Van Hulst M, Tieleman R. Cost-effectiveness of screening for atrial fibrillation in primary care with a hand-held, single-lead ECG device in the Netherlands. Circulation Conference: American Heart Association's 2016;134 | Not primary source |
| Janzic A, Kos M. Cost effectiveness of novel oral anticoagulants for stroke prevention in atrial fibrillation depending on the quality of warfarin anticoagulation control. PharmacoEconomics 2015;33:395–408 | Not primary source |
| Jones A, Krishnamurthi R, Theadom A, Barker-Collo S, McPherson K, Feigin V. Predictors of long-term health-related quality of life in stroke survivors. Neuroepidemiology 2016;47:140 | Conference abstract with insufficient detail |
| Jonsson A-C, Hoglund P, Brizzi M, Pessah-Rasmussen H. Secondary prevention and health promotion after stroke: can it be enhanced? J Stroke Cerebrovasc Dis 2014;23:2287–95 | Not available |
| Jowett S, Bryan S, Mant J, Fletcher K, Roalfe A, Fitzmaurice D, et al. Cost effectiveness of warfarin versus aspirin in patients older than 75 years with atrial fibrillation. Stroke 2011;42:1717–21 | Utility values not reported |
| Kamel H, Hegde M, Johnson DR, Gage BF, Johnston SC. Cost-effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke 2010;41:1514–20 | Not primary source |
| Kansal AR, Sorensen SV, Gani R, Robinson P, Pan F, Plumb JM, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012;98:573–8 | Not primary source |
| Kim J, Lannin N, Kilkenny M, Anderson C, Thrift A, Moss K, et al. Health-related quality of life of working-age adults in the Australian Stroke Clinical Registry. Int J Stroke 2017;12(Suppl. 1):10 | Conference abstract with insufficient detail |
| Kim S-K, Kim S-H, Jo M-W, Lee S-i. Estimation of minimally important differences in the EQ-5D and SF-6D indices and their utility in stroke. Health Qual Life Outcomes 2015;13:32 | Utility values not reported |
| Kongnakorn T, Lanitis T, Annemans L, Thijs V, Marbaix S. Cost effectiveness of apixaban versus aspirin for stroke prevention in patients with non-valvular atrial fibrillation in Belgium. Clin Drug Investig 2014;34:709–21 | Not primary source |
| Kwon S, Park J-H, Kim W-S, Han K, Lee Y, Paik N-J. Health-related quality of life and related factors in stroke survivors: data from Korea National Health and Nutrition Examination Survey (KNHANES) 2008 to 2014. PLoS ONE 2018;13:e0195713 | Population unclear |
| Lafuente-Lafuente C, Emery C, Laurendeau C, Fagnani F, Bergmann J-F. Long term treatment of atrial fibrillation in elderly patients: a decision analysis. Int J Cardiol 2012;155:102–9 | Not primary source |
| Lahr M, Freriks R, Buskens E, Pizzo E, Van Der Zee DJ, Mierau J, et al. Comparing real-world costeffectiveness of a centralized versus decentralized stroke care system; a northern Netherlands exemplar. Eur Stroke J 2018;3(Suppl. 1):280 | Conference abstract with insufficient detail |
| Lamy A, Eikelboom J, Connolly S, Bosch J, Fox KA, Tong W, et al. Costs impact rivaroxaban plus aspirin versus aspirin in the COMPASS trial. Circulation 2017;136:e456–7 | Conference abstract with insufficient detail |
| Lanitis T, Kongnakorn T, Jacobson L, De Geer A. Cost-effectiveness of apixaban versus warfarin and aspirin in Sweden for stroke prevention in patients with atrial fibrillation. Thromb Res 2014;134:278–87 | Not primary source |
| Lannin NA, Anderson CS, Kim J, Kilkenny M, Bernhardt J, Levi C, et al. Treatment and outcomes of working aged adults with stroke: results from a National Prospective Registry. Neuroepidemiology 2017;49:113–20 | EQ-5D not valued using standard methods |
| Leno Diaz C, Holguin Mohedas M, Hidalgo Jimenez N, Rodriguez-Ramos M, Lavado Garcia JM. Long-term health-related quality of life in stroke survivors. Revista Cientifica de la Sociedad Espanola de Enfermeria Neurologica 2016;44:9–15 | Not available |
| Levin L-A, Husberg M, Sobocinski PD, Kull VF, Friberg L, Rosenqvist M, et al. A cost-effectiveness analysis of screening for silent atrial fibrillation after ischaemic stroke. Europace 2015;17:207–14 | Not primary source |
| Lip GYH, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192–210.e20 | Not primary source |
| Lip GYH, Lanitis T, Mardekian J, Kongnakorn T, Phatak H, Dorian P. Clinical and economic implications of apixaban versus aspirin in the low-risk nonvalvular atrial fibrillation patients. Stroke 2015;46:2830–7 | Not primary source |
| Lopez Espuela F, Portilla Cuenca JC, Leno Diaz C, Parraga Sanchez JM, Gamez-Leyva G, Casado Naranjo I. Sex differences in long-term quality of life after stroke: influence of mood and functional status. Neurologia 2017;19:19 | Full text in Spanish |
| Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–76 | Not primary source |
| Mayer F, Stahrenberg R, Groschel K, Mostardt S, Biermann J, Edelmann F, et al. Cost-effectiveness of 7-day-Holter monitoring alone or in combination with transthoracic echocardiography in patients with cerebral ischemia. Clin Res Cardiol 2013;102:875–84 | Not primary source |
| Monreal M, Soulard S, Crespo C, Brand S, Kansal A. Apixaban, dabigatran and rivaroxaban: which direct oral anticoagulant is the most cost-efficient for the prevention of stroke in patients with atrial fibrillation in Spain? Eur Heart J 2016;37(Suppl. 1):497 | Conference abstract with insufficient detail |
| Monz BU, Connolly SJ, Korhonen M, Noack H, Pooley J. Assessing the impact of dabigatran and warfarin on health-related quality of life: results from an RE-LY sub-study. Int J Cardiol 2013;168:2540–7 | Irrelevant population |
| Moran PS, Teljeur C, Harrington P, Smith SM, Smyth B, Harbison J, et al. Cost-effectiveness of a national opportunistic screening program for atrial fibrillation in Ireland. Value Health 2016;19:985–95 | Not primary source |
| O'Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA 2005;293:699–706 | Not primary source |
| Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost-effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke 2004;35:196–203 | Utility values not reported |
| Phan H, Blizzard L, Thrift A, Cadilhac D, Sturm J, Konstantinos V, et al. Sex differences in health-related quality of life (HRQOL) in the long-term after stroke: the international stroke outcomes study. Eur Stroke J 2016;1(Suppl. 1):285 | Conference abstract with insufficient detail |
| Phan HT, Cadilhac D, Blizzard L, Lannin N, Thrift A, Anderson C, et al. Differences in stroke care and outcomes after stroke for women compared to men: Australian Stroke Clinical Registry (AuSCR). Int J Stroke 2017;12(Suppl. 1):30 | Conference abstract with insufficient detail |
| Phan HT, Gall SL, Blizzard L, Lannin NA, Thrift AG, Anderson C, et al. Lower health-related quality of life (HRQoL) at 3-6 months after stroke in both women and men compared to those without stroke: an observational study from the australian stroke clinical registry (AUSCR). Stroke Conference: American Heart Association/American Stroke Association 2018;49 | Conference abstract with insufficient detail |
| Puumalainen A, Numminen H, Elonheimo O, Roine RO, Sintonen H. Health outcomes and costs of ischemic stroke patients in Finland. Acta Neurologica Scandinavica 2016;134:42–8 | EQ-5D not used to measure HRQoL |
| Quiroz M, Wolff C, Eggington S. Insertable cardiac monitor versus standard of care for detection of atrial fibrillation in patients following cryptogenic stroke: a Dutch cost-effectiveness analysis. Value Health 2017;20:A588 | Conference abstract with insufficient detail |
| Radholm K, Arima H, Lindley RI, Wang J, Tzourio C, Robinson T, et al. Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing 2015;44:422–7 | Utility values not reported |
| Rangaraju S, Haussen D, Nogueira RG, Nahab F, Frankel M. Comparison of 3-Month stroke disability and quality of life across Modified Rankin Scale categories. Interv Neurol 2017;6:36–41 | Utility values not relevant to the pathway in the model |
| Rudberg AS, Berge E, Gustavsson A, Nasman P, Lundstrom E. Long-term health-related quality of life, survival and costs by different levels of functional outcome six months after stroke. Eur Stroke J 2018;3:157–64 | Utility values not relevant to the pathway in the model |
| Savelieva I, Paquette M, Dorian P, Lüderitz B, Camm AJ. Quality of life in patients with silent atrial fibrillation. Heart 2001;85:216–17 | EQ-5D not used to measure HRQoL |
| Schleinitz MD, Weiss JP, Owens DK. Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis. Am J Med 2004;116:797–806 | Not primary source |
| Schreuders J, van den Berg LA, Fransen PS, Berkhemer OA, Beumer D, Lingsma HF, et al. Quality of life after intra-arterial treatment for acute ischemic stroke in the MR CLEAN trial-Update. Int J Stroke 2017;12:708–12 | Utility values not relevant to the pathway in the model |
| Sorensen SV, Dewilde S, Singer DE, Goldhaber SZ, Monz BU, Plumb JM. Cost-effectiveness of warfarin: trial versus "real-world" stroke prevention in atrial fibrillation. Am Heart J 2009;157:1064–73 | Not primary source |
| Sprigg N, Selby J, Fox L, Berge E, Whynes D, Bath PMW, et al. Very low quality of life after acute stroke: data from the Efficacy of Nitric Oxide in Stroke trial. Stroke 2013;44:3458–62 | Utility values not reported |
| Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. PharmacoEconomics 2006;24:1021–33 | Not primary source |
| Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. PharmacoEconomics 2003;21:191–200 | Not primary source. EQ-5D not used to measure HRQoL |
| Thijs V, Kaffenberger T, Bernhardt J, Koehler J, Ziegler P. Early assessment of patient activity predicts functional outcome and quality of life at 6 months following cryptogenic stroke. Eur Stroke J 2017;2(Suppl. 1):168 | Conference abstract with insufficient detail |
| Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet 2000;355:956–62 | EQ-5D not used to measure HRQoL |
| Van Den Berg L, Berkhemer O, Fransen P, Beumer D, Lingsma H, Majoie C, et al. Economic evaluation alongside the multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands – MR CLEAN Trial. Eur Stroke J 2017;2(Suppl. 1):82–3 | Conference abstract with insufficient detail |
| Verhoef TI, Redekop WK, Hasrat F, de Boer A, Maitland-van der Zee AH. Cost effectiveness of new oral anticoagulants for stroke prevention in patients with atrial fibrillation in two different European healthcare settings. Am J Cardiovasc Drugs 2014;14:451–62 | Not primary source |
| Walfridsson H, Walfridsson U, Cosedis Nielsen J, Johannessen A, Raatikainen P, Janzon M, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health-related quality of life and symptom burden. The MANTRA-PAF trial. Europace 2014;17:215–21 | Utility values not relevant to the pathway in the model |
| Wisloff T, Hagen G, Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. PharmacoEconomics 2014;32:601–12 | Not primary source |
| Wright J, Bibby J, Eastham J, Harrison S, McGeorge M, Patterson C, et al. Multifaceted implementation of stroke prevention guidelines in primary care: cluster-randomised evaluation of clinical and cost effectiveness. Qual Saf Health Care 2007;16:51–9 | Not primary source |
Appendix 7 Economic data extraction tables
| Population, intervention and comparator | Perspective, discounting, cost year and model structure | Measures of diagnostic accuracy | Clinical effectiveness | Resource and cost use | HRQoL | Total costs and total QALYs | ICER and results of sensitivity analysis |
|---|---|---|---|---|---|---|---|
| De Angelis et al.89 | |||||||
|
|
|
|
|
|
|
|
| Diamantopoulos et al.90 | |||||||
|
|
|
Mortality:
|
Costs sourced from NHS Reference Costs:104
|
CRYSTAL-AF baseline 0.774 Utilities sourced from Luengo-Fernandez et al. 102 using the EQ-5D and UK population valuations:Utilities for temporary events sourced from Dorian et al. ,94 Lip et al. 95 and Sullivan et al. 103 using a UK-based catalogue:Disutilties applied in the model: |
Total discounted costs per patient (ICM; SoC)
|
ICER, cost per QALY gained £17,175 Other results:Subgroup analysis:CHADS2 scorePSATornado diagram (Diamantopoulos et al. ,90 Figure 2) illustrates the eight most sensitive parameters: |
| Maervoet et al.91 | |||||||
|
|
Diagnostic yield and accuracy based on RCTs and diagnostic accuracy studies; no further details reported | NR | Clinical actions based on clinical expert’s input, no further details reported | NR (life-years measure of benefit) |
|
ICER, US$18,500 per life-year gained Other results: |
| Quiroz et al.92 | |||||||
|
|
NR | NR | Costs were applied to each state according to occurrence of stroke, AF diagnosis and drug therapy use. Values and data sources NR | Utilities were applied to each state according to occurrence of stroke, AF diagnosis and drug therapy use. Values and data sources NR | NR |
|
| Thijs et al.93 | |||||||
|
|
Used a linked evidence approach to estimate the rates of recurrent stroke when AF detection leads to initiation of oral anticoagulation, as detected using ICM during the lifetime of the device, or as detected using conventional care. Values and data sources NR | NR | Included all diagnostic and patient management costs. Values and data sources NR | Inputs determined by literature review, no further details reported | NR |
|
| Study | Elicitation method | Valuation method | Population | Health states and utility values |
|---|---|---|---|---|
| Alvarez-Sabín et al.112 | Patients assessed their own HRQoL using the EQ-5D-3L 2 years post-stroke | NR |
|
Mean (SD) utility
|
| Bach et al.113 | Patients assessed their own HRQoL using the EQ-5D-3L (time NR) | Responses were converted into utilities using scoring algorithms for the German population (Greiner et al.187) | 3109 patients in Germany were included from the DETECT study75 MI; stroke; MI and stroke:
|
Mean (SD) utility
|
| Berg et al.131 | Patients assessed their own HRQoL using the EQ-5D-3L at baseline and at 1 year post AF | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utilityFinal model specification results for determinants of utility at follow-up, AEs during follow-up CLAD; OLS, mean (95% CI) |
| Bushnell et al.114 | Patients assessed their own HRQoL using the EQ-5D-3L at 3 and 12 months post stroke or post TIA | Responses were converted into utilities using US population-based preference weights (Rockville188) |
|
Median (IQR) utility (3 months; 12 months)
|
| Chang et al.115 | Patients assessed their own HRQoL using the EQ-5D-3L 6 months post stroke | Responses were converted into utilities using Kang 2006 |
|
Mean (SD) utility
|
| Christensen et al.116 | Patients assessed their own HRQoL using the EQ-5D-3L 3 months post stroke | Responses were converted into utilities using US population-based preference weights (Shaw et al.189 and Luo et al.190) |
|
Mean (SD) utility after ICH 0.62 (0.3) |
| Ghatnekar et al.117 | Patients assessed their own HRQoL using the EQ-5D-3L 3 months post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
Patients included in the Ris-Stroke registry in Sweden Used two time periods with patients who had experienced their first HS or IS (ICD-10: I61, I63 and I64) |
Mean (SD) utility
|
| Golicki et al.118 | Patients assessed their own HRQoL using the EQ-5D-3L and EQ-5D-5L during their index hospitalisation (median 8 days since admission) | To obtain 3L index values, the Polish EQ-5D-3L value set based on the TTO valuation technique (Golicki et al.191) was used. To obtain EQ-5D-5L index values, the Polish interim EQ-5D-5L value set (Golicki et al.192) estimated with crosswalk methodology developed by van Hout et al.193 |
|
|
| Golicki et al.119 | Patients assessed their own HRQoL using the EQ-5D-3L and EQ-5D-5L 1 week and 4 months post stroke | To obtain 3L index values, the Polish EQ-5D-3L value set based on the TTO valuation technique (Golicki et al.191) was used. To obtain 5L index values, the Polish interim EQ-5D-5L value set (Golicki et al.192) estimated with crosswalk methodology developed by van Hout et al.193 |
|
Mean (SD) EQ-5D-3L utility values
|
| Haacke et al.120 | Patients assessed their own HRQoL using the German version of the EQ-5D-3L 4 years post stroke | NR |
|
Mean (SD) utility
|
| Hallinen et al.121 | Patients with AF assessed their own HRQoL using the EQ-5D-3L (time NR) | NR | 5690 Finnish inhabitants with AF who participated in the Health 2000 study (Methodology report, Health 2000 Survey194) |
In the regression model the constant term was 1.068, the disutility associated with AF was –0.045 and the decrease in quality of life per year of age was –0.004. The AF equals 0.743 ( = 1.068 – 0.004 × 70 – 0.045), where 70 is the average age in years of patients Mean disutility |
| Lindgren et al.122 | Patients assessed their own HRQoL using the EQ-5D-3L 3, 6, 9 or 12 months post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utility
|
| Lopez-Bastida et al.123 | Patients assessed their own HRQoL using the EQ-5D-3L 1, 2 or 3 years post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utility
|
| Luengo-Fernandez et al.102 | Patients assessed their own HRQoL using the EQ-5D-3L over 5 years | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Month: 1; 6; 12; 24; 60 Mean (SD) utility |
| Lunde124 | Patients assessed their own HRQoL using the EQ-5D-3L 6 months post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utility (n = 345) 0.70 (0.30) |
| Mar et al.125 | Patients assessed their own HRQoL using the EQ-5D-3L 1 year post stroke | NR |
|
Mean (SE) utilityAutonomous 0.736 (0.069) Disabled 0.4013 (0.2213) |
| Mar et al.126 | Patients assessed their own HRQoL using the EQ-5D-3L at admission and at 3 and 12 months post stroke | Responses were converted into utilities using general Spanish population (Badía et al.195) |
|
Mean (SD) utility
|
| Pickard et al.128 | Patients assessed their own HRQoL using the EQ-5D-3L at admission and 6 months post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utility
|
| Pickard et al.127 | Patients assessed their own HRQoL using the EQ-5D-3L at baseline and 6 months post stroke | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a |
|
Mean (SD) utilityn = 98
|
| Roalfe et al.132 | Patients assessed their own HRQoL using the EQ-5D-3L (time NR) | NR |
|
Mean (SD) utility
|
| Sullivan et al.103 | Patients assessed their own HRQoL using the EQ-5D-3L (time NR) | Responses were converted into utilities using UK population tariffs developed by Dolan 1997a | 79,522 individuals taken from the US-based MEPS OLS, Tobit, and CLAD regression methods were used to estimate the ‘marginal disutility’ of each condition (ICD-9 codes, CCC codes), controlling for covariates |
EQ-5D (UK-Dolan 1997), scores by CCC CCC 100 Acute MICCC 109 acute cerebrovascular disease (stroke) |
| van Eeden et al.129 | Patients assessed their own HRQoL using the Dutch EQ-5D-3L 2, 6 and 12 months post stroke | Responses were converted into utilities using Dutch tariffs (Lamers et al.196) |
|
Mean (SD) utility
|
| Wang et al.133 | Patients assessed their own HRQoL at 3-month intervals for up to 48 months. Authors estimated the impact of different categories of bleeding events on health-state utility over 12 months following the event | Responses were converted into utilities using an algorithm developed for the US population (EuroQol197) |
10,706 patients included in the ENGAGE AFTIMI 48 trial from 46 counties to asses the prevention of stroke or systemic embolism in AF Major GI bleeding event (n = 207)Major non-GI bleeding (extracranial) event (n = 152)Clinically relevant non-major bleeding event (n = 1419)Minor bleeding event (n = 714) |
Mean (SD) utility
|
| Xie et al.130 | Patients (26% proxy) assessed their own HRQoL using the EQ-5D-3L (time NR) | Responses were converted into utilities using US population-based preference weights (Shaw et al. 2005) |
1040 patients in the USA who ‘had ever been diagnosed as having had a stroke or transient ischaemic attack’ Data obtained from the Household Component of the MEPS Age (years): 18–49, 12.6%; 50–64, 26.4%; 65–74, 25.6%; 75–84, 27.7%; ≥ 85, 7.8% Male, 43.9% Ethnicity: white, 78.0%; black, 17.7%; other, 4.3% |
Mean (SE) utility Age (years):Total: 0.69 (0.01) Male: 0.72 (0.01) Female: 0.67 (0.01) |
Glossary
- Accuracy
- The ability of a diagnostic test to identify positive and negative cases correctly. Calculated as the proportion of true positives and true negatives in all evaluated cases.
- Atrial fibrillation
- A heart condition that causes an irregular and often abnormally fast heart rate. Atrial fibrillation may be intermittent (paroxysmal) or continuous, and symptomatic (dizziness, shortness of breath, tiredness) or asymptomatic.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs per additional health gain.
- Cryptogenic stroke
- A stroke of undetermined cause or origin. Classification of cryptogenic stroke depends on the system used and may include strokes that have more than one identifiable cause or those that have not been investigated fully.
- False negative
- An incorrect negative test result for an affected individual.
- False positive
- An incorrect positive test result for an unaffected individual.
- Implantable cardiac monitor
- A small electrocardiographic device for long-term monitoring of a patient’s electrical heart activity. The device is implanted via a small incision under the skin of a patient’s chest to record and transmit detected arrhythmia episodes.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Markov model
- An analytical method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- A statistical technique used to combine the results of two or more studies and obtain a combined estimate of effect.
- Negative predictive value
- The probability that people with a negative test result truly do not have the target condition (which, in this case, is atrial fibrillation).
- Opportunity cost
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Positive predictive value
- The probability that people with a positive test result truly have the target condition (atrial fibrillation).
- Probabilistic sensitivity analysis
- A method of quantifying uncertainty in a mathematical model, such as a cost-effectiveness model.
- Reference standard
- The best currently available diagnostic test against which the index test is compared.
- Sensitivity
- The proportion of people with the target condition (atrial fibrillation) who test positive.
- Specificity
- The proportion of people without the target condition (atrial fibrillation) who test negative.
- Transient ischaemic attack
- A brief episode of neurological dysfunction caused by loss of blood flow in the brain, without an identifiable lesion on imaging. Transient ischaemic attacks have the same underlying mechanism as ischaemic strokes, and symptoms resolve within 24 hours.
- True negative
- A correct negative test result for an unaffected individual.
- True positive
- A correct positive test result for an affected individual.
List of abbreviations
- AE
- adverse event
- AF
- atrial fibrillation
- BNF
- British National Formulary
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHA2DS2-VASc
- Congestive heart failure (or left ventricular systolic dysfunction), Hypertension [blood pressure consistently > 140/90 mmHg (or treated hypertension on medication)], Age ≥ 75 years (doubled), Diabetes mellitus, prior Stroke or transient ischaemic attack or thromboembolism (doubled), Vascular disease (e.g. peripheral artery disease, myocardial infarction, aortic plaque), Age 65–74 years, Sex category (e.g. female)
- CI
- confidence interval
- CIS
- cryptogenic ischaemic stroke
- CRB
- clinically relevant (extracranial) bleed
- CRD
- Centre for Reviews and Dissemination
- CRYSTAL-AF
- Cryptogenic Stroke and underlying Atrial Fibrillation
- CS
- cryptogenic stroke
- CT
- computerised tomography
- CTA
- computed tomography angiography
- CVE
- cardiovascular event
- DAR
- Diagnostic Assessment Report
- DARE
- Database of Abstracts of Reviews of Effects
- DETECT
- Diabetes Cardiovascular Risk Evaluation: Targets and Essential Data for Commitment of Treatment
- DOAC
- directly acting oral anticoagulant
- DTA
- diagnostic test accuracy
- DVT
- deep-vein thrombosis
- EAG
- Evidence Assessment Group
- ECG
- electrocardiogram
- ECH
- extracranial haemorrhage
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HS
- haemorrhagic stroke
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracranial haemorrhage
- ICM
- implantable cardiac monitor
- INB
- incremental net benefit
- INR
- international normalised ratio
- IQR
- interquartile range
- IS
- ischaemic stroke
- ITT
- intention to treat
- KM
- Kaplan–Meier
- MI
- myocardial infarction
- MPP
- manual pulse palpation
- MRA
- magnetic resonance angiography
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NPV
- negative predictive value
- OAC
- oral anticoagulant
- ONS
- Office for National Statistics
- OR
- odds ratio
- OX-VASC
- Oxford Vascular Study
- PE
- pulmonary embolism
- PERDIEM
- Post-Embolic Rhythm Detection with Implantable versus External Monitoring
- PFO
- patent foramen ovale
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SLR
- systematic literature review
- SoC
- standard of care monitoring
- TIA
- transient ischaemic attack
- TOE
- transoesophageal echocardiography
- WTP
- willingness to pay
- XPECT
- Reveal XT Performance Trial
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Diagnostics Advisory Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
