Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/10/11. The contractual start date was in December 2016. The draft report began editorial review in April 2019 and was accepted for publication in August 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Joanna Coast reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study; and she led the development of the ICECAP (ICEpop CAPability) instruments. Claire Goodman is a NIHR Senior Investigator. Ben Hardwick reports grants from the NIHR HTA programme during the conduct of the study. Catherine Walshe reports that she was a member of the NIHR Health Services and Delivery Research programme Researcher Led Committee during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Froggatt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Dementia is a life-limiting condition, and survival time from diagnosis decreases with age, from 6.7 to 1.9 years. 1 In advanced dementia, an individual is fully dependent on others for care; they can be chair- or bedbound, doubly incontinent and no longer able to communicate verbally [assessed as having a Functional Assessment Staging Test (FAST) score of 6 or 7]. 2 For many people with advanced dementia, a move to a care home is required because they can no longer live independently at home. 3 ‘Care home’ is a generic term that refers to:
A collective institutional setting where care is provided for older people who live there, 24 hours a day, seven days a week, for an undefined period of time. The care provided includes on site provision of personal assistance with activities of daily living. Nursing and medical care may be provided on-site or by nursing and medical professionals working from an organisation external to the setting.
Froggatt and Reitinger, p. 14. 4
Nursing homes are care homes that employ on-site nurses who are present 24 hours per day, 7 days per week for people with higher levels of dependency and health-care needs. At least two-thirds of people in care homes are estimated to have dementia5 and will therefore die with, if not of, dementia. 6 In England and Wales, approximately 18% of the population die in care homes. 7 In an ageing population, the numbers of people with advanced dementia who require palliative and end-of-life care in care homes will rise. 8
Dying with advanced dementia is often prolonged and distressing, with poor quality of life and death reported,9,10 through either under- or overtreatment. 11–13 People dying with advanced dementia suffer symptoms such as pain, which leads to distressing behaviours such as agitation and sleep disturbance. 14 Evidence suggests that there is also a negative impact on carers who witness dying when there is pain, agitation and distress. 15 There is therefore a need for appropriate care that will ensure a good quality of life and a good quality of dying. 13,16
Health-care practitioners can struggle to provide appropriate care for people with advanced dementia. 17 Palliative and end-of-life care seeks to address the needs of people whose disease is not responsive to curative treatment by providing active care and treatment that addresses all physical, psychological, spiritual and social domains. 18 Challenges in providing appropriate palliative and end-of-life care for people with dementia are recognised19 and interventions to support good practice are being sought.
One intervention for those with advanced dementia that is gaining increasing currency with practitioners, but without good evidence of effect, is Namaste Care. 20 Namaste Care is a non-pharmacological, complex intervention to improve the quality of life and care at the end of life that is designed to ameliorate challenging symptoms such as agitation, pain, distress and sleep disturbance. It is proposed that this intervention, delivered by nurses and care assistants, could, if successful, enable the skilled and confident delivery of care known to improve both quality of care and quality of dying. 20 Small-scale studies have demonstrated this intervention’s potential to reduce pain, urinary tract infections and distress, improve sleep and reduce agitation. 21–25
A future full trial is urgently required to determine the efficacy of an intervention already spreading across end-of-life and nursing home care settings to ensure that only appropriate cost-effective and clinically effective technology that can be practically delivered is adopted.
Explanation of rationale
Namaste Care is based on the premise that people with advanced dementia have the right to be cared for as human beings with full moral worth23–25 and draws on principles of person-centred care. 26 There is little strong evidence for Namaste Care as a multicomponent intervention, but some disparate evidence suggests that it leads to a reduction in the severity of physical and behavioural symptoms, and changes in social interaction, agitation and delirium. 22,27,28 Wider benefits to the health economy are suggested with respect to the reduced use of psychotropic medication. 27 Family and staff report increased satisfaction with care following delivery of Namaste Care. 29
Understanding the effect (including cost-effectiveness) of Namaste Care and how best to organise care to achieve this effect will enable clear decision-making about health-care practice for those with advanced dementia, and whether or not and how to change the focus of care for those with advanced dementia nearing the end of life. This research will provide a clear specification for the delivery of Namaste Care that will feed forwards into health-care decision-making.
This trial is also part of a cohort of larger clinical studies being undertaken internationally (Canada30 and the Netherlands31) to develop a robust comparable evidence base for the efficacy of the intervention.
Study overall aim
The aim of the study is to undertake robust, evidence-based development of the Namaste Care intervention followed by a feasibility trial to determine the parameters of a full trial of Namaste Care in nursing home settings.
Research question
What is the feasibility of conducting a cluster randomised controlled trial in a nursing home context to understand the impact on quality of life, and quality of dying, of the Namaste Care intervention for people with advanced dementia, when compared with usual end-of-life care?
Aim
The main aim of the feasibility trial was to ascertain the feasibility of conducting a full trial of the Namaste Care intervention.
Objectives
The feasibility aims of the research design and data collection processes to enable the design of a full trial to determine the efficacy of Namaste Care were to:
-
understand how best to sample and recruit nursing homes into a cluster randomised controlled trial of Namaste Care
-
establish recruitment, retention and attrition rates at the level of the nursing home and of the individual resident, informal carer and nursing home staff
-
determine the most appropriate selection, timing and administration of primary and secondary outcome measures for a full cluster randomised controlled trial of Namaste Care against criteria of bias minimisation, burden and acceptability
-
assess the acceptability (to staff and family), fidelity and sustainability of the Namaste Care intervention
-
establish the willingness of a large number of nursing homes, representing the range of nursing homes with respect to provider type, size and resident care needs, to participate in a full trial.
Chapter 2 Realist review of Namaste Care and other multisensory interventions
This chapter includes text from the paper by Bunn et al. 32 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
We conducted a stakeholder-driven realist review. It was conducted and reported in accordance with the Realist and Meta-narrative Evidence Syntheses: Evolving Standards (RAMESES). 33 This review is registered as PROSPERO CRD42016047512. The aim of the review was to develop a theory-driven explanation of how the Namaste Care intervention might work, and in what circumstances, to inform the development of the Namaste Care intervention.
Rationale for using a realist approach
Realist review is a systematic, theory-driven approach that aims to make explicit the underlying processes, structures or reasoning (mechanisms) of how and why complex interventions work (or do not) in particular settings or contexts. 34,35 Namaste Care is a complex multicomponent intervention dependent on the behaviours and choices of those delivering and receiving the care. The purpose of this review was to develop an explanatory account or programme theory about Namaste Care and how it might work for people with advanced dementia living in long-term care settings, such as nursing homes. We knew when starting the review that most of the literature on Namaste Care was descriptive and experiential. However, in a realist approach the unit of analysis is the programme theory or underpinning mechanism of action, rather than the intervention. 34 This allowed us to draw on a broader range of literature rather than literature focusing solely on Namaste Care.
Programme theory comprises configurations of context (the background conditions/circumstances in which interventions are delivered and in which mechanisms are triggered), mechanism (the responses or changes that are brought about through a programme within a particular context) and outcomes. The development of these context–mechanism–outcome (CMO) configurations is iterative, involving data collection, theorising and stakeholder engagement. Stakeholders with direct experience of providing end-of-life care to people with dementia in care home settings were involved in defining the scope of the review and, later, in validating the programme theory. 33,34
Methods
Phase 1: defining the scope of the realist review – concept-mining and theory development
In phase 1, we searched the literature and consulted with stakeholders to develop provisional programme theories about how Namaste Care might work. To identify relevant literature, we searched PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for all available literature describing the implementation or use of Namaste Care, conducted forwards and backwards citation tracking and hand-searched a book by Joyce Simard, the originator of Namaste Care. 20 We searched for research studies of any design and descriptive items in non-academic journals.
In conjunction with scoping the literature, we conducted face-to-face or telephone interviews with 11 participants involved in delivering Namaste Care, training of care home staff in Namaste Care, and for research within dementia and/or palliative and end-of-life care. Participants were based in the UK, the Netherlands and the USA. Participants were recruited for their known expertise and through snowball sampling. Interviews were conducted either face to face or by telephone or Skype™ (Microsoft Corporation, Redmond, WA, USA) video call. Participants were given a copy of the study information sheet, which provided contact details of the research team, and a consent form that they were asked to read and sign. Interviews were conducted using realist principles36 and were guided by a topic guide. The interview schedules were designed to explore participants’ experiences of Namaste Care for people living with dementia and their views on what they considered to be the essential components of the intervention, and how and on what outcomes the intervention was thought to work. Research Ethics Committee approval was obtained from Lancaster University (reference number 17/wa/0378).
Findings from the literature and interviews were used to develop a preliminary theory in the form of 13 explanatory ‘if–then’ statements. 32 ‘If–then’ statements are the identification of an intervention/activity linked to outcome(s). They contain references to contexts and mechanisms, although these may not be very explicit at this stage. 37 Following this, we held a workshop to review and refine the theory. Participants for the workshop were recruited based on their expertise in Namaste Care and/or in dementia or end-of-life care. The workshop included seven external participants (three of whom had participated in interviews) and six members of the study team (one of whom was a participant and public involvement lead). At the workshop, members of the project team presented the preliminary findings from the scoping, the outcomes identified from the literature and the if–then statements. We adapted nominal group technique to facilitate the discussion of the if–then statements. The purpose was to understand what participants thought was needed for Namaste Care to work, how they thought Namaste Care changed the behaviour of residents and staff, and why/how it worked. Nominal group technique is a process that promotes the generation of ideas to develop a set of priorities and enables the participation of all group members. 38 Participants’ comments were recorded, and statements were ranked by participants in order of importance.
After the workshop, members of the project team who had attended the workshop reviewed the if–then statements, and the rankings, and grouped them into three categories:
-
how Namaste Care is introduced to the care home, including the structure of the intervention, frequency and resources
-
characteristics/approach of the care home staff and characteristics of the Namaste Care programme, for example staff providing person-centred care and engaging in biography work with residents
-
how Namaste Care is delivered, including meaningful activities involving all five senses and adaptation of activities to individual circumstances and preferences.
These categories became the basis for the three preliminary CMOs32 that were taken forward for testing in phase 2.
Phase 2: retrieval, review and synthesis
Inclusion criteria and study identification
In phase 2, we undertook systematic searches to identify sufficient evidence to test and develop the three CMOs identified in phase 1. 39 As the literature on Namaste Care is limited, we widened the searches to include studies that drew on similar principles or approaches to Namaste Care. The rationale for this was that these offered opportunities for transferable learning and allowed us to test aspects of our programme theory, such as the mechanisms of action.
The inclusion criteria for studies were as follows:
-
All or some participants with advanced dementia. This included studies in which the definition of ‘advanced dementia’ was based on the authors’ reports and studies that provided more formal definitions or used measures such as the Mini Mental State Examination.
-
People living in a long-term care institution (e.g. a care home or a nursing home).
-
Interventions that drew on similar principles to Namaste Care or included components of Namaste Care (e.g. music therapy, massage, aromatherapy) and that offered opportunities for transferable learning. This included group-based or one-to-one interventions. Interventions could be delivered by care home staff or by external facilitators.
-
Published and unpublished studies of any design.
The searches focused on papers published in the last 10 years to reflect the rapid expansion of work and interest in the research area. We searched PubMed, Scopus and CINAHL and undertook lateral searching such as forwards and backwards citation tracking.
Search terms and dates are given in Box 1.
Run 24 April 2017, focused on elements of Namaste Care intervention such as massage, music, sensory stimulation) sensory[Title/Abstract] OR touch[Title/Abstract] OR senses[Title/Abstract] OR massage[Title/Abstract] OR namaste[Title/Abstract] OR music[Title/Abstract] OR smell[Title/Abstract] OR aroma[Title/Abstract]) OR (‘massage therapy’) OR (‘sensory stimulation’) OR (‘music therapy’) OR (‘therapeutic touch’)) AND ((‘dementia’) OR (‘alzheimers’) OR (‘end of life’) OR (‘palliative’) OR (‘coma’)) Filters: published in the last 10 years; Humans
PubMed search 2Run 26 April 2017, terms relating to person-centred care) ((‘person centred care’) OR (‘person centred care’[Title/Abstract]) OR (person centred care) OR ((‘biography’) OR (biography[Title/Abstract] OR biographical[Title/Abstract]))) AND ((‘residential care’) OR (‘nursing home’) OR (‘care home’) OR (‘residential home’))
Selection and appraisal of documents
Results of the searches were imported into bibliographic software. Two researchers independently screened the title and abstract of records and the full text of articles that appeared to be relevant. Papers were assessed for inclusion on the basis of whether or not they were considered ‘good enough and relevant enough’. 40,41 This was an ongoing process that involved discussion between research team members. ‘Good enough’ was based on the reviewers’ assessment of whether or not the research was of a sufficient standard based on the detail provided, the articulation of how the intervention worked and if the claims made were considered credible. Papers were judged to be relevant if it was felt that the authors provided sufficient information and/or theoretical discussion to contribute to the programme theories being tested. For example, although many studies were not focused on Namaste Care, they could still be included if they were felt to share an underpinning mechanism of action. 34 Studies that were poorly conducted could still be included if the relevance was high, for example if they contributed to our understanding about how a programme was thought to work. We tested for conceptual saturation through regular discussion among team members involved in data extraction. 42 For example, multiple studies drew on theories of biography and person-centred care as a rationale for the intervention and to explain how they worked.
Data extraction and synthesis
In the first stage of the review, we extracted information on how Namaste Care was interpreted and delivered, including the core components, and reported outcomes. In stage 2, we extracted information on study focus, participants, setting and intervention (including method of delivery and duration), how outcomes were measured and reported, and how underlying assumptions about the intervention were articulated. In a realist review, data are not restricted to outcomes measured or results reported but also include author explanations. For example, discussions can provide a rich source of ‘data’ that helps explain how an intervention was thought to work (or why it did not). Data were extracted into a Microsoft Access (Microsoft Corporation, Redmond, WA, USA) database and the ‘query’ feature was used to create tables enabling the identification of recurrent patterns of contexts and outcomes in the data and the possible mechanisms by which these occurred. 43 In addition, we created data tables to map the most commonly reported outcomes (e.g. agitation) against data on context, mechanisms and our programme theory.
Testing and refining programme theory
To enhance the trustworthiness of our programme theory, we discussed the CMOs at a second project team workshop (n = 7) and undertook a second round of stakeholder consultation. This consultation involved discussing the CMOs and it was conducted via telephone interviews (n = 1), face to face (n = 2) and by e-mail (n = 1). In addition, findings from the review were presented to, and discussed with, a group of end-of-life care specialists (n = 40) at a community of practice meeting organised by specialist end-of-life and dementia care organisations. Many of those attending had direct experience of Namaste Care. Stakeholders were from similar groups as in phase 1 (two people took part in both sets of consultations).
Results
Description of included evidence
Phase 1
In phase 1 we found 25 papers relating to Namaste Care, 18 of which provided sufficient information for theory development. The majority were descriptive accounts of Namaste Care rather than research studies. Of the seven research studies, three included some before-and-after data,22,44,45 three were qualitative46–48 and one (reported in three papers)27,29,49 used an action research approach. Only five studies21,22,27,44,45 presented data on resident outcomes. The seven research studies and one further Namaste Care study identified during the phase 1 searches50 were taken forward for inclusion in phase 2.
The core elements of Namaste Care, derived from the literature and stakeholder accounts, are:
-
the environment (e.g. calm, warm, scented, music, group setting, gentle lighting)
-
time (done every day, performed slowly, dedicated time for each person)
-
use of loving touch, which might include massage, hair care, skin care, tactile items
-
provision of food and drink
-
pain assessment.
In phase 2 we included 86 papers. The selection process for studies is summarised in Figure 1. Further details of the Namaste Care studies are listed in additional file 3 of Bunn et al. 32
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart detailing the selection of studies for the review.
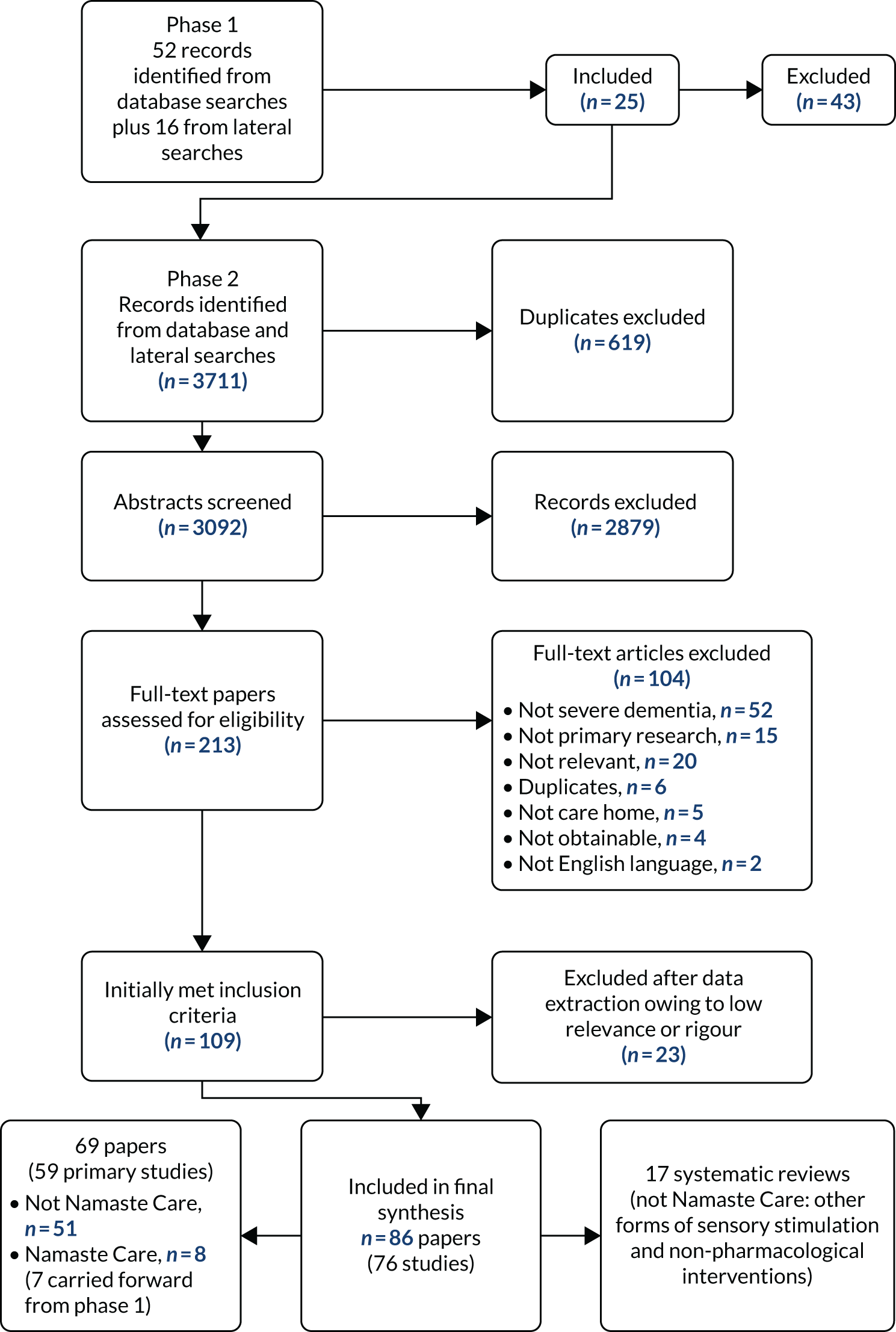
With respect to research design, the 86 papers reported:
-
59 papers reporting 51 primary studies (not Namaste Care)69–129
-
10 papers reporting 8 Namaste Care studies. 22,27,29,44–48,50,130
The designs of the 59 primary studies were:
-
24 randomised controlled trials (reported in 26 papers75–77,82,83,87,95,96,98–102,105,107–115,117,119,123,128,129)
-
four observational studies (reported in 10 papers69–74,84,86,116,126)
-
one action research study (reported in three papers27,29,130).
The remaining six studies78,80,92,104,109,127 used a variety of study designs, including retrospective and crossover.
Studies were conducted in a variety of countries including the USA (n = 1720,46,69–74,79,85,87,92,93,101,116,125,126), the UK (n = 1429,44,45,48,50,75–78,89,97,102,103,113), Australia (n = 947,88,99,100,104,114,121,127), Japan (n = 583,95,110,111,120), Spain (n = 396,109,112), Sweden (n = 384,86,106) and Taiwan (n = 3115,118,119). There were two studies in Canada,98,122 Portugal,80,81 Norway90,94 and Italy107,131 and one study in each of the Netherlands,123 France,105 Belgium124 and Ireland. 117 Studies were generally small, with 33 having < 50 participants.
Details of the interventions and how they were delivered
Studies covered a range of sensory and multisensory interventions (Table 1), with multisensory interventions and music therapy being the most common interventions included. Interventions were most commonly delivered by researchers or by outside facilitators such as music therapists. Care home staff were involved in delivering the intervention in only 1381,84,86,103,104,106,111,113,114,117,118,125,127 out of the 59 non-Namaste Care primary studies. Among Namaste Care studies, the programme was delivered by care home staff in six,44–47,50 by Namaste Care workers in two22,27 and by activity co-ordinators in one. 48
| Category | Primary studies (n) | Reviews (n) |
|---|---|---|
| Namaste Care | 822,27,44–48,50 | 0 |
| Multisensory | 2150,69–71,73,75,76,78–81,92–94,96,98,101,112,117,124–127 | 0 |
| Music | 1861,72,77,79,84,86,91,104,105,110,115,118,119,121,123,126,131 | 653,54,58,61,63,64 |
| Touch/massage | 1082,87,93,95,99,100,106,109,120,126 | 164 |
| Aromatherapy | 582,83,102,111,113 | 252,62 |
| Environment | 770,74,85,90,92,98,116 | 256,132 |
| Other (e.g. person-centred care, use of biography) | 644,59,89,103,114,122 | 555,57,59,60,68 |
The longest and most frequent sessions were reported in the Namaste Care studies, with several22,27,44 reporting that Namaste Care was delivered for 4 hours, 7 days per week.
Programme theory
Our review resulted in three CMO configurations that together provide an account of how and why Namaste Care might work for people with advanced dementia. These are presented in Figure 2 and described in the text below. Interventions were delivered by a variety of different occupational groups; we use the term ‘provider’ to encompass all of these.
FIGURE 2.
Summary of the programme theory.

Context–mechanism–outcome 1: Namaste Care provides structured access to social and physical stimulation
The programme theory is that care home interventions (e.g. Namaste Care) that provide regular and structured access to social and physical stimulation for residents with advanced dementia (C) give staff permission to engage with residents outside task-based care, and trigger responses such as familiarity, reassurance and trust in residents (M), creating a positive impact on resident behaviour and mood (O).
The evidence suggests that one of the most important aspects of programmes is how they enable meaningful relationships to form between providers and residents who are no longer able to communicate easily with speech and have other symptoms consistent with advanced dementia, for example by having the same person provide each session, by incorporating one-to-one interaction into an activity and by providers having the skills to work with people with advanced dementia. 50,64,77,84,92,96,106,121,123,131 By contrast, interventions involving providers who are unfamiliar to residents and/or do not have the appropriate skills99,100 or are unable to engage socially with people with dementia may be less effective. 127 Stakeholders at the workshop suggested that having the same person deliver Namaste Care was not always practical and that rather the aim should be to achieve a consistent approach and attitude towards programme delivery:
What they felt positive about was that they’d managed to create and access what they called a special atmosphere, an environment to practise Namaste Care.
Nam06
Social stimulation appears to be a particularly key component of interventions. In a series of studies, Cohen-Mansfield and colleagues69–74,116,126 evaluated a variety of stimuli for people with dementia living in care homes. They found that social stimulation, especially when it involved one-to-one interaction and the active participation of the resident, had the most dramatic effect on engagement and attention. 73 The importance of one-to-one attention and social stimulation was also highlighted in other studies96,105,113,123 and by stakeholders:
I think when Namaste [Care] really works is when you can create a space where people who are withdrawn can actually come out of that shell and can connect and make eye contact and maybe start to try and talk again.
Nam03
I think most of us are sort of social people, we don’t spend 24 hours a day in a room . . . by bringing them out into a shared space they’re in a room that’s set up for that, they’ve got people around them, and I think the staff there do have good connections with their residents.
Nam04
In Namaste Care, physical stimulation is provided through both the components of the intervention and the environment in which the programme is delivered. For example, scents and soft music were felt to be calming and soothing for residents. 22,27,46 Stakeholders supported this, suggesting that the right space could help overstimulated residents relax and that benefits were also seen outside the Namaste Care space:
We’ve had a lot of residents upstairs that are quite vocal and can be slightly aggressive during personal care and then that’s kind of improved over time with the Namaste and the whole calming atmosphere they’re not shouting out as much.
Nam02
Although some non-Namaste Care studies referred to the importance of environment (e.g. having a private space or a quiet room), in many studies the space was not described. However, studies92,97 did identify practical as well as therapeutic benefits to having a designated space, suggesting that sessions were less likely to be cancelled because of competing priorities and that activities could take place as and when needed by the residents. However, although space might be an important context, it is unlikely to trigger staff engagement without additional resources such as the allocation of time and management support. A study127 evaluating the use of a sensory room for people with dementia (Snoezelen®; www.snoezelen.info) found that staff missed sessions because they did not see them as a priority. Stakeholders suggested that for Namaste Care to be achievable and adopted as a core part of the work of the care home, it was important that staff were given permission, through the appropriate allocation of time and resources, to engage with it:
I think that’s a really big thing that Namaste adds is that there’s like structured time to really pay attention to the residents and yeah, and give them that extra time, and also the opportunity to make contact with the residents.
Nam09
I think it encourages them to work together and it encourages them and gives them permission to find space for their residents . . . and the fact that everybody’s doing it makes it acceptable within the care home, it’s not like somebody’s taken an hour out of their day to try and spend time with a resident and everybody else is saying you should be working, everybody’s doing a similar thing.
Nam04
The originator of Namaste Care suggests that it should be delivered twice per day, 7 days per week. 20 Three non-randomised studies22,27,44 reported that Namaste Care was delivered in this way, and this was endorsed by some stakeholders who saw it as important in normalising the approach within the day-to-day work of the care home:
When you see proper results is when it’s a programme, when it happens 7 days a week and before and after lunch and that involves a huge change in the culture of the care home.
Nam03
However, some stakeholders suggested that this intensity was unlikely to be feasible in most care homes in the UK. From the non-Namaste Care studies we found little empirical evidence on the optimal ‘dose’ of sensory interventions such as Namaste Care, although a meta-analysis53 of music therapy found that sessions provided twice per week had a more statistically significant impact on disruptive behaviours, anxiety and mood than weekly sessions.
There was little evidence on the benefits of group versus one-to-one delivery. A meta-analysis53 of music therapy for people with dementia found that group therapy had more positive effects on disruptive behaviours and anxiety than individual therapy. However, this analysis did not distinguish between those with and those without severe dementia. One randomised controlled trial115 found that group music therapy was more effective for residents with mild and moderate dementia than for those with severe dementia, and another123 suggested that it was more difficult to achieve therapeutic goals if the ratio of participants to therapist was too high (e.g. five residents to one therapist).
Context–mechanism–outcome 2: equipping staff to cope effectively with complex behaviours and variable responses
Programme theory: interventions that include a ‘toolkit’ of multisensory activities equip staff to work effectively with residents with complex behaviours and variable responses, leading to improvements in resident outcomes (e.g. reduced agitation) through triggering responses such as engagement and connection between residents and carers.
The use of ‘loving touch’ is perceived to be key to Namaste Care, with touch thought to evoke an emotional response that leads to physical engagement. 47 We found some evidence to suggest that touch (such as hand massage) can have a calming effect,95 reduce behavioural symptoms,69,120 improve sleep87 and increase engagement. 93 Hand massage may be more effective than simulated social intervention (e.g. holding a doll) because the intervention combines one-to-one social interaction with sensory stimulation. 116
Stakeholders also reported that Namaste Care had a positive impact on relationships and resident behaviour:
Our residents are calmer and eating more, we’ve got residents talking that never used to talk, we’ve got really anxious residents that would be constantly calling for somebody that will actually sit in there for an hour and not call out once . . .
Nam01
Music also appears to trigger emotional responses in people with advanced dementia. There is evidence that receptivity to music can remain until the late stages of dementia. 58 Primary studies73,86,97,107 and reviews53,58 reported that music therapy improved communication and connection, increased engagement and reduced agitation. 84,86,97,131
There is some evidence to suggest that the most effective interventions are those that equip care staff to cope effectively with the complexity of caring for people with advanced dementia. A systematic review64 of interventions to reduce agitation in people with dementia found that the complexity of behaviour associated with dementia required a multifaceted response that could be tailored to the needs of individuals. Stimulating a range of senses may be particularly important for people no longer able to verbalise,50 and as cognitive function deteriorates people with dementia can become very sensitive to sensory experiences. 78,81 Stakeholders talked about the impact of Namaste Care on care home staff and the way in which the staff perceived people with advanced dementia:
I think watching staff I think what you see is that they realise that this person that may be end of life, they may have really quite advanced dementia but we’re still reaching them . . . they’re still living, they’ve still got all the things that we have, we just need to find it in a different way and I think that changed people’s perceptions.
Nam01
The multisensory nature of an intervention such as Namaste Care means that staff have a range of activities to draw on, giving residents a choice of what is delivered and access to different stimulation (touch, auditory, olfactory and visual). 50,64,75,78,79 The assumption from this evidence is that it is the combined effect of being able to use a range of activities that triggers staff capacity and ability to respond to residents’ symptoms and behaviours, and not individual activities.
Context–mechanism–outcome 3: providing a framework for person-centred care
Programme theory: multisensory interventions that focus attention on residents’ individual biographies and that attempt to connect with residents’ reality make staff more responsive to residents’ needs and lead to improvements in resident outcomes (such as increased responsiveness).
Many studies20,60,61,64,100 suggested that, because people respond differently to the same stimuli, interventions need to be tailored to the needs of the individual. Aspects of interventions that needed to be tailored included the environment,133 the music played,84,110,115,119 the aromas used,82,83,111,124 the way someone was touched99,100,106,113 and how someone was spoken to. 93,121 It was also seen as important to consider people’s known habits and preferences, the stage of dementia that people presented with and whether or not their current preferences may be different from their previous habits. 93,97 How this might work in practice, where the intervention creates a heightened awareness of the individual’s preferences, is illustrated in this quotation from a stakeholder:
While you’re doing it and while you’re observing it you notice things that actually that might not work so well for that person . . . so it’s about thinking about your residents and how they’re changing and what we need to do to keep people involved.
Nam01
Using past and current preferences to tailor interventions was reported to reduce agitation64,69,114 and increase alertness or engagement. 72 There was also evidence (although this was largely qualitative or anecdotal) that personalised interventions made staff notice more about residents and their abilities,27,29,50,93 leading to improved communication between staff and residents,61,68,77,84,127 the development of trusting relationships between residents and caregivers89,92,106 and a shift towards a more person-centred culture of care:29,46
You’ve got to have fundamentally good nursing care and the staff need to have good dementia care training as well . . . but what I think Namaste [Care] does is to make it real for them, you know, it makes the person-centred care real for them and it then feeds into the basic care that they’re giving.
Nam03
In our original programme theory, we hypothesised that Namaste Care would have benefits for family members, either through better connection with their family member with dementia or through improved communication with staff. Few studies measured outcomes for family members, although there was some anecdotal and qualitative evidence that Namaste Care improved connections between family members and residents44,48,50,85,93,94 and between relatives and care home staff,44,46,50 for example as described by this stakeholder:
Very often I think relatives don’t see the positive relationship that carers have because when you’re visiting somebody the staff step back, it’s a visit and they just turn up and say, we need to change your mum or it’s lunchtime, will you feed her or shall I or it’s time we changed her for bed or whatever and so it’s very tasky but in Namaste the family actually see the efforts to get somebody to respond and the positive stuff.
Nam03
Conclusions
This realist review provided a coherent account of how Namaste Care, and other multisensory interventions, might work. The evidence on Namaste Care is currently limited, but we drew on a wider literature to test the evidence from a range of studies looking at sensory stimulation and implementation in care homes. The findings from the review were used to develop the Namaste Care intervention delivered in phase 2 and described in Chapter 3. The review also provides practitioners and researchers with a framework to judge the feasibility and likely success of Namaste Care in long-term settings. The proposed theoretical account of what works, why and in what circumstances is not final. As further relevant evidence emerges, it will be refined, challenged and developed further. Nevertheless, it is reasonable to conclude that the key mechanisms that Namaste Care triggers for residents are feelings of familiarity, reassurance, engagement and connection, and that for staff it gives them permission and awareness to engage with residents in a more person-centred way.
Chapter 3 Design and methods: intervention development, cluster randomised controlled trial and process evaluation
The Namaste trial is a cluster randomised controlled trial undertaken to establish if it is feasible to undertake such a trial in nursing homes and if it is possible for the intervention to be implemented as prescribed. The trial protocol134 was reviewed and published in BMJ Open in 2018.
Study design
This was a cluster randomised controlled trial with a non-blinded outcome assessment. The eight clusters were randomised 3 : 1 to the intervention or usual care arm. A cluster trial was chosen, with the nursing home defined as a cluster. This ensured that there was no contamination between the comparator with the test intervention. A qualitative process evaluation and economic analysis ran alongside the trial element of the study, preceded by a process to develop the Namaste Care intervention.
Study setting
The trial was conducted in eight nursing homes, where on-site nursing was available 24 hours per day. This setting was chosen rather than all care homes because it was necessary to have nursing oversight of the intervention delivery and components of it, for example comfort assessment.
Nursing home eligibility
The inclusion criteria were specified in the protocol. 134 The main criteria for participation were that the facility had more than 30 beds (to ensure that there were enough residents for inclusion in the study) and that it was currently engaged with palliative care delivery, as evidenced by involvement in established palliative care programmes. The facility also needed to be able to identify a space that could be dedicated to deliver the intervention. Facilities were not recruited if they had a Care Quality Commission (CQC) rating of ‘needs improvement’ or ‘inadequate’. They were excluded on these grounds because sites addressing quality issues would not necessarily be in a position to engage in research and the change that this requires.
Participants: eligibility criteria
The specific inclusion and exclusion criteria for residents, informal carers and nursing home staff are presented in the protocol. 134
People with dementia
Residents were included in the trial if they lived permanently in the nursing home (and were not present to receive respite or day care). The intervention addresses the needs of people with advanced dementia, so participants needed to have had a formal assessment of advanced dementia based on having a FAST score of 6 or 7. 2 A FAST score of 6 or 7 indicates a need for assistance with personal care and urinary and faecal incontinence, and a higher score reflects reduced mobility and a reduced ability to speak. This was assessed by the nursing home manager or another experienced member of staff. However, residents who were bedbound and unable to leave their room to join the group were not eligible to participate. All participants lacked mental capacity (as assessed and documented with the capacity assessment process in use within each site). As the study relied on proxy data collection, each resident needed to have a key worker member of staff available who was willing to provide proxy outcome data.
Informal carers
Informal carer participants were recruited if they were > 18 years, and self-identified as the relative or friend who acted as an informal carer for a participant included in the study. The informal carer could be, but was not necessarily, the person acting as personal consultee.
Nursing home staff
All nursing home staff, including managers, nurses, care assistants and activity co-ordinators, who were paid to provide care were eligible to participate.
Recruitment
Nursing homes
Recruitment of the cluster nursing homes was undertaken between August 2017 and November 2017. Commitment to participation from one small provider chain prior to the award of funding led to two nursing homes from this group participating. A wider search for participating nursing homes was undertaken using CQC databases and local ENRICH (Enabling Research in Care Homes) network contacts.
People with dementia
Senior staff in the nursing home were asked to identify residents who met the inclusion criteria. As all participants lacked mental capacity, the process of recruitment and consent involved personal consultees or, if no personal consultee was available or responded, a nominated consultee was identified.
Informal carers
Eligible informal carers of residents participating in the trial were identified by the nursing home manager or a senior staff member. This person was not necessarily the informal carer who had acted as a personal consultee for the resident.
Nursing home staff
Information about the study was distributed to all nursing home staff in information packs and at staff meetings.
Data collection
Data collection was undertaken at baseline and at 2 weeks, 4 weeks and monthly until 24 weeks (and post bereavement, if appropriate) using five methods: questionnaires, observation, interviews (individual and group), completion of a session activity log and use of an ActiGraph device (Activinsights Ltd, Kimbolton, UK).
Person with dementia measures
Demographic and clinical characteristics
For residents, data on their age, sex, ethnicity, existing medical conditions and stage of dementia (assessed using the FAST2) were collected at baseline.
Potential outcomes for main trial
We considered two contender primary outcomes: (1) quality of dying (dementia) [using the Comfort Assessment in Dying – End of Life Care in Dementia (CAD-EOLD)]16,135 and (2) quality of life [using the Quality of Life in Late Stage Dementia (QUALID)]136 (Table 2). For people with advanced dementia living in nursing homes, both quality of life and quality of dying are important outcomes. It is also not always clear which is the most appropriate outcome to measure for people living and dying with advanced dementia. Although this was designed as an end-of-life study, it was not clear if the population with advanced dementia who were eligible to receive the Namaste Care intervention would die during the study. Consequently, one of the feasibility aims was to see whether or not an outcome measure about quality of dying would be appropriate for a full trial.
| Outcome measures or rationale for data collection | Time point of data collection | ||||
|---|---|---|---|---|---|
| Baseline of the individual resident taking part in the study | 2 weeks (after the individual resident has the first Namaste Care session) | 4 weeks (after the individual resident has the first Namaste Care session) | Every 4 weeks up to 24 weeks (after the individual resident has the first Namaste Care session) | 24 weeks (after the individual resident has the first Namaste Care session) or following death | |
| Resident demographicsa | ✗ | ||||
| Quality of dying (dementia) (CAD-EOLD)a | ✗ | ✗ | ✗ | ✗ | ✗ |
| Quality of life of the person with dementia (QUALID)a | ✗ | ✗ | ✗ | ✗ | ✗ |
| NPI-Qa | ✗ | ✗ | ✗ | ||
| Pain (PAIN-AD)a | ✗ | ✗ | ✗ | ||
| EQ-5D-5La | ✗ | ✗ | ✗ | ||
| ICECAP-SCMa | ✗ | ✗ | ✗ | ||
| ICECAP-Oa | ✗ | ✗ | ✗ | ||
| CMAIa | ✗ | ✗ | ✗ | ||
| Sleep/activity (actigraphy) | Ongoing for 28 days | ||||
| Think-aloud tools (ICECAP-O and ICECAP-SCM)b | ✗ | ✗ | ✗ | ✗ | |
| Resource use (primary and secondary care)c | ✗ | ✗ | ✗ | ✗ | ✗ |
We chose 4 weeks as a primary end point because we wanted to include as many participants as possible in the analysis and recognised that, in this frail and ill participant group, intervention effects needed to be rapid to be meaningful. Attrition due to death is a limiting factor in the successful completion of studies that involve participants with advanced disease. 137 Hence, using early end points is recommended. 138 In previous work evaluating Namaste Care, early deaths (< 2 months) were not uncommon. 139 Benefits from the intervention have been reported within days,139 so by recording an early assessment at 2 weeks and 4 weeks some record of temporal change was made. Missing data and attrition were also likely to be an issue and so having an early measure of 2 weeks could be used to impute missing data.
Secondary outcome measures
A number of secondary outcome measures were also included, chosen because of their potential to measures changes in outcomes that reflect different dimensions of the Namaste Care intervention (see Table 2).
Resident measures
Secondary outcome measures for residents included physical and psychological symptoms [using the Neuropsychiatric Inventory – Questionnaire (NPI-Q)],140,141 pain [using the Pain Assessment in Advanced Dementia (PAIN-AD)]142 and agitation [using the Cohen-Mansfield Agitation Inventory (CMAI)]143 (see Table 2). Sleep and activity were measured using actigraphy, which we hoped offered an objective way to assess outcomes that would support the proxy reporting used for the other measures. Residents wore an ActiGraph device for 28 days to measure their sleep levels and patterns and activity.
Informal carer measures
Informal carer measures were used to collect proxy data about residents’ sociodemographic characteristics and satisfaction with care [Satisfaction With Care – End of Life in Dementia (SWC-EOLD)] (Table 3), as well as economic data, including quality of life (see Chapter 6).
| Outcome measures or rationale for data collection | Time point of data collection | |||
|---|---|---|---|---|
| Baseline of the individual resident taking part in the study | 2 weeks | 4 weeks | 24 weeks or following death | |
| Informal carer demographics | ✗ | |||
| SWC-EOLD | ✗ | ✗ | At least 8 weeks after death | |
| ICECAP-CPM | At least 8 weeks after death | |||
| EQ-5D-5L | ✗ | At least 8 weeks after death | ||
| Think-aloud tool (ICECAP-CPM) | At least 8 weeks after death | |||
| Resource use information (Client Service Receipt Inventory) | ✗ | |||
Nursing home staff measures
Nursing home staff completed a form that recorded their sociodemographic details and work characteristics.
Nursing home organisational measures
Organisational measures were collected to provide contextual data for the findings with respect to retention and the impact on staff. This included person-centredness [the Person-Centred Care Assessment Tool (P-CAT)144] and organisational readiness for change (Alberta Context Tool145). Nursing home staff measures and nursing home organisational measures were collected from all staff on duty on the day of the baseline visit.
Intervention
The intervention is a programme of care (Namaste Care) delivered in the intervention care homes by care staff working in the facility. It requires implementation at the organisational (cluster) level and also with individual residents (participants). The following description uses the TIDieR (Template for Intervention Description and Replication) guidelines for intervention description (items 1–9). 146
Namaste Care seeks to give comfort and pleasure to people with advanced dementia through engagement, meaningful and creative activities as well as sensory stimulation to reflect the resident’s ‘life story’. 20 Core elements of the intervention were considered to be that:
-
The Namaste Care sessions should be undertaken within a designated space in the nursing home. This space could be in a room that is used for other purposes (e.g. a dining room), but at specified times it is to be used only for the Namaste Care session.
-
The environment of the designated space must be made ‘special’. It should enable a feeling of calm (i.e. welcoming and homely, with natural or slightly dimmed lighting, perhaps attractive scents, such as lavender from an aromatherapy diffuser, and with soft music playing).
-
The Namaste Care sessions should be undertaken in a group setting.
-
Food snacks and drinks should be offered to the residents throughout the session.
-
A minimum of two nursing home staff members or volunteers should be present to run the Namaste Care sessions.
-
The duration and frequency of Namaste Care delivery as proposed by its originator (2 hours per day, twice per day, 7 days per week) was promoted, but flexibility in this was allowed as part of the feasibility objectives. 20
Namaste Care champions were appointed in each nursing care home in the intervention arm. At least two care staff (registered nurses, care assistants or activity co-ordinators) attended a 1-day workshop about Namaste Care, led by an experienced external facilitator. A follow-up training session was held at each nursing care home to train more staff and provide advice on preparing the Namaste Care space. Nursing homes were given a copy of the Namaste Care guide developed by the research team.
Intervention development and refinement
Developing, refining and clearly specifying the Namaste Care intervention to ensure that it was suitable to use in the feasibility trial was essential for a number of reasons, including training, understanding fidelity, ascribing outcomes to the intervention if a full trial was deemed feasible, and appropriate implementation. 146 Following the realist review of the literature (see Chapter 2), the components of the intervention were mapped onto the identified components (Table 4).
| Key element | Present in revised Namaste Care intervention specification |
|---|---|
| Importance of activities that enabled development of moments of connection for people with advanced dementia |
Principle outlined in Namaste Guide Multisensory activities outlined to address taste, smell, sound, sight and touch Relational care and working with family and friends present in guide |
| CMO1: providing structured access to social and physical stimulation |
Identified space Regular sessions once or twice per day up to 7 days per week Multisensory activities provided |
| CMO2: equipping care home staff to cope effectively with complex behaviours and variable responses |
Training – off-site and on-site Comfort assessment present in training and guide |
| CMO3: providing a framework for person-centred care |
Family conference to explore individual life story and preferences Identification of person-centred interests and activities |
Four iterative stages of intervention refinement were then followed, incorporating co-design of the intervention description with nursing care home staff and family carers. First, existing materials used to support Namaste Care were gathered and, together with the review results, a draft intervention description and guide were collated. Second, these materials were explored with nursing care home staff new to Namaste Care, but outside the trial homes. Third, modified nominal group techniques were used with nursing care home staff, volunteers and family carers with experience of Namaste Care in practice to refine, prioritise and re-present the information in a format suitable to be used with nursing care home staff. Fourth, our public involvement panel were involved (see Chapter 7) in final refinement of the materials.
This stage of the study was conducted with the approval of Lancaster University Faculty of Health and Medicine Research Ethics Committee (17 November 2016/FHMREC16028), and written consent was obtained from all participants.
These stages are briefly described:
-
Approaches were made to 69 organisations (2 NHS trusts, 11 hospices, 56 nursing/care homes) known to already use Namaste Care in practice. Materials received from three organisations, together with the Namaste Care book,147 were used in the preparation of a draft intervention and implementation description and guide, prioritised using findings from the realist review. The design of written materials was guided by best evidence on writing manuals and guidelines. 148–154
-
Nursing and support staff (n = 3) from two nursing care homes that had not used Namaste Care participated in a 2-hour workshop to discuss the emergent intervention guide. They amended the wording to suit a UK situation, and advised on the addition of colour-coding and infographics to aid use of the guide in practice.
-
Two consensus workshops were held with those who had experience of Namaste Care in practice in nursing care home settings (n = 17 staff, volunteers, family carers). We used modified nominal group methods including exposure to stimulus materials (realist review and emergent guide from stages 1 and 2) and silent generation of ideas, and then held a round-robin and group discussion to clarify and rank elements of the intervention. 155–158 This resulted in the addition of a section to the guide, further shortening of the guide booklet and better specification of some elements, such as intervention timing, frequency, focus and staffing. Participants also helped identify potential adverse events that may be associated with the intervention.
-
Finally, the materials were presented to the study public involvement panel, who clarified wording and recommended changes to the colours of the infographics to enhance their readability.
These changes are summarised in Table 5. The final study guide and infographics used to support the study are in Report Supplementary Material 1. 159
| Stage | Content | Presentation |
|---|---|---|
| 2: workshop (no experience of Namaste Care) |
Need for a brief overview document Materials for family members Use of graphics (infographics and images) Colour-coding of sections Wording anglicised |
|
| 3: consensus workshops (experience of Namaste Care) |
Further section, ‘preparing people and organisations for Namaste Care’, added Further detail provided on intervention timing, frequency, focus and staffing requirements Relational and philosophical aspects of the intervention emphasised Identification of potential adverse events incorporated |
Renaming the materials as a ‘guide’ rather than a ‘manual’ Guide shortened to 16 pages Guide materials to be used as basis of formal training |
| 4: PPI consultation |
Clarification of wording Recommended changes to the colours of the infographics to enhance readability |
Usual care
Usual care is the term used to describe the control arm of this trial. This is the usual care provided in a nursing home for people with dementia that addresses the key components of good palliative care practice. The study team provided no further education, training or support on care to the nursing homes in the control arm of the trial.
Outcomes for a full trial
To decide whether or not a full trial would be feasible, a number of criteria were identified before the start of the study (Table 6).
| Indicator | Data source | Achieved if |
|---|---|---|
| Recruitment rate | Researcher records | Six residents per care home recruited |
| Attrition rate | Researcher records | No more than two residents per care home cease receiving the intervention because of practical or preference issues |
| Number of Namaste Care sessions delivered by nursing home staff in 1 week | Nursing home staff completed Namaste Care pro forma | At least 7/14 sessions held per week (50% per week) |
| Average length of Namaste Care session | Nursing home staff completed Namaste Care pro forma | Average length is 1.5 hours |
| Potential primary outcome data completion | Complete CAD-EOLD and QUALID questionnaires | 80% of residents participating in the study have CAD-EOLD and QUALID questionnaires completed for them |
| Namaste Care intervention acceptability to staff and family |
Interviews (family) Focus group (staff) Nursing home staff completed Namaste Care pro forma |
Intervention described as acceptable in terms of components of care provided, timing and frequency of delivery |
| Namaste Care intervention suitable for UK nursing home environments |
Interviews (family) Focus group (staff) Observation of care delivery |
Intervention described as being suitable for this context |
| Identification of a sufficient pool of nursing homes that reflect nursing home diversity and that would be willing to participate in a full trial | ENRICH network data; CQC database | Identified a pool of nursing homes willing to participate in a future trial that exceeds the proposed sample required for a future trial |
Process evaluation
A robust process evaluation was undertaken to ensure the capture of data that directly addressed the feasibility objectives and addressed the acceptability, fidelity and sustainability of the intervention. This evaluation identified factors that influenced the implementation of the Namaste Care intervention in the context of a cluster randomised controlled trial to enable a full trial to be planned. In designing the process evaluation, we drew from a framework for designing process evaluations for cluster controlled randomised trials of complex interventions,160 and descriptors of components of process evaluations. 161 The process evaluation also provided key information to add to the programme theory developed in phase 1 in relation to how and why Namaste Care might work as a complex intervention. 162
The process evaluation involved interviews, observation of the Namaste Care intervention being delivered by care staff and the completion of activity logs. At the start of the study, individual semistructured interviews were conducted with managers. At the end of the study, individual interviews were conducted with informal carers and focus groups were held with care home staff. We assessed perceptions of Namaste Care or usual care, and the fidelity, acceptability and appropriateness of Namaste Care or of usual care. We also assessed the fidelity, acceptability and appropriateness of Namaste Care (intervention arm) or assessed the activities used in usual care (control arm) through observation in each site at 2, 4 and 24 weeks. We also asked staff to complete an activity log for each Namaste session.
Informal carer interviews
Interviews with informal carers were undertaken to assess the informal carers’ perceptions of Namaste Care (intervention arm) or informal carers’ perceptions of the activities offered in usual care (control arm). Interviews were conducted approximately 16–24 weeks after the first resident was recruited at the nursing home. The informal carer could also be interviewed if their relative died, but to reduce distress this would be at least 8 weeks after the resident’s death, as specified in the ethics application.
Manager interviews
The managers of all eight intervention and control nursing homes were invited to take part in an interview before the first resident was recruited in their nursing home. The aim was to explore their organisation’s readiness for change and the context of care. Demographic contextual data about each nursing home were also collected.
All eight managers agreed to take part in an interview, and the interviews took place in the study nursing homes. The interviews were audio-recorded and transcribed; the length of the interviews ranged from 15 to 40 minutes. The topics included in the intervention arm interview were based on the criteria for readiness for change identified by Goodman et al. 163 that addressed aspects of the care home’s readiness to take on new interventions and the fit between current care approaches and Namaste Care. The interview in the control arm covered the same topics but questions were asked in a more general way without reference to Namaste Care.
Nursing home staff interviews
Group interviews were held in each participating nursing home at the end of the study to assess staff members’ perceptions of Namaste Care (intervention arm) or perceptions of the usual care activities (control arm).
Structured observation
To assess the fidelity, acceptability and appropriateness of Namaste Care, observation of the Namaste Care sessions was undertaken at 2, 4 and 24 weeks after the first resident was recruited in the intervention homes. Observation was conducted in the control homes at 2 and 4 weeks to assess the delivery of ‘usual’ care. In control homes, the researcher observed the residents in communal spaces where ‘usual’ care activities were taking place. A structured schedule that reflected the core components of the intervention was used in both control and intervention homes, as were field notes. Observation was undertaken for up to 20 minutes at the beginning, middle and end of the sessions, and audio-recordings were made to ensure that all verbal data were captured.
Session activity log
An activity log was completed by the staff delivering the Namaste Care session at each session to assess the fidelity and appropriateness of the Namaste Care (intervention arm only).
Health economic analysis
Economic assessments combined qualitative assessments of feasibility of use for the outcome measures gained through the use of think-aloud techniques and more quantitative assessments of agreement between proxies, and assessments of construct validity for the measures164 (see Chapter 6 for further detail).
Adverse events
During the study there was a relatively high risk of death and hospitalisation and an expectation of progression of disease for participants. These were not anticipated to be related to the receipt of the intervention. These types of events were not treated as adverse events or serious adverse events as they were not unexpected in this resident population. These were to be reported only if concern was raised by anyone associated with the study that death, hospitalisation or any other medical occurrence were directly related to study participation.
As this was a feasibility study, any events reported to any personnel involved in the trial (including health professionals, informal carers or research team members) that were considered adverse events were noted on a trial event recording form, which was completed by or returned to the trial manager and/or chief investigator. The trial manager or chief investigator would investigate the event with the person who reported it, and other involved individuals, and then take appropriate action following standard operating procedures aligned with the clinical trials unit policies.
Quantitative analysis
As this was a feasibility trial, the main purpose was to undertake a descriptive statistical analysis based on the full trial indicators to see if it would be feasible to undertake a full trial. The analysis was not undertaken to determine the effect of the intervention. Analysis of the outcome data focused on recruitment, response and completion rates, and missing data. Reasons for non-consent and missing outcome data were reported. Primary and secondary outcomes at baseline and follow-up were summarised using descriptive statistics [mean, standard deviation (SD), interquartile range]. Intracluster correlation coefficients (ICCs) were calculated for a definitive trial design.
The sleep/activity data from the ActiGraph device were an important element of this study. The search for an objective measure that provides clinically meaningful data for this population, among whom there is heavy reliance on proxy data, is ongoing. The acceptability of the ActiGraph device was a key question, but also of importance was the nature of the data and how they shaped the analysis of the different variables (Table 7) such as sleep–wake ratios, total sleep time, sleep efficiency, wake after sleep onset and total activity, alongside rhythm fragmentation and synchronisation.
| Term | Explanation |
|---|---|
| Sleep–wake ratio | Ratio of total sleep time to time awake |
| Total sleep time | Refers to total time of periods of inactivity (inferred to be sleep) within the sleep period time |
| Sleep efficiency | Ratio of total sleep time to time in bed |
| Wake after sleep onset | Periods of wakefulness occurring after defined sleep onset |
| Rhythm fragmentation; intradaily variability | Frequency and extent of transitions between periods of rest and activity on an hourly basis. Quantifies how fragmented the rhythm is relative to its 24-hour amplitude; more frequent alterations between an active and an inactive state lead to a higher intradaily variability |
| Rhythm synchronisation; interdaily stability | Quantifies the rhythm’s synchronisation, the stability of the rhythm or the extent to which the profiles of individual days resemble each other |
A participant’s rhythm fragmentation and synchronisation were estimated using intradaily variability and interdaily stability. Intradaily variability quantifies the frequency and extent of transitions between periods of rest and activity on an hourly basis. Interdaily stability quantifies the extent to which the rhythms synchronise to Zeitgeber’s 24-hour day–night cycle. 165,166
Qualitative analysis
Interviews
All interviews (manager, informal carer, nursing home staff) were recorded, transcribed and anonymised. Framework analysis was used to analyse the transcripts,167,168 aided by using the qualitative analysis package NVivo 11 (QSR International, Warrington, UK).
Observation analysis and session activity log analysis
Definitions were developed for the core components of the Namaste Care session to ensure that researchers applied the level of agreement scores consistently during analysis. The data from the observation forms and recordings were compared with those from the relevant Namaste Care activity logs to analyse the extent of agreement between the different types of data. This comparison was initially carried out independently by two researchers and then cross-checked to ensure that definitions had been applied consistently across all of the nursing homes. Any discrepancies were discussed and advice sought, as appropriate, from a senior researcher in order to reach agreement.
Sample size
The sample size of eight nursing homes (six intervention and two control) with eight residents per cluster was selected as it offered a reasonable test of the intervention to assess the feasibility objectives. The numbers of residents in feasibility studies have ranged from 2128 to 6169 to 14. 170
Randomisation
The eight nursing homes were randomised to either the intervention arm or the control arm by assigning an ID to each nursing home and then randomly selecting each ID. The random allocation was carried out by a statistician who was employed by the Clinical Trials Research Centre at the University of Liverpool and not otherwise involved in the trial. A one-off computer generated randomisation procedure was used.
Blinding
The nature of the intervention and its delivery meant that it was not possible to blind nursing homes or staff to the allocation status. When possible, to minimise potential for bias, staff involved in the delivery of the Namaste Care intervention were not involved in completing the outcome measures. However, in practice the staff available on the day of data collection were, at times, the staff who had delivered Namaste Care sessions. It was also not possible to blind researchers to the allocation of nursing homes, as the intervention required changes to the nursing home environment that were visible to any researcher visiting the facility. Statisticians carrying out the analysis were blinded as to which data were from control sites and which were from intervention sites.
Ethics
The trial was approved by the Wales Research Ethics Committee 5 Bangor Research Ethics Committee (reference number 17/WA/0378) on 22 November 2017.
Changes to the protocol
Over the course of the whole trial, one amendment was submitted to the research ethics committee (22 February 2018; approval received 6 March 2018). Amendments consisted of changes to clarify the process of staff recruitment, information on how the think-aloud interviews would be conducted and a new format for questionnaire presentation.
Chapter 4 Results of cluster randomised controlled trial
The primary objective of this feasibility study is to ascertain the feasibility of conducting a full trial of the Namaste Care intervention. The feasibility aims of the research design and data collection processes to enable the design of a full trial to determine the efficacy of Namaste Care are:
-
to understand how best to sample and recruit nursing homes into a cluster randomised controlled trial of Namaste Care
-
to establish recruitment, retention and attrition rates at the level of the nursing home and individual resident, informal carer and nursing home staff
-
to determine the most appropriate selection, timing and administration of primary and secondary outcome measures for a full cluster randomised controlled trial of Namaste Care against criteria of bias minimisation, burden and acceptability
-
to assess the acceptability, fidelity and sustainability of the Namaste Care intervention
-
to establish the willingness of a large number of nursing homes representing the range of nursing homes, with respect to provider type, size and resident care needs, to participate in a full trial.
Sampling and recruitment of nursing homes
The recruitment of the nursing homes used the inclusion criteria outlined in Chapter 3.
Thirty-six facilities were approached in the north-west of England (Figure 3); nine were recruited, and eight consented to be included in the trial. During the funding application process, a regional not-for-profit provider committed to be involved in the study and provide nursing home sites for the study. However, the senior management commitment did not result in nursing homes meeting the inclusion criteria, and only two facilities participated. Engagement with ENRICH and the Clinical Research Network was key to identifying nursing homes that met the inclusion criteria and were interested in participating in a research study. A major reason why nursing homes did not participate was the difficulty of speaking to a manager about the study.
FIGURE 3.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram: summary of recruitment and follow-up.

The time taken to recruit eight nursing homes was 41 weeks, from initial contacts in August 2017 to the first baseline visit in May 2018. The average number of days from first contact to the baseline visit across the eight sites was 241 days (34 weeks), with a range of 193–255 days (Table 8). The varying lengths of time required for recruitment reflected differences in how long it took for the clinical trial agreement to be signed by the nursing home managers. Randomisation could not occur until all of the eight nursing homes had signed the agreement, thereby delaying study commencement. Delay in managers signing the form resulted from their unfamiliarity with that type of documentation, which is widely used in the NHS. This lack of knowledge needs to be taken account of by the research team as they support managers to participate.
| Length of time for nursing home recruitment | Nursing home | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n01 | n02 | n03 | n04 | n05 | n06 | n07 | n08 | Mean | |
| Number of days between initial contact and contract being sent out | 96 | 104 | 97 | 22 | 99 | 44 | 27 | 21 | 63 |
| Number of days between contract being sent out and signed by the nursing home manager | 27 | 5 | 22 | 51 | 7 | 26 | 20 | 7 | 21 |
| Number of days between contract being signed and randomisation | 34 | 56 | 39 | 10 | 54 | 35 | 41 | 48 | 40 |
| Number of days between randomisation and baseline visit | 114 | 120 | 98 | 113 | w | 137 | 105 | w | 115 |
| Total number of days from initial contact to baseline visit | 271 | 285 | 256 | 196 | N/A | 242 | 193 | N/A | 241 |
Telephone and face-to-face support was required by the study team to facilitate the signing of the clinical trial agreement between the university and the individual nursing homes.
Learning for a future trial
-
Ensure that enough time is required for the necessary agreements to be in place so that randomisation can occur promptly and this should be factored into study planning.
-
The study design should allow the study to commence as nursing homes consent, rather than waiting for all sites to be ready (randomisation in blocks of four nursing homes).
-
Research support is required for nursing home managers agreeing to participate to facilitate contracting and governance.
Recruitment, retention and attrition of participants
Cluster: nursing home
Six out of the eight clusters (nursing homes) (four in the intervention group and two in the control group) remained in the study for full analysis. The withdrawal of two nursing homes after randomisation and before the start of resident recruitment was a result of staffing issues (the resignation of a manager) and start delays (that led a manager to decide that the intervention would not work for the residents in that care home). Once the trial had commenced, no nursing homes withdrew.
Learning for a future trial
Once nursing homes consented and the study commenced, retention was excellent, but there is a need to shorten the period between engaging with nursing homes and starting the study.
Nursing homes
Facilities varied in size from 37 to 60 beds, but the number of nursing home beds provided varied from 24 to 60 (Table 9). Two nursing homes were dual registered and also provided residential care. Mean number of nursing beds was 48.5 (control) and 42.67 (intervention), slightly higher than the national average. Provider status was four private independent (intervention) and two private independent (control) and two not-for-profit (intervention). All nursing homes had undertaken either palliative care programmes [Gold Standards Framework (four sites) or Six Steps to Success in End of Life Care (five facilities)] or bespoke training with a local hospice (one site). Two nursing homes used both the Gold Standards Framework and Six Steps.
| Characteristic | Intervention | Control | Overall | National data (if available) |
|---|---|---|---|---|
| Number of nursing homes | 4 | 2 | 8 | |
| Number of beds, mean (SD) | 42.7 (11.5) | 48.5 (16.3) | 44.1 (11.8) | 40171 |
| Dual registered (nursing and residential), n | ||||
| Yes | 2 | 0 | 2 | Not known |
| No | 4 | 2 | 6 | |
| Location (number of homes), n | ||||
| Urban town/city | 4 | 1 | 4 | Density caries regionally but no specific figures172 |
| Rural village/town | 1 | 1 | 2 | |
| Coastal town | 1 | 2 | ||
| CQC rating (at time of recruitment), n | ||||
| Good (with one domain as outstanding) | 2 | 1 | 2 | 66.5% rated as being good (CQC data 3 January 2019) |
| Good | 4 | 1 | 5 | |
| Good (with one domain requires improvement) | 1 | |||
| Use of volunteers in nursing home, n | ||||
| Yes | 3 | 0 | 3 | Not known |
| No | 3 | 2 | 5 | |
| Designated GP providing care to all residents, n | ||||
| Yes | 4 | 1 | 5 | Not known |
| No | 2 | 1 | 3 | |
| Number of GP practices nursing home engages with for care of residents, mean (SD) | 3.5 (3.0) | 3.0 (1.4) | 3.3 (2.6) |
No national data: Median 7, range 1–50173 Mean 4.6, range 1–9174 |
Volunteers were present in only three of the intervention nursing homes. In four intervention nursing homes and one control nursing home, a designated general practitioner (GP) worked with the facility, as opposed to individual residents keeping their own GP once they had been admitted to the nursing home. This may explain the relatively small number of GP practices that most facilities worked with, ranging from one to eight. There are no national data regarding the number of GP practices that care homes work with, but studies identify a range from 1 to 50). 173,174 It is recognised that the number will reflect the size of the facility, and the number at the higher end of the range is from older data in 2002. A lack of national data means that comprehensive comparisons cannot be made to examine the representativeness of this sample in this trial and in any future trial.
Participant recruitment
Recruitment was undertaken over 6 months for residents between (14 February–8 August 2018), over just under 6 months for informal carers (21 February–1 August 2018) and over 10 months for nursing home staff (18 January–9 November 2018). The longer duration for staff recruitment reflects the need to continue to recruit staff for proxy data collection for residents throughout the study.
Learning for a future trial
Recruitment of all staff (not just those working on day of visit) needs to be undertaken at the start of a study, with ongoing recruitment for new staff as they join the organisation.
People with dementia
Two hundred and forty-three people with dementia were assessed for eligibility from the six participating nursing homes (see Figure 3). In the first instance, all facilities recruited four residents per facility, and then after 1 month they were able to recruit further participants. In the intervention sites this number was shaped by the availability of space within the Namaste Care area, so in only two intervention homes was a further resident recruited. The control homes were able to recruit a further two and four residents.
The time taken to recruit residents to be able to undertake the baseline visit using the consultee process varied from 29 to 79 days across the sites (Figure 4). The mean number of days between giving out the first consultee pack and consenting the fourth resident (defined as speaking to last consultee) in the six remaining homes was 32 days (range 11–44 days). Even when consent for the fourth resident had been obtained, there was a delay in the commencement of data collection, with an average of 26 days (range 2–43 days) between the fourth resident being consented and the baseline visit as a result of nursing home staffing issues. One personal consultee refused to give consent for the person with dementia to participate because of the amount of paperwork.
FIGURE 4.
Time taken to consent first four residents, by nursing home.
Learning for a future trial
A future study needs to allow sufficient time to recruit residents, but the intervention could commence and participants could be added as consent is obtained.
Of the 32 residents recruited, one resident was withdrawn from the study by the 4-week follow-up. This was because they had moved to an acute setting for further assessment. By 24 weeks, another 10 residents had been lost to follow-up data collection as staff were not available to complete the data collection tools. By the 24-week time point, four residents had died. These findings will help determine the sample size calculation for a full trial (see Chapter 8).
The resident participants in the control and intervention homes differed in terms of sex (55.6% and 35.7% female, respectively) (Table 10). The overall sex balance of the study was surprisingly weighted in favour of men, with the proportion of male residents higher than those found in other studies. 174,175 This may have been because recruitment involved a personal consultee process, and, as is discussed later (see Chapter 6), this was shaped by staff members’ views on the willingness of family members to give consent. However, in a full trial, with a larger sample size, it is anticipated that the sex difference would represent the expected proportions.
| Characteristic | Intervention | Control | Overall | National data |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 8 (44.4) | 9 (64.3) | 17 (53.1) | 76.7% female174 |
| Female | 10 (55.6) | 5 (35.7) | 15 (47.0) | 73.5% female 175 |
| Age (years), mean (SD) | 79.0 (10.5) | 84.7 (6.43) | 81.5 (9.31) | 83.2 (7.2)174 |
| Marital status, n (%) | ||||
| Married | 7 (38.9) | 11 (78.6) | 18 (56.3) | Not known |
| Single | 3 (16.7) | 0 | 3 (9.4) | |
| Widowed | 8 (44.4) | 3 (21.4) | 11 (34.4) | |
| Ethnicity, n (%) | ||||
| Black African Caribbean | 1 (5.6) | 0 | 1 (3.2) | Not known |
| White | 17 (94.4) | 14 (100) | 31 (96.9) | Not known |
| Dementia diagnosis, n (%) | ||||
| Alzheimer’s disease | 7 (38.9) | 7 (50) | 14 (43.8) | General population:176 Alzheimer’s disease, 62%; vascular dementia, 17%; mixed dementia, 10%; dementia with Lewy bodies, 4%; frontotemporal dementia, 2%; Parkinson’s dementia, 2%; other, 3% |
| Vascular dementia | 5 (27.8) | 1 (7.14) | 6 (18.8) | |
| Dementia with Lewy bodies | 0 | 2 (14.3) | 2 (6.3) | |
| Unspecified dementia | 6 (33.3) | 4 (28.6) | 10 (31.3) | |
| Length of stay (years), mean (SD) | 2.1 (1.4) | 2.93 (2.6) | 2.2 (2.0) | 94 days (28–160 days)174 |
More participants in the intervention homes were widowed, and more in the control homes were married. This was a mainly white population, with only one participant from a non-white background. All residents had a diagnosis of dementia, with no real differences by type of dementia between the two groups. The figures for types of dementia among this nursing home population differ from those among the general population, but the relatively high number of patients with ‘unspecified dementia’ in this small sample prevents firm conclusions being drawn about this. Participating residents had lived in the facility for between 0.13 and 7.67 years in control homes and between 0.27 and 5.11 years in intervention homes. Overall, the participants reflect the population living in care homes, and the findings could be extrapolated to the wider care home population given their heterogeneity.
Informal carers
Informal carers for the 32 recruited residents were invited to participate. Twenty (63%) informal carers consented to participate in the study, but only 12 (38%) provided any data at the point of data collection; the eight who provided no data did not provide reasons for this (Table 11). One informal carer withdrew on receiving the baseline questionnaire, stating that there was too much paperwork and he or she was now housebound because of health issues. This recruitment rate for informal carers is comparable with other care home research. 177,178
| Intervention | Control | Overall | |
|---|---|---|---|
| Number recruited | 12 | 8 | 20 |
| Number with data provideda | 5 | 7 | 12 |
| Sex, n (%) | |||
| Male | 3 (60.0) | 3 (42.9) | 6 (50.0) |
| Female | 2 (40.0) | 3 (42.9) | 5 (41.7) |
| Data unobtainable | 0 | 1 (14.3) | 1 (8.3) |
| Age (years), mean (SD) | 70.7 (12.5) | 69.2 (15.9) | 69.9 (13.7) |
| Ethnicity, n (%) | |||
| Black African/Caribbean | 1 (5.6) | 0 | 1 (3.2) |
| White | 17 (94.4) | 14 (100) | 31 (96.9) |
| Relationship to resident, n (%) | |||
| Husband | 1 (20.0) | 3 (42.9) | 4 (33.3) |
| Wife | 1 (20.0) | 1 (14.3) | 2 (16.7) |
| Son/daughter | 2 (40.0) | 3 (42.9) | 5 (41.7) |
| Other | 1 (20.0) | 0 | 1 (8.3) |
Informal carers were nearly equally male and female, with no difference in mean age across the two groups, of 69.88 years. Again, this was predominantly a group with white ethnicity, with only one African Caribbean relative. Apart from one, all informal carers were family members. There were no major differences between the two groups.
Nursing home staff
Data from nursing home staff at baseline (n = 97) were collected from all eight of the facilities recruited to the study. The purpose was to establish if staff data could be collected and to what extent this could be done, and if any data were not provided when requested. The findings would be used in a full trial to provide a context for the intervention implementation. After two sites withdrew, data from 67 staff at ≥ 2 weeks were analysed for the six remaining sites (four intervention and two control). This is a predominantly female workforce, with approximately 54 (80.6%) staff identifying as female (Table 12). The staff ranged in age from < 20 years to > 60 years. There was a difference in ethnicity between the groups, with the intervention group (which included homes in large urban areas) being more ethnically diverse. The majority of staff worked full-time. The workforce was relatively stable, with a mean time worked in the unit of 5.89 years and a range of time working in the facility from ‘just started’ to > 30 years. The majority of staff in both intervention and control homes held a diploma or certificate-level qualification.
| Characteristic | Group, n (%) | Overall, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Sex | |||
| Male | 8 (17.0) | 4 (20.0) | 12 (17.9) |
| Female | 38 (80.9) | 16 (80.0) | 54 (80.6) |
| Data unobtainable | 1 (2.1) | 0 | 1 (1.5) |
| Age (years) | |||
| < 20 | 1 (2.1) | 1 (5.00) | 2 (3.0) |
| 20–29 | 12 (25.5) | 4 (20.0) | 16 (23.9) |
| 30–39 | 8 (17.0) | 2 (10.0) | 10 (14.9) |
| 40–49 | 10 (21.3) | 6 (30.0) | 16 (23.9) |
| 50–59 | 12 (25.5) | 6 (30.0) | 18 (26.9) |
| 60–70 | 3 (6.4) | 1 (5.00) | 4 (6.0) |
| Data unobtainable | 1 (2.1) | 0 | 1 (1.5) |
| Ethnicity | |||
| Asian/Asian British | 5 (10.6) | 0 | 5 (7.5) |
| Black/African/Caribbean/Black British | 1 (2.1) | 0 | 1 (1.5) |
| Mixed/multiple ethnic groups | 1 (2.1) | 0 | 1 (1.5) |
| White | 38 (80.9) | 20 (100) | 58 (86.6) |
| Any other ethnic group | 1 (2.1) | 0 | 1 (1.5) |
| Data unobtainable | 1 (2.1) | 0 | 1 (1.5) |
| Employment status | |||
| Full-time | 36 (76.6) | 17 (85.0) | 53 (79.1) |
| Part-time | 9 (19.1) | 1 (5.00) | 10 (14.9) |
| Data unobtainable | 2 (4.3) | 2 (10.0) | 4 (6.0) |
| Educational qualifications | |||
| Bachelor’s degree | 5 (10.6) | 3 (15.0) | 8 (11.9) |
| Diploma/certificate | 35 (74.5) | 13 (65.0) | 48 (71.6) |
| Master’s degree | 0 | 1 (5.00) | 1 (1.5) |
| Medical degree | 1 (2.1) | 0 | 1 (1.5) |
| Missing education details | 3 (6.4) | 0 | 3 (4.5) |
| PhD or DN or DPharma | 1 (2.1) | 0 | 1 (1.5) |
| Time in unit (years), mean (SD) | 5.5 (5.6) | 6.9 (7.0) | 5.9 (6.0) |
We have shown that it is possible to collect demographic data on staff, which in a future trial will enable inferences to be drawn about the representativeness of the participating nursing homes with respect to their staffing profile.
Outcomes administration
Primary outcomes: QUALID and CAD-EOLD
Completion rates of the potential primary outcome variables were high, with only one questionnaire not completed at 4 weeks (see Appendix 1, Table 35). Three residents had missing CAD-EOLD values at baseline as a result of partially completed questionnaires, but all were completed at 4-week follow-up. The second potential primary outcome, QUALID, had an incomplete measure for one resident at 4-week follow-up, but was otherwise complete.
Secondary outcomes
Among the secondary outcomes, completion rates were also high for the CMAI short form (complete at baseline, two missing at 4 weeks) and PAIN-AD scales (all complete at both baseline and 4 weeks). The NPI-Q had greater numbers of missing data as a result of non-completion of questions about specific symptoms (see Appendix 2, Table 36). For example, staff who worked only day shifts reported that they could not comment on sleep symptoms.
Feasibility and acceptability of the Namaste Care intervention
The feasibility and acceptability of the Namaste Care intervention were assessed from the records that nursing home staff kept of the session activity logs. This was supported by data from the observations and the interviews with informal carers and nursing home staff, as reported in Chapter 5.
In total, across the four sites there were 427 Namaste Care session logs in which residents were recorded as attending. Over the study period, the proportion of days on which at least one Namaste Care session was delivered varied from 32% to 68.3% (Table 13). One facility offered two sessions per day, and 80% of the time it delivered them, reflecting the size and multiuse of the space (dining room) used to deliver sessions. The mean length of sessions across all sites was 1.33 hours, but this varied by site and by day, with the mean ranging from 0.87 to 1.91 hours (Table 14). The overall mean across all sites was 1.33 hours. The facility with the fewest sessions (n04) provided the longest mean session. The two sites with the greatest number of sessions (n02 and n01) had the shortest sessions. Therefore, it appears that one site gave two shorter sessions per day, whereas the other sites gave one longer session. The dose residents received may be similar over time, but delivered in different ways with respect to session frequency and length. There is no evidence to date of what the most effective process is.
| Nursing home | One session | Two sessions | Total sessions | Total days of data collection | Days sessions delivered (%) |
|---|---|---|---|---|---|
| n01a | 109 | 0 | 110 | 173 | 63.0 |
| n02 | 22 | 92 | 114 | 167 | 68.3 |
| n04 | 55 | 1 | 56 | 163 | 34.3 |
| n07 | 54 | 0 | 54 | 169 | 32.0 |
| Nursing home | Total number of sessions | Number of sessions with missing length | Proportion missing session data (%) | Length in hours, mean (SD) |
|---|---|---|---|---|
| n01 | 110 | 20 | 18.2 | 0.87 (0.23) |
| n02 | 206 | 13 | 6.3 | 1.08 (0.22) |
| n04 | 57 | 7 | 12.3 | 1.45 (0.40) |
| n07 | 54 | 0 | 0 | 1.91 (0.23) |
We aimed to see if we could assess the ‘dose’ of Namaste Care each resident received (Table 15) by looking at the session logs. This allowed an estimate of the amount of time residents received Namaste Care over the study period. Data were missing on the length of sessions. Overall, data on session length were missing in 8.9% of session logs. The number varied by nursing home; one site provided a full record of the sessions (n04) and others were less accurate in record-keeping. This may reflect the number of staff in each site who were involved in delivering Namaste Care, with higher completion rates when fewer staff were involved. From the activity log records, sites n04 and n07, which had smaller numbers of missing data, recorded two and three different individuals, respectively, completing the log. Sites n01 and n02, with greater numbers of missing data, show seven and five staff signing, respectively. However, the signing was not consistent, so this is only an approximation.
| Resident ID | Total number of sessions (n) | Number (%) of sessions where length not recorded | Mean length of session (minutes) | Estimated total hours of Namaste Carea |
|---|---|---|---|---|
| 01R001 (died) | 24 | 8 (33.3) | 47 | 19.0 |
| 01R002 | 110 | 20 (18.2) | 52 | 95.9 |
| 01R003 | 108 | 19 (17.6) | 53 | 94.6 |
| 01R004 | 108 | 19 (17.6) | 53 | 94.6 |
| 01R005 (replaced 01R001) | 43 | 7 (16.3) | 54 | 38.8 |
| 02R001 | 202 | 13 (6.4) | 65 | 217.9 |
| 02R002 | 199 | 12 (6.0) | 65 | 214.8 |
| 02R003 | 199 | 13 (6.5) | 65 | 214.5 |
| 02R004 | 168 | 9 (5.4) | 66 | 182.6 |
| 04R001 (withdrawn) | 0 | 0 | 0 | 0 |
| 04R002 | 51 | 5 (9.8) | 86 | 73.4 |
| 04R003 | 54 | 6 (11.1) | 88 | 78.8 |
| 04R004 | 26 | 2 (7.7) | 98 | 42.3 |
| 07R001 (died) | 20 | 0 | 119 | 39.8 |
| 07R002 | 37 | 0 | 115 | 71.0 |
| 07R003 | 43 | 0 | 116 | 83.3 |
| 07R004 | 52 | 0 | 114 | 99.0 |
| 07R005 (replaced 07R001) | 16 | 0 | 113 | 30.0 |
| Total | 1484 | 133 | 81 minutesb | 99.4 hoursb |
More clarity is needed about when individual residents start to receive the intervention and how to record this for a future study.
Among the 13 residents who received the intervention over the full trial period, the mean length of session was 81 minutes and the mean time spent in Namaste Care sessions was 99.4 hours. The data on average session length can inform future recommendations about an appropriate session length, which are based on what is practically possible in the setting.
Session log summaries by nursing home
We were also able to record how many residents attended each session; one facility (n04) provided Namaste Care for only two residents per session (Table 16; see Figure 7), whereas other facilities maintained delivery to four residents for the majority of the time (n01, n02). Where eight residents are shown (see Figure 6, site n02), this indicates that sessions were being run twice per day.
| Number of residents attending each session | Nursing home, n (%) | |||
|---|---|---|---|---|
| n01 | n02 | n04a | n07 | |
| Sessions with one resident | 1 (1) | 4 (2) | 7 (12) | 2 (4) |
| Sessions with two residents | 1 (1) | 8 (4) | 49 (86) | 10 (19) |
| Sessions with three residents | 42 (38) | 28 (14) | 0 | 22 (41) |
| Sessions with four residents | 66 (60) | 166 (81) | 0 | 20 (37) |
It was also possible to illustrate the delivery of the intervention over time (Figures 5–8). There were different seasonal and participant patterns in delivery of the intervention. During the summer there were periods of time when Namaste Care was not delivered (n04, n07), with a shorter break in September at site n01. Site n02 delivered Namaste Care consistently across the week over the time period. The number of residents attending sessions also varied over time, with some reduction in the summer months (n01, n02, n07). The facility offering more resident sessions per day was able to consistently offer these to the same residents.
FIGURE 5.
Number of residents attending sessions in nursing home 01 over time in 2018.

FIGURE 6.
Number of residents attending sessions in nursing home 02 over time in 2018.

FIGURE 7.
Number of residents attending sessions in nursing home 04 over time in 2018.

FIGURE 8.
Number of residents attending sessions in nursing home 07 over time in 2018.

Outcomes results
The study did not seek to establish efficacy, but the outcomes data were analysed so that these can inform which primary outcome measure to use in a full trial.
Resident outcomes
Primary outcomes: QUALID and CAD-EOLD
Higher values of CAD-EOLD indicate greater comfort, and lower values of QUALID indicate higher quality of life. In the Namaste Care group the mean value of CAD-EOLD increases slightly after 4 weeks and then decreases, but remains higher than at baseline at 24 weeks. The QUALID decreases slightly after 4 weeks in both groups. At 24 weeks, QUALID continues to decrease for the Namaste Care group, and increases again in the control group (Table 17; see Appendix 3, Tables 37–39, and Appendix 4, Table 40). No statistical comparisons have been made, as the uncertainty of estimates would be very high because of the small sample and the number of clusters.
| Outcome | Intervention | Control | Overall |
|---|---|---|---|
| Number of residents | |||
| Baseline | 18 | 14 | 32 |
| 4 weeks | 17 | 14 | 31 |
| 24 weeks | 10 | 7 | 17 |
| Primary outcomes, mean (SD); n missing | |||
| CAD-EOLD (higher score = greater comfort) | |||
| Baseline | 34.8 (4.0); 1 | 33.6 (4.7); 2 | 34.3 (4.2); 3 |
| 4 weeks | 36.4 (4.0); 0 | 33.4 (3.4); 0 | 35.1 (4.0); 0 |
| 24 weeks | 37.6 (2.9); 1 | 33.6 (1.9); 0 | 35.8 (3.2); 1 |
| QUALID (lower score = better quality of life) | |||
| Baseline | 24.0 (8.4); 0 | 27.1 (8.0); 0 | 25.3 (8.2); 0 |
| 4 weeks | 22.9 (7.1); 0 | 25.7 (7.4); 1 | 24.1 (7.2); 1 |
| 24 weeks | 19.9 (7.5); 1 | 28.1 (7.8); 0 | 23.5 (8.5); 1 |
| Secondary outcomes, mean (SD); n missing | |||
| NPI-Q (higher score = greater severity and distress) | |||
| NPI-Q severity | |||
| Baseline | 8.1 (8.4); 4 | 7.8 (5.2); 3 | 8.0 (7.0); 7 |
| 4 weeks | 2.9 (3.6); 4 | 8.0 (4.7); 2 | 5.4 (4.8); 6 |
| NPI-Q distress | |||
| Baseline | 8.3 (11.7); 4 | 8.5 (6.6); 3 | 8.4 (9.6); 7 |
| 4 weeks | 1.1 (1.0); 5 | 6.3 (6.1); 2 | 3.7 (5.0); 7 |
| CMAI short form (higher score = greater agitation) | |||
| Baseline | 23.0 (10.4); 0 | 23.6 (6.1); 0 | 23.3 (8.7); 0 |
| 4 weeks | 18.6 (5.5); 1 | 25.3 (7.5); 1 | 21.6 (7.2); 2 |
| PAIN-AD (higher score = greater agitation) | |||
| Baseline | 3.6 (2.1); 0 | 5.4 (3.1); 0 | 4.4 (2.7); 0 |
| 4 weeks | 2.3 (1.6); 0 | 5.3 (3.1); 0 | 3.7 (2.8); 0 |
Figures 9 and 10 are box plots of the distributions of the contender primary outcomes by nursing home. The patterns of change between baseline and 4 weeks in both measures varies between nursing homes, and, owing to the small numbers, we did not seek to establish definitive conclusions regarding effect. In terms of relevance of the two measures, although participants were identified as living with advanced dementia, they were not likely to die imminently. Only four people with dementia died during the study and the study inclusion criteria required people to be well enough to leave their rooms and enter a group space, which excluded people who were towards the end of their lives and considered too unwell to join a group activity.
FIGURE 9.
Box plot of primary outcome measure QUALID at baseline and week 4.
FIGURE 10.
Box plot of primary outcome measure CAD-EOLD at baseline and week 4.

These findings raise questions about the suitability of an end-of-life/dying measure to assess the impact of Namaste Care for individuals in a future trial. The usefulness of the CAD-EOLD as an outcome measure for the end of life is called into question by our findings.
Learning for a future trial
Outcome measures used in a future trial need to focus on the quality of life of people with advanced dementia, taking a broader palliative, rather than an end-of-life, perspective.
Secondary outcomes
All secondary outcomes had lower observed mean values after 4 weeks in the Namaste Care group (see Table 17), although no statistical comparisons were carried out. Variation between nursing homes is present. Appendices 5 (see Tables 41 and 42), 6 (see Table 43) and 7 (see Table 44) present detailed data for each measure.
The NPI-Q (see Appendix 5, Tables 41 and 42) provides two types of data: the presence of symptoms, and then, if present, the severity of a symptom and the distress this caused. The symptoms of most interest were agitation and apathy (Table 18; data on all symptoms assessed are in Appendix 5).
| Symptom | Control: baseline | Control: week 4 | Intervention: baseline | Intervention: week 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing (n) | Symptom absent (n) | Symptom present (n) | Missing (n) | Symptom absent (n) | Symptom present (n) | Missing (n) | Symptom absent (n) | Symptom present (n) | Missing (n) | Symptom absent (n) | Symptom present (n) | |
| Agitation/aggression | 0 | 2 | 12 | 0 | 3 | 11 | 1 | 6 | 11 | 1 | 9 | 7 |
| Apathy/indifference | 0 | 13 | 1 | 0 | 13 | 1 | 0 | 12 | 6 | 1 | 14 | 2 |
Apathy was present for only one resident in the control sites at baseline and week 4, whereas in the intervention sites it was reported for six residents at baseline and it then decreased to being reported for two residents at 4 weeks. Agitation was more frequently reported in both arms, for 12 residents in the control sites and for 11 residents in the intervention nursing homes at baseline, with this being reduced in the intervention sites to being present in only seven residents. At a cluster level the severity scores varied between nursing homes, with both a decrease in severity (n01, n02, n04, n03) and increases in severity (n07 and n06) reported (Figure 11).
FIGURE 11.
Box plot of secondary outcome measure NPI-Q total score at baseline and week 4.
The distress scores decreased in all clusters between baseline and 4 weeks (Figure 12). The measure of agitation from the CMAI tool showed that there was an overall decrease in all intervention sites and in one control site (n03) (Figure 13).
FIGURE 12.
Box plot of secondary outcome measure: NPI-Q distress total score at baseline and week 4.
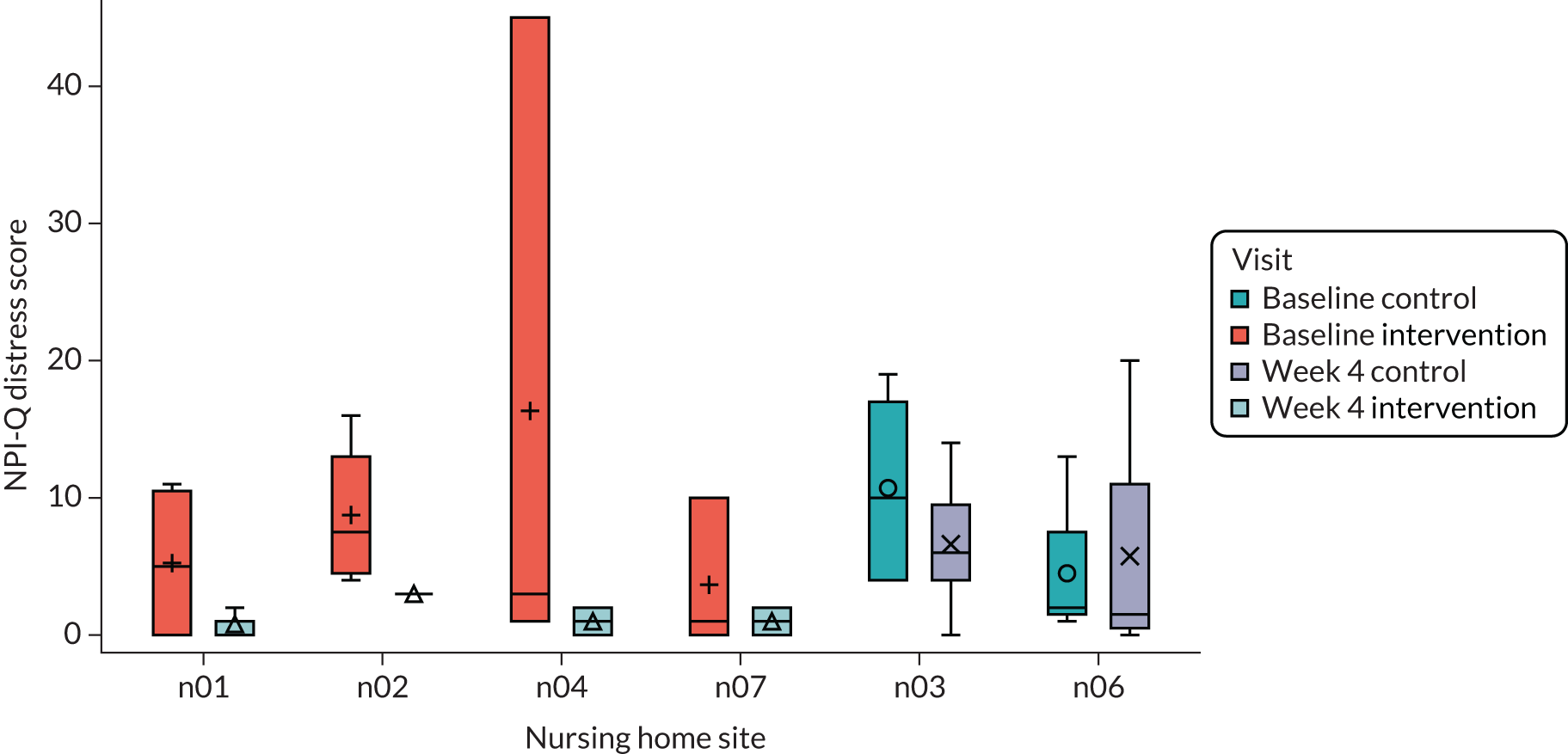
FIGURE 13.
Box plot of secondary outcome measure: CMAI total score at baseline and week 4.

Pain scores, as measured with the PAIN-AD, decreased in all sites from baseline to 4 weeks, except in one control site (n06) (Figure 14).
FIGURE 14.
Box plot of secondary outcome measure: PAIN-AD at baseline and week 4.

Actigraphy
On average, data for analyses were available for 22 days (SD 3.4 days, range 13–25 days) after 04R001 and 06R001 were excluded (Table 19). The reasons for exclusion were that one resident was temporarily admitted to hospital and so their device was removed, and one device failed. Variability at the participant level reflected different factors, such as practical decisions (e.g. the device being removed before the end of data collection) and technical aspects (e.g. the device stopped collecting data because the battery was drained).
| Resident ID | Total days (n) | Useable days, n (%) |
|---|---|---|
| 01R001 | 18 | 16 (88.9) |
| 01R002 | 28 | 24 (85.7) |
| 01R003 | 28 | 24 (85.7) |
| 01R004 | 28 | 24 (85.7) |
| 01R005 | 28 | 18 (64.3) |
| 02R001 | 28 | 25 (89.3) |
| 02R002 | 28 | 25 (89.3) |
| 02R003 | 23 | 18 (78.3) |
| 02R004 | 28 | 22 (78.7) |
| 03R001 | 23 | 21 (91.3) |
| 03R002 | 28 | 25 (89.3) |
| 03R003 | 21 | 19 (90.5) |
| 03R004 | 19 | 17 (89.5) |
| 03R005 | 28 | 25 (89.3) |
| 03R006 | 28 | 25 (89.3) |
| 03R007 | 28 | 23 (82.1) |
| 03R008 | 27 | 25 (92.6) |
| 04R001 | 28 | 3 (10.7) |
| 04R002 | 28 | 25 (89.3) |
| 04R003 | 28 | 25 (89.3) |
| 04R004 | 27 | 24 (88.9) |
| 06R001 | 3 | 0 (0.00) |
| 06R002 | 28 | 25 (89.3) |
| 06R003 | 28 | 25 (89.3) |
| 06R004 | 28 | 17 (60.7) |
| 06R005 | 28 | 22 (78.6) |
| 06R006 | 28 | 13 (46.4) |
| 07R001 | 28 | 25 (89.3) |
| 07R002 | 28 | 25 (89.3) |
| 07R003 | 26 | 23 (88.5) |
| 07R004 | 28 | 25 (89.3) |
| 07R005 | 22 | 20 (90.9) |
Using all available data (n = 31) (Table 20; see Appendix 8, Table 45), we observed that participants were generally inactive: the sample mean Euclidean norm minus one (a measure of wrist acceleration and a proxy for physical activity) was 8.6 (SD 4.3) (moderate physical activity is ≈ 100). The estimated mean sleep duration was 7.6 hours (SD 2.05 hours) and sleep efficiency was 68% (SD 14%), lower than the 78.8% sleep efficiency reported among people with earlier-stage dementia. 179 The average sleep–wake ratio was 4.08 hours (SD 3.22 hours) and the average time of wake after sleep onset was 3.5 hours (SD 1.6 hours).
| Actigraphy variable | Trial arm | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Sleep–wake ratios | Control | 4.01 | 3.22 | 3.19 | 1.57 | 13.5 |
| Intervention | 4.13 | 3.32 | 2.48 | 0.58 | 10.05 | |
| Time asleep (minutes) | Control | 462.15 | 74.81 | 467.16 | 337.5 | 574.48 |
| Intervention | 458.33 | 151.19 | 439.23 | 196.64 | 706.29 | |
| Sleep efficiency | Control | 0.71 | 0.1 | 0.72 | 0.55 | 0.9 |
| Intervention | 0.65 | 0.17 | 0.65 | 0.35 | 0.87 | |
| Wake after sleep onset | Control | 193.14 | 70.45 | 194.05 | 34.88 | 294.39 |
| Intervention | 228.26 | 95.79 | 234.22 | 95.24 | 383.69 | |
| Total activity | Control | 7.31 | 2.15 | 7.59 | 3.54 | 10.69 |
| Intervention | 9.63 | 5.23 | 8.19 | 4.52 | 25 | |
| Intradaily variability | Control | 0.78 | 0.23 | 0.77 | 0.32 | 1.18 |
| Intervention | 0.64 | 0.2 | 0.67 | 0.21 | 1.05 | |
| Interdaily stability | Control | 0.08 | 0.05 | 0.07 | 0.02 | 0.21 |
| Intervention | 0.08 | 0.06 | 0.06 | 0.01 | 0.22 |
In terms of the participants’ circadian rhythm fragmentation, the average intradaily variability was 0.7 (SD 0.2) (high intradaily variability values of close to 1 or higher can indicate the occurrence of daytime naps and/or nocturnal activity episodes). Regarding participants’ circadian rhythm synchronisation, the average interdaily stability was 0.08 (SD 0.05) (with values close to 0 reflecting a lack of synchronisation).
Data on activity demonstrated that this population was inactive, with an indication that their sleep and circadian rhythms were not as healthy as they could be, that this population had more fragmented sleep throughout the day and night and that this population had a less consistent pattern of sleep over time. This supports similar results observed in previous studies with similar populations. 170–172
Learning for a future trial
Collecting objective data using an actiwatch is feasible. Despite the low level of activity and the disturbed sleep patterns among this population, variation within and between days and within and between participants can be identified. Further analysis is required to identify how best to use these patterns in a future trial.
Other non-invasive objective measures need to be explored for this population; for example, using cortisol as a marker of ‘stress’ might be appropriate.
Informal carer secondary outcomes
The data from the informal carers on their satisfaction with care (Table 21) show a slight decline in satisfaction with care over time, but, with small numbers and missing data, this cannot be interpreted in any meaningful way.
| Outcome | Intervention | Control | Overall |
|---|---|---|---|
| Number of carers | |||
| Baseline | 5 | 7 | 12 |
| 4 weeks | 5 | 7 | 12 |
| SWC-EOLD (higher score = greater satisfaction with end-of-life care), mean (SD); missing | |||
| Baseline | 33.25 (4.86); 1 | 31.83 (4.26); 1 | 32.40 (4.30); 2 |
| 4 weeks | 31.25 (5.85); 1 | 29.5 (2.35); 2 | 30.2 (3.91); 3 |
In conclusion, our learning of how to best sample and recruit nursing homes into a cluster randomised controlled trial of Namaste Care is that this requires time and the use of wider clinical research and ENRICH networks to ensure engagement at all levels of the organisation (if part of a larger chain). However, senior management support does not necessarily ensure that individual nursing home managers feel able or are willing to participate in research.
The recruitment of residents with dementia through a proxy personal or nominated consent process also takes time and requires investment from nursing home staff and researchers. Once recruited to the study, residents with dementia had a low attrition rate, although the reliance on proxy completion for data collection did reduce the number of data collected. Informal carer recruitment rates were lower than those for other groups, but they were comparable with those of other UK studies in this setting.
Nursing home staff data are considered in Chapter 5.
Adverse events
No adverse events were reported arising from the delivery of the intervention. One adverse event arose from the use of the ActiGraph device, which was identified at the end of the study during an informal carer interview. The carer reported that she had seen bruising on her relative’s ankle. This was reviewed with the site research lead and no lasting effects were identified.
Learning for a future trial
If an ActiGraph device is used, it should be located on a wrist, not on an ankle. This will ensure that it is more visible to staff who can monitor for rubbing or bruising.
Chapter 5 Process evaluation
The process evaluation was carried out contemporaneously with the cluster randomised controlled trial and provides a greater understanding of many of the feasibility objectives, as well as the context underpinning their achievement or otherwise. The research process (recruitment, and data collection tools and processes), the organisational context (readiness for change, P-CAT), the intervention (preparation, implementation and fidelity) and the impact of the intervention are considered.
The research process
Recruitment
Two main factors appeared to influence the selection of residents across both intervention and control nursing homes: residents’ health and the perceived likelihood that the informal carer would consent to the resident participating in the study. Residents considered for participation were those with advanced dementia whose cognitive abilities were most affected:
I thought it was really nice that some people that can’t do other activities were getting so much attention and the intimacy of it as well, ‘cause when you think some people get up and probably don’t have a cuddle, don’t have their arms touched.
n07 (intervention), staff member S017, bolding added to denote theme
Usually, those informal carers who were known to be co-operative, and with whom staff members had a good relationship, were approached for consent:
I’ll be quite honest, family played a part in that as well . . . And we’ll be perfectly frank with that because there were some people that we thought oh they’d be good but no that son would be horrendous.
n03 (control), staff member S005, bolding added to denote theme
Co-operation of relatives and next of kin was a big thing, because there was a lady that I really think that she would have benefited and her brother who’s the next of kin said no. And that was sad . . .
n02 (intervention), staff member S005, bolding added to denote theme
Even though clear inclusion criteria for residents were identified for the study, it was apparent that nursing home staff applied their own informal criteria based on their judgement about family support for such an intervention, about whether a resident would enjoy the intervention, and about whether the resident’s likely deterioration or complex needs might affect the delivery of Namaste Care in a group setting. In the control sites, staff made similar judgements about family members’ interest in being involved in research.
Learning for a future trial
-
Family engagement with the research process is essential to resident recruitment, and consideration should be given to how to publicise the study widely to families within the nursing home.
-
Nursing home staff make judgements about whom they invite to participate based on health status, estimation of how the person may respond, how the person behaves in a group setting and family dynamics. Training needs to take this process into account.
Data collection tools and process
Some informal carer participants found the questionnaires relatively easy to complete:
I think it was fairly straightforward, I think with the forms . . . they were quite easy to follow.
n01 (intervention), carer C002, bolding added to denote theme
Some participants struggled with the closed-question format of the questionnaires, feeling that qualitative methods of collecting some data may be more appropriate:
I mean some of the questions were really hard to answer because there’s no like some of them wanted either a black and white answer wasn’t it and there was no area, grey area.
n04 (intervention), staff member S003, bolding added to denote theme
I think the paperwork was a bit bureaucratic and the discussion was better . . . I don’t think questionnaires really, because they’re you couldn’t put the discussion we had this morning in a questionnaire, you couldn’t frame it, yes, no, was it positive, mark it on a scale.
n01 (intervention), carer C001, bolding added to denote theme
Proxy measures were also challenging in this situation, given the difficulties of understanding how someone was feeling on a day-to-day basis, or assessing how much time a staff member had spent with that resident:
I don’t know how I am meant to scale somebody on a scale of 1 to 100 on how they are feeling . . . I can’t tell you what his health’s like, because as well as we know our residents, personally we could say they’re having a really good day, they’re about 70 . . . but in reality if they were them looking at them they’d go oh well I’ve got dementia and I’m sat in a chair, I can’t walk, I’m zero . . .
n03 (control), staff member S011
Sometimes [name of staff member S005] might have only been there for the length of the session but it asks about, the last week . . . And also [name of staff member S005] doesn’t provide direct care, she is our co-ordinator for activity, so some of the questions would have been a challenge for somebody in that role.
n01 (intervention), staff member S001
This mirrors the experiences of those who undertook the think-aloud interviews reported in Chapter 6.
Although the research training emphasised that it was necessary for the measures to be completed consistently by the same key worker, this was not always possible. To ensure reliability in a future trial, the same members of staff need to complete the outcome measures at both baseline and 4-week follow-up.
Learning for a future trial
-
Reducing the number of data collection tools for staff to complete would be helpful; qualitative data collection would provide contextual data to explain findings.
-
Proxy data completion can be challenging because staff work shifts and so they do not see residents continuously over a 24-hour period. To address this, group completion (a group of staff members completing a measure for one resident) could be considered.
-
Identifying ways to support the same staff member to complete the outcome measures at baseline and at 4-week follow-up could maximise outcome measurement reliability.
Organisational context
Readiness for change
To contextualise the findings from the study, we sought to understand the nursing home context and each site’s organisational readiness for change. This was measured using the Alberta Context Tool, which was given to staff at the start of the study. This tool has 10 domains (Table 22) and measures the culture, leadership and evaluation aspects of an organisational context that may explain why change does or does not happen. Data were available for all eight nursing homes that agreed to participate in the study.
| Domaina | Nursing home | |||||||
|---|---|---|---|---|---|---|---|---|
| n01 (Namaste Care) | n02 (Namaste Care) | n03 (control) | n04 (Namaste Care) | n05 [Namaste Care (withdrew)] | n06 (control) | n07 (Namaste Care) | n08 [Namaste Care (withdrew)] | |
| Staff completing tool (n) | 6 | 3 | 10 | 7 | 5 | 10 | 12 | 14 |
| Leadership | 4.7 (0.4) | 4.0 (0.2) | 3.87 (0.69) | 4.4 (0.3) | 3.7 (0.4) | 3.1 (1.0) | 3.7 (1.2) | 3.9 (0.5) |
| Culture | 4.6 (0.3) | 4.1 (0.5) | 3.82 (0.89) | 4.2 (0.4) | 3.8 (0.6) | 3.5 (0.7) | 3.9 (0.7) | 4.3 (0.5) |
| Evaluation | 4.0 (0.4) [1] | 3.5 (0.7) | 3.75 (0.51) | 3.8 (0.6) | 2.0 (0.8) | 3.0 (0.6) | 3.4 (1.1) | 3.9 (0.9) |
| Social capital | 4.4 (0.4) | 4.0 (0.5) | 4.55 (0.41) | 4.4 (0.4) | 4.2 (0.4) | 3.7 (0.6) | 4.0 (0.5) | 4.2 (0.4) [1] |
| Formal interactionsb | 11.8 (2.9) | 10.3 (4.5) | 9.45 (4.21) | 11.7 (2.6) | 10.0 (5.3) | 8.8 (4.0) | 8.6 (3.1) | 10.6 (3.9) [2] |
| Informal interactionsb | 7.4 (2.6) [1] | 3.8 (3.6) | 4.13 (3.22) [2] | 4.6 (3.9) | 4.9 (2.3) | 3.6 (2.2) | 3.8 (2.9) [1] | 3.4 (3.1) [4] |
| Structural/electronic resourcesb | 3.92 (2.6) | 3.7 (3.1) | 4.11 (2.64) [1] | 3.3 (2.4) [1] | 4.9 (3.1) | 3.7 (2.1) [2] | 2.8 (1.5) [1] | 2.5 (1.5) [4] |
| Organisational slack: staff | 3.2 (0.7) | 4.3 (0.6) | 3.15 (1.25) | 3.8 (0.5) | 1.8 (1.1) | 2.9 (1.5) | 2.6 (1.2) [1] | 3.8 (1.0) [1] |
| Organisational slack: space | 4.6 (0.5) | 3.8 (0.5) | 4.03 (0.55) | 4.4 (0.3) [1] | 1.6 (1.4) | 2.6 (0.9) [1] | 4.1 (0.4) | 4.4 (0.4) [1] |
| Organisational slack: time | 3.5 (0.7) | 3.8 (0.4) | 3.30 (0.95) | 3.7 (0.8) | 2.8 (0.4) | 2.8 (0.9) | 3.2 (0.7) | 3.2 (0.6) [1] |
The small numbers of staff responses from each site meant that it was not possible to compare sites statistically. There were few differences across the sites, or between the two facilities that withdrew and those that stayed in the study, or between the intervention and control nursing homes. There were slightly higher scores for formal interactions in n01, indicating that patient-centred discussions were taking place more often with other professionals within the facility. Lower scores for structural/electronic resources were seen for two nursing homes (n07 and n08) and for organisational slack factors related to staff, time and resources to do their care work. The usefulness of this tool for a future trial is not established, and a larger data set is required to enable more meaningful conclusions to be drawn. The Canadian terminology may need to be adapted to more closely align the concept with the roles and terms used in the UK, which would invalidate the tool.
Learning for a future trial
We would not recommend using the Alberta Context Tool in a future trial owing to its complexity and the differences in terminology between Canada and the UK.
Nursing home values
Person-Centred Care Assessment Tool
The P-CAT was administered at baseline in all sites and at 24 weeks in intervention sites to assess the organisational support for person-centred care (Table 23).
| P-CAT domain | Intervention | Control | Overall |
|---|---|---|---|
| Extent of personalised care, mean (SD); n (missing) | |||
| Baseline | 31.1 (3.2); 47 (0) | 28.7 (3.2); 20 (0) | 30.4 (3.2); 67 (0) |
| 24 weeks | 33.1 (2.7); 18 (0) | N/A | N/A |
| Amount of organisational support, mean (SD); n (missing) | |||
| Baseline | 15.2 (3.4); 46 (1) | 14.6 (3.1); 20 (0) | 15.0(3.3); 66 (1) |
| 24 weeks | 17.4 (2.8); 18 (0) | N/A | N/A |
| Degree of environmental accessibility, mean (SD); n (missing) | |||
| Baseline | 6.1 (1.4); 45 (2) | 5.8 (1.2); 20 (0) | 6.0 (1.3); 65 (2) |
| 24 weeks | 6.0 (1.2); 18 (0) | N/A | N/A |
It was proposed that delivering Namaste Care could improve staff’s perception of the amount of person-centred care they delivered and that the P-CAT might ascertain this. Three subscales assessed the extent to which personalised care could be delivered in the facility, how much support was available for staff and the accessibility of the environment. At baseline there were some differences between the intervention and control groups. The intervention group showed a small improvement but had small numbers per facility, with responses reduced from 47 to 18 in the intervention sites between baseline and 24 weeks, limiting the ability to infer meaning.
Staff felt that the values underpinning Namaste Care were familiar, that they fitted with their priorities and that they were synergistic with their current person-centred approaches to care:
We make it environmentally like a home that smells nice, that looks nice, that they [the residents] eat and drink whenever they want and they’re comfortable and do what they want when they want really.
n05 (intervention), manager
Managers felt that they were already carrying out elements of Namaste Care in the nursing home:
And the staff are always doing hand massage and nail care and those types of things, it’s one long meal in here, people are always eating, whether it’s a choc ice, whether it’s Quavers [Walkers, Leicester, UK], you know in between meals, a lot of snacking goes on, we have an afternoon tea as well, so yeah all those types of things are ongoing and going on anyway.
n01 (intervention), manager
The inclusion criteria for participating nursing homes meant that in all of them staff had experience of some form of training or accreditation regarding end-of-life care that underpinned their care approach:
All the staff are very interested in it because . . . we get a lot of people that are coming to the end of their life . . . most of the senior carers have done the Six Steps [an approach to end of life care management], . . . the nurses have all done the Six Steps so they’re all quite involved in, you’ve got to be because that’s where we are I’m afraid.
n08 (intervention), manager
Some of the nursing homes had regular GP ‘ward rounds’ and all accessed specialist care palliative care input as needed:
We have GP rounds twice a week, which is quite rare for a care home . . . occasionally the Macmillan nurses will pop in, or if we need any advice about symptom control or pain control, specific, that we would be struggling with and we have [name of] hospice that we could ring up as well.
n05 (intervention), manager
Despite this existing involvement of nursing homes in end-of-life care, Namaste Care involved introducing changes. Staff thought that change management was ongoing within the nursing home context, and that they already had mechanisms to discuss change, whether it be externally imposed (e.g. regulation) or internally suggested:
When we do any changes, when I present something, we’re always getting the support from my whole team, not only from my nursing team, my care team, my domestic team and my kitchen staff, they always support as a team to see can we achieve something more.
n07 (intervention), manager
Meetings were a common mechanism for discussing change, but these could be suboptimal, as the manager in nursing home 08 (intervention) explained: ‘the meetings are for everyone, nobody ever comes’. She explained that not many residents ‘can be as much involved as we’d like them to be’ but felt that the lack of family attendance was maybe ‘a good indicator that there isn’t that much to complain about’.
Implementing change in dementia care and the Namaste Care intervention
All of the managers had been involved in implementing dementia-specific projects in their nursing homes, and had received support for Namaste Care from their senior management and nursing home owners. There were concerns about possible financial commitments and the requirement for staff ‘buy-in’:
We had five or six people [staff] down, and they listened and they made the decision really. It’s not for us who within the office really, it has to be the staff who are on the floor.
n04 (intervention), manager
In most nursing homes staff members talked about the importance of a culture of wanting to provide more than basic care, whereby the care workers must understand and work to achieve a better quality of life for their residents:
I think we’ve got a chance of a good positive change in staff now that are more understanding of what it means, does that make sense, less task orientated . . . who are much more dementia friendly I would say. And I could see now more of a future of this continuing with them taking over from you.
n04 (intervention), staff member S008
I don’t think it [person-centred care] should be an option, I think it should be standard practice really, I really really do. I think there’s a lot to be said for this type of care really, I really really do.
n07 (intervention), staff member S025
Learning for a future trial
-
Discussion of change management is imperative at the start of the study, even though staff may feel that they are familiar with the concepts of Namaste Care.
The Namaste Care intervention
Initiating the Namaste Care programme
The decision about where Namaste Care would take place, who would provide it and how frequently it would be carried out was made by the nursing home managers at the start of the study. Only one nursing home (n08) planned to carry out Namaste Care twice per day, 7 days per week. The managers of nursing homes 05 and 07 expressed their concerns about providing Namaste Care with this frequency without extra staffing, given the complex needs of the other residents:
Personally I think that 2 hours in the morning and 2 hours in the afternoon every day is quite difficult for us to protect that every day because of the other needs of residents, because of the client group we’ve got we need that constant attention in the lounge all the time.
n07 (intervention), manager
Although enthusiastic about taking part in the Namaste Care study, nursing homes 05 and 08 withdrew from the study before resident data collection commenced. Nursing home 05 was unable to continue in the study because of unforeseen staffing changes implemented within the nursing home by the wider care home group. This example highlights the external factors that can influence a manager’s ability to implement an intervention within their nursing home. Nursing home 08 withdrew because of delays to the study starting, which illustrates the challenges of maintaining the motivation of nursing home managers who are keen to commence an intervention within their facility.
Training and preparation to deliver Namaste Care
Staff members talked about feeling inspired by the Namaste Care guide and the training that they received, which had given them ideas about how to run the sessions and whom they would be most relevant for:
I couldn’t fault it, it taught us everything we knew and in enough detail as well.
n02 (intervention), staff member S005
Staff providing Namaste Care used the manuals, more so at the start of the study, to help them tailor the Namaste Care programme. They adapted it flexibly to meet their own needs and those of the residents, and the nursing home setting:
Maybe to start with I’ve looked at it [guide] but not as time went on.
n04 (intervention), staff member S002
I think we’ve kind of used all the guidelines and carved it into our own to suit the people, yeah I think so, I don’t know.
n01 (intervention), staff member S006
Staff members in two of the nursing homes also mentioned the value of meeting and sharing ideas with staff from other nursing homes:
It would actually have been really nice to have got back together again in the middle to find out how other people were doing.
n04 (intervention), staff member S008
There was something about coming away from the building, coming away to learn and also coming away to experience different homes and different people, ‘cause we don’t meet people from other homes do we.
n07 (intervention), staff member S004
Creating the Namaste Care space
Each nursing home made the decision about where it would carry out Namaste Care during the trial. Three nursing homes had a separate room that could be used specifically for Namaste Care, and one used a communal area that was set aside for Namaste Care at certain times of the day. The size and availability of space affected what could be done in the sessions, how many people could take part and how often sessions could be delivered:
Well it’s just a small conservatory, very cluttered, so by the time you got four people in there in big chairs . . . and by the time you got two staff in there and maybe me, that was seven people in a very small space.
n07 (intervention), carer C001
The challenge of trying to provide Namaste Care in a room that had multiple uses was discussed by staff members and was observed during the 4-week observation in nursing home 02. This particular home had to use its dining room at certain times for Namaste Care:
That was a big restriction and there was times when we planned to do a session at say 11 o’clock and then we’d only find out there’s a meeting with social workers and things, and this is the only room to have a meeting in.
n02 (intervention), staff member S005
Namaste Care very short and feeling that rushed, sign taken down at 11.50. Sense in room that staff conscious towards the end that nearly lunchtime and have to clear up the room quickly. Had to stop someone wheeling trolley in at 11.50 before residents taken away from Namaste Care space. 2 residents left sitting in the room at the end of the session while space cleared and room prepared for lunch.
Observation field note at 4 weeks, n02 (intervention)
Learning for a future trial
-
Managers need to consider staffing requirements and the space needed to deliver Namaste Care, and implement a programme of sessions that builds on their staff-to-resident ratios.
-
Structures to facilitate a wider support network, for example a community of practice for those delivering Namaste Care, may assist ongoing implementation of the intervention.
Implementing the Namaste Care programme
Two elements of data are presented here: first, qualitative data on perceptions of Namaste Care implementation, and, second, information on the association between the staff reports of Namaste Care delivery and the intermittent observation by researchers. This enables an understanding of fidelity to the planned intervention, and an appreciation of how best to collect data on intervention delivery in any future study.
Greetings and farewells
Staff would typically have been caring for residents before and after the Namaste Care session, and hence they could feel that greetings in the Namaste Care space were ‘artificial’, even though the guide suggested that these were important. Staff did recognise that inviting residents into a new space could encourage residents to step out of their daily routine and behaviour:
The lounge is noisy and crowded and you are taking away from that in a different area and then you are giving it directly to that client, not with everyone else, or that little group, then they can relax . . . it’s a different atmosphere.
n07 (intervention), staff member S003
Changes to the activities offered and the physical environment were made towards the end of a Namaste Care session to indicate that it was ending:
Just wind it down, just maybe turn the light on or open a curtain for me and just say right and tell the resident that we were going to be finishing soon.
n04 (intervention), staff member S003
Comfort assessments
Staff described how they assessed a resident’s non-verbal cues and gestures to assess their comfort and they explained how knowing the residents well allowed them to do this. A staff member from nursing home 02 spoke about how they had also carried out a risk assessment of the residents taking part in the study before Namaste Care was commenced in the nursing home:
And we had to make sure that they didn’t have histories of like if we were going to massage their hands, have they got bone disease that might cause pain and things, so we had to . . . get doctor . . . to have a look through their records to make sure we weren’t going to make anything worse.
n02 (intervention), staff member S005
Namaste Care activities
Staff typically focused on pampering and hand massage during Namaste Care, despite the other elements being covered in the manual and training. This might have been because a hand massage demonstration formed part of the training sessions. The staff appeared to enjoy carrying out these activities with the residents and the residents generally appeared more calm and relaxed, benefiting from these touch-related activities:
Offered foot massage at the beginning of the session. Declined very strongly. Staff member gave him space and reassured him. Staff said enjoyed yesterday. Foot massage offered during the middle of the session. Resident allowed (the staff member) to do this, appeared relaxed and calm. Appeared to enjoy this very much.
Observation field note, n07 (intervention), resident R001
I think when I was doing it, that the most positive responses you got from the stimulation of the senses was the touch bit.
n02 (intervention), staff member S005
However, one resident was averse to the intimate nature of receiving a hand massage and did not feel comfortable in a room with dimmed lighting:
He would pull his hand away and he’d be like oh no . . . he was like no, and then he’d tell you get away, he didn’t like it so much . . . being in perhaps a dark, enclosed space just wasn’t right for him. He needed light and space and just to be able to move.
n04 (intervention), staff member S003
The way in which staff in the intervention nursing homes used dimmed lighting, candles, oils and music to create a calm and relaxing atmosphere was commented on in the informal carer interviews:
Well they dimmed the lighting and the different smells from the candles and stuff were quite sensory and it was relaxing, it’s very relaxing for him to be in there.
n02 (intervention), carer C002
Resident attendance at the Namaste Care sessions
Residents’ ability to attend the sessions regularly could be challenging because of illness; in addition, one resident died during the study:
. . . he started to deteriorate . . . he seemed to be sleeping a lot and I couldn’t engage with him.
n01 (intervention), carer C001
If they are unwell, if there are medical conditions, and we had that numerous experience here, one lady was quite poorly and she missed a couple of times because she was in her bed.
n07 (intervention), staff member S003
A few participants occasionally refused to go to the Namaste Care sessions, while some either wandered out or were taken out of sessions by staff because they became agitated:
. . . one time he couldn’t take part because he was very agitated and they couldn’t carry on with him that day.
n02 (intervention), carer C002
I think some days as you know he just walked out, he didn’t want to be there, so and because he didn’t speak it was hard to say why he walked out.
n07 (intervention), carer C001
Challenges associated with the implementation of Namaste Care
Resident-related factors
Staff often had to take many resident-related factors into account before deciding how and when to hold the sessions, and they could shorten the sessions to avoid challenging behaviour:
Everything’s challenging all the time, how long it’s going to last, are they going to let us do it, if it’s going to upset them, if it’s going to benefit them, it’s all day in’t it, it just depends.
n01 (intervention), staff member S005
Data indicated that staff adjusted the delivery of Namaste Care to take into account the nature of the activity, residents’ sleeping patterns and residents’ mood:
Seeing what their reaction is, ‘cause if they don’t like it [massage] on their hands they’re not going to want you to touch their head.
n02 (intervention), staff member S007
Yeah it was always an afternoon thing from really early on, because people’s moods are different at different times and all aren’t they? . . . We’ve got a lady who every day she’s been here she would sleep throughout the day, wake up at teatime and that’s her day.
n01 (intervention), staff member S006
Resource availability
The availability of resources, which included both staff and space, had an impact on how often the nursing homes were able to deliver Namaste Care sessions. This was particularly notable during periods when annual leave could be expected, such as the summer:
And we’ve had a lot of staff move around and people leaving nursing on residential.
n01 (intervention), staff member S005
Staffing levels weren’t really as great as they could have been . . . every session I felt I’m not going to fit this in today, I’ve got such and such to look after and such and such to do and such, yeah I felt really bad . . . I did me best, as I always do.
n04 (intervention), staff member S002
Learning for a future trial
-
Namaste Care is pragmatically incorporated into nursing home routines, and this should be anticipated.
-
While the person-centred nature of the intervention is acknowledged, staff need additional training on a wider range of activities to encourage their use in Namaste Care sessions.
Assessing the content of the Namaste Care or usual care sessions
Staff were asked to complete a session log each time a Namaste Care session occurred; this was to capture details of the residents attending, the time taken and the activities completed. Namaste Care sessions were observed intermittently during the study to assess the correspondence between what was observed to occur during the session and the activities recorded in the session log. There was high agreement between the Namaste Care session logs and the observation data. In a few instances the task had been completed by nursing home staff but had not been documented in the Namaste Care session log (Table 24).
| Nursing home | Observation time point | Set lighting | Music at start of session | Fragranced the room | Prepared activity items |
|---|---|---|---|---|---|
| n01 | 2 weeks | No Namaste Care | |||
| 4 weeks | 2 | 2 | 2 (not done) | 2 | |
| 24 weeks | No Namaste Care | ||||
| n02 | 2 weeks | 2 | 2 | 2 | 2 |
| 4 weeks | 2 | 2 | 2 | 2 | |
| 24 weeks | No Namaste Care | ||||
| n04 | 2 weeks | 2 | 2 | 2 | 2 |
| 4 weeks | 2 | 2 | 2 | 2 | |
| 24 weeks | No Namaste Care | ||||
| n07 | 2 weeks | 2 (not done) | 2 | 2 | 2 |
| 4 weeks | 0+ | 0+ | 2 | 2 | |
| 24 weeks | 0+ | 2 | 0 | 2 |
There was low agreement between the activity session logs and observation data for the resident being ‘individually greeted’ and also low agreement for ‘farewells’. This was explored during the staff interviews along with ‘comfort assessed’, as this measure could be assessed only partly by observation.
Whether or not nursing home staff in the control nursing homes carried out any of the Namaste Care preparation activities as part of their ‘usual care’ was documented during observation (Table 25). Table 25 lists the Namaste Care preparation activities that took place in the control (usual care) nursing homes. Like Namaste Care, the session in control nursing home 06 was carried out in a certain space and at a regular time, but it occurred only weekly for 1 hour. Although similar activities might have been carried out, the frequency of sessions differentiates this care from Namaste Care.
| Nursing home | Observation time point | Activity observed (yes/no) | |||
|---|---|---|---|---|---|
| Set lighting | Music at start of session | Fragranced the room | Prepared activity items | ||
| n03 | 2 weeks | Yes | No | No | No |
| 4 weeks | Yes | No | No | Yes | |
| n06 | 2 weeks | Yes | Yes | Yes | Yes |
| 4 weeks | Yes | Yes | Yes | Yes | |
Length of Namaste Care sessions
The length of the Namaste Care session documented in the session log was compared with the observation data to assess the agreement between the two sets of data (Table 26). The data show that the nursing homes sometimes overestimated the length of the session in the activity session logs, although they had been asked during the study training to document the time accurately. This may indicate that nursing home staff were concerned that they might have been viewed in a negative light if it was shown that they were not carrying out the length of session agreed with the research team.
| Nursing home | Observation time point | Length of session documented in the Namaste Care log | Length of session documented during observation |
|---|---|---|---|
| n01 | 2 weeks | No Namaste Care session | N/A |
| 4 weeks | 65 minutes plus 10 minutes’ set-up and 5 minutes’ clear-up time | 35 minutes; set-up and clear-up not observed | |
| 24 weeks | No Namaste Care session | N/A | |
| n02 | 2 weeks | 60 minutes plus 30 minutes’ set-up and 10 minutes’ clear-up time | 60 minutes; set-up and clear-up time not observed |
| 4 weeks | 60 minutes plus 20 minutes’ set-up; clear-up time not documented | 45 minutes; set-up and clear-up time not observed | |
| 24 weeks | No Namaste Care session | N/A | |
| n04 | 2 weeks | 120 minutes plus 20 minutes’ set-up and 15 minutes’ clear-up time | 120 minutes; set-up and clear-up time not observed |
| 4 weeks | 60 minutes plus 15 minutes’ set-up and 15 minutes’ clear-up time | 60 minutes; set-up and clear-up time not observed | |
| 24 weeks | No Namaste Care session | N/A | |
| n07 | 2 weeks | 120 minutes plus 10 minutes’ set-up and 15 minutes’ clear-up time | 115 minutes; set-up and clear-up time not observed |
| 4 weeks | 120 minutes plus 15 minutes’ set-up and 15 minutes’ clear-up time | 110 minutes; set-up and clear-up time not observed | |
| 24 weeks | 90 minutes plus 30 minutes’ set-up and 10 minutes’ clear-up time | 85 minutes; set-up and clear-up time not observed |
Learning for a future trial
-
Activity session logs represent the content of Namaste Care sessions sufficiently accurately, and observation could be reduced in a full trial, but improved recording of the start time of a session and when an individual starts to receive Namaste Care needs to be addressed.
Impact of the Namaste Care intervention
Perceived benefits of Namaste Care
Both informal carers and staff commented on the impact that Namaste Care had had on the quality of life of the residents who took part in the study, especially in terms of being more aware of and engaged with family, staff and their surroundings:
She seemed to have more eye contact rather than just kind of staring, and she would smile more occasionally you know when we’d go in and perhaps give her a kiss and a hug . . . Mum seemed to react quite a lot then with her facial expressions.
n01 (intervention), carer C002
She’s responding more as well with proper words we can understand, ‘cause I asked her the other day are you all right? She said ‘Yeah I’m fine’ and I like did a double take, I’ve never heard her talk clearly.
n02 (intervention), staff member S005
Residents were generally found to be much more relaxed and less agitated during and after a Namaste Care session:
I think he’s more relaxed, more sort of settled and looks contented.
n04 (intervention), carer C003
The people who are in there, in that time they are much calmer than while they are sitting in the communal lounge, there seems to be much relaxed.
n07 (intervention), staff member S003
This helped informal carers spend more quality time with the residents:
Well we’ve got a lady that’s quite stiff, and when we do the touch therapy she loosens up and the family have noticed it and they’ve started massaging and stuff like that . . . they think she’s better after a massage now so they do it . . .
n01 (intervention), staff member S005
Staff members’ view of Namaste Care was similar to that of informal carers, explaining that it was different from their day-to-day practice, which was of great benefit to the quality of care and life of the residents:
For me, what I see is it’s a one to one . . . something different away from the client group, away from the communal lounge, somewhere in a quiet area.
n07 (intervention), staff member S003
Many of the staff members who were involved in delivering the Namaste Care sessions appreciated the one-to-one time they spent with the residents, feeling that it helped them build a rapport with residents and provide a better quality of care:
It’s bonding as well with them in’t it I suppose, in a different way, bonding with them without actually having to do the talking and that, just the touch therapy and that . . . It’s like a softer bond, it’s very mellow and soft and gentle.
n01 (intervention), staff member S006
As a result, some staff members talked about adopting elements of Namaste Care into their day-to-day practice:
I find myself more doing little bits and bobs with other residents, you know if they’re distressed and that I think right I’ll just use that now and I’ll do a hand massage and whatever.
n04 (intervention), staff member S002
Although informal carers and staff members talked about residents being able to close their eyes and relax/sleep during the Namaste Care sessions, or being more alert after the sessions, they did not comment on residents' quality of sleep at night:
It’s also stimulation in a soothing, calming way, ‘cause some of ours went to sleep.
n02 (intervention), staff member S007
With regard to level of activity, one resident was reported to have become more mobile following a few Namaste Care sessions, while a female resident in another nursing home who was known to undress in public was reported to have stopped this after attending the sessions. Some of the benefits were perceived to be subtle:
A lot of people would assume that that sort of care is what people do anyway, and the subtlety of the difference between the intensity and the frequency, they wouldn’t see that.
n01 (intervention), carer C001
Informal carers appreciated the one-to-one attention received by the residents:
A little bit of like I say one-to-one attention and a bit of extra care . . . anything that’s going to try and make life easier for them.
n04 (intervention), carer C003
Additionally, among those informal carers who were able to attend the Namaste Care sessions, the sessions were considered an opportunity to be involved in the care of the residents and connect with them in a more intimate way:
One of the biggest impacts was on us as a family, to think more about how mum could be more involved and sort of reached somehow . . .
n01 (intervention), carer C002
Learning for a future trial
-
Further consideration is required of how to capture the different changes (increased engagement or being calmer) observed by staff in residents, using a measure or measures.
Challenges in attributing benefits to Namaste Care
Although informal carers and staff reported that Namaste Care had a positive impact on residents overall, some remained cautious about drawing a direct link between Namaste Care sessions and the changes observed in the residents:
I don’t know if it was because of the Namaste or something happened in his brain, but it’s happened during this time, the man suddenly started to walk or to recognise his daughter when she’s come to visit and when she tried to leave the building he gone to the window and tried to wave at her.
n07 (intervention), staff member S003
They noted the difficulty in determining whether changes in residents’ mood and behaviour (e.g. feeling calmer and acting less agitated) were the result of the sessions or the gradual progression of their dementia:
They’re so ill with dementia, I think it was hard to gauge how much benefit they actually got from it. I think they did benefit from it, but as to say how much, it would be very hard to say because they change so much, they change every day. The men, you know you can go in one day and they’ll be quite settled and they’ll talk to you and smile and stuff, and then the next day they don’t want to know.
n02 (intervention), carer C002
But it’s hard to assess whether it is Namaste or whether it’s a deterioration of her dementia . . . at the end part of that dementia spectrum, if they were presenting with noisy behaviour before, they go really, really calm, and that’s the deterioration, and we could be viewing it as a positive thing but it’s not.
n02 (intervention), staff member S005
They also highlighted that, in many cases, this impact was not permanent or long term, as residents’ agitation continued to fluctuate:
Well he just seemed to be not as agitated as he normally is, but this can change as you know, I mean every day is different and some days even now when I go, he can be quite agitated or sleepy, or on the other hand he can be talking.
n02 (intervention), carer C002
Without being able to ask the residents directly about their experience of Namaste Care, informal carers were also wary of overstating the positive experience and impact of the sessions:
It’s difficult ‘cause you don’t get a lot of response from him . . . I don’t know whether, I mean [resident’s name] doesn’t open his eyes really so he’s not and I don’t know how much they hear, but he must be hearing it because he’s nice and relaxed.
n04 (intervention), carer C003
Perceived impact of usual care
There was recognition of the person behind the dementia in the usual care and activities that staff members provided in the control nursing homes:
We’ve gone through a bit of a change process, due to changes in staff really, we’ve just brought on board this week a new full timer who is just looking like she’s going to be absolutely fantastic, and she’s really tailoring the activities specifically to the individual in terms of their level of disability and interaction, so she’s doing lots of sensory activities, lots of one to one . . . There’s a lot of trial and error, but what [S008] does is she does a ‘this is me’ on admission to get some idea of good ideas that we can theme activities around.
n03 (control), staff member S004
However, a staff member in one of the control nursing homes reported:
I think we do struggle with finding activities for people with advanced dementia. At the moment the activities co-ordinator in particular provides a relaxation morning so that’s quite nice with a darkened room and lights and music, which is quite nice, and there’s some hand massage, it’s difficult for interactive group work.
n06 (control), staff member S003
Satisfaction with care was also reported in control sites. The two informal carers interviewed in the control nursing homes were very appreciative of the care provided and felt that the staff were doing everything they could to engage with residents:
They’re still trying with them, they’re still trying. I’m trying to think who they’ve had a reaction out of, you don’t see much reaction out of any of them. I think they’re doing everything they possibly can to engage them really.
n06 (control), carer C003
This particular informal carer spoke about some of the activities that she had seen when visiting the nursing home:
I’ve been there when they’ve done the light activities . . . when they’ve played the nice classical music and it’s soothing . . . when they’ve had the tambourines out and that type of thing, so there’s a lot of that. And they tried to do like craft stuff with them as well . . . We went when they did the dogs as well, they bring the dogs in, the husky dogs, they do all that type of thing.
n06 (control), carer C003
Learning for a future trial
There is perceived benefit of Namaste Care as an intervention for people with advanced dementia as identified by informal carers and staff that warrants further research.
In summary, the findings from the process evaluation interviews and observations identified that staff were delivering Namaste Care in ways that mapped onto the proposed programme theory. Staff and informal carers described moments of connection with residents that had not been there before.
Chapter 6 Economic analysis
Introduction and aims
The economic component of the research aimed to inform the design of an economic evaluation alongside a full trial of the Namaste Care intervention. There were four main aims of the economic element of the study:
-
determining the excess costs associated with the provision of the Namaste Care intervention
-
exploring the feasibility of collecting relevant information on the resources used by nursing homes, primary care, secondary care and informal carers for nursing home residents suitable to take part in the Namaste Care intervention
-
carrying out a cost analysis to identify the resource use elements that make a significant contribution to the total per-patient cost of care provided under each of the assessed options, as well as the variability in resource use items across nursing homes
-
exploring the feasibility and acceptability of proxy completion of alternative outcome measures to the EuroQol-5 Dimensions (EQ-5D) for people with advanced dementia.
Aims 1–3 are covered in Feasibility of resource use data collection and valuation and aim 4 is covered in Feasibility and acceptability of economic outcome assessment.
Methods
Feasibility of resource use data collection and valuation
Detailed exploration of the costs of Namaste Care
An initial costing of the Namaste Care programme intervention itself was undertaken, the aim being to understand the costs of the intervention for use in a full trial. Information on the costs that the nursing homes incurred in implementing the Namaste Care sessions was gathered through interviews with nursing home staff members, usually the manager. Information about staffing levels and the costs of any additional items purchased for use in the Namaste Care sessions (i.e. items that would not have been purchased if usual care had been provided) were obtained directly during these interviews. These data were then combined with data on staff participating in sessions and the usage of these items, which were captured from activity session logs completed concurrently by staff carrying out the sessions. The unit costs of additional staff hours required were obtained directly from nursing homes; when this was not feasible, the Personal Social Services Unit’s Unit Costs of Health and Social Care 2017180 was used. As not all nursing homes incurred additional costs, information is presented both as means across all nursing homes/nursing home residents and as means across just those nursing homes/nursing home residents incurring additional costs.
Feasibility of obtaining information on residents’ resource use
The exploration of the feasibility of obtaining information on residents’ resource use began with an initial consultation with three nursing homes to ensure that the relevant costs were identified for inclusion in the analysis. Additional discussion took place about the way in which residents used primary care services and the best methods of accessing the data. The following relevant costs were identified and collected for the analysis.
-
Primary care use: data on the use of services by each resident during the trial were extracted from the care records kept within the nursing home.
-
Secondary care use: data on the use of services by each resident during the trial were extracted from the care records kept within the nursing home.
-
Medication use: residents’ medical records were used to gather data on medications used during the trial, both total medication used and psychotropic and pain medications used (i.e. those medications most likely to be affected by the implementation of the intervention).
-
Informal care costs: resource use by informal carers was collected using an adaptation of the Client Service Receipt Inventory, including use of any primary care services and support groups, items purchased, financial contributions to the nursing homes and productivity costs, including any working hours sacrificed.
Difficulties in collecting specific aspects of resource use data were noted so that these could be addressed in a full trial. The extent to which nursing homes varied in data accessibility and the level of detail in their records was noted. The extent of missing data across different resource use items was estimated where feasible.
Understanding cost drivers
An initial assessment of the costs of the intervention and resources used by the nursing home residents (cost analysis) was carried out to convert the resource use into cost estimates and identify the key drivers of the costs of the intervention to ensure that these costs would be collected for future trials. To expand the analysis to consider cost drivers, a valuation of the collected resource use information was undertaken. The mean differences in total cost per patient associated with each trial arm were analysed over a time horizon of 4 weeks, corresponding to the trial follow-up period. A complete-case analysis was undertaken as there were few missing data; where there were concerns about the quality of data, one-way sensitivity analyses were undertaken to explore the implications of these concerns. A one-way sensitivity analysis was also undertaken substituting recently published data on the costs of the Namaste Care intervention181 for the costs collected during the feasibility trial. No discounting of costs was necessary as the trial was conducted within a single year. All resource use data were costed in Great British pounds in values from a single year.
-
Primary care use: the costs of primary care service use were based on the Unit Costs of Health and Social Care 2017. 180
-
Secondary care use: the unit costs were obtained from published NHS reference costs. 182
-
Medication use: the Prescription Cost Analysis data set183 was used to estimate the costs of residents’ medication use.
-
Informal care costs: only a very small number of informal carers were recruited and so these data were not considered further.
Feasibility and acceptability of economic outcome assessment
The study was designed to assess the feasibility of using a number of outcome measures. The EQ-5D is the standard measure used in economic evaluation, although there is currently some uncertainty about which specific measure to choose of the new EuroQol-5 Dimensions, five-level version (EQ-5D-5L) and the older, more established, EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 184–187 The EQ-5D has been used in nursing home settings,188–191 but there is some concern about whether or not health measures such as the EQ-5D capture the outcomes important to those coming towards the end of life. 192,193 However, alternative generic measures for those in the later stages of the life-course194 are relatively new and untested, although they are included in the National Institute for Health and Care Excellence (NICE) recommendations for assessing social care interventions195 and recommended in other settings for long-term care. 196 This work explores proxy completion rates for EQ-5D and two alternative ICECAP (ICEpop CAPability) measures for those nearing the end of the life-course: the ICECAP measure for Older people (ICECAP-O)197,198 and the ICECAP Supportive Care Measure (ICECAP-SCM) for those at end of life. 199,200 Given the much smaller evidence base for these alternative capability measures, the research focuses mainly on exploring the feasibility and acceptability of these two generic capability measures.
The ICECAP-O is a generic capability-based outcome measure for older people. It has five attributes (attachment, security, role, enjoyment and control), each with four levels. The descriptive system was generated using in-depth qualitative methods198 and valuations were obtained using best–worst scaling. 197 The measure has been validated in a number of settings; its use with dementia patients, however, has been limited to translated versions,201–203 although these suggest favourable validity assessment,201 with the measure showing good convergent and discriminant validity for people with dementia. 202,203
The ICECAP-SCM is a generic capability-based outcome measure for adults at the end of life. It has seven attributes (love and friendship, choice, physical suffering, emotional suffering, dignity, support and preparation). As with the ICECAP-O, the descriptive system was generated using in-depth qualitative methods200 and valuations were obtained using best–worst scaling. 199 The measure is still very new, and published evidence so far has been limited to its use in palliative care patients in a hospice setting. 204
Completion rates
The numbers of missing values for each item of each questionnaire were assessed for all patient outcome measures across the whole patient group, the small numbers included.
Valuation of outcomes
Values were generated for each of the outcome measures collected for each resident at each time point. For the valuation of the EQ-5D-5L responses, the value set mapped from the EQ-5D-5L was used to generate index values in accordance with NICE guidelines at the time of analysis. 205 Valuation of the ICECAP measures responses was carried out using published UK tariffs,197,199 with the valuation for ICECAP-SCM using the main effects tariff. 199
Relationship between economic outcome measures and key measures likely to be taken forward in a full trial
Pearson’s correlations were used to explore the strength of the relationship between overall scores for each of EQ-5D-5L, ICECAP-O and ICECAP-SCM and each of QUALID and the CMAI. The strength of relationship was assessed based on Cohen’s criteria of interpreting correlations of 0.10–0.30 as weak, 0.30–0.50 as moderate and ≥ 0.50 as strong. 206
Think-aloud assessment of feasibility and acceptability for ICECAP-O and ICECAP-SCM
The think-aloud technique was used to assess the feasibility of proxy respondents completing the ICECAP-O and ICECAP-SCM. This involves verbalising thoughts while completing questionnaires.
Sampling
A purposive sample of nursing home staff was recruited to ensure coverage of residents at different time points and across different nursing homes. The sample size was intended to be adequate for exploring the key issues arising in completion of the measure in this context; a sample of 20–30 interviews was originally envisaged, in line with similar think-aloud studies. 204,207,208
Data collection
Each think-aloud interview began with a warm-up exercise to ensure that the person responding understood the nature of ‘thinking aloud’. The respondent was then asked to think aloud while completing the measures. After this, a semistructured interview probed areas of particular difficulty respondents encountered when completing the measures and more general views about the appropriateness of the measures.
Data analysis
The think-aloud interviews were fully transcribed. Three raters (JC, PM and GM) independently utilised segments within these transcripts, alongside the scores given by the proxy respondents, to identify errors in completion associated with comprehension, retrieval, judgement or response, or identifying a struggle in arriving at a correct response (see Appendix 9). Constant comparative analytical methods were used to provide a more in-depth assessment of the questionnaire; open coding of three transcripts was completed by three researchers (JC, PM and GM) and an initial coding structure was discussed. Analysis was then conducted by JC, who read and re-read transcripts, applied codes to the data and wrote analytic accounts for different aspects of the data. The data presented here related to feasibility issues in responding to the questionnaires, coded under ‘judgement’ in the initial analysis.
Results
Feasibility of resource use data collection and valuation
All costs are presented in 2017/18 Great British pounds.
Detailed intervention costing
Two of the four intervention homes reported that they had incurred costs associated with providing Namaste Care, namely the cost of purchasing consumable items and the costs incurred for extra staff time. Additional consumable costs were incurred for items such as massage oils, throws, diffusers, flowers and snacks (the full list and associated costs are in Appendix 10, Table 46). The mean cost per nursing home of these items across all four nursing homes was £98.35 (£196.69 across the two nursing homes incurring costs). These two facilities also required additional staffing to carry out the programme sessions. Additional staff costs over the 4 weeks of running the programme were a larger component of running the programme, amounting to a mean cost per nursing home of £396.67 (£793.34 across the two nursing homes that incurred costs). Overall, the mean cost per resident receiving the Namaste Care intervention over a 4-week period was £110.96 (£221.92 across the two nursing homes that incurred costs). For the two nursing homes that incurred costs, this equated to £8.30 per resident per session received.
Feasibility of obtaining data on residents’ resource use
Some feasibility issues were noted in collecting resource use data. Records were accessible for all of the trial participants through the nursing homes; however, the detail in these records varied. It should, for example, be noted that the nursing home that maintained the most detailed records was a control nursing home; in a full trial, using these data in an unthinking manner could potentially skew the resource use estimates in one direction. One specific area where there was clear variation in the detail on resource use was the recording of primary care services, particularly in relation to routine GP visits. Some nursing homes recorded any routine visits to the facility that resulted in any discussion regarding the resident, while others recorded only non-routine visits made to a specific patient.
Information about secondary care use and medication use was recorded in precise detail because this is a legal requirement; however, in one nursing home the medication records had already been archived by the time of data collection. Some information was available, but some had already been taken off-site. This is an issue to be aware of when planning for a full trial.
The mean per-resident use of NHS and Personal Social Services resources in each nursing home and between the trial arms is presented in Table 27.
| Service | Mean per-resident frequency of NHS service use | |||||||
|---|---|---|---|---|---|---|---|---|
| 01 | 02 | 04 | 07 | Intervention | 03 | 06 | Control | |
| GP home visit | 1.00 | 0.50 | 0.70 | 0.40 | 0.65 | 2.50 | 0.80 | 1.65 |
| GP telephone call | 0.80 | 0.00 | 0.30 | 0.40 | 0.38 | 0.00 | 0.00 | 0.00 |
| Cardiac nurse | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 0.10 |
| Parkinson’s nurse | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | 0.00 | 0.25 |
| Physiotherapist | 0.20 | 0.00 | 0.00 | 0.00 | 0.05 | 0.30 | 0.00 | 0.15 |
| Psychiatrist | 0.20 | 0.00 | 0.30 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 |
| Social worker | 0.20 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.30 | 0.15 |
| Occupational therapist | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.80 | 0.00 | 0.40 |
| Dietitian | 0.4 | 0.00 | 0.00 | 0.00 | 0.10 | 0.5 | 0.2 | 0.35 |
| Memory service | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | 0.20 | 0.35 |
| Inpatient services | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 | 0.20 | 0.25 |
| Outpatient services | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.20 | 0.10 |
| Ambulance | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.70 | 0.35 |
Learning for a future trial
-
Collecting resource use data for an economic evaluation from nursing homes is feasible.
-
Study-specific data collection is needed for information about GP visits.
-
Procedures such as regular ongoing data collection should be put in place to ensure that records for trial participants are not archived before data are collected.
-
A full economic evaluation should focus on obtaining good estimates of resource use in relation to GP visits, inpatient stays, outpatient visits, ambulance costs and medication, as these are the main drivers of costs.
Understanding cost drivers
Table 28 provides detailed information on the mean individual NHS and Personal Social Services costs, the medication costs and the intervention costs to give total cost estimates from the perspective of the NHS/Personal Social Services. These estimates include all nursing homes, but some issues with these costs should be noted. One is the greater level of detail available for primary care costs in the control arm; the second is the archiving of some medication use records for residents of nursing home n07, affecting four residents to varying degrees; and the third is the atypical use of dementia memory assessment services by three residents, all living in control nursing homes.
| Service | Mean NHS service use cost (£) per resident | |||||||
|---|---|---|---|---|---|---|---|---|
| 01 | 02 | 04 | 07 | Intervention | 03 | 06 | Control | |
| GP home visit | 38.00 | 19.00 | 25.33 | 15.20 | 24.38 | 95.00 | 31.67 | 63.34 |
| GP telephone call | 11.68 | 0.00 | 4.87 | 5.84 | 5.60 | 0.00 | 0.00 | 0.00 |
| Cardiac nurse | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 14.33 | 7.17 |
| Parkinson’s nurse | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 38.00 | 0.00 | 19.00 |
| Physiotherapist | 11.40 | 0.00 | 0.00 | 0.00 | 2.85 | 14.25 | 0.00 | 7.13 |
| Psychiatrist | 36.00 | 0.00 | 60.00 | 0.00 | 24.00 | 0.00 | 0.00 | 0.00 |
| Social worker | 11.80 | 0.00 | 0.00 | 0.00 | 2.95 | 0.00 | 19.67 | 9.84 |
| Occupational therapist | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 60.75 | 0.00 | 30.38 |
| Dietitian | 34.40 | 0.00 | 0.00 | 0.00 | 8.60 | 43.00 | 14.33 | 28.67 |
| Memory service | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 310.00 | 206.67 | 258.34 |
| Inpatient services | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 176.83 | 88.42 |
| Outpatient services | 0.00 | 0.00 | 106.33 | 0.00 | 26.58 | 0.00 | 115.83 | 57.92 |
| Ambulance | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 82.00 | 41.00 |
| Medication costs | 303.62 | 167.80 | 92.44 | 3.65 | 146.00 | 120.77 | 137.41 | 127.90 |
| Intervention costs | 0.00 | 239.14 | 0.00 | 204.70 | 110.96 | 0.00 | 0.00 | 0.00 |
| Mean total NHS/Personal Social Services costs | 446.90 | 425.94 | 288.98 | 229.39 | 344.61 | 681.77 | 798.74 | 731.90 |
Table 28 shows that the key areas of resource use for collection in a future trial, in addition to the cost of the intervention, are perhaps as would be expected: GP visits, inpatient stays, outpatient visits, ambulance costs and medication. Dementia memory services also appear to be important but may well be atypical, as discussed below.
Appendix 11 contains greater detail about some areas of resource use related to these issues. In Table 48, the total medication cost is included, but it may be that the costs associated specifically with psychotropic and pain medication use are more relevant. These costs are considerably lower on average in both intervention (psychotropic, £3.51; pain, £17.84) and control (psychotropic, £43.29; pain, £4.03) nursing homes; Table 48 also provides medication costs both including and excluding n07 from the analysis because of the problems with archiving resource use data at this nursing home. The implications of excluding n07 from the analysis are shown in Appendix 11, Table 49, namely that the total cost of all resource use in the intervention arm rises to a mean of £400.43. Appendix 11, Table 50, explores the implications of removing resource use related to memory services given the atypical use of this service by individuals already living with advanced dementia. Excluding these costs makes a large difference to the mean cost per resident for the control group, reducing it to £466.18. Appendix 11, Table 51, explores the implications of removing set-up costs for Namaste Care, which would typically be allocated over many more individuals were the service to be adopted on a long-term basis. This reduces the costs in the intervention arm to £324.16 (£395.18 excluding n07). Appendix 11, Table 52, examines the implications of applying the modelled cost estimates of Namaste Care generated by Bray et al. 181 Using these costs increases the mean cost of the Namaste sites for two reasons. First, although the cost is very similar to that generated through the trial, it is applied to all intervention sites and not just those that actually incurred costs. Second, the one nursing home that did incur costs provided a larger number of sessions than was assumed in the modelled costs.
Feasibility and acceptability of economic outcome assessment
Completion rates
All measures were completed by staff proxy respondents and had high completion rates. Across 94 completions of the measure (three completions for 31 patients and one completion for one patient), only six dimensions had any missing data: for EQ-5D-5L, pain/discomfort (n = 1) and anxiety/depression (n = 5); for ICECAP-O, security (n = 5); and for ICECAP-SCM, dignity (n = 1), support (n = 2) and preparation (n = 5). The overall completion rates were 98.7% for EQ-5D-5L, 99.4% for ICECAP-O and 98.8% for ICECAP-SCM.
Valuation of outcomes
Table 29 provides information about the mean values at each time point for each measure by trial arm.
| Trial arm | Mean (SD) EQ-5D index values | Mean (SD) ICECAP-O tariff values | Mean (SD) ICECAP-SCM tariff values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 4 weeks | Baseline | 2 weeks | 4 weeks | Baseline | 2 weeks | 4 weeks | |
| Intervention | |||||||||
| n | 16 | 14 | 15 | 16 | 16 | 15 | 17 | 16 | 14 |
| Value | 0.07 (0.23) | 0.10 (0.19) | 0.10 (0.27) | 0.50 (0.22) | 0.57 (0.16) | 0.55 (0.15) | 0.70 (0.14) | 0.74 (0.11) | 0.73 (0.11) |
| Control | |||||||||
| n | 13 | 13 | 11 | 14 | 14 | 14 | 14 | 13 | 14 |
| Value | 0.05 (0.28) | 0.04 (0.28) | 0.07 (0.25) | 0.54 (0.17) | 0.58 (0.11) | 0.56 (0.14) | 0.60 (0.20) | 0.59 (0.16) | 0.70 (0.13) |
Relationship between economic and non-economic measures likely to be taken forward to a full trial
Table 30 shows correlations between the economic outcome measures and key outcome measures likely to be taken forward to a full trial. Correlations were generally stronger between the QUALID and all of the economic outcome measures than between the CMAI and all of the economic outcome measures. For QUALID, correlations were generally stronger with ICECAP-SCM than with the other two measures, although there was some variation across time points. There was a clear pattern of strength of correlation for the economic measures with CMAI, with no or weak correlations for EQ-5D-5L, weak correlations for ICECAP-O and moderate correlations for ICECAP-SCM.
| Time point | QUALID | CMAI | ||||
|---|---|---|---|---|---|---|
| EQ-5D-5L | ICECAP-O | ICECAP-SCM | EQ-5D-5L | ICECAP-O | ICECAP-SCM | |
| Baseline | –0.31b | –0.59c | –0.63c | 0.14 | –0.19a | –0.43b |
| 2 weeks | –0.37b | –0.18a | –0.60c | –0.10a | –0.14a | –0.44b |
| 4 weeks | –0.10a | –0.33b | –0.23a | 0.10 | –0.10a | –0.35b |
Learning for a future trial
Further evidence is needed on the validity and sensitivity to change of the economic measures.
Think-aloud assessment of feasibility and acceptability of ICECAP-O and ICECAP-SCM
Twelve proxy staff respondents were included in the think-aloud study, providing think-aloud assessments of questionnaire completion and data from semistructured interviews. Residents for whom the respondent was acting as proxy were, according to the proxy respondents, aged in their early 70s to their 90s; nine were male and three were female. Residents had lived in the nursing home for periods of time that ranged from relatively short (5–6 months) to longer (4–5 years); most had lived in the nursing home for between 1 and 2 years. All proxy respondents had substantial care home experience, having worked in care homes in all cases for at least 2 years. Most had known the resident since the resident had arrived in the nursing home and most spent considerable amounts of time with the resident for whom they were proxying, usually at least 1 hour each day but often substantially more.
The numbers of errors for each of the ICECAP-O and ICECAP-SCM are given in Table 31. The overall error (struggle) rate for ICECAP-O was 6.7% (5%) and for ICECAP-SCM was 8.3% (6%). (Definitions are provided in Appendix 9.) More insight and understanding about the feasibility of completing these measures as a proxy respondent in this context came from the qualitative analysis of the data.
| Questionnaire | Error type (n) | Total errors (n) | Total struggles (n) | |||
|---|---|---|---|---|---|---|
| Comprehension | Retrieval | Judgement | Response | |||
| ICECAP-O | ||||||
| Attachment | 1 | 1 | 1 | |||
| Security | 1 | 1 | 2 | 0 | ||
| Role | 0 | 1 | ||||
| Enjoyment | 0 | 1 | ||||
| Control | 1 | 1 | 0 | |||
| Total | 0 | 1 | 1 | 2 | 4 | 3 |
| ICECAP-SCM | ||||||
| Choice | 0 | 1 | ||||
| Love and friendship | 1 | 1 | 0 | |||
| Physical suffering | 0 | 1 | ||||
| Emotional suffering | 1 | 1 | 1 | |||
| Dignity | 1 | 1 | 1 | |||
| Support | 0 | 1 | ||||
| Preparation | 2 | 2 | 4 | 0 | ||
| Total | 0 | 2 | 5 | 0 | 7 | 5 |
Problems with making judgements
Proxy respondents at times found it difficult to make judgements on behalf of residents. There were various reasons for this, including having limited knowledge of the respondent, needing to interpret and ‘stand in another’s shoes’, difficult choices about perspective, conflicts with the care role and (in just one case) a feeling of having had insufficient contact with the resident to act as an adequate proxy.
At times, when responding to the capability questions, proxy respondents struggled with limited knowledge. This arose in part because some residents had a limited ability to communicate, but also because some areas of knowledge were outside the scope of the carer relationship:
If a person isn’t able to express themselves, they’re relying on you to get it right, that bit I find a bit difficult.
06S008-baseline
. . . some like maybe we’re not in that zone to answer you know, like you’re saying the financial side of stuff . . .
03S009-baseline
Around half of respondents talked about the difficulty of making judgements on behalf of others, particularly when those others are living with dementia. Respondents used imagery such as ‘trying to get into their thoughts’ (06S008-baseline), being ‘stood in his shoes’ (02S005-2 week), having ‘to put myself into somebody else’ (02S005-2 week) and ‘answering questions as if I am looking through her eyes’ (06S007-4 week) to convey the difficulty – and responsibility – they faced in trying to make an appropriate judgement on behalf of their resident:
So, we don’t know what’s going on in their head, so we don’t know what’s going on in their thoughts, so we don’t know what’s going on in their feelings, and we have to interpret them in the best way that we can in the hope that we are spot on.
02S005-2 weeks
Particularly for the dignity question, some respondents struggled to come to a judgement because they were unsure whether to answer from the perspective that attempts were made to maintain the dignity of the resident, or from the perspective that what the resident was facing was intrinsically undignified:
. . . as carers we do try and maintain their dignity all the time, but when you’re having to give somebody a wash, it’s not very dignified, so they probably would feel like there is no dignity in it. But to us we’re doing it in the most dignified manner, so it’s a struggle to answer this question . . . It’s quite a hard question to answer because if you write it from a carer’s point of view, we would put that we do maintain their dignity and respect but they probably feel like it’s not . . .
03S011-4 weeks
A few proxy respondents at times felt a conflict when responding to the questions relating to their role as a provider of care because of the self-judgement involved:
The only thing is I have to be careful that I don’t answer them as my job and not the person, because it’s my job to think people are being treated and respected, and it’s my job to make sure people have got support, and if I thought somebody wasn’t being treated, I’d be very disappointed . . .
07S004-4 weeks
Coming to a judgement
In practice, different proxy respondents drew on a number of sources when making their judgements, with some drawing on more than one source of information. Specifically, proxy respondents drew on communication from the resident, their knowledge of the resident and their family, their knowledge of the condition and their knowledge of the nursing home and observable cues, and they also made assumptions.
In a very small number of cases, respondents were able to give proxy responses to the items in the capability instruments based on their ongoing communication with the resident:
. . . knowing this particular person makes it a lot easier, because he is able to express himself. If a person isn’t able to express themselves, they’re relying on you to get it right, that bit I find a bit difficult.
06S008-baseline
Knowledge of the resident, and indeed of the people close to the resident, was important information that the proxy respondents drew on when available:
Quite easy, we know the residents very well so.
03S011-4 weeks
For attributes that focused on relationships (attachment, love and friendship), the proxy respondent’s knowledge often came through visits from family and friends, whereas for attributes related to the functional abilities of the resident (control, choice) the proxy respondent often drew on their direct knowledge of the caring situation. For attributes related to affect (feelings), respondents also drew on their own knowledge of the resident in some circumstances. Only a few proxy respondents had direct information that enabled them to answer the question on preparation:
. . . he’s got he’s full access to be with the people he cares about most all the time, most of the time, so yes, he has.
07S004-4 weeks
. . . he’s able to be independent in a few things ‘cause when he came to us, he wasn’t even using a knife and fork and things, so he’ll hold his own cup and things now, so he’s got a little bit of independence there.
04S002-4 weeks
Enjoyment and pleasure. I’m going to have to tick two. Because he doesn’t tend to participate in one to ones or group situations, and when [wife] comes, although he appears to smile, it’s not like a happy smile, and it’s a smile that wears off and he just sort of sits there . . .
02S005-2 weeks
However, the ability of the proxy respondent to know the resident well did rely in part on the nature of the resident:
More probably because they’re a bit closed book, like I say people are different and this patient is a bit closed book, so where you’ve got more flamboyant patients or you know a bit more character, this was a bit like mind-reading so, but I think pretty much got there, me and [name of staff member] both put our heads together on it so.
03S009-baseline
Some proxy respondents used their knowledge or perceptions of illness, particularly in relation to advanced dementia, when responding to some of the capability questions. This information was used particularly for questions about aspects that were likely to be affected by cognitive issues but that were less observable than other similarly affected capabilities, for example in relation to security (thinking about the future without concern) and role (doing things that make you feel valued):
I will presume on the thinking about the future, because he’s got short-term memory loss that he doesn’t generally think about it with any concerns, in his day-to-day life . . .
01S004-2 weeks
In some cases, respondents drew on their knowledge of the nursing home when responding to the capability questions. This knowledge tended to be used most often when responding to questions about personal interaction between the resident and other individuals, including attachment, enjoyment, love and affection, dignity and support:
Yeah, so being treated with respect, being spoken to with respect, having their religious and spiritual beliefs respected, being able to be him- or herself, being clean and having privacy. Well that’s what we strive for, so I’ll put . . .
01S003-2 weeks
On some occasions respondents used observable ‘cues’ to arrive at a judgement, particularly for attributes related to feelings. These cues included both facial expressions and agitation; however, proxy respondents were also sometimes aware that these cues might be interpreted inaccurately:
Emotional suffering, worries and stress. Sometimes he’ll pull a face when he’s suffering stress or anything.
06S012-baseline
Physical suffering . . . it’s weird because he doesn’t show any outward signs of physical suffering. Now whether he can’t express physical suffering, I’m not really sure . . .
06S002-2 weeks
Finally, on occasion, and having exhausted other sources of information, proxy respondents resorted to making assumptions; this was most marked in relation to the preparation attribute:
I haven’t a clue if he’s got any of this in place or not. I’m assuming he would have had this conversation with his wife . . . so I’m going to put a few, I don’t know, I haven’t a clue, I’ll put a few ‘cause I would like to think that he’s got something in place with his wife, but I don’t know 100%.
01S003-2 weeks
Learning for a future trial
The way that proxy respondents complete measures on behalf of those with advanced dementia should be accounted for when interpreting data collected in this way.
Discussion
The economic element of the feasibility study has explored the issues with collecting resource use data from nursing homes to estimate the costs associated with Namaste Care provision, and has explored the use of new economic outcome measures, the ICECAP-O and ICECAP-SCM, when these are completed by paid nursing home staff acting as proxy respondents for residents with advanced dementia. Collecting resource use data was found to be feasible, although in some areas the quality of the data could be increased. The completion of measures by proxy respondents was explored in depth; respondents were aware of the difficulties and limitations they faced when answering questions on behalf of residents, but they worked hard to draw on available information so that they could answer the questions and reflect residents’ quality of life as best they could.
At the time this study was conducted, the costs of Namaste Care had not been estimated, but the basis of Namaste Care is that it is cost neutral;181 the intervention is intended to involve the use of existing resources in additional ways (although there is also the suggestion that some additional resources could be used to help with atmosphere and activities). 154 Therefore, the costing of Namaste Care in this study included only the additional costs associated with its provision. However, nursing homes were found to differ in the extent to which they incurred additional costs, with some incurring no additional costs and others incurring costs equivalent to an average of £222 per resident across a 4-week period. Recent research has used a modelling approach to estimate the costs of nursing home provision with and without nursing care, suggesting an additional cost per Namaste Care session per resident of £8–10. 181 The costs found here, by contrast, reflect the reality that different nursing homes will implement this form of care in different ways, resulting in different resource implications; the costs reported here also reflect that not all residents attended all available sessions. Nevertheless, when nursing homes did incur costs, the mean cost was very similar to that reported by Bray et al. 181
Other studies have conducted think-aloud research for ICECAP-O and ICECAP-SCM in different contexts. The error rate in previous research for ICECAP-O for older people with hip problems was similar (7%) to the rate in this study,207 whereas the error rate for ICECAP-SCM was found to be lower both when completed by patients (3.9%) and when completed by health professionals acting as proxy (6.7%) in a hospice. 204 These marginally higher error rates are likely to be a reflection of both the proxy completion and the residents’ lack of ability to communicate fully with care staff.
The major limitation of the work is the sample size in the quantitative element. Small numbers of residents included in the study mean that it was not possible to explore the validity or sensitivity to change of the economic measures used, beyond simple cross-sectional correlations. The think-aloud study was also smaller than planned, although saturation in understanding completion of the measures was reached in relation to the analysis of the qualitative data. Although some carer information was collected, this was of limited value given the very small numbers available, and it was not analysed further.
A full economic evaluation should focus on obtaining good estimates of resource use in relation to GP visits, inpatient stays, outpatient visits, ambulance costs and medication, as these are the main drivers of cost. A future trial should put in place study-specific data collection in relation to GP visits to ensure that these are defined and measured in a standardised manner across nursing homes. Procedures should also be put in place to ensure that records for trial participants are not archived prior to data collection. Additionally, a full trial should obtain further information about variation between nursing homes in the extent to which they incur additional resource use in providing the Namaste Care intervention. This will enable better understanding of the opportunity costs associated with providing the intervention, the role that excess capacity plays in provision, and the relationship between additional resource use and the fidelity with which Namaste Care is provided.
The strength of the correlation between those measures considered most appropriate for a full trial (here the QUALID and CMAI) and the potential economic outcome measures that could be used provides one basis for choosing between economic outcome measures to be included in a full trial. Here, for both QUALID and CMAI the correlations were strongest with ICECAP-SCM, suggesting that this measure might provide the most appropriate basis for measuring outcomes for a full economic evaluation; this measure also specifically targets the end of the life-course. Nevertheless, any decision about the outcomes to be included should also take into account the policy context, including the recommendations of organisations such as NICE for social care research, at the time that the trial is commissioned. NICE currently recommends using a quality-adjusted life-year (QALY) or a social care QALY. A further evaluation alongside can be provided using capability measures. This latter approach is considered appropriate when broader outcomes encompassing health/social care outcomes and capability well-being are relevant (p. 86). 195
Finally, the findings of the qualitative analysis have important implications for proxy measurement in advanced dementia beyond the specific economic outcome measures considered here, and may provide other researchers with useful information with which findings can be interpreted.
Chapter 7 Patient and public involvement in the study
The aim of patient and public involvement (PPI) in the study was to ensure that the experiences of family members of people with advanced dementia who had died in nursing homes informed the design and undertaking. As suggested by the reviewers during the funding application, we sought to also include people with early-stage dementia, but we were unsuccessful in identifying such potential PPI partners through either the Alzheimer’s Society or local contacts.
Methods
The PPI in the study used three main approaches: (1) working with co-applicants/co-researchers, (2) establishing a public involvement panel and (3) representation on strategic oversight groups (the Trial Steering Committee and the International Research Advisory Group).
Co-applicants/co-researchers
The two representatives from the Alzheimer’s Society Research Network who had helped design the original bid stayed on as co-applicants in the core study management group. They joined monthly teleconferences, main trial meetings and the protocol development meeting at the end of the study. This helped us to maintain a focus on making our research understandable by challenging the language we used and our underlying concepts.
Public involvement panel
We set up a public involvement panel. We recruited PPI partners from the Crewe area, as this was where our PPI co-applicants were located and where they had additional contacts through their local networks. In addition, one PPI partner lived in an assisted living facility where people with advanced dementia are cared for and an additional member was identified through this. We recruited five PPI partners including our two co-applicants. We met at the assisted living facility to ease travel for PPI partners.
The public involvement panel worked alongside the research team. It was facilitated by Nancy Preston and supported by the research associate. The panel helped to review study documentation, to support information given in care homes (in person), and to analyse and develop a further study. The group met six times during the study, as well as communicating by e-mail. At the initial meeting, the group agreed that, in their experience, current care was variable – at times excellent, but at other times inadequate – and that there is a need to improve the care for people with advanced dementia at the end of life in nursing homes.
Representation on strategic oversight groups
We ensured that there was PPI representation on both the Trial Steering Committee and the International Research Advisory Group. Members attended the relevant meetings in person or by teleconference.
Study results
Co-applicants/co-researchers
The two PPI co-applicants have been invaluable to the research. They have maintained the voice for people with dementia and families caring for people with dementia. They immersed themselves in all aspects of the research by attending training events about the Namaste Care intervention but also by taking part in consultation exercises related to phase 1 of the study (realist review). They were integral to the writing of the report and to discussing the findings and take-home messages.
Public involvement panel
The public involvement panel was very successful and we have replicated this method for engaging PPI partners in subsequent research bids. We started with six members, but over time two members dropped out. These were the members with perhaps less current involvement in the direct care of someone with dementia.
The initial meeting focused on getting to know one another and sharing expectations of what the panel could focus on. The initial meetings involved explaining our research methods and giving an account of the Namaste Care intervention. We gave plenty of time for questions and these in turn helped us to develop our methods of communicating about the trial.
A key concern for us was convincing nursing homes of the value of conducting regular Namaste Care sessions, ideally twice per day every day. One of the members of the public involvement panel was able to share how important regular activity was in the care of her husband and explained that, when this had failed, it had major repercussions. This was supported by the experience of other members of the public involvement panel. This information was then shared at the training sessions with nursing home staff to reinforce the message.
The panel reviewed all of the outcome measures; these were predominantly the questionnaires but also included the ActiGraph device. All of the questionnaires were provided in one booklet and the panel gave detailed feedback about the wording used to introduce the booklet and each questionnaire. In addition, they helped us remove wording that could cause anxiety for the carers, such as references to end of life. When reviewing the booklet of questionnaires, one PPI member became slightly distressed. He assumed that the inclusion of questions about certain symptoms meant that his partner would ultimately experience these symptoms, and this made him anxious about the future. We were able to discuss this at the meeting, but it was an important lesson for the researchers about how best to phrase requests of PPI partners and to give more explanations about the tools we use, which cover a broad range of possible symptoms and do not necessarily mean that everyone will experience these. With respect to the ActiGraph device, the members of the public involvement panel stressed the importance of knowing someone’s life story in relation to wearing this, for example, if the person wore a watch and, if so, on which wrist they wore it. In addition, they suggested clear processes for staff to follow if a person did not want or no longer wished to wear the ActiGraph device.
The public involvement panel became involved in recruitment at the nursing homes. One or two members joined researchers at all our site initiation visits. Their role was to support the researchers when discussing the trial both with care home staff and with carers of residents. This also meant that family members could speak to someone, other than the researchers, who understood not only the trial but also the experience of caring for a relative with dementia. The panel members were able to answer questions about the study using more accessible language.
Finally, the public involvement panel members were involved in looking at our analysis. Not only did they assist in ensuring the clarity of results but they also asked pertinent questions from their perspectives about what would be interesting for family carers to know. They were particularly interested in the results from the ActiGraph device and were involved in discussions about how the data could be shared in a meaningful way. We agreed that an overview of the results was most appropriate. The panel also attended the dissemination activities after the trial.
The public involvement panel members wrote their own piece for the Alzheimer’s Society research network newsletter about their involvement in the study as part of a longer article about involvement in research. 209 The relevant extract is presented below:
Our PPI brief is to comment on the research as it progresses and ask for any clarification we feel is needed, advise on communications with people with dementia and their carers, and assist with dissemination and evaluation. We have current knowledge of practice in care settings and can ground the academic theory of care research in the reality of care practice. We are well placed to suggest what is likely to be possible, which can save time, effort and money. And as we’re not intimately engaged in the research, we can stand back and call attention to the more general but equally important aspects of the work, such as making sure the findings are shared widely.
The public involvement panel members felt that, should the study lead to a main trial, an explanatory leaflet for relatives would be helpful.
Representation on strategic oversight groups
One panel member sat on the Trial Steering Committee and one PPI member sat on the International Research Advisory Group. They were able to attend three Trial Steering Committee meetings and one International Research Advisory Group meeting, respectively.
Discussion and conclusions
Above all, the public involvement panel kept the experiences of people with dementia, and of people caring for those with dementia, central to the study. They grounded an academic research study in the reality of lived experience, not least by making it possible for us to hold meetings in the assisted living facility. Time and again they reminded us of the importance of the ultimate goal of our research: to contribute to improvements in care for people with advanced dementia.
Their panel members’ feedback on outcome measures was particularly helpful in ensuring that our research tools (the questionnaires and the ActiGraph device) would be used. The questions they asked about our results helped us to refine and clarify our data analysis. Having their support for discussions at the nursing homes was also very useful. Making sure that the public involvement panel members could understand each aspect of the study as it unfolded challenged us to be really clear in our own thinking, not to make any assumptions, and to be realistic about our research methods, population and environment.
We had wanted to involve people with early-stage dementia in the public involvement panel but were unsuccessful, even with the support of the Alzheimer’s Society. This would still be our aim for future studies. We surmise that the research focus on end of life may have been discouraging for some eligible PPI partners. However, working with family carers gave us extremely useful insights, particularly as some members of the public involvement panel were caring for a partner with dementia at that time.
Reflections
University researchers are sometimes accused of being remote from their subject matter and particularly from the people/environment they are researching. The public involvement panel kept us very much grounded in the world of people living with advanced dementia; with their help, we built a bridge between academic research and the families and staff in the nursing homes whose residents constituted our research population.
-
Having a dedicated public involvement panel was very useful and provided broader PPI than drawing on the experiences of one or two individuals. We had access throughout the study to a wider and more differentiated perspective and to a range of lived experience.
-
Hosting meetings in the area local to the panel members, specifically in an assisted care facility for all of these meetings, kept the focus on the setting in a very real way. We also held some Trial Steering Committee meetings there. Although the aim of this was to allow the PPI partners to attend more easily, it had an unforeseen additional benefit: for some members of the wider research team, this was their first experience of visiting such a facility. It was another instance of connecting two otherwise unconnected worlds.
-
Involving the public involvement panel in site visits at kick-off events was a bonus in terms of engaging and reassuring family carers about the study. We learned that PPI members need some support and training/guidance beforehand for everyone to benefit fully.
-
We should be very aware of the content of the questionnaires on which we ask PPI members to comment. We now know that we need to explain the purpose of having a wide range of questions and that these are typically broad to allow all information on all potential experiences to be collected. We must not distribute by e-mail material that may give rise to anxiety. In this instance, briefing about the content needed to be given before the questionnaires were sent out for comment. We also need to be prepared to support PPI members who may become upset when they get a glimpse into a possible future.
-
In general, PPI partners need to tell their stories and we need to allow time for this. During the telling people may become upset and we need to offer support within the group. We are asking people to engage and advise on our research, which, given its focus, requires them to reflect on some very distressing experiences.
-
As with most human interaction of any kind, relationships are key. Having a PPI lead within the research team who could liaise between our co-applicants/co-researchers and the public involvement panel was greatly appreciated.
-
The benefit of patient involvement in the research is not all one way. As the project developed and deepened, the PPI members enjoyed the learning experience, the increased understanding from participating in an in-depth study, and – particularly for the co-researchers – having a valuable experience to carry into future research. In addition, at our final meeting, one public involvement panel member commented: ‘I’ll be better able to tread the path I’m on from what I’ve gained from being part of this study. And I hope my wife [living with dementia] will be a beneficiary too.’
Chapter 8 Discussion
Main findings
The aim of this feasibility cluster randomised controlled trial, preceded by a realist review and intervention refinement phase, was to ascertain the feasibility of conducting a full trial of the Namaste Care intervention. From the realist review we identified the importance of activities that developed moments of connection for people with advanced dementia. This fed us to refine the intervention by developing a resource that staff unfamiliar with Namaste Care could easily understand and offering flexibility in delivery.
The key findings of the feasibility trial are presented under the objectives outlined in Chapter 1 and in Table 32.
Sampling and recruitment of nursing homes
It was possible to recruit both nursing homes and residents, but this took several months. The length of time from initial contact to the baseline visits at the start of the study was, on average, 34 weeks, reflecting the time needed to identify facilities and then address contractual arrangements. In some sites the recruitment of the initial four residents in each nursing home took up to 2.5 months.
Recruitment, retention and attrition rates
Of the eight nursing homes recruited, six remained in the trial (two withdrew before the trial started). Only one resident withdrew after the trial began, for health reasons; four other residents died during the study. The rate of attrition among informal carers was low.
Selection, timing and administration of primary and secondary outcome measures
The choice of two primary outcome measures was to inform a future trial. The quality-of-life tool (QUALID) was appropriate, but the quality-of-dying measure (CAD-EOLD) was not, because those well enough to attend Namaste Care in a group context were not imminently dying, and their deterioration was not fast. One secondary outcome measure (CMAI) was found to be useful in this population. Data from both the QUALID and the CMAI, although not analysed for effect, did show a change in scores at 4 weeks, and in QUALID at 24 weeks, indicating an improvement for residents. For QUALID, this was sustained until measurement at 24 weeks. Measuring the primary outcome at 4 weeks was appropriate given the slow deterioration in this population. The ActiGraph device was acceptable to wear, and data were collected with minimal device failure. This mode of data collection addresses the limitations of proxy outcome measures in offering objective measurement, but there are still questions to be answered about the clinical relevance of the findings. All economic outcome measures (EQ-5D-5L, ICECAP-O and ICECAP-SCM) were feasible for proxy completion, with high completion rates and a weak to strong correlation with QUALID recorded at the different time points. Staff proxy respondents noted some difficulties in completing the ICECAP capability measures in a think-aloud study.
Intervention acceptability, fidelity and sustainability
The Namaste Care intervention was acceptable to staff delivering it, to the family members of people with dementia receiving it, and to the people with dementia receiving it, in the opinion of those staff and family members. The person-centred nature of the intervention allows residents to change their response to what is offered, allows residents to not participate on particular days or to stop participating during a session, and allows for changes in activity to better meet in-the-moment needs. This is supported by the realist review. In our programme theory (see CMO2), we concluded that because Namaste Care includes a range of activities it is easier for staff to find activities that meet individual preferences and responses.
The aspiration of the intervention’s originator was for Namaste Care to be delivered in two sessions per day, each 2 hours long. This was not consistently possible in any of the facilities. When Namaste Care was delivered twice per day, the sessions were shorter. The delivery format reflected the space available, staffing levels and routines in each facility. We understand that a forthcoming version of the originator’s book will acknowledge the challenges in delivering 14 2-hour sessions per week and will support a more flexible delivery model to suit different nursing home contexts (Joyce Simard, March 2019, personal communication).
In terms of sustainability, delivery fidelity changed over time, partly reflecting wider seasonal staffing issues. No longer taking part in a trial has allowed sites to continue to deliver Namaste Care in a way better suited to their resources and environment. We conclude that the implementation and delivery of a person-centred intervention needs to be nursing home centred.
The health economics costing analysis found that homes differed in the extent to which they incurred additional costs from providing Namaste Care, with some incurring no additional costs and others incurring costs equivalent to an average of £222 per resident across a 4-week period.
Primary and secondary outcomes for a full trial
We propose that end-of-life-specific measures may not be the most appropriate to use with people with advanced dementia receiving Namaste Care in a group context; the study criteria exclude people who are towards the end of their lives because they are too unwell to join a group activity, but such people are naturally the focus of many end-of-life measures.
Validated interview measures
Of the two contender primary outcome measures, quality of life was judged to be the more appropriate for evaluating this intervention in this population. Although people with advanced dementia were recruited, they were not near enough to the end of life for a quality-of-dying measure to be appropriate. The secondary outcome measure for agitation (CMAI) was also judged appropriate to evaluate this intervention. However, as identified in the interviews with staff about data collection and in the think-aloud interviews, staff found it challenging to complete the proxy tools in terms of having the confidence to assess the experiences of people with dementia. Caution therefore needs to be used when interpreting such results.
Actigraphy
As a device for data collection, the ActiGraph device was acceptable to and tolerated by all participants. In the light of the one adverse event identified, we would recommend that the watch be used only on the wrist in this population to ensure that it can be checked for any irritation more easily and more often. Further consideration is needed of how best to use these data to support the findings from the proxy outcome measures.
Informal carer outcomes
Data were collected from informal carers who participated. The SWC-EOLD, which focuses on care at the very end of life, was therefore not the most appropriate informal carer measure. The numbers were too small to make meaningful statements about completion rates.
Nursing home staff outcomes
Measures were less well completed than proxy measures on residents.
Additional data
Data on the organisation’s readiness for change were collected to provide context for implementation. Some items are not necessarily transferable to a UK context, particularly with respect to resources. We would therefore recommend not including this in a full trial.
Strengths and limitations
This feasibility study has demonstrated that it is possible to undertake research in nursing homes and to collect nearly complete sets of proxy and objective data on a generally under-researched and vulnerable population. Recruiting nursing homes is possible, but allowance for withdrawals needs to be made in sample size calculations. All of the participating nursing homes delivered the intervention over a 6-month period as allowed by their physical environment, their care routines and their staffing levels.
One limitation of this study was that it was designed as an end-of-life study, but the population needed to be ‘well’ enough to take part in the Namaste Care sessions and so would not necessarily be in the last weeks of life. The residents in this study were not at the end of their lives, but rather required palliative care to maximise their quality of life. This changed the parameters of any decision about primary outcomes to include measures that would help determine changes in engagement among people with dementia, either from agitation to calm or from apathy to interest.
Nursing homes were randomised in a ratio of 4 : 2 to the intervention and control arm, respectively. However, the number of residents recruited was nearly the same, at 18 and 14, respectively. This reflected the limits of the spaces used to deliver the intervention. In a future trial, this needs to be considered if a ratio of 1 : 1 is not used to randomise between arms.
Recruitment was influenced by staff ‘choosing’ who should receive the intervention based on its perceived acceptability to residents and family members. In the control nursing homes, selection was based on family members’ perceived willingness to allow their relative to take part. The trial did not recruit to target, reflecting wider organisational issues of delivering the intervention within predetermined spaces. Further recruitment of residents after the initial 4-week period was not possible in facilities with smaller spaces for Namaste Care. This was because residents who were initially recruited to the study continued to receive Namaste Care and so there was no space to introduce new residents. This limitation could be addressed by allowing facilities to determine the number of participants to be recruited, or by being more specific about the amount of space required for delivering Namaste Care.
Neither the researchers nor the staff completing the proxy measures were blinded, although the use of actigraphy aimed to offer an alternative form of data collection that would address the limitations of proxy reporting. These limitations will remain in a future trial because of the population receiving the intervention. Ensuring that the same staff member completes the proxy measures at each time point, as far as possible, would help to reduce inconsistency.
Issues were also identified among staff in nursing homes when the intervention training was conflated with research processes.
Interpretation for a future trial design
In this section we consider a review of the full trial indicators written at the start of the study, and we consider how a full trial could be undertaken reflecting the findings from phases 1 and 2 and the feasibility trial. We review the full trial indicators, present a sample size calculation for a full trial, address key methodological challenges raised in this study and how they could be resolved in a future trial, and propose a time frame for a future trial, with a modified design. We also consider modifications to the intervention specification and implementation.
Review of full trial indicators
We have reviewed the full trial indicators as articulated in the funding bid (Table 32). The feasibility trial indicators were partially met. The acceptability (to staff and family) and appropriateness of the intervention in a UK context was met, but not the dose indicator with respect to the frequency of session or length. Recruitment of residents was possible and was limited by the Namaste Care space rather than by an inability to recruit appropriate residents. Once recruited, attrition to receive the intervention was low, and only four deaths were recorded among the sample over 6 months. There was an almost 100% completion rate for the primary outcome measures and little attrition, as expected in an end-of-life study. The numbers of deaths are more in line with those in a palliative population. 210
| Indicator | Achieved if | Achieved | Commentary |
|---|---|---|---|
| Recruitment rate | Six residents per care home recruited | Partially |
Four to eight recruited Met in control sites: six and eight recruited Partially met in intervention sites: four or five in intervention homes. Further recruitment of residents after the initial 4-week period was prevented in facilities with smaller spaces for Namaste Care. This was because residents who were initially recruited to the study continued to receive Namaste care so there was no space to introduce new residents. Starting the intervention with eight residents rather than four would ensure that facilities choose large enough space. This would favour larger nursing homes |
| Attrition rate | No more than two residents per care home cease receiving the intervention because of practical or preference issues | Yes | Only one resident withdrawn from one nursing home and this was because they moved to another facility |
| Number of Namaste Care sessions delivered in a week by care home staff | At least 7/14 sessions held per week (50% per week) | No | 30% of possible sessions held, if delivered twice per day for duration of study. Note that where two sessions per day were held in one facility, the total length of Namaste Care delivery was no higher than that in other homes offering Namaste Care once per day |
| Average length of Namaste Care session | 1.5 hours | No |
Range 0.87–1.91 hours; only one facility had an average > 1.5 hours Total range 0.08–2.25 hours |
| Potential primary outcome data completion | 80% of residents participating in the study had CAD-EOLD and QUALID questionnaires completed for them | Yes |
Completion rates Baseline: 100% 4 weeks: 96.88% (one questionnaire not completed) for both tools 24 weeks: 94% (one questionnaire not completed) for both tools |
| Namaste Care intervention acceptability to staff and family | Intervention described as acceptable in terms of components of care provided, timing and frequency of delivery | Yes | Frequency of delivery acceptability shown by numbers and length of sessions delivered |
| Namaste Care intervention suitable for UK nursing home environments | Intervention described as suitable for this context | Yes | Adapted to different environments and delivered in dedicated and shared spaces |
| Identification of a sufficient pool of potential nursing homes, reflecting nursing home diversity, that would be willing to participate in a full trial | Identified a pool of nursing homes willing to participate in a future trial, which exceeds the proposed sample required for a future trial | Partially | Data available from CQC and ENRICH show that a sufficient number of homes meet our criteria. We have not established willingness, as experience of identifying nursing homes ahead of time shows that this is of limited value |
On this basis, we propose that a full trial would be possible given the following recommendations regarding the trial design, changing the intervention specification to make it more person centred and changing the implementation process so that it can be used flexibly in different nursing homes.
Sample size calculation for a definitive trial
We consider two potential primary outcome variables for a definitive trial, QUALID (quality of life) and CMAI (agitation). Analysis of the primary outcome at 4-week follow-up would use a mixed analysis of covariance model, with nursing home as a random effect and baseline value of the outcome measure as a covariate. To calculate the sample size, we need estimates of the following parameters.
Standard deviations of the outcome at follow-up
The observed SDs in the Namaste Care feasibility trial were 7.2 for QUALID and 7.2 for CMAI. We will assume a SD of 8 for both outcomes. The other trial of Namaste Care31 used QUALID as primary outcomes and assumed a much lower SD (4.9), but other reports have SDs more similar to ours.
Intracluster correlation coefficient
The observed ICC in the feasibility trial was 0.10 for QUALID and 0.26 for CMAI. Owing to the small number of clusters, these estimates are likely to be very imprecise. A recent cluster trial of 33 nursing homes in Norway211 showed ICCs of 0.15 for QUALID and 0.10 for CMAI. Required sample sizes for a range of values between 0.10 and 0.25 are presented.
Correlation between population cluster means at baseline and follow-up
This quantity is used to estimate the efficiency gain from adjusting for baseline value using analysis of covariance. This is difficult to estimate accurately with a small number of clusters and is related to the correlation between baseline and follow-up at both the participant and the cluster levels. Our observed correlation between baseline and follow-up at participant level was 0.57 for CMAI and 0.54 for QUALID. We will use a more conservative estimate of 0.30 to adjust the sample size calculation.
Minimum clinically important difference
Another trial31 used a minimum clinically important difference of 4 points for QUALID but assumed a much lower SD than we observed. Little information seems to be available on what difference on the CMAI is meaningful. Based on our assumption of a SD of 8, a difference of 4 would be a medium standardised effect size of 0.5. Sample sizes for a range of minimum clinically important differences are presented in Table 33.
| ICC | Minimum difference to detect (points on scale) | |||
|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |
| Four participants per cluster | ||||
| 0.10 | 90 (360) | 52 (208) | 34 (136) | 24 (96) |
| 0.15 | 102 (408) | 58 (232) | 38 (152) | 26 (104) |
| 0.20 | 112 (448) | 64 (256) | 42 (168) | 30 (120) |
| 0.25 | 122 (488) | 70 (280) | 44 (176) | 32 (128) |
| Eight participants per cluster | ||||
| 0.10 | 60 (480) | 36 (288) | 22 (176) | 18 (144) |
| 0.15 | 72 (576) | 42 (336) | 28 (224) | 24 (192) |
| 0.20 | 84 (672) | 48 (384) | 32 (256) | 28 (224) |
| 0.25 | 96 (768) | 56 (448) | 36 (288) | 32 (256) |
Required sample sizes (α = 0.05 and 90% power) for cluster sizes of four and eight
Values are the total number of nursing homes required, with number of participants in brackets (see Table 33). For example, four participants per nursing home to detect a difference of 4 points with an ICC of 0.10 would require 26 homes and 104 participants per group, for a total of 52 homes and 208 participants.
We propose that the sample size per cluster be eight rather than four, but we recognise that this will be determined by the size of the facility and the space available for delivering Namaste Care.
Sampling and recruitment
Nursing homes
To undertake a full trial, the funders requested data on the availability of nursing homes that would meet the inclusion criteria and represent a range of provider types, size and resident care needs. Using data from the CQC (3 January 2019) and the inclusion criteria from this feasibility trial, 3719 nursing homes were identified that were rated ‘good’ or ‘outstanding’ in their last CQC inspection, cared for service users with dementia and had at least 30 beds. The CQC data do not allow the type of provider organisation to be clearly determined, but all types (for-profit, not-for-profit and public sector) would be represented in this figure. We estimate that approximately 10% (n = 370) would be registered as not-for-profit.
Including only nursing homes rated as ‘good’ or ‘outstanding’ reduces the potential pool. Including nursing homes rated as ‘requires improvement’ would increase the number by 1116 (32.5%) to 4439 sites. To improve the generalisability of findings it is important to include a broad range of facilities, but we are mindful that nursing homes that have to address CQC requirements may not want to undertake the added activity of research. Evidence suggests that CQC rating is related to both provider type (with lower quality reported in for-profit facilities)212 and social care quality of life. 213 We could not find any studies looking at deprivation or geography and CQC ratings, but in an internal report the CQC172 identified that 10% more locations in the East of England were rated as ‘good’ or ‘outstanding’ than in the North West.
Other studies undertaken in nursing homes have demonstrated that it is possible to recruit large numbers of nursing homes nationally. The DCM-EPIC trial214 recruited 50 sites from three regions in England: Yorkshire, London and Oxfordshire. The inclusion of larger facilities (i.e. those with ≥ 40 beds) could create more economy of scale for the introduction of the intervention, but it would reduce the pool of potential facilities available.
Working with larger consortia does ensure senior management support for research, but this does not always equate to involvement from individual nursing homes. We would seek to include facilities from large private provider organisations, but we recognise that this does not always imply greater access to sites. The willingness of a nursing home manager to participate has to be established as close to the start of the study as possible. Changes in management (in the nursing home and externally when part of a larger group), or closures, cannot be predicted, but agreement close to the start should reduce late withdrawals.
We would also question whether or not the CQC quality criteria is an essential for inclusion. The facilities in this study all met the ‘good’ overall criteria, but two had elements that needed attention prior to the start of the trial, which were improved during the trial, and one during the trial had an area of improvement identified. The priority is ensuring that the ‘caring’ domain has received a ‘good’ or ‘outstanding’ rating in the last inspection by the time of recruitment. Given the intervention’s emphasis on person-centred care, for it to be effectively implemented requires a baseline quality of care delivery, which meeting this standard would ensure.
To ensure better retention of nursing homes, the research team would use two approaches. First, we would consider randomising nursing homes in blocks as they obtain the required approvals and permissions so that there is less time between this and the start of the trial. Second, we would provide all interested sites with clear information about the anticipated time frames and communicate with sites more often about the progress of study-wide approvals.
Residents
Using the FAST score helped us to identify people with advanced dementia but did not identify an end-of-life population. The requirement to be able to join a group excluded people at the very end of life and those who were bed-bound, so we conclude that Namaste Care is not an end-of-life (i.e. last weeks of life) intervention but rather a palliative one. Consequently, this will change the inclusion criteria for the study so that potential sites would need to demonstrate their approach to person-centred dementia care, rather than to end-of-life care. Further work would be required with care home staff at the point of screening to ensure that residents are not screened selectively because of staff members’ relationships with the resident and/or their family. Another way to increase resident recruitment would be to allow ongoing recruitment and consent so that individuals could join the trial when possible.
Family
Proxy consent was obtained from personal consultees in all cases except one. The reliance on proxy consent did shape who was included in the study, as informal gatekeeping by staff led to residents being included based on their having more amenable family members, rather than their needs. To address this, further support is required for nursing home staff, both prior to the study and when screening potential residents, to avoid keeping out ‘unsuitable’ residents and those family members perceived to be more difficult. Engagement with family members is required early in the study process and is a recognised challenge in research with people who have capacity issues. 215
Staff
Successful staff recruitment requires active engagement with all staff both at the start and throughout the study. Recruitment of all staff and not just those on duty at the baseline visit would be the aim in a future trial. This would ensure that more data could be collected about the organisational domains that might affect implementation (e.g. person-centred culture).
Outcomes: selection, timing and administration
The identification in the realist review of the importance of the moments of connection in the Namaste Care intervention has led to the person with dementia’s level of social engagement being considered as an outcome of interest. However, because the direction of change in response to Namaste Care can be either greater social engagement or becoming calmer, it may be necessary to identify two primary outcomes or to identify an outcome measure that would allow for measurement in both directions. The CMAI (agitation measure) as an outcome, alongside a secondary measure of apathy, is proposed. A focus on using only the QUALID, CMAI and one further measure for apathy, supported by actigraphy, or another objective measure, would also reduce the administrative burden on proxy respondents.
The limitations of proxy data collection outlined in this study for the health and economic measures will continue through to a full trial, as there is currently no better alternative. However, there is no reason to believe that the effects of proxy measurement would differ between the trial arms as long as proxy respondents are selected in a similar manner in both. The collection of actigraphy data as an objective alternative that overcomes these limitations remains a possibility. If the primary outcome measure is agitation, it may be possible to support the use of these data to corroborate proxy reports in this domain. Consequently, we would propose that the proxy outcome measures still be used, but that caution is applied when interpreting their results.
The lack of blinding to the status of sites among the individuals completing outcome measures cannot be avoided because the tools require knowledge of the person being assessed and so it would not be possible to bring in external data collectors. This limitation will carry into a full trial.
Use of observation alongside the activity log of Namaste Care is not warranted in a future trial as fidelity was good. The selection of economic outcome measure(s) should follow policy guidelines when a full trial is commissioned. Currently, NICE social care guidelines195 recommend a parallel evaluation based on capability measures, such as ICECAP, meaning that all three economic outcomes collected here (EQ-5D-5L, ICECAP-O and ICECAP-SCM) could be useful for aiding decision-making.
Data collection
A number of practical points about data collection were identified during this trial that can inform a future trial. There was some confusion among nursing home staff about the difference between the intervention delivery and the accompanying research activity. This would suggest that a high level of researcher support is needed in the nursing home context. This would enable each site’s principal investigator to fully understand and undertake their role, and it would also support the data collection during the trial. The use of the ENRICH network as a wider source of support and knowledge offers another way to support nursing homes as they engage in research.
Shortening and simplifying the data collection tools, particularly the Namaste Care activity session logs, would help improve data collection rates further.
From a health economics perspective, data collection should focus on the main cost drivers of Namaste Care (i.e. GP visits, inpatient stays, outpatient visits, ambulance costs and medication). Collecting study-specific data on GP visits and avoiding archiving patient data before these can be collected would be beneficial. A full trial should obtain further information about the variation between nursing homes in the additional resources they use in providing Namaste Care. This would enable the claim that this intervention is ‘resource neutral’ to be fully explored, considering where resource is reallocated as well as identifying what extra resources are needed and in what circumstances. This would enable us to determine if any excess treatment costs were present.
A clearer understanding of which data are collected as standard for nursing homes and where these are recorded is needed at the start of the trial. This is so the data collected that is not related to the intervention can reflect those already recorded. However, this is challenging because of the range of paper and electronic systems used across the sector.
In conclusion, we would propose that a pragmatic trial can be rigorous enough to measure efficacy in a robust way but flexible enough to allow an intervention to be delivered that would work in the nursing home setting. We anticipate that this trial would last for at least 3 years (set-up and recruitment of homes, 6–9 months; running the trial in nursing homes, 18 months; follow-up period, 6 months; and analysis and report-writing, 3–6 months). We would randomise nursing homes in blocks to reduce the waiting times for sites, to reduce the level of withdrawal.
Intervention content and implementation
We recommend that this person-centred intervention be implemented in a nursing-centred way, or in a way that allows context-level adaptation to occur. 216 Table 34 presents the learning from this trial with respect to the intervention specification and also the implications for the implementation processes to support a future trial.
| Key element | Revised Namaste Care intervention specification | How implemented | Changes in future trial |
|---|---|---|---|
| Importance of activities that enable the development of moments of connection for people with advanced dementia |
Principle outlined in page 3 of Namaste Care guide Multisensory activities outlined to address taste, smell, sound, sight and touch Relational care – with staff and family (informal carers) |
Evidence that all senses engaged with; strong emphasis on touch through hand massage Activities addressing all five senses were offered; for example:Staff and family reported changes in their connections with people with dementia |
Give broader training to ensure that staff experience a wider range of activities so that they can offer options Training and preparation of staff better links the importance of creating connection with how different activities may achieve this and how connection is understood and recognised Ensure family engagement from the start of the study Develop booklet for family members with public involvement panel |
| CMO1: providing structured access to social and physical stimulation |
Identified space Regular sessions once or twice per day up to 7 days per week Multisensory activities provided |
Space identified – dedicated room in three facilities Dedicated time in dining room in one facility Sessions run in facilities at least once per day. Staff able to deliver between 32% and 68% of available days As above |
No change: establish a dedicated space reflecting what is available in the facility Intervention is run once per day for a minimum of 60 minutes Continue to deliver |
| CMO2: equipping care home staff to cope effectively with complex behaviours and variable responses |
Training – off-site and on-site Comfort assessment |
Off-site training attended by 49 staff On-site training at three sites with between 6 and 15 staff |
Agree training location and approach with care home staff. On-site training required (with or without off-site training) with experiential approach to broad range of activities Ongoing facilitation during trial |
| CMO3: providing a framework for person-centred care |
Family conference Identification of person-centred interests and activities; creation of personal memory box |
Life history work undertaken with all residents. Person-centred activities offered, reflecting person’s interests | Further consideration of an explicit person-centred care framework217 will be used to structure activities |
The focus of the intervention varied according to the needs of the recipient and staff confidence. Staff members reported flexibly using the intervention components as ‘intuitive’, reflecting some staff members’ backgrounds in touch therapies. Hand massage was frequently used in this study, possibly reflecting what staff members had been taught during training. However, the use of ‘loving touch’ is perceived as key to Namaste Care, and the realist review found evidence to suggest that hand massage has a positive impact on resident outcomes. Such an activity may be particularly beneficial because it combines social interaction with sensory stimulation, and it is a relatively easy way to introduce this concept and practice. Future training needs to incorporate a wider range of potential activities to promote their inclusion in Namaste Care sessions.
Identifying an appropriate person-centred framework to structure activities would strengthen the way in which the person-centred focus in Namaste Care is provided. For example, McCormack and McCance’s person-centred framework217 offers five dimensions (working with a person’s beliefs and values, engagement, shared decision-making, having sympathetic presence, and providing holistic care) that map on to Namaste Care practice, but offers a theoretical and empirical basis for including these activities.
Discussions about introducing Namaste Care are important at the start of the study, even though staff members may feel that they are familiar with the concepts. Family and informal carer involvement is important not only in maintaining a person-centred focus on which activities are offered and to which residents, but also potentially as a wider resource for its delivery. The development of family-focused materials, as suggested by the public involvement panel, would support this engagement.
The setting used to deliver the intervention in a group context varied, which influenced how many people could be involved, what could be provided and for how long it could be provided. In multiuse rooms, the sessions were generally shorter. This is supported by the realist review. Studies suggested that there were practical benefits to having a designated space, as sessions were less likely to be cancelled because of competing priorities and because activities could take place as and when needed by residents. However, in our study, the site in which the Namaste Care room was used daily for other purposes delivered sessions most consistently. Flexible criteria for the Namaste Care space and an exploration of the use of volunteers and family members may create more options to ensure that the intervention can be delivered. Learning from other ongoing international studies in this area could inform recommendations. 31
In terms of implementation processes, the resources created for the intervention delivery were well received, but not greatly referred to following the initial training. Further review of the materials, including the guide, would be undertaken prior to a full trial (see below) at which time consideration of family and volunteer roles could be addressed. The 1-day training with an external facilitator and follow-up visit was well evaluated, but a need for ongoing support was identified; for example, regular meetings for care home staff providing the Namaste Care to learn and share experiences would be beneficial.
The ongoing interest in Namaste Care has led to an Alzheimer’s Society-funded implementation study of the intervention currently running. 218 As part of this study, a community of practice has been established (www.adscommunities.ning.com) that could act as a wider resource and ongoing support in a future trial. The Worcester study team has also developed its own manual and training package, which would need to be reviewed before a main trial to ensure a coherent message about Namaste Care and its delivery.
Conclusions
This feasibility trial fulfilled its main aim and objectives to ascertain the feasibility of conducting a full trial of the Namaste Care intervention, with respect to the delivery of the intervention and the conduct of research on the implementation and efficacy of the intervention in the nursing home context. The work across the three phases was supported by robust public involvement activity. The intervention is acceptable for people with advanced dementia from the perspective of their family members and staff, and also acceptable to informal carers and nursing home staff. The intervention can be delivered in a nursing home context. Delivering a complex person-centred intervention for a heterogeneous population (albeit all with advanced dementia), in different nursing home environments requires the intervention and implementation process to be ‘nursing home centred’. A full trial therefore needs to be pragmatically designed that has the flexibility to encompass the person-centred and nursing home-centred intervention delivery and implementation processes.
Implications for health care
The recognition that activities enabling the development of moments of connection for people with advanced dementia are important could be integrated more widely into service developments around person-centred care for people with dementia. We have developed a feasible intervention guide and have collected the information required to inform the design of a full trial. The intervention is feasible to deliver and no adverse effects were noted.
Recommendations for future research
Future research needs to address the following issues:
-
The feasibility study was not powered for efficacy, but some evidence from the outcome measures and interviews suggests that informal carers and staff perceived there to be benefit; this requires further research. Measurements of agitation and apathy/engagement as outcomes for a future trial, alongside quality of life, would build on the programme theory identified in this study, and further consideration is needed of other outcomes that could evaluate the reported different responses of participants.
-
Research is required to establish how to evaluate the delivery of a complex person-centred intervention in a heterogeneous population (albeit all with advanced dementia), in a group context, in different nursing home environments, which requires the intervention and implementation process to be ‘nursing home centred’. A full trial must have the flexibility to encompass the person-centred intervention delivery and a nursing home-centred implementation processes. The learning from other Namaste Care-focused studies nearing completion should be maximised to inform a future trial.
-
Further work is required to examine the actigraphy data to identify how clinically meaningful conclusions can be drawn for this population prior to a full trial, which can inform practice, as this method of data collection offers an objective complement to proxy outcome data.
Acknowledgements
We would like to thank the participating nursing home managers, nursing home staff, residents and informal carers for giving so generously of their time and enthusiasm to allow this study to happen. The contribution of Rachel Sharpe in the phase 1 realist review is acknowledged.
We would also like to thank the members of the Trial Steering Committee: Liz Sampson (chairperson), Adam Gordon (member), Paul McCrone (member), Chris Sutton (member) and Hilary Rhoden (public member).
We acknowledge the support and advice of the members of the International Advisory Group: Dawn Brooker, Sonia Dalkin, Amanda Hobson (public member), Sharon Kaasalainen, Deborah Parker, Joyce Simard, Jenny van der Steen and Ladislav Volicer.
We thank Amanda Hobson, Gladys Sarcher, Jane Spruce and Ted Thorley, who were members of the public involvement panel with experience of being family carers of people with dementia. We would like to thank the Alzheimer’s Society for providing support through the Research Network in identifying public involvement members to work with us on this study.
The support of the Clinical Research Network and ENRICH was invaluable for site recruitment and data collection. Thank you to Sara Yearsley, Margaret Broughton Smith and Philip Tinkler.
The research was designed, conducted, analysed and interpreted by the authors, entirely independently of the funding sources.
Contributions of authors
Katherine Froggatt (https://orcid.org/0000-0003-0339-3877) (Professor of Ageing and Palliative Care) was the chief investigator. She designed and co-ordinated the delivery of the study, and drafted and finalised the application, protocol and final report.
Ashley Best (https://orcid.org/0000-0002-7268-8735) (Trial Statistician) was the phase 3 feasibility trial statistician and carried out data analysis for phase 3.
Frances Bunn (https://orcid.org/0000-0002-5885-918X) (Professor of Health and Complex Conditions) co-led the phase 1 realist review. She designed and supervised the data collection for the realist review and drafted the findings.
Girvan Burnside (https://orcid.org/0000-0001-7398-1346) (Lead Statistician) was responsible for statistical aspects of trial design and supervised data analysis in phase 3.
Joanna Coast (https://orcid.org/0000-0002-3537-5166) (Professor in the Economics of Health & Care) conceived, designed and led the economic component of the research, including analysis, interpretation and writing.
Lesley Dunleavy (https://orcid.org/0000-0002-5924-8145) (Research Associate) undertook phase 3 nursing home and participant recruitment, data collection and qualitative data analysis.
Claire Goodman (https://orcid.org/0000-0002-8938-4893) (Processor of Health Care Research) co-led the phase 1 realist review and designed and supervised data collection and analysis for the realist review.
Ben Hardwick (https://orcid.org/0000-0003-1050-5777) (Supervising Trials Manager) provided trial management guidance and support to the Trial Management Group for the phase 3 feasibility trial.
Clare Jackson (https://orcid.org/0000-0002-1968-0961) (Senior Data Manager) designed the case report forms, specified the database requirements and defined the cleaning checks for the phase 3 feasibility trial.
Julie Kinley (https://orcid.org/0000-0003-0530-1489) (Clinical Associate) co-led the phase 2 intervention refinement and facilitated phase 2 data collection.
Anne Davidson Lund (PPI member) was a PPI co-applicant, a member of the Trial Management Group and the co-chairperson of the public involvement panel. She also drafted the Plain English summary.
Jennifer Lynch (https://orcid.org/0000-0002-2601-7498) (Research Fellow) was the researcher for the phase 1 realist review.
Paul Mitchell (https://orcid.org/0000-0002-7593-4460) (Research Fellow in Health Economics) supervised the collection and analysis of the economic feasibility data, conducted an error analysis of the economic outcome data, contributed to the interpretation of the economic data, and revised Chapter 5 for important intellectual content.
Gareth Myring (https://orcid.org/0000-0002-7811-2091) (Research Associate in Health Economics) collected economic data from nursing homes, conducted an analysis of economic cost data, conducted an error analysis of economic outcome data, contributed to the interpretation of the economic data, and drafted elements of the economic report.
Shakil Patel (https://orcid.org/0000-0003-3931-8684) (Trial Manager) led on the phase 3 feasibility study ethics applications and undertook recruitment and data collection in phase 2 and phase 3.
Guillermo Perez Algorta (https://orcid.org/0000-0001-6610-4951) (Lecturer) led on actigraphy use and analysis of actigraphy data.
Nancy Preston (https://orcid.org/0000-0003-2659-2342) (Professor of Supportive and Palliative Care) led the PPI activity and trial design oversight.
David Scott (PPI member) was a PPI co-applicant, a member of the Trial Management Group and the co-chairperson of the public involvement panel. He also drafted the Plain English summary.
Kate Silvera (https://orcid.org/0000-0002-8470-0164) (Data Manager) was responsible for performing database testing and centrally monitoring case report forms for missing data and inconsistent values.
Catherine Walshe (https://orcid.org/0000-0002-4531-8608) (Professor of Palliative Care) was the lead for process evaluation and co-lead of phase 2 intervention refinement. She designed and oversaw data collection and analysis for intervention refinement and process evaluation.
All authors provided a critical review and final approval of the report.
Publications
Bunn F, Lynch J, Goodman C, Sharpe R, Walshe C, Preston N, Froggatt K. Improving living and dying for people with advanced dementia living in care homes: a realist review of Namaste Care and other multisensory interventions. BMC Geriatrics 2018;18:303.
Froggatt K, Patel S, Algorta GP, Bunn F, Burnside G, Coast J, et al. Namaste Care in nursing care homes for people with advanced dementia: protocol for a feasibility randomised controlled trial. BMJ Open 2018;8:e026531.
Walshe C, Kinley J, Patel S, Goodman C, Bunn F, Lynch J, et al. A four-stage process for intervention description and guide development of a practice-based intervention: refining the Namaste Care intervention implementation specification for people with advanced dementia prior to a feasibility cluster randomised trial. BMC Geriatr 2019;19:275.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63-75.e2. https://doi.org/10.1016/j.jalz.2012.11.007.
- Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull 1988;24:653-9. https://doi.org/10.1037/t08620-000.
- Stephan A, Afram B, Koskenniemi J, Verbeek H, Soto ME, Bleijlevens MH, et al. Older persons with dementia at risk for institutionalization in eight European countries: a cross-sectional study on the perceptions of informal caregivers and healthcare professionals. J Adv Nurs 2015;71:1392-404. https://doi.org/10.1111/jan.12493.
- Froggatt K, Reitinger E. Palliative Care in Long-Term Care Settings for Older People. Report of an EAPC Taskforce 2010-2012 2013.
- Prince M, Acosta D, Ferri CP, Guerra M, Huang Y, Llibre Rodriguez JJ, et al. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet 2012;380:50-8. https://doi.org/10.1016/S0140-6736(12)60399-7.
- Cox S, Cook A, Hockley J, Clark D. Palliative Care for Older People in Care Homes. Buckingham: Open University Press; 2002.
- Broad JB, Gott M, Kim H, Boyd M, Chen H, Connolly MJ. Where do people die? An international comparison of the percentage of deaths occurring in hospital and residential aged care settings in 45 populations, using published and available statistics. Int J Public Health 2013;58:257-67. https://doi.org/10.1007/s00038-012-0394-5.
- Houttekier D, Cohen J, Bilsen J, Addington-Hall J, Onwuteaka-Philipsen BD, Deliens L. Place of death of older persons with dementia. A study in five European countries. J Am Geriatr Soc 2010;58:751-6. https://doi.org/10.1111/j.1532-5415.2010.02771.x.
- van der Maaden T, van der Steen JT, de Vet HC, Hertogh CM, Koopmans RT. Prospective observations of discomfort, pain, and dyspnea in nursing home residents with dementia and pneumonia. J Am Med Dir Assoc 2016;17:128-35. https://doi.org/10.1016/j.jamda.2015.08.010.
- Hendriks SA, Smalbrugge M, Galindo-Garre F, Hertogh CM, van der Steen JT. From admission to death: prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J Am Med Dir Assoc 2015;16:475-81. https://doi.org/10.1016/j.jamda.2014.12.016.
- Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med 2009;361:1529-38. https://doi.org/10.1056/NEJMoa0902234.
- Small N. Living well until you die: quality of care and quality of life in palliative and dementia care. Ann N Y Acad Sci 2007;1114:194-203. https://doi.org/10.1196/annals.1396.019.
- van der Steen JT, Radbruch L, Hertogh CM, de Boer ME, Hughes JC, Larkin P, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med 2014;28:197-209. https://doi.org/10.1177/0269216313493685.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry 2014;205:436-42. https://doi.org/10.1192/bjp.bp.113.141119.
- van Uden N, Van den Block L, van der Steen JT, Onwuteaka-Philipsen BD, Vandervoort A, Vander Stichele R, et al. Quality of dying of nursing home residents with dementia as judged by relatives. Int Psychogeriatr 2013;25:1697-707. https://doi.org/10.1017/S1041610213000756.
- van der Steen JT, Sampson EL, Van den Block L, Lord K, Vankova H, Pautex S, et al. Tools to assess pain or lack of comfort in dementia: a content analysis. J Pain Symptom Manage 2015;50:659-75.e3. https://doi.org/10.1016/j.jpainsymman.2015.05.015.
- Verkaik R, van Weert JC, Francke AL. The effects of psychosocial methods on depressed, aggressive and apathetic behaviors of people with dementia: a systematic review. Int J Geriatr Psychiatry 2005;20:301-14. https://doi.org/10.1002/gps.1279.
- Great Britain . Data Protection Act 1998 1998.
- Small N, Froggatt K, Downs M. Living and Dying with Dementia. Dialogues about Palliative Care. Oxford: Oxford University Press; 2007.
- Simard J. The End-of-life Namaste Care Program for People with Dementia. Baltimore, MD: Health Professions Press; 2013.
- Fullarton J, Volicer L. Reductions of antipsychotic and hypnotic medications in Namaste Care. J Am Med Dir Assoc 2013;14:708-9. https://doi.org/10.1016/j.jamda.2013.06.002.
- Simard J, Volicer L. Effects of Namaste Care on residents who do not benefit from usual activities. Am J Alzheimers Dis Other Demen 2010;25:46-50. https://doi.org/10.1177/1533317509333258.
- Kitwood T. Toward a theory of dementia care: ethics and interaction. J Clin Ethics 1998;9:23-34.
- Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing Soc 1992;12:269-87. https://doi.org/10.1017/S0144686X0000502X.
- Li J, Porock D. Resident outcomes of person-centered care in long-term care: a narrative review of interventional research. Int J Nurs Stud 2014;51:1395-415. https://doi.org/10.1016/j.ijnurstu.2014.04.003.
- Downs M, Small N, Froggatt K. Explanatory models of dementia: links to end-of-life care. Int J Palliat Nurs 2006;12:209-13. https://doi.org/10.12968/ijpn.2006.12.5.21173.
- Stacpoole M, Hockley J, Thompsell A, Simard J, Volicer L. The Namaste Care programme can reduce behavioural symptoms in care home residents with advanced dementia. Int J Geriatr Psychiatry 2015;30:702-9. https://doi.org/10.1002/gps.4211.
- Wen A, Wen A. Behavioral symptoms among patients before and after implementation of a specialized advanced dementia care program in a nursing home. J Am Med Direct Assoc 2014;15. https://doi.org/10.1016/j.jamda.2013.12.043.
- Stacpoole M, Hockley J, Thompsell A, Simard J, Volicer L. Implementing the Namaste Care Program for residents with advanced dementia: exploring the perceptions of families and staff in UK care homes. Ann Palliat Med 2017;6:327-39. https://doi.org/10.21037/apm.2017.06.26.
- Kaasalainen S, Hunter P, Dal Bello Haas V, Dolovich L, Markle-Reid M, Ploeg J, et al. Launching Namaste Care in Canada: evaluation of a facility-wide education program to improve end-of-life care in advanced dementia. JPSM 2016;52. https://doi.org/10.1016/j.jpainsymman.2016.10.065.
- Smaling HJA, Joling KJ, van de Ven PM, Bosmans JE, Simard J, Volicer L, et al. Effects of the Namaste Care Family programme on quality of life of nursing home residents with advanced dementia and on family caregiving experiences: study protocol of a cluster-randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-025411.
- Bunn F, Lynch J, Goodman C, Sharpe R, Walshe C, Preston N, et al. Improving living and dying for people with advanced dementia living in care homes: a realist review of Namaste Care and other multisensory interventions. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0995-9.
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-21.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. https://doi.org/10.1258/1355819054308530.
- Shaw J, Gray CS, Baker GR, Denis JL, Breton M, Gutberg J, et al. Mechanisms, contexts and points of contention: operationalizing realist-informed research for complex health interventions. BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0641-4.
- Manzano A. The craft of interviewing in realist evaluation. Evaluation 2016;22:342-60. https://doi.org/10.1177/1356389016638615.
- Pearson M, Brand SL, Quinn C, Shaw J, Maguire M, Michie S, et al. Using realist review to inform intervention development: methodological illustration and conceptual platform for collaborative care in offender mental health. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0321-2.
- Bartunek JM, Murninghan JK. The nominal group technique: expanding the basic procedure and underlying assumptions. Gr Organ Manag 1984;9:417-32. https://doi.org/10.1177/105960118400900307.
- Ford JA, Wong G, Jones AP, Steel N. Access to primary care for socioeconomically disadvantaged older people in rural areas: a realist review. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010652.
- Pawson R. Evidence-based policy: the promise of ‘realist synthesis’. Evaluation 2002;8:340-58. https://doi.org/10.1177/135638902401462448.
- Rycroft-Malone J, Burton C, Hall B, McCormack B, Nutley S, Seddon D, et al. Improving skills and care standards in the support workforce for older people: a realist review. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005356.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Wong G, Pawson R, Owen L. Policy guidance on threats to legislative interventions in public health: a realist synthesis. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-222.
- Manzar B, Volicer L. Effects of Namaste Care: pilot study. Am J Alzheimers Dis 2015;2:24-37. https://doi.org/10.7726/ajad.2015.1003.
- Soliman A, Hirst S. Using sensory activities to improve dementia care. Nurs Times 2015;111.
- McNiel P, Westphal J. Namaste Care™: a person-centered care approach for Alzheimer’s and advanced dementia. West J Nurs Res 2018;40:37-51. https://doi.org/10.1177/0193945916679631.
- Nicholls D, Chang E, Johnson A, Edenborough M. Touch, the essence of caring for people with end-stage dementia: a mental health perspective in Namaste Care. Aging Ment Health 2013;17:571-8. https://doi.org/10.1080/13607863.2012.751581.
- St John K, Koffman J. Introducing Namaste Care to the hospital environment: a pilot study. Ann Palliat Med 2017;6:354-64. https://doi.org/10.21037/apm.2017.06.27.
- Stacpoole M, Thompsell A. OA25 The namaste care programme can enrich quality of life for people with advanced dementia and those who care for them without additional resources. BMJ Support Palliat Care 2015;5. https://doi.org/10.1136/bmjspcare-2015-000906.25.
- Magee M, McCorkell G, Guille S, Coates V. Feasibility of the Namaste Care Programme to enhance care for those with advanced dementia. Int J Palliat Nurs 2017;23:368-76. https://doi.org/10.12968/ijpn.2017.23.8.368.
- Wu J, Wang Y, Wang Z. The effectiveness of massage and touch on behavioural and psychological symptoms of dementia: A quantitative systematic review and meta-analysis. J Adv Nurs 2017;73:2283-95. https://doi.org/10.1111/jan.13311.
- Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane Database Syst Rev 2014;2. https://doi.org/10.1002/14651858.CD003150.pub2.
- Chang YS, Chu H, Yang CY, Tsai JC, Chung MH, Liao YM, et al. The efficacy of music therapy for people with dementia: a meta-analysis of randomised controlled trials. J Clin Nurs 2015;24:3425-40. https://doi.org/10.1111/jocn.12976.
- Chatterton W, Baker F, Morgan K. The singer or the singing: who sings individually to persons with dementia and what are the effects?. Am J Alzheimers Dis Other Demen 2010;25:641-9. https://doi.org/10.1177/1533317510385807.
- Clare L. Awareness in people with severe dementia: review and integration. Aging Ment Health 2010;14:20-32. https://doi.org/10.1080/13607860903421029.
- Enmarker I, Olsen R, Hellzen O. Management of person with dementia with aggressive and violent behaviour: a systematic literature review. Int J Older People Nurs 2011;6:153-62. https://doi.org/10.1111/j.1748-3743.2010.00235.x.
- Ennis EM, Kazer MW. The role of spiritual nursing interventions on improved outcomes in older adults with dementia. Holist Nurs Pract 2013;27:106-13. https://doi.org/10.1097/HNP.0b013e318280f7f9.
- Guetin S, Charras K, Berard A, Arbus C, Berthelon P, Blanc F, et al. An overview of the use of music therapy in the context of Alzheimer’s disease: a report of a French expert group. Dementia 2013;12:619-34. https://doi.org/10.1177/1471301212438290.
- Kim SY, Yoo EY, Jung MY, Park SH, Park JH. A systematic review of the effects of occupational therapy for persons with dementia: a meta-analysis of randomized controlled trials. NeuroRehabilitation 2012;31:107-15. https://doi.org/10.3233/NRE-2012-0779.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18390.
- McDermott O, Crellin N, Ridder HM, Orrell M. Music therapy in dementia: a narrative synthesis systematic review. Int J Geriatr Psychiatry 2013;28:781-94. https://doi.org/10.1002/gps.3895.
- Nguyen QA, Paton C. The use of aromatherapy to treat behavioural problems in dementia. Int J Geriatr Psychiatry 2008;23:337-46. https://doi.org/10.1002/gps.1886.
- Raglio A, Filippi S, Bellandi D, Stramba-Badiale M. Global music approach to persons with dementia: evidence and practice. Clin Interv Aging 2014;9:1669-76. https://doi.org/10.2147/CIA.S71388.
- Randall EW, Clissett PC. What are the relative merits of interventions used to reduce the occurrences of disruptive vocalisation in persons with dementia? – a systematic review. Int J Older People Nurs 2016;11:4-17. https://doi.org/10.1111/opn.12083.
- Wall M, Duffy A. The effects of music therapy for older people with dementia. Br J Nurs 2010;19:108-13. https://doi.org/10.12968/bjon.2010.19.2.46295.
- Padilla R. Effectiveness of environment-based interventions for people with Alzheimer’s disease and related dementias. Am J Occup Ther 2011;65:514-22. https://doi.org/10.5014/ajot.2011.002600.
- Wu J, Wang Y, Wang Z. The effectiveness of massage and touch on behavioural and psychological symptoms of dementia: a quantitative systematic review and meta-analysis. J Adv Nurs 2017;73:2283-95. https://doi.org/10.1111/jan.13311.
- Conn DK, Seitz DP. Advances in the treatment of psychiatric disorders in long-term care homes. Curr Opin Psychiatry 2010;23:516-21. https://doi.org/10.1097/YCO.0b013e32833efe56.
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli?. J Am Geriatr Soc 2010;58:1459-64. https://doi.org/10.1111/j.1532-5415.2010.02951.x.
- Cohen-Mansfield J, Marx MS, Freedman LS, Murad H, Regier NG, Thein K, et al. The comprehensive process model of engagement. Am J Geriatr Psychiatry 2011;19:859-70. https://doi.org/10.1097/JGP.0b013e318202bf5b.
- Cohen-Mansfield J, Marx MS, Freedman LS, Murad H, Thein K, Dakheel-Ali M. What affects pleasure in persons with advanced stage dementia?. J Psychiatr Res 2012;46:402-6. https://doi.org/10.1016/j.jpsychires.2011.12.003.
- Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging Ment Health 2010;14:67-73. https://doi.org/10.1080/13607860902845574.
- Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of stimuli on affect in persons with dementia. J Clin Psychiatry 2011;72:480-6. https://doi.org/10.4088/JCP.09m05694oli.
- Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Murad H, Freedman LS. The relationships of environment and personal characteristics to agitated behaviors in nursing home residents with dementia. J Clin Psychiatry 2012;73:392-9. https://doi.org/10.4088/JCP.10m06605.
- Collier L. The use of multi-sensory stimulation to improve functional performance in older people with dementia: a randomised single blind trial. Br J Occup Ther 2008;71.
- Collier L, McPherson K, Ellis-Hill C, Staal J, Bucks R. Multisensory stimulation to improve functional performance in moderate to severe dementia – interim results. Am J Alzheimers Dis Other Demen 2010;25:698-703. https://doi.org/10.1177/1533317510387582.
- Hsu MH, Flowerdew R, Parker M, Fachner J, Odell-Miller H. Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: a cluster randomised controlled feasibility study. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0082-4.
- Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas 2014;77:305-10. https://doi.org/10.1016/j.maturitas.2014.01.003.
- Belgrave M. The effect of expressive and instrumental touch on the behavior states of older adults with late-stage dementia of the Alzheimer’s type and on music therapist’s perceived rapport. J Music Ther 2009;46:132-46. https://doi.org/10.1093/jmt/46.2.132.
- Cruz J, Marques A, Barbosa A, Figueiredo D, Sousa LX. Making sense(s) in dementia: a multisensory and motor-based group activity program. Am J Alzheimers Dis Other Demen 2013;28:137-46. https://doi.org/10.1177/1533317512473194.
- Cruz J, Marques A, Barbosa AL, Figueiredo D, Sousa L. Effects of a motor and multisensory-based approach on residents with moderate-to-severe dementia. Am J Alzheimers Dis Other Demen 2011;26:282-9. https://doi.org/10.1177/1533317511411177.
- Fu CY, Moyle W, Cooke M. A randomised controlled trial of the use of aromatherapy and hand massage to reduce disruptive behaviour in people with dementia. BMC Complement Altern Med 2013;13. https://doi.org/10.1186/1472-6882-13-165.
- Fujii M, Hatakeyama R, Fukuoka Y, Yamamoto T, Sasaki R, Moriya M, et al. Lavender aroma therapy for behavioral and psychological symptoms in dementia patients. Geriatr Gerontol Int 2008;8:136-8. https://doi.org/10.1111/j.1447-0594.2008.00461.x.
- Götell E, Brown S, Ekman SL. The influence of caregiver singing and background music on vocally expressed emotions and moods in dementia care: a qualitative analysis. Int J Nurs Stud 2009;46:422-30. https://doi.org/10.1016/j.ijnurstu.2007.11.001.
- Goto S, Kamal N, Puzio H, Kobylarz F, Herrup K. Differential responses of individuals with late-stage dementia to two novel environments: a multimedia room and an interior garden. J Alzheimers Dis 2014;42:985-98. https://doi.org/10.3233/JAD-131379.
- Hammar LM, Emami A, Götell E, Engström G. The impact of caregivers’ singing on expressions of emotion and resistance during morning care situations in persons with dementia: an intervention in dementia care. J Clin Nurs 2011;20:969-78. https://doi.org/10.1111/j.1365-2702.2010.03386.x.
- Harris M, Richards KC, Grando VT. The effects of slow-stroke back massage on minutes of nighttime sleep in persons with dementia and sleep disturbances in the nursing home: a pilot study. J Holist Nurs 2012;30:255-63. https://doi.org/10.1177/0898010112455948.
- Kellett U, Moyle W, McAllister M, King C, Gallagher F. Life stories and biography: a means of connecting family and staff to people with dementia. J Clin Nurs 2010;19:1707-15. https://doi.org/10.1111/j.1365-2702.2009.03116.x.
- Kupeli N, Leavey G, Moore K, Harrington J, Lord K, King M, et al. Context, mechanisms and outcomes in end of life care for people with advanced dementia. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0103-x.
- Bergland Å, Johansen H, Sellevold GS. A qualitative study of professional caregivers’ perceptions of processes contributing to mealtime agitation in persons with dementia in nursing home wards and strategies to attain calmness. Nurs Open 2015;2:119-29. https://doi.org/10.1002/nop2.24.
- Lancioni GE, O’Reilly MF, Singh NN, Sigafoos J, Grumo G, Pinto K, et al. Assessing the impact and social perception of self-regulated music stimulation with patients with Alzheimer’s disease. Res Dev Disabil 2013;34:139-46. https://doi.org/10.1016/j.ridd.2012.07.026.
- Lape JE. Using a multisensory environment: to decrease negative behaviors in clients with dementia. OT Pract 2009;14:9-13.
- Litchke LG, Hodges JS. The meaning of ‘now’ moments of engagement in yoga for persons with Alzheimer’s disease. Ther Recreat J 2014;48.
- Lykkeslet E, Gjengedal E, Skrondal T, Storjord MB. Sensory stimulation – a way of creating mutual relations in dementia care. Int J Qual Stud Health Well-Being 2014;9. https://doi.org/10.3402/qhw.v9.23888.
- Mariko A, Matsuda H, Takahashi M, Fujii M, Sasaki H. Touch on the acupoint of Shinchuu of Alzheimer’s disease patients. Geriatr Gerontol Int 2015;15:385-6. https://doi.org/10.1111/ggi.12351.
- Maseda A, Sánchez A, Marante MP, González-Abraldes I, Buján A, Millán-Calenti JC. Effects of multisensory stimulation on a sample of institutionalized elderly people with dementia diagnosis: a controlled longitudinal trial. Am J Alzheimers Dis Other Demen 2014;29:463-73. https://doi.org/10.1177/1533317514522540.
- McDermott O, Orrell M, Ridder HM. The importance of music for people with dementia: the perspectives of people with dementia, family carers, staff and music therapists. Aging Ment Health 2014;18:706-16. https://doi.org/10.1080/13607863.2013.875124.
- Milev RV, Kellar T, McLean M, Mileva V, Luthra V, Thompson S, et al. Multisensory stimulation for elderly with dementia: a 24-week single-blind randomized controlled pilot study. Am J Alzheimers Dis Other Demen 2008;23:372-6. https://doi.org/10.1177/1533317508316681.
- Moyle W, Cooke ML, Beattie E, Shum DH, O’Dwyer ST, Barrett S, et al. Foot massage and physiological stress in people with dementia: a randomized controlled trial. J Altern Complement Med 2014;20:305-11. https://doi.org/10.1089/acm.2013.0177.
- Moyle W, Cooke ML, Beattie E, Shum DH, O’Dwyer ST, Barrett S. Foot massage versus quiet presence on agitation and mood in people with dementia: a randomised controlled trial. Int J Nurs Stud 2014;51:856-64. https://doi.org/10.1016/j.ijnurstu.2013.10.019.
- Staal JA, Sacks A, Matheis R, Collier L, Calia T, Hanif H, et al. The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med 2007;37:357-70. https://doi.org/10.2190/PM.37.4.a.
- Burns A, Perry E, Holmes C, Francis P, Morris J, Howes MJ, et al. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dement Geriatr Cogn Disord 2011;31:158-64. https://doi.org/10.1159/000324438.
- Murphy JL, Holmes J, Brooks C. Nutrition and dementia care: developing an evidence-based model for nutritional care in nursing homes. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0443-2.
- Nair BK, Heim C, Krishnan C, D’Este C, Marley J, Attia J. The effect of Baroque music on behavioural disturbances in patients with dementia. Australas J Ageing 2011;30:11-5. https://doi.org/10.1111/j.1741-6612.2010.00439.x.
- Narme P, Clément S, Ehrlé N, Schiaratura L, Vachez S, Courtaigne B, et al. Efficacy of musical interventions in dementia: evidence from a randomized controlled trial. J Alzheimers Dis 2014;38:359-69. https://doi.org/10.3233/JAD-130893.
- Quell R, Skovdal K, Kihlgren M, Lökk J. Using tactile stimulation in a dementia care facility with plasma prolactin as an outcome measure – a pilot study. Arch Int J Med 2008;1:123-9.
- Raglio A, Bellandi D, Baiardi P, Gianotti M, Ubezio MC, Zanacchi E, et al. Effect of active music therapy and individualized listening to music on dementia: a multicenter randomized controlled trial. J Am Geriatr Soc 2015;63:1534-9. https://doi.org/10.1111/jgs.13558.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Addendum to ‘Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial’ (Raglio et al., 2010). Aging Ment Health 2012;16:265-7. https://doi.org/10.1080/13607863.2011.630376.
- Rodríguez-Mansilla J, González López-Arza MV, Varela-Donoso E, Montanero-Fernández J, González Sánchez B, Garrido-Ardila EM. The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin Rehabil 2015;29:683-93. https://doi.org/10.1177/0269215514554240.
- Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr 2013;25:775-84. https://doi.org/10.1017/S1041610212002256.
- Sakamoto Y, Ebihara S, Ebihara T, Tomita N, Toba K, Freeman S, et al. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. J Am Geriatr Soc 2012;60:1005-11. https://doi.org/10.1111/j.1532-5415.2012.03977.x.
- Sánchez A, Maseda A, Marante-Moar MP, de Labra C, Lorenzo-López L, Millán-Calenti JC. Comparing the effects of multisensory stimulation and individualized music sessions on elderly people with severe dementia: a randomized controlled trial. J Alzheimers Dis 2016;52:303-15. https://doi.org/10.3233/JAD-151150.
- Cameron H, Toit S, Richard G, Bearns L. Using lemon balm oil to reduce aggression and agitation in dementia: results of a pilot study. J Dement Care 2011;19:36-9.
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein-Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person-centred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8:317-25. https://doi.org/10.1016/S1474-4422(09)70045-6.
- Chu H, Yang CY, Lin Y, Ou KL, Lee TY, O’Brien AP, et al. The impact of group music therapy on depression and cognition in elderly persons with dementia: a randomized controlled study. Biol Res Nurs 2014;16:209-17. https://doi.org/10.1177/1099800413485410.
- Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behavior problems in persons with advanced dementia?. Psychiatry Res 2015;228:59-64. https://doi.org/10.1016/j.psychres.2015.03.043.
- Strøm BS, Engedal K, Benth JS, Grov EK. Effect of the Sonas Programme on communication in people with dementia: a randomized controlled trial. Dement Geriatr Cogn Dis Extra 2017;7:122-35. https://doi.org/10.1159/000468147.
- Sung HC, Chang AM, Lee WL. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J Clin Nurs 2010;19:1056-64. https://doi.org/10.1111/j.1365-2702.2009.03016.x.
- Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry 2012;27:621-7. https://doi.org/10.1002/gps.2761.
- Suzuki M, Tatsumi A, Otsuka T, Kikuchi K, Mizuta A, Makino K, et al. Physical and psychological effects of 6-week tactile massage on elderly patients with severe dementia. Am J Alzheimers Dis Other Demen 2010;25:680-6. https://doi.org/10.1177/1533317510386215.
- Tuckett AG, Hodgkinson B, Rouillon L, Balil-Lozoya T, Parker D. What carers and family said about music therapy on behaviours of older people with dementia in residential aged care. Int J Older People Nurs 2015;10:146-57. https://doi.org/10.1111/opn.12071.
- Vézina A, Robichaud L, Voyer P, Pelletier D. Identity cues and dementia in nursing home intervention. Work 2011;40:5-14. https://doi.org/10.3233/WOR-2011-1201.
- Vink AC, Zuidersma M, Boersma F, de Jonge P, Zuidema SU, Slaets JP. The effect of music therapy compared with general recreational activities in reducing agitation in people with dementia: a randomised controlled trial. Int J Geriatr Psychiatry 2013;28:1031-8. https://doi.org/10.1002/gps.3924.
- Van Vracem M, Spruytte N, Declercq A, Van Audenhove C. Agitation in dementia and the role of spatial and sensory interventions: experiences of professional and family caregivers. Scand J Caring Sci 2016;30:281-9. https://doi.org/10.1111/scs.12240.
- Ward-Smith P, Llanque SM, Curran D. The effect of multisensory stimulation on persons residing in an extended care facility. Am J Alzheimers Dis Other Demen 2009;24:450-5. https://doi.org/10.1177/1533317509350153.
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Thein K. The use and utility of specific nonpharmacological interventions for behavioral symptoms in dementia: an exploratory study. Am J Geriatr Psychiatry 2015;23:160-70. https://doi.org/10.1016/j.jagp.2014.06.006.
- Anderson K, Bird M, Macpherson S, McDonough V, Davis T. Findings from a pilot investigation of the effectiveness of a snoezelen room in residential care: should we be engaging with our residents more?. Geriatr Nurs 2011;32:166-77. https://doi.org/10.1016/j.gerinurse.2010.12.011.
- Hsu MH, Flowerdew R, Parker M, Fachner J, Odell-Miller H. Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: a cluster randomised controlled feasibility study. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0082-4.
- Staal JA, Sacks A, Matheis R, Collier L, Calia T, Hanif H, et al. The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med 2007;37:357-70. https://doi.org/10.2190/PM.37.4.a.
- Stacpoole M, Thompsell A. OA25 The namaste care programme can enrich quality of life for people with advanced dementia and those who care for them without additional resources. BMJ Support Palliat Care 2015;5. https://doi.org/10.1136/bmjspcare-2015-000906.25.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial. Aging Ment Health 2010;14:900-4. https://doi.org/10.1080/13607861003713158.
- Padilla R, Domina A. Effectiveness of sensory stimulation to improve arousal and alertness of people in a coma or persistent vegetative state after traumatic brain injury: a systematic review. Am J Occup Ther 2016;70. https://doi.org/10.5014/ajot.2016.021022.
- Bicket MC, Samus QM, McNabney M, Onyike CU, Mayer LS, Brandt J, et al. The physical environment influences neuropsychiatric symptoms and other outcomes in assisted living residents. Int J Geriatr Psychiatry 2010;25:1044-54. https://doi.org/10.1002/gps.2460.
- Froggatt K, Patel S, Perez Algorta G, Bunn F, Burnside G, Coast J, et al. Namaste Care in nursing care homes for people with advanced dementia: protocol for a feasibility randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-026531.
- Kiely DK, Volicer L, Teno J, Jones RN, Prigerson HG, Mitchell SL. The validity and reliability of scales for the evaluation of end-of-life care in advanced dementia. Alzheimer Dis Assoc Disord 2006;20:176-81. https://doi.org/10.1097/00002093-200607000-00009.
- Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc 2000;1:114-16. https://doi.org/10.1037/t00432-000.
- Preston NJ, Fayers P, Walters SJ, Pilling M, Grande GE, Short V, et al. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med 2013;27:899-907. https://doi.org/10.1177/0269216313486952.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Stacpoole M, Hockley J, Thompsell A, Simard J, Volicer L. Implementing the Namaste Care Program for residents with advanced dementia: exploring the perceptions of families and staff in UK care homes. Ann Palliat Med 2017;6:327-39. https://doi.org/10.21037/apm.2017.06.26.
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233-9. https://doi.org/10.1176/jnp.12.2.233.
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 2000;8:75-83. https://doi.org/10.1097/00019442-200002000-00010.
- Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc 2003;4:9-15. https://doi.org/10.1097/01.JAM.0000043422.31640.F7.
- Werner P, Cohen-Mansfield J, Koroknay V, Braun J. The impact of a restraint-reduction program on nursing home residents. Geriatr Nurs 1994;15:142-6. https://doi.org/10.1016/S0197-4572(09)90040-4.
- Edvardsson D, Innes A. Measuring person-centered care: a critical comparative review of published tools. Gerontologist 2010;50:834-46. https://doi.org/10.1093/geront/gnq047.
- Estabrooks CA, Squires JE, Cummings GG, Birdsell JM, Norton PG. Development and assessment of the Alberta Context Tool. BMC Health Serv Res 2009;9. https://doi.org/10.1186/1472-6963-9-234.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Simard J. The End of Life Namaste Care Program for People with Dementia. Baltimore, MD: Health Professions Press; 2013.
- Gupta S, Rai N, Bhattacharrya O, Cheng AYY, Connelly KA, Boulet LP, et al. Optimizing the language and format of guidelines to improve guideline uptake. CMAJ 2016;188:E362-E368. https://doi.org/10.1503/cmaj.151102.
- Versloot J, Grudniewicz A, Chatterjee A, Hayden L, Kastner M, Bhattacharyya O. Format guidelines to make them vivid, intuitive, and visual: use simple formatting rules to optimize usability and accessibility of clinical practice guidelines. Int J Evid Based Healthc 2015;13:52-7. https://doi.org/10.1097/XEB.0000000000000036.
- Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev 2016;8. https://doi.org/10.1002/14651858.CD010669.pub2.
- Kastner M, Bhattacharyya O, Hayden L, Makarski J, Estey E, Durocher L, et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol 2015;68:498-509. https://doi.org/10.1016/j.jclinepi.2014.12.013.
- Valenstein PN. Formatting pathology reports: applying four design principles to improve communication and patient safety. Arch Pathol Lab Med 2008;132:84-9. https://doi.org/10.1043/1543-2165(2008)132[84:FPRAFD]2.0.CO;2.
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD004398.pub3.
- Stacpoole M, Thompsell A, Hockley J. Toolkit for Implementing the Namaste Care Programme for People with Advanced Dementia Living in Care Homes. London: St Christopher’s; 2016.
- Harvey N, Holmes CA. Nominal group technique: an effective method for obtaining group consensus. Int J Nurs Pract 2012;18:188-94. https://doi.org/10.1111/j.1440-172X.2012.02017.x.
- McMillan SS, Kelly F, Sav A, Kendall E, King MA, Whitty JA, et al. Using the Nominal Group Technique: how to analyse across multiple groups. Health Serv Outcomes Res Methodol 2014;14:92-108. https://doi.org/10.1007/s10742-014-0121-1.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979-83. https://doi.org/10.2105/AJPH.74.9.979.
- Hutchings A, Raine R, Sanderson C, Black N. A comparison of formal consensus methods used for developing clinical guidelines. J Health Serv Res Policy 2006;11:218-24. https://doi.org/10.1258/135581906778476553.
- Walshe C, Kinley J, Patel S, Goodman C, Bunn F, Lynch J, et al. A four-stage process for intervention description and guide development of a practice-based intervention: refining the Namaste Care intervention implementation specification for people with advanced dementia prior to a feasibility cluster randomised trial. BMC Geriatrics 2019;19. https://doi.org/10.1186/s12877-019-1275-z.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-15.
- Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of Change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-267.
- Goodman C, Sharpe R, Russell C, Meyer J, Gordon AL, Dening T, et al. Care Home Readiness: A Rapid Review and Consensus Workshops on How Organisational Context Affects Care Home Engagement with Health Care Innovation. Hatfield: University of Hertfordshire; 2017.
- Patten S. Health measurement scales: a practical guide to their development and use, 4th edition. Can J Psychiatry 2011;56:187-8. https://doi.org/10.1177/070674371105600309.
- Gonçalves BS, Cavalcanti PR, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: an update. Sleep Sci 2014;7:158-64. https://doi.org/10.1016/j.slsci.2014.09.013.
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry 1996;40:259-70. https://doi.org/10.1016/0006-3223(95)00370-3.
- Ritchie J, Lewis J. Qualitative Research Practice. A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Res 2011;18:52-6. https://doi.org/10.7748/nr2011.01.18.2.52.c8284.
- Stow R, Ives N, Smith C, Rick C, Rushton A. A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials 2015;16. https://doi.org/10.1186/s13063-015-0952-2.
- Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Evidence-based intervention to reduce avoidable hospital admissions in residents in care homes (the Better Health in Residents in Care Homes study); protocol for a pilot cluster randomised trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026510.
- Competition and Markets Authority . Care Homes Market Study. Final Report 2017.
- Care Quality Commission (CQC) . The State of Adult Social Care Services 2014 to 2017 2017.
- Glendinning C, Jacobs S, Alborz A, Hann M. A survey of access to medical services in nursing and residential homes in England. Br J Gen Pract 2002;52:545-8.
- Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing 2014;43:97-103. https://doi.org/10.1093/ageing/aft077.
- Bowman C, Whistler J, Ellerby M. A national census of care home residents. Age Ageing 2004;33:561-6. https://doi.org/10.1093/ageing/afh177.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK Second Edition – Overview. London: Alzheimer’s Society; 2014.
- Ellis-Smith C, Higginson IJ, Daveson BA, Henson LA, Evans CJ. BuildCARE . How can a measure improve assessment and management of symptoms and concerns for people with dementia in care homes? A mixed-methods feasibility and process evaluation of IPOS-Dem. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0200240.
- Goodman C, Davies SL, Gordon AL, Dening T, Gage H, Meyer J, et al. Optimal NHS service delivery to care homes: a realist evaluation of the features and mechanisms that support effective working for the continuing care of older people in residential settings. Health Serv Deliv Res 2017;5. https://doi.org/10.3310/hsdr05290.
- Kinnunen KM, Rapaport P, Webster L, Barber J, Kyle SD, Hallam B, et al. A manual-based intervention for carers of people with dementia and sleep disturbances: an acceptability and feasibility RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22710.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Bray J, Brooker D, Latham I, Wray F, Baines D. Costing resource use of the Namaste Care Intervention UK: a novel framework for costing dementia care interventions in care homes [published online ahead of print February 21 2019]. Int Psychogeriatr 2019. https://doi.org/10.1017/S1041610218002314.
- NHS Improvement . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed 19 November 2019).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 29 November 2018).
- National Institute for Health and Care Excellence . Position Statement on the Use of the EQ-5D-5L Valuation Set 2017.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) 2018.
- Devlin N, Brazier J, Pickard AS, Stolk E. 3L, 5L, What the L? A NICE Conundrum. PharmacoEconomics 2018;36:637-40. https://doi.org/10.1007/s40273-018-0622-9.
- Round J. Once bitten twice shy: thinking carefully before adopting the EQ-5D-5L. PharmacoEconomics 2018;36:641-3. https://doi.org/10.1007/s40273-018-0636-3.
- Easton T, Milte R, Crotty M, Ratcliffe J. An empirical comparison of the measurement properties of the EQ-5D-5L, DEMQOL-U and DEMQOL-Proxy-U for older people in residential care. Qual Life Res 2018;27:1283-94. https://doi.org/10.1007/s11136-017-1777-0.
- Parker B, Petrou S, Underwood M, Madan J. Can care staff accurately assess health-related quality of life of care home residents? A secondary analysis of data from the OPERA trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-012779.
- Lung T, Howard K, Etherton-Beer C, Sim M, Lewin G, Arendts G. Comparison of the HUI3 and the EQ-5D-3L in a nursing home setting. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0172796.
- Devine A, Taylor SJ, Spencer A, Diaz-Ordaz K, Eldridge S, Underwood M. The agreement between proxy and self-completed EQ-5D for care home residents was better for index scores than individual domains. J Clin Epidemiol 2014;67:1035-43. https://doi.org/10.1016/j.jclinepi.2014.04.005.
- Coast J. Strategies for the economic evaluation of end-of-life care: making a case for the capability approach. Expert Rev Pharmacoecon Outcomes Res 2014;14:473-82. https://doi.org/10.1586/14737167.2014.914436.
- Normand C. Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs. J Pain Symptom Manage 2009;38:27-31. https://doi.org/10.1016/j.jpainsymman.2009.04.005.
- Coast J. Assessing capability in economic evaluation: a life course approach?. Eur J Health Econ 2019;20:779-84. https://doi.org/10.1007/s10198-018-1027-6.
- National Institute for Health and Care Excellence . The Social Care Guidance Manual 2013.
- Zorginstituut Nederland . Richtlijn Voor Het Uitvoeren Van Economische Evaluaties in De Gezondheidszorg 2016.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. https://doi.org/10.1016/j.socscimed.2008.05.015.
- Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities?. Soc Sci Med 2006;62:1891-901. https://doi.org/10.1016/j.socscimed.2005.08.023.
- Huynh E, Coast J, Rose J, Kinghorn P, Flynn T. Values for the ICECAP-Supportive Care Measure (ICECAP-SCM) for use in economic evaluation at end of life. Soc Sci Med 2017;189:114-28. https://doi.org/10.1016/j.socscimed.2017.07.012.
- Sutton EJ, Coast J. Development of a supportive care measure for economic evaluation of end-of-life care using qualitative methods. Palliat Med 2014;28:151-7. https://doi.org/10.1177/0269216313489368.
- Makai P, Beckebans F, van Exel J, Brouwer WB. Quality of life of nursing home residents with dementia: validation of the German version of the ICECAP-O. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0092016.
- Makai P, Brouwer WB, Koopmanschap MA, Nieboer AP. Capabilities and quality of life in Dutch psycho-geriatric nursing homes: an exploratory study using a proxy version of the ICECAP-O. Qual Life Res 2012;21:801-12. https://doi.org/10.1007/s11136-011-9997-1.
- Sarabia-Cobo CM, Parás-Bravo P, Amo-Setién FJ, Alconero-Camarero AR, Sáenz-Jalón M, Torres-Manrique B, et al. Validation of the Spanish version of the ICECAP-O for nursing home residents with dementia. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0169354.
- Bailey C, Kinghorn P, Orlando R, Armour K, Perry R, Jones L, et al. ‘The ICECAP-SCM tells you more about what I’m going through’: a think-aloud study measuring quality of life among patients receiving supportive and palliative care. Palliat Med 2016;30:642-52. https://doi.org/10.1177/0269216315624890.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge; 2013.
- Horwood J, Sutton E, Coast J. Evaluating the face validity of the ICECAP-O capabilities measure: a ‘think aloud’ study with hip and knee arthroplasty patients. Appl Res Qual Life 2014;9:667-82. https://doi.org/10.1007/s11482-013-9264-4.
- Al-Janabi H, Keeley T, Mitchell P, Coast J. Can capabilities be self-reported? A think aloud study. Soc Sci Med 2013;87:116-22. https://doi.org/10.1016/j.socscimed.2013.03.035.
- Davidson Lund A. Getting involved. Care and Cure Magazine 2017.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Lichtwarck B, Selbaek G, Kirkevold Ø, Rokstad AMM, Benth JŠ, Lindstrøm JC, et al. Targeted interdisciplinary model for evaluation and treatment of neuropsychiatric symptoms: a cluster randomized controlled trial. Am J Geriatr Psychiatry 2018;26:25-38. https://doi.org/10.1016/j.jagp.2017.05.015.
- Barron DN, West E. The quasi-market for adult residential care in the UK: Do for-profit, not-for-profit or public sector residential care and nursing homes provide better quality care?. Soc Sci Med 2017;179:137-46. https://doi.org/10.1016/j.socscimed.2017.02.037.
- Towers AM, Palmer S, Smith N, Collins G, Allan S. A cross-sectional study exploring the relationship between regulator quality ratings and care home residents’ quality of life in England. Health Qual Life Outcomes 2019;17. https://doi.org/10.1186/s12955-019-1093-1.
- Surr CA, Walwyn RE, Lilley-Kelly A, Cicero R, Meads D, Ballard C, et al. Evaluating the effectiveness and cost-effectiveness of Dementia Care Mapping™ to enable person-centred care for people with dementia and their carers (DCM-EPIC) in care homes: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1416-z.
- Surr C, Lilley-Kelly A, Graham L, Walwyn R, Cicero R, Griffiths A, et al. Complexities of trial recruitment in the care home setting: an illustration via the DCM™ epic (dementia care mapping™: to enable person-centred care in care homes) trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-S2-P96.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. https://doi.org/10.1136/bmj.328.7455.1561.
- McCormack B, McCance T, McCormack B, McCance T. Person-Centred Practice in Nursing and Healthcare. Chichester: Wiley-Blackwell; 2016.
- Bray J, Atkinson T, Latham I, Brooker D. Practice of Namaste Care for people living with dementia in the UK. Nurs Older People 2018;31:22-8. https://doi.org/10.7748/nop.2018.e1109.
Appendix 1 Primary outcome measure completion rates (all participants)
| Measure | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 2 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | |
| CAD-EOLD | ||||||||
| Total number of residents | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 | 3 | 3 | 7 | 14 | 15 |
| Number with incomplete CAD-EOLD | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Number with completed CAD-EOLD | 29 | 30 | 31 | 29 | 29 | 24 | 18 | 16 |
| % of total with completed CAD-EOLD | 91 | 94 | 97 | 91 | 91 | 75 | 56 | 50 |
| QUALID | ||||||||
| Total number of residents | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 | 3 | 3 | 7 | 14 | 15 |
| Number with incomplete QUALID | 0 | 1 | 1 | 2 | 1 | 1 | 5 | 4 |
| Number with completed QUALID | 31 | 30 | 30 | 27 | 28 | 24 | 13 | 13 |
| % of total with completed QUALID | 100 | 94 | 94 | 84 | 88 | 75 | 41 | 41 |
Appendix 2 Secondary outcome measure completion rates
| Measure | Time point | ||
|---|---|---|---|
| Baseline | Week 2 | Week 4 | |
| NPI-Q severity | |||
| Total number of residents | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 |
| Number with incomplete NPI-Q severity | 7 | 6 | 6 |
| Number with completed NPI-Q severity | 25 | 25 | 25 |
| % of total with completed NPI-Q severity | 78 | 78 | 78 |
| NPI-Q distress | |||
| Total number of residents | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 |
| Number with incomplete NPI-Q distress | 7 | 5 | 7 |
| Number with completed NPI-Q distress | 25 | 26 | 24 |
| % of total with completed NPI-Q distress | 78 | 81 | 75 |
| CMAI | |||
| Total number of residents | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 |
| Number with incomplete CMAI | 0 | 0 | 2 |
| Number with completed CMAI | 32 | 31 | 29 |
| % of total with completed CMAI | 100 | 97 | 91 |
| PAIN-AD | |||
| Total number of residents | 32 | 32 | 32 |
| Number withdrawn or lost to follow-up | 0 | 1 | 1 |
| Number with incomplete PAIN-AD | 0 | 0 | 0 |
| Number with completed PAIN-AD | 32 | 31 | 31 |
| % of total with completed PAIN-AD | 100 | 97 | 97 |
Appendix 3 Primary outcome measure data: Comfort Assessment in Dying – End of Life Care in Dementia
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| Number of resident questionnaires at each time point | |||
| Baseline | 18 | 14 | 32 |
| Week 2 | 17 | 14 | 31 |
| Week 4 | 17 | 14 | 31 |
| Week 8 | 15 | 14 | 29 |
| Week 12 | 15 | 14 | 29 |
| Week 16 | 13 | 12 | 25 |
| Week 20 | 10 | 8 | 18 |
| Week 24 | 10 | 7 | 17 |
| Bereavement | 3 | 1 | 4 |
| CAD-EOLD, mean (SD); n missing | |||
| Baseline | 34.8 (4.0); 1 | 33.6 (4.7); 2 | 34.3 (4.2); 3 |
| 2 weeks | 36.5 (2.6); 0 | 32.6 (3.8); 1 | 34.8 (3.7); 1 |
| 4 weeks | 36.4 (4.0); 0 | 33.4 (3.4); 0 | 35.1 (4.0); 0 |
| 8 weeks | 35.5 (3.6); 0 | 32.1 (2.5); 0 | 33.9 (3.5); 0 |
| 12 weeks | 36.5 (4.5); 0 | 33.9 (2.5); 0 | 35.3 (3.9); 0 |
| 16 weeks | 35.1 (2.9); 1 | 33.8 (3.7); 0 | 34.4 (3.3); 1 |
| 20 weeks | 36.5 (4.0); 0 | 35.3 (2.1); 0 | 35.9 (3.3); 0 |
| 24 weeks | 37.6 (2.9); 1 | 33.6 (1.9); 0 | 35.8 (3.2); 1 |
| Bereaved | 31.0 (6.2); 0 | 31.0 (–); 0 | 31.0 (5.1); 0 |
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| CAD-EOLD physical distress, mean (SD); n missing | |||
| Baseline | 9.9 (1.3); 0 | 9.2 (1.5); 1 | 9.6 (1.4); 1 |
| 2 weeks | 10.3 (1.0); 0 | 8.3 (1.5); 0 | 9.4 (1.6); 0 |
| 4 weeks | 9.8 (1.6); 0 | 9.1 (1.7); 0 | 9.5 (1.6); 0 |
| 8 weeks | 10.0 (1.3); 0 | 8.4 (1.0); 0 | 9.2 (1.4); 0 |
| 12 weeks | 10.7 (1.6); 0 | 9.2 (1.3); 0 | 10.0 (1.6); 0 |
| 16 weeks | 10.3 (1.3); 0 | 9.6 (1.6); 0 | 10.0 (1.5); 0 |
| 20 weeks | 11.2 (1.2); 0 | 9.9 (1.6); 0 | 10.6 (1.5); 0 |
| 24 weeks | 10.8 (1.5); 0 | 9.6 (1.3); 0 | 10.3 (1.5); 0 |
| Bereaved | 8.0 (2.6); 0 | 10.0 (.); 0 | 8.5 (2.4); 0 |
| CAD-EOLD dying symptoms, mean (SD); n missing | |||
| Baseline | 11.4 (0.9); 0 | 10.8 (1.9); 1 | 11.2 (1.4); 1 |
| 2 weeks | 11.7 (0.6); 0 | 10.9 (1.6); 0 | 11.3 (1.2); 0 |
| 4 weeks | 11.6 (1.1); 0 | 11.3 (1.7); 0 | 11.5 (1.4); 0 |
| 8 weeks | 11.9 (0.3); 0 | 10.8 (1.6); 0 | 11.4 (1.3); 0 |
| 12 weeks | 11.5 (1.1); 0 | 11.1 (1.3); 0 | 11.3 (1.2); 0 |
| 16 weeks | 11.6 (0.9); 0 | 10.8 (1.9); 0 | 11.2 (1.5); 0 |
| 20 weeks | 11.1 (1.2); 0 | 10.8 (2.2); 0 | 10.9 (1.7); 0 |
| 24 weeks | 10.0 (2.4); 0 | 11.6 (1.1); 0 | 10.6 (2.1); 0 |
| Bereaved | 10.0 (3.5); 0 | 5.0 (–); 0 | 8.8 (3.8); 0 |
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| CAD-EOLD emotional distress, mean (SD); n missing | |||
| Baseline | 10.3 (1.4); 1 | 9.8 (1.4); 0 | 10.1 (1.4); 1 |
| 2 weeks | 10.6 (1.4); 0 | 9.0 (1.5); 1 | 9.9 (1.6); 1 |
| 4 weeks | 10.8 (1.0); 0 | 9.4 (1.2); 0 | 10.2 (1.3); 0 |
| 8 weeks | 10.1 (2.3); 0 | 9.8 (1.3); 0 | 10.0 (1.9); 0 |
| 12 weeks | 10.3 (1.5); 0 | 9.9 (1.4); 0 | 10.1 (1.5); 0 |
| 16 weeks | 9.9 (1.3); 0 | 10.0 (1.2); 0 | 10.0 (1.2); 0 |
| 20 weeks | 11.0 (1.2); 0 | 10.8 (1.0); 0 | 10.9 (1.1); 0 |
| 24 weeks | 11.3 (1.0); 1 | 9.3 (0.8); 0 | 10.4 (1.4); 1 |
| Bereaved | 9.3 (1.5); 0 | 12.0 (–); 0 | 10.0 (1.8); 0 |
| CAD-EOLD well-being, mean (SD); n missing | |||
| Baseline | 5.7 (2.2); 0 | 5.7 (1.5); 0 | 5.7 (1.9); 0 |
| 2 weeks | 5.1 (1.8); 0 | 5.4 (1.6); 0 | 5.2 (1.7); 0 |
| 4 weeks | 5.0 (1.9); 0 | 5.6 (1.2); 0 | 5.3 (1.6); 0 |
| 8 weeks | 5.5 (1.5); 0 | 6.1 (1.1); 0 | 5.8 (1.3); 0 |
| 12 weeks | 5.1 (2.2); 0 | 5.5 (1.0); 0 | 5.3 (1.7); 0 |
| 16 weeks | 5.5 (2.0); 1 | 5.8 (0.9); 0 | 5.6 (1.5); 1 |
| 20 weeks | 5.9 (2.6); 0 | 5.3 (1.4); 0 | 5.6 (2.1); 0 |
| 24 weeks | 4.8 (1.6); 0 | 5.9 (0.9); 0 | 5.2 (1.4); 0 |
| Bereaved | 6.0 (0.0); 0 | 6.0 (–); 0 | 6.0 (0.0); 0 |
Appendix 4 Primary outcome measure data: Quality of Life in Late Stage Dementia
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| Number of resident questionnaires at each time point | |||
| Baseline | 18 | 14 | 32 |
| Week 2 | 17 | 14 | 31 |
| Week 4 | 17 | 14 | 31 |
| Week 8 | 15 | 14 | 29 |
| Week 12 | 15 | 14 | 29 |
| Week 16 | 13 | 12 | 25 |
| Week 20 | 10 | 8 | 18 |
| Week 24 | 10 | 7 | 17 |
| Bereavement | 3 | 1 | 4 |
| QUALID total score, mean (SD); n missing | |||
| Baseline | 24.0 (8.4); 0 | 27.1 (8.0); 0 | 25.3 (8.2); 0 |
| 2 weeks | 21.4 (3.8); 1 | 29.1 (8.6); 0 | 25.0 (7.5); 1 |
| 4 weeks | 22.9 (7.1); 0 | 25.7 (7.4); 1 | 24.1 (7.2); 1 |
| 8 weeks | 21.9 (6.3); 1 | 26.9 (5.4); 1 | 24.3 (6.3); 2 |
| 12 weeks | 22.0 (7.5); 1 | 27.3 (8.0); 0 | 24.6 (8.1); 1 |
| 16 weeks | 21.5 (5.9); 1 | 26.5 (6.7); 0 | 23.9 (6.7); 1 |
| 20 weeks | 21.2 (6.1); 3 | 28.1 (6.1); 2 | 24.3 (6.9); 5 |
| 24 weeks | 19.9 (7.5); 4 | 28.1 (7.8); 0 | 23.5 (8.5); 4 |
| Bereaved | 31.0 (5.2); 0 | 27.0 (–); 0 | 30.0 (4.7); 0 |
Appendix 5 Secondary outcome measures data: NPI-Q
| Symptom | Symptom present (n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control: baseline | Control: week 4 | Intervention: baseline | Intervention: week 4 | |||||||||
| Missing | No | Yes | Missing | No | Yes | Missing | No | Yes | Missing | No | Yes | |
| Agitation/aggression | 0 | 2 | 12 | 0 | 3 | 11 | 1 | 6 | 11 | 1 | 9 | 7 |
| Anxiety | 0 | 11 | 3 | 0 | 13 | 1 | 2 | 12 | 4 | 1 | 14 | 2 |
| Apathy/indifference | 0 | 13 | 1 | 0 | 13 | 1 | 0 | 12 | 6 | 1 | 14 | 2 |
| Appetite/eating | 1 | 8 | 5 | 0 | 10 | 4 | 0 | 13 | 5 | 1 | 13 | 3 |
| Delusions | 0 | 11 | 3 | 0 | 12 | 2 | 1 | 14 | 3 | 1 | 15 | 1 |
| Depression/dysphoria | 0 | 6 | 8 | 0 | 8 | 6 | 2 | 9 | 7 | 1 | 10 | 6 |
| Disinhibition | 0 | 11 | 3 | 0 | 9 | 5 | 1 | 14 | 3 | 1 | 14 | 2 |
| Elation/euphoria | 0 | 10 | 4 | 0 | 8 | 6 | 1 | 13 | 4 | 1 | 15 | 1 |
| Hallucinations | 0 | 12 | 2 | 0 | 12 | 2 | 1 | 12 | 5 | 1 | 11 | 5 |
| Irritability/lability | 0 | 12 | 2 | 0 | 8 | 6 | 1 | 12 | 5 | 0 | 14 | 3 |
| Motor disturbance | 0 | 8 | 6 | 0 | 7 | 7 | 1 | 9 | 8 | 0 | 13 | 4 |
| Night-time behaviours | 2 | 5 | 7 | 2 | 3 | 9 | 3 | 10 | 5 | 3 | 11 | 3 |
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| Number of resident questionnaires at each time point | |||
| Baseline | 18 | 14 | 32 |
| Week 2 | 17 | 14 | 31 |
| Week 4 | 17 | 14 | 31 |
| NPI-Q severity score, mean (SD); n missing | |||
| Baseline | 8.1 (8.4); 4 | 7.8 (5.2); 3 | 8.0 (7.0); 7 |
| 2 weeks | 3.8 (5.2); 3 | 8.4 (4.4); 3 | 6.0 (5.3); 6 |
| 4 weeks | 2.9 (3.6); 4 | 8.0 (4.7); 2 | 5.4 (4.8); 6 |
| NPI-Q distress score, mean (SD); n missing | |||
| Baseline | 8.3 (11.7); 4 | 8.5 (6.6); 3 | 8.4 (9.6); 7 |
| 2 weeks | 1.7 (1.9); 2 | 7.0 (5.2); 3 | 4.1 (4.6); 5 |
| 4 weeks | 1.1 (1.0); 5 | 6.3 (6.1); 2 | 3.7 (5.0); 7 |
Appendix 6 Secondary outcome measures data: Cohen-Mansfield Agitation Inventory
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| Number of resident questionnaires at each time point | |||
| Baseline | 18 | 14 | 32 |
| Week 2 | 17 | 14 | 31 |
| Week 4 | 17 | 14 | 31 |
| CMAI total, mean (SD); n missing | |||
| Baseline | 22.9 (10.4); 0 | 23.6 (6.1); 0 | 23.3 (8.7); 0 |
| 2 weeks | 19.4 (6.5); 0 | 25.5 (6.3); 0 | 22.2 (7.0); 0 |
| 4 weeks | 18.6 (5.5); 1 | 25.3 (7.5); 1 | 21.6 (7.2); 2 |
| CMAI aggressive behaviours, mean (SD); n missing | |||
| Baseline | 6.7 (3.7); 0 | 8.9 (3.6); 0 | 7.6 (3.7); 0 |
| 2 weeks | 6.2 (3.0); 0 | 9.2 (3.0); 0 | 7.5 (3.3); 0 |
| 4 weeks | 5.6 (2.2); 1 | 9.1 (3.5); 0 | 7.2 (3.3); 1 |
| CMAI physically non-aggressive behaviours, mean (SD); n missing | |||
| Baseline | 8.1 (4.2); 0 | 6.6 (2.1); 0 | 7.5 (3.5); 0 |
| 2 weeks | 6.4 (2.2); 0 | 7.7 (2.4); 0 | 7.0 (2.4); 0 |
| 4 weeks | 7.1 (3.9); 0 | 8.2 (4.2); 0 | 7.6 (4.0); 0 |
| CMAI verbally agitated behaviours, mean (SD); n missing | |||
| Baseline | 8.2 (3.5); 0 | 8.1 (2.3); 0 | 8.2 (3.0); 0 |
| 2 weeks | 6.8 (2.4); 0 | 8.6 (3.2); 0 | 7.6 (2.9); 0 |
| 4 weeks | 5.7 (1.4); 0 | 8.8 (3.1); 1 | 7.0 (2.7); 1 |
Appendix 7 Secondary outcome measures data: Pain Assessment in Advanced Dementia
| Time point | Intervention | Control | Overall |
|---|---|---|---|
| Resident questionnaires at each time point (n) | |||
| Baseline | 18 | 14 | 32 |
| Week 2 | 17 | 14 | 31 |
| Week 4 | 17 | 14 | 31 |
| CMAI total, mean (SD); n missing | |||
| Baseline | 3.6 (2.1); 0 | 5.4 (3.1); 0 | 4.4 (2.7); 0 |
| 2 weeks | 1.6 (1.5); 0 | 5.3 (2.7); 0 | 3.3 (2.8); 0 |
| 4 weeks | 2.3 (1.6); 0 | 5.3 (3.0); 0 | 3.6 (2.8); 0 |
Appendix 8 Actigraphy summaries
| Summary | Trial arm | Mean | SD | Median | Lower quartile | Upper quartile | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Sleep–wake ratios | Overall | 4.08 | 3.22 | 2.72 | 1.91 | 6.33 | 0.58 | 13.5 |
| Control | 4.01 | 3.22 | 3.19 | 2.47 | 3.97 | 1.57 | 13.5 | |
| Intervention | 4.13 | 3.32 | 2.48 | 1.91 | 7.21 | 0.58 | 10.05 | |
| Time asleep | Overall | 459.94 | 123.27 | 458.72 | 391.72 | 542.47 | 196.64 | 706.29 |
| Control | 462.15 | 74.81 | 467.16 | 414.58 | 517.91 | 337.5 | 574.48 | |
| Intervention | 458.33 | 151.19 | 439.23 | 391.72 | 600.45 | 196.64 | 706.29 | |
| Sleep efficiency | Overall | 0.68 | 0.14 | 0.69 | 0.56 | 0.81 | 0.35 | 0.9 |
| Control | 0.71 | 0.1 | 0.72 | 0.68 | 0.77 | 0.55 | 0.9 | |
| Intervention | 0.65 | 0.17 | 0.65 | 0.55 | 0.82 | 0.35 | 0.87 | |
| Wake after sleep onset | Overall | 213.53 | 86.58 | 204.82 | 143.5 | 275.62 | 34.88 | 383.69 |
| Control | 193.14 | 70.45 | 194.05 | 167.15 | 209.76 | 34.88 | 294.39 | |
| Intervention | 228.26 | 95.79 | 234.22 | 138.07 | 317.88 | 95.24 | 383.69 | |
| Total activity | Overall | 8.66 | 4.32 | 7.77 | 5.64 | 10.69 | 3.54 | 25 |
| Control | 7.31 | 2.15 | 7.59 | 5.79 | 9.02 | 3.54 | 10.69 | |
| Intervention | 9.63 | 5.23 | 8.19 | 5.35 | 12.56 | 4.52 | 25 | |
| Intradaily variability | Overall | 0.7 | 0.22 | 0.7 | 0.59 | 0.79 | 0.21 | 1.18 |
| Control | 0.78 | 0.23 | 0.77 | 0.66 | 0.89 | 0.32 | 1.18 | |
| Intervention | 0.64 | 0.2 | 0.67 | 0.57 | 0.73 | 0.21 | 1.05 | |
| Interdaily stability | Overall | 0.08 | 0.05 | 0.07 | 0.04 | 0.09 | 0.01 | 0.22 |
| Control | 0.08 | 0.05 | 0.07 | 0.04 | 0.09 | 0.02 | 0.21 | |
| Intervention | 0.08 | 0.06 | 0.06 | 0.04 | 0.09 | 0.01 | 0.22 |
Appendix 9 Think-aloud analysis: detailed methods
Interviews were fully transcribed and then segmented by questionnaire and item. These segments were presented to three raters along with information about the scores given by the proxy respondents for each questionnaire item. Three raters then independently assessed the transcript for errors in terms of:
-
comprehension (understanding the question in the way intended)
-
retrieval (retrieving information – in general, this is assumed to be the ability to retrieve information from long-term memory, but, for this case, with proxy respondents, it was also used to indicate errors where the respondent was unable to retrieve information that they were unaware of)
-
judgement (judging how the retrieved information should be used to answer the question), response (providing a valid response) or struggle (providing a correct response, but struggling in the process).
Definitions of each error type and examples of potential errors were available to raters.
Each rater made an independent assessment of error for each questionnaire item, for each questionnaire, for each proxy respondent. A set of rules was then used to determine whether or not a response should be classified as an error and, if so, of what type:
-
If an error type was identified by all raters, then an error of that type was recorded.
-
If no error was identified by any rater, then no error was recorded.
-
If an error was identified by one or two raters, then the raters collectively came to a final decision through discussion.
-
If an error was identified by all three raters, but there was disagreement about the nature of the error, then the raters collectively came to a final decision through discussion.
-
If raters could not come to a collective decision through discussion, then the final assessment was made based on majority choice.
Appendix 10 Phase 3 detailed nursing home costs of providing the Namaste Care intervention
The reported costs of any items the nursing homes purchased to use in the Namaste Care programme that they would not have purchased for usual care, either as fixed set-up costs or as variable costs of consumables, are shown in Table 46. One nursing home had detailed petty-cash records available; for the other nursing home that used resources to provide the intervention, information was obtained through the staff interviews. Two of the intervention nursing homes required extra items to carry out the Namaste Care sessions, while the others already had all of the necessary items as part of the nursing homes’ provision of usual care. One of these nursing homes already had a ‘sensory room’ available as part of its usual care provision, and this home simply needed to adapt the room for Namaste Care use based on the intervention protocol. For the other nursing home, it seemed that its ability to provide Namaste Care might have been compromised as a result of lack of funds and staff availability.
| Item | Fixed/variable cost | Reported cost (£) |
|---|---|---|
| Massage oils/creams | Variable | 40.00 |
| Lights | Fixed | 20.00 |
| CDs | Fixed | 3.00 |
| Aromatherapy set | Fixed | 75.00 |
| Washing bowl | Fixed | 20.00 |
| Wash bag | Fixed | 5.98 |
| Towels | Fixed | 30.00 |
| Throws/pillows | Fixed | 79.00 |
| Curtains/cosmetics | Fixed/variable | 38.40 |
| Diffuser/smells | Fixed | 3.50 |
| Chairs | Fixed | 50.00 |
| Cushions | Fixed | 3.50 |
| Flowers | Fixed | 15.00 |
| Snacks | Variable | 10.00 |
The two nursing homes that had needed to purchase additional items also required additional staffing to carry out the programme sessions. The cost that the nursing homes incurred from paying any additional staff wages as a result of increased staffing levels were combined with the costs of the items purchased to give the total cost of implementing Namaste Care to each of the homes, as shown in Table 47. The mean cost of implementing the programme across intervention homes over the 4-week intervention period was calculated. As some of the intervention nursing homes reported no additional costs, the mean intervention cost based only on the two homes that had needed additional resources is also reported in Table 47, as this provides the most useful information about the likely costs for nursing homes that lack the capacity to run Namaste Care using their existing resources. For these two nursing homes, the cost for each resident per session was £8.30 (SD £3.49).
| Nursing home | Costs (£) | ||||
|---|---|---|---|---|---|
| Item costs | Staff costs | Staff costs per resident | Programme total | Programme total per resident | |
| n01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| n02 | 63.00 | 893.56 | 223.39 | 956.56 | 239.14 |
| n04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| n07 | 330.38 | 693.12 | 138.62 | 1023.50 | 204.70 |
| Intervention mean (SD) | 98.35 (157.52) | 396.67 (465.29) | 90.50 (110.09) | 495.02 (572.25) | 110.96 (128.89) |
| Intervention mean for homes incurring costs (SD) | 196.69 (189.07) | 793.34 (141.73) | 181.01 (59.94) | 990.03 (47.33) | 221.92 (24.35) |
Appendix 11 Phase 3 detailed NHS costs associated with the Namaste Care intervention
The mean costs of the total, psychotropic and pain medication use from baseline to 4 weeks post baseline for each nursing home and by trial arm are presented in Table 48. This table contains two estimates of total costs because in nursing home n07 some records of medication use for residents had been archived; this affected four residents to varying degrees.
| Nursing home | Mean (SD) per-resident total medication use cost (£) | Mean (SD) per-resident psychotropic medication use cost (£) | Mean (SD) per-resident pain medication use cost (£) |
|---|---|---|---|
| n01 | 303.62 (130.92) | 2.40 (1.16) | 17.51 (21.30) |
| n02 | 167.80 (154.11) | 4.59 (5.35) | 24.09 (30.95) |
| n04 | 92.44 (85.85) | 9.49 (9.99) | 39.77 (31.64) |
| n07 | 3.65 (7.68) | 0.18 (0.33) | 0.00 (0.00) |
| Total intervention | 146 (156.29) | 3.51 (5.39) | 17.84 (24.85) |
| Total intervention (excluding n07) | 205.55 (149.76) | 4.90 (5.92) | 25.27 (26.33) |
| n03 | 120.77 (104.73) | 59.19 (102.48) | 7.06 (7.27) |
| n06 | 137.41 (119.86) | 22.09 (51.85) | 0.00 (0.00) |
| Control population | 127.90 (107.26) | 43.29 (83.98) | 4.03 (6.45) |
Given this issue, Table 49 provides the mean costs per resident across all services, including both values including and values excluding the costs for nursing home n07.
| Nursing home | Costs to the NHS (£) | Cost to nursing home (£) | |||
|---|---|---|---|---|---|
| Mean service use cost | Mean total medication use cost | Mean total NHS cost | Per-resident programme cost | Mean total cost | |
| n01 | 143.28 | 303.62 | 446.90 | 0.00 | 446.90 |
| n02 | 19.00 | 167.80 | 186.80 | 239.14 | 425.94 |
| n04 | 196.53 | 92.44 | 288.98 | 0.00 | 288.98 |
| n07 | 21.04 | 3.65 | 24.69 | 204.70 | 229.39 |
| Total intervention | 87.48 | 146.17 | 233.65 | 110.96 | 344.61 |
| Total intervention (excluding n07) | 115.17 | 205.55 | 320.72 | 79.71 | 400.43 |
| n03 | 561.00 | 120.77 | 681.77 | 0.00 | 681.77 |
| n06 | 661.33 | 137.41 | 798.74 | 0.00 | 798.74 |
| Total control | 604.00 | 127.90 | 731.90 | 0.00 | 731.90 |
The use of dementia memory assessment services by three residents living in control homes was unexpected given that residents were living with advanced dementia. Table 50 shows the impact of excluding this atypical and expensive resource.
| Nursing home | Costs to the NHS (£) | Cost to nursing home (£) | |||
|---|---|---|---|---|---|
| Mean service use costs | Mean total drug use costs | Mean total NHS costs | Per-resident programme cost | Mean total costs | |
| n01 | 143.28 | 303.62 | 446.90 | 0.00 | 446.90 |
| n02 | 19.00 | 167.80 | 186.80 | 239.14 | 425.94 |
| n04 | 196.53 | 92.44 | 288.98 | 0.00 | 288.98 |
| n07 | 21.04 | 3.65 | 24.69 | 204.70 | 229.39 |
| Total intervention | 87.48 | 146.17 | 233.65 | 110.96 | 344.61 |
| Total intervention (excluding n07) | 115.17 | 205.55 | 320.72 | 79.71 | 400.43 |
| n03 | 251.00 | 120.77 | 371.77 | 0.00 | 371.77 |
| n06 | 454.67 | 137.41 | 592.08 | 0.00 | 592.08 |
| Total control | 338.29 | 127.90 | 466.18 | 0.00 | 466.18 |
Items the nursing homes purchased for the Namaste Care sessions were largely associated with initial set-up costs; Table 51 provides the estimates of costs, excluding these set-up costs.
| Nursing home | Costs to the NHS (£) | Cost to nursing home (£) | |||
|---|---|---|---|---|---|
| Mean service use costs | Mean total drug use costs | Mean total NHS costs | Staff costs per resident | Mean total costs | |
| n01 | 143.28 | 303.62 | 446.90 | 0.00 | 446.90 |
| n02 | 19.00 | 167.80 | 186.80 | 223.39 | 410.19 |
| n04 | 196.53 | 92.44 | 288.98 | 0.00 | 288.98 |
| n07 | 21.04 | 3.65 | 24.69 | 138.62 | 163.31 |
| Total intervention | 87.48 | 146.17 | 233.65 | 90.50 | 324.16 |
| Total intervention (excluding n07) | 115.17 | 205.55 | 320.72 | 74.46 | 395.18 |
| n03 | 561.00 | 120.77 | 681.77 | 0.00 | 681.77 |
| n06 | 661.33 | 137.41 | 798.74 | 0.00 | 798.74 |
| Total control | 604.00 | 127.90 | 731.90 | 0.00 | 731.90 |
The costs of the Namaste Care intervention in the primary analysis were the additional costs actually incurred by the nursing homes. An alternative approach to costing the service is to apply recently published estimates of the cost of Namaste Care generated using a modelling approach to the number of sessions residents received in each home. 181 This sensitivity analysis is shown in Table 52.
| Nursing home | Costs to the NHS (£) | Costs to nursing home (£) | |||
|---|---|---|---|---|---|
| Mean service use costs | Mean total medication use costs | Mean total NHS costs | Per-resident programme cost | Mean total cost | |
| n01 | 143.28 | 303.62 | 446.90 | 117.52 | 564.42 |
| n02 | 19.00 | 167.80 | 186.80 | 370.64 | 557.44 |
| n04 | 196.53 | 92.44 | 288.98 | 153.68 | 442.66 |
| n07 | 21.04 | 3.65 | 24.69 | 171.76 | 196.45 |
| Total intervention | 87.48 | 146.17 | 233.65 | 203.40 | 437.05 |
| Total intervention (excluding n07) | 115.17 | 205.55 | 320.72 | 213.95 | 534.67 |
| n03 | 561.00 | 120.77 | 681.77 | 0.00 | 681.77 |
| n06 | 661.33 | 137.41 | 798.74 | 0.00 | 798.74 |
| Total control | 604.00 | 127.90 | 731.90 | 0.00 | 731.90 |
List of abbreviations
- CAD-EOLD
- Comfort Assessment in Dying – End of Life Care in Dementia
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMAI
- Cohen-Mansfield Agitation Inventory
- CMO
- context–mechanism–outcome
- CQC
- Care Quality Commission
- ENRICH
- Enabling Research in Care Homes
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FAST
- Functional Assessment Staging Test
- GP
- general practitioner
- ICC
- intracluster correlation coefficient
- ICECAP
- ICEpop CAPability
- ICECAP-O
- ICEpop CAPability measure for Older people
- ICECAP-SCM
- ICEpop CAPability Supportive Care Measure
- NICE
- National Institute for Health and Care Excellence
- NPI-Q
- Neuropsychiatric Inventory – Questionnaire
- PAIN-AD
- Pain Assessment in Advanced Dementia
- P-CAT
- Person-Centred Care Assessment Tool
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QUALID
- Quality of Life in Late Stage Dementia
- SD
- standard deviation
- SWC-EOLD
- Satisfaction With Care – End Of Life in Dementia
Notes
-
The Namaste Care guide
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hta/151011/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
