Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/48. The contractual start date was in November 2011. The draft report began editorial review in December 2017 and was accepted for publication in October 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Irwin Nazareth was on the Health Technology Assessment (HTA) Commissioning Board from 2012 to July 2017. For the duration of the Vitamin D and Longevity (VIDAL) trial, Irwin Nazareth’s PRIMENT Clinical Trials Unit was funded by the National Institute for Health Research (NIHR). He was a member of the HTA Disease Prevention Panel, a member of the HTA Commissioning Sub-board (Expression of Interest) and a member of the HTA Primary Care Themed Call. Benoit Aigret reported that Queen Mary University of London received a grant from the London School of Hygiene & Tropical Medicine to develop the VIDAL online application during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Rake et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The number of people aged ≥ 80 years in the UK is projected to more than double, to 6 million, by mid-2037,1 and interventions that improve quality as well as length of life are needed. 2 Cancer, cardiovascular disease, dementia, community-acquired pneumonia, falls and fractures account for much of the reduction in the quality of life (QoL) as well as overall mortality rates of older adults3–7 and impose a huge economic burden on the NHS, social services and many families. 8 There is, therefore, a need for new interventions to prevent these conditions. A large and growing body of evidence identifies vitamin D supplementation as a promising candidate to reduce morbidity and mortality in the elderly. 9 Vitamin D is a pre-pro-hormone that is synthesised in the skin by ultraviolet B radiation in sunlight, which is a major source of vitamin D. Dietary sources are limited, with oily fish being the only significant contributor. 10 At the UK’s latitude (50–58°N), sunlight can stimulate cutaneous vitamin D synthesis only between April and October. 11 Consequently, vitamin D insufficiency {defined as a serum 25-hydroxyvitamin D [25(OH)D] concentration of < 75 nmol/l} is very common, especially among older adults, who may spend less time outdoors and whose skin is less efficient at synthesising vitamin D. 9 Vitamin D insufficiency among older adults in the UK may therefore be an important and readily correctable risk factor for a variety of diseases. Offering a daily vitamin D supplement to all UK adults aged > 65 years would be inexpensive and safe and could result in significant and cost-effective improvements in QoL as well as longevity.
The diversity of the roles played by vitamin D in normal human physiology offers a plausible explanation of how a single micronutrient might ameliorate a heterogeneous collection of diseases. Humans evolved at equatorial latitudes in unlimited sunshine, and serum concentrations of the major circulating metabolite, 25(OH)D, are ≈115 nmol/l in people living traditional lifestyles near the equator,12,13 which is three times the median level of 37 nmol/l among adults aged ≥ 65 years in the UK in January to March. 14 The enzyme that converts 25(OH)D to its active metabolite, the steroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D] or calcitriol, and the cognate receptor for that metabolite [the vitamin D receptor (VDR)] are expressed in the majority of human tissues,10 not just those involved in calcium homeostasis, as was thought throughout much of the twentieth century. Ligation of VDR by calcitriol modifies expression of > 200 genes15 to support a wide range of biological responses that may have an impact on the pathogenesis of many diseases as well as falls and fractures16 (Figure 1).
FIGURE 1.
Mechanisms by which vitamin D may prevent cancer, cardiovascular disease, dementia, infections, falls and fractures. CVD, cardiovascular disease.
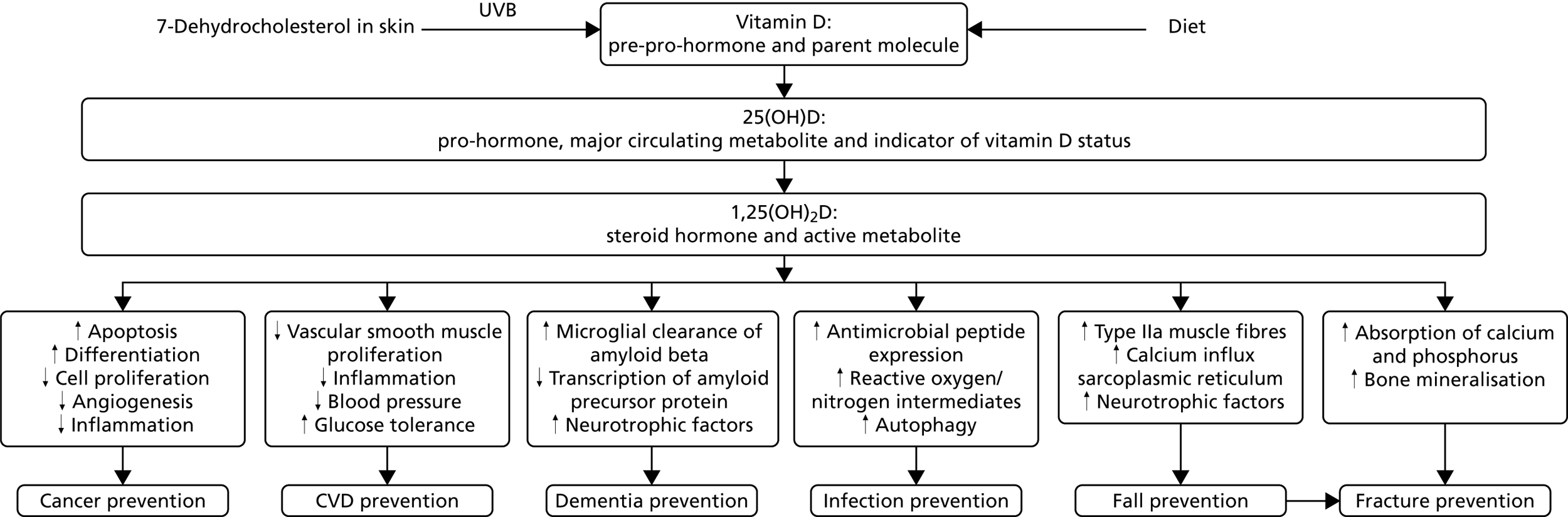
In keeping with these biological actions, observational epidemiological studies have reported associations between low 25(OH)D levels and increased incidence of several cancers (particularly of the colon), cardiovascular disease, Alzheimer’s disease and all-cause dementia, acute respiratory infection and risk of falls and fractures. 17–20 However, a recent systematic review of mortality studies21 concluded that although all-cause mortality is consistently increased in people with 25(OH)D levels below about 75 nmol/l, several uncertainties remain. The optimal 25(OH)D level is ill-defined owing to imperfect assay methods; for cancers other than colon, survival after diagnosis rather than incidence may be affected; and effects on individual cancer types and subgroups of cardiovascular disease are unclear. Last but most important, reverse causation can be excluded only by large randomised controlled trials (RCTs). Published results of RCTs of vitamin D supplementation conducted to date are inconclusive and may be subject to publication bias. Some RCTs have reported protective effects for cancer incidence,22 acute respiratory infections,23–25 fractures26 and falls27 while others have not. 28–34 Many of these trials suffered from one or more of the following limitations:
-
The dose of vitamin D administered was inadequate to elevate serum 25(OH)D concentration to > 75 nmol/l. 28–30
-
Vitamin D deficiency was not highly prevalent at baseline. 31
-
There was inadequate statistical power to detect modest but clinically significant effects of the intervention. 32
The promise of a potentially safe and cost-effective intervention to reduce the incidence of several diseases and prolong life has prompted a huge international research effort, particularly in the last decade. This has culminated in the establishment of four very large (> 5000 participants) RCTs of higher-dose vitamin D3 in older adults – the so-called ‘vitamin D megatrials’, defined here as RCTs of higher-dose vitamin D3 (≥ 2000 IU per day or equivalent) in older adults with a sample size of > 5000. The primary characteristics of these studies are summarised in PICO (Participants, Intervention, Comparator, Outcome) format in Table 1. Trials of lower doses28,29 or of vitamin D2 rather than vitamin D335 have not been listed, as such regimens do not produce an adequate increase in 25(OH)D.
| Trial | Setting | Participants | Intervention | Comparator | Outcome (primary) | Status |
|---|---|---|---|---|---|---|
| VIDA | New Zealand | n = 5110, aged 50–84 years | 100,000 IU vitamin D3 monthly p.o. | Placebo | Incidence of cardiovascular disease over 5 years | Cardiovascular and bone outcomes reported41,42 |
| VITAL | USA | n = 25,875 aged ≥ 50 years (male), ≥ 55 years (female) | 2000 IU vitamin D3 daily p.o. (2 × 2 factorial with omega-3) | Placebo | Incidence of cancer and cardiovascular disease (co-primary) over 5 years | Cardiovascular and cancer outcomes reported43 |
| TIPS-3 | Canada, India + nine other countries | n = 5713 aged ≥ 55 years (male), ≥ 60 years (female) | 60,000 IU vitamin D3 monthly p.o. (2 × 2 × 2 factorial with polypill and aspirin) | Placebo | Hip fracture (primary vitamin D outcome) over 5 years | Enrolling; due to report 2019 |
| D-Health | Australia | n = 25,000 aged 60–79 years | 60,000 IU vitamin D3 monthly p.o. | Placebo | All-cause mortality over 5 years | Enrolling; due to report 2020 |
Why then does the UK need a ‘vitamin D megatrial’ of its own?
We propose two reasons:
-
The UK represents a setting with a high prevalence of vitamin D insufficiency, where supplementation could have maximal impact. Median serum 25(OH)D concentrations among older adults in the UK (37–49 nmol/l, depending on season) are significantly lower than in the countries where large trials are currently being conducted (New Zealand, 66 nmol/l;36 Australia, 69 nmol/l;37 Canada, 70 nmol/l;38 USA, 57 nmol/l39). The efficacy of vitamin D supplementation is likely to depend on the prevalence of inadequate vitamin D status at baseline, so the results of the intervention studies conducted in these settings are likely to underestimate any effects that would be seen in older adults living in the UK and are less likely to achieve statistical significance. International differences in baseline vitamin D status may be partly attributable to the fact that many of the countries listed in Table 1 are situated at lower latitudes than the UK and, therefore, their populations have greater exposure to sunshine of sufficient intensity to stimulate cutaneous vitamin D synthesis; moreover, many of these countries routinely fortify foods with vitamin D (e.g. milk in the USA, Canada and Finland is routinely vitamin D-fortified).
-
Conduct of a further large trial of daily vitamin D supplementation in the UK will add substantially to meta-analysis of these four megatrials to detect and estimate a modest but clinically significant effect of vitamin D on all-cause mortality among participants with low serum 25(OH)D, among whom any effect is likely to be concentrated. Apart from FIND (Finnish Vitamin D Trial)40 in Finland, which stopped recruitment at 2500 participants (target 18,000 participants) with only 830 participants allocated to 3200 IU daily (see Table 1 footnote), the proposed trial would provide the only evidence on the effects of a daily dose of the order of 4000 IU. Three trials (VIDA,41,42 TIPS-3 and D-Health) are testing monthly dosing, which may be less effective. The VITamin D and OmegA-3 TriaL (VITAL), which tested 2000 IU daily, included only 3318 participants aged ≥ 75 years,43 one-third of the number proposed in the VIDAL main trial.
The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme did not support our application in 2008 to conduct a large trial of vitamin D supplementation in older adults in the UK, but invited an application for this feasibility study to establish the procedures required to conduct the main trial. Funding was awarded in 2011 and recruitment began in 2013. An application for funding for the main trial was made again in January 2015 when recruitment was complete, but by that time the trials listed in Table 1 were under way and the NIHR HTA programme decided that funding for a large British trial would not be reconsidered until the results of those trials become available. The results of the VITAL trial, which is the only large trial of daily high-dose vitamin D, are inconclusive for all-cause mortality [hazard ratio 2 to 5 years after entry 0.96, 95% confidence interval (CI) 0.84 to 1.11], so further evidence is now needed. 43
Chapter 2 Methods
Trial design
The Vitamin D and Longevity (VIDAL) feasibility trial was a four-arm multicentre RCT of 2 years’ duration of subjects aged 65–84 years. Twenty general practitioner (GP) practices in England were cluster randomised in matched pairs to either double-blind or open-label study design. The GP practices were assigned to pairs matched approximately on size, whether urban or rural, ethnic mix and ward multiple deprivation index based on practice postcode. The practices in each pair were then randomly assigned to double-blind or open-label individual randomisation. In double-blind practices participants were individually randomised to blind vitamin D (BD) or blind placebo control (BC). In open-label practices individual randomisation of participants was to open-label vitamin D (OD) or untreated open control (OC).
Ethics and regulatory approval and research governance
Ethics approval for the study was given by the London–Chelsea National Research Ethics Service in February 2012 (reference number 11/LO/1989). Clinical trial authorisation for the study was given by the Medicines and Healthcare products Regulatory Agency (MHRA) in March 2012 (reference number 17072/0006/001-0001). Appropriate site-specific assessments were obtained from the primary care trusts to confer the required management permissions for the 20 participating GP practices. The trial was registered with the International Standard Randomised Controlled Trial Register under the reference number ISRCTN 46328341 and also with the European Union Drug Regulating Authorities Clinical Trials (EudraCT) database under the reference number 2011-003699-34.
Patient and public involvement
Public opinion regarding the information being provided to participants of the trial was gauged in collaboration with Barts Clinical Trials Unit (CTU). The Queen Mary Trials Advisory Group provided valuable feedback on the patient invitation letter, information sheet and consent forms used in the trial. The lay member and consumer representative on the VIDAL Trial Management Group also contributed from the outset to the design of the feasibility study and provided feedback on trial management issues as they arose.
Eligibility
Members of the general population were recruited from 20 GP practices across England.
Inclusion criteria
Registered patients were considered for inclusion if they:
-
were aged ≥ 65 years and ≤ 84 years at enrolment
-
were contactable by telephone, able to receive recorded deliveries by post, able to attend enrolment at the GP surgery
-
had GP notes available for the previous year.
Exclusion criteria
The study design excluded anyone:
-
with known active tuberculosis, sarcoidosis, hyperparathyroidism, past or present nephrolithiasis, vitamin D intolerance, referral for suspected hepatic or renal dysfunction, terminal illness or any malignancy other than non-melanoma skin cancer not in remission for ≥ 3 years
-
planning to move from the GP practice or to emigrate within 5 years
-
with any other condition that in the principal investigator’s or chief investigator’s judgement might compromise participant safety or compliance, interfere with evaluation or preclude completion of the study
-
with a baseline corrected blood calcium level of > 2.65 mmol/l
-
taking dietary supplements or other medication containing > 400 IU (10 µg) of vitamin D per day
-
taking concomitant therapy with any of the following: carbamazepine, phenobarbital, phenytoin, primidone, digoxin, oral 1-alpha-hydroxylated vitamin D preparations (e.g. alfalcalcidol, calcitriol) or the combination of a thiazide diuretic (e.g. bendrofluazide, metolazone) with a calcium supplement
-
taking treatment with any other investigational medical product or device up to 4 months before first dose of the investigational medicinal product.
Recruitment procedure
Each of the 20 GP practices generated a list of registered patients aged 65–84 years. After excluding ineligible individuals, including those judged by the GP to lack the mental capacity to give informed consent or to be unsuitable for other reasons, study invitations were sent by post to potential participants.
Interested respondents were telephoned by a member of the research team at the GP practice to confirm eligibility and arrange a baseline assessment appointment at the GP practice, at which time informed consent was obtained from those agreeing to participate in the trial. Trial participants then provided information on current medications and conditions, diet (including dietary supplements), skin type and sun exposure, and QoL (see Appendix 1). Virtually all information was entered directly into case report forms (CRFs) accessed via the online clinical data management system [the VIDAL app (online application); see Appendix 2], with paper copies of all CRFs available as a back-up option. Systolic and diastolic blood pressure (BP), height, weight and waist circumference were recorded, and 12.5 ml of blood [9 ml ethylene diamine tetra-acetic acid (EDTA) vacutainer for measurement of 25(OH)D and 3.5 ml serum separator tube (SST) vacutainer for calcium assay] were obtained. The 3.5-ml aliquot was sent for corrected calcium assay at the practice’s local laboratory to verify eligibility. The 9-ml aliquot was sent to the Clinical Trials Service Unit in Oxford for separation of buffy coat and storage in liquid nitrogen. Circulating 25(OH)D was assayed on all stored samples (baseline and 2-year follow-up) at the end of the study on the Cobas 6000 immunoassay (Roche Molecular Diagnostics, Pleasanton, CA, USA).
Informed consent
Informed written consent was obtained during the baseline assessment from eligible participants after an explanation of the aims, methods, anticipated benefits and potential hazards of the study. The original signed and dated consent forms were held at each GP practice, with copies sent to the participant and the Trial Coordination Centre. Patients ineligible for inclusion, based on the corrected blood calcium result, were informed of this by their GP or GP nurse, who also discussed whether or not any treatment was indicated.
Randomisation, concealment and blinding
Cluster randomisation of GP practices
Prior to study commencement, 20 GP practices were matched as closely as possible in pairs based on urban/rural location, deprivation [Index of Multiple Deprivation (IMD) of the ward of the GP practice based on the GP practice postcode], practice size and ethnic mix (non-white proportion). Practices were then randomised within each pair, one to the double-blind study and one to the open-label study, by the Biostatistician and Director of the Barts CTU, a UK Clinical Research Collaboration registered Trials Unit. Four GP practices (including both in one pair) withdrew after randomisation, so these were replaced and the three pairs were re-randomised.
Individual randomisation of eligible participants within the practices
Individual participants were subsequently randomised within GP practices using the VIDAL app developed by the Barts CTU. The VIDAL app generated a random sequence of allocations for each GP practice balanced in blocks of six or eight so that the next participant’s allocation could not be predicted. Allocation of treatment was concealed from all participants, GP practices and researchers in the blind arm of the trial. Only the independent senior programmer at Barts CTU, who wrote the randomisation code on the VIDAL app, had access to this code.
On receipt of an eligible corrected blood calcium result and after verifying participant eligibility and consent, the Trial Coordination Centre telephoned potential participants to confirm their willingness to be randomised. Randomisation was then performed by the automated system on the VIDAL app.
Participants randomised to a treatment arm (BD, BC or OD) were then sent a 1-year supply of study medication by recorded delivery from the dispensing pharmacy. The second year’s study medication was allocated automatically by the VIDAL app 1 year later and sent by the same procedure. Participants allocated to OC at randomisation received a letter from the Trial Coordinating Centre explaining that they would be recontacted at 2 years for a follow-up visit and a further blood sample.
The study participants who were enrolled at the blind practices received annual study medication packs, each containing 12 monthly doses of study oil labelled as ‘vitamin D3 oil/placebo oil’. Each pack contained 12 bottles containing either 5.2 ml cholecalciferol (Vigantol® Oil; Merck Serono GmbH, Germany) – an oily solution of vitamin D3, concentration 0.5 mg/ml – or 5.2 ml placebo, a pharmacopoeia-listed mixture of palm oil and coconut oil containing medium-chain triglycerides (Miglyol® 812; Caesar & Loretz GmbH, Germany).
Study participants enrolled at open-label practices who were allocated to vitamin D (OD arm) received annual study medication packs each containing 12 monthly doses of study oil labelled as ‘vitamin D3 oil’. Each of the 12 bottles contained 5.2 ml, cholecalciferol.
The bottles of medication contained 5.2 ml to ensure delivery of 5 ml (2.5 mg of vitamin D3) because ≈ 0.2 ml of the oily solution adheres to the sides of the bottle.
Follow-up
To obtain information on their vitamin D consumption for an interim report, 121 participants who were randomised to the no treatment arm in open practices (OC arm) before May 2014 were contacted by post, e-mail or telephone in December 2014. There was no other contact after randomisation with the OC participants until they were invited to attend the 2-year final visit. All other study participants (i.e. those allocated to active treatment or placebo) were contacted at least once per month post randomisation by their preferred medium, as described in the following paragraphs. Letters were sent by CFH Docmail Ltd (Radstock, UK) on behalf of the participating GP practices, with responses sent directly back to the study participant’s GP practice.
Automated telephone call, e-mail or text message every month from month 1 to month 24
All participants except those randomised to OC (no treatment) were reminded to take their study medication every month by automated telephone call, e-mail or text message generated by the VIDAL app.
E-mail or letter follow-up at months 3, 6, 9, 12, 15, 18, 21 and 24
All participants except those who were randomised to the OC (no treatment) arm were contacted quarterly either by post from their GP practice or by e-mail from the VIDAL app (depending on the participant’s choice of medium). The participants were asked to send a short reply by the same medium. E-mails to the VIDAL app were recorded automatically. The reply recorded the dates on which the last three doses of study medication were taken or reasons for non-compliance, any planned change of address and any hospital admissions. The 3-monthly follow-up also included a reminder for participants to contact their GP if they were experiencing ongoing symptoms of hypercalcaemia (persistent nausea, vomiting, thirst, passing excessive amounts of urine or feeling generally unwell).
The Trial Coordinating Centre also monitored serious adverse events (SAEs), adverse reactions (ARs) and compliance during follow-up.
General practitioner practice visit at month 24
Two years after randomisation, all participants were invited to attend their GP practice for the 2-year visit to obtain a repeat blood sample for 25(OH)D assay, a BP measurement and responses to the same lifestyle questions as at baseline. Current consumption of any medication or supplement containing vitamin D was recorded to assess contamination.
Each GP practice also examined all treatment packs brought in at the 2-year visit, cross-checking unused study oil bottles and the study oil dose dates recorded by each participant on the exterior of the treatment pack against the compliance information supplied in the quarterly follow-up form.
Summaries of GP records for all randomised participants were extracted by the practice staff to obtain prospectively recorded information about GP visits, prescriptions and infections over the preceding 3 years (1 year pre randomisation and 2 years during the trial).
Automated follow-up
Cause-specific mortality, cancer incidence and hospital records were obtained by linking NHS number, date of birth and postcode to medical records held by NHS Digital on cancer registrations (provided by NHS Digital on behalf of Public Health England), deaths (from civil registration data and provided by NHS Digital on behalf of the Office for National Statistics) and the Hospital Episode Statistics (HES) database (from March 2012 to March 2017) on hospital admissions. 44
Interim Data Monitoring Committee reports
A Data Monitoring Committee (DMC) was convened. Interim safety analyses were conducted twice during the feasibility study. The independent statistician conducted an analysis to compare the incidence of SAEs between intervention and control arms for review by the DMC. Had there been a significant difference (p < 0.05) in the incidence of fatal or life-threatening adverse events, the DMC would have been informed and would have discussed whether or not the sponsor and ethics committee should be consulted regarding stopping the trial, but this did not arise.
Trial outcomes
Primary outcomes
The primary aim of the feasibility study was to establish the procedures required to conduct the main trial and to determine the time taken to recruit and randomise 1600 participants aged 65–84 years. The aims of the cluster randomisation of practices were to:
-
compare response (number randomised/number invited) and attrition (attendance at 2-year final visit) in blind and open practices
-
compare allocated treatment compliance among open-label (OD) participants and blind (BC or BD) participants
-
compare contamination rates (the proportion taking > 400 IU per day of vitamin D), particularly between open untreated control (OC) and blind (BC or BD) participants.
Secondary outcomes
-
Comparison of reported SAEs between vitamin D and control participants in blind practices provides a conventional safety measure. OC participants did not receive quarterly follow-ups and therefore did not report SAEs, which were recorded only retrospectively at the 2-year follow-up.
-
Comparison of numbers of infections and GP visits between vitamin D and control participants (a) in blinded practices, and (b) in open-label practices. This provides an estimate of the bias in these measures with open-label randomisation.
-
Blood 25(OH)D concentration at recruitment and at 2 years in relation to allocated treatment and other potential determinants of vitamin D status including self-reported sun exposure, latitude, consumption of oily fish and use of vitamin D supplements.
-
Comparison of change in systolic and diastolic BP from recruitment to 2 years between the vitamin D arm and the control arm.
Sample size
The aim of the feasibility study was to recruit 1600 participants aged 65–84 years through 20 GP practices [400 on OD vs. 400 on OC; 400 in the blind vitamin D (BD) arm vs. 400 in the blind placebo control (BC) arm]. The target was to randomise an average of 80 participants aged 65–84 years per GP practice with at least 9% response (number randomised/number invited). If recruitment in some GP practices fell below this target, recruitment in other practices would be continued after 80 participants had been randomised to achieve the overall target of 1600 participants.
Power
The main purpose of the feasibility study was to pilot the organisational procedures for the main trial, to demonstrate adequate recruitment and compliance and to prepare for any unexpected difficulties in running the trial. The number of practices involved was considered large enough to be representative of the diversity of practices that may participate in the main trial, so that average participation (the proportion of those invited who are randomised) could be considered a reliable estimate of what would be achieved in the main trial.
The proposed feasibility study also had adequate power to detect a 5% difference in participation between open-label and blind practices with a nominal two-sided alpha level of 5%, comparing blind and open designs in 10 pairs of practices, with each practice recruiting 80 participants. To estimate the power, we simulated the number of registered GP practice patients who one would need to approach in each practice to recruit 80 participants (negative binomial). The cluster randomisation was powered to detect a change in the mean participation rate from 10% (range 2.5% to 17.5%) to 15% (5.7% to 24.3%). Under these assumptions the probability of detecting this difference at p < 0.05 would be 92%. (If there were no heterogeneity between practices the overall recruitment rate would be estimated more precisely, e.g. 9% with standard error 0.2%.)
In addition, to detect a difference of 10% in any binary outcome, the trial had (at least) 80% power (using a nominal 5% significance level) for any overall comparison, such as vitamin D versus placebo (400 per arm), and 70% power for any between-practice comparison, such as BC versus OC (sign test with 10 pairs of practices; power = 70% for 0.45 vs. 0.55, and 88% for 0.10 vs. 0.20). The outcome might be reporting a respiratory infection (15% vs. 25%) or compliance (85% vs. 95%). The pre-specified definition of ‘composite compliance’ was that a randomised participant should attend the 2-year visit, and:
-
if allocated to vitamin D, report taking at least 19 (79%) of the 24 monthly doses of the allocated investigational medicinal product, or
-
if allocated to no vitamin D (BC or OC), report taking a total of < 300,000 IU of vitamin D supplements over the 2 years of the study. (The current UK reference nutrient intake of 400 IU per day is 292,000 IU over 2 years.)
Statistical methods
All analyses were performed on an intention-to-treat basis using Stata® version 15 (StataCorp LP, College Station, TX, USA). Wilcoxon’s signed-rank test was used for comparisons of blind versus open practices within matched pairs.
Response rates
The GP practices were able to provide anonymised data on the number of participants they approached to take part in the trial by 5-year age group and by sex. No further variables were available. From these totals, participation rates were calculated. Estimated numbers of replies are shown for two practices (1O and 3B) that did not record the number of reply slips, and for one (9B) that invited all 960 eligible patients, received 170 replies and stopped recruitment when 100 had attended the baseline visit. The numbers of replies at these three practices were estimated by assuming the same ratio of replies to baseline visits as at other practices with the same allocation (open or blind). The number of invitations required by practice 9B to give the estimated 108 replies was estimated as 960 × (108/170).
Compliance
Data from quarterly follow-ups and the 2-year visit were used to calculate the overall number of study medication doses taken. The percentage of participants taking all three doses was calculated in each quarter and tabulated by GP practice and treatment allocation. The proportion of participants taking at least one dose was also calculated in each quarter. Participants were defined as having stopped taking medication at the 2-year follow-up if they took fewer than two doses in the last quarter of the trial. The reasons for stopping study medication, as given on the withdrawal form, were tabulated by allocated treatment and study arm.
Contamination
The daily dose of any supplements containing vitamin D was self-reported at baseline and at the follow-up visit. Participants reporting taking cod liver oil were assumed to be consuming 200 IU per day of vitamin D. Details of prescriptions were also downloaded from GP records, but some did not record frequency, so daily dose could not always be calculated accurately. Doses of medication indicated from prescription data that could not be verified from self-reported medication data were assumed to be 800 IU per day. Self-reported and prescription data have been tabulated separately.
Summary measure of compliance
The single measure of ‘composite compliance’, defined above, was pre-specified to avoid multiple testing in the power calculation. This was calculated as described using the compliance and contamination data. Participants who died during the 2-year trial period were excluded from this analysis. The definition required control participants not to exceed 300,000 IU over the 2 years of the trial; however, a conservative approach was taken such that any control participants who reported taking, or were prescribed, supplements exceeding 400 IU per day at either baseline or follow-up were considered non-compliant.
Deprivation
English indices of multiple deprivation (IMD) for 2015 were downloaded for each participant’s home postcode. 45 These produce the IMD by small areas of approximately 650 households. We also used the Income Deprivation Affecting Older People Index (IDAOPI), grouping deciles of IDAOPI into quintiles.
Blood 25(OH)D
Mean blood 25(OH)D levels at baseline were categorised on demographic and lifestyle factors. Multivariate linear regression was used to calculate the adjusted means of 25(OH)D and trend p-values across categories for each factor (see Table 14). The adjusted means are the estimated marginal means, which are standardised to the observed distribution on all other variables. The suboptimal threshold was defined as blood 25(OH)D < 75 nmol/l.
Linear regression was used to assess change in blood 25(OH)D from baseline to the 2-year visit with respect to allocated treatment. As a secondary analysis, the change in season was also adjusted for, only slightly modifying the estimates. Vitamin D levels were lower in winter and spring and therefore a variable was constructed to represent change in season: summer/autumn to winter/spring, same season, winter/spring to summer/autumn.
Infections and GP visits
Data downloaded from GP notes were used to identify visits to the GP when an infection was diagnosed. Infections were categorised into five categories: upper respiratory, lower respiratory, urinary, skin/mucosal or soft tissue, and other. Multiple visits were combined by ignoring subsequent visits within the same category within 2 weeks. Infections were tabulated by allocated treatment and baseline blood 25(OH)D. Numbers of infections and visits were calculated in the year preceding the randomisation date and in the 2 years of the trial.
Cancer, mortality and hospital admissions data
Cancer incidence data were available until April 2017, just over 2 years after the last patient was randomised. The numbers of incident cancer diagnoses within 2 years of randomisation were tabulated; skin cancers and benign and in situ tumours were excluded. HES data44 were complete until March 2017, providing complete data on emergency hospital admissions within 2 years of randomisation for all patients. Numbers of admissions by treatment arm were also available for the year preceding randomisation. Mortality data were available until February 2018, 3 years after the last patient was randomised.
Other measurements
Quality of life was measured at baseline and 2 years after randomisation using the standardised EuroQol-5 Dimensions, three-level version (EQ-5D-3L), health status instrument46 consisting of two elements: (1) a simple descriptive profile comprising five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and (2) the EQ analogue scale, a single index value for health status. The descriptive dimensions each comprised three levels: no problems, some problems and extreme problems. These levels were assigned a score of 1, 2 and 3 respectively and a total score calculated from adding all five values, where a score of 5 equated to best possible health and a score of 15 equated to worst possible health. For the tables, these were categorised into five groups equating to a score of 5, 6, 7, 8 or ≥ 9. The EQ visual analogue scale records each respondent’s self-rated health that day as a score between 1 and 100, where 1 is the worst imaginable health state and 100 is the best imaginable health state. This was adapted by asking participants to give a number between 1 and 100 instead of marking their score on a scale (see Appendix 1).
Changes in BP, height, weight, body mass index (BMI) and self-reported health score (given as a percentage) were calculated. Univariate linear regression models for each of these were fitted to estimated mean changes by allocated treatment.
Chapter 3 Results
Recruitment
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram summarising the number of individuals participating at each stage of the trial is shown in Figure 2. In addition to data collected from the participants at study visits, data regarding prescriptions, infections and GP visits were downloaded from each GP database. GP data were obtained for 1554 participants (96.2%) but this varied by GP practice. Some practices did not provide data from patients who had moved GP or who had died, as these data were no longer available on their computer systems. Figure 3 shows cumulative recruitment. The pilot practice began recruiting in April 2013. Main recruitment began in October 2013 and ended in January 2015 with 1615 participants randomised (the target was 1600 participants). The recruitment period for individual practices ranged from 4 to 12 months.
FIGURE 2.
The VIDAL trial CONSORT flow diagram.
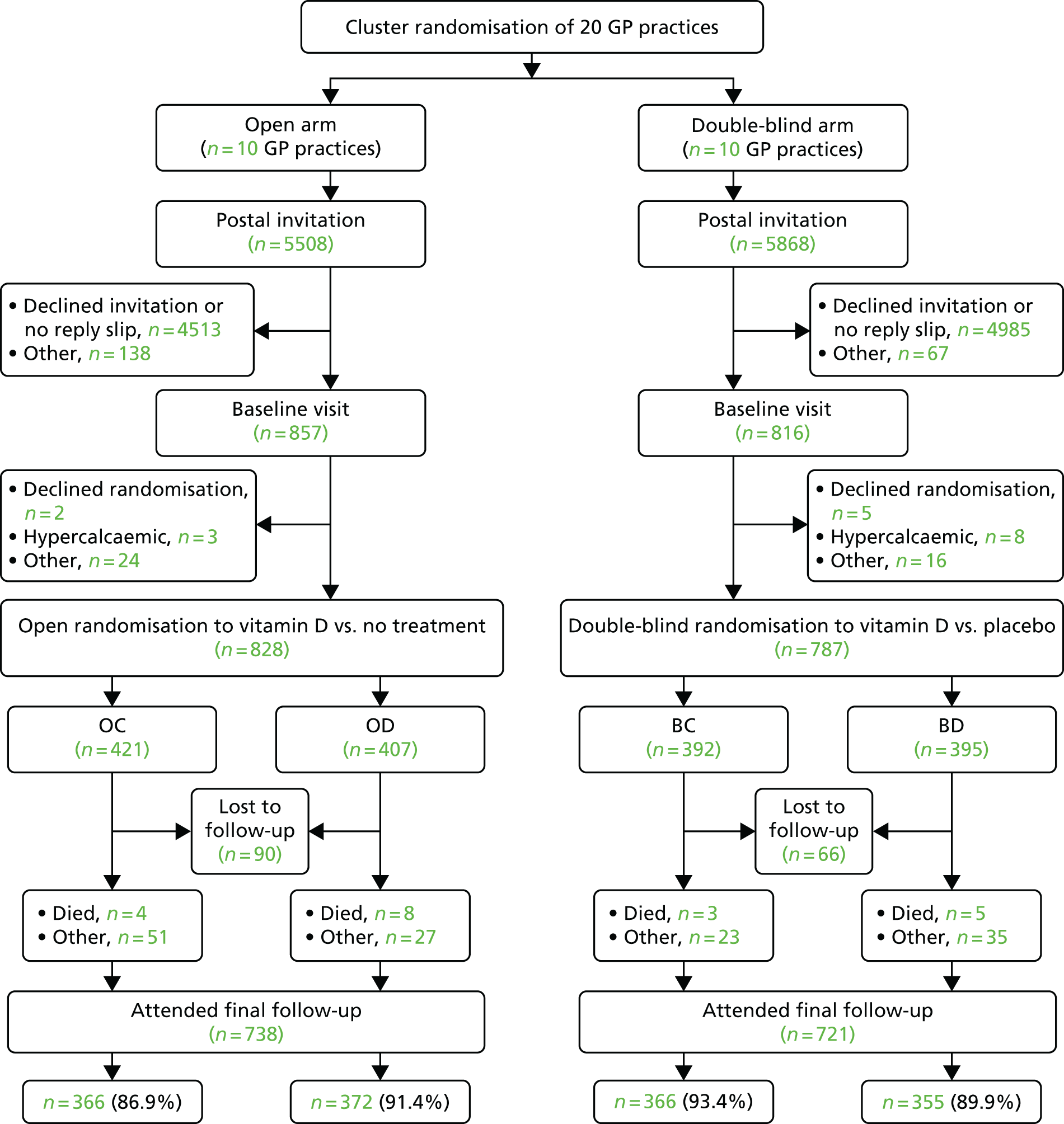
FIGURE 3.
Cumulative VIDAL recruitment by month and year.

Table 2 shows the numbers of registered patients in each practice invited, replying, attending the baseline visit and randomised. GP practice codes indicate matched pair (0–9) and whether or not randomisation was open-label or blind (O or B).
| GP practice | Number invited | Reply slips received | Attended baseline visit | Number randomised | % randomised | OC | OD | BC | BD |
|---|---|---|---|---|---|---|---|---|---|
| 0B (pilot) | 690 | 83 | 81 | 72 | 10.4 | 36 | 36 | ||
| 0O | 348 | 95 | 80 | 78 | 22.4 | 42 | 36 | ||
| 1B | 659 | 81 | 80 | 79 | 12.0 | 39 | 40 | ||
| 1O | 500 | (97) | 84 | 83 | 16.6 | 47 | 36 | ||
| 2B | 680 | 60 | 63 | 60 | 8.8 | 30 | 30 | ||
| 2O | 316 | 77 | 67 | 60 | 19.0 | 28 | 32 | ||
| 3B | 476 | (88) | 81 | 73 | 15.3 | 35 | 38 | ||
| 3O | 450 | 112 | 86 | 83 | 18.4 | 44 | 39 | ||
| 4B | 528 | 74 | 65 | 65 | 12.3 | 34 | 31 | ||
| 4O | 616 | 64 | 57 | 54 | 8.8 | 28 | 26 | ||
| 5B | 375 | 129 | 102 | 99 | 26.4 | 48 | 51 | ||
| 5O | 450 | 91 | 86 | 81 | 18.0 | 36 | 45 | ||
| 6B | 500 | 95 | 82 | 80 | 16.0 | 39 | 41 | ||
| 6O | 705 | 78 | 78 | 76 | 10.8 | 38 | 38 | ||
| 7B | 1000 | 81 | 78 | 77 | 7.7 | 39 | 38 | ||
| 7O | 805 | 176 | 159 | 156 | 19.4 | 79 | 77 | ||
| 8B | 350 | 84 | 84 | 84 | 24.0 | 43 | 41 | ||
| 8O | 479 | 90 | 80 | 78 | 16.3 | 36 | 42 | ||
| 9B | (610) | (108) | 100 | 98 | (15.5) | 49 | 49 | ||
| 9O | 839 | 115 | 80 | 79 | 9.4 | 43 | 36 | ||
| Total | 11,376 | 1878 | 1673 | 1615 | 14.2 | 421 | 407 | 392 | 395 |
The overall recruitment rate (number randomised/number invited) was 14.2%. The rate was higher in open (15.0%, range 8.8–22.4%) than in blind practices (13.4%, range 8.8–26.4%), but this did not approach statistical significance because of the wide variation between practices (Wilcoxon signed-rank test; p = 0.7). Table 3 shows that the recruitment rate was lower (p = 0.002) in participants aged 80–84 years (11.5%) than in participants aged < 80 years (14.6%), and was lower in women than in men (p = 0.002).
| Participants | Number invited | Number randomised | % randomised |
|---|---|---|---|
| Age group (years) | |||
| 65–69 | 4599 | 624 | 13.6 |
| 70–74 | 3122 | 510 | 16.3 |
| 75–79 | 2297 | 325 | 14.2 |
| 80–84 | 1358 | 156 | 11.5 |
| Sex | |||
| Male | 5631 | 857 | 15.2 |
| Female | 5745 | 758 | 13.2 |
| Total | 11,376 | 1615 | 14.2 |
Table 4 shows the 1615 randomised participants by age and sex. There were 857 (53.1%) men and 758 (46.9%) women, with similar age distributions. The majority (70.2%) were aged 65–74 years and only 9.7% were 80–84 years. Almost all were white (Table 5: 1600/1615). Table 6 shows numbers by age and sex of those who chose to receive and return quarterly follow-ups by e-mail. The proportion choosing e-mail was higher among men and declined with age, from 77.4% in those aged 65–69 years to 36.5% in those aged 80–84 years. The proportion choosing e-mail for monthly reminders to take their study medication also declined with age, from 70.5% (440/624) of those aged 65–69 years to 33.3% (52/156) of those aged 80–84 years, most of whom (79.5%) requested a monthly telephone call. The proportion requesting text message reminders declined from 40.1% (250/624) in those aged 65–69 years to 9.0% (14/156) in those aged 80–84 years (more than one medium could be chosen).
| Age group (years) | Male, n (%) | Female, n (%) | All randomised, n (%) |
|---|---|---|---|
| 65–69 | 341 (39.8) | 283 (37.3) | 624 (38.6) |
| 70–74 | 253 (29.5) | 257 (33.9) | 510 (31.6) |
| 75–79 | 177 (20.7) | 148 (19.5) | 325 (20.1) |
| 80–84 | 86 (10.0) | 70 (9.2) | 156 (9.7) |
| Total | 857 (100) | 758 (100) | 1615 (100) |
| Ethnicity | n (%) |
|---|---|
| White British | 1563 (96.8) |
| White Irish | 11 (0.7) |
| White other | 26 (1.6) |
| Caribbean | 6 (0.4) |
| Asian | 6 (0.4) |
| Mixed | 3 (0.2) |
| Total | 1615 (100) |
| Method | Males (years) | Females (years) | ||||||
|---|---|---|---|---|---|---|---|---|
| 65–69 | 70–74 | 75–79 | 80–84 | 65–69 | 70–74 | 75–79 | 80–84 | |
| Quarterly follow-up, n (%) | ||||||||
| 274 (80.4) | 182 (71.9) | 114 (64.4) | 37 (43.0) | 209 (73.9) | 163 (63.4) | 67 (45.3) | 20 (28.6) | |
| Monthly reminder, n (%) | ||||||||
| 249 (73.0) | 158 (62.5) | 105 (59.3) | 34 (39.5) | 191 (67.5) | 142 (55.3) | 60 (40.5) | 18 (25.7) | |
| Text | 135 (39.6) | 84 (33.2) | 38 (21.5) | 10 (11.6) | 115 (40.6) | 85 (33.1) | 27 (18.2) | 4 (5.7) |
| Telephone | 140 (41.1) | 123 (48.6) | 105 (59.3) | 66 (76.7) | 118 (41.7) | 148 (57.6) | 108 (73.0) | 58 (82.9) |
| Total | 341 | 253 | 177 | 86 | 283 | 257 | 148 | 70 |
Compliance
Tables 7 and 8 and Figure 4 show compliance among the 1194 participants randomised to receive study medication (the blind treatment arms or the OD arm). Excluding participants who died during the trial period, 89.9% (1059/1178) were still taking medication at the end of the study, 91.2% in the OD arm and 89.2% in the blind treatment arms. All 24 doses of study medication were taken by 80.2% (625/779) of participants in the blind treatment arms and 83.2% (332/399) of those in the OD arm. Eleven participants in the blind treatment arms (1.5%) and four (1.0%) in the OD arm did not take any study medication from the outset. Compliance was higher among men than among women and among participants aged < 75 years: all 24 doses were taken by 86.6% of men and 79.0% of women aged < 75 years, and by 83.8% of men and 69.9% of women aged ≥ 75 years.
| Practice | Total randomised | Randomised to treatment | Compliance (% of randomised participants taking all three doses) | Attended the 2-year visita | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 month | 6 month | 9 month | 12 month | 15 month | 18 month | 21 month | 24 month | n (%) | |||
| 0B | 72 | 72 | 95.8 | 93.1 | 88.9 | 90.3 | 88.9 | 86.1 | 84.7 | 80.6 | 64 (88.9) |
| 0O | 78 | 36 | 97.2 | 97.2 | 97.2 | 97.2 | 97.2 | 97.2 | 97.2 | 97.2 | 70 (89.7) |
| 1B | 79 | 79 | 96.2 | 96.2 | 94.9 | 93.7 | 92.4 | 89.9 | 88.6 | 89.9 | 74 (93.7) |
| 1O | 83 | 36 | 100.0 | 100.0 | 100.0 | 94.4 | 94.4 | 94.4 | 94.4 | 88.9 | 76 (91.6) |
| 2B | 60 | 60 | 100.0 | 98.3 | 98.3 | 98.3 | 98.3 | 95.0 | 91.7 | 90.0 | 54 (91.5) |
| 2O | 60 | 32 | 96.9 | 90.6 | 90.6 | 90.6 | 90.6 | 87.5 | 87.5 | 87.5 | 53 (88.3) |
| 3B | 73 | 73 | 98.6 | 98.6 | 91.8 | 90.4 | 89.0 | 89.0 | 89.0 | 87.7 | 64 (88.9) |
| 3O | 83 | 39 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 97.4 | 73 (89.0) |
| 4B | 65 | 65 | 96.9 | 93.8 | 89.2 | 83.1 | 81.5 | 81.5 | 81.5 | 80.0 | 52 (83.9) |
| 4O | 54 | 26 | 96.2 | 92.3 | 88.5 | 88.5 | 88.5 | 84.6 | 84.6 | 80.8 | 42 (79.2) |
| 5B | 99 | 99 | 100.0 | 99.0 | 98.0 | 97.0 | 94.9 | 93.9 | 93.9 | 93.9 | 96 (97.0) |
| 5O | 81 | 45 | 95.6 | 95.6 | 95.6 | 93.3 | 93.3 | 93.3 | 93.3 | 93.3 | 75 (93.8) |
| 6B | 80 | 80 | 98.8 | 95.0 | 95.0 | 92.5 | 92.5 | 91.3 | 90.0 | 88.8 | 73 (91.3) |
| 6O | 76 | 38 | 100.0 | 97.4 | 94.7 | 92.1 | 92.1 | 92.1 | 92.1 | 89.5 | 70 (93.3) |
| 7B | 77 | 77 | 98.7 | 98.7 | 98.7 | 97.4 | 97.4 | 97.4 | 96.1 | 96.1 | 75 (98.7) |
| 7O | 156 | 77 | 97.4 | 97.4 | 96.1 | 96.1 | 92.2 | 92.2 | 89.6 | 88.3 | 142 (92.2) |
| 8B | 84 | 84 | 97.6 | 94.0 | 94.0 | 90.5 | 84.5 | 84.5 | 83.3 | 82.1 | 75 (90.4) |
| 8O | 78 | 42 | 95.2 | 85.7 | 83.3 | 83.3 | 81.0 | 76.2 | 76.2 | 76.2 | 60 (82.2) |
| 9B | 98 | 98 | 99.0 | 96.9 | 96.9 | 95.9 | 95.9 | 94.9 | 93.9 | 90.8 | 94 (96.9) |
| 9O | 79 | 36 | 100.0 | 100.0 | 100.0 | 97.2 | 97.2 | 97.2 | 94.4 | 94.4 | 77 (98.7) |
| Total | 1615 | 1194 | 98.1 | 96.2 | 94.8 | 93.3 | 92.0 | 91.0 | 90.0 | 88.7 | 1459 (91.5) |
| Total number of doses taken | Blind treatment arms, n (%) | OD, n (%) | Total, n (%) |
|---|---|---|---|
| 0–5 | 28 (3.6) | 18 (4.4) | 46 (3.9) |
| 6–11 | 31 (3.9) | 9 (2.2) | 40 (3.4) |
| 12–17 | 21 (2.7) | 8 (2.0) | 29 (2.4) |
| ≥ 18 | 707 (89.8) | 372 (91.4) | 1079 (90.4) |
| Total | 787 | 407 | 1194 |
FIGURE 4.
Compliance of participants taking study medication in each quarter (percentage of those randomised taking all three monthly doses vs. at least one dose) by randomisation method (blind or open-label).
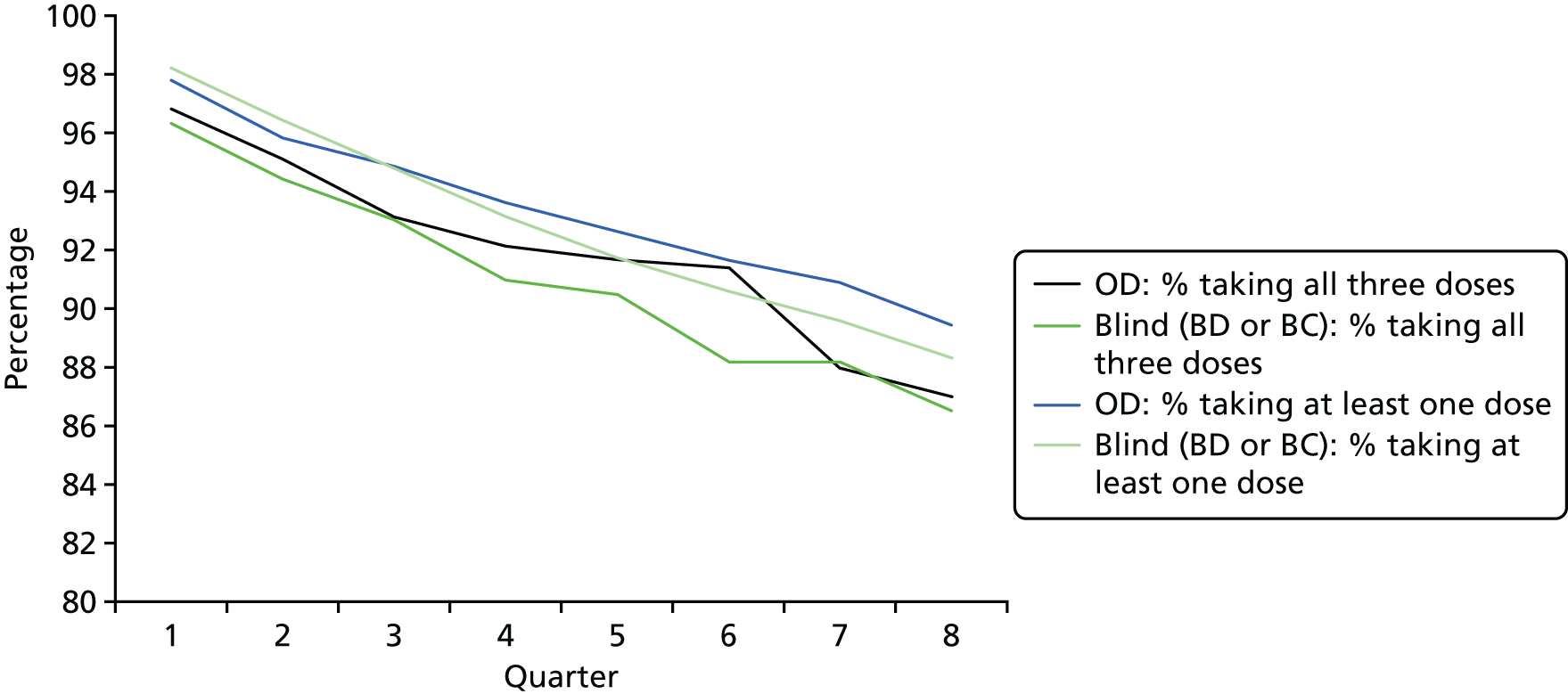
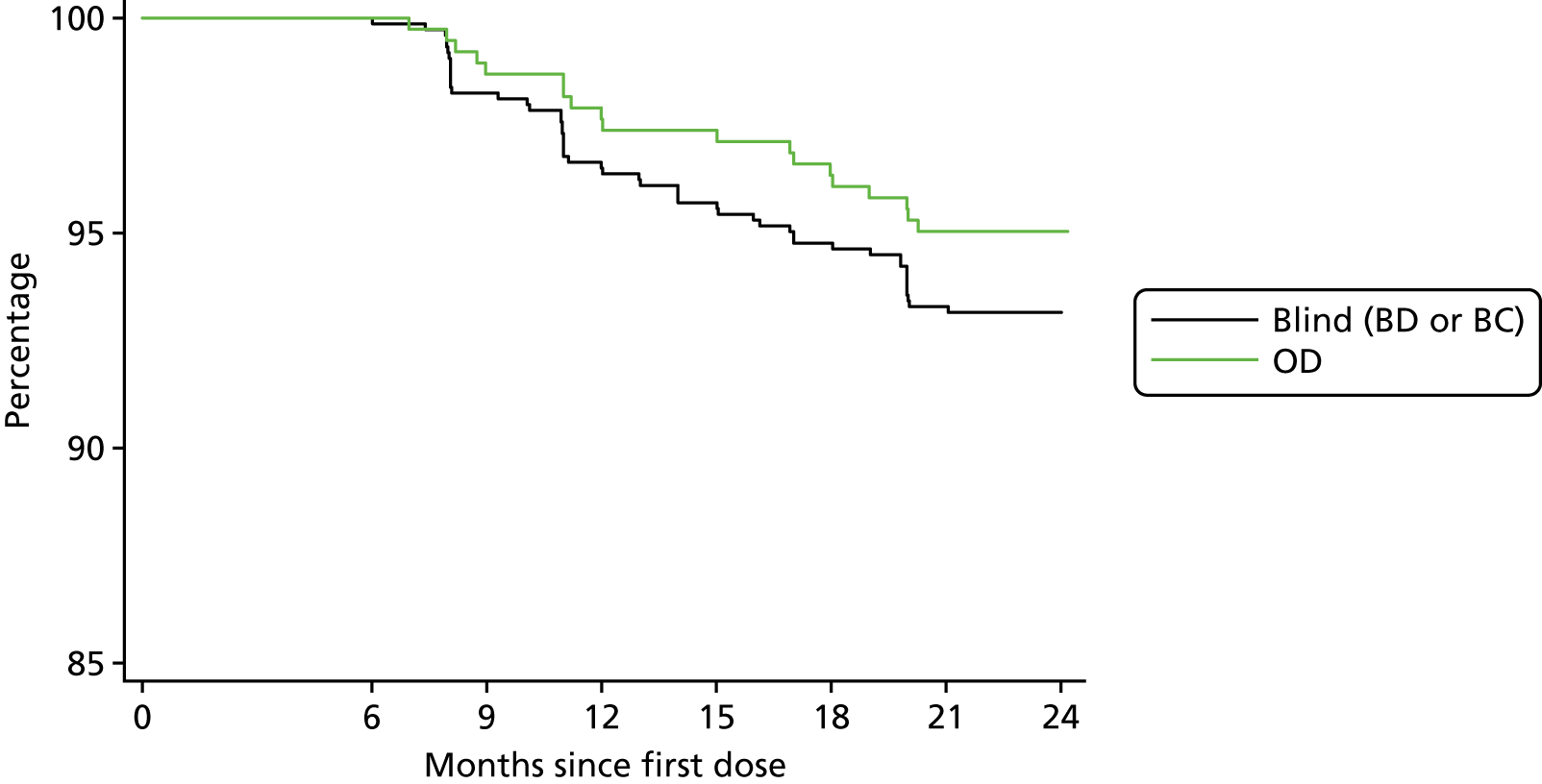
Occasional doses were missed for various reasons and so a more useful measure of compliance is the date when a participant last took their study medication. Within 6 months of entry, 16 (4.0%) participants allocated to OD and 34 (4.4%) on blind treatment (BC or BD) had stopped taking study medication. The subsequent rate of decline (Figure 5), which was slightly lower, is likely to provide a better estimate of the proportion who would continue treatment in a longer trial. Among those compliant at 6 months, 6.2% (70/1128) stopped taking medication over the remaining 18 months of the trial [5.0% (19/383) on OD and 6.8% (51/745) on blind treatment; p = 0.21], which is an overall annual attrition of 4.1% per year. If this rate of attrition continued, 81.1% of all randomised participants would still be taking allocated medication at the end of a 5-year trial.
FIGURE 5.
Proportion of participants who were compliant at 6 months still taking study medication over the remainder of the trial. Within 6 months of entry 4.0% of participants allocated to OD and 4.4% on blind treatment had stopped taking study medication.
Treatment compliance and withdrawal
For mortality (the primary end point in the main trial for which this is the feasibility study), there were no withdrawals from follow-up, because all participants will continue to be followed up through NHS Digital unless they are censored at emigration. Table 9 shows the reasons given by 13 (3.1%) of 421 OC participants who notified the practice that they wished to withdraw from the trial, either because they began taking > 400 IU per day of vitamin D or because they would not attend the 2-year follow-up (this includes two participants who started taking vitamin D during the trial but returned for the 2-year follow-up). Table 9 also shows reasons for stopping treatment for 137 (11.5%) of 1194 participants allocated to treatment who stopped taking medication before the 2-year visit, including 16 participants who died and 45 participants who attended the 2-year visit. In the blind practices, 11.0% of participants stopped taking study medication compared with 8.8% of those allocated to vitamin D in open practices (Wilcoxon signed-rank test; p-value = 0.4). The proportion of participants who stopped their medication increased with the number of days spent in hospital over the 2-year trial period according to HES data:44 8.5% of those without hospital admissions, 27% (30/109) of those spending < 10 days in hospital and 53% (16/30) of those spending > 10 days in hospital.
| Variable | Open practices | Blind practices | |||
|---|---|---|---|---|---|
| OC | OD | BC | BD | All blind patients | |
| Reason for withdrawal | |||||
| AR to oil | NA | 11 | 7 | 7 | 14 |
| Illness | 3 | 8 | 4 | 12 | 16 |
| Moved out of the area | 4 | 4 | 6 | 6 | 12 |
| Other reason | 3 | 8 | 10 | 8 | 18 |
| Decided to take vitamin D | 2 | 0 | 1 | 1 | 2 |
| Prescribed vitamin D | 1 | 3 | 9 | 5 | 14 |
| No reason given | 0 | 1 | 1 | 8 | 9 |
| Total stopping medication excluding deaths, n (%) | 35 (8.8) | 38 (9.8) | 48 (12.3) | 86 (11.0) | |
| Deceased | 4 | 8 | 3 | 5 | 8 |
| Totals | |||||
| Randomised participants | 421 | 407 | 392 | 395 | 787 |
| Continued taking medication | NA | 364 | 351 | 342 | 693 |
Table 10 shows that 91.5% (1459/1595) of all surviving randomised participants returned for the 2-year visit. A viable blood 25(OH)D result was obtained for 1456 of them. As expected, a higher proportion of surviving participants in the three study medication arms than in the OC arm attended the 2-year visit (93.2% OD vs. 87.8% OC in open practices; p = 0.008) (92.6% BD and BC in blind practices). The proportion returning for the 2-year visit decreased with increasing number of days in hospital according to HES data:44 92.6% (1318/1424) of those without stays in hospital attended the follow-up visit compared with 79.9% (119/149) of those spending < 10 days in hospital and 52.4% (22/42) of those spending ≥ 10 days in hospital within the 2-year trial period.
| GP practice visit | Open practices | Blind practices | Total | |
|---|---|---|---|---|
| OC | OD | |||
| Baseline | 421 | 407 | 787 | 1615 |
| 2-year visit | 366 | 372 | 721 | 1459 |
| % returning for 2-year visita | 87.8 | 93.2 | 92.6 | 91.5 |
| Deceased | 4 | 8 | 8 | 20 |
Contamination
Table 11 shows that almost one-quarter of participants (23.2%) reported taking self-administered daily supplements containing vitamin D at baseline, but the majority of these contained ≤ 200 IU (5 µg). GP notes abstracted at the end of the study revealed that an additional 17 (1.1%) participants were being prescribed medication containing vitamin D at baseline, including six at > 400 IU per day. These six, together with the three individuals who were taking > 400 IU (10 µg) per day (see Table 11 footnote a), were missed by the practice nurse when eligibility was checked at baseline. At follow-up, the proportion reporting self-administered supplements containing vitamin D (16.5% overall) had fallen in the OD arm and in both blind treatment arms but remained unchanged in the OC arm. The proportion of individuals taking high-dose vitamin D increased in the OC arm and the blind treatment arm, but only four individuals reported taking > 1000 IU (25 µg) per day. At 2 years, 3.6% of participants with available data were receiving > 400 IU per day of additional vitamin D (1.1% self-administered, 2.2% prescribed). Contamination data were collected by telephone from 65 participants who did not attend the 2-year visit but remained unknown for 91 participants, including 20 who died during the trial and a further three who died before the telephone follow-up call.
| Daily vitamin D in addition to study medication | Open practices | Blind practices | Total | |||
|---|---|---|---|---|---|---|
| OC | OD | BC | BD | All blind | ||
| Baseline visit | ||||||
| None | 327 | 305 | 297 | 295 | 592 | 1224 |
| Self-administered | ||||||
| ≤ 200 IU | 75 | 76 | 73 | 78 | 151 | 302 |
| 201–400 IU | 15 | 23 | 14 | 17 | 31 | 69 |
| 401–1000 IU | 1a | 0 | 2 | 0 | 2a | 3 |
| GP prescribed | ||||||
| ≤ 400 IU | 1 | 3 | 4 | 3 | 7 | 11 |
| > 400 IU | 1 | 2 | 2 | 4 | 5 | |
| Dose NKb | 1 | 1 | ||||
| % > 400 IU | 0.7 | 0.0 | 1.0 | 0.5 | 0.8 | 0.6 |
| 2-year visit | ||||||
| None | 304 | 318 | 304 | 297 | 601 | 1223 |
| Self-administered | ||||||
| ≤ 200 IU | 54 | 46 | 36 | 47 | 83 | 183 |
| 201–400 IU | 18 | 10 | 9 | 10 | 19 | 47 |
| 401–1000 IU | 8 | 2 | 4 | 3 | 7 | 17 |
| > 1000 IU | 1 | 1c | 2 | 0 | 2d | 4 |
| GP prescribed | ||||||
| ≤ 400 IU | 4 | 2 | 6 | 4 | 10 | 16 |
| > 400 IU | 10 | 2 | 6 | 3 | 9 | 21 |
| Dose NKb | 1 | 3 | 6 | 3 | 9 | 13 |
| % > 400 IU among responders | 5.0 | 2.1 | 4.8 | 2.5 | 3.6 | 3.6 |
| Totals at 2-year visit | ||||||
| Attended 2-year visit | 366 | 372 | 366 | 355 | 721 | 1459 |
| Telephoned at 2 years | 34 | 12 | 7 | 12 | 19 | 65 |
| No data | 17 | 15 | 16 | 23 | 37 | 71 |
| Deceased | 4 | 8 | 3 | 5 | 8 | 20 |
| All randomised participants | 421 | 407 | 392 | 395 | 787 | 1615 |
Composite compliance
‘Composite compliance’, as defined in Chapter 2, Methods, was calculated for all participants who did not die within 2 years of randomisation. All between-practice differences were tested using Wilcoxon‘s signed-rank test with 10 matched pairs of practices. Among the control participants, a significantly higher proportion were compliant in blind practices than in open practices (89.7% BC vs. 83.0% OC; p = 0.01). This difference was a result of the lower attendance among untreated OCs at the follow-up visit (94.1% BC vs. 87.8% OC; p = 0.01), as the proportions exceeding the permitted total dose of vitamin D supplements (300,000 IU over 2 years) were similar. There was an opposite but non-significant difference for the composite compliance variable among those allocated to vitamin D, with higher compliance among open practices (91.0% OD vs. 86.9% BD; p = 0.07) owing to slightly higher attendance at the follow-up visit (93.2% OD vs. 91.0% BD) and a slightly higher proportion taking at least 19 doses (92.7% OD vs. 89.2% BD). These opposite effects led to similar overall composite compliance of 88.3% (688/779) in the blind practices and 86.9% (709/816) in the open practices (p = 0.43).
Safety
At least one SAE was reported during the 2-year trial period for 11.6% of participants allocated to study medication (Table 12), the majority of which resulted in hospitalisation. None of the 184 reported SAEs was judged to be associated with the study medication. There were no significant differences between the SAEs reported between the vitamin D arms and the blind placebo arm. Only seven SAEs were not a cancer diagnosis, did not necessitate hospitalisation and did not result in death. The SAEs were not reported during the trial by patients in the untreated OC arm of the study, as they did not return quarterly follow-up forms. Rates of emergency hospitalisation from HES data44 are slightly higher than the self-reported episodes for the patients allocated to study medication (11.6% vs. 10.4%), and much higher for OCs (12.4% vs. 1.7%), as expected. There were no significant differences in the hospitalisation rate between the open and blind arms of the trial (11.8% in both arms spent at least one night in hospital). Very few participants were hospitalised for > 30 days in total during the 2 years following randomisation [8/813 (1.0%) in the control arms and 7/802 (0.9%) in the vitamin D arms]. Table 12 shows that the notification source for 116 (91.3%) of the 127 patients with SAEs other than death was self-report on quarterly follow-up forms (n = 101) or retrospective report at the 2-year follow-up (n = 15). GPs reported only an additional 11 non-fatal SAEs (8.7%), and none in OCs. ARs were rare, with a non-significantly higher rate on OD (2.9%) than on BD (1.1%) (Wilcoxon signed-rank test; p = 0.13). Neither SAEs nor ARs were significantly related to treatment in blind practices.
| SAEs, hospital admissions and ARs | Open practices | Blind practices | Total | ||
|---|---|---|---|---|---|
| OC | OD | BC | BD | ||
| SAEs | |||||
| Number of SAEs | |||||
| None reported | 408 | 359 | 347 | 349 | 1463 |
| 1 reported | 13 | 43 | 37 | 36 | 129 |
| 2 reported | 0 | 3 | 6 | 8 | 17 |
| 3 reported | 0 | 1 | 1 | 1 | 3 |
| 4 reported | 0 | 1 | 1 | 1 | 3 |
| Total | 421 | 407 | 392 | 395 | 1615 |
| Reporting ≥ 1 SAE, n (%) | 13 (3.1) | 48 (11.8) | 45 (11.5) | 46 (11.7) | 152 (9.4) |
| Reporting ≥ 1 life-threatening SAE, n (%) | 6 (1.4) | 10 (2.5) | 7 (1.8) | 11 (2.8) | 34 (2.1) |
| Participants with ≥ 1 SAE resulting in disability, n (%) | 3 (0.7) | 8 (2.0) | 2 (0.5) | 3 (0.8) | 16 (1.0) |
| Participants with ≥ 1 SAE not defined as cancer or resulting in death or hospitalisation,a n (%) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 3 (0.8) | 5 (0.3) |
| Reporting ≥ 1 SAE requiring hospitalisation, n (%) | 7 (1.7) | 43 (10.6) | 40 (10.2) | 41 (10.4) | 131 (8.1) |
| Source of notification for participants with non-fatal SAEs | |||||
| Quarterly follow-up | NA | 31 | 35 | 35 | 101 |
| GP practice during trial | 0 | 3 | 4 | 4 | 11 |
| At 2-year follow-up | 7 | 5 | 2 | 1 | 15 |
| Hospital admissions from HES data44 | |||||
| Reporting ≥ 1 SAE requiring hospitalisation, n (%) | 52 (12.4) | 47 (11.5) | 44 (11.2) | 48 (12.2) | 191 (11.8) |
| ARs | |||||
| Participants reporting ARs to study oil,b n (%) | NA | 12 (2.9) | 4 (1.0) | 5 (1.3) | 21 (1.8) |
| Possible | 6 | 2 | 2 | 10 | |
| Probable | 2 | 2 | 1 | 5 | |
| Definite | 0 | 0 | 1 | 1 | |
| Not assessable | 4 | 0 | 1 | 5 | |
Quality of life
The self-assessed QoL score at baseline decreased with age, as expected (see lifestyle questionnaire in Appendix 1). At baseline, 23.9% of individuals reported problems with mobility, 3.1% reported problems with self-care, 11.1% reported problems performing usual activities, 41.4% reported some pain and 12.5% reported some anxiety or depression. Overall, 49% of individuals reported no problems with mobility, self-care or performing usual activities and did not report pain, discomfort, anxiety or depression (Table 13). The lowest QoL scores (reflecting some trouble with four or five of the indicators or extreme trouble with two or more indicators) were reported by 4% of the population overall, but this varied by GP practice (0–17%).
| QoL score, n (%) | Age (years) | Total | |||
|---|---|---|---|---|---|
| 65–69 | 70–74 | 75–79 | 80–84 | ||
| 5 (best health) | 349 (55.9) | 252 (49.4) | 131 (40.3) | 56 (35.9) | 788 (48.8) |
| 6 | 157 (25.2) | 126 (24.7) | 90 (27.7) | 44 (28.2) | 417 (25.8) |
| 7 | 73 (11.7) | 82 (16.1) | 59 (18.2) | 21 (13.5) | 235 (14.6) |
| 8 | 27 (4.3) | 29 (5.7) | 34 (10.5) | 23 (14.7) | 113 (7.0) |
| ≥ 9 (worst health) | 18 (2.9) | 21 (4.1) | 11 (3.4) | 12 (7.7) | 62 (3.8) |
| Total | 624 (100) | 510 (100) | 325 (100) | 156 (100) | 1615 (100) |
Serum 25(OH)D concentrations
Blood samples valid for 25(OH)D analysis were collected for 1608 participants at baseline and 1448 participants at the year 2 visit. (Four baseline samples were not received at the laboratory and three were insufficient.) Overall, 82.6% of participants had baseline 25(OH)D below the 75 nmol/l threshold. Baseline levels by demographic and lifestyle factors reported at baseline are shown in Table 14. Levels decreased with increasing age [p(trend) = 0.01] and were lower in women (p < 0.0001). Average levels were highest in summer and autumn, lower in winter and even lower in spring (p < 0.0001) and increased with skin darkness from the fairest skin to participants who reported that they rarely burn and always tan (olive skin) (p < 0.0001). The small number of participants who described their skin colour as brown or black had lower levels than those with olive skin. Average levels increased with frequency of eating oily fish, although the mean level of 66 nmol/l in participants who reported eating oily fish more than four times a week was still below the adequacy threshold of 75 nmol/l. Levels were lower in participants living at higher latitude (p = 0.03 adjusted for IDAOPI deprivation score). The three right-hand columns in Table 14 show adjusted means and significance levels from a multiple regression including all variables in the table. The trend of reduced 25(OH)D with increasing age was virtually eliminated after adjustment for all variables [p(trend) 0.01 unadjusted, 0.6 adjusted]. The magnitude and significance of trends for other variables were slightly weakened or unaffected by adjustment.
| Demographic and lifestyle factors | Univariate analysis | Multivariate regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 25th percentile | Median | 75th percentile | Unadjusted p-value | Adjusted mean | 95% CI | Adjusted p-valuea | |
| Male | 852 | 54.2 | 33.7 | 50.3 | 69.5 | < 0.001 | 53.2 | 51.6 to 54.9 | 0.003 |
| Female | 756 | 48.5 | 29.1 | 44.3 | 64.3 | 49.6 | 47.8 to 51.3 | ||
| Blind practices | 781 | 50.2 | 29.9 | 45.9 | 64.4 | 0.07 | 50.1 | 48.2 to 52.0 | 0.06 |
| Open practices | 827 | 52.7 | 32.3 | 48.6 | 68.8 | 52.9 | 51.0 to 54.7 | ||
| Season recruited | |||||||||
| Summer (June–August) | 495 | 59.5 | 39.9 | 56.2 | 75.1 | < 0.001 | 58.1 | 55.9 to 60.2 | < 0.001 |
| Autumn (September–November) | 244 | 58.0 | 41.0 | 56.4 | 71.7 | 56.4 | 53.4 to 59.5 | ||
| Winter (December–February) | 257 | 47.1 | 28.0 | 42.7 | 62.3 | 50.5 | 47.5 to 53.6 | ||
| Spring (March–May) | 612 | 44.3 | 25.0 | 39.9 | 56.7 | 44.7 | 42.8 to 46.6 | ||
| Complexion | |||||||||
| Very fair | 80 | 39.4 | 22.3 | 37.9 | 52.1 | < 0.001 | 49.3 | 43.8 to 54.7 | 0.001 |
| Fair | 279 | 46.8 | 28.0 | 41.6 | 62.5 | 49.1 | 46.2 to 52.0 | ||
| Pale | 397 | 48.0 | 29.5 | 45.6 | 62.6 | 47.9 | 45.5 to 50.3 | ||
| Olive | 805 | 56.2 | 36.1 | 52.8 | 71.3 | 54.4 | 52.7 to 56.1 | ||
| Brown/black | 45 | 49.0 | 22.5 | 41.8 | 59.7 | 51.2 | 44.1 to 58.2 | ||
| Eating oily fish | |||||||||
| Less than once per week | 598 | 48.7 | 27.3 | 44.5 | 64.3 | < 0.001 | 49.7 | 47.7 to 51.6 | 0.001 |
| Once per week | 599 | 52.1 | 33.3 | 47.3 | 67.1 | 51.3 | 49.3 to 53.2 | ||
| 2 or 3 times per week | 382 | 53.9 | 34.4 | 50.6 | 69.1 | 53.8 | 51.4 to 56.2 | ||
| ≥ 4 times per week | 29 | 65.9 | 36.6 | 59.8 | 82.1 | 64.1 | 55.2 to 72.9 | ||
| Travel abroad in previous year | |||||||||
| No | 704 | 44.3 | 25.6 | 40.9 | 58.8 | < 0.001 | 46.8 | 45.0 to 48.7 | < 0.001 |
| Yes | 902 | 57.2 | 37.3 | 53.1 | 72.4 | 55.2 | 53.6 to 56.8 | ||
| QoL score | |||||||||
| 5 (best) | 785 | 55.6 | 36.5 | 51.6 | 70.1 | < 0.001 | 53.7 | 52.0 to 55.5 | < 0.001 |
| 6 | 414 | 51.6 | 30.8 | 46.9 | 67.9 | 51.4 | 49.0 to 53.7 | ||
| 7 | 235 | 44.4 | 24.6 | 38.6 | 60.9 | 47.0 | 43.9 to 50.2 | ||
| ≥ 8 (poorest) | 174 | 42.5 | 24.3 | 38.7 | 56.2 | 47.8 | 44.1 to 51.5 | ||
| Age (years) | |||||||||
| 65–69 | 621 | 53.2 | 35.0 | 49.8 | 67.9 | 0.013 | 51.5 | 49.6 to 53.4 | 0.6 |
| 70–74 | 509 | 51.9 | 30.8 | 47.6 | 66.4 | 52.2 | 50.1 to 54.3 | ||
| 75–79 | 324 | 49.5 | 28.3 | 43.7 | 65.3 | 50.9 | 48.3 to 53.6 | ||
| 80–84 | 154 | 47.7 | 25.7 | 43.3 | 67.2 | 50.3 | 46.5 to 54.2 | ||
| Latitude | |||||||||
| 51° | 878 | 53.8 | 32.2 | 49.7 | 69.4 | < 0.001 | 53.8 | 52.2 to 55.4 | < 0.001 |
| 52° | 220 | 48.8 | 29.6 | 44.4 | 63.5 | 50.6 | 47.0 to 54.2 | ||
| 54° | 427 | 49.5 | 31.6 | 46.0 | 65.4 | 47.5 | 45.0 to 49.9 | ||
| 55° | 83 | 44.7 | 22.8 | 42.2 | 55.4 | 50.5 | 45.0 to 55.9 | ||
| Quintile deprivation score | |||||||||
| 1 (lowest) | 91 | 39.1 | 19.9 | 32.4 | 51.7 | < 0.001 | 47.3 | 42.2 to 52.4 | 0.04 |
| 2 | 137 | 47.7 | 29.1 | 43.1 | 63.7 | 48.2 | 44.1 to 52.3 | ||
| 3 | 289 | 53.3 | 34.1 | 50.4 | 67.9 | 51.8 | 49.0 to 54.6 | ||
| 4 | 431 | 52.0 | 31.1 | 47.4 | 68.3 | 52.3 | 50.0 to 54.6 | ||
| 5 (highest) | 660 | 52.9 | 32.5 | 48.6 | 67.6 | 52.2 | 50.3 to 54.0 | ||
| Time spent outdoors per day (hours) | |||||||||
| < 1 | 309 | 37.6 | 23.0 | 33.3 | 48.4 | < 0.001 | 42.1 | 39.2 to 44.9 | < 0.001 |
| 1–2 | 543 | 48.8 | 29.6 | 45.6 | 64.0 | 49.9 | 47.9 to 52.0 | ||
| 3–4 | 332 | 54.5 | 34.7 | 50.6 | 69.3 | 53.3 | 50.7 to 55.9 | ||
| ≥ 4 | 422 | 62.8 | 42.3 | 59.7 | 78.8 | 59.0 | 56.7 to 61.4 | ||
| Use sun protection | |||||||||
| Never | 192 | 50.4 | 29.8 | 43.4 | 64.1 | 0.3 | 51.0 | 47.5 to 54.6 | 0.3 |
| Rarely | 143 | 49.7 | 30.2 | 44.1 | 63.0 | 49.5 | 45.5 to 53.6 | ||
| Sometimes | 392 | 51.5 | 32.4 | 48.3 | 67.5 | 51.1 | 48.6 to 53.5 | ||
| Often | 879 | 52.1 | 30.9 | 48.0 | 67.8 | 52.1 | 50.5 to 53.8 | ||
| Actively seek suntan | |||||||||
| Never | 1006 | 47.9 | 28.6 | 43.5 | 62.8 | < 0.001 | 48.6 | 47.1 to 50.2 | < 0.001 |
| Rarely | 242 | 50.6 | 32.2 | 49.1 | 65.3 | 51.0 | 48.0 to 54.1 | ||
| Sometimes | 215 | 58.9 | 38.6 | 52.6 | 74.3 | 58.0 | 54.7 to 61.2 | ||
| Often | 143 | 67.4 | 46.3 | 63.0 | 83.2 | 62.8 | 58.7 to 66.8 | ||
| Sunbed use in past year | |||||||||
| Never | 1592 | 51.4 | 30.9 | 47.1 | 66.4 | 0.009 | 51.4 | 50.2 to 52.6 | 0.02 |
| 1–9 times | 10 | 65.8 | 48.6 | 68.1 | 79.1 | 59.9 | 44.9 to 74.9 | ||
| ≥ 10 times | 4 | 79.1 | 51.2 | 80.1 | 107.0 | 76.7 | 52.9 to 100.5 | ||
Treatment effects
Figure 6 and Table 15 show blood 25(OH)D levels at baseline and at 2 years in control participants and in those randomised to receive vitamin D. The mean blood 25(OH)D levels at follow-up were 109.6 nmol/l in those allocated to vitamin D and 51.8 nmol/l in control participants. The proportion ≥ 75 nmol/l was 16.4% at baseline and 88.0% at 2 years in the vitamin D treatment arms but remained unchanged in the control arms (18.3% at baseline, 17.9% at 2 years). The proportion ≥ 75 nmol/l at 2 years was 51.7% (15/29) in non-compliant participants allocated to vitamin D. Among those allocated to vitamin D, mean blood levels decreased with time since last dose from 117.6 nmol/l in those tested within a month of last dose (220 participants) to 108.4 nmol/l in those tested 1–3 months after last dose (463 participants) and 80.0 nmol/l in those tested ≥ 4 months after last dose (38 participants).
FIGURE 6.
Baseline blood 25(OH)D levels by treatment arm at (a) baseline and (b) 2 years. The vertical line shows the suboptimal 75 nmol/l threshold.


| Blood 25(OH)D (nmol/l) | Open practices, n (%) | Blind practices, n (%) | ||
|---|---|---|---|---|
| OC | OD | BC | BD | |
| Baseline | ||||
| 0–24 | 65 (15.4) | 66 (16.3) | 71 (18.3) | 56 (14.3) |
| 25–49 | 153 (36.3) | 147 (36.2) | 146 (37.5) | 166 (42.4) |
| 50–74 | 126 (29.9) | 120 (29.6) | 101 (26.0) | 112 (28.6) |
| 75–99 | 56 (13.3) | 51 (12.6) | 48 (12.3) | 36 (9.2) |
| 100–149 | 19 (4.5) | 21 (5.2) | 21 (5.4) | 21 (5.4) |
| ≥ 150 | 2 (0.5) | 1 (0.3) | 2 (0.5) | 1 (0.3) |
| All participants | ||||
| < 75 | 344 (81.7) | 333 (82.0) | 318 (81.8) | 334 (85.2) |
| ≥ 75 | 77 (18.3) | 73 (18.0) | 71 (18.3) | 58 (14.8) |
| Total participants | 421 (100.0) | 406 (100.0) | 389 (100.0) | 392 (100.0) |
| 2-year visit | ||||
| 0–24 | 50 (13.7) | 0 (0.0) | 67 (18.5) | 1 (0.3) |
| 25–49 | 130 (35.7) | 6 (1.6) | 134 (37.0) | 5 (1.4) |
| 50–74 | 115 (31.6) | 42 (11.4) | 100 (27.6) | 33 (9.4) |
| 75–99 | 57 (15.7) | 106 (28.7) | 38 (10.5) | 114 (32.3) |
| 100–149 | 10 (2.8) | 180 (48.8) | 20 (5.5) | 168 (47.6) |
| ≥ 150 | 2 (0.6) | 35 (9.5) | 3 (0.8) | 32 (9.1) |
| All participants | ||||
| < 75 | 295 (81.0) | 48 (13.0) | 301 (83.2) | 39 (11.1) |
| ≥ 75 | 69 (19.0) | 321 (87.0) | 61 (16.9) | 314 (89.0) |
| Complianta | ||||
| < 75 | 43 (12.0) | 289 (83.3) | 30 (8.9) | |
| ≥ 75 | 314 (88.0) | 58 (16.7) | 306 (91.1) | |
| Non-compliantb | ||||
| < 75 | 5 (41.7) | 12 (80.0) | 9 (52.9) | |
| ≥ 75 | 7 (58.3) | 3 (20.0) | 8 (47.1) | |
| Total participants | 364 (100.0) | 369 (100.0) | 362 (100.0) | 353 (100.0) |
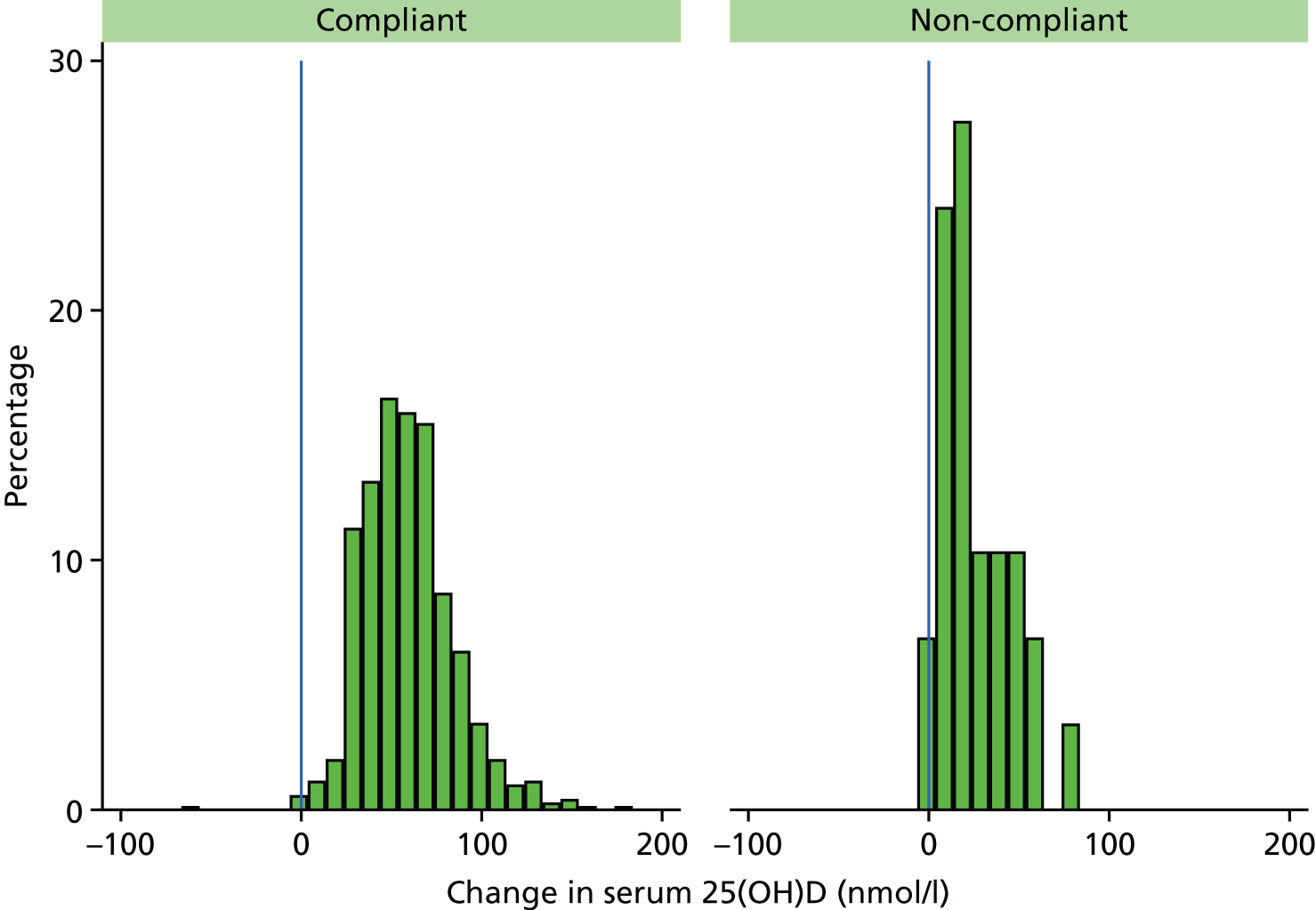
Figure 7 shows that vitamin D levels increased for almost all (99.3%) of those randomised to receive vitamin D. The estimated mean increase was 57.9 nmol/l (95% CI 56.3 to 59.4 nmol/l) in the vitamin D arms compared with 0.2 nmol/l (95% CI –1.4 to 1.7 nmol/l) in the control arms. Figure 8 shows the change in each arm and Figure 9 shows the smaller change in non-compliant (OD and OB) participants who took vitamin D for < 2 years. A larger proportion of participants in the control arms had their year 2 visit in winter/spring and baseline visit in summer/autumn than those in the vitamin D arms (8.7% vs. 6.7%, respectively) (Table 16), so the change in season was adjusted for yielding an estimated mean increase of 57.8 nmol/l (95% CI 56.1 to 59.5 nmol/l) in the vitamin D arms compared with 0.3 nmol/l (95% CI –1.4 to 2.0 nmol/l) in the control arms. In the control arms, the effect of season change was fairly small with an estimated increase of 5.7 nmol/l (95% CI 3.0 to 8.3 nmol/l) for those whose baseline blood was taken in winter/spring and follow-up was taken in summer/autumn. Conversely, the estimated decrease in vitamin D levels in control participants whose blood samples were taken in summer/autumn at baseline and winter/spring at follow-up was 8.8 nmol/l (95% CI 4.9 to 12.6 nmol/l).
FIGURE 7.
Baseline and follow-up blood 25(OH)D levels by allocated treatment arm in 1444 participants who provided two blood samples. Participants stopping their vitamin D medication early are shown as solid blue points.
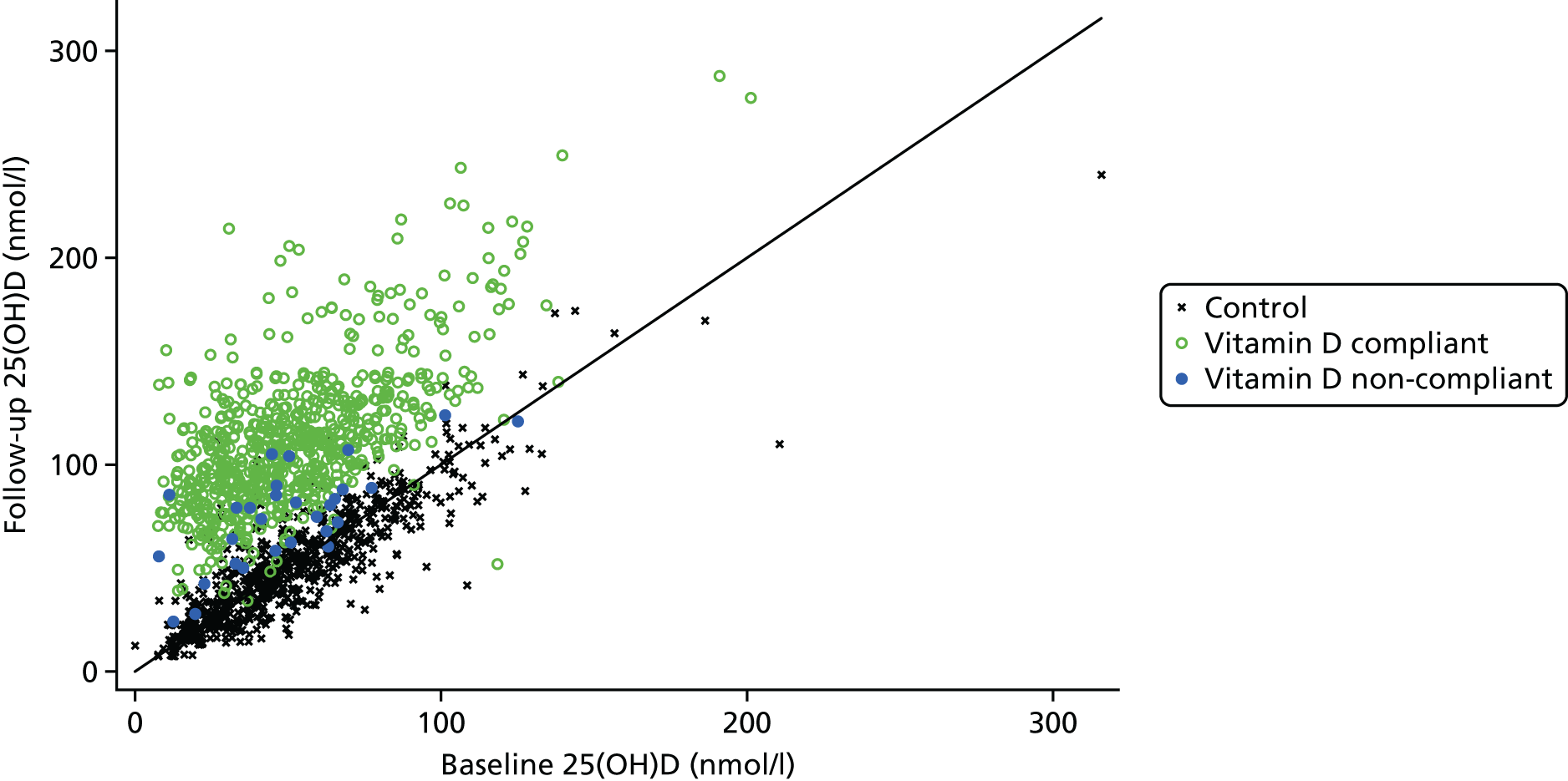
FIGURE 8.
Change from baseline to 2 years in blood 25(OH)D levels by treatment arm in 1444 participants who provided two blood samples. No change is denoted by the vertical line at zero.
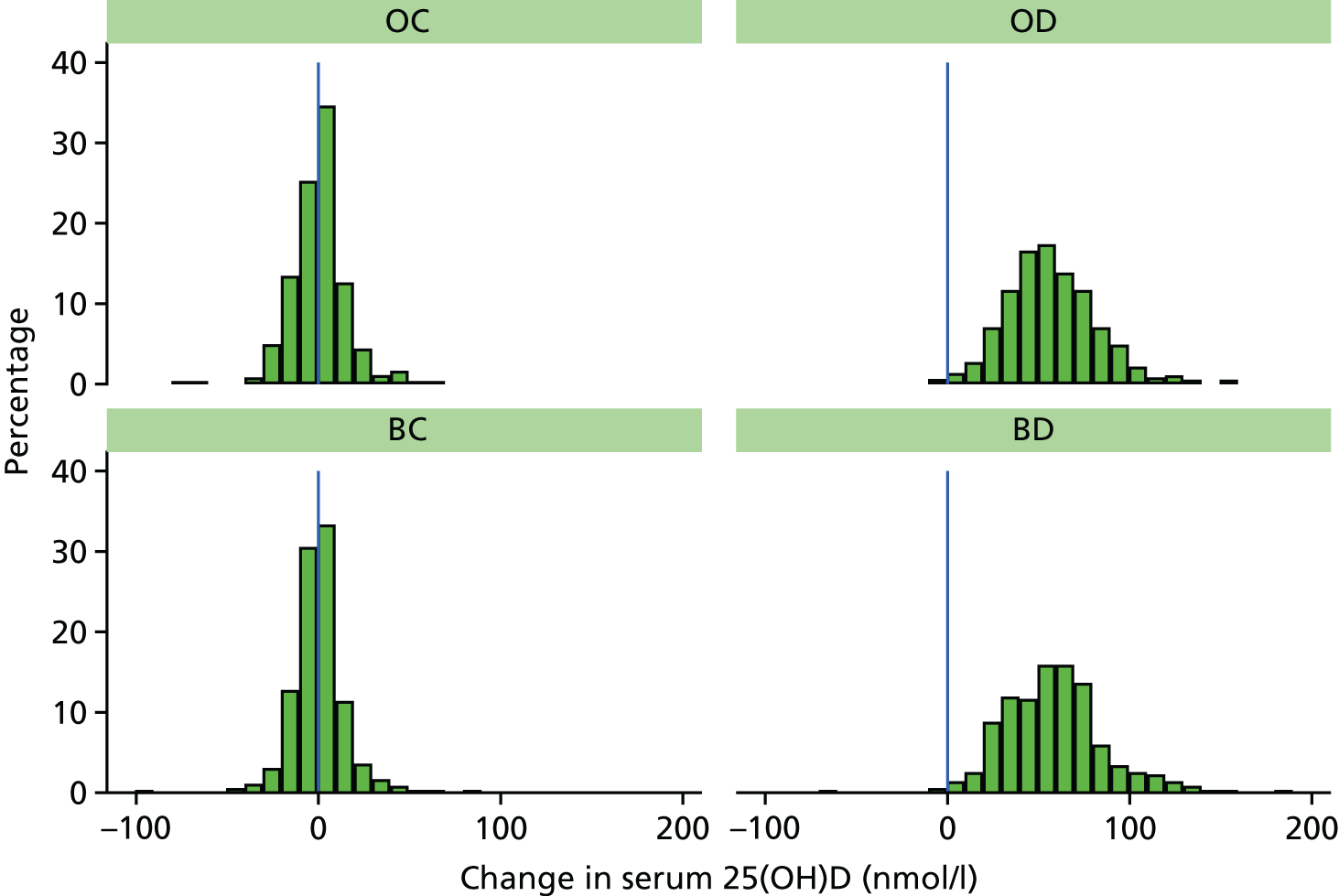
FIGURE 9.
Change in blood 25(OH)D levels in those allocated to take vitamin D who were compliant and those who stopped taking their medication (non-compliant, n = 41).
| Season change between visits | Control | Vitamin D | Total |
|---|---|---|---|
| Summer/autumn to winter/spring, n (%) | 154 (21.3) | 144 (20.0) | 298 (20.6) |
| Same season, n (%) | 507 (70.0) | 528 (73.3) | 1035 (71.7) |
| Winter/spring to summer/autumn, n (%) | 63 (8.7) | 48 (6.7) | 111 (7.7) |
| Total | 724 | 720 | 1444 |
Table 17 shows significantly lower blood 25(OH)D levels at baseline in all participants and at follow-up among control participants who did not take supplements containing vitamin D (p < 0.0001). The mean blood 25(OH)D (nmol/l) was 47.0 (95% CI 45.5 to 48.5) among those not taking supplements compared with 65.5 (95% CI 62.8 to 68.1) among those taking supplements at baseline, and similar results were seen at follow-up among the control participants. However, this higher level was still below the optimal threshold of 75 nmol/l. Additional supplementation did not change the blood 25(OH)D levels among those randomised to take vitamin D.
| Daily vitamin D supplements | n | < 75 nmol/l, n (%) | Mean | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| None | 1218 | 1055 (86.6) | 47.0 | 28.4 | 42.4 | 61.4 |
| ≤ 400 IU | 381 | 268 (70.3) | 65.3 | 47.8 | 61.2 | 79.3 |
| > 400 IU | 9 | 6 (66.7) | 71.5 | 52.8 | 66.1 | 81.7 |
| Total | 1608 | 1329 (82.6) | 51.5 | 30.9 | 47.4 | 66.6 |
| Follow-up visit | ||||||
| OC | ||||||
| None | 278 | 234 (84.2) | 49.3 | 30.5 | 46.5 | 62.3 |
| ≤ 400 IU | 67 | 49 (73.1) | 64.1 | 48.6 | 64.1 | 78.7 |
| > 400 IU | 19 | 12 (63.2) | 68.3 | 50.6 | 65.2 | 80.9 |
| Total | 364 | 295 (81.0) | 53.0 | 34.1 | 50.6 | 68.4 |
| OD | ||||||
| None | 304 | 44 (14.5) | 108.1 | 83.7 | 107.2 | 126.4 |
| ≤ 400 IU | 58 | 3 (5.2) | 121.6 | 96.2 | 116.0 | 132.6 |
| > 400 IU | 7 | 1 (14.3) | 99.1 | 79.2 | 80.6 | 120.0 |
| Total | 369 | 48 (13.0) | 110.0 | 85.3 | 108.5 | 127.0 |
| BC | ||||||
| None | 298 | 256 (85.9) | 46.6 | 26.4 | 42.5 | 62.9 |
| ≤ 400 IU | 50 | 36 (72.0) | 68.7 | 47.7 | 59.8 | 77.0 |
| > 400 IU | 14 | 9 (64.3) | 71.6 | 62.2 | 68.0 | 87.3 |
| Total | 362 | 301 (83.1) | 50.6 | 29.4 | 46.3 | 67.2 |
| BD | ||||||
| None | 284 | 32 (11.3) | 108.3 | 86.9 | 105.3 | 124.2 |
| ≤ 400 IU | 60 | 7 (11.7) | 113.6 | 91.9 | 103.3 | 128.4 |
| > 400 IU | 9 | 0 (0.0) | 106.2 | 102.9 | 106.5 | 111.4 |
| Total | 353 | 39 (11.0) | 109.2 | 88.6 | 104.9 | 123.7 |
| All control participants | ||||||
| None | 576 | 490 (85.1) | 47.9 | 28.3 | 44.7 | 62.6 |
| ≤ 400 IU | 117 | 85 (72.7) | 66.1 | 48.6 | 62.0 | 77.0 |
| > 400 IU | 33 | 21 (63.6) | 69.7 | 61.1 | 67.4 | 84.1 |
| Total | 726 | 596 (82.1) | 51.8 | 32.0 | 48.2 | 67.3 |
| All allocated to vitamin D | ||||||
| None | 588 | 76 (12.9) | 108.2 | 85.7 | 106.5 | 125.7 |
| ≤ 400 IU | 118 | 10 (8.5) | 117.5 | 92.9 | 109.9 | 132.6 |
| > 400 IU | 16 | 1 (6.3) | 103.1 | 81.8 | 103.0 | 115.7 |
| Total | 722 | 87 (12.0) | 109.6 | 87.4 | 107.0 | 126.1 |
Tables 18 and 19 show the number of infections during the 2-year trial period as reported from GP notes for all patients, comparing control participants (untreated or placebo) with vitamin D participants (open-label or blind). The overall number of infections during the 2-year trial period did not differ between control and vitamin D arms (see Table 19): 28.0% of those in the control arms had at least one infection compared with 26.8% in the vitamin D arms. A slightly higher proportion of control participants with low blood 25(OH)D had at least one infection during the trial period compared with those allocated vitamin D (31.6% vs. 25.9% in the < 25 nmol/l group and 29.1% vs. 28.4% in the 25–49 nmol/l group).
| Type of infection | Number of infections in 2 years, n (%) | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥ 4 | ||
| All infections | ||||||
| Control | 585 (72.0) | 145 (17.8) | 48 (5.9) | 18 (2.2) | 17 (2.1) | 813 |
| Vitamin D | 587 (73.2) | 142 (17.7) | 49 (6.1) | 14 (1.7) | 10 (1.2) | 802 |
| Upper respiratory infections | ||||||
| Control | 753 (92.6) | 57 (7.0) | 2 (0.2) | 1 (0.1) | 0 (0.0) | 813 |
| Vitamin D | 743 (92.6) | 53 (6.6) | 3 (0.4) | 2 (0.2) | 1 (0.1) | 802 |
| Lower respiratory infections | ||||||
| Control | 735 (90.4) | 57 (7.0) | 17 (2.1) | 3 (0.4) | 1 (0.1) | 813 |
| Vitamin D | 733 (91.4) | 53 (6.6) | 11 (1.4) | 2 (0.2) | 3 (0.4) | 802 |
| Urinary tract infections | ||||||
| Control | 772 (95.0) | 27 (3.3) | 9 (1.1) | 1 (0.1) | 4 (0.5) | 813 |
| Vitamin D | 774 (96.5) | 25 (3.1) | 2 (0.2) | 0 (0.0) | 1 (0.1) | 802 |
| Skin/mucosal or soft tissue infections | ||||||
| Control | 744 (91.5) | 54 (6.6) | 8 (1.0) | 7 (0.9) | 0 (0.0) | 813 |
| Vitamin D | 738 (92.0) | 51 (6.4) | 10 (1.2) | 1 (0.1) | 2 (0.2) | 802 |
| Other infections | ||||||
| Control | 770 (94.7) | 36 (4.4) | 6 (0.7) | 1 (0.1) | 0 (0.0) | 813 |
| Vitamin D | 761 (94.9) | 35 (4.4) | 6 (0.7) | 0 (0.0) | 0 (0.0) | 802 |
| Type of infection | Arm | Baseline blood 25(OH)D (nmol/l), n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|
| < 25 | 25–49 | 50–74 | ≥ 75 | |||
| Any infections | ||||||
| At least one infection | Control | 43 (31.6) | 87 (29.1) | 60 (26.4) | 37 (25.0) | 227 (28.0) |
| Vitamin D | 35 (25.7) | 85 (28.4) | 61 (26.9) | 33 (22.3) | 214 (26.4) | |
| At least two infections | Control | 23 (18.9) | 27 (8.6) | 23 (9.9) | 10 (7.6) | 83 (10.4) |
| Vitamin D | 12 (9.8) | 33 (10.5) | 19 (8.2) | 9 (6.9) | 73 (9.1) | |
| Upper respiratory infections | ||||||
| At least one infection | Control | 10 (7.4) | 20 (6.7) | 15 (6.6) | 15 (10.1) | 60 (7.4) |
| Vitamin D | 8 (5.9) | 21 (7.0) | 19 (8.4) | 10 (6.8) | 58 (7.2) | |
| At least two infections | Control | 1 (0.8) | 0 (0.0) | 1 (0.4) | 1 (0.8) | 3 (0.4) |
| Vitamin D | 2 (1.6) | 1 (0.3) | 3 (1.3) | 0 (0.0) | 6 (0.8) | |
| Lower respiratory infections | ||||||
| At least one infection | Control | 18 (13.2) | 28 (9.4) | 23 (10.1) | 9 (6.1) | 78 (9.6) |
| Vitamin D | 13 (9.6) | 31 (10.4) | 19 (8.4) | 6 (4.1) | 69 (8.5) | |
| At least two infections | Control | 5 (4.1) | 6 (1.9) | 9 (3.9) | 1 (0.8) | 21 (2.6) |
| Vitamin D | 5 (4.1) | 6 (1.9) | 4 (1.7) | 1 (0.8) | 16 (2.0) | |
| Urinary tract infections | ||||||
| At least one infection | Control | 12 (8.8) | 13 (4.3) | 9 (4.0) | 6 (4.1) | 40 (4.9) |
| Vitamin D | 5 (3.7) | 10 (3.3) | 8 (3.5) | 5 (3.4) | 28 (3.5) | |
| At least two infections | Control | 5 (4.1) | 4 (1.3) | 3 (1.3) | 2 (1.5) | 14 (1.8) |
| Vitamin D | 0 (0.0) | 3 (1.0) | 0 (0.0) | 0 (0.0) | 3 (0.4) | |
| Skin/mucosal or soft tissue infections | ||||||
| At least one infection | Control | 16 (11.8) | 27 (9.0) | 18 (7.9) | 8 (5.4) | 69 (8.5) |
| Vitamin D | 14 (10.3) | 24 (8.0) | 18 (7.9) | 8 (5.4) | 64 (7.9) | |
| At least two infections | Control | 4 (3.3) | 6 (1.9) | 4 (1.7) | 1 (0.8) | 15 (1.9) |
| Vitamin D | 3 (2.5) | 5 (1.6) | 2 (0.9) | 3 (2.3) | 13 (1.6) | |
| Other infections | ||||||
| At least one infection | Control | 7 (5.1) | 18 (6.0) | 10 (4.4) | 8 (5.4) | 43 (5.3) |
| Vitamin D | 5 (3.7) | 17 (5.7) | 11 (4.8) | 8 (5.4) | 41 (5.1) | |
| At least two infections | Control | 3 (2.5) | 3 (1.0) | 1 (0.4) | 0 (0.0) | 7 (0.9) |
| Vitamin D | 0 (0.0) | 3 (1.0) | 2 (0.9) | 1 (0.8) | 6 (0.8) | |
| Participants, n | ||||||
| Control | 136 | 299 | 227 | 148 | 810 | |
| Vitamin D | 122 | 313 | 232 | 131 | 798 | |
There were no significant differences between the treatment arms in changes from baseline to follow-up for systolic BP and BMI (Table 20). A small increase in diastolic BP and a small decrease in health score were seen among those allocated to take vitamin D (p = 0.03 and p = 0.04, respectively). No significant differences were seen between the treatment arms regarding changes in the five indices of QoL (Table 21). On average, participants lost 0.47 cm in height and 0.55 kg in weight over the 2 years of the trial.
| Health measures | Control (n = 732) | Vitamin D (n = 726) | Treatment difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Change in systolic BP (mmHg) | 0.14 | 1.21 | 1.07 | –0.75 to 2.88 | 0.2 |
| Change in diastolic BP (mmHg) | –0.55 | 0.63 | 1.19 | 0.11 to 2.26 | 0.03 |
| Change in height (cm) | –0.46 | –0.48 | –0.02 | –0.26 to 0.22 | 0.9 |
| Change in weight (kg) | –0.70 | –0.39 | 0.31 | –0.19 to 0.81 | 0.2 |
| Change in BMI (kg/m2) | –0.10 | 0.01 | 0.10 | –0.09 to 0.30 | 0.3 |
| Change in health score (out of 100) | 0.33 | –1.12 | –1.45 | –2.82 to –0.08 | 0.04 |
| QoL indices | Experiencing changes in health state, n (%) | |||||
|---|---|---|---|---|---|---|
| Control | Vitamin D | |||||
| N (%) | Worsened | Improved | N (%) | Worsened | Improved | |
| Mobility | ||||||
| No problem at baseline | 560 (76.6) | 82 (14.6) | NA | 575 (79.1) | 91 (15.8) | NA |
| Problems at baseline | 171 (23.4) | 0 | 52 (30.4) | 152 (20.9) | 0 | 50 (32.9) |
| Self-care | ||||||
| No problem at baseline | 712 (97.4) | 17 (2.4) | NA | 704 (96.8) | 15 (2.1) | NA |
| Problems at baseline | 19 (2.6) | 0 | 8 (42.1) | 23 (3.2) | 1 (4.3) | 10 (43.5) |
| Usual activities | ||||||
| No problem at baseline | 644 (88.1) | 69 (10.7) | NA | 665 (91.5) | 63 (9.5) | NA |
| Problems at baseline | 87 (11.9) | 2 (2.3) | 42 (48.3) | 62 (8.5) | 2 (3.2) | 33 (53.2) |
| Pain/discomfort | ||||||
| No problem at baseline | 438 (59.9) | 115 (26.3) | NA | 438 (60.2) | 135 (30.8) | NA |
| Problems at baseline | 293 (40.1) | 15 (5.1) | 104 (35.5) | 289 (39.8) | 12 (4.2) | 99 (34.3) |
| Anxiety/depression | ||||||
| No problem at baseline | 654 (89.5) | 71 (10.9) | NA | 628 (86.4) | 59 (9.4) | NA |
| Problems at baseline | 77 (10.5) | 6 (7.8) | 33 (42.9) | 99 (13.6) | 3 (3.0) | 43 (43.4) |
Table 22 shows the number of GP appointments recorded in the GP notes during the year before randomisation and during the 2-year trial period. There was a mean of 5.7 appointments in the year preceding randomisation with no significant differences between the treatment arms (i.e. 5.5 appointments in the OC arm, 5.4 in the OD arm and 5.9 in both blind treatment arms). The mean number of appointments per year increased to 6.3 during the trial, with no significant differences either between year 1 and year 2 or between treatment arms.
| Number of GP appointments | Open practices, n (%) | Blind practices, n (%) | Total, n (%) | ||
|---|---|---|---|---|---|
| OC | OD | BC | BD | ||
| Total participants | 421 | 407 | 392 | 395 | 1615 |
| No dataa | 20 | 21 | 8 | 12 | 61 |
| 1 year prior to randomisation | |||||
| 0–5 appointments | 218 (54.4) | 208 (53.9) | 195 (50.8) | 189 (49.4) | 810 (52.1) |
| 5–9 appointments | 112 (27.9) | 108 (28.0) | 117 (30.5) | 110 (28.7) | 447 (28.8) |
| 10–14 appointments | 45 (11.2) | 45 (11.7) | 42 (10.9) | 56 (14.6) | 188 (12.1) |
| ≥ 15 appointments | 26 (6.5) | 25 (6.5) | 30 (7.8) | 28 (7.3) | 109 (7.0) |
| Mean | 5.5 | 5.4 | 5.9 | 5.9 | 5.7 |
| Median | 4 | 4 | 4 | 5 | 4 |
| Year 1 of trial | |||||
| 0–5 appointments | 194 (48.4) | 176 (45.6) | 193 (50.3) | 198 (51.7) | 761 (49.0) |
| 5–9 appointments | 122 (30.4) | 114 (29.5) | 104 (27.1) | 117 (30.6) | 457 (29.4) |
| 10–14 appointments | 53 (13.2) | 66 (17.1) | 52 (13.5) | 39 (10.2) | 210 (13.5) |
| ≥ 15 appointments | 32 (8.0) | 30 (7.8) | 35 (9.1) | 29 (7.6) | 126 (8.1) |
| Mean | 6.4 | 6.5 | 6.4 | 5.8 | 6.3 |
| Median | 5 | 5 | 4 | 4 | 5 |
| Year 2 of trial | |||||
| 0–5 appointments | 195 (48.6) | 190 (49.2) | 186 (48.4) | 184 (48.0) | 755 (48.6) |
| 5–9 appointments | 109 (27.2) | 115 (29.8) | 121 (31.5) | 115 (30.0) | 460 (29.6) |
| 10–14 appointments | 63 (15.7) | 51 (13.2) | 36 (9.4) | 51 (13.3) | 201 (12.9) |
| ≥ 15 appointments | 34 (8.5) | 30 (7.8) | 41 (10.7) | 33 (8.6) | 138 (8.9) |
| Mean | 6.5 | 6.1 | 6.5 | 6.4 | 6.4 |
| Median | 5 | 5 | 5 | 5 | 5 |
| 2-year trial period | |||||
| 0–5 appointments | 93 (23.2) | 83 (21.5) | 92 (24.0) | 94 (24.5) | 362 (23.3) |
| 5–9 appointments | 98 (24.4) | 89 (23.1) | 100 (26.0) | 101 (26.4) | 388 (25.0) |
| 10–14 appointments | 74 (18.5) | 81 (21.0) | 73 (19.0) | 75 (19.6) | 303 (19.5) |
| 15–19 appointments | 56 (14.0) | 53 (13.7) | 49 (12.8) | 40 (10.4) | 198 (12.7) |
| ≥ 20 appointments | 80 (20.0) | 80 (20.7) | 70 (18.2) | 73 (19.1) | 303 (19.5) |
| Mean | 12.9 | 12.6 | 12.9 | 12.2 | 12.6 |
| Median | 10 | 11 | 9.5 | 9 | 10 |
Table 23 shows the mortality and cancer incidence for the 1615 participants in the trial. All were flagged with NHS Digital, providing complete follow-up data. The overall death rate was 4.2% in the vitamin D treatment arms and 2.8% in the untreated control arms (p = 0.12), with a cancer incidence rate over 2 years of 2.6% in those allocated to vitamin D and 3.1% among those allocated to the control arms (p = 0.6). Cause of death is shown in the lower part of the table. This is not yet available for the most recent deaths (5/57).
| Follow-up | OC, n (%) | OD, n (%) | BC, n (%) | BD, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Total participants | 421 (100) | 407 (100) | 392 (100) | 395 (100) | 1615 (100) |
| Alive at follow-upa | 408 (96.9) | 389 (95.5) | 382 (97.4) | 379 (96.0) | 1158 (96.5) |
| Died within 2 years of randomisation | 4 (1.0) | 8 (2.0) | 3 (0.8) | 5 (1.3) | 20 (1.2) |
| Died between 2 and 4 years after randomisation | 9 (2.1) | 10 (2.5) | 7 (1.8) | 11 (2.8) | 37 (2.3) |
| Total deaths | 13 (3.1) | 18 (4.4) | 10 (2.6) | 16 (4.1) | 57 (3.5) |
| Cancer incidence within 2 years of randomisation | 15 (3.6) | 12 (3.0) | 10 (2.6) | 9 (2.3) | 46 (2.8) |
| Control, n (%) | Vitamin D, n (%) | ||||
| Total participants | 813 (100) | 802 (100) | |||
| Cause of death | |||||
| Cancer | 5 (0.62) | 14 (1.75) | |||
| Circulatory | 7 (0.86) | 12 (1.50) | |||
| Other | 8 (0.98) | 6 (0.75) | |||
| Not known | 3 (0.37) | 2 (0.25) | |||
| Total deaths | 23 (2.83) | 34 (4.24) | |||
Chapter 4 Discussion
The primary aims of this study were (1) to demonstrate the feasibility of achieving adequate recruitment through GP practices in a larger trial (20,000 participants) of prolonged high-dose vitamin D in people aged 65–84 years with mortality as the primary outcome and (2) to compare the effects of open and double-blind randomisation on recruitment, contamination (self-administered or prescribed vitamin D consumption in control participants) and treatment compliance assessed both by self-report and by blood 25(OH)D concentration at the 2-year final visit, particularly the proportion in whom this figure is < 75 nmol/l. The proportion of participants with blood 25(OH)D concentration of < 75 nmol/l was 81.8% at baseline, which confirmed the high prevalence of suboptimal vitamin D status in this age group in the UK. This decreased to 22.0% at the final 2-year assessment in participants allocated to vitamin D (see Table 15) and was not significantly altered from baseline in control participants. The only substantial difference between the protocol in this feasibility study and that proposed for the main trial is the regimen of vitamin D. We used 100,000 IU monthly, but reports published after we began recruitment suggest that daily dosing is likely to prove superior. 47,48 Therefore, we propose a daily dose of 4000 IU, which was shown to achieve substantially higher serum 25(OH)D levels than 2000 IU per day in a recent study. 49,50
We believe that this is the first such trial in which open-label and double-blind randomisation have been compared by cluster randomisation. Strong opinions are held on the relative merits and disadvantages of open-label and blind allocation, and we held divergent views on the best approach for evaluating the effects of long-term vitamin D on overall mortality. Therefore, we decided that randomised evidence was needed to inform the choice of protocol for the main trial and that the best way to obtain this was by cluster randomisation of GP practices between open-label and double-blind randomisation. The 20 GP practices were situated throughout England in areas ranging from wealthy to relatively deprived.
Preliminary procedures
Study approvals occupied almost 3 years (July 2011–May 2014) before recruitment could begin at all GP practices. Research Ethics Committee and MHRA approvals were straightforward, and the major delay was in obtaining approvals for NHS research and development and service support costs in each Clinical Research Network. In view of this experience, a formal review of the costs and benefits of expediting these processes for non-commercial population-based trials of this sort might be evaluated in consultation with the relevant agencies. Protracted correspondence and discussions with NHS Digital also delayed linkage to HES and other databases to obtain follow-up data for death, cancer registration and hospital admissions. Our application to NHS Digital underwent 13 revisions for these linkages. A major focus was the wording of our informed consent, which had been reviewed and approved both by our patient advisory group and by senior staff at the Health and Social Care Information Centre before the trial began. Requested amendments included the wording of the London School of Hygiene & Tropical Medicine’s Data Protection Act registration, and the VIDAL trial website’s description of fair processing, linkage processes, data flow, research outputs, the target audience and benefits to health and social care.
Recruitment
Recruitment in the pilot practice (practice 0B) began in April 2013 and was completed in September 2013. Procedures modified over this period included improvements to the web-based clinical data management system (the VIDAL app, see Appendix 2). The recruitment period in the other 19 practices ranged from 4 to 12 months, and the last participant was randomised in January 2015. Participation was better than expected, with one in seven (14.2% compared with the protocol target of 9%) of all invited eligible patients aged 65–84 years being randomised. Despite the matching of practice pairs on region and social deprivation, recruitment varied widely within matched pairs (see Table 2), and the slightly higher overall recruitment in open practices (15.0% of invited patients randomised) than in blind practices (13.4% randomised) did not approach statistical significance.
Compliance
The proportion of participants allocated to a treatment arm who took all three doses of study medication was 88.7% in the last 3 months of the trial (see Table 7). The proportion was slightly, but not significantly, higher for OD participants than for blind participants throughout (see Figure 4), and this non-significant trend was also seen in the total number of doses taken (see Table 8) and in the proportion of participants compliant at 6 months who were still taking study medication over the remainder of the trial (see Figure 5).
Feasibility of the main trial
The recruitment target in the VIDAL main trial is 20,000 participants aged 65–84 years with equal numbers in each 5-year age group (i.e. 200 practices recruiting an average of 100 participants with 25 in each age group). The recruitment rate was 11.5% at age 80–84 years and higher at < 80 years (see Table 3), so this uniform age distribution could be achieved if the average number of registered patients aged 80–84 years in participating practices was 220 and all were invited. The average number of registered patients aged 80–84 years per practice in England is 190, so this should be easily achievable by targeting larger practices.
The proportions of participants choosing e-mail for quarterly follow-up at ages 65–69, 70–74, 75–79 and 80–84 years were 77.4%, 67.6%, 55.7% and 36.5%, respectively (see Table 6). The corresponding participation rates (see Table 3) imply that in a trial restricted to participants willing to be contacted by e-mail, the participation rates at ages 65–69, 70–74, 75–79 and 80–84 years would be 10.5%, 11.0%, 7.9% and 4.2%, respectively. To recruit an average of 25 patients in each age group, the following numbers of patients would need to be invited on average per practice: 240 patients aged 65–69 years; 230 aged 70–74 years; 320 aged 75–79 years; and 600 aged 80–84 years. The average numbers of registered patients per practice in England in these age groups are 380, 300, 250 and 190, respectively. The main trial could thus be restricted to participants willing to receive and reply to follow-ups by e-mail for those aged < 75 years, or < 80 years if larger practices were targeted, but postal contact would be required for many participants aged 80–84 years to recruit equal numbers in each age range.
A limitation of this study was the virtual absence of recruits of Asian, African or Caribbean origin (see Table 5). Our protocol for the main trial specifies that practices in areas with large numbers of people from these ethnic groups should be invited to participate, but we have no information on the participation rate that could be achieved in such practices.
A further limitation is the monthly dosing regimen, which we would not now recommend. The suggestion that daily dosing may be clinically more effective than our monthly regimen was published during the trial. 47,48 A feasibility study of daily vitamin D3 supplementation among men and women aged > 65 years at a GP practice in Oxfordshire (the ‘BEST-D’ study)49,50 showed that allocation to 4000 IU of daily vitamin D increased 25(OH)D on average by 80 nmol/l and 88% of participants achieved plasma levels of 25(OH)D > 90 nmol/l. Compliance levels were comparable to those in the current feasibility study, and we would therefore recommend that daily regimen in the main trial.
Our recruitment, compliance and contamination results are based on patients from the 20 participating practices and are inevitably limited to the 2-year treatment duration of the trial. We have no direct evidence of differences between these selected practices and the 200 practices that would be required for the main trial, or on changes that would occur over the 5-year treatment period of the main trial.
Contamination in untreated open control participants
Table 11 shows that only 20 (5.0%) of the 400 untreated control participants who were interviewed at 2 years (366 who attended the 2-year visit and 34 non-attenders who were telephoned) reported taking > 400 IU of vitamin D per day (11 prescribed and nine self-administered), and only one was taking > 1000 IU per day. This very low level of self-reported contamination is confirmed by their blood 25(OH)D levels, which were similar to their baseline results (see Table 15 and Figure 6). Significant contamination was thus negligible over the 2 years since randomisation, during which time they were not contacted. We have no reason to think that contamination was much more common among the 17 (4.1% of 417 2-year survivors allocated to OC) who could not be contacted 2 years after randomisation.
Open versus placebo-controlled trial designs
Compliance and contamination
The only significant difference between BCs and OCs was in the proportion attending the 2-year visit (94.1% and 87.8%, respectively; p = 0.01), and hence in ‘composite compliance’ (p = 0.01). Contamination was negligible among open untreated control participants, and treatment compliance was equally high for those in the OD arm and the blind treatment arms. Any real effect of 5 years of vitamin D on mortality will thus be estimated with similar power by open or by placebo-controlled randomisation, so the choice between these trial designs will be determined by other considerations.
Simplicity and cost
The advantages of open allocation with an untreated control arm include simplicity and lower trial costs. The costs of recruitment are the same for open and blind allocation, and the main additional expenditure with placebo control would be a doubling of trial office staff costs associated with participant contact and follow-up during the trial plus the costs of manufacturing, labelling, dispensing and delivering placebo. The savings that could be achieved in an open trial of 5 years duration might be substantially greater if aspects of monitoring required for a placebo-controlled trial could be reduced. We have shown that contact with participants allocated to vitamin D could be conducted entirely by e-mail for those aged < 80 years, with automated text and/or e-mail reminders to take study medication and report suspected adverse effects. Those on OD should perhaps be recalled every few years for serum calcium assay, and a sample of a few hundred in each arm should be recalled at 5 years to confirm the high compliance and low contamination seen in this trial. However, little useful additional information is obtained by recalling all participants for a final visit either for the primary aim, the analysis of mortality by allocated treatment, or for cancer incidence and reasons for hospitalisation, which were captured more completely by linkage with national databases (cancer registration and HES44). SAEs were reported retrospectively and incompletely by GPs; 96.2% (177/184) of SAEs were deaths or cancer diagnoses or involved hospitalisation (see Table 12). Our results show that these outcomes will be obtained more completely and reliably by linkage to NHS Digital databases than by GP or patient report, with no evidence of bias as a result of open randomisation. Moreover, in the large trial, for which this is a feasibility study, cancer diagnosis and hospitalisation should be included as primary end points and would therefore not be classed as SAEs. The accumulated evidence of the safety of high-dose vitamin D will be augmented by other ongoing trials with more detailed clinical monitoring. The possibility of adverse effects in those allocated to vitamin D can be managed, as in this trial, by self-report and GPs who are aware that their patient is taking study medication.
Extending treatment beyond 5 years
An important potential advantage of open allocation is that when the trial ends (subject to an application for extended funding) a further 5-year supply can be offered to participants allocated to vitamin D without recontacting control participants, provided this is specified at the outset in the patient information. A continued supply of vitamin D could be offered to those on active treatment following unblinding at the end of a 5-year double-blind trial, but power might be compromised by increased contamination in those who are informed that they have been taking placebo for 5 years, and the theoretical advantage of placebo control would be lost for the comparison of subsequent mortality. If the effects of vitamin D on mortality are transient and confined to the period of treatment, as was seen for simvastatin (Zocor©; Merck Serono GmbH, Darmstadt, Germany),51 any effect on long-term mortality would be much larger for ≥10 years than for 5 years of treatment. This would reinforce the arguments rehearsed earlier (see Chapter 1 Introduction) for the value of a large UK trial. A trial in which the majority of treated participants continue to take high-dose vitamin D is the only way to observe the effects of continuing treatment beyond 5 years. Ongoing double-blind trials cannot answer this important question and, if the effect on mortality is transient, they will also have substantially lower power to achieve statistical significance. If confirmed, the 4% reduction in overall mortality seen in the VITAL trial (hazard ratio 2–5 years after entry 0.96, 95% CI 0.84 to 1.11) might justify vitamin D supplementation, but it did not approach statistical significance. 43
The advantages of placebo control are well known. They include unbiased evidence on potential ARs and on any diagnoses that might be influenced by participants or clinicians knowing that high-dose vitamin D is being taken. A substantial placebo effect on lifestyle behaviour and hence health is, in principle, possible if implausible. Many researchers are therefore suspicious of all open trials irrespective of the end point. Perhaps the strongest reason for insisting on placebo control is the guarantee of universal acceptance of the results on incidence of non-fatal diseases and, hence, their inclusion in meta-analyses of ongoing trials. This is reflected in the Cochrane risk-of-bias tool, which downgrades quality of evidence by at least one step for RCTs when a placebo is not used.
Some co-authors felt a priori that the scientific advantages of a blind design were so great that it should be replaced by an open design only if it were shown to be substantially (and significantly) inferior in the cluster randomised comparison of participation, compliance and contamination. That has not happened, so those co-authors believe that the results reinforce the case for placebo control. Other co-authors think that an open design is simpler, substantially cheaper and facilitates extending treatment beyond 5 years and, therefore, believed a priori that the absolute need for a blind design exists only when the primary outcome is subjective. This group argued that it was only necessary to show that the open design was not inferior in terms of participation, compliance, non-contamination and retention. This trial has shown the open design to be essentially equivalent in these areas, so those co-authors believe that the main trial should use an open design.
Chapter 5 Conclusions
The study was designed to (1) assess the feasibility of conducting a large trial of vitamin D in healthy adults aged 65–84 years (n = 20,000 with equal numbers aged 65–69, 70–74, 75–79 and 80–84 years) recruited through 200 GP practices and (2) compare the effects of open-label and placebo-controlled randomisation on recruitment, compliance and contamination. Our conclusions are therefore restricted to these issues. The study was not powered to detect clinical effects of vitamin D other than elevation of blood 25(OH)D, which was the only substantial and statistically significant clinical effect observed.
Recruitment
The overall participation rate (the proportion of invited patients who were randomised) was 14.2% overall (the protocol target was 9%) and 11.5% at age 80–84 years, with no evidence of a difference between open and blind practices. This confirms the feasibility of the main trial as planned.
Compliance and contamination
Treatment compliance was high among participants openly allocated to vitamin D and among those on blind treatment, and contamination was quantitatively negligible in all arms including the open untreated control arm. A trial of 5 years of treatment would thus be equally powerful whether open or blind, so the choice of design depends on other considerations.
For the main end points (overall mortality, cancer diagnosis and reasons for hospital admission), the advantages of open-label randomisation with follow-up by linkage to national registers are:
-
A potentially substantial increase in power if those still on medication at 5 years were offered a continued supply of vitamin D, maintaining a large difference in blood 25(OH)D between treated and control participants beyond the initial 5 years of the trial. This might not be feasible in a double-blind trial when treatment is unblinded after 5 years, and in any case results beyond 5 years would not be placebo controlled.
-
A lower cost, as control participants would not be recontacted after randomisation and would not be given any medication.
The main advantages of placebo control are:
-
Reliable evidence on side effects and any potentially subjective outcomes.
-
Lifestyle changes that are affected by treatment allocation might in principle influence health, so any effect observed in an open trial could be a biased estimate of the pharmacological effect of vitamin D.
Research recommendations
-
Having established the feasibility of a large UK vitamin D trial, our main recommendation is that it should begin as soon as possible, irrespective of the pending results of ongoing trials, which are likely to be inconclusive for the reasons shown in Table 1 [bolus dosing or daily regimens ≤ 2000 IU, and higher population 25(OH)D levels than in the UK]. A substantial body of evidence from different populations will be required to decide whether or not, at what dose and for whom, this mass medication should be recommended, and results from a UK trial would be the most relevant. Increasing publicity about the potentially large benefits may make it increasingly difficult to recruit for such a trial. If the evidence from ongoing trials is encouraging but not strong enough to justify recommending mass medication, or to determine both the optimal dose and the blood 25(OH)D level below which it is worthwhile, these issues may never be satisfactorily resolved.
-
The decision on whether randomisation should be open-label, blind or a mixture of the two in a large vitamin D trial with mortality as the primary outcome will depend strongly on a priori assumptions. The NIHR HTA might consider its view on this separately from assessing specific applications to conduct such a trial.
-
The NIHR HTA in consultation with the relevant agencies might review opportunities for reducing delays in Clinical Research Network funding approvals for multicentre population-based prophylactic trials, for simplifying trial regulations for non-prescription treatments, such as vitamin D, for which extensive evidence on safety is already available, and for arranging linkage to HES and other NHS Digital databases for all non-commercial medical research.
Acknowledgements
We would particularly like to thank the study participants and the GPs, nurses, practice managers and administrative staff who provided valuable assistance throughout the study.
We would also like to thank the external members of the Trial Steering Committee: Professor Kay-Tee Khaw (chairperson, University of Cambridge), Professor Stephen Evans (London School of Hygiene & Tropical Medicine) and Professor Cyrus Cooper (University of Southampton and University of Oxford). We would also like to thank the DMC: Professor Timothy Peto (chairperson, University of Oxford), Professor Andrew Nunn (University College London) and Professor David Mant (University of Oxford). We thank Bernard North (Queen Mary University of London) as independent statistician for preparing the reports for the DMC, and Jayne Simpson (London School of Hygiene & Tropical Medicine) for contributing to data collection throughout the period of the trial.
The vitamin D study medication (Vigantol oil and Miglyol 812 oil) was provided by Merck Serono GmbH, Darmstadt, Germany.
Contributions of authors
Christine Rake (Trial Manager) managed all aspects of the conduct of the study and contributed to the design of the study and writing the report.
Clare Gilham (Medical Statistician) performed the majority of the statistical analyses and contributed to the interpretation of data and writing the report.
Laurette Bukasa (Trial Administrator) contributed to the conduct of the study, trial management, data collection and data analysis.
Richard Ostler (Senior Programmer) designed the dedicated online trial management system and contributed to trial oversight via the Trial Management Group.
Michelle Newton (Trial Administrator) contributed to the conduct of the study and data collection.
James Peto Wild (Trial Administrator) contributed to the conduct of the study, data collection and database implementation.
Benoit Aigret (Barts CTU Head) contributed to protocol development and trial oversight via the Trial Management Group and as Operation Lead of Barts CTU.
Michael Hill (Oxford Clinical Trials Service Unit Laboratory Scientific Director) provided technical support in planning and throughout the trial on sample receipt, storage and analysis.
Oliver Gillie (Medical Journalist) was our lay member and contributed to the design of the study and trial oversight via the Trial Management Group.
Irwin Nazareth (Professor of Primary Care and Population Sciences) was delegate clinical chief investigator, hosted the pilot GP practice and assisted with GP practice selection and trial oversight via the Trial Management Group.
Peter Sasieni (Professor of Biostatistics and Cancer Epidemiology) contributed to protocol development and trial oversight via the Trial Management Group and as Director of Barts CTU, which developed the online trial management system.
Adrian Martineau (Clinical Professor of Respiratory Infection and Immunity) was the clinical chief investigator and contributed to the design of the study, trial oversight and writing the report.
Julian Peto (Professor of Epidemiology) was the chief investigator with overall responsibility for the design and supervision of the study, the statistical analysis and writing the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Office for National Statistics . National Population Projections, 2012-Based Statistical Bulletin London 2013. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2013-11-06 (accessed 28 March 2019).
- Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 1980;303:130-5. https://doi.org/10.1056/NEJM198007173030304.
- Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psycho-Oncology 2008;17:891-900. https://doi.org/10.1002/pon.1300.
- Hillers TK, Guyatt GH, Oldridge N, Crowe J, Willan A, Griffith L, et al. Quality of life after myocardial infarction. J Clin Epidemiol 1994;47:1287-96. https://doi.org/10.1016/0895-4356(94)90134-1.
- Banerjee S, Smith SC, Lamping DL, Harwood RH, Foley B, Smith P, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatry 2006;77:146-8. https://doi.org/10.1136/jnnp.2005.072983.
- Salkeld G, Cameron ID, Cumming RG, Easter S, Seymour J, Kurrle SE, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ 2000;320:341-6. https://doi.org/10.1136/bmj.320.7231.341.
- Metlay JP, Fine MJ, Schulz R, Marrie TJ, Coley CM, Kapoor WN, et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med 1997;12:423-30. https://doi.org/10.1046/j.1525-1497.1997.00074.x.
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. https://doi.org/10.1016/S0140-6736(12)61689-4.
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80:1678-88. https://doi.org/10.1093/ajcn/80.6.1678S.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. https://doi.org/10.1056/NEJMra070553.
- Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007;85:860-8. https://doi.org/10.1093/ajcn/85.3.860.
- Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck-Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr 2013;52:1115-25. https://doi.org/10.1007/s00394-012-0421-6.
- Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr 2012;108:1557-61. https://doi.org/10.1017/S0007114511007161.
- Public Health England . National Diet and Nutrition Survey. Results from Years 1–4 (Combined) of the Rolling Programme (2008 2009–2011 12) 2014. www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed 28 March 2019).
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352-60. https://doi.org/10.1101/gr.107920.110.
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med 2008;29:361-8. https://doi.org/10.1016/j.mam.2008.08.008.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18-2. https://doi.org/10.1093/ajcn/84.1.18.
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503-11. https://doi.org/10.1161/CIRCULATIONAHA.107.706127.
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014;83:920-8. https://doi.org/10.1212/WNL.0000000000000755.
- Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 2013;136:321-9. https://doi.org/10.1016/j.jsbmb.2012.11.017.
- Heath AK, Kim IY, Hodge AM, English DR, Muller DC. Vitamin D status and mortality: a systematic review of observational studies. Int J Environ Res Public Health 2019;16. https://doi.org/10.3390/ijerph16030383.
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586-91. https://doi.org/10.1093/ajcn/85.6.1586.
- Camargo CA, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012;130:e561-7. https://doi.org/10.1542/peds.2011-3029.
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012;156:105-14. https://doi.org/10.7326/0003-4819-156-2-201201170-00004.
- Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Vitamin D supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015;3:120-30. https://doi.org/10.1016/S2213-2600(14)70255-3.
- Bischoff-Ferrari HA, Willett WC, Orav EJ, Oray EJ, Lips P, Meunier PJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012;367:40-9. https://doi.org/10.1056/NEJMoa1109617.
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA 2004;291:1999-2006. https://doi.org/10.1001/jama.291.16.1999.
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365:1621-8. https://doi.org/10.1016/S0140-6736(05)63013-9.
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684-96. https://doi.org/10.1056/NEJMoa055222.
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669-83. https://doi.org/10.1056/NEJMoa055218.
- Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012;308:1333-9. https://doi.org/10.1001/jama.2012.12505.
- Chandra RK. Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition 2001;17:709-12. https://doi.org/10.1016/S0899-9007(01)00610-4.
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815-22. https://doi.org/10.1001/jama.2010.594.
- Martineau AR, Hanifa Y, Witt KD, Barnes NC, Hooper RL, Patel M, et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015;70:953-60. https://doi.org/10.1136/thoraxjnl-2015-206996.
- Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women – a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 2007;46:1852-7. https://doi.org/10.1093/rheumatology/kem240.
- Vitamin D Status of New Zealand Adults: Findings from the 2008/09 New Zealand Adult Nutrition Survey. Wellington; 2012.
- Australian Bureau of Statistics . Australian Health Survey: Biomedical Results for Nutrients, 2011–12 2013. www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.006main+features12011-12 (accessed 30 May 2018).
- Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 dietary reference intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 2011;94:128-35. https://doi.org/10.3945/ajcn.111.013268.
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011;59:1-8.
- Tuomainen TP. US National Library of Medicine . Finnish Vitamin D Trial n.d. https://clinicaltrials.gov/ct2/show/NCT01463813 (accessed 30 May 2018).
- Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol 2017;5:438-47. https://doi.org/10.1016/S2213-8587(17)30103-1.
- Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study :a randomized clinical trial. JAMA Cardiol 2017;2:608-16. https://doi.org/10.1001/jamacardio.2017.0175.
- Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33-44. https://doi.org/10.1056/NEJMoa1809944.
- NHS Digital. Hospital Episode Statistics (HES) 2019. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 8 May 2019).
- Ministry of Housing Communities and Local Government . English Indices of Deprivation 2015 n.d. http://imd-by-postcode.opendatacommunities.org/ (accessed 30 May 2018).
- EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356. https://doi.org/10.1136/bmj.i6583.
- Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013;98:4619-28. https://doi.org/10.1210/jc.2013-2653.
- Clarke R, Newman C, Tomson J, Hin H, Kurien R, Cox J, et al. Estimation of the optimum dose of vitamin D for disease prevention in older people: rationale, design and baseline characteristics of the BEST-D trial. Maturitas 2015;80:426-31. https://doi.org/10.1016/j.maturitas.2015.01.013.
- Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int 2017;28:841-51. https://doi.org/10.1007/s00198-016-3833-y.
- Heart Protection Study Collaborative Group . Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 2011;378:2013-20. https://doi.org/10.1016/S0140-6736(11)61125-2.
Appendix 1 Lifestyle and quality of life questions
Appendix 2 The web-based clinical data management system: the VIDAL online application
The VIDAL app was the web-based clinical data management system developed specifically to streamline data capture and management for the VIDAL feasibility study. It was designed to be able to accommodate the 200 practices and 20,000 participants required for the main trial, to eliminate costly administration, transport and postal fees, and to optimise the process of data acquisition and data management.
The functions of the VIDAL app were to provide:
-
secure online access
-
current GP practice and trials office contact details
-
access to all current study documents (in the online library)
-
access to online CRFs for local GP staff to create, complete and view participant data
-
participant accessible follow-up data entry forms (CRFs)
-
trials office access to view participant and GP practice data
-
participant randomisation into the trial
-
online tools for ordering and tracking study medication
-
tracking tools for recording blood sample dispatch and receipt
-
automated e-mail, text message and telephone call alerts and reminders for participants, GP practices and trials office staff
-
online tools for mail merging and printing study letters and prescriptions and logging reply slips and consent forms
-
reports to monitor participant progress and compliance through the trial
-
timely completion of online SAE and AR CRFs.
List of abbreviations
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- app
- online application
- AR
- adverse reaction
- BC
- blind placebo control
- BD
- blind vitamin D
- BMI
- body mass index
- BP
- blood pressure
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- EDTA
- ethylene diamine tetra-acetic acid
- EudraCT
- European Union Drug Regulating Authorities Clinical Trials
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HTA
- Health Technology Assessment
- IDAOPI
- Income Deprivation Affecting Older People Index
- IMD
- Index of Multiple Deprivation
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NIHR
- National Institute for Health Research
- OC
- open control
- OD
- open-label vitamin D
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SST
- serum separator tube
- VDR
- vitamin D receptor
- VIDAL
- Vitamin D and Longevity