Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/48/01. The contractual start date was in August 2017. The draft report began editorial review in October 2018 and was accepted for publication in June 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Stevenson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Creutzfeldt–Jakob disease (CJD) is a progressive, fatal disease affecting the brain. CJD is caused by an abnormal transmissible protein called a prion. Once CJD is transmitted, the concentration of CJD prions varies throughout the body, but reaches high levels in the brain and posterior eye, resulting in neurological symptoms including rapidly progressive dementia, extrapyramidal signs and visual symptoms. Most people with clinically diagnosed CJD will die within 1 year of the symptoms appearing.
Four classifications of CJD exist: sporadic CJD (sCJD), variant CJD (vCJD), genetic CJD (gCJD) and iatrogenic CJD (iCJD). Referrals of suspected CJD and values for death definitely related (with neuropathological confirmation) or probably related (without neuropathological confirmation) to CJD are recorded by the National CJD Research and Surveillance Unit (NCJDRSU) in Edinburgh. 1 This source estimates that since 1990 there have been 3746 referrals for investigation and 2370 deaths from definite or probable CJD (as of 8 January 2018).
Sporadic CJD has historically been the most common type of CJD, accounting for around 85% of CJD cases. The cause of sCJD is thought to be the spontaneous generation of an abnormal isoform of prion protein (PrP). sCJD generally occurs later in life (in those with a mean age of 67 years) and has a short survival post diagnosis of around 4 months. 2 Although there is evidence of a genetic predisposition to sCJD, the precise cause of the disorder is unknown.
Genetic CJD, also known as familial or inherited CJD, is associated with a pathogenic mutation in the prion protein gene (PRNP) and includes conditions known as fatal familial insomnia (FFI) and Gerstmann–Schäussler–Scheinker (GSS) syndrome. Overall, gCJD accounts for between 5% and 15% of CJD cases or approximately 10 CJD deaths in the UK, per year.
Variant CJD was observed following the exposure of the UK population during the late 1980s and early 1990s to bovine spongiform encephalopathy (BSE), which was presumed to be transmitted to humans by eating food contaminated with the brain, spinal cord or digestive tract of infected carcasses. The vCJD epidemic peaked in 2000 with 28 deaths and has since declined, with only two ‘definite or probable’ vCJD deaths reported since 2012. The majority of cases have occurred in a younger population compared with that observed in sCJD, with a mean age of 26 years. The median disease duration post diagnosis is longer in vCJD (14 months) than that observed in sCJD. All people who have contracted clinically observed vCJD have died.
Incidences of iCJD, which is the transmission of prion disease through medical procedures or equipment, have been recorded for procedures such as dura mater grafts, electroencephalography (EEG) needles and neurosurgery, and from receipt of corneal grafts, growth hormones, gonadotrophin or packed red blood cells. 3
The current decision problem focuses on the risk of transmission of CJD (of all forms) via surgical instruments. Prions are unlikely to be completely deactivated on surgical instruments by conventional hospital cleansing and sterilisation techniques4 and, therefore, patients may be infected iatrogenically with CJD by surgical instruments resulting in a surgically transmitted CJD (stCJD) case. Iatrogenic transmission can occur when surgical instruments, endoscopes or laryngoscopes are used during high-risk neurosurgical procedures in patients who have asymptomatic CJD but who are infectious because neural tissue in particular has a high infectious load. 5 Four cases of iCJD transmitted via neurosurgery were observed between 1952 and 1974 from three sporadic index cases of CJD. 6 Stringent public health requirements are in place to limit the risk of iCJD being spread from people with an increased risk of developing CJD, or with CJD, or for whom a diagnosis of CJD is being considered or cannot be excluded.
Immediately following the recognition of vCJD, as a consequence of the BSE outbreak, the potential scale of the number of infections was uncertain; estimations incorporated potential subclinical vCJD infections identified from a histopathological survey of lymphoreticular tissue to be 237 per million [95% confidence interval (CI) 49 to 692 per million]. 7–9 Surgical transmission of CJD in this scenario was considered to pose a potential risk to public health by virtue of a self-sustaining iatrogenic epidemic. Therefore, in 2005 the National Institute for Health and Care Excellence (NICE) commissioned the School of Health and Related Research (ScHARR) at the University of Sheffield to conduct a systematic review and perform cost-effectiveness modelling of evidence on patient safety and reduction of risks of transmission of CJD. 10 This evidence, together with data collected from experts, was used to populate a mathematical model assessing the cost-effectiveness of single-use surgical instruments. 11,12 The outputs from the model and a separate risk assessment conducted by the Department of Health Economics, Statistics and Operational Research Division13 were used to inform the NICE Interventional Procedures Guidance 196 (IPG196) Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) Via Interventional Procedures. 14 The existing guidance includes recommendations on decontamination methods and guidance for set-keeping to ensure that instruments in contact with potentially high-risk tissues do not move from one set to another. Furthermore, supplementary instruments (SIs) used during high-risk procedures were recommended to either be single-use or to remain with the set with which they were introduced. An age split was also recommended with separate instruments used for people born before 1997 (and at risk of dietary exposure to BSE) and those born after 1996 (who were believed, at the time of writing IPG196, to be not infected with vCJD). High-risk procedures are regarded as intradural neurosurgical operations on the brain (excluding operations on the spine and peripheral nerves), neuroendoscopy, and posterior eye procedures that involve the retina or optic nerve. 14 Although the cost-effectiveness analysis indicated that the introduction of single-use instruments for all high-risk procedures was not cost-effective, there was great uncertainty in these results and a recommendation was made by the study authors that policy might need to be revised if new relevant data become available.
An epidemic of CJD has not occurred since the publication of IPG196 and no conclusive evidence of transmission by surgery has transpired to date. However, a number of developments have occurred since 2006 that include:
-
a finding of abnormal prion accumulation in the appendixes of low-risk cohorts (i.e. those born after 1996)15,16
-
continued evolution of high quality and less expensive single-use instruments
-
anecdotal reports of difficulties implementing the recommendation from IPG196 related to keeping instruments in their original sets across a number of units
-
anecdotal reports of problems in maintaining quarantined instruments for patients born after 1996.
A recent study has also implicated neurosurgery as a possible iatrogenic source for amyloid beta accumulation in the brain, a peptide that is associated with Alzheimer’s disease. 17 This finding underlines the potential risk associated with high-risk procedures and the importance of assessing evidence relevant to decontamination or disposal of neurosurgical equipment.
Purpose of the research
The objective of the current research is to update selected evidence from the research project conducted in 2005 (project number IP1553)18,19 that informed NICE guidance IPG19611 for the NICE Interventional Procedures (IPs) committee to review the decision problem in 2018. The aim is to review the evidence base for the current risk of transmission of CJD (any form) related to surgery in order to provide up-to-date relevant evidence to NICE, and to inform the cost-effectiveness of potential management strategies.
Research objectives
-
To perform updates of the systematic reviews completed in 2005 on the clinical evidence on patient safety and risks of transmission of CJD via surgery.
-
To update the economic model and, where necessary, seek new input from expert elicitation to make the model relevant for the decision problem today.
-
To undertake modelling to estimate the cost-effectiveness of strategies to reduce the risk of transmission of CJD via surgical procedures.
Chapter 2 Clinical evidence
Methods for systematic reviews
The protocol for this project was developed in consultation with the NICE Interventional Procedures Advisory Committee and was registered on the Centre for Reviews and Dissemination (CRD) systematic review database (PROSPERO registration number CRD42017071807). The project aimed first to update the evidence for the following eight research questions:
-
What is the incidence of CJD and what is the prevalence of CJD-related prions in humans in the UK?
-
What is the risk of secondary transmission of CJD by surgical procedure?
-
What are the incubation periods of acquired transmissible spongiform encephalopathies (TSEs)?
-
What is the infectivity of CJD?
-
What is the evidence on the efficacy of decontamination techniques for instruments infected with prions?
-
What is the evidence that instruments used for high-risk procedures remain in their original sets?
-
What is the evidence for complication rates of single-use compared with reusable instruments for high-risk procedures?
-
What is the evidence for likelihood of future surgery for a patient undergoing high-risk procedures?
Eight systematic reviews have been completed to address these research questions. These reviews adhered to best practice systematic review methodology in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 200920 standards.
Eligibility criteria
The inclusion and exclusion criteria differ for each review question. These are broadly summarised in Table 1.
| Review question | Eligibility criteria for inclusion into the review |
|---|---|
| What is the incidence of CJD and what is the prevalence of CJD-related prions in humans in the UK? |
|
| What is the risk of secondary transmission of CJD by surgical procedure? |
|
| What are the incubation periods of acquired TSEs? |
|
| What is the infectivity of CJD? |
|
| What is the evidence on the efficacy of decontamination techniques for instruments infected with CJD/TSE/prions? |
|
| What is the evidence that instruments used for high-risk procedures remain in their original sets? |
|
| What is the evidence for complication rates of single-use compared with reusable instruments for high-risk procedures? |
|
| What is the evidence for risk of future surgery for a patient undergoing high-risk procedures? |
|
Search strategy
Literature searches were conducted to retrieve relevant evidence. Electronic databases were searched on 14 August 2017:
-
MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations – via Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands), 1946 to 2017
-
EMBASE – via Ovid, 1974 to 2017
-
Science Citation Index and Conference Proceedings Citation Index– Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA), 1990 to 2017.
A date restriction from 2005 to 2017 was applied for the first seven review questions. For the final review question regarding the risk of future surgery in patients who have had high-risk procedures, because no relevant evidence was found in the previous review, the search strategy was revised and searches were performed from database inception to 2017. No language or study design limits were applied to the searches. The search strategies are presented in Appendix 1.
Members of the NICE IP’s committee were consulted as content experts for potentially relevant papers for all review questions. Papers recommended by experts were subject to bibliography checking.
The searches combined terms that would be relevant for more than one review question. Therefore, five targeted literature searches, instead of eight, for all review questions were conducted, which combined terms for:
-
Searches for the UK incidence and prevalence of CJD and the incubation period of acquired human TSEs.
Electronic literature searches were performed to identify relevant articles. Terms for ‘incidence and prevalence’ or ‘incubation’ (see Appendix 1, search strategy lines 10–15) were combined with ‘CJD’ population terms (see Appendix 1, search strategy lines 1–9). The terms applied were identical to those used in appendices 1 and 3 in the original systematic review. 10
-
Searches for the secondary transmission of CJD by invasive diagnostic or surgical procedures; infectious mass required to transmit CJD; and the decontamination of surgical, anaesthetic and diagnostic instruments, scopes and implantable devices.
Electronic literature searches were performed to identify relevant articles. Terms for ‘transmission’ and ‘transfer’ (see Appendix 1, search strategy line 27) and ‘instrument decontamination’ (see Appendix 1, search strategy lines 28–33) were combined with ‘CJD’ population terms in humans or non-human mammals (see Appendix 1, search strategy lines 18–25).
-
Searches for the extent to which surgical instruments remain in their original sets following use and decontamination.
Electronic literature searches were performed to identify articles that report on the extent to which surgical instruments remain in their original sets following use and decontamination. Terms for ‘instrument decontamination’ (see Appendix 1, search strategy lines 36–41) were combined with ‘high-risk surgical procedures’ (see Appendix 1, search strategy lines 42–56). A list of high-risk surgical procedures were taken from appendix C of NICE IPG196. 14
-
Searches for the complication rates associated with the use of single-use versus reusable anaesthetic, diagnostic or surgical instruments.
Electronic literature searches were performed to identify articles that report on complication rates associated with the use of single-use versus reusable anaesthetic, diagnostic or surgical instruments. Terms for ‘disposable’ or single-use’ instruments (see Appendix 1, search strategy lines 60–63), including specifically named instruments recommended at the NICE committee meeting in June 2017 (see Appendix 1, search strategy line 63), were combined with ‘high-risk surgical procedures’ (see Appendix 1, search strategy lines 65–79) or ‘complications’ (see Appendix 1, search strategy lines 81–84).
-
Searches for the risk of future surgery following surgery.
Electronic literature searches were performed to identify articles that report on the risk of future surgery following surgery. Terms for ‘reoperation’ or ‘repeat surgery’ were combined (see Appendix 1, search strategy lines 88–90) with ‘high-risk surgical procedures’ (see Appendix 1, search strategy lines 92–106). As the review question was reconceptualised to be more sensitive to potentially relevant studies than the previous review undertaken in 2006, no date restrictions were applied.
Cost-effectiveness searches
A literature search was undertaken to identify evidence relevant to the cost-effectiveness model such as relevant economic evaluations in CJD.
Four electronic databases were searched on 7 June 2017 from 2004 to present:
-
MEDLINE, MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations – via Ovid, 1946 to 2017
-
EMBASE – via Ovid, 1974 to 2017
-
The Cochrane Library (Wiley Online Library) Cochrane Database of Systematic Reviews, 1996 to 2017; Health Technology Assessment Database, 1995 to 2016; NHS Economic Evaluation Database, 1995 to 2015
-
Science Citation Index and Conference Proceedings Citation Index – Web of Science, 1990 to 2017.
The search strategy comprised Medical Subject Headings or Emtree thesaurus terms and free-text synonyms for ‘CJD’. Searches were translated across databases and were not limited by language. The search strategies are presented in Appendix 2. Search filters designed to identify economic evaluations were used on MEDLINE and EMBASE.
Study selection
Results from the electronic bibliographic searches were imported into reference management software, EndNote Version 8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA], and duplicates were removed. Titles and abstracts of retrieved records were examined by one reviewer (LU) and irrelevant citations were excluded. A proportion (10%) of randomly selected excluded citations were double-checked by a second reviewer (CC) and any disagreements were resolved by discussion between the reviewers. Consultation with the third designated team member (MS) was not required for any citation. At the full-paper stage, all citations excluded from a particular review question by the reviewer were double-checked by the second reviewer. Lists of these citations, with the principal reason for exclusion, are reported for each review in Appendix 3. Data identified from countries outside the UK were incorporated if deemed relevant.
Literature identified within the cost-effectiveness review was processed in a similar manner. Titles and abstracts of retrieved records were examined by one reviewer (MS) and irrelevant citations were excluded. A proportion (10%) of randomly selected excluded citations were double-checked by a second reviewer (LU). All full-text articles were independently assessed for inclusion by two reviewers (MS, and LU). No disagreements were required to be resolved through discussion or with involvement of the third designated team member (CC).
Data extraction
Bespoke data extraction forms were developed for each review question in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) to record relevant outcome data for the review question in hand. All data were extracted by one systematic reviewer (LU for reviews 1, 2 and 4; CC for reviews 3, 5, 6, 7 and 8) and independently checked by a second reviewer (LU for reviews 3, 5, 6, 7 and 8; CC for reviews 1, 2 and 4). Any disagreements were resolved by discussion and consensus or by consulting with a third member of the project team (MS).
Quality assessment
Formal quality assessment using standard checklists, such as the Cochrane risk-of-bias tool, was considered for these systematic reviews. The value of conducting quality assessment is to assess how a study has been conducted in order to balance the numerical findings (or the statistical strength of effects) against the methodological quality. There are a range of quality assessment tools available depending on the study type included; quality assessment is not only amenable to a review of RCTs. However, none of the review questions sought data that were estimating treatment effects; therefore, the typical domains of quality assessment, such as randomisation, performance bias, detection bias and attrition bias, are less relevant. Furthermore, in many cases, the included ‘studies’ in this review were not amenable to quality assessment because (1) they are surveillance reports, thereby not constituting the traditional definition of a study or (2) they are laboratory studies using highly specific scientific methods that are not amenable to the quality assessment for clinical trials. As these included studies were mainly observational in nature, the data of interest were less vulnerable to author conflicts of interest or systematic bias. Assessment of study heterogeneity is most important when performing formal synthesis to estimate treatment effects, which is not the objective of this review. Indeed, limitations to review inclusion criteria based on study design, scientific discipline, setting or context would potentially have restricted the external validity of the review. Therefore, no formal quality assessment has been undertaken and the protocol for the systematic review, registered on the PROSPERO database (CRD42017071807), was updated accordingly. 21 The purpose of the reviews was primarily to describe the relevant literature rather than to aggregate data or rank individual studies.
Data analysis/synthesis
Data were tabulated, synthesised and discussed narratively for each review question. Meta-analyses were planned to be conducted by an experienced statistician using appropriate software, and heterogeneity was to be explored using meta-regression where comparable data were available. However, no suitable data were identified for formal aggregation using meta-analysis.
Meta-biases and assessment of external validity
Owing to the complex nature of the clinical topic, the number of review questions and the diverse information required to inform the economic model, the systematic reviews were methodologically challenging. To obtain high-quality, trustworthy data and to maintain the external validity of the reviews, the inclusion criteria were kept broad until full text retrieval. After discussion within the project team and with the NICE committee experts, a decision was made to take a broad approach during the assessment of study relevance, rather than applying stringent inclusion criteria.
The risk of this approach was that the evidence generated from the reviews was less amenable to replication. However, the purpose the clinical reviews was to inform commissioners about potential risks of CJD transmission via surgery rather than estimating treatment effect. Therefore, a more inclusive methodological approach by the evidence review group in this complex clinical topic was deemed justifiable.
Literature search results
The literature searches of bibliographic databases were performed on 14 August 2017 and yielded 8466 citations. During the screening process, a citation of potential relevance to review question 2 was identified that had not been picked up by the literature searches. Therefore, the information specialist in consultation with the project team revised the search terms for review 2 to perform an additional search on 2 October 2017, resulting in a further 310 citations. A total of 41 further citations were obtained and assessed for eligibility either from recommendations from NICE’s committee members (n = 16) or through checking the reference lists of relevant citations (n = 25). After duplicates were removed, the 8549 titles and abstracts were reviewed by one reviewer (LU). In total, 10% of excluded citations were independently assessed by a second reviewer (CC) with very good agreement (κ = 0.98). Any disagreements were carried forward for further discussion but none was ultimately deemed eligible for full text inspection by either reviewer (see Appendix 3 for the table of excluded studies). A PRISMA flow diagram illustrating the process of identifying citations through to final study selection for each review question is shown in Figure 1.
FIGURE 1.
The PRISMA flow diagram of studies included in systematic reviews.

The incidence of Creutzfeldt–Jakob disease and the prevalence of Creutzfeldt–Jakob disease-related prions in humans in the UK
The purpose of this review was to identify published and unpublished evidence for:
-
the incidence of CJD (sporadic, genetic, variant and iatrogenic)
-
the prevalence of CJD-related prions in humans in the UK.
The NCJDRSU provides the most comprehensive and regularly updated figures for the UK. Globally, figures are gathered by the CJD International Surveillance Network (EuroCJD);22 however, this source was last updated in May 2015 and is therefore less up to date than the NCJDRSU. The literature searches were also used to retrieve the most recent or complete figures, incidence trends or studies regarding subclinical prevalence of CJD prions in tissue. A total of 69 published citations were identified as being relevant to the incidence of clinical CJD or the prevalence of subclinical CJD around the world.
The incidence of Creutzfeldt–Jakob disease
The global incidence of CJD is typically reported to be around 1 to 2 cases per million per year,22 based on surveillance studies published around the world from 2005 (Table 2). Higher incidence rates may be more likely to occur in areas with access to established surveillance units for referring suspected cases of prion disease. In the UK since 1990, the NCJDRSU has been mandated to actively monitor and identify all CJD cases. By contrast, a paper by Jeon et al. 28 described that CJD surveillance did not begin in Korea until 2001, and iCJD was not studied in Korea prior to 2011. This indicates geographical variation in how CJD may have been detected and reported in time globally.
| Country | Time period of estimation | CJD incidence or mortality rate per million | CJD types included | Study author/source |
|---|---|---|---|---|
| Austria | 1993–2017 | 1.49 | Sporadic | EuroCJD22 |
| Australia | 1993–2016 | 1.20 | Sporadic | EuroCJD22 |
| Belgium | 1997–2017 | 1.19 | Sporadic | EuroCJD22 |
| Canada | 1994–2017 | 1.03 | Sporadic | EuroCJD22 |
| Czech Republic | 2000–17 | 1.16 | Sporadic | EuroCJD22 |
| Denmark | 1993–2017 | 1.45 | Sporadic | EuroCJD22 |
| Estonia | 2004–17 | 0.32 | Sporadic | EuroCJD22 |
| France | 1993–2017 | 1.53 | Sporadic | EuroCJD22 |
| Germany | 1993–2017 | 1.36 | Sporadic | EuroCJD22 |
| Hungary | 1997–2017 | 1.07 | Sporadic | EuroCJD22 |
| Italy | 1993–2017 | 1.44 | Sporadic | EuroCJD22 |
| Netherlands | 1993–2017 | 1.21 | Sporadic | EuroCJD22 |
| Norway | 1995–2017 | 0.96 | Sporadic | EuroCJD22 |
| Slovakia | 1993–2017 | 0.85 | Sporadic | EuroCJD22 |
| Slovenia | 1993–2017 | 1.38 | Sporadic | EuroCJD22 |
| Spain | 1993–2017 | 1.30 | Sporadic | EuroCJD22 |
| UK | 1993–2017 | 1.19 | Sporadic | NCJDRSU 20161 |
| USA | 2016 | 1.22 | Excludes vCJD | US Centers for Disease Control and Prevention23 |
| Japan | 1999–2015 | 1.3 | All types | Yamada et al.24 |
| Australia | 1993–2014 | 1.2 | All types | Klug et al.25 |
| Finland | 1997–2013 | 1.45 | Sporadic | EuroCJD22 |
| Cyprus | 1995–2013 | 0.70 | Sporadic | EuroCJD22 |
| Germany | 1993–2013 | 1.33 | Excludes vCJD | EuroCJD22 |
| Holland | 1993–2013 | 1.21 | Excludes vCJD | EuroCJD22 |
| Hungary | 1997–2013 | 1.65 | Excludes vCJD | EuroCJD22 |
| Sweden | 1997–2013 | 1.44 | Excludes vCJD | EuroCJD22 |
| Switzerland | 1993–2013 | 1.72 | Sporadic | EuroCJD22 |
| Argentina | 2008 | 0.85 | All types | Begué et al.26 |
| Greece | 1997–2008 | 0.62 | Sporadic | EuroCJD22 |
| Taiwan | 1998–2007 | 0.55 | Sporadic | Lu et al.27 |
A study by Gao et al. 29 does not report an incidence rate per million for CJD in China, but does report that during the period from 2006 to 2010, 261 patients were diagnosed with sCJD and 23 patients were diagnosed with genetic human prion diseases out of a group of 624 suspected patients who were referred to China CJD surveillance. 29
Increase in the UK sporadic Creutzfeldt–Jakob disease incidence over time
Between 1990 and 2017, the NCJDRSU recorded figures of iCJD [from receipt of human gonadotrophin (hGN), human-derived growth hormone or dura mater) and vCJD, which were relatively low compared with sCJD. Figure 2 plots the number of deaths in the UK that have been attributed to definite or probable CJD between 1996 and 2017 (as of 2 May 2018) as reported by the NCJDRSU. An increase in sCJD cases is noted over the 27-year period, whereas iatrogenic, genetic and variant forms remain rare.
FIGURE 2.
Deaths attributed to definite or probable CJD in the UK, using data from the NCJDRSU, between 1996 and 2017. 30

Possible reasons for the increase in the detection of sCJD cases in the UK are speculated to include:
-
improved case ascertainment because of clinician awareness and/or improvements in diagnostic testing
-
population increases
-
an ageing population
-
changes to the sporadic case definition to include cerebrospinal fluid and magnetic resonance imaging (MRI) diagnostic tests.
An upwards trajectory of CJD cases may be attributable to the way that data are collected for the surveillance of CJD. Case ascertainment is likely to improve in areas where CJD surveillance is strong, where there is greater awareness among health-care professionals of CJD and where there are more neurologists who are able to diagnose CJD. As the national surveillance programme for CJD has been operating since May 1990, and is a prospective surveillance programme, there are likely to be improvements over time with respect to how this rare condition is detected, referred, investigated and reported when compared with retrospective surveillance studies. Moreover, owing to the potential for iatrogenic transmission, there has been a focused collaborative effort to examine the evidence of transmission through different exposures by examining the links to confirmed CJD cases through retrospective ‘lookback’ studies. 31
The gradual increase in sCJD but not gCJD, adds support to the ‘ageing population’ theory over merely population increase and improved case ascertainment.
Increase in sporadic Creutzfeldt–Jakob disease incidence globally
Reports of increased rates of sCJD were noted from other countries. In Finland, an increased incidence of sCJD was noted between 1974 and 1989 of 0.6 per million to 1.36–1.44 per million in 2007–13, as reported in an abstract by Isotalo et al. 32 An abstract by Chen33 reports that sCJD incidence rates in Taiwan doubled between 2008 and 2015. They also report that age at onset became younger. Chen33 speculates that the reasons for the increase in CJD cases include physician’s sensitivity in recognising CJD; improved reporting systems; concerns around vCJD, and high media coverage. A published study34 from Belgium noted a relevant trend of significantly increased age-specific incidence of sCJD patients between the age of 70 and 90 years in the period 2002–04 compared with 1998–2001, using retrospectively obtained data (1990–1997; p < 0.01). The authors conducted a clinical and biochemical analysis to investigate this increase, but could not identify any reason other than an increased vigilance for the diagnosis. Similarly, in Japan, Ae et al. 35 report in a study abstract that the annual incidence of human prion diseases has increased since 1999, particularly so in older patients (aged ≥ 70 years), with cases of rapidly developing dementia increasingly being identified by domestic physicians.
One study from Slovenia reported an apparent fluctuation of sCJD cases in 2015, with seven definite and two probable sCJD cases resulting in an incidence of 4.36 per million for the country that year. 36
Autopsy and biopsy in Creutzfeldt–Jakob disease
In the UK, confirmation of CJD from neuropathological (via autopsy or brain biopsy), immunocytochemical or biochemical examination is required for obtaining a definitive sCJD diagnosis. For vCJD, confirmation must be from neuropathology. 1 Despite the observed increase in sCJD cases over the last twenty years, autopsy is not performed routinely on sCJD cases. In the UK, almost 50% of all cases referred to the NCJDRSU undergo autopsy. 2 The most recent case of vCJD appeared in its clinical presentation and neuroimaging to be sCJD, but as the age of the patient was atypically young (aged 36 years), a pathological examination after death in February 2016 confirmed it to be vCJD despite the absence of clinical epidemiologic diagnostic criteria for probable or possible vCJD. 37 On the basis of this recent vCJD case, pathological examination of every sCJD case would be required to know the true figures of autopsy-proven sCJD and vCJD. Given this, an alternative explanation for the increasing number of sCJD cases over the last 20 years could be attributable to an altered incubation and clinical presentation of acquired CJD (variant or iatrogenic CJD) that mimics sCJD or another neurological condition. Indeed, surgery has been posited as a risk factor the transmission of sCJD by a number of epidemiological studies; a retrospective study by Urwin et al. ,38 described as ongoing, is seeking to investigate this risk factor further by reviewing UK sCJD cases.
Cursory analysis of published literature from studies on CJD around the world generates potential reasons for why autopsy is not always routinely completed in sCJD patients. Brain biopsy and autopsy of suspected CJD cases carry the risk of iatrogenic transmission to medical or pathology staff, meaning that there is an extra burden of duty to ensure that stringent infection control protocols are followed. Protocols for instrument decontamination are required for brain biopsy. For example, Shi et al. 39 state that although an intracranial biopsy procedure is invasive and carries risk of cerebral infection or hematoma, it is generally a safe and well-tolerated procedure; however, special precautions to prevent the spread of prions must be taken. Medical instruments and equipment supplies must be either destroyed by incineration or autoclaved and sterilised. Similarly, Baig and Phillips. 40 state that getting a biopsy in a timely manner is often not possible given the costly and aggressive nature of the diagnostic test and that the rigorous decontamination and sterilisation techniques for handling tissue at biopsy may make it impractical in a community setting.
Ethnic and geographical differences
Variations in CJD incidence according to ethnicity by Maddox et al. 41 and Holman et al. 42 were noted in the literature. In the USA, the age-adjusted CJD incidence for white people was reported as being 2.7 times higher than that for black people (1.04 and 0.40 per million, respectively). Similarly, the estimated incidence of CJD (0.7 per million) among Asians and Pacific Islanders in the USA between 2003 and 2009 was reported by Maddox et al. 43 as being significantly lower than that for white people (p < 0.001).
Nakatani et al. 44 noted that the occurrence of sCJD appeared to have regional variations in Japan, suggesting that the existence of genetic or region-specific factors may affect the incidence of the disease, such as hereditary background or other local factors. In this study, geographical clusters of sCJD were scattered in the western half of Japan. However, no direct evidence to support theories about the causative factors underlying this trend are presented and, therefore, this particular phenomenon remains to be explored. Klug et al. 45 conducted a spatial and epidemiological analysis of sCJD case-clusters in Australia. The authors concluded that the observed increase of sCJD cases in a geographic area is more likely to be related to better awareness of the disease by local neurologists rather than to an increase in risk factors.
Genetic forms of CJD are most often associated with a mutation at codon 200. 46 Mitrova et al. 47 report that although gCJD represents approximately 10–15% of all CJD patients in the majority of countries, in Slovakia the rate of gCJD has been higher than 65% since 1975 owing to an accumulation of gCJD incidence in two clusters in central Slovakia. The authors state that all but one of the 202 patients who had gCJD in Slovakia carried the mutation form E200K and highlight that asymptomatic carriers of this gene could contribute to iatrogenic transmission of CJD. A voluntary genetic testing study conducted by the authors showed positivity for the E200K mutation in 9 out of 2662 subjects who were unrelated to the gCJD cases both inside and outside the focal cluster. This finding indicates an unusual phenomenon of an increased prevalence of the E200K mutation linked to gCJD in the Slovak region. A study by Ladogana et al. 48 reported similar prevalence of sCJD across the UK, France, Germany, Italy, the Netherlands and Slovakia, but also reported an excess of genetic cases in Italy and Slovakia.
Geographical differences in CJD incidence are likely to be influenced by ascertainment bias in countries where access to health care is free and, moreover, when active national CJD surveillance is in place.
Diagnosis of Creutzfeldt–Jakob disease
Global differences in the culture of pursuing autopsy to confirm CJD diagnosis and subtype are likely to exist depending on national CJD surveillance protocols. For example, Tuskan–Mohar et al. 49 report that post-mortem examination was not performed in any of the five cases of CJD occurring in Croatia between 2001 and 2011 owing to patient families’ refusal of the procedure. More generally, Kosier50 state anecdotally in a US case report that the diagnosis of CJD is often delayed because of clinician bias towards more obvious possible medical or psychiatric causes. Litzroth et al. 51 highlight that in Belgium, between 1998 and 2012, on average 60% of hospitalised patients who died with suspected CJD were captured by the surveillance system. The authors also report that 11% of surveyed neurologists would not refer suspect vCJD cases for autopsy, nor contact a reference centre for diagnostic support and that 61% of surveyed neurologists were not familiar with the surveillance system.
Two studies from Ireland describe a relatively sensitive surveillance system for CJD detection but less accuracy in obtaining a final confirmatory CJD diagnosis. From a review of 21 referrals to the National CJD Centre in Ireland, Brett et al. 52 found that only five referrals were positive for CJD, with 12 being referred as part of their differential diagnosis. Brett et al. 52 cautioned that, more often than not, the clinical suspicion of CJD was not borne from the final neuropathological diagnosis and that failure by clinicians to adhere to the recommended CJD investigation algorithm impacts adversely on the neuropathology workload and causes unnecessary concern among operating theatre, laboratory and nursing personnel. Loftus et al. 53 also raised the issue that the terms ‘probable CJD’ and ‘definite CJD’ might be used indiscriminately. They highlight from an analysis of 100 cases of CJD in Ireland, that approximately half of cases (50/96 referrals) were confirmed as definite CJD via tissue samples through biopsy or autopsy. 53 The authors proposed an algorithm for CJD referrals to reduce infection control and diagnostic difficulties encountered in CJD surveillance.
Despite the fact that sCJD is a condition known to affect older people, its detection may have improved in the last 6 years. Figure 3 is taken from the 25th Annual Report of the NCJDRSU2 and shows a steep increase in the detection of CJD mortality in the UK,2 particularly in the age category of 65–69 years. However, incidence using age-adjusted data of CJD-related deaths per million will be influenced by the assumed population in each band. The mortality rates for 1995–2004 use the same census data as those for 2005–9. However, if there are proportionately more older people in the more recent age band, the incidence will be inflated.
FIGURE 3.
Age-specific mortality rates from sCJD in the UK 1970–2016: reproduced from NCJDRSU Annual Report 2016. 2 1970–1984 mortality rates calculated using mid-1981 England and Wales population estimates based on the 1981 Census. 1985–1994 mortality rates calculated using mid-1991 UK population estimates based on the 1991 Census. 1995–2004 mortality rates calculated using mid-2001 UK population estimates based on the 2001 Census. 2005–2009 mortality rates calculated using mid-2001 UK population estimates based on the 2001 Census. 2010–2016 mortality rates calculated using mid-2011 UK population estimates based on the 2011 Census. Reproduced with permission from the National CJD Research & Surveillance Unit, University of Edinburgh. 2

Owing to the median age at onset of sCJD symptoms, it is possible that CJD and prion disease cases may be concealed among cases of more commonly encountered but similarly rapidly deteriorating neurological conditions affecting older people, such as Alzheimer’s disease. In the published literature, there are numerous reports of CJD mimicking other conditions including stroke,54,55 acute neuropathy,56 hyperparathyroidism,57 dementia,51,58–61 Lewy body dementia,51 encephalitis,51 aphasia,62 Alzheimer’s disease,51,60 psychiatric decompensation50 and movement disorder. 63 The potential for CJD cases to be misdiagnosed was first demonstrated in a study in 1995 which found from an analysis of dementia autopsies that only about 60% of prion disease cases with pathologically typical spongiform encephalopathy were identified clinically during life. 64 Therefore, the observed rates of any type of CJD could still be an underestimate of the actual rate of CJD deaths in the absence of definitive pathological examination of all cases. It is also plausible that numerous cases of CJD that occur later in life, particularly where access to clinicians with experience of diagnosing CJD is limited, may result in some cases of misclassification of CJD, despite potentially improved detection. However, given the rarity of CJD presentation worldwide and consequent clinical expertise, a degree of caution should be exercised in the interpretation of the limited available data.
Disease duration
Disease duration is regarded as the time between the onset of clinical CJD symptoms and death. sCJD is commonly reported to have a disease duration of 4–7 months;2,22,65–67 however, Nagoshi et al. 68 report that duration of disease was longer for sCJD in Japan than in Western countries. The authors state that sCJD, which represented 77.0% of cases of prion disease in their surveillance network between 1999 and 2008, had a mean disease duration of 15.7 months. This longer disease duration in Japan is more akin to the median observed in the UK for vCJD, which is 14 months from the onset of symptoms to death (NCJDRSU’s 2016 annual report2) or indeed iCJD via human growth hormone (hGH), the median of which is reported as 16 months (mean 14 months) for 22 iCJD patients. 69 Nagoshi et al. 68 also report that disease duration was longer in females (19.7 months) than males (14.5 months) for sCJD and that this tendency was also true for dura mater iCJD and types of gCJD including human GSS syndrome and FFI. Nagoshi et al. 68 also report that younger onset of disease was associated with longer disease duration for all types of CJD.
Genotype: codon 129
Methionine homozygosity at codon 129 (MM) is considered the most susceptible genotype for CJD, with sCJD and vCJD occurring mostly in individuals with the MM genotype. Both methionine (MM) and valine (VV) homozygotes at codon 129 of PRNP are at an increased risk of sCJD. 70 In the north of Europe, the MM genotype represents 38% of the general population, whereas 11% of the population have the VV genotype and 51% are heterozygotes (methionine/valine; MV) at codon 129 of PRNP. 71 An epidemiological study by Giaccone et al. 72 of the PRNP genotype of 402 consecutive sCJD cases in Italy revealed that 70.4% (n = 283) had the MM genotype, 15.4% (n = 62) were MV and 14.2% (n = 57) were VV. 72 Although the numbers of MV and VV sCJD cases appear comparable in this study, the fact that over half of the population in Europe are MV indicates that the relative incidence of sCJD in heterozygotes at codon 129 is low.
In 2006, Ironside et al. 73 re-analysed three of the appendixes identified (from the 12,674 appendix and tonsil samples analysed by Hilton et al. 7) as positive for disease-associated PrP; two of the three were found to be VV genotype, which provided the first indication that the valine homozygotes are also susceptible to vCJD infection. 73 The authors suggested that people infected with vCJD who are VV may have a prolonged incubation period with subclinical infection that could cause secondary infection via blood transfusion or surgery. Additionally, detection of subclinical prion accumulation in peripheral tissue by Gill et al. 74 from 16 positive appendix samples found that eight were MM, four were MV, and four were VV at codon 129 of PRNP, indicating that genetic susceptibility for subclinical CJD was more equally distributed in the population.
Heterozygosity at codon 129 of PRNP was generally believed to confer complete resistance to both sporadic and acquired prion diseases. 75 However, the most recent case of clinical vCJD in 2016 was heterozygous37 and an additional possible vCJD case reported by Kaski et al. 76 in 2008 was also heterozygous, but this possible vCJD case was not confirmed by autopsy. Two case reports indicate that the MV genotype is susceptible to iCJD, but the cases were subclinical. First, the case of a heterozygous 73-year-old male with haemophilia whose spleen at autopsy gave a strong positive result on repeated testing for protease-resistant prion protein (PrPres) by western blot analysis, as reported by Peden et al. 77 This patient had received over 9000 units of factor VIII concentrate prepared from plasma pools known to include donations from a vCJD-infected donor. Second, a case in 2004 of subclinical vCJD from blood transfusion, who was heterozygous at codon 129, and died from a cause unrelated to CJD78 highlights the possibility of potential transmission to this genotype. A study using mice supports the notion that transmission efficiency of vCJD is greatest in MM but indicates that all genotypes are susceptible, with the MV and VV genotypes benefiting from apparent reduced transmission efficiency and longer asymptomatic incubation periods. 79
Disease duration and genotype
Prion protein–gene data from 378 of the Japanese patients diagnosed with sCJD, reported by Nagoshi et al. ,68 showed that 364 cases (96.3%) had the MM genotype but that disease duration was longest for the 11 patients (2.9%) who were MV (mean, 32.2 months for MV vs. 16.6 months for MM and 13.2 months for VV). 68 Begué et al. 26 report data for the disease duration of sCJD from 59 definite cases in Argentina. Genotype analysis indicated that the MV genotype was associated with the longest disease duration (10.9 months), followed by the VV (5.6 months) and the MM genotypes (3.6 months). 26 Data from Rudge et al. 69 relating to CJD transmission via hGH in the UK also found that MM patients had the shortest disease duration; MM patients had a mean disease duration of 7.8 months, the VV patients 17 months and MV patients had a mean disease duration of 18.6 months (range 10–32 months). In addition, the duration of disease from first symptom was significantly longer in the MV patients (p = 0.02, two-tailed t-test). Although there were only four patients who were MM, three of these had the most rapid disease progression (p = 0.04, Mann–Whitney U-test). Yamada et al. 24 state that the majority of the general Japanese population (93%) carry the MM genotype. Considering that a large share of patients in the Argentinian sample also contained the MM genotype (n = 37, 66%), genotype data at codon 129 alone cannot account for the substantial difference in disease duration for sCJD reported between Japan and other countries.
Data from Japan,68 Argentina,26 and the UK69 therefore indicate that the MV genotype is associated with the longest disease duration compared with homozygotes. Pennington and Knight80 also reported disease duration to be significantly longer in codon 129 heterozygotes for gCJD.
Variant Creutzfeldt–Jakob disease
The annual number of confirmed cases of clinical vCJD has declined since 2005. As of 2016, the NCJDRSU recorded 178 cases of vCJD in the UK. 1 The most recent vCJD case occurred in an individual who was heterozygous at codon 129. 37 A further 52 cases have been reported from other countries around the world, which brings the global total of clinical vCJD cases to 231. 81 Between 2005 and 2014, 68 vCJD cases were reported from 11 countries including the UK (n = 29), France (n = 19), Spain (n = 5), Ireland (n = 3), the USA (n = 3), Holland (n = 3), Portugal (n = 2), Italy (n = 1), Canada (n = 1), Saudi Arabia (n = 1) and Taiwan (n = 1). 22 A total of 3 out of the 178 cases in the UK that occurred up to 2016 are considered to have occurred through blood transfusion. 2 A fourth case of vCJD transmission through blood transfusion was identified in the spleen of an individual (heterozygous at codon 129) who died of a non-CJD related cause. This is considered to be preclinical vCJD. 78 Three further potential, but unconfirmed, cases of CJD transmission through blood transfusion are described by Chohan et al. 82 and Davidson et al. 83 A retrospective study by Molesworth et al.,84 which was performed to identify situations where the transplantation of organs or tissues might have occurred in any of the 177 UK vCJD cases, found no evidence of transplant-associated vCJD in the UK. 84 The remaining 175 clinical vCJD cases are presumed to be related to dietary exposure to BSE. 85
Iatrogenic Creutzfeldt–Jakob disease
The most common causes of iCJD were hGH and dura mater grafts obtained from human cadavers. A review of worldwide iCJD cases published by Brown et al. 3 identified 469 cases from dura mater grafts (n = 228), surgical instruments (n = 4), EEG needles (n = 2), corneal transplants (n = 2), hGH (n = 226), hGN (n = 4) and packed red blood cells (n = 3). 3
In the UK, 85 cases of iCJD were identified between 1970 and December 2016, and are described by the NCJDRSU. 1 In total, eight cases were from dura mater grafts, 76 from hGH and one from hGN. All cases have since died, with a mean age at death for the hGH/hGN group of 35 years (range 20–51 years) and for the dura mater cases 46.5 years (range 27–78 years).
Subsequent to the three cases of blood transfusion transmitted vCJD described above, no new cases of transfusion-associated infection have been identified since 2007, based on an epidemiological analysis of CJD cases and blood transfusion recipients by Urwin et al. 86 The Urwin et al. 86 study referenced the Davidson et al. 83 paper but not the Chohan et al. 82 paper. These two papers discuss three potential, but unconfirmed, cases of CJD transmission via blood transfusion. Ward et al. 87 studied the risks in treatment for haemophilia and concluded that it is unlikely that any of the UK vCJD clinical cases to date were infected through exposure to fractionated plasma products. 87 The evidence regarding the incidence of iCJD from surgery is discussed in the review on the risk of CJD transmission via surgery.
The estimated prevalence of subclinical variant Creutzfeldt–Jakob disease in the UK
In vCJD, prions appear to replicate extensively within lymphoid tissue; therefore, tonsil and appendix tissues are some of the earliest sites that can be used to assess abnormal prion accumulation. Such abnormal prion accumulation prior to the onset of clinical symptoms is regarded as subclinical CJD for the purposes of risk assessment and is thought to represent a potentially background, but low, level of infection in the population. 88 Immunohistochemistry staining is regarded as highly indicative of the abnormal prion protein pattern that has been observed in cases of vCJD, but not observed in other types of CJD, and is used to estimate the approximate number of individuals who may go on to develop vCJD or be asymptomatic carriers of the disease. 89
A key study conducted by Gill et al. ,74 referred to as the ‘Appendix II’ study, examined subclinical prion accumulation in excised peripheral tissues from general population cohorts born in 1941–60 and 1961–85. 74 Detection of abnormal prion accumulation in appendix samples from these two cohorts resulted in a central estimation of 1 in 2000 for populations exposed to the BSE epidemic. The Advisory Committee on Dangerous Pathogens (ACDP) TSE subgroup produced a summary of findings16 following completion of the most recent study of stored appendixes (‘Appendix III’) and calculated a rough central prevalence estimate of asymptomatic carriers of vCJD in the UK population, previously presumed unexposed to BSE, of approximately 1 in 4200 people or 240 per million people. 15,16 This estimate is based on results of immunohistochemical (IHC) staining of appendixes from two birth cohorts, which are described in Table 3.
| Appendix III cohort | IHC stain results | Central estimate |
|---|---|---|
| Appendixes removed between 1970 and 1979 and before the BSE epidemic | Two positive samples from 14,692 appendixes | 1 in 7000 |
| Appendixes removed from patients born after 1 January 1996 and after measures to remove BSE were in place | Five positive samples from 14,824 appendixes | 1 in 3000 |
Variant Creutzfeldt–Jakob disease and bovine spongiform encephalopathy
The hypothesis of zoonotic transmission through dietary exposure from the BSE outbreak is largely upheld as the most plausible route of vCJD infection in humans, and transmission has been replicated in wild-type mice. 90 Moreover, a recent study by Diack et al. 91 examined two Spanish cases of vCJD: a mother and son who resided in a BSE-endemic area, who are thought to have ingested bovine brain. 91 The strain characteristics of both individuals are similar to the UK cases, implying BSE as the source of infection and supporting the hypothesis of risk via ingestion of high-titre bovine material.
The Appendix III study75,76 highlights that abnormally stained appendixes associated with vCJD prion accumulation have been confirmed in cohorts of people who were not considered to have had significant exposure to BSE because they were either from appendixes removed before the BSE epidemic in the UK (prior to 1980) or from appendixes from patients born after food safety measures to limit BSE were implemented (after 1996). The presence of seven positive samples in these cohorts could suggest that there is low background prevalence of abnormal prion protein staining in human lymphoid tissue that may not represent subclinical vCJD or be related to the BSE outbreak, and may be unlikely to progress to vCJD. Another possible interpretation is that the duration of the BSE epidemic and subsequent ingestion by humans through the food chain was longer than the presumed duration of human exposure to the BSE epidemic (between 1980 and1996). Moreover, planned statistical analysis, as described by Gill et al. ,74 found no difference between the prevalence observed in the cohort considered to be most at risk of the BSE epidemic (people born 1961–85) and an older cohort (born 1941–60).
These two possible explanations are considered by the ACDP TSE subgroup as not necessarily being mutually exclusive nor fully satisfactory.
Previous estimates of prevalence of abnormal prion in humans
Primary studies (published after 2005) that provide estimates for subclinical CJD in the general population based on analysis of peripheral tissue are described in Table 4. Central estimates range between 0 and 493 per million people in the population. Studies providing evidence of the prevalence of vCJD prions in lymphoid tissues published prior to 2005 are described in a review published by Olsen et al. 94 This review includes the cross-sectional study by Hilton et al. 7 that estimated the prevalence in the sample population to be 120 per million from 11,228 appendixes.
| Study (first author and year of publication) | Design | Number of samples | Predicted/estimated prevalence | Description of estimation |
|---|---|---|---|---|
| Gill et al. (2013)74 | UK histological analysis of appendix samples from the 1941–60 and 1961–85 birth cohorts | 32,441 |
|
|
| de Marco (2010)92 | Two estimations based on UK tonsil tissue samples from the 1961–85 birth cohort | 10,075 |
|
|
| Clewley (2009)93 | UK estimation combining tonsil tissue samples | 63,007 (32,661 from the 1961–95 cohorts) |
|
|
Obtaining definitive prevalence estimations
Subclinical vCJD can be detected through typical PrP staining in lymphoid tissue or through observation of the presence of florid plaques in the brain at autopsy; however, systematic lymphoid or neuropathological examination is not performed routinely in post-mortems. To collect a truly accurate picture of the prevalence of CJD through abnormal prion protein in humans, the UK Health Protection Agency proposed the creation of a post-mortem tissue archive. 95 The study required tissue from a large number of post-mortems and the participation of coroners in England and Wales. However, the Coroners’ Society of England and Wales (CSEW) declined to participate in the study, citing various issues including its putative legality, cost and feasibility. 96 The CSEW concluded that to participate in the study would ‘adversely affect the independence of the coronial service and would further erode public confidence’. 97 McGowan and Viens95 describe that as death investigation systems with substantial independence are not directly answerable to central government, they cannot be instructed to participate in any disease surveillance programme, regardless of how crucial it is to the protection of human health and safety.
Discussion of the incidence and prevalence of Creutzfeldt–Jakob disease
The incidence of CJD is relatively stable around the world (between 1 and 2 cases per million people) but age-adjusted detection of sCJD is increasing in the UK as well as in other countries. Reasons posited for this increase include improved case ascertainment and an ageing population. The estimated prevalence of subclinical vCJD from lymphoid tissues of people in the UK who were exposed to the BSE epidemic was 1 in 2000 people and the estimated prevalence of CJD-related prions in lymphoid tissues in the UK population who are not thought to be exposed to the BSE epidemic was 1 in 4200 people. This suggests a potentially constant underlying rate of abnormal prion accumulation in lymphoreticular tissue in the UK population, which may or may not represent disease that will progress to clinical CJD. Estimations of prevalence are currently limited to retrospective cohort studies of anonymised tonsil or appendix samples.
The risk of Creutzfeldt–Jakob disease transmission via surgery
The literature searches retrieved no further published papers from the period 2005 to 2017 reporting confirmed cases of stCJD, further to the four neurosurgical cases which occurred between 1952 and 1974. 3 These four historical cases (three in the UK and one in France) are distinct from the known dura mater and hGH iCJD cohorts, and occurred prior to the vCJD epidemic that began in the late 1980s. The four historical surgical cases, therefore, represent a small proportion of the known iCJD cases (469 iCJD cases according to Brown et al. 3) and occurred when methods for cleaning surgical instruments were not adequate assuming current decontamination standards. Consequently, the risk of CJD transmission via surgery according to recent direct evidence appears to be low. However, the long asymptomatic incubation periods noted in some cases of CJD, the difficulties of eradicating prions from neurosurgical instruments (especially once adhered to dry instruments), the high levels of infectivity of CJD in the brain and a presumed subclinical underlying prevalence (albeit low) in the general population mean that there is a margin of uncertainty around detecting and quantifying the risk of CJD transmission via surgery.
Observational studies implicating surgery in Creutzfeldt–Jakob disease
Despite the absence of studies providing direct evidence of further cases of stCJD, a number of papers were identified which allude to a potential relationship between CJD cases and prior surgery. Papers that investigate but do not provide evidence of a direct link to surgery are listed in Table 5.
| Study (first author and year(s) of publication) | Design | Source |
|---|---|---|
| Kobayashi (2015 and 2016)98–100 | Two historical sCJD cases with neuropathological and biochemical features of plaque-type dura mater-acquired-CJD. The authors posit that these cases (a neurosurgeon and a patient with a medical history of neurosurgery without dura mater grafting) represent iCJD through cross-contamination from neurosurgical instruments or through occupational exposure as a neurosurgeon | Two published papers and a conference abstract |
| Gnanajothy (2013)101 | Case report of 64-year-old man diagnosed with CJD (type of CJD not reported) 3 months after cataract surgery. The authors discuss the possibility that the visual symptoms that prompted the surgery might have represented onset of the disease rather than it being the case that the procedure itself transmitted the disease (i.e. the patient already had CJD) | Published paper |
| Tuck (2013)102 | Case report of sCJD that was posited to be iCJD via surgery because of the patient’s young age. At 33 years of age, the patient experienced progressive deficits over 3 months. Review of medical history revealed that a ventriculoperitoneal shunt was placed at 11 years of age for hydrocephalus. Autopsy results were consistent with sCJD | Conference abstract |
| Moreno (2013)103 | A surveillance study in Meixoeiro Hospital (Spain) reported 12 cases of CJD (10 sCJD and 2 gCJD) from 1997 to 2010, which represented a high average yearly rate of 4.6 per million people (3.8 for sCJD and 0.8 for gCJD). According to the Poisson distribution for the 12 cases (with an expected annual incidence of 1.5 cases per million people), only 3.9 cases would have been expected over a 14-year period. A total of 8 out of 12 CJD cases had undergone at least one surgical or invasive medical procedure | Published paper |
| Puopolo (2011)104 | A case–control study found that ‘history of surgery’ was more frequent in sCJD cases (n = 13, 2%; neurosurgery, n = 12; cornea transplantation, n = 1) vs. no-CJD cases (n = 5, 1%; neurosurgery n = 5) and none in genetic TSE patients. A crude OR of 1.57 (95% CI 1.14 to 2.16) was reported. Results did not reach statistical significance when adjusted for a 10-year time lag | Published paper (included in de Pedro-Cuesta et al.112) |
|
de Pedro-Cuesta (2011)105 Mahillo-Fernandez (2008)106 |
A case–control study of sCJD to look for risk factors from 167 sCJD cases in Denmark and Sweden. Surgery for ‘lower risk procedures’ (i.e. surgery to veins, peritoneal cavity and lymph nodes) compared with high-risk procedures (i.e. surgery to brain, spinal cord, retina and optic nerve) carried out > 20 years before disease onset was associated with an increased risk of sCJD (OR 2.81, 95% CI 1.62 to 4.88). When tissues or structures were reclassified by hypothetical transmission risk at a latency of ≥ 1 year, surgery to the retina and optic nerve were the most strongly associated risk factors (OR 5.53, 95% CI 1.08 to 28.0) | Two published papers (included in de Pedro-Cuesta et al.112) |
| Hamaguchi (2009)107,108 | A case–control study in Japan with 753 sCJD patients and 210 controls. Surgery was not a risk factor for sCJD prior to disease onset. However, 4.5% of sCJD patients underwent surgery after onset of sCJD, including neurosurgery in 0.8% and ophthalmic surgery in 1.9% of patients. Among the neurosurgery cases, the symptoms of sCJD were misdiagnosed as those of other neurological diseases, and the surgeries were performed near disease onset. The authors concluded that, despite absence of empirical evidence of transmission via surgery, the risk of contracting CJD via surgery is still present because patients are operated on after disease onset | Two published papers (included in de Pedro-Cuesta et al.112) |
| Ruegger (2009)109 | A case–control study in Switzerland found that 69 sCJD patients, compared with 224 controls, were more likely (p < 0.05) to have travelled abroad, worked at an animal laboratory, undergone invasive dental treatment, had orthopaedic surgery, had ophthalmologic surgery after 1980, attended regular GP visits, taken medication regularly, and consumed kidney. No differences between patients and controls were found for residency, family history, and exposure to environmental and other dietary factors. Other types of surgery were not found to be a possible factor. Previous under-reporting/misdiagnosis was proposed as the most likely explanation for the increased annual mortality | Published paper (included in de Pedro-Cuesta et al.112) |
| Ward (2006)110 | Case–control study of 136 vCJD patients and 922 controls. Investigation of risk factors in the UK identified dietary exposure to contaminated beef products as the main route of infection of vCJD with no convincing evidence of increased risk through medical, surgical, or occupational exposure or exposure to animals | Published paper (included in de Pedro-Cuesta et al.112) |
| Ward (2008)111 | A case–control study in the UK of 431 sCJD patients and 454 controls, found increased risk was not associated with surgical categories chosen a priori but appeared most marked for ‘other surgery’, especially the three subcategories: (1) skin stitches, (2) nose/throat operations and (3) removal of growths/cysts/moles. No convincing evidence was found of links between cases undergoing neurosurgery or gynaecological surgery | Published paper (included in de Pedro-Cuesta et al.112) |
Issues of reliability and validity in case–control studies
Because sCJD is idiopathic, its aetiological basis is presumed to be spontaneous but this is not known with any certainty. 89 Therefore, case–control studies are a frequently encountered design in estimating possible and plausible risk factors for sCJD. de Pedro-Cuesta et al. 112 caution about the potential biases in these study designs in an assessment of 18 case–control studies of CJD. From a combined analysis of studies, the authors found that history of surgery or blood transfusion was associated with a risk of sCJD in some, but not all, recent studies using a 10-year or longer lag time, when controls were longitudinally sampled. Furthermore, they found that none of surgical history, blood transfusion, dental treatments or endoscopic examinations was linked to vCJD. However, the authors highlight that the validity of the findings in these case–control studies may be undermined by (1) the selection of control cases; (2) exposure assessment in lifetime periods of different durations; (3) disregarding ‘at-risk’ periods for exposure in the controls, or asymmetry between the case and control data; and (4) confounding by concomitant blood transfusion at the time of surgery. They also postulate that surgery at early clinical onset might be over-represented among cases.
As a retrospective study design, case–control studies are prone to bias. The source of cases and the selection of control (matched or unmatched) cannot be performed blindly or impartially; therefore, there is a high risk of selection bias on the researcher’s part. Owing to long incubation periods and the reliance on family members’ reports of medical histories, there is also substantial likelihood of recall bias. Case–control designs are also less useful when the study exposures are rare, as in the case of surgery or blood transfusion. Therefore, the utility of these studies in attempting to fairly estimate risk factors is limited. However, as CJD is rare, fatal and has a potentially long latency period, there are few plausible alternative study designs to establish potential lifetime risk factors in humans. Therefore, the use of community controls and ascertainment of surgical exposures through the use of medical records in case–control designs is currently the most feasible approach for identifying the potential association between surgery and CJD at a population level.
Risk of Creutzfeldt–Jakob disease through occupational exposure for health-care professionals
In 2009, the Spanish CJD registry was notified of a case of sCJD in an experienced general pathologist/neuropathologist, which prompted investigation into the possible risks to health-care professionals in contact with CJD patients. 113 As a result, Alcade-Cabero et al. 113 reported the data requested from the EuroCJD surveillance network, which documented 65 physicians or dentists (including two pathologists) and 137 health-care workers from 8321 registered sCJD cases from 21 countries. Control data, which used ‘non-cases’ from five countries, recorded 15 physicians and 68 other health-care professionals among 2968 controls or non-cases, and suggested that there was no relative excess of sCJD among health-care professionals. The study authors also performed a literature review examining reports (n = 12) pertaining to 66 health-care professionals with sCJD, and analytical studies on health-related occupations and sCJD (n = 5). From a range of occupations, only people working at physicians’ offices were found to be at a statistically significant risk of sCJD [odds ratio (OR 4.6, 95% CI 1.2 to 17.6)]. The authors concluded that a wide spectrum of medical specialties and health-care professions are represented in sCJD cases and that there is no evidence of an increased occupational risk for health-care professionals. The authors do caution that there may be a specific risk in some professions associated with direct contact with high human-infectivity tissue. The NCJDRSU continue to monitor occupational exposure to CJD in health-care professionals.
Risk of Creutzfeldt–Jakob disease in surgery and age
de Pedro-Cuesta et al. 114 performed a retrospective analysis of 167 cases of sCJD between 1987 and 2003. From a study of 167 probable or definite CJD cases and 835 matched controls, the authors suggest that a younger age at first surgery may increase the risks of acquiring sCJD: patients aged < 30 years (OR 12.80, 95% CI 2.56 to 64.00), patients aged 30–39 years (OR 3.04, 95% CI 1.26 to 7.33) and patients aged ≥ 40 years (OR 1.75, 95% CI 0.89 to 3.45), for anatomically classified surgical procedures. As highlighted by the same authors in a different study,112 caution should be urged when interpreting conclusions from analyses on indirect evidence in retrospective samples. Additionally, the ≥ 40-year age group contains those who are elderly and may die before clinical symptoms appear or may remain undiagnosed.
Risk of iCJD transmission through surgery can potentially occur when patients are unwittingly treated in hospital at the time of symptom onset. Cruz et al. 115 used a cross-sectional design to study surgical procedures in sCJD patients and controls to estimate subclinical and clinical risks to future surgery. The authors posit that patients with sCJD in the clinical stage undergo a considerably higher frequency of surgical procedures than non-CJD patients, including neurosurgery. The authors argue that identification of such potentially higher-risk events, where surgery is undertaken in infectious patients around the onset of clinical symptoms, but prior to CJD diagnosis, might well constitute a priority in clinical settings. A conference abstract by Kobayashi116 reinforces this concern by providing data from the Japanese CJD Surveillance registry. From an analysis of 760 CJD patients, Kobayashi116 identify that six patients had undergone neurosurgery after the onset but before the diagnosis of CJD during the period from 1999 to 2008. 116
Cases of suspected but unconfirmed Creutzfeldt–Jakob disease transmission via neurosurgery
Patients may be identified as being ‘at increased risk’ of CJD if they have had surgery using instruments that had been used on someone who went on to develop CJD or someone who was ‘at increased risk’ of CJD. 117 A study by Hall et al. 118 reports that 154 patients in the UK are considered to be ‘at increased risk’ of various forms of CJD following neurosurgery. This paper reports that of these 154 patients, only 129 have been informed that they are at an increased risk of CJD, either because of deaths before notification or because a local decision was taken not to inform the individual. Although no incidence of CJD has been reported within these 154 patients, the authors highlight that ‘at-increased-risk’ patients often have a relatively short life expectancy because of their medical conditions. Diagnosing asymptomatic infection requires testing specific tissues that are most readily available at post-mortem. Few post-mortems have been conducted when at-increased-risk individuals have died; therefore, some asymptomatic infections may have been missed.
Two published papers119,120 from the USA report instances in which potential iCJD exposure via neurosurgery was investigated in hospitals; however, no confirmed cases of transmission were subsequently identified.
Risks in surgery other than neurosurgery
Prospective risks from surgery
A study by Baig and Phillips40 describes a case report of a male patient (aged 66 years) who had surgical fixation of a hip fracture, most probably around the onset of CJD symptoms; therefore, given the lack of symptoms, the standard sterilisation method was appropriately used. The authors highlight that this standard decontamination method is typically not adequate for the eradication of the CJD prion protein, thus presenting a theoretical risk of prion protein transmission through surgical equipment. The focus of this paper is not on the implication that the patient contracted iCJD via surgical transmission but instead highlights a circumstance where subsequent iatrogenic transmission may have occurred because of a lack of high-risk decontamination procedures. However, surgery of low infective tissues in individuals diagnosed with CJD is noted to be common and,110,111 therefore, surgery that did not involve high (or medium) infectivity tissues would not be regarded as a risk of iatrogenic transmission.
A recent study by Orrú et al. 121 found infectivity in the skin of sCJD patients, albeit at prion levels 1000–100,000 times lower than that in the brain and detectable only by an extremely sensitive assay. 121,122 However, a study using humanised transgenic mouse models demonstrated that the skin prions were infectious. The study authors argue that extra precautions should be taken during non-neurosurgeries in sCJD patients, particularly when instruments will be re-used, because infectivity through skin was previously unknown.
A study by Notari et al. 123 found from a neuropathological examination of a vCJD case in the USA that as well as detection of PrPres in the brain, lymphoreticular system, pituitary and adrenal glands, and gastrointestinal tract, PrPres was also detected in the dura mater, liver, pancreas, kidney, ovary, uterus and skin. 123 The authors concluded that the number of organs affected in vCJD is greater than previously realised, and this further underscores the risk of iatrogenic transmission in vCJD.
Risks in eye surgery
Davanipour et al. 124 postulate that ocular tonometry is a risk factor for contracting sCJD from a case–control study conducted across 11 states in the USA. Contact tonometry is used by ophthalmologists to diagnose glaucoma. The authors conclude that disposable covers or non-contact tonometry should be used in the absence of adequate decontamination processes. 124
Tullo et al. 125 document that there were three recipients of either cornea or sclera from a woman who died of biopsy-proven carcinoma of the bronchus in 1997, but was later neuropathologically identified as having sCJD. 125 At the time of publication, two recipients remained symptom-free of CJD, whereas one patient had died, aged 92 years (7 years after surgery), showing some signs of dementia that were not considered indicative of iCJD.
Jirsova et al. 126 conducted an analysis of brain tissue samples from the frontal lobe of 1142 eye donors obtained from three tissue banks in the Czech Republic. As no pathogenic prions were found, the authors presume a very low risk of transmission of CJD through corneal graft transplantation. However, the authors’ conclusion can be regarded as a logical fallacy, denying the antecedent, because in the absence of sCJD cases in the analysis it is not possible to conclude on the risk of CJD transmission via surgery in corneal graft transplantation. Additionally, Maddox et al. 127 used data from corneal transplantation and CJD deaths from 1990 to 2006 in a statistical analysis, to suggest that a case of coincidental sCJD will occur among the population of corneal transplant recipients approximately every 1.5 years. 127
Risks in dentistry
Bourvis et al. 128 conducted a risk assessment of the transmission of sCJD in endodontic treatment in the absence of adequate prion inactivation. The authors developed a mathematical model, which incorporated experimental and observational data and expert consultation. They estimated that without effective prion deactivation procedures, the risk of being infected during endodontic treatment ranged between 3.4 and 13 per million procedures. The authors consider that strict respect of the official recommendations on decontamination procedures is essential in dentistry, and even suggest that the cost–benefit of single-use endodontic instruments should be re-evaluated. Everington et al. 129 found no evidence of an increased risk of vCJD associated with reported dental treatments in a case–control study of UK vCJD patients. 129 However, the authors do not rule out the possibility that some cases may have resulted from secondary transmission via dental procedures. Azarpazhooh and Fillery130 highlight that, although no definite cases of prion disease transmission have been reported, the theoretical risk from dental instruments is low but real and, as a general rule, appropriate family and medical history (including the risk for prion diseases) should be obtained from all patients before dental procedures. 130
Discussion/summary of risk of Creutzfeldt–Jakob disease transmission via surgery
Although no studies have identified a new case of stCJD in the search period covered, many speculative case–control reports of the relationship have been conducted. These analyses provide indirect retrospective evidence implicating neurosurgery or surgery as a risk factor for CJD, but their design is known to be at risk of bias and confounding. However, as CJD is rare, fatal and has a potentially extended incubation period, there are few plausible alternative study designs to establish potential lifetime risk factors for CJD in humans.
Indirect evidence points to other factors being relevant to CJD and surgery, including younger age at first exposure, increased risk of surgery around the time of symptom onset, the risk to health-care professionals, and the risk from procedures where high-risk decontamination measures are not in place. Although less relevant to the decision problem, clinical studies have recently demonstrated low levels of CJD infectivity in skin. When considering vCJD, surgical procedures (other than high-risk procedures) that could potentially be regarded as posing a risk of iatrogenic transmission include appendectomy and tonsillectomy. However, no direct evidence exits to highlight a serious risk from surgical procedures involving tissues that are not high risk.
Incubation periods of acquired transmissible spongiform encephalopathys
The purpose of this review was to identify published and unpublished evidence for the incubation periods of acquired TSEs, especially CJD, in human populations. Evidence on incubation periods has implications for determining the risk of transmission from surgical procedures. Eighteen full-text papers were identified as relevant.
Studies of incubation periods
Studies relating to incubation periods of acquired TSEs are described in Table 6.
| Study (first author and year of publication) | Design | Population | Source of infection | Location | Number of cases |
|---|---|---|---|---|---|
| Ae (2016)35 | Epidemiological surveillance | iCJD | Dura mater graft | Japan | 149 |
| Brown (2012)3 | Epidemiological surveillance | iCJD | All | International | 469 |
| Brown (2015)6 | Review | iCJD | Neurosurgery | International | 6 |
| Chohan (2010)82 | Case study | iCJD | Blood products | UK | 1 |
| Collinge (2008)131 | Epidemiological surveillance, cohort | Kuru | Ingestion | Papua New Guinea | 11 |
| Collinge (2008)132 | Review | Kuru | Ingestion | Papua New Guinea | NA |
| Collinge (2006)133 | Epidemiological surveillance, cohort | Kuru | Ingestion | Papua New Guinea | 11 |
| Collinge (2006)134 | Letter: reply regarding Collinge et al.134 | Kuru | Ingestion | Papua New Guinea | NA |
| Davidson (2014)83 | Retrospective cohort | iCJD | Blood products | UK | 9 |
| Haïk (2014)135 | Review | CJD | All | International | NA |
| Hamaguchi (2013)136 | Epidemiological surveillance | iCJD | Dura mater graft | Japan and international | 195 |
| Heath (2006)137 | Epidemiological surveillance | iCJD | Dura mater graft | UK | 8 |
| Hirst (2005)138 | Epidemiological surveillance | iCJD | hGH | UK | 1 |
| Peden (2010)77 | Case study | iCJD | Blood products | UK | 3 |
| Meissner (2009)139 | Retrospective cohort | iCJD | Dura mater graft | Germany | 10 |
| Ritchie (2017)140 | Epidemiological surveillance, retrospective cohort | iCJD | hGH, dura mater graft | UK | 37 |
| Rudge (2015)69 | Epidemiological surveillance, cohort | iCJD | hGH | UK | 22 |
| Wroe (2006)141 | Case study | iCJD | Blood products | UK | 1 |
The diagnosis of definite or probable iCJD depends on identification of the likely source of contamination to which patients have been exposed, as well as fulfilling the basic requirements for the definite or probable diagnosis of CJD. Wherever possible, only the most recent and/or up-to-date data are presented in Table 7, unless there is potential value in comparisons with data from earlier samples or earlier publications which provide relevant details that are not reproduced in the more recent papers.
| Source of infection | Number of cases (n) | Incubation periods for overall data (years) | Studies/reports (first author and year of publication) | ||
|---|---|---|---|---|---|
| Overall | UK | Mean | Range | ||
| Primary transmission | |||||
| vCJD from ingestion/BSE | 229 | 175a | 12 | – | Haik (2014);135 NCJDRSU (2017)2 |
| Kuru | – | 0 | 12b | 4–> 40 | Haik (2014);135 Collinge (2006);133 Ritchie (2017)140 |
| Secondary transmission | |||||
| Dura mater graft | 228 | 8 | 12 | 1.3–30 | Brown (2012);3 Haik (2014)135 |
| Neurosurgical instruments | 4c | 3 | 1.4 | 1–2.3 | Brown (2015);6 Brown (2012);3 Haik (2014)135 |
| EEG needles | 2 | 0 | – | 1.3–1.7 | Brown (2015);6 Brown (2012);3 Haik (2014)135 |
| Corneal transplant | 2 | 0 | – | 1.5–27 | Brown (2012);3 Haik (2014)135 |
| Growth hormone | 226 | 78 | 17 | 5–42d | Ritchie (2017)140 |
| 21 | 20 | 8–31 | Ritchie (2017)140 – online table 1 | ||
| Gonadotrophin | 4 | 0 | 13.5 | 12–16 | Brown (2012)3 |
| Packed red blood cells | 3 | 3 | – | 6.5–8.3e | Brown (2012);3 Haik (2014)135 |
| 2 | 2 | Wroe (2006);141 Peden (2004);78 Ironside (2010);146 Chohan (2010)82 | |||
| Total (secondary transmission) | 471 | 81 | |||
UK data on incubation
A summary of data on incubation periods for iCJD in the UK is presented in Table 8.
| Source of infection | Number of cases (n) | Mean incubation period (years) | Range (years) | Studies/reports (first author and year of publication) |
|---|---|---|---|---|
| Dura mater graft 1990–2012a | 8b | 7.8c | 3.8–14.8c | Heath (2006)137 |
| 3 | 11d | 8–15 | dRitchie (2017)140 | |
| Neurosurgical instruments | 3 | 1.4 | 1.3–1.6 | Brown (2015)6 |
| EEG needles | 0 | NA | NA | – |
| Corneal transplant | 0 | NA | NA | – |
| Growth hormone 1990–2012a | 78 | NR | NR | Ritchie (2017)140 |
| 65 | 20 | 7–39 | Brown (2012)3 | |
| 21 | 19 | 11–31 | dRitchie (2017)140 | |
| Gonadotrophin | 0 | NA | NA | – |
| Packed red blood cells | 3 | – | 6.5–8.3 | Brown (2012)3 |
| Haik (2014)135 |
Dura mater grafts and genotypes
Frequency data and mean incubation periods by genotype are presented in Table 8 for the UK subset of CJD cases that are known to be caused by dura mater. The worldwide number of iCJD cases due to dura mater surgery exceeds 200, and it is known that the majority have occurred in Japan (n = 149). 35 Frequency data and mean incubation periods by genotype, extracted from Brown et al.,3 are also presented in Table 9 for subsets of the worldwide (excluding Japan) and Japanese affected populations. Hamaguchi et al. 147 reported a mean incubation period of 12.1 years (range 1–30 years) for 142 patients in Japan and 11.3 years (range 1–23 years) for a subset of 53 patients with published data from the other countries, although this subset did not include a Dutch case of dura mater-related iCJD that had an incubation period of 28 years, which is the longest reported incubation period outside Japan. 148
| Location | Number of cases (n) | Genotype | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Homozygotes | Heterozygotes | ||||||||
| MM | VV | Total | MV | ||||||
| Frequency (%) | Incubation period (years) | Frequency (%) | Incubation period (years) | Frequency (%) | Incubation period (years) | Frequency (%) | Incubation period (years) | ||
| Not Japan3,136 | 54a | 65 | 12 | 15 | 12 | 80a | 12 | 20 | 16 |
| bJapan3,136 | 54c | 96 | 16 | 0 | – | 96 | 16 | 4 | 13 |
Meissner et al. 139 reported on a sample of 10 cases (nine from Germany and one from Croatia) of iCJD related to dura mater, which were identified between 1993 and 2006. The median incubation period was 18 years (range 9 to 23 years), with 90% of cases being homozygotes, the majority of which were the MM genotype (80%). This study also reviewed published evidence from the literature on 27 international patients with iCJD because of dura mater grafts who had MRI data. Data on incubation periods were available for 22 of these patients: the mean incubation period was 11.5 years (range 1.6–23 years), with 95% being homozygotes, mainly of the MM genotype (81%).
Human growth hormone and genotypes
Table 10 presents frequency data and mean incubation periods by genotype for a subset of the known CJD cases caused by hGH. It is reported that one particular preparation of hGH was most probably responsible for cases of iCJD caused by hGH in the UK, to date. 3,69
| Location | Cases with genotype data/total known cases | Genotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MM | MV | VV | Homozygotes | Heterozygotes | Study (first author and year of publication) | ||||||
| Frequency, n (%) | Incubation period (years) | Frequency, n (%) | Incubation period (years) | Frequency, n (%) | Incubation period (years) | Frequency | Incubation period (years) | Incubation period (years) | |||
| UK | 21/78 (1990–2012)a | 2 (10) | 30 | 12 (57) | 18.5 | 7 (33) | 15.7 | 43% | 19 | 19 years | bRitchie (2017)140 |
| UK | 37 | 10% | 68% | 22% | 32% | Ritchie (2017)140 | |||||
| UK | 56/65 | 8 (14) | 30.8 | 33 (59) | 23.4 | 15 (27) | 14.3 | 41% | 23 | Rudge (2015)69 | |
| UK | 22 (2000–2014) | 4 (18) | 31.8 | 17 (77) | 28.6 | 1 (5) | 20.6 | 23% | 29 | Rudge (2015)69 | |
| UK | 28 | 1 (4) | 21 | 13 (50) | 23 | 14 (46) | 18 | 54% | 20 | 23 | Brown (2012)3 |
| France | 111/119 | 54% | 12 | 15% | 9 | 69% | 11 | 17 | Brown (2012),3 Haik (2014)135 | ||
| USA | 11/29 | 55% | 21 | 18% | 18 | 73% | 20 | 23 | Brown (2012)3 | ||
As reported in Table 7, 78 cases had been reported in the literature for the UK as of 2017,140 an increase from the 65 cases reported previously. 69 Ritchie et al. 140 present data on subsets of 21 and 37 patients with available tissue samples; analysis was conducted on frozen tissue samples for the former group. Both samples demonstrated the same pattern: patients with the MM genotype were fewer and had consistently longer incubation periods, whereas the VV genotype had the shortest mean incubation period. The heterozygous genotype MV was the most frequently identified genotype in both subsets. There is potential for some crossover of data between the included studies, which cannot be accounted for in this review.
These findings were broadly similar to those reported for another subset analysis of iCJD patients using imaging, molecular and autopsy data. 69 Rudge et al. 69 present data on a subset of 22 patients from 56 patients with genotypic data available. They studied a cohort of 22 patients diagnosed between 2000 and 2014, and combined relevant data from these patients with data for 34 published cases up to 2000. In the cohort of 22 patients, Rudge et al. 69 presented a range of possible incubation times calculated from (1) the last injection of any type of growth hormone to onset of symptoms; (2) the midpoint of that series of injections to onset of symptoms; and (3) the first injection to onset of symptoms. The mean and ranges were (1) mean 25.9 years (range 18.3–33.6 years); (2) mean 29.3 years (range 20.6–37.6 years); and (3) mean 32.8 years (range 23.2–43.3 years). 69 Incubation times between the cohort of 22 patients and a large subset of the UK group as whole were also compared. 69 The mean incubation times were longer in the latter cohort, but there was a noteworthy change in the proportion of MM and VV homozygote genotypes between the two periods; there was only one case of the VV genotype in the period 2000–14 compared with 14 occurring before 1998, whereas seven of the eight MM homozygotes occurred after 2004. 69 These findings are quite distinct from those for dura mater grafts in terms of the distribution of genotypes and their incubation periods: incubation periods for iCJD caused by dura mater grafts appear shorter and are dominated by the MM genotype (see Table 9). The group with iCJD affected by hGH are equally distinct in terms of genotype, from individuals with sCJD. 140
Discussion/summary of incubation data
The incubation periods for CJD reported in the published literature range from 1 to 42 years, with the shortest durations occurring in stCJD and the longest durations occurring in kuru or iCJD via hGH. Different incubation times might occur because of the resistance of different genotypes. Evidence from kuru studies132,133 indicates that incubation times are shorter and mortality risk is significantly greater in those with the homozygous genotype (MM or VV) compared with the heterozygous genotype (MV), which has longer incubation times; older survivors are, therefore, more likely to be MV. 131,134 However, the hGH data suggest that this might not always be the case, given the longer incubation times for MM homozygote patients and shorter times for VV homozygotes.
Where the proportions of heterozygotes and homozygotes are similar across countries or groups but the incubation times are different, it has been proposed that differences in these incubation times might be because of infection with different strains or subtypes of the CJD agent. 135,140 For example, most cases of hGH iCJD in France were of the MM genotype, whereas in the UK the VV and MV genotypes predominated. Infections that appear to affect certain genotypes in a particular location may reflect an absence of genotypic resistance to a particular strain and, thus, have shorter incubation times. Hence, it is believed that the MM genotype in the UK hGH iCJD cohort had the longest incubation times because the infectious strain was of the VV or MV genotype. Other possible factors include higher infectious doses and/or differences in the actual data, for example where the precise date of likely contamination is known, incubation times appear to be shorter. 3
Diagnoses of sCJD could potentially be made that are actually iatrogenic in origin. Correct identification can be difficult if cases of iCJD initially present as sCJD. Ritchie et al. 140 and Kobayashi et al. 144 report that some of the MM1 genotype present as sCJD, but others might be able to be distinguished as iCJD based on the presence of kuru plaques: this has been demonstrated for hGH in the UK. 140 Consequently, there might be more evidence forthcoming on incubation of iCJD as more cases are identified that previously were considered to be sCJD, but revised to iCJD following neuropathological examination.
The infectivity of Creutzfeldt–Jakob disease
The purpose of this review was to identify relevant published and unpublished evidence on the infectiousness of CJD in terms of CJD type, subtype or prion strain, genotype of the recipient, infectivity of infectious tissue, and the infectious mass required to transmit CJD.
Few relevant papers addressing the research question in humans were identified; however, 38 papers from a range of scientific approaches were found to highlight themes that potentially relate to CJD infectivity. Therefore, papers were organised thematically for CJD infectivity and are presented as a narrative secondary discourse.
Studies discussing the infectivity of Creutzfeldt–Jakob disease
Infectious mass required to transmit Creutzfeldt–Jakob disease
The risk of any individual becoming infected by CJD is considered to be related to the dose of infectious material received. A quantitative estimation of infectivity in CJD is traditionally ascertained using end-point dilution titration and is expressed as median infective dose in terms of ID50.
No new evidence regarding the quantity of infective material required to transmit CJD in humans was identified in the period covered by the searches. The estimations used in the original mathematical model to estimate the risk of vCJD transmission via surgery12,13 and implemented in IPG19614 are reported in Table 11.
| Risk of CJD transmission | Surgical procedure | Infectivity |
|---|---|---|
| High | Brain and pituitary gland | 108 ID50s/g |
| Posterior eye, retina and optic nerve | ||
| Intradural spine operations | ||
| Neuroendoscopy | ||
| Medium | Spinal cord | 106 ID50s/g |
| Tonsils | 105.5 ID50s/g | |
| Spleen | 105.5 ID50s/g | |
| Lymphoid tissue | 104.5 ID50s/g | |
| Anterior eye | 103.5 ID50s/g | |
| Peripheral nerves |
Higher infectious titres than those estimated in Table 11 have been detected in animal studies using novel methods for end-point titration. For example, Makarava et al. 149 used protein misfolding cyclic amplification (PMCA) with beads to propagate abnormal prion protein scrapie (PrPSc) (infectious isoform/protease K-resistant prion protein) in Syrian hamsters. Using this method, they were able to detect infectious titres ranging from 108.6 to 1012.8. A study by Halliez et al. 150 also found that it was possible to detect higher levels of vCJD infectious titre in a human spleen using a novel bioassay than with a gold-standard immunoblot bioassay. As methods for end-point titration improve, it is therefore likely that some variation in the estimated titres used in IPG196 and noted in Table 11 will be observed in the future.
Codon 129 genotype and susceptibility
All individuals, irrespective of genotype at codon 129 (MM, MV or VV), are now known to be susceptible to sCJD and secondary transmission of vCJD through routes such as blood transfusion; however, the phenotype (or observable physical properties) for MV and VV cases has been noted to be less predictable because of reduced transmission efficiency and increased incubation periods. 79,89 The vCJD prion or agent appears to replicate in lymphoid tissues during the asymptomatic phase of the incubation period. 151 A study152 of abnormal prion protein accumulation in peripheral tissues from MV individuals has been undertaken to understand the infectivity and the risk of horizontal transmission. Bishop et al. 152 inoculated mice with brain and spleen samples from a subclinical vCJD recipient and the clinical vCJD donor. They found transmission of vCJD from the spleen to the mice but not from the brain of the subclinical vCJD recipient, whereas there was transmission from both the spleen and the brain tissues from the clinical vCJD donor. The authors concluded that spleen tissue from the MV genotype can propagate the vCJD agent and that the infectious agent can be present in the spleen without central nervous system (CNS) involvement and that ‘silent’ spread within the human population is, therefore, a possibility from heterozygous carriers. This finding was also echoed by Halliez et al. 150 in their evaluation of novel methods for end-point titration of vCJD in the human spleen. The authors posit the notion that lymphoid tissue exhibits a higher capacity than the brain to replicate prions even after low-dose infection and highlight potential silent carriers of vCJD in lymphoid tissue as a key issue. 150
Subtype or phenotype of Creutzfeldt–Jakob disease
In sCJD, an interaction between the host genotype at codon 129 and the causative agent identified as either PrPSc type 1 or PrPSc type 2 produces different clinical and histopathological phenotypic expressions, which may be influenced by other factors such as route of infection or locations of the initial PrPSc conversion. 153 In sCJD, six major subtypes carrying diverse clinical and pathological features have been identified: (1) MM1/MV1, (2) VV2, (3) MV2 with kuru plaques (MV 2K), (4) MM2-cortical (MM2C), (5) MM2-thalamic (MM2T) or sporadic fatal insomnia and (6) VV1. 154 In mice studies, all subtypes have been found to be transmissible to at least one genotype. 155 Four major prion strains have been proposed to underlie sCJD, iCJD, kuru and some gCJD cases, which are termed M1, V2, M2 and V1. 89 Variant CJD, however, can be distinguished from other categories of CJD owing to the unique PrPres biochemical glycotype referred to as type 2B or type 4, which are also found in cases of natural BSE and other BSE-related conditions. 90
Definitive information on the phenotype can be identified only following neuropathological examination, which provides the opportunity to establish whether or not CJD may have been acquired as opposed to being sporadic or genetic causes. Kobayashi et al. 98 propose the distinctive combination of the MM genotype at codon 129, kuru plaques and intermediate-type PrPSc as a reliable criterion for the identification of iatrogenically acquired CJD cases among presumed sCJD cases. Additionally, some studies highlight that, although exclusive type 1 (sCJDMM1) or type 2 (sCJDMM2) cases do exist, a frequent co-occurrence has been noted of both PrPSc type 1 and type 2 in sCJD in different areas, or the same area, of the brain from a single sCJD patient. 156 This finding complicates the diagnosis and the current classification of sCJD,153 with Parchi et al. 154 highlighting the importance of assessing the cerebral cortex from each of the four lobes (striatum, hippocampus, thalamus and cerebellum) to avoid misclassification of disease. For example, Jansen et al. 157 report from an analysis of CJD cases in the Netherlands that a ‘pure’ phenotype was demonstrated in 60.1% of patients, whereas a mixed phenotype was detected in 39.9% of all sCJD cases. Similarly, an abstract by Mackay et al. 158 reports that 26 out of 108 sCJD patients (24%) had both type 1 and type 2 proteins on Western blot analysis. Mackay et al. 158 argue that the lack of distinct clinical or pathological findings in the six discrete subtypes suggests that these groups do not represent unique strains of prions but rather groupings over a spectrum of disease. These findings underline the importance of neuropathological assessment of CJD cases to document the phenotypic variability and help to disclose the aetiology of CJD strains and efficiency of transmission, where possible.
Route of transmission
The route of transmission may also be relevant to the infectivity of iCJD. A study of five dura mater iCJD cases conducted by Iwasaki et al. 159 indicated that the initial symptoms at perceived sCJD onset appeared to be closely related to the graft site in the brain, indicating a direct transmission of CJD from the graft site to the adjacent brain. Sakai et al. 160 also support the finding of a relationship between the initial clinical manifestation and the site of graft in patients with dura mater graft-associated CJD. 160 Beringue et al. 161 demonstrated in a study of transgenic mice that prion strain divergence can occur on transmission of human primary vCJD, and that peripheral exposure in mice resulted in inefficient neuroinvasion with asymptomatic, life-long infection of the lymphoid compartment. 161
Beringue et al. 162 raise the possibility that human-to-human transmission of vCJD might produce alternative neuropathological phenotypes and that lymphoid tissue examination of CJD cases classified as sporadic might reveal an infection by vCJD-type prions. Cali et al. 163 demonstrated that novel phenotypes may arise as a result of the adaptation of heterologous prion strains of sCJD through contaminated growth hormone. 163 A conference abstract by Peden et al. 164 also described that human-to-human transmission of prion disease may affect the seeding properties of the PrPSc associated with the disease. Their analysis compared the seeding properties of iCJD tissue samples (including both hGH and dura mater) with sCJD tissue samples using a real-time quaking-induced conversion (RT-QuIC) assay, which showed lower seeding properties for secondary iCJD cases than for sCJD cases. The authors note that their findings refute the hypothesis that secondary transmission of a human prion disease results in acquired virulence (or harmfulness). This is supported by a study by Galeno et al. ,67 which found that a novel strain from an atypical CJD in a heterogeneous 69-year old woman who had been treated with phospholipids extracted from bovine brains was not transmissible to transgenic mice but transmitted exclusively to bank voles. The authors note that bank voles are susceptible to a variety of human and animal prions with an efficacy that is often higher than that observed in transgenic mice. 67
Detection of Creutzfeldt–Jakob disease
Whether or not and when asymptomatic carriers of CJD become infectious is important in understanding the potential risks of contamination during surgery. Bougard et al. 165 described an assay that detected prions 1.3 and 2.6 years before the clinical onset of disease in plasma samples from two blood donors who later developed vCJD. The authors report that the ability to identify presymptomatic (n = 2) and symptomatic (n = 18) vCJD-positives in a blinded cohort of 256 plasma samples comprising sCJD, Alzheimer’s disease, Parkinson’s, other neurological diseases and healthy controls indicates the possibility of detecting incubating or silent carriage of vCJD prions.
Identification of abnormal prion accumulation in peripheral lymphoreticular tissue is commonly considered to be a marker of subclinical vCJD that may subsequently develop into clinical vCJD. However, the reliability of this marker for representing subclinical or indeed, clinical, vCJD has been questioned. Mead et al. 166 highlight a case of clinical vCJD whose presentation, imaging findings, cerebrospinal fluid investigation results and clinical progression were typical of other vCJD cases. However, subsequent examination of multiple tissues from a biopsy and at autopsy showed minimal deposition of disease-associated prion protein in tonsil tissue. 166 This patient also received a negative score from a blood test specifically for vCJD, the direct-detection assay. The authors note that this case demonstrates that even patients with end-stage vCJD may have minimal prion colonisation in lymphoreticular tissue.
Absence versus presence of abnormal prion accumulation may occur because of the sensitivity of the CJD assay employed. Examination of 14-3-3 proteins in both the cerebrospinal fluid and a RT-QuIC assay are commonly employed tests that are considered to be sensitive and specific for sCJD detection, although less so for vCJD. 167 For example, the identified heterozygous clinical vCJD patient, aged 36 years in 2016,37 tested negative for the 14-3-3 protein, RT-QuIC assay and vCJD-focused direct-detection assay, but immunoblotting of brain homogenate at autopsy confirmed the presence of vCJD prions. Moreover, immunostaining performed in this patient for abnormal prion protein-labelled amyloid plaques highlighted a relative lack of peripheral tissue involvement, with only minute amounts detected in the spleen and no detection in the appendix or mesenteric lymph nodes. However, Douet et al. 168 used a highly sensitive PMCA assay to assess abnormal prion accumulation in the identified heterozygous subclinical vCJD patient, aged 82 years in 2017. Previous investigations had not detected abnormal prion protein or infectivity in the brain, indicating a lack of CNS involvement at the time of death. 78,152 However, using this assay they found vCJD prions in all lymphoid organs and a wide variety of other tissues, including the salivary gland, lung and liver. The authors caution that the identification of wide vCJD involvement in the peripheral tissues of a preclinical patient further indicates the potential for iatrogenic transmission of this fatal neurological condition by surgical procedures.
Transmission to and from peripheral tissues
The infectious load is known to be higher in certain tissues, such as CNS tissues,14 and, therefore, the risk of infectivity from peripheral tissue has been questioned. Studies report conflicting findings regarding the infectivity of peripheral tissues. For example, Bishop et al. 152,169 reported that spleen tissue from the MV genotype preclinical vCJD blood recipient was transmissible in a study using transgenic and wild-type mice. The authors highlight that significant levels of infectious agent are present in the spleen before CNS involvement. 152,169 However, Wadsworth et al. 170 found from an animal study of transgenic mice that, although vCJD prion infection was readily reported following inoculation with frozen vCJD brain or appendix, and formalin-fixed, paraffin-embedded (FFPE) brain, no infectivity was detected from FFPE vCJD spleen or FFPE appendix samples. 170 The authors caution that the absence of detectable infectivity in fixed, known positive vCJD lymphoreticular tissue does not definitively prove that vCJD transmission cannot occur through appendix specimens, as the assays used were not able to detect the low levels of infectivity previously found in the positive control lymphoreticular tissue following formalin fixation by Hilton et al. 7 However, in contrast, Halliez et al. 150 more recently found that lymphoid tissue exhibits higher capacity than the brain to replicate prions using novel detection methods.
Infectivity of genetic Creutzfeldt–Jakob disease
The potential for horizontal transmission of gCJD, as discussed previously, was raised by the unusually high prevalence of gCJD in Slovakia. 47 Ritchie et al. 171 report from an animal study of squirrel monkeys that no clinical or pathological signs of CJD were observed following blood transfusion of either sCJD or vCJD of the intracerebral-inoculated monkeys after euthanasia at 7 years. However, there was evidence that GSS, a form of gCJD, transmitted autopsy-proven disease to two intracerebral-inoculated monkeys after incubation periods of 34 and 39 months. Ritchie et al. 17 conclude that these results, and other studies from rodents and non-human primates, suggest that blood donations of GSS (and perhaps other familial forms of TSE) carry more risk than those from vCJD. 171 The infectiousness of CJD via blood is not directly relevant to the current decision problem of CJD risk via surgery. However, consideration of the potential differences of infectiousness of the CJD types may be relevant when considering the risks of horizontal transmission in the future and in particular localities.
MV genotype as protective: PrPSc allotype
Allotype refers to an inherited set of determinants or a sequence of amino acids and other proteins that demonstrate heterogeneity, which is specific for an individual but more common in an ethnic group. The relative contribution of each PrP allotype to the infectious disease associated with the abnormal isoform of prion protein (PrPSc) is unknown. Moore et al. 172 found from an analysis of four heterozygous cases of sCJD that the PrPSc allotype ratio is highly variable, with PrPSc (-M129 and -V129) differing markedly between different regions within the same sCJD brain. 172 However, an analysis of six heterozygous cases of iCJD found that the composition of PrPSc iCJD was more homogenous and tended to contain a higher proportion of PrPSc-V129 than heterozygous cases of sCJD. The presence of two different PrP allotypes in the same brain can often lead, in a dose-dependent manner, to inefficient PrPSc formation and increased disease incubation. However, the study authors report that in both types of CJD (sCJD and iCJD), the PrPSc allotype ratio had no correlation with CJD type, age at clinical onset or disease duration. This evidence suggests that, therefore, factors other than PrPSc allotype abundance must influence the clinical progression and phenotype of heterozygous cases of CJD.
Discussion/summary of infectivity of Creutzfeldt–Jakob disease
When opportunities for CJD transmission occur, a range of factors are likely to influence how the disease will manifest itself in terms of clinical phenotype, neuropathological pattern, incubation period and disease duration. These factors include an interaction between the genotype at PRNP codon 129, infecting prion strain, route of transmission and location of PRNP conversion. Moreover, the method of detection and the analysis of CJD is crucial in obtaining detailed and accurate neuropathological confirmation of CJD type in order to posit the most plausible explanations for acquisition of iCJD. Although few data regarding infectious dose or infectious titre in humans have been published to supersede the information used to populate the model built by ScHARR in 2005,12 some animal studies using advanced detection methods indicate that infectious doses > 108 ID50 are possible.
The evidence on the efficacy of prion decontamination procedures for surgical instruments
The purpose of this review was to identify published and unpublished evidence for the efficacy of decontamination procedures in terms of reducing the infectivity of prions adhering to steel wires or other steel materials. The review focuses principally on log-reductions in the infectious titre, that is the reduction in the load of infectivity on steel (wires) before and after the decontamination processes. Log-reductions are a common measure of decontamination and the review could inform this parameter in the health economic model. This systematic review includes studies that investigate autoclaving, the principal process currently employed in the NHS, as well as decontamination procedures that might be used in addition to autoclaving.
According to a 2014 report by the House of Commons Science and Technology Committee,173 a potentially effective decontaminant (Rely+On®; DuPont, Midland, MI, USA) to be used prior to autoclaving experienced barriers to its uptake in the NHS owing to (1) the perceived low risk of iCJD through surgical transmission and (2) resistance to the inclusion of an additional step in the decontamination process. 173 It should therefore be noted that, first, based on the number of known cases, the risk of iCJD through surgical transmission has not increased markedly since 2013–14, which suggests evidence on new decontaminants might not be taken up in practice. Second, any decontaminants identified by this systematic review as potentially being effective, but also representing an additional step, might experience the same barriers to uptake.
Decontamination studies
Studies reporting log-reductions of prion infectivity after autoclaving with/without other processes
Five studies174–178 reported log-reductions of prion infectivity after autoclaving with and without other decontamination processes (Table 12). In terms of prion strain, three studies used 10% brain homogenate of 263K hamster scrapie,174–176 two used vCJD,176,177 and the following prion strains were investigated in only a single study: 127S,177 M1000178 and BSE 6PB1. 176 All studies used steel wires contaminated with the prion (one study also used steel sheets176) and all studies investigated autoclaving at 121 or 134 °C for specified amounts of time, as a decontamination procedure.
| Study (first author and year of publication) | Prion strain(s) | Source material (% w/v) | Steel | Decontamination methods | Assay used | |
|---|---|---|---|---|---|---|
| Autoclaving | Other | |||||
| Belondrade (2016)177 | 127S scrapie and vCJD | BH (10) | Wires |
|
|
PMCA |
| Lawson (2007)178 | M1000 | BH (10) | Wires |
|
|
Tga20 mice WB |
| Lehmann (2009)174 | 263K scrapie | BH (10) | Wires |
|
|
Syrian golden hamsters |
| Lemmer (2008)175 | 263K scrapie | BH (10) | Wires |
|
|
Syrian golden hamsters |
| Rogez-Kreuz (2009)176 | 263K scrapie, vCJDa and BSE 6PB1a | BH (10 or 20) | Wires, sheetsa |
|
|
Syrian golden hamsters, WBa |
The efficiency of autoclaving was assessed alone and in combination with a range of other decontaminants. These included sodium hydroxide (NaOH), sodium hypochlorite (NaOCl), sodium dodecyl sulfate (SDS), hydrogen peroxide (H2O2) and various other enzymatic and alkaline detergents. These decontaminants were also investigated alone or in combination with other decontaminants. Selected results from these investigations are reported in Table 13.
| Study (first author and year of publication) | Prion strain | Decontamination methods | Log reduction | Transmission rate, n/N (%) | Incubation period days, n (SD) | |
|---|---|---|---|---|---|---|
| Autoclaving | Other | |||||
| Belondrade (2016)177 | 127S | 121 °C: 20 minutes | ≥ 5 | 10/12 (83) | NR | |
| 134 °C: 20 minutes | FE | 0/12 (0) | NR | |||
| vCJD | 121 °C: 20 minutes | 5 | 1/8 (12.5) | NR | ||
| 134 °C: 20 minutes | FE | 0/8 (0) | NR | |||
| Lawson (2007)178 | M1000 | 121 °C: 20 minutes | 1.6 | 100% | 106 (2) | |
| 134 °C: 3 minutes | 1.5 | 100% | 104 (3) | |||
| 134 °C: 18 minutes | 2.2 | 100% | 120 (5) | |||
| 134 °C: 3 minutes | 0.3% RMEC B: 60 °C for 30 minutes | ≥ 4.5 | 10% | 166 (NR) | ||
| 121 °C: 20 minutes | 0.3% RMEC B: 60 °C for 30 minutes | > 5 | 0% | – | ||
| Lehmann (2009)174 | 263K | 134 °C: 18 minutes | 4.11 | 57% | 140 | |
| aLemmer (2008)175 | 263K | 134 °C: 5 minutes | 0.5% alkaline cleaner: 5 minutes at 55 °C | ≥ 5.5 | NR | NR |
| 134 °C: 5 minutes | 0.5% alkaline cleaner: 10 minutes at 55 °C | ≥ 5.5 | NR | NR | ||
| 134 °C: 5 minutes | 1% alkaline cleaner: 5 minutes at 55 °C | ≥ 5.5 | NR | NR | ||
| 134 °C: 5 minutes | 1% alkaline cleaner: 10 minutes at 55 °C | ≥ 5.5 | NR | NR | ||
| 134 °C: 5 minutes | 0.2% SDS/0.3% NaOH: 5 minutes at 23 °C | ≥ 5.5 | NR | NR | ||
| 134 °C: 5 minutes | 0.2% SDS/0.3% NaOH: 10 minutes at 23 °C | ≥ 5.5 | NR | NR | ||
| 134 °C: 5 minutes | Disinfectant with 0.2% PAA/0.075–0.225% NaOH: 120 minutes at 23 °C | > 2 to < 3 | NR | NR | ||
| Rogez-Kreuz (2009)176 | 263K | 134 °C: 18 minutes | ≥ 5 to 6 | 50% | 428 ± 103 | |
| 134 °C: 18 minutes | 1 N NaOH: 60 minutes | ≥ 5 to 6 | 28% | 554 ± 197 | ||
| 134 °C: 18 minutes | 2% enzymatic detergent: 10 minutes at 37 °C | 4 | 100% | 131 ± 17 | ||
| 134 °C: 18 minutes | 1% alkaline detergent A: 10 minutes at 70 °C | ≥ 5 to 6 | 0% | 525 ± 149 | ||
| 263K in vitro | 134 °C: 18 minutes | ≥ 5.4 | NR | NR | ||
The log-reductions produced by autoclaving at 134 °C for 18 minutes for the 263K prion strain ranged from 4.11174 to > 5–6,176 with transmission rates of 57% and 50%, respectively. The log-reduction was only 2.2 (100% transmission) for the M1000 strain. Autoclaving at 134 °C for 18 minutes combined with NaOH or an alkaline detergent produced log-reductions of > 5 to 6, as well as lower transmission rates (28% for NaOH and 0% for the alkaline detergent) for the 263K prion strain. 176 Autoclaving at 134 °C for 5 minutes combined with alkaline cleaners or 0.2% SDS or 0.3% NaOH at different concentrations and/or different durations, also produced log-reductions of > 5.5 for the 263K prion strain. 175
The only process reported to have produced a log-reduction of > 5 and a transmission rate of 0% is autoclaving at 121 °C for 20 minutes plus 0.3% rapid multienzyme cleaner trial formulation (RMEC) B at 60 °C for 30 minutes. 178
The aim of some studies is development of a prion detection assay, rather than the development of the decontaminant. 177
Studies reporting log-reductions of prion infectivity after processes other than autoclaving
Eleven studies reported log-reductions of prion infectivity after various decontamination processes, principally enzymatic detergents that did not use autoclaving (Table 14). In terms of prion strain, seven studies used 10% or 20% brain homogenate of 263 K hamster scrapie,174–176,179–182 three used vCJD,177,179,180 two used ME7183,184 and the following prion strains were investigated in only a single study: Rocky Mountain Laboratory (RML),185 BSE 6PB1 and TGB1,181 M1000178 and 127S. 177 Nine studies used steel wires contaminated with the prion, one study used steel tokens183 and one used steel sheets. 176
| Study (first author and year of publication) | Prion strain | Source material (% w/v) | Steel | Decontamination methods other than autoclaving | Assay used |
|---|---|---|---|---|---|
| Beekes (2010)179 | 263K scrapie, vCJD (MM1), sCJD | BH (10) | Wires |
|
Hamsters, WB |
| Bellon (2014)180 | 263K, vCJD (mouse adapted) | BH (20) | Wires |
|
Hamsters, WB |
| Belondrade (2016)177 | 127S scrapie, vCJD | BH (10) | Wires |
|
Surf-PMCA |
| Edgeworth (2011)185 | RML | BH (10) | Wires |
|
Tga20 mice, Tg20 mice SSBA |
| Fichet (2007)181 | 263K scrapie, 6PB1 BSE, TGB1 BSE | BH (10) | Wires |
|
Animal, WB |
| aHervé (2010)182 | 263K scrapie | NR | Wires |
|
Animal |
| Hervé (2010)183 | ME7 | BH (NR) | Tokens |
|
EDIC/EF and WB |
| Howlin (2010)184 | ME7 | BH (10) | Wires |
|
EDIC/EF and WB |
| Lawson (2007)178 | M1000 | BH (10) | Wires |
|
Tga20 mice, WB |
| Lehmann (2009)174 | 263K scrapie | BH (10) | Wires |
|
Hamsters |
| Lemmer (2008)175 | 263K scrapie | BH (10) | Wires |
|
Hamsters |
| Rogez-Kreuz (2009)176 | 263K scrapie, vCJD,b BSE 6PB1b | BH (10 or 20) | Wires, sheetsb |
|
Hamsters, WB |
The efficiency of a range of decontaminants was assessed. Selected results from these investigations are reported in Table 15. It was reported by Edgeworth et al. 185 that the following processes inactivated RML prions below the detection limit of the in vitro standard steel-binding assay (SSBA), stated to be equivalent to a reduction of 8 logs: Rely-On PI (DuPont), Prionzyme plus 2 M NaOH, and 2 M NaOH. It was noted, however, that the decontaminating effect of Prionzyme (Genencor) was indistinguishable from that of the diluent in which the decontaminant was prepared (2 M NaOH solution, following the manufacturer’s instructions), that is treatment with 2 M NaOH alone also resulted in no detectable infectivity remaining on the steel surface. The only process reported to have produced a log-reduction of ≥ 5 and a transmission rate of 0% for the RML prion strain was Rely+On PI.
| Study (first author and year of publication) | Prion strain | Decontamination methods | Log-reduction | Transmission rate | Incubation period days, n (SD) | Other (e.g. TICUw) |
|---|---|---|---|---|---|---|
| Beekes (2010)179 | 263K | 0.2% SDS/0.3% NaOH in 20% n-propanol | ≥ 5.5 | 0/10 | 503 | |
| 0.2% SDS/0.3% NaOH in 30% n-propanol | ≥ 5.5 | 0/9 | 503 | |||
| vCJD | 0.2% SDS/0.3% NaOH in 20% n-propanol | 3.3 | NR | NR | ||
| sCJD | 0.2% SDS/0.3% NaOH in 20% n-propanol | 3.3 | NR | NR | ||
| Bellon (2014)180 | vCJD | 0.1–0.45 mol/l NaOH: 25–45 °C for 5–240 minutes | ≥ 3.8 | 0/8 | NR | |
| 263K | 0.45 mol/l NaOH: 4 °C for 60 minutes | 4.9 | 2/8 | 441 | ||
| 0.2 mol/l NaOH: 15 °C for 15 minutes | 5 | 1/5 | 237 | |||
| 0.2 mol/l NaOH: 15 °C for 60 minutes | ≥ 5 | 0/8 | NR | |||
| 0.45 mol/l NaOH: 15 °C for 30 minutes | ≥ 5.2 | 0/8 | NR | |||
| 0.45 mol/l NaOH: 15 °C for 60 minutes | ≥ 5.2 | 0/8 | NR | |||
| 0.15 mol/l NaOH: 25 °C for 60 minutes | 4.1 | 5/9 | 215 | |||
| 0.45 mol/l NaOH: 25 °C for 60 minutes | 4.7 | 3/10 | 382 | |||
| 0.45 mol/l NaOH: 25 °C for 240 minutes | ≥ 5.4 | 0/8 | NR | |||
| 0.45 mol/l NaOH: 40 °C for 5 minutes | ≥ 5.3 | 0/8 | NR | |||
| 0.45 mol/l NaOH: 40 °C for 15 minutes | 5.1 | 1/6 | 364 | |||
| 0.1 mol/l NaOH: 45 °C for 5 minutes | ≥ 5.4 | 0/8 | NR | |||
| 0.1 mol/l NaOH: 45 °C for 15 minutes | ≥ 5.4 | 0/8 | NR | |||
| Belondrade (2016)177 | 127S | 0.1 N NaOH: 15 minutes | ≥ 3 | 12/12 | NR | |
| vCJD | 0.1 N NaOH: 15 minutes | 3 | 6/8 | NR | ||
| Edgeworth (2011)185 | RML | Rely-On PIa | 5.5 | 0/19 | > 250 | |
| Rely-On PIb | 8 | NR | NR | < 0.003 TICUw | ||
| Prionzyme plus 2 M NaOHb | 8 | NR | NR | < 0.003 TICUw | ||
| 2 M NaOHb | 8 | NR | NR | < 0.003 TICUw | ||
| 0.8% Hamo 100b | NR | NR | NR | 0.3c | ||
| 1.6% Hamo 100b | NR | NR | NR | 0.07c | ||
| Fichet (2007)181 | 263K | 6% liquid H2O2,: 20 °C for 60 minutes | 1 | 11/11 (100%) | 114 (13) | |
| 2 mg/l gaseous H2O2: 30 °C (3 pulses) | > 5.5 | 0/8 | > 540 | |||
| 2 mg/l gaseous H2O2: 30 °C (6 pulses) | > 5.5 | 0/8 | > 540 | |||
| 6PB1 BSE | 2 mg/l gaseous H2O2: 30 °C (3 pulses) | > 5.5 | 0/9 | > 540 | ||
| TGB1 BSE | 2 mg/l gaseous H2O2: 30 °C (3 pulses) | > 5.3 | 0/9 | > 540 | ||
| dHervé (2010)182 | 263K | Cold atmospheric plasma | > 6 | NR | NR | |
| Hervé (2010)183 | ME7 | Cleaner 4 (most efficient): 50 °C for 5 minutes | 3 | NR | NR | 99.21% of initial prion amyloid load removed |
| Howlin (2010)184 | ME7 | Unspecified enzyme pretreatment (containing proteases) without presoak | Approximately 2 log greater reduction in prion amyloid than presoak alone even if allowed to dry and 3 log-reduction if process was started immediately after contamination (wet) | |||
| Unspecified enzyme pretreatment (containing proteases) with presoak | 1 log-reduction in prion amyloid if process was started immediately after contamination (wet) instead of being allowed to dry | |||||
| Unspecified enzyme pre-treatment (containing proteases) plus alkaline detergent w/d | Prion-associated amyloid concentration levels were reduced below the experimental cut-off value of 0.001 ng/mm,2 wet or dry | |||||
| Lawson (2007)178 | M1000 | 1 M NaOH: 60 minutes | 2.7 | 100% | 130 (19) | |
| 1% RMEC A: 50 °C for 30 minutes | ≥ 4.5 | 80% | 204 (18) | |||
| 0.3% RMEC B: 60 °C for 30 minutes | ≥ 3.5 | 60% | 147 (13) | |||
| Lehmann (2009)174 | 263K | H2O2: 30 minutes | ≥ 5.25 | 0% | ≥ 370 | |
| AF: 10 minutes | ≥ 5.25 | 0% | ≥ 370 | |||
| Np-Np-H2O2/Cu: 10 minutes – 5 minutes – 15 minutes | 4.55 | 43% | 133 | |||
| Dp-Dp-H2O2/Cu: 10 minutes – 5 minutes – 15 minutes | ≥ 5.25 | 20% | 159 | |||
| Np-Dp-H2O2/Cu: 10 minutes – 5 minutes – 15 minutes | ≥ 5.25 | 0% | ≥ 370 | |||
| Nmp-Nmp-PAA/Cu: 10 minutes – 5 minutes – 15 minutes, at 40 °C | 3.43 | 67% | 102 | |||
| Lemmer (2008)175 | 263K | 1.0 M NaOH: 60 minutes at 23 °C | ≥ 5.5 | NR | NR | |
| 2.5% NaOCl: 60 minutes at 23 °C | ≥ 5.5 | NR | NR | |||
| 0.5% alkaline cleaner: 5 minutes at 55 °C | ≥ 4 to < 5 | NR | NR | |||
| 0.5% alkaline cleaner: 10 minutes at 55 °C | > 5 to ≤ 5.5 | NR | NR | |||
| 1% alkaline: 5 minutes at 55 °C | > 5 to ≤ 5.5 | NR | NR | |||
| 1% alkaline cleaner: 10 minutes at 55 °C | ≥ 5.5 | NR | NR | |||
| 0.2% SDS/0.3% NaOH: 5 minutes at 23 °C | ≥ 5.5 | NR | NR | |||
| 0.2% SDS/0.3% NaOH: 10 minutes at 23 °C | ≥ 5.5 | NR | NR | |||
| Disinfectant with 0.2% PAA/0.075–0.225% NaOH: 120 minutes at 23 °C | > 5 to ≤ 5.5 | NR | NR | |||
| Rogez-Kreuz (2009)176 | 263K | H2O2: 10 minutes | ≥ 5 to 6 | 50% | 443 ± 140 | |
| H2O2: 20 minutes | ≥ 5 to 6 | 50% | 428 ± 142 | |||
| 2% enzymatic detergent: 10 minutes at 37 °C | 1.1 | 100% | 95 ± 0 | |||
| 1% alkaline detergent A: 10 minutes at 55 °C | ≥ 5 to 6 | 11% | 446 ± 153 | |||
| 1% alkaline detergent B: 10 minutes at 55 °C | ≥ 5 to 6 | 0% | 524 ± 42 | |||
| Sterrad NX1 (Advanced Sterilization Products Services Inc, Irvine, CA, USA) advanced cycle | ≥ 5 to 6 | 0% | 570 ± 18 | |||
| Sterrad NX2 continuous advanced cycles | ≥ 5 to 6 | 0% | 574 ± 0 | |||
| 1% alkaline detergent A (10 minutes at 55 °C) plus Sterrad NX1 advanced cycle | ≥ 5 to 6 | 0% | 559 ± 22 | |||
| 1% alkaline detergent B (10 minutes at 55 °C) plus Sterrad NX1 advanced cycle | ≥ 5 to 6 | 0% | 562 ± 16 | |||
| 263K in vitro | Sterrad NX1 advanced cycle | ≥ 5.4 | NR | NR | ||
The only process or combination of processes reported to have produced a log-reduction of ≥ 5 and a transmission rate of 0% for the 263K prion strain were 0.2% SDS/0.3% NaOH in 20% or 30% n-propanol;179 0.2 mol/l NaOH at 15 °C for 60 minutes; 0.45 mol/l NaOH at 15 °C for 15 or 30 minutes; 0.45 mol/l NaOH at 25 °C for 240 minutes; 0.45 mol/l NaOH at 40 °C for 5 minutes; 0.1 mol/l NaOH at 45 °C for 5 or 15 minutes;180 2 mg/l gaseous H2O2 at 30 °C for 3 or 6 pulses;181 H2O2 for 30 minutes; AF (alkaline detergent, surfactants, chelatant) for 10 minutes; and combinations of enzymatic detergents and disinfectants Np-Dp-H2O2/Cu (for 10 minutes – 5 minutes – 15 minutes),174 the alkaline detergent B at 1% for 10 minutes at 55 °C, the Sterrad NX1 advanced cycle and Sterrad NX2 continuous advanced cycles (H2O2 and gas plasma), and the alkaline detergents A or B at 1% for 10 minutes at 55 °C, in combination with the Sterrad NX1 advanced cycle,176 and cold atmospheric plasma. 182 According to Rogez-Kreuz et al. ,176 no insoluble prion (PrPres) signal was detected for BSE 6PB1 or vCJD ‘after exposure to steam in either of the two Sterrad systems’. The only process reported to have produced a log-reduction of ≥ 5 and a transmission rate of 0% for the BSE prion strains 6PB1 and TGB1 were 2mg/l gaseous H2O2 at 30 °C for three pulses. 181 None of the treatments for the ME7, vCJD, 127S or M1000 prion strains reported a log-reduction of ≥ 5 and a transmission rate of 0%. 177,178,182,184
Supplementary evidence: studies reporting outcomes other than log-reductions after autoclaving with/without other processes
Six studies185–190 reported outcomes other than log-reductions (Table 16). In terms of prion strain, two studies used 10% or 20% brain homogenate of RML186 and two studies investigated sc237 and sCJD,187,188 with the following prion strains investigated in only a single study: 263K scrapie,189 and 301 V BSE and cattle BSE. 188 Five studies used steel wires contaminated with the prions and one study189 used steel spheres. All studies investigated autoclaving at 121, 134 or 137 °C for specified amounts of time as a decontamination procedure; one study investigated autoclaving at 65 and 121 °C. 188
| Study (first author and year of publication) | Prion strain | Source material (% w/v) | Steel | Decontamination methods | Assay used | |
|---|---|---|---|---|---|---|
| Autoclaving | Other | |||||
| Baxter (2005)189 | 263K | BH (20) | Spheres |
|
|
Hamsters |
| Edgeworth (2011)185 | RML | BH (10) | Wires |
|
|
SSBA |
| Giles (2007)188 |
Sc237 sCJD |
BH (10) | Wires |
|
|
Tg7 and Tg23372 mice |
| Giles (2008)190 |
301V BSE Cattle BSE |
BH (10) | Wires |
|
|
Tg2091 mice Tg4092 mice |
| Jackson (2005)186 | RML | BH (10 and 20) | Wires |
|
|
Tg20 mice, CD-1 mice, WB |
| RML | BH (10) | Wires |
|
Enzymes | Tg20 mice, CD-1 mice, WB | |
| Peretz (2006)187 |
Sc237 sCJD |
BH (10) | Wires |
|
|
Tg7 and Tg23372 mice Micro BCA Protein assay (Pierce, Rockford, IL, USA) |
The efficiency of autoclaving was assessed alone and in combination with a range of other decontaminants. These included SDS, acetic acid (AcOH), NaOH, radiofrequency (RF) gas plasma, Trigene disinfectant and various other enzymatic detergents. Selected results from these investigations are reported in Table 17.
| Study (first author and year of publication) | Prion strain | Decontamination methods | Transmission rate | Incubation period days, n (SD) | |
|---|---|---|---|---|---|
| Autoclaving | Other | ||||
| Baxter (2005)189 | 263K | 137 °C: 18 minutes | Trigene disinfectant | 5/5 | 202 ± 28 |
| Trigene disinfectant | 0/5 | 466a | |||
| RF gas plasma | 0/5 | 466a | |||
| Edgeworth (2011)185 | RML | 134 °C: 18 minutes | NR | 5% | NR |
| bGiles (2007)188 | Sc237 in Tg7 mice | 65 °C: 30 minutes | 2% SDS + 1% AcOH | 100% | 82 ± 0.7 |
| 65 °C: 120 minutes | 2% SDS + 1% AcOH | 68% | 269 ± 3.2 | ||
| 65 °C: 18 hours | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 121 °C: 15 minutes | NR | 100% | 160 ± 7.3 | ||
| 121 °C: 30 minutes | NR | 20% | > 400 | ||
| 121 °C: 120 minutes | NR | 0% | > 400 | ||
| 121 °C: 15 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 121 °C: 30 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 121 °C: 120 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| sCJD in Tg23372 mice | 65 °C: 30 minutes | 2% SDS + 1% AcOH | 86% | 354 ± 1.6 | |
| 65 °C: 120 minutes | 2% SDS + 1% AcOH | 44% | > 500 | ||
| 65 °C: 18 hours | 2% SDS + 1% AcOH | 25% | > 500 | ||
| 121 °C: 15 minutes | NR | 22% | > 500 | ||
| 121 °C: 30 minutes | NR | 0% | > 500 | ||
| 121 °C: 120 minutes | NR | 73% | 414 ± 15 | ||
| 121 °C: 15 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| 121 °C: 30 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| 121 °C: 120 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| Giles (2008)190 | 301V | 134 °C: 15 minutes | NR | 96% | 161 |
| 134 °C: 30 minutes | NR | 57% | 438 | ||
| 134 °C: 120 minutes | NR | 14% | > 600 | ||
| NR | 1% AcOH: 65 °C for 18 hours | 100% | 117 | ||
| NR | 4% SDS: 65 °C for 18 hours | 100% | 127 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 30 minutes | 73% | 267 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 120 minutes | 33% | > 600 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 18 hours | 58% | 410 | ||
| 134 °C: 15 minutes | 4% SDS + 1% AcOH | 5% | > 600 | ||
| 134 °C: 30 minutes | 4% SDS + 1% AcOH | 0% | > 600 | ||
| 134 °C: 120 minutes | 4% SDS + 1% AcOH | 0% | > 600 | ||
| BSE | 134 °C: 15 minutes | NR | 84% | 384 | |
| 134 °C: 30 minutes | NR | 100% | 375 | ||
| 134 °C: 120 minutes | NR | 89% | 420 | ||
| NR | 1% AcOH: 65 °C for 18 hours | 91% | 354 | ||
| NR | 4% SDS: 65 °C for 18 hours | 100% | 368 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 30 minutes | 42% | > 500 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 120 minutes | 26% | > 500 | ||
| NR | 4% SDS + 1% AcOH: 65 °C for 18 hours | 4% | > 500 | ||
| 134 °C: 15 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
| 134 °C: 30 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
| 134 °C: 120 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
| Jackson (2005)186 |
RML (20% w/v) Tg20 mice |
121 °C: 20 minutes | NR | 0/6a | NR |
| 134 °C: 20 minutes | NR | 0/4b | NR | ||
| NR | LpH | 5/5 | 91 (SEM 2.6) | ||
| NR | LpHse | 3/5c | 70 (0) | ||
| NR | Endozyme Plus | 5/5 | 81 (1) | ||
| NR | Enzymes SDS-PK-Pronase: 40 °C for 60 minutes | 0/3 | NR | ||
| 121 °C: 20 minutes | Enzymes SDS-PK-Pronase: 40 °C for 60 minutes | 0/5d | NR | ||
| 134 °C: 20 minutes | Enzymes: SDS-PK-Pronase: 40 °C for 60 minutes | 0/4 | NR | ||
|
RML (20% w/v) CD-1 mice |
134 °C: 20 minutes | NR | 0/9 | NR | |
| 134 °C: 20 minutes | 2 M NaOH | 0/10 | NR | ||
| 134 °C: 20 minutes | Enzymes SDS-PK-Pronase: 40 °C for 60 minutes | 0/8 | NR | ||
| NR | Enzymes SDS-PK-Pronase: 40 °C for 60 minutes | 0/10 | NR | ||
| RML (10% w/v) Tg20 mice | 134 °C: 20 minutes | NR | 13/13c | 108 (12.4 SEM) | |
| NR | Enzymes SDS-PK-Pronase: 40 °C for 60 minutes | 1/18 (101) | NR | ||
| Peretz (2006)187 | Sc237 in Tg7 mice | 121 °C: 15 minutes | NR | n = 10, 100% | 160 ± 7.3 |
| 121 °C: 30 minutes | NR | 20% | > 400 | ||
| 121 °C: 120 minutes | NR | 0% | > 400 | ||
| 121 °C: 15 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 121 °C: 30 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 121 °C: 120 minutes | 2% SDS + 1% AcOH | 0% | > 400 | ||
| 134 °C: 15 minutes | NR | 87% | 96 ± 0.6 | ||
| 134 °C: 30 minutes | NR | 55% | 262 ± 10 | ||
| 134 °C: 120 minutes | NR | 9% | > 400 | ||
| 134 °C: 15 minutes | 4% SDS + 1% AcOH | 0% | > 400 | ||
| 134 °C: 30 minutes | 4% SDS + 1% AcOH | 4% | > 400 | ||
| 134 °C: 120 minutes | 4% SDS + 1% AcOH | 0% | > 400 | ||
| sCJD Tg23372 | 121 °C: 15 minutes | NR | n = 10, 22% | > 500 | |
| 121 °C: 30 minutes | NR | 0% | > 500 | ||
| 121 °C: 120 minutes | NR | 73% | 414 ± 15 | ||
| 121 °C: 15 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| 121 °C: 30 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| 121 °C: 120 minutes | 2% SDS + 1% AcOH | 0% | > 500 | ||
| 134 °C: 15 minutes | NR | 73% | 218 ± 4.1 | ||
| 134 °C: 30 minutes | NR | 63% | 242 ± 2.8 | ||
| 134 °C: 120 minutes | NR | 46% | > 500 | ||
| 134 °C: 15 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
| 134 °C: 30 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
| 134 °C: 120 minutes | 4% SDS + 1% AcOH | 0% | > 500 | ||
Only one study reported the outcome ‘tissue culture infectious units on wires’ (TICUw). 185 This study recorded that autoclaving at 134 °C for 18 minutes reduced the TICUw measure of the RML prion strain to 0.03, which is reported to be equivalent to a reduction of 5.5 logs (see Table 15).
The remaining studies all reported transmission rates. The transmission rates produced by autoclaving at 134 °C for 15 minutes and 30 minutes for the 301 V BSE prion strain were 96% and 57%, respectively, and for cattle BSE they were 84% and 100%, respectively. 190 The transmission rates produced by autoclaving at 134 °C for 20 minutes for the RML prion strain ranged from 25% (1/4) to 100% (13/13) in Tg20 mice and 0% (0/9) in CD-1 wild mice. 186 The unusually high transmission rate in the larger sample of Tg20 mice was explained by the autoclaving process being affected by partial sealing of the glass tubes containing the steel wires, which impaired the penetration of the steam. 186 The transmission rates produced by autoclaving at 121 °C for 15 minutes and 30 minutes for the Sc237 prion strain were 100% and 20%, respectively, and for sCJD, 22% and 0%, respectively. 187,188 Finally, the transmission rates produced by autoclaving at 134 °C for 15 minutes and 30 minutes for the Sc237 prion strain were 87% and 55%, respectively, and for sCJD 73% and 63%, respectively. 187
The following combinations of autoclaving and other processes are reported to have produced a transmission rate of 0% or ≤ 5%: autoclaving at 134 °C for 15, 30 or 120 minutes plus 4% SDS and 1% AcOH for the 301 V, cattle BSE,190 Sc237 and sCJD prions strains;187 autoclaving at 121 °C for 15, 30 or 120 minutes plus 2% SDS and 1% AcOH for the 301 V and cattle BSE prion strains;190 and autoclaving at 65 °C for 18 hours plus 2% SDS and 1% AcOH for the Sc237 and sCJD prions strains. 188 Autoclaving at 134 °C for 20 minutes plus SDS-proteinase K (PK)-Pronase at 40 °C for 60 minutes also produced a 0% transmission rate for RML prion strains. 186
Without autoclaving Trigene disinfectant and RF gas plasma, and SDS-PK-Pronase at 40 °C for 60 minutes, also produced transmission rates of 0% in the 263K and the RML prion strains, respectively. 186,189
Supplementary evidence: studies reporting evidence for levels of protein residue on surgical instruments after cleaning
Nine studies183,191–198 reported this outcome after autoclaving with and without other decontamination processes (Table 18): seven studies192–198 for surgical instruments and two studies for endoscopes. 183,191 All studies were conducted in the UK. Seven studies183,193–198 reported on protein residue on instruments acquired between one and nine NHS trusts; the number of trusts involved was not reported in two studies. 191,192 All studies reported that cleaning essentially involved conventional procedures for the equipment concerned. With the exception of two studies,192,193 the assay appears to have involved detection of protein in situ on the instruments. Where reported, the number of instruments ranged from 2 to 1000.
| Study (first author and year of publication) | Country | Source | Surgical instruments (number) | Cleaning cycle | Assay/in situ | Residual protein contamination of surgical instruments after conventional cleaning and sterilisation in a sterile service department | |
|---|---|---|---|---|---|---|---|
| Mean protein per instrument (µg) | Median protein per instrument (µg) | ||||||
| Baxter (2006)192 | UK | SSDs from a random sample of NHS trusts | Five trays (n = 120) | Routine hospital cleaning and sterilisation | Ninhydrin/acid stripping of surfaces and hydrolysing of proteins | NR | 163–756 µg (range)a |
| bLipscomb (2006)194 | UK | SSD from one NHS trust | Ranged in shape and size (n = 23) | Traditional machine washer-disinfector cleaning procedures |
SYPRO ruby protein stain and EDIC/EF microscopy Unclearc |
Results indicated that over half (56%) of the instruments inspected showed severe (classes 3–4) contamination in at least one of the sample regions, 35% were moderately contaminated (class 3), and only 9% displayed low-level deposition (class 0–2). The overall mean contamination index value for all the instruments was 2.8 | |
| bLipscomb (2006)195 | UK | SSDs from nine anonymous NHS primary care trusts | Nine sets (n = 260) | Traditional machine washer-disinfector cleaning procedures |
SYPRO ruby protein stain and EDIC/EF microscopy In situ |
Levels of soiling (scores averaged for each instrument): severe (66%: contamination index score, > 3 to 4); moderate (17%: contamination index score, > 2 to 3); low level (7%: contamination index score, 0 to 2). Across the nine trays, the mean contamination index per instrument set ranged from 2.4 to 3.6; overall mean contamination index value for all the instruments was 3.2. Contamination index: class 3 is 0.42–4.2 µg of protein/mm2 and class 4 is > 4.4 µg of protein/mm2 Statistical analysis indicated that there was significant difference in the levels of contamination between the different types of instrument, with needle holders and tissue forceps (as hinged instruments) showing contamination levels significantly higher than some other instruments |
|
| Murdoch (2006)193 | UK | Five Department of Health and Social Care hospitals | A range of instruments (n = 43) | Autoclaved | ‘Protein extraction and quantification methods’, i.e. levels of protein removed from instruments and identified in the ‘buffer’ or ‘wash’ |
71.67 µgd 8–91 µg (range)e |
NR |
| Baxter (2006)197 | UK | One NHS trust | A basic neurosurgical tray in regular use (n = 6) | ‘Conventional hospital SSD procedures (washing and autoclaving) n = 3; ‘conventional hospital SSD procedures’ plus RF gas plasma, n = 3 |
EDX Unclearc |
Protein contamination on instruments was identified after the conventional SSD procedure, but was ‘not directly quantified . . . the analyses simply show the elemental composition of these residues’ | |
| Baxter (2009)198 | UK | One NHS trust | Forceps (n = 2) | ‘Conventional hospital SSD procedures’ (washing and autoclaving) |
EDX in situ |
Measure of residual protein is by units of fluorescence after conventional SSD processes, but before and after RF gas plasma treatment | |
| Smith (2018)196 | UK | One NHS trust. Some instruments ‘artificially soiled’ with Edinburgh soil | The five most-commonly used neurosurgery sets (n = 1000) | ‘Automated washer disinfector’ in SSD (untreated), plus instruments treated with two types of wetting agents [PreKlenz (Steris, Mentor, OH, USA) and sterile water] | SDS extraction and OPA, ProReveal (Synoptics, Cambridge, UK). Unclearc |
10 craniotomy sets only: instruments, n = 305 (OPA assay, and includes 40 artificially soiled instruments): < 30 µg, except for one untreated instrument: sharp elevator: 44.02 µgf Different sets: instruments n = 187 (ProReveal assay): 87% (163/187): < 5 µg of protein per instrument side; 96% (179/187): < 10 µg per instrument side |
NR |
| Hervé (2013)183 | UK | Manufacturer, contaminated with ‘Edinburgh soil’ | Endoscopes (n = NR) | An ‘enzymatic cleaner used in a number of endoscopy units’ |
SYPRO ruby protein stain and EDIC/EF microscopy Unclear |
Contamination was < 10 ng/mm2 after standard cleaning (see figure 3 in Hervé and Keevil,183 for details) | |
| Hervé (2016)191 | UK | Unknown number of ‘hospital-based endoscopy units’ (n = 6) | Endoscopes (n = 6) | An ‘enzymatic cleaner: Enzol’ (Enzol, Johnson & Johnson, New Brunswick, NJ, USA) |
SYPRO ruby protein stain and EDIC/EF microscopy Unclear |
Level of microcontamination absorbed into the luminal surface of the endoscope: 0.1–0.9 µg of protein/m. With the exception of one endoscope channel (with protein residues equivalent to almost 33 µg), most protein residues remained under the equivalent of 1–4 µg per channel | |
There was no consistency in the measures used to quantify and report the residual protein contamination of surgical instruments after conventional cleaning and sterilisation in a sterile service department (SSD). Murdoch et al. 193 reported a mean amount of protein per instrument of 71.67 µg (range 8–91 µg); Baxter et al. 192 reported a median range of 163–756 µg per instrument; Lipscomb et al. 194,195 reported residual contamination using an unvalidated ‘contamination index’ and reported that 56% of instruments (out of a total of 23) from a single NHS trust showed severe contamination (contamination index score of > 3–4) in at least one of the sample regions, whereas 66% of instruments (n = 260) from nine NHS primary care trusts showed equivalent severe contamination (contamination index score of > 3–4). According to this contamination index, a classification of 3 represents 0.42–4.2 µg of protein/mm2 and a classification of 4 is > 4.4 µg of protein/mm2. The most recent study,196 reported residue ‘per instrument side’ for evaluated instruments from craniotomy sets (n = 187): 87% of instruments were found to have < 5 µg per instrument side and 96% were found to have < 10 µg per instrument side. Two papers did not explicitly quantify the residual protein but only noted its presence. 197,198 The studies assessing endoscopes reported either < 10 ng of protein/mm2 after processing183 or the ‘equivalent to 1–4 µg of proteins per channel, except in one channel which harboured . . . equivalent to almost 33 µg of residual proteins for the whole channel’. 191
Residual mass/protein studies
Studies reporting the impact on protein absorption and/or the relative efficacy of cleaning when keeping instruments wet or dry before processing
Four studies (five papers) reported a comparison between ‘wet’ and ‘dry’ instruments in terms of precleaning protein absorption or post soaking or cleaning protein residue (Table 19). All studies were conducted in the UK, used steel tokens or wires and the same contaminant: 1 µl drops of ME7-infected brain homogenate. Detection was made of in situ contamination using the same techniques: SYPRO® (Life Technologies Corporation, Carlsbad, CA, USA) ruby protein stain and episcopic differential interference contrast/epifluorescence microscopy. Across the studies, drying times before assessment ranged from 15 minutes200 to 24 hours. 200,201
| Study (first author and year of publication) | Country | Contaminant | Steel medium | Pretreatment | Assay/in situ | Dry | Wet | Differences in residual protein (ng/mm2) contamination of wires or tokens | |
|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | ||||||||
| Lipscomb (2007)199 | UK | 1-µl drops of ME7-infected BH | Surgical 316L grade SS tokens (10 × 25 mm) | Klenzyme®; Endozyme AW; Enzol; Liquid 52 (Hamo Liquid 52, Steris, Mentor, OH, USA) | SYPRO ruby and EDIC/EF microscopy; in situ | DT = 17 hours. No presoak/pretreatment | DT = 17 hours 5 minutes for each: Klenzyme, 8 ml/l; Endozyme AW, 4 ml/l; Enzol, 8 ml/l; liquid 52, 8 ml/l | Final residual protein contamination at 22 °C:a
|
Percentage of final residual protein contamination compared with control at 22 °C:a
|
| Howlin (2010)184 | UK | 1-µl drops of ME7-infected BH | Surgical 316L grade SS wires (5 × 0.16 mm) | Klenzyme; Pre-Klenz (presoak gel) | Western blot; in situb | DT = 16 hours followed by pretreatments | DT = 0 hours (immediate treatment) | Total protein removal (ng/mm2): immediate treatment with Pre-Klenz, produced a 2 log-reduction compared with ‘dry’ controls | |
| aSecker (2011, 2010)201,202 | UK | 1-µl drops of ME7-infected BH | Surgical 316L grade SS tokens (25 × 75 mm) | Klenzyme; Endozyme | SYPRO ruby protein stain and thioflavin T (Sigma Aldrich, St Louis, MO, USA), EDIC/EF microscopy; in situb | DT = 24 hours; air RT or F (4–8 °C) | DT = 24 hours; moist: air-tight container lined with moist tissue; RT or F (4–8 °C) |
|
|
| Secker (2015)200 | UK | 1-µl drops of ME7-infected BH (equivalent to 1 µg total protein) | Surgical 316L grade SS tokens (10 × 30 mm) | Prolystica 2 × alkaline detergent, working pH 10.1 or Progenica (Serchem Ltd, Telford, UK) detergent, working pH 10.9 | SYPRO ruby protein stain and thioflavin T; EDIC/EF microscopy; in situb | Air for (DT):
|
Wet bag: sealed bag with 35 ml of distilled water for (DT):
|
24 hours’ humidity:
|
24 hours’ humidity:
|
Dry bag: tied, clear polythene bag for (DT):
|
24 hours humidity:
|
(Repeat from above) 24 hours humidity:Protein absorption pre-cleaning (RT):Prolystica (RT):Prolystica (F):Progenica (RT):Progenica (F): |
|||||||
The process to keep steel tokens or wires ‘wet’ was different in each study: Secker et al. 98 used a ‘wet bag’ (Humibag), that is a sealed bag containing 35 ml of distilled water for set time periods; Secker et al. 201 used an air-tight container lined with moist tissue for 17 hours; Howlin et al. 184 treated steel wires immediately, rather than allowing them to dry; and Lipscomb et al. 199 treated steel tokens with one of four presoak treatments for 5 minutes, followed by 17 hours’ drying time. The dry conditions for comparison were: air dry or a ‘dry bag’ for comparable times to the ‘wet bag’;200 air dry for 24 hours;201 air dry for 16 hours;184 and air dry for 17 hours. 199 Different temperatures were evaluated but this text will focus only on the findings for room temperature (or the closest available data) across studies. Three of the four studies used the enzymatic cleaner Klenzyme (Steris, Mentor, OH, USA).
Both Secker et al. studies200,201 reported on protein residue after 24 hours at room temperature before cleaning: 324.7 ± 15.0 ng of protein/mm2 for the air dry conditions compared with 6.0 ± 3.5 ng of protein/mm2 for the wet conditions (98.2% reduction compared with air dry; p ≤ 0.001)201 and 1000 ± 205.0 ng of protein/mm2 for the air dry conditions compared with 31.9 ± 5.3 ng of protein/mm2 for wet conditions. 200 After the application of presoaks or cleaners, Lipscomb et al. 199 reported a reduction in protein between 64% and 96% on the presoaked or treated tokens compared with the dry, untreated controls and Howlin et al. 184 reported a reduction of approximately 2 logs in protein residue for tokens treated immediately (not allowed to dry) with the PreKlenz presoak compared with wires that were allowed to dry for 16 hours. Secker et al. 200 and Lipscomb et al. 199 also reported that the longer the drying times, the more difficult it was to remove the contamination.
Discussion/summary of studies on residual mass and decontamination
The published evidence suggests that standard cleaning practices within SSDs do not achieve levels of ≤ 5 µg residual protein per instrument for all instruments, as required by current guidance. 203. However, these published data are based on different assays and detection methods and the most recent data196 suggest that as much as 87% of assessed instruments might have protein residue of < 5 µg per instrument side, and 96% might have residue of < 10 µg per instrument side. Recent papers200,201 also report very large differences in protein absorption on instruments kept in dry or wet conditions, with the latter producing as much as a 98.2% reduction in protein absorption compared with dry conditions (p ≤ 0.001). 201 Standard cleaning in SSDs might, therefore, be expected to produce residual protein levels of ≤ 5 µg per instrument side for neurosurgical instruments kept in moist or wet conditions before processing. There is also some evidence for reduced contamination of endoscope channels if kept wet, although the evidence is more equivocal. 183
The findings for autoclaving at 134 °C for 15–20 minutes in the more recent sample of studies are generally similar to those previously reported for publications up to 2004: log-reductions of between 4 and 5, with highly variable transmission rates (ranging from 0% to 100%) that are generally > 50%. It is generally accepted that autoclaving alone only partially inactivates TSE prions. 119,204–207 The majority of studies published in 2004 and earlier focused on the 263K scrapie prion strain, whereas the more recent data have investigated efficiency of autoclaving on a wider range of prions, for example RML and various CJD and BSE strains. Some strains, such as the M1000 strain, appear to be more resistant to autoclaving.
Certain combinations of autoclaving and enzymatic or alkaline detergents have also been reported to achieve log-reductions of infectivity in excess of 5 and transmission rates of 0% in animal assays: for the 263K, RML, 301 V, cattle BSE, Sc237 and sCJD prions strains, alkaline detergents,176 0.2% SDS/0.3% NaOH,175 RMEC B,178 4% SDS plus 1% AcOH,187,190 H2O2 and gas plasma (Sterrad NX1 and 2 cycles). 176 It has been reported that, based on the evidence, the following should be sufficient to achieve adequate levels of inactivation and decontamination of prions bound to steel wires: a combination of an alkaline or enzymatic detergent followed by autoclaving, with each process known to produce a log-reduction of ≥ 5. 205
Within the specified requirements of dose, time and temperature of exposure, a number of decontaminants, without autoclaving, were also reported to achieve log-reductions of ≥ 5 and/or 0% transmission across a range of prion strains. These include Rely-On PI (DuPont) and Prionzyme (Genecor);185 NaOH;180,185 Trigene disinfectant and RF gas plasma;189 SDS-PK-Pronase;186 H2O2 and gas plasma;174,176; and combinations of enzymatic detergents and disinfectants. 174 However, it has also been stated that NaOH and NaOCl, although effective, ‘are not compatible with various pieces of medical equipment, and . . . present a serious handling hazard for healthcare employees’,174 although NaOH is reported to be less corrosive than NaOCl. 119,205 A combination of immersion in NaOH or NaOCl followed by autoclaving is recommended by the World Health Organization. 4,119
It has been acknowledged that these studies do not permit a direct comparison of their respective findings and that the findings are, in some cases, contradictory or discrepant because they have been conducted under different conditions (such as differing prion strains, drying times, whether in vitro or in vivo, different animal assay, infectious titre of the material used, time and temperature of the exposure to the decontaminant, dose of the decontaminant, observation period, substrate used, infectivity detection method used. 119,176,186,204,205,208 Such differences also make direct comparison between findings difficult for human prions and other TSE prions. 177 It is also noted that all are laboratory studies that do not necessarily reflect procedures used in clinical settings; the papers retrieved for this systematic review included studies of surgical instruments and SSDs,189,193,209 but they report contamination only with proteins (not prions) after standard decontamination processes. Although steel wires are generally accepted to be the most useful simulator to test prion adherence to steel surgical instruments, it is also recognised that they do not clean in the same manner as larger, more complicated surfaces. 206,209
The evidence that instruments used for high-risk procedures remain in their original sets after decontamination
The purpose of this review was to identify relevant published and unpublished evidence to determine the extent to which instruments used in neurosurgery remain in a specific set after decontamination procedures as per NICE guidance (IPG196). 14 Labelling or tracking systems may be in place to maintain the integrity of such sets and to reduce or prevent the migration of instruments between sets. Evidence for the migration of instruments between sets might have implications for the risk of transmission of disease between patients undergoing neurosurgery. A report by the ACDP TSE subgroup estimated that the likelihood of at least one instrument migrating in or out of a neurosurgical set is 50%. 117 This document contains a project report and guidance for the Department of Health and Social Care to employers on the precautions to control the risk of exposure of employees and others to TSE agents from work activities. The estimate does not appear to be supported by any evidence. However, if such a high level of migration of instruments during high-risk posterior segment surgery occurred, this could potentially promote a self-sustaining epidemic of CJD or vCJD.
Studies relating to evidence that instruments used for high-risk procedures remain in their original sets after decontamination
The number and type of included studies are shown in Table 20.
| Study (first author and year of publication) | Country | Time period | Design | Strategy | Details |
|---|---|---|---|---|---|
| Belay (2013)119 | USA | 1998–2012 | Audit and evaluation of neurosurgery performed on CJD patients | None |
An audit of the ability of specified centres to identify particular instruments and sets The study provides limited quantitative evidence on sets and set-splitting |
| NICE (2016)210 | England | NR | Qualitative and observational: interviews and a single site visit for neurosurgery | The implementation of guidance on maintaining set integrity |
Qualitative evidence on the barriers to achieving or maintaining set integrity The study provides limited qualitative evidence on sets and set-splitting |
Only two studies were identified that provided any evidence on whether or not instruments for procedures on high-risk tissues remain in their original sets. 119,210 In 2013, an article was published by Belay et al. 119 reporting an audit to identify instruments and sets of instruments that might have been used on patients known to have CJD. The sample was limited to CJD index cases from US hospitals and reported to the US Centers for Disease Control and Prevention. The aim of the audit was to identify patients who subsequently underwent neurosurgery with the same instruments or sets used on the CJD index case. There was no reported strategy in place to maintain or to evaluate set integrity. The audit reported that a single hospital could have between 1 and 12 sets of instruments for neurosurgery, that 12 of the 19 affected hospitals had multiple sets and that in 11 of these 12 hospitals those sets used on a CJD patient could not be identified (Table 21).
| Study (first author and year of publication) | Findings |
|---|---|
| Belay (2013)119 |
|
| NICE (2016)210 | Barriers that affect the implementation of the guidance on keeping instruments for high-risk surgery within specific sets:
|
The second study was an unpublished report produced for NICE in 2016. 210 The aim of the study was to explore the barriers and facilitators affecting the implementation of NICE IPG19614 on the surgical transmission of CJD. The document reported findings concerning the identification of at-risk patients and the acquisition of instruments but also covered the principal perceived barriers to the implementation of the guidance on set integrity, which required keeping all instruments for neurosurgery within their designated sets or ‘kits’. The sample was limited to four NHS trusts in the UK. The report did not provide a detailed methodology for the study. Study participants (‘clinicians and other users’) reported multiple barriers to maintaining set integrity, that is guaranteeing that instruments did not migrate between sets. These are detailed in Table 21 and included: the absence of an adequate and reliable instrument-tracking system; errors in scanning instruments that did have barcodes; the periodic inaccessibility of tracking systems; and their failure to be completely integrated with patient records. The study also reported, however, that set integrity had been improved in the sampled settings by stopping the use of SIs and the increased use of single-use instruments. It is important to note that the report provided no quantitative evidence on whether or not instruments for high-risk surgery remained in their sets, but given that participants reported many problems with identifying and tracking certain instruments, the migration of at least some instruments between neurosurgery sets is probable.
Discussion/summary of evidence on set-keeping for high-risk procedures
Very little research has been undertaken to evaluate whether or not instruments for high-risk neurosurgeries remain in their designated sets. The two studies identified for this systematic review reported only limited evidence on this question. One study was conducted in the USA and reported that instruments could not be identified in the vast majority of cases where they had been used on a patient who was later diagnosed with CJD, and where there were multiple sets in a hospital. 119 The second study210 was performed in the highly relevant setting of the NHS, but is unpublished and its methodology was poorly reported. It did not report quantitative evidence on whether or not instruments for high-risk surgery remained in their sets; rather, the evidence consisted of clinicians’ and users’ reported experiences of implementing NICE IPG196 guidance14 on keeping instruments for high-risk surgeries in their designated sets. These participants reported a range of barriers to set integrity, but also reported more frequent use of single-use instruments and anaesthetic equipment, and that SIs were no longer used. These developments reduce the absolute levels of migration of contaminated instruments between sets. Evidence to substantiate the estimated likelihood of 50% for at least one instrument migrating in or out of a neurosurgical set, posited in the Department of Health and Social Care guidance report117 is therefore limited, but indicates that there is a high probability that at least some if not all instruments in neurosurgery sets do migrate between sets.
The evidence for complication rates of single-use compared with reusable instruments for high-risk procedures
The aim of this review was to identify any published or unpublished evidence for the safety of single-use instruments compared with reusable instruments for high-risk procedures. Safety was to be determined by the relative frequency of complications. This review excluded instruments, including anaesthetic equipment, that would not normally come into contact with high-risk tissues205,211 or that are now single use. 210 Evidence on safety outcomes might have implications for the viability of single-use or disposable instruments as an alternative to reusable instruments for high-risk procedures.
Studies relating to evidence for complication rates of single-use compared with reusable instruments for high-risk procedures
No relevant papers were identified pertaining to this review question.
Discussion/summary of complication rates for single-use versus reusable instruments
An unpublished report produced for NICE in 2016210 explored the barriers to and facilitators of affecting the implementation of NICE IPG19614 on the surgical transmission of CJD. The report summarised the findings of an observational site visit and interviews with ‘clinicians and other users’ in a sample of four NHS trusts in the UK. The participants reported more frequent use of single-use instruments and anaesthetic equipment than before, and that single-use instruments were increasingly relatively inexpensive. However, no published or unpublished studies were identified by this systematic review that compared complication rates for single-use instruments with the complication rates for reusable instruments employed in the designated high-risk neurosurgeries. The relative efficacy and safety of these groups of instruments or devices are therefore unknown.
The evidence for the likelihood of future surgery for a patient undergoing high-risk procedures
The purpose of this review was to identify relevant published and unpublished evidence to determine the risk of future surgery for a patient undergoing high-risk neurosurgical procedures. A risk assessment study performed for the Department of Health and Social Care212 reported that one factor that can have a significant impact on infection dynamics is the chance of individuals having two or more operations (especially surgery to the CNS or posterior eye). The aim of this review was to assess the potential number of high-risk tissue exposures to potentially contaminated instruments, which might then have implications for the risk of transmission of disease to patients undergoing high-risk procedures.
Studies relating to evidence for the likelihood of future surgery for a patient undergoing high-risk procedures
Only one study was identified that provided any evidence on the risk or rate of neurosurgery after a first neurosurgical procedure213 (Table 22). The aim of the study was to assess the feasibility of post-mortem surveillance of patients who had undergone neurosurgical procedures at least 5 years previously, in order to explore the prevalence of subclinical vCJD. To do this, the article analysed the relationship between mortality rates and reoperation rates by procedure. The annual incidence of mortality in this cohort ≥ 5 years after the first instance of neurosurgery was as low as 3% for certain procedures that would not be considered as high risk (such as primary/revision excision of a lumbar disc), whereas a greater likelihood of mortality was associated with other procedures (e.g. brain excisions and the drainage of extra- and sub-dural haematomas).
| Study (first author and year of publication) | Country | Time period | Design | Type of surgery | Details |
|---|---|---|---|---|---|
| Bird (2009)213 | UK (Scotland) | 1993–2001 | Audit and evaluation | Neurosurgery | Neurosurgery (and proportions of patients experiencing more than one procedure) and mortality |
The article reported the extraction and analysis of patient records’ data relating to the 10 most frequent neurosurgical operations performed in Scotland in the period 1993–2001, focusing on four procedures considered to present a medium or high risk of CJD prion transmission: drainage of extra- and sub-dural haematoma; cerebral aneurysm operations; primary or revisional decompression operations; and the creation of ventricular shunts. 213 Two additional potentially relevant procedures from this paper have also been included here: unspecified excision of brain and excision of brain lesion(s); this is because of their low 5-year survival rates (41.5% and 29.9%, respectively). In terms of the current review, the aim was to document the potential for surgical transmission through contaminated instruments by establishing the rate of future high-risk procedures following an index procedure. It is not clear whether, in the Bird et al. 213 report, the future procedures are always the same as the index procedure (i.e. if the index procedure was concerned with ventricular shunts, then the reported rates of future procedures also related only to ventricular shunts) or whether they might be a neurosurgical procedure different from the index procedure. The evidence was presented as event rates for procedures deemed to be of high or medium potential risk of vCJD transmission (Table 23).
| Procedure | Only one subsequent procedure after the index procedure, % (n/N) | More than one subsequent procedure, % (n/N) | Proportion of individuals with a subsequent procedure who underwent more than one, % (n/N) |
|---|---|---|---|
| Drainage of extra- and sub-dural haematomaa | 8.3 (221/2654) | 2.4 (63/2654) | 22.2 (63/284) |
| Cerebral aneurysm operations | 14.8 (264/1782) | 7.1 (127/1782) | 32.5 (127/391) |
| Creation of ventricular shunts | 21.2 (191/900) | 28.3 (255/900) | 57.2 (255/446) |
| Excision of brain: unspecifieda | 12.1 (110/911) | 6.9 (63/911) | 36.4 (63/173) |
| Excision of brain lesion (e.g. frontala) | 13.0 (139/1072) | 5.6 (60/1072) | 30.2 (60/199) |
The data indicate that the proportion of individuals in this sample having a second or third procedure (or more) within 5–10 years after an initial neurosurgical procedure differed depending on the index procedure (see Table 23). This ranged from 10.7% for individuals having one or more additional procedures for the drainage of extra- and sub-dural haematoma to 49.5% for individuals having one or more additional procedures related to a ventricular shunt. In the case of ventricular shunts, the majority (57.2%) of those who had subsequent procedures were also likely to have more than one additional procedure.
Discussion/summary of risk of future surgery in high-risk procedures
The Bird et al. 213 paper is a UK (Scottish-based) study analysing relatively recent patient records’ data on the actual proportions of patients undergoing one or more medium- or high-risk neurosurgical procedure. This evidence indicates that, depending on the procedure, between 50% and 90% of patients are unlikely to have a second high-risk procedure within 5–10 years of the initial procedure and that the number of patients undergoing additional procedures, with their increased risks of surgical transmission, depends heavily on the procedures involved. The potential for the Bird et al. 213 paper to inform the model is limited, as the paper did not focus solely on high-risk procedures and does not compare the risk of additional procedures with control data for those who had not undergone an index high-risk procedure.
Chapter 3 Cost-effectiveness
Background
Previous modelling work assessing the risks of surgical transmission of CJD was undertaken by ScHARR, culminating in a report in 2006. 11 Henceforth, this will be known as the ScHARR report. This report was part of the evidence base appraised by the CJD Advisory Sub-Committee (CJDAS), which produced IPG196. 214 This guidance highlighted three high-risk surgical areas: neurosurgery, posterior eye and neuroendoscopy. It was recommended that migration of instruments between sets should be abolished and that single-use instruments were not recommended on the basis of cost-effectiveness with the exception of accessories for neuroendoscopy. A separate recommendation was made that separate sets of instruments should be established for patients born after 1 January 1997 (who are unlikely to have been exposed to the BSE epidemic).
An update of the previous work was undertaken by ScHARR. However, with the agreement of NICE, the current work focuses solely on surgical procedures deemed to be high risk. This update incorporates the latest evidence on key model parameters and assess a range of appropriate strategies and interventions. Reasons for updating IPG196 include the continued evolution of high-quality and less expensive single-use instruments; the lack of adoption of new decontamination methods potentially effective against human prions; the findings of abnormal prion accumulation in the appendixes of patients born after 1996; and anecdotal reports that the recommendations of IPG196 have proved to be difficult to implement, or unachievable, for a number of units. The primary deliverable was a report for a NICE committee that had been convened for the purposes of providing an update to IPG196.
The analyses undertaken assess the potential transmissions of all forms of CJD, which include sCJD, fCJD and iCJD. Throughout the report, any CJD cases that have been caused by surgical transmission will be abbreviated to stCJD.
Elicitation
Many model parameters are subject to considerable uncertainty and were populated following two elicitation sessions undertaken in 2005, one with epidemiological experts and one with decontamination experts. These elicitations were reported in Stevenson et al. 11 and the results are repeated in this report. At a meeting of the NICE interventional procedures committee and ScHARR in October 2017, it was decided that the elicitation related to epidemiological parameters should be reconducted to address possible concerns relating to the lack of potential to be misdiagnosed with a different neurodegenerative disease, and with the incubation periods previously elicited. This elicitation session was undertaken on 18 January 2018; the results of the elicitation exercise are in Appendix 4.
Elicitation methods
The 2018 elicitation session was conducted using the Sheffield Elicitation Framework (SHELF). Four experts participated in a face-to-face facilitated workshop. The experts first completed a training exercise (using a quantity known to the facilitator, but unknown to the experts) to familiarise themselves with the elicitation methodology. For each parameter, the experts first recorded their probability judgements individually without conferring. Experts were asked to separately consider lower and upper plausible limits; different scenarios that might lead to high values or low values of the parameter. Probabilities were not attached to these plausible limits; the purpose of eliciting the limits is to mitigate the effects of anchoring and overconfidence, which may occur if a ‘best guess’ is first provided, followed by some assessment of uncertainty around such a guess.
The experts were then asked to provide median values by dividing their plausible ranges into two intervals judged to be equally likely. They were then asked to divide each interval into two further equally likely intervals, hence providing their lower and upper quartiles.
Each expert then declared his/her judgements to the facilitator, who then presented a graphical comparison of all the experts’ individual judgements. Disagreements between the experts’ judgements were highlighted and the experts were invited to justify their own opinions and question each other. Following the discussion, the experts were asked to imagine a rational impartial observer (RIO): an independent observer who has heard and understood the discussion, and on that basis formed his/her own probability judgements. It was for the experts to decide how much weight a RIO would give to the different opinions/arguments that had been stated; if the experts disagreed, with no convincing experts to favour one side over the other, RIO’s uncertainty would be expected to reflect the disagreement.
The experts agreed on a median and quartiles for RIO’s distribution. The facilitator then fitted a probability distribution to these judgements, by choosing a parametric family of distributions and selecting parameter values using a least-squares fit to the cumulative distribution function. The selected distribution was presented to the experts, with feedback in the form of 5th and 95th percentiles (which had not been directly elicited). The experts were asked to comment on whether or not the fitted distribution was an acceptable representation of RIO’s uncertainty and whether or not the level of uncertainty would be justified based on the proceeding discussion. The distribution would be modified as necessary, before being adopted within the ensuing calibration and probabilistic sensitivity analysis (PSA).
Experts were recruited from within the NICE advisory committee. We believed that the workshop format was important to allow for sufficient training of and discussion between the experts. However, owing to the time scale of the project, it was possible to convene only one workshop with four experts.
Cost-effectiveness literature review
The literature searches of bibliographic databases were performed on 14 August 2017 and yielded 1108 citations. Forty-eight citations were obtained for full text retrieval. Evidence from none of the papers was directly used within the model, but some provide context or alternative values and have been detailed in the context of alternative values for the de novo model.
The conceptual model
Previously, authors of this report had undertaken work for the CJDAS to assess the cost-effectiveness of single-use instruments to reduce the risk of vCJD through surgical procedures. 11 The paper by Stevenson et al. 12 provided further information, where they had utilised a Bayesian approach to take into account data observed since the generation of the results for NICE and submission of the manuscript. As this model was used by the research team in the initial appraisal in 2005, there was a preference to use, or adapt, this model unless it was shown to be not fit for purpose.
Within the literature review by Bennett et al. 13 a publication was identified, which was not conceptually different from Stevenson et al. 12 but used a system dynamics approach. Bennet et al. 13 had three broad aims: (1) to clarify the possible scale of vCJD infection via surgical instruments, (2) to identify the most important factors contributing to this risk and (3) to help prioritise scientific research. Conclusions from the Bennet et al. 13 paper were that ‘the risk of surgical transmission of vCJD could not be dismissed’ and that improvements to decontamination ‘should be respectively cost-effective unless vCJD turned out to be a very rare disease’. As the paper by Bennett et al. 13 was published earlier than that of Stevenson et al. 12 (2005 compared with 2009), this was not preferred to the previous modelling structure.
A further paper by Garske et al. 215 was identified that reported that key determinants of future cases of CJD were the number of times an instrument is re-used, the infectivity of contaminated instruments and the effectiveness of decontamination. These results came from a differential equation model that did not consider instrument migration nor the mass transferred to a patient. The former was noted to be a key parameter in Stevenson et al. ,12 which also explored uncertainty in the mass transferred and was published later than the Garske et al. 215 paper (which was published in 2006) and thus this model was not deemed preferable to that of Stevenson et al. 12
Based on the authors’ critique of Bennett et al. 13 and Garske et al. ,215 there appeared no strong reason to diverge from a model foundation as described by Stevenson et al. 12 This model was amended in consultation with the NICE committee, most noticeably to include the possibility that patients may be an stCJD case but could be diagnosed with an alternative neurodegenerative disease.
A schematic of the conceptual model relating to infection transmission in Stevenson et al. 12 is shown in Figure 4; this model works on an individual patient level for those with CJD infection. The modelling unit was a geographical area representing a population 1/27 of the size of England, which was assumed to have a neurosurgical centre and a posterior eye centre. Population of the model is detailed in Key model parameters. Figure 4 depicts the flows of patients, instrument sets and SIs that have the potential to transmit CJD surgically. Patients have been categorised into three discrete groups: (1) patients who are not infected with CJD; (2) patients who are infected with CJD who are not infectious; and (3) patients who are infected with CJD and are infectious, but asymptomatic. Patients who have clinical CJD would not be operated on with reusable instruments and are assumed to be outside the modelling process. Across time, patients can move (1) from the non-infected state to the infectious but asymptomatic state following an operation with a contaminated instrument, and (2) to the infected and infectious state when the incubation period of the disease for that patient has been reached; additionally, patients can be removed from the model when the CJD infection becomes symptomatic. In all states, patients can die in accordance with background mortality rates applicable to the age of the hypothetical patient. The decontamination cycle removes mass from the instruments and reduces the infectious titre where applicable.
FIGURE 4.
The conceptual model relating to the infection process. Reproduced from Stevenson et al. ,12 with permission from Journal of the Operational Research Society.
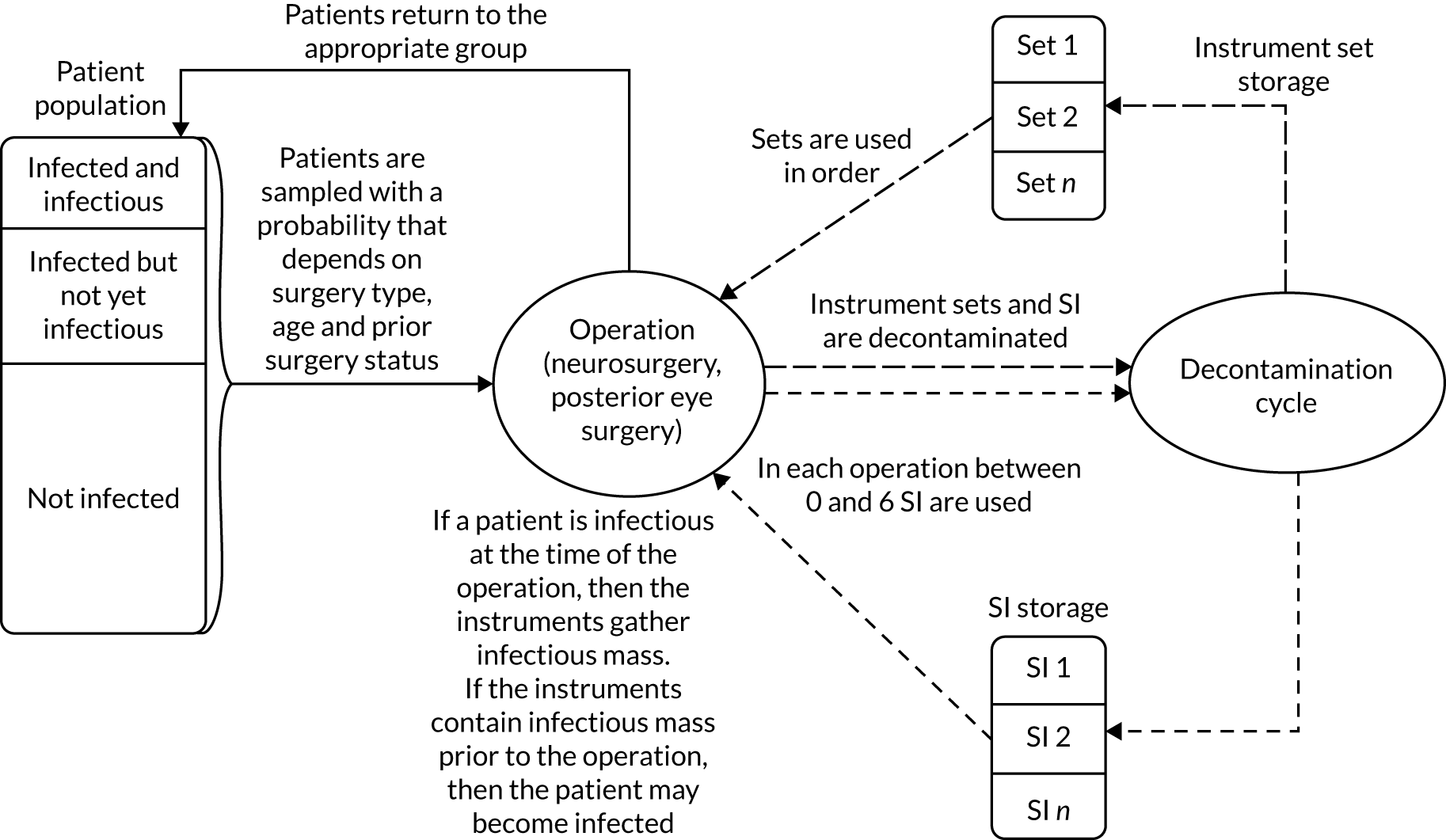
During the operation, decontamination process and instrument-storing process, instruments may migrate between sets. Furthermore, SIs cannot always be distinguished from similar items in the main instrument set and migration between SIs and instruments from the main set can occur. The rate of instrument migration is important in circumstances where there are multiple contaminated instruments in one set. Therefore, maintaining set fidelity can limit the spread of infection compared with a situation where the contaminated instruments are spread across a number of sets, which can result in a greater number of subsequent transmissions. In order to model this, dynamic SIs were modelled at an individual level, whereas sets were modelled with the possibility of instrument migration.
A key change in the methodology is that where previously patients born after 1996 were excluded from the original ScHARR model, these were explicitly included in the updated modelling work. The rationale for the change was that it may be the case that such patients can be infectious, whereas previously this was not thought possible, and that this explicitly allows an evaluation of the health and cost implications of removing the guidance that patients born after 1996 should use different instrument sets to the remainder of the population.
Figure 5 provides the conceptual model for determining the outcomes for patients who have become infected. There has been a fundamental change in this process since the initial work undertaken by ScHARR, as the possibility that patients who become symptomatic following infection with CJD are misdiagnosed as having a different neurodegenerative disease is included. Further details are provided in following sections.
FIGURE 5.
The conceptual model relating to patient outcome post infection.
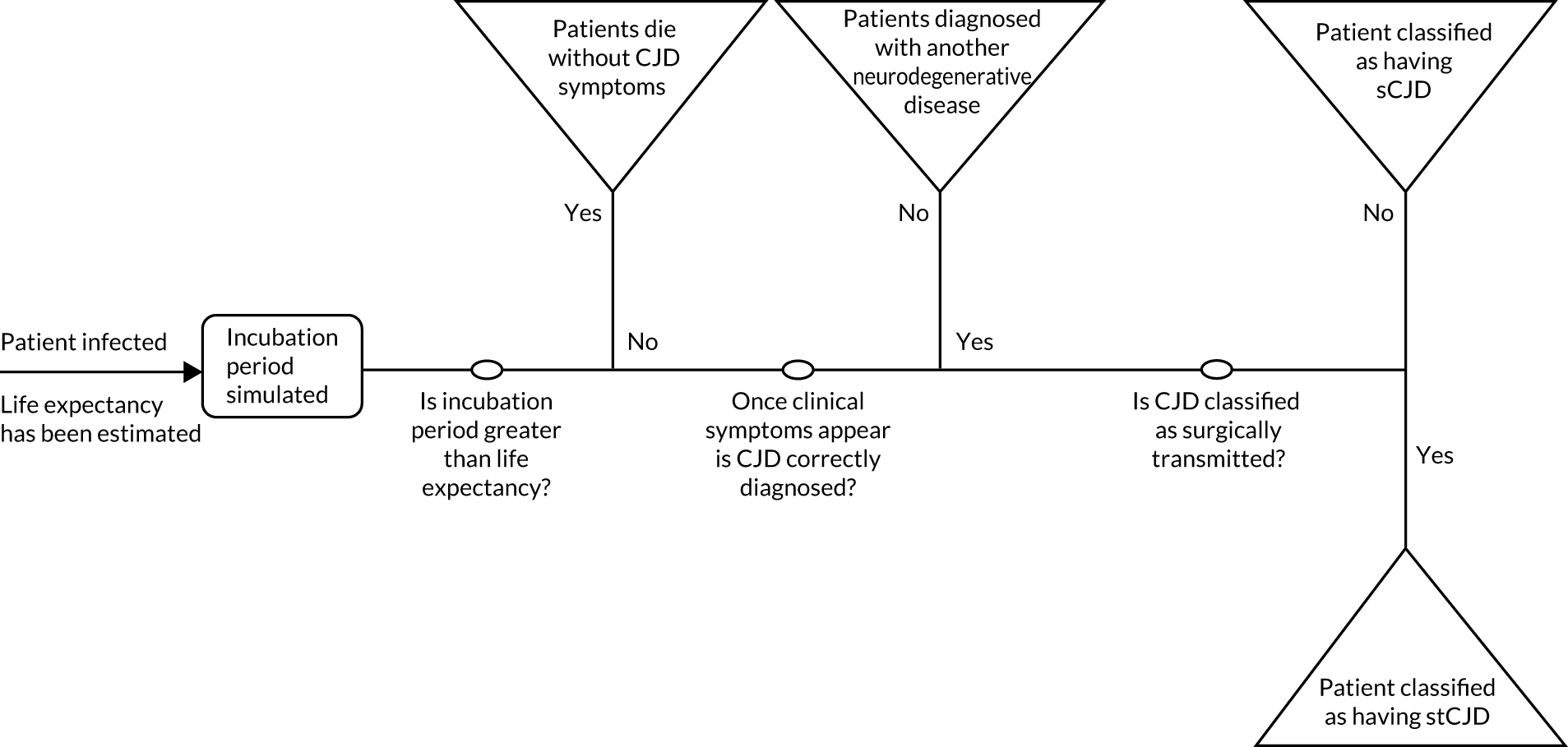
The model was run from 1 January 2004, the year at which a proportion of key distributions within the model were elicited, to 2018 in the calibration period. This duration included a 1-year warm-up period from 1 January 2004 to 1 January 2005, which allowed for the possibility that instruments were contaminated with CJD prions at the start of 2005. The expected number of modelled CJD cases (estimated based on the number of transmissions that resulted in clinical infection and the elicited probability of correct diagnosis) between 2005 and 2018 were then compared with those potentially observed in the UK to establish plausible bounds for use within the PSA and then subsequently to determine likelihood ratios for each PSA configuration. This process is described in further detail in a later section (see Appendix 7).
Having established parameter configurations that were plausibly consistent with the number of stCJD cases potentially observed, the model was run for a further 5 years to look at the potential loss of health because of stCJD associated with each strategy evaluated. The 5-year period was agreed with the NICE advisory committee to be an appropriate time period that would be sufficiently long to allow potential cases of stCJD to become apparent, but short enough that the computational time required to generate the results was not excessive and that it did not limit the committee to a decision which could not be changed in the longer term if required. The 5-year period matched the value used by Stevenson et al. 12 The measure of benefit was reported in terms of life-years gained and quality-adjusted life-years (QALYs). A lifetime perspective was undertaken for the patients simulated to have high-risk surgery within the 5-year period.
The model was constructed in Simul8 (© 2017 Professional Edition Simul8 Corporation, Glasgow, UK). A NHS and Personal Social Services perspective was taken and both costs and benefits were discounted at 3.5% per annum as recommended by NICE. 216
Key model parameters
Parameters relating to the probability and the mass of prions being transferred to surgical instruments
The underlying probability of Creutzfeldt–Jakob disease prions within central nervous tissue in the asymptomatic population
The experts in the elicitation session indicated that the previously elicited distributions relating to the prevalence of CJD prions in all tissue for patients aged 16–39 years in 2005 could still be used for the prevalence of CJD prions in central nervous tissue in 16- to 39-year-olds in the current analysis; although, they acknowledged that the range would produce an overestimate as the probability in all tissue would be greater than that confined to just the CNS. The experts disagreed with the previous experts in whether or not the prevalence would be greatest in the 16–39 years age group compared with the 0–15, 40–69 and ≥ 70 years age groups. The current experts believed that the elicited distribution should be used for all age groups. The distribution used to populate the model is shown in Figure 6 and represents a beta (1.240, 2225.393) for the prevalence. The distribution provides a 95% credible interval (CrI) ranging from 26 to 1875 people per million.
FIGURE 6.
The prevalence of CJD prions within central nervous tissue.
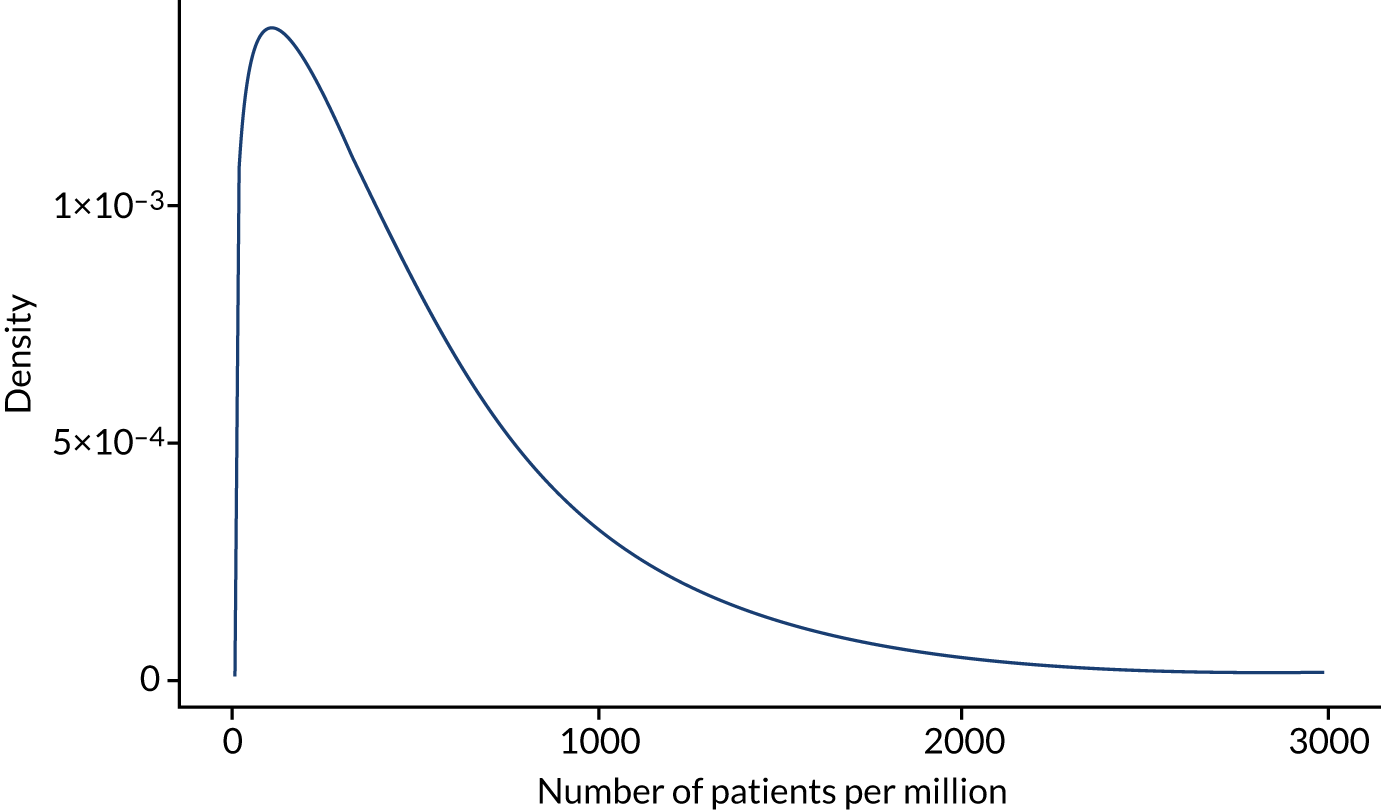
The NICE committee asked for two scenarios to be evaluated, which used different assumptions for the patients born after 1996, henceforth denoted the P96 group. In one scenario, it was assumed that the P96 group were not infectious, as they were assumed unlikely to have been exposed to the BSE epidemic; in an alternative scenario it was assumed that the P96 group had the same probability of being infectious as the general population.
The residual mass per surgical instrument
The ScHARR report11 assumed that the mass on an individual instrument was 2.88 mg of wet-tissue equivalent for instruments used for tonsillectomies, and 1.26 mg of wet-tissue equivalent for instruments used in general surgery. This mass was assumed to be independent of size and complexity. The source for this was ‘provided by Professor Baxter and colleagues from the University of Edinburgh’,11 with these data reported in a different form within Baxter et al. 192 It was assumed that the tonsillectomy value was generalisable to the residual mass on a brain and posterior eye surgery. When multiplied by the number of instruments assumed to be in each set, this equated to 51.84 mg of wet-tissue equivalent on brain surgery instrument sets and 25.92 mg of wet-tissue equivalent on posterior eye instrument sets. Each SI would have a wet-mass equivalent of 2.88 mg. These values were assumed fixed.
During discussions on the parameterisation of residual mass, a committee member highlighted a recently published article,196 which suggested that the residual protein mass is likely to be < 5 µg protein mass per instrument side. This is considerably less than that used in the previous ScHARR report,11 which was 576 µg of protein mass (2.88 mg of wet-tissue equivalent).
A preliminary inspection of articles discussing residual mass was undertaken, which indicated that protein mass ranged between 163 and 756 µg (120 instruments) in Baxter et al. 192 and between 8 and 91 µg (mean 71.67 µg; 43 instruments) in Murdoch et al. 193 Lipscomb et al. 195 presented further evidence, which was based on a set from each of nine NHS trusts (260 instruments in total), and reported that 66% of all instruments showed severe contamination in at least one sample area, equating to > 4.4 µg of protein/mm2.
Examining the data in Baxter et al. 192 and Murdoch et al. ,193 the mean residual protein mass per instrument in 2004 was set to 200 µg (95% CI 150 to 250 µg) in consultation with NICE committee members.
However, the data reported in Smith et al. ,196 and further data marked as academic-in-confidence obtained from a NICE committee member (anonymous, May 2018), indicate that there has been a reduction in mass over time for the hospitals where data has been recorded. In discussion with committee members, it was assumed that this change, which is assumed to be related to guidance on keeping instruments moist prior to decontamination, would have occurred in 2012 in line with the purchase of new instruments for those units that had adhered to IPG196. Following discussion with committee members, the mean residual mass for those units that were compliant with guidance to keep instruments moist was assumed to be 10 µg. In the 90% of units that did not adhere to IPG196, it was assumed that two-thirds of these (i.e. 60% of total units) would not keep instruments sufficiently moist and that 200 µg of residual mass would remain on each instrument, with the remaining third (i.e. 30% of total units) adequately keeping instruments moist. It was assumed that the reduction in protein residue on instruments will translate into a reduction in the possibility of transmission of stCJD.
This conceptual model was operationalised by assuming that the mass harvested from a patient from 2012 onwards was 5% (10/200) of the mass assumed to be harvested prior to 2012. Any infectious mass already on an instrument was assumed to remain on the instrument following measures to keep instruments moist.
The proportion of residual mass on brain and posterior instruments that is transferred to a patient
This value was estimated in the original elicitation exercise, which was undertaken to inform the previous work by ScHARR. 11 A depiction of the distribution for the proportion of residual mass transferred to the patient is provided in Figure 7. This has a mean of 31.5% and a 95% CrI 0.4% to 87.1%, showing considerable uncertainty.
FIGURE 7.
The proportion of residual mass transferred to a patient.
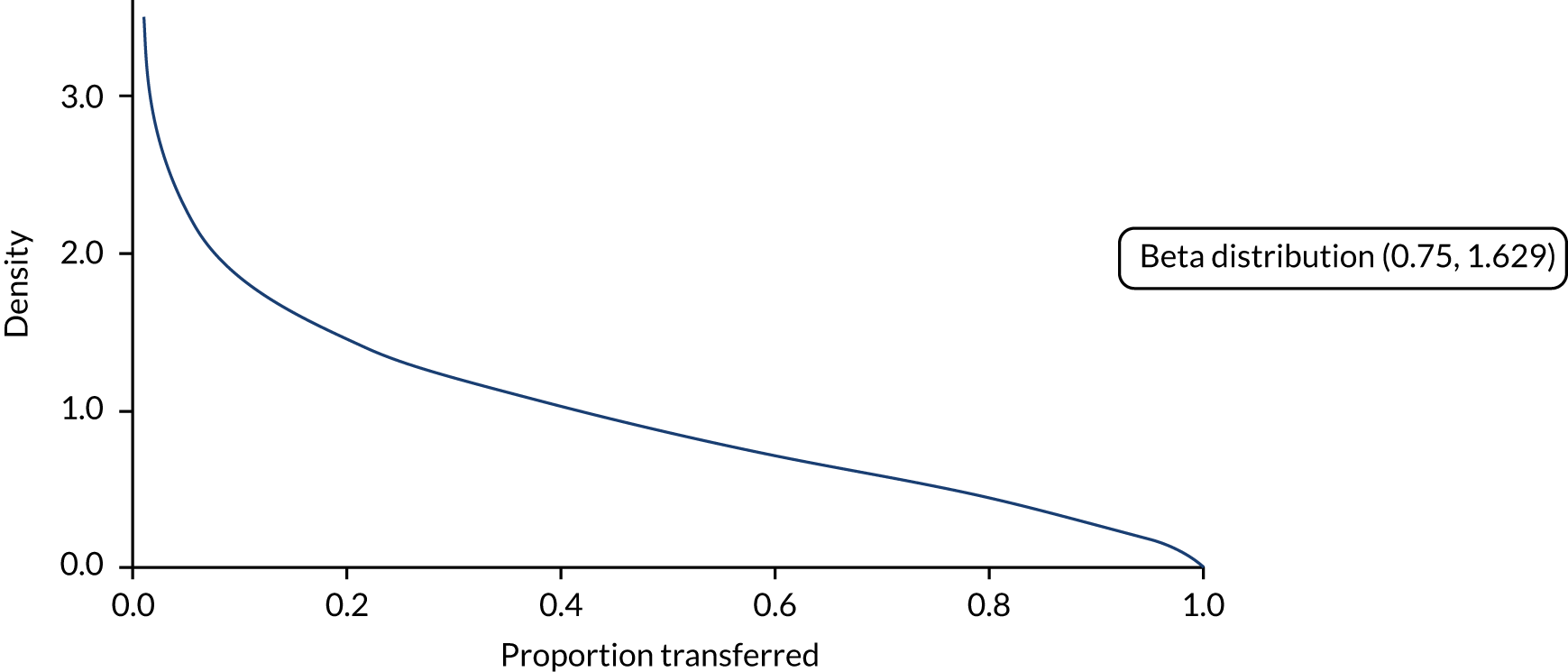
The proportion of residual mass on brain and posterior instruments that is removed in a subsequent decontamination cycle
This value was estimated in the original elicitation exercise, which was undertaken to inform the previous work by ScHARR. 11 A depiction of the distribution for the proportion of mass transferred to the patient is provided in Figure 8. This has a mean of 0.9% and a 95% CrI of 0.0% to 4.0%.
FIGURE 8.
The proportion of residual mass removed in a subsequent decontamination cycle.

The proportion of mass on instruments that is replaced with new tissue per brain or posterior eye operation
In accordance with the previous ScHARR model,11 it was assumed that the residual mass on an instrument was in steady state. Therefore, the sum of the mass transferred to the patient and the mass removed in the next decontamination cycle equals the newly acquired mass from the operation. The mean value of the proportion of the mass removed from instruments during an operation is 32.4%, with an estimated 95% CrI of 1.1% to 88.4%. Any SIs that were used were assumed to gather the same mass as instruments in the main set.
Residual mass, proportion transferred to a patient, proportion removed during the operation and the mass harvested during neuroendoscopy
The spreadsheet calculations that were performed to obtain the proportion of mass transferred to the patient and the proportion removed in the next decontamination cycle for rigid neuroendoscopes and flexible neuroendoscopes used in the previous modelling work11 could not be retrieved, but the values used in the PSA were available. These values have been re-used in the modelling, although it appears that there was a discrepancy between the mass transferred from a patient to a rigid neuroendoscope lumen used in the model and the mass that was reported in table 11 of the ScHARR report,11 with the former being 10 times smaller. For the updated work, we have erred on the side of caution and assumed that the greater mass is harvested per operation.
For information, key statistics on the proportion of mass harvested from a patient, the proportion of mass transferred to a patient and the proportion of mass removed in the next decontamination cycle are provided in Table 24.
| Type of neuroendoscope | Proportion of mass transferred to a patient, mean (95% range in the PSA) | Proportion of mass that has already been decontaminated that is removed in the next decontamination cycle, mean (95% range in the PSA) | Mass harvested from a patient (µg), mean (95% range in the PSA) |
|---|---|---|---|
| Flexible | 19.5% (3.10% to 49.73%) | 70.6% (42.10% to 91.22%) | 2.37 (0.74 to 4.21) |
| Rigid | 0.61% (0.00% to 2.99%) | 1.22% (0.00% to 5.24%) | 0.48 (0.00 to 2.24) |
Parameters relating to the decontamination of surgical instruments
The assumed infectious titre of tissues containing Creutzfeldt–Jakob disease prions
In the previous modelling undertaken,11 brain and posterior eye tissue were assumed to have 108 ID50 per gram, with this value assumed to be fixed and applied from the moment the patient became infectious to the moment when clinical symptoms of CJD were observed, in which instance reusable instruments would not be used on the patient.
The NICE committee requested, based on the collective experience of its members, that the previous assumptions were amended to allow more heterogeneity in patients who have CJD prions in high-risk tissue. First, the mean infectious titre was varied between 107 and 109 ID50 per gram, assuming a uniform distribution. Second, it was assumed that 20% of patients would have an infectious titre 1 log higher than the mean and that 20% of patients would have an infectious titre 1 log lower than the mean, with the remaining 60% of patients having the mean value. This approach incorporates uncertainty around the mean estimate as well as patient heterogeneity, with individual patient values ranging from 106 to 1010 ID50 per gram.
The effectiveness of current decontamination processes in reducing infectivity
The distributions produced from the elicitation exercise to inform the ScHARR report11 were considered appropriate by the NICE committee. These were split into three categories: (1) the effectiveness of infectivity reduction in the first decontamination cycle, (2) the effectiveness of infectivity reduction in subsequent decontamination cycles and (3) the mass removed in second and subsequent decontamination cycles. The model assumes that there have been no improvements in the reduction in infectivity since 2004.
The effectiveness of infectivity reduction in the first decontamination cycle
The distribution assumed for the infectivity reduction associated with the first cycle of autoclaving is displayed in Figure 9. This has a mean log-reduction of 2.50 and a 95% CrI log-reduction of 1.42 to 3.58.
FIGURE 9.
The reduction in infectivity in the first autoclaving cycle.
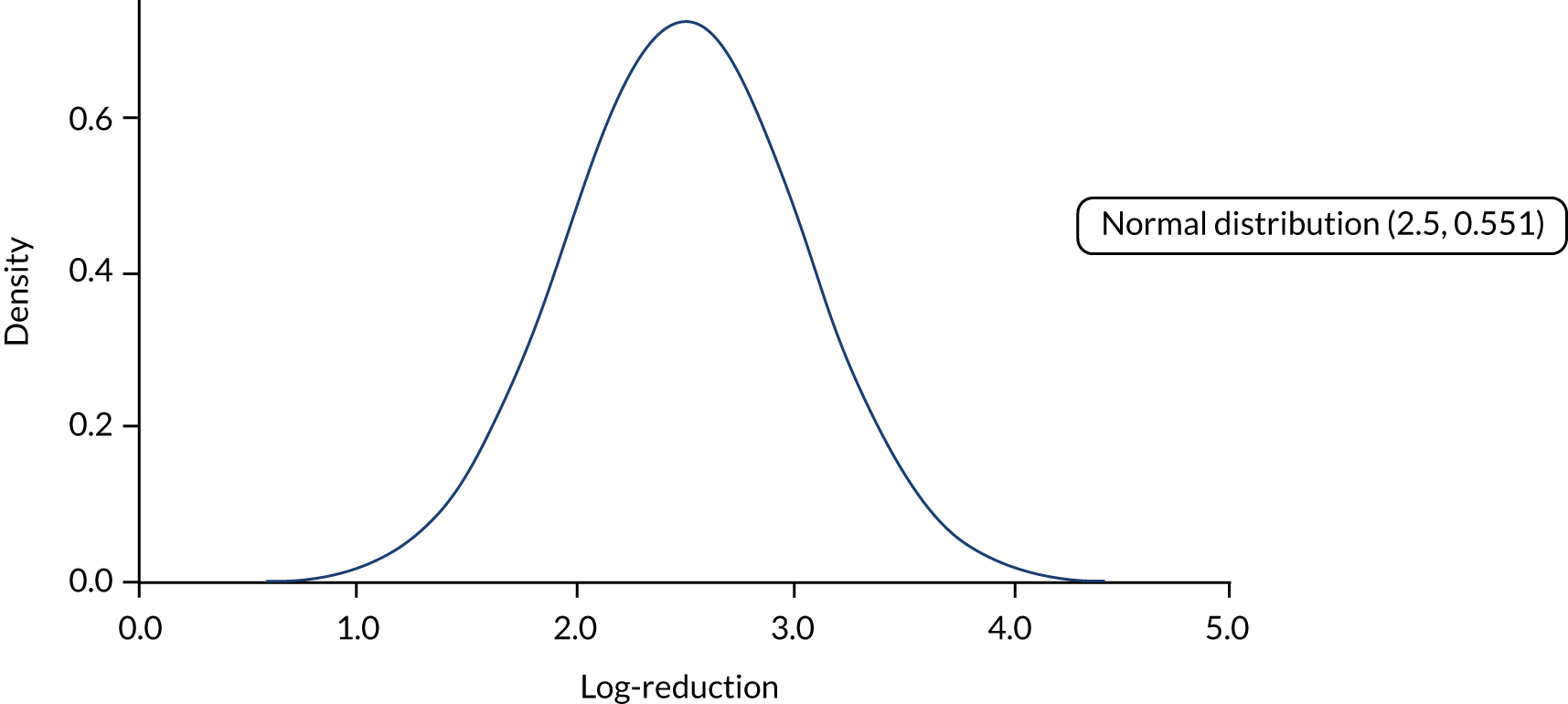
The distribution assumed for the infectivity reduction associated with the first cycle of detergents is displayed in Figure 10. This has a mean log-reduction of 0.64 and a 95% CrI log-reduction 0.04 to 2.03. Note that detergents used in cleaning neuroendoscopes were assumed to not reduce infectivity.
FIGURE 10.
The reduction in infectivity in the first detergent cycle.

The effectiveness of infectivity reduction in subsequent decontamination cycles
It was assumed in the ScHARR report11 that the second and third autoclaving cycles would reduce prion infectivity, although this would be to a lesser extent than the initial autoclaving cycle. These further autoclaving cycles would occur following a subsequent operation. The log-reduction on the second and third autoclaving cycle was expressed as a proportion of the reduction estimated in the first cycle. The distribution assumed for the multiplier is shown in Figure 11. This distribution has a mean of 0.157 with a 95% CrI of 0.043 to 0.330.
FIGURE 11.
The proportion of autoclave cycle 1 log-reduction achieved by cycles 2 and 3.
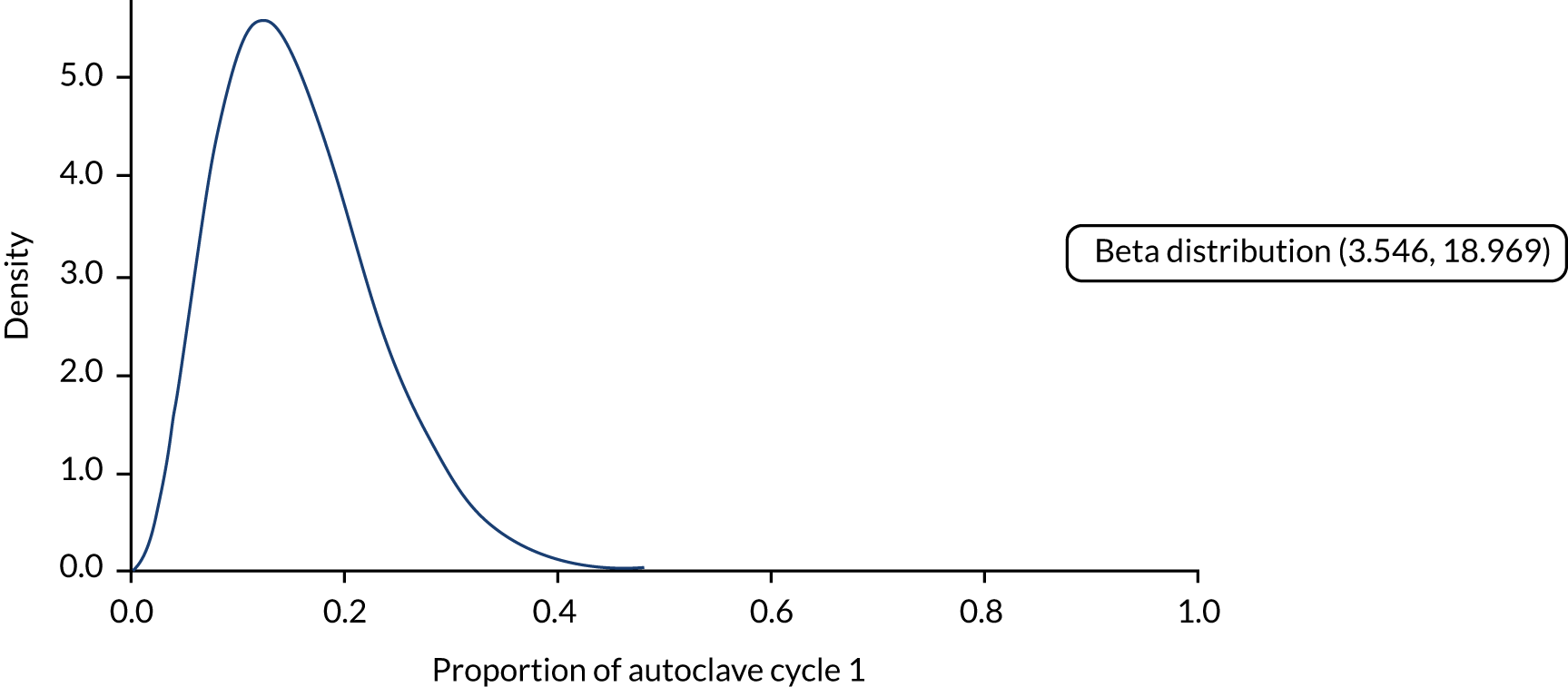
It was assumed in the ScHARR report11 that the second detergent cycle would reduce prion infectivity, although this would be to a lesser extent than the initial autoclaving cycle. The log-reduction on the second and third autoclaving cycle was expressed as a proportion of the reduction estimated in the first cycle. The distribution assumed for the multiplier is shown in Figure 12. This distribution has a mean of 0.474 with a 95% CrI of 0.047 to 0.931.
FIGURE 12.
The proportion of detergent cycle 1 log-reduction achieved by cycle 2.

The proportion of mass that has been through a decontamination cycle that is removed in subsequent decontamination cycles
This has been detailed in The proportion of residual mass on brain and posterior instruments that is removed in a subsequent decontamination cycle for brain and posterior eye instruments and in Residual mass, proportion transferred to a patient, proportion removed during the operation and the mass harvested during neuroendoscopy for neuroendoscopes.
The probability of disposing of a reusable instrument
In the ScHARR report,11 it was assumed that following use an instrument had a 1/250 probability of being disposed of (range 1/200–1/300) with all infectious load on the instrument destroyed. In discussions with the committee, it was believed that the serviceable life of a reusable instrument was longer than that previously assumed and the probability of an instrument being disposed of was reduced to 1/2500 with a range of 1/2000–1/3000.
For each instrument that was disposed of in a brain surgery set, it was assumed that between 0% and 12% (sampled from a uniform distribution) of infectious load was removed from the set. For each instrument disposed of in a posterior eye surgery set, it was assumed that between 0% and 25% (sampled from a uniform distribution) of infectious load was removed from the set. The midpoints of these distributions (6.0% and 12.5%, respectively) were chosen such that it was close to the proportion of the set that one instrument comprised. This is (see Parameters relating to instrument migration, costs and safety) a 1/14 probability (7%) for an instrument in a neurosurgery set and a 1/9 probability (11%) for an instrument in a posterior eye surgery set. Uncertainty was incorporated by allowing a range between 0% and approximately twice the midpoint value.
Parameters relating to instrument migration, costs and safety
The instruments assumed on model set-up
In the modelling undertaken for the ScHARR report,11 it was assumed that there were 12 brain surgery sets with 18 instruments assumed to come into contact with potentially infectious mass; 12 posterior eye surgery sets with nine instruments assumed to come into contact with potentially infectious mass; and one rigid neuroendoscope and one flexible neuroendoscope, both of which had a single accessory.
Following discussion with the committee, it was assumed that the number of instruments coming into contact with high-risk tissue in brain operations was lower than previously thought, with the number reduced to 14 instruments (previously 18).
For brain and posterior eye sets, the instrument sets were used in rotation. For neuroendoscopy operations, it was assumed that 75% were undertaken with rigid neuroendoscopes (which can be autoclaved) and 25% were undertaken using flexible neuroendoscopes (which cannot be autoclaved).
Brain and posterior eye sets were also complemented by six types of SI, each of which had six instruments that were used in rotation. During each operation, each SI had a 20% chance of being required.
For neuroendoscopy, IPG196214 recommended that all neuroendoscopy accessories became single use. For simplicity, however, it was assumed that this was not followed, based on the committee’s estimation of units that had adhered to IPG196 and with an assumption that one SI was used in all operations. If a large number of deaths was observed related to neuroendoscopy, this assumption would be amended.
Recommendations on instrument migration and use of supplementary instruments in IPG196
Maintaining the integrity of surgical instrument sets was shown to be a key parameter affecting the incremental cost-effectiveness ratios (ICERs) associated with the introduction of single-use surgical instruments. 12 The ScHARR report11 made the following assumptions in relation to set integrity:
-
That the probability of an instrument being swapped with a similar instrument in a separate set was 50%, while the set was undergoing the decontamination process. This value was selected following discussion with clinicians and review of evidence. When instruments migrate between sets it was assumed that between 0% and 20% (sampled from a uniform distribution) of the infectious material in ‘set A’ would move to ‘set B’, with between 0% and 20% (sampled from a uniform distribution) of the infectious material in set B being moved to set A. These values were chosen as there were approximately 10 instruments in a surgical set, which would be expected to contain 10% of all mass (infectious or not) and that there would be expected uncertainty around the proportion of mass contained on individual instruments.
-
That when a SI was used, there was a 50% chance that this instrument would join the set with a similar instrument from the set becoming the ‘new’ SI. When this occurs, all infectious load on the SI is added to the set, and between 0% and 10% of the infectious load (sampled from a uniform distribution) in the set is assumed to reside on the new SI. The distribution used to model infectious mass transference as a result of SI migration is associated with smaller mass levels than non-SI instruments.
The model has the facility to alter the levels of set migration following the publication of IPG196,214 which recommended that migration of instruments between sets should be abolished and that SIs that come into contact with high-risk tissues should either be single-use or remain with the set to which they have been introduced. However, owing to logistical and/or financial problems in implementing IPG196,214 these recommendations were not fully adhered to by all hospitals. The model has been set up so that it is assumed that after 2012 no SIs are used for those units that are assumed to adhere to IPG196.
The costs associated with single-use instruments
A NICE committee member stated that the costs of single-use sets are likely to lie in the region of £350–500 and that the cost of a single-use rigid neuroendoscope is £710; no cost was identified for a single-use flexible neuroendoscope (anonymous, May 2018).
The costs associated with reusable instruments
In the ScHARR report,11 it was assumed that a brain surgery set costs £3500 and that a posterior eye set costs £1000. Based on the number of instruments that come into contact with high-risk tissue, the cost of an individual reusable instrument is likely to be in the region of £100–200.
The ScHARR report11 assumed that a reusable rigid neuroendoscope costs £397 and a reusable flexible neuroendoscope costs £9300. More recent prices estimate that a reusable rigid endoscope set including instruments would cost approximately £8850, with a flexible endoscope costing approximately £21,000. Although there will have been inflation during the period, the increase in prices for rigid neuroendoscopes in particular, look high. Clinical advice suggests that in 2005 these were very cheap, disposable rigid neuroendoscopes, but that these have since been withdrawn. This puts downwards pressure on the costs of the reusable rigid neuroendoscopes and, furthermore, it is likely that the volume of sales of neuroendoscopes has decreased resulting in an increase in the price. Whatever the reasons underlying the increase, the NICE committee were comfortable that the prices used in this report was appropriate.
The costs associated with decontaminating reusable instruments
Data provided by a committee member indicated that the cost of decontaminating a reusable instrument was, on average, £0.60 in Scotland (personal communication, May 2018). Assuming that this result is generalisable to England, this would correspond to a decontamination cost of £8.40 for a high-risk tissue brain set and £5.40 for a high-risk tissue posterior eye set.
The costs associated with disposing single-use sets
For simplicity, we have assumed that the costs of disposing of single-use sets are included within the purchase price. Given the relatively wide range in the costs assumed for a single-use high-risk tissue set (£350–500), the authors of this report deemed that this simplifying assumption would not cause significant inaccuracy.
The costs associated with keeping instruments moist
Data reported in Smith et al. 196 state that the cost of NHS bags would be £440 per 7355 neurosurgical trays reprocessed, equating to £0.06 per bag. Calculations based on the additional savings that could be made ‘using tap water and tray liner’ suggests that the costs of these elements are also £0.06 per tray. Thus, it has been assumed that the cost of keeping instruments moist was £0.12 per set conditional on using NHS bags, tap water and a tray liner.
The assumed safety of single-use instruments
In the base case it is assumed that the complication rates and outcomes are identical for reusable instruments and single-use instruments. The NICE committee believed this assumption was reasonable.
The costs associated with systems to allow instruments to be tracked
NICE committee members provided data from an unpublished Society for British Neurosurgeons survey and from costs recorded at their own units, which indicated that £750,000 across a 5-year period, including necessary equipment, would be a reasonable estimate (anonymous, May 2018). Sensitivity analyses were intended using £500,000 and £1,000,000.
Parameters relating to the probability of infection, the incubation time and consequences if clinical symptoms appear
The conceptual model of estimating the probability of infection when prions are transferred to the patient
In the earlier ScHARR model,11 the probability of infection was estimated using the mass transferred to the patient (in grams) and the infectious titre of the mass (in terms of ID50 per gram, where an ID50 is the dose required to infect 50% of the susceptible population). It was assumed that the relationship between the number of ID50 and the probability of infection was:
such that 2 ID50 or more would result in a certain infection. In the earlier ScHARR model, the use of a geometric sequence was used, such that 2 ID50 would result in only 75% of patients being infected (1–0.52). However, the committee did not want to use this assumption because the dose to infection was not robustly known and high levels of ID50 transferred could be associated with definite, rather than a high probability of infection, and the committee wished to err on the side of caution. The linear assumption was upheld by the NICE appraisal committee.
The mass assumed to be transferred per operation is detailed in The proportion of residual mass on brain and posterior instruments that is transferred to a patient and the assumed infectious titre per gram is detailed in Parameters relating to the decontamination of surgical instruments.
In a key change from the ScHARR report,11 it was assumed that all patients, regardless of age or genotype, were susceptible to CJD infection.
The incubation period following surgically transmitted Creutzfeldt–Jakob disease infection
The incubation period associated with stCJD was elicited from clinical experts in January 2018 (see Appendix 4). The results are contained in Appendix 1, but are briefly detailed here. The elicited results differed from those previously elicited in that (1) distributions were no longer elicited for each genotype, as it was assumed that a single distribution could cover all genotypes given the incubation period would be affected by the genotype of the recipient, the infecting prion and the infectious dose provided; (2) uncertainty in the mean estimates was formally captured; and (3) it was assumed that all genotypes were susceptible to CJD.
Four incubation intervals were specified; in the base case, each interval was assumed to be equally likely. These were (1) 0.25 to 2 years, (2) 2 to 10 years, (3) 10 to 20 years and (4) 20 to 50 years. Within each time interval a uniform distribution was used on the assumption that each value was equally likely to occur. To allow for uncertainty around the mean incubation period, it was proposed that the first probability of being in the first three intervals would range between 10% and 40%, whereas the probability of being in the fourth interval (20 to 50 years) would lie between 15% and 35%.
As indicated in Figure 5, should the incubation time be less than the patient’s life expectancy (sourced from the Office for National Statistics217), the patient would display clinical symptoms. Otherwise, the patient would die without CJD symptoms. Each year a proportion of patients incubating CJD die as a result of non-CJD-related reasons, in line with data reported by the Office for National Statistics. 217 The probability of non-CJD-related death was dynamic between 2005 and 2014, using the appropriate life table, but was assumed to use life tables from 2014 to 2023.
The infectious period following surgically transmitted Creutzfeldt–Jakob disease infection
The proportion of the incubation period associated with stCJD for which a patient was considered infectious and able to pass CJD prions to instruments was taken from the elicitation session used to inform the earlier ScHARR report. 11 This distribution is shown in Figure 13. The mean of this distribution is 20.0%, indicating that the patient is infectious for only the last 20% of the incubation period. The 95% CrI for this parameter ranged from 15.3% to 25.2%. It has been assumed that the infectious titre of CJD prions is at the maximum value for the entire infectious period.
FIGURE 13.
The proportion of the incubation period during which the patient is infectious.

Estimations of the relative likelihood of returning to high-risk surgery
Patients who are infectious can return to surgery and may do so at a quicker rate than people who have not experienced prior surgery. The earlier ScHARR report11 assumed that (1) people who had previous brain surgery were 43 times more likely to have a further brain operation than people without a history of a brain operation; (2) people who had previous posterior eye surgery were 60 times more likely to have a further posterior eye operation than people without a history of a posterior eye operation; and (3) people who had previous neuroendoscopy were 761 times more likely to have a further neuroendoscopy than people without a history of a neuroendoscopy. These values were based on Hospital Episode Statistics (HES) data that were extracted by a third party (Northgate Information Solutions; Zellis, Hemel Hempstead, UK) and were assumed to be applicable for use in the updated modelling. Having performed sensitivity analyses in the construction of the model, by increasing the relative rates by 10, the model did not appear sensitive to this variable and the values were left at the values used previously.
The assumed costs and quality-adjusted life-years associated with Creutzfeldt–Jakob disease
Once clinical symptoms have developed, it is assumed that patients accrue no further QALYs as a result of the severity of the condition. The earlier ScHARR report11 used a value of £40,000 for the costs associated with treating a case of CJD. This has been updated using the inflationary indices,218,219 which estimate an inflation value of 302.3/240.9 (1.25) between 2005–6 and 2016–17 using the Hospital and Community Health Services index. Data reported in Barnett and McLean220 indicate that costs of additional care and/or equipment were approximately £10,500 per person from invoices received from 33 patients, although the authors of the paper state that ‘local agencies contributions have not been quantified’. This is lower than that assumed in the original ScHARR model, which has been maintained as the base-case value and is favourable to strategies to reduce future stCJD cases. For simplicity, we have assumed that the cost, from a NHS and Personal Social Services perspective, in 2017–18 for a CJD case was £50,000.
The probability that a person with Creutzfeldt–Jakob disease symptoms are not diagnosed with Creutzfeldt–Jakob disease
It is possible that patients with CJD may be diagnosed with another neurodegenerative disease. This possibility was not considered in the initial ScHARR report,11 but was requested following a meeting of the NICE committee. The distribution of patients who were presumed to be diagnosed with another neurodegenerative disease was elicited from experts in January 2018 (see Appendix 1 for full details) for two age bands, with the experts willing to allow the misdiagnosis in the aged 60–80 years category to be the average of the two other age bands: those aged < 60 years and those aged > 80 years. The distribution for those patients aged < 60 years is shown in Figure 14.
FIGURE 14.
The proportion of patients < 60 years with clinical CJD symptoms who are diagnosed with another neurodegenerative disease.
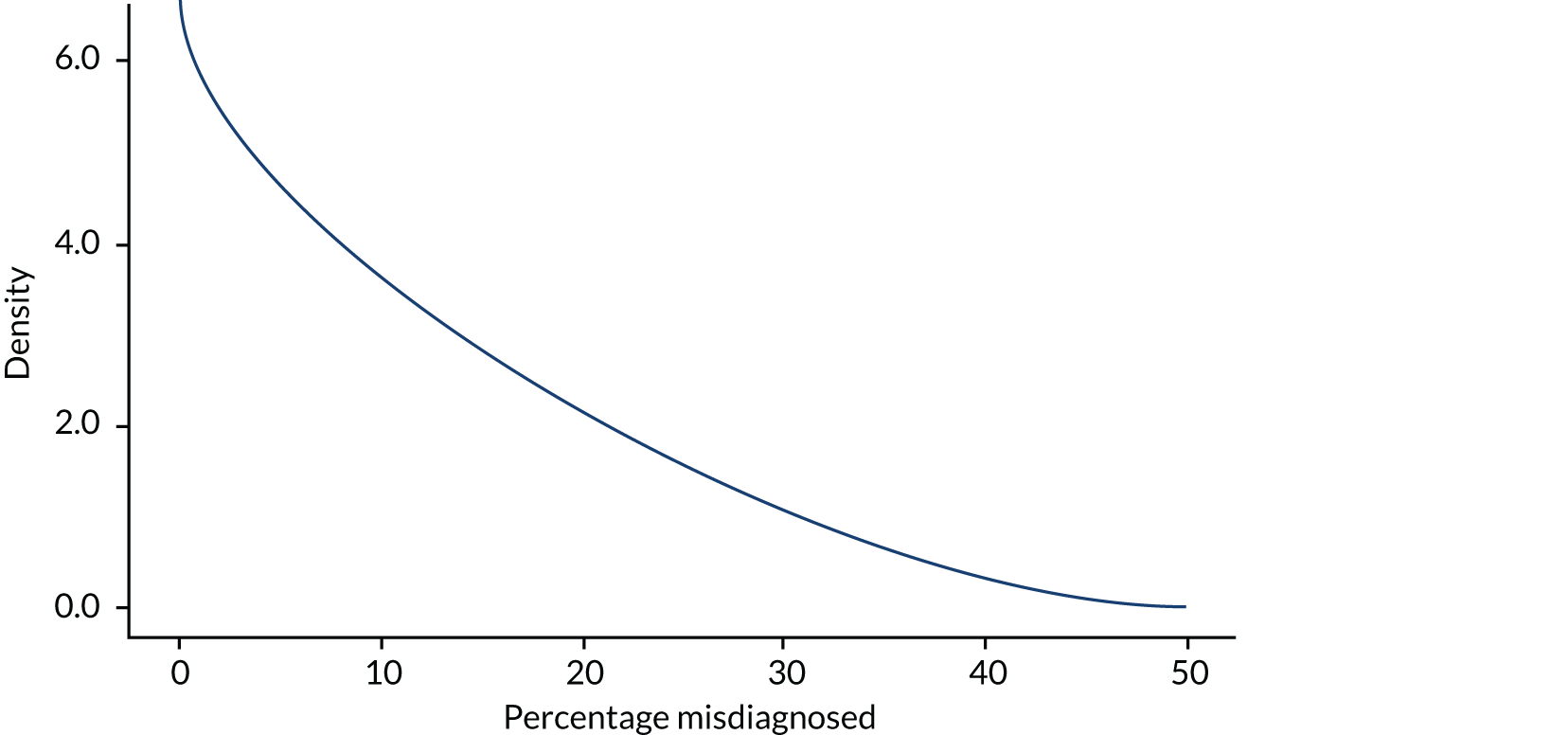
The mean value is 13.0% with a 95% CrI 0.4% to 26.8%. The distribution for those patients aged > 80 years is shown in Figure 15. The mean value is 55.0% with a 95% CrI of 18.6% to 88.4%. The simulated distribution for patients aged between 60 and 80 years of age inclusive is shown in Figure 16. This distribution has a mean of 34.0% with an estimated 95% CrI of 13.5% to 54.3%.
FIGURE 15.
The proportion of patients over 80 years with clinical CJD symptoms who are diagnosed with another neurodegenerative disease.
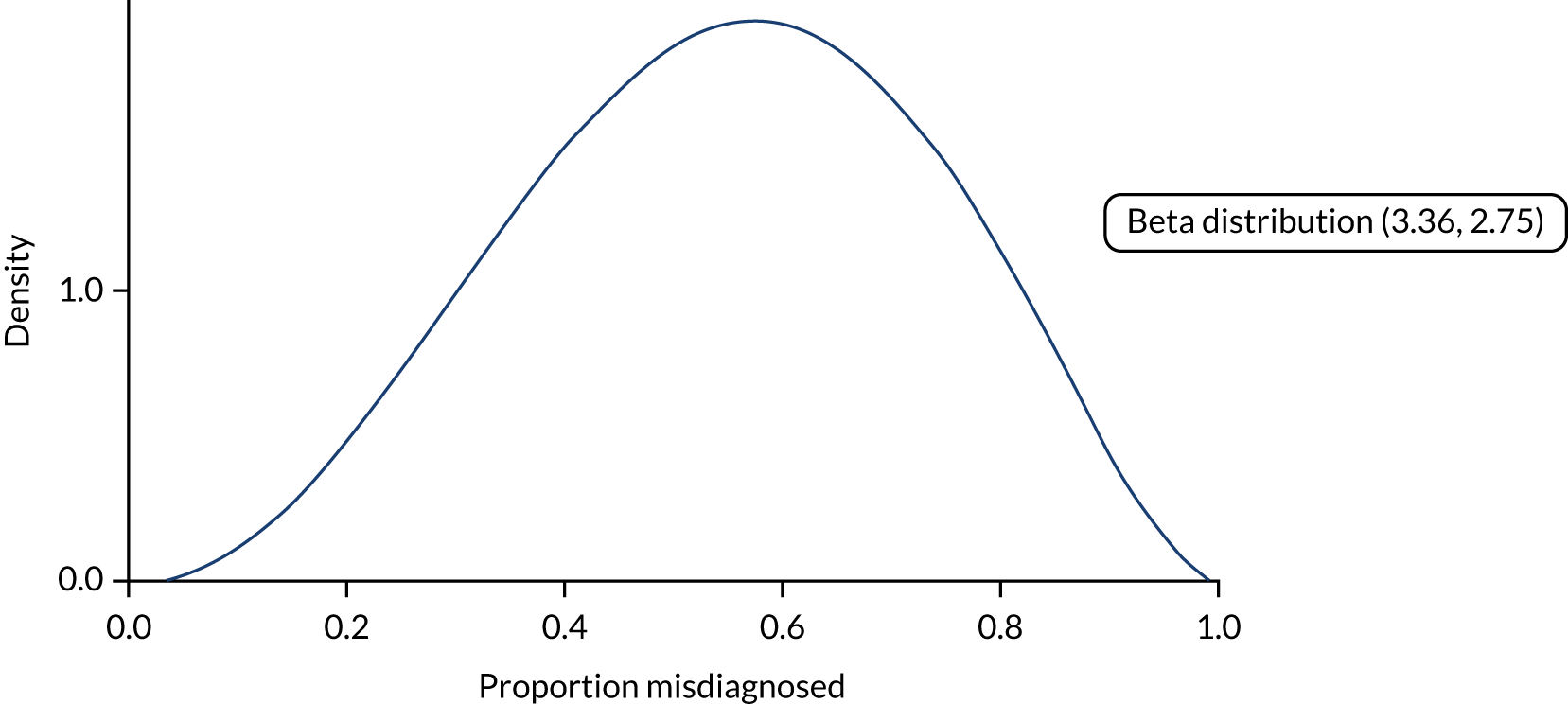
FIGURE 16.
The simulated proportion of patients aged between 60 and 80 years inclusive with clinical CJD symptoms who are diagnosed with another neurodegenerative disease.

It should be noted that based on the advice of clinical experts on the committee, there has been no change in CJD case ascertainment levels since 2005. This is partially supported by data from the NCJDRSU in the UK (25th Annual Report (see Figure 32), which showed similar age-specific mortality rates between 2005–9 and 2010–16 in those aged 60–64 years and those aged 75–79 years. However, the age-specific mortality rates were higher in the 70–74 years of age group in 2010–16 than in 2005–9, which could be indicative of better ascertainment in recent years. The assumption of equal ascertainment would favour single-use instruments.
In patients who are correctly diagnosed with CJD, the model does not explicitly distinguish between sCJD and stCJD and thus the probability node at the far right of Figure 5 is not contained in the model. However, it is appreciated that patients with stCJD may be categorised as sCJD, and these are used when calibrating the model output to the numbers of observed cases. This is described in more detail in The potentially unobserved number of surgically transmitted Creutzfeldt–Jakob disease cases between 2005 and 2018.
Parameters relating to the numbers of operations that are considered to be high-risk and the characteristics of patients undergoing these operations
The operations considered to be at risk
In consultation with NICE, only high-risk operations are modelled, which have been subdivided into those related to the brain, those related to posterior eye operations and those involving neuroendoscopy. The operations, using HES data to four characters, that are considered to be high-risk were identified by an expert on the NICE committee and are contained in Appendix 5. For brain operations, an expert on the NICE committee grouped the operations into those with normal life expectancy, those where the patient would be expected to survive 18 months, and those with a 50% probability of death at 18 months and a 50% probability of a normal life expectancy.
Only the main procedure codes have been used rather than all the procedure codes, as there is a possibility that more than one high-risk HES code is undertaken within the same operation, using the same instrument set. In the modelling, the HES data have been inflated by 15% as in the ScHARR report11 to take into consideration that not all of the additional operations (between the main procedure and all procedures) are conducted simultaneously with another high-risk code, and also to incorporate operations undertaken by the private sector in non-NHS hospitals.
The estimated number of operations reported within the HES data since 1 January 2005 is provided in Table 25. For future years, the average number of operations in the last 3 years was assumed to continue. Operations were assumed to happen at a constant rate throughout the year. It should be noted that the values in Table 25 are those reported in the HES data as main procedures and have been increased by 15% within the model in line with the earlier modelling undertaken by ScHARR.
| Year | Brain 1a | Brain 2b | Brain 3c | NE | PE |
|---|---|---|---|---|---|
| 2005–6 | 19,554 | 5346 | 1684 | 302 | 4629 |
| 2006–7 | 21,451 | 5317 | 1069 | 311 | 4098 |
| 2007–8 | 19,302 | 5517 | 1062 | 338 | 6164 |
| 2008–9 | 18,406 | 5557 | 1107 | 354 | 8415 |
| 2009–10 | 19,404 | 5706 | 1101 | 389 | 7660 |
| 2010–11 | 20,323 | 5755 | 1121 | 488 | 7796 |
| 2011–12 | 21,288 | 5889 | 1217 | 497 | 5081 |
| 2012–13 | 21,110 | 5887 | 1151 | 500 | 13,296 |
| 2013–14 | 22,497 | 5905 | 1110 | 539 | 13,060 |
| 2014–15 | 22,508 | 6013 | 1087 | 532 | 5378 |
| 2015–16 | 22,916 | 6106 | 1110 | 527 | 5226 |
| 2016–17 | 23,029 | 6114 | 968 | 518 | 5481 |
| Numbers assumed subsequent to 2017d | 22,818 | 6078 | 1055 | 526 | 5362 |
Hospital Episode Statistics data provide age breakdowns for each code, with more granularity from the year 2012 than prior to this date. Analysis of these data indicated that the age profile of patients remained relatively stable across time for each of the three brain operation groupings, for neuroendoscopy and for posterior eye operations. Therefore, for simplification, the age profile within 2016–17 was assumed to apply throughout the model. Depictions of each assumed age profile are provided in Appendix 6.
Calibration targets
The observed number of surgically transmitted Creutzfeldt–Jakob disease cases between 2005 and 2018 and the potentially unobserved number of surgically transmitted Creutzfeldt–Jakob disease cases
The observed number of surgically transmitted Creutzfeldt–Jakob disease cases between 2005 and 2018
There are no cases of CJD that have been categorised as stCJD during this period.
The potentially unobserved number of surgically transmitted Creutzfeldt–Jakob disease cases between 2005 and 2018
There are two possible ways in which patients with stCJD can be misdiagnosed. The first is that another neurodegenerative disease is diagnosed; this has been discussed in The costs associated with decontaminating reusable instruments. The second way that stCJD can be misdiagnosed is that a different form of CJD (in particular sCJD) is the presumed diagnosis, as a previous operation may not be recalled. The potential number of patients misdiagnosed as a different form of CJD was investigated.
Data were supplied to a NICE committee member by the NCJDRSU, which detailed whether or not patients who had a diagnosis of CJD since 2005 had a history of neurosurgery or posterior eye surgery, as well as a brief description of the operation. These data were reviewed by a NICE committee member who categorised each patient as having an operation that was of high risk (and, therefore, potentially a stCJD case) or not. The committee member erred on the side of caution, stating whether or not the operation could have the potential to transmit CJD prions to the patient. However, it is possible that some of the cases reviewed occurred in other parts of the UK than England, to which this guidance is limited, which would result in an overestimated calibration target.
For posterior eye surgery, there were potentially 24 individuals who had undergone surgical operations that could have transmitted CJD, although only 10 of these had operations in 2005 or later. The year of the operation is important, as we want to calibrate the model only to cases where the patient had been infected during the modelling period. For brain surgery, there were potentially 13 individuals who had undergone operations that could have transmitted CJD. There were no dates provided for the operations and thus it was assumed that the proportion of operations conducted in 2005 or later that were observed for posterior eye surgery (10/24) were applicable to neurosurgery, which equates to a possible five cases of stCJD since 2005 (rounding to the nearest integer). The sum of these calculations implies that there could have been 15 cases of stCJD transmitted since 2005 that had been misdiagnosed as another form of CJD, or just over one case per year on average.
Categorisation of surgical units, establishing probabilistic sensitivity analysis configurations that are plausible and generating likelihood functions for plausible probabilistic sensitivity analysis configurations
Categorisation of surgical units
Based on the heterogeneity in surgical units adhering to IPG196 and the analyses varying the assumption of whether or not the P96 group (patients born after 1996) could be infectious from birth, six categories of surgical units were defined (denoted S1 to S6). These were:
-
S1 – a unit adheres to IPG196 and guidance on keeping instruments moist. The P96 group are infectious from birth.
-
S2 – a unit does not adhere to IPG196 but adheres to guidance on keeping instruments moist. The P96 group are infectious from birth.
-
S3 – a unit does not adhere to IPG196 nor does it adhere to guidance on keeping instruments moist. The P96 group are infectious from birth.
-
S4 – a unit adheres to IPG196 and guidance on keeping instruments moist. The P96 group are not infectious from birth.
-
S5 – a unit does not adhere to IPG196 but adheres to guidance on keeping instruments moist. The P96 group are not infectious from birth.
-
S6 – a unit does not adhere to IPG196 nor does it adhere to guidance on keeping instruments moist. The P96 group are not infectious from birth.
Based on the opinion of members of the NICE committee it was assumed that, independent of whether or not the P96 group was assumed to be infectious, 10% of units adhered to IPG196 and guidance on keeping instruments moist, 30% of units adhered only to keeping instruments moist and 60% of units neither followed IPG196 nor kept instruments moist. These probabilities were altered in a scenario analysis.
Employing a heuristic to rule out probabilistic sensitivity analysis configurations that would produce implausible results
Owing to the time required for each run [approximately 12 seconds per ‘plausible’ (defined later) PSA configuration] and the number of PSA configurations, random number (RN) streams, scenarios and PSA configurations that would not be compatible with the observed data, heuristics were used to generate the cost-effectiveness results. At all stages, a cautious approach was employed to ensure that potentially appropriate configurations were not prohibited. Appendix 7 describes the methodology using formal mathematical notation, with a lay description provided in the main text.
The initial step was to develop a metric to exclude PSA draws that would clearly be discrepant to the observed data (known cases of CJD that could potentially be attributed to surgical transmission), without having to run these configurations.
Here, a factor to efficiently maximise the likelihood (FML) was established and any PSA configuration with a value greater than the FML value was discarded.
The FML was derived using a combination of parameters related to the infectious titre after a decontamination cycle, the mass transferred to a patient and the prevalence of prion in tissue in asymptomatic patients:
in which
-
A = mean infectious titre (in log-terms) × log-reduction in infectivity associated with the first autoclaving cycle × log-reduction associated with detergent on the first cycle
-
B = residual mass on an instrument × (1 – the proportion of residual mass transferred to the patient)
-
C = the proportion of asymptomatic individuals with CJD prions in their tissue.
In order to generate the FML threshold value, 2000 PSA configurations were drawn from the appropriate distributions and run using 12 RN streams for each of the following scenarios: S1, S2 and S3. Having assessed the likelihood of each of the 2000 PSA configurations producing results consistent with the observed data, it was decided that any draw with a FML value of > e12 would effectively have zero weight and could be discarded without affecting the results. Any draw with a value ≤ e12 could potentially be consistent with the observed data.
Running further analyses to remove probabilistic sensitivity analysis configurations that are potentially consistent with the observed data but generate an implausible number of transmissions when run through the model
In total, 2000 PSA configurations with a FML value of ≤ e12 were sampled. For each configuration, the first RN stream was run, assuming a S3 surgical unit and determining whether or not there was a violation of the permissible limit (VPL) of clinical transmissions for patients aged ≤ 60 years. It was noted that the clinical experts had stated it was implausible that the correct detection rate of CJD was below 50% in this age group and that the assumed maximum number of clinically apparent cases potentially transmitted via surgery, across all ages, was 15. If there was a VPL, the PSA configuration was deemed to be inconsistent with the observed data and the PSA run was discarded. If there was not a VPL, the next RN stream was run with this process repeated until a maximum of 27 RN streams had been run.
The VPL threshold was dynamic and changed as the number of RN streams increased. A large VPL threshold was chosen to reduce the possibility of rejecting viable PSA configurations, while acknowledging that there was also the probability that clinical transmissions had occurred in older patients. The initial threshold for VPL was 36 transmissions, which was constant for the cumulative total across the first six RN streams. From RN streams 7 to 13, the VPL threshold was increased to 40; from RN streams 14 to 17, the VPL threshold was increased to 45; from RN streams 18 to 23, the VPL threshold was increased to 55; and for RN streams 24 to 27 the VPL threshold was increased to 66. This resulted in 509 out of the 2000 PSA runs that all had an FML ≤ e12 being potentially consistent with the observed data. These are denoted ‘plausible’ PSA configurations.
Calculating the likelihood of each plausible probabilistic sensitivity analysis configuration being consistent with the observed data
Approximate Bayesian computation methods were used to estimate the likelihood of a PSA configuration being consistent with the observed data. Full details are provided in Appendix 7. A likelihood ranges from 1, where the simulated number of transmissions that are clinically detected are entirely consistent with the number of observed cases, to zero where the simulated number of transmissions that are clinically detected cannot be consistent with the number of observed cases. Within this decision problem, any PSA configuration that produces ≤ 15 transmissions that result in clinical symptoms would have a likelihood of 1, whereas any PSA configuration that produced > 30 transmissions that result in clinical symptoms, in patients than < 60 years of age, would have a likelihood of zero.
The likelihoods for each PSA configuration are shown in Figure 17. These have been ranked in descending order and have been curtailed at 250 of the 509 PSA configurations. A large proportion of the PSA configurations that were not rejected have likelihoods close to zero, which offers support to the belief that it was unlikely that potentially appropriate PSA configurations were discarded. For information, the lowest likelihood was 10–12 where the P96 group was assumed to be infectious and 10–13 where the P96 group was assumed not to be infectious.
FIGURE 17.
The likelihoods of the PSA configurations being compatible with the observed data (curves are drawn on top of each other).
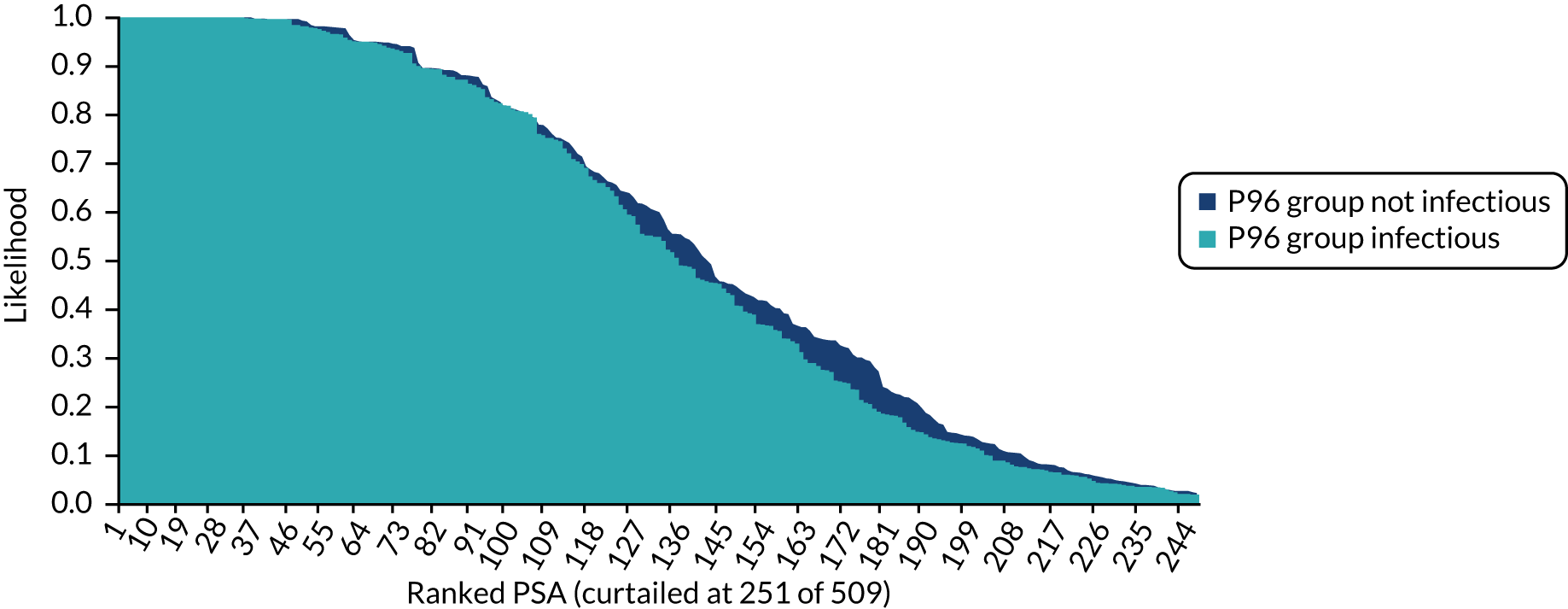
Generating estimates of the expected numbers of future surgically transmitted Creutzfeldt–Jakob disease, life-years lost and quality-adjusted life-years lost
The likelihoods associated with each PSA sample were multiplied by the results (future stCJD deaths, life-years lost and QALYs lost) produced when using that PSA sample and these were added together and divided by the sum of the likelihood to produce expectations for the combined results.
Exploring the uncertainty in the results produced within the base-case analyses
In order to explore more pessimistic scenarios, the maximum value across all of the 509 PSA configurations of the number of QALYs simulated to be lost multiplied by the likelihood of the PSA was also calculated. These values are necessarily greater than the expectations, which use the average value multiplied by the likelihood of the PSA rather than the maximum value. Generating CIs around the mean of each output was more complex owing to the use of likelihoods, as not all of the 509 scenarios were weighted equally. In order to provide an indication of the width of the CI (which would need to be halved if only looking at increasing or decreasing the value from the mean), an approximation was made, which is detailed in Appendix 7, that involved simulation to translate each PSA likelihood into either zero or 1 and then using statistical techniques to estimate a CI.
Exploring the probability that each type of surgical unit was the most cost-effective
Exploratory analyses were undertaken to provide indicative probabilities that each type of surgical unit (one of S1, S2 and S3, or S4, S5 and S6) or moving to single-use instruments were most cost-effective across a range of cost-per-QALY thresholds. This analysis assumed that a surgical centre was a S3 (S6), meaning that expenditure was required to move to S1 or S2 (S4 or S5). The probabilities were calculated assuming that the weight applied to each of the 509 PSA values would be provided to the surgical unit or single-use instrument scenario that was most cost-effective at a chosen cost-per-QALY threshold. The summated total of weights for each option was divided by the sum of the total weights to provide a probability of being most cost-effective, which summate to 1.
Exploring the changes in the results produced with alternative assumptions relating to the assumed distribution of surgical units between the assumed decontamination levels
In the base-case analyses, it was assumed that 10% of surgical units would both follow IPG196 and keep instruments moist; 30% would not follow IPG196; and 60% of surgical units neither kept instruments moist nor followed IPG196. The NICE committee requested that a scenario analysis be run that changed these proportions to 50%; 30%; and 20%, respectively. Thus, in this scenario analysis half of surgical units both followed IPG196 and kept instruments moist.
Strategies modelled
In consultation with the NICE committee, the following strategies were run:
-
Do nothing, assuming that the current situation is maintained with respect to surgical centres’ adherence to IPG196.
-
Full adherence to IPG196, and guidance on keeping instruments moist for those units where this is not followed.
-
Full adherence to keeping instruments moist for those units where this is not followed.
-
Removal of the requirements to have separate instrument sets for the P96 group.
-
Modelling interventions that prohibit the possibility of stCJD. These are likely to take the form of the introduction of single-use instruments or the introduction of a decontamination product during the sterilisation process that is completely effective.
Within this report ‘adherence to IPG196’ is a slight misnomer, as the modelled scenario does not assume that neuroendoscopy instruments are single-use. However, for brevity, we have used the term ‘adherence to IPG196’.
Based on advice provided by the NICE committee, it was assumed that the quality of single-use and reusable instruments were equivalent.
Based on advice provided by the NICE committee, no modelling of decontamination products was conducted other than that contained in strategy five. The reasons for this were multiple. First, there was a lack of homogeneity in the identified studies of decontamination products in terms of prion strains, drying times, infectious titre of the material used, time and temperature of the exposure to the decontaminant, dose of the decontaminant, observation period, substrate used, assay and infectivity detection method used. Second, the findings for some agents inevitably differed both within and between studies, owing to the described heterogeneity (see results for Rely+On depending on the assay or NaOH depending on the prion strain). Identifying the most ‘efficacious’ decontaminant, requiring comparison across agents, was, therefore, not possible. Third, as far as we could tell, the majority of the decontaminants (and combinations thereof) were not commercially available but had been developed for the laboratory tests, whereas others that did exist as distinct products were few and, in some cases, were no longer on the market, for example Rely+On. Fourth, uptake of additional decontaminant solutions might be very low in practice owing to requiring an extra step in the sterilisation process. Therefore, major concerns affected the certainty and generalisability of the evidence on decontaminants for reducing the risk of prions in surgery. The authors wanted to provide an indication of the potential prices that could be cost-effective if a completely effective decontamination product was commercially available and so explored this in strategy 5.
Epidemiological results
For each PSA scenario the number of transmissions by age group that resulted in clinical symptoms (whether correctly diagnosed as CJD or not), the number of life-years lost and the number of discounted QALYs lost were simulated through the mathematical model. These results were then weighted by the likelihood, with the sum of these values divided by the sum of the likelihoods.
The epidemiological results presented are based on an individual surgical unit. Units are denoted S1 to S6 (defined in Categorisation of surgical units) to represent the combinations of the unit’s adherence to IPG196; whether or not instruments are kept moist; and whether or not it is assumed that the P96 group is infectious. It has been assumed that there are 27 units in England.
It is assumed that the answers produced will contain Monte Carlo sampling errors and that further RN streams and PSA configurations would provide more accurate answers. However, we believe that the results presented are sufficiently robust to draw conclusions. The base-case results assume that there may have been up to 15 deaths attributable to stCJD between 2005 and 2018.
Base-case results
The base-case results are provided in Table 26 and relate to the period 2019–23, as agreed with the NICE committee. The estimated values are presented in columns two to four; these are calculated using all PSA configurations (n = 509) and all RN streams (n = 27). The values of simulated deaths as a result of CJD infection, which were weighted by their likelihood that the transmissions of CJD modelled between 2005 and 2018, matched the observed data. The final column contains a value that represents the maximum value across the PSA configurations of the simulated deaths in that PSA multiplied by the likelihood of that PSA. Note that the maximum deaths across the P96 and the non-P96 group may not equal the maximum values for both the P96 group and the non-P96 group individually. The values are per surgical unit and need to be multiplied by 27 to represent values for England.
| Surgical unit | Average number of future deaths caused by infections between 2019 and 2023, total (non-P96 group/P96 group)b | Average number of future undiscounted life-years lost caused by infections between 2019 and 2023 | Average number of future discounted QALYs lost caused by infections between 2019 and 2023 | Maximum number of future deaths across the PSAs caused by infections between 2019 and 2023 multiplied by likelihood, total (non-P96 group/P96 group)b |
|---|---|---|---|---|
| S1 | 0.052 (0.036/0.016) | 1.548 | 0.459 | 0.519 (0.519/0.000) |
| S2 | 0.087 (0.068/0.020) | 2.699 | 0.874 | 1.741 (1.481/0.259) |
| S3 | 0.430 (0.339/0.091) | 12.438 | 4.009 | 4.259 (3.704/0.556) |
| S4 | 0.038 (0.038/0.000) | 0.741 | 0.275 | 0.519 (0.519/0.000) |
| S5 | 0.078 (0.036/0.015) | 2.276 | 0.736 | 1.741 (1.481/0.259) |
| S6 | 0.389 (0.314/0.075) | 10.809 | 3.485 | 4.259 (3.704/0.556) |
Interpretation of the base-case results
As anticipated, fewer deaths as a result of stCJD were estimated when IPG196 was followed and when residual mass was reduced. Thus, in terms of future deaths as a result of stCJD, S1 had fewer deaths than S2, which had fewer deaths than S3, and S4 had fewer deaths than S5, which had fewer deaths than S6. Furthermore, as anticipated, when the P96 group was assumed not to be infectious there were fewer projected deaths as a result of stCJD; that is, S1 had more deaths than S4, S2 had more deaths than S5 and S3 had more deaths than S6.
Those units that followed IPG196 and kept instruments moist (S1 and S4) had 0.052 and 0.038 future deaths caused by stCJD, respectively. Where IPG196 was not followed but instruments were kept moist, there was an increase in future deaths as a result of stCJD of 0.035 when the P96 group was deemed infectious from birth and 0.040 when the P96 group was not deemed infectious from birth. Assuming IPG196 was not followed, failure to keep instruments moist was associated with an increase in the estimated numbers of future deaths compared with not following IPG196 increased by 0.343 when the P96 group was deemed infectious from birth and 0.310 when the P96 group was not deemed infectious from birth. From these results, it is apparent that ensuring that instruments are kept moist has a large impact on the risk of future transmissions.
It is of note that the number of potential stCJD infections in the P96 group is not necessarily zero, even when these patients are assumed not to be infectious. This can occur when a P96 patient is infected via an operation prior to 2012, the date at which the new instrument sets for the P96 patients were introduced. Such a patient could then have a further high-risk operation while in the subclinical but infectious period, which could have infected P96 patients.
The circumstances in which the maximum future deaths predicted within the model were explored. A high number of future deaths were associated with the prevalence of CJD prions in their tissue being very low: < 1 per 200,000 people had prions in their tissue. In these PSA runs, no infectious people had entered the system between 2004 and 2018; this resulted in no infections and thus these PSA runs have a likelihood of 1 of matching the observed data. In the 2019–23 period, infectious people were simulated to have an operation in some RN streams, which resulted in infections and deaths. The number of deaths was greater where IPG196 was not followed and where instruments were not kept moist. The maximum number of future deaths multiplied by the likelihood is expected to be associated with approximately 10 times more deaths than the expectation. For completeness, the best-case scenario would be that there were no further deaths, which applies for all types of surgical unit.
Uncertainty in the mean number of QALYs gained was explored as described in Exploring the uncertainty in the results produced within the base-case analyses and Appendix 7. The width of the CI around the mean estimate of QALY loss was estimated to be 0.25 for S1 units, 0.58 for S2 units, 2.07 for S3 units, 0.19 for S4 units, 0.58 for S5 units and 1.89 for S6 units. To explore the relationship between the number of PSA samples and the width of the CI, a randomly selected PSA was removed with the remaining 508 split into two groups of 254. The widths of the CIs for each of the two groups were 0.32 and 0.40 for S1; 0.87 and 0.78 for S2; 3.02 and 2.85 for S3; 0.25 and 0.29 for S4; 0.78 and 0.87 for S5; and 2.76 to 2.62 for S6. This indicated that approximately doubling the number of PSA configurations had led to a reduction in the width of the CIs by approximately 30%. The CIs produced from the 509 PSA configurations were not believed by the authors of this report to be large enough to endanger the conclusions of the analyses are endangered. Given this, it was believed that further reductions in the width of the CIs through running further PSAs were not required.
Scenario analyses using the base case as the foundation
Eight scenario analyses were run, with the change within a unit being assumed to happen instantly at midnight on the 31st December 2018. These scenarios comprised strategies to follow IPG196 and/or reduce the residual mass on instruments, and estimated the effect of removing the guidance on having different instrument sets for the P96 group from the remaining patients. The results of the scenario analyses are presented in Table 27. The results are presented in terms of surgical centres; these values would be needed to be multiplied by 27 in order to form estimates for England.
| Surgical unit | Average number of future deaths caused by infections between 2019 and 2023, total (not P96 group/P96 group)a | Average number of future undiscounted life-years lost caused by infections between 2019 and 2023 | Average number of future discounted QALYs lost caused by infections between 2019 and 2023 | Maximum number of future deaths across the PSAs caused by infections between 2019 and 2023 multiplied by likelihood, total (non-P96 group/P96 group)a |
|---|---|---|---|---|
| S2 to S1 | 0.045 (0.037/0.008) | 1.127 | 0.359 | 0.519 (0.519/0.000) |
| S3 to S1 | 0.047 (0.039/0.008) | 1.159 | 0.371 | 0.519 (0.519/0.000) |
| S3 to S2 | 0.073 (0.073/0.000) | 2.894 | 0.825 | 1.741 (1.481/0.259) |
| S5 to S4 | 0.038 (0.038/0.000) | 0.744 | 0.271 | 0.519 (0.519/0.000) |
| S6 to S4 | 0.040 (0.040/0.000) | 0.782 | 0.285 | 0.519 (0.519/0.000) |
| S6 to S5 | 0.058 (0.058/0.000) | 2.238 | 0.627 | 1.741 (1.481/0.259) |
| S1b | 0.041 (0.041/0.000) | 1.661 | 0.484 | 0.556 (0.444/0.111) |
| S4b | 0.037 (0.037/0.000) | 1.543 | 0.451 | 0.556 (0.444/0.111) |
Interpretation of the scenario analyses results using the base case as the foundation
These results are subject to Monte Carlo sampling error, particularly in relation to the RNs exhausted within a simulation. For example, in the scenario analysis that changed a unit from S2 to S1, at the start of 2019 this model run will have used significantly more RNs than a comparison with S1 alone. This is a result of the RNs required in selecting from 2012 onwards, the SIs used in an operation and the migration of instruments between sets (which is a feature of S2 but not of S1). This misalignment of RNs between runs will result in different simulated outcomes.
Despite the presence of Monte Carlo sampling error, the results generated are broadly consistent between comparable units, which offers support that the values are relatively robust. However, caution is advised in trying to interpret differences in the results of the scenario analyses (see Table 27) and the base-case results (see Table 26), as these differences could be artefacts of the RNs selected. Significantly more computational time would be required to provide an accurate comparison of the scenario analyses and the base-case results; this was beyond the time scales of the project.
Scenario analyses using an alternative distribution of surgical unit compliance with following IPG196 and keeping instruments moist
As described in Exploring the probability that each type of surgical unit was the most cost-effective, the distribution that was assumed in relation to following IPG196 and guidance on keeping instruments moist was changed to provide an indication of the sensitivity of the epidemiological results to these parameters. The results for the expected number of QALYs lost as a result infections occurring between 2019 and 2023 are shown for the base-case and the alternative scenario in Figure 18. The results are very similar, as will be the costs associated with each strategy and, as such, no analyses of the alternative scenario will be provided as these are highly comparable to those of the base case.
FIGURE 18.
Comparing the QALYs lost within the base case and when using an alternative assumption related to the distribution of surgical units following IPG196 and in keeping instruments moist. The percentages in the figure key refer to the proportion of units that are S1/S4, S2/S5 and S3/S6, respectively. S1 and S4 are assumed to follow both IPG196 and guidance on keeping instruments moist. S2 and S5 are assumed to keep instruments moist. S3 and S6 are assumed to neither follow IPG196 nor keep instruments moist.
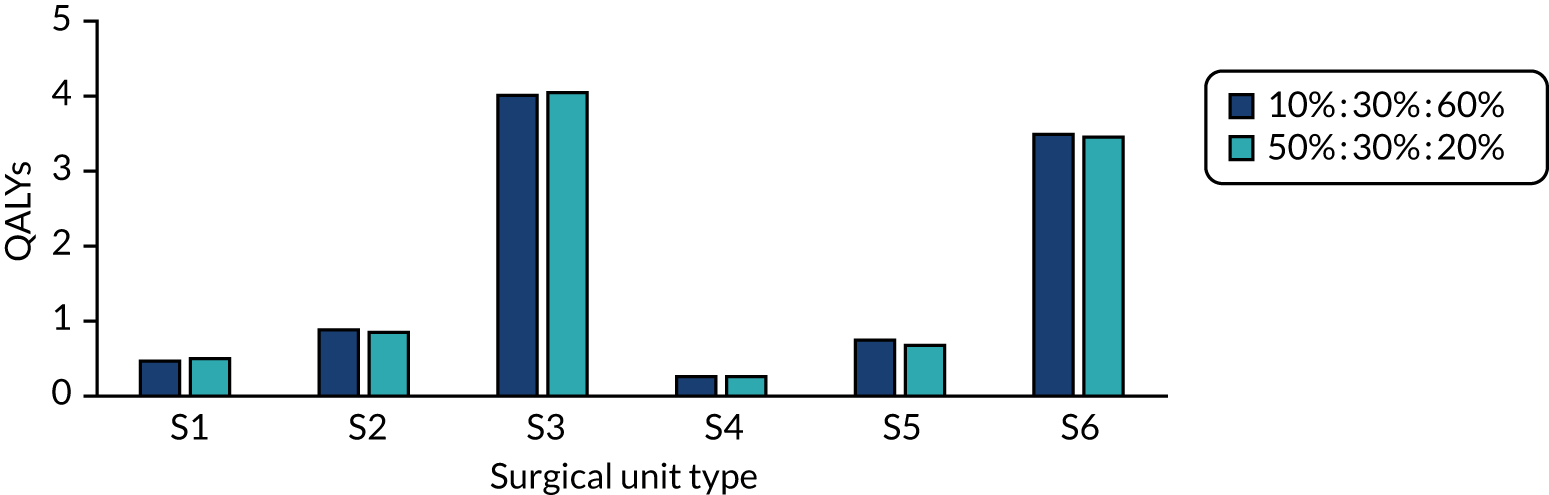
Cost-effectiveness results
The presented results have been grouped by type of surgical unit (from S1 to S6). Within each category, evaluated strategies are compared incrementally if appropriate. In addition to the base-case results, sensitivity analyses have been run that change the values of parameters and threshold analyses have been performed to determine at what price for a single-use kit, or cleaning solution that was 100% effective, the cost per QALY gained would equal the chosen cost-effectiveness threshold.
In all analyses, the additional costs have been calculated considering the following elements: the costs of single-use sets; the disposal costs of reusable instruments; the costs of autoclaving reusable instruments; and the costs associated with symptomatic stCJD.
When cost-per-QALY values have been calculated, these are compared with threshold values used within common NICE evaluations. These are £30,000 within a standard technology appraisal, although this can potentially be raised to approximately £50,000 if the end-of-life criteria are met,216 and between £100,000 and £300,000 for highly specialised technologies. 221
Parameter values within the base-case cost-effectiveness results
The parameter values used within the base-case estimate of the cost-effectiveness of various strategies are shown in Table 28. It is noted that the number of operations were discounted such that sensitivity analyses on the values could be performed without re-running the model. On completion of the runs, it was discovered that the number of instruments disposed of within the run was not saved to file. As such, an estimate of this was calculated rather than being directly taken from the model; it is unlikely that this limitation will influence the results owing to the relatively small values involved.
| Parameter | Base-case value | Intended values for use in the sensitivity analyses |
|---|---|---|
| Discounted number of brain operations performed between 2019 and 2023 | 5199.84 | Assumed fixed |
| Discounted number of posterior eye operations performed between 2019 and 2023 | 904.92 | Assumed fixed |
| Discounted number of neuroendoscopies performed between 2019 and 2023 | 58.7 | Assumed fixed |
| Cost of an average single-use set, including disposal costs | £425 | £350; £500 |
| Cost of a replacement reusable instrument | £150 | £100; £200 |
| Assumed number of new instruments bought per surgical unit between 2019 and 2023 | 32 | Assumed fixed |
| Assumed cost associated with a clinically CJD transmission (diagnosed correctly or not) | £50,000 | £30,000; £70,000 |
| Cost of an autoclaving cycle (per instrument) | £0.60 | Assumed fixed |
| Cost of keeping an instrument set moist | £0.12 | Assumed fixed |
| Cost of increasing standards to adhere to IPG196 – set-up costs | £750,000 | £500,000; £1,000,000 |
| Assumed cost-effectiveness threshold (per QALY) | £30,000 | £50,000; £100,000; £300,000 |
The base-case cost-effectiveness of strategies for reducing the likelihood of surgically transmitted Creutzfeldt–Jakob disease
Results for S1 and S4 units
For surgical units that adhere to IPG196 and keep instruments moist, the only strategy currently available to reduce the potential for stCJD is to use single-use instruments. Based on the values reported in Table 28, it is estimated for a S1 unit that the additional net cost of single-use instruments per unit would be £1,814,139, which would produce an expected 0.459 QALYs, thereby resulting in a cost per QALY gained of £4.0M. For a S4 unit, the net cost was similar (£1,814,545) with fewer QALYs gained (0.275), resulting in a cost per QALY gained of £6.7M. Both cost-per-QALY estimates are markedly higher than the thresholds commonly used by NICE.
Results for S2 and S5 units
For surgical units that do not adhere to IPG196 but keep instruments moist, two strategies are currently available to reduce the potential for stCJD: the use of single-use instruments and adhering to IPG196.
Based on the values reported in Table 28, it is estimated for a S2 unit that the additional net cost of single-use instruments per unit would be £2,562,829, which would produce an expected 0.874 QALYs, thereby resulting in a cost per QALY gained of £2,933,530. For a S5 unit, the net costs were similar (£2,563,238) with fewer QALYs gained (0.736), resulting in a cost per QALY gained of £3,484,476. Both cost-per-QALY estimates are markedly higher than the thresholds commonly used by NICE.
For a S2 unit, adherence to IPG196 is estimated to have a net cost of approximately £750,000 and provide an increase in QALYs of 0.415, resulting in a cost per QALY of approximately £1.8M. For a S5 unit, adherence to IPG196 is estimated to have a net cost of approximately £750,000, an increase in QALYs of 0.461, resulting in a cost per QALY gained in the region of £1.6M. Both cost-per-QALY estimates are markedly higher than the thresholds commonly used by NICE.
Results for S3 and S6 units
For surgical units that are neither adhering to IPG196 nor keeping instruments moist, three strategies are currently available to reduce the potential for stCJD: the use of single-use instruments; adhering to IPG196 and keeping instruments moist; and keeping instruments moist.
Based on the values reported in Table 28, it is estimated for a S3 unit that the additional costs of single-use instruments per unit would be £2,550,760, which would produce an expected 4.009 QALYs, thereby resulting in a cost per QALY gained of £636,292. For a S6 unit the costs were similar (£2,552,043) with fewer QALYs gained (3.485), resulting in a cost per QALY of £732,364. Both cost-per-QALY estimates are markedly higher than the thresholds commonly used by NICE.
For a S3 unit, keeping instruments moist is estimated to produce a cost saving (as the costs of potential prevented CJD cases outweighed those associated with keeping the instruments moist) and to provide an increase of 3.135 QALYs, suggesting that keeping instruments moist is dominant (lower costs and more QALYs produced). For a S6 unit, there was also an expected cost saving of an increase in QALYs of 2.749, resulting in keeping instruments moist being dominant.
For a S3 unit, having initially kept instruments moist, the cost-effectiveness of adhering to IPG196 would be similar to that of moving from S2 to S1, that is in the region of £1.8M per QALY gained. For a S6 unit having moved to a S5, the cost per QALY gained of adhering to IPG196 would be in the region of £1.6M.
Estimating the probabilities that each type of surgical unit or using single-use instruments are the most cost-effective strategies assuming that a centre does not currently follow IPG196 nor keep instruments moist
The probabilities of each surgical unit and using single-use instruments being the most cost-effective are provided in Figure 19 when it is assumed that the P96 group are infectious, and in Figure 20 when it is assumed the P96 group are not infectious. These results assume that all surgical units are currently S3 or S6. Both figures have similar characteristics in that S2/S5 (units that keep instruments moist but do not follow IPG196) have the highest probability of being cost-effective, followed by units that continue to ignore IPG196 and those that do not keep instruments moist. Even at high cost-per-QALY thresholds, the probability that single-use instruments are the most cost-effective is negligible. The probability of being most cost-effective accord with the scenarios (S2 and S5) that are estimated to be the most cost-effective.
FIGURE 19.
The probabilities that S1, S2, S3 and single-use instruments are the most cost-effective at a range of cost-per-QALY thresholds. SUI, single-use instrument.

FIGURE 20.
The probabilities that S4, S5, S6 and single-use instruments are the most cost-effective at a range of cost-per-QALY thresholds. SUI, single-use instrument.

Sensitivity analyses performed on the base-case results
Having observed the ICERs that were presented in terms of cost per QALY gained produced in the base case, the sensitivity analyses that was performed explored a combination of all of the values that were more favourable to single-use instruments. Thus, the cost of a CJD case was increased to £70,000; the average cost of a reusable instrument was assumed to be £200; and the cost of a single-use set was assumed to be £350. Note that these sensitivity analyses change the costs only and that the benefits in QALYs are assumed to be constant.
Sensitivity analyses results for S1 and S4 units
The ICER for single-use instruments for a S1 unit became £2.9M, whereas the ICER for a S4 unit became £4.9M. Neither value was below the commonly used NICE thresholds.
Sensitivity analyses results for S2 and S5 units
The ICER for single-use instruments for a S2 unit became £2.4M, whereas the ICER for a S5 unit became £2.9M. Neither value was below the commonly used NICE thresholds.
For a S2 unit, adherence to IPG196 is estimated to have an ICER of approximately £1.2M, whereas for a S5 unit this ICER was approximately £1.1M. Neither value was below the commonly used NICE thresholds.
Sensitivity analyses results for S3 and S6 units
For a S3 unit, keeping instruments moist remains a dominant strategy. This is also the case for a S6 unit.
For a S3 unit, having initially kept instruments moist, the cost-effectiveness of adhering to IPG196 would be similar to that of moving from S2 to S1, that is in the region of £1.2M per QALY gained. For a S6 unit, the cost-per-QALY of adhering to IPG196 would be in the region of £1.1M, which is similar to moving from a S5 to a S4 unit. These values are similar rather than identical, as there may be more infectious material on instruments in the S3 and S6 units than in S2 and S5 units.
Threshold analyses on the costs of single-use sets or a completely effective cleaning solution
Analyses were performed to indicate the cost at which a single-use set (including disposal costs) would be cost-effective at cost-per-QALY thresholds of £30,000, £50,000, £100,000 and £300,000. These results are identical to the threshold cost of a cleaning solution that was 100% effective at removing CJD prions, as both approaches (single-use instruments and the cleaning solution) are assumed to prohibit CJD infection via surgery.
The results are presented for each unit type, by four cost-per-QALY thresholds, and for the base case and for a scenario analysis that was more favourable to reusable instruments and a completely effective cleaning solution. The results are presented in Table 29. Caution must be used in interpreting these results, as options other than single-use instruments or a completely effective cleaning solution exist. For example, moving from a S6 to a S5 (or S3 to a S2) is estimated to be a dominant strategy and thus the thresholds for single-use sets for a S6 unit or a S3 unit are redundant, although these have been presented for information.
| Surgical unit | Assumptions | Cost-per-QALY threshold (£) | |||
|---|---|---|---|---|---|
| 30,000 | 50,000 | 100,000 | 300,000 | ||
| S1 | Base case | 11.21 | 12.70 | 16.43 | 31.32 |
| Favourable | 11.60 | 13.09 | 16.81 | 31.71 | |
| S2 | Base case | 13.44 | 16.28 | 23.36 | 51.71 |
| Favourable | 13.91 | 16.75 | 23.83 | 52.18 | |
| S3 | Base case | 30.66 | 43.67 | 76.19 | 206.27 |
| Favourable | 31.91 | 44.92 | 77.44 | 207.53 | |
| S4 | Base case | 10.25 | 11.14 | 13.37 | 22.30 |
| Favourable | 10.61 | 11.50 | 13.73 | 22.66 | |
| S5 | Base case | 12.70 | 15.09 | 21.06 | 44.93 |
| Favourable | 13.15 | 15.53 | 21.50 | 45.37 | |
| S6 | Base case | 27.90 | 39.21 | 67.48 | 180.55 |
| Favourable | 29.07 | 40.38 | 68.65 | 181.72 | |
It is seen that in units where instruments were kept moist, a single-use set price would need to be in the region of £50 to have a cost per QALY below £300,000; to be below a cost per QALY of £30,000, the cost of a single-use kit would need to be in the region of £10.
Threshold analyses on the costs of adhering to IPG196
For S2 and S5 units, analyses were performed to indicate the cost at which adhering to IPG196 would produce an ICER equal to a chosen threshold. These results are presented for the S2 and S5 unit types, by four cost-per-QALY thresholds, and for the base case and for the scenario more favourable to reusable instruments and a completely effective cleaning solution. The results are presented in Table 30. The estimated cost of implementing IPG196 is estimated to be £750,000, which is greater than the threshold values provided in Table 30.
| Surgical unit | Assumptions | Cost-per-QALY threshold (£) | |||
|---|---|---|---|---|---|
| 30,000 | 50,000 | 100,000 | 300,000 | ||
| S2 | Base case | 13,746 | 22,038 | 42,766 | 125,678 |
| Favourable | 14,270 | 22,561 | 43,290 | 126,202 | |
| S5 | Base case | 15,122 | 24,333 | 47,360 | 139,466 |
| Favourable | 15,645 | 24,856 | 47,882 | 139,988 | |
Estimating the cost-effectiveness of removing the need for the P96 group to be operated on with separate instrument sets
The data reported in Tables 26 and 27 indicate that there would be fewer deaths and marginally more QALYs lost when the recommendation that the P96 group are operated on using different instrument sets is removed and the P96 group is considered infectious on model entry. These results lack face validity, particularly in relation to QALYs lost as younger patients can lose more QALYs, and is caused by Monte Carlo sampling error as a result of the misalignment of RNs. Conversely, where the requirement for different instrument sets is removed, given the assumption that the P96 group are not infectious on model entry there are an additional 0.18 QALYs lost although marginally fewer deaths.
The computational time required to provide an accurate estimate for both the number of deaths (which may be equal in both scenarios) and the QALYs lost is far beyond the resources assigned to this work. As such, the results should be interpreted with caution, although currently there is no indication that removing the recommendation related to separate instrument sets would greatly influence the numbers of predicted CJD cases.
Chapter 4 Discussion and conclusions
The purpose of the systematic review was to summarise the most up-to-date published evidence about CJD with regards to the risk of transmission by surgery. As the reviews are largely descriptive rather than summative, with no attempt to rank evidence, formal critical appraisal of study quality was not deemed to be useful. Direct evidence to answer the literature review questions was limited because of the rare nature of CJD. As a result, the eight systematic reviews are heavily reliant on historical cases of stCJD, observational data, case–control study designs and animal data.
This review has included evidence from all forms of CJD, whereas the decision problem was focused on vCJD in the previous work conducted by ScHARR in 2005. 11 The apparent increase in sCJD cases noted in several papers is speculated to be due to improved case ascertainment, population increases and an ageing population. Although the vCJD epidemic appears to have subsided, with few recent clinical cases observed, CJD remains an iatrogenic risk in surgery, mainly from sporadic and genetic forms. Abnormal prion protein, detected using vCJD-specific immunostaining, has also been detected in stored anonymised appendix tissue samples in cohorts of people considered not to have had significant exposure to the BSE epidemic, as reported in the recent Appendix III study. 16 Studies using advanced detection assays also highlight wide vCJD accumulation in the peripheral tissues of a preclinical patient. However, some studies indicate that prions can accumulate in peripheral tissues such as appendixes without transmission to the CNS. Therefore, the assumption that a prevalence of non-clinical prion accumulation in peripheral tissue represents disease that will go on to become clinical CJD has yet to be substantiated. As CJD detection methods advance, more accurate confirmation of CJD pathology will be possible from autopsy and excised tissue samples. Data on the likely incubation periods of CJD are limited to retrospective data from iCJD, vCJD or kuru cases. These data indicate that very long incubation periods that exceed life expectancy are possible. Although sCJD cannot be considered to have an incubation period, as the precise time of disease onset cannot be ascertained, on the basis of having the highest incidence, sCJD (rather than vCJD or gCJD) is likely to pose the greatest risk to surgery.
In the period covered by the reviews, to our knowledge no reports of observed cases of stCJD have been published. Although many studies aim to retrospectively suggest a relationship between prior surgery and risk of developing CJD, these case–control designs are known to be prone to bias and confounding. Few data to supersede the original review conducted by ScHARR regarding infectious dose required to transmit CJD were identified, but some animal studies using advanced detection methods indicate that infectious doses greater than 108 ID50 per gram are possible.
Evidence on the decontamination of surgical instruments is fragmented with no single study assessing the efficacy of all strategies, which include reducing residual mass, keeping instruments moist, autoclaving and sterilisation. Comparison of included studies is also problematic as a result of these being conducted under different conditions and in laboratory settings that limit their external validity to the clinical setting. As empirical data on instrument set-keeping and single-use instruments were not retrieved, no evidence to substantiate or refute anecdotal claims about the drawbacks and merits of reusable versus single-use instruments is available. Data on the likelihood of future surgery in those undergoing high-risk procedures are limited in their potential to inform the model, as these did not focus solely on high-risk procedures and do not compare the risk of additional procedures with control data for those who had not undergone an index high-risk procedure.
The decision problem was complex owing to the paucity of robust data on key modelling parameters such as the efficacy of current decontamination methods and the incubation period associated with stCJD; the lack of observed stCJD cases; the possibility for patients with stCJD to be misdiagnosed as having a different neurodegenerative disease; and the number of model runs required to produce accurate results for the scenarios evaluated. In order to provide additional data to populate the model, output from elicitation sessions was used alongside heuristics that increased the efficiency of the available computational time. The results produced suggest that although there is a possibility that stCJD cases are observed between 2019 and 2023, these are unlikely to be large in number. Based on the analyses run to inform this report, the maximum number of cases of stCJD simulated that were infected between 2019 and 2023 was 47 across England, although the mean estimate was 2.36 cases. Not adhering to keeping instruments moist had a higher mean number of cases (approximately 11) and a maximum among the simulations undertaken of 115. As such, keeping instruments moist should be undertaken wherever possible.
Although simplifications were made in the modelling process, all decisions were made in consultation with the NICE advisory committee meaning that it is likely that most key aspects were included, but there remains the possibility that some were not identified by the committee and were therefore omitted. The use of a distribution for prevalence data based on prions in lymphoid tissue rather than just the central nervous system will overestimate the potential numbers used as prior distributions in the model runs used for calibration. However, this will not restrict the posterior distribution formed in the PSA that are consistent with observed information.
Given the large ICERs produced for the modelled strategies, the additional QALYs that would need to be gained through improved public perception of infection control would have to be very large to bring the ICERs for strategies below the thresholds commonly used by NICE. Similarly, the cost implications related to a potential future public inquiry would need to be very large to alter the conclusions. Both the affect of public perception of infection control and any future inquiry would need to be weighted by the probability of a large number of cases being observed, which is expected to be small given the expected number of potential stCJD deaths per unit (< 0.08; see Table 27) having kept instruments moist, noting that these cases would appear in the future and may be misdiagnosed.
Running a greater number of PSA configurations would increase the accuracy in the ICER related to uncertainty in parameter estimates, and running more RN streams would increase the accuracy for a given PSA configuration. However, it is believed that the results are suitable for robust decision-making, given that (1) the estimated uncertainty in the mean QALYs lost is relatively small and (2) that keeping instruments moist is a dominant strategy compared with not, and (3) that all other ICERs are in excess of £1 million per QALY gained; these values are greater than the cost-effectiveness thresholds reported by NICE. Keeping instruments moist is also aligned with guidance from Department of Health and Social Care. 203
Threshold analyses undertaken indicate that the cost of single-use instruments (per cycle) or the cost per set of using a completely effective decontamination method would need to be in the region of £50 to be cost-effective at a threshold of £300,000 per QALY. Assuming a lower cost-per-QALY threshold of £30,000 meant that the single-use sets or the decontamination method would need to be in the region of £10 per set. The current estimated cost of a single-use set is £425, thus it does not appear likely that costs can be reduced to the threshold levels. The additional cost per set of using a completely effective novel decontamination method is unknown and thus it is possible that standard NICE threshold levels can be achieved, for a commercially available agent that is proven to be completely effective at removing CJD prions.
Threshold analyses were also undertaken to determine the maximum cost to a unit, over a 5-year period, of following IPG196. Assuming a cost-per-QALY threshold of £300,000 and £30,000, these costs were approximately £125,000 and £15,000, respectively. Given that the estimated costs of installing a system to track surgical instruments is estimated to be £750,000 per unit, it is not expected that the prices would fall to the estimated threshold levels.
Furthermore, the analyses run indicated that there would be no marked increase in the risk of stCJD cases when the requirement that P96 patients need to be operated on with separate instruments were removed.
Within this report the authors have presented ICERs on a number of strategies. Using a cost-per-QALY gained threshold of either £30,000 or £300,000, it would appear that the following strategy would be cost-effective: implementing measures to ensure that instruments are kept moist, which is estimated to increase health and save money. Strategies to prevent instrument migration, to use different instrument sets for the P96 group and the non-P96 group, or to use single-use instruments (at current prices) do not appear cost-effective. These results appear robust to assumptions regarding the current standard of decontamination among surgical units. If a decontamination solution became commercially available that was proven to be perfectly effective at removing CJD prions, it is possible that it could be cost-effective dependent on the acquisition price. The ultimate decision in terms of any strategy recommended is, however, the responsibility of NICE.
Strengths and limitations of the work
There has been a comprehensive review of published literature of factors associated with stCJD. The modelling work considered all of the aspects deemed important by the NICE advisory committee and was calibrated to the potential number of stCJD cases that have been observed between 2005 and 2018. Limitations with the work are primarily due to the lack of evidence on key parameters, in particular the number of stCJD cases that have actually occurred in England, which was used as the calibration target. Elicitation sessions were conducted to provide an estimation of possible values where there was little published evidence. Owing to the time scale of the project, it was only possible to elicit opinion from four experts, which may be a limitation. It is highlighted that the model focused only on brain surgery, posterior eye surgery and neuroendoscopy, based on the results of previous work.
The approach was also selective on what to include in the model; these decisions were made in conjunction with the NICE advisory committee, but it is possible that pertinent costs or changes in utility have been omitted. It is acknowledged that gains that may be achieved in surgical procedures unrelated to CJD have not been included within the model.
A further limitation is that novel decontaminant cleaning solutions could not be included, although exploratory analyses were performed to estimate the maximum price that a completely effective decontaminant could command to be cost-effective.
Although the work undertaken suggests that (1) keeping instruments moist is a dominant strategy compared with not, and (2) that all other ICERs are in excess of £1 million per QALY gained, there remains a possibility of future stCJD cases using this strategy due to the uncertainty in the calibration targets and the estimated posterior distributions for each parameter. If there are multiple suspected or definite stCJD cases observed in the near future, then re-assessing the decision problem promptly would be required.
Recommendations for future work
Clinical trials in this rare disease are not a feasible recommendation for research; however, future investigations could take advantage of data from national surveillance programmes such as NCJDRSU and HES to conduct and publish well-designed studies to provide an indication of the number of CJD cases that could potentially be attributed to surgical transmission. Case–control studies using appropriately matched controls and ascertaining surgical exposures through use of medical records are likely to be the best feasible approach for identifying an association between surgery and CJD, at a population level. Considering the methodological limitations and potential for bias in these studies, it is important that the conduct of each individual case–control study is well planned, pre-registered and rigorously executed.
The accuracy of the results produced within the model can be improved through better knowledge relating to the number of stCJD cases that have been observed in England. Historical data are unlikely to provide further insight; however, prospectively assessing whether or not patients diagnosed with alternative neurodegenerative diseases have a history of high-risk surgery could improve the ascertainment rates of stCJD. Furthermore, autopsy studies of patients dying with dementia could also help to assess the extent of underdiagnoses of CJD.
It is acknowledged that surgical history data are gathered and assessed for patients with confirmed CJD by the NCJDRSU. Where family consent is given following the death of suspected, or confirmed, CJD cases, routine post-mortem analysis and publication of clinicopathological data may provide further understanding on the transmissibility and infectivity of CJD. Additionally, seeking in-life permission from those identified as being at risk of exposure to vCJD to perform an autopsy may also improve the rate of CJD confirmation. Furthermore, increasing the number of future and stored appendixes tested for prions may allow an improved estimate of the prevalence of asymptomatic CJD in the UK population.
Further studies of the effectiveness of keeping surgical instruments moist in producing log-reductions in prion load, and on reductions in transmission, would be informative to further validate understanding of the efficacy of current decontamination procedures. Currently, the information used in the model is indirect, as it assumes that reductions associated with protein residue translate to reductions in the potential for transmission of vCJD.
The analyses undertaken did not exclude the possibility that a cleaning solution could be cost-effective providing it was sufficiently efficacious at removing CJD prions, priced appropriately and was commercially available. Further research into proving the efficacy of such products may be worthwhile. It is noted that should a policy to prevent surgical instruments migrating between sets be put in place, then the threshold costs for a completely effective cleaning solution would be approximately half that contained in Table 29, as the QALYs likely to be gained are approximately halved (see Table 27).
In the event of identification of multiple stCJD cases, performing an urgent update of this review with an amended calibration target is likely to be informative.
Acknowledgements
We would like to thank the NICE committee members who provided feedback and data during the research. We would like to thank Andrea Shippam for help in extracting data and in formatting the report and Paul Tappenden for providing a review of a draft.
No patient or public involvement was directly used in producing this report. However, patient and public representatives on the NICE committee provided guidance to the authors.
Contributions of authors
Matt Stevenson (https://orcid.org/0000-0002-3099-9877) led the project, undertook the review of cost-effectiveness literature, constructed the mathematical model and interpreted the results.
Lesley Uttley (https://orcid.org/0000-0003-4603-9069) undertook the review of clinical literature and reviewed a proportion of the cost-effectiveness literature review to ensure consistency.
Jeremy E Oakley (https://orcid.org/0000-0002-9860-4093) led the elicitation session and devised the methods to produce the likelihoods for PSA configurations, and to approximate the width of the confidence intervals.
Christopher Carroll (https://orcid.org/0000-0002-6361-6182) undertook the review of clinical literature and reviewed a proportion of the cost-effectiveness literature review to ensure consistency.
Stephen E Chick (https://orcid.org/0000-0002-8026-1571) provided simulation advice throughout the project.
Ruth Wong (https://orcid.org/0000-0002-4536-4794) updated and performed the literature searches of electronic databases.
Publication
Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M. Creutzfeldt–Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis 2020;20:e2–10.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- University of Edinburgh . The National CJD Research &Amp; Surveilance Unit (NCJDRSU) 2017. www.cjd.ed.ac.uk/ (accessed 11 July 2017).
- National CJD Research & Surveillance Unit . Creutzfeldt–Jakob Disease Surveillance in the UK: 25th Annual Report 2016 2016.
- Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, et al. Iatrogenic Creutzfeldt–Jakob disease, final assessment. Emerging Infect Dis 2012;18:901-7. https://doi.org/10.3201/eid1806.120116.
- World Health Organization (WHO) . WHO Infection Control Guidelines for Transmissible Spongiform Encephalopathies: Report of a WHO Consultation, Geneva, Switzerland, 23–26 March 1999 2000.
- World Health Organization (WHO) . WHO Tables on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies 2010.
- Brown P, Farrell M. A practical approach to avoiding iatrogenic Creutzfeldt–Jakob disease (CJD) from invasive instruments. Infect Control Hosp Epidemiol 2015;36:844-8. https://doi.org/10.1017/ice.2015.53.
- Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol 2004;203:733-9. https://doi.org/10.1002/path.1580.
- Ghani AC, Ferguson NM, Donnelly CA, Anderson RM. Factors determining the pattern of the variant Creutzfeldt–Jakob disease (vCJD) epidemic in the UK. Proc R Soc Lond B 2003;270:689-98. https://doi.org/10.1098/rspb.2002.2313.
- Clarke P, Ghani AC. Projections of the future course of the primary vCJD epidemic in the UK: inclusion of subclinical infection and the possibility of wider genetic susceptibility. J R Soc Interface 2005;2:19-31. https://doi.org/10.1098/rsif.2004.0017.
- Lloyd Jones M, Stevenson M, Sutton A. Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) via Interventional Procedures. Interventional Procedure Guidance 196. London: National Institute for Health and Care Excellence, University of Sheffield, School of Health and Related Research; 2006.
- Stevenson M, Oakley J, Chick SE. Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) via Interventional procedures—final Report 2006. www.nice.org.uk/guidance/ipg196/documents/ipg196-patient-safety-and-reduction-of-risk-of-transmission-of-creutzfeldtjakob-disease-cjd-via-interventional-procedures-final-report2 (accessed 10 February 2020).
- Stevenson MD, Oakley JE, Chick SE, Chalkidou K. The cost-effectiveness of surgical instrument management policies to reduce the risk of vCJD transmission to humans. J Oper Res Soc 2009;60:506-18. https://doi.org/10.1057/palgrave.jors.2602580.
- Bennett P, Hare A, Townshend J. Assessing the risk of vCJD transmission via surgery: models for uncertainty and complexity. J Oper Res Soc 2005;56:202-13. https://doi.org/10.1057/palgrave.jors.2601899.
- National Institute for Health and Care Excellence (NICE) . NICE Interventional Procedure Guidance 196. Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) Via Interventional Procedures 2008. www.nice.org.uk/guidance/ipg196/documents/ipg196-patient-safety-and-reduction-of-risk-of-transmission-of-creutzfeldtjakob-disease-cjd-via-interventional-procedures-guidance2 (accessed 2 February 2018).
- Public Health England . Summary Results of the Third National Survey of Abnormal Prion Prevalence in Archived Appendix Specimens 2016.
- Advisory Committee on Dangerous Pathogens TSE Subgroup . Updated Position Statement on Occurrence of VCJD and Prevalence of Infection in the UK 2016. www.clinicalvirology.org/news/acdp-tse-subgroup-updated-position-statement-on-occurrence-of-vcjd-and-prevalence-of-infection-in-the-uk/ (accessed 8 January 2020).
- Jaunmuktane Z, Quaegebeur A, Taipa R, Viana-Baptista M, Barbosa R, Koriath C, et al. Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol 2018;135:671-9. https://doi.org/10.1007/s00401-018-1822-2.
- Stevenson M, Jeremy O, Chick S. Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) via Interventional Procedures 2006. www.nice.org.uk/guidance/ipg196/documents/ipg196-patient-safety-and-reduction-of-risk-of-transmission-of-creutzfeldtjakob-disease-cjd-via-interventional-procedures-final-report2 (accessed 3 October 2019).
- Lloyd Jones M, Stevenson M, Sutton A. Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob Disease (CJD) via International Procedures 2006. www.nice.org.uk/guidance/ipg196/documents/ipg196-patient-safety-and-reduction-of-risk-of-transmission-of-creutzfeldtjakob-disease-cjd-via-interventional-procedures-systematic-review2 (accessed 3 October 2019).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Uttley L, Carroll C, Wong R, Stevenson M. Update review: risk of transmission via surgical interventional procedures of Creutzfeldt–Jakob disease (CJD) and economic modelling of clinical management policies. PROSPERO 2017 CRD42017071807 n.d. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017071807 (accessed 10 February 2020).
- Creutzfeldt–Jakob Disease International Surveillance Network Formerly EuroCJD . CJD Surveillance Data 1993–2017. 2017 n.d. www.eurocjd.ed.ac.uk/surveillance%20data%201.html (accessed 23 January 2018).
- US Centres for Disease Control and Prevention . Creutzfeldt–Jakob Disease, Classic (CJD) Occurrence and Transmission 2018. www.cdc.gov/prions/cjd/occurrence-transmission.html (accessed 23 January 2018).
- Yamada M, Hamaguchi T, Sakai K, Nozaki I, Noguchi-Shinohara M, Sanjo N, et al. Epidemiological and clinical features of human prion diseases in Japan: prospective 17-year surveillance. Prion 2016;10:S10-S1.
- Klug GM, Boyd A, Sarros S, Stehmann C, Simpson M, McLean CA, et al. Creutzfeldt–Jakob disease surveillance in Australia: update to December 2015. Commun Dis Intell Q Rep 2016;40:E368-E376.
- Begué C, Martinetto H, Schultz M, Rojas E, Romero C, D’Giano C, et al. Creutzfeldt–Jakob disease surveillance in Argentina, 1997–2008. Neuroepidemiology 2011;37:193-202. https://doi.org/10.1159/000331907.
- Lu CJ, Sun Y, Chen SS. Incidence of Creutzfeldt–Jakob disease in Taiwan: a prospective 10-year surveillance. Eur J Epidemiol 2010;25:341-7. https://doi.org/10.1007/s10654-010-9446-4.
- Jeon BH, Kim J, Kim GK, Park SC, Kim S, Cheong HK. Estimation of the size of the iatrogenic Creutzfeldt–Jakob disease outbreak associated with cadaveric dura mater grafts in Korea. Epidemiol Health 2016;38. https://doi.org/10.4178/epih.e2016059.
- Gao C, Shi Q, Tian C, Chen C, Han J, Zhou W, et al. The epidemiological, clinical, and laboratory features of sporadic Creutzfeldt–Jakob disease patients in China: surveillance data from 2006 to 2010. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0024231.
- University of Edinburgh . The National CJD Research & Surveilance Unit (NCJDRSU). Creutzfeldt–Jakob disease in the UK (by calendar year) 2019. www.cjd.ed.ac.uk/sites/default/files/figs.pdf (accessed 26 November 2019).
- Molesworth A, Yates P, Hewitt PE, Mackenzie J, Ironside JW, Galea G, et al. vCJD associated with organ or tissue transplantation in the UK: a lookback study. Transplantation 2014;98:585-9.
- Isotalo J, Gardberg M, Verkkoniemi-Ahola A, Paetau A, Martikainen MH, Korpela J, et al. Phenotype and incidence of Creutzfeldt–Jakob disease in Finland in 1997-2013. Duodecim 2015;131:465-74.
- Chen SS. Surveillance of prion diseases in Taiwan. Prion 2016;10.
- Van Everbroeck B, Michotte A, Sciot R, Godfraind C, Deprez M, Quoilin S, et al. Increased incidence of sporadic Creutzfeldt–Jakob disease in the age groups between 70 and 90 years in Belgium. Eur J Epidemiol 2006;21:443-7. https://doi.org/10.1007/s10654-006-9012-2.
- Ae R, Nakamura Y, Takumi I, Sanjo N, Kitamoto T, Yamada M, et al. Epidemiologic features of human prion diseases in Japan: a prospective 15-year surveillance study. Prion 2016;10:S103-S4.
- Rus T, Caks–Jager N, Popovic M, Blasko Markic M, Kramberger Gregoric M. High incidence of sporadic Creutzfeldt–Jakob disease in Slovenia in 2015. Eur J Neurol 2016;23.
- Mok T, Jaunmuktane Z, Joiner S, Campbell T, Morgan C, Wakerley B, et al. Variant Creutzfeldt–Jakob disease in a patient with heterozygosity at PRNP codon 129. N Engl J Med 2017;376:292-4. https://doi.org/10.1056/NEJMc1610003.
- Urwin P, Mackenzie J, Knight R, Will R, Molesworth A. Is sporadic CJD an acquired disease? A review of the UK CJD cases. Prion 2016;10.
- Shi J, Chen Q, Chen X, Zhang J. Case report: clinical scenarios in Creutzfeldt–Jakob disease (CJD): report of nine cases. Int J Exp Pathol 2016;9:2744-51.
- Baig M, Phillips M. A case of Creutzfeldt–Jakob disease: diagnostic dilemmas of a rapidly fatal disease. Infect Dis Rep 2013;5. https://doi.org/10.4081/idr.2013.e10.
- Maddox RA, Holman RC, Folkema AM, Gambetti P, Zou WQ, Minino AM, et al. Creutzfeldt–Jakob disease among blacks in the United States, 1994–2007. Prion 2010;4.
- Holman RC, Belay ED, Christensen KY, Maddox RA, Minino AM, Folkema AM, et al. Human prion diseases in the United States. PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0008521.
- Maddox RA, Holman RC, Minino AM, Blevins JE, Schonberger LB, Belay ED. Prion disease among Asians and Pacific Islanders in the United States, 2003–9. Prion 2013;7.
- Nakatani E, Nishimura T, Zhou B, Kaneda H, Teramukai S, Nagai Y, et al. Temporal and regional variations in sporadic Creutzfeldt–Jakob disease in Japan, 2001–10. Epidemiol Infect 2015;143:1073-8. https://doi.org/10.1017/S0950268814001605.
- Klug GM, Wand H, Boyd A, Law M, Whyte S, Kaldor J, et al. Enhanced geographically restricted surveillance simulates sporadic Creutzfeldt–Jakob disease cluster. Brain 2009;132:493-501. https://doi.org/10.1093/brain/awn303.
- Brandel JP, Peckeu L, Haïk S. The French surveillance network of Creutzfeldt–Jakob disease. Epidemiological data in France and worldwide. Transfus Clin Biol 2013;20:395-7. https://doi.org/10.1016/j.tracli.2013.02.029.
- Mitrová E, Kosorinová D, Gajdoš M, Šebeková K, Tomečková I. A pilot study of a genetic CJD risk factor (E200K) in the general Slovak population. Eur J Epidemiol 2014;29:595-7. https://doi.org/10.1007/s10654-014-9937-9.
- Ladogana A, Puopolo M, Croes EA, Budka H, Jarius C, Collins S, et al. Mortality from Creutzfeldt–Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 2005;64:1586-91. https://doi.org/10.1212/01.WNL.0000160117.56690.B2.
- Tuskan-Mohar L, Legac M, Prunk DA, Bucuk M, Perkovic O, Antoncic I. The frequency of Creutzfeldt–Jakob disease in Primorsko-Goranska county. Acta Clin Croat Suppl 2012;51:83-4.
- Kosier N. Why won’t she talk? A case of Creutzfeldt–Jakob disease masquerading as psychiatric decompensation. J Am Geriatr Soc 2017;65.
- Litzroth A, Cras P, De Vil B, Quoilin S. Overview and evaluation of 15 years of Creutzfeldt–Jakob disease surveillance in Belgium, 1998-2012. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0507-x.
- Brett FM, Looby S, Chalissery A, Chen D, Heaney C, Heffernan J, et al. Brain biopsies requiring Creutzfeldt–Jakob disease precautions in the Republic of Ireland 2005–2016. Ir J Med Sci 2018;187:5-. https://doi.org/10.1007/s11845-017-1673-1.
- Loftus T, Chen D, Looby S, Chalissery A, Howley R, Heaney C, et al. CJD surveillance in the Republic of Ireland from 2005 to 2015: a suggested algorithm for referrals. Clin Neuropathol 2017;36:188-94. https://doi.org/10.5414/NP301016.
- Ali A, Abbas M, Ahmed S, Ejaz K. Creutzfeldt–Jakob Disease (CJD) rare stroke mimic. Cerebrovasc Dis 2015;39.
- Hirst CL. Sporadic Creutzfeldt–Jakob disease presenting as a stroke mimic590. Br J Hosp Med 2011;72:590-1. https://doi.org/10.12968/hmed.2011.72.10.590.
- Hanumanthu R, Alchaki A, Nyaboga A, Ghuman H, Chen J, Feinstein E. An unusual case of sporadic Creutzfeld Jacob disease presenting as acute neuropathy [abstract]. Mov Disord 2017;32:563-4.
- Karatas H, Dericioglu N, Kursun O, Saygi S. Creutzfeldt–Jakob disease presenting as hyperparathyroidism and generalized tonic status epilepticus. Clinical EEG Neurosci 2007;38:203-6. https://doi.org/10.1177/155005940703800404.
- Kher M, Rao MY, Acharya PT, Mahadevan A, Shankar SK. Heidenhain variant of Creutzfeldt–Jakob disease: an autopsy study from India. Ann Indian Acad Neurol 2009;12:48-51.
- Neville JL, Fichtenbaum C. ‘Rapidly progressive dementia: Sometimes it is a zebra’. J Gen Intern Med 2012;27.
- Pachalska M, Kurzbauer H, Formińska-Kapuścik M, Urbanik A, Bierzyńska-Macyszyn G, Właszczuk P. Atypical features of dementia in a patient with Creutzfeldt–Jakob disease. Med Sci Monit 2007;13:CS9-19.
- Patrawala S, Soltani M, Zulauf M. A rare case of ataxia and rapidly progressing dementia. J Gen Intern Med 2014;29:S280-S1.
- Krystina C, Abbas A, Hall A, Khadjooi K, Elhag RA, Rostami K. Sporadic CJD presenting with aphasia diagnosed in medical admissions unit. Eur J Intern Med 2011;22. https://doi.org/10.1016/S0953-6205(11)60451-2.
- Sann AA, Zaw MM, Choie TL. Creutzfeldt–Jakob disease presenting predominantly with movement disorder: a case report. Mov Disord 2016;31:S577-S8.
- Bruton CJ, Bruton RK, Gentleman SM, Roberts GW. Diagnosis and incidence of prion (Creutzfeldt–Jakob) disease: a retrospective archival survey with implications for future research. Neurodegeneration 1995;4:357-68. https://doi.org/10.1006/neur.1995.0043.
- Urwin P, Thanigaikumar K, Ironside JW, Molesworth A, Knight RS, Hewitt PE, et al. Sporadic Creutzfeldt–Jakob disease in 2 plasma product recipients, United Kingdom. Emerging Infect Dis 2017;23. https://doi.org/10.3201/eid2306.161884.
- Shepherd KJ, Barker BR. A common presentation of a rare disease: sporadic CJD. J Gen Intern Med 2016;1:S500-S1.
- Galeno R, Di Bari MA, Nonno R, Cardone F, Sbriccoli M, Graziano S, et al. Prion strain characterization of a novel subtype of Creutzfeldt–Jakob disease. J Virol 2017;91:e02390-16. https://doi.org/10.1128/JVI.02390-16.
- Nagoshi K, Sadakane A, Nakamura Y, Yamada M, Mizusawa H. Duration of prion disease is longer in Japan than in other countries. J Epidemiol 2011;21:255-62. https://doi.org/10.2188/jea.JE20100085.
- Rudge P, Jaunmuktane Z, Adlard P, Bjurstrom N, Caine D, Lowe J, et al. Iatrogenic CJD due to pituitary-derived growth hormone with genetically determined incubation times of up to 40 years. Brain 2015;138:3386-99. https://doi.org/10.1093/brain/awv235.
- Sanchez-Juan P, Bishop MT, Croes EA, Knight RS, Will RG, van Duijn CM, et al. A polymorphism in the regulatory region of PRNP is associated with increased risk of sporadic Creutzfeldt–Jakob disease. BMC Med Genet 2011;12. https://doi.org/10.1186/1471-2350-12-73.
- Brandner S, Jaunmuktane Z. Prion disease: experimental models and reality. Acta Neuropathol 2017;133:197-222. https://doi.org/10.1007/s00401-017-1670-5.
- Giaccone G, Capellari S, Ingrosso L, Ferrari S, Imperiale D, Taraglio S, et al. An update of the epidemiology of sporadic Creutzfeldt–Jakob disease in Italy based on neuropathologic and molecular typing of a large cohort of patients. Clin Neuropathol 2009;28.
- Ironside JW, Bishop MT, Connolly K, Hegazy D, Lowrie S, Le Grice M, et al. Variant Creutzfeldt–Jakob disease: prion protein genotype analysis of positive appendix tissue samples from a retrospective prevalence study. BMJ 2006;332:1186-8. https://doi.org/10.1136/bmj.38804.511644.55.
- Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ 2013;347. https://doi.org/10.1136/bmj.f5675.
- Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Beck J, et al. Genetic susceptibility, evolution and the kuru epidemic. Phil Trans R Soc B 2008;363:3741-6. https://doi.org/10.1098/rstb.2008.0087.
- Kaski D, Mead S, Hyare H, Cooper S, Jampana R, Overell J, et al. Variant CJD in an individual heterozygous for PRNP codon 129. Lancet 2009;374. https://doi.org/10.1016/S0140-6736(09)61568-3.
- Peden A, McCardle L, Head MW, Love S, Ward HJ, Cousens SN, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia 2010;16:296-304. https://doi.org/10.1111/j.1365-2516.2009.02181.x.
- Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 2004;364:527-9. https://doi.org/10.1016/S0140-6736(04)16811-6.
- Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol 2006;5:393-8. https://doi.org/10.1016/S1474-4422(06)70413-6.
- Pennington C, Knight R. The clinicopathological phenotype of genetic CJD due to the E200K mutation in the UK. Prion 2010;4.
- Coulthart MB, Geschwind MD, Qureshi S, Phielipp N, Demarsh A, Abrams JY, et al. A case cluster of variant Creutzfeldt–Jakob disease linked to the Kingdom of Saudi Arabia. Brain 2016;139:2609-16. https://doi.org/10.1093/brain/aww206.
- Chohan G, Llewelyn C, Mackenzie J, Cousens S, Kennedy A, Will R, et al. Variant Creutzfeldt–Jakob disease in a transfusion recipient: coincidence or cause?. Transfusion 2010;50:1003-6. https://doi.org/10.1111/j.1537-2995.2010.02614.x.
- Davidson LR, Llewelyn CA, Mackenzie JM, Hewitt PE, Will RG. Variant CJD and blood transfusion: are there additional cases?. Vox Sang 2014;107:220-5. https://doi.org/10.1111/vox.12161.
- Molesworth A, Yates P, Hewitt PE, Mackenzie J, Ironside JW, Galea G, et al. Investigation of variant Creutzfeldt–Jakob disease implicated organ or tissue transplantation in the United Kingdom. Transplantation 2014;98:585-9. https://doi.org/10.1097/TP.0000000000000105.
- Ward HJT, Will RG, Ghani A, Ironside JW. An update on variant CJD (vCJD), secondary transmission and prevalence. Eur J Neurol 2006;13:306-7.
- Urwin PJ, Mackenzie JM, Llewelyn CA, Will RG, Hewitt PE. Creutzfeldt–Jakob disease and blood transfusion: updated results of the UK transfusion medicine epidemiology review study. Vox Sang 2016;110:310-16. https://doi.org/10.1111/vox.12371.
- Ward HJ, MacKenzie JM, Llewelyn CA, Knight RS, Hewitt PE, Connor N, et al. Variant Creutzfeldt–Jakob disease and exposure to fractionated plasma products. Vox Sang 2009;97:207-10. https://doi.org/10.1111/j.1423-0410.2009.01205.x.
- Diack AB, Will RG, Manson JC. Public health risks from subclinical variant CJD. PLOS Pathog 2017;13. https://doi.org/10.1371/journal.ppat.1006642.
- Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW, et al. Variant CJD. 18 years of research and surveillance. Prion 2014;8:286-95. https://doi.org/10.4161/pri.29237.
- Ritchie DL, Boyle A, McConnell I, Head MW, Ironside JW, Bruce ME. Transmissions of variant Creutzfeldt–Jakob disease from brain and lymphoreticular tissue show uniform and conserved bovine spongiform encephalopathy-related phenotypic properties on primary and secondary passage in wild-type mice. J Gen Virol 2009;90:3075-82. https://doi.org/10.1099/vir.0.013227-0.
- Diack AB, Boyle A, Ritchie DL, Rabano A, de Pedro-Cuesta J, Brandel JP, et al. Variant CJD: Lessons in public health. Prion 2016;10:S82-S3.
- de Marco MF, Linehan J, Gill ON, Clewley JP, Brandner S. Large-scale immunohistochemical examination for lymphoreticular prion protein in tonsil specimens collected in Britain. J Pathol 2010;222:380-7. https://doi.org/10.1002/path.2767.
- Clewley JP, Kelly CM, Andrews N, Vogliqi K, Mallinson G, Kaisar M, et al. Prevalence of disease related prion protein in anonymous tonsil specimens in Britain: cross sectional opportunistic survey. BMJ 2009;338. https://doi.org/10.1136/bmj.b1442.
- Olsen SB, Sheikh A, Peck D, Darzi A. Variant Creutzfeldt–Jakob disease: a cause for concern. Review of the evidence for risk of transmission through abdominal lymphoreticular tissue surgery. Surg Endosc 2005;19:747-50. https://doi.org/10.1007/s00464-004-9205-2.
- McGowan CR, Viens AM. Coroners and the obligation to protect public health: the case of the failed UK vCJD study. Public Health 2011;125:234-7. https://doi.org/10.1016/j.puhe.2010.12.001.
- McGowan CR, Viens AM. Death investigation systems and disease surveillance. Epidemiol Infect 2011;139:986-90. https://doi.org/10.1017/S0950268810002840.
- Rebello A. Correspondence With the CSEW Concerning Research into Subclinical vCJD. Liverpool: Honorary Secretary of the Coroners’ Society of England and Wales; 2007.
- Kobayashi A, Parchi P, Yamada M, Mohri S, Kitamoto T. Neuropathological and biochemical criteria to identify acquired Creutzfeldt–Jakob disease among presumed sporadic cases. Neuropathology 2016;36:305-10. https://doi.org/10.1111/neup.12270.
- Kobayashi A, Parchi P, Yamada M, Brown P, Saverioni D, Matsuura Y, et al. Iatrogenic transmission of Creutzfeldt–Jakob disease. Prion 2016;10.
- Kobayashi A, Parchi P, Yamada M, Brown P, Saverioni D, Matsuura Y, et al. Transmission properties of atypical Creutzfeldt–Jakob disease: a clue to disease etiology?. J Virol 2015;89:3939-46. https://doi.org/10.1128/JVI.03183-14.
- Gnanajothy R, Umashanker D, Vega MC, Wu BJ. A case of Creutzfeldt–Jakob disease following cataract surgery: sporadic versus iatrogenic cause. Conn Med 2013;77:335-7.
- Tuck K, Mass M. Sporadic Creutzfeld-Jakob disease in a 33 year old male with prior cerebral instrumentation. Neurology 2013;80.
- Moreno MJ, Escriche D, Romero J, Maciñeiras JL, Corredera E, Castro MD, et al. Creutzfeldt–Jakob disease cluster in the health area of Meixoeiro Hospital. Acta Neurol Scand 2013;127:38-45. https://doi.org/10.1111/j.1600-0404.2012.01678.x.
- Puopolo M, Ladogana A, Vetrugno V, Pocchiari M. Transmission of sporadic Creutzfeldt–Jakob disease by blood transfusion: risk factor or possible biases. Transfusion 2011;51:1556-66. https://doi.org/10.1111/j.1537-2995.2010.03004.x.
- de Pedro-Cuesta J, Mahillo-Fernández I, Rábano A, Calero M, Cruz M, Siden A, et al. Nosocomial transmission of sporadic Creutzfeldt–Jakob disease: results from a risk-based assessment of surgical interventions. J Neurol Neurosurg Psychiatry 2011;82:204-12. https://doi.org/10.1136/jnnp.2009.188425.
- Mahillo-Fernandez I, de Pedro-Cuesta J, Bleda MJ, Cruz M, Mølbak K, Laursen H, et al. Surgery and risk of sporadic Creutzfeldt–Jakob disease in Denmark and Sweden: registry-based case-control studies. Neuroepidemiology 2008;31:229-40. https://doi.org/10.1159/000163097.
- Hamaguchi T, Noguchi-Shinohara M, Nozaki I, Nakamura Y, Sato T, Kitamoto T, et al. The risk of iatrogenic Creutzfeldt–Jakob disease through medical and surgical procedures. Neuropathology 2009;29:625-31. https://doi.org/10.1111/j.1440-1789.2009.01023.x.
- Hamaguchi T, Noguchi-Shinohara M, Nozaki I, Nakamura Y, Sato T, Kitamoto T, et al. Medical procedures and risk for sporadic Creutzfeldt–Jakob disease, Japan, 1999–2008. Emerging Infect Dis 2009;15:265-71. https://doi.org/10.3201/eid1502.080749.
- Ruegger J, Stoeck K, Amsler L, Blaettler T, Zwahlen M, Aguzzi A, et al. A case-control study of sporadic Creutzfeldt–Jakob disease in Switzerland: analysis of potential risk factors with regard to an increased CJD incidence in the years 2001–2004. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-18.
- Ward HJ, Everington D, Cousens SN, Smith-Bathgate B, Leitch M, Cooper S, et al. Risk factors for variant Creutzfeldt–Jakob disease: a case-control study. Ann Neurol 2006;59:111-20. https://doi.org/10.1002/ana.20708.
- Ward HJ, Everington D, Cousens SN, Smith-Bathgate B, Gillies M, Murray K, et al. Risk factors for sporadic Creutzfeldt–Jakob disease. Ann Neurol 2008;63:347-54. https://doi.org/10.1002/ana.21294.
- de Pedro-Cuesta J, Ruiz Tovar M, Ward H, Calero M, Smith A, Verduras CA, et al. Sensitivity to biases of case-control studies on medical procedures, particularly surgery and blood transfusion, and risk of Creutzfeldt–Jakob disease. Neuroepidemiology 2012;39:1-18. https://doi.org/10.1159/000339318.
- Alcalde-Cabero E, Almazan-Isla J, Brandel JP, Breithaupt M, Catarino J, Collins S, et al. Health professions and risk of sporadic Creutzfeldt–Jakob disease, 1965 to 2010. Euro Surveill 2012;17.
- de Pedro-Cuesta J, Mahillo-Fernandez I, Calero M, Rábano A, Cruz M, Siden Å, et al. Towards an age-dependent transmission model of acquired and sporadic Creutzfeldt–Jakob disease. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0109412.
- Cruz M, Mahillo-Fernandez I, Rábano A, Siden A, Calero M, Laursen H, et al. Late-in-life surgery associated with Creutzfeldt–Jakob disease: a methodological outline for evidence-based guidance. Emerg Themes Epidemiol 2013;10. https://doi.org/10.1186/1742-7622-10-5.
- Kobayashi A. Mechanisms of transmission of prion diseases. Clin Neurol 2016;56.
- Bryant G, Hewitt P, Hope J, Howard C, Ironside J, Knight R, et al. Minimise Transmission Risk of CJD and VCJD in Healthcare Settings. Report on the Prevention of CJD and VCJD by Advisory Committee on Dangerous Pathogens’ Transmission Spongiform Encephalopathy (ACDP TSE) Subgroup 2015. www.gov.uk/government/publications/guidance-from-the-acdp-tse-risk-management-subgroup-formerly-tse-working-group (accessed 8 January 2020).
- Hall V, Brookes D, Nacul L, Gill ON, Connor N. CJD Incidents Panel . Managing the risk of iatrogenic transmission of Creutzfeldt–Jakob disease in the UK. J Hosp Infect 2014;88:22-7. https://doi.org/10.1016/j.jhin.2014.06.002.
- Belay ED, Blase J, Sehulster LM, Maddox RA, Schonberger LB. Management of neurosurgical instruments and patients exposed to Creutzfeldt–Jakob disease. Infect Control Hosp Epidemiol 2013;34:1272-80. https://doi.org/10.1086/673986.
- Thomas JG, Chenoweth CE, Sullivan SE. Iatrogenic Creutzfeldt–Jakob disease via surgical instruments. J Clin Neurosci 2013;20:1207-12. https://doi.org/10.1016/j.jocn.2013.01.007.
- Orrú CD, Yuan J, Appleby BS, Li B, Li Y, Winner D, et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt–Jakob disease. Sci Transl Med 2017;9. https://doi.org/10.1126/scitranslmed.aam7785.
- Zou W, Orru CD, Yuan J, Appleby BS, Li Y, Rarick J, et al. PrPSc in the skin of CJD patients. Prion 2016;10.
- Notari S, Moleres FJ, Hunter SB, Belay ED, Schonberger LB, Cali I, et al. Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States. PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0008765.
- Davanipour Z, Sobel E, Ziogas A, Smoak C, Bohr T, Doram K, et al. Ocular tonometry and sporadic Creutzfeldt–Jakob disease (sCJD): a confirmatory case-control study. Br J Med Med Res 2014;4:2322-33. https://doi.org/10.9734/BJMMR/2014/7247.
- Tullo AB, Buckley RJ, Kelly T, Head MW, Bennett P, Armitage WJ, et al. Transplantation of ocular tissue from a donor with sporadic Creutzfeldt–Jakob disease. Clin Experiment Ophthalmol 2006;34:645-9. https://doi.org/10.1111/j.1442-9071.2006.01308.x.
- Jirsova K, Krabcova I, Novakova J, Hnathova I, Koukolik F, Kubesova B, et al. The assessment of pathogenic prions in the brains of eye tissue donors: 2-years experience in the Czech Republic. Cornea 2010;29:996-9. https://doi.org/10.1097/ICO.0b013e3181cc7b37.
- Maddox RA, Belay ED, Curns AT, Zou WQ, Nowicki S, Lembach RG, et al. Creutzfeldt–Jakob disease in recipients of corneal transplants. Cornea 2008;27:851-4. https://doi.org/10.1097/ICO.0b013e31816a628d.
- Bourvis N, Boelle PY, Cesbron JY, Valleron AJ. Risk assessment of transmission of sporadic Creutzfeldt–Jakob disease in endodontic practice in absence of adequate prion inactivation. PLOS ONE 2007;2. https://doi.org/10.1371/journal.pone.0001330.
- Everington D, Smith AJ, Ward HJ, Letters S, Will RG, Bagg J. Dental treatment and risk of variant CJD – a case control study. Br Dent J 2007;202. https://doi.org/10.1038/bdj.2007.126.
- Azarpazhooh A, Fillery ED. Prion disease: the implications for dentistry. J Endod 2008;34:1158-66. https://doi.org/10.1016/j.joen.2008.07.008.
- Collinge J, Whitfield J, McKintosh E, Frosh A, Mead S, Hill AF, et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Phil Trans R Soc B 2008;363:3725-39. https://doi.org/10.1098/rstb.2008.0068.
- Collinge J. Lessons of kuru research: background to recent studies with some personal reflections. Phil Trans R Soc B 2008;363:3689-96. https://doi.org/10.1098/rstb.2008.0121.
- Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, Thomas DJ, et al. Kuru in the 21st century – an acquired human prion disease with very long incubation periods. Lancet 2006;367:2068-74. https://doi.org/10.1016/S0140-6736(06)68930-7.
- Collinge J, Alpers MP. Incubation period of human prion disease - author’s reply. Lancet 2006;368:914-15. https://doi.org/10.1016/S0140-6736(06)69363-X.
- Haïk S, Brandel JP. Infectious prion diseases in humans: cannibalism, iatrogenicity and zoonoses. Infect Genet Evol 2014;26:303-12. https://doi.org/10.1016/j.meegid.2014.06.010.
- Hamaguchi T. Clinical manifestations and epidemiology of prion diseases in Japan. Rinsho Shinkeigaku 2013;23:1246-8. https://doi.org/10.5692/clinicalneurol.53.1246.
- Heath CA, Barker RA, Esmonde TF, Harvey P, Roberts R, Trend P, et al. Dura mater-associated Creutzfeldt–Jakob disease: experience from surveillance in the UK. J Neurol Neurosurg Psychiatry 2006;77:880-2. https://doi.org/10.1136/jnnp.2005.073395.
- Hirst C. Iatrogenic Creutzfeldt–Jakob disease presenting 24 years after human growth hormone administration. Br J Hosp Med 2005;66:592-3. https://doi.org/10.12968/hmed.2005.66.10.19901.
- Meissner B, Kallenberg K, Sanchez-Juan P, Ramljak S, Krasnianski A, Heinemann U, et al. MRI and clinical syndrome in dura mater-related Creutzfeldt–Jakob disease. J Neurol 2009;256:355-63. https://doi.org/10.1007/s00415-009-0026-z.
- Ritchie DL, Barria MA, Peden AH, Yull HM, Kirkpatrick J, Adlard P, et al. UK Iatrogenic Creutzfeldt–Jakob disease: investigating human prion transmission across genotypic barriers using human tissue-based and molecular approaches. Acta Neuropathol 2017;133:579-95. https://doi.org/10.1007/s00401-016-1638-x.
- Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt–Jakob disease associated with blood transfusion: a case report. Lancet 2006;368:2061-7. https://doi.org/10.1016/S0140-6736(06)69835-8.
- Cervenova L, Goldfarb LG, Garruto R, Lee HS, Gajdusek DC, Brown P. Phenotype-genotype studies in kuru: implications for new variant Creutzfeldt–Jakob disease. Proc Natl Acad Sci USA 1998;95:13239-41. https://doi.org/10.1073/pnas.95.22.13239.
- Klitzman RL, Alpers MP, Gadjusek DC. The natural incubation period of kuru and the episodes of transmission in three clusters of patients. Neuroepidemiology 1984;3:3-20. https://doi.org/10.1159/000110837.
- Kobayashi A, Teruya K, Matsuura Y, Shirai T, Nakamura Y, Yamada M, et al. The influence of PRNP polymorphisms on human prion disease susceptibility: an update. Acta Neuropathol 2015;130:159-70. https://doi.org/10.1007/s00401-015-1447-7.
- Xiao X, Yuan J, Qing L, Cali I, Mikol J, Delisle MB, et al. Comparative study of prions in iatrogenic and sporadic creutzfeldt–jakob disease. J Clin Cell Immunol 2014;5. https://doi.org/10.4172/2155-9899.1000240.
- Ironside JW. Variant Creutzfeldt–Jakob disease. Haemophilia 2010;16:175-80. https://doi.org/10.1111/j.1365-2516.2010.02317.x.
- Hamaguchi T, Sakai K, Noguchi-Shinohara M, Nozaki I, Takumi I, Sanjo N, et al. Insight into the frequent occurrence of dura mater graft-associated Creutzfeldt–Jakob disease in Japan. J Neurol Neurosurg Psychiatry 2013;84:1171-5. https://doi.org/10.1136/jnnp-2012-304850.
- Ibrahim-Verbaas C, Engelen-Lee JY, Spliet W, Mondria T, Willems S, Van Duijn C, et al. CJD with numerous Abeta plaques in a 58-year old patient 28 years after dura mater grafting. Prion 2012;6:131-2.
- Makarava N, Savtchenko R, Alexeeva I, Rohwer RG, Baskakov IV. Fast and ultrasensitive method for quantitating prion infectivity titre. Nat Commun 2012;3. https://doi.org/10.1038/ncomms1730.
- Halliez S, Reine F, Herzog L, Jaumain E, Haïk S, Rezaei H, et al. Accelerated, spleen-based titration of variant Creutzfeldt–Jakob disease infectivity in transgenic mice expressing human prion protein with sensitivity comparable to that of survival time bioassay. J Virol 2014;88:8678-86. https://doi.org/10.1128/JVI.01118-14.
- Ironside JW. Variant Creutzfeldt–Jakob disease: an update. Folia Neuropathol 2012;50:50-6.
- Bishop MT, Diack AB, Ritchie DL, Ironside JW, Will RG, Manson JC. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt–Jakob disease. Brain 2013;136:1139-45. https://doi.org/10.1093/brain/awt032.
- Cali I, Cohen I, Blevins J, Castellani R, Al-Shekhlee A, Yuan J, et al. The co-existence of PrPSc type 1 and 2 in sporadic Creutzfeldt–Jakob disease affects the phenotype and PrPSc conformation. J Neuropathol Exp Neurol 2009;68. https://doi.org/10.1093/brain/awp196.
- Parchi P, Strammiello R, Notari S, Giese A, Langeveld JP, Ladogana A, et al. Incidence and spectrum of sporadic Creutzfeldt–Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol 2009;118:659-71. https://doi.org/10.1007/s00401-009-0585-1.
- Bishop MT, Will RG, Manson JC. Defining sporadic Creutzfeldt–Jakob disease strains and their transmission properties. Proc Natl Acad Sci USA 2010;107:12005-10. https://doi.org/10.1073/pnas.1004688107.
- Uro-Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, Perret-Liaudet A, et al. Beyond PrP9res) type 1/type 2 dichotomy in Creutzfeldt–Jakob disease. PLOS Pathog 2008;4. https://doi.org/10.1371/journal.ppat.1000029.
- Jansen C, Parchi P, Capellari S, Ibrahim-Verbaas CA, Schuur M, Strammiello R, et al. Human prion diseases in the Netherlands (1998-2009): clinical, genetic and molecular aspects. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0036333.
- Mackay G, Yull H, Ironside J, Head M, Knight R. Unravelling the mysteries of sporadic CJD. J Neurol Neurosurg Psychiatry 2013;84. https://doi.org/10.1136/jnnp-2013-306573.18.
- Iwasaki Y, Mimuro M, Yoshida M, Hashizume Y, Kitamoto T, Sobue G. Clinicopathologic characteristics of five autopsied cases of dura mater-associated Creutzfeldt–Jakob disease. Neuropathology 2008;28:51-6. https://doi.org/10.1111/j.1440-1789.2007.00847.x.
- Sakai K, Hamaguchi T, Noguchi-Shinohara M, Nozaki I, Takumi I, Sanjo N, et al. Graft-related disease progression in dura mater graft-associated Creutzfeldt–Jakob disease: a cross-sectional study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003400.
- Béringue V, Le Dur A, Tixador P, Reine F, Lepourry L, Perret-Liaudet A, et al. Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0001419.
- Béringue V, Vilotte JL, Laude H. Prion agent diversity and species barrier. Vet Res 2008;39. https://doi.org/10.1051/vetres:2008024.
- Cali I, Miller CJ, Parisi JE, Geschwind MD, Gambetti P, Schonberger LB. Distinct pathological phenotypes of Creutzfeldt–Jakob disease in recipients of prion-contaminated growth hormone. Acta Neuropathol Commun 2015;3. https://doi.org/10.1186/s40478-015-0214-2.
- Peden AH, Kirkpatrick JRM, Head MW, Ironside JW. Comparison of the in vitro seeding activity of UK iatrogenic and sporadic Creutzfeldt–Jakob disease subtypes by real time quaking induced conversion. Prion 2016;10:S50-S1.
- Bougard D, Brandel JP, Bélondrade M, Béringue V, Segarra C, Fleury H, et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt–Jakob disease. Sci Transl Med 2016;8. https://doi.org/10.1126/scitranslmed.aag1257.
- Mead S, Wadsworth JD, Porter MC, Linehan JM, Pietkiewicz W, Jackson GS, et al. Variant Creutzfeldt–Jakob disease with extremely low lymphoreticular deposition of prion protein. JAMA Neurol 2014;71:340-3. https://doi.org/10.1001/jamaneurol.2013.5378.
- Peden AH, McGuire LI, Appleford NE, Mallinson G, Wilham JM, Orrú CD, et al. Sensitive and specific detection of sporadic Creutzfeldt–Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol 2012;93:438-49. https://doi.org/10.1099/vir.0.033365-0.
- Douet JY, Lacroux C, Aron N, Head MW, Lugan S, Tillier C, et al. Distribution and quantitative estimates of variant Creutzfeldt–Jakob disease prions in tissues of clinical and asymptomatic patients. Emerging Infect Dis 2017;23:946-56. https://doi.org/10.3201/eid2306.161734.
- Bishop MT, Diack A, Cancellotti E, Will R, Manson J. Variant CJD strain remains stable after secondary transmission. Prion 2010;4.
- Wadsworth JD, Dalmau-Mena I, Joiner S, Linehan JM, O’Malley C, Powell C, et al. Effect of fixation on brain and lymphoreticular vCJD prions and bioassay of key positive specimens from a retrospective vCJD prevalence study. J Pathol 2011;223:511-18. https://doi.org/10.1002/path.2821.
- Ritchie DL, Gibson SV, Abee CR, Kreil TR, Ironside JW, Brown P. Blood transmission studies of prion infectivity in the squirrel monkey (Saimiri sciureus): the Baxter study. Transfusion 2016;56:712-21. https://doi.org/10.1111/trf.13422.
- Moore RA, Head MW, Ironside JW, Ritchie DL, Zanusso G, Choi YP, et al. The distribution of prion protein allotypes differs between sporadic and iatrogenic Creutzfeldt–Jakob disease patients. PLOS Pathog 2016;12. https://doi.org/10.1371/journal.ppat.1005416.
- House of Commons, The Science and Technology Committee . After the Storm? UK Blood Safety and the Risk of Variant Creutzfeldt–Jakob Disease. Second Report of Session 2014. https://publications.parliament.uk/pa/cm201415/cmselect/cmsctech/327/32706.htm#a7 (accessed 15 March 2018).
- Lehmann S, Pastore M, Rogez-Kreuz C, Richard M, Belondrade M, Rauwel G, et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive medical equipment. J Hosp Infect 2009;72:342-50. https://doi.org/10.1016/j.jhin.2009.03.024.
- Lemmer K, Mielke M, Kratzel C, Joncic M, Oezel M, Pauli G, et al. Decontamination of surgical instruments from prions. II. In vivo findings with a model system for testing the removal of scrapie infectivity from steel surfaces. J Gen Virol 2008;89:348-58. https://doi.org/10.1099/vir.0.83396-0.
- Rogez-Kreuz C, Yousfi R, Soufflet C, Quadrio I, Yan ZX, Huyot V, et al. Inactivation of animal and human prions by hydrogen peroxide gas plasma sterilization. Infect Control Hosp Epidemiol 2009;30:769-77. https://doi.org/10.1086/598342.
- Belondrade M, Nicot S, Béringue V, Coste J, Lehmann S, Bougard D. Rapid and highly sensitive detection of variant Creutzfeldt–Jakob disease abnormal prion protein on steel surfaces by protein misfolding cyclic amplification: application to prion decontamination studies. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0146833.
- Lawson VA, Stewart JD, Masters CL. Enzymatic detergent treatment protocol that reduces protease-resistant prion protein load and infectivity from surgical-steel monofilaments contaminated with a human-derived prion strain. J Gen Virol 2007;88:2905-14. https://doi.org/10.1099/vir.0.82961-0.
- Beekes M, Lemmer K, Thomzig A, Joncic M, Tintelnot K, Mielke M. Fast, broad-range disinfection of bacteria, fungi, viruses and prions. J Gen Virol 2010;91:580-9. https://doi.org/10.1099/vir.0.016337-0.
- Bellon A, Comoy E, Simoneau S, Mornac S, Dehen C, Perrin A, et al. Decontamination of prions in a plasma product manufacturing environment. Transfusion 2014;54:1028-36. https://doi.org/10.1111/trf.12381.
- Fichet G, Antloga K, Comoy E, Deslys JP, McDonnell G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J Hosp Infect 2007;67:278-86. https://doi.org/10.1016/j.jhin.2007.08.020.
- Hervé R, Kong M, Comoy E, Deslys JP, Keevil B. Cold atmospheric plasma for the decontamination of reusable surgical instruments. Prion 2010;4.
- Hervé R, Keevil CW. Current limitations about the cleaning of luminal endoscopes. J Hosp Infect 2013;83:22-9. https://doi.org/10.1016/j.jhin.2012.08.008.
- Howlin RP, Khammo N, Secker T, McDonnell G, Keevil CW. Application of a fluorescent dual stain to assess decontamination of tissue protein and prion amyloid from surgical stainless steel during simulated washer-disinfector cycles. J Hosp Infect 2010;75:66-71. https://doi.org/10.1016/j.jhin.2009.12.023.
- Edgeworth JA, Sicilia A, Linehan J, Brandner S, Jackson GS, Collinge J. A standardized comparison of commercially available prion decontamination reagents using the standard steel-binding assay. J Gen Virol 2011;92:718-26. https://doi.org/10.1099/vir.0.027201-0.
- Jackson GS, McKintosh E, Flechsig E, Prodromidou K, Hirsch P, Linehan J, et al. An enzyme-detergent method for effective prion decontamination of surgical steel. J Gen Virol 2005;86:869-78. https://doi.org/10.1099/vir.0.80484-0.
- Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, et al. Inactivation of prions by acidic sodium dodecyl sulfate. J Virol 2006;80:322-31. https://doi.org/10.1128/JVI.80.1.322-331.2006.
- Giles K, Supattapone S, Peretz D, Glidden DV, Baron H, Prusiner SB. Disinfection of prions. New Biocides Dev 2007;967:52-74. https://doi.org/10.1021/bk-2007-0967.ch003.
- Baxter HC, Campbell GA, Whittaker AG, Jones AC, Aitken A, Simpson AH, et al. Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment. J Gen Virol 2005;86:2393-9. https://doi.org/10.1099/vir.0.81016-0.
- Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ, et al. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLOS Pathog 2008;4. https://doi.org/10.1371/journal.ppat.1000206.
- Hervé RC, Keevil CW. Persistent residual contamination in endoscope channels; a fluorescence epimicroscopy study. Endoscopy 2016;48:609-16. https://doi.org/10.1055/s-0042-105744.
- Baxter RL, Baxter HC, Campbell GA, Grant K, Jones A, Richardson P, et al. Quantitative analysis of residual protein contamination on reprocessed surgical instruments. J Hosp Infect 2006;63:439-44. https://doi.org/10.1016/j.jhin.2006.03.011.
- Murdoch H, Taylor D, Dickinson J, Walker JT, Perrett D, Raven ND, et al. Surface decontamination of surgical instruments: an ongoing dilemma. J Hosp Infect 2006;63:432-8. https://doi.org/10.1016/j.jhin.2006.02.015.
- Lipscomb IP, Sihota AK, Botham M, Harris KL, Keevil CW. Rapid method for the sensitive detection of protein contamination on surgical instruments. J Hosp Infect 2006;62:141-8. https://doi.org/10.1016/j.jhin.2005.07.008.
- Lipscomb IP, Sihota AK, Keevil CW. Comparative study of surgical instruments from sterile-service departments for presence of residual gram-negative endotoxin and proteinaceous deposits. J Clin Microbiol 2006;44:3728-33. https://doi.org/10.1128/JCM.01280-06.
- Smith A, Winter S, Lappin D, Sherriff A, McIvor I, Philp P, et al. Reducing the risk of iatrogenic CJD by improving the cleaning of neurosurgical instruments. J Hosp Infect 2018;100:e70-e76. https://doi.org/10.1016/j.jhin.2018.03.001.
- Baxter HC, Campbell GA, Richardson PR, Jones AC, Whittle IR, Casey M, et al. Surgical instrument decontamination: efficacy of introducing an argon:oxygen RF gas-plasma cleaning step as part of the cleaning cycle for stainless steel instruments. IEEE Trans Plasma Sci, IEEE Nucl Plasma Sci Soc 2006;34:1337-44. https://doi.org/10.1109/TPS.2006.878387.
- Baxter HC, Jones AC, Baxter RL. Application of epifluorescence scanning for monitoring the efficacy of protein removal by RF gas-plasma decontamination. Prion 2009;4:204-5.
- Lipscomb IP, Pinchin H, Collin R, Keevil CW. Effect of drying time, ambient temperature and pre-soaks on prion-infected tissue contamination levels on surgical stainless steel: concerns over prolonged transportation of instruments from theatre to central sterile service departments. J Hosp Infect 2007;65:72-7. https://doi.org/10.1016/j.jhin.2006.09.025.
- Secker TJ, Pinchin HE, Hervé RC, Keevil CW. Efficacy of humidity retention bags for the reduced adsorption and improved cleaning of tissue proteins including prion-associated amyloid to surgical stainless steel surfaces. Biofouling 2015;31:535-41. https://doi.org/10.1080/08927014.2015.1067686.
- Secker TJ, Hervé R, Keevil CW. Adsorption of prion and tissue proteins to surgical stainless steel surfaces and the efficacy of decontamination following dry and wet storage conditions. J Hosp Infect 2011;78:251-5. https://doi.org/10.1016/j.jhin.2011.03.021.
- Secker TJ, Hervea R, Keevil CW. Wet versus dry: do environmental conditions have an effect on prion decontamination?. Prion 2010;4:213-4.
- Department of Health and Social Care (DHSC) . CFPP-01-01: Decontamination of Surgical Instruments (HTM 01-01) Health Technical Memorandum (HTM) 01-01: Management and Decontamination of Surgical Instruments (Medical Devices) Used in Acute Care. Part B: Common Elements 2016. www.gov.uk/government/publications/management-and-decontamination-of-surgical-instruments-used-in-acute-care (accessed 8 January 2020).
- Bonda DJ, Manjila S, Mehndiratta P, Khan F, Miller BR, Onwuzulike K, et al. Human prion diseases: surgical lessons learned from iatrogenic prion transmission. Neurosurg Focus 2016;41. https://doi.org/10.3171/2016.5.FOCUS15126.
- Rutala WA, Weber DJ. Society for Healthcare Epidemiology of America . Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol 2010;31:107-17. https://doi.org/10.1086/650197.
- Dickinson J, Murdoch H, Dennis MJ, Hall GA, Bott R, Crabb WD, et al. Decontamination of prion protein (BSE301V) using a genetically engineered protease. J Hosp Infect 2009;72:65-70. https://doi.org/10.1016/j.jhin.2008.12.007.
- Fichet G, Comoy E, Duval C, Antloga K, Dehen C, Charbonnier A, et al. Novel methods for disinfection of prion-contaminated medical devices. Lancet 2004;364:521-6. https://doi.org/10.1016/S0140-6736(04)16810-4.
- Rochefort F. The role of detergents in prevention of transmission of Creutzfeldt–Jakob disease. Zentralsterilisation 2010;18:395-400.
- Hervé R, Secker TJ, Keevil CW. Current risk of iatrogenic Creutzfeld-Jakob disease in the UK: efficacy of available cleaning chemistries and reusability of neurosurgical instruments. J Hosp Infect 2010;75:309-13. https://doi.org/10.1016/j.jhin.2010.01.024.
- National Institute for Health and Care Excellence . Adoption and Impact Programme. IPG196 Adoption Scoping Report 2016.
- Department of Health and Social Care . Minimise Transmission Risk of CJD and VCJD in Healthcare Settings 2012. www.gov.uk/government/publications/guidance-from-the-acdp-tse-risk-management-subgroup-formerly-tse-working-group (accessed 23 January 2018).
- Department of Health and Social Care, Economics and Operational Research Division (EOR4) . Risk Assessment for Transmission of VCJD via Surgical Instruments: A Modelling Approach and Numerical Scenarios 2001.
- Bird SM, Merrall EL, Ward HJ, Will RG. Survival and re-operation rates after neurosurgical procedures in Scotland: implications for targeted surveillance of sub-clinical variant Creutzfeldt–Jakob disease. Neuroepidemiology 2009;33:1-11. https://doi.org/10.1159/000209281.
- National Institute for Health and Care Excellence (NICE) . Patient Safety and Reduction of Risk of Transmission of Creutzfeldt–Jakob (CJD) via Interventional Procedures [IPG196] 2006.
- Garske T, Ward HJ, Clarke P, Will RG, Ghani AC. Factors determining the potential for onward transmission of variant Creutzfeldt–Jakob disease via surgical instruments. J R Soc Interface 2006;3:757-66. https://doi.org/10.1098/rsif.2006.0142.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/article/pmg9/ (accessed 19 March 2018).
- Office for National Statistics . National Life Tables: United Kingdom 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 13 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Barnett F, McLean G. Care management of Creutzfeldt–Jakob Disease within the United Kingdom. J Nurs Manag 2005;13:111-18. https://doi.org/10.1111/j.1365-2934.2005.00449.x.
- National Institute for Health and Care Excellence (NICE) . Interim Process and Methods of the Highly Specialised Technologies Programme Updated to Reflect 2017 Changes 2017.
Appendix 1 Clinical effectiveness search strategies
MEDLINE, MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations
Date range searched: 1946 to 2017 (via Ovid).
Date searched: 14 August 2017.
Search strategy
-
exp Creutzfeldt–Jakob Syndrome/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp Prion Diseases/
-
exp Prions/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
exp Incidence/
-
exp Prevalence/
-
incidence.tw.
-
prevalence.tw.
-
or/10-13
-
incubat*.tw.
-
9 and (14 or 15)
-
limit 16 to yr=“2005 -Current”
-
exp Creutzfeldt–Jakob Syndrome/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp Prion Diseases/
-
exp Prions/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/18-25
-
((transmission or transmit* or iatrogenic or transfer*) adj5 (creutzfeldt or cjd or vcjd or v-cjd or encephalopath* or prion* or tse or prp)).tw.
-
exp Surgical Instruments/
-
exp Decontamination/
-
exp Sterilization/
-
28 and (29 or 30)
-
((surgery or surgical* or instrument* or device* or equipment*) adj5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)).tw.
-
31 or 32
-
26 and (27 or 33)
-
limit 34 to yr=“2005 -Current”
-
exp Surgical Instruments/
-
exp Decontamination/
-
exp Sterilization/
-
36 and (37 or 38)
-
((surgery or surgical* or instrument* or device* or equipment*) adj5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)).tw.
-
39 or 40
-
Neurosurgery/
-
Neurosurgical Procedures/
-
(neurosurgery or neurological surgery).tw.
-
exp Brain/su [Surgery]
-
exp Meninges/su [Surgery]
-
exp Pituitary Gland/su [Surgery]
-
Pineal Gland/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp Cranial Nerves/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
Ophthalmologic Surgical Procedures/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
Eye/su [Surgery]
-
Vitreous Body/su [Surgery]
-
exp Retina/su [Surgery]
-
or/42-56
-
41 and 57
-
limit 58 to yr=“2005 -Current”
-
(disposable or dispose* or nondispos* or non-dispos* or reus* or re-us* or “single use” or “single-use”).mp.
-
Disposable Equipment/
-
exp Equipment Reuse/
-
(ultrasonic aspirator or aneurysm clip applicator or rhoton dissectors or microsurgical scissors or upcut rongeurs or budde halo or retraction system or self-retaining retractors or neuroendoscope*).mp.
-
or/60-63
-
Neurosurgery/
-
Neurosurgical Procedures/
-
(neurosurgery or neurological surgery).tw.
-
exp Brain/su [Surgery]
-
exp Meninges/su [Surgery]
-
exp Pituitary Gland/su [Surgery]
-
Pineal Gland/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp Cranial Nerves/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
Ophthalmologic Surgical Procedures/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
Eye/su [Surgery]
-
Vitreous Body/su [Surgery]
-
exp Retina/su [Surgery]
-
or/65-79
-
complication*.mp.
-
co.fs.
-
exp Postoperative Complications/
-
exp Intraoperative Complications/
-
or/81-84
-
64 and 80 and 85
-
limit 86 to yr=“2005 -Current”
-
*Reoperation/
-
reoperat*.tw.
-
((repeat or revision) adj3 (surgery or surgical* or operat*)).tw.
-
or/88-90
-
Neurosurgery/
-
Neurosurgical Procedures/
-
(neurosurgery or neurological surgery).tw.
-
exp Brain/su [Surgery]
-
exp Meninges/su [Surgery]
-
exp Pituitary Gland/su [Surgery]
-
Pineal Gland/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp Cranial Nerves/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
Ophthalmologic Surgical Procedures/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
Eye/su [Surgery]
-
Vitreous Body/su [Surgery]
-
exp Retina/su [Surgery]
-
or/92-106
-
91 and 107
-
17 or 35 or 59 or 87 or 108
EMBASE
Date range searched: 1974 to 11 August 2017.
Date searched: 14 August 2017.
Search strategy
-
exp Creutzfeldt Jakob disease/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp prion disease/
-
exp prion/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
exp incidence/
-
exp prevalence/
-
incidence.tw.
-
prevalence.tw.
-
or/10-13
-
incubat*.tw.
-
9 and (14 or 15)
-
limit 16 to yr=“2005 -Current”
-
((transmission or transmit* or iatrogenic or transfer*) adj5 (creutzfeldt or cjd or vcjd or v-cjd or encephalopath* or prion* or tse or prp)).tw.
-
exp surgical equipment/
-
instrument sterilization/
-
19 and 20
-
((surgery or surgical* or instrument* or device* or equipment*) adj5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)).tw.
-
21 or 22
-
9 and (18 or 23)
-
limit 24 to yr=“2005 -Current”
-
exp surgical equipment/
-
instrument sterilization/
-
26 and 27
-
((surgery or surgical* or instrument* or device* or equipment*) adj5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)).tw.
-
28 or 29
-
neurosurgery/
-
(neurosurgery or neurological surgery).tw.
-
exp brain/su [Surgery]
-
exp meninx/su [Surgery]
-
exp hypophysis/su [Surgery]
-
pineal body/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp cranial nerve/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
eye surgery/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
eye/su [Surgery]
-
vitreous body/su [Surgery]
-
exp retina/su [Surgery]
-
or/31-44
-
30 and 45
-
limit 46 to yr=“2005 -Current”
-
(disposable or dispose* or nondispos* or non-dispos* or reus* or re-us* or “single use” or “single-use”).mp.
-
disposable equipment/
-
exp recycling/
-
(ultrasonic aspirator or aneurysm clip applicator or rhoton dissectors or microsurgical scissors or upcut rongeurs or budde halo or retraction system or self-retaining retractors or neuroendoscope*).mp.
-
or/48-51
-
neurosurgery/
-
(neurosurgery or neurological surgery).tw.
-
exp brain/su [Surgery]
-
exp meninx/su [Surgery]
-
exp hypophysis/su [Surgery]
-
pineal body/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp cranial nerve/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
eye surgery/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
eye/su [Surgery]
-
vitreous body/su [Surgery]
-
exp retina/su [Surgery]
-
or/53-66
-
complication*.mp.
-
co.fs.
-
exp postoperative complication/
-
exp peroperative complication/
-
or/68-71
-
52 and 67 and 72
-
limit 73 to yr=“2005 -Current”
-
*reoperation/
-
reoperat*.tw.
-
((repeat or revision) adj3 (surgery or surgical* or operat*)).tw.
-
or/75-77
-
neurosurgery/
-
(neurosurgery or neurological surgery).tw.
-
exp brain/su [Surgery]
-
exp meninx/su [Surgery]
-
exp hypophysis/su [Surgery]
-
pineal body/su [Surgery]
-
((brain or meninges or cerebral or pituitary or pineal) adj5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)).tw.
-
exp cranial nerve/su [Surgery]
-
((cranial or dura) adj5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)).tw.
-
eye surgery/
-
((eye or vitreous or retina) adj5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)).tw.
-
eye/su [Surgery]
-
vitreous body/su [Surgery]
-
exp retina/su [Surgery]
-
or/79-92
-
78 and 93
-
17 or 25 or 47 or 74 or 94
-
96 remove duplicates from 95
Science Citation Index (SCI-E) and Conference Proceedings Citation Index (CPCI)
Date range searched: 1990 to 2017 (via Web of Science).
Date searched: 14 August 2017.
Search strategy
-
TS=(creutzfeldt jakob NEAR/1 disease) OR TS=(creutzfeldt jakob NEAR/1 syndrome) OR TS=(creutzfeldt-jakob NEAR/1 disease) OR TS=(creutzfeldt-jakob NEAR/1 syndrome)
-
TS=((cjd or vcjd or v-cjd))
-
TS=(transmissible NEAR/1 encephalopath*) OR TS=(spong* NEAR/1 encephalopath*)
-
TS=(prion* or tse or prp)
-
#4 OR #3 OR #2 OR #1
-
TS= (incidence)
-
TS= (prevalence)
-
TS= (incubat*)
-
#8 OR #7 OR #6
-
#9 AND #5 Timespan=2005-2017
-
TS=(creutzfeldt jakob NEAR/1 disease) OR TS=(creutzfeldt jakob NEAR/1 syndrome) OR TS=(creutzfeldt-jakob NEAR/1 disease) OR TS=(creutzfeldt-jakob NEAR/1 syndrome)
-
TS=((cjd or vcjd or v-cjd))
-
TS=(transmissible NEAR/1 encephalopath*) OR TS=(spong* NEAR/1 encephalopath*)
-
TS=(prion* or tse or prp)
-
#14 OR #13 OR #12 OR #11
-
TS=(((transmission or transmit* or iatrogenic or transfer*) NEAR/5 (creutzfeldt or cjd or vcjd or v-cjd or encephalopath* or prion* or tse or prp)))
-
TS=(((surgery or surgical* or instrument* or device* or equipment*) NEAR/5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)))
-
#17 OR #16
-
#18 AND #15 Timespan=2005-2017
-
TS=(((surgery or surgical* or instrument* or device* or equipment*) NEAR/5 (decontaminat* or reprocess* or disinfect* or wash* or clean* or steril* or contaminat* or prerinse or pre-rinse or inactivat*)))
-
TS=((neurosurgery or neurological surgery))
-
TS=(((brain or meninges or cerebral or pituitary or pineal) NEAR/5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)))
-
TS=(((cranial or dura) NEAR/5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)))
-
TS=(((eye or vitreous or retina) NEAR/5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)))
-
#24 OR #23 OR #22 OR #21
-
#25 AND #20 Timespan=2005-2017
-
TS=((disposable or dispose* or nondispos* or non-dispos* or reus* or re-us* or “single use” or “single-use”))
-
TS=((ultrasonic aspirator or aneurysm clip applicator or rhoton dissectors or microsurgical scissors or upcut rongeurs or budde halo or retraction system or self-retaining retractors or neuroendoscope*))
-
#28 OR #27
-
TS=((neurosurgery or neurological surgery))
-
TS=(((brain or meninges or cerebral or pituitary or pineal) NEAR/5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)))
-
TS=(((cranial or dura) NEAR/5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)))
-
TS=(((eye or vitreous or retina) NEAR/5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)))
-
#33 OR #32 OR #31 OR #30
-
#34 AND #29
-
TS=(complication*)
-
#36 AND #35 Timespan=2005-2017
-
TS=(reoperat*)
-
TS=(((repeat or revision) NEAR/3 (surgery or surgical* or operat*)))
-
#39 OR #38
-
TS=((neurosurgery or neurological surgery))
-
TS=(((brain or meninges or cerebral or pituitary or pineal) NEAR/5 (surgery or surgical* or excision or lesion or ablation or operation* or neurostimulation or connection or destruction)))
-
TS=(((cranial or dura) NEAR/5 (graft* or transection or destruction or lesion or repair* or decompress* or neurostimulation or exploration or operation*)))
-
TS=(((eye or vitreous or retina) NEAR/5 (surgery or surgical* or excision or operation* or photocoagulation or destruction)))
-
#44 OR #43 OR #42 OR #41
-
#45 AND #40
-
#46 OR #37 OR #26 OR #19 OR #10
Supplementary searches
Supplementary searches were carried out in October 2017.
MEDLINE, MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations
Date range searched: 1946 to 2017 (via Ovid).
Date searched: 2 October 2017.
Search strategy
-
exp Creutzfeldt–Jakob Syndrome/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp Prion Diseases/
-
exp Prions/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
(surgery or surgical* or operat*).tw.
-
risk*.mp.
-
9 and 10 and 11
-
limit 12 to yr=“2005 –Current”
EMBASE
Date range searched: 1974 to 2017 October.
Date searched: 2 October 2017.
Search strategy
-
exp Creutzfeldt Jakob disease/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp prion disease/
-
exp prion/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
(surgery or surgical* or operat*).tw.
-
risk*.mp.
-
9 and 10 and 11
-
limit 12 to yr=“2005 -Current”
Science Citation Index (SCI-E) and Conference Proceedings Citation Index (CPCI)
Date range searched: 1990 to 2017 (via Web of Science).
Date searched: 2 October 2017.
Search strategy
-
# TS=(creutzfeldt jakob NEAR/1 disease) OR TS=(creutzfeldt jakob NEAR/1 syndrome) OR TS=(creutzfeldt-jakob NEAR/1 disease) OR TS=(creutzfeldt-jakob NEAR/1 syndrome)
-
# TS=((cjd or vcjd or v-cjd))
-
# TS=(transmissible NEAR/1 encephalopath*) OR TS=(spong* NEAR/1 encephalopath*)
-
# TS=(prion* or tse or prp)
-
# #4 OR #3 OR #2 OR #1
-
# TOPIC: ((surgery or surgical* or operat*))
-
# TOPIC: ((risk*))
-
# #7 AND #6 AND #5
-
# #7 AND #6 AND #5 Refined by: PUBLICATION YEARS: (2006 OR 2012 OR 2016 OR 2015 OR 2007 OR 2013 OR 2005 OR 2009 OR 2010 OR 2017 OR 2014 OR 2011 OR 2008)
Appendix 2 Cost-effectiveness search strategies
MEDLINE, MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations
Date range searched: 1946 to 2017 (via Ovid).
Date searched: 7 June 2017.
Search strategy
-
exp Creutzfeldt–Jakob Syndrome/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp Prion Diseases/
-
exp PRIONS/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/10-30
-
9 and 31
-
limit 32 to yr=“2004 –Current”
EMBASE 1974 to 2017 June 6
Date range searched: 1974 to 6 June 2017.
Date searched: 7 June 2017.
Search strategy
-
exp Creutzfeldt Jakob disease/
-
((creutzfeldt jakob or creutzfeldt-jakob) adj (disease or syndrome)).tw.
-
(cjd or vcjd or v-cjd).tw.
-
exp prion disease/
-
exp prion/
-
((transmissible or spong*) adj encephalopath*).tw.
-
(prion* or tse).tw.
-
prp.tw.
-
or/1-8
-
Socioeconomics/
-
Cost benefit analysis/
-
Cost effectiveness analysis/
-
Cost of illness/
-
Cost control/
-
Economic aspect/
-
Financial management/
-
Health care cost/
-
Health care financing/
-
Health economics/
-
Hospital cost/
-
(fiscal or financial or finance or funding).tw.
-
Cost minimization analysis/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
or/10-25
-
9 and 26
-
limit 27 to yr=“2004 -Current”
Science Citation Index (SCI-E) and Conference Proceedings Citation Index (CPCI)
Date range searched: 1990 to 2017 (via Web of Science).
Date searched: 11 July 2017.
Search strategy
-
# TS=(creutzfeldt jakob NEAR/1 disease) OR TS=(creutzfeldt jakob NEAR/1 syndrome) OR TS=(creutzfeldt-jakob NEAR/1 disease) OR TS=(creutzfeldt-jakob NEAR/1 syndrome)
-
# TS=((cjd or vcjd or v-cjd))
-
# TS=(transmissible NEAR/1 encephalopath*) OR TS=(spong* NEAR/1 encephalopath*)
-
# TS=(prion* or tse or prp)
-
# #4 OR #3 OR #2 OR #1
-
# TS=((cost* and (effective* or utilit* or benefit* or minimi*))) OR TI=((cost*)) OR TS=((economic* or pharmacoeconomic* or pharmaco-economic*)) OR TS=((price* or pricing*)) OR TS=((financial or finance or finances or financed)) OR TS=((economic* and (hospital or medical or nursing or pharmaceutical))) OR TS=((“quality adjusted life year” or “quality adjusted life years”)) OR TS=((qaly or qalys)) OR TS=((budget*))
-
# #6 AND #5 Refined by: PUBLICATION YEARS: ( 2015 OR 2017 OR 2011 OR 2014 OR 2016 OR 2008 OR 2013 OR 2007 OR 2009 OR 2005 OR 2012 OR 2004 OR 2010 OR 2006 )
Appendix 3 Excluded studies from the clinical reviews with reasons for exclusion
| Reference | Primary reason for exclusion |
|---|---|
| Adam AM, Akuku O. Creutzfeldt–Jakob disease in Kenya. Trop Med Int Health 2005;10:710–12 | Data from pre 2005 |
| Allen CT, Sonnen J, Leslie MJ, Kidoguchi L, Harris C, Gambetti P, Montine TJ. Washington statewide pathology surveillance for prion disease. Ann Neurol 2007;61:371–72 | Superceded data |
| Amour J. Comparison of single-use and reusable metal laryngoscope blades for orotracheal intubation during rapid sequence induction of anaesthesia: a multicenter cluster randomised study. Anaesthesiology 2010;112:325–32 | Not high-risk surgery |
| Brandel JP, Salomon D, Capek I, Vaillant V, Alperovitch A. Epidemiological surveillance of Creutzfeldt–Jakob in France. Rev Neurol 2009;165:684–93 | Review with no original data |
| Chandra SR, Issac TG, Philip M, Gadad V. Creutzfeldt–Jakob disease phenotype and course: our experience from a tertiary centre. Indian J Psychol Med 2016;38:438–42 | No usable data for any review question |
| Checchi M, Hewitt PE, Bennett P, Ward HJ, Will RG, Mackenzie JM, Sinka K. Ten-year follow-up of two cohorts with an increased risk of variant CJD: donors to individuals who later developed variant CJD and other recipients of these at-risk donors. Vox Sang 2016;111:325–32 | No usable data for any review question |
| Chen CC, Wang YH, Wu KY. Consumption of bovine spongiform encephalopathy (BSE) contaminated beef and the risk of variant Creutzfeldt–Jakob disease. Risk Anal 2013;33:1958–68 | No usable data for any review question |
| de Pedro-Cuesta J, Bleda MJ, Rabano A, Cruz M, Laursen H, Molbak K, Siden A. Classification of surgical procedures for epidemiologic assessment of sporadic Creutzfeldt–Jakob disease transmission by surgery. Eur J Epidemiol 2006;21:595–604 | Wrong outcome |
| Frontzek K, Moos R, Schaper E, Jann L, Herfs G, Zimmermann DR, et al. Iatrogenic and sporadic Creutzfeldt–Jakob disease in 2 sisters without mutation in the prion protein gene. Prion 2015;9:444–8 | No usable data for any review question |
| Graziano S and Pocchiari M. Management and prevention of human prion diseases. Curr Neurol Neurosci Rep 2009;9:423–9 | Review with no original data |
| Gregori L, Yang H, Anderson S. Estimation of variant Creutzfeldt–Jakob disease infectivity titres in human blood. Prion 2012;6:139 | No usable data for any review question |
| Gubbels S, Bacci S, Laursen H, Hogenhaven H, Cowan S, Molbak K, Christiansen M. Description and analysis of 12 years of surveillance for Creutzfeldt–Jakob disease in Denmark, 1997 to 2008. Euro Surveill 2012;17:12 | Superceded data |
| Hamaguchi T. Clinical manifestations and epidemiology of prion diseases in Japan. Rinsho Shinkeigaku 2013;23:1246–8 | Superceded data |
| Ironside JW, Head MW, Peden A, Ward H. Asymptomatic vCJD infection detected at autopsy in a UK haemophilic patient. Haemophilia 2010;16:29 | Superceded data |
| Karhade AV, Vasudeva VS, Dasenbrock HH, Lu Y, Gormley WB, Groff MW, et al. Thirty-day readmission and reoperation after surgery for spinal tumours: a National Surgical Quality Improvement Program analysis. Neurosurg Focus 2016;41(2) | Wrong outcome |
| Klug GM, Boyd A, Lewis V, McGlade A, Stehmann C, Masters CL, Collins SJ. Surveillance of Creutzfeldt–Jakob disease in Australia: 2009 update. Commun Dis Intell Q Rep 2019;33:188–91 | Superceded data |
| Kobayashi A, Matsuura Y, Iwaki T, Iwasaki Y, Yoshida M, Takahashi H, et al. Sporadic Creutzfeldt–Jakob disease MM1 + 2C and MM1 are identical in transmission properties. Brain Pathol 2016;26:95–101 | Review with no original data |
| Kobayashi A, Teruya K, Matsuura Y, Shirai T, Nakamura Y, Yamada M, et al. The influence of PRNP polymorphisms on human prion disease susceptibility: an update. Acta Neuropathol 2015;130:159–70 | Review with no original data |
| Kovacs GG, Majtenyi K. Creutzfeldt–Jakob disease in Hungary. Folia Neuropathol 2005;43:279–85 | Superceded data |
| Maddox RA, Person MK, Minino AM, Blevins JE, Schonberger LB, Belay ED. Unusually young prion disease cases in the United States, 1979–2014. Prion 2016;10:S98–S99 | No usable data for any review question |
| Maheshwari A, Fischer M, Gambetti P, Parker A, Ram A, Soto C, Hussein HM. Recent us case of variant Creutzfeldt–Jakob disease-global implications. Emerg Infect Dis 2015;21:750–9 | Superceded data |
| Mei LL, Sin HF, Suk ML, Wai CL. Effectiveness of 2D barcode tracking in recording instrument sterilisation & avoiding spread of infection in operating theatre. J Microbiol Immunol Infection 2015;48:S68 | Wrong outcome |
| Mikol J, Deslys JP, Zou WQ, Xiao W, Brown P, Budka H, Goutieres F. Creutzfeldt–Jakob disease with unusually extensive neuropathology in a child treated with native human growth hormone. Clin Neuropathol 2012;31:127–34 | Superceded data |
| Papacostas S, Malikides A, Petsa M, Kyriakides T. Ten-year mortality from Creutzfeldt–Jakob disease in Cyprus. East Mediterr Health J 2008;14:715–19 | Data from pre-2005 |
| Parchi P. Molecular-phenotypic correlation in sporadic and genetic Creutzfeldt–Jakob disease: Insights from recent studies. Clin Neuropathol 2009;28:235–36 | Review with no original data |
| Ritchie DL, Lowrie S, Le Grice M, Burns K, Ironside JW. Amyloid-beta accumulation in human growth hormone related iatrogenic CJD patients in the UK. Neuropathol Appl Neurobiol 2017;43:39 | Not CJD related |
| Rohan Z, Rusina R, Maresova M, Matej R. Human prion diseases in the Czech Republic. Epidemiol Mikrob Im 2015;64:115–20 | No usable data for any review question |
| Saba R, Booth SA. The genetics of susceptibility to variant Creutzfeldt–Jakob disease. Public Health Genomics 2013;16:17–24 | No usable data for any review question |
| Sawyer EB, Edgeworth JA, Thomas C, Collinge J, Jackson GS. Preclinical detection of infectivity and disease-specific PrP in blood throughout the incubation period of prion disease. Sci Rep 2015;5:17742 | No usable data for any review question |
| Takeuchi A, Kobayashi A, Ironside JW, Mohri S, Kitamoto T. Characterization of variant Creutzfeldt–Jakob disease prions in prion protein-humanised mice carrying distinct codon 129 genotypes. J Biol Chem 2013;288:21659–66 | No usable data for any review question |
Appendix 4 Elicitation exercise relating to epidemiological parameters (conducted 18 January 2018)
List of participants
Participating experts: in alphabetical order of surname
-
Dr David Hilton: Consultant Neuropathologist, University Hospitals Plymouth NHS Trust.
-
Professor Simon Mead: Professor of Neurology, University College London.
-
Professor Graham Medley: Professor of Infectious Disease Modelling, London School of Hygiene & Tropical Medicine.
-
Dr Katy Sinka: Creutzfeldt-Jacob disease Section Head, Public Health England.
Note that this order does not correspond to experts A, B, C and D: we have chosen to anonymise individual responses and comments in this record.
Facilitator
-
Professor Jeremy E Oakley: Professor of Statistics, University of Sheffield.
Parameters related to misdiagnoses of the cause of death in patients who die as a result of Creutzfeldt–Jakob disease
The quantity of interest is the percentage of patients whose death was due to CJD who were misdiagnosed as having died from another neurodegenerative disease, since 2005.
A separate percentage is considered for each of three age categories:
-
aged < 60 years
-
aged 60–79 years
-
aged ≥ 80 years.
It was decided to elicit distributions for age categories (1) and (3), and assume that the percentage for age category (2) would be the mean of these two.
Parameter 1 definition: the percentage of patients, aged less than 60 years, whose death was because of Creutzfeldt–Jakob disease, that are misdiagnosed as having died from another neurodegenerative disease, since 2005
Individual judgements
Without conferring, the experts made the probability judgements for parameter 1 as shown in Table 31.
| Expert | Plausible lower limit (%) | 25th percentile (%) | Median (%) | 75th percentile (%) | Plausible upper limit (%) |
|---|---|---|---|---|---|
| A | 0 | 0.5 | 1 | 3 | 10 |
| B | 0 | 2.5 | 5 | 7.5 | 15 |
| C | 0 | 10 | 20 | 30 | 50 |
| D | 0 | 1 | 5 | 10 | 20 |
Group discussion and consensus judgements
Expert C argued that correct diagnosis would be dependent on whether or not the patient was referred to Neurology; a higher misdiagnosis rate could occur if the referral rate were lower. Where patients were misdiagnosed, a possible diagnosis would be early-onset dementia.
Expert A was willing to revise their own judgements upwards somewhat, but thought that expert C’s view was pessimistic.
It was agreed that expert C’s arguments were valid, but not overwhelming; for the consensus distribution, the experts agreed on quartiles supporting higher values, but set somewhat lower than those originally proposed by expert C. Agreed percentiles for parameter 1 are provided in Table 32.
| Plausible lower limit (%) | 25th percentile (%) | Median (%) | 75th percentile (%) | Plausible upper limit (%) |
|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 50 |
Fitted distribution for parameter 1
A beta (0.952, 2.71) distribution, scaled to the interval (0, 50%), was fitted to the consensus judgements. This is shown in Figure 21. The blue shaded region indicates that, given this choice of distribution, a probability of about 0.99 has been assumed that the percentage misdiagnosed will be < 40%. Percentiles from the fitted distribution for parameter 1 are shown in Table 33.
FIGURE 21.
The distribution chosen to represent the experts’ consensus judgements for parameter 1: the percentage of patients aged < 60 years whose death was due to CJD who are misdiagnosed as having died from another neurodegenerative disease, since 2005.

| Percentile | 1st | 5th | 95th | 99th |
|---|---|---|---|---|
| Parameter value | 0.1% | 0.8% | 33.0% | 40.6% |
Parameter 2 definition: the percentage of patients aged ≥ 80 years whose death was because of Creutzfeldt–Jakob disease who are misdiagnosed as having died from another neurodegenerative disease, since 2005.
Individual judgements
Without conferring, the experts made the probability judgements for parameter 2 as shown in Table 34.
| Expert | Plausible lower limit (%) | 25th percentile (%) | Median (%) | 75th percentile (%) | Plausible upper limit (%) |
|---|---|---|---|---|---|
| A | 5 | 30 | 50 | 60 | 70 |
| B | 10 | 25 | 50 | 75 | 90 |
| C | 20 | 50 | 80 | 90 | 100 |
| D | 0 | 20 | 50 | 60 | 75 |
Group discussion and consensus judgements
Expert C argued for higher values of parameter 2, based on figures 2 and 3 from the 25th Annual Report on CJD surveillance in the UK. 2 The argument was that mortality rates from sCJD have been observed to increase over time in the higher age categories and that this is a consequence of changes in diagnostics; it is plausible that this trend will continue, suggesting that the current percentage of misdiagnoses could be high. The remaining experts accepted a higher median and 25th percentile as consensus judgements, but thought that percentages close to 100% would be unlikely, agreeing a 75th percentile closer to the median. The consensus judgements are given in Table 35.
| Plausible lower limit | 25th percentile | Median | 75th percentile | Plausible upper limit |
|---|---|---|---|---|
| 0% | 40% | 60% | 65% | 100% |
Fitted distribution for parameter 2
A beta (3.36, 2.75) distribution was fitted to the consensus judgements, as is shown in Figure 22. The blue shaded region indicates that, given this choice of distribution, a probability of about 0.98 has been assumed that the percentage misdiagnosed will be between 14% and 92%. Percentiles from the distribution fitted to parameter 2 are provided in Table 36.
FIGURE 22.
The distribution chosen to represent the experts’ consensus judgements for parameter 2: the percentage of patients aged ≥ 80 years whose death was due to CJD who are misdiagnosed as having died from another neurodegenerative disease, since 2005.
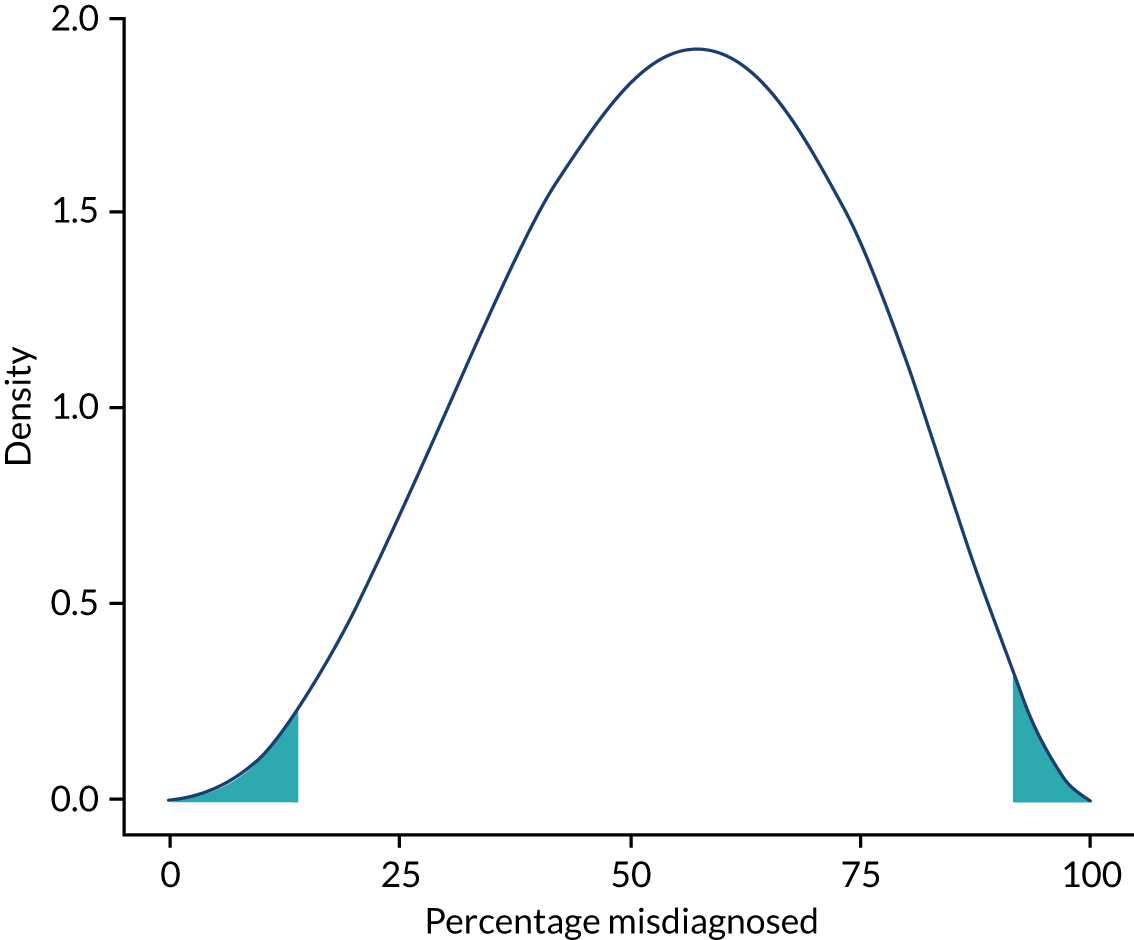
| Percentile | 1st | 5th | 95th | 99th |
|---|---|---|---|---|
| Parameter value | 14% | 23% | 85% | 92% |
Parameter 3 definition: the percentage of patients aged 60–79 years whose death was because of Creutzfeldt–Jakob disease who are misdiagnosed as having died from another neurodegenerative disease, since 2005
This parameter is assumed to be the mean of Parameters 1 and 2 (the percentages for the two age groups: aged < 60 years and ≥ 80 years). Its implied distribution can be obtained by simulation and is shown in Figure 23. Percentiles of this distribution are estimated by simulation and are provided in Table 37.
FIGURE 23.
The distribution chosen to represent the experts’ consensus judgements for parameter 3: the percentage of patients aged 60–79 years, whose death was due to CJD who are misdiagnosed as having died from another neurodegenerative disease, since 2005.

| Percentile | 1st | 5th | 95th | 99th |
|---|---|---|---|---|
| Parameter value | 10% | 16% | 51% | 58% |
Distributions related to incubation periods
Previous analysis had used different incubation periods for different recipient genotypes. It was thought that incubation period would depend on the genotypes of both host and recipient and also the infecting prion, and that a more manageable elicitation task would be to consider a single distribution of incubation periods, for genotype unspecified.
Distribution definition
The uncertain object of interest here is not a single parameter, but instead a distribution of incubation periods: the distribution of incubation period in years, in all patients, following infection with prion via surgery (i.e. posterior eye, brain, neuroendoscopy, and intradural spinal surgery), genotype unknown for each patient.
Individual estimates of the uncertain distribution
Without conferring, each expert gave estimates of three quantiles of the uncertain distribution, together with suggested lower and upper limits. These values are provided in Table 38.
| Expert | Plausible lower limit (years) | 25th percentile (years) | Median (years) | 75th percentile (years) | Plausible upper limit (years) |
|---|---|---|---|---|---|
| A | 0.5 | 2 | 4 | 10 | 50 |
| B | 0.25 | 3 | 7.5 | 10 | 40 |
| C | 0.2 | 1 | 12 | 20 | 50 |
| D | 0.5 | 3 | 12 | 30 | 70 |
Group discussion and quantifying uncertainty about the distribution
It was proposed to quantify uncertainty about the distribution of incubation periods as follows. First, four intervals were specified based on the estimates provided at the individual stage. These intervals are provided in Table 39.
| Interval 1 | Interval 2 | Interval 3 | Interval 4 |
|---|---|---|---|
| 0.25–2 years | 2–10 years | 10–20 years | 20–50 years |
As a central estimate, it was proposed that each interval describes incubation periods for 25% of the population. Incubation periods would be assumed to be uniform in each interval, giving the estimated distribution shown in Figure 24. The blue shaded region indicates that, given this choice of distribution, 98% of incubation periods will lie between 0.32 years and 48.8 years.
FIGURE 24.
An estimate of the distribution of incubation periods for all patients the distribution of incubation period in years, in all patients, following infection with prion via surgery (posterior eye, brain, neuroendoscopy, and intradural spinal surgery), genotype unknown for each patient.

To allow for uncertainty in the estimated distribution, it was proposed to allow the percentages in each interval to vary by up to 15% in intervals 1–3 and up to 10% in interval 4. For example, an alternative distribution would be as shown in Table 40 and Figure 25. The blue shaded region indicates that, given this choice of distribution, 98% of incubation periods will lie between 0.37 years and 48 years.
| Incubation period | Interval 1 | Interval 2 | Interval 3 | Interval 4 |
|---|---|---|---|---|
| 0.25–2 years | 2–10 years | 10–20 years | 20–50 years | |
| Percentage of patients | 15% | 35% | 35% | 15% |
FIGURE 25.
Alternative the distribution of incubation periods, constructed by perturbing the proportions of the population in each interval from the central estimates.
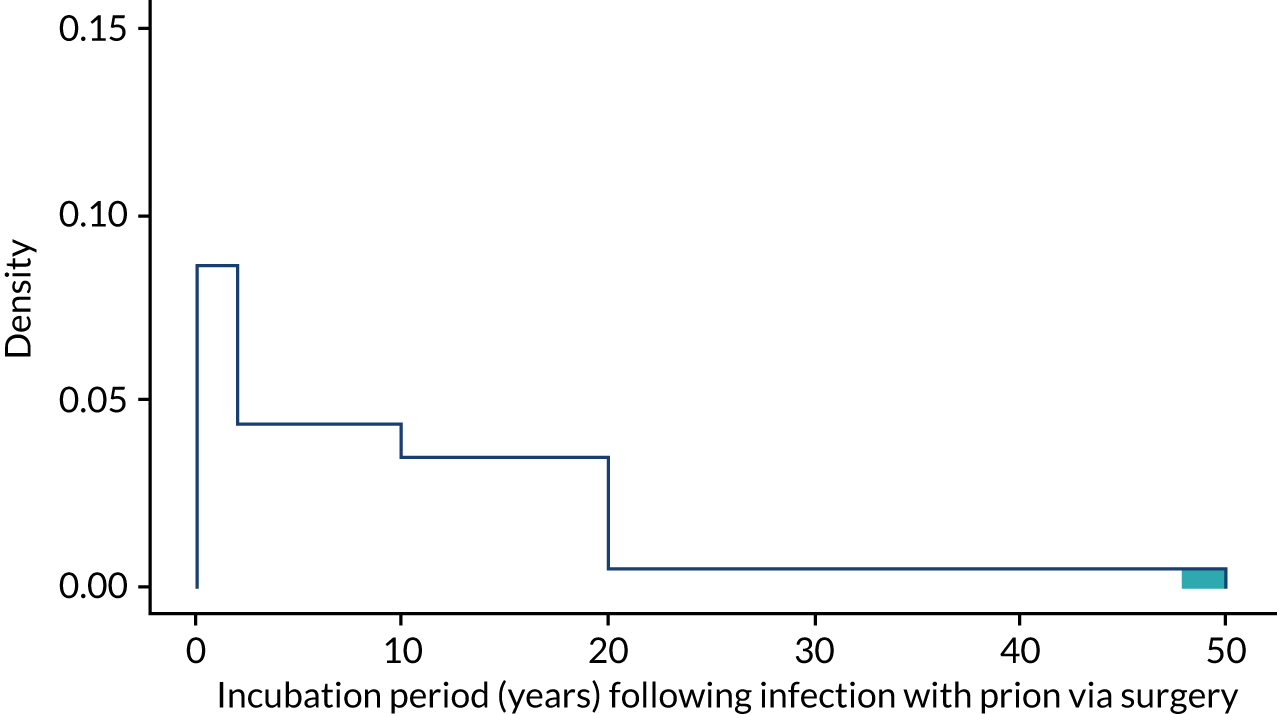
Susceptibility of patients to Creutzfeldt–Jakob disease: prion infection
The experts agreed that all patients would be susceptible to infection if a sufficient infectious load was received. This differed from the previous modelling undertaken where it was assumed that proportions of MV genotype and VV genotype at codon 129 patients were non-susceptible.
The prevalence of Creutzfeldt–Jakob disease prions in central nervous system tissue
The experts suggested that there is uncertainty in this parameter but that using different prevalence distributions for different age bands, which resulted in increased prevalence in the 16- to 39-year-old band, was not appropriate. It was commented that because sCJD increases with age but vCJD incubation could be greatest in younger ages, using the same distribution independent of age would be appropriate. The previous distribution used for 16- to 39 year-olds for prevalence per million people was a beta (1.24, 2225.393). This distribution is shown in Figure 26. The blue shaded region indicates that, given this choice of distribution, a probability of about 0.99 has been assumed that number per million will be less than 2300. Percentiles from the distribution are shown in Table 41. The experts commented that this may produce pessimistic numbers as the original elicitation was for all tissue, and not just CNS tissue, but thought that the use of the distribution was reasonable, and this was assumed appropriate for all ages. This prevalence was assumed to apply from 2005 onwards.
FIGURE 26.
The distribution chosen to represent the experts’ consensus judgements for the number of patients per million with CJD-prions in central nervous system tissue.

| Percentile | 1st | 5th | 95th | 99th |
|---|---|---|---|---|
| Prevalence (patients per million) | 12 | 46 | 1547 | 2304 |
Appendix 5 The assumed age profile of patients receiving each operation
The assumed age profiles for patients undergoing brain surgery (conditional on survival category), posterior eye surgery and neuroendoscopy are contained in Figures 27–31.
FIGURE 27.
The assumed age profile of patients undergoing brain surgery who are assumed to have normal life expectancy.

FIGURE 28.
The assumed age profile of patients undergoing brain surgery assumed to die at 18 months.
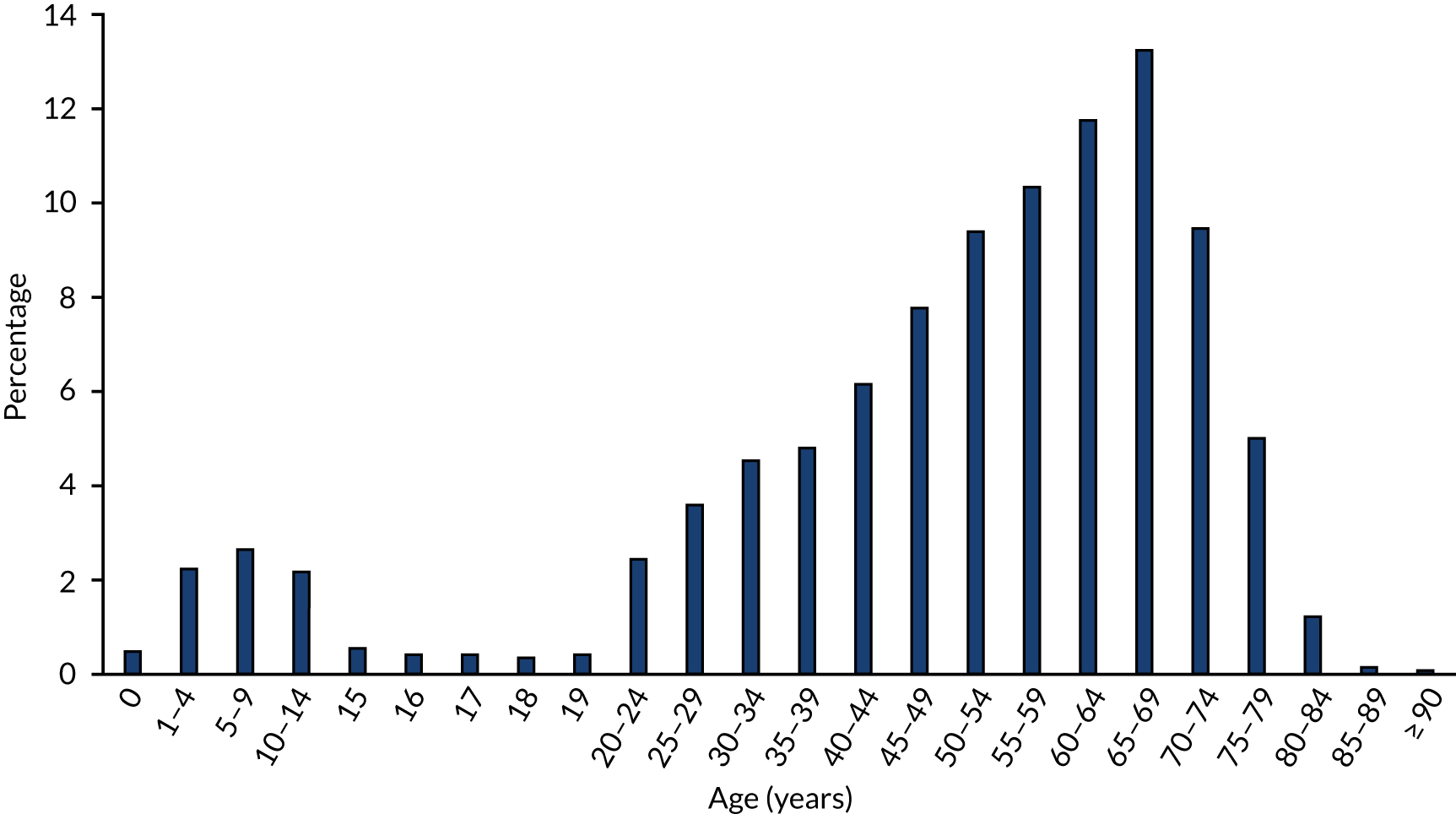
FIGURE 29.
The assumed age profile of patients undergoing brain surgery who are assumed to have a 50% chance of death at 18 months otherwise who are assumed to have normal life expectancy.

FIGURE 30.
The assumed age profile of patients undergoing neuroendoscopy.

FIGURE 31.
The assumed age profile of patients undergoing posterior eye operations.

Appendix 6 The operations considered to be at high risk
The operations considered to be high risk are contained in Tables 42–46, conditional on the type of operation and expected prognosis.
| National code | Operation |
|---|---|
| A01.1 | Hemispherectomy |
| A01.2 | Total lobectomy of brain |
| A01.3 | Partial lobectomy of brain |
| A01.8 | Other specified major excision of tissue of brain |
| A01.9 | Unspecified major excision of tissue of brain |
| A02.1 | Excision of lesion of tissue of frontal lobe of brain |
| A02.2 | Excision of lesion of tissue of temporal lobe of brain |
| A02.3 | Excision of lesion of tissue of parietal lobe of brain |
| A02.4 | Excision of lesion of tissue of occipital lobe of brain |
| A02.5 | Excision of lesion of tissue of cerebellum |
| A02.6 | Excision of lesion of tissue of brain stem |
| A02.7 | Excision of transcranial dermoid cyst |
| A02.8 | Other specified excision of lesion of tissue of brain |
| A02.9 | Unspecified excision of lesion of tissue of brain |
| A04.1 | Open biopsy of lesion of tissue of frontal lobe of brain |
| A04.2 | Open biopsy of lesion of tissue of temporal lobe of brain |
| A04.3 | Open biopsy of lesion of tissue of parietal lobe of brain |
| A04.4 | Open biopsy of lesion of tissue of occipital lobe of brain |
| A04.5 | Open biopsy of lesion of tissue of cerebellum |
| A04.6 | Open biopsy of lesion of tissue of brain stem |
| A04.8 | Other specified open biopsy of lesion of tissue of brain |
| A04.9 | Unspecified open biopsy of lesion of tissue of brain |
| A08.1 | Biopsy of lesion of tissue of frontal lobe of brain NEC |
| A08.2 | Biopsy of lesion of tissue of temporal lobe of brain NEC |
| A08.3 | Biopsy of lesion of tissue of parietal lobe of brain NEC |
| A08.4 | Biopsy of lesion of tissue of occipital lobe of brain NEC |
| A08.5 | Biopsy of lesion of tissue of cerebellum NEC |
| A08.6 | Biopsy of lesion of tissue of brain stem NEC |
| A08.8 | Other specified other biopsy of lesion of tissue of brain |
| A08.9 | Unspecified other biopsy of lesion of tissue of brain |
| National code | Operation |
|---|---|
| A03.1 | Stereotactic leucotomy |
| A03.2 | Stereotactic ablation of tissue of thalamus |
| A03.3 | Stereotactic ablation of tissue of globus pallidus |
| A03.8 | Other specified stereotactic ablation of tissue of brain |
| A03.9 | Unspecified stereotactic ablation of tissue of brain |
| A05.1 | Drainage of abscess of tissue of brain |
| A05.2 | Evacuation of haematoma from temporal lobe of brain |
| A05.3 | Evacuation of haematoma from cerebellum |
| A05.4 | Evacuation of intracerebral haematoma NEC |
| A05.8 | Other specified drainage of lesion of tissue of brain |
| A05.9 | Unspecified drainage of lesion of tissue of brain |
| A07.1 | Open division of tissue of brain |
| A07.2 | Removal of foreign body from tissue of brain |
| A07.3 | Exploration of tissue of brain |
| A07.4 | Excision of abscess of tissue of brain |
| A07.6 | Complete callosotomy |
| A07.7 | Partial callosotomy |
| A07.8 | Other specified other open operations on tissue of brain |
| A10.2 | Aspiration of abscess of tissue of brain |
| A10.3 | Aspiration of haematoma of tissue of brain |
| A10.4 | Aspiration of lesion of tissue of brain NEC |
| A10.5 | Puncture of tissue of brain NEC |
| A10.8 | Other specified other operations on tissue of brain |
| National code | Operation |
|---|---|
| A06.1 | Excision of basal encephalocele |
| A06.2 | Excision of occipital encephalocele |
| A06.3 | Excision of syncipital encephalocele |
| A06.4 | Repair of post-traumatic meningoencephalocele |
| A06.8 | Other specified other excision of lesion of tissue of brain |
| A06.9 | Unspecified other excision of lesion of tissue of brain |
| A09.1 | Implantation of neurostimulator into brain |
| A09.2 | Maintenance of neurostimulator in brain |
| A09.3 | Removal of neurostimulator from brain |
| A09.4 | Operation on neurostimulator in brain NEC |
| A09.5 | Insertion of neurostimulator electrodes into the brain |
| A09.8 | Other specified neurostimulation of brain |
| A09.9 | Unspecified neurostimulation of brain |
| A11.1 | Placement of depth electrodes for electroencephalography |
| A11.2 | Placement of surface electrodes for electroencephalography |
| A11.3 | Monitoring of pressure in tissue of brain |
| A11.4 | Cortical mapping |
| A11.8 | Other specified operations on tissue of brain |
| A12.1 | Ventriculocisternostomy |
| A12.2 | Creation of ventriculovascular shunt |
| A12.3 | Creation of ventriculopleural shunt |
| A12.4 | Creation of ventriculoperitoneal shunt |
| A12.5 | Creation of subcutaneous cerebrospinal fluid reservoir |
| A12.8 | Other specified creation of connection from ventricle of brain |
| A13.1 | Maintenance of proximal catheter of cerebroventricular shunt |
| A13.2 | Maintenance of distal catheter of cerebroventricular shunt |
| A13.3 | Insertion of antisyphon device into cerebroventricular shunt |
| A13.4 | Renewal of valve of cerebroventricular shunt |
| A13.8 | Other specified attention to component of connection from ventricle of brain |
| A13.9 | Unspecified attention to component of connection from ventricle of brain |
| A14.1 | Renewal of cerebroventricular shunt |
| A14.2 | Revision of cerebroventricular shunt NEC |
| A14.3 | Removal of cerebroventricular shunt |
| A14.4 | Irrigation of cerebroventricular shunt |
| A14.5 | Attention to cerebroventricular shunt NEC |
| A14.8 | Other specified other operations on connection from ventricle of brain |
| A14.9 | Unspecified other operations on connection from ventricle of brain |
| A16.1 | Open drainage of ventricle of brain NEC |
| A16.8 | Other specified other open operations on ventricle of brain |
| A20.1 | Drainage of ventricle of brain NEC |
| A20.2 | Ventriculography of brain |
| A20.3 | Monitoring of pressure in ventricle of brain |
| A20.8 | Other specified other operations on ventricle of brain |
| A20.9 | Unspecified other operations on ventricle of brain |
| A22.1 | Drainage of subarachnoid space of brain |
| A22.2 | Puncture of cistern of brain |
| A22.3 | Isotopic cisternography |
| A22.8 | Other specified operations on subarachnoid space of brain |
| A25.1 | Intracranial transection of optic nerve (ii) |
| A25.2 | Intracranial transection of oculomotor nerve (iii) |
| A25.3 | Intracranial transection of trigeminal nerve (v) |
| A25.4 | Intracranial transection of facial nerve (vii) |
| A25.5 | Intracranial transection of acoustic nerve (viii) |
| A25.6 | Intracranial transection of glossopharyngeal nerve (ix) |
| A25.7 | Intracranial transection of vagus nerve (x) |
| A25.8 | Intracranial transection of specified cranial nerve NEC |
| A26.1 | Intracranial destruction of optic nerve (ii) |
| A26.2 | Intracranial destruction of oculomotor nerve (iii) |
| A26.3 | Intracranial destruction of trigeminal nerve (v) |
| A26.4 | Intracranial destruction of facial nerve (vii) |
| A26.6 | Intracranial destruction of glossopharyngeal nerve (ix) |
| A26.8 | Intracranial destruction of specified cranial nerve NEC |
| A26.9 | Unspecified other intracranial destruction of cranial nerve |
| A29.1 | Excision of lesion of optic nerve (ii) |
| A29.8 | Excision of lesion of specified cranial nerve NEC |
| A29.9 | Unspecified excision of lesion of cranial nerve |
| A31.3 | Intracranial stereotactic neurolysis of trigeminal nerve (v) |
| A31.5 | Intracranial stereotactic neurolysis of acoustic nerve (viii) |
| A31.8 | Intracranial stereotactic neurolysis of specified cranial nerve NEC |
| A32.1 | Decompression of optic nerve (ii) |
| A33.1 | Introduction of neurostimulator into cranial nerve |
| A33.2 | Maintenance of neurostimulator in cranial nerve |
| A33.3 | Removal of neurostimulator from cranial nerve |
| A33.4 | Insertion of neurostimulator electrodes into the cranial nerve |
| A33.8 | Other specified neurostimulation of cranial nerve |
| A33.9 | Unspecified neurostimulation of cranial nerve |
| A34.1 | Exploration of optic nerve (ii) |
| A34.3 | Exploration of trigeminal nerve (v) |
| A34.4 | Exploration of facial nerve (vii) |
| A34.5 | Exploration of acoustic nerve (viii) |
| A34.7 | Exploration of vagus nerve (x) |
| A34.8 | Exploration of specified cranial nerve NEC |
| A34.9 | Unspecified exploration of cranial nerve |
| A36.8 | Other specified other operations on cranial nerve |
| A38.1 | Extirpation of lesion of meninges of cortex of brain |
| A38.2 | Extirpation of lesion of meninges of sphenoidal ridge of cranium |
| A38.3 | Extirpation of lesion of meninges of subfrontal region of brain |
| A38.4 | Extirpation of lesion of meninges of parasagittal region of brain |
| A38.5 | Extirpation of lesion of falx cerebri |
| A38.6 | Extirpation of lesion of tentorium cerebelli |
| A38.8 | Other specified extirpation of lesion of meninges of brain |
| A38.9 | Unspecified extirpation of lesion of meninges of brain |
| A39.1 | Repair of meningoencephalocele |
| A39.2 | Repair of dura of anterior fossa of cranium |
| A39.3 | Repair of dura of middle fossa of cranium |
| A39.4 | Repair of dura of posterior fossa of cranium |
| A39.5 | Repair of dura of vault of cranium |
| A39.8 | Other specified repair of dura |
| A39.9 | Unspecified repair of dura |
| A41.1 | Evacuation of subdural haematoma |
| A41.2 | Drainage of abscess of subdural space |
| A41.8 | Other specified drainage of subdural space |
| A41.9 | Unspecified drainage of subdural space |
| A42.1 | Creation of anastomosis of dura |
| A42.2 | Biopsy of lesion of meninges of brain |
| A42.8 | Other specified other operations on meninges of brain |
| A43.1 | Extirpation of lesion of meninges of skull base |
| A43.2 | Extirpation of lesion of meninges of skull clivus |
| A43.8 | Other specified other extirpation of lesion of meninges of brain |
| A43.9 | Unspecified other extirpation of lesion of meninges of brain |
| A44.1 | Chordectomy of spinal cord |
| A44.2 | Extirpation of lesion of spinal cord NEC |
| A44.3 | Excision of lesion of intradural intramedullary spinal cord |
| A44.4 | Excision of lesion of extradural spinal cord |
| A44.5 | Excision of lesion of intradural extramedullary spinal cord |
| A44.8 | Other specified partial extirpation of spinal cord |
| A44.9 | Unspecified partial extirpation of spinal cord |
| A45.1 | Stereotactic chordotomy of spinal cord |
| A45.2 | Open chordotomy of spinal cord NEC |
| A45.3 | Myelotomy of spinal cord |
| A45.4 | Open biopsy of lesion of spinal cord |
| A45.5 | Removal of foreign body from spinal cord |
| A45.6 | Open aspiration of lesion of spinal cord |
| A45.8 | Other specified other open operations on spinal cord |
| A47.1 | Needle destruction of substantia gelatinosa of cervical spinal cord |
| A47.2 | Radiofrequency controlled thermal destruction of spinothalamic tract |
| A47.3 | Percutaneous chordotomy of spinal cord |
| A47.8 | Other specified other destruction of spinal cord |
| A48.1 | Biopsy of lesion of spinal cord NEC |
| A48.2 | Aspiration of lesion of spinal cord |
| A48.3 | Insertion of neurostimulator adjacent to spinal cord |
| A48.4 | Attention to neurostimulator adjacent to spinal cord NEC |
| A48.6 | Removal of neurostimulator adjacent to spinal cord |
| A48.7 | Insertion of neurostimulator electrodes into the spinal cord |
| A48.8 | Other specified other operations on spinal cord |
| A49.1 | Freeing of spinal tether NEC |
| A49.2 | Closure of spinal myelomeningocele |
| A49.3 | Closure of spinal meningocele |
| A49.4 | Complex freeing of spinal tether |
| A49.8 | Other specified repair of spina bifida |
| A49.9 | Unspecified repair of spina bifida |
| A51.1 | Extirpation of lesion of meninges of spinal cord |
| A51.2 | Freeing of adhesions of meninges of spinal cord |
| A51.3 | Biopsy of lesion of meninges of spinal cord |
| A51.8 | Other specified other operations on meninges of spinal cord |
| A51.9 | Unspecified other operations on meninges of spinal cord |
| A53.1 | Cerebrospinal syringostomy |
| A53.3 | Creation of syringoperitoneal shunt |
| A57.1 | Extirpation of lesion of spinal nerve root |
| A57.6 | Reimplantation of spinal nerves into spinal cord |
| A57.8 | Other specified operations on spinal nerve root |
| A57.9 | Unspecified operations on spinal nerve root |
| B01.1 | Transethmoidal hypophysectomy |
| B01.2 | Trans-sphenoidal hypophysectomy |
| B01.4 | Transcranial hypophysectomy |
| B01.8 | Other specified excision of pituitary gland |
| B02.2 | Implantation of radioactive substance into pituitary gland |
| B04.1 | Excision of lesion of pituitary gland |
| B04.2 | Biopsy of lesion of pituitary gland |
| B04.3 | Decompression of pituitary gland |
| B04.4 | Exploration of pituitary gland |
| B04.5 | Operations on pituitary stalk |
| B04.8 | Other specified other operations on pituitary gland |
| B06.1 | Excision of pineal gland |
| B06.8 | Other specified operations on pineal gland |
| B06.9 | Unspecified operations on pineal gland |
| L33.1 | Excision of aneurysm of cerebral artery |
| L33.2 | Clipping of aneurysm of cerebral artery |
| L33.3 | Ligation of aneurysm of cerebral artery NEC |
| L33.4 | Obliteration of aneurysm of cerebral artery NEC |
| L33.8 | Other specified operations on aneurysm of cerebral artery |
| L34.1 | Reconstruction of cerebral artery |
| L34.2 | Anastomosis of cerebral artery |
| L34.3 | Open embolectomy of cerebral artery |
| L34.4 | Open embolisation of cerebral artery |
| L34.8 | Other specified other open operations on cerebral artery |
| National code | Operation |
|---|---|
| A17.1 | Endoscopic extirpation of lesion of ventricle of brain |
| A17.2 | Endoscopic third ventriculostomy |
| A17.8 | Other specified therapeutic endoscopic operations on ventricle of brain |
| A17.9 | Unspecified therapeutic endoscopic operations on ventricle of brain |
| A18.1 | Diagnostic endoscopic examination of ventricle of brain and biopsy of lesion of ventricle of brain |
| A18.9 | Unspecified diagnostic endoscopic examination of ventricle of brain |
| National code | Operation |
|---|---|
| C85.1 | Retinopexy using cryotherapy |
| C84.5 | Drainage of subretinal fluid through retina |
| C84.6 | Retinotomy NEC |
| C89.2 | Injection of steroid into posterior segment of eye |
| C85.5 | Retinopexy NEC |
| C84.1 | Epiretinal dissection |
| C85.2 | Retinopexy using diathermy |
| C89.3 | Injection of therapeutic substance into posterior segment of eye NEC |
| C84.8 | Other specified other operations on retina |
| C82.8 | Other specified destruction of lesion of retina |
| C89.1 | Insertion of sustained release device into posterior segment of eye |
| C85.8 | Other specified fixation of retina |
| C01.1 | Exenteration of orbit |
| C84.3 | Biopsy of lesion of retina |
| C84.2 | Excision of lesion of retina NEC |
| C84.9 | Unspecified other operations on retina |
| C01.2 | Enucleation of eye |
| C01.3 | Evisceration of eye |
| C82.9 | Unspecified destruction of lesion of retina |
| C89.8 | Other specified operations on posterior segment of eye |
| C83.3 | Limited macular translocation |
| C85.4 | Retinopexy using tissue adhesive |
| C85.9 | Unspecified fixation of retina |
| C01.8 | Other specified excision of eye |
| C01.9 | Unspecified excision of eye |
| C85.3 | Retinopexy using mechanical tacks |
| C89.9 | Unspecified operations on posterior segment of eye |
| C88.9 | Unspecified destruction of subretinal lesion |
Appendix 7 The calibration methodology
Notation
We define the following:
-
θ – the simulation model inputs. The true values of these inputs are uncertain; following various expert elicitation sessions, we have constructed a prior distribution p(θ) for θ.
-
T(i) – the number of transmissions of CJD via surgery that result in clinical symptoms in age category i, over the period 2005–18. The age categories are i = 1: ≤ 59 years; i = 2: 60–79 years; and i = 3: ≥ 80 years. We write T = [T(1),T(2),T(3)].
-
R(i) – the number of transmissions of CJD via surgery that result in clinical symptoms, in age category i, over the period 2005–18, that resulted in deaths recorded as being due to CJD. Note that for each i, we have R(i) ≤ T(i).
-
C – the data available for calibrating the simulation model. We know that over the period 2005–18, there were 15 recorded deaths from CJD, where the individuals were known to have had surgery. Hence, any number between 0 and 15 of these individuals could have acquired CJD from surgery. The age categories for these 15 recorded deaths are unavailable to us, so the calibration data C is the observation of the event that:(3)0≤R(1)+R(2)+R(3)≤15.
-
ϕ(i) – the percentage of patients, in age category i, whose death was due to CJD, that are misdiagnosed as having died from another neurodegenerative disease, since 2005. These percentages are unknown, and we have elicited probability distributions for them. Note that we treat these as elicited ‘posterior distributions’ p(ϕ(i)|C).
We suppose that:
We collect the ϕ(i) parameters into vector ϕ and write:
M(i) the maximum number of transmissions of CJD via surgery that result in clinical symptoms, in age category i, over the period 2005–2018, that resulted in deaths recorded as being due to CJD. We have:
and that:
for i = 1, 2, 3. Defining:
we make the assumption that:
that is, that each of the 15 potential cases were equally likely to be in any age category. This is likely to give too much weight to the oldest age category, but the assumption is conservative in the sense of minimising the risk of underestimating numbers of transmissions of CJD via surgery that result in clinical symptoms; patients in the oldest age category are judged the most likely to be misdiagnosed as having died from another neurodegenerative disease. Allocating a higher number of the 15 cases into the oldest age category will ‘permit’ higher numbers of transmissions of CJD via surgery that result in clinical symptoms, as more can be undetected.
‘S’ and ‘Y’ can be defined as follows:
-
S = (s1, . . . s27) – a vector of scenario values for each surgical centre. Two separate analyses are performed. In the first, each si is coded as an integer from 1 to 3 inclusive, and in the second, each si is coded as an integer from 4 to 6 inclusive. These correspond to the six scenarios S1 to S6 defined in Chapter 3, Categoristation of surgical units. (The P96 group are infectious from birth in scenarios S1 to S3 only.)
-
Y – the number of discounted QALYs that would be lost, as a result of transmission of CJD via surgery that result in clinical symptoms, due to surgery that took place between 2019 and 2023.
Estimating the number of quality-adjusted life-years lost owing to surgically transmitted Creutzfeld–Jakob disease caused by an operation between 2019 and 2023
The aim is to draw a sample of values Y1, . . . ,YN from the probability distribution of p(Y:C), from which we can provide an estimate of the expected value E(Y:C). This distribution can be expressed as:
Hence, we can obtain a sample Y1, . . . ,YN by obtaining a sample θ1, . . . , θN from p(θ|C), and then sampling Y1 from p(Y|θi,C). In essence, we are:
-
Calibrating the simulation model by updating the model inputs from p(θ) to p(θ|C) – we update what we know about the model inputs in light of the calibration data C.
-
Running the simulation model forward to predict Y, at input values θ sampled from p(θ|C) – input values identified to be consistent with the calibration data C.
Sampling from p(θ|C)
The method we use to sample from p(θ|C) is known as approximate Bayesian computation (ABC). This is a standard technique when we have a simulation model that can generate a random value of C given an input θ, but no formula can be obtained for the likelihood function p(C|θ). The basic ABC algorithm is as follows:
-
Generate one random value θ* from the elicited prior p(θ).
-
Given the model input θ*, run the model, and observe whether or not the event C has occurred within the model simulation.
-
If the event C has occurred within the model simulation, accept θ* as a valid draw from p(θ|C). Otherwise, reject, and return to step 1. Repeat until a candidate value θ* is accepted.
The process is repeated as many times as required to produce a sample θ1, . . . , θN from p(θ|C). For each accepted θ value, the model can be run forward to produce the desired sample Y1, . . . ,YN. We refer to this as the ‘simple rejection ABC algorithm’.
Implementing the simple rejection approximate Bayesian computation algorithm
The output quantities produced by the simulation model are T(1), T(2), T(3). To determine from these whether or not the event C has occurred, we additionally sample M, S and ϕ, so that we are in effect sampling from the joint distribution p(θ, M, S, ϕ|C). We write:
and we assume:
We have the multinomial distribution for M|C and the elicited distribution for ϕ|C, from which we can simulate values easily.
Note that:
since given:
we already know C – the total of M(1), M(2), M(3).
The ABC algorithm is then, in effect, used to sample from p(θ, S|M, ϕ), where the ‘prior’ distribution is p(θ, S|ϕ) and we assume independence between θ, S and ϕ:
-
Sample θ* from p(θ|ϕ) = p(θ).
-
Sample S* from p(S|ϕ) = p(S).
-
Run the simulation model to generate outputs T.
-
Given the outputs T, sample R, where:(16)R(i)|T(i), ϕ(i)∼Binomial[T(i), 1−ϕ(i)].
-
Observe whether or not, within the simulation model, the event:(17)R(i)≤M(i),
for i = 1, 2, 3 has occurred. If it has, we accept θ*, S* as a sample from p(θ,S|M,ϕ). Otherwise, we reject and return to step 1.
Estimation of E(Y|C)
Applying the ABC algorithm would give a sample θ(1), . . . , θ(M). Running the simulation model forward at these inputs only, we obtain an independent sample Y(1), . . . , Y(M) from the distribution of p(Y|C), from which we can estimate E(Y|C) via:(18)Y¯=1M∑i=1MY(i),
and an approximate 95% CI for E(Y|C) can be calculated as:(19)Y¯±2SY2/M,
with:(20)SY2=1M−1∑i=1M[Y(i)−Y¯]2.
We actually use a slightly different estimator for E(Y|C) which has a lower variance, but we retain the CI given above. Note also that there is a computational bottleneck in step 3 of this algorithm; running the model to observe whether or not C has occurred can be computationally expensive.
Speeding up the computation
We can speed up the computation by noting that, in some cases, it will not be necessary to simulate outcomes for all 27 surgical centres. Based on the number of simulated transmissions of CJD via surgery that result in clinical symptoms for a single surgical centre, an upper bound can be placed on the probability that the parameter value will ultimately be accepted. For example, if there were T(1) = 65 simulated transmissions of CJD via surgery that result in clinical symptoms in the age < 60 years category, no more than 50 of these could result in undetected CJD cases, and the probability of this occurring would be of the order of 10–9; the final probability of acceptance could be no more than this, regardless of what other events are simulated. (Under such a scenario, almost certainly, there would be transmissions of CJD via surgery that result in clinical symptoms in the other age groups, which would reduce the probability of acceptance by further orders of magnitude.)
Based on an understanding of the model’s behaviour and some preliminary analysis of the model outputs, we can determine parameter combinations that are guaranteed to be rejected. Specifically, we consider the term:
where θM is the mean infectious titre (in log-terms) × log-reduction in infectivity associated with the first autoclaving cycle × log-reduction associated with detergent on the first cycle; θR is the residual mass on an instrument × (1– the proportion of residual mass transferred to the patient); θP is the proportion of asymptomatic individuals with CJD prions in their tissue.
2000 parameter sets θ1, . . . , θ2000 were drawn from the appropriate distributions. Y1, . . . , Y2000 was calculated in each case, and 12 RN streams (corresponding to 12 surgical centres) were simulated for each of the following scenarios: S1, S2 and S3. We identified that for γ > e12, the final probability of acceptance would be negligible (too many transmissions of CJD via surgery that result in clinical symptoms would be simulated), and so the corresponding parameter set could be rejected without running the full simulation to produce R.
For γ > e12, it would still be possible for the candidate θ to be rejected. In other cases, we can be certain that a candidate value θ* will be rejected, based on a ‘partial’ simulation run: we do not have to simulate the full calibration output R. We used the following approach:
-
Generate a candidate value θ*, for which γ > e12.
-
Under scenario S3, first simulate the number of transmissions of CJD via surgery that result in clinical symptoms for six RN streams (six surgical centres).
-
If the total number of transmissions of CJD via surgery that result in clinical symptoms for the first six RN streams for the aged < 60 years category exceeds 36, reject θ* and return to step 1.
-
Continue simulating RN streams in batches: reject θ* if in streams 7 to 13 the rejection threshold was increased to 40; to 45 for RN streams 14 to 17; to 55 for RN streams 18 to 23; and 66 for RN streams 24 to 27.
A weighted ABC scheme
Instead of using the estimator Y¯, we can instead calculate a weight wi: the probability that the model will simulate the event T to have occurred. The estimate for E(Y|C) will then be of the form:(22)E^(Y|C)=∑i=1509w˜iYi,
with:(23)w˜i=wi∑i=1509wi.
This approach instead generates (weighted) samples directly from the marginal distribution p(θ|T), rather than joint samples from p(θ, M, S, ϕ|T). Each weight wi is estimated using the following Monte Carlo procedure. For each candidate value θi, the model simulates numbers of transmissions of CJD via surgery that result in clinical symptoms in each age band, under all scenarios for each surgical centre. The transmissions of CJD via surgery that result in clinical symptoms corresponding to the scenarios in S can then be selected.
For k = 1, . . . , 100,000:
-
Randomly sample S from its prior distribution, and denote this value by Sk. Given the model simulation run for input value θj and scenario set Sk, extract the number of transmissions of CJD via surgery that result in clinical symptoms in each age band. Denote these by:(24)Tj,k(i) for i=1,2,3.
-
Randomly sample M from its multinomial distribution. Denote the sampled values by:(25)Mk(1),Mk(2),Mk(3).
-
Randomly sample ϕ(1),ϕ(2),ϕ(3) from the three elicited prior distributions. Denote these values by:(26)ϕk(1),ϕk(2),ϕk(3).
-
Given the sampled values in step 2, we now have:(27)R(i)|Tj,k(i), ϕk(i)∼Binomial[Tj,k(i), 1−ϕk(i)].
-
Compute, from the corresponding binomial distributions in step 3:(28)wj,k=∏i=13Pr[R(i)≤Mk(i)].
-
The weight wi is estimated as:(29)w^j=1100,000∑k=1100,000wj,k.
Implementation
We started with a sample of 2000 parameter values. Applying the screening based on the calculated γ1, . . . , γ2000 values, we obtained a set θ1, . . . , θ509 that were not rejected. The weighted ABC algorithm was used to estimate E(Y|C), and the (conservative) CI using the simple rejection ABC algorithm was calculated for this estimate. Applying the simple rejection ABC algorithm reduces the sample size from 509 candidate parameter values to 119, when it was assumed that the P96 group could be infectious from birth, and 134, when it was assumed that the P96 group were not infectious from birth; the estimator Y¯ would be based on 119 and 134 model runs, respectively.
Glossary
- ID50
- The infectious dose required to infect 50% of individuals receiving the infectious agent.
- MM
- Methionine homozygosity at codon 129.
- MV
- Heterozygosity (methionine/valine) at codon 129.
- VV
- Valine homozygosity at codon 129.
List of abbreviations
- ABC
- approximate Bayesian computation
- ACDP
- Advisory Committee on Dangerous Pathogens
- BSE
- bovine spongiform encephalopathy
- CI
- confidence interval
- CJD
- Creutzfeldt–Jakob disease
- CJDAS
- Creutzfeldt–Jakob disease Advisory Sub-Committee
- CNS
- central nervous system
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CSEW
- Coroners’ Society of England and Wales
- EEG
- electroencephalography
- FFI
- fatal familial insomnia
- FFPE
- formalin fixed, paraffin embedded
- FML
- factor for efficiently maximising the likelihood
- gCJD
- genetic Creutzfeldt–Jakob disease
- GSS
- Gerstmann–Sträussler–Scheinker
- HES
- Hospital Episode Statistics
- hGH
- human growth hormone
- hGN
- human gonadotrophin
- ICER
- incremental cost-effectiveness ratio
- iCJD
- iatrogenic Creutzfeldt–Jakob disease
- IHC
- immunohistochemical
- IP
- interventional procedure
- IPG196
- Interventional Procedures Guidance 196
- MRI
- magnetic resonance imaging
- NCJDRSU
- National CJD Research and Surveillance Unit
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PK
- proteinase K
- PMCA
- protein misfolding cyclic amplification
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRNP
- prion protein gene
- PrP
- prion protein
- PrPres
- protease-resistant prion protein
- PrPSc
- prion protein scrapie
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RF
- radiofrequency
- RIO
- rational impartial observer
- RMEC
- rapid multienzyme cleaner trial formulation
- RML
- Rocky Mountain Laboratory
- RN
- random number
- RT-QuIC
- real-time quaking-induced conversion
- ScHARR
- School of Health and Related Research
- sCJD
- sporadic Creutzfeldt–Jakob disease
- SDS
- sodium dodecyl sulfate
- SI
- supplementary instrument
- SSBA
- standard steel-binding assay
- SSD
- sterile service department
- stCJD
- surgically transmitted Creutzfeldt–Jakob disease
- TICUw
- tissue culture infectious units on wires
- TSE
- transmissible spongiform encephalopathy
- vCJD
- variant Creutzfeldt–Jakob disease
- VPL
- violation of the permissible limit
