Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/42/01. The contractual start date was in February 2016. The draft report began editorial review in February 2019 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
James Prentis has received personal fees from Pharmacosmos A/S (Holbaek, Denmark) outside the submitted work. Elaine McColl was a member of the National Institute for Health Research (NIHR) Journals Library Editorial Group from 2013 to 2016 and was a member of the NIHR Clinical Trials Unit Standing Advisory Committee until 2016. Denise Howel was a member of the NIHR Health Services and Delivery Research Healthcare Delivery Research Commissioning Board from January 2012 until May 2016 and was a member of the NIHR Programme Grants for Applied Research subpanel from February 2017. Eileen Kaner was a panel member of the NIHR Public Health Research Research Funding Board until October 2016.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Snowden et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Reproduced with permission from Snowden et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Structure of this report
This report documents a feasibility study and pilot randomised controlled trial (RCT) of brief behavioural interventions (BBIs) to reduce alcohol consumption before elective orthopaedic surgery.
Chapter 1 describes the context of the issue, considering postoperative complications and the role of the preoperative assessment clinic (PAC) in identifying and addressing surgical risk in the context of reducing postoperative complications. This is followed by a review of the literature regarding brief interventions (BIs) and alcohol-focused interventions in the perioperative period.
Chapter 2 details the development of the intervention, the intervention training package and research conducted to characterise the comparator condition of ‘treatment as usual’ (TAU).
Chapter 3 presents the methods and results from the feasibility study, including qualitative interviews with health-care professionals (HCPs) and patient participants.
Chapter 4 describes the methods and results of the pilot RCT, including rates of recruitment and retention of patient participants, rates of completion of outcome measures and qualitative work exploring the acceptability of processes to both patient and HCP participants.
The key findings of the project are summarised and discussed in Chapter 5, with conclusions and recommendations for future research presented in Chapter 6.
Background
Perioperative morbidity and mortality after elective surgery is an important public health issue. 2 Although surgical mortality is reducing worldwide,3,4 even at the lowest mortality rate estimation of 1.1%, the volume of surgery being performed means that approximately 85,000 patients per year will not survive to 30 days following surgery. By contrast, postoperative morbidity or ill health is more common than mortality and remains at a relatively consistent level across sites and over time. 3 This clinical paradox has led to the accepted concept of ‘failure to rescue’, whereby the failure to identify and treat complications at an early stage following surgery leads to early mortality. 3,5
Although overall mortality rates remain important in a global sense,2 there has been a shift of focus to the reduction of morbidity after both high- and low-risk surgeries. 5 Postoperative complications have profound implications in terms of both patient well-being4 and utilisation of health-care resource increasing the costs of surgery two- to threefold. 2,6,7
The spectrum of alcohol use from abstinence to dependence is broad and can be subdivided in a number of ways. The research and clinical literature has, and still does, employ a variety of terms, often interchangeably, to describe different patterns of drinking. Many of these terms are included in the Glossary. Terms of specific interest to this work are described here. In the UK, ‘lower-risk’ drinking is considered to be the consumption of ≤ 14 units of alcohol per week (1 unit is 8 g of alcohol), with additional guidelines specifying that drinks should be spread evenly over ≥ 3 days and that ‘binge drinking’ (i.e. the consumption of ≥ 6 units on a single occasion) should be avoided. Any drinking above this level can be considered to increase the risk of experiencing alcohol-related harm. The term ‘increased risk’ drinking has replaced the term ‘hazardous drinking’ and is used to describe patterns of alcohol consumption that exceed the lower-risk drinking guidelines and are associated with an increased risk of experiencing harm as a result of alcohol consumption, although actual harm may not yet have occurred. Regular consumption in the range of 15–35 units per week for women and 15–50 units per week for men would usually fall into this category. 8 Higher-risk drinking, previously ‘harmful drinking’, is a pattern of alcohol consumption that results in harm to physical or mental health, with regular consumption in excess of 35 units per week for women and of 50 units per week for men. 8
A number of screening tools have been developed to identify different patterns of alcohol use. One of the most frequently employed in health-care settings is the Alcohol Use Disorders Identification Test (AUDIT). 9,10 The AUDIT, originally developed by the World Health Organization9 and validated for use in medical settings,11 is a 10-item questionnaire that assesses alcohol consumption alongside social and health consequences of drinking behaviour and indications of possible alcohol dependence. 12 Scores range from 0 to 40 and a cut-off point of ≥ 8 has been found to have a sensitivity of 92% and a specificity of 94% for detecting increased risk or higher-risk drinking. 9
Following the development of the AUDIT, a number of shorter variants have been developed. The Alcohol Use Disorders Identification Test Consumption (AUDIT-C) measure, which comprises the first three items of the AUDIT that focus on assessing frequency and volume of consumption as well as the frequency of high intensity or binge drinking,13 has been shown to have a similar level of accuracy,14 including validity and reliability, as the full AUDIT. 13,15 With a cut-off point of 5, this measure has been found to have a sensitivity of 74% and a specificity of 83% for detecting increased risk drinking. 16
Orthopaedic surgery and postoperative complications
Among the elective orthopaedic surgical population, average rates of mortality (0.2–0.6%)17–19 and morbidity (4–6%)17,20–22 are relatively low when compared with those for other surgical populations such as cardiac or abdominal surgery. However, orthopaedic surgery is a high-volume elective specialty,23 meaning that even low rates of mortality and morbidity can have a significant population impact. In 2017, over 100,000 primary knee replacements and 90,000 total hip replacements were performed in England and Wales. 24 The most common complications include systemic infections and cardiovascular events (e.g. pulmonary embolism), deep-vein thrombosis, wound infections, wound dehiscence, perioperative fracture and nerve injury. 17,22 In general non-cardiac surgery, any complication is associated with an average 101% increase in hospital costs. 25 In orthopaedic surgery specifically, surgical site infections are associated with, approximately, a 61% increase in costs. 26
In addition, many elective orthopaedic procedures are performed in an older population. Advancing age predisposes patients to an increased risk of acquired, multiple comorbidities, which in turn confer an increased risk of post-surgical complications. As life expectancy continues to increase, more orthopaedic procedures will be performed in older people, leading to an increase in overall surgical morbidity. 17,22,27
Any perioperative intervention that aims to reduce known preoperative risk factors that predispose patients to postoperative complications will be important in improving future postoperative outcomes.
The role of the preoperative assessment clinic
Preoperative assessment clinics aim to assess patients’ suitability for elective surgery early in the surgical pathway through the detection, optimisation and modification of coexisting conditions. Indirectly, these clinics have been shown to reduce surgical cancellations and improve day-of-surgery arrival rates. 28 Importantly, they also provide an early opportunity to identify and address modifiable risk factors associated with postoperative complications, an approach often referred to as ‘prehabilitation’. This approach has included interventions to improve nutritional status, correct diet-induced iron deficiency anaemia,29 promote smoking cessation,30 reduce anxiety,31,32 provide patients with information regarding the perioperative pathway33,34 and increase physical activity. Regarding the last, the promotion of structured exercise regimes has been shown to improve aerobic fitness35,36 and enhance functional capacity both pre- and postoperatively in total knee arthroplasty patients37 and decrease length of stay in hospital for adult surgical patients across a range of specialties. 38
Preoperative alcohol intake also represents an important target for modifiable risk factor reduction. Subsequent sections of this chapter present existing literature concerning the association of alcohol consumption and postoperative outcomes, the identification and management of alcohol consumption in the PAC and interventions that have been found to be effective in other clinical settings.
Alcohol consumption
Alcohol is a component cause of > 200 health conditions39 and has a range of negative social consequences, including familial and relationship problems,40 involvement in crime,41,42 loss of productivity and unemployment. 43,44 Alcohol consumption is a leading cause of ill health globally, accounting for an estimated 4.2% of the global burden, of disease and injury. 45 In addition, alcohol-related harm represents a large economic burden, with the combined cost of alcohol-related health harms, crime, antisocial behaviour and loss of productivity in England estimated at £21B in 2012. 46 Both volume of consumption and drinking pattern are important predictors of alcohol-related health harms. 45
With a dose–response relationship between alcohol consumption and many forms of harm, those who consume the most alcohol, including those who are alcohol dependent, undoubtedly experience the highest levels of alcohol-related harm at the individual level. However, because a far greater number of individuals drink at increased risk and higher-risk levels, it is these drinking patterns that account for the largest proportion of population-level alcohol-related harm. 47 Therefore, it is this group, who drink in excess of the lower-risk guidelines but are not alcohol dependent, who are a primary target of interventions seeking to minimise alcohol-related harm,48 a phenomenon known as the prevention paradox.
Alcohol consumption and postoperative complications
The physiological effects of alcohol (e.g. liver damage, risk of accidental injury) may directly result in a need for surgery, or may indirectly increase surgical risk. Of specific concern are systems that are inhibited by both alcohol consumption and surgery. These include the haemostatic, immune, cardiac and endocrine systems. 49 The same mechanisms by which moderate alcohol consumption can protect against the formation of blood clots50 also place those with increased levels of alcohol consumption at increased risk of prolonged bleeding during and after surgical procedures. 51 Reduced immune system function as a result of alcohol consumption is further inhibited by surgery52 and leaves the patient susceptible to both surgical site and systemic infections; subclinical cardiac insufficiency and arrhythmias53 present increased risks of cardiac complications and an altered endocrine stress response49,51 may also increase the risk of complications.
With this myriad of physiological effects of alcohol, it is not surprising that studies have identified associations between alcohol intake and perioperative complication rates across a wide range of surgical procedures (e.g. cardiac, orthopaedic and gastric). The complications most frequently associated with alcohol use are infection, sepsis, bleeding, cardiac events and deep-vein thrombosis. 51,54,55
Within this literature, a number of studies have focused on those consuming ≥ 60 g of alcohol per day and those identified as having alcohol use disorders (AUDs) or alcohol dependence. 52,56 In 1999, a review51 identified a two- to threefold increase in postoperative morbidity among individuals consuming > 60 g of alcohol per day for a minimum period of ‘several months’. However, the risk of increased postoperative complication rates may not be limited to those identified as ‘alcohol misusers’ or those with alcohol use disorder. Comparison of postoperative complication rates with scores on validated alcohol screening tools has demonstrated that the incidence of complications increases with increased screening scores among all adult drinkers. Bradley et al. 57 identified that patients with an AUDIT-C score of ≥ 5 (score range 0–12) were at increased risk of postoperative complications. Complication rates increased from 5.6% for those scoring 0–4 on AUDIT-C to 14% for those scoring 11–12 on the screening tool.
In 2013, a review conducted by Eliasen et al. 58 assessed the association between preoperative alcohol consumption and postoperative complications across a wider population of drinkers than were included in the review by Tønnesen and Kehlet. 51 They found that alcohol consumption (of 24 g per day for women and 36 g per day for men) was associated with increased rates of postoperative complications, including a 23% increased risk of wound complications, an 80% increased risk of pulmonary complications, a 24% increased risk of prolonged hospital stay and a 29% increased risk of intensive care admission. Furthermore, high, but not low to moderate (< 24 g per day for women and < 36 g per day for men), alcohol consumption was associated with increased mortality. Two similar reviews by Wåhlin and Tønnesen59 and Shabanzadeh and Sørensen60 in 2014 and 2015, respectively, concluded that the consumption of two or more standard drinks per day is enough to increase the rate of postoperative complications.
This pattern of dose-dependent association of alcohol intake with postoperative complications is also reported in orthopaedic surgical populations. Among patients undergoing total hip or knee arthroplasty, those with high levels of alcohol use showed significantly higher incidences of complications (12 out of 15 assessed),61 with an overall complication rate of 33.5% among alcohol misusers compared with 22.6% in their non-alcohol-misusing counterparts. This population was also found to have significantly longer stays in hospital. 61 Another study62 reported a 29% increase in the number of complications for every additional point on the AUDIT screening tool in patients undergoing total joint arthroplasty. This is also supported by the results of a registry-based study that considered patients who reported abstinence, low to moderate or high alcohol consumption and found a similar dose-dependent association with rates of mortality and morbidity. 63
With a strong evidence base for an association between increased alcohol consumption and increased rates of postoperative complications, it is reasonable to suggest that interventions that reduce or cease alcohol consumption in the preoperative period may result in fewer complications and better postoperative outcomes. Existing literature suggests that many of the physiological effects of increased alcohol consumption thought to explain the increased risk of postoperative complications may be improved or entirely reversed within a relatively short period of alcohol cessation (between 2 weeks and 3 months depending on the specific effect considered). 51,64,65 The effects of reduction rather than cessation of alcohol consumption are less clear.
Achieving such improvements requires two key things: first, clinicians must be able to effectively identify patients at increased risk of perioperative complications as a result of alcohol consumption and, second, they must be able to provide an acceptable and effective intervention to bring about timely changes in alcohol consumption behaviour.
Preoperative alcohol screening
Although the assessment of alcohol consumption is part of standard care in preoperative assessment (PA), the use of validated screening tools is not widespread. Until recently, the more formal validated screening tools developed to assess of alcohol consumption (e.g. AUDIT, AUDIT-C), had not been employed in the preoperative setting.
Concerning the detection of alcoholism in the preoperative period, Martin et al. 66 found that routine practice identified only 16% of alcohol-dependent patients at the first visit, although this increased to 34% if the patient was seen three or more times in the preoperative period. When a screening questionnaire was used, the rates of detection on first visit were much higher (64%).
Because alcohol-dependent patients are at highest risk of intra- and postoperative complications and are at risk of the life-threatening complication of experiencing alcohol withdrawal syndrome, it is critical that they be identified and effectively manged in the preoperative period. However, they represent only a small proportion of those who may be at increased risk as a result of alcohol consumption. Kip et al. 67 considered a wider range of AUDs and compared detection rates from screening with computerised AUDIT questionnaire with those from anaesthesiologist assessment. The findings support those of Martin et al. ,66 with only 17.4% of patients who were found to be AUDIT positive (score of ≥ 8) also detected by anaesthesiologists. In addition, although anaesthesiologists were reliably able to detect patients who showed signs of dependence (25.2%), the patients with harmful (20%) or hazardous (17.2%) drinking were not detected. Furthermore, subgroup analysis found that anaesthesiologists were significantly less likely to identify AUDs in women and those aged < 50 years. 67
Among both elective and emergency orthopaedic surgical patients in Australia, 34% were identified as having hazardous drinking behaviour using the AUDIT questionnaire but only 38% of these patients reported a history of problematic alcohol consumption,68 indicating that a majority of those drinking at hazardous levels may be unaware that their drinking could represent a risk to their health.
In combination, these findings demonstrate that utilisation of validated screening tools in the preoperative period is likely to increase detection rates of AUDs across the spectrum.
Alcohol screening using validated screening tools has been shown to have good patient acceptance in both preoperative and wider health-care settings. 57,62,68–70 Indeed, the need to improve detection rates for increased risk alcohol consumption (and smoking behaviour) in hospitals has been recognised in the UK, where the Commissioning for Quality and Innovation (CQUIN) has identified these health behaviours as a priority.
The CQUIN framework aims to improve care by providing financial incentives to health-care providers in the UK who demonstrate delivery of quality and innovation in prespecified areas. The 2017–19 CQUIN targets include a goal to support people to change their behaviour in order to reduce the risk to their health from alcohol and tobacco use through identification, advice and referral to specialist services for inpatients in all acute trusts. 71 Regarding alcohol consumption, the CQUIN promotes screening with the AUDIT-C questionnaire followed by delivery of brief advice (BA) to those identified as increased risk or higher-risk drinkers and the referral of those identified as alcohol dependent. Although PA is an outpatient appointment, patients are subsequently admitted as inpatients for the surgical procedure. Thus, the PA provides an important opportunity for the CQUIN screening, intervention and referral package to be implemented earlier in the surgical pathway.
Preoperative alcohol cessation interventions
A small number of studies have considered interventions concerned with bringing about preoperative alcohol cessation among heavy drinkers with a minimum level alcohol consumption equivalent to between 4.5 and 7.5 units per day (36–60 g of alcohol) or 31.5 and 52.5 units per week (252–420 g of alcohol). The results of three RCTs (one published,64 two unpublished72,73) that targeted alcohol cessation in the preoperative period have been included in the most recent update of the Cochrane review concerning perioperative alcohol cessation interventions. 74 All three trials employed intensive interventions, including the provision of disulfiram (Antabuse® Pliva, East Hanover, NJ, USA), and aimed to achieve between 1 and 3 months of alcohol abstinence prior to surgery. Two trials also offered chlordiazepoxide hydrochloride for the treatment of withdrawal symptoms and provided motivational counselling each week, with staff available for telephone contact if required. 72,73 All three trials identified significantly higher rates of abstinence in the intervention groups than in the control. An associated finding of lower rates of postoperative complications following intervention was not statistically significant. However, when pooled data were analysed,74 there was a significant reduction in complication rates associated with reduced alcohol consumption immediately following completion of the intervention.
It is important to consider that all three of these trials were conducted in Denmark and results cannot be assumed to be generalisable to the UK setting. These trials are all concerned with individuals drinking at more than double the current UK lower-risk drinking guidelines, levels that suggest possible dependence, and thus require the provision of specialist support including pharmacotherapy. However, such intensive interventions are unlikely to be acceptable to, or appropriate for, patients drinking at increased risk levels (> 14 units per week). Because this group also confer additional risk as a result of their alcohol consumption, present for surgery in much greater numbers and are unlikely to recognise the risks associated with their own drinking behaviour, it is necessary to consider alternative, more appropriate interventions. BIs in the form of structured advice or behaviour change counselling, have been shown to be effective in bringing about reductions in alcohol consumption in other health-care settings, including primary and emergency care,75–77 and represent a potential alternative that could be employed in the PAC at relatively low cost and with minimal burden to the patient.
Brief alcohol interventions
The terms ‘brief intervention’, ‘brief behavioural intervention’ and ‘brief advice’ are often used interchangeably in the literature to describe short sessions of advice and/or behaviour change counselling that are usually delivered by a care provider. Such methods have frequently been used to address increased and higher-risk drinking behaviours. BIs usually last between 5 and 30 minutes with key intervention content including feedback on alcohol consumption often with comparison to social norms; identification of the drinker as the only one who can change their behaviour; identification of the potential harms of drinking behaviour and the potential benefits of reducing alcohol intake; advice about how to reduce alcohol consumption; and goal-setting or the development of a plan to reduce drinking. In addition to this content, the interventionist or care provider adopts a non-judgemental, empathic approach with roots in motivational enhancement therapy, which seeks to increase motivation to change and enhance self-efficacy. These core components and the associated therapeutic approach are summarised by the FRAMES (Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy) acronym. 78
Although research trials often identify decreases in alcohol consumption in both intervention and control groups (e.g. Fleming et al. 79 and Lock et al. 80), systematic reviews and meta-analyses have consistently identified positive effects of providing BI over and above changes in comparator groups. 81–83 This approach has been most frequently studied in primary care settings, where the focus is on reducing alcohol consumption in the longer term. 75,84,85 In these settings, BIs have been shown to result in an average reduction of between 20 g75 and 38 g82 of alcohol per week (2.5–5 units) compared with control groups at 6–12 months post intervention.
However, the implementation of BIs into standard practice has been limited,86 with a number of studies documenting barriers to implementation, including lack of resources, training and support. 87
There is now a small, but growing, body of evidence considering the use of BIs for alcohol consumption in other settings including general hospital wards76,88 and outpatient clinics. 89 A recent review and meta-analysis of interventions delivered to general hospital inpatients identified a significant benefit of BIs over control conditions in terms of reduction in alcohol consumption at 6 (–70 g per week) and 9 months (–183 g per week) post intervention as well as a reduction in mortality rates at 6-month (relative risk 0.42) and 1-year (relative risk 0.60) follow-up. 76 Meanwhile, it has been shown that screening and BI in outpatient clinics can reach a large number of patients90 and can result in significant reductions in alcohol consumption and in the percentage of hazardous drinkers (intervention, reduction from 60% to 27%; control, reduction from 54% to 51%). 89
With the implementation of BIs in primary care being limited, it is important to establish when and where such interventions can best be implemented in standard care. Previous research shows that patients are more accepting of health behaviour advice when it is delivered in an appropriate setting or linked to the presenting health condition. 91,92 In some settings, such as emergency care, some level of alcohol-related harm may already be present (e.g. alcohol-related injuries). On the other hand, the PAC offers an opportunity to introduce the idea of health behaviour and behaviour change before many will have incurred any significant harm but when there is a positive, salient and temporally proximal focus (i.e. elective surgery). In other words, it offers a ‘teachable moment’. The benefit of BIs targeting shorter-term changes in alcohol consumption (e.g. preoperative alcohol reduction or cessation) has not been widely studied and thus it is unclear what magnitude of effect is likely in such contexts. However, broader intervention studies predominantly show a higher level of behaviour change post intervention and at early follow-up (e.g. ≤ 3 months) than at longer-term follow-up (e.g. 12 months). 93
Further to this, the PAC patient population is both large and diverse, with most patients who receive elective surgeries being referred for at least some level of assessment. As such, the PAC offers an opportunity to screen and intervene with a large number of patients across a wide age range, many of whom may not frequently present to primary or emergency care.
If brief alcohol interventions are to be implemented in PACs, it is likely that they will be delivered by nurses rather than anaesthetists because the clinics tend to be nurse led. Although there is some evidence to suggest that physician-delivered interventions may be better received by patients,91 others have reported that nursing staff may be best placed to deliver interventions92 and that patients view interventions delivered by nursing staff as equivalent to those delivered by general practitioners (GPs). 94
Behavioural interventions in the preoperative period
To date, four trials have considered the effectiveness of behavioural interventions (including both brief and more extended interventions) targeting alcohol reduction or cessation in the preoperative period. The majority have included alcohol interventions as part of a multimodal intervention package targeting multiple health behaviours, which precludes firm decisions on the effectiveness of isolated intervention components for individual behaviours. The only trial to assess an alcohol intervention alone was reported by Shourie et al. 95 This trial commenced as a randomised trial but difficulty in recruiting patients at least 7 days before surgery led to the adoption of a non-randomised method whereby those with ≥ 7 days to surgery received the intervention and those with < 7 days to surgery were allocated to the control condition. The intervention employed was a revised version96 of the manualised BI from the ‘Drink-less’ campaign,97 which aimed to facilitate preoperative alcohol cessation. No significant intervention effect on alcohol consumption was demonstrated. This finding was attributed the intervention being delivered too close to surgery, thereby allowing too little time for either behaviour or physiological change. Three further trials used behavioural interventions targeting preoperative alcohol consumption as part of multimodal intervention packages. McHugh et al. 98 conducted a RCT with patients awaiting coronary artery bypass surgery. Patients allocated to the intervention group had their physical and mental health needs assessed and were provided with a tailored intervention package delivered in monthly health education sessions. Interventions targeted smoking, alcohol use, physical activity, diet, hypertension and hypercholesterolemia as indicated by screening and patients’ readiness to change. In addition to providing advice and information, the interventionists used a ‘pros and cons’ approach to behaviour change, goal-setting and progress monitoring. Patients in the intervention group showed greater rates of smoking cessation, physical activity and weight loss than the control group and greater improvements in blood pressure. Alcohol consumption outcome data were not published in this original study report but were included in a subsequent review,99 which identified a significant group × time interaction whereby the intervention group decreased their weekly alcohol consumption by 1.2 standard drinks per week between baseline and follow-up, whereas the control group showed an average increase of 1.3 drinks per week over the same period. With an average of > 8 months from trial entry to surgery date, patients had adequate time to implement behaviour change and observe positive health outcomes. However, the maintenance of change over an extended period, alongside the targeting of multiple health behaviours, presents its own difficulties.
Kummel et al. 100 conducted a RCT to assess the effects of guidance and counselling delivered in individual and group sessions on health behaviours in older (> 65 years) coronary artery bypass patients. Patients received one group session in the preoperative period and four sessions postoperatively. Sessions included health counselling, guidance and adjustment education, including information on risk factors for coronary heart disease, peer support and goal-setting. The primary focus of this intervention package was outcomes in the postoperative period rather than preoperative changes in health behaviour. The intervention was found to reduce alcohol consumption relative to baseline at 3-month follow-up, whereas the control group showed greater alcohol consumption at 6- and 12-month follow-up than at baseline.
The third trial101 assessing the effectiveness of a multimodal intervention package used a quasi-experimental design with patients referred for fast-track hip and knee arthroplasty. Patients were recruited over 4 months, with those undergoing surgery in the first 2 months of 2007 allocated to the control group and those receiving surgery in the next 2 months of the same year allocated to the intervention. The intervention involved completion of a preoperative risk-screening questionnaire, followed by a motivational conversation addressing identified risks with interventions for nutritional risk, physical activity, health, medication and alcohol use and/or smoking. Interventions for smoking and alcohol consumption involved the provision of information and identification of the potential benefits of stopping or abstaining in the preoperative period. Information leaflets about the identified risks were also provided. Findings showed a reduced number of ‘unplanned’ surgical pathways (including complications, readmission and mortality in the postoperative period) among intervention patients compared with controls. However, it is notable that only 4 of the 78 participants in the intervention condition were identified ‘at risk’ in terms of drinking more than the recommendations from the Danish National Board for Health (i.e. 21 units per week for men, 14 units per week for women). 101 As a result, it is not possible to conclude what, if any, benefits were gained from identifying and intervening to address risky drinking in this population.
A further trial considered preoperative alcohol consumption as part of a multimodal ‘prehabilitation’ package and planned to provide interventions for risky drinking but did not identify any patients drinking at risky levels. 102
The four studies assessing behavioural interventions for alcohol consumption in the preoperative period have been drawn together in a narrative review conducted by Fernandez et al. 99 This review identified some positive findings, with two of the trials identifying significant reductions in alcohol consumption and one identifying a significant reduction in postoperative complications. However, it also highlighted a number of methodological weaknesses of the current evidence and made multiple recommendations for future research. They pointed to difficulties in recruitment across the trials. Specifically, the studies by Shourie et al. ,95 McHugh et al. 98 and Kummel et al. 100 took between 15 months and 3.5 years to recruit 117–136 participants. These issues were further exacerbated by the fact that rates of risky drinking in participants recruited to multimodal trials were low. For example, Hansen et al. 101 identified just 4 out of 78 intervention participants as drinking above recommended guidelines and Kummel et al. 100 identified 12 out of 49 intervention patients and 21 out of 68 control patients as using alcohol at least once per week at baseline. This is in contrast to alcohol screening data, which identify 8–43% of surgical patients as screening positive for alcohol misuse,57,62–103 and rates increase to as high as 82% when considering surgeries for conditions strongly associated with alcohol use (e.g. head and neck tumour surgeries). 103 This suggests a number of possibilities: alcohol consumption levels may be much lower in certain surgical populations, those drinking at risky levels may be less likely to participate in intervention trials or alcohol consumption may be under-reported by trial participants. Fernandez et al. 99 recommend adopting clear, precise definitions of risky drinking and using unbiased screening to detect those drinking at risky levels.
Further recommendations resulting from this review99 include assessing change in alcohol use preoperatively as well as postoperatively to allow the assessment of preoperative change on postoperative outcomes; a focus on the needs of older adults because the average age of trial participants is < 50 years; using empirically supported interventions and improving the reporting of both intervention content and fidelity of delivery; delivering interventions early enough in the preoperative care pathway to allow adequate time for behaviour change and subsequent pathophysiological improvement; and careful consideration of the sample size required to ensure adequately powered evaluations.
Feasibility and acceptability of preoperative screening and alcohol intervention
There has been limited work exploring the acceptability of screening and BI methods to patients and HCPs in the context of perioperative care. Acceptability has tended to be judged on rates of uptake of screening and interventions. 57,62,68 Considering the difficulties experienced in recruiting patients to trials of preoperative alcohol interventions in previous studies, qualitative methods may provide important insight.
To the authors’ knowledge, only two studies have used qualitative methods to provide more detailed findings regarding the acceptability of perioperative alcohol intervention methods. Pedersen et al. 104 carried out qualitative interviews to assess patients’ views of the intervention materials and methods used in their trial of postoperative alcohol interventions following surgery for ankle fractures. Findings show that patients held positive views about the intervention in relation to surgery and the proposed materials. Approximately half of the patients interviewed reported that they were open to changing their drinking behaviour. The validity of these findings is restricted by the sampling technique. Specifically, patients interviewed did not receive the intervention as part of the trial but were presented with the intervention materials and asked to provide their views. Lauridsen et al. 105 interviewed 11 participants from the ongoing ‘STOP-OP’ trial of alcohol and smoking cessation interventions delivered to patients awaiting radical cystectomy. Of those interviewed, three had received an alcohol intervention alone and two had received both the smoking and the alcohol interventions. The authors reported that the interventions were well received and that patients perceived it to be a part of their treatment package rather than something separate or distinct. A number of facilitators of effective intervention were identified, including having intervention sessions scheduled alongside other appointments or delivered over the telephone, and the empathic approach of interventionists. Facilitators of alcohol and smoking cessation were also identified; these included aspects of undergoing surgery (e.g. hospital stay, nausea) that removed either the desire or the opportunity to smoke/drink, as well as a feeling of obligation to do what they could for their own health or to please family or HCPs who were ‘invested’ in the outcome of their surgery.
Despite the limited research in perioperative care, trials of BI for alcohol reduction and cessation in other contexts have assessed the acceptability of methods that may inform work in PA. Qualitative studies in other health-care settings support the findings of Pedersen et al. 104 and Lauridsen et al. ,105 with patients and staff expressing generally positive attitudes towards alcohol screening and intervention (HCPs106,107 and patients108,109). However, they have also identified a number of barriers to alcohol screening and intervention in health-care settings. These barriers include logistical issues, having the necessary alcohol knowledge and having the required training and skills. 106,110
In combination, findings from these qualitative works suggest that alcohol screening and intervention is likely to be acceptable to patients, and that surgery may increase the salience of health behaviour change and facilitate change in the short term. However, there is a need for further exploration, and overcoming or addressing logistical barriers will be important to ensure effective implementation.
Outcomes of interest for trials of preoperative alcohol interventions
With previous research having identified that alcohol consumption in the preoperative period is associated with increased risk of postoperative complications, interventions leading to alcohol reduction or cessation in the preoperative period may reduce postoperative complications and improve outcomes. Therefore, the effectiveness of a preoperative alcohol intervention would be judged initially in terms of changes in alcohol consumption behaviour and, later, by any resultant reduction in complications or improvements in outcomes. A trial of a preoperative alcohol intervention should therefore include measures of alcohol consumption, complications and condition-specific as well as general health outcomes.
As detailed in Background, the AUDIT-C and full AUDIT tools are frequently used to screen for different patterns of drinking and to assess changes in drinking behaviour.
Regarding postoperative complications, two measures are frequently used in health-care settings: the Post Operative Morbidity Survey (POMS),111 which collects data on minor complications related to postoperative pathways, and the Clavien–Dindo tool,112 which classifies the presence of more significant postoperative complications.
Specific to the disease process requiring orthopaedic surgical intervention is the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC),113 which aims to evaluate the functional ability of patients with knee and hip osteoarthritis and includes items related to pain, stiffness and physical functioning of the affected joints. In addition, broader patient-reported health outcomes may be of interest. These are frequently assessed with quality-of-life114 questionnaires, such as the EuroQol-5 Dimensions (EQ-5D). 114,115
Characterising treatment as usual in preoperative assessment
In a setting where alcohol consumption is routinely assessed as part of TAU, it is important to clearly document the characteristics of TAU to allow accurate comparisons between that and any intervention delivered, and to ascertain whether or not the proposed intervention is in fact sufficiently different from TAU. A national survey116 of consultant anaesthetists involved in perioperative care found that the majority of PACs involve some form of alcohol screening, with almost half having alcohol intervention services available. However, the survey provided little detail of screening approach, or of the form and content of any interventions available. Although anaesthetists play a key role in leading PA units, the majority of PAs are conducted by nurses, who are therefore likely to be better placed to provide insight into the delivery of alcohol screening and intervention in PA.
Conclusions
Preoperative alcohol consumption is firmly associated with postoperative complications and poorer surgical outcomes and represents a modifiable risk factor suitable to be addressed using an appropriate presurgical intervention. Evidence exists to support the effectiveness of intensive interventions, combining pharmacological and behavioural aspects, in bringing about abstinence in heavy and dependent drinkers and leading to reduced rates of postoperative complications. 64,72–74 However, these methods are unlikely to be either appropriate or acceptable for the majority of increased risk drinkers, who represent a greater proportion of the surgical patient population. Although there is evidence for the effectiveness of BIs in other settings (e.g. primary care, emergency care), less is known about the use of such methods in the preoperative period. Studies employing preoperative behavioural interventions have been confounded by several methodological issues, including:
-
the use of multimodal interventions targeting a number of health behaviours98,100,101
-
poor reporting of the uptake, content and delivery98,100,101 of interventions
-
small numbers of risky drinkers in the overall trial population101
-
delivery of interventions close to the date of surgery, allowing little time for behaviour change95
-
the use of non-randomised study designs. 95
Given these limitations, drawing firm conclusions about the effectiveness of any intervention to reduce or cease alcohol consumption in the perioperative period is difficult, and further high-quality RCTs are required. In addition, with a number of the studies conducted to date having also identified issues with recruitment and having limited data regarding the acceptability of preoperative alcohol screening and intervention, it is important to explore the acceptability of proposed screening, recruitment and intervention methods with participants in order to optimise both research and intervention methods before seeking to assess effectiveness.
Aim and rationale
Summary of rationale
Brief behavioural interventions are effective in reducing alcohol consumption among increasing risk and risky drinkers and have been demonstrated to be acceptable to patients and HCPs in several clinical settings.
Upcoming major surgery provides an opportunity to deliver a BBI for reducing preoperative alcohol consumption. Reducing risky drinking preoperatively may have immediate benefits on short-term clinical outcomes after surgery. Sustained reductions in alcohol consumption are likely to support longer-term recovery, as alcohol has immunosuppressant effects. Furthermore, any such sustained changes would also decrease alcohol-related risk to future well-being in a predominantly older, orthopaedic population.
Aim
The overall aim of the project was to establish if a definitive trial of a BBI to reduce drinking prior to elective orthopaedic surgery is feasible.
Three overarching objectives reflected three sections of work, each of which had its own secondary objectives:
-
Characterise the intervention and comparator (TAU) conditions.
-
– Train HCPs to deliver structured screening and BBI to eligible patients in the PA setting.
-
– Assess fidelity of delivery of the BBI.
-
– Characterise TAU in the PAC for elective orthopaedic surgery at the three pilot RCT sites using focus groups.
-
– Characterise TAU for elective orthopaedic surgery at PACs nationally using a quantitative electronic survey.
-
-
Assess the feasibility and acceptability of consent, screening, intervention and outcome assessment procedures.
-
– Optimise the screening and behavioural intervention process in a way that nurses and patients find acceptable.
-
-
Estimate rates of eligibility, recruitment and retention in a pilot RCT.
-
– Assess completion rates for all data collection tools, including measures of alcohol consumption, quality of life and joint functionality.
-
– Establish response variability of proposed outcome measures for a definitive trial, which will include drinking status and quality of life.
-
– Estimate rates of secondary outcomes and perioperative complication rates, including bleeding and infections.
-
– Explore the acceptability of intervention and research methods with HCPs and patients through qualitative interviews.
-
Patient, public and professional involvement
There was patient, public and professional involvement at all stages of the study from inception to completion.
Prior to commencement of the study, formative work conducted at the primary site provided initial estimates of screening uptake and eligibility based on an AUDIT score of ≥ 8.
Prior to commencing the feasibility study, all patient materials were reviewed by members of a local patient and public involvement group, Voice North: (www.ncl.ac.uk/ageing/partners/internal_ageing/voice/; accessed 4 February 2019). Comments were used to inform amendments to the materials before they were submitted for relevant approvals.
Three members of this group expressed an interest in being involved in the Trial Steering Committee (TSC) and attended meetings during the research programme. When these members were unable to attend meetings they were sent a copy of the meeting minutes and asked to provide comments. Further to this, a member of the research team (EL) met with lay members to discuss proposed changes between the feasibility study and the pilot RCT phase to ensure that these were considered acceptable.
During the feasibility study, anecdotal feedback from researchers and HCPs involved in the screening and recruitment of patient participants was collected. With initial rates of recruitment being lower than anticipated, this information was used to inform the amendments made to the feasibility study processes. The proposed changes were then discussed with the lay members of the TSC before being submitted for approval.
Prior to commencing the feasibility study, researchers engaged with HCPs employed in PA to inform the design of the training session. The HCPs completed questionnaires identifying their training needs and the acceptability of the proposed training methods. HCPs were also asked to complete feedback questionnaires after the group training session was delivered.
Prior to commencing the pilot RCT, HCPs involved in pre-assessment at each of the three trial sites were invited to a launch meeting, where they had the opportunity to discuss the pilot RCT and consider how the research processes would be implemented at each site. Members of the research team also met with pre-assessment leads from each site. These meetings led to the decision to allow either face-to-face screening or postal invitations followed by telephone screening to minimise the impact of extended waiting times between listing for surgery and attendance at the PAC on recruitment times. With HCPs also explaining that some patients would attend satellite hospitals for follow-up appointments, the option to conduct follow-up visits over the telephone was also included in the pilot RCT.
Chapter 2 Defining the intervention and comparator
Introduction
When considering behavioural interventions to target alcohol consumption in the preoperative setting, a key limitation of the literature is the lack of information on intervention content. 99 This prevents intervention replication117 and further exploration of what makes an intervention effective. Furthermore, fidelity of intervention delivery has not been assessed in the trials reporting on behavioural interventions in the preoperative period. 99 This makes it difficult to determine whether or not the intervention was delivered as planned and, therefore, has implications for the assessment and interpretation of intervention effectiveness. Recent reviews of alcohol interventions further highlight the importance of clear reporting of intervention content. 75,118
The field of behavioural science offers a precise and systematic approach to reporting on the content of behavioural interventions. Specifically, the Behaviour Change Technique (BCT) Taxonomy v1119 provides standardised definitions of BCTs to make reporting more consistent across disciplines, allowing comparisons of interventions to be made. Accordingly, in the current study, the behavioural intervention delivered was described in terms of behavioural components (i.e. BCTs) and fidelity of delivery was assessed in terms of whether or not specific BCTs were delivered.
Although existing screening and intervention training packages are available, these are not specific to the PA setting. For this reason, adaptations were made to an existing package to provide HCPs with the knowledge and skills they required to deliver the screening and intervention during routine clinics.
This chapter provides an overview of the development of the intervention that was then delivered during the feasibility study and pilot RCT.
Aims
-
Train HCPs to deliver structured screening and BBI to eligible patients in the PA setting.
-
Assess fidelity of delivery of the BBI.
-
Characterise TAU in the PAC for elective orthopaedic surgery at the three pilot RCT sites using focus groups.
-
Characterise TAU for elective orthopaedic surgery at PACs nationally using a quantitative electronic survey.
Optimisation of screening and behavioural intervention process
Screening tool selection
Both the AUDIT67 and the AUDIT-C62 have been used in studies in PA to report on the association of alcohol consumption with postoperative complication rates, and they have shown good patient acceptability. 57,62,68 Therefore, either or both of these tools were considered appropriate for the current study.
Qualitative evidence92 has identified time and burden of new processes as key barriers to the implementation of alcohol screening and BI, and findings from alcohol screening studies conducted in PA have reported that the majority of patients drink at ‘safe’ or ‘sensible’ drinking levels. 62,103 Therefore, it is appropriate to use a short initial screen (AUDIT-C) to identify potentially eligible patients, with the longer full AUDIT being used to inform intervention delivery and, potentially, the tailoring of intervention content.
Optimisation of the intervention tools
It is appropriate to adopt and adapt an intervention package that has demonstrated effectiveness in other health-care settings. 75 The ‘How much is too much? Simple Structured Advice’ and the ‘How much is too much? Extended Brief Intervention’ tools, originally developed by the World Health Organization for use in a collaborative study on alcohol screening and BI,120 later revised for use in the UK as part of the Drink-Less Brief Intervention programme121 and subsequently updated and utilised in the Screening and Intervention Programme for Sensible Drinking (SIPS),84 were adopted to guide the BBI. In addition, the Department of Health and Social Care’s ‘How much is too much?’ booklet122 was used as a leaflet for patients to motivate and facilitate behaviour change after the session.
The most recent iteration of these intervention materials was reviewed by three members of the project team (EL, LA and CH) and revised in accordance with developments in alcohol intervention research and to maximise integration and acceptance among the orthopaedic surgical population and in the preoperative setting. The following amendments were made:
-
The term ‘unit’ was replaced with ‘standard drink’ (standard drink = 1 unit = 8 g of pure alcohol) in accordance with current research and international agreement.
-
Additional drink icons were included to indicate the alcohol content of standard drinks.
-
The terms sensible, hazardous and harmful drinking were replaced with low, increased and high risk, respectively, to reflect the updated Department of Health and Social Care terminology.
-
The graphic displaying normative drinking data was amended to show risk categories in place of alcohol disorders.
-
Information relating to the common effects of alcohol use and the benefits of ‘cutting down’ was tailored to fit the pre-surgical population by including additional information about the relationship between alcohol use and immune function, recovery time and postoperative complications.
-
The category of ‘sensible drinking’ was amended to include one single recommendation for both male and female drinkers, in line with updated guidelines.
-
Information on avoiding alcohol when pregnant was removed as patients who were pregnant would not undergo this type of elective surgical procedure.
-
Information about risks relating to alcohol consumption and the potential benefits of reducing drinking was reordered so that the items most relevant to surgery appeared first (e.g. risk of complications, potential for weight loss), followed by those most relevant to older patient populations.
The revised intervention materials were then circulated to members of the patient and public involvement group Voice North for their consideration and feedback. Comments on the patient booklet were reviewed, summarised and responded to. Changes made were as follows:
-
Addition of ‘when you get bad news’ to the list of examples of difficult times.
-
Replacement of the previous logo with a project-specific logo.
-
Specification that goal-setting and behavioural monitoring would work only if individuals were honest about their consumption.
-
Clarification that the amount of alcohol in a drink depends on the size and strength of the drink.
-
Rewording of the definition of a small glass of wine from ‘8–10% alcohol by volume in a 125 ml glass’ to ‘a 125 ml glass of wine (8–10% alcohol by volume)’.
No comments on the simple structured advice or extended BI tools were received, so no further amendments were made to these. For the purposes of this study, and to avoid confusion with previous iterations, the tools were renamed as follows:
-
BA tool – formerly the ‘How much is too much? Simple Structured Advice’ tool
-
BI tool – formerly the ‘How much is too much? Extended Brief Intervention’ tool
-
patient leaflet – formerly the Department of Health and Social Care’s ‘How much is too much?’ booklet.
Once changes had been made in response to comments, the content of the intervention tools was coded in terms of BCTs (see Appendix 1).
Description of intervention delivery
The AUDIT-C, with a cut-off point of 5, was employed as an initial screen to identify potentially eligible patients. After patients provided informed consent to participate, the full AUDIT, with a cut-off point of 8, was used to assess baseline alcohol use and identify those eligible to receive the intervention. The intervention was designed to be delivered in up to two sessions, the first to be delivered face to face in the PAC. The second, an optional booster, could be delivered either over the telephone or face to face in the PAC (as per patient preference) approximately 1 week before surgery. The screening and intervention tools were used to guide the content of the sessions, and HCPs were also trained in the use of brief motivational techniques to enhance motivation to change, which helped guide the style of delivery.
The first session lasted up to 30 minutes and was delivered following completion of the full AUDIT questionnaire. The outcome of AUDIT was used to provide patients with their screening score and feedback in relation to this (i.e. whether this related to low- or high-risk drinking behaviour). This was followed by 5 minutes of structured BA using the BA tool. The BA delivered was designed to be tailored to individual patients. As such, patients were asked about existing knowledge of alcohol consumption and its effects to avoid the delivery of information that might not be relevant or appropriate. BA could include information about the lower-risk drinking guidelines, explanation of the term ‘standard drink’, normative comparison of drinking behaviour, feedback on risk including risk of postoperative complication, the potential benefits of reducing alcohol consumption and strategies that could be used to reduce drinking (e.g. goal-setting). This component of the intervention targeted motivation by providing information to increase intention to make behavioural changes. The remainder of the intervention session (15–20 minutes) involved the delivery of a behaviour change intervention that aimed to target volition (i.e. enactment of behaviour change). This component of the intervention could cover assessment of motivation and confidence to change drinking behaviour; identification of the pros and cons of changing drinking behaviour; goal-setting, problem-solving and action planning; and identification of sources of social support. Patient participants were given copies of the BA and BI tools, as well as a copy of the patient leaflet.
As a follow-up to the intervention described above, patients were offered an optional booster session. Patient participants either consented or declined to be contacted about the booster session during initial informed consent. Those who consented were contacted approximately 1 week before surgery, when their interest in the booster session was confirmed and a suitable date and time to receive the booster session was arranged. The session could be delivered over the telephone or face to face in the PAC as per patient preference. The booster session commenced with patients completing the full AUDIT questionnaire to facilitate a review of their progress since delivery of the initial intervention. For patients who had previously set goals, these could be reviewed and revised if necessary. For those who had not yet set goals or made action plans, this session provided an opportunity to do so. The session aimed to prompt behaviour change maintenance for those who had made changes and increase self-efficacy with a view to making changes for those who had not.
The finalised BBI is summarised in Table 1.
| Item | Details |
|---|---|
| Name | Preoperative Behavioural Intervention to Reduce Drinking before elective orthopaedic Surgery (Preop BIRDS) |
| Why? | Preoperative alcohol consumption is related to increased risk of postoperative complications. The aim of the intervention is to support patients to reduce or cease alcohol consumption prior to elective orthopaedic surgery |
| What? |
Materials: HCPs are trained to screen for increased risk alcohol consumption and to deliver the following behavioural intervention materials – BA tool, BI tool, patient leaflet Training also covers use of brief motivational techniques to increase motivation for change. Training of HCPs is supported by a training manual Procedures: the intervention is delivered over two sessions (the second is optional). The first session involves provision of 5 minutes of structured advice that aims to increase motivation via use of a ‘BA sheet’. This is followed by delivery of a behaviour change intervention lasting 15–25 minutes using the BI sheet. This intervention targets volitional aspects of behaviour change. The aim of the second optional booster session is to review and/or revise behavioural goals, provide feedback on performance and discuss self-monitoring to increase self-efficacy. This session is also designed to allow those individuals who have shown an initial intention to make changes, but who have not formally set behavioural goal(s) and made plans, to do so if desired |
| Who provided? | HCPs employed in the PACs who received training in the delivery of screening and BBI |
| How? | The initial intervention session is delivered face to face during routine clinics. The second session, an optional booster, is delivered either face to face in clinic or over the telephone, depending on patient preference. All intervention sessions are delivered one to one |
| Where? | Intervention sessions are delivered during routine PACs. Where the patient opts to receive a booster session over the telephone, the HCP delivering the session calls from the PAC |
| When and how much? | The first session involving delivery of BA and BBI lasts approximately 30 minutes and is delivered during routine PACs once all clinical procedures have been completed. The second optional ‘booster’ session lasts approximately 20 minutes and is delivered around 1 week before surgery |
| Tailoring | Intervention materials incorporate specific BCTs that target intention formation and enactment of behaviour change (e.g. information on health consequences, social support, goal-setting behaviour, problem-solving, restructuring the physical environment). HCPs are trained to use these techniques to tailor the intervention to the needs and preferences of individual patients, for example providing information relevant to and requested by the patient and supporting them to set meaningful and realistic goals that fit in with their own specific circumstances. Brief motivational techniques are used by HCPs allow them to determine level of motivation to change and tailor the intervention to target motivation or volition at the appropriate times |
| How well? | Consultations with participating patients are audio-recorded to allow an assessment of skill acquisition and fidelity of delivery of the intervention post training. The aim is to improve fidelity of delivery by providing feedback to HCPs including aspects of intervention delivery that went well and where they could improve. A member of the research team provided feedback to each HCP following delivery of intervention sessions 2 and 4 |
Training development
The HCPs employed in PA at the primary site were involved in the development of the training to make sure it met their needs. The training was developed in accordance with the Behaviour Change Wheel123 and the various components were selected based on a needs assessment using the Capability, Opportunity and Motivation to perform a particular Behaviour (COM-B)124 and Theoretical Domains Framework. 125 The BCT Taxonomy version 1119 was then used to help select BCTs to help operationalise the theory within the training.
The HCPs were asked to complete an adapted version of the COM–B self-evaluation questionnaire (see Appendix 2) containing 19 items designed to assess existing levels of COM–B, in this case to deliver alcohol screening and behavioural intervention to patients. They were asked to assess what they felt needed to change for that behaviour to occur. Tick boxes were provided for each question, with free-text boxes to collect additional information and context. Twelve HCPs completed and returned questionnaires (see Appendix 3 for a summary of responses). Assessment of questionnaire responses identified that HCPs required support to increase all three domains (capability, opportunity, motivation). Having an understanding of why alcohol reduction or cessation was important in the preoperative period was seen as key to enhancing HCPs’ motivation to deliver a screening and behavioural intervention, while also providing them with the capability to motivate patients to change their behaviour.
To enhance capability, HCPs reported that they needed training in the BBI and that this should include a clear plan of how to deliver it. In addition, HCPs requested supporting materials to facilitate intervention delivery that would include visual aids to help communicate information. The need for adequate ‘protected’ time in their appointment schedule to facilitate delivery was emphasised, as was knowing that all colleagues would work together to facilitate delivery of the intervention.
The TDF125 was used to identify potential candidate techniques to address HCPs’ training needs. The provision of education, training and modelling, along with the use of persuasion, incentivisation, and enablement, was identified as a potential component of the training package. To determine the acceptability of these methods to HCPs, the lead nurse from the primary site completed an amended version of the APEASE questionnaire (see Appendix 4), which assessed the anticipated affordability, practicability, effectiveness, acceptability, safety and equity of these techniques. All suggested techniques were found to be acceptable.
These techniques were subsequently used to guide the selection of training content in terms of BCTs. 119 A draft training strategy describing how these BCTs would be delivered was developed and then expanded into a 2-hour face-to-face group training session with a supporting manual.
Training content
The group training sessions included the following components: education about alcohol consumption (i.e. recommended limits and accepted terms and definitions) and surgical outcomes; the potential benefits of delivering a BI for alcohol reduction/cessation during PAs (BCT – education about health consequences, information about social and environmental consequences, credible source); instructions on how to perform alcohol screening and intervention with demonstration videos (BCT – instructions on how to perform the behaviour, demonstration of behaviour); role-play activities to practice intervention delivery (BCT – behavioural practice/rehearsal, feedback on behaviour); brainstorming of potential barriers to delivery and formation of plans to address these (BCT – problem-solving); and a quiz and prize to test nurses’ knowledge (BCT – material incentives). To allow tailoring of the intervention to the needs and preferences of individual patients, HCPs were trained to identify and use the BCTs embedded in the intervention tools, for example providing information relevant to and requested by the patient and supporting them to set meaningful and realistic goals. In addition, the training covered the use of brief motivational techniques to allow HCPs to determine the level of motivation to change and tailor the intervention to target motivation or volition at the appropriate times.
Training delivery
During the feasibility study, the training was delivered as a group session led by three psychologists (EL, LA and Sebastian Potthoff), each of whom covered different aspects of the training based on their own areas of expertise and experience. Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) slides and video recordings of intervention delivery were used as visual aids during the training sessions. A training manual was also given to each staff member who attended the session alongside a copy of the screening and behavioural intervention tools.
When new staff members joined the study, additional training sessions were delivered by one of the psychologists (EL).
Evaluation of the training
The HCPs were asked to complete an evaluation form (see Appendix 5) on completion of the training. This asked them to indicate how strongly they agreed or disagreed with 15 statements relating to the delivery and content of the training as well as knowledge and skills gained. Responses were on a scale from 1 (strongly agree) to 5 (strongly disagree). Five of the 10 HCPs who attended completed the evaluation form. Feedback indicated that the training was enjoyable and well structured and that the content was appropriate, with adequate time allocated for questions and discussion. Although the training was considered beneficial for the delivery of screening and behavioural intervention during the study, it was not rated as beneficial for clinical practice more generally.
Fidelity of delivery: feasibility study
Fidelity of delivery was assessed during the feasibility study; the findings were used to document whether or not interventions were being delivered as intended and to inform further refinement of the intervention and training package. To facilitate skill acquisition and support effective delivery of interventions by HCPs, written feedback was provided on the initial intervention sessions delivered by each HCP. During the feasibility study, feedback was provided on sessions 1 and 4. However, following feedback from HCPs, this was amended to sessions 2 and 4 for the pilot RCT.
All intervention sessions were audio-recorded when the patient had provided consent for this. Recordings were transcribed verbatim and anonymised. Transcripts were read and independently coded by two members of the research team (EL and LA). Where applicable, feedback on skill acquisition and fidelity of delivery was constructed by the same two researchers (EL and LA). When fidelity of delivery was > 80%, written feedback was provided to the HCP. When fidelity of delivery was < 80%, the research associate (EL) contacted the HCP to arrange an additional, one-to-one, face-to-face feedback and training session, lasting up to 1 hour, to focus on components of the intervention that had been omitted when it might have been appropriate to deliver them.
When patient participants in sessions 1 and/or 4 did not consent to audio-recording, fidelity of delivery assessments were conducted and feedback was provided for the next recorded session.
Fidelity of delivery was assessed using a standardised checklist of 18 BCTs (those present in the intervention materials; see Appendix 1). As interventions were designed to be tailored, coders first rated which BCTs and counselling techniques were delivered before assessing whether or not they had been delivered appropriately (i.e. were BCTs delivered that best met the needs of the patient). This was to determine whether or not any BCTs had been delivered simply because they formed part of the intervention.
Of the 10 HCPs who consented to take part in the study, four went on to deliver intervention sessions. Staff allocation to each intervention varied based on shift patterns and availability. A total of nine initial intervention sessions were delivered during the study, with seven of these audio-recorded and assessed for fidelity of delivery.
All intervention sessions included delivery of BA, BI and the patient leaflet. Delivery of 9 of the 18 possible BCTs was coded as occurring. Specific content varied between sessions indicating tailoring of interventions. The most consistently delivered BCTs were information about health consequences (5.1), goal-setting behaviour (1.1), social support (unspecified) (3.1) and pros and cons (9.2).
Fidelity of delivery was low, with 20–50% (mean = 40%) of BCTs considered to be appropriate being successfully delivered. However, there were clear indications of attempts to deliver additional BCTs but, as delivery did not fully meet the definitions in the BCT Taxonomy v1, these were not coded.
Optimisation of the training package
Intervention and interview transcripts generated during the feasibility study were reviewed by three members of the project team in (EL, LA and CH) to identify barriers to effective intervention delivery and to inform revision of the training, screening and intervention package ahead of delivery during the pilot RCT. Barriers identified and addressed are detailed in Table 2.
| Barrier | Explanation | Addressed |
|---|---|---|
| Time lag between training and intervention | A substantial period of time elapsed between training and commencement of intervention delivery | Training in the pilot RCT phase to be delivered as close as possible to the commencement of screening and recruitment without risking delays to the project |
| Infrequent intervention delivery | With only a small number of patient participants recruited in the feasibility trial and consent rates fluctuating, intervention delivery occurred infrequently over a fairly extended period | The pilot RCT to include a greater number of patients and resolution of issues with recruitment during feasibility trial, which should lead to more consistent recruitment rates in the pilot RCT |
| Adequate time to deliver intervention | HCPs felt that they sometimes had to ‘make time’ to deliver the intervention and could be pressured if the clinic was not running to time or if other patients arrived either late or early for their appointments | Extended appointments to be arranged for research participants, with the participant attending ahead of their PA appointment to complete consent and randomisation processes and additional time for intervention delivery allocated. Each site in the pilot RCT to have a HCA available to aid facilitation of the intervention sessions |
| Identifying level of motivation | HCPs were not always able to identify how motivated patients were to change | Instruction on how to use the questions exploring motivation to change and confidence in changing behaviour, which appear in the BI tool, to identify how motivated patients are to change their behaviour. During training, explicitly identify skills HCPs already use that will help them to identify level of motivation, specifically ‘listen, ask, think’ |
| Tailoring intervention content to level of motivation | HCPs were aware that they should tailor the interventions but intervention transcripts showed they were not always successful in tailoring content to level of motivation | Additional educational aspect in the training to explain intervention aspects relevant to motivating patients or targeting volition. Addressed in expanded ‘action-planning’ section of the training |
| Tailoring screening and interventions to address screening reactivity | HCPs were not clear about how to tailor intervention sessions or how best to complete AUDIT questionnaires when patient participants had changed their drinking between initial screening and intervention delivery | Be explicit in training that initial AUDIT is concerned with drinking over the past 6 months. Include time scale for each AUDIT questionnaire on the questionnaire. Address this in the training session along with tailoring of the intervention to level of motivation |
| Delivery of BCTs attempted but not successful | BCTs ‘social support’, ‘goal-setting’ and ‘problem-solving’ were frequently attempted but not successfully delivered during interventions | Additional instruction provided on delivery of these BCTs. These examples were targeted in expanded coping planning section of the training |
Pilot randomised controlled trial training delivery
During the pilot RCT, training was delivered as group sessions led by two or three psychologists (EL, LA, and Sebastian Potthoff) (depending on availability). Training was delivered in two formats. Staff at the primary centre who had already been involved in the feasibility study received a shorter refresher version, covering aspects of the training that had been revised after the feasibility study and including revised project aims and objectives. The staff who had not participated in the feasibility trial (predominantly those from the two additional centres) received a longer training session that provided a more comprehensive introduction to the project and information about the use of the screening and intervention package before covering areas that had not translated well from training to delivery at the primary centre in the feasibility study. As with the feasibility study, additional training sessions were arranged and delivered by Ellen Lynch if required.
Pilot randomised controlled trial intervention fidelity of delivery
Of the 11 HCPs who consented to participate in the pilot RCT, nine went on to deliver at least one intervention session. One HCP conducted screening only.
During the pilot RCT, HCPs delivering the intervention sessions were asked to record whether or not the patient attended the intervention session, which aspects of the intervention were delivered and whether or not the patient set any behavioural goals. These details are provided in Table 3 and Box 1.
| Intervention aspect | Site | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| n | % | n | % | n | % | n | % | |
| Attended intervention | 8 | 100 | 16 | 94.1 | 8 | 100 | 32 | 97.0 |
| Consented to audio-recording | 19 | 100 | 28 | 82.4 | 11 | 73.3 | 58 | 85.3 |
| BA delivered | 8 | 100 | 16 | 100 | 8 | 100 | 32 | 100 |
| BI delivered | 8 | 100 | 16 | 100 | 8 | 100 | 32 | 100 |
| Patient leaflet given | 8 | 100 | 17 | 100 | 8 | 100 | 33 | 100 |
| Goals set | 4 | 66.7 | 15 | 88.4 | 8 | 100 | 27 | 87.1 |
Health, does not want to gain weight as won’t be as active, better recovery time and save money.
Weight, save money, feel better in the morning.
Healthy weight and for surgery.
Weight management and healthy lifestyle.
More money better outcome to reduce the risk of any complications in surgery and to reduce recovery time.
Volitional goalsSwap non-alcoholic drinks for usual drinks and leave environment for drinking (match day/pool nights).
Reduce drinking, at home and to start from today.
Reduce drinking from now and to try and stop drinking altogether.
Stop drinking for 2 days a week and to reduce intake by a third.
To reduce from seven pints per day to four by the surgery day.
To reduce intake to 10 units 1 week for surgery.
Try and reduce alcohol intake.
Reduce alcohol intake.
Reduce number of units of alcohol.
To still have a reduced intake of alcohol.
OtherPatient will see how it goes after intervention session.
Of the 33 patient participants allocated to the intervention arm, 32 attended the initial session and received BA, BBI and the patient leaflet. The remaining patient participant received the patient leaflet only. Twenty-seven of these participants also set goals, details of which were recorded for 16 patient participants and are provided in Box 1. The level of detail included in the goals varied. Goals can be broadly divided into two categories: motivationally focused goals, concerning the possible benefits of reduced alcohol consumption; and volitionally focused goals, which centred on methods of reducing alcohol consumption.
Patient participants were also asked to consent to their intervention session being audio-recorded to allow a fidelity of delivery assessment to be made. More than half of participants who consented to take part in the trial also consented to audio-recording of the session, and 17 of the intervention sessions delivered were audio-recorded (as patients consented to audio-recording before randomisation, the number consenting to recording exceeds the number allocated to receive the intervention).
As with the feasibility study, all audio-recorded sessions were transcribed verbatim and transcripts were assessed for fidelity of delivery. Written feedback was provided to HCPs based on early sessions (2 and 4) to facilitate skill acquisition and support effective intervention delivery. When patient participants in sessions 2 and/or 4 did not consent to audio-recording, feedback was provided for the next recorded session. The same standardised checklist developed during the feasibility study was used to code intervention content, and all transcripts were coded independently by two researchers (EL and LA).
Fidelity of delivery improved from the feasibility study to the pilot RCT (from 40% to 65.9%), with evidence of delivery of 13 out of 18 possible BCTs identified in coding and an average of 65.9% of BCTs considered to have been appropriately delivered. Specific content varied between sessions, indicating tailoring of interventions, but fidelity of delivery also varied among staff members, suggesting different levels of skill acquisition from training.
The BCTs that targeted motivation were most frequently coded as both appropriate and delivered. Specifically, those involving informing participants about the health, social and emotional consequences of alcohol consumption (5.1, 5.3, 5.6) were coded most frequently. Feedback on behaviour (2.2) in the form of an alcohol screening score was successfully delivered in the majority of sessions; however, feedback on the outcomes of behaviour (2.7), which would have been appropriate in all sessions, was never successfully delivered.
Delivery of BCTs that targeted volition (i.e. enactment of behaviour change) generated a less consistent picture: goal-setting (1.1) and social support unspecified (3.1) were frequently utilised/delivered, whereas pros and cons (9.2) and problem-solving (1.2) were frequently attempted but less often delivered successfully. Some volitionally focused BCTs were also those most frequently rated as not appropriate [restructuring the social environment (12.2), reducing exposure to cues for behaviour (12.3), behaviour substitution (8.2)], although motivationally focused social comparison (6.2) also fell into this grouping.
Characterising treatment as usual
This chapter concludes by reporting on a series of focus groups (n = 3 groups) and an electronic survey that were utilised to characterise ‘TAU’ in respect of screening and intervention for alcohol consumption in the context of PA for hip and knee arthroplasty.
Methods
A mixed-methods approach was adopted:
-
During the feasibility study, focus groups were used to provide in-depth descriptions of PA for hip and knee arthroplasty in the three centres included in the pilot RCT.
-
During the pilot RCT, a quantitative survey was used to gather data relating to alcohol screening and intervention in PACs across the UK.
Focus group methods
The HCPs were eligible to participate in focus group sessions if they were currently involved in the orthopaedic patient pathway in one of the three centres identified to take part in the pilot RCT. It should be noted that focus groups were held during the feasibility study recruitment period. As such, some HCPs from the primary site were already familiar with the project and a smaller number had been involved in screening and intervention delivery.
Potential participants were sent an e-mail inviting them to participate in one of the focus groups (one was held at each of the three sites). The HCPs who replied to indicate their intention to attend the sessions were sent an electronic version of the focus group information sheet (see Appendix 6) at least 24 hours in advance of the session.
Focus groups were conducted by the research associate (EL), who greeted each participant on arrival and provided hard copies of the information sheet for further consideration. Before the focus group discussion commenced she introduced herself to the group, providing a description of her research background and qualifications before giving a verbal introduction to the project and her role. As a result of her role in the feasibility study, the associate was already known to some HCPs from the primary site where she had been involved in training delivery and intervention feedback.
This personal introduction was followed by an explanation of the purpose of the focus group session (to establish TAU), ethics considerations and ground rules for the discussion. Participants then had the opportunity to ask any questions and to suggest any of their own ground rules for the session. Once all questions had been answered, participants completed informed consent forms (see Appendix 7), which included consent for the session to be audio-recorded.
Discussion commenced by asking the HCPs to introduce themselves and state their job role. The research associate then used a semistructured topic guide (see Appendix 8) to guide discussion to cover the preoperative care pathway, alcohol screening, alcohol intervention and referral, perceptions of the current approach to alcohol screening, previous experience of intervention delivery and acceptability of preoperative alcohol interventions.
Two hours were allocated per focus group, with the discussion itself lasting between 50 minutes and 1.5 hours.
All focus group recordings were transcribed verbatim and subject to framework analysis,126 with a deductive approach adopted to focus on the characteristics of the ‘TAU’ condition. All transcripts were dual coded, with codes discussed and agreed periodically.
Survey methods
An electronic web-based survey was used to gather data from PA staff nationally regarding TAU, with a specific focus on alcohol screening and intervention in PA.
The electronic survey included 20 questions (see Appendix 9) that collected demographic details (age and gender) brief information about job role and experience (current job role, time employed in current role, total time employed in PA) and basic information about preoperative appointment length before proceeding to explore the current approach to alcohol screening and intervention, with fixed-response questions asking participants to indicate if alcohol screening, intervention and referral were conducted as part of PA, what form these took and how patients were identified for information provision or referral. Finally, participants were asked to indicate any previous or current involvement in intervention delivery for a number of health behaviours (alcohol, smoking, physical activity, diet or nutrition). Free-text response boxes were provided for participants to provide additional detail if they chose.
The link to the electronic survey was circulated to informal groups of PA HCPs, predominantly nurses and health-care assistants (HCAs), via an e-mail mailing list (n = 22 recipients) and a social media group (n = 98 members). On following the survey link, potential participants were presented with an electronic version of the survey information sheet. The next screen presented an electronic ‘tick box’ consent form, which asked participants to indicate their agreement to each point on the consent form. Participants had to agree to each statement before being able to proceed with the survey. The subsequent screens presented the survey questions, with branching logic applied to ensure that subquestions (e.g. detail of alcohol interventions) were displayed only if applicable. At the end of the survey, participants had the option to either submit or discard their responses. Finally, an electronic thank-you and debrief screen was presented. The survey took an average of 6 minutes to complete.
Data were subject to descriptive analysis only with frequency of each response category reported.
Results
Focus group results
A total of 19 HCPs, ranging from band 5 nurse to consultant anaesthetist, took part in the focus groups; all were female (Table 4).
| Group | Participant | Job role | Site |
|---|---|---|---|
| 1 | 1 | PA nurse band 6 | 1 |
| 2 | PA nurse band 6 | 1 | |
| 3 | Student nurse | 1 | |
| 4 | PA nurse band 6 | 3 | |
| 5 | PA lead nurse | 3 | |
| 6 | Substance use lead | 1 | |
| 7 | PA nurse band 5 | 1 | |
| 2 | 8 | PA lead nurse | 2 |
| 9 | PA sister | 2 | |
| 10 | PA sister | 2 | |
| 11 | PA sister | 2 | |
| 12 | PA sister | 2 | |
| 3 | 13 | Consultant anaesthetist | 3 |
| 14 | PA lead | 3 | |
| 15 | PA nurse band 6 | 3 | |
| 16 | PA team leader | 3 | |
| 17 | PA nurse band 5 | 3 | |
| 18 | PA nurse band 6 | 3 | |
| 19 | PA nurse band 5 | 3 |
Framework analysis of focus group transcripts revealed five key themes relating to TAU in PA for hip and knee arthroplasty.
It’s a big and busy appointment
The PA appointment for hip and knee arthroplasty was described as lasting 45 minutes at one site and 1 hour at the other two sites. However, staff identified that this could vary if patients were seen as ‘walk rounds’ immediately following listing for surgery where appointment length was seen to be dictated by ‘supply and demand’. The HCPs identified a variety of tasks, tests and assessments that needed to be completed as part of PA for primary hip and knee arthroplasty. This included gathering information about health and social history as well as assessing a variety of physiological systems and considering medication management:
. . . you start off doing it’s like a ‘major form’ that we use for pre-assessment of orthopaedics if it’s a hip or a knee erm so you start off like with height, weight and obs and you do bloods and you do breathing tests and you do an ECG [electrocardiogram] and you do a full medical questionnaire, look at their drugs erm and ask them all sorts of questions . . .
ID 1, PA nurse band 6, site 1
The HCPs expanded on this to explain that although the PA appointment was a demanding one for them, the burden on the patient was higher still, with a number of additional forms and assessments. Further to this, patients also have to see a number of HCPs, including occupational and physiotherapists, as well as the PA nurse:
They go for the ECG [electrocardiography] that’s usually the last thing we do, get them to go for ECG when we’re finished, give them the paperwork, they take that with them so they’re busy looking at the paperwork and it’s quite an in-depth questionnaire, tick-box questionnaire erm and then when they’ve done that, when they come back then the OT [occupational therapist] wants to see them, she takes them somewhere else, brings them back. So it’s quite a, it seems like quite a long drawn-out assessment by that time they’ve had enough.
ID 1, PA nurse band 6, site 1
These numerous assessments and appointments were also seen to result in a large amount of information being provided to patients, both during the appointment and for them to read and consider in their own time. The HCPs expressed some concerns that this could be too much for patients and that elderly patients especially may not be able to process all of this information:
. . . you’re giving them a lot of information, you’re giving them an anaesthetic leaflet and a meds [medications] leaflet, they’ve got to sign two forms, you’re giving them a leaflet to say why you’re taking blood for transfusion and sometimes these elderly people maybe can’t see what the hell you’re talking about sometimes by the time you go away they’ve got bags of paperwork and they don’t even know what they’re doing with it . . .
ID 1, PA nurse band 6, site 1
Despite concerns about the burden placed on patients, HCPs explained that the duration and intensity of the assessment process was necessary because of the complexity of the planned surgery as well as the nature of the orthopaedic patient population, who were considered to be older and more likely to have comorbid conditions:
. . . the majority still are elderly patients who have the nature of the beast is they’ve got other medical conditions going on anyway and they’ve got a drugs list like a roll of whatever, yeah so and that’s a big thing as well we’ve got like a medication list that we instruct ‘em about what drugs they can and can’t take so those kind of things take quite a while [Interviewer: Yep] just for the type of patients they are . . .
ID 1, PA nurse band 6, site 1
. . . it is a big PAC [pre-assessment], it is a major surgery and it needs to have the time and that credence to do it properly or else you’re just skating around corners . . .
ID 1, PA nurse band 6, site 1
In line with the purpose of PACs, the assessments conducted could be seen as focusing, first, on the identification of risk and, second, on the mitigation of these risks and the optimisation of the patient ahead of surgery. Factors identified included both known conditions and previously unidentified risks, with the identification of cardiac conditions being discussed by HCPs from all three sites:
. . . we may pick up on newly diagnosed heart problems and erm things so they may have to be referred to a cardiologist . . .
ID 14, PA lead, site 3
. . . umm and as you said sometimes we see a lot of new atrial fibrillations, we see abnormal heart rhythms or they’ve got poor lung function or they’re diabetic on insulin . . .
ID 1, PA nurse band 6, site 1
Procedures to mitigate risks and optimise patients ahead of surgery addressed a number of factors; these included medication management, diabetes control and correction of anaemia:
. . . so the patients that are low in iron we transfuse them preoperatively with iron err so it optimises their post-op care . . .
ID 5, PA lead nurse, site 3
. . . prior to surgery we do some medication management as well because we might have to stop certain drugs, may have to give them some erm injections prior to surgery to help thin the blood because we’ve stopped them having a particular drug . . .
ID 14, PA lead, site 3
The only optimisation process identified that directly related to health behaviour was smoking cessation but one HCP identified that they expected to undertake further optimisation processes in the future:
. . . I know there’s obviously plans afoot, you know pre-assessment’s going to change and there’s going to be more optimisation of patients . . .
ID 1, PA nurse band 6, site 1
Having the time to identify and manage these different factors was seen to be the predominant reason for conducting PA well in advance of the date of surgery and this was explained to be important for avoiding cancellations:
The reason why we see them from same day in our same-day clinic is because it gives them weeks and weeks and weeks because in our patients we find that we’ve had a lot of brand new heart problems that weren’t there before so it saves postponing the patients as well so we get all that sorted first, optimise the patient as much as possible before we give them a date for surgery.
ID 14, PA lead, site 3
The vast majority of the PA was described as being undertaken by members of the nursing team, who clearly identified themselves as responsible for requesting information and tests, interpreting the results and identifying how to proceed:
. . . that’s really the nurse who is doing it what she does about it, whether she sees it as a red flag or not . . .
ID 1, PA nurse band 6, site 1
However, staff from all three sites discussed the fact that anaesthetists were available to offer additional support for complex patients or to conduct further assessments when required:
Also we do have a, a consultant anaesthetist or an anaesthetist allocated every single day. [Interviewer: Yep] In case there’s a patients who needs to see an anaesthetist so there’s somebody available to see that patient ‘cause others got concerns with their GAs [general anaesthetic] previous GA problems and things like that so we get them seen before they go home. And also he takes patient with complex is history via our anaesthetist to have a look and decide what to do . . .
ID 7, PA nurse band 5, site 1
Alcohol is asked about but not ‘screened for’
Health-care professionals from all three sites explained that alcohol was ‘always’ asked about as part of the PA and that ‘as long as you ask the question it needs to be answered’. However, none of the HCPs referred to this as ‘screening’ and none currently used validated alcohol screening tools. Instead, the PA form included a box to indicate how many units the patient consumed per week:
There isn’t any screening but we are mindful of their intake. We always ask that.
ID 9, PA sister, site 2
. . . we do ask them how many units they will drink in an average week . . .
ID 16, PA team leader, site 3
Alcohol consumption did not appear to be a focus of PA, and HCPs described it as ‘just another question’. There was some indication that HCPs felt that they could be doing more to assess and address alcohol consumption but that they were restricted by the amount of time available and the small amount of space on the PA form:
. . . really the space is just how many units a week it doesn’t really give you that much scope. I mean you could go on if they say yeah I drink every night and whatever but it doesn’t give you a lot of scope . . .
ID 1, PA nurse band 6, site 1
. . . you know really you could spend so long doing so much and making these things so much better but as it is, the way it is at the moment you just don’t have the time or the resource to do it but I think you’re just skipping over it . . .
ID 1, PA nurse band 6, site 1
The reliance on self-reports of alcohol consumption was seen as a potential issue. The HCPs discussed difficulties in gaining honest and accurate information from patients. They were of the opinion that some patients would be honest about their alcohol consumption whereas others would under-report or ‘lie’ about how much they drank. Reasons for under-reporting included being ‘ashamed’ and providing socially desirable responses, with some patients having a tendency to ‘tell you what you want to hear’:
But it’s only what people say how many units you know you’re just going by what they say [Interviewer: Mmm] some people will give you the minimum or just they’ll tell you what you want to hear . . .
ID 1, PA nurse band 6, site 1
You have to rely on the honesty of the patient and sometimes if you are a heavy drinker you don’t want to acknowledge it like others do. They are quite happy to tell you their units.
ID 9, PA sister, site 2
Some HCPs felt that older patients were more likely to be honest about their drinking and were often comfortable with the amount that they drank and did not see it as ‘problematic’ regardless of whether or not this was in excess of the recommended drinking guidelines. Reasons given for this included the fact that drinking was part of their routine, that it was part of their social lives, that they had been drinking in a particular way for a long time and that significant others, including previous generations, had shown similar drinking patterns:
I think a lot of the older people that we get through and we ask they’re like not proud of it but very confident they’re like I go out three times a week and I have three pints every time they don’t see that as a problem because it’s what they’ve always done for years, that’s what their dads done it’s a social event for them they don’t see that that’s excessive because it’s only three pints and it’s only three times a week . . .
ID 2, PA nurse band 6, site 1
In some cases it was explained that patients’ responses may be inaccurate not because they wanted to mislead the HCP but because they did not realise how much alcohol they were consuming until they were asked to report it day by day:
. . . then you’ve gotta say right so how many do you drink on a weekend when you add it up it’s like two bottles of wine well that’s 18 units, that’s above and they go ‘oh is it’ . . .
ID 4, PA nurse band 6, site 3
Descriptions of frequency of drinking by patients were also often inconsistent with HCPs interpretations with perceptions of what constituted ‘regular’ drinking being particularly problematic:
They are totally oblivious, they’re not bothered. I’ve had quite a few say oh I drink occasionally and you go well when would that be? ‘On a Friday and Saturday.’ Well that’s not occasionally that’s regular . . .
ID 16, PA team leader, site 3
In combination, these issues point to the need for HCPs to probe patients about alcohol consumption rather than simply ask how many units they drink per week if they are to gain accurate information. Despite identifying possible issues with self-reports of alcohol consumption, HCPs identified that patients were the only ones who would actually know how much alcohol they consumed and thus asking them was the best way to gain the required information:
. . . because nobody knows more than them if they are drinking more than they should be nobody knows more than them . . .
ID 6, substance use lead, site 1
Objective assessments of alcohol-related harm were available at all three sites in the form of liver function tests (LFTs) and HCPs from all three sites also talked about conducting LFTs as part of PA. However, this was not done for every patient but rather was an optional test that could be conducted for patients considered to have a high alcohol intake to provide a more detailed assessment of surgical risk:
We ask about their intake, how many units a week [Interviewer: Yeah, yeah] and if it was a massive amount, we would probably check their liver function.
ID 8, PA lead nurse, site 2
It’s not what you ask, it’s how you ask
The majority of HCPs were comfortable asking patients about alcohol consumption but explained that patient responses to questions about alcohol consumption varied with some being very comfortable and others being more reluctant or defensive:
. . . it’s all, its variable isn’t it?
. . . it’s different . . .
. . . you get some that are really defensive when you ask . . .
ID 1, ID 7, ID 3, site 1
I think our patient population here, err they’re very down to earth, they’re very laid back. Yeah I think it’s quite easy to ask them questions like that.
ID 13, consultant anaesthetist, site 3
Some HCPs explained that patients would laugh or offer joke responses, which they appeared to interpret as an indication of patients being comfortable with the questions but could also be considered a defensive response:
They usually laugh.
Yes.
I was gonna say laugh . . .
Or they say, ‘Do you want the truth?’.
ID 8, ID 12, ID 11, ID 9, site 2
However, HCPs explained that alcohol could be a sensitive subject for some patients and that how the questions were asked was important. Asking questions about alcohol use in among a large number of other questions and once a rapport had been established were seen as ways to reduce the possibility of adverse responses. HCPs also talked about different methods of ‘selling’ the questions to patients with techniques such as explaining that the questions were routine and pointing out the relevance of the questions to their health or the planned surgery being discussed:
I know it can be a bit of embarrassment but don’t worry we ask everybody it’ it’s just that’s how I sell it as . . .
ID 6, substance use lead, site 1
. . . it’s really important we know how much alcohol you have because that really can affect your general anaesthetic we need to know whether to bring you in a day early do we need to you know we need to look after you, we need to make sure that you’re fine you know we don’t want anything going wrong while you’re here.
ID 6, substance use lead, site 1
The fact that alcohol consumption was routinely asked about was not only a way of assuaging patients’ anxiety about the questions but also meant that HCPs became experienced in asking about alcohol consumption, which was seen to be beneficial:
I’ve done this a long time so it’s kind of you know it’s not too difficult a conversation for me to have because I’m used to broaching it . . .
ID 6, substance use lead, site 1
This experience meant that HCPs had been able to develop the skills necessary to broach the subject of alcohol consumption effectively. Skills described as important included adopting a non-judgemental approach and knowing when to ‘take a step back’:
I think if people felt you were challenging them and didn’t like it I think we would always, sort of, be aware of when to draw back a bit because if they don’t want you to know and they don’t want to tell you, you feel like you are invading their privacy . . .
ID 9, PA sister, site 2
. . . it’s about as well I think saying to people this is not about judging you . . .
ID 6, substance use lead, site 1
In addition to the experience and skills of the HCPs, patients’ acceptance of questions about alcohol use was considered to relate to the reason that they were attending the PA. The orthopaedic population were seen to have a strong desire to have their surgery and HCPs explained that many patients were in pain and would have waited a long time for surgery. This was seen to make them more compliant:
Yeah, yeah] you know they’re a [Interviewer: Ah hu] they are willing and they’re sitting there listening to you . . .
. . . they want their operation . . .
. . . they’ll do anything to get it . . .
ID 6, ID 2 and ID 1, site 1
Managing preoperative alcohol consumption
The HCPs talked about a number of different strategies that were available for the management of alcohol consumption in the preoperative period. How alcohol consumption was managed appeared to be dependent on the amount of alcohol being consumed. Patients who were drinking more than the drinking guidelines were often advised simply that it would be beneficial to reduce their alcohol consumption:
I just say someone who drinks like 30-odd units a week I just say something like ‘that’s above the national guidelines have you thought about cutting down?’ . . .
ID 5, PA lead nurse, site 3
All three sites also had alcohol specialist or alcohol liaison nurses to whom they could refer patients who required additional support to reduce their drinking. However, not all of the PA staff were aware of this service, perhaps because outpatient clinics were not focus points for identifying increased risk or dependent drinkers. Further to this, many of those who were aware reported that their patients consistently declined any offer of support from this service:
. . . we ask them if they would like any further information, you know once we’ve pointed out that that is over the national guideline limit and erm they all say no . . .
ID 14, PA lead, site 3
The HCPs reported that those patients who were viewed to be drinking to excess or those identified through LFTs would be referred on regardless of their preference. In some cases, patients would be referred to an anaesthetist who would either review the patient’s notes or see the patient in person to further assess their surgical risk and decide how to manage them:
. . . if that flags up through the like bloods or whatever you’ve taken [Interviewer: Yeah, yeah] and then alcohol erm anaesthetic review . . .
ID 2, PA nurse band 6, site 1
Alternatively, patients could be referred to the alcohol specialist nurses to help them prepare for surgery rather than to support them in reducing their drinking. Such management often involved a more in-depth assessment of alcohol consumption and then preparations to bring the patient into hospital a day before their surgery date so that withdrawal could be managed and surgery could proceed as planned:
. . . how are they when they’ve not been drinking [Interviewer: Mmhmm] because that’s gonna guide you when they’re gonna be nil by mouth and whether you’re gonna have to get some meds [medications] prescribed or whether you need to be advising this chap will need to come in early or we need to be prepared or do they need to have a conversation or do they need to come back to have a full intervention . . .
ID 6, substance use lead, site 1
This option was considered to be only for the ‘big drinkers’ who were thought to be unlikely or unable to change, indicating possible alcohol dependence:
We do have somebody but that tends to be for if they’re you know drinking loads [Interviewer: Yeah] like need detox or something. Not for your erm not for the vast majority of patients . . .
ID 1, PA nurse band 6, site 1
The option to refer patients back to their GP was also discussed by some HCPs, albeit less frequently than other options. The description of patients being ‘referred back’ to the GP suggested that surgery would be postponed until the GP had seen and reassessed the patient:
It would be referral onto the anaesthetist for him and then he would make a decision as to whether they needed referring on or back to the GP for further advice.
ID 8, PA lead nurse, site 2
It was clear from the descriptions offered by HCPs that, although measures were in place to ensure the safety of patients who showed signs of alcohol dependence, little provision was made for those drinking at ‘risky’ or ‘increased risk’ levels:
The ones I’ve referred are the ones who would be having a bottle of vodka every night, not the ones who would be having, sharing a bottle of wine with their husband every night, that just is gets ignored really. Not ignored but you would still do certain blood tests but it it’s the big drinkers like hundreds of units that get referred . . .
ID 18, PA nurse band 6, site 3
. . . there’s nothing really in place for this group of patients as it stands at the moment because they’re not so high risk that you’re concerned about their perioperative period you, you know you accept it that I think there’s such a big proportion of people that fall into this category that it becomes normal erm I think if you can say to patients we’re trying to do this because we’re worried it’s going to take your wound longer to heal or you’re more at risk of getting an infection afterwards then to be able to say something like that you know 15-minute chat and if you reduce for 6 weeks it might improve your outcome that’s very different . . .
ID 13, consultant anaesthetist, site 3
Could be more, could be better
The descriptions of alcohol screening and management in PA showed variation in practice both between and within the three sites. Although some assessment of alcohol consumption was part of formal PA at each of the three sites, there did not appear to be any clear or consistent guidelines as to what to do in response to this questioning. Individual HCPs tended to talk about ‘big drinkers’, ‘excessive drinkers’ and those consuming ‘a massive amount’ as being referred for review or identified as requiring LFTs. Otherwise, in the absence of specific guidelines and procedures, the HCPs drew on their own training and experience to make decisions about how to manage alcohol consumption in the preoperative period:
. . . it is very much what the nurse does themself but you know we are all obviously trained enough to know that someone who drank god knows how many units you would do something about it.
ID 1, PA nurse band 6, site 1
The HCPs explained that asking questions about alcohol consumption often felt purposeless as the vast majority of patients received no feedback, intervention or further testing even though their alcohol consumption exceeded the drinking guidelines. In the absence of any method of addressing risky and increasing risk drinking, HCPs described simply moving on to the next question in their list:
. . . well you work it out ‘well that’s 70-odd a week’ right have you got any allergies? you think what do you ask that for because you’re just sweeping over it . . .
ID 1, PA nurse band 6, site 1
Although all three of the sites had alcohol specialist nurses available to whom patients from any part of the hospital could be referred, the awareness of these services varied between individual HCPs and across sites, indicating that further communication and clarification of services available was required:
. . . if someone asked you that would you know who to refer them to would you know what the referral process is in [site 1]?
No.
OK so you need to know that.
Because I’ve never done that so I do think we do need a structure like that in PAC.
ID 2, ID 6, site 1
Further to this, even when staff were aware of the processes for making referrals to alcohol specialist services, they felt that they had no method of following up on this or ensuring that the patient was actually seen. Beyond this, there was some concern that referrals may not actually lead to any further treatment or support, and staff did not always agree that the guidelines were appropriate for the effective delivery of alcohol-related care:
. . . we ask the question, refer them on but nobody there follows them up and makes sure that they come or go and see [alcohol specialist nurse] . . .
ID 14, PA lead, site 3
What I’ve got concerns with is is I don’t think five should be a referral to us because five is six units once a month . . .
ID 6, substance use lead, site 1
In addition to the variations between and within sites in current practice, it was clear that practice changed over time. National incentivised programmes had influenced practice at two sites. Site 3 had previously employed the Michigan Alcoholism Screening Test (MAST)127 with BA and referral available, and site 1 was in the process of implementing alcohol screening for all inpatients; both of these were part of the CQUIN initiative:
It used to be a CQUIN target so we’ve got the questions on the pro forma like how many do you drink a week and then we’ve got like . . .
. . . the MAST score . . .
. . . the MAST score yeah nought to one and then two to four we have to fill that out and then you’ve got erm brief intervention given . . .
ID 14, ID 16, site 3
However, negative personal experiences with these processes could lead to failure to adopt or maintain them, with staff from site 3 identifying that they stopped using the full screening, intervention and referral process when patients showed little interest in the additional information and support available to them:
. . . we did used to have some written information booklets that mapped it out . . .
. . . no one none of the patients wanted to take it on [Interviewer: Yep] and have this further information provided so [Interviewer: OK] sort of fizzled out . . .
ID 18, PA nurse band 6, site 3
Survey results
Sixty-two participants (51.7% response rate) completed the survey. The majority of respondents were female (90.3%) but covered a diverse age range, the full spectrum of nursing job roles (from HCA to matron) and included those new to PA (< 1 year) as well as those with over a decade working in PACs. Demographics of the sample are summarised in Table 5. All were familiar with PA for hip and knee arthroplasty.
| Variable | Category | Respondents, n (%) |
|---|---|---|
| Gender | Female | 56 (90.3) |
| Male | 6 (9.7) | |
| Age | 21–30 | 10 (16.1) |
| 31–40 | 30 (48.3) | |
| 41–50 | 13 (21.0) | |
| 51–60 | 9 (14.6) | |
| Job role | HCA/senior HCA | 7 (11.3) |
| Band 4 | 2 (3.2) | |
| Band 5 | 33 (53.2) | |
| Band 6 | 12 (19.4) | |
| Band 7 | 1 (1.6) | |
| Team leader | 4 (6.5) | |
| Sister/senior sister | 1 (1.6) | |
| Matron | 2 (3.2) | |
| Time working in PA | < 1 year | 4 (6.5) |
| 1–5 years | 35 (56.5) | |
| 6–9 years | 11 (17.8) | |
| ≥ 10 years | 12 (19.4) |
Appointment details
The typical length of PA appointments for hip and knee arthroplasty ranged between 30 minutes and 1 hour, with the majority of respondents (knee, 61.3%; hip, 59.7%) reporting appointments lasting 45 minutes. All respondents indicated that patients were asked about alcohol consumption as part of PA, although two stated that this was not part of the formal procedure. However, the type of questions used to assess alcohol consumption varied, with only 29% using a formal screening tool or questionnaire (Table 6). Similarly, although > 50% reported that patients were given information about alcohol consumption in PA (14.5% indicated that this was part of the formal PA procedure) and 80.6% indicated that they could refer patients for additional advice or support related to alcohol consumption, what information patients received and to whom they could be referred varied (Table 7).
| Response option | Yes, standard practice/formal procedure, n (%) | Yes, but not standard practice/formal procedure, n (%) | No, n (%) | Don’t know, n (%) | Missing, n (%) |
|---|---|---|---|---|---|
| Patients asked about alcohol use | 59 (95.2) | 2 (3.2) | – (–) | 1 (1.6) | – (–) |
| Patients given information about alcohol use | 9 (14.5) | 23 (37.1) | 26 (41.9) | 3 (4.8) | 1 (1.6) |
| Referral for additional advice or support available | 34 (54.8) | 16 (25.8) | 7 (11.3) | 1 (1.6) | 4 (6.5) |
| Category | Response option | Yes, n (%) | No, n (%) | Don’t know, n (%) | Missing, n (%) |
|---|---|---|---|---|---|
| Alcohol screening (N = 62) | Yes/no indication of alcohol consumption | 34 (54.8) | 26 (41.9) | 1 (1.6) | 1 (1.6) |
| ‘How much do you drink?’ | 19 (30.6) | 42 (67.7) | – | 1 (1.6) | |
| ‘How often do you drink?’ | 25 (40.3) | 35 (56.5) | 1 (1.6) | 1 (1.6) | |
| How many units/standard drinks per week? | 27 (43.5) | 34 (54.8) | – | 1 (1.6) | |
| How many units/standard drinks per day? | 18 (29.0) | 40 (64.5) | 1 (1.6) | 1 (1.6) | |
| Screening tool or questionnaire | 18 (29.0) | 40 (64.5) | 3 (4.8) | 1 (1.6) | |
| Alcohol information (N = 32) | Information leaflet about alcohol in general | 11 (34.4) | 17 (53.1) | 4 (12.5) | – |
| Information leaflet about alcohol and surgery | 10 (31.3) | 17 (53.1) | 5 (15.6) | – | |
| Face-to-face information about alcohol in general | 7 (21.9) | 22 (68.8) | 3 (9.4) | – | |
| Face-to-face information about alcohol and surgery | 21 (65.6) | 7 (21.9) | 4 (12.5) | – | |
| Websites to access in their own time | 2 (6.3) | 24 (75.0) | 6 (18.8) | – | |
| Contact numbers for telephone information/support | 3 (9.4) | 24 (75.0) | 5 (15.6) | – | |
| Patient is told to contact their GP surgery | 1 (3.1) | 26 (81.3) | 5 (15.6) | – | |
| Referrals for additional information or support (N = 50) | Referral to consultant anaesthetist | 37 (74.0) | 9 (18.0) | 4 (8.0) | – |
| Referral to GP | 5 (10.0) | 41 (82.0) | 4 (8.0) | – | |
| Referral to alcohol liaison or nurse specialist | 28 (56.0) | 20 (40.0) | 2 (4.0) | – | |
| Referral to in-hospital addiction psychiatrist | 1 (2.0) | 42 (84.0) | 7 (14.0) | – | |
| Referral to Alcoholics Anonymous | 2 (4.1) | 43 (87.8) | 4 (8.2) | – | |
| Referral to other external organisation | 3 (6.0) | 42 (84.0) | 5 (10.0) | – |
The form of alcohol screening varied; patients were most frequently asked if they drank alcohol and to provide details of how many units they drank, or how often and how much they drank, so that HCPs could calculate the number of units consumed:
We have to record units per week so I ask if they drink, when they drink and what they have . . .
ID S4
One respondent provided a possible explanation about why patients are not always directly asked to report their consumption in units:
Patients don’t always know units so I ask what they drink and calculate . . .
ID S7
A number of respondents also used the text boxes to explain that they had previously used less structured questioning about alcohol consumption but had recently replaced this with the AUDIT-C questionnaire as part of the CQUIN initiative:
We have always asked patients if they drink and how much they drink but we recently started using the AUDIT-C questionnaire as part of the CQUIN . . .
ID S31
A number of sites appeared to provide minimal information or simple advice to cut down or avoid alcohol before surgery:
Patients told to avoid alcohol before surgery . . .
ID S2
In some cases information was provided only to those considered to be ‘heavy’ drinkers:
I generally suggest that heavy drinkers cut down before surgery and we have some booklets about alcohol that we can give them . . .
ID S11
This information was not always delivered by PA staff:
Physios [physiotherapists] provide this info . . .
ID S27
Regarding referral, respondents most frequently indicated that they could refer patients to consultant anaesthetists and alcohol liaison or alcohol nurse specialists.
As can be seen in Table 8, there was a similar level of variation in how patients were identified to receive information and to be referred for additional information and support. Responses recorded in the free-text boxes provide some further insight into this. The specialist or service to which patients were referred was often related to how they had been identified. Specifically, those identified through LFTs would be referred for consideration by an anaesthetist, whereas those identified by screening tools would be referred to alcohol liaison or nurse specialists:
If a patient asked for help I’d give them a leaflet and tell them to get in touch with their GP or arrange alcohol liaison but this has never happened. I usually give patients who score five or more on AUDIT a bit of advice and/or leaflet. Liver function tests flag and the patients notes go to anaesthetist . . .
ID S11
| Response option | Yes, n (%) | No, n (%) | Don’t know, n (%) | Prefer not to say, n (%) | |
|---|---|---|---|---|---|
| Information provided to (N = 32) | All patients | 11 (34.4) | 18 (56.3) | 2 (6.3) | 1 (3.1) |
| All patients who report drinking | 3 (9.4) | 26 (81.3) | 2 (6.3) | 1 (1.6) | |
| All patients who request information | 12 (37.5) | 17 (53.1) | 3 (9.4) | – | |
| Patients who consume over a specified number of units | 16 (50.0) | 12 (37.5) | 4 (12.5) | – | |
| Patients who state, when asked, that they would like to reduce their alcohol consumption | 5 (15.6) | 23 (71.9) | 4 (12.5) | – | |
| Patients identified through LFTs | 9 (28.1) | 17 (53.1) | 6 (18.8) | – | |
| Patients identified through other blood tests | – | 26 (81.3) | 6 (18.8) | – | |
| Other | 4 (12.5) | 23 (71.9) | 5 (15.6) | – | |
| Patients receive referral (N = 50) | All patients | – | 48 (96.0) | 2 (4.0) | – |
| All patients who report drinking alcohol | – | 49 (98.0) | 1 (2.0) | – | |
| Patients who consume over a specified number of units | 30 (60.0) | 18 (36.0) | 2 (4.0) | – | |
| Patients who state, when asked, that they would like to cut down their alcohol consumption | 10 (20.0) | 37 (74.0) | 3 (6.0) | – | |
| Patients identified through LFTs | 39 (78.0) | 8 (16.0) | 3 (6.0) | – | |
| Patients identified through other blood tests | – | 45 (90.0) | 5 (10.0) | – | |
| Patients who ask to be referred | 9 (18.0) | 37 (74.0) | 4 (8.0) | – | |
| Patients who show signs of alcohol dependence | 25 (50.0) | 21 (42.0) | 4 (8.0) | – | |
| Patients who are already identified as alcohol dependent | 31 (62.0) | 14 (28.0) | 5 (10.0) | – | |
Some respondents from sites participating in the implementation of the initial CQUIN initiative explained that this also included the provision of information and, for screen-positive patients, the option to refer to alcohol specialists:
CQUIN has referral to alcohol liaison. We can refer to [anaesthetist] . . .
ID S26
Finally, a number of staff indicated that, although patients did not routinely ask for information about alcohol, this information would be provided if it was requested:
If patients asked I’d give them information but they don’t . . .
ID S6
Methods of identification showed considerable variation, suggesting that there are not clear policies outlining which patients should receive a referral. Further to this, some respondents indicated that referrals for additional support would be made only if patients wanted to be referred, whereas referral for review by an anaesthetist would be made either way:
Patients only referred to alcohol nurse if they want to be. Notes seen by anaesthetist either way . . .
ID S1
Conclusions
The focus group and survey results both indicated that asking patients about alcohol consumption and recording their responses was a formal part of PA at almost all sites. Focus group discussions built on this, with HCPs explaining that they were experienced in asking about alcohol consumption and had the necessary skills to broach this subject with patients. As a result they were likely to be well placed to provide additional guidance on alcohol use and surgical risk. The majority of HCPs also stated that they could refer ‘heavy’ drinkers for review by an anaesthetist prior to surgery, with LFTs and units of alcohol consumed being used to assess whether a patient or their notes should be reviewed by an anaesthetist. However, few specific details were provided on how LFTs were interpreted and what, if any, cut-off points were used to decide if such a referral was appropriate.
This suggests that there are few formal guidelines regarding how to identify alcohol consumption that potentially represents a risk for surgery and when and how to address alcohol consumption as a ‘risky’ behaviour. Just over 50% of survey respondents indicated that they provided patients with some form of information related to alcohol consumption but < 15% stated that this was part of formal PA; the form of information varied from a simple leaflet about alcohol consumption to face-to-face information about alcohol consumption and surgery. Free-text responses indicated that when face-to-face information was provided, this was predominantly in the form of a brief recommendation to avoid alcohol consumption before surgery, with little or no explanation of why or guidance on how long alcohol intake should be altered.
At the point of conducting the focus groups (October–December 2016), none of the three sites used a validated alcohol screening questionnaire. This is in line with the previous survey of medical perioperative assessment leads. 116 At the time of the PRE-OP BIRDS survey (2018), 29% of respondents indicated that they used a validated screening tool. Many of these utilised the free-text response boxes to indicate that this was a recent change as a result of the CQUIN initiative, which came into effect in the period between the focus groups being completed and the survey commencing. With previous research67 demonstrating that using screening tools improves the detection of both alcohol dependence and increased risk drinking, the deployment of a validated tool may lead to a reduction in alcohol-related complications.
Awareness of in-hospital alcohol specialist services was low among PAC staff at two of the three sites included in the focus groups, with the third site requiring patients to agree to a referral to alcohol specialist nurses, something that they indicated was widely refused. Awareness appeared to be higher among survey respondents, with 45% indicating that they could refer patients to alcohol liaison or alcohol specialist nurses. However, the methods for identifying patients to receive information or referral also varied, with biological tests (LFTs), self-reported alcohol consumption, indications of alcohol dependence and previous alcohol history all being used.
Chapter 3 Feasibility study
Background
Feasibility studies offer the opportunity to work with patients and HCPs from the proposed target populations to gain insight and to explore the appropriate optimisation and acceptability of interventions, outcome measures and research processes. Pilot trials may then be performed to provide an accurate estimation of rates of eligibility, recruitment and retention to inform a decision about the feasibility of proceeding to a definitive trial.
Aims
The study aims were to:
-
assess the feasibility and acceptability of consent, screening, intervention and outcome assessment procedures.
-
optimise the screening and behavioural intervention process in a way that nurses and patients find acceptable.
Methods
Design
A mixed-methods design was used, including qualitative interviews combined with assessment of screening and recruitment rates.
During the feasibility study, focus groups (n = 3 groups) to characterise TAU and assessments of fidelity of delivery of intervention sessions were also conducted. These are reported in Chapter 2.
Setting
Patient participants were recruited from a single site, a large secondary care teaching hospital in an urban location in the north-east of England. The site has an orthopaedic preoperative surgical care pathway of 6–10 weeks from PA to surgery. Patient screening took place in a surgical outpatient clinic with the BBI delivered in the PAC, and an optional booster session was delivered either over the telephone or face to face at the PAC.
Outcome measures
Acceptability and feasibility were assessed using a combination of quantitative (eligibility, screening, recruitment and retention rates) and qualitative (themes identified in analysis of staff and patient interview transcripts) data.
Procedure
Health-care professional recruitment, training and interviews
Screening and intervention were conducted by HCPs employed in the PAC. As such, it was necessary first to recruit and train HCPs to deliver the screening and intervention processes.
Health-care professionals were eligible if they were currently employed in the PA unit and were both willing and able to attend study-specific training. HCPs were excluded if they were unwilling to consent to audio-recording of intervention sessions, which was required in order to conduct assessments of fidelity of delivery (described in Chapter 2), or if they were unwilling or unable to attend the required training. A group training session (described in detail in Chapter 2) was delivered to HCPs on 15 April 2016 and a further session was held on 1 September 2016 to train a newly appointed HCA dedicated to the role of conducting initial eligibility screening with patients.
Health-care professional interviews
All HCPs involved in screening or screening and intervention delivery were eligible to participate in a qualitative interview about their experience of being involved in the research. The research associate (EL) sent all HCPs involved in the delivery of screening and/or interventions an e-mail inviting them to take part in an interview. E-mail invitations included the HCP interview-specific information sheet (see Appendix 10) as an attachment. Interviews were arranged at a time and place that suited the HCP participant; all three were conducted in a private room at the hospital immediately following a shift in the PAC. Interviews were conducted by the research associate, who gave a verbal introduction to the interview process before completing the informed consent form with the HCP participant. A topic guide (see Appendix 11) was used to structure the interview, which included questions about participants’ experience of being involved in the study, the acceptability of the study processes, barriers and difficulties encountered during alcohol screening and/or intervention delivery and recommendations for improvements. Interviews lasted between 20 minutes and 1 hour; all were audio-recorded and transcribed verbatim.
Patient screening and recruitment
From the patient perspective, the feasibility study was based around four key points of contact or ‘visits’. This study process is summarised in Figure 1. The activities completed at each visit are displayed in Table 9 and all processes are described in detail here.
FIGURE 1.
Feasibility study process.

| Time point | Study period | |||
|---|---|---|---|---|
| Visit 1: screening | Visit 2: consent and intervention | Visit 3: booster session | Visit 4: qualitative interview | |
| Enrolment | ||||
| AUDIT-C | ✗ | |||
| Expression of interest | ✗ | |||
| Consent | ✗ | |||
| Intervention | ||||
| AUDIT | ✗ | |||
| Intervention | ✗ | |||
| Booster | ✗ | |||
| Follow-up | ||||
| Interview | ✗ | |||
Inclusion and exclusion criteria
Patients were eligible for the study if they:
-
were aged ≥ 18 years and listed for elective primary hip or knee arthroplasty
-
had capacity to provide informed consent
-
were able to write and converse in English (i.e. able to understand English sufficiently to complete the study questionnaires without the need for an interpreter)
-
had screened positive for increased risk drinking (AUDIT-C score of ≥ 5 and reported consuming ≥ 6 units in one session at least weekly).
Patients were excluded from the study if they:
-
scored < 5 on the AUDIT-C screen or did not report consuming of 6 units on a single occasion at least weekly
-
were likely to undergo sequential (on different dates) joint replacements (for bilateral disease) within the scope of the proposal (because of reduced availability for follow-up)
-
displayed current (active) withdrawal from alcohol
-
had a severe psychiatric disorder requiring medical therapy, or severe cognitive impairment or dementia, affecting their ability to interact with the intervention and increasing the likelihood of postoperative delirium.
Patient participants were eligible to receive the intervention if they reported a score of ≥ 8 and consumption of ≥ 6 units in one session at least weekly on the full AUDIT questionnaire. Patient participants were excluded from the intervention if they scored < 8 on the full AUDIT or reported consuming ≥ 6 units on a single occasion monthly or less frequently.
Visit 1: screening
Initial screening for alcohol consumption using the AUDIT-C was conducted in a surgical outpatient clinic following listing for surgery. Patients were approached by a HCP trained in delivery and scoring of the AUDIT-C tool. The HCP completed the AUDIT-C with all willing patients and immediately assessed the responses for study eligibility. Eligibility was confirmed if the patient scored ≥ 5 on the AUDIT-C and reported consuming six or more standard drinks in one session at least weekly. Eligible patients were given a verbal explanation of the study and asked if they were interested in taking part. Those who expressed an interest were provided with a copy of the participant information sheet to take away with them and were asked for permission for follow-up contact to discuss the study further. Expressions of interest, contact information and preferred method of contact were recorded in writing by the HCP on the expression of interest form. Patients were then given 1 week to consider taking part in the study. After this time, the HCP contacted them by telephone or e-mail (depending on patient preference) to establish ongoing interest in participation and to arrange intervention delivery for those patients who confirmed ongoing interest.
Visit 2: consent and intervention
Consent, completion of the full AUDIT questionnaire and intervention delivery (when patients were eligible) took place in the PAC. On arrival at the PAC, patients were met by a research nurse, who checked that the patient was still committed to taking part and, if so, confirmed their eligibility, including establishing their capacity to consent, before completing the informed consent discussion and asking patients to complete and sign the informed consent form; this included an indication of whether or not they were willing to have their intervention session audio-recorded and if they were willing to be contacted about participating in a qualitative interview at 6 weeks post surgery. The participant was given a copy of the completed consent form. After consenting to the study, patients completed the full AUDIT questionnaire with an appropriately trained HCP prior to intervention delivery. Patients who scored ≥ 8 on the full AUDIT or reported consuming six or more standard drinks on a single occasion at least weekly were eligible for intervention delivery. Patients who scored < 8 on the full AUDIT and reported drinking six or more standard drinks on a single occasion less than weekly were thanked for agreeing to take part, received positive feedback and were informed that the more in-depth measure indicated that their drinking fell within the lower-risk category and that nothing further was required of them in the preoperative period. As these patients were able to provide information about the acceptability of preoperative alcohol screening and study consent procedures, all those who provided consent to be contacted about a qualitative interview were contacted 6 weeks post surgery to gauge their interest in interview participation.
There was no upper limit of alcohol consumption or screening score that would lead to exclusion from the study. However, patients displaying current (active) withdrawal from alcohol were excluded from the study and assessed by the principal investigator to consider if a referral to an addiction psychiatrist for additional support was appropriate.
Intervention
The intervention and its development are described in detail in Chapter 2 and their content, defined in terms of BCTs, is presented in Appendix 1. The intervention tools and processes are summarised here.
The BBI was structured around three tools utilised as follows:
-
The BA tool acted as a visual aid for communicating information and provided HCPs with prompts to structure and deliver this advice to participating patients.
-
The BI tool provided prompts to guide discussion of behaviour change with participating patients. This discussion could include motivation to change, pros and cons of change, goal-setting and problem-solving. This tool also provided space to record aspects of the intervention (e.g. goals and plans) to prompt behaviour outside the clinical setting.
-
The patient leaflet106 was introduced in the intervention sessions and given to patients at the end of the intervention session along with copies of the BA and BI.
Following completion of the full AUDIT, eligible patients received the BBI. The first session was delivered face to face in the PAC by an appropriately trained HCP. The session lasted 20–30 minutes, beginning with 5 minutes of BA delivered using the BA tool as a visual aid and prompt. The remainder of the session involved providing of the behaviour change intervention, targeting motivation and volition to target alcohol reduction or cessation in the preoperative period and using the BI tool to guide the discussion. At the end of the session, participants were provided with copies of the intervention materials to take away with them, as well as a copy of the patient leaflet.
Visit 3: booster session
Patients who indicated at the time of consent that they were willing to be contacted about participating in the optional booster intervention session were contacted by their preferred method (telephone or e-mail) approximately 2 weeks prior to surgery to gauge their continued interest in the booster session. For those who agreed to participate in the booster session, arrangements were made for the session to be delivered either face to face in the PAC or over the telephone approximately 1 week before surgery. The booster session, delivered by the same HCP who delivered the initial intervention session, lasted between 10 and 20 minutes and began with completion of the full AUDIT tool. The aim of this session was to revisit any goals and plans made during the first session, to provide feedback on performance and to boost self-efficacy, supporting behaviour change maintenance. For patients who indicated a desire to change but who did not set goals and plans during the first session, this booster provided an opportunity for them to do so. For those who had set goals and plans, it served as an opportunity to make any revisions and to receive feedback to target behaviour change maintenance.
Visit 4: post-intervention interview
All patient participants who consented to take part in the feasibility study indicated on the consent form that they were willing to be contacted about participating in a qualitative interview. These interviews aimed to explore the acceptability of screening, intervention and research processes to patient participants. The research associate (EL) contacted participants by telephone approximately 5 weeks post surgery with a view to arranging interviews to take place at, or around, the 6-week post-surgical outpatient appointment. Interviews took place in a private room at the hospital site, either immediately before or immediately after their outpatient appointment (n = 10) or in their own home on a day when they were not attending the hospital (n = 3). Those wanting to take part were provided with an interview-specific patient information sheet (see Appendix 12) using their preferred method (e-mail or post) prior to the interview. On the day of the interview, the research associate gave a verbal explanation of the interview process and a brief overview of her research background. Participants then completed the interview-specific informed consent form. All interviews were conducted using a topic guide (see Appendix 13) including questions about alcohol consumption, the acceptability and feasibility of alcohol screening and BI in the preoperative setting, their views of the intervention, their experience of being involved in the study, their opinions of the study tools and the acceptability of planned methods for a subsequent RCT. Of the 15 participants in the feasibility study, 13 took part in an interview (one could not be contacted and one failed to attend interview). All interviews were audio-recorded and transcribed verbatim.
Protocol amendments during the study
Recruitment to the study was initially lower than anticipated. Assessment of initial screening and recruitment rates identified that considerable numbers of patients were being screened as potentially eligible, with many expressing interest in the study and taking away a copy of the patient information sheet. However, on further contact (when possible) the majority did not express ongoing interest in the study and only a small minority went on to take part in the study. A number of factors were identified as contributing to this issue and three amendments were made to address this:
-
A number of patients demonstrated adverse attitudes to the term ‘risky drinking’, which appeared in the initial study title and study documentation (Preoperative Behavioural Intervention for Risky Drinkers before elective orthopaedic Surgery).
-
Amendment: the wording of the study title was amended to focus on the benefits of reducing alcohol consumption rather than identification of patients as ‘risky drinkers’. The new title adopted was ‘Preoperative behavioural intervention to reduce drinking before elective orthopaedic surgery’.
-
-
Allowing 1 week for consideration of the study before the research team made contact with patients who had expressed interest in the study meant that many had lost interest. This gap also presented issues in terms of arranging consent and intervention around the PAC appointment, as many appointments had already been scheduled.
-
Amendment: to facilitate the arrangement of research procedures and intervention delivery, the time allowed for consideration of study participation was reduced from 1 week to 24 hours before the research team attempted to make contact to gauge ongoing interest.
-
-
The inclusion criteria required patients to score ≥ 5 on the AUDIT-C and to report consuming ≥ 6 units in a single session at least weekly. This significantly reduced the number of patients who were eligible for the study and meant that many frequent heavy drinkers who could potentially benefit from the intervention were being excluded from the study because they either did not report high intensity drinking (consumption of six or more standard drinks in a single session) or reported doing so less frequently than weekly.
-
Amendment: to capture both regular heavy drinkers and high intensity drinkers, the inclusion criteria were amended to an ‘and/or’ criterion rather than an ‘and’ criterion alone.
-
The amendments were approved by the funder, the Research Ethics Committee and the Health Research Authority in November 2016 and were implemented at the recruiting site shortly thereafter.
Results
Sample
Health-care professional participants
A total of nine HCPs consented to take part in the feasibility study and received the study training; four (44%) were involved in screening and intervention delivery during the study period and three went on to take part in qualitative interviews.
Patient participants
Patient screening commenced on 3 May 2016 and the first patient was recruited on 24 May 2016. The study closed to recruitment on 2 March 2017; over the 10-month screening and recruitment period, a total of 15 patients consented to and participated in the feasibility study (two were recruited in the first 5 months and, 13 were recruited in the subsequent 5 months). Patient participants were predominantly male (87%) and had an average age of 70.5 years. Details of the sample are shown in Table 10 and participant health behaviour data are displayed in Table 11.
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 13 (86.7) |
| Female | 2 (13.3) |
| Ethnicity | |
| White British | 5 (33.3) |
| Missing | 10 (66.6) |
| Operation | |
| Hip | 3 (20) |
| Knee | 12 (80) |
| AUDIT score | |
| ≥ 8 | 9 (60) |
| < 8 | 6 (40) |
| Variable | Mean | Standard deviation |
|---|---|---|
| Alcohol units per week (n = 7, missing = 8) | 19 | 8.9 |
| BMI (kg/m2) (n = 6, missing = 9) | 31.3 | 8.1 |
| Number of days active per week (n = 6, missing = 9) | 3.2 | 3.1 |
| Physical activity (n, %) | ||
| Yes | 6 | 40.0 |
| No | 1 | 6.6 |
| Missing | 8 | 53.3 |
| Smoking (n, %) | ||
| Current smoker | 0 | 0.0 |
| Ex-smoker | 1 | 6.6 |
| Never smoked | 6 | 40.0 |
| Missing | 8 | 53.3 |
Analysis
Quantitative data were subject to descriptive analysis only. Screening and recruitment rates are detailed along with mean scores on the AUDIT questionnaires completed at baseline and the booster session (when applicable).
Qualitative data from patient and HCP interviews were analysed thematically. Framework analysis was used to analyse the interview transcripts. 31 For patient participant interview data, two experienced qualitative researchers (EL and CH) repeatedly read and coded a subset of transcripts independently. Initial codes were discussed and a framework of themes and subthemes for the analyses was produced. All transcripts were then coded by EL and CH within this framework with additional emergent themes and subthemes added to the framework. Codes were discussed on an ongoing basis to inform the analysis and resolve any disagreement about the interpretation of the data. Analysis was discussed with the full research team to identify areas for closer consideration (including negative case analysis) and to enhance the credibility of the thematic framework and interpretation. 32 Finally, themes relevant to the aim of assessing the acceptability and feasibility of the study and intervention methods were selected and are discussed here.
For HCP interviews, initial descriptive codes were generated from the transcripts and these codes were then grouped into a framework that used the components and constructs of normalisation process theory (NPT)128 as themes and subthemes but was left open enough to allow the emergence of additional themes relating to the acceptability of the study research, screening and intervention methods. NPT is an implementation theory that can help us understand how research, screening and intervention processes translate into real-world practice. This translation is often key to the acceptability of processes to HCPs. It provides a theoretical framework to guide the exploration of how processes are implemented (operationalisation and organisation), embedded (routinised in day-to-day practice) and integrated (sustained). NPT proposes that it is the work of people individually and collectively to implement practices that lead to these becoming embedded in practice. The theory has four key constructs (coherence, cognitive participation, collective action and reflexive monitoring), each of which has four components.
Patient screening and recruitment
The flow of participants through the study is displayed in Figure 2. A total of 620 patients were screened, of whom 105 (17%) were potentially eligible (based on AUDIT-C score). Fifteen of these (14%) were recruited and nine of those recruited (60%) received interventions. Of the 15 patients recruited, 13 (87%) participated in qualitative interviews about their experience.
FIGURE 2.
Feasibility study Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, 75 out of 470 screened eligible based on original inclusion criteria and 30 out of 150 screened eligible based on amended inclusion criteria; b, nine received the intervention, four of whom also received the booster session.
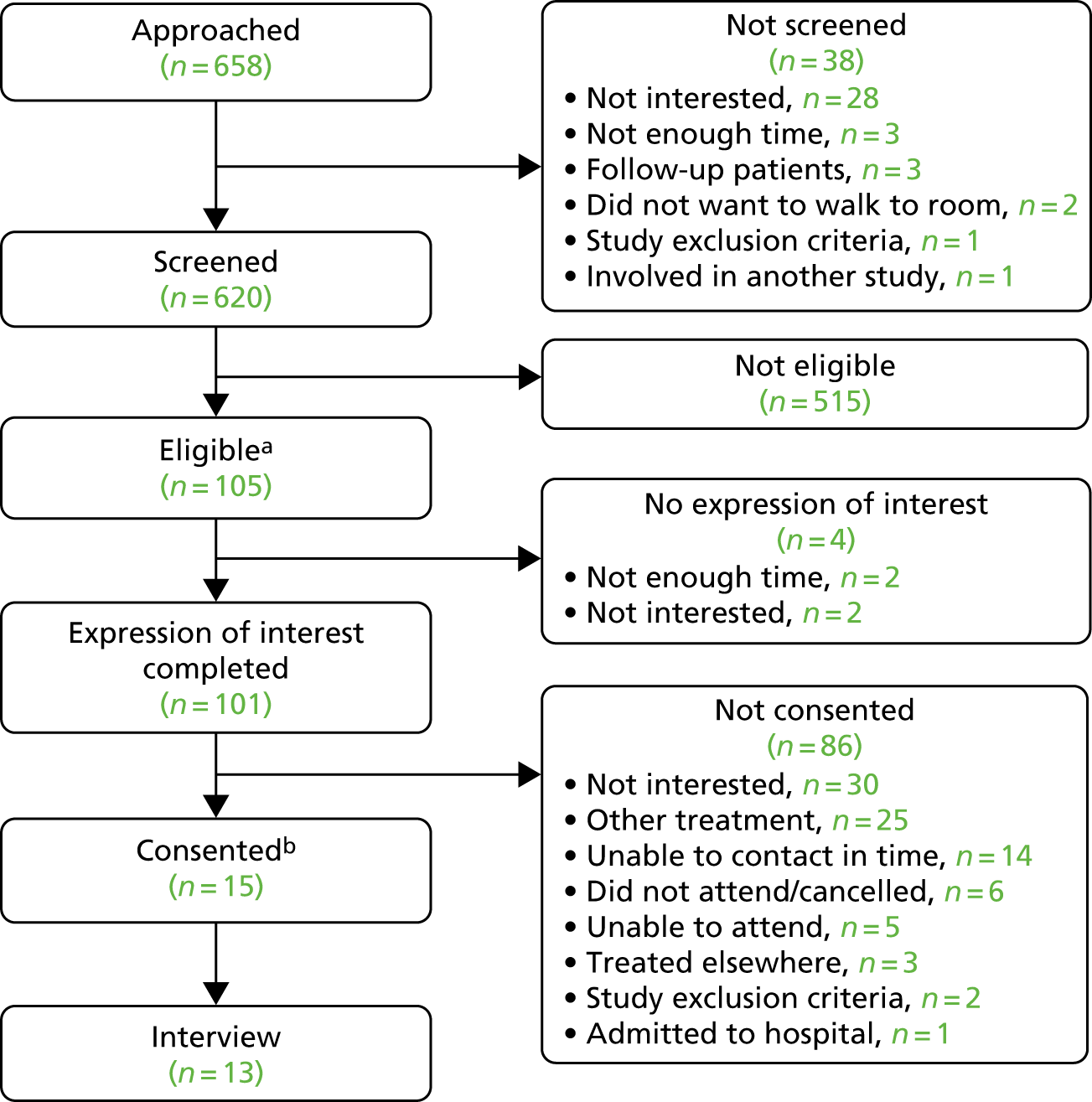
Figure 3 shows that, across the study as a whole, approximately 29% of patients screened scored ≥ 5 on the AUDIT-C, with almost 16% reporting consuming six or more standard drinks on a single occasion at least weekly and 14% of eligible patients consenting to participate in the study.
FIGURE 3.
Recruitment graph for feasibility study.
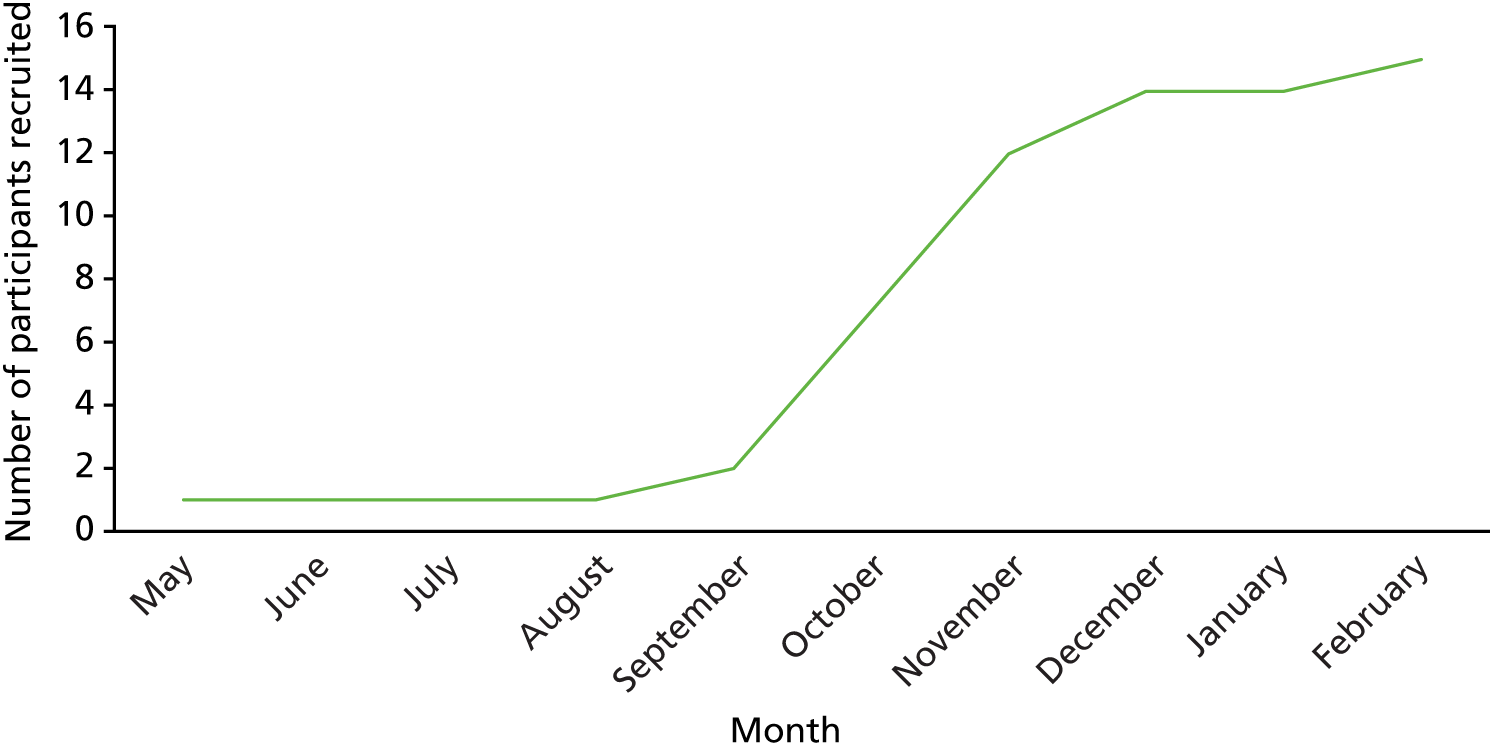
Screening and recruitment rates both before and after amendments were made to the study procedure are detailed in Table 12. These figures demonstrate that the recruitment rate improved from 9.33% of eligible patients to 26.67% following implementation of the amendments. Recruitment rates are displayed in Figure 3 and show the influence of the amendments as well as the benefit of a HCA joining the PAC team in October 2016, who, after completing the study-specific training, took ownership of the study at their site.
| Variable | Original criteria | Post-amendment criteria | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | % of eligible | n | % | % of eligible | n | % | % of eligible | |
| Screened | 470 | 100.0 | 150 | 100.0 | 620 | 100 | |||
| AUDIT-C score of ≥ 5 | 148 | 31.5 | 30 | 20 | 178 | 28.7 | |||
| AUDIT-C question 3 score of ≥ 3 | 75 | 16.0 | 22 | 14.7 | 97 | 15.7 | |||
| Eligible | 75 | 15.0 | 100.0 | 30 | 20 | 100.0 | 105 | 16.3 | 100 |
| Consented | 7a | 1.5 | 9.3 | 8 | 5.3 | 26.7 | 15 | 2.4 | 14.3 |
Patient alcohol consumption
All patients completed the full AUDIT questionnaire to assess eligibility to receive the intervention and the four participants who received a booster session also completed the full AUDIT questionnaire at this time. The mean AUDIT score at consent was 8.3 [standard deviation (SD) 3.0, n = 15] and at visit 3 (booster session) was 6 (SD 1.4, n = 4). Among AUDIT-positive patients only (≥ 8) the mean score was 10.4 (SD 1.6, n = 9) at consent.
Intervention delivery
As part of data collection, HCPs who delivered intervention sessions were asked to record whether or not patients received each of the three key intervention aspects (BA, BI and patient information leaflet) and, whether or not goals were set during the initial intervention session. Of the nine patients who screened as eligible to receive the intervention, all received BA, BI and the patient information leaflet, and 56% (n = 5) set goals.
Qualitative results
Interviews (HCPs, n = 3; patients, n = 13) found that proposed screening and intervention techniques (i.e. behaviour change and brief motivational techniques) were acceptable both to patients and to HCPs delivering the intervention. It was also found that screening and intervention could be conducted without having a negative impact on patient care.
Patient interviews
Initial framework analysis of patient interview data identified eight themes, seven of which were considered to relate to the aim of assessing the feasibility and acceptability of the research, screening and intervention processes. Themes concerned patient perceptions of their drinking behaviour (Defensive othering); the acceptability of screening [Alcohol screening is expected in the NHS but not specifically preoperative assessment; Alcohol screening (using AUDIT) is acceptable]; the acceptability and effectiveness of intervention methods (Preoperative assessment is a window of opportunity for screening and brief behavioural intervention, Brief behavioural intervention targeting alcohol consumption is acceptable although with mixed effects, Screening alone may lead to alcohol reduction) and terminology used in screening and intervention (It’s pints not units, but units rather than standard drinks). These themes, supported by quotations from the interview transcripts, are discussed below.
Defensive othering
In line with findings early in the feasibility study, which showed that patients responded adversely to the use of terminology around ‘risky drinking’ in the study materials, all participants interviewed resisted the narrative of being a ‘risky’ or ‘problem’ drinker. Instead they drew on the strategy of ‘defensive othering’, whereby they made comparisons with others who drink more or more frequently than them in order to normalise their own drinking and distance themselves from those perceived as ‘problem drinkers’. In this way participants were able to admit that they drank and that they may have drank in excess of the drinking guidelines but also to portray their drinking behaviour as acceptable. Reasons used by participants to explain why their drinking behaviour was not a concern included that it was within the old recommended limits, it was not excessive or dependent or alcoholic or alcohol abuse or binge, they were not a spirit drinker, they did not drink in the house, they drank only with meals, they drank only socially, they did not drink as much as their peers, young people or health professionals and they did not get drunk, have hangovers or drink and drive:
Well it didn’t really, it didn’t, the drinking, it didn’t apply at all really. It’s, as I say, it’s I mean that’s all I have, it’s nothing compared with some of them I’ve seen, some of them and they’ve way more than me I have me pints that’s it no more than that . . . Oh I drink a lot less than other men.
ID 120, male, 87 years
I know that I should cut down. But I also like it. I don’t have a problem in as much as I’m dependent on it. I think maybe a little bit dependent but I do like it. I don’t think about it all the time.
ID 14, female, 62 years
Alcohol screening is expected in the NHS but not specifically at preoperative assessment
Alcohol screening was generally viewed as a ‘normal’ part of health-care appointments in the NHS as a whole. As a result, just over half of participants expected to be asked about their alcohol consumption during PA:
As I say, for years I’ve had it [alcohol screening] from the NHS through the doctor’s surgery, all my life, even the chemists ask now.
ID 108, male, 77 years
However, the majority of participants did not associate alcohol use with surgery or consider that it would be relevant to the perioperative period. As such, many did not expect to be asked about their drinking behaviour at PA:
No, I didn’t think it [alcohol] had any bearing on a hip replacement basically.
ID 70, male, 68 years
However, two of the 13 participants interviewed were able to identify ‘risks with the anaesthetic and drinking’ and ‘if you cut down beforehand and afterwards your recovery should be much better’. Despite the clearly limited patient knowledge of the associations between alcohol and surgical outcomes, a couple of participants reported that they would not consume alcohol before surgery:
When I went in for surgery I wouldn’t drink any alcohol anyway really.
ID 120, male, 87 years
Alcohol screening (using AUDIT) is acceptable
Regardless of whether or not alcohol screening was expected or thought to be relevant to surgery in general and to orthopaedic surgery more specifically, it was widely considered to be acceptable. At least some of this acceptability seemed to derive from the perceived normality of alcohol screening in health-care settings, with patients explaining ‘they always ask about how much alcohol you drink’ and ‘it’s always asked in hospital’. The approach adopted by screening staff also appeared to be important in ensuring acceptability, with participants describing the process as ‘non-judgemental’, ‘not intimidating’ and ‘comfortable’:
I felt fine, because she asked it in such a non-judgemental way she didn’t put it over to me that she was criticising or judging or anything.
ID 14, female, 62 years
The AUDIT, used for screening in this study, was found to be acceptable to patients, who described it as easy to complete and understand:
Well, I think it covers every aspect, and it gives you plenty of choices. Yes, I think it’s a very good laid out questionnaire.
ID 103, male, 67 years
Although there was no indication that alcohol screening was unacceptable to even the heaviest drinkers in our sample, some participants explained that alcohol use could be a ‘sensitive subject’ for some people, with the amount of alcohol consumed being related to the acceptability of screening:
. . . people who are drinking perhaps erm to a level that is not healthy that would be a little bit uncomfortable and then you’ve got the people who would be in the excess area who probably would refuse to take part . . .
ID 126, male, 65 years
Brief behavioural intervention targeting alcohol consumption is acceptable albeit with mixed effects
All the participants who had received the BBI reported that it was acceptable. Participants explained that they were ‘comfortable with it’ and ‘happy to get the advice’ and that the intervention gave them ‘insight’:
It did focus my mind more as well because I know that I have been at risk or I am at risk but it did focus my mind on do I really want that one? No well let’s stop there . . .
ID 14, female, 62 years
However, the acceptability of the intervention did not necessarily mean that it would lead to behaviour change. Some participants explained that although they were happy to receive the information they would ‘take no notice of that’, or that it ‘didn’t change us one bit’, and some linked their lack of intent to change with their age, stating ‘I’m too old to change now’.
Despite this, over half of the participants who received the intervention reported that it had led to a reduction in their alcohol consumption. However, the magnitude of the change and the period of time during which the change was maintained varied:
I knocked a pint off each session . . . and now that I’m on the mend I still probably knocked a pint off.
ID 104, male, 70 years
From the week before I had surgery I didn’t have any drink for 6 weeks. Completely 6 weeks, possibly 7 . . . that I didn’t have any alcohol at all.
ID 108, male, 77 years
These changes in drinking behaviour were reported by some to be a result of having the consequences of alcohol consumption on surgery explained:
. . . it was explained that it was, it would help us in healing . . . and there was less chance of catching any bugs or anything.
ID 108, male, 77 years
Screening alone may lead to alcohol reduction
Although information received in the intervention was linked to behaviour change, some participants reported that it was the AUDIT screening tool that had led them to change their drinking behaviour. These participants explained that it ‘focused’ or ‘concentrated’ the mind and ‘crystallised how often I drink’:
. . . answering them and going through them and I thought ‘mmm that might be a little bit too much’ or ‘mmm I’m OK with that’ so I think the information was clear and it, it did focus my mind down on things.
ID 14, female, 62 years
The issue of honesty in self-report measures of alcohol consumption also arose here. Although one participant reported that other patients may conceal the truth when asked about their alcohol consumption in a health-care setting, another identified that the time between completing the screening and attending the intervention session allowed for reflection on the reasons for screening and led to more honest responses when completing the baseline measures (i.e. the full AUDIT):
It might be in the back of your mind that you know you’re going in for surgery, and that they might get a bit wobbly with you. Really I don’t, but it’s the syndrome where you don’t tell medical people.
ID 76, male, 64 years
. . . when they’ve had chance to think and reflect they might think that honesty is the best policy.
ID 108, male, 77 years
Preoperative assessment as a window of opportunity for screening and brief behavioural intervention
The preoperative period and the PAC were seen as an acceptable time and place for the delivery of alcohol screening and intervention. However, it was generally seen as important that screening and intervention were conducted around existing appointments, with some participants explaining that it was a case of catching them at the ‘right place, right time’ and others explicitly identifying the benefit of not having to ‘make a special trip’. One patient also explained that the time and setting would be beneficial to both the patient and the NHS:
Yes, it was probably good timing, with coming in, you know, to going to be coming in for an operation.
ID 103, male, 67 years
However, some patients indicated that the orthopaedic patient population may not be the ideal target for alcohol screening and intervention specifically because they tend to undergo surgery later in life than those attending PA for other conditions. When asked about screening and intervening with people of a similar age, participant responses varied. Some indicated that interventions may be less relevant as their alcohol consumption was already lower than it had been in their younger years:
I think there must be 60, 70 per cent of people who 10, 20 years ago were big drinkers. I’m not saying it’s like that now [Interviewer: Mmm] but most of them have come to common sense and they don’t drink as much [Interviewer: Yep] but years ago when you were younger I think most people drank far too much . . .
ID 117, male, 67 years
Others explained that older people may be less motivated to change:
I think you are wasting your time really . . . I shouldn’t say that like but ah well I don’t think at my age, I don’t think I would take a lot of notice to be honest . . . a lot of young ones would but the older ones wouldn’t I don’t think oh at my age I wouldn’t they are set in their ways.
ID 120, male, 87 years
On the other hand, some participants felt that screening and intervention with older people would be more effective, with one explaining that older people were likely to be more honest, and another that they were likely to listen more than younger people. One participant also explained that respect and a non-judgemental approach were particularly important if interventions were to be acceptable to older people.
It’s pints not units, but units rather than standard drinks
To accurately document alcohol consumption and to understand the sensible drinking guidelines, patients and HCPs must understand the amount of alcohol in different beverages and servings. The screening and intervention tools in this study used the term ‘standard drink’ to describe a drink or serving containing 8 g of alcohol. From interview transcripts, it was clear that participants had little to no understanding of this term prior to participation in the study; however, they stated that they were able to understand the term when it was explained:
I’ve not heard it before . . . my interpretation of standard drink is how many units you should have. You hear it all the time on the television, you hear it on advertising, you hear it on all sorts of things but it, until I was actually taking part in the study it didn’t really mean a lot to me.
ID 14, female, 62 years
Participants described being more familiar with the term ‘unit’, although it was clear that the understanding of what a unit comprised was limited. Some participants suggesting that further health promotion and communication relating to this terminology would be beneficial:
I think they advertise 3 units for a pint. So, if you go in a pub for a drink, or if you buy a can, do you have a standard drink, or do you have 3 units? Or is there only 2 units in it?
ID 103, male, 67 years
Overall, the consensus was that patients were most comfortable talking about the quantity of alcohol consumed in terms of pints or glasses:
No, well I, I only know, like, ‘a pint’ or ‘a pint and a half’, you know what I mean?
ID 109, male, 66 years
Health-care professional interview results
Five overarching themes were identified, four relating to components of NPT (defined in Box 2), which provide insight into the work of HCPs to implement the screening, intervention and research processes in the context of PA. Although these four themes show that interview transcripts revealed evidence of all four of the key constructs of NPT, there was no evidence that all four components were present within each construct, although findings may have emerged in these areas had the topic guide included probes for each specific construct. The final theme (Perception of patient responses) emerged from the data but did not directly fit within the NPT framework and relates to the various patient responses to screening and intervention processes.
Owing to the small pool of potential interviewees and the visibility of their involvement in the trial to others (e.g. colleagues in PA, research team members), quotations are labelled using ID numbers only.
The ‘sense-making’ work that people do individually and collectively when an intervention is delivered (e.g. How is the intervention understood by patients? Do they share a view of its purpose, understand how it will affect them personally, and grasp its potential benefits?).
Cognitive participationThe ‘relational’ work involved in engaging and legitimising an intervention and associated practice (e.g. How do patients participate in an intervention? What keeps them motivated to continue taking part?).
Collective actionThe ‘operational’ work performed by individuals to organise and enact a new practice (e.g. How do patients make the changes work? What strategies do they employ to organise and structure their lives to accommodate the new practice?).
Reflexive monitoringThe ‘appraisal’ work that people do to understand and evaluate the impact of a new practice (e.g. how it affects them and the people around them, and how patients come to decide whether or not the intervention is worthwhile over time).
Coherence
During interviews, HCPs did not directly differentiate their roles in day-to-day clinical practice from their roles in the feasibility study (differentiation). However, they did consider how the study activities fitted in with usual care and how screening and BBI may best be implemented into PA (communal specification), as well as providing clear descriptions of their own roles and tasks in the study (individual specification). They also discussed the benefits of being involved in the study as well as benefits of screening and BBI (internalisation).
Communal specification
Communal specification involves individuals working together to understand the aims and objectives of a new practice. In the case of this feasibility study, much of this understanding can be seen to relate to how screening and intervention processes can be embedded in PA. HCPs considered how the screening and intervention process fitted with existing preoperative processes from both their own perspective and that of patients. The HCPs explained that, for both them and patients, discussion about alcohol use and specifically the impact of alcohol use on surgery fitted well with the existing PA because alcohol use was already asked about. Further to this, staff felt that conducting the TAU PA with the patient before going into the intervention allowed them to develop a rapport with the patient and to anticipate how the patient was likely to react to different questions. The HCPs perceived this as facilitating effective intervention delivery:
. . . when we are doing it as part of the PAC, I did a couple that way and that actually flows quite well. Because again, you’ve got that, sort of, you know, rapport going already with them and you just, kind of, you mention the alcohol thing at the start of the pre-assessment. You go, ‘You’re part of the study but we’ll come back to that at the end,’ so actually it, kind of, filters in quite nicely that way and it did work a lot better that way . . .
HCP 4
However, owing to staff availability and involvement in the study, it was not always possible for the same HCP who conducted the TAU pre-assessment to deliver the intervention, which meant that these benefits could not be gained:
I think a few people I did the intervention with, I’d never met them before, I didn’t have the notes, it was quite difficult to go in and go, ‘All right, OK, so you’ve triggered you drink X amount of units,’ and I think sometimes that can be a little bit hostile for people coming into that.
HCP 5
The HCPs also considered the potential burden on patient participants. Arranging screening and intervention around existing appointments so that the patient did not need to travel to the hospital specifically for the study was seen as important. Making the most of time usually spent waiting for appointments by using that time to conduct screening was also beneficial:
. . . they’re already here in the hospital erm and if they are waiting for their appointment it passes a bit of time for them and they don’t mind it if they come in early and things like that.
HCP 9
Following up on initial expressions of interest in a timely manner was also seen to be beneficial both for patients and for study staff as it meant that the study information was still fresh in patients’ minds and that they were more likely to have maintained an interest in taking part:
. . . waiting a week for to do the ‘phone call, that was a bit, because by the time they’d gone home, if they’ve read it or if they didn’t read it they’d forgot about it and they just didn’t want to know but now that that’s been fixed it’s just to a day thing’s will go, go better . . .
HCP 9
Individual specification
Individual specification relates to how individuals develop an understanding of their specific tasks and responsibilities. HCPs identified that the study training had provided them with an idea of what tasks they personally would have to complete as part of the study. However, having the opportunity to practise the screening and intervention and to develop real-world experience of delivery was more important in terms of learning how to complete these tasks:
Well, again, it comes with experience, it’s good to have that initial training so you know what you’re asking, you know what you’re roughly wanting to get from it and then you just find your own way of asking the questions. I think yes, again, practice.
HCP 5
All three of the HCPs interviewed also demonstrated a clear understanding of their own role and responsibilities, including the specific activities that they needed to complete. There was also an indication that they had an understanding of how their role fitted with the roles of others in the project:
I have been interviewing people after they have already seen [HCA] and been identified so they have agreed to participate in it. We have spoken about strategies they might want to use or have thought of to try and, not abstain from drink, but to try to reduce the amount they drink prior to anaesthetics.
HCP 4
Internalisation
Internalisation is concerned with understanding the value and benefit of the processes being implemented. The HCPs demonstrated knowledge of the benefits both from involvement in the study and from the delivery of screening and intervention. The HCPs identified benefits for themselves as individuals as well as benefits for the patients involved. For HCPs, being involved in the study was viewed as ‘interesting’ and ‘enjoyable’. Similarly, being involved in new care practices was seen as beneficial:
I think just being involved in something that’s quite new and upcoming and will play a very big part in pre-assessment in the future, I think this is obviously just the benchmark for it now, so I think it’s quite good to be involved in something like that.
HCP 5
The knowledge gained from the training, which was then communicated to patients in the intervention sessions, was also seen as worthwhile and a key driver of ensuring that patients engaged with the intervention:
. . . because a lot of the patients want the surgery, so if you can say to them, ‘You know that it’s proven that it can improve your post-operative recovery,’ a lot of them are quite keen to do it. I know it was identified in a lot of our training, people hadn’t realised that alcohol can be a contributing factor to blood pressure or another issue that they’re dealing with, weight loss and that type of thing, so when you’ve actually sat and gone through stuff like that, people had responded really well to it.
HCP 5
Cognitive participation
Cognitive participation is the work individuals do to develop practice around new processes and then to sustain these. There was no specific evidence in the interviews of managers or team leaders working to drive the implementation of screening and intervention (initiation). However, there was clear evidence that the HCA responsible for screening also acted to co-ordinate the project activities, increase engagement and facilitate the roles of other staff members (enrolment). In contrast to the clear consideration that had been given to how best to implement screening and intervention in to the PA setting, there was no evidence to suggest that HCPs had considered whether or not they were the right people to be involved in delivering screening and intervention (legitimation), although the feedback provided on intervention delivery was used by one staff member as a method of ensuring that they were fulfilling their role within the study. The HCPs did discuss difficulties in maintaining engagement with the study and the limited profile of the study within the PAC (activation).
Enrolment
The HCA responsible for conducting the initial screening of patients identified that she was involved in co-ordinating other HCPs involved in the study and facilitating their delivery of intervention sessions, as well as connecting with other HCPs in the clinic to help promote screening:
I just speak to the nurse and say ‘oh I’m down here if they want to talk about some research that’s going on erm bob them down’ . . .
HCP 9
This work was also identified by one of the other HCPs, who explained that the HCA was the person to go to with questions about the study:
We’ve got [HCA] which is great, she’s doing a fantastic job and anyone just goes to her now which is great to have . . .
HCP 4
Activation
The HCPs identified time lags, both between the initial training and recruitment of the first patient and between each recruited patient, as limiting implementation of the study into PA. With practice and experience of delivering screening and intervention being identified as key to effective delivery of the study activities, such delays were likely to have an impact not only on the implementation of the study but also on the quality of intervention delivery:
There are huge gaps between doing it and then, I mean, it seemed like a massive gap between our first training and then doing it because I thought it had been such a long time. Then big gaps between times when you have seen patients and then you are all ready to see them and they just don’t turn up . . .
HCP 4
The HCPs explained that they thought that ‘there would be more going on’ as part of the study. The small number of participants recruited over a relatively extended period restricted the visibility of the study in the PAC and led to both reduced confidence and reduced capability in delivering the interventions:
I think I would just feel more comfortable and more proficient if we did it more often and if it was more visible . . . Just make it more of an established thing.
HCP 4
Collective action
Collective action is the work that people do to enact a set of new practices. Interviews included descriptions of how the HCPs and patients interacted with the screening and intervention tools (interactional workability), as well as the skills they had that enabled their delivery of the screening and intervention (skill set workability); however, there was no discussion of HCPs’ trust in the intervention or in each other’s ability to deliver the required tasks (relational integration). As with cognitive participation, where there was no evidence of higher-level management involvement in the implementation of the study, there was no discussion of support for the study from management, but there was some discussion of how staff time was allocated to the study activities (see Contextual integration).
Interactional workability
The screening and intervention processes were based around the full AUDIT and AUDIT-C screening questionnaires, the BA tool and the BBI tool. In interviews, HCPs discussed how they interacted with these tools and how patients responded to them. The screening questionnaire was described as easy to use, with the inclusion of an infographic explaining standard drinks being particularly beneficial:
It’s, it was easy, it’s easy for them to understand and me as well. Erm and with it having the little table on the top of what a, a standard drink is I can [Interviewer: Yeah] show them that and they can understand it as well as me . . .
HCP 9
Similarly, the BA and BI tools were described as ‘good’, ‘not difficult’ to use, containing all the required information and acting as effective prompts to guide intervention delivery:
The intervention is really in detail, it is all there. There is loads of information and it points you to exactly where you need to be so it is good.
HCP 4
However, having time to become familiar with the intervention materials was considered to be important in improving interaction with them, which was, in turn, important in maintaining a professional demeanour during intervention delivery:
. . . once you’ve done it a couple of times and you, kind of, get familiar with the paperwork, with the questions, with the intended outcomes, I think it becomes a lot easier to do it and a lot more fluid . . .
HCP 5
. . . you don’t want them to think you don’t know what you’re doing or looking through endless bits of paper. I don’t feel comfortable doing that.
HCP 4
The tools were also seen to aid interaction between the HCPs and patients during intervention delivery. The tools supported the communication of information, reinforced the intervention session by offering space to record key aspects of the discussion and enhanced engagement:
. . . definitely showing them something because then they lean in and they’ll start reading off the page.
HCP 5
Skill set workability
The HCPs discussed a number of skills that enabled them to deliver the screening and intervention effectively. They explained that they were experienced in and comfortable with discussing alcohol with patients and were able to do so in a non-judgemental manner:
I don’t talk to people in a judging way. I feel quite comfortable and confident to talk about that kind of thing. I don’t think I have to shy away from stuff . . .
HCP 4
Further to this, HCPs explained that they had existing skills in recognising and managing different patient responses, which was important in tailoring questioning and interventions to maintain a positive interaction with patient participants:
. . . the response of the patients if they were like a bit abrupt and you know about it then I would feel a little bit oh I’m I’m putting on to this patient a bit too much so I kinda back off a little bit and let them talk about it . . .
HCP 9
Contextual integration
Regarding contextual integration, HCPs discussed the allocation of staff time to deliver screening and intervention. There was a clear focus on wanting to ‘do things properly’, which was considered to require dedicated time:
Have you got valid time where you don’t feel like you’re going to be rushed? Because if you do feel rushed then I know I have to skim through it and I’m not going to do it the way I should do . . .
HCP 4
Effective co-ordination and organisation of the study sessions was necessary with pre-planning to ensure that staff were available and appointments were arranged to give them the additional time needed to deliver the intervention:
. . . it’s fitting everything in it’s trying to fit things in and moving other appointments around to fit the intervention in as well but if it’s planned in advance it works out good . . .
HCP 9
However, in some circumstances, for example when patients arrived late, these factors were outside HCPs’ control. This was particularly relevant for screening, which was usually conducted while patients were waiting for an appointment:
. . . if they turn up late it’s it’s hard for me to get them, they don’t always want to stay and talk or they’re too busy with something . . .
HCP 9
Similarly, if a HCP allocated time to deliver an intervention or indeed to deliver a number of interventions on one day but patients failed to attend the sessions, this was problematic:
I had a morning set aside, there were two or three people booked for the intervention . . . so when I was set aside the morning, I think only one or two people came for it . . .
HCP 5
Reflexive monitoring
Reflexive monitoring focuses on the work that individuals do to appraise new processes. The HCPs did not specifically discuss the collection of evidence to gauge the effectiveness of the intervention or the implementation of screening and intervention into the PA (systematisation). However, there was evidence that the individuals involved had drawn on both anecdotal evidence from their own experiences and the feedback provided by the research team (individual appraisal) to determine whether or not screening and intervention was effective. Two of the interviewees pointed to collaborative assessments of how effectively screening and intervention could be implemented in PA (communal appraisal). Finally, participants discussed formal modifications (made to the study procedures through the submission of an amendment) and informal modifications made to tailor delivery of the intervention (reconfiguration).
Individual appraisal
The HCPs appraised their involvement in the study and described it as enjoyable and interesting. Beyond this, they identified the benefits of the screening process in terms of raising patients’ awareness of their alcohol consumption and motivating change:
. . . a lot of them actually don’t realise that they’re drinking more than what is recommended, so if you tell them that they are, they’ll go, ‘Ah!’ [gasps], you know, and they’re completely in shock. So they want to know more . . .
HCP 5
The HCPs also identified instances when they had been able to assess whether or not the interventions had led to patients changing their alcohol consumption behaviour:
I recognised him the second time so that was quite good because usually I get moved around. So, I think it just being a longer-term thing, it is interesting to see results . . .
HCP 4
These positive appraisals of screening and intervention effectiveness had also led to some of the HCPs changing their usual practice during PA:
I’ve actually had people, not for orthopaedic surgery . . . they’ve questioned me on the alcohol and does it, sort of, pose any difference either way. So I’ve brought it in a little bit there without actually bringing it in, but just saying, ‘Well, actually yes, you know, we’re running this study for certain ones.’
HCP 5
Communal appraisal
Although HCPs did not specifically discuss participating in communal appraisal of the interventions, two of the interviewees alluded to its occurrence. The first anticipated that interviews with other HCPs would produce similar findings, suggesting that the study processes had been discussed:
I imagine that whoever is doing it has probably got similar things . . . you might get repetition for the same sort of things going on.
HCP 4
Another interviewee explained that, although NHS staff are often averse to change, discussion of positive experiences of delivering the screening and intervention processes had helped to alter this view and that this would, in turn, aid future implementation:
. . . people are reluctant to change and reluctant to change the ways that they do the practice. I think it actually has gone down a lot easier than I thought it was going to and I think it will continue to do that because I think people are now saying it’s not as bad as anybody thought it was going to be, it’s actually all right, it’s quite easy . . .
HCP 4
Reconfiguration
The study processes were formally reconfigured during the course of the feasibility study via the submission of an amendment, which had been informed by the experiences of the HCPs and research team to this point. The positive impact of this was discussed by one interviewee, who felt that the change of the study title and the reduction of the time between initial and follow-up expression of interest were both beneficial:
I think things will go better now with it just being 24 hours . . .
HCP 9
I think now that the titles changed it’s a lot better . . .
HCP 9
All three interviewees also pointed to less formal modifications that they made to the delivery of screening and intervention processes with reference to finding ‘your own way’. The key here appeared to be rewording questions and amending the terminology used to help improve the acceptability of screening and intervention to patient participants. In some instances it appeared that specific terms could be problematic, regardless of the individual patient. This specifically related to avoiding any implication that drinking behaviour may be problematic or that patients were being ‘accused’ of being a dependent drinker:
I perhaps might say pattern for that rather than behaviour because I think that gives a negative vibe to people . . .
HCP 5
I don’t know, I think just the wording of those, so it was trying to find a way around wording that so that people didn’t feel like they were being, almost accused of being an alcoholic . . .
HCP 5
In other cases, it was important to tailor the phrasing of questions and information to specific patients:
. . . it’s trying to get the wording to suit the person in front of you because again, it varies from person to person, not everybody will respond the same way to the same question.
HCP 5
Perception of patient responses
The HCPs’ perceptions of alcohol screening were that it was generally acceptable to patients, with very few declining screening and the majority being open to discussion about their alcohol consumption:
. . . good, yeah good ah I’ve never had one say ‘oh no I a can’t be bothered with it . . .
HCP 9
However, some demographic characteristics of patients were seen to make certain groups more open to screening and more likely to provide honest responses, with men and younger patients being perceived as more accepting of the screening and more open about their drinking behaviour:
I think the men are they the tend to be a bit more honest than the women erm women get quite a bit more defensive . . .
HCP 9
The HCPs identified once again that patients generally showed a favourable response to the intervention:
. . . the majority of the time it’s been really fine, people responded really well.
HCP 5
Nonetheless, specific aspects of both the screening and intervention were identified as potentially leading to adverse responses from patients. The HCPs identified individual questions and phrases as well as a view that individuals were being ‘singled out’ as potentially making patients more defensive or less open. Each of these issues appeared to relate to a potential implication of judgement that the individual’s drinking was problematic:
I think it’s just a general issue erm some people get defensive because I think they think you’re insinuating that they are drinking too much and I don’t think they kinda get that it’s for everybody on the list not just a single person . . .
HCP 9
However, it was clear that HCPs felt that the majority of patients were comfortable discussing their alcohol consumption.
Discussion
Although recruitment of patient participants initially proved to be challenging, amendments to the inclusion criteria, study title and period for consideration of participation led to a clear improvement in the percentage of eligible patients consented to the trial. This indicates that the amended study materials and processes were acceptable. Qualitative data from interviews with patient and HCP participants support this as they identify screening and intervention methods as acceptable and the PA as a suitable setting. Further to this, qualitative data provide additional insight into factors that can enhance the acceptability of methods and those that can aid behaviour change.
Qualitative data show that, despite patient participants having little to no knowledge of the association between alcohol consumption and surgical outcomes, screening and intervention in the PAC were acceptable. The finding that alcohol screening is acceptable supports findings from previous quantitative research, which also indicated good patient acceptance. 57,62,68 Meanwhile, the findings that alcohol interventions delivered in the PA are acceptable mirror those relating to interventions delivered either predominantly105 or entirely in the postoperative period. 104
This study builds on previous work by providing further detail on factors that contribute to the acceptability of screening and intervention in PA. Specifically, arranging screening and intervention around existing preoperative appointments so that no additional trips to the hospital were required was important, and the perceived normality of screening, along with the non-judgemental approach of staff, contributed to its acceptability. Although both patient and HCP participants identified alcohol as a potentially sensitive subject and suggested that some patients would not be comfortable completing the screening questionnaire, there was no evidence of this being the case even among the heaviest drinkers in our sample.
This work provides some early indications that BBIs may bring about reductions in alcohol consumption in the preoperative period, with half of the participants who received the intervention reporting at least some change to their drinking behaviour. Further to this, the subgroup of participants who did not change still reported receipt of the intervention as acceptable. This work also provides some insight into the components of screening and intervention techniques that were most useful to participants, namely information about the effects of alcohol on recovery, healing and surgical outcomes, along with effects on body weight and medication use and information about the lower-risk guidelines and health benefits of cutting down. However, the small number of patient participants and the variability in the information and advice identified as important highlights the need for interventions and interventionists in the preoperative setting to be sufficiently flexible to allow the tailoring of delivery to the individual patient. 129,130
With previous research131,132 finding that older adults are one of the least well-informed groups when it comes to alcohol measurement and terminology, it is perhaps not surprising that patient participants in this study were unfamiliar with the term ‘standard drink’. Although participants reported being able to understand the screening questionnaires that use this term, their descriptions of standard drinks and units were not always consistent with this and participants consistently reported being more comfortable describing their consumption in terms of pints or glasses. Based on these findings, providing HCPs with adequate training and guidance to ensure that they are confident in converting patient descriptions of alcohol consumed in terms of pints or glasses into standard drinks or units is likely to enhance the accuracy of screening. Further to this, interventions designed for or implemented with older patient populations should seek to include descriptions of units or standard drinks in relation to common beverage servings (e.g. pints of beer, glasses of wine) in order to enhance the interpretation of low-risk drinking guidelines.
Similarly, as participants in this study rejected any categorisation of their drinking as ‘risky’ and endorsed the ‘non-judgemental’ approach of HCPs delivering screening and interventions, future screening and intervention work should avoid the use of such categorisations and ensure that the non-judgemental approach is maintained. Furthermore, the tendency for patients to use defensive othering to distance themselves from ‘problem drinkers’ suggests that interventions that focus on individual-level, and context-specific (e.g. surgery), risks and benefits are likely to be more acceptable to patients in the preoperative setting.
Some barriers to effective delivery and receipt of screening and intervention methods were identified. Lack of adequate time to deliver screening and intervention to a high standard was identified as a barrier by the HCP participants. Scheduling of intervention sessions appropriately, providing research staff to conduct research-specific tasks (such as consent and randomisation procedures) and including guidance in intervention training that directly addresses how best to deliver intervention sessions if time is limited would go some way to minimising this issue. Nonetheless, unless additional fully trained staff are made available, and adequate space for them to work in is made available, this challenge is unlikely to be fully resolved.
Although removing the term ‘risky drinking’ from the project title and study materials appears to have aided improvements in patient recruitment, feedback following alcohol screening and information about the low-risk drinking guidelines often draw on this or similar terminology. Therefore, qualitative work in the subsequent pilot RCT sought to explore the preferred terminology in more depth.
Conclusions
The proposed research and intervention methods, once amended, were found to be acceptable to both patient and HCP participants. Although recruitment of patient participants was initially challenging, the changes made during the feasibility study greatly improved the percentage of eligible patients who consented to participate in the study. The planned pilot RCT was therefore considered justifiable.
Changes made prior to pilot randomised controlled trial
With well-documented screening effects and subject reactivity in previous research133,134 and some participants in this feasibility study reporting that completion of the screening questionnaire resulted in changes to drinking behaviour, possible changes to the screening and eligibility process were considered. Completion of an initial screen (AUDIT-C) followed by a more in-depth (full AUDIT) screening questionnaire to confirm eligibility at a later date (the method used in this work) may well induce changes in drinking behaviour between the two screens and lead to participants who are motivated, and already acting to reduce consumption, being excluded from intervention delivery. Therefore, the subsequent pilot RCT will assess eligibility relating to drinking behaviour based on the initial AUDIT-C screen. The full AUDIT will then be used at baseline and at follow-up to provide a more detailed assessment of alcohol consumption.
Chapter 4 Pilot randomised controlled trial
Introduction
The feasibility study showed that the screening and intervention methods were broadly acceptable to both patient and HCP participants. A pilot RCT was therefore considered to be justified. Pilot RCTs involve the rehearsal of full trial processes, including randomisation and follow-up, to provide estimations of rates of eligibility, recruitment, retention and data completion that can be used to inform a decision about the feasibility of proceeding to a definitive trial.
The methods of the pilot RCT are reported in the trial protocol. 1
Aims
Primary objective
To assess the feasibility of a definitive RCT by estimating rates of eligibility, recruitment and retention in a pilot RCT.
Secondary objectives
-
Assess of completion rates for all data collection tool, including measures of alcohol consumption, quality of life and joint functionality.
-
Establish response variability of proposed outcome measures for a definitive trial, which will include drinking status and quality of life.
-
Estimate rates of secondary outcomes and perioperative complication rates, including bleeding and infections.
-
Explore the acceptability of intervention and research methods with HCPs and patients in qualitative interviews.
Methods
Design
The design was a three-centre, two-arm (TAU vs. BBI), parallel-group, individually randomised pilot trial with an embedded qualitative interview study.
Setting and subject population
The pilot RCT was conducted across three secondary care hospitals in the north of England, one of which was the site of the feasibility study. All three sites have dedicated PACs with surgical care pathways of 6–10 weeks for patients undergoing hip and knee arthroplasty.
Eligible patients were those aged ≥ 18 years who were listed for elective primary hip or knee arthroplasty, met the criteria for increasing risk drinking, had the capacity to provide informed written consent and were able to write and converse in English.
Sample size
Based on recommendations for external pilot trials,135 this pilot trial aimed to obtain data from 30 patient participants in each trial arm at 6 months post recruitment. Systematic reviews of alcohol BI trials show mean follow-up rates of approximately 75%,82,136 although rates of follow-up for older adult populations may be higher. 137 It was therefore decided to allow for a loss to follow-up of 25% providing an initial recruitment target of 80 patients (40 per trial arm).
Progression criteria
The criteria for progression to a definitive trial were recruitment of 40% of patients identified as eligible and retention of 75% of participants at 6-month follow-up.
Procedure
Initial screening for alcohol consumption was conducted by a HCP trained in the use and scoring of the AUDIT-C. This initial screen was conducted after a patient had been listed for elective orthopaedic surgery, either face to face in the outpatient clinic or over the telephone, with the responses immediately assessed for eligibility. Eligibility for the study was confirmed if the patient screened positively for increased risk drinking (AUDIT-C score of ≥ 5) or reported drinking six or more standard drinks in one session at least weekly (identified from question 3 on the AUDIT-C). Patients who scored an AUDIT-C score of < 5 and who consumed six or more standard drinks in a single session less frequently than weekly were thanked for their time and received positive feedback on their low-risk drinking status. Patients were excluded if they were scheduled to undergo sequential joint replacements (bilateral replacement of both joints within a short time) during the study, displayed current (active) withdrawal from alcohol, or had a severe psychiatric disorder requiring medical treatment, cognitive impairment or dementia, affecting their ability to interact with the intervention.
All patients who screened eligible were given a verbal description of the trial and those who expressed interest in participating were provided with a copy of the participant information sheet (see Appendix 13) and were asked if they were willing to be contacted about the study. Those willing to be contacted provided either telephone or e-mail contact details (dependent on patient preference), which were recorded on the expression of interest form. Patients were then given a minimum of 24 hours to consider participation. After this time the HCP contacted them by their preferred method to gauge ongoing interest in the trial. When patients expressed ongoing interest, the PAC was informed of their likely participation and arrangements were made for the completion of the consent and randomisation process as well as potential delivery of the BBI.
Patients were met at the PAC by a member of the research team not involved in screening or intervention delivery. The researcher checked that each patient was still interested in participating in the study, confirmed their eligibility, including establishing their capacity to consent, and conducted the informed consent discussion, during which the patient completed the written consent form including indication of their willingness to have their intervention session recorded and to be contacted about participating in the optional booster session and a qualitative interview.
Once informed consent had been obtained, the researcher accessed the electronic randomisation system, which randomised patients to either TAU or BBI on a one-to-one basis. All participants were provided with copies of the WOMAC and EQ-5D questionnaires to complete while they waited to be seen by the PA HCP.
Following consent and randomisation, all participants were seen by a PA HCP. Participants allocated to the control condition received TAU and completed the AUDIT questionnaire. Participants allocated to the intervention condition received TAU and completed the AUDIT questionnaire before receiving BBI.
Brief behavioural intervention
The intervention and its development are described in detail in Chapter 2 and summarised here. The intervention commenced with completion of the full AUDIT questionnaire. This was followed by the delivery of approximately 5 minutes of structured advice relating to alcohol consumption (based on completion of the AUDIT). The HCPs used the BA tool as a visual prompt and to guide their delivery of advice about alcohol consumption. Following BA, the BI protocol was used to guide up to 25 minutes of brief behaviour change counselling targeting alcohol reduction or cessation. This aspect of the intervention was designed to be tailored to the individual patient’s needs and motivations and could include assessing motivation and confidence to change, pros and cons of changing, goal-setting, problem-solving and identifying of sources of social support. Patients were provided with copies of the intervention tools to take away with them, as well as a copy of the patient leaflet.
Patients who consented to be approached for the second booster session were contacted around 1–2 weeks before surgery to gauge their ongoing interest in the booster session and to arrange this. Where possible, booster sessions were delivered by the HCP who delivered the initial intervention session. If this was not possible, for example because of availability, another appropriately trained HCP delivered the session. Booster sessions were delivered either in clinic or over the telephone according to patient preference. As with the initial intervention session, the booster session began with completion of the full AUDIT tool. The remainder of the 10- to 20-minute session aimed to review or revise behavioural goals, provide feedback on progress and increase self-efficacy. For participants who had expressed interest in changing their drinking behaviour during the initial intervention session, but had not yet set any goals, this session allowed them to set goals and produce action plans to reach these goals and address any potentially ‘difficult’ times.
Control condition: treatment or care as usual
The comparator condition of TAU is described in detail in Chapter 2 and summarised here. Patients allocated to the TAU condition did not receive the intervention but did complete all study baseline measures. As part of the standard PA for hip or knee arthroplasty, patients were asked a variety of health-related questions, had blood samples taken and had other observations, including blood pressure and echocardiograms, completed. Patients considered by the PA HCP to be ‘heavy drinkers’ or those identified as heavy drinkers as a result of LFTs were routinely referred for consideration by a consultant anaesthetist who assessed any potential risk to anaesthesia or surgery itself.
All three sites included in the trial also had specialist alcohol support services available. However, referral of patients from PA to in-hospital alcohol services was reported to be rare, with no standard process for identifying and referring patients (see Chapter 2 for further detail). Further to this, these services were hospital wide and not designed to provide specific support for preoperative alcohol reduction or cessation.
Follow-up
From screening to final follow-up there were six or seven key points of contact between participants and members of the research team, depending on trial arm. The trial process is summarised in Figure 4 and the measures and activities completed at study visit are shown in Tables 13 and 14.
FIGURE 4.
Pilot RCT process.

| Time point | Study period | ||||||
|---|---|---|---|---|---|---|---|
| Screening | Visit 1: 6–8 weeks pre surgery | Visit 2: 1–2 weeks pre surgery (intervention arm only) | Visit 3: 1 and 3 days pre surgery | Visit 4: up to 5 days post surgery | Visit 5: 6 weeks post surgery | Visit 6: 6 months post intervention | |
| Enrolment | |||||||
| AUDIT-C | ✗ | ||||||
| Expression of interest | ✗ | ||||||
| Consent | ✗ | ||||||
| Randomisation | ✗ | ||||||
| Interventions | |||||||
| Intervention | ✗ | ||||||
| Booster | ✗ | ||||||
| TAU | ✗ | ||||||
| Assessments | |||||||
| AUDIT baseline | ✗ | ||||||
| EQ-5D baseline | ✗ | ||||||
| AUDIT outcome | ✗ | ✗ | ✗ | ✗ | |||
| EQ-5D outcome | ✗ | ✗ | |||||
| WOMAC | ✗ | ✗ | |||||
| POMS | ✗ | ||||||
| Clavien–Dindo score | ✗ | ||||||
| Interview | ✗ | ||||||
| Site | Number | Intervention | Control |
|---|---|---|---|
| 1 | 19 | 11 | 8 |
| 2 | 34 | 17 | 17 |
| 3 | 15 | 7 | 8 |
| Total | 68 | 35 | 33 |
All baseline measures were completed at visit 1. Follow-up assessments took place 1–3 days preoperatively (either over the telephone or in hospital if admitted the day before surgery); 3–5 days post surgery; at or around the 6-week postoperative outpatient appointment; and at 6 months post intervention.
Data collection
The primary outcomes of this pilot trial were the number of patients screened, the percentage of eligible patients recruited, and the percentage retained at 6-month follow-up. In addition to these outcomes to be utilised in designing a definitive trial, we collected, at the time points shown in Table 13:
-
alcohol consumption – assessed with the full AUDIT tool9
-
health-related quality of life – assessed with the EQ-5D114,115
-
joint functionality – assessed with the WOMAC. 113
Rates of postoperative complications and length of hospital stay were assessed with the Clavien–Dindo tool112 and POMS. 111
Covariate data relating to health behaviours [smoking, physical activity and body mass index (BMI), which have been shown to be associated with surgical complication rates] routinely captured during PA were also collected at visit 1.
Qualitative methods
Patient interviews
At the point of consent to the pilot RCT, patient participants indicated whether or not they were willing to be contacted about participating in a qualitative interview. At 6 months post intervention, Ellen Lynch contacted participants by telephone to conduct the final follow-up. Participants who had previously provided their consent were asked if they would be willing to take part in an interview. Those who assented were sent a copy of the interview-specific information sheet by e-mail or post (depending on participant preference) and Ellen Lynch made arrangements to conduct the interview at a time and place convenient to the participant.
On the day of the interview, the participant was met by Ellen Lynch, who provided them with a hard copy of the interview-specific patient information sheet and gave them a verbal introduction to the interview process, including ethical considerations. She also gave a verbal summary of her research experience and her role in the project. The participant then had the opportunity to ask any questions before completing the informed consent discussion and signing the interview-specific consent form. Once consent had been gained, Ellen Lynch began the interview by asking the participant what led to them coming into hospital and then utilised the questions and prompts contained within the arm-appropriate (TAU or BBI) interview topic guide (see Appendix 15) to structure the interview.
At the end of the session, the participant was thanked for their participation in the interview and in the trial as a whole, and had the opportunity to ask any further questions.
Interviews lasted between 35 minutes and 1 hour 45 minutes and were audio-recorded and transcribed verbatim. Transcripts were anonymised and were then subject to framework analysis.
Health-care professional interviews
Each HCP who consented to take part in the pilot RCT and went on to be involved in delivery of the screening or intervention was offered the opportunity to participate in a qualitative interview regarding their experience of the trial. The research associate contacted HCPs by e-mail asking if they were willing to participate in such an interview.
Those who assented to participate were sent an electronic copy of the HCP interview-specific information sheet and the research associate arranged a time and place convenient to the HCP for the interview to take place. At the beginning of each interview session, the HCP was provided with a hard copy of the interview-specific information sheet. The research associate provided a verbal summary of her research experience, her role in the project and the interview process including ethics considerations. The HCP then had the opportunity to ask any questions before completing the informed consent form. Once consent had been gained, the research associate began the interview by asking the HCP to explain their role in the project and then utilised the questions and prompts contained within the interview topic guide to structure the interview.
At the end of the session, the HCP was thanked for their participation and had the opportunity to ask any questions.
Interviews lasted between 20 minutes and 1 hour 30 minutes, and were audio-recorded and transcribed verbatim. Transcripts were anonymised and then subject to framework analysis.
Analysis
Statistical analysis
The numbers of screened and eligible patients and the rates of recruitment, randomisation and retention were reported, and any reasons for ineligibility and non-participation were summarised. The baseline characteristics and outcomes at follow-up time points were summarised, with descriptive statistics presented by arm or site (means, medians and ranges for continuous data and frequencies and proportions/rates for categorical data). We investigated the data completeness of the study tools by reporting the number of missing data by outcome and arm. This information was then used to identify the primary outcome for a definitive trial and estimate the necessary sample size.
Qualitative analysis
Qualitative data from HCP and patient interviews were analysed using framework analysis. 126 This is a recommended approach for qualitative health research with objectives linked to quantitative investigation. 138 As in the feasibility study, patient and HCP participant interviews were analysed separately.
For patient participant interview data, a subset of transcripts were repeatedly read and coded with initial codes used to develop a framework of themes and subthemes. All transcripts were then coded within this framework with additional emergent themes and subthemes added to the framework. Finally, themes relevant to the aim of assessing acceptability and feasibility of study and intervention methods were selected and are discussed here.
For HCP interviews, initial descriptive codes were generated from the transcripts before being grouped into a framework that used the components and constructs of NPT128 as themes and subthemes but was left open enough to allow the emergence of additional themes relating to the acceptability of the study research, screening and intervention methods.
Results
Screening, recruitment and retention
The flow of participants through the trial, including screening, recruitment and retention at follow-up, is presented in the CONSORT (Consolidated Standards of Reporting Trials) flow diagram in Figure 5. In total, 1117 patients were approached; 866 completed the screening questionnaire (AUDIT-C), 198 were potentially eligible based on the AUDIT-C screen (22.9% of those screened) and 68 (34.3% of those eligible) consented and were randomised (Figure 6).
FIGURE 5.
Pilot RCT CONSORT flow diagram.
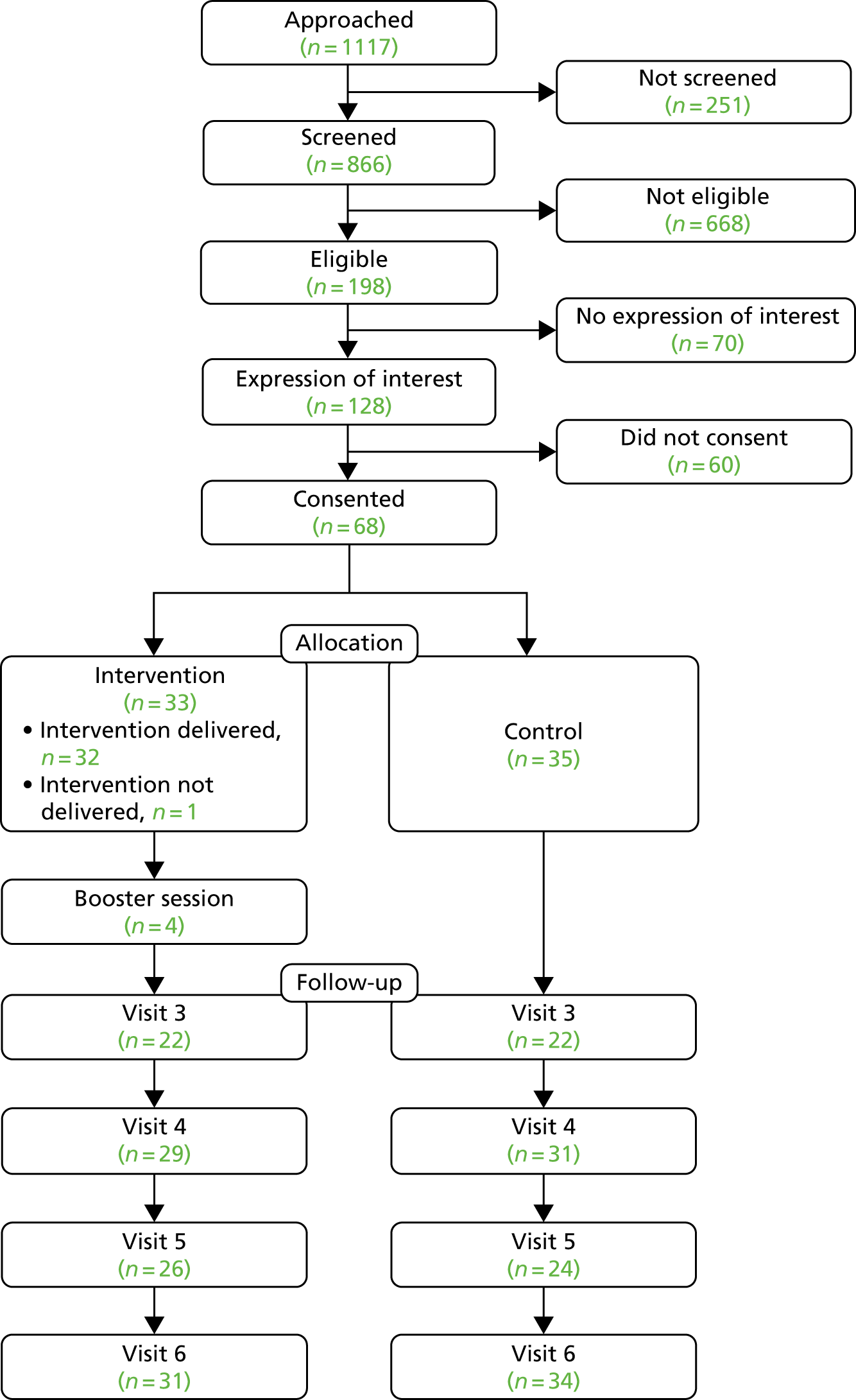
FIGURE 6.
Pilot RCT recruitment graph.
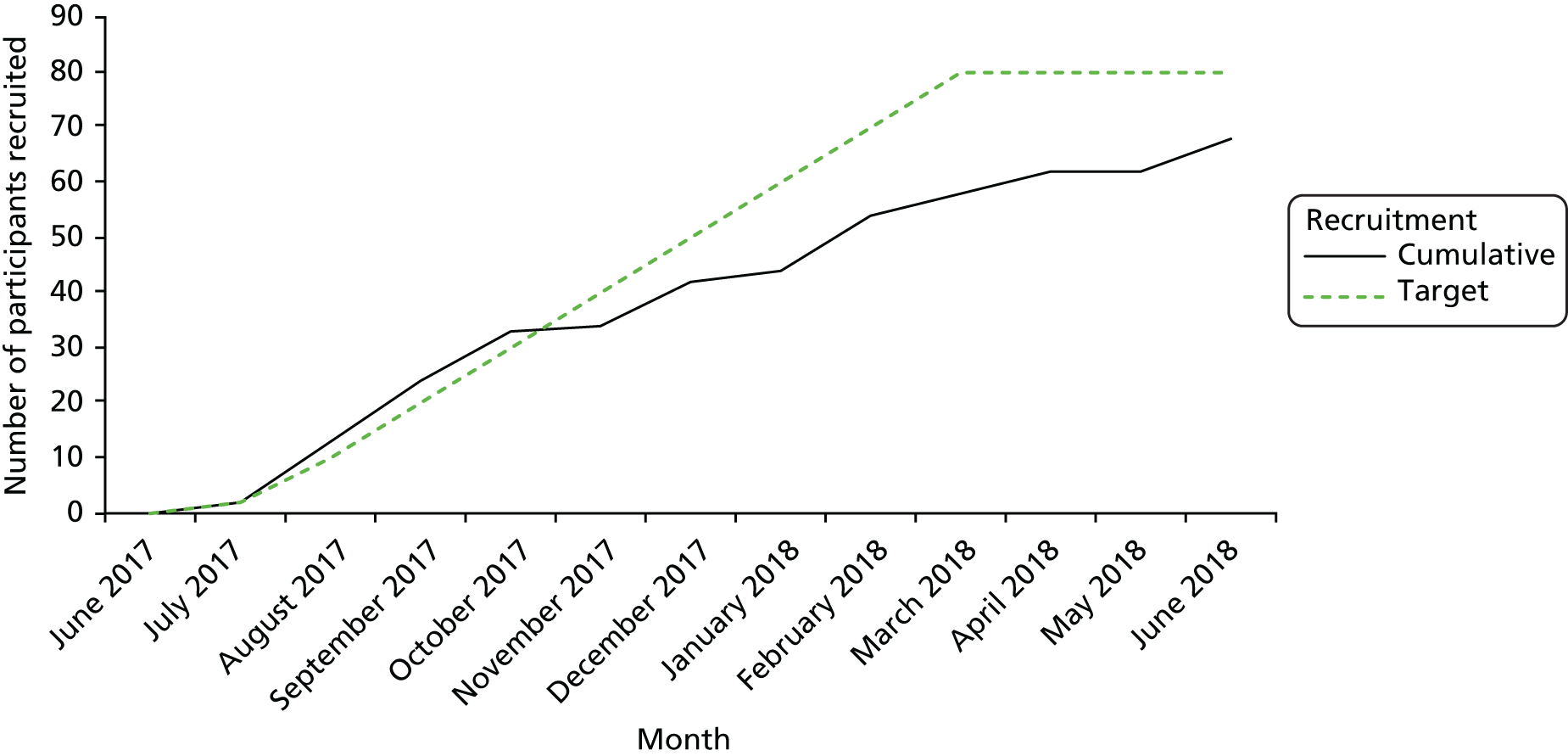
Although recruitment of eligible participants fell short of the progression criterion (40%) and the target of 80 patients recruited was not met, retention at final study visit was much higher than estimated, with 65 patients completing the final follow-up. Thus, the subsequent target of follow-up data obtained from 60 patients was exceeded.
Tables 15 and 16 show the reasons patients were found to be ineligible or declined to participate in the trial. Table 17 presents a summary of screening and recruitment data from the feasibility study and pilot RCT to allow direct comparison. Although the percentage of eligible patients expressing interest in the study was 31% lower in the pilot RCT than in the feasibility study, the percentage of eligible patients recruited increased by 20%. The most frequent reasons for ineligibility remained consistent and related to alcohol consumption behaviour, with 88% of ineligible participants scoring < 5 on the AUDIT-C questionnaire and a further 6% reporting that they did not drink alcohol at all. The most frequently cited reasons for non-participation among eligible patients were lack of interest in the study and not having enough time available to participate; 17 eligible patients had no clearly recorded reason for non-participation.
| Reason | Frequency (%) |
|---|---|
| AUDIT-C score of < 5 | 668 (88.1) |
| Did not drink at all | 46 (6.1) |
| Surgery too soon | 24 (3.2) |
| Psychiatric disorder | 6 (0.8) |
| Possible alcohol dependence | 5 (0.7) |
| Pre-assessment already complete | 4 (0.5) |
| Patient requested rescreen – not eligible | 3 (0.4) |
| Sequential surgeries | 1 (0.1) |
| Did not speak English | 1 (0.1) |
| Reason | Frequency (%) |
|---|---|
| Not interested | 44 (42.7) |
| Unclear | 17 (16.5) |
| Not enough time | 12 (15.6) |
| Unable to contact | 9 (8.7) |
| No staff available | 8 (7.8) |
| Cut down on own | 4 (3.9) |
| Left clinic | 3 (2.9) |
| Did not attend | 1 (1.0) |
| Recruitment stage | n (%) | % of eligible | |||
|---|---|---|---|---|---|
| Feasibility | Pilot | Feasibility | Pilot | Difference | |
| Approached | 658 (100) | 1117 (100) | – | – | – |
| Screened | 620 (94) | 866 (78) | – | – | – |
| Eligible | 105 (16) | 198 (18) | 100 | 100 | – |
| Expressed interest | 101 (15) | 128 (11) | 96 | 65 | –31 |
| Consented | 15 (2) | 68 (6) | 14 | 34 | + 20 |
Participant characteristics at baseline
Table 18 summarises the baseline characteristics of the trial participants. The age of the 68 patients recruited ranged from 40 to 85 years (median 67 years); the patients were predominantly male (80.9%), all but one identified as white British, and 66% attended pre-assessment in preparation for knee arthroplasty surgery.
| Demographic characteristics | Arm | Total (N = 68) | |
|---|---|---|---|
| BBI (N = 33) | TAU (N = 35) | ||
| Age (years) | |||
| Mean | 63.8 | 68.4 | 66.2 |
| Minimum | 40 | 46 | 40 |
| Median | 64 | 69 | 67 |
| Maximum | 75 | 85 | 85 |
| Missing | – | – | – |
| Gender, n (%) | |||
| Number | (N = 33) | (N = 35) | (N = 68) |
| Male | 27 (81.8) | 28 (80.0) | 55 (80.9) |
| Female | 6 (18.2) | 7 (20.0) | 13 (19.1) |
| Missing | – | – | – |
| Ethnicity, n (%) | |||
| Number | (N = 33) | (N = 35) | (N = 68) |
| White British | 33 (100) | 34 (97.1) | 67 (98.5) |
| Other | 0 | 1 (2.9) | 1 (1.5) |
| Operation type, n (%) | |||
| Number | (N = 33) | (N = 35) | (N = 68) |
| Hip | 13 (39.4) | 10 (28.6) | 23 (33.8) |
| Knee | 20 (60.6) | 25 (71.4) | 45 (66.2) |
| Missing | – | – | – |
Participant health behaviour at baseline
Table 19 shows the distribution of measures of health behaviours at baseline by trial arm. Participants’ weekly alcohol consumption as reported in units at pre-assessment ranged from 1 to 90 units per week, with a median of 18 units. Some participants reduced alcohol consumption between screening and baseline. Although only five participants identified as current smokers, almost 60% were ex-smokers. Forty per cent of patients reported no physical activity and the median BMI identifies the majority of the patient group as falling into the ‘obese’ category.
| Health factor | Arm | Total | |
|---|---|---|---|
| BBI | TAU | ||
| Units of alcohol per week | |||
| Number | (N = 32) | (N = 34) | (N = 66) |
| Mean | 24.6 | 18.5 | 21.4 |
| Minimum | 1 | 2 | 1 |
| Median | 20 | 16 | 18 |
| Maximum | 90 | 60 | 90 |
| Missing | 1 | 1 | 2 |
| Number of days physically active per week | |||
| Number | (N = 18) | (N = 18) | (N = 36) |
| Mean | 5.0 | 4.6 | 4.8 |
| Minimum | 1 | 1 | 1 |
| Median | 6 | 5 | 5 |
| Maximum | 7 | 7 | 7 |
| Missing | 15 | 17 | 32 |
| BMI (kg/m2) | |||
| Number | (N = 33) | (N = 34) | (N = 67) |
| Mean | 31.7 | 31.6 | 31.7 |
| Minimum | 24.6 | 21.9 | 21.9 |
| Median | 32.2 | 32.1 | 32.1 |
| Maximum | 44.6 | 43.6 | 44.6 |
| Missing | 0 | 1 | 1 |
| Any physical activity, n | |||
| Number | (N = 31) | (N = 34) | (N = 65) |
| Yes | 20 | 19 | 39 |
| No | 11 | 15 | 26 |
| Missing | 2 | 1 | 3 |
| Smoking history, n (%) | |||
| Number | (N = 33) | (N = 34) | (N = 67) |
| Current | 2 (6.1) | 3 (8.9) | 5 (7.5) |
| Ex | 16 (48.5) | 23 (67.7) | 39 (58.2) |
| Never | 15 (45.5) | 8 (23.5) | 23 (34.3) |
| Missing | 0 | 1 | 1 |
The distributions were comparable across trial arms.
Patient follow-up
As seen in the schedule of events (see Table 13), follow-up was based around four or five study visits (depending on trial arm). Visit 2 was an optional booster session and therefore available only to those in the intervention condition. All participants were asked to provide data at all other study visits, although the occurrence and timing of visits 3, 4 and 5 were dependent on participants undergoing surgery (eight of the 68 participants recruited did not have surgery during the study period). Rates of completion varied by visit, with the highest rates of completion at visits 1 and 6 and participation in the optional booster session being especially low (4/35).
Time interval between baseline and surgery
It was expected that surgery would take place between 6 and 10 weeks after the baseline visit. Table 20 and Figure 7 show the distribution of the time intervals between baseline and surgery observed during the pilot trial.
| Time interval (number of weeks) | Arm | Total (n = 65) | |
|---|---|---|---|
| BBI (n = 32) | TAU (n = 33) | ||
| Minimum | 0.7 | 0.3 | 0.3 |
| Mediana | 6.6 | 5.6 | 5.7 |
| Maximuma | > 70.6 | > 46 | > 70.6 |
FIGURE 7.
Number of weeks between baseline and surgery.

Eight patients did not undergo surgery before the end of the trial. Two (one in each arm) were removed from the surgical waiting list and six (three in each arm) were awaiting surgery at the close of follow-up. A small number also had the 6-month post-intervention follow-up very shortly after surgery.
Completion rates for baseline and outcome measures
If patient participants attended a study visit, completion of each questionnaire was high, especially for AUDIT and EQ-5D. The WOMAC questionnaire was less well completed, with five partially complete at visit 1, four partially complete and 18 not completed at visit 4; this instrument required contact with the patient within a short period following surgery. By contrast, measures of complication rates and length of hospital stay could be completed from patient notes by an appropriately trained HCP and did not require patient input (Table 21).
| Study visit | Measure | Completion | Arm | Total | |
|---|---|---|---|---|---|
| BBI | TAU | ||||
| 1 | AUDIT | Complete | 32 | 35 | 67 |
| Partiallya complete | 1 | – | 1 | ||
| Missingb | – | – | – | ||
| EQ-5D | Complete | 33 | 35 | 68 | |
| Partially complete | – | – | – | ||
| Missing | – | – | – | ||
| EQ-5D score | Complete | 32 | 35 | 67 | |
| Missing | 1 | – | 1 | ||
| WOMAC | Complete | 30 | 32 | 62 | |
| Partially complete | 3 | 2 | 5 | ||
| Missing | – | 1 | 1 | ||
| 2 (optional booster 1 week pre surgery) | AUDIT | Complete | 4 | 0 | 4 |
| Partially complete | – | – | – | ||
| Missing | 5 | – | 5 | ||
| 3 (1–3 days pre surgery) | AUDIT | Complete | 22 | 22 | 44 |
| Partially complete | – | – | – | ||
| Missing | 2 | 6 | 4 | ||
| 4 (in hospital 3–5 days post operative) | POMS | Complete | 29 | 31 | 60 |
| Missing | 4 | – | – | ||
| Clavien–Dindo | Complete | 29 | 31 | 60 | |
| Missing | 4 | – | – | ||
| WOMAC | Complete | 20 | 24 | 48 | |
| Partially complete | 2 | 2 | 4 | ||
| Missing | 7 | 5 | 12 | ||
| 5 (6 weeks post operative) | AUDIT | Complete | 26 | 24 | 50 |
| Partially complete | – | – | – | ||
| Missing | 1 | 5 | 6 | ||
| EQ-5D | Complete | 24 | 24 | 48 | |
| Partially complete | – | – | – | ||
| Missing | 3 | 5 | 8 | ||
| EQ-5D score | Complete | 24 | 24 | 48 | |
| Missing | 3 | 5 | 8 | ||
| 6 (6 months post intervention) | AUDIT | Complete | 31 | 34 | 65 |
| Partially complete | – | – | – | ||
| Missing | – | – | – | ||
| EQ-5D | Complete | 31 | 34 | 65 | |
| Partially complete | – | – | – | ||
| Missing | – | – | – | ||
| EQ-5D score | Complete | 31 | 34 | 65 | |
| Missing | – | – | – | ||
| Interview | Consented | 7 | 7 | 14 | |
Summary statistics for clinical measures across study visits
Tables 22–25 summarise the clinical outcomes by trial arm. Because varying numbers of participants provided data at each study visit, it is difficult to comment on the pattern over time, but typical AUDIT scores decreased in both arms until visit 6. The AUDIT scores were also summarised as whether or not they were in the ‘risky drinking’ category (AUDIT score of ≥ 8). At baseline, 55% of those in the BBI arm were risky drinkers, compared with 49% in the TAU arm. At visit 6, 45% of those in the BBI arm were risky drinkers, compared with 38% in the TAU arm. Typical EQ-5D visual analogue scale scores increased slightly over time in both arms. Typical WOMAC scores were little changed in either arm.
| Study visit | Arm | Total | |
|---|---|---|---|
| BBI | TAU | ||
| (N = 32) | (N = 35) | (N = 67) | |
| 1 | |||
| Mean | 9.7 | 7.7 | 8.6 |
| Minimum | 3 | 3 | 3 |
| Median | 9 | 8 | 8 |
| Maximum | 24 | 18 | 24 |
| Missing | 1 | – | 1 |
| (N = 4) | (N = 0) | (N = 4) | |
| 2 | |||
| Mean | 6.8 | – | 6.8 |
| Minimum | 3 | – | 3 |
| Median | 4 | – | 4 |
| Maximum | 16 | – | 16 |
| Missing | 5 | – | 5 |
| (N = 22) | (N = 22) | (N = 44) | |
| 3 | |||
| Mean | 6.3 | 4.6 | 5.4 |
| Minimum | 0 | 0 | 0 |
| Median | 5.5 | 4 | 5 |
| Maximum | 19 | 15 | 19 |
| Missing | 2 | 6 | 8 |
| (N = 26) | (N = 24) | (N = 50) | |
| 5 | |||
| Mean | 5.5 | 3.6 | 4.6 |
| Minimum | 0 | 0 | 0 |
| Median | 5 | 3 | 4 |
| Maximum | 16 | 10 | 16 |
| Missing | 1 | 5 | 6 |
| (N = 31) | (N = 34) | (N = 65) | |
| 6 | |||
| Mean | 7.6 | 6.6 | 7.1 |
| Minimum | 0 | 0 | 0 |
| Median | 7 | 7 | 7 |
| Maximum | 17 | 12 | 17 |
| Missing | – | – | – |
| Study visit | Arm | Total | |
|---|---|---|---|
| BBI | TAU | ||
| (N = 32) | (N = 35) | (N = 67) | |
| 1 | |||
| Mean | 63.0 | 60.9 | 61.9 |
| Minimum | 20 | 10 | 10 |
| Median | 65 | 70 | 65 |
| Maximum | 95 | 95 | 95 |
| Missing | 1 | – | 1 |
| (N = 24) | (N = 24) | (N = 48) | |
| 5 | |||
| Mean | 67.1 | 76.1 | 71.6 |
| Minimum | 35 | 35 | 35 |
| Median | 70 | 80 | 75 |
| Maximum | 90 | 95 | 95 |
| Missing | 3 | 4 | 7 |
| (N = 31) | (N = 34) | (N = 65) | |
| 6 | |||
| Mean | 73.2 | 71.3 | 72.3 |
| Minimum | 30 | 25 | 25 |
| Median | 80 | 80 | 80 |
| Maximum | 95 | 100 | 100 |
| Missing | – | – | – |
| Study visit | Arm | Total | |
|---|---|---|---|
| BBI | TAU | ||
| (N = 30) | (N = 32) | (N = 62) | |
| 1 | |||
| Mean | 55.1 | 54.3 | 54.7 |
| Minimum | 31 | 4 | 4 |
| Median | 52.5 | 51 | 52 |
| Maximum | 86 | 90 | 90 |
| Missing | 3 | 3 | 6 |
| (N = 20) | (N = 24) | (N = 44) | |
| 4 | |||
| Mean | 54.2 | 50.3 | 52.1 |
| Minimum | 23 | 6 | 6 |
| Median | 55 | 52 | 53 |
| Maximum | 79 | 89 | 89 |
| Missing | 10 | 7 | 17 |
| Visit 4 outcome measure | Arm, n (%) | Total, n (%) | |
|---|---|---|---|
| BBI | TAU | ||
| POMS: morbidity category | |||
| None | 26 (89.7) | 31 (100.0) | 57 (95.0) |
| Pulmonary | 1 (3.5) | – | 1 (1.7) |
| Infectious | 2 (6.9) | – | 2 (3.3) |
| Renal | – | – | – |
| Gastrointestinal | – | – | – |
| Cardiac | – | – | – |
| Neurological | – | – | – |
| Wound | – | – | – |
| Haematological | – | – | – |
| Pain | – | – | _ |
| Missing | 2 | 2 | 4 |
| Any complications (Clavien–Dindo) | |||
| No | 27 (93.1) | 29 (93.6) | 56 (93.3) |
| Yes | 2 (6.9) | 2 (6.5) | 4 (100.0) |
| Missing | 4 | – | 4 |
| Clavien–Dindo grade | |||
| 1 | 1 (33.3) | 2 (100.0) | 3 (60.0) |
| 2 | 1 (33.3) | – | 1 (20.0) |
| 3a | 1 (33.3) | – | 1 (20.0) |
| 3b | – | – | – |
| 4a | – | – | – |
| 4b | – | – | – |
| 5 | – | – | – |
| Length of stay (days) | |||
| Mean | 3.4 | 3.4 | 3.4 |
| Minimum | 2 | 1 | 1 |
| Median | 3 | 3 | 3 |
| Maximum | 13 | 18 | 18 |
Complication rates for orthopaedic surgery were relatively low, at approximately 4–5%. 17,22 In this sample, four patients developed at least one postoperative complication. Of these, one patient developed two different complications. Although the numbers are very small, this equates to approximately 6% of the overall sample, and almost 7% of those who completed visit 4. This is in line with evidence from larger trials that complication rates are higher among AUDIT-C-positive patients.
In addition to collecting details of postoperative complications, the Clavien–Dindo measure collects data relating to length of hospital stay and length of intensive care unit stay. The national average length of hospital stay is 4.3 days following primary knee arthroplasty and 4.4 days following primary hip arthroplasty. However, length of stay varies from site to site, with hospitals that employ enhanced recovery procedures reporting shorter postoperative hospital stays. This variation is reflected in our data. None of the participants for whom visit 4 was completed was admitted to intensive care following surgery; however, a number of participants had extended hospital stays, with the maximum length of stay being 18 days.
Sample size estimates for a definitive study
After a discussion of the pilot trial results, it was decided that the effectiveness of a preoperative alcohol intervention in a definitive study should have a primary outcome based on alcohol consumption behaviour, and that measures of complications would be a secondary outcome. The primary outcome would be based on AUDIT. This offers the opportunity to use either a continuous (AUDIT score) or a binary (risk group) measure. A number of considerations led us to select a binary measure of AUDIT score of ≥ 8 or < 8 at 6 months after the intervention. First, the intervals between each point on the AUDIT are not necessarily equivalent. Thus, a reduction of, for example, 2 points for someone originally scoring 20 points does not represent the same level of change or associated benefit as a reduction of 2 points for someone originally scoring 10 points. Second, there is evidence that reduction in risk levels assessed by AUDIT is associated with improvements in physical and mental health and quality of life, as well as reductions in alcohol-related consequences. 139,140 This is mirrored in the literature about PA, which shows that those drinking at least two standard drinks per day (i.e. the cut-off point for lower-risk drinking) and high but not low to moderate drinkers are at increased risk of experiencing surgical complications. 58–60 Meanwhile, no current evidence explicitly identifies what would be a clinically meaningful reduction in either alcohol consumption or AUDIT score in the preoperative setting. Third, employing a dichotomous variable for a sample size calculation will project larger numbers and thus would provide a sample size that would be more than adequate for conducting analysis on a continuous outcome should this be adopted in a future trial. Finally, it was also felt that the binary risk measure would be better understood by those less familiar with alcohol screening tools and the interpretation of screening scores. Studies of other brief alcohol interventions have used an effect size of 13% as the basis of the sample size calculation, when the outcome was whether or not the patient managed to reduce from an AUDIT score of ≥ 8 to that of < 8 over the follow-up period. A study of the PRE-OP BIRDS intervention could not use exactly the same outcome as some of the patients had reduced their alcohol consumption between screening and baseline, meaning that although they were eligible based on the initial AUDIT-C screen, they reported lower-risk drinking at baseline. We have nevertheless used 13% as the effect size, but also shown the sensitivity of the estimates by calculating for an effect size of 10% and 15%. The proportion of patients in the pilot trial with an AUDIT score of ≥ 8 at 6 months was 42%. Because the sample size is largest when the percentages are close to 50%, the differences to be detected have been calculated for 40–50%, 40–53% and 40–55%. Standard sample size calculations to compare proportions will give the number of responses needed at follow-up, using a type 1 error rate of 5% and a power of 90%.
It is also necessary to take into account other estimates from the pilot trial to work out how many patients would need to be screened, approached and recruited to the trial:
-
percentage of those screened who are eligible = 23%
-
percentage of those eligible who are recruited = 34%
-
percentage of responses to primary outcome at follow-up – this was 96% in the pilot study, but a conservative figure of 80% was used for the calculation.
Table 26 shows the total number of patients necessary for a definitive trial. To have 90% power to detect a difference between risky drinking rates of 40% and 53%, it would be necessary to screen 9843 patients, of whom 2264 would meet the eligibility criteria and 770 would be recruited (385 randomised to each arm), to obtain the primary outcome for a total of 616 patients at 6 months. Depending on the number of surgeries undertaken at the sites selected for a definitive trial, it would be possible to achieve such a sample size with 8–10 sites recruiting for between 12 and 18 months.
| Trial stage | Difference to be detected | ||
|---|---|---|---|
| 10% | 13% | 15% | |
| Number of responses to primary outcome | 1038 | 616 | 462 |
| Number of recruited patients | 1298 | 770 | 578 |
| Number of eligible patients | 3818 | 2264 | 1700 |
| Number of patients screened | 16,600 | 9843 | 7391 |
Safety reporting
No serious adverse events were identified during the pilot trial.
Qualitative results
Patient interview results
Fourteen pilot trial patient participants consented to and participated in qualitative interviews about their experience of being involved in the trial. Demographic details of these participants are provided in Table 27. Seven themes relating to the acceptability of research, screening and intervention processes were identified from the data and these are presented here.
| Participant characteristic | Arm | Total (N = 14) | |
|---|---|---|---|
| BBI (N = 7) | TAU (N = 7) | ||
| Age (years) | |||
| Mean | 67.7 | 71.3 | 69.5 |
| Minimum | 60 | 63 | 60 |
| Median | 67 | 69 | 69 |
| Maximum | 75 | 84 | 84 |
| Missing | – | – | – |
| Gender, n (%) | |||
| Number | (N = 7) | (N = 7) | (N = 14) |
| Male | 7 (100) | 6 (85.7) | 13 (92.9) |
| Female | 0 (0) | 1 (14.3) | 1 (7.1) |
| Missing | – | – | – |
| Ethnicity, n (%) | |||
| Number | (N = 7) | (N = 7) | (N = 14) |
| White British | 7 (100) | 7 (100) | 14 (100) |
| Other | – | – | – |
| Operation type, n (%) | |||
| Number | (N = 7) | (N = 7) | (N = 14) |
| Hip | 2 (28.6) | 1 (14.3) | 3 (21.4) |
| Knee | 5 (71.4) | 6 (85.7) | 11 (78.6) |
| Missing | – | – | – |
Acceptability of trial design
Interviews considered the acceptability of the trial as a whole, as well as some specific aspects of the pilot RCT design (time to consider involvement, randomisation and follow-up). Many participants stated that they took part simply because they had been asked to. However, participation in research was also viewed as a way of contributing to something that could help others in the future and was a method of ‘giving back’ to the NHS both for previous treatment and for the fact that they would be receiving a hip/knee arthroplasty:
I have had two previous involvements with the NHS, both emergencies, one 10 years ago and the other about 20 years ago. On both of those occasions I couldn’t speak too highly of the treatment I got. Here you are again and you realise what it’s all about. It’s up to you to do what you can to play your part and contribute, I think.
Male, knee, 78 years
The potential individual benefit of receiving additional information from a HCP and the possibility of reducing the risk of complications arising from surgery or improving recovery time was discussed by only one of the interviewees as a motivation for taking part:
I thought, ‘Well, yes’. With initially them saying, ‘Well, it could be beneficial for the operation and after the operation–’ I’ve never had an operation like this before. I think, ‘Well, if it’s going to help, then I’ll have a look at that’.
Male, knee, 64 years
Having the necessary free time to participate in the research also appeared to play a role in the decision. However, one participant explained that it was important that patients were not required to take part, and others indicated that there was still a limit to how much time they would be willing to give up in order to participate, which highlights the need to minimise the potential burden of research on participants:
That’s fine, you know, you don’t mind. It’s when it’s every week, people are, ‘Can we come in?’ and I think if that happened, I would just say, ‘Sorry, I’ve had enough’ . . .
Male, knee, 70 years
Although participants stated that they were ‘quite happy’ with the trial, that they understood the questions they were asked and that the study was ‘fine’ and even ‘enjoyable’ and one of the 14 interviewees expressed a desire to know more about how the analyses would be completed and what was expected of them in terms of behaviour change:
It was always a bit vague about what the nature of the trial was. Obviously, I know the aim and what it’s about, but I didn’t know what you were trying to get out of me. I didn’t know what you were trying to get from me, so it was always a bit vague, I thought . . .
Male, knee, 61 years
The majority of participants identified their participation in the trial as ‘completely independent’ from and ‘separate’ to their treatment but were clear that the trial activities and visits fitted around their existing appointments, with no indication that their participation had any negative impact on the care that they received during the perioperative period:
It’s like today, it’s fitted in absolutely brilliantly today in the hospital. You’re there. It’s not as though you’ve got to put things aside for it. No, it’s fine.
Male, knee, 64 years
When asked about particular aspects of the research design that could have influenced participation, interviewees showed little need for a specified amount of time to consider their participation (24 hours), with the majority stating that they had made the decision to participate when they were first approached or contacted:
Well I was happy anyway because when she asked me I said, ‘I’ll do it anyway’. I was happy just to go ahead with it anyway.
Male, knee, 71 years
Similarly, the randomisation process was found to be acceptable to participants and did not influence their decision to take part. Some participants expanded on this to explain that although there may be some benefit to being allocated to the intervention condition, they did not necessarily feel that they needed that additional information and support; being assigned to the control arm had its own benefits in that it took up less time and that randomisation was an important part of the research method and a requirement if evidence to inform practice was to be gained. However, one participant did feel that being allocated to the intervention arm may have helped them to further decrease their alcohol consumption:
I didn’t object to that because I knew it was a 50/50 thing. If I’m honest, well what did I think? I mean part of me thought, ‘Well that’s me off the hook. They’re not going to probe too deeply’. But on the other hand, I wouldn’t have objected to it. I thought, ‘Well that might have helped me drink even less’. I just accepted it really because that’s the basis on which I went into it.
Male, knee, 85 years
One participant also explained that although the prospect of randomisation did not influence their decision to take part, they did feel that it would be important in the future, should interventions be found to be beneficial, that all patients receive information about alcohol use and surgery:
Personally, I can see why it has to be randomised at the moment, but also I think, longer term, once the research has been pulled together, my view would be it would be great for people to receive it.
Male, knee, 78 years
Trial follow-up methods were also described as acceptable by participants. Specifically, follow-ups were not considered to occur too frequently or to take up too much time, and neither were they described as too intrusive. Participants identified that they never felt pressured to complete the follow-ups and that they felt they could easily request a callback at another time if they were busy:
I didn’t think it was too intrusive and it didn’t take too long. The questions were pretty straightforward and I gave honest answers. I haven’t found it a difficult process.
Male, knee, 85 years
A number of interviewees explained that the follow-ups were fine because they had agreed to them or had been made aware of them when they consented to the trial. One participant went further, identifying that because follow-ups were described in the trial information they would be expected and it was therefore important that they took place:
I think I would have been a little bit frustrated if I hadn’t received the ‘phone call. So going back, earlier on, you were talking about the process. If somebody said, ‘Right, I’m going to ring you in 4 weeks,’ and it’s gone 4 weeks and 2 days, I’m thinking, ‘Why haven’t you phoned me?’ So I would have been on to them just to say, ‘Hang on, you were supposed to ring me’.
Male, knee, 63 years
Acceptability of alcohol screening
In addition to exploring the acceptability of the trial design, the interviews considered the acceptability of the screening and intervention procedures to build on evidence collected in the feasibility study, which found the processes generally acceptable. As in the feasibility study, very few of the participants had identified a link between alcohol use and surgery prior to taking part in the study and thus had not considered that they would be asked about alcohol use as part of the PA:
It never even entered my mind that alcohol would affect the outcome of the operation, the recovery period . . .
Male, knee, 67 years
However, one of the participants identified that a hospital stay could be problematic for ‘heavy’ drinkers:
If anybody was going in who I knew to be a heavy drinker, I would tell them to come and see me and we’ll cut it down. Because it doesn’t go hand in hand . . . You’re only in hospital for a couple of nights. If you’re a heavy drinker, a couple of night can be a long, long time. I think anybody who was a heavy drinker would get asked a lot more questions.
Male, knee, 70 years
Despite this, four of the interviewees expected to be asked about alcohol at PA either because they considered alcohol consumption to be relevant to health generally or because alcohol use was frequently asked about in health-care settings:
Yes, I suppose so, when I look back, because when you go into hospital, you’re always asked about your current health situation, how you are at the minute and how healthy you are. I suppose alcohol would come into that, so, on reflection, possibly, yes, I think it would be part of the standard questionnaire.
Male, knee, 61 years
One participant also identified that he had not expected to be asked about alcohol because he did not consider his consumption to be problematic. However, this participant identified that he was accepting of being asked about alcohol consumption because he recognised that those drinking to excess may present problems for the HCPs:
I don’t consider myself a drinker really, so it didn’t even cross my mind. It’s something that I would never, ever have thought of . . . Doesn’t bother me in the least. They’ve got a right to know because they need to know. If the prospective patient has a problem and makes it cause them a problem. By that I’m talking about somebody who maybe uses a lot of alcohol could cause a problem in the ward . . .
Male, knee, 70 years
Indeed, all of the participants described alcohol screening as acceptable, explaining that they were not ‘bothered by it’, were ‘quite comfortable’ and ‘didn’t object to it’. However, the importance of explaining the relevance of questioning patients about their alcohol consumption in this setting, or at least explaining that it is a standard question, was raised by one interviewee who felt that he had been asked about his alcohol use specifically because he was overweight and expressed a desire to understand why HCPs felt the need to ask about alcohol consumption if the individual was otherwise well:
It’s not really an imposition, it seems to be the way slimmer people view older people or people that are overweight, that means they’ve got to be smokers, drinkers or eaters. Nobody’s ever the perfect person.
Male, knee, 71 years
Much of the acceptability of alcohol screening appeared to derive from the fact that participants were generally open to discussing their alcohol consumption and had ‘nothing to hide’. As such, they did not have a preference about whether it was a doctor, a nurse or a researcher who asked about their drinking behaviour:
It doesn’t really make any difference. No, I’m quite open to whoever it is.
Male, knee, 63 years
Despite the reported acceptability of alcohol screening, the issue of alcohol as a potentially ‘sensitive subject’ for others was raised by a subset of participants, who explained that some people would not be comfortable answering questions about their alcohol use and as a result would not provide honest answers and would not agree to take part in this type of research. However, this assumption is at least partially contradicted by two of the interviewees who self-identified as drinking more than they should. Although one explained that screening could be awkward, the other was pleased that the issue was being raised:
. . . it’s still awkward talking about something which you know is wrong. You’re trying to tell somebody that what you’re doing is not good for yourself. It’s always a bit awkward.
Male, knee, 61 years
I was actually pleased that they were concerned, you know, drinking could be a bit of a problem so I was quite happy that they did . . .
Male, knee, 70 years
Acceptability and effect of the intervention
Interviewees who received the intervention identified it as acceptable and ‘great’ and that it contained the right amount of information, and one went so far as to say ‘I love advice’. Further to this, for one participant the motivational, non-judgemental approach adopted by staff was key as it allowed him to come to his own decision about whether or not he wanted to change their drinking behaviour once he had received the intervention:
I wanted to help, but I didn’t want to be preached to when I’d already made these decisions myself . . .
Male, knee, 61 years
In contrast to the fact that very few of the participants were aware of any association between alcohol use and surgery, a number of interviewees explained that the intervention did not include any information that they were not already aware of:
I felt like I, pretty much, knew, anyway, about the drinking. I’m getting on a bit now, and I’ve seen a lot of it. I didn’t feel like I was learning a great lot. You tend to know what’s right and wrong, anyway, don’t you?
Male, hip, 67 years
Findings regarding the potential effects of the intervention varied, with some participants identifying that they had made significant, long-lasting changes to their drinking behaviour, some saying that they had ‘cut down’ and others saying that they had not changed their drinking at all. This variation was anticipated and summarised by one participant:
I think that some people would just say, ‘I’m going to do what I’m doing. I’m going to have the surgery, then I’m going to do what I’m doing’. I think there are other people who would say, ‘Yes, I can see the benefits, fair is fair’.
Male, hip, 67 years
Reasons for changing were similarly varied and included the calorific content of alcohol, the influence of alcohol on weight, interactions with medication, not ‘feeling’ like drinking in the postoperative period, the intervention confirming existing knowledge and wanting to improve recovery following surgery:
I think it was actually the research which was saying if you cut down on alcohol, you’ll recover better. Something like that.
Male, hip, 67 years
There was some indication that the messages of the intervention specifically about when alcohol consumption should be reduced may need further clarification, as four of the participants explained that they had reduced their drinking postoperatively in order to benefit their recovery:
I was still drinking up until I went into hospital, then following the operation I didn’t drink for 4 weeks, simply because I’d been told that alcohol could be a factor in how well you recovered.
Male, knee, 67 years
Findings regarding the potential benefit of a booster sessions were similarly mixed, with some participants explaining that additional contact would have been beneficial and others explaining that it would not make any difference because they were confident that they could make the changes they wanted without any further input:
I don’t think it would have made much difference to me, personally, simply because I would have said to myself I’d signed up to it or agreed to it pre-op. So I knew it was going to happen, and so I, personally, wouldn’t have needed much, perhaps, assistance or reminding of it. I don’t think I needed to do that . . .
Male, knee, 63 years
The preoperative setting, optimisation, obligation and opportunity
The PAC was considered to be an acceptable setting for the delivery of alcohol screening and intervention and for some it was simply considered to be part of the PA package:
Just as staff at the hospital talk about, for example, ‘These are the tablets you’ll need to take. These are the things you need to bring in on the day,’ ‘This is what you need to think about alcohol.’ So I can see it being part of just general, normal routine stuff that is part of that package . . .
Male, knee, 63 years
However, for those participants who did not draw an association between alcohol consumption and surgery, offering an explanation of this prior to screening could influence acceptability of the alcohol screening and intervention:
I was quite surprised because like I say, I didn’t have a clue that it would have an effect on the recovery at all until it was explained.
Male, knee, 67 years
Upcoming surgery was identified to act as a motivator for changing alcohol consumption in a number of ways. One participant explained that they had planned to reduce their drinking prior to surgery even before they had received the intervention because they wanted to be ‘be fit and right’ for surgery. Others explained that they reduced their drinking to enhance their recovery:
. . . it was mainly for the recovery of my knee; I wanted the best outcome possible for my knee. So, that sort of spurred me on just to try and keep off it.
Male, knee, 67 years
Some participants felt that getting surgery presented them with a certain amount of obligation to do their bit to ensure that the operation went well:
There’s a lot of time and money going into your hip, or whatever, and you need to play the game.
Male, hip, 67 years
Building on this sense of obligation and the desire to undergo surgery that could lead to improvements in health and quality of life, a number of participants identified that they would cut down or stop drinking if this was a requirement for them to have their surgery:
It’s like I said, they told me I couldn’t take warfarin, and it’d be exactly the same as saying, ‘Before you come in, you mustn’t have alcohol for 10 days, but we will test you when you come in’. If you’re in pain, you’re going to stop.
Male, knee, 73 years
The physical effects of surgery itself and specifically of the early postoperative phase could lead to changes in drinking behaviour because participants were not mobile enough to go out, they were taking medication or they did not feel like drinking:
It didn’t occur to me, I wasn’t really interested I suppose. I was groggy half the time anyway. I slept a lot, with all this dope inside me.
Male, hip, 67 years
Alcohol health communication
With the feasibility study identifying that terminology concerning ‘risky drinking’ had a negative impact on recruitment and that the term ‘standard drink’ was not widely understood by participants, these interviews probed the understanding and acceptability of the key terminology used in health communication about alcohol use. Four participants raised the topic of the low-risk drinking guidelines, which they broadly rejected as ‘unrealistic’, inconsistent with their normative perceptions of alcohol consumption and ‘impossible’ to stick to:
Maybe it’s just the circles I mix in, I don’t know. Maybe people do, by and large, but it doesn’t make sense to me, the units. I think they’re so low, I’m sure you’d be able to drink more than that safely.
Male, hip, 67 years
Two participants also questioned the motivation and perspective of those behind the low-risk drinking guidelines, stating that these were decided by the ‘anti-drink lobby’ and those that ‘actually have the money’.
A number of participants were able to provide accurate descriptions of a standard drink as being equivalent to a ‘unit’ and equate a ‘standard drink’ to servings such as half a pint, a small glass of wine or a single measure of spirits. However, four of the interviewees provided inaccurate descriptions of servings that would equate to a standard drink:
Is a measure where you say ‘standard drink’? My standard drink is a pint.
Male, knee, 71 years
Participants identified being more familiar with the term ‘unit’ than the term ‘standard drink’, explaining that ‘unit’ was used in alcohol labelling as well as in health promotion. Indeed, this increased familiarity is demonstrated by the fact that when participants were asked about their understanding of the term ‘standard drink’, transcripts show that a number immediately interpreted this as referring to ‘units’:
I wondered what you understood that term to mean, so what a standard drink is?
I’ve kind of given up trying to understand what a unit and . . . Personally, I don’t think they’re realistic.
Male, hip, 67 years
This increased familiarity appeared to equate to a more accurate understanding of the term ‘unit’, with more than half of participants providing accurate descriptions of servings that equate to 1 unit. However, familiarity was not considered to be universal and could potentially be lower among those who were drinking heavily:
Not everybody is aware of units and not a lot of people that drink a lot care about units.
Male, hip, 67 years
Despite the familiarity with the term ‘unit’, participants were clear that they were most comfortable talking about alcohol in terms of servings such as pints, half pints and glasses, and that talking in this form allowed them to ‘give you quicker answers’. One participant expanded on this, explaining that although they were familiar with ‘unit’, it required additional work on their part:
I’ve got to translate. It’s using Fahrenheit and celsius or kilos and pounds. You only think in one way. You’ve got to actually work out the other way.
Male, knee, 61 years
When asked what the terms ‘risky drinking’, ‘hazardous drinking’ and ‘harmful drinking’ meant to them, interviewees provided a range of descriptions relating to volume of alcohol consumed, frequency of drinking, drinking in the morning or before driving, drinking the ‘wrong type’ of drinks, short-term negative outcomes of drinking, including being involved in accidents and suffering memory loss, and longer-term health outcomes:
Well I mean it means there must be degrees, there is quite low level of risky drinking. I mean you can fall over and injure yourself. I mean any excess drinking can have long-term harm, cancer, heart attack and all this sort of thing but also social effects, domestic effects, driving, just because it frees you from inhibitions. You can act in ways which it would be better that you didn’t but it’s a matter of degree.
Male, knee, 78 years
Some participants considered the terms to be synonymous and reported no preference about which terms were used in health communication:
I’d know exactly what they were talking about when I saw either, and they would both mean exactly the same.
Male, knee, 61 years
However, others identified distinctions between harmful and hazardous drinking specifically, with hazardous considered to refer to short-term negative outcomes and harmful to longer-term health implications:
I would think hazardous drinking was risk of having an accident or getting in a situation, harmful I would put down to medical. I would just draw that difference for the two terms.
Male, hip, 67 years
Finally, the potential for ‘risky’ drinking to be interpreted in a positive manner and be something to aspire to was raised by one participant, who explained that this term may be appealing to young people specifically:
No, I don’t think it is. Especially for, like, the younger generation, because them, to take a risk is far better than being dull. So, when you say ‘risky’, they think, ‘I’ll have a go at that’.
Male, knee, 73 years
By contrast, the term ‘harmful’ drinking was identified as potentially more effective for use in health communications as it would not have positive implications:
In a way, ‘harmful’, I think, is the word that hits things home with me, because you can harm yourself in a number of ways, some of which are self-inflicted, some of which you can’t do anything about. The idea of if you say to somebody, ‘This is harmful to you’: that seems to simplify things . . .
Male, knee, 63 years
Although some participants identified that they were drinking ‘too much’, drank in excess of the guidelines, or were ‘heavy’ drinkers, when participants were asked directly whether they would use the terms ‘risky’, ‘hazardous’ or ‘harmful’ to describe their own drinking, they stated they would not. However, one participant explained that the researcher might use that term to describe his drinking:
I don’t know what you would class as risky drinking, quantity-wise. It’s probably what I’m drinking.
Male, knee, 61 years
Others also explained that, retrospectively, they would describe their previous drinking, specifically their drinking when they were younger, in these terms, although they would not have done so at the time:
In the past. Well yes, that’s an honest answer. I mean not all that much but it has happened, I’ve got to say that.
Male, knee, 78 years
Talking to older patients
Two key factors can be used to broadly characterise the participant population in this trial: they were all ‘patients’ attending for orthopaedic surgery and they were generally in later life. Interviewees were asked if specific considerations should be made when working with older adults and with patients.
Participants raised a number of reasons that older adults may choose to drink alcohol or may increase their alcohol consumption. These reasons predominantly related to changes in circumstances. Retirement was seen to provide an increased opportunity to drink and may lead to people getting ‘carried away’. Similarly, there was an increased chance of boredom, which would act as a motivator to drink. One participant also explained that older adults were less risk-averse and, another pointed to the relatively lucrative financial situation of some retirees, which might facilitate increased drinking:
I think a lot of people our age just think, ‘That’s it, I’ve retired, I’m going to enjoy it’. You can get carried away, can’t you? . . . And people tend to have a few bob in their pocket, it seems.
Male, hip, 67 years
The issue of death was seen to have the potential to influence drinking behaviour in a number of ways. Loneliness following the death of partners or friends could motivate increased drinking. Meanwhile, the increased salience of death could either lead to a ‘why not’ approach to drinking or motivate individuals to do more to ensure their health:
Loneliness. When you get to the age I’m at and a little bit further on, that’s when people die. People do get lonely and it’s very, very easy to turn to drink if you’re lonely.
Male, knee, 70 years
. . . if you are a heavy drinker you just think well so what, I’m going to die anyway. I might as well go really happy.
Male, knee, 85 years
Consideration of whether or not older adults were an appropriate target for alcohol interventions revealed split opinions. Some interviewees felt that older adults were more likely to be open and honest about their drinking, whereas others explained that older adults would not want to be asked about their alcohol consumption and would not want to be ‘educated’ about alcohol use:
I’m very careful about the words ‘educate people’ because I’m not convinced that some people like to be educated in this sort of age bracket.
Male, knee, 63 years
Some participants explained that because there was the potential for alcohol use to increase in later life it was appropriate to work with older adults to increase their awareness of alcohol issues and support them in monitoring or changing their behaviour:
It’s easy for people to say, ‘Oh, we’ll go and have a beer or whatever and sit in the garden’. Then one thing leads to another, doesn’t it? So I think maybe people our age could be aware of that.
Male, hip, 67 years
In contrast to this, a number of participants felt that alcohol interventions targeting older adults were unlikely to make any difference because the target population would not pay attention or would not be able to change their drinking behaviour:
I think people are set in their ways aren’t they? When they get to over 50, they’re either drinkers or they’re not. Then I think changing someone’s habits at my age is really asking a lot.
Male, knee, 67 years
Others pointed to the fact that there was no need to target this population because they did not have the opportunity to drink to excess and their drinking behaviour would already have decreased. One participant went so far as to state that excessive drinkers simply did not live long enough to reach the point of later life:
Big drinkers are not older because well it kills them doesn’t it?
Male, knee, 85 years
All participants were directly asked if any specific considerations should be made when working with patients; however, the majority felt that there was nothing additional that should be considered. Five participants did provide specific responses. Once again, opinion was split about whether or not alcohol would be a potentially sensitive subject; two interviewees felt that discussing drinking behaviour would not be a problem and that patients would be honest, whereas two others felt that patients, especially those drinking to excess, were unlikely to tell the truth:
I suppose the more you drink, the less likely you are to tell the truth about what you drink.
Male, knee, 73 years
This difference in opinion was summarised by one patient, who identified that responses were likely to vary from one patient to another depending on the amount of alcohol consumed and that it was important to be aware that some patients would feel less comfortable discussing alcohol use and to take this into account in the way that the subject was approached:
If there’s an issue, they probably won’t tell the truth. If they’re more than happy to say, ‘Oh, I have one glass of wine a week,’ they’d be proud of it and they’d shout it from the rooftops, but I think it’s something that you’ve just got to bear in mind that not everybody wants to talk about.
Male, knee, 61 years
One participant once again identified that he felt that people were unlikely to act on the advice or information provided:
I don’t know what you could say, really. I’m not sure how many people would actually take a great deal of notice, you know.
Male, hip, 67 years
In contrast to this view and the view of other interviewees who felt that little could be done to change the drinking behaviour of older adults, five participants identified that more should be done to address alcohol consumption in general, with a number of diverse approaches suggested. One participant felt that more should be done to remove high-volume, low-price alcoholic beverages from sale; another felt that drinking to excess should be taxed in some way; another recommended improvements to alcohol labelling to make alcohol content more visible and easier to interpret; and two considered that increasing ‘hard-line’ health communication messages and fear appeals, especially those involving personalised feedback, was more likely to lead to change:
I don’t think that the hospital makes people aware of it, of the damage it could do. Maybe if that could be made a bit more forcefully.
Male, knee, 70 years
Gender differences
With a much greater number of men than women consenting to participate in both the feasibility study and the pilot RCT, participants were asked if they could provide any insight into gender differences that might influence participation. Participants raised the issue of potential gender differences in orthopaedic surgery and in alcohol consumption that would make women less likely to meet the inclusion criteria. However, these opinions were not consistent across interviewees, with some explaining that women drank as much as or as often as men and that from their experience a greater number of women than men were undergoing surgery:
Oh God, this day and age, I don’t know. I go out and just see more women out than bloody men.
Male, knee, 64 years
Two participants also explained that women were generally less likely to want to share information about their personal lives. These views suggest that women may decline screening, refuse participation or under-report their alcohol consumption when screened:
I don’t think women like being asked invasive questions about themselves. I’m trying to picture my wife being asked the questions and I think you’d be getting a lot of. ‘Erm, but, erm’. Maybe she wouldn’t quite give you the exact amount.
Male, knee, 70 years
Other participants felt that men would be more open about their alcohol consumption than women, with one suggesting that this might be motivated by seeing friends and family members going through health scares and treatment:
Maybe they’ve come to their senses and think too much drink is doing too much damage. There are a lot of people, like I’ve got a couple of friends who are having problems and going through tests on their liver and stuff like that. Maybe it’s something to do with that, too much drink, and they realised.
Female, hip, 67 years
One participant also felt that male participants may have been motivated to take part by their female partners:
I don’t know. Probably their wives have told them to do it.
Male, knee, 70 years
Health-care professional interview results
Eleven HCPs were recruited during the pilot RCT: four each from sites 1 and 2 and three from site 3. Of these, eight went on to deliver at least one intervention session, one conducted screening only and two had no further involvement. Five HCPs (two from site 1, one from site 2 and two from site 3) consented to and participated in interviews about their experience of being involved in the trial. All five were female: three conducted both screening and intervention, one conducted the screening only and one conducted the intervention only. Owing to the small number of HCPs involved in the trial and the visibility of their involvement, quotations are identified by site only.
Coherence
Differentiation and individual specification
The HCPs interviewed were asked about both their day-to-day roles and their study-specific roles. The descriptions provided clearly demonstrated that participants had an understanding of their own tasks and responsibilities within the pilot RCT as well as how these differed from their usual preassessment role:
We do the AUDIT, the alcohol questions and then we go through the materials so reasons to cut down, why it’s important for surgery and then looking at ways to cut it down . . .
Site 3
I was doing pre-assessments for patients that were going through surgery, minor surgery. Taking heights, weights, blood pressure, bloods, stuff like that.
Site 1
Similarly, when asked how appointments differed for patients involved in the study, HCPs were able to identify key differences between the trial intervention and standard PA processes. They highlighted a focus on taking additional time to complete the AUDIT questionnaire and discuss alcohol consumption as the key aspect of the trial for those involved specifically in intervention delivery. They also mentioned that completion of screening and co-ordination were trial tasks for others:
So it’s about we take some extra time to go through the questionnaire and to talk more about alcohol. Sometimes I am doing the pre-assessment as well so I will mention it at the beginning of the assessment and say we will come back to that at the end and I think they have some more questionnaires and forms to fill out for the study . . .
Site 3
There was also some recognition that as the trial participants were taking part in something additional on a voluntary basis it was important to ensure that the experience was as positive as possible for them:
. . . people that are coming in for a booked BIRDS appointment, I never let them wait. So, if their appointment time is 2 o’clock, they won’t be in this room any later than 2 o’clock, even if the research team are not here, just give them a cuppa, ask them if they want a cuppa, and let them feel relaxed really. Whereas the other appointments, some people are waiting there a very long time . . .
Site 3
Communal specification
Interview transcripts revealed that the study processes, and specifically screening and intervention, were considered to fit well with existing PA specifically because questions about alcohol use and other health behaviours are already a standard part of PA. Trial processes could be seen to build and expand on these aspects of TAU:
. . . we do already ask about alcohol but we don’t really do much with that information. Other than maybe refer the notes to anaesthetist review if they are drinking a lot or maybe do like liver function tests and see how that comes out. So it does fit and it gives a bit something extra that we can do . . .
Site 1
From the patient perspective, the delivery of screening and intervention was also considered to fit well because it was scheduled around times when patients were already attending the hospital for appointments:
So I think it is quite good, the fact of having it in pre-assessment when they come, because they’ve got to come for their appointment anyway.
Site 3
Because the pilot RCT tasks were additional to TAU, it was considered necessary to have additional time allocated for these sessions in order to allow for implementation:
Although it fitted in well, time is a big thing in this clinic. I think they found it was a bit more time-consuming for them and they were getting held back a little bit. It went OK, just time wise. Some patients like to talk more than others.
Site 1
Further to this, even when adequate time was available to deliver the intervention, there were instances when issues emerged that had to be prioritised over intervention delivery to ensure patient safety:
I think one patient wanted to talk more about something else like they were using some other drugs as well so that was what we talked about that a lot more really but otherwise it was fine with all the others it really was fine.
Site 3
Internalisation
Interviewees identified a number of potential benefits of the trial and intervention at personal, system and societal level. Personal benefits included positive outcomes for professional development, with potential cost-saving benefits for the NHS and wider benefits of addressing alcohol consumption:
I think it will have a positive impact on the NHS. People are taking it in and cutting down on their drinking, it’s going to benefit them and us . . .
Site 1
Well, it was to move myself up and get more experience within the NHS. I know the research is a good thing to have on a CV [curriculum vitae].
Site 3
There was a clear indication that many of the potential benefits of screening and intervention were communicated during the trial training suggesting limited understanding of the association between alcohol and surgery:
. . . in the training it said about alcohol making it more likely for the patients to have complications and possibly get infections and things like that and taking longer to recover so if they can cut down then obviously that would help.
Site 3
Cognitive participation
Initiation
Only one of the five participants interviewed directly referred to managerial support of the implementation of the trial process, explaining that one of the consultant anaesthetists was ‘keen for us to be involved’ and wanted them to ‘get to the target’. This was identified as beneficial. In addition, two HCPs described instances when they discussed difficulties balancing trial work with clinical work and received support to prioritise their trial roles as well as in communicating this prioritisation with the wider clinical team:
I did tell my manager, ‘I need you to tell everybody what my priority is and it’s the study,’ and there was an e-mail sent at one point saying that my job role was the study . . .
Site 3
Despite this support, one of the interviewees identified that it ‘didn’t really make a difference’ to her workload or to others expectations of her in the subsequent weeks.
Enrolment
The HCAs who were responsible for conducting the majority of the screening also took ownership of the study and worked to push it forwards at each of the three sites:
I think I sort of took ownership of it and just went out there and did it.
Site 2
Although this was recognised by other interviewees, one of whom stated that the HCAs’ support ‘helped a lot’ with the co-ordination of study visits, balancing clinical and research work was not always easy and often the research work did not seem to be recognised by other HCPs in the PACs:
I think everybody here thinks you’re doing the same as them. They try and get you involved in what they’re doing but you don’t really have the time.
Site 3
Legitimation
Two of the interviewees clearly expressed the view that nursing staff, and specifically PA nursing staff, were best placed to deliver screening and intervention processes with patients. To some extent this was because of the content of the PAs that they were conducting:
Yes. I think when you’re doing a pre-assessment with somebody it is quite in-depth questions, so you have to feel like you’ve got that bond with the patient for them to be able to tell you things honestly, anyway. So I think if you were to give them a bit of brief advice or brief intervention included in your pre-assessment, then it would be best off a prep nurse.
Site 3
However, one HCP also identified that having nursing staff conduct the interventions would be preferable to doctors doing it as guidance from doctors would be less motivationally focused:
Obviously, a normal person doing it. Not a doctor saying, ‘Well, this is what you should be doing, and this, and this, and this’.
Site 2
Similarly, patients were thought more likely to provide truthful, accurate responses to screening questions when screening was conducted by nursing staff rather than by doctors:
I think the problem with doctors [is] that they will say, ‘Oh, how many units have you had?’ And obviously they will be like, ‘Oh, I just have 3 units a week,’ or something like that, which you know is totally a lie.
Site 3
Activation
There was limited discussion of how visible the trial was within the PACs. Although there was some indication in interviews that staff were aware of one another’s roles, with one of the HCPs describing the supporting role of the screening HCA, there were also instances, as discussed in Enrolment, when screening was not recognised as a priority:
It’s been hard because you would want to get some of your BIRDS stuff done, but you couldn’t because you were being used as a health-care assistant . . .
Site 3
However, the process of randomisation in combination with screening reactivity led to limited involvement of one HCP, despite the fact that she had undertaken training and was scheduled to conduct visit 1 with a number of participants:
I think it has just been by chance but whenever I am going to do the intervention they get allocated to the no intervention or like they are only just on the guidelines or already reduced so there isn’t that much to do . . .
Site 1
Collective action
Interactional workability
Interviewees discussed how they interacted with other staff members in the PACs, how they worked with the screening and intervention tools and how patients interacted with these tools.
Regarding interactions with other staff members, it was considered important to maintain positive relationships with PA staff not involved in the trial and to minimise any impact on the day-to-day running of the clinic:
I’ve always just said to them, ‘If you need the patient I’m here all day anyway. You just take them first of all, and I will see them afterwards’ . . . obviously you’ve got to have a good rapport with them, which obviously now we do . . .
Site 2
Both the AUDIT-C and the full AUDIT questionnaires was considered easy to use and fairly self-explanatory, and the infographic detailing standard drinks was seen as beneficial for both the HCPs and the patients:
Yes. The alcohol units are quite good. A lot of patients did like that as well.
Site 2
However, some questions on the full AUDIT questionnaire were considered more likely to generate adverse reactions specifically because they were related to potential dependence. This could in turn hinder the interaction between the HCP and the patient participant:
. . . there was a particular question that had a couple of raised eyebrows. It was, ‘How often in the last year have you needed an alcoholic drink to get you going in the morning?’ A couple of patients were all right until you got to that question and then they would kind of retract themselves back a bit as if to say, ‘Are you calling me an alcoholic?’
Site 1
The BI tools were also considered easy to use and were described as having ‘everything that you need’, with one HCP explaining ‘I don’t think they need to change’. However, familiarity with these tools and practice using them was considered important both for the effective delivery of the intervention and for assessing their benefit:
. . . there is a lot of information like they have all the things you would need I suppose I just I can’t really say more than that I don’t know what it’s like really to go through it all with a patient . . .
Site 1
Skill set workability
In terms of delivering the screening and intervention, HCPs considered themselves to be experienced in asking about alcohol use and, therefore, well placed to conduct the screening:
. . . it’s just about how you say it and you know we ask about alcohol all the time so I’m used to it and I don’t it’s not about judging them it’s about making sure they are safe for their surgery so that can help too.
Site 1
However, training was considered necessary to develop the additional knowledge and skills required to deliver the intervention, with practice needed to ensure effective delivery:
I don’t think that you could do it without having the training. I don’t think that you could just go and just reel off what was on there, because obviously there are different crib sheets that you go off.
Site 2
Additionally, one of the interviewees identified that those who were interested in and enjoyed delivering the interventions were best placed to deliver them:
I think there were a few of us doing it at the beginning but I am the only one really now doing the interventions. I enjoyed doing it so I suppose it just makes sense because some of the others they were quite nervous about It . . .
Site 3
Contextual integration
Consideration of the interview transcripts identified a number of issues relating to the allocation of resources, specifically in terms of staff and time allocation. As discussed in Chapter 3, Communal specification, the allocation of adequate time to deliver the intervention was important. However, there was a potential downside to this. Even with the work of HCAs to co-ordinate the pilot RCT, there were times when staff time was allocated to deliver sessions but the session was delayed, the participant was allocated to the TAU condition or the patient did not turn up:
It is a bit frustrating because you set the time aside and you know you’ve done the training and then you set the time aside and it’s a lot of standing around and waiting while they do the like the consent then at the end of it they don’t go in the intervention so you just do the preassessment . . .
Site 1
When staff had both clinical and research responsibilities, this could also lead to issues with HCAs having to remind other staff to complete the AUDIT screening questionnaires and return them in a timely manner to ensure that expressions of interest could be completed for eligible patients:
A lot of the nurses do forget to give us them and I had a lot of patients that were missed, because if I was working in the investigation suite the AUDITs would be popped on this desk and then I would come in and they had scored over five, but they had left the department . . .
Site 3
Reflexive monitoring
Systematisation
There was that the effectiveness of screening and intervention was formally assessed. One interviewee, however, did express a desire to see the final results of the pilot trial, although she did not demonstrate a clear understanding of the fact that a pilot trial would not be powered to detect between-group differences:
. . . it would be good to see the results because some of them they do seem positive but it would be really nice to know if it helped and if it did make a difference.
Site 3
Communal appraisal
Discussion of communal engagement in assessing of the effectiveness of research, screening and intervention processes was also limited. Although there was some evidence of HCPs discussing the trial and assessing issues around implementation, there was no suggestion that such conversations had considered the potential benefits or effectiveness of the work:
. . . other people I know had a patient booked in but then they didn’t turn up or cancelled at the last minute and that is annoying . . .
Site 1
Individual appraisal
In contrast to the limited evidence of systematisation and communal appraisal, there were numerous examples of how individuals had collected anecdotal evidence of effectiveness. Some of these related specifically to completion of the screening questionnaires, which was seen to increase patients’ awareness of their alcohol consumption:
The patients that I’ve talked to have said, ‘Actually, I didn’t realise how much I was drinking’ . . .
Site 2
Others thought that the intervention would lead to more changes in drinking behaviour than screening alone:
. . . they want the surgery and they want to get better more quickly and then like the questions they realise that maybe they are drinking more than they thought so we can then we look at cutting it down and they are quite positive so I think that some of them they will change for the surgery.
Site 3
In addition, the fact that the trial involved multiple contact points across the full course of the surgical pathway not only offered HCPs the opportunity to get an idea about whether or not the intervention was having a positive impact on patients’ alcohol consumption but was also seen as beneficial to the patient experience:
I think it was just the fact as well of having that extra bit of contact that actually made it enjoyable. Within the trust more. The patient experience was a bit better.
Site 2
The HCPs also identified a number of benefits to themselves as individuals from being involved in the trial. In addition to the anticipated benefits for their own professional development, being involved was described as ‘interesting’ and ‘enjoyable’ and for some had led to a change in their own drinking behaviour:
I know my drinking habit has changed since doing it completely. And it still has changed now. We will just have alcohol if we go out. Which is very rarely, obviously, with a young child. But we don’t have alcohol in the house anymore . . . The whole family has reduced alcohol intake, which I think has been a good thing.
Site 2
Reconfiguration
The HCPs described tailoring the delivery of the screening questionnaire to help ensure positive interactions with patients when completing them. For some, this involved ‘forewarning’ patients that some of the questions might or might not apply to them:
People do adapt a little bit to their own style, but you still have to ask them questions and get them answers.
Site 2
However, HCPs also made some suggestions about how the trial could be improved, with a recommendation to use the term ‘units’ instead of ‘standard drinks’:
. . . as a pre-assessment nurse you go by units, whereas the auditing is by standard drinks and people will get a bit confused about a unit or a standard drink.
Site 3
Conclusions
The pilot trial identified that a definitive trial is feasible. We aimed to recruit 80 patient participants to allow the collection of outcome data from a total of 60 patient participants at 6 months post recruitment. Sixty-eight patient participants were recruited (86% of target) but data were collected from 65 participants (96% of target) at 6 months post recruitment. There were very small numbers of missing data at baseline and at 6 months post recruitment in both trial arms. Therefore, the very high retention rate and high levels of data completion mitigated the fact that the initial recruitment target was not met.
To detect a between-group difference of 13% in the number of participants identified as lower-risk drinkers at 6 months post recruitment follow-up (AUDIT score of 0–7), just less than 10,000 patients would need to be screened to recruit 770 patients to the trial. With approximately 160,000 patients undergoing hip or knee arthroplasty in the UK annually24 and the majority of sites employing some form of assessment of preoperative alcohol consumption,116 this level of screening and recruitment is considered to be achievable.
Further to this, qualitative findings identified the proposed screening, intervention and research processes as broadly acceptable to both HCPs and patient participants. The randomisation process was shown to have little influence on patient participants, although, as we did not interview decliners, it is possible that they hold different views. Similarly, the burden of participation was also acceptable, with patient participants identifying themselves as having the necessary free time to participate.
The amendment of the inclusion criteria so that patients were not required to report regular high-intensity consumption (consumption of six or more standard drinks on a single occasion at least weekly) and to judge eligibility on the initial AUDIT-C screen rather than the completion of full AUDIT at baseline led to the identification of 23% of patients screened as being eligible. This also ensured that reductions in alcohol consumption following screening and invitation to participate did not lead to the exclusion of patients who were potentially eligible at initial screening.
Just over one-third of eligible patients were recruited to the pilot trial. With higher rates of recruitment seen in RCTs that conduct screening, consent and intervention on a single occasion,141 further streamlining of the trial processes may enhance recruitment rates.
A total of 68 patient participants were randomised to the BBI or TAU. Of the 33 patient participants allocated to the BBI arm, 32 (97%) received the intervention as intended and all received a copy of the patient information leaflet. However, delivery rates of the optional booster intervention session was very low, with only four out of 33 participants (12%) allocated to the intervention receiving this session. With such low rates of delivery and limited indications of any additional benefit or potential benefit of this session in qualitative data from patient participants, it is considered neither feasible nor necessary to employ a booster session in a definitive trial.
Very few problems were identified with the proposed follow-up measures, although length of hospital stay could limit collection of self-reported patient data at the in-hospital (visit 4) follow-up. Rates of completion of the AUDIT tool, the proposed primary outcome measure, were consistently high. Although qualitative interview data from HCP participants identified that some of the wording and terminology in the AUDIT tool could lead to adverse reactions among patient participants, something that they acted to mitigate by tailoring delivery, this was not something that emerged from interviews with patient participants.
The proportion of missing data was higher at interim study visits, although this never fell below 65% for required follow-up points (i.e. excluding the optional booster session). Rates of completion were lowest for visit 3, which was designed to be conducted over the telephone 1–3 days before surgery. Scheduling this visit to occur on the day of surgery, when patients present at the hospital, may improve rates of completion, but the acceptability of this should be further explored.
Alcohol use at baseline, as assessed by AUDIT and self-reported units of alcohol consumed per week, was observed to be lower in the BBI arm than in the control arm. Although no inferential statistics were conducted to assess the significance of this difference, a definitive trial may need to assess and control for such differences.
Chapter 5 Discussion
This chapter draws together and discusses the findings from the three key phases of the project (feasibility, pilot RCT and characterising TAU). Findings are discussed in order of trial processes (screening and recruitment, intervention compliance, retention and follow-up) followed by additional findings related to the implementation of trial processes and variation in perioperative pathways and TAU. Key findings and their implications for future research are summarised in Table 28.
| Issue | Findings | Implications for future research |
|---|---|---|
| Identifying potential participants | Screening for AUDIT-C score of ≥ 5 or consumption of six or more standard drinks weekly increased the number of eligible patients. Initial screening and study invitation can lead to reductions in alcohol consumption prior to intervention. Waiting time between listing for surgery and attendance at the PAC (≥ 3 months) could create a lag between screening and recruitment | Use revised screening criteria. Inclusion should be based on initial screening to avoid excluding patients who are highly motivated to change. Consider postal invitation and telephone screening |
| Consenting eligible participants | Removal of the term ‘risky drinking’ from the study title and a reduction in the period of time before expression of interest was followed up increased the proportion of patients consenting. In the pilot RCT, 34% of eligible participants were consented to the trial. The most common reasons for declining participation were lack of interest and not having enough time | Future trials must avoid any pejorative terminology in recruitment materials and streamline trial visits. Improve appeal of initial approach by including information on the potential benefits of the trial |
| Meeting recruitment target | Recruitment fell short of target, with 68 patient participants recruited against a target of 80 (85%) | Recruitment of preoperative patients to alcohol intervention trials is challenging |
| Compliance with intervention | In total, 32 out of 33 patient participants allocated to the intervention arm received the intervention and all 33 received a copy of the patient information leaflet. Only 4 out of 33 received the optional booster intervention | Delivery of BI in the PAC is feasible. Future trials need not include a booster session |
| Fidelity of delivery | Fidelity of delivery improved between the feasibility study and the pilot RCT. Motivational BCTs were well delivered but volitional ones less so | Additional training may be required for HCPs to effectively deliver volitionally focused BCTs. Alternatively, the adoption of an advice-focused intervention could be adopted to ensure consistent delivery |
| Characterising TAU | TAU relating to alcohol screening, availability of advice and availability of referral was found to vary between individuals and between sites | TAU at trial sites should be considered during selection. Standardisation of TAU across sites may also be necessary |
| Retention | Retention at 6 months post recruitment was very high (96%) | Retention unlikely to be a concern for future trials |
| Outcome assessments | There were few missing data on AUDIT and EQ-5D. WOMAC was less well completed | AUDIT and EQ-5D are acceptable outcome measures. Consider when WOMAC is completed and how patient participants are instructed to complete it |
| Safety issues | No serious adverse events were reported | No safety issues for future trials |
| Sample size calculation | Sample size calculation feasible | Indicated that 385 participants per arm to be recruited for definitive trial |
| Communication of alcohol health messages | There was limited understanding of associations between alcohol and surgical risk, as well as of the term ‘standard drink’ and to a lesser extent ‘unit’ | HCPs need adequate training to conduct screening and intervention. Training and interventions should adopt the term ‘unit’ and better explanations of alcohol use |
Screening and recruitment
Findings from the feasibility study and comparison of screening and recruitment rates from the feasibility and pilot RCT clearly demonstrate the importance of working with end-users (both HCPs and patients) to refine study processes and materials to ensure and enhance acceptability before attempting to recruit to a definitive RCT. Specifically, amendments made during and following the feasibility study led to a 20% increase in the percentage of eligible patients recruited. As a result, four times as many patient participants were recruited from screening only twice as many patients.
Initial results of screening in the feasibility trial identified that a large proportion of patients who screened AUDIT-C positive (score of ≤ 5), indicating increased risk from drinking, were judged ineligible because they did not report consumption of six or more standard drinks on a single occasion at least weekly. Amending the screening criteria to include both regular and high-intensity drinking (AUDIT-C score of ≤ 5 or consumption of six or more standard drinks on a single occasion at least weekly) increased eligibility rates from 16% to 20%. In the pilot RCT, the rate of eligibility based on alcohol screening with the AUDIT-C was approximately 34% when the criteria were set as AUDIT-C score of ≤ 5 or consumption of six or more standard drinks on a single occasion at least weekly. Taking into consideration the fact that one site employed pre-screens to avoid screening those already documented as abstainers, this is reflective of overall population rates of exceeding the lower-risk guidelines. 10 Although this means that a large number of patients (approximately five) have to be screened to identify one eligible patient, it is these patients who are most at risk of developing alcohol-related complications. Further to this, assessment of alcohol consumption is already part of the standard preoperative care pathway, and the use of validated tools is becoming more common, especially with the advent of the CQUIN programme.
Although the number of patients who declined participation is much smaller than the number of those found to be ineligible, the recruitment of eligible patients (34%) fell short of the predefined progression criteria. Taking into account the high retention rate, which indicates that progression to full trial would be feasible, it is important to consider if and how further improvements can be made to recruitment. With over 40% of those declining doing so because they were not interested in the trial, future trials should ensure that the initial approach to patients is appealing and clearly sets out the possible benefits of involvement to health and postoperative recovery.
The percentage of eligible patients who did not express an interest in being involved in the trial was higher in the pilot RCT than in the feasibility study (35% vs. 4%). This is interpreted as either resulting from the addition of the randomisation process or resulting from the increased number of study visits in the pilot RCT. This points to a need to ensure that study visits are kept to a minimum. This issue is further highlighted by the fact that 15% of participants who declined participation did so on the basis that they did not have enough time to take part or to attend the intervention session. In addition, the findings of qualitative interviews with patient participants identified that even among those who considered themselves to have a large amount of free time there was a limit to the frequency of contact that would be acceptable. Minimising the time burden placed on patients by ensuring that the number of study visits are kept to a minimum, that the intervention itself and any required research processes are as succinct as possible and that study visits are arranged around existing hospital appointments or over the telephone will be key for a successful full trial. Possible streamlining of study visits is discussed in more depth in Follow-up and retention.
Participants in qualitative interviews were specifically asked about the acceptability and importance of the number of study visits. Although time is required for all participants to consider entry into any study, many patients indicated that offering a minimum of 24 hours following screening was not necessary. This information lends itself to the possibility of conducting same-day screening, consent and randomisation. Indeed, this pragmatic approach would be more consistent with how screening and intervention would occur if the intervention were implemented as part of standard care. It would also minimise the number of ineligible patients who had already been through PA or for whom the date of surgery would not have allowed adequate time for behaviour change (the third most frequent reason for non-eligibility). However, it would also require consistent availability of staff for consent and intervention delivery and, therefore, may not be practical in the research trial.
A final point to consider concerning patient eligibility and recruitment is that there was no clear reason recorded for 16.5% of the patients who declined to participate. This points to the need to ensure accurate recording of the outcomes of all patient contact while protecting patients’ right to decline or withdraw from participation without giving a reason.
Intervention compliance and fidelity of delivery
Findings from the pilot RCT show a high level of compliance to allocation, with 96% of patient participants allocated to the intervention receiving the BA and BI elements and all receiving a copy of the patient information leaflet. However, the specific content of the intervention sessions, as documented by assessments of fidelity of delivery, showed more variability.
The AUDIT questionnaire was found to be acceptable to both patients and HCPs in the feasibility study and pilot RCT. However, some of the questions designed to assess harm experienced as a result of drinking behaviour were identified by HCPs as potentially more likely to provoke adverse reactions. This reflects early feasibility findings that the term ‘risky drinking’ was not acceptable to potential participants. BIs emphasise a non-judgemental, positive, motivational approach that can be seen to be inconsistent with some of the terminology routinely included in alcohol screening and alcohol-related health communication. Therefore, qualitative interviews conducted as part of both the feasibility study and the pilot RCT were used to further explore the issue of appropriate terminology. Findings from the pilot RCT show that although interviewees demonstrated a large amount of variation in how they interpreted the terms risky, harmful and hazardous drinking, none considered these terms to be applicable to their own current drinking behaviour. Indeed, the majority of participants identified their drinking as acceptable or moderate even if it was in excess of the low-risk drinking guidelines. Even the two participants who self-identified as ‘heavy’ drinkers did not relate to these terms. Similarly, findings from the feasibility study point to the use of ‘defensive othering’ as a technique to distinguish own drinking from the ‘problematic’ drinking of others. In combination, these findings point to a need to avoid the use of any language that categorises participants’ drinking behaviour in a negative manner (e.g. ‘risky drinking’), even when providing feedback on screening.
Fidelity of intervention delivery was low in the feasibility study but improved in the pilot RCT. This change is likely to have resulted from a combination of two factors: first, the work to optimise the training package between the feasibility and pilot RCT and, second, the greater number of patients recruited to the pilot trial. The resulting increase in frequency of intervention delivery offered HCPs the opportunity to gain additional experience of delivering the intervention and enhanced their familiarity with the intervention tools, two factors that the HCP qualitative interview data identified as contributing to the effective delivery of interventions. Nonetheless, in both phases of the study, although motivationally focused BCTs, especially those related to communicating the health, social and emotional consequences of alcohol consumption, were consistently delivered, volitionally focused BCTs such as problem-solving and pros and cons were less consistently delivered. This suggests that further refinement of the training package, and possibly a longer period of time dedicated to training, may be required to enable PA HCPs to deliver these BCTs.
Although HCPs identified alcohol consumption as a priority for health care, awareness of the link between alcohol consumption and surgery was low among both patient and HCP participants. The training was identified as increasing HCPs’ knowledge in this area and thus increasing the perceived benefit of delivering alcohol screening and BI in this setting. Similarly, findings from patient interviews identified that messages around alcohol and surgical risk are most relevant to screening and intervention in the preoperative setting, with issues regarding weight loss and medication management also considered relevant. Therefore, further amendments to the intervention to increase the focus on the perioperative setting may be beneficial.
There was very low uptake of the optional booster intervention session planned to be delivered approximately 1 week before surgery. Findings from qualitative interviews regarding the potential benefit of this session were mixed, with no clear indication that it was considered necessary or beneficial. This is in line with previous trials that have found no additional benefit from offering more extended interventions and lower rates of compliance when interventions require multiple sessions. 75
Follow-up and retention
Follow-up data from the pilot RCT demonstrate that this population is highly amenable to follow-up, with a very high retention rate (96%) at 6 months post recruitment. This means that provision for a 25% loss to follow-up rate in the setting of recruitment targets was not necessary to allow adequate data at outcome. This compares favourably with a previous trial of alcohol interventions in the preoperative setting95 and trials from primary84 and emergency84 care, which report lower but still acceptable retention rates at 6-month follow-up (72–88%). With variation in retention rates for other alcohol intervention trials, and lower completion of interim follow-ups in this pilot trial, a retention rate of 80% was employed for the sample size calculation necessary for a definitive trial. This will produce more conservative recruitment targets.
The proportion of missing data was higher at interim study visits, although it never fell below 65% for required follow-up points (i.e. excluding the optional booster session). Rates of completion were lowest for visit 3, designed to be conducted over the telephone 1–3 days before surgery. Completion of this measure face to face when patients present at the hospital prior to surgery could improve rates of completion. However, the acceptability of this to patient participants has not yet been considered and thus would need to be explored prior to implementation.
The average length of hospital stay in our study of 3–4 days is in line with national data available from Scotland. 142 Given this information, and the fact that eight patient participants did not undergo surgery during the study, the low completion rate for visit 4 (planned to take place 3–5 days postoperatively) is unsurprising. Any future trial should aim to conduct post-surgical follow-up on or before 3 days post surgery in order to maximise opportunities for measure completion. Further to this, completion of the WOMAC questionnaire at visit 4 was additionally complicated by the nature of the questionnaire. Triangulation with qualitative data shows that some items in the WOMAC require respondents to have completed certain activities (e.g. climbing the stairs) before being able to accurately assess their ability to complete these activities. Providing guidance on how to manage situations when accurate assessments cannot be made may be helpful in this regard. However, it would be more appropriate to address these issues (variation in length of stay, minimising of participant burden and lower completion rates of the WOMAC questionnaire) by ensuring that visit 4 is focused primarily on postoperative complications that could be mainly accessed from patient notes, thereby reducing patient contact.
If these changes were made, along with the completion of screening, recruitment and intervention on a single occasion, it could reduce the current seven-visit trial design to four visits.
Surgical care pathway variation
Although many hospitals have a standard care pathway of 6–10 weeks from PA, variation in pathways, even in this pilot study, was notable. Some eligible patients when identified had < 7 days before their operation, whereas eight of the 68 patients recruited to the pilot RCT had not undergone surgery before the end of the study. This variation would be difficult to control without having an adverse impact on patient care. Nevertheless, some considerations may be relevant to minimise the impact of such variation.
First, identifying and intervening with patients as early as possible in the preoperative care pathway remains a priority for preoperative intervention trials. Second, tailoring interventions to individual preoperative pathways will be important in facilitating both shorter- and longer-term behaviour change. Finally, it may be appropriate to consider including a trial follow-up visit to occur a specified length of time after surgery.
Both the focus groups and the survey responses demonstrate a large amount of variation in day-to-day practices between individuals and between sites, as well as over time. This variation was seen across processes for alcohol screening, the identification of patients whose drinking represented a potential surgical risk, the management of patients following assessment of alcohol consumption and the availability of alcohol specialist services. Despite previous research demonstrating that applying validated screening tools in PA improves the identification of both dependent and increased risk drinkers,67 findings from this work indicate that such tools remain infrequently adopted in PA, although the CQUIN initiative may change this. This is in line with a previous survey of PA leads. 10
Although many respondents identified the availability of support and potentially interventions for dependent drinkers, including anaesthetic review and referral to alcohol specialist nurses, limited options were available to address increased risk drinking either specifically in relation to surgery or more generally. However, the availability of generalised interventions may increase for sites participating in the CQUIN initiative as the second phase of this focuses on delivery of appropriate interventions. Further to this, some HCPs indicated that they provide simple advice to reduce drinking or avoid alcohol consumption prior to surgery or that such a recommendation was included in the information leaflets provided to patients. Overall, this variation points to a need for high-quality evidence to inform policy and practice around alcohol consumption and surgery. However, this very same variation means that the selection of trial sites for a future definitive trial will need to involve consideration of the method of alcohol screening, level of information and available alcohol specialist support. Work to standardise the TAU condition would also be beneficial to ensure consistency in its delivery.
Implementation of trial processes
Qualitative interviews with HCP participants identified a number of factors that could act as barriers to or facilitators of the implementation of trial processes.
It was clear that initial knowledge of associations between alcohol consumption and surgical outcomes was limited and that knowledge of the potential benefits of the research predominantly derived from the training provided. Without this understanding, it is unlikely that HCPs would volunteer to participate in the trial. Having the required time to complete trial activities, including screening, intervention, co-ordination and follow-up, was important as PACs were considered to be busy and individual appointments included a relatively extensive assessment. Having adequate staff time allocated for intervention delivery was considered vital to the effective delivery of intervention sessions but could present a problem if patients failed to attend or declined to participate on the day. Furthermore, HCPs based in PACs often struggled to balance research tasks against PA roles. In addition to having the necessary training to deliver screening and intervention, gaining ‘real-life’ experience of delivery and becoming familiar with the tools were highlighted as important to effective delivery and to maintaining a sense of professionalism. This was not considered to be possible through training or role-play activities but required experience with actual patients and within the day-to-day clinic. The application of NPT allowed further identification of issues specifically related to the implementation of trial activities. These included limited higher-level management support for trial activities, infrequent intervention delivery and limited visibility of the research within the PAC.
Chapter 6 Conclusions and recommendations
Conclusions
This work finds that preoperative alcohol screening using the AUDIT-C and full AUDIT is acceptable to patients and HCPs. Similarly, delivering BBI in the PAC is possible and the intervention methods adopted were acceptable to patients and HCPs. The evidence supports the feasibility of a definitive trial to assess the effectiveness of these methods in bringing about reductions in preoperative alcohol consumption and secondary outcomes of surgical complications if recommendations to enhance recruitment are adopted.
Recommendations
The primary recommendation from this project is that a definitive RCT is feasible. The very high levels of retention in the pilot RCT and data completion at baseline and follow-up mitigate the fact that recruitment fell slightly short of the initial target. With the background literature identifying a wealth of evidence on the associations between preoperative alcohol consumption and surgical outcomes, but a sparsity of evidence regarding the effectiveness of behavioural interventions in reducing preoperative alcohol consumption, such a definitive trial is also considered to be appropriate.
A number of secondary recommendations for the design and conduct of a definitive trial can also be made. These are arranged in priority order.
-
Evidence from both the feasibility study and the pilot RCT shows that avoiding potentially pejorative terminology in any recruitment materials and the adoption of a non-judgemental approach throughout are vital for recruitment to be successful. Specifically, a future trial should:
-
– avoid any categorisation of drinking as ‘risky’, ‘harmful’ or ‘hazardous’
-
– communicate that all patients are being invited to complete screening.
-
-
Qualitative evidence shows limited understanding of, and low familiarity with, the term ‘standard drink’; it is therefore recommended that:
-
– the term ‘units’ be adopted in all trial materials
-
– HCPs be trained and prepared to ensure that they are confident in converting patient descriptions of alcohol consumed in terms of pints or glasses into units in order to enhance the accuracy of screening
-
– interventions include a description of units to enhance interpretation of the lower-risk drinking guidelines and increase the accurate monitoring of alcohol.
-
-
Although qualitative evidence from trial participants identified that the burden of participation was acceptable, the rate of eligible participants (34%) consented may be further improved by streamlining the trial visits. Specifically:
-
– with the very low delivery of the optional booster session, this visit should be excluded from any future trials
-
– with low completion rates of visit 3 (1–3 days pre surgery), this visit could be scheduled to take place when patients arrive at the hospital on the day of surgery.
-
– with variable length of stay and some difficulties completing the WOMAC questionnaire at visit 4 (in-hospital follow-up 3–5 days post surgery), a future trial should consider either conducting this visit 1–3 days post surgery or amending it to focus only on postoperative complications that can be collected from patient notes
-
– as in this pilot RCT, all study visits should be arranged to either take place around existing hospital appointments or be conducted over the telephone or at the patients’ home in order to minimise burden and remove any requirement for additional travel to the hospital site.
-
-
With 40% of eligible patients who declined participation in the trial stating that they did so because they were ‘not interested’, future trials should ensure that the initial approach and introduction to the study is appealing and clearly sets out potential health benefits of participation.
-
With HCP interviews identifying difficulties in balancing research roles with clinical demands in busy PACs, it is recommended that future trials:
-
– arrange for all follow-ups to be conducted by research-specific staff while PA staff maintain the role of intervention delivery
-
– work with PA leads throughout the trial to raise the profile of the research in the clinic, track progress against targets and identify any issues.
-
-
With evidence from the focus groups and HCP survey showing that PA frequently involves the assessment of alcohol consumption and may include a brief recommendation to reduce drinking prior to surgery alongside qualitative evidence that some participants in both the feasibility study and pilot trial report reducing their alcohol consumption following initial screening and invitation to participate, future trials should:
-
– assess eligibility in terms of alcohol consumption based on initial screening
-
– carefully consider and characterise the comparator condition.
-
Acknowledgements
The PRE-OP BIRDS research team would like to acknowledge the contribution of all HCP and patient participants; the PA leads from the three recruiting centres, Emma McCone, Hilary Turner and Munyaradzi Vushemasimba; Elaine Stamp and Lesley Hall for their work on the feasibility study; Sebastian Potthoff for his work on the HCP training; and all members of the TSC and Data Monitoring Committee.
Contributions of authors
Christopher Snowden (https://orcid.org/0000-0001-6396-9167) (Chief Investigator and Consultant Anaesthetist) contributed to the design of the study and provided oversight.
Ellen Lynch (https://orcid.org/0000-0002-6301-4499) (Research Associate) contributed to the optimising of the intervention and training package and to data collection and analysis.
Leah Avery (https://orcid.org/0000-0003-3578-1209) (Co-applicant and Reader in Health Behaviour Change) contributed to the design of the study and the design and delivery of the training package.
Catherine Haighton (https://orcid.org/0000-0002-8061-0428) (Co-applicant and Associate Professor in Public Health and Wellbeing) contributed to the design of the study and the analysis of the qualitative work.
Denise Howel (https://orcid.org/0000-0002-0033-548X) (Co-applicant and Lead Statistician) contributed to the design of the study and the conduct of the trial, supervised the statistical component of the research, and sat on the Trial Management Group, TSC and Data Monitoring and Ethics Committee.
Valentina Mamasoula (https://orcid.org/0000-0001-6834-9610) (Statistician) carried out the statistical analyses.
Eilish Gilvarry (https://orcid.org/0000-0001-7493-1156) (Co-applicant and Consultant Psychiatrist in Addictions and Honorary Professor of Addiction Psychiatry at Newcastle University) contributed to the design of the study and provided specialist input regarding addiction.
Elaine McColl (https://orcid.org/0000-0001-8300-3204) (Co-applicant and Professor of Health Services Research) contributed to the design of the study and provided expertise in the design, conduct and interpretation of trials.
James Prentis (https://orcid.org/0000-0001-8019-5247) (Co-applicant and Consultant Anaesthetist) contributed to the design of the study and provided expertise in PA.
Craig Gerrand (https://orcid.org/0000-0003-3043-4507) (Co-applicant and Consultant Orthopaedic Surgeon) contributed to the design of the study and provided expertise in orthopaedics.
Alison Steel (https://orcid.org/0000-0003-1279-1455) (Senior Trial Manager) contributed expertise in the conduct and management of trials.
Nicola Goudie (https://orcid.org/0000-0003-1211-936X) (Trial Manager) led on the management of the trial.
Nicola Howe (https://orcid.org/0000-0001-9446-8314) (Database Manager) designed and managed the study database and randomisation system.
Eileen Kaner (https://orcid.org/0000-0002-7169-9344) (Co-applicant and Professor of Public Health Research) contributed to the design of the study and provided expertise in BI trials.
Publication
Snowden C, Lynch E, Avery L, Gerrand C, Gilvarry E, Goudie N, et al. Preoperative Behavioural Intervention versus standard care to Reduce Drinking before elective orthopaedic Surgery (PRE-OP BIRDS): protocol for a multicentre pilot randomised controlled trial. Pilot Feasibility Stud 2018;4:140.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Snowden C, Lynch E, Avery L, Gerrand C, Gilvarry E, Goudie N, et al. Preoperative Behavioural Intervention versus standard care to Reduce Drinking before elective orthopaedic Surgery (PRE-OP BIRDS): protocol for a multicentre pilot randomised controlled trial. Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-018-0330-4.
- Abbott TEF, Fowler AJ, Dobbs TD, Harrison EM, Gillies MA, Pearse RM. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth 2017;119:249-57. https://doi.org/10.1093/bja/aex137.
- Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009;361:1368-75. https://doi.org/10.1056/NEJMsa0903048.
- Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Participants in the VA National Surgical Quality Improvement Program: determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326-41. https://doi.org/10.1097/01.sla.0000179621.33268.83.
- Shah R, Attwood K, Arya S, Hall DE, Johanning JM, Gabriel E, et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg 2018;153. https://doi.org/10.1001/jamasurg.2018.0214.
- Carey K, Stefos T, Zhao S, Borzecki AM, Rosen AK. Excess costs attributable to postoperative complications. Med Care Res Rev 2011;68:490-503. https://doi.org/10.1177/1077558710396378.
- Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004;199:531-7. https://doi.org/10.1016/j.jamcollsurg.2004.05.276.
- National Institute for Health and Care Excellence (NICE) . Alcohol-Use Disorders: Preventing the Development of Hazardous and Harmful Drinking [PH24] 2010.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Cook JR, Warren M, Ganley KJ, Prefontaine P, Wylie JW. A comprehensive joint replacement program for total knee arthroplasty: a descriptive study. BMC Musculoskelet Disord 2008;9. https://doi.org/10.1186/1471-2474-9-154.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995;56:423-32. https://doi.org/10.15288/jsa.1995.56.423.
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test – Guidelines for Use in Primary Health Care. Geneva: World Health Organization; 2001.
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789-95. https://doi.org/10.1001/archinte.158.16.1789.
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: an update of research findings. Alcohol Clin Exp Res 2007;31:185-99. https://doi.org/10.1111/j.1530-0277.2006.00295.x.
- Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med 2008;23:781-7. https://doi.org/10.1007/s11606-008-0594-0.
- Rumpf HJ, Hapke U, Meyer C, John U. Screening for alcohol use disorders and at-risk drinking in the general population: psychometric performance of three questionnaires. Alcohol Alcohol 2002;37:261-8. https://doi.org/10.1093/alcalc/37.3.261.
- Belmont PJ, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am 2014;96:20-6. https://doi.org/10.2106/JBJS.M.00018.
- Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, et al. 90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet 2013;382:1097-104. https://doi.org/10.1016/S0140-6736(13)61749-3.
- Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, et al. 45-day mortality after 467,779 knee replacements for osteoarthritis from the National Joint Registry for England and Wales: an observational study. Lancet 2014;384:1429-36. https://doi.org/10.1016/S0140-6736(14)60540-7.
- Inneh IA, Lewis CG, Schutzer SF. Focused risk analysis: regression model based on 5,314 total hip and knee arthroplasty patients from a single institution. J Arthroplasty 2014;29:2031-5. https://doi.org/10.1016/j.arth.2014.05.007.
- Williams O, Fitzpatrick R, Hajat S, Reeves BC, Stimpson A, Morris RW, et al. Mortality, morbidity, and 1-year outcomes of primary elective total hip arthroplasty. J Arthroplasty 2002;17:165-71. https://doi.org/10.1054/arth.2002.29389.
- Belmont PJ, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty 2014;29:2025-30. https://doi.org/10.1016/j.arth.2014.05.015.
- NHS Digital . Hospital Episode Statistics, Admitted Patient Care, England, 2013–2014 2015.
- National Joint Registry (NJR) Editorial Board . 15th Annual Report 2018: National Joint Registry for England, Wales, Northern Ireland and the Isle of Man 2018. https://reports.njrcentre.org.uk/ (accessed 21 September 2019).
- Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 2006;21:177-80. https://doi.org/10.1007/s11606-006-0254-1.
- Peel TN, Cheng AC, Liew D, Buising KL, Lisik J, Carroll KA, et al. Direct hospital cost determinants following hip and knee arthroplasty. Arthritis Care Res 2015;67:782-90. https://doi.org/10.1002/acr.22523.
- Murphy BPD, Dowsey MM, Spelman T, Choong PFM. The impact of older age on patient outcomes following primary total knee arthroplasty. Bone Joint J 2018;100–B:1463-70. https://doi.org/10.1302/0301-620X.100B11.BJJ-2017-0753.R6.
- Snowden CP, Anderson H. Preoperative optimization: rationale and process – is it economic sense?. Curr Opin Anaesthesiol 2012;25:210-16. https://doi.org/10.1097/ACO.0b013e32834ef903.
- Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 2011;106:13-22. https://doi.org/10.1093/bja/aeq361.
- Musallam KM, Rosendaal FR, Zaatari G, Soweid A, Hoballah JJ, Sfeir PM, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg 2013;148:755-62. https://doi.org/10.1001/jamasurg.2013.2360.
- Fearon KC, Jenkins JT, Carli F, Lassen K. Patient optimization for gastrointestinal cancer surgery. Br J Surg 2013;100:15-27. https://doi.org/10.1002/bjs.8988.
- Durrand JW, Batterham AM, Danjoux GR. Pre-habilitation. I: aggregation of marginal gains. Anaesthesia 2014;69:403-6. https://doi.org/10.1111/anae.12666.
- Carli F, Charlebois P, Baldini G, Cachero O, Stein B. An integrated multidisciplinary approach to implementation of a fast-track program for laparoscopic colorectal surgery. Can J Anaesth 2009;56:837-42. https://doi.org/10.1007/s12630-009-9159-x.
- Stergiopoulou A, Birbas K, Katostaras T, Mantas J. The effect of interactive multimedia on preoperative knowledge and postoperative recovery of patients undergoing laparoscopic cholecystectomy. Methods Inf Med 2007;46:406-9. https://doi.org/10.1160/ME0406.
- Kothmann E, Batterham AM, Owen SJ, Turley AJ, Cheesman M, Parry A, et al. Effect of short-term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: a pilot study. Br J Anaesth 2009;103:505-10. https://doi.org/10.1093/bja/aep205.
- Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil 2012;93:2148-53. https://doi.org/10.1016/j.apmr.2012.07.012.
- Topp R, Swank AM, Quesada PM, Nyland J, Malkani A. The effect of prehabilitation exercise on strength and functioning after total knee arthroplasty. PM R 2009;1:729-35. https://doi.org/10.1016/j.pmrj.2009.06.003.
- Santa Mina D, Clarke H, Ritvo P, Leung YW, Matthew AG, Katz J, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy 2014;100:196-207. https://doi.org/10.1016/j.physio.2013.08.008.
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 2010;105:817-43. https://doi.org/10.1111/j.1360-0443.2010.02899.x.
- Boden JM, Fergusson DM, Horwood LJ. Alcohol misuse and relationship breakdown: findings from a longitudinal birth cohort. Drug Alcohol Depend 2013;133:115-20. https://doi.org/10.1016/j.drugalcdep.2013.05.023.
- Boden JM, Fergusson DM, Horwood LJ. Alcohol misuse and criminal offending: findings from a 30-year longitudinal study. Drug Alcohol Depend 2013;128:30-6. https://doi.org/10.1016/j.drugalcdep.2012.07.014.
- Boden JM, Fergusson DM, Horwood LJ. Alcohol misuse and violent behavior: findings from a 30-year longitudinal study. Drug Alcohol Depend 2012;122:135-41. https://doi.org/10.1016/j.drugalcdep.2011.09.023.
- Schou L, Moan IS. Alcohol use-sickness absence association and the moderating role of gender and socioeconomic status: a literature review. Drug Alcohol Rev 2016;35:158-69. https://doi.org/10.1111/dar.12278.
- Henkel D. Unemployment and substance use: a review of the literature (1990–2010). Curr Drug Abuse Rev 2011;4:4-27. https://doi.org/10.2174/1874473711104010004.
- World Health Organization (WHO) . Global Status Report on Alcohol and Health 2018.
- House of Commons Health Committee . Government’s Alcohol Strategy: Third Report of Session 2012–13. Volume I: Report, Together With Formal Minutes and Oral and Written Evidence 2012. www.publications.parliament.uk/pa/cm201213/cmselect/cmhealth/132/132.pdf.
- Anderson P. Alcohol as a key area. BMJ 1991;303:766-9. https://doi.org/10.1136/bmj.303.6805.766.
- McGovern R, Kaner EFS, Riekert KA, Ockene JK, Pbert Lori. The Handbook of Health Behavior Change. New York, NY: Springer; 2013.
- Spies C, Tønnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin Exp Res 2001;25:164-70. https://doi.org/10.1097/00000374-200105051-00028.
- Zakhari S. Alcohol and the cardiovascular system: molecular mechanisms for beneficial and harmful action. Alcohol Health Res World 1997;21:21-9.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg 1999;86:869-74. https://doi.org/10.1046/j.1365-2168.1999.01181.x.
- Spies CD, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, et al. Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology 2004;100:1088-100. https://doi.org/10.1097/00000542-200405000-00010.
- Preedy VR, Patel VB, Why HJ, Corbett JM, Dunn MJ, Richardon PJ. Alcohol and the heart: biochemical alterations. Cardiovasc Res 1996;31:139-47. https://doi.org/10.1016/S0008-6363(95)00184-0.
- Felding C, Jensen LM, Tønnesen H. Influence of alcohol intake on postoperative morbidity after hysterectomy. Am J Obstet Gynecol 1992;166:667-70. https://doi.org/10.1016/0002-9378(92)91695-7.
- Nath B, Li Y, Carroll JE, Szabo G, Tseng JF, Shah SA. Alcohol exposure as a risk factor for adverse outcomes in elective surgery. J Gastrointest Surg 2010;14:1732-41. https://doi.org/10.1007/s11605-010-1350-4.
- Tønnesen H, Petersen KR, Højgaard L, Stokholm KH, Nielsen HJ, Knigge U, et al. Postoperative morbidity among symptom-free alcohol misusers. Lancet 1992;340:334-7. https://doi.org/10.1016/0140-6736(92)91405-W.
- Bradley KA, Rubinsky AD, Sun H, Bryson CL, Bishop MJ, Blough DK, et al. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. J Gen Intern Med 2011;26:162-9. https://doi.org/10.1007/s11606-010-1475-x.
- Eliasen M, Grønkjær M, Skov-Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS, et al. Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg 2013;258:930-42. https://doi.org/10.1097/SLA.0b013e3182988d59.
- Wåhlin S, Tønnesen H. Time for ‘alcohol-free operations’. Two standard drinks a day doubles the risk of postoperative complications. Lakartidningen 2014;111:1966-9.
- Shabanzadeh DM, Sørensen LT. Alcohol consumption increases post-operative infection but not mortality: a systematic review and meta-analysis. Surg Infect (Larchmt) 2015;16:657-68. https://doi.org/10.1089/sur.2015.009.
- Best MJ, Buller LT, Gosthe RG, Klika AK, Barsoum WK. Alcohol misuse is an independent risk factor for poorer postoperative outcomes following primary total hip and total knee arthroplasty. J Arthroplasty 2015;30:1293-8. https://doi.org/10.1016/j.arth.2015.02.028.
- Harris AH, Reeder R, Ellerbe L, Bradley KA, Rubinsky AD, Giori NJ. Preoperative alcohol screening scores: association with complications in men undergoing total joint arthroplasty. J Bone Joint Surg Am 2011;93:321-7. https://doi.org/10.2106/JBJS.I.01560.
- Rotevatn TA, Bøggild H, Olesen CR, Torp-Pedersen C, Mortensen RN, Jensen PF, et al. Alcohol consumption and the risk of postoperative mortality and morbidity after primary hip or knee arthroplasty: a register-based cohort study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173083.
- Tonnesen H, Rosenberg J, Nielsen HJ, Rasmussen V, Hauge C, Pedersen IK, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ 1999;318:1311-16. https://doi.org/10.1136/bmj.318.7194.1311.
- Tønnesen H. Alcohol abuse and postoperative morbidity . Danish Med Bull 2003;50:139-60.
- Martin MJ, Heymann C, Neumann T, Schmidt L, Soost F, Mazurek B, et al. Preoperative evaluation of chronic alcoholics assessed for surgery of the upper digestive tract. Alcohol Clin Exp Res 2002;26:836-40. https://doi.org/10.1111/j.1530-0277.2002.tb02612.x.
- Kip MJ, Neumann T, Jugel C, Kleinwaechter R, Weiss-Gerlach E, Guill MM, et al. New strategies to detect alcohol use disorders in the preoperative assessment clinic of a German university hospital. Anesthesiology 2008;109:171-9. https://doi.org/10.1097/ALN.0b013e31817f5be3.
- Poon A, Owen J, Gijsbers AJ. Identification of at-risk drinkers in an orthopaedic inpatient population. Aust N Z J Surg 1994;64:775-9. https://doi.org/10.1111/j.1445-2197.1994.tb04538.x.
- Wilson GB, Wray C, McGovern R, Newbury-Birch D, McColl E, Crosland A, et al. Intervention to reduce excessive alcohol consumption and improve comorbidity outcomes in hypertensive or depressed primary care patients: two parallel cluster randomized feasibility trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-235.
- Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. Pre-operative screening for excessive alcohol consumption among patients scheduled for elective surgery. Drug Alcohol Rev 2007;26:119-25. https://doi.org/10.1080/09595230601146595.
- NHS England . CQUIN 2017 19 Guidance 2018. www.england.nhs.uk/publication/commissioning-for-quality-and-innovation-cquin-guidance-for-2017-2019 (accessed 21 September 2019).
- Egholm JWM, Pedersen B, Oppedal K, Rasmussen M, Adami J, Lauritzen JB, et al. Scand-Ankle: Effect of Patient Education for Alcohol Cessation Intervention on Acute Fracture Surgery – a Randomized Controlled Trial 2017.
- Tønnesen H. Intensive Intervention Among Alcohol Patients Prior to Elective Hip Replacement-Complications, Life-Quality, Surgical Outcome 2002.
- Egholm JW, Pedersen B, Møller AM, Adami J, Juhl CB, Tønnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database Syst Rev 2018;11. https://doi.org/10.1002/14651858.CD008343.pub3.
- Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2018;2. https://doi.org/10.1002/14651858.CD004148.pub4.
- McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev 2011;8. https://doi.org/10.1002/14651858.CD005191.pub3.
- Nilsen P, Baird J, Mello MJ, Nirenberg T, Woolard R, Bendtsen P, et al. A systematic review of emergency care brief alcohol interventions for injury patients. J Subst Abuse Treat 2008;35:184-201. https://doi.org/10.1016/j.jsat.2007.09.008.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991.
- Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA 1997;277:1039-45. https://doi.org/10.1001/jama.1997.03540370029032.
- Lock CA, Kaner E, Heather N, Doughty J, Crawshaw A, McNamee P, et al. Effectiveness of nurse-led brief alcohol intervention: a cluster randomized controlled trial. J Adv Nurs 2006;54:426-39. https://doi.org/10.1111/j.1365-2648.2006.03836.x.
- O’Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol 2014;49:66-78. https://doi.org/10.1093/alcalc/agt170.
- Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med 2005;165:986-95. https://doi.org/10.1001/archinte.165.9.986.
- Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD004148.pub3.
- Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.e8501.
- Newbury-Birch D, Bland M, Cassidy P, Coulton S, Deluca P, Drummond C, et al. Screening and brief interventions for hazardous and harmful alcohol use in probation services: a cluster randomised controlled trial protocol. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-418.
- Segura L, Anderson P, Gual A. Optimizing the delivery of interventions for harmful alcohol use in primary healthcare: an update. Curr Opin Psychiatry 2018;31:324-32. https://doi.org/10.1097/YCO.0000000000000435.
- Johnson M, Jackson R, Guillaume L, Meier P, Goyder E. Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health (Oxf) 2011;33:412-21. https://doi.org/10.1093/pubmed/fdq095.
- Emmen MJ, Schippers GM, Bleijenberg G, Wollersheim H. Effectiveness of opportunistic brief interventions for problem drinking in a general hospital setting: systematic review. BMJ 2004;328. https://doi.org/10.1136/bmj.37956.562130.EE.
- Smith AJ, Hodgson RJ, Bridgeman K, Shepherd JP. A randomized controlled trial of a brief intervention after alcohol-related facial injury. Addiction 2003;98:43-52. https://doi.org/10.1046/j.1360-0443.2003.00251.x.
- O’Neill G, Masson S, Bewick L, Doyle J, McGovern R, Stoker E, et al. Can a theoretical framework help to embed alcohol screening and brief interventions in an endoscopy day-unit?. Frontline Gastroenterol 2016;7:47-53. https://doi.org/10.1136/flgastro-2014-100519.
- Lock CA. Alcohol and brief intervention in primary health care: what do patients think?. Prim Health Care Res Dev 2004;5:162-78. https://doi.org/10.1191/1463423604pc194oa.
- Lock CA. Screening and brief alcohol interventions: what, why, who, where and when? A review of the literature. J Subst Use 2004;9:91-101. https://doi.org/10.1080/14659890410001665096.
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction 2002;97:279-92. https://doi.org/10.1046/j.1360-0443.2002.00018.x.
- Eggleston J, Gallagher J, Gallagher M, Hares T, Murray E, Naroz N, et al. Who should give lifestyle advice in general practice and what factors influence attendance at health promotion clinics? Survey of patients’ views. Br J Gen Pract 1995;45:669-71.
- Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol 2006;41:643-9. https://doi.org/10.1093/alcalc/agl059.
- Proude EM, Conigrave KM, Haber PS. Effectiveness of skills-based training using the Drink-less package to increase family practitioner confidence in intervening for alcohol use disorders. BMC Med Educ 2006;6. https://doi.org/10.1186/1472-6920-6-8.
- Gomel MK, Saunders JB, Burns L, Hardcastle D, Sumich M. Dissemination of early intervention for harmful alcohol consumption in general practice. Health Promot J Austr 1994;4:65-9.
- McHugh F, Lindsay GM, Hanlon P, Hutton I, Brown MR, Morrison C, et al. Nurse led shared care for patients on the waiting list for coronary artery bypass surgery: a randomised controlled trial. Heart 2001;86:317-23. https://doi.org/10.1136/heart.86.3.317.
- Fernandez AC, Claborn KR, Borsari B. A systematic review of behavioural interventions to reduce preoperative alcohol use. Drug Alcohol Rev 2015;34:508-20. https://doi.org/10.1111/dar.12285.
- Kummel M, Vahlberg T, Ojanlatva A, Kärki R, Mattila T, Kivelä SL. Effects of an intervention on health behaviors of older coronary artery bypass (CAB) patients. Arch Gerontol Geriatr 2008;46:227-44. https://doi.org/10.1016/j.archger.2007.04.003.
- Hansen TB, Bredtoft HK, Larsen K. Preoperative physical optimization in fast-track hip and knee arthroplasty. Dan Med J 2012;59.
- Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil 2010;24:137-48. https://doi.org/10.1177/0269215509347432.
- Harris AHS, Frey MS, DeBenedetti AF, Bradley KA. Alcohol misuse prevalence and associations with post-operative complications in US surgical patients: a review. Open Surg J 2008;2:50-8. https://doi.org/10.2174/1874300500802010050.
- Pedersen B, Alva-Jørgensen P, Raffing R, Tønnesen H. Fractures and alcohol abuse: patient opinion of alcohol intervention. Open Orthop J 2011;5:7-12. https://doi.org/10.2174/1874325001105010007.
- Lauridsen SV, Thomsen T, Kaldan G, Lydom LN, Tønnesen H. Smoking and alcohol cessation intervention in relation to radical cystectomy: a qualitative study of cancer patients’ experiences. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3792-5.
- Groves P, Pick S, Davis P, Cloudesley R, Cooke R, Forsythe M, et al. Routine alcohol screening and brief interventions in general hospital in-patient wards: acceptability and barriers. Drug-Educ Prev Polic 2010;17:55-71. https://doi.org/10.3109/09687630802088208.
- Lock CA, Kaner E, Lamont S, Bond S. A qualitative study of nurses’ attitudes and practices regarding brief alcohol intervention in primary health care. J Adv Nurs 2002;39:333-42. https://doi.org/10.1046/j.1365-2648.2002.02294.x.
- Myers B, Stein DJ, Mtukushe B, Sorsdahl K. Feasibility and acceptability of screening and brief interventions to address alcohol and other drug use among patients presenting for emergency services in Cape Town, South Africa. Adv Prev Med 2012;2012. https://doi.org/10.1155/2012/569153.
- Seigers DK, Carey KB. Screening and brief interventions for alcohol use in college health centers: a review. J Am Coll Health 2010;59:151-8. https://doi.org/10.1080/07448481.2010.502199.
- Broyles LM, Rodriguez KL, Kraemer KL, Sevick MA, Price PA, Gordon AJ. A qualitative study of anticipated barriers and facilitators to the implementation of nurse-delivered alcohol screening, brief intervention, and referral to treatment for hospitalized patients in a Veterans Affairs medical center. Addict Sci Clin Pract 2012;7. https://doi.org/10.1186/1940-0640-7-7.
- Grocott MP, Browne JP, Van der Meulen J, Matejowsky C, Mutch M, Hamilton MA, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007;60:919-28. https://doi.org/10.1016/j.jclinepi.2006.12.003.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Dowsey MM, Choong PF. The utility of outcome measures in total knee replacement surgery. Int J Rheumatol 2013;2013. https://doi.org/10.1155/2013/506518.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Bougeard AM, Brent A, Swart M, Snowden C. A survey of UK peri-operative medicine: pre-operative care. Anaesthesia 2017;72:1010-15. https://doi.org/10.1111/anae.13934.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Beyer F, Lynch E, Kaner E. Brief interventions in primary care: an evidence overview of practitioner and digital intervention programmes. Curr Addict Rep 2018;5:265-73. https://doi.org/10.1007/s40429-018-0198-7.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Centre for Drug and Alcohol Studies . The Drink-Less Programme 1993.
- McAvoy B, Kaner E, Haighton K, Heather N, Gilvarry E. Drink-less: marketing a brief intervention package in UK general practice. Fam Pract 1997;14:427-8.
- Coulton S, Perryman K, Bland M, Cassidy P, Crawford M, Deluca P, et al. Screening and brief interventions for hazardous alcohol use in accident and emergency departments: a randomised controlled trial protocol. BMC Health Serv Res 2009;9. https://doi.org/10.1186/1472-6963-9-114.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. Sutton: Silverback Publishing; 2014.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. J Admin Gov 2009;4:72-9.
- Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8. https://doi.org/10.1176/ajp.127.12.1653.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2010;3. https://doi.org/10.1002/14651858.CD005470.pub2.
- Wanyonyi KL, Themessl-Huber M, Humphris G, Freeman R. A systematic review and meta-analysis of face-to-face communication of tailored health messages: implications for practice. Patient Educ Couns 2011;85:348-55. https://doi.org/10.1016/j.pec.2011.02.006.
- Wilkinson C, Allsop S, Chikritzhs T. Alcohol pouring practices among 65- to 74-year-olds in Western Australia. Drug Alcohol Rev 2011;30:200-6. https://doi.org/10.1111/j.1465-3362.2010.00218.x.
- Office for National Statistics (ONS) . Drinking: Adults’ Behaviour and Knowledge in 2009 2010.
- Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. J Stud Alcohol 2000;61:787-93. https://doi.org/10.15288/jsa.2000.61.787.
- McCambridge J, Day M. Randomized controlled trial of the effects of completing the Alcohol Use Disorders Identification Test questionnaire on self-reported hazardous drinking. Addiction 2008;103:241-8. https://doi.org/10.1111/j.1360-0443.2007.02080.x.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j. 2002.384.doc.x.
- Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med 1997;12:274-83. https://doi.org/10.1007/s11606-006-5063-z.
- Fleming MF, Manwell LB, Barry KL, Adams W, Stauffacher EA. Brief physician advice for alcohol problems in older adults: a randomized community-based trial. J Fam Pract 1999;48:378-84.
- Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320. https://doi.org/10.1136/bmj.320.7227.114.
- Witkiewitz K, Kranzler HR, Hallgren KA, O’Malley SS, Falk DE, Litten RZ, et al. Drinking risk level reductions associated with improvements in physical health and quality of life among individuals with alcohol use disorder. Alcohol Clin Exp Res 2018;42:2453-65. https://doi.org/10.1111/acer.13897.
- Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 2017;41:179-86. https://doi.org/10.1111/acer.13272.
- Drummond C, Deluca P, Coulton S, Bland M, Cassidy P, Crawford M, et al. The effectiveness of alcohol screening and brief intervention in emergency departments: a multicentre pragmatic cluster randomized controlled trial. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0099463.
- NHS Scotland . Scottish Arthroplasty Project: Annual Report 2017 2017.
Appendix 1 Behaviour change techniques present in intervention materials
| Intervention tool | BCT |
|---|---|
| BA | 5.1 Information on health consequences |
| 5.1 Information on health consequences | |
| 2.2 Feedback on behaviour | |
| 2.7 Feedback on outcomes of behaviour | |
| 6.2 Social comparison | |
| 5.1 Information on health consequences | |
| 5.3 Information on social and environmental consequences | |
| 5.6 Information on emotional consequences | |
| 8.2 Behaviour substitution | |
| 12.1 Restructuring the physical environment | |
| 12.2 Restructuring the social environment | |
| 12.3 Avoidance/reducing exposure to cues for behaviour | |
| 1.1 Goal-setting behaviour | |
| BI | 9.2 Pros and cons |
| 9.2 Pros and cons | |
| 1.1 Goal-setting behaviour | |
| 1.2 Problem solving | |
| 3.1 Social support unspecified |
| Intervention tool | BCT |
|---|---|
| Patient information leaflet | 5.1 Information on health consequences |
| 5.3 Information on social and environmental consequences | |
| 9.2 Pros and cons | |
| 2.3 Self-monitoring of behaviour | |
| 1.2 Problem-solving | |
| 8.2 Behaviour substitution | |
| 12.3 Avoidance/reducing exposure to cues for behaviour | |
| 12.1 Restructuring the physical environment | |
| 12.2 Restructuring the social environment | |
| 1.2 Problem-solving | |
| 3.1 Social support unspecified | |
| 2.3 Self-monitoring of behaviour |
Appendix 2 Capability, Opportunity and Motivation to perform a particular Behaviour questionnaire
Appendix 3 Capability, Opportunity and Motivation to perform a particular Behaviour questionnaire responses
| Category | Item | Summary of free-text responses | Number of responses |
|---|---|---|---|
| Capability | Know more about why it was important |
Have better understanding of postoperative risks to explain to patients why they should do it. Facts and figures to back up To have a better understanding of risks related to surgery in order to allow an explanation of why it would benefit the patient(s) and encourage them to try to cut down/quit Important to understand why the intervention is targeting alcohol reduction |
9 |
| Know more about how to do it |
No previous experience in this area so increased knowledge and training are required A structured pathway for patients to be able to follow to reduce alcohol intake would help Need a guide including precise facts and information to provide to patients Can be a difficult subject to approach especially if the patient feels that their alcohol intake is not high/problematic |
1 | |
| Have practical skills to adequately deliver each part of the intervention | Guidance on what should be covered | 6 | |
| Have skills to navigate the intervention in the way that is required | Training required to enable delivery of deliver a positive/effective intervention without it being too time-consuming | 8 | |
| Have the ability to keep going despite physical barriers | 5 | ||
| Have the ability to keep going despite lacking in determination/motivation |
Can be difficult to encourage patients when they lack interest or are resistant to change or accepting help Could be a challenging role especially for patients who have had habits for a number of years |
6 | |
| Have more physical stamina | I think we have to do this anyway on lots of things and is part of working in NHS | 1 | |
| Have more mental stamina | 2 | ||
| Opportunity | Have more time to do it |
Time is short and may neglect other issues if spending time on the intervention Need longer appointment times if to deliver more interventions |
8 |
| Have the necessary materials |
Protocols and guidelines as to who you want targeted and key things to cover Visual aids often good at helping patients understand the importance of reducing alcohol/smoking from my experience |
7 | |
| Have colleagues around me doing the same thing |
Everyone working towards the same goal and outcome Working as a team advice from colleagues how they managed certain situations/support Working as team – can bounce ideas off each other/help motivate |
6 | |
| Have reminders in place to deliver the intervention |
Very basic forms would be good due to time limits These patients will hopefully be identified on set lists |
4 | |
| Have support from others | 3 | ||
| Motivation | Feel that I want to do it | 2 | |
| Feel that I should do it | 2 | ||
| Believe that it would be a good thing | 5 | ||
| Develop better plans for doing it |
Time allocated Need to be clear, concise, easy to follow I believe in it if it’s done properly with time planning ahead Need a structured plan to feel confident in talking to patients about it |
9 | |
| Develop a habit of doing it |
Would need some continuity, not ‘one-off’ sessions Set time/follow on from a questionnaire in PAC, which would automatically trigger if needed intervention |
6 | |
| Something else (please specify) |
Increase PAC time Needs to work where patients do not feel judged – patients should not be dictated to |
2 |
Appendix 4 Affordability, Practicability, Effectiveness, Acceptability, Safety and Equity questionnaire
Appendix 5 Training feedback form
Appendix 6 Focus group participant information sheet
Appendix 7 Focus group consent form
Appendix 8 Focus group discussion guide
Appendix 9 Treatment-as-usual survey
Appendix 10 Feasibility study health-care professional interview participant information sheet
Appendix 11 Feasibility study health-care professional interview topic guide
Appendix 12 Feasibility study patient interview information sheet
Appendix 13 Feasibility study patient interview discussion guide
Appendix 14 Pilot randomised controlled trial patient participant information sheet
Appendix 15 Pilot randomised controlled trial patient participant interview topic guides
Glossary
- Abstainer
- A person who does not drink alcohol.
- Abstinence
- Never consuming alcohol.
- Alcohol
- Ethanol (ethyl alcohol) the psychoactive ingredient of alcoholic drinks.
- Alcohol dependence
- A cluster of behavioural, cognitive and physiological factors including craving alcohol, tolerance, continuing to drink despite experiencing the harmful consequences and prioritising alcohol consumption over other activities and obligations.
- Alcohol misuse
- A term used to capture both alcohol dependence and higher-risk (‘harmful’) drinking.
- Alcohol use disorder(s)
- A range of alcohol-related mental health problems including alcohol dependence, higher-risk drinking (previously harmful drinking) and increased risk drinking (previously hazardous drinking) recognised in the international disease classification systems [International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)].
- Alcohol Use Disorders Identification Test
- A validated alcohol screening tool to assess the presence of increased risk and higher-risk alcohol consumption and to identify possible instances of alcohol dependence requiring further assessment.
- Alcohol Use Disorders Identification Test Consumption
- A validated alcohol screening tool consisting of the three consumption-focused questions (the first three questions) of the Alcohol Use Disorders Identification Test.
- Alcohol withdrawal
- Physical symptoms resulting from a reduction in the amount of alcohol consumed or the complete cessation of alcohol consumption.
- Alcoholic drink
- A beverage containing ethanol.
- Binge drinking
- Single-occasion high-volume alcohol consumption, specifically the consumption of six or more units of alcohol on one occasion.
- Blood alcohol concentration
- The concentration of alcohol in the blood.
- CAGE questionnaire
- A four-question validated screening tool used to identify the presence of possible alcohol dependence.
- Dependence
- See Alcohol dependence.
- Feasibility study
- A research study used to assess the practicality of planned methods.
- Harmful drinking
- Previously used to describe a pattern of alcohol consumption resulting in mental or physical harm. Now termed ‘higher-risk’ drinking.
- Hazardous drinking
- Previously used to describe a pattern of alcohol consumption that increases an individual’s risk of experiencing harm. Now termed ‘increased risk’ drinking.
- Lower-risk drinking guidelines
- Guidelines set by the UK Government detailing amounts of alcohol and patterns of alcohol consumption that can be consumed without a serious impact on health.
- Pilot randomised controlled trial
- A small-scale randomised controlled trial employed to determine the feasibility of a full trial and to inform the design of such a trial.
- Preoperative assessment
- An assessment focused on identifying factors and comorbidities that may present a risk of complications during anaesthesia, during surgery or in the postoperative period.
- Preoperative assessment clinic
- A hospital clinic dedicated to conducting assessments of patients prior to surgery.
- Randomised controlled trial
- A trial in which individuals are randomly allocated to two or more groups, one of which (the control) is ‘treatment as usual’ or no treatment.
- Randomised trial
- A trial in which individuals are randomly allocated to two or more groups.
- Risky drinking
- Drinking alcohol in a way that increases the person’s chance of experiencing physical, mental or social harm, specifically drinking in excess of the lower-risk drinking guidelines. In this report, the term it is used to capture both ‘increased risk’ and ‘higher-risk’ (formerly harmful and hazardous) drinking but not alcohol dependence.
- Standard drink
- A measurement of alcoholic drinks. In the UK, one standard drink contains 8 g or 10 ml of ethanol and is equivalent to 1 unit.
- Unit
- A standardised measurement of alcoholic drinks. One unit contains 8 g or 10 ml of ethanol.
List of abbreviations
- AUDIT
- Alcohol Use Disorders Identification Test
- AUDIT-C
- Alcohol Use Disorders Identification Test Consumption
- BA
- brief advice
- BBI
- brief behavioural intervention
- BCT
- behaviour change technique
- BI
- brief intervention
- BMI
- body mass index
- COM–B
- Capability, Opportunity and Motivation to perform a particular Behaviour
- CONSORT
- Consolidated Standards of Reporting Trials
- CQUIN
- Commissioning for Quality and Innovation
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- HCA
- health-care assistant
- HCP
- health-care professional
- LFT
- liver function test
- MAST
- Michigan Alcoholism Screening Test
- NPT
- normalisation process theory
- PA
- preoperative assessment
- PAC
- preoperative assessment clinic
- POMS
- Post Operative Morbidity Survey
- RCT
- randomised controlled trial
- SD
- standard deviation
- TAU
- treatment as usual
- TSC
- Trial Steering Committee
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index
