Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/129/183. The contractual start date was in November 2012. The draft report began editorial review in February 2018 and was accepted for publication in August 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Robert Freeman reports speaker fees [from Bard Medical (Covington, GA, USA), Astellas Pharma Inc. (Tokyo, Japan) and Pfizer Inc. (New York City, NY, USA)] and grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. John Norrie reports membership of the following NIHR boards: the Cardiopulmonary Resuscitation Decision-making Committee, HTA Commissioning Board, HTA Commissioning Sub-Board (Expression of Interest), HTA Funding Boards Policy Group, HTA General Board and HTA Post-board Funding Teleconference; the NIHR Clinical Trials Unit Standing Advisory Committee; the NIHR HTA and Efficacy and Mechanism Evaluation Editorial Board; and the Pre-exposure Prophylaxis Impact Review Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Hemming et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In 2012, the UK government’s National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme funded the Vault or Uterine prolapse surgery Evaluation (VUE) trial. This publication describes the research.
The study was a major, multicentre, UK-based randomised controlled trial (RCT) investigating the clinical effectiveness (including safety) and cost-effectiveness of surgical treatment, primarily in terms of improvement in prolapse symptoms, in women having a uterine or a vault prolapse repair.
Relationship to PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials in women with anterior or posterior pelvic organ prolapse
The PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials (PROSPECT) in women with anterior or posterior pelvic organ prolapse (POP)1 was a large HTA-funded UK-based RCT of anterior or posterior prolapse surgery with or without the use of mesh (HTA reference number 07/60/18) that was also undertaken by the research team (with long-term follow-up under way). The methods developed in PROSPECT1 were used to inform the design, conduct, analysis and reporting of the VUE trial.
Description of the underlying health problem
Pelvic organ prolapse is the descent of pelvic organs from their normal anatomical position.
Prolapse can occur in three compartments: the anterior (cystocele) vaginal wall, the posterior (rectocele) vaginal wall and the uterus or the apex (vault in those who have had a hysterectomy). Women can have prolapsed in one or more compartments at the same time.
There is little epidemiological research into this condition because it has a variety of presentations and they do not all cause symptoms, particularly in the early stages. 2 Commonly reported symptoms include a feeling of dragging or heaviness in the vagina, an uncomfortable bulge distending the introitus, urinary symptoms (e.g. incontinence and voiding difficulty), bowel symptoms (e.g. incomplete emptying) and sexual dysfunction.
The prevalence of prolapse varies depending on age, race and symptoms, with anterior prolapse being the more prevalent. 3–8
Standard management
Women with prolapses may be managed conservatively with pelvic floor muscle training (PFMT), pessaries or surgery. Management of associated conditions, such as lower urinary tract symptoms (urinary incontinence or overactive bladder syndrome), bowel problems (constipation or faecal incontinence), sexual dysfunction and local vaginal atrophy caused by oestrogen deficiency if post-menopausal, may also be required.
Conservative management for women with prolapses
There is not enough evidence to inform the use of mechanical devices (pessaries or rings), although these are often used as a first-line treatment or can be used for women who are unfit for surgery or wish to avoid surgery. 9–12
Mechanical devices can be very efficacious, but further research is required to identify the best type of device, the potential long-term effects and the use of supplementary treatments, such as oestrogen. 9,10
Conservative physical treatments, such as PFMT, are also often recommended as first-line management. The most recent reviews found evidence supporting the use of PFMT to prevent and reduce prolapse symptoms and severity, as well as improvement in urinary and bowel symptoms. 11,13
Vaginal oestrogen can also be used to reduce atrophic symptoms for post-menopausal women, or for before and after surgery. However, the evidence supporting vaginal oestrogen use is limited and inconclusive. 14,15
Surgical management for women with prolapses
Surgery for POP is common, with recent estimates indicating that women have a lifetime risk of between 6% and 20% of undergoing surgery for prolapse. 3,5,16,17
In England and Wales in 2016–17, 24,784 women were admitted to hospital with a main diagnosis of female genital prolapse, and 29,729 operations were performed (some women had more than one type of prolapse operation). 18 The majority of operations (83%) were in women having an anterior repair (n = 11,224), posterior repair (n = 6855) or both operations (n = 6502). Around 21% of women (n = 5148) had an apical procedure. It was identified, from PROSPECT1 and NHS digital data,18 that around one-third of women with prolapse have a hysterectomy for uterine prolapse.
The demand for surgical prolapse repair may increase given that projections have predicted a rise of around 1.4 million women in the UK aged 50–85 years (most likely to have surgery) between 2017 and 2027. 19
Little is known about the long-term effectiveness and safety of different types of operations for uterine or vault prolapse, although the National Institute for Health and Care Excellence (NICE) has provided some guidance. 11,20,21
Surgical management for women with uterine or vault prolapses
Women may present with an isolated uterine or vault (apical) prolapse or in combination with other compartments. Numerous surgical techniques now exist for apical prolapse, but none has been properly evaluated in terms of adequately powered multicentre RCTs. 22–24
Uterine prolapse
If there is uterine descent (however small the uterus), removal of the uterus (hysterectomy) is standard practice at the time of prolapse repair in most parts of the world. Uterine descent is the most common indication for hysterectomy;25 however, hysterectomy for uterine descent is not an evidence-based practice.
At the onset of the VUE trial, surgery for uterine prolapse was broadly divided into two approaches: uterine removal (hysterectomy) or uterine preservation.
Uterine removal (hysterectomy)
When the uterus is removed during hysterectomy, the top of the vagina (the vaginal vault) must be secured to prevent later descent, which presents as a vault prolapse. The two main options are:
-
vaginal hysterectomy with a vault support procedure, such as plication of uterosacral and cardinal ligaments
-
subtotal abdominal hysterectomy (supracervical hysterectomy) and sacrocervicopexy (attaching the cervical stump to the sacrum with mesh).
Uterine preservation
-
Amputation of the cervix with shortening and apposition of the cardinal ligaments.
-
Hysteropexy (attaching the uterus to the sacrospinous ligaments vaginally or to the sacrum abdominally with sutures or mesh, or both).
Vault prolapses
After a hysterectomy, vault prolapse occurs in women when the vaginal vault descends into or out of the vagina despite vault support procedures carried out at the time of hysterectomy.
At the onset of the VUE trial, a variety of techniques to suspend or reposition the vault were available. The techniques were broadly divided into vaginal or abdominal approaches and included the following:
-
Vaginal approaches:
-
vaginal sacrospinous fixation or sacrospinous colpopexy (vault attachment to the sacrospinous ligament, either bilaterally or on one side only; this is traditionally performed using sutures, but mesh could also be used)
-
transvaginal mesh kits that suspended the vault.
-
-
Abdominal approaches:
-
abdominal sacrocolpopexy (attachment of the vault to the sacrum, with a mesh bridge); this could be an open, laparoscopic or robotic laparoscopic procedure.
-
Current recommendations from the National Institute for Health Care and Excellence
An interventional procedures review26 and a Cochrane review22 have been conducted on the use of mesh in uterine or vault prolapse. NICE considered the evidence from independent reviews, RCTs and non-randomised studies, and in 2017 developed updated guidance on:
-
sacrocolpopexy with hysterectomy using mesh for uterine prolapse repair – see Interventional Procedures Guidance (IPG) 57723
-
uterine suspension using mesh (including sacrohysteropexy) to repair uterine prolapse – see IPG58427
-
infracoccygeal sacropexy using mesh to repair uterine prolapse – see IPG58228
-
infracoccygeal sacropexy using mesh to repair vaginal vault prolapse – see IPG58129
-
sacrocolpopexy using mesh to repair vaginal vault prolapse– see IPG583. 24
Of these procedures, only the standard operation of sacrocolpopexy using mesh for vault prolapse (IPG583) and uterine suspension using mesh (including sacrohysteropexy) to repair uterine prolapse (IPG584) are now considered to have enough evidence for safety and efficacy, such that they can be used under standard arrangements. 24 The uncertainty regarding the other procedures now means they require strict clinical governance arrangements. A clinical decision-making tool is currently being developed. 27
The decision to test uterine and vault prolapse surgical procedures
The most recent Cochrane review included 30 RCTs comparing surgical procedures for women with an apical vaginal prolapse. 22 The reviewers did not, however, separate the results for uterine and vault prolapse. The reviewers, and others, concluded that there is still insufficient information about any of the surgical options to guide management of uterine or vault prolapse and identified a need for adequately powered RCTs. 22,26,30 Thus, the evidence base for treating either of these groups of women is clearly inadequate in terms of patient-reported outcomes [e.g. subjective prolapse symptoms, effect on quality of life (QoL)], cost-effectiveness and safety.
Questions addressed by the VUE trial
Primary outcomes
To determine the optimal surgical management for women with uterine or vault prolapse, in terms of clinical effectiveness, cost-effectiveness and adverse events (AEs). The two parallel trials compared:
-
Uterine trial – in women having uterine prolapse surgery, the effects of a uterine preservation versus vaginal hysterectomy
-
Vault trial – in women having vault prolapse surgery, the effects of an abdominal vault versus a vaginal vault suspension.
The Uterine trial and Vault trial participants are reported separately as they are believed to be two distinct groups with different patient and clinical characteristics. Many studies combine results from both groups of women with ‘any’ apical prolapse, which makes interpretation of these study results problematic.
Secondary outcomes
-
To determine the differential effects on other outcomes, such as urinary, sexual and bowel function, QoL, general health, need for secondary surgery and AEs.
-
To identify possible effect modifiers (e.g. concomitant procedures, age, complex prolapse types).
The VUE trial assessed which of the most frequently employed techniques for uterine and vault prolapse are most clinically effective, safe and cost-effective. This will guide gynaecologists in their surgical practice and purchasers in their choice of provision of health care.
Given the number of uterine or vault prolapse procedures currently performed (around 5000 annually in England),18 the potential cost implications for the health service are considerable.
Chapter 2 Methods and practical arrangements
Study design
The methods developed in PROSPECT1 were used to inform the design, conduct, analysis and reporting of the VUE trial.
The VUE trial comprised two parallel RCTs (i.e. the Uterine trial and the Vault trial) to determine the clinical effectiveness (including safety) and cost-effectiveness of surgical treatment, primarily in terms of improvement in prolapse symptoms in women having either uterine or vault POP surgery.
Participating centres were asked to randomise participants as close to surgery as possible to minimise participant dropout; therefore, there was a delay from date of consent to date of randomisation. For the majority of sites, randomisation occurred around 2 weeks prior to surgery. Participant questionnaires were issued at 6 months after surgery and at 12 months after randomisation. This meant that if a woman did not receive surgery, no 6-month follow-up questionnaires were issued, but 12-month questionnaires were issued to all women. Women who received surgery were also reviewed in clinics 12 months after surgery (Figure 1).
FIGURE 1.
Flow diagram, with anticipated sample sizes. POP-Q, Pelvic Organ Prolapse Quantification System.
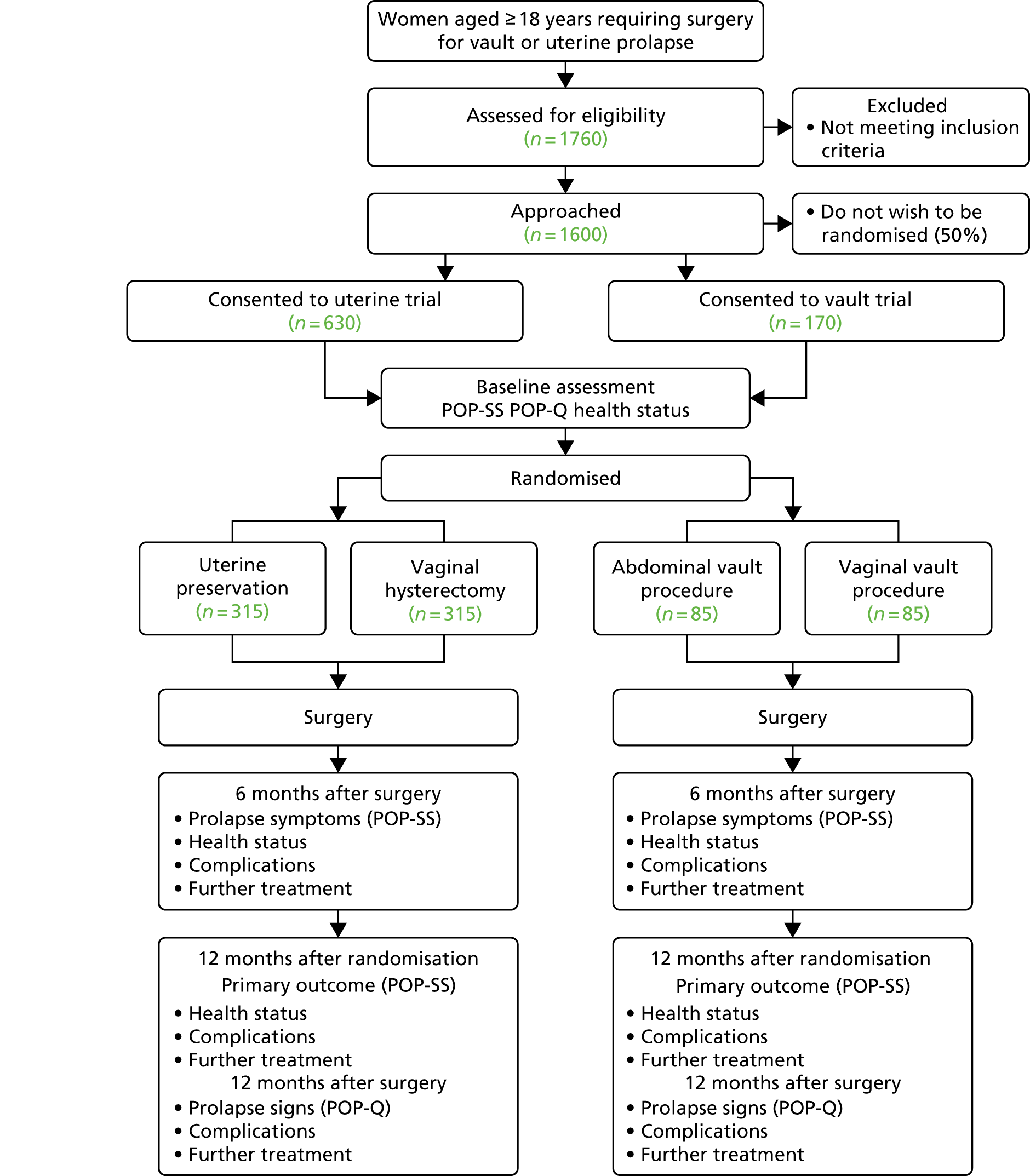
Further details of the study design, methodology and management [eligibility, consent, comparison of health technologies, treatment allocation, data collection and processing, sample size calculation, avoidance of bias (and blinding), serious adverse event (SAE) reporting] have been described previously. 31 All trial case report forms (CRFs) and participant completed questionnaires are included in the report methodology (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019).
Study outcome measures
The VUE trial used the International Urogynaecological Association (IUGA)/International Continence Society’s POP outcome recommendations and definitions. 32
Three primary outcome measures were identified (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019):
-
Women’s symptoms of prolapse [as measured using the patient-reported Pelvic Organ Prolapse Symptom Score (POP-SS)]33 at 12 months after randomisation. This scale was derived from seven questions judged to be most directly related to prolapse symptoms and has been shown to reflect the range and intensity of symptoms experienced by women, as well as being responsive to change over time. 34 Scores were determined for each of the seven items (ranging from 0 for ‘never’ to 4 for ‘all of the time’), with an overall POP-SS out of 28. Women who only partially completed the seven-item response schedule (defined as having completed six out of seven items in the scale) were assumed to have no symptoms when no response had been given to that individual item. Participants with more than one missing item were considered to have a missing overall score. Women were considered to be symptomatic if their overall score was > 0.
-
Quality of life (condition specific, measured as the woman’s rating of the overall effect of prolapse symptoms on everyday life on a 0–10 visual analogue scale in which 10 is the worst).
-
The primary economic outcome measure of cost-effectiveness was the incremental cost per quality-adjusted life-year (QALY), based on the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 35
Secondary outcomes
Other outcome measures included objective prolapse measurement; urinary, bowel and sexual symptoms [using the International Consultation on Incontinence (ICI) suite of validated questionnaires];36 intra-operative and postoperative complications, including the need for additional surgery (repeat surgery for prolapse recurrence, further prolapse surgery, or incontinence, and surgery required for AEs); cost; and cost-effectiveness (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019).
Objective prolapse measurement
Objective prolapse staging was carried out using the Pelvic Organ Prolapse Quantification (POP-Q) system37 and classified from stage 0 to 4 for anterior, posterior and apical at baseline and 12 months after surgery. The leading edge of the most descended compartment was used to define the overall stage.
The POP-Q system measures the maximum descent of each of the three prolapse compartments (anterior, posterior and apical) relative to the hymen; measurements inside the vagina are negative, whereas those outside the vagina are positive. An algorithm was employed to ensure that POP-Q staging was correctly calculated from the component measurements of the POP-Q (i.e. Aa, Ba, C, D, Bp, Ap and total vaginal length) in which common recording errors (e.g. Ba less than Aa) were corrected or queried. If data were discrepant, they were corrected by consultation with the local hospital records to obtain additional data, to achieve as complete a set of prolapse staging as possible, separately in each compartment. If the POP-Q data were missing, the surgeon’s qualitative record of stage was accepted for both overall and individual compartments (i.e. surgeons could specify the individual stage without giving the POP-Q measurements).
Usually, using the classic Bump criteria for the POP-Q system, any measurement from –1 cm (inside the hymen) to + 1 cm outside the hymen counts as stage 2. 37 Stage 2 was further subdivided into prolapse at the hymen or within (–1 cm to 0 cm, stage 2a or less) compared with prolapse at > 0 cm (stage 2b). 38,39 Thus, women were classified as having objective prolapse if the leading edge was at any point outside the hymen (measured at > 0 cm, stage 2b or more).
Urinary, bowel and sexual symptoms
Symptoms related to other aspects of pelvic floor dysfunction were measured using the ICI suite of validated questionnaires. 36
Urinary incontinence was assessed using the International Consultation on Incontinence Questionnaire (ICIQ) – urinary incontinence Short Form questionnaire (ICIQ-UI SF). Other urinary symptoms were recorded by the ICIQ – female lower urinary tract symptoms (ICIQ-FLUTS) instrument. The latter provides subscales for filling, voiding and incontinence symptoms.
The ICIQ bowel symptom questionnaire was not finalised when the VUE trial began. As in PROSPECT,1 draft questions were drafted to produce a short summary of relevant bowel symptoms in line with the ROME criteria40 to define constipation (see Appendix 1, Table 39).
Vaginal and sexual symptoms were assessed using the ICIQ – vaginal symptoms (ICIQ-VS) questionnaire. 36 The ICIQ-VS provides a brief and robust measure to assess the impact of vaginal symptoms and associated sexual matters on QoL and the outcome of treatment. The questionnaire provides subscales for vaginal symptoms, sexual matters and the overall impact of vaginal symptoms on QoL. Women who were sexually inactive were asked whether this related to their vaginal or prolapse symptoms or for another reason (including no partner).
Choice of validated outcome measures
Outcome measures were chosen to reflect the current international standards of reporting to ensure that the findings would be relevant to patients, clinicians and policy-makers. 32 The outcomes were measured at baseline to provide values for later statistical adjustments. The primary measure of prolapse symptoms was the woman’s subjective report using the POP-SS, developed and validated in a variety of populations for both research and clinical practice. 33
Temporary trial suspension
In June 2014, the Scottish Government requested that all Scottish NHS Health Boards considered suspending the use of transvaginal mesh implants in the surgical treatment of stress urinary incontinence and POP to enable an independent review to consider the ongoing debate on complication rates and AEs from the use of transvaginal mesh implants. The VUE trial was temporarily suspended (in Scottish centres only) for a period of 3 weeks during this time to ensure compliance and sponsor, Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) satisfaction with all trial processes across Scottish sites. The debate on the use of transvaginal synthetic mesh continues. 41,42
Blinding
Baseline data were reported by women before randomisation using self-completion questionnaires. Outcome assessors were blinded to randomisation; participants were unblinded if they requested the information. Surgeons were not blinded to the allocation procedure.
Sample size
Original sample size
The sample size calculations have been reproduced from that described in the original protocol publication. 31
In the Uterine trial, 268 women in each arm were required to achieve 90% power to detect a difference in the primary outcome measure (i.e. POP-SS at 12 months after randomisation) of 0.28 standard deviation (SD) at a significance level of 5% (two-sided alpha). Allowing for 15% loss to follow-up at 12 months, 315 women were required to be recruited to each arm (630 in total). The PROSPECT data indicated that a conservative estimate of the SD of the primary outcome was 7 units and a difference in means of 2 units represented a clinically important difference in POP-SS. Therefore, a standardised effect size of 2/7 = 0.28 SDs was used.
A smaller number of women were expected to be recruited to the Vault trial. Using data from the women recruited to PROSPECT,1 the expected number of recruits to the Vault trial was estimated at 27% of that recruited to the Uterine trial. Therefore, in the time that 630 women were to be recruited to the Uterine trial, an expected 85 women were to be recruited to each arm of the Vault trial (170 in total). A trial of 170 would have 80% power to detect a difference of 0.43 SDs at a 5% significance level (two-sided alpha). A standardised effect size of 0.43 equated to a difference in means of 3 units in the POP-SS measure.
In total, based on these assumptions, the number of recruits required across both trials was 800 women.
Important changes to the methods after trial commencement
Recruitment extension and increase in the Vault trial sample size
At steady state, recruitment rate of the Uterine trial was assumed to be approximately 29 women per month. Recruitment was slower than anticipated, and averaged 15 women per month.
As a result, an extension to the recruitment phase (an additional 15 months) was necessary to achieve the original target sample size (i.e. 630 women).
The PROSPECT data showed that the number of women requiring vault repair was approximately 27% of the number presenting with uterine prolapse. Therefore, during the original time period for randomising 630 women to the Uterine trial, it was anticipated that a further 170 women requiring vault repair would also be randomised to the Vault trial. 1
Recruitment rates to the Vault trial were in line with the original predictions. With an additional 15 months of recruitment, the Vault trial recruited beyond the original sample size of 170.
Conservatively assuming an average of seven women randomised per month, it was projected that a revised total of 280 Vault trial women would be recruited, which is 140 per arm or 119 allowing for 15% loss to follow-up. This gave 80% power to detect a difference of 0.36 SDs at 5% significance level (two-sided alpha). A standardised effect size of 0.36 SDs equated to a difference in means of 2.5 units in the primary outcome (POP-SS), considering a SD of 7 units. This was a smaller difference than originally calculated (i.e. 3 units with 80% power). This also equated to a relative reduction in the width of the confidence interval (CI) of 22% when compared with the precision without the extension. As the POP-SS at baseline was higher in women with vault prolapse (15.2 vs. 12.0 in women with a uterine prolapse, data from PROSPECT1) a greater difference after surgery was expected.
Statistical analysis
The predefined statistical analyses are included in the report methodology (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019). These methods apply to both the Uterine and the Vault trials, but each trial is reported and analysed separately.
A single principal analysis was undertaken 12 months after the last woman was randomised. All main analyses were based on an intention-to-treat principle in which women with observed outcome data were analysed according to their randomised allocation. All outcomes in both trials were described with the appropriate descriptive statistics when relevant: mean and SD for continuous and count outcomes, or medians and interquartile range if required for skewed data, numbers and percentages for dichotomous and categorical outcomes.
The analysis of the primary outcome POP-SS estimated the mean difference (MD; and 95% CI) between intervention and control groups at 12 months after randomisation using a linear mixed model with surgeon fitted as a random effect that adjusted for the minimisation covariates and the baseline score. The full model is available in Appendix 1, Table 40. A similar analysis was used to analyse the primary outcome at 6 months after surgery.
All secondary outcomes were analysed in a similar manner but using the appropriate generalised linear model (GLM) (logistic regression for dichotomous data, such as subjective prolapse failure) or time to event methods (Cox regression for time to further surgery). If possible, all models were adjusted for minimisation variables and baseline values.
Planned subgroup analyses
Subgroup analyses were carried out within the following groups:
-
concomitant anterior and/or posterior repair or none
-
concomitant continence procedure or not
-
age (< 60 or ≥ 60 years).
Subgroup analyses were done as exploratory analyses, including treatment-by-factor interactions in the model.
Non-compliance with allocated treatment
Non-compliance with allocated treatment was explored by producing descriptive tables of treatment received versus treatment allocated. Per-protocol analyses were undertaken to estimate treatment effects for those women who followed the protocol and received the same treatment as allocated.
Missing data
The primary analysis was undertaken using the observed outcome data for the women. If women did not have the outcome data they were excluded from the primary analysis; however, mechanisms of missingness were explored by presenting descriptive tables of baseline characteristics by missing primary outcome status. Multiple imputation was carried out for the primary outcome as a sensitivity analysis.
Missing outcome data
Although no imputation of missing participant-level outcome data was carried out in the main analysis of the primary outcome, imputation of instruments was undertaken at item level according to the rules of the specific instrument.
All randomised women were included in the analysis. Participants deemed ineligible after randomisation were considered as randomisation exclusions and excluded from the analysis.
Missing outcome baseline data
Centre mean imputation of missing baseline data for continuous variables was undertaken in order to reduce bias. For categorical variables, an additional category for the missing data was created.
Health economic evaluation
A within-trial economic evaluation assessing the costs, QoL and cost-effectiveness of the interventions according to a NHS and patient perspective was undertaken for both the Uterine and the Vault trials. All analyses were completed following the intention-to-treat principle. Trial data were used to populate a Markov decision-analytic model, developed to extrapolate the 12-month data from the Uterine trial over a longer time horizon (see Chapter 9). Methods are applicable to both the Uterine (see Chapter 5) and the Vault (see Chapter 8) trials, that is, within-trial economic evaluations unless otherwise stated.
Health services perspective costs
The NHS resource-use data were collected using CRFs completed by the recruiting officers/research nurses or gynaecologists and patient-completed questionnaires administered at baseline, at 6 months after surgery and at 12 months after randomisation (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019). The following categories of resource usage and costs were collected from a NHS perspective:
-
intervention delivery costs [based on a component costing approach for the base case and using Healthcare Resource Group (HRG) tariffs as a sensitivity analysis]
-
inpatient costs (cost of re-admissions for repeat prolapse procedures, incontinence procedures and treatment of AEs)
-
costs of consultations with health-care professionals [outpatient consultations and primary care consultations, such as general practitioners (GPs), nurses, physiotherapists, etc.]
-
medications and treatments relating to prolapse symptoms (e.g. oestrogens, pessaries, antibiotics).
Intervention costs
Two approaches to intervention costing were considered. The base-case analysis used a component costing approach. Operative details were recorded at the time of surgery using CRFs completed at each site by the recruiting officer/research nurse or gynaecologist (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019). Resource use data collected included patient time in theatre, grade of operating gynaecologist, grade of anaesthetist, other nursing staff, type of anaesthetic used and the use of prophylactic antibiotics. Details of concomitant surgery and catheterisation were also collected. Intervention resources included the type of mesh for the apical procedures and for the concomitant procedures. Detailed information regarding initial hospitalisation length of stay was available and was used to assign appropriate hospitalisation costs to each group, based on per-night costs for a gynaecology ward. The component costing approach focused on the costs that were predicted to differ across the randomised groups. Microcosting information was not collected on items of resource use that were not expected to differ across groups.
Secondary care resource use over follow-up
Inpatient resource use included further prolapse surgery, further urinary incontinence surgery, further hospitalisations for complications and any other hospital resource use deemed relevant to the VUE trial. Data on occurrence of a hospitalisation were primarily collected from participant-administered questionnaires at 6 months after surgery and at 12 months after randomisation, and supplemented by asking women about hospitalisations at their final clinic visit (12 months after surgery). The joint approach ensured the greatest possible level of detail for analysis. All reported hospitalisations were then further investigated in a post-coding exercise to (1) determine that they were related to the VUE trial, (2) obtain further details on the category of procedure (e.g. further prolapse procedures: anterior, posterior, apical, combination) and (3) ensure that procedures were not double counted. The post-coding exercise involved investigation of site notes to verify the participant-reported data. The number of attendances for outpatient visits at gynaecology clinics were collected from the patient-administered questionnaires.
Other consultations over follow-up
Participants reported contacts with primary care health professionals for prolapse-related symptoms in the 6- and 12-month questionnaires (after surgery and after randomisation, respectively). This included visits to the GP, practice nurse, district nurse, physiotherapist (at a GP practice), physiotherapist (at a hospital clinic) and any other related consultations with health professionals. When collating the data on primary care resource usage, the following assumptions were made to ensure the best use of available data:
-
when women answered ‘no’ to seeing a health professional, resource use was assumed as zero visits
-
when women returned a questionnaire and left all items of resource use blank, data were assumed to be missing
-
when women returned a questionnaire and answered one or more categories as ‘yes’, leaving the remaining blank, it was assumed that unticked boxes related to zero resource use.
Medications and treatments
Data on treatments and medications prescribed to women (i.e. oestrogens, bladder medications, catheters, pessaries and antibiotics) for prolapse-related symptoms were collected using participant questionnaires.
Total costs to the NHS
Resource use data were multiplied by the relevant national average unit cost. Health services costs were summed across categories to generate a total cost to the NHS of the intervention and the follow-up care for each trial participant. Unit cost sources were the British National Formulary (BNF)43 and the NHS Business Services Authority’s online drug tariff catalogue for medication resource use, Information Services Division (ISD) Scotland was used for intervention (ward-specific) length of stay,44 NHS Reference Costs 2015–1645 was used to provide information for secondary care procedures and visits and the Personal and Social Services Research Unit (PSSRU) provided unit costs of health and social care for other consultations with health professionals. 46 All costs were reported in 2016–17 GBP. No discounting was required because of the single-year time horizon. Detailed information on unit costs applied to each resource-use item, including any assumptions that were made are provided in Table 1.
| Resource-use itema | Unit | Cost per unit (£) | Comments | Source |
|---|---|---|---|---|
| Operation resource use | ||||
| Synthetic mesh | Per mesh | 112.97 | Average list price of synthetic mesh materials used in the PROSPECT | Glazener et al.1 |
| Biological mesh | Per mesh | 310.41 | Average list price of biological graft materials used in the PROSPECT | Glazener et al.1 |
| Mesh kits | Per mesh kit | 646.45 | Average list price of mesh kits used in the PROSPECT | Glazener et al.1 |
| Gynaecologist/anaesthetist time (consultant) | Per hour | 137.00 | If surgery was supervised, assume supervision provided by a consultant grade. Cost per working hour, includes qualification costs | Curtis and Burns46 |
| Gynaecologist/anaesthetist time (specialty doctor)b | Per hour | 71.00 | Assume registrar. Cost per working hour, includes qualification costs | Curtis and Burns46 |
| Band 5 theatre nurse | Per hour | 42.15 | Including qualification costs, cost working hour. Assume three band 5 nurses present for all procedures | Curtis and Burns;46 and Glazener et al.1 |
| Band 4 theatre nurse | Per hour | 34.98 | Including qualification costs, cost per working hour. Assume one band 4 nurse present for duration of all procedures. | Curtis and Burns;46 and Glazener et al.1 |
| General anaesthesia | Per case | 20.76 | Based on calculation (see Appendix 1, Table 41) and personal communication (Dr Christine Hemming, Aberdeen Royal Infirmary, 2017) | BNF;43 and personal communication (Dr Christine Hemming, Aberdeen Royal Infirmary, 2017) |
| Spinal anaesthesia | Per case | 2.25 | Based on calculation (see Appendix 1, Table 41) | BNF;43 and personal communication (Dr Christine Hemming, Aberdeen Royal Infirmary, 2017) |
| Local anaesthesia | Per case | 0.40 | Based on calculation (see Appendix 1, Table 41) | BNF;43 and personal communication (Dr Christine Hemming, Aberdeen Royal Infirmary, 2017) |
| Surgical antibiotics | Per case | 1.06 | Assume augmentin | BNF;43 and Glazener et al.1 |
| Theatre overheads | Per hour | 420.19 | Currently excludes consumables | ISD Scotland,47,48 |
| Cost of catheterisation | Per catheter | 6.37 | Assume Folysil® (Coloplast Ltd, Peterborough, UK) all-silicone catheters (female); EDT, April 2015. Assume no additional procedure time required if catheterised during surgery | EDT49 |
| Vaginal pack | Per pack | 4.67 | Sorbsan packing 30 cm/2 g: £3.47 + Hibitane™ (Derma UK Ltd, Stotfold, UK) obstetric cream (£1.20) | EDT49 |
| Other treatments during admission for intervention | ||||
| Return to theatre | Per case | 923.00 | No data available on time in theatre for returns; conservatively assume duration was 1 hour | Direct cost, ISD Scotland50 |
| Laxatives | Per pack of tablets | 1.96 | 5 mg of bisacodyl | BNF43 |
| Pain relief | Infusion | 5.00 | 50 mg of fentanyl | BNF43 |
| Pain relief | Tablets | 0.84 | Tramadol | BNF43 |
| Length of stay (gynaecology ward) | Per day | 179.00 | Payment by results tariff of £1433 spread over 8 days, so £179 per day | Glazener et al.1 |
| Consultations with secondary and primary health-care professionals/procedures for subsequent treatment or consultations | ||||
| New apical procedure | Per procedure | 4162.00 | Average of appropriate HRG codes for surgery for uterine and vault prolapse. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New anterior procedure | Per procedure | 2693.00 | Average of appropriate HRG codes for surgery for anterior prolapse. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New posterior procedure | Per procedure | 2231.00 | Average of appropriate HRG codes for surgery for posterior prolapse. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New anterior and posterior combined procedure | Per procedure | 3204.00 | HRG code for surgery for anterior and posterior prolapse. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New anterior and apical procedure | Per procedure | 5261.00 | Average of appropriate HRG codes for surgery for complex and major genital tract procedures. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New posterior and apical procedure | Per procedure | 5261.00 | Average of appropriate HRG codes for surgery complex and major genital tract procedures. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New anterior and posterior and apical procedure | Per procedure | 6165.00 | HRG code for very major genital tract procedures with additional comorbidities and complications to reflect the more complex procedure. See Appendix 1, Table 42 for further details | NHS Reference Costs 2015–16 45 |
| New sling incontinence procedure | Per procedure | 2096.00 | Average of elective case procedures for HRG code LB51 | NHS Reference Costs 2015–16 45 |
| Outpatient consultation | Per consultation | 131.00 | Average of gynaecology consultant and non-consultant, non-admitted face-to-face attendance | NHS Reference Costs 2015–16 45 |
| GP visit | Per visit | 36.00 | Per 9.22-minute consultation, including qualification costs | Curtis and Burns46 |
| Practice nurse | Per visit | 14.47 | Unit cost of £56 per hour of patient contact (with qualification costs); 15.5-minute consultation | Curtis and Burns46 |
| District nurse | Per visit | 38.00 | Average cost of a face-to-face contact | Curtis and Burns46 |
| Physiotherapist (community and hospital) | Per visit | 34.00 | Band 5 physiotherapist, cost per working hour (assume visit duration of 1 hour for community physiotherapist) | Curtis and Burns46 |
| Other treatments: | ||||
| Permanent/indwelling catheter | Per woman per year | 1589.00 | Based on a number of assumptions. See Appendix 1, Table 43 for calculation details | EDT49 |
| Disposable/intermittent catheter | Per woman: yearly cost | 333.84 | Based on a number of assumptions, see Appendix 1, Table 44 for more details | NHS EDT;49 and NHS Warrington Trust documentation for guidance of care51 |
| Antibiotics | Per day | 0.28 | Average costs of antibiotic drugs reported by participants. See Appendix 1, Table 45 for calculation | BNF43 |
| Oestrogen treatment | Per week | 16.72 | Vagifem® (Novo Nordisk, Bagsværd, Denmark) vaginal tablets, 10-µg estradiol vaginal pessary in disposable applicators | BNF43 |
| Ring pessary | Per pessary | 20.09 | Average across EDT products. See Appendix 1, Table 46 for calculation | EDT49 |
| Shelf pessary | Per pessary | 21.54 | Average across EDT products. See Appendix 1, Table 46 for calculation | EDT49 |
| Drug treatment for bladder problems | Per 56 tablet pack | 1.82 | Assume tolterodine tartrate, generic version to cover frequency and urgency symptoms, 2 mg twice daily dose | BNF43 |
| Participant perspective costs | ||||
| Inpatient visit | Per night | 81.70 | Time and travel costs for attendance to an inpatient procedure (including index procedure) | Glazener et al.1 |
| Outpatient visit | Per attendance | 50.82 | Time and travel costs for attendance to an outpatient department | Glazener et al.1 |
| Primary care visit | Per attendance | 13.75 | Time and travel costs for a GP appointment | Glazener et al.1 |
| Time off work | Per day | 107.72 | Assuming the average earnings/week (£538.60) and a working week of 39.1 hours and a 5-day working week | ONS52 |
| Other participant expenses | Per participant | Various | As reported directly by the trial participants | |
Participant perspective costs
This wider cost perspective identified the effect of any shifts in the balance of care between the NHS and the patients and their families. Participant perspective costs included out-of-pocket expenditure on over-the-counter medications/products and private care to treat prolapse-related symptoms. The opportunity cost to women (and companions) of attendance at primary and secondary care consultations was also included, as was the cost of time off work (for those in paid employment).
To avoid additional patient burden through completion of lengthy questionnaires, it was assumed that the unit cost of time and travel to health-care professionals was similar to PROSPECT. 1 PROSPECT data for all procedures (primary, secondary and cohort) were used to calculate unit costs of time and travel for inpatient, outpatient and primary care visits (see Table 1). These unit costs were then multiplied by the number of appropriate visits observed in the VUE trial to obtain a cost of time and travel tailored to the VUE trial participants. For time off work, the appropriate national average price of 1 hour of work is used (see Table 1). For the remaining aspects of personal incurred costs (i.e. private medical costs, over-the-counter medications, other expenses), data are sourced directly from the participant questionnaires and multiplied by the relevant participant-reported expense.
A wider cost perspective was obtained by summing together the total NHS and participant perspective costs and is considered as a sensitivity analysis.
Outcome measurement, quality of life and quality-adjusted life-years
The EQ-5D-3L generic QoL instrument was completed by participants at baseline, at 6 months after surgery and at 12 months after randomisation. 53 The EQ-5D-3L instrument divides health status into five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each of these dimensions have three levels, so 243 possible health states exist. EQ-5D-3L responses were presented in graphical format illustrating the percentage of respondents with any problems on each domain, split by randomised arms of the trial.
Responses to the EQ-5D-3L questionnaire were translated into utility values using the UK general population tariffs, based on the time trade-off method, generating utility on a scale of worst possible health state (–0.564) to best possible health state (1). 35 Respondents who died during the trial were assigned a utility of 0 at each time point following death.
The QALYs were then derived by multiplying the utility score at each time point by the length of time in that health state. An area under the curve approach was used, assuming a linear extrapolation between utility measurement time points.
Statistical analysis of cost and quality-adjusted life-year data
All components of costs were described with the appropriate descriptive statistics when relevant: mean and SD for continuous and count outcomes, and numbers and percentages for dichotomous and categorical outcomes (e.g. numbers reporting problems on EQ-5D-3L). All analyses were conducted using Stata version 14.1® (StataCorp LP, College Station, TX, USA) software. Cost data were analysed using GLMs, with adjustment for minimisation covariates specific to each trial and baseline measure when appropriate (e.g. adjustment of QALYs and costs for baseline utility score). 54 All models include a cluster effect for surgeon. GLMs allow correction for the potential for skewed cost data (i.e. a small proportion of participants incurring very high costs) by allowing specification of an appropriate distributional family and link function to best fit the data. Different distributional families offer alternative specifications to reflect the relationship between the mean and variance of the estimates under consideration. 55,56 A modified Park test was used to select the most appropriate distributional family for each analysis. Then, a combination of tests was run on the preferred family to identify the most appropriate link function (Pearson correlation test, Pregibon link test and a modified Hosmer and Lemeshow test). The preferred link function was chosen as the one that performed best on the greatest number of the three tests, confirmed by examination of the respective p-values of the tests. The process follows that outlined by Glick et al. 55 and was implemented in Stata 14.1 using the user written ‘glmdiag’ command. The coefficient on treatment in the respective analysis models represents the incremental treatment effect on costs and/or QALYs. When different link functions are used, marginal effects are estimated on the cost and QALY scale, respectively and CIs calculated using the delta method. 55,56 The base-case cost data area analysed using a gamma family with a log-link and the base-case QALY data were analysed with a Poisson family and a power equal to 0.75 link function.
Incremental cost-effectiveness analysis
Cost-effectiveness calculations and interpretation
The primary outcome for the within-trial economic evaluations is incremental cost per QALY gained over 12 months. The mean cost (ΔC) and mean QALY (ΔQ) differences between the randomised groups (see Statistical analysis of cost and quality-adjusted life-year data) were compared in order to obtain an estimate of the cost of achieving one additional QALY by implementing the new intervention compared with the control (e.g. the additional cost of uterine preservation vs. vaginal hysterectomy). This ratio between incremental costs and QALYs (i.e. ΔC/ΔQ) gives the incremental cost-effectiveness ratio (ICER) for each comparison. Estimates of the ICER are then compared with the recommended willingness-to-pay decision-making threshold in the UK, which is currently between £20,000 and £30,000 per QALY gained. 57 This means that any intervention generating additional QALYs for a cost of < £20,000 would usually be considered cost-effective. Conversely, using a similar decision rule, should an intervention deliver fewer QALYs than the comparator, then society would need to be compensated to a value of > £20,000 to justify a QALY loss. Interventions that deliver additional QALYs for lower costs (i.e. cost savings to the NHS) have a negative ICER and are said to be dominant over the comparator.
Missing data
Missing data can pose significant problems for data analysis, especially surrounding data reported using participant-administered questionnaires. A decision rule on imputation was taken as follows. If > 10% of complete-case costs or complete-case QALYs were missing, or if > 15% of complete cost and QALY pairs were missing, then imputation was considered for the base-case analysis. The analysis meets the prespecified criteria for imputation and so the base-case analysis was based on multiple imputation of missing data. 58 Data were imputed using the iterative chained equations approach within Stata 14.1’s ‘mi impute’ procedure. Missing EQ-5D-3L data were imputed using predictive mean matching (the mean of five nearest values) to account for multiple time points per respondent (i.e. at baseline, at 6 months after surgery and at 12 months after randomisation). Missing cost data were imputed at the category level (operation cost, hospitalisation cost, consultation cost and other treatment cost) using a multivariate regression approach. All imputation models were adjusted for minimisation variables specific to each trial and baseline EuroQol 5 Dimensions (EQ-5D)-derived utility score. Imputations were completed separately for the Uterine and Vault trials. Ten imputations were considered sufficient to generate stable and reliable estimates for analysis. Imputed data were then analysed using the appropriately specified GLMs described.
Assessment of uncertainty
Sampling uncertainty
Two types of uncertainty were considered for the analyses. First, the impact of sampling uncertainty on results by using non-parametric bootstrap loops of the imputed regression models to generate a probability of cost-effectiveness at commonly accepted threshold values of decision makers’ willingness to pay (WTP) for a QALY gained (£0, £10,000, £20,000, £30,000 and £50,000) was considered. In all cases, 1000 repetitions of the model are estimated, and recycled predictions are used to retrieve the mean estimates of incremental costs and QALYs. 59 The bootstrap replications of the models were further used to illustrate sampling uncertainty as follows. All 1000 replications of the bootstrapped estimates of the differences in costs and QALYs were plotted on the cost-effectiveness plane. This allows for a visual representation of the joint uncertainty in the effect sizes for cost and QALY estimates. The quadrant of the cost-effectiveness plane in which the majority of bootstrapped replications (dots) lies allows for a visualisation of the probability that the new intervention is (a) less costly and more effective (dominant, south-east quadrant) versus the comparator; (b) more costly and less effective (dominated, north-west quadrant) versus the comparator; (c) less costly and less effective (south-west quadrant); or (d) more costly and more effective (north-east quadrant). To further illustrate sampling uncertainty, cost-effectiveness acceptability curves (CEACS) were produced. CEACS and scatterplots are used to represent the probability that different interventions are cost-effective at various threshold values for society’s WTP for an additional QALY. CEACs present results when the analysis follows a net benefit approach, in which the net monetary benefit (NMB) of an intervention is calculated as a straight forward re-arrangement of the cost-effectiveness decision rule used when calculating ICERs such that:
where λ gives the threshold value of WTP for a QALY. If the above expression holds true, the intervention is considered cost-effective. The NMB is calculated for a range of plausible threshold values of λ. The resultant CEACs illustrate the probability of cost-effectiveness at different λ values.
Sensitivity analyses
A range of deterministic sensitivity analyses were conducted to assess the impact of important choices surrounding assumptions and analysis models on the cost-effectiveness findings. All sensitivity analyses were conducted using data sets with multiple imputation of missing cost and QALY data, and each analysis is subjected to the same assessment of sampling uncertainty with the production of scatterplots and CEACs for each sensitivity analysis undertaken. The following sensitivity analyses were explored.
Intervention costing approach
The base-case analysis uses component-based intervention costing using a high level of detail around resource use and staff time obtained for each trial participant. However, the use of appropriate HRG tariffs mapped to procedures in the trial was considered as a sensitivity analysis. HRG tariffs more closely reflect the current best estimate of the NHS costs of different procedures. However, the tariffs do not possess the intricate level of detail available from a trial study and, as such, may not fully consider the opportunity costs of resource use, such as consultant time, time in hospital, etc. As such, HRG tariffs may not be sufficiently sensitive to capture the different resource use for each procedure. Nonetheless, it is important to understand any potential discrepancies in intervention costing depending on the approach taken.
Choice of analysis model for costs and outcomes
The base-case analysis uses GLMs with family and link functions. To explore uncertainty in the choice of model on estimates of cost-effectiveness, an alternative seemingly unrelated regression approach (i.e. the Sureg approach) was used. 60 The Sureg approach allows an alternative approach to estimate cost-effectiveness while accounting for the underlying correlation structure between costs and QALYs.
Use of complete-case (rather than multiply imputed) analysis
The base-case analysis uses multiple imputation of missing data. Sensitivity analyses explored the impact of rerunning the analyses using complete cost and QALY pairs. Two analyses were completed on the complete-case data, first using the GLMs specified in the base case and, second, using seemingly unrelated regression as an alternative approach to account for the correlation between costs and QALYs. 60
Quality-adjusted life-year calculation approach
Participants received 6-month questionnaires triggered by the date of surgery and 12-month questionnaires triggered by the date of randomisation. The differing approach was required to ensure that appropriate clinical outcome measures that were relevant to the trial intervention were collected. This approach meant potential existed for women to receive their 12-month follow-up questionnaire prior to their 6 month and could have implications for QALY estimates, which are based on an area under the curve approach across time points. The base-case analysis uses data as reported in the questionnaires and makes no further adjustment. Sensitivity analysis explores three different configurations. First, the impact of dropping any QALY data where the 12-month questionnaire was completed before the 6-month questionnaire was explored. Second, any data when the difference between the 6- and 12-month questionnaires was < 3 months was dropped. Finally, an analysis in which the exact date of the questionnaire was used as the time point for QALY calculation was explored.
Chapter 3 Baseline characteristics: the Uterine trial
Between January 2013 and January 2017, 1544 women were identified as potential participants in the Uterine trial of the VUE trial.
This chapter describes how participating women were identified from the women considered for uterine prolapse surgery in 45 UK hospitals (see Appendix 2, Table 47) and reports the baseline characteristics up to the point of entry to the Uterine trial. The subsequent findings are described in Chapters 4 and 5.
Study recruitment
The trial outline and methodology for recruitment to the VUE trial have been described previously31 (see Chapter 2). Women attending gynaecology outpatient clinics who chose to have surgery for symptomatic uterine POP and women on the waiting list for uterine prolapse surgery were invited to participate in the Uterine trial. Women were asked if they were willing to be randomised to either a uterine preservation or a vaginal hysterectomy for their uterine prolapse. The centres and surgeons who participated in the VUE trial, the numbers recruited, and the rate of recruitment are detailed in Appendix 2 (Table 47 and Figure 19).
Non-recruited women
Of the 1544 women approached to participate in the Uterine trial, 979 did not enter the study because they declined (n = 774, 50.1%), were ineligible (n = 177, 11.5%) or not timely identified or seen (n = 28, 1.8%; see Appendix 2, Tables 48 and 49 for more details). Women’s preference (n = 431, 55.7%) for a particular surgery [most commonly the preference was for a vaginal hysterectomy (n = 324, 41.9%; see Appendix 2, Table 49)], was the most common reason for declining. Ineligible reasons included ‘a specific operation is necessary’ (n = 77, 43.5%), ‘unsuitable due to medical history’ (n = 28, 15.8%) and ‘not suitable for surgery’ (n = 13, 7.3%). Other reasons included not wanting to be randomised (n = 149, 19.3%), deciding against surgery (n = 219, 28.3%) and deciding to try a pessary (n = 34, 4.4%).
Recruited women: baseline characteristics
The baseline characteristics of the 563 women who agreed to participate in the Uterine trial and were truly eligible are described in Table 2 (see also Appendix 2, Table 50 and Figure 3).
| Characteristic | Treatment | |
|---|---|---|
| Uterine preservation | Vaginal hysterectomy | |
| Age (years), mean (SD); n | 63.4 (10.5); 280 | 63.9 (9.9); 283 |
| BMI (kg/m2), mean (SD); n | 27.7 (4.1); 233 | 27.1 (4.1); 239 |
| BMI category, n (%) | ||
| Normal weight | 60 (21.4) | 72 (25.4) |
| Overweight | 104 (37.1) | 100 (35.3) |
| Obese | 59 (21.1) | 59 (20.8) |
| Morbidly obese | 10 (3.6) | 8 (2.8) |
| Missing | 47 (16.8) | 44 (15.5) |
| Parity, median (P25–75),a n | 2 (2–3); 280 | 2 (2–3); 283 |
| Number of normal vaginal deliveries, mean (SD); n | 2.3 (1.3); 271 | 2.3 (1.1); 277 |
| Previous conservative treatment, n (%) | ||
| Vaginal pessary | 81 (28.9) | 86 (30.4) |
| Physiotherapy for prolapse | 73 (26.1) | 73 (25.8) |
| Physiotherapy for urinary incontinence | 27 (9.6) | 32 (11.3) |
| Drugs for UI | 20 (7.1) | 21 (7.4) |
| Previous surgery, n (%) | ||
| Previous anterior repair | 6 (2.1) | 12 (4.2) |
| Previous posterior repair | 8 (2.9) | 9 (3.2) |
| Vaginal repair, but compartment unknown | 2 (0.7) | 10 (3.5) |
| Previous incontinence | 5 (1.8) | 12 (4.2) |
Epidemiological characteristics
There was no difference between the randomised groups in respect of age, body mass index (BMI), parity or delivery mode history (with the majority of women having a normal vaginal delivery; see also Appendix 2, Table 50).
Previous treatment for prolapse
Overall, around one-third of women had undergone PFMT with the same proportion of women having used a vaginal pessary (ring or other type) before surgery. Fewer than 1 in 10 women had undergone previous surgery for prolapse (Table 2).
Prolapse symptoms at baseline
There were no differences in prolapse symptoms at baseline. Overall, women in the Uterine trial had been symptomatic for around 4 years, and had been bothered by their symptoms for just over 2 years (Table 3). The mean POP-SS before surgery was 13.6 out of a maximum score of 28 (Table 3) and the score ranged from 0 to 28. Using a POP-SS of > 0 to indicate presence of symptoms, around 94% of women had at least one symptom. The prolapse-related effect on QoL score (‘overall, how much do your prolapse symptoms interfere with your everyday life?’) ranged from 0 to 10 out of 10, with a mean value of 6.7 out of 10 (Table 3).
| Symptom | Treatment | |
|---|---|---|
| Uterine preservation | Vaginal hysterectomy | |
| Duration of symptoms (years), mean (SD); n | 4.1 (5.7); 264 | 4.0 (6.3); 256 |
| Duration of bother (years), mean (SD); n | 2.2 (2.8); 257 | 2.2 (2.9); 249 |
| POP-SS at baseline, mean (SD); n | 13.7 (6.4); 268 | 13.5 (5.9); 265 |
| Number of women symptomatic, n (%) | 266 (95.0) | 264 (93.3) |
| Prolapse-related effect on QoL score, mean (SD); n | 6.8 (2.7); 273 | 6.5 (2.9); 270 |
| Individual prolapse symptoms, n (%) | ||
| Something coming down (any) | 258 (92.1) | 258 (91.2) |
| Something coming down (most/all of the time) | 201 (71.8) | 204 (72.1) |
| Bladder not empty (any) | 238 (85.0) | 225 (79.5) |
| Bladder not empty (most/all of the time) | 114 (40.7) | 102 (36.0) |
| Uncomfortable feeling or pain when standing (any) | 217 (77.5) | 214 (75.6) |
| Uncomfortable feeling or pain when standing (most/all of the time) | 102 (36.4) | 109 (38.5) |
| Dragging in abdomen (any) | 213 (76.1) | 210 (74.2) |
| Dragging in abdomen (most/all of the time) | 92 (32.9) | 97 (34.3) |
| Strain to empty bladder (any) | 203 (72.5) | 187 (66.1) |
| Strain to empty bladder (most/all of the time) | 94 (33.6) | 74 (26.1) |
| Bowel not empty (any) | 199 (71.1) | 198 (70.0) |
| Bowel not empty (most/all of the time) | 67 (23.9) | 54 (19.1) |
| Dragging in back (any) | 171 (61.1) | 175 (61.8) |
| Dragging in back (most/all of the time) | 63 (22.5) | 61 (21.6) |
| Most bothersome symptom, n (%) | ||
| Something coming down | 133 (47.5) | 147 (51.9) |
| Bladder not empty | 36 (12.9) | 22 (7.8) |
| Uncomfortable feeling or pain when standing | 23 (8.2) | 27 (9.5) |
| Strain to empty bladder | 18 (6.4) | 10 (3.5) |
| Bowel not empty | 18 (6.4) | 16 (5.7) |
| Dragging in abdomen | 17 (6.1) | 13 (4.6) |
| Dragging in back | 9 (3.2) | 15 (5.3) |
| Symptom causing most bother not applicable | 12 (4.3) | 11 (3.9) |
| Missing | 26 (9.3) | 33 (11.7) |
| Actions necessitated by prolapse symptoms, n (%) | ||
| Extra hygiene measures | 88 (31.4) | 98 (34.6) |
| Fingers to ease discomfort | 46 (16.4) | 54 (19.1) |
| Digitally evacuate bowel | 10 (3.6) | 13 (4.6) |
| Fingers to help empty bladder | 4 (1.4) | 6 (2.1) |
| Fingers to help empty bowel | 2 (0.7) | 5 (1.8) |
| EQ-5D, mean score (SD); n | 0.728 (0.232); 270 | 0.775 (0.187); 266 |
| EQ-5D visual scale, mean score (SD); n | 74.2 (18.6); 266 | 76.2 (17.7); 267 |
The most common individual prolapse symptom was ‘a feeling of something coming down from or in your vagina’ reported in 91.7% of women. This was reported ‘most or all of the time’ in 72.0% of women and was the most bothersome symptom in around half (49.7%) of the women.
Around one-third (33.0%) of women found that the prolapse caused hygiene problems, and almost one in five (17.8%) needed to relieve pressure or discomfort from the prolapse using their fingers (Table 3).
Generic quality of life
The mean QoL score and visual scales (EQ-5D-3L) were 0.752 and 75.1 points, respectively.
Preoperative objective prolapse measurements
The leading edge of the most descended compartment relative to the hymen was used for the overall POP-Q stage. The majority (94.1%) of women had an overall objective prolapse and 53.5% had a uterine prolapse beyond the hymenal ring [stages 2b or more (Table 4; see also Appendix 2, Table 51)]. Around half (49.4%) of the women had an overall stage 3 prolapse of any compartment (see Appendix 2, Table 51), with 43.2% of women specifically having a stage 2 uterine prolapse.
| Stage | Treatment, n (%) | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Overall stage 2b or more | 261 (93.2) | 269 (95.1) |
| Apical stage | ||
| 0 | 1 (0.4) | 1 (0.4) |
| 1 | 89 (31.8) | 68 (24.0) |
| 2a | 43 (15.4) | 46 (16.3) |
| 2b | 76 (27.1) | 78 (27.6) |
| 3 | 57 (20.4) | 71 (25.1) |
| 4 | 4 (1.4) | 15 (5.3) |
| Missing | 10 (3.6) | 4 (1.4) |
| Stage 2b or more | 137 (48.9) | 164 (58.0) |
| Anterior prolapse stage 2b or more | 249 (88.9) | 265 (93.6) |
| Posterior prolapse stage 2b or more | 76 (27.1) | 91 (32.2) |
Planned concomitant surgery
Planned surgery was based on preoperative findings on clinical examination and women remained in the group to which they were allocated, irrespective of the actual procedure performed (Table 5). In order to take into account minimisation criteria, centres were asked to specify in advance which concomitant surgery was also thought to be necessary.
| Planned concomitant surgery | Treatment, n (%) | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| None | 40 (14.3) | 40 (14.1) |
| Anterior repair | 114 (40.7) | 135 (47.7) |
| Anterior and posterior repair (both) | 114 (40.7) | 99 (35.0) |
| Posterior repair | 11 (3.9) | 8 (2.8) |
| Enterocele repair | 13 (4.6) | 10 (3.5) |
| Concomitant continence surgery | 11 (3.9) | 10 (3.5) |
All women were expected to undergo a uterine prolapse procedure. Most women (85.5%) were expected to undergo a concomitant prolapse repair. Planned procedures were equally distributed between the two randomised groups.
Vaginal and sexual symptoms at baseline
Vaginal and sexual symptoms were measured using the ICI-validated instruments36 (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019). These symptoms were common and had effects on QoL. The majority of women were not sexually active (70.0%) and in around one in five of the women this was most often because of their prolapse symptoms. Among the women who were sexually active, or whose reason for no sex life was not ‘due to prolapse symptoms,’ a few (n = 5) had dyspareunia (pain with intercourse) at baseline (Table 6).
| Vaginal/sexual symptom | Treatment | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| ICI Vaginal Symptoms Score, mean (SD); n | 21.6 (9.9); 249 | 21.7 (8.4); 244 |
| QoL due to vaginal symptoms, mean (SD); n | 5.2 (3.4); 264 | 4.9 (3.4); 265 |
| ICI Sexual Matters Score,a mean (SD); n | 28.4 (18.1); 89 | 24.1 (16.4); 79 |
| QoL due to effect on sex life, mean (SD); n | 5.4 (3.1); 89 | 5.0 (3.2); 79 |
| Vagina too loose or lax, n (%) | 41 (14.6) | 31 (11.0) |
| Reduced sensation, n (%) | 17 (6.1) | 18 (6.4) |
| Number having intercourse, n (%) | 89 (31.8) | 80 (28.3) |
| Pain with intercourse,a n (%) | 4 (4.5) | 1 (1.3) |
| Missing, n (%) | 0 (0) | 1 (1.3) |
| Reasons for not being sexually active, n (%) | ||
| Prolapse symptoms | 59 (21.1) | 63 (22.3) |
| No partner | 51 (18.2) | 56 (19.8) |
| Vaginal symptoms | 12 (4.3) | 13 (4.6) |
| Other reasons | 45 (16.1) | 47 (16.6) |
| Reason not given | 113 (40.4) | 104 (36.7) |
Urinary and bowel symptoms at baseline
Urinary symptoms were relatively common and affected QoL. Women were counted as symptomatic if they had the symptom ‘most or all of the time’. Nocturia and urgency were common types of urinary symptom. Overall, around one in five (22.6%) women reported at least some urinary incontinence (using the ICIQ-UI SF)36 and this was slight or moderate in the majority (68.9%) of cases (see Appendix 2, Table 52). The mean QoL because of urinary symptoms was 3.9 out of 10 (Table 7).
| Symptom | Treatment | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Urinary symptoms, n (%) | ||
| Urgency | 72 (25.7) | 42 (14.8) |
| Nocturia | 71 (25.4) | 52 (18.4) |
| Frequency | 29 (10.4) | 34 (12.0) |
| All the above | 9 (3.2) | 5 (1.8) |
| Any incontinence, n (%) | 66 (23.6) | 61 (21.6) |
| Severe | 20 (7.3) | 12 (4.2) |
| Missing | 9 (3.2) | 17 (6.0) |
| Incontinence-related QoL score, mean (SD); n | 3.9 (3.3); 231 | 3.9 (3.3); 231 |
| ICI Urinary Incontinence Score, mean (SD); n | 8.0 (5.2); 223 | 7.7 (5.2); 226 |
| ICIQ-FLUTS Filling Score, mean (SD); n | 5.4 (3.0); 269 | 4.8 (2.6); 271 |
| ICIQ-FLUTS Voiding Symptoms Score, mean (SD); n | 3.4 (2.6); 272 | 3.3 (2.6); 271 |
| ICIQ-FLUTS Incontinence Score, mean (SD); n | 5.6 (4.1); 233 | 5.4 (3.9); 232 |
| Bowel symptoms | ||
| Bowel frequency, n (%) | ||
| Normal | 252 (90.0) | 255 (90.1) |
| Frequent | 11 (3.9) | 6 (2.1) |
| Infrequent | 8 (2.9) | 9 (3.2) |
| Missing | 9 (3.2) | 13 (4.6) |
| Faecal incontinence (occasionally or more often), n (%) | 93 (33.2) | 77 (27.2) |
| Passive | 77 (27.5) | 64 (22.6) |
| Active | 16 (5.7) | 13 (4.6) |
| Severe | 24 (8.6) | 25 (8.8) |
| Bowel urgency (most or all of the time), n (%) | 19 (6.8) | 14 (4.9) |
| Constipation (most or all of the time), n (%) | 17 (6.1) | 18 (6.4) |
| Bowel symptoms QoL Score, mean (SD); n | 2.8 (3.1); 269 | 2.6 (2.9); 266 |
Few women reported bowel symptoms, and these did not appear to have a large impact on QoL with the mean 2.7 out of 10 (Table 7). Around one-third (30.2%) of all women reported faecal incontinence at least occasionally [defined as loss of solid or liquid stool, but not including loss of flatus (wind)] and this was severe in < 9% of women. Few women (6.2%) reported constipation.
Discussion
Summary of findings
The participating women in the Uterine trial were around 64 years old, with a median of two previous vaginal deliveries (see Table 2). Their mean BMI was around 27 kg/m2 (although 18 women with morbid obesity were included). This was the first prolapse repair surgery in any compartment for the majority of the women.
Prolapse symptoms and measurements
Women had a mean POP-SS of 13.6 out of a maximum score of 28. The most common symptom was a feeling of ‘something coming down’ (reported in 91.7%), which was the most bothersome symptom in almost half (49.2%) of the women (see Table 3).
Objective outcome measures
When prolapse was redefined as the leading edge beyond the hymen (stage 2b or more), 94.2% of women had a protruding prolapse overall and 53.5% had a protruding uterine prolapse (see Table 4).
The majority of women (85.8%) were also expected to undergo a concomitant prolapse repair in another compartment (anterior, posterior or both) at the same time. This was most likely to be an anterior (44.2%) or both an anterior and a posterior repair (37.9%) (see Table 5).
The study was able to ascribe a prolapse stage to 94% of women at baseline (5% had no POP-Q measure). Results data were adjusted for the 10% difference in apical POP-Q staging at baseline. Just over one-quarter (28.2%) of women appeared to have no significant objective uterine prolapse (i.e. stage 0 or 1). Similarly to PROSPECT,1 it is proposed that this could be a result of these measurements being recorded:
-
without the use of provocation, such as the Valsalva manoeuvre or coughing
-
without the use of position and gravity to demonstrate the maximum descent
-
at a time when the prolapse was not evident (e.g. in the morning)
-
in theatre under anaesthetic (without manual pulling)
-
with a pessary in place or recently removed
-
successful intervening treatment (intensive physiotherapy, weight loss etc.)
-
incorrect diagnosis (full bladder or bowel).
Other clinical symptoms
Vaginal and sexual symptoms were common and had an impact on QoL. Around one-third of women were sexually active at baseline and 2.9% reported dyspareunia (see Table 6). Nocturia (21.9%) and urgency (20.3%) were common urinary symptoms. Around one-fifth (22.6%) of women reported urinary incontinence, and this was slight or moderate in the majority of cases (68.9%; see Appendix 2, Table 52). Few women were expected to undergo concomitant continence surgery or had already undergone previous continence surgery (see Tables 2 and 5). Around one-third (30.2%) of women had faecal incontinence, at least occasionally, and this was severe in around 8.7% of women (see Table 7).
These findings will serve as a benchmark for future research in women with uterine prolapse. The messages regarding symptoms and findings in relation to clinical practice may be helpful in improving prolapse assessment in the UK and internationally.
Chapter 4 Uterine trial results
This chapter reports the outcomes for women participating in the Uterine trial at 12 months.
The flow of women in the Uterine trial is shown in the CONSORT (Consolidated Standards of Reporting Trials) flow diagram (Figure 2), in line with CONSORT recommendations. 61 Two post-randomisation exclusions were not included in the study analyses (both had no significant uterine descent), leaving 563 randomised women analysed in the Uterine trial of the VUE trial.
FIGURE 2.
The CONSORT flow diagram of women recruited to the Uterine trial of the VUE study.
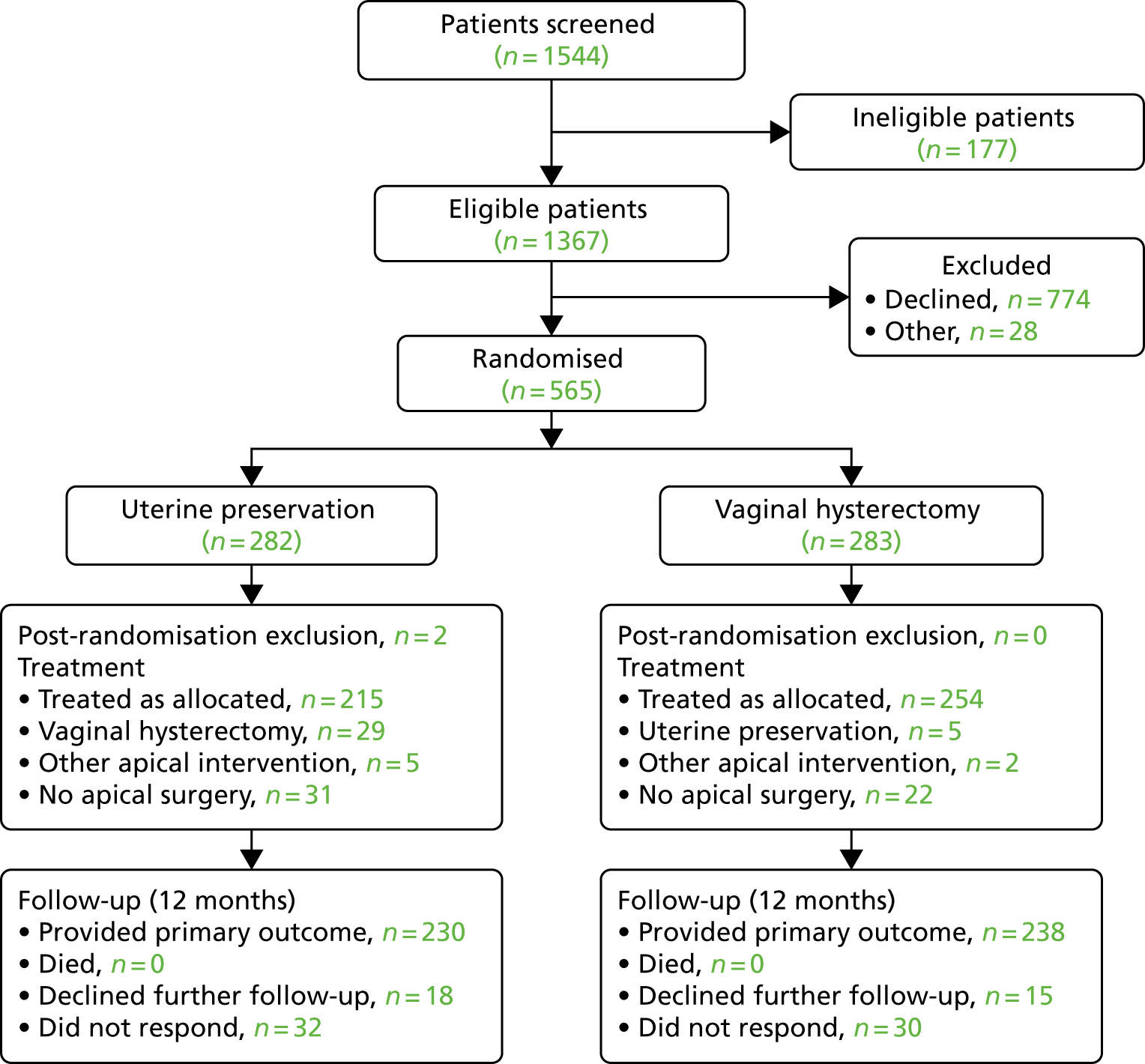
The women were recruited in 45 centres across the UK (see Appendix 2, Table 47) and received surgery as shown in Figure 3.
FIGURE 3.
Uterine trial: breakdown of the different surgeries received/or none. UIS, urinary incontinence surgery.
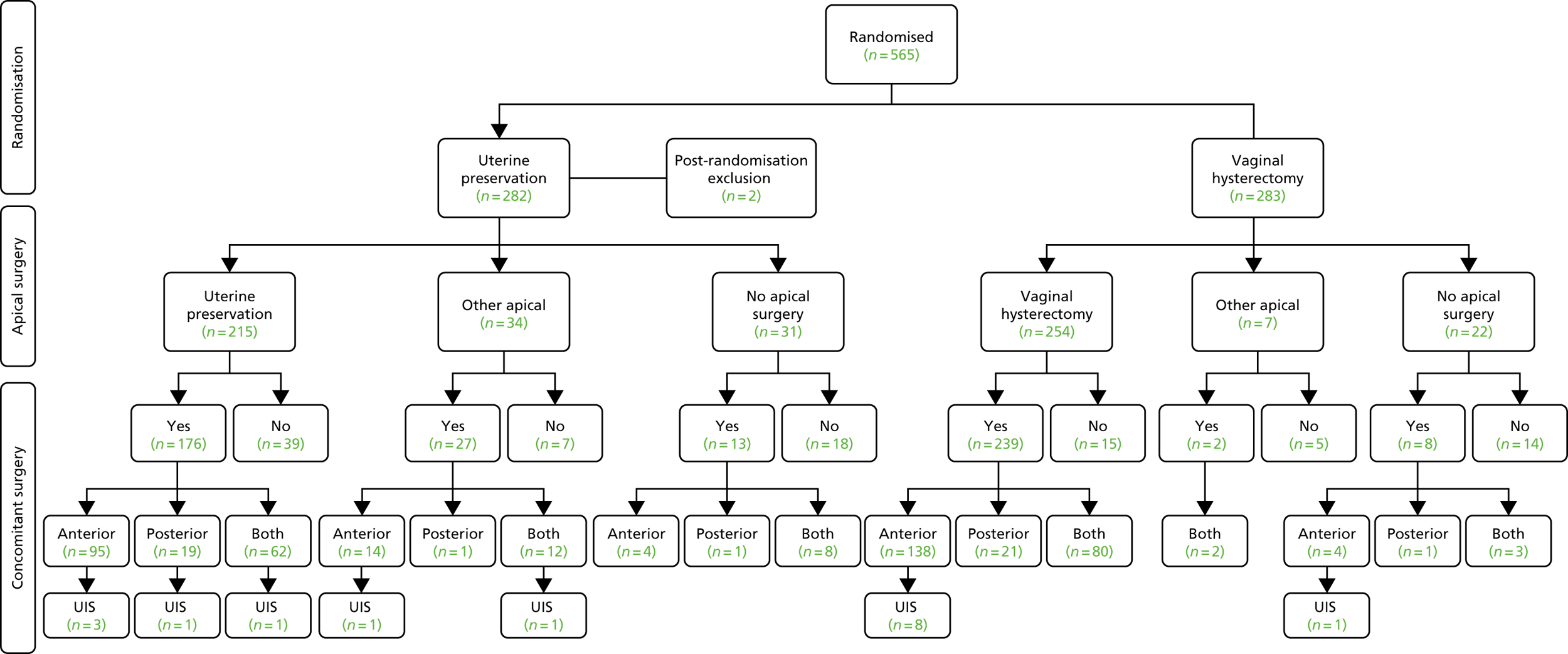
Surgery actually received
Of the 563 women included, 469 received their randomised allocation (76.8% in the uterine suspension group and 89.8% in the vaginal hysterectomy group) (Table 8). Reasons for not having the allocated treatment were the woman’s choice (n = 27), no apical descent [with alternative prolapse surgery (n = 17) and without surgery (n = 5)], alternative apical procedure (n = 7), other clinical factors (n = 10) or reason unknown (n = 28).
| Surgery | Treatment, n (%) | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Apical surgery received | ||
| Vaginal hysterectomy | 29 (10.4) | 254 (89.8) |
| Uterine suspension | 215 (76.8) | 5 (1.8) |
| Vaginal | 146 (52.1) | 3 (1.1) |
| Abdominal (laparoscopic) | 66 (23.6) | 2 (0.7) |
| Abdominal (open) | 3 (1.1) | 0 (0) |
| Other apical surgerya | 5 (1.8) | 2 (0.7) |
| No apical surgery | 31 (11.1) | 22 (7.8) |
| Concomitant surgery received | ||
| Anterior repair | 113 (40.4) | 142 (50.2) |
| Both (anterior and posterior) | 82 (29.3) | 85 (30.0) |
| Posterior repair | 21 (7.5) | 22 (7.8) |
| Continence procedure | 7 (2.5) | 9 (3.2) |
The use of mesh for the apical or concomitant procedure was recorded and is detailed in Appendix 3, Table 53.
In relation to the randomised groups, more women in the vaginal hysterectomy group received a concomitant anterior repair (50.2%) than in the uterine preservation group (40.4%). Overall, the numbers receiving a concomitant procedure in other compartments or for incontinence were similar between the groups (see Table 8).
Description of surgical characteristics
Most procedures were performed by a consultant gynaecologist (73.6%) with the patient under general anaesthesia (80.3%), and most patients received prophylactic antibiotics (92.0%) (see Appendix 3, Table 54). The operation was significantly longer in the uterine preservation group (114 minutes) than the vaginal hysterectomy group (103 minutes, MD 9.79 minutes, 95% CI 3.50 to 16.07 minutes). Women in the vaginal hysterectomy group had significantly higher blood loss (166 ml) than those randomised to uterine preservation (125 ml, MD –42 ml, 95% CI –62.68 to –21.73 ml). There was no difference in length of hospital stay (1.9 days, see Appendix 3, Table 55). There was no difference in time from the woman’s randomisation to her surgery between the groups (see Appendix 3, Table 55).
Outcomes
Response rates and clinical attendance
A total of 237 out of the 282 (84.0%) women randomised to the uterine preservation group and 241 out of the 283 (85.2%) in the vaginal hysterectomy group responded to the questionnaire at 12 months after randomisation. The clinic assessment 12 months after surgery was completed for 258 out of 262 women in the uterine preservation group who received any type of surgery (98.5%) and 262 out of 269 women in the vaginal hysterectomy group (97.4%).
Women’s prolapse symptoms and effect on everyday life
At 6 months after surgery, women reported a reduction in their POP-SS from a mean score of 13.7 out of 28 to 4.9 out of 28 in the uterine preservation group and from 13.5 out of 28 to 4.0 out of 28 in the vaginal hysterectomy group (MD 0.85, 95% CI 0.00 to 1.71). The effect of prolapse on QoL also improved at 6 months after surgery and again there was no significant difference between the groups.
The primary outcome was POP-SS at 12 months after randomisation; there was no statistically significant difference between the groups. The MD in the POP-SS for uterine preservation (4.2, SD 4.9) compared with vaginal hysterectomy (4.2, SD 5.3) adjusted for baseline variables was –0.05 (95% CI –0.91 to 0.81) (Table 9 and Figure 4).
| Symptoms and effects | Treatment, mean (SD); n | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| POP-SS | |||
| Baseline | 13.7 (6.4); 268 | 13.5 (5.9); 265 | |
| 6 months after surgery | 4.9 (5.7); 238 | 4.0 (4.7); 246 | 0.85 (0.00 to 1.71); 0.05 |
| 12 months after randomisation | 4.2 (4.9); 230 | 4.2 (5.3); 238 | –0.05 (–0.91 to 0.81); 0.91 |
| Prolapse-related effect on QoL | |||
| Baseline | 6.8 (2.7); 273 | 6.5 (2.9); 270 | |
| 6 months after surgery | 2.2 (3.1); 239 | 1.6 (2.6); 243 | 0.47 (–0.01 to 0.96); 0.06 |
| 12 months after randomisation | 1.7 (2.5); 237 | 1.5 (2.5); 239 | 0.12 (–0.26 to 0.49); 0.54 |
FIGURE 4.
Mean and standard error POP-SSs at baseline and follow-up.
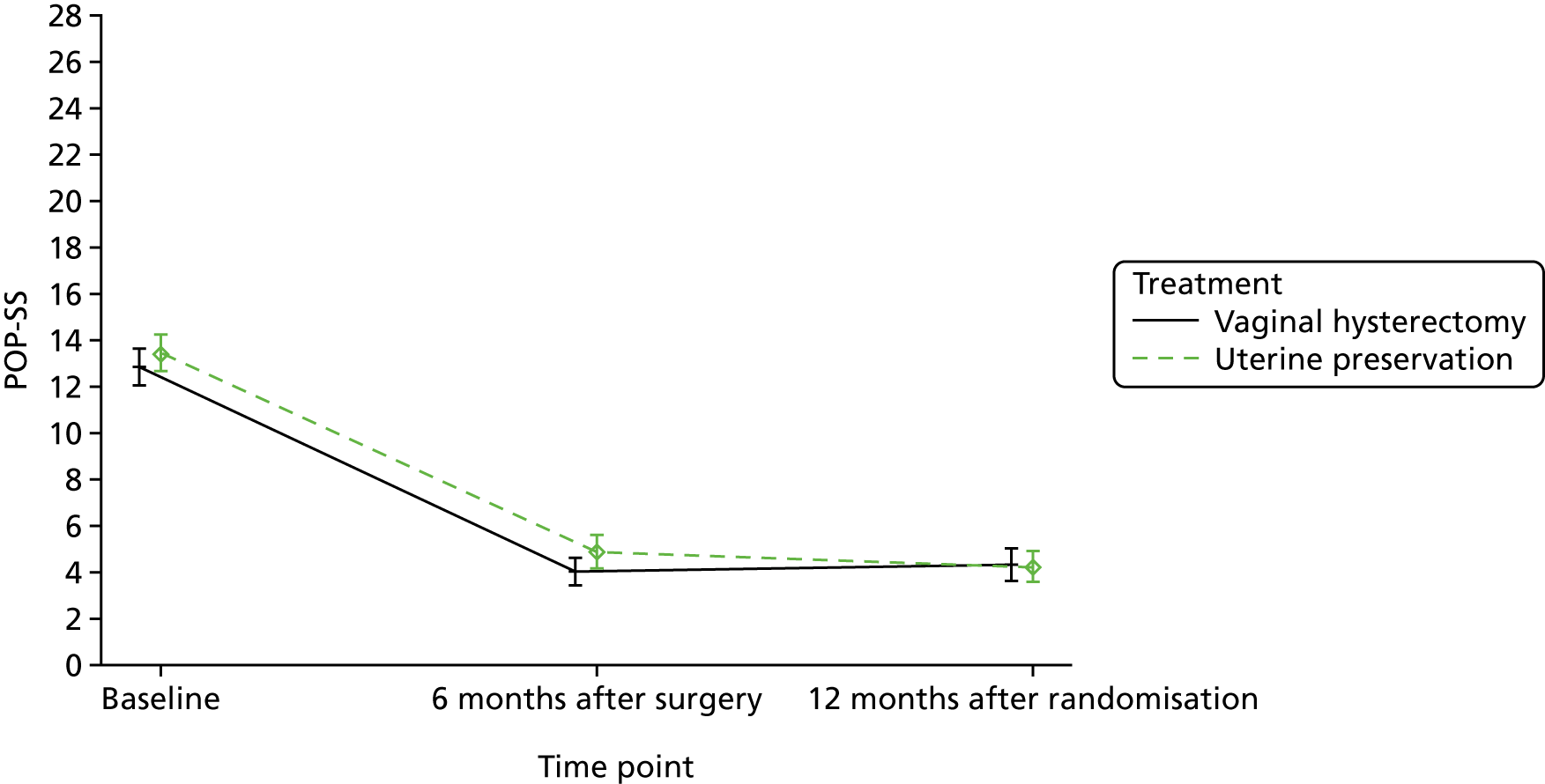
There was also no significant difference on the effect of prolapse on QoL at 12 months after randomisation [uterine preservation 1.7 (SD 2.5) vs. vaginal hysterectomy 1.5 (SD 2.5); MD 0.12, 95% CI –0.26 to 0.49]. The intracluster correlation for the surgeon was 0.04 (95% CI 0.00 to 0.44).
Distribution of individual POP-SSs and prolapse-related effects on QoL are also given in Appendix 3, Figures 20 and 21.
Each individual prolapse symptom also improved at 12 months after randomisation (Table 10) and was already apparent at 6 months after surgery (see Appendix 3, Table 56).
| Symptoms and EQ-5D | Treatment | |
|---|---|---|
| Uterine preservation | Vaginal hysterectomy | |
| Symptomatic, n (%); N | ||
| Number of women with symptoms | 178 (77.4); 230 | 188 (79.0); 238 |
| Individual prolapse symptoms, n (%); N | ||
| Bowel not empty (any) | 133 (56.6); 235 | 132 (55.5); 238 |
| Bowel not empty (most/all of the time) | 22 (9.4); 235 | 14 (5.9); 238 |
| Bladder not empty (any) | 133 (56.6); 235 | 121 (50.6); 239 |
| Bladder not empty (most/all of the time) | 21 (8.9); 235 | 24 (10.0); 239 |
| Strain to empty bladder (any) | 76 (32.6); 233 | 75 (31.5); 238 |
| Strain to empty bladder (most/all of the time) | 13 (5.6); 233 | 19 (8.0); 238 |
| Something coming down (any) | 71 (30.7); 231 | 69 (28.9); 239 |
| Something coming down (most/all of the time) | 17 (7.4); 231 | 22 (9.2); 239 |
| Dragging in abdomen (any) | 65 (28.0); 232 | 62 (26.2); 237 |
| Dragging in abdomen (most/all of the time) | 9 (3.9); 232 | 9 (3.8); 237 |
| Dragging in back (any) | 63 (27.4); 230 | 70 (29.4); 238 |
| Dragging in back (most/all of the time) | 10 (4.3); 230 | 8 (3.4); 238 |
| Uncomfortable feeling or pain when standing (any) | 40 (17.6); 227 | 50 (21.0); 238 |
| Uncomfortable feeling or pain when standing (most/all of the time) | 10 (4.4); 227 | 12 (5.0); 238 |
| Most bothersome symptom, n (%); N | ||
| Bowel not empty | 50 (36.8); 136 | 51 (40.5); 126 |
| Bladder not empty | 31 (22.3); 136 | 18 (14.3); 126 |
| Something coming down | 23 (16.9); 136 | 28 (22.2); 126 |
| Dragging in back | 10 (7.4); 136 | 11 (8.7); 126 |
| Dragging in abdomen | 10 (7.4); 136 | 4 (3.2); 126 |
| Uncomfortable feeling or pain when standing | 7 (5.1); 136 | 7 (5.6); 126 |
| Strain to empty bladder | 5 (3.7); 136 | 7 (5.6); 126 |
| Which symptom causes most bother not applicable | 82 (37.6); 218 | 96 (43.2); 222 |
| Actions necessitated by prolapse symptoms, n (%); N | ||
| Extra hygiene measures | 10 (4.4); 225 | 9 (3.9); 230 |
| Digitally evacuate bowel | 8 (3.5); 237 | 5 (2.1); 231 |
| Fingers to help empty bowel | 2 (0.9); 224 | 2 (0.9); 234 |
| Fingers to help empty bladder | 1 (0.4); 230 | 2 (0.9); 235 |
| Fingers to ease discomfort | 0 (0); 225 | 2 (0.9); 229 |
| EQ-5D, mean score (SD); N | ||
| EQ-5D | 0.865 (0.200); 227 | 0.886 (0.187); 235 |
| EQ-5D visual scale | 81.0 (15.6); 228 | 82.7 (16.3); 235 |
The most bothersome symptoms across both groups at 6 months after surgery and at 12 months after randomisation were different from those at baseline. The most bothersome symptom at baseline, ‘a feeling of something coming down’ reduced overall from 49.7% to 19.5%. Symptoms of incomplete emptying of either bladder or bowel became more common, but ‘actions necessitated by prolapse symptoms’ were improved (see Table 10, see also Appendix 3, Table 56).
Secondary outcomes
Objective prolapse outcomes
Women who had any prolapse procedure were invited back for a clinical assessment 12 months after surgery and, overall, 97.9% attended. The objective prolapse measure (POP-Q) improved in all compartments (see Appendix 3, Table 57). There was no difference in either group for objective overall prolapse stage 2b or more [uterine preservation 31.8% vs. 34.1% vaginal hysterectomy; odds ratio (OR) 0.85, 95% CI 0.55 to 1.32]. More specifically, the overall number of women with an objective apical prolapse (i.e. stage 2b or more) fell to 5.7% in the uterine preservation group compared with 5.3% in the vaginal hysterectomy group 12 months after surgery [adjusted for baseline values (Table 11)]. Again, this reduction was not significant. Objective anterior and posterior prolapse outcomes also improved.
| Stage | Treatment, n (%); N | Effect size,a OR (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| Overall stage 2b or more | 74 (31.8); 233 | 78 (34.1); 229 | 0.85 (0.55 to 1.32); 0.48 |
| Apical stage | |||
| Stage 0 | 161 (70.0); 230 | 181 (80.1); 226 | |
| Stage 1 | 51 (22.2); 230 | 30 (13.3); 226 | |
| Stage 2a | 5 (2.2); 230 | 3 (1.3); 226 | |
| Stage 2b | 9 (3.9); 230 | 3 (1.3); 226 | |
| Stage 3 | 4 (1.7); 230 | 3 (1.3); 226 | |
| Stage 4 | 0 (0); 230 | 6 (2.7); 226 | |
| Stage 2b or more | 13 (5.7); 230 | 12 (5.3); 226 | 1.18 (0.48 to 2.94); 0.72 |
| Anterior stage 2b or more | 67 (28.8); 233 | 66 (28.8); 229 | 0.97 (0.62 to 1.51); 0.88 |
| Posterior stage 2b or more | 12 (5.2); 232 | 19 (8.4); 227 | 0.61 (0.28 to 1.35); 0.22 |
| Development of new prolapse at another site | 10 (4.3); 231 | 12 (5.0); 238 | 0.86 (0.36 to 2.06); 0.74 |
Vaginal and sexual symptoms
Both the mean vaginal symptoms and the QoL scores decreased (improved) for both treatment groups (Table 12). More women were sexually active after surgery than before surgery (from around 30.0% at baseline to 44.1% in the uterine preservation group and 40.1% in the vaginal hysterectomy group), and fewer women cited prolapse symptoms as a reason for not being sexually active. For those women who were not sexually active, 17 (17.4%) women in the uterine preservation group and 12 (11.5%) in the vaginal hysterectomy group gave vaginal or prolapse symptoms as the reason.
| Symptoms | Treatment | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| ICI Vaginal Symptoms Score, mean (SD); N | 5.9 (6.7); 208 | 5.5 (7.1); 215 | 0.54 (–0.67 to 1.76); 0.38 |
| QoL due to vaginal symptoms, mean (SD); N | 1.2 (2.2); 212 | 1.1 (2.1); 218 | 0.03 (–0.36 to 0.42); 0.89 |
| ICI Sexual Matters Score, mean (SD); N | 7.8 (13.2); 93 | 8.7 (12.9); 88 | –2.47 (–6.78 to 1.85); 0.26 |
| QoL due to effect on sex life, mean (SD); N | 1.4 (2.4); 93 | 1.8 (2.6); 88 | –0.53 (–1.34 to 0.27); 0.20 |
| Reduced sensation, n (%); N | 5 (2.3); 219 | 5 (2.2); 229 | |
| Vagina too loose or lax, n (%); N | 2 (0.9); 218 | 7 (3.1); 228 | |
| Number of women having intercourse, n (%); N | 98 (44.1); 222 | 93 (40.1); 232 | |
| Pain with intercourse | 0 (0); 98 | 2 (2.2); 93 | |
| Reasons for not being sexually active, n (%); N | |||
| No partner | 49 (50.0); 98 | 57 (54.3); 105 | |
| Vaginal symptoms | 9 (9.2); 98 | 7 (6.7); 105 | |
| Prolapse symptoms | 8 (8.2); 98 | 5 (4.8); 105 | |
| Other reason | 32 (32.7); 98 | 36 (34.3); 105 | |
There were no statistically significant differences in vaginal and sexual symptoms between the uterine preservation and vaginal hysterectomy groups at 12 months after randomisation.
Urinary and bowel symptoms
Overall, at 12 months after randomisation, the proportion of women who had any urinary incontinence increased from 22.6% at baseline to 35.8%, although the proportion with severe urinary incontinence decreased from 13.0% to 1.6% (Table 13). Significantly more women reported urge incontinence at 12 months after randomisation in the uterine preservation group (n = 32, 13.9%) than in the vaginal hysterectomy group (n = 12, 5.0%; MD 2.71, 95% CI 1.32 to 5.55). Following surgery, de novo urinary incontinence was similar across both groups and reported in around one-quarter of the women. Further urinary symptoms can be found in Appendix 3, Table 58.
| Symptoms | Treatment | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| Urinary symptoms, n (%); N | |||
| Urgency | 32 (13.9); 230 | 12 (5.0); 238 | 2.71 (1.32 to 5.55); 0.01 |
| Nocturia | 34 (14.8); 229 | 29 (12.2); 238 | |
| Frequency | 11 (4.8); 230 | 9 (3.8); 238 | |
| All the above | 3 (1.3); 230 | 0 (0); 238 | |
| Persistent incontinence, n (%); N | 40 (18.0); 222 | 35 (15.8); 222 | 1.16 (0.70 to 1.91); 0.57 |
| Any incontinence, n (%); N | 87 (36.7); 237 | 84 (34.9); 241 | 1.09 (0.72 to 1.66); 0.68 |
| Severe incontinence | 3 (1.6); 184 | 3 (1.6); 187 | 1.00 (0.18 to 5.71); > 0.99 |
| De novo incontinence, n (%); N | 44 (25.6); 172 | 44 (26.2); 168 | 1.04 (0.63 to 1.70); 0.89 |
| Incontinence-related QoL Score, mean (SD); N | 1.8 (2.5); 175 | 1.6 (2.3); 176 | 0.19 (–0.27 to 0.65); 0.41 |
| ICI Urinary Incontinence Score, mean (SD); N | 5.1 (4.3); 164 | 4.6 (3.9); 168 | 0.41 (–0.36 to 1.19); 0.30 |
| ICIQ-FLUTS Filling Score, mean (SD); N | 3.7 (2.7); 228 | 3.3 (2.0); 237 | 0.18 (–0.19 to 0.54); 0.35 |
| ICIQ-FLUTS Voiding Symptoms Score, mean (SD); N | 1.6 (1.7); 230 | 1.6 (1.8); 237 | 0.00 (–0.29 to 0.30); 0.97 |
| ICIQ-FLUTS Incontinence Score, mean (SD); N | 3.7 (3.2); 184 | 3.6 (3.1); 187 | 0.23 (–0.33 to 0.79); 0.42 |
| Bowel symptoms | |||
| Bowel frequency (normal), n (%); N | 222 (96.5); 230 | 229 (95.8); 239 | 1.38 (0.46 to 4.17); 0.56 |
| Faecal incontinence (occasionally or more often), n (%); N | 59 (26.0); 227 | 61 (26.0); 235 | 0.97 (0.59 to 1.57); 0.89 |
| Passive | 43 (18.3); 235 | 38 (16.9); 225 | |
| Active | 18 (7.7); 235 | 19 (8.4); 225 | |
| Severe | 15 (6.6); 227 | 7 (3.0); 235 | 4.64 (1.49 to 14.42); 0.01 |
| Bowel urgency (most or all of the time), n (%); N | 31 (14.4); 216 | 32 (14.2); 225 | 0.97 (0.56 to 1.68); 0.90 |
| Constipation (most or all of the time), n (%); N | 9 (3.9); 231 | 10 (4.2); 238 | 0.94 (0.36 to 2.46); 0.90 |
| Bowel symptoms QoL score, mean (SD); N | 1.8 (0.9); 231 | 1.7 (0.8); 238 | 0.05 (–0.10 to 0.20); 0.51 |
Although bowel urgency increased to 14.3% across both treatment groups at 12 months after randomisation, the presence of severe faecal incontinence was slightly improved. However, significantly more women in the uterine preservation group (n = 15, 6.6%) had severe faecal incontinence than in the vaginal hysterectomy group (n = 7, 3.0%), though these were small numbers (MD 4.64, 95% CI 1.49 to 14.42).
There was no evidence of a difference in any other urinary or bowel symptoms outcomes between the randomised groups.
Satisfaction with treatment at 12 months after randomisation
At 12 months after randomisation, most women reported that they were better than before surgery, with no statistically significant difference between the groups (90.8% vs. 95.3%; OR 0.49, 95% CI 0.22 to 1.05) (Table 14). Similarly, the proportions of women completely or fairly satisfied were not statistically significantly different (85.6% vs 90.6%; OR 0.62, 95% CI 0.34 to 1.13). However, significantly more women (95.0%) in the vaginal hysterectomy group would recommend it to a friend compared with women in the uterine preservation group (88.3%) (OR 0.39, 95% CI 0.18 to 0.83).
| Recovery and satisfaction | Treatment, n (%); N | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| Prolapse is better now compared with 1 year ago | 208 (90.8); 229 | 221 (95.3); 232 | 0.49 (0.22 to 1.05); 0.07 |
| Completely or fairly satisfied | 196 (85.6); 229 | 211 (90.6); 233 | 0.62 (0.34 to 1.13); 0.12 |
| Recommend to a friend | 196 (88.3); 222 | 211 (95.0); 222 | 0.39 (0.18 to 0.83); 0.01 |
Further treatment required for failure or adverse events at 6 and at12 months
When women reported that they had been re-admitted to hospital, the information was verified with the centres and post-coded accordingly to ensure accuracy of data and resolution of discrepancies. A hospital re-admission was counted as a SAE if it was related to the initial prolapse surgery. Further surgery for prolapse (recurrence if in the same compartment), or for continence surgery, was differentiated from re-admission for surgery-related complications, such as bleeding or infection (Table 15).
| Further treatment | Treatment | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation | Vaginal hysterectomy | ||
| Further surgery, n (%); N | 18 (7.4); 244 | 11 (4.5); 242 | 1.70 (0.78 to 3.71); 0.182 |
| Further prolapse surgery, n (%); N | 15 (6.1); 244 | 8 (3.3); 242 | 2.01 (0.81 to 4.95); 0.130 |
| Anterior | 8 (3.3); 243 | 1 (0.4); 242 | 8.10 (1.00 to 65.57); 0.050 |
| Posterior | 3 (1.2); 242 | 5 (2.1); 242 | 0.60 (0.14 to 2.53); 0.484 |
| Apical | 10 (4.1); 243 | 3 (1.2); 242 | 3.43 (0.93 to 12.64); 0.064 |
| Continence surgery, n (%); N | 3 (1.2); 242 | 4 (1.7); 242 | |
| Time to further surgery (days), mean (SD); Nb | 290.1 (149.6); 16 | 263.2 (91.7); 10 | 1.66 (0.80 to 3.44); 0.177c |
| Need for conservative treatment, n (%); N | |||
| Any conservative treatment | 75 (26.8); 280 | 73 (25.8); 283 | 1.06 (0.73 to 1.54); 0.77 |
| Absorbent pads | 49 (24.3); 202 | 61 (27.4); 223 | |
| Physiotherapy | 17 (11.4); 149 | 12 (7.4); 162 | |
| Ring pessary | 14 (7.6); 185 | 9 (4.4); 205 | |
| Shelf or Gellhorn pessary | 8 (4.4); 181 | 4 (2.0); 205 | |
| Disposable or reusable catheter | 5 (2.7); 180 | 4 (2.0); 204 | |
| Permanent catheter | 1 (0.6); 177 | 2 (1.0); 201 | |
| Seen health professional, n (%); N | |||
| Gynaecology out patients (GOPD) | 73 (39.9); 183 | 49 (25.5); 192 | |
| GP for prolapse | 62 (32.5); 191 | 54 (27); 203 | |
| Practice nurse | 13 (8.6); 151 | 9 (5.5); 165 | |
| District or continence nurse | 7 (4.8); 145 | 4 (2.5); 159 | |
Overall, 29 women were re-admitted for further surgery in the first 12 months (Table 15). Twenty-three women had further prolapse surgery within 12 months [uterine preservation (n = 15) vs. vaginal hysterectomy (n = 8)]. Ten women in the uterine preservation group had a further apical procedure compared with three women in the vaginal hysterectomy group during that time. More women in the uterine preservation group (n = 8) had a re-admission for an anterior repair compared with those women in the vaginal hysterectomy group (n = 1). Overall, there was no evidence of a difference between the groups regarding further treatment.
One woman did not have her VUE procedure (or any procedure) at the time of admission (clinical decision), but pursued the apical procedure by another health-care provider 12 months later.
Serious and related adverse events in the first 12 months after surgery
Overall, there were 30 SAEs (5.7%) reported in the first 12 months following surgery (more than one SAE could be reported per woman) (Table 16). Hospitalisation was the most common type of SAE. There were no differences between the groups in any SAEs.
| Serious and related AEs | Treatment, n (%) | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation (N = 262) | Vaginal hysterectomy (N = 269) | ||
| Any SAE | 14 (5.4) | 16 (5.9) | 0.82 (0.38 to 1.75); 0.60 |
| Type of SAEb | |||
| Hospitalisation | 10 (3.8) | 9 (3.3) | |
| Considered medically significant by the investigator | 4 (1.5) | 4 (1.5) | |
| Prolongation of hospitalisation | 3 (1.2) | 5 (1.9) | |
| Life-threatening | 1 (0.4) | 0 (0) | |
| Intraoperative occurrencesc | |||
| Excessive blood lossd | 1 (0.4) | 3 (1.1) | |
| Injury to blood vessel | 0 (0) | 1 (0.4) | |
| Blood transfusion | 0 (0) | 1 (0.4) | |
| Anaesthetic complications | 0 (0) | 1 (0.4) | |
| Postoperative occurrencesc | |||
| Haematoma | 2 (0.8) | 5 (1.9) | |
| Pain | 2 (0.8) | 4 (1.5) | |
| Excessive blood loss | 1 (0.4) | 5 (1.9) | |
| Other infections | 3 (1.2) | 2 (0.7) | |
| Constipation | 2 (0.8) | 2 (0.7) | |
| Granulation tissue | 1 (0.4) | 3 (1.1) | |
| Urinary retention/voiding difficulties requiring conservative intervention | 5 (2.0) | 1 (0.4) | |
| Suture removal | 2 (0.8) | 0 (0) | |
| Urinary tract infection | 0 (0) | 2 (0.7) | |
| Bowel obstruction | 1 (0.4) | 0 (0) | |
| Thrombosis | 1 (0.4) | 0 (0) | |
| Wound infection | 0 (0) | 1 (0.4) | |
| Vaginal adhesions | 1 (0.4) | 0 (0) | |
| Urinary retention/voiding difficulties requiring surgery | 1 (0.4) | 0 (0) | |
| Mesh exposure/extrusion that requires surgical treatment | 1 (0.4) | 0 (0) | |
| New or persistent lower urinary tract symptoms | 0 (0) | 1 (0.4) | |
Specifically, of the 129 procedures (apical and concomitant) using a mesh implant one mesh exposure/extrusion was identified and required surgical treatment in the first 12 months after surgery.
Non-serious and related adverse events in the first 12 months after surgery
Non-serious AEs or complications were also recorded (Table 17). Overall, there was a total of 18 (3.6%) within the 12 months after surgery. There were no differences between the groups in any non-serious AEs.
| Non-serious AE | Treatment, n (%) | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation (N = 262) | Vaginal hysterectomy (N = 269) | ||
| Any non-serious AE | 6 (2.3) | 2 (0.7) | 3.30 (0.63 to 17.19); 0.16 |
| Intraoperative non-serious AEb | |||
| Anaesthetic complications | 1 (0.4) | 0 (0) | |
| Blood loss of > 500 ml | 1 (0.4) | 0 (0) | |
| Blood transfusion | 0 (0) | 1 (0.4) | |
| Postoperative complicationsb | |||
| Treatment for postoperative infection with antibiotics | 3 (1.2) | 1 (0.4) | |
| Infection | 2 (0.8) | 1 (0.4) | |
| Pyrexia, unknown origin | 1 (0.4) | 1 (0.4) | |
| UTI | 1 (0.4) | 0 (0) | |
| Blood transfusion | 0 (0) | 1 (0.4) | |
| Other | 3 (1.2) | 1 (0.4) | |
Subgroup analysis
There was no evidence of a difference between treatment groups in any of the planned subgroup analyses (age < 60 and ≥ 60 years, previous anterior or posterior surgery or previous incontinence surgery) (see Appendix 3, Table 60).
Sensitivity analyses
A multiple imputation model was used to impute missing values in the primary outcome, assuming a missing-at-random mechanism. No evidence of a difference between the two groups after the imputation (uterine preservation vs. vaginal hysterectomy; MD –0.02, 95% CI –0.83 to 0.78; p = 0.96) was found.
Per-protocol analysis
A per-protocol analysis including only the participants who followed protocol (i.e. received the allocated treatment) was done and no evidence of a difference between groups (uterine preservation vs. vaginal hysterectomy; MD 0.19, 95% CI –0.70 to 1.08; p = 0.68) was found.
Discussion
Summary of findings
Surgical planning and procedure
In addition to their apical surgery, concomitant surgery was planned for the majority (85.8%) of women in the Uterine trial. The randomised surgical allocation was performed in most of the women (76.8% in the uterine preservation group and 89.8% in the vaginal hysterectomy group). The woman’s choice was the most common reason for not receiving the randomised allocation. Women in the vaginal hysterectomy group were more likely to receive a concomitant anterior repair (50.2%) compared with those in the uterine preservation group (40.4%), but all other concomitant procedures were similar between the groups.
Most procedures were performed by a consultant gynaecologist under general anaesthesia with prophylactic antibiotics (92.0%). The duration of the uterine preservation surgery was significantly longer (mean duration 114 minutes, SD 50 minutes) than vaginal hysterectomy (mean duration 103 minutes, SD 33 minutes; MD 9.79 minutes, 95% CI 3.50 to 16.07 minutes)]. Women in the vaginal hysterectomy group had significantly higher blood loss (mean blood loss 166 ml) than those women randomised to uterine preservation [mean blood loss 125 ml, MD –42 ml, 95% CI –62.3 to 21.7 ml)], though postoperative hospital stay was not different.
Primary and secondary outcomes
Around 85% of women responded to the 12-month questionnaire, with more women (97.9%) attending for the 12-month clinic assessment.
The primary outcome was POP-SS at 12 months after randomisation and there was no difference between women in the uterine preservation and vaginal hysterectomy groups. The MD in the POP-SS for uterine preservation (4.2 points, SD 4.9 points) compared with vaginal hysterectomy (4.2 points, SD 5.3 points), adjusted for baseline variables, was –0.05 points (95% CI –0.91 to 0.81 points) and suggests that both are equally effective in improving uterine prolapse symptoms at 12 months.
At 12 months after randomisation, prolapse-related effects on QoL scores had improved and were apparent at 6 months after operation. The most bothersome symptom at baseline, ‘a feeling of something coming down’, reduced from 49.7% to 19.5%. Incomplete emptying of the bladder or bowel was the most bothersome symptoms at both 6 and 12 months for both randomised groups, but did not affect ‘actions necessitated by prolapse symptoms’, which were reduced from baseline.
Overall, objective prolapse stage (POP-Q) improved for both randomised groups, particularly in terms of prolapse protrusion beyond the hymen (stage 2b or more: 31.8% after uterine preservation vs. 34.1% after vaginal hysterectomy). Objective apical prolapse (stage 2b or more) fell to < 6% for both groups 12 months after surgery (adjusted for baseline values). These values were not statistically different between the groups.
More women were sexually active after surgery. There were no statistically significant differences in vaginal and sexual symptoms between randomised groups at 12 months.
Overall, at 12 months after randomisation the proportion of women who had any urinary incontinence increased from around one in five women to one in three, although severe urinary incontinence decreased. Significantly more women reported urge incontinence at 12 months in the uterine preservation group than in the vaginal hysterectomy group. De novo urinary incontinence was similar across both groups of women and reported in around one in four women. Few women had continence surgery to alleviate their symptoms within the first 12 months (n = 7).
Although bowel urgency increased to 14.3% across both randomised groups at 12 months after randomisation, the presence of faecal incontinence was slightly reduced. However, significantly more women in the uterine preservation group (n = 15, 6.6%) had severe faecal incontinence at 12 months than in those women in the vaginal hysterectomy group (n = 7, 3.0%), though these are small numbers (MD 4.64%, 95% CI 1.49% to 14.42%). There were no other significant differences in bowel symptoms between the groups of women.
At 12 months after randomisation, 93.1% of women reported that their prolapse symptoms were better compared with baseline, and the proportion of satisfaction was not statistically significantly different. However, significantly more women (95.0%) in the vaginal hysterectomy group would recommend the treatment to a friend compared with 88.3% of those in the uterine preservation group (OR 0.39, 95% CI 0.18 to 0.83).
Overall, 29 women were re-admitted for further surgery in the first 12 months after surgery (see Table 16). Twenty-three women had further prolapse surgery within 12 months [uterine preservation (n = 15) vs. vaginal hysterectomy (n = 8)] and this was not statistically significant. Ten women in the uterine preservation group had a further apical procedure compared with three women in the hysterectomy group in that time (again, this was not statistically significant). More women in the uterine preservation group (n = 8) had a re-admission for an anterior repair compared with those in the vaginal hysterectomy group (n = 1; OR 8.10, 95% CI 1.00 to 65.57).
There were 30 SAEs reported in the first 12 months following surgery and there were no differences between the groups of women in any SAEs. Of the 129 procedures (apical and concomitant), using a mesh implant there was one mesh exposure/extrusion in the first 12 months after surgery. This was treated surgically by removal of the exposed mesh.
Conclusion
Vaginal hysterectomy and uterine preservation surgeries are equally effective in relieving uterine prolapse in relation to symptoms and objective descent, although vaginal hysterectomy was more likely to be recommended to a friend. The presence of urinary incontinence may worsen after either procedure, but severe incontinence appears to remain static; however, an increased risk of re-operation for an anterior pelvic floor repair in those women having preservation procedures was identified.
Chapter 5 Uterine trial within-trial cost-effectiveness analysis
This chapter presents the results of the within-trial cost–utility analysis for women in the Uterine trial. Further details of the cost-effectiveness data are provided in Appendix 4, Tables 61–5.
NHS perspective resource use and costs
Intervention costs
Intervention costs are calculated for all women entering theatre for an operative procedure (regardless of whether or not they received a completed surgery). Intervention delivery was £92 more expensive in the uterine preservation group than in the vaginal hysterectomy group (95% CI –£5 to £178). The potential for additional costs in the uterine preservation group is because of a slightly longer operation on average (around 10 minutes) and the use of mesh in a greater number of procedures. Mesh was used in the apical procedure for 86 out of 264 (33%) women randomised to the uterine preservation group, compared with 9 out of 270 (3%) women randomised to the vaginal hysterectomy group. All other resource use contributing to the intervention cost was similar across the groups. Full details of the component costing approach underpinning these numbers can be found in Appendix 4, Table 61.
Follow-up care costs
More women required further prolapse surgery in the uterine preservation group (n = 16) than in the vaginal hysterectomy group (n = 8). No difference between the groups was evident regarding either continence procedure or SAEs requiring hospitalisation. Average hospitalisation costs (excluding the index procedure) were £320 in the uterine preservation group compared with £179 in the vaginal hysterectomy group (adjusted MD £166, 95% CI –£39 to £370). Consultations with health-care professionals (e.g. outpatient doctors, GPs, community-based nurses, physiotherapists) were similar across the groups, as were the costs of medications and devices (e.g. oestrogens, pessaries, antibiotics). Full details of all follow-up care resource use reported in the trial are provided in Appendix 4, Tables 62–5.
Total NHS costs
Overall, total costs to the NHS were calculated for 207 out of 279 (74%) and 217 out of 283 (77%) women in the uterine preservation and vaginal hysterectomy groups, respectively (randomised women after post-randomisation exclusions). Missing data are primarily because of the non-return of participant questionnaires. Based on the complete-case costs available for analysis, the total costs to the NHS were £1643 (SD £1302) in the uterine preservation group and £1345 (SD £754) in the vaginal hysterectomy group. Uterine preservation was significantly more costly to the NHS (MD £292, 95% CI £68 to £517). This cost difference is driven by higher average intervention costs and the greater proportion of women experiencing a repeat prolapse procedure over 12-months’ follow-up in the uterine preservation group.
Patient participant perspective resource use and costs
Women with prolapse symptoms have a high rate of contact with health-care professionals, including a large number of consultations in both primary and secondary care. The opportunity costs of time and travel, purchase of over-the-counter medications and other related expenses were similar across the groups. A small number of women reported private care, and the cost was higher for women in the uterine preservation group.
The number of women reporting that they had to take time off from work, as a proportion of the trial population, was small. In total, in the Uterine trial 130 out of 572 (23%) randomised women reported the need to take time off work for prolapse symptoms [64/279 (23%) and 66/283 (23%) of those randomised to the uterine preservation and vaginal hysterectomy groups, respectively]. Among those women who required sick leave from work, the average number of days of leave required was 35 days (SD 65 days) and 22 days (SD 40 days) in the uterine preservation and vaginal hysterectomy groups, respectively, but differences were not statistically significant. See Appendix 4, Table 65 for full details of all costs incurred by the women.
Among the women with complete data for participant-incurred costs (46% in the uterine preservation group vs. 54% in the vaginal hysterectomy group), the average costs were £1266 (SD £4127) and £460 (SD £1505) in the uterine preservation and vaginal hysterectomy groups, respectively (MD £609, 95% CI £47 to £1172). The large SDs in both groups indicate that some women experienced very high personal costs.
When summing participant and NHS perspective costs together to inform a wider-perspective analysis of prolapse costs, the uterine preservation group remains significantly more costly (MD £911, 95% CI £359 to £1463).
Generic quality-of-life outcomes
The proportion of women reporting any health problems on each EQ-5D-3L domain (i.e. a score of 2 or 3 points) at baseline, at 6 months after surgery and at 12 months after randomisation is presented in Figure 5. Data are presented as reported in the respective questionnaires. Missing data are not reported in the figure. At baseline, < 70% of the women in both groups reported problems related to pain and discomfort and between one-quarter and one-third of women had at least some problems with usual activities or anxiety and depression. The reporting of any pain or discomfort fell to about 30% for all women by 12 months after randomisation, with fewer than one in five women reporting problems with usual activities or anxiety/depression. The fewest number of problems were experienced in self-care, with a small proportion of women reporting any problems by 12 months after randomisation. There are no obvious differences between the groups evident from the graphical presentations. The proportion of women experiencing problems decreased in both groups, indicating that both procedures had a positive impact on generic QoL. This finding echoes the findings from the condition-specific QoL data (see Chapter 4).
FIGURE 5.
Percentage of respondents with any problems on each EQ-5D-3L domain at (a) baseline, (b) 6 months after surgery and (c) 12 months after randomisation.
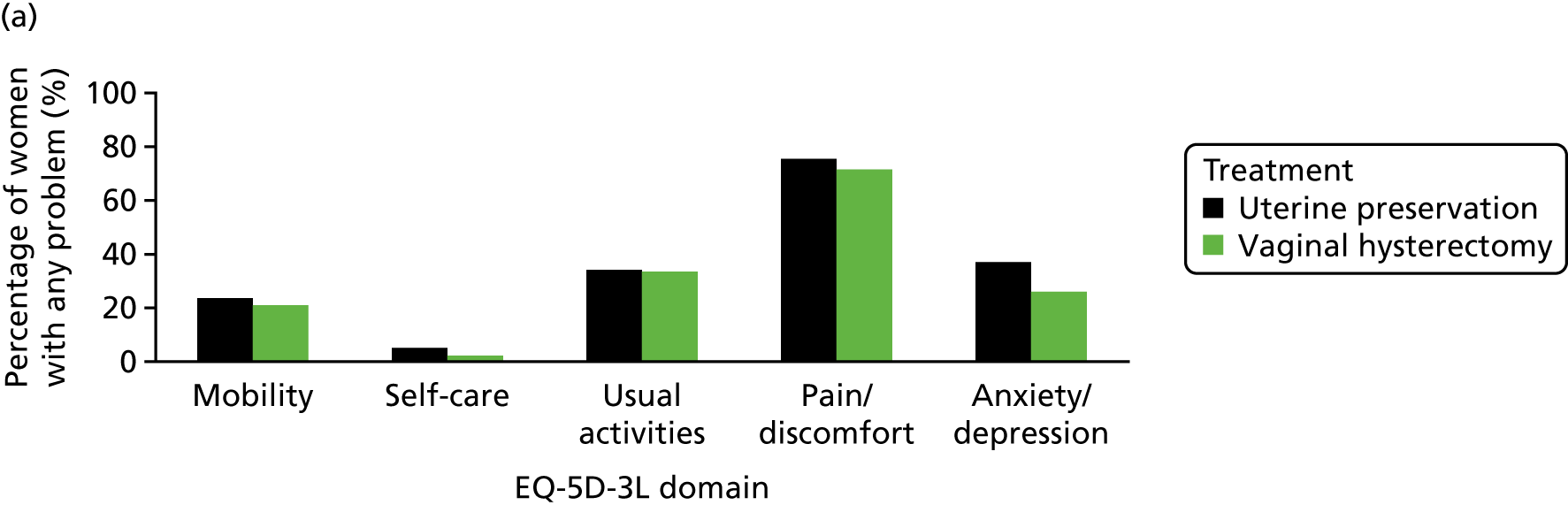

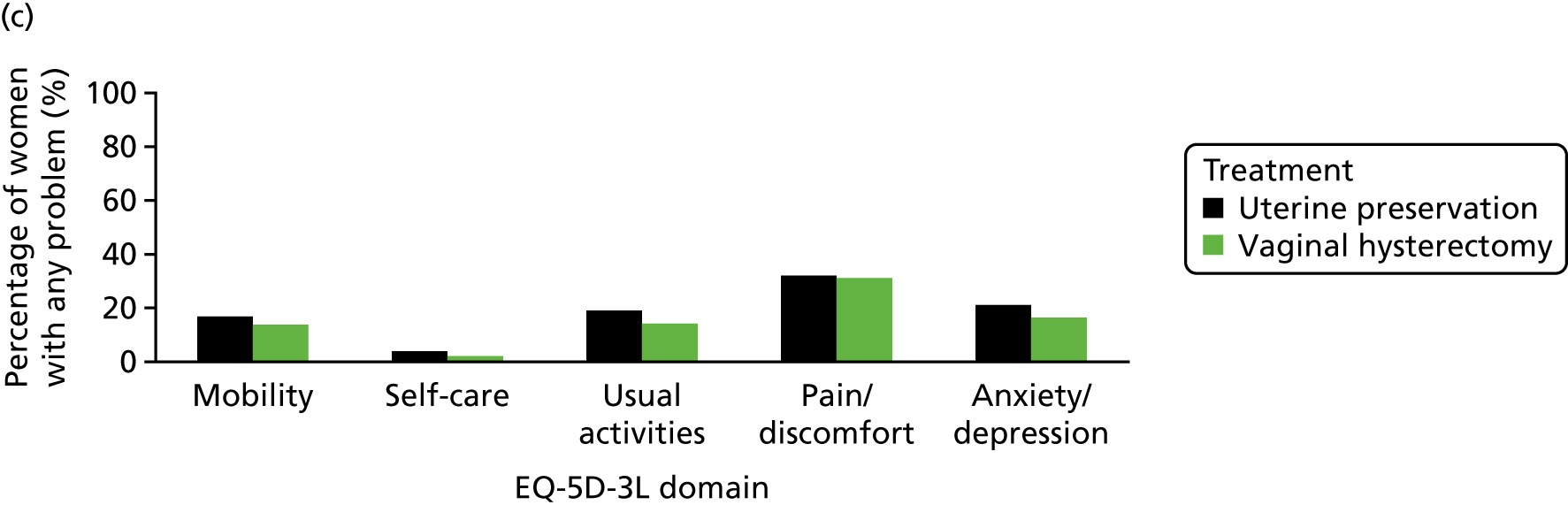
Full details of the utility scores resulting from EQ-5D-3L responses at each time point and calculated QALYs are reported in Table 18. In terms of covariates included within the analysis model, baseline utility was the only significant predictor of overall QALYs, re-enforcing the importance of adjusting for this baseline measure. 54
| Costs, QALYs and cost-effectiveness | Treatment, mean (SD); N | Uterine preservation vs. vaginal hysterectomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | MD | 95% CI | ||||||
| Costsa,b | |||||||||
| Intervention | £1213.04 (£472.08); 264 | £1116.18 (£371.28); 270 | £91.59 | £5.16 to £178.01 | |||||
| Hospital resource use | £319.69 (£1073.49); 265 | £178.71 (£839.97); 269 | £165.72 | –£39.00 to £370.45 | |||||
| Other consultations | £112.59 (£179.45); 219 | £85.83 (£169.92); 227 | £21.74 | –£17.96 to £61.45 | |||||
| Other treatments | £13.74 (£64.71); 215 | £24.22 (£108.51); 224 | –£11.66 | –£28.92 to £5.60 | |||||
| Total NHS costs | £1642.73 (£1302.29); 207 | £1344.68 (£754.37); 217 | £292.41 | £67.66 to £517.17 | |||||
| QALYsb,c | |||||||||
| EQ-5D | |||||||||
| Baseline | 0.738 (0.221); 264 | 0.781 (0.178); 263 | |||||||
| 6 monthsd | 0.855 (0.211); 225 | 0.880 (0.188); 243 | |||||||
| 12 monthsd | 0.871 (0.187); 225 | 0.886 (0.187); 235 | |||||||
| Total QALYs | 0.845 (0.158); 198 | 0.866 (0.140); 209 | 0.006 | –0.016 to 0.028 | |||||
| Cost-effectiveness | |||||||||
| N | ΔC (95% CI) | ΔE (95% CI) | ICERe | Probability that uterine preservation is cost-effective compared with vaginal hysterectomy at different WTP thresholds for a QALY gained (%) | |||||
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| Imputed data set (base case)f | 562 | £235 (£6 to £464) | –0.004 (–0.026 to 0.019) | Dominated | 2 | 5 | 10 | 15 | 20 |
| Imputed data set (SUR) | 562 | £235 (£41 to £429) | –0.002 (–0.025 to 0.021) | Dominated | 0 | 3 | 9 | 14 | 19 |
| Wider-perspective costing analysis | 562 | £770 (£23 to £1517) | –0.004 (–0.026 to 0.019) | Dominated | 2 | 2 | 4 | 5 | 8 |
| Use of HRG tariffs for intervention procedureg,h | 534 | –£395 (–£619 to –£171) | –0.004 (–0.026 to 0.019) | £98,750 | 100i | 100i | 94i | 85i | 70i |
| Assumptions regarding QALY calculation (1) | 545 | £235 (£6 to £464) | –0.003 (–0.025 to 0.020) | Dominated | 1 | 13 | 32 | 43 | 54 |
| Assumptions regarding QALY calculation (2) | 481 | £235 (£6 to £464) | –0.000 (–0.024 to 0.024) | Dominated | 1 | 10 | 25 | 34 | 42 |
| Assumptions regarding QALY calculation (3) | 378 | £235 (£6 to £464) | –0.031 (–0.071 to 0.009) | Dominated | 1 | 0 | 1 | 1 | 2 |
| Complete-case analysisi | 372 | £292 (£64 to £520) | 0.004 (–0.026 to 0.033) | £71,250 | 0 | 7 | 20 | 31 | 40 |
| Complete-case analysis, SUR | 372 | £297 (£90 to £505) | –0.002 (–0.024 to 0.020) | Dominated | 0 | 6 | 19 | 30 | 40 |
Complete data were available across domains and time points, enabling QALY calculation for 198 out of 279 (71%) and 209 out of 283 (74%) women in the uterine preservation and vaginal hysterectomy groups, respectively. There were no differences in QALYs between the uterine preservation (0.845 years) and vaginal hysterectomy (0.866 years) groups (MD 0.006 years, 95% CI –0.016 to 0.028 years). Multiple imputation of missing EQ-5D data underpinning the QALY calculation indicates a similar lack of difference across the groups.
Cost-effectiveness results
Complete-case cost and complete-case QALY data are presented (Table 18), making best use of all available data. For the base-case analysis, multiple imputed missing data are used because only 372 out of 562 (66%) participants had fully complete profiles of costs and QALYs to enable calculation of the ICER. A range of deterministic sensitivity analyses are also reported. All analyses of cost-effectiveness also indicate the probability that uterine preservation is a cost-effective use of NHS resources at five alternative threshold values (£0, £10,000, £20,000, £30,000 and £50,000) of WTP for a QALY gained.
Base-case analysis
The base-case analysis using the imputed data set shows significant additional costs for the uterine preservation group (MD £235; 95% CI £6 to £464), for no significant difference in QALYs (MD –0.004 years, 95% CI –0.026 to 0.019 years). As uterine preservation is associated with additional costs for a negative point estimate of the impact on QALYs, uterine preservation is therefore dominated by vaginal hysterectomy. The scatterplot (Figure 6) illustrates this finding visually. There is little uncertainty regarding the additional cost of uterine preservation, with almost all of the increments from the bootstrap lying above the horizontal line. However, regarding QALYs, the scatterplot indicates substantial uncertainty, with no clear evidence of one strategy being preferable.
FIGURE 6.
Scatterplot of the cost-effectiveness plane (imputed data set).
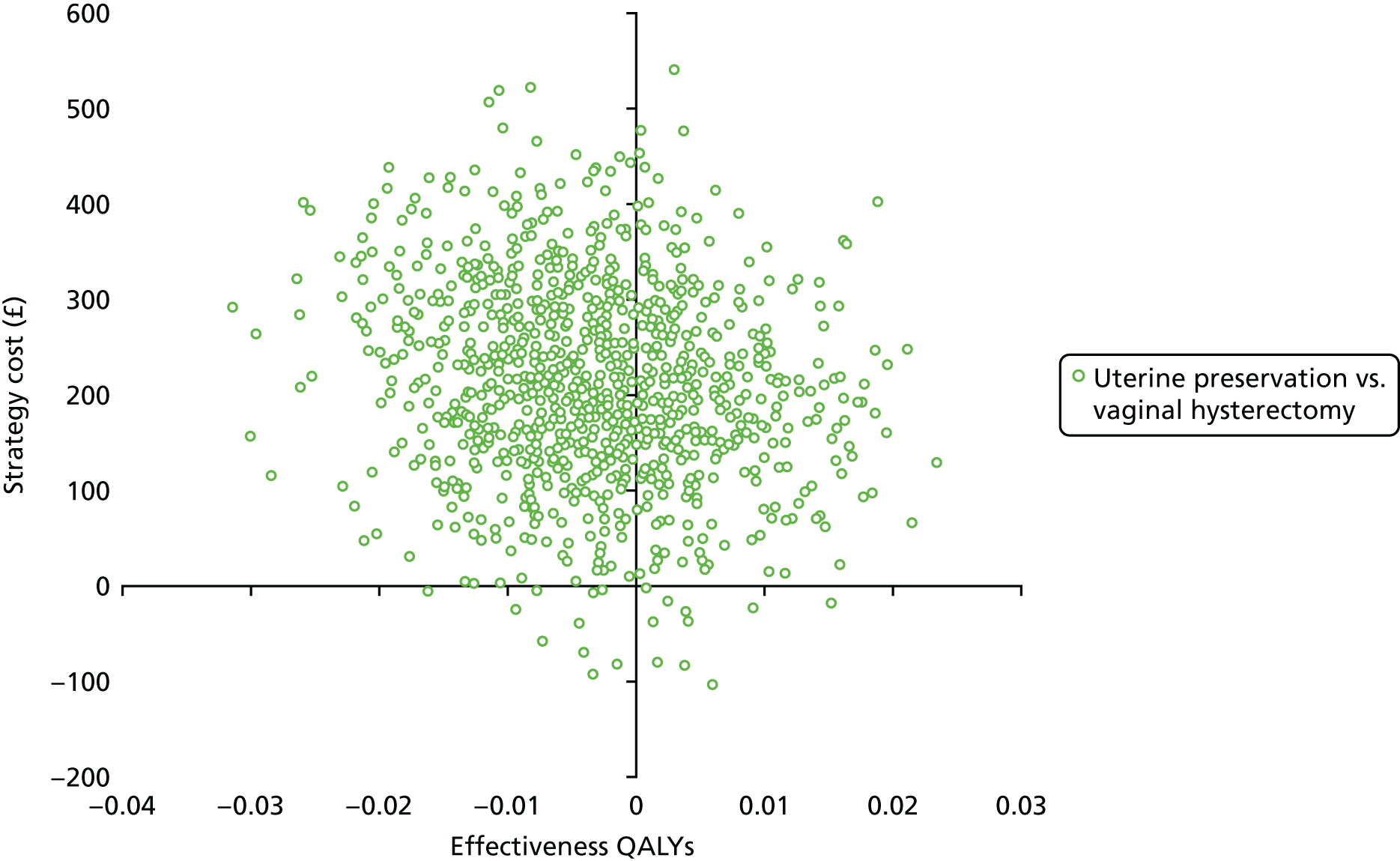
The CEAC (Figure 7) shows that, based on the NMB, there is a high probability that vaginal hysterectomy is the most cost-effective treatment option, with little evidence to support the cost-effectiveness of uterine preservation. This is driven by the additional costs of delivering the uterine preservation intervention, and the higher re-operation rate, for no QALY benefit. The probability of uterine preservation being considered to be cost-effective is 15% if society is willing to pay up to £30,000 for 1 QALY gained. A full set of scatterplots and CEACs for each analysis undertaken can be found in Appendix 4, Figures 22–9.
FIGURE 7.
Cost-effectiveness acceptability curve (imputed data set).

Deterministic sensitivity analyses
The study has produced a range of additional deterministic sensitivity analyses to address areas of uncertainty in assumptions, data collection and analysis modelling approaches. All deterministic analyses are based on the imputed data set unless otherwise stated (e.g. complete-case analysis). See Chapter 2 for the rationale behind each of the analyses. A number of observations have been made on the results.
First, the findings are not sensitive to the type of model used in the imputed data set with very similar results for the base case and a sensitivity analysis using seemingly unrelated regression, in which the probability of cost-effectiveness at a threshold of £30,000 per QALY gained reduces from 15% (base case) to 14%. Additionally, it is reassuring that the results are not sensitive to missing data assumptions, with similar findings for both the imputed and the complete-case analyses. The probability of uterine preservation being cost-effective at a threshold of £30,000 per QALY gained remains < 20% for both the imputed and the complete-case scenarios.
Second, the estimates of incremental costs are highly sensitive to the approach taken to intervention costing. The base-case component costing approach estimated additional costs because of the use of mesh and the slightly longer (around 10 minutes) operation time. However, when mapping Office of Population, Censuses and Surveys (OPCS) codes onto HRGs, using national reference cost spell-based tariffs, uterine preservation attracts a lower cost compared with vaginal hysterectomy. The use of a HRG approach to costing the intervention means that total costs to the NHS over follow-up are lower in the uterine preservation group, MD –£395; 95% CI –£619 to –£171. Considering that cost savings may be achieved for marginal (non-significant) reductions in QALYs, the base-case ICER is £98,750. The commonly accepted maximum price society would be willing to pay to get one additional QALY of benefit is between £20,000 and £30,000. The threshold could also be interpreted as the maximum amount of money that would need to be freed up in the NHS to compensate for a QALY loss. Using a HRG-based costing approach indicates that society would save £98,750 for every QALY lost, indicating a cost-effective use of resources. The probability that uterine preservation is cost-effective increases from 15% in the base case to 85% under this scenario, illustrating that the results are highly sensitive to the choice of intervention costing approach taken.
Third, because of differences in time points for questionnaire triggers (i.e. at 6 months after surgery and at12 months after randomisation) and the potential for comparatively long periods of time to elapse between randomisation and surgery, it is possible that respondents received their 12-month questionnaire before their 6-month questionnaire. The sensitivity analyses show that estimates of cost-effectiveness are not sensitive to any of the following assumptions in sensitivity analysis:
-
an analysis dropping the 6-month EQ-5D data, if the 6-month questionnaire is returned on or after the 12-month date
-
an analysis dropping any 6-month EQ-5D data within 3 months of the 12-month data
-
an analysis using the exact date of questionnaire report as the measure of time used in the calculation of QALYs.
Finally, the incorporation of a wider-perspective costing analysis, including both NHS and participant-incurred and reported costs, does not alter the estimates of cost-effectiveness. The probability that uterine preservation is cost-effective remains very low (5%) at a threshold of £30,000 per QALY.
Discussion
The findings of the economic evaluation indicate that uterine preservation is unlikely to be cost-effective compared with vaginal hysterectomy over a 12-month time horizon. Although the findings are unfavourable for uterine preservation, there are a number of factors that need to be considered when arriving at a decision on cost-effectiveness. First, the cost-effectiveness results are sensitive to the costing approach taken for the intervention. Arguments can be made for the use of either a component-costing or HRG approach. Component costing is chosen as the base case because it is grounded in the economic concepts of resource scarcity and opportunity cost. Therefore, component costing more accurately reflects the resources displaced for each surgery, and explicitly accounts for differences in operation times, length of stay and materials costs (e.g. mesh implants) between the groups observed in the trial. However, the HRG costing approach is more reflective of the way in which hospitals are paid for carrying out procedures, and it could be argued that HRGs reflect the true cost outlay to the NHS under current payment mechanisms. The disadvantage of the HRG approach is that it groups many similar surgeries together. It does not directly consider the opportunity cost of consultant time, etc. for these specific procedures. That is because HRGs are not based on the intricate level of detail that can be obtained from the trial data. Although the choice of intervention costing has a substantial impact on overall costs and cost-effectiveness for the trial-based analysis, the choice may be less influential in determining lifetime cost-effectiveness from the decision model (see Chapter 2).
Second, the validity of any policy recommendations based solely on the within-trial economic evaluation could be correctly criticised because the time horizon is very short. Indeed, it is highly unlikely that a 12-month time horizon is sufficient to capture the important costs and QoL impact of different surgical treatments. The economic modelling (see Chapter 9) aims to address this concern by extrapolating the trial results over a woman’s lifetime. Additionally, it is planned to follow-up women for a longer period to get a better understanding of the costs and the outcomes associated with treatment. These data will be used to validate and update the trial and economic modelling results in the future.
Chapter 6 Baseline characteristics: Vault trial
Between January 2013 and January 2017, 544 women were identified as potential participants for the Vault trial. 61
This chapter describes how participating women were identified from the women identified for vault prolapse surgery in UK hospitals (see Appendix 5, Table 66), 36 of which recruited women to the Vault trial of the VUE trial. Chapter 6 reports the baseline characteristics up to the point of entry to the Vault trial of the VUE trial. The subsequent findings are described in Chapters 7 and 8.
Study recruitment
The trial outline and methodology for recruitment have been described previously31 (see Chapter 2). Women who attended gynaecology outpatient clinics with symptomatic vault POP and then chose to have surgery, and women on the waiting list for vault prolapse surgery, were invited to participate in the Vault trial of the VUE trial. Women were asked if they were willing to be randomised to either a vaginal or abdominal vault procedure. The centres and surgeons who participated in the VUE trial, the numbers they recruited and the rate of recruitment are detailed in Appendix 5 (Table 66 and Figure 30).
Non-recruited women
Of the 544 women approached regarding trial participation, 335 women did not enter the study because they declined (n = 211), were ineligible (n = 115) or not timely identified or seen (n = 9) (see Appendix 5, Tables 67 and 68). Reasons for declining to participate included decided against surgery (n = 58, 27.5%), did not want mesh (n = 8, 3.8%) and preference for the route of surgery (n = 85, 40.3%), with more women preferring an abdominal (n = 53, 25.1%) rather than a vaginal procedure (n = 32, 15.2%). Clinical reasons for non-participation included ‘specific operation is necessary’ (n = 73, 63.5%) and ‘unsuitable due to medical history’ (n = 21, 18.3%).
Recruited women: baseline characteristics
The baseline characteristics of the 208 women included in the Vault trial are described in Table 19 (see also Appendix 5, Table 69).
| Characteristics | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Age (years), mean (SD); N | 65.1 (8.0); 104 | 66.4 (8.3); 104 |
| BMI (kg/m2), mean (SD); N | 28.1 (4.0); 87 | 27.8 (3.7); 86 |
| BMI category, n (%) | ||
| Underweight | 1 (1.0) | 0 (0) |
| Normal weight | 17 (16.3) | 17 (16.3) |
| Overweight | 42 (40.4) | 49 (47.1) |
| Obese | 19 (18.3) | 18 (17.3) |
| Morbidly obese | 8 (7.7) | 2 (1.9) |
| Missing | 17 (16.3) | 18 (17.3) |
| Parity, median (P25–75);a n | 2 (2–3); 101 | 2 (2–3); 104 |
| Number of normal vaginal deliveries, n (%); N | 2.4 (1.2); 100 | 2.5 (1.2); 101 |
| Previous conservative treatment, n (%) | ||
| Physiotherapy for prolapse | 38 (36.5) | 24 (23.1) |
| Vaginal pessary | 24 (23.1) | 18 (17.3) |
| Physiotherapy for urinary incontinence | 16 (15.4) | 11 (10.6) |
| Drugs for UI | 11 (10.6) | 9 (8.7) |
| Previous surgery, n (%) | ||
| Vaginal hysterectomy | 58 (55.8) | 56 (53.8) |
| For prolapse | 41 (39.4) | 35 (33.7) |
| Other reason | 8 (7.7) | 9 (8.7) |
| Reason unknown | 9 (8.7) | 12 (12.5) |
| Abdominal hysterectomy | 47 (45.2) | 49 (47.1) |
| For prolapse | 1 (1.0) | 4 (3.8) |
| Other reason | 32 (30.8) | 34 (32.7) |
| Reason unknown | 14 (13.5) | 11 (10.6) |
| Previous vault repair for prolapse | ||
| For prolapse | 3 (2.9) | 1 (1.0) |
| Other reason | 0 (0) | 1 (1.0) |
| Previous anterior repair | ||
| 1 | 28 (26.9) | 29 (27.9) |
| 2 | 4 (3.8) | 0 (0) |
| Previous posterior repair | ||
| 1 | 18 (17.3) | 14 (13.5) |
| 2 | 1 (1.0) | 0 (0) |
| Vaginal repair, but compartment unknown | 4 (3.8) | 6 (5.8) |
| Previous continence surgery | 10 (9.6) | 5 (4.8) |
Epidemiological characteristics
There was no difference between the randomised groups in respect of age, BMI, parity or delivery mode history [with the majority of women having a normal vaginal delivery (see Appendix 5, Table 69)].
Previous conservative treatment
Around one-quarter to one-third (29.8%) of women had PFMT supervised by a physiotherapist for prolapse symptoms and around one in five (20.2%) women had previously used a vaginal pessary (ring or other type) before surgery (Table 19).
Overall, around 1 in 10 women (13.0%) had previous supervised PFMT or used drug treatment (9.7%) for urinary incontinence.
Previous surgery
All women in the Vault trial had a previous hysterectomy. Slightly more women had had a vaginal hysterectomy (54.8%) than an abdominal hysterectomy (46.2%) (Table 19). Of those women with a previous vaginal hysterectomy, in just over one-third (36.6%) of the women this was for prolapse symptoms. A previous abdominal hysterectomy was rarely undertaken for prolapse reasons and was most likely due to ‘other reasons’ (31.8%), for example menorrhagia or fibroids. The reason for a previous hysterectomy (vaginal or abdominal) was unknown in around 12% of women (Table 19).
In four women, their VUE vault repair was a repeat vault procedure, having already had a previous vault repair (three of which were for prolapse and one for ‘other reasons’).
Just over one-quarter (27.4%) of all women had previously had an anterior repair and four women (3.8%) had two previous anterior repairs. Fewer women (approximately 15%) had a previous posterior repair, and one woman had two previous posterior repairs. Previous vaginal repair was noted in an additional 10 women, but the compartment of previous surgery was unknown (see Table 19).
Overall, approximately 7% of women had undergone previous continence surgery.
Prolapse symptoms at baseline
Women in the Vault trial had been symptomatic for approximately 3 years, and were bothered by their symptoms for approximately 2 years. The overall mean POP-SS was 15 out of 28 (and ranged from 1 to 28). Using a POP-SS of > 0 to indicate presence of symptoms, 100% of women who completed these questions had at least one symptom (this section was missing for seven women). The effect on QoL score (‘overall, how much do your prolapse symptoms interfere with your everyday life?’) was high and ranged from 0 to 10 out of 10, with a mean of 7 out of 10 (Table 20).
| Symptom | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Duration of symptoms (years), mean (SD); N | 3.7 (4.8); 95 | 2.4 (2.4); 98 |
| Duration of bother (years), mean (SD); N | 2.5 (3.9); 95 | 1.9 (2.4); 95 |
| POP-SS at baseline, mean (SD); N | 15.1 (6.5); 101 | 14.8 (5.7); 100 |
| Number of women symptomatic, mean (SD); N | 101 (97.1) | 100 (96.2) |
| Prolapse-related effect on QoL score, mean (SD); N | 7.1 (2.6); 100 | 7.0 (2.6); 103 |
| Individual prolapse symptoms, n (%) | ||
| Something coming down (any) | 98 (94.2) | 99 (95.2) |
| Something coming down (most/all of the time) | 81 (77.9) | 83 (79.8) |
| Bladder not empty (any) | 90 (86.5) | 94 (90.4) |
| Bladder not empty (most/all of the time) | 44 (42.3) | 43 (41.3) |
| Uncomfortable feeling or pain when standing (any) | 89 (85.6) | 92 (88.5) |
| Uncomfortable feeling or pain when standing (most/all of the time) | 50 (48.1) | 51 (49.0) |
| Dragging in abdomen (any) | 81 (77.9) | 86 (82.7) |
| Dragging in abdomen (most/all of the time) | 45 (43.3) | 40 (38.5) |
| Bowel not empty (any) | 81 (77.9) | 84 (80.8) |
| Bowel not empty (most/all of the time) | 27 (26.0) | 27 (26.0) |
| Strain to empty bladder (any) | 74 (71.2) | 76 (73.1) |
| Strain to empty bladder (most/all of the time) | 33 (31.7) | 32 (30.8) |
| Dragging in back (any) | 73 (70.2) | 75 (72.1) |
| Dragging in back (most/all of the time) | 37 (35.6) | 23 (22.1) |
| Most bothersome symptom | ||
| Something coming down | 48 (46.2) | 50 (48.1) |
| Uncomfortable feeling or pain when standing | 12 (11.5) | 19 (18.3) |
| Bladder not empty | 11 (10.6) | 8 (7.7) |
| Dragging in abdomen | 7 (6.7) | 5 (4.8) |
| Strain to empty bladder | 6 (5.8) | 6 (5.8) |
| Bowel not empty | 5 (4.8) | 6 (5.8) |
| Dragging in back | 4 (3.8) | 4 (3.8) |
| Symptoms cause most bother not applicable | 3 (2.9) | 2 (1.9) |
| Missing | 11 (10.6) | 6 (5.8) |
| Actions necessitated by prolapse symptoms | ||
| Extra hygiene measures | 34 (32.7) | 42 (40.4) |
| Fingers to ease discomfort | 17 (16.3) | 16 (15.4) |
| Fingers to help empty bladder | 5 (4.8) | 1 (1.0) |
| Fingers to help empty bowel | 3 (2.9) | 0 (0) |
| Digitally evacuate bowel | 2 (1.9) | 5 (4.8) |
| Quality of life, mean (SD); count | ||
| EQ-5D | 0.726 (0.209); 95 | 0.744 (0.197); 101 |
| EQ-5D visual scale | 74.8 (18.0); 99 | 73.4 (17.5); 103 |
The most common individual prolapse symptom was ‘a feeling of something coming down from or in your vagina’, reported in 94.7% of women. This was reported ‘most or all of the time’ in over three-quarters (78.9%) and was the most bothersome symptom for almost half (47.2%) of the women.
Over one-third (36.6%) of women found the prolapse caused hygiene problems, and 15.9% needed to relieve pressure or discomfort from the prolapse using their fingers (Table 20).
Generic quality of life
The mean score for generic QoL and visual scales (EQ-5D) were 0.735 and 74.1 points, respectively.
Preoperative objective measurements
Over one-third (38.9%) of women had a stage 0 or 1 vault prolapse. Using a more clinically meaningful definition of leading edge of prolapse beyond the hymen (POP-Q stage 2b or more), 91.4% of women had an overall objective prolapse (see Appendix 5, Table 70), and 39.4% had an objective vault prolapse [stages 2b or more (Table 21)]. The stage of vault prolapse was missing in 3.9% of women.
| Prolapse stage | Treatment, n (%) | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Overall stage 2b or more | 92 (88.5) | 98 (94.2) |
| Vault stage | ||
| 0 | 3 (2.9) | 5 (4.8) |
| 1 | 38 (36.5) | 35 (33.7) |
| 2a | 19 (18.3) | 18 (18.3) |
| 2b | 19 (18.3) | 15 (14.4) |
| 3 | 21 (20.2) | 23 (22.1) |
| 4 | 1 (1.0) | 3 (2.9) |
| Missing | 3 (2.9) | 5 (4.8) |
| Stage 2b or more | 41 (39.4) | 41 (39.4) |
| Anterior prolapse stage 2b or more | 86 (82.7) | 81 (77.9) |
| Posterior prolapse stage 2b or more | 51 (49.0) | 58 (55.8) |
More women had an objective (stage 2b or more) anterior (80.3%) than a posterior (52.4%) prolapse (Table 21).
Planned concomitant surgery
Planned surgery was based on preoperative findings on clinical examination (Table 22). The VUE trial was designed so that women would remain in the group to which they were allocated, irrespective of the actual procedure performed. In order to randomise women appropriately, taking account of minimisation criteria, gynaecologists were asked to specify in advance which concomitant surgery would also be necessary.
| Planned concomitant surgery | Treatment, n (%) | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| None | 30 (28.8) | 32 (30.8) |
| Anterior repair | 32 (30.8) | 36 (34.6) |
| Anterior plus posterior repair | 24 (23.1) | 21 (20.2) |
| Posterior repair | 14 (13.5) | 14 (13.5) |
| Enterocele repair | 7 (6.7) | 4 (3.8) |
| Concomitant UI surgery | 3 (2.9) | 3 (2.9) |
All women were expected to undergo a vault prolapse procedure from the preoperative clinical planning. A concomitant anterior prolapse repair was planned more frequently (32.7%) than either posterior repair (13.5%) or both anterior and posterior repair (21.7%). Surgery for incontinence was rarely planned (2.9%).
Planned procedures were equally distributed between the two randomised groups.
Vaginal and sexual symptoms at baseline
Vaginal and sexual symptoms were measured using the ICI-validated instruments36 (Table 23) (www.journalslibrary.nihr.ac.uk/programmes/hta/11129183/#/; accessed February 2019). These were common and had important effects on QoL. The majority of women were not sexually active (72.6%) and for about one-quarter of women this was most often because of their prolapse symptoms. No women reported dyspareunia at baseline.
| Symptom | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| ICI Vaginal Symptoms Score, mean (SD); n | 23.9 (9.5); 87 | 24.4 (9.5); 93 |
| QoL due to vaginal symptoms, mean (SD); n | 5.6 (3.1); 95 | 5.9 (3.1); 100 |
| ICI-Sexual Matters Score, mean (SD); n | 26.1 (17.3); 28 | 25.7 (17.4); 29 |
| QoL due to effect on sex life, mean (SD); n | 4.7 (3.1); 28 | 5.6 (3.1); 29 |
| Vagina too loose or lax, n (%) | 18 (17.3) | 16 (15.4) |
| Reduced sensation, n (%) | 4 (3.8) | 8 (7.7) |
| Number having intercourse, n (%) | 28 (26.9) | 29 (27.9) |
| Pain with intercoursea – most or all of the time | 0 (0.0) | 0 (0.0) |
| Reasons for not being sexually active, n (%) | ||
| Prolapse symptoms | 26 (25.0) | 27 (26.0) |
| No partner | 24 (23.1) | 19 (18.3) |
| Vaginal symptoms | 2 (1.9) | 3 (2.9) |
| Other reason | 15 (14.4) | 17 (16.3) |
| Reason not given | 37 (35.6) | 38 (36.5) |
Urinary and bowel symptoms at baseline
Urinary symptoms also had some impact on QoL (the overall mean QoL due to urinary symptoms was 4/10) (Table 24). Around one in six (16.8%) of all women reported at least some urinary incontinence, as measured using the ICIQ-UI SF;36 however, this was slight or moderate in the majority (79.3%) of cases (Appendix 5, Table 71). Urgency was the most common type of urinary symptom, reported in around 28.9% of all women. Women were counted as symptomatic if they had the symptom ‘most or all of the time’.
| Symptom | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Urinary symptoms, n (%) | ||
| Urgency | 32 (30.8) | 28 (26.9) |
| Nocturia | 26 (25.0) | 27 (26.0) |
| Frequency | 13 (12.5) | 8 (7.7) |
| Any incontinence, n (%) | 20 (19.2) | 15 (14.4) |
| Severe | 2 (1.9) | 1 (1.0) |
| Incontinence-related Qol score, mean (SD); N | 4.1 (3.3); 88 | 4.0 (3.4); 91 |
| ICI-UI score, mean (SD); N | 8.1 (5.1); 85 | 7.8 (5.1); 89 |
| ICIQ-FLUTS filling score, mean (SD); N | 5.7 (2.7); 98 | 5.5 (2.7); 101 |
| ICIQ-FLUTS voiding symptoms score, mean (SD); N | 3.5 (2.9); 102 | 3.4 (2.4); 103 |
| ICIQ-FLUTS incontinence score, mean (SD); N | 5.5 (3.5); 87 | 5.1 (3.2); 93 |
| Bowel symptoms | ||
| Bowel frequency, n (%) | ||
| Normal | 93 (89.4) | 98 (94.2) |
| Frequent | 3 (2.9) | 1 (1.0) |
| Infrequent | 6 (5.8) | 1 (1.0) |
| Missing | 2 (1.9) | 4 (3.8) |
| Faecal incontinence (occasionally or more often), n (%) | 39 (37.5) | 40 (38.5) |
| Passive | 14 (13.5) | 5 (4.8) |
| Active | 25 (24.0) | 35 (33.7) |
| Severe | 17 (16.3) | 8 (7.7) |
| Bowel urgency (most or all of the time), n (%) | 16 (15.4) | 5 (4.8) |
| Constipation (most or all of the time), n (%) | 11 (10.6) | 6 (5.8) |
| Bowel symptoms QoL score, mean (SD); N | 3.1 (3.3); 98 | 3.1 (2.9); 101 |
Over one-third (38.0%) of all women had faecal incontinence at least occasionally [defined as loss of solid or liquid stool, but not including loss of flatus (wind)], and this was severe in around 12.0% of women. Few women (8.2%) reported constipation.
Discussion
Summary of findings
The participating women in the Vault trial were around 66 years old, with a median of two babies. Their mean BMI was around 28 kg/m2 (though eight women in the abdominal group and two in the vaginal group were morbidly obese and did receive surgery). All women had a previous hysterectomy (abdominal or vaginal), with more having had a previous vaginal hysterectomy for prolapse symptoms than for other reasons. Those women with a previous abdominal hysterectomy were more likely to have had this for non-prolapse reasons.
Women were more likely to have had a previous anterior than posterior repair, and few (7%) had a previous incontinence procedure (see Table 19).
Prolapse symptoms and measurements
Women had POP-SSs of around 15 out of a maximum score of 28. The most common symptom was a feeling of something coming down, reported by 94.7%, which was most bothersome in 47.2% of women (see Table 20).
When prolapse was redefined as the leading edge beyond the hymen (i.e. stage 2b or more), most (91.3%) women had an overall protruding prolapse and 39.4% had a vault prolapse specifically (see Table 21).
In the study it was possible to ascribe a prolapse stage to 96% of women at baseline. It is difficult to explain why a small minority of women appeared not to have significant vault prolapse (i.e. stage 0 or 1) yet were listed for vault surgery (see also Chapter 3 for reasons for this).
The majority of women (70.2%) were also expected to undergo a concomitant prolapse in another compartment (anterior, posterior or both) at the same time (see Table 22). This was more likely to be an anterior repair (32.7%) rather than a posterior repair (13.5%).
Other clinical symptoms
Vaginal and sexual symptoms were common and impacted on QoL (see Table 23). Just over one-quarter (27.4%) of the women were sexually active at baseline and none reported dyspareunia (see Table 23). Urgency (28.9%) and nocturia (25.5%) were common urinary symptoms. Just under one-fifth of women had urinary incontinence, and this was severe in 1.5% of women (see Table 24). Few women were expected to undergo concomitant continence surgery or had already undergone previous continence surgery. Over one-third (38%) of women had faecal incontinence, at least occasionally, and this was severe in around 1 in 10 women (see Table 24).
The Vault trial was recruited alongside the Uterine trial, but the Vault trial was not powered in the same way. The Vault trial was considered more an opportunistic sample given the lower prevalence of vault to uterine prolapse in the population. As a result, any differences may appear larger because of the lower number of participants.
The findings in this chapter will serve as a benchmark for future research in women with a vault prolapse. The clinical messages regarding symptoms and clinical practice may be helpful in improving prolapse management in the UK and internationally.
Chapter 7 Vault trial results
This chapter reports the outcomes for women who participated in the Vault trial at 12 months.
The flow of women through the trial is shown in the CONSORT flow diagram (Figure 8), in line with CONSORT recommendations. 61
FIGURE 8.
The CONSORT flow diagram of women recruited to the Vault trial of the VUE study.

There was one post-randomisation exclusion (further review concluded that this woman had a previous subtotal hysterectomy, which was an exclusion criteria) not included in the study analyses, leaving 208 randomised women analysed in the Vault trial.
The women were recruited in 45 centres across the UK (see Appendix 5, Table 66) and received surgery as shown in Figure 9.
FIGURE 9.
Treatment received diagram for the Vault trial: breakdown of the different surgeries received or no surgery actually received. a, One participant’s surgery status is unknown. UIS, urinary incontinence surgery.

Of the 208 women included, 175 received their randomised allocation (81.8% in the abdominal group vs. 86.5% in the vaginal group) and 11 crossed over (Figure 9) (Table 25). Reasons for not having the allocated treatment were that the vault procedure was no longer appropriate (n = 12), pelvic adhesions (n = 4), other clinical reasons (n = 10), woman’s choice (n = 3) or unknown reasons (n = 3).
| Surgery received | Treatment, n (%) | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Apical surgery received | ||
| Vaginal vault suspensiona | 7 (6.7) | 90 (86.5) |
| Abdominal suspension (laparoscopicb) | 61 (58.7) | 4 (3.8) |
| Abdominal suspension (openc) | 24 (23.1) | 0 (0) |
| No apical surgery | 11 (10.6) | 10 (9.6) |
| Unknown | 1 (1.0) | 0 (0) |
| Concomitant surgery received | ||
| None | 66 (63.5) | 21 (20.2) |
| Anterior repair | 11 (10.6) | 27 (26.0) |
| Posterior repair | 16 (15.4) | 24 (23.1) |
| Both (anterior and posterior) | 9 (8.7) | 28 (26.9) |
| Continence surgery | 3 (2.9) | 2 (1.9) |
A small number of women (n = 16) had no vault surgery (Figure 9) but did receive a prolapse procedure in a different compartment [anterior (n = 8), posterior (n = 6) or both an anterior and a posterior (n = 2)].
One woman reported that she had undergone surgery (the surgery type was not detailed), but this has not been verified with the recruiting centre.
The use of mesh for the apical surgery in the abdominal group (88.0%) was higher than in the vaginal group (6.4%). Concomitant surgery was around 4% overall (see Appendix 6, Table 72).
Description of surgical characteristics
Most procedures were performed by a consultant gynaecologist (87.5%), were performed under general anaesthesia (90.4%) and most patients received prophylactic antibiotics (91.8%) (Appendix 6, Table 73).
The duration of the operation was significantly longer in the abdominal group (146 mean duration, SD 54 minutes) than in the vaginal group (82 mean duration, SD 34 minutes; MD 60.48, 95% CI 49.80 to 71.16 minutes). There was no difference in blood loss (98 ml in the abdominal group vs. 108 ml in the vaginal group) or length of hospital stay (2.1 days in the abdominal group vs. 1.8 days in the vaginal group) between the groups (Appendix 6, Table 74).
There was no difference in time from the woman’s randomisation to her surgery between the groups (Appendix 6, Table 74).
Outcomes
Response rates and clinical attendance
Of the 104 women randomised to the abdominal group, 89 (85.6%) responded to the 12 months after randomisation questionnaire, and 88 (84.6%) out of the 104 participants randomised to the vaginal group responded. Clinical outcomes were collected at 12 months after surgery for 96 out of the 104 (92.3%) women in the abdominal group and 99 out of the 104 (95.1%) women in the vaginal group.
Women’s prolapse symptoms and effect on everyday life
At 6 months after surgery, women reported a reduction in their POP-SS (maximum score 28) from an overall mean score of 15 out of 28 at baseline to 5.3 out of 28 in the abdominal group and 6.1 out of 28 in the vaginal group (Table 26). The between-group difference was not statistically significant (MD –1.17; 95% CI –2.64 to 0.31). The effect of prolapse on QoL also improved at 6 months after surgery and there was no significant difference between the groups.
| Time point | Treatment, mean (SD); N | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| POP-SS | |||
| Baseline | 15.1 (6.5); 101 | 14.8 (5.7); 100 | |
| 6 months after surgery | 5.3 (5.5); 83 | 6.1 (5.5); 93 | –1.17 (–2.64 to 0.31); 0.12 |
| 12 months after randomisation | 5.6 (5.4); 88 | 5.9 (5.4); 86 | –0.61 (–2.08 to 0.86); 0.42 |
| Prolapse-related effect on QoL | |||
| Baseline | 7.1 (2.6); 100 | 7.0 (2.6); 103 | |
| 6 months after surgery | 2.5 (3.2); 84 | 2.9 (2.9); 93 | –0.56 (–1.41 to 0.30); 0.20 |
| 12 months after randomisation | 2.3 (3.0); 87 | 2.6 (2.8); 87 | –0.25 (–1.10 to 0.59); 0.56 |
The primary outcome was POP-SS at 12 months after randomisation; there was no statistically significant difference between the groups. The MD in the POP-SS for abdominal (5.6, SD 5.4) compared with vaginal (5.9, SD 5.4) adjusted for baseline variables was –0.61 (95% CI –2.08 to 0.86) (see Figure 10, and Appendix 6, Figure 31). The intracluster correlation for surgeon was 0.04 (95% CI 0.01 to 0.12).
FIGURE 10.
The mean and standard error POP-SSs at baseline and follow-up (6 months after surgery and 12 months after randomisation).

There was also no significant difference on the effect of prolapse on QoL at 12 months after randomisation [abdominal 2.3 (SD 3.0) vs. vaginal 2.6 (SD 2.8); MD –0.25, 95% CI –1.10 to 0.59].
The distribution of individual POP-SSs and prolapse-related effects on QoL are also given in Appendix 6, Figures 31 and 32.
Each individual prolapse symptom also improved at 12 months after randomisation (Table 27) and was already apparent at 6 months after surgery (see Appendix 6, Table 75).
| Symptoms | Treatment | |
|---|---|---|
| Abdominal vault | Vaginal vault | |
| Symptomatic, n (%); N | ||
| Number of women with symptoms | 78 (88.6); 88 | 75 (87.2); 86 |
| Individual prolapse symptoms, n (%); N | ||
| Bowel not empty (any) | 62 (69.7); 89 | 56 (64.4); 87 |
| Bowel not empty (most/all of the time) | 13 (14.6); 89 | 8 (9.2); 87 |
| Bladder not empty (any) | 54 (60.7); 89 | 54 (62.1); 87 |
| Bladder not empty (most/all of the time) | 11 (12.4); 89 | 8 (9.2); 87 |
| Strain to empty bladder (any) | 38 (43.7); 87 | 37 (42.5); 87 |
| Strain to empty bladder (most/all of the time) | 7 (8.0); 87 | 6 (6.9); 87 |
| Dragging in back (any) | 36 (41.4); 87 | 39 (45.3); 86 |
| Dragging in back (most/all of the time) | 6 (6.9); 87 | 7 (8.0); 86 |
| Something coming down (any) | 31 (34.8); 89 | 41 (47.1); 87 |
| Something coming down (most/all of the time) | 13 (14.6); 89 | 15 (17.2); 87 |
| Dragging in abdomen (any) | 29 (33.3); 87 | 32 (36.8); 87 |
| Dragging in abdomen (most/all of the time) | 2 (2.3); 87 | 7 (8.0); 87 |
| Uncomfortable feeling or pain when standing (any) | 28 (32.2); 87 | 32 (37.2); 86 |
| Uncomfortable feeling or pain when standing (most/all of the time) | 7 (8.1); 87 | 7 (8.1); 86 |
| Most bothersome symptom | ||
| Bowel not empty | 27 (45.8); 59 | 18 (32.1); 56 |
| Something coming down | 6 (10.2); 59 | 19 (33.9); 56 |
| Bladder not empty | 14 (23.7); 59 | 6 (10.7); 56 |
| Strain to empty bladder | 4 (6.8); 59 | 3 (5.4); 56 |
| Uncomfortable feeling or pain when standing | 3 (5.4); 59 | 4 (7.1); 56 |
| Dragging in back | 4 (6.8); 59 | 2 (3.6); 56 |
| Dragging in abdomen | 1 (1.7); 59 | 4 (7.1); 56 |
| Which symptom causes most bother not applicable | 24 (28.9); 83 | 23 (29.1); 79 |
| Actions necessitated by prolapse symptoms | ||
| Extra hygiene measures | 6 (7.3); 82 | 6 (7.1); 84 |
| Fingers to ease discomfort | 3 (3.7); 82 | 0 (0); 84 |
| Fingers to help empty bowel | 3 (3.5); 85 | 0 (0); 85 |
| Digitally evacuate bowel | 1 (1.1); 87 | 2 (2.3); 87 |
| Fingers to help empty bladder | 1 (1.2); 85 | 0 (0); 87 |
| Quality of life, mean (SD); count | ||
| EQ-5D | 0.826 (0.217); 82 | 0.823 (0.175); 86 |
| EQ-5D visual scale | 78.2 (16.9); 83 | 77.5 (17.0); 85 |
The most bothersome symptoms across both treatment groups at both 6 months after surgery and 12 months after randomisation were different from those at baseline. The most bothersome symptom at baseline, ‘a feeling of something coming down’, reduced overall from 47.2% to 10% in the abdominal versus 34% in the vaginal group. Symptoms of incomplete emptying of either bladder or bowel became more common, but ‘actions necessitated by prolapse symptoms’ were low (see Table 27, see also Appendix 6, Table 75).
Secondary outcomes
Objective prolapse outcomes
Women who had any prolapse procedure were invited back for a clinical assessment at 12 months after surgery and, overall, 93.7% attended. The objective prolapse measure (i.e. POP-Q) improved in all compartments (Table 28).
| Compartment stage | Treatment, n (%); N | Effect size,a OR (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| Overall stage 2b or more | 28 (32.6) 86 | 38 (46.9) 81 | 0.50 (0.25 to 1.02); 0.06 |
| Apical stage | |||
| Stage 0 | 68 (81.0); 84 | 54 (69.2); 78 | |
| Stage 1 | 11 (13.1); 84 | 14 (17.9); 78 | |
| Stage 2a | 0 (0); 84 | 3 (3.8); 78 | |
| Stage 2b | 4 (4.8); 84 | 4 (5.1); 78 | |
| Stage 3 | 0 (0); 84 | 1 (1.3); 78 | |
| Stage 4 | 1 (1.2); 84 | 2 (2.6); 78 | |
| Stage 2b or more | 5 (6.0); 84 | 7 (9.0); 78 | 0.61 (0.18 to 2.08); 0.43 |
| Anterior stage 2b or more | 21 (24.4); 86 | 33 (40.7); 81 | 0.38 (0.18 to 0.79); 0.01 |
| Posterior stage 2b or more | 10 (11.6); 86 | 11 (13.6); 81 | 0.71 (0.27 to 1.85); 0.48 |
| Development of new prolapse at another site | 4 (4.4); 90 | 8 (9.2); 87 | 0.45 (0.13 to 1.56); 0.21 |
The objective overall prolapse stage 2b or more fell from 91.4% to 32.6% in the abdominal and 46.9% in the vaginal group, and this was not significant between the groups (OR 0.50, 95% CI 0.25 to 1.02; see Appendix 6, Table 76). More specifically, those with an objective vault prolapse (Stage 2b or more) fell from 39.4% to 6.0% in the abdominal group compared with 9.0% in the vaginal group after surgery (adjusted for baseline values); this was not significant (OR 0.61, 95% CI 0.18 to 2.08). Objective anterior prolapse stage 2b or more was found in 24.4% of the abdominal group and 40.7% of the vaginal group and this was statistically significant (OR 0.38, 95% CI 0.18 to 0.79). Objective posterior prolapse also improved, with no difference between the treatment groups (Table 28).
Development of new (de novo) prolapse at another site (anterior or posterior) was seen in a few cases and there was no evidence of a difference between the randomised groups (four in the abdominal group vs. eight in the vaginal group; OR 0.45, 95% CI 0.13 to 1.56).
Vaginal and sexual symptoms
There was no difference between the groups in terms of vaginal and sexual symptoms (Table 29). Both the mean vaginal symptoms and the QoL scores decreased (improved) for both groups. Slightly more women were sexually active after surgery (from around 27.4% at baseline to 36.9% in the abdominal group and 38.4% in the vaginal group), and fewer women cited prolapse symptoms as a reason for not being sexually active. From those women who were not sexually active, six (14.0%) in the abdominal and seven (17.7%) in the vaginal group gave vaginal or prolapse symptoms as the reason.
| Symptoms | Treatment | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| ICI Vaginal Symptoms Score, mean (SD); N | 8.9 (9.9); 75 | 8.4 (8.7); 79 | 0.69 (–1.89 to 3.26); 0.60 |
| QoL because of vaginal symptoms, mean (SD); N | 1.6 (2.4); 79 | 1.8 (2.3); 80 | –0.10 (–0.81 to 0.60); 0.78 |
| ICI-Sexual Matters Score, mean (SD); N | 8.8 (10.4); 31 | 14.5 (16.4); 32 | –5.13 (–13.33 to 3.07); 0.22 |
| QoL because of effect on sex life, n (%); N | 2.1 (2.7); 31 | 2.7 (3.2); 32 | –0.46 (–2.13 to 1.20); 0.59 |
| Reduced sensation, n (%); N | 4 (4.9); 82 | 2 (2.4); 82 | |
| Vagina too loose or lax, n (%); N | 3 (3.6); 83 | 2 (2.4); 83 | |
| Number having intercourse, n (%); N | 31 (36.9); 84 | 33 (38.4); 86 | |
| Pain with intercourse | 1 (3.2); 31 | 0 (0); 33 | |
| Reasons for not being sexually active, n (%); N | |||
| No partner | 21 (48.8); 43 | 17 (41.5); 41 | |
| Prolapse symptoms | 4 (9.3); 43 | 7 (17.7); 41 | |
| Vaginal symptoms | 2 (4.7); 43 | 0 (0); 41 | |
| Other reason | 16 (37.2); 43 | 17 (41.5); 41 | |
There were no statistically significant differences in vaginal and sexual symptoms between the abdominal and vaginal groups at 12 months after randomisation.
Urinary and bowel symptoms
Overall, at 12 months after randomisation the proportion of women who had any urinary incontinence increased from 16.8% at baseline to 30.8% in the abdominal versus 24.0% in the vaginal group [and this was not significantly different (OR 1.39, 95% CI 0.68 to 2.86)] (Table 30). The proportion of women with severe urinary incontinence remained low.
| Symptoms | Treatment | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| Urinary symptoms, n (%); N | |||
| Urgency | 6 (6.9); 87 | 10 (11.6); 86 | 0.49 (0.17 to 1.47); 0.20 |
| Frequency | 5 (5.7); 87 | 5 (5.9); 85 | |
| Nocturia | 8 (9.2); 87 | 15 (17.4); 86 | |
| All of the above | 0 (0); 87 | 0 (0); 85 | |
| Any incontinence, n (%); N | 32 (37.6); 85 | 25 (29.1); 86 | 1.39 (0.68 to 2.86); 0.37 |
| Severe incontinence | 2 (2.8); 71 | 2 (2.6); 78 | 1.10 (0.15 to 8.03); 0.92 |
| De novo incontinence, n (%); N | 17 (25.4); 67 | 15 (21.1); 71 | 1.27 (0.57 to 2.85); 0.56 |
| Incontinence-related QoL score, mean (SD); count | 2.0 (2.7); 70 | 2.2 (2.5); 77 | –0.03 (–0.79 to 0.72); 0.93 |
| ICI UI Score, mean (SD); count | 5.1 (4.7); 68 | 5.7 (4.6); 74 | –0.42 (–1.77 to 0.94); 0.55 |
| ICIQ-FLUTS Filling Score, mean (SD); count | 3.7 (2.2); 87 | 3.9 (2.0); 83 | –0.28 (–0.83 to 0.28); 0.33 |
| ICIQ-FLUTS Voiding Symptoms Score, mean (SD); count | 1.8 (2.0); 86 | 1.7 (2.0); 87 | 0.05 (–0.47 to 0.58); 0.84 |
| ICIQ-FLUTS Incontinence Score, mean (SD); count | 3.9 (3.4); 71 | 4.3 (3.6); 78 | –0.58 (–1.55 to 0.39); 0.24 |
| Persistent incontinence, n (%); N | 14 (16.7); 84 | 9 (10.8); 83 | 1.67 (0.66 to 4.20); 0.28 |
| Bowel symptoms | |||
| Bowel frequency (normal), n (%); N | 82 (94.3); 87 | 83 (96.5); 86 | 0.84 (0.09 to 8.01); 0.88 |
| Faecal incontinence (any), n (%); N | 30 (35.3); 85 | 29 (33.7); 86 | 1.36 (0.57 to 3.26); 0.49 |
| Passive | 17 (19.8); 86 | 15 (18.1); 83 | |
| Active | 12 (14.0); 86 | 13 (15.7); 83 | |
| Severe | 9 (10.6); 85 | 9 (10.5); 86 | 0.55 (0.16 to 1.84); 0.33 |
| Constipation | 4 (4.6); 87 | 2 (2.3); 87 | 1.85 (0.32 to 10.65); 0.49 |
| Bowel urgency | 18 (22.2); 81 | 17 (20.5); 83 | 0.75 (0.33 to 1.74); 0.51 |
| Bowel symptoms QoL score, mean (SD); count | 2.0 (1.0); 87 | 1.8 (0.9); 87 | 0.22 (–0.04 to 0.47); 0.10 |
More women reported urge incontinence at 12 months after randomisation in the vaginal group than in the abdominal group, but this was not significantly different (MD 0.49, 95% CI 0.17 to 1.47). De novo urge incontinence following surgery was similar across both treatment groups and was reported in around one-quarter of the women.
There were no statistically significant differences between the treatment groups in terms of urinary or bowel symptoms at 12 months after randomisation (see Appendix 6, Table 77).
Satisfaction with treatment at 12 months after randomisation
There were no differences between the treatment groups in terms of satisfaction with treatment at 12 months after randomisation (Table 31).
| Recovery and satisfaction | Treatment group, n (%); N | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| Prolapse is better now compared with 1 year ago | 77 (92.8); 83 | 75 (86.2); 87 | 1.97 (0.69 to 5.60); 0.20 |
| Completely or fairly satisfied | 72 (87.8); 82 | 71 (82.6); 86 | 1.56 (0.63 to 3.86); 0.33 |
| Recommend to a friend | 69 (86.3); 80 | 73 (88.0); 83 | 0.84 (0.32 to 2.18); 0.72 |
Further treatment required for failure or adverse events at 6 and at 12 months
Most women reported that they felt better than before surgery (92.8% abdominal procedure vs. 86.2% vaginal procedure) and this was not statistically significant (OR 1.97, 95% CI 0.69 to 5.60) (Table 32). The proportions of women completely or fairly satisfied were also similar (87.8% abdominal procedure vs. 82.6% vaginal procedure; OR 1.56, 95% CI 0.63 to 3.86). The majority of women would recommend the surgery to a friend (86.3% abdominal procedure vs. 88.0% vaginal procedure).
| Further treatment | Treatment group | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault | Vaginal vault | ||
| Further surgery, n (%); N | 8 (7.8); 102 | 7 (7.0); 100 | 1.08 (0.37 to 3.12); 0.892a |
| Further prolapse surgery, n (%); N | 5 (4.9); 102 | 6 (6.0); 100 | 0.79 (0.23 to 2.68); 0.702a |
| Anterior | 3 (2.9); 102 | 2 (2.0); 100 | 1.45 (0.24 to 8.89); 0.687a |
| Posterior | 3 (2.9); 102 | 0 (0); 100 | |
| Apical | 1 (1.0); 102 | 4 (4.0); 100 | 0.23 (0.02 to 2.11); 0.192a |
| Continence surgery, n (%); N | 3 (2.9); 102 | 1 (1.0); 100 | |
| Time to further surgery (days), mean (SD); Nb | 337.7 (206.3); 7 | 310.0 (190.8); 7 | 0.94 (0.31 to 2.87); 0.921c |
| Need for conservative treatment, n (%); N | |||
| Any conservative treatment | 29 (27.9); 104 | 33 (31.7); 104 | 0.83 (0.46 to 1.51); 0.54a |
| Absorbent pads | 24 (31.2); 77 | 28 (34.6); 81 | |
| Physiotherapy | 8 (13.3); 60 | 9 (18.0); 50 | |
| Disposable or reusable catheter | 3 (4.1); 74 | 4 (6.0); 67 | |
| Had a ring pessary | 3 (4.0); 75 | 3 (4.4); 68 | |
| Had a shelf or Gellhorn pessary | 0 (0.0); 73 | 3 (4.4); 69 | |
| Permanent catheter | 0 (0.0); 71 | 2 (3.1); 64 | |
| Seen health professional, n (%); N | |||
| GOPD | 27 (37.0); 73 | 39 (54.2); 72 | |
| GP for prolapse | 21 (29.2); 72 | 26 (37.7); 69 | |
| Practice nurse | 8 (14.3); 56 | 4 (8.0); 50 | |
| District or continence nurse | 2 (3.5); 57 | 5 (10.0); 50 | |
When women reported that they had been re-admitted to hospital, the information was verified with the centres and post-coded accordingly to ensure accuracy of data and resolution of discrepancies. A hospital re-admission was automatically counted as a SAE if it was related to the initial prolapse surgery. Further surgery for prolapse (failure if same compartment), or for continence surgery, was differentiated from re-admission for surgery-related complications, such as bleeding or infection.
Fewer than 1 in 10 women had a re-admission for further surgery in the first 12 months (Table 32). Eleven women had further prolapse surgery within 12 months [abdominal (n = 5) vs. vaginal (n = 6)], this was not statistically significant. Four women in the vaginal group had a further apical procedure compared with one in the abdominal group in that time (again, this was not statistically significant).
There was no evidence of a difference in further use of health services between the randomised groups of women.
Serious and related adverse events in the first 12 months
Overall, there were 12 SAEs (6.0%) reported in the first 12 months following surgery (more than one SAE could be reported per woman) (Table 33). Hospitalisation was the most common type of SAE. There were no differences between the treatment groups for any SAEs.
| Serious and related AEs | Treatment group), n (%) | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault (N = 102) | Vaginal vault (N = 100) | ||
| Any SAE | 6 (5.9) | 6 (6.0) | 0.97 (0.27 to 3.44); 0.96 |
| Type of SAE | |||
| Hospitalisation | 3 (2.9) | 4 (4.0) | |
| Prolongation of hospitalisation | 3 (2.9) | 1 (1.0) | |
| Considered medically significant by the investigator | 1 (1.0) | 2 (2.0) | |
| Intraoperative occurrences | |||
| Injury to organs (bladder or bowel) | 3 (2.9) | 1 (1.0) | |
| Postoperative occurrences | |||
| Constipation | 2 (2.0) | 1 (1.0) | |
| Urinary retention/voiding difficulties requiring conservative intervention | 1 (1.0) | 1 (1.0) | |
| Urinary tract infection | 1 (1.0) | 0 (0) | |
| Pain | 1 (1.0) | 0 (0) | |
| Catheterisation required for > 10 days post operation | 0 (0) | 1 (1.0) | |
| Vaginal adhesions | 0 (0) | 1 (1.0) | |
| Perineal scarring/tightness requiring surgery | 0 (0) | 1 (1.0) | |
| Granulation tissue | 0 (0) | 1 (1.0) | |
| Mesh exposure/extrusion that requires surgical treatment | 0 (0) | 1 (1.0)b | |
Specifically, of all the 106 procedures using a mesh implant, one concomitant continence mesh exposure/extrusion was identified and required surgical treatment in the first 12 months after surgery.
Non-serious and related adverse events in the first 12 months after surgery
Non-serious AEs/complications were also reported (Table 34). Overall, four were identified within the 12-month follow-up period. There were no differences between the treatment groups in any non-serious AEs.
| Non-serious and other related AEs | Treatment group), n (%) | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault (N = 102) | Vaginal vault (N = 100) | ||
| Any complications | 1 (1.0) | 2 (2.0) | 0.49 (0.04 to 5.44); 0.56 |
| Postoperative complications | |||
| Haematoma | 1 (1.0) | 0 (0) | |
| Treatment for postoperative infection with antibiotics | 0 (0) | 2 (2.0) | |
| Other adverse postoperative events | 0 (0) | 1 (1.0) | |
Subgroup analysis
There were no differences in any of the planned subgroup analyses [aged < 60 years and ≥ 60 years, previous anterior or posterior surgery, or previous incontinence surgery (see Appendix 6, Table 79)].
Sensitivity analyses
A multiple imputation model was used to impute missing values in the primary outcome, POP-SS, assuming a missing-at-random mechanism. No evidence of a difference between the two treatment groups after the imputation was found (abdominal vs. vaginal: MD –0.32, 95% CI –1.71 to 1.06; p = 0.65).
Per-protocol analysis
A per-protocol analysis for the primary outcome, POP-SS, including only the participants that followed protocol (received the allocated treatment) found no evidence of a difference between treatment groups (abdominal vs. vaginal: MD –0.99, 95% CI –2.59 to 0.60; p = 0.22).
Discussion
Summary of findings
Surgical planning and procedure
In addition to their apical surgery, concomitant surgery was planned for the majority (70.2%) of women in the Vault trial. The randomised surgical allocation was performed in most of the participants (81.8% in the abdominal group and 86.5% in the vaginal group). In those women who did not obtain their allocated surgery, 7.7% were found to have no apical prolapse following an anterior or posterior procedure. Women in the vaginal group were more likely to receive a concomitant anterior and/or posterior repair (76.0%) than those women in the abdominal group (34.7%).
Most procedures were performed by a consultant gynaecologist under general anaesthesia with prophylactic antibiotics (91.8%). The duration of the abdominal procedure was significantly longer (mean duration 146 minutes, SD 54 minutes) than the vaginal procedure (82 minutes, SD 34 minutes; MD 60.48 minutes, 95% CI 49.80 to 71.16 minutes). There was no difference in the level of blood loss or postoperative hospital stay between the treatment groups.
Primary and secondary outcomes
Around 85.5% of women responded to the 12-month questionnaire, with slightly more women attending for the 12-month clinic assessment.
The primary outcome was POP-SS at 12 months after randomisation, and there was no difference between an abdominal and a vaginal procedure. The MD in the POP-SS for the abdominal group was 5.6 (SD 5.4) compared with a MD of 5.9 (SD 5.4) in the vaginal group. Adjusted for baseline variables, the MD was –0.61 (95% CI –2.08 to 0.86) and suggests that both procedures are equally clinically effective in improving vault prolapse symptoms at 12 months.
At 12 months after randomisation, prolapse-related effects on QoL scores had improved and were apparent at 6 months after operation. The most bothersome symptom at baseline, ‘a feeling of something coming down’, reduced from 47.2% overall at baseline to 10% in the abdominal group and 34% in the vaginal group. Incomplete emptying of the bladder or bowel were the most bothersome at both 6 and 12 months for both randomised groups of women, but did not affect ‘actions necessitated by prolapse symptoms’, which were reduced from baseline.
Overall, objective prolapse scores (i.e. POP-Q) improved for both randomised groups, particularly in terms of prolapse protrusion beyond the hymen (stage 2b or more: 32.6% after an abdominal vs. 46.9% after a vaginal procedure; this was not statistically significant). Objective vault prolapse (stage 2b or more) fell to 6.0% in the abdominal group and 9.0% in the vaginal group 12 months after surgery (adjusted for baseline values). Again, this was not statistically significant.
More women were sexually active after surgery. There were no statistically significant differences in vaginal and sexual symptoms between either of the randomised groups of women at 12 months.
Overall, at 12 months after randomisation, the proportion of women who had any urinary incontinence increased from around one in six women to one in three in the abdominal and one in four in the vaginal group; severe urinary incontinence remained the same as baseline. In addition, de novo urinary incontinence following surgery was found in one in four women in the abdominal group and one in five in the vaginal group. Few women had continence surgery to alleviate their symptoms within the first 12 months (n = 4). There was no evidence of a difference in any urinary symptom outcomes between the randomised groups of women.
There were no other significant differences in bowel symptoms between the randomised groups of women.
There were no differences between the randomised groups of women in terms of satisfaction with treatment at 12 months after randomisation. Most women reported that they were better than before surgery (92.8% vs. 86.2%). The majority of women were completely or fairly satisfied (87.8% vs. 82.6%) and would recommend the surgery to a friend (87.2%).
Overall, 15 women were re-admitted for further surgery in the first 12 months after surgery (see Table 32). Eleven women had further prolapse surgery within 12 months [abdominal preservation (n = 5) vs. vaginal (n = 6)], and this was not statistically significant. One woman in the abdominal group had a further apical procedure compared with four women in the vaginal group in that time (again, this was not statistically significant). There was no difference in re-admission rates for anterior repair between both groups. Three women in the abdominal group had a re-admission for a posterior repair.
Overall, 12 SAEs (5.8%) were reported in the first 12 months following surgery and there were no differences between the groups in any serious adverse effects. Specifically, of all the 106 procedures using a mesh implant one mesh exposure/extrusion from a concomitant continence procedure was identified requiring surgical treatment in the first 12 months after surgery relating to a risk ratio (RR) of around 1% for mesh exposure.
Conclusion
Both abdominal and vaginal surgeries are equally clinically effective in relieving symptoms of vault prolapse in the short term. There is no statistically significant difference for the apical measurements. The objective descent measured by POP-Q is significantly different in relation to the anterior wall prolapse 12 months after surgery (though the numbers were small). This, however, did not affect re-admissions for anterior wall prolapse in the first 12 months. The presence of urinary incontinence may worsen after either procedure, but severe incontinence appears to remain static.
Chapter 8 Vault trial cost-effectiveness analysis
Overview
This chapter presents the results of the within-trial cost–utility analysis for women in the Vault trial. A single table of all results is presented (Table 35), with separate sections for summary data on costs, QALYs and cost-effectiveness (including deterministic sensitivity analysis results). Further details of the cost-effectiveness data are provided in Appendix 7, Tables 80–84.
| Costs, QALYs and cost-effectiveness | Treatment | Abdominal vault vs. vaginal vault | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | ||||||||||
| Mean | SD | n a | Mean | SD | n a | MD | 95% CI | ||||
| Costsb,c | |||||||||||
| Intervention | £1541.55 | £500.02 | 102 | £935.22 | £351.74 | 100 | £582.31 | £472.32 to £692.30 | |||
| Hospital resource use | £241.14 | £777.74 | 103 | £286.89 | £932.08 | 97 | –£19.69 | –£251.73 to £212.35 | |||
| Other consultations | £116.69 | £183.22 | 81 | £141.54 | £154.69 | 84 | –£43.21 | –£94.87 to £8.44 | |||
| Other treatments | £56.46 | £190.41 | 76 | £27.86 | £109.60 | 84 | £61.19 | –£8.25 to £130.63 | |||
| Total NHS costs | £1999.05 | £1056.23 | 74 | £1369.86 | £993.39 | 81 | £672.36 | £372.21 to £972.51 | |||
| QALYsc,d | |||||||||||
| Baseline EQ-5D | 0.735 | 0.192 | 95 | 0.760 | 0.184 | 97 | |||||
| 6 months EQ-5D | 0.835 | 0.206 | 79 | 0.828 | 0.176 | 90 | |||||
| 12 months EQ-5D | 0.833 | 0.210 | 81 | 0.832 | 0.157 | 85 | |||||
| Total QALYs | 0.824 | 0.143 | 67 | 0.825 | 0.109 | 78 | –0.009 | –0.049 to 0.032 | |||
| Cost-effectiveness | |||||||||||
| N | ΔC (95% CI) | ΔE (95% CI) | ICERe | Probability that abdominal vault is cost-effective compared with vaginal vault at different WTP for 1 QALY gained (%) | |||||||
| P(C/E) at £0 | P(C/E) at £10,000 | P(C/E) at £20,000 | P(C/E) at £30,000 | P(C/E) at £50,000 | |||||||
| Imputed data set (base case)f | 208 | £570 (£459 to £682) | 0.004 (–0.031 to 0.041) | £142,500 | 0 | 1 | 6 | 17 | 29 | ||
| Imputed data set (SUR) | 208 | £552 (£262 to £843) | 0.004 (–0.030 to 0.039) | £138,000 | 0 | 1 | 8 | 18 | 31 | ||
| Wider-perspective costing analysis | 208 | £498 (–£262 to £1259) | 0.004 (–0.031 to 0.041) | £124,500 | 6 | 8 | 14 | 21 | 32 | ||
| Use of HRG tariffs for intervention procedureg | 202 | £1212 (£916 to £1508) | 0.004 (–0.031 to 0.041) | £303,000 | 0 | 0 | 0 | 2 | 11 | ||
| Assumptions regarding QALY calculation (1) | 195 | £570 (£459 to £682) | 0.002 (–0.035 to +0.039) | £285,000 | 0 | 1 | 5 | 11 | 19 | ||
| Assumptions regarding QALY calculation (2) | 176 | £570 (£459 to £682) | 0.006 (–0.030 to 0.042) | £95,000 | 0 | 1 | 4 | 10 | 19 | ||
| Assumptions regarding QALY calculation (3) | 142 | £570 (£459 to £682) | –0.004 (–0.055 to 0.048) | Dominated | 0 | 4 | 14 | 23 | 29 | ||
| Complete-case analysish | 129 | £695 (£403 to £987) | –0.025 (–0.060 to 0.010) | Dominated | 0 | 2 | 16 | 33 | 50 | ||
| Complete-case analysis, SUR | 129 | £693 (£324 to £1064) | –0.015 (–0.050 to 0.020) | Dominated | 0 | 2 | 17 | 34 | 51 | ||
NHS perspective resource use and costs
Intervention costs
Intervention costs are calculated for all women entering theatre for an operative procedure (regardless of whether or not they received surgery). The overall intervention cost was £582 more expensive in the abdominal group compared with the vaginal group (95% CI £472 to £692) using the component costing approach. The significant additional procedure cost is predominantly driven by a longer time in surgery, but also by the additional use of apical mesh for 80% of women in the abdominal group, compared with 6% in the vaginal group. All other resource use (e.g. length of stay, use of drugs in surgery) were similar across groups. A complete list of items used for the component-based costing of each intervention is reported in Appendix 7, Table 80.
Follow-up care costs
There is no evidence of any differences across the groups in terms of re-admission to hospital for further prolapse procedures, incontinence procedures or for the treatment of SAEs. The average total cost of hospitalisation (excluding the index procedure admission) is £241 in the abdominal group and £287 in the vaginal group (MD –£19.69, 95% CI –£252 to £212). Consultations with health professionals (e.g. outpatient doctors, GPs, community-based nurses, physiotherapy) are similar across the groups, as is the cost of medications and devices (e.g. oestrogens, pessaries, antibiotics). Full details of all resource use over follow-up for hospitalisations, contact with health professionals and use of medications/devices are reported in Appendix 7, Tables 81–84.
Total NHS costs
Overall, total costs were calculated for 74 out of 104 (71%) and 81 out of 104 (78%) women in the abdominal and vaginal groups, respectively. Missing data are due primarily to participant non-return of questionnaires. Based on the complete-case costs available, the abdominal group cost £1999 and the vaginal group cost £1370. The abdominal approach was significantly more expensive overall, with a MD of £672 (95% CI £372 to £973) per patient, representing a substantial additional cost to the NHS, compared with the vaginal group over the short 12-month time horizon considered.
Patient participant perspective resource use and costs
Women with prolapse symptoms have a high rate of contact with health professionals, including a large number of consultations in both primary and secondary care. The costs to women of time spent attending appointments is substantial, with costs per women of £252 in the abdominal group compared with £201 in the vaginal group.
The number of women reporting that they had to take time off from work, as a proportion of the trial population, was small. In total, 38 women, 19 per group, representing 18% of trial participants reported needing to take sick leave. The low proportion requiring sick leave is likely to be because of the proportion in paid employment (c. 25%) and the average age of the trial participants having passed the standard retirement age. Among those women needing to take sick leave, the average number of days off work was 24, with no differences across the groups.
The costings include a few women who incurred a small amount of cost for over-the-counter medications and other related expenses. No women in the Vault trial report any use of private health-care services for prolapse-related symptoms. Full details of all costs incurred by women, collected through the study are reported in Appendix 7, Table 84.
The total average costs to women over the trial follow-up period is substantial, but there were no differences across the groups (MD –£53, 95% CI –£400 to £294). High SDs contribute to wide CIs and indicate that there is substantial variability in the experience of personal costs, with some women experiencing substantially greater financial burden than others.
When summing participant and NHS perspective costs together to inform a wider-perspective analysis of prolapse costs, the abdominal group remains significantly more costly with a MD of £825 (95% CI £208 to £1443).
Generic quality-of-life outcomes
The proportion of women reporting any health problems on each EQ-5D-3L domain (i.e. a score of two or three) at baseline, at 6 months and at 12 months is presented in Figure 11. Missing data are not reported on the graphs. At baseline, a substantial proportion of women in both groups report problems related to pain and discomfort. This would be expected given the need for surgery. Those reporting any pain or discomfort reduces to about half of all women in both groups at 6 months, with similar proportions reporting pain/discomfort at 12 months. The proportion of women reporting problems with self-care was low.
FIGURE 11.
Percentage of respondents reporting any problems on each EQ-5D domain (baseline, 6 months after surgery and 12 months after randomisation).
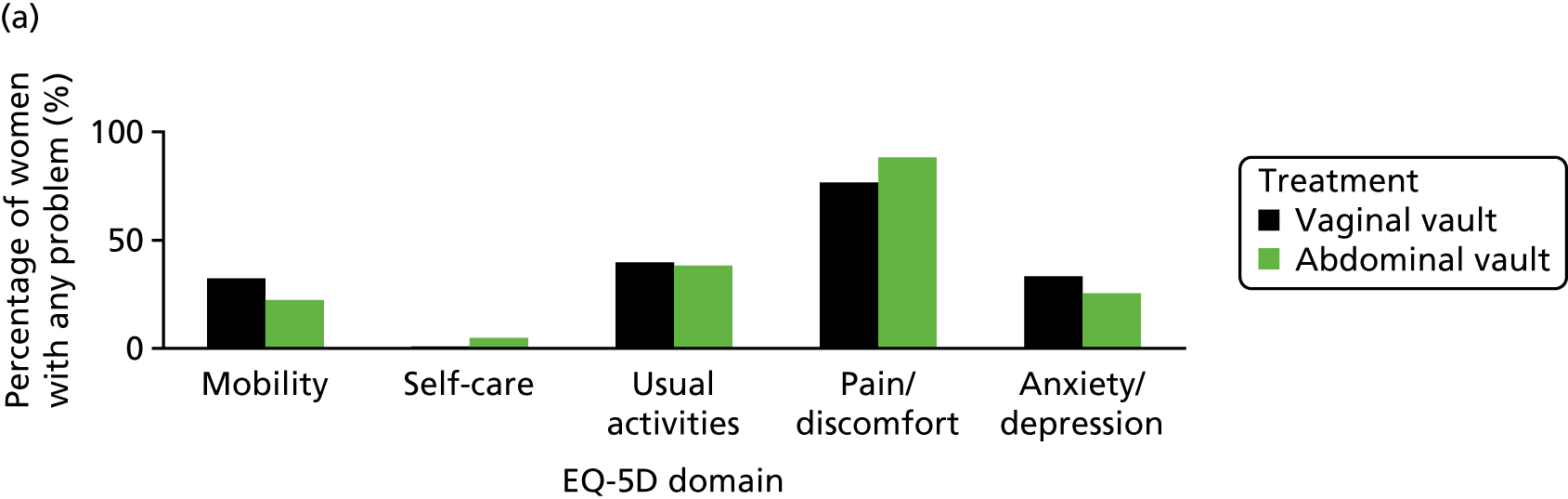
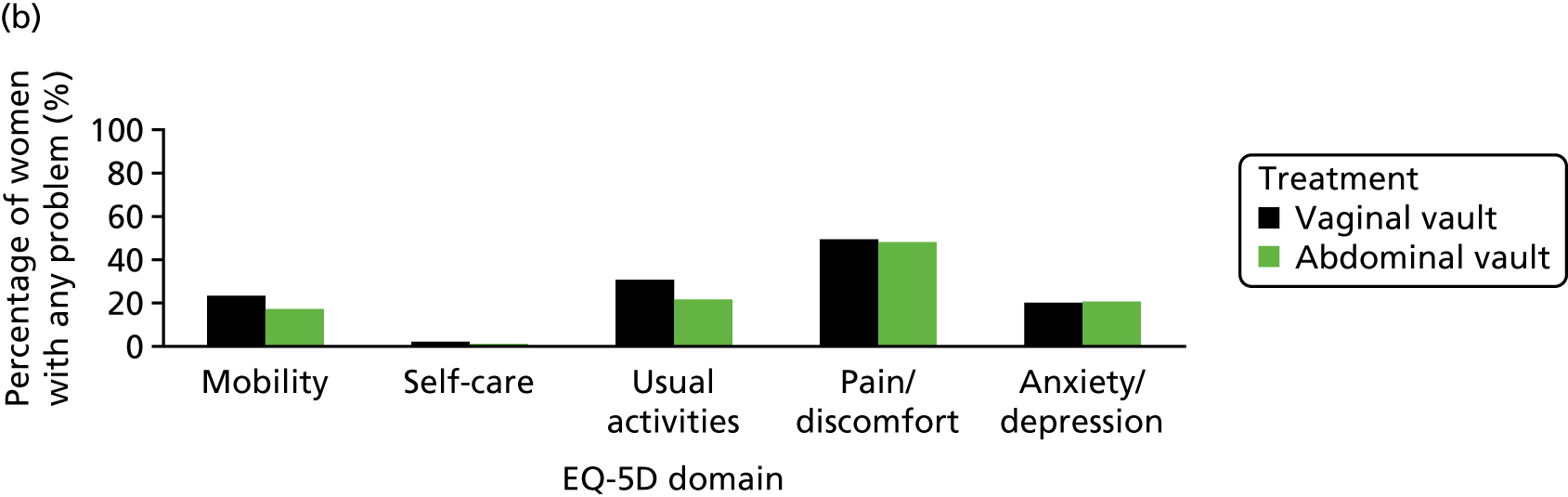

Full details of the utility scores resulting from these EQ-5D-3L responses at each time point and calculated QALYs can be found in Table 35. In terms of covariates included within the analysis model, baseline utility was the only significant predictor of overall QALYs, re-enforcing the importance of adjusting for this baseline measure.
Complete data were available across EQ-5D domains and time points (baseline, and 6 months and 12 months), enabling QALY calculation for 67 out of 104 (64%) and 78 out of 104 (75%) women randomised to the abdominal and vaginal vault groups, respectively. Based on the complete-case data, there are no statistically significant differences in QALYs between treatment groups, with the abdominal and vaginal vault groups obtaining an average of 0.824 and 0.825 QALYs per person over 12 months, respectively (MD –0.009, 95% CI –0.049 to +0.032). Multiple imputation of missing data indicates a similar lack of difference across the groups (MD 0.002, 95% CI –0.036 to 0.039).
Cost-effectiveness results
Complete-case costs and complete-case QALY data are presented, making best use of all available data (see Table 35). The base-case analysis uses multiple imputation of missing data. Imputation of missing data was chosen because (1) only 129 out of 208 (62%) participants had fully complete profiles of costs and QALYs to enable calculation of the ICER, and (2) missing data for QALYs were imbalanced across the randomised groups. A range of deterministic sensitivity analyses are also reported. All analyses of cost-effectiveness also indicate the probability that abdominal vault repair is a cost-effective use of NHS resources at five alternative threshold values of WTP for 1 QALY gained (i.e. £0, £20,000, £30,000, £50,000 and £100,000).
Base-case analysis
The multiple imputed data show significant additional costs for the abdominal group (MD £570, 95% CI £459 to £682) for no significant QALY gain (MD 0.004, 95% CI –0.030 to 0.040). The base-case cost to the NHS of obtaining one additional QALY is therefore £285,000 per QALY.
The scatterplots (Figure 12) of all bootstrapped replications further illustrates the sampling uncertainty accrued from the bootstrapped replications of the analysis models. There is little uncertainty regarding the additional cost of the abdominal procedure when using the base-case component costing approach with almost all of the increments from the bootstrap lying above the horizontal line. However, regarding QALYs, the scatterplot indicates substantial uncertainty, with no clear evidence of one strategy being preferable.
FIGURE 12.
Vault trial: scatterplot of the cost-effectiveness plane (imputed data set).
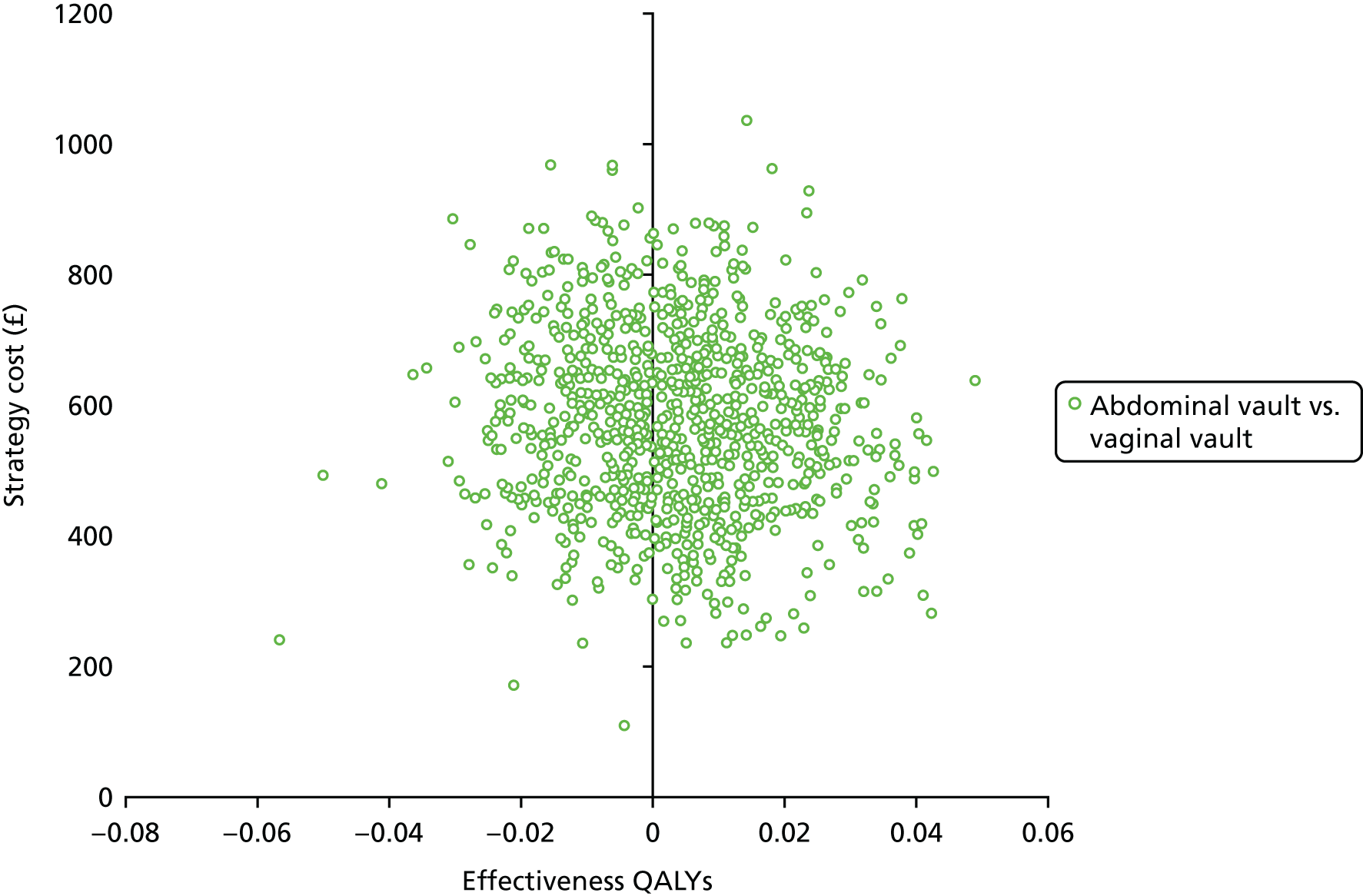
The CEAC in Figure 13 shows that at 12 months after randomisation, based on the NMB, there is a high probability that vaginal repair is the most cost-effective treatment option, with little evidence to support the cost-effectiveness of abdominal repair. The probability of abdominal repair being considered to be cost-effective is < 17%, up to a threshold value of WTP for 1 QALY gained of £30,000, increasing to a 29% probability of cost-effectiveness if the threshold was £50,000 per QALY.
FIGURE 13.
Vault trial: CEAC (imputed data set).

Deterministic sensitivity analyses
Deterministic sensitivity analyses are conducted to address areas of uncertainty in assumptions, data collection and analysis-modelling approaches. All deterministic analyses are based on the imputed data set unless otherwise stated (e.g. complete-case analysis). The rationale behind each of the analyses is described in Chapter 2 and a number of observations were made on the results. Results of all deterministic sensitivity analyses are reported in Table 35 and a full set of CEACs and scatterplots for each analysis can be found in Appendix 7, Figures 33–40.
First, based on an analysis of complete cost and QALY pair data, at the 12-month follow-up, abdominal vault repair remains significantly more costly and generates non-significantly fewer QALYs and is thus dominated by vaginal vault repair. Although the additional costs of abdominal vault repair are marginally greater for the complete-case analysis, the additional costs are surrounded by more uncertainty, with wider CIs. Probabilities of cost-effectiveness are therefore slightly higher in the complete-case group, which may initially appear counterintuitive. However, this is because of greater uncertainty around the effect size on costs in the complete-case group, with a higher proportion of simulations falling to the right of the threshold line (see Appendix 7). The important point remains that complete-case data show no evidence of changing the overall findings of the analysis.
Second, it was shown that the findings are robust to the analysis models incorporated. The use of seemingly unrelated regression as an alternative approach to account for the correlation in costs and QALYs (abdominal, –0.202; vaginal, –0.066; trial, –0.146) does not change the overall findings.
Third, the estimates of incremental costs in the Vault trial are sensitive to the approach taken to intervention costing. Using HRG reference costs for intervention procedures increases the MD (abdominal vs. vaginal) to £1212 (95% CI £916 to £1508) and increases the ICER to > £300,000 per QALY. This analysis further reduces the probability that abdominal vault repair might be considered cost-effective compared with vaginal vault repair. Arguments can be made for the use of either approach. Component costing is chosen as the base case because it is grounded in the economic concepts of resource scarcity and opportunity cost. It therefore more accurately reflects the resources displaced for each surgery, and explicitly accounts for differences in operation times, length of stay and materials costs (e.g. mesh and devices) between the groups observed in the trial. Importantly, the approach to intervention costing does not change the overall cost-effectiveness conclusions from the Vault trial.
Fourth, because of differences in time points for questionnaire triggers (i.e. at 6 months after surgery and at 12 months after randomisation), and the potential for comparatively long periods of time to elapse between randomisation and surgery, it is possible that respondents get their 12-month questionnaire before their 6-month questionnaire. The sensitivity analyses show that estimates of cost-effectiveness are not sensitive to any of the following assumptions in sensitivity analysis:
-
an analysis dropping the 6-month EQ-5D data if the 6-month questionnaire is returned on or after the 12-month date
-
an analysis dropping any 6-month EQ-5D data within 3 months of the 12-month data
-
an analysis using the exact date of questionnaire report as the measure of time used in the calculation of QALYs.
However, a note of caution on the last analysis is that some women will have QALYs greater than one over a single year, as some questionnaires were returned well after 12 months (i.e. maximum = 1.80 years).
Fifth, the incorporation of a wider-perspective costing analysis, including both NHS and participant-incurred and -reported costs, does not alter the estimates of cost-effectiveness or the probability of cost-effectiveness at alternative threshold values of WTP for 1 QALY gained.
Discussion
The findings from the Vault trial indicate that there is a low probability that abdominal vault repair is a cost-effective use of resources when compared with vaginal vault repair. However, it should be noted that the findings of the trial-based economic evaluation only partially address the question of cost-effectiveness. To fully understand the cost-effectiveness of an intervention, it is important to capture the longer-term costs and effects of a surgical intervention. For example, it may not be until many years into the future when the true impact of failure rates and complications may be realised, and any cross-group differences observed. Therefore, it is planned to have long-term follow-up of women randomised in the Vault trial to better understand the long-term costs and outcomes of surgery. The Vault trial provides a rich source of data to populate the long-term economic model of the most cost-effective treatment pathway combining the findings of the Uterine and Vault trials into one single model of the treatment pathway. The analysis will be updated as more mature data become available.
Chapter 9 Lifetime economic evaluation
Introduction and objective
Chapters 5 and 8 report the results of the within-trial economic evaluations for both the Uterine trial and the Vault trial over a 12-month time horizon. The data provide useful indications of short-term cost-effectiveness. Apical prolapse, however, is a chronic condition and the clinical effects of alternative surgical treatments may persist into the future or emerge over the longer term, well beyond the 12-month follow-up period of the VUE trial. Recent NICE guidance acknowledges that there is no conclusive evidence about the long-term clinical effectiveness or cost-effectiveness of different surgical treatments;20,21 hence, little is known about the most efficient treatment pathway. The VUE trial seeks to address this gap in the evidence base. Longer-term follow-up of women randomised to the VUE trial is ongoing. However, clinical decision-makers need best estimates of the long-term cost-effectiveness data to inform treatment decisions now. The objective of this chapter is to use a decision-analytic model to extrapolate the Uterine trial results over a lifetime horizon to determine the cost-effectiveness of uterine preservation versus vaginal hysterectomy for treating apical prolapse from a NHS perspective.
Markov structure and model description
A probabilistic Markov cohort model was developed using TreeAge Pro™ 2016 software (TreeAge Pro 2016, R1.0.; TreeAge Software, Inc., Williamstown, MA, USA) to represent the treatment pathway for women with apical prolapse. The model is structured to mimic progression through the pathway of care. A cohort of 1000 women, with an average age of 63 years (trial baseline population), suffering from symptomatic prolapse requiring surgical intervention enter the model at a treatment decision point (uterine preservation vs. vaginal hysterectomy). The women then follow a treatment pathway, based on risk of requiring further surgery. A maximum of four (vaginal hysterectomy followed by a maximum of three repeat procedures) or five (uterine preservation followed by a maximum of four repeat procedures, including vaginal hysterectomy) surgeries are modelled depending on the starting arm. Figure 14 describes the model structure for the uterine preservation arm.
FIGURE 14.
Markov model structure for treatment pathway.
The sequence of surgeries and transitions allowed in the model were developed in discussion with clinicians and trial collaborators. The model allows the cohort to have further surgery for prolapse in the following sequence: (1) uterine preservation, (2) vaginal hysterectomy, (3) vaginal vault, (4) abdominal vault and (5) repeat abdominal vault or other surgical procedure. The treatment pathway described in Figure 14 is identical for both model arms, the only difference being the number of potential surgical procedures.
After each surgical procedure, the cohort can enter into one of four mutually exclusive states:
-
Well after surgery – this reflects the proportion of the cohort in any given cycle who do not experience failures or complications requiring surgery, or die naturally. They may, however, still experience some prolapse-related symptoms or other complications (that do not require hospitalisation). Minor treatment costs may be incurred, such as physiotherapy, oestrogens, etc. Once entering this state, the cohort remains there over successive model cycles until they experience failure, complication or die.
-
Complications – the cohort may enter the complications state at any point in the model following surgery, reflecting on the fact that women may experience complication risks over both the short and the longer term following prolapse surgery. Complications have been classified in accordance with the trial reporting of SAEs. The complications state reflects major complications that require hospitalisation. The duration of the complications state is 6-month cycles, reflecting the average time required to recover from complications. The model allows the cohort to have multiple complications at any time following a surgical procedure. As the cohort progresses through successive surgeries for failures, each complication state relates to the most recent surgical procedure. For example, it is assumed that ‘complications following vaginal vault’ relate to the vaginal vault procedure, and not the index decision of uterine preservation versus vaginal hysterectomy.
-
Further surgery for failures – failure requiring a repeat surgical intervention triggers progression of the cohort along the sequence of surgeries described in Figure 14. A failure requiring surgical repair is considered to be any repeat prolapse surgery, whether it occurs in the same or a different compartment.
-
Long-term containment – the proportion of the cohort having progressed through all surgical options described and still experiencing symptoms indicative of treatment failure are assumed to be managed conservatively, and so do not receive further surgical intervention. Conservative management includes the use of pessaries, physiotherapy and regular outpatient consultation.
-
Long-term well – this state reflects the proportion of the cohort who have progressed through the full sequence of prolapse surgeries and are no longer experiencing symptoms indicative of a failure of the final procedure. In the absence of any data to confirm the proportion progressing from the final surgery state to either long-term containment or long-term well, clinical expert opinion is relied on (Dr Christine Hemming, University of Aberdeen, 2017, personal communication), assuming that approximately 75% of the cohort would have no further problematic symptoms over a 10-year period, with 25% continuing to be managed conservatively.
-
Dead – the modelled cohort may die at any point in the model, following female-specific general population all-cause mortality rates. 62 No additional surgical mortality rate is included because no women died during the surgical procedures in the VUE trial.
The cohort of 1000 women progress through the model health states in monthly Markov cycles following the sequence of surgical treatments and health-state transitions described above. A monthly cycle length has been chosen to reflect the time increments for which data regarding time of treatment failure and complications requiring surgery were available. Tunnel states were used to ensure that the proportion of the cohort in each surgery and complication state remain there for 6 monthly cycles to allow for sufficient time to recover from treatment, and experience a longer time period of lower utility (QoL). The model time horizon is 30 years’ duration (equivalent to 360 monthly cycles) in the base case, this is deemed sufficient to capture the important life-time costs and consequences of an average 63-year-old woman entering the model. Costs and QALYs occurring beyond the first year of the model are discounted at a rate of 3.5% per annum in line with current NICE guidelines. 63 All costs are reported in 2015–16 GBP and the analysis is conducted from the perspective of the UK NHS.
Model parameterisation: health-state transition probabilities
Survival analysis is used to estimate transition probabilities to further surgery and complication states using time-to-failure events observed in both the Uterine and the Vault trials. For example, failure and complications following uterine preservation are derived from the corresponding arm of the Uterine trial data. Similarly, transition probabilities following vaginal and abdominal vault procedures are derived from the corresponding arms of the Vault trial. As noted in the Introduction and objective, long-term follow-up is ongoing. Two-year follow-up data on complications and failures were available for 208 out of 562 (37%) participants randomised to the Uterine trial and 140 out of 208 (67%) participants randomised to the Vault trial. The available 2-year data supplement the existing 1-year follow-up data to generate a richer data set. The methods used to extrapolate data from both trials are similar.
Treatment failures
Event dates were available by month and so are congruent with the cycle length applied in the model. Kaplan–Meier curves illustrating the time-to-failure (requiring surgery) event data for the four surgical procedures are presented in Figure 15.
FIGURE 15.
Kaplan–Meier curves for failure event
Progression-to-treatment failure states (i.e. further prolapse surgery) in the model is based on time-dependent transition probabilities derived from a set of Weibull regression analyses of time-to-event data from the respective trials. The results of the survival model for time-to-repeat surgery event, with a Weibull distribution specified, indicate a hazard ratio of 1.610 (95% CI 0.824 to 3.146) for uterine preservation compared with vaginal hysterectomy. Treatment effects are not statistically significant, but the point estimate of the hazard ratio points towards treatment effects favouring vaginal hysterectomy, albeit it with substantial uncertainty. For the Vault trial, there is greater uncertainty driven by the smaller sample size. The hazard ratio for abdominal vault failure versus vaginal vault failure is 0.826 (95% CI 0.312 to 2.182).
A systematic approach was taken to choosing appropriate distributions for the survival analyses, in line with best practice recommendations guidance. 64,65 A range of possible survival models to fit the data was considered. Gamma, log-normal, log–logistic, exponential, Gompertz and Weibull models, as well as Cox regression models, were all considered. The Weibull model was chosen based on (1) consideration of the Akaike’s information criterion (AIC) score, with lower values indicating a preferred model fit; (2) overlaying the estimated survival curves on top of the Kaplan–Meier data; and (3) consideration of the clinical plausibility of the long-term failure probabilities. All models were estimated using Stata 14 software, using the ‘streg’ command. Cox regression models were not considered further as the proportional hazards assumption was rejected in all cases for the failure models. For both the Uterine trial and the Vault trial failure models, all parametric survival models indicated similar AIC scores. After considering the clinical plausibility of the long-run projections, it was determined that the Weibull function gives the best estimate at the tail of the curve. Output from the different survival regression models were considered (both unadjusted and adjusted for minimisation covariates and baseline EQ-5D) and extrapolated survival curves for different models are reported in detail in Appendix 8, Figures 41–4. The unadjusted regression analyses have been used to parameterise the decision model.
The survival function (S(t)), representing the probability of a success (i.e. not having a prolapse failure requiring surgery) at time t (months), is given in Equation 2:
Transition probabilities to failure states in the model are calculated for each monthly cycle according to Equation 3:
When λ is the constant parameter from the regression model, this is the scale parameter that shows the probability that a woman’s prolapse will recur in the next period given the fact that she was successful in the current period; γ is the shape parameter describing the rate of change in the probability of further surgery for prolapse failure over time. γ > 1 indicates that the rate increases over time. Conversely, γ < 1 indicates that the rate decreases over time.
Uncertainty surrounding the transition probabilities is incorporated into the model by using the Cholesky decomposition of the covariance matrix retrieved from the output of the regression models. A multinormal distribution was used to sample from the correlations between the regressions coefficients and multiplied by the coefficient of the log of the hazard ratios, lambda and gamma parameters, thus assigning treatment specific transition probabilities for failures. The process was repeated for both the Uterine and the Vault trial data.
Complications
The process for incorporating complications into the model was identical to that described for failures requiring further surgery. For the purposes of the transition probabilities, complications are defined here as any complication that required admission to hospital. Transitions to complications states were based on time since the most recent surgical procedure. For the Uterine trial, the adjusted hazard ratio for uterine preservation versus vaginal hysterectomy was 1.126 (95% CI 0.478 to 2.651); the unadjusted hazard ratio was 1.077 (95% CI 0.453 to 2.552). For the Vault trial, the hazard ratios for abdominal versus vaginal were – 0.689 for adjusted (95% CI 0.154 to 3.078) and – 0.885 for unadjusted (95% CI 0.342 to 2.294). Overall, there were no significant differences between any of the treatments in terms of complications requiring hospitalisation. CIs were wide, in part due to the relatively low number of complications in both trials.
Figure 16 presents the Kaplan–Meier curves for complications using all available 2-year follow-up data. As can be seen from the Kaplan–Meier curves and the estimated hazard ratios, there is no evidence to suggest any differences in time-to-complication events across the groups based on currently available data.
FIGURE 16.
Kaplan–Meier curves for time to complication requiring hospitalisation.
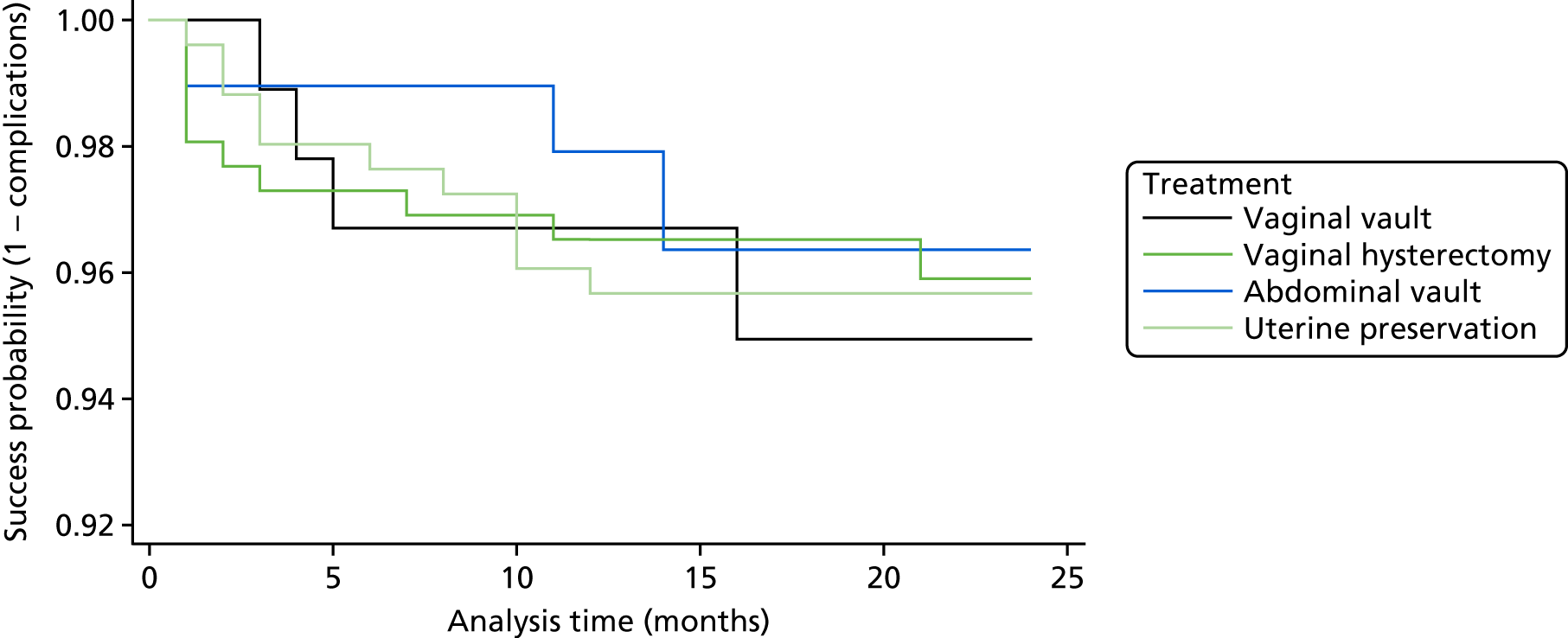
As with the analysis of time to failure, the time-to-complication event is based on Weibull regression models. A similar process for selecting the models was used. Details of the full set of models considered are available in Appendix 8, Tables 85–8. Uncertainty surrounding transition probabilities was again incorporated into the model by fitting multinormal distributions to the correlations between the relevant coefficients, obtained from the Cholesky decomposition matrix of the regression model.
Mortality parameters
When women move through the model, the chance that they might die is based on the annual rates of age-specific all-cause mortality for women (Office for National Statistics interim life tables). 62 As there were no intraoperative deaths reported during the trial, surgical mortality was not included in the model.
Model parameterisation: resource use and costs
Intervention delivery costs are based on the component costing approach used for the trial-based cost-effectiveness analysis. Sensitivity analysis considers the use of HRGs in the model given the sensitivity of findings in the trial analyses to the choice of intervention costing approach. Costs for the remaining health states (failures, complications and well) are obtained from the trial data. Definitions of failures and complications are as described in Complications for obtaining time-dependent state-transition probabilities. Women who were not categorised as having complications or failures were categorised as being in the ‘well’ health state. This does not mean that these women were asymptomatic, and so the costs of the ‘well’ state implicitly assumes a proportion of the cohort have costs of consultations with health professionals and treatments based on the data reported in the trials. To avoid double costing, all regression analyses were based on total costs over follow-up, less the initial intervention costs. GLM regression models with robust standard errors (SEs) were then used to determine the impact of health-state membership on costs. Health-state dummy variables were interacted with the randomised treatment group to obtain treatment-specific health-state costs. The marginal effects of the relevant coefficients and SEs (estimated using the delta method) were used to populate the model. Sensitivity analysis considers the impact on results of assuming that the health-state costs are not treatment specific, that is, calculating health-state costs based on all trial women, regardless of randomised treatment group. Costs obtained from the trial data are adjusted to reflect the monthly time cycle of the model. Exceptions to this include full costs applied for complication and failure states at the point of entry to capture the full resource impact of high-cost surgical/hospitalisation procedures.
All cost parameters are defined in the model as statistical distributions and are assumed to follow a gamma distribution. Table 36 reports the mean, SE (SD of the sampling distribution) and the alpha and beta parameters used to define the gamma distributions.
| Variable | Point estimate (£) | Robust SEs (£) | Parameters | |
|---|---|---|---|---|
| Alpha | Beta | |||
| Uterine trial costs | ||||
| Intervention: vaginal hysterectomy | 1116 | 22 | 2511.24 | 0.44 |
| Intervention: uterine preservation | 1213 | 28 | 1830.69 | 0.66 |
| HRG intervention: vaginal hysterectomya | 3591 | 33 | 12,171.07 | 0.30 |
| HRG intervention: uterine preservationa | 3041 | 27 | 12,444.61 | 0.24 |
| Vaginal hysterectomy: well | 82 | 14 | 36.09 | 2.27 |
| Uterine preservation: well | 105 | 20 | 27.65 | 3.80 |
| Uterine trial: wella | 93 | 12 | 59.66 | 1.56 |
| Vaginal hysterectomy: failure | 2362 | 461 | 26.22 | 90.10 |
| Uterine preservation: failure | 3160 | 419 | 56.93 | 55.51 |
| Uterine trial: failurea | 2756 | 312 | 78.06 | 35.31 |
| Vaginal hysterectomy: complication | 1164 | 438 | 7.06 | 164.76 |
| Uterine preservation: complication | 587 | 160 | 13.46 | 43.62 |
| Uterine trial: complicationa | 879 | 235 | 13.98 | 62.90 |
| Vault trial costs | ||||
| Vaginal vault: well | 187 | 54 | 12.02 | 15.55 |
| Abdominal vault: well | 125 | 35 | 12.83 | 9.74 |
| Vault trial: wella | 156 | 32 | 23.78 | 6.56 |
| Vaginal vault: failure | 2362 | 607 | 15.14 | 155.99 |
| Abdominal vault: failure | 2684 | 381 | 49.63 | 54.08 |
| Vault trial: failurea | 2525 | 357 | 50.02 | 50.47 |
| Vaginal vault: complication | 1065 | 385 | 7.65 | 139.18 |
| Abdominal vault: complication | 755 | 376 | 4.03 | 187.25 |
| Vault trial: complicationa | 908 | 269 | 11.38 | 79.81 |
| Conservative management | 167 | 20 | 66.88 | 2.50 |
| Cost of death | 0 | N/A | N/A | N/A |
Model parameterisation: utilities
The process of obtaining health-state utilities mirrors that described for the cost parameters. Health-state membership was determined (failure, complication and well). The utility measure obtained closest to the time-to-complication/time-to-failure event in the trial was used to reflect the utility of that state specific to each woman in the trial. GLM regression models were used to obtain the effect of state membership on utility and interactions between state. Treatment dummies were used to determine treatment-specific health-state utilities for the base-case analysis. Sensitivity analysis explores the impact of using health-state utilities that are not treatment specific. All utilities are adjusted for age and gender (female)-specific general population norms in accordance with best practice guidance66 to allow the average utility of health states to fall as the cohort ages. The utilities accrued in each health state are adjusted to reflect the monthly time cycle of the model. All utility data are included in the model as statistical distributions. Uncertainty in utility parameters is characterised and incorporated by sampling from beta distributions for the utility of each modelled health state. Alpha and beta parameters are calculated using the method of the moments approach and the parameters of the distribution are presented in Table 37.
| State | HSUV | SE | Parameters | |
|---|---|---|---|---|
| Alpha | Beta | |||
| Uterine trial | ||||
| Utility vaginal hysterectomy: baseline | 0.781 | 0.011 | 1103.198 | 309.348 |
| Utility uterine preservation: baseline | 0.739 | 0.014 | 726.494 | 256.583 |
| Utility Uterine trial: baseline (SA) | 0.760 | 0.009 | 1710.647 | 540.204 |
| Utility vaginal hysterectomy: well | 0.904 | 0.011 | 647.466 | 68.757 |
| Utility uterine preservation: well | 0.873 | 0.013 | 571.851 | 83.190 |
| Utility Uterine trial: well (SA) | 0.889 | 0.009 | 1082.144 | 135.116 |
| Utility vaginal hysterectomy: failure | 0.675 | 0.063 | 36.634 | 17.638 |
| Utility uterine preservation: failure | 0.728 | 0.044 | 73.733 | 27.548 |
| Utility Uterine trial: failure (SA) | 0.701 | 0.039 | 95.899 | 40.904 |
| Utility vaginal hysterectomy: complication | 0.674 | 0.064 | 35.482 | 17.162 |
| Utility uterine preservation: complication | 0.820 | 0.036 | 92.569 | 20.320 |
| Utility Uterine trial: complication (SA) | 0.746 | 0.037 | 102.508 | 34.902 |
| Vault trial | ||||
| Utility vaginal vault: well | 0.835 | 0.019 | 317.841 | 62.807 |
| Utility abdominal vault: well | 0.846 | 0.024 | 190.509 | 34.679 |
| Utility Vault trial: well (SA) | 0.841 | 0.015 | 498.971 | 94.336 |
| Utility vaginal vault: failure | 0.795 | 0.033 | 118.181 | 30.474 |
| Utility abdominal vault: failure | 0.684 | 0.105 | 12.726 | 5.879 |
| Utility Vault trial: failure (SA) | 0.740 | 0.055 | 46.326 | 16.277 |
| Utility vaginal vault: complication | 0.751 | 0.064 | 33.535 | 11.119 |
| Utility abdominal vault: complication | 0.697 | 0.094 | 15.962 | 6.939 |
| Utility Vault trial: complication (SA) | 0.724 | 0.057 | 43.804 | 16.699 |
| Other | ||||
| Utility dead | 0.000 | N/A | N/A | N/A |
| Utility long term: containment | 0.684 | 0.105 | 12.726 | 5.879 |
| Utility long term: well | 0.846 | 0.024 | 190.509 | 34.679 |
Assessment of cost-effectiveness
The model analysis was conducted using second-order Monte Carlo (probabilistic or second-order) simulation, with 1000 iterations. Each iteration resampled a value from each input parameter based on the defined distributions. The model was therefore fully probabilistic, with 1000 expected values of costs and QALYs generated for each treatment strategy from the sampling distributions. These cost and QALY values were combined into a single measure of efficiency and reported as incremental costs per QALY gained, commonly referred to as the ICER. Interventions reporting an ICER of < £20,000–30,000 per QALY gained are generally considered cost-effective. Interventions that generate cost savings and higher QALYs than a comparator are the dominant treatment and offer an even stronger case for cost-effectiveness. Conversely, interventions that cost more and generate fewer QALYs than a comparator are said to be dominated and are not considered a cost-effective use of resources.
The simulation approach allows fully probabilistic ICERs to be obtained for both the base case and all sensitivity analyses conducted. CEACs were generated by assessing the probabilistic NMB for each treatment strategy. The CEACs allow for calculation of the probability that each strategy is cost-effective at alternative threshold values of WTP for a QALY gained. Similarly, scatterplots of incremental costs and incremental QALYs were obtained for uterine preservation versus vaginal hysterectomy and report the results on the cost-effectiveness plane. Both CEACs and the scatterplots illustrate the uncertainty surrounding the optimal treatment strategy caused by the combined statistical variability in the model’s parameter estimates.
Deterministic sensitivity analysis
Cost-effectiveness acceptability curves illustrate sampling uncertainty, but do not address underlying uncertainty driven by choice of data, structural assumptions, methodological choices or heterogeneity across subgroups of the modelled cohorts. A range of deterministic sensitivity analyses were explored to address the main areas of uncertainty in the model and test the impact of important assumptions on the cost-effectiveness conclusions. Model parameters for all deterministic sensitivity analyses are sampled from the statistical distributions, ensuring that all presented ICERs are fully probabilistic. Sensitivity analyses were conducted to:
-
Explore uncertainty surrounding the choice of model utilities and costs. The base-case analysis uses treatment-specific utilities and costs for each health state in the model. These data are advantageous in that they preserve the impact of treatment on the costs and QoL in any given health state. However, for rare events, they are derived from trial data with small samples and mean values subject to rare but extremely severe health events. Sensitivity analysis explores the impact of using utilities and costs that are defined for each modelled health state, but not split according to randomised treatment in the VUE trial. This increases the sample for estimating costs and utilities, but removes any assumption about treatment-specific effects on these parameters for the model. The base-case analysis (treatment-specific utilities) assumes that baseline utility for the cohort may be different across groups. This could potentially limit generalisability to the wider population. Therefore, a further sensitivity analysis explores keeping treatment-specific utilities for health states, but ensuring that the baseline utility for the cohort is the same in both uterine preservation and vaginal hysterectomy (i.e. 0.760).
-
Determine the impact of intervention costing approach on cost-effectiveness. The trial-based analyses showed that estimates of cost-effectiveness for uterine preservation versus vaginal hysterectomy were highly sensitive to the costing approach used for the interventions. A similar sensitivity analysis is conducted for the model-based analysis to determine whether or not the implications of intervention costing approach remain an important determinant of cost-effectiveness in the longer term.
-
Determine the impact of heterogeneity in the cohort’s characteristics on cost-effectiveness. The mean age of the women in the Uterine trial was 63 years. Sensitivity analysis explores the implications for cost-effectiveness of varying the age of women at the point of entry to the model between 53 and 73 years.
-
Determine the impact of methodological uncertainty on the results. Long-term data on the time to failure and complications are lacking and so estimates have been developed for the model based on survival models, extrapolating short-term findings over the longer term. Although the models represent a good fit to the observed trial data and are clinically plausible, the models may nonetheless fail to accurately capture the true (unknown) long-term complication and failure rates of surgery. The impact of any modelling inaccuracies on cost-effectiveness is likely to be magnified over longer-term time horizons. Therefore, sensitivity analysis shortens the time horizon to 5, 10 and 20 years.
-
Determine the impact of methodological uncertainty surrounding the appropriate discount rate to apply to future costs and QALYs. Sensitivity analyses vary the discount rate between 0% and 6% in line with NICE best practice guidelines. 63
Cost-effectiveness results
Base-case results
Results from cost-effectiveness analyses are usually reported with treatments ranked in ascending order of QALYs (or costs). However, as there are only two treatment strategies in this model, to ensure ease of interpretation, all ICERs are presented for uterine preservation versus vaginal hysterectomy. The base-case and sensitivity analyses are reported in Table 38. The base-case analysis indicates that uterine preservation is not a cost-effective use of resources compared with vaginal hysterectomy. This is because uterine preservation entails an additional surgical procedure in the treatment pathway, with, on average, higher failure rates compared with vaginal hysterectomy. Therefore, it is associated with additional costs and QoL decrements associated with having an additional surgery and treatment failure in the treatment pathway.
| Sensitivity analysis | Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER | Percentage cost-effective at £30,000 (%) |
|---|---|---|---|---|---|---|---|
| 1 | Base case | ||||||
| Vaginal hysterectomy | 4428 | 12.44 | 78 | ||||
| Uterine preservation | 4814 | 386 | 12.29 | –0.15 | Dominated | 22 | |
| 2 | 0 discount rate | ||||||
| Vaginal hysterectomy | 6012 | 17.7 | 70 | ||||
| Uterine preservation | 6436 | 424 | 17.55 | –0.15 | Dominated | 30 | |
| 3 | 6 discount rate | ||||||
| Vaginal hysterectomy | 3739 | 10.10 | 83 | ||||
| Uterine preservation | 4101 | 362 | 9.96 | –0.14 | Dominated | 17 | |
| 4 | Age 53 years | ||||||
| Vaginal hysterectomy | 4963 | 14.33 | 76 | ||||
| Uterine preservation | 5357 | 394 | 14.22 | –0.11 | Dominated | 24 | |
| 5 | Age 73 years | ||||||
| Vaginal hysterectomy | 3513 | 9.11 | 86 | ||||
| Uterine preservation | 3871 | 358 | 8.96 | –0.15 | Dominated | 14 | |
| 6 | HRG-based internal costs | ||||||
| Vaginal hysterectomy | 6903 | 12.43 | 22 | ||||
| Uterine preservation | 6454 | –258 | 12.29 | –0.14 | £1214.71 | 78 | |
| 7 | Time horizon 5 years | ||||||
| Vaginal hysterectomy | 1962 | 4.02 | 96 | ||||
| Uterine preservation | 2279 | 317 | 3.91 | –0.11 | Dominated | 4 | |
| 8 | Time horizon 10 years | ||||||
| Vaginal hysterectomy | 2785 | 7.13 | 93 | ||||
| Uterine preservation | 3158 | 373 | 6.97 | –0.16 | Dominated | 7 | |
| 9 | Time horizon 20 years | ||||||
| Vaginal hysterectomy | 3979 | 11.01 | 83 | ||||
| Uterine preservation | 4342 | 363 | 10.85 | –0.16 | Dominated | 17 | |
| 10 | Removing general population utility age adjustment | ||||||
| Vaginal hysterectomy | 4428 | 13.34 | 79 | ||||
| Uterine preservation | 4814 | 386 | 13.19 | –0.15 | Dominated | 21 | |
| 11 | Turn off treatment-specific costs | ||||||
| Vaginal hysterectomy | 4614 | 12.43 | 78 | ||||
| Uterine preservation | 4605 | –9 | 12.29 | –0.14 | £128.57 | 22 | |
| 12 | Turn off treatment-specific utility | ||||||
| Vaginal hysterectomy | 4434 | 12.28 | 4 | ||||
| Uterine preservation | 4808 | 374 | 12.39 | 0.11 | £3318 | 96 | |
| 13 | Assume equal baseline utility in both arms | ||||||
| Vaginal hysterectomy | 4434 | 12.43 | 79 | ||||
| Uterine preservation | 4808 | 374 | 12.29 | –0.015 | Dominated | 21 | |
The base-case CEAC illustrating sampling uncertainty is reported in Figure 17. Scatterplots of cost-effectiveness for uterine preservation versus vaginal hysterectomy are reported in Figure 18, with 95% confidence ellipses illustrated. The results show that, although vaginal hysterectomy is the most likely cost-effective treatment at a range of plausible values of the decision-maker’s willingness to pay for 1 QALY, there remains a 20–30% chance that vaginal hysterectomy is not a cost-effective use of resources.
FIGURE 17.
Cost-effectiveness acceptability curve for base-case results.
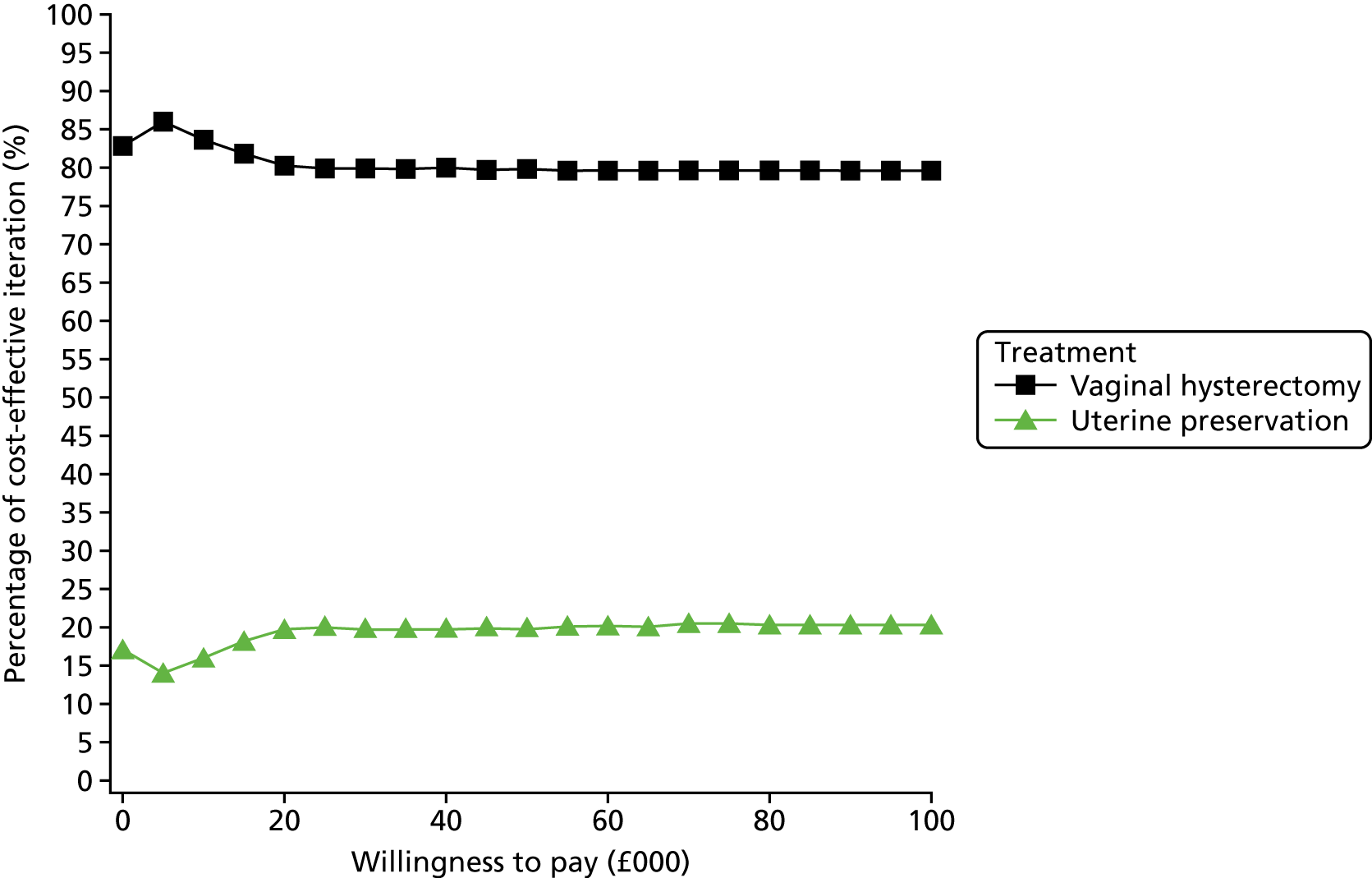
FIGURE 18.
Incremental cost-effectiveness (uterine preservation vs. vaginal hysterectomy).

Sensitivity analysis results
The results from all sensitivity analyses undertaken are reported in Table 38 alongside the base-case results. A full set of CEACs and incremental scatterplots for each analysis undertaken can be found in Appendix 8, Figures 45–68. In general, the base-case findings are robust to the sensitivity analyses around age adjustment of health-state utilities, discount rates, starting age and starting utility of the modelled cohort. As illustrated by sensitivity analyses 7–9, the uncertainty in cost-effectiveness increases over longer time horizons, when projections of the trade-offs between complications and failures become less clear. For all the time horizons considered, the model shows vaginal hysterectomy to be the most cost-effective strategy.
The cost projections from the model remain somewhat sensitive to the choice of intervention costing approach. These results echo the findings of the trial-based economic evaluation, indicating that the methodology used to assign initial treatment costs is important when projecting total costs. However, the use of HRG costing does not change the conclusions about the most cost-effective treatment option unless society were to place zero value on 1 QALY. Under the HRG costing approach, uterine preservation becomes marginally less costly, but at the expense of QALY losses. Therefore, if society would require compensation of at least £20,000–30,000 for 1 QALY lost, it is unlikely that this analysis would change the cost-effectiveness conclusions.
The model is sensitive to the choice of costs and utilities used to populate the health states, in particular the ‘well’ health state. Treatment-specific costs and utilities both tend to favour vaginal hysterectomy, whereas using averages from the Uterine trial, as a whole, favours uterine preservation. The finding is driven primarily by the treatment-specific utilities and costs for the proportion of the cohort who are in the ‘well’ state. The regression analyses indicate slightly lower costs in the ‘well’ state following vaginal hysterectomy and higher utility compared with vaginal preservation. Turning off treatment-specific costs and utilities removes this difference between the groups. As failure and complication rates are low and transient in the model (state duration for failure and complications is 6 months), the majority of the cohort spend most of the model in some form of ‘well’ state, hence the sensitivity of the results to this assumption.
Interpretation of the model results
The model describes the important long-term trade-offs between the choice of uterine preservation versus vaginal hysterectomy. The model is populated with the best available data on failures and complications following surgery. However, the maximum follow-up to date of 2 years means data are immature, and it is unlikely that all the important trade-offs between failures and complications have been observed across the groups. It is therefore crucial that the model analyses are updated once more mature data on time-to-failure and complication events become available. From a decision-making point of view, the ICER is therefore sensitive to the true long-term failure and complication rates, and in particular cross-group differences. In addition, the model describes a set of scenarios and assumptions that favours uterine preservation and vaginal hysterectomy, respectively.
Within the modelling analysis and structure of the care pathway, advantages of uterine preservation include:
-
Keeping the cohort out of a final health state in which no other options are available apart from conservative management and long-term containment for longer. This means that the cohort avoid long-term costs of containment and the QoL implications of having no other potentially curative surgical options available.
-
The use of HRG intervention costing. The implications of HRG compared with component costing have been discussed in Chapters 4 and 7.
Issues favouring the cost-effectiveness of vaginal hysterectomy include:
-
Lower intervention costs, based on a shorter surgical procedure, using the component-based costing approach described in Chapter 2.
-
Potentially lower short-term failure rates. Although differences in time to event are not statistically significant, point estimates of the hazard ratios favouring hysterectomy are propagated through the model. Fewer failures mean a lower likelihood of costly surgical procedures and the QoL benefits of experiencing a failure later than with uterine preservation.
Chapter 10 Overarching discussion
The lack of long-term information for POP surgery to improve both counselling and information for women has been highlighted by NICE, but also to guide clinicians on best practice in terms of surgical approach, particularly in relation to apical prolapse given the introduction of new techniques over the last two decades. 23,24,27–29
Both the uterine and the Vault trials presented in this report are the largest multicentre RCTs to assess surgical treatments for apical compartment prolapse to date. They provide information on outcome measures up to 12 months after randomisation and surgery in terms of women’s symptoms, their satisfaction and objective clinical assessment by POP-Q. The VUE trial will become a major contributor to current evidence and provides the opportunity to identify the most appropriate surgical care pathway for the majority of women with apical prolapse in the long term given the variability in surgical practice. 16,67–71 An update of recent evidence, including the VUE trial data, has also been completed (see Appendix 9, Figures 69–84).
Trial recruitment and treatment choice
Around 50% of parous women report symptoms of POP8,72 and have a lifetime risk of between 6% and 20% of undergoing surgery for prolapse,3,5,16,17 this is dependent on symptoms, QoL and functional impact.
Women within the VUE trial had requested surgical treatment for different reasons; the most bothersome symptom was ‘a feeling of something coming down’ (see Chapters 4 and 7). This symptom has been found to have a sensitivity of 84% and a specificity of 94% for prolapse at, or beyond, the hymenal ring. 73 Other symptoms prompted women to consider surgery, such as faecal incontinence (more common in the Vault trial), dragging in the abdomen or back or incomplete bladder emptying, although there is no clinical evidence that confirms that these symptoms have any direct relationship attributable to prolapse74 and surgery may potentially increase the risk.
A considerable number of eligible women chose not to participate in the Uterine trial during the initial planned recruitment time, which affected recruitment rate, and a 15-month recruitment extension was required. The main reason given to recruitment staff for non-participation was of the women’s wish to have their uterus removed (see Chapter 3). This finding suggests the complex nature of the decision-making process and it would be interesting to further explore reasons through a qualitative study (see Appendix 10) given that previous literature suggests that 31–60% would choose uterine preservation. 25,75,76 The recruitment rate for the Vault trial was not affected (see Chapter 6).
During the recruitment phase of the VUE trial, debate on the use of mesh implants in POP repair surgery intensified. The potential insertion of mesh, though not necessarily transvaginally, was an important decision-making point for many women. However, some women were unaware of the publicity and their knowledge depended on their own social circle. This also highlights, and reinforces, the need for high-quality standardised patient information provision on the options available for women before surgery, to allow informed decision-making and person-centred care. 41,42,77 In both the Uterine and the Vault trials, two women had a mesh exposure from 235 mesh procedures at 12 months after surgery, and both underwent surgical mesh removal. This provides a mesh exposure rate per procedure of 0.9% for the first 12 months. One of the two mesh exposures was due to a transvaginal concomitant procedure. Mesh exposure rates have been considered and reported as much greater for transvaginal mesh41,42,77 and further monitoring of the VUE trial participants is taking place to identify if the natural timeline when using mesh for apical surgery is different given the different approach. The exposure rates may also be lower than national statistical data, as the surgeons involved in the VUE trial are recognised as being highly skilled; therefore, the study rates may not accurately reflect actual rates.
Clinical findings before operation
The POP-Q scoring system for objectively quantifying prolapse is considered highly reliable for research purposes, though whether or not apical prolapse should use the same standards as anterior and posterior prolapse has been debated. It has been suggested that the scoring system does not allow sufficient detail for apical descent, such as in women with isolated cervical elongation or a large anterior wall prolapse that obscures the vault location. 78–81 At present it remains unchanged,82 as there is no unanimous view and no alternative to replace the present system. In the VUE trial, a number of women had minimal apical descent but were included in the trial (see Chapters 3 and 6), suggesting that there may be some women for whom scoring by POP-Q has been less accurate. 82 Of note, women in the Vault trial were found to have higher POP-SSs (i.e. symptoms were more bothersome), but their POP-Q scores were not necessarily as severe as those women in the Uterine trial (see Chapters 3 and 6). This may suggest a reduced tolerance to prolapse or functional symptoms, or perhaps this cohort actually suffers more from prolapse as a result of the absent uterus.
In addition to apical descent in the VUE trial population, anterior compartment descent was also present and not only led to more planned anterior repairs preoperatively, but may have also contributed to increases in baseline POP-SSs (see Chapters 3 and 6). This anatomical coexistence has been recognised previously in studies and may possibly be caused by pelvic floor dynamics, irrespective of uterine presence. However, it is possible that anterior prolapse may have been over diagnosed, particularly in those with vault prolapse, where the apical and anterior compartment are less well defined,83,84 and highlights the complexity and outpatient limitations of specific compartment preoperative surgical assessment and planning.
Some POP-Q anterior compartment and apical scoring (C+/–D; dependent on uterine presence) may have related to observer error, though the majority of clinicians had participated in the previous RCT, PROSPECT,1 and were considered experienced in the scoring process.
Diurnal variation of the prolapse presentation and bowel/bladder distension may have also affected vaginal compartments and the overall POP-Q scores.
The findings suggest that objective clinical assessment of prolapse by POP-Q is not necessarily the highest priority for surgical decision-making given that women who had low POP-Q scores did not consistently have reduced POP-SSs, and this has been reported elsewhere. 85
Overall, this preoperative clinical process and subsequent randomisation of women in the VUE trial aligns with the pragmatism of clinical day-to-day practice, enabling better insight into surgical practice for this cohort of women.
Surgical procedures and their differences
The majority of women did receive their randomly allocated surgery, though a number did not (see Chapters 4 and 7). It is possible that factors, such as lifestyle change, weight loss, oestrogen treatment and physiotherapy, could have benefited some women, causing them to change their mind about the need for surgery. It is also possible that the presence of a uterus may improve results from these conservative approaches. 86
Some women also received different surgery to that proposed preoperatively after examination under anaesthetic, suggesting that a preoperative POP-Q assessment may be useful and cost-effective if symptoms or significant lifestyle changes have occurred since surgical listing. This pre-emptive action may be useful for women whose initial examination was by a less experienced clinician. It is also possible that assessment under anaesthetic has allowed a more thorough evaluation of the vaginal compartments, but may also overestimate the degree of prolapse as the pelvic floor is paralysed. This reaffirms the need for comprehensive preoperative counselling and consent (both verbal and written) in relation to the potential change in surgical plans under anaesthesia. 39
Although both the Uterine and the Vault trials were evaluating different approaches to apical repair, associated factors, such as the use of different types of anaesthesia, are also likely to have influenced final outcome measures or surgical decisions within theatre. Procedures undertaken by a vaginal approach were more likely to involve a spinal or regional anaesthetic (see Chapters 4 and 7). Abdominal procedures were more likely to require a general anaesthetic, particularly so in those receiving laparoscopic procedures when respiratory and operating position requirements are important. Overall, abdominal procedures (open/laparoscopic) for both trials were significantly longer than vaginal procedures (see Chapters 4 and 7), and given that prolapse surgery affects the more elderly population, this approach may have exposed women to an increased anaesthetic risk (particularly in those with associated morbidity). This was not identified within the VUE trial, but the relatively healthy women in the trial and self-selected group of participating surgeons may not be representative to provide reassurance for more widespread use.
Recently, robotic laparoscopic surgery has been considered for abdominal pelvic floor procedures, although small RCTs to date have indicated that the operation is even longer and operating costs are higher. 87 These early findings are unlikely to favour its general clinical use in the current NHS and further evaluation is required.
As agreed in the VUE trial protocol,31 synthetic mesh was utilised in some of the transvaginal procedures, whether for concomitant surgery or the apical compartment. Transvaginal mesh use changed during the lifetime of the VUE trial, with a reduction in use for prolapse surgery and a consistent number (though small) for incontinence. The ongoing debate on the use of mesh implants and disseminated publications from national and professional organisations during the time of the trial was likely to be a causal factor affecting women’s and surgeons’ choice of surgical approach. 88 This trend is also in line with a recent UK national prolapse survey, and at present the use of transvaginal mesh has been paused by NHS England and NHS Northern Ireland. 23,24,27–29,77,89–91
Mesh implants were required in all abdominal procedures (see Appendices 3 and 5), though information on specific anatomical site mesh application was not requested/retrieved on the intraoperative CRF. As a result, it is not possible to identify those surgeons who indirectly or specifically repaired the anterior compartment either by an additional anterior leaf of mesh or by their individual technique during abdominal surgery. This does, however, emphasise the pragmatic approach of the variability in surgical practice in relation to mesh position for individual patients.
More concomitant anterior and posterior repairs were undertaken in the vaginal groups for both trials (see Chapters 4 and 7). This may be related to the local surgical field given the good visibility of other compartmental descent compared with abdominal procedures, and its outcome is discussed in The VUE trial of the future.
Overall, intraoperative complications and subsequent SAE rates in the VUE trial, irrespective of surgical approach, were lower than previously published rates for pelvic floor surgery. 92 This finding may be because of the surgical experience in the self-selected cohort of participating gynaecologists in the trial, as described in Trial recruitment and treatment choice.
Women’s symptoms after operation
Prolapse symptoms outcomes at 6 months after surgery, at 12 months after randomisation, with a clinic appointment for POP-Q assessment 12 months after surgery were collected (see Chapters 4 and 7).
The use of this method of data collection may be considered by some clinical investigators as controversial, but has robust statistical support and is commonly used in drug trials (Clinical Trials of an Investigational Medicinal Product). By data retrieval at 6 months after surgery, the POP-SS is very specific and aligned to time allocation for surgery. However, in routine clinical practice, women do undergo a waiting time before surgery, hence the outcome data at 12 months after randomisation is equivalent to a patient’s normal clinical pathway. This post-randomisation analysis at 12 months offers the expectations in improvement 12 months after treatment allocation occurred. The 12 months after surgery clinical assessment enables the surgeon to assess objective changes after the procedure. This time differential (c. 60 days) will become irrelevant for outcome measures over the longer term, which will be critical in informing health-care commissioners, health-care professionals and consumers to provide estimates of treatment effects currently not available in the literature.
Financial considerations for all
Women who were in paid employment and required time off work for surgery were found to require longer convalescence in the uterine preservation group in the Uterine trial (see Chapter 5). This may affect future generations in relation to surgery options given the increasing pensionable age and employment legislation, particularly in relation to unpaid leave, and will undoubtedly become integral in decision-making for the women themselves.
At 12 months, the randomised surgical approaches for each trial were found to be clinically effective in the relief of symptoms and objective surgical outcome. However, in both trials the short-term cost-effective impact and the health economic findings suggest that vaginal surgical approaches for apical prolapse may be more beneficial (see Chapters 5 and 8). The differential was based on additional re-operation rates for uterine preservation cohorts in the Uterine trial as well as longer operating times. The differential was more evident in the Vault trial when direct comparison of abdominal and vaginal approaches were made. This finding may suggest that current HRG classification for health-care costs in terms of pelvic floor surgery may require more frequent revision and amendment to accurately reflect costs and the rate of change in surgical techniques offered by clinicians. Regular long-term follow-up of women in both trials, particularly in relation to health economics, will enable more accurate costings for the present apical surgical techniques. This will identify if the predictions presented in this publication hold true for the long-term future.
The VUE trial of the future
Currently, the women participating in the VUE trial are being followed up for 6 years after randomisation, though this time frame does not provide a thorough evaluation of long-term outcomes given the expected increase in lifespan. A follow-up of up to 12 years is hoped to be achieved to enable longer-term evaluation of outcomes. This would include women who did not receive any form of surgery for prolapse.
Women participating in the VUE trial were generally in their seventh decade, with a slightly older cohort in the Vault trial (see Chapters 3 and 6). Longer-term follow-up may be more difficult because of age-related diseases and/or death. Alternative methods for data collection and outcome measures require recognition of abilities and cognitive function (i.e. age specific) over time.
Although there was no difference in POP-Q scores in the Uterine trial between the two groups, in the Vault trial there was a statistical difference in relation to the anterior wall prolapse after a vaginal vault repair (although the numbers were small). Anatomical correction does not guarantee functional improvement in neighbouring systems, with the risk of deterioration, such as de novo urinary incontinence or faecal incontinence, as was evident in both trials (see Chapters 4 and 7). Women participating in the VUE trial did experience bowel and bladder symptoms that required hygiene measures after their operation despite improved POP-Q scores (see Chapters 4 and 7). The cause of bowel and bladder dysfunction is a complex issue, but the resultant effects on QoL and finance for an elderly woman are substantial. This emphasises the need for assessment of potential short-, medium- and long-term changes made by different surgical techniques given the expected overall age-related hand–eye co-ordination, mobility changes and potential life expectancy of women in the future.
Preservation of the uterus is not without risk. Long-term information about additional investigations and treatments required for those women who had uterine preservation will be particularly important and may require analysis taking into account actual treatment received for accurate risk information. This would also enable the additional personal, financial and health impact to be scrutinised rather than prolapse symptomatology in isolation as an outcome measure. This is likely to be considered an important measure for health-care providers, clinicians and women alike.
The majority of women within the VUE trial were postmenopausal and results are generalisable for this cohort of women. It is somewhat difficult to extrapolate these outcomes for women under the age of 45 years who may also present with an apical prolapse that remains unresponsive to conservative treatments. These women are more likely to be fertile and require cervical screening, both of which may be unacceptable if uterine removal is an option. Uterine preservation prolapse surgery is possible and used presently for those women pursuing fertility preservation and future pregnancy (although excluded from the VUE trial). Although comprehensive adequately powered long-term data will not become available from the VUE trial for this age group, subanalysis of those women randomised to uterine preservation who were under 45 years of age may assist in information on its benefits or otherwise for apical prolapse.
Limitations
Although few participating women were lost to follow-up overall in both the Uterine and the Vault trials, the proportion of women who were successfully included was < 50% of those initially identified. Although not all of these women would have been eligible for the VUE trial, it may have allowed for an increased generalisability.
The surgeons who participated in the VUE trial chose the uterine preservation procedure they were most familiar with, which, although allowed pragmatic practice, did allow for greater heterogeneity in the uterine preservation arm of the Uterine trial. However, some highly skilled laparoscopic surgeons familiar with the VUE trial surgical procedures did not take part and may have influenced the uterine preservation cohort outcomes in relation to health economic and clinical outcomes. There were several reasons for this: surgical bias to particular procedures, site difficulties with timing of ethics approval, and research and development processes.
A small number of participating women did not undergo surgery and this cohort was not offered a 12-month post-surgical examination for POP-Q evaluation. The women were, however, included in the POP-SS outcomes at 12 months. The retrieval of POP-Q scores from these women who had no surgery may have impacted the POP-Q outcomes and also provided some information on the natural progression or regression of prolapse in women with apical prolapse.
The questionnaire response rate and that of clinic attendance have also identified a greater clinical attendance than questionnaire return. This suggests that women may place greater importance on objective assessment of prolapse by a health-care professional than their symptoms through questionnaire completion. However, it is possible women do not understand the importance of their feedback through serial structured questionnaire completion over time and this requires further evaluation.
Health-care costs were greater for abdominal procedures; however, the cost may still be artificially lower than the actual costings given that surgical equipment costs within each trial were assumed to be equivalent, and this may not be the case if disposable instruments were used. This information was not collected in the VUE trial other than additional costings for mesh implantation and should be considered for any further trials in the future. In addition, there was a greater exposure to health-care professionals after an operation if women had undergone uterine preservation; this may be artificially high at present given the limited experience of primary care with these procedures and the requirement of more specialist advice. If this hypothesis is true, then a trend to less health-care input will occur. Further investigation into primary health-care behaviours in relation to new interventions may be useful to inform a health-care economic analysis.
The blinding of participants was particularly difficult given the different surgical approaches and the required counselling before the operation. Blinding participants to their surgical procedure by performing sham incisions to conceal the route of surgery was not considered necessary or ethical. This would have caused falsely increased operating times, pain relief and recovery times in those having vaginal operations and unlikely to have had an impact on women’s 12-month primary outcomes. However, it was more useful to blind the POP-Q assessor at the 12-month examination, although this was sometimes difficult if the assessor had also been the surgeon or if the patient divulged the procedure before examination.
Future generations
In conclusion, this publication reports primarily on a postmenopausal population of women with apical prolapse who experienced different obstetric care from today (primarily in terms of fewer caesarean sections and forceps deliveries). The change in obstetric practice, in addition to societal changes tending towards smaller families, has a potential to reduce the pelvic floor insult associated with childbirth. 93 Linkage to increases in caesarean section rate and reduced instrumental vaginal delivery has demonstrated a reduction in lifetime risk for prolapse surgery. 67,71 In addition, the impact from reduced hysterectomy rates through changes in gynaecological practice may also improve pelvic floor health. 94–97 Over time it is therefore possible that prolapse requiring surgical treatment may be significantly reduced or delayed for several years in life. This may limit the opportunity for further large RCTs for both uterine and vault prolapse in the future because of a potential reduction in the number of women requiring treatment. However, this hypothesis does not take into account the increasing incidence of obesity worldwide, which may also affect prolapse98 and its related symptoms. 99 Further trials for assessment of uterine preservation for prolapse may also not be possible over time given the increased risk of gynaecological cancers related to obesity.
With the increasing information on the impact of mesh-related procedures worldwide, the use of synthetic mesh implants must also be scrutinised in the long term. The use of mesh and its positioning is particularly important in relation to all structures within the pelvis and abdomen. Subsequent abdominal or pelvic surgery may become more difficult if adherent mesh from a prolapse repair is nearby to the operating field, causing increased open procedures and increased lengths of hospital stay. This must also be considered and reviewed in the long term given the increasing longevity of human life.
Although evidence to date has not been favourable for biological graft support in pelvic floor surgery, some trials have shown promise. 100–103 It is therefore possible that the present synthetic mesh may also be further adapted or totally replaced by more advanced biological graft material for prolapse surgery to allow clinically effective and potentially safer treatments. This research and development will undoubtedly take many years and high resources and may await studies, such as the VUE trial, to identify their role, if any, before manufacture.
Conclusion
There was no evidence that an abdominal procedure was more clinically effective for either a uterine or vault prolapse in terms of all measured outcomes in the short term, but the abdominal procedures were slightly more expensive to perform. To identify if predictions described in this publication hold true (see Chapter 9 for long-term predictions over the next 30 years in relation to any benefit difference), long-term follow-up of the VUE trial participants will provide a unique opportunity to collect long-term information on the most clinically effective, safe and cost-effective treatment for apical prolapse.
Acknowledgements
The authors wish to thank the women who participated in the VUE trial. The authors would also like to thank Janice Cruden for her secretarial support and data management; Graeme MacLennan [Centre for Healthcare Randomised Trials (ChaRT) Director (from 2017)]; the programming team in CHaRT, led by Gladys McPherson (2012 to 2016) and Mark Forrest (2016–present); Christine Dallas, Joan Henderson and Angela Allan for recruitment and participant follow-up; members of the Project Management Group for their ongoing support and advice; Cynthia Fraser (Health Services Research Unit) and Sheila Wallace (Cochrane Incontinence Review Group) for literature searches; Danielle Pirie, Katie Gillies and Sharon McCann for a qualitative evaluation to better understand issues impacting on the decision-making process (VUE-Qual); the independent members of the TSC and DMC; and the staff at the recruiting sites who facilitated recruitment, treatment and follow-up of trial participants.
The Health Services Research Unit and the Health Economics Research Unit are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Members of the Project Management Group
Dwayne Boyers, Suzanne Breeman, Kevin Cooper, Lynda Constable, Janice Cruden, Andrew Elders, Mark Forrest, Robert Freeman, Cathryn Glazener, Beatriz Goulao, Suzanne Hagen, Christine Hemming, Mary Kilonzo, Graeme MacLennan, Alison McDonald, Isobel Montgomery, John Norrie and Anthony Smith.
Independent members of the (1) Trial Steering Group and (2) Data Monitoring Committee
-
Tony Falconer (Chairperson), Pamela Warner, Trish Emerson (Patient Representative) and Ian Ramsay.
-
Jim Thornton (Chairperson), Angela Crook, Lucia Dolan, Gill Gyte (Patient Representative) and James Neilson.
Members of the VUE trial group responsible for recruitment in the clinical centres
Aberdeen
Aberdeen Royal Infirmary
Kevin Cooper (Principal Investigator), Christine Hemming, Cathryn Glazener, Angela Allan, Christine Dallas and Joan Henderson.
Ayrshire and Arran
University Hospital Crosshouse
Wael Agur (Principal Investigator), Danielle Gilmour, Ashraf Habib, Margo Henry and Inna Sokolova.
Barnsley
Barnsley Hospital NHS Foundation Trust
Meenakshi Dass, Khaled Farag (Principal Investigators), Carol Denniss, Kerry Kay and Michelle Reid.
Basingstoke
Basingstoke and North Hampshire Hospital
Christian Phillips, Tim Sayer (Principal Investigators), Clare Rowejones, Gail Harding, Jackie Moody and Lorraine Rush.
Birmingham
Birmingham Women’s Hospital
Philip Toozs-Hobson (Principal Investigator), Paula Trinham, Pallavi Latthe, Shanteela McCooty, Fidan Israfilbayli and Supriya Bulchandani.
Bolton
Royal Bolton Hospital
Abimbola (Bim) Williams (Principal Investigator), Katrina Rhead, Raksha Mistry, Narmada Katakam, David Parkinson, Prasanta Chatterjee and Philip Chia.
Bradford
Bradford Teaching Hospitals NHS Trust
Carmel Ramage (Principal Investigator), Anne Bowyer and Carolyn Robertson.
Bristol
Bristol Royal Infirmary
Chendrimada Madhu (Principal Investigator), Carol Brain and Julie Plant.
Cambridge
Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust
Mark Slack (Principal Investigator), Julie Ellis, Helen Bowyer, Rohna Kearney, Rekha Remadevi, Patrick Forbes, Benjamin Thomas, Frances New, Alex Derpapas and Vladimir Revicky.
Chester
Countess of Chester NHS Foundation Trust
Mofid Ibraheim (Principal Investigator), Nichola Kearsley, Janet Spriggs, Hollie Delvin, Lorraine Dinardo, Helen Eccleson and Charlotte Perkins.
Cornwall
Royal Cornwall Hospital
Farah Lone (Principal Investigator), Jackie Dingle, Lisa Tembath, Lynne Graves, Rob Holmes, Stephanie Beal-Toms, Amanda Datson, Alyson Andrew, Jessica Summers, Fiona Hammonds and Abigail Weeks.
Croydon
Croydon University Hospital
Ranee Thakar (Principal Investigator), Madhu Naidu, Abdul Sultan, Dimos Sioutis and Aswini Balachandran.
Cumbria
The Cumberland Infirmary North Cumbria University Hospitals NHS Trust
Mohamed Matar (Principal Investigator), Toni Wilson, Iain Cameron, Rachel McCarthy, Beverley McGuirk, Gareth Woring and Una Poultney.
Derby
Royal Derby Hospital, Derby Hospitals NHS Foundation Trust
Jaydip Dasgupta (Principal Investigator), Elaine Coulborn and Jill Smith.
Durham
University Hospital North Durham
Rob Wood (Principal Investigator), Jean Dent, Fiona Lloyd, S. Sen, Velur Sindhu, Vicki Atkinson and David Rollins.
Exeter
Royal Devon and Exeter NHS Foundation Trust
Myles Taylor (Principal Investigator), Jacqui Tipper and Alison Potter.
Fife (Dunfermline)
Queen Margaret Hospital
Chu Lim (Principal Investigator), Linsey Burd, Carolyn McKinley, Isla Smith, Julie Aitken and Keith Boath.
Gloucester
Gloucester Royal Hospital
Mark James (Principal Investigator) and Anna Umali.
Guy’s and St Thomas’
Guy’s and St Thomas’ Hospital
Azar Khunda (Principal Investigator), Ellie Stewart, Eduard Cortes, Con Kelleher and Ramandeep Basra.
Harrogate
Harrogate & District NHS Foundation Trust
Tracy Jackson (Principal Investigator), Louise Wills, Caroline Bennett, Adrian Barnett, Joyce Guy and Michael Critchley.
Hinchingbrooke
North West Anglia (formerly Hinchingbrooke Healthcare NHS Trust)
Ashish Pradhan (Principal Investigator), Helen Bowyer, Pamela Orakci, Julie Ellis, Helen Johnson and Jo Bytham.
Huddersfield
Calderdale Royal Hospital
Yi Ling Chan (Principal Investigator), Judith Kitchingman, Anu Bondili, R Acharyya, Simone Ryan, Lisa Gledhill, S Jamau and Majed Shendy.
Hull
Hull and East Yorkshire Hospitals NHS Trust
Jagdish Ghandi (Principal Investigator), Helen Bexhell, Kim Mitchelson, Helen Brown, Sarah Wilson and Joss Cook.
Kent (Darent Valley)
Darent Valley Hospital Dartford and Gravesham NHS Trust
Rob MacDermott (Principal Investigator), Helen Woolridge, Cathy Chambers, Susan Lord, Alison Anstead, Abhishek Gupta, Andreas Lesseps, Angeli Thallon, Rachel Abrahams, Sherma Turner, Zoe Prime, Carmel Stuart and Tracy Edmunds.
Kettering
Kettering General Hospital NHS Foundation Trust
Sunil Doshi (Principal Investigator) and Carla Christie.
Leicester
Leicester General, University Hospitals Leicester NHS Trust
Douglas Tincello (Principal Investigator), Carla Christie, Katie Behan, Jennifer King, Rupa Modi, Catherine Wilkins, Christopher Mayne and Angie Dashain.
Liverpool
Liverpool Women’s NHS Foundation Trust
Ruben Trochez (Principal Investigator), Pamela Corlett, Gillian Smith, B Thomas, Elizabeth Kane, Liz Adair, Jill Bolderson, June Hanford, Alison Forsythe and Gillian Fowler.
Luton and Dunstable
Luton and Dunstable University Hospital
Abdalla Fayyad (Principal Investigator) and Rose Ann Chin.
Manchester
St Mary’s Hospital
Anthony Smith (Principal Investigator), Jayne Budd, David Iles, Clare Waters, Fiona Reid, Karen Ward, Lucy Dwyer, Julie Melville, Stephanie Bateman, Rebecca Leech, Rohna Kearney and Hannah Phillips.
Medway
Medway NHS Foundation Trust
Hasib Ahmed (Principal Investigator), Sandhu Banher, Eunice Emeakaraoha and Maya Basu.
Mid Yorkshire
Pinderfields Hospital/Dewsbury and District, Mid Yorkshire
Kathryn Fishwick (Principal Investigator), Thelma Darian, Sarah Buckley, Claire Townend, Richard Philip Assassa, Tracey Lowry, Aimee Hayton, Eloise Marsh and Ellis Burton.
Newcastle
Royal Victoria Infirmary
Karen Brown (Principal Investigator), Liz Dixon and Paul Hilton.
Norfolk
James Paget University Hospitals NHS Foundation Trust
Mumtaz Rashid (Principal Investigator), Sylvia Kishan and Sally Martin.
North Devon
North Devon District Hospital
Seumas Eckford (Principal Investigator), Geraldine Belcher, Fiona Hammonds, Amanda Skinner and Osama Eskandar.
North Staffordshire
University Hospital of North Midlands
Jason Cooper (Principal Investigator), Suzanne Jerreat, Angie Rooney, Amanda Redford, E Hubball, Fidelma O’Mahony and B Purwar.
North Tees
University Hospital of North Tees
Santhosh Puthuraya (Principal Investigator), Anne-Marie Jones, Sharon Gowans, Elaine Gouk, Rebecca Fletcher, Susan Kelsey, Sarah Croft and Jean Bage.
Nottingham
Nottingham University Hospitals
Richard Parkinson (Principal Investigator), Andy Jarvis, Paul Hooper, Mausumi Das, Bria McAllister, Sarah Farnan and Delia Bester.
Plymouth
Derriford Hospital
Robert Freeman (Principal Investigator), Heidi Hollands, Marie Weinling, Anu Dua, Amanda Carney, Beverley Cree, Sally Pedlar and Laura Jean.
Portsmouth
Queen Alexandra Hospital
Claire Burton (Principal Investigator), Deirdre Rodgers, Amanda Hungate, Glynis Hamer, Ahmad Ali, Rebecca Hardcastle, Chris Guyer, Rachel Watson and Sreebha Rajesh.
Preston
Royal Preston Hospital
Sanjeev Prashar (Principal Investigator), Sue Flintoff, Anne Gardner, Pauline Macdonald, Brice Rodriguez and Julie Butler.
Sheffield
Sheffield Teaching Hospitals NHS Foundation Trust
Swati Jha, Stephen Radley (Principal Investigators), Sheila Duffy, Chris Wragg, Clare Pye, Julie Cook and Andrew Farkas.
South Tees
Friarage Hospital/James Cook University Hospital, South Tees Hospitals
Paul Ballard (Principal Investigator), Julie Potts, Colette Anderson, Aethele Khunda, Sarah Croft, Chou Phay Lim, Callum Allison, Gulsanga Afridi, Alex Mortimer, Hazel Alexander and Helen Cuthbert.
Southport and Ormskirk
Southport and Ormskirk Hospital NHS Trust
Shaireen Aleem (Principal Investigator), Heidi Ribchester and Zena Haslam.
Sunderland
Sunderland Royal Hospital
Jonathan Chamberlain (Principal Investigator), Kirsten Herdman, Eileen Walton, Denise Milford and Judith Ormonde.
Swindon
Great Western Hospital, Swindon
Tamer Abdelrazik and Tracey Sargent.
Taunton
Musgrove Park Hospital
Adel Naguib (Principal Investigator), Paula Hill, Lisa Bowern, Sue Mahoney, Libby Graham, Debs Heard, Nicola Thorne, Kirsty O’Brien and Rachel Coe.
Torbay
Torbay Hospital
Subramanian Narayanan (Principal Investigator), Pauline Mercer, Barbara Finson, Sarah Mills, Bianca Hulance, Megan Harris, Natalie Taylor, Gabrielle de Selincourt, Josie Garfield Smith, Hannah Griffin and Michelle Allison.
West Midlands
Heart of England NHS Foundation Trust
Gurminder Matharu (Principal Investigator), Sam Stafford, Sylvia Turner and Lucy Ingram.
Wirral
Arrowe Park Hospital
Jeremy Weetch (Principal Investigator), Julie Grindey, Tom Aust and Mark Doyle.
Wolverhampton
New Cross Hospital/Royal Wolverhampton
Khaled Afifi (Principal Investigator), Ayman Elnaqa, Sharon Kempson, Nick Denyer, Marion McCormick, Maisie Orgill and Laura Gardiner.
Worcester
Worcester Royal Hospitals NHS Trust
Deepali Sinah (Principal Investigator), Dawn Kelly, Kim Powles, Sue Tohill and Angus Thomson.
Contributions of authors
Christine Hemming [Consultant Gynaecologist and Chief Investigator (April 2016 to present), NHS Grampian and University of Aberdeen] contributed to the conception and the design of the trial, recruitment of participants, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Lynda Constable [Trial Manager, Co-chief Investigator (April 2016 to present)] was responsible for the day-to-day management of the trial, the interpretation of the results and the writing/editing of the report.
Beatriz Goulao (Statistician) conducted the statistical analyses, contributed to drafting the report and reviewed the final report.
Mary Kilonzo (Health Economist) contributed to the conception and the design of the trial, conducted the analysis of the health economics data and the writing of the health economics chapters, and commented on other chapters of the report.
Dwayne Boyers (Health Economist) conducted the analysis of the health economics data and the writing of the health economics chapters and commented on other chapters of the report.
Andrew Elders (Statistician) contributed to the conception and the design of the trial, oversaw the statistical analyses and provided commentary on the draft chapters of the report.
Kevin Cooper (Consultant Obstetrician and Gynaecologist, NHS Grampian) contributed to the conception and design of the trial, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
Anthony Smith (Consultant Gynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
Robert Freeman (Consultant Gynaecologist) contributed to the conception and design of the study, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
Suzanne Breeman (Trial Manager, Triallist) contributed to the conception, design and conduct of the trial, and provided commentary on the draft chapters of the report.
Alison McDonald (Senior Trial Manager, Triallist) contributed to the conception, design and conduct of the trial, and provided commentary on the draft chapters of the report.
Suzanne Hagen (Professor of Statistics and Deputy Director of the Nursing, Midwifery and Allied Health Professionals Research Unit) contributed to the conception and the design of the trial, and provided commentary on the draft chapters of the report.
Isobel Montgomery (Patient Representative) contributed to the conception and the design of the trial, participated in trial meetings from the perspective of a patient who has undergone prolapse surgery and reviewed the report.
John Norrie [Professor of Statistics and Director of CHaRT (to 2016)] contributed to the conception, design and conduct of the trial, and provided commentary on the draft chapters of the report.
Cathryn Glazener [Chief Investigator (to April 2016) and Emeritus Professor] contributed to the conception, design and conduct of the trial, the interpretation of the results and the writing/editing of the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Publication
Glazener C, Constable L, Hemming C, Breeman S, Elders A, Cooper K, et al. Two parallel, pragmatic, UK multicentre, randomised controlled trials comparing surgical options for upper compartment (vault or uterine) pelvic organ prolapse (the VUE Study): study protocol for a randomised controlled trial. Trials 2016;17:441.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Glazener C, Breeman S, Elders A, Hemming C, Cooper K, Freeman R, et al. Clinical effectiveness and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomised controlled trials within a comprehensive cohort study – results from the PROSPECT Study. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20950.
- Hunskaar S, Burgio KL, Clark A, Lapitan MC, Nelson R, Sillen U, et al. Incontinence 3rd Edition. Bristol: International Continence Society; 2005.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501-6. https://doi.org/10.1016/S0029-7844(97)00058-6.
- Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG 2006;113:39-46. https://doi.org/10.1111/j.1471-0528.2005.00773.x.
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311-16. https://doi.org/10.1001/jama.300.11.1311.
- Samuelsson EC, Victor FTA, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol 1999;180:299-305. https://doi.org/10.1016/S0002-9378(99)70203-6.
- Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse?. Am J Obstet Gynecol 2003;189:372-7. https://doi.org/10.1067/S0002-9378(03)00698-7.
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002;186:1160-6. https://doi.org/10.1067/mob.2002.123819.
- Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD004010.pub3.
- Oliver R, Thakar R, Sultan AH. The history and usage of the vaginal pessary: a review. Eur J Obstet Gynecol Reprod Biol 2011;156:125-30. https://doi.org/10.1016/j.ejogrb.2010.12.039.
- Maher C, Baessler K, Barber M, Cheon C, Consten E, Cooper K. Pelvic Organ Prolapse Surgery (Committee 15) n.d.
- Abrams P, Cardozo L, Wagg A, Wein A. Incontinence. Bristol: International Continence Society; 2017.
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD003882.pub4.
- Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev 2010;9. https://doi.org/10.1002/14651858.CD007063.pub2.
- Weber MA, Kleijn MH, Langendam M, Limpens J, Heineman MJ, Roovers JP. Local oestrogen for pelvic floor disorders: a systematic review. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0136265.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014;123:1201-6. https://doi.org/10.1097/AOG.0000000000000286.
- Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol 2010;116:1096-100. https://doi.org/10.1097/AOG.0b013e3181f73729.
- NHS Digital . Hospital Admitted Patient Care Activity, 2016–17 2017. https://digital.nhs.uk/catalogue/PUB30098 (accessed 24 January 2018).
- Office for National Statistics . Z1 – Zipped Population Projections Data Files, UK 2017. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/z1zippedpopulationprojectionsdatafilesuk (accessed 24 January 2018).
- National Institute for Health and Care Excellence . Uterine Prolapse n.d. www.nice.org.uk/guidance/conditions-and-diseases/gynaecological-conditions/uterine-prolapse/products?Status=Published (accessed February 2019).
- National Institute for Health and Care Excellence . Vault Prolapse n.d. www.nice.org.uk/Search?q=vault+prolapse (accessed 24 January 2018).
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev 2016;10. https://doi.org/10.1002/14651858.CD012376.
- National Institute for Health and Care Excellence . Sacrocolpopexy With Hysterectomy Using Mesh to Repair Uterine Prolapse. Interventional Procedures Guidance IPG577 2017. www.nice.org.uk/guidance/ipg577 (accessed 4 July 2017).
- National Institute for Health and Care Excellence . Sacrocolpopexy Using Mesh to Repair Vaginal Vault Prolapse. Interventional Procedures Guidance IPG583 2017. www.nice.org.uk/guidance/ipg583 (accessed 23 January 2018).
- Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 2013;122:233-41. https://doi.org/10.1097/AOG.0b013e318299a6cf.
- Jia X, Glazener C, Mowatt G, MacLennan G, Bain C, Fraser C, et al. Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG 2008;115:1350-61. https://doi.org/10.1111/j.1471–0528.2008.01845.x.
- National Institute for Health and Care Excellence . Uterine Suspension Using Mesh (Including Sacrohysteropexy) to Repair Uterine Prolapse. Interventional Procedures Guidance IPG584 2017. www.nice.org.uk/guidance/ipg584 (accessed 23 January 2018).
- National Institute for Health and Clinical Excellence . Infracoccygeal Sacropexy Using Mesh to Repair Uterine Prolapse. Interventional Procedures Guidance IPG582 2017. www.nice.org.uk/guidance/ipg582 (accessed 23 January 2018).
- National Institute for Health and Care Excellence . Infracoccygeal Sacropexy Using Mesh to Repair Vaginal Vault Prolapse. Interventional Procedures Guidance IPG581 2017. www.nice.org.uk/guidance/ipg581 (accessed 23 January 2018).
- Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;4. https://doi.org/10.1002/14651858.CD004014.pub5.
- Glazener C, Constable L, Hemming C, Breeman S, Elders A, Cooper K, et al. Two parallel, pragmatic, UK multicentre, randomised controlled trials comparing surgical options for upper compartment (vault or uterine) pelvic organ prolapse (the VUE Study): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063–016–1576-x.
- Toozs-Hobson P, Freeman R, Barber M, Maher C, Haylen B, Athanasiou S, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for reporting outcomes of surgical procedures for pelvic organ prolapse. Int Urogynecol J 2012;23:527-35. https://doi.org/10.1007/s00192–012–1726-y.
- Hagen S, Glazener C, Sinclair L, Stark D, Bugge C. Psychometric properties of the pelvic organ prolapse symptom score. BJOG 2009;116:25-31. https://doi.org/10.1111/j.1471–0528.2008.01903.x.
- Hagen S, Glazener C, Cook J, Herbison P, Toozs-Hobson P. Further properties of the pelvic organ prolapse symptom score: minimally important change and test-retest reliability. Neurourol Urodyn 2010;29:1055-6.
- Dolan P. Modelling valuations for EuroQoL health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Abrams P, Avery K, Gardener N, Donovan J. ICIQ Advisory Board . The international consultation on incontinence modular questionnaire. J Urol 2006;175:1063-6. https://doi.org/10.1016/S0022-5347(05)00348-4.
- Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardisation of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10-7. https://doi.org/10.1016/S0002-9378(96)70243-0.
- Nygaard I, Brubaker L, Zyczynski HM, Cundiff G, Richter H, Gantz M, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016-24. https://doi.org/10.1001/jama.2013.4919.
- Fayyad A, Hill S, Gurung V, Prashar S, Smith AR. How accurate is symptomatic and clinical evaluation of prolapse prior to surgical repair?. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1179-83. https://doi.org/10.1007/s00192–007–0306-z.
- Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237-41.
- Scottish Government . Transvaginal Mesh Implants Independent Review: Final Report 2017. www.gov.scot/Publications/2017/03/3336/0 (accessed 1 February 2018).
- NHS England . Mesh Oversight Group Report 2017. www.england.nhs.uk/wp-content/uploads/2017/07/mesh-oversight-group-report.pdf (accessed 1 February 2018).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed February 2019).
- Information Services Division Scotland . Specialty Costs – Detailed Tables n.d. www.isdscotland.org/Health-topics/Finance/Costs/Detailed-Tables/Speciality-Costs/index.asp (accessed 6 February 2018).
- Department of Health and Social Care . NHS Reference Costs 2015–16 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 3 May 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016 n.d. www.pssru.ac.uk/project-pages/unit-costs/2016/index.php (accessed 13 May 2017).
- Information Services Division Scotland . ISD Scotland 63 n.d. www.isdscotland.org/Health-Topics/Finance/Costbook/Speciality-Costs/Overhead.asp (accessed October 2012).
- Information Services Division Scotland . R140X Theatre Services n.d. www.isdscotland.org/Health-topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 1 February 2018).
- NHS Business Services Authority . Electronic Drug Tariff n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 1 October 2018).
- Information Services Division Scotland . Costs: File Listings n.d. www.isdscotland.org/Health-Topics/Finance/Costs/File-Listings-2016.asp (accessed 11 January 2017).
- Warrington Clinical Commissioning Group . Continence and Catheter Care Formulary 2014. www.warringtonccg.nhs.uk/Page%20Images/public-info/Catheter%20Care%20Formulary%20Revised%20February%202014%20revised.pdf (accessed February 2019).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2016 Provisional Results 2016. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2016provisionalresults (accessed 11 January 2017).
- EuroQoL Group . EuroQoL: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University; 2005.
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual 2014. www.nice.org.uk/guidance/pmg20/resources/developing-nice-guidelines-the-manual-pdf-72286708700869 (accessed 18 May 2017).
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36. https://doi.org/10.1177/1536867X0500500404.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. https://doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Office for National Statistics . Interim Life Tables 2007–2009 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies#datasets (accessed February 2019).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 7 February 2018).
- Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials – Extrapolation with Patient-level Data. Manchester: National Institute for Health and Care Excellence; n.d.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. https://doi.org/10.1177/0272989X12472398.
- Ara R, Wailoo A. NICE DSU Technical Support Document 12: The Use of Health State Utility Values in Decision Models. Manchester: National Institute for Health and Care Excellence; n.d.
- Løwenstein E, Ottesen B, Gimbel H. Incidence and lifetime risk of pelvic organ prolapse surgery in Denmark from 1977 to 2009. Int Urogynecol J 2015;26:49-55. https://doi.org/10.1007/s00192–014–2413-y.
- Haya N, Baessler K, Christmann-Schmid C, de Tayrac R, Dietz V, Guldberg R, et al. Prolapse and continence surgery in countries of the Organisation for Economic Cooperation and Development in 2012. Am J Obstet Gynecol 2015;212:755.e1-755.e27. https://doi.org/10.1016/j.ajog.2015.02.017.
- Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–97. Am J Obstet Gynecol 2003;188:108-15. https://doi.org/10.1067/mob.2003.101.
- Shah AD, Kohli N, Rajan SS, Hoyte L. The age distribution, rates, and types of surgery for pelvic organ prolapse in the USA. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:421-8. https://doi.org/10.1007/s00192–007–0457-y.
- Lisonkova S, Lavery JA, Ananth CV, Chen I, Muraca G, Cundiff GW, et al. Temporal trends in obstetric trauma and inpatient surgery for pelvic organ prolapse: an age-period-cohort analysis. Am J Obstet Gynecol 2016;215:208.e1-208.e12. https://doi.org/10.1016/j.ajog.2016.02.027.
- Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol 2000;183:277-85. https://doi.org/10.1067/mob.2000.107583.
- Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:272-84. https://doi.org/10.1007/s00192-005-1314-5.
- Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol 2001;185:1332-7. https://doi.org/10.1067/mob.2001.119078.
- Frick AC, Barber MD, Paraiso MF, Ridgeway B, Jelovsek JE, Walters MD. Attitudes towards hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg 2013;19:103-9. https://doi.org/10.1097/SPV.0b013e31827d8667.
- Korbly NB, Kassis NC, Good MM, Richardson ML, Book NM, Yip S, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol 2013;209:470.e1-6. https://doi.org/10.1016/j.ajog.2013.08.003.
- National Institute for Health and Care Excellence . Transvaginal Mesh Repair of Anterior or Posterior Vaginal Wall Prolapse 2017. www.nice.org.uk/guidance/ipg599 (accessed 13 February 2018).
- Lin TY, Su TH, Wang YL, Lee MY, Hsieh CH, Wang KG, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc 2005;104:249-53.
- Neuman M, Lavy Y. Conservation of the prolapsed uterus is a valid option: medium term results of a prospective comparative study with the posterior intravaginal slingoplasty operation. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:889-93. https://doi.org/10.1007/s00192-006-0262-z.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ?. Int Urogynecol J 2012;23:625-31. https://doi.org/10.1007/s00192-011-1635-5.
- Pan K, Cao L, Ryan NA, Wang Y, Xu H. Laparoscopic sacral hysteropexy versus laparoscopic sacrocolpopexy with hysterectomy for pelvic organ prolapse. Int Urogynecol J 2016;27:93-101. https://doi.org/10.1007/s00192-015-2775-9.
- Haylen BT, Maher CF, Barber MD, Camargo S, Dandolu V, Digesu A, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J 2016;27:165-94. https://doi.org/10.1007/s00192-015-2932-1.
- Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol 2006;195:1837-40. https://doi.org/10.1016/j.ajog.2006.06.065.
- Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:137-42. https://doi.org/10.1007/s00192-007-0405-x.
- Barber MD, Brubaker L, Nygaard I, Wheeler TL, Schaffer J, Chen Z, et al. Pelvic Floor Disorders Network . Defining success after surgery for pelvic organ prolapse. Obstet Gynecol 2009;114:600-9. https://doi.org/10.1097/AOG.0b013e3181b2b1ae.
- Hagen S, Stark D, Glazener C, Dickson S, Barry S, Elders A, et al. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet 2014;383:796-80. https://doi.org/10.1016/S0140-6736(13)61977-7.
- Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomised controlled trial. Obstet Gynecol 2011;118:1005-13. https://doi.org/10.1097/AOG.0b013e318231537c.
- King AB, Goldman HB. Stress incontinence surgery at the time of prolapse surgery: mandatory or forbidden?. World J Urol 2015;33:1257-62. https://doi.org/10.1007/s00345-015-1591-7.
- Jha S, Cutner A, Moran P. The UK National Prolapse Survey: 10 years on. Int Urogynecol J 2018;29:795-801. https://doi.org/10.1007/s00192–017–3476–3.
- BBC News . Immediate Stop to NHS Mesh Operations n.d. www.bbc.co.uk/news/health-44763673 (accessed 7 July 2018).
- Smyth C. Pause in Surgical Mesh Use to Be Extended to Northern Ireland n.d. www.bbc.co.uk/news/uk-northern-ireland-44772354 (accessed 10 July 2018).
- Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair: A systematic review. Obstet Gynecol 2009;113:367-73. https://doi.org/10.1097/AOG.0b013e318195888d.
- Glazener C, Elders A, MacArthur C, Lancashire RJ, Herbison P, Hagen S, et al. Childbirth and prolapse: long-term associations with the symptoms and objective measurement of pelvic organ prolapse. BJOG 2013;120:161-8. https://doi.org/10.1111/1471-0528.12075.
- Aigmueller T, Dungl A, Hinterholzer S, Geiss I, Riss P. An estimation of the frequency of surgery for posthysterectomy vault prolapse. Int Urogynecol J 2010;21:299-302. https://doi.org/10.1007/s00192–009–1033–4.
- Marchionni M, Bracco GL, Checcucci V, Carabaneanu A, Coccia EM, Mecacci F, et al. True incidence of vaginal vault prolapse. Thirteen years of experience. J Reprod Med 1999;44:679-84.
- Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol 1988;158:872-81. https://doi.org/10.1016/0002-9378(88)90088-9.
- Cooper K, Lee A, Chien P, Raja E, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG 2011;118:1171-9. https://doi.org/10.1111/j.1471-0528.2011.03011.x.
- Bradley CS, Nygaard IE, Brown MB, Gutman RE, Kenton KS, Whitehead WE, et al. Bowel symptoms in women 1 year after sacrocolpopexy. Am J Obstet Gynecol 2007;197:642.e1-8. https://doi.org/10.1016/j.ajog.2007.08.023.
- Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol 2001;184:1496-501. https://doi.org/10.1067/mob.2001.114868.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomised controlled trial. Obstet Gynecol 2013;121:143-51. https://doi.org/10.1097/AOG.0b013e31827558dc.
- Claerhout F, De Ridder D, Van Beckevoort D, Coremans G, Veldman J, Lewi P, et al. Sacrocolpopexy using xenogenic acellular collagen in patients at increased risk for graft-related complications. Neurourol Urodyn 2010;29:563-7. https://doi.org/10.1002/nau.20805.
- Quiroz LH, Gutman RE, Shippey S, Cundiff GW, Sanses T, Blomquist JL, et al. Abdominal sacrocolpopexy: anatomic outcomes and complications with Pelvicol, autologous and synthetic graft materials. Am J Obstet Gynecol 2008;198:557.e1-5. https://doi.org/10.1016/j.ajog.2008.01.050.
- Deprest J, De Ridder D, Roovers JP, Werbrouck E, Coremans G, Claerhout F. Medium term outcome of laparoscopic sacrocolpopexy with xenografts compared to synthetic grafts. J Urol 2009;182:2362-8. https://doi.org/10.1016/j.juro.2009.07.043.
- PROACT Medical Ltd . Our Products 2018. www.proactmedical.co.uk/our-products (accessed 20 June 2019).
- Roovers JP, van der Vaart CH, van der Bom JG, van Leeuwen JH, Scholten PC, Heintz AP. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG 2004;111:50-6. https://doi.org/10.1111/j.1471-0528.2004.00001.x.
- Malandri M, Iordanidou E, Takou M, Moraitis B, Balaxis D. A Randomised Comparison of Two Vaginal Procedures for the Treatment of Stage Two, or Higher Uterine Prolapse: Hysterectomy With Mesh Versus Only Mesh Implantation n.d.
- Detollenaere RJ, Kreuwel IA, Dijkstra JR, Kluivers KB, van Eijndhoven HW. The impact of sacrospinous hysteropexy and vaginal hysterectomy with suspension of the uterosacral ligaments on sexual function in women with uterine prolapse: a secondary analysis of a randomised comparative study. J Sex Med 2016;13:213-19. https://doi.org/10.1016/j.jsxm.2015.12.006.
- Carramao S, Auge APF, Pacetta AM. A randomised comparison of two vaginal procedures for the treatment of uterine prolapse using polypropylene mesh: histateroxy versus hysterectomy. Rev Col Bras Cir 2009;36:65-72.
- Juneja M, Munday D, Kopetz V, Barry C. Hysterectomy Vs No Hysterectomy for Uterine Prolapse in Conjunction With Posterior Infracococcygeal Colpopexy – A Randomised Pilot Study 12 Months Review n.d.
- Dietz V, van der Vaart CH, van der Graaf Y, Heintz P, Schraffordt Koops SE. One-year follow-up after sacrospinous hysteropexy and vaginal hysterectomy for uterine descent: a randomised study. Int Urogynecol J 2010;21:209-16. https://doi.org/10.1007/s00192-009-1014-7.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomised pilot study. Int Urogynecol J 2015;26:1687-94. https://doi.org/10.1007/s00192-015-2761-2.
- Jeng CJ, Yang YC, Tzeng CR, Shen J, Wang LR. Sexual functioning after vaginal hysterectomy or transvaginal sacrospinous uterine suspension for uterine prolapse: a comparison. J Reprod Med 2005;50:669-74.
- Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomised study. Am J Obstet Gynecol 2004;190:20-6. https://doi.org/10.1016/j.ajog.2003.08.031.
- Maher CF, Feiner B, Decuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol 2011;204:360.e1-7. https://doi.org/10.1016/j.ajog.2010.11.016.
- Lim YN, Rosamilia A, Dwyer PL, Alvarez J, Chao F, Murray C, et al. Randomised controlled trial of post-hysterectomy vaginal vault prolapse treatment with extraperitoneal vaginal uterosacral ligament suspension with anterior mesh reinforcement vs sacrocolpopexy (open/laparoscopic). Int Urogynecol J 2012;23:S48-9.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Social Sci Med 1997;45:1337-55. https://doi.org/10.1016/S0277-9536(97)00063-4.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Fung EK, Loré JM. Randomised controlled trials for evaluating surgical questions. Arch Otolaryngol Head Neck Surg 2002;128:631-4. https://doi.org/10.1001/archotol.128.6.631.
- McCann S, Campbell M, Entwistle V. Recruitment to clinical trials: a meta-ethnographic synthesis of studies of reasons for participation. J Health Serv Res Policy 2013;18:233-41. https://doi.org/10.1177/1355819613483126.
- Jackson CJ, Dixon-Woods M, Eborall H, Kenyon S, Toozs-Hobson P, Tincello DG. Women’s views and experiences of a patient preference trial in surgery: a qualitative study of the CARPET1 trial. Clin Trials 2010;7:696-704. https://doi.org/10.1177/1740774510381286.
- Jenkins V, Farewell V, Farewell D, Darmanin J, Wagstaff J, Langridge C, et al. TTT Steering committee . Drivers and barriers to patient participation in RCTs. Br J Cancer 2013;108:1402-7. https://doi.org/10.1038/bjc.2013.113.
Appendix 1 Additional information for Chapter 2
| ROME criteria: any two of | Equivalent VUE trial questions |
|---|---|
| < 3 bowel movements per week | Stool passing once a week or less |
| Straining | Straining most or all of the time |
| Lumpy or hard stools | Hard stools |
| Sensation of incomplete defecation | Feeling that the bowel has not completely emptied most or all of the time |
| Manual manoeuvring required to defecate | Manual manoeuvre to empty bowel most or all of the time (splinting of perineum or vagina, or digital evacuation of the bowel) |
| Sensation of anorectal obstruction | No equivalent VUE trial question |
Final model (Uterine and Vault trials)
The Uterine and Vault trials had the same primary outcome and were analysed using the same statistical model. The notation followed by the model, used in both trials, is presented in Table 40.
| Yij | Continuous outcome (POP-SS at 12 months) in the j-th subject in the i-th surgeon |
| β0 | Average response in the control group |
| β1 | Treatment effect from the addition of uterine preservation/abdominal vault procedure (treatment effect) |
| β2 | Explanatory variable for baseline POP-SS |
| β3 | Explanatory variable for previous prolapse planned surgery (minimisation) |
| β4 | Explanatory variable for previous incontinence planned surgery (minimisation) |
| β5 | Explanatory variable for age category (minimisation) |
| Aij | Indicator variable equal to 1, if participant has been exposed to uterine preservation/abdominal vault procedure |
| Bij | Coefficient variable for baseline POP-SS |
| Cij | Indicator variable equal to 1, if participant has had previous prolapse planned surgery |
| Dij | Indicator variable equal to 1, if participant has had previous incontinence planned surgery |
| Eij | Indicator variable equal to 1, if participant is 60 years old or over |
| bi | Surgeon-level random effect |
| εij | Residual error term |
The final model is the following:
The surgeon-level random effects bi is assumed to be normally distributed with mean 0 and variance σA2, i.e. bi ∼ N(0, σA2). The error term was assumed to be:
Unit costs for trial-based economic evaluation
| Anaesthesia type | Drug | Unit price (£) | Price per | Resource use | Cost per average case (£) | Comments | Sources |
|---|---|---|---|---|---|---|---|
| General | 1% injection of propofol | 15.36 | 20-ml ampoule | 1 ampoule | 3.07 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 |
| 100 µg of fentanyl | 6.53 | 2-ml ampoule (50 µg/ml) | 1 ampoule | 1.31 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 | |
| Morphine | 9.36 | 1-ml vial | 1 vial | 0.94 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 | |
| Sevoflurane (volatile agent) | 125.00 | 250-ml bottle | 25 ml | 12.50 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 | |
| Laryngeal mask | 29.50 | Box of 10 masks | 1 mask | 2.95 | PROACT Medical Ltd, Corby, UK; disposable laryngeal airway PRO-breathe104 | Price list from the manufacturer’s website | |
| Total general | 20.76 | Based on cost of consumables per case | |||||
| 0.5% injection of anhydrous bupivacaine hydrochloride injection | 9.25 | 4-ml ampoule | 1 ampoule | 1.85 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 | |
| Lidocaine | 0.40 | 10-ml ampoule | 1 ampoule | 0.40 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 | |
| Total cost spinal | 2.25 | Based on cost of consumables per case | |||||
| Cost local | Lidocaine | 0.40 | 10-ml ampoule | 1 ampoule | 0.40 | Based on information used in PROSPECT | BNF;43 and Glazener et al.1 |
| Total general and local | 21.16 | Based on cost of both general and local anaesthesia | BNF;43 and Glazener et al.1 | ||||
| Total general and spinal | 23.01 | Based on cost of both general and spinal anaesthesia | BNF;43 and Glazener et al.1 | ||||
| OPCS | HRG | Average unit cost (£) | Quartile (£) | |||
|---|---|---|---|---|---|---|
| Code | Description | Code | Description | Lower | Upper | |
| Anterior or posterior prolapse | ||||||
| P221/P222/P223 | Anterior and posterior colporrhaphy and amputation of cervix uteri | MA03C | Major open lower genital tract procedures, with a CC score of ≥ 3 | 3204 | 2599 | 3690 |
| P228/P229/P231 | Anterior colporrhaphy and amputation of cervix uteri NEC | MA03D | Major open lower genital tract procedures, with a CC score of 0–2 | 2787 | 2378 | 3156 |
| P232 | Anterior colporrhaphy – NEC | MA04C | Intermediate open lower genital tract procedures, with a CC score of ≥ 3 | 2599 | 2040 | 3062 |
| P233 | Posterior colporrhaphy – NEC | MA04D | Intermediate open lower genital tract procedures, with a CC score of 0–2 | 2231 | 1913 | 2579 |
| Cost used in analysis: anterior procedures only | 2693 | 2209 | 3109 | |||
| Cost used in analysis: posterior procedures only | 2231 | 1913 | 2579 | |||
| Cost used in analysis: both anterior and posterior procedures | 3204 | 2599 | 3690 | |||
| Apical repair/hysterectomy, vault or uterine suspension | ||||||
| P24.3 | Repair of vault of vagina using abdominal approach – NEC | MA02A | Very major open, upper or lower genital tract procedures, with a CC score of ≥ 4 | 2738 | 2366 | 3119 |
| P24.4 | Repair of vault of vagina using vaginal approach – NEC | MA02B | Very major open, upper or lower genital tract procedures, with a CC score of 2 or 3 | 4133 | 3300 | 4691 |
| P24.5 | Repair of vault of vagina with mesh using abdominal approach | MA02C | Very major open, upper or lower genital tract procedures, with a CC score of 0 or 1 | 4133 | 3300 | 4691 |
| P24.6 | Repair of vault of vagina with mesh using vaginal approach | MA03C | Major open lower genital tract procedures, with a CC score of ≥ 3 | 2738 | 2366 | 3119 |
| P24.7 | Sacrospinous fixation of vagina | MA03D | Major open lower genital tract procedures, with a CC score of 0–2 | 4133 | 3300 | 4691 |
| P24.2 | Sacrocolpopexy | MA03C&D | Major open lower genital tract procedures, with a CC score of 0–2 and ≥ 3 | Same as above two rows | ||
| Q08.2 | Vaginal hysterectomy and excision of periuterine tissue – NEC | MA07E | Major open upper genital tract procedures, with a CC score of ≥ 5 | 6289 | 4308 | 7000 |
| Q54.4 | Suspension of uterus using mesh – NEC open | MA07F | Major open upper genital tract procedures, with a CC score of 3 or 4 | 4572 | 3669 | 5069 |
| MA07G | Major open upper genital tract procedures, with a CC score of 0–2 | 3745 | 3084 | 4295 | ||
| Q54.1 | Suspension of uterus – NEC | MA11 | Intermediate open upper genital tract procedures | 3008 | 2429 | 3393 |
| Q54.4 | Suspension of uterus using mesh – NEC laparoscopy | MA08A | Major, laparoscopic or endoscopic, upper genital tract procedures, with a CC score of ≥ 2 | 3719 | 3024 | 4173 |
| Q54.4 | Suspension of uterus using mesh – NEC laparoscopy | MA08B | Major, laparoscopic or endoscopic, upper genital tract procedures, with a CC score of 0–1 | 3228 | 2678 | 3685 |
| Average cost of apical procedures | 4162 | 3335 | 4692 | |||
| P241 | Repair of vault of vagina using combined abdominal and vaginal approach | MA01Z | Complex open, upper or lower genital tract procedures | 5813 | 4067 | 7961 |
| MA02A | Very major open, upper or lower genital tract procedures, with a CC score of ≥ 4a | 6165 | 4842 | 7183 | ||
| MA02B | Very major open, upper or lower genital tract procedures, with a CC score of 2 or 3 | 4875 | 4153 | 5238 | ||
| MA02C | Very major open, upper or lower genital tract procedures, with a CC score of 0 or 1 | 4189 | 3519 | 4732 | ||
| Average cost of two or more of the apical procedures (included hysterectomy, uterine preservation and vault procedures)b | 5261 | 4145 | 6279 | |||
| Urinary incontinence procedures | ||||||
| M522/M523 | Retropubic suspension of neck of bladder | LB59Z | Major, open or laparoscopic, bladder neck procedures (female) | 4037 | 2942 | 4601 |
| Urinary incontinence sling procedures | ||||||
| M533 | Introduction of tension-free vaginal tape | LB51A | Vaginal tape operations for urinary incontinence, with a CC score of ≥ 2 | 2220 | 1732 | 2713 |
| M536 | Introduction of transobturator tape | LB51B | Vaginal tape operations for urinary incontinence, with a CC score of 0 or 1 | 1972 | 1660 | 2188 |
| Average cost of sling incontinence surgery | 2096 | 1696 | 2451 | |||
| WF01A-WF02B | Consultant-led or non-consultant-led visit | 502 | Gynaecology | 130 | 96 | 145 |
| Resource | Product | Manufacturer | Pack size | Packs for 1 year | Unit cost (2017) (£) | Total cost (£) | Source |
|---|---|---|---|---|---|---|---|
| Sterile catheterisation insertion pack | Cath-It (one pack) | Richardson Healthcare Ltd, Borehamwood, UK | 1 | 4 | 1.98 | 7.92 | NHS EDT June 2017 49 |
| Sterile lubricant for instillation | OptiLube sterile lubricating jelly (1 × 11-ml syringe) | Optimum Medical Ltd, Leeds, UK | 1 | 4 | 0.96 | 3.84 | NHS EDT June 2017 49 |
| Indwelling catheter | Folysil X-tra (size 14), pack size 1 | Coloplast A/S, Humlebaek, Denmark | 1 | 6 (4 + 2 spares) | 6.37 | 38.22 | NHS EDT June 2017 49 |
| Leg bags (assumes patients have continuous drainage) | Simpla® Profile, 500 ml, 25-cm tube | Coloplast | 10 | 6 | 25.66 | 153.96 | NHS EDT June 2017 49 |
| Catheter stabilisation device | Leg bag holder – aqua-sleeve, size standard | Coloplast | 4 | 2 | 8.50 | 17.00 | NHS EDT June 2017 49 |
| Night drainage bags | Single use, Prosys® (2) | Clinisupplies Ltd, Harrow, UK | 10 | 37 | 3.06 | 113.22 | NHS EDT June 2017 49 |
| Total | Average annual cost | 334.16 | |||||
| Cost per week | 6.43 | ||||||
| Product | Manufacturer | Pack size | Packs for 1 yeara | Unit cost (2017) (£) | Total cost (£) | Source |
|---|---|---|---|---|---|---|
| hi-slip® Plus | Bullen Healthcare, Liverpool, UK | 30 | 37 | 32.86 | 1215.82 | NHS EDT 2017 49 |
| Advance catheter | Hollister Ltd, Wokingham, UK | 25 | 44 | 36.40 | 1601.60 | NHS EDT 2017 49 |
| SpeediCath® Compact | Coloplast | 30 | 37 | 46.77 | 1730.49 | NHS EDT 2017 49 |
| SpeediCath | Coloplast | 30 | 37 | 46.17 | 1708.29 | NHS EDT 2017 49 |
| HYDROSIL® | Rochester Medical, Stewartville, MN, USA | 30 | 37 | 44.06 | 1630.22 | NHS EDT 2017 49 |
| LoFric® Sense™ | Wellspect Healthcare, Mölndal, Sweden | 30 | 37 | 44.60 | 1650.20 | NHS EDT 2017 49 |
| Average cost for 12 months | 1589.44 | |||||
| Cost for 1 week | 30.56 | |||||
| Name | Dose | Cost (NHS indicative price, £) | Number | Cost per tablet (£)a |
|---|---|---|---|---|
| Nitrofurantoin, 100-mg tablets | 100 mg | 16.80 | 28 tablets | 0.60 |
| Amoxicillin, 500-mg capsules | 500 mg | 0.91 | 15 capsules | 0.06 |
| Cefalexin, 500-mg capsules | 500 mg | 1.43 | 21 capsules | 0.07 |
| Ciprofloxacin, 250-mg tablets | 250 mg | 1.50 | 20 tablets | 0.08 |
| Clindamycin, 150-mg capsules | 150 mg | 9.00 | 24 capsules | 0.38 |
| Darifenacin (as darifenacin hydrobromide), 7.5-mg tablets | 7.5 mg | 25.48 | 28 tablets | 0.91 |
| Flucloxacillin, 250-mg capsules | 250 mg | 1.31 | 28 tablets | 0.05 |
| Trimethoprim, 100-mg tablets | 200 mg | 1.15 | 14 tablets | 0.08 |
| Phenoxymethylpenicillin, 250-mg tablets | 250 mg | 1.06 | 28 tablets | 0.04 |
| Duloxetine, 20-mg gastroresistant capsules | 20 mg | 2.94 | 28 tablets | 0.11 |
| Average cost per tablet | 0.24 | |||
| Type of pessary | Price (£) for onea |
|---|---|
| Ring pessary | |
| Bioteque America (San Jose, CA, USA) | 20.00 |
| GBUK Healthcare (Selby, UK) | 19.00 |
| Milex (CooperSurgical, Inc., Trumball, CT, USA) | 21.28 |
| Average | 20.09 |
| Shelf/Gelhorn pessary | |
| Bioteque America | 21.40 |
| GBUK | 20.49 |
| Milex | 22.92 |
| Average | 21.54 |
Appendix 2 Additional information for Chapter 3
| Centres | Surgeons | Treatment (n) | |
|---|---|---|---|
| Uterine preservation (N = 282) | Vaginal hysterectomy (N = 283) | ||
| James Cook University | Paul Ballard and Aethele Khunda | 34 | 36 |
| Aberdeen | Christine Hemming and Kevin Cooper | 29 | 28 |
| Manchester | Anthony Smith, Fiona Reid, Karen Ward, Carolyn North, Melissa Bradbury and Rohna Kearney | 13 | 13 |
| University Hospital North Tees | Santhosh Puthuraya and Elaine Gouk | 14 | 11 |
| Torbay | Subramanian Narayanan | 10 | 12 |
| Calderdale Royal Hospital | Yi Ling Chan, Anu Bondili and Maged Shendy | 10 | 11 |
| Hinchingbrooke Hospital | Ashish Pradhan, Helen Johnson and Umar Hussain | 10 | 11 |
| Croydon University | Ranee Thakar, Madhu Naidu and Abdul Sultan | 9 | 12 |
| Queen Alexandra Hospital, Portsmouth | Claire Burton, Christopher Guyer, Rebecca Hardcastle and Ali Ahmed | 11 | 10 |
| South Tees | Paul Ballard and Aethele Khunda | 11 | 10 |
| Basingstoke | Christian Phillips and Tim Sayer | 10 | 11 |
| Derriford Hospital, Plymouth | Robert Freeman, Luigi Bombieri and A Dua | 10 | 7 |
| Crosshouse | Wael Agur, Ashraf Habib, Inna Sokolova, Mohamed Riad and David Rae | 7 | 10 |
| Birmingham | Philip Toozs-Hobson, Pallavi Latthe and Supriya Bulchandani | 8 | 8 |
| Royal Preston | Brice Rodriguez and Sanjeev Prashar | 8 | 8 |
| Musgrove Park | Adel Naguib | 7 | 7 |
| Leicester General | Douglas Tincello | 9 | 5 |
| Gloucester | Mark James | 6 | 7 |
| Addenbrooke | Mark Slack, Rohna Kearney, Vladimir Revicky and Alexandros Derpapas | 8 | 4 |
| Royal Derby | Jaydip Dasgupta and Victor Chilaka | 6 | 5 |
| Arrowe Park | Tom Aust, Patrick Doyle and Jeremy Weetch | 6 | 5 |
| Bradford | Carmel Ramage and Sue Calvert | 4 | 5 |
| Harrogate and District | Tracy Jackson and Adrian Barnett | 4 | 4 |
| Liverpool | Ruben Trochez, Gillian Fowler and E Adams | 5 | 3 |
| New Cross Hospital, Wolverhampton | Khaled Afifi, Ayman Elnaqa and Charles Cox | 2 | 6 |
| Jessop Wing, Sheffield | Stephen Radley and Swati Jha | 4 | 3 |
| Heart of England | Gurminder Matharu and Afshan Khaja | 3 | 3 |
| Royal Victoria Infirmary | Karen Brown, Karen Rose and Paul Hilton | 3 | 3 |
| Southport and Ormskirk | Shaireen Aleem | 2 | 3 |
| Fife | Chu Lim and Carolyn McKinley | 3 | 2 |
| North Cumbria | Mohamed Matar | 2 | 3 |
| Kettering General | Sunil Doshi | 2 | 2 |
| Countess of Chester | Mofid Ibraheim and Lorraine Dinardo | 2 | 2 |
| Barnsley | Meenakshi Dass and Khaled Farag | 1 | 2 |
| UH North Staffordshire | Jason Cooper | 2 | 1 |
| Hull and East Yorkshire | Jagdish Gandhi | 2 | 0 |
| Worcester Acute | Deepali Sinha and Angus Thomson | 0 | 2 |
| Cornwall Hospitals | Farah Lone and Rob Holmes | 0 | 2 |
| Southmead Hospital | Chendrimada Madhu | 1 | 1 |
| Royal Bolton | Philip Chia and Abimbola Williams | 1 | 1 |
| Pinderfields | Kathryn Fishwick | 1 | 1 |
| Sunderland Royal | Jonathan Chamberlain | 1 | 0 |
| Medway Maritime Hospital | Maya Basu and Hasib Ahmed | 1 | 0 |
| Dartford and Gravesham | Rob MacDermott, Angeli Thallon, Andreas Lesseps and Abhisheck Gupta | 0 | 1 |
| UH North Durham | Fiona Lloyd, Velur Sindhu, Seema Sen and Robert Wood | 0 | 1 |
| Luton and Dunstable University | Abdalla Fayyad | 0 | 1 |
FIGURE 19.
Rate of recruitment to the Uterine trial.

| Participants | n (%) |
|---|---|
| Approached | 1544 (100.0) |
| Randomised | 565 (36.6) |
| Not included | 979 (63.4) |
| Ineligible | 177 (11.5) |
| Declined | 774 (50.1) |
| Other reasons/missed appointment | 28 (1.8) |
| Reason | n (%) |
|---|---|
| Ineligible | 177 (100) |
| A specific operation is necessary | 77 (43.5) |
| No prolapse | 38 (21.5) |
| Unsuitable owing to medical history | 28 (15.8) |
| Not suitable for surgery | 13 (7.3) |
| Uterine pathology | 11 (6.2) |
| Change of prolapse type from original diagnosis | 10 (5.6) |
| Unable to give informed consent | 9 (5.1) |
| Unable to complete study questionnaires | 9 (5.1) |
| Operation cancelled | 6 (3.4) |
| Declined | 774 (100) |
| Wanted a hysterectomy | 324 (41.9) |
| Declined/refused surgery | 219 (28.3) |
| Did not want to participate in the study | 154 (19.9) |
| Did not want to be randomised | 149 (19.3) |
| Wanted a uterine preservation | 107 (13.8) |
| Wanted to try a pessary | 34 (4.4) |
| Wished to go with surgeon’s choice | 25 (3.2) |
| Wanted a vaginal procedure | 21 (2.7) |
| Did not want mesh | 21 (2.7) |
| Wanted an abdominal procedure | 16 (2.1) |
| Wanted to complete questionnaires | 9 (1.2) |
| Changed mind after initially consenting | 7 (0.9) |
| Other reasons/missed appointment | 28 (3.6) |
| Characteristics | Treatment, mean (SD); N | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Number of | ||
| Normal vaginal deliveries | 2.3 (1.3); 271 | 2.3 (1.1); 277 |
| Caesareans before labour | 0.1 (0.3); 188 | 0.1 (0.3); 191 |
| Breech vaginal deliveries | 0.0 (0.2); 188 | 0.0 (0.2); 188 |
| Forceps deliveries | 0.4 (0.6); 208 | 0.3 (0.5); 206 |
| Caesareans during labour | 0.0 (0.2); 186 | 0.1 (0.3); 197 |
| Vacuum deliveries | 0.0 (0.2); 185 | 0.0 (0.2); 187 |
| Previous anterior surgery | ||
| Mesh used | 1 (0.4) | 3 (1.1) |
| Measurement | Treatment | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| POP-Q measurement (cm), mean (SD); N [range] | ||
| Ba | 1.7 (1.8); 265 [–2.0 to 6.0] | 2.0 (1.9); 267 [–3.0 to 6.0] |
| C | –0.4 (2.6); 265 [–6.0 to 6.0] | 0.4 (2.7); 267 [–6.0 to 6.0] |
| Bp | –1.4 (1.7); 258 [–7.0 to 6.0] | –1.1 (2.3); 265 [–8.0 to 6.0] |
| Total vaginal length | 8.6 (1.4); 251 [3.0 to 13.0] | 8.5 (1.4); 250 [2.0 to 12.0] |
| Overall POP-Q stage, n (%) | ||
| 1 | 2 (0.7) | 1 (0.4) |
| 2a | 11 (3.9) | 12 (4.2) |
| 2b | 115 (41.1) | 116 (41.0) |
| 3 | 141 (50.4) | 137 (48.4) |
| 4 | 5 (1.8) | 16 (5.7) |
| Missing | 6 (2.1) | 1 (0.4) |
| Stage 2b or more | 261 (93.2) | 269 (95.1) |
| Symptom | Treatment, n (%) | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| UI | ||
| None | 24 (8.6) | 21 (7.4) |
| Slight | 109 (38.9) | 115 (40.6) |
| Moderate | 80 (28.6) | 84 (29.7) |
| Severe | 18 (6.4) | 10 (3.5) |
| Very severe | 2 (0.7) | 2 (0.7) |
| Missing | 47 (16.8) | 51 (18.0) |
| Stress UI | 37 (13.2) | 29 (10.2) |
| Missing | 43 (15.4) | 48 (17.0) |
| Urge UI missing | 9 (3.2) | 11 (3.9) |
| Mixed UI | 22 (7.9) | 10 (3.5) |
| Missing | 7 (2.5) | 13 (4.6) |
| UI for no reason | 9 (3.2) | 6 (2.1) |
| Missing | 8 (2.9) | 16 (5.7) |
| UI when asleep | 4 (1.4) | 5 (1.8) |
| Missing | 37 (13.2) | 46 (16.3) |
| UI with sex | 12 (4.3) | 5 (1.8) |
| Missing | 115 (41.1) | 118 (41.7) |
Appendix 3 Additional information for Chapter 4
| Type of surgery | Treatment, n (%); N | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Apical surgery | ||
| No mesh | 157 (63.0); 249 | 242 (92.7); 261 |
| Synthetic non-absorbable | 81 (32.5); 249 | 6 (2.3); 261 |
| Biological | 5 (2.0); 249 | 2 (0.8); 261 |
| Mesh kit | 0 (0); 249 | 1 (0.4); 261 |
| Missing | 6 (2.4); 249 | 10 (3.8); 261 |
| Concomitant surgery | ||
| Anterior repair only | ||
| No mesh | 106 (93.8); 113 | 139 (97.9); 142 |
| Synthetic non-absorbable | 2 (1.8); 113 | 0 (0); 142 |
| Biological | 2 (1.8); 113 | 2 (1.4); 142 |
| Missing | 3 (2.7); 113 | 1 (0.7); 142 |
| Posterior repair only | ||
| No mesh | 17 (81.0); 21 | 21 (95.5); 22 |
| Synthetic non-absorbable | 3 (14.3); 21 | 1 (4.5); 22 |
| Biological | 1 (4.8); 21 | 0 (0); 22 |
| Both anterior and posterior repair | ||
| Anterior compartment only | ||
| No mesh | 78 (95.1); 82 | 84 (98.8); 85 |
| Synthetic non-absorbable | 1 (1.2); 82 | 1 (1.2); 85 |
| Biological | 1 (1.2); 82 | 0 (0); 85 |
| Missing | 2 (2.4); 82 | 0 (0); 85 |
| Posterior compartment only | ||
| No mesh | 75 (91.5); 82 | 84 (98.8); 85 |
| Synthetic non-absorbable | 4 (4.9); 82 | 1 (1.2); 85 |
| Biological | 2 (2.4); 82 | 0 (0); 85 |
| Missing | 1 (1.2); 82 | 0 (0); 85 |
| Continence procedure | ||
| No mesh | 1 (14.3); 7 | 2 (22.2); 9 |
| Synthetic non-absorbable | 6 (85.7); 7 | 7 (77.8); 9 |
| Characteristic | Treatment, n (%) | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Grade of operating gynaecologist | ||
| Consultant | 225 (80.4) | 189 (66.8) |
| Trainee doctor | 24 (8.6) | 53 (18.7) |
| Speciality doctor | 15 (5.4) | 28 (9.9) |
| Missing | 16 (5.7) | 13 (4.6) |
| Grade of anaesthetist | ||
| Consultant | 195 (69.6) | 202 (71.4) |
| Specialty doctor | 47 (16.8) | 43 (15.2) |
| Trainee doctor | 19 (6.8) | 23 (8.1) |
| Missing | 19 (6.8) | 15 (5.3) |
| Anaesthetica | ||
| General | 225 (80.4) | 227 (80.2) |
| Spinal/epidural | 48 (17.1) | 48 (17.0) |
| Local | 19 (6.8) | 34 (12.0) |
| Other | 1 (0.4) | 4 (1.4) |
| Prophylatic antibiotic used | 253 (90.4) | 265 (93.6) |
| Catheter inserted in theatre | 252 (90.0) | 255 (90.1) |
| Vaginal pack | 185 (66.1) | 214 (75.6) |
| Urinary catheter | ||
| Urethral | 250 (89.3) | 255 (90.1) |
| None | 4 (1.4) | 4 (1.4) |
| Suprapubic | 1 (0.4) | 1 (0.4) |
| Missing | 25 (8.9) | 23 (8.1) |
| Details | Treatment | Effect size (95% CI); p-value | |||
|---|---|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | ||||
| Mean (SD); N | Median (P25–75)a | Mean (SD); N | Median (P25–75)a | ||
| Duration of operation (minutes) | 113.7 (50.3); 253 | 102.0 (80.0–135.0) | 103.0 (33.1); 259 | 98.0 (80.0–125.0) | 9.79 (3.50 to 16.07); 0.002b |
| Length of stay (days) | 1.9 (1.2); 262 | 2.0 (1.0–2.0) | 1.9 (1.1); 269 | 2.0 (1.0–2.0) | 0.99 (0.87 to 1.12); 0.8912c |
| Blood loss (ml) | 125.0 (110.7); 246 | 100.0 (50.0–150.0) | 166.3 (147.3); 261 | 125.0 (100.0–200.0) | –42.20 (–62.68 to –21.73); < 0.001a |
| Time to surgery (days) | 61.2 (70.8); 269 | 39.0 (10.0–85.0) | 56.7 (61.6); 261 | 40.0 (8.0–89.0) | |
FIGURE 20.
Distribution of individual POP-SSs at (a) baseline and at follow-up, that is, (b) at 6 months after surgery and (c) at 12 months after randomisation.


FIGURE 21.
Distribution of prolapse-related QoL scores (a) at baseline and at follow-up, that is, (b) at 6 months after surgery and (c) at 12 months after randomisation.
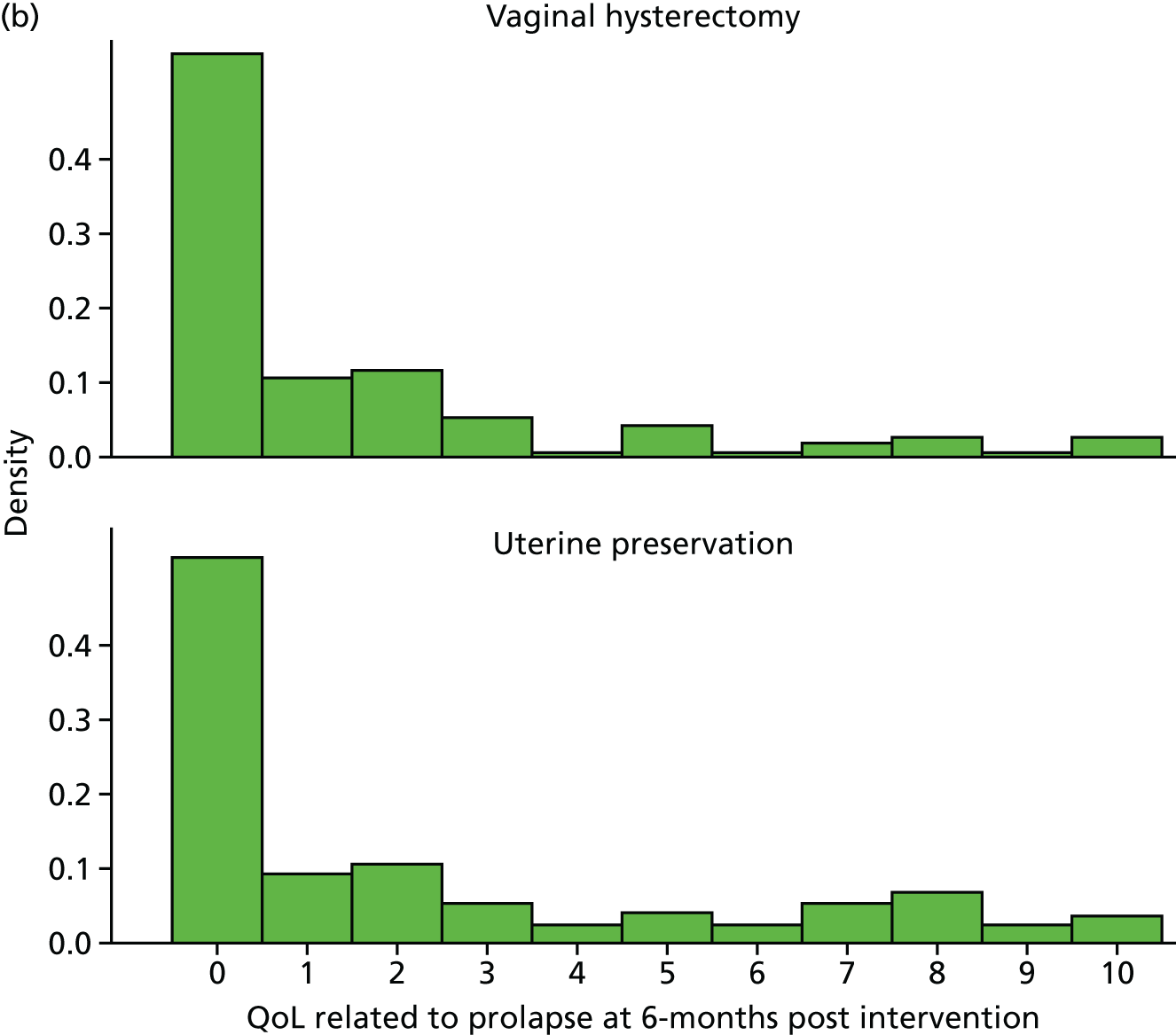

| 6 months after surgery outcomes | Treatment | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| Symptomatic, n (%); N | ||
| Number of women with symptoms | 192 (81.0); 238 | 194 (79.0); 246 |
| Number of women without symptoms | 46 (19.0); 238 | 52 (21.0); 246 |
| Individual prolapse symptoms, n (%); N | ||
| Bowel not empty (any) | 137 (100.0); 137 | 150 (100.0); 150 |
| Bowel not empty (most/all of the time) | 30 (13.0); 240 | 26 (11.0); 246 |
| Bladder not empty (any) | 127 (100.0); 127 | 117 (100.0); 117 |
| Bladder not empty (most/all of the time) | 29 (12.0); 237 | 19 (8.0); 246 |
| Strain to empty bladder (any) | 84 (100.0); 84 | 77 (100.0); 77 |
| Strain to empty bladder (most/all of the time) | 19 (8.0); 237 | 10 (4.0); 246 |
| Dragging in back (any) | 80 (100.0); 80 | 66 (100.0); 66 |
| Dragging in back (most/all of the time) | 15 (6.0); 238 | 8 (3.0); 243 |
| Dragging in abdomen (any) | 73 (100.0); 73 | 62 (100.0); 62 |
| Dragging in abdomen (most/all of the time) | 15 (6.0); 237 | 8 (3.0); 245 |
| Something coming down (any) | 72 (100.0); 72 | 61 (100.0); 61 |
| Something coming down (most/all of the time) | 26 (11.0); 236 | 16 (7.0); 245 |
| Uncomfortable feeling or pain when standing (any) | 50 (100.0); 50 | 53 (100.0); 53 |
| Uncomfortable feeling or pain when standing (most/all of the time) | 16 (7.0); 236 | 11 (4.0); 245 |
| Most bothersome symptom, n (%); N | ||
| Bowel not empty | 59 (43.0); 137 | 49 (40.0); 122 |
| Bladder not empty | 26 (19.0); 137 | 24 (20.0); 122 |
| Something coming down | 21 (15.0); 137 | 19 (16.0); 122 |
| Dragging in back | 15 (11.0); 137 | 15 (12.0); 122 |
| Strain to empty bladder | 8 (6.0); 137 | 8 (7.0); 122 |
| Dragging in abdomen | 5 (4.0); 137 | 2 (2.0); 122 |
| Uncomfortable feeling or pain when standing | 3 (2.0); 137 | 5 (4.0); 122 |
| Which symptom causes most bother not applicable | 92 (40.0); 229 | 113 (48.0); 235 |
| EQ-5D | ||
| Mean (SD); N | 0.844 (0.229); 228 | 0.878 (0.192); 244 |
| Visual Scale, mean (SD); N | 80.2 (17.0); 239 | 82.1 (16.2); 247 |
| Outcome | Treatment | |
|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |
| POP-Q measurement (cm), mean (SD); N [range] | ||
| Ba | –1.3 (1.7); 232 [–6.0 to 6.0] | –1.3 (2.0); 225 [–3.0 to 6.0] |
| C | –6.1 (2.5); 230 [–10.0 to 4.0] | –6.2 (2.9); 225 [–10.0 to 6.0] |
| Bp | –2.5 (1.1); 229 [–9.0 to 4.0] | –2.1 (1.5); 226 [–8.0 to 6.0] |
| Total vaginal length | 8.3 (1.3); 222 [4.0 to 12.0] | 7.9 (1.2); 221 [4.0 to 11.0] |
| Change in D | –3.6 (2.8); 192 [–15.0 to 3.0] | |
| Overall POP-Q individual stage, n (%); N | ||
| Stage 0 | 50 (21.5); 233 | 45 (19.7); 229 |
| Stage 1 | 68 (29.2); 233 | 59 (25.8); 229 |
| Stage 2a | 41 (17.6); 233 | 47 (20.5); 229 |
| Stage 2b | 61 (26.2); 233 | 54 (23.6); 229 |
| Stage 3 | 12 (5.2); 233 | 16 (7.0); 229 |
| Stage 4 | 1 (0.4); 233 | 8 (3.5); 229 |
| Symptom | Treatment, n (%); N | Uterine preservation vs. vaginal hysterectomy, effect size (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | ||
| SUI | 11 (5.8); 189 | 6 (3.2); 190 | 2.04 (0.72 to 5.81); 0.18 |
| Mixed UI | 9 (3.9); 232 | 1 (0.4); 238 | |
| UI for no reason | 0 (0) | 2 (0.9); 232 | |
| UI when asleep | 3 (1.5); 194 | 2 (1.0); 199 | |
| UI with sex | 3 (2.2); 137 | 1 (0.7); 137 | |
| Time to further surgery | Treatment | Effect size (95% CI); p-value | |||
|---|---|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | ||||
| Mean (SD); N | Median (P25–75)a | Mean (SD); N | Median (P25–75)a | ||
| Number of days | 290.1 (149.6); 16 | 259.0 (183.5–363.5) | 263.2 (91.7); 10 | 306.5 (231.0–325.0) | 1.66 (0.80 to 3.44); 0.177b |
| Subgroup | Treatment, mean (SD); N | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | ||
| Age group (years) | |||
| < 60 years | 5.7 (6.1); 68 | 4.0 (4.9); 62 | 1.46 (–0.17 to 3.09); 0.08 |
| ≥ 60 years | 3.6 (4.1); 162 | 4.3 (5.4); 176 | |
| Previous incontinence surgery | |||
| Yes | 3.3 (2.6); 7 | 2.9 (4.1); 8 | 0.53 (–4.31 to 5.37); 0.83 |
| No | 4.2 (4.9); 223 | 4.2 (5.3); 230 | |
| Previous anterior/posterior surgery | |||
| Yes | 4.3 (4.9); 196 | 4.2 (5.3); 205 | –0.02 (–0.95 to 0.91); 0.97 |
| No | 3.8 (4.7); 34 | 3.9 (5.0); 33 | |
Appendix 4 Additional information for Chapter 5
| Intervention | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Mean cost (SD); N | Uterine preservation vs. vaginal hysterectomy | |||||
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |||
| MD | 95% CI | |||||
| Number entering surgery | 264 (94); 280 | 270 (95); 283 | ||||
| Anaesthesia | 264 (100); 264 | 270 (100); 270 | 18.10 (6.71); 264 | 17.85 (6.93); 270 | ||
| Theatre overheads | 264 (100); 264 | 270 (100); 270 | 420.19 (0.00); 264 | 420.19 (0.00); 270 | ||
| Mesh for apical prolapse | 86 (33); 264 | 9 (3); 270 | ||||
| Mesh for concomitant anterior | 6 (2); 264 | 3 (1); 270 | ||||
| Mesh for concomitant posterior | 11 (4); 264 | 2 (1); 270 | ||||
| Mesh for prolapse cost | 52.17 (99.13); 264 | 10.50 (69.61); 270 | ||||
| Incontinence mesh | 6 (2); 264 | 7 (3); 270 | 2.52 (16.59); 264 | 2.88 (17.69); 270 | ||
| Return to theatre | 1 (0); 264 | 2 (1); 270 | 3.50 (56.81); 264 | 6.84 (79.29); 270 | ||
| Catheterisation | 252 (95); 264 | 256 (95); 270 | 6.08 (1.33); 264 | 6.04 (1.42); 270 | ||
| Pain relief | 264 (100); 264 | 270 (100); 270 | 1.79 (1.96); 264 | 1.67 (1.87); 270 | ||
| Laxatives | 167 (63); 264 | 162 (60); 270 | 1.24 (0.95); 264 | 1.18 (0.96); 270 | ||
| Antibiotics | 253 (96); 264 | 264 (98); 269 | 1.02 (0.21); 264 | 1.04 (0.13); 269 | ||
| Vaginal pack | 189 (72); 264 | 217 (80); 270 | 3.34 (2.11); 264 | 3.75 (1.86); 270 | ||
| Resource usage, mean (SD); N | Resource usage, mean (SD); N | |||||
| Gynaecologist time (minutes) | 113.05 (49.40); 265 | 103.20 (32.46); 270 | 267.49 (123.61); 264 | 251.69 (98.65); 270 | ||
| Anaesthetist time (minutes) | 113.05 (49.40); 265 | 103.20 (32.46); 270 | 229.98 (117.74); 264 | 214.16 (87.89); 270 | ||
| Nurse time (minutes) | 113.05 (49.40); 265 | 103.20 (32.46); 270 | 304.17 (132.92); 265 | 277.66 (87.34); 270 | ||
| Length of stay (days) | 1.90 (1.23); 264 | 1.89 (1.14); 270 | 339.69 (220.27); 264 | 338.77 (204.15); 270 | ||
| Total intervention costsa | 1213.04 (472.08); 264 | 1116.18 (371.28); 270 | 91.59 | –5.16 to 178.01 | ||
| Hospital resource use (postcode verified) | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Mean costs (SD); N | Uterine preservation vs. vaginal hysterectomy | |||||
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |||
| MD | 95% CI | |||||
| New prolapse procedure | 16 (6); 265 | 8 (3); 269 | 237.74 (971.53); 265 | 93.69 (570.16); 269 | 179.27 | 38.47 to 320.07 |
| Anterior only | 4 (2); 265 | 1 (0); 269 | ||||
| Posterior only | 1 (0); 265 | 4 (1); 269 | ||||
| Apical only | 7 (3); 265 | 2 (1); 269 | ||||
| Anterior and posterior | 1 (0); 265 | 0 (0); 269 | ||||
| Anterior and apical | 2 (1); 265 | 0 (0); 269 | ||||
| Posterior and apical | 0 (0); 265 | 1 (0); 269 | ||||
| Anterior and posterior and apical | 1 (0); 265 | 0 (0); 269 | ||||
| New UI procedure | 3 (1); 265 | 3 (1); 269 | 23.73 (222.17); 265 | 23.38 (220.52); 269 | –6.00 | –56.73 to 44.72 |
| Sling | 3 (1); 265 | 3 (1); 269 | ||||
| Abdominal | 0 (0); 265 | 0 (0); 269 | ||||
| Botox | 0 (0); 265 | 0 (0); 269 | ||||
| Injectable | 0 (0); 265 | 0 (0); 269 | ||||
| Hospital admission other (including SAE) | 10 (4); 282 | 9 (3); 283 | 55.30 (437.13); 279 | 58.60 (554.81); 283 | –7.45 | –113.11 to 98.21 |
| Total hospital admission costsa | 319.69 (1073.49); 265 | 178.71 (839.97); 269 | 165.72 | –39.00 to 370.45 | ||
| Other consultations (participant reported number of consultations) | Resource usage, mean (SD); na | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Mean costs (SD); nb | Uterine preservation vs. vaginal hysterectomy | |||||
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |||
| MD | 95% CI | |||||
| Outpatient consultations | 1.67 (1.04); 69 | 1.74 (1.22); 54 | 88.23 (140.33); 183 | 60.38 (133.69); 192 | ||
| GP | 1.73 (1.65); 55 | 2.06 (1.55); 51 | 18.59 (43.07); 184 | 17.91 (40.59); 199 | ||
| Practice nurse | 2.17 (1.70); 12 | 1.67 (0.87); 9 | 2.51 (10.83); 150 | 1.32 (6.15); 165 | ||
| District or incontinence nurse | 1.71 (1.11); 7 | 1.75 (0.96); 4 | 3.14 (16.46); 145 | 1.67 (11.59); 159 | ||
| Physiotherapy (practice) | 2.50 (0.71); 2 | 2.00 (N/A); 1 | 1.21 (10.33); 140 | 0.43 (5.41); 158 | ||
| Physiotherapy (hospital) | 2.21 (1.63); 14 | 3.17 (1.64); 12 | 7.12 (27.55); 148 | 7.98 (31.83); 162 | ||
| Other consultations (participant reported) | 24.10 (76.25); 126 | 16.67 (86.26); 149 | ||||
| Total cost professional consultationsc | 112.59 (179.45); 219 | 85.83 (169.92); 227 | 21.74 | –17.96 to 61.45 | ||
| Other treatments and medications (participant reported) | Resource usage, n (%); Na | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | Mean costs (SD); nb | Uterine preservation vs. vaginal hysterectomy | |||
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |||||
| MD | 95% CI | |||||
| Oestrogens | 31 (16); 191 | 31 (15); 203 | 13.02 (71.08); 176 | 25.57 (112.49); 189 | ||
| Antibiotics | 29 (15); 191 | 38 (19); 198 | 0.24 (0.73); 182 | 1.35 (8.33); 183 | ||
| Bladder medications | 11 (6); 178 | 15 (8); 188 | 0.21 (1.89); 173 | 0.21 (1.89); 177 | ||
| Reusable/intermittent catheter | 5 (3); 180 | 4 (2); 204 | 0.22 (1.65); 179 | 0.06 (0.64); 202 | ||
| Ring pessary | 14 (8); 185 | 9 (4); 205 | 1.52 (5.33); 185 | 0.88 (4.13); 205 | ||
| Shelf pessary | 8 (4); 181 | 4 (2); 205 | 0.95 (4.44); 181 | 0.42 (2.99); 205 | ||
| Permanent catheter | 1 (1); 177 | 2 (1); 201 | 0.52 (3.95); 177 | 0.15 (1.52); 201 | ||
| Total other treatmentsc | 13.74 (64.71); 215 | 24.22 (108.51); 224 | –11.66 | –28.92 to 5.60 | ||
| Participant-incurred costs | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | Mean costs (SD); N | Uterine preservation vs. vaginal hysterectomya | |||
| Uterine preservation (N = 280) | Vaginal hysterectomy (N = 283) | |||||
| MD | 95% CI | |||||
| Opportunity cost of time and travel | ||||||
| Inpatient stays | 91 (26); 262 | 88 (23); 266 | ||||
| Outpatient consultations | 33 (52); 185 | 23 (51); 194 | ||||
| Primary care consultations | 12 (25); 202 | 11 (22); 212 | ||||
| Subtotal | 127 (80); 164 | 112 (72); 174 | 18 | 5 to 31 | ||
| Purchase of over-the-counter medication | 17 (9); 197 | 12 (6); 209 | 2 (12); 190 | 1 (6); 207 | ||
| Private medical care | 3 (2); 189 | 2 (1); 200 | 43 (515); 188 | 2 (19); 200 | ||
| Other expenses | 7 (4); 190 | 2 (1); 200 | 2 (14); 188 | 2 (28); 200 | ||
| Absence from paid employment | ||||||
| Currently in employment | 74 (33); 224 | 72 (31); 234 | ||||
| Reported needing time off workb | 64 (86); 74 | 66 (91); 72 | ||||
| Days of sick leaveb,c | 35.23 (65.25); 64 | 22.36 (40.43); 66 | 1163 (4267); 207 | 707 (2591); 225 | 856 | –975 to 2688 |
| Total participant perspective costs | 1266 (4127); 129 | 460 (1505); 152 | 609 | 47 to 1172 | ||
| Total NHS perspective costs (Table 18) | 1643 (1302); 207 | 1345 (754); 217 | 292 | 68 to 517 | ||
| Wider-perspective costs (NHS and personal) | 2852 (4629); 129 | 1774 (1726); 151 | 911 | 359 to 1463 | ||
FIGURE 22.
Scatterplot (a) and CEAC (b) for a seemingly unrelated regression (imputed data set).
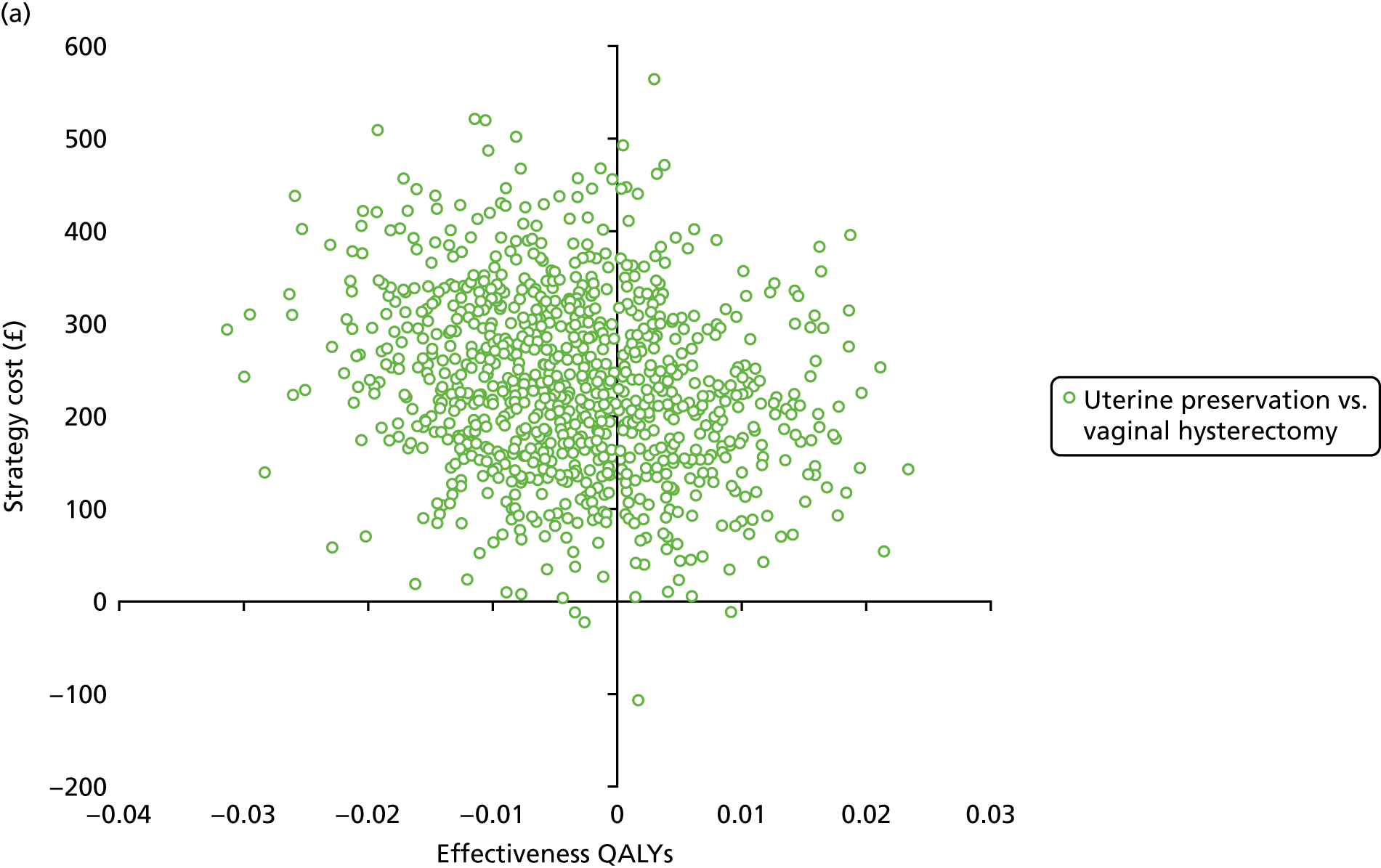

FIGURE 23.
Scatterplot (a) and CEAC (b) for a wider-perspective analysis of costs (imputed data set).
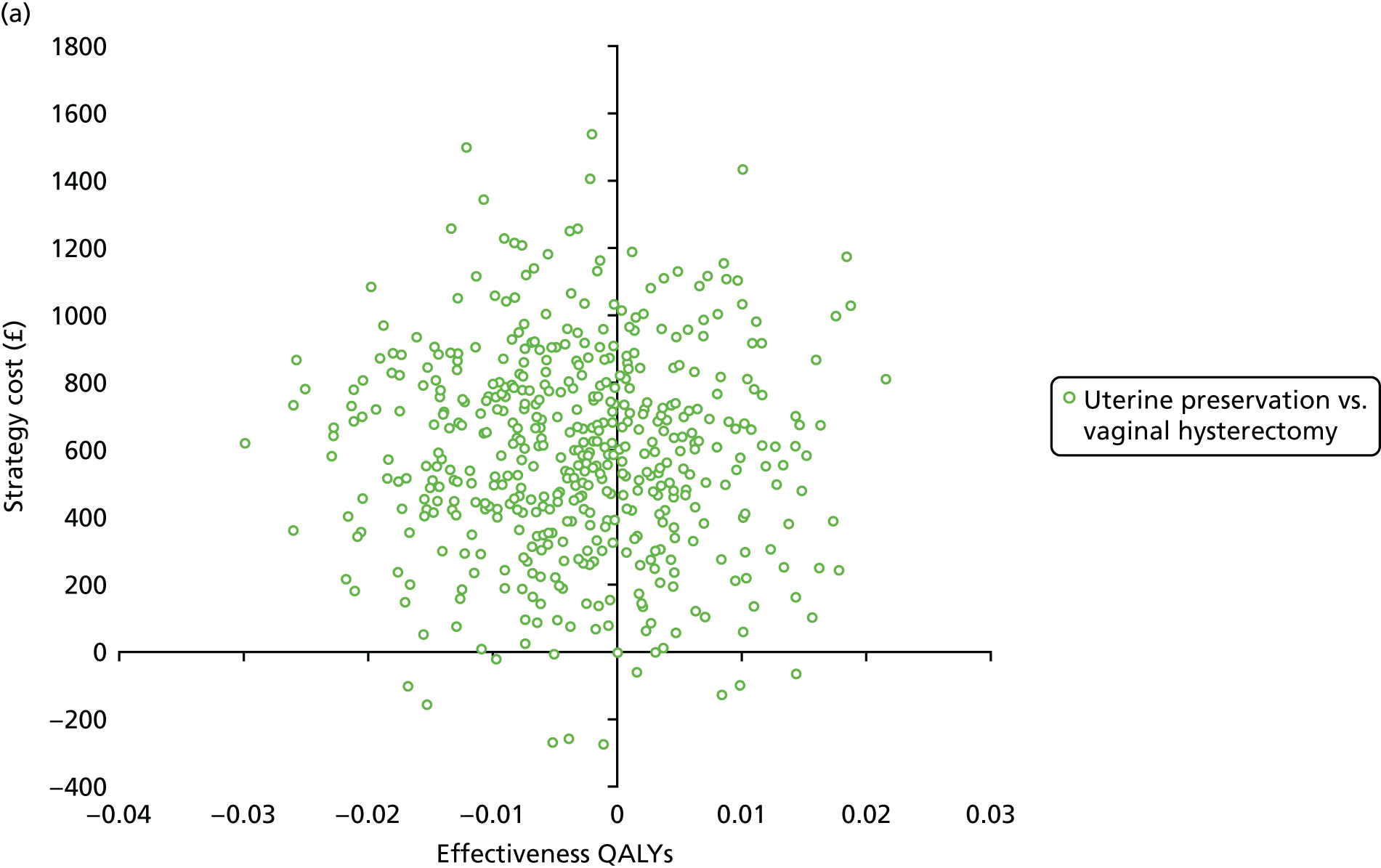
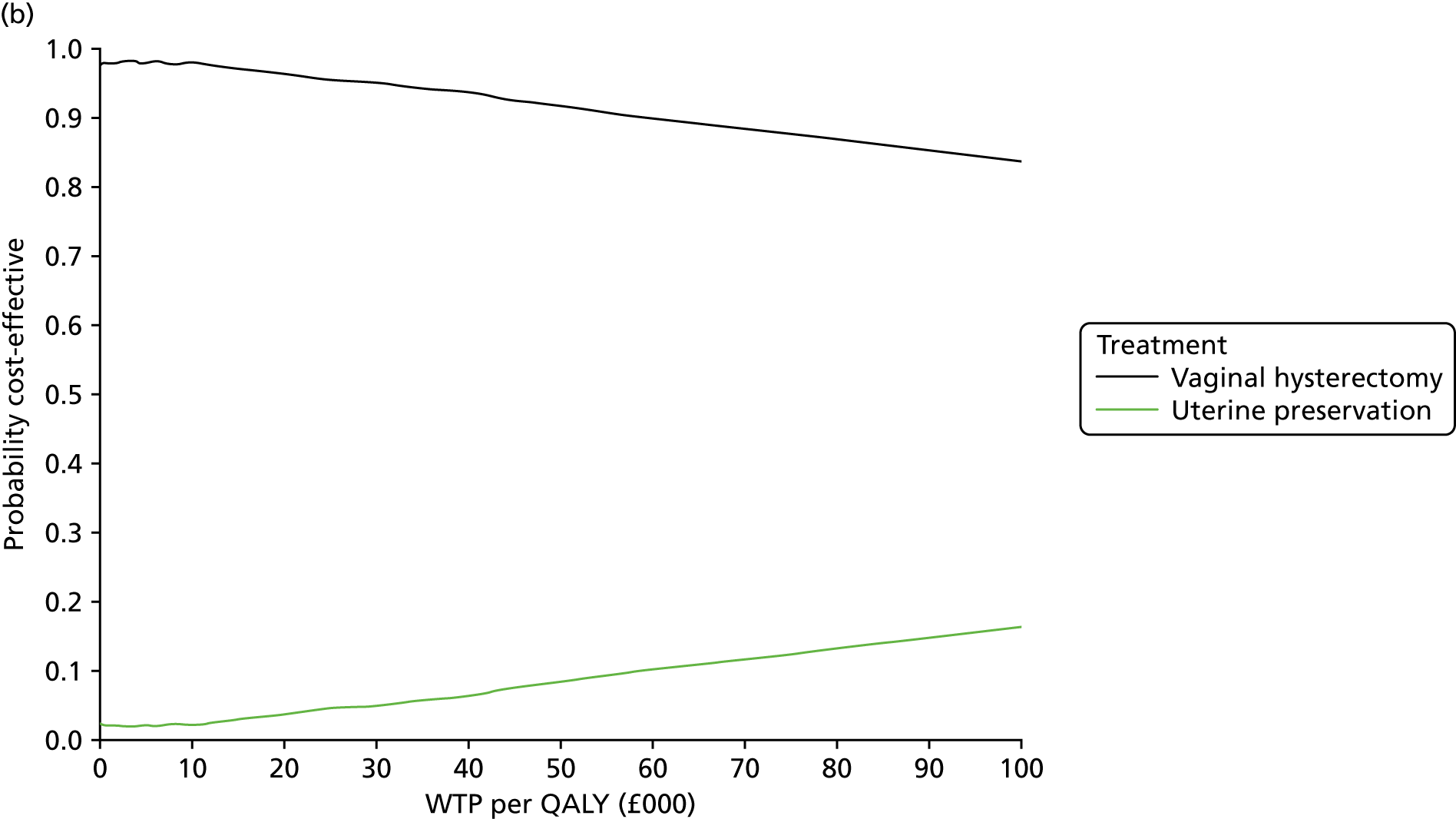
FIGURE 24.
Scatterplot (a) and CEAC (b) using HRG tariffs instead of component costing for index interventions (imputed data set).

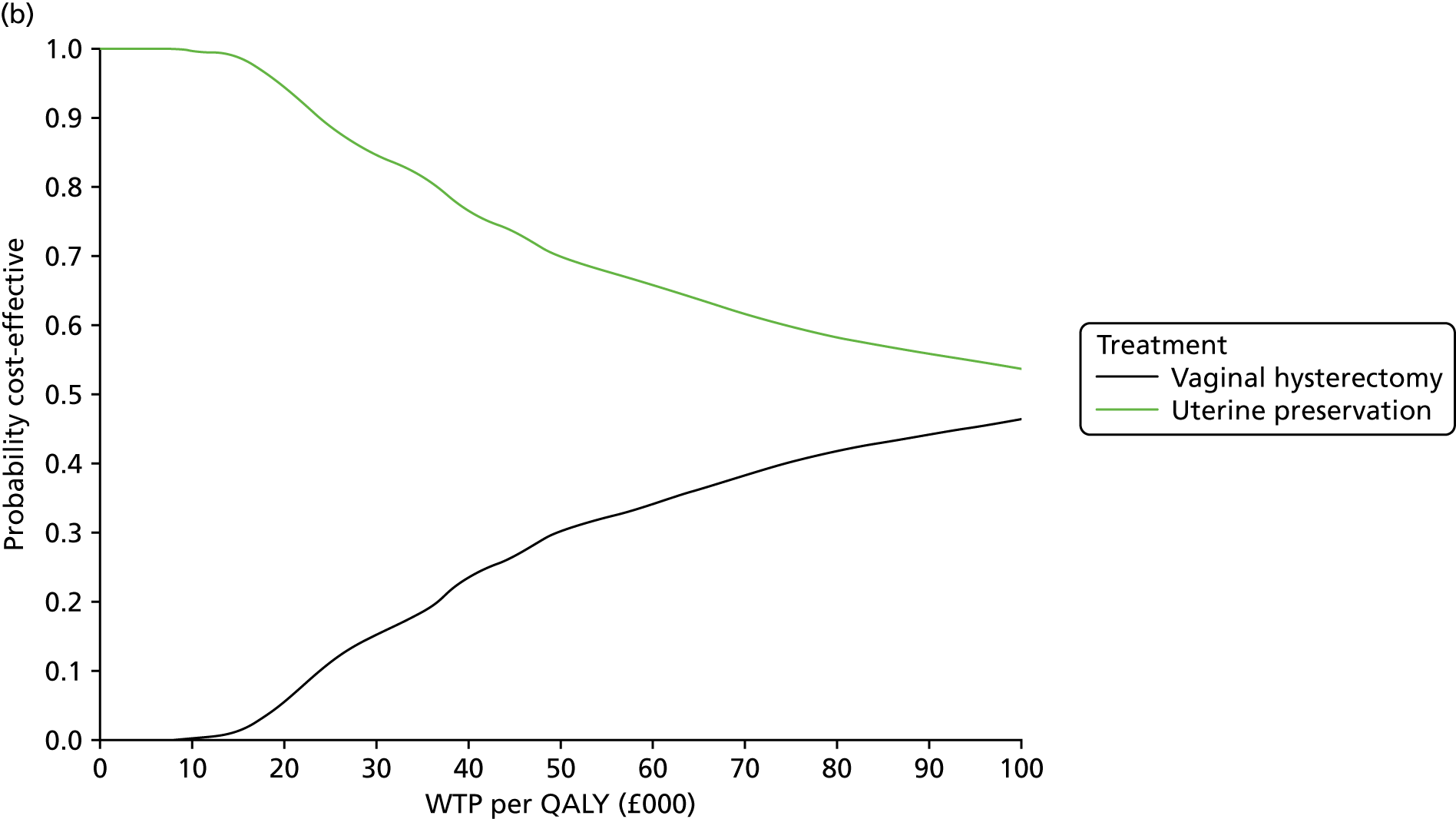
FIGURE 25.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 1 (imputed data set).
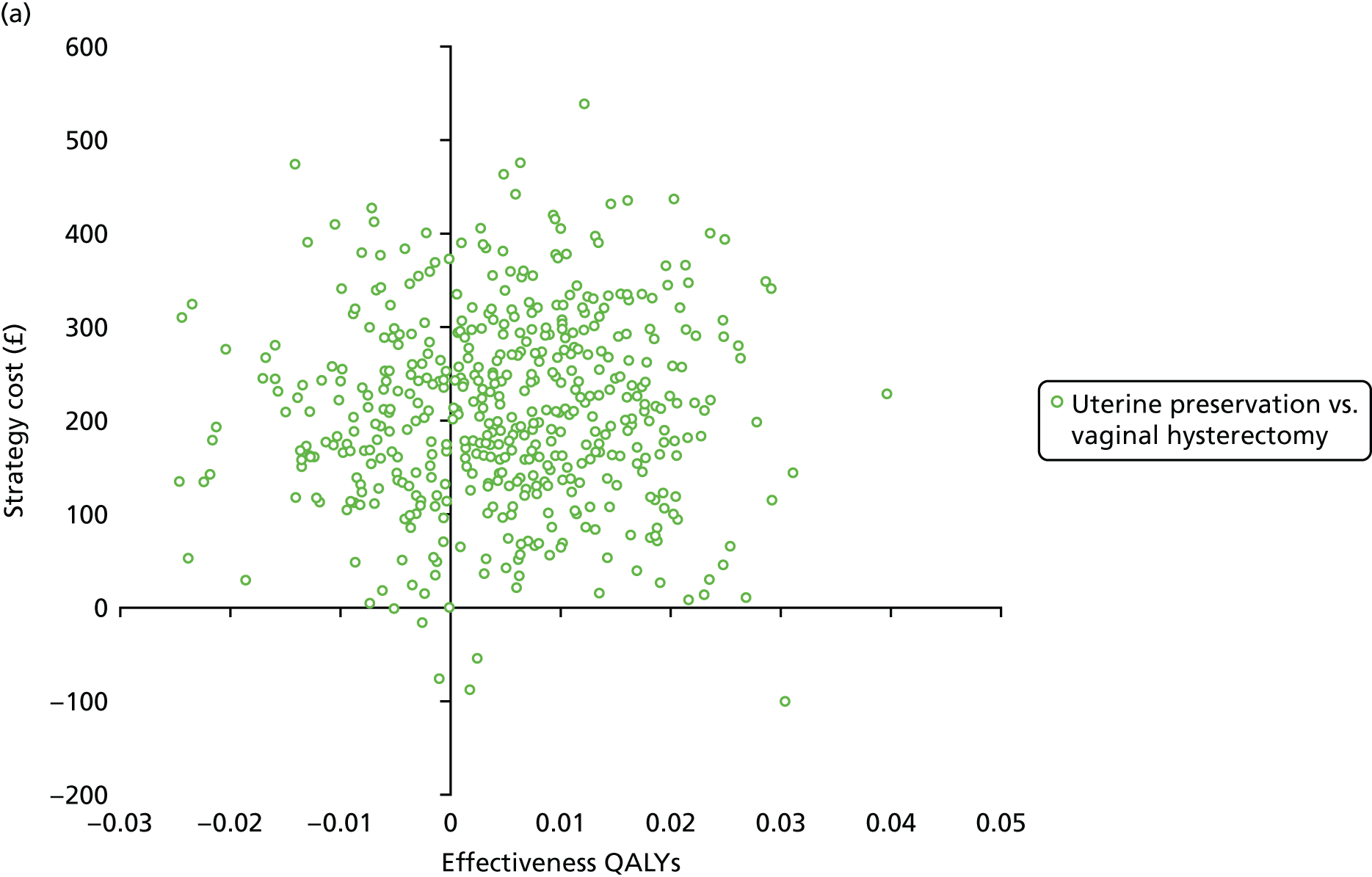
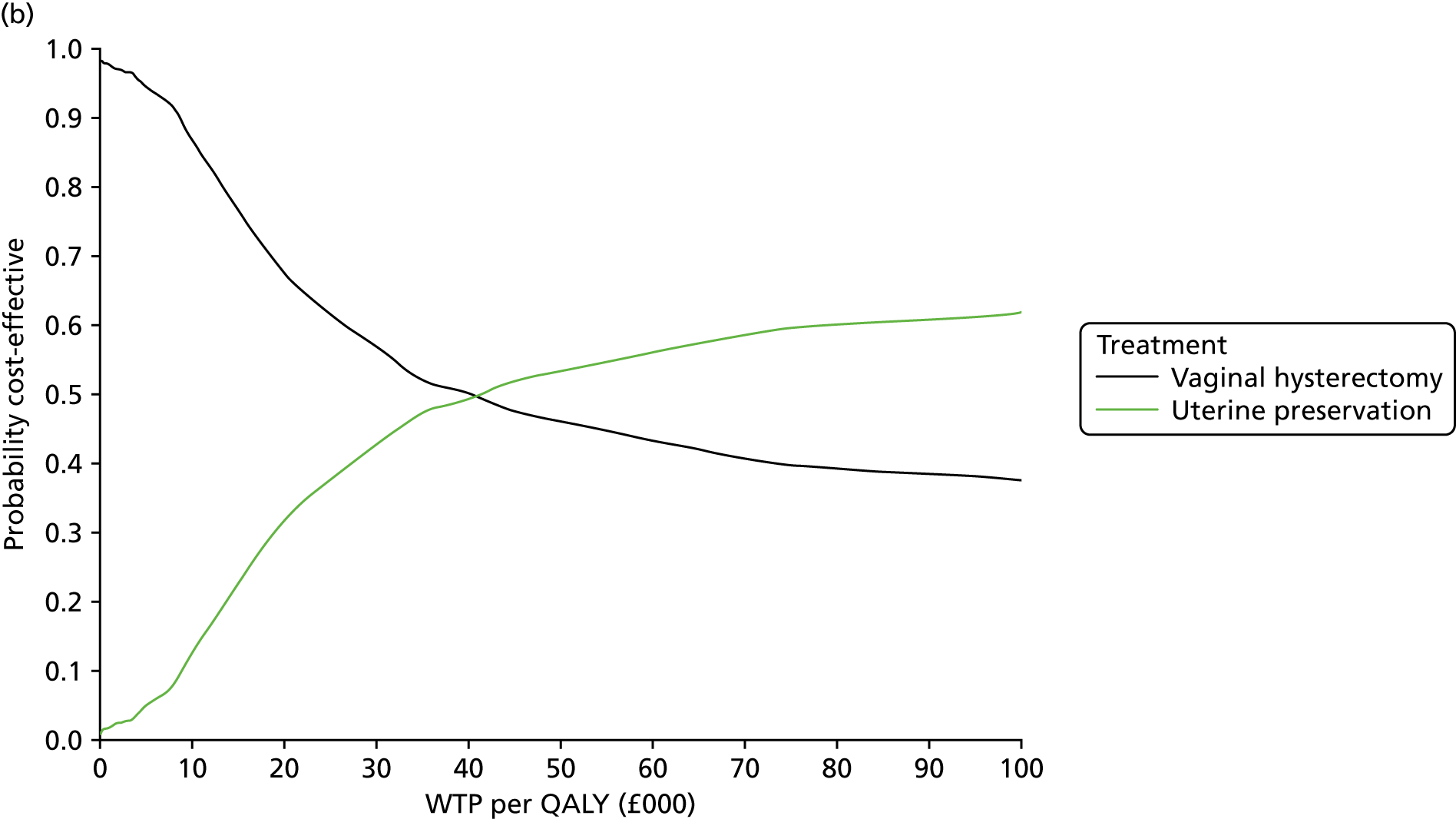
FIGURE 26.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 2 (imputed data set).
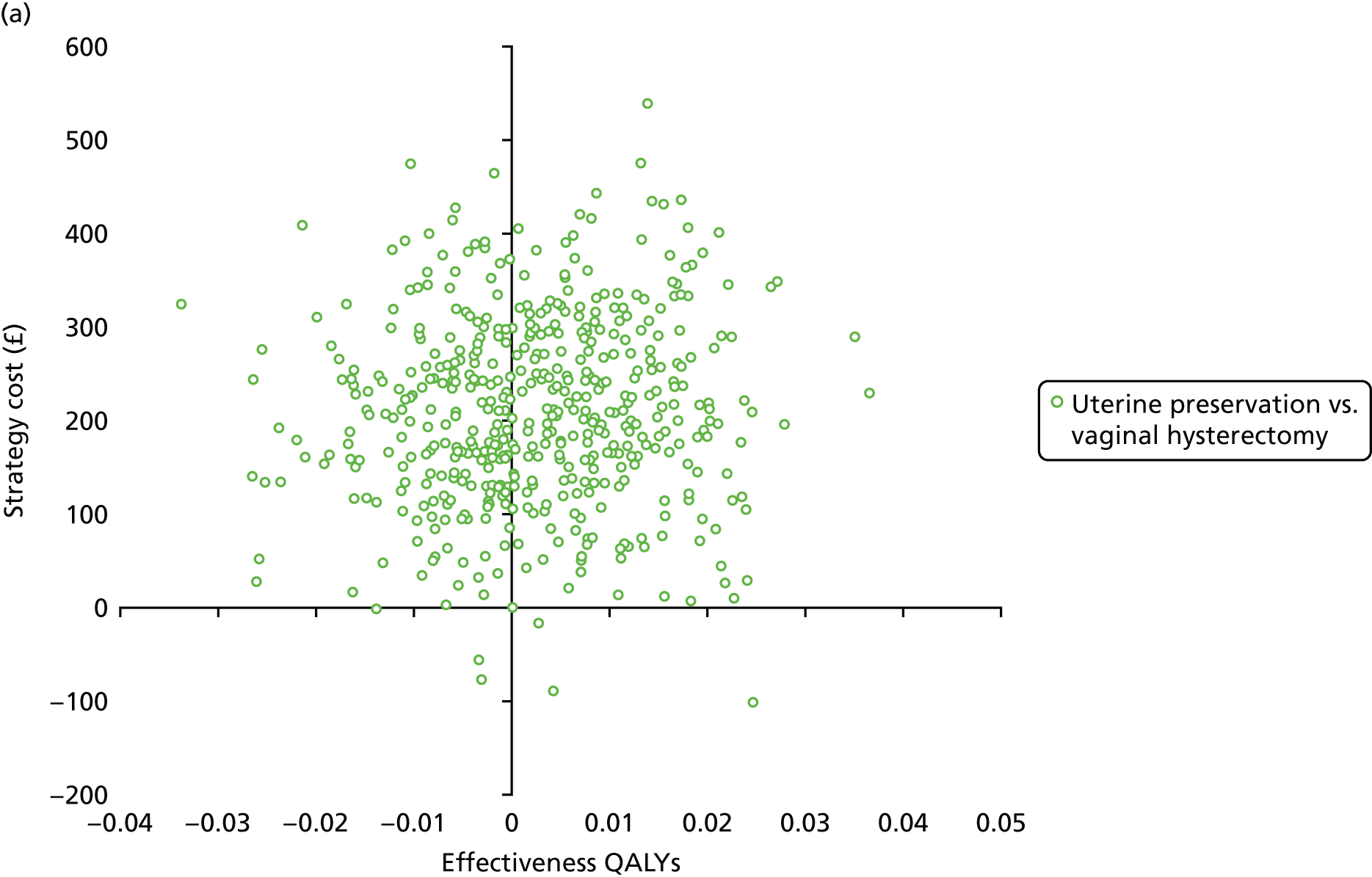
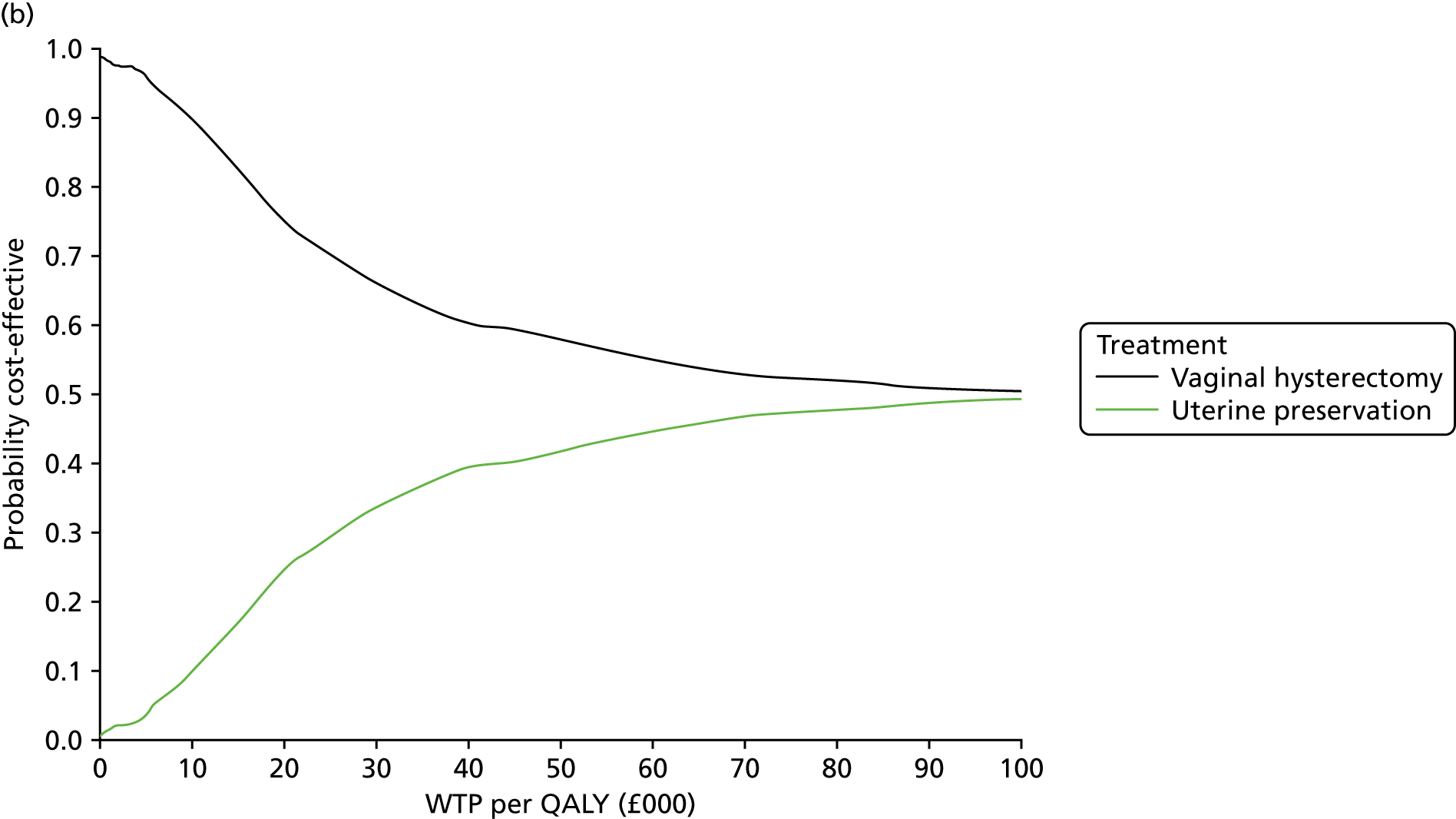
FIGURE 27.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 3 (imputed data set).
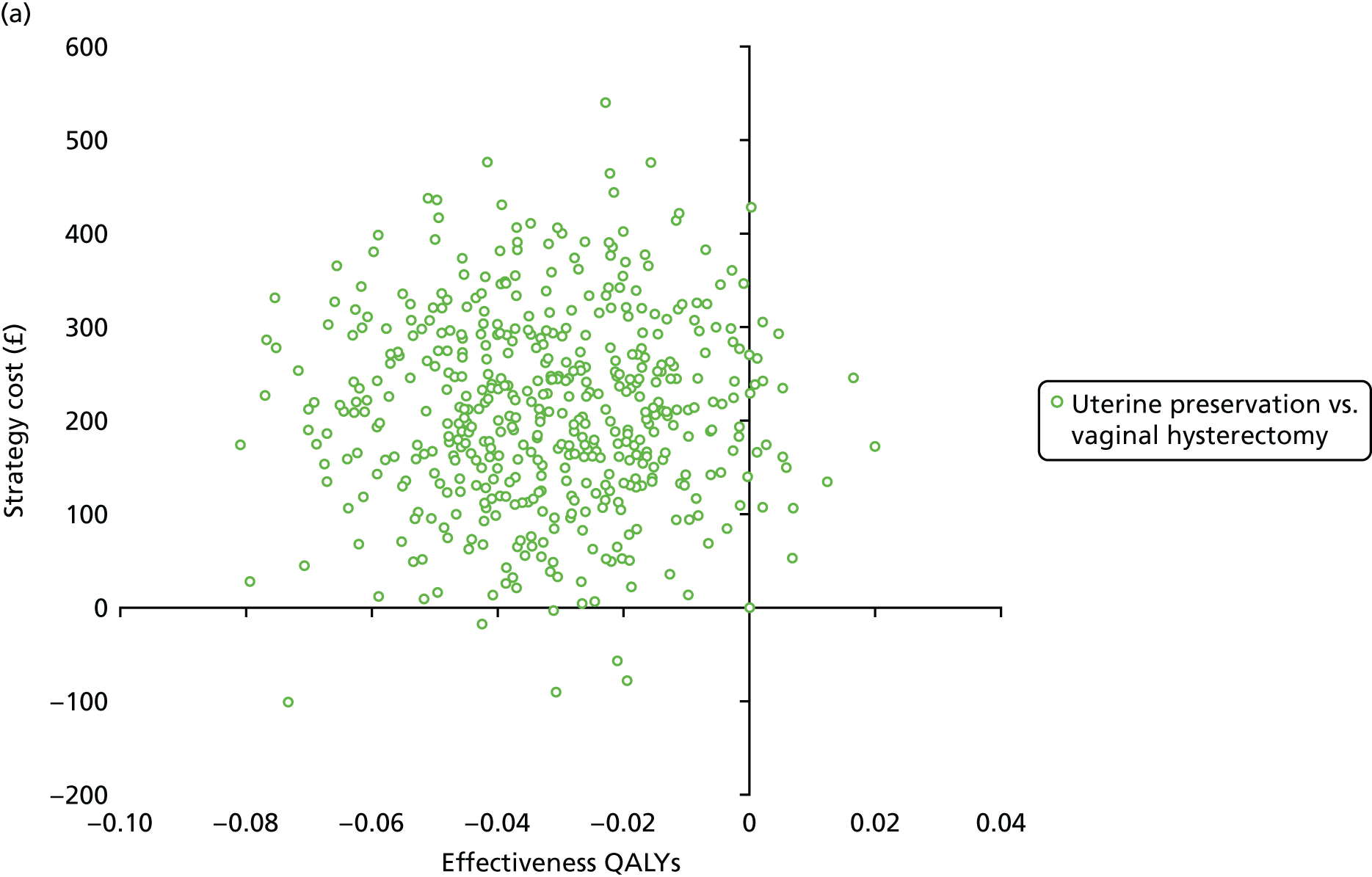
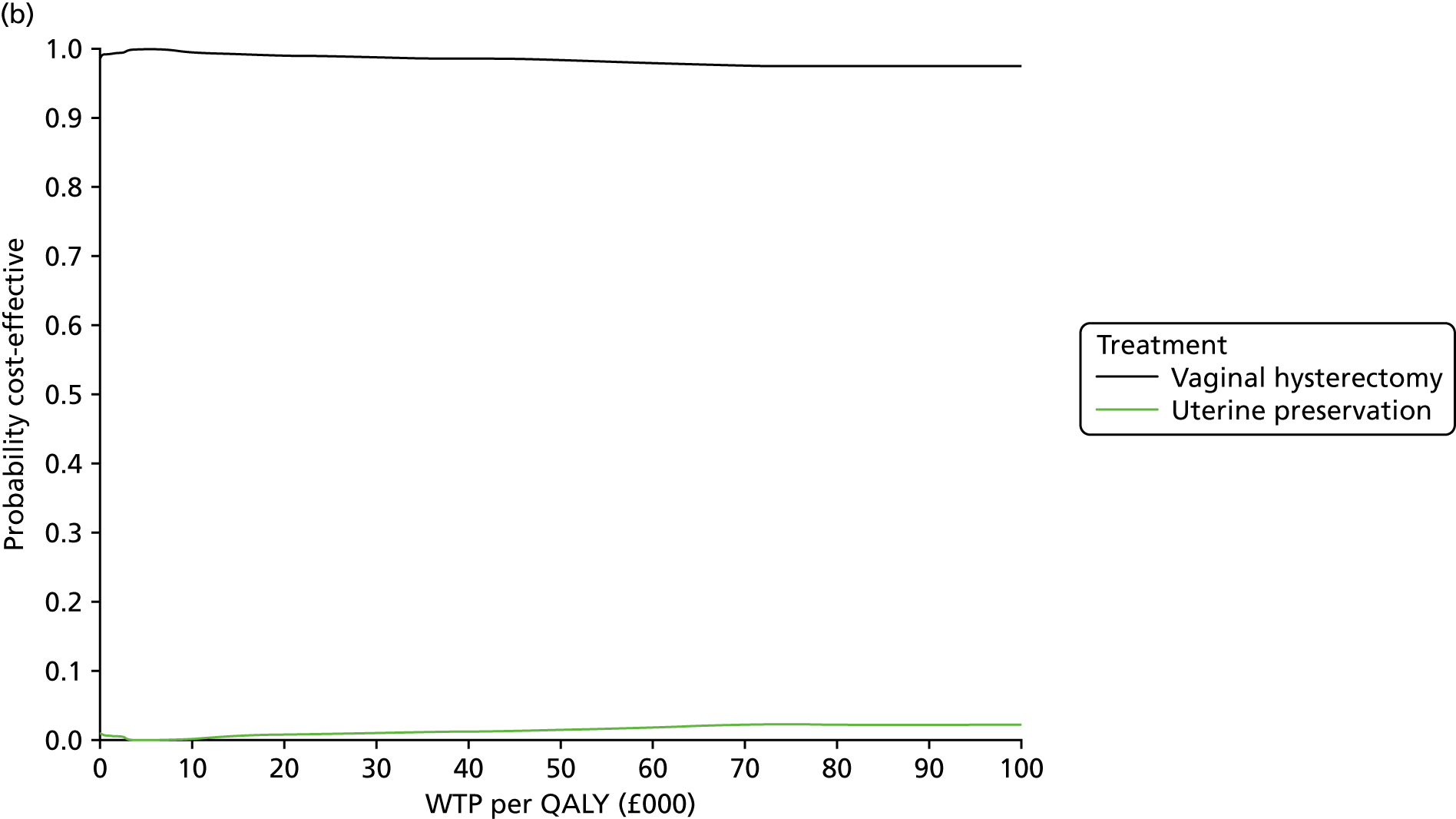
FIGURE 28.
Scatterplot (a) and CEAC (b) complete-case analysis of cost and QALY pairs.

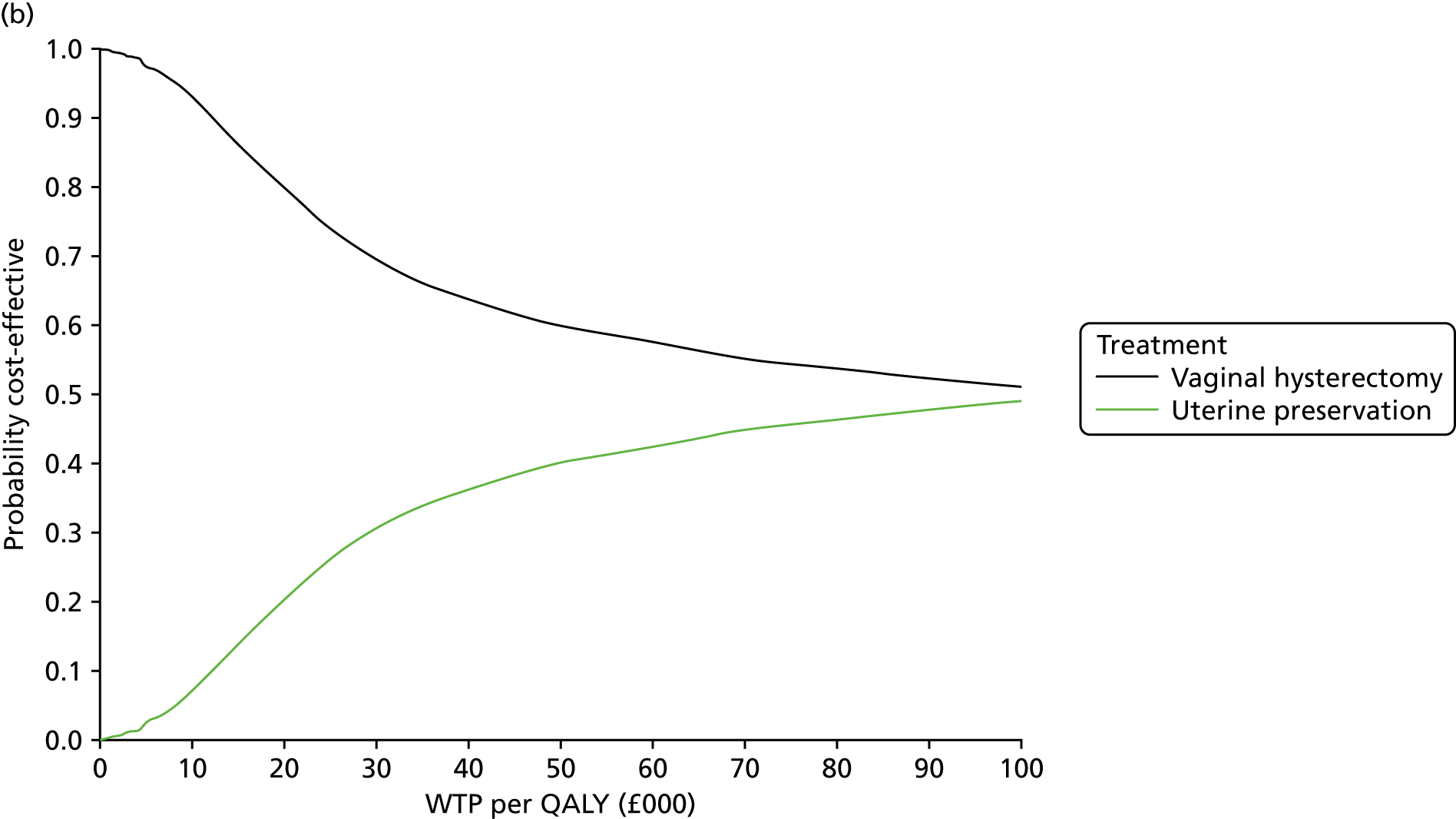
FIGURE 29.
Scatterplot (a) and CEAC (b) for a seemingly unrelated regression on complete-case analysis of cost and QALY pairs.
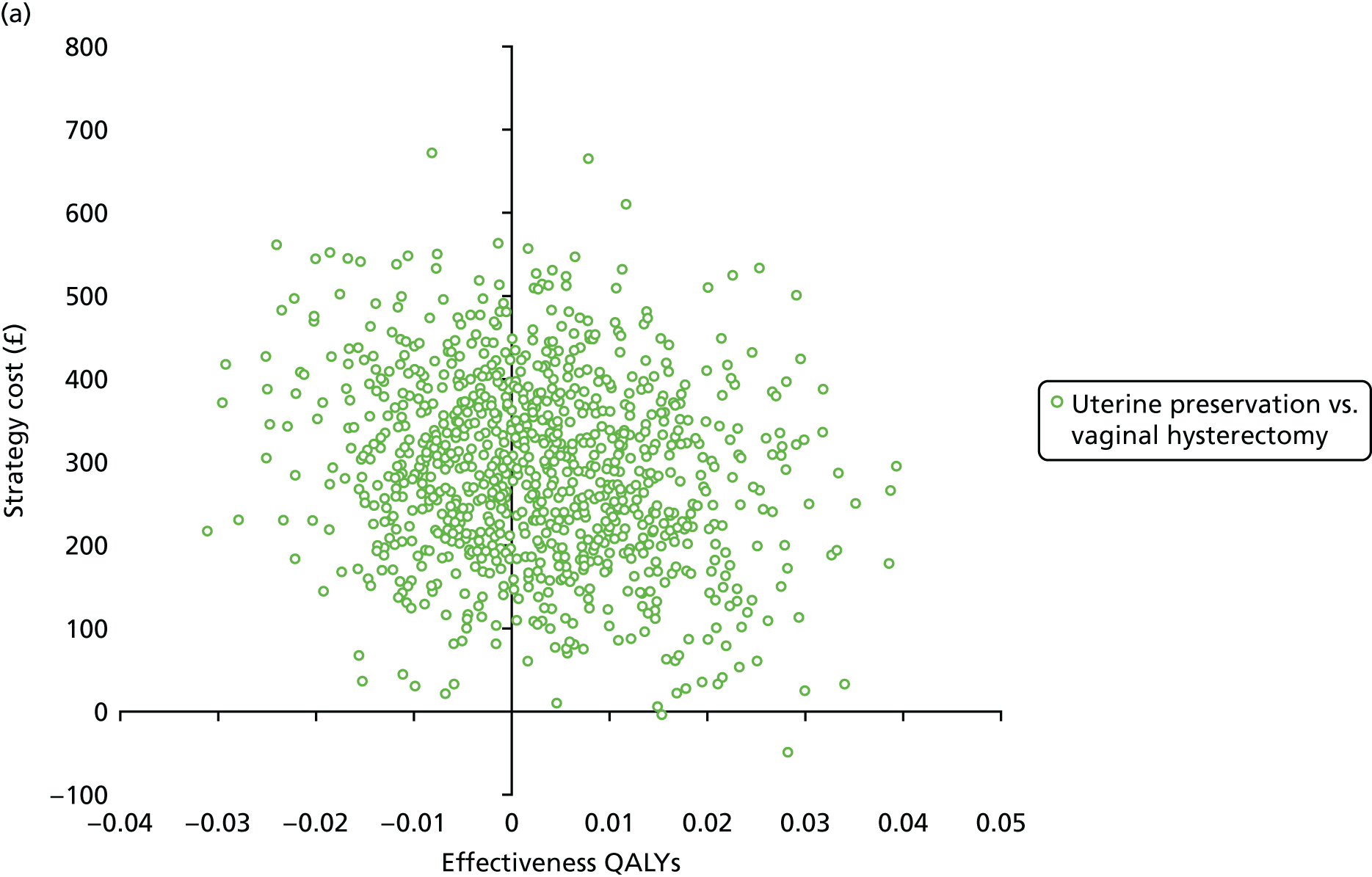

Appendix 5 Additional information for Chapter 6
| Centres recruiting | Staff | Treatment (n) | |
|---|---|---|---|
| Abdominal vault (N = 105)a | Vaginal vault (N = 104) | ||
| Basingstoke | Christian Phillips and Tim Sayer | 12 | 13 |
| Queen Alexandra Hospital, Portsmouth | Claire Burton, Christopher Guyer, Rebecca Hardcastle and Ali Ahmed | 9 | 9 |
| Manchester | Anthony Smith, Fiona Reid, Karen Ward, Carolyn North, Melissa Bradbury and Rohna Kearney | 9 | 7 |
| Aberdeen | Christine Hemming and Kevin Cooper | 8 | 7 |
| Birmingham | Philip Toozs-Hobson, Pallavi Latthe and Supriya Bulchandani | 8 | 7 |
| Hinchingbrooke Hospital | Ashish Pradhan, Helen Johnson and Umar Hussain | 4 | 7 |
| Bradford | Carmel Ramage and Sue Calvert | 4 | 6 |
| Southport and Ormskirk | Shaireen Aleem | 6 | 4 |
| Leicester General | Douglas Tincello | 4 | 5 |
| Musgrove Park | Adel Naguib | 3 | 5 |
| James Cook University | Paul Ballard and Aethele Khunda | 2 | 4 |
| Harrogate and District | Tracy Jackson and Adrian Barnett | 3 | 3 |
| Torbay | Subramanian Narayanan | 3 | 2 |
| Arrowe Park | Tom Aust, Patrick Doyle and Jeremy Weetch | 1 | 4 |
| Liverpool | Ruben Trochez, Gillian Fowler and E Adams | 2 | 3 |
| Pinderfields | Kathryn Fishwick | 3 | 2 |
| Derriford Hospital, Plymouth | Robert Freeman, Luigi Bombieri and A Dua | 3 | 1 |
| Addenbrooke’s | Mark Slack, Rohna Kearney, Vladimir Revicky and Alexandros Derpapas | 2 | 2 |
| UH North Staffordshire | Jason Cooper | 1 | 2 |
| UH North Tees | Santhosh Puthuraya and Elaine Gouk | 3 | 0 |
| Royal Preston | Brice Rodriguez and Sanjeev Prashar | 2 | 1 |
| Sunderland Royal | Jonathan Chamberlain | 2 | 1 |
| Royal Victoria Infirmary | Karen Brown, Karen Rose and Paul Hilton | 2 | 1 |
| Worcester Acute | Deepali Sinha and Angus Thomson | 2 | 1 |
| Dartford and Gravesham | Rob MacDermott, Angeli Thallon, Andreas Lesseps and Abhisheck Gupta | 1 | 1 |
| Crosshouse | Wael Agur, Ashraf Habib, Inna Sokolova, Mohamed Riad and David Rae | 1 | 1 |
| Cornwall Hospitals | Farah Lone and Rob Holmes | 1 | 0 |
| New Cross Hospital, Wolverhampton | Khaled Afifi, Ayman Elnaqa and Charles Cox | 1 | 0 |
| Heart of England | Gurminder Matharu and Afshan Khaja | 1 | 0 |
| Royal Bolton | Philip Chia and Abimbola Williams | 0 | 1 |
| Calderdale Royal Hospital | Yi Ling Chan, Anu Bondili and Maged Shendy | 0 | 1 |
| Southmead Hospital | Chendrimada Madhu | 1 | 0 |
| Hull and East Yorkshire | Jagdish Gandhi | 0 | 1 |
| Barnsley | Meenakshi Dass and Khaled Farag | 0 | 1 |
| Gloucester | Mark James | 1 | 0 |
| Jessop Wing, Sheffield | Stephen Radley and Swati Jha | 0 | 1 |
FIGURE 30.
Recruitment graph.

| Participants | n (%) |
|---|---|
| Approached | 544 (100.0) |
| Randomised | 209 (38.4) |
| Not included | 335 (61.6) |
| Ineligible | 115 (21.1) |
| Declined | 211 (38.8) |
| Other reasons/missed appointment | 9 (1.7) |
| Participants | n (%) |
|---|---|
| Ineligible | 115 (100.0) |
| A specific operation is necessary | 73 (63.5) |
| Unsuitable owing to medical history | 21 (18.3) |
| No prolapse | 10 (8.7) |
| Change of prolapse type from original diagnosis | 4 (3.5) |
| Not suitable for surgery | 3 (2.6) |
| Operation cancelled | 2 (1.7) |
| Unable to complete study questionnaires | 1 (0.9) |
| Declined | 211 (100.0) |
| Declined/refused surgery | 58 (27.5) |
| Did not want to participate in the study | 54 (25.6) |
| Wanted an abdominal procedure | 53 (25.1) |
| Did not want to be randomised | 38 (18.0) |
| Wanted a vaginal procedure | 32 (15.2) |
| Wished to go with surgeon’s choice | 13 (6.2) |
| Wanted to try a pessary | 11 (5.2) |
| Did not want mesh | 8 (3.8) |
| Did not want to complete questionnaires | 4 (1.9) |
| Wanted a hysterectomy | 2 (0.9) |
| Changed mind after initially consenting | 1 (0.5) |
| Characteristic | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Number of, mean (SD); N | ||
| Normal vaginal deliveries | 2.4 (1.2); 100 | 2.5 (1.2); 101 |
| Caesarean sections before labour | 0.1 (0.2); 61 | 0.0 (0.2); 66 |
| Breech vaginal deliveries | 0.0 (0.2); 60 | 0.1 (0.4); 64 |
| Forceps deliveries | 0.3 (0.5); 66 | 0.2 (0.5); 69 |
| Caesarean sections during labour | 0.0 (0.0); 59 | 0.0 (0.2); 64 |
| Vacuum deliveries | 0.0 (0.1); 55 | 0.0 (0.0); 63 |
| Previous anterior surgery mesh use, n (%) | 3 (2.9) | 1 (1.0) |
| Previous posterior surgery mesh use, n (%) | 0 (0) | 0 (0) |
| Measurement | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| POP-Q measurement (cm), mean (SD); N [range] | ||
| Ba | 0.0 (2.3); 100 [–3.0 to 6.0] | –0.1 (2.6); 99 [–3.0 to 6.0] |
| C | –0.4 (2.5); 99 [–3.0 to 6.0] | –0.3 (2.7); 99 [–3.0 to 6.0] |
| Bp | –0.5 (2.0); 99 [–3.0 to 4.0] | 0.0 (2.1); 96 [–3.0 to 6.0] |
| Total vaginal length | 8.1 (1.5); 91 [2.0 to 12.0] | 8.1 (1.6); 88 [4.0 to 12.0] |
| Overall prolapse stage, n (%) | ||
| 0 | 0 (0.0) | 0 (0.0) |
| 1 | 0 (0.0) | 0 (0.0) |
| 2a | 10 (9.6) | 3 (2.9) |
| 2b | 41 (39.4) | 37 (35.6) |
| 3 | 50 (48.1) | 56 (53.8) |
| 4 | 1 (1.0) | 5 (4.8) |
| Missing | 2 (1.9) | 3 (2.9) |
| Symptom | Treatment, n (%) | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Any incontinence missing | 5 (4.8) | 4 (3.8) |
| UI | ||
| None | 4 (3.8) | 8 (7.7) |
| Slight | 46 (44.2) | 45 (43.3) |
| Moderate | 35 (33.7) | 39 (37.5) |
| Severe | 2 (1.9) | 1 (1.0) |
| Missing | 17 (16.3) | 11 (10.6) |
| Stress UI | 14 (13.5) | 16 (15.4) |
| Missing | 14 (13.5) | 8 (7.7) |
| Urge UI, missing | 2 (1.9) | 1 (1.0) |
| Mixed UI | 7 (6.7) | 5 (4.8) |
| Missing | 3 (2.9) | 2 (1.9) |
| UI for no reason | ||
| Missing | 4 (3.8) | 4 (3.8) |
| UI when asleep | ||
| Missing | 13 (12.5) | 9 (8.7) |
| UI with sex | 2 (1.9) | 2 (1.9) |
| Missing | 40 (38.5) | 39 (37.5) |
Appendix 6 Additional information for Chapter 7
| Surgery | Treatment, n (%); N | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Apical surgery | ||
| No mesh | 10 (10.9); 92 | 86 (91.5); 94 |
| Synthetic non-absorbable | 71 (77.2); 92 | 4 (4.3); 94 |
| Biological | 4 (4.3); 92 | 2 (2.1); 94 |
| Mesh kit | 6 (6.5); 92 | 0 (0); 94 |
| Missing | 1 (1.1); 92 | 2 (2.1); 94 |
| Concomitant surgery | ||
| Anterior repair only | ||
| No mesh | 9 (81.8); 11 | 24 (88.9); 27 |
| Synthetic non-absorbable | 2 (18.2); 11 | 1 (3.7); 27 |
| Biological | 0 (0); 11 | 0 (0); 27 |
| Missing | 0 (0); 11 | 2 (7.4); 27 |
| Posterior repair only | ||
| No mesh | 15 (93.8); 16 | 21 (87.5); 24 |
| Synthetic non-absorbable | 1 (6.3); 16 | 2 (8.3); 24 |
| Biological | 0 (0); 16 | 0 (0); 24 |
| Missing | 0 (0); 16 | 1 (4.2); 24 |
| Both anterior and posterior repair | ||
| Anterior compartment only | ||
| No mesh | 5 (55.6); 9 | 27 (96.4); 28 |
| Synthetic non-absorbable | 4 (44.4); 9 | 0 (0); 28 |
| Biological | 0 (0); 9 | 1 (3.6); 28 |
| Posterior compartment only | ||
| No mesh | 5 (55.6); 9 | 27 (96.4); 28 |
| Synthetic non-absorbable | 4 (44.4); 9 | 0 (0); 28 |
| Biological | 0 (0); 9 | 1 (3.6); 28 |
| Continence procedure | ||
| No mesh | 1 (33.3); 3 | 1 (50.0); 2 |
| Synthetic non-absorbable | 2 (66.7); 3 | 1 (50.0); 2 |
| Characteristics | Treatment, n (%) | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Grade of operating gynaecologist | ||
| Consultant | 90 (86.5) | 92 (88.5) |
| Trainee doctor | 8 (7.7) | 4 (3.8) |
| Specialty doctor | 4 (3.8) | 4 (3.8) |
| Missing | 2 (1.9) | 4 (3.8) |
| Grade of anaesthetist | ||
| Consultant | 72 (69.2) | 60 (57.7) |
| Specialty doctor | 18 (17.3) | 27 (26.0) |
| Trainee doctor | 11 (10.6) | 8 (7.7) |
| Missing | 3 (2.9) | 9 (8.7) |
| Anaesthetic | ||
| General | 98 (94.2) | 90 (86.5) |
| Spinal/epidural | 8 (7.7) | 11 (10.6) |
| Local | 4 (3.8) | 7 (6.7) |
| Other | 0 (0) | 1 (1.0) |
| Prophylactic antibiotic used | 95 (91.3) | 96 (92.3) |
| Catheter inserted in theatre | 98 (94.2) | 87 (83.7) |
| Vaginal pack | 21 (20.2) | 81 (77.9) |
| Urinary catheter | ||
| Urethral | 96 (92.3) | 88 (84.6) |
| Suprapubic | 0 (0) | 1 (1.0) |
| Both | 1 (1.0) | 0 (0) |
| None | 1 (1.0) | 0 (0) |
| Do not know | 1 (1.0) | 0 (0) |
| Missing | 5 (4.8) | 15 (14.4) |
| Details | Treatment | Effect size (95% CI); p-value | |||
|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | ||||
| Mean (SD); N | Median (P25–75)a | Mean (SD); N | Median (P25–75)a | ||
| Duration of operation (minutes) | 145.5 (53.9); 94 | 145.0 (108.0–175.0) | 82.0 (34.1); 94 | 75.0 (61.0–93.0) | 60.48 (49.80 to 71.16); < 0.001b |
| Length of stay (days) | 2.1 (1.6); 101 | 2.0 (1.0–3.0) | 1.8 (1.2); 100 | 2.0 (1.0–2.0) | 1.21 (0.99 to 1.49); 0.059c |
| Blood loss (ml) | 97.7 (78.1); 90 | 100.0 (50.0–100.0) | 107.8 (74.8); 86 | 100.0 (50.0–150.0) | –15.25 (–35.21 to 4.71); 0.134c |
| Time to surgery (days) | 75.8 (94.4); 102 | 44.0 (14.0–105.0) | 64.3 (97.8); 100 | 36.0 (11.5–81.5) | |
FIGURE 31.
Distribution of POP-SSs (a) at baseline and at follow-up, that is, (b) at 6 months after surgery and (c) at 12 months after randomisation.
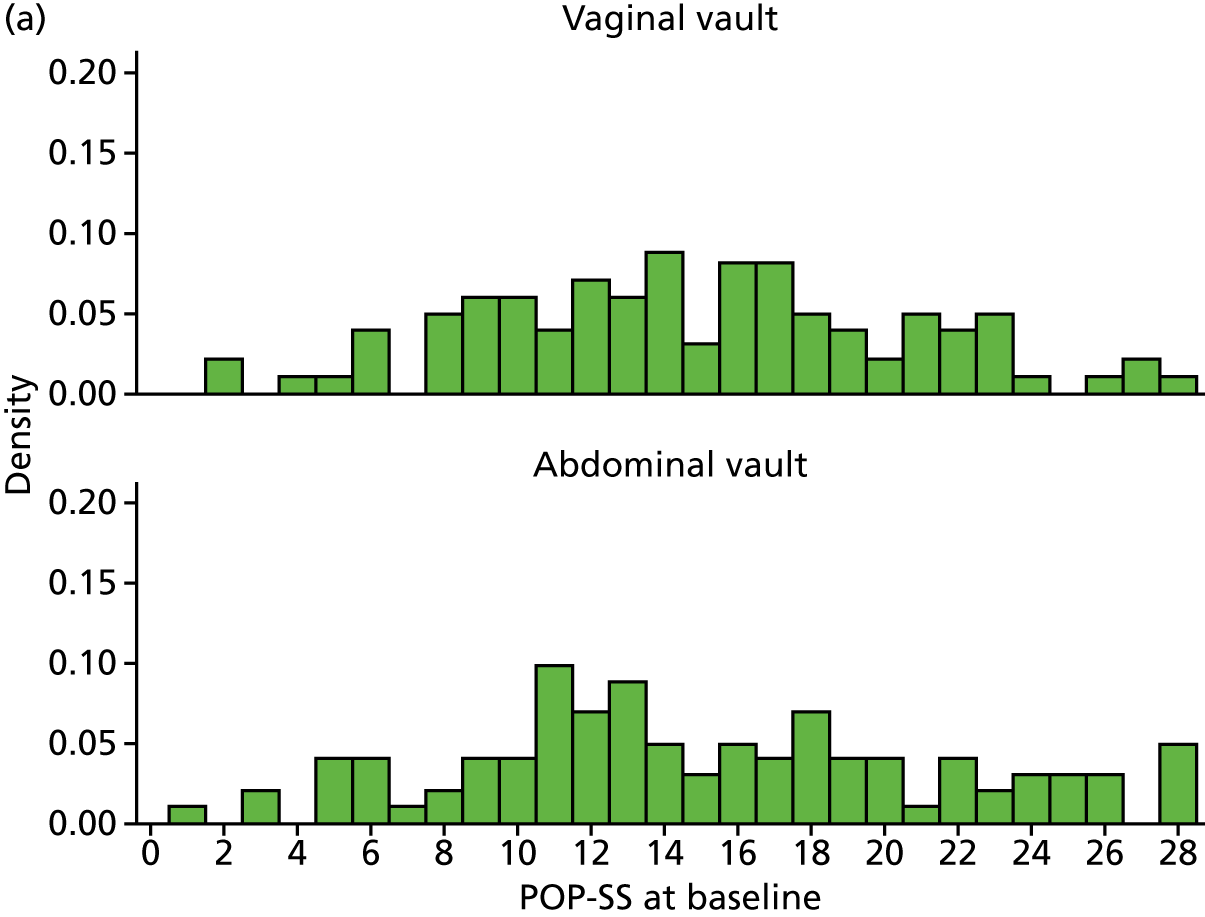
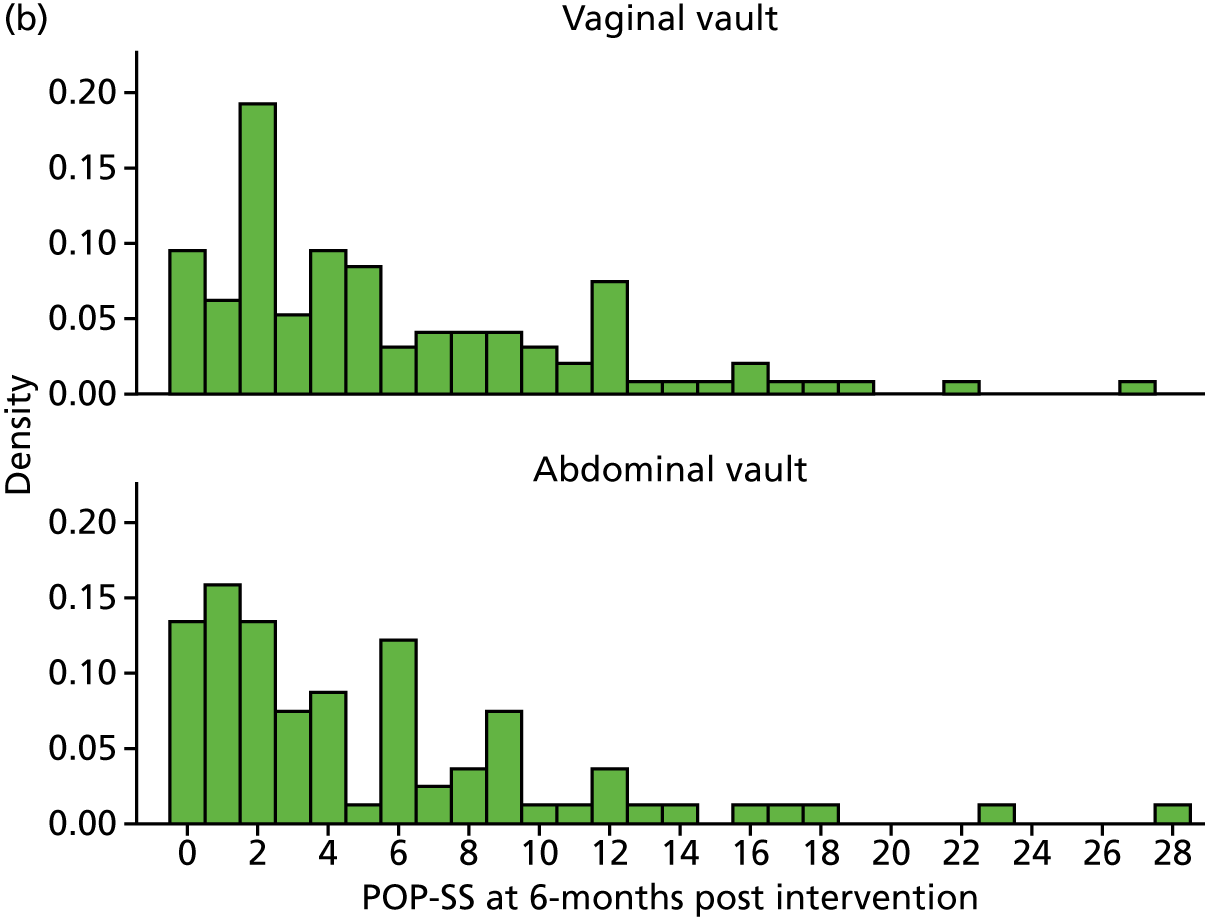
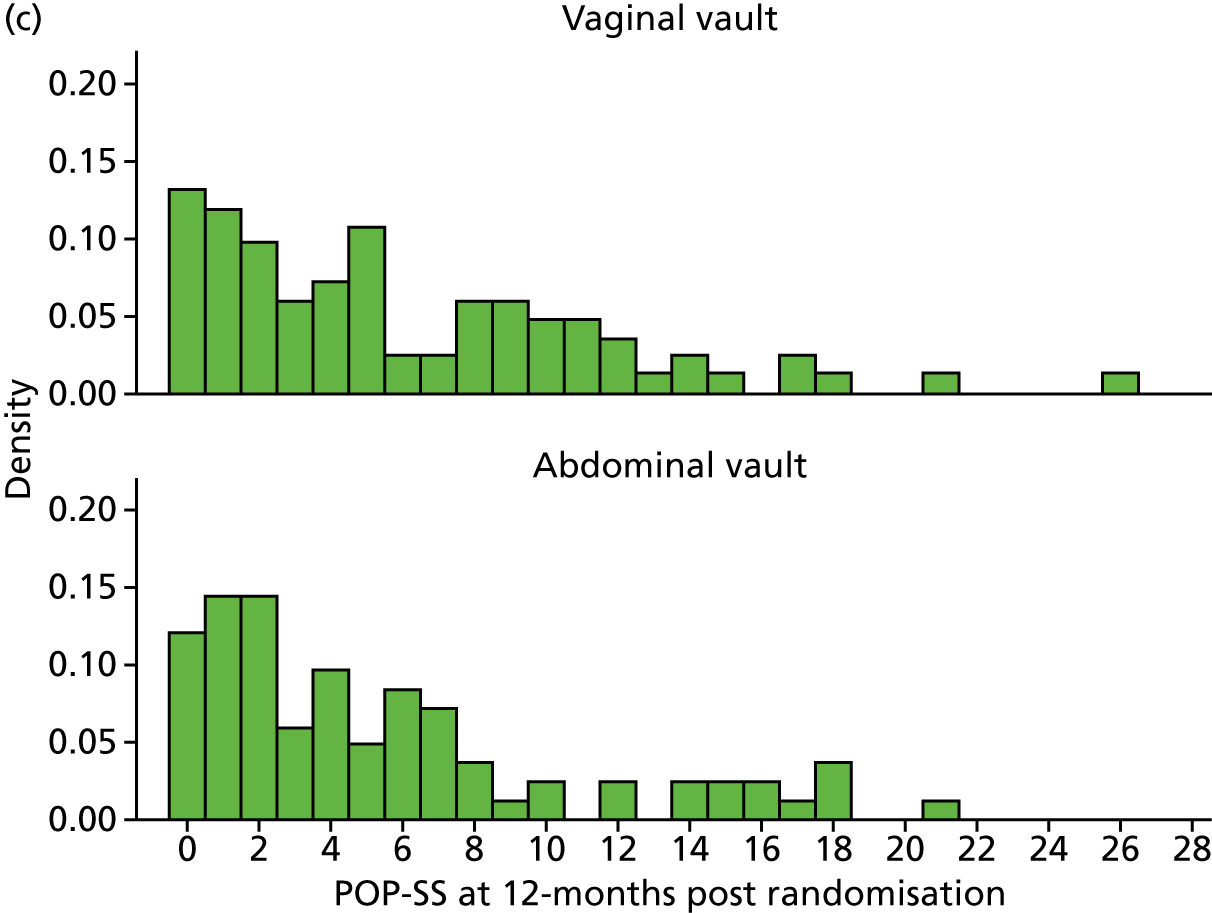
FIGURE 32.
Distribution of prolapse-related QoL scores (a) at baseline and follow-up, that is, (b) at 6 months after surgery and (c) at 12 months after randomisation.
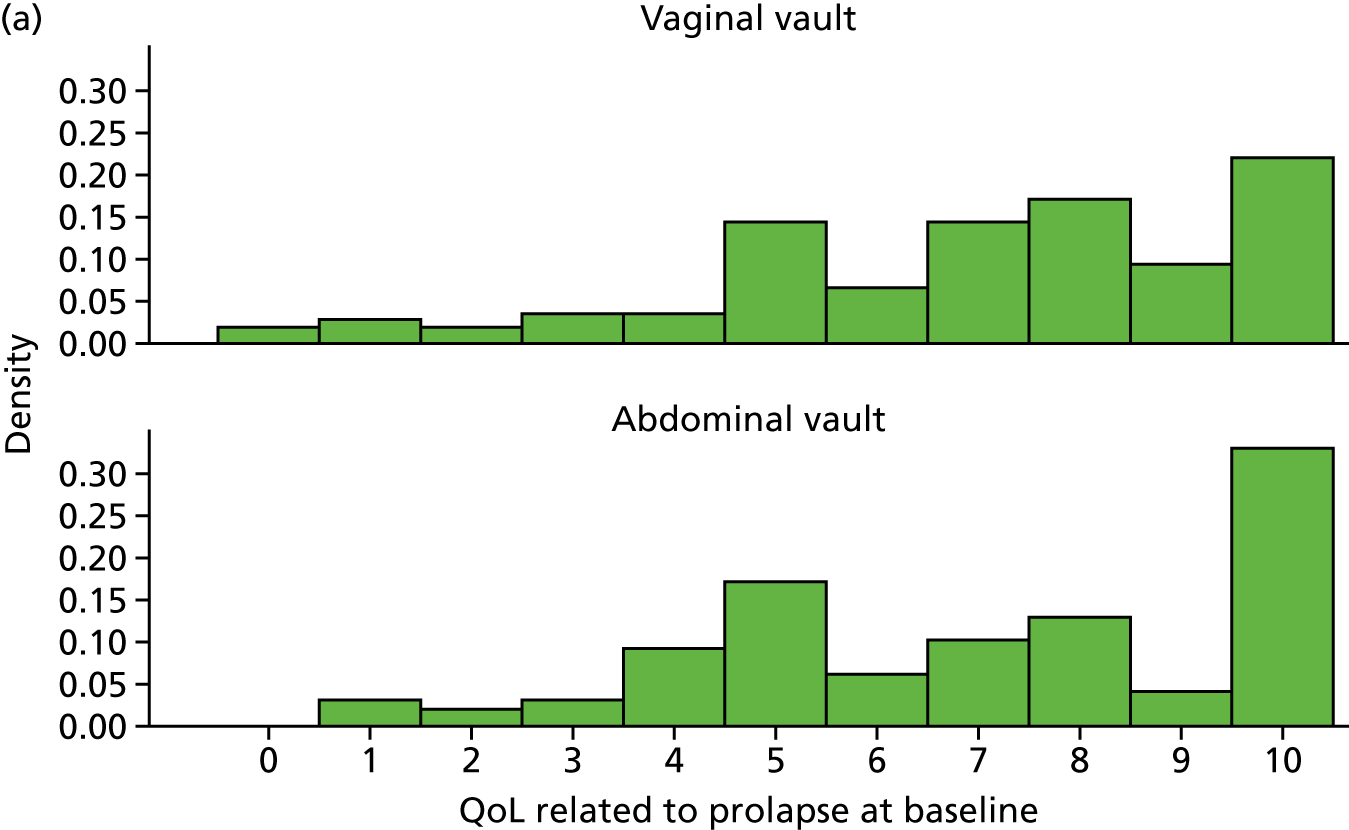
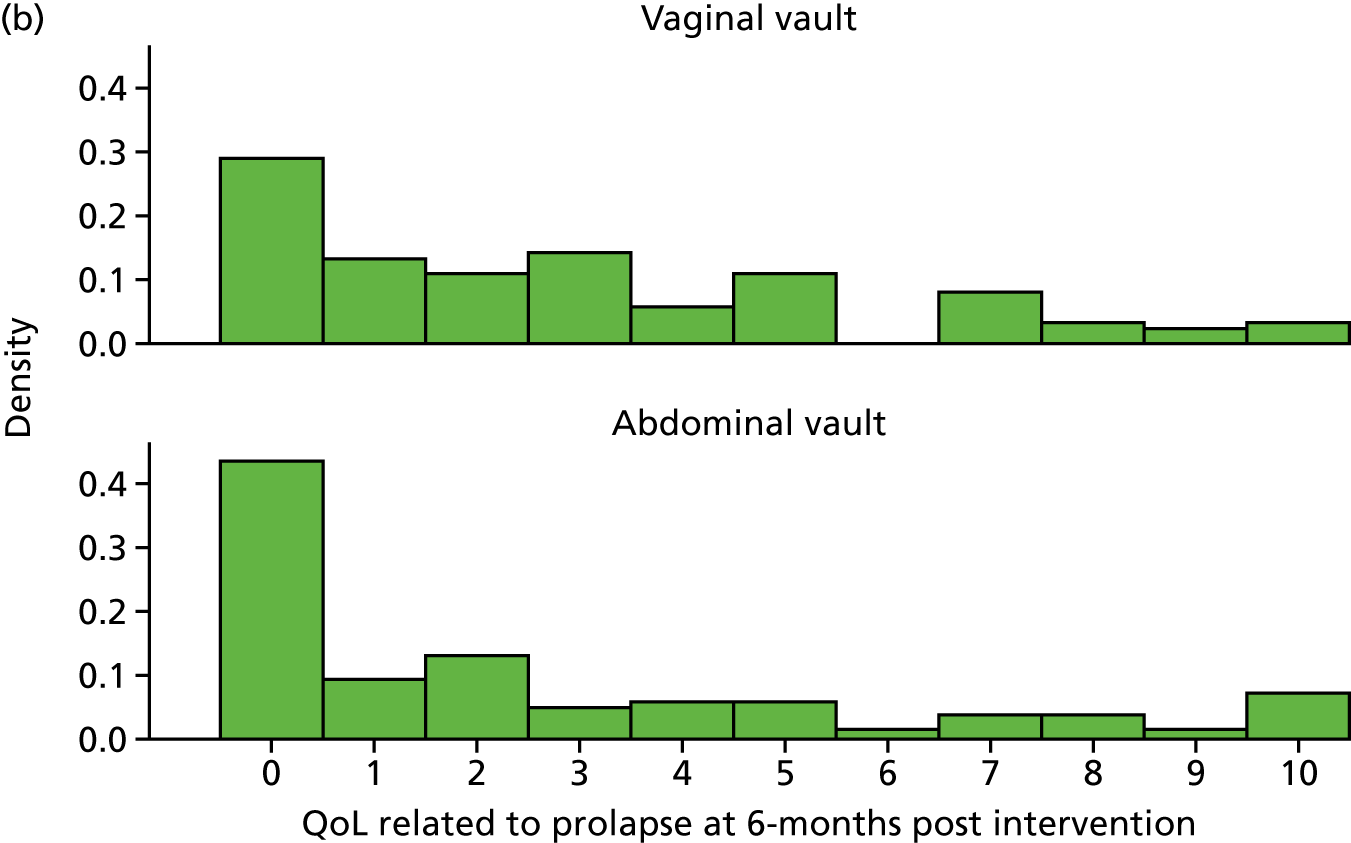
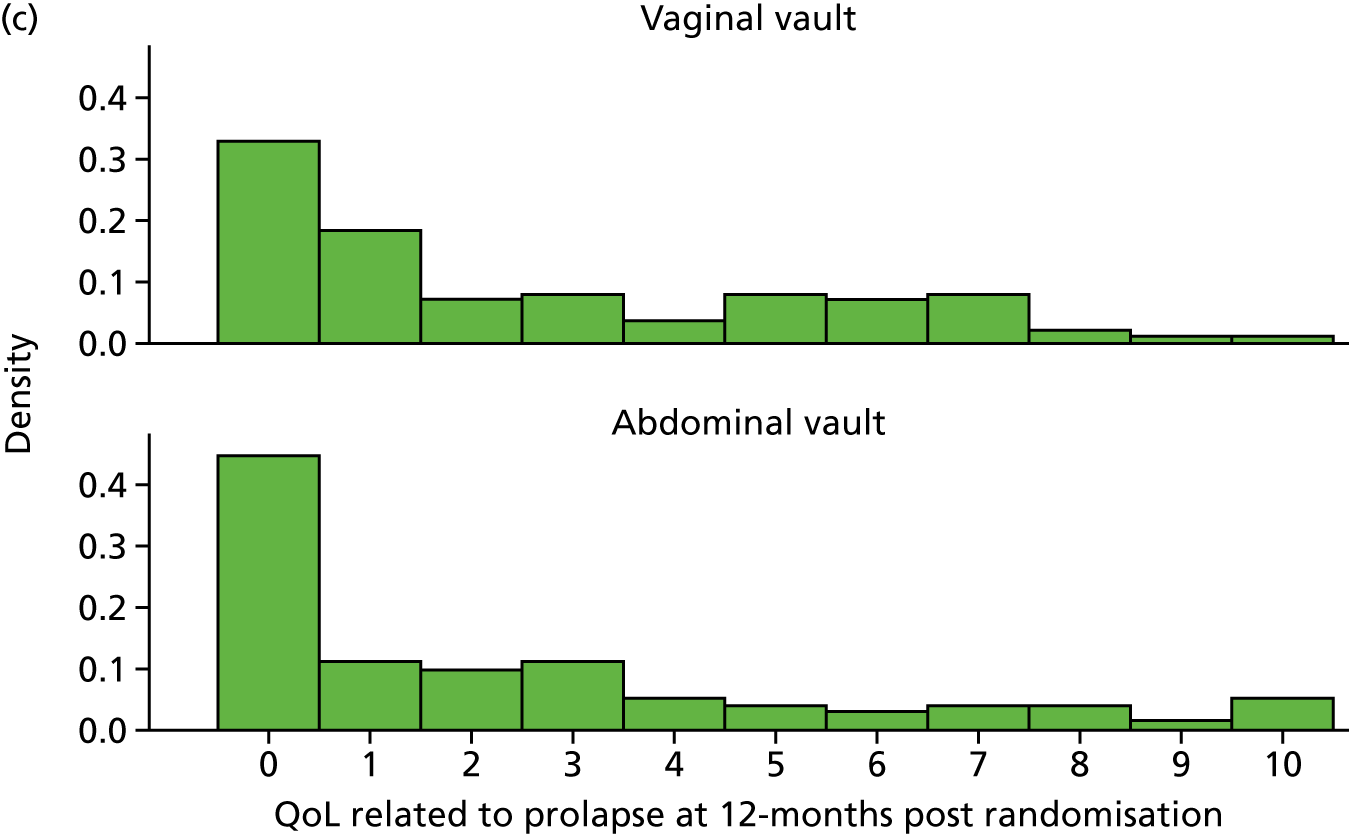
| Symptoms | Treatment | |
|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | |
| Symptomatic, n (%) N | ||
| Number of women with symptoms | 78 (89); 88 | 75 (87); 86 |
| Number of women without symptoms | 10 (11); 88 | 11 (13); 86 |
| Prolapse-related QoL score, mean (SD); count | 2.3 (3.0); 87 | 2.6 (2.8); 87 |
| Individual prolapse symptoms, n (%) N | ||
| Bowel not empty (any) | 62 (100); 62 | 56 (100); 56 |
| Bowel not empty (most/all of the time) | 13 (15); 89 | 8 (9); 87 |
| Bladder not empty (any) | 54 (100); 54 | 54 (100); 54 |
| Bladder not empty (most/all of the time) | 11 (12); 89 | 8 (9); 87 |
| Strain to empty bladder (any) | 38 (100); 38 | 37 (100); 100 |
| Strain to empty bladder (most/all of the time) | 7 (8); 87 | 6 (7); 87 |
| Dragging in back (any) | 36 (100); 36 | 39 (100); 39 |
| Dragging in back (most/all of the time) | 6 (7); 87 | 7 (8); 86 |
| Something coming down (any) | 31 (100); 31 | 41 (100); 41 |
| Something coming down (most/all of the time) | 13 (15); 89 | 15 (17); 87 |
| Dragging in abdomen (any) | 29 (100); 29 | 32 (100); 32 |
| Dragging in abdomen (most/all of the time) | 2 (2); 87 | 7 (8); 87 |
| Uncomfortable feeling or pain when standing (any) | 28 (100); 28 | 32 (100); 32 |
| Uncomfortable feeling or pain when standing (most/all of the time) | 7 (8); 87 | 7 (8); 86 |
| Most bothersome symptom, n (%) N | ||
| Bowel not empty | 27 (46); 59 | 18 (32); 56 |
| Bladder not empty | 14 (24); 59 | 6 (11); 56 |
| Something coming down | 6 (10); 59 | 19 (34); 56 |
| Strain to empty bladder | 4 (7); 59 | 3 (5); 56 |
| Dragging in back | 4 (7); 59 | 2 (4); 56 |
| Uncomfortable feeling or pain when standing | 3 (5); 59 | 4 (7); 56 |
| Dragging in abdomen | 1 (2); 59 | 4 (7); 56 |
| Which symptom causes most bother not applicable | 24 (29); 83 | 23 (29); 79 |
| EQ-5D | ||
| Mean (SD); count | 0.826 (0.217); 82 | 0.823 (0.175); 86 |
| Visual Scale, mean (SD); count | 78.2 (16.9); 83 | 77.5 (17.0); 85 |
| Outcome | Treatment | Vaginal vault (N = 104) |
|---|---|---|
| Abdominal vault (N = 104) | ||
| POP-Q measurement (cm), mean (SD); N [range] | ||
| Ba | –0.9 (1.9); 80 [–3.0 to 4.0] | –1.5 (1.7); 84 [–3.0 to 4.0] |
| C | –5.8 (2.9); 80 [–10.0 to 4.0] | –6.8 (2.7); 84 [–10.0 to 4.0] |
| Bp | –2.0 (1.4); 80 [–6.0 to 3.0] | –2.1 (1.4); 85 [–8.0 to 4.0] |
| Total vaginal length | 7.7 (1.6); 77 [2.0 to 10.0] | 8.4 (1.5); 83 [4.0 to 12.0] |
| Overall POP-Q stage, n (%); N | ||
| Stage 0 | 19 (22.1); 86 | 14 (17.3); 81 |
| Stage 1 | 25 (29.1); 86 | 16 (19.8); 81 |
| Stage 2a | 14 (16.3); 86 | 13 (16.0); 81 |
| Stage 2b | 22 (25.6); 86 | 25 (30.9); 81 |
| Stage 3 | 5 (5.8); 86 | 10 (12.3); 81 |
| Stage 4 | 1 (1.2); 86 | 3 (3.7); 81 |
| Symptom | Treatment, n (%) N | Effect sizea (95% CI); p-value | |
|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | ||
| SUI | 6 (8.1); 74 | 5 (6.3); 79 | 1.42 (0.40 to 5.10); 0.59 |
| Mixed UI | 1 (1.2); 86 | 1 (1.2); 87 | |
| UI for no reason | 3 (3.5); 85 | 2 (2.3); 86 | |
| UI when asleep | 1 (1.4); 74 | 1 (1.2); 81 | |
| UI with sex | 0 (0) | 1 (1.9); 54 | |
| Time to further surgery | Treatment | Effect sizea (95% CI); p-value | |||
|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | ||||
| Mean (SD); N | Median (P25–75)b | Mean (SD); N | Median (P25–75)b | ||
| Number of days | 337.7 (206.3); 7 | 243.0 (219.0–395.0) | 310.0 (190.8); 7 | 350.0 (152.0–367.0) | 0.94 (0.31, 2.87); 0.921 |
| Subgroup | Treatment, mean (SD); n | Effect sizea (99% CI); p-value | |
|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | ||
| Age group (years) | |||
| < 60 | 4.1 (4.2); 19 | 5.5 (5.1); 15 | –0.34 (–3.84 to 3.16); 0.85 |
| ≥ 60 | 6.1 (5.6); 69 | 6.0 (5.5); 71 | |
| Previous incontinence surgery | |||
| Yes | 3.0 (0); 1 | 11.0 (0); 1 | –1.61 (–16.29 to 13.08); 0.83 |
| No | 5.7 (5.4); 87 | 5.9 (5.4); 85 | |
| Previous anterior/posterior surgery | |||
| Yes | 5.3 (5.3); 58 | 6.2 (5.8); 59 | –1.07 (–2.88 to 0.73); 0.24 |
| No | 6.2 (5.6); 30 | 5.4 (4.5); 27 | |
Appendix 7 Additional information for Chapter 8
| Intervention cost | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | Mean costs (SD); N | Abdominal vault vs. vaginal vault | |||
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | MD | 95% CI | |||
| Anaesthesia | 102 (100); 102 | 100 (100); 100 | 20.12 (3.75); 102 | 18.96 (5.60); 100 | ||
| Theatre overheads | 102 (100); 102 | 100 (100); 100 | 420.19 (0.00); 102 | 420.19 (0.00); 100 | ||
| Mesh for apical prolapse | 82 (80); 102 | 6 (6); 100 | ||||
| Mesh for concomitant anterior | 6 (6); 102 | 2 (2); 100 | ||||
| Mesh for concomitant posterior | 5 (5); 102 | 3 (3); 100 | ||||
| Mesh for prolapse | 143.44 (150.83); 102 | 19.99 (79.82); 100 | ||||
| Incontinence mesh | 2 (2); 102 | 1 (1); 100 | 2.18 (15.48); 102 | 1.11 (11.11); 100 | ||
| Return to theatre | 0 (0); 102 | 0 (0); 100 | 0.00 (0.00); 102 | 0.00 (0.00); 100 | ||
| Catheterisation | 98 (96); 102 | 88 (88); 100 | 6.12 (1.24); 102 | 5.61 (2.08); 100 | ||
| Pain relief | 102 (100); 102 | 100 (100); 100 | 2.25 (2.25); 102 | 1.73 (1.91); 100 | ||
| Laxatives | 57 (56); 102 | 62 (62); 100 | 1.10 (0.98); 102 | 1.22 (0.96); 100 | ||
| Antibiotics | 95 (93); 102 | 96 (97); 99 | 0.99 (0.27); 102 | 1.03 (0.18); 99 | ||
| Vaginal pack | 25 (25); 102 | 81 (81); 100 | 1.14 (2.02); 102 | 3.78 (1.84); 100 | ||
| Gynaecologist’s time (minutes), mean (SD); n | 142.71 (52.24); 103 | 83.90 (33.90); 100 | 335.49 (123.91); 102 | 197.40 (79.92); 100 | ||
| Anaesthetist’s time (minutes), mean (SD); N | 142.71 (52.24); 103 | 83.90 (33.90); 100 | 279.80 (138.14); 102 | 162.59 (92.23); 100 | ||
| Nurse time (minutes), mean (SD); N | 142.71 (52.24); 103 | 83.90 (33.90); 100 | 383.96 (140.54); 103 | 225.73 (91.22); 100 | ||
| Length of stay (days); mean (SD); N | 2.15 (1.63); 102 | 1.76 (1.16); 100 | 384.32 (292.01); 102 | 315.04 (206.88); 100 | ||
| Total intervention costs | 1541.55 (500.02); 102 | 935.22 (351.74); 100 | 582.31 | 472.32 to 692.30 | ||
| Hospital resource use (postcode verified) | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | Mean costs (SD); N | Abdominal vault vs. vaginal vault | |||
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | MD | 95% CI | |||
| New prolapse procedure | 6 (6); 103 | 5 (5); 97 | 167.13 (694.31); 103 | 199.39 (870.13); 97 | –22.99 | –270.95 to 224.96 |
| Anterior | 2 (2); 103 | 1 (1); 97 | ||||
| Posterior | 2 (2); 100 | 0 (0); 97 | ||||
| Apical | 1 (1); 103 | 4 (4); 97 | ||||
| Anterior and posterior | 1 (1); 103 | 0 (0); 97 | ||||
| Anterior and apical | 0 (0); 103 | 0 (0); 97 | ||||
| Posterior and apical | 0 (0); 103 | 0 (0); 97 | ||||
| Anterior and posterior and apical | 0 (0); 103 | 0 (0); 97 | ||||
| New UI procedure | 3 (3); 103 | 1 (1); 97 | 61.05 (354.19); 103 | 21.61 (212.82); 97 | 44.70 | –53.81 to 143.21 |
| Sling | 3 (3); 103 | 1 (1); 97 | ||||
| Abdominal | 0 (0); 103 | 0 (0); 97 | ||||
| Botox | 0 (0); 103 | 0 (0); 97 | ||||
| Injectable | 0 (0); 103 | 0 (0); 97 | ||||
| Hospital admission other (including SAE) | 3 (3); 104 | 4 (4); 104 | 12.71 (74.49); 104 | 61.45 (312.51); 104 | –39.52 | –125.40 to 46.37 |
| Total hospital admission costs | 241.14 (777.74); 103 | 286.89 (932.08); 97 | –19.69 | –251.73 to 212.35 | ||
| Other consultations (participant reported number of consultations) | Resource usage, mean (SD) Na | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | Mean costs (SD); Nb | Abdominal vault vs. vaginal vault | |||
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | MD | 95% CI | |||
| Outpatient consultations | 1.60 (0.82); 25 | 1.59 (1.01); 37 | 77.51 (121.67); 73 | 111.17 (138.27); 72 | ||
| GP | 2.95 (3.14); 19 | 2.36 (2.14); 25 | 28.80 (74.71); 70 | 31.70 (62.20); 67 | ||
| Practice nurse | 2.17 (2.04); 6 | 1.00 (0.00); 3 | 3.48 (13.46); 54 | 0.89 (3.50); 49 | ||
| District or incontinence nurse | 2.00 (1.41); 2 | 2.50 (2.00); 3 | 2.67 (15.83); 57 | 7.76 (27.95); 49 | ||
| Physiotherapy | ||||||
| Practice | 1.00 (N/A); 1 | 2.00 (N/A); 1 | 0.60 (4.50); 57 | 1.48 (10.03); 46 | ||
| Hospital | 3.00 (2.45); 7 | 1.63 (0.74); 8 | 12.10 (42.71); 59 | 8.67 (22.39); 51 | ||
| Other consultations (participant reported) | 14.38 (81.71); 48 | 20.70 (49.90); 40 | ||||
| Total cost of professional consultations | 116.69 (183.22); 81 | 141.54 (154.69); 84 | –43.21 | –94.87 to 8.44 | ||
| Other treatments and medications (participant reported) | Resource usage, n (%); Na | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | Mean costs (SD); Nb | Abdominal vault vs. vaginal vault | |||
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | MD | 95% CI | |||
| Oestrogens | 17 (25); 69 | 13 (19); 69 | 66.32 (207.90); 60 | 31.90 (124.20); 65 | ||
| Antibiotics | 13 (20); 66 | 19 (26); 72 | 1.03 (6.04); 61 | 1.11 (3.58); 67 | ||
| Bladder medications | 9 (13); 67 | 6 (10); 62 | 0.94 (4.17); 62 | 0.42 (3.14); 58 | ||
| Reusable/intermittent catheter | 3 (4); 74 | 4 (6); 67 | 0.53 (3.82); 73 | 0.20 (1.12); 65 | ||
| Ring pessary | 3 (4); 75 | 3 (4); 68 | 0.80 (3.96); 75 | 0.89 (4.16); 68 | ||
| Shelf pessary | 0 (0); 73 | 3 (4); 69 | 0.00 (0.00); 73 | 0.94 (4.42); 69 | ||
| Permanent catheter | 0 (0); 71 | 2 (3); 64 | 1.27 (9.15); 72 | 0.48 (2.68); 64 | ||
| Total other treatments | 56.46 (190.41); 76 | 27.86 (109.60); 84 | 61.19 | –8.25 to 130.63 | ||
| Participant-incurred costs | Resource usage, n (%); N | Costs (£) | ||||
|---|---|---|---|---|---|---|
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | Mean costs (SD); N | Abdominal vs. vaginala (£) | |||
| Abdominal vault (N = 104) | Vaginal vault (N = 104) | MD | 95% CI | |||
| Opportunity cost of time and travel | ||||||
| Inpatient stays | 91.31 (28.84); 102 | 90.12 (24.97); 97 | ||||
| Outpatient consultations | 28.54 (44.81); 73 | 43.16 (54.02); 73 | ||||
| Primary care consultations | 18.40 (39.44); 74 | 17.82 (26.13); 71 | ||||
| Subtotal | 134.22 (90.91); 65 | 138.49 (77.17); 60 | ||||
| Purchase of over-the-counter medication | 4 (6); 68 | 6 (8); 71 | 0.43 (2.66); 66 | 4.70 (21.03); 71 | ||
| Private medical care | 0 (0); 64 | 0 (0); 66 | 0.00 (0.00); 64 | 0.00 (0.00); 66 | ||
| Other expenses | 2 (3); 63 | 3 (4); 68 | 1.27 (9.46); 63 | 9.60 (56.81); 68 | ||
| Absence from paid employment | 672.56 (2729.50); 78 | 578.50 (2658.55); 81 | ||||
| Currently in employment | 22 (27); 83 | 19 (23); 82 | ||||
| Reported needing time off workb | 19 (86); 22 | 19 (100); 19 | ||||
| Days of sick leaveb,c | 25.63 (47.14); 19 | 21.75 (46.79); 19 | ||||
| Total participant perspective costs | 864 (3141); 51 | 645 (2887); 47 | –53 | –400 to 294 | ||
| Total NHS perspective costs (Table 35) | 1542 (500); 102 | 935 (352); 100 | 582 | 472 to 692 | ||
| Wider-perspective costs (NHS and personal) | 2758 (3507); 51 | 2032 (3061); 47 | 825 | 208 to 1443 | ||
FIGURE 33.
Scatterplot (a) and CEAC (b) for seemingly unrelated regression (imputed data set).

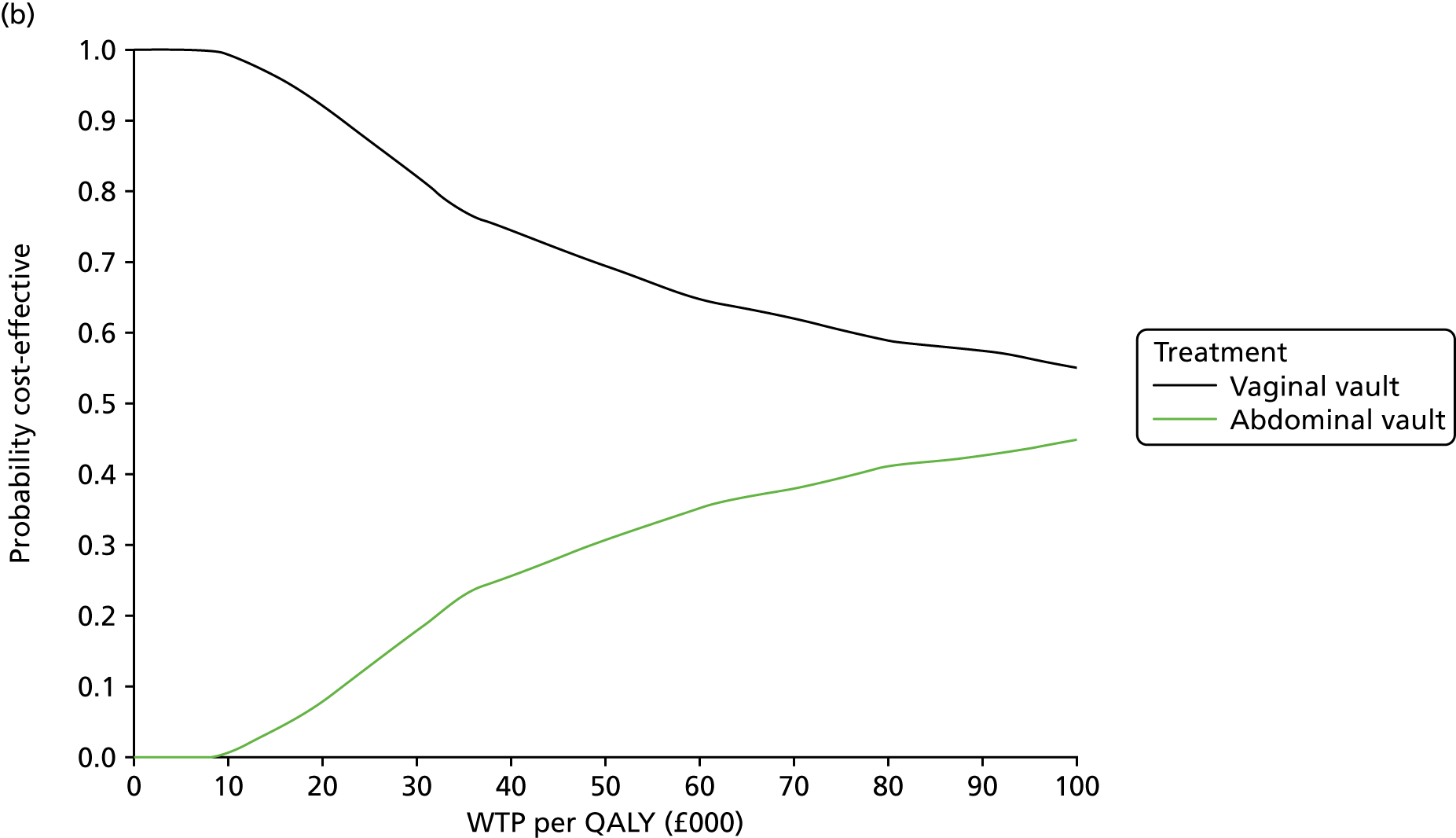
FIGURE 34.
Scatterplot (a) and CEAC (b) for wider-perspective analysis of costs (imputed data set).
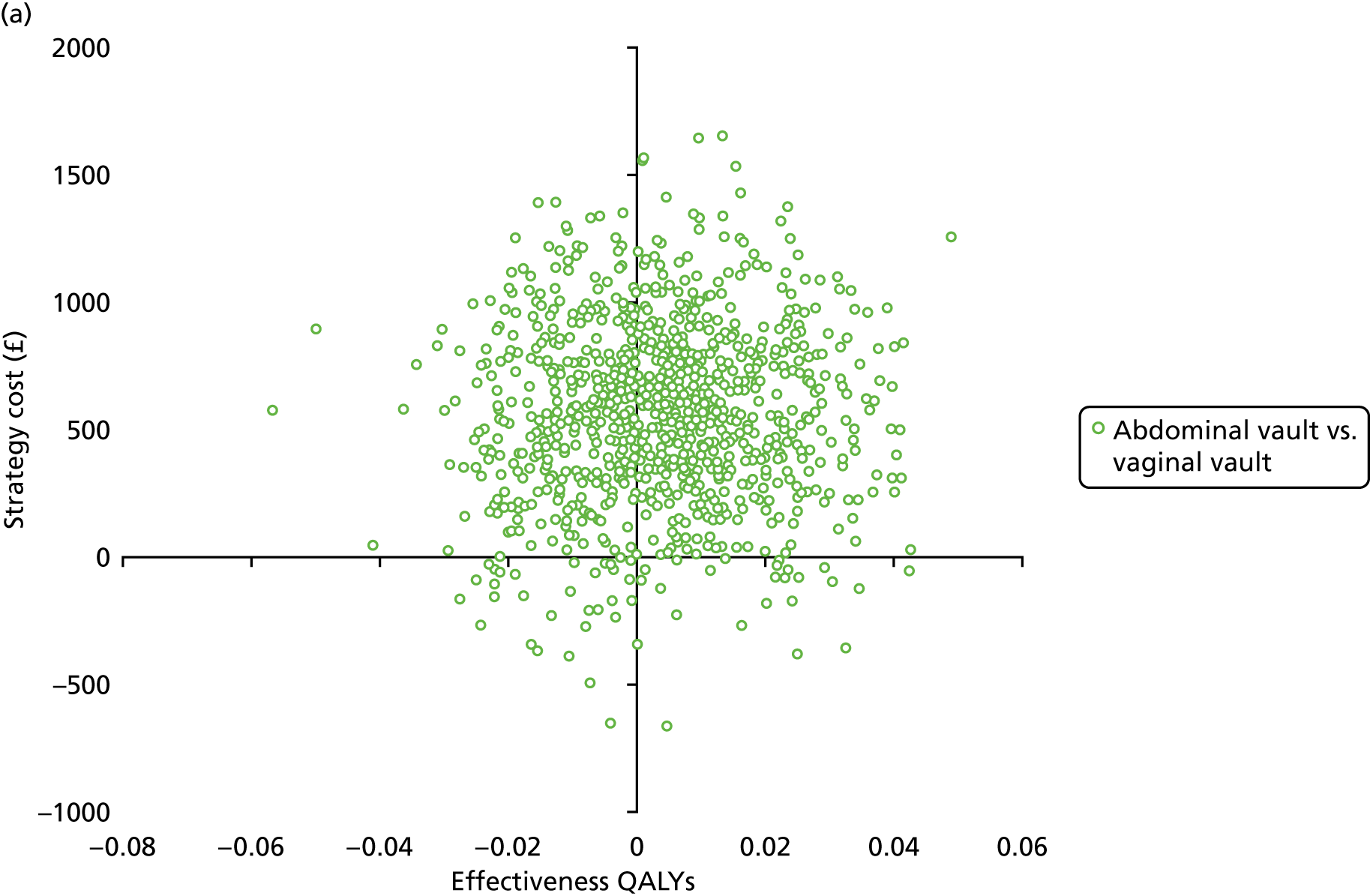
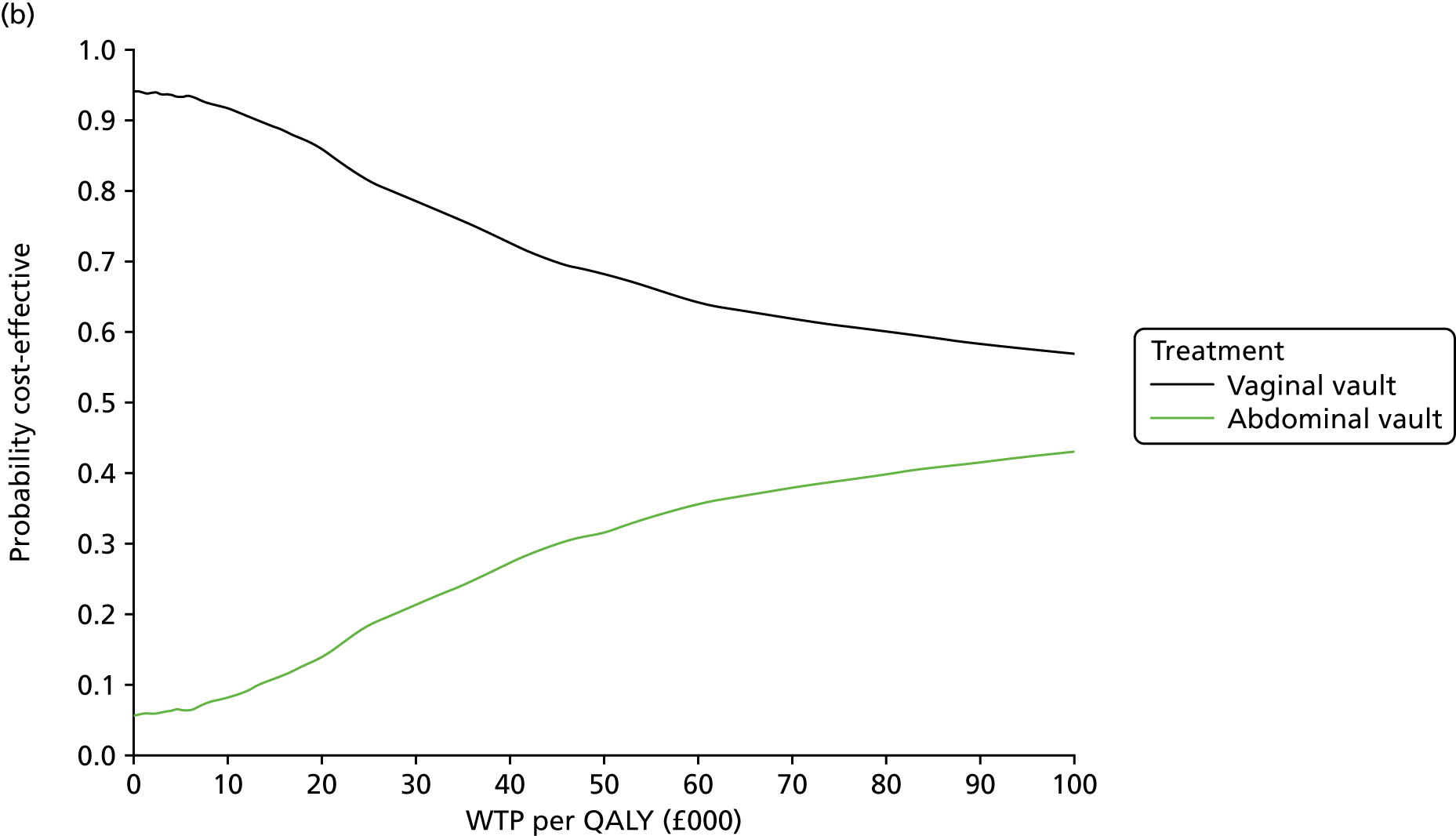
FIGURE 35.
Scatterplot (a) and CEAC (b) using HRG tariffs instead of component costing for index interventions (imputed data set).


FIGURE 36.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 1 (imputed data set).


FIGURE 37.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 2 (imputed data set).


FIGURE 38.
Scatterplot (a) and CEAC QALY calculation (b) sensitivity analysis 3 (imputed data set).
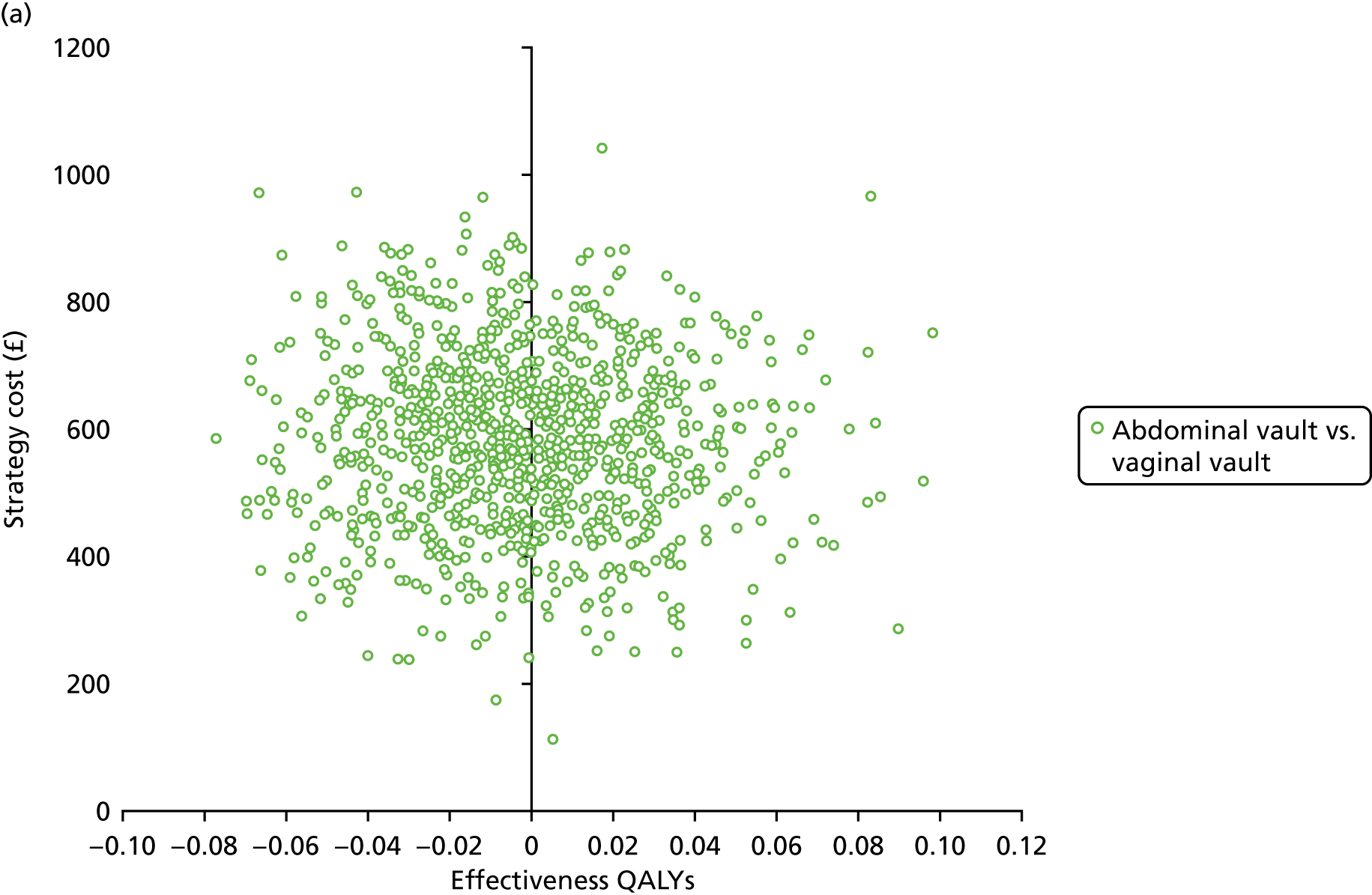
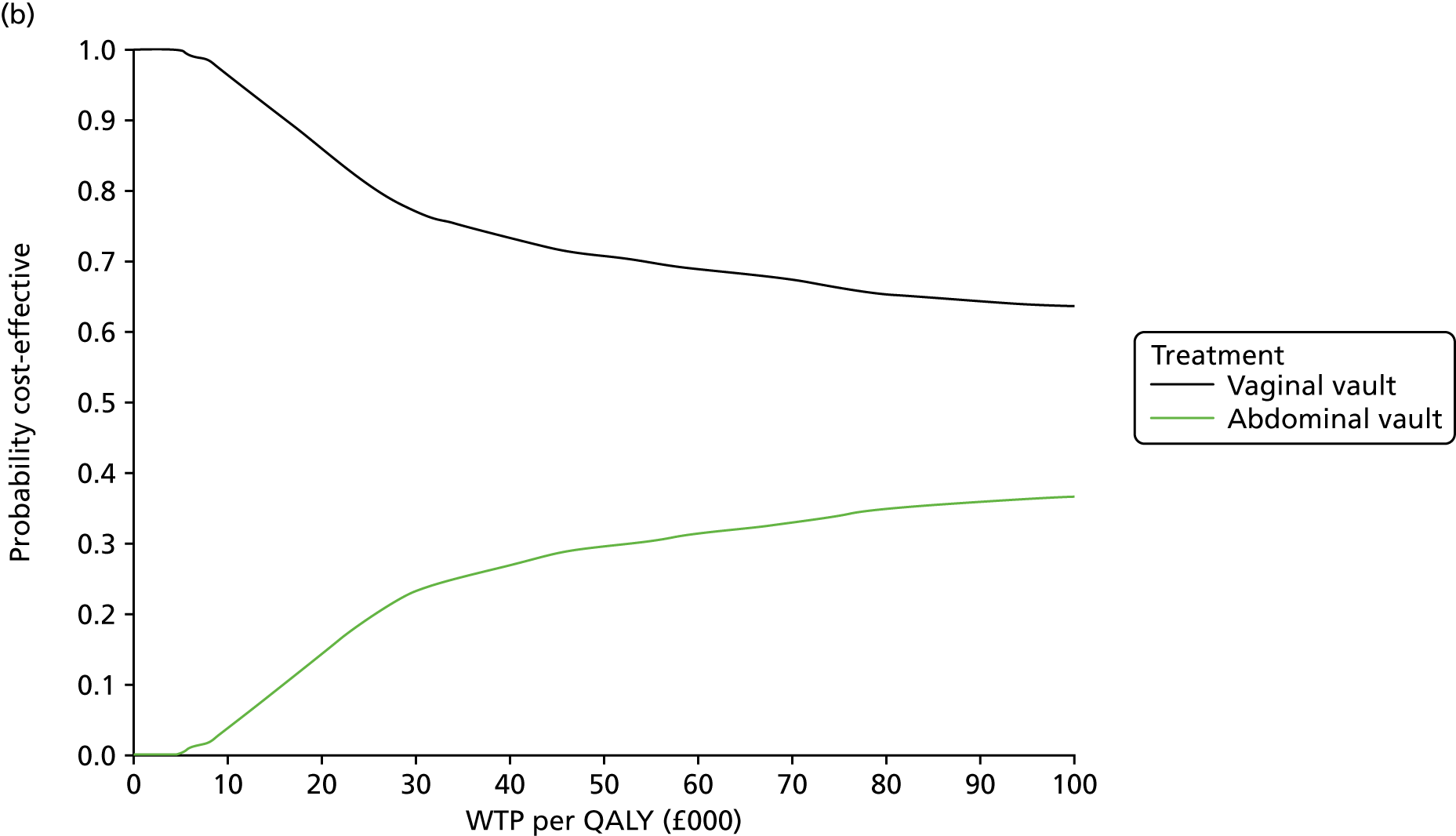
FIGURE 39.
Scatterplot (a) and CEAC (b) complete-case analysis of cost and QALY pairs.
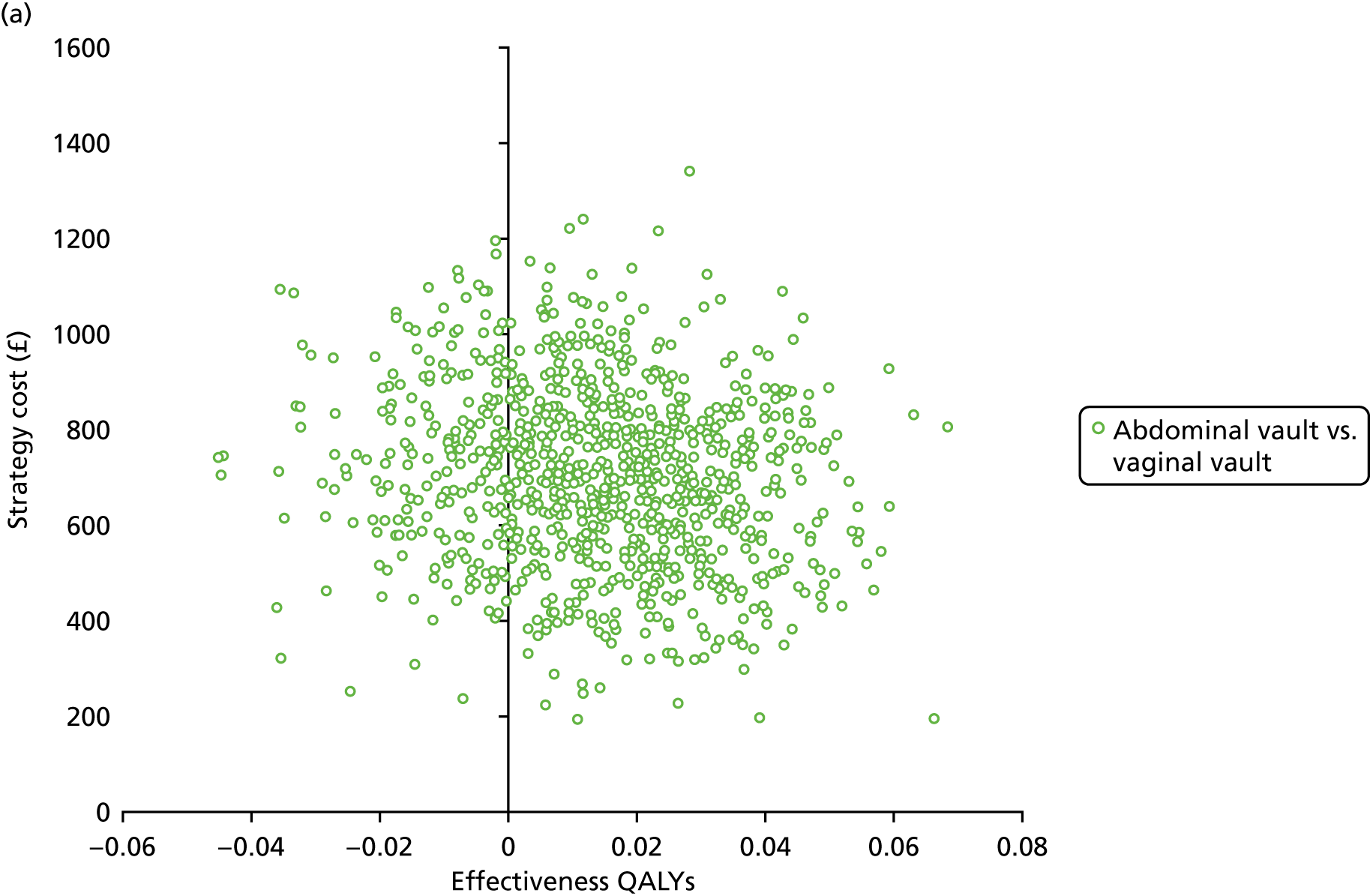
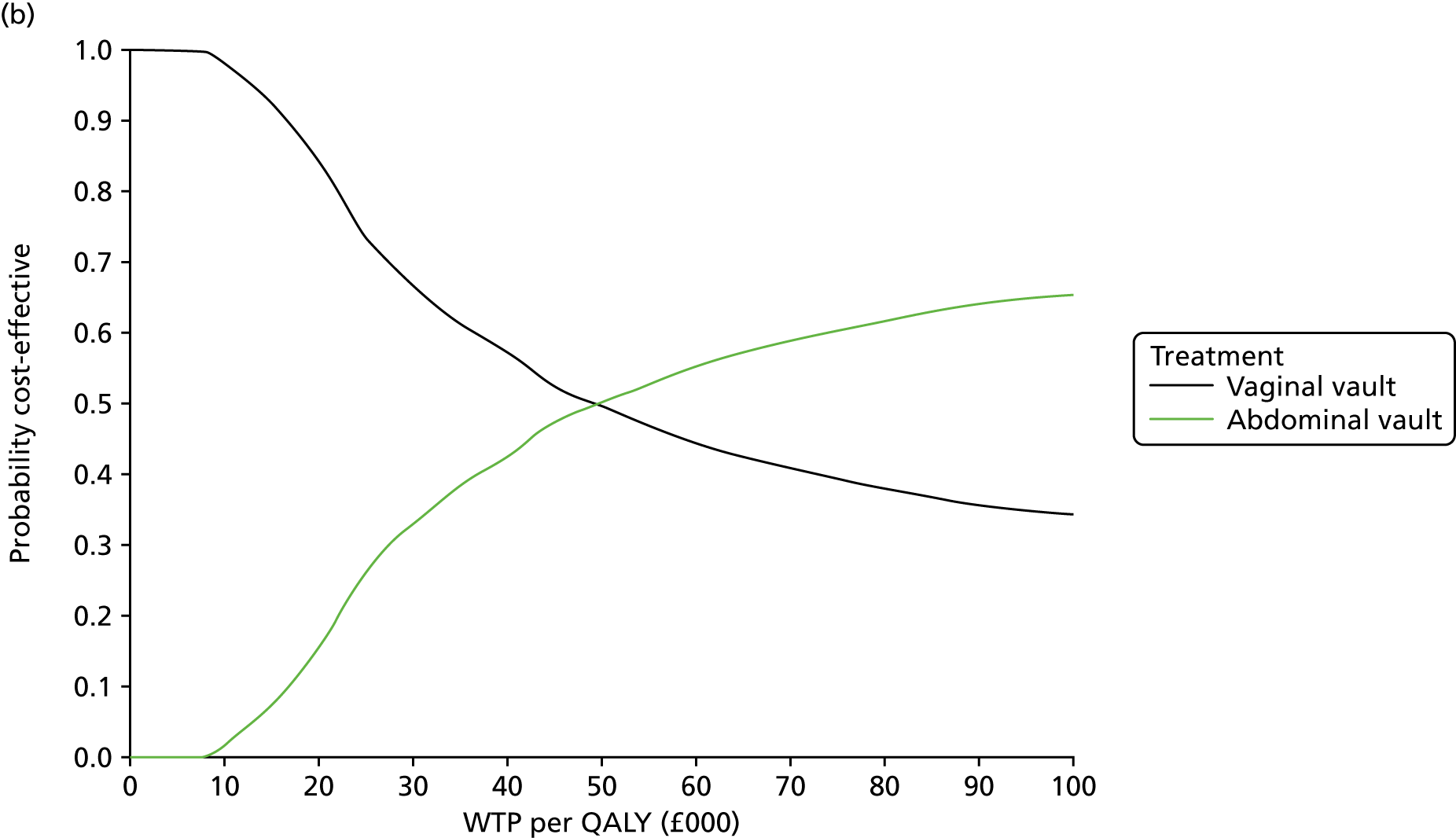
FIGURE 40.
Scatterplot (a) and CEAC (b) seemingly unrelated regression on complete-case analysis of cost and QALY pairs.


Appendix 8 Additional information for Chapter 9
| Variables | Survival function (SE) | |||||
|---|---|---|---|---|---|---|
| Coxa | Gamma | Log-logistic | Exponential | Gompertz | Weibull | |
| Adjusted models | ||||||
| Uterine preservation | 1.566 (0.575) | –0.428 (0.357) | –0.405 (0.342) | 1.556 (0.571) | 1.556 (0.572) | 1.553 (0.571) |
| Baseline EQ-5D | 0.202 (0.131) | 1.774 (0.771) | 1.631 (0.662) | 0.190 (0.124) | 0.191 (0.125) | 0.184 (0.120) |
| Age | 0.520 (0.185) | 0.637 (0.361) | 0.631 (0.338) | 0.504 (0.179) | 0.505 (0.180) | 0.496 (0.177) |
| Planned anterior surgery | 2.287 (0.966) | –0.832 (0.428) | –0.794 (0.404) | 2.308 (0.976) | 2.305 (0.975) | 2.332 (0.989) |
| Planned posterior surgery | 0.585 (0.220) | 0.454 (0.372) | 0.494 (0.352) | 0.578 (0.218) | 0.578 (0.218) | 0.574 (0.217) |
| Planned incontinence surgery | 0.735 (0.551) | 0.142 (0.843) | 0.244 (0.706) | 0.735 (0.549) | 0.735 (0.549) | 0.731 (0.545) |
| Constant | – | 4.214 (2.085) | 3.452 (1.685) | 0.027 (0.047) | 0.027 (0.048) | 0.021 (0.038) |
| Scale | – | 1.742 | 0.877 | – | –0.0019 | 1.104 |
| Kappa | – | 0.193 | – | – | – | – |
| Log-likelihood | –193.108 | –143.775 | –144.019 | –144.350 | –144.350 | –144.174 |
| AIC | 398.217 | 305.550 | 304.039 | 302.699 | 304.694 | 304.348 |
| BIC | 343.61 | 337.867 | 332.299 | 338.522 | 338.176 | |
| Unadjusted models | ||||||
| Uterine preservation | 1.615 (0.552) | –0.430 (0.363) | –0.445 (0.328) | 1.610 (0.550) | 1.610 (0.551) | 1.610 (0.550) |
| Constant | – | 6.850 (0.820) | 6.057 (0.720) | 0.002 (0.001) | 0.002 (0.001) | 0.001 (0.001) |
| Scale | – | 2.763 | 0.906 | – | –0.0095 | 1.078 |
| Kappa | – | –0.580 | – | – | – | – |
| Log-likelihood | –220.136 | –162.163 | –162.882 | –163.096 | –163.022 | –162.988 |
| AIC | 442.273 | 332.327 | 331.765 | 330.192 | 332.045 | 331.975 |
| BIC | – | 349.448 | 344.606 | 338.753 | 344.886 | 344.816 |
FIGURE 41.
Uterine trial failure: long-term extrapolation of different survival functions. H, hysterectomy; P, preservation.

| Variables | Survival function (SE) | |||||
|---|---|---|---|---|---|---|
| Coxa | Generalised gamma | Log-logistic | Exponential | Gompertz | Weibull | |
| Adjusted models | ||||||
| Abdominal vault | 0.827 (0.409) | 0.174 (0.339) | 0.111 (0.343) | 0.833 (0.411) | 0.826 (0.409) | 0.819 (0.406) |
| Baseline EQ-5D | 1.415 (1.831) | –0.211 (0.815) | –0.251 (0.906) | 1.418 (1.842) | 1.429 (1.835) | 1.434 (1.830) |
| Age | 2.324 (1.766) | –0.603 (0.537) | –0.554 (0.525) | 2.344 (1.780) | 2.354 (1.789) | 2.360 (1.794) |
| Planned anterior surgery | 1.021 (0.503) | –0.007 (0.334) | 0.007 (0.341) | 1.017 (0.500) | 0.994 (0.489) | 0.989 (0.487) |
| Planned posterior surgery | 0.296 (0.155) | 0.841 (0.403) | 0.827 (0.397) | 0.294 (0.154) | 0.292 (0.153) | 0.291 (0.152) |
| Planned incontinence Surgery | 0.058 (0.065) | 2.145 (0.763) | 2.030 (0.860) | 0.064 (0.071) | 0.052 (0.059) | 0.045 (0.051) |
| Constant | – | 0.104 | 0.262 (2.153) | 1.397 (4.240) | 1.508 (4.580) | 0.687 (2.112) |
| Scale | – | 0.262 | 0.645 | – | 0.034 | 1.481 |
| Kappa | – | 2.716 | – | – | – | – |
| Log-likelihood | –81.077 | –62.333 | –62.567 | –63.730 | –63.286 | –62.442 |
| AIC | 174.153 | 142.666 | 141.132 | 141.459 | 142.572 | 140.884 |
| BIC | 193.508 | 171.698 | 166.938 | 164.040 | 168.378 | 166.690 |
| Unadjusted models | ||||||
| Abdominal vault | 0.885 (0.430) | –0.024 | 0.087 (0.349) | 0.885 (0.430) | 0.885 (0.430) | 0.885 (0.430) |
| Constant | – | 4.992 | 4.508 (0.637) | 0.005 (0.004) | 0.004 (0.003) | 0.001 (0.002) |
| Scale | – | 1.425 | 0.683 | – | 0.028 | 1.420 |
| Kappa | – | 2.535 | – | – | – | – |
| Log-likelihood | –86.789 | –67.692 | –68.288 | –69.431 | –69.124 | –68.381 |
| AIC | 175.577 | 143.385 | 142.577 | 142.862 | 144.248 | 142.762 |
| BIC | 178.881 | 156.598 | 152.487 | 149.468 | 154.158 | 152.671 |
FIGURE 42.
Vault trial failure: long-term extrapolation of different survival functions. A, abdominal; V, vaginal.
| Variablesa | Survival function (SE) | ||||
|---|---|---|---|---|---|
| Coxb | Log-logistic | Exponential | Gompertz | Weibull | |
| Adjusted models | |||||
| Uterine preservation | 1.071 (0.472) | –0.120 (0.756) | 1.077 (0.476) | 1.070 (0.471) | 1.075 (0.474) |
| Baseline EQ-5D | 0.691 (0.689) | 0.886 (1.743) | 0.541 (0.550) | 0.666 (0.667) | 0.607 (0.611) |
| Age | 0.783 (0.363) | 0.522 (0.802) | 0.707 (0.328) | 0.771 (0.358) | 0.741 (0.344) |
| Planned anterior surgery | 0.221 (0.228) | 2.575 (1.821) | 0.219 (0.226) | 0.220 ((0.227) | 0.220 (0.227) |
| Planned posterior surgery | 1.063 (0.474) | –0.086 (0.765) | 1.019 (0.454) | 1.058 (0.472) | 1.038 (0.463) |
| Planned incontinence surgery | 0.821 (0.848) | 0.301 (1.784) | 0.878 (0.906) | 0.826 (0.853) | 0.854 (0.881) |
| Constant | – | 3.452 (4.567) | 0.039 (0.104) | 0.092 (0.244) | 0.115 (0.309) |
| Scale | – | 1.672 | – | –0.141 | 0.588 |
| Kappa | – | – | – | – | – |
| Log-likelihood | –127.103 | –116.539 | –120.405 | –113.9881 | –116.624 |
| AIC | 266.207 | 249.077 | 254.809 | 243.9761 | 249.248 |
| BIC | 291.324 | 282.567 | 284.113 | 277.466 | 282.737 |
| Unadjusted models | |||||
| Uterine preservation | 1.118 (0.489) | –0.197 (0.754) | 1.132 (0.495) | 1.132 (0.495) | 1.126 (0.492) |
| Constant | – | 8.552 (1.693) | 0.002 (0.001) | 0.002 (0.001) | 0.006 (0.005) |
| Scale | – | 1.688 | – | –0.142 | 0.585 |
| Kappa | – | – | – | – | – |
| Log-likelihood | –130.155 | –116.539 | –123.627 | –117.066 | –119.750 |
| AIC | 262.311 | 245.374 | 251.254 | 240.131 | 245.500 |
| BIC | 266.551 | 258.095 | 259.735 | 252.852 | 258.221 |
FIGURE 43.
Uterine trial complications: long-term extrapolation of different survival functions. H, hysterectomy; P, preservation.

| Variablesa | Survival function (SE) | ||||
|---|---|---|---|---|---|
| Coxb | Log-logistic | Exponential | Gompertz | Weibull | |
| Adjusted models | |||||
| Abdominal vault suspension | 0.763 (0.591) | 0.373 (1.032) | 0.750 (0.582) | 0.763 (0.591) | 0.757 (0.586) |
| Baseline EQ-5D | 4.154 (10.704) | –1.868 (3.480) | 3.985 (10.204) | 4.320 (11.202) | 4.140 (10.663) |
| Age | 1.804 (1.953) | –0.775 (1.455) | 1.781 (1.928) | 1.822 (1.972) | 1.801 (1.949) |
| Planned anterior surgery | 0.803 (0.615) | 0.307 (1.015) | 0.769 (0.588) | 0.815 (0.624) | 0.790 (0.605) |
| Planned posterior surgery | 0.705 (0.544) | 0.472 (1.042) | 0.720 (0.556) | 0.699 (0.540) | 0.711 (0.549) |
| Constant | – | 8.129 (5.227) | 0.001 (0.003) | 0.002 (0.006) | 0.002 (0.007) |
| Scale | – | 1.293 | – | –0.073 | 0.762 |
| Kappa | – | – | – | – | – |
| Log-likelihood | –34.598 | –37.480 | –37.802 | –37.047 | –37.500 |
| AIC | 79.195 | 88.960 | 87.603 | 88.095 | 89.000 |
| BIC | 94.962 | 111.033 | 106.523 | 110.168 | 111.073 |
| Unadjusted models | |||||
| Abdominal vault suspension | 0.693 (0.530) | 0.489 (1.006) | 0.684 (0.522) | 0.694 (0.530) | 0.689 (0.526) |
| Constant | – | 6.385 (1.967) | 0.003 (0.004) | 0.006 (0.008) | 0.007 (0.009) |
| Scale | – | 1.275 | – | –0.070 | 0.774 |
| Kappa | – | – | – | – | – |
| Log-likelihood | –35.518 | –38.397 | –38.682 | –38.006 | –38.414 |
| AIC | 73.037 | 82.793 | 81.364 | 82.012 | 82.828 |
| BIC | 76.268 | 92.487 | 87.826 | 91.706 | 92.522 |
FIGURE 44.
Vault trial complications: long-term extrapolation of different survival functions. A, abdominal; V, vaginal.
Detailed results from the economic model sensitivity analyses
FIGURE 45.
Cost-effectiveness acceptability curve: 0% discounting.

FIGURE 46.
Scatterplot of incremental cost-effectiveness: 0% discounting.

FIGURE 47.
Cost-effectiveness acceptability curve: 6% discounting.

FIGURE 48.
Scatterplot of incremental cost-effectiveness: 6% discounting.
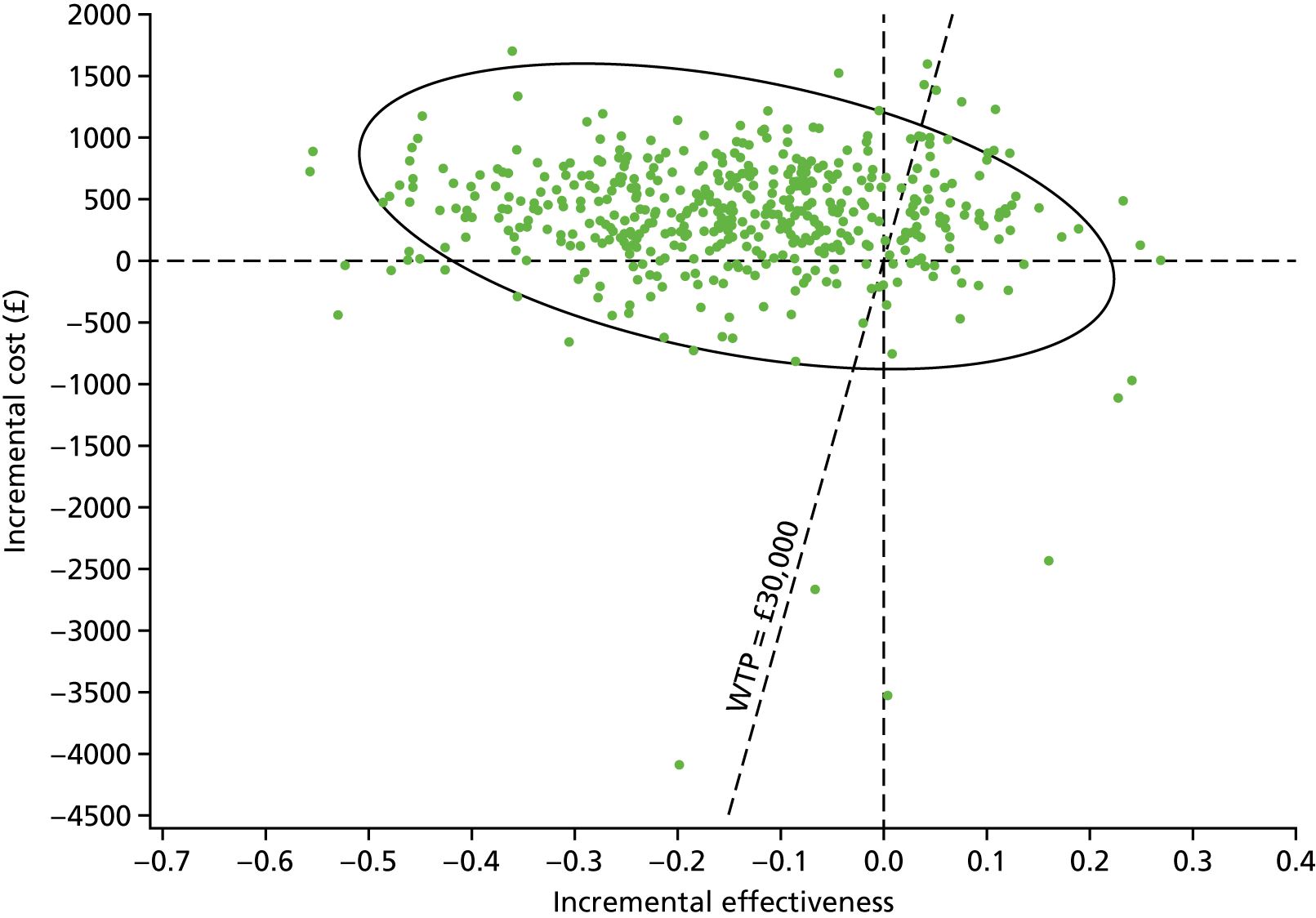
FIGURE 49.
Cost-effectiveness acceptability curve: age 53 years.
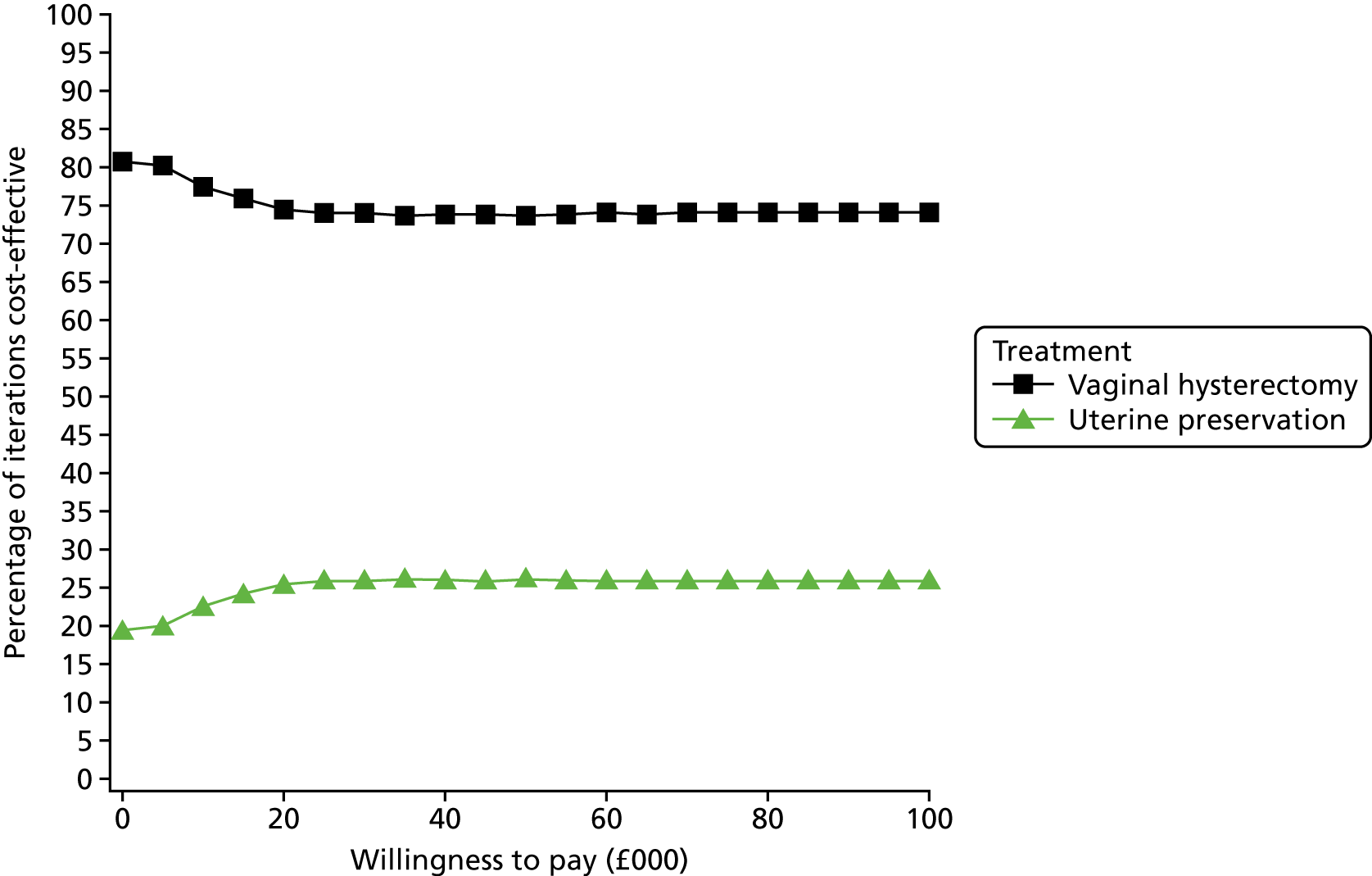
FIGURE 50.
Scatterplot of incremental cost-effectiveness: age 53 years.

FIGURE 51.
Cost-effectiveness acceptability curve: age 73 years.
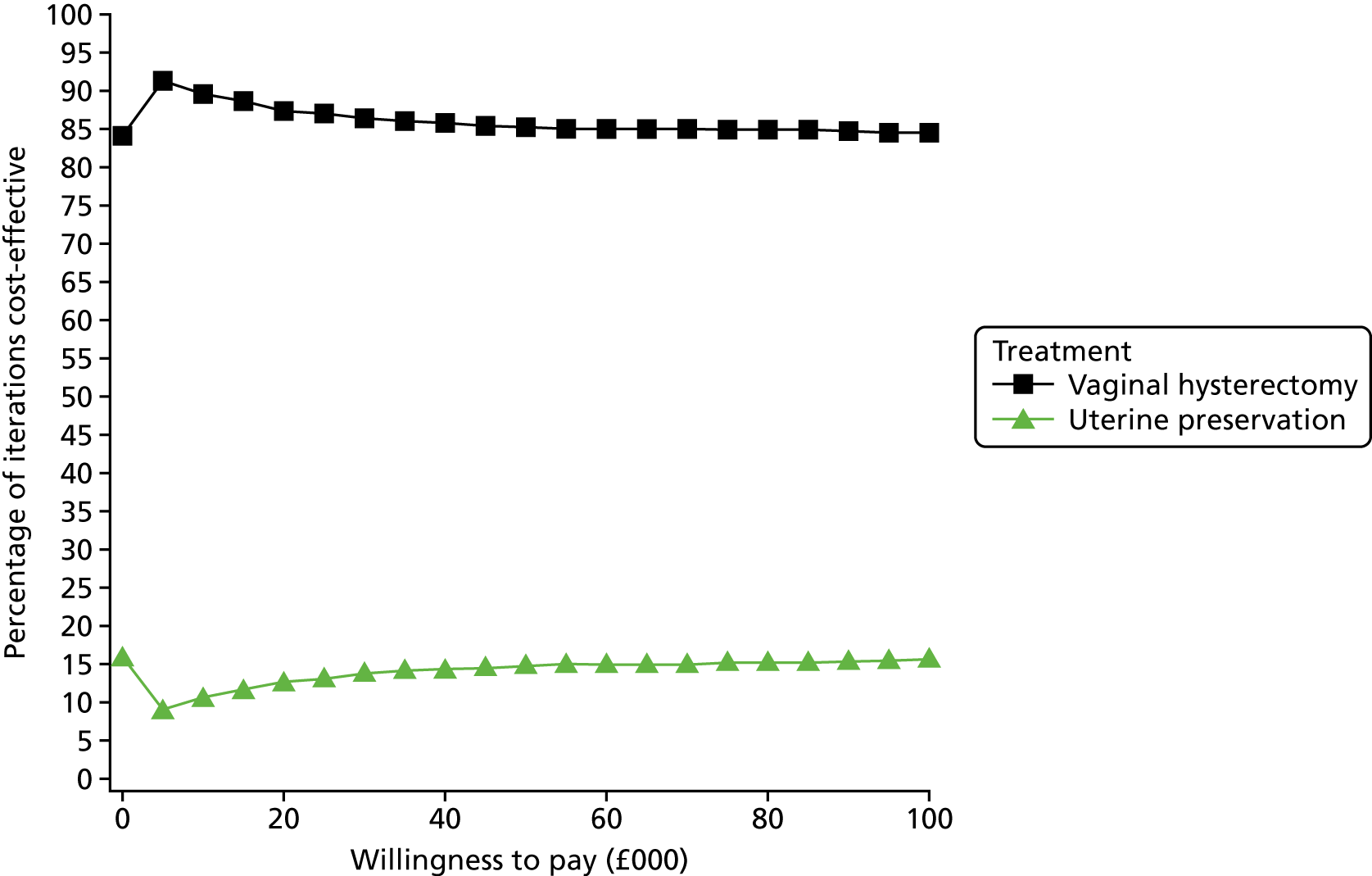
FIGURE 52.
Scatterplot of incremental cost-effectiveness: age 73 years.
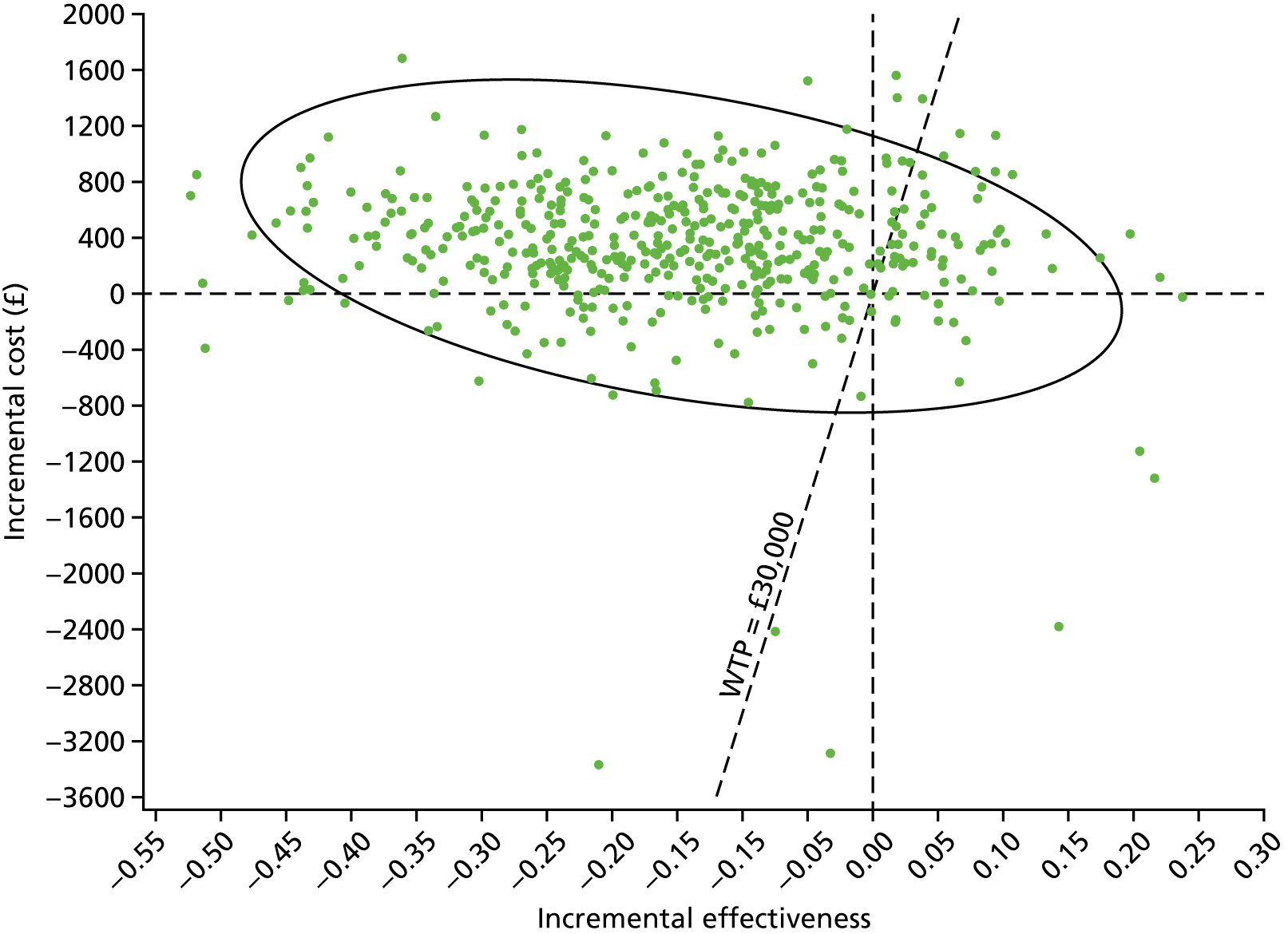
FIGURE 53.
Cost-effectiveness acceptability curve: HRG-based intervention costing.

FIGURE 54.
Scatterplot of incremental cost-effectiveness: HRG-based intervention costing.

FIGURE 55.
Cost-effectiveness acceptability curve: time horizon – 5 years.

FIGURE 56.
Scatterplot of incremental cost-effectiveness: time horizon – 5 years.
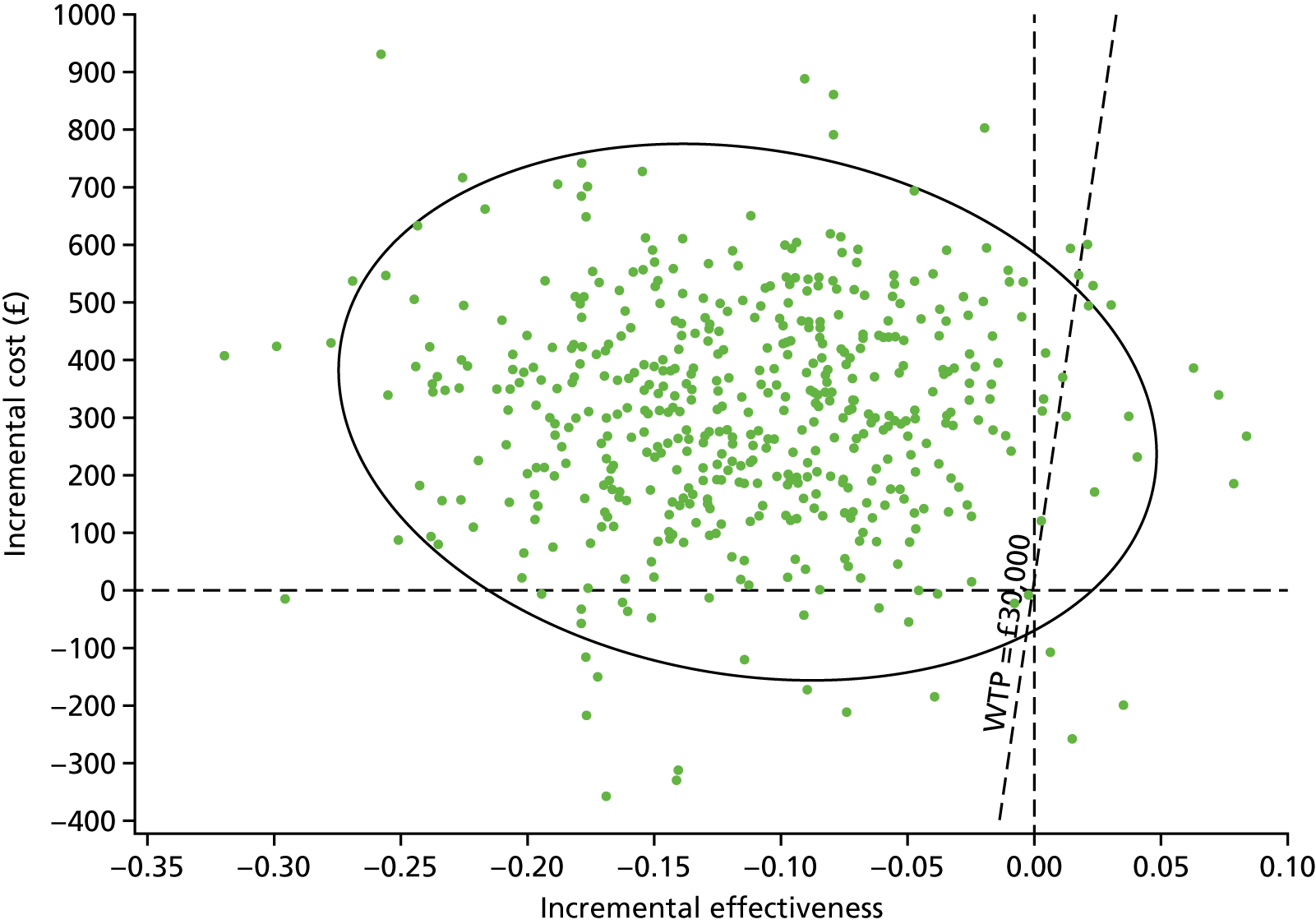
FIGURE 57.
Cost-effectiveness acceptability curve: time horizon – 10 years.
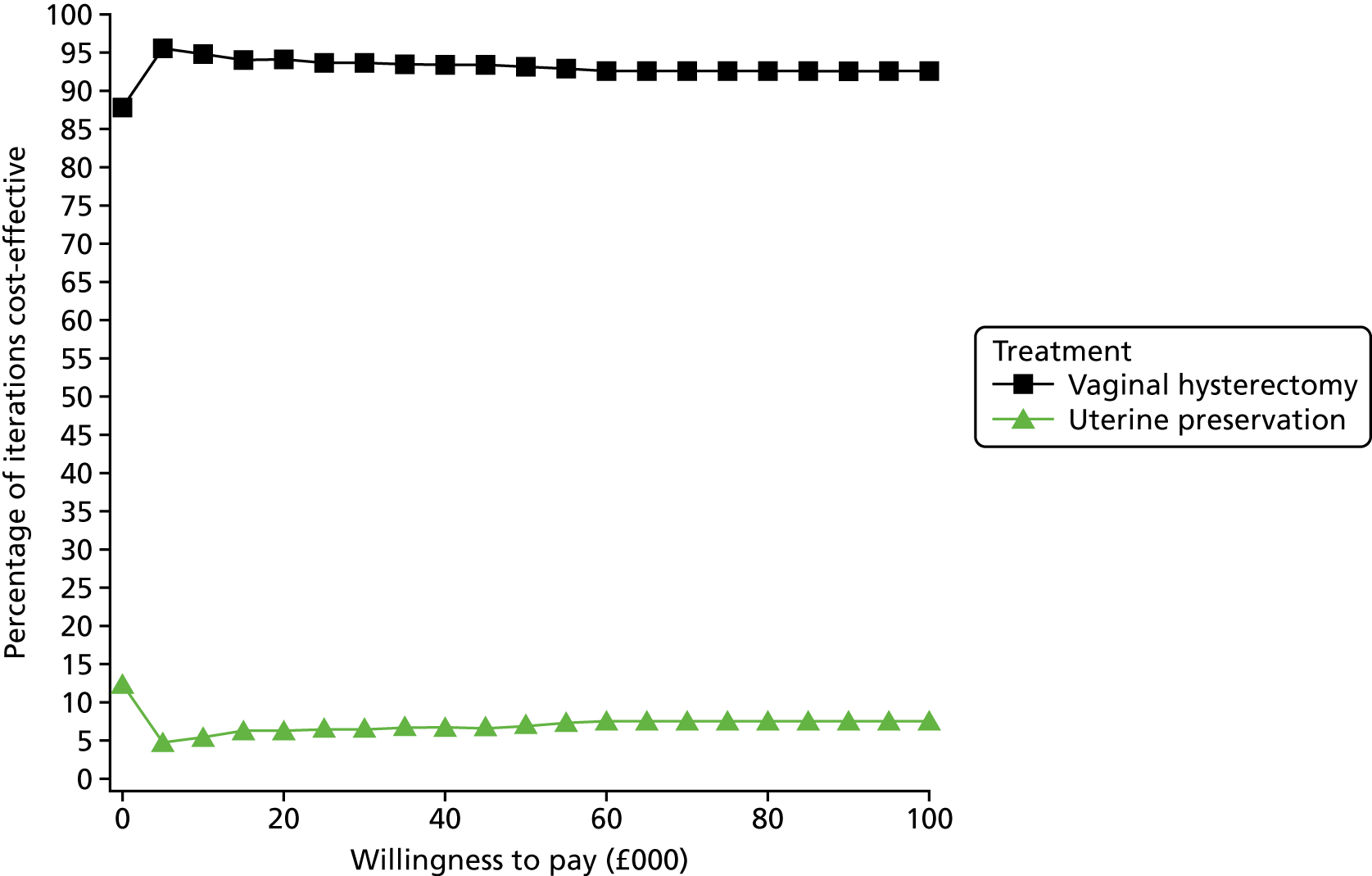
FIGURE 58.
Scatterplot of incremental cost-effectiveness: time horizon – 10 years.

FIGURE 59.
Cost-effectiveness acceptability curve: time horizon – 20 years.
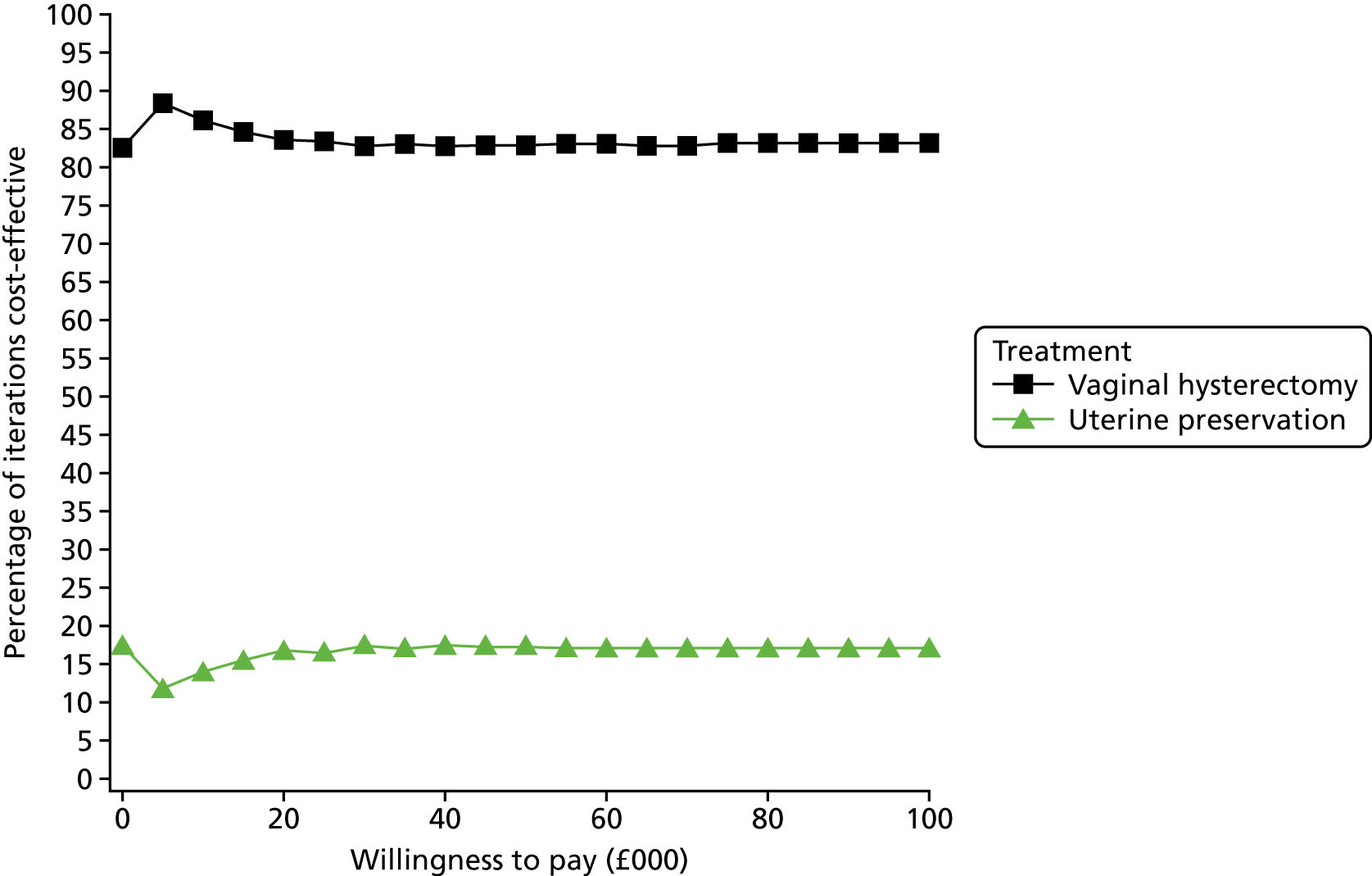
FIGURE 60.
Scatterplot of incremental cost-effectiveness: time horizon – 20 years.
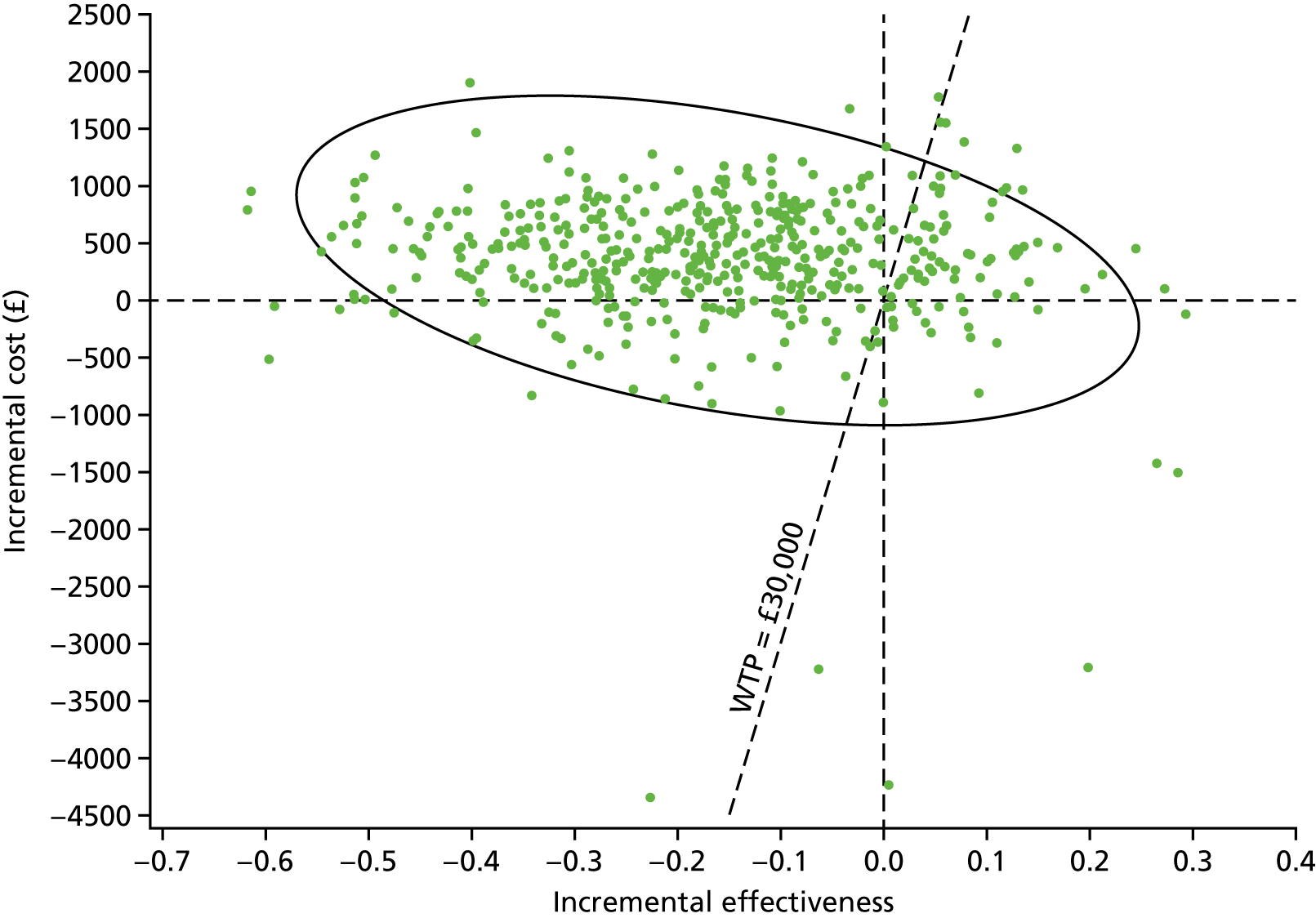
FIGURE 61.
Cost-effectiveness acceptability curve: removing general population age adjustment for utilities.

FIGURE 62.
Scatterplot of incremental cost-effectiveness: removing general population age adjustment for utilities.

FIGURE 63.
Cost-effectiveness acceptability curve: health-state costs based on trial-level rather than treatment-level data.

FIGURE 64.
Scatterplot of incremental cost-effectiveness: health-state costs based on trial-level rather than treatment-level data.
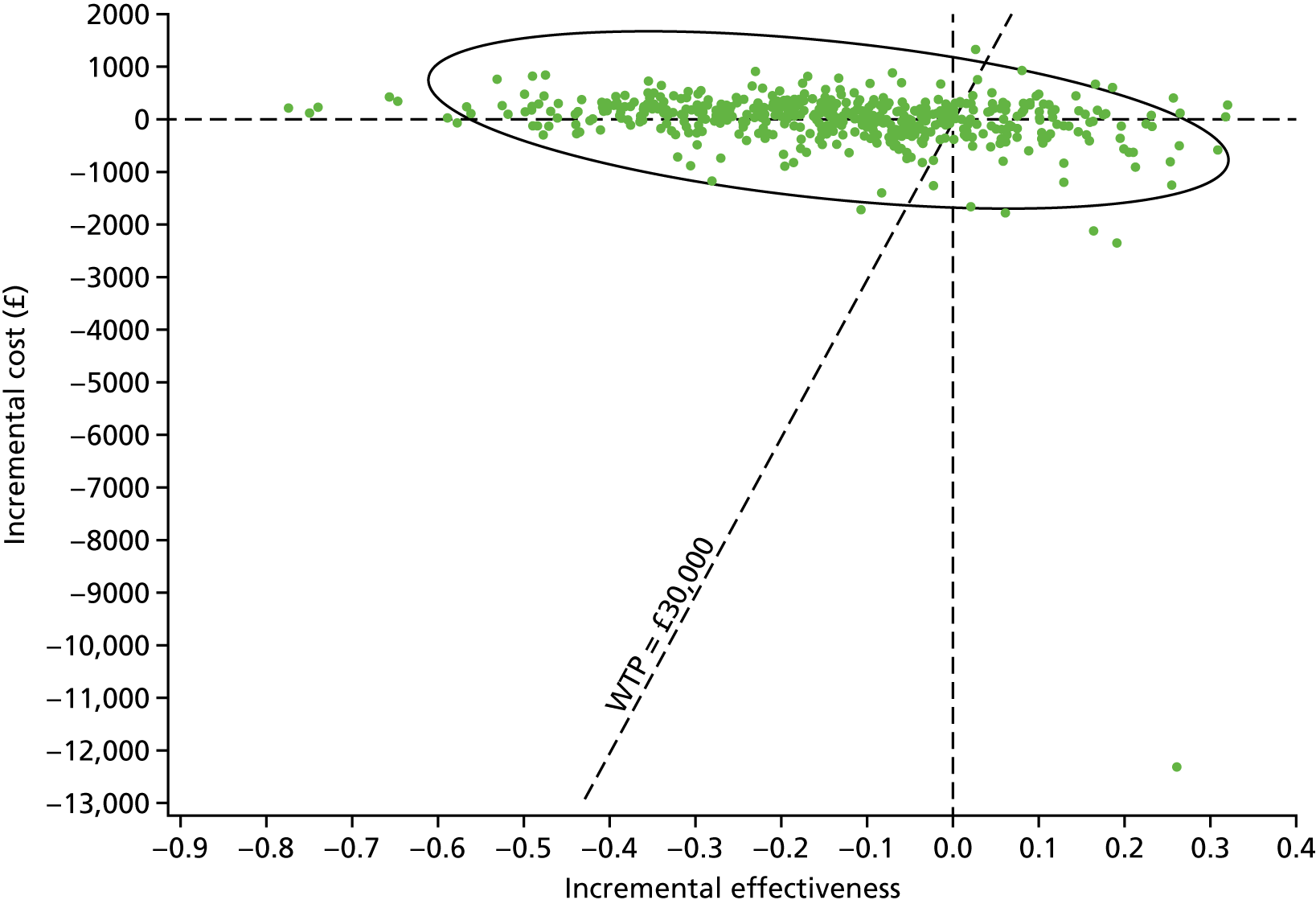
FIGURE 65.
Cost-effectiveness acceptability curve: health-state utility values based on trial-level rather than treatment-level data.

FIGURE 66.
Scatterplot of incremental cost-effectiveness: health-state utility values based on trial-level rather than treatment-level data.
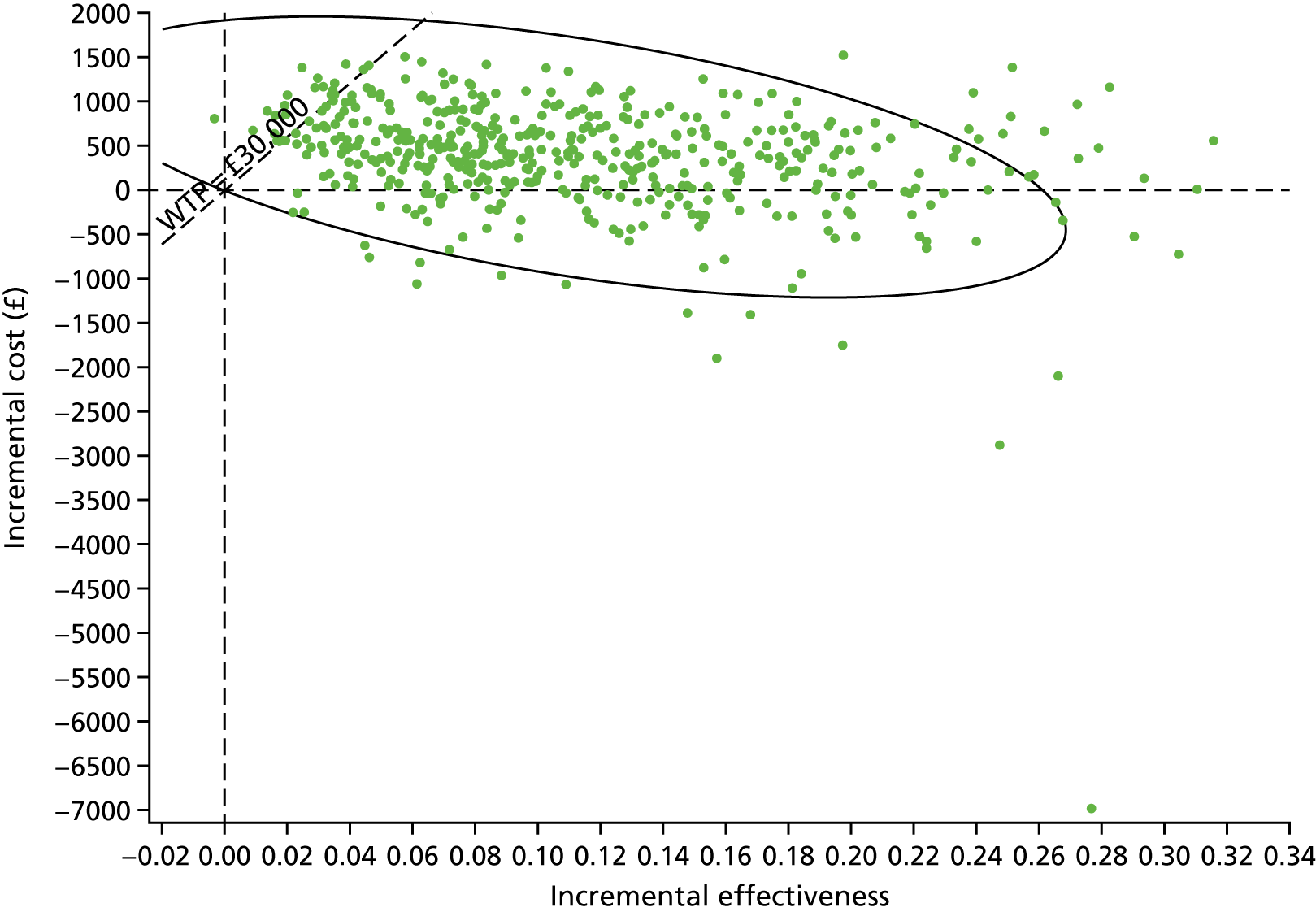
FIGURE 67.
Cost-effectiveness acceptability curve: baseline utility set equal for both uterine preservation and vaginal hysterectomy.
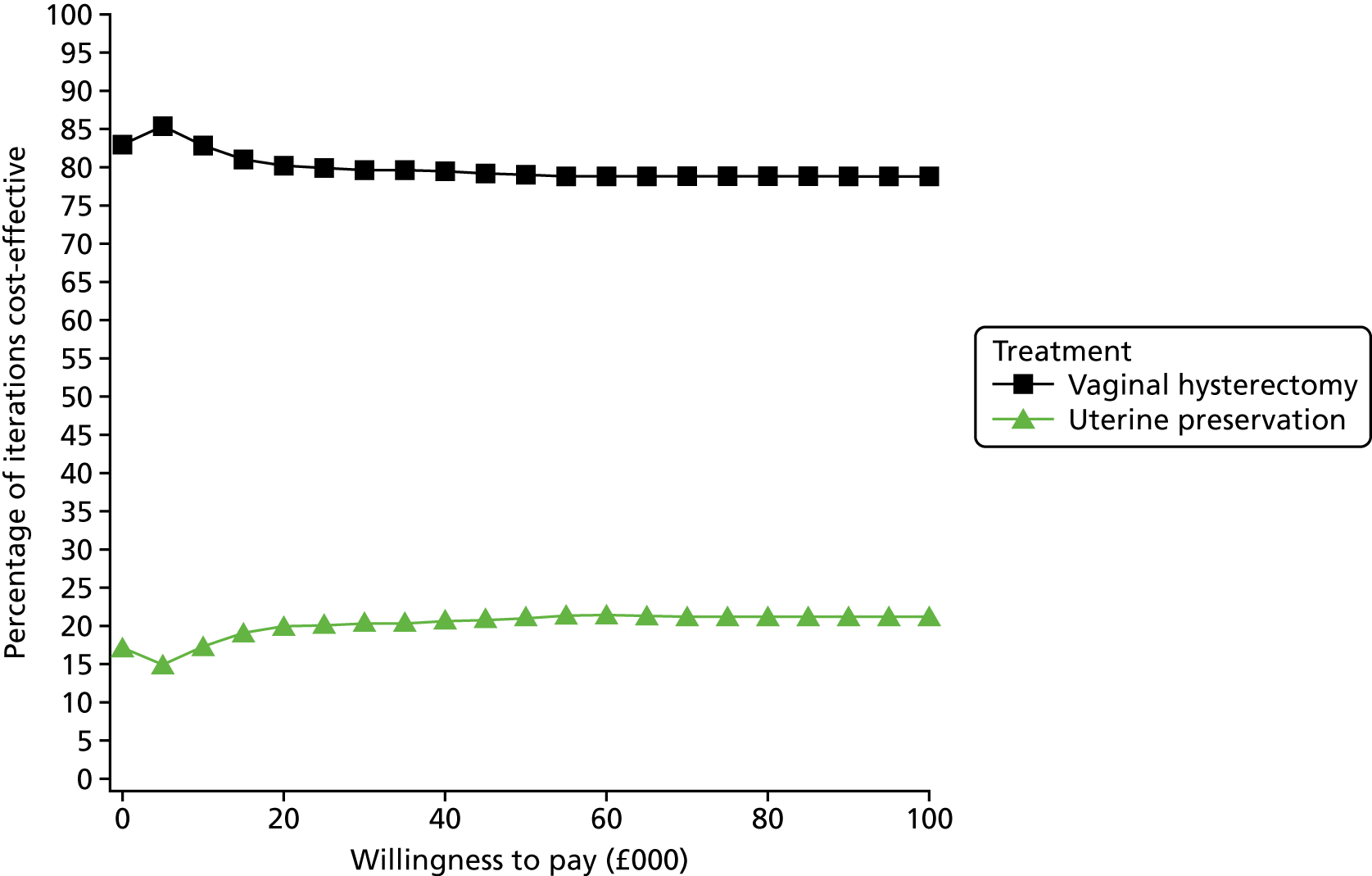
FIGURE 68.
Scatterplot of incremental cost-effectiveness: baseline utility set equal for both uterine preservation and vaginal hysterectomy.

Appendix 9 Evidence synthesis
Uterine trial
Meta-analyses: uterine preservation versus vaginal hysterectomy
This appendix contains the results of meta-analyses of eight RCTs comparing uterine preservation with vaginal hysterectomy, including new RCTs published since, or excluded from, the most recent Cochrane review22 and with the new data from the VUE Uterine trial. The eight pre-existing trials used a variety of surgical techniques and have therefore been presented separately as subgroups but combined in meta-analysis.
Meta-analyses have been undertaken for outcomes measured up to 12 months after surgery.
Prolapse symptoms: up to the 12-month follow-up
Within 12 months of surgery, and based on data from three pre-existing trials, the difference in the number of women with persistent prolapse symptoms was not statistically significant (RR 1.43, 95% CI 0.87 to 2.35), and the heterogeneity was high (I2 = 80%). The addition of the data from the VUE trial added substantially to the previous findings, increasing the proportion of participants by nearly 60%. In total, 103 out of 404 (25.5%) women had residual prolapse symptoms at up to 12 months after uterine preservation versus 91 out of 411 (22.1%) after hysterectomy (RR 1.16, 95% CI 0.91 to 1.48) (Figure 69). The difference was not statistically significant, the CI was wide, and the heterogeneity was high (I2 = 71%). The VUE trial has contributed a weighting of 75% to this meta-analysis.
FIGURE 69.
Number of women with prolapse symptoms: up to the 12-month follow-up. M–H, Mantel–Haenszel.
Any prolapse (objective failure): up to the 12-month follow-up
Within the first postoperative year, and based on data from three pre-existing trials, the difference in the risk of objective failure was not statistically significant (RR 1.40, 95% CI 0.56 to 3.53), and the heterogeneity was high (I2 = 61%). The addition of the data from the VUE trial added substantially to the previous result, increasing the proportion of participants by around 60%. In total, 83 out of 381 (21.8%) of women had residual prolapse after uterine preservation versus 84 out of 375 (22.4%) after hysterectomy (RR 0.97, 95% CI 0.76 to 1.25) (Figure 70), albeit with a CI compatible with 24% better after preservation to 25% worse. The VUE trial has contributed a weighting of 92% to this meta-analysis.
FIGURE 70.
Number of women with any prolapse (objective failure): up to the 12-month follow-up. M–H, Mantel–Haenszel.

Further prolapse surgery
FIGURE 71.
Women having further prolapse surgery: up to the 12-month follow-up. M–H, Mantel–Haenszel.

Any urinary incontinence: up to the 12-month follow up
FIGURE 72.
Number of women with any urinary incontinence: up to the 12-month follow-up. M–H, Mantel–Haenszel.

Continence surgery
FIGURE 73.
Number of women having continence surgery. M–H, Mantel–Haenszel.

Adverse events
Six pre-existing trials reported the number of women who had AEs after surgery. These included urinary tract infections, urinary retentions, buttock or sacral pain, stroke, pneumonia, ileus, atrial fibrillation and death. Although the AEs were more common in the uterine preservation group [24/320 (8%) vs. 14/320 (4%) in the hysterectomy group; RR 1.68, 95% CI 0.90 to 3.15] (Figure 74), this did not reach statistical significance. The addition of the data from the VUE trial added substantially to the previous result, increasing the proportion of participants by around 45%. In total, 38 out of 582 (6.5%) of women had AEs after uterine preservation versus 30 out of 589 (5.1%) after hysterectomy (RR 1.27, 95% CI 0.80 to 2.01) (Figure 74). Although the result was not statistically significant, the CI was compatible with 20% fewer women having AEs after uterine preservation to 101% more. The trial VUE trial has contributed a weighting of 53% to this meta-analysis.
FIGURE 74.
Number of women with AEs. M–H, Mantel–Haenszel.

Dyspareunia
Two pre-existing trials reported that around 4% of women reported dyspareunia, but there was no statistically significant difference between the groups (RR 0.81, 95% CI 0.24 to 2.73) (Figure 75). The addition of the data from the VUE trial (for sexually active women only) nearly doubled the number of participants. In total, 4 out of 208 (1.9%) of women had dyspareunia after uterine preservation versus 7 out of 202 (3.5%) after hysterectomy (RR 0.61, 95% CI 0.20 to 1.83) (Figure 75). The data were too sparse to be reliable. The VUE trial has contributed a weighting of 32% to this meta-analysis.
FIGURE 75.
Number of women with dyspareunia. M–H, Mantel–Haenszel.

Mesh exposure/erosion
Three small pre-existing trials found that around 10% of women reported mesh exposure, but there was no statistically significant difference between the groups (RR 0.89, 95% CI 0.32 to 2.42) (Figure 76). None of these trials reported whether or not the women required surgery for their mesh exposures. In the VUE trial, only one woman (out of 262 in the uterine preservation group, of whom 97 received non-absorbable mesh as part of their apical or concomitant prolapse surgery, or continence surgery) required surgery for mesh exposure, compared with none in the hysterectomy group (in 269 women of whom 16 received non-absorbable mesh). The mesh in this woman was used as part of the VUE suspension procedure (rather than for a concomitant procedure). There was no statistically significant difference between the groups (RR 1.03, 95% CI 0.40 to 2.65).
FIGURE 76.
Number of women with mesh exposure/erosion. M–H, Mantel–Haenszel.

Summary (the Uterine trial)
In summary, after the addition of the VUE trial data to the pre-existing trials, when comparing uterine suspension with hysterectomy, there was little difference between the groups in terms of prolapse symptoms or objective prolapse recurrence, but with wide CIs indicating some uncertainty. However, women were more likely to require further prolapse surgery after uterine preservation than after hysterectomy in the first 12 months after surgery. In the first 12 months after surgery, there was not enough evidence to reliably compare data for urinary incontinence or continence surgery, or SAEs, dyspareunia or mesh exposure, as few women experienced these complications.
Vault trial
Appendix 9 contains the results of meta-analyses of three pre-existing RCTs comparing abdominal vault with vaginal vault repair and with the new data from the VUE trial. There was one other new trial115 published since the last Cochrane review in 2016. 22 The four RCTs used different surgical techniques and have therefore been presented separately as subgroups, but combined in a meta-analysis.
Meta-analyses have been undertaken for outcomes measured up to 12 months after surgery.
Prolapse symptoms: up to the 12-month follow-up
The two pre-existing trials were too small to provide conclusive evidence: there was no statistically significant difference between the abdominal and vaginal approaches (RR 0.49, 95% CI 0.15 to 1.57) (Figure 77). The addition of the data from the VUE trial nearly doubled the number of participants. In total, fewer women (35/188, 18.6%) had residual prolapse symptoms after an abdominal vault suspension than after a vaginal vault suspension (49/185, 26.5%; RR 0.70, 95% CI 0.49 to 0.99) (Figure 77). This statistically significant result favours the abdominal vault approach. The VUE trial contributed a weighting of 84% to this meta-analysis.
FIGURE 77.
Number of women with prolapse symptoms: up to the 12-month follow-up. M–H, Mantel–Haenszel.

Any prolapse (objective failure): up to the 12-month follow-up
On combining data from three pre-existing trials, nearly twice as many women had residual measurable prolapse after the vaginal approach (32/137, 23%, after the abdominal route vs. 55/129, 43% after the vaginal repair; RR 0.55, 95% CI 0.38 to 0.80) (Figure 78). The addition of the data from the VUE trial increased the proportion of participants by around 40%. In total, fewer women (60/223, 26.9%) had residual objective prolapse protrusion in the 12 months after an abdominal vault suspension than after a vaginal vault suspension procedure (93/210, 44.3%; RR 0.61, 95% CI 0.47 to 0.80) (Figure 78). This statistically significant result favours the abdominal vault approach. The VUE trial contributed a weighting of 41% to this meta-analysis.
FIGURE 78.
Number of women with any prolapse (objective failure): up to the 12-month follow-up. M–H, Mantel–Haenszel.
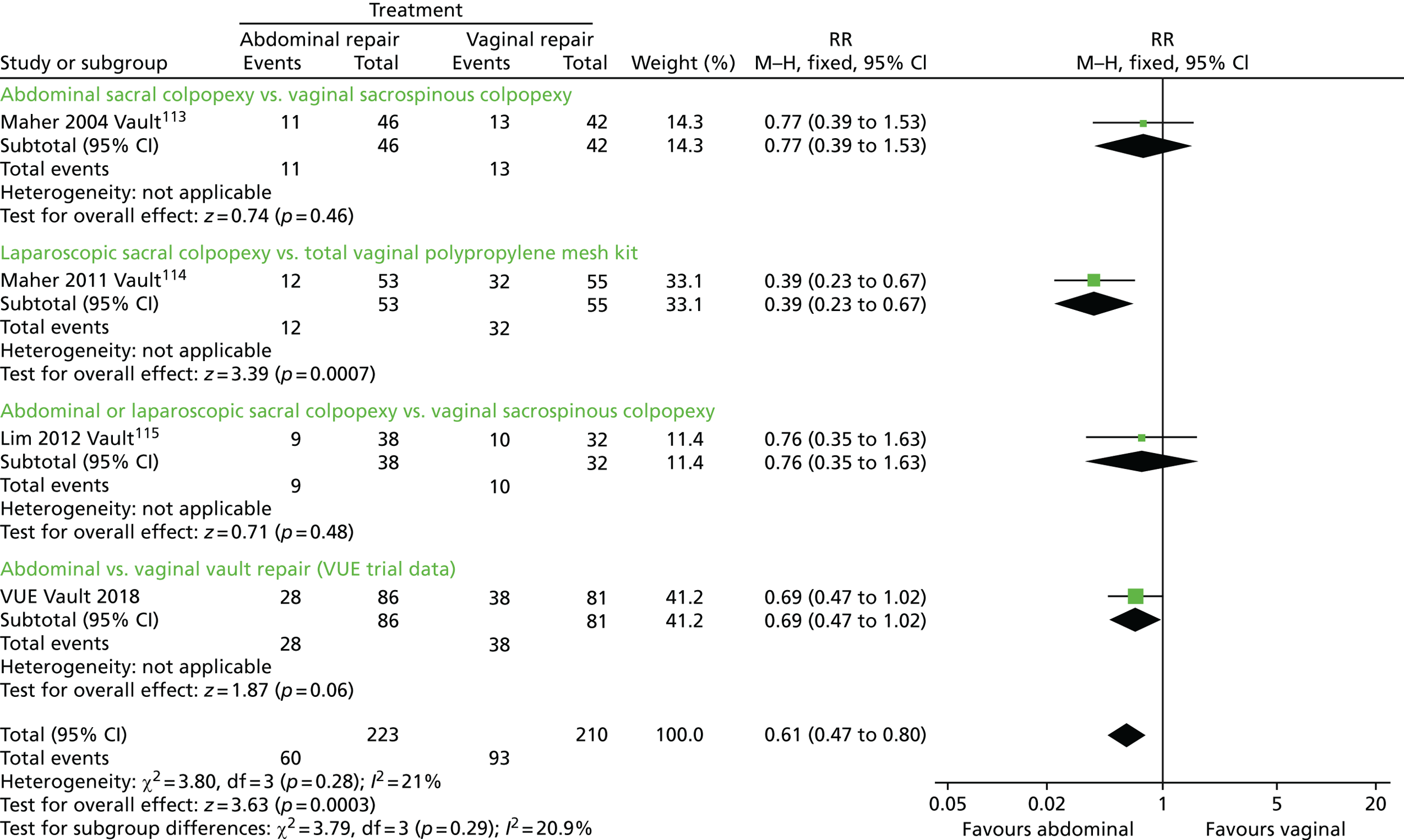
Further prolapse surgery: up to the 12-month follow-up
Too few women required further prolapse surgery to provide conclusive evidence from the two small pre-existing trials (RR 0.23, 95% CI 0.04 to 1.31) (Figure 79). The addition of the data from the VUE trial doubled the number of participants, but in total only 18 women required further prolapse surgery in the first 12 months. Although fewer women (6/201, 3%) had further surgery after an abdominal vault suspension than after a vaginal vault suspension (12/198, 6%; RR 0.51, 95% CI 0.20 to 1.29), this did not reach statistical significance (Figure 79). This result was compatible with 80% favouring the abdominal vault approach versus 29% being worse off. The VUE trial contributed a weighting of 48% to this meta-analysis.
FIGURE 79.
Number of women having further prolapse surgery: up to the 12-month follow-up. M–H, Mantel–Haenszel.

Any urinary incontinence: up to the 12-month follow-up
In two pre-existing trials, fewer women reported urinary incontinence after abdominal (12/89, 13%) than after vaginal surgery ((24/94, 26%; RR 0.53, 95% CI 0.28 to 0.99). The addition of the data from the VUE trial almost doubled the number of participants. In total, the proportion of women with urinary incontinence after an abdominal vault suspension (44/174, 25.3%) versus after a vaginal vault suspension (49/180, 27.2%) was no longer statistically different (RR 0.92, 95% CI 0.65 to 1.31) (Figure 80). The VUE trial contributed a weighting of 52% to this meta-analysis.
FIGURE 80.
Number of women with any urinary incontinence: up to the 12-month follow-up. M–H, Mantel–Haenszel.

Continence surgery
In the two pre-existing trials, there was insufficient evidence to reliably identify a difference in the number of women requiring subsequent continence surgery between the two groups (RR 0.49, 95% CI 0.13 to 1.91) (Figure 81). The addition of the data from the VUE trial doubled the number of participants, but in total only 13 women required continence surgery in the first 12 months. Few women (6/201, 3%) had continence surgery after an abdominal or after a vaginal vault suspension (7/198, 3.5%; RR 0.84, 95% CI 0.29 to 2.46), and the difference did not reach statistical significance (Figure 81). The data are still too few to be reliable. The VUE trial contributed a weighting of 14% to this meta-analysis.
FIGURE 81.
Number of women having continence surgery. M–H, Mantel–Haenszel.
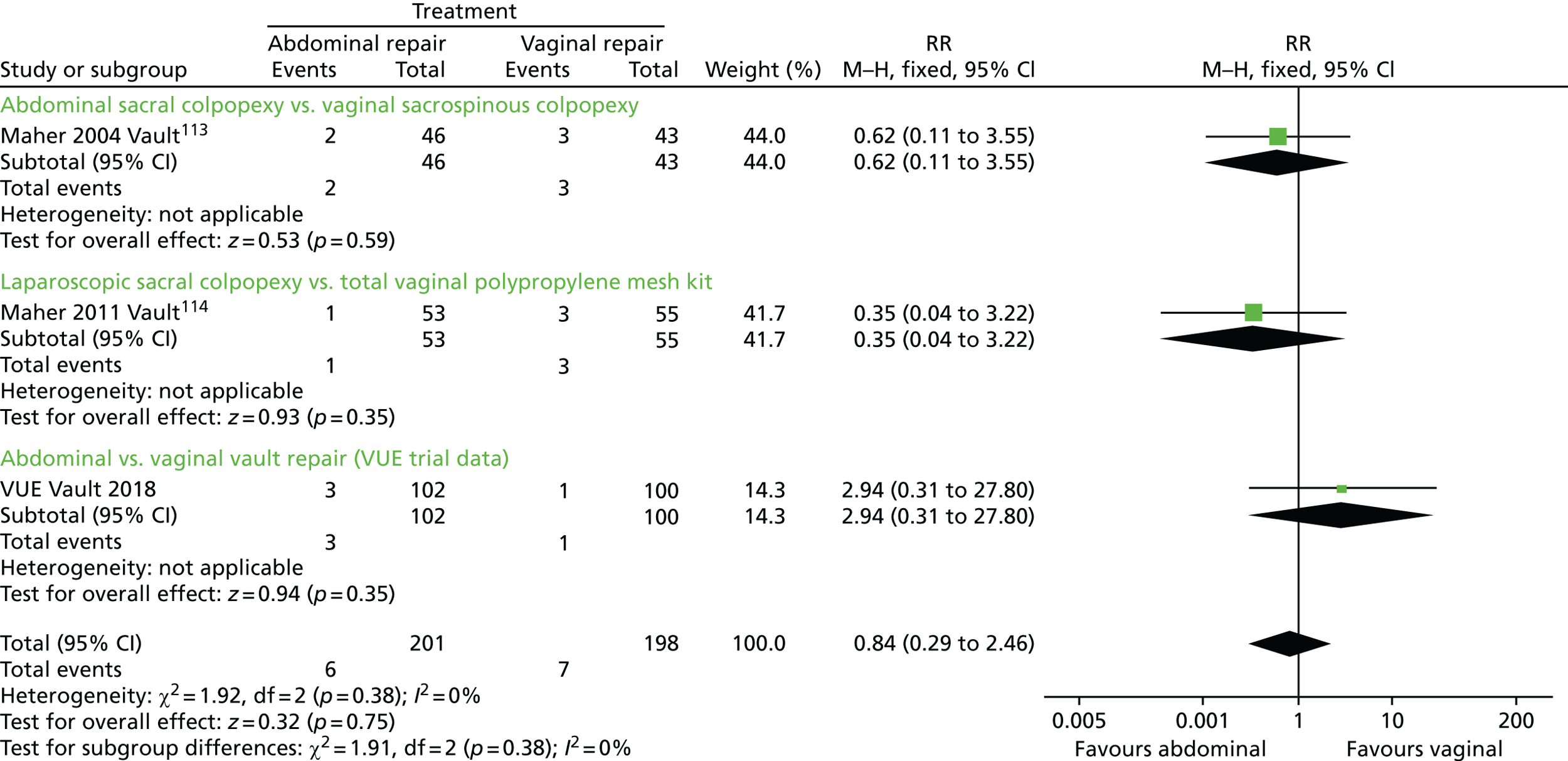
Adverse events
Only two out of the three pre-existing trials reported AEs. The AEs included cystotomy, haematoma, wound infection, voiding dysfunction (existing preoperatively), mesh infection requiring removal and incisional hernia. More women had AEs in the abdominal groups (18/84, 21%) than in the vaginal groups (7/75, 9%; RR 2.25, 95% CI 1 to 5.04) (Figure 82). The addition of the data from the VUE trial added > 50% extra participants. Although more women (24/186, 12.9%) had further surgery after an abdominal vault suspension than after a vaginal vault suspension (13/175, 7.4%; RR 1.68, 95% CI 0.89 to 3.18), this did not reach statistical significance (Figure 82). This result was compatible with 11% having fewer AEs with the abdominal approach versus 218% having more. The VUE trial contributed a weighting of 45% to this meta-analysis.
FIGURE 82.
Number of women experiencing AEs. M–H, Mantel–Haenszel.
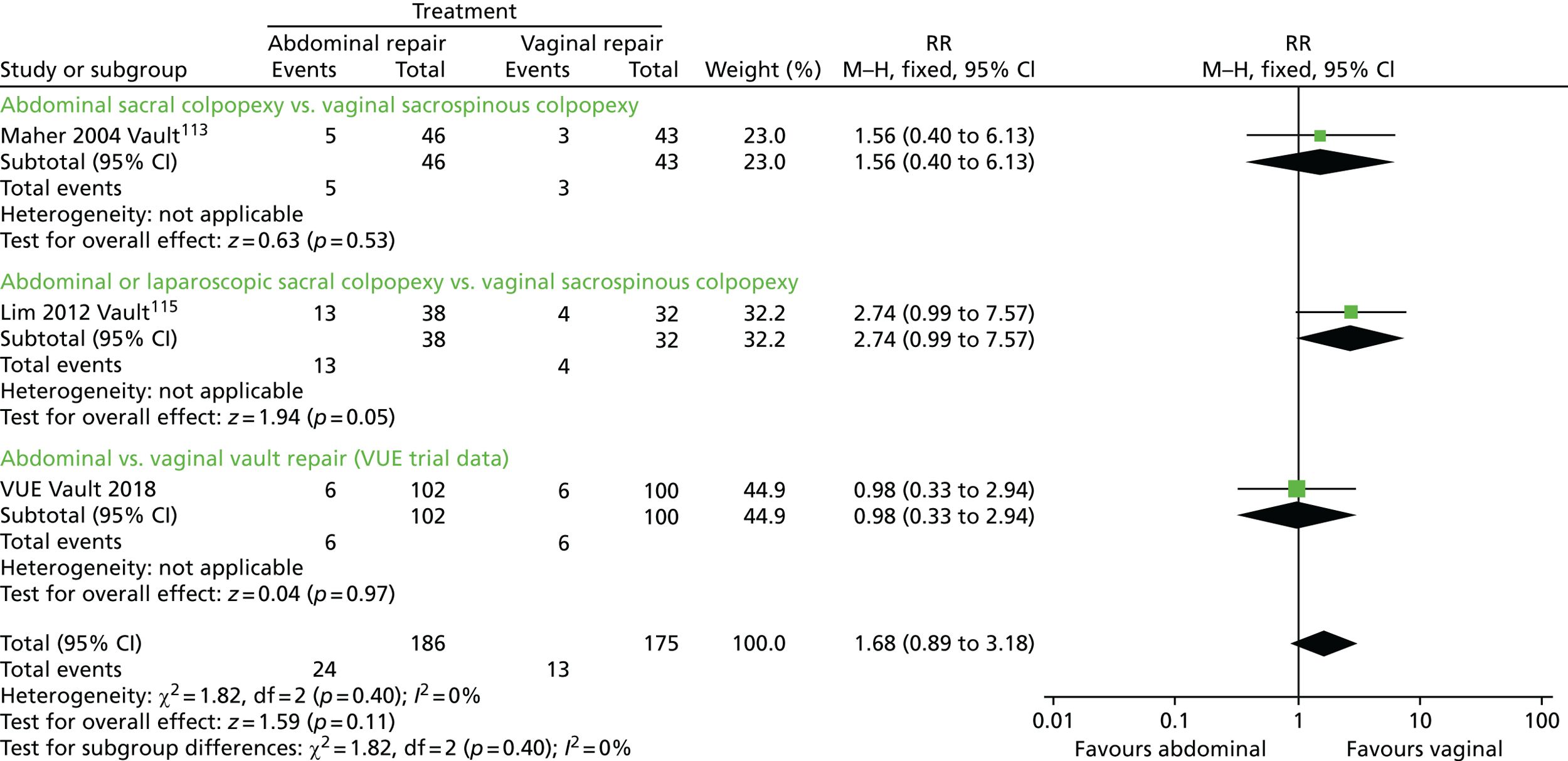
Dyspareunia
Only one of the three pre-existing trials reported dyspareunia. The trial was too small and the number of sexually active women with dyspareunia too few to provide conclusive evidence (RR 0.77, 95% CI 0.32 to 1.83) (Figure 83). The addition of the data from the VUE trial (also restricted to sexually active women) more than doubled the number of participants. In total, 7 out of 50 (14%) of the women had dyspareunia after abdominal repair versus 7 out of 42 (17%) after vaginal repair (RR 0.88, 95% CI 0.38 to 2.04) (Figure 83). The data were too sparse to be reliable. The VUE trial contributed a weighting of 7% to this meta-analysis.
FIGURE 83.
Number of women with dyspareunia. M–H, Mantel–Haenszel.

Mesh exposure/erosion
Two of the pre-existing trials reported mesh complications. 114,115 Although there were more women with mesh exposure in the vaginal group (four in the abdominal group vs. nine in the vaginal group), this did not reach statistical significance (RR 0.42, 95% CI 0.13 to 1.36) (data not shown). Only one of these trials114 reported how many women required surgery for their mesh exposure. In the VUE trial, none of the abdominal group and only one woman in the vaginal group had surgery for her mesh exposure, and that was for a concomitant mesh used for a continence procedure (Figure 84). There was no statistically significant difference between the groups (RR 0.24, 95% CI 0.04 to 1.36) (Figure 84).
FIGURE 84.
Number of women requiring surgery for mesh exposure/erosion. M–H, Mantel–Haenszel.
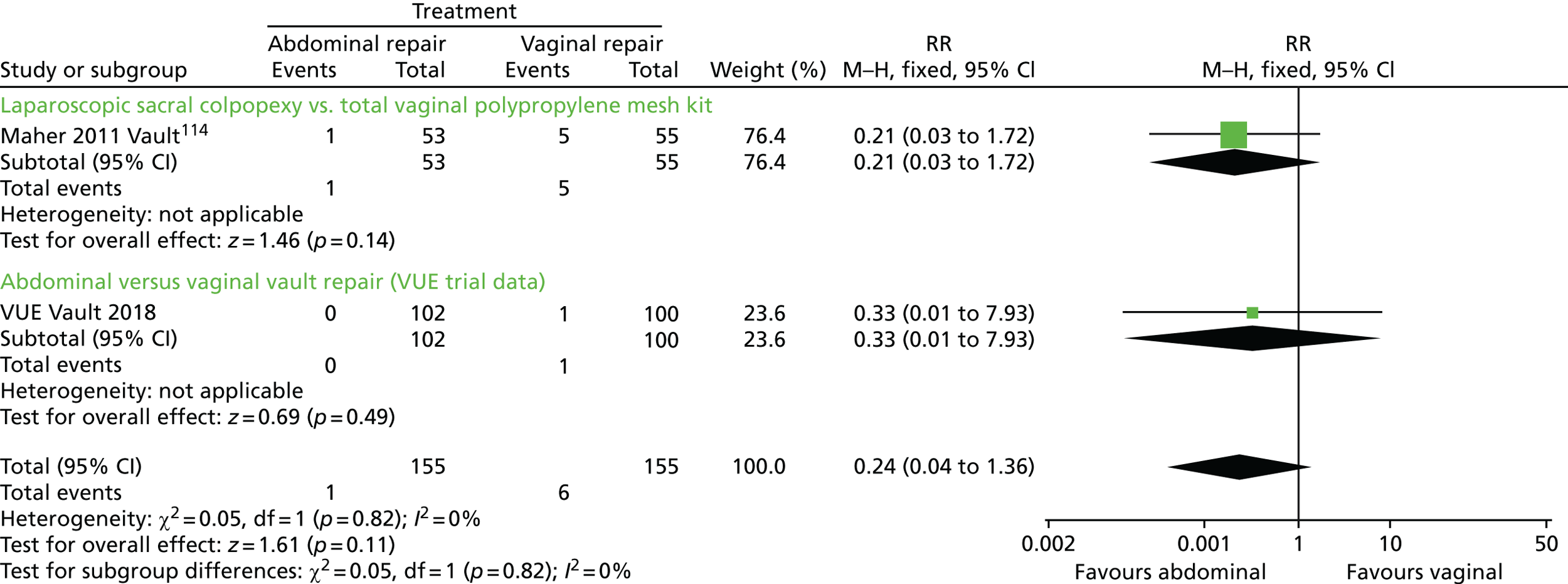
Summary (the Vault trial)
In summary, after the addition of the VUE trial data to the pre-existing trials, when comparing abdominal versus vaginal vault suspension, significantly fewer women had prolapse symptoms or objective prolapse recurrence. However, in the first 12 months there was not enough evidence to reliably compare the consequences for further prolapse surgery, urinary incontinence or continence surgery, or SAEs, dyspareunia or mesh exposure, as few women experienced these complications.
Appendix 10 VUE-Qual
Background
Recruitment to the VUE trial proved more difficult than anticipated, mainly because of patient preference for a particular treatment. A qualitative evaluation (know as VUE-Qual) was proposed to provide a better understanding of the issues impacting on the decision-making process.
Methods
Recruitment
Eligible patients were given an information sheet and consent form for VUE-Qual to allow the recruitment consultation to be audio-recorded. The patients’ decision to take part (or not) in VUE-Qual did not have an impact on their invitation to participate in the VUE trial. If the consent form had not been returned to the trial office prior to the recruitment consultation, the recruiter asked potential participants if they were happy to give verbal consent with the understanding that the consent form must be returned as soon as possible. Audio files of the recruitment consultations could not be transferred to the qualitative researchers until written consent had been received. To ensure confidentiality the files were anonymised, only identifiable by the VUE trial number, transferred and stored securely.
Data analysis
The information exchange between the recruiter and potential participant was systematically evaluated with the a priori aim of improving the informed consent process. Summary notes were made for each recording. However, owing to time restrictions transcription was not possible. A modified framework approach was used to thematically analyse the recordings to explore themes occurring throughout. 116
Results
Participants
Eight potential VUE trial participants were approached regarding participation in VUE-Qual, seven of whom were eligible for the Uterine trial and one who was eligible for the Vault trial. Of those participants approached, six provided consent and two patients refused. The one patient that was eligible for the Vault trial refused to participate in VUE-Qual.
During the recruitment consultations it became apparent that one participant was identified as having a clear preference for one of the surgical interventions, thereby making her ineligible for the VUE trial. The five other potential participants agreed to take part in the VUE trial following the recruitment consultation (giving a VUE acceptance rate of 83%).
Recruitment consultations
There were many similarities throughout the recruitment consultations in terms of how they were structured: initial introductions, establishing understanding of the VUE trial and trial processes (including completion of trial paperwork) and postoperative care. The recruiter did not spend much time discussing surgical procedures, as this would have been discussed previously in the gynaecology clinic.
Each consultation was recruiter led, although the recruiter gave participants time to talk and listened to them intently. A script was not used; however, the recruiter did have a copy of the clinical assessments of their prolapse symptoms. The recruiter established that participants had time for the consultation at the beginning of the call, with the average duration being < 22 minutes.
Randomisation
Prior to the recruitment consultation there seemed to be some uncertainty over the randomisation process. It is not clear what information potential VUE trial participants were given in the gynaecology clinic, specifically in relation to the random allocation process. However, when discussing with participants the recruiter consistently used the term ‘equally happy’ to describe how they should feel regarding the random allocation of surgical treatment. On only one occasion did the recruiter use the term ‘equipoise’ (equal uncertainty) and proceeded to explain the term. The randomisation aspect of the VUE trial was discussed with all participants. The exact method for allocation, use of a computer program, to make the selection was mentioned only twice.
The randomisation aspect of the VUE trial appeared to cause some apprehension:
That was my only concern.
Patient preferences
The participant who was identified as having a clear preference for a hysterectomy (therefore ineligible for the VUE trial) had discussed her preference with her family and they had agreed that her preferred treatment was the best option for her. The recruiter did not challenge or explore this expressed preference.
One further participant was asked if she had a treatment preference after stating the procedure she wanted. Some statements made, and issues raised, by the participants may have indicated a treatment preference but were not pursued:
If I could I would prefer to get everything, cysts can change, for safety, just want to get rid of it, will see what they want to do.
Do you mean the operation I’m away to have?
Two participants highlighted that they would want their doctor to do what they thought would be best for them.
Clinical follow-up appointment
The trial patient information leaflet clearly stated that there were no additional benefits for participating in the trial. The 12-month follow-up appointment was discussed with all participants and mentioned to half that this was not a standard NHS appointment (i.e. research appointment):
Women like the 12 months clinic appointment, not usually followed up on NHS, physically see the extent of any symptoms that are still remaining, peace of mind, opportunity to ask questions, pros and cons.
Participation in the VUE trial
All six participants expressed a willingness to participate in the VUE trial, although provided no reason for this. There was no indication, as relevant literature would suggest, that willingness to participate was owing to altruism, the desire to help others. One participant did acknowledge the benefit of the trial for women in the future, but did not pursue this as a reason for consenting to participate in the VUE trial:
Trying to find out what would be best in the long term, not for self necessarily.
Although the women were eligible for participation in the VUE trial, the recruiter clearly recognised the uncertainty of one of the participants and was very quick to put her at ease:
Don’t have to have surgery just because on study, if you’re unsure can have another discussion with Dr B.
This highlights the importance of the recruiter in the decision-making process. In addition, throughout the consultation the recruiter was able to build up a rapport with participants, making them feel more comfortable about raising their concerns. This allowed the recruiter to offer reassurance and advice to help them make their decision about whether or not to participate in the trial. A consistent example of how the recruiter attempted to reassure the participants about trial participation was how she emphasised that their personal needs would always come before the needs of the trial. This may have reassured participants that if in the operating theatre the randomised allocation was no longer deemed necessary, it would not be performed just because they were taking part in a trial.
Discussion
Qualitative methods were used to explore audio-recorded recruitment consultations within the VUE trial between potential participants and the recruiter.
Importantly, the six participants that participated in VUE-Qual were all eligible for only the Uterine trial. The trial population therefore did not give a true representation of the potential participants suitable for both RCTs within the VUE trial. It is possible that the decision-making process may be different for potential Vault trial participants, as they would have already undergone surgical treatment to remove the uterus.
In addition, the lower than expected acceptance rate for the trial was not reflected in the VUE-Qual population, as there was an 83% acceptance rate in VUE-Qual versus a 33% overall rate when VUQ-Qual was conducted in 2014. Furthermore, only one of the six participants declined randomisation because of a preference for one of the surgical procedures. However, the reasons behind this patient preference was not explored further by the recruiter.
VUE-Qual did highlight key findings of importance during the recruitment process, most importantly the overarching theme relating to the recruiter relationship and rapport-building with potential participants. This may be because the recruiter had previous experience of conducting recruitment consultations for RCTs and provided information beyond the standard requirements of the recruitment process.
There was some confusion over the randomisation process, with one participant believing the recruiter wanted to talk about the operation she was going to have. This would indicate that the participant had already agreed to a surgical treatment and had not understood the randomisation element of the trial. This has been evidenced in several other studies across a range of clinical conditions and populations in which participants consent to randomisation even though it becomes apparent during follow-up that they have not understood the process. 117 It has been established that this willingness to participate may be a way to avoid admitting to a lack of understanding. 118
A considerable amount of time during the recruitment consultation was spent discussing how to complete the paperwork. The recruiter took the time to address participants’ concerns and misunderstandings about the trial paperwork, specifically the consent form and baseline questionnaire. There was also mention of the follow-up questionnaires at 6 and 12 months for participants to self-evaluate their progress. This follow-up aspect is important, as it can help with retention of participants so they do not just feel like a number in a study. 119 Knowing what is expected of them should they participate in the trial may help participants’ decision-making process, as they will not be asked to do anything they have not already been informed of.
The importance of the relationship between recruiter and potential VUE trial participants became particularly evident when the participants were describing their prolapse symptoms. The recruiter demonstrated empathy, reassurance, advice and support when listening to their narratives. The participants were not asked for such information, but the recruiter allowed them time to express their experiences. This could have made them feel at ease and also impact on their decision to participate in the VUE trial. It has been reported previously that the relationship potential participants have with the recruiter is a contributing factor to consent to trial participation. 120
The recruiter was very supportive of the participants’ decisions to take time to consider seeking surgical treatment and did not pursue them to participate. This may have allowed the recruiter to gain the trust of the participants, which is known to be beneficial for recruitment. 121
For less experienced recruiters it may be beneficial to have a checklist for undertaking recruitment consultations. This may take away the personal aspect of the recruitment consultation, but this would be dependent on the personality of the recruiter. Having a competent and confident recruiter can reduce anxiety among potential participants (which may lead them to decline participation). 122
Strengths
The key strength of this study was the ability to examine in depth how trial information was presented to potential VUE trial participants and to understand better how to improve aspects of the recruitment process. The use of a digital audio-recorder allowed an accurate representation of the recruitment consultations and is less intrusive than having an observer present during the recruitment consultation.
Another strength was that there were no additional requirements for the potential VUE trial participants if they chose to participate in the VUE-Qual study, that is, it involved audio-recording a consultation they would be having irrespective of the qualitative investigation.
Limitations
The main limitation of this study was the low number of potential participants available to be approached regarding VUE-Qual; therefore, the themes identified may not represent the group of women eligible to participate in the VUE trial as a whole. The VUE trial was a large multicentre surgical RCT; however, VUE-Qual was conducted within only one trial centre and differences may be found in different centres, based on different recruiters and/or regional differences.
During a recruitment consultation the participant may disclose sensitive data, which may make the recruiter reluctant to record the recruitment consultations. It may also give the feeling that the recording is being used for more than its intended purpose, for example to monitor the recruiter. The recruiter may change his or her behaviour during the recruitment consultations that are being audio-recorded, which again may not give accurate representation of recruitment consultations (which would have been negated if more recordings were undertaken).
Conclusion
Throughout the recruitment consultation the recruiter was able to build a personal relationship with each of the participants. It was the forming of this relationship that allowed the recruiter to discuss the context of the trial. The relationship and rapport the recruiter had with the participants underpins the whole information exchange about participation in the VUE trial.
However, the study was undertaken within one trial centre, analysing a small sample of recruitment consultations undertaken by one recruiter. To gain a better overall representation it would have been useful to continue audio-recording the recruitment consultations with more recruiters/centres. If the VUE-Qual study was extended, this would have allowed for comparisons regionally and between recruiters to determine if the same themes were consistent throughout the VUE trial as a whole and, in particular, whether or not the rapport-building is a finding across other sites.
List of abbreviations
- AE
- adverse event
- AIC
- Akaike information criterion
- BMI
- body mass index
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidating Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GBP
- Great British pounds
- GLM
- generalised linear model
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICI
- International Consultation on Incontinence
- ICIQ
- International Consultation on Incontinence Questionnaire
- ICIQ-FLUTS
- ICIQ – female lower urinary tract symptoms
- ICIQ-UI SF
- ICIQ – urinary incontinence Short Form questionnaire
- ICIQ-VS
- ICIQ – vaginal symptoms
- IPG
- Interventional Procedures Guidance
- ISD
- Information Services Division
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OPCS
- Office of Population, Censuses and Surveys
- OR
- odds ratio
- PFMT
- pelvic floor muscle training
- POP
- pelvic organ prolapse
- POP-Q
- Pelvic Organ Prolapse Quantification system
- POP-SS
- Pelvic Organ Prolapse Symptom Score
- PROSPECT
- PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials
- PSSRU
- Personal and Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
- VUE
- Vault or Uterine prolapse surgery Evaluation
- WTP
- willingness to pay
