Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/33/12. The contractual start date was in July 2014. The draft report began editorial review in March 2018 and was accepted for publication in August 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Christopher C Butler reports that Afinion C-reactive protein devices and associated training given to participating general practices were provided by Alere Inc. (now Abbott Diagnostics, IL, USA) at no cost to the study. He has received fees for participating in a Roche Molecular Systems Advisory Board meeting on 4 and 5 February 2016 about point-of-care testing; held an investigator-initiated grant from Roche Molecular Diagnostics (Roche Molecular Systems Inc., CA, USA) to evaluate the analytic performance of the cobas® Liat® point-of-care device for detecting influenza using samples from a separately funded study; and is part of a publicly funded research consortia that includes industrial partners. He was a member of the Medical Research Council–National Institute for Health Research (MRC–NIHR) Efficacy and Mechanism Evaluation Board (2012–16). He has been a NIHR Senior Investigator since 2016. Kerenza Hood was a member of the Health Technology Assessment (HTA) programme Funding Boards Policy Group (formerly Clinical Studies Group) (2016 to present), the HTA General Board (2016 to present) and the NIHR Clinical Trials Unit Standing Advisory Committee (2014 to 2019). Gurudutt Naik reports non-financial support from Alere Inc. Carl Llor reports grants from the European Commission (Seventh Framework Programme and Horizon 2020), the Catalan Society of Family Medicine, Abbott Diagnostics and Instituto de Salud Carlos III (Spanish Ministry of Health) outside the submitted work. Rhiannon Phillips reports that her current post is a fellowship funded by Health and Care Research Wales as part of the Primary and Emergency Care Research Centre Wales research centre grant.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Francis et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Point-of-care tests (POCTs) for acute infections are being promoted by government, by industry and in clinical guidelines to reduce inappropriate antibiotic prescribing, help contain antimicrobial resistance and improve patient-reported outcomes. 1–4 Whereas POCTs are frequently subjected to evaluations of analytic performance, diagnostic strategies are often introduced into routine care before their clinical effectiveness has been determined in rigorous clinical trials and without an understanding of their cost-effectiveness using relevant health and service delivery outcomes.
Antibiotic use
Better targeting of antibiotics in acute exacerbations of chronic obstructive pulmonary disease (AECOPDs) represents a major opportunity for antimicrobial stewardship and improved patient care. Over 80% of all antibiotics are prescribed in the community,5 with high prescribing of broad-spectrum antibiotics a particular concern. AECOPD accounts for over 2 million antibiotic prescriptions each year in the UK. 6 Half of patients with chronic obstructive pulmonary disease (COPD) experience one or more exacerbations needing medical treatment each year. 7,8 Over 70% of patients presenting with AECOPD in primary care are prescribed an antibiotic, accounting for 4.6% of all primary care antibiotic prescriptions every year. 9 COPD patients are an important group who are at risk of significant mortality, morbidity and hospitalisation and, as such, are more likely to be prescribed broad-spectrum antibiotics. 10 However, many AECOPD are triggered by non-bacterial causes, such as viral infections and environmental factors including common pollutants and weather. It has been estimated that approximately 70% of AECOPDs are triggered by an infection and 30% are caused by environmental factors. Of the 70% that are triggered by an infection, potential pathogenic bacteria are isolated in 20–58% of clinical samples, while pathogenic respiratory viruses can be detected in approximately 50%. 11–13 Among COPD patients hospitalised as a result of an exacerbation, the causal infectious agents were identified as 29.7% bacterial, 23.4% viral and 25% viral/bacterial. 14
The overuse of antibiotics drives antimicrobial resistance15 and is facilitated by the unnecessary consumption of antibiotics for COPD. Antimicrobial treatment in patients with COPD decreases the infecting load but does not usually entirely eradicate organisms in the airways, increasing the risk of resistant bacteria in COPD patients. 16 Infections of antibiotic-resistant Streptococcus pneumoniae in patients with COPD are associated with antibiotic exposure. 17,18 A meta-analysis5 of seven studies of respiratory tract bacteria that comprised 2605 participants showed that the pooled odds ratio for resistance was 2.4 [95% confidence interval (CI) 1.4 to 3.9] and 2.4 (95% CI 1.3 to 4.5) within 2 and 12 months of antibiotic treatment, respectively. The unnecessary use of antibiotics for AECOPD not only contributes to the increasingly pressing public health threat of antibiotic resistance, but also poses a risk to the individual, as it may increase the risk of subsequent antibiotic-resistant exacerbations and hasten disease progression. The indiscriminate use of antibiotics in patients with COPD is particularly high risk because the respiratory tracts of those affected are frequently colonised with potential pathogens. 19 Using unnecessary antibiotics also increases the risk of patient side effects, wastes money and undermines self-care. 20
Current antibiotic prescribing recommendations for general practitioners (GPs) are generally based on symptoms alone. 21 In 1987, Anthonisen et al. 22 defined three types of exacerbation based on the presence of one, two or three of the following features: increased dyspnoea, increased sputum production and increased sputum purulence. Exacerbations with all three of these features were defined as type 1 exacerbations, those with two of these features were defined as type 2 exacerbations, and those with only one feature, in combination with an upper respiratory tract infection within the previous 5 days, a fever without another cause, increased wheezing, increased cough or a > 20% increase in respiratory rate or heart rate compared with baseline, were called type 3 exacerbations. Anthonisen et al. 22 demonstrated an association between these exacerbation types and benefit from antibiotics, and these features became widely adopted as a guide to when to prescribe antibiotics. However, the features are subjective and are insufficiently diagnostically accurate to enable clinicians to predict patients who can safely be managed without antibiotics. A Cochrane systematic review23 of the use of antibiotics for managing exacerbations of COPD comprised 16 trials (n = 2068 participants) and reported that there was insufficient evidence of effectiveness to guide antibiotic prescribing decisions in primary care. Our placebo controlled trial of antibiotics for AECOPD in primary care indicated that benefit from antibiotics for AECOPD was limited largely to those who had a raised C-reactive protein (CRP) level (CRP level refers to CRP concentration measured in serum). 24 More effective strategies are required to ensure that antibiotics are used most effectively for managing AECOPD, so that antibiotic treatment can be targeted at those most likely to benefit and effective non-antibiotic treatment can be targeted at those who are unlikely to benefit from antibiotics.
C-reactive protein
C-reactive protein is an acute-phase protein found in blood. The serum level of CRP increases rapidly during infections, particularly in severe bacterial infections. A prospective evaluation of 36 biomarkers25 found that CRP level was the most selective biomarker to confirm an AECOPD and, in combination with Anthonisen criteria, produced an area under the curve of 0.88 (95% CI 0.82 to 0.93), indicating that the biomarker had good diagnostic accuracy. High levels of serum CRP is correlated with sputum purulence and raised serum leucocyte counts, and the serum level of CRP is higher in the presence of bacterial infection. 24,26 CRP levels rise in patients with AECOPD and is correlated with the number of Anthonisen criteria present and the degree of airflow limitation in hospitalised patients. 27,28 As CRP levels are more likely to be raised when there is bacterial infection, the treatment effect of antibiotics increases with higher values of CRP. 29
In our previous study30 examining predictors of treatment with antibiotics and systemic corticosteroids for acute exacerbations of asthma and COPD, we found that > 50% of COPD patients experiencing an AECOPD had a CRP level of < 8 mg/l, and that chest examination findings, a raised CRP value and decreased oxygen saturation were stronger predictors of prescribing antibiotics and systemic corticosteroids than respiratory symptoms. We found marginal benefit from antibiotic treatment in patients with AECOPD who had only one or two Anthonisen criteria, and using Anthonisen criteria to predict benefit from antibiotic treatment produced an area under the curve of 0.708 (95% CI 0.616 to 0.801). Adding CRP increased this to an area under the curve of 0.842 (95% CI 0.760 to 0.924). 31 Based on these data, we anticipated that using a CRP test alongside clinical assessment might make it possible to safely reduce the antibiotic prescription rate for this condition to around 45%.
C-reactive protein POCTs are widely available and are already commonly used to help guide antibiotic prescribing decisions, including for lower respiratory tract infections (LRTIs) and AECOPD in primary care in a number of European countries (mostly Scandinavian). 32 In two trials33,34 evaluating the use of a CRP POCT to help target antibiotic treatment for LRTIs in primary care, antibiotics were prescribed to 53% and 48% of patients in each trial’s usual-care group, respectively, and to 31% and 33% of patients managed by clinicians using a CRP POCT (with training). However, only small numbers had COPD (< 10% in one study33 and < 20% with asthma or COPD in the other34). CRP POCT was cost-effective in reducing antibiotic prescribing for LRTIs when there was no or low willingness to pay for the tests. 35,36 Now that better and more rapid CRP POCTs are available,37 there is potential for this technology to be widely used for a variety of acute infections in primary care to better guide antibiotic prescribing and, in doing so, to help reduce unnecessary antibiotic consumption and thus contain antibiotic resistance.
The PACE randomised controlled trial: overall aim
The clinical effectiveness and cost-effectiveness of using a CRP POCT in addition to usual clinical assessment to guide antibiotic prescribing for AECOPD has not been evaluated in a well-powered, pragmatic, individually randomised controlled trial (RCT) in primary care. The PACE (Primary care use of A C-reactive protein point of care test to help target antibiotic prescribing to patients with acute Exacerbations of chronic obstructive pulmonary disease who are most likely to benefit) trial, therefore, aimed to determine whether or not using a CRP POCT in addition to usual care to guide prescribing decisions for AECOPD reduces antibiotic consumption without having a negative impact on COPD health status.
Chapter 2 Clinical effectiveness methods
Some material in this report has been reproduced from Bates et al. 38 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. No changes to the content of the material were made; sentences/paragraphs were rephrased.
Summary of trial design
The PACE trial was a multicentre, parallel-arm, open, individually randomised (1 : 1) controlled trial of the clinical effectiveness and cost-effectiveness of CRP POCT in addition to usual care to guide antibiotic treatment decisions for AECOPD on safely reducing patient antibiotic consumption. The study involved general practices that were part of primary care research networks in the UK. Participants presenting with AECOPD to participating practices were randomised to clinical management based on usual care alone (control arm) or to usual care with the addition of a CRP POCT (intervention arm) to guide antibiotic prescribing. This was an open, pragmatic trial with no blinding of participants or clinicians, as we aimed to assess the effects of the intervention in comparison with current usual practice.
Clinical effectiveness objectives
Primary objective
The primary objective was to determine whether or not the addition of a CRP POCT (with training on test use and advice on interpretation) to usual care for managing AECOPD leads to a reduction in antibiotic consumption for AECOPD without having a negative impact on COPD health status, compared with usual care alone.
Secondary objectives
The secondary objectives were to assess the effect of using a CRP POCT for AECOPD in primary care on:
-
all-cause antibiotic consumption during the first 4 weeks
-
antibiotic prescribing at the index consultation
-
use of other COPD treatments, including oral steroids, during the first 4 weeks
-
primary and secondary care consultations [including out of hours, accident and emergency (A&E) visits and hospitalisations] during the subsequent 6 months
-
incidence of pneumonia during the first 4 weeks and from the 4-week follow-up to 6 months
-
adverse effects from antibiotics and other medication prescribed for AECOPD during the first 4 weeks
-
COPD health status, as measured using the Clinical COPD Questionnaire (CCQ), at weeks 1, 2 and 4
-
health utility, as measured using the EuroQol 5-Dimensions (EQ-5D) at 1, 2 and 4 weeks and at 6 months
-
disease-specific health-related quality of life (HRQoL) and Chronic Respiratory Disease Questionnaire Self-Administered Standardized (CRQ-SAS) at 6 months
-
prevalence of potentially pathogenic bacteria cultured from sputum at 4 weeks and the proportion of bacteria that are resistant
-
prevalence of commensal organisms cultured from throat swabs at 4 weeks and the proportion of bacteria that are resistant.
Changes made to the objectives are outlined in Appendix 1, Table 30.
The objectives of the qualitative process evaluation and the health economic evaluation are outlined in Chapters 4 and 5, respectively.
Internal pilot
An internal pilot study was carried out in 15 general practices in Wales from January 2015 to August 2015. The aim of the pilot study was to assess the recruitment potential, adherence to the intervention allocation and the proportion of participants in whom both primary outcomes could be measured. The prespecified criteria for the success of the internal pilot were at least 1.5 participants recruited per open site per month (excluding the first 2 months), 80% of participants reporting receiving a finger-prick blood test concordant with their allocated intervention arm, and 80% of recruited participants for whom we could ascertain both components of the co-primary outcome. The pilot phase met these criteria, enabling progression to the main study. In addition to monitoring recruitment against projected targets, a qualitative study was embedded within the pilot phase to identify barriers to and facilitators of recruitment from the perspectives of primary care staff (n = 9) and patients (n = 10), and to pilot the qualitative topic guides. The following changes were made at the end of the pilot phase to facilitate recruitment:
-
Patient information was streamlined, and clarification was provided on when to make an appointment and on rescue packs.
-
The inclusion criteria were modified slightly (see Appendix 1, Table 31).
-
The baseline case report forms (CRFs) were simplified.
-
Guidance was provided to practices on which tasks could be delegated to members of the primary care team other than the treating clinician.
-
Owing to the more sensitive instrument becoming available at the time, the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), used during the internal pilot, was replaced with the EuroQol-5 Dimensions, five-level version (EQ-5D-5L).
Participants
During the main study (September 2015–February 2017), a total of 96 general practices in England and Wales were opened to recruitment at some point. Not all of these sites were able to recruit; not all were open during the whole study period and some sites were closed and new sites opened.
Participants were eligible if they had had an AECOPD for at least 24 hours but no longer than 21 days and did not meet any of the exclusion criteria, and they were recruited opportunistically while consulting in routine primary care. The eligibility criteria used in the pilot phase (see Appendix 1, Table 32) were modified slightly for the main trial (see Appendix 1, Table 33). The changes, which were made to facilitate recruitment, are summarised in Table 31 (see Appendix 1).
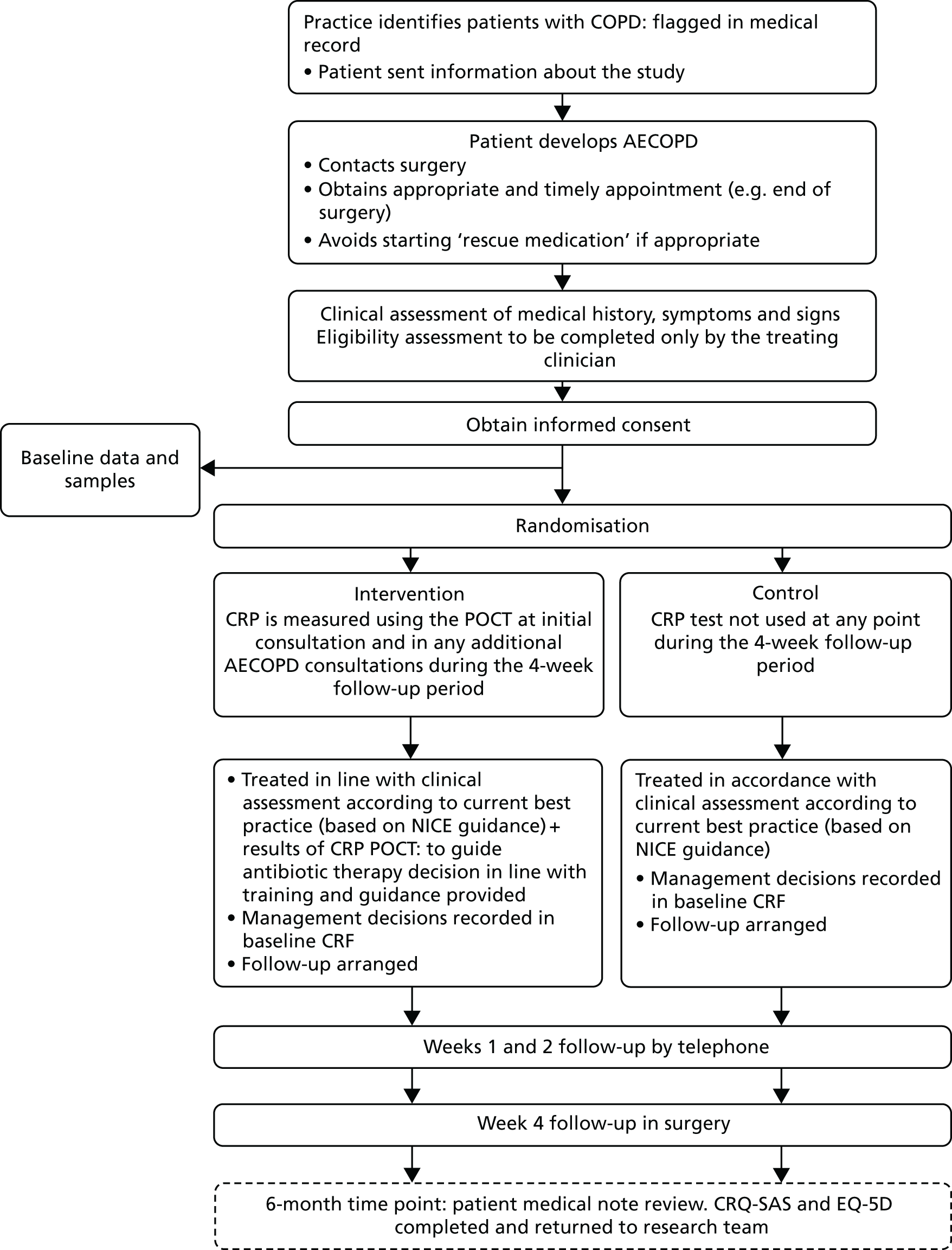
Patients’ informed consent to participate in the trial was obtained by the responsible clinician or an appropriately trained member of the practice team or a researcher. After this consent was obtained, data and sample collection were undertaken before the participants were remotely randomised to either the intervention or usual-care arm. Participant flow in the trial is summarised in Figure 1.
FIGURE 1.
Participant flow diagram. NICE, National Institute for Health and Care Excellence. Reproduced from Bates et al. 38 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Trial interventions
Intervention arm (C-reactive protein point-of-care test)
Participants randomised to the intervention arm had a CRP POCT measurement to help guide initial antibiotic prescribing decisions for their AECOPD. This was used in addition to usual clinical assessment. Clinicians were asked to use the CRP POCT during all primary care AECOPD consultations that occurred during the 4 weeks following randomisation for those participants randomised to the intervention arm.
Participating general practices were provided with a desktop CRP POCT Afinion device [Alere AfinionTM AS100 Analyzer, Alere Inc. (now Abbott Diagnostics), IL, USA] and CRP cartridges, and trained to use the device. This POCT required 1.5 µl of capillary blood (from a finger prick) and took < 4 minutes to provide a quantitative result. Other validated CE (Conformité Européene)-marked POCT devices and CRP cartridges giving a quantitative result within the range of the Alere Afinion POCT and requiring a similar volume of blood from a finger prick were also eligible to be used in the study when a practice preferred to use a CRP POCT they were already using and that was quality controlled. Only two practices used a device other than the Alere Afinion. Clinicians in participating general practices (e.g. GPs, nurse practitioners, practice nurses and health-care assistants) were provided with study-specific training, which included guidance for the clinical prescribers on interpreting CRP results in the context of AECOPD (Box 1).
The decision to prescribe antibiotics or not has to be based on a comprehensive assessment of the likely risks and benefits given:
-
the patient’s underlying health status (COPD severity, comorbidities, frailty)
-
clinical features of the current exacerbation.
Measurement of CRP can aid decision-making but is not meant to replace clinical assessment.
Patients with the following features are likely to be at an increased risk of complications:
-
severe COPD (GOLD grade 3)
-
past history of severe exacerbations (requiring hospitalisation)
-
significant comorbidities (e.g. heart failure, poorly controlled diabetes, lung cancer).
Sputum purulence is currently the best clinical predictor of bacterial infection. However:
-
patient reported sputum colour is generally not reliable
-
purulence can be increased in viral infections as well as in bacterial infections
-
try to obtain a sputum sample to objectively assess sputum purulence when possible.
Ask the patient how much the colour of their sputum has changed from its usual colour. This is particularly pertinent when it is not possible to objectively assess their sputum.
C-reactive protein measurement CRP level of < 20 mg/lAntibiotics are unlikely to be beneficial and usually should not be prescribed.
CRP level of 20–40 mg/lAntibiotics may be beneficial, mainly if purulent sputum is present. You may decide to prescribe antibiotics after taking into account the patient’s underlying health status and the features of the current exacerbation.
C-reactive protein guidance CRP level of > 40 mg/lAntibiotics are likely to be beneficial. Consider prescribing antibiotics unless the patient is assessed as being at a lower risk of complications and unlikely to have a bacterial infection (no increased sputum purulence and no features suggesting severe exacerbation).
GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Reproduced from Bates et al. 38 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
The guidance was based on a review of evidence including data from our recently published placebo-controlled trial of antibiotics for patients with acute exacerbations of mild to moderate COPD. 24 A cut-off point of 48 mg/l of CRP had previously been shown to distinguish pneumonia from non-consolidative exacerbations,39 and in our trial group we found that benefit from antibiotics was largely confined to those with a CRP level of > 40 mg/l. 24 We emphasised that CRP can take up to 24 hours to rise in a AECOPD, so duration of illness should be taken into account when interpreting the result. Our guidance, therefore, took a conservative approach and included cut-off points of < 20 mg/l, 20–40 mg/l and ≥ 40 mg/l, and placed greater emphasis on not prescribing antibiotics for those participants with a low level of CRP.
Control arm (usual care)
Participants allocated to usual care did not have a CRP POCT as part of the management of their AECOPD at any time during their participation and were managed according to usual care alone. All participating sites were provided with information on current best practice for managing AECOPD, which included a brief summary of National Institute for Health and Care Excellence (NICE) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidance delivered via the study-specific site initiation guidance, training and the PACE trial website (see Report Supplementary Material 1). No other specific guidance or instructions were given to clinicians in relation to the management of participants randomised to the usual-care arm.
Data collection
A summary of data collection is provided in Table 1. All data, including sensitive and personal data, were handled in accordance with the Data Protection Act 199840 and the subsequent General Data Protection Regulation 2016. 41 Paper CRFs were completed at all time points and then entered into the database by a member of the trial team. The database was built with internal validations and range checks. Queries arising during data entry were referred back to the site. Self-evident correction rules were developed during the trial in response to common errors of CRF completion. Central data monitoring was conducted throughout the trial, and a 10% quality control of all manually entered data was undertaken periodically. Following data cleaning, the data sets were extracted from the database, checked to ensure consistency with the paper CRFs and then provided to the statistician for analysis. All data will be retained for 15 years post trial closure in line with Cardiff University’s procedures.
| Assessment | Time point | ||||
|---|---|---|---|---|---|
| Baseline appointment | 1 week (telephone call) | 2 weeks (telephone call) | 4 weeks (face to face) | 6 months | |
| Assessment of eligibility | ✗ | ||||
| Written informed consent | ✗ | ||||
| Contact details | ✗ | ||||
| Medication history | ✗ | ||||
| Temperature | ✗ | ||||
| Oxygen saturation | ✗ | ||||
| Antibiotic prescribing | ✗ | ✗ (NS) | |||
| Antibiotics prescribed in the 12 months prior to study inclusion | ✗ (NS) | ||||
| Spirometry results (prior to inclusion or within 6 months post inclusion if no pre-inclusion results are available) | ✗ (NS) | ||||
| Most recent eosinophil count prior to inclusion | ✗ (NS) | ||||
| Other prescribed medications for current illness | ✗ | ||||
| CRP levela | ✗ | ||||
| CCQ | ✗ | ✗ | ✗ | ✗ | |
| EQ-5D | ✗ | ✗ | ✗ | ✗ | ✗ (P) |
| Sputum sample and throat swab | ✗ | ✗ | |||
| 4-week return visit date | ✗ | ||||
| Antibiotics use | ✗ | ✗ | ✗ | ✗ (NS) | |
| Other medications for AECOPD | ✗ | ✗ | ✗ | ||
| Adverse effects | ✗ | ✗ | ✗ | ||
| Adherence to use of POCT | ✗ | ✗ | ✗ | ✗ | |
| Smoking history | ✗ | ||||
| Time off paid work | ✗ | ||||
| Diagnosis of pneumonia (since baseline appointment) | ✗ | ||||
| Health-care contact and use | ✗ | ✗ (NS) | |||
| Mortalityb | ✗ | ✗ (NS) | |||
| CRQ-SAS | ✗ (P) | ||||
Baseline appointment
Baseline data collected included the number of days the participant reported experiencing AECOPD symptoms, medical history and clinical examination results (e.g. temperature, pulse, oxygen saturation, evidence of tachypnoea, crackles, wheezes, diminished vesicular sounds and evidence of consolidation). The clinicians responsible for managing the participant recorded the participants’ clinical findings. The collection of additional data could be conducted by a suitably trained member of the practice team.
A sputum sample (when participants were able to produce sputum) and a throat swab sample (using a charcoal swab) were obtained from participants at the baseline appointment (prior to randomisation) for bacterial and biological analyses. The recruiting clinicians recorded the colour of the participant’s sputum according to a BronkoTest© (London, UK) chart. 42 The BronkoTest is a colour chart that can be used by patients and clinicians to assess the colour of sputum to more effectively manage exacerbations of COPD. It is based on choosing one of five colours, and has been shown to correlate with the presence of bacterial pathogens in sputum. 43 If it was not possible to obtain sputum, participants were asked to estimate the current colour of their sputum based on a BronkoTest chart. These samples were appropriately packaged and sent to a local microbiology laboratory using first-class Royal Mail (London, UK) safe boxes at ambient temperature.
Participants were asked to self-complete the CCQ44 and the EuroQol 5-Dimensions (EQ-5D) questionnaire45–48 at their baseline appointment (prior to randomisation). The EQ-5D-3L was used for the 1-, 2- and 4-week follow-up assessments for the first 60 participants recruited; thereafter (and for all 6-month follow-ups), the EQ-5D-5L was used. CRP test results were recorded for those randomised to the intervention arm. Antibiotics prescribing and other management decisions were recorded for all participants following randomisation (as well as the outcome of the test result for those allocated to the intervention arm).
Follow-up data collection
Follow-up included telephone calls at 1 and 2 weeks, and a face-to-face consultation at 4 weeks post randomisation. In addition, the CRQ-SAS49 and the EQ-5D-5L were posted to participants for them to complete and return, using the provided and stamped addressed envelopes, to the study team at 6 months.
1- and 2-week telephone follow-up
A member of the trial team telephoned participants at weeks 1 and 2 to collect outcome data (see Table 1). Time windows for these data collection points were set at – 1/+ 2 working days for the 1-week follow-up and – 1/+ 7 working days for the 2-week follow-up.
4-week face-to-face visit
A 4-week face-to-face appointment at the general practice was arranged at the time of the baseline appointment. Appointments were conducted by a member of the clinical team in the general practice, or by a research nurse working for the local Clinical Research Network (CRN). The time window of the 4-week data collection was set at –3/+14 working days. Table 1 reports the data captured at this time point. In addition, any further CRP tests carried out since the baseline appointment were recorded. Sputum and throat swab samples were obtained when possible, and the colour of the sputum was assessed against a BronkoTest chart. If it was not possible to obtain sputum, participants were asked to estimate the current colour of their sputum based on a BronkoTest chart. These samples were sent to a local microbiology laboratory, as at baseline. If a successful appointment did not take place at week 4, the study team contacted the participant by telephone to obtain a minimum data set, including antibiotic consumption during the third and fourth week after randomisation, health-care resource use, diagnosis of pneumonia and completion of the CCQ and EQ-5D questionnaires. Minimum data set questions for the 1- and 2-week follow-up telephone calls were completed at the 4-week assessment if these had not already been completed.
Collection of relevant data from electronic medical records at 6 months
The data ascertained at the 6-month notes reviews are presented in Table 1.
Patient self-reported Chronic Respiratory Disease Questionnaire Self-Administered Standardized and EuroQol 5-Dimensions, five-level version at 6 months
Participants were sent a copy of the CRQ-SAS and EQ-5D-5L at 6 months post randomisation. The trial team telephoned participants 1 and 2 weeks after the due date to remind them to complete and return the questionnaire by post, or to offer to complete these instruments over the telephone.
Adverse events
Hospitalisation was considered an expected event in this patient population and this information was collected and reported as part of routine follow-up. All other events fulfilling the definition of a serious adverse event (SAE), including death, that occurred between the time of consent and the 4-week follow-up, were reported to the co-ordinating research centre within 24 hours of the site becoming aware of the event.
Microbiological assessment
Sputum (if available) and throat swab samples were obtained at the baseline appointment visit and at the face-to-face visit at week 4. Samples obtained were sent to the Specialist Antimicrobial Chemotherapy Unit, Public Health Wales, at the University Hospital of Wales.
Not all participants were able to produce a sputum sample on request and, therefore, we expected a lower return rate for these samples than for the throat swab samples at both baseline and week 4.
Sputum sample appearance (including colour and consistency) was noted at the laboratory, and all sputum samples were processed using the laboratory’s standard operating procedures. Potential pathogenic bacteria (including S. pneumoniae, Haemophilus influenzae/H. parainfluenzae, Moraxella catarrhalis, Pseudomonas species, Enterobacteriaceae and Staphylococcus aureus) were identified from sputum samples using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry, and semiquantitative counts were recorded. Antimicrobial susceptibilities were performed on relevant bacterial species from sputum samples by disc diffusion using European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology and break points. Throat swabs (charcoal) were added to tryptone soya broth (BO0351Y, Oxoid, Basingstoke, UK) and 50 µl was spiral plated onto a range of non-selective plates and selective plates for identification purposes [e.g. Columbia blood agar with optochin discs, chocolate agar with bacitracin, Pseudomonas agar with cetrimide, fucidin and cephaloridine plus Iso-SensitestTM agar (CM0471, Oxoid, Basingstoke, UK) with 5% defibrinated horse blood (TCS Biosciences, Buckingham, UK)] without and with the following antimicrobials: penicillin, third-generation cephalosporins, doxycycline, levofloxacin and clarithromycin at concentrations consistent with EUCAST break points. Total bacterial counts of commensal organisms were recorded on non-selective and selective agars; proportional quantification of resistant isolates was determined from the selective media. All isolated pathogens, sputum samples and the remaining broth from throat swabs were stored at –80 °C.
Nucleic acid was extracted from sputum samples using the Nuclisens® easyMAG® system (bioMérieux, Basingstoke, UK) and then analysed with the Luminex NxTAG® Respiratory Pathogen Panel (Luminex, Hertogenbosch, the Netherlands) for a variety of viral and bacterial pathogens.
Outcome measures
The co-primary outcome measure comprised:
-
Antibiotic consumption for AECOPD at any point during the 4 weeks post randomisation. Antibiotic consumption (rather than prescribing or dispensing) is the driver of antimicrobial resistance and was, therefore, selected as the outcome measure. With regard to the timing of this primary outcome, antibiotics consumed within 4 weeks of presenting with an AECOPD are likely to be related to the AECOPD and, therefore, using this time frame is more conservative in terms of demonstrating a reduction in antibiotic use related to the intervention. Data on antibiotic consumption were captured by participant self-report during the telephone interviews at weeks 1 and 2, and at the face-to-face interview at week 4. This was a binary outcome with a cut-off point of any consumption/no consumption.
-
COPD health status as measured with the CCQ via telephone interview at 2 weeks. The CCQ is a patient-centred health status measure that has been well validated, is widely used in patients with COPD and has a well-described minimal clinically important difference. 50 The CCQ is a brief questionnaire and, therefore, less burdensome to patients, and it is possible to use it with a recall period of 24 hours. CCQ scores range from 0 to 6 points, and the minimal clinically important difference is 0.4. CCQ was selected as a co-primary outcome to evaluate whether or not the intervention resulted in a meaningful reduction in antibiotic consumption (compared with usual care) without making COPD health status worse.
A 4-week time window was selected for the antibiotic consumption outcome to measure consumption of antibiotics prescribed at the initial consultation, and also antibiotics that were prescribed for the AECOPD in question but were initiated or prescribed at a later date. The CCQ outcome was measured at 2 weeks post randomisation as this is the time when most patients would be expected to have recovered and, therefore, the point at which a difference would be most indicative of delayed recovery.
Sample size
The study aimed to have sufficient power to detect a 15% reduction from an estimated 70% of patients who consume antibiotics for the AECOPD during the 4 weeks following randomisation. 9 Trials using CRP testing to reduce antibiotic prescribing for LRTI have resulted in absolute reductions in antibiotic prescribing in the region of 13–22%. 33,34,51 Even relatively small changes in prescribing are likely to have beneficial effects on bacterial resistance at a population level. 15 Detecting a difference in proportions between 0.70 and 0.55 at the 5% alpha and with 90% power required a total of 434 participants, inflated to 544 participants to account for the loss to follow-up of approximately 20% of participants. In addition, we aimed to have sufficient power to demonstrate that participants managed with the CRP POCT are no worse (non-inferior), compared with those managed without the CRP POCT, in terms of their COPD health status measured with the CCQ 2 weeks post randomisation. Assuming an expected difference between the arms of zero, a non-inferiority margin of 0.3 (this is lower than the lowest minimal clinically important difference of 0.4) and a common standard deviation (SD) of 1.1,50 based on a one-sided alpha of 5% and 90% power, the study needed 462 participants, inflated to 580 participants to account for the loss to follow-up of approximately 20% of participants.
Formulating our overall hypothesis using the intersection–union test,52 we aimed to carry out our individual sub-hypothesis tests at the 5% level and, if both were significant, conclude overall significance at the 5% level. Power will be affected by the level of correlation between the two outcomes and their corresponding effect sizes. The impact on overall power is at its greatest when there is zero correlation between outcomes and the effect sizes are identical (when this is the case, the overall power is the product of the powers for testing each individual sub-hypothesis). 53,54 Overall power decreases with increasing correlation between the outcomes and with greater difference in effect sizes. We did not expect our effect sizes to be similar, as our co-primary outcomes are two very different constructs (i.e. not two patient-reported outcome measures that are likely to yield similar effect sizes). We also anticipated that the outcomes would not be entirely independent (in those participants who do in fact require antibiotics, antibiotic consumption is likely to be related to COPD health status). We therefore aimed to recruit at least 650 participants to maintain an overall power between 81% and 90%.
Randomisation
Participants were remotely randomised, after giving their consent, using an online computerised randomisation system created by the Centre for Trials Research at Cardiff University. This was operational 24 hours a day. In addition, a telephone back-up was available from 8.30 a.m. to 6.30 p.m. if the online system failed or if the general practice had problems accessing the online site.
Participants were randomised in a 1 : 1 ratio to receive either usual care alone (control) or usual care with the addition of CRP POCT (intervention). Randomisation used minimisation, with a random element set at 80% to improve the integrity of the randomisation process. Anthonisen criteria (categorised as type 1, 2 or 3) were used as a minimisation variable to achieve balance with respect to COPD exacerbation severity. Remote allocation allowed the maintenance of allocation concealment from both the participant and the recruiting clinician up to the point of intervention, as this was an open study.
Statistical methods
Participant characteristics and clinical measures were summarised using frequencies and percentages, means and SDs or medians and interquartile ranges (IQRs), as appropriate. There was no planned interim analysis. All analyses have been presented as estimates of treatment effects (adjusted mean differences or odds ratios, as appropriate), with associated 95% CIs and p-values. The main trial analysis was based primarily on a modified intention-to-treat (MITT) population, which included all randomised participants who provided outcome data, regardless of protocol deviations or intervention received. Missing outcome data were imputed using multiple imputation to obtain a secondary analysis of the primary outcomes based on the full intention-to-treat (ITT) population. A complier average causal effect (CACE) analysis, which accounted for departures from randomised treatment while maintaining a comparison of groups as randomised, was also conducted on the primary CCQ analysis. 55 The conclusions drawn on the primary CCQ analysis were based on both the MITT and the CACE analyses [i.e. the upper limit of the one-sided 95% CI (equivalent to the two-sided 90% CI) had to exclude 0.3 in both analyses for non-inferiority to be concluded].
All planned analyses were conducted using Stata (version 13.0; StataCorp, College Station, TX, USA) described in detail in a statistical analysis plan, which was finalised prior to database lock.
Primary analysis
Our first primary analysis was to compare the odds of consuming an antibiotic for an acute exacerbation during the 4 weeks following randomisation, in each trial arm, using logistic regression. Our second primary analysis was to compare the mean CCQ score between each trial arm using linear regression, with baseline CCQ scores included as a covariate and a one-sided 95% CI constructed to assess non-inferiority. We fitted two-level regression models (using the mixed and melogit commands) to account for clustering of participants within practices. Modelling assumptions were tested, with appropriate adjustments made in the presence of any violations. Missing primary outcome data were anticipated to be minimal, but were accounted for in sensitivity analyses using multiple imputation (using the mi commands), where we assumed that primary outcome data are missing at random given observed measurements. Further sensitivity analyses considered the impact that departures from the missing at random assumption may have on any conclusions that could be drawn (using the rctmiss command).
Our second primary analysis, testing the non-inferiority of management with CRP versus no CRP with respect to the CCQ, was based on our prespecified margin of 0.3. We prespecified that if the observed difference in CCQ was between 0.3 and 0.4 (0.4 is the minimal clinically important difference for the outcome), we would further reflect on differences found in antibiotic consumption and secondary outcomes (e.g. antibiotic resistance, EQ-5D) between the two trial arms before drawing any conclusions.
Secondary analysis
Secondary outcomes were analysed in a similar manner to the primary outcomes, with linear, logistic, Poisson and negative binomial regression models fitted as appropriate (for the last two, the mepoisson and menbreg commands were used). The majority of regression models accounted for clustering effects of participants within practices. The analysis of CCQ domains and EQ-5D (both health utility and health status variables) over time involved fitting a three-level model, with responses nested within participants within practices. The antibiotic resistant organism outcomes (for both sputum and throat swabs at 4 weeks) were compared between the arms using binomial regression (using the binreg command).
Subgroup analysis
Differential intervention effects on the primary outcomes were assessed by fitting interaction terms in the primary models between trial arm and the following:
-
COPD severity (GOLD I/II/III/IV), from spirometry results (prior to inclusion or within 6 months post inclusion if no pre-inclusion results are available)
-
the severity of COPD exacerbation (Anthonisen criteria type 1/2/3)
-
the presence of a potentially pathogenic bacteria cultured from a sputum sample at baseline.
Sensitivity analysis
We determined whether or not the primary analyses were robust to the following sensitivity analyses:
-
modifying the inclusion criteria following the internal pilot
-
excluding participants on the basis of protocol violations
-
accounting for antibiotic consumption over time (rather than considering it as one single binary variable).
Patient and public involvement
The PACE trial patient and public involvement (PPI) representatives were both co-applicants and advisors on PACE and members of the Trial Management Group. They were recruited through Cynnwys Pobl (Involving People, Wales) and had relevant training and experience of contributing to national and local committees seeking to improve care for COPD sufferers. Sadly, one of our PPI representatives passed away before the end of the study. During the development of the PACE trial proposal, PPI representatives attended development team meetings, discussed the proposed research with a COPD patient group and contributed to the design of the trial. They paid special attention to plans for approaching/recruiting participants and providing participant-facing study materials, ensuring that participating patients felt safe in the knowledge that, should their acute exacerbation indicate the need for antibiotics, these would be prescribed. They were also involved in reviewing and discussing our primary outcome in relation to the best method of assessing quality of life and recovery in AECOPD. As members of our Trial Management Group, they continued to play a pivotal role in the design and conduct of the study, and our remaining PPI representative assisted with the dissemination of this research.
Ethics approval and governance
Ethics approval for the trial was given on 15 September 2014 by the Research Ethics Committee (REC) for Wales (Wales REC 6), recognised by the United Kingdom Ethics Committee Authority (REC reference 14/WA/1106). All sites received research and development approval from the appropriate Health Boards and Clinical Commissioning Groups before commencing trial procedures. The trial was registered on 20 August 2014 with the International Standard Randomised Controlled Trial Registration Number ISRCTN24346473. Cardiff University acted as a sponsor for the trial.
A summary of the changes made to the original protocol is given in Appendix 1, Table 35 (all outcome measures and changes to the outcome measures are shown in Appendix 1, Table 30).
Chapter 3 Clinical effectiveness results
Recruitment and participant flow
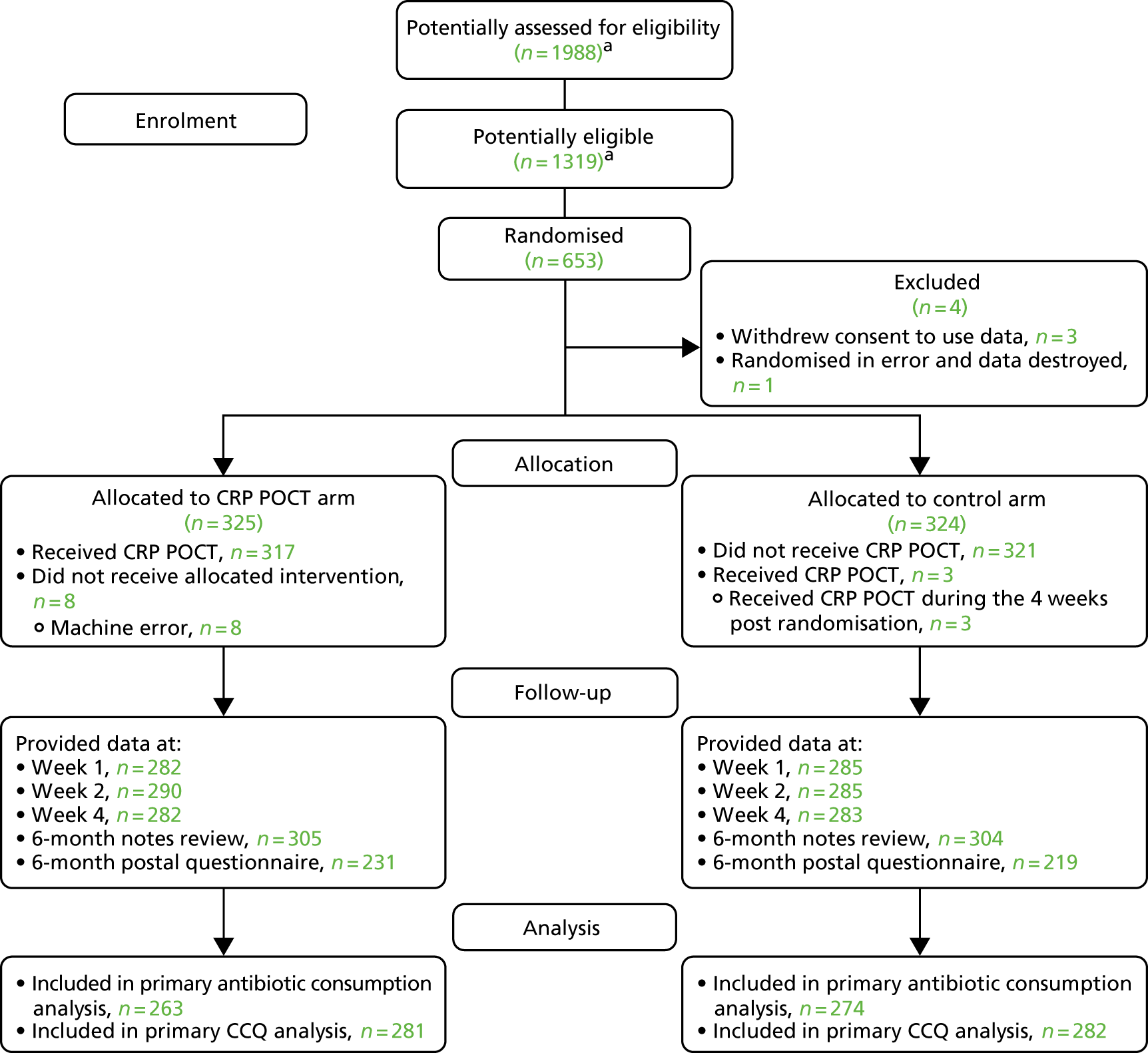
Ninety-six general practices across five research network hubs were open to recruitment at some point during the study period between January 2015 and February 2017. Ten of these practices did not recruit any participants.
It should be noted that completing screening logs is challenging for busy general practices, especially for patients with acute medical conditions. Not all of the clinicians working at each site participated in the study, worked full-time or were available to recruit each time they were on service. Fifty-six practices returned one or more screening logs, but, of these, only nine returned screening logs had data that were considered reliable (i.e. were regularly returned and consistently included details of participants’ approach in addition to those recruited). Using data from these reliable returns, we estimate that 1988 patients were approached in total, with 1319 patients being eligible for inclusion in the study (approximately 66% of those approached).
Six hundred and fifty-three participants were recruited from 86 general practices.
Table 2 provides a breakdown of the number of sites and participants from each recruitment centre. Overall, practices recruited a median of five participants, with the number per practice ranging from 1 to 40 participants.
| Recruitment centre | Number of participants | Number of sites (general practices) | Number of participants per practice | ||
|---|---|---|---|---|---|
| Mean | Median | Minimum to maximum | |||
| Cardiff and South Wales | 328 | 33 | 9.9 | 7.0 | 1 to 40 |
| London | 84 | 20 | 4.2 | 2.5 | 1 to 15 |
| Oxford and Thames Valley | 149 | 19 | 7.8 | 6.0 | 2 to 33 |
| Norfolk | 77 | 9 | 8.6 | 7.0 | 2 to 25 |
| North-west coast | 15 | 5 | 3.0 | 3.0 | 1 to 4 |
| Overall | 653 | 86 | 7.6 | 5.0 | 1 to 40 |
Three randomised participants withdrew from the study, including withdrawal of their consent for their data to be used, and one participant was randomised in error (with the recruiting clinician destroying their data). All subsequent analyses are based on a maximum of 649 participants. Figure 2 provides details of recruitment and retention throughout the study.
FIGURE 2.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Number of participants assessed for eligibility based on screening log data from 9 out of 86 practices that returned reliable screening log data (i.e. were regularly returned and consistently included details of participants’ approach in addition to those recruited). From these practices, 208 patients were approached (an average of 23 patients approached per practice), 138 patients (66.3%) were eligible and 109 patients were recruited. Potentially assessed for eligibility calculation: (208/9) × 86 = 1988. Potentially eligible calculation: (138/9) × 86 = 1319. The main reasons for patients’ ineligibility were that they had recently used, or were currently using, antibiotics (28/70), or that they had already participated in the PACE trial (13/70). The main reasons because of which eligible patients were not recruited were because the patient declined (18/29) or because there was a lack of clinical time to recruit (9/29). Data are drawn from Butler et al. 56
Baseline data
Participants in both arms were well matched at baseline. The key baseline characteristics, including participant demographics and presentation features, are provided in Tables 3–5.
| Variable | Trial arm | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||||||
| n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | |
| Age (years) | 324 | 68.3 (9.31) | 68.5 (62.0 to 75.0) | 40 to 92 | 325 | 67.8 (9.53) | 68.0 (62.0 to 75.0) | 41 to 90 | 649 | 68.1 (9.42) | 68.0 (62.0 to 75.0) | 40 to 92 |
| Number of days with symptoms prior to consultation | 324 | 7.1 (5.06) | 6.0 (3.0 to 10.0) | 1 to 21 | 325 | 6.8 (5.2) | 5.0 (3.0 to 9.0) | 1 to 28 | 649 | 6.9 (5.13) | 5.0 (3.0 to 10.0) | 1 to 28 |
| Pack years (for current smokers) | 92 | 47.8 (30.47) | 41.6 (27.5 to 56.3) | 5 to 165 | 95 | 41.0 (30.66) | 36.0 (22.2 to 49.3) | 3 to 162 | 187 | 44.4 (30.67) | 40.0 (24.6 to 53.5) | 3 to 165 |
| CCQ symptoms score (points) | 319 | 3.8 (1.09) | 3.8 (3.0 to 4.5) | 1.25 to 6.0 | 320 | 3.7 (1.14) | 3.8 (3.0 to 4.5) | 0.5 to 6.0 | 639 | 3.8 (1.11) | 3.8 (3.0 to 4.5) | 0.5 to 6.0 |
| CCQ functional state score (points) | 319 | 3.0 (1.45) | 3.0 (2.0 to 4.0) | 0.0 to 6.0 | 319 | 2.9 (1.54) | 3.0 (1.8 to 4.0) | 0.0 to 6.0 | 638 | 2.9 (1.49) | 3.0 (1.8 to 4.0) | 0.0 to 6.0 |
| CCQ mental state score (points) | 316 | 2.9 (1.60) | 3.0 (1.5 to 4.0) | 0.0 to 6.0 | 317 | 2.9 (1.64) | 3.0 (1.5 to 4.0) | 0.0 to 6.0 | 633 | 2.9 (1.62) | 3.0 (1.5 to 4.0) | 0.0 to 6.0 |
| CCQ total score (points) | 316 | 3.3 (1.11) | 3.3 (2.4 to 4.2) | 0.7 to 5.8 | 314 | 3.2 (1.16) | 3.3 (2.4 to 4.1) | 0.3 to 6.0 | 630 | 3.3 (1.14) | 3.3 (2.4 to 4.1) | 0.3 to 6.0 |
| Overall health (EQ-5D) | 289 | 48.3 (21.33) | 50.0 (30.0 to 60.0) | 0 to 100 | 288 | 48.6 (19.89) | 50.0 (40.0 to 60.0) | 0 to 98 | 577 | 48.5 (20.61) | 50.0 (35.0 to 60.0) | 0 to 100 |
| Health utility (EQ-5D) | 314 | 0.6 (0.27) | 0.6 (0.5 to 0.8) | –0.4 to 1.0 | 316 | 0.6 (0.29) | 0.7 (0.5 to 0.8) | –0.6 to 1.0 | 630 | 0.6 (0.28) | 0.7 (0.5 to 0.8) | –0.6 to 1.0 |
| Variable | Trial arm, frequency (%) | Overall, frequency (%) | |
|---|---|---|---|
| Usual care | CRP POCT | ||
| Male | 173 (53.4) | 162 (49.8) | 335 (51.6) |
| Female | 151 (46.6) | 163 (50.2) | 314 (48.4) |
| Heart failure | 15 (4.6) | 16 (4.9) | 31 (4.8) |
| Coronary heart disease | 59 (18.2) | 55 (16.9) | 114 (17.6) |
| Diabetes | 54 (16.7) | 50 (15.4) | 104 (16.0) |
| Chronic kidney disease | 32 (9.9) | 27 (8.3) | 59 (9.1) |
| Hypertension | 143 (44.1) | 124 (38.2) | 267 (41.1) |
| Other chronic disease | 70 (24.1) | 85 (28.5) | 155 (26.3) |
| Non-smoker | 22 (7.9) | 20 (7.1) | 42 (7.5) |
| Ex-smoker | 163 (58.4) | 165 (58.7) | 328 (58.6) |
| Current smoker | 94 (33.7) | 96 (34.2) | 190 (33.9) |
| 1/3 Anthonisen criteria | 81 (25.0) | 76 (23.4) | 157 (24.2) |
| 2/3 Anthonisen criteria | 98 (30.2) | 100 (30.8) | 198 (30.5) |
| 3/3 Anthonisen criteria | 145 (44.8) | 149 (45.8) | 294 (45.3) |
| Current sputum colour according to BronkoTest (clinician-assessed if sputum obtained during consultation) | |||
| 1 (light/non-purulent) | 48 (25.3) | 59 (30.6) | 107 (27.9) |
| 2 (non-purulent) | 47 (24.7) | 39 (20.2) | 86 (22.5) |
| 3 (purulent) | 36 (18.9) | 50 (25.9) | 86 (22.5) |
| 4 (purulent) | 53 (27.9) | 37 (19.2) | 90 (23.5) |
| 5 (dark/purulent) | 6 (3.2) | 8 (4.1) | 14 (3.7) |
| Current sputum colour according to BronkoTest (participant-assessed if sputum not obtained during consultation) | |||
| 1 (light/non-purulent) | 14 (11.9) | 14 (12.0) | 28 (11.9) |
| 2 (non-purulent) | 18 (15.3) | 22 (18.8) | 40 (17.0) |
| 3 (purulent) | 28 (23.7) | 22 (18.8) | 50 (21.3) |
| 4 (purulent) | 23 (19.5) | 27 (23.1) | 50 (21.3) |
| 5 (dark/purulent) | 13 (11.0) | 13 (11.1) | 26 (11.1) |
| Unable to produce sputum | 22 (18.6) | 19 (16.2) | 41 (17.4) |
| Sputum colour according to BronkoTest when not exacerbating (participant-assessed) | |||
| 1 (light/non-purulent) | 126 (44.5) | 130 (46.6) | 256 (45.6) |
| 2 (non-purulent) | 74 (26.1) | 88 (31.5) | 162 (28.8) |
| 3 (purulent) | 32 (11.3) | 23 (8.2) | 55 (9.8) |
| 4 (purulent) | 12 (4.2) | 9 (3.2) | 21 (3.7) |
| 5 (dark/purulent) | 5 (1.8) | 2 (0.7) | 7 (1.2) |
| Unable to produce sputum | 34 (12.0) | 27 (9.7) | 61 (10.9) |
| Crackles | 162 (50.0) | 158 (48.6) | 320 (49.3) |
| Wheeze | 167 (51.5) | 171 (52.6) | 338 (52.1) |
| Diminished vesicular sounds | 82 (25.5) | 71 (21.8) | 153 (23.6) |
| Evidence of consolidation | 8 (2.5) | 11 (3.4) | 19 (2.9) |
| Prescribed oral antibiotics in the past 12 months | 198 (65.6) | 205 (67.4) | 403 (66.5) |
| Using regular inhalers prior to recruitment | 290 (96.0) | 289 (95.1) | 579 (95.5) |
| Mobility | |||
| No problems | 64 (20.3) | 79 (24.7) | 143 (22.5) |
| Problems | 252 (79.7) | 241 (75.3) | 493 (77.5) |
| Self-care | |||
| No problems | 172 (54.4) | 202 (63.5) | 374 (59.0) |
| Problems | 144 (45.6) | 116 (36.5) | 260 (41.0) |
| Usual activities | |||
| No problems | 67 (21.2) | 74 (23.1) | 141 (22.2) |
| Problems | 249 (78.8) | 246 (76.9) | 495 (77.8) |
| Pain/discomfort | |||
| No problems | 102 (32.4) | 93 (29.2) | 195 (30.8) |
| Problems | 213 (67.6) | 225 (70.8) | 438 (69.2) |
| Anxiety/depression | |||
| No problems | 162 (51.3) | 156 (48.8) | 318 (50.0) |
| Problems | 154 (48.7) | 164 (51.2) | 318 (50.0) |
| No bacterial growth in sputum/not analysed | |||
| No growth | 28 (14.4) | 23 (11.4) | 51 (12.8) |
| Insufficient sample | 2 (1.0) | 4 (2.0) | 6 (1.5) |
| Sputum not processed | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Potential bacterial pathogens in sputum | |||
| Pure growth of pathogen | 22 (11.3) | 23 (11.4) | 45 (11.3) |
| Mixed growth including pathogens | 60 (30.8) | 65 (32.2) | 125 (31.5) |
| No potential bacterial pathogens in sputum | |||
| Normal respiratory flora | 83 (42.6) | 85 (42.1) | 168 (42.3) |
| Mixed growth of non-pathogens | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Variable | Trial arm | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||||||
| n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | |
| Ratio of FEV1 to FVC | 224 | 0.6 (0.13) | 0.6 (0.5 to 0.7) | 0.23 to 0.85 | 205 | 0.6 (0.12) | 0.6 (0.5 to 0.7) | 0.30 to 0.85 | 429 | 0.6 (0.13) | 0.6 (0.5 to 0.7) | 0.23 to 0.85 |
| % predicted FEV1 | 282 | 60.4 (20.73) | 59.2 (45.0 to 74.0) | 11.4 to 150.4 | 277 | 59.2 (19.33) | 58.9 (45.0 to 71.6) | 9.9 to 125.4 | 559 | 59.8 (20.04) | 59.0 (45.0 to 73.0) | 9.9 to 150.4 |
| Ratio of FEV1 to FVC of < 0.7 (% of those with a calculable ratio), frequency (%) | 181 | 80.8 | 172 | 83.9 | 353 | 82.3 | ||||||
| Ratio of FEV1 to FVC of ≥ 0.7 (% of those with a calculable ratio), frequency (%) | 43 | 19.2 | 33 | 16.1 | 76 | 17.7 | ||||||
| Mild COPD (GOLD I), frequency (%) | 20 | 11.1 | 18 | 10.5 | 38 | 10.8 | ||||||
| Moderate COPD (GOLD II), frequency (%) | 100 | 55.6 | 93 | 54.1 | 193 | 54.8 | ||||||
| Severe COPD (GOLD III), frequency (%) | 47 | 26.1 | 52 | 30.2 | 99 | 28.1 | ||||||
| Very severe COPD (GOLD IV), frequency (%) | 13 | 7.2 | 9 | 5.2 | 22 | 6.3 | ||||||
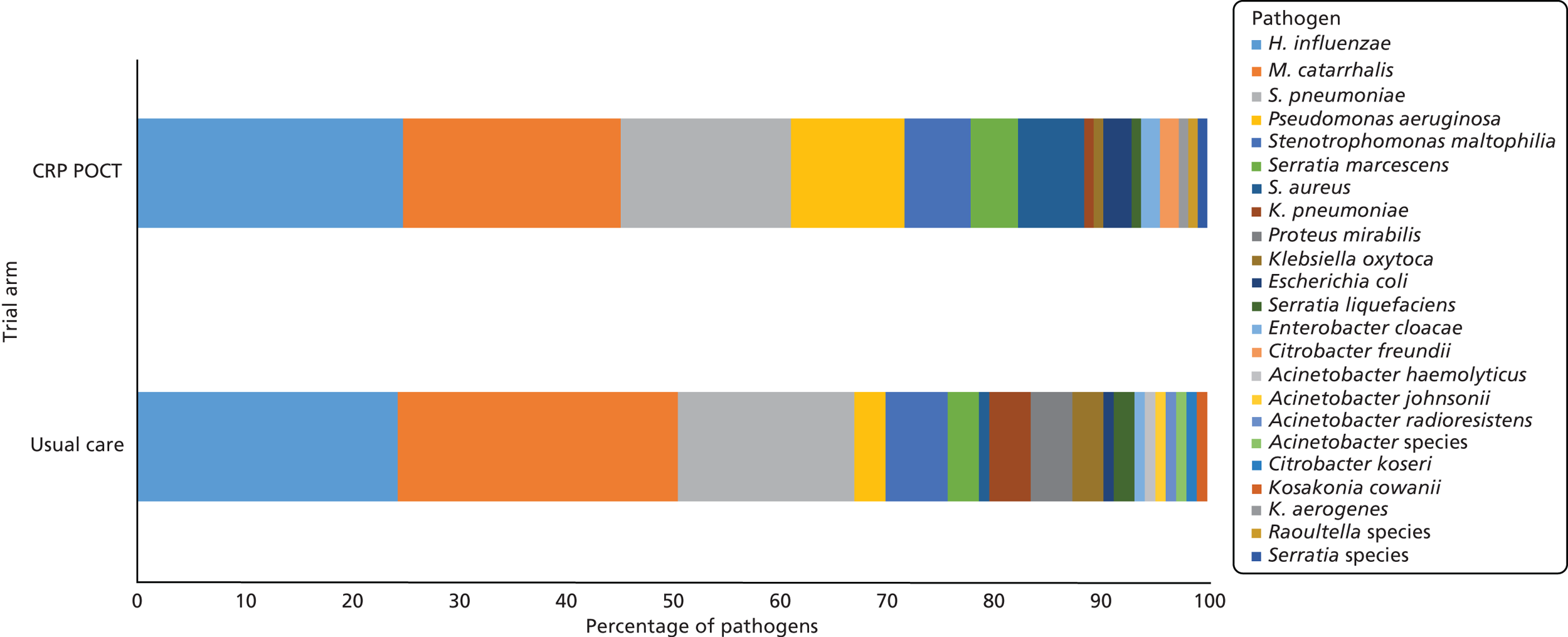
Sputum bacteriological and virological profiles at baseline
At baseline, sputum sample bacteriology data were available for 195 usual-care participants and 202 CRP POCT participants (60.2% and 62.2% of randomised participants, respectively). Of the samples with potential pathogenic bacteria, 103 organisms were cultured from usual-care participants and 113 were in sputa from participants allocated to CRP POCT. There were 23 distinct potential pathogenic species identified in total. Potential pathogen profiles were similar between the arms (Figure 3). Baseline antibiotic sensitivity data for the three most frequently cultured potentially pathogenic species are presented in Table 6.
FIGURE 3.
Potential bacterial pathogens from sputum samples at baseline. Usual care data based on 103 potential bacterial pathogens from 82 participants. CRP POCT data based on 113 potential bacterial pathogens from 88 participants.
| Bacteriaa | Antibiotic | Trial arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | ||||||
| Tested (n) | Resistant, n (%) | Tested (n) | Resistant, n (%) | Tested (n) | Resistant, n (%) | ||
| H. influenzae (n = 45) | Ampicillin | 20 | 5 (25.0) | 24 | 10 (41.7) | 44 | 15 (34.1) |
| Cefotaxime | 20 | 0 (0.0) | 24 | 0 (0.0) | 44 | 0 (0.0) | |
| Ceftazidime | 20 | 20 (100.0) | 25 | 25 (100.0) | 45 | 45 (100.0) | |
| Co-amoxiclav | 20 | 5 (25.0) | 24 | 7 (29.2) | 44 | 12 (27.3) | |
| Erythromycin | 20 | 20 (100.0) | 25 | 25 (100.0) | 45 | 45 (100.0) | |
| Tetracycline | 20 | 0 (0.0) | 24 | 0 (0.0) | 44 | 0 (0.0) | |
| M. catarrhalis (n = 44) | Ampicillin | 22 | 22 (100.0) | 21 | 21 (100.0) | 43 | 43 (100.0) |
| Cefotaxime | 22 | 1 (4.5) | 22 | 1 (4.5) | 44 | 2 (4.5) | |
| Ceftazidime | 0 | 0 | 0 | ||||
| Co-amoxiclav | 22 | 0 (0.0) | 22 | 0 (0.0) | 44 | 0 (0.0) | |
| Erythromycin | 22 | 3 (13.6) | 22 | 1 (4.5) | 44 | 4 (9.1) | |
| Tetracycline | 22 | 0 (0.0) | 22 | 0 (0.0) | 44 | 0 (0.0) | |
| S. pneumoniae (n = 36) | Ampicillin | 15 | 5 (33.3) | 10 | 1 (10.0) | 25 | 6 (24.0) |
| Cefotaxime | 0 | 0 | 0 | ||||
| Ceftazidime | 17 | 17 (100.0) | 15 | 15 (100.0) | 32 | 32 (100.0) | |
| Co-amoxiclav | 15 | 5 (33.3) | 10 | 1 (10.0) | 25 | 6 (24.0) | |
| Erythromycin | 18 | 5 (27.8) | 18 | 2 (11.1) | 36 | 7 (19.4) | |
| Tetracycline | 18 | 3 (16.7) | 18 | 3 (16.7) | 36 | 6 (16.7) | |
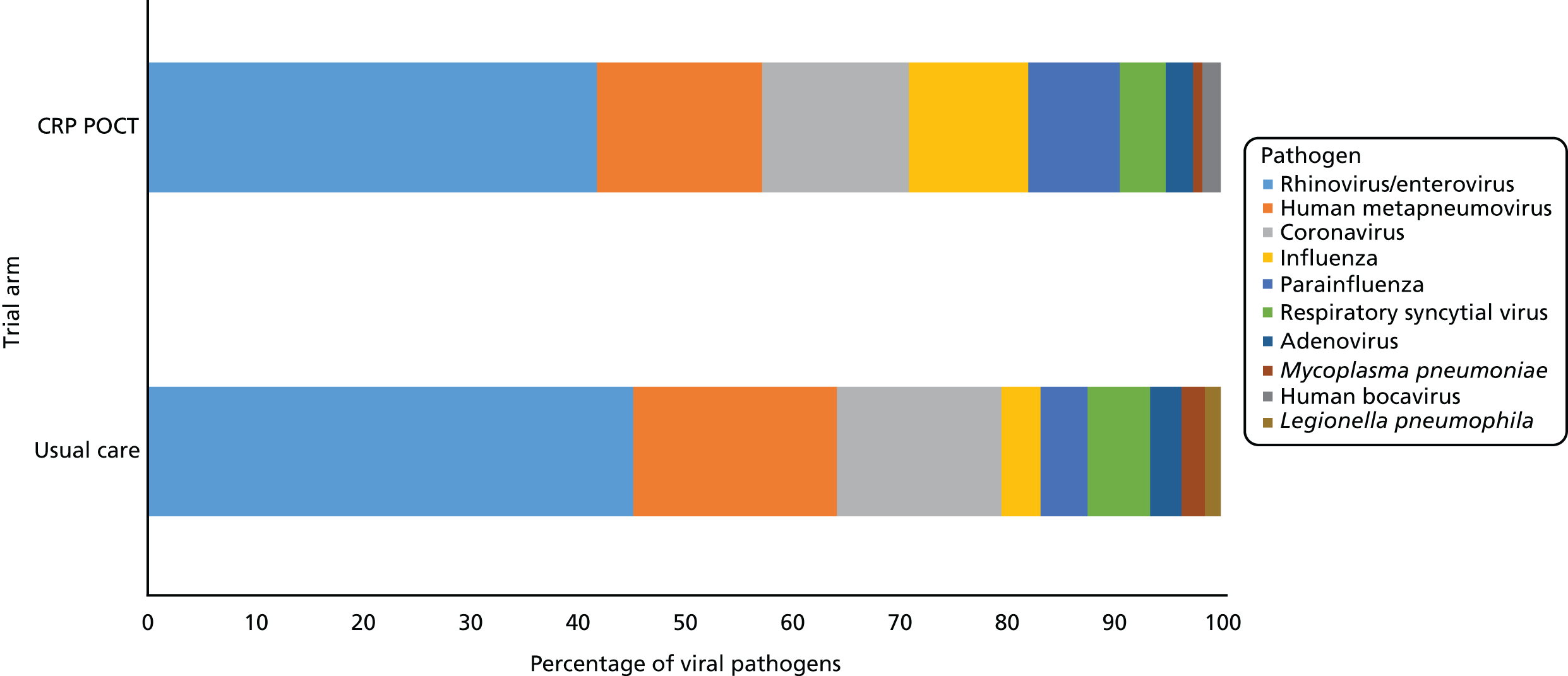
Both bacteriology and virology data from sputum samples at baseline were available for 190 usual-care participants and 196 CRP POCT participants. There was little difference between the arms regarding the general microbiological profile of sputum samples. Overall, no pathogens were detected in 95 out of 386 sputum samples (24.6%), bacterial pathogens only (i.e. no viral/atypical pathogens) were detected in 79 out of 386 cases (20.5%), viral/atypical pathogens only were detected in 123 out of 386 cases (31.9%), and both bacterial and viral/atypical pathogens were detected in 89 out of 386 cases (23.1%). Of those with viral/atypical pathogens, 137 viruses/atypical organisms were detected in samples obtained from usual-care participants and 117 in those allocated to CRP POCT. Ten distinct species were detected in total. Pathogen profiles were similar between the arms (Figure 4).
FIGURE 4.
Viral and atypical pathogens from sputum samples at baseline. Usual-care data based on 137 viral/atypical pathogens from 109 participants. CRP POCT data based on 117 viral/atypical pathogens from 103 participants. Confirmation of CRP POCT testing.
In total, 97.5% (317/325) of participants allocated to the CRP POCT arm reported receiving a CRP POCT during the recruitment consultation. For the remaining eight participants, a CRP POCT could not be carried out because of a machine error. Of the participants allocated to the usual-care arm, 13.6% (44/324) reported receiving a finger-prick blood test at some point within the 4 weeks following randomisation. However, 84% (37/44) of these reports of finger-prick blood testing were verified (by checking machine logs and 6-month notes reviews, and querying with the recruiting practice) as not being CRP tests. CRP testing was confirmed in three instances (all at the initial consultation) and unverified (i.e. no additional data to confirm or deny) in four instances. Therefore, the percentage of usual-care participants who received a CRP POCT was between 0.9% (3/324) and 2.2% (7/324).
Distribution of C-reactive protein values
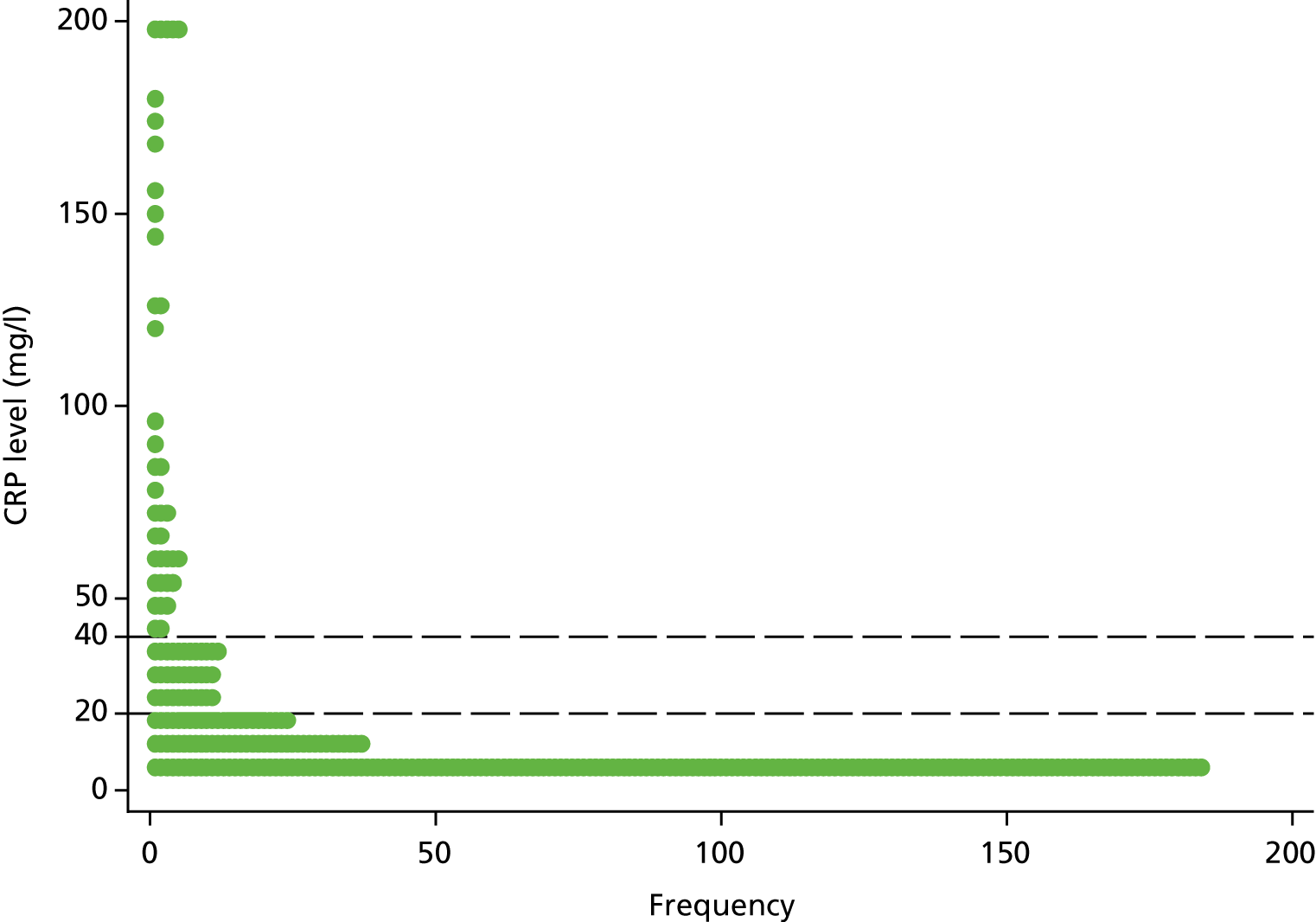
For the 317 participants allocated to the CRP POCT arm and for those who were tested with a CRP POCT at the index consultation, the median CRP value was 6 mg/l (IQR 5–18.5 mg/l). Of those participants, 76.0% (241/317) had CRP values of < 20 mg/l, 12% (38/317) had CRP values in the intermediate category (20–40 mg/l) and 12% (38/317) had CRP values in the highest range (> 40 mg/l) (Figure 5).
FIGURE 5.
Dot plot of CRP levels. Censored observations are fixed at censored value (i.e. < 5 and > 200 are set to 5 and 200, respectively). The dashed lines are at y = 20 and y = 40 to distinguish between the three categories provided to clinicians as part of the intervention.
Outcomes and estimation
Co-primary outcomes
Of the 649 participants randomised, 537 contributed to the primary analysis of antibiotic consumption (82.7%) and 563 contributed to the primary analysis of CCQ total score at 2 weeks post randomisation (86.7%).
The odds of consuming an antibiotic for AECOPD during the first 4 weeks following randomisation were 69% lower in participants allocated to the CRP POCT arm than in those in the usual-care arm (Table 7).
| Outcome measure | Time point | Trial arm | AORa (95% CI) | p-value | ICC | |||
|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||
| n | Frequency (%) | n | Frequency (%) | |||||
| Antibiotic consumption for AECOPD | During the 4 weeks post randomisation | 274 | 212 (77.4) | 263 | 150 (57.0) | 0.31 (0.20 to 0.47) | < 0.001 | 0.17 |
The adjusted mean difference in CCQ score was 0.19 (two-sided 90% CI –0.33 to –0.05) points lower in the CRP POCT arm than in the usual-care arm. The two-sided 90% CI for both CACE analyses ranged from –0.34 to –0.07 points. The upper limit of both CIs did not include the prespecified non-inferiority margin of 0.3, suggesting that a CRP-assisted management strategy is no worse than usual management (without a CRP POCT) according to COPD health status at 2 weeks (Table 8).
| Analysis set | Outcome measure | Time point | Trial arm | Adjusted mean differencea (90% CI) | ICC | |||
|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||
| n | Mean (SE) | n | Mean (SE) | |||||
| MITT | CCQ (points) | Two weeks post randomisation | 282 | 2.8 (0.07) | 281 | 2.6 (0.07) | –0.19 (–0.33 to –0.05) | 0.04 |
| CACE 1 | –0.20 (–0.34 to –0.07) | |||||||
| CACE 2 | –0.20 (–0.34 to –0.07) | |||||||
As the null hypotheses were rejected for both co-primary outcomes, our overall (composite) primary hypothesis can also be rejected, and we can conclude that we found evidence that a CRP-assisted management strategy results in reduced antibiotic use for patients presenting to primary care with AECOPD without having a negative impact on COPD health status at 2 weeks.
Secondary outcomes
The percentage of participants included in the analysis of secondary outcomes, based on data collected in the 4 weeks following randomisation, ranged from 74.0% (480/649) for the total number of days that antibiotics were consumed during the first 4 weeks outcome to 99.8% (648/649) for the antibiotic prescribing at the index consultation outcome. The percentage of participants included in the secondary outcome analysis of the CRQ-SAS domains, which were collected at 6 months via postal questionnaires, ranged between 61.5% for the dyspnoea domain (399/649) and 68.0% for the emotional function domain (441/649).
Medication use
Antibiotics were prescribed to 79 out of 241 participants with a CRP of < 20 mg/l (32.8%), 32 out of 38 participants with a CRP level between 20 and 40 mg/l (84.2%) and 36 out of 38 participants with a CRP level of > 40 mg/l (94.7%).
We found that the odds of consuming antibiotics for any reason during the 4 weeks following randomisation, receiving an antibiotic prescription at the initial consultation or receiving an antibiotic prescription during the 4 weeks following randomisation were lower in participants allocated to the CRP POCT arm than in those allocated to usual care. We found no evidence of any difference between the arms regarding the use of other COPD treatments during the 4 weeks following randomisation (Table 9). The between-arm analyses of the total number of days that antibiotics were consumed for AECOPD/any reason during the first 4 weeks following randomisation produced intervention effects consistent with the binary consumed/not consumed outcomes.
| Outcome measure | Time point | Trial arm | AORa (95% CI) | p-value | ICC | |||
|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||
| n | Frequency (%) | n | Frequency (%) | |||||
| Antibiotic consumption for any reason | During the 4 weeks post randomisation | 278 | 220 (79.1) | 267 | 158 (59.2) | 0.31 (0.20 to 0.48) | < 0.001 | 0.15 |
| Antibiotic prescribing | During index consultation | 323 | 225 (69.7) | 325 | 155 (47.7) | 0.31 (0.21 to 0.45) | < 0.001 | 0.21 |
| Antibiotic prescribing | During the 4 weeks post randomisation | 316 | 252 (79.7) | 313 | 185 (59.1) | 0.30 (0.20 to 0.46) | < 0.001 | 0.21 |
| Use of other COPD treatments | During the 4 weeks post randomisation | 290 | 268 (92.4) | 290 | 263 (90.7) | 0.79 (0.43 to 1.46) | 0.453 | 0.14 |
Potential medication side effects, consultations with primary/secondary care and pneumonia diagnoses
Symptoms commonly attributed as adverse effects from antibiotics and other COPD treatments were reported by 264 out of 289 participants in the usual-care arm (91.3%) and 255 out of 285 participants in the CRP POCT arm (89.5%) during the 4 weeks following randomisation [adjusted odds ratio (AOR) 0.79, 95% CI 0.44 to 1.39; p = 0.410]. There was no evidence of a difference between the arms regarding primary/secondary care consultations for any reason during the 6 months following randomisation (AOR 1.39, 95% CI 0.46 to 4.15; p = 0.559), or pneumonia diagnoses at 4 weeks (AOR 1.57, 95% CI 0.28 to 8.84; p = 0.608) or 6 months following randomisation (AOR 0.73, 95% CI 0.29 to 1.82; p = 0.495).
Patient-reported outcome measures
Table 10 describes the analyses of COPD health status (as measured with the CCQ) over time. For the total score, as well as the individual domains, there is a discernible reduction (improvement) in scores over the follow-up time periods. The adjusted mean difference (AMD) (averaged across follow-up time points) was lower (better) in the CRP POCT arm than in the usual-care arm for the total score (AMD –0.20, 95% CI –0.34 to –0.06; p = 0.005), symptom domain (AMD –0.19, 95% CI –0.34 to –0.05; p = 0.010) and function state (AMD 0.29, 95% CI –0.45 to –0.12; p = 0.001). There was no evidence of a difference for the mental state domain (AMD –0.08, 95% CI –0.27 to 0.10; p = 0.372). There was also no evidence of any differential intervention effect over time.
| Outcome measure | Time point (weeks) | Trial arm, mean (SE) | Time point,a coefficient (95% CI) | Intervention effectb | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | Week 2 | Week 4 | p-value | Coefficient (95% CI) | p-value | ||
| CCQ total score (points) | 1 | 2.8 (0.07) | 2.6 (0.07) | –0.08 (–0.15 to –0.01) | –0.21 (–0.29 to –0.14) | < 0.001 | –0.20 (–0.34 to –0.06) | 0.005 |
| 2 | 2.8 (0.06) | 2.6 (0.07) | ||||||
| 4 | 2.6 (0.07) | 2.4 (0.07) | ||||||
| CCQ symptom domain score (points) | 1 | 3.2 (0.07) | 3.0 (0.07) | –0.04 (–0.13 to 0.05) | –0.22 (–0.31 to –0.13) | < 0.001 | –0.19 (–0.34 to –0.05) | 0.010 |
| 2 | 3.2 (0.07) | 3.0 (0.07) | ||||||
| 4 | 3.0 (0.07) | 2.8 (0.07) | ||||||
| CCQ function state score (points) | 1 | 2.6 (0.08) | 2.3 (0.08) | –0.11 (–0.19 to –0.02) | –0.14 (–0.23 to –0.05) | 0.006 | –0.29 (–0.45 to –0.12) | 0.001 |
| 2 | 2.5 (0.08) | 2.2 (0.08) | ||||||
| 4 | 2.5 (0.08) | 2.2 (0.08) | ||||||
| CCQ mental state score (points) | 1 | 2.6 (0.09) | 2.5 (0.09) | –0.14 (–0.24 to –0.03) | –0.39 (–0.49 to –0.28) | < 0.001 | –0.08 (–0.27 to 0.10) | 0.372 |
| 2 | 2.4 (0.09) | 2.4 (0.09) | ||||||
| 4 | 2.2 (0.09) | 2.1 (0.09) | ||||||
Table 11 describes the analysis of general health utility and health status over time (measured with the EQ-5D). For health utility, there was no evidence of any difference between arms averaged across follow-up time points (AMD 0.03, 95% CI –0.04 to 0.09; p = 0.384). Although there was some evidence of a difference in health utility across time in general (i.e. averaged across usual-care arm participants and CRP POCT arm participants), there was no evidence to suggest any differential intervention effect over time. There was a difference between arms in terms of general health status, with participants allocated to the CRP POCT arm reporting a health status score > 3 points higher than that of usual-care arm participants (AMD 3.12, 95% CI 0.50 to 5.74; p = 0.019). Health status generally improved over the follow-up time points, but, similar to the health utility measure, there was no evidence to suggest any differential intervention effect over time.
| Outcome measure | Time point | Trial arm, mean (SE) | Time point,a coefficient (95% CI) | Intervention effectb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | Week 2 | Week 4 | Month 6 | p-value | Coefficient (95% CI) | p-value | ||
| EQ-5D index value | Week 1 | 0.6 (0.01) | 0.6 (0.01) | 0.04 (0.00 to 0.08) | 0.14 (0.11 to 0.18) | –0.05 (–0.09 to –0.01) | < 0.001 | 0.03 (–0.04 to 0.09) | 0.384 |
| Week 2 | 0.6 (0.01) | 0.6 (0.01) | |||||||
| Week 4 | 0.6 (0.01) | 0.7 (0.01) | |||||||
| Month 6 | 0.6 (0.01) | 0.6 (0.01) | |||||||
| EQ-5D health status | Week 1 | 54.7 (1.24) | 57.8 (1.26) | 2.94 (1.13 to 4.75) | 5.26 (3.40 to 7.11) | 5.15 (3.16 to 7.14) | < 0.001 | 3.12 (0.50 to 5.74) | 0.019 |
| Week 2 | 57.6 (1.24) | 60.7 (1.25) | |||||||
| Week 4 | 59.9 (1.25) | 63.0 (1.27) | |||||||
| Month 6 | 59.8 (1.31) | 62.9 (1.32) | |||||||
Table 12 describes the analysis of the COPD HRQoL at 6 months post randomisation (as measured by the four domains of the CRQ-SAS). There was no evidence to conclude any differences between arms for these outcomes. The AMDs were all small (ranging from –0.09 for the mastery domain to 0.15 for the emotional function domain), with no CIs containing values that would be considered to be clinically important (the literature suggests a minimally clinically important difference of between 0.4 and 0.5).
| Outcome measure | Time point | Trial arm | AMDa (95% CI) | p-value | ICC | |||
|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||
| n | Mean (SE) | n | Mean (SE) | |||||
| CRQ-SAS dyspnoea domain | Six months post randomisation | 193 | 4.2 (0.10) | 206 | 4.3 (0.10) | 0.06 (–0.20 to 0.33) | 0.636 | 0.01 |
| CRQ-SAS fatigue domain | Six months post randomisation | 215 | 3.5 (0.11) | 221 | 3.6 (0.11) | 0.13 (–0.12 to 0.38) | 0.295 | 0.11 |
| CRQ-SAS emotional function domain | Six months post randomisation | 216 | 4.3 (0.08) | 225 | 4.4 (0.08) | 0.15 (–0.04 to 0.34) | 0.129 | 0.11 |
| CRQ-SAS mastery domain | Six months post randomisation | 214 | 4.3 (0.03) | 221 | 4.2 (0.03) | –0.09 (–0.18 to 0.01) | 0.065 | 0.00 |
Sputum microbiology profile and outcomes at 4 weeks post randomisation
Sputum microbiological outcome analyses included 360 out of 649 samples for the comparison of the proportion with potentially pathogenic bacteria cultured from sputum at 4 weeks (55.5%) and 122 out of 649 samples for the comparison of the percentage of tested antibiotics to which at least one cultured, potentially pathogenic bacteria was resistant (18.8% of randomised participants; this analysis included only those with potentially pathogenic bacteria in sputum at 4 weeks).
At 4 weeks, sputum sample bacteriology data were available for 187 usual-care arm participants and 175 CRP POCT arm participants (57.7% and 53.8% of randomised participants, respectively). Among those with sputum bacteriology data, there was little difference between the arms regarding the microbiological profile. Potential pathogens (either pure or mixed) were found in 67 out of 187 samples from usual-care arm participants and in 62 out of 175 samples from CRP POCT arm participants (35.9% and 35.4%, respectively). Normal respiratory flora alone (i.e. no potential pathogens) was found in 42 out of 187 samples from usual-care arm participants and in 41 out of 175 samples from CRP POCT arm participants (22.5% and 23.4%, respectively).
Both bacteriology and virology data from sputum samples at 4 weeks were available for 178 usual-care arm participants and 167 CRP POCT arm participants (54.9% and 51.4%, respectively). There was little difference between the arms regarding the general microbiological profile of sputum samples. In 154 out of 345 participants, neither potential bacterial nor viral/atypical pathogens were detected (44.6%). Although just over one-fifth of participants provided a sample from which only a potential bacterial pathogen was detected (79/345, 22.9%), the majority of participants provided a sample from which a potential viral/atypical pathogen was detected alone (66/345, 19.1%) or in combination with a potential bacterial pathogen (46/345, 13.3%).
There was no evidence of any differences between the arms in terms of the presence of potentially pathogenic bacteria cultured from sputum at 4 weeks (AOR 0.97, 95% CI 0.63 to 1.50; p = 0.905) nor in the percentage of tested antibiotics to which at least one potentially pathogenic bacteria (cultured from sputum at 4 weeks) was resistant (Table 13).
| Outcome measure | Time point | Trial arm | Adjusted risk (proportion) differencea (95% CI) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | ||||||||||
| n | Mean (SE) number of antibiotics tested | Mean (SE) number of isolates non-susceptible to an antibiotic | Mean (SE) % | n | Mean (SE) number of antibiotics tested | Mean (SE) number of isolates non-susceptible to an antibiotic | Mean (SE) % | ||||
| Percentage of tested antibiotics to which at least one cultured, potentially pathogenic bacteria (from sputum) was resistant | Four weeks post randomisation | 64 | 17.2 (0.99) | 8.0 (0.59) | 47.1 (2.81) | 58 | 17.5 (1.06) | 8.7 (0.76) | 48.1 (3.10) | 0.04 (–0.03 to 0.11) | 0.293 |
Baseline throat swab sample data were available for 309 usual-care arm participants and 320 CRP POCT arm participants (95.4% and 98.8% of randomised participants, respectively). Resistance to antibiotics in commensal bacteria detected from throat swabs, expressed as the percentage of total bacteria load that grew on each antibiotic plate, was similar between arms (Table 14).
| Variable | Trial arm | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | |||||||||||
| n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | n | Mean (SD) | Median (IQR) | Minimum to maximum | |
| Penicillin | 309 | 48.0 (37.92) | 39.5 (10.9 to 87.5) | 0 to 100 | 320 | 50.8 (37.30) | 50.0 (12.3 to 90.0) | 0 to 100 | 629 | 49.4 (37.60) | 45.8 (11.4 to 88.9) | 0 to 100 |
| Levofloxacin | 309 | 16.0 (25.28) | 3.8 (0.4 to 18.1) | 0 to 100 | 320 | 18.3 (28.05) | 3.3 (0.2 to 25.0) | 0 to 100 | 629 | 17.1 (26.73) | 3.6 (0.3 to 20.3) | 0 to 100 |
| Clarithromycin | 309 | 43.6 (36.72) | 34.2 (8.6 to 78.6) | 0 to 100 | 320 | 47.2 (36.74) | 40.0 (11.6 to 85.9) | 0 to 100 | 629 | 45.4 (36.75) | 36.8 (10.6 to 80.8) | 0 to 100 |
| Doxycycline | 309 | 30.9 (33.09) | 16.7 (2.6 to 51.7) | 0 to 100 | 320 | 31.9 (33.36) | 20.3 (2.6 to 55.2) | 0 to 100 | 629 | 31.4 (33.20) | 18.6 (2.6 to 54.1) | 0 to 100 |
| ESBL | 309 | 2.2 (11.62) | 0.0 (0.0 to 0.0) | 0 to 100 | 320 | 5.4 (20.5) | 0.0 (0.0 to 0.0) | 0 to 100 | 629 | 3.8 (16.81) | 0.0 (0.0 to 0.0) | 0 to 100 |
At 4 weeks, throat swab sample data were available for 270 usual-care arm participants and 267 CRP POCT arm participants (83.3% and 83.1% of randomised participants, respectively). Analysis of throat swabs at 4 weeks was based on 537 out of 649 participants (82.7%). There was no evidence of any differences between the arms in the percentage of the total bacterial load from throat swabs that grew on any of the five antibiotic plates (i.e. penicillin, levofloxacin, clarithromycin, doxycycline and extended-spectrum beta-lactamases) at 4 weeks (Table 15).
| Outcome measure | Time point | Trial arm | Adjusted risk (proportion) differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | ||||||
| n | Mean (SE) % | n | Mean (SE) % | ||||
| Penicillin | 4 weeks post randomisation | 270 | 54.0 (2.24) | 267 | 55.8 (2.18) | 0.02 (–0.04 to 0.08) | 0.566 |
| Levofloxacin | 16.5 (1.55) | 16.6 (1.59) | 0.00 (–0.04 to 0.04) | 0.958 | |||
| Clarithromycin | 48.9 (2.31) | 47.4 (2.21) | –0.01 (–0.09 to 0.06) | 0.703 | |||
| Doxycycline | 35.8 (2.19) | 33.3 (2.14) | –0.02 (–0.09 to 0.04) | 0.468 | |||
| ESBL | 4.7 (1.17) | 3.6 (0.96) | –0.01 (–0.04 to 0.02) | 0.512 | |||
Sensitivity analyses
The conclusions drawn on the co-primary analyses were robust to all plausible assumptions made regarding missing outcome data, accounting for the modification of eligibility criteria following the internal pilot and excluding participants on the basis of protocol violations (see Appendix 2, Tables 36–43 and Figure 16).
Modelling medication use over time showed that antibiotic consumption displayed the greatest between-arm difference between the index consultation and 1 week post randomisation (the time during which most antibiotics were prescribed), and consumption decreased over the 4-week follow-up period in both arms (see Appendix 2, Tables 44 and 45 and Figure 17). The use of other COPD treatments decreased at a similar rate in both arms over the 4-week follow-up period (see Appendix 2, Table 46 and Figure 18).
The mean number of primary care consultations during the 6 months following randomisation was 6.3 [standard error (SE) 0.28] for participants allocated to the usual-care arm and 6.6 (SE 0.29) for participants allocated to the CRP POCT arm. The adjusted incidence rate ratio was 1.04 (95% CI 0.92 to 1.18; p = 0.504). The mean number of secondary care consultations during the 6 months following randomisation was 1.7 (SE 0.12) for participants allocated to the usual-care arm and 1.6 (SE 0.11) for participants allocated to the CRP POCT arm. The adjusted incidence rate ratio was 0.96 (95% CI 0.79 to 1.17; p = 0.719). The mean number of primary and secondary care consultations combined was, therefore, a combination of the two separate consultation variables and similarly indicated insufficient evidence of a difference between the arms (see Appendix 2, Figures 19 and 20 and Table 47).
Subgroup analyses
Table 16 provides model estimates for prespecified subgroup analyses conducted for the primary antibiotic consumption outcome.
| Subgroup analysis | Variable | AORa (95% CI) | p-value |
|---|---|---|---|
| COPD severity (GOLD category) (n = 335) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for GOLD I subgroup) | 0.119 |
| CRP POCT | 0.29 (0.06 to 1.38) | ||
| GOLD I | Reference category for COPD severity main effect (i.e. effect of GOLD subgroup for participants allocated to usual-care arm) | 0.816 | |
| GOLD II | 1.39 (0.42 to 4.64) | ||
| GOLD III | 0.91 (0.24 to 3.37) | ||
| GOLD IV | 1.18 (0.19 to 7.13) | ||
| CRP POCT × GOLD I | Reference category for trial arm × COPD severity interaction | 0.146 | |
| CRP POCT × GOLD II | 1.19 (0.21 to 6.73) | ||
| CRP POCT × GOLD III | 4.04 (0.62 to 26.54) | ||
| CRP POCT × GOLD IV | 0.39 (0.03 to 6.07) | ||
| Severity of COPD exacerbation (Anthonisen criteria) (n = 537) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for 1/3 features subgroup) | 0.506 |
| CRP POCT | 1.30 (0.60 to 2.81) | ||
| 1/3 features | Reference category for severity of COPD exacerbation main effect (i.e. effect of severity of COPD exacerbation for participants allocated to usual-care arm) | < 0.001 | |
| 2/3 features | 4.73 (2.13 to 10.48) | ||
| 3/3 features | 9.34 (4.13 to 21.13) | ||
| CRP POCT × 1/3 features | Reference category for trial arm × severity of COPD exacerbation interaction | < 0.001 | |
| CRP POCT × 2/3 features | 0.12 (0.04 to 0.35) | ||
| CRP POCT × 3/3 features | 0.14 (0.05 to 0.39) | ||
| Presence of potentially pathogenic bacteria cultured from sputum at baseline (n = 337) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for no potential pathogenic bacteria subgroup) | < 0.001 |
| CRP POCT | 0.20 (0.09 to 0.43) | ||
| No potential pathogenic bacteria | Reference category for presence of potentially pathogenic bacteria cultured from sputum at baseline main effect (i.e. effect of presence of potential pathogenic bacteria for participants allocated to usual-care arm) | 0.773 | |
| Potential pathogenic bacteria | 1.14 (0.48 to 2.72) | ||
| CRP POCT × no potential pathogenic bacteria | Reference category for trial arm presence of potential pathogenic bacteria interaction | 0.553 | |
| CRP POCT × potential pathogenic bacteria | 1.42 (0.45 to 4.49) |
There was no evidence of a differential intervention effect for participants who provided a sputum sample containing potentially pathogenic bacteria at baseline.
Although there was also insufficient evidence to conclude a differential intervention effect according to COPD severity, there were some descriptive differences between the arms. Antibiotic consumption was consistently different between the arms for participants with GOLD I, GOLD II and GOLD IV COPD. However, 28 out of 40 (70.0%) usual-care arm participants with GOLD III COPD consumed antibiotics for AECOPD, compared with 30 out of 44 (68.2%) CRP POCT arm participants with GOLD III COPD.
There was some evidence to suggest a differential intervention effect for antibiotic consumption by the severity of the COPD exacerbation (according to the Anthonisen criteria). Figure 6 illustrates this differential effect, indicating that antibiotic consumption was similar between the arms for participants who met one out of the three Anthonisen criteria and differences were only observed for participants with two or more of the symptoms.
FIGURE 6.
Differential effect of the intervention on the use of antibiotics during the first 4 weeks. Reproduced from The New England Journal of Medicine. Butler et al. , C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations, 381, 111–20. 56 Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.

There was no evidence of any differential intervention effects for the primary CCQ outcome for any of the other prespecified subgroups (see Appendix 2, Table 48).
Adverse events
Serious adverse events were collected during the first 4 weeks following randomisation. During this period, two SAEs were reported. These were both events where study participants had died. Both participants were in the usual-care arm, and the SAEs were not deemed to be related to the intervention or to trial participation.
Chapter 4 Qualitative process evaluation
Aim
The aim of the qualitative process evaluation was to facilitate the interpretation of outcome results and assist with implementation planning. 57,58
Methods
Setting and participants
Purposive sampling was used, with the aim of capturing a range of views and enabling saturation to be reached in framework analysis. 59 A sampling framework was used, with the aim of interviewing up to 20 patients in the CRP POCT trial arm and up to 20 members of the primary care teams who had carried out the CRP POCT with patients and/or used the CRP POCT result during consultations with patients. A sampling framework was used to ensure that views were captured from patients and members of the primary care teams in each of the regions where the PACE study took place (Wales, Oxford, London and Norfolk), and from approximately equal numbers of patients who had and had not been prescribed antibiotics at their initial consultation.
Procedure
Semistructured interviews were carried out over the telephone by a qualitative researcher. Topic guides (see Report Supplementary Material 2) focused on experiences of the management of AECOPD, the acceptability, implementation and potential mechanisms of the CRP POCT intervention and contextual factors that could influence future implementation.
Analysis
Interviews were audio-recorded and transcribed verbatim. NVivo 11 (QSR International, Warrington, UK) qualitative analysis software was used to assist coding. Data were analysed using framework analysis. This is a systematic approach to a thematic qualitative analysis that allows for easy comparisons between and within cases, facilitates sharing and discussion of data and allows for clear linking/access from developed themes to original data. 60–62 Framework analysis involves five stages: (1) familiarisation with the data, (2) development of a thematic framework, (3) applying thematic codes to all of the data (indexing), (4) retrieving and summarising coded data in a chart and (5) interpreting the data by drawing inferences and pulling together relevant themes. 63 Framework analysis is particularly useful when there are a number of clear research aims that have guided the questions, while allowing new themes to emerge from the data that are relevant to the research question. 60–62 This approach was used for the qualitative data gathered during the main trial phase, as opposed to a more traditional thematic analysis, to facilitate the identification of consensus and discrepancies in the themes emerging in different key groups (e.g. patients prescribed antibiotics vs. those who were not, GPs vs. other members of the primary care team) that would enable us to understand how contextual factors might interact with the acceptability and implementation of the CRP POCT.
During analysis, the qualitative researchers met regularly to discuss the coding, frameworks and themes as these developed. Data analysis was iterative, with the majority of analysis (familiarisation, development of framework and charting) taking place before the trial outcomes were known, in line with the Medical Research Council guidance on process evaluation. 58 The definition of data saturation used in this study was the point at which the ability to obtain additional new information had been attained and when further coding was not feasible. 64 Using the frameworks, the qualitative researchers (RP and HS) assessed whether or not the last five interviews with primary care staff and patients provided new information that would add to the theory being developed with regard to the mechanisms of impact for the intervention. On this basis, the judgement was made that data saturation had been achieved.
Results
Semistructured interviews were carried out with 20 patients and 20 primary care staff between October 2015 and March 2017. The interview participant characteristics are shown in Table 17.
| Participants | N | Characteristics (n) | |
|---|---|---|---|
| Patients | Prescribed antibiotics at index consultation | Not prescribed antibiotics at index consultation | |
| CRP level of < 20 mg/l | 14 | 4 | 10 |
| CRP level of > 20 mg/l | 6 | 5 | 1 |
| Total | 20 | 9 | 11 |
| Primary care staff | Made prescribing decisions guided by CRP POCT result | Carried out the CRP POCT | |
| GPs | 12 | 12 | 7 |
| Nurse practitioners | 5 | 5 | 5 |
| Non-prescribers | Made prescribing decisions guided by CRP POCT result | Carried out the CRP POCT | |
| Practice nurse | 1 | 0 | 1 |
| Research assistant | 1 | 0 | 1 |
| Pharmacist | 1 | 0 | 1 |
| Total | 20 | 17 | 15 |
The key themes identified through the framework analysis are summarised in Table 18.
| Main theme | Subtheme | Key points |
|---|---|---|
| Acceptability of the CRP POCT | General views of the CRP POCT | Patients and primary care staff generally thought that the CRP POCT was a useful addition to the consultation that would help guide antibiotic prescribing decisions. Primary care clinicians emphasised the importance of using the CRP POCT in addition to, not in place of, a thorough clinical assessment |
| Implementation of the CRP POCT | Technical aspects of the test | Primary care staff felt that the need for test cartridges to be refrigerated during storage and returned to room temperature before use, and the need for regular calibration of the machine, were barriers to using the CRP POCT in primary care |
| Time | Primary care staff acknowledged the impact on consultation length that the use of the CRP POCT had, but felt that it was worthwhile | |
| Roll-out in routine practice | Patients and primary care staff were positive about the use of the CRP POCT in routine NHS care for the management of AECOPD. There were differences of opinion about whether the CRP POCT would be used for all patients with AECOPD, or for only those patients for whom there was uncertainty about the need for antibiotics based on clinical examination. The cost of the CRP POCT machine and cartridges was seen as a potential barrier to its use in NHS primary care services | |
| Mechanisms of impact of the CRP POCT | Objective sign of illness | Patients and primary care staff felt that the CRP POCT provided an objective sign of illness that could help guide treatment. Prescribers felt that this increased their confidence in their antibiotic prescribing decisions |
| Patient education tool | Primary care staff felt that the test was useful in educating patients about antibiotic use and antimicrobial resistance. Patients, however, had a limited understanding of these issues | |
| Reinforcing prescribers’ decisions | Primary care staff perceived the CRP POCT result as being useful in reinforcing their decision about antibiotic prescribing when communicating with patients. Patients were generally passive in these consultations and did not report being involved with antibiotic treatment decisions. Continuity of care was viewed to be important by patients and clinicians in determining the quality of communication with regard to antibiotic prescribing decisions for AECOPD | |
| Contextual factors | Attitudes towards antibiotics | Patient attitudes with regard to antibiotic use for AECOPD were varied, but many did not want to take antibiotics for AECOPD unless they were required. Primary care staff were aware of the need to reduce antibiotic use for AECOPD and acknowledged that patient attitudes varied and could influence prescribing decisions |
| COPD routine care pathway | Anxiety around undertreatment through withholding antibiotics (including clinical outcomes and risk of litigation), and the use of ‘rescue packs’ that usually contain steroid and antibiotic medication that patients can start at home, could influence and be influenced by the use of the CRP POCT |
Pseudonyms have been used alongside quotations from patients to protect anonymity. CRP readings and whether or not antibiotics were prescribed during the initial consultation (at the time of recruitment) are provided as the context for patient quotations.
Acceptability
All prescribers felt that the CRP POCT provided a useful piece of information, although several felt that this affected their decision only when there was some uncertainty about whether or not antibiotics were needed. Prescribers emphasised the importance of using clinical findings to guide antibiotic prescribing decisions, and recognised that CRP POCT testing provided additional information and was not a replacement for clinical skills:
I’m not daft enough to think that this test was a right/no thing, a yes/no thing and I know that clinical suspicion and judgment and history is far more important.
General practitioner 2
It’s shown that we’re not always right when we listen in, you know. There is a possibility that this may just be a viral crackle, as opposed to bacterial, but again it’s very difficult without the reassurance of the, the CRP, to let the patient go away.
Nurse practitioner 3
Clinicians emphasised the importance of providing ‘safety-netting’ advice to patients if antibiotics were not prescribed, for example letting them know when they should reconsult or start medication that they had at home to reduce the risk of adverse clinical outcomes:
So other patients were you sort of think, well yes you have an exacerbation of your COPD, but I’m not sure if it’s an infective one. It could be that you are just getting more symptoms. So I would have a discussion with them about starting steroids straight away, but actually delaying antibiotics for maybe 1 or 2 days and obviously giving them lots of safety advice and permission for them to start the antibiotics before then if they get a temperature, if they get increased sputum, and they feel that things are worsening, because obviously these are our frail, often frail and elderly patients who end up bouncing into hospital and that’s the last thing that they want and that we want.
Nurse practitioner 2
One clinician described a case in which a CRP reading had been unexpectedly high and the effect that this had had on his perception of the value of CRP POCT testing:
I told my partner, who had seen this gentleman first this morning and I told him how high the CRP was. He was, he was as shocked as I was. Now it may be that this man has another reason for having a high CRP, you know, there may be something else going on other than infection and we’re going to follow that up. But, but I would say that it would be, you know, the point-of-care testing would be an excellent thing to have in the surgery, because it can, you know, it can give you some information which, which you would not have on a clinical examination.
General practitioner 3
One of the aspects of the CRP POCT that primary care staff liked was that they felt that the test reassured patients, particularly when the CRP reading was low, and that the test demonstrated to the patient that a thorough examination had taken place:
They [patients] feel reassured that no antibiotics have been given and the doctor’s actually checked that this was not necessary before he said ‘no’ to the antibiotics, rather than just saying ‘no you don’t need it’.
General practitioner 1
Patients felt that the CRP POCT was useful in rapidly deducing the severity of illness and/or the need for antibiotics:
I think it’s a great idea to measure really sort of how ill you are and whether you really need more treatment or not.
Participant 1, CRP < 20 mg/l, no antibiotics
Patients were generally confident that the CRP POCT would help their doctors decide whether or not they needed antibiotics, but some felt that the test result was not consistent with their subjective experience:
I wasn’t happy to be honest, because, simply because they said the test that was OK, and an ever slight inflammation which they took because of this blood test she found and she gave me 5 days of the steroids, but after the 5 days I was back to square one.
Participant 2, CRP < 20 mg/l, no antibiotics
Implementation of the C-reactive protein point-of-care test
Technical aspects of the test
Patients did not report any difficulties from a practical or technical perspective with clinicians’ use of the CRP POCT, and they were satisfied with the way in which the CRP POCT was used. Primary care staff reported that they were able to use the CRP POCT with all patients randomised to the intervention arm, and that, in general, it was easy to use.
However, the need to refrigerate cartridges and allow time for them to return to room temperature before use and the need to regularly carry out control testing were both seen as burdensome and potential barriers to implementation. Some primary care staff felt that the CRP POCT would require an operational overhaul to make it simpler and faster to use during routine care:
I think that, you know, in theory that [using the CRP POCT in routine care] could be very good, but the only thing I would say is that because it’s so cumbersome within the consultation clinicians won’t use it, I’m just being honest with you, it takes, you know, 10 minutes to go and sort the machine and calibrate it, you know, how easy is that going to be?
General practitioner 7
The portability of the device was also identified as an important factor in the future development of the CRP POCT equipment:
I think it would be nicer if it was, you know in an ideal world, if it was a hand-held machine, so you could take it with you on a, on a home visit for instance, would be a useful.
General practitioner 5
Time
Patients reported that use of the test was ‘quick’ and that it did not cause any delays. The primary care staff described an ‘investment’ of time required to use the CRP POCT, and that using the machine did make consultations slightly longer. However, they felt that this was worthwhile:
I think where there was a great degree of uncertainty about what the right thing was to do, yeah there are definitely times when you’d be willing to invest that extra bit of time to do it.
General practitioner 4
Views about roll-out in routine practice
Patients and primary care staff had a positive view about whether or not the CRP POCT should be introduced into routine NHS care for patients with AECOPD:
I think it’s an important test and if we, it’s something I’d certainly want to explore in the future after the trial is finished, getting a CRP machine for the practice.
General practitioner 5
I think they’re [GPs] doing their best, and I do think that the pinprick test is absolutely amazing and I should . . . I would like it to be done as a regular thing if you get a flare-up.
Participant 3, CRP < 20 mg/l, not prescribed antibiotics
Primary care staff discussed the advantages of using the test in routine care, mainly in terms of antibiotic stewardship and achieving better consistency in prescribing decisions:
So I think it may help to standardise the treatments that we offer, I definitely think it’s a good idea, I think it’s something that we should be doing more of, because I think we probably would end up prescribing less antibiotics because of it.
Nurse practitioner 2
Patients discussed the benefits of the test mainly in terms of reducing antibiotic use and saving money. Patients felt that the CRP POCT could ‘help’ doctors with their decisions and did not report any anxiety about having the test. Patients also recognised saving money and reducing antibiotic misuse as possible societal benefits of using the CRP POCT:
Helping decide if you need antibiotics or not, and I should imagine it would save money in the long run as well.
Participant 4, CRP < 20 mg/l, no antibiotics
Some clinicians said that they would use the CRP POCT for all patients presenting with AECOPD to ‘increase their data’. This was understood to mean that they would use it as a learning tool to improve their ability to detect patients who are likely to need antibiotic treatment. Others felt that they would not use the CRP POCT for all patients, particularly when they felt confident in making a decision based on their clinical judgement. Primary care staff felt that the CRP POCT would be introduced across a range of conditions in routine practice, rather than being used exclusively for patients with AECOPD:
I can really see that near point testing of CRP is a brilliant idea and I am sure that it is going to expand to lots of other things. I think patients really like it because it makes sense to them.
General practitioner 6
Primary care staff felt that the cost of the CRP POCT machine and cartridges was prohibitive under current funding arrangements, and that it would not be widely adopted unless additional funding was provided to cover these costs.
Mechanisms of impact of the C-reactive protein point-of-care test
Three themes emerged relating to perceptions about how using the CRP POCT might achieve the desired aims: the CRP POCT as an objective sign of illness, the CRP POCT as a patient education tool and the use of the CRP POCT to reinforce the prescriber’s decision.
The C-reactive protein point-of-care test as an objective sign of illness
Prescribers reported that the objectivity of the CRP POCT reading was used to support clinical decision-making and reduce decisional uncertainty:
I think the clinical decision was, was probably there anyway without needing the CRP test, but obviously there are some instances where, you know, if you’re not too sure, then obviously that CRP test could’ve maybe made that difference as to whether you gave the antibiotics or not.
Non-prescriber 2
Being able to share the reading with patients helped to make the treatment decision-making process more transparent, whereas some other aspects of the clinical examinations were more subjective and/or less visible. One of the clinicians described why they felt that patients liked to have objective tests carried out:
Because I think if it’s just you face to face and you have no objective evidence, it’s just your opinion and they sometimes question that.
General practitioner 8
Primary care staff felt that this enhanced their confidence and reassured both prescribers and patients about their decision with regard to antibiotic treatment:
I found writing down ‘CRP normal’, I found that that was a very powerful of reassuring me and the patient actually, it seemed to place a great deal of, you know, faith on, on blood testing.
General practitioner 2
Patients reported being able to effectively communicate the more visible symptoms of their AECOPD to their clinician. However, the less visible subjective signs of their AECOPD were more difficult to detect and communicate. Patients viewed the CRP POCT as a useful way of objectively measuring the severity of their illness:
I thought it [the CRP POCT] was excellent because it was just proving what I already knew if you know what I mean.
Participant 5, CRP 20–40 mg/l, prescribed antibiotics
The C-reactive protein point-of-care test as a patient education tool
Clinicians reported that they used the test result to help explain whether or not they thought that the patient’s AECOPD was likely to be caused by a bacterial infection and whether or not antibiotics were likely to be needed. They felt that patients had greater involvement in the consultation through the discussion of the test outcome, and that it provided them with an opportunity to educate patients about antibiotic overuse:
It allows you to talk a little bit about antibiotics, you can then, you can, we can then add and refer people to an information sheet about the duration of common symptoms for example.
General practitioner 9
From the patient perspective, there was a reasonable level of understanding of the purpose of the CRP POCT, which they typically discussed in discrete terms [presence/absence of infection (or inflammation) and requirement/no requirement of antibiotics]:
Yes, it was to see if I had an infection on my chest and the count of it was I think five, so they decided I didn’t have an infection but that the steroids would help me, which they did.
Participant 3, CRP < 20 mg/l, no antibiotics
Although this particular patient recalled her CRP result, most patients showed little evidence of remembering the actual number generated by the test or awareness of how their result compared with that of other patients. In some cases patients were also uncertain about the type of infection the CRP POCT was detecting:
They need to confirm, which is what I thought this test and that was doing, that it is, it is a proper viral infection.
Participant 6, CRP 20–40 mg/l, prescribed antibiotics
Use of the C-reactive protein point-of-care test to reinforce prescribers’ decisions
Primary care staff felt that the CRP POCT could help make the reasoning behind antibiotic prescribing decisions more transparent and less ‘secretive’ by providing patients with an objective measure. However, there were very few examples of the CRP POCT facilitating shared decision-making between the patient and the prescriber. The CRP POCT reading was generally used to articulate and justify prescribing decisions, rather than to actively involve patients in these decisions:
It gives something to justify to the patient that it’s not just your clinical judgement on the signs and things that you have actually done a test and that has, you know, given even more back up that the fact that you confidently don’t need antibiotics.
General practitioner 6
From the patient perspective, prescribers were viewed very much as being the decision-makers with regard to antibiotic treatment, and the addition of the CRP POCT appeared to reinforce this power dynamic. Patients were generally very accepting of what the doctor or nurse told them with regard to whether or not they needed antibiotics:
I would say my doctors give me sound advice about what to do, because at the end of the day I know they are very busy people and their range of knowledge is quite astounding, and at the end of the day I’m relying on him to give me the correct information to make an educated decision.
Participant 7, CRP < 20 mg/l, prescribed antibiotics
Patients felt that it was right that the doctors made the decisions about antibiotic prescribing:
Well I don’t think it comes under what the patients want, it’s the patient is ill enough to need antibiotics, you know then they should be given, other than that I don’t think they should be given, if the patient isn’t ill enough for them.
Participant 8, CRP < 20 mg/l, no antibiotics
When patients reported that they felt involved in decisions about antibiotics, they described this in terms of their agreeing with the doctor’s decision or because they felt that the doctors had explained their decision to them, rather than being actively involved in the decision-making process per se.
Patients felt that communication was better when clinicians knew them and were familiar with their case, and that a clinician’s knowledge of their patient could influence their decisions about antibiotic prescribing:
I suppose everyone is an individual, their own fears and state of mind have to be considered, whether they’re a hypochondriac or . . . there is a lot to it and I think the doctor would sort of be able to judge his patient if he knew them.
Participant 3, CRP < 20 mg/l, no antibiotics
Primary care staff also felt that their knowledge of the patient and their history was important, and that antibiotics were more likely to be overprescribed when doctors did not know their patients:
And that [the CRP POCT] would be even more important in practices where you’ve got like supermarket medicine going on where you’ve got hundreds of doctors seeing patients once and never seeing them again where there is not the trust that you get with that sort of relationship.
General practitioner 8
Contextual factors
Attitudes towards antibiotics
There was a high level of awareness among primary care staff of the need to reduce antibiotic prescribing and they acknowledged that there was a general tendency to overprescribe antibiotics. Patient anxiety, a strong patient preference for antibiotics and individual circumstances (e.g. the recent death of a spouse) were cited by primary care staff as possible reasons for still prescribing antibiotics despite a low CRP POCT result, indicating that non-medical factors continue to influence antibiotic prescribing even when an objective measure of the likely severity of infection is available.
Primary care staff had mixed views about the level of awareness of the potential risks of overuse of antibiotics among their patients; some felt that patients had a good awareness and were open to reducing their use, whereas other patients were ‘surprised’ to learn of the potential downsides of using antibiotics if they were not required. Clinicians frequently discussed the usefulness of the CRP POCT as an educational tool within this social context and talked about a range of wider interventions and resources that aim to alter antibiotic prescribing practices:
I think there are really good resources out there for GPs who do want to improve their consulting skills and to having a few ideas about how to open this discussion and to reframe the discussion around antibiotics with patients, um so one of the things that we want to do is get everyone to do those tutorials, sort of just have a few extra tools in their, in their doctor’s bag, you know for being able to help that discussion with patients around reducing antibiotic use.
General practitioner 9
Patients’ priority was to resolve their symptoms, and there were mixed feelings about when antibiotics should be prescribed. Mostly, patients recognised how valuable antibiotics were when they were needed, but did not want to take them if they were not required:
It’s not good taking antibiotics just for a minor complaint, you know, you should have it being really bad with your chest before taking antibiotics.
Participant 8, CRP < 20 mg/l, no antibiotics
Some felt that they should take antibiotics for AECOPD regardless (as a precautionary or preventative measure), others felt that people with COPD should take long-term antibiotics in winter and others saw using antibiotics as a last resort. Although the majority of patients reported no adverse effects from previous use of antibiotics, some reported having experienced drawbacks to their use, including allergic reactions, thrush and stomach upsets. A few patients perceived that the antibiotics that they had used had become less effective over time. Some patients reported that they had little or no understanding of antibiotic resistance and the need for antibiotic stewardship, whereas others appreciated the need not to overuse antibiotics:
So of course, they [doctors] are a bit concerned about giving me tablets, if I get too used to them and they don’t work.
Participant 9, CRP < 20 mg/l, no antibiotics
Patients felt that they had little understanding of how their doctor or nurse made decisions about whether or not antibiotics were required. Most often, patients thought that doctors predominantly based their decision on chest sounds:
Well in general they you know listen to my chest and do my blood pressure and that sort of thing, so they usually just do the chest, and decide then whether they think I’m rattling enough to need antibiotics or not.
Participant 1, CRP < 20 mg/l, no antibiotics
Patients discussed antibiotic resistance in terms of their body becoming resistant or ‘immune’ to the medication, blood becoming resistant or the immune system becoming damaged. A small minority of patients discussed the risk of overuse of antibiotics in terms of the bacteria becoming ‘immune’ to the medication. However, several patients reported that they were not aware of any disadvantages to antibiotic use:
There’s no drawbacks at all [to antibiotic use].
Participant 10, CRP < 20 mg/l, no antibiotics
Chronic obstructive pulmonary disease routine care pathway
Clinicians felt that the risk of undertreatment was a driver of antibiotic prescribing for AECOPD:
There’s so much pressure not to refer patients to hospital, so if you, the view is, if you treat them early, you know, when their symptoms are relatively mild, maybe we’ll be able to stop someone going to hospital unnecessarily.
General practitioner 9
Fear of litigation and how the use of the CRP can help provide objective evidence was also discussed:
I can only speak for myself, but every patient I see, when I’m writing down, I’m thinking that somebody’s going to be suing me as a result of it, which is very sad but it’s just the way the world’s going, and I think every GP is probably very similar, and I know that if I write down ‘CRP less than 5’ then anyone taking me to court over that is going to have one hell of a hard time of it to prove that that patient was ill at that point.
General practitioner 2
Patients in some practices were issued with ‘rescue packs’ containing antibiotics and oral steroids that they could use at home to manage AECOPDs. Patients had mixed views about the use of rescue packs, with some preferring to see a doctor before starting antibiotics and others preferring to start their medication without the need to see a doctor:
I’d just rather skip the appointment because you know if you’re bad or not don’t you? You know what I mean, so if, if I knew I was really ill and I had them in the drawer then I would just take them because I knows what it is now.
Participant 11, CRP > 40 mg/l, prescribed antibiotics
Clinicians also had some ambivalence about rescue packs, expressing the view that these were appropriate only for patients who were able to recognise the symptoms that indicated that antibiotics were required. They felt that there were advantages to rescue packs in terms of encouraging self-management and timely treatment:
It [having a rescue pack] improves their confidence, I think in theory it could stop people exacerbating so badly that they end up being admitted to secondary care.
General practitioner 5
Not all practices issued rescue packs to patients, and some did this only when it had been advised by secondary care. The potential for overusing antibiotics and passing on difficult decisions about when to start treatment to the patients were seen as possible disadvantages of using rescue packs:
Some patients might find it quite difficult to understand exactly when a leaflet [about use of the rescue pack] is given to them or even when they’re explained about the leaflet and when to use the antibiotics, we are leaving the patients to make their own clinical decisions.
General practitioner 1
One clinician suggested that a CRP test that could be easily administered by patients at home could help patients make decisions about when they should start their antibiotics in the future.
Qualitative evaluation summary
Patients and clinicians participating in the PACE trial’s qualitative evaluation perceived the CRP POCT as a useful tool to help guide the management of AECOPD in primary care. They described it as providing an objective sign of the severity of illness, increasing confidence and providing reassurance, being a useful tool for patient education, and being helpful in reinforcing the prescriber’s decision when communicating with patients. Previous qualitative studies in adults with acute cough have also indicated that CRP POCTs are an acceptable intervention from the perspectives of patients and clinicians, with the main perceived mechanisms of the intervention being reducing clinical uncertainty, increasing prescribing confidence and enhancing communication,65–67 and our findings were highly consistent with this.
Although CRP POCTs are widely used in a number of European countries for managing LRTIs, they have not yet become routinely used in the NHS. 68 Concerns about funding for the CRP POCT machine and cartridges, and the time needed to use the CRP POCT, were identified as potential barriers to adoption in NHS primary care services. Similar issues have been identified with regard to the routine use of CRP POCTs in the management of LRTIs in NHS primary care services. 68 The primary care staff interviewed in the current study generally felt that the additional time required to complete the test was worth the investment, and felt that the test may help practitioners be less risk-averse when deciding to manage an AECOPD without antibiotics. However, they felt that the costs of the CRP POCT testing equipment and cartridges would be prohibitive under current funding arrangements for NHS primary care services, and this would need to be addressed before it was implemented in routine practice.
There is a lack of evidence for risk stratification based on current clinical assessments in guiding antibiotic prescribing for AECOPD. 69 Our qualitative findings indicated that, in line with this, prescribers felt that the CRP POCT provided useful information in addition to their usual clinical assessment to guide their antibiotic prescribing decisions for AECOPD. They emphasised that the CRP POCT result would not replace other clinical factors in their decision-making but that, rather, it added a piece of objective information for them to consider. Clinicians reported that they would be most likely to use the CRP POCT in routine care in cases when there was clinical uncertainty, which is consistent with the views of clinicians on when the test would be used in the management of LRTIs. 67 In a study67 of primary care clinicians’ views on the potential use of CRP POCTs for LRTIs, clinicians discussed difficulties with interpreting results or being distracted from clinical reasoning as potential disadvantages of the test. The clinicians interviewed in the current study who had used the CRP POCT in consultations did not report experiencing any difficulties in interpreting the test or any negative impact on their clinical judgement.
Contextual factors can influence, and be influenced by, the introduction of an intervention aiming to alter antibiotic consumption for AECOPD, including patient attitudes towards antibiotics and clinician perception of patient preferences,70,71 and broader issues in relation to the care pathways for COPD, such as continuity of care, patient engagement, resources and communication. 72,73 The current study indicated that, in addition to informing clinicians’ decisions about the need for antibiotics, the CRP POCT was used as a tool to educate patients, reinforce clinicians’ antibiotic prescribing decisions and facilitate communication. Patients, however, reported that they were not engaging with the decisions being made about antibiotic treatment for their AECOPD. Although they did not report being dissatisfied with this consultation dynamic, their lack of involvement was reflected in their low levels of understanding of the potential disadvantages of using antibiotics and of the purpose of the CRP POCT.
Primary care clinicians’ antibiotic prescribing behaviour for acute cough in adults is influenced by their perceptions of patient expectations, but their perceptions do not always match with patient views. 71 Patients report lower levels of satisfaction with their consultation if they have an expectation of receiving antibiotics that is not met. 71 Being able to effectively elicit patient views is, therefore, an important skill for clinicians managing acute cough. 71 A study33 of the use of CRP POCT to guide antibiotic prescribing for acute respiratory tract infections in primary care indicated that both the CRP POCT and clinician training in communication skills were effective in reducing antibiotic prescribing, but the greatest reduction was seen when the CRP POCT and communication skills training interventions were combined. Patients with acute cough whose clinician had received communication skills training or had used a CRP POCT were satisfied with their consultation even when they had not received an antibiotic. 66 Patients felt positive about the clinician communication skills training, reporting that they felt that their concerns had been understood. 66 Training for prescribers in integrating the CRP POCT into consultations for AECOPD in a patient-centred way may, therefore, enhance its effects.
Patient education is likely to be an important aspect of the successful implementation of the CRP POCT, to ensure that patients understand why the test is needed, have confidence in its use and are sufficiently informed about their treatment options so that they can become more engaged with the decisions that are made about their health care. This is particularly relevant to the population of patients with COPD, as older adults and those from socioeconomically deprived backgrounds are more often affected by this disease,74 and low health literacy is more prevalent in these demographic groups. 75,76 Ensuring that there is continuity of care and that a consistent approach to antibiotic prescribing for AECOPD is adopted could also increase the quality of communication about the appropriate use of antibiotics in the management of AECOPD and facilitate the implementation of the CRP POCT in routine care.
Conclusions
Patients and clinicians perceived the addition of the CRP POCT to consultations for AECOPD as acceptable and useful. Implementation planning should include consideration of funding arrangements, training for clinicians, patient education, provision of continuity of care and promotion of a consistent approach to prescribing as ways of facilitating implementation and enhancing the effects of the CRP POCT intervention.
Chapter 5 Health economics
Introduction
This chapter presents the methods and results of the economic evaluation conducted alongside the PACE trial. A within-trial health economic analysis was undertaken from a health service perspective (the UK NHS), reflecting the trial follow-up period of 6 months and addressing the secondary trial objective of assessing the costs and cost-effectiveness of using CRP POCT to guide antibiotic prescribing for AECOPD in primary care. Wider costs resulting from patient absences from work were also considered but were reported separately. The health economic evaluation included a cost-effectiveness analysis, a cost–utility analysis (CUA) and a cost–consequences analysis. A trial-based budget impact analysis was undertaken to estimate the likely impact of using CRP POCT in the management of antibiotic prescribing for AECOPD in primary care on NHS budgets.
Methods
Before the analysis commenced, a health economic analysis plan was produced, which was reviewed by the trial team and incorporated in the statistical analysis plan. The health economic team followed this analysis plan during the conduct of the economic evaluation without deviation.
Costs included in the health economic analysis
The health economic analysis considered the following:
-
cost of the CRP POCT implementation in primary care (including staff time for training, testing and travel, and costs of CRP POCT kits and consumables)
-
costs of medications prescribed (including antibiotics, oral corticosteroids and inhaled medications)
-
cost of health-care resource use (primary and secondary care).
As the analyses were undertaken from a NHS perspective, the cost associated with productivity loss due to time off work was assessed but not included in the formal evaluation.
Costs are based on all available cases (for the most complete overview), the MITT population for antibiotic consumption and EQ-5D (for the cost-effectiveness analysis and CUA base cases) and the ITT population (using multiple imputation) for the secondary analysis. All costs are expressed in 2015/16 Great British pounds, inflated and converted appropriately where required. 77 Neither costs nor outcomes were discounted, as the duration of the trial follow-up period did not exceed 1 year.
C-reactive protein point-of-care test costs
Resource use resulting from CRP POCT implementation (including materials, consumables, staff time and training) was estimated through interviews and direct communications with general practice staff involved in the trial, the CRP POCT manufacturer and the trial team, and by using data collected during the trial (e.g. frequency of repeat testing). Unit costs of materials and consumables were obtained directly from the manufacturer and online wholesale catalogues. Staff costs were estimated using published unit costs. 78 Any assumptions were based on clinical expert opinion from the PACE trial team and the impact on the results was tested as part of the sensitivity analysis.
The number of CRP tests performed during the trial was recorded for every participant at baseline and within the first 4 weeks of follow-up.
Cost of medication prescribed for the treatment of acute exacerbations of chronic obstructive pulmonary disease
New prescriptions of medications for treating COPD were recorded routinely during the trial immediately following randomisation and for the 6-month review period. This comprised prescriptions for antibiotics, oral steroids and inhaled medication (including short- and long-acting beta-2 agonists, short- and long-acting muscarinic agonists, inhaled corticosteroids and combination inhalers). Unit costs were taken from the Monthly Index of Medical Specialities79 and the British National Formulary. 80 All medication unit costs used in the costing of the economic evaluation can be found in Tables 49 and 50 in Appendix 3.
Prescriptions were costed individually based on prescribed dose, treatment duration and number of doses per day. For antibiotics and oral steroids, in cases when information on prescribed dose, duration or frequency was missing, the most common prescription of the specific medication was assumed. In cases when no antibiotic or steroid name was reported and when it could not be extrapolated from other information (e.g. dose or duration), the most commonly prescribed antibiotic (500 mg of amoxicillin, 21 tablets) and oral steroid (5 mg of prednisolone, 56 tablets) were assumed. For inhaled medication, it was assumed that, if the medication was increased at the index consultation, a new prescription was issued at the same time. If no information on the type of inhaled medication was available, it was assumed that salbutamol metered dose inhaler (100 mg) was given, using the mean cost of all of its licensed formulations.
The costs of antibiotics, oral steroids and inhaled medications were calculated at the initial consultation, for the 6-month review period and as a total (initial consultation and 6 months combined). It was assumed, for the purpose of the cost-effectiveness analysis, that one-sixth of all medication prescriptions recorded in the 6-month review period would have occurred in the first 4 weeks. This assumption was tested in a sensitivity analysis.
Cost of health-care resource use
Health-care resource use (including primary care consultations, A&E visits, outpatient appointments and inpatient stays) was collected using data from an adapted Client Service Receipt Inventory (CSRI), integrated in the 4-week CRF and 6-month note review, to assess the differences in the profile of health-care use as a result of the intervention compared with control. If one or more items in the health-care consultations part of the CRF (i.e. the CSRI) were completed (values of ≥ 0), the CSRI was assumed to have been fully completed and any missing items were imputed with zeros. If the CRF was marked as ‘not done’ or was otherwise fully incomplete, data were considered missing and costs were based on all available cases for the base case, using multiple imputation to account for missing data in the secondary analyses.
Costs were assigned using published unit costs. 78,81 Outpatient visits and inpatient stays were costed individually according to the reasons for health-care contact, length of stay and specialty/department visited recorded in the trial CRFs. All unit costs used can be found in Table 51 in Appendix 3. The health-care costs in both trial arms were summated and the mean difference per patient in costs (including 95% CIs) was calculated.
Every patient had one initial index consultation (baseline appointment) during which they were randomised, which was excluded from the 4-week and 6-month follow-up health-care costs but included in the final health economic analysis.
Cost of work lost as a result of acute exacerbations of chronic obstructive pulmonary disease
Participants provided the number of days they had taken off paid employment as a result of AECOPD at the 4-week follow-up time point. Using the Human Capital Approach,82 days taken off work were costed using a cost of £97.77 per day based on average UK weekly gross earnings for the whole economy during the trial period. 83
Cost-effectiveness analysis
The cost-effectiveness analysis expressed the incremental cost required to reduce the number of people consuming at least one dose of antibiotics by 1%. The base-case analysis was based on the MITT population for the co-primary outcome of antibiotic consumption at 4 weeks. The total costs at 4 weeks (including costs at the initial consultation) for the MITT population only were considered. The results of the comparative analysis of incremental costs and effects can be summarised in terms of incremental cost-effectiveness ratios (ICERs). An ICER can be represented as:
where C1 and E1 are the costs and effects of the intervention arm and C0 and E0 are the costs and effects of the control arm, with ΔC and ΔE the incremental costs and effects of the intervention compared with control.
The ICER is reported to determine the cost-effectiveness of the intervention compared with competing alternatives and to aid decision-making. No established willingness-to-pay threshold for the cost-effectiveness of reducing antibiotic consumption is available. Furthermore, cost-effectiveness is a spectrum rather than a dichotomy, with the maximum threshold increasing depending on the circumstances. The reported ICERs from our analysis are presented to assist the decision-making process and are not an absolute statement on whether or not the intervention can be deemed cost-effective.
Cost–utility analysis
A within-trial CUA was undertaken to assess the incremental costs per quality-adjusted life-year (QALY) gained as a result of using CRP POCT compared with control at 6 months. Individual-level utility scores were obtained at each assessment point using the EQ-5D-5L questionnaire mapped back to the UK EQ-5D-3L valuation set, as currently recommended,84 and summated for the CRP POCT and control arms. During the internal pilot, the EQ-5D-3L version of the questionnaire was used and health states were transformed using UK EQ-5D-3L valuation sets. QALYs for each patient were calculated based on the utility scores at baseline and 6 months using the area under the curve approach and linear interpolation. The total costs at 6 months (including baseline) for the EQ-5D MITT population were used to calculate the incremental cost per QALY gained. The EQ-5D MITT population comprised all patients who had a complete baseline questionnaire and any one complete follow-up questionnaire.
Quality-adjusted life-years incorporate quantity of life (additional life-years) and quality of life in one measure. Thus, by dividing the difference in costs by the difference in QALYs, the cost per QALY can be calculated for each comparison. Generally, NICE considers an intervention cost-effective if one of the following applies:
-
The intervention is less costly and more clinically effective than all other relevant alternatives. In this case, no ICER is calculated as the strategy in question dominates the alternatives.
-
The intervention has an ICER of < £20,000 per QALY compared with the next best alternative. This means that an investment of up to £20,000 to achieve an additional QALY is considered cost-effective.
The ICER resulting from the CUA was compared with the willingness-to-pay threshold of £20,000 per QALY gained as standardised by NICE. No conditions for non-inferiority were applied in this analysis. The results are reported as ICERs showing the extra cost of producing one extra QALY or the extra savings achieved by sacrificing one additional QALY.
Sensitivity analyses
Sensitivity analyses were undertaken to test the robustness of the results of both cost-effectiveness analysis and CUA, considering the uncertainty in input parameters, such as costs and outcomes, and in different scenarios. Deterministic, univariate sensitivity analyses changed test, medication and health-care costs and outcomes individually within plausible ranges. Scenario analyses tested different assumptions and recalculated the ICER using COPD-related costs only. Furthermore, a probabilistic sensitivity analysis used non-parametric bootstrapping to address joint parameter uncertainty and assess the impact on the ICER during 1000 simulations that were undertaken using random sampling of the distributions of costs and outcomes, with results presented on cost-effectiveness planes and as cost-effectiveness acceptability curves. The cost-effectiveness plane is a scatterplot of the point estimates obtained as a result of the 1000 simulations depicted in four quadrants, representing the probability of the intervention being more or less costly and more or less effective than the control. A cost-effectiveness acceptability curve is a curve that describes the probability of the intervention being cost-effective at different willingness-to-pay thresholds based on the probabilistic sensitivity analysis.
We also undertook a secondary analysis in the ITT population using multiple imputation to account for missing data. The problems concerning missing data are particularly relevant to health economic analysis, as the main outcomes are cumulative measures collected during the trial. Missing items relating to health-care service usage may underestimate the total costs, and missing outcome data may be correlated with effects, as those individuals without information may be systematically different from those for whom all information is observed. 85 As such, using only complete-case assessments and available cases analysis could result in meaningful data being excluded. 85 We therefore adopted a multiple imputation approach as the appropriate technique to provide a comprehensive investigation of the impact of missing data on the estimations of cost-effectiveness. 86 Multiple imputation was performed using chained equations and predictive mean matching on all variables except categorical (yes/no) parameters, where multiple imputation using logistic regression was deemed appropriate given values of 0 and 1 only. Predictive mean matching for continuous variables was preferred to other methods as imputed values are taken from the set of observed values, avoiding any values outside plausible ranges (e.g. utility values of > 1 and costs of < £0). A total of 20 imputations were added. The results were combined using Rubin’s rules. 87
Cost–consequences analysis
A cost–consequences analysis presents all relevant primary and secondary outcomes alongside the costs in tabular form (without combining them into ICERs) to leave decision-makers the option to form their own view of relative importance.
Budget impact analysis
A trial-based budget impact analysis was undertaken and extrapolated to the UK population to estimate the likely impact of using CRP POCT on NHS budgets through implementation costs and changes in health-care usage. The budget impact analysis was informed by trial data supplemented by the best available published evidence when required and conducted according to recommended good practice. 88
A simple budget impact model was constructed in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA). The number of eligible patients was calculated using prevalence and yearly incidence figures and accounting for age-specific and COPD mortality. Prevalence, incidence and mortality data for COPD in the UK were obtained from published sources89 and age-specific general population mortality was derived from statistics available on deaths in England and Wales in 2016. 90 It is estimated that, since 2012,89 the yearly incidence of COPD in the UK has been steady, at around 115,000 new diagnoses, and the budget impact analysis, therefore, assumes the incidence to be constant over the first 5 years after CRP testing is introduced in primary care. It also assumes that all diagnosed patients experience one AECOPD per year and are eligible for CRP testing. The expected uptake rate is assumed to be 10% in year 1, rising to 50% depending on the installation of the required analysers in general practices. The costs of testing and subsequent health-care resource use were taken from the trial costings.
Sensitivity analyses were undertaken to estimate the range of a potential budget impact considering parameter uncertainty within plausible ranges.
Results
Four participants were excluded from all analyses (three withdrew consent to use their data and one was randomised in error).
C-reactive protein point-of-care test costs
The total cost of a CRP POCT was estimated to be £11.31 per test (Table 19). This cost comprises £5.40 for materials and consumables (including quality control tests), £0.13 for capital expenditure, £0.10 for staff training and £5.38 for staff required for sample processing, testing and reporting. During the trial baseline CRP testing, 10 out of 333 tests were invalid or resulted in an error message, requiring repeat testing. Therefore, a cost of £0.29 was added to each test to account for material and staff costs required for repeat testing.
| Cost component | List price (£) | Resource use | Cost per test (£) | Notes |
|---|---|---|---|---|
| Material costs | ||||
| Alere Afinion CRP test cartridgea | 58.00 | 1 | 3.87 | No discounts given, 15 cartridges per pack |
| Alcohol wipeb | 2.00 | 1 | 0.02 | 100 wipes per pack |
| Prick needlec | 7.50 | 1 | 0.08 | 100 lancets per pack |
| Gauze pads/swabsd | 15.30 | 1 | 0.06 | 250 pads per pack |
| Glovese | 9.19 | 2 | 0.18 | 100 per pack |
| Quality control testing kitsa | 36.00 | 0.13 | 1.20 | Four per pack. One high level and one low level control required per 15 tests |
| Repeat samples because of invalid resultsf | 58.00 | 0.03 | 0.13 | 10/333 tests at baseline gave an error (3.0%) |
| Capital costs | ||||
| Alere Afinion AS100 Analyzera | 1200.00 | 0.00011 | 0.13 | 7-year life span (five samples per day assumed, 260 working days per year) = 9100 samples |
| Maintenance contracta | None | 0.00 | No maintenance required | |
| Set-up and training | ||||
| Initial set-up, calibration and configurationa | 0.00 | Done by supplier before training | ||
| Travel and trainer cost | 0.00 | Done at GP surgery, included in purchase price | ||
| GP opportunity costg | 134.00/hour | 45 minutes | 100.50 | GMS activity including qualifications and excluding direct care staff |
| Nurse opportunity costg | 43.00/hour | 45 minutes | 32.25 | Including qualifications |
| Testing | ||||
| Analyser switch-on and self-testa,g | 43.00/hour | 1 minute | 0.14 | Nurse assumed, 1 minute needed, five tests per day assumed |
| Sample processing (including taking sample, labelling, preparation and test start) by GPg | 199.00/hour | 1.15 minutes | 3.81 | Patient contact excluding direct care staff, 1 minute and 9 seconds average processing time,a assumed 70% of cases |
| Sample processing (including taking sample, labelling, preparation and test start) by nurse | 43.00/hour | 1.15 minutes | 0.82 | Including qualifications, 1 minute and 9 seconds average processing time,a assumed 30% of cases |
| Results check and reporting (GP) | 199.00/hour | 0.7 minutes | 2.32 | |
| Dealing with testing errors and retesting | 199.00/hour | 0.03 | 0.16 | 10/333 tests at baseline required retesting |
| Total test cost | 11.31 | |||
Three participants in the control arm received CRP tests at baseline in error and eight participants in the CRP POCT arm did not have test results as a result of machine errors. Four participants in the control arm self-reported a CRP test at baseline, with no other records to clarify.
Considering all available cases (n = 649), the total cost of CRP testing at baseline in the CRP POCT arm (n = 325) was £3697.54, with a mean of £11.38 per participant (SD £1.25 per participant). Assuming that the four unresolved patients did not receive a CRP test, the total cost in the control arm (n = 324) was £33.92, with a mean of £0.10 per patient (SD £1.09 per patient), increasing to £79.16 (mean £0.24 per patient, SD £1.64 per patient) if it is assumed that the four unresolved cases did receive a CRP test.
In the first 4 weeks after randomisation, 18 patients in the CRP POCT arm received 20 CRP tests. This increases the total cost of CRP testing at the 4-week follow-up point (including baseline) to £3923.69 in the CRP POCT arm (mean cost per patient £12.08, SD £3.23).
Cost of medication prescribed for treatment of acute exacerbations of chronic obstructive pulmonary disease
A breakdown summary of the mean costs for all recorded medication prescriptions is shown in Table 20. The total medication costs for the CRP POCT and control arms are presented in Appendix 3, Table 52.
| Medication | Trial arm, mean (SD) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| CRP POCT (n = 325) | Usual care (n = 324) | |||
| Antibiotics | ||||
| Cost at index consultation (£), per patient | 0.63 (0.69) | 0.91 (0.72) | –0.28 (–0.39 to –0.17) | 0.029 |
| Cost at 6-month review (£), per patient | 2.20 (4.69) | 2.05 (2.78) | 0.15 (–0.46 to 0.77) | 0.251 |
| Oral steroids | ||||
| Cost at index consultation (£), per patient | 0.75 (0.77) | 0.74 (0.73) | 0.02 (–0.10 to 0.13) | 0.862 |
| Cost at 6-month review (£), per patient | 1.10 (1.92) | 1.26 (2.88) | –0.16 (–0.55 to 0.23) | 0.491 |
| Inhaled medications | ||||
| Cost at index consultation (£), per patient | 3.14 (8.82) | 3.10 (8.41) | 0.05 (–1.33 to 1.42) | 0.889 |
| Cost at 6-month review (£), per patient | 10.05 (18.65) | 7.74 (16.20) | 2.30 (–0.49 to 5.09) | 0.109 |
| Total medication cost | ||||
| Cost at index consultation (£), per patient | 4.51 (8.74) | 4.70 (8.39) | –0.21 (–1.57 to 1.16) | 0.905 |
| Cost at 6-month review (£), per patient | 13.35 (19.55) | 11.05 (17.00) | 2.30 (–0.62 to 5.22) | 0.341 |
Antibiotics
Considering all available cases (n = 649), 155 patients in the CRP POCT arm (47.7%) were prescribed antibiotics at their baseline general practitioner (GP) consultation following CRP testing, compared with 225 patients in the control arm (69.4%). This resulted in a cost saving of £0.28 (95% CI –£0.39 to –£0.17) per patient at baseline (see Table 20). The most commonly prescribed antibiotic was amoxicillin (59.5%), followed by doxycycline (24.0%), clarithromycin (12.8%), co-amoxiclav (1.9%), erythromycin (1.3%) and cefalexin (0.5%).
In the 6-month review period (n = 606), 173 patients in the CRP POCT arm (56.9% of 304 patients) were issued 403 antibiotic prescriptions, and 191 people in the control arm (63.2% of 302 patients) had received 404 antibiotic prescriptions. Of these, 80.6% were attributed to COPD and lung conditions (including rescue packs) in the CRP POCT arm and 79.7% were attributed to COPD and lung conditions (including rescue packs) in the control arm. Other most common indications for which antibiotics were issued were urinary tract infections, skin infections, wound infections and infected ulcers. In total, 12.8% fewer patients received any antibiotic prescriptions in the 6 months following the baseline GP consultation in the CRP POCT arm, the patients who were issued prescriptions had, on average, 0.21 prescriptions more than patients in the control arm (2.33 vs. 2.12). Furthermore, the prescriptions issued were for more expensive antibiotic formulations (mean of £1.66 per prescription, compared with £1.53 in the control arm), resulting in a £0.15 increase in cost of antibiotics per patient in the CRP POCT arm (p = 0.251).
Overall, when initial consultation and 6-month review period prescriptions were combined, the mean cost of antibiotics in the CRP POCT arm was reduced by £0.13 (95% CI –£0.72 to £0.46) per patient (p = 0.135).
Oral corticosteroids
All oral corticosteroids prescribed at baseline were prednisolone formulations. In the CRP POCT arm, 178 patients (54.9% of 324) received oral steroids compared with 179 patients (55.6% of 322) in the control arm. Patients who were issued prescriptions for oral steroids received, on average, 1.00 prescription in the control arm and 1.02 prescriptions in the CRP POCT arm, resulting in a marginal cost increase of £0.02 per patient in the CRP POCT group (see Table 20).
During the 6-month follow-up period, 130 patients in the CRP POCT arm (42.8% of 304) received 279 prescriptions for oral steroids, of which 91.4% were issued for COPD and related respiratory disease. In the control arm, 146 patients (48.3% of 302) were issued 271 steroid prescriptions, of which 95.9% were related to COPD and respiratory conditions. Although 5.5% fewer people received any oral steroid prescriptions in the CRP POCT arm, they were issued 0.29 more prescriptions for oral steroids per patient (2.15 vs. 1.86 in the control arm). However, the average cost of oral steroid prescriptions per patient was lower in the CRP POCT arm (£1.20 vs. £1.40 in the control arm), partially driven by the higher cost of dexamethasone for one patient in the control arm. The cost of oral steroids during the 6-month review period was, therefore, £0.16 per patient (95% CI –£0.55 to £0.23; p = 0.491) lower in the CRP POCT arm.
Overall, when baseline and 6-month review period prescriptions were combined, oral steroid cost was reduced by £0.12 (95% CI –£0.52 to £0.28; p = 0.482) per patient receiving the intervention.
Inhaled medications
In the CRP POCT arm, 71 patients (21.9% of 324) were prescribed new inhaled medications or had their existing prescription increased at baseline, resulting in 87 prescriptions (1.23 per patient receiving any inhaled medication prescriptions). This was similar to the 73 patients (22.7% of 321) in the control arm who were issued 86 prescriptions for inhaled medications (1.18 per patient receiving any inhaled medication prescriptions). The marginally higher cost of £0.05 per patient (see Table 20) reflects the cost of one additional prescription in the CRP POCT arm.
During the 6-month follow-up period, 93 CRP POCT arm patients (30.6% of 304) received 124 prescriptions for inhaled medications (1.33 per patient receiving any prescriptions). This was 5.4% more than in the control arm, in which 76 patients (25.2% of 302) were issued 94 inhaler prescriptions (1.24 per patient receiving a prescription). Owing to the increased use of inhalers in the CRP POCT arm and the higher acquisition cost (on average approximately £22 per prescription), the total cost of inhaled medications during the 6-month review period was £2.30 higher per patient (95% CI –£0.49 to £5.09 per patient).
Overall, when baseline and 6-month review period prescriptions were combined, inhaler cost was £2.21 (95% CI –£0.75 to £5.18; p = 0.228) per patient higher in the CRP POCT arm than in the control arm.
Cost of health-care resource use
The costs reported represent the mean cost per patient. The total health-care costs for CRP POCT and control arms are summarised in Appendix 3, Tables 53 and 54.
Primary care costs
In the first 4 weeks following randomisation, 27.6% of people in the CRP POCT arm (76/275 available cases) saw their GP at the surgery for any reason an average of 1.45 times. This represents a difference of 7.7% compared with the control arm, in which 35.4% of patients (99/280 available cases) had an average of 1.42 GP visits at their surgery, and results in a cost difference for GP visits at the surgery in the CRP POCT arm of –£3.73 per person (95% CI –£8.38 to –£0.92 per person; p = 0.07).
Patients in the CRP POCT arm accrued slightly higher total costs for nurse consultations and other health-care contacts (NHS Direct) than those in the control arm. However, this was more than offset by savings from fewer GP contacts, with an overall cost difference of –£4.58 (95% CI –£13.55 to £4.39; p = 0.132) per patient for all primary care use in the CRP POCT arm, which was mainly driven by savings in GP visits at the surgery and GP telephone consultations (Table 21).
| Health-care resource | Trial arm | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| CRP POCT (n = 275) | Usual care (n = 280) | |||
| Primary care: cost per patient in 4-week follow-up period | ||||
| GP visits at the surgery (£) (SD) | 14.40 (26.64) | 18.13 (29.08) | –3.73 (–8.38 to –0.92) | 0.070 |
| Nurse visits at the surgery (£) (SD) | 2.79 (7.43) | 2.47 (5.55) | 0.32 (–0.77 to 1.41) | 0.950 |
| GP visits at home (£) (SD) | 1.24 (10.20) | 1.52 (13.38) | –0.28 (–2.27 to 1.70) | 0.984 |
| Nurse visits at home (£) (SD) | 0.09 (1.43) | 0.17 (2.01) | –0.08 (–0.37 to 0.21) | 0.574 |
| GP telephone consultations (£) (SD) | 2.98 (10.55) | 4.01 (14.19) | –1.02 (–3.11 to 1.07) | 0.579 |
| Nurse telephone consultations (£) (SD) | 0.22 (1.20) | 0.19 (1.26) | 0.03 (–0.18 to 0.23) | 0.592 |
| Other health-care contacts (£) (SD) | 1.86 (26.47) | 1.67 (26.20) | 0.19 (–4.20 to 4.58) | 0.303 |
| Total cost of primary care use (£) per patient (SD) | 23.57 (52.34) | 28.16 (55.16) | –4.58 (–13.55 to 4.39) | 0.132 |
| Primary care: cost per patient in 6-month review period | ||||
| GP visits at the surgery (£), (SD) | 117.59 (102.44) | 114.32 (93.48) | 3.27 (–12.37 to 18.92) | 0.969 |
| Nurse visits at the surgery (£), (SD) | 18.04 (24.84) | 16.72 (29.44) | 1.32 (–3.02 to 5.67) | 0.120 |
| GP visits at home (£), (SD) | 6.99 (42.83) | 9.57 (40.45) | –2.58 (–9.23 to 4.07) | 0.364 |
| Nurse visits at home (£), (SD) | 1.80 (20.51) | 1.42 (13.76) | 0.38 (–2.41 to 3.17) | 0.521 |
| GP telephone consultations (£), (SD) | 27.90 (51.32) | 25.28 (46.39) | 2.62 (–5.18 to 10.43) | 0.540 |
| Nurse telephone consultations (£), (SD) | 1.39 (3.66) | 1.31 (4.16) | 0.08 (–0.55 to 0.70) | 0.818 |
| Other health-care contacts (£), (SD) | 0.28 (1.78) | 0.22 (1.80) | 0.06 (–0.22 to 0.35) | 0.413 |
| Total cost of primary care use (£) per patient (SD) | 174.00 (153.48) | 168.83 (138.20) | 5.16 (–18.14 to 28.47) | 0.882 |
During the 6-month review period (which includes the first 4 weeks but excludes the baseline), 259 people saw their GP at the surgery for any reason in both arms (85.7% of available cases in the control arm and 85.2% of available cases in the CRP POCT arm). Patients in the CRP POCT arm saw their GP for any condition on average 3.8 times. This was slightly more frequently than the 3.7 times in the control arm, resulting in a cost increase of £3.21 per patient (see Table 21). Considering all primary care contacts, costs for patients in the CRP POCT arm were £5.16 (95% CI –£18.14 to £28.47; p = 0.882) per person more than for control arm patients.
However, when analysing primary care contacts related to COPD only (Table 22), fewer patients had GP home visits, nurse home visits and GP telephone consultations in the CRP POCT arm than in the control arm. Furthermore, GP surgery consultations for COPD were 2.7% less frequent (58.6% vs. 61.3%), with slightly fewer visits per patient (2.18 visits in CRP POCT arm compared with 2.27 visits in the control arm), resulting in an overall difference in primary care cost due to COPD in the CRP POCT arm of £6.35 per patient (95% CI –£18.98 to £6.27; p = 0.515).
| Health-care resource | Trial arm (£), mean (SD) | Difference (£) (95% CI) | p-value | |
|---|---|---|---|---|
| CRP POCT (n = 304) | Usual care (n = 302) | |||
| Primary care related to COPD: cost per patient | ||||
| GP visits at the surgery | 45.95 (55.06) | 50.07 (59.78) | –4.12 (–13.29 to 5.05) | 0.489 |
| Nurse visits at the surgery | 7.31 (10.44) | 6.67 (11.83) | 0.64 (–1.14 to 2.42) | 0.115 |
| GP visits at home | 3.08 (18.66) | 4.50 (22.53) | –1.43 (–4.73 to 1.87) | 0.376 |
| Nurse visits at home | 0.00 (0.00) | 1.10 (13.12) | –1.10 (–2.41 to 0.37) | 0.044 |
| GP telephone consultations | 10.89 (25.96) | 11.23 (28.26) | –0.34 (–4.67 to 3.99) | 0.735 |
| Nurse telephone consultations | 0.82 (2.63) | 0.76 (2.79) | 0.06 (–0.37 to 0.49) | 0.441 |
| Other health-care contacts | 0.09 (0.94) | 0.16 (1.63) | –0.06 (–0.28 to 0.15) | 0.987 |
| Total cost of primary care use per patient | 68.13 (72.34) | 74.49 (85.37) | –6.35 (–18.98 to 6.27) | 0.515 |
Secondary care costs
Secondary care resource use is summarised in Table 23. A summary of secondary care costs can be found in Table 24.
| Health-care resource | Trial arm, n (mean, SD) | |
|---|---|---|
| CRP POCT (n = 275) | Usual care (n = 280) | |
| Secondary care resource use in the 4-week follow-up period | ||
| A&E visits | 13 (0.05, 0.21) | 9 (0.03, 0.18) |
| Outpatient visits | 35 (0.11, 0.41) | 42 (0.13, 0.37) |
| Inpatient stays | 7 (0.02, 0.15) | 4 (0.01, 0.11) |
| Secondary care resource use in the 6-month review period | n = 305 | n = 302 |
| A&E visits | 82 (0.25, 0.69) | 76 (0.23, 0.61) |
| Outpatient visits | 380 (1.17, 1.55) | 393 (1.21, 1.60) |
| Inpatient stays | 35 (0.11, 0.44) | 34 (0.10, 0.38) |
| Secondary care resource use in the 6-month review period related to COPD only | ||
| Total A&E visits related to COPD, n (% of all visits) | 35 (42.7) | 33 (43.4) |
| Total outpatient visits related to COPD, n (% of all visits) | 8 (21.1) | 11 (26.2) |
| Total inpatient stays related to COPD, n (% of all visits) | 17 (48.6) | 21 (61.8) |
| Health-care resource | Trial arm (£), mean (SD) | Difference (£) (95% CI) | p-value | |
|---|---|---|---|---|
| CRP POCT (n = 275) | Usual care (n = 280) | |||
| Secondary care cost per patient during the 4-week follow-up period | ||||
| A&E visits | 6.92 (31.44) | 5.12 (29.27) | 1.80 (–3.26 to 6.86) | 0.366 |
| Outpatient visits | 14.45 (53.23) | 20.98 (76.26) | –6.53 (–17.50 to 4.44) | 0.138 |
| Inpatient stays | 52.32 (343.41) | 31.44 (310.45) | 20.88 (–33.68 to 75.44) | 0.346 |
| Other secondary care use | 8.05 (42.51) | 10.51 (62.00) | –2.46 (–11.34 to 6.41) | 0.246 |
| Total cost of secondary care use per patient | 81.80 (369.35) | 68.05 (345.38) | 13.74 (–45.87 to 73.35) | 0.842 |
| Secondary care cost per patient in the 6-month review period for any reason | ||||
| n = 305 | n = 302 | |||
| A&E visits | 37.03 (98.02) | 34.66 (86.56) | 2.37 (–12.38 to 17.12) | 0.713 |
| Outpatient visits | 151.99 (208.14) | 157.51 (205.98) | –5.53 (–38.54 to 27.49) | 0.782 |
| Inpatient stays | 347.69 (1581.94) | 232.91 (983.98) | 115.55 (–90.35 to 322.46) | 0.802 |
| Total cost of secondary care use per patient | 536.71 (1673.02) | 424.32 (983.98) | 112.40 (–106.67 to 331.46) | 0.523 |
| Secondary care cost per patient in the 6-month review period for COPD-related reasons only | ||||
| A&E visits | 16.26 (61.07) | 14.60 (55.35) | 1.66 (–7.63 to 10.95) | 0.903 |
| Outpatient visits | 24.06 (77.74) | 36.84 (105.53) | –12.79 (–27.55 to 1.98) | 0.115 |
| Inpatient stays | 134.16 (855.00) | 123.57 (625.23) | 10.59 (–108.90 to 130.09) | 0.568 |
| Total cost of secondary care use per patient | 174.48 (911.54) | 175.01 (669.57) | –0.53 (–128.13 to 127.07) | 0.271 |
In the first 4 weeks of follow-up, the secondary care costs were £13.74 (95% CI –£45.87 to £73.35; p = 0.842) per person higher in the CRP POCT arm, mainly as a result of an increased number of inpatient stays.
In the 6-month review period, the total secondary care cost was £112.40 (95% CI –£106.67 to £331.46; p = 0.523) per person higher in the CRP POCT arm (see Table 24), mainly caused by, on average, 3.03 days (95% CI –0.08 to 6.14 days; p = 0.342) longer inpatient hospital stays in the CRP POCT arm (7.78 days vs. 4.75 days in the control arm). This was attributed to a higher number of stays exceeding 10 days in the CRP POCT arm (eight stays of > 10 days, of which three were COPD/respiratory related) than in the control arm (three stays of > 10 days, all of which were COPD/respiratory related), including two patients who experienced prolonged inpatient stays (> 20 days) as a consequence of a stroke and a fall.
Considering only COPD-related causes, the total secondary care cost was marginally lower in the CRP POCT arm despite the mean length of hospital stay being 1.68 days longer (95% CI –1.92 to 5.28 days; p = 0.617) than in the control arm (6.42 days vs. 4.74 days).
Total costs at 4 weeks and 6 months
The total costs from a NHS perspective at the 4-week follow-up time point (including baseline costs) were £143.93 (SD £348.96) and £126.34 (SD £329.37) in the CRP POCT and control arms, respectively, resulting in a difference of £17.59 per patient (95% CI –£34.80 to £69.98 per patient; p = 0.408) with higher costs in the CRP POCT arm. This was a result of the CRP testing costs and higher inpatient costs in the CRP POCT arm that could not be offset by savings in primary care (Figure 7).
FIGURE 7.
Costs accrued in the first 4 weeks of follow-up (including baseline but excluding medication costs between baseline and 4 weeks because of a lack of data).

In the 6-month review period (including baseline), the mean cost per patient for any condition in the CRP POCT arm was £732.31 (SD £1660.08) and £606.04 (SD £1009.32) in the control arm, representing a cost increase of £126.26 per patient (95% CI –£85.92 to £338.45 per patient; p = 0.680). This was mainly driven by higher inpatient costs, slightly increased primary care costs and the cost of CRP testing (Figure 8).
FIGURE 8.
Costs accrued for any condition in the 6-month review period (including baseline costs).
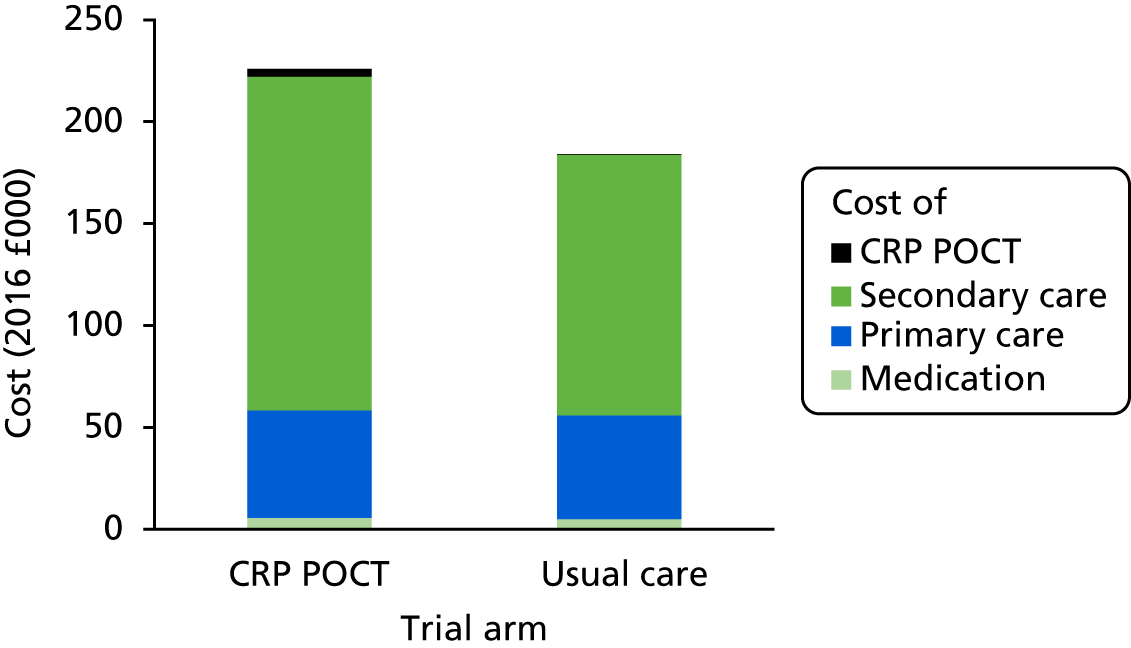
However, considering only health-care resource use related to COPD, the total cost was similar in both arms (Figure 9), with £294.14 per patient in the CRP POCT arm and £287.33 per patient in the control arm, resulting in a cost difference of £6.81 per patient (95% CI –£116.49 to £130.11 per patient; p = 0.986).
FIGURE 9.
Total costs during study period for CRP POCT and control arm, respectively. Costs associated with COPD and other respiratory conditions only (including baseline costs).
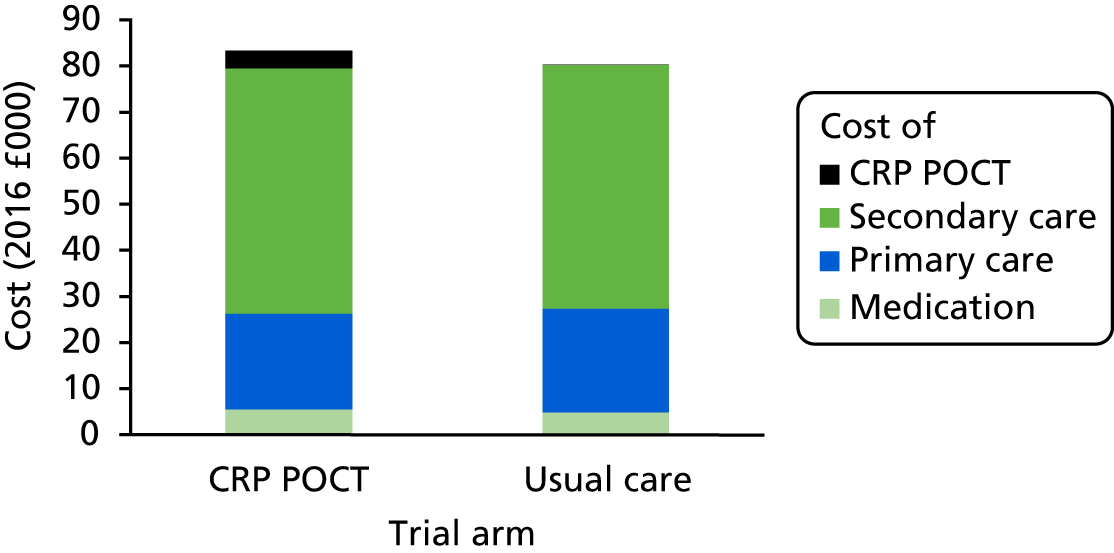
Cost of work lost due to acute exacerbations of chronic obstructive pulmonary disease
In the CRP POCT arm, 274 patients provided information on whether or not they took time off paid employment because of COPD. At the 4-week follow-up point, 30.7% were in paid employment at the time of reporting. Of these, 19.0% reported sickness leave and took an average of 4.3 days off work because of illness (a total of 69 days off work). This amounted to a cost of £421.62 per person.
By comparison, 280 people provided information in the control arm, of whom 27.5% were in paid employment at the time of reporting. Of these, 19.5% took a total of 143 days off work, which equals 9.5 days per person. This results in a cost of £932.04 per person in work lost.
Cost-effectiveness analysis
The mean cost for the MITT population (n = 537) at the 4-week follow-up point (including baseline) was £161.77 (SD £385.58) in the CRP POCT arm (n = 274) and £116.68 (SD £223.46) in the control arm (n = 263). This represents an incremental cost of £45.09 per person (95% CI £8.07 to £98.26 per person; p = 0.020) in the CRP POCT arm. Considering that 77.4% of patients in the control arm consumed antibiotics compared with 57.0% of patients in the CRP POCT arm (see Chapter 3), the mean ICER is £222 (95% CI –£42.00 to £518.14) per 1% absolute reduction in antibiotic consumption. For the base case, the four unresolved control patients who reported a CRP POCT test are assumed to not have received a test.
Sensitivity analyses
The results of the sensitivity analyses can be found in Table 25. In all analyses, ICERs ranged between £120 and £234 per 1% reduction in antibiotic consumption. ICERs were generally robust but were most affected by changes in health-care costs and the exclusion of health-care costs not related to COPD.
| Parameter | Change(s) | ICER (£) |
|---|---|---|
| Deterministic one-way sensitivity analysis | ||
| CRP POCT costs | Remove GP training cost. Assume machine used for other tests in routine practice. Assume testing carried out predominantly by nurses, change cost ±20% | 211 to 234 |
| Medication costs | Change costs ±20% | 222 (marginal change) |
| Health-care costs | Change costs ±20% | 190 to 255 |
| Antibiotic consumption | Change antibiotic consumption by ±20% | 212 to 319 |
| Scenario analysis | ||
| CRP POCT costs | Four unresolved control cases assumed to have received CRP test at baseline | 222 |
| Medication cost in 4-week follow-up period | No medication cost considered. Medication cost increased to one-third of 6-month review costs | 220 to 224 |
| Health-care costs | COPD-related health-care costs only considered | 120 |
Probabilistic sensitivity analysis gave a point estimate of the ICER of £196, with the majority of results more costly but also more effective in the CRP POCT arm, with some iterations where CRP POCT dominates control (i.e. less costly and more effective; Figures 10 and 11).
FIGURE 10.
Cost-effectiveness plane (MITT analysis) for the base case (incremental cost per percentage reduction in antibiotic consumption).
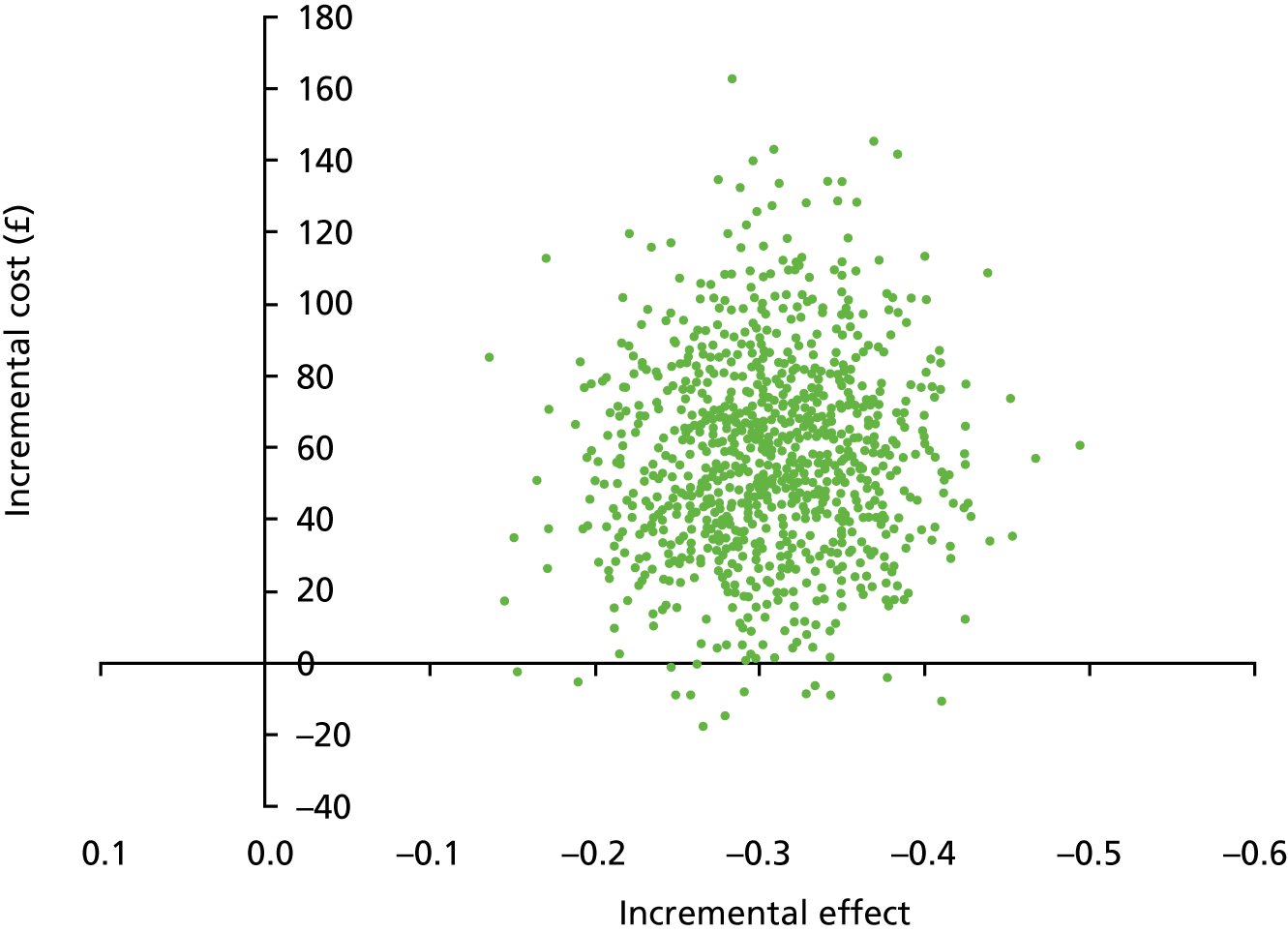
FIGURE 11.
Cost-effectiveness acceptability curve for base-case cost-effectiveness analysis.
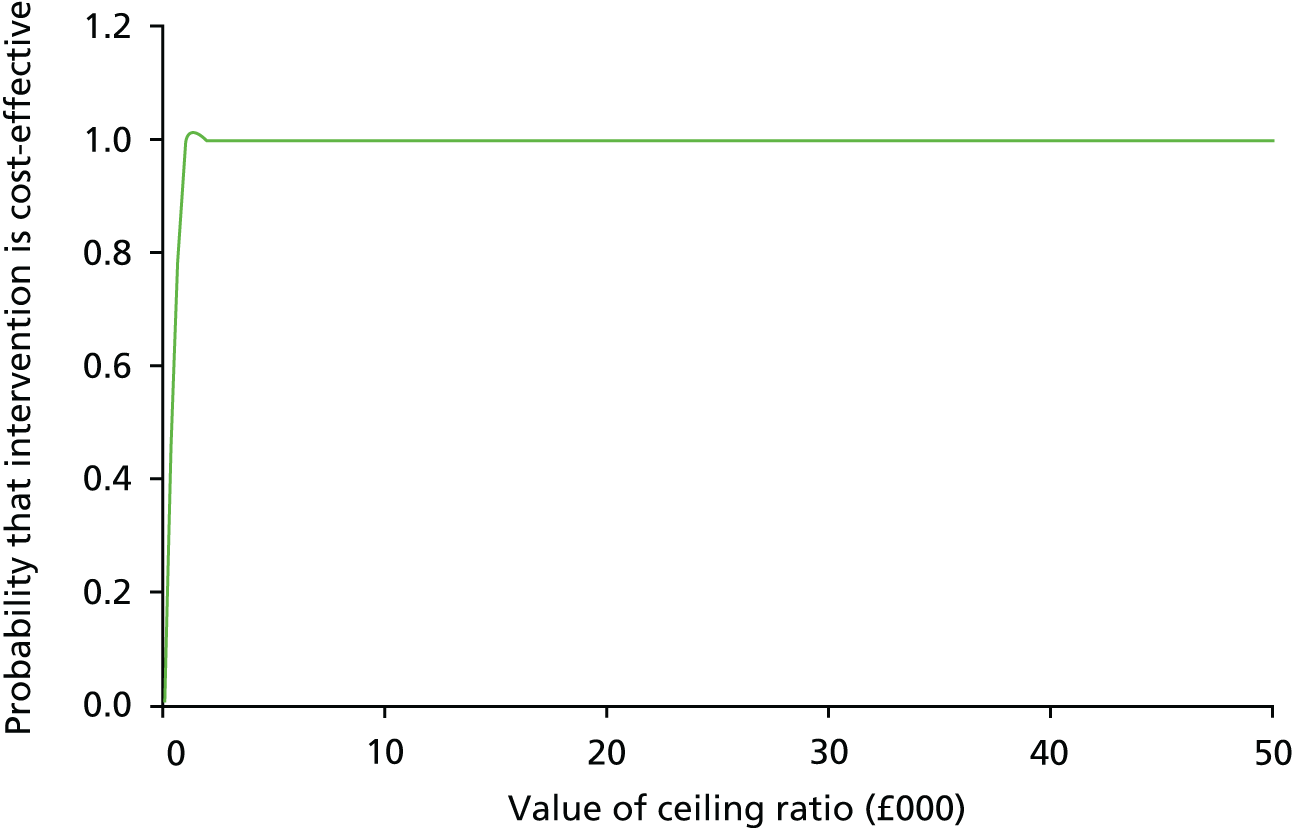
Cost–utility analysis
The total cost at 6 months (including baseline) for the EQ-5D MITT population was £759.35 (SD £1712) per person in the CRP POCT arm (n = 301) and £629.72 (SD £1036) per person in the control arm (n = 301). The number of QALYs gained over the 6-month review period was small in both arms, with a mean of 0.2915 (SD 0.1240) in the control arm and 0.3000 (SD 0.1275) in the CRP POCT arm, giving a marginal QALY increase of 0.0085 (95% CI –0.0117 to 0.0286; p = 0.760). This results in an ICER of £15,251 (95% CI £2959 to £22,813) per QALY gained.
Sensitivity analyses
Results remained reasonably robust during deterministic sensitivity analyses when subjected to changes in the cost inputs with ICERs between £12,519 and £19,063 and health-care cost as the main cost driver. Owing to the small between-arm differences in QALY gain, results were more sensitive to changes in this variable (Table 26).
| Parameter | Change(s) | ICER (£) |
|---|---|---|
| Deterministic one-way sensitivity analysis | ||
| CRP POCT costs | Remove GP training cost. Assume machine used for other tests in routine practice. Assume testing carried out predominantly by nurses, change cost ±20% | 14,980 to 15,521 |
| Medication costs | Change costs ±20% | 15,195 to 15,304 |
| Health-care costs | Change costs ±20% | 12,519 to 17,980 |
| QALY gain | Change QALY gain ±20% | 12,835 to 19,063 |
| Scenario analysis | ||
| QALY gain | All patients completing EQ-5D-3L excluded | 8444a |
| CRP POCT costs | Four unresolved control cases assumed to have received CRP test at baseline | 15,238 |
| Health-care costs | COPD-related health-care costs only considered | 1054 |
Scenario analysis showed that the ICER would be reduced to £1054 per QALY gained if COPD-related health-care costs only were included in the analysis, with a probability of the intervention being cost-effective at the £20,000 threshold of 72%.
In the probabilistic sensitivity analysis most results found CRP POCT to be more costly but also more effective. However, results are distributed across all quadrants of the cost-effectiveness plane as a consequence of the small differences in costs and QALYs between the two arms (Figure 12). Overall, the probability that CRP testing is cost-effective at a willingness-to-pay threshold of £20,000 is 56% (Figure 13).
FIGURE 12.
Cost-effectiveness plane (MITT analysis) for the base-case CUA (incremental cost per QALY gained).
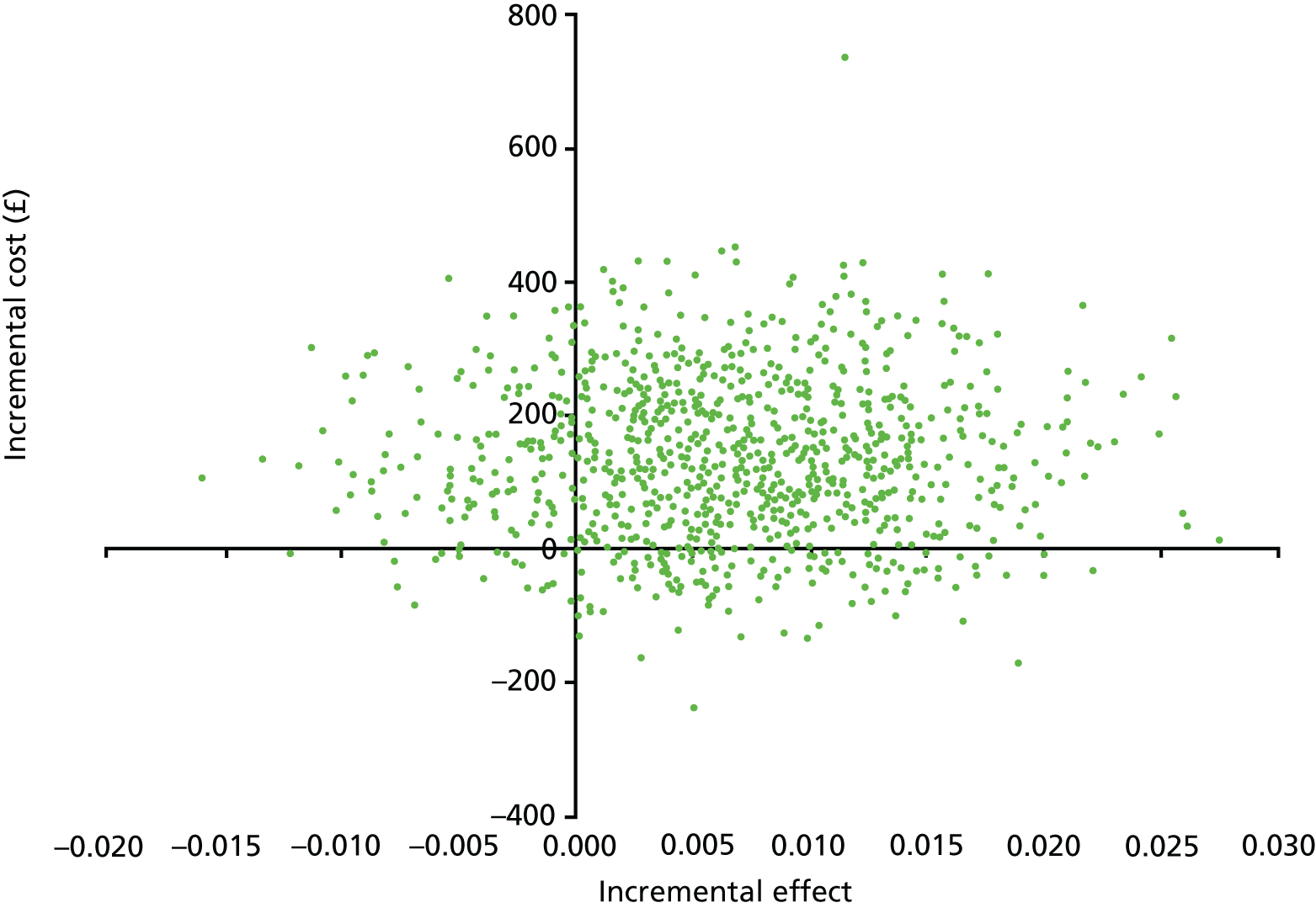
FIGURE 13.
Cost-effectiveness acceptability curve (MITT analysis) for the base-case CUA (incremental cost per QALY gained).
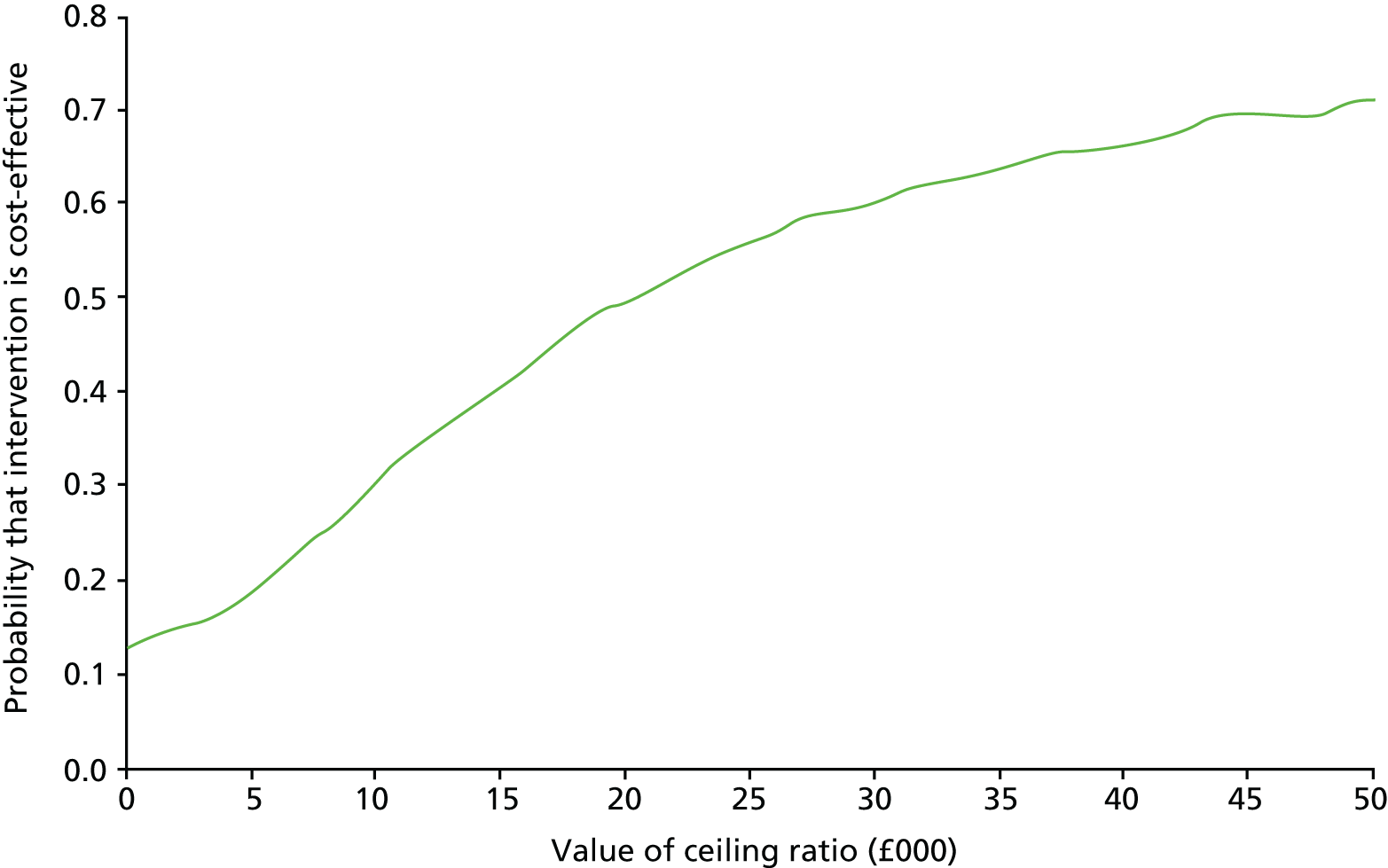
Repeating the CUA using the ITT population after multiple imputation resulted in an ICER of £14,334. Using the lower and upper bounds of the 95% CIs for the cost and QALY differences to conduct deterministic sensitivity analysis results in ICERs between £7013 and £21,151, with scenarios in which the intervention is both dominating and dominated (Table 27). This high level of uncertainty is caused by the small differences in cost and especially the effect of the intervention compared with the control.
| Analysis | Incremental | ICER (£) | Quadrant | |
|---|---|---|---|---|
| Cost (£) | Effect | |||
| Base-case multiple imputation data | 129.01 (95% CI –91.89 to 349.92) | 0.009 (95% CI –0.002 to 0.020) | 14,334 | North-east quadrant (intervention more effective and more costly) |
| 95% CI sensitivity analyses | ||||
|
Upper bound cost Upper bound QALY |
349.92 | 0.020 | 17,496 | North-east quadrant (intervention more effective and more costly) |
|
Upper bound cost Lower bound QALY |
349.92 | –0.002 |
(Not applicable) –174,959 |
North-west quadrant (intervention more costly and less effective: dominated) |
|
Lower bound cost Upper bound QALY |
–91.89 | 0.020 |
(Not applicable) –4595 |
South-east quadrant (intervention more effective and less costly: dominant) |
|
Lower bound cost Lower bound QALY |
–91.89 | –0.002 | 45,946 | South-west quadrant (intervention less effective and less costly) |
This is also illustrated by the cost-effectiveness plane (Figure 14), with point estimates distributed across all four quadrants. The majority of the point estimates indicate higher costs associated with the intervention, with most more effective than the control. The cost-effectiveness acceptability curve shows that, at a willingness-to-pay threshold of £20,000, the probability of the intervention’s cost-effectiveness is 60% (Figure 15).
FIGURE 14.
Cost-effectiveness plane (ITT analysis using multiple imputation) for the secondary CUA (incremental cost per QALY gained).

FIGURE 15.
Cost-effectiveness acceptability curve (ITT analysis using multiple imputation) for the secondary CUA (incremental cost per QALY gained).

Cost–consequences analysis
Table 28 summarises the results of the cost–consequences analysis.
| Outcome | Trial arm | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| CRP POCT | Usual care | |||
| Cost impact (£) | ||||
| Total CRP POCT implementation costs | 3924 | 34 | 3890 | |
| Total costs at 4 weeks | 46,778 | 40,935 | 5843 | |
| Total costs at 6 months | 238,000 | 196,358 | 41,642 | |
| CRP POCT implementation costs per person | 12.08 | 0.11 | 11.97 (11.60 to 12.34) | < 0.001 |
| Mean cost at 4 weeks per patient | 143.93 | 126.34 | 17.59 (–34.80 to 69.98) | 0.408 |
| Mean cost at 6 months per patient | 732.31 | 606.04 | 126.26 (–85.92 to 338.45) | 0.680 |
| Mean COPD-related cost at 6 months per patient | 294.14 | 287.33 | 6.81 (–116.49 to 130.11) | 0.986 |
| Total cost of productivity loss at 4 weeks | 6746 | 13,981 | 7235 | |
| Mean cost of productivity loss at 4 weeks per patient taking time off work | 421.62 | 932.04 | 510.42 (–989.56 to –31.28) | 0.022 |
| Health impact | ||||
| Number (%) of patients consuming antibiotics for AECOPD in first 4 weeks | 150 (57.0) | 212 (77.4) | –62; AOR 0.31 (0.20 to 0.47) | < 0.001 |
| Antibiotic prescribing at index consultation (%) | 155 (47.7) | 225 (69.7) | –70; AOR 0.31 (0.21 to 0.45) | < 0.001 |
| Mean CCQ total scores (points) | 2.6 | 2.8 | –0.20 (–0.34 to –0.06) | 0.005 |
| EQ-5D health score (VAS) | 62.9 | 59.8 | 3.12 (0.50 to 5.74) | 0.019 |
| QALYs in first 6 months | 0.3000 | 0.2915 | 0.0085 (–0.0117 to 0.0286) | 0.760 |
| CRQ-SAS dyspnoea domain | 4.3 | 4.2 | 0.06 (–0.20 to 0.33) | 0.636 |
| CRQ-SAS fatigue domain | 3.6 | 3.5 | 0.13 (–0.12 to 0.38) | 0.295 |
| CRQ-SAS emotional function domain | 4.4 | 4.3 | 0.15 (–0.04 to 0.34) | 0.129 |
| CRQ-SAS mastery domain | 4.2 | 4.3 | –0.09 (–0.18 to 0.01) | 0.065 |
| Adverse effects of treatment (%) | 255 (89.5) | 264 (91.3) | –9; AOR 0.79 (0.44 to 1.39) | 0.410 |
| Pneumonia diagnoses at 6 months (%) | 9 (3.0) | 12 (4.0) | –3; AOR 0.73 (0.29 to 1.82) | 0.495 |
Budget impact analysis
The estimated budget impact of the use of CRP POCT in primary care is summarised in Table 29. During the 6-month trial period, the total cost per patient tested with CRP POCT was £126.26 (95% CI –£85.92 to £338.45) per patient higher than in the control arm. Extrapolated to the UK population, the estimated budget impact over 5 years is £534M (95% CI –£452.8M to £1.52B; see Sensitivity analysis). This is caused by increased hospitalisation costs in the CRP POCT arm, mostly unrelated to COPD.
| Parameter | Year | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Number of eligible patients (people diagnosed with COPD) | 1,200,000 | 1,269,576 | 1,383,207 | 1,496,839 | 1,610,470 |
| Uptake estimate (%) | 10 | 20 | 30 | 40 | 50 |
| CRP-tested patients | 120,000 | 253,915 | 414,962 | 598,735 | 805,235 |
| Yearly cost of CRP testing (£) | 1,357,200 | 2,871,780 | 4,693,221 | 6,771,697 | 9,107,208 |
| Yearly incremental cost of health-care use (£) | 27,868,800 | 58,969,243 | 96,370,798 | 139,050,309 | 187,007,776 |
| Net costs (£) | 29,226,000 | 61,841,023 | 101,064,019 | 145,822,007 | 196,114,984 |
Sensitivity analysis
There is considerable uncertainty in the budget impact estimates. The 95% CIs around cost estimates are wide as a result of the general skewness of cost data. Using the 95% CI for secondary care costs as observed in the trial, the budget impact ranges from –£452.8M to £1.52B. Uptake rates are assumed as they are impossible to predict and any change will affect the budget impact of CRP testing. Assuming a stable uptake of 10%, 20%, 30%, 40% or 50% over 5 years results in budget impact estimates between £169.5M and £847.6M. Increasing the number of acute exacerbations per patient per year to two raises the budget impact estimate to £558.9M.
Finally, if COPD-related health-care costs only are considered in the budget impact analysis, CRP testing leads to a cost saving of £10.2M compared with current routine care. This is due to a health-care cost reduction of £7.98 per patient (95% CI –£138.55 to £122.59 per patient) in the CRP POCT arm as observed in the trial.
Summary
This chapter described, in detail, the methods and results of the health economic evaluation undertaken as part of the PACE trial. The results suggest that the use of CRP POCT in primary care reduces antibiotic consumption and costs without significantly affecting other COPD medication costs, health-care resource use and HRQoL. There were no significant differences in total incremental cost for all causes at 4 weeks or 6 months, or if only COPD-related health-care costs were considered in the analysis. The ICERs were £222 (95% CI –£42.00 to £518.14) per 1% reduction in antibiotic consumption and £15,251 (95% CI £2959 to £22,813) per QALY gained.
Chapter 6 Discussion
The PACE randomised controlled clinical trial found that using a CRP POCT to guide antibiotic prescribing decisions for patients presenting in primary care with an AECOPD resulted in an absolute reduction in antibiotic consumption over the 4 weeks after the initial consultation of 20%, and a marginal improvement in condition-specific health status that was smaller than the published, minimally important clinical significance for this outcome. Secondary outcomes, including antibiotic prescribing at the index consultation, antibiotic use for any reason, subsequent consultations in primary and secondary care, and COPD HRQoL and generic quality of life, were all consistent with the finding that the intervention safely reduced antibiotic use. Subgroup analyses suggest that the intervention effect was confined to patients with more than one Anthonisen criteria (the clinical features usually used to guide antibiotic treatment for AECOPD). There was no evidence of differences between the arms in the use of oral steroids or other relevant medications. Possible adverse effects from antibiotic consumption were reported by approximately 90% of participants, but there was no evidence of a difference between the trial arms. There was also no evidence that the intervention led to differences in antibiotic resistance in potential respiratory pathogens or commensal organisms isolated from sputum and throat swab samples 4 weeks after first consulting for the AECOPD.
The qualitative process evaluation found evidence that the CRP POCT was seen as broadly acceptable and useful by patients and primary care clinicians. Technical issues, such as the need to refrigerate cartridges and calibrate the machine, were seen as potential barriers to use, and concern about costs was a potential barrier to implementing the POCT in the context of NHS primary care services. Clinicians acknowledged that using the POCT increased consultation length, but they generally thought that this was offset by the perceived benefits. These benefits included reducing uncertainty in their prescribing decisions, using the test result to back up their prescribing decisions in discussions with patients and using the POCT as a tool to educate patients.
Our health economic evaluation found that the total cost from a NHS perspective at 4 weeks was < £20 more for patients managed with the addition of CRP POCT, and that there were no differences in total COPD-related costs between the two arms over 6 months.
Strengths and limitations
This pragmatic primary care trial was adequately powered to detect clinically important differences in both patient-reported antibiotic consumption and COPD health status at 2 weeks post randomisation. This is one of few studies of an antibiotic stewardship intervention to power on co-primary outcomes involving both a difference in antibiotic use and no worse (non-inferior) clinical outcomes. Patients were recruited during routine general practice consultations and from more than 80 UK general practices. Remote allocation was used and there was no evidence of breaches in allocation concealment. Participant characteristics, including age, comorbidities, smoking status, COPD health status and clinical features, were well balanced at baseline. Adherence to intervention allocation was good, with evidence that 97.5% of those allocated to CRP POCT received the test, and definitely < 3% received a CRP POCT at any point in the 4-week follow-up period. We ascertained initial antibiotic prescribing data for all but one participant. In addition, we ascertained patient-reported outcomes for > 80% of participants, with 83% and 87% of patients contributing data to the antibiotic use and COPD health status primary outcome analyses, respectively. We obtained follow-up data at 6 months for 68% of participants.
This was an open, pragmatic trial, with no blinding of participants or clinicians, as we aimed to assess the effects of the intervention in comparison with current usual practice. Introducing sham tests, for example, would have made comparison with usual care impossible. Open trials allow for a better measurement of the ‘real-world’ consequences of interventions. They are preferable for studies of cost-effectiveness as knowledge of the intervention received may influence patients’ decisions about subsequent help-seeking. Capturing subsequent resource use is critical to determining costs. However, the antibiotic prescribing reported by the responsible clinicians and captured from clinical records is consistent with patient-reported antibiotic use in the trial arms. It is possible that awareness of the intervention contributed to the beneficial impact we observed on COPD health status (e.g. through patients feeling better because they expected to feel better); if so, this effect would also occur in real-world clinical practice and, therefore, needed to be captured. 95
We chose patient reports about antibiotic consumption (over the 4 weeks after the baseline appointment for their AECOPD) to be included in the co-primary outcome, rather than clinician reports or initial antibiotic prescribing data from the primary care clinical record. Antibiotics that are actually consumed, rather than prescribed, is the critical measure. ‘Delayed’ or ‘back-up’ antibiotic prescribing is relatively common for AECOPD, and antibiotics can be obtained from several sources, including hospitals, out-of-hours services and leftover antibiotics or ‘rescue packs’ of antibiotics stored at home. We captured both antibiotic prescribing and antibiotic use over 4 weeks to determine whether or not fewer initial prescriptions might have resulted in more subsequent reconsultations and further antibiotic prescribing.
Generalisability
Participants’ age, gender, comorbidities and GOLD stage are in keeping with those of primary care patients with COPD,96 and the potential pathogens isolated from baseline sputum samples are in keeping with those in other studies of AECOPD in the community. 89 Although all patients were assessed as having COPD by their recruiting clinician, and 97.5% of patients had a diagnosis of COPD in their medical records, we were able to obtain forced expiratory volume in 1 second (FEV1) forced vital capacity (FVC) spirometry data for only 66% of participants from the primary care medical records and for many of these the spirometry was carried out many months before or after randomisation. However, clinicians tend to rely on the diagnosis in the medical records, rather than on a detailed review of spirometry results, when making treatment decisions for patients with AECOPD, hence the focus on having a COPD diagnosis in the clinical record, rather than on spirometry criteria, for trial eligibility.
The quality of spirometry is often suboptimal in primary care. 97 However, for those who had spirometry, the vast majority (85%) of patients met the criterion for the diagnosis of COPD. The decision to base inclusion on the recorded diagnosis and not on spirometry was explicit in the design. It was based on the already heavy burden of assessment on participating patients and practices and the pragmatic, ‘real-world’ nature of the trial. The low rate of recording of spirometry demonstrates how inappropriate it would have been to rely on general practice spirometry for the inclusion of patients. Most patients had purulent sputum, but only a minority (< 4%) had sputum that was rated as having the highest degree of purulence using the BronkoTest. This may be a reflection of the considerable variation of patients’ interpretations of the BronkoTest in comparison with that of clinicians. Few participants had very severe underlying COPD in this study, so the findings may not be applicable to those with very severe COPD. We also excluded patients who had characteristics that may affect CRP levels (e.g. those with chronic inflammatory diseases) and those likely to be significantly immunocompromised, so our results cannot be generalised to these groups.
Interpretation and comparison with other literature
We searched MEDLINE using the search terms ‘C-reactive protein/OR crp.mp’ AND ‘Pulmonary Disease, Chronic Obstructive/OR COPD.mp OR Respiratory Tract Infections/’ AND ‘Anti-Bacterial Agents/or antibiotic$.mp’ and found three trials that used CRP POCT and included patients with COPD as part of their population. A Norwegian cluster randomised trial that recruited 179 patients with acute cough/LRTI comprised 29 patients with COPD or asthma. 98 The authors reported an overall reduction in antibiotic prescribing, but the study was not powered to detect a difference in the COPD/asthma group.
A Dutch cluster RCT that used a factorial design to randomise patients to CRP POCT or not, and to communication skills training or not, comprised 31 patients with COPD. 33 This study also found a significant reduction in antibiotic prescribing for LRTI, but it was also not powered for a subgroup analysis of patients with COPD. A non-randomised Spanish study of a multifaceted intervention consisting of audit and feedback, education and training in CRP testing for some clinicians found lower antibiotic ‘overprescribing’ for AECOPD (prescribing to patients with ≤ 2 Anthonisen criteria) by primary care clinicians who received training in CRP testing. 99 In the hospital setting, a meta-analysis of eight trials (1062 patients) found reasonable evidence for using procalcitonin to guide antibiotic initiation and discontinuation for AECOPD, with the aim of reducing antibiotic exposure without adversely affecting clinical outcomes. 100 There is a lack of similar trial evidence for CRP testing in the hospital setting, but observational studies have shown an association between higher CRP levels and pneumonia and benefit from antibiotics. 28,39 A recent Cochrane systematic review comprised six trials with > 3000 participants, and confirmed that CRP testing can safely reduce antibiotic prescribing for respiratory tract infections in the primary care setting. 101
Although more than three-quarters of participants in our trial had ≥ 2 Anthonisen criteria, 76% of those in the intervention arm had a CRP level of < 20 mg/l, suggesting a low probability of significant bacterial infection and benefit from antibiotics. In the previous trial of antibiotics versus placebo for patients managed in the community with AECOPD from our group, we found no evidence of a between-arm difference in the proportions with clinical failure among those with a CRP level of < 40 mg/l. 24 Many patients with CRP levels in the 20–40 mg/l range, in addition to those with a CRP level of < 20 mg/l, could, therefore, probably be safely managed without antibiotics. We also found a 20% absolute reduction in antibiotic consumption despite antibiotics being prescribed for 33% of those with a CRP level of < 20 mg/l. These findings suggest, therefore, that our estimate of the potential reduction in antibiotic use from CRP POCT use is conservative.
Over half of the baseline sputa collected in our study did not yield any potential bacterial respiratory pathogen, and another one-fifth yielded both viral and bacterial pathogens (possible mixed infections). These findings are consistent with those of other studies, which have shown that bacterial infection is a likely trigger for AECOPD in a minority of patients14 and, therefore, antibiotic treatment is unlikely to benefit many of them.
Antimicrobial stewardship is often promoted on the basis of the interests of future generations and society in general. However, taking fewer antibiotics may also benefit individuals by avoiding disruption of their microbiome,16–18 including the selection of antimicrobial-resistant organisms that could lead to AECOPD that are more frequent and/or more difficult to treat. This could be especially important for patients with COPD who are at greater risk of being colonised with resistant pathogens than patients with normal lungs. At baseline, sputum sample bacteriology identified > 200 potential pathogens from the overall cohort; over one-third of the H. influenzae and S. pneumoniae strains cultured were not susceptible to ampicillin. However, in the trial, a comparison of the prevalence of resistant organisms isolated from patients at 4 weeks and resistance before treatment demonstrated no evidence of any beneficial effect of the intervention on antimicrobial resistance. Further planned analyses will explore differences in specific ‘antibiotic–bacteria’ combinations, and between those who did and those who did not consume antibiotics.
Antibiotics do benefit many people with AECOPD,24 and the underprescribing of antibiotics needs to be avoided for patients who are likely to benefit. We included COPD health status in the co-primary outcome measure to assess whether or not any reduction in antibiotics might have occurred at the expense of patient recovery and well-being. Despite the important reduction in antibiotic use, we identified a small beneficial effect on condition-specific health status in the intervention arm. Possible explanations include a psychological benefit from the perceived benefits of a blood test to guide treatment, a reduction in adverse effects from antibiotics or beneficial effects from other interventions used instead of antibiotics. The last of these is unlikely, as we found no evidence of a difference in corticosteroid or inhaler use between the study arms. Furthermore, we found no evidence of benefits in terms of primary and secondary care consultations for any reason, or in disease-specific HRQoL during the 6-month follow-up period. However, a short-term (4 weeks) intervention (and in most cases a single antibiotic prescribing decision) is unlikely to have a dramatic effect on resistance or longer-term outcomes among patients who are frequently prescribed antibiotics.
Our qualitative findings are consistent with those of previous studies on the attitudes towards using CRP POCTs to manage LRTIs. Clinicians generally have positive views about using CRP POCTs to manage LRTIs, and feel that they provide an indication of disease severity, help manage patient expectations and increase their confidence in antibiotic-prescribing decisions. 67,102,103 Previous studies have also found similar concerns about the costs and implementation of CRP POCTs in the management of LRTIs in NHS primary care services. 68
Our health economic evaluation identified reduced antibiotic costs at the initial consultation but no significant differences in the total incremental cost for all causes at 4 weeks or 6 months, or if only COPD-related health-care costs were considered in the analysis. To our knowledge, this is the first study reporting the cost-effectiveness of the use of CRP POCT in primary care to guide antibiotic prescribing for patients presenting with AECOPD based on actual antibiotic consumption. Three studies were identified that previously assessed the cost-effectiveness of CRP POCT for antibiotic prescribing in patients with LRTIs reporting similarly small cost and quality-of-life differences between groups. 35,36,104 Although these studies were conducted in different patient populations and settings, the similarities in the direction and magnitude of results confirms the robustness and accuracy of the cost-effectiveness evidence presented here.
Although we found increased all-cause health-care costs in the CRP POCT arm, these were largely attributed to a greater number of long-stay hospital patients, most of whom were admitted with conditions not related to their COPD. This might be an artefact of sampling variation or the low number of events rather than an indication of an underlying issue related to the intervention itself.
A limitation of the evaluation is the sensitivity of the cost–utility results to changes in patient quality of life. This is caused by the small differences in utility scores between the two arms. This could be caused by an insensitivity and lack of responsiveness of the generic EQ-5D instrument to small changes in the health status of specific conditions. However, EQ-5D-5L utility index values have been shown to correlate well with established disease-specific HRQoL questionnaires. 105 Furthermore, although the QALY difference at 6 months of 0.0085 does not reach the minimum important improvement of 0.05,105 the PACE trial aimed to prove non-inferiority of health and QoL outcomes despite reduction in antibiotic consumption rather than superiority. This cost-effectiveness analysis is limited by the short follow-up of the trial, which could be addressed by long-term decision-analytic modelling based on the high-quality evidence produced by the PACE trial in the future.
Impact of patient and public involvement
Public contributors were an integral part of the study team from the very beginning of the research life cycle. Even before the first grant application was submitted, public contributors living with COPD helped develop the study question, they then contributed to the grant application itself and served on the Trial Management Group and the Trial Steering Committee. Throughout, they helped refine the study questions, contributed to critical decisions about the study outcomes, including which measures were most relevant to COPD patients; and ensured that patient-facing materials were readable and user friendly (including the CRFs and patient information leaflets) and that the research materials were simplified. For example, a critically important decision to change the scale used in the co-primary outcome was based largely on public contributor input. The contributors also helped produce regular study newsletters. Public contributors have also helped develop the dissemination strategy, and are advising on framing the main key messages and the implications of the results. After the pilot phase of the study, we met with a GP and respiratory nurse from one of the best recruiting practices in the pilot study and together identified items that could be removed from the baseline CRFs and instead included in the patient diary or 6-month medical record search CRF, to make the initial assessment less burdensome for busy clinicians who were delivering acute care while also recruiting to PACE. These modifications were fundamental to our ability to implement the full study, recruit to target and follow up participants so successfully.
Implications for clinical practice and future research
This pragmatic primary care trial provides clear evidence that using a CRP POCT to guide antibiotic treatment decisions for AECOPD is both clinically effective and cost-effective in safely reducing antibiotic use in those patients for whom antibiotics are not indicated. NICE already recommends the use of CRP testing to predict pneumonia in primary care. 86 We believe that our findings provide good evidence to support the use of a CRP POCT (with the associated guidance for clinicians that we used in this trial) to guide antibiotic-prescribing decisions for AECOPD in primary care.
Further research, building on our qualitative findings, could help understand how different implementation approaches affect POCT use and acceptability to both clinicians and patients of a CRP POCT for AECOPD in primary care. In addition, implementation studies are needed to determine the effect of this intervention on antibiotic use, clinical outcomes, antibiotic resistance in commensal organisms and help-seeking behaviour over the longer term. Although we found only small differences in short-term costs and no differences in long-term costs, there is a need to evaluate the health economic implications of the implementation of this intervention. Furthermore, the source of funding for any testing would need to be addressed before this test could be implemented into routine primary care.
Further research is also required to determine the added diagnostic and prognostic value of CRP for guiding AECOPD management, over and above the diagnostic and prognostic value of clinical features such as sputum colour, sputum volume, breathlessness and other biomarkers, and how various predictors are best combined. CRP self-testing is now a realistic possibility and could have a role in directing the self-management of ‘rescue’ antibiotics for some, but this will require further development and careful evaluation prior to implementation.
Conclusions
A CRP POCT to help guide antibiotic prescribing decisions for AECOPD in primary care is clinically effective and cost-effective in safely reducing antibiotic use and prescribing for patients with AECOPD, and is thus a useful adjunct to both improving outcomes for patients and enhancing antibiotic stewardship.
Acknowledgements
The authors would like to acknowledge the use of the CRQ-SAS, authored by Dr Gordon Guyatt and Dr Holger Schünemann and under license from McMaster University, Hamilton, ON, Canada. No part of the CRQ-SAS was reproduced in this report.
Contributions of authors
Professor Nick A Francis (Professor of Primary Care Research, Primary Care and Public Health) was the co-chief investigator for the PACE trial. He contributed to the overall trial design and implementation, interpretation of findings, writing of the scientific summary and discussion section of the report, critical review of the report and final approval of the report submission.
Dr David Gillespie (Research Fellow in Statistics) was a co-investigator. He contributed to the trial design, conducted the statistical data analysis, wrote the methods and results sections, critically reviewed the report and gave final approval of the report.
Dr Patrick White (Senior Clinical Lecturer, Primary Care and Public Health Sciences) was a co-investigator. He contributed to the overall trial design and implementation, acquisition of data, interpretation of findings, critical review of the report and final approval of the report submission.
Janine Bates (Research Associate) was the trial manager, contributing to the trial design, the implementation and acquisition of data and the writing of the abstract, introduction and methods section of the report. She also co-ordinated the compilation, formatting, proofreading and final approval of the report.
Dr Rachel Lowe (Research Fellow) was the senior trials manager and the lead for trial management. She contributed to the trial design and implementation, acquisition of data, critical review of the report and final approval of the report submission.
Dr Bernadette Sewell (Senior Lecturer in Health Economics) contributed to the design of the health economics evaluation, conducted the health economics analysis, wrote the health economics evaluation section and gave final approval of the report.
Dr Rhiannon Phillips [Research Fellow, Primary and Emergency Care Research (PRIME) Centre Wales] was a co-investigator. She led the qualitative research, contributing to the study design, data analysis and interpretation, writing the qualitative evaluation section and critical review of the report and final approval of the report submission.
Helen Stanton (Research Assistant) contributed to the design and implementation of the qualitative research, acquisition and interpretation of data, critical review of the report and final approval of the report submission.
Nigel Kirby (Research Assistant) was the data manager. He contributed to the trial design, the implementation, acquisition and interpretation of data, critical review of the report and final approval of the report submission.
Dr Mandy Wootton (Lead Scientist/Operational Manager, Specialist Antimicrobial Chemotherapy Unit) was a co-investigator and the lead microbiologist. She contributed to the overall trial design, acquisition of data, interpretation of findings, writing of the microbiology sections of the report, critical review of the report and final approval of the report submission.
Dr Emma Thomas-Jones (Research Fellow) was the senior trials manager, contributing to the trial design and implementation, critical review of the report and final approval of the report submission.
Professor Kerenza Hood (Professor in Statistics and Director of Centre for Trials Research) was a co-investigator, contributed to the overall trial design and implementation, and supervised the statistical analysis and final approval of the report.
Dr Carl Llor (GP and senior researcher) was a co-investigator, contributing to the design and interpretation of the study, critical review of the report and final approval of the report submission.
Professor Jochen Cals [Professor, Department of General Practice – School for Public Health and Primary Care (CAPHRI)] was a co-investigator, contributing to the design and interpretation of the study, critical review of the report and final approval of the report submission.
Professor Hasse Melbye (Professor of General Practice, Institute of Community Medicine) was a co-investigator, contributing to the design and interpretation of the study, critical review of the report and final approval of the report submission.
Dr Gurudutt Naik (Honorary Research Fellow, Cardiff University and Clinical Research Fellow, Cardiff and Vale University Health Board) was a co-investigator, contributing to the design, implementation, acquisition and interpretation of the data, critical review of the report and final approval of the report submission.
Dr Micaela Gal [Knowledge Mobilisation and Impact Manager (PRIME)/Research Fellow (PREPARE), PRIME Centre Wales] was a co-investigator, contributing to the design, implementation, critical review of the report and final approval of the report submission.
Professor Deborah Fitzsimmons (Professor of Health Outcomes Research and Academic Director, Swansea Centre for Health Economics) supervised the health economic analyses, review of the health economics evaluation section and final approval of the report.
Dr Mohammed Fasihul Alam (Assistant Professor in Health Economics) was a co-investigator and contributed to the design of the health economics evaluation and the final approval of the report submission.
Evgenia Riga (Clinical Trial Manager) was the trials manager at the Oxford centre and contributed to the design of the study, critical review of the report and final approval of the report submission.
Ann Cochrane (Research Fellow) was the trials manager at the London centre and contributed to the design of the study, acquisition of data, critical review of the report and final approval of the report submission.
Professor Christopher C Butler (NIHR Senior Investigator and Clinical Director of the University of Oxford Primary Care and Vaccines Clinical Trials Collaboration and the NIHR Oxford Community Medical Technology and Invitro Diagnostics Co-operative) was the co-chief investigator for the study. He contributed to the conception and overall design of the study, and the implementation and interpretation of the work. He contributed to the writing of the scientific summary and discussion section of the report, critical review of the report and final approval of the report submission.
Contributions of others
Other members of the trial team
In addition to the authors, the PACE study team comprised the following, whom we would like to thank and acknowledge for their contribution to the trial implementation and management: Angela Watkins, Miguel Cossio, Stephanie Robinson, Christy Barlow and Megan Philips-Laird. Margaret Barnard (who passed away in April 2016) and Jonathan Bidmead provided patient and public representation on the Trial Management Group. Jacqueline Nuttall provided study design input.
Clinical trials units
Two UK Clinical Research Collaboration registered clinical trials units were involved in the study: Centre for Trials Research, Cardiff University; and University of Oxford Primary Care and Vaccines Clinical Trials Collaborative.
Independent members of the Trial Steering Committee and Independent Data Monitoring Committee
The authors would also like to acknowledge the contribution and continued support from the Trial Steering Committee members, namely Hilary Pinnock, Mike Thomas, William Hollingworth and Derek Cummings (patient/public representative), and also the Independent Data Monitoring Committee members: Martyn Lewis, Chris Griffiths and Charis Marwick.
Specialist Antimicrobial Chemotherapy Unit, Public Health Wales at the University Hospital of Wales
We would also like to acknowledge and thank Jennifer Richards, Leanne Davies and Joanna Diggle of the Specialist Antimicrobial Chemotherapy Unit, Public Health Wales at the University Hospital of Wales, for their dedicated help in processing and analysing the patient samples.
Research networks
We would also like to acknowledge and thank the Health and Care Research Wales Workforce, the following CRNs for their support in helping to identify sites and carry out notes reviews at these sites: Thames Valley & South Midlands CRN, East Midlands CRN, West Midlands CRN, West of England CRN, North Thames CRN, North West London CRN and South London CRN.
C-reactive protein point-of-care test supplier for the PACE trial
We would like to express out thanks to Abbott Rapid Diagnostics, formally Alere Ltd, which provided the Afinion analysers and CRP cartridges and controls to the recruitment sites at no cost. Alere provided training on the use of the CRP POCT to a number of the participating sites.
Recruitment sites
Our thanks also go to all of the primary care practices that participated in this study for their commitment in recruiting participants and engaging in all study processes. The following sites recruited at least one participant:
Albion Street Group Practice, Amersham Vale Practice, Ashfields Primary Care Centre, Bedworth Health Centre, Beech House Surgery, Bellevue Group Practice, Bicester Health Centre, Botley Medical Centre, Bradley’s Practice, Brigstock Medical Practice, Brockwell Park Surgery, Caritas Surgery, Chartfield Surgery, Church Street Practice, Churchdown Surgery, Clarence Medical Centre, Claughton Medical Centre, Clifton Surgery, Colchester Medical Practice, Corwen Family Practice, Courthouse Medical Centre, Cwm Grwydd Medical Centre, Cynon Vale Medical Practice, Ely Bridge Surgery, Eynsham Health Centre, Farnham Road Surgery, Foundry Town Clinic, Gelligaer and Gilfach Surgery, Hampstead Group Practice, Holt Medical Practice, Hurley Group Practice, Jubilee Street Practice, Keats Group Practice, Keir Hardie Practice 3, Kingsthorpe Medical Centre, Layton Medical Centre, Leicester Terrace Health Centre, Llandaff & Pentyrch Surgery, Llandaff North Medical Centre, Llanedeyrn Medical Centre, Mann Cottage Surgery, Manor Farm Medical Centre, Marches (Broughton Surgery), Mitcham Medical Centre, Morlais Health and Medical Centre, Mortimer Medical Practice, Mythe Medical Practice, Mundsley Medical Centre, Nightingale Valley Practice, North Celynen Practice, Northern Medical Centre, Open Door Surgery, Parliament Hill Medical Centre, Paston Surgery, Pen Y Bont Surgery, Plas Y Bryn Medical Centre, Portland Surgery, Preston Hill Surgery, Queen Square Medical Practice, Ridgeway View Family Practice, Riverside Surgery, Royal Arsenal Medical Centre, Rumney Primary Care Centre, Sheen Lane Health Centre, Sherringham Medical Practice, Sketty and Killay Surgeries, St Andrew’s Surgery, St Helen’s Medical Centre, Station Road Surgery, St Stephen’s Gate Medical Practice, Strawberry Place Surgery, Streatham Common Practice, The Ashgrove Surgery, The Boathouse Surgery, The Corner Surgery, The Kiltearn Medical Centre, The Nelson Medical Practice, The Practice of Health, Vauxhall Practice, Waterfront Surgery, Wells Park Practice, Whiteladies Health Centre, Winchcombe Surgery, Wymondham Medical Practice and Yorkleigh Medical Practice.
Participants
Finally, we would like to thank all patients who participated in the trial and their families, without whom this study would not have been possible.
Publication
Butler CC, Gillespie D, White P, Bates J, Lowe R, Thomas-Jones E, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med 2019;381:111–20.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Horvath AR, Lord SJ, StJohn A, Sandberg S, Cobbaert CM, Lorenz S, et al. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta 2014;427:49-57. https://doi.org/10.1016/j.cca.2013.09.018.
- US Food and Drug Administration . Medical Devices: Overview of IVD Regulation n.d. www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm123682.htm (accessed 29 June 2018).
- Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, Bossuyt PM, Deeks JJ. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ 2012;344. https://doi.org/10.1136/bmj.e686.
- O’Neill J. Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics 2015. https://amr-review.org/sites/default/files/Paper-Rapid-Diagnostics-Stopping-Unnecessary-Prescription-Low-Res.pdf (accessed 29 June 2018).
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340. https://doi.org/10.1136/bmj.c2096.
- Prescribing and Primary Care Team Health and Social Care Information Centre . Quality and Outcomes Framework, Achievements, Prevalence and Exception Data: 2011–12 2012.
- Al-ani S, Spigt M, Hofset P, Melbye H. Predictors of exacerbations of asthma and COPD during one year in primary care. Fam Pract 2013;30:621-8. https://doi.org/10.1093/fampra/cmt055.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. N Engl J Med 2010;363:1128-38. https://doi.org/10.1056/NEJMoa0909883.
- Llor C, Bjerrum L, Munck A, Hansen MP, Córdoba GC, Strandberg EL, et al. Predictors for antibiotic prescribing in patients with exacerbations of COPD in general practice. Ther Adv Respir Dis 2013;7:131-7. https://doi.org/10.1177/1753465812472387.
- Miravitlles M, Mayordomo C, Artes M, Sanchez-Agudo L, Nicolau F, Segu JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. Resp Med 1999;93:173-9. https://doi.org/10.1016/S0954-6111(99)90004-5.
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007;29:1224-38. https://doi.org/10.1183/09031936.00109906.
- Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 2003;58:37-42. https://doi.org/10.1136/thorax.58.1.37.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:150-7. https://doi.org/10.1164/rccm.200906-0837OC.
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114-21. https://doi.org/10.1164/rccm.200506-859OC.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. ESAC Project Group . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579-87. https://doi.org/10.1016/S0140-6736(05)70799-6.
- Miravitlles M. Exacerbations of chronic obstructive pulmonary disease: when are bacteria important?. Eur Respir J Suppl 2002;36:9s-19s. https://doi.org/10.1183/09031936.02.00400302.
- Pérez-Trallero E, Marimón JM, González A, Ercibengoa M, Larruskain J. In vivo development of high-level fluoroquinolone resistance in Streptococcus pneumoniae in chronic obstructive pulmonary disease. Clin Infect Dis 2005;41:560-4. https://doi.org/10.1086/432062.
- Desai H, Richter S, Doern G, Heilmann K, Dohrn C, Johnson A, et al. Antibiotic resistance in sputum isolates of Streptococcus pneumoniae in chronic obstructive pulmonary disease is related to antibiotic exposure. COPD 2010;7:337-44. https://doi.org/10.3109/15412555.2010.510162.
- Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 1999;14:1015-22. https://doi.org/10.1183/09031936.99.14510159.
- Cosby JL, Francis N, Butler CC. The role of evidence in the decline of antibiotic use for common respiratory infections in primary care. Lancet Infect Dis 2007;7:749-56. https://doi.org/10.1016/S1473-3099(07)70263-3.
- Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2016.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. https://doi.org/10.7326/0003-4819-106-2-196.
- Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12. https://doi.org/10.1002/14651858.CD010257.
- Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:716-23. https://doi.org/10.1164/rccm.201206-0996OC.
- Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:867-74. https://doi.org/10.1164/rccm.200604-506OC.
- Garcha DS, Thurston SJ, Patel AR, Mackay AJ, Goldring JJ, Donaldson GC, et al. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012;67:1075-80. https://doi.org/10.1136/thoraxjnl-2012-201924.
- Ding X, Wu X, Yu C, Hu S. Value of C-reactive protein measurement in hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease. Med J Wuhan University 2006;27:660-3.
- Weis N, Almdal T. C-reactive protein – can it be used as a marker of infection in patients with exacerbation of chronic obstructive pulmonary disease?. Eur J Internal Med 2006;17:88-91. https://doi.org/10.1016/j.ejim.2005.09.020.
- Daniels JM, Schoorl M, Snijders D, Knol DL, Lutter R, Jansen HM, et al. Procalcitonin vs. C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest 2010;138:1108-15. https://doi.org/10.1378/chest.09-2927.
- Salwan AA, Spigt M, Laue J, Melbye H. Predictors of treatment with antibiotics and systemic corticosteroids for acute exacerbations of asthma and chronic obstructive pulmonary disease in primary care. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0256-3.
- Miravitlles M, Moragas A, Hernández S, Bayona C, Llor C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment?. Chest 2013;144:1571-7. https://doi.org/10.1378/chest.13-0518.
- Oppong R, Coast J, Hood K, Nuttall J, Smith RD, Butler CC. GRACE-01 Study Team . Resource use and costs of treating acute cough/lower respiratory tract infections in 13 European countries: results and challenges. Eur J Health Econ 2011;12:319-29. https://doi.org/10.1007/s10198-010-0239-1.
- Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C-reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009;338. https://doi.org/10.1136/bmj.b1374.
- Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013;382:1175-82. https://doi.org/10.1016/S0140-6736(13)60994-0.
- Cals JW, Ament AJ, Hood K, Butler CC, Hopstaken RM, Wassink GF, et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. J Eval Clin Pract 2011;17:1059-69. https://doi.org/10.1111/j.1365-2753.2010.01472.x.
- Oppong R, Jit M, Smith RD, Butler CC, Melbye H, Mölstad S, et al. Cost-effectiveness of point-of-care C-reactive protein testing to inform antibiotic prescribing decisions. Br J Gen Pract 2013;63:e465-71. https://doi.org/10.3399/bjgp13X669185.
- Geary M, Plüddemann A, Thompson M, Wolstenholme J, Heneghan C, Price CP. Diagnostic Technology: Point-of-Care Test (POCT) for C-Reactive Protein (CRP). Horizon Scan Report 0017 2011. www.community.healthcare.mic.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/horizon-scan-reports-non-dec-funded (accessed 8 March 2019).
- Bates J, Francis NA, White P, Gillespie D, Thomas-Jones E, Breen R, et al. General practitioner use of a C-reactive protein point-of-care test to help target antibiotic prescribing in patients with acute exacerbations of chronic obstructive pulmonary disease (the PACE study): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2144-8.
- Bafadhel M, Clark TW, Reid C, Medina MJ, Batham S, Barer MR, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest 2011;139:1410-18. https://doi.org/10.1378/chest.10-1747.
- Great Britain . Data Protection Act 1998 1998.
- European Parliament, Council of the European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) 1996.
- BronkoTest n.d. www.bronkotest.co.uk/index.php?/site/bronkotest/references/ (accessed 4 December 2014).
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000;117:1638-45. https://doi.org/10.1378/chest.117.6.1638.
- van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-13.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Schünemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Austin P, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest 2003;124:1421-9. https://doi.org/10.1378/chest.124.4.1421.
- Kocks JW, Tuinenga MG, Uil SM, van den Berg JW, Ståhl E, van der Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res 2006;7. https://doi.org/10.1186/1465-9921-7-62.
- Cals JW, Schot MJ, de Jong SA, Dinant GJ, Hopstaken RM. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Ann Fam Med 2010;8:124-33. https://doi.org/10.1370/afm.1090.
- Offen W, Chuang-Sfein C, Dmitrienko A, Littman G, Maca J, Meyerson L, et al. Multiple co-primary endpoints: medical and statistical solutions – a report from the Multiple Endpoints Expert Team of the Pharmaceutical Research and Manufacturers of America. Drug Inf J 2007;41:31-46. https://doi.org/10.1177/009286150704100105.
- Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Pain 2008;139:485-93. https://doi.org/10.1016/j.pain.2008.06.025.
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Tarenflurbil Phase 3 Study Group . Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 2009;302:2557-64. https://doi.org/10.1001/jama.2009.1866.
- Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444-55. https://doi.org/10.1080/01621459.1996.10476902.
- Butler CC, Gillespie D, White P, Bates J, Lowe R, Thomas-Jones E, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med 2019;381:111-20. https://doi.org/10.1056/NEJMoa1803185.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J, Team RS. Health services research: process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332. https://doi.org/10.1136/bmj.332.7538.413.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. J Admin Governance 2009;4:72-9.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Ritchie J, Spencer L, O’Conner W, Ritchie J, Lewis J. Qualitative Research and Practice. London: Sage; 2003.
- Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. The Qualitative Report 2015;20:1408-16.
- Anthierens S, Tonkin-Crine S, Cals JW, Coenen S, Yardley L, Brookes-Howell L, et al. Clinicians’ views and experiences of interventions to enhance the quality of antibiotic prescribing for acute respiratory tract infections. J Gen Intern Med 2015;30:408-16. https://doi.org/10.1007/s11606-014-3076-6.
- Tonkin-Crine S, Anthierens S, Francis NA, Brugman C, Fernandez-Vandellos P, Krawczyk J, et al. Exploring patients’ views of primary care consultations with contrasting interventions for acute cough: a six-country European qualitative study. NPJ Prim Care Respir Med 2014;24. https://doi.org/10.1038/npjpcrm.2014.26.
- Wood F, Brookes-Howell L, Hood K, Cooper L, Verheij T, Goossens H, et al. A multi-country qualitative study of clinicians’ and patients’ views on point of care tests for lower respiratory tract infection. Fam Pract 2011;28:661-9. https://doi.org/10.1093/fampra/cmr031.
- Huddy JR, Ni MZ, Barlow J, Majeed A, Hanna GB. Point-of-care C-reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: a qualitative study of barriers and facilitators to adoption. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009959.
- Siddiqi A, Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2008;3:31-44. https://doi.org/10.2147/COPD.S1089.
- Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;4. https://doi.org/10.1002/14651858.CD003539.pub2.
- Coenen S, Francis N, Kelly M, Hood K, Nuttall J, Little P, et al. Are patient views about antibiotics related to clinician perceptions, management and outcome? A multi-country study in outpatients with acute cough. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0076691.
- Kayyali R, Odeh B, Frerichs I, Davies N, Perantoni E, D’arcy S, et al. COPD care delivery pathways in five European Union countries: mapping and health care professionals’ perceptions. Int J Chron Obstruct Pulmon Dis 2016;11:2831-8. https://doi.org/10.2147/COPD.S104136.
- Davies F, Risør MB, Melbye H, Spigt M, Brookes-Howell L, O’Neill C, et al. Primary and secondary care clinicians’ views on self-treatment of COPD exacerbations: a multinational qualitative study. Patient Educ Couns 2014;96:256-63. https://doi.org/10.1016/j.pec.2014.05.011.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. https://doi.org/10.1016/S0140-6736(07)61380-4.
- Manafo E, Wong S. Health literacy programs for older adults: a systematic literature review. Health Educ Res 2012;27:947-60. https://doi.org/10.1093/her/cys067.
- Martin LT, Ruder T, Escarce JJ, Ghosh-Dastidar B, Sherman D, Elliott M, et al. Developing predictive models of health literacy. J Gen Intern Med 2009;24:1211-16. https://doi.org/10.1007/s11606-009-1105-7.
- CCEMG-EPPI-Centre Cost Converter 2016. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 28 November 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Monthly Index of Medical Specialities . Monthly Index of Medical Specialities 2017. www.mims.co.uk/ (accessed 28 November 2017).
- Joint Formulary Committee . British National Formulary 2017. www.medicinescomplete.com/about/publications.htm?pub=bnf (accessed 28 November 2018).
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 29 June 2018).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Office for National Statistics . EARN01: Average Weekly Earnings Dataset 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/averageweeklyearningsearn01 (accessed 29 June 2018).
- NICE . Position Statement on Use of the EQ-5D-5L Valuation Set 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 3 July 2018).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- NICE . Pneumonia in Adults: Diagnosis and Management. Clinical Guideline [CG191] 2014. www.nice.org.uk/guidance/CG191 (accessed 29 June 2018).
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5-14. https://doi.org/10.1016/j.jval.2013.08.2291.
- Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017;72:919-27. https://doi.org/10.1136/thoraxjnl-2016-209023.
- Office for National Statistics . Deaths Registered in England and Wales: 2016 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2016 (accessed 29 June 2018).
- Medisupplies . Cutisoft Wipes n.d. www.medisupplies.co.uk/Syringes-Needles/Pre-Injection-Wipes/Cutisoft-Pre-Injection-Wipes (accessed 5 July 2018).
- Amazon . One Touch Ultra Soft Lancets n.d. www.amazon.co.uk/LANCETS-00-4MM-FINGER-PRICKING-DEVICES/dp/B004PU5AO2 (accessed 5 July 2018).
- Medisupplies . Multisorb Gauze Swabs n.d. www.medisupplies.co.uk/First-Aid-Safety/Gauze-Swabs/Multisorb-Gauze-Swabs (accessed 5 July 2018).
- Scientific Laboratory Supplies . GlovePlus Vinyl Gloves n.d. www.scientificlabs.co.uk/product/SAF5654 (accessed 5 July 2018).
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75. https://doi.org/10.1016/j.jclinepi.2008.12.011.
- Khan A, Dickens AP, Adab P, Jordan RE. Self-management behaviour and support among primary care COPD patients: cross-sectional analysis of data from the Birmingham Chronic Obstructive Pulmonary Disease Cohort. NPJ Prim Care Respir Med 2017;27. https://doi.org/10.1038/s41533-017-0046-6.
- White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids – implications for safety and costs: cross-sectional observational study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0075221.
- Andreeva E, Melbye H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam Pract 2014;15. https://doi.org/10.1186/1471-2296-15-80.
- Strykowski DF, Nielsen AB, Llor C, Siersma V, Bjerrum L. An intervention with access to C-reactive protein rapid test reduces antibiotic overprescribing in acute exacerbations of chronic bronchitis and COPD. Fam Pract 2015;32:395-400. https://doi.org/10.1093/fampra/cmv020.
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017;26. https://doi.org/10.1183/16000617.0073-2016.
- Aabenhus R, Jensen JU, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD010130.pub2.
- Cals JW, Chappin FH, Hopstaken RM, van Leeuwen ME, Hood K, Butler CC, et al. C-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPs. Fam Pract 2010;27:212-18. https://doi.org/10.1093/fampra/cmp088.
- Hardy V, Thompson M, Keppel GA, Alto W, Dirac MA, Neher J, et al. Qualitative study of primary care clinicians’ views on point-of-care testing for C-reactive protein for acute respiratory tract infections in family medicine. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-012503.
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Adv Ther 2015;32:69-85. https://doi.org/10.1007/s12325-015-0180-x.
- Nolan CM, Longworth L, Lord J, Canavan JL, Jones SE, Kon SS, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax 2016;71:493-500. https://doi.org/10.1136/thoraxjnl-2015-207782.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
Appendix 1 Clinical effectiveness methods
| Outcome measures | Time point(s) of evaluation of this outcome measure | Changes to outcome measures | Reason for the change |
|---|---|---|---|
| Primary | |||
| Antibiotic consumption (any consumption of antibiotics for AECOPD vs. no consumption of antibiotics for AECOPD) | First 4 weeks post randomisation | ||
| Recovery in terms of COPD health status as assessed using the CCQ total scores | 2 weeks post randomisation | ||
| Secondary | |||
| Prevalence of potentially pathogenic bacteria [including S. pneumoniae, Haemophilus species spp and Enterobacteriaceae] cultured from sputum at 4 weeks and the proportion of bacteria that are resistant | 4 weeks post randomisation | ||
| Prevalence of commensal organisms cultured from throat swabs at 4 weeks and proportion of bacteria that are resistant | 4 weeks post randomisation | ||
| COPD health status over time measured using the CCQ total score | At weeks 1, 2 and 4 post randomisation | ||
| CCQ symptoms domain | At weeks 1, 2 and 4 post randomisation | ||
| CCQ function state domain | At weeks 1, 2 and 4 post randomisation | ||
| CCQ mental state domain | At weeks 1, 2 and 4 post randomisation | ||
| Total antibiotic consumption (number of days antibiotics consumed for AECOPD/any reason) | First 4 weeks post randomisation | Total antibiotic consumption (number of days antibiotics consumed for AECOPD/any reason) during first 4 weeks post randomisation was added in to secondary outcomes | |
| Health utility measured using the EQ-5D | At weeks 1, 2 and 4 and at 6 months post randomisation |
EQ-5D was added to the 6-month postal follow-up Change from the EQ-5D-3L to the EQ-5D-5L |
Owing to the more sensitive instrument becoming available at the time, the EQ-5D-3L used during the internal pilot was replaced by the EQ-5D-5L |
| All-cause antibiotic consumption | During the first 4 weeks post randomisation | All-cause antibiotic consumption during the first 4 weeks was added to the secondary outcomes | |
| Antibiotic prescribing | At the index consultation | ||
| Antibiotic prescribing | During the first 4 weeks post randomisation | Antibiotic prescribing during the first 4 weeks post randomisation was added to secondary outcomes | |
| Use of other COPD treatments including oral steroids | During the first 4 weeks post randomisation | ||
| Adverse effects potentially attributable to antibiotics prescribed for the exacerbation | During the first 4 weeks post randomisation | ||
| Primary and secondary care consultations, including hospitalisations | At week 4 and month 6 | ||
| Costs (total NHS cost) and cost-effectiveness | At month 6 | ||
| Incidence of pneumonia (measured by patient and GP report) | At week 4 and month 6 | ||
| Disease-specific HRQoL over time measured using CRQ-SAS (dyspnoea, fatigue, emotion function, mastery and total scores) | At month 6 | ||
| Pilot study inclusion/exclusion criterion | Change made following pilot | Reason for change |
|---|---|---|
| Required spirometry confirmation of COPD, very severe (GOLD grade IV) excluded | Diagnosis of COPD in medical record: all COPD severities included, but those with a past history of respiratory failure or requiring mechanical ventilation excluded | This change occurred after the study had opened to recruitment in order to minimise the time taken for the initial assessment during times of delivering busy acute clinical care |
| Exclusion of those who had used systemic antibiotics in the past 4 weeks | Exclusion of those currently on antibiotics or who had taken antibiotics previously for the current AECOPD | Initially, those who had recently taken antibiotics were excluded as this would have had an impact on baseline assessments of antimicrobial resistance. We considered that the evidence generated by the study should be applicable to those who have recently had antibiotics |
| Exclusion of those with a life-limiting malignancy | No longer an exclusion criterion | We considered that the experimental intervention should be applicable to these patients |
| Exclusion of those who had taken systemic corticosteroids in the past week | No longer an exclusion criterion | We considered that the experimental intervention should be applicable to these patients |
| Inclusion criteria | Exclusion criteria |
|---|---|
| Has spirometry-confirmed (post-bronchodilator FEV1/FVC of < 0.7) mild, moderate or severe (GOLD grade I, II or III) COPD (FEV1 of ≥ 30% predicted) | Very severe GOLD grade IV COPD (FEV1 of < 30% predicted) or has a past history of respiratory failure or mechanical ventilation |
| Has a current AECOPD with presence of at least one of the following features: increased dyspnoea, increased sputum volume, increased sputum purulence | Has used systemic antibiotics in the last 4 weeks |
| Has AECOPD that has lasted for at least 24 hours and at most 21 days | The responsible clinician feels that urgent referral to hospital is necessary |
| Is aged ≥ 40 years | Has severe illness (e.g. suspected pneumonia, tachypnoea of > 30 breaths per minute, respiratory failure) |
| Is able to provide informed consent | Has a concurrent infection at another site (e.g. UTI, cellulitis) that is likely to produce a systemic response |
| Has a chronic inflammatory condition (e.g. rheumatoid arthritis, polymyalgia rheumatica) | |
| Has a life-limiting malignancy | |
| Has taken systemic corticosteroids (e.g. oral tablets, injections) in the past week (not including systemic corticosteroids prescribed at baseline assessment) | |
| Has cystic fibrosis, a current tracheostomy or bronchiectasis of origin other than COPD | |
| Is immunocompromised (e.g. AIDS, taking immunosuppressive therapy or is receiving anticancer radiotherapy or chemotherapy) | |
| Is currently pregnant | |
| Has previously been recruited to the PACE study |
| Inclusion criteria | Exclusion criteria |
|---|---|
| Has a current acute exacerbation (presenting with at least one of the following: increased dyspnoea, increased sputum volume, increased sputum purulence) that has lasted for at least 24 hours and no longer than 21 days | The responsible GP feels that urgent referral to hospital is necessary |
| Has a diagnosis of COPD in clinical record/on a COPD practice register | Has severe illness (e.g. suspected pneumonia, tachypnoea of > 30 breaths per minute, respiratory failure) |
| Is aged ≥ 40 years | Has concurrent infection at another site (e.g. UTI, cellulitis) that is likely to produce a systemic response |
| Is able to provide informed consent | Has a past history of respiratory failure or mechanical ventilation |
| Is able to provide the primary outcome data at 2 and 4 weeks within the expected windows | Is currently on antibiotics or has had antibiotics for this acute exacerbation of COPD |
| Has an active inflammatory condition (e.g. flare-up of rheumatoid arthritis, gout or polymyalgia rheumatica) | |
| Has cystic fibrosis, a current tracheostomy or bronchiectasis | |
| Is immunocompromised (e.g. AIDS, taking systemic immunosuppressive therapy or receiving anticancer radiotherapy or chemotherapy) | |
| Is currently pregnant |
| CRP result (mg/l) | Interpretation |
|---|---|
| < 20 | Antibiotics are unlikely to be beneficial and usually should not be prescribed |
| 20–40 | Antibiotics may be beneficial – mainly if purulent sputum is present. You may decide to prescribe antibiotics after taking into account the patient’s underlying health status and the features of the current exacerbation |
| > 40 | Antibiotics are likely to be beneficial. Consider prescribing antibiotics unless the patient is assessed as being at lower risk of complications and unlikely to have a bacterial infection (no increased sputum purulence and no features suggesting severe exacerbation) |
Assessment of sputum purulence
-
Patient-reported sputum colour is often not reliable.
-
Purulence can be increased in viral infections as well as bacterial infections.
-
Try and obtain a sputum sample to objectively assess sputum purulence when possible.
-
Ask the patient how much the colour of their sputum has changed from its usual colour. This is particularly pertinent when it is not possible to objectively assess their sputum.
-
The CRP test is particularly useful when the presence of increased purulence is uncertain.
| Amendment number | Details of changes made |
|---|---|
| 1 | Minor changes and addition of further clarification regarding the future use of the samples and the disposal of the capillary tube for the finger prick |
| 2 |
Change of primary outcome measure from CRQ-SAS at 2-week follow-up to CCQ at 2-week follow-up. Addition of CRQ-SAS at 6-month follow-up Inclusion/exclusion criteria rephrasing and some additions Expansion on qualitative evaluation process Alteration to microbiological analysis Change to patient information sheet to add that we will collect participant’s address |
| 3 |
Clarifying and defining which staff at site can assess patient eligibility Change of wording in one of the inclusion criteria from spirometry-confirmed (post-bronchodilator FEV1/FVC of < 0.7) mild, moderate or severe COPD (GOLD grade I, II and III) to spirometry-confirmed mild, moderate or severe (GOLD grade I, II or III) COPD (FEV1 of ≥ 30% predicted) Clarifying that the primary objective and primary outcome is antibiotic use for AECOPD All-cause antibiotic consumption during the first 4 weeks has been added to the secondary objectives, outcomes and analysis sections EQ-5D-5L has been added to the 6-month postal follow-up The CRP guidance has been corrected to the following categories: < 20, 20–40, > 40 mg/l Clarifying the safety reporting procedures New investigator (Carl Llor) and new administrator (Christian Barlow) have been added |
| 4 |
Clarify that the secondary outcome is prevalence of resistant bacteria in throat swab Change from the EQ-5D-3L to the EQ-5D-5L Inclusion/exclusion criteria rephrasing and some additions Clarification that the randomisation says ‘usual care’ not ‘no POCT’ Amend typographical error on back-up randomisation telephone number Amend 4-week follow-up window from –3 days to +14 days from +7 days |
| 5 |
Clarification that Alere will provide training only to those practices using an Alere CRP machine Demographic data, FEV1, clinical history and smoking status no longer collected at baseline Clarification around the contacting of patients for their 6-month questionnaire data, telephone and postal Qualitative interview topic guides, improved wording and flow, but still focused on the same topics (e.g. views on the CRP test, research processes and management of COPD) |
| 6 |
Six-month note review will also include a 12-month note review of antibiotics prescribed prior to the baseline appointment The time frame within which the qualitative interviews with participants can be carried out has been increased from 2 weeks (post 4-week follow-up date) to 4 weeks |
| 7 |
Rephrasing of the secondary outcome measures Clarifying the quantitative process evaluation Clarifying procedures occurring at baseline and at follow-ups Clarification around statistical analyses Rephrasing of microbiological analyses Rephrasing of economic evaluation |
Appendix 2 Clinical effectiveness results
Full intention-to-treat analysis (assuming data are missing at random)
A full ITT analysis was conducted for the co-primary outcomes, with participants with missing outcome data included using multiple imputation. The imputation models contained variables included in the original co-primary analyses (i.e. the Anthonisen criteria and, for the CCQ analysis, the baseline CCQ total score). Twenty imputations were run. For the primary CCQ analysis, 14 missing baseline CCQ scores were imputed using the mean of the valid responses.
The findings in Tables 36 and 37 demonstrate that the adjustment made little difference to the intervention effect estimates, and the conclusions remain unaltered on the basis of these analyses.
| Analysis set | n | AOR (95% CI) | p-value |
|---|---|---|---|
| MITT | 537 | 0.31 (0.20 to 0.47) | < 0.001 |
| Full ITT | 649 | 0.33 (0.21 to 0.52) | < 0.001 |
| Analysis set | n | AMD (90% CI) | p-value |
|---|---|---|---|
| MITT | 563 | –0.19 (–0.33 to –0.05) | 0.023 |
| MITT with missing baseline CCQ imputed via mean substitution | 577 | –0.20 (–0.34 to –0.06) | 0.017 |
| Full ITT | 649 | –0.20 (–0.36 to –0.04) | 0.016 |
Further sensitivity analysis adjusting analyses for missing data (assuming that data are missing not at random)
The observed mean CCQ total score at 2 weeks post randomisation was 2.7 points, with the mean 0.2 points higher (worse) in the control arm than in the CRP arm (Table 38). Figure 16 demonstrates that the conclusions that can be drawn from the primary CCQ analysis are robust to all but the most extreme and implausible assumptions regarding missing participants (i.e. that missing control participants are identical to those observed and that missing CRP participants were over 3 points worse than those observed).
| Trial arm | Total | ||
|---|---|---|---|
| Usual care | CRP POCT | ||
| Observed, n (% total) | 289 (89.2) | 288 (88.6) | 577 (88.9) |
| Missing, n (% total) | 35 (10.8) | 37 (11.4) | 72 (11.1) |
| Observed (mean) | 2.8 | 2.6 | 2.7 |
| Observed (SD) | 1.29 | 1.23 | 1.26 |
FIGURE 16.
Impact of different missing data assumptions on the findings of the primary CCQ analysis. The figure is based on a series of pattern mixture models with mean in unobserved outcome minus mean in observed outcome ranging from 0 (missing at random assumption) to 4 (missing not at random assumption). Black solid line is at y = 0 (no difference between trial arms) and black dashed line is at y = 0.3 (non-inferiority margin).

Table 39 demonstrates that, for all but the most extreme and implausible assumptions (i.e. that missing CRP participants all consumed antibiotics and missing control participants did not), the conclusions drawn on the primary antibiotic analysis remain robust to various missing data assumptions.
| Analysis | n | Trial arm, antibiotics, n (%) | AOR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Control | CRP POCT | ||||
| MITT | 537 | 212 (77.4) | 150 (57.0) | 0.31 (0.20 to 0.47) | < 0.001 |
| Sensitivity 1: all non-responders used antibiotics | 649 | 262 (80.9) | 212 (65.2) | 0.39 (0.27 to 0.58) | < 0.001 |
| Sensitivity 2: all non-responders did not use antibiotics | 649 | 212 (65.4) | 150 (46.2) | 0.39 (0.27 to 0.55) | < 0.001 |
| Sensitivity 3: non-responders used antibiotics if prescribed them at index consultation | 649 | 246 (75.9) | 169 (52.0) | 0.28 (0.19 to 0.41) | < 0.001 |
| Sensitivity 4: non-responders in CRP arm used antibiotics and those in control arm did not use antibiotics | 649 | 212 (65.4) | 212 (65.2) | 0.94 (0.66 to 1.33) | 0.728 |
Analysis accounting for change in eligibility criteria
The models presented in Tables 40 and 41 suggest that, although there is a suggestion of a difference in outcomes before/after the change in eligibility criteria, this is independent of trial arm. The conclusions drawn regarding differences between trial arms remain unaltered when controlling for this change in eligibility criteria, and there is insufficient evidence to suggest that the intervention worked differently pre or post change.
| Model | Variable | AOR (95% CI) | p-value |
|---|---|---|---|
| Without interaction | Before change in eligibility | Reference category for change in eligibility | 0.065 |
| After change in eligibility | 1.97 (0.96 to 4.04) | ||
| Control | Reference category for trial arm | < 0.001 | |
| CRP POCT | 0.31 (0.20 to 0.47) | ||
| With interaction | After change in eligibility (main effect) | 3.01 (1.19 to 7.59) | 0.020 |
| CRP POCT (main effect) | 0.67 (0.21 to 2.19) | 0.511 | |
| CRP POCT × after change in eligibility | 0.41 (0.12 to 1.43) | 0.162 |
| Model | Variable | AMD (95% CI) | p-value |
|---|---|---|---|
| Without interaction | Before change in eligibility | Reference category for change in eligibility | 0.034 |
| After change in eligibility | –0.32 (–0.62 to –0.03) | ||
| Control | Reference category for trial arm | 0.024 | |
| CRP POCT | –0.19 (–0.35 to –0.03) | ||
| With interaction | After change in eligibility (main effect) | –0.43 (–0.83 to –0.04) | 0.032 |
| CRP POCT (main effect) | –0.40 (–0.93 to 0.13) | 0.137 | |
| CRP POCT × after change in eligibility | 0.24 (–0.32 to 0.79) | 0.406 |
Primary analysis on per-protocol population
Excluding participants from the co-primary analyses who were not eligible at the time of recruitment, or (for the primary CCQ analysis) did not provide data within the specified time window for data collection at 2 weeks post randomisation (–1 day/+7 days), did not appreciably change the intervention effect estimates and did not alter the conclusions that could be drawn regarding the co-primary outcomes (Tables 42 and 43).
| Analysis set | n | AOR (95% CI) | p-value |
|---|---|---|---|
| MITT | 537 | 0.31 (0.20 to 0.47) | < 0.001 |
| Per protocol | 525 | 0.27 (0.18 to 0.43) | < 0.001 |
| Analysis set | n | AMD (90% CI) | p-value |
|---|---|---|---|
| MITT | 563 | –0.19 (–0.33 to –0.05) | 0.023 |
| Per protocol | 547 | –0.19 (–0.32 to –0.05) | 0.028 |
Medication consumption over time
The models in Table 44 demonstrate that the consumption of antibiotics for AECOPD declines considerably during the follow-up period and is lower in participants allocated to the CRP arm. There is also some evidence of a differential intervention effect over time. This is best illustrated in Figure 17, which indicates a steeper decline in antibiotic consumption for control participants between weeks 1 and 2 (than for CRP participants), and a further decline between weeks 2 and 4 (whereas the proportion of participants in the CRP arm consuming antibiotics remains stable). The findings from these models are similar to those from the models exploring all-cause antibiotic consumption over time (Table 45).
| Model | Variable | AOR (95% CI) | p-value |
|---|---|---|---|
| Without interaction | Week 1 | Reference category for time point | < 0.001 |
| Week 2 | 0.10 (0.07 to 0.15) | ||
| Week 4 | 0.07 (0.05 to 0.10) | ||
| Control | Reference category for trial arm | < 0.001 | |
| CRP POCT | 0.46 (0.33 to 0.65) | ||
| With interaction | Week 1 | Reference category for time point main effect | < 0.001 |
| Week 2 | 0.07 (0.05 to 0.12) | ||
| Week 4 | 0.04 (0.02 to 0.07) | ||
| Control | Reference category for trial arm main effect | < 0.001 | |
| CRP POCT | 0.29 (0.19 to 0.46) | ||
| Week 2 × CRP POCT | 1.85 (0.98 to 3.50) | 0.005 | |
| Week 4 × CRP POCT | 3.00 (1.53 to 5.86) |
FIGURE 17.
Predicted probabilities of antibiotic consumption for AECOPD over time, by trial arm.

| Model | Variable | AOR (95% CI) | p-value |
|---|---|---|---|
| Without interaction | Week 1 | Reference category for time point | < 0.001 |
| Week 2 | 0.12 (0.09 to 0.17) | ||
| Week 4 | 0.08 (0.06 to 0.12) | ||
| Control | Reference category for trial arm | < 0.001 | |
| CRP POCT | 0.48 (0.34 to 0.66) | ||
| With interaction | Week 1 | Reference category for time point main effect | < 0.001 |
| Week 2 | 0.09 (0.06 to 0.14) | ||
| Week 4 | 0.05 (0.03 to 0.08) | ||
| Control | Reference category for trial arm main effect | < 0.001 | |
| CRP POCT | 0.30 (0.19 to 0.47) | ||
| Week 2 × CRP POCT | 1.92 (1.04 to 3.52) | 0.006 | |
| Week 4 × CRP POCT | 2.79 (1.46 to 5.32) |
The model estimates presented in Table 46 indicate that, although the use of other COPD medication declines considerably over the follow-up period, there is no evidence to suggest a difference between arms and, indeed, no evidence of any differential intervention effect over time (Figure 18).
| Model | Variable | AOR (95% CI) | p-value |
|---|---|---|---|
| Without interaction | Week 1 | Reference category for time point | < 0.001 |
| Week 2 | 0.23 (0.17 to 0.33) | ||
| Week 4 | 0.06 (0.04 to 0.09) | ||
| Control | Reference category for trial arm | 0.301 | |
| CRP POCT | 0.84 (0.61 to 1.16) | ||
| With interaction | Week 1 | Reference category for time point main effect | < 0.001 |
| Week 2 | 0.24 (0.15 to 0.39) | ||
| Week 4 | 0.05 (0.03 to 0.09) | ||
| Control | Reference category for trial arm main effect | 0.357 | |
| CRP POCT | 0.77 (0.45 to 1.33) | ||
| Week 2 × CRP POCT | 0.93 (0.49 to 1.75) | 0.405 | |
| Week 4 × CRP POCT | 1.35 (0.71 to 2.59) |
FIGURE 18.
Use of other COPD treatments over time.

Rates of primary and secondary care consultations during the 6 months post randomisation
The distributions of primary and secondary care consultations during the first 6 months following randomisation for each trial arm are displayed in Figures 19 and 20.
FIGURE 19.
Distribution of number of primary care consultations by arm. (a) Usual care; and (b) CRP POCT.

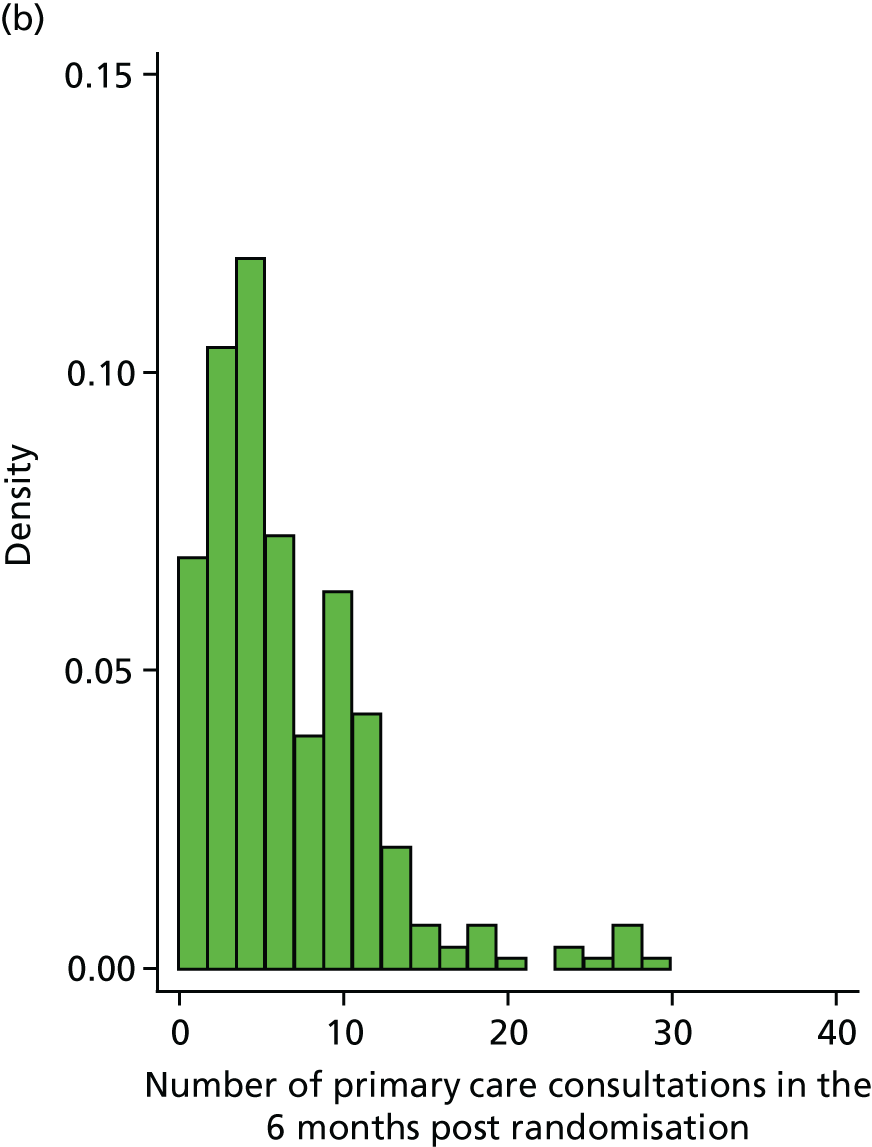
FIGURE 20.
Distribution of number of secondary care consultations by arm. (a) Usual care; and (b) CRP POCT.

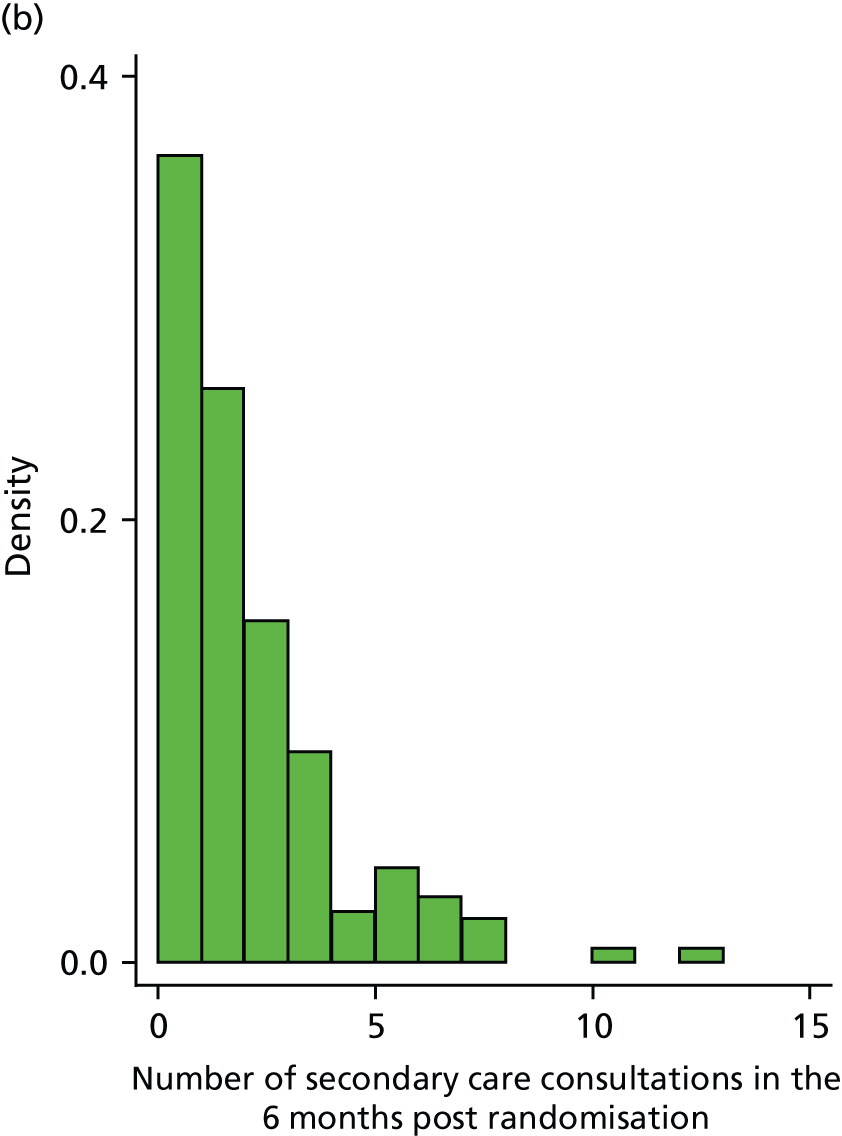
The mean number of primary care consultations during the 6 months following randomisation was 6.3 (SE 0.28) for participants allocated to the control arm and 6.6 (SE 0.29) for participants allocated to the CRP POCT arm. The adjusted incidence rate ratio was 1.04 (95% CI 0.92 to 1.18; p = 0.504). The mean number of secondary care consultations during the 6 months following randomisation was 1.7 (SE 0.12) for participants allocated to the control arm and 1.6 (SE 0.11) for participants allocated to the CRP POCT arm. The adjusted incidence rate ratio was 0.96 (95% CI 0.79 to 1.17; p = 0.719). The mean number of primary and secondary care consultations combined was, therefore, a combination of the two separate consultation variables and, similarly, indicated no evidence of a difference between the arms (Table 47).
| Outcome measure | Time point | Trial arm | Adjusted incidence risk ratioa (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | CRP POCT | ||||||
| n | Mean (SE) | n | Mean (SE) | ||||
| Primary care consultations | Six months post randomisation | 301 | 6.3 (0.28) | 304 | 6.6 (0.29) | 1.04 (0.92 to 1.18) | 0.504 |
| Secondary care consultations | Six months post randomisation | 302 | 1.7 (0.12) | 305 | 1.6 (0.11) | 0.96 (0.79 to 1.17) | 0.719 |
| Primary and secondary care consultations | Six months post randomisation | 302 | 7.9 (0.34) | 305 | 8.2 (0.35) | 1.02 (0.91 to 1.15) | 0.726 |
Prespecified subgroup analysis for the Clinical COPD Questionnaire primary outcome
There was no evidence of any differential intervention effects for the primary CCQ outcome for any of the prespecified subgroups (Table 48).
| Subgroup analysis | Variable | AMD (95% CI) | p-value |
|---|---|---|---|
| COPD severity (GOLD category) (n = 351) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for GOLD I subgroup) | 0.140 |
| CRP POCT | –0.50 (–1.16 to 0.16) | ||
| GOLD I | Reference category for COPD severity main effect (i.e. effect of GOLD subgroup for participants allocated to usual-care arm) | 0.256 | |
| GOLD II | –0.22 (–0.72 to 0.28) | ||
| GOLD III | 0.02 (–0.52 to 0.56) | ||
| GOLD IV | 0.22 (–0.45 to 0.90) | ||
| CRP POCT × GOLD I | Reference category for trial arm × COPD severity interaction | 0.793 | |
| CRP POCT × GOLD II | 0.30 (–0.42 to 1.01) | ||
| CRP POCT × GOLD III | 0.29 (–0.48 to 1.06) | ||
| CRP POCT × GOLD IV | 0.00 (–1.05 to 1.05) | ||
| Severity of COPD exacerbation (Anthonisen criteria) (n = 563) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for 1/3 features subgroup) | 0.737 |
| CRP POCT | –0.06 (–0.39 to 0.28) | ||
| 1/3 features | Reference category for severity of COPD exacerbation main effect (i.e. effect of severity of COPD exacerbation for participants allocated to the usual-care arm) | 0.564 | |
| 2/3 features | 0.16 (–0.15 to 0.48) | ||
| 3/3 features | 0.13 (–0.16 to 0.43) | ||
| CRP POCT × 1/3 features | Reference category for trial arm × severity of COPD exacerbation interaction | 0.627 | |
| CRP POCT × 2/3 feature | –0.22 (–0.66 to 0.23) | ||
| CRP POCT × 3/3 feature | –0.15 (–0.56 to 0.27) | ||
| Presence of potentially pathogenic bacteria cultured from sputum at baseline (n = 353) | Usual care | Reference category for trial arm main effect (i.e. effect of trial arm for no potential pathogenic bacteria subgroup) | 0.263 |
| CRP POCT | –0.16 (–0.43 to 0.12) | ||
| No potential pathogenic bacteria | Reference category for the presence of potentially pathogenic bacteria cultured from sputum at baseline main effect (i.e. effect of presence of potential pathogenic bacteria for participants allocated to the usual-care arm) | 0.286 | |
| Potential pathogenic bacteria | 0.16 (–0.14 to 0.46) | ||
| CRP POCT × no potential pathogenic bacteria | Reference category for trial arm presence of potential pathogenic bacteria interaction | 0.538 | |
| CRP POCT × potential pathogenic bacteria | –0.13 (–0.55 to 0.29) |
Appendix 3 Health economics
Unit costs
| Medication | Dose (mg) | Pack size | Unit cost (£) |
|---|---|---|---|
| Antibiotics | |||
| Amoxicillin | 250 | 14 | 0.69 |
| Amoxicillin | 250 | 21 | 0.97 |
| Amoxicillin | 500 | 14 | 0.90 |
| Amoxicillin | 500 | 21 | 1.26 |
| Azithromycin | 250 | 4 | 1.25 |
| Azithromycin | 500 | 3 | 1.07 |
| Cefalexin | 250 | 28 | 1.44 |
| Cefalexin | 500 | 21 | 1.64 |
| Ciprofloxacin | 100 | 6 | 1.97 |
| Ciprofloxacin | 250 | 10 | 0.68 |
| Ciprofloxacin | 500 | 10 | 0.89 |
| Ciprofloxacin | 750 | 10 | 8.00 |
| Ciprofloxacin | Eye drops | 5 ml | 4.70 |
| Ciprofloxacin/dexamethasone | Ear drops | 5 ml | 6.12 |
| Clarithromycin | 250 | 14 | 1.20 |
| Clarithromycin | 500 | 14 | 2.01 |
| Clarithromycin suspension | 250 mg/5 ml | 70 ml | 4.66 |
| Co-amoxiclav | 375 | 21 | 1.66 |
| Co-amoxiclav | 625 | 21 | 1.73 |
| Doxycycline | 50 | 28 | 1.22 |
| Doxycycline | 100 | 8 | 0.78 |
| Erythromycin | 250 | 28 | 1.30 |
| Flucloxacillin | 250 | 28 | 1.25 |
| Flucloxacillin | 500 | 28 | 1.69 |
| Macrobid | 100 | 14 | 9.50 |
| Metronidazole | 200 | 21 | 1.49 |
| Metronidazole | 400 | 21 | 4.10 |
| Metronidazole | 500 | 21 | 37.82 |
| Nitrofurantoin | 50 | 28 | 13.43 |
| Nitrofurantoin | 100 | 28 | 8.57 |
| Oxytetracycline | 250 | 28 | 0.84 |
| Phenoxymethylpenicillin | 250 | 28 | 0.97 |
| Trimethoprim | 100 | 28 | 0.85 |
| Trimethoprim | 200 | 6 | 0.40 |
| Trimethoprim | 200 | 14 | 0.93 |
| Oral corticosteroids | |||
| Dexamethasone | 2 | 50 | 16.41 |
| Prednisolone | 1 | 28 | 0.68 |
| Prednisolone | 5 | 28 | 0.74 |
| Prednisolone | 25 | 56 | 75.00 |
| Medication | Inhaler type | Dose per puff (mg) | Pack size (puffs) | Unit cost (£) |
|---|---|---|---|---|
| Aclidinium | Eklira Genuair (AstraZeneca UK Limited, Luton, UK) | 322 | 60 | 28.60 |
| Beclometasone | Clenil Modulite 50 (Chiesi Limited, Manchester, UK) | 50 | 200 | 3.70 |
| Clenil Modulite 100 | 100 | 200 | 7.42 | |
| Clenil Modulite 200 | 200 | 200 | 16.17 | |
| Clenil Modulite 250 | 250 | 200 | 16.29 | |
| Formoterol | Atimos Modulite (Chiesi Limited, Manchester, UK) | 12 | 100 | 30.06 |
| Formoterol Easyhaler [Orion Pharma (UK) Limited, Newbury, UK] | 12 | 120 | 23.75 | |
| Foradil (Novartis Pharmaceuticals UK Ltd, Camberley, UK) | 12 | 60 | 28.06 | |
| Oxis Turbohaler 6 (AstraZeneca UK Limited, Luton, UK) | 6 | 60 | 24.80 | |
| Oxis Turbohaler 12 | 12 | 60 | 24.80 | |
| Formoterol/aclidinium | Duaklir Genuair (AstraZeneca UK Limited, Luton, UK) | 12/340 | 60 | 32.50 |
| Formoterol/beclametasone | Fostair 100/6 (Chiesi Limited, Manchester, UK) | 6/100 | 120 | 29.32 |
| Fostair 200/6 | 6/200 | 120 | 29.32 | |
| Fostair NEXThaler 100/6 | 6/100 | 120 | 29.32 | |
| Fostair NEXThaler 200/6 | 6/200 | 120 | 29.32 | |
| Formoterol/budesonide | Symbicort 100/6 Turbohaler (AstraZeneca UK Limited, Luton, UK) | 6/100 | 120 | 33.00 |
| Symbicort 200/6 Turbohaler | 6/200 | 120 | 38.00 | |
| Symbicort 400/12 Turbohaler | 12/400 | 60 | 38.00 | |
| Symbicort pMDI | 6/200 | 120 | 28.00 | |
| Formoterol/budesonide | DuoResp Spiromax 160/4.5 (Teva Pharma B.V., Swensweg, the Netherlands) | 6/200 | 120 | 29.97 |
| DuoResp Spiromax 320/9 | 12/400 | 60 | 29.97 | |
| Formoterol/fluticasone | Flutiform (Napp Pharmaceuticals Limited, Cambridge, UK) | 5/50 | 120 | 14.40 |
| Flutiform | 5/125 | 120 | 28.00 | |
| Flutiform | 10/250 | 120 | 45.56 | |
| Fluticasone | Flixotide Accuhaler 50 (GlaxoSmithKline UK, Uxbridge, UK) | 50 | 60 | 4.00 |
| Flixotide Accuhaler 100 | 100 | 60 | 8.00 | |
| Flixotide Accuhaler 250 | 250 | 60 | 25.51 | |
| Flixotide Accuhaler 500 | 500 | 60 | 43.37 | |
| Flixotide Evohaler 50 | 50 | 120 | 5.44 | |
| Flixotide Evohaler 125 | 125 | 120 | 12.50 | |
| Flixotide Evohaler 250 | 250 | 120 | 20.00 | |
| Glycopyrronium | Seebri Breezhaler (Novartis Pharmaceuticals Ltd, Dublin, Ireland) | 50 | 30 | 27.50 |
| Indacaterol | Onbrez Breezhaler (Novartis Pharmaceuticals Ltd, Dublin, Ireland) | 150 | 30 | 32.19 |
| Onbrez Breezhaler | 300 | 30 | 32.19 | |
| Indacaterol/glycopyrronium | Ultibro Breezhaler (Novartis Pharmaceuticals Ltd, Dublin, Ireland) | 110 | 10 | 10.83 |
| Ultibro Breezhaler | 110 | 30 | 32.50 | |
| Ipratropium bromide | Atrovent (Boehringer Ingelheim Limited, Bracknell, UK) | 20 | 200 | 5.56 |
| Ipratropium Steri-Neb (Teva UK Ltd, Harlow, UK) | 250 ml | 1 ml | 4.71 | |
| Ipratropium Steri-Neb | 250 ml | 2 ml | 5.61 | |
| Olodaterol/tiotropium | Spiolto Respimat (Boehringer Ingelheim Limited, Bracknell, UK) | 2.5/2.5 | 60 | 32.50 |
| Salbutamol | Airomir (Teva UK Ltd, Harlow, UK) | 100 | 200 | 1.97 |
| Airomir Autohaler | 100 | 200 | 6.02 | |
| Easyhaler | 100 | 200 | 3.31 | |
| Salamol Easi-Breathe (Teva UK Ltd, Harlow, UK) | 100 | 200 | 6.30 | |
| Easyhaler | 200 | 200 | 6.63 | |
| Salbutamol nebulised | Salamol Steri-Neb | 2.5 mg/2.5 ml | 20 | 1.91 |
| Salamol Steri-Neb | 5 mg/2.5 ml | 20 | 3.82 | |
| Ventolin® Evohaler® (Glaxo Wellcome UK Limited, Uxbridge, UK) | 100 | 200 | 1.50 | |
| Ventolin® Accuhaler® (Glaxo Wellcome UK Limited, Uxbridge, UK) | 200 | 60 | 3.60 | |
| Ventolin nebulised | Ventolin® Nebules® (Glaxo Wellcome UK Limited, Uxbridge, UK) | 2.5 mg/2.5 ml | 20 | 1.65 |
| Ventolin® Nebules® | 5 mg/2.5 ml | 20 | 2.78 | |
| Salmeterol | Neovent™ [Fannin (UK) Limited, Wellingborough, UK] | 25 | 120 | 29.26 |
| Serevent® Evohaler® (Glaxo Wellcome UK Limited, Uxbridge, UK) | 25 | 120 | 29.26 | |
| Serevent® Accuhaler® (Glaxo Wellcome UK Limited, Uxbridge, UK) | 50 | 60 | 35.11 | |
| Vertine | 25 | 120 | 23.40 | |
| Salmeterol/fluticasone | Seretide 100 Accuhaler (Glaxo Wellcome UK Limited, Uxbridge, UK) | 50/100 | 60 | 18.00 |
| Seretide 250 Accuhaler | 50/250 | 60 | 35.00 | |
| Seretide 500 Accuhaler | 50/500 | 60 | 40.92 | |
| Seretide 100 Evohaler (Glaxo Wellcome UK Limited, Uxbridge, UK) | 25/100 | 120 | 18.00 | |
| Seretide 250 Evohaler | 25/250 | 120 | 35.00 | |
| Seretide 500 Evohaler | 25/500 | 120 | 59.48 | |
| Sirdupla [Generics (UK) Limited t/a Mylan, Potters Bar, UK] | 25/125 | 120 | 26.25 | |
| Sirdupla | 25/250 | 120 | 44.61 | |
| AirFluSal Forspiro (Sandoz Limited, Camberley, UK) | 50/500 | 60 | 29.97 | |
| Terbutaline | Bricanyl® Turbohaler® (AstraZeneca UK Ltd, Luton, UK) | 500 | 100 | 8.30 |
| Tiotropium | Braltus (Teva UK Limited, Eastbourne, UK) | 13 | 30 | 25.80 |
| Spiriva® (Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany) | 18 | 30 | 34.87 | |
| Spiriva Respimat | 2.5 | 60 | 23.00 | |
| Umeclidinium | Incruse Ellipta [GlaxoSmithKline (Ireland) Limited, Dublin, Ireland] | 55 | 30 | 27.50 |
| Vilanterol/umeclidinium | Anoro Ellipta [GlaxoSmithKline (Ireland) Limited, Dublin, Ireland] | 22/55 | 30 | 32.50 |
| Vilanterol/fluticasone | Relvar Ellipta [GlaxoSmithKline (Ireland) Limited, Dublin, Ireland] | 22/92 | 30 | 22.00 |
| Vilanterol/fluticasone | Relvar Ellipta | 22/184 | 30 | 29.50 |
| Aerochamber Plus | Device | 4.90 |
| Resource | Currency code | Unit cost (£) | Notes |
|---|---|---|---|
| Primary care | |||
| GP consultation at surgery | N/A | 36.00 | 9.22 minutes’ duration including direct care staff and qualifications |
| GP consultation at home | N/A | 85.00 | GP home visit (9.22 minutes) plus 20 minutes’ travel time (indirect, £147 per hour) |
| GP consultation by telephone | N/A | 27.36 | 7.1 minutes’ duration |
| Nurse consultation at surgery | N/A | 9.46 | £56.74 per hour (including qualifications), 10-minute appointment assumed |
| Nurse consultation at home | N/A | 23.79 | 10 minutes at £56.74 per hour plus 20 minutes’ travel time (indirect, £43 per hour) |
| Nurse consultation by telephone | N/A | 6.71 | 7.1 minutes’ duration |
| NHS Direct consultation | N/A | 14.60 | Costed as telephone triage (GP led) |
| Secondary care | |||
| A&E attendances | |||
| Unspecified (all) | N/A | 137.74 | Weighted across all specialties according to frequency (number of attendances) |
| Discharged | N/A | 115.10 | |
| Admitted | N/A | 194.12 | |
| Outpatient attendances | |||
| Addiction services | 721 | 21.30 | |
| Anticoagulant service | 324 | 26.26 | |
| Audiology | 840 | 58.33 | |
| Breast surgery | 103 | 141.86 | |
| Cardiology | 320 | 127.67 | |
| Chemical pathology | 822 | 89.11 | |
| Colorectal surgery | 104 | 130.32 | |
| Dermatology | 330 | 101.63 | |
| Diabetic medicine | 307 | 159.31 | |
| Diagnostic imaging | 812 | 37.30 | Assumed as X-ray |
| Endocrinology | 302 | 157.74 | |
| ENT | 120 | 96.87 | |
| Gastroenterology | 301 | 136.57 | |
| General medicine | 300 | 167.05 | |
| General surgery | 100 | 130.06 | |
| Geriatric medicine | 430 | 220.29 | |
| Gynaecology | 502 | 133.01 | |
| Haematology | 303 | 160.58 | |
| Hepatology | 306 | 255.35 | |
| Maxillofacial surgery | 144 | 118.90 | |
| Medical oncology | 370 | 151.12 | |
| Mental illness | 710 | 287.57 | |
| Nephrology | 361 | 150.78 | |
| Neurology | 400 | 175.60 | |
| Neurosurgery | 150 | 205.98 | |
| Old-age psychiatry | 715 | 171.41 | |
| Ophthalmology | 460 | 63.46 | |
| Oral surgery | 140 | 111.47 | |
| Pain management | 191 | 139.12 | |
| Plastic surgery | 160 | 99.95 | |
| Pharmacology | 305 | 114.04 | |
| Physiotherapy | 650 | 48.33 | |
| Palliative medicine | 315 | 207.43 | |
| Podiatry | 653 | 42.84 | |
| Programmed pulmonary rehabilitation | 342 | 58.69 | |
| Radiology | 811 | 84.52 | |
| Respiratory medicine | 340 | 154.77 | |
| Rheumatology | 410 | 142.74 | |
| Speech and language therapy | 652 | 116.05 | |
| Spinal surgery service | 108 | 134.83 | |
| Stroke medicine | 328 | 170.60 | |
| Thoracic surgery | 173 | 194.31 | |
| Transient ischaemic attack | 329 | 179.57 | |
| Trauma and orthopaedics | 110 | 117.01 | |
| Upper gastrointestinal surgery | 106 | 131.80 | |
| Urology | 101 | 105.19 | |
| Vascular surgery | 107 | 153.01 | |
| Inpatient stays | |||
| Non-elective inpatient stay (no further information) | 3058.19 | Weighted across all specialties according to frequency (number of episodes), mean length of stay 7.87 days | |
| Non-elective excess bed-day | 298.41 | Weighted, added per day for stays of > 8 days | |
| Elective inpatient stay (no further information) | 3749.81 | Weighted across all specialties according to frequency (number of episodes), mean length of stay 4.72 days | |
| Elective excess bed-day | 361.67 | Weighted, added per day for stays of > 5 days | |
| Non-elective short stay (no further information) | 615.83 | Weighted across all specialties according to frequency (number of episodes), mean length of stay 1 overnight stay | |
Results
| Medication | Trial arm, total cost (£) | Difference (£) | |
|---|---|---|---|
| CRP POCT (n = 325) | Usual care (n = 324) | ||
| Antibiotics | |||
| Cost at index consultation | 203.29 | 292.34 | –89.05 |
| Cost at 6-month review | 668.33 | 617.80 | 50.53 |
| Oral steroids | |||
| Cost at index consultation | 244.02 | 236.68 | 7.34 |
| Cost at 6-month review | 335.37 | 380.09 | –44.72 |
| Inhaled medications | |||
| Cost at index consultation | 1017.50 | 993.50 | 24.00 |
| Cost at 6-month review | 3053.75 | 2338.51 | 715.24 |
| Total medication cost | |||
| Cost at index consultation | 1464.81 | 1522.52 | –57.71 |
| Cost at 6-month review | 4057.45 | 3336.39 | 721.05 |
| Health-care resource | Trial arm (£) | Difference (£) | |
|---|---|---|---|
| CRP POCT (n = 275) | Usual care (n = 280) | ||
| Primary care: total cost in 4-week follow-up period | |||
| GP visits at surgery in 4 weeks’ follow-up | 3960.00 | 5076.00 | –1116.00 |
| Nurse visits at surgery in 4 weeks’ follow-up | 765.99 | 690.34 | 75.65 |
| GP visits at home in 4 weeks’ follow-up | 340.00 | 425.00 | –85.00 |
| Nurse visits at home in 4 weeks’ follow-up | 23.79 | 47.58 | –23.79 |
| GP telephone consultations in 4 weeks’ follow-up | 820.80 | 1121.76 | –300.96 |
| Nurse telephone consultations in 4 weeks’ follow-up | 60.43 | 53.71 | 6.71 |
| Other health-care contacts in 4 weeks’ follow-up | 511.00 | 467.20 | 43.80 |
| Total cost of primary care use in 4 weeks’ follow-up | 6482.01 | 7881.59 | –1399.58 |
| Primary care: total cost in 6-month review period for any reason | |||
| n = 304 | n = 302 | ||
| GP visits at surgery | 35,748.00 | 34,524.00 | 1224.00 |
| Nurse visits at surgery | 5484.87 | 5049.86 | 435.01 |
| GP visits at home | 2125.00 | 2890.00 | –765.00 |
| Nurse visits at home | 547.17 | 428.22 | 118.95 |
| GP telephone consultations | 8481.60 | 7633.44 | 848.16 |
| Nurse telephone consultations | 423.00 | 396.14 | 26.86 |
| Other contacts | 85.14 | 66.22 | 18.92 |
| Total cost of primary care use | 52,894.77 | 50,987.88 | 1906.89 |
| Primary care related to COPD: total cost in 6-month review period for COPD-related reasons | |||
| GP visits at surgery | 13,968.00 | 15,120.00 | –1152.00 |
| Nurse visits at surgery | 2222.32 | 2014.27 | 208.05 |
| GP visits at home | 935.00 | 1360.00 | –425.00 |
| Nurse visits at home | 0.00 | 333.06 | –333.06 |
| GP telephone consultations | 3310.56 | 3392.64 | –82.08 |
| Nurse telephone consultations | 248.43 | 228.28 | 20.14 |
| Other contacts | 28.38 | 47.30 | –18.92 |
| Total cost of primary care use | 20,712.68 | 22,495.55 | –1782.87 |
| Health-care resource | Trial arm (£) | Difference (£) | |
|---|---|---|---|
| CRP POCT (n = 275) | Usual care (n = 280) | ||
| Secondary care: total cost during the 4-week follow-up period | |||
| A&E visits | 1903.38 | 1433.78 | 469.60 |
| Outpatient appointments | 3988.74 | 5874.06 | –1885.32 |
| Inpatient stays | 14,389.09 | 8804.48 | 5584.62 |
| Other secondary care use | 2212.75 | 2942.83 | –730.08 |
| Total cost of secondary care use | 22,493.96 | 19,055.14 | 3438.82 |
| Secondary care: total cost in the 6-month review period for any reason | |||
| n = 305 | n = 302 | ||
| A&E visits | 11,294.68 | 10,468.24 | 826.44 |
| Outpatient appointments | 46,356.10 | 47,568.97 | –1212.87 |
| Inpatient stays | 106,046.95 | 70,106.45 | 35,940.50 |
| Total cost of secondary care use | 163,697.73 | 128,143.66 | 35,554.07 |
| Secondary care related to COPD: total cost in the 6-month review period for COPD-related reasons | |||
| A&E visits | 4958.64 | 4407.68 | 550.96 |
| Outpatient appointments | 7337.58 | 11,127.03 | –3789.45 |
| Inpatient stays | 40,919.04 | 37,317.07 | 3601.97 |
| Total cost of secondary care use | 53,215.26 | 52,851.78 | 363.48 |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AECOPD
- acute exacerbation of chronic obstructive pulmonary disease
- AMD
- adjusted mean difference
- AOR
- adjusted odds ratio
- CACE
- complier average causal effect
- CCQ
- Clinical COPD Questionnaire
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- CRN
- Clinical Research Network
- CRP
- C-reactive protein
- CRQ-SAS
- Chronic Respiratory Disease Questionnaire Self-Administered Standardized
- CSRI
- Client Service Receipt Inventory
- CUA
- cost–utility analysis
- EQ-5D
- EuroQol 5-Dimensions
- EQ-5D-3L
- EuroQol 5-Dimensions, three-level version
- EQ-5D-5L
- EuroQol 5-Dimensions, five-level version
- EUCAST
- European Committee on Antimicrobial Susceptibility Testing
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LRTI
- lower respiratory tract infection
- MITT
- modified intention to treat
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PACE
- Primary care use of A C-reactive protein point of care test to help target antibiotic prescribing to patients with acute Exacerbations of chronic obstructive pulmonary disease who are most likely to benefit
- POCT
- point-of-care test
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24150).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
