Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/15/13. The contractual start date was in September 2013. The draft report began editorial review in March 2018 and was accepted for publication in February 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Claire A Surr was previously employed by the University of Bradford, which owns the intellectual property (IP) rights to the Dementia Care Mapping™ (DCM) intervention tested in this trial. In this role, she held responsibility for DCM training and method development. She was a technical author on the British Standards Institute’s PAS 800 guide on implementing DCM in health and social care provider organisations. She declares personal fees from Hawker Publications Ltd (London, UK) outside the submitted work. Clive Ballard reports grants and personal fees from Acadia Pharmaceuticals (San Diego, CA, USA) and Lundbeck Ltd (Copenhagen, Denmark), personal fees from Hoffman-La Roche Ltd (Basel, Switzerland), Otsuka Pharmaceutical (Tokyo, Japan), Novartis International AG (Basel, Switzerland), Eli Lilly and Company (Indianapolis, IN, USA) and Pfizer Inc. (New York, NY, USA) outside the submitted work. Murna Downs works at the University of Bradford, which holds the IP rights for DCM and runs courses for practitioners and professionals who wish to learn how to use the method. David Meads was a member of the NIHR Health Technology Assessment Elective and Emergency Specialist Care methods panel from February 2013 to June 2017. Louise Robinson was a member of the NIHR Primary Care Themed Call Board until 18 February 2014.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Surr et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Of those living with dementia in the UK, 38% reside in a care home,1 and at least 80% of people living in care homes have dementia. 2 In 2017, over 16,000 care homes were registered in England, around 11,900 residential homes and 4500 nursing homes,3 the majority of which provide care for older people. Concerns have consistently been raised about care home quality. 4,5 Improvement in care quality and in staff knowledge and skills has been a consistent UK government research and practice development priority for nearly a decade. 6–9 Poor-quality care is associated with poor outcomes for people with dementia, including an increase in behaviours that staff may find challenging to support (BSC). 10,11 Developing an informed and effective care home workforce is a strategic component of improving care quality;6,12 however, there remains limited robust evidence regarding effective evidence-based staff training and practice development interventions for care homes providing care for people with dementia. 13,14 Furthermore, it is often difficult to achieve widespread implementation into real-world practice of evidence-based training interventions developed in the context of research. 14,15
Dementia Care Mapping™ (DCM)16,17 is a whole-home, practice development intervention that has been widely used in health and social care settings, nationally18 and internationally,19 to support the embedding of person-centred care in practice. There is good evidence of its use in practice settings as a quality audit and improvement tool. 20–29 This trial was designed to provide robust evidence on the clinical effectiveness and cost-effectiveness of DCM as an intervention to support care homes in sustainably transferring the learning gained from person-centred care training (PCCT) into care practice. The trial aimed to determine whether or not DCM could provide a solution for achieving widespread implementation of an approach to training and practice development that is practical for use in routine health and social care.
Behaviours that staff may find challenging to support
The behaviours that may be expressed by people with dementia in care home settings, such as agitation, aggression, restlessness, hallucinations, delusions, depression, anxiety and apathy, may be considered by staff as challenging to support. 30 These BSC are also known as ‘neuropsychiatric’ symptoms or ‘behavioural and psychological symptoms of dementia’ (BPSD). We have chosen to use the term BSC rather than BPSD, as the former reflects a more person-centred terminology that better emphasises the biopsychosocial causes of such behaviours. It also represents the terminology used by relatives and staff in care home settings. Up to 90% of people living with dementia experience one or more of these behaviours during the course of their condition30 and BSC are reported in up to 79% of care home residents at any one time. 31 BSC also cause distress to the people with dementia experiencing them,32 are associated with reduced quality of life33,34 and have a negative impact on the well-being of other residents. 35 BSC also have significant associated costs,36,37 including increased risk of hospitalisation,38,39 higher accident and emergency use37 and the production of excess disability, whereby the functional abilities of people decline more quickly than is otherwise expected. 37 Therefore, reducing BSC has the potential to improve the quality of life of people with dementia living in care homes and to reduce the costs of providing care to this group.
Agitation is the most common,31,40 the most distressing to the person with dementia32 and the most difficult to manage41 of all BSC in care home settings. Agitation includes aggressive behaviours, physically non-aggressive behaviours and verbal agitation,42 including pacing, spitting, verbal aggression, constant requests for attention, hitting, kicking, pushing, throwing things, screaming, biting, scratching, intentional falling, hurting oneself and/or others, making sexual advances and restlessness. 43 The presence of these behaviours puts the person who is agitated at risk of triggering aggressive responses from other residents44 and causes distress for other residents, the person’s family and staff. Rates of > 60% are reported of nursing-home residents with dementia displaying agitation,45,46 making it an extremely common BSC and potentially harmful for the people experiencing it, other residents and staff.
The presence of agitation is reported as highly challenging compared with other BSC in terms of clinical management. 41 Agitation places an increased burden on care staff,47,48 who feel less confident in dealing with situations in which residents are agitated than in their management of other BSC. 49 There is an association between a person with dementia experiencing agitation and fewer visits from relatives, experiencing social isolation48 and poorer quality of life. 33 The frequency of agitated behaviours, the difficulties staff have in managing these behaviours and the potential risks they pose to the person, other residents and staff mean that drug treatments such as antipsychotics and other psychotropic medications are frequently prescribed as a first-line management approach. However, antipsychotics are linked to stroke and excess deaths,50 which means that their reduced use is an ongoing priority. 4,9 There is a concern that the mandated reduction in antipsychotic prescribing may in turn lead to the prescription of other psychotropic drugs as an alternative,51,52 despite a lack of evidence of their efficacy. Investigating psychosocial approaches to reducing the incidence of agitation and supporting staff with BSC management is therefore a research priority. 5
Agitation and other BSC are not an inevitable consequence of dementia. Agitation is often exacerbated by poor care practices and a poor surrounding environment of the person with dementia,53 as well as by poorly managed physical health and pain. 41,54 Such behaviours often reflect an expression of unmet needs by a person with dementia in response to staff members’ inadequate understanding of a person’s needs or poor-quality care. 4,54,55 This is often related to a lack of stimulation of and engagement with the person with dementia. 56 For example, Brodaty et al. 57 found significant variability between care homes in terms of the proportions of residents within each setting who displayed BSC, indicating a care home-level effect that may include both admissions criteria and care practices. Likewise, Weber et al. 58 reported a significant reduction in BSC when people with dementia attended a therapeutic day hospital programme compared with when they were at home, again indicating the impact of the psychosocial environment. The presence of agitation within individuals with dementia in care home settings is, therefore, likely to be associated with organisational aspects of care and the care culture. 54 Therefore, the use of psychosocial interventions that address the quality of care practice4,59–61 are recommended, with agitation being a key treatment target area for people with dementia in care homes. 62
Person-centred care
Person-centred care is an effective psychosocial approach in dementia care63 that is considered a best-practice approach to reducing agitation and other BSC. 59 Person-centred care means providing a supportive social environment within a care setting in which people with dementia are valued and treated as individuals and staff are encouraged to see the world from their perspective. 59,64 Person-centred care, therefore, involves evaluating and responding to the unique needs of each person with dementia and offering an individualised approach. The National Institute for Health and Care Excellence (NICE) and Social Care Institute for Excellence (SCIE) dementia guideline59 recommends individualised, holistic or person-centred assessment and care planning, with regular review and individually tailored and monitored psychosocial interventions for BSC. The delivery of care that is person centred is associated with a reduction in agitated behaviours,65 and in BSC more generally,61 and with reduced use of antipsychotics. 63,66,67 Bird et al. 68 found that multifaceted, individualised interventions lead to significant reductions in BSC. Therefore, the most useful interventions to effect change identify individual causes of BSC and suggest appropriate person-centred solutions. 68–70 This approach is reliant on staff having the required knowledge, skills and confidence to deliver person-centred care. The provision of person-centred support is an element of the common induction standards71 for all social care workers in England. The provision of at least basic training to staff on person-centred care is expected within all care homes in England59 and is a regulatory requirement. 72 Currently, there are no widely implemented quality criteria for PCCT and the content, approaches, quality and efficacy of PCCT vary considerably across the sector. 73 Effective PCCT can produce immediate practice benefits;65,67 however, owing to the variability of the amount, content and quality of PCCT that staff receive across the sector, the knowledge, skills and staff confidence levels in relation to delivery of person-centred care remain a concern. 49,74 Research indicates that standardising PCCT is unlikely to address these issues14 and, therefore, evidence-based approaches to help staff sustainably embed PCCT into practice are required. 15
Although effective PCCT can produce immediate practice benefits, evidence suggests that PCCT alone might not sustain change over time13,65,67,75 and that PCCT needs to be accompanied by an additional intervention to support ongoing change. 66,76 For example, Fossey et al. 66 employed PCCT alongside a comprehensive 10-month focused intervention for training staff (FITS), including ongoing staff training and support. At post test, antipsychotic medication use had decreased by > 40% in the intervention group. Chenoweth et al. 63 provided PCCT to two staff members who then disseminated person-centred care practice across the site. Researchers provided additional individualised care planning and ongoing telephone support during a 4-month intervention period. Ten months after randomisation, agitation levels were significantly lower than in the usual-care control sites. A limitation of both of these studies is that it is unclear whether PCCT, additional support or both caused the effect. Evidence of the efficacy of PCCT after a longer follow-up period is limited;13 however, Moniz-Cook et al. 67 found that the benefits of PCCT alone were not sustained after 1 year. The PCCT programmes evaluated thus far indicate that embedding additional support into the training intervention is required to produce sustained benefits. 15,77,78 Implementing evidence-based health-care interventions in real-world practice is a recognised challenge, with barriers to implementation of research-designed interventions reported across all areas of practice. 79–81 Current successful interventions that combine staff training with ongoing support, such as the FITS,66 are resource intensive, requiring regular ongoing input from a specialist practitioner, and it has not yet been possible to implement them widely in everyday practice. 82 Interventions are required that provide staff with knowledge to support BSC management and that are cost-effective and feasible to implement. Any such intervention will need to accommodate the varying amounts, content and quality of PCCT that is a feature of the sector. DCM is an intervention that may address this issue.
Dementia Care Mapping™
This tool, DCM,16,17 is an established, routine care home/NHS practice development intervention that is recommended in the NICE/SCIE dementia guideline59 and is regularly used for ensuring that a systematic approach is used in the provision of individualised person-centred care. DCM is an observational tool set within a practice development cycle that is used to support the sustained implementation of PCCT in dementia care practice. 83 Following initial formal training of care staff to use the tool, its application includes five phases: briefing, observation, data analysis and reporting, feedback and action-planning. A detailed overview of the DCM intervention is provided in Chapter 2, Dementia Care Mapping™ (intervention arm). This cycle is repeated every 4–6 months to monitor and revise action plans. DCM implementation, therefore, requires no external input over the long term and is thus potentially less resource intensive and more closely aligned with real-world dementia practice than other interventions aiming to address BSC. 66
Although DCM has been used in dementia care for nearly 20 years, including in care home settings,25,84–87 and has strong face validity within the practice field,88 there is limited robust evidence of its effectiveness in relation to clinical outcomes such as the reduction of BSC. Reported benefits of DCM include the improvement of well-being in people with dementia,22,27,89 helping staff consider care delivery from the point of view of the person with dementia and the production of evidence to underpin action-planning that in turn motivates staff and increases their confidence to deliver person-centred care. 87,88
Evidence of the effects of Dementia Care Mapping™
Only six published studies have examined the benefits of using DCM for improving clinical outcomes in care homes: two pilot studies employing a pre-test/post-test design,90,91 one quasi-experimental controlled trial92 and three cluster randomised controlled trials (RCTs). 63,93,94 None of these was carried out in the UK. At the time of submission of the grant application for this trial, only the two pilot studies90,91 and one of the RCTs had been published. 63
A pilot study conducted in the Netherlands91 utilising a one-group pre-test/post-test design found that DCM, used alone, reduced verbal agitation and anxiety in people with dementia. It also improved care staff’s feelings of connection with their clients. A pre-test/post-test design pilot study90 conducted in three Australian care homes found that DCM led to improvements in the quality of staff interactions and reductions in agitation and depression, compared with three control homes. A quasi-experimental controlled trial conducted in Germany92 compared outcomes at 6 and at 16 months with baseline. Nine nursing homes units, located in nine nursing homes owned by the same group, were allocated not at random to one of three arms: a no-intervention control group (n = 3), a DCM-experienced intervention group (n = 3) and a DCM intervention group (n = 3). The DCM-experienced group had been exposed to two externally delivered DCM cycles annually over a number of years. The DCM intervention group had no previous exposure to DCM, but had expressed an interest in undertaking the method. Two staff members from both intervention groups received DCM training and were requested to implement three DCM cycles over 18 months. The control group received an intervention based on training staff about quality of life (QoL), followed by QoL assessment, using a standardised tool, of all care home residents at least every 6 months. The study found no significant difference between the two intervention groups and the control, and no difference between the two intervention groups, as regards QoL or BSC.
The first cluster RCT evaluating the efficacy of DCM was conducted in Australia63 in 15 care homes randomised equally between three arms [usual care (control), PCCT and DCM] and included 289 people with dementia (18% loss to follow-up at 10 months). The trial found that 10 months after randomisation, DCM, when used alone, was associated with significantly reduced agitation and falls in people with dementia compared with the control and PCCT groups, and with reduced staff feelings of burnout. 95 A three-arm cluster RCT93 was also conducted in 15 care homes in Norway, randomising equally between a control group, person-centred care framework implementation and DCM. The study recruited 446 people with dementia (29% loss to follow-up at 10 months). It found significant reductions in overall neuropsychiatric symptoms as measured by the Neuropsychiatric Inventory (NPI), significant reductions in agitation and psychosis as measured by the NPI subscales and a significant improvement in QoL compared with the usual-care control. Both trials had explanatory designs involving researcher-led cycles of DCM with variable degrees of input from trained care home staff, which restricted the generalisability of the results, as the usual implementation of DCM is practitioner led. A Dutch cluster RCT conducted in 34 units across 11 care homes compared DCM with a usual-care control. 94 It recruited 434 residents (35% loss to follow-up at 12 months) and found no difference in residents’ agitation between the DCM intervention and control homes. Positive staff outcomes were found in the intervention group, including significantly fewer reported negative emotional reactions and significantly more positive reactions towards people with dementia than in the control group. The trial authors identified potential DCM intervention fidelity issues, finding less than desirable implementation in some clusters. All three RCTs were exploratory and each included only two full cycles of DCM before the final follow-up, with follow-up periods of only 10–12 months post randomisation, reducing the time for any potential change or impacts to be seen.
The results of these existing studies are mixed in terms of the reported efficacy of DCM. The studies that included researcher-led cycles of DCM (Australia and Norway) showed efficacy for some outcomes, whereas studies with cycles of DCM led by care home staff have shown no benefits of DCM (the Netherlands and Germany). A recent systematic review of DCM implementation96 found limited research in this area, with implementation found to be challenging across a number of the published studies. There was some consensus that appropriate mapper selection, preparation and ongoing support during DCM implementation, alongside effective leadership for DCM within an organisational context of commitment to the delivery of person-centred care, could support better implementation.
In summary, the limitations of the existing studies include:
-
a relatively small number of clusters (Australia and Germany) or small numbers of care homes containing multiple clusters (the Netherlands and Norway)
-
the use of DCM alone, rather than alongside PCCT in accordance with UK best-practice guidelines83 (Australia and the Netherlands), which reflects the context within each country at the time of the trial, for example in Australia, where PCCT was the exception rather than assumed good practice
-
only two full cycles of DCM before the final follow-up, which limited the potential for impacts to be seen, owing to the length of time that changes within care home practice can take to implement, and thus limited the demonstration of potential resident benefits (Australia, Norway and the Netherlands)
-
a follow-up period of no more than 12 months after randomisation, reducing the time for potential changes and impacts to be seen (Australia and Norway)
-
explanatory trial designs (Australia and Norway) involving researcher-led cycles of DCM with variable degrees of input from trained care home staff, potentially limiting staff ownership of the DCM process, the implementation of any action plans and the longer-term sustainability of DCM use; this also restricts the generalisability of the results, as the usual implementation of DCM in care practice is practitioner led
-
no formal, published process evaluation (Australia and Norway)
-
the studies were conducted in Australia, Norway, Germany and the Netherlands, where care funding, policy, context, regulations and processes are different from those of the UK.
Despite promising results on the potential efficacy of DCM in care home settings, the conduct of these trials in countries where usual-care practices, funding and systems are different from the UK and where DCM was implemented differently from its use in the UK means that their results cannot be directly transferred. A definitive RCT evaluating the clinical effectiveness and cost-effectiveness of DCM for helping staff to implement person-centred care in UK care home settings, building on this previous work, is therefore needed to inform future UK care home practice.
Rationale for the research
The knowledge intended to be gained from this trial, beyond that gained within the existing RCTs, was:
-
Previous trials used explanatory designs. By contrast, in this trial, a pragmatic trial design reflecting the conditions of DCM implementation in usual practice in UK care home settings would be used. In particular, trained care home staff, rather than researchers, led the cycles of DCM implementation. The trial design, size and statistical power allowed definitive conclusions to be drawn regarding the effectiveness of DCM as an intervention in usual practice within UK care home settings.
-
Previous RCTs had conducted only one or two DCM cycles with a follow-up period of a maximum of 12 months. In this trial, it was intended that care homes would implement three cycles of the DCM intervention with follow-up over a period of 16 months. This is beneficial because some anticipated practice changes, for example to the underlying care culture, are likely to take time to implement. In addition, given the annual staff turnover rates of around 30%97 in care homes, which may potentially lead to longer-term implementation challenges, a longer follow-up period was necessary to investigate whether or not longer-term effects and sustainability could be achieved within this context.
-
A full economic evaluation within this pragmatic trial design was included, offering a definitive position on cost-effectiveness. Only one of the previous trials conducted an economic evaluation and, given its explanatory design and conduct in a funding system different from that of the UK, the findings cannot be confidently generalised.
The design of this trial built on existing explanatory trials to offer a definitive assessment of the clinical effectiveness and cost-effectiveness of DCM as a standard clinical intervention in care home settings.
Aims and objectives
The aim of the DCM Enhancing Person-centred care In Care homes (EPIC) cluster RCT was to evaluate the clinical effectiveness and cost-effectiveness of DCM implemented in addition to usual care (intervention), compared with the usual care (control) for people with dementia living in care homes in the UK.
It aimed to answer the following primary and secondary research questions.
Primary research questions
At 16 months after the randomisation of care homes, is the intervention:
-
more effective than the control in reducing agitation in residents with dementia as measured by the total Cohen-Mansfield Agitation Inventory (CMAI) score?
-
more cost-effective than the control?
Secondary research questions
Is the intervention more effective than the control at 6 and at 16 months after randomisation in:
-
reducing BSC in people with dementia over time?
-
reducing the use of antipsychotics and other psychotropic drugs in residents with dementia?
-
improving mood and QoL in residents with dementia?
-
improving care home staff well-being and role efficacy?
-
improving the quality of staff–resident interactions over time?
Other questions the trial sought to explore related to:
-
the safety profile of the intervention as assessed by the number and types of adverse events
-
any differential predictors of the effects of the intervention
-
the process, challenges, benefits and impact of implementing the intervention.
Chapter 2 Trial design and methods
Trial design
This section outlines the trial design and procedures at the commencement of trial recruitment. The original trial protocol is published elsewhere. 98 Subsequent amendments to the original trial protocol, after trial commencement, are highlighted throughout this section and then reported in detail in Summary of changes to project protocol.
This trial was a pragmatic, multicentre, cluster RCT of DCM plus usual care (intervention) versus usual care alone (control) in residential, nursing and dementia care homes across West Yorkshire, Oxfordshire and South London for people with dementia.
Owing to greater than expected loss to follow-up during the trial, a design change was approved to move from a closed-cohort to an open-cohort design, with additional residents recruited at the 16-month follow-up and the cross-sectional sample of residents used within the primary analysis (see Summary of changes to project protocol). The cross-sectional sample of residents was used in the primary statistical analysis (and a secondary health economic analysis), defined at baseline and at 16 months, respectively, as all residents registered at care home randomisation and at 16 months. The closed-cohort sample of residents was used in the primary health economic analysis (a supportive statistical analysis and all analyses of 6-month outcomes), defined simply as all residents registered at care home randomisation.
As the aim of DCM is to change practice across the whole care home setting and it is not possible to limit the potential effects to the care provided to only a sample of people with dementia living in the care home, a cluster design was justified. This influenced the decision to consider two important sources of clustering: cluster randomisation and DCM treatment provision, with care homes nested within treatment arms. Owing to this, we anticipated that the clustering effect would vary across arms, with a higher intracluster correlation coefficient (ICC) in the intervention arm. Therefore, an unequal allocation of care homes on a ratio of 3 : 2 to intervention and control groups, respectively, was implemented. An integral cost-effectiveness analysis and a nested qualitative process evaluation were included.
Ethics approval, research governance and study oversight
Ethics approval for the study was granted by National Research Ethics Service (NRES) Committee Yorkshire and the Humber – Bradford Leeds on 14 February 2013, Research Ethics Committee (REC) reference number: 13/YH/0016. Care home insurance and indemnity applied to trained mappers who implemented the intervention within the care home setting. Appropriate site-specific approvals were obtained from the three participating hubs: Yorkshire (Bradford Teaching Hospitals NHS Foundation Trust), Oxford (Oxford Health NHS Foundation Trust) and London (Guy’s and St Thomas’ NHS Foundation Trust). The trial was registered with the International Standard Randomised Controlled Trial Register (ISRCTN) reference 82288852. Day-to-day management of the trial was undertaken by a Trial Management Group (TMG) comprising the co-applicants, trials researchers and staff, as well as a patient and public involvement (PPI) representative. This group met twice before the official start of the project, monthly during trial set-up and then bi-monthly or quarterly. Updates on trial progress were provided by e-mail between meetings. A Lay Advisory Group was established and contributed to TMG decisions (see Patient and public involvement).
Trial Steering Committee
The trial was overseen by a Trial Steering Committee (TSC) comprising five independent members (three academic members, one PPI representative and one care home representative). The TSC monitored trial recruitment, retention, timelines, intervention adherence, data return and quality and considered new issues. It also provided advice and approval for changes to the protocol or trial procedures. It met approximately every 6 months throughout the trial.
Data Monitoring and Ethics Committee
An independent Data Monitoring and Ethics Committee (DMEC), comprising four academic members, met approximately every 6 months during the trial. It reviewed unblinded data, recruitment, retention, intervention implementation and safety by group. The DMEC also undertook an annual review of any serious adverse events (SAEs).
Participants
It was intended that 750 residents with dementia from a random sample of 50 care homes would be recruited, along with participants’ relatives and care home staff.
Care home eligibility, recruitment and consent
Care home eligibility
To be eligible for the trial, a care home was required to:
-
have a sufficient number of permanent residents with dementia [based on a formal diagnosis or Functional Assessment Staging Test of Alzheimer’s Disease (FAST) score of 4+] eligible to participate, achieving a minimum of 10 residents registered to the trial prior to care home randomisation
-
have a manager or nominated person agreeing to sign up to the trial protocol as the research lead for the duration of the project
-
have agreed to release staff for DCM training and subsequent mapping processes
-
be within the trial catchment area.
Care homes were not eligible for the trial if they:
-
were subject to Care Quality Commission (CQC) enforcement notices, admission bans or relevant moderate or major CQC compliance breaches
-
were receiving other special support for specific quality concerns, such as being currently subject to, or having pending, any serious safeguarding investigations, or receiving voluntary or compulsory admission bans or local commissioning special support, owing to quality concerns
-
had used DCM as a practice development tool within the 18 months prior to randomisation or were planning to use DCM over the course of trial involvement
-
were currently in, had recently taken part in or were planning to take part in another trial that conflicted with DCM or data collection.
If a care home was a large multisite or multifloor establishment, the one or two units with the largest percentage of residents with dementia, or in which the manager felt that DCM implementation would be most beneficial, were selected to participate as one home.
Care home recruitment
Catchment areas for each recruitment hub (Leeds Beckett University, King’s College London and Oxfordshire Health NHS Trust) were established based on postcode districts/boroughs in West Yorkshire, South London and Oxfordshire, respectively. All care homes in the catchment areas were identified and screened for initial eligibility via publicly available information (home type, number of beds and CQC status). Care homes that were deemed eligible were randomly ordered within catchment areas and divided into batches. The first batch of homes from each hub were sent the care home information sheet [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)] by post. A researcher then contacted the care homes by telephone within 1 to 3 weeks to determine their interest in taking part. If a care home expressed interest in taking part, the researcher conducted initial eligibility screening ahead of visiting to determine full eligibility and to initiate care home consent and management permissions [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. If the researcher was unable to make contact with the care home following several attempts, a decision was made to cease attempting to contact. Once all care homes within a batch had been contacted, or deemed uncontactable, the next batch was approached until sufficient homes were recruited.
Dementia training audit and provision of dementia awareness training
As person-centred care is considered best practice within UK care homes,59 it was expected that homes would have routinely provided staff with appropriate PCCT. 72 As the quality of PCCT is variable across the sector in the UK, to ensure that each care home met at least minimum dementia awareness training standards, a training audit was developed by the research team (its content and the minimum standards required in the trial are reported elsewhere). 99 The training audit was completed in each care home prior to baseline data collection. The researcher completed this by reviewing training records and having discussions with home managers and/or other relevant staff (e.g. the training lead). When homes fell below the minimum standard, they received a half-day dementia awareness course modified in consultation with service users from an existing resource developed by Bupa Care Services and the University of Bradford. 100 The course was delivered by an experienced trainer/mentor who coached a member of the care home staff to be able to deliver the course to additional staff. Care homes were expected to deliver the training to at least 20% of permanent direct care staff prior to baseline data collection and to complete paperwork detailing how many staff members received the training and when. Based on CQC data,101 we expected up to 20% of homes to require this dementia awareness package.
Resident eligibility, recruitment and consent
Residents were recruited to the trial at baseline, prior to care home randomisation. Additional residents were recruited 16 months after a design change to the study, owing to larger than anticipated loss to follow-up (see Design change).
Resident eligibility
At baseline, residents were eligible for the trial if they:
-
were a permanent resident in the care home and not present for receipt of respite or day care
-
had a formal diagnosis of dementia or a score of 4+ on the FAST102 (indicating mild to severe dementia) as rated by the home manager or another experienced member of staff
-
were appropriately consented (in accordance with the Mental Capacity Act 2005103 and clinical trials guidance on informed consent104,105)
-
had an allocated member of staff willing to provide proxy data
-
had sufficient proficiency in English to contribute to the data collection required for the research.
At baseline, residents were not eligible for the trial if they were:
-
known by the care home manager and/or relevant senior staff member to be terminally ill (e.g. formally admitted to an end-of-life care pathway)
-
permanently bed-bound/cared for in bed
-
currently in, had recently taken part in or were planning to take part in another trial that conflicted with DCM or with the data collected in the trial.
Resident screening
The researcher, along with the care home manager and/or a relevant member of senior staff, screened all care home residents to identify eligible people with dementia to be approached to take part in the trial. The basic demographics of all residents and their eligibility or reasons for ineligibility at screening were recorded, using only the screening number.
Resident informed consent
In accordance with the Mental Capacity Act 2005,103 all eligible residents were assumed to have capacity to consent unless assessed otherwise. The manager/senior staff member approached each eligible resident and sought their permission for the researcher to speak with them. If the resident had capacity and gave verbal consent to speak to the researcher, this was documented and the researcher approached them to discuss the study. If the resident was deemed to lack the capacity to make this decision, then the process for appointing a consultee was followed (see Consent for those without capacity).
The researcher approached each resident who had capacity and agreed to speak to them, explaining the trial using the appropriate documentation and undertaking a further documented assessment of capacity to give informed consent. The resident was provided with the resident information sheet [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)] and, at least 24 hours later, they were given the opportunity to ask any further questions and then, for those with capacity, formal consent to participate in the trial was sought. If the resident was deemed by the researcher at any point to lack the capacity to consent, the process for appointing a consultee was followed (see Consent for those without capacity).
Consent for those with capacity
Residents who were able to give informed consent were asked to sign, or make a mark on, the trial consent form [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. For those who were not able to sign, a witness confirmed that informed consent had been given. Given the progressive nature of dementia, a further capacity assessment was conducted at each data collection point by the researcher to assess continued capacity. In the case of residents who lost capacity during the trial, appropriate guidance on consent in the light of changed capacity was followed,106 involving the appointment of a consultee (see Consent for those without capacity). When a resident had capacity and consented to taking part in the trial, consent to approach his or her main carer (relative/friend) was sought regarding their participation as a proxy informant.
Consent for those without capacity
When a resident was assessed and found to lack the capacity to give informed consent, a ‘Personal Consultee’ was appointed to give advice on the resident’s wishes. This was usually a relative or a close friend. When the resident had no close family or friends able or willing to act as Personal Consultee, a member of staff in the care home who knew them well but who was not actively involved in any elements of the research process (e.g. as a mapper or in giving proxy data on the resident) was appointed as a ‘Nominated Consultee’.
If the proposed Personal Consultee was present in the care home, they were approached by the researcher and given all of the appropriate documentation [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)] in person and asked for written consent to hold their personal details to enable the researcher to directly contact them thereafter. The proposed Personal Consultee was given at least 24 hours to talk to the resident and other relatives/friends about the resident’s wishes. The Personal Consultee was then asked to return the declaration form by post, within a week, expressing their advice on the resident’s wishes regarding taking part in the trial. If the Personal Consultee was not present in the care home, the documentation was sent to them by post, via the care home, on the researcher’s behalf. Details were provided on how to contact the researcher should they wish to discuss the role. For both methods of approach, if the declaration form had not been returned after 1 week, a follow-up reminder was sent by post by the researcher informing the Personal Consultee that a Nominated Consultee would be identified if no response was received within 1 week. If, after a further week, the declaration form had not been returned, the process of appointing a Nominated Consultee was followed.
A Nominated Consultee identified by the manager was approached using the appropriate documentation [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)] to discuss the potential involvement in the trial with the resident, other staff members who knew them well and any relatives/friends. The Nominated Consultee was then asked to complete the declaration form, providing advice on the resident’s wishes.
Personal and Nominated Consultees were advised that they could approach the researchers at any time to indicate if they felt that the person they were representing had changed their mind about participating in the trial and to withdraw them from participation. Given the potential frailty of those serving as Personal Consultees, a review of Personal Consultees’ capacity was undertaken by the researcher via the care home manager at 6- and 16-month follow-ups, where feasible.
Staff roles, eligibility, recruitment and consent
Staff roles
There were five staff roles within the trial, some of which were mutually exclusive (Table 1):
-
to act as a Nominated Consultee for residents (see Consent for those without capacity)
-
to provide data on standardised measures relating to their role (see Staff measures)
-
to provide proxy informant data on residents they know well (see Proxy informant eligibility, recruitment and consent)
-
to become a trained DCM mapper (see Mapper identification, eligibility and consent)
-
to participate in the trial’s process evaluation (see Process evaluation and assessment of treatment implementation).
| Staff roles | Nominated Consultee | DCM mapper | Proxy informant |
|---|---|---|---|
| Nominated Consultee | ✗ | ✗ | |
| Staff measures | ✗ | ✓ | ✓ |
| Proxy informant | ✗ | ✗ | |
| DCM mapper | ✗ | ✗ | |
| Process evaluation | ✗ | ✓ | ✓ |
Staff measures
To be eligible to complete a staff measures booklet, staff were required to be a permanent, contracted, agency or bank member of staff at the time of data collection and have sufficient proficiency in English. Consent to participate in this role was assumed through staff return of the booklet. The staff measures booklets and accompanying information sheets [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)] were distributed to all eligible staff members at each data collection visit, by either the researcher or the care home manager. Booklets were returned anonymously by the staff member either via a sealed envelope to a locked box located within the care home or posted directly to the research office in the stamped return envelope provided.
Proxy informant eligibility, recruitment and consent
To be eligible to act as a proxy informant and to provide proxy data on a resident, staff had to be a permanent or contracted member of staff who knew the resident well. Bank or agency staff were not eligible for this role. Potential proxy informants were identified by the care home manager/senior member of staff using the appropriate trial documentation [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. Where possible, the same proxy informant was used for each resident throughout the trial, although this was not always possible owing to staff turnover, annual leave and shift patterns.
Relative/friend eligibility, recruitment and consent
Where possible, a relative or friend who visited the care home regularly was identified for each participating resident to provide proxy data. The relative/friend proxy was identified in discussion with either the resident or the care home manager/senior member of staff. They could also act in the role of Personal Consultee. To be eligible to provide proxy data, relatives/friends were required to have visited the resident at least once per week over the previous month, be willing to provide data by either telephone or post during the data collection week and have sufficient proficiency in English to contribute to the data collection required. Relatives/friends were approached either in person by the care home manager or researcher or by post, depending on visiting patterns and times, using the appropriate trial documentation [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)].
Relative/friend recruitment took place at baseline and continued at the 6-month follow-up in some homes until December 2015, when the decision to cease further recruitment was made owing to low overall relative/friend recruitment. Data continued to be collected from consented relatives/friends throughout the trial. Their continuing eligibility for participation was reassessed at each subsequent data collection point because of changing patterns of visiting over time. When relative/friend proxies withdrew from the trial, additional participant relatives/friends were not recruited.
Registration, randomisation and blinding
Registration of residents
Residents recruited at baseline were registered with the Clinical Trials Research Unit (CTRU) at the University of Leeds following care home recruitment, training review (see Dementia training audit and provision of dementia awareness training), eligibility confirmation, obtaining informed consent and resident-level baseline data collection, but prior to care home randomisation. Following a design change (see Summary of changes to project protocol), additional residents were recruited at 16 months and were registered with the CTRU after confirmation of eligibility, informed consent and collection of their resident-level data.
Randomisation, stratification and blinding
Immediately following baseline, once all residents, staff and relatives/friends were recruited and registration was complete, care homes were randomised using the 24-hour automated randomisation system at the CTRU. Care homes fulfilling eligibility criteria were randomised on a 3 : 2 basis to either the intervention or the control group, respectively. A computer-generated minimisation programme was used,107 incorporating a random element to ensure that the arms were balanced for the following care home characteristics:
-
home/unit type (general residential/nursing or specialist dementia care)
-
size (large, ≥ 40 beds; or medium/small, < 40 beds)
-
provision of dementia awareness training by research team (yes or no)
-
recruiting hub (West Yorkshire, London or Oxford).
To maintain blinding of trial researchers collecting data within care homes, randomisation was performed by the CTRU Data Management team, who were therefore not blind to treatment allocation. Following randomisation, the CTRU informed the care home manager of the treatment allocation, by telephone call or e-mail. The intervention lead was notified of homes allocated to the intervention, so that arrangements could be confirmed for training with consented mappers and contact with the DCM expert mapper could be initiated. Researchers were not informed of treatment allocation and agreed procedures were applied to maintain blinding throughout the trial. Other CTRU staff were informed of treatment allocation only if this was required to undertake their role. All occurrences of unblinding and the reasons for and method of unblinding were recorded.
Because researchers were blinded, they were unaware of the identity of trained mappers. Therefore, a text message was sent to mappers in the intervention homes by CTRU trial management staff, ahead of data collection at 6 and at 16 months, to remind them not to provide proxy informant data if requested to do so.
Procedure
Usual care (both arms)
Usual care was defined as the care routinely delivered within the setting and was continued in all participating care homes with no restrictions imposed on current practices or on homes undertaking additional development or training. The exception was that control-arm homes were required not to implement DCM during the trial period. Details regarding any changes in usual-care practice during the course of the trial (e.g. new staff roles, change of ownership, new practice initiatives or training programmes) were documented by the researcher at follow-up visits.
To facilitate a person-centred primary care response to BSC in case care homes sought support, all general practitioners (GPs) who served each care home were provided with generic best-practice guidance about the implementation of person-centred care and managing BSC, irrespective of whether or not the residents they provided services to were participating in the trial. We did not inform individual GPs about which residents were participating in the trial.
Dementia Care Mapping™ (intervention arm)
The intervention followed standard procedures as set out in the DCM manual and guidance. 17,108 Two staff members from each intervention care home were trained to use DCM, followed by implementation of (ideally) three standard DCM cycles (each comprising briefing; observation; data analysis, reporting and feedback; and action-planning), in accordance with the British Standard best-practice guideline. 83 In addition, care homes were provided with fidelity guidelines, which included standardised templates for recording attendance at briefing and feedback sessions and for DCM reporting and action-planning. Other mechanisms for ensuring adherence to the intervention and for supporting mapper engagement were implemented, including support from a DCM expert mapper during cycle 1 (see Expert mapper support for cycle 1) and sending short message service (SMS) reminders to mappers ahead of each cycle.
Mapper identification, eligibility and consent
Two staff members in each home were identified by the manager as suitable to be trained in the use of DCM (mappers). To ensure timely progression from care home randomisation to DCM training, and to avoid selection bias, two mappers were identified in every consenting home at care home recruitment and their informed consent to undertake the mapper role was gained. To be eligible, staff had to be a permanent or contracted staff member, had to have the right skills and qualities as assessed by the home manager against a mapper role descriptor provided by the research team [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)], had to agree to implement DCM per protocol and had to take part in the process evaluation, if required.
Potential mappers were initially approached by the manager with reference to the written mapper role description. Once verbal consent was obtained, the researcher discussed the role and responsibilities of mappers again with reference to the role descriptor and mapper information sheet, before gaining their written informed consent [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. If a mapper withdrew or left the care home before the end of the trial, where feasible, another suitable member of staff was identified, consented and trained using a similar procedure, to ensure continuity of DCM implementation in the home.
Training
Following randomisation, care homes allocated to the intervention group received DCM training as soon as their mappers were able, depending in part on the course schedule.
All trial mappers attended a standard 4-day DCM training course held in Bradford or London and provided by the University of Bradford. It included an assessment of competency in the use of DCM. One additional attempt at the assessment was permitted for those attendees who failed to achieve a pass mark at the first attempt. The course trainers were informed in advance of which attendees were EPIC trial mappers. They provided data on which mappers had successfully completed and passed the course.
Implementation
Following completion of the formal assessed training course, implementation of DCM commenced, comprising a practice development cycle of briefing the staff team; observation over a number of hours; data analysis, reporting and feedback to the staff team; and action-planning. Re-mapping at regular intervals forms part of the standard process to monitor progress and to set new action plans. Intervention homes were scheduled to complete their first cycle 3 months after randomisation (or as soon as practicable), their second cycle at 8 months and their final cycle at 13 months. Ahead of each mapping cycle, the trial manager at the CTRU contacted mappers individually via SMS to remind them of the upcoming cycle. Paper documents were posted to them to prompt completion of the cycle.
Briefing
Mappers were asked to run at least one briefing session 1–2 weeks prior to undertaking the mapping observations. Briefing sessions informed the care home staff about DCM and the process of implementation, and provided an opportunity for staff to ask questions and for mappers to address any staff concerns.
Observation
Mappers used the standard DCM procedure. They were asked to observe as many individuals as they felt confident to, up to a total of five, for up to 6 consecutive hours on a single day if possible. Alternatively, they could observe for as long as possible on consecutive days up to a total of 6 hours. A detailed description of the DCM tool is published elsewhere83,109 and summarised here: every 5 minutes, the mapper records two pieces of information about each person they are observing, namely a behaviour category code (BCC) and a mood/engagement (ME) value. There are 23 possible BCCs for the mapper to choose from and they capture what the person with dementia is doing within that 5-minute period. The ME value encapsulates the associated mood and engagement level of the person with dementia and is chosen from a six-point scale (+5, +3, +1, –1, –3, –5). A set of rules is used to determine which BCC and ME value to choose. The mapper also records instances when a person with dementia is ‘put down’ by a care worker, known as personal detractions, and examples of excellent care, called personal enhancers. These are recorded as and when they occur. As DCM is grounded in person-centred care, for reasons of privacy and dignity, observations take place only in communal living areas, such as the lounge, the dining room and corridors. Mappers do not observe in bedrooms or bathrooms.
Data analysis, reporting and feedback
For the purposes of trial data analysis, reporting and feedback were considered as a single phase, rather than as the two separate phases of implementation described in the DCM literature. Once the data had been collected, they were analysed by the mappers and presented in a standardised report format for the purposes of feedback to the care team. In the trial, a standard template for DCM reporting was given to the mappers by the research team [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. DCM feedback sessions provided an opportunity for mappers to share their observations with the staff team and for collective discussion about good care practices and areas for improvement. In the trial, mappers were requested to run one or more feedback sessions with as many members of the staff team as possible within 1 month of conducting the observations.
Action-planning
Action plans of ways to improve care were then produced. As part of the feedback session, or in a subsequent meeting, staff and mappers were asked to jointly develop agreed, achievable group (care home-level) and individual resident-level action plans containing short-, medium- and longer-term goals that they wished to implement. Mappers were asked to monitor progress on these actions during the next mapping cycle.
Resident consent for mapping
Prior to mapping, residents were selected to be mapped through discussions between the care team and mappers, during the briefing session or on the day of mapping. Mappers followed DCM guidance, which states that residents may be selected for observation because they display a range of abilities or have particular care needs that staff members have difficulties meeting or understanding. Residents selected for mapping observations did not need to be consenting trial participants, as DCM was implemented as a whole-home intervention. Consent was gained verbally by the mappers, either from the resident or in discussion with their relative prior to observations taking place, in accordance with the usual consent process utilised in DCM. Any resident data collected as part of the DCM process, which was subsequently used for monitoring intervention fidelity or for any other purposes in the trial, was anonymised by the mappers before being sent to the research team.
Expert mapper support for cycle 1
This pragmatic trial aimed to ensure that DCM implementation reflected what is possible in a typical UK care home, maximising relevance to practice. However, the first cycle of mapping was supported by an expert in the use of DCM (a DCM expert mapper), who was assigned by the research team. This is not standard practice, as trained mappers would usually engage in DCM without further support following training completion. However, it was implemented in the trial to support implementation fidelity across clusters (see Process evaluation and assessment of treatment implementation), provide coaching for care home mappers, encourage implementation and support establishment of inter-rater reliability of DCM coding between trained mappers in each care home. The DCM expert mapper worked alongside the mappers during their first DCM cycle, spending 3 days in the care home supporting the establishment of inter-rater reliability on DCM coding frames, briefing, mapping observations and delivery of the feedback and action-planning session. Two additional days of desk-based support were provided on the preparation of briefing documentation, the feedback report and action plans. Telephone and e-mail support for DCM implementation from the DCM intervention lead was available to all intervention homes thereafter, if required.
Outcomes
Primary end point
The primary end point was agitation at 16 months following randomisation, measured by the CMAI, as rated by staff proxy. The Pittsburgh Agitation Scale (PAS) and a modified observational CMAI (CMAI-O), rated by independent researchers, provided a means of assessing concurrent validity, addressing the issue of potential detection bias owing to the inability to blind staff to intervention allocation status.
Health economic end points
The primary health economic end point was cost per quality-adjusted life-year (QALY) at 16 months. A secondary end point was cost per unit of improvement in CMAI score at 16 months. Both of these adopted the health and personal social service provider perspective.
Secondary end points
Secondary end points relating to residents were:
-
BSC (NPI)
-
mood (NPI)
-
QoL [Quality of Life in Late-Stage Dementia (QUALID) scale, Quality of Life in Alzheimer’s Disease (QOL-AD) measure, Dementia Quality of Life (DEMQOL) measure and EuroQol-5 Dimensions, five-level version (EQ-5D-5L)]
-
prescribed medication
-
safety (SAEs and safeguarding).
Secondary end points relating to staff were:
-
the Sense of Competence in Dementia Care Staff (SCIDS) scale.
Secondary end points relating to homes were:
-
the Quality of Interactions Schedule (QUIS).
Furthermore, intervention fidelity was assessed. All other data are potential mediators or moderators of the treatment effect. Measures, collection time points and methods of completion are summarised in Table 2.
| Assessment | Method of completion (completed with/on) | Purpose | Level | Timeline | |||
|---|---|---|---|---|---|---|---|
| Screening | Baseline | 6 months | 16 months | ||||
| Resident demographics | Researcher assessment (CM, CR) | Individual | ✗ | ✗ | ✗ | ||
| CMAI | Researcher interview (SP) | Primary end point | Individual | ✗ | ✗ | ✗ | |
| CMAI-O | Independent researcher observations (R) | Independent assessment of concurrent validity of CMAI for detection of potential bias | Individual | ✗ | ✗ | ✗ | |
| PAS | Independent researcher observations (R) | Independent assessment of concurrent validity of CMAI for detection of potential bias | Individual | ✗ | ✗ | ✗ | |
| NPI – nursing home version | Researcher interview (SP) | Secondary end point | Individual | ✗ | ✗ | ✗ | |
| DEMQOL measure – proxy version | Researcher interview (SP, RF) | Health economics end point | Individual | ✗ | ✗ | ✗ | |
| EQ-5D-5L/EQ-5D-5L – proxy version | Researcher interview (R, RF, SP) | Health economics end point | Individual | ✗ | ✗ | ✗ | |
| QUALID scale | Researcher interview (SP, RF) | Secondary end point | Individual | ✗ | ✗ | ✗ | |
| QOL-AD measure (care home) | Researcher interview (R) | Secondary end point | Individual | ✗ | ✗ | ✗ | |
| Health-care resource use | Researcher assessment (CR) | Health economics end point | Individual | ✗ | ✗ | ✗ | |
| Prescription medications | Researcher assessment (CR) | Secondary end point | Individual | ✗ | ✗ | ✗ | |
| Resident comorbidities | Researcher assessment (CR) | Individual | ✗ | ✗ | ✗ | ||
| Clinical Dementia Rating scale | Researcher interview (SP) | Process measure | Individual | ✗ | ✗ | ✗ | |
| FAST | Researcher interview (SP) | Individual | ✗ | ✗ | ✗ | ||
| General Health Questionnaire – 12 itemsa | Self-completed (S) | Secondary end point | ✗ | ✗ | |||
| SCIDS scale | Self-completed (S) | Secondary end point | ✗ | ✗ | ✗ | ||
| QUIS | Researcher observations (R, S) | Process measure | Cluster | ✗ | ✗ | ✗ | |
| Care home demographics | Researcher interview (CM) | Cluster | ✗ | ✗ | ✗ | ||
| Environmental Audit Tool | Researcher observations (CH) | Process measure | Cluster | ✗ | ✗ | ✗ | |
| Group Living Home Characteristics questionnaire | Researcher assessment (CH) | Secondary end point | Cluster | ✗ | ✗ | ✗ | |
| Assessment of Dementia Awareness and Person-Centred Care Training audit | Researcher assessment (CM, CR) | Pre-baseline benchmarking for provision of additional person-centred dementia awareness training and usual-care monitoring | Cluster | ✗ | ✗ | ✗ | |
| Safety reporting | Researcher assessment (CM) | Safety | Monthly following randomisation | ||||
| Reporting unexpected SAEs | Researcher assessment (CM) | Safety | As highlighted | ||||
To ensure that a consistent data set was available for each resident at each time point, the main informant for the primary outcome and for proxy-completed secondary outcomes was a staff proxy informant. These data were supplemented, where possible, by information provided by the resident (when able) and by their relative/friend (when available).
Resident measures
Primary outcome measure: Cohen-Mansfield Agitation Inventory42,43
The CMAI measures 29 agitated or aggressive behaviours. 110 The frequency of each symptom is rated on a seven-point scale (1–7) ranging from ‘never’ to ‘several times an hour’, based on observations over the previous 2 weeks. A total score is obtained by summing the individual frequency scores, yielding a total score ranging from 29 to 203. The CMAI has good psychometric properties111 when used in a care home setting. Data from previous similar studies provide an expected points change to inform the sample size calculation. The CMAI was completed via researcher interview with the staff proxy informant, in accordance with the CMAI manual. 43
As blinding staff to intervention allocation was not possible, two independent observational measures of agitation were collected to assess potential bias in completion of the CMAI by staff proxy informants [see Agitation measures (supportive outcomes)]. Observation scales have been shown to have good convergence with informant measures of agitation. 112 Observations were completed by an independent blinded researcher who was not involved in any other data collection in the care home.
Agitation measures (supportive outcomes)
Cohen-Mansfield Agitation Inventory – Observational113
The CMAI-O was developed by the trial team, with the permission of the CMAI’s author, to provide an observational measure of agitation. It is rated on a four-point scale (1–4) ranging from ‘never’ to ‘several times an hour’, based on observations over 1 day. The CMAI-O data collection was completed on participating trial residents in communal areas between approximately 10.00–12.00 and 14.00–17.00 (dependent on meal times in each care home). A copy is available from the authors on request.
Pittsburgh Agitation Scale114
The PAS is an established observational rating of agitation. The scale has good reported reliability and validity. 114 Observations are conducted for between 1 and 8 hours. PAS data were collected on participating trial residents in communal areas between 10.00 and 12.00 and between 14.00 and 17.00.
Neuropsychiatric Inventory – nursing home version115
The Neuropsychiatric Inventory – nursing home version (NPI-NH) records a broader range of BSC including delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviour, sleep and night-time behaviour disorders and appetite/eating disorders. The NPI-NH is a 12-item version designed for use with nursing-home/care home populations and has good reported reliability and validity. 115
Quality of life
Dementia Quality of Life measure proxy version116
The DEMQOL measure proxy version (DEMQOL-proxy) is a QoL questionnaire designed specifically for use in people with dementia. It has 32 items covering mood, behavioural symptoms, cognition and memory, physical and social functioning, and general health. It is administered by interview with a carer (formal or family) of the person with dementia. The DEMQOL-proxy has acceptable psychometric properties for measuring QoL in dementia117 and is modelled to enable the derivation of preference-based indices (utility values), with the latter employed in the secondary cost–utility analyses. 118
EuroQol-5 Dimensions, five-level version – self-report and proxy versions119
The EQ-5D-5L is an accepted standardised, five-item measure of health outcomes that provides a single index value for health status120 covering usual activities, self-care, mobility, pain and anxiety/depression, each with five response options (no problems, slight problems, moderate problems, severe problems and unable to do the task). Both the self-report and the proxy versions were used in the trial.
Quality of Life in Late Stage Dementia121
The QUALID scale is an 11-item scale that rates the presence and frequency of QoL indicators over the previous 7 days using proxy report. It is a reliable and valid scale for rating QoL in people with moderate to severe dementia and has good internal consistency, test–retest reliability and inter-rater reliability. 121
Quality of Life in Alzheimer’s Disease (care home)122
The QOL-AD measure is a 13-item self-report measure of QoL with good reported internal reliability, test–retest reliability and convergent validity. 122 It is reported to be reliable in use with people with mild to severe dementia. 123–125 The adapted version of the QOL-AD measure126 is a 15-item questionnaire developed for use in care homes and it uses simple language and a four-response answer that is consistent across all questions (poor, fair, good or excellent). It includes minor changes to the standard QOL-AD measure to ensure relevance to those living in long-term care (e.g. an amendment of the wording of existing items, the removal of questions on management of money and marriage status, and the addition of questions relating to relationships with staff, one’s ability to take care of oneself, one’s ability to live with others and one’s ability to make choices). It has good reported internal consistency. 126
Demographics, health and health-care resource use
Resident demographics
Standardised demographic information (sex, date of birth, etc.) was collected by the researcher via interview with the care home manager or other senior member of staff and a review of the resident’s care records.
Health-care resource use
This measure was adapted from one developed for a care home feasibility trial. 127 The measure captured the use of primary and secondary care, including hospital-based care [e.g. hospital and accident and emergency (A&E) visits and stays], community-based care (e.g. GP visits and contact with other health-care professionals such as physiotherapists and psychiatrists) and other costs (e.g. adapted beds and other aids) incurred during the previous 3 months.
Prescription medications
The prescription of medications within categories of interest (e.g. antipsychotic, benzodiazepine, non-benzodiazepine anxiolytic, non-benzodiazepine antipsychotic, memantine, antidepressant, cholinesterase inhibitor, anticonvulsant, mood stabiliser and pain relief), and the administration of these if prescribed on an as required (PRN) basis, was recorded on a standardised case report form (CRF). This was completed by the researcher through a review of residents’ medication records for the previous month.
Resident comorbidities
These were collected by the researcher using a standardised CRF through a review of residents’ care records.
Dementia severity
Clinical Dementia Rating scale128
The Clinical Dementia Rating (CDR) scale is a well-utilised, standardised scale for rating the severity of dementia, ranging from no cognitive impairment to severe or advanced dementia. 129 Impairment on six cognitive categories is rated and an algorithm is used to calculate the overall severity rating. Severity is rated by a trained assessor via informal interview/conversation with the person, or with a proxy who knows the person well. In this study, the CDR scale was completed by the researcher through interview with a staff proxy who knew the resident well.
Functional Assessment Staging Test102
The FAST is a scale designed to record the functional severity of dementia. Scores range from 1 (no dementia) to 7 (severe dementia) with levels 6 and 7 each having five sublevels. It is designed for use particularly in more moderate to severe dementia. It is completed by proxy report from a caregiver. 102
Staff measures
Staff work stress
General Health Questionnaire – 12 items130
The General Health Questionnaire – 12 items (GHQ-12) is a measure of stress/psychological well-being used in the general population. It has good reported psychometric properties. 131 It contains 12 items related to mental health, each scored on a four-point scale of the frequency of symptoms or behaviours (‘less than usual’ to ‘much more than usual’). Owing to poor return rates, the collection of GHQ-12 data ceased during the trial (see Summary of changes to project protocol for further details).
Job or role efficacy
Sense of Competence in Dementia Care Staff scale132
The SCIDS scale is a user-friendly, self-complete, 17-item scale measuring staff members’ competence in caring for people with dementia across four subscales (professionalism, building relationships, care challenges and sustaining personhood). Each item is rated on a four-point scale of confidence (‘not at all’ to ‘very much’). It has acceptable internal consistency and test–retest reliability. 132
Organisational measures
Care quality
Quality of Interactions Schedule133
The QUIS is an observational measure of the quality and quantity of staff interactions with residents during care delivery, at the care home level. It records five types of interactions (positive social, positive care, neutral, negative protective and negative restrictive) and has reported adequate inter-rater reliability and sensitivity. 134 The QUIS was completed via researcher observation, using a time-sampling technique in each setting. In accordance with QUIS guidelines,133,135 observations of interactions at 5-minute intervals were conducted in communal areas in the care home and recorded, then summarised into 15-minute intervals. One-hour observations were completed at two time points (a.m. and p.m.) over 2 days within the same week (7-day period) in line with care home activities (e.g. morning coffee break) in the most populated communal area in the home. For the purposes of analysis in this trial, the proportion of interactions that were positive (positive social and positive care) was used.
Care home environment and characteristics
Care home demographics questionnaire
This questionnaire, designed by the study team, collected organisational data regarding each care home (size, type, ownership, geography, staff turnover, staff ratios, resident demographics, etc.) and its manager (qualifications, length of time in post, leadership style, etc.).
Environmental Audit Tool136
The Environmental Audit Tool (EAT) is an instrument with reported adequate reliability and validity used to differentiate between the quality of the physical environment in various types of dementia care facilities. 136 It was completed by the researcher with the assistance of a staff member if required.
Group Living Home Characteristics questionnaire137
The Group Living Home Characteristics (GLHC) questionnaire is a measure of the style of care being delivered in the care home. It examines how ‘home-like’ care delivered is. It includes four subscales (physical environment, residents, relatives/other visitors and staff), each containing at least three related statements answered according to a five-point scale (‘never’ to ‘always’). It was completed by the researcher.
Assessment of Dementia Awareness and Person-Centred Care Training audit99
For information on the Assessment of Dementia Awareness and Person-Centred Care Training (ADAPT) audit, see Dementia training audit and provision of dementia awareness training.
Safety reporting and reported unexpected serious adverse events
For information, see Resident safety.
Sample size
The sample size was calculated to detect a moderate standardised effect size of 0.4 on the primary outcome: the between-group difference in mean CMAI scores at 16 months. We assumed that the standard deviation (SD) would be similar to that observed in a recently completed trial in UK care homes (7.5 points). 66 The moderate effect size translated into a minimum difference of 3 points. If greater variation in CMAI scores was observed (SDs ranging from 15 to 20 points as reported by Zuidema et al. 138) then, for the same effect size, a difference of 6 to 8 points could be detected, respectively. A difference of 8 points on the CMAI score is seen as indicative of real behavioural change. 138 Fifty care homes, each recruiting 15 participants, provide 90% power at a 5% significance level to detect a clinically important difference of 3 points (SD 7.5 points), assuming 25% loss to follow-up (as seen in Chenoweth et al. 63) and an inflation factor of 2.0 (i.e. a cluster size of 11 participants available for analysis after loss to follow-up and an ICC no greater than 0.166).
As provision of care is a further source of clustering and because the ICC was anticipated to be higher in the intervention arm (based on clinical opinion), an allocation ratio of 3 : 2 was used, resulting in there being 30 (450) and 20 (300) care homes (residents) in the intervention and control arms, respectively, namely 50 (750) care homes (residents) overall.
During the trial, the TMG, DMEC and TSC monitored loss to follow-up. This was higher than the anticipated maximum of 25%, mainly owing to death rates. To maintain a statistical power close to 90% and to preserve our ability to detect an effect size of 0.4 SDs, to maintain validity and to increase the generalisability of the trial, we recruited additional, newly eligible, consenting residents from the randomised care homes 16 months after randomisation and performed a cross-sectional analysis of the data (see Summary of changes to project protocol).
Statistical and health economic methods
A comprehensive statistical and health economic analysis plan was developed and approved following the approval of the design change. All analyses were performed once at final analysis in SAS® v9.4 (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.) or Stata® v14 (StataCorp LP, College Station, TX, USA).
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram has been used to display care home and resident pathways from registration to final follow-up (see Chapter 3, Recruitment and randomisation).
Analysis populations
The principal analyses were intention to treat (ITT), including all randomised care homes and all registered residents, regardless of whether or not they received or adhered to their allocated intervention. A further supportive analysis was planned of ‘compliers’, defined as care homes that would have received at least one cycle of DCM to an acceptable level (with all components of the cycle completed) had it been offered to them. Other thresholds of compliance that we considered were exploratory. Safety was summarised on the closed-cohort sample of residents, as we were unable to obtain timely NHS Digital data on the cross-sectional sample. The samples of staff and relatives/friends providing data (other than staff proxy data) were so small that analyses based on them are descriptive and provided in the appendices only (see Appendix 1, Table 31).
Missing data
In general, if there were no instructions in the manual on how to handle missing items (for the PAS, NPI-NH, QUALID scale, SCIDS scale and EAT), prorating was used if < 25% of items were missing (based on adopting a more conservative approach than that proposed by Staquet et al. 139), otherwise the score was assigned as missing. As the proportion of residents with notable missing primary outcomes was low, we prorated for simplicity, despite assumptions underlying prorating not always being met.
The primary intended method for handling missing scale data in the cross-sectional analyses was to analyse complete cases, under the assumption that data are missing completely at random (MCAR). For completeness, we also report a sensitivity analysis using multiple imputation (MI), under the assumption that cross-sectional data are missing at random (MAR). If the MCAR assumption were found not to hold true, then the primary analysis would use the multiple-imputed data, assuming that data are MAR. The proportion of residents missing in analyses of the closed-cohort sample was sizeable at 6 months and substantial at 16 months. As death and moving care home were expected to be the most important predictors of missing closed-cohort data, we expected that these data would be missing not at random (MNAR). We considered a range of approaches to handling missing closed-cohort data (see Tables 9, 47 and 48), but report a tipping point analysis140 for the primary analysis, which indicates the assumptions that would be required about the missing data to change the conclusions.
For completeness, we also report an analysis using MI, under the assumption that closed-cohort data are MAR, which assumes that residents registered at care home randomisation do not die during the duration of the trial. The same variables were included in the imputation models, apart from the baseline questionnaire scores: the baseline questionnaire score used in the imputation model was always the same as the outcome questionnaire score. The imputation model was done separately for different analysis populations: in the cross-sectional analysis, baseline variables were care home summaries, whereas, in the closed cohort, individual-level baseline information was used.
Screening, baseline, treatment and outcome summaries
The numbers of care homes approached, screened, eligible, consenting and participating, along with the numbers of residents in the closed-cohort sample and the cross-sectional sample, were summarised in a CONSORT flow diagram. Reasons for exclusion and the characteristics of screened residents were also presented overall and across samples.
Baseline characteristics of care homes, care home managers and residents (closed-cohort and cross-sectional samples) were summarised overall and for the intervention and control groups. In accordance with the TIDieR checklist,141 summaries of treatment receipt were given by intervention component for DCM and by parallel-group for usual care.
Baseline, 6- and 16-month outcomes were summarised for the intervention and control groups and additionally for residents in the closed-cohort and cross-sectional samples at 16 months.
Primary effectiveness analysis
The continuous primary outcome of agitation (CMAI score) was analysed on the cross-sectional sample of residents using a linear two-level heteroscedastic regression model that allowed the cluster and resident-level random effects to vary by arm. The model adjusted for minimisation factors (care home type, size, provision of dementia awareness training and recruiting hub) and average care home-level baseline characteristics (dementia severity via CDR scale, age and CMAI score) as fixed effects. These variables were prespecified in the protocol and age was added as an additional covariate. Unadjusted and adjusted ICCs, estimates and corresponding 95% confidence intervals (CIs) were presented, by arm. A negative mean difference in outcome favours the intervention. The primary analysis model was decided a priori in the statistical analysis plan (SAP) before the data were unblinded and without reference to the data. It was consistent with/followed on from the trial design.
Sensitivity effectiveness analyses
The robustness of the conclusions of the primary effectiveness analysis was assessed via a number of sensitivity analyses. The primary effectiveness analysis was repeated:
-
with an additional covariate categorising care homes by whether they were recruited before or after the eligibility criteria change
-
including care home size as a continuous covariate
-
assuming that clustering is homogeneous across arms
-
using CMAI-O and PAS scores in place of the CMAI
-
using the closed-cohort sample in place of the cross-sectional sample, allowing dementia severity, age and CMAI score to be included as covariates at the resident level.
Supportive effectiveness analyses
The treatment effect among ‘compliers’ was estimated using a series of complier-average causal effect (CACE) models. Our main supportive analysis defined ‘compliers’ as care homes that would have received at least one cycle of DCM to an acceptable level if it had been offered. Other thresholds were exploratory. CACE treatment estimates were obtained from two-stage least squares instrumental variable regressions (using the Stata command ‘ivreg’), using robust standard errors to allow for clustering effects. The model adjusted for the same baseline variables as the primary analysis model, with the addition of the binary variable ‘treatment received’ (number of DCM cycles received to a prespecified level).
For our mediation analysis, we used a parametric causal mediation approach to allow for interactions between mediators and treatments, which the typical Baron and Kenny142 approach does not. We reported the natural indirect effect, which is the effect on outcomes of having the mediator present compared with it not being present, for a number of prespecified intermediate variables (potential mediators). Analysis was done on an ITT basis on the cross-sectional cohort. Each mediator was analysed separately assuming that there was no unobserved confounding in treatment–outcome, mediator–outcome and treatment–mediator relationships and that any mediator–outcome relationship confounders were not affected by treatment allocation. We used parametric regression models using the Stata command ‘paramed’. A linear regression model was fitted for the outcome variable. Logistic regression was used for the mediator variable. In the MI, we also included the potential mediators.
In our moderator analyses, we explored whether or not the treatment effect differed depending on prespecified baseline characteristics of either the care home or the resident. The primary analysis was repeated, including each potential moderator, alongside the interaction between treatment and the potential moderator. Analyses were performed on the ITT cross-sectional sample, subject to the availability of data for each potential moderator.
Secondary effectiveness analyses
Secondary analyses were undertaken using the same principles as the analysis of the primary outcome. For secondary outcomes (BSC, use of antipsychotic drugs and other psychotropic drugs, mood, resident QoL, staff role efficacy, care quality and the quality of staff–resident interactions), three analyses were performed:
-
cross-sectional analysis at 16 months
-
closed-cohort analysis at 6 months
-
closed-cohort analysis at 16 months.
The same covariates were included as for the primary analysis (for closed-cohort analyses, individual resident-level covariates were used as appropriate). Cluster-specific linear two-level heteroscedastic regressions were fitted in which outcomes were continuous (resident QoL). Population-average logistic regressions were fitted, using generalised estimating equations (GEE), in which outcomes were binary (BSC, use of antipsychotic drugs and mood).
Safety analysis
The number and proportion of residents in the closed cohort who died from any cause between randomisation and 16 months was summarised by arm. The cause and place of death were also reported. The number of hospital admissions per resident, the mean number of hospital admissions per resident, the average length of hospital admission, the overall number of hospital admissions reported and the admissions by ward type were summarised by arm and overall. No formal statistical comparison was undertaken between arms.
Health economic analysis
The economic evaluation was a within-trial analysis. We chose not to develop a decision-analytic model for the evaluation. Although a model may have been useful in extrapolating any costs and health benefits beyond the end of the trial, we felt that the measure of future effectiveness would be highly uncertain and would require additional assumptions (e.g. about the duration of effect). The analysis followed the reference case guidance for technology appraisals set out by NICE. 143 The primary analysis was a cost–utility analysis and it presented outcomes as QALYs using a health and personal social service provider perspective (although some of these costs might, in practice, be paid for by residents themselves, this was not accounted for in the analysis because it was deemed not to have any impact on the incremental costs of the DCM intervention). A secondary cost-effectiveness analysis based on cost per unit of improvement in CMAI score was also conducted.
Deviations from the statistical analysis plan
The following deviations from the SAP were decided on during data analysis. The primary health economic analysis assumed that the Local Authority pays for the provision of care for residents (NHS and social care perspective). We had planned to conduct an analysis in which we assumed that some proportion of residents paid for their own care home stay. Following further discussions with the research team, it was decided that this element would be removed. The justification for this was that care homes are paid even when residents are hospitalised and hence the source of payments for residency would not have an impact on the results.
In the SAP we stated that: ‘The validity of reports will be assessed by correlating scores between EQ-5D-5L and those on the alternative measures (QUALID, QOL-AD) and by exploring the ability of the measure to distinguish between known groups (for example, based on CDR)’ (Holloway I, Walwyn REA, Martin A, Meads D, Farrin A, Surr C. Statistical and Health Economic Analysis Plan, University of Leeds, 2017). This was not included as part of the required analysis within the original grant application. This analysis is still planned as additional methodological research. For the trial analysis, we took the pragmatic approach and based the primary analysis on staff proxy measures, as this was by some margin the most complete data.
Resource use and costs
Unit costs for health service staff and resources were obtained from national sources such as the Personal Social Services Research Unit (PSSRU),144 the eMIT national database145 and the NHS reference cost database146 (see Appendix 1, Table 66, for a summary of unit costs).
Cost of intervention
The DCM intervention consisted of two components: (1) delivery and receipt of DCM training and (2) implementation of the DCM process in care homes.
It was assumed that both components would require DCM mappers in the trial (two per intervention site) to take dedicated time away from their usual care duties during the working day. The amount of time required for the DCM training course was 4 days. The estimated amount of time required for the DCM process is reported in existing DCM guidelines,83 based on the experiences of experts using DCM in practice settings. Data were also collected during the trial to assess the validity of these estimates and, when this was shown to exceed the assumed average, the impact of any additional staff time was assessed in sensitivity analyses. It was assumed that additional time was not required for other care home staff to attend DCM briefing and feedback sessions, but that these were arranged at handover and other convenient times for staff to attend as part of their usual duties.
To calculate the total cost of staff time, an hourly wage was estimated for a typical DCM mapper. This incorporated data from the trial on the proportion of DCM mappers in particular roles (e.g. a care home worker and a care home manager) and data published by the PSSRU on the hourly wages (or annual salaries converted to hourly wages using standard methods) of workers in these roles. When relevant wage data were not available from the PSSRU, we reviewed alternative sources, including recent job advertisements.
Additional costs of the delivery and receipt of DCM training included the course fees, training materials, accommodation, meals, subsistence and travel. These were estimated using information from the DCM course provider and data on the costs incurred in running the trial. A further additional cost of implementing the DCM process in care homes was the consultancy fees, travel and subsistence expenses incurred through employment of external DCM expert mappers to support the intervention implementation and fidelity during the first DCM mapping cycle in each of the intervention sites. The primary analysis assumed that the intervention was delivered as planned and that all cycles were implemented and costed. A sensitivity analysis costed only the cycles that had been partially or fully implemented.
The primary analysis assumed that the Local Authority paid for the provision of care home care for residents. As such, these costs were included in the health-care provider (NHS) and social care cost perspective.
Health-care resource use
Data on health-care resource use incurred during the previous 3 months were collected for each resident at baseline, at 6 months and at 16 months. Medication use during the past month was captured at the same time points (see Demographics, health and health-care resource use).
Quality of life/utility
Quality of life was measured in the trial at baseline, at 6 months and at 16 months using the EQ-5D-5L, completed by care home residents, and the EQ-5D-5L-proxy, completed by staff and relatives. 120 A recently generated UK general population tariff147 was used to calculate the utility scores and a (5L to 3L) mapping algorithm was used as a sensitivity analysis. 148
Utility values were also calculated using the DEMQOL-proxy tool (DEMQOL-proxy-U), which was completed by staff and relatives. A UK population tariff was used to calculate utility scores. 118 The main cost–utility analyses were based on the EQ-5D-5L utility, but sensitivity analyses were conducted based on DEMQOL-proxy-U.
Taking a pragmatic approach, we elected to base the primary analysis on EQ-5D-5L data from the staff proxy at all three time points, as this represented the most complete set of responses. However, we conducted a sensitivity analysis employing resident-completed EQ-5D-5L data when they were available at all three time points. When such data were not available, we used data from relative proxies (if available at all three time points) and, finally, when this was unavailable, from staff proxies.
Analysis
The primary economic analysis was a cost–utility analysis over 16 months presenting incremental cost-effectiveness ratios (ICERs) for intervention versus control, with effects expressed in terms of QALYs. As the clinical efficacy analyses used agitation as the primary end point, a secondary cost-effectiveness analysis based on change in CMAI score over 16 months was also conducted.
Total QALYs were calculated using an area under the curve approach between adjacent utility measure completions using EQ-5D-5L and DEMQOL-proxy utilities captured at baseline, at 6 months and at 16 months. If residents died, their utility value was assumed to be zero and their data were retained in the analyses. Quality of life was assumed to change from last completion value to zero in a linear fashion.
Total costs were estimated using the resource use questionnaires at 6 and 16 months. It was assumed that reported resource use during a 1-month (for medications) or 3-month (other costs) period remained constant between time points in the trial (e.g. the 10-month period between follow-up at 6 and 16 months). To capture the costs incurred prior to death, a daily cost was estimated based on each resident’s previous resource consumption (at baseline or at 6 months) and applied until the date of death.
Incremental costs and QALYs (or CMAI scores) were estimated using a seemingly unrelated regression (SUR) approach, which consisted of a system of regression equations that can recognise the correlation between individual costs and outcomes:
Model 1 (cost):
Model 1A (cost-sensitivity analysis):
Model 2 (QALYs):
where T0 = baseline and CDR = CDR score.
So that the economic analysis was consistent with the statistical analysis, age and baseline CDR scores were included in the regression model, in addition to baseline QALYs (Model 2 above). Although costs were not significantly different at baseline, these same baseline characteristics were included in the SUR for costs in a sensitivity analysis.
Incremental cost-effectiveness ratios were calculated for both cost per QALY gain and cost per unit improvement in CMAI score. We used the NICE willingness-to-pay per incremental QALY threshold [£20,000 = lambda (λ)] to determine whether or not the intervention was cost-effective. Interventions with an ICER under £20,000 per QALY are generally considered cost-effective. There is no such willingness-to-pay threshold to aid the interpretation of changes in CMAI score, but we framed this in the context of other study results.
Discounting at the NICE preferred rate of 3.5% per annum for costs and effects was conducted for values after 12 months (i.e. for the final 4 months of the trial).
Net-benefit analysis
A net-benefit regression framework was also employed to allow parametric analysis of the incremental costs and benefits of the intervention. Net monetary benefit (NMB) is calculated using individual-level total costs, total QALYs and the cost-effectiveness threshold (λ = £20,000):
Linear regression models were used to regress treatment allocation on individual-level (‘i’ for individuals in the trial) estimates of NMB, while controlling for other observable trial-arm imbalances (e.g. dementia severity, agitation levels or sociodemographics):
where RandTrt is the treatment allocation and X is a vector of observable characteristics.
We examined heterogeneity in the treatment effect by compliance in a multilevel model accounting for clustering at the care home level:
where C is a categorical variable representing care home compliance with the intervention as measured by the number of cycles in which all four DCM components were completed to an ‘acceptable’ level (at the care home level): zero, one, or two to three cycles.
Control-arm care homes were in the reference category, with the coefficient (β) being a measure of incremental net benefit.
To be consistent with the statistical analysis, we also conducted a CACE analysis on NMB, which is designed to account for the potential endogeneity of compliance using a two-stage least squares instrumental variables approach.
Missing data
We ran the resident-level analysis on complete cases [complete-case analysis (CCA)] initially, which required data on total QALYs (based on various EQ-5D-5L or DEMQOL measures, depending on the analysis) and total costs. However, owing to the extent of missing data, the primary analysis was based on data for which missing values were imputed using MI. This assumed that the data were MAR. The first stage of the imputation process used mean imputations to estimate the baseline values of each EQ-5D-5L, DEMQOL-proxy measures, CMAI score and time-invariant characteristics (age/date of birth at baseline), following guidance in a paper by Faria et al. 149
Second, MIs by chained equations (MICE) was used to impute missing EQ-5D-5L, DEMQOL-proxy measure (index values rather than individual items) and CMAI score at 6 months and at 16 months, and individual components of total costs at all three time points. The number of individual components of total costs (n = 15) used in the imputation process was decided, taking a pragmatic approach. As a general rule, at each time point, high-cost and common resource items (e.g. hospital visits and stays) were imputed individually and less common items were imputed on a bundled basis.
The number of imputations (n = 48) reflected the ratio of missing to complete data. We accounted for clustering within care homes. Rubin’s150 rules were used to combine parameter estimates of the MIs.
Cross-sectional cohort analysis
The change in the trial to an open-cohort design meant that additional data for some residents were available at 16 months, despite them not being in the trial at baseline or at the 6-month follow-up. For the primary analysis, we used data only from those residents consented into the trial at baseline (the original cohort). However, to be consistent with the statistical analysis, an additional analysis was conducted incorporating the costs and QALYs for those residents providing data only at 16 months (the cross-sectional cohort).
When data on both costs and EQ-5D-5L were available only at 16 months in the cross-sectional cohort, we imputed the total cumulative costs and total QALYs for the whole trial period using a two-stage imputation process. First, mean values of the total costs and total QALYs generated in the imputations described above (n = 48) were used to replace the missing data on total costs and total QALYs in the closed cohort. Second, data on total costs and total QALYs for each individual in the closed cohort (n = 726) (including the values that had been imputed in the first stage) were used to impute the total costs and QALY data for all individuals in the cross-sectional cohort, using the MICE method and Rubin’s rules described above, accounting for recorded data at 16 months, including costs and QoL. This enabled calculation of an ICER for the cross-sectional cohort. As this approach relied on an unusual two-stage imputation process for individuals who had no recorded data at baseline, the results should be considered illustrative only and be treated with due caution. This approach also relied on an assumption that survival was independent of the intervention and the time spent in the care home, as residents providing data only at 16 months would have survived until 16 months had they been in the care home for that duration.
Sensitivity analyses
Deterministic sensitivity analysis of the ICER was undertaken to test the robustness of the results to changes in the analytical approach and to assumptions made. For example, we re-ran analyses exploring the impact on results of different approaches to costing, of handling missing data and of employing alternative utility capture methods.
A non-parametric bootstrapping analysis was also conducted to determine the level of sampling uncertainty around the ICER estimates by generating 10,000 estimates of incremental costs and benefits, using the combined estimates of the multiple-imputed data sets (n = 48) using Rubin’s150 rules, and accounting for clustering in care homes. The bootstrapped estimates were used to generate the cost-effectiveness plane and the cost-effectiveness acceptability curve (CEAC). 151
Process evaluation and assessment of treatment implementation
Aims and research questions
The process evaluation was designed to examine the process, challenges, benefits and impacts of the trial to identify the processes and factors associated with degrees of successful and unsuccessful intervention implementation.
The aims of the process evaluation included:
-
describing adherence to the required components of the intervention and the quality (or fidelity) of intervention delivery
-
understanding staff members’, residents’ and relatives’ perceptions of the impacts of the intervention
-
understanding the barriers to and facilitators of implementing DCM in practice.
The process evaluation answered research questions aligned to the Medical Research Council guidelines on process evaluations152 and included implementation, mechanisms of impact and context.
-
What was implemented?
-
What was the process of setting up the intervention in each care home?
-
Did this differ, and, if so, how did it differ, from the intended process as outlined in the protocol?
-
How many cycles of DCM were delivered in each care home? (Dose + Reach)
-
To what extent did each cycle in each care home meet the planned delivery as set out in the protocol? (Fidelity + Reach)
-
Did care homes deviate from the delivery of the intervention as set out in the protocol and, if so, how?
-
-
How did participants react to the intervention?
-
What were mappers’, managers’, residents’, relatives’ and staff members’ experiences of the intervention and its implementation?
-
What were mappers’, managers’, residents’, relatives’ and staff members’ perceptions of the impact of the intervention?
-
Did the intervention have any perceived or unexpected impacts or consequences?
-
For the perceived impacts, through what mediators/processes did each group perceive the intervention to have operated?
-
Did the intervention or its mechanisms of impact operate in any unexpected ways?
-
-
What contextual factors shaped if, and how, the intervention was implemented or worked?
-
What were the perceived barriers to and facilitators of intervention implementation, mechanisms of impact and the perceived impact from the perspective of mappers, DCM expert mappers, managers, staff members, residents and relatives?
-
How did care homes that demonstrated different degrees of intervention implementation manage and address barriers to and facilitators of intervention implementation?
-
The process evaluation and implementation assessment was intended to support the refinement and improvement of intervention efficacy and the sustainable implementation of the intervention over time, if the intervention was found to be effective. 153
Design of the process evaluation
A mixed-methods approach to data collection was used, involving quantitative and qualitative components to embed the process evaluation as part of the main trial data set.
The quantitative data set included an assessment of the levels of adherence and fidelity in each care home, utilising data provided by the mappers from each care home at each cycle. These data included details on the ‘dose’ and quality of DCM use in relation to briefing (the number of briefing sessions held and the proportion of care home staff receiving briefing), mapping cycles (the number of mapping sessions, the number of residents observed, the length of the mapping period and the number of mappers taking part), feedback sessions (the number of feedback sessions held and the proportion of care home staff receiving feedback) and DCM and action-planning documentation (successful completion of standard mapping documents during each cycle using the standard templates provided and the number of action plans developed per resident and at the home level).
The qualitative data were collected from a subset of 18 intervention homes using semistructured interviews with residents, the care home manager, mappers, staff members, relatives and residents. Homes that had achieved varying degrees of success with DCM implementation (no full cycles, at least one full cycle, two or more full cycles) were purposefully selected to explore the factors associated with successful and unsuccessful implementation. Although the selection of care homes took place before the final follow-up data collection point, the process-evaluation interviews took place after all outcome data had been collected in each home (i.e. at the end of the 16-month follow-up data collection). Semistructured interviews were also conducted with the DCM expert mappers to explore their experience of supporting the implementation of DCM within the intervention homes. To enable links between the qualitative and quantitative data, researchers undertaking the qualitative data collection were provided with implementation data by the CTRU from the first two cycles in the home prior to the interviews.
Sampling for the quantitative and qualitative data collection
For the quantitative data analysis, frequency data from the mapping cycles in all intervention homes were used to assess dose, adherence and fidelity, and to understand the variation in the levels of DCM implementation across homes.
For the qualitative data collection, purposive sampling was used to select a subset of 18 homes that had achieved varying degrees of success with DCM implementation in order to explore factors associated with this in greater detail. Owing to the staggered recruitment of care homes and the need to set up the process-evaluation data collection dates with home managers ahead of time, participating homes had to be identified before all three cycles of mapping were due to have been completed. These homes were stratified into three equal groups (six per group) according to if they were considered likely to be ‘successful implementers’ (more than two cycles completed), ‘partial implementers’ (one or two cycles completed) or ‘unsuccessful implementers’ (fewer than one cycle completed) of DCM.
Homes that differed according to key characteristics with the potential to affect DCM implementation, including location (six from each hub), size (large ≥ 40 beds vs. medium or small < 40 beds) and type of home (nursing, dementia or general residential), were also accounted for in the sampling.
Participant eligibility
Residents from homes taking part in the process evaluation were eligible if they were deemed to have the capacity to consent and were able to take part in a brief interview. Staff were eligible to take part if they were a permanent or contracted member of staff. Relatives/friends were eligible if they had visited the care home at least once a month during the trial.
Identifying staff and relatives/friends to approach was undertaken in conjunction with the home manager and included identification of the staff members who had played a key role in intervention delivery. All potential participants were provided with verbal and written information about the interview, were given time to consider taking part and signed a consent form if they were willing to participate [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019)]. Mappers had already provided consent to take part in the process evaluation as part of their initial consent to become mappers.
Data collection, transcription and storage
All researchers were trained in qualitative interviewing ahead of data collection to ensure consistency in the approach. Resident interviews were brief, using a conversational style informed by a flexible interview schedule. Staff and relative/friend interviews were conducted using a semistructured format informed by a topic guide. The interviews focused on experiences of DCM implementation, with prompts to encourage interviewees to discuss the various stages of DCM implementation, the successes, challenges and impacts of implementation, and any changes required to improve DCM implementation or impact in the care home, as well as future plans for DCM within the care home. Mappers who had left the home during the trial were not interviewed. Relatives/friends of resident participants who had died during the trial were not contacted regarding the process-evaluation interviews. Interviews were conducted within the care homes, in a private room with no other individuals present, and an alternative method of telephone interviews was offered to all relatives/friends [see www.journalslibrary.nihr.ac.uk/programmes/hta/111513/#/ (accessed July 2019) for copies of interview topic guides].
The interviews were audio-recorded using a digital audio-recording device and were professionally transcribed by a researcher independent to the study. Any potentially identifying information about the participants was anonymised or removed during transcription. Audio files were securely transferred in encrypted format and stored securely on computers in university offices.
Data analysis
Data analysis utilised a framework analysis approach. 154 The initial data analysis by all researchers involved in data collection informed the development of a coding matrix, which guided and created a structure for further data analysis. The focus of the coding matrix (and therefore of the data analysis) was on experiences of utilising and implementing DCM, particularly on identifying patterns and variations in implementation, barriers to and facilitators of implementation, and the impacts of DCM implementation. The coding matrix helped to assimilate the development of coding categories between the team of researchers that undertook the analysis. Each transcript was independently analysed by two researchers to ensure that key themes were identified. Development of the coding categories continued throughout the data analysis, informed by the emerging themes and analytic thoughts of the researchers. Codes and themes were compared and contrasted across homes and between different types of respondents to develop an in-depth, nuanced and contextualised understanding of the implementation and the impacts of DCM.
The quantitative data that informed the process evaluation (measures of adherence and fidelity in each home) were collected and analysed as part of the main trial data set (as described in Screening, baseline, treatment and outcome summaries). Findings from the quantitative data were integrated with the qualitative data to provide an in-depth understanding of DCM implementation and the issues surrounding implementation.
Measurement of adherence
Adherence to the prescribed processes for intervention delivery was monitored from randomisation to check that both mappers attended DCM training on time and passed the assessment. At each expected round of mapping, adherence to the processes was monitored to check that mappers delivered all components of the DCM cycle as intended and to the required quality (fidelity) and delivered three full cycles (dose). Anonymised copies of all observation data collection sheets, feedback reports and action plans were collected to assess fidelity. Data were also collected from the DCM expert mapper about cycle 1 completion, following their support of mappers through their first cycle of mapping. For the purposes of the trial, DCM was considered as comprising four required components: (1) briefing, (2) observation, (3) data analysis, reporting and feedback and (4) action-planning.
Care homes were classified according to their compliance with the intervention at each cycle, namely as ‘acceptable’, ‘partial’ or ‘none’.
For a cycle to be classified as:
-
acceptable, the care home must have completed all four components
-
partial, the care home must have completed one to three components
-
none, the care home must have completed none of the components.
If paperwork was not received for specific components and the researchers had been unable to ascertain verbally from mappers if particular cycle components had been completed, the following rules were used to determine whether a component had been completed:
-
If there was paper documentation for observation, it was assumed that briefing had also taken place (at least two components were completed).
-
If there was paper documentation for feedback, it was assumed that briefing and observation had taken place (at least three components were completed).
-
If there was paper documentation for action-planning, it was assumed that briefing, observation and feedback had taken place (all components were completed).
An assessment of the quality of each component was also conducted when paperwork had been returned, including whether or not all of the required DCM coding frames and accompanying qualitative notes had been used during mapping; if the standard feedback report format had been used and all parts of this had been completed (group data summary and individual data summary for each resident); and whether or not the standard action-planning template had been used, and if there were action plans developed at the care home level and for each resident mapped.
Summary of changes to project protocol
Ten substantial amendments to the protocol and associated trial documentation were made during the trial.
Internal pilot
Initially, two homes were recruited to the study early to allow internal piloting and review of trial processes, procedures, measures and tools ahead of recruitment of further care homes. Data from these homes were included in the trial. Changes to the original project protocol, implemented following this pilot, are reported in detail in the published protocol,98 in Table 3 and Appendix 2.
| Amendment number | Date | Summary of amendment |
|---|---|---|
| SA1 | 10 January 2014 | Modification to method and content of health resource data to be collected, including from medical records and NHS Digital (previously Health and Social Care Information Centre) |
| SA2 | 22 April 2014 | Modifications to care home information sheet to improve clarity and provide additional information following review by the PPI panel |
| SA3 | 26 June 2014 | Modifications to care home recruitment process; resident, staff and relative eligibility criteria; screening of proxy informants; translation of trial documentation; process for completion of independent assessments; monitoring of DCM implementation; relative/friend withdrawal; resident safety monitoring; and information included on participant information sheets and consent forms (for mappers, staff proxy and residents including consultees). In addition, clarification of mutually exclusive staff roles, amendment of assessment measures to be used, establishment of a DMEC and development of a short form of the resident information sheet |
| SA4 | 10 September 2014 | Personal Consultee introductory letter and reminder letter, and relative/friend proxy informant introductory letter approved |
| SA5 | 15 January 2015 | GP letter to accompany guidance on antipsychotic prescribing approved |
| SA6 | 15 January 2015 | Change of sponsor, modification to care home eligibility criteria, modification to resident eligibility criteria and modification to randomisation stratification criteria |
| SA7 | 22 October 2015 | Modification to requirements for witnessing resident consent, addition of SMS reminders for mappers and modifications to participant information sheets and consent forms |
| SA8 | 4 February 2016 | Detail added to the protocol on conduct of the process evaluation, modifications to staff measures booklet, modification to continued attempts to recruit relative/friend proxy informants post baseline and modifications to participant information sheets |
| SA9 | 15 April 2016 | Change to open-cohort design, with additional recruitment of resident participants at the 16-month follow-up and associated changes to trial documentation approved; modification to staff proxy informant consent processes; modification regarding requirements to check care home indemnity insurance; introduction/modification of documents to support process evaluation and to proposed process evaluation methods and processes; and modification to process for assessing ongoing capacity of Personal Consultees |
| SA10 | 25 July 2016 | Modification to data collected during process evaluation and additional text messages to remind mappers about mutually exclusive staff roles |
Design change
Our original sample size estimation, to detect a clinically important difference of 3 points (SD 7.5 points) in the primary end point of agitation using the CMAI questionnaire, assumed a 25% loss to follow-up 16 months after care home randomisation. If loss to follow-up was higher than anticipated (but no greater than 35%), our intended sample size of 750 residents still provided more than 85% power at a two-sided 5% significance level to detect a moderate effect size, equating to 0.4 SDs.
Through monitoring loss to follow-up within the trial, we determined by November 2015 that the rate would exceed our lower limit of 25%. Using data from care homes randomised into the trial up to 27 November 2015, we predicted that loss to follow-up at 16 months would be in the range of 32% to 48% (see Appendix 3, Figures 10 and 11). As such, continuing the trial as planned would not provide sufficient power for statistical analysis of the primary end point. An amendment to the trial design was required to ensure that the results of the trial were robust and generalisable. After considering all of the available options, we proposed recruiting additional residents at follow-up (i.e. a move to an open-cohort design) (see Appendix 3). All those consenting to take part (residents already participating in the trial and consented at baseline, as well as additional residents consenting at 16 months) provided data at 16 months.
The key impact of this design change was to increase the size of the cohort at follow-up to maintain the power of the trial and its ability to detect the effect size of 0.4 with 90% power (see Appendix 3, Table 67).
Sample size calculations
With an estimated 48% loss to follow-up, we expected to lose 360 residents before the 16-month follow-up, resulting in data at all three time points from 388 residents. All of the other parameters (significance level, two-sided test and an ICC of 0.1) remained the same. Consideration was given to recruiting only a proportion of eligible residents at each home at 16 months.
Three possible scenarios of additional recruitment were considered (an average of three additional residents per care home, recruiting 35% of residents lost to follow-up in each care home or replacing only 25% of residents lost to follow-up in each care home) and all provided sufficient power to detect the effect size of 0.4 (89%, 91% and 90% power, respectively). The TMG, the oversight committees (TSC and DMEC) and the funder agreed that imposing a recruitment ceiling at 16 months would be open to selection bias and that statistical power and the ability to generalise could be limited. Recruitment processes could also be protracted as a result of allowing time for decision-making via a Personal Consultee (i.e. should this be a refusal to take part, further resident–consultee dyads would then need to be approached, thereby considerably lengthening the recruitment process, researcher workload and thus cost).
Researchers were therefore instructed to recruit as many residents as possible to minimise bias. Numbers were monitored to ensure that at least three extra residents from each remaining care home were recruited.
The benefits of the design change were:
-
An ability to detect intervention effects at the care home level (as the intervention is aimed at the whole care home).
-
Conclusions could be generalised to a broader population of residents (i.e. not just to those still residing in the care home 16 months following randomisation).
-
We would be able to analyse the data for a cross-sectional (i.e. open-cohort) and closed-cohort (longitudinal) design.
-
We minimised selection bias by providing an objective criterion for inclusion (all eligible consenting residents).
-
Recruitment processes were resource-efficient, as all eligible residents were approached to participate at a single time point.
-
We would be less reliant on assumptions regarding imputation for missing data.
As well as maintaining power and increasing generalisability, the agreed design change incurred minimal additional cost.
Three of the authors (RW, AF and CS) have since secured additional funding from the Medical Research Council155 to conduct a methodology ‘bolt on’ to the EPIC trial regarding the use of open-cohort designs in clinical trials. This recognises the importance of considering alternative trial designs for the conduct of studies in populations with potential large loss to follow-up rates.
Resident eligibility (16 months after randomisation)
The following inclusion criteria were applied for additional residents recruited at the 16-month follow-up:
-
a permanent resident within the care home or unit(s) taking part in the trial
-
a formal diagnosis of dementia or a score of 4+ on the FAST,102 rated by the home manager or another experienced member of staff
-
sufficient proficiency in English to contribute to the data collection required for the research.
Residents were not eligible if they:
-
were already a DCM EPIC trial participant
-
declined (personally or via their Personal or Nominated Consultee) to participate in the trial at baseline
-
moved to the care home (or participating EPIC unit) fewer than 3 months prior to screening
-
were known by the care home manager and/or relevant senior staff member to be terminally ill (e.g. formally admitted to an end-of-life care pathway)
-
were permanently bed-bound/cared for in bed
-
were taking part in or had recently taken part in another trial that conflicted with the DCM intervention or with data collection for the DCM EPIC trial.
Resident safety
Given that the intervention was at the care home level, was very low risk and was non-invasive, and that trial consent was for data collection, minimal reporting of safety data was required. Given that the trial population was care home residents with dementia, adverse events were expected as part of usual care and, therefore, data on SAEs were collected only on consented trial residents.
A SAE was defined as an untoward event that resulted in death, was life-threatening, required or prolonged existing hospitalisation, was significantly or permanently disabling or incapacitating, or was otherwise considered medically significant by a clinician. It was expected that residents would be admitted to hospital in the event of a SAE; therefore, the safety reporting form collected information on hospitalisation, including the reason, the duration and the outcome. All deaths occurring from the date of consent to the final data collection visit were recorded on a trial death form and reported electronically to the CTRU within 1 working day of becoming aware. These data were collected by the researcher monthly, via a telephone call to the care home manager/research lead, from the point of randomisation to the completion of the 16-month follow-up. Summaries of SAEs were reviewed annually by the trial DMEC.
Any SAE occurring to a resident that, in the opinion of the care home manager/lead and chief investigator, was related to research procedures and was unexpected needed to be reported to the main REC.
Safeguarding
It was possible that the researchers might observe poor or potentially abusive practice while visiting care homes participating in the trial. The definition of abuse detailed in the Department of Health156 guidance was utilised. In the case of observing suspected abuse, the relevant Local Authority ‘Safeguarding Adults’ processes were followed after a discussion of the incident between the researcher and the recruitment centre lead/chief investigator.
Patient and public involvement
Patient and public involvement was embedded in both the design and the conduct of the trial through lay advisors on the investigator team and a Lay Advisory Group (LAG). The main focus was ensuring that PPI input was meaningful and that a PPI strategy was written at the beginning of the trial to outline how their contribution was envisaged.
Lay advisors
Three dedicated lay advisors were part of the investigator team: one individual as a member of the TSC and two as members of the TMG (one of whom was also a co-applicant). These individuals provided a user perspective on the design and conduct of the trial. They attended regular meetings throughout the trial and ensured that the TMG considered issues of importance to people living with dementia, their families and people working in care homes. Examples of advice included simplification of participant information and provision of assistance to the researchers to do this, and a suggestion that a short, pictorial version of the resident information sheet was developed. These individuals also reviewed newsletters before they were circulated, making suggestions such as increased font size to improve the accessibility of these documents to the families of people living in care homes. The lay advisors collaborated on the development and writing of a trial summary that was prepared for care home managers. They also supported the preparation of this section on PPI.
Lay Advisory Group
The LAG was recruited through a partnership agreement with the Alzheimer’s Society, which hosted the LAG meetings. The LAG consisted of eight members: a person living with dementia, relatives of people living with dementia, the manager of a care home, a person working for a care organisation and a representative from the Alzheimer’s Society. The LAG met three times during the trial to discuss progress, initial results and dissemination strategies. A fourth meeting was held to discuss final trial outputs in February 2018, following completion of the trial in December 2017.
Alongside attendance at LAG meetings, individuals reviewed trial documents such as information sheets and consent forms prior to ethics approval being sought. Individuals from the group also reviewed the intervention protocols. All trial newsletters were reviewed by the LAG prior to distribution. Members of the LAG had the opportunity to review the publication plan and be involved with all publications arising from the study. The decision on whether or not to be involved in each publication was based on if, as a group, members considered that it would be beneficial for a PPI representative to be involved and if it was relevant for them to provide input.
The LAG was responsible for devising the non-academic dissemination strategy for the trial. Such avenues for dissemination included practitioner articles, a lay article for the Alzheimer’s Society magazine, infographics and radio interviews, as well as dissemination on social media. The LAG will continue to be involved in the design and dissemination of these publications, including the design of the trial results summaries, as well as posters for care homes and for individual trial participants (i.e. residents, relatives/friends, staff members, etc.).
Chapter 3 Results
Recruitment and randomisation
Cluster recruitment
The numbers of care homes randomised and residents registered are summarised in Figure 1 by treatment arm, at baseline, at 6 months and at 16 months following randomisation for the original cohort and for the cross-sectional sample.
FIGURE 1.
Care home and resident CONSORT flow diagram. Max., maximum; min., minimum.
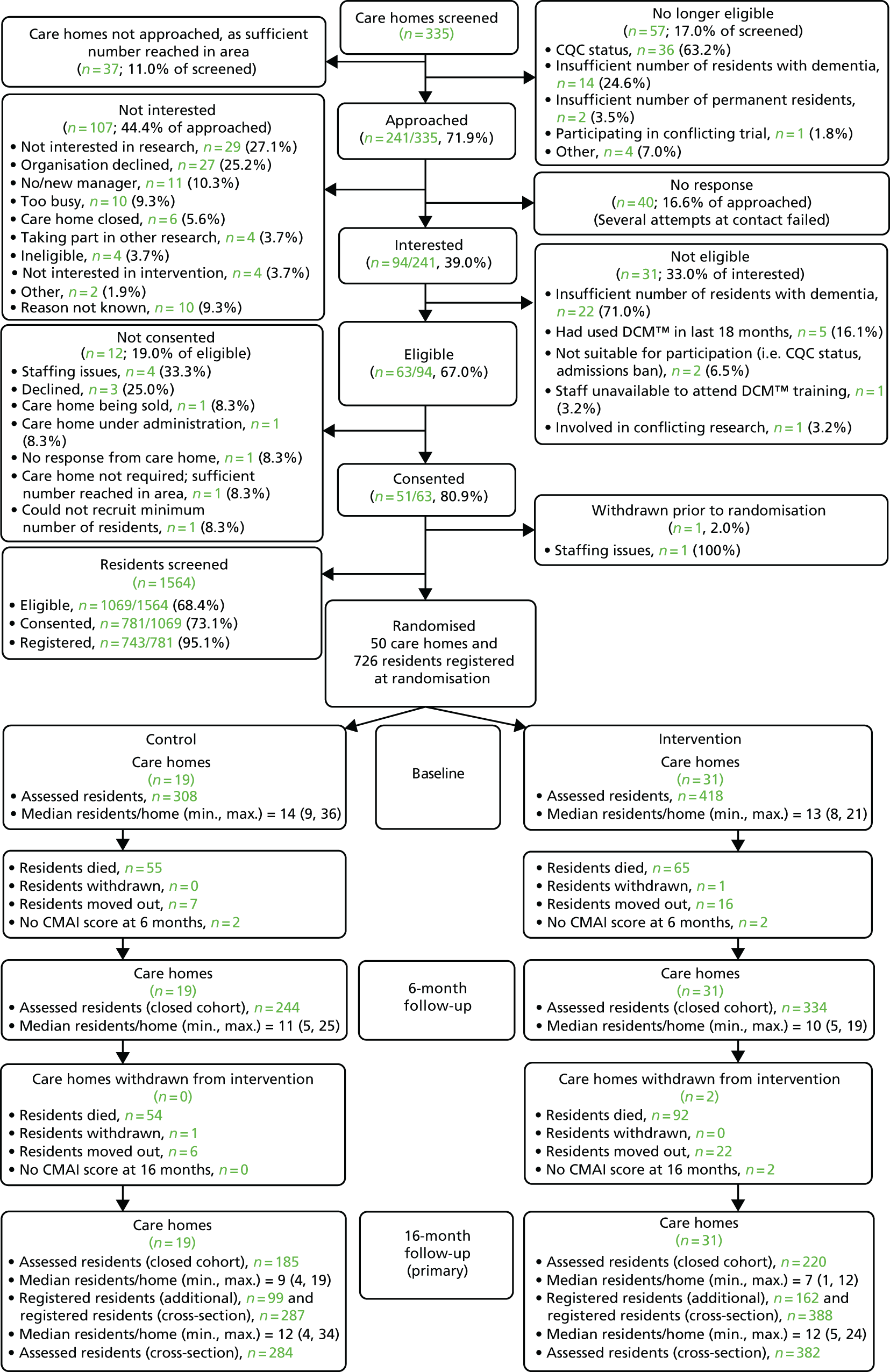
A total of 335 care homes were screened for entry into the trial. Of these, 241 randomly sampled care homes were approached and 94 homes expressing interest were formally assessed using the eligibility criteria. Of the 63 eligible care homes, 51 consented to take part and, following one consent withdrawal, 50 care homes were randomised into the trial (21 from Yorkshire, 15 from London and 14 from Oxford; see Appendix 1, Table 26). Nineteen care homes (38.0%) were randomised to the control group and 31 (62.0%) to the intervention group. Care homes were randomised over 16 months, from October 2014 to January 2016.
Resident participant flow and recruitment
Original cohort
A total of 1564 residents were screened for eligibility from consenting care homes, of whom 1069 (68.4%) were eligible. Of those who were eligible, 781 (73.1%) were consented and, of those, 743 (95.1%) were registered. Finally, of those registered, 726 (97.7%) were consented and registered at the point of care home randomisation. The reasons for exclusion from the trial are summarised overall and by hub in Appendix 1, Table 27. Residents in the original cohort were registered over 15 months, from October 2014 to December 2015.
Additional resident recruitment at 16 months
Following the approved design change, a further 1444 residents were screened from 48 care homes 16 months after randomisation (see Appendix 1, Table 27). This included all residents already participating and those who had declined to take part when approached at baseline, who were then recorded as ineligible, alongside participants failing to meet other eligibility criteria. The first two care homes randomised did not screen additional residents, as agreement for the design change was received after these care homes had completed the 16-month follow-up. Of the 1444 residents, 421 were eligible, 266 consented and 261 residents were subsequently registered (99 residents in control homes and 162 in intervention homes). A lower proportion of residents in London were ineligible as a result of being permanently bed-bound or terminally ill.
There was a higher proportion of ineligible residents of those screened (owing to not having a formal diagnosis of dementia) and of consent refusals in the intervention arm than in the control arm (see Appendix 1, Table 28). The additional residents were screened over 12 months, from June 2016 to May 2017.
Cross-sectional sample
Overall, at 16 months, a total of 675 residents were included in the cross-sectional sample: 414 residents from the original cohort who reached 16 months, and 261 additionally recruited residents. There were regional differences between hubs in resident ethnicity and funding type, with London reporting the lowest proportion of white residents and Oxford reporting the highest proportion of Local Authority funding (see Appendix 1, Table 29).
Investigation into potential recruitment bias of additional residents
As the additional residents for the cross-sectional sample were recruited following care home randomisation, age, gender, ethnicity, length of stay in care home and funding type were compared for all screened and registered residents (Table 4). Overall, there was a shorter length of stay in the additional cohort than in the original cohort, as was expected. Of the 726 residents included in the original cohort, 145 (20.0%) consented themselves, 263 (36.2%) were consented by a Personal Consultee and 318 (43.8%) were consented by a Nominated Consultee (see Appendix 1, Table 30). By contrast, of the 261 residents recruited at 16 months, 58 (22.2%) consented themselves, 73 (28.0%) were consented by a Personal Consultee and 130 (59.8%) were consented by a Nominated Consultee. There was no difference by arm in the proportion of residents who consented themselves, but a higher proportion were consented by Nominated Consultees in the intervention arm (n = 87, 53.7%) than in the control arm (n = 43, 43.4%).
| Characteristic | Original cohort | Additional residents | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screened | Registered | Screeneda | Registered | |||||||
| Total (N = 1564) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 275) | Intervention (N = 602) | Total (N = 877) | Control (N = 99) | Intervention (N = 162) | Total (N = 261) | |
| Age at registration (years), mean (SD) | 85.1 (8.18) | 85.2 (7.37) | 85.9 (7.83) | 85.6 (7.64) | 85.1 (7.51) | 85.1 (8.36) | 85.1 (8.10) | 84.6 (7.69) | 85.9 (8.09) | 85.4 (7.95) |
| Length of stay in care home (years), mean (SD) | 2.3 (2.48) | 2.3 (2.14) | 2.4 (2.47) | 2.3 (2.34) | 1.3 (1.84) | 1.7 (2.29) | 1.6 (2.17) | 1.2 (1.01) | 1.5 (1.72) | 1.4 (1.50) |
| Sex, number of females (%) | 1140 (72.9) | 244 (79.2) | 292 (69.9) | 536 (73.8) | 202 (73.5) | 423 (70.3) | 625 (71.3) | 68 (68.7) | 118 (72.8) | 186 (71.3) |
| Ethnicity, n (%) missing data | ||||||||||
| White | 1483 (94.8) 26 | 302 (98.1) | 400 (95.7) | 702 (96.7) | 271 (98.5) 2 | 575 (95.5) 4 | 846 (96.5) 6 | 99 (100.0) | 158 (97.5) | 257 (98.5) |
| Other | 55 (3.5) | 6 (1.9) | 18 (4.3) | 24 (3.3) | 2 (0.7) | 23 (3.8) | 25 (2.9) | 0 (0.0) | 4 (2.5) | 4 (1.5) |
| Funding type, n (%) | ||||||||||
| Local Authority | 741 (47.4) | 128 (41.6) | 224 (53.6) | 352 (48.5) | 113 (41.1) | 291 (48.3) | 404 (46.1) | 52 (52.5) | 74 (45.7) | 126 (48.3) |
| Continuing health care | 115 (7.4) | 28 (9.1) | 20 (4.8) | 48 (6.6) | 5 (1.8) | 16 (2.7) | 21 (2.4) | 1 (1.0) | 1 (0.6) | 2 (0.8) |
| Self-funded | 555 (35.5) | 133 (43.2) | 156 (37.3) | 289 (39.8) | 94 (34.2) | 224 (37.2) | 318 (36.3) | 33 (33.3) | 75 (46.3) | 108 (41.4) |
| Local Authority and self-funded | 69 (4.4) | 17 (5.5) | 17 (4.1) | 34 (4.7) | 26 (9.5) | 42 (7.0) | 68 (7.8) | 13 (13.1) | 12 (7.4) | 25 (9.6) |
| Missing | 84 (5.4) | 2 (0.6) | 1 (0.2) | 3 (0.4) | 37 (13.5) | 29 (4.8) | 66 (7.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Staff recruitment
There was a very poor return rate of staff questionnaire booklets (see Appendix 1, Table 31), despite the changes made to encourage return rates [i.e. removal of the GHQ-12 and personal data (see Chapter 2, Summary of changes to project protocol)]. Following consultation with oversight committees, it was agreed that persistence with obtaining staff data was important, as the intervention was designed to effect a ‘whole-home’ change. However, owing to low return rates, planned statistical analyses could not be conducted.
Relative/friend recruitment
At baseline, 197 relatives/friends were registered to the trial, with 96 in the control arm and 101 in the intervention arm. This reflects a larger proportion in the control arm, given the 2 : 3 randomisation allocation. The total number of relatives/friends registered to the trial reduced at 6 months (N = 170; control, n = 85; intervention, n = 85) and 16 months (N = 118; control, n = 63; intervention, n = 55) (see Appendix 1, Table 32), as might be expected with the high loss to follow-up rates. It was agreed by the oversight committees that, given the low percentage of data received, these data would not be useful when undertaking statistical analyses, with the exception of some of the health economic analyses (see Chapter 2, Health economic analysis). New relative/friend informants were therefore not identified at follow-up. When relatives/friends agreed to take part at baseline, we continued to request their follow-up data.
Baseline data
Care home characteristics
At baseline, on average, the intervention-arm homes were larger than the control-arm homes. However, the average proportion of permanent residents with dementia was higher in the control arm. Care home managers had similar work experience and training across both arms (Table 5).
| Characteristic | Control (N = 19) | Intervention (N = 31) | Total (N = 50) |
|---|---|---|---|
| Unit type, n (%) missing data | |||
| General residential/nursing home | 11 (57.9) 0 | 20 (64.5) 0 | 31 (62) 0 |
| Specialist dementia care home/unit | 8 (42.1) 0 | 11 (35.5) 0 | 19 (38) 0 |
| Care home | |||
| More than one unit, n (%) missing data | 3 (15.8) 0 | 3 (9.7) 0 | 6 (12) 0 |
| DCM used between 18 months and 5 years, n (%) missing data | 11 (57.9) 0 | 20 (64.5) 0 | 31 (62) 0 |
| Residents’ meeting held within the last 6 months, n (%) missing data | 17 (89.5) 0 | 30 (100) 1 | 47 (95.9) 1 |
| Relatives’ meeting held within the last 6 months, n (%) missing data | 18 (94.7) 0 | 29 (96.7) 1 | 47 (95.9) 1 |
| Number of beds in the care home, mean (SD) missing data | 28.8 (8.97) 2 | 36.8 (14.28) 1 | 33.9 (13.1) 3 |
| Number of permanent residents, mean (SD) missing data | 30 (11.27) 0 | 32.9 (14.02) 1 | 31.8 (12.98) 1 |
| Percentage of permanent residents with dementia, mean (SD) missing data | 83.1 (21.21) 0 | 74.2 (22.48) 1 | 77.7 (22.21) 1 |
| Percentage of self-funded residents, mean (SD) missing data | 52.8 (28.12) 0 | 37.9 (21.12) 1 | 43.7 (24.89) 1 |
| Cost of a self-funded place per year (£), mean (SD) missing data | 44,553 (13,291) 0 | 41,638 (13,003) 1 | 42,768 (13,056) 1 |
| Number of residents per staff member (daytime), median (range) missing data | 5.2 (3.0–8.8) 0 | 4.7 (2.5–10.5) 1 | 4.8 (2.5–10.5) 1 |
| Number of residents per staff member (night-time), median (range) missing data | 9.5 (3.3–17.5) 0 | 9.7 (2.9–15.3) 1 | 9.7 (2.9–17.5) 1 |
| Care home manager | |||
| Time in current role (years), median (range) | 2.5 (0.3–37.0) | 2.9 (0.3–25.0) | 2.6 (0.3–37.0) |
| Length of time working in care homes, n (%) | |||
| Up to 10 years | 3 (15.8) | 7 (22.6) | 10 (20.0) |
| More than 10 years | 16 (84.2) | 24 (77.4) | 40 (80.0) |
| Length of time in a manager role, n (%) | |||
| Up to 2 years | 3 (15.8) | 7 (22.6) | 10 (20.0) |
| Up to 5 years | 5 (26.3) | 4 (12.9) | 9 (18.0) |
| Up to 10 years | 2 (10.5) | 5 (16.1) | 7 (14.0) |
| More than 10 years | 9 (47.4) | 15 (48.4) | 24 (48.0) |
| Manager dementia training/education, n (%) | |||
| Previously trained as a dementia care mapper by University of Bradford | 3 (15.8) | 4 (12.9) | 7 (14.0) |
| Dementia-specific qualification | 4 (21.1) | 10 (32.3) | 14 (28.0) |
| Dementia covered in one part of a qualification | 10 (52.6) | 18 (58.1) | 28 (56.0) |
| Attended a dementia-specific training course | 19 (100.0) | 31 (100.0) | 50 (100.0) |
A slightly higher than anticipated number of care homes (n = 13, 26%)99 needed PCCT ahead of baseline data collection, owing to not meeting minimum criteria on the ADAPT audit tool.
Resident characteristics
In the closed-cohort sample, the mean resident age at randomisation was similar between intervention and control arms (85.3 years in control, 86.0 years in intervention) (Table 6). A higher proportion of residents in the intervention arm were male (n = 126, 30.1%) than in the control arm (n = 64, 20.8%) and the median number of comorbidities was two in both arms, with the proportion of residents with no comorbidities similar across arms.
| Original cohort at baseline | Control (N = 308) | Intervention (N = 418) | Total (N = 726) |
|---|---|---|---|
| Age at randomisation (years), mean (SD) missing data | 85.3 (7.38) 0 | 86 (7.83) 0 | 85.7 (7.64) 0 |
| Sex, number of males (%) | 64 (20.8) | 126 (30.1) | 190 (26.2) |
| Number of comorbidities per resident, median (range) | 2 (0–10) | 2 (0–14) | 2 (0–14) |
| Selected comorbidities,a n (%) | |||
| Anxiety | 34 (11.0) | 23 (5.5) | 57 (7.9) |
| Depression | 62 (20.1) | 55 (13.2) | 117 (16.1) |
| Psychosis | 16 (5.2) | 24 (5.7) | 40 (5.5) |
| Sleep disturbance | 6 (1.9) | 7 (1.7) | 13 (1.8) |
| Delirium | 3 (1.0) | 2 (0.5) | 5 (0.7) |
| FAST stage (out of completed scores), n (%) | (N = 306) | (N = 391) | (N = 697) |
| 1 | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| 2 | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| 3 | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| 4 | 39 (12.7) | 56 (14.3) | 95 (13.6) |
| 5 | 26 (8.5) | 48 (12.3) | 74 (10.6) |
| 6 | 166 (54.2) | 214 (54.7) | 380 (54.5) |
| 7 | 70 (22.9) | 72 (18.4) | 142 (20.4) |
| Cross-section at 16 months | Control (N = 287) | Intervention (N = 388) | Total (N = 675) |
| Age at randomisation (years), mean (SD) missing data | 83.7 (7.77) 0 | 85.2 (7.79) 0 | 84.6 (7.81) 0 |
| Gender, number of males (%) | 71 (24.7) | 110 (28.4) | 181 (26.8) |
| Number of comorbidities per resident, median (range) | 2 (0–7) | 3 (0–12) | 2 (0–12) |
| Selected comorbidities,a n (%) | |||
| Anxiety | 26 (9.1) | 27 (7.0) | 53 (7.9) |
| Depression | 64 (22.3) | 66 (17.0) | 130 (19.3) |
| Psychosis | 11 (3.8) | 21 (5.4) | 32 (4.7) |
| Sleep disturbance | 2 (0.7) | 5 (1.3) | 7 (1.0) |
| Delirium | 2 (0.7) | 2 (0.5) | 4 (0.6) |
| FAST stage (out of completed scores), n (%) | (N = 284) | (N = 384) | (N = 668) |
| 4 | 22 (7.7) | 35 (9.1) | 57 (8.5) |
| 5 | 20 (7.0) | 21 (5.5) | 41 (6.1) |
| 6 | 168 (59.2) | 238 (62.0) | 406 (60.8) |
| 7 | 74 (26.1) | 90 (23.4) | 164 (24.6) |
In the cross-sectional sample, control residents were slightly younger than intervention residents (83.7 and 85.2 years, respectively). There was a higher proportion of residents with no reported comorbidities in the control arm than in the intervention arm. Similar levels of dementia severity were observed in both arms, as measured with the FAST, although a lower proportion of residents had moderately severe to severe dementia (FAST stages 6 or 7) in the closed cohort (74.9%) than in the cross-sectional sample (85.4%), owing to the worsening of dementia of residents in the closed cohort over time.
Treatment summaries
Control
Organisational and staff changes reflecting usual care at a care home level are summarised in Table 7 for both arms, each compared with the previous time point. A higher proportion of care homes had experienced management changes in the intervention arm than in the control arm at 6 months, and a higher proportion of care homes had new staff roles introduced in the unit in the intervention arm than in the control arm. At both follow-up points, a higher proportion of intervention care homes achieved or completed standard quality assessments than control-arm homes. Compared with control homes, a smaller proportion of intervention care homes reported having staff with higher-level dementia-specific qualifications at 6 months but, by 16 months, a higher proportion of intervention homes reported having staff with higher-level dementia-specific qualifications.
| Change | At 6 months (from baseline), n (%) number unknown | At 16 months (from 6 months), n (%) number unknown | ||||
|---|---|---|---|---|---|---|
| Control (N = 19) | Intervention (N = 31) | Total (N = 50) | Control (N = 19) | Intervention (N = 31) | Total (N = 50) | |
| Any organisational changes | 4 (21.1) 0 | 6 (19.4) 0 | 10 (20.0) 0 | 4 (21.1) 0 | 6 (19.4) 0 | 10 (20.0) 0 |
| Any care home management changes | 5 (26.3) 0 | 12 (38.7) 0 | 17 (34.0) 0 | 8 (42.1) 0 | 13 (41.9) 0 | 21 (42.0) 0 |
| Any new staff roles | 1 (5.3) 0 | 6 (19.4) 0 | 7 (14.0) 0 | 3 (15.8) 0 | 7 (22.6) 0 | 10 (20.0) 0 |
| Any new projects or initiatives | 5 (26.3) 0 | 9 (29.0) 0 | 14 (28.0) 0 | 6 (31.6) 0 | 12 (38.7) 0 | 18 (36.0) 0 |
| Any new voluntary measures to improve standards | 1 (5.3) 0 | 3 (9.7) 0 | 4 (8.0) 0 | 0 (0.0) 0 | 3 (9.7) 0 | 3 (6.0) 0 |
| Any standard quality assessments achieved | 3 (15.8) 0 | 9 (29.0) 0 | 12 (24.0) 0 | 2 (10.5) 0 | 6 (19.4) 0 | 8 (16.0) 0 |
| Currently subject to any CQC notifications | 2 (10.5) 0 | 6 (19.4) 0 | 8 (16.0) 0 | 1 (5.3) 0 | 1 (3.2) 0 | 2 (4.0) 0 |
| PCCT available in unit | 18 (94.7) 0 | 29 (93.5) 0 | 47 (94.0) 0 | 16 (84.2) 1 | 31 (100.0) 0 | 47 (94.0) 1 |
| Staff with higher-level dementia-specific qualification | 10 (52.6) 0 | 12 (38.7) 1 | 22 (44.0) 1 | 10 (52.6) 0 | 20 (64.5) 0 | 30 (60.0) 0 |
Intervention
Adherence to the intervention is reported by cycle and number of components completed (i.e. briefing; observation; analysis, reporting and feedback; and action-planning) in Figure 2 (see Appendix 1, Table 33, for further details on adherence by care home), with the furthest reported component through the DCM cycle presented when the full cycle was not completed. Intervention fidelity is also reported in detail elsewhere. 169 Based on documented evidence, 16 care homes (51.6%) in the intervention arm completed only one cycle to an acceptable level, four (12.9%) completed two cycles to an acceptable level and four (12.9%) completed all three cycles to an acceptable level. Seven care homes (22.6%) did not complete a full intervention cycle, with three (9.7%) of these not completing any of the intervention components. Additional intervention component summaries can be found in Appendix 1 (see Tables 34–40 and Figure 7). Owing to challenges in a complete set of adherence data being received from care homes, it was not possible to ascertain how many care home staff had engaged with the DCM process during each cycle and thus to assess intervention ‘dose’ in terms of reach.
FIGURE 2.
Completion of intervention components by cycle. Reproduced from Surr et al. 158 This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
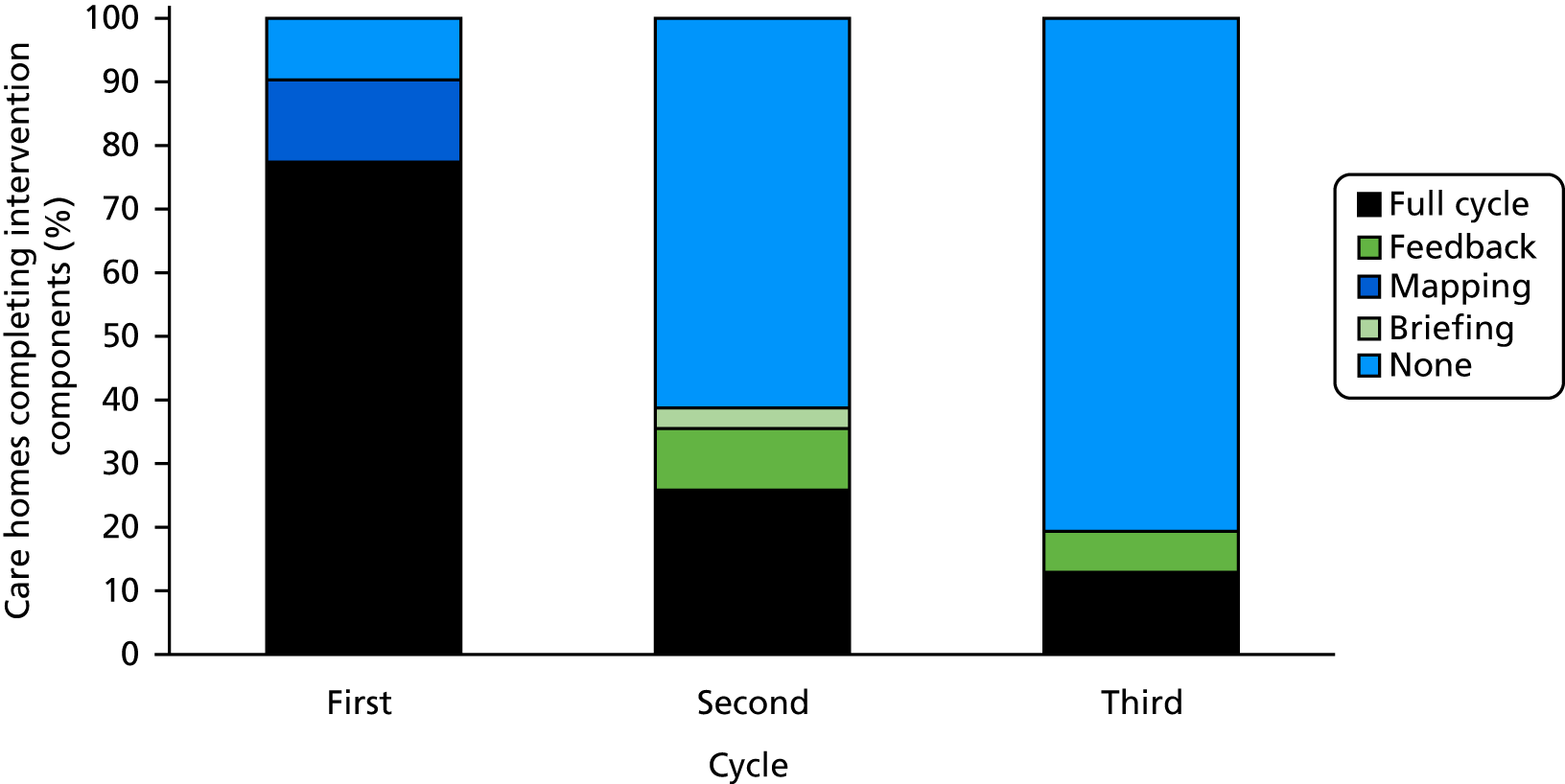
Losses and exclusions after randomisation
Withdrawals
Two care homes in the intervention arm withdrew from further trial treatment but not from further data collection: one in month 11 and one in month 12. One resident from the closed cohort withdrew consent for all data collection in the intervention arm (withdrawn by Personal Consultee in month 2). There were four staff-proxy withdrawals, one in each arm at each of the the 6- and 16-month follow-ups. There were four relative/friend withdrawals, one in the control arm (at the 16-month follow-up) and three in the intervention arm (one at the 6-month and two after the 16-month follow-up).
Protocol violations
There were two care home eligibility violations identified and reported in the first 2 months following randomisation (one in each arm). Both related to changed CQC status between recruitment and randomisation. In both cases, the chief investigator agreed to the care homes continuing in the trial and so they were included in the ITT analysis. Five staff eligibility violations were reported in the intervention arm, involving individuals who undertook both mapper and staff-proxy roles.
Resident deaths in closed cohort
Seventeen residents died between care home registration and randomisation (see Appendix 1, Table 41); the remaining 726 residents constituted the original cohort. Overall, there were 272 (37.5%) deaths reported between randomisation and the end of the 16-month follow-up in the original cohort, 111 (36.0%) in the control arm and 161 (38.5%) in the intervention arm [primary outcome data were available for two residents who died (1.8%) in the control arm and for four residents who died (2.5%) in the intervention arm]. The majority of these deaths occurred in the care home [224/272, 82%: 89/111 (80.2%) in the control arm and 135/161 (83.9%) in the intervention arm]. The mean proportion of deaths per care home in the control arm was 0.36 (SD 0.12) and in the intervention arm it was 0.39 (SD 0.14).
Clinical effectiveness of the intervention
Analyses of the primary outcome
Analyses were conducted on the cross-sectional sample (primary) and the closed cohort. Unadjusted scores are presented in Table 8 for the primary outcome (staff proxy-completed CMAI), and the change in unadjusted scores from baseline is presented graphically in Appendix 1, Figures 8 and 9. At baseline, the mean total CMAI score was higher in the control arm (48.4 points) than in the intervention homes (45.4 points). In the closed cohort at 6 months, the gap had closed, with the mean total CMAI scores being 44.9 points and 43.6 points, respectively, in control and intervention homes [however, 148/726 (20.4%) residents were lost to follow-up]. By 16 months, the gap had widened again in the closed cohort [although, by this time, 321/726 (44.2%) residents were lost to follow-up], with mean total CMAI scores of 46.4 points and 41.4 points, respectively, in control and intervention care homes. The gap was slightly narrower in the cross-sectional sample [9/675 (1.3%) lost to follow-up], with mean total CMAI scores of 46.1 points and 42.8 points in the control and intervention homes, respectively. Differences in mean total CMAI scores between the control and intervention homes are therefore small in both resident samples, largely arising from changes carried through from baseline.
| Closed cohort | Control (n = 308), mean (SD) missing data | Intervention (n = 418), mean (SD) missing data | Total (n = 726) |
|---|---|---|---|
| Baseline total score | 48.4 (19.53) 2 | 45.4 (15.95) 2 | 46.7 (17.6) 4 |
| Subscales | |||
| Aggressive behaviour | 14.3 (8.10) 2 | 12.6 (6.28) 2 | 13.3 (7.16) 4 |
| Physically non-aggressive | 11.6 (6.47) 8 | 11.3 (6.08) 10 | 11.4 (6.25) 18 |
| Verbally agitated | 10.4 (6.23) 5 | 9.9 (5.94) 2 | 10.1 (6.06) 7 |
| Other | 12 (4.58) 0 | 11.5 (3.73) 2 | 11.7 (4.12) 2 |
| 6-month total score | 44.9 (16.75) 64 | 43.6 (14.32) 84 | 44.2 (15.39) 148 |
| Subscales | |||
| Aggressive behaviour | 13.3 (7.21) 64 | 12.4 (6.14) 84 | 12.8 (6.62) 148 |
| Physically non-aggressive | 10.5 (5.88) 68 | 10.6 (5.28) 100 | 10.6 (5.54) 168 |
| Verbally agitated | 9.4 (5.42) 64 | 9.6 (5.42) 87 | 9.5 (5.42) 151 |
| Other | 11.7 (3.95) 64 | 11 (3.26) 84 | 11.3 (3.58) 148 |
| 16-month total score | 46.4 (16.54) 123 | 41.4 (14.73) 198 | 43.7 (15.76) 321 |
| Subscales | |||
| Aggressive behaviour | 14 (7.66) 123 | 12.3 (5.9) 196 | 13 (6.8) 319 |
| Physically non-aggressive | 11 (5.82) 124 | 9.2 (4.85) 205 | 10 (5.38) 329 |
| Verbally agitated | 9.7 (5.55) 123 | 9 (5.63) 197 | 9.3 (5.60) 320 |
| Other | 11.8 (4.05) 123 | 10.8 (3.08) 199 | 11.3 (3.59) 322 |
| Cross-section | Control (n = 287), mean (SD) missing data | Intervention (n = 388), mean (SD) missing data | Total (n = 675) |
| 16-month total score | 46.1 (16.78) 3 | 42.8 (15.79) 6 | 44.2 (16.29) 9 |
| Subscales | |||
| Aggressive behaviour | 13.7 (7.93) 3 | 12.2 (5.87) 4 | 12.9 (6.86) 7 |
| Physically non-aggressive | 11 (6.01) 4 | 9.9 (5.36) 15 | 10.4 (5.67) 19 |
| Verbally agitated | 9.8 (5.79) 3 | 9.7 (6.16) 5 | 9.7 (6.00) 8 |
| Other | 11.5 (3.73) 3 | 11 (3.49) 7 | 11.2 (3.60) 0 |
All 675 residents in the cross-sectional sample at 16 months were included in the primary analysis, of whom complete data were available for 666. There was no evidence of a difference in agitation levels between arms. The mean difference in total CMAI score from the two-level heteroscedastic linear regression model fitted to the multiply imputed data (assuming that data were MAR) was –2.11 points, being lower in the intervention arm than in the control arm (adjusted means: 45.47 points in the control arm and 43.35 points in the intervention arm; 95% CI –4.66 to 0.44 points; p = 0.104). The unadjusted ICC was 0 in the control and 0.058 in the intervention arm, but the adjusted ICC was 0 in the control and 0.001 in the intervention arm, indicating that between-cluster heterogeneity in the intervention arm was explained by the covariates in the model. Using the complete cases, the mean difference was –2.19 points, being lower in the intervention arm than in the control homes (95% CI –4.81 to 0.43 points), and the adjusted ICC was zero in both treatment arms, indicating that the treatment effect was neither clinically meaningful nor statistically significant at the 5% level (p = 0.099) (see Appendix 1, Table 45). The primary analysis is summarised in Table 9.
| Analysis | Adjusted mean in control arm | Adjusted mean in intervention arm | Estimated mean difference | 95% CI | p-value | Adjusted ICC for intervention arm | Adjusted ICC for control arm | n |
|---|---|---|---|---|---|---|---|---|
| Primary analysis, CMAI score on ITT sample | 45.47 | 43.35 | –2.11 | –4.66 to 0.44 | 0.104 | 0.001 | 0.000 | 675 |
| Sensitivity analyses | ||||||||
| Key sensitivity analysis (hub omitted from the model), CMAI score on ITT sample | 46.02 | 43.78 | –2.24 | –4.91 to 0.42 | 0.099 | 0.010 | 675 | |
| 1. Adjusting for before–after eligibility change,a CMAI score on ITT sample | 44.82 | 42.69 | –2.13 | –4.71 to 0.45 | 0.105 | 0.002 | 0.000 | 675 |
| 2. Care home size as a continuous variable, CMAI score on ITT sample | 45.59 | 43.21 | –2.38 | –5.00 to 0.25 | 0.076 | 0.000 | 0.000 | 675 |
| 3. Homogeneous clustering across arms, CMAI score on ITT sample | 45.41 | 43.32 | –2.09 | –4.61 to 0.44 | 0.105 | 0.001 | 675 | |
| 4a. CMAI-O score (a.m.) | 31.00 | 30.41 | –0.58 | –1.62 to 0.45 | 0.269 | 0.215 | 0.006 | 675 |
| 4b. CMAI score on subset with CMAI-O (a.m.) | 47.49 | 43.43 | –4.06 | –7.55 to –0.57 | 0.023 | 0.016 | 0.001 | 365 |
| 4c. CMAI-O score (p.m.) | 31.34 | 31.11 | –0.22 | –1.52 to 1.08 | 0.737 | 0.220 | 0.013 | 675 |
| 4d. CMAI score on subset with CMAI-O (p.m.) | 47.49 | 43.43 | –4.06 | –7.55 to –0.57 | 0.023 | 0.016 | 0.001 | 365 |
| 4e. PAS score (a.m.) | 0.93 | 0.73 | –0.20 | –0.67 to 0.27 | 0.402 | 0.166 | 0.011 | 675 |
| 4f. CMAI score on subset with PAS (a.m.) | 47.49 | 43.43 | –4.06 | –7.55 to –0.57 | 0.023 | 0.016 | 0.001 | 365 |
| 4g. PAS score (p.m.) | 1.17 | 0.89 | –0.28 | –0.96 to 0.41 | 0.429 | 0.299 | 0.018 | 675 |
| 4h. CMAI score on subset with PAS (p.m.) | 47.49 | 43.43 | –4.06 | –7.55 to –0.57 | 0.023 | 0.016 | 0.001 | 365 |
| 5. CMAI score at 16 months (closed cohort) | 46.4 | 43.16 | –3.25 | –6.13 to –0.37 | 0.027 | 0.013 | 0.001 | 726 |
Supportive and sensitivity analyses
Unadjusted scores for the CMAI-O and PAS outcomes, which were used in place of the CMAI, by resident sample and time point, are presented in Table 10 (cross-section) and in Appendix 1, Tables 42 (closed cohort) and 43 and 46 and 47 (complete cases). A similar pattern of differences was found for these supportive outcomes completed by the blinded independent researcher. The mean CMAI-O scores were consistently very slightly higher in the afternoon than in the morning. The same is the case for PAS scores. Loss to follow-up was higher for these supportive outcomes [about 276/726 (38.0%) at baseline, 358/726 (49.3%) at 6 months and 495/726 (68.2%) and 310/675 (45.9%) at 16 months in the closed cohort and cross-section, respectively] than for the primary outcome.
| Outcome | a.m., mean (SD) number completed | p.m., mean (SD) number completed | ||||
|---|---|---|---|---|---|---|
| Control (n = 287) | Intervention (n = 388) | Total (n = 675) | Control (n = 287) | Intervention (n = 388) | Total (n = 675) | |
| 16-month CMAI-O total score | 31.1 (3.8) 156 | 30.5 (3.3) 209 | 30.8 (3.5) 365 | 31.4 (3.8) 148 | 31.1 (3.9) 206 | 31.2 (3.9) 354 |
| Subscales | ||||||
| Aggressive behaviour | 9.3 (0.9) 156 | 9.3 (1.0) 209 | 9.3 (1.0) 365 | 9.3 (1.1) 148 | 9.3 (1.2) 206 | 9.3 (1.1) 354 |
| Physically non-aggressive | 6.7 (1.4) 156 | 6.5 (1.5) 209 | 6.6 (1.4) 365 | 6.9 (1.5) 148 | 6.8 (1.9) 206 | 6.9 (1.8) 354 |
| Verbally agitated | 5.8 (2.2) 156 | 5.5 (1.5) 209 | 5.6 (1.8) 365 | 5.8 (1.9) 148 | 5.7 (1.7) 206 | 5.7 (1.8) 354 |
| Other | 9.3 (1.0) 156 | 9.2 (0.7) 209 | 9.2 (0.8) 365 | 9.3 (0.9) 148 | 9.3 (0.9) 206 | 9.3 (0.9) 354 |
| 16-month PAS score | 1.1 (1.9) 156 | 0.8 (1.7) 209 | 0.9 (1.8) 365 | 1.2 (1.9) 148 | 0.9 (1.8) 205 | 1.0 (1.8) 353 |
The sensitivity and supportive analyses are summarised in Table 9 and Appendix 1, Table 44, respectively. The key sensitivity analysis simplified the model fitted to ensure complete convergence and was added post hoc. Sensitivity analyses on the CMAI score for the subset of residents included in the analyses of the CMAI-O and PAS were also added post hoc. The equivalent analyses on the complete cases are provided in Appendix 1, Tables 18–20. The key sensitivity analysis and the first three planned sensitivity analyses supported the results found in the primary analysis.
Sensitivity analyses of the CMAI-O and the PAS indicated a potential overestimation of the treatment effect from the primary analysis, as the mean differences are reduced when a blinded independent observation is made (see the analyses in rows 4a and 4c in Table 9). However, we would expect the CMAI-O and PAS to potentially underestimate agitation levels, as they are conducted over only two observation periods in a single week, in public areas of the home, during restricted daytime hours. The staff-proxy rating is made over 2 weeks and includes consideration of agitation during personal care in the evening and at night-time. The sensitivity analysis conducted on the closed cohort gave a mean difference of –3.25 (95% CI –6.13 to –0.37; p = 0.027), apparently contradicting the conclusion of the primary analysis. However, the sensitivity analysis is not robust, as it relies on multiply-imputed data for 45% of the sample. It has a different interpretation too, as this is the treatment effect estimated for residents who remain in the care home from baseline to 16 months. A sensitivity analysis on the closed cohort assumed that data are MNAR. This explores the impact of assumptions about the missing data, looking at a range of plausible and potentially implausible scenarios in which there was a shift in the CMAI score at 16 months of up to 40 points either way for residents that died, withdrew or moved away. This assumes that the scores for all residents with missing data would have shifted by the same number of points. The conclusions of the closed-cohort analysis remain unchanged for shifts of –40 to 5 points from the average CMAI score at 16 months for those who died, and any shift for those who withdrew (see Appendix 1, Table 48).
Supportive analyses of the closed cohort at 6 and 16 months (see Appendix 1, Table 44) indicate that there were no differences in CMAI, CMAI-O or PAS scores at 6 months and no differences in CMAI-O and PAS scores at 16 months. Overall, these analyses confirm that the intervention is not superior to the control.
A CACE analysis of the cross-sectional sample, comparing care homes in the intervention arm that completed at least one cycle to an acceptable level with care homes that would have completed at least one cycle had the intervention been offered to them, gave a mean difference in CMAI score at 16 months of –2.5 points (95% CI –5.4 to 0.4 points; p = 0.089). This indicates that the ITT estimate from the primary analysis is not dissimilar to the effect of completing at least one cycle to an acceptable level. The 95% CIs are wider than in the primary analysis and the CACE estimate is not statistically significant at the 5% level (p = 0.089) (Table 11). The exploratory CACE analyses using other definitions of adherence indicate that the treatment effect may increase if care homes complete at least two DCM cycles to an acceptable level, compared with completing only one cycle. Although these analyses are suggestive of a dose–response relationship in which supporting adherence to the second and third cycles might result in a clinically meaningful effect, this would need to be confirmed by further research.
| Analyses | Model | Treatment effect (standard error) | 95% CI | p-value |
|---|---|---|---|---|
| CACE analyses (documented and expert evidence), MI | At least one cycle to an acceptable level | –2.5 (1.5) | –5.4 to 0.4 | 0.089 |
| At least one cycle to a partial level | –2.2 (1.3) | –4.8 to 0.3 | 0.087 | |
| One cycle only to an acceptable level | –3.6 (2.2) | –7.9 to 0.8 | 0.106 | |
| At least two cycles to an acceptable level | –8.5 (5.3) | –18.9 to 2.0 | 0.112 | |
| Complete-case CACE analyses, sensitivity analyses | At least one cycle to an acceptable level | |||
| At least two cycles to an acceptable level | –2.6 (1.4) | –5.4 to 0.2 | 0.068 | |
| At least one cycle to a partial level | –2.2 (1.3) | –4.8 to 0.3 | 0.087 |
The change in unadjusted CMAI scores for the care homes between baseline and 16 months is presented by intervention adherence (the number of cycles completed to an acceptable level) in Figure 3. There was considerable variation in CMAI score changes between care homes completing zero, one, two and three acceptable cycles.
FIGURE 3.
Change in CMAI score between baseline and 16 months by adherence to the intervention.

Analyses of the secondary outcomes
Analyses of the NPI-NH, PRN prescription medications, QoL and quality of staff interactions were conducted on the closed cohort at 6 months and on the cross-sectional sample (primary) and the closed cohort (supportive) at 16 months. Unadjusted scores are presented in Tables 12–15 by resident sample and time point and in Appendix 1, Tables 49–60.
| Closed cohort | Total NPIa scores, mean (SD) missing data | Number experiencing BSC, n (%) number completed | ||||
|---|---|---|---|---|---|---|
| Control (n = 308) | Intervention (n = 418) | Total (n = 726) | Control (n = 308) | Intervention (n = 418) | Total (n = 726) | |
| Baseline | 13 (13.95) 0 | 11.7 (12.35) 0 | 12.2 (13.06) 0 | 236 (76.6) 308 | 325 (77.8) 418 | 561 (77.3) 726 |
| Subscalesb | ||||||
| Agitation/aggression | 5.0 (2.85) 2 | 4.7 (2.86) 0 | 4.8 (2.85) 2 | 145 (47.1) 308 | 192 (46.0) 417 | 337 (46.5) 725 |
| Depression/dysphoria | 4.1 (2.77) 0 | 3.6 (2.63) 2 | 3.8 (2.70) 2 | 92 (30.0) 307 | 129 (30.9) 418 | 221 (30.5) 725 |
| Anxiety | 5.2 (3.16) 2 | 3.9 (2.32) 3 | 4.5 (2.80) 5 | 80 (26.0) 308 | 98 (23.5) 417 | 178 (24.6) 725 |
| Apathy/indifference | 5.4 (3.30) 1 | 5.2 (3.07) 1 | 5.3 (3.16) 2 | 91 (29.5) 308 | 130 (31.2) 417 | 221 (30.5) 725 |
| Disinhibition | 5.0 (3.29) 0 | 3.8 (2.62) 0 | 4.3 (2.97) 0 | 51 (16.6) 308 | 65 (15.6) 416 | 116 (16.0) 724 |
| Irritability/lability | 5.3 (3.16) 3 | 4.4 (2.85) 0 | 4.8 (3.01) 3 | 117 (38.0) 308 | 153 (36.7) 417 | 270 (37.2) 725 |
| 6 months | 11.3 (12.35) 0 | 9.7 (10.14) 0 | 10.4 (11.17) 0 | 186 (76.2) 244 | 238 (74.4) 320 | 424 (75.2) 564 |
| Subscalesb | ||||||
| Agitation/aggression | 5.4 (3.24) 0 | 4.4 (2.62) 0 | 4.9 (2.98) 0 | 120 (49.0) 245 | 125 (39.2) 319 | 245 (43.4) 564 |
| Depression/dysphoria | 3.7 (2.66) 0 | 3.5 (2.35) 1 | 3.6 (2.47) 1 | 63 (26.0) 242 | 101 (31.6) 320 | 164 (29.2) 562 |
| Anxiety | 4.6 (2.92) 2 | 4.0 (2.71) 1 | 4.3 (2.81) 3 | 47 (19.3) 244 | 57 (17.9) 319 | 104 (18.5) 563 |
| Apathy/indifference | 5.7 (3.39) 1 | 4.3 (2.91) 1 | 4.9 (3.16) 2 | 73 (29.9) 244 | 116 (36.3) 320 | 189 (33.5) 564 |
| Disinhibition | 4.9 (3.08) 0 | 5.3 (3.44) 0 | 5.1 (3.23) 0 | 35 (14.3) 244 | 30 (9.4) 320 | 65 (11.5) 564 |
| Irritability/lability | 4.5 (3.12) 0 | 4.4 (2.89) 0 | 4.5 (2.99) 0 | 83 (33.9) 245 | 99 (30.9) 320 | 182 (32.2) 565 |
| 16 months | 10.4 (9.25) 0 | 7.7 (9.36) 0 | 8.9 (9.4) 0 | 146 (78.9) 185 | 154 (69.4) 222 | 300 (73.7) 407 |
| Subscalesb | ||||||
| Agitation/aggression | 4.5 (2.30) 0 | 4.5 (3.00) 0 | 4.5 (2.65) 0 | 82 (44.3) 185 | 76 (34.2) 222 | 158 (38.8) 407 |
| Depression/dysphoria | 3.5 (2.09) 1 | 3.1 (1.87) 1 | 3.3 (1.99) 2 | 63 (34.1) 185 | 55 (24.8) 222 | 118 (29.0) 407 |
| Anxiety | 4.5 (2.28) 0 | 4.4 (2.83) 1 | 4.4 (2.56) 1 | 29 (15.7) 185 | 34 (15.3) 222 | 63 (15.5) 407 |
| Apathy/indifference | 5.5 (3.33) 0 | 5.2 (3.40) 0 | 5.3 (3.36) 0 | 73 (39.5) 185 | 62 (27.9) 222 | 135 (33.2) 407 |
| Disinhibition | 3.6 (2.43) 1 | 3.6 (2.59) 0 | 3.6 (2.48) 1 | 24 (13.0) 185 | 24 (10.8) 222 | 48 (11.8) 407 |
| Irritability/lability | 4.5 (2.30) 0 | 4.0 (2.83) 0 | 4.2 (2.58) 0 | 65 (35.1) 185 | 66 (29.7) 222 | 131 (32.2) 407 |
| Cross-section | Control (n = 287) | Intervention (n = 388) | Total (n = 675) | Control (n = 287) | Intervention (n = 388) | Total (n = 675) |
| 16 months | 10 (10.46) 0 | 8.4 (10.25) 0 | 9.1 (10.36) 0 | 219 (77.1) 284 | 269 (70.1) 384 | 488 (73.1) 668 |
| Subscalesb | ||||||
| Agitation/aggression | 4.7 (2.48) 0 | 4.7 (2.67) 2 | 4.7 (2.58) 2 | 116 (40.8) 284 | 141 (36.7) 384 | 257 (38.5) 668 |
| Depression/dysphoria | 3.5 (2.35) 2 | 3.2 (2.03) 1 | 3.3 (2.19) 3 | 95 (33.5) 284 | 105 (27.3) 384 | 200 (29.9) 668 |
| Anxiety | 4.0 (2.45) 0 | 4.0 (2.57) 2 | 4.0 (2.51) 2 | 48 (17.0) 283 | 72 (18.8) 384 | 120 (18.0) 667 |
| Apathy/indifference | 5.5 (3.41) 0 | 4.6 (3.06) 0 | 5.0 (3.25) 0 | 95 (33.5) 284 | 108 (28.1) 384 | 203 (30.4) 668 |
| Disinhibition | 3.8 (2.70) 1 | 4.4 (3.22) 0 | 4.1 (2.99) 1 | 35 (12.3) 284 | 42 (10.9) 384 | 77 (11.5) 668 |
| Irritability/lability | 4.5 (2.44) 0 | 4.0 (2.66) 1 | 4.2 (2.58) 1 | 94 (33.1) 284 | 127 (33.1) 384 | 221 (33.1) 668 |
| Closed cohort | Control (n = 308), number prescribed (%) | Intervention (n = 418), number prescribed (%) | Total (n = 726), number prescribed (%) |
|---|---|---|---|
| Baseline | |||
| Antipsychotic | 5 (1.6) | 5 (1.2) | 10 (1.4) |
| Non-benzodiazepine hypnotic | 2 (0.6) | 4 (1.0) | 6 (0.8) |
| Pain relief | 109 (35.4) | 123 (29.4) | 232 (32.0) |
| 6 months | |||
| Antipsychotic | 4 (1.3) | 2 (0.5) | 6 (0.8) |
| Non-benzodiazepine hypnotic | 1 (0.3) | 3 (0.7) | 4 (0.6) |
| Pain relief | 89 (28.9) | 132 (31.6) | 221 (30.4) |
| 16 months | |||
| Antipsychotic | 2 (0.6) | 2 (0.5) | 4 (0.6) |
| Non-benzodiazepine hypnotic | 4 (1.3) | 3 (0.7) | 7 (1.0) |
| Pain relief | 59 (19.2) | 83 (19.9) | 142 (19.6) |
| Cross-section | Control (n = 287), number prescribed (%) number completed | Intervention (n = 388), number prescribed (%) number completed | Total (n = 675), number prescribed (%) number completed |
| 16 months | |||
| Antipsychotic | 2 (0.7) | 4 (1.0) | 6 (0.9) |
| Non-benzodiazepine hypnotic | 6 (2.1) | 3 (0.8) | 9 (1.3) |
| Pain relief | 90 (31.4) | 138 (35.6) | 228 (33.8) |
| Time point | Control, mean score (SD) n | Intervention, mean score (SD) n | Total, mean score (SD) n |
|---|---|---|---|
| Closed cohort | (N = 308) | (N = 418) | (N = 726) |
| Baseline | |||
| QUALIDa staff proxy | 20.9 (7.19) 308 | 20.1 (6.76) 418 | 20.5 (6.95) 726 |
| QUALID relative proxy | 22.5 (7.49) 82 | 21.6 (6.86) 81 | 22.0 (7.18) 163 |
| QOL-ADb resident | 42.7 (5.13) 155 | 41.7 (7.11) 189 | 42.1 (6.31) 344 |
| 6 months | |||
| QUALID staff proxy | 20.7 (6.88) 245 | 19.3 (6.04) 319 | 19.9 (6.45) 564 |
| QUALID relative proxy | 21.6 (7.18) 62 | 22.1 (8.89) 65 | 21.8 (8.07) 127 |
| QOL-AD resident | 43.0 (5.09) 92 | 41.3 (5.97) 137 | 42.0 (5.68) 229 |
| 16 months | |||
| QUALID staff proxy | 19.9 (6.38) 185 | 19.5 (6.06) 222 | 19.7 (6.20) 407 |
| QUALID relative proxy | 23.0 (6.24) 38 | 23.1 (8.41) 31 | 23.0 (7.24) 69 |
| QOL-AD resident | 43.2 (6.17) 65 | 42.8 (5.47) 81 | 43.0 (5.77) 146 |
| Cross-section | (N = 287) | (N = 388) | (N = 675) |
| 16 months | |||
| QUALID staff proxy | 19.5 (6.44) 284 | 19.5 (6.20) 384 | 19.5 (6.30) 668 |
| QUALID relative proxy | 23.0 (6.15) 39 | 23.1 (8.41) 31 | 23.0 (7.18) 70 |
| QOL-AD resident | 43.4 (5.69) 113 | 42.2 (6.61) 156 | 42.7 (6.25) 269 |
| Total interactions (% positive) missing data | Control (n = 19) | Intervention (n = 31) | Total (n = 50) |
|---|---|---|---|
| Baseline | 2065 (74.9) 0 | 2405 (81.7) 1 | 4470 (78.6) 1 |
| 6 months | 1766 (81.7) 0 | 2291 (88.6) 0 | 4057 (85.6) 0 |
| 16 months | 1578 (83.7) 0 | 2320 (83.7) 0 | 3898 (83.7) 0 |
As can be seen in Table 12, at baseline, the proportions of residents experiencing BSC (defined as the following behaviours in the NPI-NH: agitation/aggression, depression/dysphoria, anxiety, apathy/indifference, disinhibition or irritability/lability) were similar across the intervention and control arms. However, the average NPI-NH score was higher in the control than in the intervention arm. Agitation/aggression was experienced by the highest proportion of residents across all time points and in both samples. At 16 months, the proportion of residents experiencing BSC was smaller in the intervention arm than in the control arm for both the cross-sectional and the closed-cohort samples. The average NPI-NH score was similar in both arms for both samples, having reduced more in the control arm than in the intervention arm from baseline.
The percentage of residents prescribed antipsychotics on a PRN basis was low across time points, at less than 1.6% (see Table 13), making it difficult to detect any differences between the arms. QoL was primarily measured using the staff-proxy QUALID scale. Data are presented on the resident-rated QOL-AD measure and the relative-proxy QUALID scale; however, this is for comparison only, owing to the poor completion rates (see Table 14). There are no notable differences in the QUALID scores provided by staff proxies at baseline, 6 months or 16 months in either resident sample. This pattern is supported by the resident-rated QOL-AD measure and the relative-proxy QUALID scale.
The proportion of positive interactions as measured by the QUIS (see Table 15) differed between arms at baseline and at 6 months, with a higher proportion of interactions experienced in the intervention arm than in the control arm; this difference in proportions was not evident at 16 months.
All 726 residents in the closed cohort were included in analyses of the resident-level secondary outcomes at 6 months; all 49 care homes in which the QUIS was completed were included in the analysis at 6 months (Table 16). The odds ratio for the presence versus absence of one or more of the six domains of the NPI-NH describing BSC is 0.95 (95% CI 0.61 to 1.48), indicating that there was no difference in the odds of residents experiencing these domains across arms (at a population or cluster-specific level). The odds of residents being prescribed antipsychotics on a PRN basis in the intervention arm was 0.46 times the odds in the control arm. However, the 95% CI (0.09 to 2.24) was wide, which reflects uncertainty from the small number of prescriptions made. The odds of experiencing depression/dysphoria and apathy/indifference in the intervention arm were both approximately 1.32 times the odds in the control arm; however, the 95% CIs both overlapped one (0.87 to 2.0 and 0.85 to 2.07, respectively), so the differences are not statistically significant. The odds ratio for the presence or absence of anxiety was 1.01 (95% CI 0.62 to 1.66), indicating that there was no difference in the odds of residents experiencing anxiety across arms.
| Secondary outcome | Analysis | Treatment effect (intervention – control) | 95% CI | p-value | n |
|---|---|---|---|---|---|
| Resident related | |||||
| BSC | Population-average logistic model (GEE) | 0.950 | 0.612 to 1.476 | 0.820 | 726 |
| Cluster-specific logistic model (REML) | 0.951 | 0.584 to 1.547 | 0.838 | 726 | |
| Antipsychotic medication | Population-average logistic model (GEE) | 0.455 | 0.093 to 2.236 | 0.331 | 726 |
| Mood (NPI domain) | |||||
| Depression/dysphoria | Population-average logistic model (GEE) | 1.320 | 0.872 to 1.999 | 0.190 | 726 |
| Anxiety | Population-average logistic model (GEE) | 1.011 | 0.617 to 1.656 | 0.967 | 726 |
| Apathy/indifference | Population-average logistic model (GEE) | 1.330 | 0.853 to 2.073 | 0.208 | 726 |
| Quality of life | |||||
| QUALID (staff proxy) | Linear model (REML) | –0.740 | –1.910 to 0.430 | 0.214 | 726 |
| Care home related | |||||
| Quality of staff interactions | |||||
| QUIS: proportion of positive interactions | Linear regression | 0.039 | –0.023 to 0.101 | 0.210 | 49 |
The mean QUALID staff-proxy score was 0.74 points lower in the intervention arm than in the control arm (95% CI –1.91 to 0.43 points), indicating no difference in QoL between arms. Therefore, no statistically significant differences were found in the closed cohort between arms on any resident-level secondary outcome at 6 months. Similarly, there was insufficient evidence that proportions of positive staff interactions with residents, observed using the QUIS, differed by treatment arm.
All 675 residents in the cross-sectional sample were included in the primary analyses and all 726 residents in the closed cohort were included in the supportive analyses of the resident-level secondary outcomes at 16 months. All 49 care homes in which the QUIS was completed were included in its analysis at 16 months (Table 17). In the cross-sectional sample, the odds of residents experiencing one or more of the six domains of the NPI-NH describing BSC in the intervention arm were 0.72 (95% CI 0.48 to 1.08) times the odds in the control arm, indicating that, although there was no statistically significant difference in the odds of residents experiencing these domains across arms (at a population or cluster-specific level), the trend was in favour of the intervention. In the closed cohort, the odds in the intervention arm were 0.57 (95% CI 0.34 to 0.95) times the odds in the control arm, a result that is statistically significant (at a population or cluster-specific level) at the 5% level.
| Secondary outcome | Analysis | Treatment effect (intervention – control) | 95% CI | p-value | n |
|---|---|---|---|---|---|
| Cross-section: resident related | |||||
| BSC | Population-average logistic model (GEE) | 0.720 | 0.479 to 1.083 | 0.115 | 675 |
| Cluster-specific logistic model (REML) | 0.681 | 0.400 to 1.158 | 0.156 | 675 | |
| Antipsychotic medication | Population-average logistic model (GEE) | 1.191 | 0.216 to 6.559 | 0.841 | 675 |
| Mood (NPI domain) | |||||
| Depression/dysphoria | Population-average logistic model (GEE) | 0.757 | 0.511 to 1.123 | 0.167 | 675 |
| Anxiety | Population-average logistic model (GEE) | 1.133 | 0.670 to 1.916 | 0.642 | 675 |
| Apathy/indifference | Population-average logistic model (GEE) | 0.810 | 0.525 to 1.249 | 0.340 | 675 |
| Quality of life | |||||
| QUALID (staff proxy) | Linear model (REML) | –0.050 | –1.120 to 1.020 | 0.922 | 675 |
| Closed cohort: resident related | |||||
| BSC | Population-average logistic model (GEE) | 0.570 | 0.343 to 0.948 | 0.031 | 726 |
| Cluster-specific logistic model (REML) | 0.577 | 0.334 to 0.996 | 0.048 | 726 | |
| Antipsychotic medication | Population-average logistic model (GEE)a | 0.783 | 0.114 to 5.368 | 0.802 | 726 |
| Mood (NPI domain) | |||||
| Depression/dysphoria | Population-average logistic model (GEE) | 0.592 | 0.369 to 0.950 | 0.030 | 726 |
| Anxiety | Population-average logistic model (GEE) | 1.037 | 0.588 to 1.830 | 0.900 | 726 |
| Apathy/indifference | Population-average logistic model (GEE) | 0.601 | 0.380 to 0.952 | 0.030 | 726 |
| Quality of life | |||||
| QUALID (staff proxy) | Linear model (REML) | –0.070 | –1.260 to 1.110 | 0.902 | 726 |
| Closed cohort: care home related | |||||
| Quality of staff interactions | |||||
| QUIS: proportion of positive interactions | Linear regression | –0.001 | –0.081 to 0.078 | 0.972 | 49 |
In the individual domains, in the cross-sectional sample, the odds that residents experienced depression/dysphoria or apathy/indifference in the intervention arm were both around 0.76 times the odds in the control arm (95% CIs 0.51 to 1.12 and 0.53 to 1.25, respectively), but this was not statistically significant. In the closed cohort, however, the odds in the intervention were both around 0.59 times the odds in the control (95% CIs 0.37 to 0.95 and 0.38 to 0.95, respectively), which were statistically significant at the 5% level in favour of the intervention. Like at 6 months, the odds ratios for the presence or absence of anxiety were close to 1 in both the cross-sectional and the closed-cohort samples, indicating no difference across arms. Overall, although no statistically significant differences were found between arms in the primary cross-sectional sample at 16 months, trends in favour of the intervention in BSC and mood were found in the closed cohort.
On the staff proxy-completed QUALID scale, there was no difference in mean scores between arms, indicating no difference in QoL at 16 months. There was no evidence of a difference between treatment arms in the proportion of positive staff interactions with residents, observed using the QUIS.
The CIs for residents being prescribed antipsychotics on a PRN basis in the cross-sectional and closed-cohort samples were wide, making them difficult to interpret (see Tables 16 and 17).
Additional summaries of secondary outcomes can be found in Appendix 1, Tables 49–52 and 61 (unadjusted scores), Tables 26–30 (output from additional models) and Tables 58–60 (summary of medications).
Analyses of safety
There were no reported unexpected serious adverse events (RUSAE). The majority of care home residents in the closed cohort did not have any hospital admissions: 231 (75.0%) in the control arm and 308 (73.7%) in the intervention arm (Table 18). On average, hospital admissions lasted 3.7 days in the control arm and 2.9 days in the intervention arm. The majority of hospital admissions were to general wards.
| Admissions | Control (N = 308) | Intervention (N = 418) | Total (N = 726) |
|---|---|---|---|
| Number of hospital admissions per resident, n (%) | |||
| 0 | 231 (75) | 308 (73.7) | 539 (74.2) |
| 1 | 64 (20.8) | 77 (18.4) | 141 (19.4) |
| 2 | 11 (3.6) | 25 (6) | 36 (5) |
| 3 | 2 (0.6) | 7 (1.7) | 9 (1.2) |
| Number of hospital admissions per resident, mean (SD) | 0.3 (0.57) | 0.4 (0.71) | 0.3 (0.65) |
| Length of hospital admission (days), mean (SD) | 3.7 (12.33) | 2.9 (9.65) | 3.2 (10.86) |
| Overall number of hospital admissions reported, n (%) | 92 (25) | 153 (26.3) | 245 (25.8) |
| Admission ward type, n (%) | |||
| General | 77 (83.7) | 132 (86.3) | 209 (85.3) |
| Intensive care unit | 2 (2.2) | 4 (2.6) | 6 (0.2) |
| High-dependency unit | 0 (0) | 0 (0) | 0 (0) |
| Other | 9 (9.8) | 11 (7.2) | 20 (8.2) |
Deaths are reported in Resident deaths in closed cohort and in Appendix 1, Table 41.
Chapter 4 Cost-effectiveness
Missing data
Figure 4 outlines the data available for the economic evaluation and the level of MI conducted. Data from 389 (intervention, n = 214; control, n = 175) residents were available for the original cohort CCA and from 726 (intervention, n = 418; control, n = 308) residents were available for the imputed data set (and the primary analysis sample).
FIGURE 4.
Data completion rates for the complete-case sample (baseline resource use not required for CCA).

Costs
The costs of the DCM intervention and the assumptions behind this are described in Table 19. These were agreed with the research team and cover the DCM training and implementation. The total cost of the DCM intervention was estimated to be £421.07 per resident (£9290.30 per care home). Control-arm costs were assumed to be zero.
| Description of costs | Cost (£) | Key assumptions and sources |
|---|---|---|
| Training course fee | 975.00 | DCM course booking form.159 Inclusive of lunch, refreshments and course materials |
| Accommodation (4 nights) | 300.00 | Based on review of DCM EPIC trial records |
| Meals/other subsistence | 70.00 | Based on review of DCM EPIC trial records |
| Travel to/from the course | 100.00 | Based on review of DCM EPIC trial records |
| Staff time | 434.77 |
Assumed that there are four categories of care staff (hourly wage and proportion of staff in each category shown in brackets): care home worker (£7.38, 20%) and senior care home worker (£8.20, 25%) (hourly wages reported in PSSRU 2016144), nurse (£12.45, 20%) (based on £25,902 annual salary for band-5 nurse reported in PSSRU 2016144 and converted to hourly rate) and care home manager (£21.63, 35%) (assumed median annual salary of £45,000, based on a review of recent job advertisements) The proportion of staff in each category was based on a review of DCM EPIC trial records. Assumed that course participation required 4 full working days (8 hours per day) |
| Delivery and receipt of training (for each DCM mapper) | 1879.77 |
Assumed that two staff were trained in each intervention home and that there were no staff in the trial who did not require training (e.g. because they had previously received it) Assumed that there were no last minute cancellations (which may have incurred additional costs if rebooking) |
| Staff time per mapping cycle for DCM mapper | 543.46 | Using data on the cost of staff time listed above and assuming that each mapping cycle required 5 full working days (based on DCM mapper guidance document83 and some verification using DCM EPIC trial data) |
| Implementation costs (for each DCM mapper) | 1630.38 | Assumed that there were three mapping cycles per DCM mapper (conducted in accordance with DCM mapper guidance based on published standards83) and that additional time was not required for other staff to attend DCM briefing and feedback sessions, but that these were arranged at handover or at other convenient times as part of usual duties (as per protocol) |
| Consultancy fees for external DCM expert | 2100.00 | To support intervention implementation and fidelity in the first cycle of DCM mapping, assumed to be for 5 days (£420.00 per day) |
| Travel and subsistence expenses for DCM expert mapper | 170.00 | Based on review of DCM EPIC trial data |
| Implementation costs (for each DCM expert mapper) | 2270.00 | Assumed that each care home received one full cycle of DCM supported by the expert mapper |
| Total costs | ||
| Per care home | 9290.30 | Assumed that there were two DCM mappers and one external DCM expert per care home |
| Per resident | 421.07 | Assumed that there were 22.06 residents per care home (calculation based on DCM EPIC trial data) |
Table 65 in Appendix 1 includes descriptive statistics on resource use across time points and by trial arm, based on data taken from resident care plans and care home records. Owing to changes to the consent requirements to access NHS Digital data between baseline recruitment and the request for a data download at 16 months, we were unable to receive the data and thus were unable to use it to check the accuracy of the data on hospital admissions obtained from the care home records. The health-care resource costs are described in Table 20. Costs are presented in GBP (2017 prices). Total costs were £3539.00 and £2059.58, on average, per resident in the intervention and control arms, respectively. The t-tests suggest that these costs were significantly different for the imputed (p < 0.001) and complete-case (p < 0.05) samples.
| Costs (£) | Intervention (n = 418) | Control (n = 308) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard error | Minimum | Maximum | Mean | Standard error | Minimum | Maximum | |
| Intervention costs | 421.07 | N/A | N/A | N/A | 0.00 | N/A | N/A | N/A |
| Primary care costs | 1522.32 | 81.37 | 0.00 | 19,559.93 | 1568.13 | 85.58 | 0.00 | 8544.83 |
| Secondary care costs | 1547.34 | 315.41 | 0.00 | 67,346.67 | 436.96 | 99.98 | 0.00 | 14,220.38 |
| Medication costs | 46.40 | 3.64 | 0.00 | 405.38 | 53.67 | 4.76 | 0.00 | 459.25 |
| Total cost | 3539.00 | 337.00 | 421.00 | 73,944.00 | 2059.58 | 146.71 | 0.66 | 18,032.06 |
Primary care costs were similar across arms while secondary care costs were noticeably higher in the intervention arm. The intervention arm included a few high-cost individuals. There were six residents in the control arm whose costs exceeded the maximum, with long periods of hospital stays or one-to-one care; these were excluded, along with seven other high-cost individuals (generated in the imputation) in a sensitivity analysis.
Utility
Staff proxies represented the greatest proportion of completed QoL measures (n = 453; 62%). This was followed by relative/friend proxies (n = 176; 24%) and resident self-report (n = 168; 23%). Table 21 includes the utility values (with MI) for each trial arm across assessment mode and questionnaire.
| Assessment | Baseline | 6 months | 16 months | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |||||||||||||
| n | Mean | Standard error | n | Mean | Standard error | n | Mean | Standard error | n | Mean | Standard error | n | Mean | Standard error | n | Mean | Standard error | |
| EQ-5D-5La – staff MI; primary analysis | 418 | 0.663 | 0.011 | 308 | 0.676 | 0.011 | 418 | 0.573 | 0.015 | 308 | 0.569 | 0.019 | 418 | 0.421 | 0.018 | 308 | 0.395 | 0.019 |
| EQ-5D-5L – staff MI; death not recoded | 418 | 0.663 | 0.011 | 308 | 0.676 | 0.011 | 366 | 0.654 | 0.013 | 261 | 0.672 | 0.015 | 277 | 0.636 | 0.017 | 204 | 0.596 | 0.017 |
| EQ-5D-5La – staff MI mapped to 3L | 418 | 0.435 | 0.016 | 308 | 0.469 | 0.019 | 418 | 0.363 | 0.018 | 308 | 0.374 | 0.020 | 418 | 0.262 | 0.019 | 308 | 0.229 | 0.017 |
| DEMQOLa – staff MI | 418 | 0.759 | 0.006 | 308 | 0.746 | 0.007 | 418 | 0.669 | 0.013 | 308 | 0.623 | 0.016 | 418 | 0.746 | 0.018 | 308 | 0.736 | 0.021 |
| EQ-5D-5La – staff CCAb | 214 | 0.663 | 0.016 | 175 | 0.682 | 0.018 | 214 | 0.554 | 0.021 | 175 | 0.531 | 0.025 | 214 | 0.364 | 0.025 | 175 | 0.349 | 0.025 |
| EQ-5D-5La – patient/relative or staff CCA | 215 | 0.702 | 0.016 | 176 | 0.716 | 0.019 | 215 | 0.596 | 0.022 | 176 | 0.555 | 0.026 | 215 | 0.383 | 0.025 | 176 | 0.370 | 0.027 |
The primary analysis was based on the utility values reported in the top row of Table 21 (i.e. the imputed EQ-5D-5L completed by staff proxies and scored using the standard UK tariff). Other analyses presented in this study used the alternative utility values reported in other rows of Table 21. The first four rows of this table show the imputed utility scores for EQ-5D-5L (rows 1–3) and DEMQOL (row 4), whereas the final two rows report the utilities that were used in the CCA (i.e. prior to MI).
In the primary analysis, there was a slight baseline imbalance, with the control arm having marginally higher QoL. As we might anticipate, mean EQ-5D-5L scores declined during the trial over 16 months with resident longevity. There was a trend apparent in most of the approaches, namely that the decline in QoL was greater in the control arm than in the intervention arm. Using all approaches, QoL was higher in the intervention arm than in the control arm at 16 months.
The baseline imbalance in QoL was a relatively consistent finding across assessments and scoring methods. Adjustment for this was made in the calculation of QALYs.
Cost-effectiveness
The cost-effectiveness results are also published in Meads et al. 157 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Table 22 includes the ICERs for the primary and secondary analyses and for the various sensitivity analyses. In the base-case cost–utility analysis, the intervention was more costly (by £1479) and more effective (0.024 QALYs) than the control. This yielded an ICER of £60,627, well above the £20,000 NICE threshold, indicating that DCM is not cost-effective. The CCA had similar costs to the imputed sample but higher incremental QALYs for the intervention than for the control. With the exception of the analyses that excluded high-cost outliers, the ICERs from various sensitivity analyses [including those that restricted the intervention sample to intervention-compliant care homes (i.e. those completing at least one cycle)] also all exceeded £20,000. These analyses included additional costs associated with the intervention of over £1600 and an incremental benefit ranging from 0.024 to 0.036. The cross-sectional cohort analysis yielded lower incremental costs and higher incremental benefits for the intervention than those found in the imputed sample.
| Analysisa | Costs (£) | QALYs/benefits | ICER | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Intervention | n | Control | Incremental | n | Intervention | n | Control | Incremental | ||
| Base case | |||||||||||
| EQ-5D-5L – staff MI | 418 | 3539 | 308 | 2060 | 1479 | 418 | 0.718 | 308 | 0.708 | 0.024 | £60,627 |
| CMAI MI | 219 | 3318 | 185 | 2345 | 974 | 219 | –1.767 | 185 | –0.557 | –3.37 | £288.88b |
| Sensitivity analyses | |||||||||||
| EQ-5D-5L CCAb | 214 | 3380 | 175 | 2073 | 1307 | 214 | 0.682 | 175 | 0.665 | 0.029 | £45,674 |
| EQ-5D-5L – staff MI, implemented cycle costs | 418 | 3463 | 308 | 2060 | 1403 | 418 | 0.718 | 308 | 0.708 | 0.024 | £57,509 |
| EQ-5D-5L – staff MI, excluding intervention cost outliers in the imputations | 412 | 3046 | 308 | 2060 | 533 | 412 | 0.722 | 308 | 0.708 | 0.027 | £36,371 |
| EQ-5D-5L CCA excluding intervention cost outliersb | 208 | 2437 | 175 | 2073 | 364 | 208 | 0.688 | 175 | 0.665 | 0.033 | £10,975 |
| EQ-5D-5L – staff MI mapped to 3L | 418 | 3539 | 308 | 2060 | 1479 | 418 | 0.457 | 308 | 0.459 | 0.026 | £57,208 |
| DEMQOL – staff MI | 418 | 3539 | 308 | 2060 | 1479 | 418 | 0.836 | 308 | 0.799 | 0.032 | £45,918 |
| EQ-5D-5L – staff MI open cohortc | 523 | 2830 | 394 | 1608 | 1222 | 523 | 0.577 | 394 | 0.548 | 0.028 | £42,953 |
| DEMQOL – staff MI open cohortc | 523 | 2830 | 394 | 1608 | 1222 | 523 | 0.665 | 394 | 0.629 | 0.036 | £34,234 |
| EQ-5D-5L – staff MI (intervention arm; only those who completed at least two DCM cycles to an acceptable level) | 100 | 2856 | 308 | 2060 | 796 | 100 | 0.734 | 308 | 0.708 | 0.026 | £30,447 |
| EQ-5D-5L – staff MI (intervention arm; only those who completed at least one DCM cycle to an acceptable level)d | 328 | 3833 | 308 | 2060 | 1774 | 328 | 0.744 | 308 | 0.708 | 0.044 | £40,062 |
| CMAI CCA | 129 | 2768 | 101 | 2424 | 344 | 129 | –1.78 | 101 | 1.06 | –5.12 | £67.20b |
| EQ-5D-5L – staff MI, with adjustment for baseline costs | 262 | 3366 | 225 | 1924 | 1464 | 262 | 0.732 | 225 | 0.692 | 0.061 | £24,139 |
In the sensitivity analyses that excluded the high-cost outliers in the intervention arm (six were excluded from the CCA and prior to conducting MIs for an analysis using MI data), incremental costs reduced dramatically and the ICER approached the cost-effectiveness threshold (£36,371/QALY) in the base case and fell below it in the complete-case scenario (£10,975/QALY). The ICER also decreased in line with greater intervention compliance. An analysis adjusting for baseline costs yielded an ICER below £25,000, but this was based on a dramatically reduced sample and cannot be considered a robust estimate.
The cost-effectiveness analyses based on improvement in CMAI score indicate that, although the intervention was more costly, it was also more clinically effective. Incremental cost per unit improvement in CMAI score was £289 and £67 for the intervention and control arms, for the imputed and complete-case samples, respectively.
Figures 5 and 6 are the cost-effectiveness plane and the CEAC, respectively, for the base-case cost–utility analysis. The plane indicates that the greatest uncertainty lies in the benefits of the intervention. All of the simulations lie above the willingness-to-pay threshold, suggesting that, using the base-case analysis, DCM is unlikely to be cost-effective. The CEAC confirms this and indicates that, when λ = £20,000, there is a very low probability that the intervention will be cost-effective.
FIGURE 5.
Cost-effectiveness plane. Reproduced from Meads et al. 157 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The figure includes minor additions and formatting changes to the original figure.
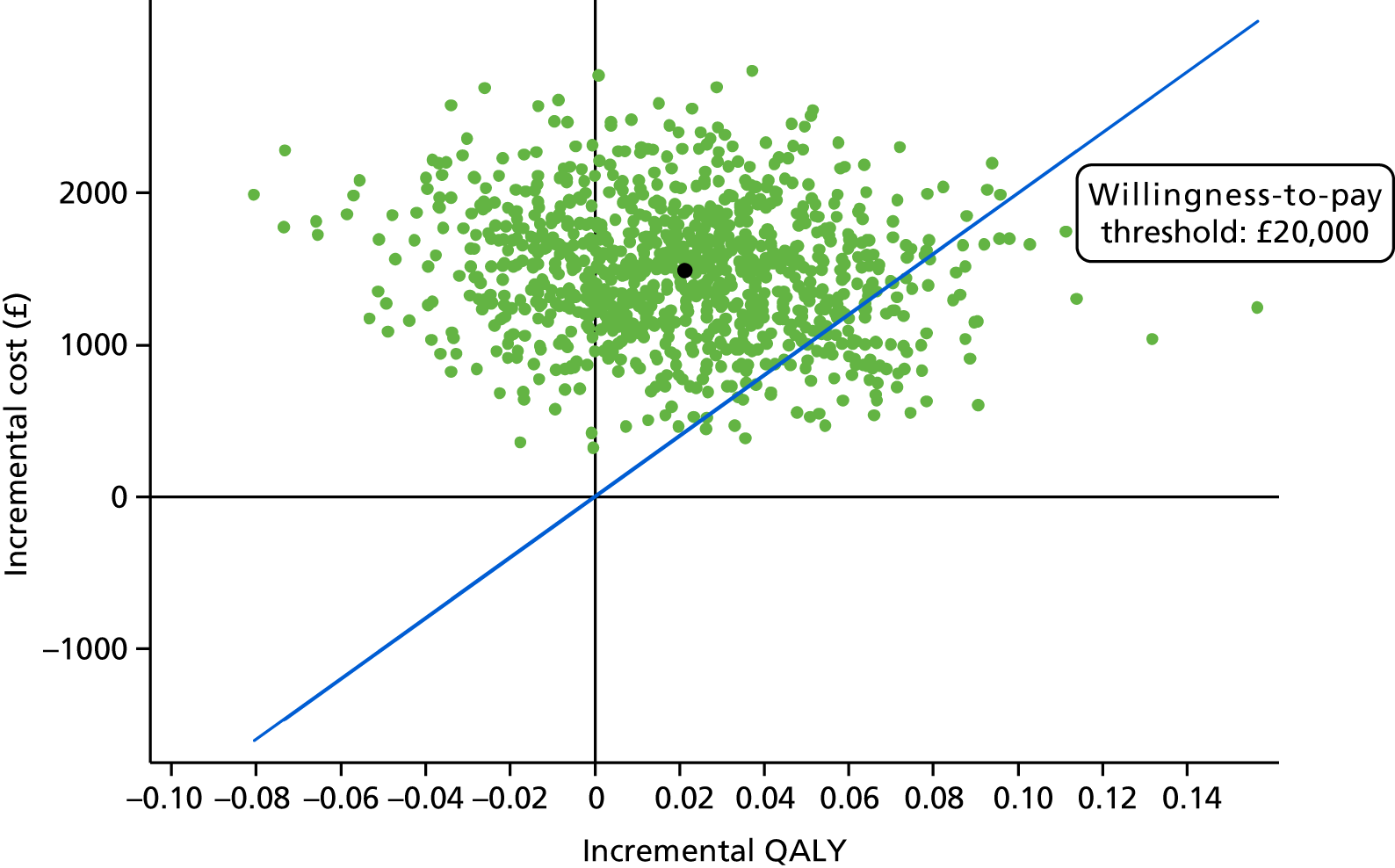
FIGURE 6.
Cost-effectiveness acceptability curve. Reproduced from Meads et al. 157 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The figure includes minor additions and formatting changes to the original figure.
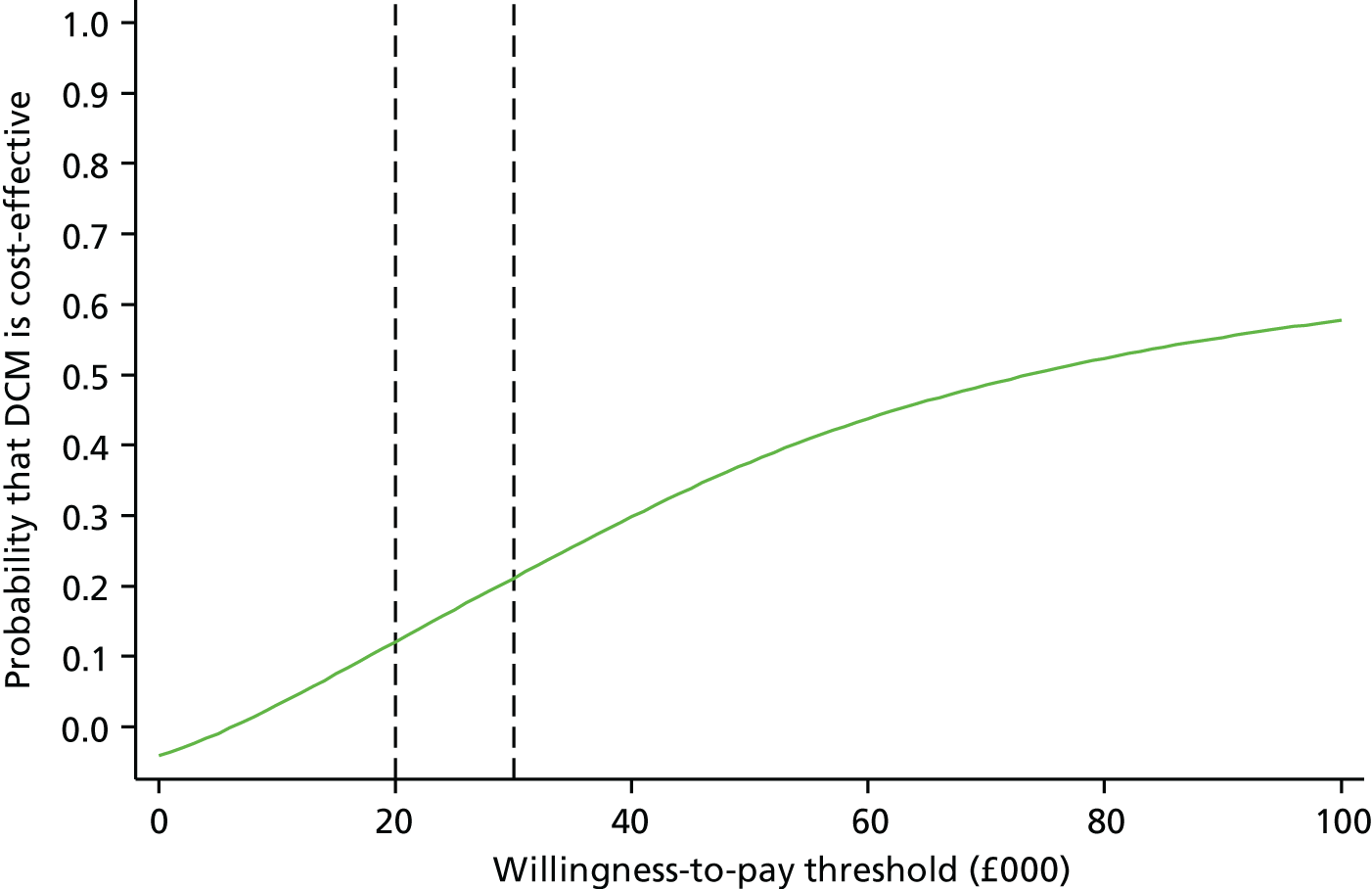
Table 23 reports the outcomes from the net-benefit regression model including the covariates employed in the main statistical model and an interaction between trial-arm and compliance indicator variables. The only significant predictors of net benefit were baseline EQ-5D-5L (a higher QoL leads to a higher net benefit) and CDR score (lower values lead to a higher net benefit). In this model, neither the intervention nor the compliance × intervention interaction terms are statistically significant. The CACE analysis yielded similar results in that the active treatment variable including only intervention care homes that complied with the intervention (having completed at least one acceptable cycle) was not statistically significant.
| Variable | Coefficient | Robust standard error | p-value | Lower confidence limit | Upper confidence limit |
|---|---|---|---|---|---|
| Treatment × compliance = 0 | –1617.88 | 840.149 | 0.061 | –3311.72 | 75.96 |
| Treatment × compliance = 1 | –1427.81 | 1159.62 | 0.225 | –3762.80 | 907.18 |
| Treatment × compliance = 2 | 177.53 | 1139.85 | 0.88 | –2117.28 | 2472.34 |
| Date of birth | 0.16 | 0.09 | 0.115 | –0.04 | 0.35 |
| Baseline EQ-5D-5L score | 14,628.21 | 1381.11 | 0.000* | 11,847.35 | 17,409.07 |
| Baseline CDR score | –807.31 | 389.57 | 0.044* | –1592.07 | –22.56 |
| Care home type | –463.22 | 835.22 | 0.582 | –2144.57 | 1218.125 |
| Care home size | 856.64 | 1032.51 | 0.411 | –1221.49 | 2934.77 |
| Care home training | 353.93 | 1107.15 | 0.751 | –1874.86 | 2582.727 |
| Care home hub = 2 | 23.62 | 1359.16 | 0.986 | –2713.0556 | 2760.31 |
| Care home hub = 3 | –1152.31 | 1215.883 | 0.348 | –3599.056 | 1294.44 |
| Constant | 5282.33 | 1726.75 | 0.004 | 1802.20 | 8762.46 |
Chapter 5 Process evaluation
Participants
In total, 75 interviews were conducted. Of these interviews, 67 were with staff members, who had undertaken various roles during the trial. Interviews took place with 17 managers, 25 mappers (two of whom were also managers) and 27 ‘other’ members of staff, who reflected a range of roles in the care home and varying degrees of involvement with the intervention. Owing to the high losses to follow-up and the requirement of having to be able to provide informed consent to participate in an interview, only two residents participated. Six relatives agreed to participate in interviews. Interviews ranged greatly in duration (from 3 to 38 minutes) depending on the interviewee’s knowledge and awareness of the intervention.
What was implemented?
Each care home was asked to implement DCM as set out in the protocol and described above (see Chapter 3, Intervention). There was considerable variation in implementation across the 31 intervention homes, as well as variable compliance with returning the required trial documentation to provide evidence of DCM implementation. A range of approaches was used to increase return rates of trial documentation, including multiple telephone and e-mail reminders from the intervention lead and CTRU staff and, in some cases, through unblinded researchers attending the care home to collect copies of documentation. In some care homes, documented evidence of all components of intervention completion (e.g. attendance sheets for briefing and feedback sessions, mapping data, feedback reports and action plans) were not always available, even though mappers or managers reported that a cycle of mapping had occurred. We made the assumption that undocumented earlier phases of a DCM cycle (e.g. briefing session) had been completed if documentation for later phases was provided (e.g. mapping data or feedback report). We also recorded a component of a cycle as complete only if we had documentary evidence for completion of it or for a later stage of the process. In some cases, mappers reported verbally to the intervention lead or CTRU staff that a DCM cycle or components of it had been completed, but failed to provide documentary evidence of this. Therefore, our final compliance data may be subject to inaccuracies of both under- and over-reporting of the components of each cycle that actually occurred.
Mapper training and retention
Mapper training was delivered per protocol (within 2 months of randomisation) in 21 out of 31 (68%) homes. There were delays in training mappers from nine care homes (29%), and in one home (3%) no mappers were trained. In two homes (6%), only one mapper was trained, rather than the stipulated two. Withdrawal of one or both of the mappers occurred in 17 homes (55%). The reasons for withdrawal were resignation from the care home, ill-health/long-term sickness and maternity leave and, in one home, both mappers withdrew because of a lack of management support to mapping. At the 16-month follow-up, 14 homes (45%) had two trained mappers still in post, seven homes had one mapper (23%) and 10 homes (32%) had no mappers. Although there was funding to train additional mappers, this occurred in only one home, owing to insufficient time before the end of the trial to train further mappers, being unable to identify a suitable replacement mapper or the consented mapper being unable to attend scheduled DCM training for personal or organisational reasons.
Mapping cycles
As is reported in Chapter 3, Resident characteristics, DCM implementation was considerably less than that required, namely three per-protocol acceptable cycles, in the majority of the 31 intervention homes, with only four homes completing three full cycles. The first cycle of mapping was commenced per protocol (within 3 months of randomisation) in 22 out of 31 homes (71%). The DCM expert mappers reported spending considerable time contacting care homes to rearrange mapping dates following cancellations by the care home and in prompting the production of feedback reports and action plans during the first supported cycle.
How did participants react to the intervention?
Experiences of the intervention
As was the case in the implementation of the intervention, experiences of the intervention and its success varied between homes and also between stakeholder groups (e.g. mappers, managers, staff, relatives and residents). The discussion about experiences of the intervention predominantly focused on the impacts of DCM and the challenging and facilitating factors experienced when implementing DCM. Experiences of the intervention are therefore explored under these two broad themes: Perceptions of intervention impact focuses on the perceptions of DCM’s impact and What were the perceived barriers to and facilitators of intervention implementation, the mechanisms of impact and the perceived impacts from the perspective of mappers, DCM expert mappers, managers, staff, residents and relatives? focuses on the barriers to and the facilitators of DCM implementation and its impact.
Perceptions of intervention impact
In keeping with the findings of the statistical analyses (see Chapter 3, Clinical effectiveness of the intervention), which identified a variability in impacts between care homes, the process evaluation identified variability in how much participants felt that DCM had an impact within their care home. In the following sections, examples of positive impacts are first considered, before moving on to consider examples of when DCM was felt to have variable or little impact.
Perceptions of impacts for people with dementia
A range of impacts of DCM were identified for people with dementia at both an individual and a home level, as indicated in the key themes below.
Improved responses to individuals’ needs, personalities and interests
A repeated positive experience was the ability of DCM, and the observational element in particular, to help staff to identify, and so respond to, residents’ individual needs, personalities and interests:
One of our gentleman that we did the observation on, we found that he made his own well-being by playing with food and chucking it. So then I could go to the chef and say . . . ‘This gentleman plays with his food, what can we do?’ We saw him doing it before but because of the mapping it makes you look into it a bit more . . . He was happier, he’d have a lot more things that he could play with.
Mapper 50028/10394
When you’re mapping somebody and you see that they’re not joining the group activities you, we thought, right, let’s just try and see if we can do an activity that’s just for her.
Manager/mapper 50069/10475
Dementia Care Mapping was repeatedly cited as enabling individualised tailoring of care and activities, which helped staff to better meet residents’ preferences, needs and interests. This ability to better identify individual needs extended to groups of residents that staff could find more difficult to care for, as discussed in the following sections.
Improved anticipation and understanding, and prevention of complex behaviours
Examples of better identification of individual needs were related to BSC, which included agitation (the primary trial outcome measure), aggression and distress:
I’m finding this really interesting because we can just observe all of the behaves of our residents, and then we can just think about this, what can we change? How can we make them more happy? . . . How can we reduce of their really low behaves, which make them distract or distressed?
Mapper 50028/10637
In terms of challenging behaviours . . . it became predictable, but then it is preventable through your interventions. The mapping itself helped us identify the individual needs and once that is identified we tried to set up plans and how to deal with or approach the challenge, the behaviour that is challenging.
Mapper 50011/10160
Participants repeatedly described how mapping helped to identify, and so to anticipate, preventable patterns of challenging behaviour by recognising antecedents, warning signs and early points of intervention. This supports the trial’s hypothesis that DCM would have an impact on agitation, although the preceding quotations suggest that reducing agitation would have been a focus only for residents who were identified by staff or mappers as being agitated, rather than a blanket intervention for all residents.
Increased quality and quantity of interactions
Alongside impacts at an individual level, staff also spoke of impacts for all residents at the care home level. The impact most frequently referred to was improvements in the quantity and quality of staff–resident and resident–resident interactions:
You know, they [staff] try and engage with people more.
Staff 50010/10095
We’ve got another lady who’s end-stage dementia who’s just by people chatting with her: she’s actually started speaking again! Now, whether that would have happened anyway I don’t know, but she’s not spoken for ever such a long time, but now odd words are coming out.
Manager 58930/40001
Increases in staff–resident interactions were repeatedly cited, as in the previous example, as having a visible impact on the person’s mood:
Sometimes even one little smile to residents, one little joke, or one interaction can make a big change, for the rest of the day even . . . It’s like our lunchtimes there is around 30 something residents plus six carers in one room, and how someone can still feel lonely, and one interaction can change that.
Mapper 50028/10637
One of our care staff, he just went to her [lady with dementia] with a bright smile and started joking and how that changed her mood! . . . She was much more brighter, she was much more involved in all the situation.
Mapper 50028/10637
These and other quotations included throughout the process-evaluation results suggest a potential link between increased interaction and activity as a result of DCM implementation, and improved well-being for residents in intervention homes.
Increased provision of activities and occupation
Alongside improvements in resident interaction, increased provision of social and therapeutic activities and meaningful occupation was another common impact of the DCM process. These activities were typically instigated in response to recognition from mapping observations that residents were spending large proportions of their time without these types of stimuli:
My activities budget is off the scale! But at least I know if I do a map now on a particular day I know that there’s going to be stuff going on, and I know that if I’m sat there I’m not going to be bored silly.
Manager/mapper 50069/10475
Now we introduce lots of sensory activities . . . all the residents have got some sort of activities . . . because we’ve been observing . . . and we’ve been thinking about what could improve their well-being.
Mapper and manager/mapper 50018/10268 and 10277
In the 2 years that we’ve been here . . . the level of stimulus, activities, has grown.
Relative 58747/40007
Dementia Care Mapping highlighted the importance of providing care that addressed not only the physical needs of residents, but also their social and emotional needs and well-being:
I have to say, that first map I was bored silly, and that made me think we are not doing anywhere near enough for these residents. Yes, we’re ticking all the boxes in terms of care, they’re well looked after, you know, everything is up to date in terms of that person, but what are we doing here to keep their well-being sort of on a good level?
Manager/mapper 50069/10475
This quotation again suggests a link between increased occupation for residents as a result of DCM and improvements in well-being.
Improved responses to the needs of particular groups of residents
Some staff perceived that DCM had a greater impact on certain subgroups of residents. Residents with more advanced dementia or with more limited verbal communication abilities were considered, by some staff, to be more likely to benefit from DCM, as it provided a useful method of identifying their unique needs:
Especially those with end-stage dementia, I think they do tend to get more attention possibly than they did before. I think staff are more considerate towards them and give them a bit more empathy . . . A lot of the residents we have that can still interact . . . they seem to be already getting quite a lot of attention . . . I think it has had more impact on the residents that weren’t getting the attention, possibly.
Mapper 59830/40002
So many of our residents have severe dementia and, you know, their comprehension is very limited so [mapper X] helped us in there to make changes.
Manager/mapper 50069/10475
Residents who were included in DCM mapping were another group that staff considered as gaining particular benefit from DCM participation, as their involvement in mapping provided a focus on identifying their care needs.
Other impacts for people with dementia
Other impacts for people with dementia that were reported by staff included giving people with dementia a voice, enhancements to the environment and improvements to the equipment to better meet residents’ needs at an individual or a care home level:
As we observe them [people with dementia], it gives them a lot of chance and opportunity to express themselves.
Mapper 50011/10160
One lady, she couldn’t lift up the cup and we decided to change from a plastic beaker to two handles, which has helped her a lot.
Mapper 58747/10447
We changed many things, even changed the place where they sit. We try to make them comfortable; those who are watching TV switch off the radio when the TV’s on, because the first time [first mapping] it was kind of noisy. So we try to make it better.
Mapper 58747/10447
These examples collectively illustrate how DCM gave staff the ability to understand experiences within the care home from the perspective of residents with dementia, and so to identify how those experiences might be improved. The ability of DCM to uncover the ‘emic’ perspective of residents is explored further in Perceptions of impacts for staff.
Summary
As the previous examples show, the impacts of DCM for people with dementia reported by staff at both an individual and a care home level indicate that DCM could lead to an increase in staff–resident and resident–resident interactions, an increase in meaningful resident occupation and an improvement in staff identification of individual residents’ needs. Some staff reported that impacts were more likely for particular groups of residents, namely those with more advanced dementia or communication difficulties, or residents who underwent DCM mapping.
Perceptions of impacts for staff
The perceived impacts of DCM for managers, mappers and other care home staff included increased awareness of residents’ needs, of communication of these needs and of care quality, as well as greater confidence among staff in caring for residents with dementia.
Improved awareness and understanding of residents’ needs and care quality
Impacts on staff predominantly related to an improved understanding of the residents under their care and, as a result, improved awareness of the quality of care being provided in the home:
You don’t realise what you’re doing sometimes and it makes you look at things to say I wouldn’t like that type of thing.
Staff 58930/40005
I think the benefits were just along the lines of highlighting to staff a little bit more about the needs of dementia clients.
Manager 50013/60005
Numerous references were made to DCM helping staff to better understand and respond to the needs and behaviours of people with dementia, indicating that this was a key impact for staff. DCM also allowed staff to see the perspectives and experiences of residents with dementia and provided a powerful reminder of the importance of understanding these:
Sometimes you just forget about, you know, the actual person. And to sit in that lounge and that dining room for 6 hours, you go through what they go through every day. If that isn’t the message of Dementia Care Mapping, I don’t know what is.
Manager/mapper 50069/10475
We were looking at it from the residents’ point of view, so we could see what they like, what they didn’t like.
Mapper 50031/10456
Comments from other staff members echoed these suggestions that, prior to DCM implementation, staff were less cognisant of residents’ experiences of care in the home.
Improved understanding of embodied communication
An improved understanding of embodied communication was a repeatedly cited impact. Staff, managers and mappers all referred to an increased awareness of, and response to, non-verbal cues and communication from residents with dementia as a result of DCM:
We’re more attuned to looking for non-verbal cues and very small changes.
Manager 58930/40001
It’s like offering somebody a drink and then, when you are observing it, actually they’re wanting to do it for themselves. So it’s about watching that hand movement isn’t it, and making carers aware.
Mapper 50019/10181
. . . by holding hands or by touch, there is, you can see the difference. The person will be quiet, or they needed that attention.
Mapper and manager/mapper 50018/10268 and 10277
Staff recognition of non-verbal cues of residents’ needs was important, as they helped the staff to identify the resident’s unique personality, abilities, preferences and requirements. Improving embodied communication was therefore particularly important for residents who rarely or never communicated verbally.
Increased confidence and positive feedback for staff
Staff were often reported to feel more confident in their care practices as a result of DCM taking place in the home:
They’re more confident now than they were.
Manager 50019/10195
Care assistants are now confident about doing things with the residents in there . . . I think they’re enjoying their jobs more, I think they’re enjoying being in that unit more.
Manager 50167/10711
Increases in confidence appeared to stem from several sources. These included feedback from the DCM process about the needs of residents, examples of the positive impact on residents when their needs were well met and DCM providing an opportunity to celebrate the sometimes overlooked positive actions of staff:
Sometimes even though you’re seeing the staff are doing very good things to the residents, sometimes you don’t appreciate . . . you don’t get the time to do that . . . but this was the time that we could be able to appreciate the staff.
Mapper and manager/mapper 50018/10268 and 10277
Increases in staff confidence and knowledge could also result from having staff who have been trained as mappers available in the home, as they are a perceived source of expertise and support in relation to problems and approaches to caring for residents with dementia:
After talking with the mappers it presents a greater awareness of what you need to do with and for your clients.
Staff 50010/40010
She’s [mapper] got that extra knowledge that she’ll go, well, it could be this, or it could be that.
Staff 10666/40015
The potential increases in staff confidence and knowledge that could arise from having access to the expertise of a mapper within the home suggests that some impact may have been possible in intervention homes that did not actually implement any DCM cycles. However, this is not borne out in the main trial results.
Summary
The most commonly cited impacts of DCM on staff were an increased awareness and understanding of the needs of residents with dementia, including the embodied communication of residents with limited verbal communication, and increased confidence among staff in providing care for people with dementia.
Changes in care practices and culture
Related to impacts on staff were wider changes in the practice and culture of care across the home. The magnitude of the changes referred to could vary greatly, from small changes in staff behaviours to more significant changes that could require managerial or financial support.
Smaller, achievable changes to care practices and culture
Relatively small, and therefore achievable, changes in staff behaviour were often considered by participants to make a big difference, despite the relatively little time, cost or effort that they took to implement:
Just tiny little things, for instance, when a staff member walked through the foyer . . . and acknowledged the residents, their faces lit up. That split second, and even a smile, it made a lot of difference to the residents.
Mapper 50031/10456
Now if I’m dealing with anybody, I have a conversation while I’m washing and dressing them. And that way I’m finding out little bits about them . . . about their likes and dislikes, what they used to do in their past life.
Staff 50018/60002
Examples of these small, achievable changes often involved staff making better use of the opportunities available to them to interact with residents, for example as they undertook care tasks or were passing through the home. Despite these examples signifying relatively small changes to practice, they were felt by staff, especially if collectively adopted, to have a significant impact on residents:
It doesn’t have to be a major functional change of the home, these [changes spoken of] are all really, really small things but, holistically and collectively, they make a massive difference.
Manager 50011/10611
The little things can make a big difference for someone who is just, who is not involved in the situation, even in the big group where they are sitting.
Mapper 50028/10637
The significance attached to such changes was also an example of the low levels of baseline interaction seen in some homes during the QUIS observations, in which residents could spend long periods of time with no one to interact with.
Larger, formal changes to care practices and culture
Larger changes to more formal care practices and processes were also reported, such as changes to staff inductions, ‘in-house’ training and care planning approaches. These changes required more effort to implement and could necessitate support at a managerial level or agreement across multiple care homes:
We have made it into a holistic type of care planning, wherein again we have brought in person-centred care.
Mapper 50011/10160
The main difference that it’s had so far is altering our training, ‘X’ does our dementia training across both homes, and we also do dignity training between both homes. And we’ve changed those courses quite a lot, so that it delivers a lot more of the language we learnt across Dementia Care Mapping.
Mapper and manager/mapper 50018/10268 and 10277
Changes to care home culture requiring managerial support were also spoken about, for example in relation to changing the previously held assumptions at a home level that talking to residents did not constitute ‘proper work’:
There’s this culture shift where it’s OK to sit down and have a chat with them [residents], it’s OK to be seen to do that . . . If she [care home owner] saw a carer sitting down [before DCM] it would be like ‘What the hell are you doing? You’re being lazy!’. And actually there’s a massive shift now, if you walk in and see a carer sitting and joking around with residents, that’s a really good responsive service.
Manager 50011/10611
This final example indicates the importance of having senior management that understands and supports the need to change care practices and culture in the home.
A tool for identifying and evaluating changes to care practices and culture
Many of the preceding responses indicate that DCM was used as a tool to identify areas for improvement across a range of care home practices and processes, including training needs, the quantity and quality of interactions with residents and care planning. Managers were particularly aware of the potential benefits of DCM as a structured tool for identifying and providing evidence for practice improvements:
Whereas before we would try to improve but we didn’t really know how, so it was a bit like running around headless . . . I think one of the most positive things about mapping is that it gives you a structure to sort of put dementia and dementia care in . . . So before [DCM], you sort of, you want to improve but it’s very difficult to know how to improve.
Mapper and manager/mapper 50018/10268 and 10277
From the cycles that the girls have done, they’ve identified and can share information with the rest of the employees, to actually improve in any way we can the care that’s delivered.
Manager 50031/11187
Dementia Care Mapping was also used as a means of providing evidence of the impact of improvements to care practices, and thus provided a means of both motivating and maintaining changes to the practice and culture of care:
Until you’ve sat there for a few hours and actually seen someone gain enjoyment from just holding something, it’s something that’s very easy to ignore because it’s very small. So it [DCM] meant we could actually start making small changes, people could see the difference.
Mapper and manager/mapper 50018/10268 and 10277
I’ve always said it’s [sitting and chatting with residents] a legitimate activity, but it is, now it’s been pointed out to them that it actually does have an impact on that person’s health and well-being, then, you know, it’s done more.
Manager 58930/40001
Having managers who were also mappers facilitated the process of getting senior staff to understand, and to provide financial support for, any changes required to care practices in the home. In some instances, significant changes were made to care practices as a result of DCM implementation:
That whole unit is light years away from before it was, before you started doing the mapping, before we started the project.
Manager 50167/10711
Summary
The DCM intervention enabled care homes to achieve change in some of the daily care practices of their staff, most noticeably in relation to the level of interactions with residents. Changes were also noted to formal care practices such as approaches to care planning and staff induction and training. DCM was perceived to be a useful tool through which the need for these changes was identified, with managerial or across-site support required for changes to be made at a care home level.
Impacts on relatives
Some impacts from DCM were noted for relatives of residents in the home. The most common impacts cited for relatives were an increased involvement in the home and better provision of information by staff to relatives about their family member’s care:
It has involved not just the home staff; it has involved families.
Manager 50010/10755
I found it really interesting for the residents that we mapped to let their families know what we’d noticed . . . This is what we found when we were doing [DCM], and this is what we’re going to do.
Manager/mapper 50069/10475
There is a suggestion here that impacts may be greater for residents who were mapped, and also for their relatives. It should, however, be noted that these perceived impacts were reported by staff and not by relatives who, as discussed previously in Impacts on relatives, could struggle to identify the impacts of DCM on themselves:
I don’t know, it’s really hard to say . . . Overall I’m really happy so I can’t say there’s been anything specific that I’ve noticed that’s any different.
Relative 50010/40009
Examples of limited impacts
Although many participants identified positive impacts resulting from the implementation of DCM within their home, they sometimes struggled to provide examples or identify specific ways in which change had occurred:
I do think there is an impact there generally yes.
Manager 50010/10755
When the interviewer asked ‘Can you give us any examples of specific action plans that came from the first cycle, which was a while ago?’, manager 50011/10611 responded ‘It was ages ago, erm . . . I can’t specifically’.
In addition, some participants considered that DCM had asserted little influence over care practices in the home or over the experiences of residents. When the interviewer asked ‘Has there been any impact, there might not have been, on the residents do you think as the result of mapping?’, mapper 50010/10096 responded ‘No. No I don’t think so’.
In addition, when the interviewer asked ‘Has there been anything for staff; have they changed kind of their routines at all?’, manager/mapper 50069/10475 responded ‘Erm, not as a whole, no’.
It might be expected that staff from homes who implemented fewer cycles of DCM would struggle to identify impacts as a result. This tendency is supported to a degree by the previous quotations, all of which came from homes that experienced problems with DCM implementation and completed only one or two cycles of DCM as a result. However, some participants from homes completing three cycles still struggled to identify definitive impacts from the implementation of DCM:
I must admit I have seen some improvement but I’m not here every day.
Mapper 58930/40002
When the interviewer followed up with ‘Have you seen improvement for the residents’ quality of life as well, do you think?’, this participant responded ‘I think so, yeah. Especially those with end-stage dementia, I think they do tend to get more attention possibly than they did before’.
I suppose I haven’t necessarily seen any real changes, but I was happy in the first place.
Relative 50018/20107
Collectively, and in line with the main trial results, these quotations suggest that implementing DCM did not uniformly lead to positive impacts for care home residents or for staff. Issues were also experienced with unexpected, and sometimes negative, impacts and with maintaining positive impacts over time, as is explored next.
Unexpected impacts or consequences
A small number of potentially unexpected, and sometimes negative, impacts or inappropriate uses of DCM were identified during the interviews.
Conflict among staff
Disagreements over the findings of mapping sessions were reported to lead to conflict among staff in one home, although these differences were subsequently resolved:
There was arguments as well, because you know they say sometimes that you don’t see the residents and how they are being . . . and we cleared everything and they did take it in a positive way eventually!
Mapper and manager/mapper 50018/10268 and 10277
Although this was the only reference made to arguments, some other homes also reported initially negative responses from staff to DCM feedback, highlighting the importance of ensuring that staff understand the DCM process and the importance of providing feedback that celebrates positive examples of care as well as highlighting areas for care improvement.
Fear of scrutiny from past negative experiences
As a result of a home beginning to use DCM, some staff felt scrutinised and fearful, predominantly because of past negative experiences of other forms of care scrutiny, such as CQC inspections:
In most cases when it [feedback on care] happens it’s a negative experience because there’s inspectors from various organisations, so I think it wasn’t until we started giving feedback and there was quite a bit of positives in there that the staff really engaged with the process.
Mapper and manager/mapper 50018/10268 and 10277
The staff . . . it didn’t matter how much time we spent explaining that it wasn’t about spying on them, that’s how they felt about it.
Mapper 50019/10181
It was like being spied on.
Staff 50010/40010
These feelings appeared to be more common in staff members who did not fully understand the purposes or processes of DCM. Such feelings typically, but not always, lessened or went away as the processes involved in implementing DCM became more familiar to staff and better understood.
Inappropriate use of Dementia Care Mapping
Some misunderstandings about the purpose of DCM also appeared to lead to it being used in ways that appeared to be inappropriate or not in line with its recommended use. One home reported using evidence from DCM to assess potential new members of staff and as part of a fee review to provide evidence that a resident’s needs had changed and their fee should be increased:
We ask all new members of staff to come in for training where we dementia map them . . . We also use it [DCM] for fee review, so if we have someone whose needs have really drastically increased I can go to them and say she needs X amount of day care hours a week and there is the evidence.
Manager 50011/10611
In addition, in another home, DCM was perceived as a method for staff to highlight errors in each other’s care practices:
The idea is that if one carer’s working with another they can turn to them and say you shouldn’t have done that you should do this.
Mapper and manager/mapper 50018/10268 and 10277
It is potentially relevant that both these examples came from managers who had not been trained in DCM and appeared, from the content of their interviews, to not fully appreciate the purpose of DCM. This reconfirms the importance of ensuring that care home staff who hold key leadership roles, such as managers, have a clear understanding of DCM for it to be implemented appropriately and effectively.
Summary
In summary, some unexpected and negative consequences of DCM implementation were also identified, including conflict between staff over the results of mapping sessions, fear of being scrutinised and inappropriate uses of DCM. These undesirable consequences were noted more frequently among homes and staff (and particularly managers) for which DCM was poorly understood. In addition, the impacts of DCM were not always easy to identify nor uniformly positive. Some participants struggled to identify any impacts as a result of DCM implementation or to definitively attribute any impacts they did identify to DCM implementation. Participants who could not identify or attribute impacts were often, but not always, from homes that had struggled or failed to implement the trial’s recommended dose of DCM.
What contextual factors shaped if and how the intervention was implemented or worked?
What were the perceived barriers to and facilitators of intervention implementation, the mechanisms of impact and the perceived impacts from the perspective of mappers, expert mappers, managers, staff, residents and relatives?
The data indicated that implementing DCM in care homes is complex and that there are many factors that may facilitate or prevent successful implementation. Barriers and facilitators were identified by managers, mappers, expert mappers and staff members and related to three main themes: care home-level barriers and facilitators, intervention barriers and facilitators, and trial barriers and facilitators. 160
Care home-level barriers and facilitators
Care home context
Contextual features of care homes affected the degree to which DCM was implemented within each care home. This included broad issues, such as the type of setting and staffing levels or losses, and more specific issues, such as the availability of computers in the home and funds to support implementation.
The type of care home may have influenced implementation, with additional complications present in nursing homes. However, the value of DCM in more complex settings was acknowledged:
I think it’s just the workload, really. The amount of work there is sometimes, and with it being a nursing home – the intensity of the workload. Obviously, we have a lot of very poorly people sometimes.
Mapper 58930/40002
Managers of residential homes felt that they were disadvantaged by a lack of qualified staff members with the expertise to help to facilitate implementation:
Because we are only a residential home, erm, y’know, we haven’t got nurses and stuff so my staff aren’t that confident anyway . . . I’m glad we got involved because we got a lot out of it, I’m just disappointed that we weren’t able to continue.
Manager 10666/10722
Larger care homes that were well staffed were able to build time for DCM into their rotas, whereas smaller care homes with fewer staff members could struggle to accommodate the cover required to facilitate DCM:
That’s the reason we pulled out, because they [mappers] couldn’t carry on doing their deputy manager role, or senior care role, and be a mapper with the amount of reports . . . So I think it’s just a bit unrealistic.
Manager 50011/10611
Across all care home settings, high levels of staff turnover were an issue. A level of consistency in staff involvement is needed for understanding the change over time for residents and also to implement changes as a result of DCM:
Care homes are really, really busy. Turnover of staff in care homes can be quite dramatic at times, and the realities are there’s other pressures on them isn’t there. But that’s, that’s it though isn’t it. That’s the reality of anywhere though I suppose.
DCM expert 70005
Particularly important in relation to the staffing of care homes was the turnover of mappers. Not only did this lead to delays in implementing DCM while additional mappers were recruited and trained, but this also had an impact on the confidence of the remaining mappers, leaving some feeling overwhelmed by what was required of them:
I think where I struggled and like with the report and things was because I was the only mapper, they were like ‘I need you to map three people’. And it was like ‘ahh . . . my first map’. And I’m mapping three people whereas if there was somebody with me, then we could’ve both done that together.
Mapper 50028/10394
Care homes with limited access to computers experienced difficulties in completing the computer-based elements of DCM:
We’re not always the most IT literate in care homes. Having access to computers and time to analyse can be quite difficult.
Mapper and manager/mapper 50018/10268 and 10277
Some care homes also had high workloads or competing priorities at the time, such as CQC reports or problems with staffing levels:
It was mainly the home, the crisis that the home was in . . . Knowing the staff we had at the time and the difficulties we had . . . I struggled just to get them to do the health and safety training, the basics.
Manager/mapper 50009/10104
These findings suggest that DCM implementation may be easier in larger nursing or dementia-specific care homes with greater numbers of qualified staff, where there may be greater access to computers and to funds and larger staffing pools to provide cover for mappers to undertake DCM.
Manager
The care home manager was a key individual in the success of DCM. Although managers were not always involved in the implementation of DCM, as they generally were responsible for rotas, allocating staff workloads and supervising the mappers, their engagement either ensured that it ran efficiently or created barriers for the mappers:
I think management support, you know, it can either be amazing when it’s amazing or it can be a real difficulty if the manager isn’t supportive.
DCM expert 70006
Generally, there was thought to be a lack of support from managers. Managers needed to have awareness of the time required for their mappers to be involved and willing to support this process:
The managers delegated all aspects – all of it – to the mappers, and didn’t take any responsibility for ensuring the process. I think the odd manager was supportive, again from the office, but not really understanding about making time.
DCM expert 70004
When there were difficulties in the relationship between managers and mappers, issues arose for mappers, particularly at the feedback stage. The hierarchical nature of care homes sometimes acted as a barrier in the process, meaning that mappers were unwilling or felt unable to challenge the care home manager:
It’s mainly from a confidence perspective, [mappers] were clearly not confident to challenge a manager who was not supporting.
DCM expert 70003
Conversely, when managers were engaged with DCM, this facilitated the process and helped mappers to make changes based on what was observed during the cycles. Furthermore, when managers valued DCM, they could see clear benefits from implementing it. For example, one manager believed that it was a key tool in helping to improve the CQC rating of the care home:
They were very clear that they thought DCM was fantastic, because they saw it as a way of improving the quality of their care to take their home CQC rating from good to outstanding.
DCM expert 70004
Managers referred to the adaptations required to make DCM fit into their home. This included suggested or actual adaptations to the process of DCM itself, such as shorter maps, and hypothetical or actual adaptations to the work of staff, such as changes to rotas and over time:
We’re going to be having to change shifts so they can be on shift at the same time every month because we can do some mapping.
Manager 50010/10755
These findings suggest that managers are key in the implementation of DCM and can act as either a barrier or a facilitator. A good relationship between the manager and the mappers is crucial to successful implementation.
Motivation and enthusiasm for Dementia Care Mapping
Motivation and enthusiasm were key factors when implementing DCM. Expert mappers emphasised that, when managers and staff teams were motivated to be involved in the DCM process, mappers were more likely to implement DCM within the home:
The manager would come in and, you know, be really enthusiastic. They came to the briefing; everybody was at the briefing, the whole home, the manager of the home, do you know what I mean. The company really bought, really bought in to DCM. And the two girls, the two mappers were just really enthusiastic about it . . . and really, really tried their hardest.
DCM expert 70005
To capitalise on this motivation and confidence, some care homes undertook the first cycle of DCM soon after the training session, and this appeared to have benefits, with greater difficulties experienced if mapping was undertaken or attempted a while after attendance at the training session:
They went for that training down in London then there was a gap and I kind of think if they had just gone straight in and done the mapping, they might have done it. But I feel that when a few weeks passed, they were struggling to say how we do this . . . maybe they didn’t have the confidence, you know what, to roll it out.
Manager 10666/10722
The motivation of mappers was sometimes overshadowed by the time constraints within care homes, meaning that the mappers were able to complete the mapping hours, but often struggled to find the time to discuss what had been observed:
When I was actually there we had lots of, you know, creative really, very inspiring conversations about care practice. But it’s trying to nab them, it’s almost like it’s impossible to nab, sit the person down and really discuss what’s going on.
DCM expert 70002
In summary, having motivation and enthusiasm for making changes to practice was key in the success of DCM. However, the challenges faced, such as time constraints, sometimes overshadowed the motivation of individuals.
Staff engagement
As DCM is a home-level intervention, effective engagement with care home staff influenced the extent to which DCM was implemented. Particularly important was having staff who were open to feedback based on the observations and were willing to contribute towards formulating action plans. In some care homes, the mappers were able to engage a large proportion of staff in feedback sessions, which was seen as positive by the DCM experts. Mappers who were in more senior roles may have found it easier to encourage staff to attend feedback sessions, owing to their status within the home:
I was so impressed how they just gathered people up, at busy times as well. And they really saw the worth of that, and great discussion. I was, and that was the first home, so I thought wow this really works.
DCM expert 70002
To implement a care home-level intervention, the involvement of all staff roles was crucial. The importance of staff members in a range of roles attending feedback sessions was highlighted:
There was a really big crowd actually, and it did include lots of different disciplines of staff, including the painter and decorator and maintenance man, which was great.
DCM expert 70002
The degree of engagement of the wider care home staff with DCM influenced the implementation of DCM. High levels of engagement with staff led to more of a ‘whole home’ approach to considering DCM feedback and agreeing on action plans:
You really have to get quite a few people across the organisation thinking in the same way to sort of drive that change.
Mapper and manager/mapper 50018/10268 and 10277
Staff engagement was achieved through multiple strategies. These included providing feedback in staff meetings to ensure good coverage; a focus on ensuring that staff understood DCM, its purpose and the outputs of mapping; and a focus on providing positive, as well as negative, feedback. The latter strategy, in particular, helped to ensure that staff were engaged and that the benefits of DCM were demonstrated:
We sort of ended up picking two or three very small examples of people who were very happy or very sad and just focusing on those, describing in laymen’s terms . . . They did take it in a positive way because they’d been, initially we said it’s for all our residents’ well-being.
Mapper and manager/mapper 50018/10268 and 10277
The selection of mappers influenced how engaged the staff team were. When mappers were not seen to be popular staff members or people to be respected, it was difficult for them to engage with the staff team to implement change:
The second time around we held a meeting and nobody came . . . We did try like, you know, individual [meetings], a few minutes at a time, but I don’t think they took it seriously enough, do you know what I mean?
Mapper 50010/10096
However, when mappers were respected, engagement was facilitated by the fact that implementation and feedback were peer led, as opposed to being conducted by an external person:
It’s people that you know and peer-led, it’s, you know, it’s not like somebody from outside coming and talking with them, it engages the staff.
Manager 58930/40001
In one home, there was a division in the work environment between staff who did and staff who did not support the mapper, which made feedback sessions particularly difficult. This led to further difficulties in implementing DCM, as staff were not willing to make changes to practice. This may have been reflective of the culture in the care home, highlighting underlying issues that existed prior to involvement in the trial:
I would say in that home there’s two very definite groups of staff: the ones who want to see progress, who would support the mapper, who would want to encourage her and make it work, and there was also a very strong group of people who say, you know, ‘what does she think she’s telling us’.
DCM expert 70001
Negative attitudes towards DCM from both staff and managers also acted as a barrier to engagement with DCM. If DCM was not perceived to be a priority, staff often did not take the time to learn about and understand the process:
I felt that the ways that people had been working prior to that, the culture of the place, whilst there was a lot about it which I would really commend it for, there were definitely some things that needed to be looked at. And I felt that there was a reluctance to look at that. And there was quite a lot of defensive response.
DCM expert 70001
Some staff questioned the validity of DCM when the presentation of residents was changeable or they considered that DCM did not suit the residents they provided care for:
. . . some of our residents are quite, quite poorly so it doesn’t work for them, it just depends how well they are.
Staff 58747/40008
However, gathering together to collectively reflect on DCM feedback sometimes made staff feel a part of the process and helped to break down potential barriers and mistrust, for example in relation to being observed and receiving feedback, of which staff may have had past negative experiences:
In most cases when it happens, it’s a negative experience because there’s inspectors from various organisations, so I think it wasn’t until we started giving feedback and there was quite a bit of positives in there that the staff really got engaged with the process.
Mapper and manager/mapper 50018/10268 and 10277
In summary, staff engagement was crucial to the implementation of DCM. Without the support of the staff team, mappers struggled to make practice changes. The mappers needed to be respected by the staff team for DCM to have any influence in the care home. The importance of receiving feedback from peers rather than from external individuals was highlighted.
Mapper qualities
The choice of mappers, including whether or not they had the required qualities, was a key indicator of implementation success. The qualities valued in mappers, namely those qualities considered by managers to facilitate DCM implementation, included confidence in undertaking the mapping and feedback sessions, leadership skills to motivate and influence action-planning in the home, pragmatism, dedication, an interest in and an enthusiasm for DCM and improving the care of people with dementia, and a keenness to learn. Managers were asked to select mappers; this was done based on the skills required to become a mapper but also based on the staff members that were available to choose from in each home, who were deemed likely to remain working at the home for the duration of the trial:
Two team leaders stuck out as being really passionate about people living with dementia.
Manager 50019/10611
Attending the training and implementing DCM improved the confidence of some mappers:
I never thought I’d be able to do it, but when we got back here, and after the training we actually put it into practice . . . It all made sense.
Mapper 50031/10456
Mappers having the motivation to improve the quality of care for people with dementia helped facilitate the implementation of DCM. However, for one mapper, the challenges of implementing DCM over-ruled her motivation and she became disengaged with the process:
I think it impacted on how they felt about it. It became a chore and one lady I can think of in particular was very excited and motivated about it, and became less so because of the challenges. And that’s really sad to see. Someone who had that real passion to just go ‘do you know, it’s just too hard’, but initially is like ‘I’m happy to come in on my day off because I think it’s marvellous’, but when you’re not then getting that support it, you know, wears you out really. Wears you down.
DCM expert 70003
Certain skills and abilities were also perceived as being central to enabling mappers to undertake the various processes involved in implementing DCM. These skills and abilities included computer literacy, writing high-quality reports, fluency in English and sufficient academic ability to undertake the more complex components. Conversely, mappers who did not possess some of the aforementioned qualities or skills, despite the trial processes used to identify and recruit mappers with the required skills, could struggle to implement DCM. In particular, a lack of IT skills, low confidence levels and insufficient fluency in English were cited as barriers to DCM implementation:
For me it was quite difficult because English is not my native language.
Mapper 58930/40002
The selection of mappers in senior roles was perceived to have both positive and negative impacts on DCM implementation. Although senior staff could possess academic, writing and leadership skills that facilitated DCM implementation, it was more difficult to free up staff in these roles to undertake mapping and they could be subject to multiple competing demands on their time, which challenged their ability to implement DCM:
[I chose] two quite strong team leaders that I knew would be able to get staff on their side and would be able to manage the feedback, because they can be quite difficult sometimes.
Manager 50019/10611
I was disappointed that my staff couldn’t continue with the mapping, but I think I made the error in the staff I chose . . . their level of responsibility in the home was too high, so it didn’t enable them to have enough time.
Manager 50167/10711
Although the qualities and skills mentioned previously were identified as important, in reality it could be difficult for managers to identify staff members who possessed many or all of the skills required to implement DCM in a care home context:
If I look at the whole team there are few other people who would have been possible, academically capable of completing that project. And that’s a difficulty.
Manager 50167/10711
An important component of mapper choice was commitment from the potential mapper. Agreement was not, however, always forthcoming, given the length of the DCM course and the often distant geographical locations in which the courses were held. These were logistical issues that were especially problematic for staff with caring or other commitments:
We need someone who would agree to do it, and promise that when they come back they’re going to get the job done.
Manager 50021/11082
Furthermore, although managers recognised the qualities that were important for mappers to possess, in reality the choice of mapper often came down to who was willing and available to undertake the 4-day course, particularly if this would involve staying in another area:
When we found out they would have to do 4 days’ training in London, [mapper initially chosen] wasn’t able to do that. And because we found out almost at the last minute, we just had to grab somebody else that was free really.
Manager 10666/10722
A further example of availability being prioritised above ability was seen in another care home in which, following a mapper leaving, the manager did not pick a staff member to attend DCM training based on their abilities. Instead, the new mapper was selected based on their availability to attend the course:
I think when in one case where a manager . . . didn’t have a clue about who to nominate, she was just, she was looking at the off-duty and sort of picking names off the off-duty.
DCM expert 70005
Mappers who were less qualified or experienced found it harder to implement DCM. The DCM process asked mappers who were care assistants to develop and utilise skills that they were not familiar with using. Having the skills to ask questions as part of feedback sessions, which allowed staff members to give opinions rather than just yes or no responses, was particularly challenging for some mappers:
It was about time, it was about access, it was about computer literacy. And the, for some of the care workers, writing anything was a real challenge. You know, they’re just not, not used to putting descriptions down, let alone sort of feedback-type questions to ask.
DCM expert 70002
There were many conflicting priorities placed on mappers, particularly if they were staff with additional responsibilities, such as completing the medication rounds or conducting assessments for potential new residents. This had an impact on the time available to complete the stages of DCM:
Well it was all just such a squeeze in the day, you know, and I remember being at one home where one of the mappers was late, one of the other mappers was busy doing the drugs, you know, and that was quite a familiar scenario.
DCM expert 70004
In summary, the selection of mappers had a significant impact on the delivery of DCM as an intervention. Recruiting mappers with the appropriate skills facilitates the delivery of DCM, as difficulties with stages of the mapping process, such as analysis and report writing, can result in much more time than anticipated needing to be dedicated to the completion of cycles. For mappers to undertake the DCM cycles, a degree of effort, commitment and time was needed that some mappers had not anticipated or appreciated when agreeing to take on the role. The amount of time that staff would need to be away from their usual roles to undertake DCM meant that it was not viewed by some, in its current form, as a tenable intervention in a care home setting. Ensuring that managers understand what skills are particularly important for mappers helps to reduce the likelihood of these acting as a barrier to the delivery of DCM.
Intervention barriers and facilitators
A number of barriers to and facilitators of the DCM intervention itself were identified.
Understanding Dementia Care Mapping
The extent to which mappers, managers and staff valued and understood the benefits of DCM influenced whether or not it was successfully implemented or facilitated. When DCM was perceived as a tool and a process that could improve the quality of care being delivered, managers and mappers were more engaged. In care homes in which DCM was not understood, particularly in terms of the time commitments required, there were issues with the completion of cycles:
The manager that clearly didn’t get it, I think was just so busy with everything else. Absolutely, you know, I did see her running around like this, yeah.
DCM expert 70003
An understanding of the DCM process and its potential for changing the care delivered in care homes is crucial to successful implementation. When some of the trained mappers did not fully understand the process, they struggled to explain it to others:
The trouble is, when they came back [from the training], they weren’t able to explain properly what they had to do. So, you know, they were trying to explain it to us and we were finding difficulty understanding what was actually involved.
Manager 10666/10722
As a result of a lack of understanding of DCM, managers and staff did not always engage with the process:
I still don’t understand it . . . no one has been able to explain it to me fully . . . Every time I asked them [the mappers] to explain they were struggling. So I never got a full grasp of what it was all about.
Manager 10666/10722
When managers did not understand the process or value of DCM, it was perceived as a distraction and it became particularly difficult for mappers to be released from their duties:
I would say the challenges outweigh everything else really.
Manager/mapper 50009/10104
However, for the majority of mappers, the value of DCM was clear and easily understood, even when this was not clear to the managers:
You can see a big difference. You can actually see what goes on through their [the residents’] eyes. When you sit there and watch them for about 3 hours.
Mapper 50019/10180
These findings indicate the importance of mappers, managers and staff having a clear understanding of the DCM process before attempting to implement it. Without this understanding, mappers are unable to be released from their duties to complete mapping tasks, as it is not seen as a priority or a valuable tool within the care home.
Complexity and time demands of Dementia Care Mapping
Dementia Care Mapping was felt to be complex and time-consuming by some participants, with the nature of DCM felt by these participants to be a barrier to its implementation in a care home context. Various aspects of DCM were felt to be too complex, including the observation phase and associated coding, the report writing and the language used:
So the report writing, yeah, was horrific to be honest. Very time-consuming. Obviously we both had different roles at that point so quite demanding, so getting time, and it’s not a very quick process. Like I say it took quite a lengthy period of time. So that were quite bad to be honest, it was very demanding.
Mapper 50069/10476
Particular components of the process were identified as time-consuming or overly onerous, including the length of the training course and the paperwork and report-writing requirements:
Some of the things that certainly I picked up on, some of the things they found more difficult, was around the kind of data analysis and report writing. That was the area that people seemed to find most difficult.
DCM expert 70006
For some mappers, there were delays between them attending the training course and completing their first cycle of DCM, which might have led to them forgetting some of the more detailed parts of the process, such as the observational coding framework. The DCM experts had to give additional, unexpected time to help mappers ‘revise’ some parts of their training before starting the mapping cycle:
I mean one person I worked with, we did our first IRR [inter-rater reliability], our first kind of check of her accuracy and I think we got, our agreement was kind of in the 40s. Like it was very, very low. And that was mapping one person for an hour.
DCM expert 70006
The time required to undertake DCM meant that mappers had to be taken away from their usual roles and defined as ‘off the floor’ and were therefore removed from the core business of care delivery in the case of direct care staff:
The mappers were also carers and nurses and had, you know, activities and tasks and jobs to do as well as the mapping. Yeah, I think they found it quite overwhelming.
Manager 58930/40001
In addition, some managers felt that once the training course was completed they were then left to implement DCM on their own, although in reality every home had access to a DCM expert for 5 days to support implementation of their first cycle. Such views raise questions about the fit of DCM for care homes and suggest the need to consider adapting standard DCM processes for care home staff in the future development of the tool.
Trial barriers and facilitators
Expectations of Dementia Care Mapping and the trial
Expectations of the trial and of what was required to support the implementation of DCM did not meet the realities experienced by participants. In particular, the time and costs exceeded those expected by the managers and mappers. This had an impact on the schedules in place for each care home and led to the expert mappers having to consistently renegotiate schedules:
But from start to finish, although we renegotiated, kind of, schedules for me going down there, it was difficult, I think they would say that they weren’t aware of the time commitments to it.
DCM expert 70005
Some managers were not aware that the mappers could not be included as members in the staff team and thus could not provide direct care on the days when they were mapping. These managers did not appreciate that the mappers were unable to stop mapping to assist residents with any care needs that they had during the mapping process. This led to tensions between some managers, mappers and expert mappers:
They were definitely not aware of that because they were not normally on the part of the numbers, so they didn’t realise that they would have to be off the numbers to do the, you know, preparing the map, for the mapping, for the map itself and to do the rest of their work.
DCM expert 70005
The range of processes and tasks involved in participation in the trial, as well as those involved in implementing DCM, such as the completion of trial and DCM paperwork, seeking consents, undertaking interviews and identification of staff participants, were not anticipated by managers or mappers prior to taking part:
They struggled with the copious amounts of paperwork, they told me that if they knew what was involved that wouldn’t have gone for it.
Manager 50019/10195
In summary, the conduct of the trial may have negatively influenced perceptions of the tenability of implementing DCM in care home settings, with the combined burden of trial and DCM participation proving difficult for some care homes to manage. Mismatches occurred between the expectations of what the DCM intervention entailed and the additional work that was required by managers and staff during the trial, despite having been provided with detailed written and verbal explanations of the processes and the time involved by the research team. Care home managers and mappers were not fully aware of the expectations of them during the trial, particularly in relation to the time involved in each stage and component of the trial, and the requirement of mappers to focus on all aspects of the DCM intervention while in the mapper role, with the consequence being that they were unable to attend to their usual care work at these times. This had a negative impact on the ability of mappers to implement DCM, as they were frequently not released from the staff roster to complete the DCM procedures.
Expert mapper support
Expert mappers viewed themselves as incredibly valuable to the implementation of DCM, suggesting that, without their input and support, DCM would not have been successfully implemented in the majority of care homes:
If the expectations had remained the same, I don’t think it would have worked without the expert mappers.
DCM expert 70006
However, two DCM experts felt that the mappers would have completed the cycles regardless of whether they supported the mappers or not. They thought, instead, that the observation data or the implementation process would have been of a lower quality without the support they provided to the mappers:
I think some of the classic mistakes that can be made in DCM would’ve been made . . . and if they hadn’t been picked up and supported or changed, it can have a really devastating effect on DCM.
DCM expert 70002
Support provided by the expert mappers helped to clarify any uncertainties and alleviate mapper doubts:
It is nice to have somebody sat with you whilst you’re actually doing it practically, to be able to say ‘Am I using this code or that code?’, ‘Am I observing this right?’
Mapper 58930/40002
When DCM expert mapper support was delivered flexibly and with a friendly manner, it was valued by care homes. There were, however, also times when support was perceived as problematic:
The expert mapper was a little full on. Knew her subject, very passionate, but very, erm, timescale orientated. Which kind of pushed, I think, added to the stress.
Manager 58930/40001
The DCM expert mappers believed that they went above and beyond their expected roles to provide support within care homes. They were allocated 5 days of time to support each care home; however, they felt that much more time than this was required. Certain situations led to an increased need for DCM expert mapper support, such as a care home having only one mapper, or tensions in the relationship between mappers:
I’ve tried to support her individually because the other mapper hasn’t supported her in the individual care summaries. So I’ve tried to support her extra by phone and do that, but I don’t think she was, she had the skills to do that by herself.
DCM expert 70005
Despite support from the DCM expert mapper being provided to all homes during the first cycle of DCM, not all homes felt supported:
I feel as if we were, had the training and then left to our own devices really.
Mapper 50024/10349
Conversely, some mappers felt that they did not need the support and that, as they knew the residents well, they had a better insight into the residents than the DCM expert:
When you learn anything really you just want to go and do it on your own don’t you. You don’t want someone looking over your shoulder going ‘yeah, yeah you’ve not done that right’ or ‘I didn’t get that’ or ‘why did you put that’ . . . well I know that resident and I know.
Mapper 50069/10476
For other homes, the mappers benefited from DCM expert mapper support during the first cycle, but felt that they required more than what was provided to continue to undertake DCM cycles:
When she’d gone the support had gone.
Mapper 50010/10096
One DCM expert mapper suggested that, for future DCM research, research assistants should support mappers in completing DCM paperwork. However, this does not represent the standard use of DCM within care homes and thus the pragmatic trial design employed in the present trial:
I think you would’ve really struggled if they hadn’t had someone going in. Be that an expert mapper or be that a research assistant, to go in and support them with doing the paperwork and completing that, which obviously would unblind the researchers. But they would need some kind of support to be able to engage with the research.
DCM expert 70006
These findings suggest that DCM expert mappers felt that their influence had a positive impact on DCM delivery and resulted in substantially more cycles being completed than would have been without their input. However, this support was not always appreciated by the mappers. The implementation data, which show only 26% of intervention homes completed further acceptable DCM cycles after the first cycle supported by the expert, highlight the value of expert mappers’ input in supporting DCM implementation in care home settings.
Summary
There were many barriers to and facilitators of implementing DCM, owing to the complex nature both of the intervention and of care home settings. The selection of appropriate staff as mappers was key, as it was important to ensure that they had the necessary skills to implement all aspects of DCM, including suitable language skills, the time to undertake all aspects of DCM within their day-to-day role, being well respected by the staff team and having leadership capabilities and influence among the staff. It was crucial that the expectations of DCM were understood by both care home managers and mappers before training was completed. Implementation was easier in larger care homes, where there was a larger staff budget to allow mappers to be released from their usual roles. The support of expert mappers was felt to be particularly important in the beginning to implement DCM. Although this is not a standard component of DCM, unless purchased as an addition to standard training, it was a necessary feature of mapper support during the trial. These findings have implications for considering the way that mappers are currently trained and the support that may be required to implement DCM in practice (i.e. to fully engage mappers in the four phases of a DCM practice development cycle).
Specific barriers to and facilitators of identifying, achieving or maintaining impacts
Alongside the barriers to intervention implementation (and so to impacts) identified in the previous section, there were some specific barriers to and facilitators of identifying, achieving or maintaining impacts from DCM.
Barriers to identifying impacts
Challenges to identifying impacts arose primarily from the perceived difficulties in accurately identifying the impacts of any care improvements on people with dementia. For example, some staff and relatives felt that people with dementia would not be able to recognise the impact of any changes made, and some relatives (who may have been involved in completing outcome measures) felt that it was difficult for them to identify changes in their family member, owing to the infrequency of their contact with the resident:
They [people with dementia] will not acknowledge it [DCM] as having an impact on them.
Mapper 50011/10160
I think their life has perhaps been improved by it, but I don’t know whether they would be able to express that or realise that.
Manager 58930/40001
I think it’s amazing and probably essential, and you know, it’s hard to get data because . . . the residents themselves aren’t particularly reliable.
Relative 50016/20114
Barriers to achieving positive impacts
Interviewees spoke of multiple challenges to achieving positive impacts from DCM. Some of the more predictable barriers included staffing, the costs of making changes and competing priorities for staff, such as high workloads or emergencies. For example, if competing priorities meant that action plans were not always carried out, then the potential impacts from DCM were not always realised:
You are trying to carry action plans out, but the day-to-day everything means that you can’t carry them out as much as you’d like to because, like I say, you end up with short staff, you end up with emergencies.
Mapper 59830/40002
The understanding and perceptions of DCM (e.g. of its purpose, quality and reliability) and the perceptions of the current quality of care in the home appeared to shape the degree to which the outputs of mapping sessions were attended to or seen as indicating a need for change:
They [staff] don’t understand what it is.
Mapper 50010/10095
The main issues [with DCM] are, some of the things we got on the feedback were, well, you were looking at so and so, they hadn’t slept last night so that’s why they’ve been nodding off the whole time. So even for that resident it sometimes doesn’t give you an accurate picture.
Mapper and manager/mapper 50018/10268 and 10277
[Answering a question about whether changes to care have occurred] No I don’t think so, because they’re all pretty good anyway. The staff here are pretty good. So we do sort of pride ourselves on person-centred care.
Mapper 50031/10456
Additional barriers to achieving positive impacts included staff who were not open to change and a lack of managerial or financial support for changes proposed as a result of DCM cycles:
Obviously you always get a few who don’t want to take on board anything.
Mapper 58930/40002
When we do the briefing we, let’s say, decide to do some things a different way, and they agree. But later on they found some difficulties, like I said, to change the chairs or something. And then maybe that’s cost then.
Mapper 58757/10446
Barriers to maintaining positive impacts
Some care homes experienced challenges in maintaining positive impacts from DCM over time. These challenges included difficulties in maintaining staff engagement with the DCM process, in particular with the feedback and action-planning sessions, and difficulties maintaining momentum as staffing teams changed over time:
People stopped turning up . . . The first time around . . . we had maybe eight or something in here, and they did, you know, we had a good meeting. But then the second time around we held a meeting and no one came . . . we put posters up all over and we let everyone know that we were doing these feedback sessions . . . and nobody turned up.
Mapper 50010/10095
I think sustained changes certainly from the staff who were here then, but the staff who haven’t actually had that form of training, the momentum has waned actually.
Mapper 50024/10349
Of note in relation to achieving and then maintaining positive impacts for care home residents generally is the fact that many examples of impacts for residents were specific to the individuals who had been mapped. These findings suggest that the impacts of DCM may be greater for mapped individuals than for residents who were not involved in mapping:
The ones that we mapped, I’d like to think are more gainfully employed with their time.
Manager/mapper 50069/10475
We have observed a resident then we have made a care plan specific to that resident’s needs.
Mapper 50011/10160
As mapped individuals were a small minority of the trial sample, producing and maintaining a positive effect on residents more generally may have been difficult for those homes that focused predominantly on action-planning for mapped individuals and focused less on the development of home-level action plans. Given that mappers could select any care home residents to be observed during DCM cycles, those mapped were not necessarily trial participants. In addition, a focus in some homes on addressing the needs of mapped individuals may have reduced the longer-term impact of DCM, as the high rates of death and transfer to other care settings made it likely that many mapped individuals were no longer residing in the homes at follow-up:
Even to be observed, for them [mapped individuals], was kind of benefitting . . . but unfortunately most of them are not here anymore . . . so we can’t say ‘oh it’s brilliant, working . . .’
Mapper 58747/10446
Action plans and impacts for mapped residents were not necessarily transferable to other residents, or were not viewed as such by staff, which may have affected the degree to which positive impacts from DCM were able to be maintained over time.
Facilitators of achieving and maintaining impacts
As well as identifying challenges to achieving positive outcomes from DCM, interviewees reported a number of factors that facilitated the achievement or maintenance of impacts. Changing staff perceptions of the quality of care they were providing and/or their perceptions of people with dementia and their needs was a key impact facilitator:
You don’t realise, when you’re walking through the room, that you’ve passed 10 people and you haven’t even spoke to them.
Mapper 50010/10095
It encourages the staff to think more of them as people . . . because obviously they [people with more advanced dementia] don’t respond as much . . . so it has helped in that way, to make them more aware that they still have to have the same contact, the same explanations for them.
Mapper 58930/40002
As creating change in care practices was dependent on staff recognising the need for change, mappers needed to clearly demonstrate the issues with the current care for these to be recognised and addressed by staff:
It’s really tempting to go in gung-ho and start talking about PEs [personal enhancers] and the different codes, and it’s like trying to sit the staff down and talk about trigonometry. It’s not something interesting that makes much sense to them . . . We sort of ended up picking two or three very small examples of people who were very happy or very sad and just focusing on those, describing it in layman’s terms.
Mapper and manager/mapper 50018/10268 and 10277
It was good and clear to see, you know, which areas we really needed to improve on.
Manager/mapper 50069/10475
Making DCM feedback accessible helped staff to understand the need for changes in their care practices, and the purpose and value of DCM, a lack of understanding of which was identified as a barrier to impacts. Creating a shared understanding of the need for improvements was felt to be an important driver for change:
You really have to get quite a few people across the organisation thinking in the same way to sort of drive that change.
Mapper and manager/mapper 50018/10268 and 10277
One thing I am more aware of is how staff, certain staff, sometimes talk to residents . . . in the inductions now that we do, we make it really clear about what we want a new member of staff, how we want them to interact, how we want them to speak . . . I go through how I would like people to speak to residents.
Manager/mapper 50069/10475
Embedding DCM data, feedback and action plans into the work of the care home, through their inclusion in care plans, handovers and staff meetings, and engaging staff across the home in identifying care improvements were strategies through which mappers tried to ensure a home-level approach to care improvement:
We implemented it in our handovers as well as through all the team leaders.
Mapper 50019/10181
Putting together a dementia group which has carers, cleaners, people across the organisation, and you talk to them and try and actually get them on board. You try and sort of instil in them what person-centred care looks like.
Mapper and manager/mapper 50018/10268 and 10277
So then I could . . . say to the chef ‘This gentleman plays with food, what can we do?’
Mapper 50028/10394
Some of these actions to embed DCM into usual-care practice also helped to ensure that changes were maintained. In addition, the identification of achievable changes, such as when staff were encouraged to interact more with residents on a routine basis, was considered by participants as a good strategy for facilitating impact.
Summary
Multiple barriers to and facilitators of identifying, achieving and maintaining impacts were identified by participants. These included difficulties in measuring impacts for people with dementia; competing care priorities; levels of managerial, financial and home-level support for change; and staff members’ understanding and perceptions of DCM, of current care quality, of the need for change and of people with dementia. A focus on care improvements for mapped individuals can limit impacts for other residents and the maintenance of impacts over time.
Mechanisms of action
In this section, we have drawn on the available evidence to assess if the anticipated mechanisms of action or logic models through which we expected DCM to have an impact on outcomes were present.
Ancillary analyses (moderator/mediator analyses)
Complete cases of the cross-sectional sample were included in the analysis of care home-level moderators identified a priori (Table 24). Moderators were measured at baseline and were assessed by including an interaction between the treatment arm and the moderator variable in the primary analysis of CMAI score, one at a time. There was no evidence of moderation of any prespecified baseline characteristics on CMAI score at 16 months. The results are exploratory and should be treated with caution.
| Moderator | Unadjusted CMAI score at 16 months (95% CI) | p-value for interactiona | |
|---|---|---|---|
| Control | Intervention | ||
| 1. Care home size | 0.7672 | ||
| < 40 residents | 45.1 (42.54 to 47.63) | 42.8 (40.71 to 44.98) | |
| ≥ 40 residents | 47.0 (44.06 to 50.03) | 42.9 (40.45 to 45.26) | |
| 2. Care home type | 0.8713 | ||
| Independent | 46.9 (42.68 to 51.20) | 40.9 (38.67 to 43.12) | |
| Chain | 45.8 (43.58 to 48.01) | 44.4 (42.16 to 46.61) | |
| 3. Agency staff use | 0.1815 | ||
| Below or equal to median | 45.5 (42.15 to 48.94) | 42.2 (40.19 to 44.16) | |
| Above median | 46.3 (43.89 to 48.69) | 43.7 (41.13 to 46.34) | |
| 4. Bank staff use | 0.2249 | ||
| Below or equal to median | 48.3 (45.19 to 51.38) | 42.0 (40.01 to 43.96) | |
| Above median | 44.3 (41.81 to 46.83) | 43.8 (41.25 to 46.33) | |
| 5. Self-funding (proportion of self-funded places) (continuous) | 0.8230 | ||
| Below or equal to mean | 46.1 (43.52 to 48.68) | 42.6 (40.68 to 44.51) | |
| Above mean | 46.1 (43.04 to 49.05) | 43.3 (40.42 to 46.11) | |
| 6. Care home facilities (EAT score) (continuous) | 0.4339 | ||
| Below or equal to mean | 45.0 (42.11 to 47.92) | 41.9 (39.80 to 43.98) | |
| Above mean | 47.0 (44.29 to 49.64) | 44.0 (41.58 to 46.47) | |
| 7. GLHC score (continuous) | 0.9756 | ||
| Below or equal to mean | 45.3 (42.67 to 47.87) | 42.4 (40.38 to 44.43) | |
| Above mean | 47.1 (44.09 to 50.12) | 43.4 (40.85 to 45.93) | |
| 8. Care home manager’s experience (length of time in care home) (continuous) | 0.9961 | ||
| Below or equal to mean | 45.9 (43.79 to 48.06) | 44.2 (42.21 to 46.18) | |
| Above mean | 46.8 (41.72 to 51.78) | 39.8 (37.22 to 42.30) | |
| 9. QUIS (proportion of positive interactions) | 0.0737 | ||
| Below or equal to mean | 46.8 (43.85 to 49.81) | 44.4 (41.67 to 47.11) | |
| Above mean | 45.4 (42.79 to 48.02) | 41.7 (39.83 to 43.65) | |
| 10. Staff-to-resident ratio (continuous) | 0.3592 | ||
| Below or equal to mean | 48.0 (45.03 to 50.90) | 44.5 (42.41 to 46.48) | |
| Above mean | 44.2 (41.64 to 46.85) | 39.4 (37.07 to 41.82) | |
| 11. Average baseline CDR score (continuous) | 0.3601 | ||
| Below or equal to mean | 44.3 (41.58 to 46.95) | 39.9 (37.71 to 42.12) | |
| Above mean | 47.8 (44.99 to 50.69) | 44.9 (42.69 to 47.06) | |
| 12. Average baseline CMAI score (continuous) | 0.7150 | ||
| Below or equal to mean | 42.8 (40.43 to 45.07) | 40.3 (38.21 to 42.32) | |
| Above mean | 49.5 (46.40 to 52.61) | 45.8 (43.37 to 48.19) | |
All 675 residents in the cross-sectional sample were included in the exploratory analyses of care home-level mediators of the randomised effect of intervention versus control on CMAI score at 16 months. The ‘natural indirect’ or mediated effects of each potential mediator (and their 95% CIs) are given in Table 25, adjusted for all of the covariates included in the primary analysis of CMAI score. It can be seen that no potential mediator was found to dominate the mediation of the effect of randomised treatment on the primary outcome, and none of the mediated effects was statistically significant at the 5% level. Additional analyses are planned (outside the scope of the final analyses reported) to explore whether or not our a priori potential mediators have a clearer role in mediating the treatment received on the primary outcome.
| Potential mediator | Adjusted natural indirect effect (standard error) | 95% CI |
|---|---|---|
| Potential care home-level mediators (at 6 months) | ||
| Change in care home manager (yes/no) | 0.27 (0.22) | –0.16 to 0.70 |
| QUIS (proportion of positive interactions) | 0.18 (0.34) | –0.48 to 0.84 |
| Improved EAT score in privacy and community (yes/no) | 0.21 (0.39) | –0.56 to 0.98 |
| Improved EAT score in community links (yes/no) | 0.00 (0.37) | –0.72 to 0.73 |
| Improved EAT score in domestic activity (yes/no) | 0.39 (0.27) | –0.13 to 0.91 |
| Improved GLHC score (yes/no) | –0.67 (0.55) | –1.75 to 0.41 |
| Potential care home-level mediators (at 16 months) | ||
| Change in care home manager (yes/no) | –0.00 (0.11) | –0.23 to 0.22 |
| QUIS (proportion of positive interactions) | 0.00 (0.06) | –0.11 to 0.11 |
| Improved EAT score in privacy and community (yes/no) | 0.12 (0.23) | –0.34 to 0.58 |
| Improved EAT score in community links (yes/no) | – | – |
| Improved EAT score in domestic activity (yes/no) | 0.15 (0.17) | –0.18 to 0.47 |
| Improved GLHC score (yes/no) | –0.67 (0.42) | –1.49 to 0.16 |
Unadjusted scores of predictive and process measures are given in Appendix 1, Tables 62–64.
Interview data
Drawing on interviewees’ perceptions of the DCM implementation process and its impacts, alongside the quantitative trial data, we had intended to propose a model for the intervention’s mechanisms of impact. This model was intended to set out the processes through which the implementation of DCM may lead to change, and the barriers and facilitators that may enable or inhibit the achievement and maintenance of those changes. Although the results set out some of the contextual features required to facilitate DCM implementation and the challenges that need to be overcome to implement DCM effectively, given the negative trial result and the great variability in DCM implementation observed, we have been unable to come to any conclusions about potential mechanisms of action. Specific potential barriers to mechanisms of action included poor implementation of DCM, particularly beyond the first supported mapping cycle, which meant that exposure to DCM over the trial period was limited to one or fewer cycles over the 15-month period for three-quarters of the intervention homes.
Chapter 6 Discussion
Key findings
The DCM EPIC trial was a pragmatic, multicentre, cluster RCT of DCM’s clinical effectiveness and cost-effectiveness compared with a usual-care control in UK care home settings. The trial evaluated whether or not DCM led to reductions in agitation, other BSC and PRN antipsychotic and other tranquilliser use and to improved QoL for care home residents with dementia and improved quality of staff interactions with residents. It also sought to determine whether or not DCM was cost-effective. Thirty-one care homes were randomised to the DCM intervention arm and 19 were randomised to the control arm. A total of 987 residents were recruited and registered: 726 at baseline (308 in the control arm and 418 in the intervention arm) and a further 261 at the 16-month follow-up (99 in the control arm and 162 in the intervention arm). A total of 675 residents were included in the final cross-sectional sample (287 in the control arm and 388 in the intervention arm) used for the primary analysis: 414 from the original sample and 261 who were recruited at 16 months.
Primary outcomes
Care home residents in the intervention-arm care homes did not demonstrate any clinically meaningful or statistically significant reduction in agitation compared with control-arm residents.
Secondary outcomes
There were no clinically meaningful or statistically significant differences in BSC, QoL or PRN use of prescription medications for care home residents with dementia at either the 6- or the 16-month follow-up. However, trends for BSC and mood (depression/apathy) were found to be in favour of the intervention arm at 16 months in the closed cohort. The prescription rates of PRN medications were low across both arms at all time points and this, alongside the wide CIs within the secondary analyses, makes the results difficult to interpret. The quality of staff interactions did not differ between arms at either time point.
Given the poor return rates for staff outcome measures, we were unable to evaluate any potential impact of DCM on staff health-related QoL (GHQ-12) or feelings of confidence in caring for people with dementia (SCIDS).
Economic evaluation
We conducted an economic evaluation alongside a clinical trial adhering, where possible, to the NICE reference case for technology appraisals. 143 The primary analysis was a cost–utility (cost per QALY) evaluation and the secondary analysis was a cost-effectiveness (cost per unit improvement in CMAI score) evaluation.
Costs for the intervention per person were £421.07. This depended on a number of assumptions, including the number of staff involved, the number of cycles implemented and the number of residents who might benefit. In general, our assumptions regarding these costs were conservative. We also conducted a sensitivity analysis that accounted for the different costs incurred by care homes as a result of their compliance with the intervention.
The costs of resource use were substantially higher in the intervention arm, and this was driven by higher secondary (hospital) care costs. This resulted from the presence of several high-cost individuals in the intervention arm (six residents in the intervention arm had higher costs than the highest cost individual in the control arm). We conducted sensitivity analyses in which we removed these six individuals (in a CCA and prior to conducting MIs for an analysis using MI data) to examine the impact of these outliers on our cost–utility estimates.
Regardless of the utility measure used and the analytical approach adopted, QoL appeared to be higher in the intervention arm than in the control arm at 16 months. Although QoL declined over 16 months, in general this decline was lower in the active treatment arm.
The base-case ICER was £60,627 and, being substantially over the NICE threshold of £20,000, this suggests that DCM would not be an efficient use of health/social service resources. The sensitivity analyses were consistent in finding that the intervention was more costly, but also more clinically effective, than the control. With the exception of the analyses that excluded the high-cost individuals, ICERs from the sensitivity analyses ranged from £24,139 to £57,509.
The analyses that excluded high-cost individuals in the intervention arm yielded ICERs that were either below (£10,975/QALY for the CCA) or closer to (£36,371/QALY for the MI analysis) the NICE threshold. When we examined the data on comorbidities and the reason for hospital admission for the six high-cost individuals, it was not possible to conclude that these higher secondary care costs could have been the result of chance rather than attributable to the intervention. Therefore, there was no reasonable justification for removing these individuals from the main analyses. ICERs from analyses adjusting for baseline costs or including only more compliant care homes also approached cost-effectiveness. However, these estimates were based on reduced samples and are considered less robust.
Consistent with the main cost-effectiveness analysis, the net-benefit regression analyses indicated that DCM did not represent value for money when compared with usual care. Furthermore, the net-benefit regression and CACE analyses also showed no indication that intervention compliance may have had a mediating effect. This was despite the likelihood that these analyses were biased by the failure to control for (unobserved) factors related to potential differences in care home quality (which might be positively related to the likelihood of compliance and to resident QoL).
We found the cost per unit improvement in CMAI score to be between £67 and £289, depending on the analysis. This lower figure, although not our base case, is roughly in line with previously generated estimates of comparable interventions. 161,162
Safety
Undertaking DCM was not detrimental to care home residents. No unexpected SAEs occurred in the trial and the majority of residents did not have any hospital admissions over the trial period, with admissions figures and length of stay similar across the intervention and control arms.
Comparison with other trials of Dementia Care Mapping in care home settings
The efficacy of DCM has been evaluated in three previous exploratory cluster RCTs63,93,94 and one quasi-experimental trial. 92 The RCT conducted by Chenoweth et al. 63 found that researcher-led cycles of DCM led to significant reductions in agitation and falls for care home residents with dementia compared with those in the usual-care control. Likewise, the Norwegian study carried out by Rokstad et al. 93 found a significant reduction in overall BSC, agitation and psychosis and a significant improvement in QoL for care home residents with dementia in the DCM intervention arm, compared with the usual-care control. This study also used researcher-led cycles of DCM implementation. Conversely, the cluster RCT conducted by van de Ven et al. ,94 with cycles of DCM led by care home staff, found no significant difference in agitation between the DCM intervention arm and the usual-care control. This trial did find a significant improvement in staff emotional reactions towards people with dementia in the DCM intervention arm compared with the control. The quasi-experimental trial conducted by Dichter et al. 92 adopted cycles of DCM led by care home staff and also found no significant benefits of the DCM intervention in comparison with the control as regards resident QoL or BSC.
The DCM EPIC trial is the only pragmatic, explanatory trial conducted on the clinical effectiveness and cost-effectiveness of DCM to date. It did not replicate the findings of the exploratory trials conducted by Chenoweth et al. 63 or Rokstad et al. ,93 in which significant benefits of DCM over usual-care controls as regards resident agitation, falls and QoL were indicated. It did support the findings of the exploratory trial by van de Ven et al. 94 and the quasi-experimental trial of Dichter et al. ,92 in which no significant benefits of DCM were found for agitation, BSC or QoL in comparison with the control. Unlike the economic evaluation of DCM conducted by van de Ven et al. ,163 which found that DCM was cost neutral, the DCM EPIC trial found that DCM was not cost-effective. The costliness of DCM as an intervention was also identified by Chenoweth et al. ,63 who found that the costs of DCM per CMAI point averted in usual care were markedly higher than those in PCCT. Owing to poor return rates on staff measures, we were unable to assess any potential effects of DCM on staff outcomes in the DCM EPIC trial.
The comparison between the outcomes of the DCM EPIC trial and those of previous trials requires caution, given the pragmatic, explanatory design of this trial compared with the exploratory designs of the previous studies. Likewise, this is the only trial to have been conducted in the UK and thus the care home resident population, care systems and costs differ from those of previous trials. Nevertheless, a common feature emerges in that all trials adopting cycles of DCM led by care home staff, even with support from a DCM expert or lead, recorded implementation challenges and no significant benefits of DCM over usual-care controls. In the two trials in which significant benefits of DCM were reported over the control, DCM was led by researchers and few implementation challenges were identified. This indicates that consideration needs to be given to the model of DCM implementation and leadership, with all trials to date adopting cycles led by care home staff failing to find any clinical effectiveness of the intervention over the control, in contrast with the trials in which efficacy was found through external- or researcher-led cycles.
The potential benefits of externally supported interventions is confirmed by other intervention trials in care home settings. The WHELD (Improving Wellbeing and Health for People with Dementia) trial,76 which combined staff training with support from a WHELD therapist who provided coaching, supervision and regular review over a 9-month period, found that the intervention had statistically significant benefits as regards QoL, agitation and neuropsychiatric symptoms and positive care interactions, compared with treatment as usual. The benefits were greater for those with moderately severe dementia.
This trial is the first randomised controlled study of DCM in the UK and it reflects the largely practice-led development and evolution of the method in the UK. Although the current eighth edition of DCM was produced following a thorough review process, only the revised observational tool was evaluated using formal research methods, with the additional guidance on DCM implementation developed through a series of working groups involving practitioners. 109 A recent systematic review96 of the published research evidence on the process of DCM implementation, when used as a practice development methodology, found only 12 papers representing nine research studies that reported on this area. Only six papers used formal research methods to gather data and all were published from 2014 onwards, indicating that there has been limited published research in this area to date, despite DCM’s use in practice for over 20 years. The review concluded that more research is required.
The formal process evaluation that is reported as part of this trial is the largest study of DCM implementation to be conducted to date internationally. Therefore, in addition to the process-evaluation results reported earlier, a number of in-depth papers discussing DCM implementation from the perspective of mappers, care home staff, care home managers and expert mappers are being prepared to contribute to this body of evidence.
Strengths and limitations of the study
The DCM EPIC trial is the largest and only definitive trial of the clinical effectiveness and cost-effectiveness of DCM to date (worldwide). It successfully recruited on time and to target, adding to the relatively limited body of research on the conduct of pragmatic, cluster RCT studies in care home settings. Our use of random sampling to approach care homes within specific geographical regions permitted recruitment of a number of care homes that had not participated in research previously. This has increased the pool of care homes that have been exposed to research and in particular to clinical trials and thus the numbers of homes that may be considered ‘research ready’. The EPIC trial gave care home staff and managers an opportunity to participate in research, and a number of the staff members who trained as mappers discussed the value they placed on being able to access this training for their own professional development. Some of the care homes have expressed a desire to be involved in future research projects with the local recruitment hubs.
The DCM EPIC trial has also provided a valuable opportunity to increase the number of researchers with expertise in conducting dementia research within care home settings, across a range of trial roles. Some research assistants employed on the trial have taken up permanent PhD or post-doctoral positions in dementia research or are commencing clinical psychology training, ensuring their expertise is retained within the field.
Study design
The EPIC trial followed the Medical Research Council guidance on evaluating a complex intervention. A cluster RCT design was utilised, appropriate outcome measures were selected, a full economic evaluation was conducted and a full, integrated process evaluation was undertaken.
However, loss to follow-up was larger than had been anticipated (close to 50%, in comparison with the estimated 25–30%), owing mainly to resident deaths because of the frailty of this population, and this resulted in the need to implement a design change and to adopt an open-cohort design mid-trial. This is not an established design for cluster RCT studies and three of the co-applicants (RW, AF and CS) have been successful in gaining additional funding to conduct methodological research on the use of open-cohort designs.
Cluster blinding to allocation was not feasible within the trial, as care home staff were responsible for intervention delivery. Therefore, this could have led to reporting bias. Independent observational measures of agitation (PAS and CMAI-O) were therefore collected by an independent, blinded researcher to permit analysis of potential reporting bias by arm. However, observational measures do not capture agitation that may occur outside public areas, for example during personal care, and the set observation days and time meant that agitation that occurred outside the observational hours could not be assessed, for example during the evening and night-time, and over more than 2.5 days during a week. Therefore, the comparison between observational and proxy-reported measures must be considered with some caution.
Researchers were all blinded to cluster allocation and were not permitted to collect data in homes to which they became unblinded. This required flexibility within the research teams and some cross-working between research hubs to provide cover when researchers became unblinded. The independent researcher who collected observational PAS, CMAI-O and QUIS data was both blinded and independent, and so had collected no other data in the care home apart from these observational measures. Independent researchers and researcher blinding to the cluster allocation of homes in which they collected data were maintained throughout the trial.
Owing to the variability in the ability of care home residents with dementia to self-report on measures of BSC and QoL, the primary and secondary analyses were conducted using staff proxy-completed measures. It was not possible to use relative or supporter proxy measures, as many residents did not have a proxy informant recruited, either because their relatives/supporters were visiting less frequently than was required for the measures used (at least once a fortnight) or because relatives/supporters did not wish to take part. Proxy-completed measures are reported to have some but not full correlation with self-report164 and therefore it is a limitation of this study that outcomes are reliant on proxy views. Although we attempted to use the same staff proxy respondent at each time point, this was not always feasible owing to staff turnover, sickness and annual leave. There is no reason to conclude that these issues affected one arm of the trial more than or differently from the other. However, the issues associated with use of staff proxy informants in both arms of the trial must be considered when interpreting the results.
The poor intervention adherence beyond the first expert-supported cycle of DCM is a further limitation. Given the pragmatic trial design aiming to implement DCM in ‘real world’ conditions, the findings are important for highlighting implementation challenges and for informing future use in such settings.
Health economic analysis
The health economic analysis has a number of limitations. The number of missing data was large and the evaluation was heavily reliant on imputation. Given the difficulties in incorporating the cross-sectional cohort approach in the economic evaluation framework, in particular the requirement to have baseline data to calculate changes in costs and QALYs, it was not possible to fully capitalise on the increased sample size in a robust way.
The adoption of a health and social care perspective meant that some societal costs were not accounted for in the analysis (e.g. informal care); however, it is highly unlikely that these would have had a substantial impact on total costs and collecting such data would have presented significant challenges.
Additional consideration is needed regarding how to deal with high-cost outliers165 and when it may be appropriate to exclude them from analyses. Research should identify the most appropriate way to measure and combine QoL estimates in this group.
Finally, future research should explore the maintenance of the health benefits of the DCM intervention identified here.
Generalisability and sources of bias
Random selection of care homes from the large pool of eligible homes from three geographically wide recruitment hub areas ensured a good representation of different care home settings and thus good generalisability of the trial across care home settings in England. This helped to minimise selection bias. Our exclusion of care homes that were subject to admissions bans, supportive input or other improvement measures, due to issues or concerns regarding care quality means that a small proportion (c. 3%)166 of the care home sector was not represented in the trial. Following randomisation, the characteristics of the clusters were found to be balanced. No clusters were lost during the trial. There was a higher variation in cluster sizes in the control than in the intervention arm; however, the median cluster size was similar in both arms and in both cohorts.
The recruitment of residents was carefully designed to minimise selection bias at various levels. Resident recruitment commenced following the recruitment of care homes but prior to their randomisation. All residents who were identified as eligible and who consented to take part in the trial were recruited. Following the change in design, the recruitment at 16 months of all eligible residents with dementia who were not already participating in the trial or who had previously declined to take part contributed to a minimisation of selection bias. Researchers independent of the care home were involved in resident recruitment. Characteristics of the screened and registered residents were well balanced across arms and cohorts, demonstrating a lack of selection bias in resident recruitment.
Allocation concealment during the researchers’ visits to care homes was not always successful; however, every effort was made to ensure that researchers collected no further data in care homes to whose allocation they were unblinded. Research blinding to the allocation of care homes in which they collected data was able to be maintained throughout the trial.
Implementation of a complex intervention
As a pragmatic trial, the DCM intervention was implemented as it would usually be in UK care home settings, with some components enhanced from standard practice, but still within the scope of what would be feasible in usual practice. This included (1) selection of care homes on the basis of criteria that would ensure that there were no setting conditions that were likely to reduce their ability to engage with the trial (e.g. quality concerns or competing research studies), (2) selection of mappers using criteria of required qualities and skills, (3) provision of a standard 4-day DCM training course with assessment, (4) provision of support for the first cycle of DCM from an expert mapper, (5) provision of standardised documentation for DCM implementation (e.g. a report template and an action plan template), (6) ongoing telephone and e-mail support from the DCM intervention lead and (7) prompts to conduct mapping cycles at the required intervals sent to mappers by SMS and through the post.
Dementia Care Mapping training was provided at standard training locations (Bradford and London) for ‘open’ DCM courses (those open to any trainees and not purchased by a single provider organisation for their own staff). However, evidence gathered during the mapper recruitment phase, subsequent efforts to recruit further mappers to replace those who had withdrawn and the process evaluation all indicated that this was difficult for many care home staff and thus restricted who could be recruited as a mapper. For the majority of those recruited as mappers, the 4-day training course had to be completed on a residential basis or it required significant daily travel. Some of those identified as potential mappers indicated that they would be unable to attend the training because of child care or other responsibilities, whereas others did not wish to or were concerned about travelling and/or attending the training on a residential basis.
Although overall commencement of DCM training within the planned 2 months after randomisation was adequate, 29% of homes (n = 9) experienced delays in training mappers and one home failed to train any mappers during the trial period. Mapper withdrawal was also high, with over half of homes having one or more mappers withdraw during the trial period and one-third of homes having no trained mappers in post by the 16-month follow-up. Reasons for withdrawal were mainly personal (leaving the organisation, ill-health, maternity leave or change of role within the home). Finding suitable replacement mappers who could be trained during the trial period was not possible in the majority of homes. These issues had an impact on the care home’s ability to implement DCM over the trial period and they raise questions regarding the long-term sustainability of DCM as an intervention within care home settings.
Given that DCM is an established intervention, piloting of its implementation was not considered a requirement within this trial. However, given the lack of robust evidence on the implementation of DCM that was available at the time of trail design and the subsequent implementation challenges identified, it may have been beneficial to undertake some feasibility work to assess intervention adherence in care home settings and potential barriers to and facilitators of this. Published and practitioner evidence regarding best practice in DCM implementation was consulted in designing the study, and experts in the use of DCM were involved in the trial design and delivery. DCM implementation within the trial included the range of supports and prompts for mapping described earlier, which are over and above what would normally be received by a mapper following completion of DCM training. Nevertheless, considerable DCM implementation difficulties and problems with compliance were still encountered.
Intervention compliance
Dementia Care Mapping implementation was poorer than expected and, even with DCM expert mapper support, 10% of intervention care homes failed to undertake any DCM activity and 23% did not complete one full cycle. A further 52% of homes completed only their first expert mapper-supported cycle, leaving just over one-quarter of homes (26%) that completed more than one cycle and only 13% (n = 4) that completed the three full, per-protocol cycles to an acceptable level. This was despite a range of methods for tracking and supporting intervention compliance being implemented during the trial. Tracking intervention compliance was challenging and it required considerable effort. Despite this, there was missing documentation, particularly in terms of that associated with briefing and feedback sessions, and assumptions had to be made that previous components of the cycle had been completed if documented evidence for later components was submitted (e.g. we assumed that a briefing session had occurred if there was documented evidence of mapping observations having taken place).
Two homes withdrew from the DCM intervention during the trial period, one because it was felt that DCM was not of value and the other because it was unable to identify any suitable mappers following withdrawal of the trained mappers for personal reasons. The poor intervention compliance was disappointing given our adoption of established DCM training and implementation processes and the introduction of enhanced support for the trained care home mappers, which was above what would be expected in usual DCM practice. This has implications for considering the implementation of DCM in the future, in particular in considering models of implementation that are not reliant on care home staff.
Integral process evaluation (separate papers in preparation)
An integral process evaluation was conducted within the DCM EPIC trial. It investigated the perceptions of DCM implementation and impacts from the perspective of mappers, care home managers and staff, care home residents with dementia, their relatives and friends, and the DCM expert mappers. The process evaluation results have provided a valuable context within which to understand and interpret the DCM EPIC trial findings and will be presented in detail in additional papers that are currently in preparation.
Interpretation of results
The results of this trial may potentially be attributed to poor intervention compliance. Although DCM implementation was successful in a number of sites and the process evaluation was able to identify factors associated with successful implementation, as well as barriers to this, the proportion of intervention homes that failed to complete any, or more than, the initial expert-supported cycle was disappointing. This indicates that, although DCM was a well-used intervention within care homes prior to this trial and it was assumed, therefore, to be acceptable and feasible to use in these settings, this may not be the case. Although the exploratory CACE analyses indicated that the treatment effect may increase if care homes complete at least two DCM cycles to an acceptable level, compared with completing only one cycle, and are thus suggestive of a dose–response relationship, further research would need to be undertaken to explore this potential relationship.
Economic evaluation
We estimated the mean resident cost of DCM to be £421.07 and the most costly components of this were attendance at the DCM training session and DCM expert mapper support. Although there appeared to be incremental health (QALY) benefits for the intervention over the control, these were relatively modest and were outweighed by the additional costs. As such, DCM did not appear to represent value for money in the cost–utility framework. Cost per reduction in CMAI cost-effectiveness values were generated and these should be interpreted alongside previous studies reporting the same metric.
The results were largely driven by a small number of high-cost outliers in the intervention arm and sensitivity analyses removing these reduced the ICER substantively. As we cannot definitively state that these cost outliers were random and not associated with the intervention, they are retained in the main analysis. The conclusions were robust to sensitivity analyses. However, efforts to reduce the cost of the intervention and to improve compliance may improve estimates of value for money. However, given the DCM implementation challenges identified in this study, it seems unlikely that greater adherence would be feasible to achieve with DCM cycles led solely by care home staff. Therefore, the costs of potential alternative models of delivery would need to be considered carefully in future research.
Overall evidence
Systematic reviews have identified DCM as clinically effective in reducing agitation immediately and at 6 months post randomisation in care home residents with dementia62 and in presenting benefits for care home staff. 167 However, the number of published studies is low, their outcomes are varied and robust evidence to guide effective DCM implementation is extremely limited. 96 Trials demonstrating the efficacy of DCM have, to date, included only researcher-led cycles of DCM. The DCM EPIC trial sought to examine whether or not DCM implemented within care home settings, following usual UK models of cycles led by care home staff, was clinically effective and cost-effective. It is the largest and only explanatory trial of DCM conducted internationally and the only UK-based trial. Recruiting 978 residents across 50 care homes, and randomising 31 clusters to the DCM intervention arm, the DCM EPIC trial is the largest trial of DCM conducted to date (the largest trial previous to this94 recruited 268 residents in 33 units across 14 care home locations and randomised 13 units in seven care homes to DCM). The DCM EPIC trial has provided conclusive evidence that implementing cycles of DCM led by care home staff is not clinically effective in reducing agitation, BSC or the use of PRN antipsychotic or other tranquillising medications, or in improving QoL for care home residents with dementia compared with the control. Neither is it cost-effective.
The findings of the process evaluation indicate that, despite a range of methods to support DCM implementation within the trial, cycles of DCM led by care home staff result in poor intervention compliance, with the vast majority of care homes (74%) failing to complete more than the first DCM expert-supported cycle. Barriers to DCM implementation were found at the individual mapper level, the DCM intervention level and the care home level. Additional barriers caused by the burdens of trial participation were also identified. Considering these results alongside the findings from previous exploratory trials of DCM indicates that externally led or supported implementation of DCM may provide a more beneficial and sustainable format for DCM delivery. This aligns with the broader contextual challenges faced by care homes in implementing complex interventions that are staff led. These include, but are not limited to, high staff turnover rates; low staff literacy, numeracy, IT skills and confidence; and a lack of time and resources. Future research will need to consider mechanisms for addressing these wider issues within the context of intervention design and delivery. Utilising ‘bottom-up’ approaches to intervention design that involve care home staff, managers and providers may provide a mechanism to identify and address potential challenges within the development process.
Chapter 7 Conclusions
This trial indicates that, as an intervention led by care home staff, DCM is not clinically effective or cost-effective in reducing agitation or improving QoL and care outcomes for residents with dementia living in care home settings. This outcome may be associated with the poor DCM implementation we experienced during the trial, despite efforts to support care home mappers in implementing the intervention. Providing support for the first DCM cycle, in the form of an external expert mapper, enabled 77.4% of intervention homes to complete that cycle; however, DCM implementation reduced greatly after the first cycle when this support ended. Given the picture emerging from trials of DCM internationally, in which, on the one hand, cycles of DCM led by care home staff have consistently produced negative trials results and, on the other hand, researcher-led cycles have produced significant outcomes, further investigation is warranted into models of DCM implementation that do not rely solely on care home staff to implement them. The process evaluation revealed the challenges that care home staff faced when trying to implement DCM. These included mappers not having the required skills and qualities to lead change or feeling unconfident to do so; a lack of time, resources and management support; and difficulties in engaging colleagues in supporting the change process. Staff turnover, sickness and other personal issues that affected mappers’ ability to continue in the role were also challenging, with over half of the intervention homes having at least one mapper withdraw during the study period. Nevertheless, one-quarter of intervention care homes did complete two or more DCM cycles, and staff within the process evaluation reported a range of benefits that they felt DCM had for residents and staff, as well as for care practices more broadly. This trial suggests that the majority of care home settings may not provide the right setting conditions for a costly intervention such as DCM and that externally led models may provide a more practical and resource-effective method of implementation. However, further research is needed to evaluate this. Our findings have implications for future complex intervention trials in care home settings. Future research should more carefully consider the setting conditions needed for effective intervention implementation and thus the most appropriate models for delivering these interventions given the available resources and cultural and organisational contexts of care home settings.
Acknowledgements
General acknowledgements
We would like to thank all of the care homes, individuals with dementia, family members and care home staff for taking part in this study and for freely giving their time.
We would like to thank the following people who contributed to the successful completion of this trial: Chris Albertyn, Heather Blakey, Marie Crabbe, Elyse Couch, Cara Gates, Layla Hamadi, Stephanie Jones, Baber Malik, Harriet Maunsell, Kirsty Nash, Sahdia Parveen, Luisa Rabanal, Bina Sharma, Victoria Simons, Emily Smeaton, Alyma Somani, Emily Standell, Rebecca Thomas, Ingelin Testad and Miguel Vasconcelos Da Silva, the researchers who collected the data; Robert Cicero, who supported the development of the SAP; Kayleigh Burton, who undertook trial management; Madeline Harms, Alison Fergusson and Laura Stubbs, who undertook data management; Benjamin Thorpe, who assisted with statistical programming; Sharon Jones, Lisa Heller, Juniper West, Judith Farmer, Maria Scurfield and Lisa Breame, who supported DCM intervention implementation activities; Jan Robins, Clare Mason and Lindsey Collins, who delivered dementia awareness training; Barbara Carlton, Sandra Duggan, Jane Ward, Daniella Watson and Connie Williams, who were members of the LAG; Sue Fortescue, who was a member of the Trial Management Group and LAG; Ian Wheeler, who provided administrative support for the trial; and Matt Murray from the Alzheimer’s Society, who provided oversight for the LAG. Graham Stokes would like to acknowledge Bupa UK, his employing organisation during the majority of the study period. Jane Fossey would like to acknowledge the NIHR Oxford Health Biomedical Research Centre, a partnership between Oxford Health NHS Foundation Trust and the University of Oxford.
Contributions of authors
Claire A Surr (Professor of Dementia Studies and Director of the Centre for Dementia Research) conceived and designed the study and was involved in the analysis of the qualitative data, interpretation of the data and drafting of this paper (URL: www.leedsbeckett.ac.uk/school-of-health-and-community-studies/epic-trial/).
Ivana Holloway (Senior Medical Statistician) was involved in the analysis of the statistical data, interpretation of the data and drafting of this paper.
Rebecca EA Walwyn (Principal Statistician) conceived and designed the study and was involved in the analysis of the statistical data, interpretation of the data and drafting of this paper.
Alys W Griffiths (Research Fellow) was involved in the data acquisition, analysis of the qualitative data, interpretation of the data and drafting of this paper.
David Meads (Associate Professor of Health Economics) helped design the study and was involved in the analysis of the health economic data, interpretation of the data and drafting of this paper.
Rachael Kelley (Research Fellow) designed the process evaluation and was involved in the analysis of the qualitative data, interpretation of the data and drafting of this paper.
Adam Martin (Senior Research Fellow in Health Economics) was involved in the analysis of the health economic data, interpretation of the data and drafting of this paper.
Vicki McLellan (Senior Trial Co-ordinator) was involved in the data acquisition, management of the trial and drafting of this paper.
Clive Ballard (Pro-Vice-Chancellor of Exeter Medical School) helped design the study, was involved in the data acquisition and commented on the draft of this paper.
Jane Fossey (Associate Director of Psychological Services) helped design the study, was involved in the data acquisition and commented on the draft of this paper.
Natasha Burnley (Research Assistant) was involved in the data acquisition, analysis of the qualitative data and interpretation of the data and commented on the draft of this paper.
Lynn Chenoweth (Professor of Nursing) helped design the study and commented on the draft of this paper.
Byron Creese (Senior Research Fellow) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Murna Downs (Professor of Dementia Studies) helped design the study and commented on the draft of this paper.
Lucy Garrod (Research Therapist) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Elizabeth H Graham (Trial Manager) helped design the study, was involved in the data acquisition and commented on the draft of this paper.
Amanda Lilley-Kelley (Trial Manager) helped design the study, was involved in the data acquisition and commented on the draft of this paper.
Joanne McDermid (Research Therapist) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Holly Millard (Assistant Psychologist) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Devon Perfect (Senior Clinical Research Assistant) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Louise Robinson (Director of the Newcastle University Institute for Ageing and Professor of Primary Care) helped design the study and commented on the draft of this paper.
Olivia Robinson (Research Assistant) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Emily Shoesmith (Research Assistant) was involved in the data acquisition and analysis of the qualitative data and commented on the draft of this paper.
Najma Siddiqi (Clinical Senior Lecturer in Psychiatry) helped design the study and commented on the draft of this paper.
Graham Stokes (Director of Memory Care Services) helped design the study and commented on the draft of this paper.
Daphne Wallace (Expert by Experience) helped design the study and commented on the draft of this paper.
Amanda J Farrin (Professor of Clinical Trials and Evaluation of Complex Interventions and Director of the Complex Interventions Division) conceived and designed the study and was involved in the interpretation of the data and drafting of this paper.
Publications
Surr CA, Walwyn RE, Lilley-Kelley A, Cicero R, Meads D, Ballard C, et al. Evaluating the effectiveness and cost-effectiveness of Dementia Care Mapping™ to enable person-centred care for people with dementia and their carers (DCM-EPIC) in care homes: study protocol for a randomised controlled trial. Trials 2016;17:300.
Griffiths AW, Creese B, Garrod L, Chenoweth L, Surr C. The development and use of the assessment of dementia awareness and person-centred care training tool in long-term care. Dementia 2018;18:3059–70.
Griffiths A, Kelley R, Garrod L, Perfect D, Robinson O, Shoesmith E, et al. Barriers and facilitators to implementing Dementia Care Mapping in care homes: results from the DCM™ EPIC trial process evaluation. BMC Geriatr 2019;19:37.
Griffiths AW, Albertyn CP, Burnley NL, Creese B, Walwyn R, Holloway I, et al. Development and validation of an observational version of the Cohen-Mansfield Agitation Inventory [published online ahead of print April 10 2019]. Int Psychogeriatr 2019.
Surr CA, Griffiths AW, Kelley R, Holloway I, Walwyn REA, Martin A, et al. The implementation of Dementia Care Mapping™ in a randomised controlled trial in long-term care: results of a process evaluation. Am J Alzheimers Dis Other Demen 2019;34:390–8.
Surr CA, Griffiths A, Woodward-Carlton B. Does DCM improve care in care homes? The EPIC trial. J Dementia Care 2019;27:24–7.
Griffiths AW, Surr CA, Alldred DP, Baker J, Higham R, Spilsbury K, Thompson CA. Pro re nata prescribing and administration for neuropsychiatric symptoms and pain in long-term care residents with dementia and memory problems: a cross-sectional study [published online ahead of print July 24 2019]. Int J Clin Pharm 2019;41:1814–22.
Martin A, Meads D, Griffiths A, Surr CA. How should we capture health state utility in dementia? Comparison of DEMQOL-Proxy-U and of self- and proxy-completed EQ-5D-5L. Value Health 2019;22:1417–26.
Meads DM, Martin A, Griffiths A, Kelley R, Creese B, Robinson L, et al. Cost-effectiveness of Dementia Care Mapping in care home settings – evaluation of a randomised controlled trial [published online ahead of print November 8 2019]. Appl Health Econ Health Policy 2019.
Surr C, Griffiths A, Kelley R, Holloway I, Walwyn R, Martin A, et al. The implementation of Dementia Care Mapping™ in a randomised controlled trial in long-term care: results of a process evaluation. Am J Alzheimers Dis Other Dement 2019;34:390–8.
Surr CA, Shoesmith E, Griffiths AW, Kelley R, McDermid J, Fossey J. Exploring the role of external experts in supporting staff to implement psychosocial interventions in care home settings: results from the process evaluation of a randomized controlled trial. BMC Health Serv Res 2019;19:790.
Surr CA, Holloway I, Walwyn REA, Griffiths AW, Meads D, Martin A, et al. Effectiveness of Dementia Care MappingTM to reduce agitation in care home residents with dementia: an open-cohort cluster randomised controlled trial. Aging Ment Health 2020; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Update. London: Alzheimer’s Society; 2014.
- Alzheimer’s Society . Low Expectations: Attitudes on Choice, Care and Community for People With Dementia in Care Homes 2013.
- Care Quality Commission . The State of Adult Social Care Services 2014 to 2017 2017.
- All-Party Parliamentary Group on Dementia . Always a Last Resort: Inquiry into the Prescription of Antipsychotic Drugs to People With Dementia Living in Care Homes 2008.
- Department of Health and Social Care (DHSC) . Quality Outcomes for People With Dementia: Building on the Work of the National Dementia Strategy 2010.
- Department of Health and Social Care (DHSC) . Living Well With Dementia: A National Dementia Strategy 2009.
- Department of Health and Social Care (DHSC) . Prime Minister’s Challenge on Dementia: Delivering Major Improvements in Dementia Care and Research by 2015 2012.
- Department of Health and Social Care (DHSC) . Prime Minister’s Challenge on Dementia 2020 2015.
- Ministerial Advisory Group on Dementia Research . The Ministerial Advisory Group on Dementia Research: Headline Report 2011.
- Bowie P, Mountain G. The relationship between patient behaviour and environmental quality for the dementing. Int J Geriatr Psychiatry 1997;12:718-23. https://doi.org/10.1002/(SICI)1099-1166(199707)12:7<718::AID-GPS608>3.0.CO;2-3.
- Ballard C, O’Brien J, James I, Mynt P, Lana M, Potkins D, et al. Quality of life for people with dementia living in residential and nursing home care: the impact of performance on activities of daily living, behavioral and psychological symptoms, language skills, and psychotropic drugs. Int Psychogeriatr 2001;13:93-106. https://doi.org/10.1017/S1041610201007499.
- All-Party Parliamentary Group on Dementia . Prepared to Care. Challenging the Dementia Skills Gap 2009.
- Kuske B, Hanns S, Luck T, Angermeyer MC, Behrens J, Riedel-Heller SG. Nursing home staff training in dementia care: a systematic review of evaluated programs. Int Psychogeriatr 2007;19:818-41. https://doi.org/10.1017/S1041610206004352.
- Surr CA, Gates C, Irving D, Oyebode J, Smith SJ, Parveen S, et al. Effective dementia education and training for the health and social care workforce: a systematic review of the literature. Rev Educ Res 2017;87:966-1002. https://doi.org/10.3102/0034654317723305.
- Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797-80. https://doi.org/10.1002/gps.4072.
- Evaluating Dementia Care. The DCM Method. Bradford: University of Bradford; 1997.
- Bradford Dementia Group . DCM 8 User’s Manual 2005. https://doi.org/10.1111/j.174-1617.1970.tb00694.x.
- Cox S, Cantley C. A Handbook of Dementia Care. Buckingham: Open University Press; 2001.
- Innes A. Dementia Care Mapping: Applications Across Cultures. Baltimore, MD: Health Professions Press; 2003.
- Bredin K, Kitwood T, Wattis J. Decline in quality of life for patients with severe dementia following a ward merger. Int J Geriatr Psychiatry 1995;10:967-73. https://doi.org/10.1002/gps.930101109.
- Brooker D. Auditing outcome of care in in-patient and day patient settings using Dementia Care Mapping. Can it be done?. PSIGE Newsletter 1994;51:18-22.
- Brooker D, Foster N, Banner A, Payne M, Jackson L. The efficacy of Dementia Care Mapping as an audit tool: report of a 3-year British NHS evaluation. Aging Ment Health 1998;2:60-7. https://doi.org/10.1080/13607869856957.
- Clare M. Spreading DCM far and wide in Suffolk. J Dement Care 2006;14:10-1.
- Edwards P, Brotherton S. DCM 8 in Cheshire. J Dement Care 2006;14.
- Jacques I. Evaluating care services for people living with dementia. Elder Care 1996;8:10-3.
- Martin GW, Younger D. Anti oppressive practice: a route to the empowerment of people with dementia through communication and choice. J Psychiatr Ment Health Nurs 2000;7:59-67. https://doi.org/10.1046/j.1365-2850.2000.00264.x.
- Martin GW, Younger D. Person-centred care for people with dementia: a quality audit approach. J Psychiatr Ment Health Nurs 2001;8:443-8. https://doi.org/10.1046/j.1351-0126.2001.00427.x.
- Wilkinson AM. Dementia Care Mapping: a pilot study of its implementation in a psychogeriatric service. Int J Geriatr Psychiatry 1993;8:1027-9. https://doi.org/10.1002/gps.930081211.
- Williams J, Rees J. The use of ‘dementia care mapping’ as a method of evaluating care received by patients with dementia – an initiative to improve quality of life. J Adv Nurs 1997;25:316-23. https://doi.org/10.1046/j.1365-2648.1997.1997025316.x.
- Ballard C, Corbett A. Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 2010;24:729-39. https://doi.org/10.2165/11319240-000000000-00000.
- Margallo-Lana M, Swann A, O’Brien J, Fairbairn A, Reichelt K, Potkins D, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry 2001;16:39-44. https://doi.org/10.1002/1099-1166(200101)16:1<39::AID-GPS269>3.0.CO;2-F.
- Davison TE, Hudgson C, McCabe MP, George K, Buchanan G. An individualized psychosocial approach for ‘treatment resistant’ behavioral symptoms of dementia among aged care residents. Int Psychogeriatr 2007;19:859-73. https://doi.org/10.1017/S1041610206004224.
- Banerjee S, Smith SC, Lamping DL, Harwood RH, Foley B, Smith P, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatry 2006;77:146-8. https://doi.org/10.1136/jnnp.2005.072983.
- Liperoti R, Pedone C, Corsonello A. Antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD). Curr Neuropharmacol 2008;6:117-24. https://doi.org/10.2174/157015908784533860.
- Deudon A, Maubourguet N, Gervais X, Leone E, Brocker P, Carcaillon L, et al. Non-pharmacological management of behavioural symptoms in nursing homes. Int J Geriatr Psychiatry 2009;24:1386-95. https://doi.org/10.1002/gps.2275.
- Herrmann N, Lanctôt KL, Sambrook R, Lesnikova N, Hébert R, McCracken P, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry 2006;21:972-6. https://doi.org/10.1002/gps.1594.
- Finkel S. Introduction to behavioural and psychological symptoms of dementia (BPSD). Int J Geriatr Psychiatry 2000;15:2-4. https://doi.org/10.1002/(SICI)1099-1166(200004)15:1+<S2::AID-GPS159>3.0.CO;2-3.
- Majic T, Pluta JP, Mell T, Aichberger MC, Treusch Y, Gutzmann H, et al. The pharmacotherapy of neuropsychiatric symptoms of dementia: a cross-sectional study in 18 homes for the elderly in Berlin. Dtsch Arztebl Int 2010;107:320-7. https://doi.org/10.3238/arztebl.2010.0320.
- Tunis SL, Edell WS, Adams BE, Kennedy JS. Characterizing behavioral and psychological symptoms of dementia (BPSD) among geropsychiatric inpatients. J Am Med Dir Assoc 2002;3:146-51. https://doi.org/10.1016/S1525-8610(04)70457-0.
- Ballard CG, Margallo-Lana M, Fossey J, Reichelt K, Myint P, Potkins D, et al. A 1-year follow-up study of behavioral and psychological symptoms in dementia among people in care environments. J Clin Psychiatry 2001;62:631-6. https://doi.org/10.4088/JCP.v62n0810.
- Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d4065.
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol 1989;44:M77-84. https://doi.org/10.1093/geronj/44.3.M77.
- Cohen-Mansfield J. Instruction Manual for the Cohen-Mansfield Agitation Inventory (CMAI). Rockville, MD: The Research Institute of the Hebrew Home of Greater Washington; 1991.
- Hindley N, Gordon H. The elderly, dementia, aggression and risk assessment. Int J Geriatr Psychiatry 2000;15:254-9. https://doi.org/10.1002/(SICI)1099-1166(200003)15:3<254::AID-GPS103>3.0.CO;2-T.
- Burgio LD, Butler FR, Roth DL, Hardin JM, Hsu CC, Ung K. Agitation in nursing home residents: the role of gender and social context. Int Psychogeriatr 2000;12:495-511. https://doi.org/10.1017/S104161020000661X.
- Zuidema S, Koopmans R, Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatry Neurol 2007;20:41-9. https://doi.org/10.1177/0891988706292762.
- Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs 2010;31:246-53. https://doi.org/10.1016/j.gerinurse.2010.01.002.
- Cohen-Mansfield J. Agitated behavior in persons with dementia: the relationship between type of behavior, its frequency, and its disruptiveness. J Psychiatr Res 2008;43:64-9. https://doi.org/10.1016/j.jpsychires.2008.02.003.
- Hughes J, Bagley H, Reilly S, Burns A, Challis D. Care staff working with people with dementia: training, knowledge and confidence. Dementia 2008;7:227-38. https://doi.org/10.1177/1471301208091159.
- Banerjee S. The Use of Antipsychotic Medication for People with Dementia: Time for Action. London: Department of Health and Social Care; 2009.
- Bishara D, Taylor D, Howard RJ, Abdel-Tawab R. Expert opinion on the management of behavioural and psychological symptoms of dementia (BPSD) and investigation into prescribing practices in the UK. Int J Geriatr Psychiatry 2009;24:944-54. https://doi.org/10.1002/gps.2200.
- Raju J, Sikdar S, Krishna T. What happened to patients with behavioural and psychological symptoms of dementia (BPSD) after the Committee on Safety in Medicines (CSM) guidelines?. Int J Geriatr Psychiatry 2005;20:898-9. https://doi.org/10.1002/gps.1377.
- Cohen-Mansfield J, Werner P, Marx MS. The social environment of the agitated nursing home resident. Int J Geriatr Psychiatry 1992;7:789-98. https://doi.org/10.1002/gps.930071104.
- Testad I, Auer S, Mittelman M, Ballard C, Fossey J, Donabauer Y, et al. Nursing home structure and association with agitation and use of psychotropic drugs in nursing home residents in three countries: Norway, Austria and England. Int J Geriatr Psychiatry 2010;25:725-31. https://doi.org/10.1002/gps.2414.
- Stokes G, Woods RT. Handbook of the Clinical Psychology of Ageing. Chichester: Wiley; 1996.
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli?. J Am Geriatr Soc 2010;58:1459-64. https://doi.org/10.1111/j.1532-5415.2010.02951.x.
- Brodaty H, Draper B, Saab D, Low LF, Richards V, Paton H, et al. Psychosis, depression and behavioural disturbances in Sydney nursing home residents: prevalence and predictors. Int J Geriatr Psychiatry 2001;16:504-12. https://doi.org/10.1002/gps.382.
- Weber K, Meiler-Mititelu C, Herrmann FR, Delaloye C, Giannakopoulos P, Canuto A. Longitudinal assessment of psychotherapeutic day hospital treatment for neuropsychiatric symptoms in dementia. Aging Ment Health 2009;13:92-8. https://doi.org/10.1080/13607860802154523.
- Social Care Institute for Excellence . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care – NICE Clinical Guideline 42 2006.
- Salzman C, Jeste DV, Meyer RE, Cohen-Mansfield J, Cummings J, Grossberg GT, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry 2008;69:889-98. https://doi.org/10.4088/JCP.v69n0602.
- Moniz-Cook E, Stokes G, Agar S. Difficult behaviour and dementia in nursing homes: five cases of psychosocial intervention. Clin Psychol Psychother 2003;10:197-208. https://doi.org/10.1002/cpp.370.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry 2014;205:436-42. https://doi.org/10.1192/bjp.bp.113.141119.
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein-Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person-centred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8:317-25. https://doi.org/10.1016/S1474-4422(09)70045-6.
- Brooker D. What is person centred care for people with dementia?. Rev Clin Gerontol 2004;13:215-22. https://doi.org/10.1017/S095925980400108X.
- Chrzescijanski C, Moyle W, Creedy D. Reducing dementia-related aggression through a staff education intervention. Dementia 2007;6:271-86. https://doi.org/10.1177/1471301207080369.
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756-61. https://doi.org/10.1136/bmj.38782.575868.7C.
- Moniz-Cook E, Agar S, Silver M, Woods R, Wang M, Elston C, et al. Can staff training reduce behavioural problems in residential care for the elderly mentally ill?. Int J Geriatr Psychiatry 1998;13:149-58. https://doi.org/10.1002/(SICI)1099-1166(199803)13:3<149::AID-GPS746>3.0.CO;2-Q.
- Bird M, Llewellyn-Jones RH, Korten A. An evaluation of the effectiveness of a case-specific approach to challenging behaviour associated with dementia. Aging Ment Health 2009;13:73-8. https://doi.org/10.1080/13607860802154499.
- Turner J, Snowdon J. An innovative approach to behavioral assessment and intervention in residential care: a service evaluation. Clin Gerontol 2009;32:260-75. https://doi.org/10.1080/07317110902895291.
- Bird M, Jones RH, Korten A, Smithers H. A controlled trial of a predominantly psychosocial approach to BPSD: treating causality. Int Psychogeriatr 2007;19:874-91. https://doi.org/10.1017/S1041610206004790.
- Skills for Care . Common Induction Standards (2010 ‘Refreshed’ Edition) 2010.
- Care Quality Commission . Guidance about Compliance: Essential Standards of Quality and Safety 2010.
- Commission for Social Care Inspection . See Me, Not Just the Dementia: Understanding People’s Experiences of Living in a Care Home 2008.
- Furåker C, Nilsson A. The competence of certified nurse assistants caring for persons with dementia diseases in residential facilities. J Psychiatr Ment Health Nurs 2009;16:146-52. https://doi.org/10.1111/j.1365-2850.2008.01347.x.
- Lintern T, Woods RT, Phair L. Training is not enough to change care practice. J Dement Care 2000;8:15-6.
- Ballard C, Corbett A, Orrell M, Williams G, Moniz-Cook E, Romeo R, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002500.
- Visser SM, McCabe MP, Hudgson C, Buchanan G, Davison TE, George K. Managing behavioural symptoms of dementia: effectiveness of staff education and peer support. Aging Ment Health 2008;12:47-55. https://doi.org/10.1080/13607860701366012.
- Ballard C, Powell I, James I, Reichelt K, Myint P, Potkins D, et al. Can psychiatric liaison reduce neuroleptic use and reduce health service utilization for dementia patients residing in care facilities. Int J Geriatr Psychiatry 2002;17:140-5. https://doi.org/10.1002/gps.543.
- Andrews S, McInerney F, Robinson A. Realizing a palliative approach in dementia care: strategies to facilitate aged care staff engagement in evidence-based practice. Int Psychogeriatr 2009;21:64-8. https://doi.org/10.1017/S1041610209008679.
- Brodribb W. Barriers to translating evidence-based breastfeeding information into practice. Acta Paediatr 2011;100:486-90. https://doi.org/10.1111/j.1651-2227.2010.02108.x.
- Godley SH, Garner BR, Smith JE, Meyers RJ, Godley MD. A large-scale dissemination and implementation model for evidence-based treatment and continuing care. Clin Psychol 2011;18:67-83. https://doi.org/10.1111/j.1468-2850.2011.01236.x.
- Brooker DJ, Latham I, Evans SC, Jacobson N, Perry W, Bray J, et al. FITS into practice: translating research into practice in reducing the use of anti-psychotic medication for people with dementia living in care homes. Aging Ment Health 2016;20:709-18. https://doi.org/10.1080/13607863.2015.1063102.
- British Standards Institution (BSI) . PAS 800:2010: Use of Dementia Care Mapping for Improved Person-Centred Care in a Care Provider Organization – Guide 2010.
- Innes A. Changing the Culture of Dementia Care: A Systematic Exploration of the Process of Culture Change in Three Care Settings. Bradford: University of Bradford; 2000.
- Innes A, Surr C. Measuring the well-being of people with dementia living in formal care settings: the use of Dementia Care Mapping. Aging Ment Health 2001;5:258-68. https://doi.org/10.1080/13607860120065023.
- Wylie K, Madjar I, Walton JA. Dementia Care Mapping. A person-centred, evidence-based approach to improving the quality of care in residential care settings. Geriaction 2002;20:5-9.
- Mansah M, Coulon L, Brown P. A mapper’s reflection on Dementia Care Mapping with older residents living in a nursing home. Int J Older People Nurs 2008;3:113-20. https://doi.org/10.1111/j.1748-3743.2007.00108.x.
- Beavis D, Simpson S, Graham I. A literature review of dementia care mapping: methodological considerations and efficacy. J Psychiatr Ment Health Nurs 2002;9:725-36. https://doi.org/10.1046/j.1365-2850.2002.00508.x.
- Brooker D. Dementia care mapping: a review of the research literature. Gerontologist 2005;45:11-8. https://doi.org/10.1093/geront/45.suppl_1.11.
- Chenoweth L, Jeon YH. Determining the efficacy of Dementia Care Mapping as an outcome measure and a process for change: a pilot study. Aging Ment Health 2007;11:237-45. https://doi.org/10.1080/13607860600844226.
- Kuiper D, Dijkstra GJ, Tuinstra J, Groothoff JW. The influence of Dementia Care Mapping (DCM) on behavioural problems of persons with dementia and the job satisfaction of caregivers: a pilot study. Tijdschr Gerontol Geriatr 2009;40:102-12. https://doi.org/10.1007/BF03079572.
- Dichter MN, Quasdorf T, Schwab CG, Trutschel D, Haastert B, Riesner C, et al. Dementia care mapping: effects on residents’ quality of life and challenging behavior in German nursing homes. A quasi-experimental trial. Int Psychogeriatr 2015;27:1875-92. https://doi.org/10.1017/S1041610215000927.
- Rokstad AM, Røsvik J, Kirkevold Ø, Selbaek G, Saltyte Benth J, Engedal K. The effect of person-centred dementia care to prevent agitation and other neuropsychiatric symptoms and enhance quality of life in nursing home patients: a 10-month randomized controlled trial. Dement Geriatr Cogn Disord 2013;36:340-53. https://doi.org/10.1159/000354366.
- van de Ven G, Draskovic I, Adang EM, Donders R, Zuidema SU, Koopmans RT, et al. Effects of dementia-care mapping on residents and staff of care homes: a pragmatic cluster-randomised controlled trial. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0067325.
- Jeon YH, Luscombe G, Chenoweth L, Stein-Parbury J, Brodaty H, King M, et al. Staff outcomes from the caring for aged dementia care resident study (CADRES): a cluster randomised trial. Int J Nurs Stud 2012;49:508-18. https://doi.org/10.1016/j.ijnurstu.2011.10.020.
- Surr CA, Griffiths AW, Kelley R. Implementing Dementia Care Mapping as a practice development tool in dementia care services: a systematic review. Clin Interv Aging 2018;13:165-77. https://doi.org/10.2147/CIA.S138836.
- Skills for Care . The State of the Adult Social Care Sector and Workforce in England 2017.
- Surr CA, Walwyn RE, Lilley-Kelly A, Cicero R, Meads D, Ballard C, et al. Evaluating the effectiveness and cost-effectiveness of Dementia Care Mapping™ to enable person-centred care for people with dementia and their carers (DCM-EPIC) in care homes: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1416-z.
- Griffiths AW, Creese B, Garrod L, Chenoweth L, Surr C. The development and use of the Assessment of Dementia Awareness and Person-centred Care Training (ADAPT) tool in long-term care [published online ahead of print 9 April 2018]. Dementia 2018. https://doi.org/10.1177/1471301218768165.
- Bupa . Person First . Dementia Second: The Essentials Workbooks 2010.
- Care Quality Commission . The State of Health Care and Adult Social Care in England. An Overview of Key Themes in Care 2009 10 2010.
- Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull 1988;24:653-9. https://doi.org/10.1037/t08620-000.
- Great Britain . Mental Capacity Act 2005 2005.
- Medical Research Council . MRC Guidelines for Good Clinical Practice in Clinical Trials 1998.
- Department of Health and Social Care, Welsh Assembly Government . Guidance on Nominating a Consultee for Research Involving Adults Who Lack Capacity to Consent 2008.
- Medical Research Council . Medical Research Involving Adults Who Cannot Consent 2007.
- Kuznetsova OM, Tymofyeyev Y. Preserving the allocation ratio at every allocation with biased coin randomisation and minimisation in studies with unequal allocation. Stat Med 2012;31:702-23. https://doi.org/10.1002/sim.4447.
- Brooker D, Surr C. Dementia Care Mapping: Principles and Practice. Bradford: University of Bradford; 2005.
- Brooker DJ, Surr C. Dementia Care Mapping (DCM): initial validation of DCM 8 in UK field trials. Int J Geriatr Psychiatry 2006;21:1018-25. https://doi.org/10.1002/gps.1600.
- Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I. A conceptual review. J Am Geriatr Soc 1986;34:711-21. https://doi.org/10.1111/j.1532-5415.1986.tb04302.x.
- Shah A, Evans H, Parkash N. Evaluation of three aggression/agitation behaviour rating scales for use on an acute admission and assessment psychogeriatric ward. Int J Geriatr Psychiatry 1998;13:415-20. https://doi.org/10.1002/(SICI)1099-1166(199806)13:6<415::AID-GPS788>3.0.CO;2-A.
- Cohen-Mansfield J, Libin A. Assessment of agitation in elderly patients with dementia: correlations between informant rating and direct observation. Int J Geriatr Psychiatry 2004;19:881-91. https://doi.org/10.1002/gps.1171.
- Griffiths AW, Albertyn CP, Burnley NL, Creese B, Walwyn R, Holloway I, et al. Validation of the Cohen-Mansfield Agitation Inventory Observational (CMAI-O) tool [published online ahead of print 10 April 2019]. Int Psychogeriatr 2019. https://doi.org/10.1017/S1041610219000279.
- Rosen J, Burgio L, Kollar M, Cain M, Allison M, Fogleman M, et al. A user-friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry 1994;2:52-9. https://doi.org/10.1097/00019442-199400210-00008.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/WNL.44.12.2308.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9:1-93. https://doi.org/10.3310/hta9100.
- Rowen D, Mulhern B, Banerjee S, Hout Bv, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. https://doi.org/10.1016/j.jval.2011.10.016.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc 2000;1:114-16. https://doi.org/10.1037/t00432-000.
- Logsdon RG, Albert SM. Assessing quality of life in Alzheimer’s disease: conceptual and methodological issues. J Ment Health Aging 1999;5:3-6.
- Hoe J, Katona C, Roch B, Livingston G. Use of the QOL-AD for measuring quality of life in people with severe dementia – the LASER-AD study. Age Ageing 2005;34:130-5. https://doi.org/10.1093/ageing/afi030.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 2003;17:201-8. https://doi.org/10.1097/00002093-200310000-00002.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19. https://doi.org/10.1097/00006842-200205000-00016.
- Edelman P, Fulton BR, Kuhn D, Chang CH. A comparison of three methods of measuring dementia-specific quality of life: perspectives of residents, staff, and observers. Gerontologist 2005;45:27-36. https://doi.org/10.1093/geront/45.suppl_1.27.
- Siddiqi N, Cheater F, Collinson M, Farrin A, Forster A, George D, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing 2016;45:652-61. https://doi.org/10.1093/ageing/afw091.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. https://doi.org/10.1192/bjp.140.6.566.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. https://doi.org/10.1212/WNL.43.11.2412-a.
- Goldberg DP, Williams PA. User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Penninkilampi-Kerola V, Miettunen J, Ebeling H. A comparative assessment of the factor structures and psychometric properties of the GHQ-12 and the GHQ-20 based on data from a Finnish population-based sample. Scand J Psychol 2006;47:431-40. https://doi.org/10.1111/j.1467-9450.2006.00551.x.
- Schepers AK, Orrell M, Shanahan N, Spector A. Sense of competence in dementia care staff (SCIDS) scale: development, reliability, and validity. Int Psychogeriatr 2012;24:1153-62. https://doi.org/10.1017/S104161021100247X.
- Dean R, Proudfoot R, Lindesay J. The Quality of Interactions Schedule (QUIS): development, reliability and use in the evaluation of two domus units. Int J Geriatr Psychiatry 1993;8:819-26. https://doi.org/10.1002/gps.930081004.
- Coates CJ. The Caring Efficacy Scale: nurses’ self-reports of caring in practice settings. Adv Pract Nurs Q 1997;3:53-9.
- Lindesay J, Skea D. Gender and interactions between care staff and elderly nursing home residents with dementia. Int J Geriatr Psychiatry 1997;12:344-8. https://doi.org/10.1002/(SICI)1099-1166(199703)12:3<344::AID-GPS504>3.0.CO;2-I.
- Smith R, Fleming R, Chenoweth L, Jeon YH, Stein-Parbury J, Brodaty H. Validation of the Environmental Audit Tool in both purpose-built and non-purpose-built dementia care settings. Australas J Ageing 2012;31:159-63. https://doi.org/10.1111/j.1741-6612.2011.00559.x.
- te Boekhorst S, Depla MF, Pot AM, de Lange J, Eefsting JA. The ideals of group living homes for people with dementia: do they practice what they preach?. Int Psychogeriatr 2011;23:1526-7. https://doi.org/10.1017/S1041610211000858.
- Zuidema SU, Buursema AL, Gerritsen MG, Oosterwal KC, Smits MM, Koopmans RT, et al. Assessing neuropsychiatric symptoms in nursing home patients with dementia: reliability and Reliable Change Index of the Neuropsychiatric Inventory and the Cohen-Mansfield Agitation Inventory. Int J Geriatr Psychiatry 2011;26:127-34. https://doi.org/10.1002/gps.2499.
- Staquet MJ, Hays RD, Fayers PM. Quality of Life Assessment in Clinical Trials: Methods and Practice. Oxford: Oxford University Press; 1998.
- Yan X, Lee S, Li N. Missing data handling methods in medical device clinical trials. J Biopharm Stat 2009;19:1085-98. https://doi.org/10.1080/10543400903243009.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. https://doi.org/10.1037/0022-3514.51.6.1173.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit; 2016.
- Department of Health and Social Care (DHSC) . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2017.
- Department of Health and Social Care (DHSC) . National Schedule of Reference Costs 2015–16 2017.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England – OHE Research Paper 16/01. London: Office of Health Economics; 2016.
- The EuroQol Group . EQ-5D-5L: Valuation – Crosswalk Index Value Calculator n.d. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/ (accessed 19 October 17).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Res 2011;18:52-6. https://doi.org/10.7748/nr2011.01.18.2.52.c8284.
- Walwyn R, Copas A, Farrin A, Surr C. Open Cohort Designs for Cluster Randomised Trials in Institutional Settings: A Methodology Bolt-on to DCM-EPIC. London: Medical Research Council; n.d.
- Department of Health and Social Care (DHSC) . No Secrets: Guidance on Developing and Implementing Multi-Agency Policies and Procedures to Protect Vulnerable Adults from Abuse 2000.
- Meads DM, Martin A, Griffiths A, Kelley R, Creese B, Robinson L, et al. Cost-effectiveness of Dementia Care Mapping in care home settings – evaluation of a randomised controlled trial [published online ahead of print November 8 2019]. Appl Health Econ Health Policy 2019. https://doi.org/10.1007/s40258-019-00531-1.
- Surr C, Griffiths A, Kelley R, Holloway I, Walwyn R, Martin A, et al. The implementation of Dementia Care Mapping™ in a randomised controlled trial in long-term care: results of a process evaluation. Am J Alzheimers Dis Other Dement 2019;34:390-8. https://doi.org/10.1177/1533317519845725.
- University of Bradford . DCM™ for Realising Person Centred Care Booking Form 2017–18 2017. www.bradford.ac.uk/health/dementia/training/training-courses/dementia-care-mapping-for-realising-person-centred-care/ (accessed September 2017).
- Griffiths AW, Kelley R, Garrod L, Perfect D, Robinson O, Shoesmith E, et al. Barriers and facilitators to implementing dementia care mapping in care homes: results from the DCM™ EPIC trial process evaluation. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1045-y.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18390.
- Zwijsen SA, Bosmans JE, Gerritsen DL, Pot AM, Hertogh CM, Smalbrugge M. The cost-effectiveness of grip on challenging behaviour: an economic evaluation of a care programme for managing challenging behaviour. Int J Geriatr Psychiatry 2016;31:567-74. https://doi.org/10.1002/gps.4360.
- van de Ven G, Draskovic I, van Herpen E, Koopmans RT, Donders R, Zuidema SU, et al. The economics of dementia-care mapping in nursing homes: a cluster-randomised controlled trial. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0086662.
- Moyle W, Murfield JE, Griffiths SG, Venturato L. Assessing quality of life of older people with dementia: a comparison of quantitative self-report and proxy accounts. J Adv Nurs 2012;68:2237-46. https://doi.org/10.1111/j.1365-2648.2011.05912.x.
- Wen YW, Tsai YW, Wu DB, Chen PF. The impact of outliers on net-benefit regression model in cost-effectiveness analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0065930.
- Care Quality Commission . The State of Health Care and Adult Social Care in England 2016 17 2017.
- Barbosa A, Lord K, Blighe A, Mountain G. Dementia Care Mapping in long-term care settings: a systematic review of the evidence. Int Psychogeriatr 2017;29:1609-18. https://doi.org/10.1017/S1041610217001028.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
Appendix 1 Supporting tables
Screening
| Residents, n (%) | Hub | Total (50 care homes) | ||
|---|---|---|---|---|
| Yorkshire (21 care homes) | London (15 care homes) | Oxford (14 care homes) | ||
| Screened | 656 | 419 | 489 | 1564 |
| Eligible (out of those screened) | 451 (68.8) | 297 (70.9) | 321 (65.6) | 1069 (68.4) |
| Not eligible (out of those screened) | 205 (31.2) | 122 (29.0) | 168 (34.4) | 495 (31.6) |
| Does not have formal diagnosis of dementia | 133 (64.9) | 67 (54.9) | 98 (58.3) | 298 (60.2) |
| Permanently bed-bound | 27 (13.2) | 32 (26.2) | 30 (17.9) | 89 (18.0) |
| Terminally ill | 22 (10.7) | 18 (14.8) | 18 (10.7) | 58 (11.7) |
| Not a permanent resident | 38 (18.5) | 2 (1.6) | 21 (12.5) | 61 (12.3) |
| Insufficient proficiency in English | 2 (1.0) | 6 (4.9) | 4 (2.4) | 12 (2.4) |
| Consented (out of those eligible) | 366 (81.2) | 199 (67.0) | 216 (67.3) | 781 (73.1) |
| Not consented (out of those eligible) | 85 (18.8) | 98 (33.0) | 105 (32.7) | 288 (26.9) |
| Consent refused | 69 (81.2) | 87 (88.8) | 82 (78.1) | 238 (82.6) |
| By resident | 24 (66.7) | 4 (11.1) | 8 (22.2) | 36 (15.1) |
| By Personal Consultee | 33 (24.8) | 37 (27.8) | 63 (47.4) | 133 (55.9) |
| By Nominated Consultee | 12 (17.4) | 46 (66.7) | 11 (15.9) | 69 (29.0) |
| Died | 5 (5.9) | 5 (5.1) | 8 (7.6) | 18 (6.3) |
| Unwilling to engage with researcher | 4 (4.7) | 2 (2.0) | 5 (4.8) | 11 (3.8) |
| Transferred elsewhere | 7 (8.2) | 2 (2.0) | 7 (6.7) | 16 (5.6) |
| No consultee available to consent | 0 (0.0) | 0 (0.0) | 2 (1.9) | 2 (0.7) |
| Other | 1 (1.2) | 2 (2.0) | 2 (1.9) | 5 (1.7) |
| Registered (out of those consented) | 339 (92.6) | 191 (96.0) | 213 (98.6) | 743 (95.1) |
| Not registered (out of those consented) | 27 (7.4) | 8 (4.0) | 3 (1.4) | 38 (4.9) |
| Died | 16 (59.3) | 7 (87.5) | 3 (100.0) | 26 (68.4) |
| Withdrew | 1 (3.7) | 0 (0.0) | 0 (0.0) | 1 (2.6) |
| No longer eligible | 3 (11.1) | 0 (0.0) | 0 (0.0) | 3 (7.9) |
| Moved out of care home | 7 (25.9) | 0 (0.0) | 0 (0.0) | 7 (18.4) |
| Other | 1 (3.7) | 1 (12.5) | 0 (0.0) | 2 (5.3) |
| Registered at randomisation (out of those registered) | 330 (97.3) | 185 (96.9) | 211 (99.1) | 726 (97.7) |
| Died between registration and care home randomisation (out of those registered) | 9 (2.7) | 6 (3.1) | 2 (0.9) | 17 (2.3) |
| Residents, n (%) | Hub | Total care homes | ||
|---|---|---|---|---|
| Yorkshire care homes | London care homes | Oxford care homes | ||
| Screened | 569 | 396 | 479 | 1444 |
| Currently participating in the EPIC trial (out of those screened) | 185 (32.5) | 109 (27.5) | 131 (27.3) | 425 (29.4) |
| Screened and not participating in the EPIC trial (out of those screened) | 384 (67.5) | 287 (72.5) | 348 (72.7) | 1019 (70.6) |
| Screened at baseline but consent refused (out of those screened and not participating in the EPIC trial) | 43 (11.2) | 57 (19.9) | 42 (12.1) | 142 (13.9) |
| Eligible (out of those screened) | 189 (33.2) | 90 (22.7) | 142 (29.6) | 421 (29.2) |
| Not eligible (out of those screened)a | 152 (26.7) | 140 (35.4) | 164 (34.2) | 456 (31.6) |
| Does not have formal diagnosis of dementia | 93 (61.2) | 57 (40.7) | 103 (62.8) | 253 (55.5) |
| Permanently bed-bound | 9 (5.9) | 38 (27.1) | 7 (4.3) | 54 (11.8) |
| Terminally ill | 6 (3.9) | 13 (9.3) | 2 (1.2) | 21 (4.6) |
| Not a permanent resident | 19 (12.5) | 1 (0.7) | 8 (4.9) | 28 (6.1) |
| Insufficient proficiency in English | 1 (0.7) | 4 (2.9) | 2 (1.2) | 7 (1.5) |
| Moved to the unit < 3 months ago | 44 (28.9) | 42 (30.0) | 48 (29.3) | 134 (29.4) |
| Missing information | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Consented (out of those eligible) | 120 (63.5) | 47 (52.2) | 99 (69.7) | 266 (63.2) |
| Not consented (out of those eligible)a missing data | 68 (36.0) 1 | 42 (46.7) 1 | 43 (30.3) 0 | 153 (36.3) 2 |
| Consent refused | 65 (95.6) | 36 (85.7) | 39 (90.7) | 140 (91.5) |
| By resident | 14 (21.5) | 1 (2.8) | 13 (33.3) | 28 (20.0) |
| By Personal Consultee | 19 (29.2) | 10 (27.8) | 14 (35.9) | 43 (30.7) |
| By Nominated Consultee | 32 (49.2) | 25 (69.4) | 12 (30.8) | 69 (49.3) |
| Died | 1 (1.5) | 2 (4.8) | 2 (4.7) | 5 (3.3) |
| Unwilling to engage with researcher | 1 (1.5) | 1 (2.4) | 1 (2.3) | 3 (2.0) |
| No response from Personal Consultee | 0 (0.0) | 2 (4.8) | 0 (0.0) | 2 (1.3) |
| Transferred elsewhere | 1 (1.5) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Other | 1 (1.5) | 1 (2.4) | 1 (2.3) | 3 (2.0) |
| Registered (out of those consented) | 119 (99.2) | 45 (95.7) | 97 (98.0) | 261 (98.1) |
| Not registered (out of those consented) missing data | 1 (0.8) 0 | 2 (4.3) 0 | 1 (1.0) 1 | 4 (1.5) 1 |
| Does not have formal diagnosis of dementia | 0 (0.0) | 1 (2.1) | 0 (0.0) | 1 (0.4) |
| Moved out of care home | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Died | 0 (0.0) | 0 (0.0) | 1 (0.8) | 1 (0.4) |
| In hospital | 0 (0.0) | 1 (2.1) | 0 (0.0) | 1 (0.4) |
| Residents, n (%) | Control (19 care homes) | Intervention (31 care homes) | Total (48 care homes) |
|---|---|---|---|
| Screened | 494 | 950 | 1444 |
| Currently participating in the EPIC trial (out of those screened) | 185 (37.4) | 240 (25.3) | 425 (29.4) |
| Screened and not participating in the EPIC trial (out of those screened) | 309 (62.6) | 710 (74.7) | 1019 (70.6) |
| Screened at baseline but consent refused (out of those screened and not participating in the EPIC trial) | 34 (11.0) | 108 (15.2) | 142 (13.9) |
| Eligible (out of those screened) | 147 (29.8) | 274 (28.8) | 421 (29.2) |
| Not eligible (out of those screened)a | 128 (25.9) | 328 (34.5) | 456 (31.6) |
| Does not have formal diagnosis of dementia | 61 (47.7) | 192 (58.5) | 253 (55.5) |
| Moved to the unit < 3 months ago | 15 (11.7) | 39 (11.9) | 54 (11.8) |
| Permanently bed-bound | 6 (4.7) | 15 (4.6) | 21 (4.6) |
| Terminally ill | 3 (2.3) | 25 (7.6) | 28 (6.1) |
| Not a permanent resident | 3 (2.3) | 4 (1.2) | 7 (1.5) |
| Insufficient proficiency in English | 51 (39.8) | 83 (25.3) | 134 (29.4) |
| Missing information | 1 (0.8) | 0 (0.0) | 1 (0.2) |
| Consented (out of those eligible) | 100 (68.0) | 166 (60.6) | 266 (63.2) |
| Not consented (out of those eligible)a missing data | 46 (31.3) 1 | 107 (39.1) 1 | 153 (36.3) 2 |
| Consent refuseda | 40 (87.0) | 100 (93.5) | 140 (91.5) |
| By resident | 9 (22.5) | 19 (19.0) | 28 (20.0) |
| By Personal Consultee | 10 (25.0) | 33 (33.0) | 43 (30.7) |
| By Nominated Consultee | 21 (52.5) | 48 (48.0) | 69 (49.3) |
| Died | 2 (4.3) | 3 (2.8) | 5 (3.3) |
| Unwilling to engage with researcher | 1 (2.2) | 2 (1.9) | 3 (2.0) |
| No response from Personal Consultee | 2 (4.3) | 0 (0.0) | 2 (1.3) |
| Transferred elsewhere | 1 (2.2) | 0 (0.0) | 1 (0.7) |
| Other | 1 (2.2) | 2 (1.9) | 3 (2.0) |
| Registered (out of those consented) | 99 (99.0) | 162 (97.6) | 261 (98.1) |
| Not registered (out of those consented) missing data | 1 (1.0) 0 | 3 (1.8) 1 | 4 (1.5) 1 |
| Does not have formal diagnosis of dementia | 1 (1.0) | 0 (0.0) | 1 (0.4) |
| Moved out of care home | 0 (0.0) | 1 (0.6) | 1 (0.4) |
| Died | 0 (0.0) | 1 (0.6) | 1 (0.4) |
| In hospital | 0 (0.0) | 1 (0.6) | 1 (0.4) |
| Characteristic | Original cohort (N = 1564) | Additional residents (N = 877)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Yorkshire (N = 656) | London (N = 419) | Oxford (N = 489) | Total (N = 1564) | Yorkshire (N = 341) | London (N = 230) | Oxford (N = 306) | Total (N = 877) | |
| Age at registration (years), mean (SD) | 85.3 (8.00) | 84.7 (8.43) | 85.1 (8.20) | 85.1 (8.18) | 85.0 (7.64) | 84.2 (8.65) | 86.0 (8.11) | 85.1 (8.10) |
| Length of stay in care home (years), mean (SD) | 2.1 (2.29) | 2.4 (2.44) | 2.5 (2.70) | 2.3 (2.48) | 1.4 (1.79) | 1.7 (2.63) | 1.7 (2.17) | 1.6 (2.17) |
| Sex, number of females (%) | 483 (73.6) | 301 (71.8) | 356 (72.8) | 1140 (72.9) | 248 (72.7) | 164 (71.3) | 213 (69.6) | 625 (71.3) |
| Ethnicity, n (%) | ||||||||
| White | 642 (97.9) | 367 (87.6) | 474 (96.9) | 1483 (94.8) | 338 (99.1) | 208 (90.4) | 300 (98.0) | 846 (96.5) |
| Other | 7 (1.1) | 40 (9.5) | 8 (1.6) | 55 (3.5) | 0 (0.0) | 20 (8.7) | 5 (1.6) | 25 (2.9) |
| Missing | 7 (1.1) | 12 (2.9) | 7 (1.4) | 26 (1.7) | 3 (0.9) | 2 (0.9) | 1 (0.3) | 6 (0.7) |
| Funding type, n (%) | ||||||||
| Local Authority | 297 (45.3) | 179 (42.7) | 265 (54.2) | 741 (47.4) | 145 (42.5) | 92 (40.0) | 167 (54.6) | 404 (46.1) |
| Continuing health care | 58 (8.8) | 35 (8.4) | 22 (4.5) | 115 (7.4) | 4 (1.2) | 16 (7.0) | 1 (0.3) | 21 (2.4) |
| Self-funded | 226 (34.5) | 141 (33.7) | 188 (38.4) | 555 (35.5) | 116 (34.0) | 80 (34.8) | 122 (39.9) | 318 (36.3) |
| Local Authority and self-funded | 59 (9.0) | 1 (0.2) | 9 (1.8) | 69 (4.4) | 57 (16.7) | 7 (3.0) | 4 (1.3) | 68 (7.8) |
| Missing | 16 (2.4) | 63 (15.0) | 5 (1.0) | 84 (5.4) | 19 (5.6) | 35 (15.2) | 12 (3.9) | 66 (7.5) |
| Person who gave consent | Original cohort, n (%) | Additional residents, n (%) | ||
|---|---|---|---|---|
| Total (N = 726) | Control (N = 99) | Intervention (N = 162) | Total (N = 261) | |
| Resident | 145 (20.0) | 22 (22.2) | 36 (22.2) | 58 (22.2) |
| Personal Consultee | 263 (36.2) | 34 (34.3) | 39 (24.1) | 73 (28.0) |
| Nominated Consultee | 318 (43.8) | 43 (43.4) | 87 (53.7) | 130 (49.8) |
Staff and relative/friend
| Staff measures | Baseline | 6 months | 16 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 86) | Intervention (n = 260) | Total (n = 346) | Control (n = 84) | Intervention (n = 112) | Total (n = 196) | Control (n = 50) | Intervention (n = 132) | Total (n = 182) | |
| Total SCIDS score, mean (SD) missing data | 53.2 (8.96) 1 | 53.7 (9.24) 5 | 53.6 (9.16) 6 | 55 (8.64) 1 | 53.5 (8.56) 2 | 54.1 (8.6) 3 | 58.4 (7.97) 1 | 56.8 (8.3) 1 | 57.2 (8.22) 2 |
| Professionalism | 16.7 (2.61) 0 | 17 (2.75) 4 | 16.9 (2.72) 4 | 17.2 (2.72) 3 | 16.8 (2.52) 2 | 17 (2.6) 5 | 18 (2.17) 1 | 17.6 (2.4) 2 | 17.7 (2.34) 3 |
| Building relationships | 11.7 (2.37) 0 | 11.8 (2.36) 4 | 11.8 (2.36) 4 | 12.3 (2.24) 0 | 11.9 (2.18) 1 | 12.1 (2.21) 1 | 13 (2.39) 1 | 12.6 (2.24) 1 | 12.7 (2.28) 2 |
| Core challenges | 11.9 (2.84) 1 | 11.9 (2.9) 6 | 11.9 (2.88) 7 | 12.2 (2.71) 1 | 12 (2.63) 3 | 12.1 (2.66) 4 | 13.6 (2.51) 1 | 12.9 (2.61) 1 | 13.1 (2.59) 2 |
| Sustaining personhood | 12.9 (2.31) 0 | 13 (2.43) 5 | 13 (2.39) 5 | 13.4 (2.27) 2 | 12.8 (2.47) 1 | 13.1 (2.4) 3 | 13.8 (1.96) 1 | 13.6 (2.11) 2 | 13.6 (2.07) 3 |
| Number of booklets circulated to staff | 525 | 1143 | 1668 | 546 | 848 | 1394 | 526 | 1108 | 1634 |
| QUALID relative/proxy | Baseline | 6 months | 16 months (original cohort) | 16 months (cross-sectional cohort) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 96) | Intervention (n = 101) | Total (n = 197) | Control (n = 85) | Intervention (n = 85) | Total (n = 170) | Control (n = 63) | Intervention (n = 55) | Total (n = 118) | Control (n = 64) | Intervention (n = 55) | Total (n = 119) | |
| Mean (SD) missing data | 22.5 (7.49) 14 | 21.6 (6.86) 20 | 22 (7.18) 34 | 21.6 (7.18) 23 | 22.1 (8.89) 20 | 21.8 (8.07) 43 | 23 (6.24) 25 | 23.1 (8.41) 24 | 23 (7.24) 49 | 23 (6.15) 25 | 23.1 (8.41) 24 | 23 (7.18) 49 |
| Median (interquartile range) | 21 (17–28) | 21 (17–25) | 21 (17–26) | 20.9 (15–25) | 20 (14.3–30) | 20.9 (15–27) | 23 (18–26) | 22 (16.5–29) | 22 (18–28) | 23 (18–26) | 22 (16.5–29) | 22.5 (18–28) |
Intervention
| Hub | Care home | Cycle 1 | Cycle 2 | Cycle 3 | Number of cycles completed to at least an acceptable level | Number of cycles completed to at least a partial level |
|---|---|---|---|---|---|---|
| Yorkshire | 1 | Acceptable | None | None | One cycle | One cycle |
| 2 | None | None | None | No cycles | No cycles | |
| 3 | Acceptable | Acceptable | None | Two cycles | Two cycles | |
| 8 | None | None | None | No cycles | No cycles | |
| 9 | Acceptable | Partial | None | One cycle | Two cycles | |
| 17 | Acceptable | Partial | Partial | One cycle | Three cycles | |
| 24 | Acceptable | None | None | One cycle | One cycle | |
| 32 | Acceptable | Acceptable | None | Two cycles | Two cycles | |
| 33 | Partial | None | None | No cycles | One cycle | |
| 34 | None | None | None | No cycles | No cycles | |
| 38 | Partial | None | None | No cycles | One cycle | |
| 44 | Acceptable | None | None | One cycle | One cycle | |
| 48 | Acceptable | Acceptable | Acceptable | Three cycles | Three cycles | |
| Oxford | 4 | Acceptable | None | None | One cycle | One cycle |
| 5 | Acceptable | Partial | None | One cycle | Two cycles | |
| 6 | Acceptable | None | None | One cycle | One cycle | |
| 11 | Acceptable | None | None | One cycle | One cycle | |
| 12 | Partial | None | None | No cycles | One cycle | |
| 14 | Partial | None | None | No cycles | One cycle | |
| 16 | Acceptable | Acceptable | Acceptable | Three cycles | Three cycles | |
| 23 | Acceptable | Partial | Partial | One cycle | Three cycles | |
| 25 | Acceptable | Acceptable | None | Two cycles | Two cycles | |
| London | 10 | Acceptable | None | None | One cycle | One cycle |
| 19 | Acceptable | None | None | One cycle | One cycle | |
| 20 | Acceptable | Partial | Partial | One cycle | Three cycles | |
| 26 | Acceptable | None | None | One cycle | One cycle | |
| 31 | Acceptable | Acceptable | None | Two cycles | Two cycles | |
| 39 | Acceptable | None | None | One cycle | One cycle | |
| 40 | Acceptable | Acceptable | Acceptable | Three cycles | Three cycles | |
| 42 | Acceptable | None | None | One cycle | One cycle | |
| 47 | Acceptable | Acceptable | Acceptable | Three cycles | Three cycles |
Briefing
| Characteristic | Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) |
|---|---|---|---|
| Number of formal sessions, n (%) | |||
| 1 | 9 (29.0) | 7 (22.6) | 3 (9.7) |
| 2 | 4 (12.9) | 1 (3.2) | 1 (3.2) |
| 3 | 2 (6.5) | 1 (3.2) | 1 (3.2) |
| Missing | 16 (51.6) | 22 (71.0) | 26 (83.9) |
| Length of formal sessions (minutes) | |||
| Mean (SD) missing data | 71.7 (61.72) 16 | 66.9 (34.94) 23 | 93.8 (41.31) 27 |
| Median (range) | 40 (20–240) | 60 (30–140) | 97.5 (45–135) |
| Total number of staff members who attended | |||
| Mean (SD) missing data | 10.1 (4.52) 18 | 15.8 (7.44) 23 | 18.0 (8.19) 28 |
| Median (range) | 10 (3–20) | 14.5 (8–28) | 20 (9–25) |
| Number of direct care staff members who attended | |||
| Mean (SD) missing data | 11.4 (6.23) 26 | 15.3 (6.08) 27 | 14.0 (5.29) 28 |
| Median (range) | 13.0 (3.0–17.0) | 13.5 (10.0–24.0) | 16.0 (8.0–18.0) |
| Time between formal session and randomisation (months) | |||
| Mean (SD) missing data | 2.8 (0.98) 16 | 8.7 (2.79) 23 | 13.5 (1.19) 26 |
| Median (range) | 2.7 (1.0–4.9) | 8.1 (4.4–13.0) | 13.6 (12.4–15.3) |
| Informal briefing sessions held, n (%) missing data | |||
| Yes | 15 (48.4) 15 | 10 (32.3) 20 | 3 (9.7) 26 |
| No | 1 (3.2) 0 | 1 (3.2) 0 | 2 (6.5) 0 |
| Number of staff informally briefed | |||
| Mean (SD) missing data | 10.5 (7.51) 17 | 13.1 (10.89) 23 | 19.3 (1.15) 28 |
| Median (range) | 8.5 (2.0–30.0) | 7.0 (4.0–31.0) | 20.0 (18.0–20.0) |
FIGURE 7.
Time between care home randomisation and briefing sessions.
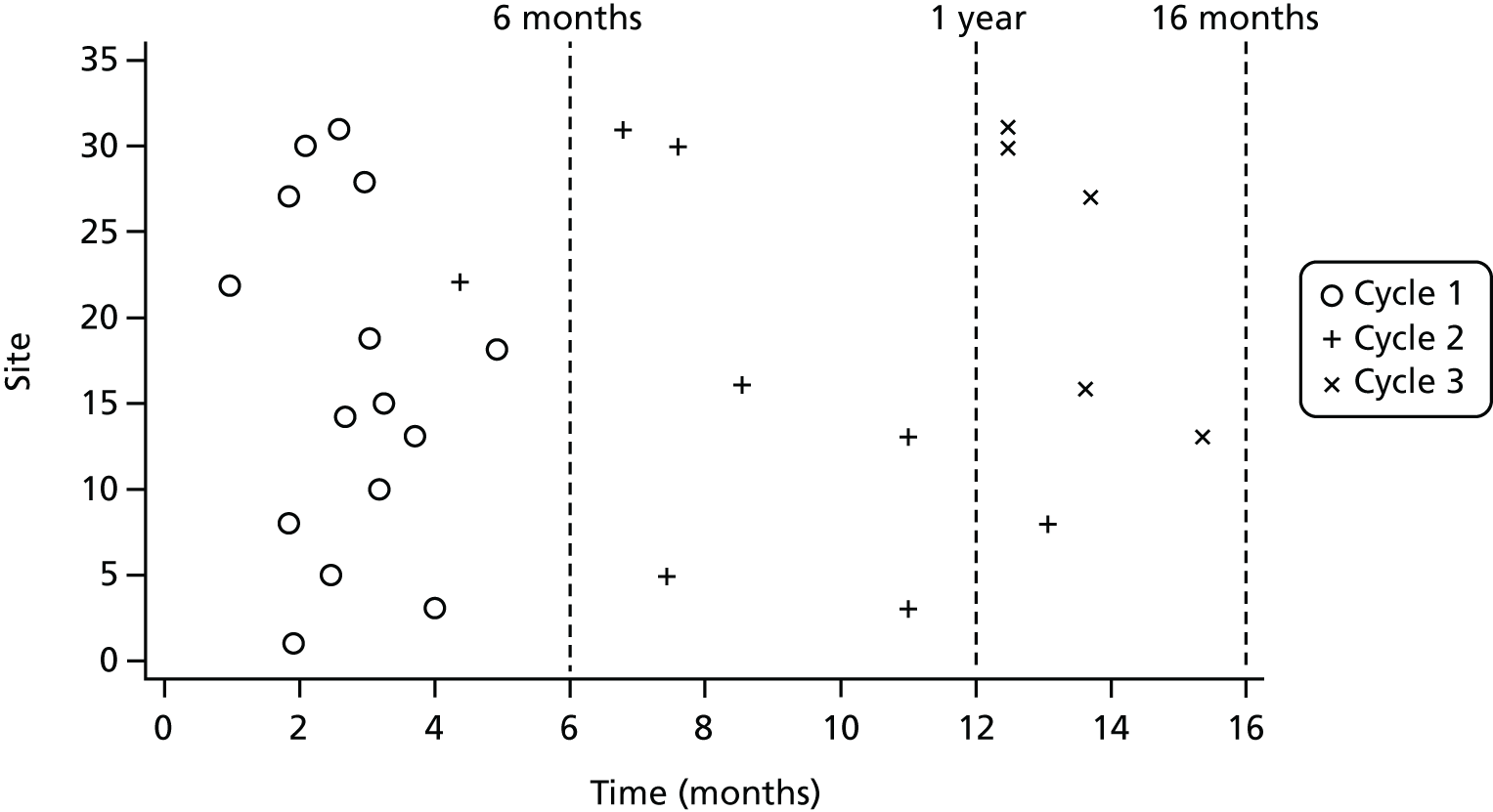
Observation
| Characteristic | Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) |
|---|---|---|---|
| Number of mappers who made observations, n (%) | |||
| 1 | 1 (3.2) | 1 (3.2) | 1 (3.2) |
| 2 | 18 (58.1) | 10 (32.3) | 4 (12.9) |
| Missing | 12 (38.7) | 20 (64.5) | 26 (83.9) |
| Number of observation periods | |||
| Mean (SD) missing data | 4.3 (2.05) 16 | 3.9 (1.89) 23 | 2.0 (0.00) 28 |
| Median (range) | 4 (2–8) | 4 (2–8) | 2 (2–2) |
| Time between first and last observation (days) | |||
| Mean (SD) missing data | 3.0 (7.05) 13 | 4.5 (14.75) 20 | 0.0 (0.00) 26 |
| Median (range) | 0 (0–29) | 0 (0–49) | 0 (0–0) |
| Total mapping time (hours) | |||
| Mean (SD) missing data | 8.9 (2.76) 16 | 9.4 (2.30) 23 | 7.8 (0.43) 28 |
| Median (range) | 9.2 (4.0–12.4) | 9.9 (6.5–12.3) | 8.0 (7.3–8.0) |
| Use of all codes, n (%) missing data | |||
| Yes | 10 (32.3) 13 | 7 (22.6) 20 | 2 (6.5) 26 |
| Total number of residents observed | |||
| Mean (SD) missing data | 5.4 (1.79) 13 | 5.7 (2.41) 20 | 5.2 (1.79) 26 |
| Median (range) | 5 (2–8) | 6 (2–10) | 4 (4–8) |
| Number of residents observed with less than 3 hours of observations | |||
| Mean (SD) missing data | 2.2 (1.72) 13 | 1.1 (2.39) 20 | 1.0 (1.41) 26 |
| Median (range) | 2 (0–5) | 0 (0–8) | 0 (0–3) |
| Number of residents observed with at least 3 hours of observations | |||
| Mean (SD) missing data | 3.3 (1.81) 13 | 4.6 (2.06) 20 | 4.2 (1.48) 26 |
| Median (range) | 3 (0–6) | 5 (2–8) | 4 (2–6) |
| Percentage of residents observed with at least 3 hours of observations | |||
| Mean (SD) missing data | 58.7 (33.75) 13 | 86.0 (25.13) 20 | 82.5 (24.37) 26 |
| Median (range) | 60 (0–100) | 100 (20–100) | 100 (50–100) |
| Quality | Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) |
|---|---|---|---|
| Two mappers completed at least 4 hours of observations over a 1-week period, n (%) | |||
| Yes, completed fully | 13 (41.9) | 7 (22.6) | 3 (9.7) |
| Completed partially | 6 (19.4) | 4 (12.9) | 2 (6.5) |
| Not completed | 12 (38.7) | 20 (64.5) | 26 (83.9) |
| At least five residents observed in total with at least 3 hours of available data on each resident, n (%) | |||
| Yes, completed fully | 2 (6.5) | 4 (12.9) | 1 (3.2) |
| Completed partially | 16 (51.6) | 7 (22.6) | 4 (12.9) |
| Not completed | 13 (41.9) | 20 (64.5) | 26 (83.9) |
| Mappers using all four of the coding frames and making at least minimal qualitative notes, n (%) | |||
| Yes, completed fully | 9 (29.0) | 5 (16.1) | 2 (6.5) |
| Completed partially | 9 (29.0) | 6 (19.4) | 3 (9.7) |
| Not completed | 13 (41.9) | 20 (64.5) | 26 (83.9) |
Feedback
| Characteristic | Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) |
|---|---|---|---|
| Number of mappers participating in the feedback process, n (%) | |||
| 1 | 1 (3.2) | 2 (6.5) | 0 (0) |
| 2 | 13 (41.9) | 7 (22.6) | 3 (9.7) |
| Missing | 17 (54.8) | 22 (71.0) | 28 (90.3) |
| Formal feedback sessions held, n (%) missing data | |||
| Yes | 12 (38.7) 17 | 8 (25.8) 21 | 3 (9.7) 27 |
| No | 2 (6.5) 0 | 2 (6.5) 0 | 1 (3.2) 0 |
| Total number of formal feedback sessions | |||
| Mean (SD) missing data | 1.8 (0.83) 19 | 1.4 (0.79) 24 | 1.0 (0.00) 28 |
| Median (range) | 2 (1–3) | 1 (1–3) | 1 (1–1) |
| Total length of formal feedback sessions (hours) | |||
| Mean (SD) missing data | 2.0 (2.26) 20 | 1.2 (0.67) 25 | 0.8 (0.29) 28 |
| Median (range) | 1.2 (0.5–8.4) | 1.0 (0.5–2.3) | 1.0 (0.5–1.0) |
| Time between first and last feedback session (days) | |||
| Mean (SD) missing data | 2.8 (5.75) 19 | 1.3 (2.98) 24 | 0.0 (0.00) 28 |
| Median (range) | 0 (0–20) | 0 (0–8) | 0 (0–0) |
| Total number of staff members who attended formal feedback sessions | |||
| Mean (SD) missing data | 9.6 (4.56) 19 | 12.3 (4.46) 25 | 12.3 (4.51) 28 |
| Median (range) | 9.0 (2–17) | 11.5 (7–18) | 12.0 (8–17) |
| Total number of direct care staff members who attended formal feedback sessions | |||
| Mean (SD) missing data | 8.0 (2.65) 28 | 8.5 (2.12) 29 | 12.0 (–) 30 |
| Median (range) | 9 (5–10) | 8.5 (7–10) | 12 (12–12) |
| Characteristic | Cycle 1 (n = 31) | Cycle 2 (n = 31) | Cycle 3 (n = 31) |
|---|---|---|---|
| Number of care home feedback points | |||
| Mean (SD) missing data | 5.0 (3.06) 21 | 3.7 (1.21) 25 | 6.0 (5.72) 27 |
| Median (range) | 4.5 (2–13) | 3 (3–6) | 5.5 (0–13) |
| Total number of residents with feedback points | |||
| Mean (SD) missing data | 4.4 (1.78) 19 | 4.2 (2.23) 25 | 3.5 (1.73) 27 |
| Median (range) | 4.5 (1–7) | 5 (1–6) | 4 (1–5) |
| Number of resident feedback points | |||
| Mean (SD) missing data | 3.2 (2.12) 20 | 2.5 (0.93) 25 | 2.3 (0.96) 27 |
| Median (range) | 2.8 (0.8–7.8) | 2.9 (1.0–3.3) | 2.4 (1.3–3.3) |
| Cycle 1 to cycle 2 | Cycle 2 to cycle 3 | ||
| Percentage of achieved resident action plans set in previous cycle | |||
| Mean (SD) missing data | 51.6 (41.75) 22 | 73.8 (43.38) 26 | |
| Median (range) | 64.7 (0–100) | 100.0 (0–100) | |
| Percentage of achieved care home action plans set in previous cycle | |||
| Mean (SD) missing data | 54.8 (44.72) 22 | 79.2 (25.00) 27 | |
| Median (range) | 60.0 (0–100) | 83.3 (50–100) | |
Action-planning
| Characteristic | Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) |
|---|---|---|---|
| Care home action plan received, n (%) missing data | |||
| Yes | 13 (41.9) 12 | 6 (19.4) 20 | 4 (12.9) 26 |
| No | 6 (19.4) | 5 (16.1) | 1 (3.2) |
| Number of care home action points | |||
| Mean (SD) | 4.9 (3.20) 18 | 5.2 (4.83) 25 | 5.0 (2.16) 27 |
| Median (range) | 4 (2–14) | 3 (3–15) | 4.5 (3–8) |
| Resident action plans received, n (%) missing data | |||
| Yes | 13 (41.9) 12 | 6 (19.4) 20 | 3 (9.7) 26 |
| No | 6 (19.4) | 5 (16.1) | 2 (6.5) |
| Total number of residents with action points | |||
| Mean (SD) | 5.5 (1.85) 18 | 5.8 (2.86) 25 | 4.7 (1.15) |
| Median (range) | 5 (3–8) | 5.5 (2–10) | 4 (4–6) |
| Number of resident action points | |||
| Mean (SD) | 2.0 (1.95) 18 | 2.0 (1.24) 25 | 1.8 (1.77) 28 |
| Median (range) | 1.6 (0.1–7.8) | 2.2 (0.1–3.3) | 1.3 (0.3–3.8) |
| Cycle 1 (N = 31) | Cycle 2 (N = 31) | Cycle 3 (N = 31) | |
|---|---|---|---|
| Standard care home template used, n (%) missing data | |||
| Yes | 13 (41.9) 18 | 6 (19.4) 25 | 3 (9.7) 27 |
| No | 0 (0) | 0 (0) | 1 (3.2) |
| Standard resident template used, n (%) missing data | |||
| Yes | 12 (38.7) 18 | 6 (19.4) 25 | 2 (6.5) 28 |
| No | 1 (3.2) | 0 (0) | 1 (3.2) |
| At least one action point per observed resident, n (%) missing data | |||
| Yes | 5 (16.1) 18 | 4 (12.9) 25 | 1 (3.2) 28 |
| No | 8 (25.8) | 2 (6.5) | 2 (6.5) |
Resident deaths
| Deaths | Control (N = 308) | Intervention (N = 418) | Total (N = 726) |
|---|---|---|---|
| Died, n (%) | 111 (36.0) | 161 (38.5) | 272 (37.5) |
| Place of death, n (%) | |||
| Care home | 89 (80.2) | 135 (83.9) | 224 (82.4) |
| Hospital | 22 (19.8) | 26 (16.1) | 48 (17.6) |
| Percentage of deaths per care home at 16 months | |||
| Mean (SD) | 36 (12.3) | 39 (14.0) | 37 (13.4) |
| Median (range) | 41 (7–60) | 36 (10–75) | 36 (7–75) |
Outcomes
Residents
| a.m., mean (SD) number completed | p.m., mean (SD) number completed | |||||
|---|---|---|---|---|---|---|
| Control (n = 308) | Intervention (n = 418) | Total (n = 726) | Control (n = 308) | Intervention (n = 418) | Total (n = 726) | |
| Baseline CMAI-O total score | 31.1 (3.1) 184 | 30.5 (2.7) 266 | 30.8 (2.9) 450 | 32.0 (3.7) 198 | 31.5 (3.8) 272 | 31.7 (3.8) 470 |
| Subscales | ||||||
| Aggressive behaviour | 9.2 (0.6) 185 | 9.1 (0.5) 266 | 9.1 (0.6) 451 | 9.4 (1.1) 198 | 9.3 (1.0) 272 | 9.3 (1.1) 470 |
| Physically non-aggressive | 7.2 (1.8) 184 | 6.9 (1.7) 265 | 7.0 (1.8) 449 | 7.6 (2.1) 198 | 7.3 (2.0) 272 | 7.4 (2.0) 470 |
| Verbally agitated | 5.5 (1.3) 184 | 5.3 (0.9) 266 | 5.4 (1.1) 450 | 5.6 (1.4) 198 | 5.6 (1.6) 272 | 5.6 (1.6) 470 |
| Other | 9.3 (0.9) 184 | 9.2 (0.7) 266 | 9.2 (0.8) 450 | 9.4 (1.1) 198 | 9.3 (0.8) 272 | 9.3 (0.9) 470 |
| 6-month CMAI-O total score | 31.1 (4) 159 | 31.3 (3.6) 209 | 31.2 (3.8) 368 | 31.6 (3.6) 151 | 32.0 (3.9) 206 | 31.8 (3.8) 357 |
| Subscales | ||||||
| Aggressive behaviour | 9.3 (1.0) 159 | 9.2 (0.6) 209 | 9.2 (0.8) 368 | 9.3 (0.9) 151 | 9.3 (0.8) 206 | 9.3 (0.9) 357 |
| Physically non-aggressive | 6.8 (1.8) 159 | 6.9 (1.8) 209 | 6.9 (1.8) 368 | 7.1 (2.0) 151 | 7.4 (2.0) 206 | 7.3 (2.0) 357 |
| Verbally agitated | 5.7 (1.7) 159 | 5.6 (1.7) 209 | 5.6 (1.7) 368 | 5.8 (1.7) 151 | 5.8 (1.9) 206 | 5.8 (1.8) 357 |
| Other | 9.4 (1.0) 159 | 9.6 (1.2) 209 | 9.5 (1.1) 368 | 9.4 (1.0) 151 | 9.5 (1.1) 206 | 9.5 (1.1) 357 |
| 16-month CMAI-O total score | 31.2 (3.8) 102 | 30.4 (3.2) 129 | 30.7 (3.5) 231 | 31.3 (4.1) 97 | 31 (3.9) 124 | 31.1 (4.0) 221 |
| Subscales | ||||||
| Aggressive behaviour | 9.3 (1.1) 102 | 9.3 (1.0) 129 | 9.3 (1.0) 231 | 9.3 (1.2) 97 | 9.4 (1.3) 124 | 9.4 (1.3) 221 |
| Physically non-aggressive | 6.7 (1.5) 102 | 6.5 (1.5) 129 | 6.6 (1.5) 231 | 6.8 (1.5) 97 | 6.7 (1.9) 124 | 6.8 (1.8) 221 |
| Verbally agitated | 5.8 (2.2) 102 | 5.4 (1.4) 129 | 5.6 (1.8) 231 | 5.8 (2.0) 97 | 5.5 (1.5) 124 | 5.6 (1.7) 221 |
| Other | 9.4 (1.0) 102 | 9.2 (0.7) 129 | 9.3 (0.8) 231 | 9.4 (1.0) 97 | 9.3 (1.0) 124 | 9.4 (1.0) 221 |
| Baseline PAS score | 1.0 (1.5) 185 | 0.8 (1.5) 266 | 0.8 (1.5) 451 | 1.3 (1.6) 197 | 1.3 (2.2) 271 | 1.3 (2.0) 468 |
| 6-month PAS score | 0.9 (1.9) 159 | 0.9 (1.4) 209 | 0.9 (1.7) 368 | 1.1 (1.9) 151 | 1.2 (1.8) 204 | 1.2 (1.8) 355 |
| 16-month PAS score | 1.0 (1.8) 102 | 0.7 (1.6) 129 | 0.9 (1.7) 231 | 1.2 (2.1) 97 | 0.9 (1.9) 123 | 1.0 (2.0) 220 |
FIGURE 8.
Graphical depiction of change in average CMAI scores in care homes (cross-sectional), by treatment arm (16 months–baseline).
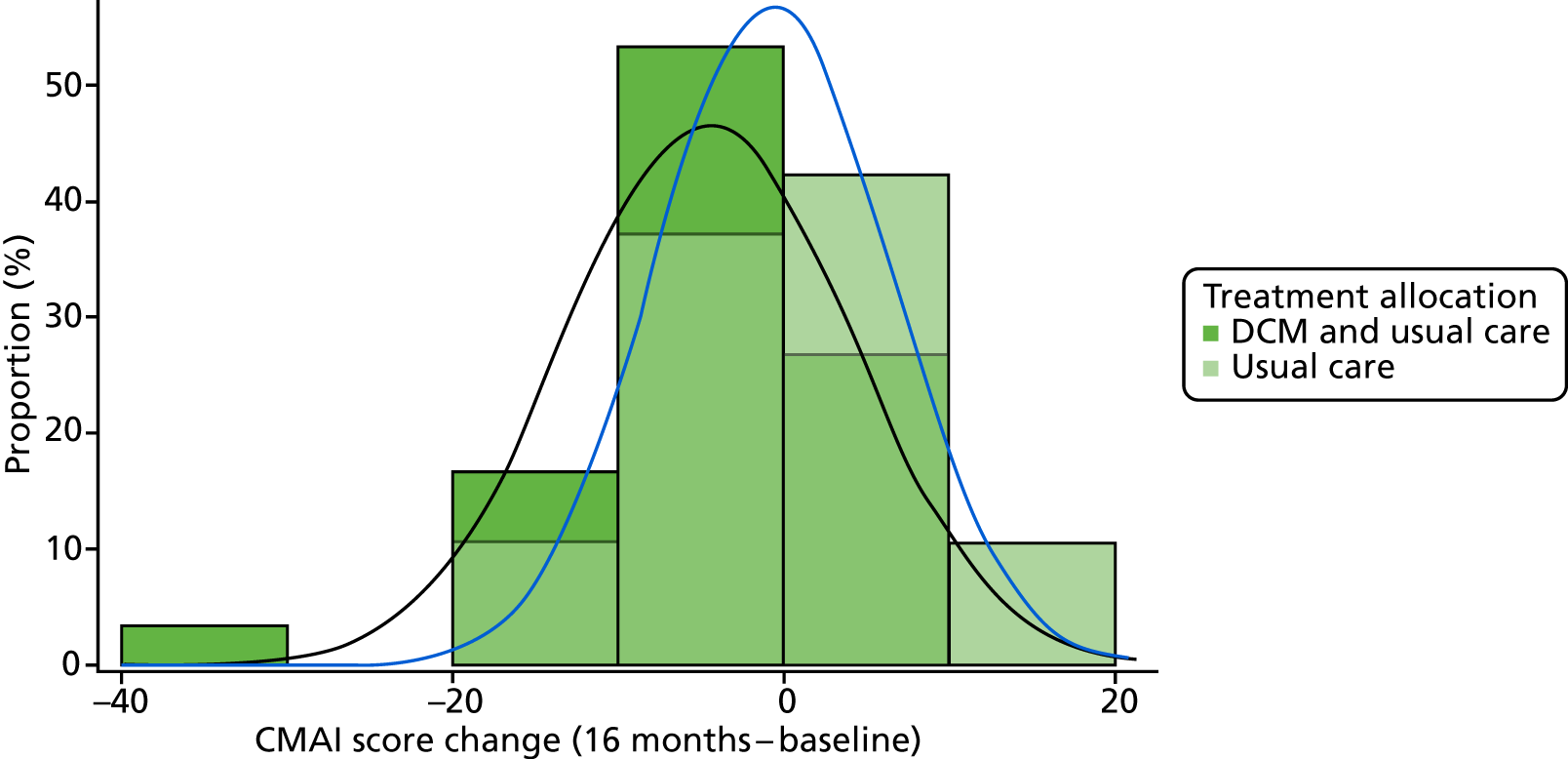
FIGURE 9.
Graphical depiction of change in CMAI scores (closed cohort), by treatment arm: (a) 16 months–baseline; and (b) 6 months–baseline.
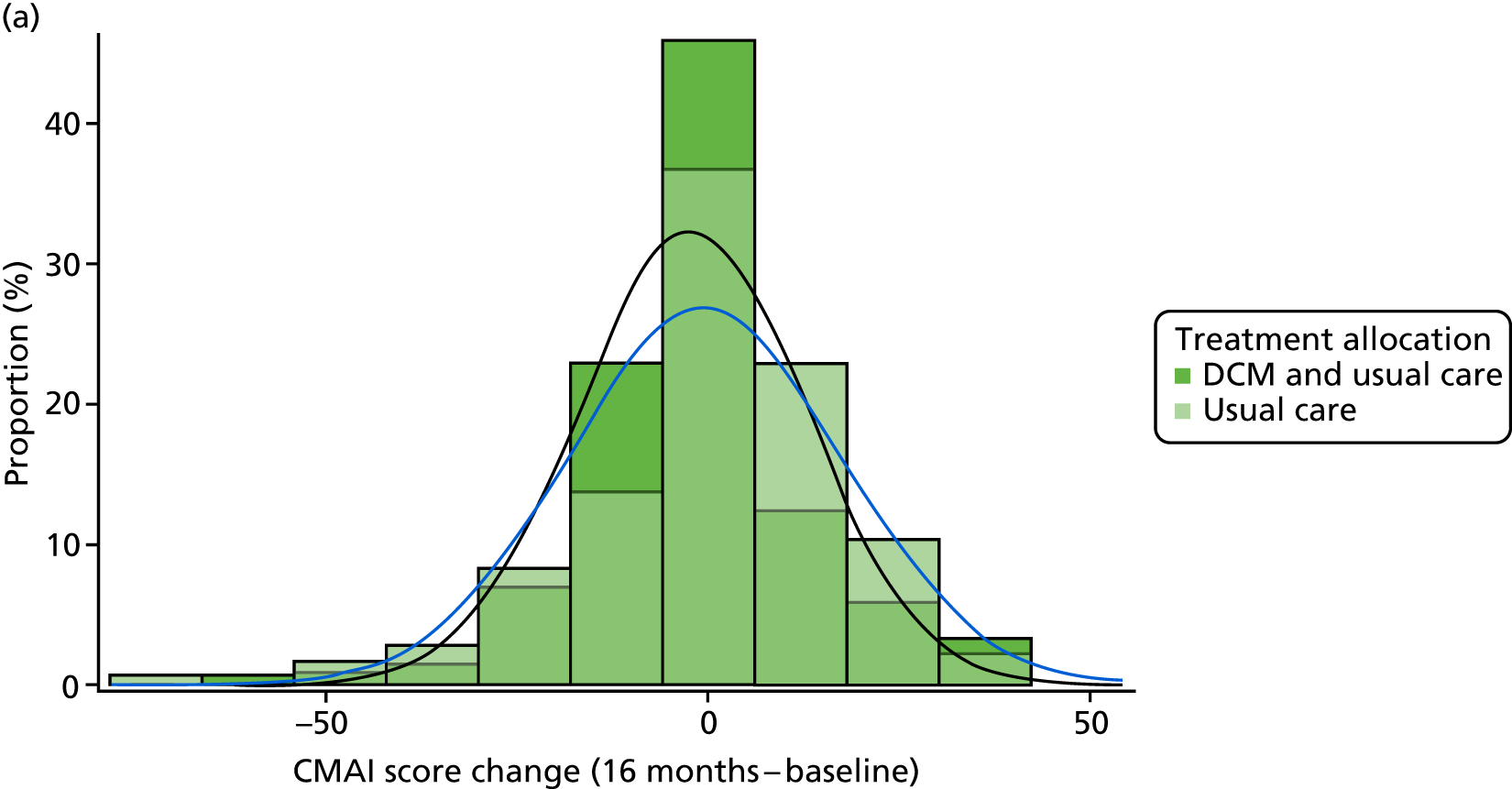

| Baseline, mean (SD) n | 6 months, mean (SD) n | 16 months (original cohort), mean (SD) n | 16 months (cross-sectional cohort), mean (SD) n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 287) | Intervention (N = 388) | Total (N = 675) | |
| Total CMAI-O score (a.m.) | 31.1 (3.1) 184 | 30.5 (2.7) 266 | 30.8 (2.9) 450 | 31.1 (4) 159 | 31.3 (3.6) 209 | 31.2 (3.8) 368 | 31.2 (3.8) 102 | 30.4 (3.2) 129 | 30.7 (3.5) 231 | 31.1 (3.8) 156 | 30.5 (3.3) 209 | 30.8 (3.5) 365 |
| CMAI-O subscales (a.m.) | ||||||||||||
| Verbally agitated | 5.5 (1.3) 184 | 5.3 (0.9) 266 | 5.4 (1.1) 450 | 5.7 (1.7) 159 | 5.6 (1.7) 209 | 5.6 (1.7) 368 | 5.8 (2.2) 102 | 5.4 (1.4) 129 | 5.6 (1.8) 231 | 5.8 (2.2) 156 | 5.5 (1.5) 209 | 5.6 (1.8) 365 |
| Physically non-aggressive | 7.2 (1.8) 184 | 6.9 (1.7) 265 | 7 (1.8) 449 | 6.8 (1.8) 159 | 6.9 (1.8) 209 | 6.9 (1.8) 368 | 6.7 (1.5) 102 | 6.5 (1.5) 129 | 6.6 (1.5) 231 | 6.7 (1.4) 156 | 6.5 (1.5) 209 | 6.6 (1.4) 365 |
| Other | 9.3 (0.9) 184 | 9.2 (0.7) 266 | 9.2 (0.8) 450 | 9.4 (1) 159 | 9.6 (1.2) 209 | 9.5 (1.1) 368 | 9.4 (1) 102 | 9.2 (0.7) 129 | 9.3 (0.8) 231 | 9.3 (1) 156 | 9.2 (0.7) 209 | 9.2 (0.8) 365 |
| Aggressive behaviour | 9.2 (0.6) 185 | 9.1 (0.5) 266 | 9.1 (0.6) 451 | 9.3 (1) 159 | 9.2 (0.6) 209 | 9.2 (0.8) 368 | 9.3 (1.1) 102 | 9.3 (1) 129 | 9.3 (1) 231 | 9.3 (0.9) 156 | 9.3 (1) 209 | 9.3 (1) 365 |
| Total CMAI-O score (p.m.) | 32 (3.7) 198 | 31.5 (3.8) 272 | 31.7 (3.8) 470 | 31.6 (3.6) 151 | 32 (3.9) 206 | 31.8 (3.8) 357 | 31.3 (4.1) 97 | 31 (3.9) 124 | 31.1 (4) 221 | 31.4 (3.8) 148 | 31.1 (3.9) 206 | 31.2 (3.9) 354 |
| Median (interquartile range) | 31 (29–34) | 30 (29–32.6) | 30 (29–33) | 30 (29–33) | 30 (29–34) | 30 (29–33) | 29 (29–32) | 29 (29–32) | 29 (29–32) | 29 (29–32) | 29 (29–32) | 29 (29–32) |
| CMAI-O subscales (p.m.) | ||||||||||||
| Verbally agitated | 5.6 (1.4) 198 | 5.6 (1.6) 272 | 5.6 (1.6) 470 | 5.8 (1.7) 151 | 5.8 (1.9) 206 | 5.8 (1.8) 357 | 5.8 (2) 97 | 5.5 (1.5) 124 | 5.6 (1.7) 221 | 5.8 (1.9) 148 | 5.7 (1.7) 206 | 5.7 (1.8) 354 |
| Physically non-aggressive | 7.6 (2.1) 198 | 7.3 (2) 272 | 7.4 (2) 470 | 7.1 (2) 151 | 7.4 (2) 206 | 7.3 (2) 357 | 6.8 (1.5) 97 | 6.7 (1.9) 124 | 6.8 (1.8) 221 | 6.9 (1.5) 148 | 6.8 (1.9) 206 | 6.9 (1.8) 354 |
| Other | 9.4 (1.1) 198 | 9.3 (0.8) 272 | 9.3 (0.9) 470 | 9.4 (1) 151 | 9.5 (1.1) 206 | 9.5 (1.1) 357 | 9.4 (1) 97 | 9.3 (1) 124 | 9.4 (1) 221 | 9.3 (0.9) 148 | 9.3 (0.9) 206 | 9.3 (0.9) 354 |
| Aggressive behaviour | 9.4 (1.1) 198 | 9.3 (1) 272 | 9.3 (1.1) 470 | 9.3 (0.9) 151 | 9.3 (0.8) 206 | 9.3 (0.9) 357 | 9.3 (1.2) 97 | 9.4 (1.3) 124 | 9.4 (1.3) 221 | 9.3 (1.1) 148 | 9.3 (1.2) 206 | 9.3 (1.1) 354 |
| Total PAS score (a.m.) | 1 (1.5) 185 | 0.8 (1.5) 266 | 0.8 (1.5) 451 | 0.9 (1.9) 159 | 0.9 (1.4) 209 | 0.9 (1.7) 368 | 1 (1.8) 102 | 0.7 (1.6) 129 | 0.9 (1.7) 231 | 1.1 (1.9) 156 | 0.8 (1.7) 209 | 0.9 (1.8) 365 |
| Total PAS score (p.m.) | 1.3 (1.6) 197 | 1.3 (2.2) 271 | 1.3 (2) 468 | 1.1 (1.9) 151 | 1.2 (1.8) 204 | 1.2 (1.8) 355 | 1.2 (2.1) 97 | 0.9 (1.9) 123 | 1 (2) 220 | 1.2 (1.9) 148 | 0.9 (1.8) 205 | 1 (1.8) 353 |
Sensitivity analyses
| Analysis | Adjusted mean in control | Adjusted mean in intervention | Estimated mean difference | 95% CI | p-value | Adjusted ICC for intervention | Adjusted ICC for control | n |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| CMAI score | 43.44 | 44.04 | 0.59 | –1.98 to 3.17 | 0.653 | 0.049 | 0.001 | 726 |
| CMAI-O score (a.m.) | 31.40 | 31.86 | 0.46 | –0.37 to 1.30 | 0.276 | 0.019 | 0.000 | 726 |
| CMAI-O score (p.m.) | 31.64 | 32.20 | 0.57 | –0.27 to 1.40 | 0.182 | 0.023 | 0.001 | 726 |
| PAS score (a.m.) | 1.04 | 1.18 | 0.14 | –0.24 to 0.52 | 0.473 | 0.022 | 0.001 | 726 |
| PAS score (p.m.) | 1.05 | 1.23 | 0.18 | –0.20 to 0.57 | 0.350 | 0.021 | 0.001 | 726 |
| 16 months | ||||||||
| CMAI-O score (a.m.) | 30.90 | 30.50 | –0.40 | –1.27 to 0.46 | 0.361 | 0.014 | 0.001 | 726 |
| CMAI-O score (p.m.) | 31.17 | 31.05 | –0.13 | –1.09 to 0.84 | 0.795 | 0.012 | 0.001 | 726 |
| PAS score (a.m.) | 0.91 | 0.79 | –0.12 | –0.52 to 0.28 | 0.547 | 0.008 | 0.001 | 726 |
| PAS score (p.m.) | 1.08 | 0.91 | –0.17 | –0.67 to 0.33 | 0.502 | 0.018 | 0.001 | 726 |
| Analysis | Estimated mean in control | Estimated mean in intervention | Estimated mean difference | 95% CI | p-value | Unadjusted ICC for intervention arm | Unadjusted ICC for control arm | Adjusted ICC for intervention arm | Adjusted ICC for control arm | n |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary analysis | 45.52 | 43.33 | –2.19 | –4.81 to 0.43 | 0.099 | 0.0546 | 0.0002 | 0 | 0 | 666 |
| Sensitivity analyses | ||||||||||
| Key sensitivity analysis | 46.01 | 43.73 | –2.28 | –4.98 to 0.42 | 0.095 | 0.0497 | 0.007 | 666 | ||
| 1. Adjusting for before–after eligibility change | 44.85 | 42.65 | –2.2 | –4.82 to 0.43 | 0.099 | 0.0546 | 0.0002 | 0 | 0 | 666 |
| 2. Care home size as a continuous variable | 45.48 | 43.16 | –2.32 | –5.03 to 0.38 | 0.090 | 0.0546 | 0.0002 | 0 | 0 | 661 |
| 3. Assuming homogeneous clustering across arms | 45.45 | 43.30 | –2.16 | –4.75 to 0.43 | 0.100 | 0.0497 | 0 | 666 | ||
| Analysis | Estimated mean in control | Estimated mean in intervention | Estimated mean difference | 95% CI | p-value | Unadjusted ICC for intervention | Unadjusted ICC for control | Adjusted ICC for intervention | Adjusted ICC for control | n |
|---|---|---|---|---|---|---|---|---|---|---|
| CMAI score | 46.00 | 42.44 | –3.57 | –6.65 to –0.48 | 0.025 | 0.0779 | 0.0003 | 0.0261 | 0.002 | 400 |
| PAS score (a.m.) | 1.10 | 0.66 | –0.44 | –1.04 to 0.15 | 0.140 | 0.0882 | 0.0012 | 0.0031 | 0.0024 | 170 |
| PAS score (p.m.) | 1.40 | 0.75 | –0.65 | –1.4 to 0.09 | 0.084 | 0.2394 | 0.0108 | 0.2265 | 0.0151 | 174 |
| CMAI-O score (a.m.) | 31.08 | 30.04 | –1.04 | –2.25 to 0.17 | 0.089 | 0.1189 | 0.0009 | 0.0251 | 0.0079 | 169 |
| CMAI-O score (p.m.) | 31.42 | 30.71 | –0.72 | –2.12 to 0.69 | 0.310 | 0.0985 | 0.0003 | 0.0272 | 0.0018 | 176 |
| Analysis | Estimated mean in control | Estimated mean in intervention | Estimated mean difference | 95% CI | p-value | Unadjusted ICC for intervention | Unadjusted ICC for control | Adjusted ICC for intervention | Adjusted ICC for control | n |
|---|---|---|---|---|---|---|---|---|---|---|
| CMAI score | 43.32 | 43.73 | 0.41 | –2.6 to 3.42 | 0.784 | 0.1356 | 0.011 | 0.0892 | 0 | 572 |
| CMAI-O score (a.m.) | 31.41 | 31.79 | 0.38 | –0.66 to 1.42 | 0.468 | 0.09 | 0.0001 | 0.0418 | 0.0006 | 270 |
| CMAI-O score (p.m.) | 31.79 | 32.34 | 0.55 | –0.73 to 1.83 | 0.393 | 0.121 | 0.0127 | 0.1445 | 0.0353 | 278 |
| PAS score (a.m.) | 0.90 | 1.08 | 0.18 | –0.29 to 0.66 | 0.446 | 0.112 | 0.0018 | 0.0862 | 0.0022 | 268 |
| PAS score (p.m.) | 1.09 | 1.23 | 0.14 | –0.42 to 0.7 | 0.621 | 0.1001 | 0 | 0.0779 | 0.0077 | 275 |
| Treatment-effect p-values | Deaths shifted by | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| –40 | –35 | –30 | –25 | –20 | –15 | –10 | –5 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | |
| Withdrawals and moves shifted by | |||||||||||||||||
| –40 | 0.028* | 0.025* | 0.023* | 0.022* | 0.02* | 0.019* | 0.019* | 0.018* | 0.018* | 0.018* | 0.018* | 0.019* | 0.02* | 0.021* | 0.023* | 0.025* | 0.028* |
| –35 | 0.029* | 0.027* | 0.025* | 0.023* | 0.021* | 0.02* | 0.02* | 0.019* | 0.019* | 0.019* | 0.019* | 0.02* | 0.021* | 0.022* | 0.024* | 0.026* | 0.029* |
| –30 | 0.03* | 0.028* | 0.026* | 0.024* | 0.023* | 0.022* | 0.021* | 0.02* | 0.02* | 0.02* | 0.021* | 0.021* | 0.022* | 0.024* | 0.026* | 0.028* | 0.03* |
| –25 | 0.032* | 0.03* | 0.027* | 0.026* | 0.024* | 0.023* | 0.022* | 0.022* | 0.021* | 0.021* | 0.022* | 0.023* | 0.024* | 0.025* | 0.027* | 0.029* | 0.032* |
| –20 | 0.034* | 0.031* | 0.029* | 0.027* | 0.026* | 0.024* | 0.024* | 0.023* | 0.023* | 0.023* | 0.023* | 0.024* | 0.025* | 0.027* | 0.029* | 0.031* | 0.034* |
| –15 | 0.036* | 0.033* | 0.031* | 0.029* | 0.027* | 0.026* | 0.025* | 0.025* | 0.025* | 0.025* | 0.025* | 0.026* | 0.027* | 0.029* | 0.031* | 0.033* | 0.036* |
| –10 | 0.038* | 0.036* | 0.033* | 0.031* | 0.03* | 0.028* | 0.027* | 0.027* | 0.027* | 0.027* | 0.027* | 0.028* | 0.029* | 0.031* | 0.033* | 0.036* | 0.039* |
| –5 | 0.041* | 0.038* | 0.036* | 0.034* | 0.032* | 0.031* | 0.03* | 0.029* | 0.029* | 0.029* | 0.029* | 0.03* | 0.032* | 0.033* | 0.036* | 0.038* | 0.041* |
| 0 | 0.044* | 0.041* | 0.038* | 0.036* | 0.034* | 0.033* | 0.032* | 0.032* | 0.031* | 0.031* | 0.032* | 0.033* | 0.034* | 0.036* | 0.038* | 0.041* | 0.044* |
| 5 | 0.047* | 0.044* | 0.041* | 0.039* | 0.037* | 0.036* | 0.035* | 0.034* | 0.034* | 0.034* | 0.035* | 0.036* | 0.037* | 0.039* | 0.041* | 0.044* | 0.048* |
| 10 | 0.05* | 0.047* | 0.045* | 0.042* | 0.04* | 0.039* | 0.038* | 0.037* | 0.037* | 0.037* | 0.038* | 0.039* | 0.041* | 0.042* | 0.045* | 0.048* | 0.051 |
| 15 | 0.054 | 0.051 | 0.048* | 0.046* | 0.044* | 0.042* | 0.041* | 0.041* | 0.041* | 0.041* | 0.041* | 0.043* | 0.044* | 0.046* | 0.049* | 0.052 | 0.055 |
| 20 | 0.058 | 0.055 | 0.052 | 0.05* | 0.048* | 0.046* | 0.045* | 0.045* | 0.044* | 0.045* | 0.045* | 0.046* | 0.048* | 0.05* | 0.053 | 0.056 | 0.06 |
| 25 | 0.062 | 0.059 | 0.056 | 0.054 | 0.052 | 0.05* | 0.049* | 0.049* | 0.048* | 0.049* | 0.049* | 0.051 | 0.052 | 0.055 | 0.057 | 0.061 | 0.065 |
| 30 | 0.067 | 0.064 | 0.061 | 0.058 | 0.056 | 0.055 | 0.054 | 0.053 | 0.053 | 0.053 | 0.054 | 0.055 | 0.057 | 0.059 | 0.062 | 0.066 | 0.07 |
| 35 | 0.072 | 0.069 | 0.066 | 0.063 | 0.061 | 0.06 | 0.058 | 0.058 | 0.058 | 0.058 | 0.059 | 0.06 | 0.062 | 0.064 | 0.067 | 0.071 | 0.075 |
| 40 | 0.077 | 0.074 | 0.071 | 0.068 | 0.066 | 0.065 | 0.064 | 0.063 | 0.063 | 0.063 | 0.064 | 0.065 | 0.067 | 0.07 | 0.073 | 0.077 | 0.081 |
| Scores | Number experiencing the behaviour, n (%) number completed | Mean (SD) missing data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency score | Severity score | Caregiver distress score | Total domain score | ||||||||||||
| Control (N = 308) | DCM™ intervention (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | |
| Total score | 308 (100) | 417 (99.8) | 725 (99.9) | 3.4 (4.72) 0 | 3.2 (4.37) 0 | 3.3 (4.52) 0 | 13 (13.95) 0 | 11.7 (12.35) 0 | 12.2 (13.06) 0 | ||||||
| Subscales | |||||||||||||||
| Delusions | 59 (19.2) 308 | 69 (16.5) 417 | 128 (17.7) 725 | 2.7 (1.12) 0 | 2.7 (1.13) 2 | 2.7 (1.12) 2 | 1.8 (0.71) 0 | 1.8 (0.68) 2 | 1.8 (0.69) 2 | 1.9 (1.28) 1 | 1.5 (1.41) 3 | 1.7 (1.35) 4 | 5.3 (3.41) 0 | 4.9 (3.04) 2 | 5.1 (3.21) 2 |
| Hallucinations | 47 (15.3) 307 | 59 (14.2) 416 | 106 (14.7) 723 | 2.6 (1.04) 0 | 2.6 (1.04) 1 | 2.6 (1.04) 1 | 1.4 (0.58) 0 | 1.5 (0.63) 1 | 1.5 (0.61) 1 | 0.8 (0.91) 0 | 1.2 (1.25) 1 | 1 (1.13) 1 | 3.8 (2.46) 0 | 4.2 (2.83) 1 | 4 (2.66) 1 |
| Agitation/aggression | 145 (47.1) 308 | 192 (46) 417 | 337 (46.5) 725 | 3 (0.95) 1 | 2.9 (0.94) 0 | 2.9 (0.95) 1 | 1.6 (0.67) 1 | 1.6 (0.69) 0 | 1.6 (0.68) 1 | 1.7 (1.23) 0 | 1.8 (1.28) 1 | 1.7 (1.25) 1 | 5 (2.85) 2 | 4.7 (2.86) 0 | 4.8 (2.85) 2 |
| Depression/dysphoria | 92 (30) 307 | 129 (30.9) 418 | 221 (30.5) 725 | 2.6 (0.94) 0 | 2.3 (1.01) 1 | 2.4 (0.98) 1 | 1.5 (0.67) 0 | 1.4 (0.6) 2 | 1.5 (0.63) 2 | 1.3 (1.04) 0 | 1.1 (1.14) 1 | 1.2 (1.1) 1 | 4.1 (2.77) 0 | 3.6 (2.63) 2 | 3.8 (2.7) 2 |
| Anxiety | 80 (26) 308 | 98 (23.5) 417 | 178 (24.6) 725 | 2.8 (0.94) 2 | 2.6 (0.96) 3 | 2.7 (0.96) 5 | 1.7 (0.71) 2 | 1.5 (0.6) 3 | 1.6 (0.66) 5 | 1.6 (1.19) 2 | 1.5 (1.25) 3 | 1.6 (1.23) 5 | 5.2 (3.16) 2 | 3.9 (2.32) 3 | 4.5 (2.8) 5 |
| Elation/euphoria | 25 (8.1) 308 | 34 (8.2) 416 | 59 (8.1) 724 | 2.6 (0.96) 0 | 2.8 (1.07) 0 | 2.7 (1.02) 0 | 1.3 (0.44) 1 | 1.5 (0.62) 0 | 1.4 (0.56) 1 | 0.4 (1) 0 | 0.7 (1.04) 0 | 0.6 (1.02) 0 | 3.4 (2.16) 1 | 4.4 (2.81) 0 | 4 (2.59) 1 |
| Apathy/indifference | 91 (29.5) 308 | 130 (31.2) 417 | 221 (30.5) 725 | 3.1 (0.89) 1 | 3.1 (0.9) 1 | 3.1 (0.89) 2 | 1.6 (0.69) 1 | 1.6 (0.67) 1 | 1.6 (0.67) 2 | 0.8 (1.03) 1 | 0.8 (0.99) 1 | 0.8 (1.01) 2 | 5.4 (3.3) 1 | 5.2 (3.07) 1 | 5.3 (3.16) 2 |
| Disinhibition | 51 (16.6) 308 | 65 (15.6) 416 | 116 (16) 724 | 2.8 (1.05) 0 | 2.6 (0.94) 0 | 2.7 (0.99) 0 | 1.7 (0.71) 0 | 1.4 (0.61) 0 | 1.5 (0.67) 0 | 1.5 (1.3) 1 | 1.2 (1.15) 0 | 1.3 (1.22) 1 | 5 (3.29) 0 | 3.8 (2.62) 0 | 4.3 (2.97) 0 |
| Irritability/lability | 117 (38) 308 | 153 (36.7) 417 | 270 (37.2) 725 | 2.9 (1.04) 3 | 2.7 (0.92) 0 | 2.8 (0.98) 3 | 1.7 (0.67) 3 | 1.5 (0.65) 0 | 1.6 (0.66) 3 | 1.7 (1.21) 4 | 1.3 (1.23) 0 | 1.5 (1.23) 4 | 5.3 (3.16) 3 | 4.4 (2.85) 0 | 4.8 (3.01) 3 |
| Aberrant motor behaviour | 94 (30.5) 308 | 135 (32.5) 416 | 229 (31.6) 724 | 3.6 (0.73) 0 | 3.4 (0.77) 0 | 3.5 (0.76) 0 | 1.6 (0.68) 0 | 1.6 (0.7) 0 | 1.6 (0.69) 0 | 1.1 (1.21) 0 | 1.1 (1.31) 0 | 1.1 (1.27) 0 | 5.9 (3) 0 | 5.7 (3.04) 0 | 5.8 (3.02) 0 |
| Sleep and night-time behaviour disorders | 48 (15.6) 307 | 77 (18.4) 418 | 125 (17.2) 725 | 3.1 (0.78) 0 | 2.9 (0.95) 5 | 3 (0.89) 5 | 1.5 (0.65) 0 | 1.7 (0.74) 5 | 1.6 (0.71) 5 | 1.8 (1.39) 0 | 2 (1.43) 5 | 1.9 (1.42) 5 | 4.9 (2.64) 0 | 5 (2.73) 5 | 5 (2.68) 5 |
| Appetite and eating changes | 57 (18.5) 308 | 98 (23.6) 415 | 155 (21.4) 723 | 3.3 (0.85) 3 | 3.3 (0.79) 4 | 3.3 (0.81) 7 | 1.9 (0.63) 2 | 1.8 (0.72) 4 | 1.8 (0.69) 6 | 1.3 (1.18) 2 | 1.3 (1.19) 4 | 1.3 (1.18) 6 | 6.4 (2.92) 3 | 6.1 (3.26) 4 | 6.2 (3.13) 7 |
| Scores | Number experiencing the behaviour, n (%) number completed | Mean (SD) missing data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency score | Severity score | Caregiver distress score | Total domain score | ||||||||||||
| Control (N = 308) | DCM™ intervention (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | |
| Total score | 244 (79.2) | 320 (76.6) | 564 (77.7) | 3 (4.03) 0 | 2.4 (3.26) 0 | 2.6 (3.62) 0 | 11.3 (12.35) 0 | 9.7 (10.14) 0 | 10.4 (11.17) 0 | ||||||
| Subscales | |||||||||||||||
| Delusions | 33 (13.5) 245 | 42 (13.2) 319 | 75 (13.3) 564 | 2.5 (1) 2 | 2.4 (1.01) 0 | 2.4 (1) 2 | 1.7 (0.68) 2 | 1.7 (0.67) 0 | 1.7 (0.67) 2 | 1.7 (1.19) 2 | 1.6 (1.19) 0 | 1.6 (1.19) 2 | 4.7 (3.07) 2 | 4.3 (2.99) 0 | 4.4 (3.01) 2 |
| Hallucinations | 26 (10.6) 245 | 29 (9.1) 319 | 55 (9.8) 564 | 2.8 (0.88) 0 | 2.6 (0.98) 0 | 2.7 (0.94) 0 | 1.5 (0.71) 0 | 1.4 (0.57) 0 | 1.5 (0.63) 0 | 1.2 (1.12) 0 | 0.7 (0.84) 0 | 0.9 (1) 0 | 4.5 (2.72) 0 | 3.7 (1.91) 0 | 4.1 (2.34) 0 |
| Agitation/aggression | 120 (49) 245 | 125 (39.2) 319 | 245 (43.4) 564 | 2.9 (0.89) 0 | 2.6 (0.94) 0 | 2.8 (0.93) 0 | 1.8 (0.71) 0 | 1.6 (0.66) 0 | 1.7 (0.69) 0 | 1.8 (1.17) 0 | 1.7 (1.2) 0 | 1.7 (1.18) 0 | 5.4 (3.24) 0 | 4.4 (2.62) 0 | 4.9 (2.98) 0 |
| Depression/dysphoria | 63 (26) 242 | 101 (31.6) 320 | 164 (29.2) 562 | 2.4 (1.01) 0 | 2.4 (0.97) 1 | 2.4 (0.98) 1 | 1.4 (0.59) 0 | 1.4 (0.57) 1 | 1.4 (0.57) 1 | 1.2 (1.17) 0 | 0.9 (0.9) 1 | 1 (1.02) 1 | 3.7 (2.66) 0 | 3.5 (2.35) 1 | 3.6 (2.47) 1 |
| Anxiety | 47 (19.3) 244 | 57 (17.9) 319 | 104 (18.5) 563 | 2.7 (0.99) 2 | 2.5 (0.87) 1 | 2.6 (0.93) 3 | 1.6 (0.74) 2 | 1.5 (0.66) 1 | 1.6 (0.7) 3 | 1.6 (1.2) 2 | 1.2 (1.06) 1 | 1.4 (1.13) 3 | 4.6 (2.92) 2 | 4 (2.71) 1 | 4.3 (2.81) 3 |
| Elation/euphoria | 15 (6.1) 244 | 19 (6) 319 | 34 (6) 563 | 2.8 (1.01) 0 | 2.4 (0.9) 0 | 2.6 (0.96) 0 | 1.4 (0.63) 0 | 1.3 (0.67) 0 | 1.4 (0.65) 0 | 0.3 (0.8) 0 | 0.3 (0.58) 0 | 0.3 (0.68) 0 | 4.3 (3.27) 0 | 3.4 (2.81) 0 | 3.8 (3.01) 0 |
| Apathy/indifference | 73 (29.9) 244 | 116 (36.3) 320 | 189 (33.5) 564 | 3.1 (0.87) 1 | 2.8 (0.98) 1 | 2.9 (0.95) 2 | 1.7 (0.77) 1 | 1.5 (0.62) 1 | 1.6 (0.7) 2 | 0.7 (0.93) 1 | 0.7 (0.91) 1 | 0.7 (0.91) 2 | 5.7 (3.39) 1 | 4.3 (2.91) 1 | 4.9 (3.16) 2 |
| Disinhibition | 35 (14.3) 244 | 30 (9.4) 320 | 65 (11.5) 564 | 2.7 (1.07) 0 | 2.9 (0.88) 0 | 2.8 (0.99) 0 | 1.7 (0.67) 0 | 1.7 (0.83) 0 | 1.7 (0.74) 0 | 1.7 (1.39) 0 | 1.6 (1.45) 0 | 1.6 (1.41) 0 | 4.9 (3.08) 0 | 5.3 (3.44) 0 | 5.1 (3.23) 0 |
| Irritability/lability | 83 (33.9) 245 | 99 (30.9) 320 | 182 (32.2) 565 | 2.6 (0.92) 0 | 2.7 (0.88) 0 | 2.7 (0.9) 0 | 1.6 (0.66) 0 | 1.6 (0.69) 0 | 1.6 (0.68) 0 | 1.5 (1.14) 0 | 1.3 (1.18) 1 | 1.4 (1.17) 1 | 4.5 (3.12) 0 | 4.4 (2.89) 0 | 4.5 (2.99) 0 |
| Aberrant motor behaviour | 71 (29.1) 244 | 90 (28.2) 319 | 161 (28.6) 563 | 3.4 (0.73) 0 | 3.4 (0.69) 0 | 3.4 (0.7) 0 | 1.6 (0.62) 0 | 1.7 (0.67) 0 | 1.7 (0.65) 0 | 1.1 (0.98) 0 | 1.1 (1.12) 0 | 1.1 (1.06) 0 | 5.6 (2.58) 0 | 6 (2.98) 0 | 5.8 (2.81) 0 |
| Sleep and night-time behaviour disorders | 39 (15.9) 245 | 51 (16) 319 | 90 (16) 564 | 3 (1) 0 | 2.9 (0.97) 0 | 3 (0.98) 0 | 1.5 (0.79) 0 | 1.6 (0.66) 0 | 1.6 (0.72) 0 | 1.7 (1.28) 0 | 2 (1.26) 0 | 1.8 (1.27) 0 | 4.9 (3.55) 0 | 5 (3.01) 0 | 5 (3.24) 0 |
| Appetite and eating changes | 48 (19.6) 245 | 46 (14.4) 319 | 94 (16.7) 564 | 3.3 (0.7) 1 | 3.2 (0.88) 1 | 3.3 (0.79) 2 | 1.9 (0.62) 1 | 1.8 (0.63) 1 | 1.8 (0.62) 2 | 1.4 (1.21) 1 | 1.6 (1.3) 1 | 1.5 (1.25) 2 | 6.3 (2.87) 1 | 5.8 (2.71) 1 | 6 (2.79) 2 |
| Scores | Number experiencing the behaviour, n (%) number completed | Mean (SD) missing data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency score | Severity score | Caregiver distress score | Total domain score | ||||||||||||
| Control (N = 308) | DCM™ intervention (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | Control (N = 308) | DCM (N = 418) | Total (N = 726) | |
| Total score | 185 (60.1) | 222 (53.1) | 407 (56.1) | 1.8 (3.12) 0 | 1.6 (2.79) 0 | 1.7 (2.94) 0 | 10.4 (9.25) 0 | 7.7 (9.36) 0 | 8.9 (9.4) 0 | ||||||
| Subscales | |||||||||||||||
| Delusions | 18 (9.7) 185 | 20 (9) 222 | 38 (9.3) 407 | 2.8 (1.26) 0 | 2.6 (1.1) 0 | 2.7 (1.16) 0 | 1.7 (0.57) 0 | 1.5 (0.51) 0 | 1.6 (0.55) 0 | 0.9 (1.16) 0 | 1.5 (1.07) 1 | 1.2 (1.13) 1 | 5.2 (3.03) 0 | 3.9 (2.28) 0 | 4.5 (2.71) 0 |
| Hallucinations | 15 (8.1) 185 | 20 (9) 222 | 35 (8.6) 407 | 2.3 (1.18) 0 | 3 (1.08) 0 | 2.7 (1.15) 0 | 1.4 (0.63) 0 | 1.3 (0.44) 0 | 1.3 (0.53) 0 | 0.2 (0.41) 0 | 0.6 (0.82) 0 | 0.4 (0.7) 0 | 3.3 (2.32) 0 | 3.8 (1.99) 0 | 3.6 (2.12) 0 |
| Agitation/aggression | 82 (44.3) 185 | 76 (34.2) 222 | 158 (38.8) 407 | 3 (0.92) 0 | 2.8 (1.02) 0 | 2.9 (0.97) 0 | 1.5 (0.55) 0 | 1.5 (0.64) 0 | 1.5 (0.59) 0 | 1.4 (1.17) 0 | 1.6 (1.17) 0 | 1.4 (1.17) 0 | 4.5 (2.3) 0 | 4.5 (3) 0 | 4.5 (2.65) 0 |
| Depression/dysphoria | 63 (34.1) 185 | 55 (24.8) 222 | 118 (29) 407 | 2.6 (0.9) 1 | 2.5 (0.95) 1 | 2.5 (0.92) 2 | 1.3 (0.49) 1 | 1.2 (0.49) 1 | 1.3 (0.49) 2 | 0.6 (0.94) 1 | 0.6 (0.77) 1 | 0.6 (0.86) 2 | 3.5 (2.09) 1 | 3.1 (1.87) 1 | 3.3 (1.99) 2 |
| Anxiety | 29 (15.7) 185 | 34 (15.3) 222 | 63 (15.5) 407 | 2.9 (0.84) 0 | 2.7 (0.94) 1 | 2.8 (0.9) 1 | 1.5 (0.51) 0 | 1.5 (0.67) 1 | 1.5 (0.59) 1 | 1 (0.98) 0 | 1 (1.16) 1 | 1 (1.07) 1 | 4.5 (2.28) 0 | 4.4 (2.83) 1 | 4.4 (2.56) 1 |
| Elation/euphoria | 7 (3.8) 185 | 14 (6.3) 222 | 21 (5.2) 407 | 3.1 (0.9) 0 | 2.6 (1.02) 0 | 2.8 (1) 0 | 1.3 (0.49) 0 | 1.2 (0.43) 0 | 1.2 (0.44) 0 | 0 (0) 0 | 0.2 (0.58) 0 | 0.1 (0.48) 0 | 4.1 (2.19) 0 | 3.3 (2.09) 0 | 3.6 (2.11) 0 |
| Apathy/indifference | 73 (39.5) 185 | 62 (27.9) 222 | 135 (33.2) 407 | 3.3 (1.01) 0 | 3 (1) 0 | 3.2 (1.01) 0 | 1.6 (0.69) 0 | 1.6 (0.73) 0 | 1.6 (0.71) 0 | 0.4 (0.63) 0 | 0.5 (0.88) 0 | 0.4 (0.76) 0 | 5.5 (3.33) 0 | 5.2 (3.4) 0 | 5.3 (3.36) 0 |
| Disinhibition | 24 (13) 185 | 24 (10.8) 222 | 48 (11.8) 407 | 2.5 (1.04) 1 | 2.5 (1.14) 0 | 2.5 (1.08) 1 | 1.3 (0.47) 1 | 1.3 (0.56) 0 | 1.3 (0.52) 1 | 0.8 (1.03) 1 | 1.3 (1.3) 0 | 1.1 (1.19) 1 | 3.6 (2.43) 1 | 3.6 (2.59) 0 | 3.6 (2.48) 1 |
| Irritability/lability | 65 (35.1) 185 | 66 (29.7) 222 | 131 (32.2) 407 | 3 (0.76) 0 | 2.6 (1.04) 0 | 2.8 (0.92) 0 | 1.5 (0.56) 0 | 1.4 (0.61) 0 | 1.5 (0.59) 0 | 1.2 (1.09) 0 | 1 (1.1) 0 | 1.1 (1.1) 0 | 4.5 (2.3) 0 | 4 (2.83) 0 | 4.2 (2.58) 0 |
| Aberrant motor behaviour | 54 (29.2) 185 | 38 (17.1) 222 | 92 (22.6) 407 | 3.4 (0.74) 0 | 3.5 (0.73) 1 | 3.4 (0.73) 1 | 1.4 (0.56) 0 | 1.5 (0.56) 2 | 1.4 (0.56) 2 | 0.5 (0.84) 0 | 0.9 (1.15) 2 | 0.7 (0.98) 2 | 4.7 (2.41) 0 | 5.2 (2.35) 2 | 4.9 (2.38) 2 |
| Sleep and night-time behaviour disorders | 22 (11.9) 185 | 27 (12.2) 222 | 49 (12) 407 | 2.7 (1.08) 0 | 2.8 (1.03) 4 | 2.8 (1.04) 4 | 1.1 (0.35) 0 | 1.4 (0.59) 4 | 1.3 (0.51) 4 | 0.8 (1.01) 0 | 1.7 (1.47) 4 | 1.2 (1.32) 4 | 3.1 (1.58) 0 | 4 (2.1) 4 | 3.6 (1.9) 4 |
| Appetite and eating changes | 30 (16.2) 185 | 25 (11.3) 222 | 55 (13.5) 407 | 3 (0.96) 4 | 3.2 (0.77) 4 | 3.1 (0.88) 8 | 1.8 (0.61) 4 | 1.7 (0.78) 4 | 1.8 (0.69) 8 | 1.2 (1.23) 4 | 1.5 (0.93) 4 | 1.3 (1.11) 8 | 5.9 (2.96) 4 | 5.8 (3.22) 4 | 5.8 (3.05) 8 |
| Scores | Number experiencing the behaviour, n (%) number completed | Mean (SD) missing data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency score | Severity score | Caregiver distress score | Total domain score | ||||||||||||
| Control (N = 287) | DCM™ intervention (N = 388) | Total (N = 675) | Control (N = 287) | DCM (N = 388) | Total (N = 675) | Control (N = 287) | DCM (N = 388) | Total (N = 675) | Control (N = 287) | DCM (N = 388) | Total (N = 675) | Control (N = 287) | DCM (N = 388) | Total (N = 675) | |
| Total score | 284 (99) | 384 (99) | 668 (99) | 1.6 (2.86) 0 | 2 (3.77) 0 | 1.9 (3.41) 0 | 10 (10.46) 0 | 8.4 (10.25) 0 | 9.1 (10.36) 0 | ||||||
| Subscales | |||||||||||||||
| Delusions | 24 (8.5) 284 | 50 (13) 384 | 74 (11.1) 668 | 2.9 (1.26) 0 | 2.6 (1.13) 1 | 2.7 (1.17) 1 | 1.7 (0.62) 0 | 1.5 (0.62) 1 | 1.6 (0.62) 1 | 0.8 (1.1) 0 | 1.5 (1.15) 2 | 1.3 (1.17) 2 | 5.3 (3.28) 0 | 4 (2.66) 1 | 4.5 (2.92) 1 |
| Hallucinations | 29 (10.2) 284 | 37 (9.6) 384 | 66 (9.9) 668 | 2.5 (1.09) 0 | 2.8 (1.12) 0 | 2.7 (1.11) 0 | 1.5 (0.69) 0 | 1.3 (0.53) 0 | 1.4 (0.6) 0 | 0.4 (0.78) 0 | 0.8 (0.95) 0 | 0.6 (0.89) 0 | 3.9 (3) 0 | 3.8 (2.38) 0 | 3.8 (2.65) 0 |
| Agitation/aggression | 116 (40.8) 284 | 141 (36.7) 384 | 257 (38.5) 668 | 3 (0.92) 0 | 2.9 (0.95) 2 | 2.9 (0.94) 2 | 1.5 (0.57) 0 | 1.5 (0.58) 1 | 1.5 (0.57) 1 | 1.3 (1.14) 0 | 1.6 (1.22) 2 | 1.4 (1.19) 2 | 4.7 (2.48) 0 | 4.7 (2.67) 2 | 4.7 (2.58) 2 |
| Depression/dysphoria | 95 (33.5) 284 | 105 (27.3) 384 | 200 (29.9) 668 | 2.5 (0.94) 2 | 2.4 (0.96) 1 | 2.5 (0.95) 3 | 1.3 (0.53) 2 | 1.3 (0.52) 1 | 1.3 (0.52) 3 | 0.6 (0.92) 2 | 0.8 (1) 1 | 0.7 (0.96) 3 | 3.5 (2.35) 2 | 3.2 (2.03) 1 | 3.3 (2.19) 3 |
| Anxiety | 48 (17) 283 | 72 (18.8) 384 | 120 (18) 667 | 2.6 (0.98) 0 | 2.6 (0.95) 2 | 2.6 (0.96) 2 | 1.5 (0.62) 0 | 1.5 (0.61) 2 | 1.5 (0.61) 2 | 0.7 (0.96) 0 | 1.1 (1.22) 2 | 1 (1.14) 2 | 4 (2.45) 0 | 4 (2.57) 2 | 4 (2.51) 2 |
| Elation/euphoria | 13 (4.6) 283 | 22 (5.7) 384 | 35 (5.2) 667 | 2.8 (0.93) 0 | 2.6 (0.9) 0 | 2.7 (0.9) 0 | 1.4 (0.51) 0 | 1.3 (0.55) 0 | 1.3 (0.53) 0 | 0 (0) 0 | 0.2 (0.5) 0 | 0.1 (0.4) 0 | 3.8 (1.88) 0 | 3.6 (2.61) 0 | 3.7 (2.34) 0 |
| Apathy/indifference | 95 (33.5) 284 | 108 (28.1) 384 | 203 (30.4) 668 | 3.3 (1) 0 | 2.9 (1) 0 | 3.1 (1.01) 0 | 1.6 (0.71) 0 | 1.5 (0.65) 0 | 1.5 (0.68) 0 | 0.3 (0.63) 0 | 0.5 (0.85) 0 | 0.4 (0.76) 0 | 5.5 (3.41) 0 | 4.6 (3.06) 0 | 5 (3.25) 0 |
| Disinhibition | 35 (12.3) 284 | 42 (10.9) 384 | 77 (11.5) 668 | 2.7 (1.09) 1 | 2.6 (1.03) 0 | 2.6 (1.05) 1 | 1.3 (0.53) 1 | 1.5 (0.71) 0 | 1.4 (0.64) 1 | 0.7 (0.93) 1 | 1.5 (1.38) 0 | 1.2 (1.26) 1 | 3.8 (2.7) 1 | 4.4 (3.22) 0 | 4.1 (2.99) 1 |
| Irritability/lability | 94 (33.1) 284 | 127 (33.1) 384 | 221 (33.1) 668 | 3 (0.84) 0 | 2.6 (0.96) 1 | 2.8 (0.93) 1 | 1.5 (0.58) 0 | 1.4 (0.57) 1 | 1.5 (0.58) 1 | 1.1 (1.05) 0 | 1.1 (1.13) 1 | 1.1 (1.09) 1 | 4.5 (2.44) 0 | 4 (2.66) 1 | 4.2 (2.58) 1 |
| Aberrant motor behaviour | 83 (29.2) 284 | 74 (19.3) 384 | 157 (23.5) 668 | 3.4 (0.8) 0 | 3.4 (0.72) 1 | 3.4 (0.76) 1 | 1.4 (0.59) 0 | 1.5 (0.58) 2 | 1.5 (0.58) 2 | 0.5 (0.85) 0 | 0.9 (1.15) 2 | 0.7 (1.01) 2 | 4.9 (2.53) 0 | 5.2 (2.54) 2 | 5.1 (2.53) 2 |
| Sleep and night-time behaviour disorders | 28 (9.9) 284 | 49 (12.8) 384 | 77 (11.5) 668 | 2.9 (1.07) 1 | 2.8 (0.94) 5 | 2.9 (0.98) 6 | 1.2 (0.42) 1 | 1.5 (0.59) 5 | 1.4 (0.55) 6 | 0.8 (0.97) 1 | 1.7 (1.49) 5 | 1.4 (1.38) 6 | 3.7 (2.11) 1 | 4.3 (2.14) 5 | 4 (2.13) 6 |
| Appetite and eating changes | 41 (14.4) 284 | 44 (11.5) 384 | 85 (12.7) 668 | 3.1 (1.01) 7 | 3.3 (0.74) 4 | 3.2 (0.88) 11 | 1.9 (0.69) 7 | 1.6 (0.67) 4 | 1.7 (0.69) 11 | 1.1 (1.23) 7 | 1.4 (1.03) 4 | 1.3 (1.12) 11 | 6.1 (3.39) 7 | 5.3 (2.75) 4 | 5.7 (3.07) 11 |
| Analyses | Logistic regression models | Treatment odds ratio (treated control) | 95% CI | p-value | n |
|---|---|---|---|---|---|
| BSC | Complete cases only | 0.941 | 0.598 to 1.479 | 0.7921 | 558 |
| Missing data imputed assuming they are MAR | 0.95 | 0.612 to 1.476 | 0.8196 | 726 | |
| Cluster specific – complete cases only | 0.939 | 0.561 to 1.57 | 0.8088 | 558 | |
| Cluster specific – missing data imputed assuming they are MAR | 0.951 | 0.584 to 1.547 | 0.8381 | 726 | |
| PRN antipsychotic medication | Complete cases only | 0.454 | 0.114 to 1.815 | 0.2640 | 581 |
| Missing data imputed assuming they are MAR | 0.455 | 0.093 to 2.236 | 0.3314 | 726 | |
| Complete cases only without hub | 0.494 | 0.093 to 2.629 | 0.4084 | 581 | |
| Missing data imputed assuming they are MAR without hub | 0.533 | 0.095 to 2.997 | 0.4743 | 726 | |
| Mood | |||||
| Depression/dysphoria | Complete cases only | 1.34 | 0.862 to 2.082 | 0.1932 | 558 |
| Missing data imputed assuming they are MAR | 1.32 | 0.872 to 1.999 | 0.1895 | 726 | |
| Anxiety | Complete cases only | 1.023 | 0.59 to 1.774 | 0.9343 | 558 |
| Missing data imputed assuming they are MAR | 1.011 | 0.617 to 1.656 | 0.9668 | 726 | |
| Apathy/indifference | Complete cases only | 1.319 | 0.79 to 2.2 | 0.2897 | 559 |
| Missing data imputed assuming they are MAR | 1.33 | 0.853 to 2.073 | 0.2075 | 726 | |
| Analyses | Logistic regression models | Treatment odds ratio (treated control) | 95% CI | p-value | n |
|---|---|---|---|---|---|
| BSC | Complete cases only | 0.605 | 0.339 to 1.079 | 0.0886 | 403 |
| Missing data imputed assuming they are MAR | 0.57 | 0.343 to 0.948 | 0.0305 | 726 | |
| Cluster specific – complete cases only | 0.591 | 0.308 to 1.133 | 0.1131 | 403 | |
| Cluster specific – missing data imputed assuming they are MAR | 0.577 | 0.334 to 0.996 | 0.0484 | 726 | |
| PRN antipsychotic medication | Complete cases only without hub | 0.766 | 0.132 to 4.457 | 0.7666 | 406 |
| Missing data imputed assuming they are MAR without hub | 0.783 | 0.114 to 5.368 | 0.8019 | 726 | |
| Mood | |||||
| Depression/dysphoria | Complete cases only | 0.614 | 0.345 to 1.094 | 0.0980 | 404 |
| Missing data imputed assuming they are MAR | 0.592 | 0.369 to 0.95 | 0.0298 | 726 | |
| Anxiety | Complete cases only | 1.027 | 0.51 to 2.069 | 0.9395 | 403 |
| Missing data imputed assuming they are MAR | 1.037 | 0.588 to 1.83 | 0.9004 | 726 | |
| Apathy/indifference | Complete cases only | 0.601 | 0.322 to 1.124 | 0.1109 | 403 |
| Missing data imputed assuming they are MAR | 0.601 | 0.38 to 0.952 | 0.0302 | 726 | |
| Analyses | Logistic regression models | Treatment odds ratio (treated control) | 95% CI | p-value | n |
|---|---|---|---|---|---|
| BSC | Complete cases only | 0.723 | 0.481 to 1.088 | 0.1198 | 668 |
| Missing data imputed assuming they are MAR | 0.720 | 0.479 to 1.083 | 0.1146 | 675 | |
| Cluster specific – complete cases only | 0.683 | 0.4 to 1.166 | 0.1619 | 668 | |
| Cluster specific – missing data imputed assuming they are MAR | 0.681 | 0.4 to 1.158 | 0.1561 | 675 | |
| PRN antipsychotic medication | Complete cases only | 1.166 | 0.127 to 10.688 | 0.892 | 413 |
| Missing data imputed assuming they are MAR | 1.28 | 0.153 to 10.685 | 0.8189 | 675 | |
| Mood | |||||
| Depression/dysphoria | Complete cases only | 0.757 | 0.51 to 1.123 | 0.1666 | 668 |
| Missing data imputed assuming they are MAR | 0.757 | 0.511 to 1.123 | 0.1672 | 675 | |
| Anxiety | Complete cases only | 1.134 | 0.667 to 1.928 | 0.6422 | 667 |
| Missing data imputed assuming they are MAR | 1.133 | 0.67 to 1.916 | 0.6423 | 675 | |
| Apathy/indifference | Complete cases only | 0.81 | 0.525 to 1.249 | 0.3402 | 668 |
| Missing data imputed assuming they are MAR | 0.81 | 0.525 to 1.249 | 0.3403 | 675 | |
| Analysis | Estimated mean difference | 95% CI | p-value | Unadjusted ICC for intervention | Unadjusted ICC for control | Adjusted ICC for intervention | Adjusted ICC for control | n |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| QUALID (staff) – complete cases only | –0.62 | –1.91 to 0.67 | 0.334 | 0.1357 | 0.0173 | 0.0627 | 0.0001 | 560 |
| QUALID (staff) – missing data imputed assuming they are MAR | –0.74 | –1.91 to 0.43 | 0.214 | 0.129 | 0.005 | 0.035 | 0.001 | 726 |
| 16 months | ||||||||
| QUALID (staff) – complete cases only | –0.04 | –1.24 to 1.16 | 0.948 | 0.0838 | 0.0064 | 0 | 0 | 404 |
| QUALID (staff) – missing data imputed assuming they are MAR | –0.07 | –1.26 to 1.11 | 0.902 | 0.07 | 0.004 | 0.004 | 0 | 726 |
| Analysis at 16 months | Estimated mean difference | 95% CI | p-value | Unadjusted ICC for intervention | Unadjusted ICC for control | Adjusted ICC for intervention | Adjusted ICC for control | n |
|---|---|---|---|---|---|---|---|---|
| QUALID (staff) – complete cases only | –0.06 | –1.14 to 1.02 | 0.910 | 0.0788 | 0.0089 | 0.0119 | 0.0015 | 668 |
| QUALID (staff) – missing data imputed assuming they are MAR | –0.05 | –1.12 to 1.02 | 0.922 | 0.082 | 0.01 | 0.015 | 0.002 | 675 |
| Medication | Baseline, n (% sample) | 6 months, n (% sample) | ||||
|---|---|---|---|---|---|---|
| Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | |
| Antipsychotic medication | 44 (14.3) | 51 (12.2) | 95 (13.1) | 35 (11.4) | 37 (8.9) | 72 (9.9) |
| Benzodiazepine medication | 20 (6.5) | 21 (5.0) | 41 (5.6) | 14 (4.5) | 14 (3.3) | 28 (3.9) |
| Non-benzodiazepine anxiolytic medication | 0 (0) | 4 (1.0) | 4 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-benzodiazepine hypnotic medication | 22 (7.1) | 14 (3.3) | 37 (5.1) | 20 (6.5) | 15 (3.6) | 35 (4.8) |
| Memantine medication | 26 (8.4) | 28 (6.7) | 54 (7.4) | 21 (6.8) | 27 (6.5) | 48 (6.6) |
| Antidepressant medication | 127 (41.2) | 135 (32.3) | 262 (36.1) | 107 (34.7) | 113 (27.0) | 220 (30.3) |
| Cholinesterase inhibitor medication | 47 (15.3) | 61 (14.6) | 108 (14.9) | 40 (13.0) | 54 (12.9) | 94 (12.9) |
| Anticonvulsant medication | 14 (4.5) | 20 (4.8) | 34 (4.6) | 13 (4.2) | 17 (4.1) | 30 (4.1) |
| Mood stabiliser medication | 1 (0.3) | 2 (0.5) | 3 (0.4) | 1 (0.3) | 4 (1.0) | 5 (0.7) |
| Pain relief medication | 143 (46.4) | 213 (51.0) | 356 (49.0) | 105 (34.1) | 160 (38.3) | 265 (36.5) |
| Total number of medications prescribed on the medication record administration chart over the reporting period | ||||||
| Mean (SD) number taken/month | 8.7 (4.3) 304 | 8.7 (4.01) 414 | 8.7 (4.13) 718 | 8.5 (3.73) 240 | 9.2 (4.4) 336 | 8.9 (4.15) 576 |
| Median (Q1, Q3) | 8 (6, 11) | 8 (6, 11) | 8 (6, 11) | 8.5 (6, 11) | 9 (6, 12) | 9 (6, 12) |
| Medication | Original cohort, n (% sample) | Cross-sectional cohort, n (% sample) | ||||
|---|---|---|---|---|---|---|
| Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 287) | Intervention (N = 388) | Total (N = 675) | |
| Antipsychotic medication | 29 (9.4) | 27 (6.5) | 56 (7.7) | 41 (14.3) | 46 (11.9) | 87 (12.9) |
| Benzodiazepine medication | 11 (3.6) | 9 (2.2) | 20 (2.8) | 18 (6.3) | 14 (3.6) | 32 (4.7) |
| Non-benzodiazepine anxiolytic medication | 0 (0.0) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| Non-benzodiazepine hypnotic medication | 14 (4.5) | 12 (2.9) | 26 (3.6) | 21 (7.3) | 22 (5.7) | 43 (6.4) |
| Memantine medication | 17 (5.5) | 21 (5.0) | 38 (5.2) | 31 (10.8) | 44 (11.3) | 75 (11.1) |
| Antidepressant medication | 80 (26.0) | 68 (16.3) | 148 (20.4) | 119 (41.5) | 131 (33.8) | 250 (37.0) |
| Cholinesterase inhibitor medication | 28 (9.1) | 33 (7.9) | 61 (8.4) | 50 (17.4) | 71 (18.3) | 121 (17.9) |
| Anticonvulsant medication | 9 (2.9) | 10 (2.4) | 19 (2.6) | 9 (3.1) | 15 (3.9) | 24 (3.6) |
| Mood stabiliser medication | 1 (0.3) | 0 (0.0) | 1 (0.1) | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| Pain relief medication | 84 (27.3) | 121 (28.9) | 205 (28.2) | 140 (48.8) | 201 (51.8) | 341 (50.5) |
| Total number of medications prescribed on the MAR over the reporting period | ||||||
| Mean (SD) number taken/month | 8.9 (3.82) 165 | 8.9 (4.61) 214 | 8.9 (4.28) 379 | 8.7 (3.71) 260 | 8.8 (4.74) 368 | 8.7 (4.34) 628 |
| Median (Q1, Q3) | 9 (6, 11) | 8 (6, 12) | 9 (6, 12) | 9 (6, 11) | 8 (5, 11) | 8 (6, 11) |
| Medication | Baseline, n (% sample) | 6 months, n (% sample) | 16 months (original cohort), n (% sample) | 16 months (cross-sectional cohort), n (% sample) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 287) | Intervention (N = 388) | Total (N = 675) | |
| Antipsychotic medication | 1 (0.3) | 1 (0.2) | 2 (0.3) | 2 (0.6) | 1 (0.2) | 3 (0.4) | 1 (0.3) | 0 (0.0) | 1 (0.1) | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Benzodiazepine medication | 9 (2.9) | 8 (1.9) | 17 (2.3) | 9 (2.9) | 9 (2.2) | 18 (2.5) | 4 (1.3) | 2 (0.5) | 6 (0.8) | 10 (3.5) | 6 (1.5) | 16 (2.4) |
| Non-benzodiazepine anxiolytic medication | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-benzodiazepine hypnotic medication | 2 (0.6) | 3 (0.7) | 5 (0.7) | 1 (0.3) | 1 (0.2) | 2 (0.3) | 4 (1.3) | 1 (0.2) | 5 (0.7) | 6 (2.1) | 1 (0.3) | 7 (1.0) |
| Anticonvulsant medication | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mood stabiliser medication | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pain relief medication | 67 (21.8) | 69 (16.5) | 136 (18.7) | 74 (24) | 93 (22.2) | 167 (23.0) | 48 (15.6) | 40 (9.6) | 88 (12.1) | 71 (24.7) | 67 (17.3) | 138 (20.4) |
Care homes
| All interactions (% positive) missing data | Baseline | 6 months | 16 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | ||
| a.m. | 0–15 minute interval | 255 (75.3) 0 | 297 (83.8) 2 | 552 (79.9) 2 | 268 (89.2) 0 | 283 (91.9) 0 | 551 (90.6) 0 | 180 (86.7) 0 | 376 (83.5) 0 | 556 (84.5) 0 |
| 15–30 minute interval | 288 (71.9) 0 | 334 (88.9) 2 | 622 (81) 2 | 250 (84) 0 | 271 (89.7) 0 | 521 (86.9) 0 | 165 (86.1) 0 | 254 (85.4) 0 | 419 (85.7) 0 | |
| 30–45 minute interval | 213 (68.5) 0 | 296 (85.1) 1 | 509 (78.2) 1 | 224 (88.4) 0 | 280 (88.6) 0 | 504 (88.5) 0 | 204 (86.3) 0 | 285 (81.8) 1 | 489 (83.6) 1 | |
| 45–60 minute interval | 264 (83.7) 0 | 303 (87.1) 1 | 567 (85.5) 1 | 258 (85.7) 0 | 226 (81.9) 0 | 484 (83.9) 0 | 231 (84) 0 | 276 (81.2) 1 | 507 (82.4) 1 | |
| p.m. | 0–15 minute interval | 298 (81.2) 0 | 317 (79.5) 2 | 615 (80.3) 2 | 217 (78.3) 0 | 341 (90) 0 | 558 (85.5) 0 | 211 (80.6) 0 | 324 (83) 0 | 535 (82.1) 0 |
| 15–30 minute interval | 264 (75.4) 0 | 312 (76.9) 2 | 576 (76.2) 2 | 168 (81.5) 0 | 272 (86.8) 0 | 440 (84.8) 0 | 216 (80.6) 0 | 316 (81) 0 | 532 (80.8) 0 | |
| 30–45 minute interval | 246 (72.4) 0 | 291 (74.9) 1 | 537 (73.7) 1 | 188 (69.7) 0 | 319 (89.7) 0 | 507 (82.2) 0 | 200 (83) 0 | 256 (86.3) 0 | 456 (84.9) 0 | |
| 45–60 minute interval | 237 (67.9) 0 | 255 (76.1) 1 | 492 (72.2) 1 | 193 (70.5) 0 | 299 (88.6) 0 | 492 (81.5) 0 | 171 (83) 0 | 233 (89.3) 0 | 404 (86.6) 0 | |
| Both a.m. and p.m. | 0–15 minute interval | 553 (78.5) 0 | 614 (81.6) 4 | 1167 (80.1) 4 | 485 (84.3) 0 | 624 (90.9) 0 | 1109 (88) 0 | 391 (83.4) 0 | 700 (83.3) 0 | 1091 (83.3) 0 |
| 15–30 minute interval | 552 (73.6) 0 | 646 (83.1) 4 | 1198 (78.7) 4 | 418 (83) 0 | 543 (88.2) 0 | 961 (86) 0 | 381 (82.9) 0 | 570 (83) 0 | 951 (83) 0 | |
| 30–45 minute interval | 459 (70.6) 0 | 587 (80.1) 2 | 1046 (75.9) 2 | 412 (79.9) 0 | 599 (89.1) 0 | 1011 (85.4) 0 | 404 (84.7) 0 | 541 (83.9) 1 | 945 (84.2) 1 | |
| 45–60 minute interval | 501 (76.2) 0 | 558 (82.1) 2 | 1059 (79.3) 2 | 451 (79.2) 0 | 525 (85.7) 0 | 976 (82.7) 0 | 402 (83.6) 0 | 509 (84.9) 1 | 911 (84.3) 1 | |
| All interactions | 2065 (74.9) 0 | 2405 (81.7) 1 | 4470 (78.6) 1 | 1766 (81.7) 0 | 2291 (88.6) 0 | 4057 (85.6) 0 | 1578 (83.7) 0 | 2320 (83.7) 0 | 3898 (83.7) 0 | |
Predictive and process measures
| Scores | Baseline | 6 months | 16 months (original cohort) | 16 months (cross-sectional cohort) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 308) | Intervention (N = 418) | Total (N = 726) | Control (N = 287) | Intervention (N = 388) | Total (N = 675) | |
| Global CDR score categories, n (%) | ||||||||||||
| 0 | 1 (0.3) | 2 (0.5) | 3 (0.4) | 0 (0.0) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| 0.5 | 17 (5.5) | 23 (5.5) | 40 (5.5) | 6 (1.9) | 7 (1.7) | 13 (1.8) | 5 (1.6) | 0 (0.0) | 5 (0.7) | 11 (3.8) | 6 (1.5) | 17 (2.5) |
| 1 | 79 (25.6) | 101 (24.2) | 180 (24.8) | 37 (12.0) | 77 (18.4) | 114 (15.7) | 27 (8.8) | 49 (11.7) | 76 (10.5) | 55 (19.2) | 101 (26.0) | 156 (23.1) |
| 2 | 111 (36.0) | 160 (38.3) | 271 (37.3) | 110 (35.7) | 145 (34.7) | 255 (35.1) | 54 (17.5) | 89 (21.3) | 143 (19.7) | 90 (31.4) | 151 (38.9) | 241 (35.7) |
| 3 | 98 (31.8) | 130 (31.1) | 228 (31.4) | 92 (29.9) | 92 (22.0) | 184 (25.3) | 99 (32.1) | 83 (19.9) | 182 (25.1) | 128 (44.6) | 125 (32.2) | 253 (37.5) |
| Missing | 2 (0.6) | 2 (0.5) | 4 (0.6) | 63 (20.5) | 96 (23.0) | 159 (21.9) | 123 (39.9) | 196 (46.9) | 319 (43.9) | 3 (1.0) | 4 (1.0) | 7 (1.0) |
| Global CDR score | ||||||||||||
| Mean (SD) missing data | 1.97 (0.85) 2 | 1.98 (0.84) 2 | 1.98 (0.84) 4 | 2.19 (0.74) 63 | 2.01 (0.77) 96 | 2.09 (0.76) 159 | 2.35 (0.79) 123 | 2.14 (0.77) 196 | 2.24 (0.79) 319 | 2.2 (0.83) 3 | 2.03 (0.8) 4 | 2.1 (0.82) 7 |
| Median (interquartile range) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (2–3) | 2 (1–3) | 2 (2–3) | 3 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (1–3) | 2 (1–3) |
| Subscales, mean (SD) missing data | ||||||||||||
| Memory (primary category) | 1.95 (0.81) 2 | 1.91 (0.84) 2 | 1.93 (0.83) 4 | 2.23 (0.73) 63 | 2.03 (0.75) 96 | 2.12 (0.75) 159 | 2.34 (0.7) 123 | 2.14 (0.76) 196 | 2.23 (0.74) 319 | 2.21 (0.78) 3 | 2.04 (0.79) 4 | 2.11 (0.79) 7 |
| Orientation | 1.98 (0.86) 1 | 1.87 (0.93) 3 | 1.92 (0.9) 4 | 2.17 (0.78) 63 | 1.96 (0.86) 95 | 2.05 (0.83) 158 | 2.32 (0.79) 124 | 2.12 (0.82) 196 | 2.21 (0.81) 320 | 2.2 (0.85) 4 | 2.02 (0.86) 4 | 2.1 (0.86) 8 |
| Judgement and problem solving | 1.85 (0.91) 2 | 1.89 (0.95) 3 | 1.88 (0.93) 5 | 2.12 (0.83) 63 | 1.95 (0.87) 92 | 2.03 (0.86) 155 | 2.29 (0.83) 123 | 2.12 (0.84) 196 | 2.2 (0.84) 319 | 2.14 (0.88) 3 | 1.98 (0.87) 4 | 2.05 (0.87) 7 |
| Community affairs | 1.86 (0.76) 0 | 1.9 (0.78) 2 | 1.88 (0.77) 2 | 2.04 (0.64) 64 | 1.95 (0.68) 90 | 1.99 (0.66) 154 | 2.17 (0.67) 123 | 2.09 (0.69) 196 | 2.13 (0.68) 319 | 2.07 (0.69) 3 | 2 (0.72) 4 | 2.03 (0.71) 7 |
| Home and hobbies | 1.79 (0.87) 0 | 1.86 (0.85) 3 | 1.83 (0.86) 3 | 2.09 (0.75) 63 | 1.94 (0.77) 90 | 2 (0.77) 153 | 2.15 (0.82) 123 | 2.04 (0.76) 196 | 2.09 (0.79) 319 | 2.02 (0.84) 3 | 1.92 (0.79) 4 | 1.96 (0.81) 7 |
| Personal care | 2.29 (0.86) 2 | 2.3 (0.83) 1 | 2.3 (0.84) 3 | 2.36 (0.8) 63 | 2.45 (0.72) 90 | 2.41 (0.76) 153 | 2.59 (0.74) 123 | 2.55 (0.68) 196 | 2.57 (0.71) 319 | 2.41 (0.9) 3 | 2.39 (0.82) 5 | 2.4 (0.85) 8 |
| Total EAT score (%) | Baseline | 6 months | 16 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | |
| Mean (SD) missing data | 53.5 (9.17) 1 | 53 (10.26) 2 | 53.2 (9.76) 3 | 58.9 (6.04) 4 | 52.9 (8.57) 3 | 55 (8.22) 7 | 54.7 (9.28) 3 | 55.3 (8.52) 4 | 55.1 (8.7) 7 |
| Median (interquartile range) | 53.2 (47.2–62.3) | 52.5 (46–61) | 52.9 (46.1–62.3) | 61.5 (56.5–63.3) | 52.1 (46.6–61) | 56.5 (48.2–62.5) | 55.2 (48.4–62.3) | 55.8 (51.3–60.7) | 55.8 (49.8–60.7) |
| Subscales, mean (SD) missing data | |||||||||
| Safety | 47.8 (13.83) 0 | 48.4 (16.08) 1 | 48.2 (15.1) 1 | 52.8 (16.68) 0 | 57.9 (13.56) 0 | 56 (14.86) 0 | 59.3 (14.58) 0 | 56.2 (16.28) 0 | 57.4 (15.57) 0 |
| Size | 30.7 (23.74) 0 | 23.9 (24.64) 1 | 26.5 (24.28) 1 | 29.8 (21.93) 0 | 19.9 (26.67) 0 | 23.7 (25.22) 0 | 33.3 (27.78) 0 | 22.8 (27.85) 1 | 26.9 (28.02) 1 |
| Visual access features | 23.1 (11.52) 0 | 25.4 (12.88) 1 | 24.5 (12.3) 1 | 19.6 (12.13) 1 | 21.2 (13.41) 1 | 20.6 (12.84) 2 | 17.6 (12.38) 0 | 24.1 (15.55) 0 | 21.6 (14.65) 0 |
| Highlighting useful stimuli | 91.8 (13.65) 0 | 86.3 (13.59) 1 | 88.4 (13.74) 1 | 89.5 (13.97) 0 | 89.1 (11.52) 0 | 89.2 (12.37) 0 | 91.3 (10.8) 0 | 93 (9.02) 0 | 92.4 (9.66) 0 |
| Wandering | 48.5 (35.95) 0 | 45.6 (38.08) 1 | 46.7 (36.92) 1 | 66 (32.27) 2 | 43.1 (38.02) 2 | 51.6 (37.34) 4 | 52.3 (33.05) 2 | 53.9 (30.81) 0 | 53.4 (31.27) 2 |
| Familiarity | 71.1 (16.74) 0 | 74.4 (24.44) 1 | 73.2 (21.65) 1 | 73.5 (16.09) 0 | 80.2 (12.82) 0 | 77.7 (14.38) 0 | 70.4 (18.87) 0 | 79.6 (16.02) 1 | 76 (17.58) 1 |
| Privacy and community | 76.6 (19.13) 0 | 81.5 (14.25) 1 | 79.6 (16.3) 1 | 77.1 (20) 0 | 75.8 (18.83) 0 | 76.3 (19.09) 0 | 76.8 (23.54) 0 | 72.2 (18.17) 0 | 73.9 (20.27) 0 |
| Community links | 51.3 (48.93) 0 | 48.3 (49.97) 1 | 49.5 (49.08) 1 | 69.4 (42.49) 1 | 46.8 (49.89) 0 | 55.1 (48.14) 1 | 36.8 (46.67) 0 | 46.7 (50.74) 1 | 42.9 (48.95) 1 |
| Domestic activity | 35 (9.36) 1 | 33.2 (11.43) 2 | 33.9 (10.62) 3 | 35.9 (11.62) 0 | 32.5 (11.46) 0 | 33.8 (11.52) 0 | 34.9 (10.22) 0 | 33.1 (10.19) 0 | 33.8 (10.14) 0 |
| Total GLHC score | Baseline | 6 months | 16 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | Control (n = 19) | Intervention (n = 31) | Total (n = 50) | |
| Mean (SD) missing data | 32.2 (4.09) 0 | 31.1 (4.19) 2 | 31.5 (4.14) 2 | 29.9 (5.13) 0 | 30.2 (5.25) 0 | 30.1 (5.15) 0 | 29.9 (3.91) 0 | 30.8 (4.29) 0 | 30.4 (4.13) 0 |
| Median (interquartile range) | 31 (28.5–36) | 31 (28–33) | 31 (28–35.2) | 29 (26–34) | 30 (27–33) | 29.5 (26.5–33) | 29 (27–34) | 31 (27–34) | 31 (27–34) |
Health economic analysis
| Health-care resource item | Month | Intervention | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | ||
| Primary care | |||||||||||
| GP face-to-face visit | 0 | 214 | 1.61 | 2.17 | 0 | 12 | 175 | 1.54 | 1.63 | 0 | 8 |
| 6 | 214 | 1.31 | 2.10 | 0 | 13 | 175 | 1.46 | 1.69 | 0 | 8 | |
| 16 | 214 | 0.84 | 1.43 | 0 | 8 | 175 | 0.93 | 1.69 | 0 | 9 | |
| GP telephone call | 0 | 214 | 0.72 | 1.62 | 0 | 12 | 175 | 0.71 | 1.24 | 0 | 6 |
| 6 | 214 | 0.49 | 0.99 | 0 | 5 | 175 | 0.39 | 0.92 | 0 | 7 | |
| 16 | 214 | 0.36 | 0.89 | 0 | 5 | 175 | 0.29 | 0.71 | 0 | 4 | |
| District nurse visit | 0 | 214 | 1.22 | 7.02 | 0 | 90 | 175 | 1.79 | 5.29 | 0 | 43 |
| 6 | 214 | 0.36 | 1.24 | 0 | 13 | 175 | 1.53 | 4.88 | 0 | 41 | |
| 16 | 214 | 0.75 | 3.44 | 0 | 39 | 175 | 0.57 | 2.68 | 0 | 27 | |
| District nurse telephone call | 0 | 214 | 0.08 | 0.44 | 0 | 3 | 175 | 0.13 | 0.44 | 0 | 2 |
| 6 | 214 | 0.03 | 0.19 | 0 | 2 | 175 | 0.14 | 0.53 | 0 | 4 | |
| 16 | 214 | 0.03 | 0.24 | 0 | 2 | 175 | 0.13 | 1.25 | 0 | 16 | |
| Secondary care | |||||||||||
| Nights spent in hospital | 0 | 214 | 0.72 | 4.06 | 0 | 43 | 175 | 0.66 | 3.81 | 0 | 37 |
| 6 | 214 | 0.64 | 3.02 | 0 | 28 | 175 | 0.29 | 1.58 | 0 | 15 | |
| 16 | 214 | 0.14 | 1.14 | 0 | 12 | 175 | 0.01 | 0.08 | 0 | 1 | |
| Hospital day centre visit | 0 | 214 | 0.01 | 0.10 | 0 | 1 | 175 | 0.02 | 0.13 | 0 | 1 |
| 6 | 214 | 0.00 | 0.07 | 0 | 1 | 175 | 0.00 | 0.00 | 0 | 0 | |
| 16 | 214 | 0.00 | 0.07 | 0 | 1 | 175 | 0.03 | 0.20 | 0 | 2 | |
| Hospital outpatient clinic visit | 0 | 214 | 0.14 | 0.61 | 0 | 7 | 175 | 0.14 | 0.46 | 0 | 4 |
| 6 | 214 | 0.08 | 0.27 | 0 | 1 | 175 | 0.06 | 0.31 | 0 | 3 | |
| 16 | 214 | 0.07 | 0.35 | 0 | 3 | 175 | 0.01 | 0.15 | 0 | 2 | |
| Hospital A&E visit | 0 | 214 | 0.15 | 0.83 | 0 | 11 | 175 | 0.10 | 0.39 | 0 | 2 |
| 6 | 214 | 0.07 | 0.27 | 0 | 2 | 175 | 0.06 | 0.29 | 0 | 2 | |
| 16 | 214 | 0.01 | 0.12 | 0 | 1 | 175 | 0.01 | 0.08 | 0 | 1 | |
| Resource item | Unit cost (£) | Assumptions and source |
|---|---|---|
| Advanced nurse practitioner | 77.24 | Reference Costs 2015–16 146 |
| Advanced nurse practitioner (telephone) | 33.08 | Reference Costs 2015–16 146 |
| Counsellor | 62.03 | PSSRU 2011/12168 |
| District nurse | 37.98 | Reference Costs 2015–16 146 |
| District nurse (telephone) | 16.16 | Reference Costs 2015–16 146 |
| GP | 132.69 | PSSRU 2009/10169 |
| GP (telephone) | 28.39 | PSSRU 2014/15170 |
| Health visitor | 64.81 | Reference Costs 2015–16 146 |
| Health visitor (telephone) | 26.38 | Reference Costs 2015–16 146 |
| Hospital A&E | 137.74 | Reference Costs 2015–16 146 |
| Hospital outpatient clinic | 136.79 | Reference Costs 2015–16 146 |
| Hospital overnight stay | 464.83 | Reference Costs 2015–16 146 |
| Member of community health team | 43.00 | PSSRU 2015/16144 |
| Physiotherapist | 48.94 | Reference Costs 2015–16 146 |
| Psychiatrist or psychologist | 142.98 | PSSRU 2011/12168 |
| Social worker | 39.50 | PSSRU 2015/16144 |
| Speech and language therapist | 88.02 | Reference Costs 2015–16 146 |
Appendix 2 Summary of substantial amendments
Summary of substantial amendments to trial
Substantial amendment 1: approved 10 January 2014
Collection of data from medical records/the Health and Social Care Information Centre (now NHS Digital)
The proposed plan for collection of resource use data (prescription medication usage and repeat hospital attendances/admissions/safety data) was to obtain all of the required information from a review of each resident’s care home records. Having undertaken some consultation with other researchers doing care home research and collecting similar data, we have been informed that these data are often incomplete/ambiguous and so further clarification needs to be sought from residents’ medical records.
To minimise missing data and to ensure a meaningful data set is obtained, we therefore propose to amend section 13.5.5 of the protocol and the following participant information and consent/declaration forms to include consent for researchers to access residents’ medical records [either via direct searching or remotely, via the Health and Social Care Information Centre (now NHS Digital)]:
-
resident information sheet
-
short form of resident information sheet
-
resident consent form
-
Personal Consultee declaration form
-
Nominated Consultee declaration form.
Full NHS research and development permissions will be obtained from the relevant trusts and the study researchers will apply for research passports and approval to access these notes.
Substantial amendment 2: approved 22 April 2014
Changes to the care home information sheet
The care home information sheet has been amended to incorporate comments following the PPI review. The content has also been updated to correct inaccuracies and to provide additional information and clarification regarding trial processes.
Amendments to the approved document are highlighted using tracked changes. The following is a brief summary of the key changes:
-
clarification of the abbreviated title – ‘The EPIC trial’
-
the addition of the trade mark (DCM™) throughout
-
clarification of ‘What will happen in the study’
-
care home selection
-
confirming care home eligibility
-
participant consent
-
care home allocation
-
DCM training
-
data collection – researcher interview/questionnaires
-
-
clarification of ‘What do I do if I am interested in taking part?’.
Substantial amendment 3: approved 26 June 2014
Protocol amendments
Updated care home selection process
The proposed plan for care home selection has been revised during consultations with the statistical team and researchers who are experienced in recruitment in the care home setting to minimise the burden on care home staff. To maximise response rates while retaining a representative sample of care homes in an attempt to maximise the generalisability of trial results, we propose to amend section 7.2 of the protocol to incorporate the following key changes:
-
Care homes within the hub catchment area are screened for eligibility and randomly ordered for subsequent contact.
-
Invitation information is sent to ordered samples of eligible care homes.
-
Researchers contact all of the invited care homes (via telephone) to determine their interest – the care home reply slip is no longer required.
-
If interested care homes will complete the eligibility assessments via researcher interview, the eligibility screening questionnaire is no longer required.
Eligibility criteria
English proficiency
Following discussions with the TMG and the TSC, we intend to update the eligibility criteria for residents, staff (completing staff measures only) and residents’ relatives/friends to include the following: ‘Has sufficient proficiency in English to contribute to the data collection required for the research.’
We propose this change for staff completing staff measures, as this questionnaire will be self-completed by members of staff with no assistance from trial researchers. Therefore, to ensure that staff understand the questions being asked, they must have sufficient proficiency in written English. Validated translations of assessments are also not available; therefore, the TMG agreed that it was not appropriate to use translated versions because of the potential impact on the validity of the data collected. Consultation with care home managers and staff suggested that the majority of staff working within UK care homes should have sufficient English proficiency as a requirement for employment.
The proposed change has been suggested for residents and their relatives/friends (if applicable), as assessments are completed via researcher interview; therefore, sufficient English proficiency is required to develop a meaningful dialogue. It was deemed infeasible by the TMG/TSC for translated discussions to be both available and accurate.
Proxy informant
As outlined in the protocol, the primary outcome for analysis is based on completion of the CMAI by a proxy informant (staff member). Therefore, we propose to update section 8 of the protocol to incorporate the following inclusion criteria for residents: ‘Has an allocated member of staff willing to provide proxy data.’
Screening questionnaires
Proxy informants (staff and relatives/friends) were initially required to demonstrate their willingness to participate by completing and returning a screening questionnaire. However, following a review of the process, the TMG have confirmed that it would be more appropriate to collect proxy informant data via researcher interview. It is hoped that this will decrease the burden on proxy informants and increase responses. We therefore propose to amend the relevant section of the protocol (sections 10.1–10.2).
Mutually exclusive roles
The protocol outlines roles that staff members can undertake within the trial and highlights any that are mutually exclusive (e.g. a mapper cannot act as a proxy informant). However, to clarify this further, we propose to update the exclusion criteria by role to ensure that eligibility is assessed ahead of consent. This update will also clarify that a Nominated Consultee (staff member) cannot actively participate in the trial in any way (e.g. providing staff or proxy measures).
Translation of trial documentation (information sheets/questionnaires)
Following consultation with the TMG and suggested updates regarding sufficient English proficiency, the TMG agreed that translation of trial documentation would no longer be required. Discussions regarding the variety of translations required by region (hub) also suggested that this process would not be feasible. Therefore, references to translation of materials have been removed from the protocol.
Data collection/assessments
Assessments
We propose to amend data collection assessments used within the trial, following a review with the TMG, as summarised below:
-
DEMQOL is to be replaced with QOL-AD: the TMG agreed that this was more appropriate to the trial population.
-
Caring Efficacy Scale is to be replaced with SCIDS: the TMG agreed that this was more appropriate to the trial population.
-
Bristol Activities of Daily Living Scale is to be removed: TMG agreed that it is not appropriate to collect this in the trial population.
The overall quantity of data to be collected, and therefore the perceived participant burden, remain the same.
Completion of assessments (Pittsburgh Agitation Scale/Quality of Interactions Schedule)
The proposed plan for collection of independent assessments (PAS/QUIS) suggested that the PAS and QUIS would be completed on a random 25% of registered residents. However, as these assessments are completed following observations made within communal areas, the TMG agreed that it would not be appropriate to restrict observations to a random sample of residents, because if they were not available within communal areas at the time of observation, the data collection could not be completed, affecting the integrity of the data for analysis. Thus, it was agreed that PAS/QUIS data would be collected for all registered residents. The protocol has been updated to incorporate these changes.
Monitoring: recording sessions
Dementia Care Mapping intervention: feedback sessions
The protocol outlines the recording sessions planned for DCM feedback sessions within a sample (a minimum of 10 care homes) of randomised care homes (n = 30). Following discussions with DCM experts, it was agreed that the feasibility and accuracy of a standardised review would not be sufficiently robust and therefore it should not be undertaken. Therefore, we propose to update section 12.7 of the protocol to remove references to audio recording.
Withdrawal
Proxy informant: relative/friend
We propose to amend the planned process for data collection following relative/friend withdrawal. The protocol currently suggests that, in the event of a relative/friend withdrawing, researchers would encourage the continuation of a subset of assessments. However, following a review with the statistical team, discussions concluded that this process would not be feasible and does not have a significant impact on the validity of the data for analysis. Therefore, we propose to amend section 12.12 of the protocol to outline that, in the event of a relative/friend withdrawing, a new proxy informant will be identified to complete all assessment measures.
Resident safety
Following consultation with the trial DMEC, we propose changes to the protocol to ensure that sufficient safety data are collected for ongoing safety monitoring. The proposed changes include:
-
proactive (monthly) reporting of adverse events that fulfil the SAE criteria (i.e. hospitalisation)
-
an annual summary of hospitalisations for registered residents collected from the Health and Social Care Information Centre.
Suggested amendments to safety reporting have been reviewed by external experts (DMEC/TSC) to ensure that reporting is commensurate with the risk for this population in the context of this trial.
Data Monitoring Ethics Committee
In accordance with guidance from the trial funder (NIHR HTA programme), a trial DMEC has been established and responsibilities have been agreed. We therefore propose changes to the protocol to incorporate the DMEC.
Participant information sheet and informed consent form amendments
Study title
Following consultation with PPI groups and experts by experience, the TMG have agreed to amend the study title in publicly available information to remove the acronym ‘DCM’ (Dementia Care Mapping). We therefore include information sheets and consent forms with the title amended throughout.
Participant consent
Mapper
We propose to add an additional statement to the mapper consent form to reference the DCM training course schedule, to make it clear to mappers that we are asking them to be available for training, as follows: ‘I agree to attend the next scheduled DCM training course if my care home is randomly allocated to DCM + UC. <Insert course date>‘. As the DCM training course is a publicly available course, dates are scheduled in advance and cannot be changed, so we need to be sure that mappers are able to attend on specified dates. This will reflect the implementation of the intervention in practice.
Staff proxy
The proposed plan for staff proxy informant consent was vague in the protocol, with no previous staff proxy informant consent form being submitted for REC approval. Therefore, in accordance with the proposed protocol update that removes references to the screening questionnaire (implied consent following return of data), a staff proxy informant consent form has been produced and is submitted for approval. This document will be version 4.0 (dated 30 May 2014) to match existing documentation following approval of this amendment.
Residents (including Nominated and Personal Consultees)
As data on residents and on residents’ relatives/friends are not used as part of the primary analysis, the TMG have agreed that consent to obtain information from residents and their relatives/friends can be optional. We therefore propose to update the relevant information sheets to incorporate these optional statements.
Short form of participant information sheet
Following consultation with PPI groups and experts by experience, we have developed shortened versions of the information sheets for staff (measures), staff proxies and relative/friend proxies. These short versions summarise the key information from the existing information sheets in a simple to understand format. It is intended that these information sheets will be used in addition to existing participant information to ensure that informed consent is obtained.
The existing short form of the resident information sheet has also been amended to reflect the format of the new short-form information sheets. These documents will be version 4.0 (dated 30 May 2014) to reflect existing documentation following approval of this amendment.
Substantial amendment 4: approved 10 September 2014
Protocol amendments
Submission of a new document for approval (Personal Consultee introductory letter)
The protocol (version 4.0) states in section 8.3.2, ‘Consent for those (residents) without capacity’, that, if an identified potential Personal Consultee is not present within the care home during participant (resident) recruitment, they may be sent information in the post regarding taking part (acting as a Personal Consultee) by the care home. We therefore enclose a proposed introductory letter template to be sent by care homes with the relevant (REC-approved) information sheets. As this letter is designed to be sent by the care home, it will be used as a template and added to where appropriate by the care home to personalise it for the person in question.
Submission of a new document for approval (Personal Consultee reminder letter)
The protocol (version 4.0) states in section 8.3.2, ‘Consent for those (residents) without capacity’, that a reminder will be sent to a potential Personal Consultee within 1 week of being approached to complete the relevant (REC-approved) declaration form. We therefore enclose a proposed reminder letter template for researchers to send within 1 week of initial approach (if required). As this letter is designed to be sent after initial discussions with the researcher, it will be used as a template and added to where appropriate by the researcher.
Submission of a new document for approval (relative/friend proxy informant introductory letter)
The protocol (version 4.0) states in section 10.1, ‘Relative/friend and informants’, that, if an identified potential relative/friend is not present in the care home during participant recruitment, information regarding taking part can be sent to them in the post (by the care home). We therefore enclose a proposed introductory letter template to be sent by the care home with the relevant (REC-approved) information sheets. As this letter is designed to be sent by the care home, it will be used as a template and added to where appropriate by the care home to personalise it for the relative/friend.
Substantial amendment 5: approved 15 January 2015
Participant information sheet and informed consent form amendments
Submission of a new document for approval (general practitioner letter)
The protocol (version 4.0) states in section 12.1, ‘Intervention details – usual care’, that all GPs that deliver care in a consenting care home will be provided with current best-practice guidelines for managing BSC. We therefore enclose a proposed GP letter template to be sent to GP practices with a copy of current antipsychotic prescribing guidance (Alzheimer’s Society). Please note that, in accordance with the protocol, this information will not give details of residents currently participating in the study.
Substantial amendment 6: approved 15 January 2015
Protocol amendments
Change of sponsor
Following acceptance of a professorship role at Leeds Beckett University, Claire Surr, DCM EPIC chief investigator, will be transferring from the University of Bradford to Leeds Beckett University in February 2015. Therefore, the study sponsor will be transferred to reflect this move.
The following documents have therefore been updated:
-
NHS research and development and REC form
-
A3–1. Chief investigator
-
A4. Sponsor contact
-
A64. Details of research sponsor
-
A76. Insurance and/or indemnity
-
-
Protocol (version 5.0) section 20.4, ‘Clinical governance issues’
-
Protocol (version 5.0) section 23, ‘Statement of indemnity’
-
Protocol (version 5.0) section 24, ‘Trial organisational structure’.
Care home eligibility criteria
Based on experience from ‘pilot’ care home recruitment and consultation with the trial oversight committees (TMG/TSC), we propose to amend the care home eligibility criteria to clarify the requirements for having a sufficient population of permanent residents living with dementia to recruit (register), namely a minimum of 10 residents. This wording will reduce the exclusion of care homes that would otherwise be eligible but do not achieve the criteria as currently worded.
The protocol has also been updated to clarify the minimum and maximum resident recruitment limits. As previously stated, a minimum of 10 registered (eligible, consented and complete-data) residents is required per care home. In line with the experiences of care home recruitment to date, the trial team have also investigated whether a maximum recruitment limit is required. However, after a review of the impact of cluster size variability on the power calculations for analysis with the trial oversight committees (TMG/TSC), they have confirmed that no maximum limit for resident recruitment is required.
Therefore, the protocol (version 5.0, section 7.1, ‘Care home eligibility’) has been updated to reflect the suggested changes summarised above.
Resident eligibility criteria
During care home screening, it became apparent that the potential for co-enrolment to other studies is relevant not only to care homes, but also to residents. For example, a trial may be recruiting a large number of homes within the DCM EPIC hub catchment areas (London, Oxford and West Yorkshire), but may be recruiting only a small proportion of residents in the participating care home. Therefore, it would not be appropriate to exclude the care home, owing to the associated impact on care home recruitment, but it would be appropriate to exclude the resident, owing to the potential for confounding factors and the associated participant burden and research fatigue.
Therefore, the protocol (version 5.0, section 8.1) has been updated to include the following: ‘involvement in another trial that conflicts with DCM or with the data collection during the course of their involvement in the EPIC study’.
Randomisation
Following randomisation of the first two ‘pilot’ homes, the team has reviewed the stratification factors [external factors (other than the intervention) that could have an impact on the trial outcome] for care home randomisation with the trial oversight committees (TMG/TSC). It was noted that the four current stratification factors do not include stratification by hub (London, Oxford and West Yorkshire). However, it was noted that ‘previous use of DCM’ might vary by hub, as Oxford care homes have introduced DCM at a local level.
Therefore, the team concluded, in consultation with the trial oversight committees, that the care home randomisation stratification factors should be updated, with ‘previous use of DCM’ replaced by ‘recruiting hub’. Protocol version 5.0, section 11.2, has been updated to reflect the suggested changes summarised above.
Substantial amendment 7: approved 22 October 2015
Protocol amendments
Witnessing consent
Recently, we have had a few instances in which we have had resident signatures that are almost illegible – some can pass for a signature; others are more of a mark. We have discussed this with the chief investigator, who is happy that any form of signature stands as informed consent, and notes that we must respect residents’ dignity by not asking for a witness countersignature just because their handwriting is not clear. In the current version of the protocol (section 8.3.1) we say:
Residents who are able to give informed consent will sign the trial consent form. Where a resident is unable to sign his/her name, s/he will be asked to make a mark on a consent form that will be witnessed by an independent observer (staff member, relative or friend).
However, on checking Health Research Authority guidance and the clinical trials toolkit, it seems that any form of mark is acceptable and that we would expect to need a witness only when a participant cannot write at all.
After verbal confirmation from the REC manager that following the Health Research Authority guidance on this issue is acceptable, we have removed this statement from the protocol and clarified that witnessing by an independent observer is required only when a resident is unable to make any kind of mark on the form. Therefore, section 8.3.1 has been updated as follows:
Residents who are able to give informed consent will sign or make a mark on the trial consent form. Where a resident is unable to sign, or make a mark, s/he will be asked to indicate his/her consent verbally. This will be witnessed by an independent observer (staff member, relative or friend) and recorded on the trial consent form.
Text messages to mappers
To assist the mappers in planning subsequent cycles, ahead of each of the three DCM mapping cycles, we will send a short text message to each mapper. The standard wording for these text messages can be found in the attached document (Mapper Text Reminders_V1.0_28/09/2015).
The following statement has been added to section 12.2.3 to reflect this process: ‘Ahead of each mapping cycle the CTRU will contact each mapper via SMS to remind them of the upcoming cycle’.
Participant information sheet and informed consent form amendments
The table below summarises the substantial amendments made to the participant information sheets, consent forms and covering letters. All amendments can be reviewed in the tracked change versions of the relevant documents.
| Document | Amendment details |
|---|---|
| Relative/friend proxy informant introductory letter for Personal Consultees | We have drafted a new letter to be used in instances in which the Personal Consultee is also invited to act as the relative/friend proxy informant for the resident. The current relative/friend proxy informant covering letters previously approved by the REC are aimed at relatives/friends who have no prior knowledge of the EPIC study, and so are not appropriate in these circumstances |
| Personal Consultee reminder letter – postal template (approach by care home manager) | The current Personal Consultee reminder letter previously approved by the REC is aimed at Personal Consultees who have previously spoken with the researcher at the care home regarding the EPIC study. In some instances, the potential Personal Consultee is approached via post as opposed to face to face in the care home (i.e. in cases in which their visits do not coincide with the researcher’s time in the care home) and, therefore, the wording of the current letter is not appropriate. This new letter is aimed at Personal Consultees who have had no prior contact with the researcher and, therefore, the initial approach would be by the care home manager/research lead |
| Personal Consultee reminder letter – postal template (approach by researcher) | This letter will be used for circumstances similar to the one outlined above; however, this letter will be for cases in which a potential Personal Consultee has already given consent to be contacted by the researcher directly and therefore the letter is from the researcher, rather than the care home manager/research lead |
| Relative/friend proxy consent form |
Updated to include date of birth (for identification purposes). Address and telephone number of relative/friend proxy added and a sentence regarding why this is collected added to page 2 |
| Personal Consultee declaration form |
Optional consent questions amended from initials to ‘Y’ or ‘N’ to aid completion There had been some confusion highlighted by the researchers over question 12; therefore, an additional question (Q12) has been added for clarification. The additional question confirms if the Personal Consultee is happy to be asked questions about their relative/friend (i.e. acting as a proxy) Owing to the addition of Q12, Q13 has been reworded to confirm that, if the Personal Consultee is not willing to be a relative/friend proxy, they are happy for other relatives/friends to take on this role Address and telephone number of Personal Consultee added and a sentence regarding why this is collected added to page 2 |
Substantial amendment 8: approved 4 January 2016
Protocol amendments
Process evaluation
More detail has been added to the protocol on how the process evaluation associated with the trial will work in practice. The design of the process evaluation remains the same (integrating data from the main trial data set/documentation with qualitative data from interviews and focus groups), but we have simply provided more detail on the participant information sheets and consent forms, data collection methods, sampling and data analysis that will be used.
Summaries of the extra detail provided are as follows.
Data collection
More detail has been provided on the data that will be extracted from the main trial data set and trial documentation. Topic guides have been developed to indicate the kinds of questions that will be asked of participants during the qualitative data collection. The topic guides are enclosed with this amendment application.
Sampling
To explore the implementation of the intervention with sufficient depth, we plan to conduct the qualitative data collection in a subset of homes. Homes will be primarily selected according to the degree of intervention implementation so that the factors affecting implementation can be thoroughly explored. More details on the sampling strategy are included in the amended protocol. A more basic evaluation of implementation (utilising data from the main trial data set and trial documentation) will still take place across all homes.
Data analysis
More detail is provided on the approach to the qualitative data analysis (framework analysis) and how the qualitative and quantitative analyses will be integrated.
Staff measures booklet
There has been a poor return rate for the staff measures booklet, despite multiple efforts to increase compliance. Following consultation and discussion with the DMEC and the TSC, it has been agreed that persistence in relation to staff data is important because DCM (the intervention) is designed to effect a ‘whole home’ change. To try and increase compliance, the TSC has suggested reducing the length and the identifiable nature of the staff booklet. To this end, we are proposing to remove the GHQ-12 and the request for personal data from the booklet. We would also like to improve the aesthetics of the booklet to ensure that it is as easy as possible for staff to complete.
Relative/friend informants
There has been poor trial participation by relatives/friends despite efforts to encourage uptake. It has been agreed by the oversight committees that the low percentage of data received will not be sufficient for quantitative analyses. Therefore, new relative/friend informants will not be identified at any follow-up time points, as this would utilise significant researcher resources but would be unlikely to result in much additional uptake or data. However, we will continue to request follow-up data for relative/friends who provided data at baseline because data from different time points could still be usefully analysed (e.g. to allow an analysis to be undertaken of the agreement between staff, resident and relative/friend completed measures and to augment the process evaluation). Relatives/friends who completed these baseline measures also indicated that they valued the opportunity to share their experiences and so would be likely to continue to take part. It seems unethical to exclude their data because of poor participation from other relatives.
Participant information sheet and informed consent form amendments
We have developed new participant information leaflets and consent forms for the three groups that will be asked to participate in the process evaluation: staff, residents and relatives. The information leaflets and consent forms have been developed with PPI input.
Substantial amendment 9: approved 15 April 2016
Protocol amendments
Design change
We propose a change to the design of the EPIC trial, such that additional residents will be recruited at the 16-month follow-up time point from each care home, in order to minimise bias (owing to higher than anticipated loss to follow-up) and to maintain the power and validity of the trial. The trial conduct will be affected in the following ways:
-
additional resident screening, recruitment and registration will be needed
-
new staff proxies will need to be identified
-
additional data collection from staff proxies will be needed
-
data management will be affected
-
statistical analyses will be affected.
Therefore, the relevant sections of the protocol have been updated and the following new supporting documents have been produced to support the recruitment process:
-
16M Resident Information Sheet_SHORT_v1.0 18 March 2016
-
16M Resident Information Sheet_v1.0 18 March 2016
-
16M Resident Consent Form_v1.0 18 March 2016
-
16M Personal Consultee Introductory Letter_v1.0 18 March 2016
-
16M Personal Consultee Declaration Form_v1.0 18 March 2016.
Staff proxy informant consent
We propose an alternative method of documenting staff agreement to provide data about the resident they know well. In a similar trial in care homes run by the CTRU, the REC has agreed that provision of information to staff proxies followed by verbal consent to take on the role is sufficient. Staff agreement to keep their name on record for follow-up purposes is documented by the researcher in the data-collection booklets. It is felt that this process is fit for purpose, given that we are not collecting any other personal data relating to the staff member.
We propose that this process be adopted for the involvement of all staff proxies recruited at 16 months in the EPIC trial, and we will adjust the data-collection booklets accordingly.
Care home indemnity
We propose to remove the statement ‘Possession of the appropriate insurance will be checked at point of recruitment of the care home to the study.’ This is in line with new guidance received following the change of the study sponsor. The sponsor has advised that this statement be removed, as EPIC is a trial of a low-risk intervention, with care home employees delivering the intervention. Therefore, it is appropriate to assume that standard care home insurance will cover the activities of the employees and additional checks are not required.
Staff measures data collection
Following a review of the data collection process, we have amended the trial protocol (section 9, ‘Staff roles, eligibility, recruitment and consent’) to include collection of the ‘current pattern of work’. This information will be used to determine the impact of shift patterns on staff training and exposure to the trial intervention.
Process evaluation: relative/friend recruitment
We propose to introduce a new document, ‘RF Introductory Letter – PE’, to support postal invitations sent to relatives/friends to ask them to participate in the process evaluation. This document would be sent with a copy of the relevant information sheet and consent form to relatives/friends currently participating in the main trial that are not available in the care home during researcher visits. EPIC researchers would confirm with the care home manager (or delegate) that postal contact is appropriate, prior to contacting the relative/friend.
In addition to the new introductory letter, we also propose to amend the relative/friend consent form so that those completing and returning it by post can outline their availability for discussions. This information would be useful, as it would help researchers to schedule their time and ensure availability for relative/friend feedback.
Following comments from the trial funder, we also propose to amend the number of residents and staff members approached to participate in the process evaluation. We had originally planned to include two or three residents and eight members of staff; however, we now propose to recruit up to 5 residents and up to 10 members of staff. This amendment will allow for flexibility in homes that have limited numbers of residents; it could also result in the emergence of key themes from fewer interviews.
General practitioner information for residents recruited at 16 months
We propose to update the protocol (section 12) to clarify that we will be sending generic best-practice guidance to GPs only for residents recruited at baseline and not for those additional residents recruited at 16 months (associated with the design change summarised above). This is because of the timelines for circulation of information to GPs and the potential confusion regarding active care home participation in the project, which ceases after the 16-month data collection. The guidance information would therefore also have a limited impact on trial outcomes at this stage (i.e. supporting person-centred care).
Personal Consultee capacity
Following a review of trial processes, we propose to update the protocol (section 8.1.2, ‘Consent for those without capacity’) to clarify the process for confirming the ongoing capacity of Personal Consultees. As a Personal Consultee is not required to visit a care home with any frequency, and has the ability to provide postal ascent for trial participation, trial researchers may never have face-to-face contact with a Personal Consultee. Therefore, it is not feasible to determine any changes in capacity over time in accordance with the Mental Capacity Act. 103 In these instances, it is essential to obtain input from care home staff, who may have more frequent interactions with the Personal Consultee and may be best placed to identify changes in capacity over time.
Substantial amendment 10: approved 25 July 2016
Protocol amendments
Process evaluation: participant demographics
We are proposing an additional data collection of participant demographics (age/gender) for those consented to participate in the process evaluation to aide with summarising the population sampled at analysis. As participants in the process evaluation are not required to have taken part in the main trial (as the intervention affects the entire care home irrespective of individual trial participation), we are not able to summarise demographics as a subset of the main trial population. Therefore, we have amended the relevant sections of the EPIC protocol (section 14, ‘Process evaluation’).
We have also updated the topic guides to include prompts to confirm participant details (identification, role) at the start of the interview to assist with identification of recordings, as is best practice for qualitative interviews. Any personal identifiers (e.g. name) will be removed from all transcriptions.
Text messages to mappers
We propose to introduce an additional text message to be sent to staff members acting as DCM mappers to highlight the mutually exclusive roles in the EPIC trial ahead of follow-up (at 6 and 16 months). In the EPIC trial, researchers completing follow-up data collection (at 6 and 16 months post randomisation) are blinded to care home allocation and are therefore not aware of any changes to staff members delivering the trial intervention [researchers recruit staff to act as mappers at baseline in all homes (n = 50); however, owing to high staff turnover, these often change during the course of the trial for those homes randomised to deliver the intervention (n = 31)]. This has therefore led to instances of inappropriate members of staff (i.e. mappers – those delivering the trial intervention) providing data (staff proxy informant) for participating residents.
We would therefore like to circulate the following text message ahead of follow-up (at 6 and 16 months) to staff currently acting as a consented mapper:
EPIC researchers will be visiting your home shortly to collect some more data. Please remember not to provide data on behalf of any residents during this visit. Do not tell the researcher you are acting as a DCM mapper. Regards, the EPIC team!
The following statement has been added to the protocol (section 9, ‘Staff roles, eligibility, recruitment and consent’):
A text message will be sent to trained DCM mappers ahead of data collection (6 and 16 months post randomisation) to remind mappers not to provide proxy data relating to residents.
Appendix 3 Rationale for design change
Health Technology Assessment programme extension application 11/13/15: the EPIC trial (March 2016)
Justification
In our original sample size estimation, we anticipated a 25% loss to follow-up rate of residents at 16 months (our primary outcome) following care home randomisation, to detect a clinically important difference of 3 points (SD 7.5) in agitation using the CMAI. If loss to follow-up was higher than anticipated (but no greater than 35%), our sample size of 750 residents would still provide more than 85% power at a two-sided 5% significance level to detect the moderate effect size, equating to 0.4 SDs.
By monitoring loss to follow-up within the trial, we are now confident that the rate will exceed our lower limit of 25%. Using data from care homes randomised into the trial up to 27 November 2015, we predict that loss to follow-up at 16 months will be within the range of 32.4% to 48.1%. As such, continuation of the trial as currently planned is unlikely to provide sufficient power for statistical analysis of the primary end point and so an amendment is required to ensure that the results of the trial are robust and generalisable. Therefore, based on consideration of all the available options, we propose recruiting more residents at follow-up (i.e. move to an ‘open-cohort’ design).
As of 27 November 2015, there were 42 care homes randomised, with 638 registered residents. Residents are registered before care home randomisation. Overall, there were 11 residents lost before the care homes were randomised, so, at the point of randomisation, 627 residents were included in the trial. None of the care homes had reached the 16-month follow-up time point and there were two care homes currently at 13 months following randomisation.
Loss to follow-up rates were estimated using the number of residents who died or moved care home between randomisation and 27 November 2015. The rate was then extrapolated to 16 months. Figure 10 summarises the actual and predicted loss to follow-up rates by number of months since randomisation. The same information is displayed graphically in the Kaplan–Meier curve in Figure 11.
FIGURE 10.
Predicted loss to follow-up. The x-axis represents the number of months that care homes have been randomised; the numbers lost to follow-up are grouped by care home and month.
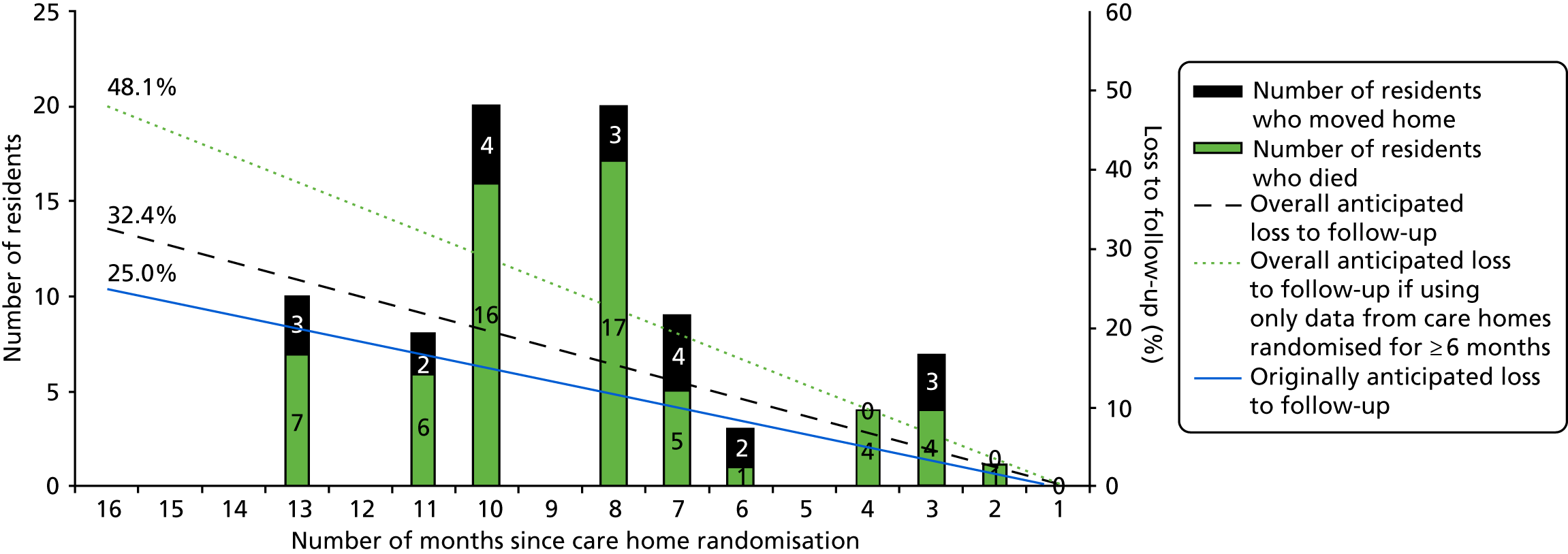
FIGURE 11.
Kaplan–Meier survival curve stratified by the length of time the care home has been in the trial. Number of residents at risk at care home randomisation and at 3, 6, 9 and 12 months. Note that 16 months is 487 days’ ‘survival’.
To provide a robust evaluation of the trial, we propose to move to an open-cohort design in which all eligible residents who (1) have resided in the care home for 3 months or more, 16 months after care home randomisation and (2) are not already taking part in the trial or have not already declined to take part will be approached to provide consent for trial participation at the 16-month follow-up visit. All those consenting to take part (residents already participating in the trial and consented at baseline, as well as additional residents consenting at 16 months) will provide data at 16 months.
The key impact of this option will be an increase in the size of the cohort at follow-up, which will maintain the power of the trial and its ability to detect the effect size of 0.4 with 90% power.
Sample size calculations
With a current estimated 48.2% loss to follow-up, we expect to lose 360 residents before the 16-month follow-up, meaning that we will have data at all three time points from 388 residents. All of the other parameters – significance level, two-sided test and ICC of 0.1 – are the same. We have done sample size calculations for three different scenarios of additional recruitment and all provide sufficient power to detect the effect size of 0.4.
If we recruit, on average, an additional three residents per care home at the 16-month follow-up (from the remaining 48 care homes) the sample size will be 388 + (48 × 3) = 532 residents (i.e. 10.64 residents/care home). The design effect will be 1 + (10.64 – 1) × 0.1 = 1.964. We will achieve 89% power to detect the effect size of 0.4.
By replacing residents with 35% recruited residents, the overall number of residents available for the analysis would be 388 + 254 (additional recruits) = 642. The mean number of residents/care home (cluster size) would be 12.8. The design effect is now 1 + (12.8 – 1) × 0.1 = 2.18. The power to detect the effect size of 0.4 would be 91% (or with 90% power, we can detect a smaller effect size of 0.39).
By replacing residents with 25% recruited residents, the overall number of residents available for the analysis would be 388 + 182 (additional recruits) = 570. The mean number of care home residents/care home (cluster size) will be 11.4. The design effect is now 1 + (11.4 – 1) × 0.1 = 2.04. The power to detect the effect size of 0.4 would be 90%.
All scenarios will achieve the desired effect size with sufficient power. The message to researchers should still be to recruit as many residents as possible to minimise bias. We will need to monitor recruitment to ensure that we have at least three extra residents from each remaining care home.
The benefits of this design change are:
-
We will be able to detect intervention effects at the care home level (as the intervention is aimed at the whole care home).
-
Our conclusions can be generalised to a broader population of residents (i.e. not just to those still residing in the care home 16 months after randomisation).
-
We will be able to conduct analyses based on both a cross-sectional (i.e. open-cohort) and a closed-cohort (longitudinal) design.
-
We will minimise selection bias by providing an objective criterion for inclusion (all eligible consenting residents).
-
Our recruitment process will be resource-effective, as all eligible residents can be approached to participate at a single time point.
-
We will be less reliant on assumptions regarding missing data mechanisms.
Consideration was given to recruiting only a proportion of eligible residents at each home at 16 months (to increase resident numbers to 75% baseline recruits, in line with originally predicted loss to follow-up rates). However, the team and oversight committees (the TSC and the DMEC) agreed that such an option would be open to selection bias and that statistical power and the ability to generalise could be limited by including a limit to the number of residents recruited at baseline. Recruitment processes could also be protracted by virtue of allowing time for Personal Consultee response (i.e. should this be negative, further resident–consultee dyads would then need to be approached, thus considerably lengthening the recruitment process and adding to researcher workload and thus cost).
As well as maintaining power and increasing generalisability, this design change incurs minimal additional cost compared, for example, with recruiting additional clusters.
This application for extension and the included options have been discussed in detail at the DMEC and TSC meetings in November and December 2015, respectively, based on the figures presented here. Those committee members supported the open-cohort design, with the DMEC recommending it, provided that we address the risk of selection bias. It should be noted that, as of the beginning of January 2016, we have met our target of randomising 50 care homes but the patterns of loss to follow-up remain unchanged.
We believe that approaching all eligible residents best addresses the potential threat of selection bias. With the additional recruitment of eligible residents, we will be able to achieve a power of over 90%, even if loss to follow-up in the original sample of residents was 50%. Moving to an open-cohort design will require additional funding and time to complete the trial – we are requesting an additional 3-month extension to the trial (to the end of December 2017) to allow for the additional analysis and write-up time that will be needed if the design change is approved. We are not requesting additional funding for all co-applicants and trial staff for this period.
Impact if approved
The design change involves recruiting additional residents only from care homes that are already randomised and aware of the requirements of the trial. We envisage that additional trial processes will result in minimal additional burden on care homes.
Researchers will be able to combine 16-month follow-up visits to existing care homes (to see existing residents) with recruitment and data collection for newly eligible residents. This reduces researcher burden (when compared with recruiting entirely new care homes), although it does involve additional time at each care home.
By implementing an open-cohort design, we will be able to generalise trial results to a broader group of dementia residents and complete the trial robustly with sufficient power.
Impact if not approved
If the request is not approved, high attrition rates may decrease the statistical power, introduce bias in trial reporting and pose a threat to the validity and generalisability of the trial.
If we continue with the trial with its current design, based on current data, the anticipated proportion of residents lost to follow-up (died or moved care home) would be at least 32%. However, only 17/42 (40.5%) care homes have been randomised for more than 6 months. If only those randomised for more than 6 months were included in the estimation of overall loss to follow-up (as this would allow more precise estimates), the predicted loss at 16 months would be 48%.
Loss of entire cluster(s) is also a realistic scenario if the request is not approved, with small clusters being most likely to be lost. Loss of clusters in addition to loss of residents induces further bias, as loss of cluster(s) as a unit of randomisation has a greater influence in cluster randomised trial analysis than loss of individual residents.
| Number of registered residents at randomisation | 750 | |||
|---|---|---|---|---|
| Loss to follow-up | 32% | 48% | ||
| Design effect | 1.96 | 1.72 | ||
| Power | 90% | 80% | 90% | 80% |
| Ability to detect the effect size | 0.41 | 0.36 | 0.45 | 0.39 |
The design effect (due to clustering of resident outcomes within care homes) is lower, with higher loss to follow-up because the available mean cluster size at follow-up is smaller. However, high losses to follow-up with loss of entire clusters threaten the validity of the trial, introduce bias and affect generalisability.
Glossary
- Agency staff
- Temporary staff who are provided by an external organisation (an agency) to cover staff shortages/absences when these cannot be met by the care home’s own staff pool.
- Bank staff
- A pool of staff employed by the care home on non-substantive contracts and who are drawn on when the care home is unable to cover absences or shortages with staff who have contracted hours.
- DCM expert mapper
- An experienced user of DCM appointed by the trial to support trial mappers in completing cycle 1 of DCM in each intervention home.
- DCM intervention lead
- A member of the trial team who is responsible for oversight and leadership of DCM implementation across the intervention care homes and co-ordination of the DCM expert mappers.
- Independent researcher
- A member of the research team who is independent of the care home by virtue of not having previously collected any outcomes data there.
- Mapper
- A member of care home staff trained to use DCM.
List of abbreviations
- A&E
- accident and emergency
- ADAPT
- Assessment of Dementia Awareness and Person-Centred Care Training
- BCC
- behaviour category code
- BPSD
- behavioural and psychological symptoms of dementia
- BSC
- behaviours that staff may find challenging to support
- CACE
- complier-average causal effect
- CCA
- complete-case analysis
- CDR
- Clinical Dementia Rating
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CMAI
- Cohen-Mansfield Agitation Inventory
- CMAI-O
- Cohen-Mansfield Agitation Inventory Observational
- CONSORT
- Consolidated Standards of Reporting Trials
- CQC
- Care Quality Commission
- CRF
- case report form
- CTRU
- Clinical Trials Research Unit
- DCM™
- Dementia Care Mapping
- DEMQOL
- Dementia Quality of Life
- DEMQOL-proxy
- Dementia Quality of Life – proxy version
- DMEC
- Data Monitoring and Ethics Committee
- EAT
- Environmental Audit Tool
- EPIC
- Enhancing Person-centred care In Care homes
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-5D-5L-proxy
- EuroQol-5 Dimensions, five-level version, proxy version
- FAST
- Functional Assessment Staging Test of Alzheimer’s Disease
- FITS
- focused intervention for training staff
- GEE
- generalised estimating equations
- GHQ-12
- General Health Questionnaire-12
- GLHC
- Group Living Home Characteristics
- GP
- general practitioner
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LAG
- Lay Advisory Group
- MAR
- missing at random
- MCAR
- missing completely at random
- ME
- mood/engagement
- MI
- multiple imputation
- MICE
- multiple imputations by chained equations
- MNAR
- missing not at random
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NPI
- Neuropsychiatric Inventory
- NPI-NH
- Neuropsychiatric Inventory – nursing home version
- PAS
- Pittsburgh Agitation Scale
- PCCT
- person-centred care training
- PPI
- patient and public involvement
- PRN
- pro re nata (as required)
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QOL-AD
- Quality of Life in Alzheimer’s Disease
- QUALID
- Quality of Life in Late-Stage Dementia
- QUIS
- Quality of Interactions Schedule
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RUSAE
- reported unexpected serious adverse event
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SCIDS
- Sense of Competence in Dementia Care Staff
- SCIE
- Social Care Institute for Excellence
- SD
- standard deviation
- SMS
- short message service
- SUR
- seemingly unrelated regression
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
