Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/01/25. The contractual start date was in February 2013. The draft report began editorial review in February 2019 and was accepted for publication in September 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jane Abbott, Janet Berrington, Elaine Boyle, Ursula Bowler, Jon Dorling, Nicholas Embleton, Kenny McCormick, William McGuire, Edmund Jaszczuk, Samantha Johnson, Madeleine Hurd, Oliver Hewer, Andrew King, Alison Leaf, Louise Linsell, Christopher Partlett, David Murray, Ben Stenson, Judith Rankin and Tracy Roberts report funding from the National Institute for Health Research (NIHR) for the trial. Jon Dorling, Janet Berrington, Elaine Boyle, Nicholas Embleton, Edmund Jaszczuk, Samantha Johnson, Andrew King, Louise Linsell, William McGuire, Christopher Partlett and Tracy Roberts report receipt of funding from NIHR, outside the submitted work. Jon Dorling reports grants from Nutrinia (Nazareth, Israel) outside the submitted work; specifically, he was funded for part of his salary to work as an expert advisor on a trial of enteral insulin. Furthermore, he was a member of the NIHR Health Technology Assessment (HTA) General Board (2017–18) and the NIHR HTA Maternity, Newborn and Child Health Panel (2013–18). Elaine Boyle reports grants from the Medical Research Council and East Midlands Specialised Commissioning Group outside the submitted work. Janet Berrington reports grants and personal fees from Danone Early Life Nutrition (Paris, France) and grants from Prolacta Biosciences US (Duarte, CA, USA) outside the submitted work. Nicholas Embleton reports grants from Prolacta Biosciences US and Danone Early Life Nutrition and personal fees from Nestlé Nutrition Institute (Vevey, Switzerland), Baxter (Deerfield, IL, USA) and Fresenius Kabi (Bad Homburg vor der Höhe, Germany) outside the submitted work. Samantha Johnson reports grants from Action Medical Research (Horsham, UK), EU Horizon 2020 (Brussels, Belguim), the Medical Research Council (London, UK), Sparks (London, UK) and the Nuffield Foundation (London, UK) outside the submitted work. William McGuire is a member of the NIHR HTA Commissioning Board (2013 to present) and the HTA and Efficacy and Mechanism Evaluation Editorial Board (2012 to present). Edmund Juszczak was a member of the NIHR HTA General Board from 2016 to 2017 and the HTA funding committee (commissioning) from 2013 to 2016.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Dorling et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced and adapted from Abbott et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Outcomes affected by feeding strategies
In the UK, 1–2% of newborn infants are very preterm or have a very low birthweight (VLBW). Preterm birth is the major risk factor for infant mortality, with 73% of neonatal deaths in the UK occurring in infants born before 37 completed weeks of gestation. 2 As survival, especially of very preterm infants, has increased in recent years,3 the high prevalence of morbidity associated with preterm birth means that the assessment of long-term outcomes has become increasingly important. 4 Short- and long-term outcomes for preterm infants are affected by strategies that reduce infection rates, lower necrotising enterocolitis (NEC) rates, promote adequate growth and maintain access to tertiary-level facilities.
Optimising infant feeding strategies offers the potential to improve all of these outcomes. Benefits are likely to arise from both the individual and the combined effects of identifying the optimum feeding strategy, as the rates of such complications in very preterm infants are high. NEC that is severe enough to cause death or require surgery affects approximately 7.5% of infants born before 29 weeks of gestation and is the cause of death in 11% of the deaths of infants born before 32 weeks’ gestation. 5 Late-onset sepsis (LOS) affects around 25% of very preterm infants and is responsible for 10% of deaths in the same population. Long-term data following LOS or NEC suggest that these conditions almost double the risk of poor neurodevelopmental outcome. 6 Preterm infants are at significant risk of poor long-term neurodevelopmental problems: almost 12% of infants have moderate or severe disability,7 with both sepsis and NEC dramatically increasing this risk. 8–13
Nutritional support of preterm infants and speed of increasing milk feeds
Every year in the UK, around 8000 infants are born so preterm that they cannot initially be fed milk and, therefore, require intravenous nutrition. Milk feeding is gradually increased as the immature gut begins to tolerate milk and intravenous nutrition is correspondingly reduced, but there are few data determining how quickly this is best achieved. 14 One of the most serious complications of intravenous feeding is LOS, which occurs in 27% of infants born weighing < 1500 g at birth or under 29 weeks’ gestation. 14 LOS is known to cause poor long-term cognitive outcomes, liver damage and sudden death from cardiac problems resulting from misplaced catheters. 15–17 One of the most common late-onset infections is ‘catheter-related bloodstream infection’; the risk of bloodstream infection is directly related to the time the catheter is indwelling in the bloodstream. 18–20 The more rapid advancement of enteral feeds described in this study will, in principle, reduce exposure to intravenous nutrition by causing infants to reach full milk feeds (tolerating 150 ml/kg/day for 3 consecutive days) approximately 4 days earlier than the slower advancement. Reducing exposure by this amount could reduce the number of infections by between 5 and 15 cases per 250 infants, which is an absolute risk reduction of 4%. This is possibly an underestimate of the reduction, as infection risk increases with the length of time a catheter is in place. 21,22
However, faster increases in milk feed volumes may increase the likelihood of NEC that, as well as being potentially fatal, may provoke intolerance of feeds or gut dysfunction, which could result in longer times to achieve full feeds rather than shorter. Survivors of NEC also have significantly worse long-term outcomes across multiple developmental domains than those who are unaffected. 6,23 Therefore, although emerging data suggest that better health outcomes may be achieved with faster feeding increments, there are possible disadvantages of this and a randomised controlled trial (RCT) is required to support a change in clinical practice. 14
Existing evidence
Existing trial data are insufficient to determine whether or not advancing enteral feed volumes slowly (typically < 24 ml/kg/day) or more quickly (daily increments of 30–40 ml/kg) affects outcomes, including the risk of neurological impairment, LOS or NEC in very preterm or VLBW infants. 14,24–32 The Cochrane review14 included nine RCTs with a total of 949 participants (Box 1). None of the studies prior to The Speed of Increasing milk Feeds Trial (SIFT) published neurodevelopmental outcomes in early childhood and the Cochrane review authors concluded ‘that advancing enteral feed volumes at daily increments of 30 to 40 ml/kg (compared to 15 to 24 ml/kg) does not increase the risk of NEC or death in VLBW infants’. 14 They also concluded that ‘advancing the volume of enteral feeds at slow rates results in several days of delay in establishing full enteral feeds and increases the risk of invasive infection’. 14 ‘The applicability of these findings to extremely preterm, extremely low-birthweight or growth-restricted infants is limited’ due to the participants studied and ‘further randomised controlled trials in these populations may be warranted to resolve this uncertainty’. 14 SIFT provided this information by recruiting a large number of infants, including those at highest risk.
-
Typical RR 1.46, 95% CI 1.03 to 2.06; typical RD 0.07, 95% CI 0.01 to 0.13; number needed to harm 14, 95% CI 8 to 100; six trials, 553 participants.
-
Typical RR 1.02, 95% CI 0.64 to 1.62; typical RD –0.00, 95% CI –0.03 to 0.03; nine trials, 949 participants.
-
Typical RR 1.18, 95% CI 0.90 to 1.53; typical RD 0.03, 95% CI –0.02 to 0.08; eight trials, 791 participants.
CI, confidence interval; RD, risk difference; RR, risk ratio.
Objective
The study aimed to assess if faster (30 ml/kg/day) or slower (18 ml/kg/day) daily feed increments improve survival without moderate or severe disability at 24 months of age [corrected for gestational age (CGA)] and other morbidity and mortality in very preterm and/or VLBW infants.
Chapter 2 Methods
Parts of this chapter have been reproduced and adapted from Abbott et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Design
The SIFT was a multicentre, two-arm, non-blinded, parallel-group RCT in very preterm and/or VLBW infants in the UK and the Republic of Ireland. 1,33
Ethics approval and research governance
The SIFT protocol33 was approved by the National Research Ethics Service (NRES) Committee East Midlands – Nottingham 2 on 31 January 2013 (reference 13/EM/0030). Local approval and site-specific assessments were obtained from the NHS trusts for trial sites. The trial was registered with the International Standard Randomised Controlled Trial Register (https://doi.org/10.1186/ISRCTN76463425).
Patient and public involvement
The planning and delivery of the SIFT was facilitated by close engagement with infant and family representatives who were experienced in service-user representation. Bliss (www.bliss.org.uk/; accessed 29 August 2019), the UK national charity for ‘babies born premature or sick’, was the most heavily involved charity. Parents of children who had received neonatal intensive care contributed directly and via Bliss to both the development of trial materials [e.g. parent information leaflets (PILs) and consent forms] and training research staff (e.g. in simulated ‘consent-seeking’ sessions). INVOLVE good practice guidelines were followed to ensure service-user leadership in the trial delivery and dissemination of the findings. INVOLVE is a national advisory group established and funded by the National Institute for Health Research (NIHR) to support active public involvement in NHS, public health and social care research (www.invo.org.uk/about-involve/; accessed 9 December 2019).
Participants
Inclusion criteria
-
Gestational age at birth of < 32 weeks and/or birthweight of < 1500 g.
-
Receiving ≤ 30 ml/kg/day of milk at randomisation.
-
Written informed parental consent.
Exclusion criteria
-
Severe congenital anomaly.
-
No realistic prospect of survival.
-
Unlikely to be traceable for follow-up at 24 months of age (e.g. infants of non-UK residents).
Setting
The setting was neonatal units caring for very preterm infants in the UK and the Republic of Ireland:
-
Recruiting sites – parental consent was obtained, infants were enrolled by randomisation and participation in the trial was commenced (n = 55; see Appendix 1).
-
Continuing care sites – clinicians continued to administer the intervention and collect data if a participant was transferred from a recruiting or another continuing care site (n = 78; see Appendix 2).
Infants were able to participate in other clinical trials at the same time as taking part in the SIFT, depending on the nature of the interventions in the other trials. The Enteral Lactoferrin In Neonates (ELFIN) trial was designed alongside the SIFT to allow enrolment of infants into both trials. 1,34 The SIFT and the ELFIN trial shared some procedures including some joint data collection forms and other documents. Other trials running concurrently were discussed by the chief investigators or their delegated representative, who agreed whether or not joint recruitment was appropriate.
Screening and eligibility assessment
The local health-care team identified potential participants who met the eligibility criteria. Exclusion criteria were defined and assessed by clinicians. Assessment of eligibility was accepted to be within the scope of competency of appropriately trained and experienced neonatal nurses, as no specific medical assessments were required. Competency was formally delegated by the principal investigator (PI) on the delegation log.
Informed consent and recruitment
Parents of potential participants were approached only after receiving a PIL, which gave a full verbal and written explanation of the trial. Parents who did not speak English were approached only if an adult interpreter was available and if they were likely to be resident in the UK or the Republic of Ireland for at least 2 years.
The consent-seeking process included informing parents of the possible benefits and risks as a staged process. 35 If the anticipated infant was likely to be eligible to participate in the trial, preliminary verbal information and the PIL were offered prior to birth and this was followed up after birth. For infants who were not identified antenatally or if other issues took precedence, information was provided after birth.
Written informed parental consent was obtained by means of a dated parental signature and the signature of the person who obtained the informed consent; this was the PI or the health-care professional with delegated authority. The parents were given a copy of the signed informed consent form. A copy was retained both in the infant’s medical notes and in the site file by the PI and the original copy was posted to the trials unit co-ordinating centre.
No financial or material incentive or compensation was given to the participants or parents to take part. It was highlighted to parents that they were free to withdraw their infant from the trial at any time without the need to provide an explanation or reason. The PIL explained that such a decision would not affect any aspect of clinical care.
The trial entry form was completed after informed consent was received. Information on the form was then entered in the randomisation website hosted by the National Perinatal Epidemiology Unit (NPEU) Clinical Trial Unit (CTU) (https://rct.npeu.ox.ac.uk/; accessed 30 May 2015). Randomisation took place when the clinicians were ready to increase the feeds to > 30 ml/kg/day. Infants were considered to have been enrolled once they were allocated a study number and one of the two rates of feeding increment.
Interventions
Trial participants were allocated randomly to receive daily increments in milk feed volume of either 30 ml/kg or 18 ml/kg.
All other aspects of feeding and care followed routine clinical practice in the individual units, including the capacity to stop or alter the rate of the increase in feeds if clinically indicated.
Randomisation
Randomisation was performed by computer through a secure website hosted by the NPEU CTU, University of Oxford. A minimisation algorithm was used to balance prognostic factors: hospital, multiple birth, gestational age ranges and birthweight of < 10th centile for gestational age. The algorithm included a random component that minimises with 80% probability of reducing predictability. Multiple births were allocated to the same feeding increment rate.
Allocation concealment and blinding
The allocation sequence was concealed from those who were assigning participants by the web-based randomisation. It was not possible to safely and completely blind caregivers and parents to the feed rate. This was because nurses, doctors and parents indicated the need to know how much milk was being given as part of feeding practice and care-giving. Blinded end-point reviewers were not aware of the allocation for any participant when reviewing the outcome data.
Primary and secondary outcomes
The primary outcome was survival without moderate or severe neurodevelopmental disability at 24 months of age CGA. Moderate or severe neurodevelopmental disability was defined as any of:
-
moderate or severe visual impairment (i.e. reduced vision uncorrected with aids, blind in one eye with good vision in the contralateral eye or blind/perceives light only)
-
moderate or severe hearing impairment (i.e. hearing loss corrected with hearing aids, some hearing loss but not corrected by hearing aids, or deaf)
-
moderate or severe gross motor impairment (i.e. unable to walk or sit independently)
-
moderate or severe cognitive impairment, assessed using the Parent Report of Children’s Abilities – Revised (PARCA-R).
A total PARCA-R score of < 44 was used to identify children with moderate or severe cognitive impairment. 38 The definition is summarised in Box 2.
Secondary outcomes included:
-
mortality
-
moderate or severe neurodevelopmental disability at 24 months CGA
-
death before discharge home
-
microbiologically confirmed (Box 3) or clinically suspected late-onset invasive sepsis (Box 4) from trial entry to discharge home
-
NEC (Bell’s stage 2 or 3) from trial entry to discharge home40–42
-
time taken to reach full milk feeds (tolerating 150 ml/kg/day for 3 consecutive days)
-
growth (change in weight and head circumference z-score for gestational age) from birth to discharge home
-
duration of parenteral feeding
-
duration of time in intensive care
-
duration of hospital stay to discharge home
-
diagnosis of cerebral palsy by a doctor or other health professional
-
the individual components of the definition of moderate or severe neurodevelopmental disability.
Diagnoses of moderate or severe neurodevelopmental disability, LOS and NEC were confirmed by the blinded end-point review committee (BERC) using standard definitions (see Appendix 3 and the published protocol1,33). All of the data collection forms were assessed independently by pairs of clinicians who were unaware of allocation. We noted that owing to ‘rounding’ of the feed rate to the nearest 0.5 ml or to small changes in a daily weight in the clinical setting, some infants on ‘full feeds’ received only 146–149 ml/kg/day. We therefore considered an infant to be on full feeds if ≥ 145 ml/kg/day was tolerated for 3 consecutive days. Infants who did not meet these criteria were reviewed by the BERC to determine if a sustained level of feeding at a level below this had been achieved before discharge. Examples of this included feeds being stopped during transfer or for a procedure, use of higher-calorie formula or fluid restriction after 150 ml/kg/day had been reached.
For live infants, a parent-report questionnaire was used to assess sensory and gross motor impairment and standardised measures were used to assess cognitive function in order to identify children with:
-
Moderate/severe visual impairment (reduced vision uncorrected with aids, blind in one eye with good vision in the contralateral eye or blind/perceives light only).
-
Moderate/severe hearing impairment (hearing loss corrected with aids, some hearing loss but not corrected by aids, or deaf).
-
Moderate/severe gross motor impairment (unable to walk or sit independently).
-
Moderate/severe cognitive impairment assessed using the PARCA-R. Total PARCA-R scores of < 44 were used to identify children with moderate/severe cognitive impairment. 36,37
A child who has any one or more of these impairments will be classified as having a moderate/severe disability.
Definitions for motor and sensory impairments described above are as defined in the report published by, and reproduced with permission from, the British Association of Perinatal Medicine in 2008. 38
Microbiological culture of potentially pathogenic bacteria (including coagulase-negative staphylococci species, but excluding probable skin contaminants such as diphtheroids, micrococci, propionibacteria, or a mixed flora) or fungi from blood or cerebrospinal fluid sampled aseptically more than 72 h after birth, and treatment, or clinician intention to treat, for 5 days or more with intravenous antibiotics (excluding antimicrobial prophylaxis) after investigation was done. If the infant died or was discharged or transferred before the completion of 5 days of antibiotics, this condition would still be met if the intention was to treat for at least 5 days.
Reproduced from The ELFIN Trial Investigators Group. 39 (© The authors. Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. See https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Absence of positive microbiological culture, or culture of a mixed microbial flora or of probable skin contaminants (ie,diphtheroids, micrococci, propionibacteria) only, and treatment or clinician intention to treat for 5 days or more with intravenous antibiotics (excluding antimicrobial prophylaxis) after the investigation was undertaken for an infant who presents at least 3 of the following clinical or laboratory features of invasive infection:
-
increase in oxygen requirement or ventilatory support
-
increase in frequency of episodes of bradycardia or apnoea
-
temperature instability
-
ileus or enteral feeds intolerance or abdominal distension
-
reduced urine output to less than 1 mL/kg per h
-
impaired peripheral perfusion (capillary refill time longer than 3 seconds, skin mottling or core-peripheral temperature gap greater than 2°C)
-
hypotension (clinician-defined as needing volume or inotrope support)
-
irritability, lethargy, or hypotonia (clinician-defined))
-
increase in serum C-reactive protein concentrations to more than 15 mg/L or in procalcitonin concentrations to 2 ng/mL or more
-
white blood cells count smaller than 4×109/L or greater than 20×109/L
-
platelet count less than 100×109/L
-
glucose intolerance (blood glucose smaller than 40 mg/dL or greater than 180 mg/dL)
-
metabolic acidosis (base excess less than –10 mmol/L or lactate concentration greater than 2 mmol/L).
Reproduced from The ELFIN Trial Investigators Group. 39 (© The authors. Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. See https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Sample size
It was estimated that 80% of infants would survive to 24 months of age and 11% of survivors would have moderate or severe neurodevelopmental disability. 7 It was expected that the proportion with the primary outcome would be 71% in the comparator (slower increment) group. With a total sample size of 2500 and allowing for a questionnaire response rate of 80%, there would be 90% power to detect an absolute difference of 6.3% with a two-sided 5% significance level. Similarly, a sample size of 2500 infants would have 90% power to detect an absolute difference of 5.4% (from 25.0% in the comparator group) in the incidence of LOS43 and an absolute difference of 3.5% (from 6.0% in comparator group) in the incidence of NEC (Bell’s stage 2 or 3). 40–42
Subsequently, an inflation factor of 1.12 was applied to the sample size to allow for multiple births as they received the same allocation and would probably have correlated outcomes. This adjustment assumed the proportion of multiple births to be 25% and an intraclass correlation coefficient of 0.9 for the primary outcome at 24 months CGA, based on a previous study. 44 The total target sample size was therefore increased to 2800.
Statistical analyses
Demographic factors, baseline clinical characteristics and outcomes were summarised with counts and percentages for categorical variables, means [standard deviations (SDs)] for normally distributed continuous variables and medians [interquartile ranges (IQRs) or simple ranges] for other continuous variables. Outcomes were analysed according to allocation, using the slower fed group as the comparator.
Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated for the primary outcome at 24 months CGA and for the discharge outcomes of LOS and NEC, with a 99% CI used for all other dichotomous outcomes to allow for multiple comparisons. For normally distributed continuous outcomes, the mean difference (99% CI) was presented; for skewed continuous variables, the median difference (99% CI) was presented. Adjusted risk ratios were estimated using log-binomial regression, or log-Poisson regression with a robust variance estimator if the binomial model failed to converge. Linear regression was used for normally distributed continuous variables and quantile regression was used for skewed continuous variables. The primary inference was based on the analysis adjusting for the minimisation factors at randomisation. Centre was fitted as a random effect and all other factors were fitted as fixed effects. The mother’s identification was nested within centre to take account of the additional level of clustering due to multiples and siblings. This adjusts the standard error to allow for the lack of independence in trial allocation and the potential correlation in outcome.
The consistency of the effects of advancing milk feeds on the incidence of the primary outcome, LOS and NEC across specific subgroups of infants was assessed using the statistical test of interaction. Prespecified subgroup analyses included (1) week of gestation at birth, (2) birthweight of < 10th centile versus ≥ 10th centile for gestational age and (3) type of milk received during the hospital stay (i.e. breast milk only/formula only/mixed) (see Figures 3 and 4). A non-prespecified analysis assessed the effect of the speed increments on sepsis and NEC in infants with abnormal Doppler ultrasounds (see Table 4). Other deviations from the protocol included the use of quantile regression instead of Cox regression to analyse time to full feeds (as the Cox proportional hazard assumption was not satisfied) and mixed-effect log-binomial-Poisson models instead of generalised estimating equations (owing to the ease and flexibility of these methods, which were not in common use when the study was conceived). We performed a sensitivity analysis to examine the affect of missing data at 24 months on the primary outcome by considering different scenarios departing from the assumption that data were missing completely at random.
Data collection
All outcome data for this trial were routinely recorded clinical items that could be obtained from the clinical notes or local microbiology laboratory records. Information was collected using the data collection forms (see Appendix 5).
A BERC, masked to participant allocation, reviewed all case report forms (CRFs) that reported moderate or severe impairment at 24 months of age CGA, episodes of LOS, episodes of NEC or episodes of other gastrointestinal pathology. Two members who were blind to allocation independently assessed adherence to case definitions and resolved any disagreements or discrepancies by discussion or referral to a third committee member, or both. Persisting uncertainties were discussed with the site PI or research nurse or both until resolved.
We noted that owing to ‘rounding’ of the feed rate to the nearest 0.5 ml or to small changes in a daily weight in the clinical setting, some infants on ‘full feeds’ received only 146–149 ml/kg/day. We therefore considered an infant to be on full feeds if ≥ 145 ml/kg/day was tolerated for 3 consecutive days. Infants who did not meet this criterion were reviewed by the BERC to determine if a sustained level of feeding at a level below this had been achieved before discharge. Examples of this included feeds being stopped during transfer or for a procedure, use of higher-calorie formula or fluid restriction after 150 ml/kg/day had been reached.
Adverse event reporting
Adverse events were defined as serious if they:
-
resulted in death
-
were life-threatening
-
required inpatient hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability/incapacity
-
were a congenital anomaly/birth defect.
The term ‘life-threatening’ refers to an event in which the participant was at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death if it were more severe. Serious adverse events (SAEs) were to be reported from randomisation to discharge home.
Expected SAEs were those that could be reasonably expected to occur in the population of eligible infants during the course of the trial or form part of the outcome data. These did not require reporting by the SIFT co-ordinating centre and referred to the following SAEs:
-
death (unless unexpected in this population)
-
NEC or focal intestinal perforation
-
microbiologically confirmed or clinically suspected late-onset invasive infection
-
bronchopulmonary dysplasia (mechanical ventilator support or supplemental oxygen at 36 weeks’ postmenstrual age)
-
intracranial abnormality (i.e. haemorrhage, parenchymal infarction or white matter damage) on cranial ultrasound scan or other imaging
-
pulmonary haemorrhage
-
patent ductus arteriosus requiring treatment (non-steroidal anti-inflammatory drugs or surgery)
-
retinopathy of prematurity.
Reporting procedures
All expected SAEs (detailed above) were recorded on a CRF and were reviewed by the Data Monitoring Committee (DMC) at regular intervals throughout the trial. Any unexpected SAEs (a SAE that was not included in the list of expected SAEs) were reported by trial sites to the SIFT co-ordinating centre as soon as possible after the event had been recognised. Information on each SAE was recorded on a SAE reporting form, which was faxed to the SIFT co-ordinating centre. Additional information received for a case (follow-up or corrections to the original case) were faxed to the SIFT co-ordinating centre on a new SAE CRF. A standard operating procedure (SOP) that outlined the reporting procedure for clinicians was provided with the SAE form and in the trial handbook. The SIFT co-ordinating centre processed and reported the events, as specified in the CTU SOPs. The chief investigator informed all of the investigators concerned of the relevant information about unexpected SAEs that could adversely affect the safety of participants. Once per year throughout the recruiting period of the trial, a safety report was submitted to the sponsor and ethics committee.
Economic analysis
A health economic analysis of the two speeds of milk feed increments was performed in this study and is described in Chapter 4.
Governance and monitoring
Structured training for site investigators, local research nurses and other clinical staff was provided during initiation meetings. Training covered areas such as seeking consent, protocol details and processes and governance requirements. These events were supported with bespoke written and online training materials that were available to all staff via the trial website (www.npeu.ox.ac.uk/sift/neonatal-staff; accessed 9 January 2020). Staff in continuing care sites were directed to online training and advised to access support from the trial team as needed.
Ongoing monitoring included review of the investigator site files that contained delegation logs, good clinical practice certificates and research curricula vitae of staff. Best-practice data management procedures and data monitoring at the study data centre and trial centres were followed to achieve quality assurance. Data management was in accordance with SOPs at the trial co-ordinating centre (NPEU CTU) and a prespecified plan. Data monitoring included review of consent forms and participant eligibility. Additional validation checks of data were carried out regularly, with data queries issued to study sites for resolution. Final data validation checks were carried out before database lock, with questions being resolved by discussion with the site PI or local research nurse where possible.
During the trial, the study statisticians produced reports for the independent Trial Steering Committee (TSC) and the independent DMC. Data quality concerns that were identified by study statisticians were reported to study data management staff and were queried when appropriate or included in future routine data validation checks, or both. Opportunities for external, independent review of summary data were provided by the DMC and the TSC meetings.
Summary of changes to the study protocol
A summary of the changes made to the original protocol is presented in Appendix 7.
Chapter 3 Results
Recruitment and retention
Patient flow, including recruitment to and retention in the trial, is detailed in Figure 1. The trial recruited infants from June 2013 to June 2015 in 55 hospitals. In total, the trial recruited 2804 infants; 1400 infants were allocated to faster daily feed increments (30 ml/kg/day) and 1404 were allocated to receive slower feed increments (18 ml/kg/day). The trial was closed on reaching the sample size. All infants received the allocated intervention, but 69 infants discontinued the intervention as a result of clinician or parental preference (see Figure 1). For 11 of these infants, parental consent was withdrawn and their data were not available for analysis, but the remainder were included in the intention-to-treat analysis. Outcome data at discharge home were not available for eight infants; their data were included in analyses except when knowledge of discharge or the date of discharge was required. In total, 68 (4.9%) infants in the faster increment group and 77 (5.5%) in the slower increment group died before 24 months CGA. Outcome data on disability at 24 months CGA were available for 1156 (87.2%) surviving infants in the faster increment group and 1169 (88.4%) in the slower increment group. The primary outcome (mortality or disability) was therefore known for 1224 (87.8%) infants in the faster increment group and 1246 (89.0%) in the slower increment group (see Figure 1 and Appendix 8).
FIGURE 1.
Flow of participants through the trial. Adapted from New England Journal of Medicine, Dorling et al. 45 Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434–43. Copyright © 2019 Massachusetts Medical Society.

Demographic and other baseline characteristics
The baseline characteristics and demographic features of the participating infants were well balanced between the two feeding increment groups (Table 1). The median gestational age was 29 weeks in both groups (36% at < 28 weeks). The mean birthweight was 1144 g in the faster increment group and 1142 g in the slower increment group. Overall, 60% of infants were born via caesarean section; 24% of infants were born following rupture of maternal amniotic membranes for > 24 hours; and 16% of infants had evidence of absent or reversed end diastolic flow in the fetal umbilical arteries. The allocation groups were well balanced in individual recruiting sites, as per the minimisation algorithm (see Appendix 9).
| Characteristic | Faster feed increment group (30 ml/kg/day) (N = 1394) | Slower feed increment group (18 ml/kg/day) (N = 1399) |
|---|---|---|
| Number of centres,a n | 55 | 54 |
| Male sex, n/N (%) | 739/1394 (53.0) | 726/1398 (51.9) |
| Missing, n | 0 | 1 |
| Infant age at randomisation (days) | ||
| Median (IQR) | 4 (3–6) | 4 (3–6) |
| Birthweight of < 10th centile for gestational agea | ||
| Total, n/N (%) | 295/1394 (21.2) | 291/1398 (20.8) |
| Missing, n | 0 | 1 |
| Gestation at delivery (completed weeks),a n/N (%) | ||
| Median (IQR) | 29 (27–30) | 29 (27–30) |
| 23+0 to 23+6 | 30/1394 (2.2) | 31/1399 (2.2) |
| 24+0 to 24+6 | 72/1394 (5.2) | 69/1399 (4.9) |
| 25+0 to 25+6 | 103/1394 (7.4) | 101/1399 (7.2) |
| 26+0 to 27+6 | 291/1394 (20.9) | 297/1399 (21.2) |
| 28+0 to 29+6 | 377/1394 (27.0) | 383/1399 (27.4) |
| 30+0 to 31+6 | 432/1394 (31.0) | 432/1399 (30.9) |
| 32+0 to 36+6 | 88/1394 (6.3) | 86/1399 (6.1) |
| ≥ 37 +0 | 1/1394 (0.1) | 0/1399 (0.0) |
| Birthweight (g), n/N (%) | ||
| Mean (SD) | 1144.2 (339.3) | 1142.3 (328.9) |
| < 500 | 10/1394 (0.7) | 7/1399 (0.5) |
| 500 to 749 | 178/1394 (12.8) | 164/1399 (11.7) |
| 750 to 999 | 316/1394 (22.7) | 345/1399 (24.7) |
| 1000 to 1249 | 348/1394 (25.0) | 349/1399 (24.9) |
| 1250 to 1499 | 313/1394 (22.5) | 328/1399 (23.4) |
| ≥ 1500 | 229/1394 (16.4) | 206/1399 (14.7) |
| Infant heart rate > 100 beats per minute at 5 minutes | ||
| Total, n/N (%) | 1263/1374 (91.9) | 1265/1381 (91.6) |
| Missing, n | 20 | 18 |
| Infant temperature on admission (°C) | ||
| Mean (SD) | 36.8 (0.7) | 36.8 (0.8) |
| Missing, n | 8 | 8 |
| Infant worst base excess within the first 24 hours of birth (mEq/l) | ||
| Mean (SD) | –6.1 (4.0) | –6.1 (3.9) |
| Missing, n | 29 | 26 |
| Infant ventilated via endotracheal tube at randomisation | ||
| Total, n/N (%) | 316/1392 (22.7) | 293/1397 (21.0) |
| Missing, n | 2 | 2 |
| Infant had absent or reversed end diastolic flow | ||
| Total, n/N (%) | 209/1372 (15.2) | 226/1380 (16.4) |
| Missing, n | 22 | 19 |
| Time from trial entry to first feed (days) | ||
| Median (IQR) | 0 (0–0) | 0 (0–1) |
| Missing, n | 5 | 4 |
| Mother’s age at randomisation (years) | ||
| Mean (SD) | 30.5 (6.2) | 30.7 (6.2) |
| Missing, n | 0 | 1 |
| Multiple pregnancy | ||
| Multiple pregnancy,a,b n/N (%) | 412/1394 (29.6) | 411/1399 (29.4) |
| Singlesc | 3 | 5 |
| Twinsd | 358 | 359 |
| Tripletse | 51 | 47 |
| Caesarean section delivery | ||
| Total, n/N (%) | 841/1393 (60.4) | 847/1399 (60.5) |
| Missing, n | 1 | 0 |
| Membranes ruptured before labour | ||
| Total, n/N (%) | 496/1373 (36.1) | 486/1380 (35.2) |
| Missing, n | 21 | 19 |
| Membranes ruptured > 24 hours before delivery | ||
| Total, n/N (%) | 323/1377 (23.5) | 338/1380 (24.5) |
| Missing, n | 17 | 19 |
Adherence
All of the infants received the allocated intervention but 69 infants discontinued the intervention: 66 from clinician or parental preference and three from transfer to a non-participating hospital (see Figure 1). For 11 of these 66 infants, parental consent was withdrawn and their data were not available for analysis. The remainder were included in intention-to-treat analyses. Outcome data at discharge home were not available for eight infants; their data were included in analyses except when knowledge of discharge or the date of discharge was required. In total, 68 (4.9%) infants in the faster increment group and 77 (5.5%) in the slower increment group died before 24 months CGA. Primary outcome classification at 24 months CGA was possible in 1224 (87.8%) infants in the faster increment group and 1246 (89.0%) in the slower increment group.
Outcomes
The estimates of effect for the primary and secondary outcomes are presented in Table 2 for outcomes at 24 months of age CGA and Table 3 for outcomes at hospital discharge.
| Outcome at 24 months of age CGA | Faster feed increment group (30 ml/kg/day) (N = 1394) | Slower feed increment group (18 ml/kg/day) (N = 1399) | Unadjusted effect measure (CI)a,b | Adjusted effect measure (CI)a,b,c | p-valued |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Survival without moderate or severe disability,e n/N (%) | 802/1224 (65.5) | 848/1246 (68.1) | 0.96 (0.91 to 1.02) | 0.96 (0.92 to 1.01) | 0.16 |
| Missing, n | 170 | 153 | |||
| Survival, n/N (%) | 1326/1394 (95.1) | 1322/1399 (94.5) | 1.01 (0.99 to 1.02) | 1.01 (0.99 to 1.03) | 0.55 |
| Missing, n | 0 | 0 | |||
| Moderate or severe disability, n/N (%) | 354/1156 (30.6) | 321/1169 (27.5) | 1.12 (0.98 to 1.28) | 1.10 (0.97 to 1.25) | 0.12 |
| Missing, n | 238 | 230 | |||
| Secondary outcome | |||||
| Moderate or severe visual impairment, n/N (%) | 21/1156 (1.8) | 16/1171 (1.4) | 1.33 (0.57 to 3.10) | 1.28 (0.43 to 3.83) | 0.57 |
| Missing, n | 238 | 228 | |||
| Moderate or severe hearing impairment, n/N (%) | 58/1143 (5.1) | 41/1172 (3.5) | 1.45 (0.86 to 2.46) | 1.43 (0.79 to 2.57) | 0.12 |
| Missing, n | 251 | 227 | |||
| Moderate or severe motor impairment, n/N (%) | 87/1164 (7.5) | 59/1177 (5.0) | 1.49 (0.96 to 2.32) | 1.48 (1.02 to 2.14) | 0.007 |
| Missing, n | 230 | 222 | |||
| Moderate or severe cognitive impairment, n/N (%) | 307/1156 (26.6) | 289/1170 (24.7) | 1.08 (0.89 to 1.30) | 1.06 (0.89 to 1.27) | 0.39 |
| Missing, n | 238 | 229 | |||
| PARCA-R | |||||
| Composite score | |||||
| Mean (SD) | 72.5 (38.3) | 73.9 (37.8) | –1.46 (–6.31 to 3.39) | –0.62 (–4.82 to 3.59) | 0.71 |
| Median (IQR) | 69 (40–100) | 70 (43–101) | |||
| Missing, n | 419 | 392 | |||
| Non-verbal cognition scale score | |||||
| Mean (SD) | 25.1 (6.2) | 25.5 (5.7) | –0.45 (–1.18 to 0.29) | –0.36 (–1.01 to 0.29) | 0.15 |
| Median (IQR) | 27 (23–29) | 27 (23–29) | |||
| Missing, n | 414 | 390 | |||
| Vocabulary subscale score | |||||
| Mean (SD) | 39.3 (29.7) | 40.3 (30.1) | –0.99 (–4.81 to 2.83) | –0.37 (–3.71 to 2.97) | 0.78 |
| Median (IQR) | 34 (13–60) | 35 (14–62) | |||
| Missing, n | 412 | 383 | |||
| Sentence complexity subscale score | |||||
| Mean (SD) | 7.9 (5.7) | 7.9 (5.4) | –0.09 (–0.79 to 0.61) | –0.05 (–0.73 to 0.64) | 0.86 |
| Median (IQR) | 7 (3–12) | 8 (4–11) | |||
| Missing, n | 405 | 379 | |||
| Diagnosis of cerebral palsy by a doctor or other health professional | |||||
| Total, n/N (%) | 58/1084 (5.4) | 35/1099 (3.2) | 1.68 (0.97 to 2.91) | 1.66 (0.97 to 2.84) | 0.015 |
| Missing, n | 310 | 300 | |||
| Outcome from trial entry to discharge home | Faster feed increment group (30 ml/kg/day) (N = 1394) | Slower feed increment group (18 ml/kg/day) (N = 1399) | Unadjusted effect measure (CI)a,b | Adjusted effect measure (CI)a,b,c | p-valued |
|---|---|---|---|---|---|
| Primary discharge outcome | |||||
| Microbiologically confirmed or clinically suspected LOS, n/N (%) | 414/1389 (29.8) | 434/1397 (31.1) | 0.96 (0.85 to 1.08) | 0.96 (0.86 to 1.07) | 0.43 |
| Missing, n | 5 | 2 | |||
| NEC (Bell’s stage 2 or 3), n/N (%) | 70/1394 (5.0) | 78/1399 (5.6) | 0.90 (0.66 to 1.24) | 0.88 (0.68 to 1.16) | 0.37 |
| Missing, n | 0 | 0 | |||
| Secondary outcome | |||||
| Death before discharge, n/N (%) | 60/1392 (4.3) | 65/1393 (4.7) | 0.92 (0.59 to 1.45) | 0.91 (0.55 to 1.53) | 0.65 |
| Missing, n | 2 | 6 | |||
| Time taken to reach full milk feeds (days) (145 ml/kg/day for 3 consecutive days), median (IQR) and median difference (99% CI) | 7 (7–10) | 10 (9–13) | –3.0 (–3.3 to –2.7) | –2.7 (–3.1 to –2.4) | < 0.001 |
| Missing, n | 72 | 102 | |||
| Weight SD score at discharge home,d mean (SD) and mean difference (99% CI) | –1.5 (1.1) | –1.5 (1.1) | –0.04 (–0.15 to 0.08) | –0.02 (–0.11 to 0.08) | 0.67 |
| Missing, n | 75 | 77 | |||
| Head circumference SD score at discharge home,d mean (SD) and mean difference (99% CI) | –0.8 (1.5) | –0.7 (1.7) | –0.09 (–0.27 to 0.09) | –0.07 (–0.24 to 0.10) | 0.31 |
| Missing, n | 258 | 228 | |||
| Duration of parenteral feeding (days) from trial entry to discharge home, median (IQR) and median difference (99% CI) | 9 (7–14) | 11 (9–16) | –2.0 (–2.4 to –1.6) | –2.2 (–2.7 to –1.6) | < 0.001 |
| Length of time in intensive care (days) from trial entry to discharge home, median (IQR) and median difference (99% CI) | 7 (4–21) | 8 (4–21) | –1.0 (–2.6 to 0.6) | –0.4 (–1.5 to 0.6) | 0.30 |
| Length of hospital stay (days) from trial entry to discharge home,e median (IQR) and median difference (99% CI) | 54 (37–81) | 55 (38–78) | –1.0 (–5.2 to 3.2) | 0.1 (–1.9 to 2.0) | 0.94 |
| Missing, n | 62 | 71 | |||
Primary outcome
Data were available for 2470 infants (88%) at 24 months of age CGA. In the faster increment group, 802 out of 1224 (65.5%) infants survived to 24 months of age CGA without moderate or severe disability, compared with 848 out of 1246 (68.1%) infants in the slower increment group [adjusted risk ratio (ARR) 0.96, 95% CI 0.92 to 1.01]. There were also no significant differences in the separate components of the composite outcome, with survival occurring in 1326 out of 1394 (95.1%) infants in the faster increment group and 1322 out of 1399 (94.5%) infants in the slower increment group, and moderate or severe disability in 354 out of 1156 (30.6%) infants in the faster increment group and 321 out of 1169 (27.5%) infants in the slower increment group.
Secondary outcomes at 24 months of age corrected for gestational age
For one of the components of the definition of moderate or severe neurodevelopmental disability at 24 months CGA, there was evidence of a significant difference between groups after adjustment for the factors used in the minimisation algorithm. Moderate or severe motor impairment occurred in 87 out of 1164 (7.5%) infants in the faster increment group and 59 out of 1177 (5.0%) infants in the slower increment group (ARR 1.48, 99% CI 1.02 to 2.14; p = 0.007) (see Table 2).
There was, however, no evidence of a significant difference between groups in the other three components of the disability definition (moderate or severe visual, hearing or cognitive impairment). However, numerically more adverse outcomes were seen in the faster increment group for each of these components; this was also the case for the diagnosis of cerebral palsy by a doctor or other health professional, which occurred in 5.4% of the faster increment group and 3.2% of the slower increment group (ARR 1.66, 99% CI 0.97 to 2.84; p = 0.015).
Other secondary outcomes
In total, 414 out of 1389 (29.8%) infants in the faster increment group had microbiologically confirmed or clinically suspected LOS, compared with 434 out of 1397 (31.1%) infants in the slower increment group (ARR 0.96, 95% CI 0.86 to 1.07; p = 0.43). Bell’s stage 2 or 3 NEC occurred in 70 out of 1394 (5.0%) infants in the faster increment group and 78 out of 1399 (5.6%) infants in the slower increment group (ARR 0.88, 95% CI 0.68 to 1.16; p = 0.37) (see Table 3).
The faster increment group reached full milk feeds significantly sooner: median 7 days from trial entry (IQR 7–10 days), compared with 10 days (IQR 9–13 days) in the slower increment group (adjusted median difference –2.7 days, 99% CI –3.1 to –2.4 days; p < 0.001). Significantly fewer days of parenteral nutrition from trial entry were seen in the faster increment group: 9 days (IQR 7–14 days), compared with 11 days (IQR 9–16 days) in the slower increment group (adjusted median difference –2.2 days, 99% CI –2.7 to –1.6 days; p < 0.001).
There was no evidence of between-group differences for (1) death during hospitalisation, (2) weight and head circumference SD scores at discharge home, (3) duration of time in intensive care from trial entry or (4) duration of hospital stay from trial entry (see Table 2).
Subgroup analyses
A subgroup analysis showed a significant interaction (p = 0.045) with the primary outcome for the type of enteral milk received: human, formula or both (Figure 2). No significant interaction was seen with the primary outcome for completed weeks of gestation at birth (p = 0.076) or birthweight < 10th centile or ≥ 10th centile for gestational age (p = 0.18). A post hoc analysis was also undertaken to assess the interaction of the presence of absent or reversed antenatal umbilical Doppler studies with the two incremental feed rates (see Tables 4 and 5).
FIGURE 2.
Subgroup analyses for survival without moderate or severe disability to 24 months of age CGA. n/N refers to the number of infants with the primary outcome/number of infants in that category. a, The primary outcome was survival without moderate or severe neurodevelopmental disability (CGA). p-values for interaction were adjusted for minimisation factors: collaborating hospital, single or multiple birth, gestational age at birth, and whether or not the birthweight was below the 10th percentile for gestational age, when technically possible. p-values and CIs were not adjusted for multiple comparisons and should not be used to infer definitive treatment effects. Adapted from New England Journal of Medicine, Dorling et al. 45 Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434–43. Copyright © 2019 Massachusetts Medical Society.

The subgroup analyses did not show any significant interactions with NEC (Figure 3) for:
-
completed weeks of gestation at birth (p = 0.63)
-
birthweight < 10th centile or ≥ 10th centile for gestational age (p = 0.25)
-
the type of enteral milk received (human, formula or both) (p = 0.53)
-
the presence of absent or reversed antenatal umbilical Doppler studies (p = 0.09). This was a post hoc analysis.
FIGURE 3.
Subgroup analyses for NEC from trial entry to discharge from hospital. a, n/N refers to the number of infants with one or more episodes of NEC from trial entry to hospital discharge/number of infants in that category. b, Combined with the 30+0 to 31+6 weeks’ category for calculation of RR. c, Combined with mixed category for calculation of RR. ARRs (faster/slower) and p-values for an interaction between allocation and category are shown. Adapted from New England Journal of Medicine, Dorling et al. 45 Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434–43. Copyright © 2019 Massachusetts Medical Society.

The subgroup analyses did not show any significant interactions with confirmed or suspected LOS (Figure 4) for:
-
completed weeks of gestation at birth (p = 0.07)
-
birthweight < 10th centile or ≥ 10th centile for gestational age (p = 0.51)
-
type of enteral milk received (human, formula or both) (p = 0.56)
-
presence of absent or reversed antenatal umbilical Doppler studies (p = 0.16). This was a post hoc analysis.
FIGURE 4.
Subgroup analyses for confirmed or suspected LOS from trial entry to discharge from hospital. a, n/N refers to the number of infants with one or more episodes of LOS from trial entry to hospital discharge/number of infants in that category. Adapted from New England Journal of Medicine, Dorling et al. 45 Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434–43. Copyright © 2019 Massachusetts Medical Society.

Adjusted risk ratios (faster/slower) and p-values for an interaction between allocation and presence of absent or reversed antenatal umbilical Doppler studies are shown in Tables 4 and 5.
| Necrotising enterocolitis | Faster feed increment group (30 ml/kg/day) (N = 1394) | Slower feed increment group (18 ml/kg/day) (N = 1399) | Adjusted relative risk (95% CI) |
|---|---|---|---|
| Absent or reversed end diastolic flow in the umbilical arteries identified, n/N (%) | |||
| No | 62/1163 (5.3) | 62/1154 (5.4) | 1.00 (0.73 to 1.35) |
| Yes | 8/209 (3.8) | 16/226 (7.1) | 0.49 (0.23 to 1.06) |
| Unknown | 22 | 19 | |
| Late-onset sepsis | Faster feed increment group (30 ml/kg/day) (N = 1394) | Slower feed increment group (18 ml/kg/day) (N = 1399) | Adjusted relative risk (95% CI) |
|---|---|---|---|
| Absent or reversed end diastolic flow in the umbilical arteries identified, n/N (%) | |||
| No | 337/1159 (29.1) | 361/1152 (31.3) | 0.94 (0.84 to 1.05) |
| Yes | 69/208 (33.2) | 66/226 (29.2) | 1.10 (0.89 to 1.36) |
| Unknown | 27 | 1 | |
Safety and adverse events
Sixty-two SAEs were reported, including follow-up reports, relating to 52 separate incidents. Of these 52 events, 34 were not related or relevant to the trial. Fourteen were deemed ‘possibly’ related, but were listed in the protocol as expected SAEs. 33
Four SAEs were deemed ‘possibly’ trial related and were not on the list of expected SAEs. All were reported to the Research Ethics Committee (REC), the DMC and the TSC in previous communications. These four SAEs were two cases of intracardiac thrombosis, one case of prolonged conjugated jaundice and one case of dehydration when a central venous line extravasated. Table 6 summarises the reported adverse events (definitions of adverse reactions and events are presented in Appendix 6).
| Group | Age at SAE (days) | Brief description of event | Severity | Related to trial |
|---|---|---|---|---|
| Faster increment group (30 ml/kg/day) (n = 1394) | 55 | Intracardiac thrombus, superior vena cava occluded; deteriorating renal function | Moderate | Possibly |
| 52 | Infant developed prolonged conjugated jaundice | Moderate | Possibly | |
| Slower increment group (18 ml/kg/day) (n = 1399) | 36 | Intracardiac thrombus | Moderate | Possibly |
| 9 | Central venous line extravasated, dehydration and lack of fluids | Mild | Possibly |
Chapter 4 Economic evaluation
Parts of this chapter have been reproduced and adapted from Tahir et al. 47 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
This chapter reports the economic evaluation conducted as part of SIFT. The objective of the economic evaluation was to compare the relative cost-effectiveness of two rates of enteral feed advancement, faster feed increments (30 ml/kg/day) with slower feed increments (18 ml/kg/day), on the principal outcome of survival without moderate or severe disability at 24 months of age CGA.
Methods
A within-trial cost-effectiveness analysis (CEA) was performed from the perspective of the NHS and Personal Social Services (PSS) in line with recommended practice. 48 The CEA results are expressed in terms of additional cost per survivor without disability at 24 months of age CGA.
Outcomes
The primary outcome of the CEA was disability-free survival at 24 months of age CGA. Secondary outcomes of the trial included microbiologically confirmed or clinically suspected LOS and NEC. These secondary clinical outcomes are presented here as part of a cost–consequence analysis (CCA). All statistical analyses were conducted in Stata® version 15 (StataCorp LP, College Station, TX, USA).
Data
Resource use and costs
Under the NHS and PSS perspective, only the direct costs to the health service provider incurred within the time horizon of the trial were included. Costs incurred during the initial hospital stay and interactions associated with the health service from discharge home to 24 months of age (CGA) were also included. Resource use data were collected prospectively from centres participating in the trial. All centres completed a total of eight different data collection forms that included specific items measuring health-care use. Where SAEs were reported, the associated resource use was collected on an additional form by the relevant participating centres. For instance, the severity of the event and any subsequent additional hospitalisation were recorded, as well as the use of concomitant medication. Health service use until 24 months of age (CGA) was measured through a parent questionnaire (URL: www.npeu.ox.ac.uk/downloads/files/sift/SIFT%202%20Year%20Form%20-%20V2_21%20Sept%202015.pdf; accessed 7 March 2019), which included health-care-related resource use items such as use of primary care services and hospital readmissions.
We also measured out-of-pocket costs to families during the 2-year follow-up period to capture the broader costs that were associated with each trial group, which had the purpose of presenting an additional analysis from a wider perspective. The parent questionnaire included specific items measuring personal financial costs, such as purchasing special equipment, home changes and the travel costs resulting from hospital or outpatient visits. Indirect non-medical costs, referring to income or productivity loss, were also collected. These included both paid and unpaid time off work as a result of the infant’s health. 7 This was valued by multiplying the gross wage rate with the time lost (measured in days) as a result of the infant’s health.
Valuation of resource use
‘Top-down’ methods of costing were used to value resource use. Aggregate cost data were taken from standard published sources to assign costs to resource use variables such as inpatient days. Relevant unit costs were obtained from several sources, but predominantly NHS Reference Costs 2017/1849 and the most recently published Unit Costs of Health and Social Care. 50 Medication prices were extracted from the British National Formulary (BNF)51 and some unit costs were also extracted from existing literature in this area. 52,53 Unit costs were then combined with resource volumes in order to calculate the costs of health service use for each feeding allocation. Table 7 presents the relevant items of resource use, their associated unit costs and the source from which these costs were obtained. All costs were expressed in Great British pounds (GBP) and in 2016–17 prices. Costs were inflated where necessary, using the Hospital and Community Health Services Pay and Prices Index. 50
| Resource use items | Unit cost (£)a | Source |
|---|---|---|
| Intervention | ||
| Cost per day on parenteral nutrition | 45.00 | Walter et al.55 |
| Intensive care: cost per day in intensive care differentiated by level of care required | ||
| Level 1: intensive care | 1295.00 | NHS Reference Costs 2017/18 49 |
| Level 2: high-dependency care | 1032.00 | |
| Level 3: special care | 510.00 | |
| Initial hospital stay | ||
| Cost per pulmonary haemorrhage | 1485.00 | NHS Reference Costs 2017/18 49 |
| Cost per intraventricular haemorrhage by severity | ||
| Grade 1 IVH/germinal matrix haemorrhage | 862.00 | NHS Reference Costs 2017/18 49 |
| Grade 2 IVH | 1472.00 | NHS Reference Costs 2017/18 49 |
| Grade 3/4 IVH | 1519.00 | NHS Reference Costs 2017/18 49 |
| Course of shunts for hydrocephalus | 2608.00 | NHS Reference Costs 2017/18 49 |
| Bronchopulmonary dysplasias | 5954.00 | NHS Reference Costs 2017/18 49 |
| Periventricular leukomalacias | 1341.00 | NHS Reference Costs 2017/18 49 |
| Retinopathy treated medically or surgically | 1603.00 | NHS Reference Costs 2017/18 49 |
| Patent ductus arteriosus treated with NSAID | 1152.00 | BNFC56 |
| Surgeries due to gut signs | 6629.00 | NHS Reference Costs 2017/18 49 |
| Cost of antibiotic medication per day | 3.00 | BNFC56 |
| Cost of antifungal treatment per day | 1.06 | BNFC56 |
| Cost per ml of preterm milk formula | 0.02 | Ganapathy et al.52 |
| Cost per packet of breast milk fortifier | 0.93 | Ganapathy et al.52 |
| Cost per litre of donor breast milk | 335.00 | Renfrew et al.53 |
| Cost per 200 ml of term formula milk | 2.00 | Renfrew et al.53 |
| Resource use during 2-year follow-up | ||
| Cost per outpatient day | 199.00 | NHS Reference Costs 2017/18 49 |
| Cost per inpatient day | 635.00 | NHS Reference Costs 2017/18 49 |
| Cost per operation | 2247.00 | NHS Reference Costs 2017/18 49 |
| Cost per general practitioner visit | 33.00 | PSSRU50 |
| Cost per health visitor visit | 75.00 | PSSRU50 |
| Cost per community nurse visit | 36.00 | PSSRU50 |
| Cost per home visitor/volunteer visit | 19.00 | PSSRU50 |
| Cost per community paediatrician visit | 407.00 | NHS Reference Costs 2017/18 49 |
| Cost per physiotherapist visit | 95.00 | NHS Reference Costs 2017/18 49 |
| Cost per social worker visit | 39.00 | PSSRU50 |
| Cost per speech and language therapist visit | 95.00 | NHS Reference Costs 2017/18 49 |
| Cost per dietitian visit | 85.00 | NHS Reference Costs 2017/18 49 |
| Cost per other health-care professional visit | 135.00 | NHS Reference Costs 2017/18 49 |
Economic analysis
A CCA was conducted as a preliminary measure to compare the disaggregated costs with the outcomes for both feeding increments. CCA is a form of economic evaluation where disaggregated costs and outcomes (consequences) are presented in their natural units. 57 To calculate costs, the quantity of resource use per infant was multiplied by unit costs. Mean costs per infant were estimated and the mean cost differences between the two feeding allocations were calculated. To address the skewness often present in cost data, a bootstrapping approach58 was carried out to calculate CIs around the mean costs. If the CIs of the difference in mean resource use and the costs between groups do not cross zero, this indicates a significant difference. In bootstrapping, repeated random samples of the same size as the original sample are drawn with replacement from the data. 58 The statistic of interest (mean) is calculated from each resample and these bootstrap estimates of the original statistic are then used to build up an empirical distribution for the statistic. 58
The primary base-case economic analysis took the form of a CEA from the perspective of the NHS and PSS. A CEA is a method for assessing the gains in health relative to the costs of different health interventions. 59 In the current study, health consequences are measured as a clinical outcome rather than in the form of health-related utilities, such as quality-adjusted life-years (QALYs). An incremental analysis was conducted, comparing incremental (additional) costs with the outcomes between the two feeding allocations. Costs and clinical outcomes associated with each feeding allocation were combined by calculating incremental cost-effectiveness ratios (ICERs). An ICER is expressed as the incremental cost (£) per incremental gain in a natural unit. 60 Cost data were discounted at 3.5% but discounting is not applied to outcomes in natural units; thus, outcomes were not discounted. Cost-effectiveness was based on the principal outcome of additional cost per survival without moderate to severe disability at 24 months of age CGA and from the perspective of the NHS and PSS.
The cost-effectiveness estimates are presented on a cost-effectiveness plane, to illustrate the incremental cost and effect of the intervention (faster feed increments). The cost-effectiveness plane has four quadrants, each with a different implication for the decision of implementing the intervention. 61 This was used to determine the relative position of the results. For example, faster feed increments might be said to ‘dominate’ slower feed increments if its position on the plane showed that the cost of the intervention was lower and the effectiveness in achieving the outcome was greater, when compared with slower feed increments. In other words, if faster feed increments were cheaper and more effective than slower feed increments, faster feed increments would be said to dominate.
Sensitivity analysis
Statistical uncertainty around the difference in ICERs was estimated by 5000 bootstrap replications, represented as scatter points on the cost-effectiveness plane. Results were presented using cost-effectiveness acceptability curves (CEACs) to reflect sampling variation and uncertainties in the cost-effectiveness value where appropriate. A CEAC shows the probability that an intervention (e.g. faster feed increments) is cost-effective compared with the alternative (e.g. slower feed increments) given the observed data, for a range of maximum monetary values (thresholds) that decision-makers might be willing to pay for a particular unit change in outcome. 61 Unlike economic evaluations, in which outcomes are expressed in QALYs, there is no set monetary threshold here for the primary outcome. Therefore, we examined cost-effectiveness at a range of monetary willingness-to-pay (WTP) thresholds (£0 to £40,000 per additional prematurity-adjusted survivor without disability at 24 months).
To explore the cost of missing resource use data for those infants who did not complete follow-up (15.4%), multiple imputation was performed. Multiple imputation replaces each missing observation with a set of plausible imputed (predicted) values, drawn from the posterior predictive distribution of the missing data given the observed data. 62 Because the missing values are replaced with predicted values, calculated in this way, this method is more favourable than simpler methods, such as replacing missing values with the means of all available values. Costs were imputed at the total cost level and the imputation model used 500 imputations.
Results
Participants
In total, 2804 infants were recruited into the trial, of whom 1400 were randomised to faster feed increments and 1404 were randomised to slower feed increments. Consent was withdrawn for six infants in the faster increment group and for five infants in the slower increment group. A total of 129 deaths occurred before discharge home during infants’ initial hospital stay. Of these deaths, 62 occurred in the faster increment group and the remaining 67 were in the slower increment group.
Follow-up rates at 24 months CGA in survivors were 84.3% (1175 infants) and 85.0% (1189 infants) in the faster increment group and slower increment group, respectively.
Resource use
Average volumes of resource use per infant during the initial hospital stay and the 2-year follow-up are presented in Table 8. On average, infants receiving slower feed increments spent more days in intensive care and in hospital. There was very little variation in resource use during the initial hospital stay across a number of variables, including number of surgeries owing to gut signs or intracranial abnormalities.
| Resource items | Faster feed increment group (N = 1394), mean (SD) | Slower feed increment group (N = 1399), mean (SD) | Bootstrap difference, adjusted mean difference (95% CI) |
|---|---|---|---|
| Days receiving faster or slower feed increments | 13.24 (16.22) | 15.04 (14.31) | –1.80 (–2.87 to –0.55)a |
| Days in intensive care | |||
| Level 1: intensive care | 15.06 (18.43) | 14.72 (17.48) | 0.34 (–0.96 to 1.71) |
| Level 2: high-dependency care | 20.71 (24.79) | 21.12 (19.65) | –0.41 (–1.96 to 1.51) |
| Level 3: special care | 30.13 (15.28) | 29.60 (15.05) | 0.53 (–0.58 to 1.68) |
| Initial hospital stay | |||
| Days in hospital | 91.00 (94.77) | 94.44 (103.76) | –3.25 (–11.07 to 3.30) |
| Grade 1 IVH/germinal matrix haemorrhage, proportion of daysa | 0.15 (0.38) | 0.16 (0.44) | –0.01 (–0.04 to 0.02) |
| Grade 2 IVH, proportion of days | 0.10 (0.36) | 0.09 (0.34) | 0.006 (–0.02 to 0.03) |
| Grade 3 IVH, proportion of days | 0.04 (0.25) | 0.03 (0.25) | 0.007 (–0.01 to 0.02) |
| Grade 4 IVH, proportion of days | 0.04 (0.24) | 0.03 (0.25) | 0.007 (–0.02 to 0.02) |
| Shunts for hydrocephalus, proportion of days | 0.01 (0.13) | 0.01 (0.18) | –0.002 (–0.02 to 0.009) |
| Bronchopulmonary dysplasias, proportion of days | 0.32 (0.89) | 0.31 (0.90) | 0.01 (–0.04 to 0.06) |
| Periventricular leukomalacias, proportion of days | 0.03 (0.27) | 0.02 (0.20) | 0.009 (–0.006 to 0.02) |
| Retinopathies treated medically or surgically, proportion of days | 0.07 (0.36) | 0.06 (0.32) | 0.02 (–0.002 to 0.04) |
| PDA treated with NSAID or surgery, proportion of days | 0.16 (0.49) | 0.17 (0.47) | –0.001 (–0.03 to 0.03) |
| Surgeries as a result of gut signs, proportion of days | 0.04 (0.25) | 0.04 (0.23) | 0.001 (–0.02 to 0.02) |
| Days on antibiotic medication | 5.60 (11.13) | 5.55 (10.85) | 0.05 (–0.70 to 0.10) |
| Days treated with antifungals | 1.20 (5.90) | 1.59 (7.52) | –0.39 (–0.86 to 0.10) |
| Days receiving preterm milk formula | 1.74 (3.93) | 1.88 (4.14) | –0.14 (–0.44 to 0.15) |
| Days receiving breast milk fortifier | 0.86 (2.47) | 0.83 (2.45) | 0.02 (–0.19 to 0.20) |
| Days receiving donated breast milk | 1.35 (3.34) | 1.43 (3.67) | –0.12 (–0.41 to 0.11) |
| Days receiving term formula milk | 0.24 (1.35) | 0.24 (1.57) | –0.001 (–0.11 to 0.11) |
| Resource use during 2-year follow-up | |||
| Readmissions, proportion of days | 0.34 (0.48) | 0.34 (0.48) | –0.006 (–0.04 to 0.03) |
| Operations, proportion of days | 1.47 (15.71) | 1.46 (0.36) | 0.02 (–0.99 to 1.10) |
| Days as an inpatient, proportion of days | 3.23 (17.33) | 2.82 (11.84) | 0.41 (–0.60 to 1.73) |
| Routine hospital follow-up visits as a day patient, proportion of days | 3.08 (6.82) | 3.17 (6.78) | –0.1 (–0.59 to 0.41) |
| Other hospital outpatient visits as a day patient, proportion of days | 0.39 (0.49) | 0.37 (0.49) | 0.02 (–0.02 to 0.06) |
| Paediatrician visits as a day patient, proportion of days | 1.55 (3.96) | 1.58 (3.57) | –0.03 (–0.29 to 0.26) |
| Number of general practitioner visits | 2.68 (6.06) | 2.42 (5.60) | 0.26 (–0.17 to 0.70) |
| Number of health visitor appointments | 2.05 (6.27) | 1.88 (5.86) | 0.17 (–0.26 to 0.61) |
| Number of community nurse visits | 2.25 (21.38) | 1.28 (6.40) | 0.97 (0.10 to 2.36)a |
| Number of home visitor/volunteer visits | 0.05 (0.21) | 0.04 (0.20) | 0.004 (–0.01 to 0.02) |
| Number of community paediatrician visits | 0.27 (1.46) | 0.29 (1.75) | –0.01 (–0.14 to 0.11) |
| Number of physiotherapist visits | 2.03 (8.00) | 1.93 (8.21) | 0.1 (–0.48 to 0.71) |
| Number of social worker visits | 0.22 (2.02) | 0.19 (2.46) | 0.03 (–0.14 to 0.19) |
| Number of speech and language therapist visits | 0.54 (2.70) | 0.53 (2.87) | 0.006 (–0.21 to 0.20) |
| Number of dietitian visits | 0.68 (3.47) | 0.73 (3.16) | –0.05 (–0.27 to 0.21) |
For the follow-up data, there was very little variation in mean resource use between groups. Infants who had received faster feed increments reported a slightly higher number of days as inpatients, community nurse visits, physiotherapist visits and health visitor visits than the slower feed increment group did.
Costs
Mean resource use was combined with unit costs (see Table 7) to derive the health service costs accruing from each feeding strategy (Table 9). Average costs of the initial hospital stay did not differ very much between the two groups; this is not surprising given that mean resource use was very similar between groups. Mean costs throughout the 2-year follow-up are also presented in Table 9. The most sizeable cost difference was in the cost of inpatient stays; this cost £267 more in the faster increment group. Throughout the follow-up period, health service costs were generally slightly higher for the faster increment group, particularly for primary care services, such as general practitioner, health visitor and community nurse visits.
| Resource items | Faster feed increment group (n = 1394), mean (SD) | Slower feed increment group (n = 1399), mean (SD) | Bootstrap difference, adjusted mean difference (95% CI) |
|---|---|---|---|
| Days receiving faster or slower feeds | 597 (731) | 678 (645) | –80 (–126 to –30) |
| Days in intensive care | |||
| Level 1: intensive care | 19,506 (23,863) | 19,063 (22,631) | 443 (–1272 to 2277) |
| Level 2: high-dependency care | 21,378 (25,578) | 21,798 (20,280) | –420 (–2016 to 1566) |
| Level 3: special care | 15,375 (7793) | 15,102 (7676) | 273 (–315 to 887) |
| Initial hospital stay | |||
| Grade 1 IVH/germinal matrix haemorrhage, proportion of days | 127 (331) | 134 (381) | –11 (–38 to 15) |
| Grade 2 IVH, proportion of days | 143 (532) | 136 (494) | 7 (–28 to 46) |
| Grade 3 IVH, proportion of days | 60 (378) | 50 (381) | 10 (–19 to 40) |
| Grade 4 IVH, proportion of days | 54 (364) | 51 (366) | 4 (–23 to 30) |
| Shunts for hydrocephalus, proportion of days | 24 (348) | 28 (477) | –4 (–40 to 24) |
| Bronchopulmonary dysplasias, proportion of days | 2475 (3970) | 2392 (4112) | 83 (–214 to 362) |
| Periventricular leukomalacias, proportion of days | 48 (305) | 36 (240) | 12 (–7 to 32) |
| Retinopathies treated medically or surgically, proportion of days | 137 (499) | 103 (453) | 35 (–1 to 70) |
| PDA treated with NSAID or surgery, proportion of days | 202 (509) | 202 (537) | 0.58 (–37 to 43) |
| Surgeries as a result of gut signs, proportion of days | 237 (1567) | 231 (1536) | 5 (–116 to 114) |
| Days on antibiotic medication | 16 (33) | 16 (32) | 0.16 (–2 to 3) |
| Antifungals | 1 (6) | 2 (8) | –0.41 (–0.89 to 0.14) |
| Preterm milk formula | 0.04 (0.08) | 0.04 (0.09) | –0.003 (–0.009 to 0.004) |
| Breast milk fortifier | 0.79 (2) | 0.78 (2) | 0.02 (–0.17 to 0.20) |
| Donated breast milk | 438 (1120) | 480 (1232) | –41 (–127 to 51) |
| Term formula milk | 0.37 (2) | 0.37 (2) | –0.001 (–0.19 to 0.15) |
| Resource use during 2-year follow-up | |||
| Number of operations | 3316 (35,294) | 3273 (29,567) | 42 (–2259 to 2612) |
| Number of inpatient stays | 2150 (11,057) | 1883 (7577) | 267 (–326 to 1076) |
| Number of outpatient visits | 1067 (1971) | 1082 (1827) | –18 (–162 to 112) |
| Number of general practitioner visits | 88 (200) | 80 (185) | 9 (–6 to 24) |
| Number of health visitor appointments | 154 (471) | 141 (440) | 13 (–23 to 45) |
| Number of community nurse visits | 81 (770) | 46 (230) | 35 (5 to 94) |
| Number of home visitor/volunteer visits | 0.86 (4) | 0.77 (4) | 0.09 (–0.20 to 0.40) |
| Number of community paediatrician visits | 112 (596) | 117 (712) | –6 (–59 to 39) |
| Number of physiotherapist visits | 193 (760) | 183 (780) | 9 (–55 to 61) |
| Number of social worker visits | 9 (79) | 8 (96) | 1 (–6 to 7) |
| Number of speech and language therapist visits | 51 (257) | 50 (273) | 0.59 (–19 to 18) |
| Number of dietitian visits | 58 (295) | 62 (269) | –4 (–23 to 19) |
Mean total costs for each group are presented in Table 10. Faster feed increments cost approximately £1043 less per infant than slower feed increments during infants’ initial hospital stay; however, within the 2-year follow-up the faster increment group was more costly by approximately £349 per infant. Overall, the intervention of faster feed increments was less costly.
| Cost category | Faster increments (n = 1224) | Slower increments (n = 1246) | Bootstrap mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Total cost | Mean | SD | Total cost | Mean | SD | ||
| Costs of initial hospital care | 124,386,552 | 101,623a | 80,759 | 126,923,790 | 101,865 | 80,498 | –242 (–6307 to 6251) |
| Costs from initial discharge from hospital up to 24 months corrected age | 10,151,856 | 8,294 | 49,585 | 9,698,864 | 7,784 | 38,473 | 510 (–2864 to 4508) |
| Total costs of health service use after initial discharge from hospital and up to 24 months corrected age CGA | 134,538,408 | 109,917 | 98,040 | 136,623,900 | 109,650 | 94,788 | 267 (–6928 to 8117) |
Mean total costs
Mean total costs are detailed in Table 10.
Cost–consequence analysis
The disaggregated CCA results (Table 11) show that the faster feed increment (the intervention) was slightly more costly and also less effective in terms of achieving the primary outcome of survival without moderate or severe neurodevelopmental disability at 24 months of age CGA.
| Costs/consequences | Faster feed group (N = 1224) | Slower feed group (N = 1246) |
|---|---|---|
| Total costs of health service use for 2 years | £134,538,408 | £136,623,900 |
| Costs of initial hospital care | £124,386,552 | £126,923,790 |
| Costs from initial discharge from hospital up to 24 months of age | £10,151,856 | £9,698,864 |
| Survival at hospital discharge, n (%) | 1332/1394 (95.6) | 1337/1399 (95.2) |
| Death before discharge home, n (%) | 60/1392 (4.4) | 65/1393 (4.8) |
| Disability-free survival at 24 months (corrected for prematurity), n (%) | 802/1224 (65.5) | 848/1246 (68.1) |
| Survival at 24 months (corrected for prematurity), n (%) | 1326/1394 (95.1) | 1322/1399 (94.5) |
| Moderate to severe neurodevelopmental disability, n (%) | 473/1394 (33.9) | 405/1399 (28.9) |
| Neonatal late-onset invasive infection, n (%) | 414/1398 (29.8) | 434/1397 (31.1) |
| Necrotising enterocolitis, n (%) | 70/1394 (5) | 78/1399 (5.6) |
The total estimated costs of health service use for the first 2 years after birth were approximately £134.5M for those allocated faster feed increments compared with £136.6M for those allocated slower feed increments. In Table 11, total costs are broken down into mean costs during initial hospital stay and during follow-up for both groups. Although there were fewer deaths in the faster feed increment group, for the primary outcome of survival without moderate or severe neurodevelopmental disability at 24 months (CGA), the intervention (faster feed increment) was less effective than the comparator (slower feed increment). Increasing milk feeds at a faster rate was associated with 46 (2.6%) more infants with moderate to severe disability compared with the slower feed group.
The breakdown of costs shows that faster feed increments compared with slower feed increments are less costly on average during infants’ initial hospital stay, and the 2-year follow-up data show that during follow-up, costs are lower in the faster feeding group, on average. This is primarily due to greater resource use associated with those in the faster increment group, in terms of hospital inpatient stays and primary care visits (see Tables 8 and 9).
Cost-effectiveness analysis
Faster feeds are shown to cost more per infant on average and are also less effective in achieving the primary outcome, thus they are dominated by the comparator (slower feeds) (see Figure 1). There is therefore no ICER to present in this circumstance.
Non-health-service costs
Non-health-service costs measured from a broader societal perspective have not been incorporated into the main results, given that the intervention leads to more cases of disability. Parents’ out-of-pocket costs were measured and analysed (see Tables 13 and 14).
Sensitivity analysis
The scattered points on the cost-effectiveness plane (Figure 5) show that the vast majority of points lie in the south-west and north-west quadrants. This shows that at higher monetary thresholds, faster feed increments become the more expensive strategy but remain less effective. At the higher thresholds (north west), the existing treatment (slower feed increments) dominates the intervention. This finding is confirmed by the CEAC (Figure 6), which shows a decreased probability that the intervention is cost-effective as the WTP threshold increases. This is because the more monetary value that is placed on a disability-free life, the less we value this intervention, given its detrimental effects for this outcome.
FIGURE 5.
Cost-effectiveness plane (faster feed increments vs. slower feed increments).

FIGURE 6.
The CEAC (faster feed increments vs. slower feed increments).
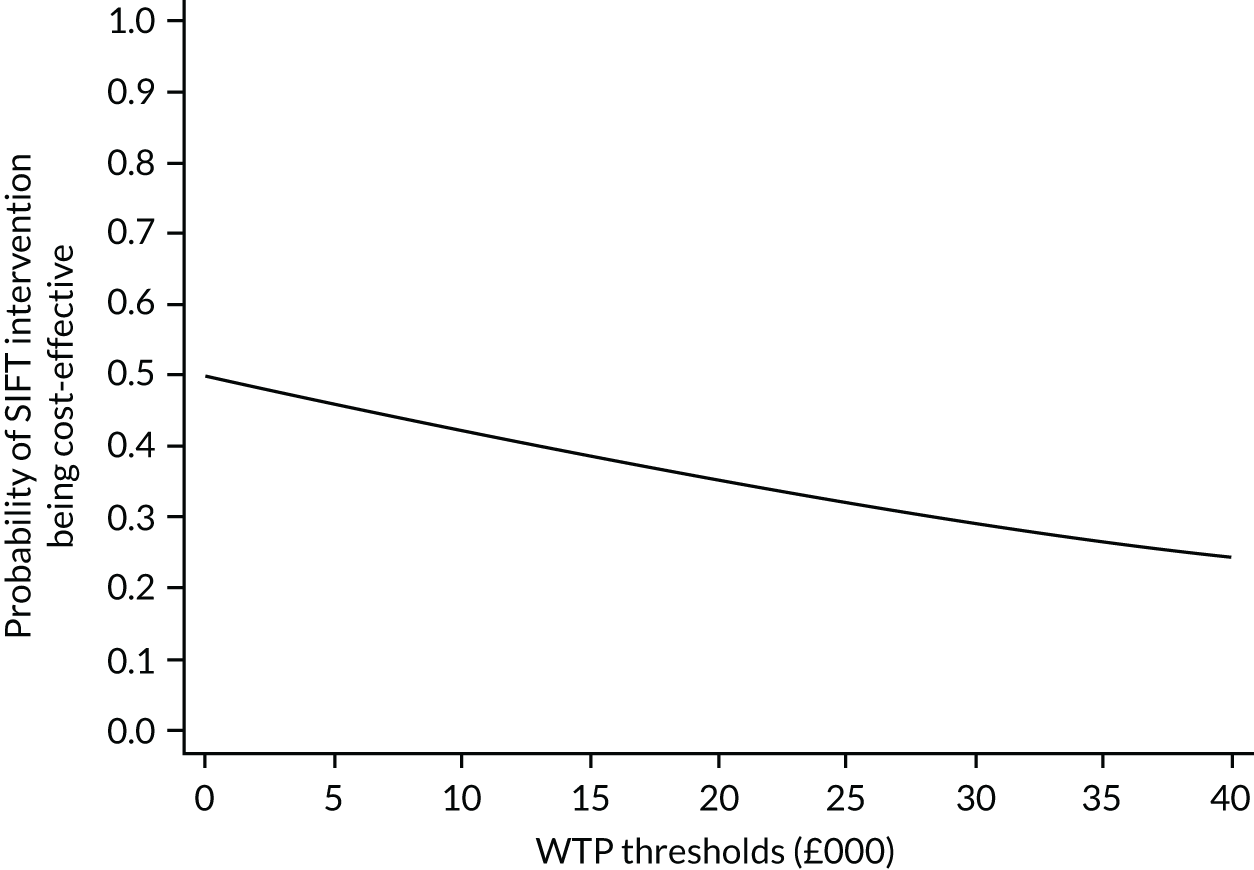
Table 12 shows the mean resource use for all infants once missing data were accounted for using multiple imputation. When the missing values were accounted for, faster feed increments were slightly more costly than slower feed increments (£378 more per infant). This is in contrast to the baseline results that showed the reverse; however, given the poorer clinical outcomes associated with the faster feed increment, this finding is in line with the expectations. We would expect more resource use in the faster increment group as a result of more health-care utilisation, for example primary care visits and readmissions.
| Cost | Faster feed increment group (n = 1224), mean (SD) | Slower feed increment group (n = 1246), mean (SD) |
|---|---|---|
| Total costs of health service use after initial discharge from hospital to 24 months of age | 109,410 (92,266) | 109,032 (89,763) |
Discussion
Principal findings
The results of the economic evaluation suggest that for very preterm or VLBW infants, daily increments in milk volume at a faster increment rate (30 ml/kg) are per infant more costly and less cost-effective compared with a slower increment rate (18 ml/kg). In terms of initial hospital and post-discharge costs between the two feeding increment groups evaluated, a faster increment was shown to be more costly on average compared with feeding with a slower increment at 24 months corrected age. The average cost per infant for faster increments was £109,917, compared with £109,650 for slower increments.
However, in terms of the clinical data, faster feed increments were less effective than slower feed increments in achieving the principal outcome of survival without moderate or severe neurodevelopmental disability at 24 months of corrected age. Fewer infants in the faster feed increment group (n = 802) than in the slower feed increment group (n = 848) achieved the primary clinical outcome of survival without moderate to severe neurodevelopmental disability at 24 months of age CGA.
When the uncertainty around all of the estimates is incorporated into the analysis, the results suggest that the faster feeding increment is dominated by the slower feeding increment, as it is, on average, more costly and less effective than the slower increment. The cost-effectiveness plane (see Figure 5) that incorporates the uncertainty around each point estimate in the results shows that relative to the comparator the faster feeding increment is likely to be less cost-effective than the slower feeding increment (with the scatterplot being predominantly to the left of the origin in Figure 5). The CEAC also shows the low probability of the intervention being cost-effective, which clearly decreases as the willingness to pay increases. Thus, in summary, for very preterm or VLBW infants, a faster rate of daily increments in milk volumes (30 ml/kg) was more costly on average at 24 months of age and also less effective, with fewer infants achieving the principal outcome of survival without disability, and this result is supported by analysis of the cost-effectiveness plane and the CEAC.
One of the key principles of a health economic analysis is to maximise the health benefits from and ensure the most efficient allocation of scarce resources. It is plausible to incur analyses that suggest that a potentially small decrement in health outcome is acceptable on cost-effectiveness grounds if the potential cost saving is great enough to more than offset the loss in health outcome and if the saved resources can be used to better effect elsewhere. However, this interpretation does not apply in the current analysis owing to the serious uncertainty in a number of areas. First, regarding costs, the probabilistic cost-effectiveness analysis and in particular, the cost-effectiveness plane suggests that faster feeds could be either more or less costly, compared with slower feeds. Second, with respect to the clinical effectiveness, the probabilistic sensitivity analysis represented in the cost-effectiveness plane (see Figure 5) indicates that a potential risk for harm is associated with the faster feeding increment relative to the slower feeding increment and that this is based on the uncertainty in the data at 24 months. Finally, the neurological development of infants at 24 months of age is a serious outcome on which the smallest decrement can have lifetime consequences and associated costs. 63
There are also broader societal consequences that could result from the clinical outcome, namely productivity loss, stemming from both the time off work and the lost earnings of parents of children with disabilities. 7,64 The trial did not collect resource and outcome data beyond 24 months of age . It is noteworthy that, although the costs of initial hospital care were lower in those allocated faster feed increments than in those allocated to slower feed increments and overall lower in the specific period from initial discharge to the 24 months of age assessment, the costs incurred by those allocated to faster feeding increments for this period were slightly greater on average than for those allocated to slower feeding increments.
In summary, based on the neurological outcome at 24 months of age and given the uncertainty in both the cost and effectiveness, the faster feeding increment (30 ml/kg/day) that was tested in the trial cannot be advocated on cost-effectiveness grounds.
A secondary analysis that was based on cost per case of neonatal sepsis avoided was initially proposed, but this was deemed misleading in the final analysis because this is an intermediate outcome only and does not relate to the ultimate outcome of the infant. Although outcomes in terms of LOS and NEC were more favourable in the intervention group, these outcomes occurred at one time end point that was intermediate and one time end point that was ultimately undermined by the 2-year outcome.
Strengths and weaknesses of the study
To our knowledge, this was the first economic evaluation conducted alongside the largest RCT comparing enteral feeding practices in infants. Economic evidence regarding enteral feeding regimes prior to this study had been scarce. A previous clinical systematic review65 identified only four RCTs that compared enteral feed volumes in very preterm and VLBW infants. All reviewed studies had limited their outcome measures to events prior to discharge home, such as the time taken to advance to full feeds, NEC, LOS and length of hospital stay. These studies generally had a very short follow-up period (e.g. 2 weeks)25 or no follow-up period at all. 23,31 Given that NEC and LOS are major causes of late neonatal mortality and long-term neurodevelopmental disability, it is possible that some relevant consequences were excluded in previous studies given they would not have been captured within short study durations. Thus, only intermediate health service costs were considered and potentially sizeable cost consequences accruing in the future were ignored. Furthermore, none of these studies included economic evaluations; thus, inferences cannot be made regarding the most cost-effective feeding strategy and uncertainty remains. The SIFT was one of the largest studies of any intervention ever carried out in the neonatal age group and, thus, there is broad applicability of this economic evaluation, for both policy and practice.
The rigorous analysis fills a gap in the literature and provides empirical evidence that shows the broader economic (as well as clinical) consequences if faster feeding practices were to be adopted. The effectiveness of feeding practices across a number of outcomes has been evaluated, showing that although faster feeding increments may be effective in the short term, with reduced NEC and LOS, the detrimental consequences in terms of impairments may not manifest until after infancy.
One of the key weaknesses of this study is that the follow-up period was only 2 years. Had this been longer, there would be greater scope for an observation of the economic consequences of parenteral feeding strategies. In particular, the costs of the disabilities/impairments present and the degree of differentiation in health service use between the two feeding groups would have been informative. Our analyses required some pragmatic assumptions, specifically regarding the proportion of milk volume and antibiotic use during infants’ initial hospital stay. Assumptions were necessary owing to excessive staff burden in collecting data, as getting precise volumes would have meant recording use of these items for each individual infant every day (as this would vary day to day). Our assumptions are, however, validated by existing literature and guidelines, that is antibiotic use is based on the doses that are recommended in the BNF for Children56 and are not likely to have had a significant effect on the results. Assumptions were also required for some of the resource use that occurred during follow-up. For items such as inpatient stays, it was not possible to determine the level of care that had been received by the infant; thus, when assigning a cost to this variable, a weighted average of costs for different care episodes was calculated. For infants treated in Scottish and Irish hospitals, we assumed that costs from English sources such as the NHS Reference Costs 2017/1849 would be reflective and applicable.
A further potential limitation of this study is the confusion that might arise given the reported clinical results for the base case, which suggested that the difference in effectiveness between faster feeding increments and slower feeding increments was not significantly significant. Whereas, the health economics analysis suggests that faster feeds have broadly equivalent costs but poorer outcome and may be harmful. This contrasting interpretation of the results relates to a requirement in the recommendations for health economic analysis to estimate and quantify the uncertainty around the clinical end points (based on appropriate distributions applied to the CIs surrounding the point estimate and using probabilistic sensitivity analysis). 66,67 This recommended and widely endorsed approach to conducting robust economic analysis is recognised as potentially challenging and has been widely debated and explained elsewhere. 67–69
Meaning of the study
Based on the results of this within-trial economic evaluation, increasing the milk feed volumes at the faster rate (30 ml/kg/day) in very preterm or VLBW infants does not appear to be a cost-effective strategy. 68
This work highlights an ongoing debate and also reveals the impact of the difference in paradigms between the statistical approach and the economic approach. 68,69
Recommendations for future research
Given that the SIFT was not less effective in terms of lower rates of NEC and LOS, it is important for future research to investigate the cause of the higher rate of disabilities in the intervention group. The findings suggest that there is an alternative effect of faster increment feeding strategies that leads to impairments at a later stage in children in this study. Further randomised trials and examination of feeding data sets (either existing or prospectively collected) may provide further information.
Supplementary data
Non-health-service costs
Details of non-health-service costs are provided in Table 13.
| Cost | Faster feed increment group (n = 1394), mean (SD) | Slower feed increment group (n = 1399), mean (SD) | Bootstrapped difference, adjusted mean difference (95% CI) |
|---|---|---|---|
| Costs to parents (£) | |||
| Transport | 63.26 (717.34) | 34.63 (164.54) | 28.53 (–0.16 to 80) |
| Home changes | 67.79 (1645.65) | 196.68 (4915.92) | –129 (–451 to 83) |
| Special equipment | 27.21 (318.74) | 14.98 (155.62) | 12 (–3 to 35) |
| Other costs | 161.07 (5001.13) | 58.82 (1150.32) | 102 (–73 to 417) |
| Days taken off work | |||
| Number of days off work | |||
| Without pay for respondent | 6.13 (45.78) | 5.66 (48.10) | 0.473 (–3 to 4) |
| With pay for respondent | 1.85 (11.27) | 3.04 (27.80) | –1 (–3 to 0.20) |
| Without pay for respondent’s partner | 1.83 (9.45) | 1.78 (7.82) | 0.047 (–0.56 to 0.71) |
| With pay for respondent’s partner | 1.89 (12.72) | 1.11 (5.50) | 0.785 (0.19 to 1.72) |
| Cost of days taken off work (£) | |||
| With pay for respondent | 199.60 (1213.74) | 327.69 (2995.36) | –128 (–311 to 16) |
| With pay for respondent’s partner | 203.69 (1370.55) | 119.14 (592.95) | 85 (16 to 181) |
| Total out-of-pocket costs from initial hospital discharge to 2 years | 722.53 (5789.46) | 751.94 (5991.44) | –29 (–442 to 429) |
Chapter 5 Parental experiences of being approached to join multiple neonatal clinical trials: a qualitative study, ‘PARENT’
Background
Complex neonatal care must be underpinned by a strong evidence base. This requires RCTs, often with enrolment shortly after birth when parents are likely to be extremely anxious and where there may be concerns about longer-term survival and prognosis. Health-care professionals must weigh up the additional stress caused to parents by being approached for RCT enrolment against their desire to support trial recruitment; there are likely to be a range of factors that may affect recruitment. 70 Preterm infants are a vulnerable group because they are unable to consent for themselves and, from an ethics perspective, proxy decisions about enrolment by parents and health-care professionals should always be made in the infant’s best interests. The vast majority of parents want to be involved in decisions about whether or not their infant should participate in a RCT, although it is common for parents to also seek guidance from health-care professionals. 71 Studies of such parents suggest that most feel that they made well-informed decisions, although there is debate as to whether or not fully informed consent can really be achieved in this context. 72–74 The anticipated level of risk and benefit from each individual RCT has been established as a key driver of parental desire to consent. 75,76 In many large, research-active neonatal intensive care units (NICUs), it is common for parents to be approached to enrol their infant in more than one trial or study. This presents important ethical and scientific challenges, many of which have been outlined in a recent review focused on neonatal practice,77 although similar challenges do exist in adult intensive care settings. 78 To achieve high-quality research that determines best practice of relevance to these families, it is important for research teams to understand the parental perspective when planning, designing, conducting and analysing research studies. Where co-enrolment does not compromise scientific integrity, it might be considered unethical to deny parents the choice of supporting studies and co-enrolment may also make studies more representative of the populations and questions that they aim to address. 77
Research aim
The aim of this study was to describe the perceptions and experiences of parents of infants who were approached to participate in at least one UK NIHR collaborative RCT, as well as one or more additional RCT or observational study. This study was designed to explore the experiences of parents whose infants had been approached to join either or both of two clinical trials of investigational medicinal products (CTIMPs), which were both funded by the UK NIHR Health Technology Assessment (HTA) programme. These trials were (1) the Speed of Increasing milk Feeds Trial (SIFT),1 a non-blinded trial comparing two different rates of increase in the amount of enteral milk feeds the infant received and (2) the Enteral Lactoferrin in Neonates (ELFIN) trial that was a double-blinded, placebo-controlled trial of supplemental enteral bovine lactoferrin (a milk protein) added to milk feeds. 39 Both of these trials recruited preterm infants who were born at < 32 weeks’ gestation and who were free of major congenital anomalies in the first few days of life. At the development stage of both trials, it appeared likely that there would be considerable overlap of these two trials, although owing to other factors, in the end there was in fact minimal overlap during recruitment. However, at all of the three units that took part in this qualitative study (PARENT), a number of NIHR or local RCTs and/or observational studies were under way,79,80 including one further HTA-funded RCT, Baby-OSCAR (see www.npeu.ox.ac.uk/baby-oscar; accessed 9 January 2020). At the time of writing, the Baby-OSCAR trial is still actively recruiting preterm infants who were born at < 29 weeks’ gestation and is a double-blinded, placebo-controlled study of the use of ibuprofen in the treatment of patent ductus arteriosus. We took advice from parents when planning this study and only sought ethics permission to interview parents whose infants had joined at least one trial or study, even if they had declined enrolment in other studies or trials. This meant that we did not approach parents who had declined participation in all of the studies or trials that they had been offered.
Literature search/review
Where the prospect of enrolment to more than one RCT or study exists, additional medical, practical and ethical issues arise for parents, clinical staff and research teams. 77,81 Large NICUs may be actively recruiting to several studies at any one time, which may include multicentre collaborative or single-centre RCTs and observational studies. Recent studies with parents suggest that many are receptive to being approached to be involved in more than one RCT or study, but this area has not been subject to detailed qualitative study in the context of multiple RCTs in neonates, despite the importance to future research progress. 82,83 A recent study in a paediatric intensive care unit setting suggested that co-enrolment does not adversely affect recruitment, but this did not include direct exploration of the reasons behind parental decision-making. 84
Methodology (including any changes to the protocol) and data sources
There were no changes to the original protocol.
Sampling
Parents were recruited when their infant was no longer receiving intensive care and prior to their infant being discharged home from one of three NICUs in the north of England. We included parents who had been approached to join at least one RCT that was a CTIMP and one additional RCT or observational study and who had subsequently consented for their infant to join at least one trial or study. We used purposive sampling based on actual RCT participation, age and educational or employment factors in an attempt to reflect a broad range of opinions and experiences. The initial approach for this study was made by a research nurse who had knowledge of study approaches and enrolment.
Interviews
We reviewed the existing literature, had discussions with doctors and nurses on a NICU and developed an initial interview topic guide with the aim of provoking discussion rather than seeking specific answers. The guide was iterative and flexible, which allowed participants to have space to define the issues that they regarded as significant and to articulate their experiences in their own words. 85 We estimated that we would need 15–20 interviews to reach thematic saturation, that is the point at which no new themes emerged from the data, and we reached this after 17 interviews, which was similar to our previous work with parents of preterm infants. 86 Interviews were tape-recorded, lasted approximately 1 hour and took place in a location convenient for the participants, either in hospital or in their own home. They were conducted by a single trained qualitative researcher (Judy Richards) who had experience of interviewing parents of preterm infants but who was not a health-care professional. Interviews were conducted either shortly before or shortly after the infant was discharged home. Interview recordings were transcribed verbatim and fully anonymised and participants were offered the opportunity to read and review the interview transcript.
Data analysis
Data analysis was carried out using a thematic approach85 in order to identify patterns of meaning across our data that are relevant to the conduct of research on a neonatal unit. The interview transcripts were analysed by two research team members to ensure that there was standardisation of thematic coding. Significant themes and subthemes were extracted from the data and their inter-relationships were considered alongside contradictions and overlap that emerged between them. The themes were discussed and agreed with three members of the research team (Judy Richards, Judith Rankin and Nicholas Embleton) and we include illustrative quotations for the identified themes. Participants are identified as P1, P2, etc.
Governance
This SIFT received approval from the NRES Committee East Midlands, Nottingham, and this PARENT substudy received approval from the Office for Research Ethics Committees Northern Ireland: reference 15/NI/0021, 2 February 2015.
Results
Seventeen semistructured qualitative interviews were carried out with parents. There were eight parents of twins, eight parents of singletons and one set of triplets. Nine interviews were with the mother alone, one was with the mother and a grandparent and seven interviews were with both parents. Interviews took place either in the hospital or at home. Data analysis identified four overarching themes, which outlined factors that influenced parents’ decisions regarding their infants’ participation in more than one RCT or study.
Theme 1: ‘just another little thing’
The main finding that emerged from the data was that parents did not perceive the enrolment of their infants in more than one study as a major issue in the context of their infant’s daily medical care (Box 5). Similarly, the actual number of studies or trials (range 1–5 studies or trials) that their infant participated in was typically not raised as significant for parents. For the most part, being introduced to several trials or studies soon after the birth was not seen as problematic. The majority of parents held a strong belief in the benefits of research and had faith that the health-care professionals caring for their infant would ensure that trial participation would not compromise care in any way. Parents generally viewed their infant’s participation in more than one study positively. For parents of some sick infants where survival was uncertain, trial participation was perceived as a ‘gift’ or a way of making their infant’s potentially short life matter. A small minority of parents were concerned about perceived issues that may arise when two trial interventions take place concurrently. However, most often, parents focused on the daily medical care that their infant was receiving and many forgot about the trials or studies once they were under way.
In the beginning I did feel a bit bombarded, but that is only because I was going through hell and I didn’t really want to think about anything else. They [trials] have to happen in order for things to develop and for new procedures and new ways of treating things to work and come into practice full time, so it is necessary.
P1
I yeah, it didn’t bother me being asked. I think because I had been bombarded with so much stuff that day that it was just kind of ‘it’s another little thing’.
P3
They [trials] just weren’t important . . . You were more worried about whether they’d poo’d their nappy, whether they’d wee’d, you know, or how they were doing with their breathing, whether their oxygen support had gone down. So, trials were just not even . . .
P11
. . . it was just very small variations in the care they [babies] were going to have already and so we thought ‘oh well if it’s just tiny little things like this’ . . .
P5
At the time, coz it seemed almost certain that she was gonna die, it felt like such a gift because it felt like we were being given an opportunity for her life to matter . . . We knew that if she were to die, she wouldn’t just have been our baby who we have loved, she’d made an impact, yeah . . . and for us to have the opportunity to contribute.
P4
We would have been in a hundred studies, I mean obviously you make a decision about whether it will be good or bad for [baby] but as long as I was satisfied that you know . . . I was happy for the risk to [baby], I would have been in a hundred like coz they are doing such an amazing job for these babies and the only reason they are doing it is to try and make these babies better and when you are in that yourself you just want to make things better for any other baby that comes after her.
P4
And also how would they know which one [NIHR RCT 1 or RCT 2] was having an effect? That was kind of . . . and I didn’t sort of voice my concerns to the nurse . . . because I’d sort of made my mind up by that point . . . I thought that em . . . the RCT 2 one was just one step too far for me personally. I just felt like if they are messing around with her feeding, the amount of feed and then they are putting something new in as well then I thought, I do not want that.
P1
Every time they spoke to us about a trial, I’d said, ‘Would it affect their care?’. That was my biggest concern. I almost did not mind them being part of any trial, as long as it did not change . . . If they changed somehow, it would not affect their care in the future.
P11
Theme 2: information gathering
Most parents focused on the specific details of each individual trial and attempted to gain information in order to make a decision that was as fully informed as possible (Box 6). Parents preferred a succinct PIL that provided just enough information in a clear ‘jargon-free’ fashion and also valued the opportunity to talk through any concerns they had with a health-care professional. On the whole, parents tended not to seek advice from other parents on the ward but did look for ‘stickers’ found on an infant’s cot or incubator that signified trial participation. Conversely, others did not read the PIL in detail and simply agreed to participate, driven largely by feelings of moral obligation to take part in trials for the benefit of future infants; the perception that their infant was benefiting from past research; or parents’ desire to repay the health-care professionals for the care their infant was receiving.
Although parents felt that they were given all of the information that they needed regarding each trial, some parents admitted that they sometimes felt too upset or unwell to fully take in trial details, especially very soon after the birth of their infant. Indeed, some parents suggested retrospectively that they could not recall the trial names and did not fully understand the goals of the studies or processes involved, but none suggested that they had any regrets about the decisions they made. Parents often used their own names for trials to help them remember specific details of each one (the most common being the ‘poo and wee trial’).
Parents not only placed importance on the information they received from the PIL but also on the manner of the health-care professional who initially approached them, an issue that they regarded as very influential in their decision-making process. They appreciated in particular a friendly and informal, but confident, approach. The majority of parents said that they would have disliked feeling ‘pressured’ (although most did not say that they felt pressured) into making a decision and always appreciated being given as much time as feasibly possible to think about the trial implications. Although parents often could not remember the names, numbers and nature of the trials or studies, they remembered clearly the health-care professional or researcher who introduced them to a trial and supported them throughout.
. . . his [health professional] manner certainly helped. I think if somebody had come in and said ‘right we are doing this trial and we need people’ I would have thought, ‘oh my goodness me, I’m not sure’, but it was definitely the way he broached it, yeah, absolutely that helped.
P12
You know when he asked he was saying ‘have you had a chance to think about it’ you know he was not saying ‘I want your decision now’, so it was, yeah it felt like they were not rushing us but they helped us understand that we needed to make a decision by a certain point you know, coz obviously they had to choose how to progress her feeds.
P4
Obviously the people who were asking the questions were nice which helps, yeah, they weren’t pushy like, like I say or anything like that, it was always my decision so . . . coz I think the one person who used to come and . . . he used to come and talk to us every single day . . . and he was lovely as well, but . . . he just wore normal clothes . . . but he was dead funny.
P3
I think we did three but – this sounds really awful – I’m not sure of the second one, because you know they collect the wee and poo . . . and I do not know if that’s just because they collect it for the hospital, or if it’s something that we have agreed to. So I cannot really remember.
P16
So, we did sign up to it [trial], but then when we came here the other week, they were like, ‘Oh, we need to do a brain scan’, because it was part of the RCT 3 trial, and I thought, ‘Well, I didn’t know that’. You know? So, those little things that, kind of came out . . .
P11
Yes to be honest I wasn’t even bothered about reading the information because for me, anything research wise that can be done to help another baby, because we knew we were ultimately going to lose [other twin baby] for me it did not matter what she went through as long as it was not going to physically harm her it did not make any difference me reading the information about what it was about so I actually just turned round to [nurse] and said ‘yes that’s fine’ and she actually told me to take the information away and read it.
P7
Yes, to be honest at that point, again being under a lot of medication I thought just answer ‘yes’. [Doctor] was very good, he came back afterwards and said ‘can you remember when we talked?’.
P15
. . . coz the leaflets were very well set out and quite sort of succinct which is quite important when you are in such a distractible kind of mood constantly.
P5
Theme 3: making decisions – ‘weighing up the pros and cons’
Parental decisions around participation were not based on the number of trials or studies, but rather on the perceived risks of each individual trial that they were asked to join (Box 7). Parents considered issues such as the well-being of their infant at the time of enrolment, the invasiveness of the trial, the perceived importance of the trial goals and a number of personal motivational factors. Most parents acknowledged that it was not possible to make a fully informed decision on behalf of their infant and so in the absence of certainty they drew on more personal, non-medical risk assessments in order to make a decision. These included wondering what decision ‘baby’ would make; perceiving that trial participation would help their infant to get well; welcoming ‘another pair of eyes’ over their infant’s care; a sense of purpose that helped them to overcome feelings of helplessness; a way of getting their infant home sooner; and, as mentioned above, a debt of gratitude.
We were talking about [baby’s] little character and just how she arrived, we could not have children and then she arrived in my tummy and she just arrived at 23 weeks and then she lived after 23 weeks and so we were just thinking she’s not, she would not, sounds silly, but it feels that she’s not a person who would make that decision based on fear so we felt that we would make the decision based on hope.
P1
I think what we were trying to do in our head was almost imagine, ‘do we think a slower feeding would be better than a quicker feeding . . . or do we think she would be better off somewhere in the middle?’ And when we talked that through, we realised, research doesn’t know so we do not know so we might as well, we cannot make an informed decision about whether one is better than the other so we might as well put her in the trial.
P4
You know you should be the one changing and feeding your baby, you should be the main caregiver and actually your time with them is limited. I think it was just a comfort to know that at least we were doing the one thing that we could do, we were doing, by allowing [baby on trial] . . .
P6
The lady at the [hospital] caught me when I was just washing the bottles em after expressing some milk [laughs] again, very informally, ‘we’ve got a trial, I think [baby] would be perfect for it and it about coming on a err with a . . . feeding tube‘ em. So again there was a positive light to it because obviously babies don’t come home with feeding tubes, you have to wait until you know until you know, they are on bottles or on the breast so I was thinking ‘aah, get her home soon’.
P1
Well they did get on the fast arm of the feeds and we just thought that obviously if they were getting more milk quicker it wouldn’t be detrimental to them because why would they be doing it? But you do think don’t you if they are getting more food in their bellies they hopefully will be getting bigger and stronger especially as they were getting breast milk as well.
P6
Yes. ‘It’s a pair of eyes over the baby’, that’s the way I’m thinking of it as. I thought of it as anyone else, if she’s under someone else’s care, that someone else looking after my baby, sort of makes you feel – not special – but a bit more reassured I was thinking of it as . . . I was just like- we did not even know what was happening, I was just like ‘anything that might actually help her, just do, do, do’.
P9
. . . in my logical mind I knew that I should probably take part in this kind of thing because if people hadn’t done that 40 or 50 years ago then my babies would not be here now. I thought, ‘It is my duty to think about the future’.
P13
Theme 4: saying ‘no’
Although parents were happy to be approached about multiple trials or studies, this did not mean that they agreed to enrol their infant on all of them (Box 8). Some parents admitted that they felt a degree of guilt attached to declining participation, largely because they felt that they owed it to future infants to take part in research. Although very few parents could remember how many trials their infant had participated in, taking part in several trials did provide perceived justification to refuse future trials, as it gave the impression to health-care professionals that they had ‘done their bit’. Parents who did say ‘no’ to a trial were divided in whether or not they felt it necessary to give a reason to justify their decision.
Parents articulated a number of specific reasons for refusing an individual trial; one of the main reasons concerned the time that they were approached about participation. If parents had a possible discharge home date for their infant, they were often keen to safeguard this and saw enrolment on a trial close to discharge as a possible threat to their infant leaving the hospital. In addition, a small minority of parents were approached when their infant was unwell; in these instances they felt that it was inappropriate that they had been approached and that hospital notes had not been read properly. Importantly, parents highlighted that it takes confidence to say ‘no’ to health-care professionals who are caring for their infant. Some parents were relieved to be supported by nursing staff in their decision to refuse a trial, whereas others felt that their decision was not initially accepted by health-care professionals, which left them feeling slightly pressurised and guilty.
I think it was because he’d [health professional] probably asked me quite a lot. I felt that there was maybe a real need from his side, so I think if it had just been a, ‘Yes, that’s fine. Don’t worry about it’, I wouldn’t have felt guilty, but because it was a, ‘Well, should I speak to your husband?’. And he kept coming back to me. You know? That’s why I felt guilty. I felt like I was, kind of, letting him down a little bit.
P11
Perhaps I was more wary of doing anything that would hinder her coming home at that stage coz I felt like we were so close to that final hurdle em, even though she was obviously bigger and stronger, you know . . . I mean I do feel bad because I know good things will come of the research but I think by that point, we were so close to just getting her home anyway that I did not want to put a spanner in the works.
P1
. . . a lot of mums would feel like it, and probably couldn’t have the confidence to say ‘I’m gonna just say no’. I certainly don’t think [name] would, my husband, I don’t think he would kind of say ‘look I want to stop this’.
P15
. . . it felt almost like we’d done our bit, if that make sense.
P18
I think if I’d been asked perhaps for any more than three, maybe like four would have been the limit really, because I think at that point you just kind of it’s a bit, probably it’s a bit too much. I felt that three was fine em . . . and in a funny way being offered three trials made it easier to say no to one of them.
P1
I don’t like having to say ‘no’ to people, and I think because, because with the whole study 2 one that we just talked about, that did make me feel a little bit like, ‘Oh no, what happens if I don’t like the trial and I have to say no again? Will I feel a bit awkward you know?’.
P11
Yes I think from my own point of view, if I was saying no to a trial, it would have to have been a justified reason as well, it can’t just have been ‘no I can’t be bothered’ because that is not a reason to say ‘no’. Yeah from my personal perspective ‘no I can’t be bothered’ is not a reason to say no to research that is potentially going to help somebody and you have got such a sick baby, there’s potentially thousands of babies before you who’ve done research which is helping the treatment that your baby is getting, unless it’s going to damage or disturb your baby’s progress, I personally do not think you have got a right to say ‘no’, but that is purely my own personal perspective on the situation . . .
P7
Discussion including the robustness of the results and limitations
Enrolment of preterm infants into more than one RCT is a common challenge on many research-active neonatal units. 77 Previous studies have suggested that this may not be a significant issue for many parents; however, there are few data or in-depth qualitative studies. 75,81,82,87 Our study provides an in-depth analysis of parental experiences and motivations as well as supporting the idea that enrolment into multiple studies is not necessarily problematic for most parents. Although most parents offered complex personal reasons that motivated them to see research in a positive light and subsequently enrol their infant, others provided important insights into their decision-making and how it made them feel, including their feelings of guilt or obligation.
Our study has several strengths. The semistructured nature of the interviews gave parents the space and time to reflect on their experiences of enrolling their infant on multiple trials and the initial approach to parents to take part in this qualitative study typically took place several weeks after their infant was born and enrolled into these studies. Participants were enabled to articulate in their own words the issues that were important to them. A further strength was the impartial nature of the qualitative researcher (Judy Richards) who was not a health-care professional, had no prior involvement in clinical trials and was able to provide valuable insight as a ‘naive observer’. Nevertheless, the findings of the study require careful interpretation. The design and parameters of the research that may affect the generalisability to other populations, settings and situations include the:
-
specifics of the hospital sites
-
timing of approach for research
-
interventional nature of the trials and studies
-
parent experience of having an infant who survived and had participated in at least one study
-
attitudes, behaviours and beliefs of the clinical and research teams involved.
Although we utilised purposive sampling, we did not record detailed demographic, socioeconomic or educational information from the parents and did not explore how those factors, or other factors such as ethnicity or religion, affect decision-making. Around half of our interviewees were parents of twins or triplets, yet we do not explore in this report how that may affect decision-making. This may be important, as recent studies suggest that such parents have important views on the co-randomisation of their infants. 88 In addition, we did not seek the views of parents who had declined participation in all of the studies or trials that they had been offered or the feelings of parents whose infants had enrolled in a study or trial but had subsequently died. The recent BRACELET study89 explored some of these issues for parents when bereavement occurs in the context of RCTs in neonatal and paediatric intensive care.
Our study supports existing data that suggest that parents make separate decisions about each individual trial, judging each by its own perceived ‘pros and cons’. 75,90 Our findings emphasise that parents want to make final decisions about their infant’s participation and contrast with earlier data that suggested that some parents wanted or expected health-care professionals to make the final decision. 71,91 Although most parents felt that they made good decisions that they did not later regret, some parents suggested that they had not fully taken in or understood the trial information at the time that they were approached and, as a result, some subsequent trial procedures came as a surprise. This highlights the challenges of gaining fully informed consent and the importance of continued and regular involvement of the research team to explain the nature and conduct of the study to parents, as well as the rights of parents to withdraw or to decline specific procedures. 71,74,75,82,91 Importantly, however, most parents acknowledged that despite feeling stressed, they were still happy with their decision to participate, frequently citing their faith in health-care professionals to protect their infant from harm. Many parents in our study suggested that they would have liked information about the trials and studies that were available before their infant was born and although many acknowledged that there were time restraints on decision-making, they did not perceive these decisions to have been made in haste or inadvisably either at the time or with hindsight.
Conclusions including implications for health care and recommendations for additional research
This in-depth exploration of parents’ views on enrolling their infants into more than one RCT or study highlights the need for research teams to be aware of a range of factors when approaching parents on the NICU, especially in the context of co-enrolment. Although parents felt that they had made good decisions and had few regrets, our study highlights that for some this may have been motivated by guilt, a debt of gratitude or a lack of confidence to refuse. However, although parents acknowledged that fully informed consent was not possible, they were still happy that their decisions were ‘good enough’ given the situation they were in. A deeper awareness of these factors would potentially enable health-care professionals to support parents more fully throughout the process of enrolling their infants on multiple trials.
The themes identified here could been used as part of training in good clinical practice and as part of ongoing education for researchers, students, nurses, doctors and allied health professionals who are, or may in the future be, involved in designing or supporting research. The study also suggests that careful consideration is needed when PILs are developed and emphasises the importance of involving patients, parents and/or members of the public in all aspects of research design. Modifications to the PIL may be appropriate where co-enrolment is likely. The study also highlights the confusing and ‘overwhelming’ nature of having a sick infant who requires intensive care and the additional confusion when parents are approached to join research studies. Providing such parents with additional opportunities to further discuss and learn about the research objectives and procedures after they have consented and eliciting their continued assent for their infants’ participation in the study will enhance their satisfaction and understanding of the need for research that aims to improve patient care. Future research could assess these modifications to see if they improve patient satisfaction and experience.
Chapter 6 Evaluation of the effectiveness of an incentive strategy on the response rate in parents of preterm infants: a randomised controlled study within a trial nested within SIFT
Background
Loss to follow-up has a detrimental effect on research studies. It can compromise internal and external validity of results for a number of reasons. These include reduction of statistical power owing to smaller sample size, negative impact on generalisability and potential to bias results. 92 Therefore, it is important to implement strategies to effectively maintain retention of participants.
Organising clinical assessments for follow-up can often be very costly in perinatal trials, so research questionnaires are often used to capture follow-up data, including the primary outcome. Therefore, the need to mitigate the loss to follow-up and maximise the return rate of questionnaires is essential.
Systematic reviews suggest that incentives are effective at improving response rates for research questionnaires in clinical trials. 93,94 Brueton et al. 93 and Khadjesari et al. 95 both reported that offering a monetary incentive improved questionnaire return rates compared with no incentive, in both postal and online settings. Edwards et al. 94 reported that unconditional incentives (i.e. a reward given in advance as a goodwill gesture) led to superior response rates compared with conditional incentives (i.e. the promise of a reward on receipt of a questionnaire). However, there was significant heterogeneity among these results (p < 0.00001). Dillman96 and Singer et al. 97 also reported results that favoured unconditional over conditional incentives.
However, use of financial incentives is costly and studies may not have the funds or resources available to provide unconditional incentives. A conditional incentive, promised on receipt of a completed questionnaire, could be a more cost-effective means of enhancing retention. Both direct costs and resource costs could be reduced by rewarding only those participants who respond. It would minimise wastage created by the monetary incentives sent out to non-responding participants. In studies of incentive versus no incentive, similar gains are reported for both conditional and unconditional incentives. Khadjesari et al. 95 reported an increase of 9% when using a conditional incentive, whereas Dillman96 described increases of 7–14% in studies using unconditional incentives. Furthermore, Brueton et al. 93 reported two studies (297 participants) that suggested that unconditional monetary incentives provided no greater effect than conditional incentives (entry into a prize draw).
There is also a lack of evidence for the most effective incentive methods in the context of perinatal RCT follow-up. There is sensitivity around the population (parents of vulnerable infants) and often a lengthy time period between recruitment and longer-term outcomes that are captured at follow-up (in this case 2 years). Hardy et al. 98 successfully carried out a study of incentives at 1-year follow-up and reported it to be the only known study of incentives in this population. Kenyon et al. 99 investigated the effects of incentives in a perinatal trial; however, this was for follow-up when the children were aged 7 years. It is, therefore, prudent to use the available opportunities to narrow the evidence gap as to what works best. 100 Given the significant heterogeneity in results in previous trials94 and the potential serious effects of loss to follow-up, the need is still present to investigate which method of incentive provides the greatest return, particularly in perinatal RCTs.
The main objective of the SIFT study within a trial (SWAT) was to establish in the parents of preterm infants whether offering an unconditional incentive in advance (with the first mailing of a questionnaire) or promising an incentive (in the first mailing) on completion of a 2-year follow-up postal questionnaire (conditional) improves the response rate. This randomised controlled SWAT was nested within the SIFT: a multicentre RCT carried out in neonatal units in the UK and Republic of Ireland caring for very preterm or VLBW infants. The primary outcome of the SIFT was survival without moderate or severe disability at 24 months of age (CGA). This primary outcome was assessed by a questionnaire sent directly to parents (principally to infants’ mothers). Questionnaires were sent both by post and by a link to an online submission form via e-mail and SMS (short message service) message where these contact details were available. The SWAT investigated whether or not an unconditional incentive (a monetary voucher given before completion of a questionnaire) was more effective than a conditional incentive (the promise of a monetary voucher on receipt of a completed questionnaire).
Changes to the SIFT protocol
The SIFT protocol was amended in 2015 to account for the use of financial incentives. The amendment was approved by the NRES Committee and implemented in 2016. Funding was used from surplus funding for direct research nurse funds on the trial, as recruitment finished 11 months early.
Consent
No additional consent from parents was sought for this SWAT. The PIL and original consent process clearly indicated that the trial primary outcome was to be ascertained by a questionnaire at 24 months of age CGA.
Sample size
Recruitment for the SIFT was completed on 30 June 2015, with 2804 infants randomised. Given that there was an expected overall mortality rate of 5% for the population, it was estimated that 1250 of these infants would survive to the projected start date of the study (originally 1 December 2016) and that about 10% of the parents of these infants would be excluded from the follow-up owing to withdrawal or to a lack of information on contact details or survival status. This would result in approximately 1100 infants eligible for the SWAT, giving 550 infants per group.
It was estimated that the response rate with no incentives would be approximately 66%, based on past experience from the BOOST-II UK trial,101 which had a similar patient population and 2-year follow-up method.
Based on the most applicable studies that investigated incentives, it was anticipated that the addition of an incentive would result in an absolute increase of 10% in the response rate. 95,99 In total, 550 infants per group would allow detection of an absolute difference in response rate of around 7% at 90% power and a two-sided 5% level of significance.
Methods
Eligible participants were those who were recruited to the SIFT who were due to be sent a questionnaire at the age of 24 months (CGA). Participants were recruited to SIFT in 55 recruiting centres in the UK and the Republic of Ireland. Eligibility criteria for inclusion in SIFT have been described elsewhere. 1 Participants were traced to confirm survival and current residence. Where these details could not be ascertained, parents were not contacted. In addition, parents who had withdrawn consent to the 2-year follow-up were excluded.
Participants were allocated randomly to one of two groups:
-
After group – the first paper letter to parents would include a promise of an incentive (£15 gift voucher redeemable at high-street shops) after receipt of a completed form.
-
Before group – the first paper letter to parents would enclose the incentive (£15 gift voucher redeemable at high-street shops) before the receipt of a completed form.
Participants were randomised in a 1 : 1 allocation ratio by permuted block randomisation (using variable block sizes) and stratified by original SIFT allocation (faster feed increment/slower feed increment) and by singleton/multiple birth. Infants from multiple births were allocated to the same incentive group. Vouchers were allocated per questionnaire, so parents of multiple births received a voucher for each infant.
The SIFT office staff at the NPEU CTU were aware of participant allocation owing to the nature of the interventions and the practicalities involved in sending out the letters and the vouchers.
The incentive was a high-street shop voucher valued at £15 (€15 for participants recruited in the Republic of Ireland), which was sent via post. Reminder letters in both groups mentioned the incentives. Letters to those allocated to the after group reiterated the promise of an incentive. Letters to those allocated to the before group tactfully mentioned the incentive that was sent with the first letter. Parents were also contacted via text and/or e-mail to give reminders during the follow-up, although these contacts were not included as part of the analysis of the number of reminders sent. All of the parents were provided with an option of completing the questionnaire online or, as a last resort, via the telephone.
The incentives SWAT was implemented midway through the SIFT follow-up. All SIFT participants who had returned their follow-up questionnaire prior to the incentives study being implemented were sent a £15 voucher out of courtesy to ensure that there was fairness.
Outcomes
The primary outcome was the rate of questionnaire return, defined as receipt of a completed or partially completed questionnaire at the SIFT office. A questionnaire was considered completed or partially completed if the first three out of the five subsections of the questionnaire were completed (as these sections were applicable to the derivation of the primary outcome for the main SIFT).
The secondary outcomes included the:
-
primary method of completion (paper, online or telephone)
-
total cost
-
number of reminders.
The total cost included the postage, receipt of material via prepaid Freepost, cost of envelopes, supplementary materials (e.g. sticker sets sent with questionnaires for infants to play with) and value of gift vouchers. It did not include Freepost licence fee, printing, telephone calls and trial staff time. All costs for participants were calculated in GBP. The cost of the €15 vouchers that were sent to the participants in the Republic of Ireland was converted to GBP using the exchange rate (via xe.com) on 10 May 2017: the date of the invoice for these vouchers. Costs for these participants also included the higher air mail postage fees.
Statistical analysis
The baseline demographic information was summarised by randomised group using frequency counts and percentages for categorical data, means and SDs for normally distributed continuous data or medians with IQRs for other continuous data.
The comparative analysis entailed calculating the absolute difference in the proportion responding with the corresponding 95% CI and the difference in mean cost (plus 95% CI). In addition, the cost in GBP per 1% increase in response rate was calculated factoring in administration costs such as the number of reminder letters, as well as the monetary value of the incentive. For other outcomes relating to the method of completion and the reminder letters, a similar strategy was used based on the distributions/type of data collected.
The principal comparison was the incentivised before group versus the incentivised after group.
The prespecified subgroup analysis examined the consistency of effect of the timing of the incentive for SIFT original allocation (slower feed increment vs. faster feed increment) and singleton versus multiple births using the statistical test of interaction.
The prespecified exploratory analysis examined the response rate in the period prior to the incentives study starting, during the incentives study and overall (i.e. irrespective of incentive group allocation) with a 95% CI. In addition, an analysis exploring ‘regional’ variation was performed.
No adjustment was planned for multiple testing as this SWAT involves a very small number of focused hypothesis tests.
Results
Participant flow and baseline characteristics
In total, 923 infants were randomised to the SWAT (799 women). A total of 459 infants were allocated to receive the incentive before completion. Out of these, three were not sent questionnaires because addresses and survival status could not be confirmed. A total of 464 infants were allocated to receive the incentive after completion. Out of these, 11 were not sent questionnaires because addresses and survival status could not be confirmed and two of the infants were randomised in error. In these two cases, both were later found to have died after they were randomised to the SWAT. All 923 infants were included in analysis (Figure 7). Baseline comparability was balanced across the infant and maternal characteristics at trial entry (Table 14).
FIGURE 7.
Flow of participants through the incentives SWAT. a, Included in the analysis where data are available.

| Characteristic | Before group (N = 459) | After group (N = 464) |
|---|---|---|
| Number of centres, n | 51 | 51 |
| Allocated to faster group in SIFT,a n/N (%) | 226/459 (49.2) | 231/464 (49.8) |
| Male sex, n/N (%) | 229/459 (49.9) | 257/463 (55.5) |
| Missing, n | 0 | 1 |
| Infant age at randomisation (days) | ||
| Median (IQR) | 4 (3–6) | 4 (3–6) |
| Birthweight of < 10th centile for gestational age, n/N (%) | 106/459 (23.1) | 77/463 (16.6) |
| Missing, n | 0 | 1 |
| Gestation at delivery (weeks) | ||
| Median (IQR) | 29 (27–31) | 29 (27–30) |
| 23+0 to 23+6, n/N (%) | 4/459 (0.9) | 7/464 (1.5) |
| 24+0 to 24+6, n/N (%) | 21/459 (4.6) | 26/464 (5.6) |
| 25+0 to 25+6, n/N (%) | 31/459 (6.8) | 44/464 (9.5) |
| 26+0 to 27+6, n/N (%) | 101/459 (22.0) | 107/464 (23.1) |
| 28+0 to 29+6, n/N (%) | 129/459 (28.1) | 117/464 (25.2) |
| 30+0 to 31+6, n/N (%) | 146/459 (31.8) | 145/464 (31.3) |
| 32+0 to 36+6, n/N (%) | 27/459 (5.9) | 18/464 (3.9) |
| Birthweight (g) | ||
| Mean (SD) | 1139.5 (331.8) | 1131.8 (319.4) |
| < 500 g, n/N (%) | 0/459 (0.0) | 2/464 (0.4) |
| 500 to 749 g, n/N (%) | 59/459 (12.9) | 57/464 (12.3) |
| 750 to 999 g, n/N (%) | 111/459 (24.2) | 114/464 (24.6) |
| 1000 to 1249 g, n/N (%) | 124/459 (27.0) | 114/464 (24.6) |
| 1250 to 1499 g, n/N (%) | 91/459 (19.8) | 115/464 (24.8) |
| ≥ 1500 g, n/N (%) | 74/459 (16.1) | 62/464 (13.4) |
| Infant heart rate > 100 b.p.m. at 5 minutes, n/N (%) | 421/455 (92.5) | 418/456 (91.7) |
| Missing, n | 4 | 8 |
| Infant temperature on admission (°C) | ||
| Mean (SD) | 36.8 (0.7) | 36.8 (0.8) |
| Missing, n | 4 | 2 |
| Infant worst base excess within the first 24 hours after birth (mEq/l) | ||
| Mean (SD) | –6.0 (3.9) | –6.1 (4.1) |
| Missing, n | 8 | 10 |
| Infant ventilated via endotracheal tube at randomisation, n/N (%) | 90/458 (19.7) | 107/463 (23.1) |
| Missing, n | 1 | 1 |
| Infant had absent or reversed end diastolic flow, n/N (%) | 76/452 (16.8) | 70/455 (15.4) |
| Missing, n | 7 | 9 |
| Mother’s age at randomisation (years) | ||
| Mean (SD) | 30.7 (5.8) | 31.2 (6.5) |
| Multiple pregnancy,a,b n/N (%) | 144/459 (31.4) | 139/464 (30.0) |
| Singles,c n | 0 | 1 |
| Twins,d n | 136 | 116 |
| Triplets,e n | 8 | 22 |
| Caesarean section delivery, n/N (%) | 284/459 (61.9) | 272/464 (58.6) |
| Membranes ruptured before labour, n/N (%) | 167/453 (36.9) | 159/460 (34.6) |
| Missing, n | 6 | 4 |
| Membranes ruptured > 24 hours before delivery, n/N (%) | 119/454 (26.2) | 104/458 (22.7) |
| Missing, n | 5 | 6 |
Primary outcome
In total, 381 infants allocated to the before group had a questionnaire returned (out of 459, 83.0%) compared with 353 infants allocated to the after group (out of 464, 76.1%) (Table 15). An unconditional £15 incentive upfront (before group) led to a statistically significantly higher response rate than a conditional incentive (after group), with an absolute difference of 6.8% (95% CI 1.6% to 12.0%; p = 0.01), adjusted for stratification factors (trial allocation and single or multiple birth).
| Outcome | Before group (N = 459) | After group (N = 464) | Unadjusted effect measure (95% CI)a | p-value | Adjusted effect measure (95% CI)a,b | p-value |
|---|---|---|---|---|---|---|
| Questionnaire received (at 24 months of age CGA),c n/N (%) | 381/459 (83.0) | 353/464 (76.1) | 6.9 (1.7 to 12.1) | 0.009 | 6.8 (1.6 to 12.0) | 0.010 |
| Method of completion, n/N (%) | ||||||
| Paper questionnaire | 326/459 (71.0) | 295/464 (63.6) | 0.061d | |||
| Online completion | 50/459 (10.9) | 53/464 (11.4) | ||||
| Completion via telephone | 5/459 (1.1) | 5/464 (1.1) | ||||
| Total cost of the strategye (£) | ||||||
| Mean (SD) | 17.97 (1.7) | 15.00 (6.7) | 2.97 (2.33 to 3.60) | < 0.001 | 2.98 (2.34 to 3.61) | < 0.001 |
| Median (IQR) | 18.22 (17.41–18.40) | 18.08 (18.08–18.88) | ||||
| Increase in cost compared with prior to commencement of incentives study (95% CI) | 14.80 (14.68 to 14.94) | 11.84 (11.51 to 12.12) | ||||
| Reminders required,f n/N (%) | 248/456 (54.4) | 264/452 (58.4) | ||||
| 0 | 208/456 (45.6) | 188/452 (41.6) | ||||
| 1 | 104/456 (22.8) | 95/452 (21.0) | 0.027g | |||
| 2 | 43/456 (9.4) | 31/452 (6.9) | ||||
| 3 | 101/456 (22.1) | 138/452 (30.5) | ||||
| Missing, n | 3 | 12 | ||||
Secondary outcomes
Method of completion
In total, 326 questionnaires were completed (and returned) on paper in the before group (out of 459, 71.0%) compared with 295 questionnaires in the after group (out of 464, 63.6%) (see Table 15). Completion rates online were broadly similar between groups, with 50 questionnaires (out of 459, 10.9%) returned in the before group and 53 questionnaires (out of 464, 11.4%) returned in the after group. Five questionnaires were completed over the telephone in each group (1.1%). The difference in response rates appears to be dominated by paper completion.
Cost
The mean cost of the incentive strategy per infant was £17.97 (SD £1.7) in the before group and £15.00 (SD £6.7) in the after group (see Table 15). The mean cost of the unconditional incentive scheme was £21.65 per response compared with £19.72 per response for the conditional incentive. The mean cost per 1% increase in response is £1.35 per infant in the before group compared with £2.95 per infant in the after group.
The additional cost of the incentives strategies per infant compared with prior to the incentives study was £14.80 for the before group and £11.84 for the after group. Unsurprisingly, providing the incentive upfront was more costly; however, the mean difference in the cost per infant was only £2.99 (95% CI £2.33 to £3.61).
Number of reminders
There was evidence to suggest that fewer reminders were required in the before group (54.4%) than in the after group (58.4%) (Table 16). Furthermore, a higher proportion reached the third (final) reminder stage in the after group (138/452, 30.5%) than in the before group (101/456, 22.1%).
| Questionnaire received | Before group (N = 459) | After group (N = 464) | Unadjusted risk difference (95% CI) | p-valuea | Adjusted risk difference (95% CI)b | p-valuea |
|---|---|---|---|---|---|---|
| Trial allocation, n/N (%) | 0.530 | 0.529 | ||||
| Faster feed increment | 188/226 (83.2) | 180/231 (77.9) | 5.3 (–2.0 to 12.5) | 5.2 (–2.0 to 12.5) | ||
| Slower feed increment | 193/233 (82.8) | 173/233 (74.2) | 8.6 (1.2 to 16.0) | 8.5 (1.1 to 16.0) | ||
| Multiple birth, n/N (%) | 0.102 | 0.102 | ||||
| Singleton | 258/315 (81.9) | 253/325 (77.8) | 4.1 (–2.1 to 10.3) | 4.0 (–2.2 to 10.2) | ||
| Multiple | 123/144 (85.4) | 100/139 (71.9) | 13.5 (4.0 to 22.9) | 13.4 (4.0 to 22.8) |
Prespecified subgroup analysis
There was no evidence of a differential effect of the incentives strategy between the original SIFT allocations (faster feed increment/slower feed increment) or between single and multiple births. However, the response rate from parents of multiples was higher in the before group than the after group (see Table 16 and Figure 8), but the test of interaction is not statistically significant and the finding may be, in part, simply due to the increased amount (double or triple) received unconditionally.
FIGURE 8.
Subgroup analyses for response rate at 2 years.
Prespecified exploratory analysis
We examined the response rate prior to and during the incentives study and overall (i.e. irrespective of allocation) (Table 17). Again, not surprisingly, the response rate following the implementation of the vouchers was statistically significantly higher (79.5%, 95% CI 76.8% to 82.0%) than before the implementation of the vouchers (72.1%, 95% CI 69.9% to 74.1%).
| Questionnaire received | Prior to the incentives study (N = 1756) | During the incentives study (N = 923) | ||
|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | |
| Questionnaire received (at 24 months of age CGA) | 1266/1756 (72.1) | 72.1 (69.9 to 74.1) | 734/923 (79.5) | 79.5 (76.8 to 82.0) |
In addition, we examined the response rate in terms of regional variation during the incentives study (Table 18). Unfortunately, the vast majority of the infants were randomised in England and so these results were difficult to interpret. In the Republic of Ireland the trend is reversed, whereas in Scotland response rates were lower in both groups.
| Questionnaire received | Before group (N = 459) | After group (N = 464) | Unadjusted risk difference (95% CI) | p-valuea | Adjusted risk difference (95% CI)b | p-valuea |
|---|---|---|---|---|---|---|
| Region, n/N (%) | 0.636 | 0.640 | ||||
| England | 308/367 (83.9) | 295/381 (77.4) | 6.5 (0.9 to 12.1) | 6.5 (0.9 to 12.2) | ||
| Scotland | 37/48 (77.1) | 26/43 (60.5) | 16.6 (–2.2 to 35.5) | 16.6 (–2.2 to 35.5) | ||
| Wales | 14/16 (87.5) | 10/13 (76.9) | 10.6 (–17.5 to 38.6) | 10.6 (–17.5 to 38.6) | ||
| Northern Ireland | 11/13 (84.6) | 11/14 (78.6) | 6.0 (–23.1 to 35.1) | 6.1 (–23.1 to 35.2) | ||
| Republic of Ireland | 11/15 (73.3) | 11/13 (84.6) | –11.3 (–41.0 to 18.5) | –11.2 (–41.1 to 18.6) |
Discussion
In this SWAT, an unconditional incentive (before group) produced a significantly more favourable response rate than a conditional incentive (after group), with an absolute increase of 6.8% (83.0% vs. 76.1%, respectively). This mirrors results from previous studies. 94,97 The overall response rate was significantly higher after the SWAT was implemented (79.5%, 95% CI 76.8% to 82.0%) than before it was implemented (72.1%, 95% CI 69.9% to 74.1%).
The majority of questionnaires were returned by post and this method saw the significant increase between groups. There was little difference between groups for the online or telephone-supplied questionnaires but there were relatively small numbers of responses overall via these two methods.
Both of the incentive methods are cost-effective in terms of increasing response rate. However, providing an unconditional incentive comes with an additional cost, in this case almost £3 per infant. The before group led to an absolute increase in response rate of 11%, at an average additional cost of £14.80 per infant. The after group led to an absolute increase in response rate of 4%, at an average additional cost of £11.84 per infant. Therefore, although the unconditional incentive is more expensive, it represents good value for money with a cost of £1.35 per infant for every 1% increase in response rate. This is in contrast to the conditional incentive that costs an additional £2.95 per infant for every 1% increase in response rate.
There was no evidence of a differential response rate between trial allocations or between single and multiple births; however, there was a greater response from parents of multiples in the before group. We could postulate that there is a cumulative factor involved here, as parents of multiples received greater upfront incentives (£30 for twins, £45 for triplets) than parents of singletons (£15 for one infant). Previous systematic reviews have reported that higher incentive amounts lead to higher response rates. 93
Strengths and limitations
This SWAT, nested within a perinatal RCT, contributes to the evidence base for methods of incentive in this context. This study was run efficiently and required minimal resource, as it was added to the existing SIFT study and was run without additional staff time. Delays in ethics approval and logistical management meant that the SWAT study did not begin until February 2017, which resulted in fewer infants due to receive their questionnaire and thus participate. Despite this, results showed a significant increase in the response rate in the group allocated an unconditional incentive (before group).
This study is limited to a particular study population: parents of infants born prematurely. It is also limited to the follow-up period of 2 years. Given the scarcity of studies in the context of perinatal RCTs, it is important to bear this in mind. Longer follow-up periods (e.g. at school age), different modes of data collection and assessment (e.g. clinical assessment) and other incentive amounts may produce different results.
Implications
This SWAT provides strong evidence that incentives can be a cost-effective strategy to maximise follow-up in perinatal RCTs. It also demonstrates that giving the incentive voucher before receiving the questionnaire is more effective than giving the incentive voucher after. Indeed, an unconditional incentive strategy, although more expensive than a conditional incentive strategy, pays dividends with a significantly higher response rate. This SWAT gives a clear indication of the potential benefit, which may be helpful to other triallists considering the same approach.
Chapter 7 Discussion and conclusions
Summary of main findings
The SIFT shows that advancing enteral feed volumes at daily increments of 18 ml/kg versus 30 ml/kg does not affect survival without moderate or severe neurodevelopmental disability at 24 months CGA or affect the risks of LOS or NEC in very preterm or VLBW infants. Advancing feeds more quickly reduced the duration of parenteral nutrition by 2 days but was associated with an unexpected increase in the frequency of abnormal motor outcomes at 24 months CGA.
To our knowledge, the SIFT is the first study to assess the neurodevelopmental outcome in relation to the speed of increasing feeds. Although the composite primary outcome was not statistically different between the two groups, the unexpected finding of an observed increase in the risk of moderate or severe motor impairment in the faster feed increment group requires consideration. This makes it unclear which increment to adopt in clinical practice and was notable as we anticipated that those infants in the faster increment group would have better outcomes at 24 months CGA than those infants in the slower group, unless there was an increased risk of NEC that was not outweighed by a reduction in LOS for faster-fed infants. The result is more perplexing in that there were numerically fewer cases of LOS and NEC in the faster group. It is possible that this is a chance finding, but there are biologically plausible mechanisms that could explain it, such as increased cardiorespiratory events as a result of pressure on the diaphragm,102 impaired cerebral blood flow autoregulation103 or the inability to digest and absorb enteral nutrition. 104–107
The quality and power of the trial enhances the validity of the findings. Practices were used to limit bias, including central web-based randomisation for allocation concealment. We obtained a high rate of follow-up and assessed the trial cohort with intention-to-treat analyses based on a prespecified statistical analysis plan. The trial achieved recruitment of 2804 participants, as per protocol based on the a priori sample size estimation. This sample was inflated before the trial commenced to allow for correlation in the primary outcome between twins and multiple siblings who received the same intervention as their siblings. This approach was taken after consulting parents of multiple birth siblings and representative groups who raised concerns about feeding siblings at different rates.
At randomisation, demographic and prognostic characteristics were well balanced between the two groups, with a minimisation algorithm ensuring that there was balance for major known or anticipated prognostic indicators (i.e. hospital, multiple birth, gestational age ranges and birthweight of < 10th centile for gestational age) or potential confounding influences, such as recruiting site. Interim analyses by the trial’s independent DMC used strict criteria to minimise the chances of spurious findings due to data fluctuations before a sufficient sample size was achieved. Adherence to the allocated interventions was high, the incidence of protocol violations was low and primary outcome data were available for 89.8% of the trial cohort.
The PARCA-R is a parent-completed assessment of cognitive and language development at 24 months of age. 37 It has good concurrent validity and test–retest reliability and, using published cut-off scores for identifying preterm children at risk of developmental delay, it has excellent diagnostic utility (sensitivity and specificity of > 80%) for identifying those with moderate to severe delay, as determined by scores of < –2 SD on a gold-standard developmental test. 37 It is widely used both as an assessment in routine developmental follow-up (e.g. as recommended for use in neonatal follow-up in the NICE guideline108 for ‘developmental follow-up of children and young people born preterm’ in the UK) and as an outcome measure in observational studies and clinical trials. 37 The resources that are required to carry out gold-standard tests prohibit their use in large-scale studies, especially where there are large geographically dispersed cohorts as in SIFT. Thus, the PARCA-R provides a cost-efficient alternative to examiner-administered tests for assessing cognitive and language outcomes at 24 months of age. However, like many developmental screening tools, lower positive predictive values (PPVs) may result in over-referrals or false positives. In studies comparing the PARCA-R with a gold-standard test, PPVs have ranged from 23% to 65% in samples of children born preterm. As such, a higher rate of positive screens is found on the PARCA-R than the rate of children identified by an examiner using a standardised test. 37,109,110 Therefore, the proportion of children with moderate to severe developmental delay as measured by scores of < –2 SD is likely to be lower than observed in SIFT. However, studies have shown that preterm children with false-positive responses represent a high-risk group and have significantly poorer developmental outcomes than those with true-negative screens and, thus, have a higher risk of long-term intellectual disability. 37,110,111
Maximising developmental follow-up and minimising participant attrition is a key concern for researchers, given that selective loss to follow-up can bias results. 111,112 In the UK this is increasingly challenging and completion rates for questionnaire-based follow-up have recently been reported at approximately ≤ 60%. 44,113,114 Intensive efforts were therefore made to maximise response rates at 24 months of age CGA in SIFT, as detailed in Appendix 10, which shows the return rate of questionnaires alongside the timings of the interventions. The 72% response rate of completed, classifiable, SIFT questionnaires, with the addition of classifications of outcomes obtained from blinded end-point review of routine clinical follow-up data (11.2%), meant that we achieved an excellent overall follow-up rate of 83.2%, where a classification of disability was possible in survivors at 24 months of age.
Event rates for the primary and secondary outcomes were different from those we anticipated when estimating the sample size required for the trial. 2,33 There were fewer deaths than expected, which was probably a result of the timing of randomisation that took place in a median of 4 days of age for both groups, by which time most neonatal deaths have already occurred. 115 The rates of LOS and NEC were similar to previous studies; however, NEC occurred in 5.3% of SIFT participants compared with 6.95% of participants in previous studies and invasive (confirmed) infection was seen in 18.4% versus 18.8%, respectively. 13
To our knowledge, almost three times as many infants participated in SIFT than in all of the existing trials combined; this suggests that we were able to produce more precise estimates of effect size than those available before SIFT. Analysis of secondary outcomes demonstrated that the number of days to reach full milk feeds and the number of days of parenteral nutrition were reduced with faster increments. Although the 95% CI for the relative risk estimate for the primary outcome was not statistically significant, it excludes a > 1% reduction in risk and a ≥ 18% increase in risk for those fed at 30 ml/kg/day compared with those fed at 18 ml/kg/day.
These results substantially outweigh data from previous trials,24–32 as large numbers of high-risk infants were recruited, including 1020 extremely low-birthweight infants, 994 extremely preterm infants and 435 infants with absent or reversed end diastolic flow in the umbilical artery on antenatal Doppler studies.
Limitations
A limitation of the trial is that is was not blinded, as it would have been difficult to safely and completely blind caregivers and parents to the feed rate. This is unlikely to have influenced the ascertainment of the most important outcomes, which were reviewed by BERCs. It is possible that knowledge of allocation could alter clinician practice, for example stopping feeds more often or diagnosing suspected NEC in faster increment infants. We did, however, see fewer cases of NEC in the faster increment group, suggesting that this did not occur often.
Infants were a median age of 4 days at commencement of the intervention and some clinicians may have been less likely to enrol the highest-risk infants. The trial does not, therefore, allow conclusion about the safety of different feed advancement increments in the first few days after birth.
Subgroup analyses
The subgroup analyses were informative, as they did not show significant findings but, as with all such analyses, they are inherently underpowered. The one statistically significant finding in the subgroup analysis was evidence of excess adverse outcome in the small number of faster increment infants (18/30) who received formula milk alone compared with slower increment infants (12/40). Higher-risk infants (including those with abnormal Dopplers) did not do worse with faster feed increments (see Figures 3 and 4 and Table 4). As the size of the group that received formula alone comprised 93 infants only, with 24-month outcomes not being known in 23 infants and including 16 infants in the faster increment group, it is likely that survival without moderate or severe disability being lower in the faster group is a chance finding. Further analysis is planned given that 1666 infants received a mix of breast and formula milk. It is possible that a higher proportion or volume of breast milk could protect against a poor outcome through anti-infective or anti-inflammatory mechanisms. It was a limitation of the study that additional granularity was not used in this subgroup analysis.
In post hoc analyses, we did not show any evidence that absent or reversed end diastolic flow that was detected by antenatal Dopplers of the umbilical arteries increased the risk of NEC with the faster feed increment.
Cost analyses
Given the apparently worse motor outcome at 24 months CGA, increasing the milk feed volumes at a faster rate in very preterm or VLBW infants is not a cost-effective strategy. Although it may reduce the burden on scarce health-care resources in the short term, the cost–consequences of this strategy in the long term are likely to be too severe to recommend this clinical practice.
Qualitative analysis of parent views
This in-depth exploration of parents’ views on enrolling their infants into more than one RCT or study highlights the need for research teams to be aware of a range of factors when approaching parents about multiple studies on the NICU. Although parents felt that they had made good decisions and had few regrets, our study highlights that, for some, this may be motivated by guilt, a debt of gratitude or a lack of confidence to refuse. A deeper awareness of these factors would enable health-care professionals to support parents more fully through the process of enrolling their infants on multiple trials.
The study also suggests that careful consideration is needed when PILs are developed and emphasises the importance of involving patients, parents and/or members of the public in all aspects of research design. The study also highlights the confusing and ‘overwhelming’ nature of having a sick infant requiring intensive care and the additional confusion when they are approached to join research studies. Providing such parents with additional opportunities to further discuss and learn about the research objectives and procedures after they have consented and eliciting their continued assent for their infants’ participation in the study may be important mitigation.
Incentives trial
The incentives study was an important part of increasing the follow-up rate in the trial to ensure that the primary outcome was known in > 90% of cases after other data sources were also taken into account. The unconditional incentive given before receiving the questionnaire produced a more favourable response rate than a conditional incentive, with an absolute increase of 6.8% (83.0% vs. 76.1%, respectively). The overall response rate was significantly higher after the SWAT was implemented (79.5%, 95% CI 76.8% to 82.0%) than before it was implemented (72.1%, 95% CI 69.9% to 74.1%), suggesting that both methods of incentives can be an effective strategy in maximising return rates.
Applicability
The SIFT findings are likely to be applicable both in the UK and internationally. Participants were enrolled in 55 neonatal units across the UK and in the Republic of Ireland, which gave a broad geographical, social and ethnic representation. Many infants who were enrolled in a recruiting site were subsequently transferred to another neonatal unit of ongoing care, usually closer to home. Trial participation continued in another 78 neonatal units, which mirrored care pathways for preterm infants in managed clinical networks in the UK.
The trial population was representative of very preterm or VLBW infants who were cared for within health-care facilities in well-resourced health services and included a substantial proportion of extremely preterm infants (36%) and of infants with other putative risk factors for neonatal morbidity, such as prolonged rupture of maternal amniotic membranes (24%) and evidence of absent or reversed end diastolic flow in the umbilical artery (16%). Overall, 30.4% of participants acquired a microbiologically confirmed or clinically suspected late-onset infection and, in total, 18.4% had a microbiologically confirmed infection, which was consistent with rates reported from cohort studies and other RCTs. 18–20,22,24–32 Similarly, the incidence of NEC (5.3%) was similar to rates reported in large, population-based surveillance and cohort studies and RCTs. 72
Implications for practice
The SIFT does not conclusively support the routine use of faster or slower enteral feed increments at daily increments of 18 ml/kg versus 30 ml/kg. Neither rate affected the primary outcome of survival without moderate or severe neurodevelopmental disability at 24 months of age or the risk of LOS or NEC in very preterm or VLBW infants. Advancing feeds more quickly reduced the duration of parenteral nutrition use by 2 days but was associated with an unexpected increase in the frequency of abnormal motor outcomes at 24 months CGA. Clinicians reviewing these data will need to weigh up the relative importance of the secondary outcomes when deciding how fast to increase the milk feeds of infants in their care.
Implications for research
Further randomised trials and examination of feeding data sets (either existing or prospectively collected) may provide further information. Research efforts should continue to investigate the aetiology, epidemiology and pathogenesis of complications from faster and slower feeding, with a particular focus on neurodevelopmental outcomes in relation to milk-feeding practices. They should also aim to develop, refine and assess feeding interventions that may prevent or reduce adverse acute and long-term consequences for very preterm infants and their families. Assessing different incremental feeding increments may be of benefit, as different groups of infants may respond differently to different increments (e.g. extremely premature infants). It may also be worthwhile to assess different increments in other groups, as these increments may also be better for those exposed to associated risk factors such as formula milk or placental dysfunction. Further detailed analysis of the association between milk types and outcomes is planned using data from this trial. Interventions to improve parental understanding, experience and acceptance of multiple research studies also warrant study.
Patient and public involvement
The SIFT was facilitated by close engagement with infant and family representatives experienced in service-user representation. Bliss, the UK national charity for ‘babies born premature or sick’, and parents of children who had received neonatal intensive care contributed to development of trial materials (e.g. PILs and consent forms) and to training of research staff (e.g. in simulated ‘consent-seeking’ sessions). This assistance was extremely beneficial and an important part of the successful delivery of the study.
Acknowledgements
We are grateful to the parents of participating infants and staff and carers in recruiting and continuing care sites. We thank the members of the independent DMC and the TSC and the administrative and support colleagues at the NPEU CTU.
Funding and sponsorship
The SIFT was funded by the NIHR Health Technology Assessment programme (11/01/25) and sponsored by the University of Oxford. The funder provided advice and support and monitored study progress but did not have a role in study design or data collection, analysis and interpretation.
Independent Trial Steering Committee
The members of the TSC were Denis Azzopardi (chairperson), Alison Baum, Mike Bradburn, Kate Costeloe, Mark Dolman, Andrew Ewer, Fan Hutchison and Michael Millar.
Independent Data Monitoring Committee
The members of the DMC were Richard Cooke (chairperson), Steve Kempley and Andrea Marshall.
Contributions of authors
Jon Dorling (https://orcid.org/0000-0002-1691-3221) (Chief Investigator, Neonatologist) was responsible for data collection and management, study design, data interpretation and writing the report.
Oliver Hewer (https://orcid.org/0000-0002-6251-7174) (Trial Manager) was responsible for data collection and management and writing the report.
Madeleine Hurd (https://orcid.org/0000-0002-2797-2358) (Administrator and Data Co-ordinator) was responsible for data collection and management and writing the report.
Vasha Bari (https://orcid.org/0000-0001-8183-2455) (Administrator and Data Co-ordinator) was responsible for data collection and management.
Beth Bosiak (https://orcid.org/0000-0002-6856-879X) (Trial Manager) was responsible for data collection and management and study design.
Ursula Bowler (https://orcid.org/0000-0002-0100-0155) (Senior Trials Manager) was responsible for data collection and management and study design.
Andrew King (https://orcid.org/0000-0001-7175-2718) (Head of Trials Programming) was responsible for data collection and management.
Louise Linsell (https://orcid.org/0000-0003-3205-6511) (Lead Medical Statistician) was responsible for study design, data analysis and report writing.
David Murray (https://orcid.org/0000-0001-9010-2905) (Senior Trials Programmer) was responsible for data collection and management.
Omar Omar (https://orcid.org/0000-0001-7582-8337) (Trial Statistician) was responsible for study design and data analysis.
Christopher Partlett (https://orcid.org/0000-0001-5139-3412) (Trial Statistician) was responsible for data analysis.
Catherine Rounding (https://orcid.org/0000-0002-1376-7572) (Trial Manager) was responsible for data collection and management and study design.
John Townend (https://orcid.org/0000-0001-5455-2562) (Trial Statistician) was responsible for data analysis.
Jane Abbott (https://orcid.org/0000-0002-5847-6824) (PPI representative) was responsible for study design.
Janet Berrington (https://orcid.org/0000-0002-6185-2843) (Co-investigator, Neonatologist) was responsible for study design and data interpretation.
Elaine Boyle (https://orcid.org/0000-0002-5038-3148) (Co-investigator, Neonatologist) was responsible for study design.
Nicholas Embleton (https://orcid.org/0000-0003-3750-5566) (Co-investigator, Neonatologist) was responsible for study design and data interpretation.
Samantha Johnson (https://orcid.org/0000-0001-8963-7881) (Co-investigator, Developmental Psychologist) was responsible for study design and data interpretation.
Alison Leaf (https://orcid.org/0000-0002-6068-7188) (Co-investigator, Neonatologist) was responsible for study design and data interpretation.
Kenny McCormick (https://orcid.org/0000-0001-8378-2690) (Co-investigator, Neonatologist) was responsible for study design and data interpretation.
William McGuire (https://orcid.org/0000-0001-8572-3467) (Co-investigator, Neonatologist) was responsible for data collection and management, study design, data interpretation and writing the report.
Mehali Patel (https://orcid.org/0000-0001-6824-0551) (Patient and public involvement representative) was responsible for study design and data interpretation.
Tracy Roberts (https://orcid.org/0000-0002-0624-0537) (Co-investigator, Health Economist) was responsible for study design and data interpretation.
Ben Stenson (https://orcid.org/0000-0003-2645-7458) (Co-investigator, Neonatologist) was responsible for study design, data interpretation and writing the report.
Warda Tahir (https://orcid.org/0000-0002-4410-1757) (Health Economist) was responsible for data collection and management, data analysis, data interpretation and writing the report.
Mark Monahan (https://orcid.org/0000-0002-1175-9421) (Health Economist) was responsible for data collection and management, data analysis, data interpretation and writing the report.
Judy Richards (https://orcid.org/0000-0003-1287-5923) (Sociologist) was responsible for data collection and management, data analysis, data interpretation and writing the report.
Judith Rankin (https://orcid.org/0000-0001-5355-454X) (Epidemiologist) was responsible for data collection and management, study design, data analysis and data interpretation.
Edmund Juszczak (https://orcid.org/0000-0001-5500-2247) (NPEU CTU Director) was responsible for data collection and management, study design, data analysis, data interpretation and writing the report.
All of the authors approved the final draft of the manuscript.
Publications
Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, et al. Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434–43.
Tahir W, Monahan M, Dorling J, Hewer O, Bowler U, Linsell L, et al. Economic evaluation alongside the Speed of Increasing milk Feeds Trial (SIFT) [published online ahead of print April 2 2020]. Arch Dis Child 2020.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Please note exclusive use will be retained until the publication of major outputs. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Abbott J, Berrington J, Bowler U, Boyle E, Dorling J, Embleton N, et al. The Speed of Increasing milk Feeds: a randomised controlled trial. BMC Pediatr 2017;17. https://doi.org/10.1186/s12887-017-0794-z.
- Healthcare Quality Improvement Partnership (HQIP) . CMACE Report – Perinatal Mortality 2009 2014. www.hqip.org.uk/resource/cmace-and-cemach-reports/ (accessed 16 January 2019).
- Field DJ, Dorling JS, Manktelow BN, Draper ES. Survival of extremely premature babies in a geographically defined population: prospective cohort study of 1994–9 compared with 2000–5. BMJ 2008;336:1221-3. https://doi.org/10.1136/bmj.39555.670718.BE.
- Hack M, Costello DW. Trends in the rates of cerebral palsy associated with neonatal intensive care of preterm children. Clin Obstet Gynecol 2008;51:763-74. https://doi.org/10.1097/GRF.0b013e3181870922.
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. J Pediatr 2012;160:49-53.e1. https://doi.org/10.1016/j.jpeds.2011.06.046.
- Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 2007;92:F193-8. https://doi.org/10.1136/adc.2006.099929.
- Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123:e312-27. https://doi.org/10.1542/peds.2008-1827.
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss national cohort of extremely premature infants. Pediatrics 2011;128:e348-57. https://doi.org/10.1542/peds.2010-3338.
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153:170-5. https://doi.org/10.1016/j.jpeds.2008.02.033.
- Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. NICHD Neonatal Network . Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics 2005;115:673-80. https://doi.org/10.1542/peds.2004-0667.
- Murphy DJ, Hope PL, Johnson A. Neonatal risk factors for cerebral palsy in very preterm babies: case-control study. BMJ 1997;314:404-8. https://doi.org/10.1136/bmj.314.7078.404.
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292:2357-65. https://doi.org/10.1001/jama.292.19.2357.
- Dobson B, Middleton S. Paying to Care: The Cost of Childhood Disability 1998. www.jrf.org.uk/report/paying-care-cost-childhood-disability.
- Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD001241.pub6.
- Hsu JF, Tsai MH, Huang HR, Lien R, Chu SM, Huang CB. Risk factors of catheter-related bloodstream infection with percutaneously inserted central venous catheters in very low birth weight infants: a center’s experience in Taiwan. Pediatr Neonatol 2010;51:336-42. https://doi.org/10.1016/S1875-9572(10)60065-4.
- Kelly DA. Preventing parenteral nutrition liver disease. Early Hum Dev 2010;86:683-7. https://doi.org/10.1016/j.earlhumdev.2010.08.012.
- Nadroo AM, Lin J, Green RS, Magid MS, Holzman IR. Death as a complication of peripherally inserted central catheters in neonates. J Pediatr 2001;138:599-601. https://doi.org/10.1067/mpd.2001.111823.
- Adams-Chapman I, Stoll BJ. Prevention of nosocomial infections in the neonatal intensive care unit. Curr Opin Pediatr 2002;14:157-64. https://doi.org/10.1097/00008480-200204000-00003.
- Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109:34-9. https://doi.org/10.1542/peds.109.1.34.
- Nagata E, Brito AS, Matsuo T. Nosocomial infections in a neonatal intensive care unit: incidence and risk factors. Am J Infect Control 2002;30:26-31. https://doi.org/10.1067/mic.2002.119823.
- Chathas MK, Paton JB, Fisher DE. Percutaneous central venous catheterization. Three years’ experience in a neonatal intensive care unit. Am J Dis Child 1990;144:1246-50. https://doi.org/10.1001/archpedi.1990.02150350078030.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285-91. https://doi.org/10.1542/peds.110.2.285.
- Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012;345. https://doi.org/10.1136/bmj.e7961.
- Caple J, Armentrout D, Huseby V, Halbardier B, Garcia J, Sparks JW, et al. Randomized, controlled trial of slow versus rapid feeding volume advancement in preterm infants. Pediatrics 2004;114:1597-600. https://doi.org/10.1542/peds.2004-1232.
- Karagol BS, Zenciroglu A, Okumus N, Polin RA. Randomized controlled trial of slow vs rapid enteral feeding advancements on the clinical outcomes of preterm infants with birth weight 750–1250 g. J Parenter Enteral Nutr 2012;37:223-8. https://doi.org/10.1177/0148607112449482.
- Krishnamurthy S, Gupta P, Debnath S, Gomber S. Slow versus rapid enteral feeding advancement in preterm newborn infants 1000-1499 g: a randomized controlled trial. Acta Paediatr 2010;99:42-6. https://doi.org/10.1111/j.1651-2227.2009.01519.x.
- Modi M, Ranji S, Jain A, Sharma P, Gupta N. A Randomised Trial of Aggressive Feeding Regimen in Infants With Birthweight ≤ 1250 Grams n.d.
- Raban S, Santhakumaran S, Keeran Q, Joolay Y, Uthaya S, Horn A. A Randomised Controlled Trial of High or Low Volume Initiation and Rapid or Slow Advancement of Milk Feeds for Infants ≤ 1000 G n.d.
- Jain S, Mukhopadhyay K, Jain V, Kumar P. Slow versus rapid enteral feed in preterm neonates with antenatal absent end diastolic flow. J Matern Fetal Neonatal Med 2016;29:2828-33. https://doi.org/10.3109/14767058.2015.1105954.
- Raban S, Santhakumaran S, Keraan Q, Joolay Y, Uthaya S, Horn A, et al. A randomised controlled trial of high vs low volume initiation and rapid vs slow advancement of milk feeds in infants with birthweights ≤ 1000 g in a resource-limited setting. Paediatr Int Child Health 2016;36:288-95. https://doi.org/10.1179/2046905515Y.0000000056.
- Rayyis SF, Ambalavanan N, Wright L, Carlo WA. Randomized trial of ‘slow’ versus ‘fast’ feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. J Pediatr 1999;134:293-7. https://doi.org/10.1016/S0022-3476(99)70452-X.
- Salhotra A, Ramji S. Slow versus fast enteral feed advancement in very low birth weight infants: a randomized control trial. Indian Pediatr 2004;41:435-41.
- Dorling J, Abbott J, Berrington J, Bowler U, Boyle E, Embleton N, et al. Protocol For The Speed of Increasing Milk Feed Trial (SIFT) n.d. www.npeu.ox.ac.uk/sift/protocols (accessed 23 December 2016).
- The ELFIN . Trial Investigators Group. Summary protocol for a multi-centre randomised controlled trial of Enteral Lactoferrin Supplementation in Newborn Very Preterm Infants (ELFIN). Neonatology 2018;114:142-8. https://doi.org/10.1159/000488927.
- Allmark P, Spedding M. Clinical trials in neonates: ethical issues. Semin Fetal Neonatal Med 2007;12:318-23. https://doi.org/10.1016/j.siny.2007.01.023.
- Johnson S, Wolke D, Marlow N. Preterm Infant Parenting Study Group. Developmental assessment of preterm infants at 2 years: validity of parent reports. Dev Med Child Neurol 2008;50:58-62. https://doi.org/10.1111/j.1469-8749.2007.02010.x.
- Johnson S, Marlow N, Wolke D, Davidson L, Marston L, O’Hare A, et al. Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol 2004;46:389-97. https://doi.org/10.1017/s0012162204000635.
- British Association of Perinatal Medicine . Report of a BAPM RCPCH Working Group. Classification of Health Status at 2 Years As a Perinatal Outcome. 2008. www.networks.nhs.uk/nhs-networks/staffordshire-shropshire-and-black-country-newborn/documents/2_year_Outcome_BAPM_WG_report_v6_Jan08.pdf (accessed 29 January 2020).
- The ELFIN Trial Investigators Group . Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019;393:423-33. https://doi.org/10.1016/S0140-6736(18)32221-9.
- Kamoji VM, Dorling JS, Manktelow B, Draper ES, Field DJ. Antenatal umbilical Doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatr 2008;97:327-31. https://doi.org/10.1111/j.1651-2227.2008.00671.x.
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. https://doi.org/10.1056/NEJMra1005408.
- Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol 2006;20:498-506. https://doi.org/10.1111/j.1365-3016.2006.00756.x.
- Vermont Oxford Network . Vermont Oxford Network Database. Data for 31 Neonatal Units in the United Kingdom 2011. https://public.vtoxford.org/manuals-forms/members-area/ (accessed 29 September 2011).
- Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed 2015;100:F301-8. https://doi.org/10.1136/archdischild-2014-307684.
- Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, et al. Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434-43. https://doi.org/10.1056/NEJMoa1816654.
- Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 1995;73:17-24. https://doi.org/10.1136/adc.73.1.17.
- Tahir W, Monahan M, Dorling J, Hewer O, Bowler U, Linsell L, et al. Economic evaluation alongside the Speed of Increasing milk Feeds Trial (SIFT) [published online ahead of print April 2 2020]. Arch Dis Child 2020. https://doi.org/10.1136/archdischild-2019-318346.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Department of Health and Social Care . NHS Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 17 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Joint Formulary Committee . British National Formulary 2016.
- Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med 2012;7:29-37. https://doi.org/10.1089/bfm.2011.0002.
- Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al. Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13400.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Walter E, Liu FX, Maton P, Storme T, Perrinet M, von Delft O, et al. Cost analysis of neonatal and pediatric parenteral nutrition in Europe: a multi-country study. Eur J Clin Nutr 2012;66:639-44. https://doi.org/10.1038/ejcn.2011.225.
- National Institute for Health and Care Excellence (NICE) . BNF for Children 2019. https://bnfc.nice.org.uk/ (accessed 17 January 2019).
- Drummond MF, Sculpher MJ, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM 1999;92:177-82. https://doi.org/10.1093/qjmed/92.3.177.
- Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Priorities in Health. Washington, DC: The International Bank for Reconstruction and Development/ The World Bank; 2006.
- Brown M, Bennett P. Clinical Pharmacology – 11th Edition. London: Churchill Livingstone; 2012.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3 (accessed 9 January 2020).
- Petrou S, Henderson J, Bracewell M, Hockley C, Wolke D, Marlow N. EPICure Study Group . Pushing the boundaries of viability: the economic impact of extreme preterm birth. Early Hum Dev 2006;82:77-84. https://doi.org/10.1016/j.earlhumdev.2006.01.002.
- Chapko MK, Liu CF, Perkins M, Li YF, Fortney JC, Maciejewski ML. Equivalence of two healthcare costing methods: bottom-up and top-down. Health Econ 2009;18:1188-201. https://doi.org/10.1002/hec.1422.
- Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2011;3. https://doi.org/10.1002/14651858.CD001970.pub3.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Whitehurst DG, Bryan S. Trial-based clinical and economic analyses: the unhelpful quest for conformity. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-421.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. https://doi.org/10.1016/S0167-6296(98)00039-3.
- Bayarri MJ, Berger JO. The interplay of Bayesian and frequentist analysis. Stat Sci 2004;19:58-80. https://doi.org/10.1214/088342304000000116.
- Wilman E, Megone C, Oliver S, Duley L, Gyte G, Wright JM. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the empirical research. Trials 2015;16. https://doi.org/10.1186/s13063-015-0957-x.
- Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics 1997;99. https://doi.org/10.1542/peds.99.1.e6.
- Burgess E, Singhal N, Amin H, McMillan DD, Devrome H. Consent for clinical research in the neonatal intensive care unit: a retrospective survey and a prospective study. Arch Dis Child Fetal Neonatal Ed 2003;88:280-6. https://doi.org/10.1136/fn.88.4.F280.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997;45:1337-55. https://doi.org/10.1016/S0277-9536(97)00063-4.
- Mason S. Obtaining informed consent for neonatal randomised controlled trials — an ‘elaborate ritual’?. Arch Dis Child 1997;76:143-5. https://doi.org/10.1136/fn.76.3.F143.
- Hoehn KS, Wernovsky G, Rychik J, Gaynor JW, Spray TL, Feudtner C, et al. What factors are important to parents making decisions about neonatal research?. Arch Dis Child Fetal Neonatal Ed 2005;90:267-9. https://doi.org/10.1136/adc.2004.065078.
- Tooher RL, Middleton PF, Crowther CA. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC Pregnancy Childbirth 2008;8. https://doi.org/10.1186/1471-2393-8-36.
- Davis JM, Baer GR, Portman R, Nelson R, Storari L, Aranda JV, et al. Enrolment of neonates in more than one clinical trial. Clin Ther 2017;39:1959-69. https://doi.org/10.1016/j.clinthera.2017.09.006.
- Randolph AG. The unique challenges of enrolling patients into multiple clinical trials. Crit Care Med 2009;37:107-11. https://doi.org/10.1097/CCM.0b013e3181921c9d.
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al. Mechanisms affecting the gut of preterm infants in enteral feeding trials. Front Nutr 2017;4. https://doi.org/10.3389/fnut.2017.00014.
- Embleton ND, Turnbull E, Turner S, Berrington JE. Successful blood salvaging from preterm infants: maximizing opportunities, minimizing interventions. Acta Paediatr 2013;102:e527-9. https://doi.org/10.1111/apa.12373.
- Brocklehurst P. Randomised controlled trials in perinatal medicine: 2. Recruitment of a pregnant woman or her newborn child into more than one trial. Br J Obstet Gynaecol 1997;104:765-7. https://doi.org/10.1111/j.1471-0528.1997.tb12016.x.
- Beardsall K, Brocklehurst P, Ahluwalia J. Should newborn infants be excluded from multiple research studies?. Lancet 2008;372:503-5. https://doi.org/10.1016/S0140-6736(08)61200-3.
- Ward Platt M. Participation in multiple neonatal research studies. Arch Dis Child Fetal Neonatal Ed 2005;90. https://doi.org/10.1136/adc.2004.067371.
- Harron K, Lee T, Ball T, Mok Q, Gamble C, Macrae D, et al. CATCH team . Making co-enrolment feasible for randomised controlled trials in paediatric intensive care. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0041791.
- Silverman D. Interpreting Qualitative Data: Methods for Analyzing Talk, Text and Interaction. London: SAGE Publications Ltd; 2001.
- Richards J, Graham R, Embleton ND, Campbell C, Rankin J. Mothers’ perspectives on the perinatal loss of a co-twin: a qualitative study. BMC Pregnancy Childbirth 2015;15. https://doi.org/10.1186/s12884-015-0579-z.
- Stenson BJ, Becher JC, McIntosh N. Neonatal research: the parental perspective. Arch Dis Child Fetal Neonatal Ed 2004;89:321-4. https://doi.org/10.1136/adc.2002.021931.
- Bernardo J, Nowacki A, Martin R, Fanaroff JM, Hibbs AM. Multiples and parents of multiples prefer same arm randomization of siblings in neonatal trials. J Perinatol 2015;35:208-13. https://doi.org/10.1038/jp.2014.192.
- Embleton ND, Rankin J. The BRACELET study: implications for the design of randomised controlled trials in neonatal and paediatric intensive care. Arch Dis Child Fetal Neonatal Ed 2015;100:F97-8. https://doi.org/10.1136/archdischild-2014-307103.
- Morley CJ, Lau R, Davis PG, Morse C. What do parents think about enrolling their premature babies in several research studies?. Arch Dis Child Fetal Neonatal Ed 2005;90:225-8. https://doi.org/10.1136/adc.2004.061986.
- Harth SC, Thong YH. Parental perceptions and attitudes about informed consent in clinical research involving children. Soc Sci Med 1995;40:1573-7. https://doi.org/10.1016/0277-9536(94)00412-M.
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ 2012;344. https://doi.org/10.1136/bmj.e2809.
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003821.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. https://doi.org/10.1002/14651858.MR000008.pub4.
- Khadjesari Z, Murray E, Kalaitzaki E, White IR, McCambridge J, Thompson SG, et al. Impact and costs of incentives to reduce attrition in online trials: two randomized controlled trials. J Med Internet Res 2011;13. https://doi.org/10.2196/jmir.1523.
- Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons Inc; 2007.
- Singer E, Cong Y. The use and effects of incentives in surveys. Ann Am Acad Pol Soc Sci 2013;645:112-41. https://doi.org/10.1177/0002716212458082.
- Hardy P, Bell JL, Brocklehurst P. Epidural and Position Trial Collaborative Group. Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0180-9.
- Kenyon S, Pike K, Jones D, Taylor D, Salt A, Marlow N, et al. The effect of a monetary incentive on return of a postal health and development questionnaire: a randomised trial [ISRCTN53994660]. BMC Health Serv Res 2005;5. https://doi.org/10.1186/1472-6963-5-55.
- Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-399.
- Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med 2013;368:2094-104. https://doi.org/10.1056/NEJMoa1302298.
- Stein HM, Wilmoth J, Burton J. Electrical activity of the diaphragm in a small cohort of term neonates. Respir Care 2012;57:1483-7. https://doi.org/10.4187/respcare.01650.
- Kooi EMW, Verhagen EA, Elting JWJ, Czosnyka M, Austin T, Wong FY, et al. Measuring cerebrovascular autoregulation in preterm infants using near-infrared spectroscopy: an overview of the literature. Expert Rev Neurother 2017;17:801-18. https://doi.org/10.1080/14737175.2017.1346472.
- Martin CR, Cheesman A, Brown J, Makda M, Kutner AJ, DaSilva D, et al. Factors determining optimal fatty acid absorption in preterm infants. J Pediatr Gastroenterol Nutr 2016;62:130-6. https://doi.org/10.1097/MPG.0000000000000934.
- Østergaard MV, Cilieborg MS, Skovgaard K, Schmidt M, Sangild PT, Bering SB. Preterm birth reduces nutrient absorption with limited effect on immune gene expression and gut colonization in pigs. J Pediatr Gastroenterol Nutr 2015;61:481-90. https://doi.org/10.1097/MPG.0000000000000827.
- Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care 2010;13:314-20. https://doi.org/10.1097/MCO.0b013e328337bbf0.
- Neu J, Koldovsky O. Nutrient absorption in the preterm neonate. Clin Perinatol 1996;23:229-43. https://doi.org/10.1016/S0095-5108(18)30240-9.
- NICE . Developmental Follow-up of Children and Young People Born Preterm n.d. https://www.nice.org.uk/guidance/NG72 (accessed February 2020).
- Cuttini M, Ferrante P, Mirante N, Chiandotto V, Fertz M, Dall’Oglio AM, et al. Cognitive assessment of very preterm infants at 2-year corrected age: performance of the Italian version of the PARCA-R parent questionnaire. Early Hum Dev 2012;88:159-63. https://doi.org/10.1016/j.earlhumdev.2011.07.022.
- Blaggan S, Guy A, Boyle EM, Spata E, Manktelow BN, Wolke D, et al. A parent questionnaire for developmental screening in infants born late and moderately preterm. Pediatrics 2014;134:e55-62. https://doi.org/10.1542/peds.2014-0266.
- Tin W, Fritz S, Wariyar U, Hey E. Outcome of very preterm birth: children reviewed with ease at 2 years differ from those followed up with difficulty. Arch Dis Child Fetal Neonatal Ed 1998;79:F83-7. https://doi.org/10.1136/fn.79.2.F83.
- Wolke D, Söhne B, Ohrt B, Riegel K. Follow-up of preterm children: important to document dropouts. Lancet 1995;345. https://doi.org/10.1016/S0140-6736(95)90425-5.
- Field D, Spata E, Davies T, Manktelow B, Johnson S, Boyle E, et al. Evaluation of the use of a parent questionnaire to provide later health status data: the PANDA study. Arch Dis Child - Fetal Neonatal Ed 2015;101:F304-308. https://doi.org/10.1136/archdischild-2015-309247.
- Lynn P, Burton J, Kaminska O, Knies G, Nandi A. An Initial Look at Non-Response and Attrition in Understanding Society. n.d. www.understandingsociety.ac.uk/sites/default/files/downloads/working-papers/2012-02.pdf (accessed 29 January 2020).
- Draper ES, Manktelow BN, Cuttini M, Maier RF, Fenton AC, Van Reempts P, et al. Variability in very preterm stillbirth and in-hospital mortality across Europe. Pediatrics 2017;139. https://doi.org/10.1542/peds.2016-1990.
- British Association of Perinatal Medicine . Categories of Care (2011). A BAPM Framework for Practice n.d. www.bapm.org/resources/34-categories-of-care-2011 (accessed 29 January 2020).
Appendix 1 Recruiting neonatal units
| Recruiting site | Principal investigator | Research staff |
|---|---|---|
| Altnagelvin Area Hospital, UK | Damien Armstrong | Julie Brown |
| Arrowe Park Hospital, Wirral, UK | Srinivasarao Babarao/Adrian Hughes | Sharon Hughes, Lucy Lewis and Rachael Rice |
| Birmingham City Hospital, UK | Fiona Chambers/Shanmugasundaram Sivakumar | Lara Alamad, Rania M El Beltagy, Lisa Charles and Sarah Potter |
| Birmingham Heartlands Hospital, UK | Imogen Storey | Francesca Bisso, Juliet Hopkins and Lucy Ingram |
| Birmingham Women’s Hospital, UK | Alison Bedford Russell/Gemma Holder | Heather Barrow, Rachel E. Jackson and Elizabeth Simcox |
| Bradford Royal Infirmary, UK | Sunita Seal | Liz Ingram, Rachel Wane and Kelly Young |
| Calderdale Royal Hospital, UK | Eilean Crosbie | Salamiah Burgess, Amanda Smith and Kristy Somerville |
| Countess of Chester Hospital, UK | Stephen Brearey | Caroline Burchett, Sarah de-Beger and Yvonne Farmer |
| Craigavon Area Hospital, UK | Philip Quinn/Michael Smith | Sara Gilpin and Judith Ratcliffe |
| Croydon University Hospital, UK | John Chang | Aline Cook and Vana Wardley |
| Derbyshire Children’s Hospital, UK | John McIntyre/Mal Ratnayaka | Coral J Smith and Vanessa Unsworth |
| Derriford Hospital, UK | Oladipo Aworinde/Nicola Maxwell | Louise Meredith, Sarah-Jane Sharman and Susan Tyson |
| Gloucestershire Royal Hospital, UK | Miles H Wagstaff | Susan Beames |
| Great Western Hospital, Swindon, UK | Girish Gowda | Rebecca Elliott-Jones |
| Hull Royal Infirmary, UK | Hassan Gaili | Gail Bartley, David Bolton and Sarah Trufhitt |
| James Cook University Hospital, Middlesbrough, UK | Mithilesh Lal | Suzanne Bell, Mandy Forster and Helena Smith |
| Jessop Wing, Sheffield, UK | Robert Coombs | Pauline Bayliss, Julie Cook and Clare Pye |
| John Radcliffe Hospital, Oxford, UK | Kenny McCormick | Sheula Barlow and Sara Davis |
| Kettering General Hospital, UK | Jasmina Marinova | Harsha Bilolikar and Sonia White |
| King’s Mill Hospital, Sutton-in-Ashfield, UK | Vibert Noble/Simon Rhodes | Yvonne Lisseman-Stones, Caroline Moulds and Karen Whysall |
| Leeds General Infirmary/St James’s University Hospital, Leeds, UK | Kathryn Johnson | Marjorie Allen, Deborah Burton, Stephanie Heath, Suzanne Laing, Charlotte Reilly, Collette Spencer and Lindsay Uryn |
| Leicester Royal Infirmary, UK | Elaine Boyle | Rosalind Astles and Marie Hubbard |
| Leighton Hospital, Crewe, UK | Arumugavelu Thirumurugan | Sarah Bramhall, Sally A Smith and Samantha Tapscott |
| National Maternity Hospital, Dublin, Republic of Ireland | Anne Twomey | Breda Coronella |
| New Cross Hospital, Wolverhampton, UK | Babu Kumararatne/Alyson Skinner | Rebecca Denyer and Sharon Kempson |
| Northampton General Hospital, UK | Subodh Gupta/Fiona Thompson | Hannah Graham |
| Nottingham City Hospital/The Queen’s Medical Centre, Nottingham, UK | Jon Dorling | Dushyant Batra, Sarah Craig, T’ng Chang Kwok, Julie Lynch, Alison Paton, Jodie Sibert and Rachel Smitheram |
| Pinderfields General Hospital, Wakefield, UK | Kathryn Deakin | Gail Castle and David Gibson |
| Princess Anne Hospital, Southampton, UK | Mark J Johnson/Alison Leaf | Philippa Crowley, Charlotte Oates, Jenny Pond and Jane Rhodes |
| Princess Royal Maternity, Glasgow, UK | Helen Mactier | Isobel Crawford |
| Queen Alexandra Hospital, Portsmouth, UK | Tim Scorrer | Michelle Pople |
| Queen’s Hospital, Romford, UK | Wilson Lopez/Khalid Mannan | Helen Smith |
| Royal Berkshire Hospital, Reading, UK | Nicola Pritchard | Sue Hallett and Morag Zelisko |
| Royal Cornwall Hospital, Truro, UK | Yadlapalli Kumar | Barbara Bromage, Gillian Craig and Hannah Osborn |
| Royal Devon and Exeter Hospital, UK | David Bartle | Julia Halpin, Nicola Jones, Jackie Massey, Aileen Roberts, Jacqui Tipper and Sue Ward |
| Royal Infirmary of Edinburgh, UK | Ben Stenson | Lynn Clark |
| Royal Maternity Hospital, Belfast, UK | Stanley Craig | Patricia McCreesh, Muriel Millar, Mary O’Neill and Alison Walker |
| Royal Shrewsbury Hospital, UK | Sanjeev Deshpande | Sarah Kirk and Charlotte Owen |
| Royal Stoke University Hospital, UK | Lee Abbott | Anne Harrison, Julie Hollins, Ruth Jones, Katharine Lewney, Rachel Pringle, Viki Riches and Eric Roe |
| Royal Victoria Infirmary, Newcastle, UK | Janet Berrington/Nicholas Embleton | Tracey Downes, Julie Groombridge, Alison Kimber, Julie Pirnie, Lynda Shah, Thomas Skeath, Linda Smith and Stefan Zalewski |
| Singleton Hospital, Swansea, UK | Sujoy Banerjee | Amanda Cook, Helen Goldring and Malini Ketty |
| Southern General Hospital, Glasgow, UK | Colin Peters | Lorna McKay |
| Southmead Hospital, Bristol, UK | Paul Mannix | Anne Gay and Diane Stubbs |
| St George’s University Hospitals NHS Foundation Trust, London, UK | Nigel Kennea | Naomi Hayward |
| St Michael’s Hospital, Bristol, UK | Pamela Cairns/Jonathan Davis | Joanne Innoles |
| St Peter’s Hospital, Chertsey, UK | Peter Reynolds | Nicola Holland and Karen Wells |
| Sunderland Royal Hospital, UK | Majd Abu-Harb | Donna Coppard, Kathryn Marshall and Eileen Turnbull |
| United Lincolnshire Hospitals Trust, UK | Narasimha Kollipara | Susie Butler, Ruchika Gupta, Amanda Roper and Dougie Thomas |
| University Hospital Coventry, UK | Richard C de Boer | Kathryn Blake and Geraldine Ward |
| University Hospital of North Tees, UK | Sundaram Janakiraman | Wendy Cheadle and Alex Ramshaw |
| Warrington Hospital, UK | Satish Hulikere/Delyth Webb | Natalie Rogers |
| William Harvey Hospital, Ashford, UK | Vimal Vasu | Shermi George, Jodie Harrison and Stephanie O’Brien |
| Wishaw General Hospital, UK | Samuel Ibhanesebhor | Denise Vigni |
| Worcestershire Royal Hospital, UK | Andrew Gallagher | Dawn Kelly and Catherine Townsend |
| York Hospital, UK | William McGuire/Guy Millman | William McGuire and Anna Clayton |
Appendix 2 Continuing care sites
The continuing care sites were as follows:
-
Airedale NHS Foundation Trust, Keighley, UK
-
Alder Hey Children’s Hospital, Liverpool, UK
-
Barnet Hospital, London, UK
-
Basingstoke and North Hampshire Hospital, Basingstoke, UK
-
Birmingham Children’s Hospital, Birmingham, UK
-
Borders General Hospital, Melrose, UK
-
Burnley General Hospital, Burnley, UK
-
Chelsea and Westminster Hospital NHS Foundation Trust, London, UK
-
Chesterfield Royal Hospital NHS Foundation Trust, Chesterfield, UK
-
Cumberland Infirmary, Carlisle, UK
-
Darlington Memorial Hospital, Darlington, UK
-
Diana, Princess of Wales Hospital, Grimsby, UK
-
Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust, Doncaster, UK
-
Dorset County Hospital, Dorchester, UK
-
Dumfries and Galloway Royal Infirmary, Dumfries, UK
-
East Surrey Hospital, Redhill, UK
-
Forth Valley Royal Hospital, Larbert, UK
-
Frimley Park Hospital, Frimley, UK
-
George Eliot Hospital, Nuneaton, UK
-
Harrogate District Hospital, Harrogate, UK
-
Hereford County Hospital, Hereford, UK
-
Homerton Hospital, London, UK
-
The Horton General Hospital, Banbury, UK
-
Jersey General Hospital, Jersey, UK
-
King’s College Hospital, London, UK
-
Lister Hospital, Stevenage, UK
-
Liverpool Women’s Hospital, Liverpool, UK
-
Luton and Dunstable University Hospital, Luton, UK
-
Macclesfield District General Hospital, Macclesfield, UK
-
Walsall Manor Hospital, Walsall, UK
-
Medway Maritime Hospital, Gillingham, UK
-
Midland Regional Hospital, Mullingar, Republic of Ireland
-
Milton Keynes University Hospital, Milton Keynes, UK
-
Musgrove Park Hospital, Taunton, UK
-
NIHR Clinical Research Network West Midlands, UK
-
NIHR Clinical Research Network Yorkshire and Humber, UK
-
Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK
-
North Devon District Hospital, Barnstaple, UK
-
North Middlesex University Hospital, London, UK
-
Northumbria Specialist Emergency Care Hospital, Cramlington, UK
-
Ormskirk and District General Hospital, Ormskirk, UK
-
Peterborough City Hospital, Peterborough, UK
-
Pilgrim Hospital, Boston, UK
-
Poole Hospital, Poole, UK
-
Portiuncula Hospital, Co. Galway, Republic of Ireland
-
Princess Elizabeth Hospital, Le Vanquiedor, Guernsey, UK
-
Princess of Wales Hospital, Bridgend, UK
-
Queen Elizabeth Hospital, Gateshead, UK
-
Queen Elizabeth Hospital, King’s Lynn, UK
-
Queen’s Hospital, Burton-on-Trent, UK
-
Raigmore Hospital, Inverness, UK
-
Rotherham General Hospital, Rotherham, UK
-
Royal Alexandra Children’s Hospital, Brighton, UK
-
Royal Hampshire County Hospital, Winchester, UK
-
The Royal London Hospital, London, UK
-
Royal United Hospital, Bath, UK
-
Russells Hall Hospital, Dudley, UK
-
Salisbury District Hospital, Salisbury, UK
-
Scarborough General Hospital, Scarborough, UK
-
Scunthorpe General Hospital, Scunthorpe, UK
-
Sligo University Hospital, Sligo, Republic of Ireland
-
South Tyneside District Hospital, South Shields, UK
-
St Helens and Knowsley Teaching Hospitals NHS Trust, Whiston, UK
-
St Helier Hospital, Carshalton, UK
-
St John’s Hospital, Livingston, UK
-
St Mary’s Hospital, Isle of Wight, UK
-
St Richard’s Hospital, Chichester, UK
-
Stoke Mandeville Hospital, Aylesbury, UK
-
Torbay Hospital, Torquay, UK
-
University Hospital Crosshouse, Kilmarnock, UK
-
University Hospital Lewisham, London, UK
-
University Hospital of North Durham, Durham, UK
-
Victoria Hospital, Kirkcaldy, UK
-
Wansbeck General Hospital, Ashington, UK
-
Warwick Hospital, Coventry, UK
-
West Cumberland Hospital, Whitehaven, UK
-
Wexham Park Hospital, Slough, UK
-
Worthing Hospital, Worthing, UK.
Appendix 3 Case definition of survival without moderate or severe disability
For the purposes of the blinded end-point review of follow-up data, five outcomes will be considered:
-
Survival without moderate or severe disability at 24 months of age CGA. This is the primary outcome.
-
Survival without moderate or severe vision impairment.
-
Survival without moderate or severe hearing impairment.
-
Survival without moderate or severe gross motor impairment.
-
Survival without moderate or severe cognitive/language impairment.
Review conventions
The four components will be defined as follows:
-
Moderate/severe vision impairment – reduced vision uncorrected with aids, blind in one eye with good vision in the contralateral eye or is blind or can perceive light only.
-
Moderate/severe hearing impairment – hearing loss corrected with aids, some hearing but loss not corrected by aids or deaf.
-
Moderate/severe gross motor impairment – unable to walk independently or unable to sit independently.
-
Moderate/severe cognitive impairment – child’s development is between 6 and 12 months behind corrected age, child’s development > 12 months behind corrected age, child has fewer than five meaningful words, vocalisations or signs or child is unable to understand words or signs.
These definitions are taken from the protocol apart from the definition of moderate/severe cognitive impairment. In the protocol it was stated that moderate/severe cognitive impairment would be assessed using the PARCA-R, a parent-reported measure of non-verbal cognitive and language development. Total PARCA-R scores of < 44 would be used to identify children with moderate/severe cognitive impairment. Participants whose data are reviewed will be unlikely to have a fully completed valid PARCA-R questionnaire.
If the child is classed as having moderate/severe impairment in one of the four domains above, then they will be classed as having moderate/severe disability in terms of the primary outcome.
If the only data available for the child are scores on the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III), from an assessment performed between 22 and 28 months of age (CGA), these should be interpreted as follows:
-
Standardised composite score of ≤ 84 (equivalent to a scaled score of ≤ 6) in any domain denotes moderate to severe impairment.
-
If the child was able to complete the Bayley-III, particularly the cognitive and language scales, with scores in the normal range, it should be assumed that the child has mild/no visual impairment (even if this is not stated).
If Bayley-III scores are available in addition to clinical information, the scores should be interpreted in the light of that information, particularly where scores are close to the cut-off points for moderate/severe impairment listed above. Bayley-III is not the primary outcome measure and the cut-off points above are not formally agreed standards. Thus, reviewers should interpret in the light of other information where scores are close to the cut-off.
Where there is a detailed clinic letter that provides evidence of no concerns regarding developmental progress (i.e. cognitive, language, motor), it should be assumed that there is also no moderate to severe hearing impairment, unless otherwise stated.
Appendix 4 British Association of Perinatal Medicine categories of care
Definitions are reproduced with permission from British Association of Perinatal Medicine. 116
Intensive care
General principle
This is care provided for infants who are the most unwell or unstable and have the greatest needs in relation to staff skills and staff-to-patient ratios.
Definition of intensive care day
-
Any day when an infant receives any form of mechanical respiratory support via a tracheal tube.
-
Both non-invasive ventilation [e.g. nasal continuous positive airway pressure (CPAP), synchronised inspiratory positive airway pressure (SIPAP), bilevel positive airway pressure (BIPAP) and Vapotherm (Vapotherm Inc., Exeter, NH, USA)] and parenteral nutrition (PN).
-
Day of surgery [including laser therapy for retinopathy of prematurity (ROP)].
-
Day of death.
-
Any day receiving any of the following:
-
presence of an umbilical arterial line
-
presence of an umbilical venous line
-
presence of a peripheral arterial line
-
insulin infusion
-
presence of a chest drain
-
exchange transfusion
-
therapeutic hypothermia
-
prostaglandin infusion
-
presence of replogle tube
-
presence of epidural catheter
-
presence of silo for gastroschisis
-
presence of external ventricular drain
-
dialysis (any type).
-
High-dependency care
General principle
This is the care that is provided for infants who require highly skilled staff but where the nurse-to-patient ratio is less than that in intensive care.
Definition of high-dependency care day
A high-dependency care day is any day when an infant does not fulfil the criteria for intensive care where any of the following apply:
-
Any day when an infant receives any form of non-invasive respiratory support (e.g. nasal CPAP, SIPAP, BIPAP, humidified high-flow nasal cannula).
-
Any day receiving any of the following:
-
parenteral nutrition
-
continuous infusion of drugs (except prostaglandin and/or insulin)
-
presence of a central venous or long line (peripherally inserted central catheter)
-
presence of a tracheostomy
-
presence of a urethral or suprapubic catheter.
-
Special care
General principle
Special care is provided for infants who require additional care delivered by the neonatal service but do not require either intensive care or high-dependency care.
Definition of special care day
A special care day is any day when an infant does not fulfil the criteria for intensive or high-dependency care and requires any of the following:
-
oxygen by nasal cannula
-
feeding by nasogastric, jejunal tube or gastrostomy
-
continuous physiological monitoring (excluding apnoea monitors only)
-
care of a stoma
-
presence of intravenous cannula
-
phototherapy
-
special observation of physiological variables at least 4-hourly.
Appendix 5 Data collection forms
| Name of form | Description |
|---|---|
| Form 1: trial entry | Completed before, during and after randomisation, collecting data on baseline characteristics and eligibility/exclusion criteria |
| Form 2: daily feed log | Completed daily starting at randomisation. Records data for each day in the study until the infant reaches full feeds (150 ml/kg/day) for 3 consecutive days, or until their participation in the trial stops for other reasons. Also records daily use of antibiotics and feed stops for ≥ 4 hours |
| Form 3: late-onset infection | Completed for each episode of microbiologically confirmed or clinically suspected late-onset invasive infection, from trial entry to discharge home |
| Form 4: gut signs | Completed when the infant received > 5 days of treatment for gut signs (e.g. feeds withheld), from trial entry to discharge home |
| Form 5: hospital transfer and discharge | Completed when the infant is transferred to another hospital, is discharged home or if the infant died |
| Form 6: discontinuation of intervention | Completed when a parent or clinician decided to stop the allocated SIFT feeding regimen permanently |
| Form 7: serious adverse events | Completed for all reportable SAEs as defined in the trial protocol |
| Form 8: incident reporting | Completed in the event of any deviation from the trial protocol, other trial-specific procedures, good clinical practice or other regulations and legislation |
| 2-year follow-up questionnaire | Sent directly to parents of surviving infants shortly before the infant’s 24-month corrected age. The form collected data on the primary outcome (survival without moderate or severe disability), individual components of the primary outcome, the PARCA-R, and health service access and resource use |
Copies of SIFT data collection forms are available at www.npeu.ox.ac.uk/sift/dcfs (accessed 29 January 2020).
Appendix 6 Safety reporting definitions
Serious adverse event
Adverse events are defined as serious if they:
-
result in death
-
are life-threatening
-
require inpatient hospitalisation or prolongation of existing hospitalisation
-
result in persistent or significant disability/incapacity
-
are a congenital anomaly/birth defect.
The term ‘life-threatening’ refers to an event in which the participant was at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death if it were more severe.
The SAEs are to be reported from randomisation to discharge home.
Expected serious adverse events
The following are SAEs that could be reasonably expected to occur in this population of infants during the course of the trial or form part of the outcome data. They do not require reporting by the SIFT co-ordinating centre as SAEs:
-
death (unless unexpected in this population)
-
NEC or focal intestinal perforation
-
microbiologically confirmed or clinically suspected late-onset invasive infection
-
bronchopulmonary dysplasia (mechanical ventilator support or supplemental oxygen at 36 weeks’ postmenstrual age)
-
intracranial abnormality (haemorrhage, parenchymal infarction or white matter damage) on cranial ultrasound scan or other imaging
-
pulmonary haemorrhage
-
patent ductus arteriosus requiring treatment (non-steroidal anti-inflammatory drugs or surgery)
-
retinopathy of prematurity.
Appendix 7 Summary of changes to the study protocol
| Date | Summary of changes | Version number |
|---|---|---|
| 12 December 2012 | Version 1 of the protocol33 was submitted to the REC on 20 December 2012 and approved on 31 January 2013 | 1 |
| 8 July 2013 | A protocol amendment was submitted to the REC on 8 July 2013 and approved on 19 August 2013. Changes were as follows:
|
2 |
| 15 September 2014 |
|
3 |
| 24 June 2015 | Changed reference to reimbursement in preparation for possible incentives SWAT (subject to HTA and ethics approval) | 4 |
| 7 December 2015 | Never released: clarified methods of capturing outcome data. Added survival to discharge home as secondary outcome | 5 |
| 4 February 2016 | Additional clarification of methods of capturing outcome data. Supersedes v5 that was never submitted to ethics; numbered v5.1 to keep track of alterations internally | 5.1 |
Appendix 8 Withdrawals from intervention by randomisation group
| Reason for withdrawal | Faster (30 ml/kg/day) (N = 1400),a n | Slower (18 ml/kg/day) (N = 1404),a n |
|---|---|---|
| Clinical decision | ||
| Total | 12 | 23 |
| Consent remains | 12 | 21 |
| Consent completely withdrawn | 0 | 2 |
| Parental wish | ||
| Total | 17 | 14 |
| Consent remains | 11 | 11 |
| Consent completely withdrawn | 6 | 3 |
| Overall total | 29 | 37 |
Appendix 9 Group allocation per recruiting site
| Centre | Faster increment group (N = 1394), n (%) | Slower increment group (N = 1399), n (%) |
|---|---|---|
| Jessop Wing, Sheffield | 38 (2.7) | 39 (2.8) |
| Royal Infirmary of Edinburgh | 63 (4.5) | 61 (4.4) |
| Princess Royal Maternity Hospital, Glasgow | 37 (2.7) | 46 (3.3) |
| Southern General Hospital, Glasgow | 34 (2.4) | 32 (2.3) |
| Wishaw General Hospital | 27 (1.9) | 28 (2.0) |
| Royal Maternity Hospital, Belfast | 15 (1.1) | 13 (0.9) |
| James Cook University Hospital, Middlesbrough | 48 (3.4) | 54 (3.9) |
| Nottingham City Hospital | 33 (2.4) | 33 (2.4) |
| Queen’s Medical Centre University Hospital | 23 (1.6) | 23 (1.6) |
| Birmingham Heartlands Hospital | 33 (2.4) | 34 (2.4) |
| Birmingham Women’s Hospital | 70 (5.0) | 73 (5.2) |
| Birmingham City Hospital | 28 (2.0) | 29 (2.1) |
| Royal Berkshire Hospital, Reading | 8 (0.6) | 8 (0.6) |
| Derbyshire Children’s Hospital | 13 (0.9) | 10 (0.7) |
| Sunderland Royal Hospital | 30 (2.2) | 30 (2.1) |
| Altnagelvin Area Hospital, Londonderry | 7 (0.5) | 5 (0.4) |
| Worcestershire Royal Hospital | 8 (0.6) | 7 (0.5) |
| Royal Shrewsbury Hospital | 25 (1.8) | 25 (1.8) |
| King’s Mill Hospital, Sutton-in-Ashfield | 1 (0.1) | 0 |
| New Cross Hospital, Wolverhampton | 10 (0.7) | 10 (0.7) |
| University Hospital Coventry | 45 (3.2) | 44 (3.1) |
| Royal Victoria Infirmary, Newcastle | 59 (4.2) | 57 (4.1) |
| Arrowe Park Hospital, Wirral | 22 (1.6) | 23 (1.6) |
| University Hospital of North Tees | 40 (2.9) | 35 (2.5) |
| John Radcliffe Hospital, Oxford | 45 (3.2) | 41 (2.9) |
| Lincoln County Hospital | 3 (0.2) | 6 (0.4) |
| Hull Royal Infirmary | 23 (1.6) | 23 (1.6) |
| Bradford Royal Infirmary | 62 (4.4) | 61 (4.4) |
| Calderdale Royal Hospital | 20 (1.4) | 17 (1.2) |
| Countess of Chester Hospital | 3 (0.2) | 3 (0.2) |
| Derriford Hospital, Plymouth | 1 (0.1) | 2 (0.1) |
| Gloucestershire Royal Hospital | 7 (0.5) | 6 (0.4) |
| Kettering General Hospital | 4 (0.3) | 4 (0.3) |
| Leeds General Infirmary | 58 (4.2) | 58 (4.1) |
| Leicester Royal Infirmary | 54 (3.9) | 55 (3.9) |
| Leighton Hospital, Crewe | 1 (0.1) | 4 (0.3) |
| Northampton General Hospital | 3 (0.2) | 7 (0.5) |
| Pinderfields General Hospital, Wakefield | 4 (0.3) | 4 (0.3) |
| Princess Anne Hospital, Southampton | 25 (1.8) | 26 (1.9) |
| Queen Alexandra Hospital, Portsmouth | 82 (5.9) | 80 (5.7) |
| Royal Cornwall Hospital, Truro | 7 (0.5) | 10 (0.7) |
| Royal Devon and Exeter Hospital | 25 (1.8) | 26 (1.9) |
| Singleton Hospital, Swansea | 29 (2.1) | 33 (2.4) |
| Southmead Hospital, Bristol | 24 (1.7) | 23 (1.6) |
| St George’s Hospital, London | 25 (1.8) | 24 (1.7) |
| St James’s University Hospital, Leeds | 22 (1.6) | 18 (1.3) |
| St Michael’s Hospital, Bristol | 15 (1.1) | 15 (1.1) |
| St Peters Hospital, Chertsey | 32 (2.3) | 35 (2.5) |
| Warrington Hospital | 4 (0.3) | 3 (0.2) |
| William Harvey Hospital, Ashford | 24 (1.7) | 23 (1.6) |
| York Hospital | 4 (0.3) | 5 (0.4) |
| University Hospital of North Staffordshire | 18 (1.3) | 14 (1.0) |
| Croydon University Hospital | 13 (0.9) | 11 (0.8) |
| National Maternity Hospital, Dublin | 28 (2.0) | 32 (2.3) |
| Queen’s Hospital, Romford | 12 (0.9) | 11 (0.8) |
Appendix 10 Strategies to enhance follow-up return rates
A number of strategies were adopted to enhance the return of questionnaires. These included:
-
Contacting parents via telephone or text message earlier in the follow-up process – making a telephone call to parents to remind them of their child’s participation in SIFT and check their contact details, 4 days before their follow-up questionnaire was due to be posted out.
-
Contacting parents via telephone as well as by post if a second reminder was required (rather than waiting until a third reminder to begin telephoning).
-
Where parents had supplied a mobile telephone number and/or e-mail address, sending e-mail/SMS reminders in addition to postal letters.
-
Requesting that recruiting sites remind parents of the follow-up questionnaire if and when they attended a clinical appointment at 24 months of age.
-
Supplying recruiting and continuing care sites with a poster to display in clinic waiting rooms reminding parents of the follow-up.
-
Offering parents the option of completing the questionnaire online via a direct link, using a new product called OpenClinica Participate 3.3 to 3.13 (OpenClinica LLC, Waltham, MA, USA). SIFT was part of the piloting for this new software.
-
Having the follow-up promoted on a quarterly basis via social media platforms through the trial’s service-user advocate partner, the charity Bliss.
-
An embedded SWAT offering incentives to participants to complete the questionnaire (see Chapter 6). This SWAT investigated the effects of giving an incentive (£15/€15 high-street shop voucher) before completion compared with promising an incentive on receipt of a completed questionnaire.
Figure 9 displays the changes in follow-up rate throughout the process.
FIGURE 9.
Interventions and changes in SIFT follow-up rate.

List of abbreviations
- ARR
- adjusted risk ratio
- BERC
- blinded end-point review committee
- BIPAP
- bilevel positive airway pressure
- BNF
- British National Formulary
- CCA
- cost–consequence analysis
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CGA
- corrected for gestational age
- CI
- confidence interval
- CPAP
- continuous positive airway pressure
- CRF
- case report form
- CTIMP
- clinical trial of an investigational medicinal product
- CTU
- clinical trials unit
- DMC
- Data Monitoring Committee
- ELFIN
- Enteral Lactoferrin In Neonates
- GBP
- Great British pounds
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LOS
- late-onset sepsis
- NEC
- necrotising enterocolitis
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- NPEU
- National Perinatal Epidemiology Unit
- NRES
- National Research Ethics Service
- PARCA-R
- Parent Report of Children’s Abilities – Revised
- PI
- principal investigator
- PIL
- parent information leaflet
- PPV
- positive predictive value
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SIFT
- Speed of Increasing milk Feeds Trial
- SIPAP
- synchronised inspiratory positive airway pressure
- SOP
- standard operating procedure
- SWAT
- study within a trial
- TSC
- Trial Steering Committee
- VLBW
- very low birthweight
- WTP
- willingness to pay
