Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/199/14. The contractual start date was in January 2016. The draft report began editorial review in March 2019 and was accepted for publication in June 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Matthew L Costa is a National Institute for Health Research (NIHR) Senior Investigator and a member of the General Board for the NIHR Health Technology Assessment funding stream. Julie Bruce receives personal fees as a consultant for Medtronic plc (Dublin, Ireland) (an unrelated study). Jagdeep Nanchahal receives personal fees and non-financial support from Smith & Nephew plc (London, UK) and Orthofix Holdings Inc. (Lewisville, TX, USA).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Costa et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Major trauma is the leading cause of death worldwide in people aged < 45 years and a significant cause of short- and long-term morbidity. The UK National Audit Office (NAO) estimates that there are at least 20,000 cases of major trauma each year in England, resulting in 5400 deaths, and many survivors suffer permanent disabilities requiring long-term care. 1 The NAO estimates that trauma costs the NHS between £0.3B and £0.4B per year for immediate treatment. 1 This excludes the cost of subsequent hospital treatments, rehabilitation, home care support or informal carers. The NAO also estimates an annual lost economic output from traumatic injury of between £3.3B and £3.7B. 1
Fractures of the limbs are extremely common injuries, with 85% of major trauma patients sustaining serious limb injuries. 2 In ‘open’ fractures of the lower limb, in which the broken bone is exposed to the environment by a breach in the skin, the risk of wound infection is particularly high. 2 However, it has been shown that even in ‘closed’ high-energy injuries associated with major trauma, the rate of wound or surgical site infection (SSI) remains high because of extensive damage to the soft tissues overlying the fracture. For example, tibial plateau fractures are associated with average infection rates of up to 27%,3–7 and pilon fractures have an incidence of infection ranging from 5% to 40%. 8–11 If deep SSI does occur, treatment frequently continues for years after the initial injury. This treatment often involves prolonged courses of antibiotics, with the attendant risk of antibiotic resistance in chronic wounds, as well as hospital re-admissions for further surgery in many cases. The cost associated with such injuries is huge. A US study12 found that the average lifetime cost associated with reconstruction was US$163,282, but was three times higher if amputation was necessary; this lifetime cost represented only the health-care burden, excluding social and personal costs.
Major trauma patients are at greater risk of infection owing to several factors, including the presence of antibiotic-resistant organisms in the intensive therapy unit and high-dependency environment. Furthermore, the presence of a wound haematoma or postoperative wound leak may predispose to infection in wounds created by surgical incisions. One of the factors that may reduce the risk of SSI is the type of dressing applied over the closed incision at completion of the operative procedure. Dressings may reduce bacterial ingress into the wound. The published literature13 suggests that the type of dressing applied to the wound may also influence the healing process itself. This trial deals with the type of dressing that is applied to the closed surgical incision at the end of the operation.
Traditionally, the surgical incision is covered with an adhesive dressing to protect the wound from contamination from the external environment. These ‘standard dressings’ have been used throughout the NHS and in most health-care systems around the world for many years. Incisional negative-pressure wound therapy (NPWT), also known as topical negative pressure, is an alternative form of dressing that may be applied to closed surgical incisions. In this treatment, a non-adherent absorbent dressing covered with a semipermeable membrane overlies the incision, which is permeable to gas only. A sealed tube is used to connect the dressing to a pump, which creates a partial vacuum over the wound. Incisional NPWT provides a sealed environment, preventing bacterial ingress and removes blood and serous fluid exuding from the wound. The application of negative pressure to the dressing leads to the application of positive pressure to the wound bed and has been shown to reduce the incidence of wound haematoma. 14 A recent systematic review13 suggests that NPWT shifts the cytokine profile to being less inflammatory, and also potentially promotes the production of proangiogenic growth factors and enzymes responsible for matrix remodelling, leading to improved wound healing.
However, NPWT for surgical incisions is considerably more expensive than traditional wound dressings. Despite its perceived benefits over standard dressings, at the beginning of this study, there had been only one randomised trial14 (n = 249 participants) comparing standard wound dressing with incisional NPWT for patients with closed surgical wounds following major trauma to the lower limb. This trial demonstrated a reduction in the rate of deep wound infection (described in the report as ‘late’ infection) in the group of patients treated with incisional NPWT (9%) versus the standard dressing group (15%). However, the reduction was of borderline statistical significance (p = 0.049), and the study was criticised in the subsequent Cochrane review15 for methodological flaws.
The recent Cochrane review for surgical wounds concluded that ‘it is still not clear whether incisional NPWT promotes faster healing and reduces complications’. 15 They concluded that ‘Given the cost and widespread use of incisional NPWT, there is an urgent need for suitably powered, high-quality trials to evaluate the effects of the newer incisional NPWT products that are designed for use on clean, closed surgical incisions’15 and that ‘such trials should focus initially on wounds that may be difficult to heal.’15 The Wound Healing in Surgery for Trauma (WHiST) trial aimed to address this evidence gap.
Since the start of the WHiST trial, one further small randomised trial16 of incisional NPWT versus standard dressings has reported on surgical wounds following trauma. In this trial, 66 patients having surgery for fixation of an acetabular fracture were randomised to incisional NPWT versus standard gauze dressings. There was no evidence of a difference in the rate of deep infection, although the number of deep infections was small: two patients (6.1%) in the standard dressing group and five (15.2%) in the incisional NPWT group reported a deep infection. The only other trial reported since the WHiST trial started is a mechanistic study17 involving 20 patients; in this study, ultrasonography was used to assess wound seroma formation following the use of incisional NPWT in patients receiving surgery for spinal fractures.
Relevance of project
Wound healing complications are clearly a major problem for the NHS. New techniques for wound management are being developed but are often implemented without sufficient evidence. Incisional NPWT has provided promising preliminary results in different patient groups, including patients with surgical wounds associated with major trauma. This pragmatic randomised controlled trial (RCT) was designed to compare the clinical effectiveness and cost-effectiveness of standard dressings with incisional NPWT on wound-related outcomes in adults undergoing surgical incisions associated with major trauma to the lower limb.
The trial was carried out in accordance with Medical Research Council Good Clinical Practice18 and applicable UK legislation.
Objectives
The primary objective of the RCT was to:
-
estimate differences in the rate of deep SSI of the lower limb in the 30 days after randomisation between treatment groups of standard wound dressing and incisional NPWT. Any wound infection that required continuing medical intervention or had already led to amputation at the 30-day review would be considered a deep infection.
The secondary objectives were to:
-
estimate differences in the Disability Rating Index (DRI) and health-related quality of life in the 6 months after surgery for the major trauma
-
estimate differences in general health-related quality of life in the 6 months after surgery for the major trauma
-
estimate differences in the quality of wound healing using a validated, patient-reported assessment of the scar
-
determine the number and nature of postoperative complications, including further surgical interventions related to the injury, in the first 6 months after surgery for the major trauma
-
investigate, using appropriate statistical and economic analysis methods, the resource use, and thereby the cost-effectiveness, of incisional NPWT versus standard dressing for wounds associated with major trauma to the lower limbs.
Chapter 2 Methods
The final protocol for this trial has been published in Achten et al. 19 and some of the content has been reproduced in this monograph. © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Some content from this chapter has also been reproduced from Knight et al. 20 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Trial design
This was a multicentre, pragmatic, superiority, parallel-arm RCT recruiting patients with a lower-limb fracture with 1 : 1 random allocation to receive either a standard wound dressing or incisional NPWT after lower-limb surgery following major trauma.
Internal pilot summary
The internal pilot took place at six recruitment centres over a period of 6 months using the same eligibility criteria and methods as the main study. The aim of this initial phase was to determine whether or not the number of eligible and recruited patients in the trauma recruitment centres over the course of 6 months would enable the delivery of a successful full trial.
The trial successfully recruited during the pilot phase and therefore continued to the main trial without any pause. Participants from the internal pilot were included in the final analysis.
Main randomised controlled trial summary
All adult patients presenting to hospital within 72 hours of sustaining major trauma and who required a surgical incision to treat a fractured lower limb were potentially eligible for inclusion. Randomisation, stratified by trial recruitment centre, open or closed fracture at presentation, and Injury Severity Score (ISS) of ≤ 15 versus ≥ 16 was generated and administered via a secure web-based service using minimisation in a 1 : 1 ratio. Participants were randomly allocated to either standard wound management or incisional NPWT.
The participants had clinical follow-up at the local fracture clinic for a minimum of 6 months, as per standard NHS practice after these injuries. Photographs of the wound were taken at 30 days, and a validated patient-reported questionnaire21 was used to assess the surgical scar. Functional and quality-of-life outcome data were collected using the DRI and the EuroQol-5 Dimensions (EQ-5D) questionnaire at 3 months and 6 months post surgery. Postoperative complications, including self-reported chronic pain [measured using the Douleur Neuropathique 4 Questionnaire (DN4)] and any further surgery related to the injury or the wound, were collected at the same time points, along with a resource use questionnaire. Completed case report forms (CRFs) were received centrally by a data administrator at the University of Oxford who entered the information into a secure password-protected database.
Participants
Patients were screened in the emergency department or trauma unit of participating trial recruitment centres. Throughout the trial, screening logs were kept at each recruitment centre to determine the number of potentially eligible patients and reasons for any exclusion. Patients who declined to participate or who withdrew from the trial were given the opportunity to discuss/inform the research team of their reasoning behind their decision not to take part.
A patient’s routine imaging on admission was used, including any ‘Major Trauma CT scan’, and associated ‘secondary survey’ to identify the patient’s injuries and to calculate the ISS (≤ 15 vs. ≥ 16) prior to the randomisation process. All major trauma patients in England are automatically considered for entry into the national Trauma Audit and Research Network (TARN) database, which requires the calculation of the ISS. As a result, all recruitment centres were familiar with the use of this major trauma scoring system.
Inclusion criteria
Patients were eligible for the WHiST trial if they:
-
were aged ≥ 16 years
-
presented to hospital within 72 hours of injury
-
had a major trauma injury and/or injury defined by eligibility for the UK TARN database
-
had a lower-limb fracture requiring a surgical incision.
Some patients had major trauma affecting just one limb (e.g. heel, pilon and tibial plateau fractures). As the wounds associated with these injuries are always at risk of infection, we included these injuries even if the patient was subsequently not included in the TARN database.
Exclusion criteria
Patients were excluded from participation in the WHiST trial if:
-
they had an open fracture of the lower limb that could not be closed primarily. Patients with open fractures that cannot be closed at the first surgery are at the highest risk of surgical site infection but incisional NPWT cannot be applied to these wounds
-
there was evidence that the patient would be unable to adhere to trial procedures or complete questionnaires.
Patients who sustained injuries to other areas of the body as well as the lower limbs that could affect the primary outcome measure had their other injuries documented but were still included. For patients with more than one lower-limb injury, only the most severe wound was included as the ‘WHiST’ wound in the trial. It was at the surgeon’s discretion which injury was the most severe.
Consent
The consent procedure for this trial reflected that of the surgery, with the attending clinician assessing capacity before taking consent for the surgical procedure; this capacity assessment was then used to inform the appropriate approach to consenting to the WHiST study. A process approved by the National Research Ethics Service was used to gain consent from the patient or agreement from an appropriate consultee by an appropriately delegated member of the research team.
Conducting research in this ‘emergency setting’ is regulated by the Mental Capacity Act 2005 (MCA). 22 As patients may have lacked capacity, and the urgent nature of the treatment may have limited access to appropriate discussion with personal consultees, action was taken in accordance with section 32, subsection 9b of the MCA. This involved the clinical team making an assessment of capacity as per their usual procedures for obtaining consent for a surgical procedure. The clinical team then provided guidance to the research team as to whether the patient had capacity to consent prospectively or if consultee agreement needed to be sought.
Throughout the trial, best efforts were made to involve participants who, temporarily or permanently, lacked the capacity to decide to be involved in the trial. The clinical team made a judgement about the amount and complexity of the information that the participant was able to understand and retain. Appropriate information was communicated to the participant and updated as their understanding changed. At all times, the trial team acted in accordance with the participants’ best interests.
When the clinical team advised that prospective patient consent was appropriate, this was sought by the research team. If the clinical team advised that prospective patient consent was not appropriate, the research team approached an appropriate consultee. The main responsibility of a consultee was to advise the research team as to whether or not they thought that the participant would be happy to take part in the trial if they had capacity to consent. When a personal consultee was available, they were provided with the trial information. The personal consultee was someone who had a personal relationship with the patient, such as a family member, carer or friend. The personal consultee was given the opportunity to ask questions and discuss the trial, after which their agreement for the patient’s inclusion in the trial was recorded. When a personal consultee was not available, then a nominated consultee was identified to advise the research team. In most cases, a patient’s senior treating surgeon acted as the nominated consultee. If that surgeon was a member of the research team, another independent surgeon was identified. The nominated consultee was asked, after reviewing the trial documentation, to agree that the patient participated fully in the trial and all trial procedures; this was recorded during the electronic randomisation process.
Data collection, including linkage to routine NHS data sets, commenced as soon as consent or agreement by personal/nominated consultee had been obtained.
Patients who were able to consent before their operation were always approached. For patients who did not consent prior to surgery, the research associate provided them with all of the trial information at the first appropriate time when the patient had regained capacity. The patients were given the opportunity to ask questions and discuss the trial with their family and friends. They were then asked to provide written consent for continuation in the trial. Patients who did not consent prior to surgery and preferred not to be actively involved in the trial follow-up were asked if they were willing to consent to the research team using their routinely collected NHS data for the trial.
Patients were asked to consent to long-term follow-up (reported separately) and data linkage to routine NHS data sets. For patients who did not prospectively consent or who had a nominated consultee give prospective agreement and still lacked capacity after their surgery, every effort was made to contact a personal consultee to advise the research team about the patient’s continued participation in the trial.
If the consultee was present, they were asked to sign a consultee agreement form. When the consultee was not present at the agreement discussion (e.g. when they were being contacted via telephone), verbal agreement was recorded by the research associate on an informed agreement checklist. Personal consultees who preferred not to be actively involved in the trial follow-up were asked if they were willing to agree to the research team using the patient’s routinely collected NHS data for the trial. If no personal consultee was identified, the participant remained in the trial under the nominated consultee’s agreement provided at the time of enrolment.
Agreement for a participant to be involved in the trial was recorded in a patient’s notes. All original signed consent forms were kept in the investigator site file. Three copies of the consent forms were made: one was held in a patient’s medical notes, one was for the participant and one copy was for the trial team.
Responsibility for recording and dating both oral and written informed consent or agreement was with the investigator, or persons designated by the investigator, who conducted the informed consent discussion. Designated responsibility was recorded on the recruitment centre delegation log. Permission was sought to inform the participant’s general practitioner (GP) of their participation in the trial.
Randomisation
The treating surgeon confirmed a patient’s eligibility at the end of the operative procedure but before the wound dressing was applied. Randomisation was on a 1 : 1 basis using a validated computer randomisation program managed centrally by the Oxford Clinical Trials Research Unit. A minimisation algorithm was used to ensure a balanced allocation of participants across the two treatment groups, stratified by trial recruitment centre, ISS of ≤ 15 versus ISS of ≥ 16 and open or closed fracture at presentation (only those open fractures for which the wound could be closed primarily after the first surgical wound debridement were eligible for inclusion because incisional NPWT cannot be applied to wounds that are left open). The first 30 participants were randomised using simple randomisation to seed the minimisation algorithm (allocations generated by the trial statistician). Thereafter, each participant was allocated to the treatment that minimised imbalance between the groups with probability 0.9 and to the opposite treatment with probability 0.1. This probabilistic element was introduced to ensure unpredictability of the treatment assignment. 23
In February 2018, it was discovered that randomisation by minimisation was not being implemented correctly. The probabilistic element of the randomisation schedule had not been transferred to the live randomisation system. Therefore, all participants were being randomised to treatment groups by simple randomisation without reference to their minimisation factors. The decision was taken to set the probability of being immediately allocated to the treatment that minimised imbalance to 0.95 for the remainder of the trial in order to maximise balance across minimisation factors without making the randomisation entirely deterministic. This change was made to address the slight imbalance observed in the recruitment centre strata. This change was made on 27 February 2018, at which point 1477 participants had been randomised (90.7% of the total).
Allocation of treatment
All modern operating theatres include a computer with internet access, so a secure, 24-hour, web-based randomisation system was used to generate the treatment allocation intraoperatively. After the confirmation of randomisation and treatment allocation was received electronically by the surgical team, the allocated treatment could be administered immediately.
Blinding
As the wound dressings and topical devices were clearly visible, the treating surgeon and trial participants could not be blinded to treatment allocation. However, the treating surgeons were not involved in trial follow-up assessments or data collection for the trial. Data from clinical reporting forms were entered into a central database administered by a data clerk in the trial central office. Wound photographs taken at an outpatient clinic at approximately 30 days post surgery were reviewed independently by two experienced assessors (tissue viability specialists) blinded to the treatment allocation.
Interventions
Patients with a fracture of the lower limb associated with major trauma usually have surgery on the next available trauma operating list. Some patients may be transferred to a major trauma centre for definitive care within the first 48 hours of injury but will still have their surgery as soon as possible. All participants received general or regional anaesthesia at the discretion of the treating anaesthetist. Details of the fracture type and operative treatment were as per standard practice, with relevant details recorded by the research team. At the end of the operation, a dressing was applied to the surgical wound. The WHiST trial compared two types of wound dressing: standard dressing versus incisional NPWT.
Standard dressing
The standard dressing for a surgical wound comprises a non-adhesive layer applied directly to the wound, which is then covered by a sealed dressing or bandage. The standard dressing does not use ‘negative pressure’. The exact details of the materials used were left to the discretion of the treating surgeon as per their routine practice, but the details of each dressing applied were recorded.
Incisional negative-pressure wound therapy
This uses a silicone contact layer with a silicon-based adhesive, an airlock layer, a superabsorbent layer and a polyurethane (semipermeable) layer on the top, which makes the system waterproof while allowing water vapour to pass. A sealed tube connects the dressing to a built-in mini-pump that creates a partial vacuum (–80 mmHg of negative pressure) over the wound.
It was applied to the wound at the end of the operation as per the treating surgeon’s normal practice and according to the dressing manufacturer’s instructions. The wound could be redressed again on the ward at the discretion of the clinical team; any further wound dressing was recorded and followed the allocated treatment unless otherwise clinically indicated.
Post-randomisation decline to consent and exclusions
Participants could decline to continue to take part in the trial at any time without prejudice. A decision to decline consent or withdraw did not affect the standard of care the patient received.
Participants had two options for withdrawal:
-
Participants could withdraw from completing any further questionnaires but allow the trial team to still view and retain, anonymously, any relevant hospital data that were recorded as part of normal standard of care, for example radiographs and further surgery information.
-
Participants could withdraw completely from the trial but data obtained up until the point of withdrawal were included in the final analysis of the trial; thereafter, no further data were collected for that participant.
Once withdrawn, a patient was advised to discuss their further care plan with their surgeon.
Participants were excluded in the post-randomisation phase if it was later established that they were ineligible for the trial (e.g. if they did not fulfil the criteria for ‘major trauma’), if they had a fracture that was not part of the lower limb (e.g. the spine), if they had an incision for which incisional NPWT could not be applied (e.g. with an external fixator) or if they were unable to adhere to trial procedures or complete questionnaires. In the context of major trauma, patients are sometimes taken directly to the operating theatre before their past medical history and full extent of their injuries can be assessed.
Participant care pathway
Participants were usually reviewed at 30 days, 3 months and 6 months, as per routine practice after this type of injury. Details about rehabilitation and additional follow-up appointments were recorded but left entirely to the discretion of the treating clinicians, as the type of injury varied between patients.
Primary outcome
The primary outcome measure for this study was deep SSI; we used the Centers for Disease Control and Prevention (CDC) definition of a ‘deep infection’, that is a wound infection involving the tissues deep to the skin that occurs within 30 days of injury. 24 Of note, shortly after the start of the WHiST trial, the CDC updated its criteria for a deep SSI in patients treated for fracture fixation. Specifically, the end point for wounds involving an implant was changed from 30 days to 90 days. Therefore, to facilitate future evidence synthesis, and in consultation with the Trial Steering Committee (TSC) and Data Safety and Monitoring Committee (DSMC), we included a second assessment of deep SSI at 90 days, as per the new CDC criteria, as a secondary outcome.
The treating clinical team recorded any signs or symptoms of wound-related infection in the medical record, as per routine clinical practice. The treating clinicians were not part of the research team. The participant was assessed and the medical records were reviewed by an independent research associate. Information was collected on a CRF to include any wound infection up to 30 days, according to the specific criteria used by the CDC to define a deep SSI. Any infection that required continuing medical intervention or had already led to amputation by 30 days was considered a deep infection.
According to the CDC criteria, an individual was classed as having a deep infection if he/she belonged in one or more of the following categories within 30 days of injury:
-
Fluid was leaking from the wound, and the fluid was pus.
-
At least one criterion from each of the following lists was satisfied –
-
the wound was gaping open (dehisced) or a surgeon had deliberately opened the wound
-
the area around the wound was painful or tender or the participant had a fever of > 38 °C.
-
-
There was any sign of abscess or infection on direct examination or imaging (e.g. ultrasonography).
Participants were confirmed to not have a deep infection if they met none of the above criteria.
As the CDC definition changed to include deep infections up to 90 days shortly after the trial started, signs of infection at 90 days were patient-reported only and fewer variables were recorded. Participants were defined as having a deep infection within 90 days if they had a deep infection within 30 days, or met one of the following criteria after 30 days but before 90 days:
-
Fluid was leaking from the wound, and the fluid was pus.
-
Increasing pain or discomfort in the area around the wound and one of the following –
-
the edges of any part of the wound had separated or gaped open
-
participant had further surgery because of their fracture, and the operation note confirmed that this was for, or revealed, a deep infection.
-
Secondary outcomes
The secondary outcome measures in this trial were as follows.
Disability Rating Index and general health-related quality of life
The DRI is measured using a self-administered, 12-item visual analogue scale (VAS) questionnaire assessing the participants’ own rating of their disability (range 0–100 in which higher scores indicate more disability). 25 This measure was chosen as it addresses gross body movements rather than specific joints or body segments. Therefore, it captured function and disability associated with different fractures and injuries of the lower limbs.
The EQ-5D is a validated measure of health-related quality of life, consisting of a five-dimension health status classification system and a separate VAS (range –0.594 to 1, with 0 equating to death and a higher score relating to better quality of life). 26 An updated version of the EQ-5D, the EuroQol-5 Dimensions, five-level version, (EQ-5D-5L) has been developed to enhance the responsiveness of the instrument to changes in patient health. 27
Responses to the health status classification system were converted into multiattribute utility (MAU) scores using tariffs developed for England. 28 These MAU scores were combined with survival data to generate quality-adjusted life-year (QALY) profiles for the purposes of the economic evaluation. The EQ-5D has been validated to be completed by a patient’s proxy in case of continued impaired capacity.
Table 1 shows the collection times for all of the trial outcome questionnaires.
| Outcome measure | Pre injurya | Post injury | 30 days | 3 months | 6 months |
|---|---|---|---|---|---|
| DRI | ✗ | ✗ | ✗ | ✗ | ✗ |
| EQ-5D | ✗ | ✗ | ✗ | ✗ | |
| Resource questionnaire | ✗ | ✗ | |||
| Postoperative complications | ✗ | ✗ | ✗ | ✗ | |
| Neuropathic pain | ✗ | ✗ | |||
| Scar self-assessment | ✗ | ✗ | ✗ |
Complications: wound healing and scar
The quality of wound healing was self-reported using the patient scale from the Patient and Observer Scar Assessment Scale (POSAS). 21 This consists of six questions regarding different aspects of the scar, as well as an overall assessment, to provide a subjective patient assessment of wound healing complications. An objective assessment of wound healing was also recorded using a standardised photograph of the surgical site taken at the 30-day review. The photograph was evaluated by two independent wound specialists who were blinded to the treatment allocation.
Complications: chronic pain
Chronic pain after surgery and trauma is common and disabling, but no previous studies have assessed the prevalence of persistent painful neuropathic characteristics after lower-limb fracture. Shortly after the start of the trial, and with the permission of the Research Ethics Committee, we added such an assessment. The proportion of participants reporting chronic pain with neuropathic characteristics post surgery was measured using the DN4. 29 The DN4 is a short validated neuropathic pain screening tool. The full tool includes an assessment by a clinician (10 questions, with a score of ≥ 4 indicating neuropathic pain). For the purposes of this trial, we used the patient self-reported component of the DN4 comprising seven questions, with a score of ≥ 3 indicating the presence of neuropathic pain. This screening tool is recommended for use by the International Association for the Study of Pain. 30
Complications: further surgical interventions and complications
Participants were also asked to self-report (or a consultee on their behalf, in the case of continued impaired capacity) at each of the follow-up points regarding any medical/surgical intervention they received related to their surgical wound. Any self-report of surgical treatment for infection was cross-referenced with the participant’s medical record. This allowed us to report deep infection at later time points, for example at 90 days. All other postoperative complications and surgical interventions related to the index wound were recorded in the CRFs completed in clinic or by the participant.
Health resource use
Health resource use data were collected at 3 and 6 months post surgery via patient-reported (or consultee-reported) questionnaires, which have been shown to be accurate in terms of the use of different services. 31 Unit cost data were obtained from the latest available national databases including the British National Formulary (BNF),32 the Personal Social Services Research Unit’s (PSSRU’s) Unit Costs of Health and Social Care,33 NHS Reference Costs34 and NHS Supply Chain Catalogue. 35
Data management: questionnaire completion
Completed CRFs were delivered to the trial co-ordinating centre by secure e-mail or Royal Mail (London, UK). When sending any confidential and/or sensitive personal data collected for research, secure NHS e-mail was used rather than the post whenever possible.
Participants were routinely followed up by the recruitment centres at 30 days post surgery. The research associate made a record of any early complications and took a photograph of the wound using a standardised protocol. The participant completed the ‘scar assessment’ questionnaire. These data were returned securely to the trial co-ordinating centre. If participants did not have an appointment, recruitment centres were requested to contact the participant and complete the 30-day report form over the telephone. If a participant could not be contacted, the research associate was requested to check for medical records and call the participant’s GP to check if there had been any wound-related complications. If no wound complications were recorded, it was assumed that there had not been any complications related to the wound.
The number and timing of any subsequent follow-up appointments were at the discretion of the treating surgeon, as per routine clinical practice. For the 3- and 6-month CRFs, if the participant had appointments scheduled, the report forms were completed in clinic. Participants who did not have a follow-up appointment at an appropriate time received the questionnaire by post. If the participant did not return the questionnaire, a reminder was sent by post after 3 weeks. If the participant did not return the second questionnaire, the participant was called on the telephone to complete the CRF. If the 6-month CRF was not obtained, the WHiST office requested recruitment centres to check whether or not the participant attended hospital/clinic from the time of discharge and, if so, when. Depending on the date the participant was seen, the recruitment centre was requested to complete the wound complications section of the 3-month (if not received) or the 6-month CRF. If a recruitment centre confirmed that there was no record of the participant attending hospital since discharge, no wound-related complications were assumed.
Approval for main trial
On completion of the 6-month pilot study period, results were reported to National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. Ninety-seven per cent of the recruitment target rate was achieved, indicating that it was feasible to proceed with the main trial. Therefore, no changes to the protocol or recruitment targets were made and the NIHR HTA programme granted approval for progression to the main phase of the trial. Participants recruited during the pilot phase were included in the main trial analysis data set.
Adverse event management
Serious adverse events (SAEs) were recorded in the SAE form and reported to the central trial team. However, some adverse events were foreseeable as part of the proposed treatment and therefore were reported as a complication in the CRFs. These events included any complications of anaesthesia or surgery (wound infection; bleeding or damage to adjacent structures such as nerves, tendons and blood vessels; delayed unions/non-unions; delayed wound healing; further surgery to remove/replace metalwork; and thromboembolic events). All participants who experienced SAEs were followed up as per protocol until the end of the trial.
Statistical analysis
Sample size
There had been only one previous randomised trial14 that compared incisional NPWT with standard dressings for surgical incisions associated with major trauma to the lower limb prior to this trial. This trial indicated that the rate of ‘late’ (deep) infection, that is, those not occurring during the initial hospitalisation, was 15% (18/122) in the standard dressing group versus 9% (13/141) in the incisional NPWT group. 14
In the absence of a ‘minimum clinically important difference’ for deep wound infection, we surveyed surgeons in the UK Orthopaedic Trauma Society who perform surgery for major trauma to the limbs. The survey showed that those who responded considered that a 6% reduction in the rate of ‘deep infection’ would, universally, be sufficient to change clinical practice with regard to the choice of wound dressing.
Therefore, assuming a reduction in the proportion of patients having a deep infection from 15% to 9%, 615 patients would be required in each group to provide 90% power at the 5% level. Our previous experience in clinical trials of lower-limb fracture surgery for major trauma indicated that up to 20% of primary outcome data may be lost during the follow-up period owing to death and loss to follow-up. Therefore, we aimed to recruit 1540 participants in total for this trial.
Analysis plan
General analysis principles
Two analysis populations were considered, the intention-to-treat (ITT) population and the per-protocol (PP) population. The ITT population included all participants randomised with the exception of those who (1) were randomised in error, (2) declined consent post randomisation or (3) withdrew and requested that all their data were removed. Participants were analysed according to the group to which they were randomised. The PP population were analysed according to the treatment they actually received. Participants with major protocol deviations or violations were excluded from the PP population. Major protocol deviations were those participants who (1) did not receive either of the trial interventions (e.g. those participants whose wound could not be closed primarily) or (2) those participants for whom the intervention was not recorded.
In addition, two analysis data sets were defined: (1) the available-case data set, comprising all observed data, and (2) the imputed data set, in which missing outcome data were imputed. Missing data were imputed using multiple imputation (MI) under the missing-at-random (MAR) assumption.
A two-sided significance level of 0.05 was used throughout, and 95% confidence intervals (CIs) were reported. The primary conclusion of the trial was based on the results from the ITT analysis of the primary outcome. Sensitivity analyses of the primary outcome were performed to assess whether or not these results were robust. All secondary outcomes were considered as supporting the primary analysis, and conclusions of the trial were not based on these outcomes. All analyses were undertaken using Stata® 15.0 (StataCorp, College Station, TX, USA).
Descriptive analyses
The numbers of potentially eligible individuals screened, randomised to each group, receiving allocated treatment and included in the primary analysis were summarised using a Consolidated Standards of Reporting Trials (CONSORT) flow chart. Reasons for ineligibility, loss to follow-up and exclusion from the primary analysis were also summarised, as well as the number of patients who declined consent both prospectively and retrospectively.
The baseline comparability of the two randomised groups in terms of minimisation factors was assessed. This was done for all randomised participants, including those who declined consent, and for the ITT population. The comparability of the two randomised groups in terms of baseline characteristics and operative procedure details was also summarised for the ITT population. Numbers with percentages were used to compare binary and categorical variables, means and standard deviations (SDs) were used for normally distributed continuous variables, and medians and interquartile ranges (IQRs) were used for non-normally distributed continuous variables. Approximate normality was established by visual assessment of histograms of the relevant variables. No tests of statistical significance or CIs for differences between the two randomised groups at baseline were calculated.
The numbers and percentages of losses to follow-up and withdrawals along with reasons for these were reported by intervention group at each time point. Absolute risk differences (with 95% CIs) and chi-squared tests were used to test whether or not there were differential losses between the groups. Deaths were reported separately.
The patterns of availability of data for primary and key secondary outcomes, from baseline until 6-month follow-up, were summarised for the two treatment groups as number and percentage of individuals missing. Reasons for missingness, when known, were also summarised. Differentiation was made between partially completed and fully missing outcome data.
The randomised intervention in this trial was the dressing (standard or incisional NPWT) applied to the surgically closed fracture wound at the end of surgery. The intervention occurred at a single time point, and compliance was therefore defined as the proportion of participants in each group receiving the treatment to which they were randomised. The number and percentage of participants receiving the assigned dressing, receiving an alternative dressing or receiving no dressing in each group was summarised. Reasons for not receiving the randomised treatment and details of what was provided instead were also summarised.
Analysis of the primary outcome
The numbers and proportion of deep SSI occurring up to 30 days post randomisation in the two intervention groups were calculated and reported. The rates of deep infection in the two groups were compared using a mixed-effects logistic regression model. The model included a random effect to account for any heterogeneity in response due to recruitment centre, and fixed effects to adjust for open versus closed fractures at presentation, ISS (≤ 15 vs. ≥ 16), participant age and participant gender. The odds ratio (OR), 95% CI and associated p-value were used to compare the two treatment groups. The adjusted risk difference between the two treatment groups and associated 95% CIs were calculated. The unadjusted OR and associated 95% CIs were also reported.
This analysis was conducted for the ITT population using the available-case data set. As sensitivity analyses, the analysis was repeated for (1) the ITT population using an imputed data set (MI) and (2) the PP population using the available-case data set. MI was used instead of best-case–worst-case imputation because of the lower than anticipated deep infection rate and the small number of missing primary outcome data (< 2%).
As < 5% of participants had died prior to the 30-day time point, the planned sensitivity analysis taking account of the competing risk of death36 was not conducted.
As a significant treatment effect of incisional NPWT was not identified in the primary analysis, the planned exploratory subgroup analysis to investigate whether or not this effect was moderated by the underlying risk level of the wound was not conducted.
Analysis of the secondary outcomes
The main analysis of the primary outcome (ITT population using the available-case data set) was repeated using the revised CDC definition of deep infection, that is including infections occurring up to 90 days after surgery. As none of the sensitivity analyses conducted for the primary end point (30 days) demonstrated substantially different results from the primary analysis, none of these analyses was repeated for the rates of deep infection up to 90 days.
For each continuous secondary outcome, mean scores and SDs for both intervention groups at each follow-up time point (30 days, 3 months and 6 months, as appropriate) were reported. Multilevel, mixed-effects linear regression models, using repeated measures (level 1) nested within participants (level 2), were used to compare the two intervention groups. The models included a random effect to account for any heterogeneity in response due to recruitment centre (level 3). The models also included fixed effects to adjust for open versus closed fractures at presentation, ISS (≤ 15 vs. ≥ 16), participant age, participant gender and, for the DRI and EQ-5D-5L, pre-injury values. Trends over time were examined, and an interaction between treatment and time was included in the model. The adjusted difference between the treatment groups at each time point was reported. This analysis was conducted for the ITT population using the available-case data set. The residuals from each model were plotted to ensure that the assumption of approximate normality was appropriate. The analysis of the DRI was repeated using a data set imputed using MI under the MAR assumption.
In addition, supplementary analyses of the DRI and EQ-5D utility variables were conducted using area under the curve (AUC) summary statistics. 37 The parameter estimates from the mixed-effects models were used to calculate the AUC from 3 to 6 months for the DRI and from post injury to 6 months for the EQ-5D in each intervention group. The difference between the two groups was calculated and compared using a t-test.
Pain was assessed using a 0–10 VAS. Median pain scores and IQRs were presented for each intervention group at each time point. Pain scores were compared across the two treatment groups using the Wilcoxon rank-sum test.
The DN4 results were analysed using similar methods to those outlined for the primary outcome. The number and proportion of individuals deemed to have neuropathic pain (DN4 score of ≥ 3) or non-neuropathic pain (DN4 score of < 3) were reported for each treatment group at each time point (3 and 6 months). A multilevel, mixed-effects logistic regression model with repeated measures (level 1) nested within participants (level 2) was used. The model was adjusted for recruitment centre as a random effect (level 3), and fixed effects were included to adjust for open versus closed fractures at presentation, ISS (≤ 15 vs. ≥ 16), participant age and participant gender. Trends over time were examined and, based on this, an interaction between treatment and time was included. Results were presented as ORs and associated CIs at each time point. The unadjusted OR and its associated CI were also reported. This analysis was conducted for the ITT population using the available-case data set. As this outcome measure was introduced while the trial was ongoing, there was a significant number of missing data at the 3-month time point.
Similar methods were also used to analyse wound-healing complications other than infection, and other local complications. The number and percentage of people experiencing each complication in each treatment group were reported and mixed-effects logistic regression models were used to compare the rates of complications between intervention groups. The model included a random effect for recruitment centre and fixed effects for open versus closed fractures at presentation, ISS (≤ 15 vs. ≥ 16), participant age and participant gender. This analysis was conducted for the ITT population using the available-case data set.
Temporal details of complications were reviewed; however, in the light of the limited data on the timing of complications, temporal patterns are not presented graphically. In addition, there were insufficient data for a time-to-event analysis of complications.
The number of related and unrelated SAEs was summarised by intervention group, as well as the number and percentage of participants experiencing at least one SAE. The rates of related and unrelated SAEs were compared between the two intervention groups using logistic regression models as outlined for other binary outcomes.
Health economic analysis plan
In the base-case (primary) analysis, a within-trial economic evaluation that consisted of direct medical costs and direct non-medical costs was conducted from the recommended NHS and Personal Social Services (PSS) perspective. 38
Collection of health resource data
As randomisation of the trial occurred after the surgery, only health and social service resources used after randomisation were included in the economic evaluation. In other words, resource use data related to the fracture fixation or concurrent surgery (e.g. head, chest, abdomen, pelvis or spine), labour (e.g. surgeon) or wound closure (e.g. skin clips, sutures, glue) were not collected.
Direct medical costs were separated into two groups: costs associated with the intervention and costs incurred for other reasons attributable to the intervention. The cost of the intervention was captured by the trial CRFs and consisted of the costs of wound management, inpatient care (i.e. hospitalisation and further treatment procedures) and antibiotics. Other health-care costs, such as inpatient care, outpatient care, primary and community care, and medications, were determined by means of health resource use questionnaires as filled out by the participants (or their consultees). Direct non-medical costs, such as aids and adaptations and PSS, were captured in the same patient-reported health resource use questionnaire. Other direct non-medical costs such as travel, child care and help with housework, as well as indirect costs (lost productivity) that would be used in the sensitivity analysis, were also obtained from the same patient-reported health resource use questionnaire. The health resource use questionnaires were administered at 3 and 6 months, with a recall period of 3 months.
Free-text responses (applicable to all the ‘other’ options) were reclassified in the appropriate cost category, were removed if deemed unrelated/irrelevant to the trial by clinical experts (e.g. cardiology, renal management) or were analysed collectively as ‘other’ in the descriptive analysis and excluded in the cost analysis. Items not listed as one of the prespecified options were not included in the cost analysis because the most frequently utilised resource item in each cost category would have been listed as one of the options in our questionnaire, so the exclusion of such miscellaneous items will not materially affect findings.
Collection of unit cost
Unit direct medical costs associated with the intervention were obtained from the NHS Supply Chain Catalogue 2018/201935 (see Appendix 1, Table 37). The unit cost of standard dressing was assumed to be the mean cost of permeable or semipermeable and non-permeable film/soft polymer dressings [e.g. OpSite (Smith & Nephew plc, London, UK), Mepore (Mölnlycke Health Care, Gothenburg, Sweden), Leukomed (BSN Medical, Hull, UK), Tegaderm (3M Health Care Ltd, Loughborough, UK), Cosmopor E (Paul Hartmann Ltd, Heywood, UK), Softpore (Richardson Healthcare, Elstree, UK), Mepitel (Mölnlycke Health Care), Hydrofilm (Paul Hartmann Ltd)]. The costs of orthotic cast (i.e. backslab cast, full cast and air cast/boot) was included as part of the intervention cost when there was a clinical need. Likewise, an additional component of intervention costs was the cost associated with dressing change, which occurred in both groups when there was a clinical need. This cost was estimated from the number of dressing changes and the time taken for a band 5 hospital nurse to replace the dressing. The number and type of dressing changes were captured in the CRFs; the time taken to change a dressing was estimated to be 5 minutes per change for both types of dressing. The cost per working hour of the nurse was obtained from the PSSRU 2018. 39
The cost of inpatient care consisted of two components: the cost of hospitalisation after the initial operation and the cost of further procedures that were related to the trial (e.g. debridement, metalwork removal and revision of internal fixation). These costs were derived using the NHS Digital HRG4+ 2017/18 Reference Costs Grouper40 and the NHS Reference Costs 2017/18. 34
Unit costs of medical items other than those directly attributable to the intervention (e.g. subsequent inpatient care, outpatient care or primary and community care utilised by a patient post surgery) were sourced from the NHS Reference Costs 2017/18. 34 Medication costs were sourced from the BNF;32 classes of medications deemed related to the trial by clinical experts included analgesic, antibiotic, anticoagulant, antidepressant, bisphosphonate, corticosteroid, hypnotic and anxiolytic, nausea, supplements and vitamins.
Unit costs for direct non-medical cost items such as PSS were obtained from PSSRU, whereas the costs of aids and adaptations were obtained from the NHS Supply Chain Catalogue35 (see Appendix 1, Table 1). The total cost per patient for additional (private) cost items incurred by patients and their next of kin, such as travel expenditure, child care and help with housework, were obtained from the patients directly via the patient-reported questionnaires. To estimate indirect costs, the daily median wage was obtained from the Office for National Statistics41 to compute the cost of absenteeism.
Cost per participant
Costs were calculated by multiplying resource use by the unit cost per resource and were expressed in 2017/18 Great British pounds. Unit costs were adjusted to 2017/18 prices using the NHS Hospital and Community Health Services (HCHS) index39 for health service resources when required. As the HCHS index has been revised in 2018 and the new HCHS index was available only from 2014/15 onwards, unit costs that were earlier than 2014/15 were inflated to 2014/25 levels using the older version of the HCHS (as the HCHS index for 2014/15 in the previous and current versions are the same), and then inflated to 2017/18 levels using the new HCHS index. No discounting of costs was applied because cost-effectiveness was determined within a time horizon of < 1 year (i.e. 6 months).
Medication costs over the trial period were computed using the cost per dose for each product and the mean quantity taken per day during the reported number of days. All medications were assumed to be in tablet form unless stated otherwise. If the dose of the medication was not recorded, the defined daily dose for each medication was taken from the World Health Organization website using the relevant anatomical therapeutic chemical code. 42 For the base-case analysis, which is from the NHS and PSS perspective, only medications that were prescribed were included, as we assumed that patients bought the medications out of pocket if it was used without a prescription.
Cost of absenteeism was computed using the human capital approach in which the daily median wage was multiplied by the number of days taken off work that were attributable to the injury.
Health utilities
Responses to the EQ-5D-5L were converted into MAU scores using the algorithm developed to reflect societal preferences in England. 28,43 Cross-walking algorithms developed by van Hout et al. 44 were employed to generate supplementary utility values comparable with those derived from the EQ-5D-3L instrument. QALYs were calculated as the area under the baseline-adjusted utility curve of EuroQol-5 Dimensions, three-level version (EQ-5D-3L) utility scores from baseline, 3- and 6-month data using the trapezoidal rule. 45 As the time horizon was < 1 year, no discounting was required for health utilities.
Data analysis
All analysis was based on ITT. Means and SDs of resource use and cost values for each cost category, at each time point, within each trial allocation were calculated. Differences between health resource utilisation, the means of costs and utility scores were calculated and tested for statistically significant differences from zero using t-tests. For differences in mean costs, the bootstrap 95% CI was computed based on 1000 replications. Differences in the proportion of resource use between treatment groups were examined using chi-squared tests.
Cost-effectiveness analysis
An incremental cost-effectiveness analysis comparing the cost-effectiveness of standard dressing with that of incisional NPWT, expressed in terms of incremental cost-per-QALY gained, was performed from the NHS and PSS perspective for the base-case analysis. Results were presented using incremental cost-effectiveness ratios (ICERs) and cost-effectiveness acceptability curves (CEACs) generated via non-parametric bootstrapping with 1000 replicas. This accommodates sampling (or stochastic) uncertainty and varying levels of willingness to pay for an additional QALY. The ICER was compared with willingness-to-pay thresholds of £20,000 and £30,000 per QALY, which are commonly assumed in the UK by bodies such as the National Institute for Health and Care Excellence. 46 An additional £15,000 cost-effectiveness threshold was also included to reflect recent trends in health-care decision-making. 47 The net monetary benefit (NMB) of standard dressing versus incisional NPWT was also computed and presented in a graph across different cost-effectiveness thresholds, for which a positive incremental NMB indicated that incisional NPWT is cost-effective compared with standard dressing at the given cost-effectiveness threshold.
Missing data
Under the MAR assumption, mean imputation was used for missing baseline covariate, whereas multiple imputation by chained equations (MICE) was implemented for missing cost and QALYs in order to produce unbiased estimates of costs and health outcomes. This assumption was tested using logistic regressions of missingness of costs and QALYs against baseline covariates. Inverse probability weighting was not adopted as our data were non-monotonic (i.e. patients who did not complete the 3-month questionnaire could have completed the 6-month questionnaire).
Mean imputation was used to fill in each missing value of the baseline EQ-5D utility score with the mean of the observed values, as it ensured that imputation was done in an arm-independent way. 48
Multiple imputation for QALYs was done at the score level, whereas costs were imputed at the total cost level in each follow-up time point using the Stata® command mi impute chained. Independent variables included in the imputation model consisted of baseline EQ-5D score, whether the fracture was open or closed at presentation, age at randomisation and smoking, education and employment status. The imputation was run 60 times in line with a ‘rule of thumb’, suggesting that the number of imputations should be similar to the percentage of incomplete cases. 49 A seemingly unrelated regression model was fitted to the imputed data to estimate total costs and total QALYs in each treatment group over the 6-month follow-up period. This approach allows for correlation between costs and outcomes and estimates the two regression equations jointly, potentially improving the precision of the estimates. Estimates obtained from each imputed data set were then combined using Rubin’s rules48 to obtain an overall mean estimate of the costs or QALYs.
Sensitivity analysis
Several sensitivity analyses were executed to explore the effects of alternative perspectives or scenarios on the cost-effectiveness results. First, a societal perspective that included medications bought out of pocket and additional (private) costs incurred by patients and next of kin, as well as the cost of absenteeism, was considered. Second, a complete-case analysis in which only patients with completed data on all cost and outcome data at all follow-up time points were included, after adjusting for the covariates, was performed.
Ethics approval and monitoring
Ethics committee approval
The National Research Ethic Committee approved this study on 16 February 2016 (reference number 16/WM/0006).
Trial Management Group
The day-to-day trial management was the responsibility of the trial manager, based at the Oxford Trauma Unit/Oxford Clinical Trials Research Unit of the University of Oxford and supported by administrative staff. The Trial Management Group (TMG) met monthly to assess overall trial progress. It was also the responsibility of the trial manager to train the research associates at each of the trial recruitment centres.
Trial Steering Committee
A TSC was appointed and was responsible for oversight, monitoring and supervising trial progress. The TSC consisted of two independent experts, a lay member and the chief investigator. Membership is listed in the Acknowledgements.
Data Safety and Monitoring Committee
The trial DSMC adopted a DAMOCLES charter, which defines its terms of reference and operation in relation to oversight of the trial. The DSMC was tasked with monitoring ethics, safety and data integrity. The trial statistician provided data and analyses requested by the DSMC so that they could review accruing data and summaries of the data presented by treatment group, and assess the screening algorithm against the eligibility criteria. They also considered emerging evidence from other related trials or research and reviewed related SAEs that had been reported. Membership of the DSMC is listed in the Acknowledgements.
Audit of primary outcome data collection
The DSMC recommended that an audit be performed on the rate of deep SSI based on the information obtained from medical notes, that is without contacting the patient, up to 6 months post surgery. This audit was conducted to assess whether or not the deep infection data captured on the CRF matched the data recorded in the clinical notes.
The audit was conducted when the data entry of the 30-day CRF had been completed for 1000 participants. Participants were eligible for inclusion in the audit if (1) the data entry of their 30-day CRF had been completed, (2) their deep infection status at 30 days was known and (3) they did not withdraw < 4 weeks after entering the trial.
Participants to be included in the audit were randomly sampled from all recruitment centres that had at least 10 participants eligible for inclusion in the audit. From each included recruitment centre, a simple random sample of participants was taken. This sample was approximately 20% of the total number of eligible participants at the recruitment centre, with a minimum sample size of 10 participants and a maximum sample size of 20 participants. The sample was enriched by including all participants with a deep infection diagnosis. This sampling strategy was designed to ensure a spread of participants across recruitment centres both with and without deep infections. No adjustment to ensure a spread across the recruitment time frame was included, because there was no reason to believe that recruitment centres might get better at recording infection diagnoses over time.
Each recruitment centre was provided with a list of included participants and asked to review the medical record for these participants for prespecified signs of infection and the dates these were recorded. This was done by a suitably trained surgical professional who would have not previously completed the CRFs. One recruitment centre declined to take part in the audit.
Patient and public involvement
Prior to the commencement of the trial, the research question under investigation was refined through discussions with members of a patient and public involvement group. Throughout the trial, a patient with direct experience of sustaining a lower-limb fracture and the subsequent recovery path reviewed participant documents prior to submission to the sponsor and the ethics committee. Advice was sought from this lay collaborator during management meetings, specifically when issues directly related to participant engagement were discussed. Independent lay representation was present on the TSC.
Chapter 3 Results
Screening and randomisation
Patient screening for potential trial participants was open from September 2016 to April 2018. A total of 3572 patients were screened, of whom 1509 were not eligible and 274 declined to participate in the trial. The most common reason for ineligibility was that an open fracture could not be primarily closed. Table 2 shows the reasons given for ineligibility or for declining consent.
| Reason | n |
|---|---|
| Not eligible | 1509 |
|
269 |
|
392 |
|
319 |
|
518 |
|
6 |
|
5 |
| Declined to participate a | 274 |
|
13 |
|
213 |
|
7 |
|
41 |
| Total | 1783 |
Of the potentially eligible patients, two were not randomised because of a lack of equipment (incisional NPWT not available) and 162 were not included because of surgeon’s preference. Table 3 shows the reasons given by the surgeons.
| Reason | n |
|---|---|
| Wound not suitable for NPWT | 7 |
| No equipoise – treatment preference not provided | 40 |
| No equipoise – preference for NPWT | 18 |
| No equipoise – preference for standard dressing | 12 |
| Unknown | 85 |
| Total | 162 |
Recruitment
A total of 1629 patients were randomised. However, four patients had prospectively declined to consent but were randomised in error because of communication breakdown at the recruitment centre, 58 were recruited under consultee agreement but subsequently declined to consent and 19 were found to be ineligible after randomisation under consultee agreement but prior to giving consent, leaving 1548 participants.
Recruitment by centre
Participants were recruited from 24 recruitment centres in England and Wales, representing the UK Major Trauma Network. Table 4 shows the number of participants recruited per recruitment centre and details on the participant’s sex, wound at presentation (open or closed), ISS (≤ 15 or ≥ 16) and type of consent at randomisation.
| Recruitment centre | Sex (n) | Consent (n) | Wound (n) | ISS | Randomisations (n) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Patient | Professional consultee | Personal consultee | Open | Closed | ≤ 15 | ≥ 16 | ||
| ADD | 49 | 21 | 61 | 5 | 4 | 6 | 64 | 38 | 32 | 70 |
| AUH | 51 | 19 | 63 | 7 | 0 | 8 | 62 | 45 | 25 | 70 |
| PLY | 14 | 14 | 17 | 11 | 0 | 4 | 24 | 25 | 3 | 28 |
| HEY | 14 | 9 | 23 | 0 | 0 | 0 | 23 | 20 | 3 | 23 |
| SMH | 13 | 5 | 15 | 3 | 0 | 9 | 9 | 17 | 1 | 18 |
| JCU | 38 | 18 | 39 | 16 | 1 | 14 | 42 | 53 | 3 | 56 |
| OUH | 79 | 58 | 67 | 66 | 4 | 45 | 92 | 108 | 29 | 137 |
| KCH | 136 | 49 | 5 | 176 | 4 | 50 | 135 | 132 | 53 | 185 |
| LGI | 30 | 23 | 38 | 10 | 5 | 1 | 52 | 45 | 8 | 53 |
| LRI | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| NHT | 27 | 33 | 38 | 22 | 0 | 1 | 59 | 59 | 1 | 60 |
| NGH | 35 | 16 | 25 | 25 | 1 | 11 | 40 | 39 | 12 | 51 |
| NUH | 59 | 47 | 57 | 48 | 1 | 11 | 95 | 82 | 24 | 106 |
| QEH | 20 | 10 | 24 | 5 | 1 | 5 | 25 | 23 | 7 | 30 |
| RLH | 114 | 42 | 108 | 48 | 0 | 45 | 111 | 134 | 22 | 156 |
| RSH | 38 | 51 | 22 | 66 | 1 | 6 | 83 | 86 | 3 | 89 |
| RSC | 23 | 22 | 45 | 0 | 0 | 5 | 40 | 34 | 11 | 45 |
| RVI | 17 | 11 | 12 | 16 | 0 | 11 | 17 | 27 | 1 | 28 |
| SRH | 9 | 13 | 16 | 6 | 0 | 3 | 19 | 18 | 4 | 22 |
| NBT | 51 | 41 | 53 | 39 | 0 | 8 | 84 | 66 | 26 | 92 |
| SGH | 50 | 18 | 56 | 11 | 1 | 18 | 50 | 41 | 27 | 68 |
| UHC | 61 | 25 | 32 | 53 | 1 | 11 | 75 | 57 | 29 | 86 |
| UHW | 14 | 18 | 28 | 3 | 1 | 2 | 30 | 28 | 4 | 32 |
| UHS | 22 | 20 | 37 | 5 | 0 | 7 | 35 | 33 | 9 | 42 |
| Total | 965 | 583 | 882 | 641 | 25 | 281 | 1267 | 1211 | 337 | 1548 |
| Percentage of the total | 62% | 38% | 57% | 41% | 2% | 18% | 82% | 78% | 22% | |
For those patients who lacked capacity to consent pre surgery (45%), consent for continuation in the trial was made at the first appropriate time point in the postoperative period. Table 5 shows the final type of consent/agreement obtained for all the participants recruited.
| Type of consent | Standard dressing | NPWT | Total (N) |
|---|---|---|---|
| Patient consent | 697 | 718 | 1415 |
| Prospective/retrospective informed agreement from a personal consultee | 19 | 22 | 41 |
| Patient consent/personal consultee confirmation of informed agreement to routine data only | 37 | 28 | 65 |
| Professional consultee agreement (routine data only) | 15 | 12 | 27 |
| Total | 768 | 780 | 1548 |
The planned overall required recruitment rate for the WHiST trial was approximately six participants per recruitment centre per month, based on 1540 participants recruited and consented over 22 months at 24 recruitment centres. Overall recruitment across recruitment centres was 4.2 participants per month. This was lower than the planned rate based on the original recruitment period; therefore, the trial recruitment was extended by 3 months to the end of April of 2018, to reach the target of 1540.
Minimisation factors by intervention group
The minimisation factors (recruitment centre, ISS and open or closed fracture at presentation) are summarised by treatment group for all randomised participants (Table 6). These factors were well balanced across treatment groups.
| Minimisation factor | Standard dressing (N = 816), n (%) | NPWT (N = 813), n (%) | Total (N = 1629), n (%) |
|---|---|---|---|
| Type of fracture | |||
| Open | 157 (19.2) | 153 (18.8) | 310 (19.0) |
| Closed | 659 (80.8) | 660 (81.2) | 1319 (81.0) |
| ISS | |||
| ≤ 15 | 634 (77.7) | 633 (77.9) | 1267 (77.8) |
| ≥ 16 | 182 (22.3) | 180 (22.1) | 362 (22.2) |
| Recruitment centres | |||
| ADD | 34 (4.2) | 36 (4.4) | 70 (4.3) |
| AUH | 38 (4.7) | 35 (4.3) | 73 (4.5) |
| QEH | 16 (2.0) | 14 (1.7) | 30 (1.8) |
| NBT | 49 (6.0) | 49 (6.0) | 98 (6.0) |
| UHC | 48 (5.9) | 49 (6.0) | 97 (6.0) |
| PLY | 12 (1.5) | 19 (2.3) | 31 (1.9) |
| HEY | 10 (1.2) | 13 (1.6) | 23 (1.4) |
| SMH | 9 (1.1) | 9 (1.1) | 18 (1.1) |
| KCH | 91 (11.2) | 95 (11.7) | 186 (11.4) |
| LGI | 26 (3.2) | 27 (3.3) | 53 (3.3) |
| LRI | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| JCU | 31 (3.8) | 27 (3.3) | 58 (3.6) |
| NHT | 33 (4.0) | 27 (3.3) | 60 (3.7) |
| NUH | 60 (7.4) | 61 (7.5) | 121 (7.4) |
| OUH | 73 (8.9) | 71 (8.7) | 144 (8.8) |
| RSC | 20 (2.5) | 25 (3.1) | 45 (2.8) |
| RVI | 14 (1.7) | 17 (2.1) | 31 (1.9) |
| SRH | 14 (1.7) | 10 (1.2) | 24 (1.5) |
| NGH | 28 (3.4) | 29 (3.6) | 57 (3.5) |
| UHS | 21 (2.6) | 21 (2.6) | 42 (2.6) |
| SGH | 39 (4.8) | 31 (3.8) | 70 (4.3) |
| RSH | 50 (6.1) | 51 (6.3) | 101 (6.2) |
| RLH | 83 (10.2) | 81 (10.0) | 164 (10.1) |
| UHW | 16 (2.0) | 16 (2.0) | 32 (2.0) |
Post-consent eligibility errors
After consent, 27 participants were found to be ineligible for the trial. These patients were followed up as per ITT but were excluded from the PP population. Table 7 shows the reasons for ineligibility post consent.
| Reasons for ineligibility post randomisation | Standard dressing (n) | NPWT (n) |
|---|---|---|
| Followed up as ITT | ||
| Acetabular fracture fixed using intrapelvic approach | 4 | 3 |
| Ankle fracture dislocation | 3 | 3 |
| Presented to hospital > 72 hours post injury | 1 | 1 |
| Wound unable to be closed | 4 | 8 |
Participants and interventions
Consented and non-consented participants
Of the 1548 participants randomised and consented, one withdrew immediately after surgery, requesting not to provide any of the baseline data. Therefore, this participant was excluded from the ITT population. Figure 1 shows the study CONSORT flow diagram.
FIGURE 1.
The WHiST trial CONSORT flow diagram. a, One participant who prospectively declined and was randomised in error; b, three participants who prospectively declined and were randomised in error.

Treatment allocation
Participants who did and those who did not receive their allocated intervention are summarised in Table 8. There were 100 crossovers in total, 92 from incisional NPWT to standard dressing and eight from standard dressing to incisional NPWT. Reasons for crossovers are also summarised in Table 8. Most crossovers in the standard dressing group [n = 7 (87.5%)] were due to surgeon’s choice, whereas most crossovers in the incisional NPWT group [n = 47 (51.1%)] were due to ‘other’ reasons: the surgical team forgot to apply the allocated treatment, or the allocated treatment was not communicated to the surgical team.
| Dressing received | Standard dressing (N = 763), n (%) | NPWT (n = 784a), n (%) |
|---|---|---|
| Received allocated dressing | 755 (99.0) | 692 (88.3) |
| Received other dressing | 8 (1.0) | 92 (11.7) |
| Reason for thisb | ||
| Surgeon choice | 7 (87.5) | 33 (35.9) |
| Lack of equipment | 0 (0.0) | 12 (13.0) |
| Otherc | 1 (12.5) | 47 (51.1) |
Details of treatment compliance following application of the dressings are summarised in Table 9. Incisional NPWT was in place for a median of 7 days (as recommended by the manufacturer). Participants were classed as having changed their treatment if they had a different dressing applied after < 7 days.
| Compliance | Standard dressing (N = 763) | NPWT (N = 784) |
|---|---|---|
| NPWT days in placea | – | 7.0 (7.0–8.0) |
| Treatment subsequently changed,b n (%) | 17 (2.2) | 85 (10.8) |
| Treatment changed to:c n (%) | ||
| Standard | 3 (17.6)d | 84 (98.8) |
| NPWT | 14 (82.4) | 1 (1.2)d |
| Reason treatment changed:c n (%) | ||
| Surgeon choice | 8 (47.1) | 26 (30.6) |
| Other | 9 (52.9) | 59 (69.4) |
Available data
Primary outcome data for 197 participants (13.0% of the ITT population) were completed retrospectively by checking medical records, as these participants did not attend their follow-up appointment and could not be contacted by telephone. A total of 92 participants declined to have data collected after the primary outcome point of 30 days; therefore, data obtained from the 3- and 6-month follow-up points could be collected for a maximum of only 1456 participants (711 and 745 participants in the standard and incisional NPWT groups, respectively). Table 10 shows the follow-up rate at 3 and 6 months post surgery.
| Follow-up completions | Standard dressing (N = 711) | NPWT (N = 745) |
|---|---|---|
| 3-month follow-up | ||
| Completed, n (%) | 590 (83) | 630 (85) |
| Questionnaire by participant/consultee (n) | 483 | 535 |
| Complications reported in medical records only (n) | 107 | 95 |
| Not completed, n (%) | 121 (17) | 115 (15) |
| Withdrawn (n) | 26 | 22 |
| Dead (n) | 15 | 12 |
| Lost to follow-up (n) | 1 | 0 |
| Missing (n) | 79 | 81 |
| 6-month follow-up | ||
| Completed, n (%) | 647 (91) | 672 (90) |
| Questionnaire by participant/consultee (n) | 456 | 492 |
| Complications reported in medical records only (n) | 191 | 180 |
| Not completed, n (%) | 64 (9) | 73 (10) |
| Withdrawn (n) | 40 | 51 |
| Dead (n) | 19 | 18 |
| Lost to follow-up (n) | 2 | 2 |
| Missing (n) | 3 | 2 |
Details of the completion of the primary and key secondary outcome measures (DRI and EQ-5D utility scores) at each time point are summarised by intervention group in Table 11. Outcomes were expected to be completed for all participants who had not withdrawn or died by each time point. For participants who answered some, but not all, parts of the DRI, pro-rated scores were calculated if more than half of the questions (i.e. at least 7) had been answered. Pro-rated scores were an average across all answered questions. Eleven pre-injury DRI scores, 40 3-month DRI scores and 33 6-month DRI scores were imputed in this way.
| Outcome measure | Standard dressing | NPWT | ||||
|---|---|---|---|---|---|---|
| Expecteda (n) | Received (n) | Compliance (%) | Expecteda (n) | Received (n) | Compliance (%) | |
| Deep infection | ||||||
| 30 days | 753 | 749 | 99.5 | 778 | 770 | 99.0 |
| 3 months | 670 | 578 | 86.3 | 711 | 628 | 88.3 |
| DRI | ||||||
| Baseline | 763 | 693 | 88.4 | 784 | 722 | 90.8 |
| 3 months | 670 | 464 | 69.3 | 711 | 517 | 72.7 |
| 6 months | 650 | 438 | 67.4 | 674 | 480 | 71.2 |
| EQ-5D utility score | ||||||
| Pre injury | 763 | 704 | 89.8 | 784 | 740 | 92.3 |
| Post injury | 763 | 701 | 89.4 | 784 | 735 | 91.9 |
| 3 months | 670 | 473 | 70.6 | 711 | 530 | 74.5 |
| 6 months | 650 | 448 | 68.9 | 674 | 488 | 72.4 |
All withdrawals up to 6 months after randomisation are summarised by intervention group in Table 12. The median time to withdrawal and the reasons for withdrawals are summarised. The proportion of withdrawals was slightly higher in the incisional NPWT group (7.4%) than in the standard dressing group (5.9%); however, the median time to withdrawal was longer in the incisional NPWT group. The main reason for withdrawal was that participants (or their consultee) did not want to complete questionnaires. Withdrawals > 6 months after randomisation will be reported separately as part of the long-term follow-up of the WHiST trial.
| Details of withdrawals | Standard dressing (N = 763) | NPWT (N = 784) |
|---|---|---|
| Withdrawals, n (%) | 40 (5.2) | 50 (6.4) |
| Time to withdrawal (days)b | 108.5 (45.5–154.5) | 140.0 (61.0–202.0) |
| Reasons for withdrawal,c n (%) | ||
| Does not like being part of research | 8 (20.0) | 3 (6.0) |
| Does not want to complete questionnaires | 19 (47.5) | 28 (56.0) |
| No reason given | 0 (0.0) | 5 (10.0) |
| Other | 13 (32.5) | 14 (28.0) |
Baseline characteristics
Baseline participant characteristics
The descriptive characteristics of the participants included in the ITT population are summarised by intervention group and overall in Table 13. These values are presented as numbers and percentages for categorical factors and means and SDs or medians and IQRs, as appropriate, for continuous variables. These variables all appear well balanced across the two treatment groups. The distribution of participant ages at enrolment is shown in Figure 2. This distribution has two peaks (i.e. it is bimodal): one in younger adults and one in older adults.
| Characteristics | Standard dressing (N = 763) | NPWT (N = 784) | Total (N = 1547) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 482 (63.2) | 482 (61.5) | 964 (62.3) |
| Female | 281 (36.8) | 302 (38.5) | 583 (37.7) |
| Age (years), n (%) | |||
| ≤ 40 | 278 (36.4) | 283 (36.1) | 561 (36.3) |
| > 40 | 485 (63.6) | 501 (63.9) | 986 (63.7) |
| BMI (kg/m2), mean (SD) | 26.7 (6.0) | 26.4 (5.9) | 26.5 (5.9) |
| Mechanism of injury, n (%) | |||
| Low-energy fall | 252 (33.0) | 275 (35.1) | 527 (34.1) |
| High-energy fall | 145 (19.0) | 139 (17.7) | 284 (18.4) |
| Road traffic accident | 273 (35.8) | 298 (38.0) | 571 (36.9) |
| Crush injury | 16 (2.1) | 16 (2.0) | 32 (2.1) |
| Contact sports | 12 (1.6) | 10 (1.3) | 22 (1.4) |
| Other | 61 (8.0) | 42 (5.4) | 103 (6.7) |
| Not recorded | 4 (0.5) | 4 (0.5) | 8 (0.5) |
| Days from injury to randomisation, median (IQR) | 1.0 (1.0–3.0) | 1.0 (1.0–3.0) | 1.0 (1.0–3.0) |
| Patient transferred, n (%) | |||
| Yes | 155 (20.3) | 167 (21.3) | 322 (20.8) |
| No | 604 (79.2) | 614 (78.3) | 1218 (78.7) |
| Not recorded | 4 (0.5) | 3 (0.4) | 7 (0.5) |
| Any other injuries, n (%) | |||
| Yes | 427 (56.0) | 454 (57.9) | 881 (56.9) |
| No | 332 (43.5) | 327 (41.7) | 659 (42.6) |
| Not recorded | 4 (0.5) | 3 (0.4) | 7 (0.5) |
| Diagnosed with diabetes mellitus, n (%) | |||
| Yes | 85 (11.1) | 63 (8.0) | 148 (9.6) |
| No | 665 (87.2) | 712 (90.8) | 1377 (89.0) |
| Not recorded | 13 (1.7) | 9 (1.1) | 22 (1.4) |
| Regular smoker, n (%) | |||
| Yes | 216 (28.3) | 218 (27.8) | 434 (28.1) |
| No | 524 (68.7) | 544 (69.4) | 1068 (69.0) |
| Not recorded | 23 (3.0) | 22 (2.8) | 45 (2.9) |
| Alcohol consumption per week (units), n (%) | |||
| 0–7 | 514 (67.4) | 507 (64.7) | 1021 (66.0) |
| 8–14 | 95 (12.5) | 120 (15.3) | 215 (13.9) |
| 15–21 | 53 (6.9) | 58 (7.4) | 111 (7.2) |
| > 21 | 68 (8.9) | 71 (9.1) | 139 (9.0) |
| Not recorded | 33 (4.3) | 28 (3.6) | 61 (3.9) |
| Regular analgesia before injury, n (%) | |||
| Yes | 137 (18.0) | 151 (19.3) | 288 (18.6) |
| No | 605 (79.3) | 624 (79.6) | 1229 (79.4) |
| Not recorded | 21 (2.8) | 9 (1.1) | 30 (1.9) |
| Other medication before injury, n (%) | |||
| Yes | 380 (49.8) | 392 (50.0) | 772 (49.9) |
| No | 367 (48.1) | 383 (48.9) | 750 (48.5) |
| Not recorded | 16 (2.1) | 9 (1.1) | 25 (1.6) |
| Ethnicity, n (%) | |||
| White | 667 (87.4) | 701 (89.4) | 1368 (88.4) |
| Black Caribbean | 6 (0.8) | 9 (1.1) | 15 (1.0) |
| Black African | 13 (1.7) | 15 (1.9) | 28 (1.8) |
| Black other | 4 (0.5) | 2 (0.3) | 6 (0.4) |
| Indian | 11 (1.4) | 7 (0.9) | 18 (1.2) |
| Pakistani | 14 (1.8) | 7 (0.9) | 21 (1.4) |
| Bangladeshi | 3 (0.4) | 1 (0.1) | 4 (0.3) |
| Chinese | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Other | 22 (2.9) | 31 (4.0) | 53 (3.4) |
| Not recorded | 22 (2.9) | 11 (1.4) | 33 (2.1) |
| Training post school, n (%) | |||
| No | 296 (38.8) | 308 (39.3) | 604 (39.0) |
| Formal training through work | 143 (18.7) | 127 (16.2) | 270 (17.5) |
| Qualification from college/university (not degree) | 156 (20.4) | 194 (24.7) | 350 (22.6) |
| Degree from college/university | 115 (15.1) | 112 (14.3) | 227 (14.7) |
| Not recorded | 43 (5.6) | 53 (6.9) | 96 (6.2) |
| Employment status, n (%) | |||
| Full-time employed | 287 (37.6) | 310 (39.5) | 597 (38.6) |
| Part-time employed | 56 (7.3) | 53 (6.8) | 109 (7.0) |
| Self-employed | 70 (9.2) | 77 (9.8) | 147 (9.5) |
| Unpaid work | 7 (0.9) | 5 (0.6) | 12 (0.8) |
| Unemployed | 89 (11.7) | 83 (10.6) | 172 (11.1) |
| Full-time student | 22 (2.9) | 19 (2.4) | 41 (2.7) |
| Retired/look after home/inactive | 201 (26.3) | 206 (26.3) | 407 (26.3) |
| Not recorded | 31 (4.1) | 31 (4.0) | 62 (4.0) |
FIGURE 2.
Distribution of participant age in years at randomisation.
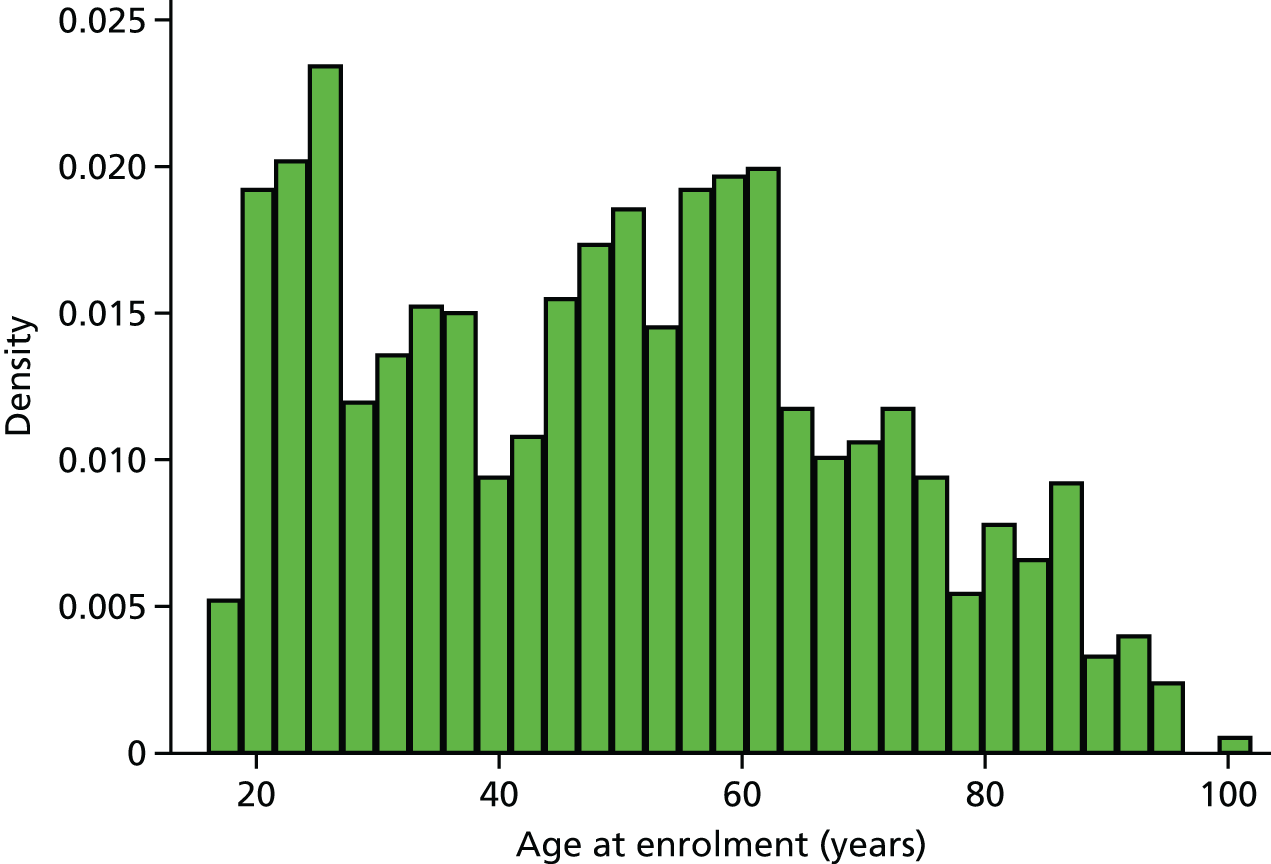
Operative procedures
Table 14 summarises operative procedure details for the ITT population by group and overall. These details are well balanced across the two treatment groups.
| Operative procedure details | Standard dressing (N = 763) | NPWT (N = 784) | Total (N = 1547) |
|---|---|---|---|
| ISS, n (%) | |||
| ≤ 15 | 598 (78.4) | 609 (77.7) | 1207 (78.0) |
| ≥ 16 | 165 (21.6) | 175 (22.3) | 340 (22.0) |
| Wound at presentation, n (%) | |||
| Open | 141 (18.5) | 147 (18.8) | 288 (18.6) |
| Closed | 622 (81.5) | 637 (81.3) | 1259 (81.4) |
| Lead surgeon grade, n (%) | |||
| Consultant | 525 (68.8) | 526 (67.1) | 1051 (67.9) |
| Staff grade/associate specialist | 47 (6.2) | 46 (5.9) | 93 (6.0) |
| Specialist trainee | 162 (21.2) | 176 (22.4) | 338 (21.8) |
| Other | 23 (3.0) | 33 (4.2) | 56 (3.6) |
| Not recorded | 6 (0.8) | 3 (0.4) | 9 (0.6) |
| Number of surgeons, median (IQR) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| Wound limb, n (%) | |||
| Right | 390 (51.1) | 390 (49.7) | 780 (50.4) |
| Left | 368 (48.2) | 390 (49.7) | 758 (49.0) |
| Not recorded | 5 (0.7) | 4 (0.5) | 9 (0.6) |
| Wound location, n (%) | |||
| Hip | 126 (16.5) | 145 (18.5) | 271 (17.5) |
| Femur | 284 (37.2) | 290 (37.0) | 574 (37.1) |
| Patella | 8 (1.0) | 14 (1.8) | 22 (1.4) |
| Tibia/fibula | 293 (38.4) | 282 (36.0) | 575 (37.2) |
| Foot | 19 (2.5) | 25 (3.2) | 44 (2.8) |
| Acetabulum | 29 (3.8) | 25 (3.2) | 54 (3.5) |
| Not recorded | 4 (0.5) | 3 (0.4) | 7 (0.5) |
| Duration of operation (hours), mean (SD) | 2.71 (1.56) | 2.79 (1.50) | 2.75 (1.53) |
| Type of fixation, n (%) | |||
| Nail | 256 (33.6) | 260 (33.2) | 516 (33.4) |
| Plate and screws | 362 (47.4) | 379 (48.3) | 741 (47.9) |
| Wires/tension band wires | 5 (0.7) | 16 (2.0) | 21 (1.4) |
| External – half-pin | 12 (1.6) | 13 (1.7) | 25 (1.6) |
| External – fine wire | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Other | 123 (16.1) | 111 (14.2) | 234 (15.1) |
| Not recorded | 4 (0.5) | 5 (0.6) | 9 (0.6) |
| Type of closure, n (%) | |||
| Interrupted sutures | 200 (26.2) | 203 (25.9) | 403 (26.1) |
| Skin clips | 216 (28.3) | 198 (25.3) | 414 (26.8) |
| Subcuticular suture | 220 (28.8) | 242 (30.9) | 462 (29.9) |
| Other skin closure | 120 (15.7) | 131 (16.7) | 251 (16.2) |
| Not recorded | 7 (0.9) | 10 (1.3) | 17 (1.1) |
| Any adjuncts,a n (%) | |||
| Yes | 609 (79.8) | 646 (82.4) | 1255 (81.1) |
| No | 145 (19.0) | 129 (16.5) | 274 (17.7) |
| Not recorded | 9 (1.2) | 9 (1.1) | 18 (1.2) |
| Intraoperative complications, n (%) | |||
| Yes | 18 (2.4) | 22 (2.8) | 40 (2.6) |
| No | 741 (97.1) | 757 (96.6) | 1498 (96.8) |
| Not recorded | 4 (0.5) | 5 (0.6) | 9 (0.6) |
| Type of intraoperative complication, n (%) | |||
| Nerve injury | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Vascular injury | 0 (0.0) | 2 (0.3) | 2 (0.1) |
| Tendon injury | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extension of fracture | 6 (0.8) | 4 (0.5) | 10 (0.6) |
| Other | 11 (1.4) | 16 (2.0) | 27 (1.7) |
| Other surgery, n (%) | |||
| Yes | 161 (21.1) | 177 (22.6) | 338 (21.8) |
| No | 598 (78.4) | 604 (77.0) | 1202 (77.7) |
| Not recorded | 4 (0.5) | 3 (0.4) | 7 (0.5) |
| Type of other surgery, n (%) | |||
| Head | 2 (0.3) | 5 (0.6) | 7 (0.5) |
| Chest | 1 (0.1) | 4 (0.5) | 5 (0.3) |
| Abdomen | 2 (0.3) | 0 (0.0) | 2 (0.1) |
| Pelvis | 21 (2.8) | 20 (2.6) | 41 (2.7) |
| Spine | 4 (0.5) | 3 (0.4) | 7 (0.5) |
| Upper limbs | 55 (7.2) | 53 (6.8) | 108 (7.0) |
| Ipsilateral limb | 81 (10.6) | 83 (10.6) | 164 (10.6) |
| Contralateral limb | 46 (6.0) | 55 (7.0) | 101 (6.5) |
| Prophylactic antibiotics, n (%) | |||
| Yes | 736 (96.5) | 760 (96.9) | 1496 (96.7) |
| No | 21 (2.8) | 21 (2.7) | 42 (2.7) |
| Not recorded | 6 (0.8) | 3 (0.4) | 9 (0.6) |
| Standard dressing type, n (%) | |||
| Permeable/semipermeable | 191 (25.0) | NA | NA |
| Non-permeable | 382 (50.1) | NA | NA |
| Other | 173 (22.7) | NA | NA |
| Not recorded | 17 (2.2) | NA | NA |
| Orthotic/cast used, n (%) | |||
| Yes | 194 (25.4) | 166 (21.2) | 360 (23.3) |
| No | 562 (73.7) | 614 (78.3) | 1176 (76.0) |
| Not recorded | 7 (0.9) | 4 (0.5) | 11 (0.7) |
Patient-reported outcome measures at baseline
Baseline values of patient-reported outcome measures (DRI and EQ-5D) are summarised by intervention group using medians and IQRs in Table 15. For the EQ-5D variables, both immediate pre- and post-injury values are summarised. These are all similar across the two treatment groups.
| Intervention group | Standard dressing (n = 763), median (IQR) | NPWT (n = 784), median (IQR) | Total (n = 1547), median (IQR) |
|---|---|---|---|
| Pre-injury DRI scores | 1.4 (0.0–19.2) | 1.5 (0.0–19.3) | 1.5 (0.0–19.3) |
| Pre-injury EQ-5D (utility) scores | 1.0 (0.8–1.0) | 1.0 (0.8–1.0) | 1.0 (0.8–1.0) |
| Post-injury EQ-5D (utility) scores | 0.0 (–0.2–0.2) | –0.0 (–0.2–0.2) | –0.0 (–0.2–0.2) |
| Pre-injury EQ-5D (VAS) scores | 85.0 (70.0–95.0) | 85.0 (70.0–95.0) | 85.0 (70.0–95.0) |
| Post-injury EQ-5D (VAS) scores | 40.0 (20.0–60.0) | 40.0 (25.0–60.0) | 40.0 (20.0–60.0) |
Primary outcome
Analysis of primary outcome
The number and percentage of participants with a deep infection at up to 30 days is reported by intervention group in Table 16. The deep infection rate in the control group (6.7%, 50/749) was substantially lower than anticipated: the sample size calculation for this study was based on a control group deep infection rate of 15%. The deep infection rate within 30 days in the intervention group was 5.8% (45/770).
| Analysis | Standard dressing | NPWT | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Total (N) | n (%) | Total (N) | n (%) | Raw | Adjusted | ||
| ITT (available cases) | 749 | 50 (6.7) | 770 | 45 (5.8) | 0.87 (0.57 to 1.32) | 0.87 (0.57 to 1.33) | 0.52 |
| PP (available cases) | 731 | 48 (6.6) | 668 | 41 (6.1) | 0.93 (0.60 to 1.43) | 0.93 (0.60 to 1.44) | 0.76 |
| ITT (MI) | 763 | 51 (6.7) | 784 | 46 (5.8) | 0.86 (0.57 to 1.31) | 0.86 (0.57 to 1.31) | 0.49 |
The logistic regression analysis indicated that there was no statistically significant difference between the intervention groups in terms of deep infection rate (OR 0.87, 95% CI 0.57 to 1.33; see Table 16). The adjusted absolute risk difference between the control and intervention groups was –0.77% (95% CI –3.19% to 1.66%). This analysis was also repeated for the PP population to assess the impact of deviations from the planned trial protocol; it indicated no statistically significant difference between the two groups.
The rate of deep infection was lower than anticipated (6.3% across the whole trial) and the number of missing primary outcome data was very small [n = 28 (< 2%)]; therefore, MI was considered to be the most robust sensitivity analysis for best versus worst case.
Potential prognostic factors were investigated, and wound location (above or below knee) was included in the MICE model, along with all variables from the fitted model. Ten imputed data sets were created and combined using Rubin’s rules. No significant differences between groups were identified by this analysis (see Table 16).
Secondary analyses of the primary outcome
The number and percentage of participants with a deep infection at up to 90 days is reported in Table 17. The deep infection rate was higher than the rate reported at 30 days (13.2% in the control group and 11.4% in the intervention group) and similar to that expected in the sample size calculation. The rate at 90 days may be slightly overestimated because those with a deep infection before 30 days were counted as a deep infection before 90 days regardless of the availability of 3-month data for these individuals, whereas individuals were counted as not having a deep infection at 90 days only if they did not have a deep infection at either 30 days or 90 days. A mixed-effects logistic regression model was again used to compare the two trial groups for the ITT population using available cases. There was no significant difference in the rates of deep infection at up to 90 days between the two intervention groups (OR 0.86, 95% CI 0.61 to 1.23).
| Standard dressing | NPWT | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Total (N) | n (%) | Total (N) | n (%) | Raw | Adjusted | |
| 590 | 78 (13.2) | 629 | 72 (11.4) | 0.85 (0.60 to 1.19) | 0.84 (0.59 to 1.19) | 0.32 |
Secondary outcomes
Other wound healing complications
There were 1452 participants who did not have a deep infection at up to 30 days. This includes those who were confirmed not to have a deep infection status using the criteria outlined previously, and the small number (n = 28) whose deep infection status was missing. The number and proportion of these individuals experiencing other wound-related symptoms that did not fit the CDC definition for deep SSI is summarised by intervention group in Table 18. For each outcome, the proportion of participants experiencing them were compared across intervention groups using mixed-effects logistic regression models, as outlined for the primary analysis. The results of fitting these models are summarised in Table 18; no significant differences between the two groups were identified for any wound healing complications.
| Wound complication | Standard dressing (N = 713) | NPWT (N = 739) | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n (%) | Total (N) | n (%) | Total (N) | Raw | Adjusted | ||
| Red and inflamed | 90 (13.2) | 684 | 76 (10.6) | 715 | 0.78 (0.57 to 1.09) | 0.76 (0.55 to 1.07) | 0.11 |
| Swollen | 137 (20.0) | 684 | 147 (20.6) | 715 | 1.03 (0.80 to 1.34) | 1.03 (0.79 to 1.36) | 0.81 |
| Painful/tender | 188 (27.6) | 682 | 173 (24.4) | 710 | 0.85 (0.67 to 1.08) | 0.84 (0.65 to 1.08) | 0.18 |
| Fever of > 38°C | 67 (9.8) | 681 | 61 (8.6) | 713 | 0.86 (0.60 to 1.23) | 0.82 (0.56 to 1.19) | 0.30 |
| Fluid leaking (not pus) | 60 (8.7) | 687 | 50 (7.0) | 715 | 0.79 (0.53 to 1.16) | 0.76 (0.51 to 1.14) | 0.18 |
| Wound dehisced | 7 (1.0) | 687 | 2 (0.3) | 714 | 0.27 (0.06 to 1.32) | 0.27 (0.05 to 1.34) | 0.11 |
| Surgeon deliberately opened | 2 (0.3) | 688 | 2 (0.3) | 715 | 0.96 (0.14 to 6.85) | 1.05 (0.14 to 7.64) | 0.96 |
| Trial wound complications treated surgicallya | 2 (0.3) | 575 | 1 (0.2) | 573 | 0.50 (0.05 to 5.54) | 0.39 (0.03 to 5.06) | 0.47 |
| Wound complications treated with antibiotic | 27 (3.9) | 689 | 25 (3.5) | 724 | 0.88 (0.50 to 1.53) | 0.87 (0.50 to 1.51) | 0.61 |
There were 1088 participants who did not have a deep infection at up to 90 days; that is, they did not meet the criteria for deep infection up to 90 days and a 3-month CRF was available for review. The number and proportion of these individuals experiencing other wound healing complications between 30 and 90 days is summarised by intervention group in Table 19. Fewer wound complications were recorded at this time point. The intervention groups were again compared using mixed-effects logistic regression models, and no significant differences were identified (see Table 19).
| Wound complication | Standard dressing (N = 525) | NPWT (N = 563) | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n (%) | Total (N) | n (%) | Total (N) | Raw | Adjusted | ||
| Red and inflamed | 28 (5.3) | 525 | 24 (4.3) | 563 | 0.79 (0.45 to 1.38) | 0.80 (0.45 to 1.39) | 0.42 |
| Swollen | 28 (5.3) | 525 | 23 (4.1) | 563 | 0.76 (0.43 to 1.33) | 0.75 (0.43 to 1.33) | 0.33 |
| Painful/tender | 39 (7.4) | 525 | 32 (5.7) | 563 | 0.75 (0.46 to 1.22) | 0.75 (0.46 to 1.21) | 0.23 |
| Fluid leaking (not pus) | 15 (2.9) | 519 | 17 (3.0) | 563 | 1.05 (0.52 to 2.12) | 1.04 (0.51 to 2.13) | 0.91 |
| Wound dehisced | 2 (0.4) | 525 | 2 (0.4) | 563 | 0.93 (0.13 to 6.64) | 0.93 (0.13 to 6.67) | 0.95 |
Continuous outcomes (repeated-measures models)
For each continuous outcome (DRI, EQ-5D utility and VAS, POSAS total score and overall opinion), mean scores with SDs are reported for each intervention group at each time point (Table 20). Figure 3 provides plots of each of the continuous variables over time by intervention group. The DRI and EQ-5D variables demonstrate a trend of improvement over time, whereas the POSAS variables appear relatively constant over time. Adjusted differences between the two treatment groups at each time point were calculated using multilevel mixed-effects linear regression models. These analyses were performed for the ITT population using the available-case data set, and the results are presented in Table 20. No significant differences between the groups on any of the outcomes were identified.
| Outcome | Time point | Standard dressing | NPWT | Adjusted difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Total (N) | Mean (SD) | Total (N) | ||||
| DRI | 3 months | 51.1 (23.92) | 456 | 51.6 (23.46) | 507 | –0.01 (–2.79 to 2.78) | 1.00 |
| 6 months | 40.2 (26.73) | 432 | 40.6 (24.98) | 469 | 0.03 (–2.82 to 2.88) | 0.98 | |
| EQ-5D (utility) | 3 months | 0.5 (0.30) | 470 | 0.5 (0.29) | 528 | 0.00 (–0.03 to 0.04) | 0.84 |
| 6 months | 0.6 (0.29) | 446 | 0.6 (0.28) | 486 | 0.00 (–0.03 to 0.04) | 0.86 | |
| EQ-5D (VAS) | 3 months | 64.7 (22.78) | 478 | 64.1 (22.24) | 531 | –0.73 (–3.30 to 1.84) | 0.58 |
| 6 months | 69.4 (21.76) | 449 | 69.7 (21.15) | 489 | 0.08 (–2.57 to 2.74) | 0.95 | |
| Scar assessment total score | 30 days | 22.9 (11.65) | 607 | 21.4 (11.38) | 648 | –1.16 (–2.40 to 0.08) | 0.07 |
| 3 months | 23.4 (12.40) | 452 | 23.1 (12.87) | 513 | –0.36 (–1.73 to 1.01) | 0.61 | |
| 6 months | 21.2 (11.84) | 432 | 21.4 (12.47) | 476 | 0.15 (–1.26 to 1.55) | 0.84 | |
| Scar assessment overall opinion | 30 days | 4.6 (2.65) | 616 | 4.4 (2.65) | 657 | –0.18 (–0.46 to 0.10) | 0.22 |
| 3 months | 4.9 (2.73) | 470 | 4.7 (2.77) | 523 | –0.11 (–0.41 to 0.20) | 0.51 | |
| 6 months | 4.5 (2.69) | 437 | 4.6 (2.80) | 483 | 0.11 (–0.21 to 0.42) | 0.52 | |
FIGURE 3.
Summary plots of continuous outcome measures over time by intervention group. (a) DRI scores from pre injury to 6 months. Scores range from 0 to 100, with higher scores indicating more disability. (b) EQ-5D utility scores from pre injury to 6 months. Scores range from –0.594 to 1, with higher scores indicating better quality of life; 0 is equivalent to death. (c) EQ-5D VAS scores from pre injury to 6 months. Scores range from 0 to 100, with higher scores indicating better quality of life. (d) POSAS total scores from 30 days to 6 months. Scores range from 6 to 60 with lower scores indicating a better-healed scar. (e) POSAS overall opinion scores from 30 days to 6 months. Scores range from 1 to 10 with lower scores indicating a better opinion of the scar.



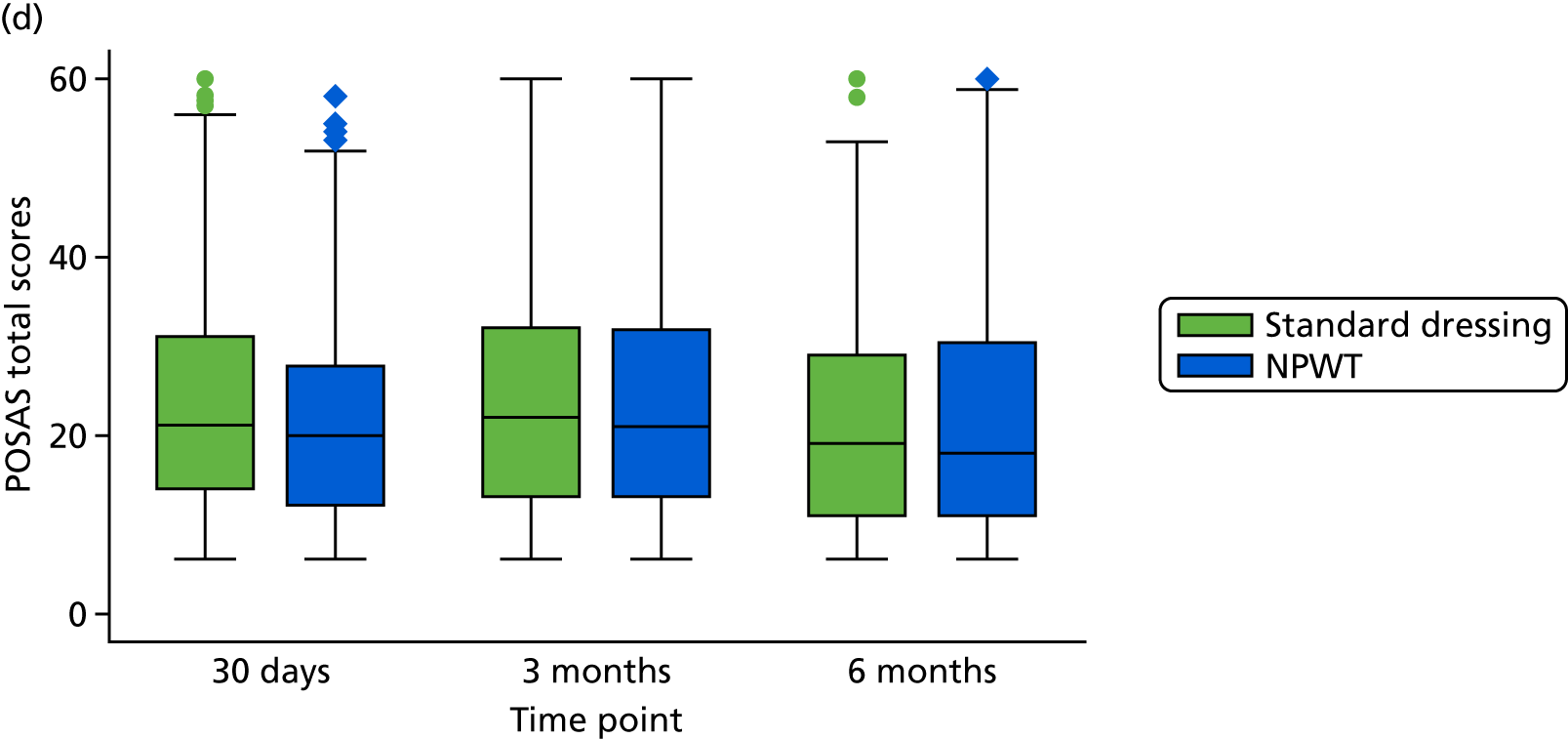

Residuals from each fitted model were used to confirm that the assumption of approximate normality was appropriate.
A sensitivity analysis of the DRI data using MI under the MAR assumption was performed; this did not change the results.
Continuous outcomes
Supplementary analyses of the DRI and EQ-5D utility variables were performed using AUC summary statistics. Parameter estimates from the mixed-effects models were used to calculate the AUC from 3 to 6 months for the DRI and from post injury to 6 months for the EQ-5D utility scores for each intervention group. This provided an overall estimate of recovery over time in each group (Table 21). The differences between the groups were also compared using a t-test; however, there was no significant difference between the two groups for either outcome.
| Outcome | Standard dressing | NPWT | Difference (95% CI) | p-value |
|---|---|---|---|---|
| AUC (95% CI) | AUC (95% CI) | |||
| DRI | 116.38 (108.84 to 123.92) | 116.41 (108.98 to 123.84) | 0.03 (–7.36 to 7.43) | 0.99 |
| EQ-5D utility | 2.89 (2.73 to 3.05) | 2.86 (2.71 to 3.02) | –0.02 (–0.17 to 0.12) | 0.73 |
Pain outcomes
Postoperative pain was assessed using a 0–10 VAS at 3 and 6 months post surgery. Median and IQR pain scores for each intervention group at each time point are reported in Table 22; pain intensity remained fairly constant over time. The assumption of normality of residuals could not be made when fitting a mixed-effects linear regression model; therefore, the two groups were compared using a non-parametric alternative: the Wilcoxon rank-sum test. There was no significant difference in pain intensity between the two treatment groups at either time point (see Table 22).
| Outcome | Time point (months) | Standard dressing | NPWT | Difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Total (N) | Total (N) | Raw | Adjusted | |||||
| Pain VAS, median (IQR) | 3 | 4.0 (2.0–5.0) | 339 | 3.0 (1.0–6.0) | 365 | – | – | 0.13 |
| 6 | 3.0 (1.0–5.0) | 368 | 3.0 (1.0–5.0) | 419 | – | – | 0.96 | |
| DN4 positive, n (%) | 3 | 109 (32.2) | 339 | 113 (31.2) | 362 | 0.96 (0.70 to 1.32) | 0.94 (0.68 to 1.31) | 0.72 |
| DN4 negative, n (%) | 3 | 230 (67.8) | 339 | 249 (68.8) | 362 | – | – | – |
| DN4 positive, n (%) | 6 | 117 (31.9) | 367 | 117 (28.3) | 414 | 0.84 (0.62 to 1.14) | 0.84 (0.61 to 1.15) | 0.27 |
| DN4 negative, n (%) | 6 | 250 (68.1) | 367 | 297 (71.7) | 414 | – | – | – |
The number and proportion of individuals experiencing neuropathic pain (i.e. with a DN4 score of ≥ 3) was reported for each treatment group (see Table 22). These were compared using a multilevel, mixed-effects logistic regression model with repeated measures (level 1) nested within participants (level 2). The model was adjusted for recruitment centre as a random effect (level 3), and for open versus closed fractures at presentation, ISS (≤ 15 vs. ≥ 16), participant age and participant gender. An interaction between treatment and time was included in the model, and ORs at 3 and 6 months are reported (see Table 22). No significant differences were identified.
These analyses were performed for the ITT population using available cases. As these outcomes were added while the trial was ongoing, the number of available cases is lower than for other outcomes.
Other complications
The number and proportion of participants experiencing other local complications (further surgery, deep-vein thrombosis, other) up to 6 months after injury is summarised by intervention group in Table 23. For each complication, the rate in the two intervention groups was compared using a mixed-effects logistic regression model. More participants in the incisional NPWT group [n = 83 (14.4%)] had further surgery than in the standard dressing group [n = 56 (10.5%)]. This was statistically significant (p = 0.04); however, the 95% CI for the OR is close to 1 (1.01 to 2.10). No other significant differences were identified.
| Local complications | Standard dressing | NPWT | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n (%) | Total (N) | n (%) | Total (N) | Raw | Adjusted | ||
| Further surgery not related to the wounda | 56 (10.5) | 534 | 83 (14.4) | 578 | 1.43 (1.00 to 2.05) | 1.46 (1.01 to 2.10) | 0.04 |
| Deep-vein thrombosis | 32 (5.6) | 573 | 31 (5.1) | 611 | 0.90 (0.54 to 1.50) | 0.94 (0.56 to 1.59) | 0.82 |
| Other complication | 235 (41.7) | 563 | 268 (42.7) | 627 | 1.04 (0.83 to 1.31) | 1.01 (0.79 to 1.28) | 0.95 |
Serious adverse events were classified as related to the surgery or unrelated to the surgery. Related SAEs occurring up to 6 months after surgery are summarised by intervention group in Table 24; no participants experienced more than one related SAE. The number of participants experiencing related SAEs was compared across intervention groups; however, there was no significant difference between the groups. Unrelated SAEs occurring up to 6 months after surgery are summarised in the same way (Table 25). A small number of participants had more than one unrelated SAE. No significant difference between the groups was identified.
| SAE | Standard dressing, n (%) | NPWT, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Raw | Adjusted | ||||
| Total number of related SAEs | 12 (–) | 9 (–) | – | – | – |
| Number of participants with related SAEs | 12 (1.6) | 9 (1.2) | 0.73 (0.30 to 1.73) | 0.71 (0.29 to 1.70) | 0.44 |
| SAE | Standard dressing | NPWT | OR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | Raw | Adjusted | ||
| Total number of unrelated SAEs | 45 | – | 40 | – | – | – | – |
| Number of participants with unrelated SAEs | 40 | 5.2 | 37 | 4.8 | 0.90 (0.57 to 1.42) | 0.89 (0.56 to 1.42) | 0.63 |
Image analysis
Participants consented to have a photograph taken of their wound at their 30-day follow-up appointment; 1123 out of 1547 (72.6%) participants did so. These images were independently assessed by two tissue viability specialists blinded to treatment allocation to establish whether or not the wounds appeared ‘healed’, and also whether or not the wounds appeared ‘infected’. A small number of photographs were of insufficient quality to be assessed; therefore, 1113 out of 1547 photographs were assessed in terms of wound healing and 1107 were assessed in terms of signs of infection. Agreement between the two tissue viability specialists was calculated after the initial assessment. Agreement for SSI was 89% and 87% for wound healing. A final agreement was reached through a joint discussion between the assessors.
Table 26 summarises the number and proportion of wounds healed by intervention group as assessed by the photographs. The proportion of wounds deemed ‘healed’ in the incisional NPWT group was 86.6% (498/575) compared with 83.8% (451/538) in the standard dressing group. No significant difference was identified. The number and proportion of wounds categorised as showing signs of ‘infection’ by this assessment is also summarised by intervention group in Table 26. The proportion of wounds showing signs of infection in the incisional NPWT group was 11.4%, versus 13.6% in the standard dressing group. When the rates were compared using a mixed-effects logistic regression model, no significant differences were identified.
| Wound assessment | Standard dressing, n (%) | NPWT, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Raw | Adjusted | ||||
| Wound ‘healed’ | 451 (83.8) | 498 (86.6) | 1.25 (0.90 to 1.74) | 1.21 (0.85 to 1.71) | 0.29 |
| Wound appears ‘infected’ | 73 (13.6) | 65 (11.4) | 0.81 (0.57 to 1.16) | 0.83 (0.58 to 1.20) | 0.33 |
This assessment of wound infection was compared to the assessment of deep infection, based on the CDC criteria, determined from the 30-day assessment. The agreement between the two variables (Table 27) was 87.8%; this was significantly higher than would be expected by chance (p < 0.001). Those individuals who met only one of the definitions of infection, that is, either the CDC criteria or the photographic assessment but not both, were further investigated. There were 102 participants who met the photographic criteria only; 62 of them had at least one sign of wound healing complications other than deep infection listed on their 30-day CRF. Discrepancies of this nature were expected because the criteria for infection based on the images were based only on superficial signs, and were less stringent than the CDC definition for deep SSI. There were also 33 individuals who had deep infection based on 30-day data in the CRFs, but not by assessment of the images. Twenty-four of these individuals had signs of infection recorded since randomisation, but these were not present on the day that the photographic image was taken, that is, the infection was treated earlier than the 30-day follow-up, such that signs would not be expected to be present or identified from the image assessment. For the other nine participants, details of the tissue viability specialists’ comments are provided in Table 28. In several cases, the nurses noted that the quality of the image was poor.
| Infection suspected from wound image | Deep infection diagnosed as per CDC definition using CRFs (n) | |
|---|---|---|
| Yes | No | |
| Yes | 36 | 102 |
| No | 33 | 935 |
| Participant | Comments |
|---|---|
| 1 | None |
| 2 | None |
| 3 | Poor-quality photo |
| 4 | Dressing still in situ |
| 5 | None |
| 6 | Poor-quality image. Slough |
| 7 | Scab on incisional line |
| 8 | Scab on incisional line. Wound edge separation |
| 9 | Several wounds, some healed others not |
The assessment of wound healing from the images was compared with the two specific wound healing variables recorded as part of the 30-day CRF. These separately asked the clinician and the patient whether or not they felt that the wound had healed. Table 29 summarises the agreement between these three variables.
| Which assessments match | n (%) |
|---|---|
| Image, clinician and patient | 843 (77.3) |
| Image and clinician | 55 (5.0) |
| Image and patient | 26 (2.4) |
| Clinician and patient | 166 (15.2) |
Infection audit results
Deep infection status based on the main trial data and deep infection status based on the audit data were compared for the sample of participants included in the audit (Table 30). Five participants were found to have a deep infection based on the review of the hospital medical record, but which occurred > 6 weeks after surgery. Four of these participants also had a deep infection based on the main trial data and, as all infections occurred within 8 weeks of surgery, we assumed that these referred to the same infection. One individual did not have a deep infection by 6 weeks based on the main trial data; however, they did have one recorded before 3 months.
| Deep infection as per audit | Deep infection as per CRF (n) | |
|---|---|---|
| Yes | No | |
| Yes | 14 | 4 |
| No | 49 | 241 |
For the purposes of this audit, the participants of interest were those who had a deep infection by only one of the two measures; that is, either in the trial data set or in the review of the hospital medical record, but not both.
Four participants had a deep infection based on the review of the medical record only. On further investigation of the medical notes, it was discovered that two of these individuals had not met the criteria for deep infection based on either data source. This highlights potential concern about the extraction of data from medical notes.
Forty-nine participants had a deep infection based on the main trial data but not based on the review of the medical record. For several of these participants [n = 24 (49.0%)], at least one wound healing complication not defined as infection was identified in the audit (Table 31); however, the remaining participants (n = 25) had no indication at all of a wound complication in their medical record. This indicates that not all signs of infection are captured by clinical notes.
| Sign of infection | n |
|---|---|
| Fluid leaking (not pus) | 10 |
| Pain or fever | 4 |
| Dehisced or deliberately opened | 14 |
Chapter 4 Health economic evaluation
Completion rate
A total of 1548 participants consented to be randomised to the WHiST trial, of whom 763 were randomised to the standard dressing group and 785 were randomised to incisional NPWT group. Those who completed at least the baseline form [n = 1540 (99.5%)] formed the baseline trial population for the economic evaluation. This included 759 participants randomised to standard dressing and 781 randomised to incisional NPWT. A complete profile of both EQ-5D and resource use values (from the NHS and PSS perspectives) from baseline to 6 months was collected for 623 participants (40.5%). Completion rate of all the health resource items for each time point is detailed in Table 32 and the completion rate of each domain of the EQ-5D-5L is presented in Appendix 1, Table 38.
| Time point | Standard dressing (N = 759), n (%) | NPWT (N = 781), n (%) |
|---|---|---|
| Baseline to discharge | ||
| Inpatient care | 739 (97.4) | 763 (97.7) |
| Antibiotics | 713 (93.9) | 742 (95.0) |
| Dressing change | 677 (89.2) | 684 (87.6) |
| Discharge to 3 months | ||
| Subsequent inpatient care | 474 (62.5) | 532 (68.1) |
| Outpatient care | 474 (62.5) | 534 (68.4) |
| Community care | 470 (61.9) | 528 (67.6) |
| Medications | 422 (55.6) | 481 (61.6) |
| PSS | 472 (62.2) | 526 (67.3) |
| Aids and adaptations | 473 (62.3) | 527 (67.5) |
| Additional costsa | 467 (61.5) | 525 (67.2) |
| Time off work | 434 (57.2) | 504 (64.5) |
| 3–6 months | ||
| Subsequent inpatient care | 446 (58.8) | 488 (62.5) |
| Outpatient care | 447 (58.9) | 488 (62.5) |
| Community care | 447 (58.9) | 485 (62.1) |
| Medications | 416 (54.8) | 427 (54.7) |
| PSS | 447 (58.9) | 484 (62.0) |
| Aids and adaptations | 447 (58.9) | 485 (62.1) |
| Additional costsa | 441 (58.1) | 483 (61.8) |
| Time off work | 377 (49.7) | 416 (53.3) |
| Complete cases – EQ-5D and resource use (NHS and PSS perspective) | ||
| Baseline to 6 months | 301 (39.7) | 322 (41.2) |
Health resource use
Table 33 shows the available-case analysis of the observed health resource use for participants at each time point by treatment group. The complete-case analysis across all time points of this health resource use is presented in Appendix 1, Table 39.
| Resource items | Standard dressing | NPWT | p-value |
|---|---|---|---|
| Hospitalisation, mean LoS in days (SD) | |||
| Intensive care | 0.76 (2.96) | 1.05 (4.53) | 0.14 |
| Acute trauma | 10.92 (9.73) | 11.74 (10.59) | 0.12 |
| Rehabilitation | 1.23 (6.00) | 1.16 (4.82) | 0.81 |
| Other | 6.72 (9.14) | 12.64 (15.95) | 0.10 |
| Antibiotic (other than prophylactic antibiotics), proportion of patients | 0.07 | 0.06 | 0.32 |
| Dressing change to, mean number (SD) | |||
| Standard | 0.79 (1.14) | 0.62 (0.82) | < 0.001 |
| NPWT | 0.05 (0.34) | 0.21 (0.68) | < 0.001 |
| Discharge to 3 months | n = 590 | n = 630 | |
| Subsequent inpatient care, mean number of days (SD) | |||
| Orthopaedics (leg) | 0.24 (2.25) | 0.97 (7.00) | 0.02 |
| Orthopaedics (other bones) | 0.05 (0.59) | 0.13 (1.38) | 0.23 |
| Rehabilitation unit | 0.37 (3.58) | 0.74 (5.25) | 0.19 |
| Other surgery | 0.01 (0.19) | 0.10 (1.06) | 0.06 |
| Other non-surgery | 0.15 (1.73) | 0.30 (4.72) | 0.48 |
| Outpatient care, mean number of visits (SD) | |||
| Orthopaedics | 1.81 (1.69) | 1.75 (1.70) | 0.60 |
| Pathology | 0.09 (0.42) | 0.15 (1.17) | 0.25 |
| Radiology | 1.19 (1.43) | 1.12 (1.35) | 0.42 |
| Physiotherapy (NHS) | 1.88 (4.81) | 1.80 (3.27) | 0.76 |
| Physiotherapy (private) | 0.68 (4.27) | 0.50 (2.00) | 0.40 |
| Emergency department (related to fracture or wound) | 0.05 (0.28) | 0.05 (0.28) | 0.74 |
| Emergency department (any other reason) | 0.05 (0.33) | 0.03 (0.20) | 0.18 |
| Other | 0.18 (1.18) | 0.12 (0.62) | 0.28 |
| Community care, mean durationa (SD) | |||
| GP surgery consultation | 6.69 (29.35) | 7.91 (32.48) | 0.53 |
| GP home visit | 2.00 (11.36) | 1.14 (6.51) | 0.15 |
| GP telephone call | 1.63 (6.69) | 2.02 (12.28) | 0.52 |
| Practice nurse | 3.30 (19.17) | 7.48 (90.27) | 0.30 |
| District nurse | 11.00 (71.74) | 11.26 (44.19) | 0.95 |
| Community physiotherapy | 31.82 (145.85) | 24.01 (115.15) | 0.35 |
| Calls to NHS Direct (or NHS 111) | 0.05 (0.32) | 0.07 (1.10) | 0.64 |
| Calls for an ambulance or paramedic | 0.07 (0.61) | 0.02 (0.22) | 0.12 |
| Occupational therapy | 3.35 (29.30) | 7.79 (60.34) | 0.13 |
| Other | 7.27 (149.89) | 1.76 (26.25) | 0.17 |
| Medications, proportion of participants | |||
| At least one type prescribed | 0.27 | 0.31 | 0.85 |
| PSS, mean durationa (SD) | |||
| Meal delivery (frozen, daily) | 0.00 (0.00) | 0.14 (3.22) | 0.32 |
| Meal delivery (hot, daily) | 0.03 (0.64) | 0.00 (0.00) | 0.29 |
| Laundry services | 0.02 (0.25) | 0.05 (1.04) | 0.54 |
| Social worker | 3.17 (34.03) | 0.54 (6.82) | 0.10 |
| Care worker/home help | 89.96 (661.58) | 237.62 (1899.64) | 0.09 |
| Other | 2.58 (46.79) | 11.09 (325.46) | 0.40 |
| Aids and adaptations, mean number (SD) | |||
| Crutch | 0.98 (1.01) | 1.05 (1.02) | 0.32 |
| Stick | 0.19 (0.49) | 0.15 (0.45) | 0.26 |
| Walking frame | 0.30 (0.52) | 0.35 (0.58) | 0.15 |
| Grab rail | 0.18 (0.56) | 0.14 (0.45) | 0.29 |
| Dressing aid | 0.11 (0.63) | 0.21 (1.55) | 0.18 |
| Long-handled shoehorn | 0.06 (0.25) | 0.08 (0.28) | 0.23 |
| Other | 0.20 (0.44) | 0.21 (0.48) | 0.55 |
| Additional cost,b proportion of participants | 0.33 | 0.38 | 0.59 |
| Time off, mean number of days (SD) | |||
| Days off work | 54.03 (42.41) | 58.11 (42.68) | 0.23 |
| 3–6 months | n = 647 | n = 672 | |
| Subsequent inpatient care, mean number of days (SD) | |||
| Orthopaedics (leg) | 0.43 (3.42) | 0.32 (2.57) | 0.60 |
| Orthopaedics (other bones) | 0.01 (0.11) | 0.09 (1.01) | 0.06 |
| Rehabilitation unit | 0.27 (5.25) | 0.38 (5.50) | 0.76 |
| Other surgery | 0.07 (0.84) | 0.10 (1.40) | 0.70 |
| Other non-surgery | 0.04 (0.51) | 0.12 (1.52) | 0.28 |
| Outpatient care, mean number of visits (SD) | |||
| Orthopaedics | 1.01 (1.45) | 1.19 (1.67) | 0.07 |
| Pathology | 0.12 (0.52) | 0.14 (0.55) | 0.54 |
| Radiology | 0.62 (1.09) | 0.75 (1.22) | 0.08 |
| Physiotherapy (NHS) | 2.51 (5.67) | 2.01 (4.06) | 0.13 |
| Physiotherapy (private) | 0.57 (2.69) | 0.69 (2.89) | 0.53 |
| Emergency department (related to fracture or wound) | 0.04 (0.26) | 0.03 (0.20) | 0.64 |
| Emergency department (any other reason) | 0.03 (0.22) | 0.02 (0.21) | 0.53 |
| Other | 0.19 (1.24) | 0.16 (0.92) | 0.72 |
| Community care, mean durationa (SD) | |||
| GP surgery consultation | 6.98 (44.92) | 6.53 (24.62) | 0.85 |
| GP home visit | 0.52 (3.75) | 0.73 (4.94) | 0.47 |
| GP telephone call | 1.49 (9.01) | 1.12 (9.02) | 0.53 |
| Practice nurse | 7.44 (127.88) | 1.97 (13.07) | 0.37 |
| District nurse | 5.49 (73.25) | 5.97 (48.40) | 0.91 |
| Community physiotherapy | 23.96 (106.09) | 18.85 (79.23) | 0.41 |
| Calls to NHS Direct (or NHS 111) | 0.04 (0.50) | 0.03 (0.39) | 0.87 |
| Calls for an ambulance or paramedic | 0.03 (0.23) | 0.02 (0.16) | 0.43 |
| Occupational therapy | 3.84 (40.85) | 3.91 (28.98) | 0.97 |
| Other | 1.48 (28.20) | 1.67 (21.13) | 0.84 |
| Medications, proportion of participants | |||
| Prescribed | 0.15 | 0.17 | 0.50 |
| PSS, mean durationa (SD) | |||
| Meal delivery (frozen, daily) | 0.00 (0.05) | 0.10 (2.27) | 0.33 |
| Meal delivery (hot, daily) | 0.00 (0.00) | 0.00 (0.00) | – |
| Laundry services | 0.03 (0.57) | 0.00 (0.00) | 0.32 |
| Social worker | 0.93 (10.91) | 4.97 (68.90) | 0.20 |
| Care worker/home help | 212.87 (2501.19) | 50.54 (543.76) | 0.18 |
| Other | 6.19 (145.73) | 16.16 (374.10) | 0.44 |
| Aids and adaptations, mean count (SD) | |||
| Crutch | 0.27 (0.78) | 0.32 (0.74) | 0.34 |
| Stick | 0.14 (0.41) | 0.12 (0.40) | 0.58 |
| Walking frame | 0.10 (0.33) | 0.10 (0.35) | 0.90 |
| Grab rail | 0.11 (0.53) | 0.13 (0.65) | 0.60 |
| Dressing aid | 0.03 (0.22) | 0.05 (0.52) | 0.34 |
| Long-handled shoehorn | 0.04 (0.20) | 0.04 (0.20) | 0.91 |
| Other | 0.06 (0.32) | 0.06 (0.27) | 0.80 |
| Additional cost,b proportion of participants | 0.20 | 0.28 | 0.01 |
| Time off, mean number of days (SD) | |||
| Days off work | 40.46 (56.71) | 48.95 (60.00) | 0.10 |
Three patients had further procedures that were captured in the 6-week CRFs. One participant in the incisional NPWT group had removal of metalwork, one in the standard dressing group had debridement and revision of internal fixation and the other one had revision of internal fixation only.
Between discharge and 3 months, there were no statistically significant differences in resource use between treatment groups in any health resource category, with the exceptions of the number of dressing changes and the number of inpatient episodes related to further orthopaedic surgery to the injured leg. This was true in both available-case and complete-case analyses. Participants randomised to the standard dressing group had more frequent dressing changes than those in the incisional NPWT group (t-test p < 0.001). The mean length of inpatient stay during the first 3 months after discharge because of the need for ‘further orthopaedic surgery to the injured leg’ was significantly longer in the incisional NPWT group (0.97 days; SD 7.00 days) than that of the standard dressing group (0.24 days; SD 2.25 days; t-test, p = 0.02). This difference was driven by a small number of participants having extended inpatient stays in the incisional NPWT group (n = 29; range 0–105 days) compared with the standard dressing group (n = 11; range 0–42 days). If log transformation was performed before the t-test to account for positive skew in the length of stay, this difference would become insignificant (p = 0.09).
Health-care costs
Table 34 summarises the mean costs of the key resource inputs associated with the trial in the available-case analysis (on all observed data) and the sensitivity analysis in which the societal perspective was considered. Appendix 1, Table 40, summarises the same cost categories but for the complete cases only.
| Cost category | Standard dressing | NPWT | Mean difference | p-value | Bootstrap 95% CI |
|---|---|---|---|---|---|
| Baseline to 6 months | |||||
| Initial intervention costa | 4774.15 (4633.18) | 5420.66 (5559.95) | 646.51 | 0.01 | 140.49 to 1166.10 |
| Subsequent inpatient care | 982.54 (7447.93) | 2108.42 (13436.28) | 1125.88 | 0.04 | 102.12 to 2250.52 |
| Outpatient care | 413.34 (549.43) | 434.89 (526.62) | 21.55 | 0.43 | –30.27 to 75.08 |
| Community care | 97.16 (299.49) | 96.28 (257.75) | –0.89 | 0.95 | –29.05 to 26.39 |
| Medications | 15.39 (125.25) | 13.28 (73.26) | –2.11 | 0.69 | –13.22 to 7.20 |
| PSS | 86.53 (925.35) | 95.32 (779.62) | 8.78 | 0.84 | –81.63 to 89.24 |
| Aids and adaptations | 90.92 (1303.61) | 53.01 (231.36) | –37.91 | 0.43 | –147.05 to 28.63 |
| Total cost, NHS and PSS | 6460.05 (9521.96) | 8221.87 (15,234.65) | 1761.81 | < 0.01 | 535.64 to 3054.11 |
| Medications (out of pocket) | 15.39 (125.25) | 13.28 (73.26) | –2.11 | 0.69 | –13.22 to 7.20 |
| Additional costb | 263.29 (1623.61) | 316.82 (1478.76) | 53.53 | 0.50 | –106.04 to 202.97 |
| Productivity loss | 1704.96 (9922.39) | 1650.04 (4671.19) | –54.91 | 0.89 | –930.24 to 614.89 |
| Total cost, societal | 8443.70 (14,266.17) | 10,202.01 (16,285.05) | 1758.32 | < 0.01 | 268.31 to 3344.51 |
| Breakdown: baseline to discharge | |||||
| Inpatient care | 4817.22 (4594.14) | 5280.90 (5550.15) | 463.68 | 0.08 | –41.67 to 975.13 |
| Antibiotics | 12.31 (110.95) | 9.69 (97.46) | –2.62 | 0.63 | –13.45 to 7.95 |
| Dressing change | 11.69 (51.96) | 35.86 (103.29) | 24.17 | < 0.001 | 16.13 to 32.80 |
| Total cost | 4834.11 (4631.24) | 5317.07 (5562.50) | 482.96 | 0.06 | –25.54 to 993.70 |
| Breakdown: discharge to 3 months | |||||
| Subsequent inpatient care | 658.29 (4947.18) | 2315.59 (14444.52) | 1657.30 | 0.01 | 464.93 to 3071.30 |
| Outpatient care | 392.15 (404.73) | 359.70 (311.08) | –32.45 | 0.16 | –77.17 to 11.31 |
| Community care | 92.37 (246.19) | 85.51 (224.49) | –6.86 | 0.65 | –36.72 to 21.64 |
| Medications | 30.81 (82.40) | 33.04 (88.16) | 2.24 | 0.80 | –15.26 to 19.19 |
| PSS | 45.27 (301.14) | 111.18 (858.88) | 65.92 | 0.10 | –4.34 to 151.86 |
| Aids and adaptations | 49.03 (236.06) | 54.35 (205.58) | 5.32 | 0.70 | –24.46 to 31.27 |
| Total cost, NHS and PSS | 1242.31 (4975.50) | 2921.63 (14,441.26) | 1679.31 | 0.01 | 505.04 to 3138.51 |
| Medications (out of pocket) | 12.55 (54.65) | 13.98 (59.54) | 1.43 | 0.70 | –6.10 to 8.68 |
| Additional costb | 302.99 (1935.80) | 313.33 (1521.49) | 10.34 | 0.93 | –222.23 to 215.22 |
| Productivity loss | 1371.08 (3578.39) | 1431.42 (3540.91) | 60.34 | 0.79 | –385.93 to 506.80 |
| Total cost, societal | 2833.39 (6804.71) | 4612.02 (14,924.19) | 1778.63 | 0.01 | 484.42 to 3302.58 |
| Breakdown: 3–6 months | |||||
| Subsequent inpatient care | 966.70 (7166.37) | 843.51 (5735.62) | –123.20 | 0.77 | –976.44 to 684.96 |
| Outpatient care | 283.66 (350.43) | 301.01 (387.22) | 17.35 | 0.47 | –29.55 to 64.79 |
| Community care | 67.30 (249.34) | 61.63 (170.27) | –5.67 | 0.69 | –34.45 to 20.51 |
| Medications | 56.42 (302.51) | 33.39 (148.48) | –23.03 | 0.47 | –91.93 to 32.34 |
| PSS | 98.62 (1126.76) | 32.68 (261.74) | –65.95 | 0.23 | –190.31 to 17.72 |
| Aids and adaptations | 101.97 (1607.47) | 26.14 (133.87) | –75.83 | 0.32 | –242.98 to 16.19 |
| Total cost, NHS and PSS | 1519.89 (7488.80) | 1263.65 (5787.87) | –256.24 | 0.56 | –1147.81 to 600.27 |
| Medications (out of pocket) | 14.84 (156.62) | 8.12 (74.35) | –6.72 | 0.42 | –24.62 to 7.51 |
| Additional costb | 130.75 (613.12) | 170.68 (879.39) | 39.92 | 0.42 | –51.12 to 142.52 |
| Productivity loss | 1754.56 (12569.29) | 1294.08 (3726.51) | –460.49 | 0.49 | –2006.70 to 532.82 |
| Total cost, societal | 3165.20 (13,951.22) | 2559.96 (7039.39) | –605.24 | 0.41 | –2096.53 to 687.58 |
In terms of the absolute cost from baseline to 6 months, the main cost driver was the initial intervention, which consisted of the cost of the dressing, orthotic/cast, initial inpatient care (i.e. hospitalisation and further surgery), antibiotics and dressing changes. The mean cost for initial intervention cost was £4774 for standard dressing and £5421 for incisional NPWT; mean unadjusted cost was £647 higher for incisional NPWT than for standard dressing (t-test, p = 0.01).
In terms of relative differences in mean cost from baseline to 6 months, the mean cost of subsequent inpatient care (or re-admission) was £1126 higher in the incisional NPWT group than in the standard dressing group (t-test, p = 0.04).
Health utilities
Table 35 shows the summary statistics of the EQ-5D utility scores for available cases across all time points by treatment group. There was no evidence of a difference in the EQ-5D utility between treatment groups at any time point and the mean QALY gain in the standard dressing group [0.41 (SD 0.24)] was also not statistically significant from that in the incisional NPWT group [0.40 (SD 0.22); t-test, p = 0.49].
| Time point | Standard dressing | NPWT | p-value |
|---|---|---|---|
| Pre injury | 0.83 (0.24) | 0.84 (0.24) | 0.89 |
| Post injury | 0.04 (0.31) | 0.01 (0.29) | 0.09 |
| 3 months | 0.49 (0.30) | 0.50 (0.29) | 0.92 |
| 6 months | 0.58 (0.29) | 0.57 (0.29) | 0.89 |
Cost-effectiveness results
Table 36 depicts the incremental cost-effectiveness results for incisional NPWT in the base-case analysis and in each of the sensitivity analyses. The probability that incisional NPWT is cost-effective at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY gained is also presented. The NMB, CEAC and cost-effectiveness plane with the confidence ellipse and the base-case analysis and in each of the sensitivity analysis is presented in Figures 4–6.
| Analysis | Incremental cost (£) (95% CI) | Incremental QALYs (95% CI) | ICER (£/QALY) | Probability of cost-effectiveness (willingness-to-pay threshold) per QALY | ||
|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||
| Base-case analysis | ||||||
| NHS and PSS perspective: imputed costs and QALYs, covariate adjusted | 2037 (349 to 3724) | 0.005 (–0.018 to 0.028) | 396,531 | 0.015 | 0.019 | 0.028 |
| Sensitivity analysis | ||||||
| (1) Societal perspective: imputed costs and QALYs, covariate adjusted | 1794 (–448 to 4036) | 0.003 (–0.022 to 0.027) | 679,482 | 0.071 | 0.077 | 0.090 |
| (2) NHS and PSS perspective: complete-case costs and QALYs, covariate adjusted | 1065 (–654 to 2784) | 0.002 (–0.026 to 0.031) | 454,903 | 0.14 | 0.15 | 0.17 |
FIGURE 4.
Base-case analysis of NPWT vs. standard dressing. (a) NMB; (b) CEAC; and (c) confidence ellipse on the cost-effectiveness plane.
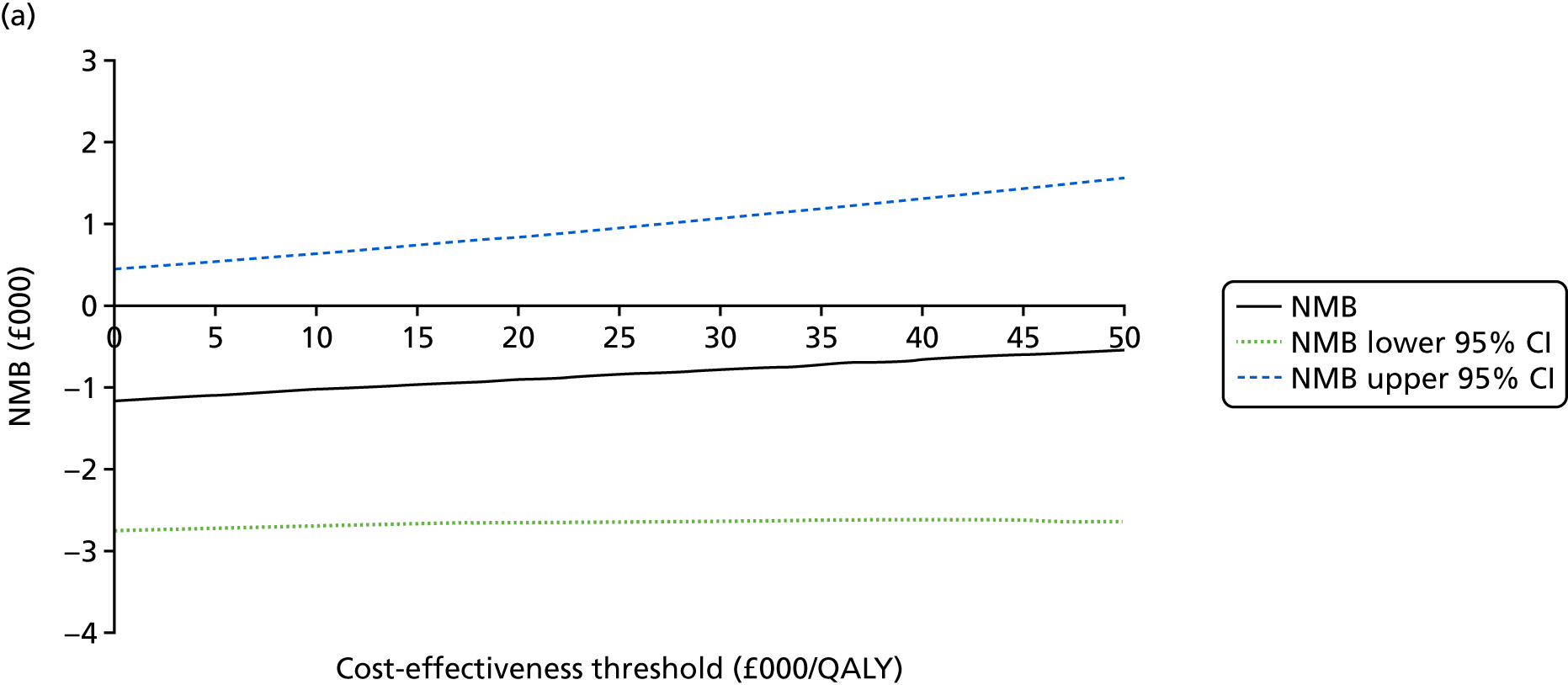
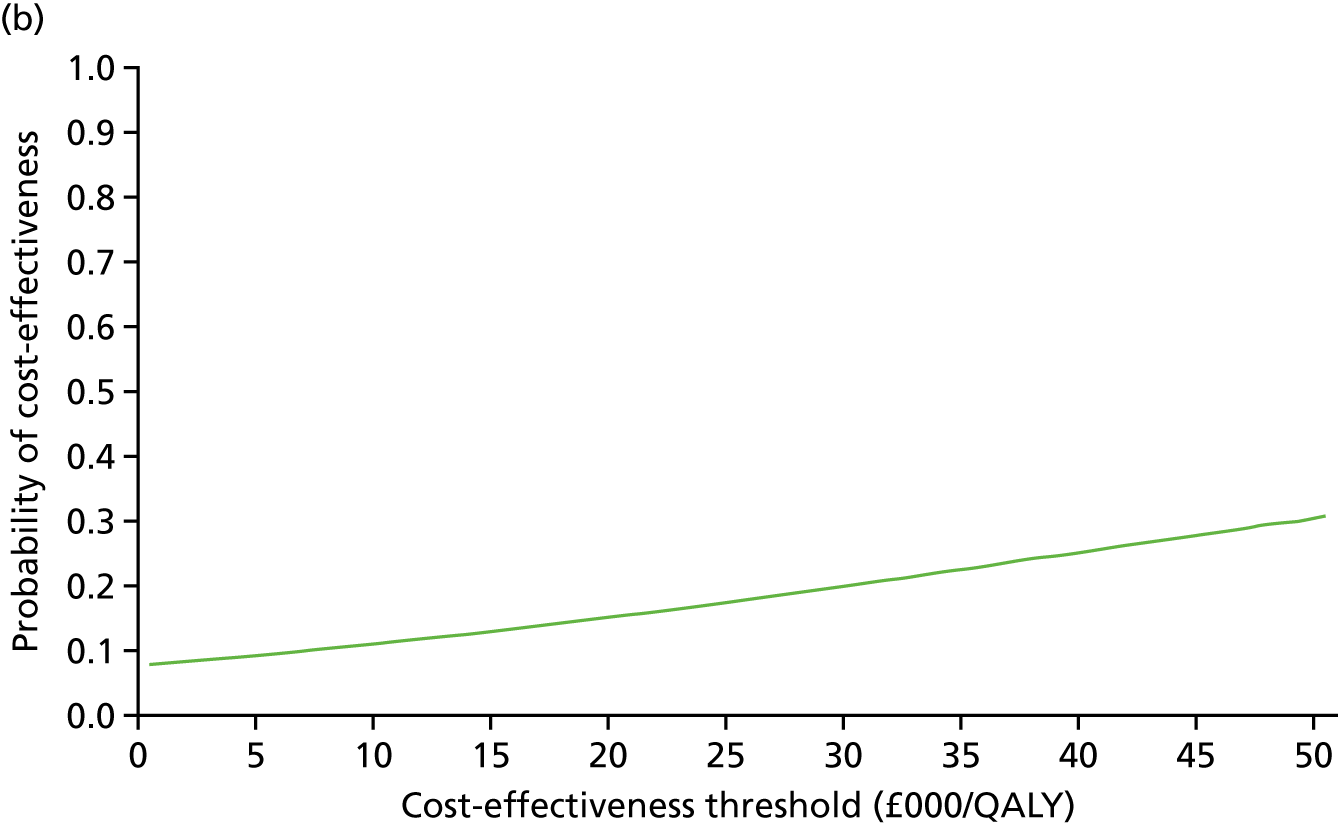

FIGURE 5.
Sensitivity analysis (societal perspective) of NPWT vs. standard dressing. (a) NMB; (b) CEAC; and (c) confidence ellipse on the cost-effectiveness plane.
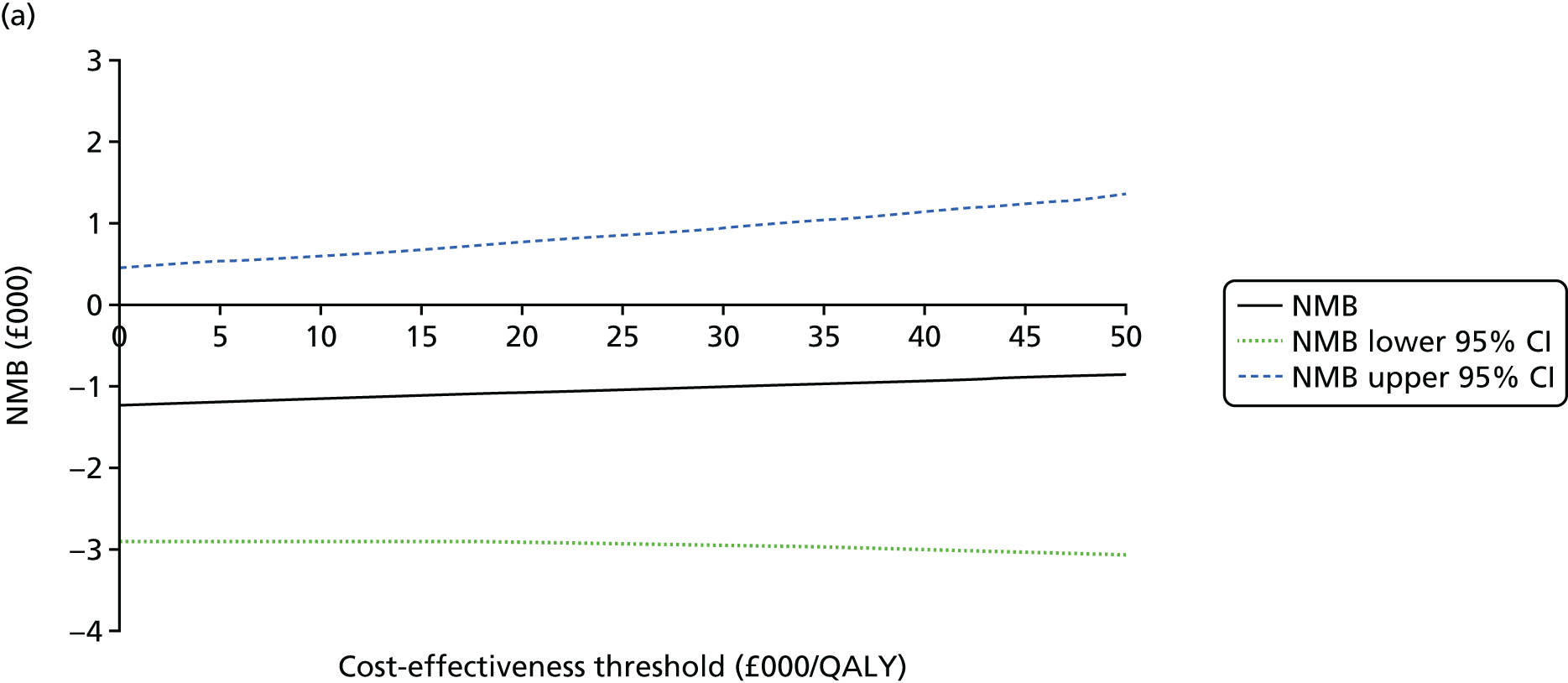


FIGURE 6.
Sensitivity analysis (complete case) of NPWT vs. standard dressing. (a) NMB; (b) CEAC; and (c) confidence ellipse on the cost-effectiveness plane.
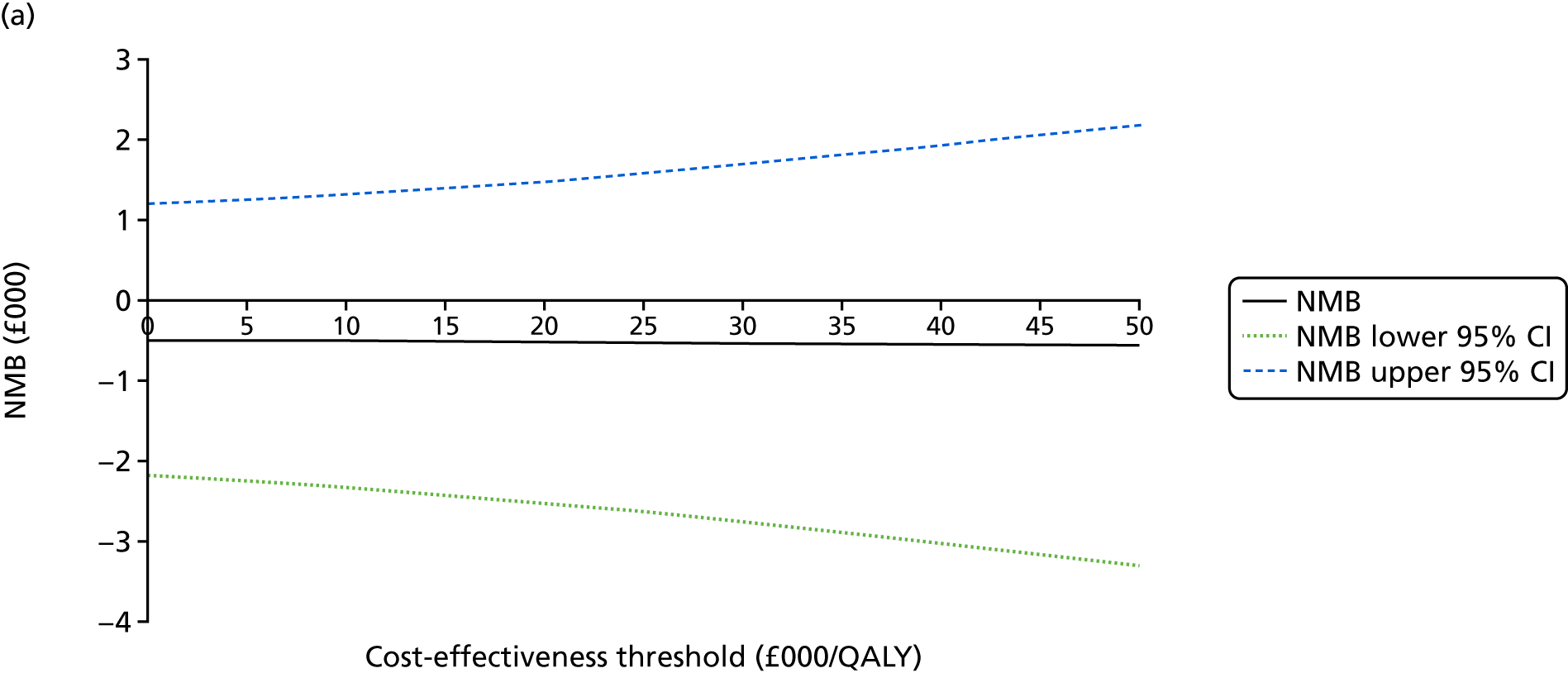
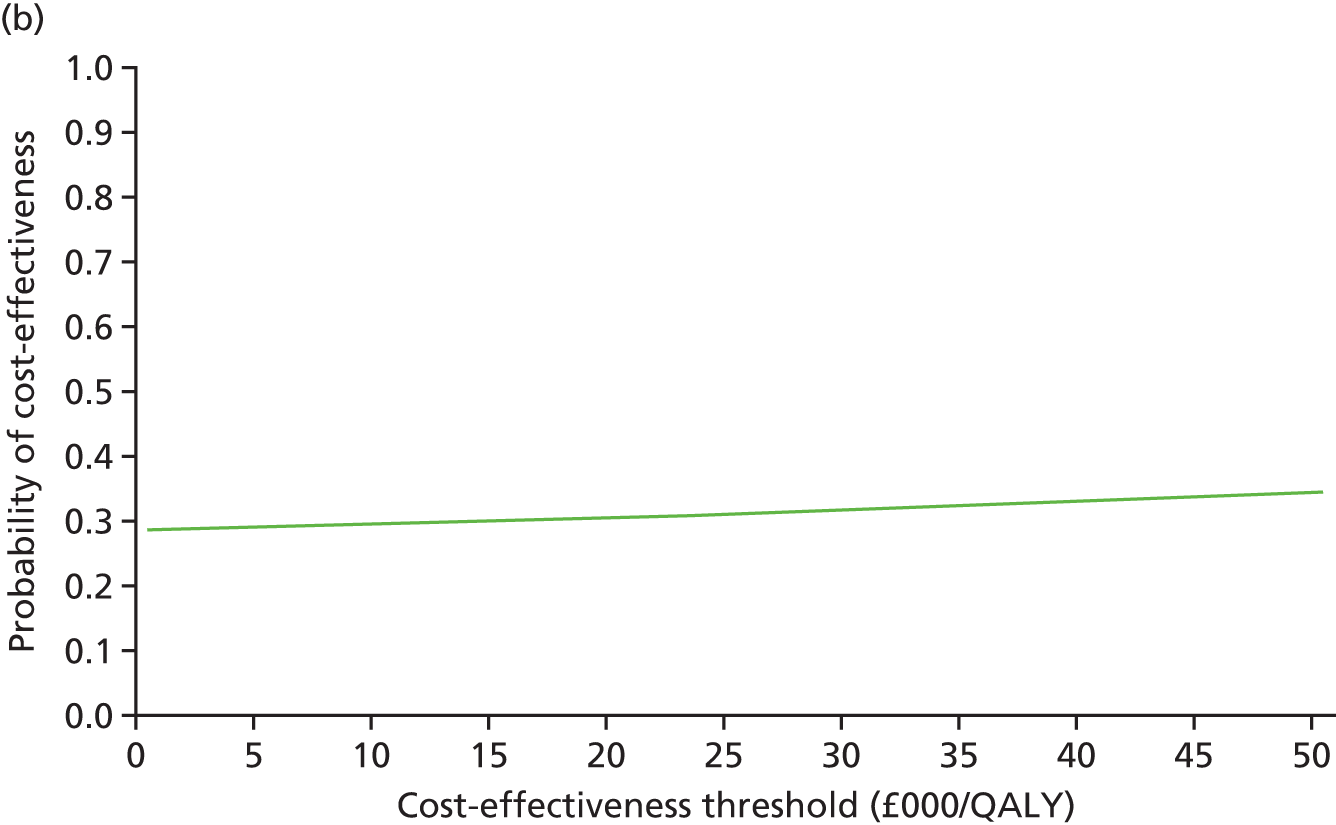
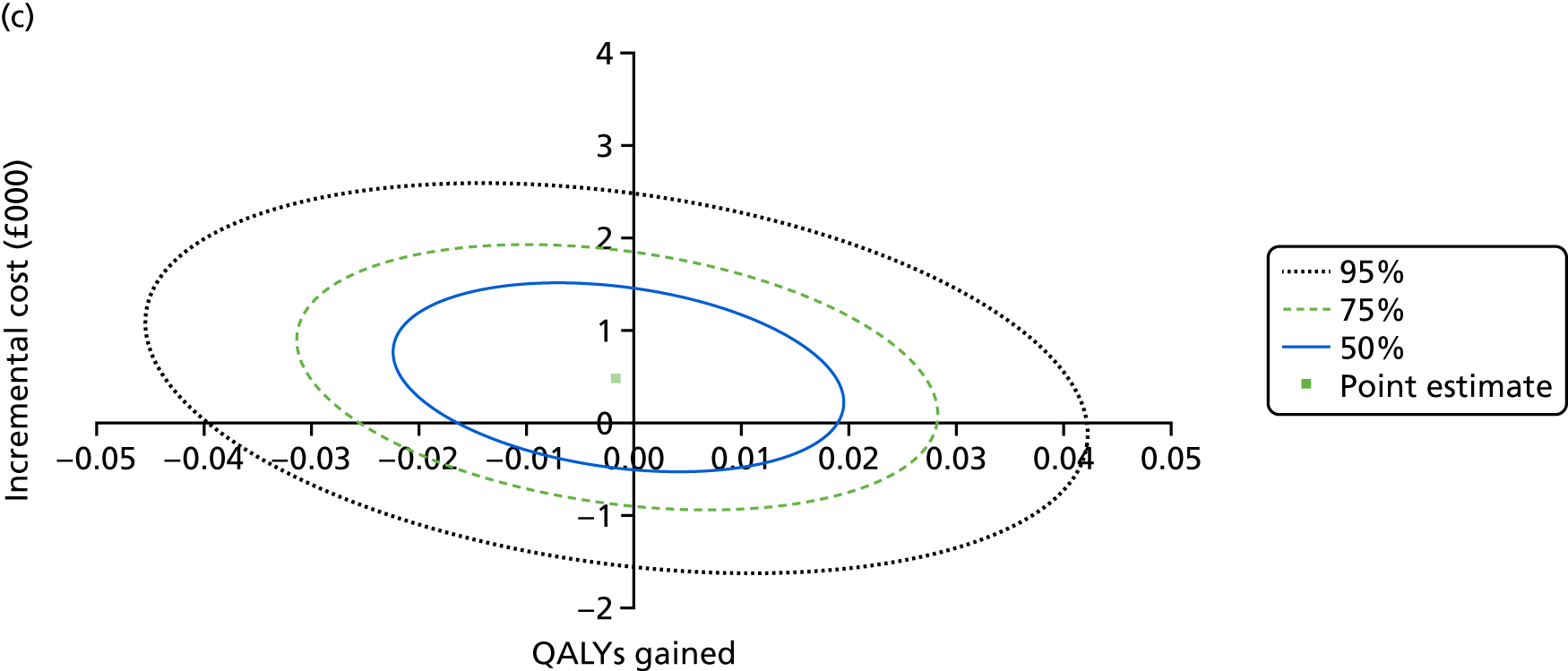
The base-case analysis, which involved imputed data, produced an ICER of £396,531 per QALY gained from the NHS and PSS perspective. Based on the assumed cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY, the probability of incisional NPWT being cost-effective ranged from 0.015 to 0.028 and its NMB was negative. Therefore, the base-case analysis indicates that incisional NPWT is highly unlikely to be cost-effective among the studied population.
Both sensitivity analyses showed similar results that supported the base-case finding. The ICER in which the societal perspective was adopted was £679,482 per QALY, whereas the ICER when the complete-case analysis was implemented was £454,903 per QALY.
Chapter 5 Discussion
Recruitment
The UK Major Trauma Network, through which this trial was delivered, is built around regional major trauma centres each with a catchment population of around 2.5 million people. Therefore, we anticipated that the number of potentially eligible patients presenting at each centre would be similar. The overall number of patients screened in the trial was in keeping with the estimated rate of recruitment at the recruitment centres. However, the number of patients actually recruited varied considerably by recruitment centre; the key determinant of recruitment was the availability of research staff. The overall rate of recruitment was a little lower than anticipated, leading to a 3-month extension of the recruitment window.
Some patients (1509) with major trauma to the lower limb were excluded according to the pre-determined eligibility criteria. Approximately 15% of the excluded patients presented to the recruitment centre more than 72 hours after their injury. This exclusion criterion was based on the current UK Major Trauma Network guidelines, which dictate that, if required, patients with major trauma are transferred to a major trauma centre within 72 hours of their injury. 50 Clearly this was not always possible, as 269 patients failed to meet this criterion. As anticipated, the more common reason for exclusion (47%) was that the patient did not have a lower-limb fracture requiring a surgical incision. As well as the small number of patients with major trauma who were treated non-operatively, surgeons used this criterion in which the surgical wound was too small/it was impossible to apply incisional NPWT, most commonly because of the use of an external fixator or a percutaneously inserted intramedullary nail. The other key exclusion criterion was that the patient had an open fracture of the lower limb that could not be closed primarily, which was used 518 times (34%). In contrast, 288 fractures that were open at presentation could be closed primarily at the first debridement and were included in the WHiST trial. This is in keeping with the findings of the WOLLF trial of open lower-limb fractures, which found that 60% of open fractures could not be closed at the time of the first surgical debridement. 51,52
Reassuringly, only 274 potentially eligible patients prospectively declined to take part in the trial, the most common reason being that the patient did not want to be part of a research project.
All but one of the English major trauma centres agreed to take part in the WHiST trial, but inevitably some individual surgeons were more committed to randomising patients than others. Fortunately, in terms of the external validity of the trial, only 162 patients were excluded because of surgeon preference for one dressing or another.
Overall, we can be confident that the patients who took part in the trial are broadly representative of those patients with major trauma to the lower limb requiring surgical fixation of a fracture.
Many patients with major trauma are operated on immediately or on the next available trauma operating list. Some patients are unconscious, all were distracted by their injury and its subsequent treatment and all had large doses of opiates for pain relief, potentially affecting their ability to process trial-related information. Similarly, patients’ next of kin, carers and friends are often anxious at this time and may have difficulty in considering the large amounts of information that they are given about the injury and plan for treatment. In this emergency situation, the focus was on obtaining consent for surgery (when possible) and informing the patient and any next of kin about immediate clinical care. As anticipated, a large proportion of patients (42%) lacked capacity to prospectively give consent to enter the trial and were entered under consultee agreement in a process advised by the Research Ethics Committee. In setting up this sort of emergency trial, there is always a concern that patients recruited under consultee agreement will subsequently decline to consent to continue their participation when they have regained capacity. It is testament to patients’ enthusiasm for taking part in research, even under very difficult circumstances, that only 58 patients declined to consent after having been randomised into the trial. 53 Only 19 patients were found to be ineligible after being randomised, most commonly because of permanent cognitive impairment that could not have been diagnosed before surgery. We can therefore be very confident that the trial results are not affected by the small number of patients who were not able to take part post randomisation.
Participants and interventions
In total, 1548 participants consented to take part in the trial. However, 27 patients were found to be ineligible after randomisation, most commonly as a result of having surgical approaches (intrapelvic approaches for acetabular fractures) that were excluded from the trial or because they had an open fracture wound that could not be closed primarily. Although a change in the surgical plan is not uncommon – responding to unexpected findings or events during surgery – the protocol for the WHiST trial was designed to account for this by having the randomisation performed at the end of the surgical procedure but before wound closure. It seems that some surgeons randomised the participant earlier in the operation. This is understandable, given the preference for surgeons to have the dressings out ready for wound closure, but was in breach of the trial protocol. These 27 participants were included in the ITT analysis but excluded from the PP analysis.
Of the 1548 participants, 763 were randomised to standard dressings and 785 to incisional NPWT. We anticipated that some patients would cross over following randomisation and, indeed, 92 patients received standard dressings despite being randomised to incisional NPWT, and eight patients received incisional NPWT despite being randomised to standard dressings. The imbalance was mostly (47 cases) because of human/administrative errors: the ‘newer’ incisional NPWT intervention was forgotten in the busy emergency operating theatre environment and the more commonly used standard dressing was used as a ‘default’ position. These crossovers were unlikely to create a systematic error in the trial. However, 40 crossovers (seven from the standard dressing group and 33 from the incisional NPWT group) were because of surgeon preference. This imbalance could potentially pose a threat to the integrity of the trial but, because the number of such crossovers was small in the context of a trial of 1548 participants, this is very unlikely to have affected the results, particularly as the loss to follow-up for the primary end point was much less than anticipated.
Compliance with the interventions was good. In particular, the median period of application of the incisional NPWT dressings was 7 days, which is in keeping with the manufacturer’s instructions. Only 10.8% of patients had their dressing changed from incisional NPWT to standard dressings before 7 days, most commonly because of technical issues with the dressing or patient compliance.
The two groups were well balanced in terms of both injury factors (open vs. closed fractures at presentation and ISSs of ≤ 15 or ≥ 16), baseline patient factors and surgical interventions. As expected, there was a bimodal distribution according to age, with younger patients sustaining their fractures due to high-energy trauma and older patients with osteoporosis sustaining more injuries due to falls from a standing height. Falls and road traffic accidents were the most common mechanisms of injury, in keeping with the epidemiology of major trauma to the lower limbs. The tibia and femur were the most commonly injured bones; therefore, it is no surprise that intramedullary nails, and plates and screws, were the most common methods of fracture fixation. External fixation was rare in this population with < 2% of patients having either half-pin or fine-wire frames. Wound closure was roughly equally split between interrupted sutures, skin clips and subcuticular sutures. In the standard dressing group, 50% of participants had impermeable dressings, 25% had permeable or semipermeable dressings and 25% had their dressing either not recorded or described as ‘other’. Of note, 23% of patients had a plaster cast of orthotic applied at the end of the operation. These devices inevitably covered the incisional NPWT dressings, which may have reduced the clinical effectiveness of the evaporation of fluid through the semipermeable surface of the dressing. However, plaster casts and orthotics are commonly used after surgery for major trauma of the lower limbs and so this reflects normal clinical practice.
In terms of the primary outcome measure of deep infection at 30 days, there were 98% complete data in both groups; considerably more than the 80% accounted for in the sample size calculation and ensuring that the trial had > 90% power.
For secondary outcome measures, 84% of participants completed their 3-month questionnaire and 90% completed their 6-month questionnaire, thereby ensuring adequate power for the secondary analyses as well. Of those patients who did not complete the 6-month follow-up for the trial, 91 participants withdrew and 37 patients had died.
Results
Primary outcome
This trial showed no evidence of a difference between standard dressings and incisional NPWT in the management of major trauma surgical wounds of the lower limb. The rate of deep infection at 30 days was 6.7% (50/749) in the control group and 5.8% (45/770) in the incisional NPWT group (ITT OR 0.87, 95% CI 0.57 to 1.33; p = 0.52).
Similarly, there was no evidence of a difference in the PP analysis (analysis by treatment received). The number of missing data at the primary end point was very low (< 2%); therefore, a sensitivity analysis using MI for missing data also showed no evidence of a difference between the two groups in terms of the rate of deep infection.
Secondary outcomes
After the start of the trial, the CDC changed their definition of deep infection to include any SSI up to 90 days after surgery rather than the original 30 days. To facilitate future evidence synthesis, we prespecified a secondary analysis using this 90-day time point. The rate of deep infection reported at 90 days was much closer to that anticipated at the beginning of the trial (in keeping with the existing literature for major trauma), with 13.2% (78/590) in the control group and 11.4% (72/629) in the intervention group. However, there was no evidence of a difference between groups (OR 0.84, 95% CI 0.59 to 1.19; p = 0.32).
As part of the trial data set, we recorded details of any other wound healing complications that did not fulfil the CDC criteria for a deep SSI. There was no evidence of difference in any of these complications. Interestingly, despite the wounds not fulfilling the criteria for a deep infection, 3.7% of patients were treated with antibiotics. This number is low in comparison with other related studies,54 but still suggests that clinicians are failing to follow guidance regarding the empirical use of antibiotics for surgical wounds.
In keeping with the primary analysis of deep infection, this trial found no evidence of a difference in the patients’ self-reported DRI nor in their health-related quality of life at either 3 months or 6 months. Other trials51,54 of serious lower-limb injuries have found that patients continue to report serious disability and poor quality of life even 6 months after the surgical fixation of their fractures. Similarly, the WHiST trial found that patients reported a 40-point disability score (in which 100 points represents complete disability) and similar loss of quality of life at 6 months. This is further powerful evidence of the very severe and lasting effects of these injuries on patients.
Chronic pain has previously been described as a substantial problem for patients after major trauma injuries to the lower limb,55 and this was also the case in the WHiST trial. Visual analogue pain scores were still 3/10 (in which higher scores indicate more pain) at 6 months in both groups of patients. Neuropathic pain is particularly difficult to manage with standard analgesia. The proportion of patients reporting neuropathic pain according to the DN4 was 32% (117/367) in the standard dressing group and 28% (117/414) in the incisional NPWT group (p = 0.27).
The trial found no evidence of difference in the rate of deep-vein thrombosis between the two groups of patients, nor in the rate of other local complications. There was a statistically significant difference in the rate of further surgery to the broken bone (not related to the wound), with more patients requiring further surgery in the incisional NPWT group, but the OR was close to 1 (95% CI 1.01 to 2.10).
There was no evidence of a difference in the subjective POSAS at any time during the 6 months of follow-up.
The WHiST trial also offered the opportunity to evaluate the use of photographs as an objective assessment of wound healing. Of the 1548 participants, 1123 had a photograph of their wound taken at, or shortly after, 30 days. These were assessed by independent tissue viability specialists who were blinded to the treatment allocation. There was no evidence of a difference between the two groups in terms of the proportion of wounds deemed ‘fully healed’: 84% in the standard dressing group and 87% in the incisional NPWT group. Nor was there a difference in the proportion of wounds showing signs of infection: 13.6% in the standard dressing group versus 11.4% in the incisional NPWT group. Although the agreement between the primary outcome measure of infection using the CDC criteria and this photographic assessment was significantly higher than would be expected by chance (p < 0.001), this estimate of infection using photographs (12%) was twice as high as that reported using the CDC criteria (6%). Photographic assessment is an attractive method, in that the images can be assessed in a standardised objective fashion, but it has several drawbacks. The tissue viability specialists commented that their ability to assess for signs of infection was very dependent on the quality of the photographs. In some cases, it was difficult to distinguish between ‘slough’ on the wound surface and signs of infection, which may have led to an overestimation of the rate of infection. The photographs provide only a two-dimensional assessment, which makes it difficult to appreciate swelling and fluctuance around the wound. Also, the photographs clearly provide only a single moment in time. It is possible that the patient had a clear deep infection in week 1, which, if treated successfully, may have all but resolved at 30 days. In summary, photographs provide an objective means of assessing aspects of wound healing but are probably not sufficiently reliable to replace clinical assessment in the diagnosis of infection.
Infection audit
The WHiST trial DSMC recommended that the trial team audit the medical records of patients with a CDC diagnosis of infection in the trial data set and a random sample of those who did not have a diagnosis of infection. The audit took place when the first 1000 patients had been recruited to the trial and reached their 30-day assessment.
For the large majority of patients, there was agreement between the trial data set and the routine hospital medical record. At the first review of the medical records, four further potential cases of deep infection were highlighted that had not been captured in the trial data set. However, a further detailed review of the medical records indicated that two of these patients did not fulfil the criteria for deep infection. Conversely, 49 patients were identified as having met the criteria for a deep SSI according to the trial data set, but were not identified in the routine medical record. A further detailed review of these medical records by the local clinicians showed that half (n = 24) of these participants had at least one documented sign of deep infection in the medical record but did not have documentation that fulfilled all the criteria for the CDC definition. For example, there was documentation of gaping at the wound edges but not of associated pain and/or fever. The other 25 patients had no documentation of a deep infection in the medical record at all, despite having a clear diagnosis of infection in the trial data set, for example, pus leaking from the wound at the 30-day follow-up appointment.
In summary, this audit found 49 cases in which signs and symptoms of infection were poorly documented, or indeed completely absent, from the routine medical record. These cases would have been missing from the trial data set if the medical record alone was used to collect evidence of infection. In the trial data set, the research associates reviewed the medical records of each participant and also spoke to and assessed the patient directly regarding their wound healing. A higher rate of infection was therefore documented in the trial data set. Even so, two cases of deep infection that were identified from the routine medical record were ‘missed’ in the trial data set.
Clearly, there is no perfect system for collecting evidence of infection in surgical wounds. A degree of caution is warranted in interpreting the audit data as the interpretation was not part of the pre-planned analysis of the WHiST trial but was implemented during the trial on the advice of the DSMC. However, the audit does suggest that a review of the routine medical record alone is likely to lead to an underestimation of the rate of deep infection in surgical wounds.
This has implications for the interpretation of reports of infection using routinely collected data. In the context of a randomised trial, it could be argued that any difference between interventions would still be detected using routine data, if the trial was large enough, the assumption being that any underestimation of the rate of deep infection would be equally balanced in the two groups of participants. However, in the case of observational studies, the use of routine data in isolation is likely to lead to an underestimation of the incidence of important complications such as infection.
Health economic evaluation
The health economic evaluation in the WHiST trial indicates that incisional NPWT is highly unlikely to be cost-effective among patients with a surgical incision for major trauma of the lower limb. This finding was consistent across the pre-planned sensitivity analyses.
The only statistically significant differences in resource use relate to the number of dressing changes, which were higher in the standard dressing group, and the length of unplanned re-admissions for orthopaedic surgery to the injured leg, which was higher in the incisional NPWT group.
In keeping with the manufacturer’s instructions, which advise that, when possible, the incisional NPWT dressing is left in situ for the first 7 days, the number of dressing changes in the standard dressing group was statistically higher than that in the incisional NPWT group. However, given that the unit cost of incisional NPWT is almost one hundred times higher than that of the standard dressing, this did not make a significant difference to the overall cost.
Based on the base-case analysis, incisional NPWT involved substantially higher costs (£2037) per patient than a standard dressing from the NHS and PSS perspective. However, this difference in costs is largely driven by differences in re-admission cost between discharge and 3 months because of the significantly longer length of stay for a small number of patients in the incisional NPWT group. Although it is plausible that the type of wound dressing may have altered the rate of SSI, leading to a difference in re-admission for wound healing complications, the recorded re-admissions were actually for orthopaedic surgery to the injured limb, that is for failure of fixation or to promote fracture union. It seems very improbable that these re-admissions were related to the type of wound dressing applied, that is this difference is most probably due to chance.
There was no meaningful difference in QALYs between groups and this is consistent with the main clinical findings. The ICER in the base-case analysis was £396,531 per QALY gained, which indicated that incisional NPWT had higher costs and slightly better outcomes than standard dressing. Given its substantial extra cost, incisional NPWT is highly unlikely to be cost-effective under commonly assumed thresholds.
Limitations
Recruiting patients to clinical trials in the context of urgent surgery is difficult. A concern before this trial started was that patients and surgeons would not be willing to take part. However, only 274 potentially eligible patients prospectively declined to take part in the WHiST trial, whereas 1548 did take part. Similarly, only 58 patients declined to consent having been randomised to the trial under consultee agreement. Therefore, the trial is likely to be strongly representative of the population of patients having surgery for fractures associated with major trauma to the lower limb. A further 162 patients were excluded because of surgeon preference for one dressing or another. This creates a selection bias in the trial but, again, these numbers are small and unlikely to affect the external validity of the results.
A further anticipated limitation was crossover from the allocated trial treatment; indeed, 100 patients did not receive their allocated intervention. As expected, when testing a relatively new intervention such as incisional NPWT, the majority of the crossovers were from the incisional NPWT group to the standard dressing group (n = 92). The number is relatively small in a trial of this size and the PP analysis, that is by the treatment given, confirmed the result of the primary analysis, that is there was no evidence of a difference between the two groups of participants.
In terms of assessing the primary outcome of infection, the event rate at 30 days was lower than anticipated during the trial development. In mitigation of this limitation, the loss to follow-up at 30 days was considerably lower than anticipated, at < 2%, so the primary end-point analysis remains robust. The secondary analysis of deep infection at up to 90 days – as per the change in the CDC criteria after the trial started – found an event rate much closer to that used in the sample size calculation for the WHiST trial. It is reassuring that there was also no evidence of a difference between treatment groups at this time point. However, this estimate at 90 days is inevitably less precise than that at 30 days. Our prespecified trial data set did not cover all the parameters that contribute to the identification of a deep infection at this time point, for example having a ‘fever of > 38 °C’ or an ‘abscess confirmed on imaging’ was not part of the data set at 90 days. Our results could, therefore, underestimate the infection rate at 90 days, although any such effect is likely to be balanced between treatment groups.
The secondary outcomes reported in the trial provide strong corroborating evidence of no difference between the two interventions in this population. The health economic analysis indicates that it is highly improbable that incisional NPWT is cost-effective in patients having surgery associated with major trauma to the lower limb, albeit with some small caveats. The mean incremental cost from baseline to 6 months (from the NHS and PSS perspective) was different when MI was used in the base-case analysis (£2037) instead of the complete-case analysis (£1065) (as depicted in Table 36). The latter analysis assumes that patients with complete data are representative of those with missing data. However, under the MAR assumption, it is the MI analysis that can produce unbiased estimates of treatment effect. 48
Chapter 6 Conclusions
Among patients with lower-limb fractures associated with major trauma, use of incisional NPWT, compared with standard wound dressing, resulted in no significant difference in the rate of deep surgical site infections. The findings do not support the use of NPWT in this setting.
Our work suggests that the use of incisional NPWT dressings in other at-risk surgical wounds requires further investigation. Future research may also investigate different approaches to reduce postoperative infections, for example the use of topical antibiotic preparations in surgical wounds and the role of orthopaedic implants with antimicrobial coatings when fixing the associated fracture.
Acknowledgements
Trial team
Trial management team
Professor Matthew Costa, chief investigator; Dr Ruth Knight and Mr Karan Vadher, trial statisticians; Mrs Susan Dutton, senior trial statistician; Dr Juul Achten, senior research fellow; Dr Julie Bruce, principal research fellow; Mrs Louise Spoors and Dr Marta Campolier, trial managers; Mr Damian Haywood, senior trial manager; Mr James Masters, specialist registrar; Dr May Ee Png and Dr Melina Dritsaki, trial health economists; Professor Jagdeep Nanchahal, consultant reconstructive plastic surgeon; Dr Jason Madan, senior health economist; Mrs Suzanne Jones, public member; Miss Bojana Selinšek, Dr Scott Parsons and Miss Cheridan Morgan, trial administrators; and Mrs Kylea Draper, Miss Alice Brealy, Miss Kinzah Abbasi, Mr Hugo Strachwitz and Miss Catherine Thompsett, data clerks.
Trial applicants
Professor Matthew Costa, chief investigator.
Co-applicants: Dr Juul Achten, Dr Nick Parsons, Dr Julie Bruce, Professor Jagdeep Nanchahal, Mr Miguel Fernandez, Mrs Suzanne Jones, Dr Jason Madan and Mr Richard Grant.
Trial steering committee
Professor Matthew Costa, chief investigator; Mr Tom Pinkney, consultant colorectal surgeon and Mr Dan Perry, consultant paediatric orthopaedic surgeon (chairpersons); Mr Tim White, consultant orthopaedic and trauma surgeon (independent member), Ms Jo Dumville (independent member); and Ms Deb Smith, lay member (independent member).
Data Safety and Monitoring Committee
Professor Lee Shepstone, Professor of Medical Statistics (chairperson); Professor Simon Donell, consultant orthopaedic surgeon (independent member); and Dr Jean Craig, research methodologist (independent member).
Statisticians
Dr Ruth Knight, Mr Karan Vadher and Mrs Susan Dutton.
Health economists
Dr May Ee Png, Dr Melina Dritsaki and Dr Jason Madan.
Programming team
Ms Lucy Eldridge, Mr Malcolm Hart, Mr Patrick Julier and Dr Simon Shayler.
Research team
Tissue viability specialists
Ms Ria Betteridge and Ms Julie Brown.
Principal investigators
Peter Hull, Simon Scott, Sharon Scott, David Melling, Javed Salim, William Eardley, Peter Giannoudis, Jitendra Mangwani, Andrew Ridick, Paul Harnett, Edward Mills, Mike Reed, Ben Ollivere, Xavier Griffin, Mark Brinsden, Ravichandran Karthikeyan, Peter Bates, Benedict Rogers, Haroon Majeed, Damian McClelland, Sharad Bhatnagar, Caroline Hing, Rajarshi Bhattacharya, Usman Butt, George Cox and Khitish Mohanty.
Associate principal investigators
Majid Chowdhry, Sharon Scott, David Melling, Hermant Sharma, Alex Sims, Paul Baker, Ruth Varrall, Edward Massa, Ines Reichert, George Kleftouris, Hany Saleeb, Paul Harwood, Brett Rocos, Edward Hollowry, Ian Baxter, Mich Pennison, Michael Petzle, Paul Brewer, Tanvir Khan, Tim Coughlin, Matthew Baldwin, Paul Guyuer, Siddhant Kadoor, Nima Heidari, David Crone, Richard Freeman, Andreas Fontalis, John Dabis, Michalis Kaminaris, Graham Lawton, Majed Nayjar, Richard Unsworth, Tanya Tordoff, Tom Collins, Jonathan Young, Robert Jordan, Tarek Boutefnouchet, Bob Jennings, Christopher Jordan, Laura Beddard, Gareth Roberts, James Lewin, Lars Tiesl and Manhal Yasseen.
Research associates
Leila Dehghani, Simone Hargreaves, John Moorehead, Kim Dearnley, Lisa Wilson, Alanna Milne, Charlotte Bousted, Juliet James, Abdulkerim Gokturk, Marta Santamaria, Bernadette Cook, Mehvish Hussain, Tracey Lee, Tracy Bear, Elisabeth Barnett, Katherine Coates, Lizzy Shaw, Lucille Hooper, Ruth Halliday, Steven Barnfield, Carol Peel, Helen Ruth Foot, Joanne Badlok, Julie Walker, Rachel Sellars, Chris Herriott, Christine Dobb, Deborah Bunn, Elizabeth Corbishley, Helen Shirley, Kerry Third, Charlotte Bentley Jess Nightingale, Doreen Muller, Kathryn Lewis, Maria Mestre, Martin Austin, Sangeetha Prasath, Yuhan Zhang, Zoey Warnok, Rosalyn Squire, Laura Mee, Liesl Despy, Catherine Hilton, Peter May, Shanaz Ahmad, Sophie Young, William Stephens, Holly Maguire, Jonathan Sweetman, Francis Alepo, Holly Maguire, Ida Marje Ponce, Joanne Hiden, Nenette Abano, Racquel Carpio, Susan Lyjko, Donna Simpson, Karen Smith, Stuart Watson, Alexandra Ball, Elizabeth Cruddas, Isabel Carballo Horton, Jocelyn Nocerino, Kameka Angulo, Sherrin Gotru, Isabella Wilkes, Jo-Anna Conyngham, Karolina Zimmerman, Louise Young, Anne Keen, Zin Nwe Naing, Greg Scott, Helen Sharples, Joanna Teuke, Kerri McGown, Sylvia Turner, Charlie Smith, David Baker, Dima Ivanova, Maria Letts, Heather Javis and Matthew Williams.
Other acknowledgements
The WHiST trial was supported by the NIHR Oxford Biomedical Research Centre.
Smith & Nephew plc provided incisional NPWT dressings (PICO Single Use Negative Pressure Wound Therapy System) to recruiting centres for the purposes of the trial, but had no part in the design, conduct or reporting of the trial.
Contributions of authors
Matthew L Costa (https://orcid.org/0000-0003-3644-1388) was the chief investigator. He had clinical responsibility for the trial, developed the protocol, and was involved in the trial conception and design, and the writing and reviewing of the report.
Juul Achten (https://orcid.org/0000-0002-8857-5743) developed the protocol, was a member of the TMG and was involved in the trial conception and design, and the writing and reviewing of the report.
Ruth Knight (https://orcid.org/0000-0001-6810-2845) was a member of the TMG, was responsible for the statistical analysis of the trial and was involved in writing and reviewing the report.
May Ee Png (https://orcid.org/0000-0001-5876-9363) was a member of the TMG, was responsible for the health economics analysis and was involved in writing and reviewing the report.
Julie Bruce (https://orcid.org/0000-0002-8462-7999) developed the protocol, provided quality assurance of the photographic review, was a member of the TMG and was involved in reviewing the report.
Susan Dutton (https://orcid.org/0000-0003-4573-5257) developed the protocol, was a member of the TMG, was responsible for the statistical analysis of the trial and was involved in writing and reviewing the report.
Jason Madan (https://orcid.org/0000-0003-4316-1480) developed the protocol, was responsible for the health economics analysis, was a member of the TMG and was involved in writing and reviewing the report.
Karan Vadher (https://orcid.org/0000-0002-6287-5596) developed the protocol and was a member of the TMG.
Melina Dritsaki (https://orcid.org/0000-0002-1673-3036) developed the protocol, was a member of the TMG and was involved in reviewing the report.
James Masters (https://orcid.org/0000-0001-9663-8858) provided an independent clinical review of the outcome data and was involved in writing and reviewing the report.
Louise Spoors (https://orcid.org/0000-0003-0488-0087) was involved in trial management and was a member of the TMG.
Marta Campolier (https://orcid.org/0000-0002-7168-2534) was involved in trial management and writing and reviewing the report, and was a member of the TMG.
Nick Parsons (https://orcid.org/0000-0001-9975-888X) was involved in the trial conception and design, and reviewed the report.
Miguel Fernandez (https://orcid.org/0000-0003-1533-8752) was involved in the trial conception and design, and reviewed the report.
Suzanne Jones (https://orcid.org/0000-0001-6605-160X) was involved in the trial conception and design, reviewed the report and was a member of the TMG.
Richard Grant (https://orcid.org/0000-0001-7041-0273) was involved in the trial conception and design, and reviewed the report.
Jagdeep Nanchahal (https://orcid.org/0000-0002-9579-9411) was involved in the trial conception and design, reviewed the report, developed the protocol and provided an independent clinical review of the outcome data.
Publications
Achten J, Vadher K, Bruce J, Nanchahal J, Spoors L, Masters JP, et al. Standard wound management versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for major trauma to the lower limb – a two-arm parallel group superiority randomised controlled trial: protocol for Wound Healing in Surgery for Trauma (WHIST). BMJ Open 2018;8:e022115.
Knight R, Spoors LM, Costa ML, Dutton SJ. Wound Healing In Surgery for Trauma (WHIST): statistical analysis plan for a randomised controlled trial comparing standard wound management with negative pressure wound therapy. Trials 2019;20:186.
Costa ML, Achten J, Knight R, Bruce J, Dutton SJ, Madan J, et al. Effect of incisional negative pressure wound therapy vs standard wound dressing on deep surgical site infection after surgery for lower limb fractures associated with major trauma: the WHIST randomized clinical trial. JAMA 2020;323:519–26.
Png ME, Madan JJ, Dritsaki M, Achten J, Parsons N, Fernandez M, et al. Cost-utility analysis of standard dressing compared with incisional negative-pressure wound therapy among patients with closed surgical wounds following major trauma to the lower limb. Bone Joint J 2020;102-B:1072–81.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Audit Office (NAO) . Major Trauma Care in England: Report by the Comptroller and Auditor General 2010.
- The Trauma Audit and Research Network (TARN) . The Trauma Audit and Research Network 2015 n.d. www.tarn.ac.uk/ (accessed 25 September 2018).
- Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev 1994;23:149-54.
- Stokel EA, Sadasivan KK. Tibial plateau fractures: standardized evaluation of operative results. Orthopedics 1991;14:263-70.
- Stannard JP, Martin SL, Stannard JP, Schmidt AH, Kregor PJ. Surgical Treatment of Orthopaedic Trauma. New York, NY: Thieme; 2007.
- Mallik AR, Covall DJ, Whitelaw GP. Internal versus external fixation of bicondylar tibial plateau fractures. Orthop Rev 1992;21:1433-6.
- Koval KJ, Helfet DL. Tibial plateau fractures: evaluation and treatment. J Am Acad Orthop Surg 1995;3:86-94. https://doi.org/10.5435/00124635-199503000-00004.
- Wyrsch B, McFerran MA, McAndrew M, Limbird TJ, Harper MC, Johnson KD, et al. Operative treatment of fractures of the tibial plafond. A randomized, prospective study. J Bone Joint Surg Am 1996;78:1646-57. https://doi.org/10.2106/00004623-199611000-00003.
- Teeny SM, Wiss DA. Open reduction and internal fixation of tibial plafond fractures. Variables contributing to poor results and complications. Clin Orthop Relat Res 1993;292:108-17. https://doi.org/10.1097/00003086-199307000-00013.
- McFerran MA, Smith SW, Boulas HJ, Schwartz HS. Complications encountered in the treatment of pilon fractures. J Orthop Trauma 1992;6:195-200. https://doi.org/10.1097/00005131-199206000-00011.
- Blauth M, Bastian L, Krettek C, Knop C, Evans S. Surgical options for the treatment of severe tibial pilon fractures: a study of three techniques. J Orthop Trauma 2001;15:153-60. https://doi.org/10.1097/00005131-200103000-00002.
- MacKenzie EJ, Jones AS, Bosse MJ, Castillo RC, Pollak AN, Webb LX, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am 2007;89:1685-92. https://doi.org/10.2106/JBJS.F.01350.
- Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 2014;101:1627-36. https://doi.org/10.1002/bjs.9636.
- Stannard JP, Volgas DA, McGwin G, Stewart RL, Obremskey W, Moore T, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma 2012;26:37-42. https://doi.org/10.1097/BOT.0b013e318216b1e5.
- Webster J, Scuffham P, Sherriff KL, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 2012;4. https://doi.org/10.1002/14651858.CD009261.pub2.
- Crist BD, Oladeji LO, Khazzam M, Della Rocca GJ, Murtha YM, Stannard JP. Role of acute negative pressure wound therapy over primarily closed surgical incisions in acetabular fracture ORIF: a prospective randomized trial. Injury 2017;48:1518-21. https://doi.org/10.1016/j.injury.2017.04.055.
- Nordmeyer M, Pauser J, Biber R, Jantsch J, Lehrl S, Kopschina C, et al. Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J 2016;13:1176-9. https://doi.org/10.1111/iwj.12436.
- Medical Research Council . MRC Ethics Series. Good Research Practice: Principles and Guidelines n.d. https://mrc.ukri.org/publications/browse/good-research-practice-principles-and-guidelines/ (accessed 15 October 2019).
- Achten J, Vadher K, Bruce J, Nanchahal J, Spoors L, Masters JP, et al. Standard wound management versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for major trauma to the lower limb-a two-arm parallel group superiority randomised controlled trial: protocol for Wound Healing in Surgery for Trauma (WHIST). BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022115.
- Knight R, Spoors LM, Costa ML, Dutton SJ. Wound Healing In Surgery for Trauma (WHIST): statistical analysis plan for a randomised controlled trial comparing standard wound management with negative pressure wound therapy. Trials 2019;20. https://doi.org/10.1186/s13063-019-3282-y.
- Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004;113:1960-5. https://doi.org/10.1097/01.PRS.0000122207.28773.56.
- Great Britain . Mental Capacity Act 2005 2005.
- Brown S, Thorpe H, Hawkins K, Brown J. Minimization – reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med 2005;24:3715-27. https://doi.org/10.1002/sim.2391.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. https://doi.org/10.1016/j.ajic.2008.03.002.
- Salén BA, Spangfort EV, Nygren AL, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol 1994;47:1423-35. https://doi.org/10.1016/0895-4356(94)90086-8.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380-7. https://doi.org/10.1016/j.pain.2007.08.013.
- Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152:14-27. https://doi.org/10.1016/j.pain.2010.07.031.
- Van den Brink M, Van den Hout WB, Stiggelbout AM, Van de Velde CJ, Kievit J. Self-reports of health care utilization: diary or questionnaire?. Int J Technol Assess Health Care 2005;35:298-304. https://doi.org/10.1017/S0266462305050397.
- Joint Formulary Committee . British National Formulary (Online) London n.d. www.medicinescomplete.com (accessed 3 January 2019).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care n.d. www.pssru.ac.uk/project-pages/unit-costs/ (accessed 12 December 2018).
- Department of Health and Social Care . NHS Reference Costs 2017 to 2018. Using Costing Information to Support Better Outcomes 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 7 January 2019).
- NHS Business Services Authority . NHS Supply Chain Catalogue 2018 19. 2018. www.supplychain.nhs.uk/ (accessed 7 January 2019).
- Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care 2010;48:96-105. https://doi.org/10.1097/MLR.0b013e3181d99107.
- Bell ML, King MT, Fairclough DL. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data: summary measures versus summary statistics. SAGE Open 2014;4. https://doi.org/10.1177/2158244014534858.
- National Institute for Health and Care Excellence . The Social Care Guidance Manual 2016. www.nice.org.uk/process/pmg10/chapter/incorporating-economic-evaluation (accessed 7 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- NHS Digital . HRG4+ 2017 18 Reference Costs Grouper n.d. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing-hrg4-2017-18-reference-costs-grouper (accessed 13 February 2019).
- Office for National Statistics . Employee Earnings in the UK: 2018 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2018 (accessed 7 January 2019).
- World Health Organization Collaborating Centre for Drug Statistics Methodology (WHOCC) . Guidelines for ATC Classification and DDD Assignment, 2019 2018.
- Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health 2014;17:445-53. https://doi.org/10.1016/j.jval.2014.04.002.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Drummond MF, Sculpher MJ, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- NHS England . NHS Standard Contract for Major Trauma Service (All Ages) n.d. www.england.nhs.uk/wp-content/uploads/2014/04/d15-major-trauma-0414.pdf (accessed 7 January 2019).
- Costa ML, Achten J, Bruce J, Tutton E, Petrou S, Lamb SE, et al. UK WOLLF Collaboration . Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb: The WOLLF randomized clinical trial. JAMA 2018;319:2280-8. https://doi.org/10.1001/jama.2018.6452.
- Costa ML, Achten J, Bruce J, Davis S, Hennings S, Willett K, et al. Negative-pressure wound therapy versus standard dressings for adults with an open lower limb fracture: the WOLLF RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22730.
- Tutton E, Achten J, Lamb SE, Willett K, Costa ML. UK WOLLF Research Collaborators . Participation in a trial in the emergency situation: a qualitative study of patient experience in the UK WOLLF trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2722-4.
- Costa ML, Achten J, Griffin J, Petrou S, Pallister I, Lamb SE, et al. FixDT Trial Investigators . Effect of locking plate fixation vs intramedullary nail fixation on 6-month disability among adults with displaced fracture of the distal tibia: the UK FixDT randomized clinical trial. JAMA 2017;318:1767-76. https://doi.org/10.1001/jama.2017.16429.
- Tutton E, Achten J, Lamb SE, Willett K, Costa ML. A qualitative study of patient experience of an open fracture of the lower limb during acute care. Bone Joint J 2018;100–B:522-6. https://doi.org/10.1302/0301-620X.100B4.BJJ-2017-0891.R1.
- Management Sciences for Health . International Medical Products Price Guide n.d. http://mshpriceguide.org/en/home/ (accessed 7 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- The Physiotherapy Centre . Prices n.d. www.thephysiocentre.co.uk/how_much/ (accessed 7 January 2019).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Financial Times . NHS to Trial Artificial Intelligence App in Place of 111 Helpline 2017. www.ft.com/content/aefee3b8-d1d8-11e6-b06b-680c49b4b4c0 (accessed 7 January 2019).
- NHS Business Services Authority . Prescription Cost Analysis (PCA) Data n.d. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data (accessed 7 January 2019).
- Merton Voluntary Service Council . Group Details – Meals On Wheels (LBM) n.d. www.mvsc.co.uk/civicrm/profile/view?reset=1&id=94191&gid=1/ (accessed 7 January 2019).
- North Yorkshire County Council . Paying for Care at Home n.d. www.northyorks.gov.uk/paying-care-home/ (accessed 7 January 2019).
Appendix 1 Health economic supplementary data
| Resource item | Unit type | Unit cost (£) | Source |
|---|---|---|---|
| Direct medical cost associated with trial | |||
| Wound management | |||
| NPWT | Each | 149.52 | NHS Supply Chain Catalogue 35 |
| Standard dressing | Each | 1.87 | NHS Supply Chain Catalogue 35 |
| Band 5 nurse | Hour | 37.00 | PSSRU33 |
| Back slab cast | Each | 3.31 | NHS Supply Chain Catalogue 35 |
| Full cast | Each | 3.54 | NHS Supply Chain Catalogue 35 |
| Air boot/cast | Each | 68.66 | NHS Supply Chain Catalogue 35 |
| Hospitalisation | |||
| Intensive care | Per session | 759.00 | NHS Reference Costs 2017/18 34 |
| Acute trauma | Per day | 346.00 | NHS Reference Costs 2017/18 34 |
| Rehabilitation | Per session | 374.00 | NHS Reference Costs 2017/18 34 |
| Antibiotics | |||
| Amoxicillin, 500 mg | Pack of 21 | 1.11 | BNF32 |
| Ceftriaxone, 2000 mg | Pack of 1 | 19.18 | BNF32 |
| Cefuroxime, 1500 mg | Pack of 1 | 4.70 | BNF32 |
| Chloramphenicol, 1% | Pack of 4 | 2.02 | BNF32 |
| Ciprofloxacin, 400 mg | Pack of 10 | 10.00 | BNF32 |
| Ciprofloxacin, 500 mg | Pack of 10 | 0.93 | BNF32 |
| Ciprofloxacin, 750 mg | Pack of 10 | 8.00 | BNF32 |
| Clarithromycin, 250 mg | Pack of 14 | 1.18 | BNF32 |
| Clarithromycin, 500 mg | Pack of 7 | 6.72 | BNF32 |
| Clindamycin, 150 mg | Pack of 24 | 3.90 | BNF32 |
| Clindamycin, 300 mg | Pack of 5 | 29.50 | BNF32 |
| Co-amoxiclav, 250 mg | Pack of 21 | 1.77 | BNF32 |
| Co-amoxiclav, 500 mg | Pack of 21 | 2.05 | BNF32 |
| Co-amoxiclav, 625 mg | Pack of 21 | 2.05 | BNF32 |
| Co-amoxiclav, 1000 mg | Pack of 10 | 27.50 | BNF32 |
| Co-amoxiclav, 1200 mg | Pack of 10 | 10.60 | BNF32 |
| Daptomycin, 500 mg | Pack of 1 | 88.57 | BNF32 |
| Dicloxacillin, 500 mg | Pack of 1 | 0.81a | (http://mshpriceguide.org/en/home)56 2015 £1: US$1.5618 |
| Doxycycline, 100 mg | Pack of 8 | 0.76 | BNF32 |
| Flucloxacillin, 250 mg | Pack of 28 | 1.08 | BNF32 |
| Flucloxacillin, 500 mg | Pack of 28 | 2.01 | BNF32 |
| Flucloxacillin, 1000 mg | Pack of 10 | 49.00 | BNF32 |
| Flucloxacillin, 2000 mg | Pack of 1 | 6.00 | BNF32 |
| Fucidin, 250 mg | Pack of 10 | 6.02 | BNF32 |
| Gentamicin, 80 mg | Pack of 20 | 40.17 | BNF32 |
| Gentamicin, 240 mg | Pack of 20 | 122.58 | BNF32 |
| Gentamicin, 360 mg | Pack of 20 | 174.07 | BNF32 |
| Lymecycline, 408 mg | Pack of 28 | 4.22 | BNF32 |
| Meropenem, 500 mg | Pack of 10 | 84.70 | BNF32 |
| Meropenem, 1000 mg | Pack of 10 | 169.30 | BNF32 |
| Metronidazole, 400 mg | Pack of 21 | 2.44 | BNF32 |
| Metronidazole, 500 mg | Pack of 21 | 38.39 | BNF32 |
| Phenoxymethylpenicillin, 250 mg | Pack of 28 | 0.90 | BNF32 |
| Rifampicin, 300 mg | Pack of 100 | 123.89 | BNF32 |
| Rifampicin, 600 mg | Pack of 1 | 9.20 | BNF32 |
| Teicoplanin, 400 mg | Pack of 1 | 7.32 | BNF32 |
| Vancomycin, 1000 mg | Pack of 1 | 11.25 | BNF32 |
| Tazocin, 4 g/500 mg | Pack of 10 | 15.75 | BNF32 |
| Other direct medical cost | |||
| Subsequent inpatient care | |||
| Orthopaedics: leg | LoS of 1 day | 2004.00 | NHS Reference Costs 2017/18 34 |
| Orthopaedics: other bones | LoS of 4 days | 2990.00 | NHS Reference Costs 2017/18 34 |
| Per excess bed-day | 365.00 | NHS Reference Costs 2017/18 34 | |
| Rehabilitation unit | Per session | 374.00 | NHS Reference Costs 2017/18 34 |
| Outpatient care | |||
| Orthopaedics | Per session | 124.00 | NHS Reference Costs 2017/18 34 |
| Physiotherapist (NHS) | Per hour | 38.53a | PSSRU 201557 p. 217 |
| Physiotherapist (private) | Per hour | 75.00 | The Physio Centre website58 |
| Pathology (blood tests) | Per test | 2.51 | NHS Reference Costs 2017/18 34 |
| Radiology (radiography) | Per test | 31.49 | NHS Reference Costs 2017/18 34 |
| Emergency department: fracture or wound | Per session | 136.00 | NHS Reference Costs 2017/18 34 |
| Emergency department: others | Per session | 136.00 | NHS Reference Costs 2017/18 34 |
| Community care | |||
| GP surgery | Per minute | 4.00 | PSSRU 201833 |
| GP home visit | Per minute | 5.25a | PSSRU 201059 p. 167 |
| GP telephone call | 7.1 minutes | 27.38a | PSSRU 201557 p. 177 |
| Practice nurse | Per hour | 42.00 | PSSRU 201833 |
| District nurse | Per hour | 50.70a | PSSRU 201557 p. 169 |
| Physiotherapist | Per hour | 36.83a | PSSRU 201460 p. 179 |
| Occupational therapist | Per hour | 47.00 | PSSRU 201833 |
| Calls to NHS 111 | Per call | 14.00 | Financial Times, 201761 |
| Calls for ambulance or paramedic | Per call | 7.00 | PSSRU 201833 |
| Medication | |||
| Analgesic | |||
| Algesal cream, 50 g | Each | 2.98 | PCA Oct 201862 |
| Aspirin, 75 mg | Pack of 28 | 0.61 | BNF32 |
| Co-codamol, 8 mg/500 mg | Pack of 100 | 2.63 | BNF32 |
| Co-codamol, 30 mg/500 mg | Pack of 100 | 4.03 | BNF32 |
| Solpadeine, 12.8 mg/500 mg | Pack of 100 | 3.57 | BNF32 |
| Codeine, 15 mg | Pack of 28 | 0.79 | BNF32 |
| Codeine, 30 mg | Pack of 28 | 0.94 | BNF32 |
| Codeine, 60 mg | Pack of 28 | 1.63 | BNF32 |
| Co-dydramol, 10 mg/500 mg | Pack of 30 | 0.75 | BNF32 |
| Diclofenac, 50 mg | Pack of 28 | 7.41 | BNF32 |
| Voltarol, 100 mg | Pack of 10 | 3.64 | BNF32 |
| Dihydrocodeine, 30 mg | Pack of 28 | 0.93 | BNF32 |
| Fentanyl, 25 µg/hour | Pack of 5 | 17.99 | BNF32 |
| Gabapentin, 100 mg | Pack of 100 | 2.13 | BNF32 |
| Gabapentin, 300 mg | Pack of 100 | 4.86 | BNF32 |
| Gabapentin, 600 mg | Pack of 100 | 7.25 | BNF32 |
| Ibuprofen, 200 mg | Pack of 24 | 0.93 | BNF32 |
| Ibuprofen, 400 mg | Pack of 24 | 0.80 | BNF32 |
| Ibuprofen, 600 mg | Pack of 84 | 4.07 | BNF32 |
| Naproxen, 250 mg | Pack of 56 | 2.46 | BNF32 |
| Naproxen, 500 mg | Pack of 56 | 5.19 | BNF32 |
| Buprenorphine, 5 µg/hour | Pack of 4 | 17.60 | BNF32 |
| Buprenorphine, 10 µg/hour | Pack of 4 | 31.55 | BNF32 |
| Buprenorphine, 15 µg/hour | Pack of 4 | 49.15 | BNF32 |
| Meptazinol, 200 mg | Pack of 112 | 22.11 | BNF32 |
| Methadone, 5 mg | Pack of 50 | 2.84 | BNF32 |
| Methadone, 30 mg | Pack of 50 | 139.62 | PCA Oct 201862 |
| Morphine, 5 mg | Pack of 60 | 3.29 | BNF32 |
| Morphine, 10 mg | Pack of 60 | 5.20 | BNF32 |
| Morphine, 15 mg | Pack of 60 | 9.10 | BNF32 |
| Morphine, 20 mg | Pack of 56 | 10.61 | BNF32 |
| Morphine, 30 mg | Pack of 60 | 12.47 | BNF32 |
| Morphine, 60 mg | Pack of 60 | 24.32 | BNF32 |
| Morphine, 10 mg/1 ml | Pack of 10 | 9.36 | BNF32 |
| Zomorph, 30 mg | Pack of 60 | 8.30 | BNF32 |
| Zomorph, 60 mg | Pack of 60 | 16.20 | BNF32 |
| Oxycodone, 5 mg | Pack of 28 | 12.52 | BNF32 |
| Oxycodone, 10 mg | Pack of 56 | 25.04 | BNF32 |
| Oxycodone, 15 mg | Pack of 56 | 38.12 | BNF32 |
| Paracetamol, 500 mg | Pack of 100 | 1.56 | BNF32 |
| Paracetamol, 1000 mg | Pack of 100 | 2.50 | BNF32 |
| Tramadol, 50 mg | Pack of 60 | 4.60 | BNF32 |
| Tramadol, 100 mg | Pack of 60 | 14.47 | BNF32 |
| Tramadol, 150 mg | Pack of 60 | 21.71 | BNF32 |
| Antibiotic | |||
| Amoxicillin, 250 mg | Pack of 21 | 0.97 | BNF32 |
| Amoxicillin, 500 mg | Pack of 21 | 1.11 | BNF32 |
| Ciprofloxacin, 250 mg | Pack of 10 | 0.83 | BNF32 |
| Ciprofloxacin, 750 mg | Pack of 10 | 8.00 | BNF32 |
| Clarithromycin, 500 mg | Pack of 7 | 6.72 | BNF32 |
| Clindamycin, 150 mg | Pack of 24 | 3.90 | BNF32 |
| Co-amoxiclav, 250 mg/125 mg | Pack of 21 | 1.77 | BNF32 |
| Doxycycline, 100 mg | Pack of 8 | 0.76 | BNF32 |
| Flucloxacillin, 250 mg | Pack of 28 | 1.08 | BNF32 |
| Flucloxacillin, 500 mg | Pack of 28 | 2.01 | BNF32 |
| Flucloxacillin, 1000 mg | Pack of 10 | 49.00 | BNF32 |
| Fucidin, 500 mg | Pack of 1 | 20.90 | BNF32 |
| Metronidazole, 500 mg | Pack of 21 | 38.39 | BNF32 |
| Nitrofurantoin, 50 mg | Pack of 28 | 8.23 | BNF32 |
| Phenoxymethylpenicillin, 250 mg | Pack of 28 | 0.90 | BNF32 |
| Rifampicin, 150 mg | Pack of 100 | 50.49 | BNF32 |
| Teicoplanin, 400 mg | Pack of 1 | 7.32 | BNF32 |
| Trimethoprim, 100 mg | Pack of 28 | 0.87 | BNF32 |
| Trimethoprim, 200 mg | Pack of 14 | 1.00 | BNF32 |
| Anticoagulant | |||
| Apixaban, 2.5 mg | Pack of 60 | 57.00 | BNF32 |
| Apixaban, 5 mg | Pack of 56 | 53.20 | BNF32 |
| Clopidogrel, 75 mg | Pack of 28 | 1.47 | BNF32 |
| Dabigatran, 150 mg | Pack of 60 | 51.00 | BNF32 |
| Dalteparin, 2500 units/0.2 ml | Pack of 10 | 18.58 | BNF32 |
| Dalteparin, 18,000 units/0.72 ml | Pack of 5 | 50.82 | BNF32 |
| Edoxaban, 15 mg | Pack of 10 | 17.50 | BNF32 |
| Edoxaban, 60 mg | Pack of 28 | 49.00 | BNF32 |
| Enoxaparin, 20 mg/0.2 ml | Pack of 10 | 20.86 | BNF32 |
| Enoxaparin, 40 mg/0.4 ml | Pack of 10 | 30.27 | BNF32 |
| Rivaroxaban, 10 mg | Pack of 30 | 54.00 | BNF32 |
| Rivaroxaban, 15 mg | Pack of 28 | 50.40 | BNF32 |
| Rivaroxaban, 20 mg | Pack of 28 | 50.40 | BNF32 |
| Tinzaparin, 4500 units/0.45 ml | Pack of 10 | 35.63 | BNF32 |
| Tinzaparin, 12,000 units/0.6 ml | Pack of 10 | 71.40 | BNF32 |
| Warfarin, 1 mg | Pack of 28 | 0.53 | BNF32 |
| Antidepressant | |||
| Amitriptyline, 10 mg | Pack of 28 | 0.99 | BNF32 |
| Amitriptyline, 25 mg | Pack of 28 | 0.76 | BNF32 |
| Citalopram, 10 mg | Pack of 28 | 0.89 | BNF32 |
| Citalopram, 20 mg | Pack of 28 | 1.08 | BNF32 |
| Duloxetine, 60 mg | Pack of 28 | 4.67 | BNF32 |
| Fluoxetine, 10 mg | Pack of 30 | 44.00 | BNF32 |
| Fluoxetine, 20 mg | Pack of 30 | 0.64 | BNF32 |
| Mirtazapine, 30 mg | Pack of 28 | 1.18 | BNF32 |
| Sertraline, 50 mg | Pack of 28 | 0.81 | BNF32 |
| Sertraline, 100 mg | Pack of 28 | 1.08 | BNF32 |
| Bisphosphonate | |||
| Alendronic acid, 10 mg | Pack of 28 | 1.63 | BNF32 |
| Corticosteroid | |||
| Clobetasone, 30 g | Each | 1.86 | BNF32 |
| Hypnotic/anxiolytic | |||
| Chlordiazepoxide, 10 mg | Pack of 100 | 17.80 | BNF32 |
| Diazepam, 10 mg | Pack of 28 | 0.65 | BNF32 |
| Temazepam, 10 mg | Pack of 28 | 1.46 | BNF32 |
| Zolpidem, 10 mg | Pack of 28 | 0.97 | BNF32 |
| Zopiclone, 3.75 mg | Pack of 28 | 0.88 | BNF32 |
| Zopiclone, 7.5 mg | Pack of 28 | 0.89 | BNF32 |
| Muscle relaxant | |||
| Methocarbamol, 750 mg | Pack of 100 | 13.09 | BNF32 |
| Nausea | |||
| Domperidone, 10 mg | Pack of 30 | 0.97 | BNF32 |
| Metoclopramide, 10 mg | Pack of 28 | 0.55 | BNF32 |
| Ondansetron, 4 mg | Pack of 10 | 0.85 | BNF32 |
| Supplement | |||
| Calcium, 750 mg | Pack of 112 | 2.95 | BNF32 |
| Calcium, 1000 mg | Pack of 28 | 16.07 | BNF32 |
| Calcium, 1250 mg | Pack of 100 | 9.33 | BNF32 |
| Calcium, 1500 mg | Pack of 100 | 8.70 | BNF32 |
| Glucosamine, 1500 mg | Pack of 30 | 18.20 | BNF32 |
| Iron, 210 mg | Pack of 84 | 3.50 | BNF32 |
| Vitamin | |||
| Calcitriol, 250 ng | Pack of 100 | 18.04 | BNF32 |
| Colecalciferol, 800 units | Pack of 30 | 3.60 | BNF32 |
| Colecalciferol, 20,000 units | Pack of 30 | 29.00 | BNF32 |
| Colecalciferol, 50,000 units | Pack of 10 | 36.00 | BNF32 |
| Multivitamins | Pack of 30 | 0.46 | PCA Oct 201862 |
| Thiamine, 50 mg | Pack of 100 | 4.93 | BNF32 |
| Thiamine, 100 mg | Pack of 100 | 7.15 | BNF32 |
| Wound-related | |||
| Bio-Oil® (Perrigo Company plc, Dublin, Ireland) 60 ml | Each | 3.65 | PCA Oct 201862 |
| Dermatix 15 g | Each | 16.66 | PCA Oct 201862 |
| Direct non-medical cost | |||
| PSS | |||
| Frozen meal delivery | Per meal | 3.17 | Meals on Wheels (LBM) 63 |
| Hot meal delivery | Per meal | 6.75 a | PSSRU 201460 p. 127 |
| Laundry services | Per load | 4.60 | North Yorkshire County Council,64 2019 |
| Social worker | Per hour | 60.00 | PSSRU 201833 |
| Care worker/help at home | Per hour | 27.00 | PSSRU 201833 |
| Aids and adaptations | |||
| Crutch | Each | 8.24 | NHS Supply Chain Catalogue 35 |
| Stick | Each | 4.10 | NHS Supply Chain Catalogue 35 |
| Walking frame | Each | 18.60 | NHS Supply Chain Catalogue 35 |
| Grab rail | Each | 13.80 | PSSRU 201833 |
| Dressing aid | Each | 7.26 | NHS Supply Chain Catalogue 35 |
| Long-handled shoe horn | Each | 2.72 | NHS Supply Chain Catalogue 35 |
| Indirect cost | |||
| Median wage | Per week | 569.00 | Employee earnings in the UK: 201841 (37.5 hours per week assumed) |
| Domain | Levels | Pre injury, n (%) | Post injury, n (%) | 3 months, n (%) | 6 months, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Standard dressing (n = 763) | NPWT (n = 784) | Standard dressing (n = 763) | NPWT (n = 784) | Standard dressing (n = 590) | NPWT (n = 630) | Standard dressing (n = 647) | NPWT (n = 672) | ||
| Mobility | 1 | 537 (70.4) | 562 (71.7) | 7 (0.9) | 9 (1.1) | 54 (9.1) | 81 (12.8) | 118 (18.2) | 128 (19.0) |
| 2 | 70 (9.2) | 72 (9.2) | 39 (5.1) | 25 (3.2) | 166 (28.1) | 149 (23.5) | 158 (24.4) | 154 (22.9) | |
| 3 | 52 (6.8) | 51 (6.5) | 70 (9.2) | 61 (7.8) | 139 (23.5) | 166 (26.2) | 96 (14.8) | 114 (17.0) | |
| 4 | 39 (5.1) | 52 (6.6) | 168 (22.0) | 171 (21.8) | 78 (13.2) | 93 (14.7) | 58 (9.0) | 73 (10.9) | |
| 5 | 7 (0.9) | 4 (0.5) | 418 (54.8) | 473 (60.3) | 39 (6.6) | 44 (7.0) | 20 (3.1) | 20 (3.0) | |
| Self-care | 1 | 613 (80.3) | 642 (81.9) | 66 (8.7) | 53 (6.8) | 235 (39.8) | 240 (37.9) | 268 (41.4) | 284 (42.3) |
| 2 | 36 (4.7) | 39 (5.0) | 118 (15.5) | 99 (12.6) | 117 (19.8) | 156 (24.6) | 94 (14.5) | 112 (16.7) | |
| 3 | 37 (4.8) | 39 (5.0) | 168 (22.0) | 195 (24.9) | 85 (14.4) | 98 (15.5) | 61 (9.4) | 67 (10.0) | |
| 4 | 15 (2.0) | 19 (2.4) | 162 (21.2) | 187 (23.9) | 29 (4.9) | 28 (4.4) | 22 (3.4) | 20 (3.0) | |
| 5 | 4 (0.5) | 2 (0.3) | 188 (24.6) | 204 (26.0) | 9 (1.5) | 11 (1.7) | 4 (0.6) | 7 (1.0) | |
| Usual activities | 1 | 564 (73.9) | 585 (74.6) | 10 (1.3) | 15 (1.9) | 40 (6.8) | 56 (8.8) | 107 (16.5) | 119 (17.7) |
| 2 | 47 (6.2) | 75 (9.6) | 20 (2.6) | 16 (2.0) | 134 (22.7) | 127 (20.1) | 136 (21.0) | 133 (19.8) | |
| 3 | 50 (6.6) | 38 (4.8) | 50 (6.6) | 45 (5.7) | 132 (22.3) | 158 (25.0) | 113 (17.5) | 123 (18.3) | |
| 4 | 33 (4.3) | 35 (4.5) | 123 (16.1) | 118 (15.1) | 95 (16.1) | 103 (16.3) | 51 (7.9) | 72 (10.7) | |
| 5 | 11 (1.4) | 8 (1.0) | 499 (65.4) | 544 (69.4) | 75 (12.7) | 89 (14.1) | 43 (6.6) | 43 (6.4) | |
| Pain | 1 | 489 (64.1) | 498 (63.5) | 45 (5.9) | 36 (4.6) | 40 (6.8) | 62 (9.8) | 60 (9.3) | 70 (10.4) |
| 2 | 116 (15.2) | 124 (15.8) | 122 (16.0) | 118 (15.1) | 201 (34.0) | 201 (31.8) | 187 (28.9) | 191 (28.4) | |
| 3 | 68 (8.9) | 72 (9.2) | 248 (32.5) | 289 (36.9) | 163 (27.6) | 186 (29.4) | 142 (21.9) | 161 (24.0) | |
| 4 | 26 (3.4) | 38 (4.8) | 187 (24.5) | 181 (23.1) | 55 (9.3) | 71 (11.2) | 49 (7.6) | 57 (8.5) | |
| 5 | 6 (0.8) | 8 (1.0) | 100 (13.1) | 114 (14.5) | 16 (2.7) | 12 (1.9) | 12 (1.9) | 11 (1.6) | |
| Anxiety/depression | 1 | 500 (65.5) | 539 (68.8) | 309 (40.5) | 318 (40.6) | 209 (35.4) | 235 (37.1) | 215 (33.2) | 220 (32.7) |
| 2 | 98 (12.8) | 77 (9.8) | 184 (24.1) | 175 (22.3) | 118 (20.0) | 151 (23.9) | 104 (16.1) | 134 (19.9) | |
| 3 | 61 (8.0) | 87 (11.1) | 114 (14.9) | 147 (18.8) | 97 (16.4) | 102 (16.1) | 85 (13.1) | 91 (13.5) | |
| 4 | 36 (4.7) | 28 (3.6) | 59 (7.7) | 62 (7.9) | 37 (6.3) | 29 (4.6) | 39 (6.0) | 30 (4.5) | |
| 5 | 9 (1.2) | 9 (1.1) | 35 (4.6) | 36 (4.6) | 13 (2.2) | 13 (2.1) | 6 (0.9) | 14 (2.1) | |
| Resource items | Standard dressing (n = 301) | NPWT (n = 322) | p-value |
|---|---|---|---|
| Baseline to discharge | |||
| Hospitalisation, mean LoS in days (SD) | |||
| Intensive care | 0.51 (2.17) | 0.90 (3.85) | 0.12 |
| Acute trauma | 10.27 (8.52) | 10.04 (8.69) | 0.73 |
| Rehabilitation | 0.99 (4.47) | 1.10 (4.39) | 0.76 |
| Other | 9.80 (12.65) | 14.00 (18.62) | 0.53 |
| Antibiotic, proportion of patients | 0.07 | 0.06 | 0.82 |
| Dressing change to, mean number (SD) | |||
| Standard | 0.83 (1.19) | 0.58 (0.82) | < 0.001 |
| NPWT | 0.04 (0.27) | 0.23 (0.75) | < 0.001 |
| Discharge to 3 months | |||
| Subsequent inpatient care, mean number of days (SD) | |||
| Orthopaedics (leg) | 0.15 (1.11) | 0.56 (3.46) | 0.04 |
| Orthopaedics (other bones) | 0.03 (0.42) | 0.08 (0.96) | 0.42 |
| Rehabilitation unit | 0.35 (3.94) | 0.33 (2.97) | 0.95 |
| Other surgery | 0.01 (0.17) | 0.16 (1.34) | 0.05 |
| Other non-surgery | 0.03 (0.28) | 0.01 (0.14) | 0.32 |
| Outpatient care, mean number of visits (SD) | |||
| Orthopaedics | 1.81 (1.50) | 1.82 (1.51) | 0.92 |
| Pathology | 0.06 (0.31) | 0.12 (0.53) | 0.08 |
| Radiology | 1.19 (1.26) | 1.17 (1.33) | 0.83 |
| Physiotherapy (NHS) | 1.80 (2.65) | 2.14 (3.79) | 0.20 |
| Physiotherapy (private) | 0.51 (2.23) | 0.61 (2.26) | 0.60 |
| Emergency department (related to fracture or wound) | 0.04 (0.21) | 0.04 (0.21) | 0.88 |
| Emergency department (any other reason) | 0.05 (0.33) | 0.02 (0.15) | 0.24 |
| Other | 0.26 (1.47) | 0.13 (0.70) | 0.18 |
| Community care, mean durationa (SD) | |||
| GP surgery consultation | 3.56 (11.99) | 6.77 (25.87) | 0.04 |
| GP home visit | 2.36 (13.37) | 1.43 (7.76) | 0.29 |
| GP telephone call | 1.68 (6.66) | 2.64 (14.66) | 0.29 |
| Practice nurse | 3.60 (16.30) | 10.96 (115.40) | 0.26 |
| District nurse | 10.66 (50.15) | 13.70 (47.72) | 0.44 |
| Community physiotherapy | 36.56 (163.06) | 24.75 (103.64) | 0.28 |
| Calls to NHS Direct (or NHS 111) | 0.06 (0.38) | 0.02 (0.12) | 0.05 |
| Calls for an ambulance or paramedic | 0.03 (0.24) | 0.02 (0.15) | 0.28 |
| Occupational therapy | 3.55 (30.06) | 8.68 (69.99) | 0.23 |
| Other | 10.26 (187.37) | 2.31 (32.81) | 0.21 |
| Medications, proportion of participants | |||
| At least one type prescribed | 0.30 | 0.37 | 1.00 |
| PSS, mean durationa (SD) | |||
| Meal delivery (frozen, daily) | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Meal delivery (hot, daily) | 0.05 (0.81) | 0.00 (0.00) | 0.29 |
| Laundry services | 0.03 (0.32) | 0.00 (0.00) | 0.14 |
| Social worker | 2.86 (36.24) | 0.76 (8.45) | 0.33 |
| Care worker/home help | 55.70 (327.23) | 75.65 (664.94) | 0.63 |
| Other | 1.23 (29.40) | 17.30 (415.97) | 0.33 |
| Aids and adaptations, mean number (SD) | |||
| Crutch | 1.07 (1.02) | 1.09 (0.99) | 0.80 |
| Stick | 0.22 (0.53) | 0.16 (0.49) | 0.21 |
| Walking frame | 0.34 (0.56) | 0.36 (0.58) | 0.59 |
| Grab rail | 0.16 (0.51) | 0.14 (0.45) | 0.61 |
| Dressing aid | 0.13 (0.63) | 0.13 (0.70) | 0.94 |
| Long-handled shoe horn | 0.08 (0.28) | 0.10 (0.30) | 0.32 |
| Other | 0.23 (0.48) | 0.22 (0.48) | 0.81 |
| Additional costb, proportion of participants | 0.42 | 0.45 | 1.00 |
| Time off, mean number of days (SD) | |||
| Days off work | 53.87 (40.45) | 62.08 (40.87) | 0.04 |
| 3–6 months | |||
| Subsequent inpatient care, mean number of days (SD) | |||
| Orthopaedics (leg) | 0.32 (3.35) | 0.38 (2.95) | 0.83 |
| Orthopaedics (other bones) | 0.01 (0.13) | 0.04 (0.44) | 0.24 |
| Rehabilitation unit | 0.02 (0.29) | 0.00 (0.00) | 0.32 |
| Other surgery | 0.11 (1.02) | 0.05 (0.40) | 0.39 |
| Other non-surgery | 0.04 (0.59) | 0.07 (1.18) | 0.73 |
| Outpatient care, mean number of visits (SD) | |||
| Orthopaedics | 1.00 (1.43) | 1.10 (1.62) | 0.42 |
| Pathology | 0.10 (0.44) | 0.12 (0.50) | 0.57 |
| Radiology | 0.63 (1.06) | 0.74 (1.27) | 0.23 |
| Physiotherapy (NHS) | 2.79 (6.33) | 2.15 (4.32) | 0.14 |
| Physiotherapy (private) | 0.74 (3.13) | 0.63 (2.61) | 0.64 |
| Emergency department (related to fracture or wound) | 0.03 (0.22) | 0.02 (0.19) | 0.76 |
| Emergency department (any other reason) | 0.04 (0.26) | 0.01 (0.10) | 0.05 |
| Other | 0.22 (1.43) | 0.21 (1.08) | 0.92 |
| Community care, mean durationa (SD) | |||
| GP surgery consultation | 6.76 (53.06) | 5.76 (18.88) | 0.76 |
| GP home visit | 0.78 (4.57) | 0.79 (5.20) | 0.98 |
| GP telephone call | 1.13 (6.30) | 0.50 (2.50) | 0.11 |
| Practice nurse | 1.51 (15.11) | 2.55 (14.60) | 0.39 |
| District nurse | 6.83 (87.22) | 2.44 (22.58) | 0.40 |
| Community physiotherapy | 27.60 (117.13) | 19.47 (84.77) | 0.32 |
| Calls to NHS Direct (or NHS 111) | 0.01 (0.08) | 0.04 (0.47) | 0.25 |
| Calls for an ambulance or paramedic | 0.01 (0.08) | 0.02 (0.15) | 0.35 |
| Occupational therapy | 3.75 (42.25) | 4.47 (33.37) | 0.81 |
| Other | 1.72 (32.30) | 2.08 (24.55) | 0.79 |
| Medications, proportion of participants | |||
| Prescribed | 0.21 | 0.20 | 0.22 |
| PSS, mean durationa (SD) | |||
| Meal delivery (frozen, daily) | 0.003 (0.06) | 0.00 (0.00) | 0.32 |
| Meal delivery (hot, daily) | 0.00 (0.00) | 0.00 (0.00) | – |
| Laundry services | 0.04 (0.69) | 0.00 (0.00) | 0.32 |
| Social worker | 0.00 (0.00) | 0.31 (5.04) | 0.27 |
| Care worker/home help | 252.87 (2989.52) | 32.51 (528.77) | 0.21 |
| Other | 7.95 (176.98) | 6.95 (170.56) | 0.92 |
| Aids and adaptations, mean count (SD) | |||
| Crutch | 0.24 (0.66) | 0.28 (0.71) | 0.43 |
| Stick | 0.14 (0.43) | 0.12 (0.38) | 0.45 |
| Walking frame | 0.08 (0.31) | 0.07 (0.30) | 0.73 |
| Grab rail | 0.09 (0.50) | 0.11 (0.61) | 0.67 |
| Dressing aid | 0.03 (0.20) | 0.03 (0.28) | 0.94 |
| Long-handled shoehorn | 0.04 (0.22) | 0.04 (0.19) | 0.72 |
| Other | 0.06 (0.34) | 0.05 (0.26) | 0.60 |
| Additional cost,b proportion of participants | 0.29 | 0.38 | 0.06 |
| Time off, mean number of days (SD) | |||
| Days off work | 39.63 (57.28) | 48.71 (62.01) | 0.16 |
| Cost category | Standard dressing (£), mean (SD) | NPWT (£), mean (SD) | Mean difference (£) | p-value | Bootstrap 95% CI |
|---|---|---|---|---|---|
| Baseline to 6 months | |||||
| Initial intervention costa | 4336.52 (3715.32) | 4778.90 (4745.08) | 442.38 | 0.19 | –203.39 to 1109.52 |
| Subsequent inpatient care | 1110.59 (7261.11) | 2111.52 (11141.91) | 1000.93 | 0.18 | –384.42 to 2575.24 |
| Outpatient care | 685.05 (538.11) | 679.74 (574.63) | –5.30 | 0.91 | –95.20 to 80.92 |
| Community care | 155.78 (387.14) | 143.85 (283.69) | –11.93 | 0.66 | –66.84 to 39.66 |
| Medications | 31.38 (196.80) | 22.75 (107.60) | –8.63 | 0.50 | –35.73 to 14.61 |
| PSS | 145.69 (1359.32) | 59.00 (512.02) | –86.70 | 0.30 | –270.01 to 45.49 |
| Aids and adaptations | 202.34 (2069.72) | 83.97 (304.01) | –118.37 | 0.33 | –398.03 to 46.15 |
| Total cost, NHS and PSS | 6667.35 (9137.26) | 7879.73 (12,417.22) | 1212.38 | 0.05 | –427.45 to 2975.08 |
| Medications (out of pocket) | 31.38 (196.80) | 22.75 (107.60) | –8.63 | 0.50 | –35.73 to 14.61 |
| Additional costb | 465.96 (2362.73) | 318.70 (991.19) | –147.26 | 0.32 | –468.99 to 96.47 |
| Productivity loss | 3199.06 (15189.92) | 2806.36 (6044.07) | –392.70 | 0.68 | –2513.55 to 1083.26 |
| Total cost, societal | 10,363.75 (18,495.49) | 11,027.54 (14,333.13) | 663.79 | 0.07 | –2001.00 to 3144.90 |
| Breakdown: baseline to discharge | |||||
| Inpatient care | 4312.86 (3685.77) | 4576.32 (4719.28) | 263.46 | 0.44 | –379.36 to 927.28 |
| Antibiotics | 9.46 (78.58) | 13.48 (118.82) | 4.02 | 0.62 | –10.82 to 20.97 |
| Dressing change | 10.18 (41.42) | 37.95 (114.33) | 27.77 | < 0.001 | 15.58 to 42.45 |
| Total cost | 4332.47 (3715.46) | 4627.66 (4745.25) | 295.19 | 0.39 | –351.05 to 961.93 |
| Breakdown: discharge to 3 months | |||||
| Subsequent inpatient care | 451.12 (2742.14) | 1331.85 (7213.94) | 880.73 | 0.04 | 115.03 to 1810.89 |
| Outpatient care | 382.37 (312.50) | 387.39 (312.72) | 5.02 | 0.84 | –45.65 to 53.67 |
| Community care | 90.69 (255.88) | 89.45 (225.16) | –1.24 | 0.95 | –39.69 to 36.82 |
| Medications | 32.41 (97.41) | 32.54 (96.72) | 0.14 | 0.99 | –24.49 to 23.63 |
| PSS | 29.05 (151.44) | 39.84 (317.69) | 10.79 | 0.58 | –24.13 to 53.45 |
| Aids and adaptations | 61.23 (293.99) | 55.84 (194.19) | –5.39 | 0.79 | –48.44 to 30.33 |
| Total cost, NHS and PSS | 1026.84 (2867.27) | 1918.71 (7279.04) | 891.88 | 0.04 | 114.73 to 1837.70 |
| Medications (out of pocket) | 12.38 (62.08) | 14.35 (66.11) | 1.97 | 0.70 | –8.13 to 11.88 |
| Additional costb | 336.64 (2247.04) | 217.94 (905.54) | –118.70 | 0.40 | –425.98 to 103.71 |
| Productivity loss | 1425.82 (3187.41) | 1715.73 (4079.18) | 289.92 | 0.33 | –291.20 to 877.97 |
| Total cost, societal | 2722.52 (5589.60) | 3808.12 (8691.96) | 1085.60 | 0.06 | –1.03 to 2267.24 |
| Breakdown: 3–6 months | |||||
| Subsequent inpatient care | 659.47 (6720.56) | 779.67 (5919.47) | 120.20 | 0.81 | –904.87 to 1083.33 |
| Outpatient care | 302.68 (363.93) | 292.35 (391.15) | –10.32 | 0.73 | –70.86 to 49.55 |
| Community care | 65.09 (270.30) | 54.40 (138.16) | –10.69 | 0.54 | –48.62 to 19.48 |
| Medications | 65.74 (341.08) | 36.54 (174.95) | –29.19 | 0.49 | –118.97 to 44.69 |
| PSS | 116.64 (1346.12) | 19.16 (247.33) | –97.48 | 0.22 | –275.35 to 17.20 |
| Aids and adaptations | 141.11 (1965.29) | 28.13 (157.14) | –112.98 | 0.32 | –364.44 to 23.24 |
| Total cost, NHS and PSS | 1303.99 (7266.89) | 1182.12 (5963.54) | –121.88 | 0.82 | –1185.20 to 906.22 |
| Medications (out of pocket) | 19.00 (185.04) | 8.40 (84.84) | –10.60 | 0.36 | –35.73 to 9.36 |
| Additional costb | 133.12 (649.70) | 101.08 (308.13) | –32.04 | 0.44 | –120.16 to 40.54 |
| Productivity loss | 2140.62 (15210.07) | 1335.94 (3643.52) | –804.68 | 0.41 | –3018.49 to 572.30 |
| Total cost, societal | 3304.70 (16039.57) | 2440.51 (6966.18) | –864.19 | 0.39 | –3052.63 to 827.37 |
Appendix 2 Changes to the protocol
All protocol versions can be found on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419914#/ (accessed 22 May 2019). Table 41 shows the summary of changes implemented with each protocol version.
| Version and date | Summary of changes |
|---|---|
| 2–8 February 2016 |
|
| 3–13 October 2016 |
|
| 4–21 February 2017 |
|
| 5–27 June 2017 |
|
List of abbreviations
- AUC
- area under the curve
- BNF
- British National Formulary
- CDC
- Centers for Disease Control and Prevention
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DN4
- Douleur Neuropathique 4 Questionnaire
- DRI
- Disability Rating Index
- DSMC
- Data Safety and Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HCHS
- Hospital and Community Health Service
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISS
- Injury Severity Score
- ITT
- intention to treat
- MAR
- missing at random
- MAU
- multiattribute utility
- MCA
- Mental Capacity Act 2005
- MI
- multiple imputation
- MICE
- multiple imputation by chained equations
- NAO
- National Audit Office
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NPWT
- negative-pressure wound therapy
- OR
- odds ratio
- POSAS
- Patient and Observer Scar Assessment Scale
- PP
- per protocol
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SSI
- surgical site incision
- TARN
- Trauma Audit and Research Network
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WHiST
- Wound Healing in Surgery for Trauma
