Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/15. The contractual start date was in January 2014. The draft report began editorial review in March 2018 and was accepted for publication in February 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Abrams reports grants and personal fees from Astellas Pharma Inc. (Tokyo, Japan), and personal fees from Pfizer Inc. (Walton Oaks, UK), Ipsen (Paris, France), Ferring Pharmaceuticals (Saint Prex, Switzerland), Pierre Fabre (Paris, France), Coloplast UK (Orton, UK) and Sun Pharmaceuticals Industries Ltd (Mumbai, India), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Worthington et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter are reproduced from Worthington et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced from Hashim et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Scientific background and review of current literature
The prostate gland sits at the exit of the bladder like a collar, and as men get older their prostates enlarge, causing benign prostatic enlargement and benign prostatic obstruction (BPO). The resulting urethral obstruction can cause either urinary retention (the inability to pass urine) or bothersome voiding lower urinary tract symptoms (LUTS) secondary to BPO, such as slow and intermittent urinary stream. These conditions can severely affect a man’s quality of life, resulting in worsening physical and social functioning, vitality and mental health. 3 Bothersome LUTS secondary to BPO with an International Prostate Symptom Score (IPSS) of ≥ 11 points and a maximum urine flow rate (Qmax) of < 15 ml/second affects 2.5 million men aged 40–79 years in the UK, with 44,000 new cases diagnosed annually. 4 If medical therapy fails to improve LUTS, men often request surgery to reduce these. The aim of the surgery is to relieve the obstruction to allow men to void better, and to prevent the complications associated with BPO. These can include renal failure, urinary tract infections and bladder stones, as well as the persistence of bothersome LUTS.
Around 25,000 prostate operations are performed annually in the UK to relieve BPO. Transurethral resection of the prostate (TURP), the gold-standard operation, accounts for around 80% of these. TURP has been used widely for the past 40 years, and, although generally a successful procedure, it is associated with small but significant risks. It has a 30-day mortality rate of 0.3%, as well as a range of morbidities, including transurethral resection (TUR) syndrome (1%), which is due to the absorption of irrigating fluid leading to confusion and collapse; haemorrhage during the operation (transfusion rate 5%); and subsequent urinary tract infections (up to 20%). 5 These morbidities result in delayed discharge and increased re-admissions, increased primary care resource utilisation, considerable distress to patients and additional costs to the NHS. 1
The well-known risks of both mortality and morbidity from TURP have meant that many alternatives have been assessed. Various laser alternatives have been marketed, but uptake has been slow, owing in part to a long learning curve, or to inferior performance in terms of clinical outcomes. However, although the uptake of laser techniques has been slow historically, there does seems to have been a significant increase over the past few years. Hospital Episode Statistics data do not provide a clear indication of the proportion of procedures carried out using laser techniques as there is variation and overlap in coding. Considering only operations specifically coded as laser cases [OPCS Classification of Interventions and Procedures (OPCS-4) M65.46], the number increased in England by 45% from 2341 in 2012/13 to 3387 in 2016/17. However, this is still a relatively small proportion (≈14%) of the total number of cases of endoscopic resection of the prostate, which has remained reasonably constant at around 25,000. This is despite the commonly accepted advantages of laser prostatectomy, including lower risk of perioperative complications, shorter catheterisation time and reduced hospital stay. 7
National Institute for Health and Care Excellence (NICE) clinical guidelines 978 currently recommend offering TURP or holmium laser enucleation of the prostate (HoLEP) as BPO surgery. However, since the last update to the guidelines, in 2016 NICE recommended the use of the GreenLight XPSTM Laser Therapy System (Boston Scientific Corporation, Marlborough, MA, USA) for certain patients. 9 According to the current guidelines, any other lasers should be subject to a clinical trial before being used widely.
The current form of the HoLEP technique was originally described by Gilling et al. 10 in 1998. In the HoLEP procedure, the holmium laser is used to remove the prostatic lobes, which are then morcellated in the bladder before being removed. Although HoLEP is a long-established, effective procedure (recommended by NICE since 200311), with the key benefit that it can be undertaken regardless of prostate size, it has a recognised limitation in that it has a long learning curve, which reduces its generalisability. NICE8 recommends that this procedure be performed only in centres specialising in the technique or that have mentorship arrangements in place.
The GreenLight XPS laser involves the photoselective vaporisation of prostatic tissue. NICE has recommended it only since 2016 in its medical technologies guidance 29. 9 Currently NICE has deemed that there is insufficient evidence to recommend its use in high-risk patients, such as those with an increased risk of bleeding, prostates larger than 100 ml, or urinary retention. The NICE review concludes that the GreenLight XPS is at least as effective as TURP but can more often be carried out as a day-case procedure, given appropriate service redesign.
This study has evaluated a new laser technique called thulium laser transurethral vaporesection of the prostate (ThuVARP). Thulium is a continuous laser technology and has a tunable wavelength of between 1.75 and 2.22 µm, which is similar to that of holmium technology. 12 However, holmium is pulsed, making vaporesection time-consuming. This thulium laser technique was chosen for evaluation in this trial because it vaporises and resects the prostate, thereby using a surgical technique similar to TURP, enabling a short learning curve and meaning that it can be quickly put into widespread use.
The thulium laser was first made available in the UK in 2004 and it had been compared with TURP in one randomised controlled trial (RCT) in China at the time that the UNBLOCS trial was funded. 13 Based on this RCT and on one non-randomised prospective controlled trial in patients with small and medium-sized prostates,14 the European Association of Urology (EAU) guidelines on laser technologies in 2012 stated that ThuVARP showed efficacy equivalent to that of TURP. 15 However, the thulium patients had shorter catheterisation and hospitalisation times and fewer adverse events than patients undergoing TURP (intraoperative and postoperative bleeding), with level 1b evidence [individual RCTs conducted with narrow confidence intervals (CIs)]. 1 Subsequent EAU guidelines on the treatment of non-neurogenic male LUTS, published in 2017, reviewed the 4-year follow-up of the RCT conducted in China,16 as well as other, new studies. The guidelines concluded that thulium enucleation may be an alternative to TURP and HoLEP in men with moderate to severe LUTS, leading to both immediate and medium-term objective and subjective improvements (level of evidence 1b).
Zhang et al. 17 reported equal outcomes for ThuVARP and HoLEP in treatment of urinary tract symptoms, and similar efficacy and safety. ThuVARP was statistically superior to HoLEP in blood loss and inferior to HoLEP in operation time; however, this was clinically negligible.
In its guidelines on male LUTS (updated in 2015),8 NICE stated that the evidence base was inadequate to allow clear guidance in terms of the clinical effectiveness and cost-effectiveness of laser vaporesection techniques. NICE identified that research in this area, in the form of a RCT, would help to inform future guidance on the use of laser vaporesection techniques in men with LUTS or urinary retention who need surgery. 1
Rationale for the trial
The general population has an increased life expectancy, resulting in an ageing population. As BPO is a disease seen in older men, the number of patients with the condition is expected to grow by almost 50% by the year 2025, increasing the need for BPO surgery. 4 Furthermore, the age of men undergoing the operation is increasing (41% of the TURP operations in 2016–17 were carried out on patients aged > 75 years); therefore, the risks of surgery associated with TURP will continue to grow. There is, therefore, sustained interest in the condition and an increasing need to find safer techniques than TURP. The potential advantages of reduced blood loss, shorter hospital stay and earlier return to normal activities make laser vaporesection techniques attractive to both patients and health-care providers. A potential advantage of ThuVARP is that it would allow urologists to operate on a wider range of men, including those men who are more frail and older, but with less risk. However, there is uncertainty about the extent of improvement to symptoms and quality of life in the short and longer term, which this trial addresses, and the procedure has not yet been widely taken up across the UK.
An additional reason for the early evaluation of ThuVARP is the promise it offers to convert BPO surgery from an inpatient operation to a day-case procedure. Shortened stay is becoming more important to the NHS because of the growing cost of inpatient beds, a shortage of inpatient beds owing to an ageing and increasingly comorbid population, and the risk that longer hospital stays present for these patients, for example of hospital-acquired infections.
In summary, although there has been little existing work on ThuVARP, the promising initial evidence from one RCT suggested that ThuVARP has equivalent clinical effectiveness to TURP, although this was in a single centre in China. Our randomised study was designed to provide the high-quality evidence, in a NHS setting with a range of patient-reported, clinical effectiveness and cost-effectiveness outcomes, to underpin and inform future NICE guidance. 1
Study aims and objectives
The key aim of the UNBLOCS (UriNary oBstruction relieved by Laser Or Conventional Surgery) trial was to determine whether or not ThuVARP is equivalent to TURP in men with BPO treated in the NHS in terms of a patient-reported symptom severity score (IPSS) and the clinical measure of Qmax.
The following primary question was addressed:
-
What is the relative clinical effectiveness of ThuVARP and TURP in improving patient-reported LUTS as measured by the IPSS patient-reported questionnaire, and the objective measure of maximum urine flow rate (Qmax), 12 months after surgery?
The secondary research questions were:
-
How do the two procedures compare in terms of perioperative outcomes?
-
What is the cost-effectiveness of ThuVARP compared with that of TURP in terms of quality-adjusted-life-years (QALYs: the primary economic outcome), IPSS and Qmax?
-
What is the comparative impact of each treatment on patient-reported LUTS, erectile function, quality of life and general health?
-
What is the comparative satisfaction of men with each type of surgery?
-
What is the comparative effectiveness of these operations in men who present with LUTS as opposed to men who present with urinary retention?
-
What are men’s experiences of both procedures, including those men presenting with LUTS and those men presenting with urinary retention?
Chapter 2 Methods
Parts of this chapter are reproduced from Worthington et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced from Hashim et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial design
The UNBLOCS trial is a multicentre, pragmatic, randomised controlled, parallel-group trial of ThuVARP versus standard TURP. The trial was conducted in four university teaching hospitals and three district general hospitals in the UK, and recruited patients from June 2014 to December 2016. Men with BPO who were suitable for surgery were randomised to one of the two trial surgical treatment arms. The main aim of the study was to establish equivalence in clinical improvement, 12 months after the two surgical techniques. Both clinical and patient-reported outcomes were used to demonstrate equivalence: Qmax and IPSS.
Ethics approval and research governance
Approval from the National Research Ethics Service Committee South Central – Hampshire B Ethics Committee (13/SC/0644) was received on 15 January 2014 and applied to all NHS sites who took part in the study.
The trial is registered at the International Standard Randomised Controlled Trial Number registry with the reference ISRCTN00788389.
All patients provided their written informed consent to participate before entering the study. Consent was taken by the consultant, a dedicated research nurse or a designated team member. All adverse events were recorded, and serious adverse events that were categorised as unexpected and related were notified to the sponsor and Research Ethics Committee within 15 days.
A number of minor protocol changes were made during the trial. 1 The secondary outcome measures were reviewed and updated to include:
-
post-void residual urine at 12 months post surgery
-
change in haemoglobin and serum sodium levels postoperatively as measures of blood loss and the absorption of irrigation fluid
-
postoperative catheterisation time.
These measures were added to broaden the picture of surgical outcomes reported and were prespecified in the statistical analysis plan. 18 The protocol and study documentation were also updated during the trial to make clarifications to study procedures, particularly for catheterised participants, and improve the formatting of health economic questionnaires, as well as making other adjustments to procedures to improve questionnaire return, and the addition of one other study site.
Participants
Patients presenting with either bothersome LUTS or urinary retention (secondary to BPO), who were suitable for TURP surgery, were recruited to the trial. Patients were excluded if they met any of the following criteria:
-
had neurogenic LUTS
-
had a prostate cancer diagnosis, or a prostate-specific antigen test outside the normal range without prostate cancer excluded
-
had undergone previous prostate or urethral surgery
-
were unable to give informed consent or complete trial documentation.
Interventions
Patients were randomised in a 1 : 1 ratio to receive either TURP or ThuVARP. ThuVARP uses laser technology to vaporise and resect the prostate, whereas TURP uses an electric current to resect the prostate. The procedures are otherwise similar in that a cystoscope (telescope) goes through the urethra into the bladder and irrigation fluid is used (saline or glycine). The prostate is resected in small ‘chips’ and these fragments are then evacuated with a device called an ‘Ellick’. The procedures are therefore minimally invasive, with no incisions made in the skin, and utilise the urethral meatus to enter the body and reach the prostate. During ThuVARP, everyone in the operating room needs to wear special goggles to protect the eyes. In this trial, all surgeons conducting the procedures were at consultant level, and mentored by the chief investigator or another principal investigator already certified as competent in the ThuVARP technique, as described in Surgeon training in using the laser. Given the pragmatic nature of the study, centres continued to follow their usual practices (e.g. monopolar or bipolar TURP). Concomitant procedures during BPO surgery were allowed and details of these were recorded.
It should be noted that, owing to the trial design, surgeons needed to list patients as either day case or inpatient before randomisation, which took place when the patient was already anaesthetised. Two centres listed the majority of both their TURP and laser cases as day-case procedures, whereas the remaining five sites listed all TURP and laser cases as inpatient procedures.
Surgeon training in using the laser
ThuVARP essentially uses the same surgical skill set as that for the TURP procedure, which is part of core clinical practice for all urologists in the UK, including the trial surgeons who performed both procedures. The experience of the chief investigator and of other urologists both in the UK and Europe indicates that a maximum of 15 ThuVARP laser cases can assure a surgeon’s competence in the ThuVARP laser procedure.
All surgeons were mentored by the chief investigator or another principal investigator already certified as competent in the ThuVARP technique, and certified by an independent assessor, using standard criteria, before the official study commenced. First, surgeons observed the chief investigator or principal investigator performing one or two procedures. The chief investigator or principal investigator then observed the surgeons in each centre perform 2–5 procedures during site visits. The surgeons then performed 5–10 procedures without supervision, following their own trust’s clinical governance and audit guidelines. Competency was assessed by an independent assessor using the Intercollegiate Surgical Curriculum Programme work-based assessment. If competency had not been achieved by this stage, then further procedures would have been observed and further training would have been provided by the chief investigator until the competency criteria were met. However, in practice, all surgeons were signed off as competent at their first assessment. 1
Outcome measures
Primary outcome
International Prostate Symptom Score
The IPSS, a well-established and validated patient-reported outcome, was collected at 12 months. Participants filled in a questionnaire concerning their LUTS, which produced a score from 0 to 35, with higher scores indicating more severe symptoms.
Maximum urine flow rate (ml/second)
The Qmax was measured at participants’ 12-month follow-up assessment.
These measures were not collected if the participant had an indwelling catheter or was conducting intermittent catheterisation, if he was unable to void.
These outcomes address the primary research question for the trial. The IPSS and Qmax are internationally accepted, and are the most frequently used primary outcomes in BPO studies, thereby making results from this study comparable with those from others. No core outcome measures for BPO are listed on the COMET (Core Outcome Measures in Effectiveness Trials) Initiative website.
Secondary outcomes
Surgical complications
Any surgical complications were recorded and graded using the Clavien–Dindo classification. 19 The number of complications experienced per participant was explored, along with the worst event per participant. There are five gradings:
-
deviation from normal postoperative course (e.g. analgesics) but not requiring pharmaceutical or surgical intervention
-
requiring pharmaceutical treatment
-
requiring surgical, endoscopic or radiological intervention (3a, not under general anaesthetic; 3b, under general anaesthetic)
-
life-threatening complication that requires intensive care
-
death of the participant.
Surgical outcomes
The length of hospital stay was measured as the number of days that the participant remained an inpatient in hospital after the procedure. The binary blood transfusion rate, time to successful trial without catheter, catheter use, post-void residual urine, blood loss during surgery (change in haemoglobin level) and absorption of irrigation fluid (change in serum sodium) were also collected for each arm.
Trial participants were asked to complete the following instruments.
International Consultation on Incontinence Questionnaire – Male Lower Urinary Tract Symptoms (ICIQ-MLUTS)
Both a voiding and incontinence score were generated from this questionnaire, as were daytime and night-time voiding frequency data.
International Consultation on Incontinence Questionnaire – Male Sexual Matters associated with Lower Urinary Tract Symptoms (ICIQ-MLUTSsex), and International Index of Erectile Function-5 (IIEF-5)
An overall erectile dysfunction score was generated from the IIEF-5 questionnaire, and the ICIQ-MLUTSsex questionnaire provided data on erection and ejaculation quality and how bothersome these aspects were for the participant.
International Consultation on Incontinence Questionnaire – Lower Urinary Tract Symptoms Quality of Life (ICIQ-LUTSqol)
This is a 20-item questionnaire about patients’ urinary problems, based on the King’s Health Questionnaire. Subscores were created for role limitations, physical implications, social limitations, personal relationships, emotions, sleep/energy and severity measures.
International Consultation on Incontinence Questionnaire – Satisfaction (ICIQ-satisfaction)
This is a 20-item questionnaire on patients’ satisfaction with surgery. Overall satisfaction (0–10) and pain suffered were explored.
Equivalence margin
In the light of the existing literature, TURP and ThuVARP were expected to be broadly similar in terms of the primary outcomes, with potentially more differences in the secondary outcomes. 13 Therefore, an equivalence trial was chosen to allow greater focus on CIs, so that any clinically important difference would not be ruled out.
The sample size calculation was based on the consideration that patients randomised to the ThuVARP arm should have clinical outcomes that are equivalent to the outcomes of those patients who are randomised to the TURP arm. For the primary outcomes, a difference in LUTS score of no more than 2.5 points on the IPSS and of 4 ml/second in Qmax were hypothesised as suggesting equivalence. These were deemed appropriate for the following reasons:
-
The minimal clinically important difference for the IPSS is generally accepted to be 3 points;13,20 however, a previous trial of ThuVARP versus TURP used a minimal clinically important difference of 2 points. The team felt that a level between these would be more suitable.
-
There is no minimal clinically important difference in flow rate that is accepted in the literature; however, 2 ml/second has been quoted previously. 8
-
Discussions between clinicians, both in the trial team and with other urologists, led to an overall consensus that differences of no more than 4 ml/second in Qmax and of 2.5 points on the IPSS would be deemed clinically equivalent.
Sample size
This study is powered to establish equivalence in clinical improvement (measured with the IPSS and Qmax). A Chinese trial13 observed differences of 0.4 ml/second (95% CI –2.0 to 2.8 ml/second) in Qmax and 0.4 points (95% CI –0.7 to 1.5 points) in the IPSS between ThuVARP and TURP. Variability (standard deviation; SD) in data at 12 months was approximately 6.0 ml/second (Qmax) and 3.0 units (IPSS), but previous trials of TURP have reported greater variability of around 9 ml/second (Qmax) and 5 units (IPSS). 21,22
After considerable discussions between clinicians both inside and outside the trial, we specified differences of 4 ml/second in Qmax and 2.5 units in IPSS as demonstrating equivalence. Equivalence studies often use an alternative hypothesis of a difference of zero between treatments. However, the Chinese trial observed differences of around 0.4 ml/second and 0.4 units for Qmax and IPSS, respectively. Incorporating these as alternative hypotheses ensured adequate power to demonstrate equivalence if the treatments were indeed similar but not identical.
Assuming SDs of 9 ml/second for Qmax and 5 units for IPSS, the target sample size needed to complete the 12-month follow-up was 163 participants in each arm. Using nQuery Advisor (Statistical Solutions Ltd, Cork, Ireland), this provided 85% power to demonstrate equivalence for Qmax and just over 90% power for IPSS, at a two-sided alpha of 5%. Assuming 20% loss to follow-up following randomisation, it would be necessary to recruit 410 participants in total. This loss to follow-up was a conservative estimate from our experience of previous trials. 1
Randomisation and implementation
Men identified as eligible by a consultant or a research nurse were introduced to the trial at their clinical appointment or over the telephone. Eligible men who consented to take part in the trial were referred for LUTS surgery, if they had not already been listed. Randomisation took place in the anaesthetic room once the patient had been anaesthetised. An automated web/telephone randomisation system, provided by the Bristol Randomised Trials Collaboration, was used to randomly allocate patients to ThuVARP or TURP. Randomisation was stratified by centre and patient eligibility classification at baseline (bothersome LUTS or urinary retention). Patients were randomised in random blocks of two, four and six.
Blinding
One of the main strengths of this study was the successful blinding of participants, which is unusual for a trial involving surgical procedures. As all surgeons were able to carry out ThuVARP and TURP, the surgeon was blinded to the randomised allocation right up until the time of surgery. Following surgery, participants were not informed of their allocation but they were aware that this information was available from their general practitioner (GP) should they want to request it. Participants were asked at their 12-month follow-up which treatment they thought they had received and whether or not they had become unblinded at any point during the trial; these data were explored and utilised in a sensitivity analysis.
All investigators remained blinded to aggregate data throughout the recruitment and analysis of participants. The senior statistician, Chris Metcalfe, had not seen any data while writing the statistical analysis plan and remained blinded until the analysis had been finalised. The junior statistician (GY) had access to a random small subset of participants (n = 20) while writing the analysis plan to allow her to become familiar with the question and answer layouts. The protocol was written before recruitment ended and was published in Trials. 1 The statistical analysis plan18 was written and agreed by the trial team in October 2017, post recruitment end but prior to any statistical analysis. The junior statistician became unblinded with full access to the data on commencement of analysis in January 2018.
Data collection
Case report forms (CRFs) were collected at baseline, perioperatively, postoperatively, 3 months after surgery and 12 months after surgery (Table 1). Questionnaires were given to participants to fill in at their baseline clinic, by post at 6 weeks, and at their 3- and 12-month clinics.
| Data | Study period | |||||
|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post allocation | ||||
| Time point | Baseline | Day of surgery | Postoperative | 6 weeks | 3 months | 12 months |
| CRF | ✓ | ✓ | ✓ | ✓ | ✓ | |
| ICIQ bladder diary | ✓ | ✓ | ✓ | |||
| Qmax | ✓ | ✓ | ✓ | |||
| Post-void residual and voided volume | ✓ | ✓ | ✓ | ✓ | ||
| Full blood count | ✓ | ✓ | ||||
| Urea and electrolytes | ✓ | ✓ | ||||
| IPSS | ✓ | ✓ | ✓ | ✓ | ||
| ICIQ-MLUTS | ✓ | ✓ | ✓ | ✓ | ||
| ICIQ-MLUTSsex/IIEF-5 | ✓ | ✓ | ✓ | ✓ | ||
| ICIQ-LUTSqol | ✓ | ✓ | ✓ | ✓ | ||
| EQ-5D-5L | ✓ | ✓ | ✓ | ✓ | ||
| ICIQ-satisfaction | ✓ | ✓ | ✓ | |||
Participants with indwelling catheters were unable to complete urine flow tests; participants using intermittent catheterisation provided flow tests if they were able to void.
The trial team became aware that quite a few CRFs and questionnaires were being filled in much earlier or later than their scheduled follow-up. Therefore, before analysis the team established a time frame for which CRFs/questionnaires would be included in the analysis, as detailed in Table 2.
| Data collected | Date | |
|---|---|---|
| Minimum | Maximum | |
| Baseline questionnaire | – | Day of surgery |
| Baseline flow rate | – | Day of surgery |
| Baseline blood sample | 4 months before surgery | Day of surgery |
| Post operative blood sample | Day of surgery | 2 weeks after surgery |
| 6-week questionnaire | Day of surgery | 3 months after surgery |
| 3-month questionnaire | 2 months after surgery | 6 months after surgery |
| 3-month flow rate | 2 months after surgery | 6 months after surgery |
| 12-month questionnaire | 6 months after surgery | 18 months after surgery |
| 12-month flow rate | 6 months after surgery | 18 months after surgery |
Patient and public involvement
We involved patient and public involvement representatives at all stages of the project. We discussed the design of the trial with several men awaiting prostate surgery for BPO before we submitted the grant proposal. Discussion subjects included the comparative nature of the trial and the acceptability of randomisation, and the burden of the questionnaires.
Throughout the trial, two patient representatives were members of the UNBLOCS Trial Steering Committee, and they attended all meetings. They advised on recruitment and retention, provided feedback on patient-facing materials and newsletters and were directly involved in creating a press release to raise the profile of the trial.
Statistical methods
The main statistical analyses were prespecified using a statistical analysis plan. 18 Final data analysis started in January 2018 and finished in March 2018. Stata version 15.1 (StataCorp LP, College Station, TX, USA) was used for all statistical and health economic analyses in this trial. Binary outcomes were presented as numbers and percentages, whereas continuous outcomes were presented as mean and SD or median and interquartile range (IQR), as appropriate.
Primary analysis
Maximum urine flow rate was collected in clinic approximately 12 months after randomisation, while the IPSS was patient reported and collected using questionnaires sent to the patient approximately 12 months after randomisation. The primary analyses were conducted under the intention-to-treat principle using multivariable linear regression and adjusting for the stratification variables used in randomisation (centre and baseline LUTS/retention). The team originally planned to impute missing 12-month IPSS and Qmax data using data from 6 weeks and 3 months; however, the statistical nature of these data meant that this was not possible (see Multiple imputation). Instead, a conditional multiple imputation model using chained equations was employed and used data from baseline, including all relevant IPSS data at baseline, baseline flow, comorbidities, age, baseline diagnosis and arm. At baseline and 12 months, conditional imputation allowed the model to impute only for participants with intermittent catheters or no catheters. Participants unable to void with indwelling catheters were unable to complete urine flow tests and the IPSS questions were not relevant.
The results were based on the equivalence margin prespecified in the trial design process. As the primary analysis tested equivalence, interpretation of primary analysis results focused on observed differences and 95% CIs for the between-group comparisons. When CIs lay within the equivalence margins, the two arms were deemed equivalent. When equivalence was not achieved, the team tested for superiority, as this does not incur a statistical penalty. 23
-
H0: For IPSS and Qmax, ThuVARP is better or worse than TURP by more than 2.5 points or 4 ml/second, respectively.
-
H1: For IPSS and Qmax, ThuVARP is not better or worse than TURP by more than 2.5 points or 4 ml/second, respectively.
Secondary outcomes
All secondary analyses, unlike the primary analyses, tested for superiority. Therefore, p-values were presented alongside observed differences and CIs. All analyses were adjusted for centre and diagnosis at baseline (LUTS/retention), apart from the Clavien–Dindo19 grading of complications (based on the small number of events).
-
Prespecified surgical complications were collected in CRFs both at the perioperative stage and at each time point when a clinic CRF was administered. The Clavien–Dindo (1–5) classification scale of surgical complications was used to grade the severity of each complication. The number of complications experienced per participant was explored, along with the worst event per participant, using ordinal logistic regression. Given that complications were repeatedly reported across CRFs, the data were not explored at the event level. The complications were graded using the following system:
-
deviation from normal postoperative course without the need for further interventions (pharmaceutical, surgical, etc.)
-
requiring pharmacological treatment
-
requiring surgical intervention (a) not under general anaesthetic or (b) under general anaesthetic
-
life-threatening complication: (a) single-organ or (b) multiorgan failure
-
death of the participant.
-
-
The number of hours that the participant remained in hospital after surgery and the drop in blood haemoglobin/sodium levels were compared between the two arms using linear regression. When the distributions of continuous variables were skewed, medians and IQRs were presented alongside regression models (after checking residuals). The p-value was also compared with the one achieved from the Mann–Whitney U-test to ensure consistency. The number of hours until successful trial without catheter was analysed using a Cox proportional hazards model, whereby the assumption of proportional hazards was checked using a Schoenfeld residuals test. 24 The requirement of a blood transfusion was recorded perioperatively, postoperatively and 3 months and 12 months after surgery. The proportion of participants requiring a transfusion within 12 months of surgery was compared between the arms using logistic regression. Catheter use was recorded at 3 months and 12 months and the proportion of men using a catheter was compared using logistic regression, for both time points. We intended to compare post-void residual at 12 months using linear regression; however, after inspecting the results, the decision was made to compare the groups using logistic regression (zero vs. non-zero residuals) and ordinal logistic regression, as a large proportion of participants had post-void residuals of zero.
-
Each of the following patient-reported outcome measures was compared between the arms at 12 months using linear and logistic regression as appropriate. When the distributions of continuous variables were skewed, medians and IQRs were presented alongside regression models (after checking residuals). The p-value was also compared with that achieved from the Mann–Whitney U-test to ensure consistency:
-
IPSS quality of life
-
ICIQ-MLUTS
-
ICIQ-MLUTSsex
-
ICIQ-LUTSqol
-
ICIQ-satisfaction
-
IIEF-5.
-
A voiding and incontinence score can be created from the ICIQ-MLUTS questionnaire and an overall erectile dysfunction score can be created from the IIEF-5. Similar to previous studies, the following dichotomous variables were created for ease of reporting25 (Table 3). The results were also compared on an ordinal scale to ensure that they remained the same.
| New variable | Question | Coded as 0 | Coded as 1 |
|---|---|---|---|
| Daytime frequency (> 8 times) | ICIQ-MLUTS question 13a: how often do you pass urine during the day? | If the participant ticked ‘1–6 times’ or ‘7 or 8 times’ | If the participant ticked ‘9 or 10 times’, ’11 or 12 times’ or ‘≥ 13 times’ |
| Nocturia (> 1 times per night) | ICIQ-MLUTS question 14a: during the night, how many times do you get up to urinate, on average? | If the participant ticked ‘none’ or ‘one’ | If the participant ticked ‘two’, ‘three’ or ‘four or more’ |
| Erections (reduced or none) | ICIQ-MLUTSsex question 2a: do you get erections? | If the participant ticked ‘yes, with normal rigidity’ | If the participant ticked ‘yes, with reduced rigidity’, ‘yes, with severely reduced rigidity’ or ‘no, erection not possible’ |
| Ejaculation (reduced or none) | ICIQ-MLUTSsex question 3a: do you have an ejaculation of semen? | If the participant ticked ‘yes, normal quantity’ | If the participant ticked ‘yes, reduced quantity’, ‘yes, significantly reduced quantity’ or ‘no ejaculation’ |
| Painful ejaculation | ICIQ-MLUTSsex question 4a: do you have pain or discomfort during ejaculation? | If the participant ticked ‘no’ | If the participant ticked ‘yes, slight pain/discomfort’, ‘yes, moderate pain/discomfort’ or ‘yes, severe pain/discomfort’ |
| Urinary symptoms affected sex life? | ICIQ-MLUTSsex question 5a: to what extent do you feel that your sex life has been spoilt by your urinary symptoms? | If the participant ticked ‘not at all’ | If the participant ticked ‘a little’, ‘somewhat’ or ‘a lot’ |
Sensitivity analyses
Several sensitivity analyses were conducted to test the robustness of the results to different assumptions made.
-
Complete-case analysis: the primary analysis was repeated without imputation for missing variables. This set was then used to compute the following sensitivity analyses.
-
Per-protocol analysis: using complete cases, the per-protocol analysis included only those patients who received their randomised treatment. Those who had converted from ThuVARP to TURP were not included.
-
Complier average causal effect (CACE) analysis: using complete cases, the CACE analysis utilised all data but, unlike the primary analysis, incorporated the treatment received variable as the independent variable and randomisation as an instrumental variable using the ‘iv regress 2sls’ command in Stata.
-
Removal of participants: nine participants found out their allocation at their 12-month appointment (not including those patients who guessed correctly) before they completed the 12-month questionnaire. As the site confirmed that these participants had become unblinded, they were removed in a sensitivity analysis.
-
Adjustment for baseline: as baseline IPSS and Qmax were not available for those patients who had an indwelling catheter, the primary analysis did not adjust for these baseline measures to avoid a large number of missing or imputed data. In a sensitivity analysis, participants with an indwelling catheter at baseline were given an imputed Qmax value of zero. For IPSS, there was no specific single value that made sense for catheterised participants. Instead, the baseline scores were categorised, in accordance with the American Urological Association classification,26 into mild (0–7), moderate (8–19) or severe (20–35). Those participants with an indwelling catheter were placed in the ‘severe’ category.
-
Adjustment for imbalance at baseline: covariates that differed at baseline by more than half a SD (or by 10%) were adjusted for in an additional model to investigate their effect on the difference observed between the two groups.
-
Type of TURP/surgery: ThuVARP was compared separately with monopolar and with bipolar TURP. The two TURP procedures were also compared in terms of IPSS and Qmax, and therefore as three two-way tests. However, the results were interpreted with caution as this analysis was exploratory.
-
Surgeon effects: a mixed-effects model was conducted that included the surgeon as a random effect and the centre as a fixed effect in the primary analyses.
-
As a post hoc sensitivity analysis, bootstrap regression (4999 replications) was performed to allow for the slightly skewed distribution of IPSS and Qmax.
-
As a post hoc sensitivity analysis, given the slightly skewed distribution of IPSS and Qmax, log-transformations were carried out. For the IPSS, 1 was added to all scores before being log-transformed (natural log). Qmax scores were directly log-transformed (natural log). The equivalence margins were also log-transformed to aid interpretation of CIs, 0.92 and 1.39 for IPSS and Qmax, respectively.
Subgroup analyses
Prespecified subgroups were used to test whether or not the difference between ThuVARP and TURP was more pronounced in certain subgroups of patients. Although underpowered, tests of interaction between the dichotomised/categorical variables and treatment were carried out to explore whether or not treatment effect differed between subgroups; the focus of interpretation is on the CIs rather than the p-values. These interaction terms were added to the primary analysis model.
Subgroup analyses included:
-
baseline diagnosis of LUTS or retention
-
age (split by median age)
-
preoperative prostate size in ml, estimated during digital rectal examination (small, < 40 ml; medium, 40–60 ml; large, 60–80 ml; and very large, > 80 ml)
-
patients with or without comorbidities at baseline.
In the protocol we had specified that we would look at the length of stay of procedures (day case or inpatient). However, it was later decided that this would be more suitable as an outcome only, as the baseline intention would be unlikely to alter the treatment effect on IPSS/Qmax.
Multiple imputation
Multiple imputation by chained equations was used to impute missing values for the primary outcomes. Originally, as prespecified in the analysis plan, the model intended to use data collected at 6 weeks and 3 months to inform the 12-month data. However, when these data were included, the model failed to converge owing to the high collinearity between the measures. On inspection of the CONSORT (Consolidated Standards of Reporting Trials) flow chart (see Figure 1), the team felt that this was not detrimental to the study as few participants had completed 3-month questionnaires but not 12-month questionnaires. Instead, the model included baseline and 12-month follow-up variables to inform imputation. Trial arm, baseline diagnosis of LUTS or urinary retention, baseline comorbidities and age were included as complete variables. Indwelling catheter status (yes/no) was imputed at baseline and 12 months, while Qmax and IPSS individual items at baseline and 12 months were imputed conditionally when no indwelling catheter was present at the corresponding time point. The general rule of thumb is that the number of imputations should exceed the percentage of incomplete cases; therefore, for this trial, 40 individual imputations were created and combined using Rubin’s rules in Stata 15.1. 27 The prespecified randomisation seed 525 was used to create reproducible imputations.
Chapter 3 Results
Participant flow
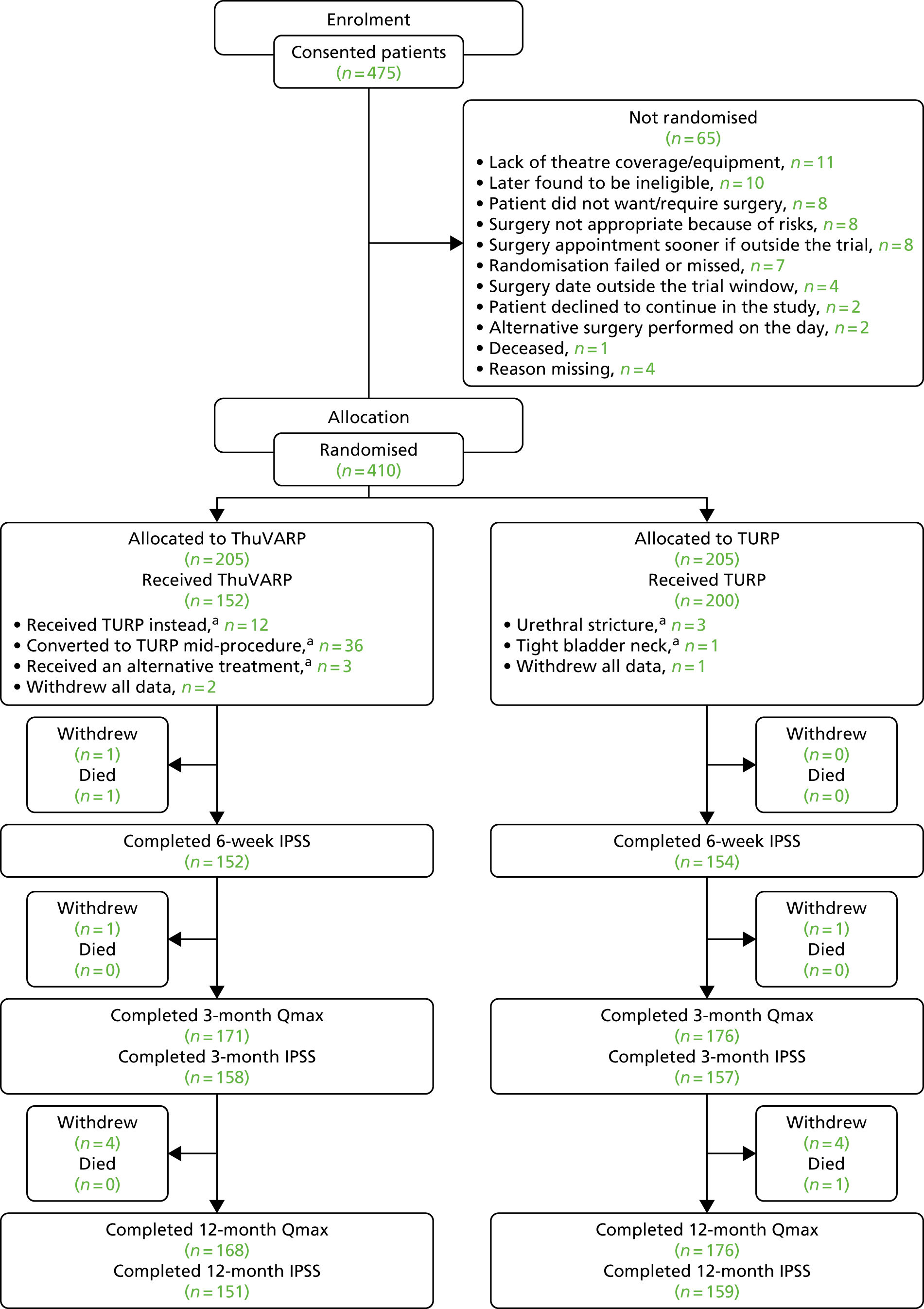
Figure 1 shows the layout of the trial and the different levels of dropout and analysis. Overall, 16 randomised patients withdrew from the study before their 12-month end point (with three requesting complete data withdrawal), with a median withdrawal time of 3.8 months (of the 13 recorded). The IPSS was able to be analysed for 74% and 78% at 12 months in the ThuVARP and TURP arms, respectively. Qmax was able to be analysed for 82% and 86% of participants, respectively.
FIGURE 1.
The UNBLOCS trial CONSORT flow chart. a, Reasons for change are recorded in Table 11. Adapted from Hashim et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Recruitment
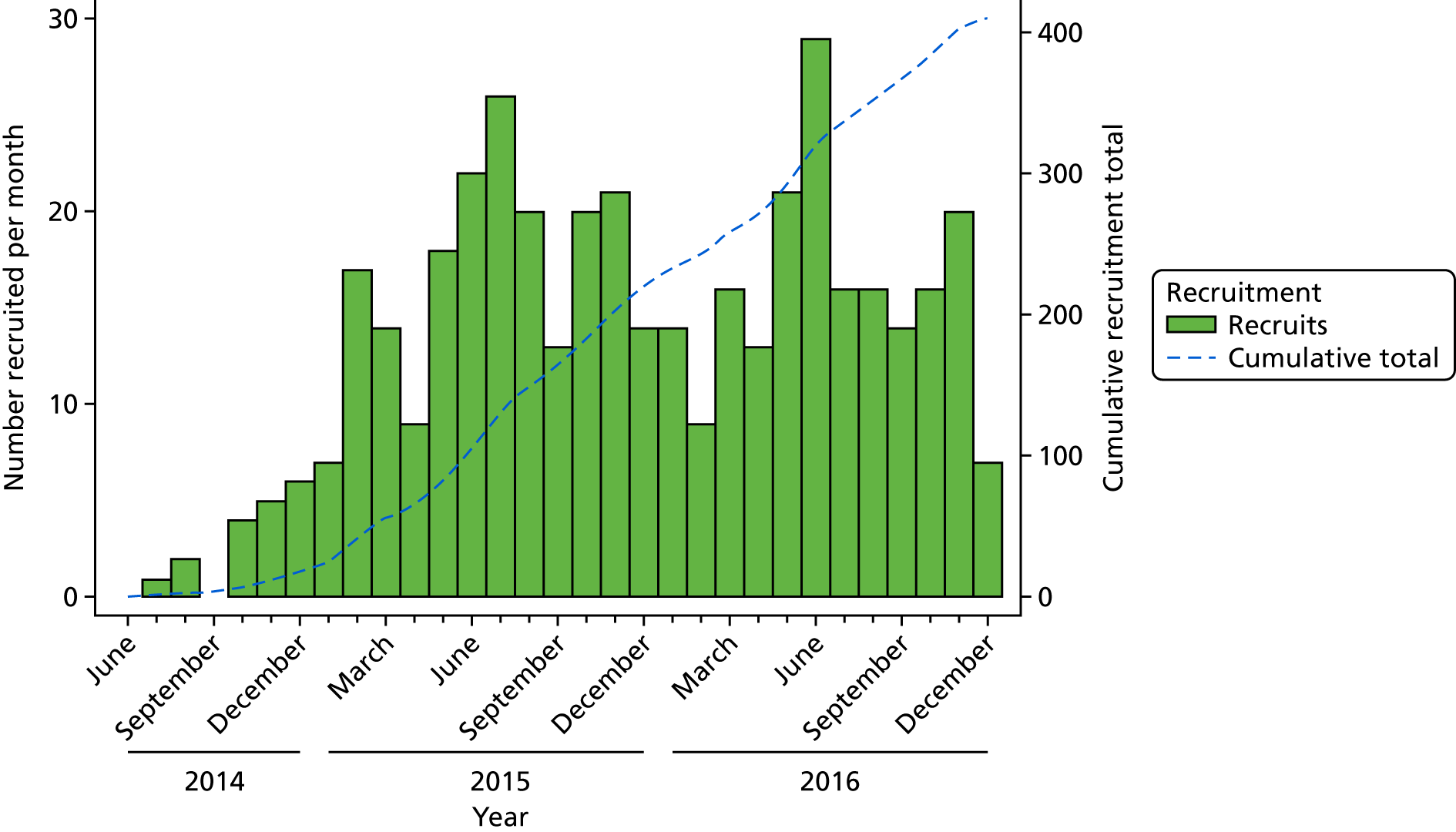
Overall, 410 patients were randomised to receive either ThuVARP or TURP, 205 in each arm (Figure 2). The first patient to receive surgery was randomised in July 2014 and the final randomised patient had surgery in December 2016.
FIGURE 2.
Recruitment.
Concomitant procedures
Concomitant procedures are shown in Table 4.
| Concomitant procedure | Trial arm (n) | |
|---|---|---|
| ThuVARP (as treated) | TURP (as treated) | |
| Urethrotomy | 9 | 16 |
| Removal of bladder stones/cystolitholapaxy | 8 | 11 |
| Urethral dilatation | 7 | 8 |
| Bladder neck incision | 2 | 6 |
| Bladder biopsy | 2 | 3 |
| Transurethral resection of bladder tumour | 1 | 1 |
| Meatal dilatation | 0 | 2 |
| Cystoscopy | 2 | 1 |
| Drainage of hydrocele | 0 | 1 |
| Circumcision | 1 | 0 |
| Ileostomy | 0 | 1 |
| Guide wire to insert catheter | 0 | 1 |
| Open cystotomy | 0 | 1 |
| Dorsal slit of foreskin | 0 | 1 |
| Excision epidydmal cyst | 0 | 1 |
| Left JJ stent removal | 0 | 1 |
| Penile and urethral strictures dilated | 0 | 1 |
| Left rigid ureteroscopy and stone fragmentation | 0 | 1 |
Baseline data
Baseline comparisons for the UNBLOCS trial are presented in Tables 5–7. Baseline information on flow rates and urinary/sexual symptoms and quality of life was not available for those patients with an indwelling catheter; therefore, the figures are rather low for some baseline variables.
| Characteristic | Trial arm | |||
|---|---|---|---|---|
| ThuVARP | TURP | |||
| N a | n (%) | N a | n (%) | |
| Age (years), mean (SD) | 203 | 70.85 (7.85) | 204 | 69.22 (7.91) |
| Eligibility: bothersome LUTS | 203 | 94 (46) | 204 | 102 (50) |
| Eligibility: urinary retention | 203 | 109 (54) | 204 | 102 (50) |
| Recruiting centre | ||||
| 1 | 66 (32) | 66 (32) | ||
| 2 | 44 (21) | 44 (21) | ||
| 3 | 33 (16) | 32 (16) | ||
| 4 | 205 | 20 (10) | 205 | 20 (10) |
| 5 | 20 (10) | 19 (9) | ||
| 6 | 15 (7) | 16 (8) | ||
| 7 | 7 (3) | 8 (4) | ||
| Ethnicity | ||||
| White | 199 | 196 (98) | 201 | 197 (98) |
| Other | 3 (2) | 4 (2) | ||
| Characteristic | N a | ThuVARP, n (%) | N a | TURP, n (%) |
|---|---|---|---|---|
| BMI on day of surgery (kg/m2), mean (SD) | 193 | 28.54 (4.16) | 194 | 27.83 (4.32) |
| Comorbidities at baseline (from the Charlson Comorbidity Index) | ||||
| None | 113 (56) | 115 (56) | ||
| One | 203 | 57 (28) | 204 | 58 (28) |
| More than one | 33 (16) | 31 (15) | ||
| Catheterisation status (on the day of surgery) | ||||
| No catheter | 202 | 96 (48) | 204 | 97 (48) |
| Catheterised | 106 (52) | 107 (52) | ||
| Intermittent | 4 (4) | 10 (9) | ||
| Indwelling | 93 (88) | 92 (86) | ||
| Type not disclosedb | 9 (8) | 5 (5) | ||
| Urinary measures (not measured in those patients with indwelling catheter) | ||||
| Qmax,c mean (SD) | 92 | 8.90 (5.90) | 102 | 8.00 (6.00) |
| Median post-void residual (IQR)c | 96 | 157 (53–285) | 99 | 140 (80–300) |
| Median voided volume (IQR)c | 97 | 186 (110–251) | 100 | 181 (117–244) |
| Patient has had urodynamics | 192 | 37 (19) | 191 | 44 (23) |
| Outcome measure | N a | ThuVARP, n (%) | N a | TURP, n (%) |
|---|---|---|---|---|
| IPSS: symptom severity, mean (SD) | ||||
| Incomplete emptying | 89 | 3.12 (1.72) | 97 | 3.29 (1.60) |
| Frequency | 90 | 3.63 (1.25) | 96 | 3.83 (1.42) |
| Intermittency | 90 | 2.94 (1.61) | 97 | 2.99 (1.58) |
| Urgency | 90 | 2.97 (1.64) | 96 | 3.30 (1.44) |
| Weak stream | 90 | 3.91 (1.36) | 97 | 3.80 (1.30) |
| Straining | 90 | 2.20 (1.77) | 93 | 2.37 (1.79) |
| Nocturia | 90 | 2.97 (1.34) | 94 | 2.83 (1.35) |
| Total IPSS | 86 | 21.74 (6.37) | 89 | 22.56 (6.78) |
| IPSS quality of life | 90 | 4.89 (1.11) | 97 | 5.01 (1.01) |
| ICIQ-MLUTS | ||||
| Voiding score,b mean (SD) | 91 | 11.62 (4.35) | 96 | 11.78 (3.92) |
| Incontinence score,c mean (SD) | 89 | 5.75 (3.42) | 97 | 6.10 (3.85) |
| Daytime frequency (> 8 times) | 91 | 42 (52) | 97 | 56 (58) |
| Nocturia (> 1 times per night) | 91 | 75 (82) | 97 | 81 (84) |
| ICIQ-MLUTSsex | ||||
| Erections (reduced or none) | 86 | 65 (76) | 91 | 65 (71) |
| Ejaculation (reduced or none) | 85 | 73 (86) | 89 | 75 (84) |
| Painful ejaculation (yes) | 72 | 13 (18) | 85 | 30 (35) |
| Urinary symptoms affected sex life? (yes) | 82 | 56 (68) | 89 | 62 (70) |
| IIEF-5, mean (SD) | ||||
| Erectile dysfunction scored | 65 | 14.11 (6.51) | 74 | 16.49 (6.17) |
| IPSS, mean (SD) | ||||
| Quality of life | 90 | 4.89 (1.11) | 97 | 5.01 (1.01) |
| ICIQ-LUTSqol | ||||
| Presence of limitations | ||||
| Role limitations | 88 | 73 (83) | 98 | 79 (81) |
| Physical limitations | 91 | 77 (85) | 97 | 84 (87) |
| Social limitations | 89 | 57 (64) | 95 | 76 (80) |
| Personal relationships | 75 | 63 (84) | 83 | 67 (81) |
| Emotions | 88 | 68 (77) | 94 | 84 (89) |
| Sleep/energy | 90 | 89 (99) | 95 | 91 (86) |
| Severity measures | 87 | 78 (90) | 95 | 82 (86) |
| Urinary symptom effect on . . . | ||||
| Getting embarrassed | 90 | 59 (66) | 97 | 66 (68) |
| Overall interference with everyday life, mean (SD) | 99 | 6.02 (2.81) | 101 | 6.47 (3.00) |
Overall, participants in the TURP arm appeared to be very slightly worse off in terms of patient-reported measures of symptom burden. However, no large differences were seen between the arms at baseline in terms of sociodemographic (see Table 5) or clinical characteristics (see Table 6). Painful ejaculation was the only patient-reported outcome measure that differed by more than an absolute difference of 10% (see Table 7).
Numbers analysed
When comparing our analysable sample with those patients who withdrew or were lost to follow-up (i.e. those patients who did not complete the 12-month Qmax or the IPSS), we can see differences that met the criteria used to look for imbalance between the arms (> 10% or 0.5 SDs). These differences were seen in centre, daytime urinary frequency and the effect on participants’ sex lives (Table 8). A difference was also seen in comorbidities, but this did not reach the 10% absolute difference. All variables not presented in Table 8 were balanced between those patients who were and those patients who were not analysed at 12 months. However, in general, those patients who withdrew or were lost to follow-up had slightly worse symptoms at baseline.
| Characteristic | Analysable sample | Non-responders/withdrawn | ||
|---|---|---|---|---|
| N a | n (%) | N a | n (%) | |
| Comorbidities at baseline | ||||
| None | 161 (56) | 67 (55) | ||
| One | 286 | 87 (30) | 121 | 28 (23) |
| More than one | 38 (13) | 26 (21) | ||
| Recruiting centre | ||||
| 1 | 92 (32) | 40 (32) | ||
| 2 | 64 (22) | 24 (19) | ||
| 3 | 54 (19) | 11 (9) | ||
| 4 | 286 | 26 (9) | 124 | 14 (11) |
| 5 | 24 (8) | 15 (12) | ||
| 6 | 19 (7) | 12 (10) | ||
| 7 | 7 (2) | 8 (6) | ||
| ICIQ-MLUTS | ||||
| Daytime frequency (> 8 times) | 132 | 112 (85) | 56 | 40 (71) |
| Urinary symptoms affected sex life? | 125 | 92 (74) | 46 | 26 (57) |
The numbers of patients who had outcome data were also relatively balanced between the arms, as are the numbers of withdrawals. The numbers of patients undergoing their assigned treatment differed between the arms, with only 75% of those patients randomised to ThuVARP actually receiving their treatment (p < 0.001) (Table 9).
| Trial arm, n/N (%) | p-valuea | ||
|---|---|---|---|
| ThuVARP | TURP | ||
| Numbers of withdrawals and lost to follow-up | |||
| Number randomised | 205 | 205 | – |
| Number who did undergo their randomised surgery | 152/203 (75) | 200/204 (98) | < 0.001 |
| Withdrew from the study | 9/205 (4) | 7/205 (3) | 0.610 |
| Analysable sample | |||
| MI: Qmax at 12 monthsb | 197/205 (96) | 199/205 (97) | 0.587 |
| MI: IPSS at 12 monthsb | 197/205 (96) | 199/205 (97) | 0.587 |
| CC: Qmax at 12 months | 168/205 (82) | 176/205 (86) | 0.282 |
| CC: IPSS at 12 months | 151/205 (74) | 159/205 (78) | 0.354 |
Reasons for withdrawal were relatively similar between the arms (Table 10). A large proportion of changes in treatment were because of equipment failure in the ThuVARP arm, with 18 participants being changed to TURP straight away or converting mid-procedure (Table 11). Prostate size also resulted in nine conversions to TURP. The proportion of participants receiving conversions compared with receiving ThuVARP was relatively balanced across the centres and over time. Between 6% and 25% of participants in each centre received a conversion from ThuVARP to TURP. When breaking the recruitment period per surgeon into halves, the conversion rate in the first half was 11%, whereas it increased to 28% in the second half.
| Reason for withdrawal | Trial arm, n/N (%) | |
|---|---|---|
| ThuVARP | TURP | |
| Other health problems became a priority | 5/205 | 0/205 |
| Death of the participant | 1a/205 | 1b/205 |
| Reason not given | 1/205 | 0/205 |
| Did not wish to attend clinics or have questionnaires | 1/205 | 3/205 |
| Not happy with surgery outcome | 0/205 | 1/205 |
| Experienced adverse event | 0/205 | 1/205 |
| New diagnosis → no surgeryc | 1/205 | 1/205 |
| Treatment received | Reason | Number of participants |
|---|---|---|
| Change in treatment from TURP | ||
| Urethral stricture | Prostate reasonable size | 2 |
| Urethral stricture | Unable to access urethra | 1 |
| Bladder neck incision | Tight bladder neck | 1 |
| Change in treatment from ThuVARP | ||
| TURP | Equipment failure (no treatment with ThuVARP) | 9 |
| TURP | Anaesthetic complications | 1 |
| TURP | No laser-trained nursing staff available | 1 |
| TURP | Start time delayed, so proceeded with TURP | 1 |
| Conversion (TV-TP) | Equipment failure (converted to TURP mid-procedure) | 9 |
| Conversion (TV-TP) | Very large prostate | 9 |
| Conversion (TV-TP) | Bleeding | 5 |
| Conversion (TV-TP) | To collect remaining fragments of prostate | 4 |
| Conversion (TV-TP) | Failed to progress with ThuVARP | 4 |
| Conversion (TV-TP) | Poor visibility | 3 |
| Conversion (TV-TP) | Incidental finding of tumour | 1 |
| Conversion (TV-TP) | No details found | 1 |
| Optical urethrotome | No details found | 1 |
| Prostatic embolism | Prostate too big for ThuVARP/TURP | 1 |
| Transurethral resection of bladder tumour | Risk of seeding tumour cells into prostatic urethra so no bladder outlet procedure performed | 1 |
Statistical outcomes and estimation
International Prostate Symptom Score
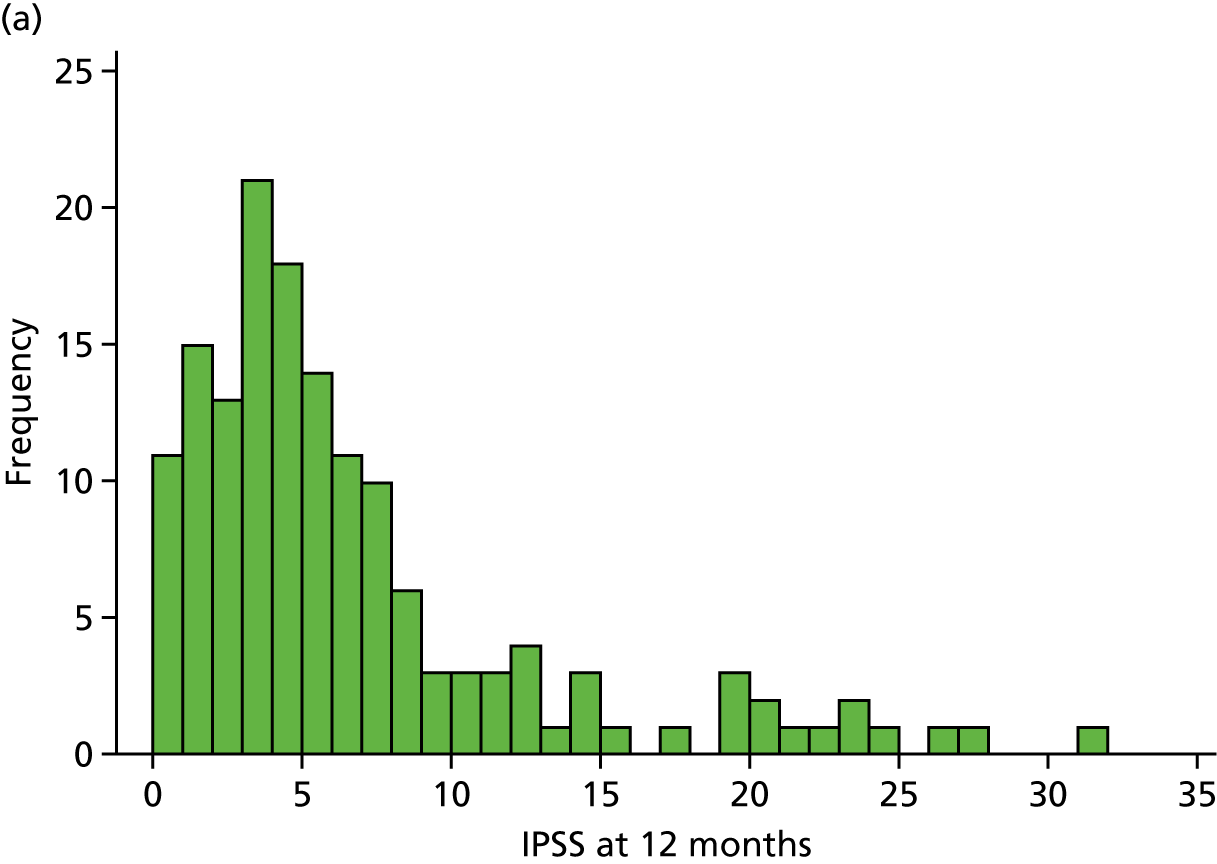
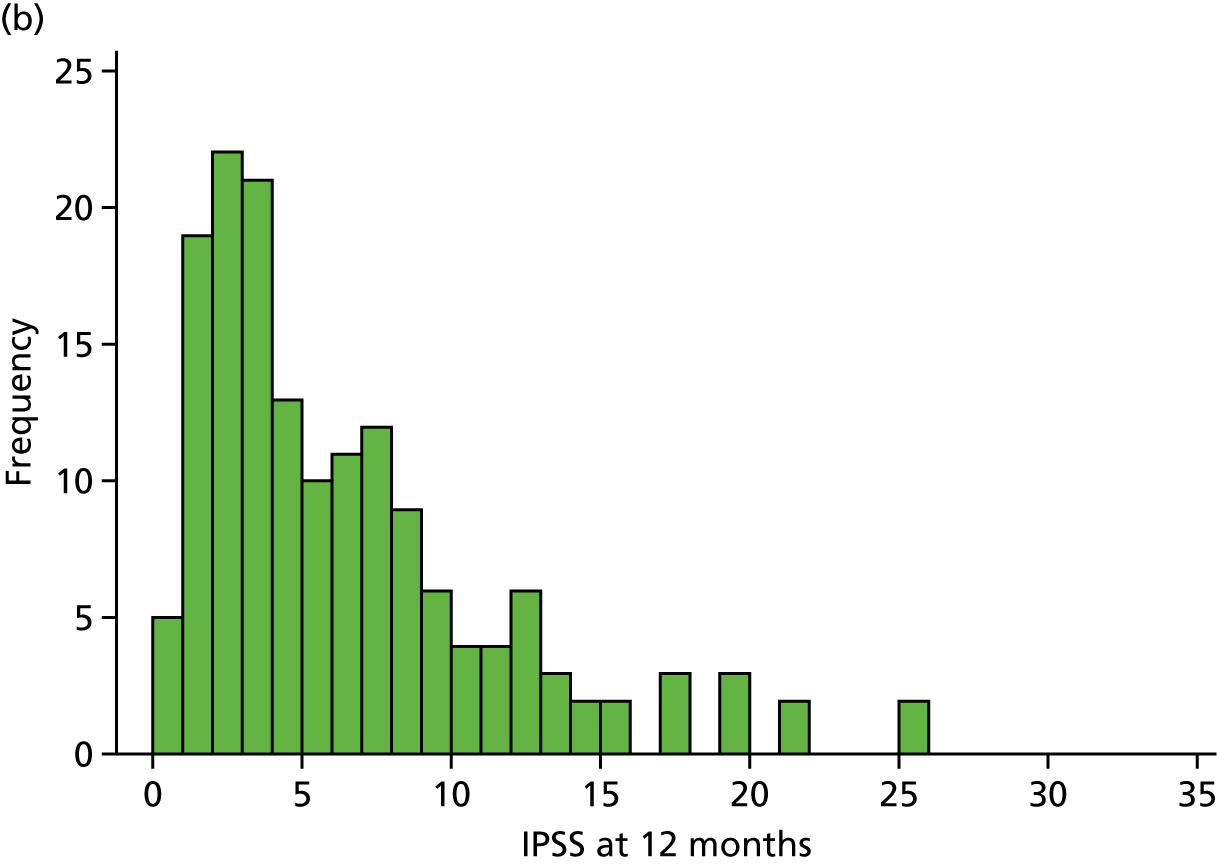
The first of two primary outcomes was the IPSS at 12 months. The null hypothesis was that the two surgical procedures differed by at least 2.5 points, while the alternative hypothesis was that the two procedures were equivalent. At the 12-month point from surgery, IPSS (overall median 4.0 points) was much lower than that recorded in baseline questionnaires (overall median 23.0 points). However, it was possible to obtain IPSS at baseline only for those patients who did not have an indwelling catheter (n = 175). The distribution of scores at 12 months is presented in Figure 3.
FIGURE 3.
Distribution of IPSS at 12 months for (a) ThuVARP; and (b) TURP.
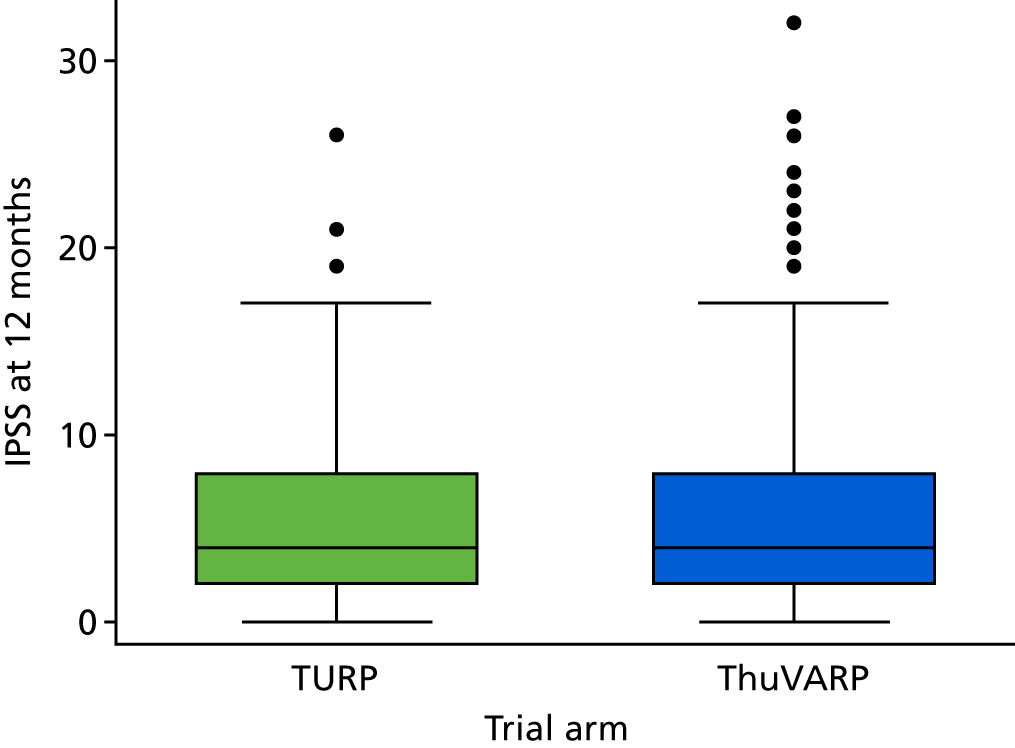
The box plot in Figure 4 shows the distribution of IPSS for each arm. They are very similar, both sharing a median score of 4 and an IQR of 2–8 points.
FIGURE 4.
Box plot of IPSS for TURP vs. ThuVARP. The box plot indicates the median (central line in the box), 25th percentile (bottom line of the box), 75th percentile (top line of the box) and the whiskers are for the lowest and highest values (excluding outliers). The dots beyond these whiskers are the outliers, which are observations that lie an abnormal distance from other values. Outliers are defined as those higher than (1.5 × IQR) + (75th percentile) or lower than (25th percentile) – (1.5 × IQR).
Given the skewed nature of the data, as well as the prespecified linear regression model, a bootstrap regression model was calculated as a post hoc sensitivity analysis.
Maximum urine flow rate values
The second of the two primary outcomes was the maximum urine flow rate at 12 months. The null hypothesis was that the two surgical procedures differed by at least 4 ml/second, while the alternative hypothesis was that the two procedures were equivalent. As with IPSS, 12 months after surgery, Qmax values were much improved from those values recorded at baseline (Figure 5). However, baseline Qmax values were possible to collect from only patients without an indwelling catheter.
FIGURE 5.
Distribution of Qmax values at 12 months for (a) ThuVARP; and (b) TURP.

The box plot in Figure 6 shows the maximum urine flow rates by trial arm. The results show that those patients in the ThuVARP arm had a median 12-month Qmax of 17 ml/second (IQR 11–27 ml/second), whereas those in the TURP arm had a median Qmax of 20 ml/second (IQR 13–32 ml/second).
FIGURE 6.
Box plot of Qmax values for TURP vs. ThuVARP. The box plot indicates the median (central line in the box), 25th percentile (bottom line of the box), 75th percentile (top line of the box) and the whiskers are for the lowest and highest values (excluding outliers). The dots beyond these whiskers are the outliers, which are observations that lie an abnormal distance from other values. Outliers are defined as those higher than (1.5 × IQR) + (75th percentile) or lower than (25th percentile) – (1.5 × IQR).

As with the IPSS, given the slightly skewed nature of the data, a bootstrap regression model was calculated as a post hoc sensitivity analysis.
Primary analysis results
The multiple imputation model included all randomised patients, apart from those patients who withdrew all data (n = 3). It imputed the catheter status, IPSS and Qmax at baseline and 12 months where data were incomplete. It employed a conditional imputation, whereby initially catheter status was imputed followed by IPSS/Qmax values only for those patients without a catheter. For participants who died during the trial period, we did not impute any data after their time of death (Table 12).
| Variable | N (TV:TR) | Trial arm, mean (SD) | Crude difference in means (95% CI) | Adjusted difference in meansa (95% CI) | |
|---|---|---|---|---|---|
| ThuVARP | TURP | ||||
| Primary analysis (MI) | |||||
| IPSS | 197:199 | 6.43 (6.79) | 6.26 (5.79) | 0.16 (–1.08 to 1.41) | 0.28 (–0.92 to 1.49) |
| Qmax | 197:199 | 20.16 (16.88) | 23.24 (13.28) | –3.08 (–5.75 to –0.41) | –3.12 (–5.79 to –0.45) |
| Primary analysis (CC) | |||||
| IPSS | 151:159 | 6.29 (6.22) | 6.03 (5.21) | 0.26 (–1.02 to 1.54) | 0.43 (–0.78 to 1.64) |
| Qmax | 168:176 | 20.19 (12.43) | 23.47 (12.82) | –3.28 (–5.96 to –0.60) | –3.42 (–6.10 to –0.73) |
The equivalence margin for IPSS was prespecified as 2.5 points. The difference between the arms, using the imputed model, was 0.28 points. The two procedures appear to be equivalent for the IPSS as the CIs are within the range –2.5 to 2.5 points; therefore, this blinded trial has demonstrated that a patient’s perception of urinary tract symptoms after treatment is equivalent for the ThuVARP and TURP procedures.
The equivalence margin for the Qmax levels was prespecified as 4 ml/second. The ThuVARP procedure gives a lower maximum urine flow rate at 12 months than TURP (just over 3 ml/second). The CIs are outside the range –4 to 4 ml/second, with the lower reaching almost 6 ml/second, deeming the treatments non-equivalent with respect to Qmax. Changing the test to superiority, which does not carry a statistical penalty after a non-inferiority or equivalence trial, did lead us to conclude that TURP is superior to ThuVARP in terms of maximum urine flow rate for both the complete-case and the imputation analyses. 28
Success of blinding
In their 12-month questionnaire, participants were asked if they thought they knew which type of surgery they had undergone. Overall, 70% (238/339) of patients said that they did not know which operation they received. When asked to predict their surgery, 40% (138/346) of patients did so. Of those who predicted ThuVARP, 54% were correct; of those who predicted TURP, 82% were correct. However, of those who were correct, 80% went on to say that they did not actually know/they had guessed. Based on this, the team felt that blinding had been successful. Unfortunately, a research nurse unblinded nine participants at their 12-month clinic appointment (before they had completed their 12-month questionnaire). In a sensitivity analysis, these participants were removed to avoid any potential bias from the IPSS results.
Secondary outcomes: surgical complications
Surgical complications were recorded during surgery, postoperatively and at the 3-month and 12-month clinics. Where participants did not attend follow-up clinic, details of complications were extracted from the participants’ medical notes.
Perioperative complications
Overall, there were 28 complications in theatre or during the recovery period: 17 in the ThuVARP arm and 11 in the TURP arm (Table 13).
| Variable | Trial arm, n (%) | |
|---|---|---|
| ThuVARP | TURP | |
| Perioperative complications | ||
| Anaesthetic complications | 8/203 (4) | 4/204 (2) |
| Bleeding requiring haemoglobin measurement | 4/203 (2) | 3/204 (1) |
| Blood transfusion | 0/203 (0) | 1/204 (< 1) |
| TUR syndrome | 0/203 (0) | 0/204 (0) |
| Perforation/extravation | 4/203 (2) | 3/204 (1) |
| Catheter misplacement | 1/203 (< 1) | 0/204 (0) |
Although there appeared to be more events in the ThuVARP arm, when looking at treatment received we could establish that only 8 of those 17 complications were experienced during ThuVARP, whereas 2 were from TURP and 7 were during conversions from ThuVARP to TURP. ‘Other’ complications were not presented as these were reported differently across sites and did not always reflect true complications (e.g. broken laser fibre).
Postoperative complications
Data on surgical outcomes (Table 14) and postoperative complications (Table 15) were collected from postoperative, 3-month and 12-month CRFs completed by a clinician. The average length of stay was 48 hours in both arms of the trial. Transfusion and catheter requirement rates were low and similar. There was no evidence to suggest that one arm was better than the other for surgical outcomes. There was some evidence to suggest that there might have been a trend towards higher post-void residuals in the ThuVARP arm; however, when comparing the number of men with zero post-void residual volume, there did not appear to be a difference.
| Variable | N (TV:TR) | Trial arm | Adjusted comparison,a (95% CI) | p-value | |
|---|---|---|---|---|---|
| ThuVARP | TURP | ||||
| Surgical outcomes | |||||
| Length of hospital stay (hours), median (IQR) | 198:198 | 48 (29 to 58) | 48 (29 to 61) | –3.28 (–9.61 to 3.06)b | 0.310 |
| Transfusion required (yes/no), n (%) | 200:202 | 3 (2) | 4 (2) | 0.79 (0.17 to 3.62)c | 0.765 |
| Postoperative catheter time (days), median (IQR) | 195:198 | 2 (1 to 5) | 2 (1 to 4) | 1.02 (0.83 to 1.26)d | 0.830 |
| Catheter required at 3 months, n (%) | 196:201 | 5 (3) | 5 (2) | 0.99 (0.28 to 3.49)c | 0.988 |
| Catheter required at 12 months, n (%) | 192:195 | 4 (2) | 2 (1) | 1.95 (0.35 to 10.82)c | 0.446 |
| Haemoglobin: blood loss (g/l),e median (IQR) | 146:138 | –6 (–13 to –1) | –8 (–16 to –2) | 0.88 (–2.14 to 3.89)b | 0.568 |
| Serum sodium (mmol/l),e median (IQR) | 141:138 | –2 (–4 to –1) | –3 (–4 to –1) | 0.40 (–0.34 to 1.14)b | 0.290 |
| Post-void residual, n (%) | 169:176 | 1.02 (0.58 to 1.78)f | 0.950 | ||
| Quintile 1 (0) | 39 (23) | 39 (22) | |||
| Quintile 2 (1–34) | 21 (12) | 39 (22) | |||
| Quintile 3 (35–71) | 35 (21) | 35 (20) | 1.46 (1.00 to 2.15)g | 0.053 | |
| Quintile 4 (72–140) | 32 (19) | 36 (20) | |||
| Quintile 5 (≥ 141) | 42 (25) | 27 (15) | |||
| Variableb | Trial arm, n (%) | ORc (95% CI) | p-value | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Bleeding requiring haemoglobin measurement | ||||
| Not experienced | 188 (94) | 189 (94) | 1.00 (0.42 to 2.35) | 0.992 |
| Clavien–Dindo grade I | 10 (5) | 8 (4) | ||
| Clavien–Dindo grade II | 0 (0) | 2 (1) | ||
| Clavien–Dindo grade IIIb | 1 (1) | 1 (1) | ||
| TUR syndrome | ||||
| Not experienced | 203 (100) | 203 (100) | – | – |
| Catheter misplacement | ||||
| Not experienced | 198 (100) | 199 (> 99) | – | – |
| Clavien–Dindo grade II | 0 | 1 (1) | ||
| Clot retention | ||||
| Not experienced | 190 (95) | 189 (94) | 0.74 (0.30 to 1.79) | 0.498 |
| Clavien–Dindo grade I | 9 (5) | 9 (4) | ||
| Clavien–Dindo grade II | 0 (0) | 2 (1) | ||
| Clavien–Dindo grade IIIb | 0 (0) | 1 (< 1) | ||
| Urethral stricture | ||||
| Not experienced | 191 (96) | 195 (98) | 1.43 (0.45 to 4.59) | 0.546 |
| Clavien–Dindo grade I | 0 (0) | 2 (1) | ||
| Clavien–Dindo grade II | 0 (0) | 0 (0) | ||
| Clavien–Dindo grade IIIa | 4 (2) | 0 (0) | ||
| Clavien–Dindo grade IIIb | 3 (2) | 3 (2) | ||
| Urinary tract infection | ||||
| Not experienced | 131 (68) | 136 (68) | 1.02 (0.67 to 1.55) | 0.938 |
| Clavien–Dindo grade I | 10 (5) | 11 (6) | ||
| Clavien–Dindo grade II | 51 (26) | 53 (27) | ||
| Clavien–Dindo grade IVb | 1 (1) | 0 (0) | ||
| Pyrexia of unknown region | ||||
| Not experienced | 188 (97) | 190 (98) | 1.50 (0.42 to 5.41) | 0.533 |
| Clavien–Dindo grade I | 2 (1) | 0 (0) | ||
| Clavien–Dindo grade II | 4 (2) | 4 (2) | ||
| Sepsis/septicaemia/abscess | ||||
| Not experienced | 190 (99) | 189 (98) | 0.50 (0.09 to 2.76) | 0.427 |
| Clavien–Dindo grade II | 1 (1) | 3 (2) | ||
| Clavien–Dindo grade IVa | 0 (0) | 1 (1) | ||
| Clavien–Dindo grade IVb | 1 (1) | 0 (0) | ||
| Other infection | ||||
| Not experienced | 186 (97) | 187 (96) | 0.71 (0.22 to 2.29) | 0.570 |
| Clavien–Dindo grade I | 1 (1) | 0 (0) | ||
| Clavien–Dindo grade II | 4 (2) | 7 (4) | ||
The numbers of events in each arm were extremely similar (see Table 15). Rows have been omitted when certain grades were not experienced by a single participant; for example, the odds ratio for urinary tract infections suggested that those patients in the ThuVARP arm were at a 2% higher odds of being in a higher Clavien–Dindo grade (0–5) than those patients in the TURP arm (p = 0.938). The highest Clavien–Dindo score recorded was IVb (a life-threatening complication requiring intensive care – multiorgan dysfunction). This was experienced twice by the same participant during a urinary tract infection and sepsis episode. This participant died 1 month later, but this was deemed unrelated (see Serious adverse events).
If we look at the total number of events experienced per participant, we see that the majority do not experience an event: 56% in the ThuVARP arm and 53% in the TURP arm (Table 16). There was no evidence to suggest that there are more events in one arm of the trial.
| Variable | Trial arm, n (%) | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Number of complications experienced | ||||
| 0 | 107 (53) | 113 (56) | 1.10 (0.75 to 1.63) | 0.623 |
| 1 | 28 (14) | 27 (13) | ||
| 2 | 40 (20) | 26 (13) | ||
| 3 | 10 (5) | 15 (7) | ||
| 4 | 9 (4) | 9 (4) | ||
| 5 | 4 (2) | 4 (2) | ||
| 6 | 0 (0) | 6 (3) | ||
| 7 | 2 (1) | 0 (0) | ||
| 8 | 1 (< 1) | 1 (< 1) | ||
| 9 | 0 (0) | 2 (1) | ||
| 10 | 1 (< 1) | 1 (< 1) | ||
| 11 | 1 (< 1) | 0 (0) | ||
Secondary outcomes: patient-reported outcomes
Overall patient-reported outcomes were similar in the two arms. Although urinary symptoms were generally worse in the ThuVARP arm, all differences could be explained by chance (Table 17). For nocturia (getting up to urinate more than once per night), there was some evidence to suggest that TURP was more effective in reducing the proportion of men reporting this outcome, which was strengthened when looking at this on an ordinal scale (p = 0.031). However, given the large number of secondary outcomes, we cannot rule out that this may have been a chance finding. Sexual symptoms were similar but with reduction in painful ejaculation still slightly in favour of ThuVARP; however, this difference had been already present at baseline.
| Variable | N (TV:TR) | Trial arm, n (%) | Adjusted differencea (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| ThuVARP | TURP | ||||
| Secondary analysis (ICIQ-MLUTS) | |||||
| ICSmaleVS (voiding scale),b mean (SD) | 164:173 | 3.14 (3.40) | 3.00 (3.07) | 0.15 (–0.53 to 0.82) | 0.666 |
| ICSmaleIS (incontinence scale),c mean (SD) | 165:175 | 2.40 (2.65) | 2.23 (2.37) | 0.22 (–0.30 to 0.75) | 0.406 |
| Daytime frequency (> 8 times) | 164:175 | 20 (12) | 18 (10) | 1.20 (0.61 to 2.39) | 0.597 |
| Nocturia (> 1 times per night) | 164:172 | 72 (44) | 63 (37) | 1.47 (0.93 to 2.34) | 0.102 |
| Secondary analysis (ICIQ-MLUTSsex) | |||||
| Erections (reduced or none) | 145:152 | 101 (70) | 113 (74) | 0.79 (0.47 to 1.31) | 0.356 |
| Ejaculation (reduced or none) | 139:148 | 129 (93) | 136 (92) | 1.13 (0.47 to 2.71) | 0.780 |
| Painful ejaculation (yes) | 118:139 | 8 (7) | 17 (12) | 0.55 (0.22 to 1.32) | 0.179 |
| Urinary symptoms affected sex life? | 133:145 | 74 (56) | 88 (61) | 0.81 (0.50 to 1.31) | 0.339 |
| Secondary analysis (IIEF-5), mean (SD) | |||||
| Erectile dysfunction scored | 100:118 | 14.18 (7.46) | 15.14 (7.34) | –0.95 (–2.95 to 1.05) | 0.348 |
Participants in both arms seemed generally satisfied with their treatment, with 207 out of 340 (61%) of all patients giving the maximum score of 10 (Table 18). Participants in the ThuVARP arm were at a lower odds of saying that they would have the same treatment again if they had the same problem in the future, but there was only very weak statistical evidence to support this finding.
| Variable | N (TV:TR) | Trial arm, n (%) | p-value | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Satisfaction with treatment | ||||
| Overall how satisfied were youa (0–10)?, mean (SD) | 163:177 | 8.67 (2.42) | 8.88 (1.92) | 0.338b |
| Same treatment again?c | ||||
| Yes, definitely/probably | 165:174 | 150 (91) | 165 (95) | 0.156d |
| Not sure | 11 (7) | 5 (3) | ||
| No, definitely/probably not | 4 (2) | 4 (2) | ||
Overall, quality of life was very high in both arms at 12 months (Table 19). Fifty-eight per cent (193/335) of all participants answered ‘not at all’ to the question ‘Overall, how much do urinary symptoms interfere with your everyday life?’ on the ICIQ-LUTSqol. This was reflected in the IPSS quality of life question, to which 170 (50%) participants responded that they would be delighted if they were to spend the rest of their lives with their current urinary condition.
Looking at specific scores, we see weak evidence to suggest that those in the ThuVARP arm are at a lower odds of both getting embarrassed by their urinary problem and scoring at least 1 on the King’s Health Questionnaire severity measures scale (which includes wearing pads, changing underclothes because of leakage, worrying in case of smell and being careful of fluid intake).
| Variable | N (TV:TR) | Trial arm, n (%) | Adjusted differencea (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| ThuVARP | TURP | ||||
| Quality of life, mean (SD) | |||||
| IPSS quality of life | 164:175 | 1.22 (1.67) | 1.08 (1.46) | –0.17 (–0.15 to 0.49) | 0.294 |
| Presence of limitations | |||||
| Role limitations | 165:172 | 40 (24) | 39 (23) | 1.11 (0.66 to 1.87) | 0.690 |
| Physical limitations | 165:172 | 59 (36) | 55 (32) | 1.24 (0.77 to 2.00) | 0.374 |
| Social limitations | 164:172 | 30 (18) | 33 (19) | 0.97 (0.56 to 1.69) | 0.911 |
| Personal relationships | 115:128 | 76 (66) | 81 (63) | 1.18 (0.69 to 2.02) | 0.555 |
| Emotions | 163:176 | 42 (26) | 52 (30) | 0.86 (0.52 to 1.42) | 0.552 |
| Sleep/energy | 162:174 | 116 (72) | 129 (74) | 0.91 (0.55 to 1.50) | 0.710 |
| Severity measures | 161:168 | 76 (47) | 97 (58) | 0.65 (0.41 to 1.03) | 0.067 |
| Urinary symptom effect on . . . | |||||
| Getting embarrassed | 164:175 | 23 (14) | 37 (21) | 0.61 (0.34 to 1.11) | 0.108 |
| Overall interference with everyday life, mean (SD) | 162:173 | 1.33 (2.39) | 1.42 (2.27) | –0.07 (–0.55 to 0.41) | 0.778 |
Statistical ancillary analyses
Subgroup analyses
Formal tests of interaction were employed to explore potential effect modifiers. Looking at the interaction tests for the prespecified subgroups (Tables 20 and 21), we can see that those participants diagnosed with LUTS benefited from a greater increase in Qmax if they were in the TURP arm, whereas there was little difference between the arms in those patients diagnosed with urinary retention. Younger men were also more likely to benefit from TURP than from ThuVARP in terms of Qmax. However, all 95% CIs were consistent with no interaction effects, although these analyses are likely to be underpowered.
| Variable | IPSS at 12 months | p-value | |||
|---|---|---|---|---|---|
| Trial arm, mean (SD); n | Subgroup-specific MD (95% CI)a | Interaction MD (95% CI)b | |||
| ThuVARP | TURP | ||||
| Subgroup analyses | |||||
| Baseline diagnosis | |||||
| LUTS | 8.19 (7.38); 64 | 7.63 (5.72); 78 | 0.52 (–1.63 to 2.67) |
– –0.17 (–2.61 to 2.27) |
0.888 |
| Urinary retention | 4.90 (4.80); 87 | 4.49 (4.16); 81 | 0.32 (–1.03 to 1.68) | ||
| Age (years) | |||||
| < 70 | 6.83 (7.04); 75 | 6.27 (5.59); 90 | 1.00 (–0.83 to 2.83) |
– –0.79 (–3.23 to 1.66) |
0.519 |
| ≥ 70 | 5.76 (5.30); 76 | 5.72 (4.70); 69 | –0.07 (–1.68 to 1.53) | ||
| Perioperative prostate size (ml) | |||||
| Small (< 40) | 6.21 (5.39); 68 | 6.38 (5.69); 63 | –0.18 (–2.10 to 1.74) | – | 0.614c |
| Medium (40–60) | 6.68 (6.63); 41 | 5.72 (5.34); 53 | 0.54 (–1.64 to 2.72) | 0.90 (–2.00 to 3.81) | |
| Large (60–80) | 5.40 (7.22); 20 | 4.65 (3.33); 17 | –0.25 (–3.97 to 3.47) | 1.20 (–2.85 to 5.24) | |
| Very large (> 80) | 6.67 (7.35); 12 | 5.50 (3.07); 8 | 3.76 (–3.08 to 10.59) | 2.61 (–2.71 to 7.93) | |
| Comorbidities at baseline | |||||
| With | 6.27 (5.56); 67 | 6.77 (5.39); 64 | –0.60 (–2.43 to 1.23) |
– –1.58 (–4.05 to 0.89) |
0.202 |
| Without | 6.31 (6.74); 84 | 5.54 (5.06); 95 | 0.92 (–0.73 to 2.56) | ||
| Variable | Qmax at 12 months | p-value | |||
|---|---|---|---|---|---|
| Trial arm, mean (SD); n | Subgroup-specific MD (95% CI)a | Interaction MD (95% CI)b | |||
| ThuVARP | TURP | ||||
| Subgroup analyses | |||||
| Baseline diagnosis | |||||
| LUTS | 18.68 (10.11); 78 | 23.81 (12.36); 95 | –5.11 (–8.53 to –1.69) | – | 0.189 |
| Urinary retention | 21.51 (14.06); 90 | 23.07 (13.42); 81 | 1.56 (–5.77 to 2.65) | 3.54 (–1.84 to 8.91) | |
| Age (years) | |||||
| < 70 | 22.33 (13.41); 85 | 26.69 (12.62); 99 | –5.26 (–9.10 to –1.43) | – | 0.114 |
| ≥ 70 | 18.00 (11.00); 83 | 19.33 (11.93); 77 | –1.06 (–4.60 to 2.47) | 4.17 (–1.09 to 9.43) | |
| Perioperative prostate size (ml) | |||||
| Small (< 40) | 18.18 (9.87); 78 | 24.08 (13.13); 73 | –6.17 (–9.95 to –2.39) | – | 0.774c |
| Medium (40–60) | 22.60 (12.69); 42 | 23.70 (12.85); 57 | –1.09 (–6.34 to 4.15) | 4.84 (–1.74 to 11.42) | |
| Large (60–80) | 20.25 (12.35); 21 | 23.19 (12.04); 18 | 0.64 (–8.23 to 9.50) | 2.83 (–6.36 to 12.02) | |
| Very large (> 80) | 20.43 (20.08); 15 | 24.68 (18.98); 9 | –1.38 (–22.52 to 19.76) | 1.64 (–9.84 to 13.13) | |
| Comorbidities at baseline | |||||
| With | 19.17 (12.33); 75 | 21.02 (11.44); 76 | –1.80 (–5.63 to 2.02) | – | 0.307 |
| Without | 21.02 (12.52); 93 | 25.33 (13.55); 100 | –4.91 (–8.67 to –1.15) | 2.79 (–2.66 to 8.25) | |
Sensitivity analyses
Several sensitivity analyses were conducted to test the robustness of the primary outcome results. The per-protocol and CACE analyses (although prone to bias) strengthened the intention-to-treat results. In the imputation primary analysis, the IPSS differed by 0.28, whereas in the per-protocol and CACE analyses the differences were –0.04 and 0.05, respectively, with CIs indicating equivalence (Figure 7 and Table 22).
FIGURE 7.
Testing equivalence for IPSS.

| Variable | N (TV:TR) | Trial arm, mean (SD) | Difference in meansa (95% CI) | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Sensitivity: IPSS symptom score | ||||
| ITT complete-case analysis | 151:159 | 6.29 (6.22) | 6.03 (5.21) | 0.43 (–0.78 to 1.64) |
| Per protocolb | 114:156 | 5.78 (5.63) | 5.99 (5.25) | –0.04 (1.28 to 1.21) |
| CACE analysisc | 0.05 (–1.22 to 1.32) | |||
| Removal of participantsd | 147:157 | 6.04 (5.84) | 5.97 (5.13) | 0.26 (–0.92 to 1.44) |
| Adjusted for baselinee | 143:146 | 6.05 (5.78) | 6.07 (5.23) | 0.13 (–1.08 to 1.34) |
| Adjusted for imbalancef | 52:67 | 8.00 (6.71) | 7.79 (5.93) | 0.28 (–1.98 to 2.53) |
| Surgeon effectsg | 149:157 | 6.29 (6.26) | 6.03 (5.24) | 0.44 (–0.76 to 1.65) |
| Post hoc: bootstraph | 0.43 (–0.77 to 1.64) | |||
| Post hoc: log-transformationi | 151:159 | 1.67 (0.81) | 1.69 (0.73) | 0.00 (–0.16 to 0.17) |
| Sensitivity: Qmax level | ||||
| ITT complete-case analysis | 168:176 | 20.19 (12.43) | 23.47 (12.82) | –3.42 (–6.10 to –0.73) |
| Per protocolb | 123:172 | 19.30 (11.01) | 23.75 (12.83) | –4.61 (–7.39 to –1.83) |
| CACE analysisc | –4.67 (–7.56 to –1.78) | |||
| Removal of participantsd | 163:173 | 20.12 (12.19) | 23.51 (12.87) | –3.47 (–6.16 to –0.77) |
| Adjusted for baselinee | 155:162 | 19.81 (11.87) | 23.39 (12.42) | –3.87 (–6.57 to –1.16) |
| Adjusted for imbalancef | 60:80 | 19.93 (11.16) | 23.90 (12.61) | –3.95 (–8.07 to 0.17) |
| Surgeon effectsg | 165:175 | 20.28 (12.46) | 23.53 (12.84) | –3.44 (–6.11 to –0.78) |
| Post hoc: bootstraph | –3.42 (–6.06 to –0.78) | |||
| Post hoc: log-transformationj | 168:176 | 2.83 (0.61) | 2.99 (0.60) | –0.17 (–0.29 to –0.04) |
In the imputation primary analysis, Qmax levels differed between the arms by –3.12 in favour of TURP. For the per-protocol and CACE analyses, the differences were –4.61 and –4.67, respectively, indicating a larger improvement for the TURP arm (Figure 8 and see Table 22). The multiple imputation, complete-case, per-protocol and CACE analyses all demonstrated non-equivalence. Changing the hypothesis to superiority, not penalised after non-inferiority or equivalence, the results demonstrated that TURP was superior to ThuVARP. 28
FIGURE 8.
Testing equivalence for Qmax levels.

All sensitivity analyses were in agreement with the results from the main analyses. Adjusting for imbalance (painful ejaculation) reduced the sample size substantially as those patients with a catheter had not answered a questionnaire at baseline. The post hoc bootstrap analysis, and log-transformed analyses, conducted to ensure that the slightly skewed distribution did not affect the outcome, were also in agreement.
The type of TURP procedure was included as a prespecified subgroup analysis in the trial protocol paper;1 however, given that an interaction term was not possible, the team chose to use it in an exploratory analysis instead. Figure 9 shows how each of the centres differed in their use of monopolar and bipolar TURP.
FIGURE 9.
Monopolar and bipolar TURP use across the trial centres.
The IPSS results were equivalent when comparing ThuVARP separately with monopolar TURP and bipolar TURP. The monopolar technique showed the greatest improvement when looking at Qmax, resulting in non-equivalence (Table 23).
| Variable | N (TV:TR) | Trial arm, mean (SD) | Difference in meansa (95% CI) | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Sensitivity: IPSS symptom score | ||||
| ThuVARP vs. monopolar TURP | 113:84 | 5.81 (5.65) | 6.43 (5.78) | –0.74 (–2.49 to 1.01) |
| ThuVARP vs. bipolar TURP | 113:72 | 5.81 (5.65) | 5.47 (4.53) | 0.92 (–0.75 to 2.59) |
| Sensitivity: Qmax level | ||||
| ThuVARP vs. monopolar TURP | 121:93 | 19.09 (10.94) | 23.30 (13.49) | –5.41 (–9.19 to –1.64) |
| ThuVARP vs. bipolar TURP | 121:79 | 19.09 (10.94) | 24.27 (12.07) | –3.67 (–7.38 to 0.05) |
Looking at the Qmax results, we can see that the results from bipolar TURP versus ThuVARP comparison included the zero difference in its CI. When comparing monopolar with bipolar directly (Table 24), we can see that IPSS scores are slightly reduced in patients receiving the bipolar TURP procedure than in those patients receiving the monopolar procedure, whereas the monopolar technique fared better than the bipolar technique in terms of Qmax levels. However, the CIs suggested that these observations could have been a result of chance. These analyses were exploratory and were hypothesis generating rather than confirmatory.
| Variable | N (BT:MT) | TURP | Difference in meansa (95% CI) | |
|---|---|---|---|---|
| Bipolar | Monopolar | |||
| Baseline diagnosis, n (%) | ||||
| Baseline diagnosis of LUTS | 116:126 | 52 (45) | 68 (54) | – |
| Sensitivity: IPSS symptom score, mean (SD) | ||||
| Bipolar vs. monopolar TURP | 72:84 | 5.47 (4.53) | 6.43 (5.78) | –3.66 (–8.38 to 1.06) |
| Sensitivity: Qmax level, mean (SD) | ||||
| Bipolar vs. monopolar TURP | 79:93 | 24.27 (12.07) | 23.30 (13.49) | –3.25 (–15.16 to 8.65) |
Serious adverse events
Pathological findings
Pathology findings (including the weight of the resected prostate) were collected from 3-month CRFs and were available for 386 (94%) of the randomised patients. Cases of high-grade prostatic intraepithelial neoplasia (PIN) were excluded from analyses comparing benign and prostate cancer diagnoses. Although exploratory, our results suggest that participants in the ThuVARP arm were at a 65% lower odds of finding prostate cancer than those patients in the TURP arm (p = 0.007) (Table 25). This is probably due to the prostate weight available after resection, which was, on average, 15 ml higher in the TURP arm than in the ThuVARP arm (p < 0.001).
| Variable | N (TV:TR) | Trial arm | Difference (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| ThuVARP | TURP | ||||
| Prostate histology | |||||
| Resection weight (ml), median (IQR) | 149:162 | 7.0 (2.0–15.0) | 20.0 (11.0–35.0) | –15.4a (–19.3 to –11.5) | < 0.001 |
| Benign, n (%) | 193:193 | 182 (94) | 166 (86) | ||
| Prostate cancer, n (%) | 193:193 | 10 (5) | 25 (13) | 0.35b (0.16 to 0.75) | 0.007 |
| High-grade PIN, n (%) | 193:193 | 1 (1) | 2 (1) | ||
Looking at this on a treatment-received basis, we can see that the gap between ThuVARP and TURP becomes wider (Table 26). Conversions from ThuVARP to TURP gave results that were very similar to those for TURP alone.
| Variable | Trial arm | Conversion (TV to TR) | Alternative procedure | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Prostate histology | ||||
| Resection weight (ml), median (IQR) | 4.3 (2.0–10.0) | 20.0 (11.4–35.0) | 19.0 (10.0–36.0) | 13.0 (0.0–26.0) |
| Benign, n (%) | 142 (97) | 175 (86) | 29 (85) | 2 (100) |
| Prostate cancer, n (%) | 4 (3) | 27 (13) | 4 (12) | 0 (0) |
| High-grade PIN, n (%) | 0 (0) | 2 (1) | 1 (3) | 0 (0) |
Serious adverse events
There were a total of 53 serious adverse events across 41 participants in the ThuVARP arm and 53 adverse events across 45 participants in the TURP arm.
Overall, 20% and 22% of patients in ThuVARP and TURP arms, respectively, suffered from a serious adverse event (Table 27). Two unrelated deaths occurred during the follow-up period, one in each arm. In the ThuVARP arm, this occurred 34 days after the operation (bowel ischaemia and subsequent organ failure) and the post-mortem results confirmed that it was unrelated to treatment. In the TURP arm, this occurred 298 days after the operation, when the participant died from an acute myocardial infarction.
| Variable | Trial arm, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Number of SAEs reported | ||||
| 0 | 164 (80) | 160 (78) | ||
| 1 | 32 (16) | 39 (19) | 0.93 (0.57 to 1.49)a | 0.747 |
| ≥ 2 | 9 (4) | 6 (3) | ||
There is a slightly larger difference between the arms when we look at the actual treatment received, with 19% of participants receiving ThuVARP suffering a serious adverse event, compared with 23% of participants receiving TURP (Table 28). Conversions to TURP resulted in fewer serious adverse events, with only 14% of participants suffering from an event; however, there were very few participants to base this on and even fewer in the alternative treatment group.
| Variable | Trial arm, n (%) | Conversion, n (%) | Alternative treatment, n (%) | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Number of SAEs reported | ||||
| 0 | 123 (81) | 163 (77) | 31 (86) | 4 (57) |
| 1 | 22 (15) | 43 (20) | 4 (11) | 2 (29) |
| ≥ 2 | 7 (5) | 6 (3) | 1 (3) | 1 (14) |
Of the 106 events in total, 39 occurred in participants receiving ThuVARP surgery, 57 occurred in participants receiving TURP, six occurred in participants receiving a conversion from ThuVARP to TURP and four occurred in participants receiving an alternative treatment (see Table 28).
The majority of events were unrelated to treatment (Table 29), with 25 events listed as ‘probably’ related. Most of these were haematuria (ThuVARP, n = 2; TURP, n = 5), urinary retention (ThuVARP, n = 3; TURP, n = 4) and infections (ThuVARP, n = 3; TURP, n = 6). An additional six serious adverse events (including four deaths) were collected after 365 days of follow-up. These have not been included here to avoid potential bias from differential reporting across sites. All six were confirmed as unrelated or unlikely to be related to treatment.
| Variable | Trial arm, n (%) | Conversion, n (%) | Alternative treatment, n (%) | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| Relatedness to surgery | ||||
| Probably | 11 (28) | 13 (23) | 1 (17) | 0 (0) |
| Possibly | 8 (21) | 15 (26) | 2 (33) | 2 (50) |
| Unlikely | 6 (15) | 4 (7) | 1 (17) | 0 (0) |
| Unrelated | 14 (36) | 25 (44) | 2 (33) | 2 (50) |
Statistical results summary
-
Primary analysis results demonstrate that TURP and ThuVARP are equivalent in terms of IPSS at 12 months following treatment, but not in terms of Qmax, with TURP showing additional benefit over ThuVARP.
-
Sensitivity analyses, including a complete-case, a per-protocol and a CACE analysis, demonstrated that these findings were robust to the statistical assumptions made.
-
No differences were observed between TURP and ThuVARP in terms of complications.
-
Other surgical outcomes, such as length of hospital stay, transfusion rates and blood levels, were also very similar between the arms.
-
Patient-reported outcomes for urinary symptoms showed no differences between the arms at 12 months.
-
Patient-reported outcomes for sexual symptoms showed no differences between the arms at 12 months.
-
Quality of life and satisfaction with treatment were high in both arms of the trial, with no evidence to suggest that one arm was better.
-
There was no evidence to suggest any subgroup effects, although these analyses are likely to be underpowered.
-
The number of participants undergoing their randomised procedure differed between the two groups, with fewer participants randomised to ThuVARP receiving their allocated treatment.
-
A post hoc exploratory analysis of the pathology data suggested that those patients in the TURP arm were more likely to be diagnosed with prostate cancer than those patients in the ThuVARP arm.
Chapter 4 Economic evaluation
Parts of this chapter are reproduced from Noble et al. 29 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The objective of this chapter is to report the within-trial economic evaluation of the UNBLOCS trial, conducted from randomisation to 12-month follow-up, from two perspectives: an NHS secondary care perspective and a wider NHS perspective. This economic evaluation is also reported in Noble et al. 29 A cost–consequences analysis of ThuVARP versus TURP in men with BPO in relation to QALYs, the IPSS and Qmax is reported. Furthermore, cost-effectiveness analyses of ThuVARP versus TURP from the two perspectives in relation to QALYs are reported. Costs are derived from resources used by patients in relation to the interventions and the use of NHS services in relation to the treatment of LUTS, or urinary retention secondary to BPO. QALYs are determined from the EuroQol-5-Dimensions, five-level version (EQ-5D-5L), questionnaire and the cross-walk valuation set. 30
Methods
Measurement and valuation of relevant resource use
Resource use was collected from randomisation for 12 months. Data on resource use for this analysis came from three main sources: trial CRFs, hospital patient-linked information costing systems (PLICS) and patient-completed questionnaires.
Case report forms were completed by UNBLOCS nurses from the treating hospital and captured the operation, including time (which was calculated from time of start of resection until the time the participant entered recovery) and disposable equipment; and the postoperative stay, including complications, returns to theatre and time spent on different wards (calculated as the number of days from the time leaving recovery). The complete details of the resources collected are in Table 30.
| Resource | Unit cost (£) | Source of cost |
|---|---|---|
| Theatre time | 15.70 per minuteb | Finance department of a treating hospital |
| Recovery ward | 12.71 per minute | Finance department of a treating hospital |
| Laser capital and reusable equipmentc | 93.61a | Manufacturer |
| Laser consumablesd | Varies | Manufacturer |
| TURP capital and reusable equipmente | 15.81a | Manufacturer |
| TURP consumablesf | Varies | Manufacturer |
| Blood transfusion | 498.26 | NHS Reference Costs 2016/17 31 |
| Ward | 360 per day | Finance departments of a treating hospital |
| HDU/ITU | 1300 per day | Finance departments of a treating hospital |
| Subsequent inpatient stays | Varies | NHS Reference Costs 2016/17;31 Curtis and Burns, 201732 |
| Day cases | Varies | NHS Reference Costs 2016/17 31 |
| Outpatient visits | Varies | NHS Reference Costs 2016/17 31 |
| Outpatient procedures | Varies | NHS Reference Costs 2016/17 31 |
| Accident and emergency attendances (no admission) | 147.80 | NHS Reference Costs 2016/17 31 |
| GP surgery visit | 29g,h | Curtis and Burns, 201732 |
| GP home visit | 89.44g,h,i | Curtis, 201333 |
| GP telephone call | 14.60 | Curtis and Burns, 201732 |
| GP nurse visit | 5.53h,j | Curtis and Burns, 201732 |
| District nurse visit | 38.68i | Curtis and Burns, 201534 |
| Community continence nurse visit | 83 | NHS Reference Costs 2016/17 31 |
| NHS 111 call | 12.26 | Pope et al., 201735 |
| Car mileage | 0.45 | HM Revenue and Customs36 |
| Community-based urology service visit | 103 | NHS Reference Costs 2016/17 31 |
| Medication | Varies | NHS Drug Tariff 37 |
Informatics or similar departments in all sites were contacted and asked if they would be able to provide electronic information from PLICS for inpatient stays and day cases in the form of International Classification of Diseases, Tenth Revision (ICD-10);38 OPCS-4;6 and Healthcare Resource Group (HRG) codes; admission and discharge dates; and, for outpatient visits and procedures, service codes, HRG/currency codes and attendance dates. For all centres for which this was possible, a Microsoft Excel spreadsheet containing a list of study identifiers (IDs) and the dates of randomisation and 12-month follow-up were sent to the on-site study research nurses. The nurses added NHS and hospital numbers to the study IDs, which were then forwarded to the relevant contact. Data on all inpatient stays and outpatient attendances between the two given dates were then added to this spreadsheet and returned to the research nurse, who anonymised the data before transferring them to the co-ordinating centre. For one centre for which this was not possible, the local principal investigator manually extracted data on all inpatient stays and outpatient visits from the hospital systems.
All inpatient admissions occurring on the randomisation date were deleted, as were events that, according to clinician opinion, could not be related to the treatment of LUTS or urinary retention secondary to BPO. When HRG codes were not supplied, the ICD-1038 and OPCS-46 were used to map the event to a relevant HRG code.
The resource use questionnaire was administered to participants at 3- and 12-month follow-up. Either it was posted or nurses provided it to the participant at clinic to be completed in their own time and then returned by post. The questionnaires were used to collect information on NHS and private community-based health-care use (e.g. GP visits, district nurse visits), other NHS hospital health-care use, medications, Personal Social Services resource use in addition to travel, time off work/usual activities and any other expenses resulting from their treatment.
All resource use and the unit costs used to value the resources are given in Table 30. The 2016/17 costs excluding VAT (value-added tax) were used to value the resource use.
Any costs given in an earlier year were upgraded using the Hospital and Community Health Services Index.
A form of microcosting was used to cost the operation and the initial hospital stay. The trust finance department of one of the participating hospitals and equipment manufacturers were approached to obtain values for the initial NHS resources used. Any equipment that differed between the two procedures was costed. For ThuVARP this included the cost of the laser itself and for TURP this included the cost of the generator. For both procedures, the costs of any consumables and working elements required for the operations were included. To obtain a cost per procedure for the capital equipment, an ‘annual equivalent cost’, which took into account capital charges/depreciation over the useful life of the equipment,39 was calculated for the two operations. The annual maintenance costs for the laser machine and the generator were then added to these respective costs. These costs were divided by the number of TURP procedures (n = 260) carried out in a single operating theatre in 1 year. If one procedure had been converted to another, then both capital equipment costs were assigned to the participant. The cost per minute of a medi room (a room in which patients are admitted to prior to surgery and return to following surgery) was used to value time in recovery.
The NHS Reference Costs 2016/1731 were used to value inpatient stays and outpatient visits and procedures. The elective inpatient unit cost related to the relevant HRG code was used. For outpatient procedures, the unit cost relating to the service code and HRG code for each procedure was used. A consultant-led unit cost relating to the relevant service and currency code was used for outpatient appointments. When the outpatient currency code was missing, a value based on the unit cost for that service code for total outpatient attendances was applied.
Outcome data collection and valuation
The primary economic outcome in the UNBLOCS trial is the QALY as recommended by NICE. The EQ-5D-5L was given to participants at baseline and at the 3- and 12-month follow-up to either complete at clinic or take home and return by post. At the 6-week follow-up, the EQ-5D-5L was administered by post. The participants’ self-reported EQ-5D-5L values at baseline and at the 6-week and 3- and 12-month follow-up were transformed into utility scores using the cross-walk valuation set as currently recommended by NICE. QALYs for each participant are calculated from the utility scores using the area under the curve approach, and this takes into account any deaths that have occurred during the study. 40 The co-primary outcomes of the effectiveness analysis, IPSS and Qmax, are also evaluated in the cost–consequences analysis.
Data cleaning and missing costs and outcomes
The health economists were blinded to the randomisation allocation until all of the data cleaning and valuation of resources had taken place. Simple imputation was used for a few missing data items occurring during the operation. If the start of resection time was missing (n = 35), then the anaesthetic start time was used. If the time leaving recovery ward or discharge time (for day cases) was missing (n = 52), then a 3-hour duration was used, based on information given by one of the hospitals. If the type of laser fibre used was missing (n = 5), then a reusable fibre was assumed; if the number of laser fibres was missing (n = 14), then one was assumed. In relation to the missing items from the resource use questionnaires, if the questionnaire had been returned and an item was missing, then it was assumed that no resource had been used. In relation to medications, if a dose was missing, then the usual dose was obtained from the British National Formulary. 41 In relation to incontinence pads/pads costs, the cost of the most commonly used pad was imputed.
Multiple imputation by chained equations was used. The model included baseline, 6-week and 3- and 12-month utility variables, trial arm, baseline diagnosis of LUTS or urinary retention, baseline comorbidities, age and centre. The typical approach is that the number of imputations should surpass the percentage of incomplete cases; thus, 54 individual imputations were conducted and combined using Rubin’s rules27 in Stata 15.1 in relation to both the secondary care and the NHS perspective. The randomisation seed of 12678 was employed to create reproducible imputations.
Analysis
The economic analyses are conducted using an intention-to-treat approach, that is, analysing participants in the arm to which they were randomised, irrespective of any post-randomisation changes. As the trial period does not fall beyond 1 year, no costs and effects are discounted. No modelling has been specified within this evaluation, as the work is seen as a definitive trial, and experience has shown that most of the uncertainty in relation to cost differences are captured within the first 12 months, the duration of this trial.
The cost of each item of resource used during the 12 months of follow-up is evaluated as the resource use (e.g. number of minutes in theatre) multiplied by its unit cost. The total cost for each resource category (e.g. theatre equipment, subsequent inpatient stays), the NHS secondary care and NHS perspective for each individual patient was calculated as the sum of the cost of resource use items. The mean resource use and costs were estimated and presented by trial arm for each resource use category (e.g. outpatient visits, equipment costs). The mean EQ-5D-5L domain scores by time point were estimated and presented by arm. The method of seemingly unrelated regressions, which accounts for the correlation between costs and QALYs, was used to estimate adjusted mean costs and QALYs and the differences in adjusted mean costs, QALYs (and their associated 95% CIs) between the trial arms in relation to secondary care NHS costs and NHS costs. Costs and QALYs were adjusted for the stratification variables used in the randomisation process [centre and patient eligibility classification at baseline (bothersome LUTS or urinary retention)].
Quality-adjusted life-years were adjusted for the stratification variables and baseline utility. 42
Cost–consequences analysis
A cost–consequences analysis was conducted in which adjusted secondary care costs and all NHS costs were compared with adjusted QALYs, IPSS and Qmax scores.
Cost-effectiveness analysis
Cost-effectiveness analyses were conducted in which the secondary care NHS costs and all NHS costs were compared with QALYs. Incremental cost-effectiveness ratios were created using seemingly unrelated regressions if neither treatment was dominant (i.e. less expensive and more effective). Seemingly unrelated regression outputs were used to estimate the incremental net monetary benefit (INMB) statistic at the standard NICE willingness to pay threshold of £20,000 per QALY. Cost-effectiveness acceptability curves, which show the probability that ThuVARP is the cost-effective option compared with TURP at different willingness-to-pay-per-QALY thresholds, were created to explore uncertainty.
Sensitivity analyses
A series of one-way sensitivity analyses were conducted on the NHS secondary perspective analysis (as this contained the most complete data and the greatest cost drivers) to test the robustness of different parameter estimates and assumptions made in relation to resource use and costs. The sensitivity analyses included a complete-case analysis; the exclusion of prostate cancer-related hospital resource use; the addition of TURP capital costs to those patients randomised to ThuVARP to account for TURP equipment that needed to be available because of laser equipment failure; the application of the average times of theatre for each arm from the last 25% of cases in each centre to all other respective cases in order to examine the learning curve; the exclusion of post-recovery ward costs for those participants who underwent a ThuVARP procedure in the five centres where day-case TURP procedures were not conducted to examine the cost implication of being able to conduct the ThuVARP procedure as a day case; the exclusion of the capital equipment costs from both operations to reflect the fact that manufacturers often give capital equipment free provided that enough consumables are purchased; and adjustment for the number of people who would have had the operation in 1 year.
Results
Complete resource use and cost data for the secondary care perspective were obtained for 385 participants (95% of those patients randomised who did not withdraw their data). In the case of the NHS perspective, the completeness of the data declined to 47%. The resource use questionnaires administered at 3 and 6 months were not completed adequately to allow a patient or a societal perspective to be conducted. The number of complete cases from a patient perspective would have been 127. The number of complete cases and the means and costs of these resource use items are given in Appendix 1. EQ-5D-5L data were complete for 89% of participants at baseline, 82% of participants at 6 weeks, 78% of participants at 3 months and 81% of participants at 12 months; however, because there was a great deal of intermittent missingness, complete QALY data were obtained for only 212 (52%) participants. The seemingly unrelated regression analyses were conducted on all randomised participants who did not withdraw consent for their data to be used (n = 407).
In terms of resources used (Table 31), the ThuVARP procedure took, on average, 21 minutes longer than the TURP procedure. Participants in the TURP arm spent longer in recovery (14 minutes) and they spent more time in high dependency units/intensive therapy units, and they had slightly more inpatient stays and outpatient visits and slightly fewer day cases. Participants in the TURP arm also had slightly more community-based health service contacts and medications.
| Resource category | Trial arm | |||||
|---|---|---|---|---|---|---|
| ThuVARP | TURP | |||||
| n | Mean resource use (SD) | Mean cost (SD) (£) | n | Mean resource use (SD) | Mean cost (SD) (£) | |
| Theatre time (minutes) | 191 | 82.48 (33.57) | 1283.08 (522.39) | 196 | 61.50 (28.57) | 958.55 (453.16) |
| Recovery ward (minutes) | 191 | 143.97 (138.43) | 1789.24 (1465.17) | 196 | 157.58 (170.75) | 1928.45 (1736.43) |
| Laser reusable equipment (number of uses) | 203 | 0.93 (0.26) | 85.70 (24.27) | 204 | 0 | 0.00 |
| Laser consumables (number of consumables) | 202 | 2.23 (0.88) | 42.02 (21.94) | 204 | 0 | 0.00 |
| TURP reusable equipment (number of uses) | 203 | 0.24 (0.43) | 4.55 (8.20) | 204 | 0.98 (0.14) | 18.50 (2.73) |
| TURP consumables (number of consumables) | 203 | 0.42 (0.83) | 50.82 (99.62) | 204 | 2.54 (0.72) | 218.98 (91.75) |
| Blood transfusion (number of units) | 203 | 0.00 | 0.00 | 204 | 0.01 (0.70) | 2.44 (34.89) |
| Ward (days) | 203 | 1.55 (1.21) | 559.63 (434.74) | 204 | 1.67 (1.43) | 601.81 (514.37) |
| HDU/ITU (days) | 203 | 0.01 (0.07) | 6.40 (91.24) | 203 | 0.02 (0.28) | 25.62 (364.97) |
| Subsequent inpatient stays (number of stays) | 203 | 0.064 (0.26) | 176.84 (789.78) | 204 | 0.078 (0.32) | 230.26 (1188.75) |
| Day cases (number of cases) | 203 | 0.28 (0.66) | 111.37 (281.49) | 204 | 0.24 (0.48) | 99.48 (227.05) |
| Outpatient visits (number of visits) | 203 | 1.18 (1.73) | 125.70 (177.58) | 204 | 1.29 (1.72) | 143.23 (194.12) |
| Outpatient procedures (number of procedures) | 203 | 0.26 (0.56) | 33.05 (71.75) | 204 | 0.25 (0.61) | 32.20 (76.04) |
| Secondary care NHS costs (unadjusted) | 190 | 4274.33 (2033.97) | 195 | 4241.02 (2372.14) | ||
| Inpatient stays at other NHS hospitals (number of stays) | 144 | 0.00 (0.00) | 0.00 (0.00) | 151 | 0.01 (0.11) | 6.93 (60.98) |
| Outpatient visits at other NHS hospitals (number of visits) | 144 | 1.12 (1.70) | 21.52 (118.86) | 151 | 0.71 (1.48) | 7.53 (35.46) |
| A&E visits (number of visits) | 139 | 0.09 (0.53) | 12.76 (78.54) | 145 | 0.08 (0.41) | 11.21 (60.55) |
| Face-to-face GP contacts (number of contacts) | 128 | 0.57 (1.66) | 16.54 (48.10) | 136 | 0.79 (1.85) | 24.59 (59.90) |
| Telephone calls with GP (number of calls) | 116 | 0.16 (0.57) | 2.27 (8.30) | 119 | 0.33 (1.47) | 4.78 (21.51) |
| District nurse visit (number of visit) | 117 | 0.15 (0.98) | 5.62 (37.76) | 122 | 0.16 (0.53) | 6.02 (20.52) |
| Community-based health service contacts (number of contacts) | 110 | 0.12 (0.60) | 0.65 (3.33) | 117 | 0.27 (1.26) | 2.63 (12.42) |
| Medications (number of medications) | 123 | 0.03 (0.25) | 9.11 (40.68) | 138 | 0.05 (0.33) | 9.68 (45.25) |
| NHS costs (unadjusted) | 88 | 4198.61 (2188.11) | 103 | 3930.59 (1969.54) | ||
In the secondary care analysis, the total adjusted mean costs in the ThuVARP arm were slightly higher (£4252) than those in the TURP arm (£4244), a cost difference of just £9 (see Table 33). The higher cost of the time in theatre has, to some extent, been offset by the higher cost of disposable equipment in the TURP arm and the greater use in the TURP arm of specific resources outlined above. In the NHS analysis, this cost difference had decreased slightly to £4 as a result of the slightly greater community care NHS use among participants in the TURP arm.
The EQ-5D-5L domain scores (Table 32) show an improvement (i.e. the mean score decreases) between baseline and 12 months in both arms, with the exception in the ThuVARP arm of mobility and self-care.
| Domain | Score, mean (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | 3 months | 12 months | |||||
| ThuVARP | TURP | ThuVARP | TURP | ThuVARP | TURP | ThuVARP | TURP | |
| Mobility | 1.47 (186) | 1.47 (184) | 1.49 (169) | 1.37 (169) | 1.45 (155) | 1.40 (164) | 1.54 (159) | 1.38 (170) |
| Self-care | 1.10 (187) | 1.17 (185) | 1.17 (169) | 1.11 (168) | 1.10 (157) | 1.09 (165) | 1.15 (159) | 1.09 (171) |
| Usual activity | 1.57 (184) | 1.57 (185) | 1.61 (165) | 1.54 (170) | 1.42 (156) | 1.34 (163) | 1.42 (159) | 1.34 (171) |
| Pain/discomfort | 1.87 (185) | 1.96 (183) | 1.64 (169) | 1.68 (170) | 1.53 (156) | 1.50 (162) | 1.47 (158) | 1.46 (169) |
| Anxiety/depression | 1.44 (187) | 1.51 (183) | 1.33 (169) | 1.40 (169) | 1.29 (156) | 1.29 (164) | 1.25 (159) | 1.28 (170) |
Participants who had complete QALY data had a similar number of adjusted mean QALYs (0.86, 95% CI 0.83 to 0.89 in the TURP arm and 0.84, 95% CI 0.81 to 0.87 in the ThuVARP arm). For the multiple imputation analysis, this was still the case (0.84 TURP vs. 0.83 ThuVARP) (Table 33); the difference is equivalent to an extra 4 days of perfect health. TURP therefore weakly dominates ThuVARP in that it is very slightly more effective and less costly, but this could be a result of chance. This is also the case for the two primary outcomes.
| Variable | n (TV:TR) | Trial arm, adjusted, mean (95% Cl) | Adjusted difference in means (95% Cl) | |
|---|---|---|---|---|
| ThuVARP | TURP | |||
| QALY | 203:204 | 0.83 (0.81 to 0.85) | 0.84 (0.82 to 0.86) | –0.01 (–0.04 to 0.01) |
| IPSS | 197:199 | 6.43 (6.79) | 6.26 (5.79) | 0.28 (–0.92 to 1.49) |
| Qmax level | 197:199 | 20.16 (16.88) | 23.24 (13.28) | –3.12 (–5.79 to –0.45) |
| NHS secondary care costs | 203:204 | £4252.92 (£3992.29 to £4513.54) | £4244.12 (£3985.12 to £4503.11) | £8.80 (–£358.64 to £376.24) |
| NHS costs | 203:204 | £4309.45 (£4046.08 to £4572.82) | £4305.23 (£4043.75 to £4566.71) | £4.22 (–£366.60 to £375.04) |
No incremental cost-effectiveness ratios were created because TURP weakly dominated ThuVARP. The INMB at a threshold of £20,000 per QALY in relation to the secondary care perspective was –£236.24 (95% CI –£891.96 to £419.48) and in relation to the NHS perspective was –£231.57 (95% CI –£892.46 to £429.32). Figure 10 illustrates the cost-effectiveness acceptability curves for the two perspectives and shows that, at the willingness-to-pay threshold of £20,000 per QALY, the probability that ThuVARP was the cost-effective treatment compared with TURP was 24% for the NHS secondary care perspective and 25% for the NHS perspective.
FIGURE 10.
Cost-effectiveness acceptability curves from (a) an NHS secondary care perspective; and (b) an NHS perspective.
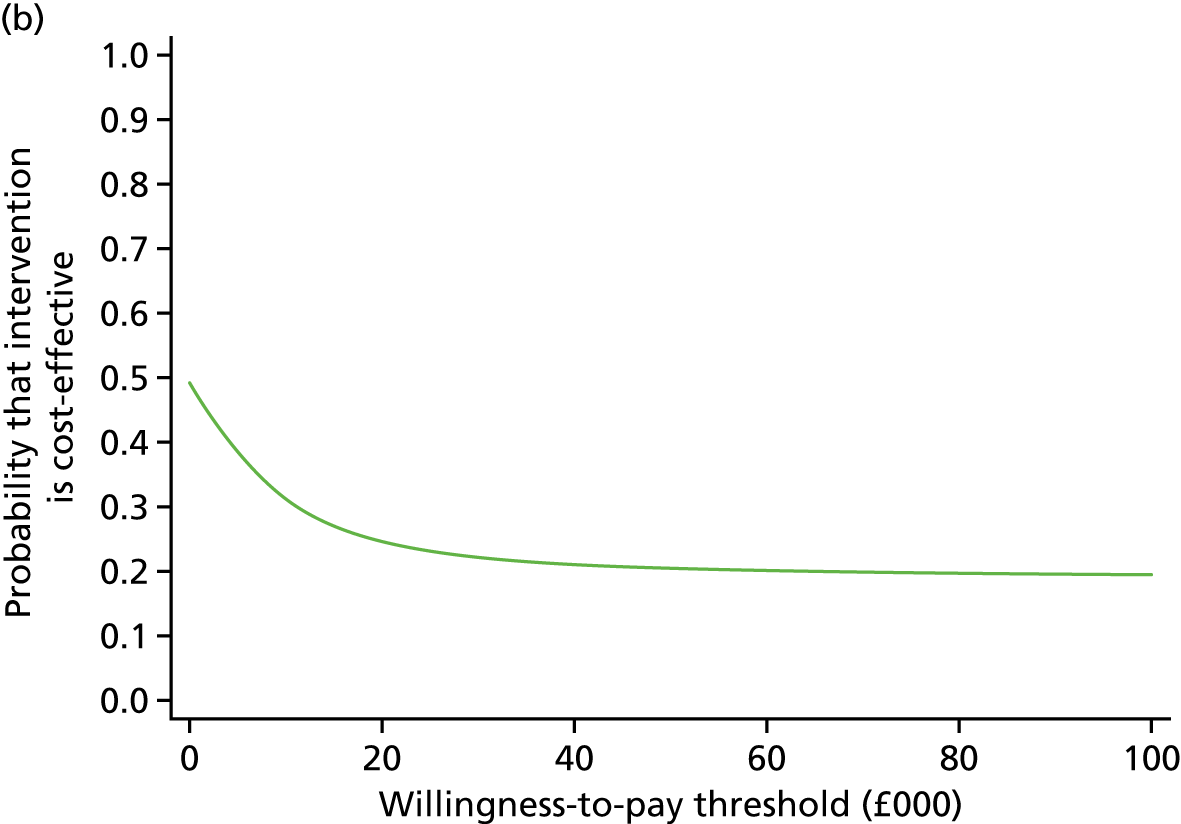
The sensitivity analyses (Table 34) show the initial results to be robust; only when costing ThuVARP as day-case surgery did ThuVARP have a positive INMB (£98.95) at a threshold of £20,000 per QALY.
| Trial arm | n a | Adjusted, mean (95% CI) | Incremental, mean (95% CI) | ICER (£/QALY) | INMB (£) at £20,000/QALY (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Costsb (£) | QALYsb | Costs (£) | QALYs | ||||
| Complete-case analysis | |||||||
| ThuVARP | 75 | 4085.53 (3735.04 to 4436.02) | 0.84 (0.81 to 0.87) | ThuVARP dominated by TURP | –1185.53 (–2111.18 to –259.87) | ||
| TURP | 77 | 3528.74 (3182.88 to 3874.61) | 0.87 (0.84 to 0.90) | 556.79 (61.94 to 1051.63) | –0.03 (–0.072 to 0.009) | ||
| The exclusion of prostate cancer-related hospital resource use | |||||||
| ThuVARP | 203 | 4192.06 (3934.95 to 4449.16) | 0.83 (0.81 to 0.85) | £2270.32d | –215.93 (–883.15 to 451.29) | ||
| TURP | 204 | 4219.71 (3963.99 to 4475.43) | 0.84 (0.82 to 0.86) | –27.65 (–390.65 to 335.35) | –0.01 (–0.04 to 0.01) | ||
| Application of the average times of theatre from the last 25% of cases by arm in each centre, all other casesc | |||||||
| ThuVARP | 183 | 4252.56 (3986.43 to 4518.70) | 0.83 (0.81 to 0.85) | ThuVARP dominated by TURP | –1382.08 (–2038.85 to –725.31) | ||
| TURP | 185 | 4179.60 (3915.71 to 4443.49) | 0.84 (0.82 to 0.86) | 72.96 (–301.50 to 447.42) | –0.01 (–0.04 to 0.02) | ||
| ThuVARP costed as a day case | |||||||
| ThuVARP | 203 | 3909.32 (3654.55 to 4164.09) | 0.83 (0.81 to 0.85) | ||||
| TURP | 204 | 4244.57 (3988.76 to 4500.39) | 0.84 (0.82 to 0.86) | –335.25 (–696.05 to 25.54) | –0.01 (–0.04 to 0.01) | 28,375.12 | 98.95 (–546.90 to 744.81) |
| Excluding the cost of the laser machine and TURP generator | |||||||
| ThuVARP | 203 | 4186.80 (3927.69 to 4445.91) | 0.83 (0.81 to 0.85) | 4534.61d | –175.55 (–832.10 to 481.00) | ||
| TURP | 204 | 4238.27 (3979.01 to 4497.53) | 0.84 (0.82 to 0.86) | –51.47 (–418.20 to 315.26) | –0.01 (–0.04 to 0.01) | ||
| Capital and reusable equipment costs based on 100 uses | |||||||
| ThuVARP | 203 | 4400.13 (4137.97 to 4662.29) | 0.83 (0.81 to 0.85) | ThuVARP dominated by TURP | |||
| TURP | 204 | 4278.79 (4018.28 to 4539.30) | 0.84 (0.82 to 0.86) | 121.34 (–247.22 to 489.91) | –0.01 (–0.04 to 0.01) | –343.87 (–986.70 to 298.96) | |
| Capital and reusable equipment costs based on 500 uses | |||||||
| ThuVARP | 203 | 4209.23 (3949.04 to 4469.42) | 0.83 (0.81 to 0.85) | 2671.38d | |||
| TURP | 204 | 4239.92 (3981.04 to 4498.79) | 0.84 (0.82 to 0.86) | –30.69 (–398.49 to 337.12) | –0.01 (–0.04 to 0.01) | –199.04 (–849.47 to 451.38) | |
| The need for TURP equipment to be available because of failures in ThuVARP equipment | |||||||
| ThuVARP | 203 | 4261.36 (4003.38 to 4519.33) | 0.83 (0.81 to 0.85) | ThuVARP dominated by TURP | |||
| TURP | 204 | 4243.68 (3983.94 to 4503.43) | 0.84 (0.82 to 0.86) | 17.68 (–348.73 to 384.08) | –0.01 (–0.04 to 0.01) | –268.38 (–910.59 to 373.83) | |
Discussion
The total mean secondary care costs in the ThuVARP arm were £9 higher than those costs in the TURP arm, which reduced to £4 when all NHS costs were included; however, this could be down to chance. A slightly higher QALY score in the TURP arm meant that weak dominance was shown for both perspectives.
Strengths
The use of PLICS to obtain information on follow-up outpatient visits (including procedures) and inpatient stays meant that these data can be assumed to be complete, and it is likely that more complete information was obtained this way than would have been the case through a medical note review. It was also less burdensome on the research nurses. Detailed data collection during the operation meant that a form of microcosting to establish the difference between the two operations could be used.
Limitations
In relation to the use of PLICS, the lack of diagnosis codes in the outpatients’ information may have meant an overestimate of the number of urological appointments related to the treatment of LUTS or urinary retention secondary to BPO; however, this would have been the same in both trial arms. There may have been an underestimate of uncertainty around the theatre costs resulting from the use of simple imputation methods. Resection time missingness did not vary by arm. Missingness of recovery time is slightly higher for TURP (34 vs. 24), which could have led to a slight overestimate of the costs in the TURP arm. Equipment was costed using a bipolar list of TURP equipment, as this was the most common procedure. Even if the monopolar fixed equipment cost per procedure had been double that of the bipolar equipment (£15.64), this would not have had a significant effect on the results.
There is uncertainty with the NHS analysis. The number of complete cost cases was < 50% and the percentage of complete cases differed by arm; furthermore, the unadjusted NHS costs were lower than the secondary care perspective costs, potentially a reflection of healthier participants completing more of the resource use questions. Although the results using multiple imputation showed that the difference between the two arms diminished because of the higher number of community-based contacts that was reflected in community-based resources use, the results from the NHS perspective need to be treated with caution compared with those from the NHS secondary care perspective. The resource use questions were in a separate questionnaire, and therefore perhaps less likely to have been completed than if they had been in the main trial questionnaire. The need to have additional questions to have a wider-than-NHS perspective may have led to non-completion. Additionally, because of previous research,43 the participants had been given a resource use log to complete throughout the study to act as an aide-memoire, but they may have felt that they were being asked to do the same thing twice. It was therefore not appropriate to conduct an analysis from a patient or societal perspective as originally planned because of the incompleteness of the data.
Conclusions
The results indicate that, from an NHS secondary care perspective, participants in the ThuVARP arm had slightly higher costs and fewer QALYs than those in the TURP arm, but these differences were consistent with chance. At a willingness-to-pay threshold of £20,000 per QALY, there is only a 24% probability that ThuVARP is the cost-effective intervention compared with TURP.
Chapter 5 Qualitative study
Introduction
Qualitative methods are increasingly being used to develop a thorough understanding of the views and experiences of patients involved in RCTs44 and to provide an insight into the patient experience that could be missed when using quantitative measures alone. 45 It is reported that successful clinical outcome may not necessarily equate to patient satisfaction. 46 Thus, it is important to gain an insight into the meaning attributed to success, satisfaction and outcomes when patients discuss their experiences. The qualitative study nested within the UNBLOCS trial explored participants’ views of their symptom experience and outcomes from the interventions. The specific aims were to investigate the participants’ experiences of:
-
LUTS prior to trial participation
-
the intervention
-
the recovery period
-
the outcome from intervention.
The qualitative study was included as a vital component of the trial to contextualise the quantitative findings and provide greater understanding of the experiential difference between the two treatment options.
Methods
Recruitment and sampling
All trial participants who had consented at baseline to being approached by a qualitative researcher were identified, and potential interviewees were purposively sampled to represent both surgical interventions, both presentations for surgery (LUTS and urinary retention) and the varied geographical areas involved in the study. Researchers contacted participants by telephone to explain the study and participants then received a specific information leaflet describing the qualitative study by post. Written informed consent was sought in advance of the interview and a convenient time was arranged for it to take place. Forty-four participants were invited to take part and 37 took part in an interview. The seven study participants who declined to take part in an interview provided reasons for this, including preferring not to be interviewed, having difficulty hearing over the telephone, having poor health and considering the interviews too personal. As study participants were already involved in the main trial, we did not experience many difficulties in making contact as current contact details were well documented. We took a proactive approach and varied the times of day to make calls as well as making repeat calls where initial contact had failed.
Interviews
Participants were interviewed on one occasion between 3 and 6 months following surgery. This was deemed a suitable time frame for the participants’ recovery to have stabilised but also for their recall of experiences to be optimised. A topic guide (see Appendix 2) was developed to explore the key qualitative aims and this was pilot tested during initial interviews and amended iteratively as interviews progressed to ensure all newly emerging elements of the patient experience were explored. The questions in the topic guide were open-ended and followed a common structure to explore all issues of relevance. Exploration of the study aims and issues newly emerging were investigated to provide a comprehensive understanding of the patients’ perspective. Participants were interviewed from all of the seven sites included in the study and only those participants from the Bristol site were interviewed face to face. Participants were purposefully sampled to ensure balance in the presenting symptoms experienced, LUTS and urinary retention, in order to reflect the breadth of indications for surgery. Interviews were conducted between July 2015 and January 2017. The interviews lasted between 20–60 minutes. Participants were recruited until data saturation was achieved and no further themes were emerging.
Analysis of the interviews
All of the interviews were audio-recorded and transcribed verbatim, for which consent had been provided. Transcripts were prepared in parallel with data collection, and analyses were conducted on an ongoing basis for emerging findings to inform the focus of future interviews. Transcripts were read and re-read for familiarisation, and a coding frame was devised to thematically analyse their content. 47 Transcript data were imported into the software package NVivo 10 (QSR International, Warrington, UK) to facilitate formal electronic coding and data management. Data were coded according to the framework identifying themes and subthemes to highlight patterns within the data of relevance to the research aims. Data were continuously coded until data saturation was reached, at which point very few new codes were emerging. 48 Table 35 describes the key topic areas explored during the interviews.
| Topic area | Areas of investigation |
|---|---|
| LUTS/retention experience | Symptoms prior to surgery |
| Symptom impact | |
| Use of urinary management aids, medications | |
| Health-seeking drivers | |
| Expectations for outcome | |
| Perioperative experience | Physical health surrounding hospital admission |
| Psychological health surrounding hospital admission | |
| Experience of surgery and hospital stay | |
| Complications from surgery | |
| Short-term postoperative experience | Initial recovery period symptom experience |
| Impact/return to usual activities | |
| Symptom improvement | |
| Unresolved/new symptoms | |
| Outcome | Symptoms following surgery |
| Changes in symptoms through recovery period | |
| Impact on daily life and comparison with pre-surgical period | |
| Factors influencing recovery | |
| Satisfaction with surgery | |
| Comparison with expectations | |
| Discontent with surgery | |
| Suggestions for overall improvement of experience |
Results
In total, 37 participants aged between 61 and 83 years (median age 70 years) were interviewed (Table 36). Of these participants, 19 had undergone ThuVARP and 18 had undergone TURP. Of these participants, 19 had presented with urinary retention and 18 had experienced LUTS; however, at least five of those participants presenting with retention had also experienced various forms of LUTS over the years. Following the analysis of data, overarching themes were identified that reflected the participants’ experience and journey to recovery. Within these themes, perspectives on satisfaction were embedded. As the qualitative team had been unblinded to the randomised procedure, analyses were also undertaken with this knowledge in order to identify if any patterns emerged according to procedure. Figure 11 shows the overall conceptual framework devised to summarise the themes identified in the qualitative study according to stage of participants’ journey.
| Characteristic | Trial arm | |
|---|---|---|
| ThuVARP | TURP | |
| Centre (n) | ||
| 1 | 6 | 6 |
| 2 | 2 | 3 |
| 3 | 3 | 2 |
| 4 | 2 | 2 |
| 5 | 4 | 0 |
| 6 | 2 | 3 |
| 7 | 0 | 2 |
| Age range (years) | 61–83 | 64–82 |
| Presenting symptoms (n) | ||
| LUTS | 10 | 8 |
| Retentiona | 9 | 10 |
FIGURE 11.
Conceptual framework of qualitative findings from participants in the UNBLOCS trial.
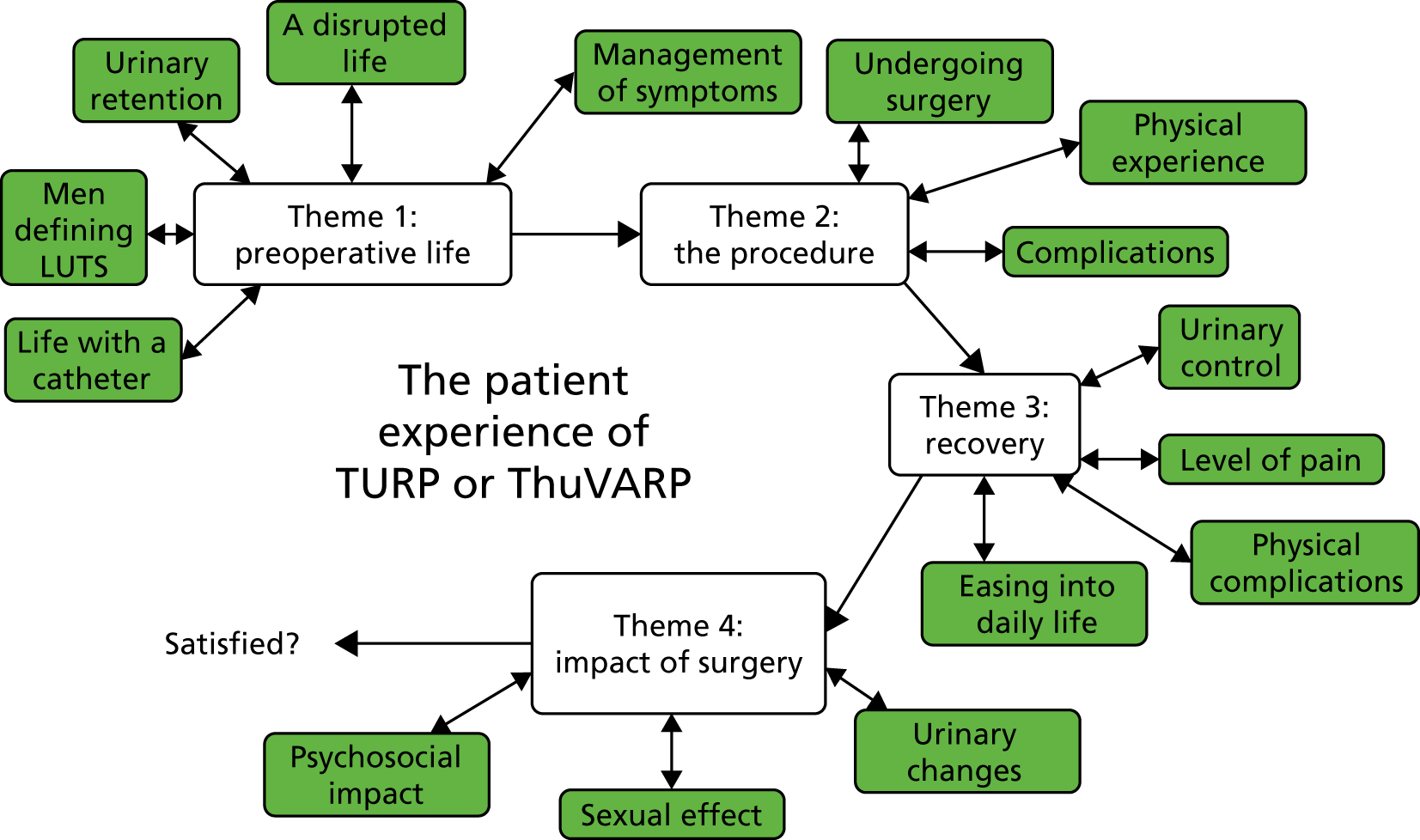
Preoperative life
Defining lower urinary tract symptoms
The participants interviewed had presented with a range of LUTS that were typically summarised as ‘old men’s’ problems’ (Figure 12).
FIGURE 12.
Participants’ descriptions of commonly occurring LUTS.

The onset of these symptoms was described as slow and gradual by the men interviewed who had constructed LUTS as intrinsic to the ageing process. In some ways, this had allowed participants to normalise their symptoms rather than perceive them as a major concern requiring medical attention and this had continued for some years. For some men, the eventual recognition that the issues were indicative of a larger urinary problem was accompanied by a sudden escalation of symptom severity. Participants reported an overwhelming feeling that suggested a lack of control as the challenges imposed by these symptoms became increasingly problematic. This was perceived as detrimental to the quality of life of individuals as the disruptive nature of LUTS began to manifest:
It had happened gradually I suppose, over, I don’t know, 10 years or so I hadn’t really noticed . . . so I hadn’t put it down to anything other than just old age until this, until I peed a bit of blood.
62 years, urinary retention presentation, ThuVARP
Eventual urinary retention
Typically, the somewhat mild urinary symptoms had started to surface some years before patients participated in this study and, for some, other health problems would lead to the eventual deterioration of urinary function. Interestingly, most participants with retention described a significant lack of urinary issues before their urinary retention emerged, and this was repeatedly described as a ‘switch’ by one participant. This signified the rapid, unexpected nature of urinary retention:
I didn’t have any [urinary symptoms] at all other than I got up, um, one morning and just couldn’t go.
69 years, urinary retention presentation, ThuVARP
Other participants believed urinary retention to be the outcome of years spent overlooking their LUTS, although they had acknowledged this only after the onset of retention. Participants appeared unable to recall a specific pattern to this symptom, as it was largely unpredictable:
We were on a coach tour and I felt I needed to go the toilet so I went in the one on the coach and I had the feeling I wanted to go but I couldn’t pass and I felt like that all the way down to Yorkshire through the day, the following day it was no better and my stomach was all swollen.
74 years, urinary retention, TURP
Suddenly I’m not able to go to the toilet and even when I go there’s just the measliest dribble it’s like losing the pressure off and I mean the whole thing was definitely very different and why this suddenly happened I’m not sure.
74 years, urinary retention presentation, ThuVARP
Participants reporting urinary retention appeared eager to pass urine as they stressed that the feeling of needing to do so would become increasingly urgent. They continued to emphasise the need for ‘relief’, which was finally achieved through catheterisation. Furthermore, participants reflected on their emotional relief once they had become aware of the underlying problem. Generally, urinary retention was described as an unsettling experience, and this was largely because of the uncertainty that participants had felt when attempting to determine the cause of their pain. It was an experience largely shaped by a feeling of not knowing, which resulted in some anxiety and worry:
I was obviously concerned, you know, I thought it may have been something more sinister.
74 years, urinary retention, TURP
A disrupted life
The effect of urinary symptoms varied daily, and this depended on the specific symptoms with which participants presented. Travelling was particularly difficult for those participants with LUTS, as the frequent need to pass urine meant that they required regular breaks. This resulted in some participants reducing their use of public transport, and those participants who chose to drive long distances recalled that the time taken to do so was much greater because of the frequency of toilet breaks. This was embarrassing for these participants and it could also be embarrassing for their partners:
Travelling was very difficult obviously because you’re often travelling for more than an hour.
61 years, LUTS, ThuVARP
Any sort of long drive is a bit of a problem.
63 years, LUTS/urinary retention presentation, ThuVARP
This inconvenience was also reflected in participants’ discussions of their experiences of being in public. All men with LUTS described a routine of pre-planning before any social outings to ensure that a toilet would be nearby. Despite this, some men continued to encounter problems, describing their fear of having urge incontinence when toilet facilities were in use. This was a source of embarrassment and it also reduced confidence, and participants detailed the way in which these issues had made them self-conscious; for example, any form of incontinence could result in visible marks on their trousers. Furthermore, those participants who used incontinence pads or catheters described the embarrassment of having to use specific cubicles that would allow them to use these without others seeing; this was frustrating and time-consuming:
You were always searching for toilets, you know having to plan things, just generally a bit of a drag, I mean it’s not great quality of life, really, when you’re doing that.
69 years, LUTS/urinary retention presentation, ThuVARP
The other side of it or downside of it was me wanting to go and someone using the toilet, well a couple of times I nearly had an accident with it.
66 years, LUTS presentation, ThuVARP
Therefore, it appeared that such urinary symptoms required a constant state of vigilance, which meant that participants were unable to enjoy many things. This resulted in a reduced quality of life, and participants continued to discuss the unpleasant nature of these symptoms and the way that these affected both their lives and their psychological well-being:
You really couldn’t do nothing properly because I had to keep running backwards and forwards to the toilet when I was doing my jobs.
75 years, LUTS presentation, ThuVARP
It was, you know, embarrassing for my wife and embarrassing to me to explain to people. I explained it because rather than think ‘what’s wrong with this guy?’ You know, so you just handled it the best way you could.
66 years, LUTS presentation, ThuVARP
It became in the end that I’d probably became a bit of a recluse. I didn’t want to go out.
66 years, LUTS presentation, ThuVARP
It’s very embarrassing because you obviously had to change your trousers and you couldn’t come out and people said, well, why have you just changed your trousers? You had to tell them but luckily I’m quite thick-skinned that way.
69 years, urinary retention presentation, ThuVARP
Within the home, the time taken to complete everyday activities was also negatively affected by the frequency with which they needed to pass urine and also by incontinence, resulting in the need to wash and change far more often:
I was out in the garden for instance, I think I’d only last about half an hour and then I had to run in, washing my hands [because they] were all dirty and change your shoes and by the time I got in there I wetted myself.
75 years, LUTS presentation, ThuVARP
The daily tiredness caused by nocturia also resulted in irritable moods and negatively affected relationships; for example, one participant discussed his decision to sleep in a separate bedroom to his partner to avoid disturbing her:
I could be up four or five times, tired. I get cross with my wife poor love, she’d say I was bad tempered.
76 years, LUTS presentation, TURP
Management of symptoms
Some of the men interviewed had discussed their initial reluctance to report their urinary symptoms and to visit the doctor. This appeared to be a prominent issue among these men, which some speculated was shaped by perceptions of manhood. Instead, they had adopted strategies to assist them as they attempted to cope with their symptoms:
I would wear the shirt outside the trousers just in case anything did happen so it would maybe help cover it up. I had to be careful where I went to and if I went to [place name] I would make sure I knew where the toilets were, that sort of thing.
68 years, LUTS presentation, ThuVARP
I carry a little plastic pot that’s just in case. In fact that’s had to be used. It was a sort of portable loo.
76 years, LUTS presentation, TURP
If you were on a long journey you had to make sure you had the stops or take something with you to relieve yourself, I used to take a bottle with us.
68 years, LUTS presentation, TURP
As the symptoms became more severe, participants became more amenable to the idea of seeking medical guidance. Before being involved in this trial, a number of participants had sought to alleviate some symptoms by using non-surgical interventions. Some had been offered a surgical procedure at an earlier stage but had instead made the decision to explore options that were considered less invasive. Medication was believed to have helped for a short period, but these improvements were not permanent. This was considered disappointing, as were the side effects that the participants had experienced as a result of this medication:
Finasteride particularly has a dulling effect [on] one’s sexual appetite.
65 years, LUTS presentation, ThuVARP
I was given a choice of a drug medication or the operation so we decided to go with the drug medication first of all so I went on that for about a year but it didn’t really make any difference.
71 years, LUTS presentation, TURP
Other participants found that incontinence pads were necessary to help them manage and cope with unexpected urinary leakage. Such accounts were met with some humour as the men recognised that these products were important. Although pads were accepted as useful, they were also noted to be a financial burden, which meant that participants were eager to find ways to spread this cost. An example of this was one participant cutting his pads in half and another using his wife’s sanitary products instead. Participants also highlighted that pads could be demeaning in some ways, as they were constantly conscious of any possible smells and whether or not the pads were visible. Finally, it appeared that, for these participants, surgery was considered a last option for dealing with these problematic symptoms.
Life with a catheter
A number of participants were also required to use a catheter, and perspectives differed on this form of symptom management. Generally, the catheter was considered a substantially inconvenient part of daily life as it often prevented participants from fully engaging in many aspects of life. Physically, participants felt unable to exercise, to have sex, to travel, to sleep comfortably and to socialise for extended periods of time. The catheter was uncomfortable, and in some cases it resulted in pain:
Horrendous absolutely horrendous, it was really, really traumatic, well it was just constant, you know, you were aware it was there all the time. I was unable to drive properly, it just interfered. I was having to empty this thing every couple of hours this bag you know. Ah, I hated it, absolutely hated it.
74 years, LUTS presentation, TURP
Such restrictions could be considered a psychological phenomenon in some ways, as some participants explicitly stated that actually participating in certain activities was less challenging than they had believed it would be. Nonetheless, the catheter significantly influenced the reduction in these participants’ psychological quality of life. Depression, embarrassment, fear and low self-esteem meant participants were further restricted in what they were able to do on a daily basis. This had major implications for the identity of these men, as it became evident that the catheter had become a threat to their sense of masculinity. As such, the narrative around catheter use was intrinsically linked to the notion that the catheter was far more problematic for participants as men:
I had to sort of carry these catheters around. I was due to go away four times that year and three holidays I couldn’t go on because I didn’t feel confident about, you know, anything.
69 years, LUTS/urinary retention presentation, ThuVARP
It just wasn’t me.
71 years, urinary retention presentation, TURP
These negative experiences were pivotal in participants’ decisions to undergo surgery, as the participants stressed that the possibility of a future without a catheter had motivated them. The extent to which these men were dissatisfied with catheters, and the resulting effect on their life, was highlighted as they depicted a sense of enthusiasm for an invasive procedure rather than proceed with their life as it was:
If I didn’t, I’d have to have a catheter for in for the rest of my life, it was enough for me . . . That was enough for me to make the decision.
74 years, LUTS presentation, TURP
The thought of the rest of my life with catheters was a bit off-putting to be honest.
82 years, urinary retention presentation, TURP
Importantly, not all participants viewed their catheter as a problem. For some, it had come to symbolise relief, as it eased the pain and obstruction caused by retention. Furthermore, it allowed some participants to freely socialise and to move beyond the constraints of their home, as they were provided with a feeling of secureness, although this depended on the type of catheter used. Some found that the catheter prevented them from the embarrassment of having to frequently go to pass urine when they were socialising. It also enabled them to pass urine with ease, without interrupting any activities. However, the participants noted that in these situations they became conscious of the need to empty their catheter, which was a task that made them self-conscious, as did the visibility of the catheter:
I had a catheter fitted, that was a bit of a lifesaver for me because I actually got my life back together. Although it was an embarrassment it gave me freedom of movement of where I wanted to go and do what I wanted to do . . . My life became more tolerable, I could do what I wanted to do, if I wanted to go out I could go out and I did go out. I felt safer with the catheter and going out and doing what I wanted to do.
74 years, urinary retention presentation, ThuVARP
Discussion
This qualitative exploration of men’s lived experiences of LUTS and urinary retention revealed a number of significant themes. First and foremost, participants attempted to define their urinary symptoms. Moreover, it was revealed that participants had normalised their symptoms, believing them to be simply a feature of ageing. This has been noted elsewhere in the literature by Irwin et al. ,49 who found that most participants believed their symptoms to be a normal part of ageing. This showed that men may require information about prostatic obstructions and treatment options. It also highlighted that men need to be encouraged to visit health professionals about LUTS.
Urinary symptoms appeared to play a vital role in reducing well-being as participants experienced countless disruptions to all aspects of their life, including social, leisure, physical and, in some instances, work. An awareness of the impact on partners and family was also highlighted. Gradual isolation was also identified as a consequence of ongoing symptoms.
These findings also increase our understanding of how men view their catheters, which formed the focal point of many discussions. The dissatisfaction reported by some participants about living with a catheter contrasted with previous findings by Cobussen-Boekhorst et al. ,50 who reported that individuals could be satisfied with some elements of the experience; this was also borne out by the views of some participants. A number of participants interviewed identified the catheter as a symbolic barrier that restricted their functioning. However, this may have been because this was a cohort of men who had sought surgery for their symptoms and were unprepared to continue using a catheter. This view may not necessarily be shared by men who are satisfied with using a catheter to manage their symptoms.
The procedure
Undergoing surgery
Prior to discussing the experience of undergoing an invasive surgical procedure, participants discussed their reasons for requiring the operation and, in doing so, also described their personal expectations and aims. This highlighted the subjective nature of surgical success, with most hoping that their symptoms would reduce to a more controllable level. In these discussions, participants also relayed their expectations about their surgical experience and their preconceived perceptions of recovery. This was especially relevant when they were discussing the length of their hospital stay and any delays experienced when they were admitted. Some participants were sympathetic about this, referencing the strains on the NHS as a contributing factor, and appeared to construct this as a normal feature of being hospitalised for surgery. Some participants described delays between agreeing to participate in the trial and having their specific procedure. This was received negatively by a cohort of participants who were eager to improve their quality of life, and were deemed particularly problematic as they prolonged disruptive LUTS and retention and sometimes allowed the symptoms to increase in severity. Those participants using a catheter had also expressed their concerns that an extended wait could ultimately have a harmful effect on their recovery. Specifically, they speculated that relying on a catheter could result in an underactive bladder, which would require further attention and ‘training’ in the future:
I waited 10 months from when I first saw my doctor to when I finally had the operation or when I first contacted my doctor, it took 10 months and by then it was beginning to become obviously much more of a nuisance.
65 years, LUTS presentation, ThuVARP
I shouldn’t have had the catheter in this long and I had trouble because they knew I had trouble with my prostate. I should have been took straight in for an operation straight away, no ifs, no buts.
71 years, urinary retention presentation, TURP
Those participants interviewed thought that the surgical experience had begun just as they were anaesthetised. Discussions regarding this perioperative period were brief, and participants disclosed their psychological approach to being in a medical environment. Understandably, some presented a distinct aversion to hospitals, a feeling that was often strengthened when the individual lacked experience of being in that setting. This contrasted with the experiences of participants who had been hospitalised before and had undergone successful procedures, who were able to use their positive experiences to develop a pragmatic viewpoint of hospitals in general that provided them with assurance and comfort in a situation embroiled with uncertainties:
I was sort of anxious and nervous about going in to hospital because I’d been in to see various family members who’d been in to hospital, never liked it at all and never wanted to go in to hospital.
63 years, LUTS presentation, ThuVARP
It don’t worry me hospitals, I’ve been in before . . . I mean hospitals, they really don’t worry me but it’s just that people seem to be frightened of hospitals but I don’t seem to be frightened of them for some reason. I just let them do what they want to do and that’s it.
63 years, urinary retention presentation, ThuVARP
Some discussions about preoperative fears also became evident at this stage as feelings of anxiety were heightened by an awareness of potential side effects. Perceptions and worries about surgical success were thus interlinked with the prevalence of any side effects. Although some men were confident, they had been expected to provide consent immediately before the procedure, which had resulted in them becoming somewhat nervous about the possibility of something going wrong:
I can’t really say for sure what I was concerned about. I mean it was explained that the percentage of successful surgery, you know, was quite high, that there was a possibility of complications and a damage to the urethra and scarring and various other problems but I thought, well, on balance you know the, um, the odds were quite good for a successful outcome which is why I decided to go ahead with it.
82 years, urinary retention presentation, TURP
I didn’t know whether it would succeed or not . . . I read all these leaflets and they said, you know, you’ve got a 10% chance of this and a 5% chance of that and a 2% of this that and the other and I thought, um, you know, hopefully, you know, I won’t fall in any of those categories.
63 years, LUTS presentation, ThuVARP
Those participants who were anxious before surgery preferred to undergo a general anaesthetic rather than remain conscious during the surgery. Some had expressed a preference for an epidural as this allowed them to feel far less drowsy than they would have done after a general anaesthetic; these participants believed that this reduced the recovery period in hospital as they were more likely to feel better much sooner. The epidural had also meant that the men were unable to recall any feelings of pain during that particular stage, apart from some general discomfort:
My legs started to go to stone you know just limp like, I couldn’t move them around which was fine and that was it I was awake throughout the operation. The only thing I felt the only pain I felt during the operation was from the blood pressure monitor on my left arm.
65 years, urinary retention presentation, TURP
Anaesthesia also influenced what participants were able to remember about the procedure and so very few were able to estimate how long surgery had lasted. However, one participant did note that his surgical time had been much quicker than he had estimated, which resulted in him stating with certainty that he had undergone ThuVARP. In fact, this perspective was shared by all participants who felt that the surgical period had been particularly efficient regardless of the actual procedure that they had undergone. This did also raise an important point, namely that those men who were satisfied with the duration of surgery were adamant that they had undergone a ThuVARP procedure:
My operation time was very quick so when I came round, I knew which one I’d had. I’d had the laser one so I was very pleased with it because it was wonderful as far as I was concerned.
66 years, LUTS presentation, ThuVARP
Participants discussed various elements of their inpatient care that helped make the experience much more bearable. This included the level of care that they received from members of staff, which all participants were eager to emphasise had been excellent:
The hospital [staff] were so nice and helpful and attentive, you know, a very good hospital experience.
72 years, LUTS presentation, TURP
The majority of participants interviewed who had undergone ThuVARP recalled an average of two nights’ stay, whereas those participants undergoing a TURP reported being in hospital for an average of three nights. Longer stays could often be for a number of reasons, including perioperative complications that were reported by both TURP and ThuVARP participants. Nonetheless, participants appeared to be satisfied with the length of their hospital stay when this had been much shorter than they had expected:
This one day thing it’s just amazed me because I took stuff in for 5 days or something like they put with the booklet.
63 years, urinary retention presentation, ThuVARP
Physical experience
Immediately following surgery, most participants were distinctly unaware of any physical effects from treatment as that particular period was characterised by a range of overwhelming sensations that were indicative of recovery. Participants were attached to a catheter that was visibly filled with blood, and they were in some discomfort; however, they emphasised that this had been expected and was considered a normal part of having an invasive procedure. Some participants also reported some drowsiness, which they attributed to general anaesthesia. Those participants who had received an epidural reported numbness in their legs, which lasted a few hours:
I probably spent longer recovering from the epidural than I did from the operation.
66 years, LUTS presentation, ThuVARP
I felt pretty good actually at that point and then I went to the ward and none of it was too painful really or uncomfortable amazingly.
65 years, LUTS presentation, ThuVARP
Nonetheless, most participants agreed that this stage was largely pain-free, and this appeared to be the case regardless of procedure. However, a few participants who had been randomised to undergo ThuVARP described some soreness and discomfort, with one man requiring morphine. This was later believed to be the result of an external complication. Similarly, one participant who underwent a TURP reported a similar experience, which was believed to be the result of an infection. In fact, any differences between experiences that had emerged at this stage were often perceived to be the result of other complications, such as unrelated illnesses and infections. In general, however, men undergoing either procedure reported very little pain and some bleeding.
It’s more pain than I could sort out with paracetamol, that’s the only way I can describe [it]. You know, it was quite bad pain but the morphine sorted it out and after I don’t know how many hours, probably about 12 hours or so, I came off the morphine and was on just normal painkillers.
64 years, LUTS presentation, ThuVARP
I was suffering pain and discomfort even when I was discharged it was a bit uncomfortable if you understand, you know what I mean? Obviously in the lower area there, it wasn’t massive but I knew there was some pain there.
72 years, LUTS presentation, TURP
Bleeding was reported by all participants at this stage and this was visibly noticeable in their catheter. Although this had been expected, as all participants had been counselled regarding this, some did describe their surprise at the amount of blood that they had passed. The presence of blood clots was also recalled:
They did tell me after that there would be blood clots but the thing that was worrying me was the amount of old blood that I was amassing in my catheter bag.
65 years, urinary retention presentation, TURP
I was generating a very rich red Bordeaux wine type blood. I was on the wash with the catheter and washed to clear out particles and just the bag would fill up with really fairly deep red. I’d been a blood donor in my young army days. I’m not fazed by the sight of blood, even my own, but I felt, oh dear, I didn’t expect this and it went on Tuesday, Wednesday.
83 years, LUTS presentation, ThuVARP
These participants continued to describe some blood as the catheter was finally removed before they were discharged. It was also noted that the actual removal of the catheter was somewhat painful and uncomfortable. Some even specified that this was the most painful part of their entire hospital experience as they referred to the discomfort that it had caused. For some men, it was also followed by the reinsertion of the catheter, which was fairly difficult and problematic. This was usually because the participants were unable to pass a substantial amount of urine, which in itself resulted in a longer hospital stay. Despite the discomfort, the majority of participants specified that the procedure had been painless, which was a strong determinant of surgical satisfaction. This was particularly the case for the participants who had expected some pain, as it meant that their procedure exceeded their expectations in some ways:
When they took that out there was a lot of blood came away with it as well but it was the only discomfort really was removing it.
64 years, LUTS/retention presentation, ThuVARP
They got the catheter reinserted. Some of the instruments they used the first time I’d been hanging on to a bed for dear life but the staff nurse and the doctor were very good, but nobody could explain why it wouldn’t go in . . . I thought oh God it’s going to damage me or something but no, everything’s fine.
66 years, LUTS presentation, ThuVARP
When I came out from the anaesthetic I had no pain, nothing really, I was surprised actually because I thought oh I’m going to have all sorts of symptoms and pains and this, that and the other but I hadn’t.
79 years, urinary retention presentation, TURP
Complications
Perioperative complications reported by some participants influenced their perspective on the procedure. Such issues were recalled by six participants who had undergone ThuVARP and seven who had undergone TURP. Most commonly, these meant that the participants experienced a longer than expected hospital stay, which was somewhat disappointing for them. However, these participants also stressed that they believed that their issue was unique and not a result of their treatment, which they felt had been relatively successful:
I’m quite satisfied with the surgery, I don’t know how much of the other effects are due to that or other things but no the surgery did what it was intended to do.
63 years, LUTS/retention presentation, ThuVARP
A wide range of specific complications were reported and it became evident that it was in fact difficult for participants to determine whether they thought that these had been caused by the procedure or were the result of underlying health issues. Nonetheless, most participants gave the impression that these issues had emerged as a result of external issues that were unrelated to the procedure. One particular incident included the diagnosis of myasthenia gravis and two participants recalled issues relating to their blood pressure.
Other men reported how their hospital stay had been extended and this was sometimes a result of infections, which appeared to be equally prevalent among those participants undergoing TURP and those participants undergoing ThuVARP. Overall, infections were described by three participants who had undergone ThuVARP and three participants who had undergone TURP. These infections could be problematic, as one participant described how he had been given the wrong antibiotics, which increased his recovery time to much longer than anticipated. Other participants also agreed that such surgical complications resulted in a longer recovery period. One participant described how he had been re-hospitalised immediately after discharge as a result of norovirus:
Twelve hours after I’d come home, I woke up being violently sick and had diarrhoea. I think – well fairly sure it was the norovirus that I’d picked up while I’d been in hospital so for the next 3 or 4 hours I got weaker and weaker.
65 years, LUTS presentation, ThuVARP
Discussion
Participants’ discussions about surgery were briefer than those about other elements of their journey. It became clear that some participants considered the period of being hospitalised anxiety-inducing, which had been shaped by a feeling of ‘not knowing’. Although participants did not claim that they had been uninformed, there was a sentiment of surprise regarding events that had not been expected, for example the amount of postoperative bleeding experienced. Thus, the question arises of how to fully inform an informed patient group to appropriately manage expectations. However, participants largely considered any complications to be unrelated to the type of surgery specifically and simply viewed them as unfortunate incidents.
The period waiting for surgery was cause for concern as there was a perception that symptoms might worsen. The ability to achieve a full recovery was considered to be potentially hampered. Individuals understood this is a frustration with the NHS but had concern for their own potential health decline resulting from the ‘system’.
Recovery
Urinary control
Participants reported a number of effects during the weeks following surgery, describing the ways that their bodies attempted to recover. It was during this stage that participants reported fluctuations in their urinary symptoms, which meant that they found this particular period somewhat unsatisfactory. In total, seven participants who had undergone TURP reported LUTS during recovery, and this was also reported by nine participants who had undergone ThuVARP. The specific type of LUTS differed, as did the severity. Some urgency was described by these participants; however, most commonly, participants reported different types of incontinence, which was the case irrespective of the specific procedure they had undergone. Two participants who reported urge incontinence during this period after ThuVARP also noted that this was an ongoing issue. One participant who had undergone TURP also described some urge incontinence, which was mostly prevalent during the night; however, this had subsided after a few weeks. Interestingly, all three of these participants had initially presented with urinary retention and had used a catheter preoperatively. As a result, they felt that their bladder was simply becoming accustomed to being used:
It was more a case of there was a little night incontinence with a little bit of dribbling and that, you know, where it was shutting down properly which they said was going to happen so readjusting to that.
64 years, urinary retention presentation, TURP
Some participants also reported total incontinence, which was an issue for three participants who had undergone a TURP and five who had undergone ThuVARP. Two of these participants who had received a TURP and three who had received ThuVARP had initially presented with LUTS. The two participants who had undergone TURP had also experienced complications during their procedure:
When I came home the first afternoon, I put on my white jogging trousers and I thought I’ll just go to my bed . . . I woke up and I had just a puddle of urine just by my left hip, my right hip it was totally drenched and the blanket was just totally drenched. I’m straight away depressed to say the least.
65 years, urinary retention presentation, TURP
It made me feel dirty. You know, that’s all I can say, you know, that’s when I started wearing these pads again.
73 years, urinary retention presentation, ThuVARP
These participants utilised and incorporated into their daily lives a range of aids to ease the negative effects of this procedure. These included pads, incontinence pants, medication and, in one case, the use of a penile clamp. However, some participants specified that they had continued to use pads for security rather than necessity. Participants noted that they continued to practise pelvic floor exercises, which for one man were uncomfortable and difficult. Some participants also noted that this iatrogenic effect had led to depression and had a detrimental effect on their self-esteem as well as their mood while interacting with others:
I was still leaking heavily into pads, I think because at that stage I’d been leaking for a year or so I was just used to leaking.
72 years, LUTS presentation, TURP
I cut them in half so that I don’t waste sort of half the pad because as I say it’s like double incontinence pads so I cut them in two so that’s two pads instead of the one and I’m using maybe a pad every couple of days.
65 years, urinary retention presentation, TURP
In addition to this, two participants who had undergone ThuVARP had been catheterised throughout the recovery period, and both had experienced a problem relating to their temperature during the procedure. One of these participants stressed that his retention had been the result of a stretched bladder rather than solely due to a prostatic obstruction. As a result of this, he appeared to present a complacent attitude and to have accepted this particular outcome as his reality as it continued to be ongoing. The second participant, however, highlighted that he had contracted norovirus, which had resulted in catheterisation for two and a half weeks following his procedure. Following this, he expressed satisfaction at his ability to pass urine, which was slowly improving; however, he also noted that his urine had been somewhat cloudy during this stage:
My prostate was not particularly large. What I had was an extremely large bladder or stretched bladder where the muscles were horribly damaged due to the size of it and that’s the main problem with not being able to pee afterwards and this remains to be the same thing at the moment, but it is improving as the weeks and months go by.
69 years, urinary retention presentation, ThuVARP
Other participants saw immediate improvements; one man described how he had realised this while still in hospital when he attempted to drink a caffeinated drink. Before undergoing ThuVARP, he had restricted the type of liquids he would drink and had made the decision to drink only non-alcoholic, decaffeinated drinks. This participant no longer experienced negative effects of having drinks he enjoyed and he described his happiness at being able to drink beer for the first time in 12 years. Other participants who experienced similar immediate improvements described the surgery as ‘life-changing’, and this was again equally the case whether participants had undergone TURP or ThuVARP. It was noted that the ability to pass urine was often immediate and that the flow became stronger as the weeks passed. Furthermore, participants noted that they had been passing urine fairly frequently but during this period this subsided. Participants were elated with these changes and discussed their satisfaction at being able to pass urine in a way that reminded them of being younger:
When I come out of hospital I was going to [the] toilet as you know not as normal, more often but I was, I was quite happy with the whole thing.
71 years, urinary retention presentation, TURP
They flushed it through I went to the toilet and the nurse on duty she says ‘I’m going to take your catheter out and then we’ll see what progress is’, and from then it’s been champion.
71 years, urinary retention presentation, TURP
Level of pain
Although most participants had declared that the procedure itself had been painless, they described their period of recovery as somewhat painful. As a result, pain became an important feature of the recovery experience, and a range of descriptions were provided. In general, 12 out of 18 TURP participants felt some level of physical discomfort, whether this was while passing urine or general soreness throughout the day; 12 out of 19 ThuVARP participants reported similar experiences of pain. Most common was a burning sensation when passing urine, a severely unpleasant feeling that continued for some time:
Bit like peeing acid or peeing ground glass if you could imagine either sensation, that’s kind of where it sits between something very chemically hot like acid or something that’s friction. It would be like ground glass or even sand or something, you know, it’s not a natural feeling.
61 years, LUTS presentation, ThuVARP
This intense pain caused some participants to think negatively about their surgical procedure, a view that, for some, was shaped by their lack of awareness of this particular effect. This was specifically highlighted by participants as they stressed the importance of being warned about the pain associated with recovery. A number of participants described this pain as unbearable, although in some instances it was short-lived. Participants also explained that the information they had received about pain had been from other men who had undergone TURP in the past; this highlighted the necessity of sharing valuable information from lived experiences as it enabled participants to realise that their recovery was normal:
It only lasted 3 days but that pain was excruciating. It wasn’t worth taking any tablets for because the only pain I had was when I went to the toilet and afterwards for about a couple of minutes. The burning sensation and the pain was horrendous. You say the men don’t take pain very well but that was horrendous and I wasn’t told that. OK it would have been nice to have been told that that was pretty painful.
69 years, LUTS presentation, TURP
Some participants felt that the pain was due to the insertion of catheters and surgical equipment in their urethra, with some speculating that this may have resulted in scarring. The men stated that their urethra felt tender during the recovery period and for some this feeling had continued until the point of interview. In attempting to identify and make sense of this pain, participants largely attributed it to the invasiveness of the procedure:
The catheter that they use for the operation is bigger than the normal one because it carries a camera as well as the equipment, you know, so that’s probably what’s causing the burning. It’s as if there’s urine in my urethra up to the head of the penis and it creates a stinging, quite a strong stinging, so I need to go to relieve the stinging more than the urine.
68 years, LUTS presentation, ThuVARP
Physical complications
The participants interviewed reported that bleeding, and, in some instances, clots, when passing urine continued to be common following discharge. Although participants had been aware that this particular effect may occur, it nonetheless elicited some fear and worry. In particular, the visibility of blood and flesh had been disconcerting:
They told me that there’d be some sort of residual bleeding for a while and then it seemed to stop and then one evening, I felt the need to pee and they did warn me that a clot might come out but I wasn’t expecting it to be quite – it was sort of oh that size [comparison with a coin] and it was a bit like a bit of cloth obviously soaked in blood and that frightened me a bit.
63 years, LUTS presentation, ThuVARP
This was considered normal by some participants who had passed blood for up to 2 weeks after their procedure. In fact, for a number of participants who had undergone ThuVARP, the bleeding had subsided within a few days. Perspectives on this did vary and it was deemed concerning largely among those participants who felt that they had not been told about it beforehand. This difference in perspective can be observed when analysing the views of two participants who had a similar experience of postoperative bleeding, only one of whom had been aware that this could happen. This suggested that participants who were more informed about their recovery were more likely to be satisfied as they were aware that the bleeding would subside:
There was a little blood and clots the like, I was passing in the first couple of weeks after the operation. I didn’t think anything of it because I’d been warned that could be the case and I had seen that in my own reading and then when it was explained to me.
71 years, LUTS presentation, TURP
I was bleeding for a couple of days after that and when I got back in touch and I said I’m bleeding, you know, and they said don’t worry that’s quite normal. She said that will clear up on its own within a couple of days which it did and then after that everything’s been fine you know the colour of the urine and everything has been normal.
79 years, urinary retention presentation, TURP
Postoperative bleeding continued to dominate discussions as a number of participants noted that it had continued for an extended period, which, ultimately, had resulted in a prolonged recovery. This was especially problematic for those participants who had reported larger blood clots that were painful to pass. Although some reported ‘slithers’ of flesh when passing urine, others reported severe urinary retention due to blockages that resulted in further hospitalisations. This erratic physical effect had meant that participants were anxious and feared that it would happen again. As these repeated incidents of painful urinary retention had led to hospitalisations, the participants noted that their fears had prevented them from planning many trips, which highlighted the disruptive effect that this had on their lives. However, a combination of medication and time appeared to alleviate this problem:
I says to my wife ‘I got a lot of bleeding’, so I left it that night. The next morning I got up and I felt terrible you know because I’d worn out my stomach and it was retention so I goes to the hospital in [location] right and he says well we can’t do a lot and I felt terrible mind and I thought I could feel the blood clots . . . I couldn’t plan anything really I couldn’t plan after the holiday because you’re frightened you’re going to have another bleed.
70 years, LUTS/retention presentation, TURP
I didn’t expect it to be so bad for me but I don’t know if everybody else had the same problems, I don’t know. I had massive bleeds about 3 weeks afterwards, then I had another bleed again 2 weeks after that and I had another bleed again 2 weeks after that, so it’s been quite an experience. I had to go into hospital to be flushed out each time, it was blood that was clotting. I had blood clots all the time.
68 years, LUTS presentation, TURP
Easing into daily life
These fears had not only disrupted the plans of these participants but also affected their ability to maintain many daily activities. In general, participants were instructed to rest for some time, which they considered frustrating. Nonetheless, they stressed the need to rest, recalling trial instructions that recommended a 2-week rest period. However, some participants noted that they had found the instructions somewhat vague as they were unable to understand what the appropriate rest period would be. Some participants were able to return to daily activities almost immediately without any major problems; in these situations, they ensured that they rested during the instructed period, although they felt that they had essentially recovered before that:
You had to take things easy and not do any strenuous exercise, but if no one had told me that if someone had said, ‘Oh you can just go back to normal as soon as you know as soon as you feel fit’, it would have been about 5 days.
69 years, LUTS/retention presentation, ThuVARP
For participants who had undergone ThuVARP, the time taken to return to normal daily activities, including work, was estimated to be between 4 and 5 weeks, similar to that reported by those patients who had undergone TURP. Therefore, it appeared that for most participants, this time depended on their subjective preparedness to continue with specific activities rather on than which surgical procedure they had received. During this period, participants avoided a number of physical and leisure activities, including sport, long walks, driving and sexual intercourse. Some noted that they were able to participate in light physical activities within 2–3 weeks, usually walking the dog or shopping. More strenuous activities, such as physical exercise and sport, took much longer, and some men noted during their interviews that they had only begun to play golf again between 3 and 6 months following the procedure:
I still wanted to do my own shopping but I could hardly like walk. I’m still stubborn like that.
63 years, urinary retention presentation, ThuVARP
I do weight training and sort of gym work and I would say after 4 or 5 weeks, say 5 weeks, I was back to normal.
69 years, LUTS/retention presentation, ThuVARP
Return to daily life was delayed for participants who experienced incontinence during recovery and they described how this led to depression. Interviews revealed that this was one of the most important phases in reshaping and shifting perspectives on satisfaction. It was during this period that participants reported the profound effect that their fluctuating physical symptoms had on their daily life as they struggled to adapt to the restrictions that their surgery had imposed. Some who were catheterised described discomfort, and it became apparent that, overall, this period was distinctly characterised by psychological difficulties, as feelings of fear, anxiety and depression dominated their accounts:
I thought once I was discharged I was going to be fine you know but I was quite rough. It must have been a couple of months, you know, I couldn’t get myself together like. Not very nice it made me quite depressed actually and I don’t usually get like that.
73 years, urinary retention presentation, ThuVARP
Detrimental to an extent because you know I didn’t do any gardening and I didn’t do much walking about for up to 6 weeks after the operation so I tended to put on weight.
63 years, LUTS presentation, ThuVARP
I mean I probably didn’t go out so much when I was at the beginning of the recovery. I gradually did, we’ll go anywhere now it’s not a problem.
64 years, LUTS presentation, ThuVARP
Discussion
This theme illustrated the importance of recovery in shaping participants’ perspectives of their surgical procedure overall. During this stage, some participants continued to experience many fluctuating symptoms that placed them in ‘limbo’ and had some implications for their sense of self. These participants reported a range of negative urinary symptoms, including a frequent need to pass urine, urgency and incontinence. Further to this, bleeding and retention due to clots and pain when attempting to pass urine were commonly noted by participants regardless of procedure. In some instances, these issues led to limited function, which, in turn, caused participants to question and re-evaluate their social roles as they became somewhat reliant on the help of others, which some seemed to resent.
A range of factors became apparent during these discussions that revealed key facets of patient dissatisfaction that were specific to both procedures. This was particularly prevalent when participants described their period of recovery as many shifting determinants of dissatisfaction were embedded within participants’ journeys. For example, the subjective recollections of blood loss, pain and postoperative LUTS and urinary retention were perceived as problematic and upsetting, especially when they were viewed as prolonging their recovery. Some participants had pursued further information about the clinical aspects of their recovery, which had reassured them that their recovery was ‘normal’. However, others felt that this information was provided only postoperatively, which led to some dissatisfaction with the overall health service. Although knowledge of potentially negative effects enabled participants to psychologically cope rather than endure a feeling of uncertainty, participants emphasised that this had not made the lived experience any easier. These factors were generally viewed negatively and resulted in feelings of regret for a period following surgery as they encountered a range of difficulties and reported limitations to the activities that they were able to engage in.
Despite the perceived negative elements of their recovery, most participants were satisfied with the efficiency with which they were able to return to their normal daily activities. Factors that resulted in patient satisfaction included the efficiency of the procedure, a lack of pain, swift improvements to quality of life, alleviation of symptoms and diminished catheter dependency. Some participants had in fact felt that their symptoms had improved immediately as they detailed their satisfaction at being able to pass urine with a strong flow. Moreover, they had recognised that their ability to pass urine would generally improve and highlighted that this had indeed appeared to be the case. Recovery was essentially shorter for these participants than for others, as they described the way in which they had felt able to participate in many activities much sooner than they had expected. Others considered that previous catheter use would result in a prolonged recovery; this did appear to be the case for most as they described their need to ’re-train’ their bladders. This was problematic for those participants for whom urinary symptoms were just one of a list of other, overlapping, health issues; some discussed other life concerns that had interfered with their recovery, including the death of a partner and other operations. Ultimately, these external elements had also affected their perceptions of recovery.
Outcome of surgery
Urinary changes
During the interviews, participants were able to identify the impact that their procedure had had on their lives by discussing its eventual effect on their pre-trial symptoms. Most participants emphasised an absence of urinary symptoms at the point of interview; however, those participants who had some symptomatic issues stressed their belief that this would improve. In general, those participants who had presented with LUTS reported eventually regaining control over their bladder and urinary functions, with many specifying that they had experienced improvements to their flow rate, a reduction in nocturia and frequency and a distinct lack of incontinence. Such improvements were evident across all interviews, as men undergoing either procedure expressed their satisfaction and amazement at the changes:
The beauty of it is that I’m able to empty my bladder in one go whereas before I couldn’t sometimes. I’d have to have a wee and then 10 minutes I’d have to have another one because I couldn’t empty it but now I can.
76 years, LUTS presentation, TURP
For those men who had undergone TURP while presenting with LUTS, it was reported that their urinary symptoms gradually subsided after a period of 2–3 weeks. During this period, participants reported some urgency and, as previously noted, some suffered from incontinence. However, during their interviews participants highlighted how this had drastically improved, and this was largely attributed to general recovery and a combination of pelvic floor exercises, rest and medication. At the point of interview, it was agreed that participants were essentially free of bothersome daytime LUTS, although it was noted that they continued to pass urine during the night. However, the frequency with which this occurred had reduced:
He stressed to me, pelvic floor muscle exercises and put me on Vesicare which has worked wonders. My bladder control has gone back to like it was when I was a young man.
72 years, LUTS presentation, TURP
Similarly, some participants who had presented with LUTS and had been randomised to ThuVARP discussed their immediate ability to pass urine without difficulty and noted that their urinary frequency had reduced within 1 month. This immediate improvement was also highlighted by one participant who had been catheterised for his incontinence prior to surgery and discussed his satisfaction at regaining control over his bladder and ability to pass urine. For these men, this ability was largely improving:
When the consultant came round or whoever it was that came round looked at the bag and then said right we can take the catheter out . . . I was blown away when I heard that. It’s fantastic now. When we go out I don’t have the inconvenience. I can last for longer now than I’ve lasted for years I think.
66 years, LUTS presentation, ThuVARP
I came home, I was catheterising away every 4 hours maybe and that was working fine there was no problems with that whatsoever and then I suddenly found that I was starting to pee a little bit on my own and that was a maybe an eggcup full or half a cup full.
69 years, urinary retention presentation, ThuVARP
Those participants who had experienced urinary retention and were subsequently catheterised prior to surgery presented a range of postoperative experiences. Most men who had been randomised to ThuVARP reported immediate improvements; however, one participant did describe a continuation of the urinary frequency and nocturia that he had experienced before surgery. Another two participants discussed their incontinence during their interviews, with one expressing a stronger dissatisfaction with that particular outcome:
I’m still wearing these pants. I was three a day but I now have an appliance which is basically a clamp which I put on my penis to stop it dripping so one pair of pants is security gets me through the day but I’m still leaking. I’m still doing my five-a-day pelvic floor. It is better but it’s a very slow process.
83 years, LUTS presentation, ThuVARP
Positive outcomes were also highlighted by men who had undergone TURP for their urinary retention as some were delighted with the symptomatic outcome. These men had found that any initial LUTS had reduced and they were eager to recommend the procedure. However, other men noted that they experienced some hesitancy, frequency and urgency. This was thought to be a result of their ongoing attempts to regain control over a bladder that had become reliant on a catheter:
I’m pretty pleased with what I’ve had done so I would recommend it to any one of my friends.
75 years, urinary retention presentation, TURP
Almost normal, apart from the occasional times when there’s a little bit of a problem starting or it’s hesitant but that’s not a great problem.
82 years, urinary retention presentation, TURP
Interestingly, some participants noted that their recovery period had been fraught with a number of urinary symptoms that had resulted in dissatisfaction. However, the eventual improvements and reduction in urinary-related issues caused participants to change their initial perspective on their procedure. In doing so, participants highlighted the fragmented elements of patient satisfaction, as they had been dissatisfied with components of their experience and yet extremely satisfied with their outcome. As such, it became apparent that the surgical procedure was perceived as separate from the overall experience, and discussions on satisfaction reflected this:
Satisfied with the operation, just not satisfied with the after-effects.
68 years, LUTS presentation, TURP
I’m happy with the outcome now, yeah. It seemed to take a long time to get right.
64 years, LUTS presentation, ThuVARP
Sexual effect
Some participants reported sexual changes that they thought had resulted from the procedure. This outcome was reported broadly across both arms as participants disclosed their sexual dysfunction and sexual discomfort. Perspectives on these changes varied as participants recognised that they had been aware of potential sexual problems prior to participation and felt that this was simply the cost of having an otherwise beneficial procedure. Nonetheless, it was deemed a concerning after-effect, as participants appeared to describe some regret. Furthermore, age appeared to play a key role in shaping perceptions of this negative effect; older participants noted that sexual intercourse was no longer an important feature of their lives:
I wish it wasn’t like that [limitation to sexual matters] but if it’s the price to pay for the other issues I’d pay that price.
69 years, LUTS/retention presentation, ThuVARP
We’re both in our middle 70s and it doesn’t worry me that much I’ve had my share so [laughs] I had four children so sometimes it’s frustrating but it don’t worry me that much.
73 years, urinary retention presentation, ThuVARP
Ejaculatory issues were described by many participants, as they began to experience retrograde ejaculation following surgery. However, some participants said that this had been an issue prior to participation and perceived it to be a result of medication they had taken previously for LUTS. Participants also provided descriptive accounts of visible changes in their semen and speculated that they were no longer producing sperm:
What comes out isn’t fully loaded with sperm so you can’t in theory have children but as I said at 61 already having done that I don’t feel the sudden need to do it now. There’s no effect at all in terms of the social side of what you’re referring to.
61 years, LUTS presentation, ThuVARP
I do actually ejaculate but [unsure] whether it’s semen that I’m ejaculating because it’s quite a clear fluid.
74 years, urinary retention presentation, TURP
I was taking Tamsulosin before. I think I had a partial sort of retrograde ejaculation but now it’s total so that means, you know, it’s not as good as it was.
69 years, LUTS/retention presentation, ThuVARP
Two participants had also reported erectile dysfunction, which was particularly concerning, and expressed a desire to seek treatment. Participants had in fact felt that sexual issues had been sidelined as they noted that these concerns had not been discussed before the interview and appeared to welcome the opportunity to do so. For some participants, severe discomfort and pain during their recovery had affected their participation in sexual activities. Furthermore, passing blood and clots had resulted in fear and anxiety surrounding sex, which was evident in some accounts:
That’s almost non-existent because that’s another problem I’ve got and that’s keeping an erection so that hasn’t improved. I don’t know if it’s got worse but that hasn’t really changed no and I don’t know what I can do about that, I’ve been living with that.
64 years, LUTS presentation, ThuVARP
You’re frightened, after that first bleed you think well I can’t because or else I’ll bleed . . . I still get the urge but there’s just that feeling in the back of your mind saying that you might have a bleed.
70 years, LUTS/retention presentation, TURP
Psychosocial impact
Among the participants interviewed, it became apparent that their pre-trial symptoms had had a detrimental effect on the quality of their life. As a result, a key aim of all participants had been to improve their daily lives as it had become evident that they had hoped for a positive psychosocial effect from their particular procedure. The majority of participants felt that this had been achieved, discussing the impact of regaining control over their urinary function; for them, surgery had been ‘life-changing’. They described how relieved they were that their symptoms no longer had control over their lives:
I was coping with it before, it’s just I don’t have to think about it now. I suppose that’s the difference it’s made.
64 years, LUTS presentation, ThuVARP
Mentally it was a weight lifted off my mind. I was going back to normality, what other people did.
66 years, LUTS presentation, ThuVARP
It feels a lot better, I haven’t had any problems. I can go to the toilet and I feel fine and to me from using the self-catheter four or five times a day to going on my own is wonderful so all in all that’s been a success it has to me. I can start planning, start my life again.
70 years, LUTS/retention presentation, TURP
Participants discussed their delight at being able to participate in activities that had been impossible prior to surgery as they recalled being able to partake in long journeys, family holidays and sport with ease and without the inconvenience of stopping to pass urine. This had allowed them to regain the confidence that had been severely hindered by their urinary symptoms. They specified that the anxiety that had previously engulfed their thoughts before socialising was no longer a concern and, importantly, they were now happy:
I don’t have to worry about going to the toilet every 10 minutes, you know. I can go out and I can enjoy myself without worrying about where the next toilet is and stuff like that.
64 years, LUTS/retention presentation, ThuVARP
It was like I had another 10 years put on my life.
63 years, urinary retention presentation, ThuVARP
My happiness is like I am a new person . . . I can smile again.
75 years, urinary retention presentation, TURP
I have a life back. I’ve probably gone back almost 20 years ago. I’m a different person altogether.
66 years, LUTS presentation, ThuVARP
This reflected a process of psychological rejuvenation as participants repeatedly asserted that the surgery and its subsequent positive outcome had allowed them to regain elements of their youth. Some participants specified that they felt much younger and others noted that it had changed their entire outlook on life. As such, the difference that the surgery had made to their lives was visible and the participants noted that it had been a ‘miracle’. In fact, this level of satisfaction was also shared by the participants who had described their recovery period as problematic, as they noted that the eventual outcome meant that the challenges had been worth it:
In the first questionnaire I filled out and sent off I put that that I wasn’t very satisfied you know but at that moment that’s how I was feeling. But now when I look back I think why was I getting like that?
73 years, urinary retention presentation, ThuVARP
Almost all participants suggested that they would recommend the procedure and those participants who experienced a less than pleasing outcome felt that this was a result of external issues rather than the surgery. For these participants the effect had been unique and they speculated that this would not be the case for others. It was also highlighted that the surgery had in fact dealt with the initial symptoms, although it was believed to have resulted in new symptoms. For some men, this had resulted in depressive feelings as their lives were now restricted and dominated by urinary symptoms. However, these particular participants also described their hope and optimism that this would improve with time and as such appeared to construct their present symptoms as part of a prolonged recovery process.
Discussion
This theme aimed to provide a greater insight into the overall outcome from TURP and ThuVARP on participants who had presented with LUTS and urinary retention. Although all participants agreed that the surgery had successfully treated their prostatic obstruction, some also revealed a number of new urinary symptoms and some sexual dysfunction. The impact on urinary symptoms varied greatly and appeared to be dependent on participants’ initial symptoms. Some of those participants who had been catheterised before their surgery revealed that their recovery was ongoing as they continued to regain control over their bladder while suffering from incontinence.
The interview findings revealed that surgery had a specific effect on sexual functioning. However, it was also found that most participants had experienced some form of sexual issue before participating in this trial. This was often perceived to have been due to previous drug interventions that were thought to have failed to treat their urinary symptoms. This particular effect could have implications for participants’ perceptions of masculine identity as they often drew on notions of manhood while discussing the barriers that they encountered. When discussing the improvements to their psychological well-being following their satisfactory procedure, participants stressed that it had enabled them to feel confident and young again.
It also appeared that successful procedures that had reduced symptoms had also altered participants’ perceptions of their own bodies. Their initial LUTS and urinary retention had pushed bladder and urinary functioning to the fore of their consciousness and the final symptomatic outcomes meant that this heightened awareness had subsided along with their symptoms. This had reduced the anxious feelings that had been so prevalent. This can perhaps be interpreted using phenomenological theories relating to the concept of embodiment. In particular, the concept of the ‘lived body’, as proposed by Gadow,51 could be applicable, as it suggests that a conscious lack of awareness of one’s body or body parts results in improvements in psychological well-being.
Discussion
Strengths and limitations of the qualitative study
The fact that the participants interviewed were already involved in a surgical trial meant that we were sampling from a select group who may have had particular views, given their willingness to take part in a RCT. In addition, we interviewed only those men who had consented to participate, which may have meant that we interviewed participants with a particularly positive perspective. However, we did collect both positive and negative accounts, and the reasons for non-participation do not suggest a particular skew towards the interviewed population.
Interviews were conducted at only one time point and largely when symptoms were expected to have resolved, which may have resulted in recall bias. If we had interviewed participants during the immediate postoperative period, which appears to capture the most negative experiences overall, we might have gathered more accurate accounts of that time without the resulting improvements that have led to more positive reflections. Descriptions were fairly balanced and certainly included negative aspects, which suggests that the disadvantages of the pragmatic approach taken to reduce participant burden by interviewing once only is mitigated by the evidence in the data obtained.
The majority of interviews were conducted over the telephone rather than in person, but there is no evidence to suggest that this influenced participants’ candour. It is recognised that robustly conducted telephone interviews can be as effective as face-to-face interviews, and using the telephone enabled participants at all of the study sites to be included and allowed regional perspectives to be reflected. 52
Although approximately half of participants had experienced LUTS that may have been evident for a number of years, a strength of the study from a qualitative perspective is that patients were included who presented with urinary retention, which in some cases had a very sudden onset. The participants with urinary retention included those men who had been dismissing their symptoms for some time but now found themselves in a health-seeking situation because of their circumstances, as well as those men who had not had reason to seek help previously. The opportunity to interview these participants would not often arise and this has helped to investigate opinions related to health-seeking, for example that LUTS are ‘just part of the ageing process’.
Summary of the findings and implications
A general finding of importance is that male LUTS continue to be viewed as a ‘normal part of ageing’, which can prevent individuals from seeking treatment that could significantly benefit their lives. Health promotion activities and engagement with this population is required to alter this perspective and facilitate active treatment-seeking behaviours.
Interviews confirmed that both procedures resulted in a fairly equal patient experience, with participants reporting similar journeys of recovery and outcomes. Descriptions of a period of uncertainty that preceded satisfactory outcomes were also common. The few differences that did emerge were largely perceived as individual, unique experiences rather than representative of the surgical procedure, and this was particularly the case for participants who reported complications during the perioperative stage. These complications were thought to have had a negative impact on recovery, as some reported a prolonged period of recovery that resulted in some feelings of depression. During this stage, some participants had experienced urinary symptoms, bleeding and pain, although these were thought to have subsided by the time of the interview. In general, most participants were able to continue with their daily lives within 1 month, although some had begun to slowly reintroduce light exercise and work into their lives much earlier.
A number of participants also reported urinary retention due to clots and this was similarly noted by those undergoing either procedure. For other participants, this period was characterised by a range of LUTS that essentially delayed their return to normality. Those men who had initially presented with urinary retention appeared more likely to have experienced some incontinence during this period and discussed their need for medication and pelvic-floor exercises, which helped them to regain bladder control. These symptoms were thought to be due to previous catheter use as participants speculated that their bladder had poorer function. This was supported by the fact that some participants who had been catheterised previously for incontinence also experienced LUTS. Most found that these problems did resolve, and others hoped there would be a gradual improvement. Concerns about the length of time waiting for surgery were also linked to perceptions of the potential for full symptom resolution.
In general, almost all participants were satisfied with the final outcome as both procedures had essentially met their aims. The participants had hoped and expected to have their initial symptoms treated and, as this had happened, most discussed their delight at the improvements in their quality of life. Those participants who were less satisfied with their eventual outcome noted that they were satisfied with the procedure itself, but that outcome and, indeed, recovery had been trying. Furthermore, they described a lack of knowledge about specific side effects and suggested that the level of information they had received played a large role in shaping their feelings of satisfaction. However, it must be stressed that all participants had received the same information about the trial and any potential outcomes, yet some had described themselves as less informed. Although clinical interactions contributed to this, the personal characteristics of patients must also play a large role. Some appeared more receptive to the information and to actively pursue additional information than others. It is therefore important that clinicians and health-care staff ensure that all participants receive accurate and good-quality information that can ultimately enhance their experience.
As the majority of participants did specify that their expectations regarding the outcome of surgery had been met, most appeared generally satisfied with the outcome and the extent to which their initial symptoms had alleviated. The subjective determinants of dissatisfaction reported during recovery were particularly informative as they revealed factors that affect perceptions of outcome. Differences between the procedures in terms of blood loss, pain, large blood clots, and LUTS and retention are aspects that may influence individuals when they are considering potential treatment options, and will provide a further perspective on the quantitative findings. These symptoms were reported to contribute to a prolonged recovery, which was also considered to have had a negative influence on outcome.
Participants also stressed their need for further information, and the findings of this study will be key to strengthening the advice and guidance provided to future patients. These findings also support Burgio et al. ,53 who highlighted the importance of preoperative counselling as a determinant of satisfaction for those men undergoing stress incontinence surgery. It was noted that this would enable patients to set realistic expectations about surgical outcomes, which could ultimately minimise their postoperative impact.
The need to be aware of participants’ concerns about sexual matters when undergoing these procedures was highlighted. Participants raised concerns about their ability to conduct a satisfactory sex life, which were perceived to be related to, and to predate, surgery. The opportunity to discuss this during the patient journey is important and would provide an opportunity to signpost patients to treatment if desired. During interviews, participants commented that this was the first time they had been asked and that they were glad to have the opportunity to discuss these personal issues, something that has implications for practice going forward.
These findings extend on other studies that have explored determinants of patient satisfaction and apply them specifically to surgical interventions for BPO. Previously it has been reported following joint replacement surgery that overall satisfaction has been determined by meeting expectations, achieving pain relief, and a satisfactory hospital experience. 54 This was indeed also found to be key among this cohort as participants described resolution of symptoms and the procedure experience as pivotal to their satisfaction.
This study has provided unique insight into the lived experiences of men undergoing TURP or ThuVARP for the treatment of LUTS or urinary retention. This will provide a deeper understanding of the patient perspective when interpreting the quantitative trial data and when counselling patients in clinical practice. The ability to counsel future patients using insight from previous patients will help men to understand the patient journey and help inform decision-making. Most participants were ultimately satisfied with their surgical outcome following the recovery period and would appreciate robust information about the procedures and their recovery, along with active enquiry regarding any concerns with their sexual matters.
Chapter 6 Discussion and conclusions
Parts of this chapter are reproduced from Hashim et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary and interpretation of main findings
The UNBLOCS trial compared two technologies for treating BPO in the UK NHS: ThuVARP and the gold-standard TURP.
Patients were randomised between the two arms at the point of surgery. The two arms were generally well balanced at baseline for sociodemographic and clinical characteristics, with the groups also equally divided between those presenting with urinary retention and those with bothersome LUTS secondary to BPO. Participants had a mean age of 70 years. The proportion of participants receiving their randomised treatment was much higher in the TURP arm (98%) than in the ThuVARP arm (75%), with eight participants requiring a conversion to TURP mid-procedure.
Primary outcomes
The primary aim of the trial was to determine whether ThuVARP and TURP are equivalent in terms of the co-primary outcomes of patient-reported IPSS and Qmax at 12 months after surgery.
The two procedures appear to be equivalent for symptom relief measured by the IPSS, and therefore this blinded trial has shown that patients’ perceptions’ of urinary tract symptoms are equivalent when the actual treatment is unknown. Both procedures achieved a much improved IPSS from that reported at baseline, dropping from a mean score of around 21 for participants with LUTS (baseline data could not be collected for retention participants) to a mean score of around 6 at 12 months post surgery for all participants. This is as expected from BPO surgery, demonstrating that both procedures improve subjective symptom scores.
Maximum urine flow rate improved after both procedures, from a mean of < 10 ml per second at baseline for participants with LUTS (baseline data could not be collected for retention participants) to a mean of > 20 ml per second at 12-month follow-up for all participants. ThuVARP gives a lower maximum urinary flow rate at 12 months than TURP, deeming the treatments non-equivalent in Qmax. Changing the test to superiority provided evidence to suggest that TURP is superior to ThuVARP with respect to Qmax.
The result for Qmax is based on the assumption that a difference of > 4 ml per second has clinical significance. As discussed in Chapter 2, Equivalence margin, no minimal clinically important difference in Qmax is accepted in the literature. A figure of 2 ml per second has been quoted previously; however, following discussion between clinicians, in this trial the higher value of 4 ml per second was chosen. Therefore, the finding of non-equivalence and superiority is in the context of uncertainty about what is considered as a clinically significant difference for Qmax, and superiority was demonstrated for TURP against a relatively high value of clinical significance. However, the improvement in Qmax from baseline seen in both arms would be considered a clinically successful outcome from BPO surgery.
Secondary outcomes
The ThuVARP procedure did not lead to fewer complications, as had been expected, with results suggesting that the procedures were similar in this respect. Both procedures were equally safe perioperatively, with a risk of no more than 4% for any individual complication, and no incidence of TUR syndrome (dilutional hyponatraemia due to fluid absorption). In the 12 months post surgery, a similar incidence of clot retention (≈5%) and urethral stricture (≈3%) was observed, as well as of infections including urinary tract infection (≈35%), sepsis (≈1%) and pyrexia of unknown origin (≈3%). Overall, ≈20% of participants in both arms suffered a serious adverse event.
Of particular note when considering complications is the level of clinically significant bleeding, which was experienced equally between the two arms; around 5% of participants experienced bleeding to the point that their haemoglobin had to be measured, and around 2% of participants required a blood transfusion. One of the advantages quoted in the literature of laser procedures over standard treatment is a reduction in the risk of bleeding and transfusion rates; however, no such difference was observed in this trial. A further advantage of laser procedures that has previously been reported is a reduction in the length of hospital stay. However, in this trial the length of stay was 48 hours in both arms, which is the national average, and certainly shorter than that in the Chinese trials of the thulium laser published in the literature. 13,14
Other surgical outcomes, such as the length of indwelling catheterisation time and change in levels of haemoglobin and serum sodium pre- and postoperatively, were also very similar between the arms. Serum sodium did not reduce significantly after surgery, suggesting that serum sodium checks may not be required postoperatively, as have previously been conducted. Both groups had a median indwelling catheterisation time postoperatively of approximately 2 days.
In terms of urinary measures on the whole, taking into account IPSS, International Consultation on Incontinence Questionnaire, Qmax and post-void residual, all results were in favour of the TURP procedure on visual appraisal, albeit by a small amount and without any statistical significance. Patient-reported outcome measures in this trial looked at urinary symptoms, quality of life, sexual symptoms and satisfaction with surgery, and no significant difference was found between the arms in these. However, we cannot rule out a clinically important difference in patient-reported nocturia, with indication of an increased incidence at 12 months post surgery in the ThuVARP arm. This result is difficult to rationalise, although it may be a heat-induced effect; however, the other storage symptoms do not reflect this. It is also possible that charring from the laser could cause irritation and urinary frequency/nocturia. It should be noted that satisfaction with surgery and quality of life after surgery were rated highly in both arms, with no significant difference between the arms.
Subgroup analyses suggest a greater improvement in Qmax after receiving TURP than after receiving ThuVARP among patients diagnosed with LUTS, with no difference between the arms indicated in participants diagnosed with urinary retention. In addition, there is a suggestion that men aged < 70 years may benefit more from TURP than from ThuVARP in terms of Qmax. Further exploration of these potential effects would be of interest for future research.
Of note is the finding that only 75% of participants randomised to ThuVARP received their complete randomised treatment, as opposed to 98% of participants who underwent TURP procedures. A large proportion of changes in treatment were because of equipment failure in the ThuVARP arm, with 18 participants being changed to TURP straight away or converting mid-procedure. The size of the prostate also resulted in nine conversions to TURP. The proportion of participants receiving conversions, compared with those receiving ThuVARP, was relatively balanced across the centres, with between 6% and 25% of participants in each centre receiving a conversion from ThuVARP to TURP. The high rate of conversions may have been due to several factors. As surgeons had generally completed a mean of only seven cases before proceeding to trial cases, there may have been an element of ‘teething problems’ with the laser, and a continued learning curve past the assessment of competence. However, this is unlikely to be the cause, as, when breaking the recruitment period per surgeon into halves, the conversion rate in the first half was 11%, whereas it increased to 28% in the second half. Potentially, as surgeons became more experienced with the laser, their judgement of when procedures needed to be converted may have altered. Equally, some surgeons expressed a reluctance to operate on large prostates with the laser early in the trial, with some exclusions of patients with larger prostates from the trial. The increased number of conversions could therefore also have reflected the increasing confidence of surgeons to operate on larger prostates with the laser. Future research into the comparative effectiveness of ThuVARP and TURP in large prostates would also be of interest.
An interesting finding, although one not prespecified, was that the treatments differed in pathology diagnostic detection. Following the procedure, 193 participants in each arm had a prostate histology, among whom 13% in the TURP arm were diagnosed with prostate cancer, compared with only 5% in the ThuVARP arm. When looking at the histology samples, pathologists were usually alerted to laser TURP cases by the fact there was charring of the tissue. On reviewing the weight of resected tissue, the amount of tissue available for histology was noted to be also significantly reduced in those who had laser procedures. This is due to the vaporesection conducted by the thulium laser, which vaporises a proportion of the tissue as well as resecting. The difference in histological finding may therefore be due to both a reduction in tissue and the tissue being damaged by charring from the laser. The significance of this finding is that prostate cancer diagnoses may be missed as a result of the limited histology available, although TURP is not part of the diagnostic pathway for prostate cancer. The results of this study may therefore have implications that the National Cancer review55 may wish to consider. Further work into this area could include categorising the prostate cancer findings by stage and grade from pathological reports to further establish the clinical relevance of the findings.
The trial was conducted across four teaching hospitals and three district general hospitals in the UK, and included a variety of hospitals to increase the generalisability of the results. The majority of recruitment occurred in the teaching university affiliated hospitals, which suggests that research is easier to conduct in these hospitals because there are more resources available. However, this does not mean that smaller hospitals cannot participate in research, as demonstrated by successful recruitment across all sites.
Economic evaluation
The results of the economic evaluation indicate that, from an NHS secondary care perspective and an NHS perspective, participants in the ThuVARP arm had slightly higher costs and fewer QALYs than those in the TURP arm, but these differences were consistent with chance. The probability that ThuVARP is the cost-effective option compared with TURP at a threshold of £20,000 per QALY is only 24%, which increases slightly to 25% when all NHS costs are taken into account.
The expected lower costs in the ThuVARP arm resulting from the fact that this can be carried out as a day case procedure did not materialise. This is to some extent an artefact of the trial, in that participants had to be listed as a day-case or an inpatient stay prior to admission. According to Hospital Episode Statistics data from 2013/14,56 the mean length of stay for TURP procedures was 2.9 days, as opposed to 1.8 days for laser procedures already in use in the NHS. However, Hospital Episode Statistics data for 2016/1757 report a reduction in the mean length of stay for TURP to 2 days, equal to that for laser procedures. Indeed, a shift was made in two of our study sites to conduct TURP procedures as day case during the trial. No difference in actual length of stay was observed between the TURP and ThuVARP arms during the trial. The effect on costs of being able to carry out ThuVARP as a day-case procedure in the other five centres was examined; this showed that ThuVARP was cheaper than TURP in this scenario (£3909 vs. £4245), which led to a positive INMB of £99 (95% CI –£547 to £745). This implies that if TURP did not become a day-case procedure, and if ThuVARP was always a day-case procedure, then the two operations would not differ in cost-effectiveness.
It is also worth noting that all hospitals will have a TURP machine. Therefore, although an annual equivalent cost (including machine maintenance) was calculated for both procedures, there would be an upfront cost to purchase the laser machine that would not in reality be mirrored by a need to purchase a TURP generator, until the generator required replacing. The cost of purchasing a generator is approximately 10% of the purchase cost of a laser machine and therefore trusts may not be inclined to fund this upfront higher laser cost. The majority of the equipment cost for the TURP procedure is in the consumables used, contrary to the laser cost, the majority of which results from the reusable equipment, including the laser machine. There is a high rate of conversions from ThuVARP to TURP, which during this trial meant that both sets of equipment were required for 24% of procedures. The effect of this in relation to the overall costs in a sensitivity analysis showed that the need to have TURP capital and reusable equipment always available increased the cost in the laser arm by only £9.
The finding that the ThuVARP procedure took on average 21 minutes longer than the TURP procedure is important to consider. This is the case both from a patient perspective, in terms of increased time under anaesthetic, and from the perspective of service delivery. It is possible that a continued learning curve of the ThuVARP procedure past assessment of competence had an impact on this finding; however, there was no evidence of any learning curve effect. If this difference is maintained, then it is possible that an additional small case could be added on to an operating list if TURPs are performed, which could have implications for reducing waiting lists.
Qualitative study
The qualitative study investigated the participants’ experiences of both procedures in more depth and found that participants reported satisfactory outcomes in the main, regardless of procedure. Similar journeys were reported following both procedures. Participants described a period of uncertainty about whether or not their expectations for outcome would be met, which preceded a largely satisfactory outcome. The period of uncertainty was characterised by concerns regarding pain, bleeding and residual urinary symptoms. Some negative influence on recovery of the participants’ mood was reported, but this had largely resolved by the time of interview. Participants reported satisfaction overall with the outcome but highlighted that understanding and being appropriately informed about what to expect during recovery were essential to helping them cope with the recovery period.
Slower recovery associated with previous catheter use was intimated, which was perceived to be because their bladder had had poorer function before the operation. Supported by conservative strategies, such as pelvic floor exercises and medication where necessary, bladder function was perceived to have improved or to be improving.
Most participants were delighted that their quality of life had been restored and for some this was quicker than they had expected. A sentiment of returning to a more ‘youthful’ form was discussed. This exploration highlighted that perceptions of male LUTS as ‘an old man’s problem’ remain entrenched. Engagement with this group (the study population) to educate about these perceptions may be indicated in order to encourage men to seek treatment that could benefit their lives.
Active enquiry about sexual matters was made. Participants who had experienced issues with their sex lives either as a result of the surgery or that predated the intervention reported that they had been rarely asked about this aspect and would like the opportunity to disclose this in order to receive appropriate signposting.
With regard to future practice, accurate information provision is key to patients’ satisfaction and expectations about the outcome. Participants achieved their expected outcome regardless of procedure, with no evidence of preference for one type of surgery. Exploration of de novo symptoms, such as those relating to sexual matters, is welcome so that patients can receive appropriate guidance rather than feeling that they simply have to ‘put up’ with their situation.
Strengths and limitations
The main strength of this study is the successful recruitment to target, despite the challenges of consenting patients to a trial in which randomisation happened at the point of surgery, and particularly at a time when NHS trusts were experiencing significant pressures, with delays to elective surgery as a result of operating theatre availability and hospital bed shortages, particularly during the winter months. In addition, the number of patients who withdrew from the trial was small, and follow-up rates were exceptional.
In terms of blinding, this seems to have been successful as participants were not able to guess which procedure they had received. This reduced the bias in subjective improvement of symptoms and quality of life, and we would recommend this method of randomising at the point of patient anaesthesia for blinding in future surgical trials where logistically possible.
An additional strength of this study was the inclusion of catheterised participants, who are logistically a difficult population to include in trials of BPO surgery. The inclusion of these participants meant that adjustment for baseline for the primary analysis was not possible; however, the trial team believe that this is outweighed by the increased generalisability.
Limitations lay with the reporting of complications, with different qualities of reporting across staff and sites. Interpretation of reporting criteria may have varied across sites and there is always an element of clinical judgement, for example in defining what is a normal expectation for surgery and what is a complication. Bleeding is a good example of where the level that constitutes a complication is subjective, as some degree of bleeding would be expected during surgery. Therefore, the team focused on reporting bleeding to the extent that haemoglobin levels needed to be checked for a possible blood transfusion, and whether or not a blood transfusion was conducted. The team analysed the complications according to the main tick boxes provided in the CRFs. The ‘other’ section was completed to varying degrees and therefore the decision was taken to abandon these, while considering any serious adverse events which were reported separately. However, it should be noted that the data on serious adverse events were collected for the purposes of safety reporting during the trial and not as outcome data. Therefore, categorisation of expectedness and relatedness has been determined by the principal investigators at the sites and not independently verified, and levels of reporting also significantly varied across sites.
A further limitation lay in the measurement of prostate size, which was obtained by surgeons carrying out digital rectal examination in the operating theatre before surgery but once the patient was anaesthetised. We are aware that digital rectal examination is not an accurate measure of prostate size,58 and that a transrectal ultrasound would have provided more accurate data. However, this trial has taken the pragmatic approach of following routine care in the NHS, which usually involves only a digital rectal examination before surgery. It should be noted that, in general, this did not affect clinical outcome, with only one participant sent for emobilisation because he had a very large prostate. In general, rectal examination can reveal a very large prostate, which would have deemed such patients as unsuitable for TURP and also may indicate prostate cancer.
An additional limitation of randomising patients at the point of surgery was that the patients had to be listed as either day case or inpatient before randomisation. Therefore, no differential could be made between the two operations prior to randomisation. At two trial sites, a move had already been made to conduct TURP procedures as day case, and therefore the majority of both procedures were listed as day case. The other five trial sites listed all procedures as inpatient procedures. This trial was therefore not set up so that sites could make a judgement on whether they wished to conduct either procedure as day case or inpatient, but rather decisions had to be made that applied to both operations. In some respects this is a limitation, as sites that would not have conducted TURP as a day-case procedure may have chosen to conduct ThuVARP as a day-case procedure. However, in other respects it is a strength, as sites could not apply any bias in whether they perceived one or the other operation to be conducted as day case. Ultimately, length of stay, after the patient has been listed as day case or inpatient, is determined by the patient’s health status and therefore we believe that this trial presents a fair comparison of the two procedures.
A statistical limitation was that the data collected at 3 months could not be made use of in the imputation model, as originally planned, owing to modelling issues (collinearity and lack of convergence).
Overall evidence and generalisability
The results of this trial will undoubtedly play a part in future decision-making for doctors and patients involved with treatment for LUTS. NICE in 2010 suggested that if offering surgery for managing voiding LUTS presumed to be secondary to BPO, to only consider laser vaporisation techniques, bipolar transurethral vaporisation of the prostate (TUVP) or monopolar or bipolar transurethral vaporisation resection of the prostate as part of a RCT that compares these techniques with TURP. The UNBLOCS trial has undertaken this recommendation for the ThuVARP technique.
The trial has shown that the ThuVARP and TURP procedures are equally effective in improving IPSS, and although TURP is superior in improving maximum urine flow rate, both procedures improved urinary flow to what is considered a clinically successful level. The two procedures also demonstrated very similar results across virtually all other clinical and patient-reported outcomes. The results of the economic evaluation indicate differences in costs and QALYs between the two arms, which could be a result of chance, with the total adjusted mean secondary care cost for the ThuVARP arm only £9 more than the TURP arm, and a QALY difference of 0.01 between the arms in favour of TURP. This marginal cost difference supports the acceptability of both techniques within the NHS. It should be noted, however, that ThuVARP took on average 21 minutes longer than TURP, which has implications for patients, in terms of an increased time under anaesthetic, and for service delivery within the NHS. In addition, a higher proportion of ThuVARP cases required conversion to TURP, which may indicate that both sets of equipment would need to be available for conducting ThuVARP. However, the data overall suggest that both procedures are viable in the UK NHS from a clinical and cost perspective.
The finding of a smaller improvement in Qmax levels after ThuVARP than after TURP is consistent with a recent systematic review and meta-analysis59 comparing ThuVARP with TURP, as is a longer operating time for ThuVARP. The anticipated benefits of the laser of reduced catheterisation time, length of hospital stay and bleeding were not observed in this trial, contrary to the recent evidence review. However, the review is based on the limited evidence available, it includes non-RCTs due to the lack of RCTs, and the majority of its studies were conducted in Asia across different health systems. 59
The National Institute for Health and Care Excellence also recommends monopolar transurethral vaporisation of the prostate or HoLEP at centres specialising in the technique with mentorship arrangements in place due to a long learning curve, reducing their generalisability. The short period of training that surgeons undertake to achieve competence in ThuVARP demonstrates that the two techniques are similar in terms of the operative skills required, and therefore its comparative ease of generalisability.
The trial also supports NICE’S recommendation of offering monopolar and bipolar transurethral resection surgery of the prostate for managing voiding LUTS presumed secondary to BPO. There is no strong clinical finding suggesting that ThuVARP is not an effective procedure for BPO, although TURP may have marginal benefits. Findings from the UNBLOCS trial provide evidence that could be used to update the current NICE guidelines on the efficacy of new technologies in the NHS.
The trial provides evidence about the risks of both procedures that can be used to update the literature, allowing patients to be more informed at the point of consent about the risks of these procedures, especially with regard to side effects. In addition, the result that serum sodium levels did not reduce significantly after surgery indicates that the routine review of urea and electrolytes and full blood counts post TURP may not be necessary. This would have cost-saving implications for the NHS, as traditional teaching suggests that these blood tests should be carried out after TURP. The trial will help to define a pathway for patients undergoing endoscopic resections of the prostate.
Recommendations for research
-
Longer-term follow-up of trial participants would demonstrate whether or not there is any sustained difference between the arms in reoperation rates over time. This would provide a more comprehensive perspective on whether or not the lower Qmax achieved by ThuVARP had any relevance in terms of requiring further future interventions for LUTS especially at an earlier time span than TURP.
-
Of interest would be future research into the comparative effectiveness of ThuVARP and TURP for patients with large prostates of > 80 ml that are operable by specialists, which may extend the scope of the operation further than current EAU guidelines.
-
Comparison of the enucleation procedures using thulium and holmium lasers, as ThuVARP is easier to learn and adopt compared with laser enucleation techniques.
Implications for health care
The trial provides evidence for the clinical effectiveness and cost-effectiveness of laser vaporesection of the prostate compared with the gold-standard TURP in men with moderate to severe bothersome LUTS, including urinary retention, who are considering surgery for BPO. The potential advantages of reduced blood loss, shorter hospital stay and earlier return to normal activities have been thought to make laser prostate resection techniques attractive to both patients and health-care providers, although there is uncertainty about the extent of symptom improvement, and about improvement in quality of life in both the short and the long term. The UNBLOCS RCT addressed these questions for the ThuVARP technique.
Both operations were shown to be effective for treating BPO. Although they were equivalent in terms of IPSS, their equivalence could not be demonstrated in terms of Qmax, for which, despite a relatively small difference in resulting flow rate, TURP was shown to be superior. The results generally suggest little difference between this procedure and the standard TURP procedure from a clinical and an overall cost point of view, although marginal benefits of TURP were indicated in terms of reduced operative time, increased available histology for incidental cancer detection and a higher rate of patients undergoing their randomised procedure. Both operations achieved a high level of patient satisfaction with surgery, and improved quality of life, which is supported by the qualitative element of this study. Overall, the results suggest that it may be appropriate that new treatment alternatives continue to be compared with TURP.
This study also provides the added benefit of up-to-date information on the patient-reported and clinical outcomes associated with standard TURP, which can be used to inform clinical decisions and patient expectations, the importance of which was highlighted in the qualitative element of this study.
Acknowledgements
The authors would like to thank all of the patients who participated in the UNBLOCS trial. We also thank the members of the Trial Management Group, Trial Steering Committee and Data Monitoring and Ethics Committee for their ongoing advice and support for the trial, and the principal investigators, research nurses and their teams at the trial sites for all of their hard work and commitment. We would also like to thank our independent assessor, Graham Watson (Consultant Urological Surgeon, Eastbourne), for independently verifying the competence of all trial surgeons in the laser technique. Finally, we would like to thank the National Institute for Health Research and the Health Technology Assessment programme for funding the UNBLOCS trial.
This study was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration, a UKCRC-registered clinical trials unit that, as part of the Bristol Trials Centre, is in receipt of National Institute for Health Research clinical trials unit support funding. The study data were collected and managed using REDCap,60 hosted at the University of Bristol.
UNBLOCS Trial Group
Principal investigators: Rupert Beck, Christopher Blake, Kim Davenport, Hashim Hashim, Oliver Kayes, Tobias Page, Jonathan Sullivan and Satchi Swami.
Trial manager: Jo Worthington.
Trial research associate: Hilary Taylor.
Senior trial manager/Bristol Randomised Trials Collaboration co-director: J Athene Lane.
Trial statisticians: Sara Brookes, Chris Metcalfe and Grace Young.
Urologists: Nicholas Cohen, Mathialagan Murugesan and Anthony Timoney.
Research nurse lead: Lyndsey Johnson.
Site research nurses: Benita Adams, Angela Allan, Carol Brain, Fiona Hammonds, Joan Henderson, Paula Hilltout, Bernadette Kilbane, Leigh Morrison, Wendy Robson, Lorraine Wiseman and Vivian Zinyemba.
Qualitative researchers: Nikki Cotterill, Rafiyah Khan and Alan Uren.
Health economists: Aideen Ahern, Aida Moure Fernandez and Sian Noble.
Administrative support: Tom Steuart-Feilding, Christopher Pawsey, Julie Plant and Barbara Warnes.
Data management: Mai Baquedano and David Carmichael.
Trial sites and principal investigators
The NHS trusts participating as sites in the trial were:
-
Gloucestershire Hospitals NHS Foundation Trust (principal investigator, Mr Jonathan Sullivan/Kim Davenport)
-
Great Western Hospitals NHS Foundation Trust (principal investigator, Mr Rupert Beck)
-
North Bristol NHS Trust (principal investigator, Professor Hashim Hashim)
-
NHS Grampian (principal investigator, Mr Satchi Swami)
-
Royal Cornwall Hospitals NHS Trust (principal investigator, Mr Christopher Blake)
-
Leeds Teaching Hospital NHS Trust (principal investigator, Mr Oliver Kayes)
-
Newcastle upon Tyne Hospitals NHS Foundation Trust (principal investigator, Mr Tobias Page).
Study oversight committees
We thank all the members of the study oversight committees for their valued contributions.
UNBLOCS Trial Steering Committee: Professor Tom McNicholas (Chairperson), Mr Malcolm Lucas, Dr Catrin Tudur-Smith, Dr Gordon Taylor and Dr Glyn Hayes.
Data Monitoring and Ethics Committee: Dr Jonathan Cook, Mr Mohammed Belal and Mr Mark Stott.
Trial Management Group: Professor Hashim Hashim, Professor Athene Lane, Dr Sara Brookes, Professor Chris Metcalfe, Dr Sian Noble, Mr Toby Page, Professor Paul Abrams, Dr Nikki Cotterill, Dr Jo Worthington, Lyndsey Johnson and Hilary Taylor.
Contributions of authors
Jo Worthington (Trial Manager) was responsible for managing the trial throughout and contributed to aspects of trial design, as well as preparing the first draft of the report.
J Athene Lane (Professor of Trials Research, Co-Director of the Bristol Randomised Trials Collaboration) was a co-applicant. She contributed to the trial design, was involved in management of the study, and contributed to writing the report.
Hilary Taylor (Research Associate) contributed to aspects of the trial design, and was involved in the day-to-day management of the trial.
Grace Young (Senior Research Associate in Medical Statistics) was the trial statistician. She wrote the statistical analysis plan, conducted the analysis and contributed to writing the report.
Sian M Noble (Senior Lecturer, Health Economics) was a co-applicant, and the lead health economist for the trial. She was involved in designing, managing and conducting the health economic evaluation, and contributed to writing the report.
Paul Abrams (Professor, Consultant Urological Surgeon) was a co-applicant. He contributed to the trial design, was involved in management of the study, and contributed to writing the report.
Aideen Ahern (Research Associate, Health Economics) was involved in the conduct and analysis of the economic evaluation.
Sara T Brookes (Senior Lecturer, Health Services Research and Medical Statistics) was a co-applicant. She contributed to trial design, was the lead statistician for the majority of the trial, and contributed to writing the report.
Nikki Cotterill (Associate Professor in Continence Care) was a co-applicant, and the lead qualitative researcher on the trial. She was involved in designing, managing and conducting the qualitative analysis, and contributing to writing the report.
Lyndsey Johnson (Lead Research Nurse, Urology) was the research nurse lead for the trial, as well as being involved in conducting the study at her respective study site.
Rafiyah Khan (Research Assistant, Qualitative Research) was involved in the conduct of the qualitative study.
Aida Moure Fernandez (Research Associate, Health Economics) was involved in the design and conduct of the economic evaluation.
Tobias Page (Consultant Urological Surgeon) was a co-applicant. He contributed to the trial design and management, as well as being a principal investigator responsible for implementation at his respective study site.
Satchi Swami (Consultant Urological Surgeon) was a co-applicant. He contributed to trial conduct issues, as well as being a principal investigator responsible for implementation in his respective study site.
Hashim Hashim (Professor, Consultant Urological Surgeon) was chief investigator and clinical lead for the trial and, as such, participated in its design, was responsible for oversight of the trial and was involved in writing the report.
All authors read and approved and/or commented on the final report.
Publications
Ahern A, Worthington J, Hashim H, Lane JA, Taylor H, Young G, et al. The cost-effectiveness of thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate. Conference abstract, The British Association of Urological Surgeons Annual Scientific Meeting 2019, 24–26 June 2019, Glasgow, UK.
Cotterill N, Khan R, Uren A, Abrams P, Brookes S, Lane A, et al. “I have a life back” – the patient experience of urinary obstruction interventions in the UNBLOCS randomised controlled trial. Podium presentation, International Continence Society Annual Meeting 2019, 3–6 September 2019, Gothenburg, Sweden.
Hashim H, Lane JA, Worthington J, Noble S, Brooks S, Cotterill N, et al. Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate: results of the UNBLOCS randomized controlled trial. Poster presentation, EAU Conference (EAU 2019), 15–19 March 2019, Barcelona, Spain.
Hashim H, Lane JA, Worthington J, Taylor H, Young G, Noble SM, et al. Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate: results of the UNBLOCS randomised controlled trial. Podium presentation, American Urological Association Annual Meeting, 3–6 May 2019, Chicago, IL, USA.
Hashim H, Worthington J, Abrams P, Young G, Taylor H, Noble SM, et al. Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate for men with lower urinary tract symptoms or urinary retention (UNBLOCS): a randomised controlled trial. Lancet 2020;396:50–61.
Noble SM, Ahern AM, Worthington J, Hashim H, Taylor H, Young GJ, et al. The cost-effectiveness of transurethral resection of the prostate vs thulium laser transurethral vaporesection of the prostate in the UNBLOCS randomised controlled trial for benign prostatic obstruction. BJUI Int 2020.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Worthington J, Taylor H, Abrams P, Brookes ST, Cotterill N, Noble SM, et al. A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral vaporesection of the prostate (ThuVARP) versus transurethral resection of the prostate (TURP) in the National Health Service (NHS) – the UNBLOCS trial: a study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1916-5.
- Hashim H, Worthington J, Abrams P, Young G, Taylor H, Noble SM, et al. Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate for men with lower urinary tract symptoms or urinary retention (UNBLOCS): a randomised controlled trial. Lancet 2020;396:50-61. https://doi.org/10.1016/S0140-6736(20)30537-7.
- Hunter DJ, McKee M, Black NA, Sanderson CF. Health status and quality of life of British men with lower urinary tract symptoms: results from the SF-36. Urology 1995;45:962-71. https://doi.org/10.1016/S0090-4295(99)80116-2.
- McNicholas TA. Management of symptomatic BPH in the UK: who is treated and how?. Eur Urol 1999;36:33-9. https://doi.org/10.1159/000052347.
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP) – incidence, management, and prevention. Eur Urol 2006;50:969-79. https://doi.org/10.1016/j.eururo.2005.12.042.
- NHS Digital . NHS Classifications OPCS-4 n.d. https://isd.digital.nhs.uk/trud3/user/guest/group/0/pack/10 (accessed 18 September 2019).
- Zhang X, Shen P, He Q, Yin X, Chen Z, Gui H, et al. Different lasers in the treatment of benign prostatic hyperplasia: a network meta-analysis. Sci Rep 2016;6. https://doi.org/10.1038/srep23503.
- National Institute for Health and Care Excellence (NICE) . Lower Urinary Tract Symptoms in Men: Management 2010.
- National Institute for Health and Care Excellence (NICE) . GreenLight XPS for Treating Benign Prostatic Hyperplasia 2016.
- Gilling PJ, Kennett K, Das AK, Thompson D, Fraundorfer MR. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: an update on the early clinical experience. J Endourol 1998;12:457-9. https://doi.org/10.1089/end.1998.12.457.
- National Institute for Health and Care Excellence (NICE) . Holmium Laster Prostatectomy 2003. www.nice.org.uk/Guidance/IPG17 (accessed 19 September 2019).
- Fried NM, Murray KE. High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol 2005;19:25-31. https://doi.org/10.1089/end.2005.19.25.
- Xia SJ, Zhuo J, Sun XW, Han BM, Shao Y, Zhang YN. Thulium laser versus standard transurethral resection of the prostate: a randomized prospective trial. Eur Urol 2008;53:382-89. https://doi.org/10.1016/j.eururo.2007.05.019.
- Fu WJ, Zhang X, Yang Y, Hong BF, Gao JP, Cai W, et al. Comparison of 2-microm continuous wave laser vaporesection of the prostate and transurethral resection of the prostate: a prospective nonrandomized trial with 1-year follow-up. Urology 2010;75:194-9. https://doi.org/10.1016/j.urology.2009.07.1266.
- Herrmann TR, Liatsikos EN, Nagele U, Traxer O, Merseburger AS. EAU Guidelines Panel on Lasers, Technologies . EAU guidelines on laser technologies. Eur Urol 2012;61:783-95. https://doi.org/10.1016/j.eururo.2012.01.010.
- Cui D, Sun F, Zhuo J, Sun X, Han B, Zhao F, et al. A randomized trial comparing thulium laser resection to standard transurethral resection of the prostate for symptomatic benign prostatic hyperplasia: four-year follow-up results. World J Urol 2014;32:683-9. https://doi.org/10.1007/s00345-013-1103-6.
- Zhang F, Shao Q, Herrmann TR, Tian Y, Zhang Y. Thulium laser versus holmium laser transurethral enucleation of the prostate: 18-month follow-up data of a single center. Urology 2012;79:869-74. https://doi.org/10.1016/j.urology.2011.12.018.
- Young GMC, Worthington J, Cook J, Hashim H. Statistical Analysis Plan for the UNBLOCS Trial: Version 1.0 (22.11.17) n.d. https://research-information.bristol.ac.uk/files/140090744/UNBLOCS_Statistical_Analysis_Plan_v1.0_22.11.17_FINAL.pdf (accessed 19 September 2019).
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Barry MJ, Cantor A, Roehrborn CG. CAMUS Study Group . Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. J Urol 2013;189:987-92. https://doi.org/10.1016/j.juro.2012.08.257.
- Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology 2003;61:1166-71. https://doi.org/10.1016/S0090-4295(03)00109-2.
- Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, et al. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;172:1926-9. https://doi.org/10.1097/01.ju.0000140501.68841.a1.
- Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-106.
- Schoenfeld D. Chi-squared goodness of fit tests for the proportional hazards regression model. Biometrika 1980;67:145-53. https://doi.org/10.1093/biomet/67.1.145.
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425-37. https://doi.org/10.1056/NEJMoa1606221.
- International Prostate Symptom Score (IPSS) n.d. www.urospec.com/uro/Forms/ipss.pdf (accessed 18 March 2018).
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. https://doi.org/10.1001/jama.2012.87802.
- Noble SM, Ahern AM, Worthington J, Hashim H, Taylor H, Young GJ, et al. The cost-effectiveness of transurethral resection of the prostate vs thulium laser transurethral vaporesection of the prostate in the UNBLOCS randomised controlled trial for benign prostatic obstruction. BJUI Int 2020. https://doi.org/10.1111/bju.15138.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- NHS Improvement . NHS Reference Costs 2016 17 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 18 March 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014815.
- HM Revenue and Customs . Travel – Mileage and Fuel Rates and Allowances n.d. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed 12 September 2018).
- NHS Business Services Authority . NHS Drug Tariff n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 13 September 2018).
- World Health Organization . International Classification of Diseases, Tenth Revision 2016. www.who.int/classifications/icd/icdonlineversions/en/ (accessed 18 September 2019).
- Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Joint Formulary Committee . British National Formulary n.d. https://bnf.nice.org.uk/ (accessed 13 September 2018).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Marques E, Johnson EC, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Value Health 2013;16:195-201. https://doi.org/10.1016/j.jval.2012.09.008.
- Al-Busaidi ZQ. Qualitative research and its uses in health care. Sultan Qaboos Univ Med J 2008;8:11-9.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Hudak PL, McKeever PD, Wright JG. Understanding the meaning of satisfaction with treatment outcome. Med Care 2004;42:718-25. https://doi.org/10.1097/01.mlr.0000132398.11342.a8.
- Braun V, Clark V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep 2015;20.
- Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306-14. https://doi.org/10.1016/j.eururo.2006.09.019.
- Cobussen-Boekhorst H, Hermeling E, Heesakkers J, van Gaal B. Patients’ experience with intermittent catheterisation in everyday life. J Clin Nurs 2016;25:1253-61. https://doi.org/10.1111/jocn.13146.
- Gadow S. Body and self: a dialectic. J Med Philos 1980;5:172-85. https://doi.org/10.1093/jmp/5.3.172.
- Taylor AW, Wilson DH, Wakefield M. Differences in health estimates using telephone and door-to-door survey methods – a hypothetical exercise. Aust N Z J Public Health 1998;22:223-6. https://doi.org/10.1111/j.1467-842X.1998.tb01177.x.
- Burgio KL, Brubaker L, Richter HE, Wai CY, Litman HJ, France DB, et al. Patient satisfaction with stress incontinence surgery. Neurourol Urodyn 2010;29:1403-9. https://doi.org/10.1002/nau.20877.
- Hamilton DF, Lane JV, Gaston P, Patton JT, Macdonald D, Simpson AH, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002525.
- NICE . NICE Guideline [NG131] 2019.
- NHS Digital . Hospital Admitted Patient Care Activity, 2013–14 n.d. https://webarchive.nationalarchives.gov.uk/20190305041047/https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/hospital-episode-statistics-admitted-patient-care-england-2013-14 (accessed 18 September 2019).
- NHS Digital . Hospital Admitted Patient Care Activity, 2016–17 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2016-17 (accessed 18 September 2019).
- Loeb S, Han M, Roehl KA, Antenor JA, Catalona WJ. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J Urol 2005;173:63-5. https://doi.org/10.1097/01.ju.0000145883.01068.5f.
- Deng Z, Sun M, Zhu Y, Zhuo J, Zhao F, Xia S, et al. Thulium laser VapoResection of the prostate versus traditional transurethral resection of the prostate or transurethral plasmakinetic resection of prostate for benign prostatic obstruction: a systematic review and meta-analysis. World J Urol 2018;36:1355-64. https://doi.org/10.1007/s00345-018-2287-6.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
Appendix 1 Patient- and productivity-related resource use
| Resource category (resource use unit) | Trial arm | |||||
|---|---|---|---|---|---|---|
| ThuVARP | TURP | |||||
| n | Mean resource use (SD) | Mean cost (SD) (£) | n | Mean resource use (SD) | Mean cost (SD) (£) | |
| Car miles (number) | 109 | 8.97 (27.78) | 4.04 (12.50) | 113 | 10.74 (28.99) | 4.83 (13.05) |
| Car parking events (number) | 109 | 0.06 (0.23) | 0.35 (1.62) | 113 | 0.05 (0.22) | 0.46 (2.06) |
| Fares (number) | 109 | 0.02 (0.14) | 0.32 (2.38) | 113 | 0.00 (0.00) | 0.00 (0.00) |
| Prescriptions (number) | 137 | 0.03 (0.24) | 0.95 (9.14) | 143 | 0.05 (0.32) | 1.40 (14.00) |
| Over-the-counter medications (number) | 144 | 0.08 (0.28) | 0.08 (0.28) | 151 | 0.11 (0.35) | 0.11 (0.35) |
| Incontinence pads or pants | 116 | 11.75 (33.58) | 9.56 (43.53) | 129 | 8.63 (24.64) | 3.48 (11.89) |
| Major expenses (number) | 144 | 0.12 (0.54) | 5.67 (29.51) | 151 | 0.02 (0.18) | 4.37 (38.36) |
| Home care visits (number of minutes) | 144 | 0.35 (4.17) | 0.10 (1.25) | 150 | 4.05 (48.99) | 1.22 (14.70) |
| Unpaid time off work (number of days) | 104 | 0.00 (0.00) | 0.00 (0.00) | 114 | 0.00 (0.00) | 0.00 (0.00) |
| Paid time off work (number of days) | 105 | 0.03 (0.29) | 1.50 (15.37) | 114 | 0.00 (0.00) | 0.00 (0.00) |
| Time off leisure (number of hours) | 132 | 15.70 (50.37) | 117.73 (377.77) | 137 | 20.85 (70.71) | 156.35 (530.35) |
| Support from relatives/friends (number of hours) | 131 | 24.96 (223.07) | 187.21 (1673.03) | 138 | 7.19 (29.61) | 53.91 (222.09) |
Appendix 2 Qualitative interview schedule
Interview schedule: version 4 – 9 May 2016
Lower urinary tract symptoms/retention experience
-
Begin with a description of your urinary symptoms before surgery? (LUTS/retention/nocturia).
-
What effect did this have on your daily life? How did you feel about that?
-
What about other aspects of your life? (Socialising, work-life?)
-
Were you using any aids to manage these symptoms? (Medication, catheter, pads).
-
What influenced your decision to get surgery? How did you expect the procedure and recovery to go? Why?
Perioperative experience
-
Describe the day of your surgery? How did you feel?
-
Following the procedure how did you feel (physically/psychologically)?
-
What was the remainder of your hospital stay like? (Complications?) Did you feel that the surgery had gone well at that point? Why?
Immediate postoperative experience
-
During the first few days following discharge, how did you feel? What were your symptoms like? Were you satisfied at this stage?
-
Were you able to continue with your daily life?
-
At what point did you feel that your symptoms had improved? Was this expected?
-
Following on from that, describe the first few weeks following the procedure? Were you able to maintain many activities? Social, physical, etc (pinpoint specific point at which they were able to do certain things).
Outcome
-
Describe current symptoms at a few months post-surgery. (Explore how this has changed from the previous two answers.)
-
Throughout the recovery, were there many changes to your symptoms? In what way did these affect you? Did you feel satisfied during these stages? Why?
-
In what way have different aspects of your life been affected since then?
-
Are there any factors that could have affected your recovery? (further illness, life changes, etc.)
Satisfaction
-
Are you satisfied with how your surgery went? Did it meet your expectations?
-
What in particular makes you happy with the outcome?
-
What would have made you satisfied with the outcome?
-
-
Are you happy with your symptoms now going forward?
-
Do you have any idea which procedure you were randomised to? Why do you think this?
-
How would you describe the experience to a family member about to go through the same operation?
-
Do you have any suggestions that would have improved your overall surgery experience?
List of abbreviations
- BPO
- benign prostatic obstruction
- CACE
- complier average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- EAU
- European Association of Urology
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HoLEP
- holmium laser enucleation of the prostate
- HRG
- Healthcare Resource Group
- ICD-10
- International Classification of Diseases, Tenth Revision
- ICIQ-LUTSqol
- International Consultation on Incontinence Questionnaire – Lower Urinary Tract Symptoms Quality of Life
- ICIQ-MLUTS
- International Consultation on Incontinence Questionnaire – Male Lower Urinary Tract Symptoms
- ICIQ-MLUTSsex
- International Consultation on Incontinence Questionnaire – Male Sexual Matters associated with Lower Urinary Tract Symptoms
- ICIQ-satisfaction
- International Consultation on Incontinence Questionnaire – Satisfaction
- IIEF-5
- International Index of Erectile Function-5
- INMB
- incremental net monetary benefit
- IPSS
- International Prostate Symptom Score
- IQR
- interquartile range
- LUTS
- lower urinary tract symptoms
- NICE
- National Institute for Health and Care Excellence
- OPCS-4
- OPCS Classification of Interventions and Procedures
- PIN
- prostatic intraepithelial neoplasia
- PLICS
- hospital patient-linked information costing system
- QALY
- quality-adjusted life-year
- Qmax
- maximum urine flow rate
- RCT
- randomised controlled trial
- SD
- standard deviation
- ThuVARP
- thulium laser transurethral vaporesection of the prostate
- TURP
- transurethral resection of the prostate
- UNBLOCS
- UriNary oBstruction relieved by Laser Or Conventional Surgery
