Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 17/109/19. The protocol was agreed in March 2019. The assessment report began editorial review in September 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jai Patel attended a product training course for using TheraSphere™ [BTG Ltd, London, UK (now Boston Scientific, Marlborough, MA, USA)] in Essen, Germany, in 2016, which was sponsored by Biocompatibles UK Ltd (Farnham, UK) [acquired by BTG Ltd], and is a member of the National Institute for Health and Care Excellence Medical Technologies Advisory Committee (June 2015 to present). Ian Rowe reports personal fees from AbbVie Inc. (North Chicago, IL, USA) and personal fees from Norgine BV (Amsterdam, the Netherlands), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Walton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Description of health problem
Liver cancer is the fifth most common cancer and the second most frequent cause of cancer-related death globally. 1 Hepatocellular carcinoma (HCC) is the most common type of liver cancer, representing around 90% of primary liver cancers. 1 Around 90% of HCCs are associated with a known underlying aetiology, most frequently chronic viral hepatitis B or C, or overconsumption of alcohol (alcoholic liver disease). Long periods of chronic liver disease, characterised by hepatic inflammation, fibrosis and aberrant hepatocyte regeneration, can cause scarring of the liver (cirrhosis). 2 One-third of patients with cirrhosis will develop HCC during their lifetime. 1
In the UK, the underlying aetiology of HCC is commonly alcoholic liver disease and non-alcoholic fatty liver disease, with 50% of cases attributable to these factors. Hepatitis infection (hepatitis B or C) is also a common cause in the UK but, in contrast with non-Western populations, represents only 15% of cases. Viral hepatitis is the primary cause of HCC in non-Western populations, with up to 90% of cases directly attributable to the hepatitis B and C virus. 3
Underlying liver cirrhosis and the burden of a growing tumour results in an often substantially reduced liver function in HCC patients, with consequences for morbidity and mortality. Liver dysfunction associated with chronic liver disease is commonly assessed using the Child–Pugh scoring system, which classifies patients into three groups: A, B or C (least severe disease, moderate liver disease and severe/end-stage liver disease). Treatment options available to HCC patients are in part dictated by liver function, with choices becoming more limited with increasing liver dysfunction. The Barcelona Clinic Liver Cancer (BCLC) staging system is used to establish prognosis and enable the selection of appropriate treatment based on both the underlying liver dysfunction and the cancer stage. 1 A modified version of the BCLC staging system is presented in Table 1. The BCLC staging system classifies patients into five stages (0, A, B, C and D) according to tumour burden, liver function and Eastern Cooperative Oncology Group (ECOG) performance status,4 which must all be considered when selecting appropriate treatment. 1
| Prognostic stage | Tumour burden | Liver function | Performance status | Recommended treatment | Survival |
|---|---|---|---|---|---|
| Very early stage (BCLC 0) | Single < 2-cm nodule | Preserved liver function | 0 | Ablation or resection | > 5 years |
| Early stage (BCLC A) | 1–3 nodules of < 3 cm in size | Preserved liver function | 0 | Ablation, resection or transplant | > 5 years |
| Intermediate stage (BCLC B) | Multinodular, unresectable | Preserved liver function | 0–1 | Conventional transarterial therapies (TAE, TACE and DEB-TACE) | > 2.5 years |
| Advanced stage (BCLC C) | PVI/extrahepatic spread | Preserved liver function | 0–2 | Systemic therapy [sorafenib,a lenvatinibb or regorafenibc (for patients who have previously had sorafenib)] | ≥ 10 months |
| Terminal stage (BCLC D) | Non-transplantable HCC | End-stage liver function | 3–4 | Best supportive care | 3 months |
Epidemiology
The incidence of HCC is higher in men than in women, with 2128 men and 586 women diagnosed with HCC in England in 2017. 5 The majority of cases occur in adults aged > 60 years. 5 The average age of patients at HCC diagnosis is 66 years, reflecting the long-term nature of most chronic liver disease underlying HCC. 6 Approximately 30% of European patients are diagnosed with early-stage (BCLC stage 0 or A) HCC, approximately 10% are diagnosed with intermediate-stage (BCLC stage B) HCC, approximately 50% are diagnosed with advanced-stage (BCLC stage C) HCC and approximately 10% are diagnosed with terminal (BCLC stage D) HCC. 7 The majority of patients are, therefore, diagnosed with advanced disease, for which treatment options are more limited (see Current service provision).
Prognosis
Prognosis of patients with HCC is heavily dependent on the stage of disease, and is summarised in Table 1. In very early-stage and early-stage disease, a range of potentially curative treatment options are typically available and, thus, the long-term prognosis of these patients can be good. In very early-stage disease, 5-year survival is between 70% and 90%, and it is between 50% and 70% in early-stage disease. 8 In intermediate- and advanced-stage disease, treatment options are more limited and are primarily delivered to prolong survival and reduce the burden of symptoms. Length of survival is, therefore, significantly shorter; prognosis in patients with advanced disease is particularly poor, with a median survival of < 12 months. 8
Current service provision
Clinical management of HCC is complex. There are a range of treatment options available, which depend on the location and stage of the cancer and liver function. Clinical practice guidelines published by the European Association for the Study of the Liver (EASL) summarise treatment recommendations according to BCLC classification. 1 These recommendations are presented in Table 1, with some modifications, reflecting entry criteria to pivotal clinical trials.
The primary aim of therapy in patients diagnosed with early-stage HCC is typically curative, and there are a number of available treatment options with curative potential. These include radiofrequency ablation (which uses the heat generated by alternating currents to destroy solid tumour tissue), resection (in which the tumour-containing portions of the liver are removed) and liver transplantation. 1 Owing to the limited availability of suitable donors, liver transplant is typically reserved for patients with a poor prognosis owing to impaired liver function, and in whom resection is inappropriate, for example in patients with multifocal tumours. Suitability for transplant is assessed against the Milan criteria,9 which require patients to have a single lesion of < 5 cm, or up to three lesions of < 3 cm each, without macroscopic vascular invasion (MVI). 1 Typically, patients not meeting these criteria are ineligible for a transplant, but increasingly patients whose disease has been ‘downstaged’ may be considered for transplant. Downstaging is when patients whose tumours fall outside the limits permitted by the Milan criteria9 are brought within the criteria, typically through the use of conventional transarterial therapies (CTTs) (see below) to reduce tumour burden. Patients waiting for a transplant may also receive CTT as a ‘bridging therapy’, in which the intent is to control the progression of disease to keep patients within the Milan criteria. 9 However, as transplant waiting times in the UK are typically relatively short, with a median time for HCC patients of approximately 50 days, the use of bridging therapy is limited.
Conventional transarterial therapies are the standard care in intermediate HCC if resection or other curative treatment modalities are unsuitable. CTT includes transarterial chemoembolisation (TACE), drug-eluting bead transarterial chemoembolisation (DEB-TACE) and transarterial embolisation (TAE) without chemotherapy. Blood is primarily supplied to the liver via the hepatic portal vein, whereas most tumours are supplied by the hepatic artery. All three forms of CTT work by administering an embolising agent into the hepatic artery to block blood vessels feeding the tumours within the liver. This process preferentially interrupts the blood supply to the tumours, while allowing blood to continue to reach the remaining healthy tissue. In the case of TACE, Lipiodol® (Guerbet, Villepinte, France) is combined with a chemotherapy agent, typically doxorubicin or cisplatin, which is administered directly to the tumour, allowing for much higher concentrations of the drug to be achieved than could be tolerated systemically. In DEB-TACE, drug-eluting beads typically bound with doxorubicin or epirubicin are administered to the tumour via the hepatic artery. This allows the release of the chemotherapeutic agent over a prolonged period of time, thereby reducing systemic concentrations (and thus any side effects) compared with TACE. 10 TAE, or bland TACE, involves only the physical occlusion of blood vessels, with no addition of chemotherapy. Because the primary therapeutic effect of CTT is the embolisation of the hepatic artery, the use of these techniques is typically limited to patients with good portal vein flow, so as to maintain a good blood supply to the liver. Therefore, patients with portal vein thrombosis (PVT) or tumour invasion of the portal vein are typically considered contraindicated to CTT.
In patients who have advanced HCC, or for whom CTT has previously failed, the current standard of care consists of systemic chemotherapy. Current National Institute for Health and Care Excellence (NICE) guidance in this population recommends sorafenib (Nexavar®; Bayer plc, Leverkusen, Germany) as an option for people with Child–Pugh class A liver impairment (TA474). 11 Lenvatinib (Kisplyx®, Eisai Ltd, Tokyo, Japan) is also recommended as an option for people with Child–Pugh class A liver impairment and an ECOG performance status of 0 or 1 (TA551). 12 A recent technology appraisal on regorafenib (Stivarga®; Bayer plc, Leverkusen, Germany) for treating advanced unresectable HCC (TA555)13 recommends regorafenib as an option for people who have previously been treated with sorafenib and have Child–Pugh class A liver impairment and an ECOG performance status of 0 or 1. Best supportive care (BSC) is offered to patients when CTTs or systemic therapy are not available or appropriate, including patients with terminal-stage disease.
Description of the technology under assessment
Selective internal radiation therapy (SIRT), also known as transarterial radioembolisation (TARE), is a complex intervention that delivers radiation directly to liver tumours via microspheres that are injected into the hepatic artery via a catheter inserted into the femoral artery. The most likely position for SIRT in the HCC treatment pathway is for patients with intermediate-stage (BCLC stage B) or advanced-stage (BCLC stage C) HCC as a non-curative option, as the use of SIRT is not precluded by reduced liver function as strictly as CTTs. However, SIRT is unlikely to be suitable for patients with more limited liver function (Child–Pugh class ≥ B8) or extrahepatic tumour spread. There may also be a role for SIRT as a bridging therapy for BCLC stage A patients awaiting transplant (see Current service provision) as an alternative to CTTs.
The NICE interventional procedures guidance 46014 states that current evidence on the efficacy and safety of SIRT for primary HCC was adequate to permit routine use of the technology. However, significant uncertainties remain about its comparative effectiveness relative to conventional transarterial and systemic therapeutic options. 14 Clinicians have been encouraged by NICE to enter eligible patients into trials comparing the procedure against other forms of treatment and to enrol all patients into the UK SIRT registry (launched in 2013). 14
The present appraisal concerns three SIRTs: SIR-Spheres® (Sirtex Medical Ltd, Woburn, MA, USA), TheraSphere™ [BTG Ltd, London, UK (now Boston Scientific, Marlborough, MA, USA)] and QuiremSpheres® (Quirem Medical BV, Deventer, the Netherlands). SIR-Spheres [manufactured by Sirtex Medical Ltd (hereafter Sirtex)] is a Conformité Européenne (CE)-marked class III active medical device comprising resin microspheres containing yttrium-90; SIR-Spheres is indicated for the treatment of inoperable liver tumours. TheraSphere [manufactured by BTG Ltd (hereafter BTG)] is a CE-marked class III active medical device comprising glass microspheres containing yttrium-90; TheraSphere is indicated for the treatment of hepatic neoplasia. QuiremSpheres [manufactured by Quirem Medical BV and distributed by Terumo Europe NV (Leuven, Belgium)] is a CE-marked class III active medical device comprising poly-L-lactic acid (PLLA) microspheres containing holmium-166; QuiremSpheres is indicated for the treatment of unresectable liver tumours.
In preparation for SIRT, patients undergo preliminary angiography of the hepatic artery, and protective coiling of extrahepatic branches to reduce extrahepatic radiation uptake. For TheraSphere and SIR-Spheres, technetium-99m-macroaggregated albumin is used as an imaging surrogate and injected into the hepatic artery using the same catheter position chosen for the scheduled SIRT session. Calculation of the radiation dose to the tumour and adjacent liver, hepatopulmonary shunt fraction and tracer distribution are evaluated with single-photon emission computerised tomography (SPECT) imaging. This is known as the ‘work-up’ procedure, and is ultimately what decides whether or not patients are eligible to receive SIRT. A high level of lung shunt or extrahepatic uptake contraindicate the SIRT procedure. When SIRT is not contraindicated following work-up, patients are later readmitted for the SIRT procedure, which is performed in a lobar, sectorial or segmental approach according to tumour size and location. 1 When tumours are present in both lobes, patients may receive a separate administration of SIRT to each lobe on separate occasions (often several weeks apart), to allow clinicians to monitor the liver’s response to radiation and prevent damage.
The work-up procedure for QuiremSpheres exploits the properties of holmium-166 microspheres, which, unlike yttrium-90, can be visualised with SPECT and magnetic resonance imaging (MRI) even at low concentrations. Therefore, a lower dose of holmium-166 is used for evaluating dose distribution [known as QuiremScout® (Quirem Medical BV)], rather than a surrogate, which may allow for a more accurate assessment of radiation distribution and dosimetry.
Table 2 presents an overview of the main characteristics of each therapy.
| Technique | SIR-Spheres | TheraSphere | QuiremSpheres |
|---|---|---|---|
| Radioactive isotope | Yttrium-90 | Yttrium-90 | Holmium-166 |
| Microsphere material | Resin | Glass | PLLA |
| Therapeutic mode of action | Beta radiation | Beta radiation | Beta radiation |
| Mean diameter of the microsphere (µm) | 32.5 | 20–30 | 30 |
| Half-life of the radioactive isotope (hours) | 64.1 | 64.1 | 26.8 |
| Specific activity per microsphere (Bq) | 50 | 2500 | 350 |
| Typical administered activity (GBq) | 1.4–2.0 | – | – |
| Typical number of microspheres administered (millions) | 30–40 | 4 | 20–30 |
| Time for 90% of dose to be deposited (days) | 11 | 11 | 4 |
Chapter 2 Definition of the decision problem
The decision problem in terms of participants, interventions, comparisons, outcomes, study design and other key issues
The decision problem relates to the use of the three SIRTs, TheraSphere, SIR-Spheres and QuiremSpheres, within their approved indications for the treatment of HCC. Relevant comparators are each other, CTTs (i.e. TAE, TACE and DEB-TACE) or, for people for whom any transarterial therapies are inappropriate, established clinical management without SIRT, such as systemic therapy (sorafenib, lenvatinib or regorafenib) or BSC.
Overall aims and objectives of the assessment
This appraisal will assess the clinical effectiveness and cost-effectiveness of the SIRT (TheraSphere, SIR-Spheres and QuiremSpheres) for treating HCC.
The objectives of the assessment are to evaluate the:
-
clinical effectiveness of each intervention
-
adverse effect profile of each intervention
-
incremental cost-effectiveness of each intervention compared with (1) each other, (2) CTTs, (3) systemic therapy and (4) BSC.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
A systematic review of the clinical effectiveness evidence on SIRT was undertaken following the general principles outlined in the Centre for Reviews and Dissemination (CRD)’s guidance on undertaking systematic reviews15 and reported in accordance with the general principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 16 The research protocol is registered on PROSPERO, the international prospective register of systematic reviews in health and social care (registration number CRD42019128383).
Search strategy
A comprehensive search was undertaken to systematically identify clinical effectiveness and cost-effectiveness literature relating to TheraSphere, SIR-Spheres and QuiremSpheres for HCC. In addition, a search for randomised controlled trials (RCTs) of comparator therapies was undertaken to strengthen the network of evidence on SIRT.
Search strategy for selective internal radiation therapy studies
A search strategy was developed in Ovid MEDLINE by an information specialist (MH), with input from the review team. The strategy consisted of a set of terms for HCC combined with terms for SIRT, and was limited to studies from 2000 onwards. The 2000 date limit was applied as scoping searches had identified controlled studies of SIR-Spheres and TheraSphere published after the year 2000; earlier studies were preliminary uncontrolled studies so have limited value for addressing the decision problem. In addition, clinical advice confirmed that the treatment environment for patients with HCC was different prior to 2000 in terms of comparator treatment options. The searches were not limited by language or study design. The MEDLINE strategy was adapted for use in all other resources searched.
The following databases were searched on 28 January 2019:
-
MEDLINE (all) (via Ovid)
-
EMBASE (via Ovid)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus
-
Science Citation Index (via Web of Science)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley)
-
Cochrane Database of Systematic Reviews (via Wiley)
-
Database of Abstracts of Reviews of Effects (via CRD databases)
-
Health Technology Assessment (HTA) database (via CRD databases)
-
NHS Economic Evaluation Database (NHS EED) (via CRD databases)
-
EconLit (via Ovid).
In addition, information on studies in progress, unpublished research or research reported in grey literature was sought by searching a range of relevant resources:
-
ClinicalTrials.gov
-
World Health Organization (WHO) International Clinical Trials Registry portal
-
European Union Clinical Trials Register
-
PROSPERO
-
Conference Proceedings Citation Index – Science (via Web of Science)
-
ProQuest Dissertations & Theses A&I (via ProQuest).
A search of the NICE website and NHS Evidence for relevant guidelines was undertaken on 8 May 2019.
Company submissions and relevant systematic reviews were also hand-searched to identify further relevant studies. Clinical advisors were consulted for any additional studies.
Search results were imported into EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) and de-duplicated. Full search strategies can be found in Appendix 1.
Search strategy for comparator therapies
A search for RCTs of comparator therapies was undertaken to strengthen the network of evidence on SIRT. In view of time and resource limitations, it was decided to identify RCTs of CTTs (i.e. TAE, TACE and DEB-TACE) by searching existing relevant systematic reviews and meta-analyses and undertaking update searches if necessary.
Evidence on systemic therapies for HCC was identified from the recent NICE single technology appraisals of sorafenib,11 lenvatinib12 and regorafenib. 13
The search strategy for systematic reviews and meta-analyses of CTTs was developed in Ovid MEDLINE by an information specialist (MH), with input from the review team. The strategy consisted of a set of terms for HCC combined with terms for embolisation or chemoembolisation, and was limited to studies from 2010 onwards to identify the most recent reviews. A search strategy to limit retrieval to systematic reviews or meta-analyses was added in MEDLINE and EMBASE. 17 The MEDLINE strategy was adapted for use in all resources searched.
The following databases were searched on 7 May 2019:
-
MEDLINE (all) (via Ovid)
-
EMBASE (via Ovid)
-
Cochrane Database of Systematic Reviews (via Wiley)
-
Database of Abstracts of Reviews of Effects (via CRD databases)
-
HTA database (via CRD databases).
In addition, PROSPERO was searched to identify any unpublished or ongoing systematic reviews or meta-analyses.
Search results were imported into EndNote X9 and de-duplicated. Full search strategies can be found in Appendix 2.
Inclusion criteria
Inclusion criteria were defined in line with the final scope provided by NICE and are outlined below. Studies were initially assessed for relevance using titles and abstracts. One reviewer examined titles and abstracts, with a second reviewer checking 10% of records. Full manuscripts of any titles/abstracts that appeared to be relevant were obtained if possible and the relevance of each study was assessed independently by two reviewers in accordance with the criteria outlined in the following sections. Any discrepancies were resolved through consensus and, if necessary, a third reviewer was consulted. Relevant foreign-language studies were translated and assessed for inclusion in the review. Studies available only as abstracts were included and attempts were made to contact authors for further data.
Study design
Randomised controlled trials were eligible for inclusion in the clinical effectiveness review. However, where RCT evidence was insufficient to address the decision problem, non-randomised comparative studies (including retrospective studies) and non-comparative studies of SIRT were considered for inclusion. The evidence was scoped before deciding what level of evidence would be included for data extraction and quality assessment.
Participants
Studies of people with early-stage HCC in whom curative treatment is contraindicated (BCLC stage A), and with intermediate-stage (BCLC stage B) or advanced-stage (BCLC stage C) HCC, with or without PVT/portal vein invasion (PVI), were included in the review. Studies of people with secondary liver metastases or other types of liver cancer (such as cholangiocarcinoma) were not included unless they also included people with primary HCC, and results were reported separately for people with HCC.
Interventions
The interventions under consideration were the selective internal radiation therapies TheraSphere, SIR-Spheres and QuiremSpheres. Studies in which more than one type of SIRT was used were included only if results were reported separately for the different types of SIRT. Where studies did not state which type of SIRT or radioembolisation technology was used, authors were contacted to identify the specific technology used.
Evidence on combined treatments (e.g. SIRT plus sorafenib) was also considered for inclusion, and evidence was scoped before deciding which trials would be included for data extraction and quality assessment.
Comparators
Relevant comparators were:
-
alternative SIRT interventions (i.e. TheraSphere, SIR-Spheres and QuiremSpheres)
-
conventional transarterial therapies (i.e. TAE, TACE and DEB-TACE)
-
established clinical management without SIRT, such as systemic therapy (i.e. sorafenib, lenvatinib and regorafenib) or BSC, for people for whom any TAE therapies are inappropriate.
To strengthen the network of evidence on SIRT, we considered undertaking comparisons of CTTs (i.e. TAE, TACE and DEB-TACE), systemic therapies (i.e. sorafenib, lenvatinib and regorafenib) and BSC, using RCT evidence. The evidence was scoped and criteria for inclusion were developed. Relevant RCTs were assessed for quality and key outcome data were extracted, based on requirements for the model.
Outcomes
The outcome measures to be considered included:
-
overall survival (OS)
-
progression-free survival (PFS)
-
time to progression (TTP)
-
response rates
-
rates of liver transplant or surgical resection
-
adverse effects of treatment
-
health-related quality of life (HRQoL)
-
time on treatment/number of treatments provided.
Data extraction
Data were extracted by one reviewer using a standardised data extraction form and independently checked for accuracy by a second reviewer. Disagreements were resolved through consensus and, if necessary, a third reviewer was consulted. If multiple publications of the same study were identified, data were extracted and reported as a single study.
Critical appraisal
The methodological quality of the included studies was assessed using criteria relevant to the study design. RCTs were assessed using the most recent version of the Cochrane risk-of-bias tool. 18 Quality-assessment tools for other study designs were developed using relevant criteria, such as those outlined in the CRD’s guidance on undertaking systematic reviews. 15 Quality assessment was undertaken by one reviewer and independently checked by a second reviewer. Any disagreements were resolved through consensus and, if necessary, a third reviewer was consulted. Details of the quality of the included studies are presented in descriptive tables and their impact on the reliability of results is discussed.
Methods of data analysis/synthesis
Characteristics of the included SIRT studies (such as participant and intervention characteristics, results and trial quality) were tabulated and described in a narrative synthesis. Where sufficient clinically and statistically homogenous data were available, data were pooled using appropriate meta-analytic techniques using WinBUGS software (Medical Research Council Biostatistics Unit, Cambridge, UK). Clinical, methodological and statistical heterogeneity were investigated, with sensitivity or subgroup analyses undertaken where appropriate and where available data permitted.
Where the data allowed, a network meta-analysis (NMA) using Bayesian statistical methods with WinBUGS software was undertaken to estimate the relative effectiveness of the different treatments. Results are summarised using point estimates and 95% credible intervals (CrIs) of the effect of each treatment relative to the reference treatment. Where possible, consistency between direct and indirect estimates of treatment effect in the NMA was assessed. The results of the NMA are described in Chapter 4 of this report and were used in the economic model described in Chapter 7.
Clinical effectiveness results
Quantity and quality of research available
Studies of selective internal radiation therapy
The electronic searches for clinical effectiveness evidence on SIRT interventions (i.e. TheraSphere, SIR-Spheres and QuiremSpheres) identified a total of 4755 records (after de-duplication between databases). The 4755 records were inserted into an EndNote library. Reviewer 1 (RW) screened 2615 titles and abstracts, and reviewer 2 (SS) screened 2617 titles and abstracts. A total of 477 records (10% of the library) were double-screened; discrepancies were resolved through consensus or in consultation with a third reviewer (AE).
Of the 4755 records in the library, 3670 were excluded from the clinical effectiveness review after title and abstract screening as they did not include patients with unresectable HCC, did not assess TheraSphere, SIR-Spheres or QuiremSpheres, did not report relevant patient outcomes or were not a primary study. A total of 1085 records appeared to meet the study selection criteria based on title and abstract (where an abstract was available).
In view of the large number of potentially eligible records, the evidence was scoped before deciding which studies to order for full-paper screening. Records were coded, using titles and abstracts (where available), in terms of the intervention (type of SIRT and whether the study focused on the delivery of SIRT or the work-up procedure), the study design (prospective or retrospective, comparative or not) and the number of HCC patients included in the study. A large number of records were conference/meeting abstracts (n = 603) rather than full publications (n = 482); reviewer 1 (RW) coded the full publications and reviewer 2 (SS) coded the conference/meeting abstracts. Studies marked as a ‘RCT’ (n = 47; 43 full publications and four conference/meeting abstracts) or as ‘prospective comparative’ (n = 26; 18 full publications and eight conference/meeting abstracts) or ‘retrospective comparative’ (n = 103; 61 full publications and 42 conference/meeting abstracts) studies were ordered for full-paper screening as comparative studies (total n = 176) were prioritised over non-comparative studies. However, it was clear that there were no comparative studies of QuiremSpheres; therefore, all studies considered to relate to QuiremSpheres (referring to holmium as the intervention) were ordered for full-paper screening (n = 11). In addition, large non-comparative studies that included > 500 patients were also ordered for full-paper screening (n = 6). One additional non-comparative study, in which BCLC subgroups and subsequent treatments were reported and which was considered to be particularly relevant for the economic model, was ordered. Therefore, a total of 194 records were ordered for full-paper screening.
Of the 194 records ordered, 130 were excluded based on full-paper screening and 64 were considered to be potentially relevant records to be included in the clinical effectiveness review and/or NMA (55 studies plus nine associated publications).
A total of 130 records were coded at the title and abstract stage as systematic reviews. Reviewer 1 (RW) screened systematic reviews from 2015 onwards for relevance; there were 25 relevant systematic reviews (plus one associated erratum). The reference lists of these systematic reviews were screened to check for additional potentially relevant studies; no additional studies were identified.
Separate searches of guideline databases (the NICE website and NHS Evidence), conducted in May 2019, identified a total of 23 records after de-duplication against the original library, none of which were considered relevant for inclusion in the systematic review. The reference lists of relevant guidelines were screened to check for additional potentially relevant studies; no additional studies were identified.
Clinical advisors were not aware of any additional studies other than those already identified from electronic searches.
A PRISMA flow diagram is presented in Figure 1. In total, 27 of the 55 studies were prioritised for data extraction, as they were considered to be the most relevant for the assessment of clinical effectiveness and/or the proposed NMAs; these studies are summarised in Table 3. One non-comparative study was included in the clinical effectiveness review because this was the only study of QuiremSpheres;51 the other 26 studies were comparative studies.
FIGURE 1.
The PRISMA flow diagram of the study selection process for the clinical effectiveness review.

| Study (first author and year) | Intervention | Comparator | Location | Population |
|---|---|---|---|---|
| RCTs of SIR-Spheres (n = 5) | ||||
|
Vilgrain 201719 and Bouattour 201720 SARAH |
SIR-Spheres | Sorafenib | France | Adults with locally advanced HCC (BCLC C) or new HCC not eligible for surgical resection, transplant or thermal ablation after a previously cured HCC (cured by surgery or thermoablative therapy) or HCC with two unsuccessful rounds of TACE |
|
Chow 201821 SIRveNIB |
SIR-Spheres | Sorafenib | Asia-Pacific region | Adults with locally advanced HCC (BCLC B or C) not amenable to curative treatment |
|
Kolligs 201522 SIRTACE |
SIR-Spheres | TACE | Germany and Spain | Adults with unresectable liver-only HCC (without portal vein occlusion) |
| Pitton 201523 | SIR-Spheres | DEB-TACE | Germany | Adults with unresectable N0, M0 HCC (BCLC stage B) |
|
Ricke 201524 SORAMIC |
SIR-Spheres plus sorafenib | Sorafenib alone | Germany | Adults with unresectable intermediate or advanced HCC (BCLC stage B or C), with preserved liver function (Child–Pugh class ≤ B7) and ECOG < 2, who were poor candidates for TACE (including those failing TACE) |
| RCTs of TheraSphere (n = 2) | ||||
|
Salem 2016,25 Gabr 201726 and Gordon 201627 PREMIERE |
TheraSphere | TACE | USA | Adults with BCLC stage A/B unablatable/unresectable HCC with no vascular invasion, Child–Pugh class A/B |
| Kulik 2014,28 Lewandowski 201629 and Vouche 201330 | TheraSphere | TheraSphere plus sorafenib | USA | Adults with Child–Pugh class ≤ B8 and potential candidates for orthotopic liver transplant |
| Prospective comparative studies of TheraSphere (n = 7) | ||||
| Kirchner 201931 | TheraSphere | TACE/DEB-TACE | Germany | Adults with unresectable HCC |
| El Fouly 201532 | TheraSphere | TACE | Germany and Egypt | Adults with intermediate-stage (BCLC B) unresectable HCC and good liver function (Child–Pugh class < B7) |
| Salem 201333 | TheraSphere | TACE | USA | Adults with treatment-naive HCC with ECOG 0–2 |
| Memon 201334 | TheraSphere | TACE | USA | Adults with HCC that progressed after intra-arterial locoregional therapies (TACE and SIRT) |
| Hickey 201635 | TheraSphere | TACE | USA | Adults with unresectable HCC and bilirubin ≤ 3.0 mg/dl |
| Maccauro 201436 | TheraSphere plus sorafenib | TheraSphere alone | Italy | Adults with unresectable HCC (Child–Pugh class A) |
| Woodall 200937 | TheraSphere | BSC | USA | Adults with unresectable HCC (including both patients with and patients without PVT) |
| Retrospective comparative studies of SIR-Spheres vs. TheraSphere (n = 5) | ||||
| Biederman 201538 | SIR-Spheres | TheraSphere | USA | Adults with HCC with PVT |
| Biederman 201639 | SIR-Spheres | TheraSphere | USA | Adults with HCC with PVI |
| Van Der Gucht 201740 | SIR-Spheres | TheraSphere | Switzerland | Adults with unresectable HCC |
| Bhangoo 201541 | TheraSphere | SIR-Spheres | USA | Adults with unresectable HCC |
| d’Abadie 201842 | SIR-Spheres | TheraSphere | Belgium | Adults with HCC |
| Retrospective comparative studies of SIR-Spheres (n = 4) | ||||
| Cho 201643 | SIR-Spheres | Sorafenib | Korea | Adults with BCLC stage C HCC with PVT |
| de la Torre 201644 | SIR-Spheres | Sorafenib | Spain | Adults with HCC with PVI |
| Gramenzi 201545 | SIR-Spheres | Sorafenib | Italy | Adults with HCC unfit for other effective therapies, Child–Pugh class A/B, performance status ≤ 1, no metastases and no previous systemic chemotherapy |
| Soydal 201646 | TACE | SIR-Spheres | Turkey | Adults with BCLC B or C HCC |
| Retrospective comparative studies of TheraSphere (n = 3) | ||||
| Salem 201147 | TheraSphere | TACE | USA | Adults with unresectable HCC and bilirubin 3.0 mg/dl |
| Moreno-Luna 201348 | TheraSphere | TACE | USA | Adults with unresectable HCC |
| Akinwande 201649,50 | TheraSphere | DEB-TACE | USA | Adults with unresectable HCC (with or without PVT) |
| Non-comparative studies of QuiremSpheres (n = 1) | ||||
| Radosa 201951 | QuiremSpheres | N/A | Germany | Adults with HCC |
The 28 lower-priority studies are summarised in Appendix 7 along with the reason for not including them in the systematic review of clinical effectiveness or the proposed NMAs (e.g. consultation with clinical advisors confirmed that the comparators used were not applicable to current UK practice). 52–55
Thirty-four records were coded at the title and abstract stage as potentially relevant economic studies (seven of which were also coded as includes for the clinical effectiveness review). A separate flow diagram of the study selection process for these economic studies is presented (see Figure 7).
Studies of comparator therapies
Randomised controlled trials of comparator therapies were sought to strengthen the network of evidence on SIRT (see Chapter 5). The search for systematic reviews and meta-analyses of CTTs (TAE, TACE and DEB-TACE) identified 989 records. The records were inserted into an EndNote library and one reviewer (RW) screened the titles and abstracts. Records were put in reverse date order and screened started at the year 2019 and worked backwards until no new relevant RCTs were identified from the reviews and meta-analyses. A total of 319 records were screened, published between 2017 and 2019. Twenty-four of the 319 records were relevant systematic reviews or meta-analyses; full papers were obtained and reference lists were checked for RCTs comparing TAE, TACE or DEB-TACE with each other. Eleven relevant RCTs (reported in 12 publications) were identified, which are summarised in Table 4. In view of the recency of the relevant systematic reviews and meta-analyses and the age of the RCTs of CTTs (published between 1992 and 2016), it was decided that update searches were not necessary.
| Study (first author and year) | Intervention | Comparator | Population |
|---|---|---|---|
|
PRECISION V |
DEB-TACE | TACE | Adults with HCC unsuitable for resection or percutaneous ablation (BCLC A/B without portal invasion or extrahepatic spread) |
| Golfieri 201458 | DEB-TACE | TACE | Adults with HCC unsuitable for curative treatment or had failed/recurred after resection/ablation |
| Sacco 201159 | DEB-TACE | TACE | Adults with previously untreated unresectable HCC not suitable for ablative treatment, Child–Pugh class A or B and ECOG score of 0/1, absence of PVT and extrahepatic metastases |
| van Malenstein 201160 | DEB-TACE | TACE | Adults with HCC who were not candidates for curative treatments, Child–Pugh class A or B cirrhosis and an ECOG score of 0 or ECOG score of < 3 if the restriction in status was not because of the HCC |
| Llovet 200261 | TACE | TAE | White patients with unresectable HCC not suitable for curative treatment, or Child–Pugh class A or B and Okuda stage I or II |
| Kawai 199262 | TACE | TAE | HCC patients |
| Chang 199463 | TACE | TAE | Untreated patients with inoperable HCC |
| Meyer 201364 | TACE | TAE | Patients aged ≥ 16 years with HCC not eligible for surgical resection |
| Yu 201465 | TACE | TAE | Unresectable HCC |
| Malagari 201066 | DEB-TACE | TAE | HCC patients unsuitable for curative treatments, with potentially resectable lesions but at high risk for surgery and patients with HCC suitable for RFA but of high risk because of location |
| Brown 201667 | DEB-TACE | TAE | Adults with HCC with ECOG score of 0 to 1 and Okuda stage I or II |
Evidence on systemic therapies for HCC was identified from the recent NICE single technology appraisals of sorafenib,11 lenvatinib12 and regorafenib. 13
Assessment of clinical effectiveness
This section describes the seven RCTs and seven prospective comparative studies of SIR-Spheres and TheraSphere, the five retrospective comparative studies comparing SIR-Spheres with TheraSphere and the non-comparative case series of QuiremSpheres. The additional seven retrospective comparative studies of SIR-Spheres or TheraSphere (see Table 3) and studies of comparator therapies (see Table 4) that were selected, as they were considered to be potentially relevant for the NMAs, are described in Chapter 5.
Risk of bias
Results of the risk-of-bias judgements are presented in Appendix 5.
The SorAfenib versus Radioembolization in Advanced Hepatocellular Carcinoma (SARAH) and Selective Internal Radiation Therapy Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma (SIRveNIB) RCTs were both rated as having a low overall risk of bias. 19–21 There were some concerns regarding bias for the trials undertaken by Pitton et al. 23 and Kulik et al. 28 Concerns related to the randomisation process for the study by Pitton et al. 23 There were concerns related to the randomisation process, potential deviations from the intended interventions and measurement of the outcome for the study by Kulik et al. 28 The SIRTACE,22 SORAMIC24 and Prospective Randomized study of chEmoeMbolization versus radIoEmbolization for the tReatment of hEpatocellular carcinoma (PREMIERE)25–27 trials were all rated as being at a high overall risk of bias; the SIRTACE trial was rated as being at a high risk of bias arising from the randomisation process, missing outcome data and measurement of the outcome,22 the SORAMIC trial was rated as being at a high risk of bias in relation to deviations from the intended interventions as well as some concerns arising from the randomisation process,24 and the PREMIERE trial was rated as being at a high risk of bias arising from the randomisation process and concerns arising from deviations from the intended interventions. 25–27
The prospective comparative studies were all rated as being at a high risk of bias. 31–37 In particular, allocation to treatment groups was either inadequately described or inappropriate, resulting in differences in prognostic factors between treatment groups at baseline. Outcome assessors do not appear to have been blinded in any of the prospective comparative studies.
Four of the retrospective comparative studies were rated as being at a high risk of bias. 38–40,42 The two studies by Biederman et al. 38,39 appear to have included many of the same patients, although one of the studies was reported only as a conference abstract, with very limited study details. 38 Each of the studies rated as being at a high risk of bias appeared to include patients with different prognostic characteristics at baseline in the two different treatment groups. It was unclear whether or not outcome assessors were blinded in any of the studies. The study by Bhangoo et al. 41 was rated as being at an unclear risk of bias; it was unclear whether or not treatment groups were similar at baseline, whether or not outcome assessors were blinded and whether or not missing outcome data were balanced across treatment groups.
The small case series undertaken by Radosa et al. 51 should be considered to be at a high risk of bias; it is unclear whether or not patients were representative of all those who would be eligible for SIRT in clinical practice, outcome assessors were not blinded to the participants’ intervention and outcome measures were not consistently assessed.
Efficacy and safety of SIR-Spheres
As discussed in Study design, RCTs were eligible for inclusion in the clinical effectiveness review, with non-randomised comparative studies and non-comparative studies considered for inclusion, in the absence of sufficient RCT evidence. Five RCTs of SIR-Spheres were identified, comparing SIR-Spheres with established therapies available to patients with intermediate (TACE/DEB-TACE) and advanced (sorafenib) HCC. Other studies of SIR-Spheres identified also compared with sorafenib or TACE (see Table 3); therefore, they were not included in the review.
This section focuses on the two large good-quality RCTs (SARAH and SIRveNIB) and also presents a brief summary of the three lower-quality RCTs of SIR-Spheres.
The SARAH and SIRveNIB randomised controlled trials
Two large RCTs compared SIR-Spheres with sorafenib in patients who were not suitable for curative treatments: the SARAH trial was conducted in France19,20 and the SIRveNIB trial was conducted in the Asia-Pacific region. 21 Both trials were considered to have a low overall risk of bias (see Appendix 5). Further details of these trials are presented in Table 5.
| Characteristic | SARAH19 | SIRveNIB21 | ||
|---|---|---|---|---|
| Trial characteristic | ||||
| Study design | Multicentre open-label RCT | Multicentre open-label RCT | ||
| Location | France (25 centres) | Asia-Pacific region (11 countries) | ||
| Source of funding | Sirtex | Sirtex | ||
| Inclusion criteria | Locally advanced HCC (BCLC stage C) or new HCC not eligible for surgery/ablation after previously cured HCC (cured by surgery or thermoablative therapy) or HCC with two unsuccessful rounds of TACE. Life expectancy of > 3 months, ECOG performance status 0 or 1, Child–Pugh class A or B score of ≤ 7 | Locally advanced HCC (BCLC stage B or C without extrahepatic disease) with or without PVT, not amenable to curative treatment modalities | ||
| Intervention |
SIR-Spheres (n = 237) Patients underwent angiography, protective coiling and MAA-SPECT/computerised tomography scan and were readmitted for SIRT 1 or 2 weeks later. In bilobar tumours, the first treatment was delivered to the hemiliver with the greatest tumour burden and the contralateral hemiliver was scheduled for treatment 30–60 days after the first treatment. If the tumour progressed, SIRT could be repeated 184/237 patients received SIR-Spheres:53/237 (22%) patients did not receive SIRT |
SIR-Spheres (n = 182) Patients underwent angiographic and MAA assessment of suitability for SIRT. Eligible patients received a single delivery of SIRT 52/182 (28.6%) patients did not receive SIRT |
||
| Comparator |
Sorafenib (n = 222) Continuous oral sorafenib (400 mg twice daily) |
Sorafenib (n = 178) Continuous oral sorafenib (400 mg twice daily) |
||
| Primary outcome | OS | OS | ||
| Secondary outcomes |
|
|
||
| Baseline patient characteristic (ITT population) | ||||
| SIR-Spheres | Sorafenib | SIR-Spheres | Sorafenib | |
| Number of patients |
237 (ITT) 174 (per protocol) |
222 (ITT) 206 (per protocol) |
182 (ITT) 130 (per protocol) |
178 (ITT) 162 (per protocol) |
| Median/mean age (years) | 66 (IQR 60–72) | 65 (IQR 58–73) | 59.5 (SD 12.9) | 57.7 (SD 10.6) |
| Proportion male (%) | 89 | 91 | 80.8 | 84.8 |
| Cirrhosis present, n (%) | 211 (89) | 201 (91) | NR | NR |
| Cause of HCC, n (%) | ||||
| Alcohol | 147 (62)a | 124 (56)a | NR | NR |
| Non-alcoholic steatohepatitis | 49 (21)a | 60 (27)a | NR | NR |
| Hepatitis B | 13 (5)a | 15 (7)a | 93 (51.1) | 104 (58.4) |
| Hepatitis C | 55 (23)a | 49 (22)a | 26 (14.3) | 19 (10.7) |
| Hepatitis B and C | NR | NR | 4 (2.2) | 5 (2.8) |
| Other/unknown | 45 (19)a | 41 (18)a | NR | NR |
| BCLC classification, n (%) | ||||
| Stage A | 9 (4) | 12 (5) | 0 | 1 (0.6) |
| Stage B | 66 (28) | 61 (27) | 93 (51.1) | 97 (54.5) |
| Stage C | 162 (68) | 149 (67) | 88 (48.4) | 80 (44.9) |
| Child–Pugh classification, n (%) |
A5 + A6: 196 (83) B7: 39 (16) Unknown: 2 (1) |
A5 + A6: 187 (84) B7: 35 (16) Unknown: 0 (0) |
A: 165 (90.7) B: 14 (7.7) |
A: 160 (89.9) B: 16 (9.0) |
| ECOG performance status, n (%) | ||||
| 0 | 145 (61) | 139 (63) | 135 (74.2) | 141 (79.2) |
| 1 | 92 (39) | 83 (37) | 47 (25.8) | 37 (20.8) |
| Tumours, n (%) | ||||
| Single | 110 (46) | 96 (43) | NR | NR |
| Multiple | 127 (54) | 126 (57) | ||
| Tumour involvement, n (%) | ||||
| Unilobar | 187 (79) | 187 (84) | NR | NR |
| Bilobar | 50 (21) | 35 (16) | ||
| MVI, n (%) | 149 (63) | 128 (58) | NR | NR |
| PVT, n (%) | NR | NR | 56 (30.8) | 54 (30.3) |
| Portal venous invasion, n/N (%) | ||||
| Main portal vein | 49/143 (34) | 38/118 (32) | NR | NR |
| Main portal branch (right or left) | 65/143 (46) | 59/118 (50) | ||
| Segmental | 29/143 (20) | 21/118 (18) | ||
| Portal vein occlusion, n/N (%) | ||||
| Complete | 18/48 (38) | 18/38 (47) | NR | NR |
| Incomplete | 30/48 (62) | 20/38 (53) | ||
| Previously received TACE, n (%) | 106/237 (45) | 94/222 (42) | NR | NR |
| Trial results | ||||
| Median OS (months) | 8.0 (95% CI 6.7 to 9.9) | 9.9 (95% CI 8.7 to 11.4) | 8.8 | 10.0 |
|
HR 1.15, 95% CI 0.94 to 1.41; p = 0.18 (ITT) HR 0.99, 95% CI 0.79 to 1.24 (per protocol) |
HR 1.12, 95% CI 0.9 to 1.4; p = 0.36 (ITT) HR 0.86, 95% CI 0.7 to 1.1; p = 0.27 (per protocol) |
|||
| Median PFS (months) | 4.1 (95% CI 3.8 to 4.6) | 3.7 (95% CI 3.3 to 5.4) | 5.8 | 5.1 |
| HR 1.03, 95% CI 0.85 to 1.25; p = 0.76 (ITT) |
HR 0.89, 95% CI 0.7 to 1.1; p = 0.31 (ITT) HR 0.73, 95% CI 0.6 to 0.9; p = 0.0128 (per protocol) |
|||
| TTP (months) | NR | 6.1 | 5.4 | |
| Tumour response rate | 36/190 (19%) evaluable patients achieved a complete (n = 5) or partial (n = 31) response | 23/198 (12%) evaluable patients achieved a complete (n = 2) or partial (n = 21) response | 16.5% (all partial response, 0% achieved a complete response) | 1.7% (all partial response, 0% achieved a complete response) |
| Rates of subsequent liver transplantation or resection |
6/237 (2.5%) had tumour ablationb 3/237 (1.3%) had liver surgeryb 2/237 (0.8%) had liver transplantation |
2/222 (0.9%) had tumour ablation 1/222 (0.5) had liver transplantation |
1/182 (0.5%) had radiofrequency ablation 2/182 (1.1%) had surgery |
2/178 (1.1%) had radiofrequency ablation 1/178 (0.6%) had surgery |
| HRQoLc | Global health status subscore was significantly better in the SIRT group than in the sorafenib group (group effect p = 0.0048; time effect p < 0.0001) and the between-group difference tended to increase with time (group × time interaction p = 0.0447) | There were no statistically significant differences in the EQ-5D index between the SIRT and sorafenib groups throughout the study in either the ITT or the per-protocol populations | ||
| Number of patients reporting treatment-related adverse events, n/N (%) | 173/226 (77) | 203/216 (94) | 78/130 (60) | 137/162 (84.6) |
| Number of patients reporting grade ≥ 3 adverse events, n/N (%) | 92/226 (41) | 136/216 (63) | 36/130 (27.7) | 82/162 (50.6) |
As shown in Table 5, there were methodological differences between the SARAH and the SIRveNIB trials. In the SIRveNIB trial, patients could receive only one SIRT delivery, whereas in the SARAH trial patients could receive more than one delivery of SIRT; 69 out of 184 (37.5%) patients who received SIRT received more than one delivery to either the ipsilateral or the contralateral lobe.
The SARAH trial was conducted in France and the SIRveNIB trial was conducted in the Asia-Pacific region. This has implications for the generalisability of the SIRveNIB trial results to the UK population. HCC in European patients is more likely to be caused by alcohol or hepatitis C, whereas in Asia it is more likely to be caused by hepatitis B. The natural history of these diseases is different. Treatment options are also different, as hepatitis B-related liver disease is often less advanced than in alcohol-related or hepatitis C-related disease; therefore, patients may have had more treatment prior to receiving systemic therapy.
The Sirtex submission stated that patient selection in the SARAH trial did not reflect UK clinical practice, as the trial included patients with a poor survival prognosis who would be considered for only systemic therapy or BSC [e.g. because of a high tumour burden, main PVT or impaired liver function (Child–Pugh class B)]. Therefore, this has implications for the generalisability of the SARAH trial results to the UK population who would be eligible for SIRT in clinical practice.
In both trials, patients were assessed for suitability of SIRT after randomisation. In the SARAH trial, 53 out of 237 (22.4%) patients allocated to SIR-Spheres did not receive SIRT, 26 of whom were treated with sorafenib. In the SIRveNIB trial, 52 out of 182 (28.6%) patients allocated to SIR-Spheres did not receive SIRT, three of whom were treated with sorafenib (where reported; subsequent treatments were not reported for 31/52 patients). Results were presented for both the intention-to-treat (ITT) and the per-protocol populations; patients who did not receive their allocated treatment were excluded from the per-protocol analysis (those who received sorafenib instead of SIRT were not included in the sorafenib arm in the per-protocol analysis).
The SARAH and SIRveNIB trial publications reported baseline characteristics for both the ITT and the per-protocol populations. 19,21 The SIR-Spheres and sorafenib groups were generally similar at baseline in the ITT populations (see Table 5). However, in the per-protocol population, patients in the sorafenib arm appeared to have slightly worse disease characteristics than those in the SIR-Spheres arm in the SARAH trial (BCLC stage C: 69.4% vs. 65.5%; Child–Pugh class B7: 14.6% vs. 11.5%; median tumour burden: 20% vs. 12.5%, respectively) and in the SIRveNIB trial (BCLC stage C: 45.1% vs. 38.5%; PVT: 29.6% vs. 23.1%; tumour size > 50% of liver: 21.6% vs. 17.7%, respectively).
Neither trial found a statistically significant difference in OS between SIR-Spheres and sorafenib in either the ITT or the per-protocol analyses, as shown in Table 5.
Both trials undertook subgroup analyses according to baseline characteristics. The SIRveNIB trial reported a statistically significant difference in OS favouring SIR-Spheres in the subgroup of patients with BCLC stage C disease in the per-protocol analysis [median 9.2 vs. 5.8 months, hazard ratio (HR) 0.67, 95% confidence interval (CI) 0.4 to 1.0; p = 0.0475]. The SARAH trial demonstrated a statistically significant difference in OS favouring sorafenib in the subgroup of patients with complete occlusion in the main portal vein in the per-protocol analysis (HR 2.44, 95% CI 1.01 to 5.88); however, the number of patients included in this subgroup analysis was very small, so the result should be interpreted with caution.
In the SARAH trial, PFS was defined as the time from the closest date of radiological examination before first administration of study treatment to disease progression, in accordance with Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria,68 or death. In the SIRveNIB trial, PFS was defined as the time from the date of randomisation to tumour progression at any site in the body, or death, whichever is earlier. Tumour progression was assessed in accordance with RECIST 1.1 criteria. 68
Progression-free survival was not statistically significantly different between treatment groups in the ITT analyses of either the SARAH or the SIRveNIB trials. However, in the SIRveNIB trial, PFS was statistically significantly improved with SIR-Spheres in the per-protocol analysis (HR 0.73, 95% CI 0.6 to 0.9; p = 0.0128).
Tumour response was statistically significantly greater in the SIR-Spheres arm than in the sorafenib arm in both the SARAH and the SIRveNIB trials (SARAH: 19% vs. 12%, p = 0.0421; SIRveNIB: 16.5% vs. 1.7%, p < 0.001). However, in the SARAH trial, only 190 SIR-Spheres patients and 198 sorafenib patients were evaluable and included in the analysis.
A very small proportion of patients in both treatment arms of the SARAH and the SIRveNIB trials went on to have subsequent liver transplantation (< 1%), liver surgery (0.6–1.3%) or tumour ablation (0.5–2.5%).
The SARAH trial reported statistically significantly better HRQoL in the SIR-Spheres treatment group than in the sorafenib group for both the ITT and the per-protocol populations, assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30. However, the proportion of patients who completed questionnaires was 71% in the SIR-Spheres group (169/237) and 84% (186/222) in the sorafenib group at baseline, reducing with time to only 29% (26/90 patients at risk) in the SIR-Spheres group and 32% (29/92 patients at risk) in the sorafenib group at 12-month follow-up. There was no statistically significant difference in HRQoL between the treatment groups in the SIRveNIB trial, assessed using the EuroQol-5 Dimensions (EQ-5D) index.
The proportion of patients reporting at least one treatment-related adverse event (TRAE) and the proportion reporting at least one grade ≥ 3 adverse event (AE) was higher in the sorafenib group than in the SIR-Spheres group in both trials, as shown in Table 5.
In the SARAH trial, the most frequent grade ≥ 3 AEs were fatigue (SIR-Spheres 9% vs. sorafenib 19%), liver dysfunction (11% vs. 13%), increased laboratory liver values (9% vs. 7%), haematological abnormalities (10% vs. 14%), diarrhoea (1% vs. 14%), abdominal pain (3% vs. 6%), increased creatinine (2% vs. 6%) and hand–foot skin reaction (< 1% vs. 6%).
In the SIRveNIB trial, the most frequent grade ≥ 3 AEs of interest were anaemia (SIR-Spheres 0% vs. sorafenib 2.5%), fatigue (0% vs. 3.7%), diarrhoea (0% vs. 3.7%), abdominal pain (2.3% vs. 1.2%), ascites (3.8% vs. 2.5%), hypertension (0% vs. 1.2%), upper gastrointestinal haemorrhage (0.8% vs. 1.9%), jaundice (0.8% vs. 1.2%), radiation hepatitis (1.5% vs. 0%) and hand–foot skin reaction (0% vs. 16.7%).
The AE profiles of SIRT and sorafenib are very different. Sorafenib is a continuous treatment, whereas most patients receive only one delivery of SIRT [37.5% patients in the SARAH trial received more than one delivery, either to the ipsilateral or to the contralateral lobe (primarily because of bilobar tumours or a large central tumour requiring bilateral treatment), whereas in the SIRveNIB trial patients received only one delivery]. AE rates were not reported separately for patients who received more than one delivery of SIRT; therefore, it is not possible to compare AE rates for patients who received one delivery with those who received more than one delivery. In the SARAH trial, patients with bilobar tumours received the first treatment in the hemiliver with the greatest tumour burden, and treatment of the contralateral hemiliver was scheduled 30–60 days after the first treatment. No patient had a whole-liver treatment approach in one session. Clinical advisors confirmed that this is reflective of their experience; patients would not receive whole-liver treatment in one session to reduce the risk of radioembolisation-induced liver disease (REILD). However, the Sirtex submission states that SIR-Spheres can be administered to both lobes of the liver during the same procedure [based on observational data in which 95.9% patients in the European Network on Radioembolisation with Yttrium-90 Resin Microspheres (ENRY) register received whole-liver treatments in a single session69]; neither the SARAH trial nor the SIRveNIB trial administered SIR-Spheres to both lobes during the same procedure. This variance is probably because of the clinical indication for SIRT; the ENRY register is likely to include a majority of patients with colorectal cancer liver metastases, who do not have underlying cirrhosis, whereas in HCC patients the cirrhotic liver is likely to be more susceptible to REILD.
A relatively large proportion of patients who undergo work-up for SIRT, to assess their suitability for the procedure, are unable to receive SIRT (e.g. owing to liver-to-lung shunting or unfavourable hepatic arterial anatomy) [42/226 (18.6%) in SARAH and 37/182 (20.3%) in SIRveNIB]. The work-up of patients who are unable to undergo SIRT delivery has cost implications.
The SARAH randomised controlled trial subgroup analysis (low tumour burden/low albumin–bilirubin grade)
The Sirtex company submission selected a subgroup of patients from the SARAH trial with ≤ 25% tumour burden and albumin–bilirubin (ALBI) 1 for their base-case analysis in the economic model; the company stated that these patients are considered the most appropriate candidates for SIR-Spheres in clinical practice, as they are the most likely to benefit from SIRT. This is not a clinically recognised subgroup and was based on a post hoc analysis; therefore, these results should be prospectively validated before being considered relevant for clinical practice.
This subgroup included 37 (16%) patients in the SIRT group and 48 (22%) patients in the sorafenib group; 92% of those allocated to SIRT received treatment after work-up. Baseline characteristics were relatively well balanced between treatment groups, although more patients in the SIRT arm had BCLC stage B disease, single tumours and received previous TACE (these patients generally have a better prognosis than patients who are diagnosed at a later stage and are not eligible for TACE) than in the sorafenib arm. More patients in the sorafenib arm had an ECOG performance status of 0 and unilobar liver involvement. Table 6 presents the baseline characteristics and results for the full ITT population and the low tumour burden/low ALBI grade subgroup of the SARAH trial.
| Characteristic | ITT population | Low tumour burden/low ALBI grade subgroup | ||
|---|---|---|---|---|
| SIR-Spheres | Sorafenib | SIR-Spheres | Sorafenib | |
| Baseline patient characteristic | ||||
| Number of patients | 237 | 222 | 37 | 48 |
| Median age (years) | 66 | 65 | NR | NR |
| Age group (years) (%) | ||||
| ≥ 65 | NR | NR | 43 | 48 |
| < 65 | NR | NR | 57 | 52 |
| BCLC classification (%) | ||||
| Stage A | 4 | 5 | 3 | 6 |
| Stage B | 28 | 27 | 43 | 35 |
| Stage C | 68 | 67 | 54 | 58 |
| Child–Pugh classification (%) |
A5 + A6: 83 B7: 16 Unknown: 1 |
A5 + A6: 84 B7: 16 Unknown: 0 |
A: 95 B: 5 |
A: 98 B: 2 |
| ECOG performance status (%) | ||||
| 0 | 61 | 63 | 62 | 79 |
| 1 | 39 | 37 | 38 | 21 |
| Tumours (%) | ||||
| Single | 46 | 43 | 43 | 33 |
| Multiple | 54 | 57 | 57 | 67 |
| Tumour involvement (%) | ||||
| Unilobar | 79 | 84 | 76 | 85 |
| Bilobar | 21 | 16 | 24 | 15 |
| MVI (%) | 63 | 58 | 54 | 52 |
| Portal venous invasion, n/N (%) | ||||
| Main portal vein | 49/143 (34) | 38/118 (32) | 11 | 10 |
| Main portal branch | 65/143 (46) | 59/118 (50) | ||
| Segmental | 29/143 (20) | 21/118 (18) | ||
| Previously received TACE (%) | 45 | 42 | 51 | 44 |
| Trial results | ||||
| Median OS (months) | 8.0 (95% CI 6.7 to 9.9) | 9.9 (95% CI 8.7 to 11.4) | 21.9 (95% CI 15.2 to 32.5) | 17.0 (95% CI 11.6 to 20.8) |
| HR 1.15, 95% CI 0.94 to 1.41; p = 0.18 | HR 0.73, 95% CI 0.44 to 1.21; p = 0.22 | |||
| Median PFS (months) | 4.1 (95% CI 3.8 to 4.6) | 3.7 (95% CI 3.3 to 5.4) | NR | NR |
| HR 1.03, 95% CI 0.85 to 1.25; p = 0.76 | HR 0.65, 95% CI 0.41 to 1.02; p = 0.06 | |||
| Tumour response rate | 36/190 (19%) evaluable patients achieved a complete (n = 5) or partial (n = 31) response | 23/198 (12%) evaluable patients achieved a complete (n = 2) or partial (n = 21) response | NR | NR |
| Rates of subsequent liver transplantation or resection |
6/237 (2.5%) had tumour ablationa 3/237 (1.3%) had liver surgerya 2/237 (0.8%) had liver transplantation |
2/222 (0.9%) had tumour ablation 1/222 (0.5%) had liver transplantation |
14% (subsequent curative therapy) | 2% (subsequent curative therapy) |
| HRQoLb | Global health status subscore was significantly better in the SIRT group than in the sorafenib group (group effect p = 0.0048; time effect p < 0.0001) and the between-group difference tended to increase with time (group*time interaction p = 0.0447) | NR | ||
| Number of patients reporting TRAEs, n/N (%) | 173/226 (77) | 203/216 (94) | NR | NR |
| Number of patients reporting grade ≥ 3 AEs, n/N (%) | 92/226 (41) | 136/216 (63) | NR | NR |
As shown in Table 6, median OS and PFS appeared to be better in the SIR-Spheres arm than in the sorafenib arm in the post hoc subgroup analysis, although the difference between treatment groups was not statistically significant. The proportion of patients who went on to have potentially curative therapy was higher in the SIR-Spheres arm than in the sorafenib arm, although numbers were very low (five and one patients, respectively). Tumour response rate, HRQoL and AEs were not reported separately for the low tumour burden/low ALBI grade subgroup.
Prespecified and post hoc subgroup analysis results were presented in the SARAH trial publication for OS. 19 Tumour burden was included as a post hoc subgroup. However, neither the ALBI grade nor the combination of low tumour burden and low ALBI grade was presented.
The SIRveNIB trial did not report subgroup analysis results for the subgroup of low tumour burden/low ALBI grade patients. However, ALBI grade was included in the OS subgroup analysis. Results favoured SIR-Spheres in the subgroup of ALBI 1 patients (HR 0.89, 95% CI 0.6 to 1.4; p = 0.58), whereas results favoured sorafenib for the subgroup of patients with ALBI 2/3 (HR 1.24, 95% CI 0.9 to 1.7; p = 0.14).
Other randomised controlled trials of SIR-Spheres
The SIRTACE is a small RCT rated as being at a high risk of bias that compared SIR-Spheres (n = 13) with TACE (n = 15) in patients with unresectable HCC without portal vein occlusion. 22 A higher proportion of patients in the SIRT group had BCLC stage A disease (38.5% vs. 26.7%) and Child–Pugh liver function class A (92.3% vs. 86.7%) than in the TACE group. The average number of tumour nodules was higher in the TACE group (5.0 vs. 3.5). Therefore, patients in the SIR-Spheres treatment arm had a better prognosis than those in the TACE arm.
At 6 months, 69.2% of SIRT patients and 86.7% of TACE patients were still alive. At 12 months, 46.2% of SIRT patients and 66.7% of TACE patients were still alive. PFS, disease control rate and the proportion of patients who went on to have potentially curative therapy were similar between treatment groups. The proportion of patients with a partial response was higher in the SIRT group than in the TACE group (30.8% vs. 13.3%), although patient numbers were very small.
There were no statistically significant differences between treatment groups in HRQoL by week 12, despite Functional Assessment of Cancer Therapy Hepatobiliary-Pancreatic Symptom Index (FACT-Hep) scores being lower in the SIRT group at baseline (indicating lower quality of life). However, 10 out of 28 patients had missing baseline data and were excluded from HRQoL analyses. The proportion of patients reporting TRAEs was higher in the TACE group than in the SIRT group (33.3% vs. 23.1%), although the proportion of patients reporting at least one AE was higher in the SIRT group (92.3% vs. 66.7%), as was the number of patients with grade ≥ 3 AEs (three vs. two patients) and serious AEs requiring hospitalisation (seven vs. five patients).
A small RCT by Pitton et al. ,23 with some concerns regarding bias, compared SIR-Spheres (n = 12) with DEB-TACE (n = 12) in patients with unresectable intermediate (BCLC stage B) HCC with preserved liver function (Child–Pugh class A–B7). Treatment groups appeared reasonably similar at baseline, although more patients in the SIRT group had received prior local ablation (four vs. one) and more patients in the DEB-TACE group had received prior resection (five vs. three). Median OS and PFS were longer in the DEB-TACE arm than in the SIR-Spheres arm (788 days vs. 592 days and 216 days vs. 180 days, respectively), although the difference between groups was not statistically significant. Median TTP was 371 days in the SIRT arm and 336 days in the DEB-TACE arm. AEs were not reported.
The SORAMIC RCT compared SIR-Spheres followed by sorafenib with sorafenib alone in patients with unresectable intermediate or advanced (BCLC stage B or C) HCC with preserved liver function (Child–Pugh class ≤ B7) and ECOG performance status of < 2, who were poor candidates for TACE. Only safety and tolerability data for the first 40 patients have been published to date, rated as being at a high risk of bias. 24 More patients in the sorafenib-alone group had PVT (35% vs. 15%) and BCLC stage C disease (70% vs. 60%), indicating poorer prognosis in this group. There were 196 treatment-emergent AEs reported in the SIRT plus sorafenib arm and 222 events in the sorafenib-alone arm, of which 21.9% and 21.2%, respectively, were considered to be grade ≥ 3. The most common grade 3 or 4 AEs (hypertension, hand–foot skin reaction and diarrhoea) were reported in a similar number of patients in both treatment arms. Grade 3 or 4 fatigue appeared more common in patients receiving SIRT plus sorafenib (20% vs. 10%). Grade 3 or 4 infection and anorexia appeared more common in patients receiving sorafenib alone (20% vs. 5% and 0% vs. 10%, respectively). Grade 3 or 4 laboratory-related events were more common in patients receiving sorafenib alone (elevated gamma-glutamyltransferase level 45% vs. 30%, elevated aspartate aminotransferase level 15% vs. 0% and elevated alanine aminotransferase level 10% vs. 0%). One patient experienced a grade 3 gastric ulcer that was probably (but not proven to be) related to SIRT microspheres deposition.
Further details of each of these trials are presented in Appendix 6.
Ongoing studies
There are three ongoing studies of SIR-Spheres including patients with HCC: the Austrian Cardiovascular and Interventional Radiological Society of Europe Registry for SIR-Spheres Therapy (CIRT),70 the RESIN tumour registry in the USA71 and the RESIN tumour registry in Taiwan. 72 The CIRT study was completed in January 2020, the RESIN tumour registry study in the USA is due to be completed in August 2022 and the RESIN tumour registry study in Taiwan was due to be completed in December 2019.
There is also an ongoing individual patient data prospective meta-analysis of patients from the SIRveNIB and SARAH trials: VESPRO. 73
Efficacy and safety of TheraSphere
As discussed in Study design, RCTs were eligible for inclusion in the clinical effectiveness review. Non-randomised comparative studies (including retrospective studies) and non-comparative studies were considered for inclusion in the absence of sufficient RCT evidence. Only two small RCTs of TheraSphere were identified. Therefore, prospective non-randomised comparative studies were also included in the clinical effectiveness review; seven non-RCTs were included, most of which compared TheraSphere with TACE/DEB-TACE. The retrospective comparative studies of TheraSphere that were identified also compared against TACE/DEB-TACE (see Table 3); therefore, they were not included in the review as they were considered to be lower quality than the prospective comparative studies.
One small RCT rated as being at a high risk of bias (PREMIERE) compared TheraSphere (n = 24) with TACE (n = 21) as a bridge to transplant in patients with BCLC stage A or B unresectable HCC with no vascular invasion and Child–Pugh liver function class A or B. 25–27 The proportion of patients with Child–Pugh class A was much higher in the TACE arm than in the TheraSphere arm (71% vs. 50%) and the proportion of patients with portal hypertension was much lower in the TACE arm (52% vs. 83%), suggesting better prognosis in the TACE arm. OS was slightly longer in the TheraSphere arm (18.6 months vs. 17.7 months) and the rate of liver transplant/resection was also higher in the TheraSphere arm (87% vs. 70% of ‘listed patients’), although time to transplant/resection was slightly longer in the TheraSphere arm (8.8 months vs. 7.6 months). TTP was significantly longer in the TheraSphere arm: overall median TTP was not reached in the TheraSphere arm (> 26 months) and was 6.8 months in the TACE arm (HR 0.112, 95% CI 0.027 to 0.557; p = 0.007); TTP in the non-transplanted patients was also significantly longer in the TheraSphere arm (median > 26 months vs. 4.8 months). AEs and HRQoL were not reported.
One small RCT by Kulik et al. ,28–30 which caused some concerns regarding bias, compared TheraSphere plus sorafenib (n = 10) with sorafenib alone (n = 10) as a bridge to transplant in patients with Child–Pugh liver function class ≤ B8 HCC who were potential candidates for liver transplant. A higher proportion of patients in the TheraSphere plus sorafenib arm were male (80% vs. 50%) and had BCLC stage A disease (70% vs. 50%), with more patients in the TheraSphere-alone arm having BCLC stage C disease (40% vs. 20%). More patients in the TheraSphere plus sorafenib arm had ECOG performance status 0 (80% vs. 60%) and Child–Pugh liver function class A (80% vs. 60%). Three patients died in the TheraSphere arm, compared with two patients in the TheraSphere plus sorafenib arm. The proportion of patients receiving liver transplant or resection was 90% in each treatment arm. Most AEs were more common in the TheraSphere-alone arm (fatigue 90% vs. 40%, diarrhoea 20% vs. 10%, pain 50% vs. 0%, nausea 70% vs. 20% and vomiting 20% vs. 0%), although grade ≥ 3 hand–foot skin reaction was more common in the TheraSphere plus sorafenib arm (20% vs. 0%).
Five prospective comparative studies, all rated as being at a high risk of bias, compared TheraSphere with TACE/DEB-TACE in patients with HCC. 31–35 Two studies assessed OS. In one small study (n = 86), OS appeared slightly longer with TACE than with TheraSphere in patients with intermediate-stage disease (median 18 months vs. 16.4 months). 32 In a much larger study (n = 765) in which survival outcomes were stratified by BCLC stage and Child–Pugh liver function class, survival was longer in the TACE arm for patients with early- and intermediate-stage disease but longer in the TheraSphere arm for patients with advanced-stage disease. 35 Two small studies (n = 86 and n = 96) assessed TTP, which was longer with TheraSphere than with TACE (median 13.3 months vs. 6.8 months and median 13.3 months vs. 8.4 months). 32,34 Two small studies (n = 67 and n = 86) assessed complete or partial response rate; results were conflicting, with one study31 favouring TACE (2.3% vs. 0%, using RECIST criteria68) and the other32 favouring TheraSphere (75% vs. 50%, using modified RECIST criteria68). Two small studies (n = 67 and n = 56) assessed HRQoL, both favouring TheraSphere. 31,33 Only one study (n = 86) reported AEs; the most commonly reported AE (unspecific abdominal pain) was more frequent in TACE patients than in SIRT patients (83% vs. 5%). 32
One small prospective matched case–control study by Maccauro et al. ,36 rated as being at a high risk of bias, compared TheraSphere plus sorafenib (n = 15) with TheraSphere alone (n = 30) in patients with predominantly BCLC stage C (due to PVT) unresectable HCC with Child–Pugh liver function class A. The study was published only as a conference abstract; therefore, very limited data are available. Results were similar between treatment groups for OS (median 10 months in each treatment arm), PFS (median 6 months vs. 7 months in the TheraSphere plus sorafenib and TheraSphere-alone arms, respectively) and response rate, using modified RECIST criteria68 (45.5% vs. 42.8%). However, response rate using EASL criteria1 was better in the TheraSphere-alone arm (40% vs. 10%).
One small prospective comparative study by Woodall et al. ,37 rated as being at a high risk of bias, compared TheraSphere in HCC patients without PVT (n = 20) with TheraSphere in HCC patients with PVT (n = 15) and a no-treatment control (BSC) in HCC patients who were not eligible for SIRT owing to substantial extrahepatic disease or hepatopulmonary shunt or underlying liver insufficiency (n = 17). OS was significantly longer in patients without PVT who received TheraSphere (median 13.9 months) than in patients with PVT who received TheraSphere (median 3.2 months) and patients who received BSC (median 5.2 months). AEs were more common in TheraSphere patients who had PVT than in those who did not have PVT (33% vs. 25%). No other outcomes were reported.
Further details of each of these studies are presented in Appendix 6.
Ongoing studies
There is one ongoing RCT of TheraSphere in patients with HCC: STOP-HCC, which has an estimated study completion date of February 2020; final results are not anticipated before at least December 2020. 74
The BTG submission presents 12 additional ongoing or planned studies of TheraSphere.
Efficacy and safety of QuiremSpheres
Only one study of QuiremSpheres has been completed in patients with HCC: a small case series undertaken by Radosa et al. 51 Nine patients with HCC were retrospectively identified from a prospectively maintained database of patients who received QuiremSpheres between March 2017 and April 2018 at a single centre. It is unclear whether or not patients were representative of all those who would be eligible for SIRT in clinical practice. The available data are too limited to draw any conclusions about the safety or efficacy of QuiremSpheres. Study details are presented in Appendix 6.
Direct comparisons of different selective internal radiation therapies
Five small retrospective comparative studies, all rated as being at a high or unclear risk of bias, compared SIR-Spheres with TheraSphere. No studies were identified that directly compared QuiremSpheres with either SIR-Spheres or TheraSphere. Further details of each of the five studies are presented in Appendix 6. The two studies by Biederman et al. (n = 9738 and n = 9039) included patients who all had PVT and appear to have included some of the same patients, although one of the studies was published only as a conference abstract,38 so it is unclear how much overlap there was. The study by d’Abadie et al. 42 (n = 58 procedures) aimed to investigate the difference in efficacy per Gy of resin versus glass spheres and whether or not the difference could result from the different degrees of heterogeneity in sphere distribution; limited patient outcomes were reported.
Overall survival was reported in four studies [n = 97,38 n = 90 (possibly with some overlap),39 n = 7740 and n = 1741]. OS was longer in the TheraSphere arm in three of the studies,38,39,41 two of which included patients who all had PVT. 38,39 Median OS in the SIR-Spheres arm ranged from 3.7 to 7.7 months. Median OS in the TheraSphere arm ranged from 7.0 to 15 months.
Progression-free survival was reported in only one study (n = 77), in which it was longer in the SIR-Spheres arm (6.1 months vs. 5.0 months). 40 However, TTP was reported for the two treatment arms separately in one other study (n = 90 patients with PVT), in which it was longer in the TheraSphere arm (5.9 months vs. 2.8 months). 39
Tumour response rate was reported for the two treatment arms separately in only one study (n = 90 patients with PVT), in which a higher proportion of evaluable patients had a complete (8.8% vs. 0%) or partial (31.6% vs. 13.3%) response in the TheraSphere arm. 39
None of the studies reported HRQoL outcomes.
Adverse events were reported separately for the two treatment arms in two studies. The study by Biederman et al. 39 (n = 90 patients with PVT) reported no significant difference in pain (41.2% vs. 30.8%), fatigue (17.6% vs. 18.5%), nausea (17.6% vs. 3.1%) or anorexia (0% vs. 9.2%) between the SIR-Spheres and TheraSphere arms, respectively. In the very small study by Bhangoo et al. 41 (n = 17), all clinical toxicities reported were more frequent in the SIR-Spheres arm than in the TheraSphere arm (fatigue 67% vs. 45%, abdominal pain 33% vs. 27%, nausea/vomiting 67% vs. 55%, anorexia/weight loss 33% vs. 9%, diarrhoea 17% vs. 0% and gastric ulcer 17% vs. 0%).
An addendum, in the form of an academic-in-confidence manuscript, was received from Terumo Europe NV (hereafter Terumo) in August 2019. The manuscript described a retrospective pilot study of (confidential information has been removed) patients treated with QuiremSpheres, TheraSphere or SIR-Spheres at two centres in Germany and the Netherlands. OS and response were assessed at 6 months for all three interventions and at 12 months for QuiremSpheres and SIR-Spheres. Median OS was similar between the treatment groups at 6 months (confidential information has been removed) and 12 months (confidential information has been removed). The most commonly reported AEs were (confidential information has been removed) abdominal pain, fatigue and nausea; other AEs were rarely reported. This was a very small pilot study with unclear patient selection; patients in the TheraSphere group had poorer prognosis at baseline than did the other two treatment groups. The authors acknowledge that the study carries several methodological limitations. 78
Clinical effectiveness summary and conclusions
SIR-Spheres
There are two large good-quality RCTs comparing SIR-Spheres with sorafenib (SARAH19,20 and SIRveNIB21).
There was no statistically significant difference in OS (HR 1.15, 95% CI 0.94 to 1.41 SARAH, and HR 1.12, 95% CI 0.9 to 1.4 SIRveNIB) or PFS (HR 1.03, 95% CI 0.85 to 1.25 SARAH, and HR 0.89, 95% CI 0.7 to 1.1 SIRveNIB) in the SARAH or SIRveNIB trials in the ITT populations. However, tumour response rate was significantly greater in the SIR-Spheres arm than in the sorafenib arm in both trials (of patients who were evaluable and included in the analyses). The SARAH trial reported significantly better HRQoL in the SIR-Spheres arm than in the sorafenib arm, assessed using the EORTC QLQ-C30, although the proportion of patients who completed the questionnaires was low, particularly at later time points. The SIRveNIB trial found no significant difference in HRQoL assessed using the EQ-5D index. The AE profiles of SIR-Spheres and sorafenib are very different, although the most common AEs generally occurred more frequently in the sorafenib arm in both trials.
There are some concerns regarding the generalisability of the SARAH and SIRveNIB trials to patients who would be eligible for SIRT in UK practice. The SIRveNIB trial was conducted in the Asia-Pacific region, where the aetiology of HCC differs from that in European patients; HCC is predominantly caused by hepatitis B in Asia, whereas it is predominantly caused by alcohol or hepatitis C in Europe. The SARAH trial included patients with a poorer prognosis than those who would be considered for SIRT in UK practice (e.g. high tumour burden, main PVT or impaired liver function).
Around one-fifth of patients in the SARAH and SIRveNIB trials were not suitable for SIRT after work-up (e.g. due to liver-to-lung shunting or unfavourable hepatic arterial anatomy); a proportion of patients assessed for suitability for SIRT in clinical practice would also be considered unsuitable, with associated cost implications.
Patients with bilobar disease may require more than one administration of SIRT. In the SARAH trial, patients with bilobar tumours received the first treatment in the hemiliver with the greatest tumour burden, and treatment of the contralateral hemiliver was scheduled 30–60 days after the first treatment. However, the Sirtex submission states that SIR-Spheres can be administered to both lobes of the liver during the same procedure; neither the SARAH trial nor the SIRveNIB trial administered SIR-Spheres to both lobes during the same procedure. Clinical advisors confirmed that this is reflective of their experience, in which patients would not receive whole-liver treatment in one session to reduce the risk of REILD.
The Sirtex company submission selected a subgroup of patients from the SARAH trial with ≤ 25% tumour burden and ALBI 1 for its base-case analysis in the economic model; the company stated that these patients are considered the most appropriate candidates for SIR-Spheres in clinical practice, as they are the most likely to benefit from SIRT. This is not a clinically recognised subgroup and was based on a post hoc analysis; therefore, these results should be prospectively validated before being considered relevant to clinical practice. Median OS (HR 0.73, 95% CI 0.44 to 1.21) and PFS (HR 0.65, 95% CI 0.41 to 1.02) appeared better in the SIR-Spheres arm than in the sorafenib arm in the subgroup analysis, although the difference between treatment groups was not statistically significant. The proportion of patients who went on to have potentially curative therapy was higher in the SIR-Spheres arm than in the sorafenib arm, although numbers were very low (five and one patients, respectively).
Three very small poorer-quality RCTs compared SIR-Spheres with TACE,22 DEB-TACE23 or SIR-Spheres plus sorafenib versus sorafenib alone. 24 The trials comparing SIR-Spheres with TACE or DEB-TACE appeared to favour the chemoembolisation procedure over SIRT in terms of survival outcomes. 22,23 The addition of SIR-Spheres to sorafenib did not appear to increase the number of treatment-emergent AEs. 24
TheraSphere
Two small RCTs25–30 and seven prospective comparative studies31–37 of TheraSphere were included in the clinical effectiveness review; one of the RCTs (PREMIERE) and all of the non-RCT studies were rated as being at a high risk of bias, and the other RCT caused some concerns regarding bias. Therefore, all of these results should be interpreted with caution.
Both RCTs assessed TheraSphere as a bridge to transplant. The PREMIERE RCT reported longer TTP, a higher proportion of patients undergoing transplant and slightly longer OS in the TheraSphere arm than in the TACE arm. 25–27 Kulik et al. 28–30 reported similar survival and transplant/resection rates between patients receiving TheraSphere plus sorafenib or sorafenib alone.
Five prospective comparative studies compared TheraSphere with TACE or DEB-TACE; OS appeared better with TheraSphere in patients with early- and intermediate-stage disease. 32,35 TTP was longer with TheraSphere than with TACE. 32,34 Results relating to response rates were conflicting. 31,32 HRQoL appeared better with TheraSphere. 31,33 One study reported that the most common AE was more frequent with TACE than with SIRT. 32
One prospective comparative study compared TheraSphere plus sorafenib with TheraSphere alone, with similar results between treatment groups. 36 The other study compared TheraSphere in patients with or without PVT with no treatment in patients unsuitable for TheraSphere; OS was significantly longer in patients without PVT who received TheraSphere compared with those with PVT who received TheraSphere and those who received only BSC. 37
QuiremSpheres
Only one study of QuiremSpheres has been completed in patients with HCC: a small case series undertaken by Radosa et al. 51 The available data are too limited to draw any conclusions about the safety or efficacy of QuiremSpheres.
Direct comparison of different selective internal radiation therapies
Five small retrospective comparative studies, all rated as being at a high or unclear risk of bias, compared SIR-Spheres with TheraSphere. Two of the studies included patients who all had PVT and appear to have included some of the same patients. 38,39 OS was reported in four studies, including the two studies of patients with PVT; OS was longer in the TheraSphere arm in three of the studies. 38,39,41 One study assessed PFS, which was longer with SIR-Spheres,40 and another study assessed TTP, which was longer with TheraSphere (in patients with PVT). 39 Tumour response rate was higher in the TheraSphere arm than in the SIR-Spheres arm in patients with PVT. 39 One very small study reported more frequent clinical toxicities in the SIR-Spheres arm than in the TheraSphere arm. 41 In patients with PVT, there was no difference in the frequency of fatigue, but pain and nausea appeared more frequent with SIR-Spheres, and anorexia appeared more frequent with TheraSphere. 39
No studies were identified that directly compared QuiremSpheres with either SIR-Spheres or TheraSphere.
The BTG submission described a systematic review by Kallini et al. ,79 supported by funding from BTG, which aimed to compare the AE profiles of TheraSphere and SIR-Spheres for the treatment of unresectable HCC. Twenty-two observational studies of TheraSphere and nine observational studies of SIR-Spheres were included in the review and the number of AEs and number of patients across studies were summed to calculate the proportion of patients experiencing each AE. No studies directly comparing TheraSphere with SIR-Spheres were included in the review. AE reporting appears to have been variable between studies, with many AEs being reported by very few of the included studies (e.g. hepatobiliary and respiratory AEs). Baseline characteristics of patients were poorly reported in many of the included studies. Gastric ulcers were reported more frequently with SIR-Spheres than with TheraSphere [3.1% (six studies) vs. 0.1% (nine studies)], but the proportion of patients reporting ascites was higher with TheraSphere than with SIR-Spheres [9.2% (10 studies) vs. 4.7% (5 studies)]. Nausea (13 studies in total), fatigue (16 studies in total) and abdominal pain (18 studies in total) occurred in similar proportions of patients for both interventions. 79
An addendum, in the form of an academic-in-confidence manuscript, was received from Terumo in August 2019. OS and response were similar between the treatment groups. The most commonly reported AEs were (confidential information has been removed) abdominal pain, fatigue and nausea; other AEs were rarely reported. This was a very small pilot study with several methodological limitations. 78
Conclusions
There is a large body of evidence on the clinical effectiveness and safety of SIRT compared with sorafenib or TACE. Only two studies were considered to have a low risk of bias: SARAH19,20 and SIRveNIB,21 which both compared SIR-Spheres with sorafenib. However, there are some concerns regarding the generalisability of the results of these two RCTs to the UK HCC population, particularly the SIRveNIB trial, which was conducted in the Asia-Pacific region where the aetiology of HCC differs from that in Europe.
Both RCTs found no significant difference in OS or PFS between SIR-Spheres and sorafenib, despite statistically significantly greater tumour response rate in the SIR-Spheres arm of both trials. The SARAH trial reported a significant difference between groups in HRQoL, favouring SIR-Spheres; however, the proportion of patients who completed the questionnaires was low. AEs, particularly grade ≥ 3 events, were more frequent in the sorafenib group in both trials.
The Sirtex company submission selected a subgroup of patients from the SARAH trial with ≤ 25% tumour burden and ALBI 1 for its base-case analysis in the economic model. Although results appeared more promising in this subgroup of patients with a better prognosis, these post hoc subgroup analysis results should be prospectively validated before being considered relevant to clinical practice.
In studies comparing the different SIRT, patients with PVT appeared to have better survival outcomes with TheraSphere than with SIR-Spheres; however, this result was from a small retrospective comparative study rated as being at a high risk of bias and, therefore, may not be reliable. Other studies comparing TheraSphere with SIR-Spheres that did not include only patients with PVT had conflicting results. The only study that compared QuiremSpheres with SIR-Spheres and TheraSphere was provided by Terumo as an addendum in August 2019. Clinical outcomes appeared to be similar between treatment groups; however, this was a very small pilot study with several methodological limitations.
Chapter 4 Evidence synthesis to inform the relative efficacy of the interventions
Overview
Studies assessing the clinical effectiveness of SIRT for patients with unresectable HCC have been discussed and summarised in Chapter 3. The PRISMA flow diagram describing the selection process is shown in Figure 1. Treatment options vary greatly for patients with unresectable HCC according to the stage and severity of cancer and liver disease, as described in Chapter 1, Current service provision. Therefore, three NMA models were produced to represent the different populations of unresectable HCC patients. The 26 comparative studies and RCTs included in the systematic review of clinical effectiveness (see Table 3) and the 11 RCTs of CTTs (see Table 4) were screened for inclusion in each of the three NMA models. Alongside this, two studies of systemic therapies were identified from recent NICE single technology appraisals of sorafenib and lenvatinib: Llovet et al. 80 and Kudo et al. 81 Therefore, 39 studies were screened for inclusion in each of the three NMAs.
Network meta-analysis of adults with unresectable hepatocellular carcinoma who are eligible for transplant and of those eligible for conventional transarterial therapies
Meta-analysis using mixed treatment comparisons enables the estimation of different parameters when direct evidence on comparisons of interest is absent or sparse. The statistical synthesis method of NMA enables the comparison of multiple treatment options using both direct comparisons of interventions from RCTs and indirect comparisons across trials based on a common comparator. 82 As suggested by the term, NMA needs a ‘network of evidence’ to be established between all the interventions of interest.
Network 1: adults with unresectable hepatocellular carcinoma who are eligible for transplant
The first model (network 1) included patients with early/intermediate-stage unresectable HCC who were eligible for transplant. SIRT could potentially be used as a bridging treatment for patients awaiting transplant as described in Chapter 1, Description of the technology under assessment. These patients are generally classed as BCLC stage A patients, with preserved liver function and performance status 0–1. To ensure consistency in the compared studies, studies were included only if ≥ 70% of the recruited population had early-stage HCC or if results were split by disease stage. Only 2 out of 39 studies were selected for network 1. This included two small RCTs: PREMIERE25 and Kulik et al. 28 The main reason for the exclusion of studies was patients having advanced-stage disease and, therefore, not being eligible for transplant. The reasons for including and excluding each study are reported in Table 7.
| Study (first author and year) | n | Intervention | Comparator | Study design | Reason for inclusion/exclusion |
|---|---|---|---|---|---|
| Studies included in the network (n = 2) | |||||
| Salem 201625–27 (PREMIERE) | 45 | TheraSphere | TACE | RCT | Patients with early/intermediate HCC with no vascular invasion. The intent of therapy was bridge to transplant |
| Kulik 201428 | 20 | TheraSphere | TheraSphere plus sorafenib | RCT | Adults with Child–Pugh class ≤ B8 and potential candidates for orthotopic liver transplant. BCLC stage C patients (30%) were symptomatic only |
| Studies excluded from this network (n = 37) | |||||
| Kolligs 201522 (SIRTACE) | 28 | SIR-Spheres | TACE | RCT | Mixed population of early- and intermediate-stage patients, without portal vein occlusion. Pilot trial funded by Sirtex. Results split for transplantable patients were requested but not provided |
| Chow 201821 (SIRveNIB) | 360 | SIR-Spheres | Sorafenib | RCT | Adults with locally advanced HCC (BCLC B or C) not amenable to curative treatment |
| Vilgrain 201719,84 (SARAH) | 459 | SIR-Spheres | Sorafenib | RCT | Adults with locally advanced HCC (BCLC C) or new HCC not eligible for surgery/ablation after previously cured HCC or HCC with two unsuccessful rounds of TACE. Only a few patients received curative therapy |
| Pitton 201523 | 24 | SIR-Spheres | DEB-TACE | RCT | Adults with intermediate-stage HCC (BCLC stage B). Patients eligible for curative therapy were excluded |
| Ricke 201524 (SORAMIC) | 40 | SIR-Spheres plus sorafenib | Sorafenib | RCT | Adults with unresectable intermediate or advanced HCC (BCLC stage B or C). No patients received transplant |
| Kudo 201881 (REFLECT) | 289 (subgroup of 954 patients) | Lenvatinib | Sorafenib | RCT | Subgroup of adults with advanced-stage HCC, majority had PVI or extrahepatic spread – ineligible for transplant |
| Llovet 200880 (SHARP) | 602 | Sorafenib | Placebo | RCT | Adults with intermediate- and advanced-stage HCC, majority had extrahepatic spread/vascular invasion. Patients ineligible for transplant |
| Malagari 201066 | 87 | DEB-TACE | TAE | RCT | Patients unsuitable for curative treatments with potentially resectable lesions but at high risk for surgery |
| Brown 201667 | 101 | DEB-TACE | TAE | RCT | Mixed population and some patients with PVI, ineligible for transplant |
| Lammer 201056,57 (PRECISION) | 212 | DEB-TACE | TACE | RCT | No relevant outcomes reported |
| Golfieri 201458 | 177 | DEB-TACE | TACE | RCT | Adults with early-, intermediate- and advanced-stage HCC without PVT. The population is too varied to include |
| Sacco 201159 | 67 | DEB-TACE | TACE | RCT | Patients with early- and intermediate-stage HCC, ineligible for transplant |
| van Malenstein 201160 | 30 | DEB-TACE | TACE | RCT | No relevant outcomes reported |
| Llovet 200261 | 112 | TACE | TAE | RCT | Adults with intermediate- and advanced-stage HCC, ineligible for transplant |
| Kawai 199262 | 289 | TACE | TAE | RCT | Patients with early/intermediate-stage HCC but no relevant transplant results reported |
| Chang 199463 | 46 | TACE | TAE | RCT | Patients with inoperable HCC |
| Meyer 201364 | 86 | TACE | TAE | RCT | Patients with early-, intermediate- and advanced-stage HCC, ineligible for transplant |
| Yu 201465 | 98 | TACE | TAE | RCT | Adults with early-, intermediate- and advanced-stage HCC, ineligible for transplant |
| Kirchner 201931 | 94 | TheraSphere | TACE/DEB-TACE | Prospective comparative | No relevant outcomes reported |
| Hickey 201635 | 765 | TheraSphere | TACE | Prospective comparative | Includes patients potentially eligible for transplant, but no transplant outcomes were reported |
| El Fouly 201532 | 86 | TheraSphere | TACE | Prospective comparative | Adults with intermediate-stage (BCLC B) unresectable HCC. Patients eligible for curative therapy were excluded |
| Salem 201333 | 56 | TheraSphere | TACE | Prospective comparative | No relevant outcomes were reported |
| Woodall 200937 | 52 | TheraSphere | BSC | Prospective comparative | Patients with advanced-stage HCC, ineligible for transplant |
| Memon 201483 | 96 | TheraSphere | TACE | Prospective comparative | No relevant outcomes reported |
| Maccauro 201436 | 45 | TheraSphere plus sorafenib | TheraSphere | Matched case–control study | Patients with intermediate/advanced HCC with PVT, not appropriate for transplant |
| Salem 201147 | 245 | TheraSphere | TACE | Retrospective comparative | Majority of patients had early/intermediate-stage HCC (88.1%) and 39% were within Milan transplant criteria (T2) but there were no relevant outcomes reported |
| Bhangoo 201541 | 17 | TheraSphere | SIR-Spheres | Retrospective comparative | Patients with intermediate/advanced unresectable HCC who either failed or had disease not amenable to alternative locoregional therapies |
| Cho 201643 | 63 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with BCLC stage C HCC with PVT, not appropriate for transplant |
| de la Torre 201644 | 73 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with HCC with PVI, not appropriate for curative therapy |
| Van Der Gucht 201740 | 77 | SIR-Spheres | TheraSphere | Retrospective comparative | Patients with early, intermediate and advanced HCC, not appropriate for curative therapy |
| Biederman 201639 | 90 | SIR-Spheres | TheraSphere | Retrospective comparative | Patients with unresectable HCC with main or lobar PVT, not appropriate for curative therapy |
| Akinwande 201649,50 | 96 (matched cohort of 358 patients) | TheraSphere | DEB-TACE | Retrospective comparative | Adults with unresectable HCC (with or without PVT), unlikely transplant intent |
| Soydal 201646 | 80 | SIR-Spheres | TACE | Retrospective comparative | Patients with intermediate/advanced-stage HCC, some patients with extrahepatic metastases |
| Gramenzi 201545 | 137 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with intermediate/advanced HCC, not appropriate for curative therapy |
| Moreno-Luna 201348 | 116 | TheraSphere | TACE | Retrospective comparative | Excluded patients eligible for curative therapy |
| Biederman 201538 | 97 | TheraSphere | SIR-Spheres | Retrospective comparative | Adults with advanced HCC with PVT, not eligible for curative therapy |
| d’Abadie 201842 | 45 | SIR-Spheres | TheraSphere | Retrospective comparative | Unclear population |
However, clinical advice was that there are short transplant waiting times in the UK (< 2 months), whereas the two trials in the network had transplant waiting times of roughly 7–9 months (mean 7.8 months in Kulik et al. 28 and median 8.8 months in Salem et al. 25). Therefore, the network may not be generalisable to the UK and there may be limited opportunity for benefit in the UK given the short waiting times. Clinicians advised that, in the UK, bridging treatment is also used during the work-up phase, before the patient goes on to the waiting list. However, TACE rather than SIRT is more commonly used in this context. Furthermore, the two RCTs included in the network have very small sample sizes and, therefore, any efficacy estimates produced would be highly uncertain. Therefore, network 1, of patients with early/intermediate-stage HCC, was not conducted as it was deemed unsuitable for decision-making.
Network 2: adults with unresectable hepatocellular carcinoma who are eligible for conventional transarterial therapies
The second model was for patients with unresectable HCC who are eligible for CTTs. Patients in this population tend to have intermediate-stage HCC (BCLC B); however, patients with advanced-stage HCC (BCLC C) can also be eligible if they do not have PVT/PVI or extrahepatic spread. Studies in which the majority of patients had intermediate-stage HCC (BCLC B) and ≤ 30% of patients had advanced disease (BCLC C) were included. If studies reported results split by disease stage, they were included. A small proportion of patients in this population may also be eligible for downstaging to transplant; however, there was very little evidence to inform this. Furthermore, clinicians advised that the role of downstaging HCC for liver transplantation is currently under evaluation in the UK and SIRT is not specifically required for downstaging as this can be achieved using existing therapies, most commonly TACE.
After screening the 39 studies described in the previous section, seven studies were identified as relevant for the population of patients who are eligible for CTT: six RCTs and one retrospective comparative study. The reasons for inclusion and exclusion are listed in Table 8. The main reason for exclusion was the population being substantially mixed in terms of stage of HCC disease or patients having advanced-stage disease, which made them ineligible for CTT. SIRTACE,22 which is a RCT comparing SIR-Spheres and TACE described in Chapter 3, Efficacy and safety of SIR-Spheres, included a mixed population of patients with early-, intermediate- and advanced-stage HCC. The trial was funded by Sirtex; therefore, data split by disease stage were requested. However, Sirtex was unable to provide the data as it did not have access to them, so the trial could not be included in the NMA.
| Study (first author and year) | n | Intervention | Comparator | Study design | Reason for inclusion/exclusion |
|---|---|---|---|---|---|
| Studies included in this network (n = 7) | |||||
| Pitton 201523 | 24 | SIR-Spheres | DEB-TACE | RCT | Patients with intermediate-stage HCC (BCLC stage B) |
| Yu 201465 | 98 | TACE | TAE | RCT | Patients with unresectable HCC, Child–Pugh class A or B, ECOG < 2 |
| Malagari 201066 | 87 | DEB-TACE | TAE | RCT | Patients unsuitable for curative treatments with potentially resectable lesions but at high risk for surgery |
| Sacco 201159 | 67 | DEB-TACE | TACE | RCT | Patients with untreated HCC, Child–Pugh class A or B, ECOG 0–1 |
| Chang 199463 | 46 | TACE | TAE | RCT | Patients with inoperable HCC, Child–Pugh class A or B |
| Meyer 201364 | 86 | TACE | TAE | RCT | Patients with untreated, unresectable HCC, Child–Pugh class A or B, ECOG 0–2 |
| Van Der Gucht 201740 | 35 (subgroup of 77 patients) | SIR-Spheres | TheraSphere | Retrospective comparative | Subgroup of early/intermediate-HCC patients |
| Studies excluded from this network (n = 32) | |||||
| Kolligs 201522 (SIRTACE) | 28 | SIR-Spheres | TACE | RCT | Mixed population of early- and intermediate-stage patients, without portal vein occlusion. Pilot trial funded by Sirtex. Data for intermediate patients were requested but not provided |
| Vilgrain 201719,84 (SARAH) | 459 | SIR-Spheres | Sorafenib | RCT | Patients with locally advanced HCC or new HCC not eligible for surgery/ablation after previously cured HCC or HCC with two unsuccessful rounds of TACE. Poor candidates for TACE |
| Salem 201625 (PREMIERE) | 45 | TheraSphere | TACE | RCT | Patients with early/intermediate-HCC with no vascular invasion. The intent of therapy was bridge to transplant |
| Kulik 201428 | 20 | TheraSphere | TheraSphere plus sorafenib | RCT | Intent of therapy was bridge to transplant |
| Chow 201821 (SIRveNIB) | 360 | SIR-Spheres | Sorafenib | RCT | Sorafenib is an irrelevant comparator in this population |
| Lammer 201056,57 (PRECISION) | 212 | DEB-TACE | TACE | RCT | No relevant outcomes reported |
| Ricke 201524 (SORAMIC) | 40 | SIR-Spheres plus sorafenib | Sorafenib | RCT | Poor candidates for TACE |
| van Malenstein 201160 | 30 | DEB-TACE | TACE | RCT | No relevant outcomes reported |
| Brown 201667 | 101 | DEB-TACE | TAE | RCT | Mixed population and some patients have PVI |
| Golfieri 201458 | 177 | DEB-TACE | TACE | RCT | Patients with early-, intermediate- and advanced-stage HCC without PVT. The population is too varied to include |
| Llovet 200261 | 112 | TACE | TAE | RCT | Patients with intermediate/advanced-stage HCC without PVI/extrahepatic disease but no relevant outcomes reported |
| Kawai 199262 | 289 | TACE | TAE | RCT | Patients with early/intermediate-stage HCC but no relevant outcomes reported |
| Kudo 201881 (REFLECT) | 289 (subgroup of 954 patients) | Lenvatinib | Sorafenib | RCT | Subgroup of patients with advanced-stage HCC, majority had PVI or extrahepatic spread – ineligible for TACE |
| Llovet 200880 (SHARP) | 602 | Sorafenib | Placebo | RCT | Adults with intermediate/advanced-stage HCC, majority had extrahepatic spread/MVI. Patients ineligible for TACE |
| Hickey 201635 | 765 | TheraSphere | TACE | Prospective comparative | Adults with early-, intermediate- and advanced-stage HCC but significant baseline imbalances in age, PVI, number of lesions and CP class |
| Kirchner 201931 | 94 | TheraSphere | TACE/DEB-TACE | Prospective comparative | No relevant outcomes reported |
| Memon 201334 | 96 | TheraSphere | TACE | Prospective comparative | No relevant outcomes reported |
| Salem 201333 | 56 | TheraSphere | TACE | Prospective comparative | No relevant outcomes reported |
| El Fouly 201532 | 86 | TheraSphere | TACE | Prospective comparative | Patients with intermediate-stage HCC but systematic selection bias and baseline imbalances in age, tumour size and tumour number were detected |
| Woodall 200937 | 52 | TheraSphere | BSC | Prospective comparative | Patients with advanced-stage HCC, ineligible for TACE |
| Maccauro 201436 | 45 | TheraSphere plus sorafenib | TheraSphere | Matched case–control study | Patients with intermediate/advanced HCC, poor candidates for TACE |
| Akinwande 201649 | 96 (subgroup of 358 patients) | TheraSphere | DEB-TACE | Retrospective comparative | Mixed population of patients with unresectable HCC with or without PVT, results not split by disease stage |
| Bhangoo 201541 | 17 | TheraSphere | SIR-Spheres | Retrospective comparative | Patients ineligible for TACE (patients had either failed or were not amenable to other locoregional therapies) |
| Moreno-Luna 201348 | 116 | TheraSphere | TACE | Retrospective comparative | Patients with unresectable HCC not eligible for transplant but significant baseline imbalances between groups in ECOG status, Child–Pugh class, number of tumours and BCLC stage |
| Cho 201643 | 63 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients ineligible for TACE |
| de la Torre 201644 | 73 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients ineligible for TACE |
| Biederman 201639 | 90 | SIR-Spheres | TheraSphere | Retrospective comparative | Patients ineligible for TACE |
| Gramenzi 201545 | 137 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients were ineligible or unsuitable for TACE |
| Biederman 201538 | 97 | SIR-Spheres | TheraSphere | Retrospective comparative | Patients with unresectable, advanced-stage HCC with PVT, poor candidates for TACE |
| d’Abadie 201842 | 45 | SIR-Spheres | TheraSphere | Retrospective comparative | Population unclear. Appears to include both patients eligible and non-eligible for TACE |
| Salem 201147 | 245 | TheraSphere | TACE | Retrospective comparative | Mixed population of patients with HCC without PVT or extrahepatic metastases but results not stratified by BCLC stage |
| Soydal 201646 | 80 | TACE | SIR-Spheres | Retrospective comparative | Patients with intermediate/advanced-stage HCC, some patients with extrahepatic metastases |
The studies included in network 2 were a RCT directly comparing SIR-Spheres with DEB-TACE,23 five RCTs comparing different CTTs59,63–66 and one retrospective comparative study comparing SIR-Spheres with TheraSphere. 40 The RCT that compared SIR-Spheres with DEB-TACE23 included only 24 patients (described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres) and was the only direct evidence between SIR-Spheres and CTT. There were no studies comparing TheraSphere with CTT. The retrospective study comparing SIR-Spheres with TheraSphere40 was rated as being at a high risk of bias, as described in Chapter 3, Efficacy and safety of SIR-Spheres.
The five RCTs comparing different CTTs, which were deemed relevant for this population, were included to inform the network. This includes three RCTs comparing TACE and TAE. 63–65 The risk-of-bias assessment reported some concerns regarding bias in the randomisation process for all three trials. The assessment also highlighted concerns regarding protocol deviations from the intended interventions for Chang et al. 63 Both Yu et al. 65 and Meyer et al. 64 showed no significant differences in OS or PFS. Chang et al. 63 reported only survival rates between groups but did not find any significant differences.
There was one RCT comparing DEB-TACE and TAE: Malagari et al. 66 The risk-of-bias assessment reported some concerns with this study regarding bias in the randomisation process and in protocol deviations from the intended interventions. The trial was conducted in 95 patients and found that TTP was significantly longer in the DEB-TACE arm (42.4 ± 9.5 weeks) than in the TAE arm (36.2 ± 9.0 weeks). The remaining RCT compared DEB-TACE and TACE: Sacco et al. 59 This trial was rated as being at a high overall risk of bias owing to an open randomisation process. The trial found no significant differences in survival rates or other relevant outcomes between the two groups. Full results of the risk-of-bias judgements are presented in Appendix 9 and the study details and results are presented in Appendix 10.
The network diagram representing the model is shown in Figure 2. There are missing direct comparisons and there is no common comparator in the evidence base for both OS and PFS outcomes in this population; therefore, it forms a ‘disconnected network’. Implementing a NMA in this population would produce very uncertain results as it relies on a single small trial by Pitton et al. 23 to connect SIR-Spheres in the network. Furthermore, it would not provide reliable evidence on TheraSphere comparisons with CTT as there is only one small, retrospective, low-quality study connecting TheraSphere in the network. Therefore, network 2, of patients with unresectable HCC who are eligible for CTT, was not conducted as it was deemed unsuitable for decision-making.
FIGURE 2.
Network 2: patients eligible for CTTs.

Network 3: adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapies
The third model was for patients with unresectable HCC who are ineligible for CTT. Patients in this population tend to have advanced-stage HCC (BCLC C) with or without PVT/PVI. This population may, however, include some patients with intermediate-stage disease (BCLC B) who are ineligible for CTT or who have previously failed CTT.
There were 26 comparative studies included in the systematic review of clinical effectiveness, which were identified as potentially eligible for the third network; the 11 RCTs comparing different CTTs were not screened as they are not relevant for this population. A further two studies of systemic therapies identified from previous technology appraisals were additionally screened for inclusion in this network. Out of 28 studies, three RCTs and five retrospective comparative studies were initially selected as relevant for this population. Twenty studies were excluded, mainly because of irrelevant comparisons or not reporting relevant outcomes. The NMA diagram is illustrated in Figure 3.
FIGURE 3.
Network 3: adults with unresectable HCC who are ineligible for CTTs.
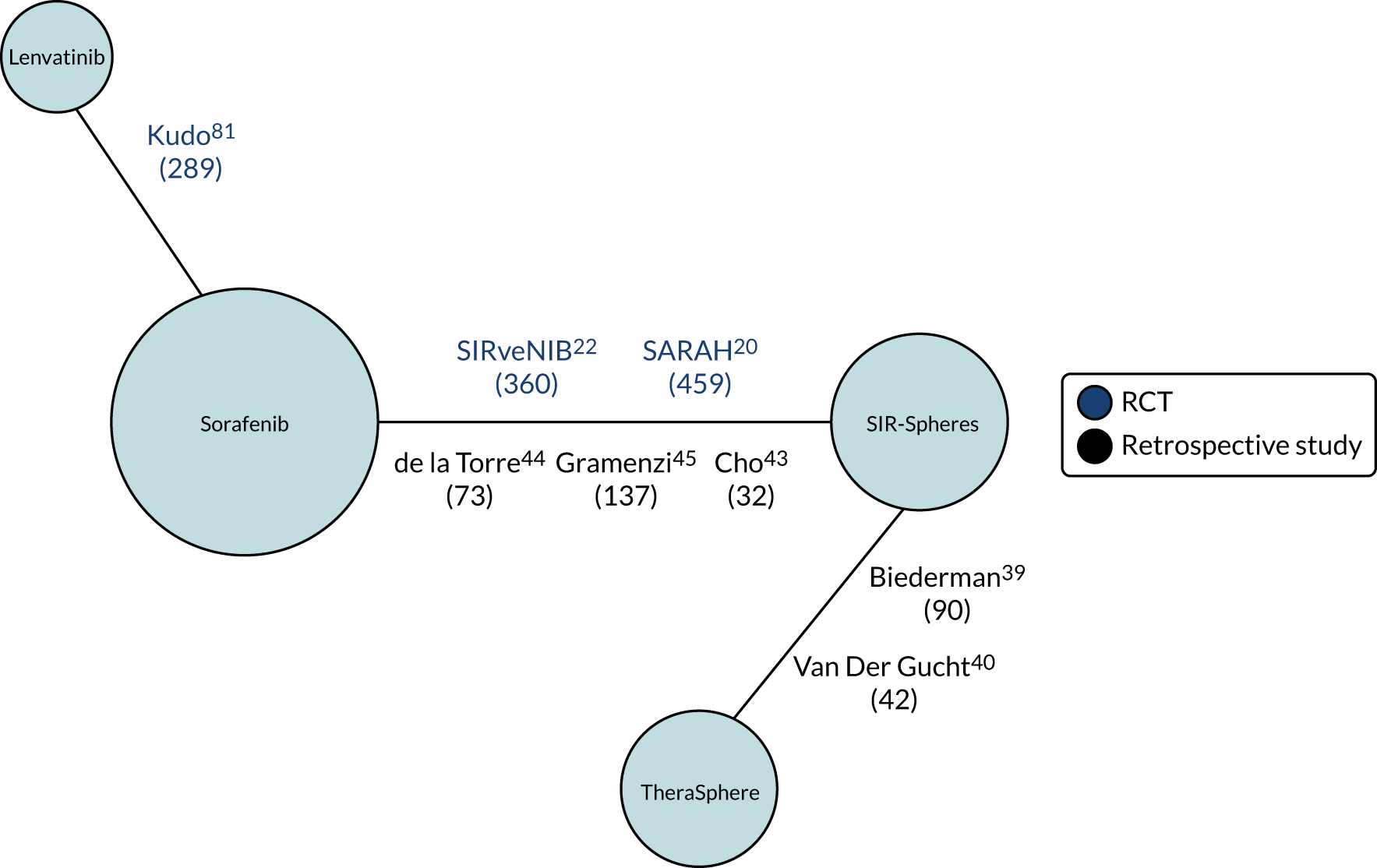
The network includes robust direct evidence between SIR-Spheres and sorafenib from the two large RCTs SARAH84 and SIRveNIB,21 which are described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. There are also three smaller retrospective comparative studies comparing SIR-Spheres and sorafenib. 44,45,85 On closer examination, all three of these studies were rated as being at a high risk of bias owing to an imbalance in baseline characteristics, unclear reporting of missing data and unblinded outcome assessors (see Appendix 8). Therefore, owing to already having identified high-quality RCTs comparing SIR-Spheres and sorafenib, these three retrospective studies were removed. Including low-quality studies where there is already reliable evidence may invalidate the NMA and consequently the results. Furthermore, the two retrospective studies, Biederman et al. 39 and Van Der Gucht et al. ,40 were also considered to have a high risk of bias, as described in Chapter 3, Direct comparisons of different selective internal radiation therapies. However, these studies were included as a sensitivity analysis as they are the only studies with direct evidence between TheraSphere and SIR-Spheres.
The network was updated and the final NMA of patients ineligible for CTT includes two RCTs comparing SIR-Spheres and sorafenib,19,21 one RCT comparing lenvatinib and sorafenib81 and two retrospective comparative studies comparing SIR-Spheres and TheraSphere (included as a sensitivity analysis) (Figure 4). 38,40 The decisions for including and excluding each study are detailed in Table 9. The study selection process for this NMA (updated network 3) is illustrated in Figure 5.
FIGURE 4.
Updated network 3: adults with unresectable HCC who are ineligible for CTTs.

| Study (first author and year) | n | Intervention | Comparator | Study design | Reason for inclusion/exclusion |
|---|---|---|---|---|---|
| Studies included in this network (n = 5) | |||||
| Chow 201821 (SIRveNIB) | 360 | SIR-Spheres | Sorafenib | RCT | Patients with locally advanced HCC |
| Vilgrain 201719,84 (SARAH) | 459 | SIR-Spheres | Sorafenib | RCT | Adults with locally advanced HCC (BCLC C) or new HCC not eligible for surgery/ablation after previously cured HCC or HCC with two unsuccessful rounds of TACE |
| Kudo 201881 (REFLECT) | 289 (subgroup of 954 patients) | Lenvatinib | Sorafenib | RCT | Subgroup of adults with advanced-stage HCC, majority had PVI or extrahepatic spread |
| Van Der Gucht 201740 | 42 (subgroup of 77 patients) | SIR-Spheres | TheraSphere | Retrospective comparative | Subgroup of advanced-stage HCC patients |
| Biederman 201639 | 90 | SIR-Spheres | TheraSphere | Retrospective comparative | Patients with unresectable HCC and main or lobar PVT |
| Studies excluded from this network (n = 23) | |||||
| Ricke 201524 (SORAMIC) | 40 | SIR-Spheres plus sorafenib | Sorafenib | RCT | Adults with unresectable intermediate or advanced HCC, poor candidate for TACE. Only safety analyses are published. Data were requested from company but, as this is an investigator-initiated trial, the data were not available |
| Llovet 200880 (SHARP) | 602 | Sorafenib | Placebo | RCT | Adults with intermediate/advanced-stage HCC, majority had extrahepatic spread/vascular invasion. This study was not required for the NMA as it did not provide any extra information and was not needed for the cost-effectiveness model |
| Salem 201625 (PREMIERE) | 45 | TheraSphere | TACE | RCT | Compared TACE – irrelevant comparison in this population |
| Kolligs 201522 (SIRTACE) | 28 | SIR-Spheres | TACE | RCT | Compared TACE – irrelevant comparison in this population |
| Pitton 201523 | 24 | SIR-Spheres | DEB-TACE | RCT | Compared DEB-TACE – irrelevant comparison in this population |
| Kulik 201428 | 20 | TheraSphere | TheraSphere plus sorafenib | RCT | Mixed population with the intent to bridge to transplant |
| Kirchner 201931 | 94 | TheraSphere | TACE/DEB-TACE | Prospective comparative | Compared TACE – irrelevant comparison in this population |
| Hickey 201635 | 765 | TheraSphere | TACE | Prospective comparative | Compared TACE – irrelevant comparison in this population |
| El Fouly 201532 | 86 | TheraSphere | TACE | Prospective comparative | Compared TACE – irrelevant comparison in this population |
| Woodall 200937 | 52 | TheraSphere | BSC | Prospective comparative | Patients with advanced-stage HCC. Excluded owing to systematic selection bias and significant baseline imbalances |
| Memon 201334 | 96 | TheraSphere | TACE | Prospective comparative | No relevant outcomes reported |
| Salem 201333 | 56 | TheraSphere | TACE | Prospective comparative | No relevant outcomes reported and compared TACE – irrelevant comparison in this population |
| Maccauro 201436 | 45 | TheraSphere plus sorafenib | TheraSphere | Matched case–control study | Patients with intermediate/advanced-stage HCC. No relevant outcomes reported |
| Cho 201643 | 63 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with BCLC stage C HCC and PVI. However, study of low quality and high risk of bias, and therefore excluded from updated network |
| de la Torre 201644 | 73 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with unresectable HCC and PVI. However, study of low quality and high risk of bias and therefore excluded from updated network |
| Gramenzi 201545 | 137 | SIR-Spheres | Sorafenib | Retrospective comparative | Patients with intermediate/advanced-stage HCC unfit for other effective therapies. However, study of low quality and high risk of bias, and therefore excluded from updated network |
| Akinwande 201649 | 96 | TheraSphere | DEB-TACE | Retrospective comparative | Compared TACE – irrelevant comparison in this population |
| Moreno-Luna 201348 | 116 | TheraSphere | TACE | Retrospective comparative | Compared TACE – irrelevant comparison in this population |
| Salem 201147 | 245 | TheraSphere | TACE | Retrospective comparative | Compared TACE – irrelevant comparison in this population |
| d’Abadie 201842 | 45 | SIR-Spheres | TheraSphere | Retrospective comparative | Population unclear. Appears to include patients both eligible and non-eligible for TACE |
| Bhangoo 201541 | 17 | TheraSphere | SIR-Spheres | Retrospective comparative | Mixed population of patients with unresectable HCC, who had either failed or were not amenable to other locoregional therapies. No relevant outcomes reported |
| Biederman 201538 | 97 | SIR-Spheres | TheraSphere | Retrospective comparative | Adults with unresectable HCC with PVT. No relevant outcomes reported |
| Soydal 201646 | 80 | TACE | SIR-Spheres | Retrospective comparative | Compared TACE – irrelevant comparison |
FIGURE 5.
Flow diagram of the study selection process for the NMA of adults ineligible for CTTs.
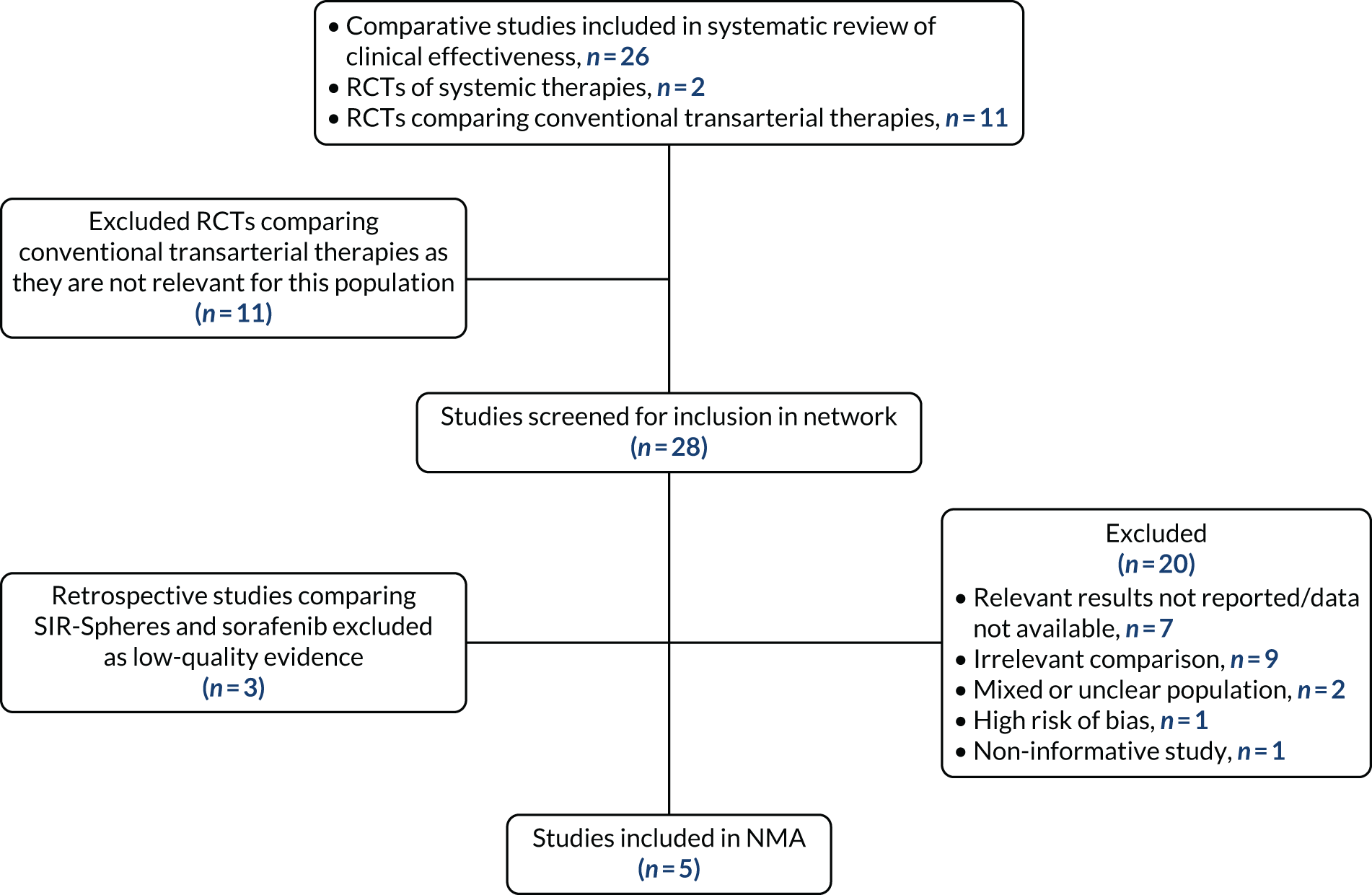
Methods of data analysis
This section describes a NMA of all relevant RCTs (Table 10) and a NMA of RCTs that included only patients with Child–Pugh class A liver function. Currently, in the UK, systemic therapy, such as sorafenib and lenvatinib, is licensed for only Child–Pugh class A patients with unresectable HCC. However, results for all patients in the ITT population are reported in Appendix 12, Tables 39 and 40.
| Study (first author and year) | Treatment | n | Median age (years) | Male, n (%) | PVT/PVI, n (%) | BCLC classification, n (%) | ||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Vilgrain 201719 (SARAH) | SIR-Spheres | 174 | 66.3 ± 9.4 | 158 (90.8) | 29 (16.7)a | 7 (4.0) | 53 (30.5) | 114 (65.5) |
| Sorafenib | 206 | 64.6 ± 9.5 | 186 (90.3) | 37 (18.0)a | 9 (4.4) | 54 (26.2) | 143 (69.4) | |
| Chow 201821 (SIRveNIB) | SIR-Spheres | 130 | 60.9 (SD 11.5) | 107 (82.3) | 30 (23.1)b | 0 (0) | 79 (60.8) | 50 (38.5) |
| Sorafenib | 162 | 57.5 (SD 10.6) | 138 (85.2) | 48 (29.6)b | 1 (0.6) | 88 (54.3) | 73 (45.1) | |
| NICE 201812 (REFLECT)c | Lenvatinib | 369 | – | – | 0 (0) | – | – | – |
| Sorafenib | 386 | – | – | 0 (0) | – | – | – | |
| Retrospective comparative studies | ||||||||
| Biederman 201639 | SIR-Spheres | 21 | 60 ± 11.5 | 20 (95.2) | 100%d | – | – | – |
| TheraSphere | 69 | 65.6 ± 11.3 | 54 (78.3) | 100%d | – | – | – | |
| eVan Der Gucht 201740 | SIR-Spheres | 24 | – | – | – | 0 (0) | 0 (0) | 24 (100) |
| TheraSphere | 18 | – | – | – | 0 (0) | 0 (0) | 18 (100) | |
In the SARAH19 and SIRveNIB21 trials, 22.4% and 28.6% of patients allocated to SIR-Spheres did not receive SIRT. Patients who did not receive their allocated treatment were excluded from the per-protocol analysis. Therefore, the NMA of Child–Pugh class A patients with unresectable HCC who are ineligible for CTT in the per-protocol population is the base-case scenario. However, the ITT results are used for the REFLECT trial. 12 Therefore, the results for the ITT population are also reported. Both OS and PFS were assessed as outcomes. However, PFS in Child–Pugh class A patients was not reported for the SIRveNIB study21 or for patients in the Biederman et al. 39 study. Therefore, PFS could not be assessed in the base-case population or in the sensitivity analyses.
The NMA was estimated using Bayesian Markov chain Monte Carlo techniques in WinBUGS, using code obtained from the NICE Decision Support Unit (DSU)’s Technical Support Document. 86 An initial burn-in of at least 50,000 simulations was used, and convergence was confirmed through visual inspection of the Brook–Gelman–Rubin diagnostic and history plots. This was followed by 100,000 simulations on three chains to estimate the sampled parameters. Where available, Kaplan–Meier (KM) data were extracted using methods reported by Guyot et al. 87 When KM data were not available, HRs and their variance were extracted, and log-hazard ratios synthesised. To synthesise HRs across studies, it is required that the proportional hazards assumption holds. Therefore, the deviation from proportional hazards was tested and the Schoenfeld residuals, survival curves and piecewise hazards visually inspected. It was decided to conduct more complex time-varying models only if simple models were not a good fit to the data. A model was chosen by visually inspecting the development of the hazard over time for the different trials and then by comparing deviance information criterion (DIC) values for the competing models. It was decided that a hierarchical model with classes of treatments composed of individual treatments, which would allow each treatment effect to be estimated as well as the overall class mean, was not possible owing to the small number of studies in the NMA. 86 Finally, both fixed- and random-effects models were evaluated and between-trial heterogeneity was assessed using the between-study standard deviation (SD). Inconsistency did not need to be examined, as there were no loops in the network.
Model selection
A Bayesian evidence synthesis approach was employed. With a Bayesian framework, prior belief about a treatment effect is combined with a likelihood distribution that summarises the data to obtain a posterior distribution reflecting the belief about the treatment effect after incorporating the evidence. Normal identity link models were used for this NMA. 86 The Schoenfeld residuals were visually inspected and statistically tested for each survival curve except for the REFLECT study because only a subgroup of the data were used, for which there was no KM curve (see Appendix 11). Although the KM curves for each study cross over, which suggests that there are some concerns about the proportional hazards assumption, there is no clear statistical evidence that the assumption is violated for all of the included studies. 12 The viability of the network depends on the proportional hazards assumption. Therefore, HRs were synthesised across studies. The choice of prior distributions for the between-study variance was explored. A half-normal (0, 0.192) prior was chosen as a uniform (0, 3) prior was too influential. The justification for the half-normal prior is that it expresses the prior belief that 95% of trials will give HRs within a factor of 2 from the estimated median HR. However, owing to the small number of studies, there was little evidence to inform the between-study heterogeneity. The half-normal prior was also influential, although less so than the uniform prior. According to DIC and total residual deviance statistics, the fixed-effects model provided a better fit to the data than did the random-effects counterpart. The fixed-effects model had both a lower DIC and fewer parameters. This is again because of the small number of studies and the influence of the prior on the between-study heterogeneity. Owing to both models having similar results, the fixed-effects model was chosen as it is a simpler model. Results from both are presented for comparison.
Scenario and subgroup analyses
Scenario analyses including the two low-quality retrospective studies, by Biederman et al. 39 and Van Der Gucht et al. ,40 were carried out, as discussed in Chapter 3, Network 3: adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapies. For the first scenario, the Biederman et al. 39 study was added to the base-case NMA: adults with unresectable HCC who are Child–Pugh class A and ineligible for CTT in both the per-protocol population and the ITT population. There were no available data on Child–Pugh class A patients in the Van Der Gucht et al. 40 study; therefore, it was not included. For the second scenario, which is reported in Appendix 12, both the Biederman et al. 39 and Van Der Gucht et al. 40 studies were added to the NMA of all adults who are ineligible for CTT in the ITT population. Biederman et al. 39 did not report PFS outcomes; therefore, the second scenario was used for the OS outcome only.
A sensitivity analysis that excluded the RCT SIRveNIB21 was conducted. Patients in the SIRveNIB trial are from the Asia-Pacific region and, thus, have different HCC disease aetiology and consequently differing treatments to those from Europe. This is discussed in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. Therefore, a scenario was conducted in which SIRveNIB was excluded from the base-case NMA.
It was not possible to conduct a subgroup analysis in Child–Pugh class A patients with PVT or in patients with PVI. The only available data for this subgroup of patients were from the two RCTs comparing SIR-Spheres and sorafenib: SARAH19,20 and SIRveNIB. 21 However, SIRveNIB reported results for only the subgroup of patients with PVT, and SARAH reported results for only patients with PVI.
Results
Results of the base-case network meta-analysis in the per-protocol population: adults with unresectable hepatocellular carcinoma who are Child–Pugh class A and ineligible for conventional transarterial therapy
Three studies were included in the base-case analysis: two RCTs comparing SIR-Spheres and sorafenib and one RCT comparing lenvatinib and sorafenib. The baseline characteristics of these studies are detailed in Table 10. The REFLECT trial,81 which compares lenvatinib and sorafenib, included patients with extrahepatic spread (61% in the lenvatinib arm and 62% in the sorafenib arm). All the other trials excluded patients with extrahepatic spread; therefore, the subgroup of patients without extrahepatic spread or PVI was used for the REFLECT trial. A more appropriate subgroup was not reported.
The results of both the fixed-effects analysis and the random-effects analysis are shown in Table 11.
| Intervention | Comparator | HR (95% CrI) | |
|---|---|---|---|
| Fixed effects | Random effects | ||
| SIR-Spheres | Sorafenib | 0.94 (0.77 to 1.14) | 0.94 (0.68 to 1.26) |
| SIR-Spheres | Lenvatinib | 0.91 (0.63 to 1.26) | 0.92 (0.52 to 1.51) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) | 1.08 (0.68 to 1.64) |
| SD | – | 0.13 (0.005 to 0.380) | |
| DIC | –1.38 | 0.40 | |
| pD | 2.0 | 2.5 | |
The results provide no evidence that the random-effects model should be preferred. The DIC is marginally higher (–0.40 for the random-effects model, compared with –1.38 for the fixed-effects model; lower DIC values are preferred, with differences of 2–5 considered important). 86 In addition, the high level of uncertainty around the random-effects CrI indicates that there is little information to inform the random-effects parameter. Therefore, the results of the fixed-effects model will be used for the base-case and all scenario analyses. Both fixed-effects and random-effects results are reported in Appendix 13, Tables 43–46, for comparison.
There were no meaningful differences in OS in the per-protocol population between any of the three treatments and all treatments appear to have a similar effect. SIR-Spheres shows a marginal improvement in OS when compared with sorafenib (HR 0.94, 95% CrI 0.77 to 1.14) and lenvatinib (HR 0.91, 95% CrI 0.63 to 1.26); however, the treatment effects are uncertain as the CrI crosses 1. Lenvatinib shows a marginal reduction in OS when compared with sorafenib (HR 1.06, 95% CrI 0.79 to 1.40), although again the CrI crosses 1 (Table 12). Figure 6 presents the cumulative ranking curves for each treatment, with rank 1 being the best and rank 3 being the worst. SIR-Spheres was ranked as the most efficacious therapy, with a probability of being the best of 0.61. Lenvatinib was ranked as the worst treatment, with a probability of being best of 0.22. Sorafenib was ranked as the second best, with a probability of being best of 0.16.
| Sorafenib | 1.07 (0.88 to 1.29) | 0.96 (0.72 to 1.27) |
| 0.94 (0.77 to 1.14) | SIR-Spheres | 0.91 (0.63 to 1.26) |
| 1.06 (0.79 to 1.40) | 1.14 (0.79 to 1.58) | Lenvatinib |
FIGURE 6.
Cumulative ranking probability plots for each treatment in the base-case NMA for the per-protocol population.
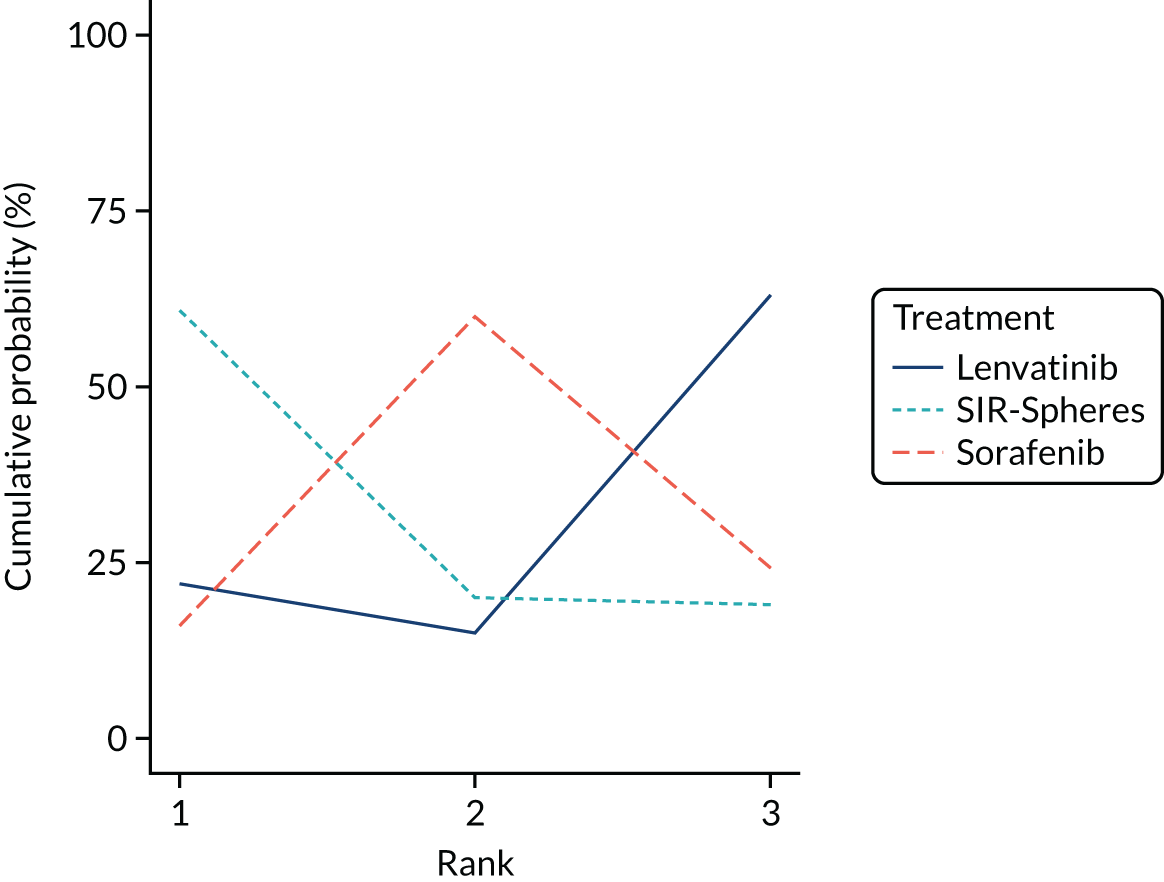
Results of the base-case network meta-analysis in the intention-to-treat population: adults with unresectable hepatocellular carcinoma who are Child–Pugh class A and ineligible for conventional transarterial therapy
Similar to the per-protocol population, there were no significant differences between treatments in the base-case NMA in the ITT population (Table 13).
| Intervention | Comparator | HR (95% CrI) | |
|---|---|---|---|
| Fixed effects | Random effects | ||
| SIR-Spheres | Sorafenib | 1.13 (0.96 to 1.32) | 1.13 (0.86 to 1.47) |
| SIR-Spheres | Lenvatinib | 1.09 (0.77 to 1.48) | 1.10 (0.66 to 1.74) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) | 1.07 (0.70 to 1.59) |
| SD | – | 0.11 (0.004 to 0.352) | |
| DIC | –3.04 | –0.86 | |
| pD | 2.00 | 2.00 | |
SIR-Spheres appears to increase mortality when compared with sorafenib and lenvatinib (HR 1.13, 95% CrI 0.96 to 1.32 and 1.09, 95% CrI 0.77 to 1.48, respectively). However, the CrIs indicate that these results are uncertain. Lenvatinib also shows a reduction in OS when compared with sorafenib (1.06, 95% CrI 0.79 to 1.40); however, the 95% CrI crosses 1, indicating that there is not a significant treatment effect.
The HRs for all patients in the ITT population for OS and PFS are shown in Appendix 12, Tables 41 and 42, respectively.
Scenario 1: inclusion of Biederman et al. into the base-case network meta-analysis
The Biederman et al. 39 study was added to the base-case NMA in a scenario analysis, which allowed for a comparison to be made against TheraSphere. Biederman et al. 39 reports a very strong treatment effect on OS with TheraSphere compared with SIR-Spheres (HR 0.40, 95% CrI 0.20 to 0.78). However, as discussed earlier, Biederman et al. 39 is a retrospective, poor-quality study; therefore, these results may either in part or in full reflect the impact of bias. Furthermore, all patients in the Biederman et al. 39 study have PVT, which is much higher than the proportion of patients who have PVT/PVI in the other included studies. Adding this study has a substantial effect on the NMA results. In the per-protocol population, TheraSphere shows a substantial significant improvement in OS when compared with SIR-Spheres (HR 0.44, 95% CrI 0.20 to 0.84), sorafenib (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.40, 95% CrI 0.18 to 0.78). There were no significant differences in OS between any of the other treatments (Table 14).
| Intervention | Comparator | HR (95% CrI) (fixed effects) | |
|---|---|---|---|
| Per-protocol population | ITT population | ||
| SIR-Spheres | Sorafenib | 0.94 (0.77 to 1.13) | 1.13 (0.96 to 1.32) |
| SIR-Spheres | Lenvatinib | 0.91 (0.63 to 1.26) | 1.09 (0.77 to 1.48) |
| TheraSphere | SIR-Spheres | 0.44 (0.20 to 0.84) | 0.41 (0.20 to 0.77) |
| TheraSphere | Sorafenib | 0.41 (0.20 to 0.77) | 0.47 (0.21 to 0.88) |
| TheraSphere | Lenvatinib | 0.40 (0.18 to 0.78) | 0.45 (0.20 to 0.89) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) | 1.06 (0.79 to 1.40) |
| DIC | 0.30 | –1.32 | |
| pD | 3.00 | 3.00 | |
Similarly, in the ITT population, there was a significant improvement in OS with TheraSphere compared with sorafenib (HR 0.47, 95% CrI 0.21 to 0.88), SIR-Spheres (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.45, 95% CrI 0.20 to 0.89). There were no significant differences in OS between SIR-Spheres, sorafenib and lenvatinib (see Table 14).
Sensitivity analysis
Exclusion of the SIRveNIB study from the base-case network meta-analysis
The SIRveNIB trial,21 which compares SIR-Spheres and sorafenib, was conducted in the Asia-Pacific region. This has implications for the generalisability of the SIRveNIB trial results to the UK population. The aetiology of HCC and the consequent treatment in the Asia-Pacific region are different, as described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. A sensitivity analysis was therefore implemented, in which the SIRveNIB study was excluded from the base-case NMA. Excluding SIRveNIB had very little impact on the results for OS in the ITT population compared with the base-case NMA. All treatment effects for all comparisons were similar to the base-case NMA (Table 15). The OS results in the per-protocol population, however, showed a slight change after excluding SIRveNIB. The treatment effect estimate for SIR-Spheres versus sorafenib increased (1.02, 95% CrI 0.79 to 1.29) compared with the base-case NMA (0.94, 95% CrI 0.77 to 1.14). This showed a reduction in OS with SIR-Spheres rather than an improvement, as seen in the base-case per-protocol population, although neither were statistically significant.
| Intervention | Comparator | OS HR (95% CrI) | |
|---|---|---|---|
| ITT population | Per-protocol population | ||
| SIR-Spheres | Sorafenib | 1.14 (0.90 to 1.41) | 1.02 (0.79 to 1.29) |
| SIR-Spheres | Lenvatinib | 1.09 (0.75 to 1.55) | 0.98 (0.66 to 1.40) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) | 1.06 (0.79 to 1.40) |
| DIC | –0.52 | –0.34 | |
| pD | 2.0 | 2.0 | |
Summary of findings of relative efficacy from network meta-analysis
Treatment options and outcomes vary greatly for patients with unresectable HCC according to the severity of cancer and liver disease. Therefore, three NMA models were produced to represent the different populations of unresectable HCC patients: patients eligible for transplant, patients ineligible for transplant but eligible for CTT and patients ineligible for CTT.
The NMA in patients eligible for transplant was not conducted. Clinical advice was that there are short transplant waiting times in the UK, whereas these were much longer in the trials in the NMA. Therefore, the network may not be generalisable to the UK and there may be limited opportunity for benefit, given the short waiting times. Furthermore, the two RCTs included in the network have very small sample sizes and, therefore, any efficacy estimates produced would be highly uncertain. The NMA of patients eligible for CTT was also not conducted because of the lack of good-quality evidence in this population. There was only one RCT of 24 patients directly comparing SIR-Spheres and the comparator therapies of interest. There were no studies comparing TheraSphere and CTT. Therefore, with missing direct comparisons and only one small study to connect the network, results produced would be very uncertain and unsuitable for decision-making.
Several NMAs of patients who are ineligible for CTT were conducted for both OS outcomes and PFS outcomes in the per-protocol and ITT populations.
The base-case NMA was in adults with unresectable HCC who have Child–Pugh class A liver disease and are ineligible for CTT in the per-protocol population. Three studies were included in the base-case analysis: two RCTs comparing SIR-Spheres and sorafenib and one RCT comparing lenvatinib and sorafenib. The results provided no evidence that the random-effects model should be preferred. In addition, the high level of uncertainty around the random-effects CrI indicated that there is little information to inform the random-effect parameter. Therefore, the results of the fixed-effects model were used for the base-case and scenario analyses.
There were no meaningful differences in OS between any of the three treatments in the per-protocol or ITT populations. All treatments appear to have a similar effect. In the per-protocol population, SIR-Spheres showed a non-significant marginal improvement in OS when compared with sorafenib (HR 0.94, 95% CrI 0.77 to 1.14), although the CrI indicates that this result is uncertain. SIR-Spheres was ranked as the most efficacious therapy, with a probability of being the best of 0.61. Lenvatinib was ranked as the worst treatment, with a probability of being best of 0.22. Sorafenib was ranked as the second best, with a probability of being best of 0.16.
To produce an efficacy estimate for TheraSphere, the only two studies that directly compared TheraSphere and SIR-Spheres for patients ineligible for CTT, Biederman et al. 39 and Van Der Gucht et al. ,40 were included as a sensitivity analysis. Both are low-quality retrospective studies, which reported strong treatment effects on OS with TheraSphere compared with SIR-Spheres (HR 0.40, 95% CrI 0.20 to 0.78, and HR 0.77, 95% CrI 0.27 to 2.18, respectively). Adding these studies had a substantial effect on the NMA results. In the per-protocol population, TheraSphere showed a substantial and statistically significant improvement in OS when compared with SIR-Spheres (HR 0.44, 95% CrI 0.20 to 0.84), sorafenib (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.40, 95% CrI 0.18 to 0.78). In the ITT population, there was also a significant improvement in OS with TheraSphere when compared with sorafenib (HR 0.53, 95% CrI 0.31 to 0.84), SIR-Spheres (HR 0.46, 95% CrI 0.28 to 0.72) and lenvatinib (HR 0.51, 95% CrI 0.28 to 0.86). A sensitivity analysis, which excluded the SIRveNIB study from the base-case NMA was also conducted. The SIRveNIB trial, which compared SIR-Spheres and sorafenib, was conducted in the Asia-Pacific region. This has implications for the generalisability of the SIRveNIB trial results to the UK population. Excluding SIRveNIB, however, had very little impact on the results for OS and PFS in the per-protocol and ITT populations compared with the base-case NMA. There were no significant differences in treatment effects for any comparisons.
Chapter 5 Assessment of existing cost-effectiveness evidence
Systematic review of existing cost-effectiveness evidence
This section presents a systematic review of previous economic evaluations of SIRT and provides an overview of these assessments and a discussion of their relevance to the UK NHS. The findings from the review were used to help inform the development of a new decision-analytic model, which is reported in Chapter 7.
Methods
Systematic searches for relevant literature were completed as part of the search used to identify clinical effectiveness studies. These searches included a broad set of terms aimed at identifying any evidence relating to SIRT, including studies evaluating the cost-effectiveness of SIRT. Details of the searches undertaken are reported in Chapter 3, Search strategy, and the full search strategy is reported in Appendix 1.
Study selection was conducted in two stages: (1) titles and abstracts identified by the search strategy were examined and screened as part of the clinical effectiveness review for any study potentially relevant to the cost-effectiveness review, and (2) full texts were then obtained and screened for inclusion. Screening of titles and abstracts, therefore, aligned with the selection approach outlined in Chapter 3, Search strategy; a single reviewer screened all studies, with 10% checked by a second reviewer. Full-text screening was conducted independently by two reviewers, with disagreements resolved by consensus. All studies meeting the inclusion criteria were summarised and used to identify potential structural issues, assumptions and key drivers of cost-effectiveness. The quality of the cost-effectiveness studies was assessed using a modified version of the Philips checklist88 (see Appendix 14, Table 47).
Studies were included in the review if they assessed the cost-effectiveness of a SIRT versus any other therapy in a HCC population. A broad range of studies were considered for inclusion in the review, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations comparing two or more options including both costs and consequences (cost-effectiveness, cost–utility or cost–benefit analyses) were included.
Results of the review of existing cost-effectiveness evidence
As described in Chapter 3, Quantity and quality of research available, a total of 34 records were identified as being potentially relevant to cost-effectiveness. The full-text articles of these records were assessed for eligibility, with a total of seven studies (eight publications) found to meet the inclusion criteria. Three studies were reported as full papers and four were reported as abstracts only. A PRISMA flow diagram of the review of studies identified in the main systematic review is presented in Figure 7.
FIGURE 7.
Flow diagram of the study selection process for the cost-effectiveness review.
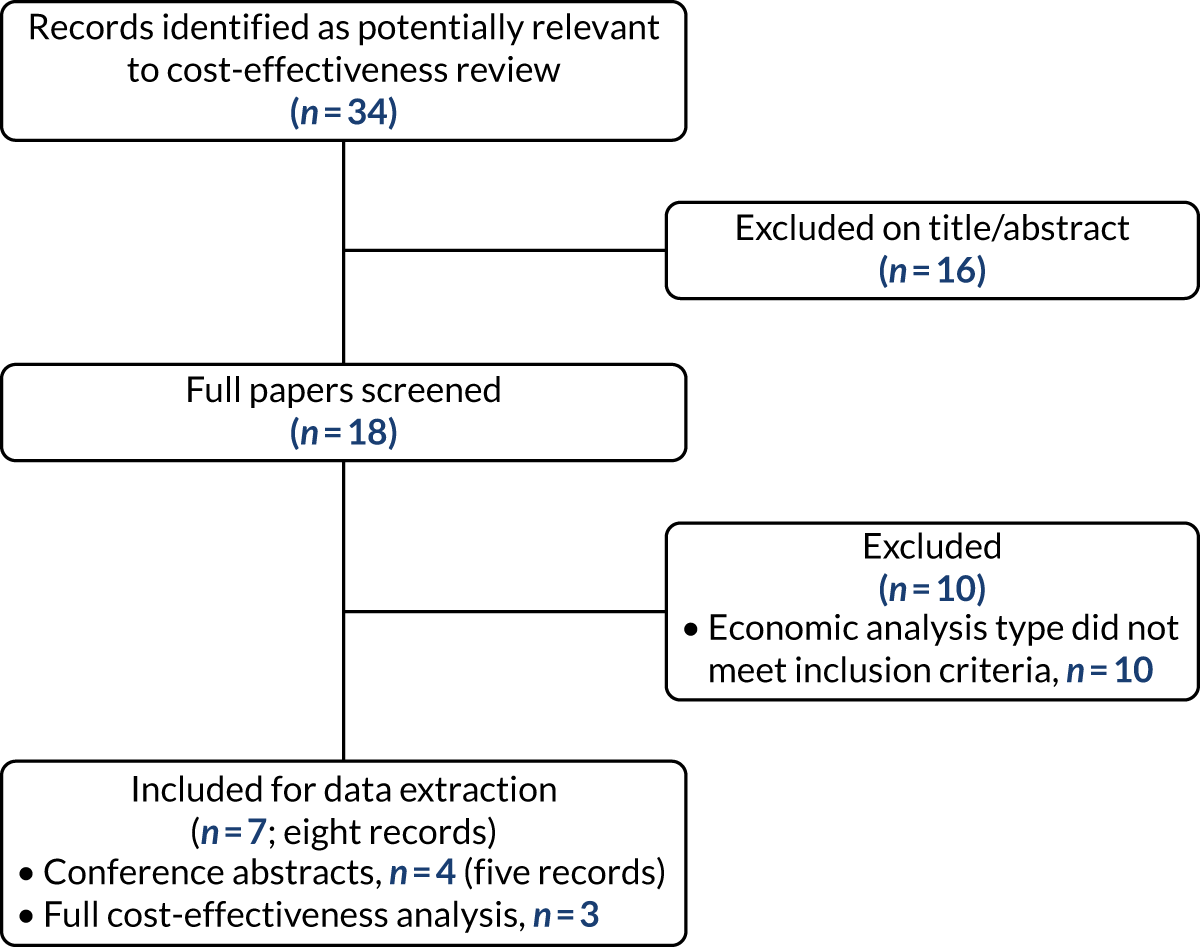
The following sections provide a summary of the Assessment Group (AG)’s critique of the three studies reported in full-paper format,89–92 including an assessment of the studies’ quality and relevance to an NHS perspective. Details of the quality assessment implemented are included in Appendix 14, Table 47. For the four studies identified that were reported only as conference abstracts,93–96 a brief overview is presented along with reported results. Given the limited nature of the reporting of study details, no formal quality assessment of the abstracts was undertaken.
Review of Rognoni et al. (2017 and 2018)90,97
Overview
Two studies by Rognoni et al. 90,97 reported on the cost-effectiveness of SIRT in HCC from an Italian heath service perspective. Both studies used the same basic model design and inputs, but investigated different treatment strategies. One study97 compared SIRT with sorafenib in two HCC subpopulations: intermediate (BCLC B) and intermediate-advanced (BCLC C) disease. The other study90 compared SIRT followed by TACE and possibly sorafenib with SIRT followed by sorafenib in patients with intermediate disease (BCLC B).
Both studies presented a probabilistic Markov model consisting of up to five health states: stable disease, progression, posttransplant, death from disease and death from other causes. The post-transplant health state was used only for the comparison of SIRT with sorafenib in patients with intermediate disease. Transition probabilities were drawn from three Italian oncology centres, which were compared using propensity score matching. HRQoL measures were not reported in this cohort; utilities were, therefore, derived from cost-effectiveness analysis registries. Utilities were assumed to be the same across the patient populations. Italy-specific costs were used in the model, and were derived primarily from official local tariffs and reference costs.
For intermediate-stage patients, the estimated incremental cost-effectiveness ratio (ICER) for SIRT compared with sorafenib was €3302 per quality-adjusted life-year (QALY) gained. In advanced-stage patients, SIRT was found to dominate sorafenib. These results appear to be driven primarily by the relatively low costs of the SIRT procedure relative to the acquisition costs of sorafenib, combined with significant clinical benefits of SIRT resulting in additional life-years gained (LYG). In the comparison of SIRT followed by TACE and possibly sorafenib, with SIRT followed by sorafenib, SIRT-TACE-sorafenib was found to dominate SIRT-sorafenib.
Commentary
The two studies appear to be comprehensive and well implemented, accounting for all major sources of costs and benefits, including long-term benefits in patients receiving liver transplant. However, the fitting and selection of parametric functions to survival data were poorly described and explored. Variability in cost-effectiveness estimates was explored using a one-way sensitivity analysis, showing that the results were robust to a wide range of assumptions.
However, the two studies suffered from a number of potential limitations. Foremost among these is the use of non-randomised data to produce estimates of relative effectiveness. Although propensity scoring was used to adjust for baseline imbalances, this process may have affected the results. The comparison between SIRT and sorafenib in the BCLC C subgroup is of particular concern, as a significant survival benefit was predicted for patients receiving SIRT. This is inconsistent with the results of the SARAH19 and SIRveNIB21 trials reported in Chapter 3, Efficacy and safety of SIR-Spheres, which show no such benefit. The HRQoL values used were generally not reflective of the population under consideration, and matched poorly with those used in previous NICE technology appraisals (TAs) in this indication. The study was also limited in its capacity to inform the present appraisal as the costs and resource use evidence reflected an Italian health-care setting, and the choice of comparators does not represent current UK practice.
Review of Rostambeigi et al. (2014)91,92
Overview
The study by Rostambeigi et al. 91,92 (also presented as a conference abstract) sought to assess the cost-effectiveness of SIRT versus conventional TACE in three subgroups (BCLC A, B and C) of patients with HCC from a US Medicare perspective.
The model presented was a patient simulation that followed 750 patients (split evenly between BCLC A, B and C) through a treatment pathway comprising treatment with either SIRT or TACE. The simulation was repeated for each treatment type and patient subgroup over a time horizon of 3 or 5 years. The model structure adopted is not clearly reported, but appeared to allow for disease recurrence, mortality and liver transplant.
Probabilities for each outcome were drawn from the literature for each patient subgroup according to BCLC stage. Exponential curves were used to estimate survival based on reported survival rates, with a 10% increase in mortality for 1 month following recurrence of HCC and re-treatment. Transplant rates of 29%, 16% and 5% were applied for patients in BCLC stages A, B and C, respectively, although is it unclear how this affected model outcomes. The model assumed disease ‘recurrence’ rates of 40%, 60% and 80% every 10 months for SIRT patients, whereas TACE patients had a recurrence rate of 60%, and could receive 4 to 10 procedures. An assumed probability of 0.5 was used for SIRT re-treatment at the beginning of every 10-month treatment interval, and patients were assumed to receive a maximum of two or three SIRT treatments depending on the scenario. Costs applied in the model were obtained from Medicare reimbursement costs; HRQoL was not considered.
The ICERs presented were estimated using an unconventional approach, calculated by dividing the incremental mean cost per month of survival (i.e. total costs divided by OS in months) by the overall incremental survival in months. The authors did not account for dominance in their calculations, presenting a number of negative ICERs without sufficient interpretation of their different meanings. ICERs in which SIRT was less costly and less effective, less costly and more effective, and more costly but less effective than TACE were presented without further distinction.
In the main analysis in which each procedure could be repeated every 10 months for up to 5 years, the AG calculated SIRT to increase mean survival by 3.80 months in BCLC C patients at a reduced cost. In the scenario in which procedures are repeated every 6 months for up to 3 years, SIRT was more effective (2.90 months incremental survival), with reduced costs compared with TACE in BCLC C patients. In all other patient groups and treatment regimens, SIRT was dominated by TACE.
Commentary
The limited reporting of the model structure and assumptions adopted prevents a detailed critique or discussion of the appropriateness of the model to estimate the relative costs and benefits of SIRT and sorafenib. A number of key structural assumptions appear to have been made arbitrarily, and poor reporting of model inputs limits the generalisability of this study to other settings. As the resource use and costs are specific to the USA, they are unlikely to be relevant to an NHS setting. The choice of comparators and outcome measures (e.g. LYG) further limits comparison with UK practice.
Review of Marqueen et al. (2018)93
Marqueen et al. 93 (conference abstract only) estimated the cost-effectiveness of SIRT with yttrium-90 resin microspheres versus sorafenib in patients with advanced HCC, from a US Medicare perspective. The authors constructed a multistate Markov model (health states not reported) to estimate incremental costs and QALYs over a 5-year time horizon. Hazard rates for disease progression and death were based on a pooled analysis of individual patient data (IPD) from the SARAH19 and SIRveNIB21 RCTs. The clinical data used in the model were not summarised in the abstract, although the authors stated that there was no statistically significant difference in OS, and SIRT was better tolerated and with a higher quality of life than sorafenib. Trial data were also used to inform the parameter values for AEs, treatment adherence and quality-of-life utility weights.
Costs were US$135,256 versus US$90,911 and QALYs were 0.63 versus 0.60 for sorafenib versus SIRT, respectively. The resulting ICER of sorafenib was US$1,479,020 per QALY gained. A probabilistic sensitivity analysis (PSA) demonstrated that the likelihood that sorafenib would be cost-effective did not exceed 1% in cost-effectiveness thresholds up to US$200,000 per QALY. If the monthly price of sorafenib decreased from US$16,390 to US$7250, the ICER of sorafenib fell below US$200,000, and an ICER of < US$100,000 was reached if the monthly price fell below US$6500. Similar results were found using SARAH and SIRveNIB results separately.
Review of Chaplin et al. (2015)94
Chaplin et al. 94 (conference abstract only) conducted a cost-effectiveness analysis of TheraSphere versus sorafenib in patients with advanced HCC in the UK. 94 The authors constructed a Markov model comprising stable disease, progression and death health states, estimating incremental costs and QALYs over a 10-year time horizon. Clinical outcomes for TheraSphere and sorafenib were drawn from two separate RCTs. For TheraSphere, clinical outcomes were based on Salem et al. ,47 a non-randomised comparative effectiveness analysis of radioembolisation with TheraSphere (n = 123) versus chemoembolisation (n = 122). The study enrolled a range of patients, including 39% who were BCLC A, 50% who were BCLC B and 9% who were BCLC C. For sorafenib, outcomes were based on Llovet et al. ,98 a Phase III RCT that included 299 sorafenib patients and 303 patients on placebo, who had not received previous systemic treatment: 82% patients were BCLC C and 18% were BCLC B. Details of data synthesis were not reported in the abstract, but a comparison of median PFS and OS reported in the trial manuscripts with the model predictions suggests that the authors undertook adjustments to account for population differences.
The model estimated that TheraSphere increased TTP (6.2 vs. 4.9 months) and median survival (13.8 vs. 9.7 months). Yttrium-90 was associated with higher QALYs than sorafenib (1.12 vs. 0.85), with lower lifetime costs (£21,441 vs. £34,050). The model also included a scenario in which OS and TTP were assumed to be equivalent, in which TheraSphere remained a dominant treatment option.
Review of Parikh et al. (2018)95
Parikh et al. 95 (conference abstract only) estimated the cost-effectiveness of SIRT with SIR-Spheres versus sorafenib in patients with unresectable HCC and Child–Pugh class A cirrhosis, from a US payer perspective. The authors constructed a Markov simulation model. Clinical inputs for survival and AEs were derived from the SARAH19 and SIRveNIB21 trials. Costs were derived from a literature review, Red Book pharmacy data99 and SEER - Medicare data. 100 Although methods for estimating clinical outcomes were not reported, the authors stated that both trials failed to demonstrate a survival difference between SIRT and sorafenib, although patient-reported outcomes were superior in the SIRT groups. The authors reported results of the model using data from the SARAH trial only, data from the SIRveNIB trial only and an analysis in which data from both studies were pooled.
In all scenarios, SIRT was associated with lower total QALYs than sorafenib was. Using data from SARAH,19 SIRT was associated with increased costs compared with sorafenib and, therefore, sorafenib was the dominant treatment option. Using data from SIRveNIB,21 sorafenib was associated with an ICER of >US$100,000, owing to lower SIRT costs. When combining data from both trials, sorafenib was cost-effective compared with SIRT, with an ICER of US$19,534 per QALY gained. In the combined scenario, lifetime costs were US$63,333 for sorafenib and US$61,897 for SIRT, and there were 0.88 QALYs gained for sorafenib and 0.81 QALYs gained for SIRT. The authors concluded that sorafenib is cost-effective compared with SIRT for patients with unresectable HCC, and that SIRT should not be used as first-line therapy in patients with advanced HCC who are eligible for sorafenib.
Review of Palmer et al. (2017)96
Palmer et al. 96 (conference abstract only) built a cost-minimisation model to evaluate the cost-effectiveness of SIR-Spheres versus sorafenib for patients with BCLC C HCC. This model assumed equal efficacy between SIR-Spheres and sorafenib based on data from the SARAH RCT. 19 AE data were collected from Llovet et al. 98 for sorafenib and Sangro et al. 69 for SIR-Spheres. Costs were derived from ‘standard UK sources’ and data from a UK hospital.
SIR-Spheres dominated sorafenib in this analysis, generating 0.0079 (95% CI 0.0046 to 0.0111) more QALYs than sorafenib, and providing a cost saving of £8909 (95% CI £3257 to £14,570). One-way sensitivity analyses showed that the primary drivers were time on treatment for sorafenib and the costs of work-up and administration for SIR-Spheres. The authors concluded that SIRT using SIR-Spheres is a cost-effective option for BCLC C HCC patients in the UK.
Discussion
The review of existing cost-effectiveness evidence identified three full studies along with four evaluations reported only in abstract form. The three studies reported as full texts compared SIRT with TACE, SIRT with sorafenib, and two alternative treatment sequences: SIRT followed by TACE and possibly sorafenib against SIRT followed by sorafenib. All studies reported in abstract form compared SIRT with sorafenib.
Selective internal radiation therapy versus sorafenib
Only one study comparing SIRT with sorafenib was reported as a full text (i.e. Rognoni et al. 89,90), with the remainder reported as conference abstracts (i.e. Chaplin et al. ,94 Marqueen et al. ,93 Palmer et al. 96 and Parikh et al. 95).
The Rognoni et al. 89,90 study has a number of important limitations, most notably the use of non-randomised evidence to estimate the relative effectiveness of SIRT and sorafenib. The survival gains achieved on SIRT in this study were not reflected in the much larger SARAH19 and SIRveNIB21 trials. A further limitation of the Rognoni et al. 89,90 study was the questionable source of utility values, which do not reflect HRQoL values used in a number of previous TAs in advanced HCC. The Rognoni et al. 89,90 study also adopts a non-UK perspective, which further limits the relevance of the model results to UK decision-makers.
Except for Chaplin et al. ,94 which used non-randomised sources of efficacy data, the conference abstracts drew data from the SARAH and/or SIRveNIB trials. This may mean that these studies are more relevant to NHS decision-making. However, their results were inconsistent. Marqueen et al. 93 and Palmer et al. 96 both reported small QALY gains in favour of SIRT with lower incremental costs. Parikh et al. ,95 in contrast, reported sorafenib to be more clinically effective with higher costs for sorafenib. The source of this inconsistency is unclear given that all three studies derived clinical effectiveness data from the same trials, but this may be reflective of differences in cost and HRQoL assumptions. In these three models, the differences in incremental QALYs between sorafenib and SIRT are small, suggesting that the results may be very sensitive to different assumptions around survival or HRQoL. Marqueen et al. 93 and Palmer et al. 96 noted that model predictions were sensitive to treatment cost assumptions. Palmer et al. 96 specifically highlighted SIRT work-up costs and time on treatment for sorafenib as particular drivers of cost-effectiveness.
Because of these inconsistencies, it is difficult to draw conclusions on the cost-effectiveness of SIRT based on existing analysis of the SARAH and SIRveNIB trials. Limited reporting also prevents meaningful validation of the assumptions and input parameters used in each model, and only Palmer et al. 96 was conducted from a UK perspective.
Selective internal radiation therapy versus transarterial chemoembolisation
One study, reported as a full text by Rostambeigi et al. ,91,92 evaluated the cost-effectiveness of SIRT versus TACE. However, the model structure and inputs used in the analysis were inadequately reported and justified. This is reflected in the AG’s quality assessment (see Appendix 5), in which the majority of elements were scored as unclear. In particular, the source of the clinical effectiveness data used to populate the model is unclear. The evidence identified in the systematic review presented in Chapter 3, however, suggests that it was probably based on non-randomised comparative studies, as little RCT evidence was identified in a CTT-eligible population.
Previous National Institute for Health and Care Excellence guidance
There have been three previous NICE TAs in HCC, although none was for SIRT. These include the evaluations of sorafenib (TA47411), lenvatinib (TA55112) and regorafenib (TA55513). These appraisals are all for systemic therapies for the treatment of advanced unresectable HCC, which forms a subpopulation of that outlined in the scope of the present appraisal of SIRT. This section discusses the key issues and sources of data in each appraisal.
A summary of relevant NICE technology appraisals completed prior to July 2019 is presented in Table 16.
| Characteristic | Sorafenib (TA474)11 | Lenvatinib (TA551)12 | Regorafenib (TA555)13 |
|---|---|---|---|
| Model structure | Markov model, using three health states: progression free, progressed and dead | A partitioned survival model, using three health states: progression free, progressed and dead | A partitioned survival model, using three health states: progression free, progressed and dead. Cycle length of 28 days |
| Population | Patients with advanced-stage HCC, who have failed or are unsuitable for surgical or locoregional therapies | Untreated, advanced or unresectable HCC patients who had Child–Pugh class A status. This was in line with the NICE scope for this appraisal. The ERG evaluated efficacy results for the Western subgroup, but ultimately used the full population results | Adults with advanced, unresectable HCC who had previously received sorafenib |
| Intervention and comparators |
Sorafenib, administered orally at a dose of 400 mg twice daily The comparator was BSC Dosing based on mean dose received in the SHARP trial,69 assuming no wastage |
The intervention was lenvatinib, which is orally administered. The starting dose was 12 mg for patients weighing > 60 kg, and 8 mg for patients weighing < 60 kg Dosing was based on mean dose received by the Western subgroup of the REFLECT trial,12 assuming no wastage. The ERG implemented dosing based on full pack usage (no wastage) The comparator was sorafenib, administered orally at a daily dose of 800 mg |
Regorafenib, administered orally at a dose of 160 mg once daily for the first 21 days of each 28-day treatment cycle The comparator was BSC, consisting of symptomatic therapies only The company used mean doses from RESORCE101 to estimate regorafenib usage. The ERG implemented dosing based on full-pack usage (no wastage) |
| Perspective, time horizon and discounting | NHS perspective (PSS in sensitivity analysis). Time horizon of 14 years; discount rate of 3.5% applied to both costs and QALYs | NHS and PSS perspective. Time horizon of 20 years; discount rate of 3.5% applied to both costs and QALYs | NHS and PSS perspective. Time horizon of 15 years; discount rate of 3.5% was applied to both costs and QALYs |
| Source of clinical outcomes data | SHARP trial.69 A Phase III trial comparing sorafenib with BSC, enrolling patients with an ECOG score of 0–2 and Child–Pugh class A liver disease | REFLECT trial.12 A Phase III trial comparing lenvatinib with sorafenib enrolling patients with unresectable BCLC stage B (those who were ineligible for TACE) or BCLC stage C HCC, and Child–Pugh class A liver disease | RESORCE trial.101 A Phase III trial comparing regorafenib with BSC. This study excluded patients who discontinued treatment with sorafenib due to toxicity, those with Child–Pugh class B liver disease, and those with an ECOG performance score of ≥ 2 |
| Effectiveness extrapolation |
For PFS, the company fit a log-normal model For OS, the company fit a log-normal model. Weibull was considered equally plausible by the committee |
For PFS, the company fit a log-normal model to each treatment group independently. The ERG applied a gamma distribution for PFS in its base-case analysis For OS, a log-logistic function was fitted to each treatment group independently. The ERG preferred adjusted OS analyses, controlling for rates of subsequent therapy |
For PFS, observed KM curves were used directly For OS, the company used a log-normal function fitted to IPD for regorafenib group in RESORCE,101 with the relative effect for BSC modelled using a HR The ERG preferred independent Weibull functions to model OS |
| HRQoL |
Mapping from FACT-G collected during the SHARP69 study to a set of time trade-off utility values using a published algorithm A treatment effect was not included |
Estimated based on EQ-5D-3L data collected in the REFLECT trial12 A linear mixed model was used to generate health state utilities from the EQ-5D data, controlling for prior treatment, age, sex, geographical region, baseline EQ-5D score and baseline ECOG performance status. A treatment effect was not included. Disutilities associated with AEs were not explicitly modelled |
Estimated based on EQ-5D-3L data collected in the RESORCE trial101 A tobit regression model was fitted to the data: progression status and TEAEs were included as covariates. Treatment effect was not included as a covariate |
| Resources and costs |
Costs and health-care resource use considered included drug acquisition, disease management and AEs Disease management costs were estimated from pooling two surveys used in the sorafenib appraisals (2007 and 2015) |
Costs and health-care resource use considered included drug acquisition, disease management, AEs and end-of-life costs Unit costs were from national sources. Disease management costs were estimated from pooling two surveys used in the sorafenib appraisals (2007 and 2015) |
The company’s model included costs of (1) drug acquisition for regorafenib, (2) health state resource use and (3) the management of AEs. Unit costs were from national sources Resource use consisted of visits, tests and hospitalisations, and was estimated from the sorafenib resource use survey conducted in 2015, as no further sources of medical resource use data were identified The ERG preferred the use of combined 2007 and 2015 survey costs |
| Time on treatment and subsequent therapies |
The cost of post-progression sorafenib treatment was removed from the model, but the analysis submitted for Cancer Drugs Fund reconsideration included these costs Patients received BSC after treatment discontinuation |
Time to treatment discontinuation KM data were used directly in the model to estimate the proportion of patients on treatment at a given time Subsequent therapies applied after discontinuation in the company model included sorafenib and regorafenib. The REFLECT trial12 included other therapies post progression. The ERG preferred a scenario whereby post-progression therapy costs were removed; however, the committee concluded that it was reasonable to apply these costs as the benefits of post-progression treatment was reflected in the OS model |
Discontinuation probability applied for patients while progression free and post progression, from RESORCE. 101 Progression-free: based on proportion of patients discontinuing regorafenib for more than one cycle prior to disease progression and median PFS. Post progression: based on proportion of patients who continued to receive regorafenib after disease progression and post-progression treatment rate The ERG preferred to fit a log-logistic model to the time to treatment discontinuation KM data No subsequent therapies were applied after discontinuation |
| AEs | Grade 3 or 4 TEAEs occurring in ≥ 10% of patients in the sorafenib arm of SHARP69 | Grade 3 or 4 TEAEs occurring in ≥ 5% of patients in either arm of REFLECT,12 or if identified as being clinically or economically significant by UK clinical experts (diarrhoea, asthenia and fatigue) | Grade 3 or 4 TEAEs occurring in ≥ 5% of patients in either arm of RESORCE101 |
| Results (ICER, Δ£/ΔQALY) |
Company base case (TA189): £64,754 Updated company base case (TA474): £39,162 DSU (TA474): between £51,208 and £71,276 |
Company base case: lenvatinib dominated sorafenib ERG base case: lenvatinib dominated sorafenib |
Company base case: £33,437 per QALY gained ERG base case: £81,081 per QALY gained |
The modelling approach taken across all three appraisals was similar, with each using a model based on three health states: progression free, progressed disease and death. The sorafenib appraisal differed slightly in its approach and used a Markov model, whereas a partitioned survival modelling approach was used in the other two appraisals.
Clinical data for TA47411 (sorafenib), TA55112 (lenvatinib) and TA55513 (regorafenib) were drawn respectively from the relevant pivotal trials SHARP,69 REFLECT12 and RESORCE. 101 Because of the availability of directly relevant RCT data, no meta-analysis was undertaken in any of the three appraisals. Modelling of clinical effectiveness was, therefore, undertaken by extrapolating available KM data. The committee’s preferred approach in all three appraisals was to independently fit parametric functions to each of the treatment arms on the grounds that proportional hazards did not hold. The parametric function adopted varied across appraisals, with the log-normal and Weibull functions considered the best fitting and most clinically plausible in the appraisal of sorafenib, and the log-logistic function was considered the most appropriate in the lenvatinib appraisal. In the regorafenib appraisal, the Weibull function was considered the best fit, with the exponential and Gompertz functions being plausible alternatives.
Modelled HRQoL across all three appraisals was based on data collected in the respective pivotal trials. In each appraisal, health state utilities were determined by the presence/absence of progressive disease, with no treatment effect included. Progression-free utilities in TA474 and TA551 were similar (0.69 and 0.693, respectively). However, progressive disease values differed, with 0.71 used in TA474 and 0.63 used in TA551. Utility values used in TA555 were generally higher than those in TA474 and TA551. The progression-free utility value used was 0.81, with a utility decrement of –0.048 applied in progression. The Evidence Review Group questioned the face validity of the utility values used, noting the inconsistency with TA474 and TA551, which appraised first-line systemic therapy, whereas regorafenib is positioned as a second-line therapy used after discontinuation of sorafenib. Costs were broadly similar across each appraisal.
Time on treatment was sourced from the relevant pivotal trials through extrapolation of KM data. In TA474, time on treatment was considered to be associated with significant uncertainty, as observational data collected during the Cancer Drugs Fund period presented in the Cancer Drugs Fund reconsideration showed that median time on treatment was much shorter than observed in the SHARP trial. 69 The committee also heard from NHS England that patients are treated for a shorter period of time than was standard in 2007, trading a sizeable decrease in AEs for a small drop in effectiveness. Despite this, the committee preferred to model time on treatment based on that observed in the SHARP trial69 to retain consistency with other clinical inputs.
Health state resource use across all three appraisals was based on two surveys of clinical experts conducted in the appraisals for sorafenib (TA189 and TA474), with unit costs updated in subsequent appraisals. Health state costs included medical staff visits, laboratory and radiological tests, and inpatient costs (including general ward, intensive care unit and accident and emergency admission). The committee preferred to pool the original and revised estimates of resource use, as it was noted that resource use data estimates varied widely.
Review of economic evidence submitted by companies
The Sirtex102 and BTG103 submissions included health economic evaluations assessing the cost-effectiveness of SIR-Spheres and TheraSphere for the treatment of HCC, together with fully executable health economic models. The Terumo submission104 included a budget impact analysis but did not include any further economic evidence.
The Sirtex and BTG company submissions each present the methods and results of two separate economic evaluations that split the population potentially eligible for SIRT into two main groups. The two populations considered in each submission were (1) those eligible for CTT, referred to by Sirtex as TACE, and BTG as TAE, assumed to consist primarily of BCLC B patients, and (2) those who are ineligible for CTT, assumed to consist primarily of BCLC C patients.
Sirtex submission: conventional transarterial therapy-eligible analysis
A cost-minimisation analysis (CMA) was conducted by Sirtex to compare SIR-Spheres, TheraSphere, TACE [referred to by Sirtex as conventional transarterial chemoembolisation (cTACE) in its company submission] and DEB-TACE in the CTT-eligible population. A summary of the key features of the Sirtex model is presented in Table 17. A CMA assumes that the treatments being compared are equivalent in terms of their clinical effectiveness, and considers only the costs associated with each treatment. The presented analysis, therefore, compares only the respective costs associated with each technology. Sirtex’s justification for implementing a CMA rather than a cost–utility analysis was the lack of comparative evidence available, and the uncertainty of the results of its NMA in this population.
| Model component | Description |
|---|---|
| Population | The patient population that is the focus of the cost-effectiveness analysis includes patients matching the following criteria:
|
| Intervention | SIRT:
|
| Comparator | Established clinical management without SIRT, consisting of CTT. These are:
|
| Analysis type | CMA |
| Economic outcome | Total treatment-related cost |
| Perspective | NHS and PSS |
| Time horizon | N/A |
| Discount rate | N/A |
Evidence used to inform the company’s model
The presented CMA considered the following costs: (1) initial treatment, (2) hospitalisation and (3) management of AEs.
Treatment costs of transarterial chemoembolisation and drug-eluting bead transarterial chemoembolisation
Sirtex provided three alternative scenarios for the cost of TACE and DEB-TACE. In one scenario, these costs were based on those estimated by Fateen et al. ,105 a single-centre retrospective database study from the UK. This study collected cost data for 101 procedures in 43 patients between 2006 and 2012 at a centre in Nottingham, UK. In this study, 25% of patients received DEB-TACE and the remaining 75% of patients received TACE. Costs reported in Fateen et al. 105 were for the 2012 cost year: these were inflated to 2018 costs. 106
A second scenario used unit costs from National Schedule of Reference Costs 2017–2018107 for hospitalisation, applied to resource use as estimated in the Fateen et al. 105 study. The mean cost per day of hospitalisation was estimated as £1757 (from Elective Inpatient, Percutaneous, Chemoembolisation or Radioembolisation, of Lesion of Liver, YR57Z), and was assumed to include the cost of delivering TACE.
A third scenario incorporated the results of the resource use survey commissioned by Sirtex, which were used to estimate the number of TACE and DEB-TACE procedures received by each patient, and the proportion of patients receiving DEB-TACE and TACE. The resource use survey was completed by five medical professionals from UK hospitals, comprising two oncologists, one hepatologist and two specialist nurses. This scenario was presented to reflect that resource use might have changed since the time that the Fateen et al. 105 study was undertaken. The survey estimated that a greater proportion of CTT patients receive DEB-TACE in the survey than in the earlier-conducted Fateen et al. 105 study (63% vs. 25%), and that, on average, there are fewer procedures undertaken for a given TACE patient (2.5 vs. 3.03) but a greater number of DEB-TACE procedures (2.83 vs. 1.43).
The costs of providing CTT, estimated as a weighted average of DEB-TACE and TACE costs, ranged from £8792.59 in the scenario based on the Fateen et al. 105 study (scenario 1), to £13,702.37 in the scenario incorporating the results of the resource use survey for the number of TACE and DEB-TACE procedures (scenario 3). A full breakdown of costs is provided in Appendix 15, Table 48.
Treatment costs of selective internal radiation therapy
Procedure costs relating to the administration of SIR-Spheres were assumed to comprise the device costs, the cost of work-up and the SIRT administration procedure (see Appendix 15, Table 49, for a detailed breakdown).
The acquisition cost for a single administration of SIR-Spheres and TheraSphere was assumed to be £8000.
Sirtex provided a range of scenarios to explore work-up and procedure costs, using alternative sources and assumptions to provide a range of plausible costs. Work-up costs were based on the number of work-ups and the total length of hospital stay for a work-up. SIRT procedure costs were based on the number of procedures and the total length of inpatient stay. If the hospital stay was < 1 day, the cost of an outpatient visit was instead applied.
Unit costs of outpatient visits and the inpatient cost for one night were obtained from two different sources. These were from either National Schedule of Reference Costs 2017–2018107 or a microcosting derived from a specialist nurse interview. The inpatient cost from the microcosting exercise was lower than that from National Schedule of Reference Costs 2017–2018107 (£1178 compared with £1757).
Two alternative sources of data were provided for the number of work-up procedures and the length of stay for the work-up. In one source, these figures were informed by a clinician survey, which did not differentiate between the resource use for TheraSphere and SIR-Spheres, which estimated a mean of 1.05 work-ups required per patient. An alternative source was from The Christie NHS Foundation Trust (The Christie NHS Foundation Trust, data on file, 2019, personal communication), which estimated a greater number of work-ups at (confidential information has been removed) per patient for SIR-Spheres and (confidential information has been removed) for TheraSphere, and longer length of stay for each SIRT, equivalent to an inpatient admission.
Data were taken from the clinician survey and elicited from The Christie NHS Foundation Trust (personal communication) to define the number of procedures and length of stay involved in an average SIRT procedure. Sangro et al. 69 provided an alternative source for the number of SIR-Spheres procedures, and two studies by Salem et al. 25,108 were used for TheraSphere. The mean number of procedures ranged from 1.20 to (confidential information has been removed) for TheraSphere, and from 1.08 to 1.20 for SIR-Spheres. Although the SIRT procedure was provided on an inpatient basis in these scenarios, Sirtex also explored the provision of SIRT on an outpatient basis.
Adverse event costs
The unit costs applied in the CTT-eligible model are reproduced in Appendix 15, Table 50. Sirtex derived the unit costs for treating each event from previous NICE TAs, and AE rates were obtained from Salem et al. ,25 a Phase II RCT that compared TheraSphere with TACE in a population of early-stage HCC patients with intent to transplant. Rates of AEs for SIR-Spheres were assumed to be equivalent to those for TheraSphere. This study estimated a higher burden of AEs in CTT patients, in particular neutropenia and elevated aspartate aminotransferase. Consequently, a higher cost was applied in the model (£346 for CTT vs. £109 for TheraSphere).
Results of the economic analysis
Sirtex provided three alternative scenarios for the costs of CTT, which estimated a total cost of providing CTT ranging between £9257 and £14,167 per patient (Table 18).
| Scenario | Total costs (£) | |
|---|---|---|
| CTT costing | ||
| CTT cost from literature | 9257 | |
| CTT resource use from literature with National Schedule of Reference Costs 2017–2018107 | 11,919 | |
| CTT resource use from survey, literature with National Schedule of Reference Costs 2017–2018107 | 14,167 | |
| With microcosting | With National Schedule of Reference Costs 2017–2018107 | |
| SIR-Spheres costing | ||
| Survey results | 12,279 | 13,419 |
| Survey results with outpatient procedures | 12,026 | 12,261 |
| The Christie NHS Foundation Trust results (personal communication) | Confidential information has been removed | Confidential information has been removed |
| Sangro et al.,69 Salem et al.25 for number of procedures, rest survey | 11,185 | 12,222 |
| Sangro et al.,69 Salem et al.108 for number of procedures, rest survey | 11,185 | 12,222 |
| TheraSphere costing | ||
| Survey results | 12,279 | 13,419 |
| Survey results with outpatient procedures | 12,026 | 12,261 |
| The Christie NHS Foundation Trust results (personal communication) | Confidential information has been removed | Confidential information has been removed |
| Sangro et al.,69 Salem et al.25 for number of procedures, rest survey | 13,244 | 14,474 |
| Sangro et al.,69 Salem et al.108 for number of procedures, rest survey | 15,800 | 17,269 |
A range of costing scenarios were presented for TheraSphere and SIR-Spheres based on the alternative methods for delivering the SIRT. Total costs ranged from £12,026 to (confidential information has been removed) for TheraSphere, and from £11,185 to (confidential information has been removed) for SIR-Spheres. In the scenarios that differentiated costs between TheraSphere and SIR-Spheres, TheraSphere costs were slightly higher than SIR-Spheres owing to an increased number of procedures per patient.
Rather than selecting a preferred scenario, Sirtex noted that the range of costs associated with CTT, TheraSphere and SIR-Spheres overlapped, demonstrating the comparability of treatment costs. Total costs comprised mostly those directly related to the primary treatment, with treatment for AEs and hospitalisation constituting a small proportion of total costs.
Assessment Group critique of the Sirtex conventional transarterial therapy-eligible model
Cost-minimisation analysis
The AG considered the presentation of a CMA for this population to be inappropriate and potentially misleading. Such an analysis is appropriate only if there is compelling and unambiguous evidence for equivalent efficacy between interventions. When a CMA is considered by NICE in other appraisals, it is typically accompanied by an extensive and conclusive assessment of equivalence between treatment arms. 109–111 Clinical equivalence is a dynamic concept and any demonstration of clinical equivalence should be sustained over time. Therefore, it is important to assess whether or not the two therapies are equivalent not just in response rate, but in terms of if PFS and OS are also similar.
Results of the AG systematic review found no high-quality evidence in this population. As discussed in Chapter 3, Clinical effectiveness results, the RCTs directly comparing SIR-Spheres with TACE and DEB-TACE were very small and of poor quality, and appeared to favour the chemoembolisation procedure over SIRT in terms of survival outcomes. Although one RCT comparing TheraSphere with TACE reported longer TTP, a higher proportion of patients undergoing transplant and a small but non-significant OS benefit in the TheraSphere arm, this study enrolled a small number of patients and was rated as having a high risk of bias. 22
Therefore, although the AG acknowledges the cited limitation in the effectiveness evidence for this population, and agrees that the development of a cost–utility model is inappropriate, the AG does not consider the identified evidence sufficient to make the strong assumption of equivalence between CTT and SIRT. Furthermore, a focus on treatment costs excludes possible important outcomes regarding people who are downstaged after treatment and become eligible to receive curative therapy, or who receive subsequent therapy after progression of disease.
Cost of treatment with conventional transarterial therapy
The cost analysis of CTT highlighted significant uncertainties in the number of CTT treatments that are typically given, and the impact on the total costs. The applicability of the available sources was limited, and included the only single UK centre collecting data between 2006 and 2012,105 and a survey of five UK-based clinicians. These two sources were used to provide a range of the number of treatments that CTT patients might receive in practice. For TACE, the estimated range was narrow and estimated at between 2.5 and 3.03 treatments. A much wider range was, however, estimated for DEB-TACE (1.43 to 2.83). To consider the plausibility of the presented estimates, the AG searched for alternative estimates of the number of TACE and DEB-TACE procedures. The AG identified two alternative sources of representative data: a UK-based multicentre trial of DEB-TACE enrolling patients between 2010 and 2015 found that a mean of 2.18 DEB-TACE treatments were given,22 and clinicians at a centre in the UK with experience in delivering TACE reported that patients (up to 2010) received a mean of 2.56 treatments with TACE (Dr Jai Patel, Leeds Teaching Hospitals NHS Trust, 2019, personal communication). These estimates both fall within the ranges presented by Sirtex.
Number of selective internal radiation therapy procedures
Sirtex explored the cost impact from using a range of sources to estimate the number of procedures with SIR-Spheres and with TheraSphere. Patients receiving treatment with SIRT typically receive multiple procedures on the basis of their tumour burden (i.e. bilobar involvement requiring sequential treatment visits), with patients not typically re-treated with SIRT on disease progression. Therefore, the number of procedures required would not be expected to differ between treatment arms, and the range of total treatment costs for SIR-Spheres and TheraSphere estimated by this analysis might be expected to be more similar.
Sirtex submission: conventional transarterial therapy-ineligible analysis
The cost–utility model developed by Sirtex evaluates SIR-Spheres for the treatment of HCC in patients currently ineligible to receive TACE, and assesses the incremental cost-effectiveness of SIR-Spheres compared with sorafenib, as well as lenvatinib in a scenario analysis. Clinical inputs in the model are largely based on a subgroup analysis of the SARAH trial. 19 The scope of the company’s model is summarised in Table 19. The model uses a lifetime (15-year) time horizon and takes an NHS perspective. Costs and health outcomes are discounted at a rate of 3.5% per annum, with cost-effectiveness expressed in terms of the incremental cost per QALY gained as per the NICE reference case. Costs were valued at 2017/18 prices. The population considered in the company’s model is limited to those patients who are currently ineligible to receive CTT, and focuses on a subgroup of patients with a low tumour burden and good liver function. Sirtex defines this as a maximum tumour size of 25% of the liver volume, with ALBI 1. The AG noted that this population is far narrower than the population that would be eligible for SIRT within the ‘CTT-ineligible’ population, and it does not match the population defined in the NICE scope. It is also important to note that this subgroup represents a post hoc subgroup analysis of the SARAH trial. 19 The company submission also presented a health economic analysis of the broader CTT-ineligible population as a scenario analysis.
| Model component | Description |
|---|---|
| Population | The patient population that is the focus of the cost-effectiveness analysis includes patients matching the following criteria:
|
| Intervention | SIRT:
|
| Comparator | Established clinical management without SIRT (including but not limited to target chemotherapy). Established clinical management is limited to systemic therapy with sorafenib or lenvatinib in UK clinical practice |
| Analysis type | Cost-effectiveness (cost–utility) analysis |
| Economic outcome | Incremental cost per QALY gained |
| Perspective | NHS and PSS |
| Time horizon | 20 years |
| Discount rate | Annual rate of 3.5% applied to costs and QALYs |
Model structure
The structure of the economic model developed by Sirtex takes the form of a cohort-level partitioned survival model. The main model includes three health states: (1) progression free, (2) post progression and (3) dead. In addition to the main partitioned survival component, the model also permits patients to receive curative therapy, assuming that a proportion of patients are downstaged and receive liver transplant, resection or ablation. Patients who receive curative therapies do not enter the main model, but instead effectively move into a separate two-state model, which comprises the health states (1) alive/received curative therapy and (2) dead. The proportion of patients downstaged to receive curative therapy is based on the numbers downstaged in the low tumour burden/ALBI 1 subgroup of the SARAH trial. 19 Figure 8 presents an overview of the model structure. Both submodels use a lifetime time horizon of 15 years and monthly model cycle with a half-cycle correction applied.
FIGURE 8.
Model structure for the CTT-ineligible population. Reproduced with permission from Sirtex Medical Ltd. 102

In the partitioned survival submodel, the transitions between the three health states were determined directly from the survival models of PFS and OS. Given the incomplete KM data available, parametric functions were fitted to KM curves for OS and PFS from the low tumour burden subgroup of the SARAH trial. 19 Log-normal functions were selected to model both OS and PFS, assuming independent (non-proportional) hazards between treatment groups.
In the partitioned survival model, health state utilities are determined based on the presence or absence of disease and the therapy received, with utility values drawn from the low tumour burden/ALBI 1 subgroup of the SARAH trial. 19 The model does not separately account for loss of QALYs as a result of AEs, as these were assumed to be accounted for through the direct use of trial-based utility values. Utility values used for patients receiving curative therapy were the same as those for pre-progression in the SIR-Spheres arm of the main partitioned survival model.
The model includes the following costs: (1) procedural costs relating to the administration of SIR-Spheres and liver transplant, (2) sorafenib/lenvatinib drug acquisition and administration costs, (3) monitoring for participants receiving non-curative care and (4) costs associated with AEs.
The model employs the following structural assumptions:
-
Health-related quality of life is determined according to the presence/absence of disease progression and the therapy received.
-
Progression-free survival and OS are modelled using Weibull functions assuming independent (non-proportional) hazards.
-
Survival models for PFS and OS were fitted to the low tumour burden/ALBI 1 subgroup of the SARAH trial. 19
-
Adverse events are assumed to affect costs only, with HRQoL assumed to be captured by the use of trial-based utility values.
-
Utility values were assumed to differ according to therapy received in both the pre-progression and post-progression health states.
-
Patients downstaged to receive curative therapy were assumed not to have recurrence of disease, with mortality outcomes determined from a US cohort study comparing outcomes for patients receiving palliative and non-palliative care. 112
Evidence used to inform the company’s model
Overall survival
Overall survival for patients downstaged and in receipt of palliative care was modelled separately, with the proportion of patients downstaged based on observed values in the low tumour burden/ALBI 1 subgroup of the SARAH trial. 19
Overall survival for patients who are not downstaged to curative therapies in the economic model was based on observed survival in the SARAH trial,19 using data on the low tumour burden/ALBI 1 subgroup of patients, including 37 SIRT patients and 48 sorafenib patients.
Before fitting parametric functions to the available KM data, diagnostic plots were used to assess the plausibility of assumption of proportional hazards. The plots revealed some evidence to suggest that the proportional hazards assumption may not hold, as in some instances the lines were not parallel and indeed crossed in some cases. 102 The Schoenfeld residuals, however, suggest no significant deviation from the proportion hazards assumption. Given this uncertainty, Sirtex opted to fit separate parametric functions to the KM data.
The following parametric survival models were fitted to the observed KM data: Weibull, log-normal, log-logistic, exponential and gamma functions. Assessment of the most appropriate parametric extrapolation was made with reference to statistical goodness of fit, visual fit to the observed data and assumptions made in previous TAs. 11–13 Assessment of statistical fit (see Sirtex company submission,102 appendix F) revealed a similar statistical fit for the majority of curves, with the exponential curve observed to have the highest statistical fit. In assessing visual fit, Sirtex noted that the generalised gamma, Weibull and Gompertz curves crossed, which is not seen in the KM curves until the last few patients, whereas the log-normal and log-logistic curves did not cross. Sirtex further noted that in previous TAs of sorafenib (TA47419) and lenvatinib (TA55112), the log-logistic and log-normal curves were considered the most appropriate, and in the analysis of the SARAH19 ITT population the log-normal distribution fitted best, in terms of both goodness-of-fit statistical criteria and visual inspection. On these grounds, Sirtex therefore selected the log-normal function for its base-case analysis. Assessment of uncertainty in curve selection was also partially explored in two scenario analyses considering the log-logistic and Weibull distributions.
Overall survival outcomes for patients downstaged to curative therapy were not drawn from the SARAH trial,19 as OS data were censored on receipt of curative therapy. Survival outcomes for these patients were, therefore, based on a US cohort study,112 which reported the outcomes for patients who did and did not receive curative therapy. The survival HR for downstaged patients was 0.29 (95% CI 0.18 to 0.47). To model survival in the downstaged patients, this HR was applied to the treatment-specific survival curves for SIR-Spheres and sorafenib patients. Importantly, because this HR was applied to the individual survival curves for SIR-Spheres and sorafenib, the model implies differential OS following receipt of curative therapies depending on the initial treatment received.
Progression-free survival
Progression-free survival was defined as the time from the closest date of radiological examination before the first administration of the study treatment to disease progression (per investigator assessment), or death from any cause. Because progression events were observed across patients who were and were not downstaged to receive curative therapy, a common PFS curve was assumed for all patients irrespective of whether or not they received subsequent curative therapy. Sirtex’s base-case analysis drew PFS data from the low tumour burden/ALBI 1 subgroup of the SARAH trial. 19
Assessment of the proportional hazards suggested a degree of uncertainty in whether or not this assumption holds. Assessment of statistical fit based on Akaike information criterion (AIC) and Bayesian information criterion (BIC) of the jointly fitted data found that the (assuming proportional hazards) log-logistic and log-normal, as well as the independently fitted (no proportional hazards) log-normal, distributions had the best statistical fit. Aligning with assumptions made for OS, Sirtex’s base-case analysis used independently fitted log-normal distributions. Uncertainty in curve selection was partially explored in a scenario analysis in which the log-logistic and Weibull distribution were used.
Health-related quality of life
The primary source of utility data used by Sirtex was the SARAH trial,19 which measured HRQoL using the EORTC QLQ-C30 questionnaire. There were a significant number of missing responses over the course of the study, ranging from 19% at baseline to 56.8% at 18 months, with an overall rate of missing data of 38.5%. To calculate health state utilities from this data set, the mapping algorithm by Longworth et al. 113 was used to generate EQ-5D scores adjusted to reflect UK population weights. Sirtex did not consider the SARAH trial19 to show evidence of an independent treatment effect on utility, and there was no significant difference between the HRQoL of those treated with SIR-Spheres and those treated with sorafenib. The company submission,102 however, also notes a statistically significant difference in reported global health scores between treatment arms, and applies treatment-specific utility values based on the subgroup of patients with a tumour burden of ≤ 25% and ALBI 1. The values used in the base-case model are reported in Appendix 15, Table 51.
Selective internal radiation therapy procedure costs
Procedure costs relating to the administration of SIR-Spheres were assumed to comprise the device costs and cost of the work-up and treatment procedures. All patients in the SIRT arm of the model were assumed to undergo at least one work-up procedure, with 5% of patients also assumed to undergo a second work-up based on clinical opinion. To account for the fact that not all patients will go on to receive SIRT (e.g. owing to excess shunting), only a proportion of patients were assumed to receive SIRT. Sirtex’s base case used the low tumour burden/ALBI 1 subgroup of the SARAH trial19 to derive this figure. The model also permitted SIRT patients to be re-treated with SIRT. Sirtex did not consider the average number of SIRT treatments in the SARAH trial19 to represent likely UK practice, as the SARAH trial19 mandated separate administrations where bilobar disease was present. Sirtex instead used data from the CIRT114 (Belgium, France, Germany, Italy, Spain and Switzerland) as well as the ENRY study showing that patients with bilobar disease typically receive a single administration of SIRT with both lobes treated simultaneously. 69 The number of SIRT administrations was, therefore, based broadly on the CIRT, with 1.20 treatments assumed per patient. Uncertainty in the number of SIRT administrations was also explored in scenario analyses based on the SARAH trial,19 the SIRveNIB trial,21 the ENRY study69 and The Christie NHS Foundation Trust data (personal communication).
Costs relating to the work-up and SIRT procedures were based on National Schedule of Reference Costs 2017–2018,107 with the cost of SIR-Spheres assumed to be £8000 per administration. Appendix 15, Table 52, summarises the assumptions and costs of the SIRT procedure.
Drug acquisition costs: systemic therapies
Drug acquisition costs for sorafenib and lenvatinib were taken from the British National Formulary (BNF). 115 Dosing of sorafenib was based on the SARAH trial,19 assuming that 24% of patients received an 800-mg dose and 76% received a 600-mg dose. In scenarios in which lenvatinib was included as a comparator, dosing was based on TA551,12 with 65% assumed to receive an 8-mg dose and 35% assumed to receive a 12-mg dose. 12 Duration of sorafenib therapy was based on the time-to-discontinuation curve from the SARAH trial,19 which was extrapolated using a log-normal function. Duration of lenvatinib therapy was estimated by applying a HR to the sorafenib time-to-discontinuation curve taken from TA551. 12
Subsequent treatments
Modelled subsequent treatments without curative intent were based on expert elicitation, as the subsequent treatments received in the SARAH trial19 were not considered reflective of NHS practice. Drug costs were taken from the electronic market information tool (eMIT)116 and the BNF. 115
For patients downstaged to receive curative therapies, the modelled therapies were based on those received in the ITT population of the SARAH trial,19 consisting of resection, liver transplantation and tumour ablation. The proportion receiving each type of therapy is summarised in Appendix 15, Table 53. Costs of resection were based on NICE TA474,11 and costs of ablation and liver transplantation were based on National Schedule of Reference Costs 2017–2018. 107
Health state costs
Resource use estimates were based on a survey of clinical experts, and included medical staff contacts [e.g. general practitioner (GP) appointments], diagnostic procedures, inpatient care and Personal Social Services (PSS) contacts. Unit costs were derived from National Schedule of Reference Costs 2017–2018. 107 Total costs by health state are reported in Appendix 15, Table 54.
Adverse event costs
The costs of grade 3 or 4 TRAEs experienced by ≥ 5% of the population were modelled, with rates drawn from the SARAH19 and REFLECT81 trials. Costs for each AE were sourced from previous TAs and inflated to the 2018 cost year as appropriate. See Appendix 15, Table 55, for a summary of included AE costs.
Model results
The headline results presented in the Sirtex company submission102 are based on the deterministic version of the model. Uncertainty surrounding model parameters was explored using deterministic sensitivity analysis (DSA) and PSA. The probabilistic results were estimated from 1000 Monte Carlo samples. Uncertainty was represented using tornado diagrams, cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs).
Table 20 presents the base-case estimates of cost-effectiveness using the list price for sorafenib. Based on the probabilistic version of the company’s model, SIR-Spheres are expected to generate an additional 0.682 QALYs at an incremental cost of –£1979 compared with sorafenib; SIR-Spheres were, therefore, estimated to be dominant, producing greater health benefits at lower overall cost. The deterministic version of the model produces similar results, with SIR-Spheres estimated to dominate sorafenib.
| Treatment | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| SIR-Spheres | 2.009 | 24,456 | 0.682 | –1979 | Dominant |
| Sorafenib | 1.408 | 26,435 | |||
| Deterministic model | |||||
| SIR-Spheres | 1.982 | 29,143 | 0.601 | –1784 | Dominant |
| Sorafenib | 1.381 | 30,927 | |||
Figure 9 presents the results of the company’s DSA. The most influential parameters (of those assessed by the company) relate to predicted OS (SIR-Spheres and sorafenib) and the proportion of patients downstaged to receive curative therapy. Additional scenario analyses presented by the company showed that the estimated ICER was generally robust to a range of alternative assumptions, including alternative extrapolations of survival data. However, this analysis also showed that estimated ICERs increased very significantly when the source of effectiveness estimates was changed from the low tumour burden/ALBI 1 subgroup to the ITT or per-protocol population from the SARAH trial,19 which yielded ICERs of £58,763 and £680,276 per QALY gained, respectively.
Critique of the Sirtex conventional transarterial therapy-ineligible model
Relevance of modelled population
The company’s health economic analysis is limited to a subpopulation of patients with a tumour burden of ≤ 25% and with preserved liver function (ALBI 1). The company cited clinical opinion and published literature in its justification for focusing on this group, stating that the ITT and per-protocol population recruited to the SARAH trial19 was unreflective of that eligible in the UK, while also highlighting that the trial included patients with high tumour volume, PVT and poor liver function. The company also outlined that this subpopulation increased the probability of receiving SIRT and the probability of going on to access curative therapy, citing figures from the SARAH trial. 19
Consultation with the AG’s clinical experts confirmed that this subgroup could be identified prospectively and treated with SIRT. However, they also noted that ALBIs are not routinely used to assess liver function in UK practice, and that this definition did not represent a widely accepted clinically distinct subgroup of patients.
The AG is further concerned that the selection of this subgroup is based on a post hoc analysis of a relatively small subgroup of the SARAH trial,19 representing < 20% of the total trial population. Comparison of the results for this subgroup on key outcomes, such as PFS and OS, revealed no statistically significant differences between this group and the remaining population. Furthermore, the randomisation procedure for the SARAH trial19 did not stratify by these baseline characteristics, increasing the risk of baseline imbalances. This can be observed in the sample size of this group between treatment arms, with 37 patients in the SIRT arm and 48 patients in the sorafenib arm. A further consequence of using this subgroup is that potentially relevant data from the SIRveNIB trial21 cannot be used, as data on this subgroup were not available to the company. This is important for two reasons: (1) it reduces the available sample size with consequences for precision and (2) it does not allow for a confirmatory analysis of the PFS and OS benefits observed in this subgroup.
The AG is, therefore, concerned that the purported treatment effects in this subgroup are potentially an artefact of imbalances in characteristics between treatment arms. Available data do not allow further analysis to establish the validity of the observed PFS and OS gains in this subgroup.
Model structure and clinical plausibility of downstaging
The company’s model allows a proportion of patients to move on to receive curative therapy. This is a significant driver of the model results, as 66% of incremental QALYs are generated by patients who received curative therapies.
The SARAH trial19 was used to support the downstaging paradigm used in the model, in which a small number of patients went on to receive curative therapy. The plausibility of downstaging at such high rates in UK practice is unclear. The AG was advised that downstaging of patients with advanced HCC to transplant and other curative options is rare in UK clinical practice, with very few if any of these patients receiving curative therapies. It is also notable that the SIRveNIB trial,21 which recruited a similar population, makes no mention of any patients going on to receive curative therapy. Similarly, none of the previous TAs that assessed systemic cancer treatments for advanced HCC modelled the possibility of curative therapies. The AG is, therefore, concerned that the very sizable benefits resulting from curative therapy would not be realised in practice, and that the rarity of downstaging means that any resulting incremental benefits are subject to very considerable uncertainty.
Modelling of overall survival
The company fit independent parametric survival functions to the observed data from the SARAH trial. 19 This method makes fewer assumptions than a treatment–covariate-based approach, and is in line with NICE DSU guidance on survival analysis. 117 However, the AG does not accept the company’s rationale for selecting the log-normal curve, which was based primarily on visual fit and its use in previous HCC appraisals. The AG notes that the log-normal is the most optimistic of all the fitted parametric curves, and has among the worst statistical fit. The log-normal also has a much longer tail, and, in the AG’s view, fits poorly to the tail of the observed data for the SIR-Spheres arm of the SARAH trial. 19 Clinical advice to the AG indicated a preference for the Weibull function, which predicts substantially shorter survival gains and also has better statistical fit.
In addition to the above, the AG is concerned that the parametric functions were fitted to the observed data that had not been censored to exclude those patients downstaged to receive curative therapy. In the economic model, the outcomes for these patients are modelling independently and, therefore, using the uncensored data means that the OS benefits experienced by these patients are double-counted. The impact of this double-counting is significant, and leads to a substantial overestimation of survival gain. For example, based on a log-normal extrapolation (used in the Sirtex base case) and using the uncensored data, estimated OS gain on SIR-Spheres is 8.27 months. Using the log-normal function on the same data censored for downstaging results in a much reduced predicted OS gain of 1.55 months.
Further to the above issues regarding the plausibility of downstaging, the AG has concerns around the methods used to model the OS benefits associated with curative therapy. Postcurative OS is modelled by using the HR from the Kanwal et al. 112 cohort study to the OS curve for each treatment. This HR is assumed to reflect the improvement in survival outcomes post curative therapy. The application of this HR is treatment specific (i.e. is applied to the SIR-Spheres OS curve for SIR-Spheres patients and to the sorafenib OS curve for sorafenib patients). This implies that OS postcurative therapy will differ depending on the initial treatment received, and thus favours SIR-Spheres. Expert advice received by the AG, however, considers this implausible and that outcomes will be the same post curative therapy regardless of previous therapy received.
Furthermore, the application of a HR to the log-normal curve is inappropriate, as the log-normal function is an accelerated failure time model and does not make assumptions about proportional hazard assumptions. Consequently, survival times are considerably over estimated. The AG also questions the appropriateness of the HR of 0.29 used by the company, noting that this figure was not based on the primary analysis presented in the cited study, but on a scenario analysis in which classification of patients was based on both BCLC stage and ECOG performance status.
Modelling of progression-free survival
The company’s approach to modelling PFS was similar to that of modelling OS, with independent parametric survival functions fitted to the observed data.
The AG is satisfied that the company’s approach of using independent curves was appropriate given the presented evidence to support the non-proportionality of hazards. The AG, however, questions the appropriateness of fitting parametric functions to PFS data at all, given that the available KM data are all but complete; no patients remain at risk in the sorafenib arm and only one remained in the SIRT arm. The company could, therefore, have used the observed data directly, avoiding any uncertainty in the choice of parametric function.
The AG is also concerned that the modelled data were not censored for downstaging events and, therefore, double-count patients who were downstaged to receive curative treatment. As with OS, this results in PFS gains being overestimated, although to a lesser degree than OS. Mean PFS gain assuming a log-normal function was 3.7 months using the uncensored data and 2.35 months using the censored data.
Concerns regarding costs of selective internal radiation therapy
It is assumed in the Sirtex model that patients with bilobar tumours receive SIRT in both liver lobes during the same treatment session. This is in contrast with how patients were treated in the SARAH trial,19 which mandated that patients receive separate treatments with a delay between the first and the second administration. Sequential treatment is implemented to mitigate the risk of REILD, which is more likely to occur if both lobes are treated simultaneously. The company put forward evidence from the European CIRT, and suggested that (confidential information has been removed).
The impact of this assumption is to reduce the costs of providing SIR-Spheres, as sequential treatment involves additional administration and acquisition costs. However, clinical advisors to the AG disagree with the assertion that simultaneous treatment would be implemented in the UK, and contend that in UK practice it is likely that sequential treatment would be used as per the SARAH trial. 19 Furthermore, the AG notes that, although the company adjusts costs to account for the use of simultaneous treatment, no corresponding adjustment is made to health outcomes to account for the increased risks associated with simultaneous treatment.
Failed work-up procedures
In the Sirtex model, a proportion of patients are assumed to fail the work-up procedure and are thus ineligible to receive SIR-Spheres. The proportion of patients receiving work-up who do not go on to receive SIRT was drawn from the low tumour burden/ALBI 1 subgroup of the SARAH trial,19 which was substantially lower than for the population as a whole (8.1% vs. 18.6%). The AG is concerned about the appropriateness of this figure, given the post hoc nature of the analysis. The primary reason patients become ineligible for SIRT following work-up is a high rate of shunting of radioactive material to the lungs. Although this may be plausibly linked to tumour volume and liver status, any such association has not been demonstrated, and it is not clear that the proportion of patients who experience excessive lung shunt will vary substantially between patient groups.
Furthermore, the company’s model assumes that patients who fail work-up will move to the sorafenib arm of the model. The AG considers this inappropriate as only 62% of patients in the SARAH trial19 who failed work-up subsequently received sorafenib. The outcomes of patients in the SARAH trial19 who received work-up but no SIRT were inferior to those who successfully received SIR-Spheres or were randomised to the sorafenib arm. Assuming that patients who fail work-up receive sorafenib outcomes is therefore likely to overestimate the PFS and OS for those allocated to receive SIR-Spheres.
Subsequent therapy costs
The company noted in its submission that the subsequent treatments received by patients in the SARAH trial19 included a number of therapies (e.g. capecitabine and doxorubicin) not used in UK practice. The treatments received following primary therapy in the model were, therefore, based on a survey of 12 clinicians instead.
The AG considers the proportions of patients receiving subsequent therapies in the model to be subject to substantial uncertainty, and notes that these differ substantially from those reported in the SARAH trial. 19 The proportion of patients assumed to receive sorafenib following SIR-Spheres is higher than that observed in SARAH,19 as is the proportion of patients receiving further treatments post sorafenib. The AG also notes that post-sorafenib treatment is based on the ITT population of the SARAH trial19 and, therefore, does not reflect the modelled low tumour burden/ALBI 1 subgroup. Given that the low tumour burden/ALBI 1 subgroup represents a particularly healthy population, it may be anticipated that a much higher proportion of these patients would go on to receive subsequent systemic therapies. As no figures on subsequent therapy in the low tumour burden/ALBI 1 subgroup are reported, this cannot be verified.
Duration of subsequent sorafenib and lenvatinib therapy was drawn from the REFLECT trial,12 and subsequent regorafenib was based on the RESORCE trial. 101 The approach taken to define time on treatment was inconsistent, as median values were used for sorafenib and lenvatinib, whereas a mean value was used for regorafenib. The AG considers mean values more appropriate than the medians used by the company, as the aim of the model is to calculate the mean costs of subsequent therapy. The AG is also concerned that the REFLECT trial12 considers the use of sorafenib and lenvatinib in a first-line setting, particularly as this implies that patients receiving sorafenib as a subsequent therapy will receive treatment for much longer than those who received it as a first-line therapy. The AG, therefore, considers that these values are likely to overestimate time on treatment, and that it may be better to base duration of subsequent therapy on the RESORCE trial,101 which considers systemic therapy use in a second-line setting.
Omission of palliative care costs
The Evidence Review Group notes that the company model does not include end-of-life costs to account for palliation at the end of life. However, the impact of this omission is small, as fewer than 1% of patients remain alive at the end of the modelled time horizon, meaning that nearly all modelled patients incur this cost.
BTG submission: conventional transarterial therapy-eligible analysis
For the comparison with transarterial therapies, the company presented a cohort-based Markov model, comparing TheraSphere, SIR-Spheres and QuiremSpheres with TACE (referred to by the company as cTACE), DEB-TACE and TAE (referred to by the company as bland embolisation). Outcomes were assessed over a time horizon of 20 years using 4-week cycles, and were discounted at a rate of 3.5%. The scope of the company’s model is summarised in Table 21.
| Model component | Description |
|---|---|
| Population | The patient population that is the focus of the cost-effectiveness analysis includes patients matching the following criteria:
|
| Intervention | SIRT:
|
| Comparator | Established clinical management without SIRT (including but not limited to target chemotherapy). The target chemotherapies are:
|
| Analysis type | Cost-effectiveness (cost–utility) analysis |
| Economic outcome | Incremental cost per QALY gained |
| Perspective | NHS and PSS |
| Time horizon | 20 years |
| Discount rate | Annual rate of 3.5% applied to costs and QALYs |
Model structure
The model presented by BTG for the CTT-eligible population was based on a Markov structure, and contained the following health states: (1) watch and wait, (2) pre transplant, (3) post transplant (a series of three tunnel states), (4) no HCC post transplant, (5) pharmacological management and (6) dead. The model schematic is illustrated in Figure 10.
FIGURE 10.
Model structure for the CTT-eligible population. Reproduced with permission from BTG Ltd. 103

Patients who are eligible for SIRT enter the model in the ‘watch and wait’ health state, following initial treatment. Patients remain in this state until they (1) are downstaged and become eligible for transplant, moving on to the pre-transplant state (equivalent to a transplant waiting list), (2) transition to the pharmacological management state owing to not entering remission and being ineligible for liver transplant, or (3) die.
Although the model includes the functionality for patients to receive resection after being downstaged or achieving remission, these transitions are not included in the base-case analysis.
The pre-transplant state captures the time when patients are on the donor organ waiting list. Patients remain in this state until they (1) receive a transplant, and move to the post-transplant state, (2) experience disease progression or become ineligible for a liver transplant, after which they move to the pharmacological management state, or (3) die.
Following transplant, patients spend a single cycle in each of the post-transplant states before arriving in the no-HCC post-transplant state, where they remain until death. The three tunnel states allow for differing resource use over the time following the transplant. In addition, the model assumed that patients would not experience a tumour recurrence after transplantation.
Patients entered the pharmacological management pathway from either the ‘watch and wait’ health state or the pre-transplant health state. Patients remain in this health state until death, although the impact of further disease progression is implicitly captured by assuming a 50 : 50 mix of patients who are in a pre-progressed or a progressed HCC state. This split is used to estimate the mean utility value and treatment-related costs. The patients in the pre-progression part of this health state received either sorafenib (33%) or BSC (67%), and the patients in the progression portion of this health state received BSC.
Evidence used to inform the company’s model
Downstaging outcomes
In this model, it was assumed that the impact of treatment with SIRT compared with CTT was limited to differences in the likelihood of patients being downstaged and becoming eligible for curative therapy.
Non-mortality outcomes for the ‘watch and wait’ health state were estimated from a single-centre, non-randomised comparison of TACE and TheraSphere patients. 118 The study was undertaken in a population of unresectable HCC patients who did not meet the Milan criteria9 at presentation, specifically including patients who were of T3 United Network for Organ Sharing (UNOS) status. This is defined as patients with either a single nodule of > 5.0 cm or two or three nodules, at least one of which is > 3.0 cm in size,119 and downstaging was defined as a decrease in the maximal tumour dimension to 3.0 cm.
The probability of remaining in the watch and wait health state for all therapies was estimated by the company using the median time to downstaging in the TheraSphere arm of the Lewandowski et al. 118 study. The company assumed that the median time to downstaging represented the median time to ‘prognosis’ (i.e. either to downstaging or to pharmaceutical management). The median time to downstaging in the study for TheraSphere patients was 3.1 months; the median time to downstaging in the TACE arm of the study had not been reached. The company converted the median time of 3.1 months to a per-cycle probability of leaving the watch and wait health state of 18.6%, resulting in a per-cycle probability of remaining in this health state of 81.4%.
Of the proportion who leave the watch and wait health state in each cycle, the company used the probability of downstaging from the Lewandowski et al. 118 study to estimate the transition of patients to the pre-transplant state. The remaining living patients entered the pharmacological management health state. The study reported a probability of downstaging from TheraSphere treatment of 58% (25/43), compared with 31% (11/35) downstaged from TACE.
The efficacy of SIR-Spheres and QuiremSpheres was assumed to be equal to that of TheraSphere, and the efficacy of DEB-TACE and TAE was assumed to be equal to that of TACE.
Owing to a lack of data specific to this outcome, the probability of death in each model cycle for the ‘watch and wait’ health state was assumed to be equivalent to that of patients on the wait list, which was estimated from a cohort of NHS patients awaiting liver transplant (see Transplant wait list outcomes). The mortality rate was assumed to be equal between all treatment arms. The greater predicted benefits of SIRT in this model are, therefore, entirely attributable to a greater proportion of patients being successfully downstaged.
Appendix 15, Tables 56 and 57, summarise the transition probability values and mortality rates, respectively, used in the model.
Transplant wait list outcomes
The probability of successfully receiving a transplant once on the wait list was calculated by the company using the median wait time of 130 days for a liver transplant in the UK. 120 This data set is based on a cohort of 2706 NHS patients who were registered for a liver transplant between April 2013 and March 2016, and is not specific to an indication of HCC. This was converted to a per-cycle probability of 13.9%. The probability of transplantation was not conditional on initial treatment.
Patients could transition from the pre-transplant state to pharmacological management, in the case that a patient becomes ineligible for transplant while on the wait list. The probability of this happening was informed by clinical advice to the company, with 16 cases of patients leaving the wait because of disease progression for every 103 transplants (National Audit for Liver Transplant, incomplete source provided by the company).
Mortality in the pre-transplant wait list health state was estimated from a figure quoted in an NHS service specification for Liver Transplantation Service in Adults,121 in which ‘up to 18% of patients die while on the liver transplant waiting list’ (reproduced with permission; contains public sector information licensed under the Open Government Licence v3.0), and converted to a per-cycle mortality rate using the median time to transplant of 130 days.
Pharmacological management outcomes
Patients entering the pharmacological management health state are assumed to remain there until death. The mortality rate applied was based on the median OS of BSC patients reported in the NICE sorafenib submission (34.4 weeks). 11 Per-cycle mortality was estimated assuming that OS followed an exponential distribution; the applied per-cycle mortality rate was 7.7%. This rate was applied to patients in this health state regardless of their initial treatment.
Post-transplant outcomes
Mortality in the three cycles (12 weeks) following transplant was estimated using data from a study of early-stage HCC patients, Bellavance et al. ,122 which reported a 30-day mortality probability of 1.5%.
The post-transplant mortality rate beyond these three cycles was assumed to be lower, and was estimated from NHS 5-year survival rates following transplantation120 of liver patients who had a transplant between 2010 and 2012, which was estimated at 81%. These data reflect a general liver transplant population and are not specific to those who have HCC. Furthermore, for the patients in the population who did have HCC, they are also not specific to patients who had been downstaged after having previously been ineligible for transplant before active treatment for HCC. The company justified the assumption that the mortality rates for a downstaged population can be assumed to be equivalent to a population who were not originally downstaged, on the basis of a systematic review by Gordon-Weeks et al. 123
Adverse events
For TheraSphere and SIR-Spheres, data on grade 3 and 4 TRAEs were sourced from a systematic review of AEs. 79 Event rates for QuiremSpheres were assumed to be the same as for SIR-Spheres. Rates of TRAEs for TACE and DEB-TACE were sourced from a RCT of DEB-TACE versus TACE in HCC. 58 The company’s model included severe TRAEs that occurred in > 5% of patients in at least one arm.
Total TRAE utility decrements and treatment costs were applied in the first model cycle. The estimates of utility decrements were based on the assumption that grade 3 and 4 AEs were associated with a utility decrement of 0.012, which was multiplied by AE rates reported for each event. The total TRAE disutility for TheraSphere, SIR-Spheres, QuiremSpheres and TACE was estimated as –0.002, with –0.009 for TAE and 0.000 for DEB-TACE. Total TRAE costs ranged from £5.59 for DEB-TACE, to £111.33 for SIR-Spheres, and £384.15 for sorafenib. Further details of TRAE rates and associated costs are provided in Appendix 15, Tables 58 and 61, respectively.
Health-related quality of life
BTG drew on a variety of external sources for the utility values in its economic model (see Appendix 15, Table 59). Utility values for all health states with the exception of the post-transplant tunnel states were the same as the pre-progression values used in the TA551124 submission for lenvatinib (equal to 0.75), which were estimated from EQ-5D data collected from patients in the REFLECT trial. 81 The utility applied to the ‘pharmacological management’ state is taken to be an average of the pre-progression and post-progression health state values, as BTG states that this population comprises patients in both progression states equally. Post-transplant utilities were derived from a study by Lim et al. ,125 which used an average of literature-derived utilities equal to 0.69. A scenario analysis was carried out using significantly lower pre- and post-liver transplant utilities from Ratcliffe et al. ;126 however, these values were taken from a primarily non-HCC population.
Utilities were adjusted according to age and gender norms reported in Kind et al. ;127 however, this adjustment was applied incorrectly, which resulted in patients experiencing a much lower HRQoL than reported in the cited sources. When this was highlighted to the company, it stated that this was intentional, and considered the use of lower utility values appropriate and consistent with methods reported in Kind et al. 127
Costs of selective internal radiation therapy treatment
Procedure costs relating to the administration of SIRT were assumed to comprise microsphere (SIRT) acquisition costs, the cost of the work-up and procedure costs relating to the administration of SIRT. The mean number of SIRT treatments per patient was informed by an elicitation exercise undertaken by BTG. Each patient was estimated as having an average of 1.2 SIRT treatments, with one work-up per patient. Only patients who are eligible for SIRT enter the model, and so the costs of work-ups that did not result in treatment with SIRT were not included.
The work-up procedure costs were based on a microcosting from The Christie NHS Foundation Trust, Manchester, and were estimated as being £467.91. These costs included the time of the personnel involved with the work-up (a technician, clinical scientist and radiologist) and a macroaggregated albumin (MAA) body SPECT. The AG requested additional details of this microcosting; however, little further granularity was provided. In addition, BTG identified further relevant cost items in the work-up procedure, which increased the cost to £860.32 per work-up. The company assumed that the resources required for the work-up associated with TheraSphere, SIR-Spheres and QuiremSpheres would be the same.
Costs relating to the administration of the SIRT work-up and the SIRT procedure were based on National Schedule of Reference Costs 2017–2018,107 and the cost of each SIRT was assumed to be £8000 per procedure. Further details are provided in Appendix 15, where Table 60 summarises the assumptions and costs of the SIRT work-up procedure and Table 62 summarises the associated unit costs.
Treatment costs of conventional transarterial therapy
Each patient in the TACE and TAE arms was assumed to have three initial treatments in their respective arms, and patients in the DEB-TACE arm had an average of 1.5 initial treatments. The unit cost and the frequency of their use was informed by clinician input.
The cost of administration involved in each CTT was assumed to be captured in the Healthcare Resource Group (HRG) code for the embolisation procedure (£2790, National Schedule of Reference Costs 2017–2018,107 HRG code YR57Z).
Second-line treatment
After patients move into the pharmacological management health state, they were assumed to receive sorafenib (33% of patients) or BSC (67% of patients). Patients remain in this state until death. The unit cost of sorafenib was obtained from the BNF,115 with the total per-cycle cost estimated assuming a posology of 400 mg twice daily. It was assumed that sorafenib would not be associated with administration costs and that patients would orally self-administer this treatment. It was unclear whether or not the costs of treating AEs associated with sorafenib treatment were captured within the model. Costs associated with BSC were assumed to be captured within the health state resource use.
Health state resource use
Owing to an absence of evidence from published literature for resource use for the CTT-eligible health states, expert opinion was sought from The Christie NHS Foundation Trust (see Appendix 15, Table 63, for a summary of health state costs). These consisted of the following:
-
physician visits (oncologist, hepatologist, Macmillan nurse, gastroenterologist, radiologist, clinical nurse specialist and palliative care physician)
-
laboratory tests [alpha-fetoprotein test, liver function test, international normalised ratio (INR), complete blood count, biochemistry and endoscopy]
-
radiological tests [computerised tomography (CT) scan, MRI scan and ultrasound scan]
-
hospitalisation
-
hospital follow-ups (specialist, GP and nurse)
-
transplant aftercare (immunosuppressants).
Unit costs for each of these items, plus the cost of a transplant procedure, were obtained from national sources. 106,107
The AG requested additional details of how these resource use estimates were obtained. BTG clarified that resource use estimates were provided by a single clinical expert whose role is consultant interventional radiologist at a centre in the UK that uses SIRT. Opinion was elicited via an unstructured telephone conversation, and the estimates were given verbally and were entered directly into the model; no transcripts of this conversation were collected. Therefore, the AG cannot verify the estimation of the resource use inputs.
Additional one-off costs were applied at the point of progression, relating to laboratory and radiological tests (estimated as £95.32 in total) and were obtained from TA555. 13
Palliative care costs
The company’s model also included a cost of £8191 to account for costs of palliation at the end of life, which was applied on death. This was derived from a joint Nuffield Trust and Marie Curie report into end-of-life cancer care and inflated to 2017/18 prices. 128
Model results
Base-case results
Results of the base-case analysis are summarised in Table 22. In the company’s main analysis, TheraSphere, SIR-Spheres and QuiremSpheres were associated with virtually identical numbers of QALYs, owing to the assumption of equal efficacy between interventions. They were all estimated to have similar total costs, with TheraSphere estimated to have marginally lower costs owing to lower rates of AEs requiring treatment.
| Treatment | Total costs (£) | Total QALYs | ΔCosts (£) | ΔQALYs | ICER (£) |
|---|---|---|---|---|---|
| Probabilistic analysis (estimated by the AG) | |||||
| DEB-TACE | 39,505 | 1.377 | – | – | – |
| TAE | 43,634 | 1.384 | 4129 | 0.007 | 621,795 |
| TACE | 43,525 | 1.373 | 4020 | –0.004 | Dominated |
| TheraSphere | 57,334 | 2.089 | 17,829 | 0.712 | 25,051.73 |
| QuiremSpheres | 57,395 | 2.092 | 17,890 | 0.715 | 25,032.69 |
| SIR-Spheres | 57,415 | 2.093 | 17,910 | 0.716 | 25,008.53 |
| Deterministic analysis | |||||
| DEB-TACE | 39,435 | 1.393 | – | – | – |
| TAE | 43,470 | 1.392 | 4035 | –0.001 | Dominated |
| TACE | 43,488 | 1.393 | 4053 | 0.000 | Dominated |
| TheraSphere | 57,338 | 2.119 | 17,903 | 0.726 | 24,647 |
| QuiremSpheres | 57,361 | 2.119 | 17,925 | 0.726 | 24,647 |
| SIR-Spheres | 57,361 | 2.119 | 17,925 | 0.726 | 24,647 |
Similarly, for TACE, DEB-TACE and TAE, marginal differences were observed owing to assumed differences in AE rates and unit costs of treatment.
DEB-TACE was estimated as being the strategy with the lowest costs owing to the fewer procedures required, and was used as the reference treatment in the incremental analysis. This resulted in an ICER of £24,647 for each of the SIRT versus DEB-TACE, and TACE and TAE being dominated versus DEB-TACE.
The probabilistic version of the model produced similar results, with the ICER relative to DEB-TACE being £25,052 per QALY.
Probabilistic results
Uncertainty surrounding model parameters was explored using scenario analyses and PSA; the executable model also included a number of DSAs that were not presented in the company submission or appendices. The company’s probabilistic results were estimated from 1000 Monte Carlo samples and were presented using CEACs and cost-effectiveness acceptability frontiers (CEAFs) only, with no ICERs from the probabilistic model presented in the company submission.
Figure 11 presents the results of the company’s PSA. Up to a threshold of approximately £25,000 per QALY, the company model estimated the treatment with the highest likelihood of being cost-effective to be DEB-TACE. After this point, the probability of being cost-effective was highest for the three SIRTs, which had similar probabilities of cost-effectiveness.
FIGURE 11.
Cost-effectiveness acceptability curve (CTT-eligible population). Reproduced with permission from BTG Ltd. 103
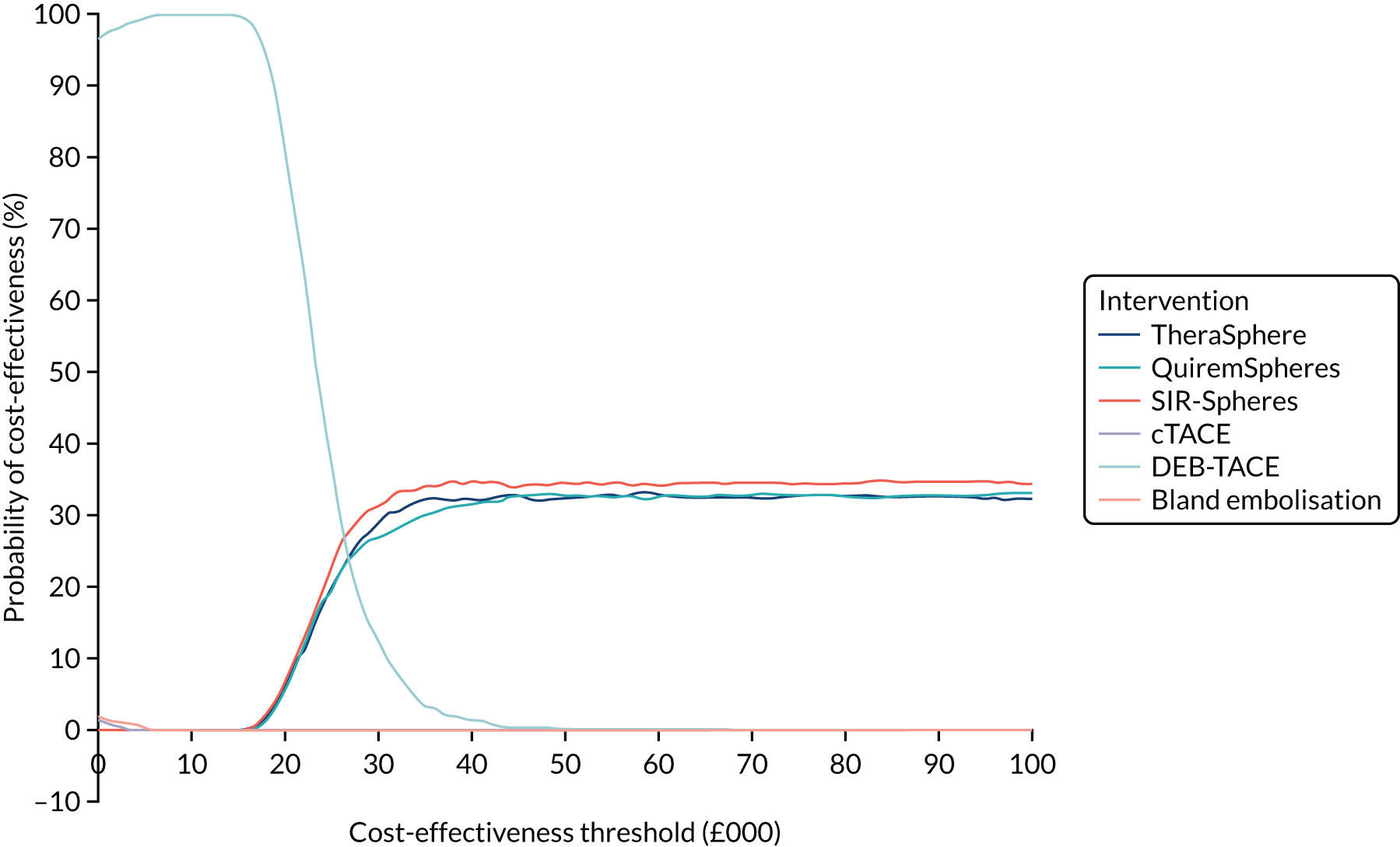
Scenario analyses
Table 23 presents the results of the company’s scenario analysis. The most influential parameters, of those assessed by the company, relate to the proportion of patients who transition to resection, and the proportion of patients who were downstaged after treatment with TheraSphere. Although the amount by which the proportion of patients was varied was arbitrary, and the ICER does not specifically represent a potential upper bound, this analysis showed that the model was most sensitive to this parameter.
| Scenario | ICER (£) |
|---|---|
| CTT-eligible scenarios: base case | 24,647 |
| 50% discount on TheraSphere | 18,039 |
| TheraSphere treatment free when more than one treatment needed | 21,676 |
| 50% of downstaged patients transition to resection rather than transplant | 31,112 |
| Removal of SIRT work-up costs | 23,773 |
| Alternative utility values | 25,003 |
| Alternative downstaging rates for SIRT (relative efficacy of SIRT decreased vs. TACE/TAE) | 38,203 |
| Alternative downstaging rates for SIRT (relative efficacy of SIRT increased vs. TACE/TAE) | 20,561 |
| Alternative post-transplant mortality rates (increased) | 26,744 |
Assessment Group critique of the BTG conventional transarterial therapy-eligible model
Downstaging and role of transplant in the UK
The company assumed that patients who are successfully downstaged become eligible for transplantation, and that no patients receive any other kind of curative therapy including resection or ablation. This was justified on the basis that few patients are expected to receive these other therapies. The company provided two sources in support of this assumption. In these studies, of the patients who received radical curative therapy after downstaging, the proportion who received resection ranged from approximately 5.9%129 to 10%. 118
Clinical advice received by the AG also suggested that at least a proportion of these patients would go on to receive resection rather than a transplant. This AG therefore considers the assumption that all patients will go on to receive a transplant to be unreasonable and likely to favour SIRT, as outcomes following resection have been demonstrated to be associated with poorer outcomes (recurrence and survival) than those following transplantation. 122 The relevance of downstaging to transplantation in UK practice is also unclear. Eligibility for transplantation in the UK has historically been defined by the Milan criteria,130 and only recently has a service evaluation been introduced in which eligibility criteria have been expanded to permit downstaged patients to receive a transplant. 131,132 Furthermore, at the time of writing, this study has recruited only a small number of patients, and does not represent established national practice.
Modelling of pharmacological management
The progression status of patients in the pharmacological management health state was estimated as a 50 : 50 average of patients in the pre-progressed and post-progression states. This split is arbitrary and unlikely to accurately reflect the actual proportion of patients in each health state. A visual comparison of the PFS and OS extrapolation plots for sorafenib and BSC in the SHARP study69 appears to show that a greater proportion of time is spent in the post-progressed health state. A more reasonable estimate of the ratio of patients in each group is likely to be 33 : 67. Furthermore, given that the PFS and OS plots for SHARP are available, time in state could have been explicitly modelled, avoiding the need for such an assumption. The implications of this assumption are important and may lead to overly pessimistic estimates for patients in this health state, as this split is used to estimate utility and cost of active treatment. Based on the 50 : 50 split assumed, this will tend to overestimate total QALYs as too many patients are assumed to be in the pre-progressed state, as well as overestimating costs associated with time on sorafenib, where treatment duration is linked to progression.
Exclusion of patients who received selective internal radiation therapy work-up procedure but not treatment with selective internal radiation therapy
An important omission from the economic analysis is the costs and outcomes associated with patients who receive work-up associated with SIRT but who subsequently do not receive SIRT. These costs should be included in the economic analysis because work-up costs will be incurred by the NHS if SIRT was to be implemented in practice. Furthermore, patients who fail the work-up procedure are likely to be different from those who go on to receive treatment, as demonstrated in the SARAH trial,19 in which patients who failed work-up had significantly poorer outcomes than those who went on to receive SIRT. Excluding these patients from the analysis therefore underestimates total costs in the SIRT treatment arms and is likely to overestimate treatment benefits.
Modelling of comparator treatments
The company assumed equivalent efficacy between the SIRT treatments due to the paucity of comparative data, which the AG considered reasonable given the lack of data, and similarities in the treatment modalities. However, the BTG company submission103 states that it considers this assumption to be conservative, and that it might be expected that TheraSphere would provide superior outcomes. The AG notes that no plausible clinical argument or clinical evidence was provided in support of this statement.
Downstaging outcomes
The key benefit of SIRT in this analysis was through the increased proportion of patients who achieved downstaging after treatment, which indirectly led to increased numbers of patients receiving curative therapy. The probability of downstaging was estimated using data from a study of TheraSphere and TACE patients. 29 The AG had concerns relating to the robustness and generalisability of this study. The study was retrospective and single centre, with non-randomised cohort arms, which could have left it open to confounding bias. Furthermore, the study retrospectively identified patients who were most likely to be downstaged to curative therapies and therefore the modelled population is not representative of the broad CTT-eligible population in the scope of the analysis, and predicts higher rates of downstaging than would otherwise be observed for this broader population.
There are also issues regarding the generalisability of the downstaging criteria applied in the Lewandowski et al. 118 study, which were based on tumour dimensions only. However, UK criteria, used in the UK service evaluation of downstaging, also take into account alpha-fetoprotein level. 131 This may mean that there are differences between these patients and those considered eligible for transplant in the NHS.
To estimate the transition of patients to the pre-transplant wait list, the observed probability of downstaging from the Lewandowski et al. 118 study was applied to the proportion of patients who remained in the ‘watch and wait’ health state, rather than being applied directly in the model. As a result, this method underestimated the proportion of patients who were downstaged: for TheraSphere, the model predicted that 48% patients were downstaged, compared with 58% reported by Lewandowski et al. ,118 and for TACE, the modelled versus observed proportion who were downstaged was 26% vs. 35%.
The company assumed that the mortality rate of patients in the ‘watch and wait’ health state was equivalent to that of the pre-transplant mortality rate, citing a lack of data to model this specific outcome. However, the Lewandowski et al. 118 study reported mortality rates that were censored to curative therapies, and it was unclear why these were not leveraged in the model. The same mortality rate was applied to both treatment arms, thereby assuming that the only impact of treatment on mortality is through the bridging of patients to transplant. Furthermore, the data used to estimate pre-transplant mortality was from a cohort of patients,121 of whom only a proportion had HCC. The Lewandowski et al. 118 study also reported progression outcomes, which again were not used in the economic analysis.
The use of different sources for downstaging, progression and mortality outcomes also means that the evidence was derived from very different study populations, which led to a lack of internal consistency, and made it more difficult to validate the predictions of the model.
Transplant wait list outcomes
The data source used to estimate the time spent on the transplant wait list was estimated for a cohort of patients not specific to HCC. Patients on the transplant wait list are prioritised by their Model for End-Stage Liver Disease (MELD) score;133 however, the presence of HCC adds ‘exception points’ to MELD, meaning that the wait list time is generally shorter for HCC patients. The AG obtained data from a report on the 1-year outcomes following the introduction of the National Liver Offering Scheme, which was implemented on 20 March 2018. 134 The median waiting time under the old offering scheme may not accurately reflect how long patients may wait under the new offering scheme. The median waiting time to transplant for HCC patients who received a transplant between 20 March 2018 and 19 March 2019 was 49.5 days, which is substantially lower than the value for the overall cohort.
The company provided an incomplete reference on the source of the data used to estimate the transition to pharmacological management, and so it was not possible to comment on the suitability of this source. In an interim report on a service evaluation of transplantation following downstaging of HCC patients in the UK,132 of 27 patients enrolled in the programme to date, only one was removed from the wait list owing to the deterioration of their condition. This provides a much lower estimate of dropout than that estimated by the company, although the AG acknowledges that it is based on a smaller subset of patients.
The AG questions whether or not it is appropriate to apply the same transition probabilities and mortality rate to patients regardless of their initial treatment; however, the AG is not aware of any directly applicable evidence for a differential rate. There are many factors that determine the rate at which patients receive transplant; some of these will not be treatment dependent, including the availability of donor grafts, and some are dependent on treatment. Previous studies of SIRT and CTT with intent to downstage have demonstrated differential outcomes of transplantation and progression between treatment arms; although these are based on very small patient numbers, there does appear to be a small benefit in favour of SIRT. 25,47 Although TheraSphere and TACE were given as downstaging rather than bridging therapies in the Lewandowski et al. 118 study and so are not directly applicable to outcomes for patients on the transplant wait list, OS censored to curative therapies was also significantly different between arms in favour of SIRT, particularly after 2 and 3 years. Similarly, the rate at which patients receive curative therapy following downstaging is also likely to differ between arms, as evidenced in the Lewandowski et al. 118 study. As such, the AG considers it unlikely that outcomes would be equivalent across different treatment modalities, although it is not possible to estimate directly without estimates of survival conditional on downstaging.
Pharmacological management
Outcomes for patients in the pharmacological management health state were based on the BSC arm of the SHARP trial;69 the company stated that this was to avoid applying any benefit associated with a particular HCC treatment in the model, as patients modelled to receive pharmacological management would be given different treatments. This is not representative of patients in this health state, as a proportion of these patients would receive further active therapy, assumed by the company to be sorafenib. Because patients receiving sorafenib experience better outcomes than patients on BSC (as demonstrated by a HR of 0.69 for OS in SHARP69), this approach underestimates survival for patients in this health state. A more accurate approach would be to calculate outcomes separately for sorafenib and BSC and then weight according to the proportion of patients in the health state over time.
Furthermore, the SHARP trial69 is unrepresentative of the patients who would receive BSC in this population for a number of reasons. Approximately 50% of patients in SHARP had extrahepatic spread, and would thus be contraindicated for SIRT treatment. A subgroup analysis of SHARP patients demonstrated that the sorafenib treatment effect was higher in patients with no extrahepatic spread (HR of 0.55 compared with 0.69 in the ITT population). Data from REFLECT,12 which compared lenvatinib with sorafenib, also demonstrated that the prognosis for patients with extrahepatic spread is worse than for those without: in the ITT population, the median OS was 12.3 months, compared with 18.0 months in a population with no extrahepatic spread. In addition, the SHARP trial enrolled only patients who had not received previous treatment with systemic therapy, so BSC patients in SHARP do not represent the patients in the pharmacological management health state who previously received TACE or SIRT. The AG was advised that patients who present with HCC and are eligible for sorafenib are typically associated with a more rapidly progressing form of the disease and will have a higher mortality rate.
As a result, the cost-effectiveness analysis is biased in favour of SIRT through the selection of unrepresentative comparator data. The use of these data from SHARP69 underestimates survival in the pharmacological management health state, thereby further inflating the relative treatment effect of SIRT, as fewer patients on SIRT than on other therapies enter this health state.
Post-transplant outcomes
The AG has concerns about the applicability of the sources used to estimate mortality following liver transplantation, and considers it uncertain whether or not the assumed treatment pathway is reflective of clinical practice.
The data set used to estimate long-term mortality after transplant is not specific to patients with HCC. Patients with HCC are at risk of tumour recurrence, which is linked to increased mortality. 122 This can be illustrated by a comparison of survival in the general liver transplant population and in a HCC population. The AG obtained a HCC-specific data set of survival outcomes for liver transplant recipients in the UK since 1994. 134 In this data set, patients with HCC (restricted to those aged > 60 years as a proxy for intermediate-HCC patients) had a 5-year survival of 71%. This was lower than for those in the general liver transplant data set, whose 5-year survival was estimated as 81%. Therefore, benefits estimated by the company model are likely to be overestimated.
By excluding tumour recurrences, the treatment pathway is also misrepresented by the model. Both the Bellavance et al. 122 and Lewandowski et al. 118 studies report on recurrences that occur after transplantation: approximately 20% of patients in the Lewandowski et al. 118 study and 14% of patients in the Bellavance et al. 122 study experienced recurrence after transplantation, with a 1-year relapse-free survival rate of between 73% and 89%. In addition, the AG found that, in its analysis of the HCC-specific transplant data set, > 10% of transplant recipients in the UK in this population experienced a recurrence in the first 5 years post transplant. The patients who experience a recurrence are at an elevated risk of death,122 and these patients often experience a reduced quality of life and additional treatment-related costs. 135 By excluding recurrence after transplant, the model overestimates the QALYs and underestimates costs generated for transplant recipients, which biases the results in favour of the SIRT arm owing to a higher proportion of patients being downstaged.
Health-related quality of life
The total number of QALYs generated by the model is likely to be underestimated, owing to the source chosen and an error in how age-related disutility was applied.
Health state utility values were estimated from a range of sources, but were primarily based on the NICE appraisal of lenvatinib (TA551),12 which enrolled patients with advanced HCC, of whom approximately 60% had extrahepatic spread. This population therefore had more advanced disease and does not reflect the model population of intermediate-HCC patients. Therefore, the utilities drawn from TA55112 are likely to underestimate the quality of life for a CTT-eligible population, and disadvantages any treatment arm associated with increased life-years.
The AG considers the company to have incorrectly implemented age-related disutilities in the model, although the company contends that the application was appropriate. This ‘error’ has an impact on all health states, and results in patients experiencing much lower utilities than those observed in the cited sources. In the company’s model, the decrement associated with ageing is estimated by estimating an absolute utility decrement for each health state relative to full health (i.e. 1 minus the reported health state utility) and then subtracting this decrement from the age- and gender-adjusted population norm from Kind et al. 127 For example, as patients enter the model at the age of 65 years, the age-adjusted utility started at 0.78, and the literature-derived absolute utility for ‘watch and wait’ patients was 0.75. 12 This meant that the age-adjusted utility for patients in the ‘watch and wait’ health state was 0.53 (0.78 – 0.25). The application of age-adjusted utilities in this way is inappropriate and ignores the fact that each health state utility is derived from an age-appropriate source, and thus already accounts for any age-related decline in HRQoL. Furthermore, this method is inconsistent with previous TAs136–138 in which age-related disutilities have been applied, where age-related decrements are applied as a multiplier to health state utilities rather than as an absolute decrement.
Resource use estimates
Resource use was estimated in the model based on feedback from a single clinician at a centre in the UK that uses SIRT. As the company could not provide details of the questionnaire or transcript of the interview, it has not been possible to verify how these data were estimated. Therefore, there are a number of uncertainties regarding which treatment costs are included, such as AEs relating to subsequent therapy (sorafenib) or to transplant, or whether or not any bridging therapy was provided for patients on the transplant wait list.
The company’s clinical expert advised that TACE and TAE patients had around three initial treatments in their respective arms, whereas patients in the DEB-TACE arm had an average of 1.5 initial treatments. As described in Chapter 5, Sirtex submission: conventional transarterial therapy-eligible analysis, there is apparent variation in the number of treatments that patients receive in practice, with values identified between 1.43 and 2.83 per patient for DEB-TACE and between 2.5 and 3.03 for TACE patients. The uncertainty in these numbers was not explored by the company. By implementing a single embolisation cost for each CTT procedure, the company also did not explore any differences in the length of hospital stay between the different CTT treatments.
A proportion of patients in the pharmacological management health state receive sorafenib. This was estimated using data obtained from a survey of clinicians; as there were limited details provided on how the proportion was estimated, the underlying assumptions could not be validated. It appears that the cost of sorafenib was applied for the time that patients were in the pre-progression health state; however, this would overestimate the cost of treatment, because mean time on treatment with sorafenib is less than mean TTP. 11 The analysis also excludes patients who receive lenvatinib instead of sorafenib, and the proportion of patients who progress on sorafenib and receive subsequent treatment with regorafenib; clinical advisors to the AG suggest that this would be approximately 20% of patients.
The company assumed that the work-up procedure for each SIRT would be associated with the same resource use. This underestimates the costs for QuiremSpheres, as the use of QuiremScout is required and is associated with an additional procurement cost.
BTG submission: conventional transarterial therapy-ineligible analysis
The second model submitted by the company assessed the incremental cost-effectiveness of SIRT compared with systemic therapy for the treatment of HCC in patients ineligible for TACE. The SIRTs assessed in this analysis were TheraSphere, SIR-Spheres and QuiremSpheres. The systemic therapies assessed were sorafenib, lenvatinib and regorafenib. Clinical inputs in the model were drawn primarily from a NMA of comparative studies and a single-arm Phase 2 trial of TheraSphere. 139 The scope of the company’s model is summarised in Table 24. The time horizon considered in the model is 20 years and adopts an NHS and PSS perspective in line with the NICE reference case. Costs and health benefits in the model were discounted at a rate of 3.5%. The price year used in the model was 2017/18. The BTG company submission103 states that the model aimed to consider patients who are considered to have later-stage HCC, which the company defines as patients who either are ineligible for or have previously failed TACE.
| Model component | Description |
|---|---|
| Population | The patient population that is the focus of the cost-effectiveness analysis includes patients matching the following criteria:
|
| Intervention | SIRT:
|
| Comparators | Established clinical management without SIRT (including but not limited to target chemotherapy). The target chemotherapies are:
|
| Analysis type | Cost-effectiveness (cost–utility) analysis |
| Economic outcome | Incremental cost per QALY gained |
| Perspective | NHS and PSS |
| Time horizon | 20 years |
| Discount rate | Annual rate of 3.5% applied to costs and QALYs |
Model structure
The model is a cohort-level partitioned survival model, which includes three health states: (1) progression free, (2) post progression and (3) dead. The model does not allow for downstaging to curative therapies. Figure 12 presents an overview of the adopted model structure. The proportion of patients in each health state is determined as a function of the TTP and OS. The proportion of patients in the progression-free health state was based on the TTP curve, and the post-progression state was estimated as the difference between the OS and TTP curves. The proportion of patients in the dead state was determined by the OS curve.
For OS, the estimated treatment effect was drawn from a NMA of studies identified in the presented systematic review. This was then applied to parametric survival models fitted to KM data from a single-arm Phase II trial of TheraSphere. 139 A Weibull function was selected as the most appropriate survival model. TTP was modelled based on a naive comparison of relevant TTP data, and was assumed to follow an exponential survival function.
Health state utilities in the model are primarily determined by the presence or absence of disease progression, with values based on those used in TA551. 12 The model also separately accounts for loss of QALYs as a result of AEs. The model attempts to account for the impact of ageing by implementing an age adjustment factor; however, this was implemented incorrectly (see Application of age-adjusted utilities for further discussion).
The model includes the following resource costs: (1) procedural costs relating to the administration of SIRT, (2) drug acquisition and administration costs associated with systemic therapy, (3) monitoring and disease management costs, (4) costs associated with AEs and (5) palliative care costs.
The model employs the following structural assumptions:
-
Health-related quality of life is determined according to the presence or absence of disease progression and the therapy received.
-
Patients were not permitted to be downstaged to receive curative therapy; all patients were, therefore, assumed to receive palliative care.
-
Time to progression for TheraSphere was modelled using an exponential function fitted to a single-arm study; comparator TTP was modelled based on median PFS extracted from trial and observational evidence identified as relevant by the company.
-
Overall survival was modelled using a Weibull function fitted to a single-arm study of TheraSphere with a HR derived from a NMA to determine OS for other therapies.
-
Adverse events are assumed to affect both costs and HRQoL.
-
Palliative care costs are assumed to be incurred only during the final month of life.
Evidence used to inform the company’s model
Overall survival
Overall survival for patients receiving TheraSphere was based on a single-arm Phase II trial of 52 patients with intermediate and advanced HCC. 139
The following standard parametric survival models were fitted to the observed data: Weibull, log-normal, log-logistic, exponential and gamma functions. Assessment of the most appropriate parametric extrapolation was made with reference to statistical goodness of fit and clinical plausibility of survival estimates. The log-logistic and log-normal curves were eliminated on this basis, as they predicted that a small proportion of patients would not die within the time horizon of the model. The Weibull function was selected for the base-case analysis; no other extrapolations were explored in scenario analysis.
Estimation of OS for comparator therapies was based on a NMA of studies identified in the presented clinical effectiveness review. The NMA drew evidence from RCTs as well as non-comparative studies. The primary NMA reported better survival for TheraSphere than for sorafenib [HR (confidential information has been removed), 95% CrI (confidential information has been removed)], although this was not statistically significant.
Progression-free survival
Modelling of TTP for TheraSphere was implemented by fitting standard parametric functions to reported KM data from the same Phase II study used to model OS. 139 TTP was defined from first SIRT to first progression at any site. TTP, therefore, excluded mortality events, as the model only permits death following progression. As with OS, standard parametric curves were fitted to available KM data and the exponential function was selected as the most appropriate survival model based on the clinical plausibility of predicted outcomes. No other parametric functions were explored in the presented scenario analyses.
Owing to inconsistent reporting of TTP in the studies identified in the systematic review, a NMA for TTP was not feasible. Time-to-progression outcomes for comparator therapies were, therefore, based on a naive comparison, generated via median TTP and PFS data from relevant sources, which were converted to survival curves by assuming that TTP followed an exponential function. Median TTP for SIR-Spheres was based on a retrospective cohort study of patients who received SIR-Spheres,45 with TTP assumed to be the same for QuiremSpheres owing to a lack of appropriate data. Median TTP for sorafenib was based on a weighted average of values reported in TA474,11 TA55112 and a retrospective cohort study. 45 Lenvatinib TTP was sourced from TA551,12 and median TTP for regorafenib was sourced from TA555. 13 Note that all values sourced from TAs were based on PFS rather than TTP.
Health-related quality of life
The primary source of utility data used by BTG was TA551,12 which drew evidence from the REFLECT trial81 comparing lenvatinib with sorafenib, which collected EuroQol-5 Dimensions, three-level version (EQ-5D-3L), values from participants. The values used assume no differences in HRQoL between treatment arms, but do not attempt to account for differences in HRQoL as a result of AEs. This was carried out by applying a one-off utility decrement in the first cycle of the model, which was estimated by applying a 0.012 decrement per grade 3 or 4 event. Note that the BTG company submission103 erroneously reports that a 0.014 decrement was applied in the model and miscalculates the decrement to be applied in the executable model.
In addition to the above, adjustments were also made to the health state utilities to account for the impact of ageing. This was undertaken by applying a decrement to every model cycle. The decrement applied was estimated by subtracting 1 from the age- and gender-adjusted population norm. Note that the BTG company submission103 erroneously reports the decrements applied as 0.26 for the progression health state and 0.32 for the progressive disease health state, when the model applies a common decrement to both health states, which changes over time to reflect the increased age of the cohort. General population utility norms were sourced from Kind et al. 127 Utility values applied in the base-case analysis along with utility decrements are reported in Appendix 15, Table 64.
Selective internal radiation therapy procedure costs
See the review of the CTT-eligible population model (Chapter 5, Evidence used to inform the company’s model) for details of SIRT procedure costs.
Drug acquisition costs: systemic therapies
Drug acquisition costs for sorafenib, lenvatinib and regorafenib were taken from the BNF. 115 Respective dosing was 800 mg, 12 mg and 160 mg per day. Dosing was based on recommended doses for HCC patients, described in their respective European Medicines Agency summary of product characteristics (SmPC). Duration of systemic therapy was based on progression, with patients assumed to continue systemic therapy until either progressive disease or death. Appendix 15, Table 65, summarises the drug acquisition costs applied in the model.
Subsequent treatments
A proportion of the patients receiving SIRT were assumed to receive sorafenib therapy following SIRT, with patients assumed to receive sorafenib after cycle 1 until disease progression or death. In the base-case analysis, the proportion of patients assumed to receive sorafenib was 33%, based on ‘data on file’. Patients not receiving concomitant sorafenib were assumed to receive BSC. No subsequent therapies were modelled following disease progression in either model arm (SIRT or systemic therapy).
Health state costs
Resource use estimates were based on a survey of clinical experts conducted to inform resource use in TA189,140 TA47411 and TA551. 12 These included physician visits, laboratory and radiological tests, and hospital stays. Unit costs were derived from TA189,140 and updated using National Schedule of Reference Costs 2017–2018. 107
In addition to the above, a one-off cost was applied on treatment progression based on the costs applied in TA551. 124 This comprised additional laboratory and radiological tests (see Appendix 15, Table 67).
Total costs by health state are reported in Appendix 15, Table 66, along with a summary of one-off progression costs.
Adverse event costs
Unit costs associated with AEs were drawn from National Schedule of Reference Costs 2017–2018107 and are summarised in Appendix 15, Table 68. No information or justification was presented with regard to how the specific costs used were selected.
Palliative care costs
The company’s model includes a cost of £8191 to account for costs of palliation at the end of life. This was derived from a joint Nuffield Trust and Marie Curie report128 into end-of-life cancer care and inflated to 2017/18 prices. This cost was applied on a patient’s death and was applied for all modelled interventions.
Model results
The headline results presented in the BTG company submission103 are based on the deterministic version of the model. Uncertainty surrounding model parameters was explored using scenario analysis and a PSA. The executable model also included a number of DSAs that were not presented in the company submission or appendices. The company’s probabilistic results were estimated from 1000 Monte Carlo samples and were presented using CEACs and CEAFs only, with no ICERs from the probabilistic model in the company submission.
Table 25 presents the company’s base-case estimates of cost-effectiveness using the corrected version of the model at the list price for sorafenib, lenvatinib and regorafenib. Based on the probabilistic version of the company’s model, regorafenib was estimated to be the most cost-effective therapy. The results of the fully incremental analysis suggested that SIR-Spheres, QuiremSpheres and lenvatinib were dominated by one or more therapies, whereas sorafenib was extendedly dominated by TheraSphere. The estimated ICER for TheraSphere compared with regorafenib was £69,070 per QALY and estimated that TheraSphere generates an additional 0.185 QALYs at an additional cost of £12,778. The deterministic version of the model produces similar results, with an ICER relative to regorafenib of £66,624 per QALY.
| Treatment | Absolute | Incremental (relative to regorafenib) | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model (calculated by the Evidence Review Group) | |||||
| TheraSphere | 0.681 | 49,574 | 0.185 | 12,778 | 69,070 |
| QuiremSpheres | 0.466 | 37,446 | –0.030 | 650 | Dominated |
| SIR-Spheres | 0.465 | 37,406 | –0.031 | 610 | Dominated |
| Sorafenib | 0.496 | 38,977 | 0.000 | 2181 | Extendedly dominated |
| Lenvatinib | 0.526 | 61,282 | 0.030 | 24,486 | Dominated |
| Regorafenib | 0.496 | 36,796 | |||
| Deterministic model | |||||
| TheraSphere | 0.695 | 49,984 | 0.200 | 13,331 | 66,624 |
| QuiremSpheres | 0.470 | 37,496 | –0.025 | 843 | Dominated |
| SIR-Spheres | 0.470 | 37,496 | –0.025 | 843 | Dominated |
| Sorafenib | 0.500 | 39,059 | 0.005 | 2406 | Extendedly dominated |
| Lenvatinib | 0.530 | 62,647 | 0.035 | 25,995 | Dominated |
| Regorafenib | 0.495 | 36,653 | |||
Figure 13 presents the results of the DSA generated by the AG. The most influential parameters (of those assessed by the company) relate to the OS HR for regorafenib and the proportion of patients assumed to go on to receive post-SIRT sorafenib. Additional scenario analysis presented by the company showed that the estimated ICER was influenced significantly by assumptions made about post-SIRT treatment. In the presented scenario analysis, in which no concomitant sorafenib was assumed, TheraSphere was estimated to be the most cost-effective intervention, with a deterministic ICER of £5870 per QALY.
The AG questioned the face validity of the utility values applied, and were concerned that the company had made a calculation error with respect to the calculation of the utility decrements. After clarification from the company, BTG confirmed that the utility decrements applied in the model were as intended by the company. See below for further critique of the utility values applied.
Critique of the BTG conventional transarterial therapy-ineligible model
Inappropriate inclusion of regorafenib as a comparator
The base-case analysis presented in the BTG economic analysis includes three systemic therapies: sorafenib, lenvatinib and regorafenib. The AG is of the view that regorafenib should not have been included as a comparator, as it is used only as a second-line therapy following sorafenib. This is stated in the SmPC for regorafenib and NICE’s recommendation13 for regorafenib, which restricts use to patients who have been previously treated with sorafenib. 141 The AG considers it entirely reasonable to model subsequent regorafenib use following sorafenib, but it should not have been directly compared with SIRT and the other systemic therapies.
Work-up without selective internal radiation therapy procedure
An important omission from the BTG economic analysis is the costs associated with patients who received work-up but did not continue on to the SIRT procedure. In the SARAH19 and SIRveNIB21 trials, 18.6% and 28.6% of patients, respectively, received work-up but did not continue on to receive SIRT. The AG considers the cost of patients who do not proceed to SIRT treatment important, as they constitute part of the incremental costs of implementing SIRT in the NHS. The AG further notes that many of these patients will receive other active therapies instead of SIRT, and it is therefore appropriate to model the associated costs and outcomes. For example, in the SARAH trial,19 62% of patients for whom work-up failed went on to receive sorafenib. The AG, therefore, considers that the costs associated with the administration of these alternatives should also be included in the economic analysis. The AG also notes that the clinical effectiveness data used to populate the model were based on the ITT population and, therefore, the clinical outcomes of these patients for whom work-up failed are implicitly included. This is inconsistent with BTG’s stated position that only patients receiving therapy were considered.
Network meta-analysis and estimation of relative overall survival benefits
BTG conducted a NMA to compare TheraSphere with sorafenib for the treatment of unresectable HCC patients. Seven studies formed the primary network: two RCTs, one prospective study and four retrospective studies. There are differences in the studies included in the NMAs conducted by BTG and the AG. The BTG network included only studies conducted outside Asia, owing to known differences in both aetiology and treatment patterns in Asian populations. The AG identified additional studies that the company did not include or identify in its systematic literature review. 40 Unlike the AG, the company did not split the NMA into different populations of patients with differing stages of HCC disease. Therefore, the baseline BCLC stage, Child–Pugh status and the proportion of patients with PVT differed across studies. However, the population in the primary network was mostly advanced-stage HCC patients.
The validity of results from the NMA relies on the quality of the studies that make up the evidence base. However, there are considerable concerns regarding the quality of the prospective and retrospective studies. The prospective observational study by Woodall et al. ,37 comparing TheraSphere with BSC, which was excluded from the AG’s NMA, presented significant baseline imbalances and evidence of selection bias, as patients who failed to meet the pre-treatment TheraSphere requirements formed the ‘no-treatment’ arm. In addition, the retrospective studies39,44,45 were all associated with a high risk of bias as there are significant baseline imbalances, unclear reporting of blinding and missing outcome data, and were excluded from the AG’s primary NMA for these reasons.
Although the NMA reports better survival for TheraSphere than for sorafenib, this appears to be on the basis of the inclusion of a particular retrospective study, Biederman et al. ,39 which reports a very strong treatment effect on OS with TheraSphere compared with SIR-Spheres (HR 0.40, 95% CrI 0.20 to 0.78). As discussed in Chapter 3, Risk of bias, the four retrospective studies (including Biederman et al. 39) and the prospective observational study are poor quality and were rated as being at a high risk of bias, which reduces the reliability of the NMA results.
Limited exploration of uncertainty surrounding survival functions
The BTG company submission103 does not include any consideration of the uncertainty surrounding the range of potentially plausible survival functions for OS. Although a number of parametric functions were fitted to the available data for OS, the impact of alternative functions was not explored in the company’s presented scenario analyses. Furthermore, there is no functionality in the presented executable model to implement alternative survival functions.
Omission of downstaging
The AG notes that the BTG economic model did not consider the possibility that patients may be downstaged to receive curative therapy. As stated in relation to the Sirtex CTT-ineligible model, the relevance of downstaging in an advanced-HCC population is unclear, with the AG’s clinical experts suggesting that this would be a very rare occurrence in UK practice. However, downstaging was observed in a small number of patients in the SARAH trial19 and, therefore, the potential benefits of downstaging represent an important uncertainty. Therefore, although the AG recognises that the inclusion of downstaging in the company’s base case may be inappropriate, this uncertainty should have been explored in scenario analysis.
Modelling of progression-free survival
The BTG company submission states that it was not possible to obtain estimates of relative PFS from the NMA and, therefore, PFS was based on a naive comparison of reported estimates from studies identified as relevant by the company. The AG considers there to be a number of significant weaknesses in the company’s approach, and that the selected median PFS for TheraSphere lacks face validity. Although the AG acknowledges that a NMA could not be run for PFS outcomes, based on the studies included in the company’s network, the AG does not agree that a relevant network could not have been constructed (see Chapter 4). Importantly, as reported in Chapters 3 and 4, there are randomised comparisons of SIRT (SIR-Spheres) and systemic therapies (sorafenib) on which estimates of median PFS could have been based. The AG would consider such an approach preferable to the company’s naive comparison, which used populations poorly matched with the modelled population. The AG further notes that this randomised evidence was ignored in favour of studies used in the relevant NICE appraisals, which focused on populations including a significant proportion of patients with extrahepatic spread, and, with respect to regorafenib, had already failed previous sorafenib therapy.
Further to the above, the AG also questions the plausibility of the modelled median PFS for TheraSphere. The modelled value of 11 months is 3.5 times longer than the value used for SIR-Spheres (3 months) and longer than the median OS reported in the SARAH trial19 for both SIR-Spheres and sorafenib. Given the broad clinical similarity between TheraSphere and SIR-Spheres, and the lack of high-quality comparative evidence, the AG considers that it is unreasonable to assume such a large disparity in PFS.
Dosing and time on systemic therapy
Dosing of systemic therapies in the BTG economic analysis was based on the relevant SmPC, with a dose of 800 mg, 12 mg and 160 mg assumed for sorafenib, lenvatinib and regorafenib, respectively. These figures are likely to overestimate the dose received for all three drugs, as dose reductions and interruptions are common in patients receiving systemic therapy, and were observed in all relevant trial data. For example, the mean dose of sorafenib received in the SARAH trial19 was 648 mg, not 800 mg. The company’s model also does not account for the fact that the dosing of lenvatinib is weight dependent, with patients < 60 kg receiving 8 mg daily; 13% of patients in the Western subgroup of the REFLECT trial81 weighed < 60 kg.
Time on systemic treatment in the BTG economic analysis is assumed to align with PFS. This is consistent with the SmPC for both sorafenib and lenvatinib, both of which indicate that therapy should continue for as long as clinical benefit is observed, or until toxicity becomes unacceptable. However, sorafenib, lenvatinib and regorafenib are all associated with significant tolerability issues, which means that many patients discontinue therapy prior to disease progression. This is seen in the pivotal trials, in which time on systemic therapy is always less than PFS. For example, median time on sorafenib in the SARAH trial19 was 2.8 months, whereas median PFS was 3.7 months. Using PFS as an indicator of treatment discontinuation, therefore, may produce overestimates of time on treatment and consequently total drug acquisition costs for sorafenib, lenvatinib and regorafenib.
Subsequent therapy costs
The BTG economic analysis assumes that a proportion of patients receiving SIRT treatment (TheraSphere, SIR-Spheres or QuiremSpheres) move on to receive subsequent systemic therapy immediately following initial SIRT. These patients are assumed to continue therapy until disease progression. The AG considers the modelling of subsequent therapy in this way to be inconsistent with likely NHS practice and the supporting trial evidence, and that initiation of systemic therapy following SIRT would typically happen following disease progression. The AG acknowledges that, in the SARAH trial,19 a proportion (11/52, 21%) of patients did receive subsequent systemic therapy prior to progression. However, there is no evidence to suggest that this was initiated immediately following SIRT; indeed, the SARAH19 and SIRveNIB21 trial protocols stipulated that further therapy should not commence until disease progression.
A further issue relating to the company’s modelling of subsequent therapy is the assumption that patients receiving first-line sorafenib therapy will not receive further active therapy following progression. This is inconsistent with clinical practice, in which a proportion of patients will receive second-line regorafenib as per NICE’s recommendations. 13 It is also not consistent with the modelled trial evidence, as a proportion of patients in the SARAH and SIRveNIB trials went on to receive subsequent therapy following discontinuation of sorafenib.
Application of age-adjusted utilities
Similar to the BTG economic analysis in the CTT-eligible population, the estimation of age-related disutility was implemented incorrectly, resulting in health state utilities being applied that are inconsistent with values used in previous TAs, as well as values reported in the SARAH trial. 19 For further details of this error, see Chapter 5, Evidence used to inform the company’s model.
Further to the above, the AG considers age adjustment unnecessary in an advanced population in which the majority of patients die within 5 years; the application of age-adjusted utilities is unnecessary and not in keeping with norms for this type of model.
Calculation errors
A small number of calculation errors were identified and corrected as part of the AG’s assessment of the BTG economic analysis. These errors related to:
-
the estimation of the comparator TTP, which used an incorrectly estimated HR
-
the calculation of per-cycle mortality and progression, which were estimated using a monthly cycle, whereas the rest of the model used a 4-week cycle.
These errors have only a marginal effect on the reported ICER, increasing the deterministic ICER from £64,693 to £66,624 per QALY.
Conclusions from the Assessment Group’s assessment of the company’s economic evidence
Conclusions from the company submissions provided by Sirtex and BTG are provided in the following sections. Please note that Terumo did not submit any economic evidence, and so a critique is not provided.
Sirtex submission: conventional transarterial therapy-eligible population
The Sirtex submission included a CMA of SIR-Spheres, TheraSphere, TACE and DEB-TACE in the CTT-eligible population. A cost–utility analysis was not undertaken for the CTT-eligible population owing to a lack of comparative evidence available for this group of patients. The CMA considered the costs of initial treatment, hospitalisation and management of AEs. The company presented a range of scenarios for the costs of each treatment option, using alternative sources and assumptions to provide a range of plausible costs. Rather than selecting a preferred scenario, the company noted that the range of costs associated with CTT, TheraSphere and SIR-Spheres overlapped, demonstrating the comparability of treatment costs.
The AG considered the presentation of a CMA for this population to be inappropriate and potentially misleading. Such an analysis is appropriate only if there is compelling and unambiguous evidence for equivalent efficacy between interventions. Results of the AG systematic review found very little high-quality evidence in this population, and the data identified were not sufficient to demonstrate clinical equivalence or a clinical difference between treatments. A focus on treatment costs only excludes possible important outcomes regarding people who are downstaged after treatment and become eligible to receive curative therapy, or receive subsequent therapy after progression of disease.
Sirtex submission: conventional transarterial therapy-ineligible population
The Sirtex submission also included a de novo model-based health economic evaluation of SIR-Spheres versus sorafenib in the restricted low tumour burden/ALBI 1 subgroup for CTT-ineligible patients. An economic analysis for the broader population of patients with intermediate or advanced HCC was also presented in scenario analysis. The company’s model suggested that SIR-Spheres dominates sorafenib, producing more QALYs at a lower cost. The AG notes several concerns relating to the company’s submitted model, in particular (1) the questionable relevance and validity of an analysis based on the low tumour burden/ALBI 1 subgroup, (2) the relevance and methods used to model the downstaging of patients to curative therapies, (3) the modelling of OS and in particular the use of data that was not censored for downstaging to curative therapy, (4) the questionable assumptions regarding the modelling of patients who underwent work-up but did not receive SIR-Spheres, (5) the number of SIRT treatments received, particularly the assumption that patients with bilobar tumours will have both lobes treated in one session, and (6) the duration of treatment on subsequent treatment.
Given the consistent direction of bias in the issues described in the sections above, the AG considers it probable that the incremental cost-effectiveness of SIR-Spheres compared with sorafenib is considerably higher than the estimates presented in the Sirtex company submission. 102
BTG submission: conventional transarterial therapy-eligible population
For the CTT-eligible population, the BTG submission included a de novo model-based health economic evaluation of TheraSphere compared with two other SIRTs (SIR-Spheres and QuiremSpheres) and with TAE, TACE and DEB-TACE. The key benefit of SIRT assumed by this analysis was through the increased proportion of patients who achieved downstaging after treatment, which indirectly led to an increased number of patients receiving curative therapy. These outcomes were based on Lewandowski et al. ,29 a retrospective analysis of TheraSphere and TACE in patients identified as being candidates for downstaging. SIR-Spheres and QuiremSpheres were assumed to have equivalent efficacy to TheraSphere, and TAE and DEB-TACE were assumed to be equivalent to TACE.
The model estimated that the cheapest strategy was DEB-TACE, which dominated TAE and TACE. TheraSphere, QuiremSpheres and SIR-Spheres had a probabilistic ICER of £25,052 per QALY gained, compared with DEB-TACE.
The AG notes several concerns relating to the company’s analysis, in particular (1) the relevance of downstaging to transplant in this population to UK clinical practice and the use of a non-HCC-specific data set to model outcomes in these patients, (2) the failure to properly account for patients who fail the work-up procedure and do not go on to receive SIRT, (3) the significant limitations in the clinical evidence used to model the relative effectiveness of TheraSphere with other therapies, (4) the inappropriate and incorrect implementation of age-adjusted utility values and (5) the inaccurate representation of patients in the pharmacological management health state. The net effect of these issues on the estimated ICER is unclear, as many issues work in opposing directions.
BTG submission: conventional transarterial therapy-ineligible population
For the CTT-ineligible population, the BTG submission included a de novo model-based health economic evaluation of TheraSphere compared with two other SIRTs (SIR-Spheres and QuiremSpheres) and three systemic therapies (sorafenib, lenvatinib and regorafenib). The corrected version of the company’s submitted model suggests that the probabilistic ICER for TheraSphere versus regorafenib is approximately £64,513 per QALY gained.
The AG has several concerns relating to the company’s submitted model, which serve to critically undermine the validity of the presented model. Many of these concerns were also present in the CTT-eligible model presented by BTG. These concerns include (1) the inclusion of regorafenib as a direct comparator at first-line when it is licensed only for use following sorafenib therapy, (2) the failure to properly account for patients who fail the work-up procedure and do not go on to receive SIRT, (3) the significant limitations in the clinical evidence used to model the relative effectiveness of TheraSphere with other therapies, (4) the inappropriate and incorrect implementation of age-adjusted utility values, (5) the questionable assumptions regarding the modelling of time on systemic therapies and (6) the assumptions made regarding subsequent therapies received following SIRT. As with the CTT-eligible model, the net effect of these issues on the estimated ICER is unclear, as many issues work in opposing directions.
Chapter 6 Independent economic assessment: scope of analysis
As described in Chapter 2, the scope of the systematic review conducted by the AG into the relative effectiveness of SIRT covered a broad population, which the AG split into three distinct populations based on the intent of treatment and the eligibility to receive CTTs. These three populations largely corresponded to early, intermediate and advanced HCC.
Assessment of the available clinical evidence to support an economic analysis in each of these three populations, however, revealed that much of the available evidence is from poor-quality observational studies, with only a very small number of high-quality randomised trials. These limitations in the availability of evidence have a number of important implications for the scope of the economic evaluation undertaken by the AG.
As described in Chapter 3, Clinical effectiveness results, only three studies were identified for the population with early HCC (patients who are eligible for transplant and CTT). The intent of treatment in this population is primarily to act as a bridge to transplantion and, therefore, to control disease so as to allow patients to remain within transplant criteria until a donor organ becomes available. The primary benefit of SIRT or CTT in this population would, therefore, be through its capacity to sustain a greater proportion of patients through to receiving a transplant. In this context, waiting time to transplant is of crucial importance, and is a determining factor in the proportion of patients who are ultimately likely to receive transplant. However, studies identified by the AG on bridging treatment efficacy were from a US setting, where waiting list residence times are significantly longer than in the UK: roughly 6–12 months in the USA,25–28 compared with an average waiting time of approximately 50 days for HCC patients in the UK. 134 The relevance of the available data on bridging to transplant was therefore limited, and basing estimates of the relative proportion of patients successfully bridged to transplant in this context would provide potentially misleading estimates of the relative effectiveness of SIRT and CTT. Furthermore, in the UK, where wait times for transplant are relatively short, there is relatively limited scope for SIRT to offer significant health benefits and, therefore, it is unclear whether or not any additional costs associated with a SIRT procedure would be justified in this setting.
In the intermediate, CTT-eligible population, the evidence base was also considered too limited to inform a NMA (see Chapter 3, Clinical effectiveness results), with only one available randomised study providing comparative evidence on the effectiveness of SIRT with CTT. This RCT recruited 24 patients and compared SIR-Spheres with DEB-TACE. 23 In the intermediate-HCC population, the primary aim of therapy is to maintain locoregional control of the tumour to prevent progression to advanced disease, for which treatment options are more limited and survival outcomes are poor. There may also be a role for the use of locoregional therapy to downstage certain patients to make them eligible for potentially curative therapies such as liver transplant or resection. Key outcomes in this population are, therefore, TTP, as patient survival is largely dictated by progression to advanced disease, and the proportion of patients who are downstaged to curative therapy. However, the identified RCT23 provided very limited data on TTP and PFS and did not report any downstaging events. Moreover, evidence on the relative effectiveness of alternative CTT was largely limited to survival outcomes. As a consequence, any economic analysis implemented in the CTT-eligible population would have had to rely on the Pitton et al. 23 RCT alone. A model based on this single small study would, however, have generated significant challenges in populating key clinical inputs, and it would not have permitted the model to address the potential role of downstaging in this population. Furthermore, any estimates of relative benefit would have been subject to very considerable uncertainty, meaning that the results of any model would have limited value for decision-making. The AG, therefore, considered it inappropriate to develop a full economic analysis in the CTT-eligible population. The AG notes that Sirtex reached a similar conclusion regarding the availability of evidence to inform a full economic analysis, and opted instead to present a CMA. As outlined in Chapter 5, Sirtex submission: conventional transarterial therapy-eligible analysis, the AG considers the value of such an approach limited, as a CMA relies on the assumption of equal efficacy, for which there was not sufficient evidence.
In contrast with early and intermediate populations, the systematic review identified two large RCTs comparing SIR-Spheres with sorafenib in the advanced-HCC population. 19,21 The focus of the AG economic analysis is, therefore, on the CTT-ineligible population. Details of the AG’s economic analysis are outlined in Chapter 7.
Chapter 7 Independent economic assessment: conventional transarterial therapy-ineligible population
A summary of the key features of the AG economic analysis for the CTT-ineligible population is presented in Table 26. The population covered by the AG base-case analysis is Child–Pugh class A patients, who are ineligible or who have failed CTT. Scenario analysis considers two further subgroups: (1) patients who have a low tumour burden and are ALBI 1 and (2) patients with MVI.
| Model component | Description |
|---|---|
| Population | The patient population that is the focus of the cost-effectiveness analysis includes patients matching the following criteria:
|
| Intervention | SIRT:
|
| Comparator | Established clinical management without SIRT using the following targeted systemic therapies:
|
| Analysis type | Cost-effectiveness (cost–utility) analysis |
| Economic outcome | Incremental cost per QALY gained, incremental net monetary benefit |
| Perspective | NHS and PSS |
| Time horizon | Lifetime (10 years) |
| Discount rate | Annual rate of 3.5% applied to costs and QALYs |
It should be noted that these analyses are limited in that they do not include all patients who are ineligible to receive or have failed CTT, as they do not cover Child–Pugh class B patients ineligible for CTT. In practice, these patients would be ineligible to receive systemic therapy as they are not covered by the relevant NICE recommendations and, therefore, in practice would receive BSC. The clinical evidence available comparing SIRT with BSC in an advanced-HCC population is, however, very limited, and as such it is not possible to extend the economic analysis to cover this population.
The interventions considered in the AG analysis were the three SIRTs (QuiremSpheres, SIR-Spheres and TheraSphere) and the comparators were the systemic therapies sorafenib and lenvatinib. Regorafenib was not included as a comparator in the AG’s analysis as the NICE recommendation and SmPC for regorafenib in HCC permits use only in patients who have previously failed sorafenib therapy. Patients in the AG model are, however, permitted to move on to regorafenib following discontinuation of sorafenib.
In all analyses, cost-effectiveness is evaluated in terms of the incremental cost per QALY gained over a lifetime time horizon from an NHS and PSS perspective. In line with the NICE reference,142 case costs and health benefits were discounted at a rate of 3.5% per annum. Costs in the model were based on the 2017/18 price year.
Model structure
The structure of the AG model is presented in Figure 14. The AG model consists of a three-state partitioned survival model and decision tree for those intended to receive SIRT. Also presented is the structure of the downstaging scenario (see dashed lines), for which the outcomes of patients successfully downstaged to receive curative therapy are modelled separately. In the AG model, those allocated to receive SIRT enter a decision tree representing the work-up procedure. A proportion of these patients go on to receive SIRT following work-up, whereas others are not considered suitable for SIRT or otherwise withdraw consent, so can go on to receive either BSC or a systemic therapy. In the AG base case, patients then move into the main partitioned survival model.
FIGURE 14.
Overview of the CTT-ineligible population AG model structure (with dashed curative therapy scenario). (a) Work-up outcome decision tree; and (b) post-SIRT Markov model.
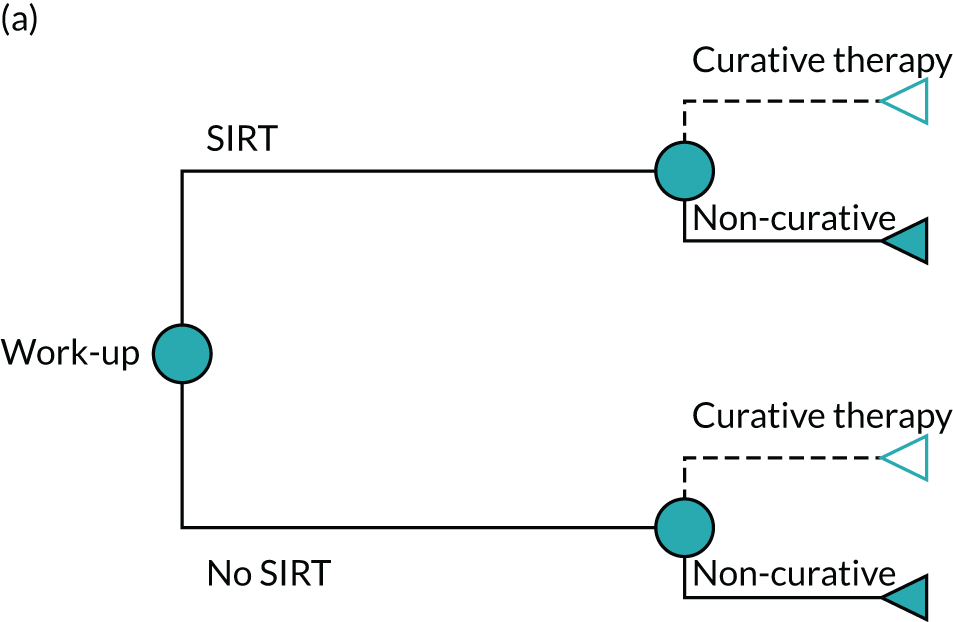

The proportion of patients who receive work-up in the AG base case is based on the SARAH trial,19 from which efficacy outcomes for these patients are drawn. Of the 226 patients who underwent work-up, 42 (18.6%) did not receive SIRT. Two further scenarios are presented in Scenario analyses, which explore the effect of using the lower and upper bounds of work-up ‘failure’ identified in the literature (5%143 to 28.6%21).
The model uses a lifetime (10-year) time horizon (< 0.1% of patients alive at 10 years in the most optimistic scenario), and takes an NHS and PSS perspective. Costs and health outcomes are discounted at a rate of 3.5% per annum, with cost-effectiveness expressed in terms of the incremental cost per QALY gained and incremental net monetary benefit (NMB). Costs were valued at 2017/18 prices.
As shown in Figure 14, the structure of the partitioned survival model is broadly similar to that adopted within both the BTG and Sirtex models (see Chapter 5, Review of economic evidence submitted by companies), consisting of three health states: (1) progression free, (2) post progression and (3) dead. For any time, t, the probability that a patient is alive and progression free is given by the cumulative survival probability for PFS, whereas the probability that a patient is alive is given by the cumulative survival probability for OS. The probability that a patient is in the post-progression state at any time, t, is given by the difference between the cumulative survival probabilities for PFS and OS. Health and cost outcomes from the partitioned survival models for each intervention were multiplied by the proportion of patients who received each within the particular treatment arm as per the decision tree.
As with the Sirtex model, HRQoL is defined according to the presence or absence of disease progression as well as treatment received. The model includes costs associated with SIRT procedures (work-up costs, acquisition costs and procedure costs), drug acquisition, health-state costs (consultant-led outpatient visits, nurse-led outpatient visits, electrocardiography, blood tests and CT scans), costs associated with managing grade 3 or 4 AEs, BSC-related costs (consultant-led outpatient visits, CT scans, MRI scans, specialist palliative care visits and palliative radiotherapy) and end-of-life care costs.
Model input parameters
A summary of the data sources used to populate the AG’s base-case model is presented in Table 27. These are discussed in greater depth over the following sections.
| Model parameter | Evidence source |
|---|---|
| OS | Parametric survival models fitted to pooled OS data from the SARAH19 and SIRveNIB21 trials for both SIR-spheres (per protocol) and sorafenib (ITT). A HR from the AG’s NMA was applied to the sorafenib OS curve to estimate OS for lenvatinib. The OS for patients who received work-up but were ineligible to receive SIRT was modelled using the observed KM data from SARAH19 |
| PFS | Parametric survival models fitted to pooled PFS data from the SARAH19 and SIRveNIB21 trials for both SIR-spheres and sorafenib. A HR from the AG’s NMA was applied to the sorafenib PFS curve to estimate OS for lenvatinib |
| Health state utilities |
Utilities were generated by Sirtex from SARAH trial19 data, and were applied by treatment class (SIRT/systemic therapy) Pre progression: EORTC QLQ-C30 scores taken from the post hoc analyses of the SARAH trial19 for the per-protocol population were mapped to EQ-5D using a mapping algorithm developed by Longworth et al. 113 Post progression: EORTC QLQ-C30 scores taken from the post hoc analyses of the SARAH trial19 for the per-protocol population were mapped to EQ-5D using the algorithm developed by Longworth et al. 113 |
| Proportion receiving SIRT | The proportion receiving SIRT after work-up was based on the full SARAH trial19 population. Number of administrations of SIRT was based on the SARAH trial19 |
| SIRT costs |
Acquisition cost: Sirtex company submission,102 BTG company submission103 and Terumo company submission104 Work-up costs: BTG-elicited values from The Christie NHS Foundation Trust (personal communication) Procedure costs: National Schedule of Reference Costs 2017–2018107 |
| Systemic therapies costs |
Sorafenib and lenvatinib: BNF115 Dosing of sorafenib: SARAH trial19 Dosing of lenvatinib: REFLECT81 Western subgroup Duration of sorafenib: SARAH trial19 Duration of lenvatinib: PFS HR from REFLECT. 81 Applied to SARAH,19 sorafenib time on treatment |
| Subsequent treatment costs | BNF,115 eMIT116 and TA555 (regorafenib)13 |
| AE costs | AEs experienced by ≥ 5% of the population were modelled, with rates drawn from the SARAH19 and REFLECT81 trials. Costs were drawn from National Schedule of Reference Costs 2017–2018,107 with cost categories based on NICE TA47411 and 55112 |
| Health state costs | Sirtex survey of clinical experts and National Schedule of Reference Costs 2017–2018107 |
Treatment effectiveness
The base-case analysis used data from the SARAH,19 SIRveNIB21 and REFLECT trials. 81 Scenario analyses also drew on a number of observational comparisons of SIR-Spheres and TheraSphere (see Chapter 4, Network 3: adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapies, for details).
The comparison of SIR-Spheres with sorafenib was based on pooled data from the SARAH and SIRveNIB trials. Modelled data from SARAH were supplied by Sirtex for both PFS and OS, and data were extracted from published literature sources from SIRveNIB.
The source of modelled survival data from the SARAH and SIRveNIB trials differed according to therapy received. For patients receiving sorafenib, OS and PFS outcomes were based on the ITT populations (sorafenib, n = 400), whereas OS and PFS outcomes for patients receiving SIR-Spheres are modelled based on the per-protocol population of each trial (SIR-Spheres, n = 304). This is done to account for the proportion of patients who fail the SIRT work-up procedure, and subsequently do not undergo the main SIRT procedure. The outcomes of patients who fail the work-up procedure are modelled independently, and are based on near-complete KM data from the SARAH trial (work-up failures, n = 42). The proportion of patients failing the work-up procedure is based on the SARAH trial. The DSA included a range of estimates for work-up failure, based on the number of work-up failures reported in SARAH and SIRveNIB and other estimates provided by Sirtex. To avoid the double-counting of patients who are downstaged to receive curative therapies, the data included from SARAH, for both SIR-Spheres and sorafenib, are censored for downstaging. There was no downstaging reported in the SIRveNIB trial publication21 and no patients received subsequent therapies that could be considered ‘curative’, so it was assumed that no patients were downstaged to receive curative therapies in these data.
The comparative effectiveness of lenvatinib was drawn from the NMA presented in Chapter 4, Results. The HR for lenvatinib versus sorafenib was applied to the Weibull curve fitted to the sorafenib data drawn from the SARAH and SIRveNIB trials. Proportional hazards is, therefore, assumed between sorafenib and lenvatinib.
In the AG’s base-case analysis, equivalence is assumed between the SIRTs owing to a lack of randomised evidence on the relative effectiveness of each SIRT. An exploratory scenario analysis is also presented in which the effectiveness of TheraSphere was based on two non-randomised comparative studies39,40 (SIR-Spheres, n = 34; TheraSphere, n = 78), with a HR versus SIR-Spheres drawn from the NMA. In this scenario, the HR is applied to the modelled parametric functions fitted to the pooled SIR-Spheres data and, therefore, proportional hazards is assumed for this comparison (see Extrapolation of progression-free survival and overall survival evidence for consideration of the plausibility of this assumption).
In addition to the base-case analysis in which the modelled population was based on pooled analysis of the SARAH and SIRveNIB trials, additional scenario analysis was implemented in a number of alternative populations. To account for uncertainties in the relevance of the Asia-Pacific population to UK practice, a scenario was implemented using data only from the SARAH trial. Two further subgroup analyses based on the SARAH trial were also considered: the restricted low-tumour burden and ALBI 1 subgroup (SIR-Spheres, n = 28; sorafenib, n = 44), and patients with MVI (SIR-Spheres, n = 64; sorafenib, n = 81). In both subgroup analyses, the comparison between SIR-Spheres and sorafenib is made using data drawn from the relevant subgroup of the SARAH trial only. Appropriate IPD were requested by the AG for these subgroups of the SIRveNIB trial but Sirtex had only limited access to the IPD from the SIRveNIB trial and did not have subgroup data from all enrolling centres. Subgroup data were not available to support the comparative effectiveness of lenvatinib and TheraSphere. This scenario, therefore, uses only data for SIR-Spheres and sorafenib, assuming equivalent efficacy across SIRTs and between lenvatinib and sorafenib.
Extrapolation of overall survival and progression-free survival evidence
For each data set, model selection was conducted in line with the process described in the NICE DSU Technical Support Document 14. 117 To assess the appropriateness of alternative parametric models, log-cumulative hazard plots were produced to illustrate and assess the hazards observed in the trial. Curve fitting was conducted using the ‘survival’ and ‘flexsurv’ packages in R (The R Foundation for Statistical Computing, Vienna, Austria). Exponential, Weibull, Gompertz, log-normal, log-logistic, gamma and generalised gamma models were considered.
The AIC and BIC fit statistics were examined to assess the comparative internal validity of competing models. The final choice of models for the economic analysis was made on the basis of fit to the observed data as well as consideration of the clinical plausibility of candidate models.
Overall survival
The analysis of OS for the base-case analysis was based on time-to-event data from the SARAH trial supplied by Sirtex, and KM curves from the SIRveNIB trial. 21 Pooled KM curves for the base-case population are presented in Appendix 16, Figures 26 and 27. Survival estimates can be found in Appendix 16, Table 71.
Standard parametric survival functions were fitted to the survival data available for each of the considered populations, and log-cumulative hazard plots were generated to assess any changes in hazards over time (see Appendix 16, Figure 28). Plots of each of the fitted parametric models with the observed KM OS curves are presented in Figures 15 (SIR-Spheres) and 16 (sorafenib). Model fit statistics are summarised in Appendix 16, Table 72, which showed that the generalised gamma model had the best fit, with the log-normal and log-logistic curves also having similar statistical fit, thereby providing little justification to discriminate between these models on this basis of fit statistics. The generalised gamma, log-normal and log-logistic models are, however, all accelerated failure time models and, as such, a HR cannot be applied to estimate outcomes for lenvatinib patients, and would likewise not permit scenarios in which differential outcomes are assumed for TheraSphere, which would similarly require the application of a HR. To accommodate the use of HRs, the AG base-case analysis, therefore, selected the Weibull function, which has the best statistical fit from the remaining curves, and was considered the most clinically plausible. The AG considered this reasonable given the limited data to accommodate accelerated failure time functions and the small variation in predicted incremental survival across all six functions, but acknowledges this as a limitation of the presented base-case analysis. Scenario analysis is, therefore, presented, in which the generalised gamma, log-normal and log-logistic functions are used to model OS. In these scenarios, equivalence is assumed between sorafenib and lenvatinib.
FIGURE 15.
Extrapolation of OS: SIR-Spheres.

FIGURE 16.
Extrapolation of OS: sorafenib.
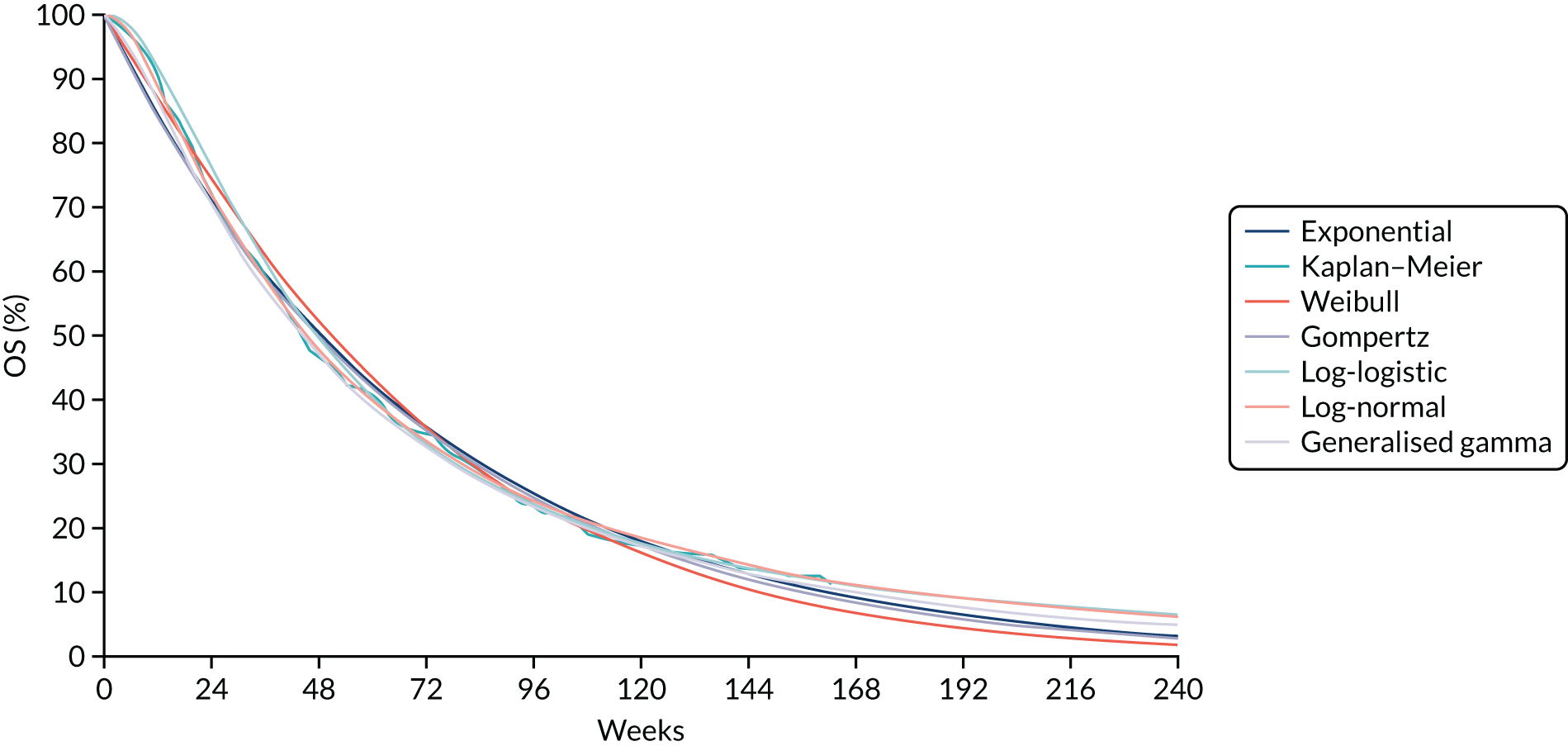
For scenarios run on the SARAH trial19 subpopulations described previously, the Weibull function was retained to model OS outcomes. Fit statistics for the SARAH trial whole population, low tumour burden/ALBI 1 subgroup and no-MVI subgroup are reported in Appendix 16, Table 74. Plots of each of the fitted parametric models with the observed KM OS curves are presented in Appendix 16, Figures 30 and 31 (SIR-Spheres) and Figures 32 and 33 (sorafenib). In all three scenarios, the Weibull function had a good statistical and visual fit to the observed data.
Progression-free survival
The analysis of PFS for the base-case analysis was based on supplied time-to-event data from the SARAH trial19 and KM curves from the SIRveNIB trial. 21
Similar to the approach previously described for OS, standard parametric survival functions were fitted to the survival data available for each of the considered populations (Figures 17 and 18), and log-cumulative hazard plots generated to consider the change in hazards over time (see Appendix 16, Figure 29). Plots of each of the fitted parametric models with the observed KM OS curves are presented in Appendix 16, Figures 34 and 35 (SIR-Spheres) and Figures 36 and 37 (sorafenib). Similar to OS, model fit statistics for the generalised gamma, log-normal and log-logistic functions were superior to other functions (see Appendix 16, Table 73). These functions were, however, rejected to accommodate the application of a HR for lenvatinib and the implementation of scenarios assuming differential effectiveness for TheraSphere. The Weibull function was, therefore, selected in the AG base-case analysis as this had the best statistical and visual fit to the observed data and was considered clinically plausible.
FIGURE 17.
Extrapolation of PFS: SIR-Spheres.

FIGURE 18.
Extrapolation of PFS: sorafenib.

Overall survival for patients downstaged to curative therapy
The base-case analysis does not allow for downstaging to curative therapies, owing to uncertainties over whether or not this is realistic in a population of patients with advanced disease. A number of scenarios are presented in which downstaging is allowed for. The proportion of patients downstaged is based on the values reported in the SARAH trial19 and varied depending on the efficacy subgroup used (see Appendix 16, Table 69). Outcomes for patients downstaged to curative therapy were based on a US prospective cohort study,112 which recruited 267 patients with HCC, including 191 with intermediate and advanced disease. This study compared outcomes for patients who had received palliative care with those who received potentially curative therapies (liver transplantation, surgical resection or tumour ablation). Using Cox multivariate proportional hazards, the HR for OS with potentially curative treatments versus non-curative treatment was 0.29 (95% CI 0.18 to 0.47). This HR was applied to the pooled sorafenib ITT arms of the SARAH and SIRveNIB trials in all scenarios. This was carried out to prevent the outcomes of downstaged patients varying depending on the patient population selected or by treatment arm; advice from clinical advisors to the AG suggested that outcomes post-curative therapy would be similar regardless of patient characteristics or treatment received to achieve downstaging. The sorafenib ITT arm was used as this was considered to best match care received in the analysed patient cohort, and is most representative of the current standard of care in UK practice.
Adverse event rates
The probability of experiencing grade 3 or 4 AEs for SIR-Spheres and sorafenib was taken directly from the per-protocol population of the SARAH trial. 19 Based on clinical advice received by the AG, AE rates for TheraSphere and QuiremSpheres were assumed to be the same as for SIR-Spheres. AE rates for lenvatinib were drawn from the REFLECT trial. 81 See Appendix 16, Table 70, for rates applied.
Health-related quality of life
Literature review and mapping of health-related quality-of-life estimates
A targeted review of published studies reporting utility estimates for patients with HCC or cirrhosis was undertaken to supplement data extracted from studies on SIRT and its comparators. Details of the search strategy used are described in Appendix 3. The objective of these searches was to identify health state utilities of patient populations that may not have been captured in studies included in the main systematic reviews. The required utilities included:
-
decompensated cirrhosis (any cause)
-
post-CTT disutility
-
post-resection disutility
-
pre- and post-transplant utilities.
The identified studies recorded HRQoL using a number of tools, namely Short Form questionnaire-36 items (SF-36) and EORTC QLQ-C30. NICE prefers the use of generic preference-based measures (i.e. EQ-5D) for the calculation of health state utilities. Therefore, mapping algorithms typically based on multinomial regression model coefficients can be used to transform disease-specific measures of health status into a EQ-5D-based utility score. Domain scores for relevant populations were mapped onto EQ-5D using the two-part beta model as developed by Woodcock and Doble144 for EORTC QLQ-C30 scores, and a model developed by Rowen et al. 145 was used to transform SF-36 outcomes.
Modelled health state utilities
The AG’s base-case model for CTT-ineligible patients applies different health state utilities based on the type of therapy received to reflect any differences in their respective AE burdens. Because utilities were drawn from patients in the SARAH trial, disutilities associated with type and length of any AEs were assumed to have been captured, and thus were not considered separately. In the absence of any evidence suggestive of a difference in HRQoL between the three SIRTs, the AG has assumed that patients experience the same quality of life regardless of whether they received SIR-Spheres, TheraSphere or QuiremSpheres. Likewise, the HRQoL estimates associated with the systemic therapies, namely sorafenib and lenvatinib, are assumed to be the same as one another, but marginally lower than those applied to SIRT, as observed in the SARAH trial19 (see Table 28). An additional scenario in which health state utilities from the lenvatinib technology appraisal are applied is presented in Scenario analyses.
Age-related disutilities
Age-adjusted UK population norms from Szende et al. 146 were applied to the utility values included in the model. Age-related decrements were estimated in the form of a multiplier, with decrements applied relative to the populations on entering the model. This allows for the trial-derived utilities applied in the model to account for age-related decline in HRQoL as the population ages over time.
Selective internal radiation therapy health state utilities
The health state utilities associated with SIRT in the CTT-ineligible model were based on the per-protocol subgroup of the SARAH trial as calculated by Sirtex in its evidence submission (see Chapter 5, Evidence used to inform the company’s model, for details). EORTC QLQ-C30 summary scores were mapped to EQ-5D using the algorithm developed by Longworth et al. ,113 and utilities were calculated based on UK general population weights.
The per-protocol utilities were considered to better reflect the HRQoL associated with SIRT than those derived from the ITT population, as 22.4% of patients randomised to SIRT did not receive SIRT in the SARAH trial. These patients may have received other systemic therapies or BSC, or were otherwise too unwell to receive SIRT; thus, the ITT utility values may not have represented those of a SIRT-treated population. There were no further utility decrements applied to these utilities as these are likely to have been captured in the SARAH trial results. The health state utilities applied in the model are presented in Table 28.
| Health state | Utility | ||
|---|---|---|---|
| SIRT | Systemic therapy | Work-up: no SIRT | |
| PFS | 0.710 | 0.699 | 0.703 |
| Progressive disease | 0.668 | 0.657 | 0.661 |
| Post transplanta | 0.710 | 0.710 | 0.710 |
Systemic therapy health state utilities
Health state utilities applied to modelled patients receiving the systemic therapies sorafenib and lenvatinib were taken from the per-protocol subgroup of sorafenib patients in the SARAH trial. 19 The difference in utility between SIRT and sorafenib in this subgroup was 0.011, which the AG considered to account sufficiently for the ostensibly greater burden of AEs associated with these drugs. Utilities applied to patients who received work-up but ultimately did not receive SIRT were weighted by the proportion on systemic therapy versus BSC (61.9% and 38.1%, respectively). This assumes that patients not on systemic therapy had a utility equivalent to those on SIRT, which may overestimate the HRQoL of BSC patients, as a proportion were likely to have been too unwell to receive systemic therapy.
Post-transplant health state utilities
The AG scenarios 6 and 10 include the possibility for downstaging; therefore, post-transplant utilities were considered for use in the model. Pre-transplant health state utilities are assumed to be equal to those experienced in pre-progression for SIRT, systemic therapies and BSC. Post-transplant health state utilities are assumed to be equal to those experienced on SIRT, regardless of which treatment a patient received before downstaging to transplant. However, it is likely that patients who received a transplant may have a better HRQoL than the per-protocol population of the SARAH trial.
Despite multiple studies showing that recipients of liver transplant enjoy increased HRQoL post transplant in comparison with pre transplant,113,147–149 a lack of generalisability between these studies and the population included in the model renders the absolute utility values reported in the literature too uncertain for inclusion. Studies also show that HRQoL remains lower for liver transplant recipients than for healthy patient controls. 150–152 However, as with the pre- and post-transplant utilities, there is insufficient evidence to suggest that these studies are generalisable to the modelled population. Given the lack of evidence to definitively suggest that utility values in the post-transplant HCC population are lower than in the general population, the AG believes that the utility values observed in the general population represent the upper bound of the utility expected in the post-transplant population.
Sources of resource utilisation and cost data
A targeted review of published studies reporting resource use and cost data for patients with HCC or cirrhosis was undertaken. Details of the search strategy used are described in Appendix 4. This review, however, identified little in the way of published literature. Resource use and cost inputs used in the AG’s economic model were, therefore, derived primarily from targeted literature searches, previous NICE technology appraisals and the estimates presented in the companies’ evidence submissions for the present appraisal. Overall costs are determined by treatment costs (acquisition, procedures and monitoring), changes in health service utilisation driven by disease status (i.e. progression free, progressed disease and death) and AE management. The assumptions applied to each category are discussed in the following sections. Note that confidential Patient Access Scheme (PAS) discounts are available but not included here for QuiremScout, sorafenib, lenvatinib and regorafenib. Please refer to Appendix 17 for results including all PAS discounts. A summary of the AG model cost inputs is presented in Summary of Assessment Group base-case analysis inputs and assumptions.
Treatment costs and resource use
Work-up costs and number of procedures
Patients allocated to receive SIRT must first undergo a work-up procedure to assess their suitability for treatment with SIRT, and to plan the procedure through angiographic evaluation and occlusion of any vessels that could carry microspheres away from the liver to the gut. Although work-up is a one-off procedure, those patients who required a second SIRT procedure owing to an unsuccessful or incomplete first procedure are likely to need a second work-up.
In the SARAH trial,19 17 of the 184 patients who received SIRT required re-treatment owing to an unsuccessful or incomplete first procedure (nine received a second work-up but were not re-treated). Therefore, patients who received any of the SIRTs incurred the cost of 1.09 work-up procedures to account for re-treatment. As the model independently considered the costs and outcomes for patients who underwent work-up but ultimately did not receive SIRT, these individuals were assumed to receive 1.0 work-up procedures. The AG’s base case assumed that 18.6% of patients who underwent work-up did not go on to receive SIRT in line with the SARAH trial19 data. However, in recognition of the uncertainty around this value, a number of alternative scenarios are presented in Sensitivity analyses results.
Work-up costs used in the AG base case were based on the values BTG elicited from The Christie NHS Foundation Trust (see Appendix 15, Table 60). The largest expenditures were staff costs and SPECT/CT. The total cost of a single work-up procedure for SIR-Spheres and TheraSphere used in the AG model was £860.32, and the work-up cost of £5178.32 for QuiremSpheres comprised the list price of QuiremScout and the BTG-elicited value excluding the £74 cost of the technetium-99m MAA agent. This does not include the PAS discount available for QuiremScout.
Selective internal radiation therapy treatment costs and number of procedures
Patients in the AG model received an average of 1.21 SIRT procedures. This is based on the assumption that patients requiring bilobar treatment will require two separate SIRT procedures, separated by a few weeks (as per the SARAH protocol153), and that patients will be re-treated owing to an incomplete or unsuccessful first treatment. The clinical advisors to the AG stated that it would be very unlikely that both lobes would be treated in the same treatment session in UK practice owing to an increased risk of REILD. SIRT patients in the SARAH study19 had 1.28 separate SIRT treatments on average [222 treatments, 173 patients (one or two treatments only)]. This broadly reflects the results of the Sirtex resource use survey (1.2 treatments per patient). This value excludes the 11 patients who had three separate SIRT treatments, and includes only one procedure for the nine patients who received a second treatment owing to disease progression, as it was unclear whether or not this would be permitted in UK practice.
The acquisition cost of a single SIRT treatment was taken from each company submission: SIR-Spheres, £8000; TheraSphere, £8000; and QuiremSpheres, £9896.
The cost of the SIRT procedure applied in the AG model was taken from National Schedule of Reference Costs 2017–2018107 (YR57Z). The average cost of ‘Percutaneous, Chemoembolisation, or Radioembolisation, of Lesion of Liver’ was £2790. This cost was incurred for each separate SIRT administration for patients receiving TheraSphere and QuiremSpheres in the AG model. The Sirtex company submission102 stated that SIR-Spheres administration procedures use intermittent contrast-medium injection to assess the distribution of the microspheres under radiography over the course of approximately 1 hour. The AG, therefore, included an additional cost of £209 for the SIR-Spheres administration procedure (RD32Z – Contrast Fluoroscopy Procedures with duration of more than 40 minutes), for a total of £2999.
Costs of systemic therapies
The pack costs for sorafenib (£3576.56), lenvatinib (£1437.00) and regorafenib (£3744.00) were taken from the BNF. 115 The confidential PAS discounts available for sorafenib, lenvatinib and regorafenib are not included in this report. For results of the AG’s economic analysis that include these discounts, please refer to Appendix 17.
The daily dose of sorafenib used in the AG base case was based on the SARAH trial19 (648.5 mg), and the mean time on treatment was calculated by applying an exponential function to the median time on treatment reported in the SARAH trial19 (exponential mean 122.95 days).
The base-case daily dose of lenvatinib was 10.2 mg per day, based on the Western subgroup of the REFLECT trial81 for lenvatinib. This value was considered by the technology appraisal committee in TA55112 to better represent the average weight-based dose used in UK practice. The AG considered the time on treatment reported in the REFLECT trial81 for lenvatinib to be excessively long compared with SARAH,19 and reflective of differences in the baseline characteristics of the populations recruited to these trials. To avoid inflating the relative cost of lenvatinib, the AG applied the reported HR of PFS between lenvatinib and sorafenib in REFLECT to the SARAH time on treatment to produce an estimate of 124.07 days on treatment.
Wastage was accounted for in the AG model using the simple assumption that if a new pack was started then in the case of treatment discontinuation, the remainder could not be used to treat other patients. However, this may be a conservative assumption, as it was reported in TA55513 that many centres have measures in place to reduce wastage of expensive cancer treatments, such as issuing only a 1-month supply of tablets at a time (approximately one pack of sorafenib). However, as it generally cannot be predetermined when therapy will be discontinued owing to AEs, death or non-compliance, it can be reasonably assumed that some wastage will occur.
Cost of subsequent treatment
The interventions used following first-line treatment in the SARAH trial19 were not representative of current UK practice; however, as the efficacy data used in the model are derived from these patients, the trial values are most appropriate. Therefore, the proportion of patients who received subsequent systemic therapy (98% sorafenib) following SIRT in the SARAH trial19 (28.8%) was used to estimate the size of this population in the AG model. The AG was advised that current NICE recommendations mean that lenvatinib is rarely used in practice, as this would preclude second-line use of regorafenib. Therefore, 95% of patients continuing to subsequent systemic therapies following SIRT treatment are assumed to receive sorafenib, and 5% are assumed to receive lenvatinib.
As a number of chemotherapeutic/systemic agents administered to patients following sorafenib in the SARAH trial19 have now been displaced in practice by regorafenib, or are otherwise no longer in use, the AG model assumes that the proportion of those who received systemic therapies after sorafenib in the trial (12.04%) would receive regorafenib in UK practice. A small proportion (3.47%; i.e. 12.04% of 28.8%) of SIRT patients also receive regorafenib following second-line sorafenib treatment. Duration of therapy and dose intensity of each of the three systemic agents modelled is assumed to be the same as first-line treatment, whereas regorafenib is assumed to have the same time on treatment as sorafenib (122.95 days), with a mean daily dose of 160 mg (RESORCE trial). 101
Disease management costs
There are a number of issues with the health state unit costs used in previous technology appraisals in this indication, which precluded their use in the AG base case. The primary concern with these costs is that the original resource use surveys given to clinicians were based on the ongoing costs associated with sorafenib treatment. The resource use implications for systemic therapies may be very different with regard to monitoring and diagnostic testing to those for SIRT as a one-off procedure; therefore, these values may overestimate the disease management costs associated with the PFS health state for SIRT patients. Furthermore, the committee-preferred resource use data used in TA551124 were collated from two resource use surveys conducted 10 years apart, generating very different estimates that may reflect differences in practice, costs and experience. As targeted therapies such as sorafenib were not yet in use at the time of this first survey, it is unlikely that these values are sufficiently representative of current practice.
In the light of these limitations, the AG used the results of a resource use survey conducted by Sirtex, which elicited information from 11 clinicians on the frequency and type of medical staff contact, monitoring and follow-up, hospitalisation frequency and length, and any use of PSS. Resource use pre progression, post progression and on progression were reported separately. Unit costs for each resource use item were derived from National Schedule of Reference Costs 2017–2018107 and Personal Social Services Research Unit (PSSRU). 106 Differential costs were applied for systemic therapy patients during pre-progression, reflecting higher levels of ongoing diagnostic testing and additional follow-up contact.
The per-cycle post-progression costs applied in the AG model are significantly lower than those used in TA551124 (£229.69 vs. £1268.16). This was driven primarily by greatly reduced use of hospital- and social care-based palliative care on progression since the original resource use survey. The health state costs used in the AG model are presented in Table 29.
| Cost item | Cost (£) | |||
|---|---|---|---|---|
| Pre-progression post SIRT (per cycle) | Pre-progression on systemic therapy (per cycle) | On progression (one off) | Progressive disease (per cycle) | |
| Medical staff contact | 47.30 | 58.18 | 54.51 | 102.55 |
| Diagnostic procedures | 59.92 | 61.90 | 41.07 | 2.83 |
| Inpatient care | 3.13 | 9.33 | 0.00 | 36.11 |
| PSS | 2.68 | 2.68 | 0.00 | 88.20 |
| Total | 113.03 | 132.10 | 95.57 | 229.69 |
A scenario that instead uses the committee-preferred costs from the lenvatinib appraisal is presented in Sensitivity analyses results.
Adverse event costs
Costs associated with the management of AEs were derived from previous NICE TAs of HCC,11–13 using the latest National Schedule of Reference Costs 2017–2018107 values or costs inflated to the 2018 cost year, where applicable. The AG base case used AE incidence rates from the SIR-Spheres arm of the SARAH trial19 for the three SIRTs, and from the sorafenib arm of this trial for sorafenib. AE rates for lenvatinib were taken from the REFLECT trial. 81 For patients who received work-up but did not progress onto SIRT, the proportion of patients who received sorafenib incurred sorafenib AE management costs.
A full list of AE costs used in the AG model is presented in Appendix 16, Table 75.
Summary of Assessment Group base-case analysis inputs and assumptions
A summary of the resource use assumptions and costs applied in the AG base-case analysis is presented in Table 30.
| Parameter | Treatment | Model input | Reference |
|---|---|---|---|
| Proportion of work-ups leading to SIRT | SIR-Spheres | 81.4% | SARAH19 |
| TheraSphere | 81.4% | SARAH19 | |
| QuiremSpheres | 81.4% | SARAH19 | |
| Treatment of SIRT work-up failure patients | Sorafenib | 61.9% | SARAH19 |
| BSC | 38.1% | AG assumption | |
| Mean number of work-ups (treated patients) | SIR-Spheres | 1.09 | SARAH19 |
| TheraSphere | 1.09 | SARAH19 | |
| QuiremSpheres | 1.09 | SARAH19 | |
| Mean number of SIRT procedures | SIR-Spheres | 1.28 | SARAH19 |
| TheraSphere | 1.28 | SARAH19 | |
| QuiremSpheres | 1.28 | SARAH19 | |
| Subsequent systemic therapies | |||
| Post SIRT | Sorafenib | 27.4% | SARAH19/AG assumption |
| Lenvatinib | 1.4% | AG assumption | |
| Regorafenib (third line) | 3.3% | AG assumption | |
| BSC | 71.2% | AG assumption | |
| Post sorafenib | Regorafenib | 12.0% | AG assumption |
| BSC | 88.0% | AG assumption | |
| Post lenvatinib | BSC | 100% | AG assumption |
| Subsequent curative therapies | |||
| Liver transplant | £16,556.07 | National Schedule of Reference Costs 2017–2018 107 | |
| Resection | £9676.59 | National Schedule of Reference Costs 2017–2018 107 | |
| Ablation | £2344.55 | National Schedule of Reference Costs 2017–2018107 (YG01A/YG01B) | |
| Treatment cost inputs | |||
| Work-up | SIR-Spheres | £860.32 | BTG elicitation (The Christie NHS Foundation Trust, personal communication) |
| TheraSphere | £860.32 | BTG elicitation (The Christie NHS Foundation Trust, personal communication) | |
| QuiremSpheres | £5178.32 | BTG elicitation (The Christie NHS Foundation Trust, personal communication); Terumo submission104 | |
| Procedure | SIR-Spheres | £2999.00 | National Schedule of Reference Costs 2017–2018107 (YR57Z and RD32Z) |
| TheraSphere | £2790.00 | National Schedule of Reference Costs 2017–2018107 (YR57Z) | |
| QuiremSpheres | £2790.00 | National Schedule of Reference Costs 2017–2018107 (YR57Z) | |
| Acquisition (list price) | SIR-Spheres | £8000.00 | Sirtex submission102 |
| TheraSphere | £8000.00 | BTG submission103 | |
| QuiremSpheres | £9896.00 | Terumo submission104 | |
| Sorafenib | £3576.56 | BNF115 | |
| Lenvatinib | £1437.00 | BNF115 | |
| Regorafenib | £3744.00 | BNF115 | |
| Management costs | |||
| AE costs (total) | SIR-Spheres | £477.69 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 |
| TheraSphere | £477.69 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 | |
| QuiremSpheres | £477.69 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 | |
| Sorafenib | £932.79 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 | |
| Lenvatinib | £542.08 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 | |
| Sorafenib/BSC (work-up/no SIRT) | £577.40 | NICE TA474,11 TA514,13 TA535,154 TA551;124 National Schedule of Reference Costs 2017–2018107 | |
| Health state costs (per cycle) | PFS (SIRT) | £113.03 | Sirtex expert elicitation; National Schedule of Reference Costs 2017–2018,107 PSSRU 2018106 |
| PFS (systemic therapies) | £132.10 | Sirtex expert elicitation; National Schedule of Reference Costs 2017–2018,107 PSSRU 2019106 | |
| On progression | £95.57 | Sirtex expert elicitation; National Schedule of Reference Costs 2017–2018,107 PSSRU 2020106 | |
| Post progression | £229.69 | Sirtex expert elicitation; National Schedule of Reference Costs 2017–2018,107 PSSRU 2021106 | |
| End of life | £8191.00 | Georghiou and Bardsley128 | |
| Postcurative therapy (scenario) | £113.03 | Sirtex expert elicitation; National Schedule of Reference Costs 2017–2018107 | |
Analytic methods
Base-case analysis
The AG produced fully incremental ICERs for each strategy included in the model; however, this approach generated a number of ICERs expressed in terms of dominance owing to the close similarity of health outcomes predicted for the SIRTs.
The AG, therefore, considered a net benefit framework to be the most appropriate approach to present the relative cost-effectiveness of the three SIRTs with existing practice. This method is often preferred when there are a number of technologies under comparison, particularly when incremental costs and benefits are very similar. Technologies with identical health outcomes and marginal differences in costs are often labelled as ‘dominant/dominated’ using incremental cost-effectiveness analysis with conventional decision rules. Considering net health benefit instead permits a more informative comparison of the effect of alternative strategies.
Net monetary benefit is calculated using a rearrangement of the ICER formula, but inherently compares the incremental health gain with the comparator with a willingness-to-pay (WTP) threshold. The NMB formula thereby assigns a value to the additional QALYs generated by an intervention, and considers the opportunity cost associated with generating these health benefits. The formula used to define NMB is λ × ΔE – ΔC, where the difference in health effects (ΔE) is multiplied by the selected WTP threshold (λ) minus the difference in costs (ΔC) (i.e. £30,000 in the results presented below). Using this approach, if an intervention has an incremental NMB of > 0, then it would be considered more cost-effective than the baseline option, in this case the least costly option. NMB results (including PAS discounts) at a £20,000 and £30,000 threshold are also presented in Appendix 17.
The AG model accounted for uncertainty using probabilistic and deterministic sensitivity analyses. PSA was undertaken using simple Monte Carlo sampling methods, using 20,000 samples for the AG base case and 5000 samples in the primary scenario analyses. The choice of distribution to reflect uncertainty around each parameter was selected for each according to its statistical suitability. To account for uncertainty around the parametric survival models fitted to OS and PFS, outcomes were sampled via Cholesky decomposition using the variance–covariance matrices produced during survival modelling. When a HR was used to estimate PFS and OS outcomes, alternate values were drawn in each model iteration from the NMA output from WinBUGS (CODA) to model uncertainty in the predicted treatment effects.
Model validation
The AG adopted a number of approaches to ensure the credibility and validity of the model. These included scrutiny of the implemented model coding and formulae by two modellers, black-box testing in which the predictive validity of parameter inputs (e.g. that increasing effectiveness of the treatment lowers cost-effectiveness) was assessed, checking the accuracy of all model inputs against the original sources and consultation with clinical experts on key assumptions (see Acknowledgements).
Results of the independent economic assessment
Base-case results
The deterministic and probabilistic fully incremental results of the AG’s base-case analysis (excluding confidential PAS discounts for QuiremScout, sorafenib, lenvatinib and regorafenib) are presented in Table 31. The probabilistic results were based on 20,000 model iterations.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| AG deterministic base case | ||||||||
| TheraSphere | 29,888 | 1.110 | 0.764 | |||||
| Lenvatinib | 30,005 | 1.183 | 0.805 | 117 | 0.04 | 2911 | 1090 | 2911 |
| SIR-Spheres | 30,107 | 1.110 | 0.764 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Sorafenib | 32,082 | 1.243 | 0.841 | 2194 | 0.076 | 28,728 | 97 | 57,488 |
| QuiremSpheres | 36,503 | 1.110 | 0.764 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| AG probabilistic base case | ||||||||
| Lenvatinib | 29,658 | 1.202 | 0.825 | |||||
| TheraSphere | 30,014 | 1.111 | 0.765 | 356 | –0.060 | Dominated | –2154 | Dominated |
| SIR-Spheres | 30,196 | 1.111 | 0.765 | 538 | –0.060 | Dominated | –2323 | Dominated |
| Sorafenib | 32,444 | 1.244 | 0.841 | 2786 | 0.016 | 174,320 | –2306 | 174,320 |
| QuiremSpheres | 36,613 | 1.111 | 0.765 | 6955 | –0.060 | Dominated | –8741 | Dominated |
The AG’s base case was based on the following assumptions and data sources:
-
SIR-Spheres efficacy based on a pooled survival analysis of SARAH19 and SIRveNIB21 data (per-protocol population)
-
QuiremSpheres and TheraSphere efficacy equal to SIR-Spheres
-
for patients who received work-up but no SIRT, OS and PFS based on SARAH19 KM
-
sorafenib efficacy based on a pooled survival analysis of SARAH19 and SIRveNIB21 data (ITT population)
-
lenvatinib HR derived from the AG’s NMA (ITT population)
-
OS and PFS extrapolated using a Weibull model
-
decision tree transition probabilities estimated using data from the SARAH19 trial
-
no downstaging to curative therapy permitted
-
bilobar treatments performed in two separate procedures
-
work-up costs from The Christie NHS Foundation Trust elicitation (as per the BTG economic analysis)
-
health state utilities from the SARAH19 per-protocol subgroup, based on therapeutic class (SIRT and systemic therapy).
Based on the probabilistic version of the AG model, the three SIRTs are each expected to generate fewer QALYs than sorafenib or lenvatinib, but were associated with higher costs. SIRT generated 0.765 QALYs; this was 0.076 QALYs fewer than generated by sorafenib and 0.060 fewer than by lenvatinib. TheraSphere and SIR-Spheres had very similar total costs, and QuiremSpheres was the most costly owing to the additional costs associated with procurement of QuiremScout.
Figure 19 presents CEACs for the fully incremental results of the AG model. Lenvatinib has the highest likelihood of being cost-effective across any WTP threshold of < £100,000. Assuming a WTP threshold of £30,000 per QALY gained, TheraSphere had an incremental NMB of –£2154, and this was –£2323 for SIR-Spheres. The NMB for QuiremSpheres versus lenvatinib was –£8741. All three SIRTs were dominated by lenvatinib. Disaggregated deterministic results show that just under half of the QALY gain in both groups is accrued in the post-progression health state.
FIGURE 19.
Cost-effectiveness acceptability curve for the AG probabilistic base-case analysis.
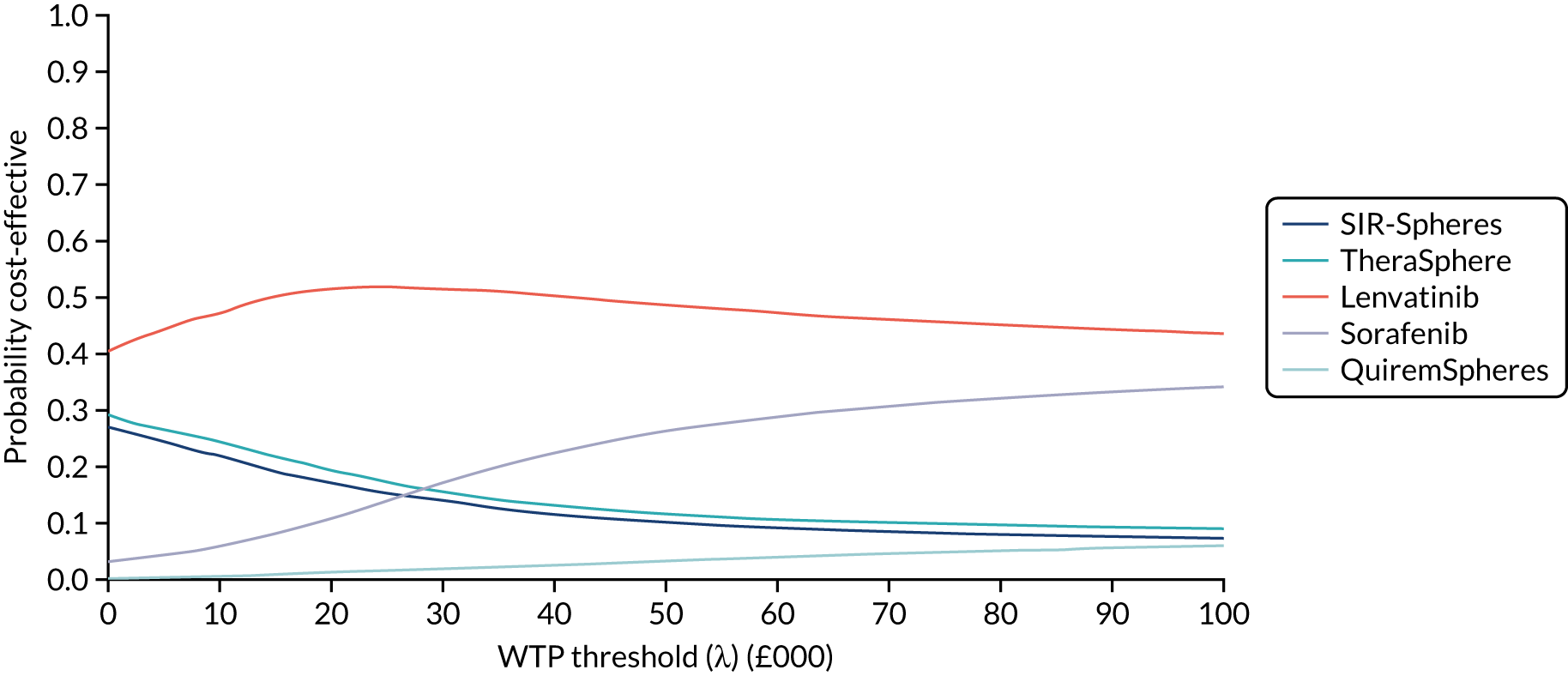
For results including the confidential PAS discounts for sorafenib, lenvatinib, regorafenib and QuiremSpheres, see Appendix 17.
Sensitivity analyses results
Scenario analyses
Scenario 1: efficacy data from SARAH only
The first scenario analysis explores the effect of using only data from the European SARAH trial19 to inform efficacy estimates for SIRT and sorafenib, on the basis that these might better represent the patient population and clinical practice in the UK. Deterministic and probabilistic results are presented in Table 32. The probabilistic results are based on 5000 model iterations. As with the AG base case, each SIRT is associated with almost the same number of life-years and QALYs; however, this scenario predicts lower OS (and thus life-years/QALYs) than in the base case, which makes SIR-Spheres marginally cheaper than lenvatinib.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| Deterministic scenario 1: efficacy data from SARAH only | ||||||||
| TheraSphere | 29,395 | 0.976 | 0.671 | |||||
| SIR-Spheres | 29,614 | 0.976 | 0.671 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Lenvatinib | 29,893 | 1.150 | 0.782 | 498 | 0.111 | 4475 | 2840 | 4475 |
| Sorafenib | 31,951 | 1.209 | 0.817 | 2556 | 0.147 | 17,424 | 1845 | 58,080 |
| QuiremSpheres | 36,010 | 0.976 | 0.671 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Probabilistic scenario 1: efficacy data from SARAH only | ||||||||
| Lenvatinib | 29,413 | 1.171 | 0.805 | |||||
| TheraSphere | 29,476 | 0.978 | 0.672 | 62 | –0.133 | Dominated | –4044 | Dominated |
| SIR-Spheres | 29,660 | 0.977 | 0.671 | 246 | –0.134 | Dominated | –4267 | Dominated |
| Sorafenib | 32,300 | 1.213 | 0.818 | 2887 | 0.014 | 212,505 | –2479 | 212,505 |
| QuiremSpheres | 36,064 | 0.977 | 0.670 | 6650 | –0.134 | Dominated | –10,684 | Dominated |
Scenario 2: low tumour burden/albumin–bilirubin 1 subgroup (SARAH)
This scenario explores the use of the company’s preferred post hoc grouping of patients from the SARAH trial19 as the source of efficacy data for SIRT and sorafenib. Further changes from the AG base case are the use of the higher low tumour burden/ALBI 1 subgroup utilities from the SARAH trial,19 and the significantly lower proportion of patients who receive work-up but not SIRT (8.1% vs. 18.6%). Note that although Sirtex used a proportion of 2.9% for work-up failures in this population, it was unclear how this figure was reached. Increasing the number of work-up failures, however, increases the cost-effectiveness of SIRT.
This scenario predicts the cost-effectiveness of an optimised decision in which only patients who have a tumour burden of ≤ 25% and a preserved liver function would be eligible to receive SIRT. As there is no equivalent evidence available for lenvatinib, this scenario assumes that the HR between sorafenib and lenvatinib remains the same as in the base-case population.
Table 33 shows that although the systemic therapies were less costly than SIRT in this scenario, SIR-Spheres generated an additional 0.139 QALYs compared with lenvatinib and 0.117 compared with sorafenib in the probabilistic model. This resulted in fully incremental ICERs of £20,926 per QALY gained for TheraSphere compared with lenvatinib, and £119,562 for SIR-Spheres compared with TheraSphere. However, the two technologies were distinguished only by the additional fluoroscopy cost associated with the SIR-Spheres procedure, resulting in very similar NMB at a £30,000 threshold. This is notably the only scenario in which TheraSphere and SIR-Spheres have a positive incremental NMB versus lenvatinib at a WTP threshold of £30,000 (excluding scenario 4). This is illustrated by the CEAC in Figure 20, which shows lenvatinib to have the highest likelihood of being cost-effective up to a WTP threshold of approximately £27,000, at which point it is surpassed by TheraSphere and by SIR-Spheres at a WTP threshold of ≥ £32,000.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| Deterministic scenario 2: low tumour burden/ALBI 1 subgroup | ||||||||
| Lenvatinib | 31,388 | 1.366 | 1.000 | |||||
| Sorafenib | 33,388 | 1.420 | 1.037 | 2000 | 0.038 | 53,320 | –875 | Extendedly dominated |
| TheraSphere | 34,021 | 1.542 | 1.153 | 2633 | 0.153 | 17,175 | 1966 | 17,175 |
| SIR-Spheres | 34,267 | 1.542 | 1.153 | 2879 | 0.153 | 18,783 | 1720 | Dominated |
| QuiremSpheres | 40,931 | 1.542 | 1.153 | 9543 | 0.153 | 62,257 | –4945 | Dominated |
| Probabilistic scenario 2: low tumour burden/ALBI 1 subgroup | ||||||||
| Lenvatinib | 31,233 | 1.397 | 1.024 | |||||
| Sorafenib | 33,834 | 1.436 | 1.048 | 2601 | 0.024 | 109,709 | –1890 | Extendedly dominated |
| TheraSphere | 34,086 | 1.552 | 1.161 | 2854 | 0.136 | 20,926 | 1237 | 20,926 |
| SIR-Spheres | 34,389 | 1.553 | 1.163 | 3156 | 0.139 | 22,725 | 1010 | 119,562 |
| QuiremSpheres | 41,088 | 1.552 | 1.162 | 9855 | 0.138 | 71,372 | –5712 | Extendedly dominated |
FIGURE 20.
Cost-effectiveness acceptability curve for AG scenario 2: low tumour burden/ALBI 1 subgroup.
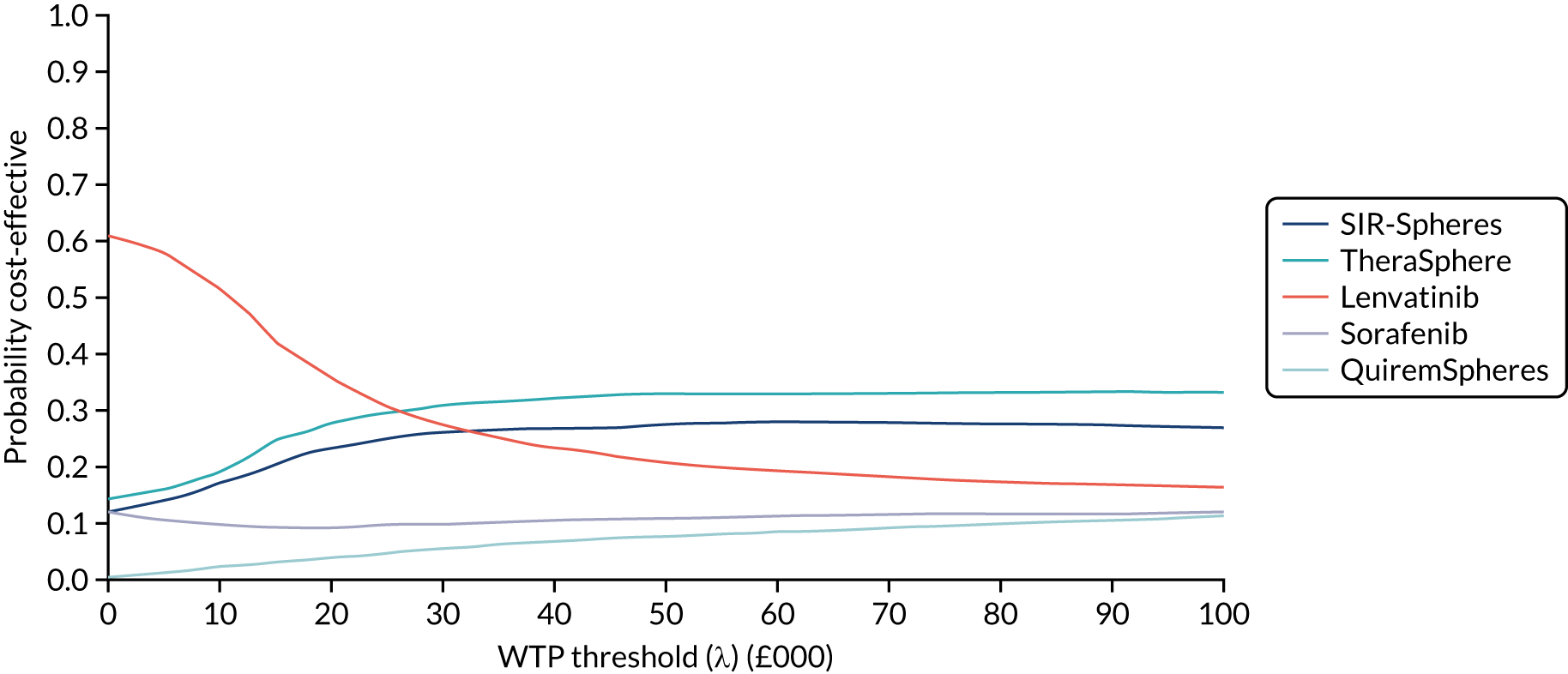
Results including the confidential PAS discounts for sorafenib, lenvatinib, regorafenib and QuiremSpheres can be found in Appendix 17.
Scenario 3: no macroscopic vascular invasion (SARAH)
This scenario limits the patient population to only those who had no MVI, referred to elsewhere as PVI, at baseline. These patients may be expected to benefit more from SIRT owing to a more favourable positioning and spread of their tumour, and were thus defined as a subgroup of interest in NICE’s scope. As there is no equivalent evidence for lenvatinib, this scenario assumes that the HR between sorafenib and lenvatinib remains the same as in the base-case population.
The probabilistic analysis in Table 34 found all three SIRTs to be dominated by lenvatinib, with a significantly lower NMB than either systemic therapy. Notably, the gap in QALYs produced by SIRT versus sorafenib widened in this analysis versus the base case, implying a reduced benefit of SIRT in this population.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| Deterministic scenario 3: no MVI (SARAH) | ||||||||
| TheraSphere | 29,949 | 1.078 | 0.740 | |||||
| SIR-Spheres | 30,167 | 1.078 | 0.740 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Lenvatinib | 30,399 | 1.272 | 0.865 | 451 | 0.125 | 3594 | 3310 | 3594 |
| Sorafenib | 32,452 | 1.326 | 0.897 | 2503 | 0.157 | 15,923 | 2213 | 64,437 |
| QuiremSpheres | 36,563 | 1.078 | 0.740 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Probabilistic scenario 3: no MVI (SARAH) | ||||||||
| Lenvatinib | 29,985 | 1.295 | 0.888 | |||||
| TheraSphere | 30,094 | 1.086 | 0.746 | 109 | –0.142 | Dominated | –4382 | Dominated |
| SIR-Spheres | 30,314 | 1.086 | 0.746 | 329 | –0.143 | Dominated | –4616 | Dominated |
| Sorafenib | 32,876 | 1.335 | 0.905 | 2890 | 0.017 | 170,117 | –2381 | 170,117 |
| QuiremSpheres | 36,662 | 1.086 | 0.745 | 6676 | –0.143 | Dominated | –10,965 | Dominated |
Scenario 4: TheraSphere hazard ratio from the Biederman et al. and Van Der Gucht et al. network meta-analysis scenario
The results presented in Table 35 use the HR derived from the AG’s NMA scenario, which included the low-quality retrospective studies by Biederman et al. 39 and Van Der Gucht et al. 40 The patient population in Biederman et al. 39 was particularly mismatched with the others included in this analysis, as it included only patients with MVI, which appeared to have a substantial impact on the treatment effect associated with TheraSphere.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| Deterministic scenario 4: TheraSphere HR from Biederman et al. and Van Der Gucht et al. NMA scenario | ||||||||
| Lenvatinib | 30,005 | 1.183 | 0.805 | |||||
| SIR-Spheres | 30,107 | 1.110 | 0.764 | 101 | –0.040 | Dominated | –1308 | Dominated |
| Sorafenib | 32,082 | 1.243 | 0.841 | 2077 | 0.036 | 57,488 | –993 | Extendedly dominated |
| TheraSphere | 33,373 | 1.883 | 1.297 | 3368 | 0.493 | 6835 | 11,413 | 6835 |
| QuiremSpheres | 36,503 | 1.110 | 0.764 | 6497 | –0.040 | Dominated | –7705 | Dominated |
| Probabilistic scenario 4: TheraSphere HR from Biederman et al. and Van Der Gucht et al. NMA scenario | ||||||||
| Lenvatinib | 29,601 | 1.197 | 0.822 | |||||
| SIR-Spheres | 30,242 | 1.110 | 0.764 | 641 | –0.058 | Dominated | –2387 | Dominated |
| Sorafenib | 32,477 | 1.244 | 0.843 | 2876 | 0.021 | 140,205 | –2260 | Extendedly dominated |
| TheraSphere | 33,670 | 1.931 | 1.330 | 4068 | 0.507 | 8017 | 11,156 | 8017 |
| QuiremSpheres | 36,616 | 1.111 | 0.765 | 7014 | –0.058 | Dominated | –8746 | Dominated |
A HR of 0.46 versus SIR-Spheres was applied for both OS and PFS outcomes for TheraSphere. Based on the probabilistic analysis (5000 iterations), TheraSphere is expected to generate an additional 0.507 QALYs compared with lenvatinib, at an additional cost of £4068, producing an ICER of £8017 per QALY gained, and a NMB of £11,413. TheraSphere was associated with higher costs than SIR-Spheres owing to the increased disease management costs associated with lower mortality, but it also produced an additional 0.566 QALYs, yielding an ICER of £6060 per QALY gained.
Further scenario analyses
Table 36 presents a number of other scenarios on the AG base case that explore the impact of alternative assumptions, including sources of utilities, downstaging to curative therapy, resource use and survival models.
| Intervention | Total | Incremental (vs. baseline) | ICER (£) (fully incremental) | |||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | Life-years | QALYs | Costs (£) | QALYs | ICER (£) | NMB (£) | ||
| Scenario 5: utilities from lenvatinib TA551124 | ||||||||
| TheraSphere | 29,888 | 1.110 | 0.791 | |||||
| Lenvatinib | 30,005 | 1.183 | 0.846 | 117 | 0.055 | 2113 | 1546 | 2113 |
| SIR-Spheres | 30,107 | 1.110 | 0.791 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Sorafenib | 32,082 | 1.243 | 0.881 | 2194 | 0.091 | 24,145 | 532 | 58,615 |
| QuiremSpheres | 36,503 | 1.110 | 0.791 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 6: downstaging to curative therapy possible (SARAH19 ITT proportions) | ||||||||
| TheraSphere | 28,990 | 1.217 | 0.842 | |||||
| SIR-Spheres | 29,208 | 1.217 | 0.842 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Lenvatinib | 29,817 | 1.212 | 0.826 | 827 | –0.016 | Dominated | –1292 | Dominated |
| Sorafenib | 31,850 | 1.271 | 0.862 | 2860 | 0.020 | 142,238 | –2256 | 142,238 |
| QuiremSpheres | 35,605 | 1.217 | 0.842 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 7: bilobar disease treated in same procedure | ||||||||
| TheraSphere | 29,159 | 1.110 | 0.764 | |||||
| SIR-Spheres | 29,364 | 1.110 | 0.764 | 204 | 0.000 | More costly | –204 | Extendedly dominated |
| Lenvatinib | 30,005 | 1.183 | 0.805 | 846 | 0.040 | 21,026 | 361 | 21,026 |
| Sorafenib | 32,082 | 1.243 | 0.841 | 2923 | 0.076 | 38,274 | –632 | 57,488 |
| QuiremSpheres | 35,646 | 1.110 | 0.764 | 6486 | 0.000 | More costly | –6486 | Extendedly dominated |
| Scenario 8: work-up costs from National Schedule of Reference Costs 2017–2018107 (Sirtex assumption) | ||||||||
| Lenvatinib | 30,005 | 1.183 | 0.805 | |||||
| TheraSphere | 30,170 | 1.110 | 0.764 | 165 | –0.040 | Dominated | –1372 | Dominated |
| SIR-Spheres | 30,389 | 1.110 | 0.764 | 383 | –0.040 | Dominated | –1590 | Dominated |
| Sorafenib | 32,082 | 1.243 | 0.841 | 2077 | 0.036 | 57,488 | –993 | 57,488 |
| QuiremSpheres | 36,864 | 1.110 | 0.764 | 6859 | –0.040 | Dominated | –8066 | Dominated |
| Scenario 9: disease management costs taken from TA551124 | ||||||||
| Lenvatinib | 48,033 | 1.183 | 0.805 | |||||
| TheraSphere | 48,186 | 1.110 | 0.764 | 152 | –0.040 | Dominated | –1360 | Dominated |
| SIR-Spheres | 48,404 | 1.110 | 0.764 | 371 | –0.040 | Dominated | –1578 | Dominated |
| Sorafenib | 53,682 | 1.243 | 0.841 | 5649 | 0.036 | 156,367 | –4565 | 156,367 |
| QuiremSpheres | 54,800 | 1.110 | 0.764 | 6767 | –0.040 | Dominated | –7974 | Dominated |
| Scenario 10: low tumour burden/ALBI 1 subgroup including possibility of downstaging | ||||||||
| Lenvatinib | 31,072 | 1.404 | 1.029 | |||||
| TheraSphere | 31,467 | 1.736 | 1.303 | 395 | 0.274 | 1440 | 7826 | 1440 |
| SIR-Spheres | 31,713 | 1.736 | 1.303 | 641 | 0.274 | 2339 | 7579 | Dominated |
| Sorafenib | 33,007 | 1.457 | 1.066 | 1935 | 0.037 | 52,685 | –833 | Extendedly dominated |
| QuiremSpheres | 38,377 | 1.736 | 1.303 | 7305 | 0.274 | 26,660 | 915 | Dominated |
| Scenario 11: Gompertz OS | ||||||||
| TheraSphere | 30,015 | 1.127 | 0.776 | |||||
| Lenvatinib | 30,066 | 1.188 | 0.808 | 51 | 0.033 | 1555 | 926 | 1555 |
| SIR-Spheres | 30,234 | 1.127 | 0.776 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Sorafenib | 32,190 | 1.255 | 0.849 | 2174 | 0.073 | 29,634 | 27 | 52,020 |
| QuiremSpheres | 36,630 | 1.127 | 0.776 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 12: exponential OS | ||||||||
| Lenvatinib | 30,239 | 1.215 | 0.826 | |||||
| TheraSphere | 30,245 | 1.160 | 0.798 | 5 | –0.028 | Dominated | –860 | Dominated |
| SIR-Spheres | 30,463 | 1.160 | 0.798 | 224 | –0.028 | Dominated | –1078 | Dominated |
| Sorafenib | 32,379 | 1.285 | 0.868 | 2139 | 0.042 | 50,493 | –868 | 50,493 |
| QuiremSpheres | 36,859 | 1.160 | 0.798 | 6620 | –0.028 | Dominated | –7474 | Dominated |
| Scenario 13: generalised gamma OS (lenvatinib OS equal to sorafenib) | ||||||||
| TheraSphere | 30,992 | 1.277 | 0.875 | |||||
| Lenvatinib | 31,148 | 1.357 | 0.919 | 155 | 0.044 | 3561 | 1154 | 3561 |
| SIR-Spheres | 31,211 | 1.277 | 0.875 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Sorafenib | 32,854 | 1.357 | 0.916 | 1862 | 0.040 | 46,103 | –650 | Extendedly dominated |
| QuiremSpheres | 37,607 | 1.277 | 0.875 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 14: log-normal OS (lenvatinib OS equal to sorafenib) | ||||||||
| TheraSphere | 30,208 | 1.156 | 0.795 | |||||
| SIR-Spheres | 30,426 | 1.156 | 0.795 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Lenvatinib | 31,480 | 1.408 | 0.952 | 1273 | 0.158 | 8078 | 3454 | 8078 |
| Sorafenib | 33,187 | 1.408 | 0.949 | 2979 | 0.154 | 19,311 | 1649 | Extendedly dominated |
| QuiremSpheres | 36,822 | 1.156 | 0.795 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 15: log-logistic OS (lenvatinib OS equal to sorafenib) | ||||||||
| TheraSphere | 30,301 | 1.169 | 0.804 | |||||
| SIR-Spheres | 30,519 | 1.169 | 0.804 | 218 | 0.000 | More costly | –218 | Extendedly dominated |
| Lenvatinib | 31,543 | 1.420 | 0.960 | 1242 | 0.156 | 7962 | 3439 | 7962 |
| Sorafenib | 33,249 | 1.420 | 0.956 | 2949 | 0.153 | 19,303 | 1634 | Extendedly dominated |
| QuiremSpheres | 36,915 | 1.169 | 0.804 | 6614 | 0.000 | More costly | –6614 | Extendedly dominated |
| Scenario 16: 5% work-up/no SIRT | ||||||||
| Lenvatinib | 30,005 | 1.183 | 0.805 | |||||
| Sorafenib | 32,082 | 1.243 | 0.841 | 2077 | 0.036 | 57,488 | –993 | 57,488 |
| TheraSphere | 32,603 | 1.183 | 0.816 | 2597 | 0.011 | 239,222 | –2272 | Extendedly dominated |
| SIR-Spheres | 32,858 | 1.183 | 0.816 | 2852 | 0.011 | 262,683 | –2526 | Extendedly dominated |
| QuiremSpheres | 39,601 | 1.183 | 0.816 | 9596 | 0.011 | 883,746 | –9270 | Extendedly dominated |
| Scenario 17: SIRveNIB21 work-up/no SIRT (28.57%) | ||||||||
| TheraSphere | 27,898 | 1.056 | 0.727 | |||||
| SIR-Spheres | 28,090 | 1.056 | 0.727 | 192 | 0.000 | More costly | –192 | Extendedly dominated |
| Lenvatinib | 30,005 | 1.183 | 0.805 | 2107 | 0.078 | 27,118 | 224 | 27,118 |
| Sorafenib | 32,082 | 1.243 | 0.841 | 4184 | 0.114 | 36,757 | –769 | 57,488 |
| QuiremSpheres | 34,232 | 1.056 | 0.727 | 6333 | 0.000 | More costly | –6333 | Dominated |
Scenarios 6 and 10 include the possibility for downstaging; in these scenarios, the distribution of the three liver-targeted treatments was derived from the SARAH trial. 19 Patients who received TACE or radiation therapy were excluded as these would not be permitted options in this population in UK practice. Liver transplant was undergone by 1.09% of SIRT patients and 0.46% of sorafenib patients; 1.63% of SIRT patients and 0% of sorafenib patients underwent liver resection, and 3.26% of SIRT patients and 0.92% of sorafenib patients received ablation therapy.
Only the deterministic results are produced for these analyses.
Table 37 presents the results of the base-case and selected scenario analyses in terms of their effect on the NMB ranking of the five technologies at list price. This shows lenvatinib to be consistently ranked first in terms of incremental NMB, except in those scenarios that use more favourable assumptions in favour of SIRT. As SIRT produces QALYs above the WTP threshold, increasing the proportion of patients who fail work-up (scenario 17) and do not go on to receive SIRT increases its cost-effectiveness, as overall costs are reduced and the more cost-effective QALYs produced on BSC and sorafenib are up-weighted.
| Intervention | Incremental NMB rank (vs. baseline) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | |
| SIR-Spheres | 4 | 4 | 2 | 4 | 4 | 4 | 2 | 3 | 4 | 3 | 2 | 4 | 4 | 3 | 4 | 4 | 4 | 3 |
| TheraSphere | 2 | 3 | 1 | 3 | 1 | 3 | 1 | 2 | 3 | 2 | 1 | 3 | 3 | 2 | 3 | 3 | 3 | 2 |
| QuiremSpheres | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Lenvatinib | 1 | 1 | 3 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sorafenib | 3 | 2 | 4 | 2 | 3 | 2 | 4 | 4 | 2 | 4 | 5 | 2 | 2 | 4 | 2 | 2 | 2 | 4 |
Deterministic sensitivity analysis
Results of the DSAs are presented in Figures 21–25 for the AG base-case scenario and the four scenarios presented in Scenario analyses. The tornado diagrams present the 10 most influential parameters in each analysis. SIR-Spheres was compared with sorafenib because sorafenib was considered the most relevant comparator and had direct evidence compared with SIR-Spheres.
FIGURE 23.
Tornado diagram: SIR-Spheres vs. sorafenib; low tumour burden/ALBI 1 subgroup (scenario 2).

FIGURE 24.
Tornado diagram: SIR-Spheres vs. sorafenib; no MVI subgroup (scenario 3).
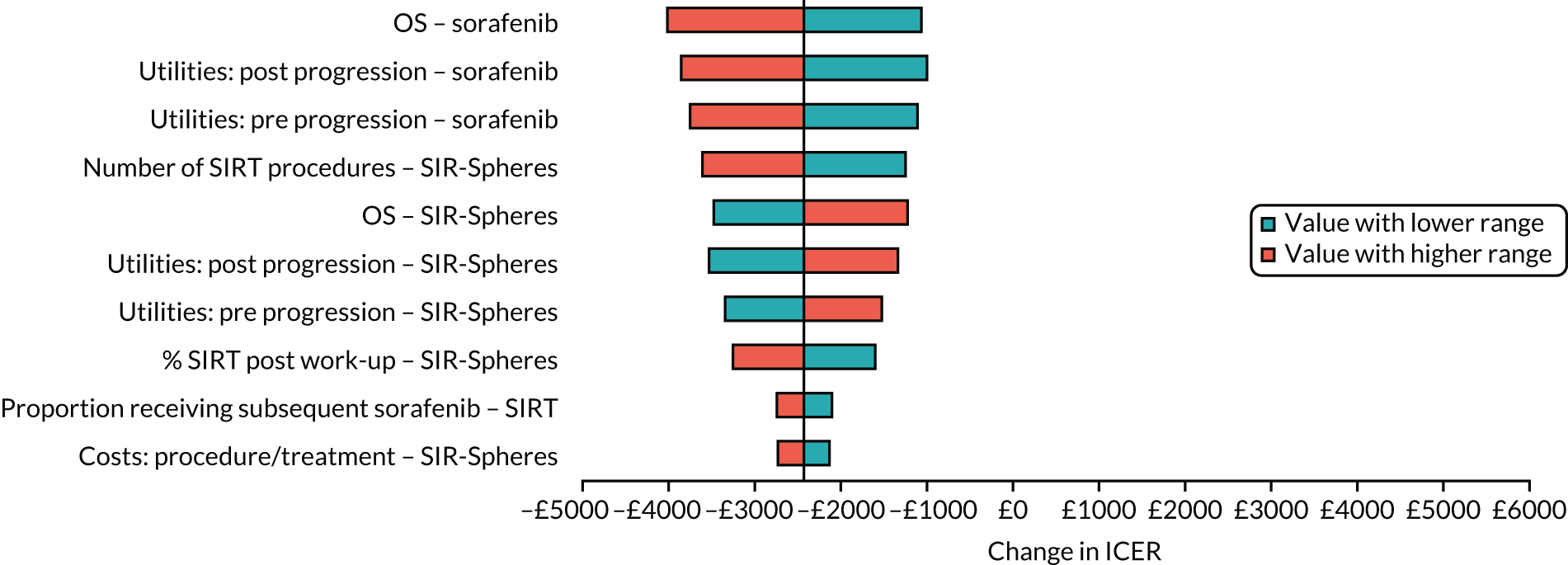
The AG base-case analysis (see Figure 21) was robust to a range of parameters, with the most influential parameters providing a range of NMBs between approximately –£1600 and £1000, with the base-case NMB as –£315. The most influential parameters were the health state utilities, the number of SIRT procedures and the proportion of patients receiving SIRT after work-up. In these scenarios, SIR-Spheres became cost-effective compared with sorafenib for some of the range of values of the parameter (i.e. SIR-Spheres had a positive incremental NMB). However, when the confidential PAS for sorafenib was applied, this was no longer the case.
In scenario 1, with efficacy data based only on SARAH19, varying the parameters in the DSA had a larger impact on NMB than in the base-case analysis, although the variation remains small (see Figure 22). Similarly to the base-case analysis, the results were most sensitive to health state utilities and SIRT procedures; however, in this analysis, OS for sorafenib and SIR-Spheres was also an influential parameter. There were no scenarios in which SIR-Spheres was estimated to be cost-effective compared with sorafenib.
The most influential parameter in the low tumour burden/ALBI 1 subgroup was OS for both SIR-Spheres and sorafenib (see Figure 23). SIR-Spheres remained cost-effective compared with sorafenib over the range of parameters; however, when the confidential PAS for sorafenib was applied, this was no longer the case.
In the ‘no-MVI’ subgroup, the most influential parameters were the health state utilities and OS for sorafenib and SIR-Spheres (see Figure 24). There were are no scenarios in which SIR-Spheres was estimated to be cost-effective compared with sorafenib.
In Figure 25, TheraSphere was compared with sorafenib. In this scenario, the results of the analysis were robust to the range of parameters, and found TheraSphere to be cost-effective across all scenarios.
Discussion of the independent economic assessment
In the light of the AG’s concerns regarding the relevance of the economic analyses identified in the review of cost-effectiveness studies and highlighted limitations in the economic evaluations developed by BTG and Sirtex, the AG developed a de novo health economic model. The AG model evaluated the three SIRTs and current UK practice for the treatment of advanced HCC in Child–Pugh class A patients ineligible to receive (or who had previously failed) CTT. Results were generated as fully incremental ICERs and in terms of incremental NMB, which allows for easier comparison of ‘dominated’ results with small differences in cost and efficacy. The AG model used a three-state partitioned survival model approach with a decision tree, which determined the proportion of patients who did not continue on to receive SIRT following the work-up procedure. The model utilises all currently available RCT evidence to generate estimates of clinical effectiveness, using data directly drawn from the SARAH19 and SIRveNIB21 trials, and HRs generated in the AG’s NMA.
Based on the AG’s probabilistic base-case analysis at list price, none of the three SIRTs is expected to be cost-effective at any WTP threshold, being more costly and less effective than lenvatinib. When the modelled population was limited to only those with a low tumour burden and preserved liver function, the ICERs for TheraSphere and SIR-Spheres were £17,165 and £18,783 per QALY gained versus the most cost-effective systemic therapy. The most optimistic ICERs were generated in the scenario presented for the low tumour burden and preserved liver function in which downstaging to curative therapy was permitted. In this scenario, the ICERs for TheraSphere and SIR-Spheres decreased to £1440 and £2339, respectively. However, there was no scenario in which SIRT was predicted to be cost-effective at a WTP threshold of £30,000 when confidential PAS discounts were included (see Appendix 17). In all scenarios, QuiremSpheres was not cost-effective compared with other SIRTs owing to higher work-up and acquisition costs (see below for further discussion of QuiremSpheres in relation to the limitations of the model).
The AG scenario 4 (including the Biederman et al. 39 and Van Der Gucht et al. 40 studies) found TheraSphere to be cost-effective versus lenvatinib when the confidential PAS prices were used. However, the AG considers the data used to model comparative effectiveness to be of low quality and inconsistent with the wider body of evidence on the comparative effectiveness of SIR-Spheres and TheraSphere. The AG, therefore, does not consider this scenario to represent a realistic estimate of the relative benefits of TheraSphere.
The results of the AG’s base-case analysis are robust to a wide range of assumptions, reflecting the completeness and quality of the included studies, and the substantial differences seen in costs and QALYs between the SIRTs and current UK practice (including confidential PAS). The AG’s analyses predicted lenvatinib to rank first in terms of NMB in all scenarios (excluding scenario 4), whereas sorafenib was a cost-effective alternative, producing more QALYs at a higher cost. There are a number of differences between the AG model and those presented by the companies, which primarily concern the issues highlighted in the critique of these models in Chapter 5, Review of economic evidence submitted by companies. Strengths of the AG model include (1) all available high-quality RCT data were used to model the outcomes of the most relevant patient population to UK practice, (2) analyses included all appropriate comparators, (3) independent modelling of the costs and outcomes of patients who receive work-up but were ineligible to receive SIRT and (4) preserved randomisation and greater internal consistency with regard to the use of subsequent and curative therapies.
Insurmountable limitations in the evidence base meant that the AG was unable to address the question of the cost-effectiveness of SIRT in patients with early or intermediate HCC. The evidence for TheraSphere and QuiremSpheres in advanced HCC was extremely limited, and a lack of head-to-head evidence prevented a meaningful comparison of SIR-Spheres, TheraSphere and QuiremSpheres with one another in terms of clinical effectiveness. This essentially limits this particular comparison to that of a cost-minimisation, with a full comparison of the cost-effectiveness of SIRT versus sorafenib and lenvatinib. Although it is therefore not possible to discern which of the SIRTs offers the best value for money, the increased cost of the QuiremSpheres work-up procedure meant that it was consistently positioned last by some way in terms of NMB. The structure of the AG model and a lack of supporting evidence on the comparative effectiveness of QuiremSpheres, however, meant that there were no means by which the concept of ‘suboptimal SIRT’, as proposed by Terumo,104 could realistically be explored. This includes the ostensibly greater selectivity of QuiremScout, and any quantifiable improvement in treatment effect resulting from optimisation of patient selection.
Chapter 8 Assessment of factors relevant to the NHS and other parties
End-of-life considerations
In the early- and intermediate-HCC populations, life expectancy reported in the most recent European Society For Medical Oncology guidelines155 is > 24 months, with reported expected survival of > 5 years in the early population and > 2.5 years in the intermediate population. There is insufficient reliable evidence to indicate whether or not SIRT provides an extension to life of > 3 months.
The NICE end-of-life supplementary advice142 outlines that end-of-life criteria should be applied when both of the criteria below are satisfied:
-
The treatment is indicated for patients with a short life expectancy, normally < 24 months.
-
There is sufficient evidence to indicate that the treatment offers an extension to life, normally of at least an additional 3 months, compared with current NHS treatment.
Undiscounted LYG predicted in the AG’s base-case analysis are presented in Table 38. These indicate that normal life expectancy for patients ineligible for CTT is < 24 months, with expected mean survival of 14.72 months on lenvatinib and 15.49 months on sorafenib. This conclusion remains consistent irrespective of the subgroup considered or the choice of parametric model used to represent OS.
| Treatment | Survival estimates (months) | |||||
|---|---|---|---|---|---|---|
| AG base case | Low tumour/ALBI 1 subgroup | MVI subgroup | ||||
| No downstaging | With downstaging | No downstaging | With downstaging | No downstaging | With downstaging | |
| Undiscounted LYG: lenvatinib | 14.72 | 15.12 | 16.98 | 17.49 | 15.80 | 16.14 |
| Undiscounted LYG: sorafenib | 15.49 | 15.89 | 17.68 | 18.17 | 16.49 | 16.82 |
| Incremental undiscounted LYG: SIRT vs. lenvatiniba | –0.95 | 0.11 | 2.80 | 5.30 | –2.49 | –1.51 |
| Incremental undiscounted LYG: SIRT vs. sorafeniba | –1.73 | –0.65 | 2.11 | 4.61 | –3.18 | –2.19 |
Regarding the criterion relating to > 3 months’ life extension, the AG’s base-case analysis suggests that SIRT is marginally inferior to both systemic therapies (sorafenib and lenvatinib), indicating that this criterion is not met. The results for the subgroup with no MVI similarly suggest that sorafenib produces marginally greater LYG than SIRT. In the low tumour burden/ALBI 1 subgroup, SIRTs are predicted to provide an extension to life of 2.11 months compared with sorafenib and 2.80 months compared with lenvatinib. These predicted survival gains, however, exclude potential gains from downstaging. In scenarios conducted in the low tumour burden/ALBI 1 subgroup that allow for downstaging, predicted survival gains increase to 4.61 months compared with sorafenib and 5.30 months compared with lenvatinib. These predicted gains are, however, subject to significant uncertainty owing to the small sample sizes and the fact that this is a post hoc subgroup analysis. There are also very significant uncertainties regarding the plausibility of downstaging patients in this population.
Chapter 9 Discussion
Statement of principal findings
Treatment options vary for patients with unresectable HCC according to the stage of the cancer and the underlying liver disease. The AG, therefore, considered three distinct unresectable HCC patient populations, defined with respect to the aim of therapy and eligibility for comparator treatments. These three populations were as follows: (1) patients eligible for transplant, (2) patients ineligible for transplant but eligible for CTT and (3) patients ineligible for CTT. These three populations largely correspond to early-, intermediate- and advanced-stage HCC.
There is a large body of evidence on the clinical effectiveness and safety of SIRT compared with sorafenib or TACE; seven RCTs, seven prospective comparative studies, five retrospective comparative studies and one non-comparative case series were included in the review of clinical effectiveness. However, only two studies were considered to have a low risk of bias, the SARAH19 and SIRveNIB21 RCTs, which both compared SIR-Spheres with sorafenib. These studies enrolled patients with locally advanced HCC not amenable to curative treatment modalities and ineligible for CTT; the evidence for the early- and intermediate-HCC populations was significantly more limited. Both RCTs found no significant difference in OS or PFS between SIR-Spheres and sorafenib, despite a statistically significantly greater tumour response rate in the SIR-Spheres arm of both trials. The SARAH trial19 reported a significant difference between groups in HRQoL, favouring SIR-Spheres; however, the proportion of patients who completed the questionnaires was low. AEs, particularly grade ≥ 3 events, were more frequent in the sorafenib group in both trials. There are some concerns regarding the generalisability of the results of these two RCTs to the UK HCC population, particularly the SIRveNIB trial,21 which was conducted in the Asia-Pacific region, where the aetiology and treatment of HCC differ from those in Europe.
The Sirtex company submission102 selected a subgroup of patients from the SARAH trial19 with ≤ 25% tumour burden and preserved liver function, defined as having ALBI 1, for the base-case analysis in its economic analysis. Although results appeared more promising in this subgroup of patients with a better prognosis, the results of this post hoc subgroup analysis should be prospectively validated before being considered relevant for clinical practice.
In studies that directly compared the different SIRTs, patients with PVT appeared to have better survival outcomes with TheraSphere than with SIR-Spheres; however, this result was from a small retrospective comparative study rated as being at a high risk of bias and, therefore, may not be reliable. Other studies comparing TheraSphere with SIR-Spheres that were not restricted to patients with PVT had conflicting results. The only study that compared QuiremSpheres with SIR-Spheres and TheraSphere was provided by Terumo as an addendum to its submission. 104 Clinical outcomes appeared to be similar between treatment groups; however, this was a very small pilot study with several methodological limitations.
Three NMA models were produced to represent the three different populations of unresectable HCC patients described above. Both the NMA in patients eligible for transplant and the NMA in patients eligible for CTT were not conducted owing to the uncertainty of using SIRT for bridging to transplant and downstaging in the UK, and a lack of good-quality evidence in patients eligible for CTT.
The base-case NMA was conducted in adults with unresectable HCC who have Child–Pugh class A liver function and are ineligible for CTT. There were no meaningful differences in OS between SIR-Spheres, sorafenib and lenvatinib in the per-protocol or ITT populations. All treatments appeared to have similar efficacy. There was only one low-quality retrospective study that directly compared TheraSphere with SIR-Spheres in the base-case population. 39 Adding this study as a sensitivity analysis had a substantial effect on the NMA results: TheraSphere showed a significant improvement in OS when compared with SIR-Spheres, sorafenib and lenvatinib. However, these results may be biased and unreliable as they rely on only one low-quality retrospective study.
The limitations in the effectiveness evidence had an important role in shaping the economic analysis and restricted the focus of the AG’s economic analysis to the population ineligible for CTT; this was the only population for which there were reliable estimates of the comparative effectiveness of SIRT with comparator technologies. The structure of the AG’s model was broadly similar to the structures of the models developed by BTG and Sirtex for this population and was designed around a decision tree and partitioned survival model. The decision tree was used to model the fact that some patients eligible to receive SIRT will fail the work-up procedure and will not receive SIRT treatment; in a scenario analysis, the decision tree was also used to allow a proportion of patients to go on to receive curative therapies. The partitioned survival model developed was based on three health states: PFS, progressive disease and death.
The results of the AG’s base-case analysis (probabilistic analysis), which assumed equal efficacy across all three SIRTs, suggested that TheraSphere is cost saving relative to both SIR-Spheres and QuiremSpheres. However, the incremental costs between TheraSphere and SIR-Spheres are < £300 and result from the additional cost of angiography required as part of the SIR-Spheres administration procedure. Pairwise NMB, assuming a £30,000 WTP threshold, for SIR-Spheres compared with TheraSphere was, therefore, close to zero (–£182). QuiremSpheres is associated with an incremental cost of £6955 relative to TheraSphere (exclusive of PAS). Pairwise NMB between QuiremSpheres and TheraSphere in the AG’s base case was –£6599, exclusive of PAS. In the analysis including the confidential PAS for QuiremScout, QuiremSpheres remained more costly than both TheraSphere and SIR-Spheres and, as such, the pairwise NMB remained negative (see Appendix 17 for full results).
In a fully incremental analysis, exclusive of the PAS discounts available for QuiremScout, sorafenib, lenvatinib and regorafenib, lenvatinib was the most cost-effective therapy and dominated TheraSphere (the lowest-costing SIRT treatment). Predicted NMB for lenvatinib compared with TheraSphere was –£2154. In a pairwise comparison of sorafenib with TheraSphere, the ICER for sorafenib was £31,974 per QALY, with an estimated NMB of –£150 (implying that TheraSphere is cost-effective compared with sorafenib at a WTP threshold of £30,000). In a fully incremental analysis inclusive of all confidential PAS discounts, lenvatinib remained the most cost-effective therapy across all scenarios, and dominated all three SIRTs, generating greater health benefits at lower costs. In pairwise comparisons of sorafenib with each SIRT, sorafenib also dominated all three SIRTs. Lenvatinib remained the most cost-effective option across 15 of the 17 AG scenarios when PAS discounts were included.
The results of the scenario analyses presented at list price showed that SIRTs were more likely to be cost-effective in the low tumour burden and ALBI 1 subgroup of patients, and when downstaging was permitted. The results of analyses conducted including PAS discounts for QuiremScout, sorafenib, lenvatinib and regorafenib, however, showed that the results of the AG’s economic analysis were robust to a range of alternative parameter values and assumptions, with a negative incremental NMB predicted for all SIRTs at a £30,000 WTP threshold (see Appendix 17 for details).
The AG’s economic analysis suggests that, although current life expectancy in patients ineligible for CTT is likely to be < 24 months, the predicted life extension generated by SIRT is likely to be < 3 months.
Strengths and limitations of the assessment
The key strengths of this assessment are as follows:
-
The reviews of clinical effectiveness and cost-effectiveness were based on comprehensive searches of the literature, which were supplemented by data identified in recent systematic reviews of CTT treatments.
-
The review of clinical effectiveness evidence included a detailed mapping and quality assessment of all comparative evidence on SIRT treatments across a range of alternative positions in the treatment pathway.
-
The AG’s economic evaluation includes a fully incremental analysis of the three SIRTs (SIR-Spheres, TheraSphere and QuiremSpheres) and relevant systemic therapies (sorafenib and lenvatinib) in patients with CTT-ineligible HCC.
-
The AG appropriately accounts for the fact that some patients eligible for SIRT treatment will fail the work-up procedure and will not go on to receive SIRT. Importantly, it recognises that patients who fail work-up are different from patients who successfully receive SIRT and tend to have inferior progression and survival outcomes.
-
The AG’s economic analysis includes an exploratory analysis of two potentially plausible prospective subgroups: (1) low tumour burden/ALBI 1 and (2) no MVI.
-
The AG’s economic analysis includes an exploration of the impact of downstaging in CTT-ineligible patients. The AG economic analysis also avoids double-counting the outcomes of patients who are downstaged to curative therapies.
The main weaknesses of the assessment are largely a consequence of weaknesses and gaps in the clinical evidence base:
-
There is very limited evidence on the comparative effectiveness of SIRT with CTT in patients with either early- or intermediate-stage HCC. The AG did not consider the identified clinical evidence sufficient to produce an economic analysis and, therefore, the presented independent economic assessment covers only part of the NICE scope. The BTG company submission included an economic analysis of downstaging in CTT-eligible patients, whereas Sirtex presented a cost-minimisation model. The limits of the clinical evidence supporting these analyses and uncertainties regarding the equivalence of SIRT and CTT in this population mean that these analyses may be of limited relevance for decision-making.
-
The AG did not have access to IPD from the SIRveNIB trial;21 instead, PFS and OS outcomes were replicated using a published algorithm. Although the precision of this replication is likely to be good, this process may have introduced a small loss of accuracy relative to the use of IPD directly. Furthermore, the lack of IPD meant that the SIRveNIB trial could not be included in scenario analyses exploring the low tumour burden/ALBI 1 and no-MVI subgroups.
-
Lack of IPD for the REFLECT trial,81 comparing lenvatinib with sorafenib, meant that there were limited options for including lenvatinib in the economic analysis and the modelled HRs were based on a subgroup that did not fully align with the population eligible for SIRT. Furthermore, the AG’s base case makes the assumption of proportional hazards between lenvatinib and sorafenib despite some evidence presented in previous technology appraisals that this assumption may not hold.
-
There was limited evidence on the relative effectiveness of TheraSphere compared with other SIRTs or systemic therapy, with the limited studies identified all rated as being at a high risk of bias.
-
There is no evidence on the comparative effectiveness of QuiremSpheres, with the exception of one small, methodologically weak pilot study provided as a late addendum by Terumo.
-
There is limited evidence on the long-term outcomes of patients who receive therapy with curative intent. The AG’s analysis and the Sirtex model present data from a historical US cohort study; these data are now several years old and potentially reflect a broader population of patients with HCC.
Uncertainties
The main uncertainties associated with the appraisal are as follows:
-
The comparative effectiveness of SIRT in patients eligible for transplant or eligible for CTTs, such as DEB-TACE, TACE and TAE, is highly uncertain, with identified evidence limited to a small number of mainly observational studies.
-
The comparative effectiveness of alternative SIRT (SIR-Spheres, TheraSphere and QuiremSpheres) in all HCC populations is largely unknown. The limited evidence available suggests that TheraSphere may be superior to SIR-Spheres for advanced HCC with PVI. The identified evidence is, however, of very low quality and, therefore, it is unknown whether or not the observed effects are the result of confounding bias. There is also no evidence on the comparative effectiveness of QuiremSpheres with any therapy, other than a very small pilot study with several methodological limitations that was provided as an addendum. This is significant, as QuiremSpheres uses a different work-up procedure and different radioactive isotope and therefore it is plausible that QuiremSpheres may have differential effectiveness when compared with SIR-Spheres and TheraSphere.
-
The Sirtex submission102 puts forward a subgroup of patients with a low tumour burden and preserved liver function as a potential subgroup of patients who may benefit from treatment with SIR-Spheres. This subgroup was, however, not prespecified and the randomisation procedure did not stratify for these characteristics. The subgroup analysis is also based on very few patients. The extent of any benefits in this subgroup are, therefore, subject to considerable uncertainty and a confirmatory study would be required to be confident that the observed benefits are not spurious.
-
The role of downstaging in a CTT-ineligible population is currently unclear. In the SARAH trial,19 a small proportion of patients were successfully downstaged to curative therapies. Advice received by the AG from clinical experts, however, suggests that downstaging in this population is likely to be very rare, and it is unclear whether or not the SARAH trial is representative of UK practice in this regard.
-
In the SARAH trial,19 patients with bilobar HCC had each lobe treated in separate SIRT administrations to avoid the risk of REILD. The Sirtex submission, however, suggests that, in UK practice, patients with bilobar HCC would have both lobes treated simultaneously. The impact of sequential versus simultaneous treatment is largely unknown and it is not fully clear what practice would be adopted in the UK; advice received from the AG’s clinical advisors, however, suggests that sequential treatment would be more likely to be used in the UK.
-
There is currently only limited evidence on the comparative effectiveness of combination therapy (SIRT combined with a systemic therapy). The searches of trial registration databases completed as part of the clinical effectiveness review, however, identified that a large RCT, STOP-HCC,74 is set to report shortly. This RCT compares TheraSphere plus sorafenib with sorafenib alone and will provide new evidence on this comparison.
-
In the NHS, systemic therapies are recommended only for those with Child–Pugh class A liver function; thus, the current standard of care for those with Child–Pugh class B liver function is BSC. There is a potential place for SIRT in a Child–Pugh class B7 population, who were represented in in the SARAH19 and SIRveNIB21 trials. However, there is currently no direct evidence on the comparative effectiveness of SIRT with BSC in this population, and currently no means of comparing them indirectly.
Chapter 10 Conclusions
The existing evidence cannot provide decision-makers with clear guidance on the comparative effectiveness of treatments in early- and intermediate-stage HCC. All of the identified studies were rated as being at a high risk of bias and included highly heterogeneous populations, limiting the conclusions that can be drawn from these results. The results of individual studies varied considerably, with some showing that CTT was superior to SIRT and vice versa. However, the available evidence suggests that SIRT may be beneficial in this population, with moderate improvements in PFS and transplantation rates.
The very limited evidence on the effectiveness of SIRT in early- and intermediate-HCC patients means that the AG was not able to generate a meaningful analysis of the value of SIRT in these populations. The focus of the AG’s economic assessment was therefore on the advanced-HCC population who are ineligible to receive CTT. In this population, two large randomised trials (SARAH19 and SIRveNIB21) have assessed the comparative effectiveness of SIR-Spheres with sorafenib. The results of these trials show that SIRT has similar effectiveness to sorafenib; notably, these studies were not designed as non-inferiority or equivalence trials. The systematic review also identified further evidence from a large RCT of the comparative effectiveness of the alternative systemic therapy lenvatinib with sorafenib, as well as observational evidence on the comparative effectiveness of TheraSphere with SIR-Spheres. The results of these studies were combined in a NMA, which showed no meaningful differences in OS between SIR-Spheres, sorafenib and lenvatinib. TheraSphere showed a significant improvement in OS when compared with SIR-Spheres, sorafenib and lenvatinib. However, there were only two retrospective studies that directly compared TheraSphere and SIR-Spheres and both were rated as being at a high risk of bias. Therefore, there is considerable uncertainty regarding the efficacy of TheraSphere, and the AG elected to assume equal efficacy across each SIRT technology in its base-case analysis.
The AG’s economic analysis showed that SIRTs are very unlikely to be cost-effective up to a threshold of £30,000 per QALY. The fully incremental analysis, including confidential PAS discounts, showed that lenvatinib was the most cost-effective therapy, dominating all three SIRTs (i.e. producing more QALYs at a lower cost). Pairwise comparisons of sorafenib with each SIRTs also showed that sorafenib dominated all three SIRTs. The results of DSA and scenarios analysis, considering a variety of alternative assumptions, including the modelling of two alternative subgroups (low tumour burden/ALBI 1 and no MVI), showed that the results of the AG’s economic analysis were generally robust to alternative parameter values and assumptions.
The AG’s economic analysis suggests that NICE’s criteria142 for life-extending therapies given at the end of life are not met for SIRT in the broad advanced population as they do not meet the required 3-month extension to life. In the low tumour burden/ALBI 1 subgroup, there is a possibility that SIRT treatments may meet this threshold. However, the ICER for the most cost-effective SIRT technology in this scenario remains > £50,000 when PAS discounts are considered.
Implications for service provision
In the event that SIRT was recommended for use in the NHS, the AG does not anticipate that any substantial changes to service provision would be required, as SIRT (SIR-Spheres and TheraSphere) is already routinely administered across a number of specialist liver units.
Suggested research priorities
As discussed above, no strong conclusions should be drawn in the early- and intermediate-HCC populations owing to considerable uncertainty in estimates of effectiveness and a high risk of bias. A priority for further research is, therefore, the conduct of studies in these populations. In designing any evaluations, careful consideration should be given to the recruited population, and, where possible, studies should avoid combining these heterogeneous populations as the aims of therapy and range of treatments available vary considerably. Careful consideration should also be given to the outcomes measured. Many studies reported on TTP, but this was rarely defined within the study report and there were concerns regarding whether or not these data had been properly analysed. Few studies also reported on downstaging outcomes; these potentially play an important role in determining patient outcomes and downstaging is increasingly becoming a realistic option for some patients with intermediate-stage HCC.
The low tumour burden and preserved liver function subgroup potentially represents a group of prospectively identifiable patients for whom SIRT may be beneficial when compared with sorafenib. However, the evidence in support of these observed benefits is weak, because the observed results are based on a post hoc analysis of the SARAH trial,19 which included only a small proportion of the total number of recruited patients. Future work considering this subgroup may, therefore, be useful. Of priority would be a similar analysis of the results of the SIRveNIB trial;21 this could not be undertaken as part of the current appraisal as IPD were unavailable. A confirmatory trial in this subgroup may also be desirable depending on the results of any analysis of the SIRveNIB trial. 21
There is currently only very limited evidence on the comparative effectiveness of the three SIRTs with one another. Future randomised prospective studies evaluating the alternative SIRTs would, therefore, be useful.
Acknowledgements
We would like to thank Dr Daniel Swinson (Consultant Clinical Oncologist, Leeds Teaching Hospitals NHS Trust) for advice throughout the project. We would also like to thank Professor Sofia Dias for support with the NMA, and Peter Murphy, who supported the development of the economic analysis. We would like to thank the companies (BTG, Terumo and Sirtex) for responding to requests for additional data, and NHS Blood and Transplant for providing data from the UK Transplant Registry for this project.
Contributions of authors
Matthew Walton (https://orcid.org/0000-0003-1932-3689) (Research Fellow, Health Economics) contributed to the protocol and the review of economic analyses. He developed the economic model and undertook the analysis and interpretation of the results, and contributed to the writing of the report.
Ros Wade (https://orcid.org/0000-0002-8666-8110) (Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, data extraction, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report, and had overall responsibility for the clinical effectiveness sections.
Lindsay Claxton (https://orcid.org/0000-0002-1795-7568) (Research Fellow, Health Economics) contributed to the protocol and undertook the review of economic studies. She developed the economic model and contributed to the analysis and interpretation of the results, and the writing of the report.
Sahar Sharif-Hurst (https://orcid.org/0000-0001-6885-0456) (Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, data extraction and validity assessments. She developed the synthesis models and undertook the analysis. She also contributed to the interpretation of the results and the writing of the report.
Melissa Harden (https://orcid.org/0000-0003-2338-6869) (Information Specialist) contributed to the protocol development, developed the search strategies, conducted a range of searches to locate studies and wrote the sections of the report relating to the literature searches.
Jai Patel (https://orcid.org/0000-0001-9248-0125) (Consultant Radiologist) provided expert clinical advice, contributed to the protocol and interpretation of the results and commented on drafts of the report.
Ian Rowe (https://orcid.org/0000-0003-1288-0749) (Honorary Consultant Hepatologist) provided expert clinical advice, contributed to the protocol and interpretation of the results and commented on drafts of the report.
Robert Hodgson (https://orcid.org/0000-0001-6962-2893) (Research Fellow, Health Economics) contributed to the development of the protocol and all aspects of the cost-effectiveness work. He also contributed to the interpretation of the results and the writing of the report, and had overall responsibility for the cost-effectiveness sections.
Alison Eastwood (https://orcid.org/0000-0003-1079-7781) (Professor, Evidence Synthesis) contributed to the protocol, study selection, validity assessments and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report, and had overall responsibility for the project.
Data-sharing statement
This is a systematic review that includes published and confidential data supplied by the participating manufacturers. No primary data were created for this report. The majority of data used are as reported in the publications listed throughout this report. Further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
- Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450-62. https://doi.org/10.1056/NEJMra1713263.
- Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am 2010;24:899-91. https://doi.org/10.1016/j.idc.2010.07.004.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-56. https://doi.org/10.1097/00000421-198212000-00014.
- Office for National Statistics . Cancer Registration Statistics, England: 2017 2019. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland (accessed 29 August 2019).
- Ryder SD. British Society of Gastroenterology . Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003;52:iii1-8. https://doi.org/10.1136/gut.52.suppl_3.iii1.
- Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. https://doi.org/10.1111/liv.12818.
- Cancer Research UK. Liver Cancer Survival. Cancer Research UK; n.d.
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. https://doi.org/10.1056/NEJM199603143341104.
- Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 2017;9:808-14. https://doi.org/10.4254/wjh.v9.i18.808.
- National Institute for Health and Care Excellence (NICE) . Sorafenib for Treating Advanced Hepatocellular Carcinoma [TA474] 2017. www.nice.org.uk/guidance/ta474 (accessed 29 May 2019).
- National Institute for Health and Care Excellence (NICE) . Lenvatinib for Untreated Advanced Hepatocellular Carcinoma [TA551] 2018. www.nice.org.uk/guidance/ta551 (accessed 29 May 2019).
- National Institute for Health and Care Excellence (NICE) . Regorafenib for Previously Treated Advanced Hepatocellular Carcinoma [TA555] 2019. www.nice.org.uk/guidance/ta555 (accessed 29 May 2019).
- National Institute for Health and Care Excellence (NICE) . Selective Internal Radiation Therapy for Primary Hepatocellular Carcinoma. Interventional Procedures Guidance [IPG460] 2013. www.nice.org.uk/guidance/ipg460 (accessed 8 May 2019).
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Centre for Reviews and Dissemination . Search Strategies for DARE 2015. www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 7 May 2019).
- Higgins JP, Savovic J, Page MJ, Sterne JA. ROB2 Development Group . Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) 2019. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 29 May 2019).
- Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled Phase 3 trial. Lancet Oncol 2017;18:1624-36. https://doi.org/10.1016/S1470-2045(17)30683-6.
- Bouattour M, Assenat E, Guiu B, Ilonca Alina D, Pageaux GP, Sibert A, et al. LBA-001 Efficacy, tolerability and impact on quality of life of selective internal radiation therapy (with yttrium-90 resin microspheres) or sorafenib in patients with locally advanced hepatocellular carcinoma: the SARAH trial. Ann Oncol 2017;28:iii150-3.
- Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913-21. https://doi.org/10.1200/JCO.2017.76.0892.
- Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int 2015;35:1715-21. https://doi.org/10.1111/liv.12750.
- Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2015;38:352-60. https://doi.org/10.1007/s00270-014-1012-0.
- Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, et al. Safety and toxicity of radioembolization plus sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int 2015;35:620-6. https://doi.org/10.1111/liv.12622.
- Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 Radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016;151:1155-63.e2. https://doi.org/10.1053/j.gastro.2016.08.029.
- Gabr A, Kallini JR, Lewandowski RJ, Salem R. Fluoroscopic radiation exposure in chemoembolization and radioembolization: results from a prospective randomized study. J Vasc Interv Radiol 2017;28:1272-3. https://doi.org/10.1016/j.jvir.2017.05.005.
- Gordon A, Lewandowski R, Hickey R, Kallini J, Gabr A, Sato K, et al. Prospective randomized phase 2 study of chemoembolization versus radioembolization in hepatocellular carcinoma: results from the PREMIERE trial. J Vasc Interv Radiol 2016;27:S61-2. https://doi.org/10.1016/j.jvir.2015.12.168.
- Kulik L, Vouche M, Koppe S, Lewandowski RJ, Mulcahy MF, Ganger D, et al. Prospective randomized pilot study of Y90+/-sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol 2014;61:309-17. https://doi.org/10.1016/j.jhep.2014.03.023.
- Lewandowski RJ, Andreoli JM, Hickey R, Kallini JR, Gabr A, Baker T, et al. Angiogenic response following radioembolization: results from a randomized pilot study of yttrium-90 with or without sorafenib. J Vasc Interv Radiol 2016;27:1329-36. https://doi.org/10.1016/j.jvir.2016.03.043.
- Vouche M, Kulik L, Atassi R, Memon K, Hickey R, Ganger D, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90 +/− sorafenib. Hepatology 2013;58:1655-66. https://doi.org/10.1002/hep.26487.
- Kirchner T, Marquardt S, Werncke T, Kirstein MM, Brunkhorst T, Wacker F, et al. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol 2019;44:1554-61. https://doi.org/10.1007/s00261-018-1802-y.
- El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization?. Liver Int 2015;35:627-35. https://doi.org/10.1111/liv.12637.
- Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013;11:1358-65.e1. https://doi.org/10.1016/j.cgh.2013.04.028.
- Memon K, Kulik L, Lewandowski RJ, Gupta R, Ryu RK, Miller FH, et al. Prospective evaluation of patients with early-/intermediate-stage hepatocellular carcinoma with disease progression following arterial locoregional therapy: candidacy for systemic treatment or clinical trials. J Vasc Interv Radiol 2013;24:1189-97.e2. https://doi.org/10.1016/j.jvir.2012.12.025.
- Hickey R, Mouli S, Kulik L, Desai K, Thornburg B, Ganger D, et al. Independent analysis of albumin-bilirubin grade in a 765-patient cohort treated with transarterial locoregional therapy for hepatocellular carcinoma. J Vasc Interv Radiol 2016;27:795-802. https://doi.org/10.1016/j.jvir.2016.03.005.
- Maccauro M, Sposito C, Chiesa C, Romito R, Spreafico C, Morosi C, et al. Trans-arterial radioembolization (TARE) with Y90 glass microspheres plus sorafenib versus tare alone for the treatment of unresectable hepatocellular carcinoma (HCC): a matched case-control study. Eur J Nucl Med Mol Imaging 2014;41.
- Woodall CE, Scoggins CR, Ellis SF, Tatum CM, Hahl MJ, Ravindra KV, et al. Is selective internal radioembolization safe and effective for patients with inoperable hepatocellular carcinoma and venous thrombosis?. J Am Coll Surg 2009;208:375-82. https://doi.org/10.1016/j.jamcollsurg.2008.12.009.
- Biederman DM, Tabori NE, Titano JJ, Pierobon ES, Fischman AM, Patel RS, et al. Outcomes of yttrium-90 therapy in the treatment of hepatocellular carcinoma (HCC) with portal vein thrombosis (PVT): resin-based vs. glassbased microspheres. J Vasc Interv Radiol 2015;26:S109-10. https://doi.org/10.1016/j.jvir.2014.12.296.
- Biederman DM, Titano JJ, Tabori NE, Pierobon ES, Alshebeeb K, Schwartz M, et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J Vasc Interv Radiol 2016;27:812-21.e2. https://doi.org/10.1016/j.jvir.2016.01.147.
- Van Der Gucht A, Jreige M, Denys A, Blanc-Durand P, Boubaker A, Pomoni A, et al. Resin versus glass microspheres for 90Y transarterial radioembolization: comparing survival in unresectable hepatocellular carcinoma using pretreatment partition model dosimetry. J Nucl Med 2017;58:1334-40. https://doi.org/10.2967/jnumed.116.184713.
- Bhangoo MS, Karnani DR, Hein PN, Giap H, Knowles H, Issa C, et al. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol 2015;6:469-78. https://doi.org/10.3978/j.issn.2078-6891.2015.056.
- d’Abadie P, Hesse M, Jamar F, Lhommel R, Walrand S. 90Y TOF-PET based EUD reunifies patient survival prediction in resin and glass microspheres radioembolization of HCC tumours. Phys Med Biol 2018;63. https://doi.org/10.1088/1361-6560/aaf205.
- Cho YY, Lee M, Kim HC, Chung JW, Kim YH, Gwak GY, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0154986.
- de la Torre MA, Buades-Mateu J, de la Rosa PA, Lué A, Bustamante FJ, Serrano MT, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int 2016;36:1206-12. https://doi.org/10.1111/liv.13098.
- Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int 2015;35:1036-47. https://doi.org/10.1111/liv.12574.
- Soydal C, Arslan MF, Kucuk ON, Idilman R, Bilgic S. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona Clinic Liver Cancer stage B-C hepatocellular cancer patients. Nucl Med Commun 2016;37:646-9. https://doi.org/10.1097/MNM.0000000000000486.
- Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2. https://doi.org/10.1053/j.gastro.2010.10.049.
- Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol 2013;36:714-23. https://doi.org/10.1007/s00270-012-0481-2.
- Akinwande O, Philips P, Scoggins C, Martin RC. Radioembolization versus chemoembolization (DEBDOX) for the treatment of unresectable hepatocellular carcinoma: a propensity matched study. Anticancer Res 2016;36:239-46.
- Akinwande O, Martin R. Is radioembolization equivalent to chemoembolization (DEBDOX) for the treatment of HCC? A propensity matched observational study. J Vasc Interv Radiol 2016;27. https://doi.org/10.1016/j.jvir.2015.12.633.
- Radosa CG, Radosa JC, Grosche-Schlee S, Zöphel K, Plodeck V, Kühn JP, et al. Holmium-166 radioembolization in hepatocellular carcinoma: feasibility and safety of a new treatment option in clinical practice. Cardiovasc Intervent Radiol 2019;42:405-12. https://doi.org/10.1007/s00270-018-2133-7.
- Steel J, Baum A, Carr B. Quality of life in patients diagnosed with primary hepatocellular carcinoma: hepatic arterial infusion of Cisplatin versus 90-Yttrium microspheres (Therasphere). Psycho-Oncology 2004;13:73-9. https://doi.org/10.1002/pon.725.
- Moroz P, Anderson JE, Van Hazel G, Gray BN. Effect of selective internal radiation therapy and hepatic arterial chemotherapy on normal liver volume and spleen volume. J Surg Oncol 2001;78:248-52. https://doi.org/10.1002/jso.1162.
- Maccauro M, Spreafico C, Chiesa C, Mira M, De Nila M, Romito R, et al. Personalized treatment planning in radioembolization of hepatocarcinoma with 90 Y glass microspheres: update of clinical outcomes in the Milan experience. Eur J Nucl Med Mol Imaging 2016;43.
- Pellerito RE, Codegone A, Tabone M, Richetta E, Miranti A, Stasi M. Intrahepatic treatment of advanced hepatocellular carcinoma with 90Y Sirtex or 131I Lipiodol: preliminary results of a case control study. Clin Transl Imaging 2013;1:S126-7.
- Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. https://doi.org/10.1007/s00270-009-9711-7.
- Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol 2011;197:W562-70. https://doi.org/10.2214/AJR.10.4379.
- Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. https://doi.org/10.1038/bjc.2014.199.
- Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2011;22:1545-52. https://doi.org/10.1016/j.jvir.2011.07.002.
- van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, van Pelt J, et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie 2011;34:368-76. https://doi.org/10.1159/000329602.
- Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. https://doi.org/10.1016/S0140-6736(02)08649-X.
- Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, et al. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma – a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). Cancer Chemother Pharmacol 1992;31:S1-6. https://doi.org/10.1007/BF00687096.
- Chang JM, Tzeng WS, Pan HB, Yang CF, Lai KH. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer 1994;74:2449-53. https://doi.org/10.1002/1097-0142(19941101)74: 9<2449::aid-cncr2820740910>3.0.co;2-4.
- Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer 2013;108:1252-9. https://doi.org/10.1038/bjc.2013.85.
- Yu SC, Hui JW, Hui EP, Chan SL, Lee KF, Mo F, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology 2014;270:607-20. https://doi.org/10.1148/radiol.13130498.
- Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. https://doi.org/10.1007/s00270-009-9750-0.
- Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol 2016;34:2046-53. https://doi.org/10.1200/JCO.2015.64.0821.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). EJC 2009;45:228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
- Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. https://doi.org/10.1002/hep.24451.
- Cardiovascular and Interventional Radiological Society of Europe . CIRSE Registry for SIR-Spheres Therapy (CIRT) 2014. https://ClinicalTrials.gov/show/NCT02305459 (accessed 1 February 2019).
- Vanderbilt-Ingram Cancer Center . Yttrium Y 90 Resin Microspheres Data Collection in Unresectable Liver Cancer: The RESIN Study 2016. https://ClinicalTrials.gov/show/NCT02685631 (accessed 1 February 2019).
- Taipei Veterans General Hospital . REgistry of Selective Internal Radiation Therapy in TaiwaN (RESIN) 2017. https://ClinicalTrials.gov/show/NCT03292991 (accessed 1 February 2019).
- Gebski V, Gibbs E, Gandhi M, Chatellier G, Dinut A, Pereira H, et al. VESPRO: an individual patient data prospective meta-analysis of selective internal radiation therapy versus sorafenib for advanced, locally advanced, or recurrent hepatocellular carcinoma of the SARAH and SIRveNIB trials. JMIR Res Protoc 2017;6. https://doi.org/10.2196/resprot.7016.
- BTG Ltd . Efficacy Evaluation of Therasphere in Patients With Inoperable Liver Cancer (STOP-HCC) 2012. https://clinicaltrials.gov/show/nct01556490 (accessed 28 January 2019).
- UMC Utrecht . HEPAR Primary: Holmium-166-Radioembolization in Hepatocellular Carcinoma Patients 2017. https://ClinicalTrials.gov/show/NCT03379844 (accessed 1 February 2019).
- Leiden University Medical Center . Holmium Radioembolization As Adjuvant Treatment to RFA for Early Stage HCC: Dose Finding Study (HORA EST HCC) 2018. https://ClinicalTrials.gov/show/NCT03437382 (accessed 1 February 2019).
- Terumo Europe NV. QuiremSpheres Observational Study (Hope166). Bethesda, MD: National Library of Medicine (US); 2018.
- Reinders MT, Hoffmann RT, Lam MG, Radosa C. First Results of Homium-166 Radioembolisation Compared with Yttrium-90 Radioembolisation in HCC Patients. Leuven: Terumo Europe NV; 2019.
- Kallini JR, Gabr A, Thorlund K, Balijepalli C, Ayres D, Kanters S, et al. Comparison of the adverse event profile of TheraSphere® with SIR-Spheres® for the treatment of unresectable hepatocellular carcinoma: a systematic review. Cardiovasc Intervent Radiol 2017;40:1033-43. https://doi.org/10.1007/s00270-017-1594-4.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. https://doi.org/10.1056/NEJMoa0708857.
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. https://doi.org/10.1016/S0140-6736(18)30207-1.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. https://doi.org/10.1002/sim.1875.
- Memon K, Kulik LM, Lewandowski RJ, Wang E, Wang J, Ryu RK, et al. Comparative study of staging systems for hepatocellular carcinoma in 428 patients treated with radioembolization. J Vasc Interv Radiol 2014;25:1056-66. https://doi.org/10.1016/j.jvir.2014.01.010.
- Vilgrain V, Bouattour M, Sibert A, Lebtahi R, Ronot M, Pageaux GP, et al. SARAH: a randomised controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma. J Hepatol 2017;66:S85-6. https://doi.org/10.1016/S0168-8278(17)30436-1.
- Cho MT, Kessler J, Park J, Singh G, Chen YJ, Ituarte PHG, et al. A single institute retrospective trial of concurrent chemotherapy with SIR-Sphere versus SIR-Sphere alone in patients with chemotherapy-resistant colorectal cancer liver metastases. J Clin Oncol 2016;34. https://doi.org/10.1200/jco.2016.34.4_suppl.770.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-analysis of Randomised Controlled Trials. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2014.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Rognoni C, Ciani O, Sommariva S, Tarricone R. Real-world data for the evaluation of transarterial radioembolization versus sorafenib in hepatocellular carcinoma: a cost-effectiveness analysis. Value Health 2017;20:336-44. https://doi.org/10.1016/j.jval.2016.09.2397.
- Rognoni C, Ciani O, Sommariva S, Tarricone R. Treatment sequence in intermediate stage hepatocellular carcinoma: a cost-effectiveness analysis of two approaches with trans-arterial radioembolization. Value Health 2017;20. https://doi.org/10.1016/j.jval.2016.09.2397.
- Rostambeigi N, Dekarske A, Austin E, Golzarian J, Cressman E. Simulation study on cost-effectiveness of radioembolization compared with trans-arterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:S104-5. https://doi.org/10.1016/j.jvir.2013.12.292.
- Rostambeigi N, Dekarske AS, Austin EE, Golzarian J, Cressman EN. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1075-84. https://doi.org/10.1016/j.jvir.2014.04.014.
- Marqueen KE, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost-effectiveness analysis of selective internal radiation therapy with yttrium-90 resin microspheres versus sorafenib in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2018;102. https://doi.org/10.1016/j.ijrobp.2018.07.1185.
- Chaplin S, Taylor M, Lapon J, White J. Economic evaluation of glass yttrium-90 microspheres versus sorafenib for the treatment of advanced hepatocellular carcinoma: cost effectiveness analysis in the United Kingdom. Cardiovasc Intervent Radiol 2015;38:S279-80.
- Parikh ND, Singal AG, Kulik LM, Hutton D. Cost-effectiveness of sorafenib versus selective internal radiation therapy for patients with advanced hepatocellular carcinoma. Hepatology 2018;68:532A-3A.
- Palmer D, Ross P, Shah T, Yu D, Shergill S, Patterson K, et al. Cost effectiveness of selective internal radiation therapy (SIRT) with Y- 90 resin microspheres versus sorafenib in Barcelona Clinic Liver Cancer (BCLC) stage C hepatocellular carcinoma patients in the UK. Ann Oncol 2017;28:239-40. https://doi.org/10.1093/annonc/mdx369.087.
- Rognoni C, Ciani O, Sommariva S, Tarricone R. Cost-effectiveness analysis of treatments involving radioembolization in intermediate-stage hepatocellular carcinoma. J Comp Eff Res 2018;7:209-21. https://doi.org/10.2217/cer-2017-0050.
- Llovet JM, Finn RS. Negative phase 3 study of 90Y microspheres versus sorafenib in HCC. Lancet Oncol 2018;19. https://doi.org/10.1016/S1470-2045(18)30025-1.
- IBM . IBM Micromedex With Watson n.d. www.ibm.com/products/micromedex-with-watson (accessed 6 September 2019).
- National Institutes of Health . SEER – Medicare Linked Database n.d. https://healthcaredelivery.cancer.gov/seermedicare/ (accessed 6 September 2019).
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-6. https://doi.org/10.1016/S0140-6736(16)32453-9.
- Sirtex Medical Ltd . Sirtex Medical Company Evidence Submission: SIR-Spheres Y-90 Resin Microspheres 2019.
- BTG . BTG Company Evidence Submission for TheraSphere 2019.
- Terumo . Terumo Company Evidence Submission: Selective Internal Radiation Therapy (SIRT) With Holmium-166 Microspheres (QuiremSpheres) for Treating Unresectable Hepatocellular Carcinoma and Holmium-166 Microspheres Work-up Procedure (QuiremScout) 2019.
- Fateen W, Khan F, O’Neill RJ, James MW, Ryder SD, Aithal GP. Healthcare costs of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Hepatocell Carcinoma 2017;4:123-30. https://doi.org/10.2147/JHC.S144068.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- NHS Improvement . National Schedule of Reference Costs 2017–2018 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 27 August 2019).
- Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68:1429-40. https://doi.org/10.1002/hep.29691.
- National Institute for Health and Care Excellence (NICE) . Guselkumab for Treating Moderate to Severe Plaque Psoriasis 2018. www.nice.org.uk/guidance/ta521 (accessed 21 August 2019).
- National Institute for Health and Care Excellence (NICE) . Golimumab for Treating Non-Radiographic Axial Spondyloarthritis 2018. www.nice.org.uk/guidance/ta497 (accessed 21 August 2019).
- National Institute for Health and Care Excellence (NICE) . Aflibercept for Treating Choroidal Neovascularisation 2017. www.nice.org.uk/Guidance/TA486 (accessed 21 August 2019).
- Kanwal F, Befeler A, Chari RS, Marrero J, Kahn J, Afdhal N, et al. Potentially curative treatment in patients with hepatocellular cancer – results from the liver cancer research network. Aliment Pharmacol Ther 2012;36:257-65. https://doi.org/10.1111/j.1365-2036.2012.05174.x.
- Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18090.
- CIRSE Registry for SIR-Spheres Therapy . CIRSE Registry for SIR-Spheres Therapy (CIRT): Preliminary Data on Patients With HCC 2019.
- Joint Formulary Committee . British National Formulary (Online) 2019. www.medicinescomplete.com (accessed 17 April 2019).
- Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT 2019. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 20 August 2019).
- Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials - Extrapolation with Patient-Level Data. Sheffield: Decision Support Unit, School of Health and Related Research, University of Sheffield; 2013.
- Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. https://doi.org/10.1111/j.1600-6143.2009.02695.x.
- Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. https://doi.org/10.1111/j.1600-6143.2007.01965.x.
- NHS Blood and Transplant . Annual Report on Liver Transplantation 2018.
- NHS England . Liver Transplantation Service (Adults) 2017.
- Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation?. J Gastrointest Surg 2008;12:1699-708. https://doi.org/10.1007/s11605-008-0652-2.
- Gordon-Weeks AN, Snaith A, Petrinic T, Friend PJ, Burls A, Silva MA. Systematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteria. Br J Surg 2011;98:1201-8. https://doi.org/10.1002/bjs.7561.
- National Institute for Health and Care Excellence (NICE) . Lenvatinib for Untreated Advanced Hepatocellular Carcinoma [TA551] - Evidence 2018. www.nice.org.uk/guidance/ta551/evidence (accessed 28 August 2019).
- Lim KC, Wang VW, Siddiqui FJ, Shi L, Chan ES, Oh HC, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology 2015;61:227-37. https://doi.org/10.1002/hep.27135.
- Ratcliffe J, Buxton M, Young T, Longworth L. Determining priority for liver transplantation: a comparison of cost per QALY and discrete choice experiment-generated public preferences. Appl Health Econ Health Policy 2005;4:249-55. https://doi.org/10.2165/00148365-200504040-00007.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life. London: Nuffield Trust; 2014.
- Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. https://doi.org/10.1053/j.gastro.2009.09.006.
- Bryce K, Tsochatzis EA. Downstaging for hepatocellular cancer: harm or benefit?. Transl Gastroenterol Hepatol 2017;2. https://doi.org/10.21037/tgh.2017.11.18.
- Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94. https://doi.org/10.1053/j.gastro.2012.05.052.
- NHS Blood and Transplant Organ Donation and Transplantation Directorate – Liver Advisory Group . Update on the HCC Down-Staging Service Evaluation 2019.
- Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1110-y.
- NHS Blood and Transplant . UK Transplant Registry Data 2019.
- de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol 2015;21:11185-98. https://doi.org/10.3748/wjg.v21.i39.11185.
- National Institute for Health and Care Excellence (NICE) . Midostaurin for Untreated Acute Myeloid Leukaemia [TA523] 2018. www.nice.org.uk/Guidance/TA523 (accessed 3 September 2019).
- National Institute for Health and Care Excellence (NICE) . Tisagenlecleucel for Treating Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia in People Aged up to 25 Years [TA554] 2018. www.nice.org.uk/guidance/ta554 (accessed 3 September 2019).
- National Institute for Health and Care Excellence (NICE) . Gemtuzumab Ozogamicin for Untreated Acute Myeloid Leukaemia [TA545] 2018. www.nice.org.uk/guidance/ta545 (accessed 3 September 2019).
- Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826-37. https://doi.org/10.1002/hep.26014.
- National Institute for Health and Care Excellence (NICE) . Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma [TA189] 2010.
- European Medicines Agency . Stivarga 40mg Film-Coated Tablets: Summary of Product Characteristics 2018. www.medicines.org.uk/emc/product/1263/smpc/history#gref (accessed 6 September 2019).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- White JD, Dale M, Morgan H, Sewell B, Carolan-Rees G. Comissioning Through Evaluation: Selective Internal Radiation Therapy (SIRT). Cardiff: CEDAR Healthcare Technology Research Centre; 2017.
- Woodcock F, Doble B. CANCER 2015 Consortium . Mapping the EORTC-QLQ-C30 to the EQ-5D-3L: an assessment of existing and newly developed algorithms. Med Decis Making 2018;38:954-67. https://doi.org/10.1177/0272989X18797588.
- Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship?. Health Qual Life Outcomes 2009;7. https://doi.org/10.1186/1477-7525-7-27.
- Szende A, Janssen B, Cabasés J. Self-Reported Population Health: An International Perspective Based on EQ-5D 2014. https://doi.org/10.1007/978-94-007-7596-1.
- Russell RT, Feurer ID, Wisawatapnimit P, Lillie ES, Castaldo ET, Pinson CW. Profile of health-related quality of life outcomes after liver transplantation: univariate effects and multivariate models. HPB 2008;10:30-7. https://doi.org/10.1080/13651820701883106.
- Estraviz B, Quintana JM, Valdivieso A, Bilbao A, Padierna A, de Urbina JO, et al. Factors influencing change in health-related quality of life after liver transplantation. Clin Transplant 2007;21:481-99. https://doi.org/10.1111/j.1399-0012.2007.00672.x.
- Telles-Correia D, Barbosa A, Mega I, Mateus E, Monteiro E. When does quality of life improve after liver transplantation? A longitudinal prospective study. Transplant Proc 2009;41:904-5. https://doi.org/10.1016/j.transproceed.2009.01.051.
- Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol 2008;48:567-77. https://doi.org/10.1016/j.jhep.2007.12.013.
- Younossi ZM, McCormick M, Price LL, Boparai N, Farquhar L, Henderson JM, et al. Impact of liver transplantation on health-related quality of life. Liver Transpl 2000;6:779-83. https://doi.org/10.1053/jlts.2000.18499.
- Bryan S, Ratcliffe J, Neuberger JM, Burroughs AK, Gunson BK, Buxton MJ. Health-related quality of life following liver transplantation. Qual Life Res 1998;7:115-20. https://doi.org/10.1023/a: 1008849224815.
- Vilgrain V, Abdel-Rehim M, Sibert A, Ronot M, Lebtahi R, Castéra L, et al. SARAH Trial Group . Radioembolisation with yttrium-90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-474.
- National Institute for Health and Care Excellence (NICE) . Lenvatinib and Sorafenib for Treating Differentiated Thyroid Cancer After Radioactive Iodine 2018.
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-55. https://doi.org/10.1093/annonc/mdy308.
- Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville JM. Performance of Ovid MEDLINE search filters to identify health state utility studies. Int J Technol Assess Health Care 2017;33:472-80. https://doi.org/10.1017/S0266462317000897.
- Canadian Agency for Drugs and Technologies in Health (CADTH) . CADTH Database Search Filters 2016. www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters (accessed 7 March 2019).
- Liver Advisory Group – NHS Blood and Transplant . Update on the HCC Down-Staging Service Evaluation 2018.
- She WH, Cheung TT, Yau TC, Chan AC, Chok KS, Chu FS, et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr 2014;3:185-93. https://doi.org/10.3978/j.issn.2304-3881.2014.07.09.
- Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:224-30. https://doi.org/10.1016/j.jvir.2009.10.013.
- Kwok PC, Leung KC, Cheung MT, Lam TW, Szeto LT, Chou SQ, et al. Survival benefit of radioembolization for inoperable hepatocellular carcinoma using yttrium-90 microspheres. J Gastroenterol Hepatol 2014;29:1897-904. https://doi.org/10.1111/jgh.12621.
- Song JE, Jung KS, Kim DY, Song K, Won JY, Lee HW, et al. Transarterial radioembolization versus concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma: a propensity score matching analysis. Int J Radiat Oncol Biol Phys 2017;99:396-40. https://doi.org/10.1016/j.ijrobp.2017.05.049.
- Oladeru OT, Miccio JA, Yang J, Xue Y, Ryu S, Stessin AM. Conformal external beam radiation or selective internal radiation therapy-a comparison of treatment outcomes for hepatocellular carcinoma. J Gastrointest Oncol 2016;7:433-40. https://doi.org/10.21037/jgo.2015.10.04.
- Rühl R, Seidensticker M, Peters N, Mohnike K, Bornschein J, Schütte K, et al. Hepatocellular carcinoma and liver cirrhosis: assessment of the liver function after yttrium-90 radioembolization with resin microspheres or after CT-guided high-dose-rate brachytherapy. Dig Dis 2009;27:189-99. https://doi.org/10.1159/000218352.
- D’Avola D, Lñarrairaegui M, Bilbao JI, Martinez-Cuesta A, Alegre F, Herrero JI, et al. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 2009;56:1683-8.
- Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14. https://doi.org/10.1002/cncr.24884.
- Kallini JR, Gabr A, Ali R, Abouchaleh N, Riaz A, Baker T, et al. Pretransplant intra-arterial liver-directed therapy does not increase the risk of hepatic arterial complications in liver transplantation: a single-center 10-year experience. Cardiovasc Intervent Radiol 2018;41:231-8. https://doi.org/10.1007/s00270-017-1793-z.
- Gabr A, Abouchaleh N, Ali R, Vouche M, Atassi R, Memon K, et al. Comparative study of post-transplant outcomes in hepatocellular carcinoma patients treated with chemoembolization or radioembolization. Eur J Radiol 2017;93:100-6. https://doi.org/10.1016/j.ejrad.2017.05.022.
- Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol 2009;27:5734-42. https://doi.org/10.1200/JCO.2009.23.1282.
- Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, et al. Radiation segmentectomy versus selective chemoembolization in the treatment of early-stage hepatocellular carcinoma. J Vasc Interv Radiol 2018;29:30-7.e2. https://doi.org/10.1016/j.jvir.2017.08.026.
- Ahmad J, Rhee J, Carr BI. The effects of hepatic artery chemotherapy on viral hepatitis in patients with hepatocellular carcinoma. Dig Dis Sci 2005;50:331-5. https://doi.org/10.1007/s10620-005-1606-0.
- Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol 2017;28:777-85.e1. https://doi.org/10.1016/j.jvir.2017.02.018.
- Padia SA, Johnson GE, Horton KJ, Kwan S, Vaidya S, Ingraham CR, et al. Segmental yttrium-90 radioembolization versus chemoembolization for localized hepatocellular carcinoma. J Clin Oncol 2016;34. https://doi.org/10.1200/JCO.2016.34.15_suppl.4084.
- Newell PH, Wu Y, Hoen H, Uppal R, Thiesing JT, Sasadeusz K, et al. Multimodal treatment of unresectable hepatocellular carcinoma to achieve complete response results in improved survival. HPB 2015;17:454-60. https://doi.org/10.1111/hpb.12377.
- Taussig MD, Irene Koran ME, Mouli SK, Ahmad A, Geevarghese S, Baker JC, et al. Neutrophil to lymphocyte ratio predicts disease progression following intra-arterial therapy of hepatocellular carcinoma. HPB 2017;19:458-64. https://doi.org/10.1016/j.hpb.2017.01.013.
- McDevitt JL, Alian A, Kapoor B, Bennett S, Gill A, Levitin A, et al. Single-center comparison of overall survival and toxicities in patients with infiltrative hepatocellular carcinoma treated with yttrium-90 radioembolization or drug-eluting embolic transarterial chemoembolization. J Vasc Interv Radiol 2017;28:1371-7. https://doi.org/10.1016/j.jvir.2017.05.017.
- Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, et al. Is radioembolization (90Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg 2015;24:270-5. https://doi.org/10.1016/j.suronc.2015.06.008.
- Philips P, Edwards J, Russell B, Scoggins CR, Martin RC. A comparative analysis of transarterial therapy for unresectable hepatocellular carcinoma with portal vein thrombosis: radioembolization 90Y versus doxorubicin drug eluting beads (DEB-DOX). Ann Surg Oncol 2015;22.
- Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology 2017;283:895-90. https://doi.org/10.1148/radiol.2016160718.
- Biederman D, Titano J, Bishay V, Durrani R, Dayan E, Schwartz M, et al. Radiation segmentectomy vs. microwave ablation for unresectable solitary hepatocellular carcinoma < 3 cm: a propensity score matching study. J Vasc Interv Radiol 2016;27:S5-6. https://doi.org/10.1016/j.jvir.2015.12.030.
- Padia SA, Chewning RH, Kogut MJ, Ingraham CR, Johnson GE, Bhattacharya R, et al. Outcomes of locoregional tumor therapy for patients with hepatocellular carcinoma and transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol 2015;38:913-21. https://doi.org/10.1007/s00270-014-1009-8.
- Radunz S, Saner FH, Treckmann J, Rekowski J, Theysohn JM, Müller S, et al. Hepatic artery and biliary complications in liver transplant recipients with radioembolization bridging treatment for hepatocellular carcinoma. Clin Transplant 2017;31. https://doi.org/10.1111/ctr.13096.
- Ali R, Gabr A, Abouchaleh N, Al Asadi A, Mora RA, Kulik L, et al. Survival analysis of advanced HCC treated with radioembolization: comparing impact of clinical performance status versus vascular invasion/metastases. Cardiovasc Intervent Radiol 2018;41:260-9. https://doi.org/10.1007/s00270-017-1791-1.
- Kudo M. Systemic therapy for hepatocellular carcinoma: 2017 update. Oncology 2017;93:135-46. https://doi.org/10.1159/000481244.
- National Institute for Health and Care Excellence (NICE) . Pertuzumab With Trastuzumab and Docetaxel for Treating HER2-Positive Breast Cancer 2018.
- NHS England . Schedule 2 – The Services. Liver Transplantation Service (Adults). Service Specification 170003 S 2017.
- NHS Blood and Transplant . Organ Donation and Transplantation 2018.
- National Institute for Health and Care Excellence (NICE) . SIR-Spheres for Treating Inoperable Hepatocellular Carcinoma. Medtech Innovation Briefing [MIB63] 2016. www.nice.org.uk/advice/mib63 (accessed 8 May 2019).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2016–17 2017.
Appendix 1 Search strategies for clinical effectiveness and cost-effectiveness
The search strategies below were used to identify studies for the systematic reviews of the clinical effectiveness and cost-effectiveness of SIRT.
Database search strategies
MEDLINE all
Includes Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE.
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to 25 January 2019.
Date searched: 28 January 2019.
Records retrieved: 1790.
Search strategy
-
Carcinoma, Hepatocellular/ (77,414)
-
Liver Neoplasms/ (137,452)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (131,703)
-
hepatocarcinoma$.ti,ab. (3749)
-
hepatoma$.ti,ab. (27,351)
-
or/1-5 (207,214)
-
(Therasphere$ or Thera-sphere$).ti,ab. (66)
-
(SIR-Sphere$ or SIRSphere$).ti,ab. (100)
-
(QuiremSphere$ or Quirem-Sphere$).ti,ab. (0)
-
or/7-9 (142)
-
6 and 10 (127)
-
Microspheres/ (27,127)
-
(microsphere$ or sphere$).ti,ab. (67,569)
-
(microbead$ or bead$).ti,ab. (49,738)
-
or/12-14 (123,972)
-
Yttrium Radioisotopes/ (2861)
-
Yttrium/ (2899)
-
Yttrium Isotopes/ (708)
-
(Yttrium$ or 90Yttrium$ or Y90 or Y-90 or 90Y or 90-Y).ti,ab. (8538)
-
Holmium/ (806)
-
(Holmium$ or 166Holmium$ or Ho-166 or Ho166 or 166Ho or 166-Ho).ti,ab. (2939)
-
Radiopharmaceuticals/ (47,137)
-
or/16-22 (60,317)
-
15 and 23 (1616)
-
((radioactiv$ or radio-activ$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$ or radiolabel$ or radio-label$ or radiopharmaceutic$ or radio-pharmaceutic$) adj2 (sphere$ or microsphere$ or bead$ or microbead$)).ti,ab. (4140)
-
(radiomicrosphere$ or radio-microsphere$).ti,ab. (31)
-
or/24-26 (5660)
-
6 and 27 (1020)
-
Brachytherapy/ (18,640)
-
(brachytherap$ or brachy-therap$ or microbrachytherap$).ti,ab. (16,214)
-
Embolization, Therapeutic/ (29,974)
-
or/29-31 (53,284)
-
32 and (23 or 25 or 26) (1603)
-
6 and 33 (815)
-
(radioemboli$ or radio-emboli$ or radioembolotherap$ or radio-embolotherap$).ti,ab. (1365)
-
TARE.ti,ab. (158)
-
(internal$ adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (2182)
-
((intra-arterial$ or intraarterial$) adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (276)
-
((intra-arterial$ or intraarterial$) adj2 (brachytherap$ or brachy-therap$)).ti,ab. (19)
-
SIRT.ti,ab. (1120)
-
(SIR adj2 (therap$ or treatment$)).ti,ab. (80)
-
(radiation adj2 (segmentectom$ or lobectom$)).ti,ab. (32)
-
or/35-42 (4675)
-
6 and 43 (1675)
-
11 or 28 or 34 or 44 (1978)
-
exp animals/not humans/ (4,541,052)
-
45 not 46 (1915)
-
limit 47 to yr = “2000 -Current” (1790).
Key:
-
/ = indexing term [Medical Subject Heading (MeSH)]
-
exp = exploded indexing term (MeSH)
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
adj3 = terms within three words of each other (any order).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 25 January 2019.
Date searched: 28 January 2019.
Records retrieved: 3440.
Search strategy
-
liver cell carcinoma/ (137,127)
-
liver cancer/ (28,908)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (185,054)
-
hepatocarcinoma$.ti,ab. (4972)
-
hepatoma$.ti,ab. (30,720)
-
or/1-5 (242,887)
-
(Therasphere$ or thera-sphere$).ti,ab,dv. (320)
-
(SIR-Sphere$ or SIRSphere$).ti,ab,dv. (479)
-
(QuiremSphere$ or Quirem-Sphere$).ti,ab,dv. (2)
-
brachytherapy device/ (555)
-
or/7-10 (1167)
-
6 and 11 (487)
-
microsphere/ (28,744)
-
(microsphere$ or sphere$).ti,ab. (73,618)
-
(microbead$ or bead$).ti,ab. (71,652)
-
or/13-15 (148,521)
-
yttrium/ (4631)
-
yttrium 90/ (7567)
-
(Yttrium$ or 90Yttrium$ or Y90 or Y-90 or 90Y or 90-Y).ti,ab. (11,105)
-
holmium/ (1495)
-
(Holmium$ or 166Holmium$ or Ho-166 or Ho166 or 166Ho or 166-Ho).ti,ab. (4761)
-
radiopharmaceutical agent/ (26,611)
-
or/17-22 (46,979)
-
16 and 23 (2924)
-
radioactive microsphere/ (937)
-
((radioactiv$ or radio-activ$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$ or radiolabel$ or radio-label$ or radiopharmaceutic$ or radio-pharmaceutic$) adj2 (sphere$ or microsphere$ or bead$ or microbead$)).ti,ab. (4430)
-
(radiomicrosphere$ or radio-microsphere$).ti,ab. (39)
-
or/24-27 (7517)
-
6 and 28 (1922)
-
brachytherapy/ (34,809)
-
(brachytherap$ or brachy-therap$ or microbrachytherap$).ti,ab. (27,633)
-
artificial embolization/ (6954)
-
or/30-32 (44,694)
-
33 and (23 or 25 or 26 or 27) (869)
-
6 and 34 (221)
-
radioembolization/ (1554)
-
selective internal radiation.dq. (258)
-
intra arterial brachytherapy.dq. (1)
-
transarterial radioembolization.dq. (72)
-
(radioemboli$ or radio-emboli$ or radioembolotherap$ or radio-embolotherap$).ti,ab. (2887)
-
TARE.ti,ab. (416)
-
(internal$ adj3 (radiation$ or radiotherap$ or radio-therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (3166)
-
((intra-arterial$ or intraarterial$) adj3 (radiation$ or radiotherap$ or radio-therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (363)
-
((intra-arterial$ or intraarterial$) adj2 (brachytherap$ or brachy-therap$)).ti,ab. (18)
-
SIRT.ti,ab. (2238)
-
(SIR adj2 (therap$ or treatment$)).ti,ab. (185)
-
(radiation adj2 (segmentectom$ or lobectom$)).ti,ab. (77)
-
or/36-47 (8358)
-
6 and 48 (3229)
-
12 or 29 or 35 or 49 (3651)
-
(animal/or animal experiment/or animal model/or animal tissue/or nonhuman/) not exp human/ (5,653,185)
-
50 not 51 (3560)
-
limit 52 to yr = “2000 -Current” (3440).
Key:
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
dv = terms in the device trade name field
-
dq = terms in the candidate term word field
-
adj3 = terms within three words of each other (any order).
Cumulative Index to Nursing and Allied Health Literature Plus
Via EBSCOhost (www.ebscohost.com/).
Date range searched: inception to 28 January 2019.
Date searched: 28 January 2019.
Records retrieved: 724.
Search strategy
-
S1 (MH “Carcinoma, Hepatocellular”) (7801)
-
S2 (MH “Liver Neoplasms”) (12,189)
-
S3 TI ((liver or hepato* or hepatic*) N3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)) OR AB ((liver or hepato* or hepatic*) N3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)) (14,708)
-
S4 TI hepatocarcinoma* OR AB hepatocarcinoma* (173)
-
S5 TI hepatoma* OR AB hepatoma* (649)
-
S6 S1 OR S2 OR S3 OR S4 OR S5 (20,300)
-
S7 TI (Therasphere* or Thera-sphere*) OR AB (Therasphere* or Thera-sphere*) (19)
-
S8 TI (SIR-Sphere* or SIRSphere*) OR AB (SIR-Sphere* or SIRSphere*) (33)
-
S9 TI (QuiremSphere* or Quirem-Sphere*) OR AB (QuiremSphere* or Quirem-Sphere*) (0)
-
S10 S7 OR S8 OR S9 (46)
-
S11 S6 AND S10 (42)
-
S12 TI (microsphere* or sphere*) OR AB (microsphere* or sphere*) (3575)
-
S13 TI (microbead* or bead*) OR AB (microbead* or bead*) (2272)
-
S14 S12 OR S13 (5795)
-
S15 (MH “Radioisotopes”) (3321)
-
S16 TI (Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y) OR AB (Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y) (1061)
-
S17 TI (Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho) OR AB (Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho) (281)
-
S18 (MH “Radiopharmaceuticals”) (6050)
-
S19 S15 OR S16 OR S17 OR S18 (9807)
-
S20 S14 AND S19 (356)
-
S21 TI ((radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) N2 (sphere* or microsphere* or bead* or microbead*)) OR AB ((radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) N2 (sphere* or microsphere* or bead* or microbead*)) (104)
-
S22 TI (radiomicrosphere* or radio-microsphere*) OR AB (radiomicrosphere* or radio-microsphere*) (1)
-
S23 S20 OR S21 OR S22 (440)
-
S24 S6 AND S23 (261)
-
S25 (MH “Brachytherapy”) (3045)
-
S26 TI (brachytherap* or brachy-therap* or microbrachytherap*) OR AB (brachytherap* or brachy-therap* or microbrachytherap*) (2956)
-
S27 (MH “Embolization, Therapeutic”) (5975)
-
S28 S25 OR S26 OR S27 (10,145)
-
S29 S19 OR S21 OR S22 (9890)
-
S30 S28 AND S29 (603)
-
S31 S6 AND S30 (309)
-
S32 (MH “Radioembolization”) (29)
-
S33 TI ((radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap*) OR AB ((radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap*) (654)
-
S34 TI TARE OR AB TARE (49)
-
S35 TI (internal* N3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) OR AB (internal* N3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) (327)
-
S36 TI ((intra-arterial* or intraarterial*) N3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) OR AB ((intra-arterial* or intraarterial*) N3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) (45)
-
S37 TI ((intra-arterial* or intraarterial*) N2 (brachytherap* or brachy-therap*)) OR AB ((intra-arterial* or intraarterial*) N2 (brachytherap* or brachy-therap*)) (5)
-
S38 TI SIRT OR AB SIRT (187)
-
S39 TI (SIR N2 (therap* or treatment*)) OR AB (SIR N2 (therap* or treatment*)) (37)
-
S40 TI (radiation N2 (segmentectom* or lobectom*)) OR AB (radiation N2 (segmentectom* or lobectom*)) (15)
-
S41 S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 (1140)
-
S42 S6 AND S41 (639)
-
S43 S11 OR S24 OR S31 OR S42 (727)
-
S44 TI (animal or animals or rat or rats or mouse or mice or rodent or rodents or porcine or murine or sheep or lamb or lambs or ewe or ewes or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or rabbit or rabbits or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*) (87,260)
-
S45 S43 NOT S44 (724)
-
S46 S43 NOT S44
-
Limiters - Published Date: 20000101-20191231 (724).
Key:
-
MH = indexing term (CINAHL heading)
-
* = truncation
-
TI = terms in the title
-
AB = terms in the abstract
-
N3 = terms within three words of each other (any order).
Science Citation Index
Via Web of Science, Clarivate Analytics (https://clarivate.com/).
Date range searched: 1900 to 25 January 2019.
Date searched: 28 January 2019.
Records retrieved: 2242.
Search strategy
-
# 38 2242 #35 NOT #36
-
Indexes=SCI-EXPANDED Timespan=2000-2019
-
# 37 2347 #35 NOT #36
-
# 36 2,811,336 TI=(animal or animals or rat or rats or mouse or mice or rodent or rodents or porcine or murine or sheep or lamb or lambs or ewe or ewes or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or rabbit or rabbits or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*)
-
# 35 2419 #34 OR #24 OR #20 OR #9
-
# 34 2106 #33 AND #4
-
# 33 7874 #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25
-
# 32 48 TS=(radiation NEAR/2 (segmentectom* or lobectom*))
-
# 31 205 TS=(SIR NEAR/2 (therap* or treatment*))
-
# 30 1676 TS=SIRT
-
# 29 20 TS=((intra-arterial* or intraarterial*) NEAR/2 (brachytherap* or brachy-therap*))
-
# 28 289 TS=((intra-arterial* or intraarterial*) NEAR/3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*))
-
# 27 3822 TS=(internal* NEAR/3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*))
-
# 26 883 TS=TARE
-
# 25 2096 TS=(radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap*)
-
# 24 263 #23 AND #4
-
# 23 533 #22 AND #21
-
# 22 47,345 #18 OR #17 OR #15
-
# 21 24,888 TS=(brachytherap* or brachy-therap*or microbrachytherap*)
-
# 20 1517 #19 AND #4
-
# 19 4871 #18 OR #17 OR #16
-
# 18 19 TS=(radiomicrosphere* or radio-microsphere*)
-
# 17 2262 TS=((radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) NEAR/2 (sphere* or microsphere* or bead* or microbead*))
-
# 16 2721 #15 AND #12
-
# 15 45,198 #14 OR #13
-
# 14 7124 TS=(Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho)
-
# 13 38,768 TS=(Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y)
-
# 12 310,417 #11 OR #10
-
# 11 81,252 TS=(microbead* or bead*)
-
# 10 235,358 TS=(microsphere* or sphere*)
-
# 9 216 #8 AND #4
-
# 8 283 #7 OR #6 OR #5
-
# 7 0 TS=(QuiremSphere* or Quirem-Sphere*)
-
# 6 172 TS=(SIR-Sphere* or SIRSphere*)
-
# 5 145 TS=(Therasphere* or Thera-sphere*)
-
# 4 199,180 #3 OR #2 OR #1
-
# 3 31,512 TS=(hepatoma*)
-
# 2 3551 TS=(hepatocarcinoma*)
-
# 1 173,805 TS=((liver or hepato* or hepatic*) NEAR/3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)).
Key:
-
TS = topic tag; searches in title, abstract, author keywords and keywords plus fields
-
TI = search in title field
-
* = truncation
-
NEAR/2 = terms within two words of each other (any order).
Cochrane Central Register of Controlled Trials
Via Wiley (http://onlinelibrary.wiley.com/).
Date range searched: issue 1 of 12, January 2019.
Date searched: 28 January 2019.
Records retrieved: 144.
The strategy below was used to search both the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews.
Search strategy
-
#1 MeSH descriptor: [Carcinoma, Hepatocellular] this term only (1483)
-
#2 MeSH descriptor: [Liver Neoplasms] this term only (2218)
-
#3 ((liver or hepato* or hepatic*) near/3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)):ti,ab,kw (6211)
-
#4 hepatocarcinoma*:ti,ab,kw (57)
-
#5 hepatoma*:ti,ab,kw (119)
-
#6 [OR #1-#5] (6287)
-
#7 (Therasphere* or Thera next sphere*):ti,ab,kw (9)
-
#8 (SIRSphere* or SIR next Sphere*):ti,ab,kw (43)
-
#9 (QuiremSphere* or Quirem next Sphere*):ti,ab,kw (0)
-
#10 [OR #7-#9] (52)
-
#11 #6 AND #10 (42)
-
#12 MeSH descriptor: [Microspheres] this term only (216)
-
#13 (microsphere* or sphere*):ti,ab,kw (1202)
-
#14 (microbead* or bead*):ti,ab,kw (948)
-
#15 [OR #12-#14] (2109)
-
#16 MeSH descriptor: [Yttrium Radioisotopes] this term only (78)
-
#17 MeSH descriptor: [Yttrium] this term only (123)
-
#18 MeSH descriptor: [Yttrium Isotopes] this term only (8)
-
#19 (Yttrium* or 90Yttrium* or “Y90” or “Y-90” or “90Y” or “90-Y”):ti,ab,kw (1147)
-
#20 MeSH descriptor: [Holmium] this term only (27)
-
#21 (Holmium* or 166Holmium* or “Ho-166” or “Ho166” or “166Ho” or “166-Ho”):ti,ab,kw (334)
-
#22 MeSH descriptor: [Radiopharmaceuticals] this term only (1425)
-
#23 [OR #16-#22] (2844)
-
#24 #15 AND #23 (117)
-
#25 ((radioactiv* or (radio next activ*) or radionuclide* or (radio next nuclide*) or radioisotope* or (radio next isotope*) or radiolabel* or (radio next label*) or radiopharmaceutic* or (radio next pharmaceutic*)) near/2 (sphere* or microsphere* or bead* or microbead*)):ti,ab,kw (15)
-
#26 (radiomicrosphere* or (radio next microsphere*)):ti,ab,kw (0)
-
#27 #24 OR #25 OR #26 (123)
-
#28 #6 AND #27 (94)
-
#29 MeSH descriptor: [Brachytherapy] this term only (653)
-
#30 (brachytherap* or brachy next therap* or microbrachytherap*):ti,ab,kw (1583)
-
#31 MeSH descriptor: [Embolization, Therapeutic] this term only (340)
-
#32 [OR #29-#31] (1919)
-
#33 #32 AND (#23 OR #25 OR #26) (46)
-
#34 #6 AND #33 (21)
-
#35 (radioemboli* or (radio next emboli*) or radioembolotherap* or (radio next embolotherap*)):ti,ab,kw (95)
-
#36 TARE:ti,ab,kw (105)
-
#37 (internal* near/3 (radiation* or radiotherap* or (radio next therap*) or radionuclide* or (radio next nuclide*) or radioisotope* or (radio next isotope*))):ti,ab,kw (116)
-
#38 ((intraarterial* or (intra next arterial)) near/3 (radiation* or radiotherap* or (radio next therap*) or radionuclide* or (radio next nuclide*) or radioisotope* or (radio next isotope*))):ti,ab,kw (17)
-
#39 ((intraarterial* or (intra next arterial*)) near/2 (brachytherap* or (brachy next therap*))):ti,ab,kw (2)
-
#40 SIRT:ti,ab,kw (99)
-
#41 (SIR near/2 (therap* or treatment*)):ti,ab,kw (10)
-
#42 (radiation near/2 (segmentectom* or lobectom*)):ti,ab,kw (1)
-
#43 [OR #35-#42] (336)
-
#44 #6 AND #43 (133)
-
#45 #11 OR #28 OR #34 OR #44 (150)
-
#46 #11 OR #28 OR #34 OR #44 with Cochrane Library publication date Between Jan 2000 and Jan 2019, in Cochrane Reviews, Cochrane Protocols (3)
-
#47 #11 OR #28 OR #34 OR #44 with Publication Year from 2000 to 2019, in Trials (144).
Key:
-
MeSH descriptor = indexing term (MeSH)
-
* = truncation
-
ti,ab,kw = terms in either title or abstract or keyword fields
-
near/3 = terms within three words of each other (any order)
-
next = terms are next to each other.
Cochrane Database of Systematic Reviews
Via Wiley (http://onlinelibrary.wiley.com/).
Date range searched: issue 1 of 12, January 2019.
Date searched: 28 January 2019.
Records retrieved: 3.
See above under Cochrane Central Register of Controlled Trials for search strategy used.
Database of Abstracts of Reviews of Effects
URL: www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2015.
Date searched: 28 January 2019.
Records retrieved: 13.
The strategy below was used to search all three of the CRD databases: Database of Abstracts of Reviews of Effects, the HTA database and NHS EED.
Search strategy
-
MeSH DESCRIPTOR Carcinoma, Hepatocellular (385)
-
MeSH DESCRIPTOR Liver Neoplasms (567)
-
((liver or hepato* or hepatic*) NEAR3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)) (850)
-
((carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) NEAR3 (liver or hepato* or hepatic*)) (587)
-
(hepatocarcinoma*) (8)
-
(hepatoma*) (7)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 (891)
-
(Therasphere* or Thera-sphere*) (2)
-
(SIR-Sphere* or SIRSphere*) (5)
-
(QuiremSphere* or Quirem-Sphere*) (0)
-
#8 OR #9 OR #10 (5)
-
#7 AND #11 (4)
-
MeSH DESCRIPTOR Microspheres (16)
-
(microsphere* or sphere*) (44)
-
(micro-sphere* or sphere*) (16)
-
(microbead* or bead*) (34)
-
#13 OR #14 OR #15 OR #16 (74)
-
MeSH DESCRIPTOR Yttrium Radioisotopes (16)
-
MeSH DESCRIPTOR Yttrium (1)
-
MeSH DESCRIPTOR Yttrium Isotopes (0)
-
(Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y) (43)
-
MeSH DESCRIPTOR Holmium (9)
-
(Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho) (43)
-
MeSH DESCRIPTOR Radiopharmaceuticals (276)
-
#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 (350)
-
#17 AND #25 (10)
-
((radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) NEAR2 (sphere* or microsphere* or bead* or microbead*)) (5)
-
((sphere* or microsphere* or bead* or microbead*) NEAR2 (radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*)) (3)
-
(radiomicrosphere* or radio-microsphere*) (0)
-
#26 OR #27 OR #28 OR #29 (11)
-
#7 AND #30 (11)
-
MeSH DESCRIPTOR Brachytherapy (133)
-
(brachytherap* or brachy-therap* or microbrachytherap*) (205)
-
MeSH DESCRIPTOR Embolization, Therapeutic (145)
-
#32 OR #33 OR #34 (348)
-
#25 OR #27 OR #28 (351)
-
#35 AND #36 (13)
-
#7 AND #37 (9)
-
(radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap*) (17)
-
(TARE) (2)
-
(internal* NEAR3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) (15)
-
((radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) NEAR3 internal*) (2)
-
((intra-arterial* or intraarterial*) NEAR3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*)) (0)
-
((radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) NEAR3 (intra-arterial* or intraarterial*)) (2)
-
((intra-arterial* or intraarterial*) NEAR2 (brachytherap* or brachy-therap*)) (0)
-
((brachytherap* or brachy-therap*) NEAR2 (intra-arterial* or intraarterial*)) (0)
-
(SIRT) (9)
-
(SIR NEAR2 (therap* or treatment*)) (0)
-
((therap* or treatment*) NEAR2 SIR) (1)
-
(radiation NEAR2 (segmentectom* or lobectom*)) (0)
-
((segmentectom* or lobectom*) NEAR2 radiation) (0)
-
#39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 (34)
-
#7 AND #52 (25)
-
#12 OR #31 OR #38 OR #53 (29)
Key:
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
NEAR3 = terms within three words of each other (order specified).
Health Technology Assessment database
URL: www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2018.
Date searched: 28 January 2019.
Records retrieved: 14.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
NHS Economic Evaluations Database
URL: www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2015.
Date searched: 28 January 2019.
Records retrieved: 2.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
EconLit
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1886 to 17 January 2019.
Date searched: 28 January 2019.
Records retrieved: 0.
Search strategy
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (17)
-
hepatocarcinoma$.ti,ab. (0)
-
hepatoma$.ti,ab. (0)
-
or/1-3 (17)
-
(Therasphere$ or Thera-sphere$).ti,ab. (0)
-
(SIR-Sphere$ or SIRSphere$).ti,ab. (0)
-
(QuiremSphere$ or Quirem-Sphere$).ti,ab. (0)
-
5 or 6 or 7 (0)
-
4 and 8 (0)
-
(microsphere$ or sphere$).ti,ab. (2659)
-
(microbead$ or bead$).ti,ab. (12)
-
10 or 11 (2671)
-
(Yttrium$ or 90Yttrium$ or Y90 or Y-90 or 90Y or 90-Y).ti,ab. (3)
-
(Holmium$ or 166Holmium$ or Ho-166 or Ho166 or 166Ho or 166-Ho).ti,ab. (1)
-
13 or 14 (4)
-
12 and 15 (0)
-
((radioactiv$ or radio-activ$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$ or radiolabel$ or radio-label$ or radiopharmaceutic$ or radio-pharmaceutic$) adj2 (sphere$ or microsphere$ or bead$ or microbead$)).ti,ab. (0)
-
(radiomicrosphere$ or radio-microsphere$).ti,ab. (0)
-
16 or 17 or 18 (0)
-
4 and 19 (0)
-
(brachytherap$ or brachy-therap$ or microbrachytherap$).ti,ab. (6)
-
21 and (15 or 17 or 18) (0)
-
4 and 22 (0)
-
(radioemboli$ or radio-emboli$ or radioembolotherap$ or radio-embolotherap$).ti,ab. (0)
-
TARE.ti,ab. (2)
-
(internal$ adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (1)
-
((intra-arterial$ or intraarterial$) adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (0)
-
((intra-arterial$ or intraarterial$) adj2 (brachytherap$ or brachy-therap$)).ti,ab. (0)
-
SIRT.ti,ab. (1)
-
(SIR adj2 (therap$ or treatment$)).ti,ab. (0)
-
(radiation adj2 (segmentectom$ or lobectom$)).ti,ab. (0)
-
or/24-31 (4)
-
4 and 32 (0)
-
9 or 20 or 23 or 33 (0).
Key:
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
adj3 = terms within three words of each other (any order).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
URL: https://clinicaltrials.gov/.
Date searched: 1 February 2019.
Records retrieved: 157.
Advanced search screen used. Ten separate searches were used, retrieving 681 records in total, which were imported into EndNote X9 and deduplicated.
Search strategy
-
93 studies found for: (Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere) | (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumour OR tumor OR malignancy)
-
73 studies found for: (Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere) | (hepatocarcinoma OR hepatoma)
-
103 studies found for: (Microsphere OR sphere OR microbead OR bead) AND (Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho) | (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumour OR tumor OR malignancy)
-
77 studies found for: (Microsphere OR sphere OR microbead OR bead) AND (Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho) | (hepatocarcinoma OR hepatoma)
-
38 studies found for: (brachytherapy OR brachy-therapy OR microbrachytherapy) AND (Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho) | (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumour OR tumor OR malignancy)
-
26 studies found for: (brachytherapy OR brachy-therapy OR microbrachytherapy) AND (Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho) | (hepatocarcinoma OR hepatoma)
-
123 studies found for: (radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR) | (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumour OR tumor OR malignancy)
-
94 studies found for: (radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR) | (hepatocarcinoma OR hepatoma)
-
32 studies found for: selective AND internal AND (radiation OR radiotherapy OR radio-therapy) | (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumour OR tumor OR malignancy)
-
22 studies found for: selective AND internal AND (radiation OR radiotherapy OR radio-therapy) | (hepatocarcinoma OR hepatoma)
World Health Organization International Clinical Trials Registry Platform
URL: www.who.int/ictrp/search/en/.
Date searched: 1 February 2019.
Records retrieved: 68.
Advanced search screen used. Ten separate searches were used, retrieving 103 records in total, which were imported into EndNote X9 and deduplicated.
Search strategy
-
Condition: hepatocellular carcinoma OR liver cancer AND Intervention: Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere (11 hits)
-
Condition: hepatocarcinoma OR hepatoma AND Intervention: Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere (4 hits)
-
Condition: hepatocellular carcinoma OR liver cancer AND Intervention: Microsphere OR sphere OR Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho 45 records (37 trials)
-
Condition: hepatocarcinoma OR hepatoma AND Intervention: Microsphere OR sphere OR Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho (6 hits)
-
Condition: hepatocellular carcinoma OR liver cancer AND Intervention: brachytherapy OR brachy-therapy OR microbrachytherapy (21 hits)
-
Condition: hepatocarcinoma OR hepatoma AND Intervention: brachytherapy OR brachy-therapy OR microbrachytherapy (6 hits)
-
Condition: hepatocellular carcinoma OR liver cancer AND Intervention: radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR (23 records for 15 trials)
-
Condition: hepatocarcinoma OR hepatoma AND Intervention: radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR (2 hits)
-
Condition: hepatocellular carcinoma OR liver cancer AND Intervention: selective internal radiation OR selective internal radiotherapy OR selective internal radio-therapy (1 hit)
-
Condition: hepatocarcinoma OR hepatoma AND Intervention: selective internal radiation OR selective internal radiotherapy OR selective internal radio-therapy (0 hits).
European Union Clinical Trials Register
URL: www.clinicaltrialsregister.eu/ctr-search/search.
Date searched: 1 February 2019.
Records retrieved: 62.
Search strategy
-
3 result(s) found for: hepatocellular carcinoma AND (Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere)
-
3 result(s) found for: liver cancer AND (Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere
-
5 result(s) found for: hepatocellular carcinoma AND (Microsphere OR sphere OR Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho)
-
12 result(s) found for: liver cancer AND (Microsphere OR sphere OR Yttrium OR 90Yttrium OR Y90 OR Y-90 OR 90Y OR 90-Y OR Holmium OR 166Holmium OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho)
-
1 result(s) found for: hepatocellular carcinoma AND (brachytherapy OR brachy-therapy OR microbrachytherapy)
-
7 result(s) found for: liver cancer AND (brachytherapy OR brachy-therapy OR microbrachytherapy)
-
10 result(s) found for: hepatocellular carcinoma AND (radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR)
-
19 result(s) found for: liver cancer AND (radioembolisation OR radioembolization OR radio-embolisation OR radio-embolization OR TARE OR SIRT OR SIR)
-
1 result(s) found for: hepatocellular carcinoma AND selective internal radiation
-
1 result(s) found for: liver cancer AND selective internal radiation.
PROSPERO
URL: www.crd.york.ac.uk/PROSPERO/.
Date searched: 1 February 2019.
Records retrieved: 23.
Search strategy
-
#1 MeSH DESCRIPTOR Carcinoma, Hepatocellular (107)
-
#2 MeSH DESCRIPTOR Liver Neoplasms (158)
-
#3 (liver or hepato* or hepatic*) adj3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) (342)
-
#4 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) ADJ3 (liver or hepato* or hepatic*) (206)
-
#5 hepatocarcinoma* (8)
-
#6 hepatoma* (11)
-
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 (411)
-
#8 Therasphere* or Thera-sphere* (1)
-
#9 SIR-Sphere* or SIRSphere* (1)
-
#10 QuiremSphere* or Quirem-Sphere* (0)
-
#11 #8 OR #9 OR #10 (1)
-
#12 #11 AND #7 (1)
-
#13 MeSH DESCRIPTOR Microspheres (4)
-
#14 microsphere* or sphere* (87)
-
#15 microbead* or bead* (33)
-
#16 #13 OR #14 OR #15 (118)
-
#17 MeSH DESCRIPTOR Yttrium Radioisotopes (4)
-
#18 MeSH DESCRIPTOR Yttrium (3)
-
#19 MeSH DESCRIPTOR Yttrium Isotopes (0)
-
#20 Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y (13)
-
#21 MeSH DESCRIPTOR Holmium (1)
-
#22 Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho (11)
-
#23 MeSH DESCRIPTOR Radiopharmaceuticals (10)
-
#24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 (32)
-
#25 #24 AND #16 (6)
-
#26 (radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) adj2 (sphere* or microsphere* or bead* or microbead*) (0)
-
#27 (sphere* or microsphere* or bead* or microbead*) adj2 (radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) (0)
-
#28 radiomicrosphere* or radio-microsphere* (0)
-
#29 #26 OR #27 OR #28 (0)
-
#30 #25 OR #29 (6)
-
#31 #30 AND #7 (6)
-
#32 MeSH DESCRIPTOR Brachytherapy (14)
-
#33 brachytherap* or brachy-therap* or microbrachytherap* (76)
-
#34 MeSH DESCRIPTOR Embolization, Therapeutic (27)
-
#35 #32 OR #33 OR #34 (104)
-
#36 #24 OR #26 OR #27 OR #28 (32)
-
#37 #35 AND #36 (0)
-
#38 #37 AND #7 (0)
-
#39 radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap* (14)
-
#40 TARE (10)
-
#41 internal* adj3 (radiation* or radiotherap* or radio therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) (10)
-
#42 (radiation* or radiotherap* or radio therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) adj3 internal* (3)
-
#43 (intra-arterial* or intraarterial*) adj3 (radiation* or radiotherap* or radio therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) (1)
-
#44 (radiation* or radiotherap* or radio therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*) adj3 (intra-arterial* or intraarterial*) (3)
-
#45 (intra-arterial* or intraarterial*) adj2 (brachytherap* or brachy-therap*) (0)
-
#46 (brachytherap* or brachy-therap*) adj2 (intra-arterial* or intraarterial*) (0)
-
#47 SIRT (5)
-
#48 SIR adj2 (therap* or treatment*) (0)
-
#49 (therap* or treatment*) adj2 SIR (0)
-
#50 radiation adj2 (segmentectom* or lobectom*) (0)
-
#51 (segmentectom* or lobectom*) adj2 radiation (0)
-
#52 #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 (35)
-
#53 #52 AND #7 (23)
-
#54 #53 OR #38 OR #31 OR #12 (23).
Key:
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
adj3 = terms within three words of each other (order specified).
National Institute for Health and Care Excellence website
URL: www.nice.org.uk/.
Date searched: 8 May 2019.
Records retrieved: 6.
Search terms entered into main search box of the website:
-
5 results for Therasphere OR Thera-sphere OR SIR-Sphere OR SIRSphere OR QuiremSphere OR Quirem-Sphere
-
10 results for SIRT OR “SIR therapy” OR “SIR treatment” – browsed for any relevant to HCC – 3 results found
-
5 results for radioembolisation OR radioembolization OR radioembolotherapy OR TARE - browsed for any relevant to HCC – 2 results found
-
60 results found for hepatocellular carcinoma – browsed for any relevant to SIRT – 4 results found
Browsed the NICE guidance for liver cancers section of the website (www.nice.org.uk/guidance/conditions-and-diseases/cancer/liver-cancers): 3 results found relevant to SIRT.
The above search results were deduplicated, leaving six results in total retrieved from searches of this website.
NHS Evidence
URL: www.evidence.nhs.uk/.
Date searched: 8 May 2019.
Records retrieved: 18.
The following search strings were entered into the search box with the inbuilt guidance filters box checked to limit results to guidelines:
-
1. Therasphere OR “Thera sphere” OR “Thera-sphere” OR “SIR Sphere” OR “SIR-Sphere” OR SIRSphere OR QuiremSphere OR “Quirem Sphere” OR “Quirem-Sphere”.
Two results.
-
2. “hepatocellular carcinoma” AND (SIRT OR “SIR therapy” OR “SIR treatment”).
Nine results.
-
3. “hepatocellular carcinoma” AND (radioembolisation OR radioembolization OR radioembolotherapy OR TARE).
13 results.
-
4. “hepatocellular carcinoma” AND (microsphere OR yttrium or holmium).
12 results.
-
5. “hepatocellular carcinoma” AND (brachytherapy OR microbrachytherapy).
Four results.
The above search results were imported into EndNote X9 and deduplicated, leaving 18 results in total.
Conference Proceedings Citation Index – Science
Via Web of Science, Clarivate Analytics (https://clarivate.com/).
Date range searched: 1990 to 25 January 2019.
Date searched: 28 January 2019.
Records retrieved: 377.
Search strategy
-
# 38 (377) #35 not #36 Timespan=2000-2019
-
# 37 (391) #35 NOT #36
-
# 36 (257,731) TI=(animal or animals or rat or rats or mouse or mice or rodent or rodents or porcine or murine or sheep or lamb or lambs or ewe or ewes or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or rabbit or rabbits or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*)
-
# 35 (398) #34 OR #24 OR #20 OR #9
-
# 34 (316) #33 AND #4
-
# 33 (1585) #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25
-
# 32 (4) TS=(radiation NEAR/2 (segmentectom* or lobectom*))
-
# 31 (24) TS=(SIR NEAR/2 (therap* or treatment*))
-
# 30 (333) TS=SIRT
-
# 29 (4) TS=((intra-arterial* or intraarterial*) NEAR/2 (brachytherap* or brachy-therap*))
-
# 28 (52) TS=((intra-arterial* or intraarterial*) NEAR/3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*))
-
# 27 (755) TS=(internal* NEAR/3 (radiation* or radiotherap* or radio-therap* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope*))
-
# 26 (180) TS=TARE
-
# 25 (357) TS=(radioemboli* or radio-emboli* or radioembolotherap* or radio-embolotherap*)
-
# 24 (11) #23 AND #4
-
# 23 (48) #22 AND #21
-
# 22 (8066) #18 OR #17 OR #15
-
# 21 (6589) TS=(brachytherap* or brachy-therap*or microbrachytherap*)
-
# 20 (193) #19 AND #4
-
# 19 (606) #18 OR #17 OR #16
-
# 18 (2) TS=(radiomicrosphere* or radio-microsphere*)
-
# 17 (153) TS=((radioactiv* or radio-activ* or radionuclide* or radio-nuclide* or radioisotope* or radio-isotope* or radiolabel* or radio-label* or radiopharmaceutic* or radio-pharmaceutic*) NEAR/2 (sphere* or microsphere* or bead* or microbead*))
-
# 16 (468) #15 AND #12
-
# 15 (7929) #14 OR #13
-
# 14 (1346) TS=(Holmium* or 166Holmium* or Ho-166 or Ho166 or 166Ho or 166-Ho)
-
# 13 (6670) TS=(Yttrium* or 90Yttrium* or Y90 or Y-90 or 90Y or 90-Y)
-
# 12 (44,967) #11 OR #10
-
# 11 (10,567) TS=(microbead* or bead*)
-
# 10 (34,955) TS=(microsphere* or sphere*)
-
# 9 (34) #8 AND #4
-
# 8 (56) #7 OR #6 OR #5
-
# 7 (0) TS=(QuiremSphere* or Quirem-Sphere*)
-
# 6 (29) TS=(SIR-Sphere* or SIRSphere*)
-
# 5 (30) TS=(Therasphere* or Thera-sphere*)
-
# 4 (22,436) #3 OR #2 OR #1
-
# 3 (1675) TS=(hepatoma*)
-
# 2 (305) TS=(hepatocarcinoma*)
-
# 1 (20,826) TS=((liver or hepato* or hepatic*) NEAR/3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)).
Key:
-
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields
-
TI = search in title field
-
* = truncation
-
NEAR/3 = terms within three words of each other (any order).
ProQuest Dissertations & Theses A&I
Via ProQuest (www.proquest.com/).
Date searched: 28 January 2019.
Records retrieved: 25.
Six separate searches were run in this database, giving 38 hits in total, which were then imported into EndNote X9 for deduplication.
-
1. (TI,AB,IF(Therasphere* OR Thera-sphere*) OR TI,AB,IF(SIR-Sphere* OR SIRSphere*) OR TI,AB,IF(QuiremSphere* OR Quirem-Sphere*)) AND (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)).
0 hits
-
2. (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)) AND (((TI,AB,IF(microsphere* OR sphere*) OR TI,AB,IF(microbead* OR bead*)) AND (TI,AB,IF(Yttrium* OR 90Yttrium* OR Y90 OR Y-90 OR 90Y OR 90-Y) OR TI,AB,IF(Holmium* OR 166Holmium* OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho))) OR TI,AB,IF((radioactiv* OR radio-activ* OR radionuclide* OR radio-nuclide* OR radioisotope* OR radio-isotope* OR radiolabel* OR radio-label* OR radiopharmaceutic* OR radio-pharmaceutic*) NEAR/2 (sphere* OR microsphere* OR bead* OR microbead*)) OR TI,AB,IF(radiomicrosphere* OR radio-microsphere*)) date limit 2000-2019.
15 hits
-
3. (TI,AB,IF(brachytherap* OR brachy-therap*or microbrachytherap*) AND ((TI,AB,IF(Yttrium* OR 90Yttrium* OR Y90 OR Y-90 OR 90Y OR 90-Y) OR TI,AB,IF(Holmium* OR 166Holmium* OR Ho-166 OR Ho166 OR 166Ho OR 166-Ho)) OR TI,AB,IF((radioactiv* OR radio-activ* OR radionuclide* OR radio-nuclide* OR radioisotope* OR radio-isotope* OR radiolabel* OR radio-label* OR radiopharmaceutic* OR radio-pharmaceutic*) NEAR/2 (sphere* OR microsphere* OR bead* OR microbead*)) OR TI,AB,IF(radiomicrosphere* OR radio-microsphere*))) AND (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)) date limit 2000-2019.
One hit
-
4. (TI,AB,IF(radioemboli* OR radio-emboli* OR radioembolotherap* OR radio-embolotherap*) OR TI,AB,IF(TARE)) AND (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)) date limit 2000-2019.
0 hits
-
5. (TI,AB,IF(internal* NEAR/3 (radiation* OR radiotherap* OR radio-therap* OR radionuclide* OR radio-nuclide* OR radioisotope* OR radio-isotope*)) OR TI,AB,IF((intra-arterial* OR intraarterial*) NEAR/3 (radiation* OR radiotherap* OR radio-therap* OR radionuclide* OR radio-nuclide* OR radioisotope* OR radio-isotope*))) AND (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)) date limit 2000-2019.
12 hits
-
6. (TI,AB,IF((intra-arterial* OR intraarterial*) NEAR/2 (brachytherap* OR brachy-therap*)) OR TI,AB,IF(SIRT) OR TI,AB,IF(SIR NEAR/2 (therap* OR treatment*)) OR TI,AB,IF(radiation NEAR/2 (segmentectom* OR lobectom*))) AND (TI,AB,IF((liver OR hepato* OR hepatic*) NEAR/3 (carcinoma* OR cancer* OR neoplas* OR tumour* OR tumor* OR malign*)) OR TI,AB,IF(hepatocarcinoma*) OR TI,AB,IF(hepatoma*)) date limit 2000-2019.
10 hits
Key:
-
TI,AB,IF = terms in title or abstract or keywords field.
-
* = truncation
-
NEAR/3 = terms within three words of each other (any order).
Appendix 2 Search strategies for comparator therapies
MEDLINE all
Includes Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE.
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to 3 May 2019.
Date searched: 7 May 2019.
Records retrieved: 449.
Lines 25–104 below are to limit the search to systematic reviews or meta-analyses, taken from a previous search strategy for finding reviews in MEDLINE developed by the CRD. 17 The strategy has been updated to include new MeSH terms and terminology relating to systematic reviews and NMA.
Search strategy
-
Carcinoma, Hepatocellular/ (78,688)
-
Liver Neoplasms/ (139,353)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (133,795)
-
hepatocarcinoma$.ti,ab. (3798)
-
hepatoma$.ti,ab. (27,491)
-
or/1-5 (209,848)
-
Chemoembolization, Therapeutic/ (5314)
-
(chemo-emboli$ or chemoemboli$).ti,ab. (7127)
-
(chemoembolotherap$ or chemo-embolotherap$).ti,ab. (4)
-
TACE.ti,ab. (4674)
-
cTACE.ti,ab. (87)
-
(DEBTACE or DEB-TACE).ti,ab. (157)
-
(eluting adj2 bead$).ti,ab. (500)
-
DC bead$.ti,ab. (95)
-
or/7-14 (9758)
-
6 and 15 (7632)
-
Embolization, Therapeutic/ (30,350)
-
(embolization$ or embolisation$ or embolize$ or embolise$ or embolizing$ or embolising$ or embolotherap$).ti,ab. (46,678)
-
TAE.ti,ab. (2173)
-
or/17-19 (56,670)
-
6 and 20 (6182)
-
((locoregional or loco-regional) adj2 (therap$ or intervention$ or treatment$)).ti,ab. (2545)
-
6 and 22 (914)
-
16 or 21 or 23 (12,277)
-
“systematic review”/ (105,413)
-
systematic$ review$.ti,ab. (145,034)
-
meta-analysis as topic/ (16,900)
-
network meta-analysis/ (771)
-
meta-analytic$.ti,ab. (6484)
-
meta-analysis.ti,ab,pt. (150,374)
-
metanalysis.ti,ab. (186)
-
metaanalysis.ti,ab. (1505)
-
meta analysis.ti,ab. (125,205)
-
meta-synthesis.ti,ab. (731)
-
metasynthesis.ti,ab. (277)
-
meta synthesis.ti,ab. (731)
-
meta-regression.ti,ab. (6437)
-
metaregression.ti,ab. (577)
-
meta regression.ti,ab. (6437)
-
(synthes$ adj3 literature).ti,ab. (2958)
-
(synthes$ adj3 evidence).ti,ab. (8954)
-
integrative review.ti,ab. (2486)
-
data synthesis.ti,ab. (10,362)
-
(research synthesis or narrative synthesis).ti,ab. (2491)
-
(systematic study or systematic studies).ti,ab. (11,184)
-
(systematic comparison$ or systematic overview$).ti,ab. (3075)
-
evidence based review.ti,ab. (1870)
-
comprehensive review.ti,ab. (13,081)
-
critical review.ti,ab. (14,731)
-
quantitative review.ti,ab. (638)
-
structured review.ti,ab. (759)
-
realist review.ti,ab. (252)
-
realist synthesis.ti,ab. (173)
-
((mixed or multiple or indirect) adj treatment$ comparison$).ti,ab. (672)
-
or/25-54 (310,742)
-
review.pt. (2,507,320)
-
medline.ab. (102,777)
-
pubmed.ab. (94,743)
-
cochrane.ab. (69,813)
-
embase.ab. (75,244)
-
cinahl.ab. (23,088)
-
psyc?lit.ab. (913)
-
psyc?info.ab. (28,630)
-
(literature adj3 search$).ab. (52,835)
-
(database$ adj3 search$).ab. (52,049)
-
(bibliographic adj3 search$).ab. (2270)
-
(electronic adj3 search$).ab. (19,250)
-
(electronic adj3 database$).ab. (25,028)
-
(computeri?ed adj3 search$).ab. (3402)
-
(internet adj3 search$).ab. (2953)
-
included studies.ab. (19,694)
-
(inclusion adj3 studies).ab. (14,219)
-
inclusion criteria.ab. (74,336)
-
selection criteria.ab. (28,289)
-
predefined criteria.ab. (1803)
-
predetermined criteria.ab. (1001)
-
(assess$ adj3 (quality or validity)).ab. (71,198)
-
(select$ adj3 (study or studies)).ab. (60,541)
-
(data adj3 extract$).ab. (55,029)
-
extracted data.ab. (12,670)
-
(data adj2 abstracted).ab. (4907)
-
(data adj3 abstraction).ab. (1520)
-
published intervention$.ab. (160)
-
((study or studies) adj2 evaluat$).ab. (169,641)
-
(intervention$ adj2 evaluat$).ab. (10,195)
-
confidence interval$.ab. (373,846)
-
heterogeneity.ab. (149,380)
-
pooled.ab. (79,714)
-
pooling.ab. (11,224)
-
odds ratio$.ab. (244,194)
-
(Jadad or coding).ab. (169,547)
-
or/57-91 (1,312,289)
-
56 and 92 (226,468)
-
review.ti. (419,930)
-
94 and 92 (121,453)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$ or evaluation$)).ti,ab. (169,610)
-
55 or 93 or 95 or 96 (514,084)
-
letter.pt. (1,024,828)
-
editorial.pt. (488,807)
-
comment.pt. (769,090)
-
98 or 99 or 100 (1,719,142)
-
97 not 101 (502,003)
-
exp animals/not humans/ (4,576,104)
-
102 not 103 (489,196)
-
24 and 104 (587)
-
limit 105 to yr = “2010 -Current” (449).
Key:
-
/ = indexing term (MeSH)
-
exp = exploded indexing term (MeSH)
-
$ = truncation
-
? = optional wildcard – stands for zero or one character
-
ti,ab = terms in either title or abstract fields
-
adj3 = terms within three words of each other (any order)
-
pt. = publication type.
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 3 May 2019.
Date searched: 7 May 2019.
Records retrieved: 826.
Lines 26–122 below are to limit the search to systematic reviews or meta-analyses, taken from a previous search strategy for finding reviews in EMBASE developed by the CRD. 17 The strategy has been updated to include terminology relating to NMA.
Search strategy
-
liver cell carcinoma/ (139,370)
-
liver cancer/ (29,412)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (188,432)
-
hepatocarcinoma$.ti,ab. (5049)
-
hepatoma$.ti,ab. (30,865)
-
or/1-5 (246,579)
-
chemoembolization/ (14,765)
-
(chemo-emboli$ or chemoemboli$).ti,ab. (12,156)
-
(chemoembolotherap$ or chemo-embolotherap$).ti,ab. (6)
-
TACE.ti,ab. (9522)
-
cTACE.ti,ab. (242)
-
(DEBTACE or DEB-TACE).ti,ab. (563)
-
(eluting adj2 bead$).ti,ab,dq. (1254)
-
DC bead$.ti,ab. (291)
-
or/7-14 (20,050)
-
6 and 15 (14,882)
-
artificial embolization/ (7551)
-
(embolization$ or embolisation$ or embolize$ or embolise$ or embolizing$ or embolising$ or embolotherap$).ti,ab. (68,834)
-
arterial embolization/ (2817)
-
TAE.ti,ab. (3247)
-
or/17-20 (72,488)
-
6 and 21 (6603)
-
((locoregional or loco-regional) adj2 (therap$ or intervention$ or treatment$)).ti,ab,dq. (4421)
-
6 and 23 (1805)
-
16 or 22 or 24 (19,749)
-
systematic$ review$.ti,ab. (179,774)
-
systematic$ literature review$.ti,ab. (13,292)
-
“systematic review”/ (201,979)
-
“systematic review (topic)”/ (23,396)
-
meta analysis/ (161,490)
-
“meta analysis (topic)”/ (39,538)
-
network meta-analysis/ (1756)
-
meta-analytic$.ti,ab. (7595)
-
meta-analysis.ti,ab. (162,787)
-
metanalysis.ti,ab. (506)
-
metaanalysis.ti,ab. (7350)
-
meta analysis.ti,ab. (162,787)
-
meta-synthesis.ti,ab. (789)
-
metasynthesis.ti,ab. (328)
-
meta synthesis.ti,ab. (789)
-
meta-regression.ti,ab. (7989)
-
metaregression.ti,ab. (948)
-
meta regression.ti,ab. (7989)
-
((mixed or multiple or indirect) adj treatment$ comparison$).ti,ab. (1407)
-
(synthes$ adj3 literature).ti,ab. (3468)
-
(synthes$ adj3 evidence).ti,ab. (9985)
-
(synthes$ adj2 qualitative).ti,ab. (2510)
-
integrative review.ti,ab. (2400)
-
data synthesis.ti,ab. (12,440)
-
(research synthesis or narrative synthesis).ti,ab. (2765)
-
(systematic study or systematic studies).ti,ab. (11,923)
-
(systematic comparison$ or systematic overview$).ti,ab. (3381)
-
(systematic adj2 search$).ti,ab. (27,836)
-
systematic$ literature research$.ti,ab. (306)
-
(review adj3 scientific literature).ti,ab. (1709)
-
(literature review adj2 side effect$).ti,ab. (17)
-
(literature review adj2 adverse effect$).ti,ab. (3)
-
(literature review adj2 adverse event$).ti,ab. (15)
-
(evidence-based adj2 review).ti,ab. (3512)
-
comprehensive review.ti,ab. (15,039)
-
critical review.ti,ab. (15,755)
-
critical analysis.ti,ab. (7854)
-
quantitative review.ti,ab. (732)
-
structured review.ti,ab. (1026)
-
realist review.ti,ab. (267)
-
realist synthesis.ti,ab. (168)
-
(pooled adj2 analysis).ti,ab. (18,168)
-
(pooled data adj6 (studies or trials)).ti,ab. (2772)
-
(medline and (inclusion adj3 criteria)).ti,ab. (23,061)
-
(search adj (strateg$ or term$)).ti,ab. (34,448)
-
or/26-70 (501,726)
-
medline.ab. (127,052)
-
pubmed.ab. (120,450)
-
cochrane.ab. (90,230)
-
embase.ab. (95,039)
-
cinahl.ab. (26,915)
-
psyc?lit.ab. (992)
-
psyc?info.ab. (26,334)
-
lilacs.ab. (7057)
-
(literature adj3 search$).ab. (67,451)
-
(database$ adj3 search$).ab. (65,231)
-
(bibliographic adj3 search$).ab. (2672)
-
(electronic adj3 search$).ab. (23,469)
-
(electronic adj3 database$).ab. (33,807)
-
(computeri?ed adj3 search$).ab. (4093)
-
(internet adj3 search$).ab. (3981)
-
included studies.ab. (24,875)
-
(inclusion adj3 studies).ab. (17,595)
-
inclusion criteria.ab. (128,601)
-
selection criteria.ab. (33,810)
-
predefined criteria.ab. (2418)
-
predetermined criteria.ab. (1252)
-
(assess$ adj3 (quality or validity)).ab. (94,916)
-
(select$ adj3 (study or studies)).ab. (79,681)
-
(data adj3 extract$).ab. (75,259)
-
extracted data.ab. (16,453)
-
(data adj2 abstracted).ab. (8082)
-
(data adj3 abstraction).ab. (2225)
-
published intervention$.ab. (204)
-
((study or studies) adj2 evaluat$).ab. (242,677)
-
(intervention$ adj2 evaluat$).ab. (14,361)
-
confidence interval$.ab. (448,335)
-
heterogeneity.ab. (190,795)
-
pooled.ab. (111,807)
-
pooling.ab. (14,826)
-
odds ratio$.ab. (306,423)
-
(Jadad or coding).ab. (200,705)
-
evidence-based.ti,ab. (130,860)
-
or/72-108 (1,828,351)
-
review.pt. (2,433,403)
-
109 and 110 (227,600)
-
review.ti. (477,956)
-
109 and 112 (151,152)
-
(review$ adj10 (papers or trials or trial data or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (501,852)
-
(retriev$ adj10 (papers or trials or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (26,856)
-
71 or 111 or 113 or 114 or 115 (945,210)
-
letter.pt. (1,060,080)
-
editorial.pt. (598,624)
-
117 or 118 (1,658,704)
-
116 not 119 (927,165)
-
(animal/or nonhuman/) not exp human/ (5,382,670)
-
120 not 121 (894,026)
-
25 and 122 (1410)
-
limit 123 to yr = “2010 -Current” (1141)
-
limit 124 to conference abstracts (315)
-
124 not 125 (826).
Key:
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
$ = truncation
-
? = optional wildcard – stands for zero or one character
-
ti,ab = terms in either title or abstract fields
-
dq = terms in the candidate term word field
-
adj3 = terms within three words of each other (any order)
-
pt. = publication type.
Cochrane Database of Systematic Reviews
Via Wiley (http://onlinelibrary.wiley.com/).
Date range searched: issue 5 of 12, May 2019.
Date searched: 7 May 2019.
Records retrieved: 19.
Search strategy
-
#1 MeSH descriptor: [Carcinoma, Hepatocellular] this term only (1552)
-
#2 MeSH descriptor: [Liver Neoplasms] this term only (2259)
-
#3 ((liver or hepato* or hepatic*) near/3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)):ti,ab,kw (8211)
-
#4 hepatocarcinoma*:ti,ab,kw (74)
-
#5 hepatoma*:ti,ab,kw (141)
-
#6 [OR #1-#5] (8301)
-
#7 MeSH descriptor: [Chemoembolization, Therapeutic] this term only (289)
-
#8 (chemo next emboli* or chemoemboli*):ti,ab,kw (1252)
-
#9 (chemoembolotherap* or chemo next embolotherap*):ti,ab,kw (0)
-
#10 TACE:ti,ab,kw (991)
-
#11 cTACE:ti,ab,kw (35)
-
#12 (DEBTACE or DEB next TACE):ti,ab,kw (46)
-
#13 (eluting near/2 bead*):ti,ab,kw (100)
-
#14 DC next bead*:ti,ab,kw (32)
-
#15 [OR #7-#14] (1478)
-
#16 #6 and #15 (1332)
-
#17 MeSH descriptor: [Embolization, Therapeutic] this term only (345)
-
#18 (embolization* or embolisation* or embolize* or embolise* or embolizing* or embolising* or embolotherap*):ti,ab,kw (2276)
-
#19 TAE:ti,ab,kw (3688)
-
#20 [OR #17-#19] (5858)
-
#21 #6 and #20 (521)
-
#22 ((locoregional or loco next regional) near/2 (therap* or intervention* or treatment*)):ti,ab,kw (426)
-
#23 #6 and #22 (122)
-
#24 #16 or #21 or #23 (1641)
-
#25 #16 or #21 or #23 with Cochrane Library publication date Between Jan 2010 and May 2019, in Cochrane Reviews, Cochrane Protocols (19).
Key:
-
MeSH descriptor = indexing term (MeSH)
-
* = truncation
-
ti,ab,kw = terms in either title or abstract or keyword fields
-
near/3 = terms within three words of each other (any order)
-
next = terms are next to each other.
Database of Abstracts of Reviews of Effects
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2015.
Date searched: 7 May 2019.
Records retrieved: 78.
Search strategy
-
MeSH DESCRIPTOR Carcinoma, Hepatocellular IN DARE,HTA (316)
-
MeSH DESCRIPTOR Liver neoplasms IN DARE,HTA (459)
-
(((liver or hepato* or hepatic*) NEAR3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*))) IN DARE, HTA (627)
-
((carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) NEAR3 (liver or hepato* or hepatic*)) IN DARE, HTA (457)
-
(hepatocarcinoma*) IN DARE, HTA (3)
-
(hepatoma*) IN DARE, HTA (3)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 (652)
-
MeSH DESCRIPTOR Chemoembolization, Therapeutic IN DARE,HTA (74)
-
((chemo-emboli* or chemoemboli*)) IN DARE, HTA (98)
-
(chemoembolotherap* or chemo-embolotherap*) IN DARE, HTA (0)
-
(TACE) IN DARE, HTA (23)
-
(cTACE) IN DARE, HTA (0)
-
(DEBTACE or DEB-TACE) IN DARE, HTA (2)
-
(eluting NEAR2 bead*) IN DARE, HTA (10)
-
(bead* NEAR2 eluting) IN DARE, HTA (0)
-
(DC bead*) IN DARE, HTA (3)
-
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 (101)
-
#7 AND #17 (98)
-
MeSH DESCRIPTOR Embolization, Therapeutic IN DARE,HTA (106)
-
((emboli* or embolotherap*)) IN DARE, HTA (759)
-
(TAE) IN DARE, HTA (12)
-
#19 OR #20 OR #21 (767)
-
#7 AND #22 (39)
-
((locoregional or loco-regional) NEAR2 (therap* or intervention* or treatment*)) IN DARE, HTA (17)
-
((therap* or intervention* or treatment*) NEAR2 (locoregional or loco-regional)) IN DARE, HTA (6)
-
#24 OR #25 (19)
-
#7 AND #26 (7)
-
#18 OR #23 OR #27 (119)
-
(#28) IN DARE, HTA FROM 2010 TO 2019 (96)
-
(#29) IN DARE (78)
-
(#29) IN HTA (18)
Key:
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
NEAR3 = terms within three words of each other (order specified).
Health Technology Assessment database
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2018.
Date searched: 7 May 2019.
Records retrieved: 18.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
PROSPERO
URL: www.crd.york.ac.uk/PROSPERO/.
Date searched: 7 May 2019.
Records retrieved: 63.
Search strategy
-
#1 MeSH DESCRIPTOR Carcinoma, Hepatocellular (119)
-
#2 MeSH DESCRIPTOR Liver Neoplasms (172)
-
#3 (liver or hepato* or hepatic*) adj3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) (378)
-
#4 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) adj3 (liver or hepato* or hepatic*) (224)
-
#5 hepatocarcinoma* (9)
-
#6 hepatoma* (12)
-
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 (452)
-
#8 MeSH DESCRIPTOR Liver Neoplasms EXPLODE ALL TREES (183)
-
#9 MeSH DESCRIPTOR Chemoembolization, Therapeutic (14)
-
#10 chemo-emboli* or chemoemboli* (47)
-
#11 chemoembolotherap* or chemo-embolotherap* (0)
-
#12 TACE (41)
-
#13 cTACE (1)
-
#14 DEBTACE or DEB-TACE (6)
-
#15 eluting adj2 bead* (7)
-
#16 bead* adj2 eluting (0)
-
#17 DC bead* (0)
-
#18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 (59)
-
#19 #18 AND #7 (54)
-
#20 #18 NOT #19 (5)
-
#21 MeSH DESCRIPTOR Chemoembolization, Therapeutic EXPLODE ALL TREES (14)
-
#22 MeSH DESCRIPTOR Embolization, Therapeutic (29)
-
#23 embolization* or embolisation* or embolize* or embolise* or embolizing* or embolising* or embolotherap* (173)
-
#24 TAE (64)
-
#25 #22 OR #23 OR #24 (238)
-
#26 #25 AND #7 (34)
-
#27 (locoregional or loco-regional) adj2 (therap* or intervention* or treatment*) (20)
-
#28 #27 AND #7 (6)
-
#29 #28 OR #26 OR #19 (63).
Key:
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
adj3 = terms within three words of each other (order specified).
Appendix 3 Search strategies for quality-of-life studies
The aim of the search was to identify published studies reporting utility estimates for patients with HCC or cirrhosis. A search strategy was developed in MEDLINE (Ovid), consisting of terms for HCC or cirrhosis combined with a study design search filter to restrict retrieval to health state utility studies. 156 Specific named instruments used to measure HRQoL in HCC patients were also included in the strategy. No language or date restrictions were applied to the searches. The MEDLINE strategy was translated to run appropriately on the other databases searched.
The following databases were searched in February 2019: MEDLINE all (Ovid), Cost-Effectiveness Analysis Registry, EMBASE (Ovid), HTA database (CRD databases), NHS EED (CRD databases) and ScHARRHUD database.
Search results were imported into EndNote X9 and deduplicated.
MEDLINE all
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to 25 February 2019.
Date searched: 26 February 2019.
Records retrieved: 1837.
A study design search filter developed by Arber et al. 156 designed to restrict retrieval to health state utility studies was included in the strategy. The sensitivity-maximising version of the filter was used; see lines 13–35 below.
Search strategy
-
Carcinoma, Hepatocellular/ (77,760)
-
Liver Neoplasms/ (137,948)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (132,386)
-
hepatocarcinoma$.ti,ab. (3764)
-
hepatoma$.ti,ab. (27,397)
-
or/1-5 (208,036)
-
exp Liver Cirrhosis/ (84,653)
-
(cirrhos$ or cirrhot$).ti,ab. (93,295)
-
((liver or hepatic$) adj3 fibros$).ti,ab. (22,118)
-
(biliary adj3 (cirrhos$ or cirrhot$ or cholangitis)).ti,ab. (9992)
-
or/7-10 (132,914)
-
6 or 11 (311,502)
-
quality-adjusted life years/ (10,727)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (14,531)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (9350)
-
(illness state$1 or health state$1).ti,ab,kf. (5828)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (1350)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (814)
-
(utility adj3 (score$1 or valu$ or health$ or cost$ or measur$ or disease$ or mean or gain or gains or index$)).ti,ab,kf. (13,429)
-
utilities.ti,ab,kf. (6374)
-
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eur qol5d or eur?qul or eur?qul5d or euro$ quality of life or european qol).ti,ab,kf. (9564)
-
(euro$ adj3 (5 d or 5d or 5 dimension$ or 5dimension$ or 5 domain$ or 5domain$)).ti,ab,kf. (3329)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (20,320)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (1743)
-
quality of life/and ((quality of life or qol) adj (score$1 or measure$1)).ti,ab,kf. (10,526)
-
quality of life/and ec.fs. (9271)
-
quality of life/and (health adj3 status).ti,ab,kf. (8092)
-
(quality of life or qol).ti,ab,kf. and Cost-Benefit Analysis/ (11,091)
-
((qol or hrqol or quality of life).ti,kf. or *quality of life/) and ((qol or hrqol$ or quality of life) adj2 (increas$ or decrease$ or improv$ or declin$ or reduc$ or high$ or low$ or effect or effects or worse or score or scores or change$1 or impact$1 or impacted or deteriorat$)).ab. (32,288)
-
Cost-Benefit Analysis/and (cost-effectiveness ratio$ and (perspective$ or life expectanc$)).ti,ab,kf. (2980)
-
*quality of life/and (quality of life or qol).ti. (48,595)
-
quality of life/and ((quality of life or qol) adj3 (improv$ or chang$)).ti,ab,kf. (23,881)
-
quality of life/and health-related quality of life.ti,ab,kf. (27,802)
-
models,economic/ (9191)
-
or/13-34 (146,623)
-
12 and 35 (1437)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (29,854)
-
disutili$.ti,ab,kf. (405)
-
(short form$ or shortform$).ti,ab,kf. (29,550)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (4154)
-
or/37-40 (61,362)
-
12 and 41 (709)
-
36 or 42 (1801)
-
“European Organization for Research and Treatment of Cancer Quality of Life”.ti,ab. (830)
-
“European Organisation for Research and Treatment of Cancer Quality of Life”.ti,ab. (336)
-
EORTC quality of life.ti,ab. (412)
-
(EORTC QLQ$ or EORTCQLQ$).ti,ab. (3173)
-
(QLQ-C30$ or QLQC30$ or QLQ-C-30$ or QLQC-30$).ti,ab. (3609)
-
(FACT-Hep or FACTHep).ti,ab. (35)
-
FACT-hepatobiliary.ti,ab. (10)
-
Functional Assessment of Cancer Therapy Hepatobiliary.ti,ab. (45)
-
(FHSI-8 or FHSI8).ti,ab. (6)
-
(FACT-G or FACTG).ti,ab. (554)
-
FACT-General.ti,ab. (69)
-
Functional Assessment of Cancer Therapy General.ti,ab. (452)
-
(QLQ-LC$ or QLQLC$).ti,ab. (114)
-
(QLQ-HCC18$ or QLQHCC18$ or QLQ-HCC-18$ or QLQHCC-18$).ti,ab. (11)
-
(QLQ-PAN$ or QLQPAN$).ti,ab. (40)
-
(Gastrointestinal Quality of Life adj (index$ or indices)).ti,ab. (387)
-
GIQLI$.ti,ab. (329)
-
or/44-60 (5833)
-
12 and 61 (132)
-
43 or 62 (1837).
Key:
-
/ = indexing term (MeSH)
-
exp = exploded indexing term (MeSH)
-
$ = truncation
-
$1 = limited truncation – restricts to one character only after word
-
ti,ab = terms in either title or abstract fields
-
ec.fs. = floating economics subheading search
-
kf = author keywords field
-
adj3 = terms within three words of each other (any order).
Cost Effectivieness Analysis Registry
URL: http://healtheconomics.tuftsmedicalcenter.org/cear2n/search/search.aspx.
Date searched: 26 February 2019.
Records retrieved: 124.
The Cost-Effectiveness Analysis Registry was searched using the basic search interface using a set of simple searches for the population. Duplicates were removed before exporting records.
Search strategy
-
hepatocellular carcinoma (86)
-
hepatocellular cancer (1)
-
hepatocellular neoplasm (0)
-
hepatocellular tumor (0)
-
hepatocellular tumour (0)
-
hepatocellular malignancy (0)
-
hepatocarcinoma (0)
-
hepatoma (1)
-
liver cancer (12)
-
liver carcinoma (0)
-
liver neoplasm (6)
-
liver tumor (2)
-
liver tumour (1)
-
liver malignancy (0)
-
liver cirrhosis (21)
-
liver fibrosis (15).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 25 February 2019.
Date searched: 26 February 2019.
Records retrieved: 2415.
Retrieval was restricted to health state utility studies using terms based on a study design search filter developed by Arber et al. 156 for use in Ovid MEDLINE. This was translated for use in Ovid EMBASE; see lines 13–42 below.
-
liver cell carcinoma/ (136,695)
-
liver cancer/ (28,869)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (184,856)
-
hepatocarcinoma$.ti,ab. (4990)
-
hepatoma$.ti,ab. (30,679)
-
or/1-5 (242,352)
-
exp liver cirrhosis/ (141,130)
-
(cirrhos$ or cirrhot$).ti,ab. (135,400)
-
((liver or hepatic$) adj3 fibros$).ti,ab. (36,133)
-
(biliary adj3 (cirrhos$ or cirrhot$ or cholangitis)).ti,ab. (13,554)
-
or/7-10 (194,904)
-
6 or 11 (388,577)
-
quality adjusted life year/ (23,009)
-
(quality adjusted or adjusted life year$).ti,ab,kw. (21,303)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kw. (17,652)
-
(illness state$1 or health state$1).ti,ab,kw. (10,032)
-
(hui or hui1 or hui2 or hui3).ti,ab,kw. (2027)
-
(multiattribute$ or multi attribute$).ti,ab,kw. (1040)
-
(utility adj3 (score$1 or valu$ or health$ or cost$ or measur$ or disease$ or mean or gain or gains or index$)).ti,ab,kw. (21,358)
-
utilities.ti,ab,kw. (10,356)
-
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eur qol5d or eur?qul or eur?qul5d or euro$ quality of life or european qol).ti,ab,kw. (17,622)
-
(euro$ adj3 (5 d or 5d or 5 dimension$ or 5dimension$ or 5 domain$ or 5domain$)).ti,ab,kw. (5144)
-
short form 36/ (24,680)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kw. (34,476)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kw. (2512)
-
quality of life/and ((quality of life or qol) adj (score$1 or measure$1)).ti,ab,kw. (22,209)
-
“quality of life”/and pe.fs. (8003)
-
“quality of life”/and de.fs. (300)
-
“quality of life”/and (health adj3 status).ti,ab,kw. (14,248)
-
(quality of life or qol).ti,ab,kw. and “cost benefit analysis”/ (5014)
-
((qol or hrqol or quality of life).ti,kw. or *”quality of life”/) and ((qol or hrqol$ or quality of life) adj2 (increas$ or decrease$ or improv$ or declin$ or reduc$ or high$ or low$ or effect or effects or worse or score or scores or change$1 or impact$1 or impacted or deteriorat$)).ab. (49,462)
-
“cost benefit analysis”/and (cost-effectiveness ratio$ and (perspective$ or life expectanc$)).ti,ab,kw. (726)
-
*”quality of life”/and (quality of life or qol).ti. (74,391)
-
“quality of life”/and ((quality of life or qol) adj3 (improv$ or chang$)).ti,ab,kw. (65,833)
-
“quality of life”/and health-related quality of life.ti,ab,kw. (50,090)
-
economic model/ (1547)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kw. (45,473)
-
disutili$.ti,ab,kw. (802)
-
(short form$ or shortform$).ti,ab,kw. (39,683)
-
short form 12/ (5132)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kw. (7154)
-
or/13-41 (294,270)
-
12 and 42 (3994)
-
“European Organization for Research and Treatment of Cancer Quality of Life”.ti,ab. (1083)
-
“European Organisation for Research and Treatment of Cancer Quality of Life”.ti,ab. (445)
-
EORTC quality of life.ti,ab. (678)
-
(EORTC QLQ$ or EORTCQLQ$).ti,ab. (6855)
-
(QLQ-C30$ or QLQC30$ or QLQ-C-30$ or QLQC-30$).ti,ab. (7303)
-
(FACT-Hep or FACTHep).ti,ab. (88)
-
FACT-hepatobiliary.ti,ab. (21)
-
Functional Assessment of Cancer Therapy Hepatobiliary.ti,ab. (58)
-
(FHSI-8 or FHSI8).ti,ab. (14)
-
(FACT-G or FACTG).ti,ab. (1231)
-
FACT-General.ti,ab. (112)
-
Functional Assessment of Cancer Therapy General.ti,ab. (678)
-
(QLQ-LC$ or QLQLC$).ti,ab. (254)
-
(QLQ-HCC18$ or QLQHCC18$ or QLQ-HCC-18$ or QLQHCC-18$).ti,ab. (21)
-
(QLQ-PAN$ or QLQPAN$).ti,ab. (77)
-
(Gastrointestinal Quality of Life adj (index$ or indices)).ti,ab. (526)
-
GIQLI$.ti,ab. (550)
-
or/44-60 (11,272)
-
12 and 61 (236)
-
43 or 62 (4054)
-
(animal/or animal experiment/or animal model/or animal tissue/or nonhuman/) not exp human/ (5,661,185)
-
63 not 64 (3979)
-
limit 65 to conference abstracts (1564)
-
65 not 66 (2415).
Key:
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
$ = truncation
-
$1 = limited truncation – restricts to one character only after word
-
ti,ab = terms in either title or abstract fields
-
pe.fs = floating pharmacoeconomics subheading search
-
de.fs = floating device economics subheading search
-
kw = terms in the author keywords field
-
adj3 = terms within three words of each other (any order).
Health Technology Assessment database
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2018.
Date searched: 26 February 2019.
Records retrieved: 188.
Search strategy
-
MeSH DESCRIPTOR Carcinoma, Hepatocellular IN NHSEED,HTA (97)
-
MeSH DESCRIPTOR Liver Neoplasms IN NHSEED,HTA (174)
-
((liver or hepato* or hepatic*) NEAR3 (carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*)) IN NHSEED, HTA (343)
-
((carcinoma* or cancer* or neoplas* or tumour* or tumor* or malign*) NEAR3 (liver or hepato* or hepatic*)) IN NHSEED, HTA (202)
-
(hepatocarcinoma*) IN NHSEED, HTA (8)
-
(hepatoma*) IN NHSEED, HTA (5)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 (365)
-
MeSH DESCRIPTOR Liver Cirrhosis EXPLODE ALL TREES IN NHSEED,HTA (129)
-
(cirrhos* or cirrhot*) IN NHSEED, HTA (340)
-
((liver or hepatic*) NEAR3 fibros*) IN NHSEED, HTA (43)
-
(fibros* NEAR3 (liver or hepatic*)) IN NHSEED, HTA (11)
-
(biliary NEAR3 (cirrhos* or cirrhot* or cholangitis)) IN NHSEED, HTA (14)
-
((cirrhos* or cirrhot* or cholangitis) NEAR3 biliary) IN NHSEED, HTA (8)
-
#8 OR #9 OR #10 OR #11 OR #12 OR #13 (350)
-
#7 OR #14 (540)
-
(#15) IN NHSEED (352)
-
(#15) IN HTA (188).
Key:
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
NEAR3 = terms within three words of each other (order specified).
NHS Economic Evaluations Database
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2015.
Date searched: 26 February 2019.
Records retrieved: 352.
See above under Health Technology Assessment database for search strategy used.
ScHARRHUD
URL: www.scharrhud.org/.
Date searched: 26 February 2019.
Records retrieved: 11.
Search strategy
-
liver OR hepato* OR hepatic*
-
cirrhos* OR cirrhot*
-
biliary AND cholangitis
-
(#1 OR #2 OR #3).
Key:
-
* = truncation.
Appendix 4 Search strategies for resource use and cost evidence
The aim of the search was to identify published studies relating to costs or resource use in patients with HCC. A search strategy was developed in MEDLINE (Ovid), comprising a set of terms for HCC combined with terms relating to costs or resource use. The terms included for costs were based on a search strategy developed by the Canadian Agency for Drugs and Technologies in Health (CADTH). 157 Retrieval was restricted to studies published from 2010 onwards in any language. The MEDLINE strategy was translated to run appropriately on the other databases searched.
The following databases were searched on 7 March 2019: MEDLINE all (Ovid) and EMBASE (Ovid). The previous results obtained for the health utilities search from the HTA database and NHS EED were added to the results from MEDLINE and EMBASE.
Search results were imported into EndNote X9 and deduplicated.
MEDLINE all
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to 6 March 2019.
Date searched: 7 March 2019.
Records retrieved: 2153.
Lines 7–19 below are based on a search strategy developed by CADTH to identify studies about costs/economics. 157
Search strategy
-
Carcinoma, Hepatocellular/ (77,885)
-
Liver Neoplasms/ (138,136)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (132,179)
-
hepatocarcinoma$.ti,ab. (3767)
-
hepatoma$.ti,ab. (27,406)
-
or/1-5 (207,882)
-
economics/ (27,006)
-
exp “costs and cost analysis”/ (222,429)
-
economics, dental/ (1901)
-
exp “economics, hospital”/ (23,378)
-
economics, medical/ (9002)
-
economics, nursing/ (3986)
-
economics, pharmaceutical/ (2843)
-
exp “Fees and Charges”/ (29,616)
-
exp Budgets/ (13,465)
-
budget*.ti,ab,kf. (27,124)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. (209,622)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (258,034)
-
or/7-18 (523,885)
-
6 and 19 (1325)
-
Health Resources/ (12,010)
-
Healthcare Financing/ (695)
-
(resource$ adj2 (“use” or utilis$ or utiliz$ or consum$ or usage)).ti,ab. (25,314)
-
((healthcare or health-care) adj2 (“use” or utilis$ or utiliz$ or consum$ or usage)).ti,ab. (25,383)
-
21 or 22 or 23 or 24 (56,988)
-
6 and 25 (134)
-
Length of Stay/ (80,203)
-
(cost$ adj2 (illness$ or disease$ or sickness$)).ti,ab. (4600)
-
(burden$ adj2 (disease$ or illness$ or sickness$)).ti,ab. (22,257)
-
((length or hospital$ or duration) adj2 stay$).ti,ab. (120,889)
-
((extended or prolonged) adj stay$).ti,ab. (1013)
-
((hospitali?ation$ or hospitali?ed) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing)).ti,ab. (6753)
-
economic consequenc$.ti,ab. (3229)
-
or/27-33 (190,256)
-
6 and 34 (2349)
-
20 or 26 or 35 (3467)
-
exp animals/not humans/ (4,553,712)
-
36 not 37 (3454)
-
limit 38 to yr = “2010 -Current” (2153).
Key:
-
/ = indexing term (MeSH)
-
exp = exploded indexing term (MeSH)
-
$ = truncation
-
? = optional wild card – stands for zero or one character within a word
-
ti,ab = terms in either title or abstract fields
-
ab./freq = 2 = frequency operator – term must appear at least twice in the abstract for the record to be retrieved
-
kf = author keywords field
-
adj3 = terms within three words of each other (any order).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 6 March 2019.
Date searched: 7 March 2019.
Records retrieved: 3913.
Lines 7–14 below are based on a search strategy developed by CADTH to identify studies about costs/economics. 158
Search strategy
-
liver cell carcinoma/ (136,950)
-
liver cancer/ (28,936)
-
((liver or hepato$ or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (185,215)
-
hepatocarcinoma$.ti,ab. (5000)
-
hepatoma$.ti,ab. (30,696)
-
or/1-5 (242,760)
-
Economics/ (231,508)
-
Cost/ (56,142)
-
exp Health Economics/ (783,424)
-
Budget/ (26,815)
-
budget*.ti,ab,kw. (35,333)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw. (253,689)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (357,407)
-
or/7-13 (1,153,032)
-
6 and 14 (4962)
-
health care utilization/ (63,300)
-
health care financing/ (12,931)
-
(resource$ adj2 (“use” or utilis$ or utiliz$ or consum$ or usage)).ti,ab. (39,541)
-
((healthcare or health-care) adj2 (“use” or utilis$ or utiliz$ or consum$ or usage)).ti,ab. (36,926)
-
16 or 17 or 18 or 19 (122,638)
-
6 and 20 (501)
-
disease burden/ (8049)
-
Length of Stay/ (159,340)
-
(cost$ adj2 (illness$ or disease$ or sickness$)).ti,ab. (6874)
-
(burden$ adj2 (disease$ or illness$ or sickness$)).ti,ab. (33,648)
-
((length or hospital$ or duration) adj2 stay$).ti,ab. (204,289)
-
((extended or prolonged) adj stay$).ti,ab. (1581)
-
((hospitali?ation$ or hospitali?ed) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing)).ti,ab. (11,727)
-
economic consequenc$.ti,ab. (4245)
-
or/22-29 (313,622)
-
6 and 30 (3966)
-
15 or 21 or 31 (8470)
-
(animal/or animal experiment/or animal model/or animal tissue/or nonhuman/) not exp human/ (5,667,672)
-
32 not 33 (8389)
-
limit 34 to yr = “2010 -Current” (6403)
-
limit 35 to conference abstracts (2490)
-
35 not 36 (3913)
Key:
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
$ = truncation
-
? = optional wild card – stands for zero or one character within a word
-
ti,ab = terms in either title or abstract fields
-
ab./freq = 2 = frequency operator – term must appear at least twice in the abstract for a record to be retrieved
-
kw = terms in the author keywords field
-
adj3 = terms within three words of each other (any order).
Health Technology Assessment database
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2018.
Date searched: 26 February 2019.
Records retrieved: 188.
To view the search strategy, see under HRQoL search strategies in Appendix 3.
NHS Economic Evaluations Database
Via www.crd.york.ac.uk/CRDWeb/.
Date range searched: inception to 31 March 2015.
Date searched: 26 February 2019.
Records retrieved: 352.
To view the search strategy, see under HRQoL search strategies in Appendix 3.
Appendix 5 Risk-of-bias assessment results
Risk-of-bias assessment results for randomised controlled trials
| Trial (first author and year) | Risk of bias arising from the randomisation process | Risk of bias owing to deviations from the intended interventions | Missing outcome data (primary outcome) | Risk of bias in measurement of the outcome | Risk of bias in selection of the reported result | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|
| Vilgrain 201719,84 | Low | Low | Low | Low | Low | Low |
| SARAH | ||||||
| Chow 201821 | Low | Low | Low | Low | Low | Low |
| SIRveNIB | ||||||
| Kolligs 201522 | High | Low | High | High | Low | High |
| SIRTACE | ||||||
| Pitton 201523 | Some concerns | Low | Low | Low | Low | Some concerns |
| Ricke 201524 | Some concerns | High | Low | Low | Low | High |
| SORAMIC | ||||||
| Salem 201625–27 | High | Some concerns | Low | Low | Low | High |
| PREMIERE | ||||||
| Kulik 201428–30 | Some concerns | Some concerns | Low | Some concerns | Low | Some concerns |
Risk-of-bias assessment results for prospective comparative studies
| Trial (first author and year) | Inclusion criteria clearly defined | Allocation to treatment groups adequately described/appropriate | Groups similar at baseline | Clearly described and consistently delivered intervention | Clearly described and consistently delivered comparator | Outcome assessors blinded | Missing outcome data balanced across groups | Free from suggestion of selective reporting | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Kirchner 201931 | No | No | No | Yes | No | No | Yes | Yes | High |
| El Fouly 201532 | Yes | No | No | Yes | Yes | No | Yes | Yes | High |
| Salem 201333 | Yes | No | No | Yes | Yes | No | Yes | Yes | High |
| Memon 201334 | Yes | No | Yes | Yes | Yes | No | Yes | Unclear | High |
| Hickey 201635 | Yes | No | No | Yes | Yes | No | Yes | Yes | High |
| Maccauro 201436 | No | No | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Woodall 200937 | Yes | No | No | Yes | Yes | No | Yes | Yes | High |
Risk-of-bias assessment results for retrospective comparative studies
| Trial (first author and year) | Inclusion criteria clearly defined | Representative sample from relevant population | Groups similar at baseline | Clearly described and consistently delivered intervention | Clearly described and consistently delivered comparator | Outcome assessors blinded | Missing outcome data balanced across groups | Free from suggestion of selective reporting | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Biederman 201538 | No | Unclear | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Biederman 201639 | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Unclear | High |
| Van Der Gucht 201740 | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes | High |
| Bhangoo 201541 | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Unclear |
| d’Abadie 201842 | No | Unclear | No | No | No | Unclear | Unclear | Yes | High |
Risk-of-bias assessment results for non-comparative studies
| Trial (first author and year) | Inclusion criteria clearly defined | Representative sample from relevant population | Clearly described and consistently delivered intervention | Outcome measures prespecified, reliable and consistently assessed | Outcome assessors blinded | Attrition low and accounted for in analysis | Incomplete outcome data minimal/dealt with in analysis | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|---|---|
| Radosa 201951 | Yes | Unclear | Yes | No | No | N/A (retrospective database of treated patients) | Yes | High |
Appendix 6 Study details and results for all studies included in the systematic review of clinical effectiveness (n = 20)
| Study (first author, year, name and location) | Study design and funding source | Population | Intervention | Comparator | Main results | Risk of bias | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
SARAH France |
Multicentre open-label RCT Funding: Sirtex |
Locally advanced HCC (BCLC C), or new HCC not eligible for surgery/ablation after previously cured HCC, or HCC with two unsuccessful rounds of TACE. Life expectancy of > 3 months, ECOG performance status of 0 or 1, Child–Pugh class A or B score of ≤ 7 | SIR-Spheres (n = 237) | Sorafenib (400 mg twice daily orally, administered until the occurrence of radiological progression, unacceptable AEs or death) (n = 222) |
OS: SIR-Spheres: median 8.0 months (95% CI 6.7 to 9.9 months); 196/237 (83%) patients died; 1-year OS: 39.5% (95% CI 33.3% to 45.9%) Sorafenib: median 9.9 months (95% CI 8.7 to 11.4 months); 177/222 (80%) patients died; 1-year OS: 42.1% (95% CI 35.6% to 48.7%) Comparison between groups: ITT population HR 1.15 (95% CI 0.94 to 1.41; p = 0.18) Per-protocol population HR 0.99 (95% CI 0.79 to 1.24) PFS: SIR-Spheres: median 4.1 months (95% CI 3.8 to 4.6 months); 218/237 (92%) had progression events Sorafenib: median 3.7 months (95% CI 3.3 to 5.4 months).; 205/222 (92%) had progression events Comparison between groups: ITT population HR 1.03 (95% CI 0.85 to 1.25; p = 0.76) Complete or partial response rate: SIR-Spheres: 36/190 (19%) evaluable patients Sorafenib: 23/198 (12%) evaluable patients HRQoL: The global health status subscore was significantly better in the SIRT group than in the sorafenib group (group effect p = 0.0048; time effect p < 0.0001) and the between- group difference tended to increase with time (group*time interaction p = 0.0447) for both the ITT and per-protocol populations AEs: SIR-Spheres: 173/226 (77%) patients reported at least one AE; 19 treatment-related deaths (six did not receive SIRT and subsequently received sorafenib) Sorafenib: 203/216 (94%) patients reported at least one AE; 12 treatment-related deaths; 139/216 (64%) patients discontinued sorafenib owing to drug-related toxicity, 108 of whom permanently discontinued Time on treatment/number of treatments: SIR-Spheres: 53/237 (22%) did not receive SIRT. Of 184 patients who received SIRT, 115 (63%) received a single administration, 58 patients received two treatments, 11 patients received three treatments Sorafenib: median dose intensity: 800 mg/day (IQR 585–800 mg/day). Median cumulative time of sorafenib intake 2.8 months (IQR 1.0–5.8 months); 82/216 (38%) required a dose reduction. Permanent discontinuation occurred in 132 (61%) patients; 49 (37%) patients discontinued sorafenib before tumour progression |
Low | ||||||||||||||||||||
|
Chow 201821 SIRveNIB Asia-Pacific region |
Multicentre open-label RCT Funding: Sirtex |
Locally advanced HCC (BCLC B or C without extrahepatic disease) with or without PVT, not amenable to curative treatment modalities | SIR-Spheres (n = 182) | Sorafenib (400 mg twice daily orally, administered until the occurrence of treatment failure, complete response, initiation of other HCC therapies, unacceptable AEs, patient request to stop treatment or death) (n = 178) |
OS: SIR-Spheres: median 8.8 months (95% CI 7.5 to 10.8 months) Sorafenib: median 10.0 months (95% CI 8.6 to 13.8 months) Comparison between groups: ITT population HR 1.12 (95% CI 0.9 to 1.4; p = 0.36) Per-protocol population HR 0.86 (95% CI 0.7 to 1.1; p = 0.27) PFS: SIR-Spheres: median 5.8 months (95% CI 3.7 to 6.3 months) Sorafenib: median 5.1 months (95% CI 3.9 to 5.6 months) Comparison between groups: ITT population HR 0.89 (95% CI 0.7 to 1.1; p = 0.31) Complete or partial response rate: SIR-Spheres: 16.5% Sorafenib: 1.7% HRQoL: There were no statistically significant differences in the EQ-5D index between the SIR-Spheres and sorafenib groups throughout the study in either the ITT or the treated populations AEs: SIR-Spheres: 78/130 (60.0%) patients reported at least one AE; 36/130 (27.7%) reported at least one grade ≥ 3 AE; 27/130 (20.8%) reported at least one serious AE Sorafenib: 137/162 (84.6%) patients reported at least one AE; 82/130 (50.6%) reported at least one grade ≥ 3 AE; 57/162 (35.2%) reported at least one serious AE Time on treatment/number of treatments: SIR-Spheres: 52/182 (28.6%) did not receive SIRT. All 130 patients who received SIRT received a single administration Sorafenib: 16/178 (9%) did not receive sorafenib. Median treatment duration was 13.8 weeks and mean daily dose was 644.5 mg |
Low | ||||||||||||||||||||
|
Kolligs 201522 SIRTACE Germany and Spain |
Multicentre open-label RCT Funding: Sirtex |
Unresectable HCC with preserved liver function (Child–Pugh class ≤ B7; total bilirubin ≤ 2 mg/dl), an ECOG performance status of ≤ 2, and absence of any form of vascular invasion or extrahepatic spread | SIR-Spheres (n = 13) | TACE (n = 15) |
Overall survival: Not reported PFS: SIR-Spheres: median 3.6 months (95% CI 2.3 to 6.2 months) TACE: median 3.7 months (95% CI 1.6 to 11.0 months) Complete or partial response rate: SIR-Spheres: 4/13 (30.8%) TACE: 2/15 (13.3%) HRQoL: HRQoL data were analysed for 18 patients (8 SIRT and 10 TACE). Higher scores reflect higher functioning and fewer symptoms. At baseline, median scores were lower for patients receiving SIRT than for patients receiving TACE, particularly for subscales of physical functioning (82.0 vs. 96.0; p = 0.04) by Kruskal–Wallis test This manifested in the lower scores with SIRT throughout the first 12 weeks after treatment, although the differences between the treatment groups by week 12 were not statistically significant for either FACT-Hep total or its subscales AEs: SIR-Spheres: 12/13 (92.3%) patients reported at least one AE; 3/13 reported at least one grade ≥ 3 AE; 7/13 reported at least one serious AE requiring hospitalisation TACE: 10/15 (66.7%) patients reported at least one AE; 2/15 reported at least one grade ≥ 3 AE; 5/15 reported at least one serious AE requiring hospitalisation Time on treatment/number of treatments: SIR-Spheres: 7/13 (53.8%) received whole-liver SIRT, 5 (38.5%) received lobar and 1 (7.7%) received segmental treatment. All patients received one course of treatment TACE: on average, patients received 3.4 (SD 2.9, median 2.0) separate sessions during the study. three patients received one course of TACE, five patients received two courses, three patients received four courses, three patients received five courses and one patient received 11 courses |
High | ||||||||||||||||||||
|
Pitton 201523 Germany |
Single-centre open-label RCT Funding: Johannes Gutenberg University of Mainz (Mainz, Germany) |
Unresectable N0, M0 HCC (BCLC stage B) | SIR-Spheres (n = 12) | DEB-TACE (n = 12) |
OS: SIR-Spheres: median 592 days (Q1: 192 days, Q3: –) Mean 437 days (SE 72 days). Cause of death was predominantly liver failure (n = 4), with only one death because of tumour progression DEB-TACE: median 788 days (Q1: 178 days, Q3: 950 days) Mean 583 days (SE 119 days). Cause of death was predominantly tumour progression (n = 4), with only one death because of liver failure PFS: SIR-Spheres: median 180 days (Q1: 120 days, Q3: 414 days) Mean 266 days (SE 55 days) DEB-TACE: median 216 days (Q1: 88 days, Q3: 355 days) Mean 237 days (SE 49 days) Complete or partial response rate: Not reported HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: SIR-Spheres: patients received either one (n = 4) or two (n = 8) treatment sessions. Eight patients had a bilobar approach DEB-TACE: the mean number of treatment sessions was 3.8 ± 2.6 (range 1–10). Embolisation was unilobar in five and bilobar in seven patients |
Some concerns | ||||||||||||||||||||
|
Ricke 201524 SORAMIC Germany |
Multicentre open-label RCT Funding: Sirtex and Bayer HealthCare (Leverkusen, Germany) |
Unresectable intermediate or advanced HCC (BCLC stage B or C) with preserved liver function (Child–Pugh class ≤ B7) and ECOG performance status of < 2, who were poor candidates for TACE (including those failing TACE) | SIR-Spheres plus sorafenib (n = 20) | Sorafenib alone (n = 20) |
OS: Not reported PFS: Not reported Complete or partial response rate: Not reported HRQoL: Not reported AEs: SIR-Spheres plus sorafenib: there were 196 AEs reported; 43/196 (21.9%) were grade 3 or worse Sorafenib alone: there were 222 AEs reported; 47/222 (21.2%) were grade 3 or worse Time on treatment/number of treatments: SIR-Spheres plus sorafenib: SIRT was administered as a sequential lobar treatment in 10/20 patients, and 10 patients received unilobar treatment. Patients received a median daily sorafenib dose of 614 mg (range 45–793 mg) over a median of 8.5 months Sorafenib alone: patients received a median daily sorafenib dose of 557 mg (range 284–792 mg) over a median of 9.6 months |
High | ||||||||||||||||||||
|
PREMIERE USA |
Single-centre open-label RCT Funding: National Institutes of Health grant (in part) |
BCLC stage A/B unablatable/unresectable HCC with no vascular invasion. Child–Pugh class A/B | TheraSphere (n = 24) | TACE (n = 21) |
Overall survival: TheraSphere: median 18.6 months (95% CI 7.4 to 32.5 months) TACE: median 17.7 months (95% CI 8.3 to not calcuable months) TTP: TheraSphere: not reached (> 26 months) TACE: 6.8 months Complete or partial response rate: TheraSphere: 20/23 (87%) achieved EASL response, 12/23 (52%) achieved WHO response TACE: 14/19 (74%) achieved EASL response, 12/19 (63%) achieved WHO response HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: TheraSphere: SIRT treatment was performed in 17/24 patients; seven were lobar treatments TACE: selective chemoembolisation was performed in 16/19 patients; three were lobar treatments |
High | ||||||||||||||||||||
|
USA |
Single-centre open-label RCT pilot study Funding: Bayer/Onyx (Novato, CA, USA) and a Northwestern University (Evanston, IL, USA) departmental pilot grant programme |
HCC, Child–Pugh class ≤ B8 and potential candidates for orthotopic liver transplantation | TheraSphere (n = 10) | TheraSphere plus sorafenib (n = 10) |
OS: TheraSphere: three patients died TheraSphere plus sorafenib: two patients died PFS: Not reported Complete or partial response rate: Not reported HRQoL: Not reported AEs: The most commonly reported AEs were fatigue (9/10 TheraSphere patients and 4/10 TheraSphere plus sorafenib patients), pain (5/10 TheraSphere patients and 0 TheraSphere plus sorafenib patients) and nausea (7/10 TheraSphere patients and 2 TheraSphere plus sorafenib patients) Time on treatment/number of treatments: TheraSphere: 2/10 patients had more than one SIRT treatment; one patient had two SIRT treatments and one patient had three SIRT treatments plus one TACE TheraSphere plus sorafenib: 3/10 patients had more than one SIRT treatment; one patient had three SIRT treatments, one patient had a second SIRT treatment plus TACE and one patient had a second SIRT treatment plus radiofrequency ablation |
Some concerns | ||||||||||||||||||||
|
Kirchner 201931 Germany |
Prospective single-centre comparative study Funding: none |
All patients undergoing initial TACE or TARE due to HCC between November 2014 and March 2016 agreed to participate (n = 94). Twenty-seven patients failed to answer the questionnaire; therefore, quality of life after 67 interventions was analysed | TheraSphere (n = 21) |
cTACE (n = 33) DEB-TACE (n = 13) |
OS: Not reported PFS: Not reported Complete or partial response rate (RECIST): TheraSphere: 0/19 (0%) evaluable patients TACE: 1/44 (2.3%) evaluable patients Complete or partial response rate (WHO): TheraSphere: 1/19 (5.3%) evaluable patients TACE: 3/44 (6.8%) evaluable patients HRQoL: Before the intervention, the mean global health status/QoL in the SIRT group (50.8%) was significantly lower than in the TACE group (62.5%, p = 0.029) After treatment, the mean absolute decrease in global health status/QoL was higher in the TACE group (–10.5%) than in the SIRT group (–4.8%), which was not statistically significant (p = 0.396). The absolute increase in fatigue after initial treatment was significantly higher with TACE (+19.1%) than with SIRT (+7.9%) (p = 0.021) The SIRT group showed the highest changes in financial difficulties (14.3% increase), role functioning (12.7% decrease) and dyspnoea (11.1% increase), C30 role functioning (12.7% decrease), social functioning (10.3% decrease) and QLQ-HCC18 nutrition (10.2% increase). The TACE group showed the highest changes in QOL-C30 physical functioning (14.1% decrease), role functioning (21.7% decrease), emotional functioning (10.2% decrease), social functioning (17.4% decrease) and fatigue (19.1% increase). It also showed an 11.6% increase in pain, QLQ-HCC18 fatigue (11.6% increase), body image (11.2% increase) and sex life (11.6% increase) Relative pre/post change in global health status was –16.8% in the TACE group and –9.4% in the SIRT group AEs: Not reported Time on treatment/number of treatments: Not reported |
High | ||||||||||||||||||||
|
El Fouly 201532 Germany and Egypt |
Prospective multicentre comparative study Funding: not reported |
Intermediate-stage (BCLC B) HCC and good liver function (Child–Pugh class B < 7) | TheraSphere (n = 44) | TACE (n = 42) |
OS: TheraSphere: median 16.4 months (95% CI 7.9 to 25.3 months); 1-year OS: 59%, 2-year OS: 40%, 3-year OS: 31% TACE: median 18 months (95% CI 12.1 to 25.5 months); 1-year OS: 64%, 2-year OS: 36%, 3-year OS: 11% TTP: TheraSphere: median 13.3 months (95% CI 3.4 to 23.1 months) TACE: median 6.8 months (95% CI 3.9 to 8.8 months) Complete or partial response rate: TheraSphere: 7% complete response, 68% partial response TACE: 5% complete response, 45% partial response HRQoL: Not reported AEs: The most commonly reported AE was unspecific abdominal pain, which was found in 83% of TACE patients (vs. 5% of SIRT patients) Time on treatment/number of treatments: TheraSphere: total number of sessions = 63, with a mean of 1.4 sessions per patient (median 1) TACE: total number of sessions = 93, with a mean of 2.2 sessions per patient (median 2 sessions) |
High | ||||||||||||||||||||
|
Salem 201333 USA |
Prospective comparative study Funding: Dimitrovich Family Foundation and National Institutes of Health (in part) |
Treatment-naive HCC patients with ECOG performance status 0–2 | TheraSphere (n = 29) | TACE (n = 27) |
OS: Not reported PFS: Not reported Complete or partial response rate: Not reported HRQoL: Overall, most of the FACT-Hep scales showed a reduction in score in the TACE group, with stability or increase in the SIRT group between baseline and 4-week assessments Despite more advanced disease at baseline (regression analysis incorporating BCLC stage), SIRT patients showed significantly better quality of life relative to TACE in social well-being (p = 0.019), functional well-being (p = 0.031) and embolotherapy-specific score (p = 0.018). Strong trends favouring SIRT were noted in overall quality of life (p = 0.055), the Trial Outcome Index (p = 0.05) and FACT-Hep (p = 0.071) Differences in physical well-being, hepatobiliary cancer subscale and FACT-Hep were less pronounced. The only subscale that appeared to favour TACE was emotional well-being (p = 0.656) In terms of specific variables, 2 weeks after treatment, SIRT patients reported greater closeness to friends (p = 0.035), and TACE patients reported a greater feeling of sadness (p = 0.034). At 4 weeks, TACE patients complained of being bothered by treatment side effects (p = 0.029) and nervousness (p = 0.047). SIRT patients experienced greater satisfaction with coping with illness (p = 0.019) and good appetite (p = 0.045) AEs: Not reported Time on treatment/number of treatments: Not reported |
High | ||||||||||||||||||||
|
Memon 201334 USA |
Prospective follow-up to a retrospective comparative study Funding: National Institutes of Health (in part) |
HCC that progressed after intra-arterial locoregional therapies: TACE and SIRT | TheraSphere (n = 42) | TACE (n = 54) |
OS: Not reported TTP: TheraSphere: median 13.3 months (range 9.3–25.0 months) TACE: median 8.4 months (range 7.3–10.6 months) Complete or partial response rate: Not reported HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: Not reported |
High | ||||||||||||||||||||
|
Hickey 201635 USA |
Prospective single-centre comparative study Funding: not reported |
Unresectable HCC and bilirubin ≤ 3.0 mg/dl | TheraSphere (n = 428) | TACE (n = 337) |
OS: Survival outcomes (months) were stratified by Child–Pugh class and BCLC stage: TheraSphere, median (95% CI)TACE, median (95% CI)BCLC A and Child–Pugh A21.4 (9.8 to 33.1)Not evaluable (most patients still alive at study termination)BCLC A and Child–Pugh B27.6 (11.6 to 43.6)BCLC B and Child–Pugh A18.3 (12.3 to 24.3)19.2 (16.0 to 22.4)BCLC B and Child–Pugh B12.2 (8.1 to 16.3)17.4 (8.8 to 26.0)BCLC C and Child–Pugh A9.5 (7.0 to 11.9)8.6 (5.1 to 12.0)BCLC C and Child–Pugh B5.6 (4.1 to 7.1)3.5 (2.6 to 4.4) PFS: Not reported Complete or partial response rate: Not reported HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: Not reported |
TheraSphere, median (95% CI) | TACE, median (95% CI) | BCLC A and Child–Pugh A | 21.4 (9.8 to 33.1) | Not evaluable (most patients still alive at study termination) | BCLC A and Child–Pugh B | 27.6 (11.6 to 43.6) | BCLC B and Child–Pugh A | 18.3 (12.3 to 24.3) | 19.2 (16.0 to 22.4) | BCLC B and Child–Pugh B | 12.2 (8.1 to 16.3) | 17.4 (8.8 to 26.0) | BCLC C and Child–Pugh A | 9.5 (7.0 to 11.9) | 8.6 (5.1 to 12.0) | BCLC C and Child–Pugh B | 5.6 (4.1 to 7.1) | 3.5 (2.6 to 4.4) | High | |
| TheraSphere, median (95% CI) | TACE, median (95% CI) | |||||||||||||||||||||||||
| BCLC A and Child–Pugh A | 21.4 (9.8 to 33.1) | Not evaluable (most patients still alive at study termination) | ||||||||||||||||||||||||
| BCLC A and Child–Pugh B | 27.6 (11.6 to 43.6) | |||||||||||||||||||||||||
| BCLC B and Child–Pugh A | 18.3 (12.3 to 24.3) | 19.2 (16.0 to 22.4) | ||||||||||||||||||||||||
| BCLC B and Child–Pugh B | 12.2 (8.1 to 16.3) | 17.4 (8.8 to 26.0) | ||||||||||||||||||||||||
| BCLC C and Child–Pugh A | 9.5 (7.0 to 11.9) | 8.6 (5.1 to 12.0) | ||||||||||||||||||||||||
| BCLC C and Child–Pugh B | 5.6 (4.1 to 7.1) | 3.5 (2.6 to 4.4) | ||||||||||||||||||||||||
|
Maccauro 201436 Location not reported |
Prospective matched case–control study Funding: not reported |
Unresectable HCC, Child–Pugh class A. 80% patients in both groups were BCLC stage C because of PVT | TheraSphere plus sorafenib (n = 15) | TheraSphere alone (n = 30) |
OS: TheraSphere plus sorafenib: median 10 months TheraSphere alone: median 10 months PFS: TheraSphere plus sorafenib: median 6 months TheraSphere alone: median 7 months Complete or partial response rate: TheraSphere plus sorafenib: 45.5% mRECIST, 10% EASL TheraSphere alone: 42.8% mRECIST, 40% EASL HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: TheraSphere plus sorafenib: patients started sorafenib at a median time of 2 months prior to SIRT; median time on sorafenib = 9 months and median dose = 600 mg/day |
High | ||||||||||||||||||||
|
Woodall 200937 USA |
Prospective comparative study Funding: MDS Nordion (Ottawa, ON, Canada) (maker of TheraSphere) |
Unresectable HCC, including patients with and those without PVT |
TheraSphere in patients without PVT (n = 20) TheraSphere in patients with PVT (n = 15) |
BSC/no treatment (n = 17) |
OS: TheraSphere: HCC patients without PVT: median 13.9 months; HCC patients with PVT: median 3.2 months BSC/no treatment: median 5.2 months PFS: Not reported Complete or partial response rate: Not reported HRQoL: Not reported AEs: TheraSphere: AEs were reported by 25% of patients without PVT and 33% of patients with PVT Time on treatment/number of treatments: TheraSphere: median 2 treatments per patient (range 1–3) |
High | ||||||||||||||||||||
|
Biederman 201538 Location not reported |
Retrospective comparative study Funding: not reported |
BCLC stage C HCC with PVT | TheraSphere (n = 72) | SIR-Spheres (n = 25) |
OS: TheraSphere: median 15 months (95% CI 8.6 to 19.5 months) SIR-Spheres: median 4.1 months (95% CI 2.7 to 6.6 months) TTP: Median 9.1 months (95% CI 5.4 to 11.7 months) – not reported for separate treatment groups Complete or partial response rate: 4/40 (10%) evaluable patients had complete response, 16/40 (40%) evaluable patients had partial response – not reported for separate treatment groups HRQoL: Not reported AEs: Clinical toxicities included grade 1/2: fatigue = 30%, abdominal pain = 28%, nausea = 17%, ascites = 7% – not reported for separate treatment groups Laboratory toxicities included grade 1/2: bilirubin = 37%, AST = 64%, ALT = 46% and grade 3/4: bilirubin = 17%, AST = 15%, ALT = 2% – not reported for separate treatment groups Time on treatment/number of treatments: A total of 101 treatments (across both treatment arms) were administered |
High | ||||||||||||||||||||
|
Biederman 201639 USA |
Retrospective comparative study Funding: not reported |
Unresectable HCC with associated main or lobar PVT | SIR-Spheres (n = 21) | TheraSphere (n = 69) |
OS: SIR-Spheres: median 3.7 months (95% CI 2.3 to 6.0 months) TheraSphere: median 9.5 months (95% CI 7.6 to 15.0 months) Comparison between groups: HR 0.39 (95% CI 0.23 to 0.67, p < 0.001) TTP: SIR-Spheres: median 2.8 months (95% CI 1.9 to 4.3 months) TheraSphere: median 5.9 months (95% CI 4.2 to 9.1 months) Complete or partial response rate: SIR-Spheres: 0/15 (0%) evaluable patients had complete response, 2/15 (13.3%) had partial response, 4/15 (26.7%) had stable disease, 9/15 (60%) had progressive disease TheraSphere: 5/57 (8.8%) evaluable patients had complete response, 18/57 (31.6%) had partial response, 8/57 (14%) had stable disease, 26/57 (45.6%) had progressive disease HRQoL: Not reported AEs: Grade 3/4 bilirubin: 39% SIR-Spheres vs. 14% TheraSphere group Grade 3/4 AST: 44% SIR-Spheres vs. 9% TheraSphere group Grade 3/4 ALT: 0% SIR-Spheres vs. 4% TheraSphere group Grade 3/4 alkaline phosphatase: 0% SIR-Spheres vs. 7% TheraSphere group Grade 3/4 albumin: 0% SIR-Spheres vs. 2% TheraSphere group Abdominal pain (32.9%) and fatigue (18.3%) were the most common clinical toxicities experienced; clinical toxicities were not significantly different between treatment groups Reported in supplementary data file (online): Pain: 41.2% SIR-Spheres vs. 30.8% TheraSphere group Fatigue: 17.6% SIR-Spheres vs. 18.5% TheraSphere group Nausea: 17.6% SIR-Spheres vs. 3.1% TheraSphere group Anorexia: 0% SIR-Spheres vs. 9.2% TheraSphere group Time on treatment/number of treatments: A total of 100 treatments (across both treatment arms) were administered, with 10 (11.1%) patients undergoing staged treatment |
High | ||||||||||||||||||||
|
Van Der Gucht 201740 Switzerland |
Retrospective comparative study Funding: not reported |
Unresectable HCC, ECOG performance status of < 2 and life expectancy of > 3 months | SIR-Spheres (n = 41) | TheraSphere (n = 36) |
OS: SIR-Spheres: median 7.7 months (95% CI 7.2 to 8.2 months) OS at 6 months = 63%, 1 year = 22%, 2 years = 11% TheraSphere: median 7.0 months (95% CI 1.6 to 12.4 months) OS at 6 months = 57%, 1 year = 29%, 2 years = 14% PFS: SIR-Spheres: median 6.1 months (95% CI 4.7 to 7.4 months) PFS at 6 months = 52%, 1 year = 7%, 2 years = 0% TheraSphere: median 5.0 months (95% CI 0.9 to 9.2 months) PFS at 6 months = 47%, 1 year = 18%, 2 years = 6% Complete or partial response rate: Not reported HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: Not reported |
High | ||||||||||||||||||||
|
Bhangoo 201541 USA |
Retrospective comparative study Funding: not reported |
Unresectable HCC patients who either had failed or had disease not amenable to alternative locoregional therapies. ECOG performance status of < 2, serum total bilirubin < 2 mg/dl | TheraSphere (n = 11) | SIR-Spheres (n = 6) |
OS: TheraSphere: median 8.4 months (95% CI 1.3 to 21.1 months) SIR-Spheres: median 7.8 months (95% CI 2.3 to 12.5 months) OS results presented for 15 out of the full 17-patient cohort, as two patients still alive PFS: Not reported Complete or partial response rate: 0/17 patients had complete response, 4/17 (24%) had partial response, 4/17 (24%) had stable disease, 6/17 (35%) had progressive disease and 3/17 (18%) had no data – not reported for separate treatment groups HRQoL: Not reported AEs: Grade 3/4 bilirubin: 18% TheraSphere vs. 0% SIR-Spheres group Grade 3/4 albumin: 11% TheraSphere vs. 0% SIR-Spheres group Grade 3/4 alkaline phosphatase: 0% TheraSphere vs. 17% SIR-Spheres group Fatigue: 45% TheraSphere vs. 67% SIR-Spheres group Abdominal pain: 27% TheraSphere vs. 33% SIR-Spheres group Nausea/vomiting: 55% TheraSphere vs. 67% SIR-Spheres group Anorexia/weight loss: 9% TheraSphere vs. 33% SIR-Spheres group Diarrhoea: 0% TheraSphere vs. 17% SIR-Spheres group Gastric ulcer: 0% TheraSphere vs. 17% SIR-Spheres group Time on treatment/number of treatments: 65% of patients received one treatment and 35% received two treatments (across both treatment arms) |
Unclear | ||||||||||||||||||||
|
d’Abadie 201842 USA |
Retrospective comparative study Funding: not reported |
HCC imaged by yttrium-90 TOF-PET | TheraSphere (n = 33 procedures) | SIR-Spheres (n = 25 procedures) |
OS: Not reported (KM curves for different equivalent uniform doses presented in publication) PFS: Not reported Complete or partial response rate: Not reported HRQoL: Not reported AEs: Not reported Time on treatment/number of treatments: Not reported |
High | ||||||||||||||||||||
|
Radosa 201951 Germany |
Single-centre retrospective case series Funding: none |
HCC | QuiremSpheres (n = 9) | Not applicable |
OS: Not reported PFS: Not reported Complete or partial response rate: 60 days: 0 complete response, 5/9 (56%) partial response, 3/9 (33%) stable disease, 1/9 (11%) progressive disease 6 months: 1/9 (11%) complete response, 4/9 (45%) partial response, 3/9 (33%) stable disease, 1/9 (11%) progressive disease HRQoL: Not reported AEs: Presence of REILD at 60 days: 0 Median MELD score (range) 1 day before SIRT: 8 (7–13) Median MELD score (range) 1 day after SIRT: 8 (6–11) Median MELD score (range) 60 days after SIRT: 8 (6–14) There were 16 reportable AEs in the nine patients, but no grade 3/4 AEs. Most common AEs were nausea (n = 3), fatigue (n = 3), vomiting (n = 3), abdominal pain (n = 2) and ascites (n = 2) Time on treatment/number of treatments: Not reported |
High |
Appendix 7 Lower-priority studies not included in the systematic review of clinical effectiveness or considered for the network meta-analyses (n = 28)
| Study (first author and year) | Intervention | Comparator | Reason for not including in the systematic review |
|---|---|---|---|
| Moroz 200153 | SIR-Spheres plus hepatic arterial chemotherapy | Hepatic arterial chemotherapy | Clinical advice that hepatic arterial chemotherapy is not applicable to current UK practice |
| Pellerito 201355 | SIR-Spheres | 131-iodine Lipiodol | Clinical advice that 131-iodine Lipiodol is not applicable to current UK practice |
| Steel 200452 | TheraSphere | Hepatic arterial infusion of cisplatin | Clinical advice that hepatic arterial infusion of cisplatin is not applicable to current UK practice |
| Maccauro 201654 | Standard-dose TheraSphere | Personalised treatment planning TheraSphere | Clinical advice that standard-dose TheraSphere is not applicable to current UK practice |
| She 2014159 | SIR-Spheres | TACE | Group imbalances make comparison meaningless (patients were allocated to SIRT if they were not eligible for TACE, e.g. had previously failed on TACE) |
| Kooby 2010160 | SIR-Spheres | TACE | Study included a more advanced population than in other studies in the NMA of patients eligible for CTTs and there was a baseline imbalance between groups in relation to PVI |
| Kwok 2014161 | SIR-Spheres | No SIR-Spheres | All patients included in the study opted for SIRT, but 16 were ineligible (primarily owing to lung shunt); the study compares those who received it with those who did not |
| Song 2017162 | SIR-Spheres | Concurrent chemoradiation therapy | Clinical advice that concurrent chemoradiation therapy is not applicable to current UK practice |
| Oladeru 2016163 | SIR-Spheres | External beam radiotherapy | Clinical advice that external beam radiotherapy is not applicable to current UK practice |
| Rühl 2009164 | SIR-Spheres | High-dose-rate brachytherapy | Clinical advice that high-dose-rate brachytherapy is not applicable to current UK practice |
| D’Avola 2009165 | SIR-Spheres | No SIRT (combination of conventional or experimental therapies or no therapy) | Comparator was a combination of conventional or experimental therapies or no therapy; conventional therapy patients were not reported separately; therefore, the trial was not informative for the NMA |
| Carr 2010166 | TheraSphere | TACE | All patients had ECOG performance status of > 2 and therefore were a more advanced population than in other studies in the NMA of patients eligible for CTTs |
| Kallini 2018167 | TheraSphere | TACE | No OS or PFS outcomes reported and therefore not informative for the NMA |
| Gabr 2017168 | TheraSphere | TACE | Population of patients who had received a transplant; therefore, not comparable population with other studies in the NMA of patients eligible for CTTs |
| Riaz 2009169 | TheraSphere | TACE | Group imbalances make comparison meaningless |
| Biederman 2018170 | TheraSphere | TACE | Patients within Milan criteria, therefore not comparable population to other studies in the NMA of patients eligible for CTTs |
| Lewandowski 2009118 | TheraSphere | TACE | No HRs or KM curves presented, therefore not informative for the NMA. In addition, patients received SIRT or TACE for downstaging; therefore, not comparable population to other studies in the NMA of patients eligible for CTTs |
| Ahmad 2005171 | TheraSphere | TACE | No OS or PFS outcomes reported; therefore, not informative for the NMA |
| Padia 2017172,173 | TheraSphere | TACE or DEB-TACE | Mixed population of BCLC A, B and C (70% within Milan criteria); therefore, not informative for the NMA of patients eligible for CTTs |
| Newell 2015174 | TheraSphere | TACE or DEB-TACE | Mixed population of BCLC B and C patients; therefore, not informative for the NMA of patients eligible for CTTs |
| Taussig 2017175 | TheraSphere | TACE or DEB-TACE | No OS or PFS outcomes reported; therefore, not informative for the NMA |
| McDevitt 2017176 | TheraSphere | DEB-TACE | Mixed population of BCLC B and C patients, therefore not informative for the NMA of patients eligible for CTTs. Patients without main PVI could receive DEB-TACE; those with PVI received SIRT and therefore group imbalances |
| Akinwande 2015177,178 | TheraSphere | DEB-TACE | Unclear population, but all patients had PVT; therefore, not informative for the NMA of patients eligible for CTTs |
| Biederman 2017179,180 | TheraSphere | TACE combined with microwave ablation | Clinical advice that TACE combined with microwave ablation is not widely practised in the UK |
| Padia 2015181 | TheraSphere | Ablation, chemoembolisation or BSC | Comparator was a combination of ablation, chemoembolisation and BSC. Chemoembolisation patients were not reported separately; therefore, the trial was not informative for the NMA of patients eligible for CTTs |
| Radunz 2017182 | TheraSphere | TACE, radiofrequency ablation or no bridging therapy | Patients were eligible for transplant and received SIRT or TACE for bridging; therefore, not comparable population to other studies in the NMA of patients eligible for CTTs |
| Salem 2018108 | TheraSphere | N/A | Non-comparative study |
| Ali 2018183 | TheraSphere | N/A | Non-comparative study |
Appendix 8 Risk-of-bias assessment results for retrospective comparative studies used in the network meta-analysis
| Study (first author and year) | Inclusion criteria clearly defined | Representative sample from relevant population | Groups similar at baseline | Clearly described and consistently delivered intervention | Clearly described and consistently delivered comparator | Outcome assessors blinded | Missing outcome data balanced across groups | Free from suggestion of selective reporting | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Biederman 201538 | No | Unclear | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Biederman 201639 | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Unclear | High |
| Van Der Gucht 201740 | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes | High |
| Bhangoo 201541 | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes | Unclear |
| d’Abadie 201842 | No | Unclear | No | No | No | Unclear | Unclear | Yes | High |
| Gramenzi 201545 | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | High |
| de la Torre 201644 | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | High |
| Cho 201643 | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes | High |
Appendix 9 Risk-of-bias assessment results for randomised controlled trials of comparative therapies used in the network meta-analysis
| Trial (first author and year) | Risk of bias arising from the randomisation process | Risk of bias owing to deviation from the intended interventions | Missing outcome data (primary outcomes) | Risk of bias in measurement of the outcomes | Risk of bias in selection of the reported result | Overall judgement of risk of bias |
|---|---|---|---|---|---|---|
| Yu 201465 | Some concerns | Low | Low | Low | Low | Some concerns |
| Chang 199463 | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Meyer 201364 | Some concerns | Low | Low | Low | Low | Some concerns |
| Malagari 201066 | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Sacco 201159 | High | Low | Low | Low | Low | High |
Appendix 10 Study details and results for studies of comparators included in the network meta-analysis
| Study (first author, year and location) | Study design and funding source | Population | Intervention | Comparator | Main results |
|---|---|---|---|---|---|
|
Yu 201465 China |
Parallel-group RCT Funding: not reported |
Patients with unresectable HCC with Child–Pugh class A or B and ECOG performance status of < 2 | TAE (n = 45) | TACE (n = 45) |
OS: TAE: median 24.3 months (95% CI 12.8 to 32.7 months) TACE: median 20.1 months (95% CI 9.3 to 31.2 months) PFS: TAE: median 6.5 months (95% CI 7.8 to 9.2 months) TACE: median 4.4 months (95% CI 1.6 to 7.2 months) TTP: TAE: median 8.4 months (95% CI 5.3 to 11.4 months) TACE: median 4.4 months (95% CI 1.7 to 7.1 months) |
|
Malagari 201066 Greece |
RCT Funding: not reported |
Patients with HCC unsuitable for curative therapy and at high risk for surgery | DEB-TACE (n = 48) | TAE (n = 47) |
OS: DEB-TACE: 100% were alive at 6 months and 85.3% at 12 months TAE: 100% were alive at 6 months and 86% at 12 months PFS: Not reported TTP: DEB-TACE: 42.4 ± 9.5 weeks TAE: 36.2 ± 9.0 weeks |
|
Sacco 201159 Italy |
Single-centre RCT Funding: not reported |
Patients with unresectable HCC with Child–Pugh class A or B, ECOG performance status of 0–1 and unsuitable for ablative treatments | TACE (n = 34) | DEB-TACE (n = 33) |
OS: TACE: 83.6% were alive at 24 months DEB-TACE: 86.8% were alive at 24 months PFS: TACE: 80.1% were disease progression free DEB-TACE: 82.5% were disease progression free TTP: TACE: mean 24.2 months DEB-TACE: mean 15.6 months |
|
Meyer 201364 UK |
Phase II/III RCT Funding: National Institute for Health Research, Experimental Cancer Medicine Centre Network |
Patients with unresectable HCC with Child–Pugh class A or B and ECOG performance status of 0–2 | TAE (n = 42) | TACE (n = 44) |
OS: HR 0.91, 95% CI 0.51 to 1.62 TAE: median 17.3 months TACE: median 16.3 months PFS: HR 0.87, 95% CI 0.52 to 1.45 TAE: median 7.2 months TACE: median 7.5 months TTP: Not reported |
|
Chang 199463 China |
Single-centre RCT Funding: not reported |
Patients with inoperable HCC and Child–Pugh class A or B | TACE (n = 22) | TAE (n = 24) |
OS: TACE: 52.5% were alive at 1 year and 26.2% were alive at 2 years TAE: 72.5% were alive at 1 year and 39.5% were alive at 2 years PFS: Not reported TTP: Not reported |
Appendix 11 Schoenfield residual plots for the studies included in the network meta-analysis for adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapy
Studies: (a) and (b) SARAH;19 (c) and (d) SIRveNIB;21 (e) Biederman et al. ;39 (f) and (g) Van Der Gucht et al. 40

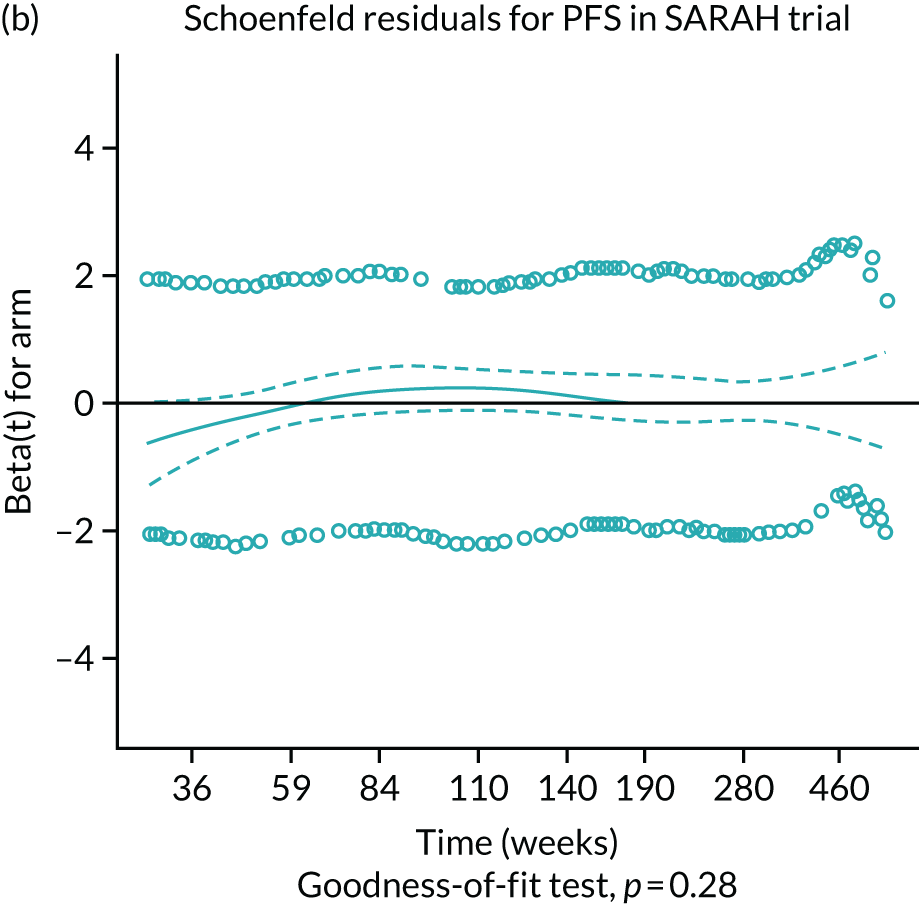




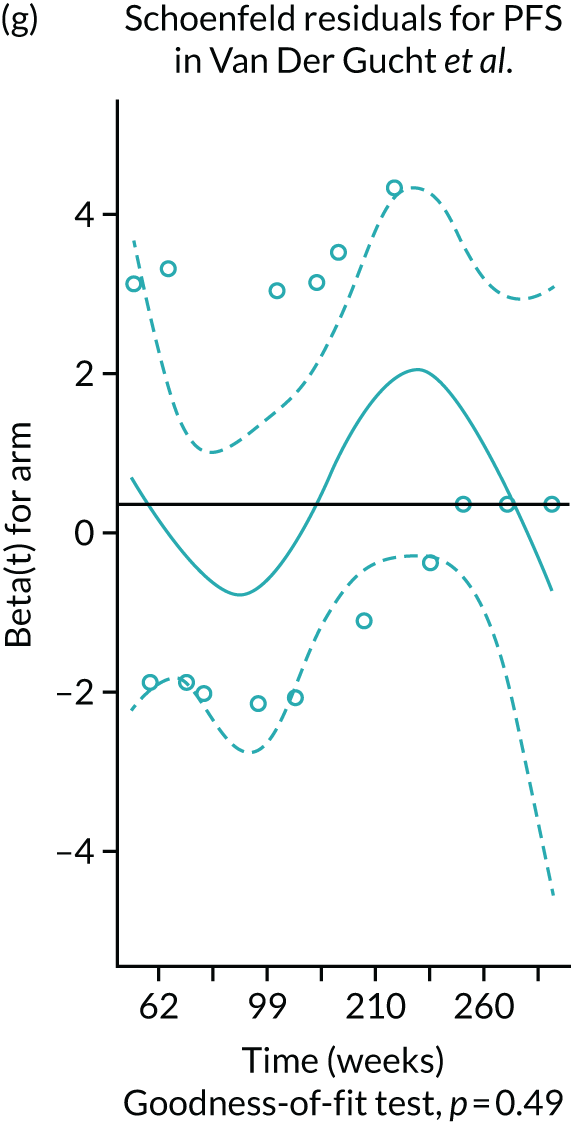
Appendix 12 Results of the network meta-analysis for all patients in the intention-to-treat population
There were three studies included in the NMA of all adults with unresectable HCC who are ineligible for CTT: SARAH,19 SIRveNIB21 and Kudo. 184 There were no significant differences in OS between the treatments when all patients, not just Child–Pugh A patients, were included (see Table 39). SIR-Spheres showed a non-significant improvement in PFS when compared with sorafenib (HR 0.97, 95% CrI 0.84 to 1.12) but showed a significant reduction in PFS compared with lenvatinib (HR 1.34, 95% Crl 1.01 to 1.73). The HR estimates for each treatment comparison are presented in Tables 41 and 42.
| Intervention | Comparator | HR (95% CrI) | |
|---|---|---|---|
| OS | PFS | ||
| SIR-Spheres | Sorafenib | 1.14 (0.98 to 1.32) | 0.97 (0.84 to 1.12) |
| SIR-Spheres | Lenvatinib | 1.10 (0.78 to 1.49) | 1.34 (1.01 to 1.73) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) | 0.73 (0.58 to 0.91) |
| DIC | –3.22 | –4.86 | |
| pD | 2.00 | 2.00 | |
Scenario 2: inclusion of Biederman et al. and Van Der Gucht et al. into network meta-analysis for all adults in the intention-to-treat population
The two retrospective comparative studies, Biederman et al. 39 and Van Der Gucht et al. ,40 were added to the NMA of all patients with unresectable HCC, who are ineligible for CTT, which allowed a comparison to be made with TheraSphere. A subgroup of 42 patients with advanced-stage HCC was used from the Van Der Gucht et al. 40 study. The fixed-effects model was chosen as the DIC and the number of parameters were lower. There was a significant improvement in OS with TheraSphere when compared with sorafenib (HR 0.53, 95% CrI 0.31 to 0.84), SIR-Spheres (HR 0.46, 95% CrI 0.28 to 0.72) and lenvatinib (HR 0.51, 95% CrI 0.28 to 0.86). As discussed earlier, Biederman et al. 39 and Van Der Gucht et al. 40 both have large treatment effects and, therefore, result in TheraSphere being significantly better for OS in the NMA. There were no notable differences between any of the other treatments for OS (see Table 40).
| Intervention | Comparator | OS HR (95% CrI), fixed effects |
|---|---|---|
| SIR-Spheres | Sorafenib | 1.14 (1.01 to 1.28) |
| SIR-Spheres | Lenvatinib | 1.10 (0.80 to 1.48) |
| TheraSphere | SIR-Spheres | 0.46 (0.28 to 0.72) |
| TheraSphere | Sorafenib | 0.53 (0.31 to 0.84) |
| TheraSphere | Lenvatinib | 0.51 (0.28 to 0.86) |
| Lenvatinib | Sorafenib | 1.06 (0.79 to 1.40) |
| Sorafenib | 0.88 (0.78 to 0.99) | 0.96 (0.71 to 1.27) |
| 1.14 (1.01 to 1.28) | SIR-Spheres | 1.1 (0.80 to 1.48) |
| 1.06 (0.79 to 1.40) | 0.93 (0.67 to 1.25) | Lenvatinib |
| Sorafenib | 1.04 (0.89 to 1.20) | 1.61 (0.45 to 4.15) |
| 0.97 (0.84 to 1.12) | SIR-Spheres | 1.56 (0.43 to 4.07) |
| 0.86 (0.24 to 2.22) | 0.89 (0.25 to 2.31) | Lenvatinib |
Appendix 13 Random-effects network meta-analysis results
| Intervention | Comparator | HR (95% CrI) | |
|---|---|---|---|
| ITT | Per protocol | ||
| SIR-Spheres | Sorafenib | 0.94 (0.68 to 1.26) | 1.13 (0.86 to 1.46) |
| SIR-Spheres | Lenvatinib | 0.92 (0.52 to 1.51) | 1.11 (0.66 to 1.74) |
| TheraSphere | SIR-Spheres | 0.46 (0.19 to 0.94) | 0.42 (0.19 to 0.82) |
| TheraSphere | Sorafenib | 0.42 (0.18 to 0.83) | 0.48 (0.20 to 0.97) |
| TheraSphere | Lenvatinib | 0.41 (0.15 to 0.89) | 0.46 (0.17 to 1.02) |
| Lenvatinib | Sorafenib | 1.07 (0.67 to 1.63) | 1.07 (0.70 to 1.58) |
| SD | 0.11 (0.004 to 0.352) | 0.13 (0.005 to 0.378) | |
| DIC | 0.9 | 2.1 | |
| pD | 3.4 | 3.4 | |
| Intervention | Comparator | HR (95% CrI), random effects | |
|---|---|---|---|
| OS | PFS | ||
| SIR-Spheres | Sorafenib | 0.97 (0.73 to 1.26) | 1.15 (0.89 to 1.45) |
| SIR-Spheres | Lenvatinib | 1.58 (0.40 to 4.21) | 1.12 (0.68 to 1.73) |
| Lenvatinib | Sorafenib | 0.87 (0.23 to 2.33) | 1.07 (0.70 to 1.57) |
| SD | 0.11 (0.004 to 0.352) | 0.12 (0.005 to 0.367) | |
| DIC | –1.69 | 2.18 | |
| pD | 2.4 | 2.5 | |
| Intervention | Comparator | OS HR (95% CrI) |
|---|---|---|
| SIR-Spheres | Sorafenib | 1.15 (0.89 to 1.45) |
| SIR-Spheres | Lenvatinib | 1.11 (0.68 to 1.73) |
| TheraSphere | SIR-Spheres | 0.50 (0.26 to 0.89) |
| TheraSphere | Sorafenib | 0.58 (0.29 to 1.06) |
| TheraSphere | Lenvatinib | 0.56 (0.24 to 1.13) |
| Lenvatinib | Sorafenib | 1.07 (0.70 to 1.57) |
| Intervention | Comparator | OS HR (95% CrI) | |
|---|---|---|---|
| ITT population | Per-protocol population | ||
| SIR-Spheres | Sorafenib | 1.16 (0.71 to 1.78) | 1.03 (0.63 to 1.61) |
| SIR-Spheres | Lenvatinib | 1.13 (0.55 to 2.09) | 1.02 (0.49 to 1.88) |
| Lenvatinib | Sorafenib | 1.08 (0.65 to 1.71) | 1.08 (0.65 to 1.71) |
| SD | 0.15 (0.006 to 0.426) | 0.15 (0.006 to 0.426) | |
| DIC | 0.92 | 1.1 | |
| pD | 2.0 | 2.0 | |
Appendix 14 Quality assessment of identified economic evidence
| Assessment criteria | Study | |
|---|---|---|
| Rostambeigi et al.91,92 | Rognoni et al.89,90 | |
| Structure | ||
| 1. Is there a clear statement of the decision problem? | Yes | Yes |
| 2. Is the perspective and scope of the model stated clearly? | No | Yes |
| 3. Are the model inputs consistent with the stated perspective? | N/A | Yes |
| 4. Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | N/A | Yes |
| 5. Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | No | Yes |
| 6. Is there a clear definition and justification for the alternative options under evaluation? | Yes | Yes |
| 7. Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | No | Yes |
| 8. Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and appropriately justified? | No | Yes |
| 9. Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | No | Yes |
| 10. Is the cycle length defined and justified in terms of the natural history of disease? | No | Yes |
| Data | ||
| 11. Are the data identification methods transparent and appropriate given the objectives of the model? | Yes | Yes |
| 12. Has the quality of the data been assessed appropriately? | No | N/A |
| 13. Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Partial | Yes |
| 14. Is the choice of baseline data described and justified? | N/A | Yes |
| 15. Are transition probabilities calculated appropriately? | N/A | Yes |
| 16. Has a half-cycle correction been applied to both costs and outcomes? | N/A | No |
| 17. If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | No | N/A |
| 18. Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Partial | Partial |
| 19. Have alternative assumptions been explored through sensitivity analysis? | Partial | Yes |
| 20. Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | No | N/A |
| Costs and discounting | ||
| 21. Are the costs incorporated into the model described and justified? | Yes | Yes |
| 22. Has the source for all costs been described? | Yes | Yes |
| 23. Have discount rates been described and justified given the target decision-maker? | N/A | Yes |
| 24. Were currency, price date and price adjustments/currency conversion information stated? | No | Yes |
| HRQoL | ||
| 25. Are the utilities incorporated into the model appropriate? | N/A | Yes |
| 26. Is the source for the utility weights referenced? | N/A | Yes |
| Validation | ||
| 27. Has heterogeneity been dealt with by running the model separately for different subgroups? | Yes | N/A |
| 28. Have the results of the model been compared with those of previous models and any differences in results explained? | No | Partial |
Appendix 15 Model parameters from submitted economic models
Sirtex model parameters: conventional transarterial therapy-eligible model
| Input | Inflated value | Source |
|---|---|---|
| Scenario 1: CTT cost from literature | ||
| Proportion of CTT with DEB-TACE | 25% | Fateen et al.105 |
| TACE cost | £9801.00 | Fateen et al.105 |
| DEB-TACE cost | £5727.03 | Fateen et al.105 |
| CTT cost (literature) | £8792.59 | Calculated |
| Scenario 2: CTT resource use from literature, with National Schedule of Reference Costs 2017–2018107 | ||
| Drug-eluding beads | £594.30 | Fateen et al.105 |
| TACE length of stay (days) | 2.37 | Fateen et al.105 |
| DEB-TACE length of stay (days) | 2.81 | Fateen et al.105 |
| Mean number of TACE procedures | 3.03 | Fateen et al.105 |
| Mean number of DEB-TACE procedures | 1.43 | Fateen et al.105 |
| Proportion of CTT with DEB-TACE | 25% | Fateen et al.105 |
| TACE cost | £12,620.41 | Calculated |
| DEB-TACE cost | £7911.80 | Calculated |
| CTT cost (reference costs) | £11,454.91 | Calculated |
| Scenario 3: CTT resource use from survey, literature with National Schedule of Reference Costs 2017–2018107 | ||
| Drug-eluding beads | £594.30 | Fateen et al.105 |
| TACE length of stay (days) | 2.37 | Fateen et al.105 |
| DEB-TACE length of stay (days) | 2.81 | Fateen et al.105 |
| Mean number of TACE procedures | 2.5 | Sirtex resource use survey (personal communication) |
| Mean number of DEB-TACE procedures | 2.83 | Sirtex resource use survey (personal communication) |
| Proportion of CTT with DEB-TACE | 63% | Sirtex resource use survey (personal communication) |
| TACE cost | £10,412.88 | Calculated |
| DEB-TACE cost | £15,676.06 | Calculated |
| CTT cost | £13,702.37 | Calculated |
| Parameter | SIR-Spheres | TheraSphere | ||
|---|---|---|---|---|
| Value | Source | Value | Source | |
| Outpatient costs for code YR57Z | £1123.15 | National Schedule of Reference Costs 2017–2018 107 | £1123.15 | National Schedule of Reference Costs 2017–2018 107 |
| Inpatient cost/day for YR57Z | £1757.45 | £1757.45 | ||
| SIRT | £8000.00 | Sirtex102 | £8000.00 | Sirtex102 |
| Survey results | ||||
| Number of work-ups | 1.05 | Survey | 1.05 | Assumed same as SIR-Spheres |
| Length of stay for work-up (days) | 0.69 | 0.69 | ||
| Number of procedures | 1.20 | 1.20 | ||
| Length of stay for procedure (days) | 1.19 | 1.19 | ||
| Cost of work-up | £1175.56 | – | £1175.56 | – |
| Cost of procedure | £2500.13 | – | £2500.13 | – |
| Total cost | £13,239.33 | – | £13,239.33 | – |
| Survey results with outpatient procedures | ||||
| Number of work-ups | 1.05 | Survey | 1.05 | Assumed same as SIR-Spheres |
| Length of stay for work-up (days) | Outpatient | Outpatient | ||
| Number of procedures | 1.20 | 1.20 | ||
| Length of stay for procedure (days) | Outpatient | Outpatient | ||
| Cost of work-up | £1175.56 | – | £1175.56 | – |
| Cost of procedure | £1342.67 | – | £1342.67 | – |
| Total cost | £12,081.87 | – | £12,081.87 | – |
| The Christie NHS Foundation Trust results | ||||
| Number of work-ups | Confidential information has been removed | The Christie NHS Foundation Trust data (personal communication) | Confidential information has been removed | The Christie NHS Foundation Trust data (personal communication) |
| Length of stay for work-up (days) | Confidential information has been removed | Confidential information has been removed | ||
| Number of procedures | Confidential information has been removed | Confidential information has been removed | ||
| Length of stay for procedure (days) | Confidential information has been removed | Confidential information has been removed | ||
| Cost of work-up | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cost of procedure | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Total cost | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Sangro et al.69 and Salem et al.108 for number of procedures, rest survey | ||||
| Number of work-ups | 1.05 | Survey | 1.05 | Survey |
| Length of stay for work-up (days) | 0.69 | Survey | 0.69 | Survey |
| Number of procedures | 1.08 | ENRY reigster69 | 1.58 | PREMIERE108 |
| Length of stay for procedure (days) | 1.19 | Survey | 1.19 | Survey |
| Cost of work-up | £1175.56 | – | £1175.56 | – |
| Cost of procedure | £2252.24 | – | £3298.08 | – |
| Total cost | £12,043.19 | – | £17,089.64 | – |
| AE | TACE (n = 19) | TheraSphere (n = 24) | Unit costs | Source for unit cost |
|---|---|---|---|---|
| Abdominal pain | 0% | 4% | £42.19 | NICE TA47411 sorafenib technology appraisal |
| Elevated aspartate aminotransferase level | 11% | 0% | £634.50 | NICE TA55112 lenvatinib technology appraisal |
| Hypoalbuminemia | 0% | 4% | £634.50 | Assumed average of elevated aspartate aminotransferase and blood bilirubin |
| Increased blood bilirubin | 5% | 8% | £916.47 | NICE TA55112 lenvatinib technology appraisal |
| Leukopenia | 0% | 4% | £215.00 | NICE TA509 pertuzumab185 |
| Neutropenia | 11% | 0% | £2097.50 | National Schedule of Reference Costs 2017–2018107 (WJ11Z) |
| Total costs | £346.34 | £108.99 |
Sirtex model parameters: conventional transarterial therapy-ineligible model
| Comparator | Utility value: mean (standard error) | Reference |
|---|---|---|
| Pre progression: SIR-Spheres | 0.762 (0.078) | Post hoc analyses of the SARAH trial19 for the low tumour burden/ALBI 1 subgroup |
| Pre progression: sorafenib | 0.746 (0.076) | |
| Post progression: SIR-Spheres | 0.738 (0.075) | |
| Post progression: sorafenib | 0.722 (0.074) | |
| After subsequent treatment with curative intent | 0.762 (0.078) | Assumed same as the pre-progression utilities with SIR-Spheres |
| Cost item | Value | Source |
|---|---|---|
| Outpatient costs for code YR57Z | £1123.15 | National Schedule of Reference Costs 2017–2018 107 |
| Inpatient cost/day for YR57Z | £1757.45 | |
| SIR-Spheres | £8000.00 | Sirtex102 |
| Number of work-ups per patient | 1.05 | Resource use survey |
| Length of stay for work-up (days) | 0.69 | |
| Number of treatments per patient | 1.20 | |
| Length of stay for treatment (days) | 1.19 | |
| Cost of a single work-up | £1175.56 | Subtotal |
| Cost of a single treatment | £2500.13 | Subtotal |
| Total cost | £13,239.33 | – |
| Proportions | After SIRT | After sorafenib |
|---|---|---|
| % of liver resection among treatments with curative intent | 33.3 | 0.0 |
| % of liver transplantation among treatments with curative intent | 16.7 | 33.3 |
| % of ablation among treatments with curative intent | 58.3 | 66.7 |
| Cost item | Pre-progression post SIRT (per month) | Pre-progression on sorafenib/lenvatinib (per month) | At progression (one off) | Progressive disease (per month) |
|---|---|---|---|---|
| Medical staff contact | £102.84 | £126.49 | £118.50 | £222.96 |
| Diagnostic procedures | £130.26 | £134.58 | £89.28 | £6.15 |
| Inpatient care | £6.80 | £20.29 | – | £78.50 |
| PSS | £5.83 | £5.83 | – | £191.76 |
| Total | £245.74 | £287.19 | £207.79 | £499.37 |
| AE | Inflated cost | Reported costs | Costing year | Source |
|---|---|---|---|---|
| Abdominal pain | £42.19 | £40.15 | 2014/15 | NICE TA47411 sorafenib TA |
| Alopecia | £18.59 | £17.69 | 2014/15 | NICE TA47411 sorafenib TA |
| Anaemia | £1319.84 | £1283.67 | 2015/16 | NICE TA51413 regorafenib TA |
| Anorexia | £657.86 | £639.83 | 2016/17 | NICE TA535154 lenvatinib and sorafenib |
| Ascites | £1713.98 | £1667.00 | 2015/16 | NICE TA51413 regorafenib TA |
| Aspartate aminotransferase level increased | £634.50 | £617.11 | 2016/17 | NICE TA551124 lenvatinib TA |
| Asthenia | £677.68 | £659.11 | 2016/17 | NICE TA551124 lenvatinib TA |
| Blood bilirubin level increased | £916.47 | £891.35 | 2016/17 | NICE TA551124 lenvatinib TA |
| Cardiac failure, congestive | £1979.71 | £1979.71 | 2017/18 | National Schedule of Reference Costs 2017–2018:107 weighted-average HRG codes EB03A and EB03E |
| Diarrhoea | £605.13 | £588.54 | 2016/17 | NICE TA551124 lenvatinib TA |
| Fatigue | £677.68 | £659.11 | 2016/17 | NICE TA551124 lenvatinib TA |
| Gamma-glutamyl transferase level increased | £634.50 | £617.11 | 2016/17 | NICE TA551124 lenvatinib TA |
| Haematological biological abnormalities | £1319.84 | £1283.67 | 2015/16 | NICE TA51413 regorafenib TA |
| Haemorrhage | £0.00 | £0.00 | 2014/15 | NICE TA47411 sorafenib TA |
| Hand–foot skin reaction | £897.98 | £873.37 | 2015/16 | NICE TA51413 regorafenib TA |
| Hypertension | £888.12 | £863.78 | 2016/17 | NICE TA551124 lenvatinib TA |
| Hypophosphataemia | £1297.52 | £1261.96 | 2015/16 | NICE TA51413 regorafenib TA |
| Liver dysfunction | £1713.98 | £1667.00 | 2015/16 | NICE TA51413 regorafenib TA |
| Nausea/vomiting | £82.18 | £78.20 | 2014/15 | NICE TA47411 sorafenib TA |
| Other increase in liver function | £634.50 | N/A | N/A | NICE TA551124 lenvatinib TA |
| Palmar–plantar erythrodysesthesia syndrome | £443.80 | £431.64 | 2016/17 | NICE TA551124 lenvatinib TA |
| Platelet count decreased | £634.50 | £617.11 | 2016/17 | NICE TA551124 lenvatinib TA |
| Proteinuria | £812.04 | £789.78 | 2016/17 | NICE TA551124 lenvatinib TA |
| Rash/desquamation | £71.09 | £67.65 | 2014/15 | NICE TA47411 sorafenib TA |
| Weight decreased | £665.35 | £647.11 | 2016/17 | NICE TA551124 lenvatinib TA |
BTG model parameters: conventional transarterial therapy-eligible model
| Parameter | Per-cycle transition probability | Source |
|---|---|---|
| ‘Watch and wait’ to pre transplant |
SIRT = 10.8% CTT = 5.8% |
Lewandowski et al.118 |
| ‘Watch and wait’ to pharmacological management |
SIRT = 7.8% CTT = 12.8% |
Calculation |
| ‘Watch and wait’ to ‘watch and wait’ | 81.4% | Lewandowski et al.118 |
| Pre transplant to pharmacological management | 2.2% | National Audit for Liver Transplant (personal communication) |
| Pre transplant to post transplant | 13.9% | NHS Annual Report on Liver Transplantation 2017/18120 |
| Pre transplant to pre transplant | 84.0% | Calculation |
| Health state | Mortality rate (per cycle) | Source |
|---|---|---|
| Watch and wait | 3.88% | Assumed the same as pre transplant |
| Pre transplant | 3.88% | NHS England. Schedule 2 – The Services. A. Service Specifications. 170003/S. Liver Transplantation service (Adults)186 |
| Pharmacological management | 7.74% | Derived from the median OS for BSC from the NICE sorafenib submission (TA47411) |
| Post transplant 1 | 1.39% | Bellavance et al.122 |
| Post transplant 2 | 1.39% | Bellavance et al.122 |
| Post transplant 3 | 1.39% | Bellavance et al.122 |
| No HCC (post transplant) | 0.29% | NHS Survival rates following transplantation187 |
| AE | Rate (%) | |||||
|---|---|---|---|---|---|---|
| TheraSphere | SIR-Spheres | Quirem Spheres | TACE | DEB-TACE | TAE | |
| Aspartate aminotransferase level increase | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Proteinuria | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Blood bilirubin level increase | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.0 |
| Diarrhoea | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fatigue | 1.9 | 2.3 | 2.3 | 0.0 | 0.0 | 8.0 |
| Gamma-glutamyl transferase level increase | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.0 |
| Hypertension | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Weight decrease | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Platelet count decrease | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Palmar–plantar erythrodysesthesia syndrome | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ascites | 6.1 | 2.3 | 2.3 | 0.0 | 0.0 | 0.0 |
| Cholecystitis | 1.9 | 5.0 | 5.0 | 0.0 | 1.1 | 0.0 |
| Hepatic encephalopathy | 2.8 | 8.0 | 8.0 | 0.0 | 0.0 | 0.0 |
| Post-procedural pain | 1.9 | 1.2 | 1.2 | 18.2 | 0.0 | 21.0 |
| Health state | Source utility | Applied utilitya | Source |
|---|---|---|---|
| Watch and wait | 0.75 | 0.534 | TA535154 (pre progression) |
| Pre transplant | 0.75 | 0.534 | TA535154 (pre progression) |
| Post transplant 1 | 0.69 | 0.474 | Lim et al.125 |
| Post transplant 2 | 0.69 | 0.473 | Lim et al.125 |
| Post transplant 3 | 0.69 | 0.473 | Lim et al.125 |
| No HCC post transplant | 0.75 | 0.534 | TA535154 (pre progression) |
| Pharmacological management | 0.72 | 0.499 | TA535154 (calculated as an average of pre-progression and post-progression utilities) |
| Work-up factors: costs included in the BTG analysis | Cost |
|---|---|
| Band 6 technician: 30 minutes (unit cost per hour: £15.96) | £7.98 |
| Band 7 clinical scientist: 30 minutes (unit cost per hour: £19.06) | £9.53 |
| MAA body SPECTa | £353.00 |
| Lung shunt calculation – band 7 clinical scientist: 10 minutes (unit cost per hour: £19.06) | £3.18 |
| Volumetary – band 7 clinical scientist: 1 hour (unit cost per hour: £19.06) | £19.06 |
| Volumetary band radiologist: 1 hour (unit cost per hour: £75.16) | £75.16 |
| Total cost | £467.91 |
| Additional costs provided by BTG following the company submission | |
| One radiologist: 2 hours (unit cost per hour: £75.16) | £150.30 |
| Two band 6 nurses: 3 hours (unit cost per hour: £23.82) | £142.92 |
| One band 6 radiographer: 3 hours (unit cost per hour: £23.82) | £71.46 |
| One band 4 co-ordinator: 1 hour (unit cost per hour: £16.30) | £16.30 |
| Blood work | £11.35 |
| Total cost | £860.32 |
| Item | Unit cost | Source |
|---|---|---|
| Aspartate aminotransferase level increase | £615.76 | National Schedule of Reference Costs 2017–2018.107 Hospitalisation. Average non-elective short stay |
| Proteinuria | £657.76 |
National Schedule of Reference Costs 2017–2018. 107 Average non-elective short stay (for hospitalisation) at £615.76 Plus a nurse visit (GP practice): £42 (PSSRU 2018106 – cost per hour including qualifications) |
| Blood bilirubin level increase | £886.56 |
National Schedule of Reference Costs 2017–2018. 107 Average non-elective short stay (for hospitalisation) at £615.76 Plus CT scan at £103.95. Weighted average of RD10Z–RD28Z. Adults only. National Schedule of Reference Costs 2017–2018107 Plus £166.85: outpatient consultant-led, non-admitted face-to-face attendance, follow up (medical oncology). Code WF01A. National Schedule of Reference Costs 2017–2018107 |
| Diarrhoea | £561.30 | National Schedule of Reference Costs 2017–2018107 – FD10K. Non-Malignant Gastrointestinal Tract Disorders without Interventions, with CC Score 6–10 – non-elective short stay |
| Fatigue | £657.76 |
National Schedule of Reference Costs 2017–2018. 107 Average non-elective short stay (for hospitalisation) at £615.76 Plus a nurse visit (GP practice) £42 (PSSRU 2018106 – cost per hour including qualifications) |
| Gamma-glutamyltransferase level increase | £615.76 | National Schedule of Reference Costs 2017–2018.107 Average non-elective short stay |
| Hypertension | £856.61 |
National Schedule of Reference Costs 2017–2018. 107 Average non-elective short stay (for hospitalisation) at £615.76 Plus 2 GP appointments (9.22 minutes) at £37 each (PSSRU 2018106 – cost per hour including qualifications) Plus £166.85: outpatient consultant-led, non-admitted face-to-face attendance, follow up (medical oncology). Code WF01A. National Schedule of Reference Costs 2017–2018107 |
| Weight decrease | £646.76 |
Hospitalisation: National Schedule of Reference Costs 2017–2018,107 average cost of non-elective short stay (£615.76) Plus dietitian (PSSRU 2018106) – dietician band 4 cost per working hour (£31) |
| Platelet count decrease | £615.76 | National Schedule of Reference Costs 2017–2018.107 Hospitalisation. Average non-elective short stay |
| Palmar–plantar erythrodysesthesia syndrome | £413.03 | National Schedule of Reference Costs 2017–2018107 – JD07J Skin Disorders without Interventions, with CC score 2–5 – non-elective short stay |
| Ascites | £615.76 | National Schedule of Reference Costs 2017–2018.107 Hospitalisation. Average non-elective short stay |
| Cholecystitis | £507.81 | Weighted average of GA07C-E. Intermediate, Hepatobiliary or Pancreatic Procedures, with CC Score 0–3 + |
| Hepatic encephalopathy | £615.76 | National Schedule of Reference Costs 2017–2018.107 Hospitalisation. Average non-elective short stay |
| Post-procedural pain | £615.76 | National Schedule of Reference Costs 2017–2018.107 Hospitalisation. Average non-elective short stay |
| Item | Unit cost | Source |
|---|---|---|
| Treatment and aftercare costs | ||
| TheraSphere | £8000 | Clinician informed |
| QuiremSpheres | £8000 | Assumed the same as TheraSphere |
| SIR-Spheres | £8000 | NICE Medtech Innovation Briefing188 |
| Sorafenib | £3576.56 | NICE BNF115 |
| BSC | £0.00 | Assumed |
| Doxorubicin (loaded on to DEB-TACE) | £109 | Clinician informed |
| Drug-eluting beads (DEB-TACE) | £550 | |
| Lipiodol (TACE) | £250 | |
| Bland beads (TAE) | £40 | |
| Ciclosporin immunosuppressants | £68.28 | NICE BNF115 |
| Admissions and procedure costs | ||
| Hospitalisation (general) | £1928 | National Schedule of Reference Costs 2017–2018.107 Weighted average of HRG GC12C–GC12K |
| Outpatient attendance | £167 | National Schedule of Reference Costs 2017–2018.107 Consultant-led: first attendance non-admitted face to face. Code 105 hepatobiliary and pancreatic surgery |
| Embolisation procedure | £2790 | National Schedule of Reference Costs 2017–2018.107 HRG code YR57Z |
| SIRT work-up | £467.91 | The Christie NHS Foundation Trust (personal communication) |
| Liver transplant procedure | £17,340 | National Schedule of Reference Costs 2017–2018.107 HRG code GA15A |
| Liver resection procedure | £4994 | National Schedule of Reference Costs 2017–2018.107 Weighted average of HRG code GA06 |
| Physician costs | ||
| Oncologist | £166.85 | National Schedule of Reference Costs 2017–2018.107 Code WF01A. Non-Admitted Face-to-Face Attendance, Follow-up. Medical oncology |
| Hepatologist | £262.40 | National Schedule of Reference Costs 2017–2018.107 WF01A Consultant-led, Non-Admitted Face-to-Face Attendance, Follow-up (hepatology) |
| Macmillan nurse | £42 | PSSRU Unit Costs of Health and Social Care 2018.106 Nurse (GP practice). Cost per hour, including qualifications |
| Gastroenterologist | £146.29 | National Schedule of Reference Costs 2016/17.189 WF01A Consultant-led, Non-Admitted Face-to-Face Attendance, Follow-up (gastroenterology) |
| Radiologist | £152.27 | National Schedule of Reference Costs 2016/17.189 WF01A Consultant-led, Non-Admitted Face-to-Face Attendance, Follow-up (interventional radiology) |
| Clinical nurse specialist | £42 | PSSRU Unit Costs of Health and Social Care 2018.106 Nurse (GP practice). Cost per hour, including qualifications |
| Palliative care physician/care | £42 | |
| GP | £37 | PSSRU Unit Costs of Health and Social Care 2018.106 Cost per 9.22-minute session, including qualifications |
| Laboratory tests | ||
| Full blood count | £2.32 | National Schedule of Reference Costs 2017–2018.107 Weighted average of DAPS03, DAPS05 and DAPS08 (integrated blood services, haematology and phlebotomy) |
| Liver function tests | £20.07 | National Schedule of Reference Costs 2017–2018.107 Weighted average of DAPS01 and DAPS02 |
| Alpha fetoprotein test | £20.07 | |
| INR | £2.32 | National Schedule of Reference Costs 2017–2018.107 Weighted average of DAPS03, DAPS05 and DAPS08 (integrated blood services, haematology and phlebotomy) |
| Biochemistry | £1.11 | National Schedule of Reference Costs 2017–2018.107 DAPS04 (clinical biochemistry) |
| Endoscopy | £499.51 | National Schedule of Reference Costs 2017–2018.107 FE50A (Wireless Capsule Endoscopy, 19 years and over). Outpatient procedures |
| CT scan | £103.95 |
National Schedule of Reference Costs 2017–2018. 107 Weighted average of RD10Z–RD28Z Adults only |
| MRI scan | £145.56 | National Schedule of Reference Costs 2017–2018.107 Weighted average of all MRI currency codes (adult only, excluding cardiac magnetic resonance imaging) (RD01A, RD02A, RD03Z, RD04Z, RD05Z, RD06Z and RD07Z) |
| Ultrasound scan | £52.06 |
National Schedule of Reference Costs 2017–2018. 107 HRG codes RD40Z and RD41Z Ultrasound scan with duration < 20 mins, weighted average of cost with/without contrast |
| Item | Cost per cycle |
|---|---|
| Total watch and wait | £539.16 |
| Total pre transplant | £577.42 |
| Total post transplant 0–1 | £971.71 |
| Total post transplant 1–2 | £1049.22 |
| Total post transplant 2–3 | £516.42 |
| No HCC post transplant | £502.49 |
| Resection | £345.07 |
| No HCC other | £306.50 |
| Pharmacological management | £1308.57 |
BTG model parameters: conventional transarterial therapy-ineligible model
| Health state | Absolute utility | Source | Utility decrement |
|---|---|---|---|
| Progression free | 0.75 | Lenvatinib NICE submission12 | 0.26 |
| Progressed | 0.68 | Lenvatinib NICE submission12 | 0.32 |
| Item | Unit cost | Source |
|---|---|---|
| Treatment and aftercare costs | ||
| TheraSphere | £8000.00 | Clinician informed |
| QuiremSpheres | £8000.00 | Assumed the same as TheraSphere |
| SIR-Spheres | £8000.00 | NICE Medtech Innovation Briefing188 |
| Sorafenib | £3576.56 | NICE BNF115 |
| Lenvatinib | £1437.00 | |
| Regorafenib | £3744.00 | |
| BSC | £0.00 | Assumed |
| Item | Unit cost | Cost per cycle | |
|---|---|---|---|
| Progression free | Progressed | ||
| Physician visits | |||
| Oncologist | £166.85 | £115.51 | £58.53 |
| Hepatologist | £262.40 | £41.18 | £121.11 |
| Macmillan nurse | £42.00 | £19.38 | £38.77 |
| Gastroenterologist | £146.29 | £10.80 | £0.00 |
| Radiologist | £152.27 | £11.24 | £0.00 |
| Clinical nurse specialist | £42.00 | £19.38 | £9.69 |
| Palliative care physician/care | £42.00 | £5.04 | £29.08 |
| Laboratory tests | |||
| Full blood count | £2.32 | £1.61 | £1.07 |
| Liver function tests | £20.07 | £6.21 | £4.63 |
| Alpha fetoprotein test | £20.07 | £11.53 | £7.04 |
| INR | £2.32 | £0.72 | £0.00 |
| Biochemistry | £1.11 | £0.51 | £0.26 |
| Endoscopy | £499.51 | £38.04 | £0.00 |
| Radiological tests | |||
| CT scan | £103.95 | £23.12 | £27.32 |
| MRI scan | £145.56 | £12.42 | £18.81 |
| Hospitalisation | |||
| Hospitalisation | £1928.00 | £130.99 | £341.70 |
| Hospital follow-ups | |||
| Hepatologist | £262.40 | £60.55 | £262.40 |
| GP | £37.00 | £51.23 | £37.00 |
| Clinical nurse specialist | £42.00 | £67.85 | £42.00 |
| Total cycle costs | £627.31 | £999.40 | |
| Resource item | Mean cost |
|---|---|
| Physician visits | £0.00 |
| Laboratory tests | £82.86 |
| Radiological tests | £12.46 |
| Hospitalisation | £0.00 |
| Hospital follow-ups | £0.00 |
| Total | £95.32 |
| Intervention | Total AE cost |
|---|---|
| TheraSphere | £88.65 |
| SIR-Spheres | £111.33 |
| QuiremSpheres | £111.33 |
| cTACE | £112.07 |
| DEB-TACE | £5.59 |
| TAE | £483.88 |
| Sorafenib | £384.15 |
| Lenvatinib | £502.93 |
| Regorafenib | £559.69 |
Appendix 16 Model parameters and plots independent economic assessment
| Population | After SIR-Spheres | After sorafenib |
|---|---|---|
| Base case (whole population) | ||
| Liver transplant | 1.09% | 0.46% |
| Resection | 1.63% | 0.00% |
| Ablation | 3.26% | 0.92% |
| Low tumour burden and ALBI 1 | ||
| Liver transplant | 2.25% | 0.70% |
| Resection | 4.50% | 0.00% |
| Ablation | 7.87% | 1.40% |
| Grade 3/4 AEs (significant/≥ 5% of patients) | Rate (%) | ||||
|---|---|---|---|---|---|
| SIR-Spheres | TheraSphere | QuiremSpheres | Sorafenib | Lenvatinib | |
| Abdominal pain | 3.0 | 3.0 | 3.0 | 6.0 | 0.0 |
| Alopecia | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Anaemia | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Anorexia | 3.0 | 3.0 | 3.0 | 5.0 | 0.0 |
| Ascites | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Aspartate aminotransferase level increase | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 |
| Blood bilirubin level increase | 4.0 | 4.0 | 4.0 | 4.0 | 6.5 |
| Cardiac failure, congestive | 1.0 | 1.0 | 1.0 | 5.0 | 0.0 |
| Diarrhoea | 1.0 | 1.0 | 1.0 | 14.0 | 4.2 |
| Fatigue | 9.0 | 9.0 | 9.0 | 19.0 | 3.8 |
| Gamma-glutamyltransferase level increase | 0.0 | 0.0 | 0.0 | 0.0 | 5.5 |
| Haematological biological abnormalities | 10.0 | 10.0 | 10.0 | 13.0 | 0.0 |
| Haemorrhage | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hypophosphataemia | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hand–foot skin reaction | 0.0 | 0.0 | 0.0 | 6.0 | 2.9 |
| Hypertension | 0.0 | 0.0 | 0.0 | 2.0 | 23.3 |
| Liver dysfunction | 8.0 | 8.0 | 8.0 | 13.0 | 0.0 |
| Nausea/vomiting | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Other increased liver values | 9.0 | 9.0 | 9.0 | 7.0 | 0.0 |
| Platelet count decreased | 0.0 | 0.0 | 0.0 | 0.0 | 5.5 |
| Proteinuria | 1.0 | 1.0 | 1.0 | 4.0 | 5.7 |
| Rash/desquamation | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Weight loss | 0.0 | 0.0 | 0.0 | 3.0 | 7.6 |
| Cholecystitis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hepatic encephalopathy | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
FIGURE 26.
Kaplan–Meier plot of OS, for SIR-Spheres and sorafenib, from the pooled SARAH and SIRveNIB data set.
FIGURE 27.
Kaplan–Meier plot of PFS, for SIR-Spheres and sorafenib, from the pooled SARAH and SIRveNIB data set.

| Survival | SIR-Spheres | Sorafenib |
|---|---|---|
| OS (weeks) | ||
| Median (95% CI) | 42.86 (39.86 to 51.14) | 44.38 (40.68 to 50.82) |
| Interquartile range | 26.43–84.00 | 21.99–90.96 |
| PFS (weeks) | ||
| Median (95% CI) | 22.99 (19.00 to 26.77) | 20.52 (16.29 to 23.73) |
| Interquartile range | 12.76–41.14 | 12.09–39.49 |
FIGURE 28.
Log-cumulative hazard plot of OS, for SIR-Spheres and sorafenib, from the pooled SARAH and SIRveNIB data set.

FIGURE 29.
Log-cumulative hazard plot of PFS, for SIR-Spheres and sorafenib, from the pooled SARAH and SIRveNIB data set.

| Model | SIR-Spheres | Sorafenib | ||
|---|---|---|---|---|
| AIC | BIC | AIC | BIC | |
| Generalised gamma | 2343.50 | 2354.54 | 3146.87 | 3158.84 |
| Weibull | 2394.10 | 2401.46 | 3168.12 | 3176.10 |
| Exponential | 2412.02 | 2415.70 | 3173.08 | 3177.08 |
| Log-logistic | 2357.55 | 2364.91 | 3144.28 | 3152.26 |
| Log-normal | 2350.14 | 2357.50 | 3146.02 | 3154.00 |
| Gompertz | 2412.72 | 2420.08 | 3175.06 | 3183.04 |
| Model | SIR-Spheres | Sorafenib | ||
|---|---|---|---|---|
| AIC | BIC | AIC | BIC | |
| Generalised gamma | 2225.88 | 2236.91 | 3120.26 | 3132.24 |
| Weibull | 2312.97 | 2320.33 | 3182.16 | 3190.15 |
| Exponential | 2337.34 | 2341.02 | 3195.35 | 3199.34 |
| Log-logistic | 2254.74 | 2262.10 | 3129.63 | 3137.61 |
| Log-normal | 2245.68 | 2253.04 | 3120.23 | 3128.21 |
| Gompertz | 2338.53 | 2345.89 | 3197.35 | 3205.33 |
| Model | PFS | OS | ||
|---|---|---|---|---|
| AIC | BIC | AIC | BIC | |
| Per-protocol population (SARAH19 only) | ||||
| Log-normal | 1881.7 | 1897.4 | 2181.2 | 2196.8 |
| Exponential | 1977.8 | 1985.6 | 2233.6 | 2241.4 |
| Weibull | 1953.4 | 1969 | 2213.8 | 2229.4 |
| Generalised gamma | 1874.7 | 1898.1 | 2183.9 | 2207.3 |
| Gompertz | 1976.3 | 1991.9 | 2231.3 | 2246.9 |
| Log-logistic | 1895.1 | 1910.8 | 2190 | 2205.6 |
| Low tumour burden and ALBI 1 subgroup | ||||
| Log-normal | 386.3 | 395.4 | 427.6 | 436.7 |
| Exponential | 394.4 | 398.9 | 442.6 | 447.1 |
| Weibull | 393.8 | 402.9 | 429.6 | 438.7 |
| Generalised gamma | 389.3 | 403 | 431.3 | 445 |
| Gompertz | 397.4 | 406.5 | 435.2 | 444.3 |
| Log-logistic | 389.4 | 398.5 | 428.4 | 437.5 |
| No macrovascular invasion subgroup | ||||
| Log-normal | 783.4 | 795.3 | 846.2 | 858.1 |
| Exponential | 815.5 | 821.4 | 872.6 | 878.6 |
| Weibull | 805.6 | 817.6 | 855 | 866.9 |
| Generalised gamma | 786.2 | 804.1 | 848.8 | 866.7 |
| Gompertz | 817.1 | 829 | 866.8 | 878.8 |
| Log-logistic | 789.5 | 801.5 | 848.7 | 860.6 |
FIGURE 30.
Extrapolation of OS: low tumour burden and ALBI 1 subgroup – SIR-Spheres.

FIGURE 31.
Extrapolation of OS: no-MVI subgroup – SIR-Spheres.
FIGURE 32.
Extrapolation of OS: low tumour burden and ALBI 1 subgroup – sorafenib.
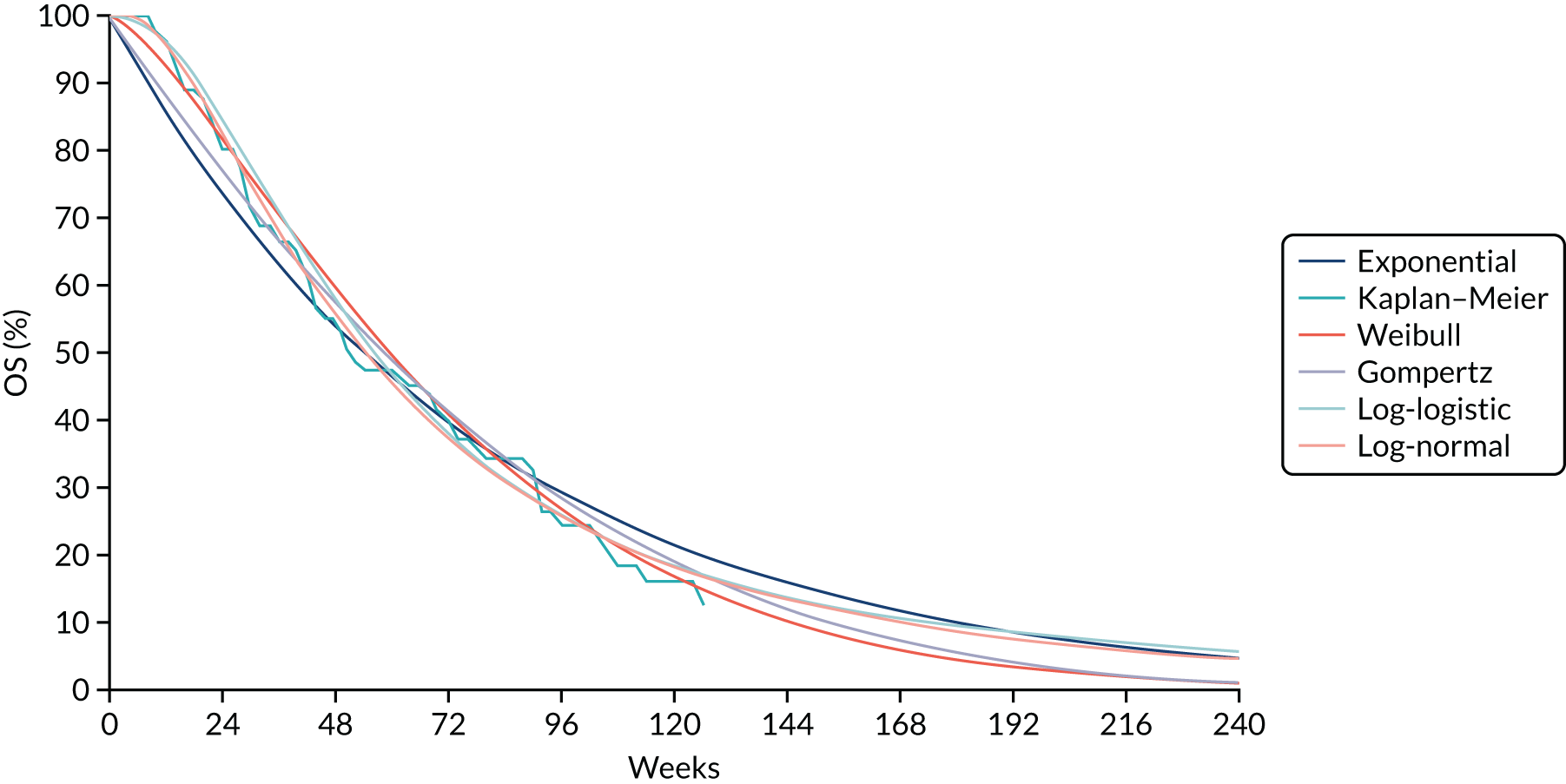
FIGURE 33.
Extrapolation of OS: no-MVI subgroup – sorafenib.

FIGURE 34.
Extrapolation of PFS: low tumour burden and ALBI 1 subgroup – SIR-Spheres.

FIGURE 35.
Extrapolation of PFS: no-MVI subgroup – SIR-Spheres.

FIGURE 36.
Extrapolation of PFS: low tumour burden and ALBI 1 subgroup – sorafenib.
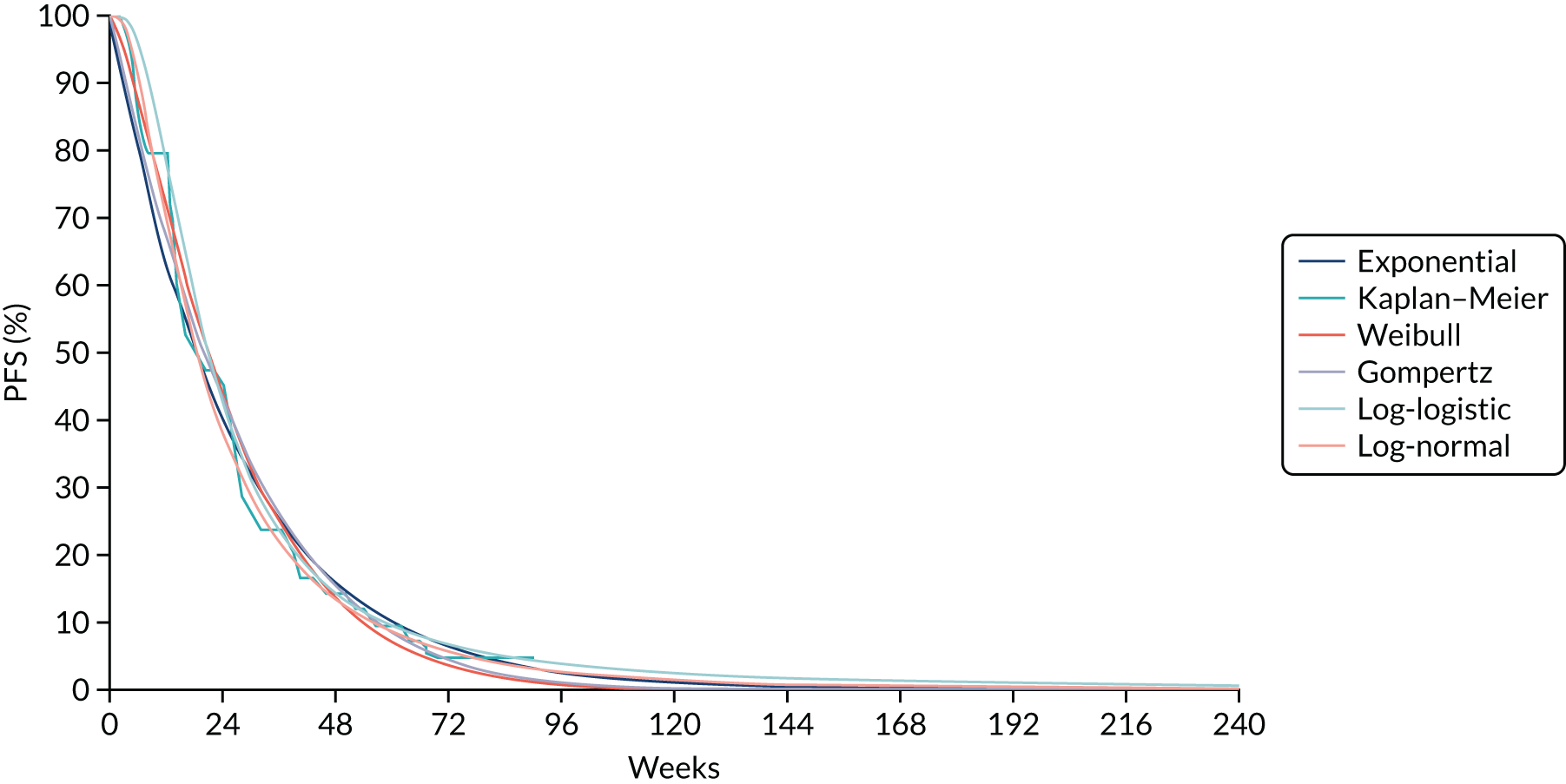
FIGURE 37.
Extrapolation of PFS: no-MVI subgroup – sorafenib.
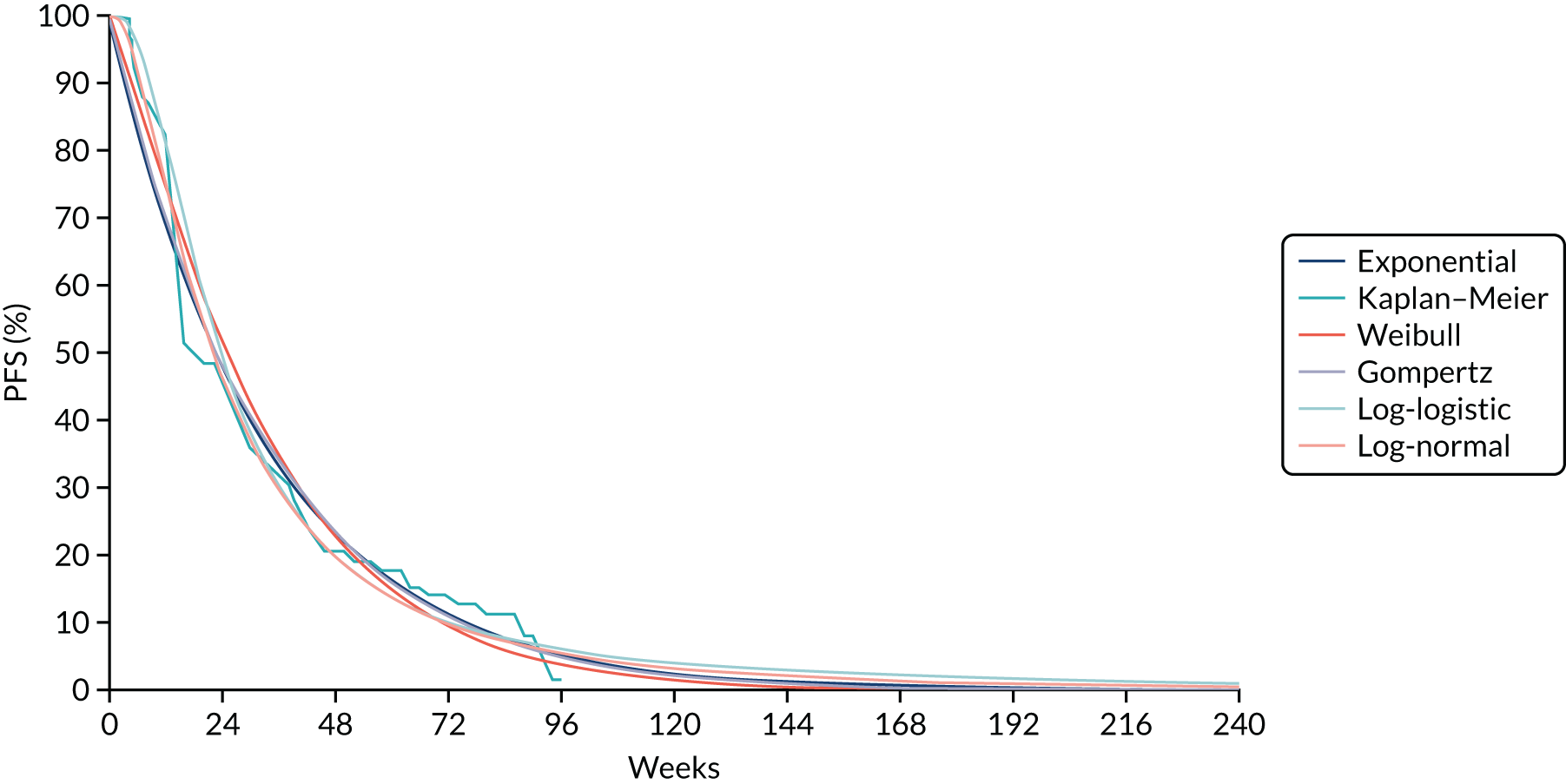
| AE | Unit cost per episode | Source |
|---|---|---|
| Abdominal pain | £42.19 | Sirtex submission102 (inflated from TA47411) |
| Alopecia | £18.59 | Sirtex submission102 (inflated from TA47411) |
| Anaemia | £615.76 | National Schedule of Reference Costs 2017–2018107 (hospitalisation) (TA535154) |
| Anorexia | £657.86 | Sirtex submission102 (inflated from TA535154) |
| Ascites | £615.76 | National Schedule of Reference Costs 2017–2018107 (hospitalisation) (TA535154) |
| Aspartate aminotransferase level increase | £634.50 | Sirtex submission102 (inflated from TA551124) |
| Blood bilirubin level increase | £916.47 | Sirtex submission102 (inflated from TA535154) |
| Cardiac failure, congestive | £1979.71 | National Schedule of Reference Costs 2017–2018 107 |
| Diarrhoea | £605.13 | Sirtex submission102 (inflated from TA551124) |
| Fatigue | £677.68 | Sirtex submission102 (inflated from TA551124) |
| Gamma-glutamyltransferase level increase | £634.50 | Sirtex submission102 (inflated from TA551124) |
| Haematological biological abnormalities | £1713.98 | Assumed same as anaemia (TA51413) |
| Haemorrhage | £0.00 | Sirtex submission102 (TA47411) |
| Hypophosphataemia | £1297.52 | Sirtex submission102 (inflated from TA551124) |
| Palmar–plantar erythrodysesthesia syndrome | £897.98 | Sirtex submission102 (inflated from TA535154) |
| Hypertension | £888.12 | Sirtex submission102 (inflated from TA551124) |
| Liver dysfunction | £1207.13 | Sirtex submission102 (inflated from TA535154) |
| Nausea/vomiting | £82.18 | National Schedule of Reference Costs 2017–2018107 (hospitalisation) (TA535154) |
| Other increased liver values | £634.50 | Sirtex submission102 (inflated from TA551124) |
| Platelet count decrease | £634.50 | Sirtex submission102 (inflated from TA551124) |
| Proteinuria | £812.04 | Sirtex submission102 (inflated from TA551124) |
| Rash/desquamation | £71.09 | Sirtex submission102 (inflated from TA47411) |
| Weight loss | £665.35 | Sirtex submission102 (inflated from TA551124) |
Appendix 17 Confidential information has been removed
Confidential information has been removed.
Glossary
- Adverse effect
- An adverse outcome that occurs during or after exposure to a drug or other intervention and that may or may not be caused by the intervention.
- Assessment Group
- An independent academic group commissioned by the National Institute for Health Research on behalf of the National Institute for Health and Care Excellence to appraise the clinical effectiveness and cost-effectiveness of selective internal radiation therapies.
- Confidence interval
- A measure of uncertainty around the results of a statistical analysis that describes the range of values within which we can be reasonably sure that the true effect lies. For example, a 95% confidence interval is based on the notion that if a study were repeated many times in other samples from the same population, 95% of the confidence intervals from those studies would include the true value of the effect being measured. Wider intervals indicate lower precision; narrow intervals indicate greater precision.
- Conventional transarterial therapies
- Includes transarterial chemoembolisation, drug-eluting bead transarterial chemoembolisation and transarterial embolisation without chemotherapy. All three forms of conventional transarterial therapy work by administering an embolising agent into the hepatic artery to block blood vessels feeding the tumours in the liver. In the case of transarterial chemoembolisation, also known as conventional transarterial chemoembolisation, Lipiodol® (Guerbet, Villepinte, France) is combined with a chemotherapy agent, typically doxorubicin or cisplatin, which is administered directly to the tumour. In drug-eluting bead transarterial chemoembolisation, drug-eluting beads typically bound with doxorubicin or epirubicin are administered to the tumour via the hepatic artery. Transarterial embolisation, or bland transarterial chemoembolisation, involves only the physical occlusion of blood vessels, with no addition of chemotherapy.
- Cost–benefit analysis
- An economic analysis that converts the effects or consequences of interventions into the same monetary terms as the costs and compares them using a measure of net benefit or a cost–benefit ratio.
- Cost-effectiveness acceptability curve
- A graph describing the impact of uncertainty on the result of a cost-effectiveness model. The graph plots a range of cost-effectiveness thresholds on the horizontal axis against the probability that the intervention will be cost-effective at that threshold on the vertical axis. It can usually be drawn directly from the results of a probabilistic sensitivity analysis.
- Cost-effectiveness analysis
- A type of economic analysis that compares the relative costs and outcomes (effects) of different courses of action. It compares an intervention with another intervention(s) (or the current standard of care) by estimating how much it costs to gain a unit of a health outcome, such as a life-year gained or a death prevented.
- Cost-effectiveness model
- A cost-effectiveness or decision model seeks to answer questions about how to deploy resources in a health-care system. A model is a simplified representation of a real-world condition and treatment pathway, which aims to estimate the costs and consequences arising from making a particular policy decision (i.e. whether or not the NHS should fund a new procedure or drug). All relevant alternative courses of action and their long-term costs and consequences are compared to inform a decision on which option to adopt.
- Cost-effectiveness threshold
- This represents the maximum amount a health-care system is willing to pay to provide a new technology or intervention. National Institute for Health and Care Excellence guidance typically considers interventions with an incremental cost-effectiveness ratio of between £20,000 and £30,000 per quality-adjusted life-year to be cost-effective.
- Cost–utility analysis
- The same as a cost-effectiveness analysis, but the effects or consequences of interventions are expressed in generic units of health gain, usually quality-adjusted life-years.
- Credible interval
- In Bayesian statistics, a credible interval is a posterior probability interval estimation that incorporates problem-specific contextual information from the prior distribution. Credible intervals are used for the purposes similar to those of confidence intervals in frequentist statistics.
- Cycle
- The time horizon in a model is split into cycles that represent the smallest period of time measured in the economic model.
- Deterministic sensitivity analysis
- Explores the impact on model results of varying one or two input parameters at a time.
- Dominance
- In the field of health economics, a treatment option is said to be ‘dominant’ when it both is less costly and produces better health outcomes than the comparator strategy. Thus, a treatment that both is more expensive and results in poorer health outcomes is referred to as ‘dominated’.
- EuroQol-5 Dimensions
- A generic measurement of quality of life used in many clinical trials. This instrument is easy to use and has been extensively validated across many disease areas. The benefit of the EuroQol-5 Dimensions is the availability of utility scores (generated through large population surveys) for each possible combination of questionnaire responses; these can be combined with the time individuals reside in particular health states to calculate the quality-adjusted life-years associated with an intervention.
- Fixed-effect model
- A statistical model that stipulates that the units under analysis (e.g. people in a trial or study in a meta-analysis) are the ones of interest and, thus, constitute the entire population of units. Only within-study variation is taken to influence the uncertainty of results (as reflected in the confidence interval) of a meta-analysis using a fixed-effect model.
- Heterogeneity
- In systematic reviews, heterogeneity refers to variability, or differences, between studies in the estimates of effects. A distinction is sometimes made between ‘statistical heterogeneity’ (differences in the reported effects), ‘methodological heterogeneity’ (differences in study design) and ‘clinical heterogeneity’ (differences between studies in key characteristics of the participants, interventions or outcome measures).
- Incremental cost-effectiveness ratio
- A measure that represents the economic value of an intervention compared with an alternative; it is generally the primary outcome of an economic evaluation. It is calculated by dividing the difference in costs between two interventions by the difference in quality-adjusted life-years. It is the cost of generating an additional quality-adjusted life-year using the intervention we are interested in versus an alternative (usually current clinical practice).
- Intention-to-treat analysis
- An analysis in which all participants enrolled in a trial are analysed according to the intervention to which they were initially allocated, regardless of whether they went on to receive it or not.
- Network meta-analysis
- A meta-analysis in which three or more treatments are compared using both direct comparisons of interventions within trials and indirect comparisons across trials, based on a common comparator.
- Probabilistic sensitivity analysis
- Assesses the joint uncertainty across all input parameters in the model. This is carried out by assigning probability distributions to each input parameter and making random draws from each of these distributions. This process is then repeated many thousands of times, resulting in a distribution of outputs that describe the uncertainty in the results of the model.
- Quality-adjusted life-year
- An index of health gain where survival duration is weighted or adjusted according to the patient’s quality of life over the time they are alive. Quality-adjusted life-years are based on utilities, which are valuations of quality of life measured on a scale between full health (1) and death (0). These valuations are multiplied by the number of years that an individual spends in a health state with that particular utility score, and the quality-adjusted life-years are summed over the modelled time horizon.
- Quality of life
- A broad concept incorporating all of the factors that might have an impact on an individual’s physical, mental and social well-being. Health-related quality of life refers to the specific impact that a medical condition or treatment has on an individual’s functioning and general well-being. Health-related quality of life is generally measured in clinical trials alongside other outcomes to assess the impact of an intervention from a patient’s perspective, typically using questionnaires completed by patients, their families or clinicians, such as the EuroQol-5 Dimensions.
- Random-effects model
- A statistical model sometimes used in meta-analysis in which both within-study sampling error (variance) and between-study variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Randomised controlled trial
- An experiment in which investigators randomly allocate eligible people into groups that are each assigned a different intervention in order to compare their relative effectiveness and safety.
- Relative risk (synonym: risk ratio)
- The ratio of risk in the intervention group to the risk in the control group. The risk (proportion, probability or rate) is the ratio of people with an event in a group to the total number in the group. A relative risk of 1 indicates no difference between comparison groups. For undesirable outcomes, a relative risk of < 1 indicates that the intervention was effective in reducing the risk of that outcome.
- Scenario analysis
- A process of exploring alternative future outcomes by selection of different assumptions used in the economic model. Scenarios can represent outcomes ranging from optimistic (where input variables are changed to their most optimistic value) to pessimistic (where they are changed to their most pessimistic). These types of analyses test the cost-effectiveness and safety of an intervention in the best and worst cases, and in other plausible ‘alternative worlds’.
- Statistical significance
- A result is described as statistically significant when the reported p-value falls below the selected significance level; this value represents the probability that the observed result could have occurred owing of chance alone if the ‘null hypothesis’ is true (i.e. there was no true difference between the groups).
- Time horizon
- The time horizon of an economic model is the duration over which costs and health outcomes are calculated. The choice of time horizon is important, and generally depends on the nature of the condition for which an intervention is being assessed. A long time horizon is preferred in chronic or long-term conditions for which there are likely to be important ongoing management costs and consequences well into the future. The use of a long-term time horizon often involves the extrapolation of short-term data into the future and the use of assumptions about the persistence of treatment effects due to a lack of long-term data.
List of abbreviations
- AE
- adverse event
- AG
- Assessment Group
- AIC
- Akaike information criterion
- ALBI
- albumin–bilirubin
- BCLC
- Barcelona Clinic Liver Cancer
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- BSC
- best supportive care
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CE
- Conformité Européenne
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CIRT
- Cardiovascular and Interventional Radiological Society of Europe Registry for SIR-Spheres Therapy
- CMA
- cost-minimisation analysis
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CT
- computerised tomography
- cTACE
- conventional transarterial chemoembolisation
- CTT
- conventional transarterial therapy
- DEB-TACE
- drug-eluting bead transarterial chemoembolisation
- DIC
- deviance information criterion
- DSA
- deterministic sensitivity analysis
- DSU
- Decision Support Unit
- EASL
- European Association for the Study of the Liver
- ECOG
- Eastern Cooperative Oncology Group
- eMIT
- electronic market information tool
- ENRY
- European Network on Radioembolisation with Yttrium-90 Resin Microspheres
- EORTC QLQ
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FACT-Hep
- Functional Assessment of Cancer Therapy Hepatobiliary-Pancreatic Symptom Index
- GP
- general practitioner
- HCC
- hepatocellular carcinoma
- HRG
- Healthcare Resource Group
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INR
- international normalised ratio
- IPD
- individual patient data
- ITT
- intention to treat
- KM
- Kaplan–Meier
- LYG
- life-years gained
- MAA
- macroaggregated albumin
- MELD
- Model for End-Stage Liver Disease
- MeSH
- medical subject heading
- MRI
- magnetic resonance imaging
- MVI
- macroscopic vascular invasion
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NMB
- net monetary benefit
- OS
- overall survival
- PAS
- Patient Access Scheme
- PFS
- progression-free survival
- PLLA
- poly-L-lactic acid
- PREMIERE
- Prospective Randomized study of chEmoeMbolization versus radIoEmbolization for the tReatment of hEpatocellular carcinoma
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
- international prospective register of systematic reviews
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PVI
- portal vein invasion
- PVT
- portal vein thrombosis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumours
- REILD
- radioembolisation-induced liver disease
- SARAH
- SorAfenib versus Radioembolization in Advanced Hepatocellular Carcinoma
- SD
- standard deviation
- SIRT
- selective internal radiation therapy
- SIRveNIB
- Selective Internal Radiation Therapy Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma
- SmPC
- summary of product characteristics
- SPECT
- single-photon emission computerised tomography
- TA
- technology appraisal
- TACE
- transarterial chemoembolisation
- TAE
- transarterial embolisation
- TARE
- transarterial radioembolisation
- TRAE
- treatment-related adverse event
- TTP
- time to progression
- WHO
- World Health Organization
- WTP
- willingness to pay
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in its deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.