Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number NIHR128164. The protocol was agreed in December 2018. The assessment report began editorial review in September 2019 and was accepted for publication in February 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rob Riemsma is a member of the National Institute for Health Research Health Technology Assessment and Efficacy and Mechanism Evaluation Editorial Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Armstrong et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Platelets play a critical role in haemostasis, a process that causes bleeding to stop. A reduction in platelets circulating in the blood is referred to as thrombocytopenia. It is usually defined as a platelet count of < 150,000 per µl of blood. 1
Thrombocytopenia occurs frequently in chronic liver disease (CLD), either directly or as a result of interferon-based antiviral treatment of liver infection. Severe thrombocytopenia, because it increases the risk of excessive bleeding during and after surgery, can significantly affect the clinical management of CLD, leading to delay and, potentially, to increased morbidity and mortality. 1
Adults with thrombocytopenia associated with CLD can undergo various types of elective procedure. These procedures might be classified by associated bleeding risk, based on the published literature, into one of three categories:2
-
low risk (paracentesis, thoracentesis, gastrointestinal endoscopy)
-
moderate risk (liver biopsy, bronchoscopy, ethanol ablation therapy, chemoembolisation)
-
high risk (vascular catheterisation, transjugular intrahepatic portosystemic shunt, dental procedures, renal biopsy, biliary interventions, nephrostomy tube placement, radiofrequency ablation, laparoscopic interventions).
Between 2016 and 2017, Hospital Episode Statistics showed that 27,927 people were admitted to hospital with liver disease in England. 3 The prevalence of thrombocytopenia among people with CLD varies from 15% to 70% depending on the stage of liver disease and the platelet count cut-off value used to define thrombocytopenia.
Current service provision
Until this assessment, no licensed treatment options had been recommended by the National Institute for Health and Care Excellence (NICE) for treating thrombocytopenia in people with CLD requiring surgery. Typical therapies include stimulation of megakaryocyte maturation and platelet production. Treatment for severe thrombocytopenia can include platelet transfusion, splenic artery embolisation and surgical splenectomy.
The NICE clinical guideline CG244 recommends that for anyone having an invasive procedure or surgery, apart from those with a low risk of bleeding, a platelet transfusion is considered in order to raise the platelet count to above:
-
50,000/µl in any type of patient
-
50,000–75,000/µl in patients with a high risk of bleeding, depending on procedure, aetiology, if platelet count is stable and any other cause of abnormal haemostasis
-
100,000/µl ‘. . . in critical sites, such as the central nervous system (including the posterior segment of the eyes)’ (reproduced with permission © NICE 2015. Blood Transfusion. Available from www.nice.org.uk/guidance/ng24. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.). 4
Description of the technology under assessment
Avatrombopag (Doptelet®; Dova Pharmaceuticals, Durham, NC, USA) is a small-molecule thrombopoietin receptor agonist (TPO-RA) that targets the c-MpI thrombopoietin cell surface receptor on megakaryocytes to stimulate platelet production. Avatrombopag is administered orally. It has been compared in clinical trials with placebo in people with thrombocytopenia associated with CLD requiring an elective procedure. It received marketing authorisation in the UK on 25 June 2019. 5 The full indication is ‘Doptelet is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo an invasive procedure’ (reproduced with permission; © European Medicines Agency). 5 The European Medicines Agency6 recommends that avatrombopag be administered for 5 days at a dose of:
-
60 mg if the baseline platelet count is < 40,000/µl
-
40 mg if the baseline platelet count is 40,000–< 50,000/µl.
The elective procedure should be performed 10–13 days after treatment initiation.
Lusutrombopag (Mulpleta®; Shionogi Inc., London, UK) is a small-molecule TPO-RA that targets the c-MpI thrombopoietin cell surface receptor on megakaryocytes to stimulate platelet production. Lusutrombopag is administered orally. It has been compared in clinical trials with placebo in people with thrombocytopenia with a platelet count of < 50 × 109/µl associated with CLD requiring elective invasive surgery. It received marketing authorisation on 14 March 2019. 7 The following indication was agreed: ‘Treatment of severe thrombocytopenia in adult patients with chronic liver disease undergoing invasive procedures’ (reproduced with permission; © European Medicines Agency). 7 The European Medicines Agency recommends a dose of 3 mg once daily for 7 days and that the elective procedure be performed from day 9 after treatment initiation. 8
Patient and public involvement
There was no patient and public involvement in the study because this was a multiple technology appraisal for NICE, which does not require patient and public involvement. However, patient and public involvement is part of decision-making by NICE, including during appraisal committee meetings with invited patient experts.
Chapter 2 Definition of the decision problem
The purpose of this chapter is to specify the decision problem and to translate it into research objectives. Where Chapter 1 provided an overall summary of the topic, this chapter states the key factors to be addressed and the scope of the assessment of the key factors as defined through the NICE scoping process.
Decision problem
Interventions
-
Avatrombopag, dose as reported in trials, although the focus will be on the licensed dose:
-
60 mg if the baseline platelet count is < 40,000/µl
-
40 mg if the baseline platelet count is 40,000–< 50,000/µl.
-
-
Lusutrombopag, dose as reported in trials, although the focus will be on the licensed dose (i.e. 3 mg).
Population
-
Adults with thrombocytopenia associated with CLD needing an elective procedure, although the focus will be on platelet count of < 50,000/µl and, to match to the licenced dose of avatrombopag, within the subgroups, platelet count of < 40,000/µl and 40,000–< 50,000/µl.
Relevant comparators
-
Established clinical management without avatrombopag and lusutrombopag (including, but not limited to, platelet transfusion).
Outcomes
-
Platelet count.
-
Response rate (by some definition related to change in platelet count).
-
Number of platelet transfusions.
-
Number of blood transfusions.
-
Return to operating theatre.
-
Need for rescue treatments.
-
Use of concurrent treatments.
-
Bleeding score.
-
Mortality.
-
Adverse effects of treatment.
-
Health-related quality of life (HRQoL).
Overall aims and objectives of the assessment
The review aims to evaluate the:
-
clinical effectiveness of each intervention
-
adverse effect profile of each intervention
-
incremental cost-effectiveness of each intervention compared with –
-
each other
-
established clinical management without avatrombopag or lusutrombopag.
-
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing effectiveness
Throughout this review, the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions9 and by the Centre for Reviews and Dissemination (CRD),10 York, were applied to reduce the risk of bias and error. All methods were in accordance with a protocol registered on PROSPERO as record number CRD42019125311.
Identification of studies
Literature searches were conducted to identify relevant information on the clinical effectiveness, safety and cost-effectiveness of avatrombopag and lusutrombopag. The searches also identified studies of the clinical effectiveness, safety and cost-effectiveness of established clinical management of thrombocytopenia in people with CLD, including platelet transfusion, stimulation of megakaryocyte maturation and platelet production, splenic artery embolisation and surgical splenectomy. All literature searches were undertaken to the highest standard to meet the best practice requirements of the CRD10 and the Cochrane Collaboration. 9
The search strategies combined relevant search terms comprising indexed keywords [e.g. medical subject heading (MeSH) terms and EMTREE] and free-text terms appearing in the title and/or abstract of database records. Search terms were identified from discussions with the review team, by scanning background literature and ‘key articles’ already known to the review team, and by browsing database thesauri. Search strategies were developed specifically for each database and the keywords were adapted for the configuration of each database. Only studies conducted in humans were sought. Searches were not limited by language, publication status (i.e. unpublished or published) or date of publication. Methodological study design filters were not included in the search strategies to ensure sensitivity and the optimal identification of clinical effectiveness, safety and cost-effectiveness studies.
Full details of the search strategies are presented in Appendix 1.
The following databases and resources were searched:
-
MEDLINE (via Ovid) – 1946–week 3 January 2019
-
MEDLINE In-Process Citations, Daily Update and Epub Ahead of Print (via Ovid) – 22 January 2019
-
PubMed (National Library of Medicine) – up to 24 January 2019
-
EMBASE (via Ovid) – 1974 to week 3 2019
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley) – issue 1 of 12, January 2019
-
Cochrane Database of Systematic Reviews (CDSR) (via Wiley) – issue 1 of 12, January 2019
-
Kleijnen Systematic Reviews (KSR) Evidence (https://ksrevidence.com/) – database last updated 24 January 2019
-
Epistemonikos (www.epistemonikos.org/) – up to 24 January 2019
-
Database of Abstracts of Reviews of Effects (DARE) (via CRD) – up to 31 March 2015*
-
Health Technology Assessment (HTA) database (via CRD) – up to 31 March 2018*
-
NHS Economic Evaluation Database (NHS EED) (via CRD) – up to 31 March 2015*
-
PROSPERO (via CRD) – up to 24 January 2019
-
Science Citation Index (SCI) (via Web of Science) – 1988–23 January 2019
-
CINAHL (via EBSCOhost) – 1982–23 January 2019
-
LILACS (BIREME) – 1982 to 24 January 2019
-
Northern Light Life Sciences Conference Abstracts (via Ovid) – 2010–19/week 02
-
Transfusion Evidence Library (www.transfusionevidencelibrary.com) – up to 23 January 2019
-
Research Papers in Economics (RePEc) (repec.org/) – up to 23 January 2019.
*DARE and NHS EED have ceased; records were published until 31 March 2015. HTA database records were added until 31 March 2018.
Supplementary searches of the following clinical trials registers were conducted to identify completed and ongoing trials:
-
ClinicalTrials.gov (www.clinicaltrials.gov/) – up to 23 January 2019
-
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) – up to 23 January 2019.
Grey literature was identified from searches of the following resources:
-
US Food and Drug Administration (FDA) (www.fda.gov/) – up to 23 January 2019
-
European Medicines Agency (www.ema.europa.eu/ema/) – up to 23 January 2019
-
OAIster (https://oaister.worldcat.org/) – up to 23 January 2019
-
OpenGrey (www.opengrey.eu/) – up to 23 January 2019
-
Copac (https://copac.jisc.ac.uk/) – up to 23 January 2019.
Relevant organisation websites were also searched, including the British Society for Haematology, the European Hematology Association, the International Society on Thrombosis and Haemostasis, and the American Society of Hematology.
Reference checking
The bibliographies of identified research and review articles were checked for relevant studies.
Handling of citations
Identified references were downloaded into EndNote X8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] bibliographic management software for further assessment and handling. Individual records in the EndNote library were tagged with searching information, such as searcher, date searched, database host, database searched, strategy name and iteration, theme and search question. This enabled the information specialist to track the origin of each individual database record and the record’s progress through the screening and review process.
Quality assurance within the search process
For all searches undertaken by the KSR information team, the main EMBASE strategy was independently peer reviewed by a second KSR information specialist. The search strategy peer review was informed by items based on the Canadian Agency for Drugs and Technologies in Health checklist. 11,12
Inclusion criteria
The following inclusion criteria were applied for the systematic review.
Population
-
Adults with thrombocytopenia associated with CLD needing an elective procedure.
Intervention
-
Avatrombopag.
-
Lusutrombopag.
Comparator
-
Any comparator or none.
Outcomes
-
Platelet count.
-
Response rate.
-
Number of platelet transfusions.
-
Number of blood transfusions.
-
Return to operating theatre.
-
Need for rescue treatments for bleeding (referred to as ‘rescue therapy’).
-
Use of concurrent treatments.
-
Bleeding score.
-
Mortality.
-
Adverse effects of treatment.
-
HRQoL.
Study design
-
Randomised controlled trials (RCTs).
-
Observational studies (cohort or case series) of at least 20 participants.
Abstraction strategy
Study selection
Titles and abstracts identified from electronic database and other searches were independently screened by two reviewers. During this initial phase of the screening process any references that obviously did not meet the inclusion criteria were excluded. Full-paper copies of all of the remaining references were obtained. These were then independently examined in detail by two reviewers to determine whether or not they met the inclusion criteria. All papers excluded at this second stage of the screening process along with the reasons for their exclusion are listed in Table 35 (see Appendix 2). These reasons were categorised as follows:
-
not relevant population (i.e. not thrombocytopenia associated with CLD needing an elective procedure)
-
not relevant intervention
-
not relevant outcome data (i.e. did not assess at least one of the specified outcomes or did not report relevant data or information that would allow the calculation of relevant data)
-
not relevant study (i.e. not a RCT, cohort study or case series)
-
insufficient study size (< 20 participants).
At both screening stages, any discrepancies between reviewers were resolved through discussion or by the intervention of a third reviewer.
A flow diagram of the numbers of studies included and excluded at each stage has been provided following guidance in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Data extraction
Data extraction sheets were individually designed and piloted using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). The extraction process was performed by two reviewers, with one checking the extraction of the other. Any discrepancies were resolved through discussion or by the intervention of a third reviewer. Studies are identified by the trial name. To avoid the duplication of data where studies (or study populations) have multiple publications, the most complete report is used as the main reference, but additional details have been extracted from the other publications as necessary. The following general information and data were extracted from each study, regardless of review topic:
-
EndNote ID
-
study ID or name (if reported; otherwise, surname of first author)
-
year of publication
-
other related publications
-
study group (if reported)
-
study country/countries
-
recruitment dates (if relevant)
-
location/setting
-
study funding (public/pharmaceutical/not reported)
-
study aim
-
sample size
-
study design
-
study methods
-
patient characteristics
-
treatment characteristics
-
results (all outcomes reported in Chapter 2, Decision problem)
-
study conclusions.
Critical appraisal strategy
The quality of each individual study was assessed using the Cochrane Collaboration Quality Assessment Tool for RCTs. 13
The findings of the quality assessment were used to ensure that the conclusions and findings of these reviews were based on the best available evidence and that any potential sources of bias in the data were identified.
Methods of data synthesis
Data are summarised in the context of population variation in aetiology of liver disease, degree of thrombocytopenia, bleeding risk and type of elective procedure. Subgroup analysis by degree of thrombocytopenia is also presented.
Quantitative analysis and meta-analysis methods (direct ‘head-to-head’ methods)
Forest plots of effect sizes are presented for each of the main efficacy outcomes. Dichotomous outcomes (e.g. proportion of patients experiencing each type of outcome) are reported as relative risks (RRs) and odds ratios (ORs) with 95% confidence intervals (CIs).
Pooled effect sizes and 95% CIs using random-effects models are presented where two or more trials are considered to be clinically and statistically homogeneous.
The judgement of clinical homogeneity is based on the baseline characteristics of the trial populations (i.e. age, sex, aetiology of liver disease, degree of thrombocytopenia, bleeding risk and type of elective procedure). Statistical homogeneity is assessed using the I2 statistic. 14 This measures the degree of inconsistency between the study results that is due to genuine heterogeneity rather than chance. The value of I2 lies between 0% and 100%. For the purposes of this review, a simplified categorisation of heterogeneity is used: low (0–25%), moderate (26–75%) and high (> 75%). Studies will be considered to be sufficiently similar for the purposes of pooling only if I2 < 75%. 14
Publication bias could not be assessed given that there were too few trials to use funnel plots of the point estimate plotted against the standard error (SE). 15
Indirect comparisons
Where the intervention and comparator were not compared in the same RCT (i.e. ‘head-to-head’ trials of A vs. B), but instead were separately compared with a common comparator, for example placebo, an indirect comparison of these was performed. Point estimates (with 95% CIs) were estimated using ‘indirect’ methods, for example from A versus C and B versus C, where C is a common control group (e.g. placebo). All methods are applied with consideration of the basic properties of homogeneity, similarity and consistency as reported in Dias et al. 16 All indirect comparisons are consistent with NICE methodological guidance for the conduct of direct and indirect meta-analysis,17 which includes indirect comparisons using the Bucher method. 18
Indirect meta-analysis was performed in Microsoft Excel using the Bucher method. 18 RRs and ORs with 95% CIs were calculated for each outcome and available treatment comparison.
Heterogeneity was investigated using the I2 statistic for each of the pairwise comparisons. 14 If there were concerns about heterogeneity, or if any trials appeared to have results that differed substantially from the others, then one or more trials were removed in a sensitivity analysis.
Bayesian network meta-analysis
As its outputs can be directly integrated into a probabilistic cost-effectiveness analysis (CEA) framework, network meta-analysis using WinBUGs version 1.4.3 (www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml) (Medical Research Council Biostatistics Unit, Cambridge, UK) was applied using a Bayesian Markov chain Monte Carlo (MCMC) approach consistent with international recommendations. This method generates a set of simulated values in the form of a posterior distribution for each of the ORs between each TPO-RA and no TPO-RA. The specification of an evidence-based baseline average risk with its SE then permits the simulation of an absolute risk for each of the three treatments, namely lusutrombopag, avatrombopag and no TPO-RA, as described in NICE Technical Support Document 2. 16 Note that the simulation from the Bayesian posterior distribution provided both statistical estimation and inference, as well as a platform for probabilistic decision-making under uncertainty. Each of the simulated absolute risks from the Bayesian MCMC was consistent and coherent and was used as an input in the CEA model to calculate the expected values of cost and quality-adjusted life-years (QALYs) using a Monte Carlo simulation.
Posterior distribution parameter estimates were obtained from 100,000 simulations after a burn-in period of 30,000 MCMC simulations, using two chains. Vague normal priors (mean 0, variance 10,000) were used for treatment effects and a vague uniform prior (0, 5) was used for the between-study standard deviation. Convergence and auto-correlation were assessed by monitoring the trace, Gelman–Rubin statistics (BGR plot) and autocorrelation plots in WinBUGS. The ORs estimated using this method were almost identical to those estimated using the Bucher method.
Results
Quantity and quality of research available
As a result of all searching, after deduplication, 11,305 records were screened at the title and abstract stage. From these, 91 were selected to be rescreened at the full-paper stage. After full-paper screening of the 91 records was complete, 35 references were included that fulfilled the inclusion criteria. No additional references were found by reference checking. Therefore, in total, 35 references pertaining to six studies were included. The results of screening are shown in Figure 1. The list of included studies is shown in Table 1: ADAPT-1,37 ADAPT-2,37 L-PLUS 139 (Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures), L-PLUS 254 (Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures 2), Study 20251 and the study registered by Japic Clinical Trials Information (JAPIC) as CTI-121944. 53 Note that the studies referred to as ADAPT-1, ADAPT-2, L-PLUS 1 and L-PLUS 2 are mentioned more than once to indicate that some references report on only one of the studies whereas others report on two of them.
FIGURE 1.
Summary of study flow.

| Trial name | NCT (or other register) number | Study authors, year |
|---|---|---|
| ADAPT-1 | NCT01972529 | Eisai Inc., 2014–1719 |
| ADAPT-2 | NCT01976104 | Eisai Co. Ltd, 201420 |
| Eisai Inc., 2013–1721 | ||
| ADAPT-1, ADAPT-2 | NCT01972529, NCT01976104 | Caldwell et al., 201822 |
| Center for Drug Evaluation and Research, 201723 | ||
| Center for Drug Evaluation and Research, 201724 | ||
| Center for Drug Evaluation and Research, 201825 | ||
| Frelinger et al., 201726 | ||
| Poordad et al., 201827 | ||
| Poordad et al., 201828 | ||
| Poordad et al., 201829 | ||
| Reau et al., 201830 | ||
| Saab et al., 201831 | ||
| Saab et al., 201832 | ||
| Sammy et al., 201833 | ||
| Sammy et al., 201834 | ||
| Terrault et al., 201735 | ||
| Terrault et al., 201736 | ||
| Terrault et al., 201837 | ||
| Vredenburg et al., 201838 | ||
| L-PLUS 1 | JapicCTI-132323 | Hidaka et al., 201839 |
| Izumi et al., 201540 | ||
| L-PLUS 2 | NCT02389621 | Afdhal et al., 201741 |
| Afdhal et al., 201742 | ||
| Peck-Radosavljevic et al., 201743 | ||
| Shionogi Inc., 201744 | ||
| L-PLUS-1, L-PLUS 2 | JapicCTI-132323, NCT02389621 | Alkhouri et al., 201745 |
| Brown et al., 201746 | ||
| Brown et al., 201747 | ||
| Center for Drug Evaluation and Research, 201748 | ||
| Study 202 | NCT00914927 | Eisai Inc., 201149 |
| Terrault et al., 201250 | ||
| Terrault et al., 201451 | ||
| Not reported | JapicCTI-121944 | Izumi et al., 201452 |
| Tateishi et al., 201753 |
All studies were generally rated as being at low risk of bias, as shown in Table 2. In addition, both sets of main trials for each of the TPO-RAs (ADAPT-1, ADAPT-2, L-PLUS 1 and L-PLUS 2) were of high quality, being found to be at low risk of bias on all criteria.
| Study authors, year | Trial | Randomisation | Allocation concealment | Participant blinding | Blinding | Blinding of outcome assessors | Incomplete outcome | Selective reporting | Other biases | Criteria ‘low’ | Criteria ‘unclear’ | Criteria ‘high’ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terrault et al., 201837 | ADAPT–1 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 8 | 0 | 0 |
| Terrault et al., 201837 | ADAPT–2 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 8 | 0 | 0 |
| Hidaka et al., 201939 | L-PLUS 1 | Unclear risk | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | 4 | 4 | 0 |
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 8 | 0 | 0 |
| Tateishi et al., 201953 | JapicCTI-121944 | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | 7 | 1 | 0 |
| Terrault et al., 201451 | Study 202 | Low risk | Unclear risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | 6 | 2 | 0 |
Study characteristics
As shown in Table 3, all of the studies were multicentre, placebo-controlled, double-blind, parallel RCTs. Participation was restricted to adults. Three of these trials compared avatrombopag with placebo (Study 202,51 ADAPT-137 and ADAPT-237), and the other three trials compared lusutrombopag with placebo (L-PLUS 1,39 L-PLUS 254 and JAPIC CTI-12194453). Patients were recruited worldwide, with the exception of three studies: one of avatrombopag, Study 20251 (solely based in USA), and two of lusutrombopag, L-PLUS 139 and JAPIC CTI-12194453 (solely based in Japan). Time was limited to between 3 and 5 weeks. With the exception of Study 202,51 which was carried out in 2014, all studies were carried out in 2018 or later. 37,39,53,54 As shown in Table 6, the number of participants in individual arms of the included studies ranged from 15 to 108. The trials studying avatrombopag reported on a total of 467 participants and the trials studying lusutrombopag reported on a total of 342 participants.
| Trial name | Study authors, year | Countries | Number of centres | Age range (lower; upper) | Study start date | Study end date | Follow-up (weeks) | Intervention | Comparator | NCT/other trial number |
|---|---|---|---|---|---|---|---|---|---|---|
| Study 202 | Terrault et al., 201451 | USA | 27 | 18; NR | May 2009 | November 2011 | 5 | Avatrombopag | Placebo | NCT00914927; E5501-G000-202 |
| ADAPT-2 | Terrault et al., 201837 | Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Czech Republic, France, Germany, Hungary, Israel, Italy, Japan, Mexico, Republic of Korea, Romania, Russia, Poland, Portugal, Spain, Taiwan, Thailand, UK, USA | 74 | 18; NR | December 2013 | January 2017 | 5 | Avatrombopag | Placebo | NCT01976104 |
| ADAPT-1 | Terrault et al., 201837 | 75 | 18; NR | February 2014 | January 2017 | 5 | NCT01972529 | |||
| L-PLUS 1 | Hidaka et al., 201939 | Japan | 81 | 20; NR | October 2013 | May 2014 | 5 | Lusutrombopag | Placebo | JapicCTI-132323 |
| L-PLUS 2 | Peck-Radosavljevic et al., 201954 | Argentina, Australia, Austria, Belgium, Canada, Czech Republic, France, Germany, Hungary, Israel, Italy, Poland, Republic of Korea, Romania, Russia, Spain, Taiwan, Thailand, Turkey, Ukraine, UK, USA | 138 | 18; NR | June 2015 | April 2017 | 3 | Lusutrombopag | Placebo | NCT02389621 |
| JapicCTI-121944 | Tateishi et al., 201953 | Japan | 63 | 20; NR | August 2012 | April 2013 | 5 | Lusutrombopag | Placebo | JapicCTI-121944 |
Degree of thrombocytopaenia
As described in Table 4, all six studies restricted patients to those with a platelet count of < 50,000/µl. ADAPT 1 and ADAPT 237 differed from the other studies in that results were published only according to the subgroups < 40,000 and 40,000–< 50,000/µl, given the variation in dose of avatrombopag according to these subgroups. Given the need to compare lusutrombopag with avatrombopag, data in these subgroups were requested of Shionogi and are presented in Chapter 3, Results, Subgroup analyses.
| Trial name | Study authors, year | Population – liver disease | Study aim | Study conclusions | Inclusion criteria |
|---|---|---|---|---|---|
| Study 202 | Terrault et al., 201451 | Mixed | To investigate the efficacy and safety of avatrombopag (E5501), an investigational second-generation TPO-RA, administered 1 week prior to elective procedures to patients with thrombocytopenia secondary to CLD | Avatrombopag was generally well tolerated and increased platelet counts in patients with CLD undergoing elective invasive procedures | Age ≥ 18 years of age; thrombocytopenia (defined as a platelet count ≥ 10,000 to ≤ 50,000 (+ 15%)/mm3); Model for End-Stage Liver Disease (MELD) scores of ≤ 24; chronic liver diseases due to chronic viral hepatitis, NASH or alcoholic liver disease; scheduled to undergo an elective invasive procedure between 1 and 4 days post last dose of study drug; adequate renal function as evidenced by a calculated creatinine clearance ≥ 50 ml/minute per the Cockcroft and Gault formula; life expectancy ≥ 3 months |
| ADAPT-1 | Terrault et al., 201837 | Mixed | To evaluate the safety and efficacy of avatrombopag in increasing platelet counts in patients with thrombocytopenia and CLD undergoing scheduled procedures | In two Phase III randomised trials, avatrombopag was superior to placebo in reducing the need for platelet transfusions or rescue procedures for bleeding in patients with thrombocytopenia and CLD undergoing a scheduled procedure | CLD (MELD score 24); thrombocytopenia with a mean baseline platelet count of < 50,000/µl; scheduled to undergo a procedure with an associated risk of bleeding that would require a platelet transfusion, unless there was a clinically significant increase in platelet count from baseline |
| ADAPT-2 | |||||
| L-PLUS 1 | Hidaka et al., 201939 | Mixed | To evaluate the superiority of lusutrombopag over placebo in efficacy in thrombocytopenic patients with CLD receiving 3 mg of lusutrombopag as a pre-treatment for invasive procedures based in the proportion of patients who required no platelet transfusion prior to invasive procedures | In a placebo-controlled trial, lusutrombopag was effective in achieving and maintaining the target platelet count in patients with CLD and thrombocytopenia undergoing invasive procedures. No significant safety concerns were raised | Male or female patients aged ≥ 20 years; with thrombocytopenia associated with CLD; with a platelet count of < 50,000/µl; undergoing invasive procedures (excluding laparotomy, thoracotomy, craniotomy, open-heart surgery, organ resection or partial organ resection) between 9 and 14 days after initiation of study treatment; of Eastern Cooperative Oncology Group performance status grade 0 or 1; and agreeing to use an appropriate method of contraception during the study |
| L-PLUS 2 | Peck-Radosavljevic et al., 201954 | Mixed | To compare the efficacy of lusutrombopag with placebo for the treatment of thrombocytopenia in patients with CLD who are undergoing elective invasive procedures | None posted on ClinicalTrials.gov (L-Plus 2) | Able to understand the study and comply with all study procedures; willing to provide written informed consent prior to screening; male or female; ≥ 18 years of age at the time of signing informed consent; platelet count < 50,000/µl at baseline on day 1 prior to randomisation; undergoing an elective invasive procedure; in the opinion of the investigator, able to meet study requirements; male patients who are sterile or who agree to use an appropriate method of contraception (including use of a condom with spermicide) from screening to completion of the post-treatment period; female patients who are not postmenopausal or surgically sterile need to agree to use a highly effective contraception [including contraceptive implant, injectable contraceptive, combination hormonal contraceptive (including vaginal rings), intrauterine contraceptive device or vasectomised partner] from screening to completion of the post-treatment period. Barrier method with or without spermicide, double-barrier contraception and oral contraceptive pill are insufficient methods on their own |
| JapicCTI-121944 | Tateishi et al., 201953 | HCC | To estimate the appropriate dose and evaluate the efficacy and safety of lusutrombopag for the treatment of thrombocytopenia before percutaneous liver RFA for primary hepatic cancer in patients with CLD | Lusutrombopag 3 mg once per day for 7 days was effective without leading to concerns about excessive increases in platelet count | Men or women aged ≥ 20 years; thrombocytopenia CLD, platelet count of < 50,000/µl; undergoing RFA for primary hepatic carcinoma; Eastern Cooperative Oncology Group performance status grade 0 or 1; able to remain hospitalised between 5 and 14 days after the initiation of the study treatment |
Disease type
As shown in Table 4, in terms of the type of CLD reported by each study, one study53 reported including a single type of disease (hepatocellular carcinoma; JapicCTI-121944), whereas five studies37,39,51,54 reported on a mixed CLD population (ADAPT-1, ADAPT-2, L-PLUS 1, L-PLUS 2, Study 202). Three studies37,51 (ADAPT-1, ADAPT-2, Study 202) reported on a CLD definition based on a model for end-stage liver disease score of ≤ 24. Two studies39,54 (L-PLUS 1, L-PLUS 2) reported on a CLD definition based on Child–Pugh class A or B; of note, the exclusion criteria reported in the L-PLUS 1 study39 implied that inclusion was based on Child–Pugh class A or B, but this was not stated explicitly. By contrast, the percentage of participants in the ADAPT trials who were in Child–Pugh class C was above zero. 37 The proportion was generally low in ADAPT-137 (i.e. no higher than 8.6% in the avatrombopag arm of the 40,000–< 50,000/µl subgroup), although it was as high as 15.2% in the placebo arm of the same subgroup in ADAPT-2. 37
Elective procedure type
Elective procedures reported in each study were quite varied (Table 5). Only one study53 reported a single type of procedure (liver radiofrequency ablation; JapicCTI-121944). The other five studies37,39,51,54 reported including mixed types of elective procedures. Only ADAPT-1 and ADAPT-2 explicitly mentioned risk of bleeding, stating that they included both ‘low-risk’ procedures, for example liver biopsy, and ‘high-risk’ procedures, for example radiofrequency ablation. Both L-PLUS 139 and L-PLUS 2,54 also according to this definition, included mixed-risk procedures, such as liver biopsy and radiofrequency ablation.
| Procedure | ADAPT-137 | ADAPT-237 | L-PLUS 139 | L-PLUS 254 | JapicCTI-12194453 | Study 20251 | Number of RCTs reported |
|---|---|---|---|---|---|---|---|
| Argon plasma coagulation | No | No | Yes | No | No | No | 1 |
| Biliary interventions | Yes | Yes | No | No | No | No | 2 |
| Biopsy (renal) | Yes | Yes | No | No | No | No | 2 |
| Biopsy (bone marrow) | No | No | No | Yes | No | No | 1 |
| Biopsy (liver) | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Bronchoscopy | Yes | Yes | No | No | No | Yes | 3 |
| Catheterisation (heart) | No | No | No | No | No | Yes | 1 |
| Catheterisation (vascular) | Yes | Yes | No | No | No | Yes | 3 |
| Cervical polyp removal | No | No | No | Yes | No | No | 1 |
| Chemoembolisation | Yes | Yes | No | No | No | Yes | 3 |
| Colonoscopy | No | No | No | No | No | Yes | 1 |
| Colonoscopy plus endoscopy | No | No | No | No | No | Yes | 1 |
| Colonoscopy plus polypectomy | No | No | No | No | No | Yes | 1 |
| Cystoscopy and biopsy of urinary bladder | No | No | No | Yes | No | No | 1 |
| Dental extraction | No | No | No | Yes | No | No | 1 |
| Dental implant | No | No | No | Yes | No | No | 1 |
| Dental procedures | Yes | Yes | No | No | No | Yes | 3 |
| Periodontal scaling/root planning | No | No | No | No | No | Yes | 1 |
| EGD | No | No | No | No | No | Yes | 1 |
| EGD with banding | No | No | No | No | No | Yes | 1 |
| Endonasal maxillectomy | No | No | No | Yes | No | No | 1 |
| Endoscopic injection sclerosis/sclerotherapy | No | No | Yes | Yes | No | No | 2 |
| Endoscopic variceal ligation | No | No | Yes | Yes | No | No | 2 |
| Endoscopy | No | No | No | No | No | Yes | 1 |
| Endoscopy (gastrointestinal) – operative or diagnostic | No | No | No | Yes | No | No | 1 |
| Endoscopy (upper gastrointestinal) and chemoembolisation | No | No | No | No | No | Yes | 1 |
| Endoscopy with banding | No | No | No | No | No | Yes | 1 |
| Endoscopy with possible oesophageal banding | No | No | No | No | No | Yes | 1 |
| Ethanol ablation therapy | Yes | Yes | No | No | No | No | 2 |
| Hernia (inguinal) | No | No | No | Yes | No | No | 1 |
| Hernia repair (prosthetic inguinal) | No | No | No | Yes | No | No | 1 |
| Hernia repair (umbilical) | No | No | No | No | No | Yes | 1 |
| Laparocentesis (diagnostic) | No | No | No | Yes | No | No | 1 |
| Laparoscopy (any) | Yes | Yes | No | No | No | No | 2 |
| Liver-related procedures | No | No | No | Yes | No | No | 1 |
| Mastoidectomy/tympanoplasty | No | No | No | Yes | No | No | 1 |
| Nephrostomy tube placement | Yes | Yes | No | No | No | No | 2 |
| Paracentesis | No | No | No | No | No | Yes | 1 |
| Paracentesis (diagnostic) | No | No | No | Yes | No | No | 1 |
| Percutaneous ethanol injection therapy | No | No | Yes | No | No | No | 1 |
| Percutaneous RFA/microwave coagulation therapy | No | No | No | Yes | No | No | 1 |
| Pleurocentesis/pleural biopsy | No | No | No | No | No | Yes | 1 |
| RFA | Yes | Yes | Yes | No | Yes | Yes | 5 |
| Septoplasty | No | No | No | Yes | No | No | 1 |
| Splenic artery aneurysm embolisation | No | No | No | Yes | No | No | 1 |
| Thoracentesis (diagnostic) | No | No | No | Yes | No | No | 1 |
| Transcatheter arterial chemoembolisation | No | No | Yes | Yes | No | Yes | 3 |
| TIPS | Yes | Yes | No | No | No | Yes | 3 |
| Trial name | Study authors, year | Trial number | Arm name | Population – liver disease | Lower/upper platelets | Number of patients randomised to study arm | Mean age (years) | SD (years) | Age range (lower; upper) | Male (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Study 202 | Terrault et al., 201451 | NCT00914927; E5501-G000-202 | Avatrombopag 40 mg | Mixed | 10,000–50,000 | 16 | 52.8 | 7.78 | NR; NR | 81.3 |
| Placebo | 16 | 54.2 | 6.87 | NR; NR | 68.8 | |||||
| ADAPT-1 | Terrault et al., 201837 | NCT01972529 | Avatrombopag 40 mg | 40,000–50,000 | 59 | 57.5 | 10.1 | 19; 77 | 62.7 | |
| Placebo 40 mg | 34 | 57.8 | 11.1 | 30; 76 | 70.6 | |||||
| Avatrombopag 60 mg | < 40,000 | 90 | 55.6 | 9.1 | 29; 78 | 72.2 | ||||
| Placebo 60 mg | 48 | 55.1 | 11 | 25; 76 | 66.7 | |||||
| ADAPT-2 | NCT01976104 | Avatrombopag 40 mg | 40,000–50,000 | 58 | 57.9 | 11.1 | 29; 77 | 56.9 | ||
| Placebo 40 mg | 33 | 59.2 | 10.3 | 39; 81 | 51.5 | |||||
| Avatrombopag 60 mg | < 40,000 | 70 | 58.6 | 14.2 | 20; 86 | 71.4 | ||||
| Placebo 60 mg | 43 | 57.3 | 12 | 27; 77 | 62.8 | |||||
| L-PLUS 1 | Hidaka et al., 201839 | JapicCTI-132323 | Lusutrombopag 3 mg | < 50,000 | 48 | 68.9 | 6.6 | 51; 40 | 43.8 | |
| Placebo | 48 | 66.8 | 10.2 | 81; 88 | 62.5 | |||||
| L-PLUS 2 | Peck-Radosavljevic et al., 201954 | NCT02389621 | Lusutrombopag 3 mg | < 50,000 | 108 | 55.2 | 11.6 | NR; NR | 60.2 | |
| Placebo | 107 | 56.1 | 11 | NR; NR | 64.5 | |||||
| JapicCTI-121944 | Tateishi et al., 201953 | JapicCTI-121944 | Lusutrombopag 3 mg | HCC | < 50,000 | 16 | 66.8 | 8.1 | NR; NR | 56.3 |
| Placebo | 15 | 70.9 | 8.6 | NR; NR | 53.3 |
Decision rule for determining treatment dose
There appeared to be some variation in the decision rules for administering platelet transfusion prior to the elective procedure. The L-PLUS39,54 studies mandated this on the basis of a drop in platelet count below the 50,000/µl threshold, whereas this rule was not explicitly reported in the ADAPT trials. 37 However, because those eligible for the ADAPT studies37 were at ‘. . . risk of bleeding that would require a platelet transfusion, unless there was a clinically significant increase in platelet counts from baseline’, it seems likely that the same rule would be applied. There was also a difference in the decision rules for administering the intervention. In the ADAPT trials,37 all patients received avatrombopag for 5 days, whereas in the L-PLUS trials39,54 lusutrombopag was administered for between 5 and 7 days depending on platelet count (i.e. if the platelet count was at least 50 × 109/l with an increase of at least 20 × 109/l then no additional dose was given). The implication of this difference is that lusutrombopag was administered, on average, over a longer period than avatrombopag.
Assessment of effectiveness
Not all studies had precisely the same primary outcome (Table 7). In two studies39,53 (JapicCTI-12194453 and L-PLUS 139) the proportion of patients who did not require platelet transfusion before the elective procedure was the primary outcome. Three studies (ADAPT-1,37 ADAPT-237 and L-PLUS 254) reported a composite outcome of the proportion of patients who did not require platelet transfusion or a rescue procedure for bleeding from randomisation up to 7 days following the elective procedure as the primary outcome. One study51 (Study 202) reported the percentage of participants with an increase in platelet count of ≥ 20,000/µl above baseline and at least one platelet count of > 50,000/µl from days 4 to 8 as the primary outcome.
| Intervention | Trial name | Study authors, year | Primary outcome |
|---|---|---|---|
| Lusutrombopag | L-PLUS 1 | Hidaka et al., 201839 | Proportion of patients who did not require platelet transfusion prior to the primary invasive procedure |
| L-PLUS 2 | Peck-Radosavljevic et al., 201954 | Percentage of patients who did not require platelet transfusion prior to the primary invasive procedure and no rescue therapy for bleeding from randomisation to 7 days after the primary elective procedure | |
| JapicCTI-121944 | Tateish et al., 201953 | Proportion of patients who did not require platelet transfusion prior to the primary invasive procedure | |
| Avatrombopag | Study 202 | Terrault et al., 201451 | Proportion of participants with an increase in platelet count ≥ 20 × 109/l above baseline; and at least one platelet count > 50 × 109/l from days 4 to 8 |
| ADAPT-1, ADAPT-2 | Terrault et al., 201837 | Proportion of patients who did not require platelet transfusion or rescue procedure for bleeding after randomisation and up to 7 days after a scheduled procedure |
Despite the differences in primary outcome, both avatrombopag (for both platelet subgroups) and lusutrombopag were clearly effective in comparison with no TPO-RA in terms of the primary outcome (Table 8). 37,54 The difference between the intervention and comparator groups in the proportion of patients receiving neither platelet transfusion nor rescue therapy following procedure was generally greater for avatrombopag at any dose than for lusutrombopag, the only exception being in ADAPT-237 in the < 40,000/µl subgroup where the difference was lowest. However, it should be noted that the extent to which the outcomes in the two sets of trials are comparable is unclear. There appears to be a difference in the timing of measurements of platelet transfusion avoided, with the JapicCTI-12194453 and L-PLUS 139 studies specifying that this was prior to the elective procedure and the ADAPT-137 and L-PLUS 254 studies specifying that it was up to 7 days following randomisation. As the primary outcome is also a composite of the number of platelet transfusions and the number of rescue procedures in the ADAPT-137 and L-PLUS 254 studies, the independent contributions of these two variables are also unclear. As shown in Table 9, lusutrombopag was effective in both the international study,54 L-PLUS 2, and the Japanese study,39 L-PLUS 1, in avoiding platelet transfusion. However, no such data were reported in the ADAPT trials37 and no data were reported for rescue procedure separately for either TPO-RA. However, as described in Chapter 3, Results, Subgroup analyses, these data were obtained by request for clarification. 56,57
| Study authors, year – trial name | Outcome | Lower/upper platelets (per µl) | Arm name | n | % with event | Size of effect | 95% CI | p-value | Arm favoured |
|---|---|---|---|---|---|---|---|---|---|
| Terrault et al., 201837 – ADAPT-1 | Percentage difference in patients who did not require a platelet transfusion or rescue procedure for bleeding after randomisation and up to 7 days after a scheduled procedure | < 40,000 | Avatrombopag 60 mg | 90 | 65.6 | 42.6 | 27.2 to 58.1 | < 0.0001 | Avatrombopag 60 mg |
| Placebo 60 mg | 48 | 22.9 | |||||||
| 40,000–< 50,000 | Avatrombopag 40 mg | 59 | 88.1 | 49.9 | 31.6 to 68.2 | < 0.0001 | Avatrombopag 40 mg | ||
| Placebo 40 mg | 34 | 38.2 | |||||||
| Terrault et al., 201837 – ADAPT-2 | < 40,000 | Avatrombopag 60 mg | 70 | 68.6 | 33.7 | 15.8 to 51.6 | 0.0006 | Avatrombopag 60 mg | |
| Placebo 60 mg | 43 | 34.9 | |||||||
| 40,000–< 50,000 | Avatrombopag 40 mg | 58 | 87.9 | 54.6 | 36.5 to 72.7 | < 0.0001 | Avatrombopag 40 mg | ||
| Placebo 40 mg | 33 | 33.3 | |||||||
| Peck-Radosavljevic et al., 201954 – L-PLUS 2 | Percentage difference in participants who required no platelet transfusion prior to the primary invasive procedure and no rescue therapy for bleeding from randomisation through 7 days after the primary elective procedure | < 50,000 | Lusutrombopag | 108 | 64.8 | 36.7 | 24.9 to 48.5 | < 0.0001 | Lusutrombopag |
| Placebo | 107 | 29.0 |
| Study authors, year – trial name | Outcome | Arm name | Time (weeks) | n | % with event | Type of effect size | Size of effect | 95% CI | p-value | Arm favoured |
|---|---|---|---|---|---|---|---|---|---|---|
| Hidaka et al., 201839 – L-PLUS 1 | Proportion of patients who received no platelet transfusion during the study | Lusutrombopag | NR | 48 | 79.2 | RRa | 6.16 | 2.92 to 13.00 | < 0.0001 | Lusutrombopag |
| Placebo | 48 | 12.5 | ||||||||
| Peck-Radosavljevic et al., 201954 – L-PLUS 2 | Percentage of participants who required no platelet transfusion during the study | Lusutrombopag | 5 | 108 | 63 | Difference | 34.8 | 22.8 to 46.8 | < 0.0001 | Lusutrombopag |
| Placebo | 5 | 107 | 29 | |||||||
| Tateishi et al., 201953 – JapicCTI-121944 | Proportion of patients who received no platelet transfusion prior to RFA | Lusutrombopag 3mg | NR | 16 | 81.2 | NR | NR | NR | NR | |
| Placebo | NR | 15 | 20 | NR | NR | NR | NR |
Both avatrombopag and lusutrombopag were reported to increase the proportion of patients with increased platelet counts, as shown in Table 10, in terms of the primary outcome for Study 202. 51 For lusutrombopag, this was observed in both of the L-PLUS trials. 39,54 It was also observed in the Japanese study53 in patients with hepatocellular carcinoma. The ADAPT trials37 did not use this outcome, but avatrombopag was shown to be effective in achieving the target platelet level of 50 × 109/µl.
| Study authors, year – trial name | Arm name | Time (weeks) | n | % with event | Type of effect size | Size of effect | 95% CI | p-value | Arm favoured |
|---|---|---|---|---|---|---|---|---|---|
| Tateishi R, et al., 201953 – JapicCTI-121944 | Lusutrombopag | 5 | 16 | 68.8 | NR | NR | NR | NR | Lusutrombopag |
| Placebo | 5 | 15 | 6.7 | NA | NA | NA | NA | ||
| Terrault et al., 201451 – Study 202 | Avatrombopag 40mg | 1 | 16 | 31.3 | NR | NR | NR | 0.1719 | Avatrombopag 40 mg |
| Placebo | 1 | 16 | 6.3 | NA | NA | NA | NA | ||
| Hidaka et al., 201839 – L-PLUS 1 | Lusutrombopag | NR | 48 | 77.1 | RR | 11.9 | 4 to 35.4 | < 0.0001 | Lusutrombopag |
| Placebo | 48 | 6.3 | NA | NA | NA | NA | |||
| Peck-Radosavljevic et al., 201954 – L-PLUS 2 | Lusutrombopag | 5 | 108 | 64.8 | Difference | 52.5 | 42 to 62.9 | < 0.0001 | Lusutrombopag |
| Placebo | 5 | 107 | 13.1 | NA | NA | NA | NA |
Safety
As shown in Table 11, neither avatrombopag nor lusutrombopag was unequivocally better than no TPO-RA in terms of adverse events (AEs). In particular, L-PLUS 254 showed a higher percentage of deaths with lusutrombopag (3/107; 2.8%) than with placebo (0/107; 0%). However, it was judged by the investigator that none of these deaths was related to treatment with lusutrombopag. Indeed, one death was a result of a protocol violation in a patient with Child–Pugh class C liver disease, which does imply a much higher mortality rate. The second patient died from a progression of hepatic cirrhosis, and the third patient died because of procedurally related vessel perforation. ADAPT–137 also showed more deaths with avatrombopag 40 mg in the 40,000–< 50,000/µl subgroup, although, again, the investigator deemed that these deaths were not associated with the study drug. One patient suffered from hepatic coma, which was due to underlying cirrhosis, and the other patient was stated to have died from multiorgan system failure. However, the clinical study report (CSR) revealed that the individual had suffered a bleeding event: ‘Bleeding oesophageal varices/Oesophageal varices’. 58 On the other hand, there was only one death in this subgroup in ADAPT-237 and this was in the placebo arm. There were no deaths in the < 40,000/µl subgroup.
| Main category | Study authors, year | Trial name | Trial number | Lower/upper platelets (per µl) | Follow-up time point (weeks) | Arm name | Number of patients with event | Number of patients analysed or ‘NR’ | % with event or ‘NR’ |
|---|---|---|---|---|---|---|---|---|---|
| Any death | Hidaka, et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | NR/unclear | Lusutrombopag | 0 | 48 | 0.0 |
| Placebo | 0 | 48 | 0.0 | ||||||
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | NR/unclear | Lusutrombopag | 3 | 107 | 2.8 | |
| Placebo | 0 | 107 | 0.0 | ||||||
| Tateishi et al., 201953 | NR | JapicCTI-121944 | < 50,000 | NR/unclear | Lusutrombopag | 0 | 16 | 0.0 | |
| Placebo | 0 | 15 | 0.0 | ||||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 0 | 89 | 0.0 | |
| Placebo 60 mg | 0 | 48 | 0.0 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 2 | 58 | 3.4 | ||||
| Placebo 40 mg | 0 | 32 | 0.0 | ||||||
| ADAPT-2 | NCT01976104 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 0 | 70 | 0.0 | ||
| Placebo 60 mg | 0 | 43 | 0.0 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 0 | 57 | 0.0 | ||||
| Placebo | 1 | 33 | 3.0 | ||||||
| Terrault et al., 201451 | Study 202 | NCT00914927; E5501-G000–202 | < 50,000 | NR/unclear | Avatrombopag 40 mg | 0 | 16 | 0.0 | |
| Placebo | 0 | 16 | 0.0 | ||||||
| Any serious adverse event | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | NR/unclear | Lusutrombopag | 1 | 48 | 2.1 |
| Placebo | 4 | 48 | 8.3 | ||||||
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | NR/unclear | Lusutrombopag | 7 | 107 | 6.5 | |
| Placebo | 7 | 107 | 6.5 | ||||||
| Tateishi et al., 201953 | NR | JapicCTI-121944 | < 50,000 | 5 | Lusutrombopag | 1 | 16 | 6.3 | |
| Placebo | 1 | 15 | 6.7 | ||||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 10 | 89 | 11.2 | |
| Placebo 60 mg | 11 | 48 | 22.9 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 8 | 58 | 13.8 | ||||
| Placebo 40 mg | 1 | 32 | 3.1 | ||||||
| ADAPT-2 | NCT01976104 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 1 | 70 | 1.4 | ||
| Placebo 60 mg | 1 | 43 | 2.3 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 1 | 57 | 1.8 | ||||
| Placebo 40 mg | 1 | 33 | 3.0 | ||||||
| Drug withdrawal/discontinuation AE | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | NR/unclear | Lusutrombopag | 0 | 107 | 0.0 |
| Placebo | 1 | 107 | 0.9 | ||||||
| Tateishi et al., 201953 | NR | JapicCTI-121944 | < 50,000 | 5 | Lusutrombopag | 0 | 16 | 0.0 | |
| Placebo | 0 | 15 | 0.0 | ||||||
| Terrault et al., 201451 | Study 202 | NCT00914927; E5501-G000–202 | 10,000–50,000 | 6 | Avatrombopag 40 mg | 0 | 16 | 0.0 | |
| Placebo | 0 | 16 | 0.0 | ||||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 2 | 89 | 2.2 | |
| Placebo 60 mg | 0 | 48 | 0.0 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | 0 | 32 | 0.0 | ||||||
| ADAPT-2 | NCT01976104 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 0 | 70 | 0.0 | ||
| Placebo 60 mg | 0 | 43 | 0.0 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 0 | 57 | 0.0 | ||||
| Placebo 40 mg | 0 | 33 | 0.0 | ||||||
| Any AE | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | NR/unclear | Lusutrombopag | 45 | 48 | 93.8 |
| Placebo | 48 | 48 | 100.0 | ||||||
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | NR/unclear | Lusutrombopag | 51 | 107 | 47.7 | |
| Placebo | 52 | 107 | 48.6 | ||||||
| Tateishi et al., 201953 | NR | JapicCTI-121944 | < 50,000 | 5 | Lusutrombopag | 16 | 16 | 100.0 | |
| Placebo | 15 | 15 | 100.0 | ||||||
| Terrault et al., 201451 | Study 202 | NCT00914927; E5501-G000–202 | 10,000–50,000 | 6 | Avatrombopag 40 mg | 11 | 13 | 84.6 | |
| Placebo | 9 | 12 | 75.0 | ||||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 53 | 89 | 59.6 | |
| Placebo 60 mg | 31 | 48 | 64.6 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 31 | 58 | 53.4 | ||||
| Placebo 40 mg | 18 | 32 | 56.3 | ||||||
| ADAPT-2 | NCT01976104 | < 40,000 | NR/unclear | Avatrombopag 60 mg | 36 | 70 | 51.4 | ||
| Placebo 60 mg | 22 | 43 | 51.2 | ||||||
| 40,000–< 50,000 | NR/unclear | Avatrombopag 40 mg | 28 | 57 | 49.1 | ||||
| Placebo 40 mg | 15 | 33 | 45.5 |
The outcome with regard to serious adverse events (SAEs) was a little more favourable towards lusutrombopag, with more SAEs reported in the placebo arm in L-PLUS 139 and equal percentages in L-PLUS 2. 54 The outcome for avatrombopag was mixed; there were higher percentages of SAEs in the placebo arm, except in the 40,000–< 50,000/µl subgroup in ADAPT-1,37 where this was reversed. Discontinuation as a result of AEs was reported only in the < 40,000/µl subgroup in ADAPT-137 for avatrombopag (2/89; 2.2%) compared with placebo (0/48; 0%). There was no clear difference in the percentage of AEs (of any severity) between TPO-RAs and no TPO-RA. 37,39,51,53,54 Specific SAEs were too rare to allow any inference to be made about the effect of the intervention (see Appendix 3).
Subgroup analyses
As the dose of avatrombopag varies by platelet count, to make a comparison between avatrombopag and lusutrombopag the outcomes needed to be estimated by subgroup analysis. Therefore, the assessment group (AG) requested these data from Shionogi and they were provided in its response. They were first used to estimate the RRs versus placebo, which are summarised in Tables 12–15. What can be observed is that, for both subgroups, both avatrombopag and lusutrombopag are superior to placebo and mostly with a statistically significant difference [i.e. 95% confidence intervals (CIs) do not overlap the point of no difference], the only exception being for the very small JapicCTI-121944 study. 53 This interpretation does not vary with the use of the OR scale (see Appendix 4). Study 20251 was excluded from these analyses, and therefore from those reported in Chapter 3, Results, Meta-analysis, because of the lack of collection of the necessary data, as revealed in the CSR. 59
| Study | Arm name | n/Na | Patients with event (%) | RR of lusutrombopag 3 mg vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Subgroup with baseline platelet count 40,000–< 50,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Study | Arm name | n/Na | Patients with event (%) | RR of lusutrombopag 3 mg vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Subgroup with baseline platelet count 40,000–< 50,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | 100.0 | 3.75 (1.26 to 11.13) |
| Placebo | Confidential information has been removed | 22.2 | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Study | Arm name | n/N | Patients with event (%) | RR of avatrombopag vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| ADAPT-137 | Avatrombopag 60 mg | 59/90 | 65.6 | 2.86 (1.67 to 4.91) |
| Placebo | 11/48 | 22.9 | ||
| ADAPT-237 | Avatrombopag 60 mg | 48/70 | 68.6 | 1.97 (1.27 to 3.05) |
| Placebo | 15/43 | 34.9 | ||
| Subgroup with baseline platelet count 40,000–< 50,000/µl | ||||
| ADAPT-137 | Avatrombopag 40 mg | 52/59 | 88.1 | 2.31 (1.49 to 3.57) |
| Placebo | 13/34 | 38.2 | ||
| ADAPT-237 | Avatrombopag 40 mg | 51/58 | 87.9 | 2.64 (1.61 to 4.31) |
| Placebo | 11/33 | 33.3 | ||
| Study | Arm name | n/N | Patients with event (%) | RR of avatrombopag vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| ADAPT-137 | Avatrombopag 60 mg | 71/90 | 78.9 | 1.46 (1.10 to 1.93) |
| Placebo | 26/48 | 54.2 | ||
| ADAPT-237 | Avatrombopag 60 mg | 58/70 | 82.9 | 1.62 (1.19 to 2.21) |
| Placebo | 22/43 | 51.2 | ||
| Subgroup with baseline platelet count 40,000–< 50,000/µl | ||||
| ADAPT-137 | Avatrombopag 40 mg | 55/59 | 93.2 | 1.86 (1.32 to 2.63) |
| Placebo | 17/34 | 50.0 | ||
| ADAPT-237 | Avatrombopag 40 mg | 55/58 | 94.8 | 1.74 (1.27 to 2.39) |
| Placebo | 18/33 | 54.5 | ||
In addition to these outcomes, the proportions of those who required no rescue therapy who received platelet transfusion were estimated, and these are shown in Tables 20 and 21. These numbers were calculated by dividing the number who had received neither platelet transfusion nor rescue therapy by the number who had received no platelet transfusion prior to the elective procedure, and show that the lusutrombopag trials differ from the avatrombopag trials in frequency of rescue therapy, regardless of treatment arm. The explanation for this is not obvious. Very few patients received rescue therapy in the lusutrombopag trials: only two patients and only in the 40,000–< 50,000/µl subgroup. In addition, the only type of rescue other than platelet transfusion was red blood cells. 56 This contrasts with the ADAPT trials,37 in which as few as 42.3% patients did not receive rescue therapy and, in addition, any of the following rescue therapies was administered:
-
platelet
-
whole blood, or packed red cell transfusions
-
plasma
-
cryoprecipitate
-
vitamin K
-
desmopressin
-
recombinant activated factor VII
-
aminocaproic acid
-
tranexamic acid
-
surgical intervention
-
interventional radiology.
Regardless of the difference in absolute risk, Table 16 shows that there is no statistically significant difference between lusutrombopag and placebo. However, there is a difference for avatrombopag in the < 40,000/µl subgroup of ADAPT-137 and the 40,000–< 50,000/µl subgroup of ADAPT-237 (Table 17). This interpretation is similar with the use of the OR scale, although the OR for lusutrombopag in the < 40,000/µl subgroup is not estimable and there is also a statistically significant difference for avatrombopag in both ADAPT trials37 in the 40,000–< 50,000/µl subgroup (see Appendix 4).
| Study | Arm name | n/Na | Patients with event (%) | RR of lusutrombopag 3 mg vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Subgroup with baseline platelet count 40,000–< 50,000/µl | ||||
| JapicCTI-12194453 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 139 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| L-PLUS 254 | Lusutrombopag 3 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | ||
| Study | Arm name | n/N | Patients with event (%) | RR of avatrombopag vs. placebo (95% CI) |
|---|---|---|---|---|
| Subgroup with baseline platelet count < 40,000/µl | ||||
| ADAPT-137 | Avatrombopag 60 mg | 59/71 | 83.1 | 1.96 (1.24 to 3.11) |
| Placebo | 11/26 | 42.3 | ||
| ADAPT-237 | Avatrombopag 60 mg | 48/58 | 82.8 | 1.21 (0.89 to 1.65) |
| Placebo | 15/22 | 68.2 | ||
| Subgroup with baseline platelet count 40,000/µl–< 50,000/µl | ||||
| ADAPT-137 | Avatrombopag 40 mg | 52/55 | 94.5 | 1.24 (0.94 to 1.62) |
| Placebo | 13/17 | 76.5 | ||
| ADAPT-237 | Avatrombopag 40 mg | 51/55 | 92.7 | 1.52 (1.04 to 2.21) |
| Placebo | 11/18 | 61.1 | ||
The proportion of those who received no rescue therapy given receipt of platelet transfusion was not available to the AG.
Meta-analysis
In the absence of head-to-head clinical trials of avatrombopag and lusutrombopag, the indirect comparison approach was used to assess the relative effect of these treatment interventions. On the basis of the published trials, placebo was used as the common comparator. As the dose of avatrombopag varies by platelet count, subgroup analyses were performed. Forest plots of each of the interventions compared with placebo are presented in Appendix 4.
As shown in Tables 18 and 19, the outcome on the RR scale was a little more favourable towards lusutrombopag in both outcomes that counted platelet transfusions prior to the elective procedure in all cases regardless of therapies required prior to the procedure and regardless of the subgroups. Only one statistically significant difference was identified between avatrombopag and lusutrombopag. This was in a fixed-effect analysis of the ratio of patients who required no platelet transfusion prior to elective procedure in the subgroup in which patients’ baseline platelet count was < 40,000/µl. It was in favour of lusutrombopag (RR 1.93, 95% CI 1.15 to 3.22). On the OR scale, there was no statistically significant difference in any subgroup, although there was a reversal in the point estimate to an advantage for avatrombopag in the 40,000–< 50,000/µl subgroup in terms of both outcomes.
| Comparison | Type of effect | RR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) | OR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) |
|---|---|---|---|
| Platelet count < 40,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 60 mg | Fixed | 1.29 (0.722 to 2.31) | 1.22 (0.49 to 3.06) |
| Random | 1.63 (0.61 to 4.37) | 2.03 (0.37 to 11.20) | |
| Platelet count 40,000–< 50,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 40 mg | Fixed | 1.02 (0.62 to 1.66) | 0.59 (0.21 to 1.68) |
| Random | 1.13 (0.61 to 2.11) | 0.68 (0.20 to 2.39) | |
| Comparison | Type of effect | RR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) | OR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) |
|---|---|---|---|
| Platelet count < 40,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 60 mg | Fixed | 1.93 (1.15 to 3.22) | 1.68 (0.67 to 4.20) |
| Random | 2.43 (0.95 to 6.27) | 2.77 (0.50 to 15.36) | |
| Platelet count 40,000/µl–< 50,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 40 mg | Fixed | 1.31 (0.86 to 2.01) | 0.53 (0.17 to 1.68) |
| Random | 1.62 (0.63 to 4.18) | 0.68 (0.15 to 3.12) | |
By contrast, Table 20 shows an advantage of avatrombopag in terms of avoidance of rescue therapy, but, again, this is not statistically significant except in the fixed-effect analysis in the < 40,000/µl subgroup. On the OR scale, the value for the < 40,000/µl subgroup was not estimable and, as for the RR scale and the other outcomes, there was an advantage for avatrombopag in the 40,000–< 50,000/µl subgroup.
| Comparison | Type of effect | RR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) | OR of lusutrombopag 3 mg vs. avatrombopag 60 mg/40 mg (95% CI) |
|---|---|---|---|
| Platelet count < 40,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 60 mg | Fixed | 0.71 (0.54 to 0.93) | Not estimablea |
| Random | 0.67 (0.41 to 1.08) | Not estimablea | |
| Platelet count 40,000–< 50,000/µl | |||
| Lusutrombopag 3 mg vs. avatrombopag 40 mg | Fixed | 0.81 (0.62 to 1.05) | 0.53 (0.04 to 6.87) |
| Random | 0.81 (0.62 to 1.05) | 0.53 (0.04 to 6.87) | |
Heterogeneity
There was clinical heterogeneity in terms of invasive procedures that patients were undergoing. In both of the L-PLUS trials39,54 patients were not restricted to the elective procedure, whereas in the study by Tateishi et al. 53 only patients who were undergoing radiofrequency ablation were included. However, sensitivity analysis by exclusion of this study increased the heterogeneity in all cases. In addition, there was moderate statistical heterogeneity within each subgroup regardless of the outcome, for example for no platelet transfusion prior to the elective procedure I2 = 53% and 34% in the < 40,000/µl and 40,000–< 50,000/µl subgroups, respectively (see Appendix 4). Sensitivity analysis revealed that the removal of one of the L-PLUS studies would remove this heterogeneity and reduce the I2 to 0%. However, the study that needed to be removed to reduce the heterogeneity depended on the subgroup. More specifically, it was the L-PLUS 1 study39 in the < 40,000/µl subgroup and the L-PLUS 2 study54 in the 40,000–< 50,000/µl subgroup. Most importantly, this did not make any substantial change to the results.
For no rescue therapy, there was no statistical heterogeneity in the L-PLUS trials,39,54 but there was moderate heterogeneity in the < 40,000/µl subgroup. Nevertheless, given no obvious clinical difference between the ADAPT-1 and ADAPT-2 studies,37 the AG did not consider that exclusion of either was warranted. As already discussed in Results, Subgroup analyses, the lusutrombopag trials also appear to be quite different from the ADAPT trials in the much lower frequency of rescue therapy, regardless of treatment arm. This highlights that caution needs to be exercised in comparing avatrombopag with lusutrombopag.
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of avatrombopag and lusutrombopag for treating thrombocytopenia in people with CLD needing an elective procedure.
For this purpose, in Systematic review of existing cost-effectiveness evidence, the systematic review of the existing cost-effectiveness, cost/resource use and HRQoL evidence is summarised. In Review of the company evidence, the summary and critique of the industry submissions to NICE on the cost-effectiveness of avatrombopag and lusutrombopag are provided. Finally, in Independent economic assessment, the AG provides its own independent economic assessment on the cost-effectiveness of avatrombopag and lusutrombopag.
Systematic review of existing cost-effectiveness evidence
Search methods
The literature searches described in Chapter 3, Methods for reviewing effectiveness, Identification of studies, were used to identify cost-effectiveness studies. Identified cost-effectiveness studies were critically assessed using a published critical appraisal checklist for economic evaluations. 60
Additional searches were conducted to identify HRQoL and resource use data related to thrombocytopenia. Methodological search filters designed to identify HRQoL and resource use data were combined with search terms for thrombocytopenia. The search strategies were developed using the same methods described in Chapter 3, Methods for reviewing effectiveness, Identification of studies. Searches were not limited by language, publication status (i.e. unpublished or published) or date of publication.
Full details of the search strategies are presented in Appendix 1.
The following databases and resources were searched:
-
MEDLINE (via Ovid) – 1946–week 3 2019
-
MEDLINE In-Process Citations, Daily Update and Epub Ahead of Print (via Ovid) – 22 January 2019
-
PubMed (via National Library of Medicine) – up to 24 January 2019
-
EMBASE (via Ovid) – 1974 to week 3 2019
-
NHS EED (via CRD) – up to 31 March 2015
-
HTA database (via CRD) – up to 31 March 2018
-
Science Citation Index (SCI) (via Web of Science) – 1988–23 January 2019
-
CINAHL (via EBSCOhost) – 1982–23 January 2019
-
LILACS (via BIREME) – 1982–24 January 2019
-
Northern Light Life Sciences Conference Abstracts (via Ovid) – 2010–19/week 2
-
CEA Registry (www.cearegistry.org) – up to 24 January 2019
-
ScHARR Health Utilities Database (www.scharrhud.org/) – up to 24 January 2019.
Grey literature was identified from searches of the following resources:
-
OAIster (https://oaister.worldcat.org/) – up to 23 January 2019
-
OpenGrey (www.opengrey.eu/) – up to 23 January 2019
-
Copac (https://copac.jisc.ac.uk/) – up to 23 January 2019
-
ISPOR (www.ispor.org/) – up to 23 January 2019
-
HTAi (https://htai.org/).
Supplementary searches were conducted to identify data to help populate the economic model:
-
PubMed search for National Institute for Health Research (NIHR) Health Technology Assessment reports with similar economic models
-
literature searches to identify rates of procedures with bleeding risk in patients with CLD
-
literature searches to identify UK mortality data associated with platelet transfusion
-
literature searches to identify platelet transfusion refractoriness studies
-
literature searches to identify CLD/thrombocytopenia cost of illness studies.
Handling of citations
Identified references were downloaded into EndNote bibliographic management software for further assessment and handling. Individual records in the EndNote library were tagged with searching information, such as searcher, date searched, database host, database searched, strategy name and iteration, and theme or search question. This enabled the information specialist to track the origin of each individual database record and its progress through the screening and review process.
Quality assurance within the search process
For all searches undertaken by the KSR information team, the main EMBASE strategy was independently peer reviewed by a second KSR information specialist. The search strategy peer review was informed by items based on the Canadian Agency for Drugs and Technologies in Health checklist. 11,12
Inclusion criteria
Table 21 presents an overview of the inclusion criteria used for the review.
| Criterion | Inclusion |
|---|---|
| Patients | Studies including CLD adult (aged ≥ 18 years) patients with thrombocytopenia, eligible for elective surgery |
| Interventions | No restrictions |
| Comparators | No restrictions |
| Outcomes |
|
| Geography | No restrictions |
| Language | English only |
Results
The cost-effectiveness search identified 3518 records. However, none of the identified records fulfilled the inclusion criteria. The potentially relevant studies (n = 5) were economic evaluation studies in other populations [e.g. interferon-based treatment-induced thrombocytopenia of patients with hepatitis C virus (HCV)], and these were excluded after full-text screening.
The HRQoL search identified 2429 records. However, none of the identified records fulfilled the inclusion criteria; all of these records were excluded during title/abstract screening.
The resource use/costs search identified 5358 records, from which seven studies fulfilled the inclusion criteria. Three of these studies were available only as conference abstracts,29,61,62 whereas the other four were available as full texts; these are summarised in Identified resource use/costs studies.
The PRISMA flow diagrams in Figure 2 depict the flow of the studies through the cost-effectiveness, HRQoL and resource use/costs search processes.
FIGURE 2.
The PRISMA flow chart for (a) cost-effectiveness, (b) HRQoL and (c) resource use/cost searches. Data from the systematic literature review performed by the AG.
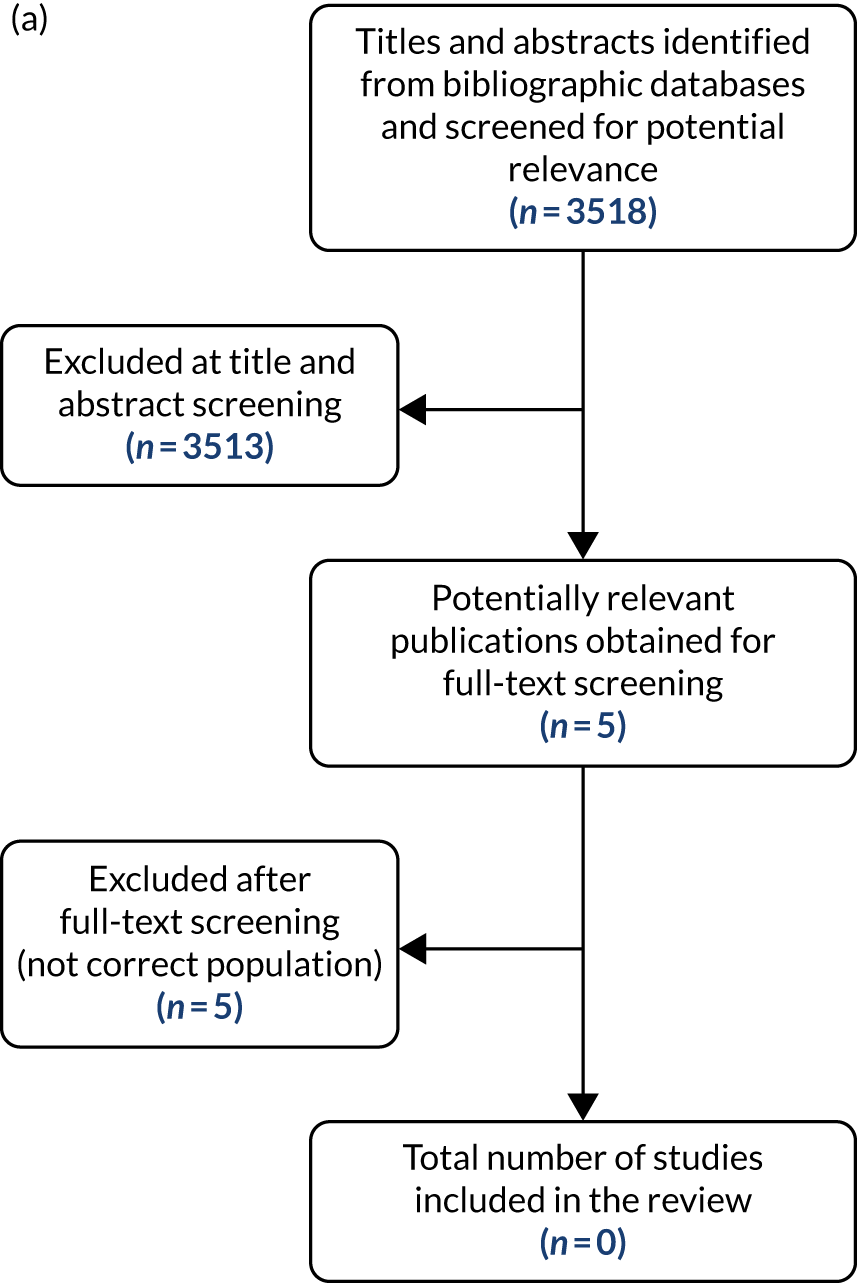
Identified resource use/costs studies
The systematic review of resource use/costs identified four full-text articles63–66 and three conference abstracts,29,61,62 discussing five separate studies. Two of the conference abstracts have since been published as full-text publications (the Poordad 2007 abstract61 corresponds to the Poordad et al. 201263 article and the Poordad et al. 2008 abstract62 is covered by the Poordad et al. 2011 article64), and, therefore, only the full-text publications of these studies are discussed. For the remaining conference abstract, no full-text publication was available and therefore only the content of the abstract is discussed. 67
Barnett et al. 65 conducted a study to estimate the cost of platelet transfusion for CLD patients with thrombocytopenia undergoing elective procedures in the USA. The authors developed a conceptual framework aiming to identify all direct, indirect and intangible costs of platelet transfusion. They then estimated the costs using the developed framework and cost data from the literature. The framework included the cost of generating the supply of platelets, the transfusion itself, the adverse events associated with platelet transfusion and refractoriness. The total direct cost obtained from considering all framework categories of platelet transfusion in CLD patients with thrombocytopenia scheduled to undergo an elective procedure was estimated to be in the range of US$5258–13,117. The majority of costs were attributable to the transfusion itself (US$3723–4436), followed by the cost of refractoriness (which included the opportunity cost of a delayed procedure and subsequent transfusions with human leucocyte antigen-matched platelets) (US$874–7578). A potential limitation of this study is that it is literature based, drawing cost elements from different sources with different study designs. These sources were not based on CLD patients with thrombocytopenia, as the authors could not identify published sources on this population. Therefore, the estimate may not well reflect the target population if differences exist in the costs of transfusion and the rates of related AEs and refractoriness in a CLD thrombocytopenia population in the UK. It is also noted that this study was funded by Dova Pharmaceuticals, the owner of avatrombopag.
Brown66 published a review article discussing the pharmacoeconomic analysis of thrombocytopenia in CLD. The review discussed the negative impact that thrombocytopenia and its treatment can have on costs and treatment outcomes in CLD. The impact of thrombocytopenia on patient outcomes was discussed in terms of the increased likelihood of complications during routine medical procedures as well as the cancellation, delay or prolongation of procedures, which can increase morbidity and mortality. The negative patient outcomes that can arise from platelet transfusions, such as refractoriness, infection, allergic reaction, iron overload and other transfusion reactions, were also outlined. The review also discussed the economic burden of costs associated with platelet transfusion and resulting AEs that can require further treatment and increased utilisation of health-care resources.
In a conference abstract, Poordad et al. 29 conducted a case–control study examining the economic burden of platelet transfusion in CLD patients with thrombocytopenia. A retrospective analysis was conducted in a large national US administrative claims database to examine the impact of platelet transfusion on health resource utilisation and expenditure, including hospitalisations, accident and emergency (A&E) visits and outpatient visits among CLD patients with thrombocytopenia. Data from 2012 to 2015 were used to match adult CLD patients with thrombocytopenia who received a platelet transfusion 1 : 2 based on age and sex with CLD patients with thrombocytopenia who did not receive a platelet transfusion. Among the 1173 CLD patients with thrombocytopenia included in the analysis, those with thrombocytopenia who received a platelet transfusion had a statistically significantly higher probability of having an additional outpatient office visit (1.04; p = 0.021), a non-significantly higher probability of hospitalisation (1.08; p = 0.174) and a significantly lower probability of an A&E visit (0.86; p = 0.001) than those who did not receive a platelet transfusion. Platelet transfusions were associated with significantly increased hospitalisation costs (US$25,802, 95% CI US$11,220 to US$40,660), outpatient office costs (US$3367, 95% CI US$1082 to US$5652) and total costs (US$29,717, 95% CI US$15,096 to US$44,339) and non-significantly decreased A&E costs (–US$371, 95% CI –US$1019 to US$277) compared with no transfusion.
In Poordad et al. ,64 the aim was to examine medical resource utilisation and health-care costs in HCV patients with and without thrombocytopenia from a longitudinal administrative claims database using International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM), diagnosis codes. The prevalence of thrombocytopenia in HCV patients identified was found to be 3.6%, and the prevalence of thrombocytopenia in the subset of patients for whom platelet count laboratory results were available was 10.8%. HCV patients diagnosed with thrombocytopenia had a higher incidence of bleeding events (27.3% vs. 9.9%) and platelet transfusions (8.5% vs. < 1%). HCV patients diagnosed with thrombocytopenia also had a higher incidence of liver disease-related ambulatory visits (10.4% vs. 4.4%; OR 2.3, p < 0.001), emergency room visits (OR 8.6, p < 0.01) and inpatient hospital stays (OR 17.7, p < 0.01) during the year before and the year after HCV diagnosis than HCV patients without a thrombocytopenia diagnosis. HCV patients diagnosed with thrombocytopenia had significantly higher overall health-care costs (US$37,924 vs. US$12,174; p < 0.001) and liver disease-related costs (US$14,569 vs. US$4107; p < 0.001) than those without thrombocytopenia. Overall health-care and liver disease-related costs in the subset of HCV patients with complete laboratory results also found significantly higher costs among HCV patients diagnosed with thrombocytopenia than among those without thrombocytopenia (overall health-care costs US$25,482 vs. US$16,412, p < 0.001; liver disease-related costs US$23,608 vs. US$7354, p < 0.001). Where results are presented according to the two different strategies for identifying thrombocytopenia (i.e. coding identification and laboratory results), they differ quite substantially.
Poordad et al. 63 estimated the prevalence of thrombocytopenia and evaluated medical resource use and costs associated with thrombocytopenia in CLD patients. A retrospective study was performed on a longitudinal administrative claims database that included 56,445 patients with an ICD-9-CM diagnosis code for CLD in the period January 2001 to December 2003. For patients with available laboratory results, including platelet counts (35.7%), the numbers of bleeding events or platelet transfusions were also determined. The annual prevalence of thrombocytopenia among patients with CLD ranged from 3.3% to 4.1%. In comparison with patients without a thrombocytopenia diagnosis, the group of patients with a thrombocytopenia diagnosis included more males (62.6% vs. 49.4%) and experienced more anaemia (54.2% vs. 18.5%), more neutropenia (20.8% vs. 1.7%), more liver cancer (5.7% vs. 1.5%), more liver transplants (2.1% vs. < 1%) and more bleeding events (27.8% vs. 10.0%). They also received more interferon therapy (5.9% vs. 2.0%) and more platelet transfusions (8.1% vs. < 1%) and, on average, each one had more platelet count assessments (mean 3.68 vs. 2.47). Patients with a thrombocytopenia diagnosis had 2.5 times more liver disease-related ambulatory visits, 3.9 times more liver disease-related emergency room visits and 12.9 times more liver disease-related inpatient hospital stays than patients without a thrombocytopenia diagnosis. Overall medical care costs were 3.5-fold higher in patients with a thrombocytopenia diagnosis, with liver disease-related costs being 7-fold higher in patients with a thrombocytopenia diagnosis than in patients without a thrombocytopenia diagnosis. Similar results were obtained for patients with a platelet count that indicated thrombocytopenia.
In summary, the findings from the literature review that were presented above indicate that the health-care costs of patients with CLD and thrombocytopenia are substantial. Most notably, the costs of, and associated with, platelet transfusions make a relatively large contribution to those costs. This emphasises the importance of evaluating how an alternative strategy through the (additional) use of TPO-RAs compares with platelet transfusions as the current standard treatment for thrombocytopenia in patients with CLD.
Review of the company evidence
Review of the avatrombopag submission
In the company submission by Dova, no cost-effectiveness analysis was presented, and no cost-effectiveness model was provided by the company. 68
Relevant details were provided for the costs of thrombocytopenia with references to studies that were also identified by the AG (see Systematic review of existing cost-effectiveness evidence, Results). These include the study by Brown66 on increased direct and indirect costs due to thrombocytopenia and its associated complications, and the studies by Poordad et al. 63,64 on costs of HCV patients with thrombocytopenia compared with those without, and costs of CLD patients with thrombocytopenia compared with those without (respectively). Subsequently, details were provided on the costs of platelet transfusions. It was argued that the costs of platelet transfusions are high owing to a combination of specific storage requirements, a short shelf life and the unpredictability of the demand for platelets, which causes a high degree of wastage due to expiration issues. 69,70 It was also noted that platelet transfusion refractoriness (i.e. the repeated failure to achieve the desired level of blood platelets in a patient following a platelet transfusion) generally occurs after multiple transfusions. 71,72 Finally, an estimate of the costs of a platelet transfusion was provided with reference to Barnett et al. ,65 which was also identified by the AG in its literature review as outlined in Systematic review of existing cost-effectiveness evidence, Results.
Review of the lusutrombopag submission
The lusutrombopag submission included a model-based cost-effectiveness analysis, which compared lusutrombopag (once per day at a dose of 3 mg for 7 days) with no TPO-RA for CLD patients with severe thrombocytopenia (platelet count < 50,000/µl) who were scheduled to undergo an elective invasive procedure. The efficacy data incorporated into the decision-tree model were based on the results from the three controlled trials of lusutrombopag (L-PLUS 1, L-PLUS 2 and Phase 2b). 55 In the base-case analysis, the company pooled the results of the three trials. In a scenario analysis the model efficacy data were based solely on the L-PLUS 2 international trial,54 excluding the other two studies, both of which were undertaken in Japan.
The model combined a short-term decision tree (Figure 3), considering costs and QALYs over a 35-day period (matching the trial time horizons), and a long-term Markov model, assessing QALYs and mortality over a lifetime time horizon of 50 years. The short-term decision tree model had the following binary (i.e. yes/no) chance nodes: receiving platelet transfusion (trial data), death following platelet transfusion (literature), receiving elective invasive procedure within study period (trial data), death before rescheduled procedure (literature), bleeding following invasive procedure (trial data), rescue therapy following bleeding (trial data), death from bleeding for those not receiving rescue therapy (literature) and death from bleeding for those receiving rescue therapy (literature).
FIGURE 3.
Structure of the short-term decision tree from the lusutrombopag submission. Data from Shionogi company’s submission for lusutrombopag. 55
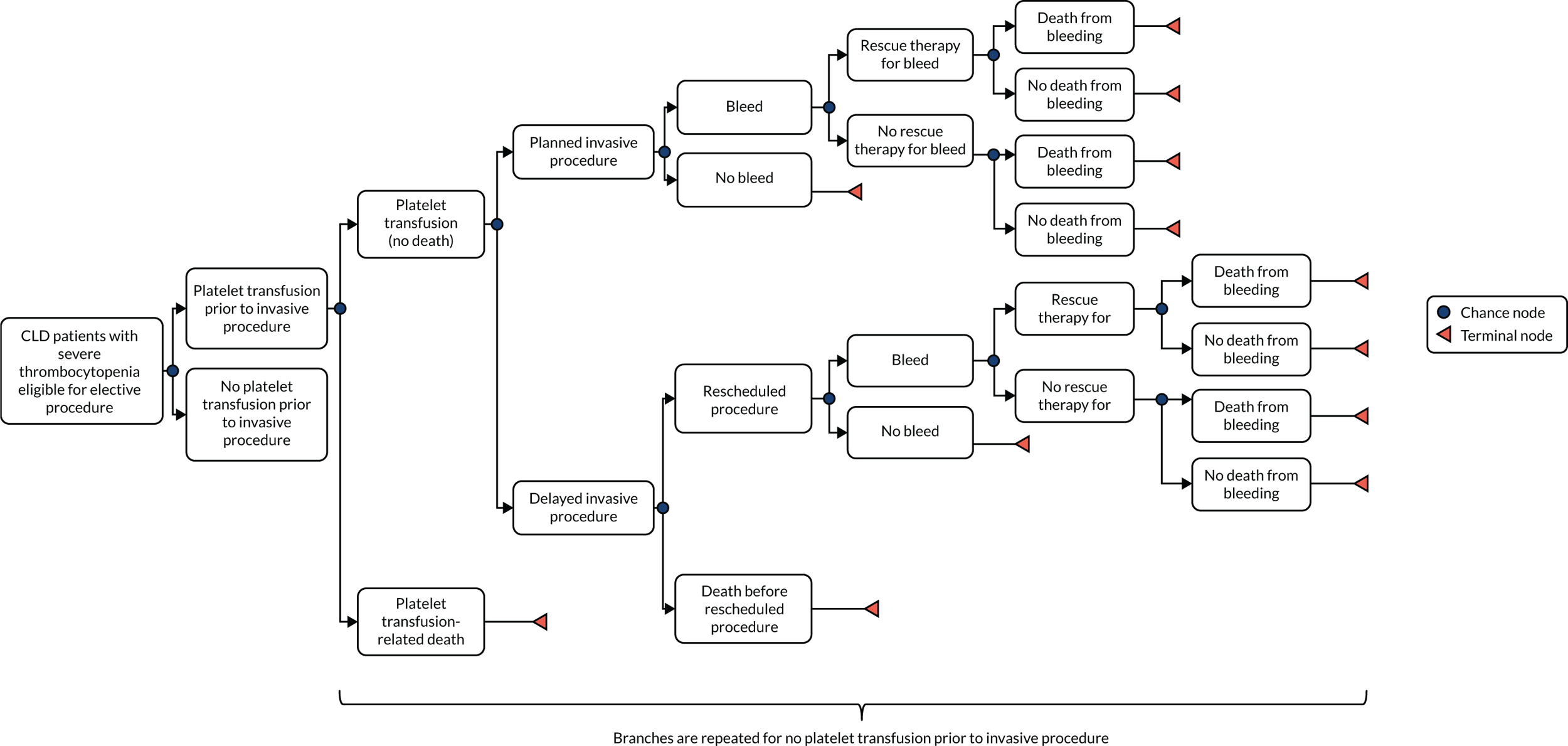
In the short-term model, costs were attributed to any platelet transfusions, procedures and rescue therapies given, drug acquisition and administration, and AE monitoring. One-off QALY decrements were included for platelet transfusions, bleeding events, rescue therapies and AEs.
In the long-term Markov model, data from the literature regarding CLD-related mortality and utility values were used to estimate the number of QALYs that would be accrued over the expected remaining life of the patient with a cycle length of 1 year. QALYs in the long-term model are discounted at a rate of 3.5%. No cost discounting was incorporated as costs were included only in the short-term model, in which discounting was inappropriate.
Efficacy summary
Efficacy inputs in the model included the following for each treatment arm:
-
proportion of patients receiving a platelet transfusion prior to the elective invasive procedure
-
proportion of patients experiencing bleeding events following an elective invasive procedure
-
proportion of patients not receiving their elective invasive procedure during the trial period (conditional on receipt of prior platelet transfusion)
-
proportion of patients receiving rescue therapy following bleeding (conditional on receipt of prior platelet transfusion and receipt of elective invasive procedure).
For efficacy inputs 1 and 2, the proportion of patients achieving each outcome in the placebo/platelet transfusion arm was taken directly from the placebo arm of the pooled lusutrombopag clinical trials (or from L-PLUS 254 only in scenario analysis). For the lusutrombopag arm, ORs for lusutrombopag compared with placebo were estimated from the pooled trials (or from L-PLUS 254 alone in scenario analysis) and were applied to the placebo/platelet transfusion arm data. Inputs 3 and 4 were calculated as conditional probabilities in the base-case analysis using individual patient-level data from the pooled lusutrombopag trials. In a scenario analysis, these conditional probabilities could be turned off and replaced with unconditional inputs calculated using ORs, as seen for inputs 1 and 2.
In the base-case analysis, the company assumed, contrary to evidence from the lusutrombopag trials, that 100% of patients in the placebo/platelet transfusion arm would receive a platelet transfusion prior to an elective invasive procedure as a result of less intensive monitoring of platelet count prior to procedures in clinical practice. This assumption was based on clinical expert opinion. In the trials (confidential information has been removed) of placebo arm patients in the pooled trials and (confidential information has been removed) in the L-PLUS 2 trial54 received a platelet transfusion prior to surgery.
Mortality in the short-term model could occur as a result of platelet transfusion or bleeding events. The company identified two different sources for the probability of platelet transfusion-related mortality. In the base-case analysis, the company adopted values from a study by van Eerd et al. ,73 in which the base-case mortality risk associated with transfusion was estimated to be 0.3315%. The company also identified an alternative source of mortality data, from a study by Vamvakas et al. ,74 that estimated an incidence of transfusion-related death of 0.0004% from UK Serious Hazards of Transfusion (SHOT).
In the base-case analysis, bleeding-related mortality was taken from a study by Takaki et al. ,75 which estimated that the rate of death from either major or minor bleeding following radiofrequency ablation (RFA) was 0.83%. Two alternative sources of estimates of bleeding-related mortality were included in the model. Lo et al. 76 estimated a mortality rate of 6% from upper gastrointestinal haemorrhage and oesophageal variceal bleeds (assumed to be a major bleed) and Triantos and Kalafateli77 estimated a 20% mortality rate from acute variceal bleeding (assumed major bleed).
Chronic liver disease-related mortality was incorporated into the long-term model to estimate lifetime QALYs for those patients surviving the short-term model. In the base-case model, data were used from a systematic review by D’Amico et al. ,78 with 1-year survival estimated at 84%.
The model included AEs relating to the treatment and to platelet transfusion. SAEs that were possibly or probably related to the drug were included in the model. Thrombus-related AEs are particularly relevant to TPO-Ras; therefore, any severe thrombus-related events in any of the three lusutrombopag trials79–81 (3 mg dose) were included in the model. In its submission, the company states that comprehensive data for all platelet transfusion-specific AEs were not available. Therefore, data for platelet transfusion AEs were taken from the van Eerd et al. 73 study, which reports the incidence of AEs per unit of fresh-frozen plasma transfused. 73
Health-related quality-of-life summary
Health-related quality-of-life data were not collected in the trials. The base-case analysis adopted a baseline utility value of 0.544 in both treatment groups, estimated for patients with CLD/cirrhosis. This utility value is from a study by Sullivan et al. 82 that provides EuroQol-5 Dimensions (EQ-5D) index scores for a wide variety of chronic conditions based on UK community preferences (using US-based panel survey data). One-off disutilities were included in the model for platelet transfusions, bleeding events, rescue therapy and AEs. In the base-case analysis, a disutility of 0.1 for patients experiencing serious platelet transfusion-related AEs was applied for one model cycle (4 weeks). This value was taken from TA293,83 a previous NICE appraisal of eltrombopag for thrombocytopenic purpura. In the base-case analysis, the company assumed the same disutility for rescue therapy as for platelet transfusion, stating that clinical experts advised that platelet transfusion would be most common in clinical practice.
Utilities summary
Disutilities for bleeds were also identified from the literature. The literature provided separate disutilities for bleeds classified as major and those classified as minor. The company assumed that all bleeds were major, stating that no studies were identified that reported the proportion of bleeds classified as major or minor following an elective invasive procedure in this population, and that minor bleeds would be expected to have a minor impact on costs and QALYs. Therefore, a disutility associated with a major bleeding event of 0.397 for a duration of 1 week was adopted from Jugrin et al. 84 For thrombus-related AEs, the company incorporated a disutility of 0.029, applied over 1 week, estimated by Jugrin et al. 84 for related thrombotic events (index deep-vein thrombosis and index pulmonary embolism).
The baseline utility value for CLD/cirrhosis patients adopted in the short-term model was also used to calculate QALYs throughout the long-term model. Utility values were adjusted to incorporate the natural decline in utility observed with ageing using the Ara and Wailoo85 equation to generate utility multipliers by age and sex.
Costs summary
The drug acquisition cost of (confidential information has been removed) for 7 days of 3 mg of lusutrombopag was included in the model. As lusutrombopag is an oral medication, no administration costs were required. The base-case cost of platelet transfusion was based on the TA293 appraisal83 of eltrombopag. In the eltrombopag appraisal, this cost was assumed to comprise the cost of blood transfusion (weighted average cost of £57.72 in 2011/12, code 821 blood transfusion) and the cost of 2 units of platelets (2 × £230.393 in 2011/12), which resulted in a cost per transfusion of £517.28 in 2011/12. The company used expert opinion to inform the average number of units of platelets that would be received per transfusion. The expert stated that most often platelet transfusions would contain either 2 or 4 units and, therefore, it was assumed that an average of 3 units of platelets would be received per transfusion. This resulted in a base-case cost of £812.61 (inflated to 2017/18), which included both administration and platelet acquisition. Two alternative costs of platelet transfusion were included in the model. One alternative was based on NHS Reference Costs 2017–1886 for single plasma exchange or other intravenous blood transfusion. Here it was assumed that a single transfusion was sufficient to transfuse the required number of units of platelets, which resulted in a cost per transfusion of £517.28. The final option was based on a poster by Varney and Guest,87 which estimated the cost per unit of adult platelet concentrate to be £347 in 2002/3, resulting in a cost per transfusion of £1493.21 (inflated to 2017/18).
The costs associated with treating transfusion-related complications were based on the costs of complications from fresh-frozen plasma transfusion, reported in van Eerd et al. 73 The cost of managing portal vein thrombosis (PVT) in lusutrombopag patients was assumed to be £958.95, based on NHS Reference Costs 2017–1886 for percutaneous transluminal, embolectomy or thrombolysis, of blood vessel, with a CC (complication) score of 0–4 in a day-case setting. The same cost of one platelet transfusion was assumed for all rescue therapies.
All patients in both treatment arms were assumed to have received an elective invasive procedure and to incur the relevant costs. Although the short-term model allowed for the possibility of delaying the procedure beyond the 35-day cycle, all patients were assumed to receive their procedure at some point. Base-case procedural costs were estimated using the pooled proportion of patients receiving each procedure in the three trials and the relevant NHS Reference Costs 2017–1886 in the elective inpatient setting. In the base-case analysis, the company included a sunk cost for cancelled or delayed procedures, assuming that there may not be enough time to reallocate a pre-assigned clinician or hospital bed to another patient procedure, thus wasting clinician time. A sunk cost of £566.05 for delayed elective invasive procedures was included, which was based on a study based on an NHS reference cost that the company had stated had been removed from subsequent years’ NHS reference costs. 88
Critique
The AG generally agreed with the model structure and input values included. However, the AG considered the model to have the following limitations:
-
The model did not consider subgroups in terms of thrombocytopenia (a baseline platelet count of either < 40,000/µl or 40,000–< 50,000/µl), which is relevant because different doses of avatrombopag are required for each of these two subgroups.
-
The model did not incorporate other available drugs such as avatrombopag.
-
The AG could not trace back the numbers from the CSRs79–81 to understand from where the probabilities for bleeding, conditional probability of surgery rescheduling and conditional probabilities of receiving rescue therapy were derived.
-
Considering the lack of a clear definition of the bleeding events used in the Shionogi economic model, as well as the extremely small numbers and lack of difference between the World Health Organization grade 2 bleeding rates between two groups from L-PLUS39,54 data (appendix c.5.3 of the Shionogi submission),55,56 the AG was doubtful about using these conditional probabilities and also doubtful about incorporating bleeding and rescue events as separate chance nodes of the decision tree.
-
The company assumed that 100% of the placebo arm would receive a platelet transfusion prior to the elective invasive procedure in the base-case analysis. This is contrary to the evidence from L-PLUS 1,39 L-PLUS 254 and the JapicCTI-121944 trial,53 in which 12.5%, 29% and 20%, respectively, of placebo patients did not require platelet transfusion prior to the elective invasive procedure (see Table 13).
-
The company did not follow standard meta-analysis approaches while deriving the transition probabilities in the economic model; instead the transition probabilities were obtained from simple pooling of the data without being weighted.
-
The model considered that the only mortality due to a surgery is the bleeding-associated mortality, whereas there are other causes of death (such as infection).
-
Platelet transfusion-related mortality can also occur after surgery.
-
Two potential values were identified from the literature73,74 for platelet transfusion-related mortality. Neither study was specific to CLD patients or to patients with thrombocytopenia. In addition, neither study actually estimated the mortality associated with platelet transfusion, with one investigating fresh-frozen plasma transfusion and the other investigating whole-blood transfusion. These studies resulted in substantially different estimates of transfusion-related mortality of 0.33% and 0.0004%. The choice to go with the higher value was justified as recommended by expert opinion.
-
It was unclear why data regarding AEs experienced as a result of platelet transfusion during the trials were not available to the company. AEs would have had to have been noted and monitored and therefore data should have been available. Again, by using the van Eerd et al. 73 study as a source for input values, the model used values not specific to the population or to platelet transfusion.
-
By assuming that all bleeds were major, the company may be overestimating the utility loss resulting from bleeding events. The AG did not consider that stating that minor bleeds would be expected to have a minor impact on costs and QALYs was a sufficient justification for assuming that all bleeds were major.
-
The company assumed an average of 3 units of platelets per transfusion. Data were not provided by the company on the average number of units used per transfusion in the lusutrombopag trials. The company stated in its clarification response that there is a lack of standardisation across countries (and potentially even centres) regarding the size of a ‘unit’ in terms of what volume of platelets this equates to or how this relates to definitions of units in UK clinical practice. 56 Therefore, although information on the number of units of platelets transfused was collected, the variation in reporting led the company to question the data’s reliability and their relevance to UK definitions and practice. The company therefore used expert opinion and the median number of units per transfusion from the eltrombopag ELEVATE trial,56 both of which resulted in the expectation that an average of 3 units of platelets would be used per platelet transfusion. The AG understood this issue of variation in the definition of ‘units’ of platelets, which was further supported through contact with its own clinical expert. In response to clarification questions, both companies provided additional information on the number of units of platelets transfused per platelet transfusion. 56,57 However, only the data provided by Shionogi came with accompanying information on the content of a unit by providing the mean number of platelets per platelet transfusion. In the case of the data provided by Dova Pharmaceuticals, it was not clear the number of platelets to which a unit would correspond. Therefore, only the data from Shionogi on the mean number of platelets per platelet transfusion could be translated into a mean number of adult therapeutic doses (ATDs) and were used for the calculation of the costs of a platelet transfusion.
-
The company included a sunk cost for delayed elective invasive procedures. It is considered unlikely that, in the case of a procedure delay, a clinician could not find another useful way to fill this time. The fact that this cost was removed from the NHS Reference Costs almost 10 years ago suggests that this cost is no longer considered appropriate.
Independent economic assessment
The AG decided to adapt the model submitted by Shionogi owing to the limitations discussed in Review of the company evidence.
Methods
Patient population
The patient population considered is CLD patients with severe thrombocytopenia (i.e. a platelet count of < 50,000/µl) who are scheduled to undergo an elective invasive procedure.
The patient population is divided into two subgroups:
-
patients with a platelet count of < 40,000/µl
-
patients with a platelet count of 40,000–< 50,000/µl.
This immediate division of the population into platelet count subgroups is necessitated by the fact that each of these subgroups receives a different dose of avatrombopag, as described below. Therefore, it is not possible to conduct a direct comparison between lusutrombopag and avatrombopag without this subgroup separation.
Interventions
Lusutrombopag is administered orally once per day at a dose of 3 mg for up to 7 days, with the first dose taken a minimum of 9 days prior to the scheduled procedure. 8
Avatrombopag for patients with a platelet count of < 40,000/µl is administered orally once per day at a dose of 60 mg (three tablets of 20 mg), with the first dose administered 10–13 days prior to the scheduled procedure and the regimen for 5 days (i.e. the procedure is scheduled 5–8 days after the last dose). For patients with a platelet count of 40,000–< 50,000/µl, the administration and timing of avatrombopag are the same, but the dose is reduced to 40 mg (two tablets of 20 mg).
Standard of care entails patients being given a platelet transfusion if their platelet count fails to reach ≥ 50,000/µl on the day of the scheduled procedure.
Model structure
The AG model is based on the structure for lusutrombopag submitted by Shionogi. Similar to that model, the AG model combines a short-term decision tree considering costs and QALYs over a 35-day period (matching the time horizon of all trials, as shown in Table 7), during which severely thrombocytopenic CLD patients are scheduled to undergo an elective invasive procedure. Those patients alive at the end of the short-term model enter the long-term Markov model, which assesses QALYs and mortality over a lifetime time horizon of 50 years. The AG short-term decision tree model has the following chance nodes:
-
receiving/not receiving platelet transfusion (taken from the avatrombopag and lusutrombopag trials)
-
receiving/not receiving the elective invasive procedure within the 35-day study period
-
rescue therapy/no rescue therapy (taken from avatrombopag and lusutrombopag trials)
-
death/no death due to platelet transfusion, surgery or rescue therapy (taken from the literature).
The structure of the AG short-term decision tree model, shown in Figure 4, differs in several ways from that of the original Shionogi model discussed in Review of the lusutrombopag submission. In the Shionogi model, a chance node for death due to platelet transfusion was placed directly after the receipt of transfusion before the chance node for undergoing an elective invasive procedure. In the AG model, both mortality due to platelet transfusion prior to elective invasive procedure and mortality due to surgical complications were considered after the chance nodes for undergoing surgery and requiring rescue therapy.
FIGURE 4.
Structure of the short-term decision tree model. Data from AG model.
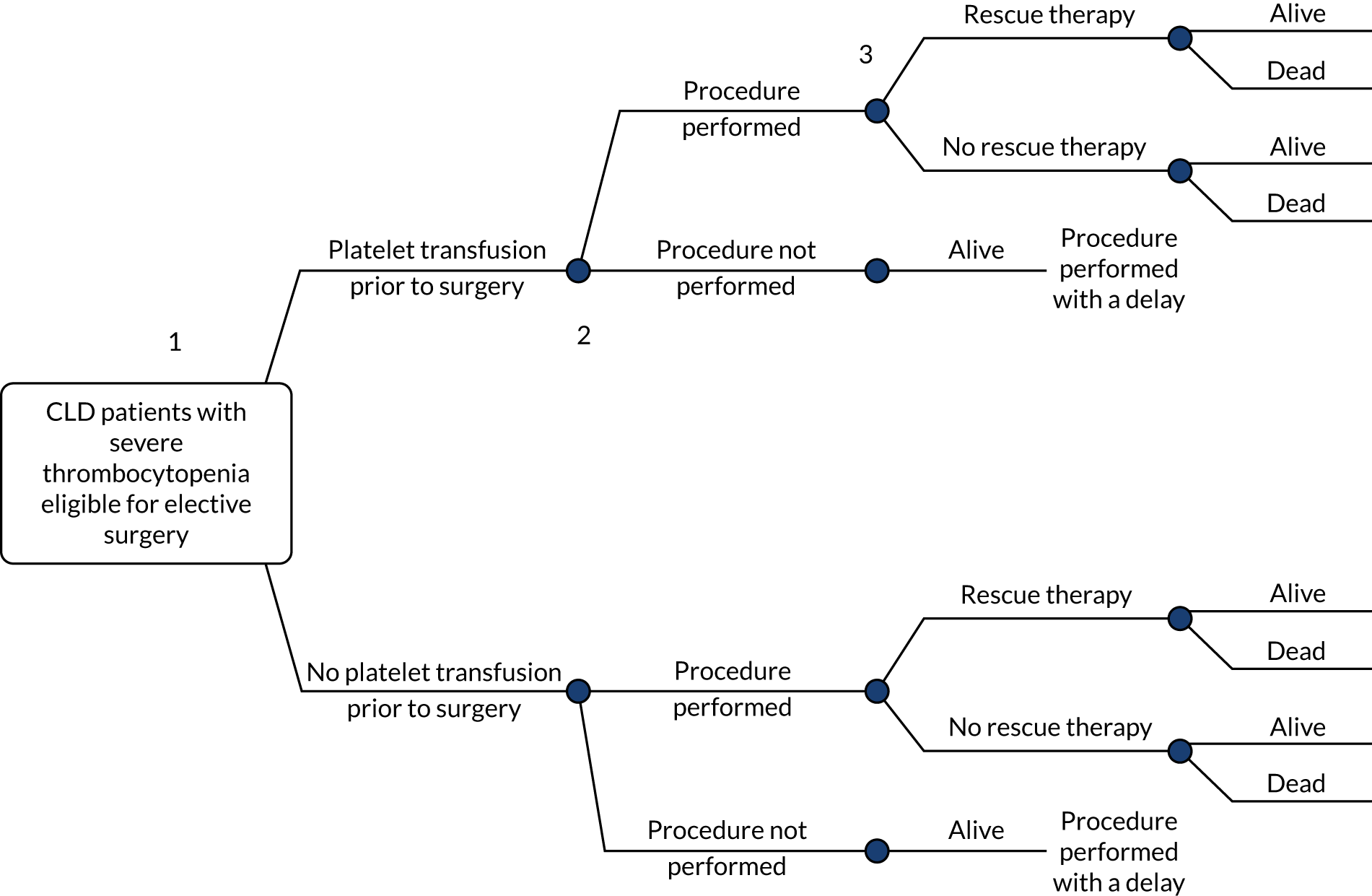
The Shionogi model also allowed for the probability of delays to scheduled procedures and modelled the potential impact of delays on quality of life and mortality and the additional costs that may be incurred as a result of such delays. Additional costs resulting from surgery delays included a possible additional platelet transfusion, as well as sunk costs resulting from last-minute delays leading to wasted surgeon and surgical theatre time. The AG did not feel that the inclusion of a sunk cost was necessary, as surgical theatre slots would usually be filled by other procedures and surgeons could effectively fill their time with other tasks. In addition, the fact that Shionogi identified a sunk cost unit cost from the NHS Reference Costs from 2009/10 but this was subsequently removed from the reference costs suggests that it is no longer considered an appropriate cost to include in a model. The Shionogi model also contained a chance node for death due to surgery delay. However, this was assumed to carry a probability of 0 in the base-case analysis and was removed by the AG.
The Shionogi model structure contained a separate chance node for bleeding events and a subsequent chance node for the requirement of rescue therapy. However, the AG had concerns regarding this structure and the data it was based on. The AG was unable to trace back the numbers used to calculate bleeding event efficacy to the lusutrombopag trials’ CSRs. 79–81 On clarification request, the company provided data on the number of bleeding events in each trial and treatment group. 56,57 However, in contrast to how it was implemented in the original Shionogi submission model, these numbers did not suggest that lusutrombopag substantially reduced the odds of bleeding. In addition, these conditional probabilities were not available for avatrombopag. The small number of World Health Organization grade 2 bleeding events and the rescue events seen in the trials led to concerns about the confidence that can be placed in conditional probabilities based on such data. Therefore, the AG felt that bleeding events were better modelled as a surgical complication rather than as a separate event. Therefore, bleeding events and their impact on the mortality and quality of life of patients were modelled as a surgical-related AE and a source of mortality. The chance node for requiring rescue therapy was retained.
The long-term Markov model presented by Shionogi was utilised without changes in the AG model. In the long-term model, data from the literature regarding CLD-related mortality and utility values were used to estimate the number of QALYs that would accrue over the expected remaining life of the patient with a cycle length of 1 year. QALYs in the long-term model were discounted at a rate of 3.5%. No cost discounting was incorporated as costs are included only in the short-term model, in which discounting is inappropriate.
Assessment group input parameters
Baseline characteristics
The AG calculated pooled baseline characteristics from the three included lusutrombopag trials (L-PLUS 1,39 L-PLUS 254 and the Phase 2b trial79–81) and two avatrombopag trials (ADAPT-137 and ADAPT-237). The overall average of each baseline characteristic was obtained from reported trial-specific means, weighted proportionally to the trial population size. These baseline characteristics, including age, sex and Child–Pugh category, are outlined in Table 22. As the AG could not find better UK-specific data for the baseline characteristics of the thrombocytopenic CLD patients in the UK, these values were used in deliberation with the clinical expert.
| Baseline characteristic | Age (years) | Sex | Child–Pugh category | |||
|---|---|---|---|---|---|---|
| Mean | SD | Male | A | B | C | |
| Pooled | 58.6 | 10.8 | 62.7% | 57.5% | 38.9% | 3.6% |
Based on the characteristics of patients in all of the trials pooled, mean patient age was 58.6 years (SD 10.8 years), 62.7% of the patients were male and patients were categorised as Child–Pugh A, B or C in proportions of 57.5%, 38.9% and 3.6%, respectively.
Efficacy
As lusutrombopag and avatrombopag were not directly compared in a head-to-head trial, indirect comparisons had to be made. This was possible because both had been compared with placebo. The methods of the data synthesis of the efficacy outcomes of interest for the short-term model are described in Chapter 3, Methods for reviewing effectiveness, Methods of data synthesis, of this report and the results are provided in Chapter 3, Results, Meta-analysis.
From the response to the clarification letters submitted by each company, the AG had data on the number of patients in each treatment arm and platelet count subgroup who did not require:
-
platelet transfusion prior to invasive procedure
-
rescue therapy given that there was no platelet transfusion prior to invasive procedure.
From these data, for each outcome, an indirect treatment comparison was performed using Bayesian meta-analysis methods to obtain estimates for the proportions/probabilities of each of the above outcomes. First, the proportions for the placebo group (all trials pooled) were obtained for each platelet count subgroup in a separate Bayesian meta-analysis. As the AG could not find better UK-specific data for the natural history of the thrombocytopenic CLD patients in the UK, these values were used in deliberation with the clinical expert. The recommendations from NICE DSU Technical Support Document 589 (Evidence Synthesis in the Baseline Natural History Model) were followed in this step. In line with the recommendations, the predictive mean and the standard deviation of the log-odds from the random-effects model were used to inform the baseline probabilities for the natural history (i.e. for no TPO-RA). They were also combined in a Bayesian evidence synthesis model, with ORs estimated using a logit function to calculate the corresponding probabilities (absolute risks) for avatrombopag and lusutrombopag.
Owing to the MCMC framework of the statistical software, such a Bayesian model ensures that the generated probabilities for each of the TPO-RAs remain between 0 and 1 without additional programming. This could not be guaranteed if an OR was estimated using the frequentist statistical method reported in Chapter 3, Results, Meta-analysis, and applied to the baseline probability. In addition, ORs were not estimable in the frequentist analysis for the proportion of patients in the < 40,000/µl subgroup who required no rescue therapy; however, the Bayesian MCMC model was able to provide stable results for this subgroup.
Both fixed-effect and random-effects models were run in all cases. Random-effects models were used in the base-case analysis because they provide a better statistical fit. (When assessing the statistical fit of a model, the global deviance information criteria statistics and the posterior mean residual deviance statistics are consulted. It is assumed that the model with lower values for these statistics provide a better fit.) The suggestions for numerical stability, on a WinBUGS (MRC Biostatistics Unit, Cambridge, UK) convergence error due to the presence of the zero cells in several trials, as outlined in NICE DSU Technical Support Document 216 (section 6.3), were followed (e.g. using less vague priors for the variance parameter or continuity correction by adding 0.5/1 to the numerator/denominator). The WinBUGS code used in the Bayesian fixed-effect and random-effects analyses is provided in Appendix 5. It should be noted that the base-case Bayesian model ORs were very similar to those presented in Tables 18 and 19.
| Platelet count subgroup | No TPO-RA, mean (95% CrI) | Avatrombopag, mean (95% CrI) | Lusutrombopag, mean (95% CrI) | Source | |||
|---|---|---|---|---|---|---|---|
| < 40 × 109/l | 40–< 50 × 109/l | < 40 × 109/l | 40–< 50 × 109/l | < 40 × 109/l | 40–< 50 × 109/l | ||
| Proportion requiring platelet transfusion prior to surgery (random effects)a | 0.699 (0.302 to 0.945) | 0.615 (0.347 to 0.837) | 0.439 (0.023 to 0.957) | 0.114 (0.022 to 0.320) | Confidential information has been removed | Confidential information has been removed | ITC |
| Proportion requiring platelet transfusion prior to surgery (fixed effects)a | 0.700 (0.301 to 0.945) | 0.615 (0.348 to 0.837) | 0.431 (0.095 to 0.831) | 0.115 (0.023 to 0.309) | Confidential information has been removed | Confidential information has been removed | ITC |
| Proportion requiring platelet transfusion prior to surgery (international trials only)a,b | 0.700 (0.299 to 0.944) | 0.615 (0.348 to 0.837) | 0.438 (0.019 to 0.964) | 0.114 (0.022 to 0.317) | Confidential information has been removed | Confidential information has been removed | ITC |
| Proportion procedure not performed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | L-PLUS 254 |
| Proportion requiring rescue procedure (random effects)a | 0.181 (0.002 to 0.817) | 0.184 (0.010 to 0.664) | 0.077 (0.0004 to 0.531) | 0.044 (0.001 to 0.252) | Confidential information has been removed | Confidential information has been removed | ITC |
| Proportion requiring rescue procedure (fixed effects)a | 0.180 (0.812 to 0.002) | 0.183 (0.655 to 0.010) | 0.075 (0.522 to 0.0004) | 0.044 (0.250 to 0.001) | Confidential information has been removed | Confidential information has been removed | ITC |
The first chance node in the model requires the probability or proportion of patients in each group who require platelet transfusion prior to an elective invasive procedure. In the base-case analysis, the proportion of patients in each treatment arm (for each subgroup) not requiring platelet transfusion prior to an elective invasive procedure was estimated from the posterior distribution parameter estimates of the Bayesian meta-analysis, derived from the baseline placebo proportions and the ORs obtained from the random-effects model, using the number of patients who received platelet transfusion before an elective invasive procedure, as provided in Table 23. These proportions were then subtracted from 1 to provide the proportion of patients in each treatment arm who did not require platelet transfusion prior to an elective invasive procedure.
For the second chance node, data on the proportion of elective invasive procedures not performed during the trial period were provided in tables 11–13 of the L-PLUS 254 CSR,81 which stated that (confidential information has been removed) and (confidential information has been removed) of lusutrombopag and placebo patients, respectively, did not receive their planned procedure during the trial period. L-PLUS 2 was the only trial that provided these data. Therefore, the lusutrombopag value of (confidential information has been removed) was also assumed for avatrombopag, and the same values were assumed for both platelet count subgroups. Patients were assumed to go on to receive their procedure at some point in the near future. Therefore, these patients were assumed to be at risk of receiving an additional platelet transfusion just before their postponed procedure, and they were also assumed to be at risk of requiring rescue therapy or of death during the postponed procedure. These risks of an additional platelet transfusion before the postponed procedure were assumed to be identical to the risks for placebo patients whose procedures were not postponed. Although these postponed procedures did not necessarily occur in the first cycle, the costs and impacts on mortality and quality of life were assigned in the first cycle for simplicity.
Platelet transfusion
There is substantial uncertainty about the mean number of units of platelets in each platelet transfusion patients received in the trials. This uncertainty is in large part caused by a lack of standardisation in terminology and definitions used across countries and centres regarding the size of a ‘unit’ in terms of number of platelets. When Shionogi56 provided, on request, data on the number of platelets transfused per platelet transfusion, the company pointed out that it became apparent during analysis that, although all trial centres collect this information, definitions and terms vary among trial centres. There was no way to standardise this or to understand how these varying definitions related to UK clinical and unit costs. Therefore, the company felt that it had no better solution than to use expert clinical opinion. The experts approached by Shionogi55 stated that patients would receive either 2 or 4 units and, therefore, an average of 3 units per transfusion was assumed. This assumption was used in the estimation of the safety and cost of platelet transfusion, with platelet transfusion AE incidents and unit costs multiplied by 3 in both cases. Given the importance of the cost of platelet transfusion in the model, the AG sought to validate this assumption of 3 further units.
First, the AG consulted its own clinical expert (S Ryder, Faculty of Medicine and Health Sciences, Queen’s Medical Centre, Nottingham, 2019, personal communication). When asked how many units of platelets he would expect to be used per platelet transfusion, the clinician stated that he was unfamiliar with the definition/term ‘unit’ in the context of platelets, as in his experience they were referred to as ‘pools’. He was not aware of the number of platelets in a pool but stated that one pool was usually sufficient to increase platelet levels by the required amount. This increased the concern within the AG about the lack of consistency in the number of platelets usually transfused in a platelet transfusion.
The AG then turned to the literature to investigate UK platelet transfusion practice. The Handbook of Transfusion Medicine,90 produced in conjunction with the Joint UK Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee and NHS Blood and Transplant, provides some useful information about UK practice. This publication states that an adult therapeutic dose (ATD) of platelets could comprise either a pool of four units of platelets derived from whole blood or a single-donor apheresis unit. The handbook also notes that UK blood services aim to provide > 80% of platelet doses by apheresis to reduce patients’ exposure to multiple donors (a measure taken to reduce the risk of variant Creutzfeldt–Jakob disease). Therefore, the AG assumed that, in UK practice, patients would receive platelets by apheresis.
An ATD is described in the publication as containing > 240,000/µl platelets per transfusion, whereas the mean number of platelets in a unit of platelets donated by apheresis is 280,000 (range 165,000–510,000). 90 Although Shionogi had been unable to supply data on the mean number of units of platelets transfused per platelet transfusion, it was able to supply estimates of the mean number of platelets (i.e. platelet content per transfusion) transfused across the lusutrombopag trials for each treatment group and platelet subgroup both prior to surgery and as a rescue therapy. These estimates of mean number of platelets per transfusion ranged from (confidential information has been removed) to (confidential information has been removed). 56 This suggests an estimate of (confidential information has been removed) ATDs per transfusion. The NICE blood transfusion guideline4 states that clinicians should not routinely transfuse more than a single dose of platelets per transfusion, suggesting that one ATD may be sufficient per transfusion.
Dova did provide data on the mean number of units transfused per platelet transfusion for each platelet subgroup and treatment group prior to an elective invasive procedure in the ADAPT trials. 37 However, these means, ranging from 3.9 to 7.5, did not correspond well with the aforementioned expectations of UK clinical practice definitions, and no information was provided on the assumed platelet content within a unit. Therefore, these data were not used in the calculation of the costs of a platelet transfusion.
Therefore, in calculating the mean number of ATDs included in each platelet transfusion prior to surgery, the AG utilised the data provided by Shionogi56 detailing that the mean number of platelets transfused per transfusion divided by the mean number of platelets in a unit of platelets donated by apheresis, which is 280,000/µl according to the Handbook of Transfusion Medicine. 90 This provided an estimate of the number of ATDs per transfusion (as the handbook also stated that an ATD was equivalent to a single-donor apheresis unit). This calculation resulted in mean numbers of ATDs for lusutrombopag and no TPO-RA patients in each platelet count subgroup, both prior to surgery and as a rescue therapy, as shown in Table 24. No clear pattern was seen in these data to suggest to the AG that the content of platelet transfusions varied substantially according to treatment group, subgroup or reason for transfusion. Therefore, the AG assumed a pooled estimate of (confidential information has been removed) ATDs per transfusion across all transfusions given in the model. This figure corresponds well with recommendations from clinical expert opinion and the NICE blood transfusion guideline4 that a single ATD should be sufficient per platelet transfusion. This assumed number of ATDs per transfusion will be tested in a scenario analysis.
| Number of ATDs per transfusion | Platelet count subgroup | ||
|---|---|---|---|
| < 40,000/µl | 40,000–< 50,000/µl | Both subgroups | |
| Prior to elective invasive procedure | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Rescue therapy | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Overall | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
Mortality
The short-term AG model includes sources of mortality due to:
-
platelet transfusion prior to the surgery
-
surgery
-
rescue therapy.
In the following paragraph, more detail is provided for each of these sources of mortality.
In the Shionogi submission, the probability of death due to platelet transfusion was based on the Vamvakas and Blajchman study. 74 This study estimated the number of deaths due to allogenic blood transfusions using the SHOT data from 1996 to 2004. There were 167 transfusion-related deaths during this period, resulting in an incidence of 0.00035%. 74 The alternative value for platelet transfusion-related mortality provided in the Shionogi submission of 0.3315% was obtained from a study by van Eerd et al. ,73 which in turn cites the incidence of complications due to fresh-frozen plasma transfusion and associated mortality in critically ill patients on an intensive care unit. 91 This value was considered inappropriate by the AG as it is approximately 1000 times higher than the value obtained by the SHOT data. The AG felt that this high estimate was probably a result of the critical health status of participants in the Gajic et al. study91 (all of whom were admitted to an intensive care unit), which does not match this trial population and would probably lead to an overestimation of the mortality rate among our population.
The AG decided to use neither of these mortality rates, the second being unrealistically high for the current population and the first being outdated. The Vamvakas and Blajchman study74 used SHOT data from 1996 to 2004, so the AG decided to also use SHOT data, but from 2012 to 2017 instead (see Table 29). 92–96 As a first step, the probability of an early transfusion reaction was determined (the transfusion-transmitted infections, which manifest later, do not lead to mortality). FAHR (febrile, allergic, hypotensive reactions) and pulmonary complications (transfusion-related acute lung injury, transfusion-associated circulatory overload, transfusion-associated dyspnoea) were selected as relevant. Probabilities were obtained using the following steps:
-
The numbers of reactions per year from 2012 to 2017 were taken and added up. They were split up into FAHR and pulmonary complications (transfusion-related acute lung injury, transfusion-associated circulatory overload, transfusion-associated dyspnoea). FAHR were reported for platelets specifically; unspecified reactions were not included. Pulmonary reactions were reported over all components issued.
-
Overall numbers were divided by the total number of platelet units issued (FAHR) or the total number of blood components issued (pulmonary complications) to get the probability of the reaction per component issued.
-
These probabilities were divided by the average survey participation to correct for this.
The resulting probability of FAHR was 0.0288% and of pulmonary reactions was 0.00395% per transfusion. The probability of death from a transfusion reaction was estimated using the number of deaths reported in the early transfusion reactions by SHOT UK. FAHR resulted in no mortality over 2012–17, so mortality was based on deaths from pulmonary complications. The probability of dying from an early transfusion reaction was estimated using the following steps:
-
Take the number of deaths from pulmonary reactions over 2012–17 and divide it by the total number of pulmonary reactions to get mortality rate from pulmonary reactions.
-
Calculate the proportion of pulmonary reactions in early transfusion reactions and multiply by the mortality rate from pulmonary reactions to get the probability for death from an early transfusion reaction.
This yielded a mortality probability, given a transfusion reaction, of 1.4%. By combining this with the probability of a transfusion reaction, we find an overall mortality rate due to platelet transfusion of 0.0004592% (see Table 29).
There have been arguments in the literature that hemovigilance systems under-report transfusion-related morbidity and mortality. 97 Therefore, in scenario analyses, under-reporting factors were included for transfusion-related mortality to adjust the base-case estimate of 0.0004592%.
As the rescue therapies given in the trials often took the form of additional platelet transfusions, the estimate of platelet transfusion-related mortality was also applied to those receiving rescue therapy. The mortality associated with platelet transfusion is reapplied each time patients receive a transfusion in the model.
The probability of surgical-related mortality in this population was estimated from the trial mortality data. As suggested in NICE DSU Technical Support Document 5,89 a binomial likelihood model was used to estimate the baseline mortality risk using a random-effects model with the predictive distribution (see Appendix 5 for the statistical code used). The mortality figures from the five studies are used, which report mixed types of elective procedures, and the mortality risk from the predictive distribution, which resulted in the pooled risk of 0.0195 (95% CI 0.0004 to 0.13), was used in the base-case analysis (Table 25). As this was a scenario analysis, the mortality risk from the posterior distribution, which resulted in the pooled risk of 0.006955 (95% CI 0.0004 to 0.019), was used (see Table 29). This risk was incorporated into the model for patients in both platelet count subgroups who received their planned surgery.
| Parameters | Value | Source | Analysis |
|---|---|---|---|
| Mortality platelet transfusion | 0.0004592% | SHOT 2012–1792 | Base case |
| Mortality surgery | 1.95% | Predictive distribution of the baseline random-effects model | Base case |
| Mortality surgery (alternative) | 0.7% | Posterior distribution of the baseline random-effects model | Scenario |
| CLD mortality | Multiple valuesa | D’Amico et al.78 | Base case |
| CLD mortality (alternative) | Multiple valuesa | UKMi98 | Scenario |
Chronic liver disease-related mortality was incorporated into the long-term model to allow estimation of lifetime QALYs for those patients surviving the short-term model in the same way as in the Shionogi submission. 55 In the base-case analysis, data were used from a systematic review by D’Amico et al. ,78 in which survival at 1 and 2 years for each Child–Pugh grade was used to estimate an extrapolated survival curve, weighted based on the proportions of patients with each Child–Pugh grade. An alternative data source was also investigated by Shionogi using data from UK Medicines Information (UKMi), for which linear interpolation was used to estimate survival per year based on reported survival at 1, 5 and 10 years for each Child–Pugh category, with survival again weighted according to the proportions of patients with each Child–Pugh score. 98 The D’Amico et al. 78 estimate was chosen for the base-case analysis as Shionogi’s clinical experts considered the UKMi estimates too low, with 1-year survival estimated at 84%. The AG concurred with this assessment.
Safety
Adverse events due to treatment, platelet transfusion and surgery were included in the model (Table 26). In the company submission,55 Shionogi stated that comprehensive data for all platelet transfusion-specific AEs were not available. In the AG model, estimates for the probability of experiencing transfusion-related AEs were taken from the SHOT reports 2012–17. 92–96 Earlier, the probabilities of FAHR and pulmonary reactions were presented, at 0.0288% and 0.00395% per transfusion, respectively. However, not all FAHR events are major. SHOT92–96 data show that only 25.6% of all FAHR responses are major, thus inducing an effect on costs and quality of life. Furthermore, the transfusion-transmitted infections were extracted from the SHOT reports,92–96 yielding some very small probabilities of bacterial infections, hepatitis A, B and E virus infection and parvovirus infection. The incidences of the remaining transfusion-related AEs were multiplied by the assumed number of ATDs per transfusion [(confidential information has been removed) units, calculated by the AG; the details are explained in Platelet transfusion]. Patients were assumed to be at equal risk of experiencing a transfusion-related AE each time they underwent a platelet transfusion, with the risk repeated in the model.
| AE | Treatment | Source | |||||
|---|---|---|---|---|---|---|---|
| Placebo | Avatrombopag | Lusutrombopag | |||||
| Platelet count subgroup | < 40 × 109/l | 40–< 50 × 109/l | < 40 × 109/l | 40–< 50 × 109/l | < 40 × 109/l | 40–< 50 × 109/l | |
| Treatment-emergent AEs | |||||||
| PVT, median (95% CrI)a | 0.0009 (0.0000 to 0.1326) | 0.0011 (0.0000 to 0.1575) | 0.0005 (0.000 to 0.2030) | 0.0039 (0.0000 to 0.8962) | 0.0005 (0.0000 to 0.1244) | 0.0019 (0.0000 to 0.3685) | ITC |
| Surgery-related AEs | |||||||
| Bleeding events (grades 2 and 3), median (95% CrI)a | 0.0286 (0.0029 to 0.2279) | 0.0287 (0.0029 to 0.0760) | 0.0256 (0.0013 to 0.3715) | 0.0104 (0.0013 to 0.0817) | 0.0085 (0.0004 to 0.1374) | 0.0802 (0.0004 to 0.5768) | ITC |
| Proportion of grade 3 bleeding events | 30% (6/20) | Pooled from all trials | |||||
| Platelet transfusion-related AEs | |||||||
| Pneumological | 0.0039500% | SHOT reports 2012–1792–96 | |||||
| FAHR (major) | 0.0073831% | ||||||
| Bacteria | 0.0000063% | ||||||
| Hepatitis A virus | 0.0000063% | ||||||
| Hepatitis B virus | 0.0000063% | ||||||
| Hepatitis E virus | 0.0000634% | ||||||
| Parvovirus | 0.0000063% | ||||||
All SAEs that were experienced by at least 1% of the patients in any treatment arm of any of the randomised lusutrombopag and avatrombopag trials can be found in Appendix 3, Table 36. A large number of AEs is expected given the severity of the underlying condition. The only AE in Table 36 that was experienced by > 5% of patients in any treatment arm was transfusion reaction, which was assumed to be accounted for in the transfusion-related AE data outlined above. Thrombus-related AEs have been judged particularly relevant to TPO-RAs. 55 Therefore, any severe thrombus-related events possibly or probably related to treatment were included in the model. Cases of PVT that were judged to be severe, possibly or probably related, thrombus-related treatment-emergent AEs were seen across the trials. Given the severity and probable relationship with the drugs, PVT AEs were included in the model. The incidence of PVT in each treatment arm (for each subgroup) was estimated from the posterior distribution parameter estimates of the WinBUGS code derived from the baseline placebo proportions and the ORs obtained from the random-effects model.
Bleeding events of ≥ grade 2 were incorporated into the model as surgical adverse events. Bleeding data were provided by both companies in their clarification responses, clarifying the number of bleeds according to severity in each treatment arm of each trial for each platelet subgroup. The AG interpreted the moderate/severe bleeding categorisations provided by the companies as in line with the bleeding severity scale used by Shionogi. 55,56 Again, the incidence of bleeding in each treatment arm (for each subgroup) was estimated from the posterior distribution parameter estimates of the WinBUGS code derived from the baseline placebo proportions and the ORs obtained from the random-effects model. It is assumed that around 30% of bleeding events at ≥ grade 2 were ≥ grade 3, because 6 out of 20 bleeding events of ≥ grade 2 were grade 3.
Utilities
Health-related quality-of-life data were not collected in any of the lusutrombopag or avatrombopag trials. As in the Shionogi submission, the base-case analysis adopts a baseline EuroQol-5 Dimensions, three-level version (EQ-5D-3L), utility value in both treatment groups, as estimated for patients with CLD/cirrhosis in a study by Sullivan et al. 82 An alternative EQ-5D-3L utility value was incorporated into the Shionogi model based on a study by Scalone et al. ,99 which compared the performances of the EQ-5D-3L and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), in patients with chronic hepatic diseases. This was considered in the scenario analysis. 99
One-off disutilities were included in the model for platelet transfusions, not receiving a planned procedure, bleeding events, rescue therapy and AEs (Table 27). In the base-case analysis, a disutility of 0.1 for patients experiencing serious platelet transfusion-related AEs was applied for one model cycle (4 weeks). This value, included in the Shionogi model, was taken from TA293,83 a previous NICE appraisal of eltrombopag for thrombocytopenic purpura. An alternative disutility for platelet transfusion of 0.17 was available from van Eerd et al. 73 However, the company selected the disutility of 0.1 for the base-case analysis as it had been previously accepted by the committee in NICE TA29383 and TA221100 and was more conservative than the alternative value available. The AG concurred with this decision. An incidence of serious transfusion-related reactions of 0.0114% was assumed based on the sum of all reactions listed in Table 30. The disutility of 0.1 for a duration of 4 weeks was multiplied by the incidence of 0.0114%, which equated to a total QALY decrement of 0.000000876. This QALY decrement was multiplied by the number of times in the model that a patient received a platelet transfusion.
| Utilities and disutilities | Value | Source |
|---|---|---|
| Baseline utilities | ||
| CLD utility (base case) | 0.544 | Sullivan et al.82 |
| CLD utility (alternative) | 0.801 | Scalone et al.99 |
| Treatment-emergent AE disutility and duration | ||
| PVT disutility | 0.029a | Jugrin et al.84 |
| PVT duration | 1 week | Clinical expert validation consulted by Shionogi55 |
| Platelet transfusion-related AE disutilities | ||
| Serious reaction (base case) | 0.1 | NICE TA29383 |
| TRALI (alternative) | 0.4 | van Eerd et al.73 |
| Severe allergic reactions (alternative) | 0.4 | van Eerd et al.73 |
| Platelet transfusion-related AE durations | ||
| Serious reaction (overall, alternative) | 4 weeks | NICE TA29383 |
| TRALI (alternative) | 4 weeks | Clinical expert validation consulted by Shionogi55 |
| Severe allergic reactions (alternative) | 4 weeks | Clinical expert validation consulted by Shionogi55 |
| Surgery-related AE disutility and duration | ||
| Bleeding events (grade 3) disutility | 0.397 | Jugrin et al.84 |
| Bleeding events (grade 3) duration | 1 week | Assumption |
| Bleeding events (grade 2) disutility (only in scenario analysis) | 0.122 | Jugrin et al.84 |
| Bleeding events (grade 2) duration (only in scenario analysis) | 1 week | Assumption |
| Delay of procedure-related disutility and duration | ||
| Delay of procedure-related disutility | 0.072 | Assumption101 |
| Delay of procedure-related disutility duration | 4 weeks | Assumption |
| Age-related utility adjustments | ||
| Sex | 0.0212126 | Ara and Wailoo85 |
| Age | –0.0002587 | |
| age2 | –0.0000332 | |
| _cons | 0.9508566 | |
The AG felt that the delay of an elective invasive procedure outside the first cycle would have an impact on patients’ HRQoL. No established value could be found from the literature for the disutility associated with surgery delay or cancellation. Therefore, the AG assumed that, although the impact on the HRQoL of patients could be seen in a number of domains of the EQ-5D, it was most likely that lengthy delays would increase patients’ worry about their surgery and condition, and therefore would increase patients’ anxiety/depression. Therefore, the AG investigated the decrements associated with anxiety and depression in the UK EQ-5D-5L value set. 101 The average decrement for a one-level increase in anxiety and depression was 0.072 (note that the average decrement for a one-level reduction in any item is 0.064). The AG felt that this value was reasonable as an expected impact of surgery delay on patients’ HRQoL. In the base-case analysis this value was applied for 4 weeks. This duration was assumed as it approximated the cycle length and therefore accounted for the fact that patients would not receive the surgery in this cycle but would receive it one cycle later. These values will be adjusted in Scenario analysis results.
In its response to clarification, the company clarified that, in L-PLUS 2,54 rescue therapies included platelet transfusion, other blood product transfusion and volume expanders, whereas in the remaining two trials (L-PLUS 139 and the Phase 2b trial), platelet transfusion was the only permitted rescue therapy (despite this, one patient in the lusutrombopag group of L-PLUS 2 received thrombin, and one patient in the placebo group received thrombin and red blood cells, in addition to platelet transfusion as rescue therapies). 56 In the ADAPT trials,37 rescue therapies included platelet transfusion, fresh-frozen plasma transfusion, adrenaline injections and tranexamic acid. In the model submitted by Shionogi, the disutility set for rescue therapy was equal to that of platelet transfusion, following on from the argument that rescue therapy would be most likely to take the form of platelet transfusion. Although the AG does not agree with this assumption, especially given the range of rescue therapies seen in the trial, the disutility of 0.1 was felt to be reasonable to cover the disutility of rescue therapy in general, and this value was applied.
Disutilities for bleeding events and thrombotic events were also identified from the literature by Shionogi. Disutilities of 0.397 for major bleeding events and of 0.122 for clinically relevant non-major bleeding events were identified from Jugrin et al. 84 The AG base-case model included only bleeding AEs of ≥ grade 3, which were assumed to be equivalent to major bleeding events. Therefore, the disutility of 0.397 for major bleeds was incorporated into the model base case, with a duration of 1 week. When grade 2 bleeding events were included in the model in scenario analysis, the disutility of 0.122 for clinically relevant non-major bleeding events was applied to these events for a duration of 1 week. For thrombus-related AEs the company incorporates a disutility of 0.029, applied over 1 week, estimated by Jugrin et al. 84 for related thrombotic events (index deep-vein thrombosis and index pulmonary embolism).
The baseline utility value for CLD/cirrhosis patients adopted in the short-term model was also used to calculate QALYs throughout the long-term model. Utility values were adjusted to incorporate the natural decline in utility observed with ageing using the Ara and Wailoo85 equation to generate utility multipliers by age and sex.
Costs
Costs were attributed to any platelet transfusions, procedures and rescue therapies given, drug acquisition and administration and AE monitoring (Table 28).
| Value | Source | |
|---|---|---|
| Treatment costs | ||
| Lusutrombopag (3 mg, pack of 7 tablets) | Confidential information has been removed | Shionogi55 |
| Avatrombopag (20-mg tablet) | – | Dova Pharmaceuticals68 |
| Treatment dosage | ||
| Lusutrombopag (3 mg): all patients | 1 tablet per day for 7 days | EMA8 |
| Avatrombopag (20 mg): patients with platelet count of < 40 × 109/l | 3 tablets per day for 5 days | EMA6 |
| Avatrombopag (20 mg): patients with platelet count of 40–< 50 × 109/l | 2 tablets per day for 5 days | EMA6 |
| Platelet transfusion | ||
| Cost of administering first unit of platelets | £64.18 | Stokes et al.102 |
| Cost of administering subsequent units of platelets | £42.16 | Stokes et al.102 |
| Apheresis-derived platelets per ATD | £219.30 | NHSBT Pricing Proposals 2017/18103 |
| Number of ATDs transfused per platelet transfusion | Confidential information has been removed | L-PLUS 1,39 L-PLUS 2,54 Phase 2b trial |
| Cost of platelet transfusion (base case) | £313.83 | Calculation by AG |
| Cost of platelet transfusion (scenario) | £812.61 | Based on Shionogi submission model |
| Average number of platelet transfusions for patients on lusutrombopag who were transfused prior to procedure and with a platelet count of < 40 × 109/l | Confidential information has been removed | Calculated from data provided in response to clarification questions |
| Average number of platelet transfusions for patients on lusutrombopag who were transfused prior to procedure and with a platelet count of 40–< 50 × 109/l | Confidential information has been removed | Calculated from data provided in response to clarification questions |
| Average number of platelet transfusions for patients on avatrombopag, who were transfused prior to procedure and with a platelet count of < 40 × 109/l | 1.0000 | Calculated from data provided in response to clarification questions |
| Average number of platelet transfusions for patients on avatrombopag who were transfused prior to procedure and with a platelet count of 40–< 50 × 109/l | 1.0000 | Calculated from data provided in response to clarification questions |
| Average number of platelet transfusions for patients on no TPO-RA, who were transfused prior to procedure and with a platelet count of < 40 × 109/l | 1.1207 | Calculated from data provided in response to clarification questions |
| Average number of platelet transfusions for patients on no TPO-RA who were transfused prior to procedure and with a platelet count of 40–< 50 × 109/l | 1.1084 | Calculated from data provided in response to clarification questions |
| Treatment-emergent AE costs (£) | ||
| Management of PVT | 958.95 | NHS reference code YR23B86 Percutaneous Transluminal, Embolectomy or Thrombolysis, of Blood Vessel, with CC Score 0–4; day-case setting |
| Platelet transfusion-related AE costs (£) | ||
| Pneumological | 2640.00 | Whiting et al.104 |
| FAHR (major) | 1134.00 | |
| Bacteria | 2024.00 | |
| HAV | 6488.00 | |
| HBV | 8971.00 | |
| HEV | 6488.00 | Assumed to be same as HAV |
| Parvovirus | 1095.00 | Whiting et al.104 |
| Surgical procedures: costs (£) | ||
| Percutaneous RFA | 2309.03 | NHS Reference Costs86 Percutaneous Ablation of Lesion of, Liver or Pancreas, with CC Score 0–1 |
| Endoscopic variceal ligation | 4202.11 | NHS Reference Costs86 Major, Oesophageal, Stomach or Duodenum Procedures, 19 years and over, with CC Score 0–1 |
| Endoscopic injection sclerotherapy | 2410.75 | NHS Reference Costs86 Endoscopic, Sclerotherapy or Rubber Band Ligation, of Lesion of Upper Gastrointestinal Tract, with CC Score 0–2 |
| Transcatheter arterial chemoembolisation | 2921.50 | NHS Reference Costs86 Minor, Hepatobiliary or Pancreatic Procedures, with CC Score 0 |
| Liver biopsy | 1546.72 | NHS Reference Costs86 Percutaneous Transvascular Biopsy of Lesion of Liver |
| Dental extraction | 680.04 | NHS Reference Costs86 Minor Extraction of Tooth, 19 years and over |
| Vascular catheterisation | 1125.62 | NHS Reference Costs86 Peripheral Insertion of Central Venous Catheter, 19 years and over |
| Endoscopy with/without polypectomy/biopsy | 1213.27 | NHS Reference Costs86 Therapeutic Endoscopic Upper Gastrointestinal Tract Procedures, 19 years and over |
| Percutaneous RFA/microwave coagulation therapy | 2309.03 | NHS Reference Costs86 Percutaneous Ablation of Lesion of, Liver or Pancreas, with CC Score 0–1 |
| Paracentesis | 1090.43 | NHS Reference Costs86 Percutaneous Drainage of Hepatobiliary System |
| Other liver procedures | 2921.50 | NHS Reference Costs86 Minor, Hepatobiliary or Pancreatic Procedures, with CC Score 0 |
| Others | 2309.03 | NHS Reference Costs86 Percutaneous Ablation of Lesion of, Liver or Pancreas, with CC Score 0–1 |
| Surgical procedures: incidence (%) | ||
| Percutaneous RFA | 8.6 | All lusutrombopag and avatrombopag trials |
| Endoscopic variceal ligation | 10.2 | |
| Endoscopic injection sclerotherapy | 0.4 | |
| Transcatheter arterial chemoembolisation | 13.1 | |
| Liver biopsy | 3.4 | |
| Dental extraction | 8.6 | |
| Vascular catheterisation | 2.0 | |
| Endoscopy with/without polypectomy/biopsy | 36.8 | |
| Percutaneous RFA/microwave coagulation therapy | 6.3 | |
| Paracentesis | 0.7 | |
| Other liver procedures | 0.8 | |
| Others | 8.7 | |
| Rescue procedures for bleeding cost estimates (£) | ||
| AG (base case) | 370.73 | Calculated by AG based on clinical expert opinion |
| Shionogi (scenario) | 812.61 | Shionogi55 |
The cost of a 7-day course of lusutrombopag is (confidential information has been removed). Although not all patients in the trials received the full 7-day treatment course [L-PLUS 1,39 10/96 (10.4%); L-PLUS 2,54 45/215 (20.9%)], the European Medicines Agency recommends that lusutrombopag be administered for 7 days. 8 In addition, in real-world practice it is likely that the full 7-day course would be dispensed and so remaining tablets would be wasted. Therefore, the full cost of 7 days was included in the model.
Avatrombopag is administered orally once per day. For patients with a platelet count of < 40,000/µl the daily dose is 60 mg (three tablets of 20 mg), with the first dose administered 10–13 days prior to the scheduled procedure and treatment continuing for 5 days (i.e. the procedure is scheduled 5–8 days after the last dose). For patients with a platelet count between ≥ 40,000/µl and < 50,000/µl the administration and timing thereof are the same, but the dose is reduced to 40 mg (two tablets of 20 mg). No price has yet been provided for avatrombopag. Wastage will again be taken into account, with full pack costs charged. As both treatments are provided as tablets to be taken orally, no administration costs are required.
The estimated costs of a platelet transfusion consist of (1) the costs of the platelets and (2) the costs of the administration of the platelets. This estimate is multiplied by the number of platelet transfusions a patient receives prior to the elective invasive procedure, which was calculated from the data provided in response to the clarification letter for each treatment arm for each subgroup.
For the costs of platelets, the cost price for one ATD of apheresis-derived platelets was sourced from the NHS Blood and Transplant (NHSBT) Pricing Proposals 2017/18. 105 This was multiplied by the estimate of (confidential information has been removed) ATDs per transfusion (see Platelet transfusion), which led to a cost of £244.15 per transfusion.
The costs of the administration of the platelets were sourced from Stokes et al. ,102 who provided separate cost estimates for the first unit administered and for subsequent units administered. The costs of administration were inflated from 2014/15 to 2017/18 using the Hospital & Community Health Services indices provided by Curtis and Burns. 106 This led to a transfusion cost estimate of £68.96.
In the Shionogi submission, the base-case cost of platelet transfusion was based on the TA29383 appraisal of eltrombopag. In the eltrombopag appraisal this was assumed to comprise the cost of a blood transfusion (weighted average cost of £57.72 in 2011/12, code 821 blood transfusion) and the cost of 2 units of platelets (2 × £230.393 in 2011/12). The company used expert opinion to inform the average number of units of platelets that would be received per transfusion. The expert stated that most often platelet transfusions would contain either 2 or 4 units and, therefore, it was assumed that an average of 3 units of platelets would be received per transfusion. This resulted in a base-case cost of £812.61 (inflated to 2017/18), which included both administration and platelet acquisition. This assumption will be tested in scenario analysis.
The AG estimated a weighted cost of procedures conducted across all the trials, calculated using NHS Reference Costs86 in the elective inpatient setting. The procedure-specific cost estimates and their frequency are provided in Table 32. This cost was incorporated into the AG model for all treatment arms for all patients, as they were all assumed to receive their planned procedure at some point in time.
In the Shionogi model, it was assumed that, in clinical practice, rescue therapy would be an additional platelet transfusion. The AG noted that this assumption was not matched by the data presented by the companies, which showed that other methods of rescue were also used by clinicians. However, in the face of uncertainty surrounding what would actually be given in UK practice, the AG cost of platelet transfusion of £313.83 was used in the base-case analysis.
The AG clinical expert stated that he would consider giving a combination of platelet transfusion, clotting factors and tranexamic acid. An alternative value for scenario analysis was calculated by the AG based on this assumed combination. For platelet transfusions given as rescue procedures, a dosage of one ATD of platelets was costed using the NHSBT Pricing Proposals 2017/18,105 including administration costs sourced from Stokes et al. 102 For clotting factors, recombinant thrombin was costed using a price (US$104 in 2009) from Plesca,107 which was converted using purchasing power parities, and inflated from 2009/10 to 2017/18 using the Hospital & Community Health Services indices from Curtis and Burns. 106,108 A dose of 5000 units was assumed (i.e. 5 ml of 1000 units per ml). For tranexamic acid, a dosage of 2 g was assumed based on CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage-2)109 and costed using the July 2019 NHS reference price sourced from the eMIT database. 110 The sum of these costs yielded an alternative unit rescue procedure cost estimate of £370.73. This unit cost is multiplied by the number of platelet transfusions required per rescue therapy for each treatment arm in each subgroup, calculated from the pooled estimates from the trials. The remaining alternative value was based on the Shionogi base-case cost of platelet transfusion of £812.61.
Costs associated with treating transfusion-related AEs were taken from the report by Whiting et al. 104 and inflated from 2013 to 2019 (see Table 32). These costs were multiplied by the incidences of transfusion-related reactions estimated from the SHOT data. 92–96 This resulted in an estimated cost of treating transfusion-related reactions of £0.22 per transfusion. This was added to the cost of platelet transfusion, creating a base-case total cost of platelet transfusion of £313.83.
In the AG model the proportion of each treatment group experiencing PVT was found for each subgroup. This was multiplied by the unit price of £958.95 based on the NHS reference code YR23B: Percutaneous Transluminal, Embolectomy or Thrombolysis, of Blood Vessel, with CC Score 0–4 in the day-case setting. 86 This provided a treatment group-specific expected cost of treating PVT.
Probabilistic sensitivity analysis and scenario analyses
Given the parametric uncertainty surrounding the input parameters utilised in the model, probabilistic sensitivity analysis, consisting of 2000 iterations, was run to test parameter uncertainty in the model. All parameters except drug prices, drug doses and discount rates were included in the probabilistic sensitivity analysis (see Appendix 6). As is standard practice, appropriate distributions were fitted to included parameters. Beta distributions were used for probabilities, proportions, risks and utilities, gamma distributions were used for costs, beta tree was used for Child–Pugh categories and normal distributions were used for age and the number of ATDs per transfusion. Where SEs were unknown, they were estimated as 20% of the mean value. For efficacy parameters obtained from WinBUGS, probabilistic values were drawn from CODA (convergence diagnostic and output analysis) output. Cost-effectiveness planes and cost-effectiveness acceptability curves will be provided to examine the uncertainty related to the decision.
Given the structural uncertainty surrounding the input parameters utilised in the model, the AG conducted a series of scenario analyses for various efficacy, mortality, safety, cost and utility parameters. These scenario analyses are listed below and explained in more detail in the following section:
-
drug prices
-
number of ATDs per platelet transfusion
-
cost of platelet transfusion
-
cost of rescue therapy
-
inclusion of grade 2 bleeding AEs
-
probability of requiring platelet transfusion, estimated from international trials only
-
efficacy model input parameters derived from fixed-effect meta-analysis models
-
literature source for long-term Child–Pugh grade-specific mortality
-
under-reporting factor for SHOT data platelet transfusion-specific mortality
-
alternative literature source for surgery-related mortality
-
alternative literature source for baseline CLD utility
-
alternative literature source for bleeding disutility
-
alternative literature source for PVT disutility
-
alternative literature source for transfusion-related AE disutilities
-
alternative values for elective invasive procedure delay disutility and duration.
Scenarios explained
-
Drug prices.
Given that the AG does not have a price for avatrombopag (with the base-case analysis assuming the same price as lusutrombopag for both doses of avatrombopag), some scenarios around drug pricing were thought to be of value. In this scenario analysis, the price of avatrombopag was lowered.
-
Number of ATDs per platelet transfusion.
Given the substantial uncertainty surrounding the number of units/ATDs transfused in each platelet transfusion, which has already been explained in this chapter, the AG felt that it was important to examine the impact of different assumptions of number of units/ATDs on the results.
The calculation of the AG base-case assumption of each platelet transfusion containing (confidential information has been removed) ATDs was explained in the Platelet transfusion section of Methods. This value was used to calculate the cost of each platelet transfusion, as well as the cost of expected platelet transfusion AEs, by multiplying the unit cost of platelets and the incidence of AEs per unit of platelets by the number of ATDs. In the Shionogi model, clinical expert opinion led to the assumption of an average of 3 units of platelets transfused per platelet transfusion. The AG included this as an upper bound scenario, although given that the base-case unit cost of platelets identified from the NHSBT pricing proposals105 is per ATD, the AG notes that a 3-unit assumption will probably overestimate the costs of platelet transfusion. Scenarios of one and two ATDs per transfusion will also be included to provide a range of estimates and to investigate the impact on the model results.
-
Cost of platelet transfusion.
In the AG base-case analysis, the cost of platelet transfusion is calculated from Stokes et al. ,111 whereas the unit cost of an ATD of platelets (obtained from apheresis) is taken from the NHSBT pricing proposals. 105 The cost of treating transfusion-related reactions was estimated at £0.22 per transfusion, using costs from Whiting et al. 104 and incidences from the SHOT data. 92–96 This resulted in a cost per platelet transfusion of £313.83. Two alternative sources of costs were taken from the Shionogi model.
The first scenario will use the Shionogi base-case cost of platelet transfusion. This estimate was obtained from the TA293 appraisal, which estimated a cost of blood transfusion from code 821, blood transfusion, of £57.72 in 2011/12 and a cost per unit of platelets of £230.393 in 2011/12. The company used expert opinion to obtain the average number of units of platelets that would be received per transfusion. The expert stated that most often platelet transfusions would contain either 2 or 4 units and, therefore, it was assumed that an average of 3 units of platelets would be received per transfusion. This resulted in a cost of £812.61 (inflated to 2017/18), which will be tested in this scenario.
The second scenario provided by Shionogi used the Healthcare Resource Group (HRG) codes for single plasma exchange or other intravenous blood transfusion for day-case and elective inpatient transfusions. These were weighted by the proportions of transfusions that have been conducted as day-case and elective inpatient cases, resulting in a weighted cost of £517.28.
-
Cost of rescue therapy.
In the Shionogi model, it was assumed that, in clinical practice, rescue therapy would be an additional platelet transfusion. The AG noted that this assumption was not matched by the data presented by the companies, which showed that other methods of rescue were also used by clinicians. However, in the face of uncertainty surrounding what would actually be given in UK practice, the AG cost of platelet transfusion of £313.83 was used in the base-case analysis. The AG clinical expert stated that he would consider giving a combination of platelet transfusion, clotting factors and tranexamic acid. The cost of this combination was used as an alternative, with a value of £370.73. The remaining alternative value was based on the Shionogi base-case cost of platelet transfusion of £812.61.
-
Inclusion of grade 2 bleeding AEs.
The AG base-case analysis includes only bleeding events of ≥ grade 3 (severe). In the scenario analysis, grade 2 (moderate) bleeding events are also included, with a disutility for clinically relevant, non-major bleeding events attached.
-
Probability of requiring platelet transfusion prior to surgery, estimated from international trials only.
In the AG base-case analysis the probability of requiring platelet transfusion was calculated from all pooled trials. To investigate whether or not there is a difference in efficacy between the two trials conducted in Japan only and the international trials, the probability of requiring platelet transfusion will be estimated from only international trials in this scenario. This scenario would have also been relevant for the following probabilities: grade 3 bleeding events and rescue therapy required. However, the numbers of events in these cases were too small to generate reliable results from only the international trials. Therefore, only the probability of requiring platelet transfusion prior to surgery was adjusted.
-
Efficacy parameters obtained from fixed-effects meta-analysis model.
In the base-case analysis, the efficacy input parameters (i.e. proportion of patients receiving no platelet transfusion and proportion of patients who did not require a request therapy) were obtained from random-effects meta-analysis models. In this scenario analysis, the impact of using efficacy parameters from fixed-effects models will be elaborated.
-
Literature source for long-term Child–Pugh grade-specific mortality.
In the base-case analysis, long-term CLD mortality was estimated using data from a systematic review by D’Amico et al. ,78 which used survival at 1 and 2 years for each Child–Pugh grade to estimate an extrapolated survival curve. This was weighted based on the proportions of patients with each Child–Pugh grade, pooled from all trials.
For the scenario analysis, the alternative data source identified by Shionogi using data from the UKMi98 to estimate survival, again using the Child–Pugh categories pooled from the trials, was utilised.
-
Under-reporting factor for SHOT data platelet transfusion-specific mortality.
In the AG base-case analysis, platelet transfusion-related mortality was estimated by the AG from SHOT data from 2012 to 2017. There have been concerns in the literature that the SHOT data under-report deaths due to transfusion-related acute lung injury. 97 Therefore, the AG included an under-reporting factor relating to this parameter in the model. In the base-case analysis, the estimate from the SHOT data was unadjusted. However, in the scenario analysis, this value was multiplied by 2, 5 and 10 to investigate the impact on the model results.
-
Alternative literature source for surgery-related mortality.
The probability of surgical-related mortality was estimated from the trial mortality data. In the base-case analysis, a binomial likelihood model was used to estimate the baseline mortality risk using a random-effects model with the predictive distribution, which resulted in pooled risk of 0.0195 (95% CI 0.0004 to 0.13). As this was a scenario analysis, the mortality risk from the posterior distribution was used, which resulted in pooled risk of 0.006955 (95% CI 0.0004 to 0.019).
-
Alternative literature source for baseline CLD utility.
In the base-case analysis, a baseline EQ-5D-3L utility value estimated for patients with CLD/cirrhosis was adopted from a study by Sullivan et al. 82 In its original model, Shionogi provided an alternative baseline utility value from a study by Scalone et al. ,99 which was used as the scenario analysis value.
-
Alternative literature source for bleeding disutility.
The AG could not find any alternative literature sources for the disutility of a major bleed. Therefore, the base-case value was increased and decreased by 25%.
-
Alternative literature source for PVT disutility.
The AG could not find any alternative literature sources for the disutility of PVT. Therefore, the base-case value was increased and decreased by 25%.
-
Alternative literature source for transfusion-related AE disutilities.
In the base-case analysis, a disutility of 0.1 for patients experiencing serious platelet transfusion-related AEs was applied for one model cycle (4 weeks). This value was taken from TA293,83 a previous NICE appraisal of eltrombopag for thrombocytopenic purpura. In its model, Shionogi provided an alternative disutility for platelet transfusion of 0.17, taken from van Eerd et al. 73 This value was used in the scenario analysis.
-
Alternative values for elective invasive procedure delay disutility and duration.
In the base-case the AG assumed a disutility for the delay of the planned procedure of 0.072 (calculated from the average decrement associated with a one-level increase in anxiety and depression on the EQ-5D-5L UK value set). 101 This disutility was varied between 0 and 0.144 by halving and doubling the assumed decrement, as well as by assuming no decrement. In the base-case analysis, this decrement was assumed for 4 weeks to account for elective invasive procedures being delayed beyond the 35-day initial cycle. This duration was varied between 2 and 6 weeks to investigate the impact on model results.
Results
Assessment group base-case deterministic results
The base-case deterministic model results from the AG model are shown in Table 29. The price of avatrombopag for both subgroups is assumed to be (confidential information has been removed), equal to the price of lusutrombopag.
| Technologies | Total costs (£) | Total LYGs | Total QALYs | Incremental costs (£) | Incremental LYGs | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|---|
| Platelet count < 40,000/µl subgroup | |||||||
| No TPO-RA | Confidential information has been removed | 7.3961 | 3.3626 | ||||
| Lusutrombopag | Confidential information has been removed | 7.3961 | 3.3627 | 592 | 0.00002 | 0.00017 | 3,422,801 |
| Avatrombopag 60 mg | Confidential information has been removed | 7.3961 | 3.3627 | 49 | –0.000006 | –0.000079 | Dominated |
| Platelet count 40,000–< 50,000/µl subgroup | |||||||
| No TPO-RA | Confidential information has been removed | 7.3961 | 3.3625 | ||||
| Lusutrombopag | Confidential information has been removed | 7.3961 | 3.3625 | 624 | 0.00002 | 0.000000007 | 84,890,361,589 |
| Avatrombopag 40 mg | Confidential information has been removed | 7.3961 | 3.3629 | 9 | 0.00000 | 0.00041 | 21,947 |
In both subgroups, no TPO-RA incurred the lowest costs and fewest QALYs. In the < 40,000/µl subgroup, lusutrombopag is the next cheapest option, with an incremental cost compared with no TPO-RA of £592 and incremental QALYs of 0.00017 (which is equivalent to a gain of 1.5 quality-adjusted life-hours), resulting in a deterministic incremental cost-effectiveness ratio (ICER) of around £3,400,000. Avatrombopag 60 mg is the most expensive option in this subgroup but incurs a lower QALY gain than lusutrombopag, with an incremental QALY of –0.000079. Avatrombopag 60 mg is therefore dominated by lusutrombopag in the < 40,000/µl subgroup. In the 40,000–< 50,000/µl subgroup, lusutrombopag is the cheapest option after no TPO-RA, with an incremental cost of £624 and an incremental QALY of 0.000000007, resulting in an ICER of > £84,000,000,000 compared with no TPO-RA. Avatrombopag 40 mg is the most expensive option in this subgroup but provides a higher QALY gain, with an incremental QALY gain of 0.00041 over lusutrombopag. This results in an ICER of £21,947 for avatrombopag 40 mg compared with lusutrombopag. However, it should be noted that the incremental QALYs are extremely small, and in both subgroups all treatments resulted in almost identical QALYs.
The disaggregated cost results in Table 30 show that, although the costs of platelet transfusion, AE management and rescue therapy are higher for no TPO-RA than for lusutrombopag and avatrombopag (except for AE costs in the 40,000–< 50,000/µl subgroup), the combined difference in cost is still substantially lower than the drug costs for lusutrombopag and avatrombopag. This results in incremental costs of > £500 for both treatments compared with no TPO-RA. In the face of such small incremental QALYs, this incremental cost has a large impact on the ICER. In both subgroups, the dominance of one treatment over the other is mostly due to the differences in the QALY decrements as a result of bleeding, which lead to small but important differences in the total QALYs (Table 31).
| Treatment | Cost (£) | |||||
|---|---|---|---|---|---|---|
| Drug | Platelet transfusion | AE | Elective invasive procedure | Rescue therapy | Total | |
| Platelet count < 40,000/µl subgroup | ||||||
| No TPO-RA | 0 | 265 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Lusutrombopag | Confidential information has been removed | 91 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Avatrombopag 60 mg | Confidential information has been removed | 148 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||
| No TPO-RA | 0 | 231 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Lusutrombopag | Confidential information has been removed | 64 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Avatrombopag 40 mg | Confidential information has been removed | 44 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Disaggregated QALYs | QALY decrement | Total long-term discounted QALYs | |||
|---|---|---|---|---|---|
| Platelet transfusion | Bleeding | Rescue therapy | AEs | ||
| Platelet count < 40,000/µl subgroup | |||||
| No TPO-RA | 0.0000007 | 0.0000315 | 0.0000002 | 0.0000085 | 3.310993 |
| Lusutrombopag | 0.0000002 | 0.0000241 | 0.0000001 | 0.0000071 | 3.311002 |
| Avatrombopag 60 mg | 0.0000004 | 0.0001003 | 0.0000001 | 0.0000066 | 3.310999 |
| Platelet count 40,000–< 50,000/µl subgroup | |||||
| No TPO-RA | 0.0000006 | 0.0000744 | 0.0000002 | 0.0000079 | 3.310994 |
| Lusutrombopag | 0.0000002 | 0.0002274 | 0.0000001 | 0.0000182 | 3.311002 |
| Avatrombopag 40 mg | 0.0000001 | 0.0000481 | 0.0000000 | 0.0000482 | 3.311004 |
Probabilistic sensitivity analysis results
The probabilistic results in Table 32 for the < 40,000/µl subgroup follow the same pattern as that of the deterministic results. Lusutrombopag is more expensive than no TPO-RA by £600 [i.e. (confidential information has been removed) more expensive] and more effective by 0.0001 QALYs, resulting in an ICER of approximately £4,000,000. Avatrombopag 60 mg is slightly more expensive and slightly less effective than lusutrombopag and is therefore dominated. In the 40,000–< 50,000/µl subgroup, no TPO-RA is again the cheapest option. Lusutrombopag is the next cheapest and most effective option, with an incremental cost of £626 and incremental QALYs of 0.0004. Avatrombopag 40 mg is £10 more expensive than lusutrombopag and –0.00054 QALYs less effective and is therefore dominated by lusutrombopag.
| Treatment | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Platelet count < 40,000/µl subgroup | |||||
| No TPO-RA | Confidential information has been removed | 3.5681 | |||
| Lusutrombopag | Confidential information has been removed | 3.5683 | 600 | 0.0001 | 4,006,891 |
| Avatrombopag 60 mg | Confidential information has been removed | 3.5682 | 38 | –0.0000 | Dominated |
| Platelet count 40,000–< 50,000/µl subgroup | |||||
| No TPO-RA | Confidential information has been removed | 3.5551 | |||
| Lusutrombopag | Confidential information has been removed | 3.5555 | 626 | 0.0004 | 1,555,549 |
| Avatrombopag 40 mg | Confidential information has been removed | 3.5550 | 10 | –0.0005 | Dominated |
The cost-effectiveness planes (Figures 5 and 6) for both subgroups show that, for the majority of iterations, both treatments are more costly and more effective than no TPO-RA. However, each diagram also shows that a substantial proportion of iterations fall in the north-west quadrant, where the treatments are more expensive but less effective than no TPO-RA. This can be seen most prominently for avatrombopag in the 40,000–< 50,000/µl subgroup, for which it appears that approximately half of the iterations suggest that avatrombopag is less effective than no TPO-RA (orange points). This indicates that, given the uncertainties in the model, the treatments should be regarded as having equivalent effectiveness in terms of QALYs.
FIGURE 5.
Cost-effectiveness plane for subgroup: platelet count of < 40,000/µl (lusutrombopag and avatrombopag 60 mg vs. placebo).
FIGURE 6.
Cost-effectiveness plane for subgroup: platelet count of 40,000–< 50,000/µl (lusutrombopag and avatrombopag 40 mg vs. placebo).
The cost-effectiveness acceptability curves in turn (Figures 7 and 8) show that, for all threshold ICERs up to £100,000, no TPO-RA has 100% probability of being the most cost-effective treatment.
FIGURE 7.
Cost-effectiveness acceptability curve for platelet count of < 40,000/µl.
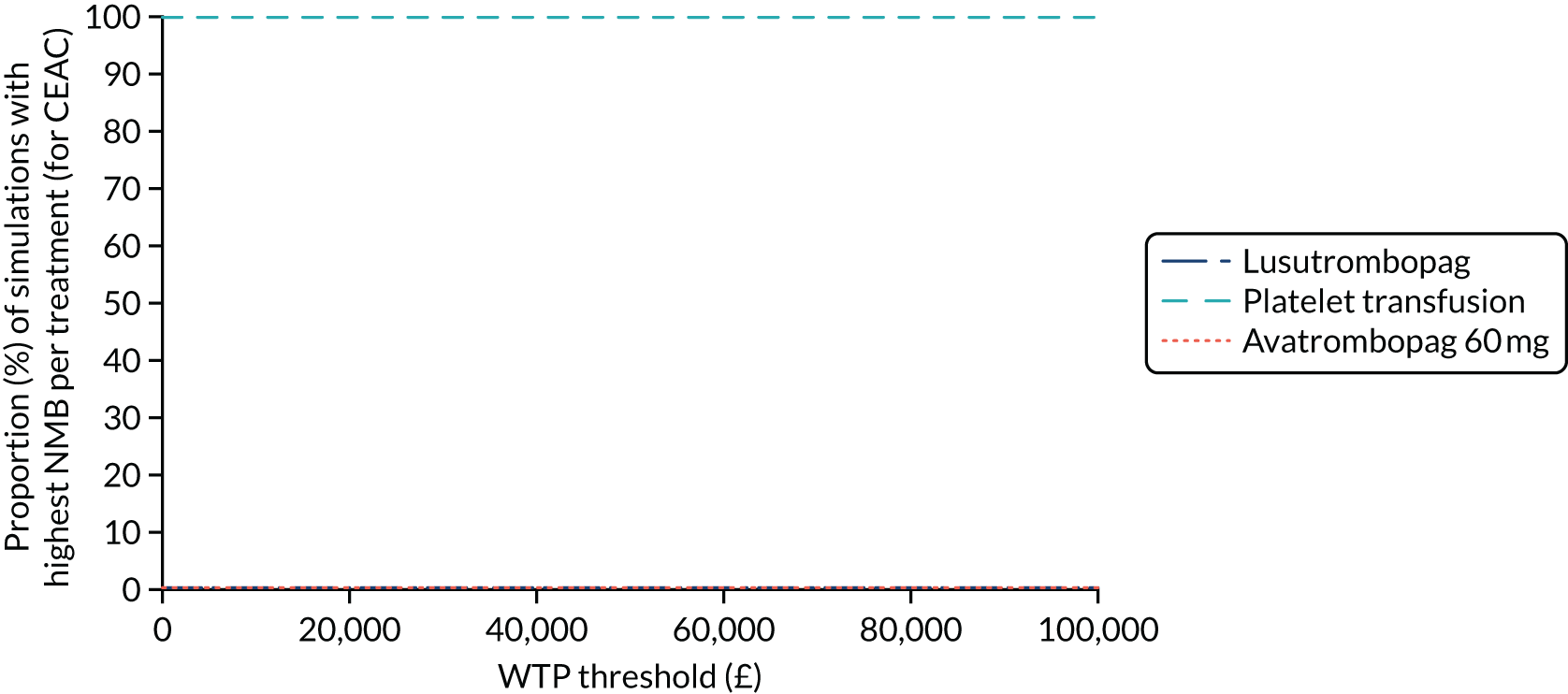
FIGURE 8.
Cost-effectiveness acceptability curve for platelet count of 40,000–< 50,000/µl.
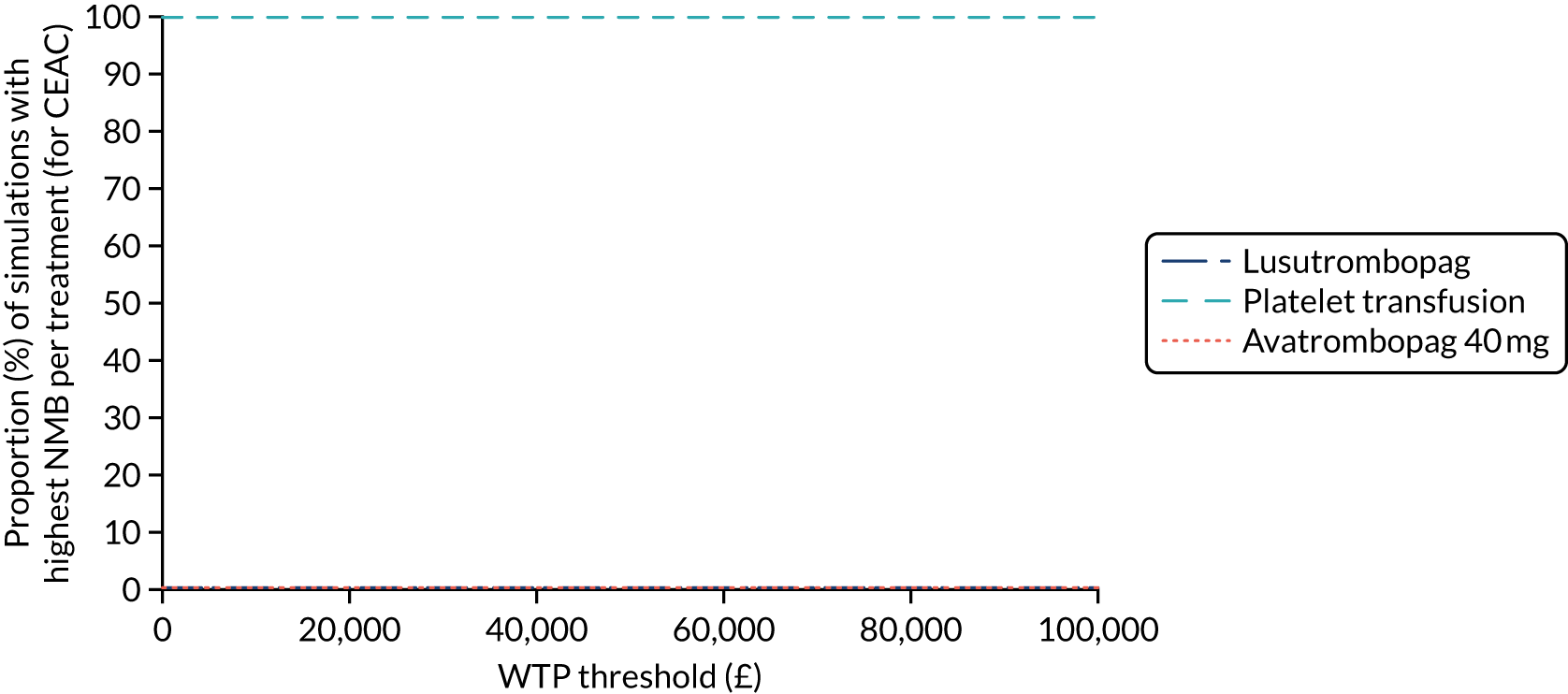
Scenario analysis results
Given the uncertainty surrounding the input parameters utilised in the model, the AG conducted a series of scenario analyses using various efficacy, mortality, safety, cost and utility parameters. These scenario analyses are listed below and the results of each are provided in Operational validation efforts on the assessment group model.
-
drug prices
-
number of ATDs per platelet transfusion
-
cost of platelet transfusion
-
cost of rescue therapy
-
inclusion of grade 2 bleeding AEs
-
probability of requiring platelet transfusion, estimated from international trials only
-
cost of elective invasive procedure taken from international trials only
-
literature source for long-term Child–Pugh grade-specific CLD mortality
-
under-reporting factor for SHOT data platelet transfusion-specific mortality
-
alternative method for calculating surgery-related mortality
-
alternative literature source for baseline CLD utility
-
alternative literature source for bleeding disutility
-
alternative literature source for PVT disutility
-
alternative literature source for transfusion-related AE disutilities
-
alternative values for elective invasive procedure delay disutility and duration
-
cost of elective invasive procedure cancellation
-
proportion of patients requiring platelet transfusion hospitalised the day before elective invasive procedure.
The description and the results of these analyses are presented in Appendix 7. Among these scenarios, only the first three (i.e. using different drug prices, different number of ATDs per platelet transfusion and different platelet transfusion costs) had a substantial impact on the incremental results.
Operational validation efforts on the assessment group model
The AG conducted the following validation efforts:
-
comparing the clinical outcomes of the AG economic model with those of clinical trials
-
comparing the economic and health outcomes of the AG economic model and the Shionogi economic model.
Comparison of the clinical outcomes from the model with clinical trials
The model primary clinical outcomes (i.e. the proportion of patients who did not receive a platelet transfusion and the proportion of patients who received neither platelet transfusion nor rescue therapy) is compared with the minimum–maximum ranges from the clinical trials (Table 33). The model generates outputs within the range of the clinical trial results for lusutrombopag and no TPO-RA for both outcomes. However, for avatrombopag, the model underestimates both the clinical trial outcomes for the platelet count < 40,000/µl subgroup and the proportion of patients who did not receive a platelet transfusion outcome in the platelet count 40,000–< 50,000/µl subgroup.
| Platelet count subgroup | No TPO-RA patients who received no PT (%) | Lusutrombopag patients who received no PT (%) | Avatrombopag patients who received no PTa (%) | |||
|---|---|---|---|---|---|---|
| Model | Trials (minimum–maximum) | Model | Trials (minimum–maximum) | Model | Trials (minimum–maximum) | |
| < 40,000/µl | 30.55 | 5.3–54.2 | 76.93 | Confidential information has been removed | 57.09 | 78.9–82.9 |
| 40,000–< 50,000/µl | 38.82 | 17.9–54.5 | 83.44 | Confidential information has been removed | 89.92 | 93.2–94.8 |
| No TPO-RA patients who received no PT and no rescue (%) | Lusutrombopag patients who received no PT and no rescue (%) | Avatrombopag patients who received no PT and no rescuea (%) | ||||
| < 40,000/µl | 25.20 | 5.3–34.9 | 69.93 | Confidential information has been removed | 52.71 | 65.6–68.6 |
| 40,000–< 50,000/µl | 31.90 | 17.9–40.5 | 74.17 | Confidential information has been removed | 86.36 | 87.9–88.1 |
This gap between the model and trial outcomes can be explained by the fact that in the model the proportion of patients experiencing each clinical outcome was obtained from meta-analyses. For each outcome in each subgroup, a common baseline proportion for the placebo arm was required, which pooled the corresponding placebo proportions from all trials. As the placebo proportions for the two clinical outcomes from ADAPT-137 and ADAPT-237 were different from those in the lusutrombopag trials, this difference is accentuated in the difference between the clinical trial outcomes and the model results based on the meta-analysis results.
Comparison of the clinical outcomes from the assessment group economic model and the Shionogi economic model
For cross-validity, the model outcomes from the AG model and the Shionogi model are compared. The placebo arm platelet transfusion proportions were updated to reflect the lusutrombopag trials to improve the comparability (i.e. in the base-case analysis, the Shionogi model considered 100% platelet transfusion for placebo arm patients). The resulting differences in model outcomes are shown in Table 34.
| Treatment arm | AG (< 40,000/µl) | AG (40,000–< 50,000/µl) | Shionogi modela |
|---|---|---|---|
| Total LYs (discounted) | |||
| Lusutrombopag | 7.3961 | 7.3961 | 7.7709 |
| Placebo | 7.3961 | 7.3961 | 7.7496 |
| Total QALYs (discounted) | |||
| Lusutrombopag | 3.3627 | 3.3625 | 4.0354 |
| Placebo | 3.3626 | 3.3625 | 4.0236 |
| Proportion receiving no platelet transfusion prior to elective invasive procedure (%) | |||
| Lusutrombopag | 76.93 | 83.44 | Confidential information has been removed |
| Placebo | 30.55 | 38.82 | Confidential information has been removed |
| Proportion receiving no rescue therapy and no platelet transfusion (%) | |||
| Lusutrombopag | 69.93 | 74.17 | Confidential information has been removed |
| Placebo | 25.2 | 69.93 | Confidential information has been removed |
| Proportion not receiving their elective invasive procedure during the trial period | |||
| Lusutrombopag | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Short-term proportion alive | |||
| Lusutrombopag | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
The AG model results in fewer life-years and fewer short-term alive proportions than the Shionogi model. This is because of differing surgery mortality inputs for two models.
The platelet transfusion and recue therapy-related model outputs differ substantially between the Shionogi and AG models. These differences are mostly due to the difference in how the chance node probabilities were obtained. The AG model used formal meta-analysis methods, whereas the Shionogi model used simple pooling.
The QALY difference between the two models is a little more accentuated than the difference in life-years.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Given that both avatrombopag and lusutrombopag are taken orally and would be expected to be administered in addition to established clinical practice, no additional change in clinical practice aside from their administration is expected. Indeed, as shown in the cost-effectiveness analysis (see Chapter 4, Independent economic assessment, Results), there would be a reduction only in the resources currently allocated to this established practice, most notably platelet transfusion.
Chapter 6 Discussion
Statement of principal findings
From a comprehensive search that retrieved 11,305 records, and after screening, 35 references pertaining to six studies were included. All six studies,37,39,51,53,54 including both sets of main trials for each of the TPO-RAs, ADAPT-1, ADAPT-2, L-PLUS 1 and L-PLUS 2, were at low risk of bias.
The main finding was that both avatrombopag (for both platelet subgroups) and lusutrombopag were clearly effective in comparison with no TPO-RA in terms of primary outcome, including that of three of the main trials, ADAPT-1,37 ADAPT-237 and L-PLUS 2,54 namely avoidance of platelet transfusion or rescue procedure for bleeding. Both avatrombopag and lusutrombopag were also shown to increase the proportion of patients who had increased platelet counts or achieved a particular target (i.e. ≥ 20,000/µl above baseline and at least one platelet count of > 50,000/µl from days 4 to 8). 37,39,51,53,113
Neither avatrombopag nor lusutrombopag was unequivocally better than no TPO-RA in terms of AEs, and there was some small amount of evidence to show a higher percentage of deaths with both TPO-RAs. 37,54
When the main outcomes of the avoidance of the composite outcome of no platelet transfusion before the elective procedure or rescue therapy or the avoidance of platelet transfusion only were analysed according to the subgroups that matched the expected licensed doses of avatrombopag (< 40,000/µl for 60 mg or 40,000–< 50,000/µl for 40 mg), both avatrombopag and lusutrombopag were superior to placebo and mostly with a statistically significant difference (i.e. 95% CIs did not overlap the point of no difference). The exception was the very small Japic CTI-121944 study. 53 However, when the outcome of avoidance of rescue therapy was considered alone, albeit only in those who did not receive platelet transfusion before the elective procedure, the lusutrombopag trials were revealed to have a much lower frequency than the ADAPT trials37 regardless of treatment arm, and the explanation for this is not obvious. The trials also show that there is no statistically significant difference between lusutrombopag and placebo. However, there was a statistically significant difference for avatrombopag between the < 40,000/µl subgroup of ADAPT-137 and the 40,000–< 50,000/µl subgroup in ADAPT-2. 37 This did imply an advantage of avatrombopag over lusutrombopag from the indirect comparison, but this was statistically significant only in the fixed-effect analysis of the < 40,000/µl subgroup. The proportion of those who received no rescue therapy who received platelets was not available to the AG.
The implications of these results are that both TPO-RAs are effective in reducing platelet transfusion prior to the elective procedure. However, there seems to be little difference between them and no TPO-RAs in AEs, including death or in the avoidance of rescue therapy due to bleeding. Neither was there much difference between the two TPO-RAs in any outcome that included avoidance of platelet transfusion and in any of the two main platelet subgroups (i.e. < 40,000/µl or 40,000–< 50,000/µl). It is interesting to note that this was not the case for the avoidance of rescue therapy given no receipt of platelet transfusion: there was some evidence of an advantage to avatrombopag. However, the underlying rate of rescue therapy was much higher in the avatrombopag trials and so this cannot be ruled out as a confounding factor.
When the cost-effectiveness of both TPO-RAs was compared with that of no TPO-RA, it was clear that, in terms of QALYs, TPO-RAs have only marginal benefit over care as usual. When uncertainty is taken into account, both lusutrombopag and avatrombopag have about a 50% chance of being more effective than no TPO-RA in terms of QALYs gained. This essentially reduces the cost-effectiveness analysis to a cost-minimisation analysis. For both subgroups, no TPO-RA clearly has the lowest costs, even when taking uncertainties into account. Lusutrombopag is about (confidential information has been removed) more costly than no TPO-RA in the < 40,000/µl subgroup and avatrombopag (confidential information has been removed) more costly. For the 40,000–< 50,000/µl subgroup, avatrombopag and lusutrombopag are (confidential information has been removed) and (confidential information has been removed) more expensive than no TPO-RA, respectively. In the probabilistic sensitivity analysis, it was shown that, for all thresholds < £100,000, no TPO-RA had a 100% probability of being cost-effective.
Various scenario analyses showed that the results are most sensitive to the (currently unknown) price of avatrombopag. If the price of avatrombopag were to be (confidential information has been removed) below the price of lusutrombopag, avatrombopag would become cost saving in the 40,000–< 50,000/µl subgroup.
Three of the 15 other scenarios, namely ‘number of ATDs per platelet transfusion’, ‘cost of platelet transfusion’ and ‘under-reporting factor for SHOT data platelet transfusion specific mortality’ had a substantial impact on the cost-effectiveness results. In each of these cases the avatrombopag costs would decrease in the 40,000 < 50,000/µl subgroup to values of around 10% more than no TPO-RA in the most extreme scenarios. However, even in these four scenarios, the ICERs would remain very high and clearly out of the range of acceptable ICERs.
Strengths and limitations of the assessment
Throughout this review, the methods recommended in the Cochrane Collaboration Handbook9 and by the CRD10 were applied to reduce the risk of bias and error. This included the search strategy, which was designed to be highly sensitive to ensure the lowest risk of missing any relevant studies in either the clinical effectiveness or the cost-effectiveness section. In addition, all published outcomes in terms of effectiveness and AEs were extracted. Furthermore, the AG sought and obtained further data from the companies responsible for each of the interventions to inform subgroup analyses necessary to compare the interventions in meta-analyses. All available data were pooled in these meta-analyses, and robustness was tested by comparing fixed- and random-effects analyses as well as sensitivity analyses to test the effect of excluding particular studies.
The review was limited initially by the lack of many of the data needed to make the comparison of lusutrombopag with avatrombopag in the < 40,000/µl and 40,000–< 50,000/µl subgroups. However, this has been largely resolved by the company response to the AG request for clarification. 56,57 Nevertheless, some of the rescue therapy data for lusutrombopag were not provided in those subgroups. In addition, there are inconsistencies in the avatrombopag data, as discussed in Uncertainties. There was also clinical heterogeneity between the lusutrombopag trials as well as between the lusutrombopag and avatrombopag sets of trials. However, statistical heterogeneity was no more than moderate, and the robustness of outcomes in terms of the extent of the difference between TPO-RA and no TPO-RA and between both TPO-RAs was demonstrated in sensitivity analyses.
From the cost-effectiveness point of view, there were several additional important gaps in the evidence required to conduct the analysis. Most notably, Dova Pharmaceuticals declined to provide a price for avatrombopag. This severely hindered the AG’s ability to fairly compare the two treatments in terms of cost-effectiveness, as it was necessary to assume that the price of avatrombopag was the same as the price of lusutrombopag. There was also a lack of consistent reporting and data provision on the content of platelet transfusions, which led to substantial uncertainty when calculating costs and safety related to platelet transfusion and rescue therapy. This will be discussed further in Uncertainties.
Uncertainties
There appeared to be a difference in the timing of platelet transfusion avoided, with the L-PLUS studies39,54 specifying prior to the elective procedure and the ADAPT studies37 specifying up to 7 days following randomisation. It is also not clear what independent contributions are made by platelet transfusion and rescue procedure, given that nature of the composite outcome.
In the ADAPT trials37 all patients received avatrombopag for 5 days, whereas in the L-PLUS trials39,54 lusutrombopag was administered for between 5 and 7 days depending on platelet count, that is, if the platelet count was at least 50,000/µl with an increase of at least 20,000/µl then no additional dose was given. The implications of this difference are that lusutrombopag was administered over a longer period on average than avatrombopag. However, the implications for clinical practice would depend on the stopping rule applied in clinical practice. Indeed, it was stated in the European Public Assessment Report for lusutrombopag8 that there was ‘. . . no clear difference in platelet response for patients without platelet transfusion was found between the group receiving a fixed dosing regimen of 7 days and the group where a stopping criterion was applied’ (reproduced with permission; © European Medicines Agency). However, this same document8 stated ‘The presented data indicate a slightly improved efficacy of lusutrombopag at a fixed 7-day treatment regimen. Conversely, comparative assessment of safety data is uncertain due to the sparsity of data. However, it is considered that the data presented do not implicate a substantial safety issue with regard to a 7-day treatment with lusutrombopag without the application of a stopping criterion’ (reproduced with permission; © European Medicines Agency). Nevertheless, this same document8 refers to the absence of a stopping rule in the summary of product characteristics. 114 The European Public Assessment Report for avatrombopag6 states a fixed time of 5 days, as in the ADAPT trials,37 and so, essentially, no stopping rule would apply to both drugs in clinical practice. In addition, Dova Pharmaceuticals responded to our question regarding this by saying that it is expected that all patients who are treated will receive 5 days of dosing and that patients who have been treated in the USA have all received 5 days of treatment with the drug. 57 It therefore seems plausible that, should no stopping rule apply, the effectiveness of lusutrombopag might be greater than was observed in the L-PLUS trials. However, a compromise in terms of safety cannot also be ruled out.
The proportion of patients who received no rescue therapy who received platelet transfusion was not available to the AG. Shionogi did provide the number of patients who received platelet transfusion as rescue therapy in each of the subgroups (see Table 5), but it provided only the number of those who received any rescue therapy per trial arm (i.e. not in each subgroup). 56 Dova Pharmaceuticals appeared superficially to have provided these numbers in each subgroup, but there was a large discrepancy between the numbers used to inform Table 23 and those reported in the response to clarification. For example, the number of patients calculated to receive rescue therapy in the avatrombopag arm of the < 40,000/µl subgroup of ADAPT 137 is 71 – 59 = 12. However, the number reported to have received rescue therapy in table ‘Summary of Rescue Therapy – FAS’ in the response to clarification is 1. 57 Similarly, the number of patients calculated to receive rescue therapy in the placebo arm of the < 40,000/µl subgroup of ADAPT 137 is 26 – 11 = 15, but the corresponding number in the response to clarification is 4. 57
Although there appeared to be little difference in mortality between each of the TPO-RAs and no TPO-RA, as reported in Table 17, follow-up specifically for mortality was unclear and total trial follow-up was short, at no more than 5 weeks (see Table 8). Therefore, the longer-term outcomes remain uncertain.
The cost-effectiveness analysis was subject to a range of structural and parameter uncertainties. In terms of cost-effectiveness parameters, one of the biggest uncertainties was the content, and therefore the cost, of platelet transfusion. The lack of consistent reporting internationally, as well as between centres, on definitions of terms such as ‘units’ and ‘pools’, and on the number of platelets these terms correspond to and how these link to UK practice and reference prices led to substantial uncertainty regarding this parameter. Although the AG was able to estimate a cost based on ATDs through searching UK guidelines, consulting its clinical expert and using data on the number of platelets transfused provided by Shionogi in its clarification response, it notes that this cost is much lower than that estimated by Shionogi in its model. 56 As can be seen from scenario analyses of the cost and size of platelet transfusions, assumptions surrounding these aspects have a large impact on the ICER. Given the very small QALY gains associated with these treatments, cost minimisation becomes important. As the main source of efficacy for these treatments is that they reduce the need for platelet transfusions, this is where the majority of the drug costs are offset. However, the issue is compounded further by the fact that the other main area of the model in which costs can be avoided is the reduction in the number of rescue therapies required, whose cost is also largely dependent on the chosen cost of platelet transfusion. Therefore, the price of platelet transfusion is crucial in determining the price at which these drugs will be cost-effective.
An additional source of uncertainty in the model is the effectiveness of the TPO-RA agents in reducing the probability of delays to surgery and the implication that this would have in terms of costs and QALYs. The treatment group-specific probabilities of delay to surgery were obtained from a single trial (L-PLUS 254), which provided only overall probabilities for lusutrombopag and no TPO-RA that were not separated by subgroup. Furthermore, it was not clear if the reason for surgery postponement was solely thrombocytopenia. Therefore, the AG had to assume that the probability of procedure delay was the same for both TPO-RAs and across subgroups, which may not be a true reflection of reality. In addition, assumptions had to be made about the implication of delays to surgery for costs and utility. The AG assumed a disutility associated with lengthy delays to a procedure as it assumed that this would have an impact on patients by increasing their worry and anxiety. However, ideally, this assumption would be based on evidence, as it is uncertain. The AG also felt it inappropriate to include a sunk cost for cancelled surgeries in the base-case analysis, given that this cost was removed from the reference costs over 10 years ago and the assumption that surgeon and theatre time would still be efficiently used for other procedures. Scenario analyses were conducted to examine the impact of assumptions surrounding sunk costs and disutilities associated with delays to surgery as well as the impact of additional hospitalisation before surgery due to the platelet transfusion. The cost scenario had a limited impact on results. The surgery delay disutility and the pre-surgery hospitalisation scenarios reduced the ICERs; however, the ICERs after these reductions remained outside acceptable ranges. When combined with the assumption that all patients who require platelet transfusion will be hospitalised before surgery, a higher cost to the NHS of procedure cancellation or rescheduling or a more substantial disutility associated with delays would mean that the cost-effectiveness of TPO-RAs, if they are indeed effective in reducing the probability of delay, would increase. However, this would probably not be sufficient to make them cost-effective, as the main difference in costs is drug related.
Chapter 7 Conclusions
Implications for service provision
If the aim of service provision is to reduce platelet transfusion prior to elective procedures in those with CLD, then both lusutrombopag 3 mg and avatrombopag 60 mg or 40 mg for the < 40,000/µl or 40,000–< 50,000/µl subgroups, respectively, would seem to be able to do that safely. The evidence suggests that avatrombopag might also be able to reduce the need for rescue therapy for bleeding. However, given the large difference between the rates of rescue therapy between the lusutrombopag and avatrombopag trials, it is uncertain under what circumstances this might be observed in clinical practice.
Similarly, from the cost-effectiveness point of view, given the lack of difference in long-term QALYs between TPO-RA options and no TPO-RA, the aim of service provision may become important in the decision. If the aim is to reduce reliance on platelet transfusion, evidence suggests that TPO-RAs are successful in safely achieving this. Therefore, careful consideration must be given to the costs of platelet transfusion compared with TPO-RA drug costs. If the focus is on long-term QALY benefits rather than reducing reliance on platelet transfusion, the results suggest that the TPO-RA options assessed are not cost-effective given the current assumptions surrounding costs and effects.
Suggested research priorities
Given the need to compare the two TPO-RAs and the potential lack of comparability of the extant trials, a head-to-head trial is warranted. This should ideally measure all relevant outcomes, including risk of platelet transfusion separate from rescue therapy and with a longer follow-up at least of mortality. The trial should be of a size that permits subgroup analysis according to baseline platelet count as well as in terms of CLD type and elective procedure.
Any future trials in this area should focus on consistently collecting data on the content of platelet transfusions in terms of the number of platelets transfused or consistent and clear definitions such as ATDs so that accurate costs can be calculated. This is particularly important given that the avoidance of platelet transfusion does not seem to translate into differences in QALYs. Therefore, accurate costing is crucial for decision-making.
Acknowledgements
We are very grateful to Dr Stephen Ryder, Consultant Hepatologist at Nottingham University Hospital Trust, who offered clinical advice and comments on the draft report.
We also gratefully acknowledge the work of David Smalbrugge, Master Student Health Economics, Policy and Law at the Erasmus School of Health Policy & Management, for data extraction from SHOT reports and estimation of input from those data.
Contributions of authors
Nigel Armstrong (https://orcid.org/0000-0002-7443-4798) is the corresponding author and acted as project lead, devised the clinical effectiveness methods and evidence and economic model and contributed to the writing of the report.
Nasuh Büyükkaramikli (https://orcid.org/0000-0002-2021-9574) acted as health economic project lead, devised the economic model, critiqued the economics literature and contributed to the writing of the report.
Hannah Penton (https://orcid.org/0000-0001-9492-7875) and Pim Wetzelaer (https://orcid.org/0000-0002-7450-0046) devised the economic model, critiqued the company submissions and contributed to the writing of the report.
Rob Riemsma (https://orcid.org/0000-0001-8892-0861), Vanesa Huertas Carrera (https://orcid.org/0000-0002-1740-8796), Stephanie Swift (https://orcid.org/0000-0002-8545-8322), Thea Drachen (https://orcid.org/0000-0003-4760-5536) and Heike Raatz (https://orcid.org/0000-0001-7015-3465) acted as systematic reviewers, devised the clinical effectiveness methods and evidence and contributed to the writing of the report.
Steve Ryder (https://orcid.org/0000-0003-1177-416X), Dhwani Shah (https://orcid.org/0000-0002-3015-4348) and Titas Buksnys (https://orcid.org/0000-0002-8383-7732) acted as health economists and systematic reviewers, devised the clinical effectiveness methods and evidence and economic model and contributed to writing of the report.
Gill Worthy (https://orcid.org/0000-0003-1463-5413) acted as statistician and contributed to the writing of the report.
Steven Duffy (https://orcid.org/0000-0001-7335-8167) acted as information specialist, devised the searches and contributed to the writing of the report.
Maiwenn Al (https://orcid.org/0000-0001-9763-0436) devised the economic model, contributed to the writing of the report and provided general health economic guidance.
Jos Kleijnen (https://orcid.org/0000-0003-2787-7091) contributed to the writing of the report and supervised the project.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence . Avatrombopag and Lusutrombopag for Treating Thrombocytopenia in People With Chronic Liver Disease Needing an Elective Procedure. Final Scope 2018. www.nice.org.uk/guidance/gid-ta10444/documents/final-scope (accessed 18 December 2018).
- Malloy PC, Grassi CJ, Kundu S, Gervais DA, Miller DL, Osnis RB, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 2009;20:240-9. https://doi.org/10.1016/j.jvir.2008.11.027.
- NHS Digital . Hospital Episode Statistics Admitted Patient Care England 2016–17 2017. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2016-17 (accessed 21 November 2018).
- National Institute for Health and Care Excellence . Blood Transfusion. NICE Guideline 24 2015. www.nice.org.uk/guidance/ng24 (accessed 11 April 2019).
- European Medicines Agency . Committee for Medicinal Products for Human Use Summary of Opinion. Doptelet: Avatrombopag 2019. www.ema.europa.eu/en/medicines/human/summaries-opinion/doptelet (accessed 29 April 2019).
- European Medicines Agency . EPAR Assessment Report. Doptelet. International Non-Proprietary Name: Avatrombopag. Procedure No. EMEA H C 004722 0000 2019. www.ema.europa.eu/en/documents/assessment-report/doptelet-epar-public-assessment-report_en.pdf (accessed 26 June 2019).
- European Medicines Agency . Committee for Medicinal Products for Human Use Summary of Opinion. Lusutrombopag Shionogi: Lusutrombopag 2018. www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-lusutrombopag-shionogi_en.pdf (accessed 25 April 2019).
- European Medicines Agency . EPAR Assessment Report. Lusutrombopag Shionogi. International Non-Proprietary Name: Lusutrombopag. Procedure No. EMEA H C 004720 0000 2018. www.ema.europa.eu/en/documents/assessment-report/lusutrombopag-shionogi-epar-public-assessment-report_en.pdf (accessed 25 April 2019).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. http://handbook.cochrane.org/ (accessed 21 November 2018).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 21 November 2018).
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40-6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
- Canadian Agency for Drugs and Technologies in Health (CADTH) . PRESS – Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&Amp;E) 2016. www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_2016.pdf (accessed 21 November 2018).
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. https://doi.org/10.1136/jech.52.6.377.
- National Institute for Health and Care Excellence . Proposed Multiple Technology Appraisal. Avatrombopag for Treating Thrombocytopenia in People With Chronic Liver Disease Needing Elective Surgery. Draft Scope (Pre-Referral) 2018. www.nice.org.uk/guidance/gid-ta10348/documents/draft-scope-pre-referral (accessed 21 November 2018).
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. www.nicedsu.org.uk (accessed 8 December 2016).
- National Institute for Health and Care Excellence . Guide to the Processes of Technology Appraisal 2014. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/technology-appraisal-processes-guide-sept-2014.pdf (accessed 21 November 2018).
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. https://doi.org/10.1016/S0895-4356(97)00049-8.
- Eisai Inc . Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing an Elective Procedure n.d.:2014-17. https://ClinicalTrials.gov/show/NCT01972529 (accessed 23 January 2019).
- Eisai Co. Ltd . Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing an Elective Procedure 2014. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-142746 (accessed 23 January 2019).
- Eisai Inc . Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing an Elective Procedure n.d.:2013-17. https://ClinicalTrials.gov/show/NCT01976104 (accessed 23 January 2019).
- Caldwell S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Shah N. Characterization of baseline thrombopoietin levels in patients with chronic liver disease: results from 2 pooled clinical studies in patients with thrombocytopenia and liver disease. Hepatology 2018;68:487A-8A. https://doi.org/10.14309/00000434-201810001-00877.
- Center for Drug Evaluation and Research, US FDA . Doptelet/Avatrombopag./Multi-Discipline/Review/Summary,/Clinical,/Non-Clinical 2017. www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000MultidisciplineR.pdf (accessed 23 January 2019).
- Center for Drug Evaluation and Research, US FDA . Doptelet/Avatrombopag./Other/Review(s) 2017. www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000OtherR.pdf (accessed accessed 23 January 2019).
- Center for Drug Evaluation and Research, US FDA . Doptelet (Avatrombopag). Drug Approval Package 2018. www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000TOC.cfm (accessed 23 January 2019).
- Frelinger AL, Koganov ES, Forde EE, Carmichael SL, Michelson AD. Avatrombopag, a novel thrombopoietin receptor agonist, increases platelet counts without increasing platelet activation in patients with thrombocytopenia due to chronic liver disease. Blood 2017;130.
- Poordad F, Allen LF, Aggarwal K, Vredenburg M, Alkhouri N. Superiority of avatrombopag to placebo in increasing platelet counts and reducing platelet transfusions in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures: pooled analysis of 2 randomized phase 3 studies. Res Pract Thromb Haemost 2018;2. https://doi.org/10.1016/S0016-5085(18)31985-1.
- Poordad F, Allen L, Aggarwal K, Vredenburg M, Tian W, Terrault N. Exploratory analyses of the efficacy of avatrombopag versus placebo from 2 phase 3 studies using alternate baseline platelet count cohorts and an alternate secondary efficacy endpoint. Res Pract Thromb Haemost 2018;2.
- Poordad F, Vredenburg M, Allen LF, Aggarwal K, Alkhouri N. Superiority of avatrombopag to placebo in increasing platelet counts and reducing platelet transfusions in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures-pooled analysis of 2 randomized phase 3 studies. Gastroenterology 2018;154. https://doi.org/10.1016/S0016-5085(18)31985-1.
- Reau NS, Sammy S, Allen LF, Aggarwal K, Vredenburg M, Kim WR. Avatrombopag decreases need for platelet transfusion in patients chronic liver disease and thrombocytopenia undergoing medical procedures with low to high associated bleeding risks. J Hepatol 2018;68. https://doi.org/10.1016/S0168-8278(18)31767-7.
- Saab S, Allen LF, Aggarwal K, Vredenburg M, Terrault N. Consistent efficacy of avatrombopag compared to placebo in patients with thrombocytopenia and chronic liver disease undergoing procedures across various liver disease severities and etiologies. Gastroenterology 2018;154:S1247-8. https://doi.org/10.1016/S0016-5085(18)34098-8.
- Saab S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Tian W. Efficacy of avatrombopag compared with placebo across various mean baseline platelet count subgroups: pooled data from 2 phase 3 studies. Gastroenterology 2018;154. https://doi.org/10.1016/S0016-5085(18)34103-9.
- Sammy S, Allen LF, Aggarwal K, Vredenburg M, Terrault N. Consistent efficacy of avatrombopag compared to placebo in patients with thrombocytopenia and chronic liver disease undergoing procedures across various disease severities and etiologies. J Hepatol 2018;68. https://doi.org/10.1016/S0168-8278(18)31769-0.
- Sammy S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Tian W, et al. Efficacy of avatrombopag compared with placebo across various mean baseline platelet count subgroups-pooled data from 2 Phase 3 studies. J Hepatol 2018;68. https://doi.org/10.1016/S0168-8278(18)31768-9.
- Terrault N, Kuter DJ, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Superiority of avatrombopag to placebo in increasing platelet counts in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures: results from 2, Phase 3 randomized studies. Blood 2017;130.
- Terrault N, Bibbiani F, Chen YC, Izumi N, Kayali Z, Soto JRL, et al. Superiority of Avatrombopag (AVA) to Placebo (PBO) for the Treatment of Chronic Liver Disease (CLD)-Associated Thrombocytopenia (TCP) in Patients Undergoing Scheduled Procedures: Results of 2 Randomized, PBO-Controlled Phase 3 Studies n.d.
- Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018;155:705-18. https://doi.org/10.1053/j.gastro.2018.05.025.
- Vredenburg M, Reau N, Allen LF, Aggarwal K, Poordad F. Consistent efficacy of avatrombopag over placebo in the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures across demographic subgroups: pooled results of two phase 3 studies. Gastroenterology 2018;154. https://doi.org/10.1016/S0016-5085(18)31992-9.
- Hidaka H, Kurosaki M, Tanaka H, Kudo M, Abiru S, Igura T, et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol 2019;17:1192-200. https://doi.org/10.1016/j.cgh.2018.11.047.
- Izumi N, Osaki Y, Yamamoto K, Kurokawa M, Tanaka K, Kano T, et al. A phase 3, randomized, double-blind, placebo-controlled study of lusutrombopag for thrombocytopenia in patients with chronic liver disease undergoing elective invasive procedures in Japan (L-PLUS 1). Hepatology 2015;62:1397A-8A.
- Afdhal N, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Platelet response to lusutrombopag, a thrombopoietin receptor agonist, in patients with chronic liver disease and thrombocytopenia undergoing non-emergency invasive procedures: results from a phase 3 randomized, double-blind, placebo-controlled study. Blood 2017;130.
- Afdhal NH, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Lusutrombopag for Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Who Are Undergoing Non-Emergency Invasive Procedures: Results from an International Phase 3, Randomized, Double-Blind, Placebo-Controlled Study (L-PLUS 2) n.d.
- Peck-Radosavljevic M, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Lusutrombopag For Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Who Are Undergoing Non-Emergency Invasive Procedures: Results from an International Phase 3, Randomized, Double-Blind, Placebo-Controlled Study (L-PLUS 2) n.d.
- Shionogi Inc . Safety and Efficacy Study of Lusutrombopag for Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Elective Invasive Procedures (L-PLUS 2) n.d.:2015-17. https://ClinicalTrials.gov/show/NCT02389621 (accessed 23 January 2019).
- Alkhouri N, Imawari M, Izumi N, Osaki Y, Ochiai T, Bentley R, et al. Use of the Thrombopoietin Receptor Agonist Lusutrombopag For Management of Thrombocytopenia in Patients With Hepatocellular Carcinoma Undergoing Planned Invasive Procedures n.d.
- Brown RS, Imawari M, Izumi N, Osaki Y, Bentley R, Baykal T, et al. Lusutrombopag Reliably Increases Platelet Counts for up to 3 Weeks in Chronic Liver Disease Patients With Thrombocytopenia Undergoing Invasive Procedures Regardless of Baseline Platelet Counts: Results from Two Phase 3 Trials n.d.
- Brown RS, Imawari M, Izumi N, Osaki Y, Ochiai T, Kano T, et al. Lusutrombopag Is a Safe and Efficacious Treatment Option for Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures: A Pooled Analysis of Two Phase 3 Trials n.d. https://doi.org/10.1097/01.HS9.0000561072.99657.03.
- Center for Drug Evaluation and Research, US FDA . Mulpleta (lusutrombopag). Multi-Discipline Review Summary, Clinical, Non-Clinical 2017. www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210923Orig1s000MultidisciplineR.pdf (accessed 23 January 2019).
- Eisai Inc . Once-Daily Oral Avatrombopag Tablets Used in Participants With Chronic Liver Diseases and Thrombocytopenia Prior to Elective Surgical or Diagnostic Procedures n.d.:2009-11. https://ClinicalTrials.gov/show/NCT00914927 (accessed 23 January 2019).
- Terrault N, Hassanein T, Joshi S, Lake JR, Sher LS, Vargas HE, et al. Once-Daily Oral Avatrombopag (E5501) Prior to Elective Surgical or Diagnostic Procedures in Patients With Chronic Liver Disease and Thrombocytopenia: Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled Study (Study 202) n.d.
- Terrault NA, Hassanein T, Howell CD, Joshi S, Lake J, Sher L, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol 2014;61:1253-9. https://doi.org/10.1016/j.jhep.2014.07.007.
- Izumi N, Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, et al. Once-daily oral lusutrombopag, alternative to platelet transfusion in thrombocytopenic patients with chronic liver disease undergoing radiofrequency ablation: results from a phase 2B, randomized, double-blind study. J Hepatol 2014;60. https://doi.org/10.1016/S0168-8278(14)61094-1.
- Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, Katsube T, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol 2019;54:171-81. https://doi.org/10.1007/s00535-018-1499-2.
- Peck-Radosavljevic M, Simon K, Iacobellis A, Hassanein T, Kayali Z, Tran A, et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L-PLUS 2). Hepatology 2019;70:1336-48. https://doi.org/10.1002/hep.30561.
- Shionogi Ltd . Avatrombopag and Lusutrombopag for Treating Thrombocytopenia in People With Chronic Liver Disease Needing an Elective Procedure [ID1520] 2019.
- Shionogi Ltd . Avatrombopag and Lusutrombopag for Treating Thrombocytopenia in People With Chronic Liver Disease Needing an Elective Procedure [ID1520] 2019.
- Dova Pharmaceuticals . Avatrombopag and Lusutrombopag for Treating Thrombocytopenia in People With Chronic Liver Disease Needing an Elective Procedure [ID1520] 2019.
- Eisai Inc . Clinical Study Report: E5501-G000-310. A Randomized, Global, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Once-Daily Oral Avatrombopag for the Treatment of Adults With Thrombocytopenia Associated With Liver Disease Prior to an Elective Procedure 2017.
- Eisai Inc . Clinical Study Report: E5501-G000-202. A Phase 2, Randomized, Multicenter, Placebo-Controlled, Double-Blind, Parallel-Group Study to Evaluate the Efficacy, Safety, and Population Pharmacokinetics of Once-Daily Oral E5501 Tablets Used up to 7 Days in Participants With Chronic Liver Diseases and Thrombocytopenia Prior to Elective Surgical or Diagnostic Procedures 2013.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Poordad F. Review article: thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther 2007;26:5-11. https://doi.org/10.1111/j.1365-2036.2007.03510.x.
- Poordad FF, Dalal MR, Grotzinger KM. Prevalence and medical resource utilization in HCV patients with thrombocytopenia. Gastroenterology 2008;134. https://doi.org/10.1016/S0016-5085(08)63898-6.
- Poordad F, Theodore D, Sullivan J, Grotzinger K. Evaluating medical resource utilization and costs associated with thrombocytopenia in chronic liver disease patients. J Med Econ 2012;15:112-24. https://doi.org/10.3111/13696998.2011.632463.
- Poordad F, Theodore D, Sullivan J, Grotzinger K. Medical resource utilisation and healthcare costs in patients with chronic hepatitis C viral infection and thrombocytopenia. J Med Econ 2011;14:194-206. https://doi.org/10.3111/13696998.2011.562266.
- Barnett CL, Mladsi D, Vredenburg M, Aggarwal K. Cost estimate of platelet transfusion in the United States for patients with chronic liver disease and associated thrombocytopenia undergoing elective procedures. J Med Econ 2018;21:827-34. https://doi.org/10.1080/13696998.2018.1490301.
- Brown RS. Review article: a pharmacoeconomic analysis of thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther 2007;26:41-8. https://doi.org/10.1111/j.1365-2036.2007.03505.x.
- Poordad F, Loo N, Han X, Aggarwal K. Burden of Platelet Transfusions in Chronic Liver Disease Patients with Thrombocytopenia: A Case-Control Study. Orlando, FL, USA: Academy of Managed Care Pharmacy Nexus; 2018.
- Dova Pharmaceuticals . NICE Dossier: DOPTELET® (Avatrombopag) for Thrombocytopenia in Patients With Chronic Liver Disease 2019.
- Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol 2017;23:3228-39. https://doi.org/10.3748/wjg.v23.i18.3228.
- Fontaine MJ, Chung YT, Rogers WM, Sussmann HD, Quach P, Galel SA, et al. Improving platelet supply chains through collaborations between blood centers and transfusion services. Transfusion 2009;49:2040-7. https://doi.org/10.1111/j.1537-2995.2009.02236.x.
- Kerkhoffs JL, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008;48:1959-65. https://doi.org/10.1111/j.1537-2995.2008.01799.x.
- Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategies. Drugs 2015;75:1981-92. https://doi.org/10.1007/s40265-015-0480-0.
- van Eerd MC, Mario Ouwens JN, de Peuter MA. Cost-effectiveness study comparing pharmaceutically licensed plasma for transfusion (OctaplasLG®) versus fresh frozen plasma (FFP) in critically Ill patients in the UK. Transfus Apher Sci 2010;43:251-9. https://doi.org/10.1016/j.transci.2010.09.019.
- Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009;113:3406-17. https://doi.org/10.1182/blood-2008-10-167643.
- Takaki H, Yamakado K, Nakatsuka A, Yamada T, Shiraki K, Takei Y, et al. Frequency of and risk factors for complications after liver radiofrequency ablation under CT fluoroscopic guidance in 1500 sessions: single-center experience. AJR Am J Roentgenol 2013;200:658-64. https://doi.org/10.2214/AJR.12.8691.
- Lo GH, Chen WC, Wang HM, Lin CK, Chan HH, Tsai WL, et al. Low-dose terlipressin plus banding ligation versus low-dose terlipressin alone in the prevention of very early rebleeding of oesophageal varices. Gut 2009;58:1275-80. https://doi.org/10.1136/gut.2008.165910.
- Triantos C, Kalafateli M. Endoscopic treatment of esophageal varices in patients with liver cirrhosis. World J Gastroenterol 2014;20:13015-26. https://doi.org/10.3748/wjg.v20.i36.13015.
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31. https://doi.org/10.1016/j.jhep.2005.10.013.
- Shionogi Ltd . Study M0626. Clinical Study Report: 1208M0626. A Phase 2b Study of S-888711 in Thrombocytopenic Patients With Chronic Liver Disease 2013.
- Shionogi Ltd . L-PLUS 1. Clinical Study Report: 1304M0631. A Phase 3 Study of S-888711 in Thrombocytopenic Patients With Chronic Liver Disease 2014.
- Shionogi Ltd . L-Plus 2. Clinical Study Report: 1423m0634. A Phase 3 Randomised, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of S-888711 (Lusutrombopag) for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Elective Invasive Procedures (L-Plus 2 2017.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- National Institute for Health and Care Excellence . Eltrombopag for Treating Chronic Immune (Idiopathic) Thrombocytopenic Purpura. NICE Technology Appraisal Guidance 293 2013. www.nice.org.uk/guidance/ta293 (accessed 25 April 2019).
- Jugrin AV, Ustyugova A, Urbich M, Lamotte M, Sunderland T. The cost-utility of dabigatran etexilate compared with warfarin in treatment and extended anticoagulation of acute VTE in the UK. Thromb Haemost 2015;114:778-92. https://doi.org/10.1160/TH14-12-1027.
- Ara R, Wailoo A. NICE DSU Technical Support Document 12: The Use of Health State Utility Values in Decision Models 2011. www.nicedsu.org.uk (accessed 25 April 2019).
- NHS Improvement . NHS Reference Costs 2017–18. 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 25 April 2019).
- Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med 2003;13:205-18. https://doi.org/10.1046/j.1365-3148.2003.00443.x.
- Cookson G, Jones S, Laliotis I. Cancelled procedures in the English NHS: evidence from the 2010 tariff reform. Health Econ 2017;26:e126-39. https://doi.org/10.1002/hec.3486.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 5: Evidence Synthesis in the Baseline Natural History Model. Last Updated April 2012 2011. www.nicedsu.org.uk (accessed 23 July 2019).
- Norfolk D. United Kingdom Blood Services. Handbook of Transfusion Medicine 2014. www.transfusionguidelines.org/transfusion-handbook (accessed 23 July 2019).
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 2007;176:886-91. https://doi.org/10.1164/rccm.200702-271OC.
- Bolton-Maggs PHB, Thomas D, Cohen H, Watt A, Poles D, Davies T, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2012 Annual SHOT Report 2013. www.shotuk.org/wp-content/uploads/myimages/SHOT-Annual-Report-20121.pdf (accessed 23 July 2019).
- Bolton-Maggs PHB, Thomas D, Cohen H, Watt A, Poles D, Davies T, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2013 Annual SHOT Report 2014. www.shotuk.org/wp-content/uploads/myimages/2013.pdf (accessed 23 July 2019).
- Bolton-Maggs PHB, Thomas D, Cohen H, Watt A, Poles D, Davies T, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2014 Annual SHOT Report 2015. www.shotuk.org/wp-content/uploads/myimages/report-2014.pdf (accessed 23 July 2019).
- Bolton-Maggs PHB, Thomas D, Watt A, Poles D, Davies T, Mistry H, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2015 Annual SHOT Report 2016. www.shotuk.org/wp-content/uploads/myimages/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked-1.pdf (accessed 23 July 2019).
- Bolton-Maggs PHB, Thomas D, Watt A, Poles D, Mistry H, Ball J, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2016 Annual SHOT Report 2017. www.shotuk.org/wp-content/uploads/myimages/SHOT-Report-2016_web_11th-July.pdf (accessed 23 July 2019).
- Hendrickson JE, Roubinian NH, Chowdhury D, Brambilla D, Murphy EL, Wu Y, et al. Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication. Transfusion 2016;56:2587-96. https://doi.org/10.1111/trf.13730.
- NHS Specialist Pharmacy Service . What Is the Child–Pugh Score? 2017. www.sps.nhs.uk/articles/what-is-the-child-pugh-score/ (accessed 18 December 2018).
- Scalone L, Ciampichini R, Fagiuoli S, Gardini I, Fusco F, Gaeta L, et al. Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Qual Life Res 2013;22:1707-16. https://doi.org/10.1007/s11136-012-0318-0.
- National Institute for Health and Care Excellence . Romiplostim for the Treatment of Chronic Immune (Idiopathic) Thrombocytopenic Purpura. NICE Technology Appraisal Guidance 221 n.d. www.nice.org.uk/guidance/ta221 (accessed 25 April 2019).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Stokes EA, Wordsworth S, Staves J, Mundy N, Skelly J, Radford K, et al. Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom National Health Service. Transfusion 2018;58:846-53. https://doi.org/10.1111/trf.14493.
- NHS Blood and Transplant . NHSBT Pricing Proposals for 2017–18. NHSBT Board September 2016 2016. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/2188/16-80.pdf (accessed 11 April 2019).
- Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19580.
- NHS Blood and Transplant . Risk Factors for Bleeding in Haematology Patients With Low Platelet Counts. ISRCTN81226121 2012. http://isrctn.com/ISRCTN81226121 (accessed 23 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Plesca D. A Review Of Topical Thrombin. Cleveland, OH: Cleveland Clinic; n.d.
- Organisation for Economic Co-operation and Development . Purchasing Power Parities (PPP) (Indicator) n.d. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart (accessed 23 July 2019).
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17100.
- Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2011. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 23 July 2019).
- Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, Rupnow MF, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-135.
- BresMed . Lusutrombopag for the Treatment of Thrombocytopenia in Chronic Liver Disease Patients Prior to an Elective Procedure: Cost-Effectiveness Model 2019.
- Li L, Yang F, Xuan J. The cost-effectiveness analysis of RHTPO versus IL-11 on the treatment of chemotherapy-induced thrombocytopenia patients in China. Value Health 2018;21:17-8. https://doi.org/10.1016/j.jval.2018.04.105.
- European Medicines Agency . Lusutrombopag Shionogi 3mg Film-Coated Tablets. Annex 1. Summary of Product Characteristics 2019. www.ema.europa.eu/en/documents/product-information/lusutrombopag-shionogi-epar-product-information_en.pdf (accessed 25 April 2019).
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818-27. https://doi.org/10.1093/ije/dys041.
Appendix 1 Literature search strategies
Clinical effectiveness, cost-effectiveness and safety search strategies
| Database/resource | Host | Date range | Results (n) | Date searched |
|---|---|---|---|---|
| MEDLINE | Ovid | 1946 to week 3 January 2019 | 805 | 24 January 2019 |
| MEDLINE Epub Ahead of Print; MEDLINE In-Process & Other Non-Indexed Citations; MEDLINE Daily Update | Ovid | 23 January 2019 | 89 | 24 January 2019 |
| PubMed | National Library of Medicine | Up to 24 January 2019 | 255 | 24 January 2019 |
| EMBASE | Ovid | 1974 to week 3 2019 | 1614 | 24 January 2019 |
| Cochrane Database of Systematic Reviews | Cochrane Library: Wiley | Issue 1 of 12, January 2019 | 8 | 24 January 2019 |
| Cochrane Central Register of Controlled Trials | Cochrane Library: Wiley | Issue 1 of 12, January 2019 | 138 | 24 January 2019 |
| KSR Evidence | www.ksrevidence.com | Database last updated 24 January 2019 | 68 | 24 January 2019 |
| Epistemonikos | www.epistemonikos.org/en/ | Up to 24 January 2019 | 212 | 24 January 2019 |
| Database of Abstracts of Reviews of Effects | www.crd.york.ac.uk/CRDWeb/ | Up to 31 March 2015 | 19 | 24 January 2019 |
| HTA database | www.crd.york.ac.uk/CRDWeb/ | Up to 31 March 2015 | 7 | 24 January 2019 |
| NHS Economic Evaluation Database | www.crd.york.ac.uk/CRDWeb/ | Up to 31 March 2018 | 11 | 24 January 2019 |
| PROSPERO | www.crd.york.ac.uk/PROSPERO/ | Up to 24 January 2019 | 39 | 24 January 2019 |
| Science Citation Index Expanded | Web of Science | 1988 to 23 January 2019 | 722 | 24 January 2019 |
| Cumulative Index to Nursing and Allied Health Literature | EBSCOhost | 1982 to 23 January 2019 | 122 | 24 January 2019 |
| Latin American and Caribbean Health Sciences | http://lilacs.bvsalud.org/en/ | 1982 to 24 January 2019 | 157 | 24 January 2019 |
| Northern Light Life Sciences Conference Abstracts | Ovid | 2010–19 week 2 | 227 | 24 January 2019 |
| Transfusion Evidence Library | www.transfusionevidencelibrary.com/ | Up to 23 January 2019 | 40 | 23 January 2019 |
| RePEc: Research Papers in Economics | http://repec.org/ | Up to 23 January 2019 | 14 | 23 January 2019 |
| ClinicalTrials.gov | http://clinicaltrials.gov/ct2/search/advanced | Up to 23 January 2019 | 319 | 23 January 2019 |
| World Health Organization International Clinical Trials Register Portfolio | www.who.int/ictrp/search/en/ | Up to 23 January 2019 | 207 | 23 January 2019 |
| US FDA | www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm | Up to 23 January 2019 | 4 | 23 January 2019 |
| European Medicines Agency | www.ema.europa.eu | Up to 23 January 2019 | 2 | 23 January 2019 |
| OAIster | http://oaister.worldcat.org | Up to 23 January 2019 | 37 | 23 January 2019 |
| OpenGrey | www.opengrey.eu/ | Up to 23 January 2019 | 41 | 23 January 2019 |
| Copac | https://copac.jisc.ac.uk/ | Up to 23 January 2019 | 90 | 23 January 2019 |
| Total records retrieved | 5247 | |||
| Duplicate records removed | 1729 | |||
| Total records to screen | 3518 | |||
MEDLINE
Date ranges searched:
MEDLINE (via Ovid) – 1946–week 3 January 2019.
MEDLINE Epub Ahead of Print (via Ovid) – 22 January 2019.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) – 23 January 2019.
MEDLINE Daily Update (via Ovid) – 22 January 2019.
Date searched: 24 January 2019.
Search strategy
-
(avatrombopag or doptelet or AKR 501 or AKR501 or AS 1670542 or AS1670542 or E 5501 or E5501 or oralE 5501 or oralE5501 or YM 477 or YM477 or 570406-98-3 or 677007-74-8).af. (33)
-
(lusutrombopag or mulpleta or S 888711 or S888711 or 1110766-97-6).af. (14)
-
or/1-2 (46)
-
exp Thrombocytopenia/ (45,457)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot,hw. (69,081)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot,hw. (574)
-
(jacobsen adj3 syndrome$).ti,ab,ot,hw. (129)
-
paris trousseau.ti,ab,ot,hw. (30)
-
kasabach merritt.ti,ab,ot,hw. (704)
-
(hemangioma or haemangioma).ti,ab,ot,hw. (32,339)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot,hw. (3354)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot,hw. (7663)
-
gasser$.ti,ab,ot,hw. (1689)
-
HELLP Syndrome/ (1709)
-
(HELLP adj2 syndrome$).ti,ab,ot,hw. (2561)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot,hw. (7)
-
May Hegglin.ti,ab,ot,hw. (221)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot,hw. (1411)
-
moschcowitz.ti,ab,ot,hw. (107)
-
werlhof.ti,ab,ot,hw. (120)
-
Wiskott-Aldrich Syndrome/ (1428)
-
(wiskott and Aldrich).ti,ab,ot,hw. (3312)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot,hw. (44)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot,hw. (22,231)
-
or/4-24 (132,417)
-
exp Liver Diseases/ (521,414)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$)).ti,ab,ot,hw. (163,004)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab,ot,hw. (123,945)
-
(chronic adj3 destructive cholangitis).ti,ab,ot,hw. (98)
-
((fibrosis or fibroses or scar$) adj3 (liver$ or hepat$)).ti,ab,ot,hw. (23,356)
-
((hepatitis or hepatopath$) adj3 (chronic or acute or persistent or long stand$ or long term or recurr$)).ti,ab,ot,hw. (76,827)
-
((liver$ or hepat$ or intrahepat$) adj3 inflam$).ti,ab,ot,hw. (13,126)
-
(haemochromatosis or hemochromatosis or bronze$ diabet$ or recklinghausen applebaum or siderochromatosis).ti,ab,ot,hw. (10,335)
-
primary biliary cholangitis.ti,ab,ot,hw. (552)
-
((liver$ or hepat$ or intrahepat$) adj3 carcinoma$).ti,ab,ot,hw. (110,103)
-
(hepatocarcinoma or hepatoma$).ti,ab,ot,hw. (30,671)
-
or/26-36 (614,221)
-
25 and 37 (9693)
-
Receptors, Thrombopoietin/ (1355)
-
((thrombopoietin$ or c-Mpl) adj3 (agonist$ or agent$ or mimetic$ or receptor$)).ti,ab,ot,hw. (1939)
-
(eltrombopag or promacta or revolade or SB 497115 or SB497115 or 496775-61-2).ti,ab,ot,hw,rn. (631)
-
(romiplostim or nplate or remiplistim or amg 531 or amg531 or 267639-76-9).ti,ab,ot,hw,rn. (521)
-
promegapoietin.ti,ab,ot,hw,rn. (12)
-
Platelet Transfusion/ (6808)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$)).ti,ab,ot,hw. (12,351)
-
Splenectomy/ (21,173)
-
(splenectom$ or (spleen adj3 (resect$ or remov$ or surg$))).ti,ab,ot,hw. (30,967)
-
Splenic Artery/ and Embolization, Therapeutic/ (667)
-
((spleen or splenic or eria lienalis or lienal) adj3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap$ or therap$ occlus$)).ti,ab,ot,hw. (999)
-
Megakaryocytes/ (7273)
-
((megakaryocyte$ or karyocyte$) adj3 (stimul$ or maturat$ or produc$)).ti,ab,ot,hw. (1186)
-
Thrombopoiesis/ (848)
-
(thrombopoiesi$ or thrombocytopoies$ or megakaryocytopoies$).ti,ab,ot,hw. (2678)
-
((platelet$ or thrombocyt$) adj3 (produc$ or formation or stimulat$)).ti,ab,ot,hw. (155,25)
-
Portasystemic Shunt, Transjugular Intrahepatic/ (2365)
-
(transjugular intrahepatic portosystemic shunt$ or transjugular intrahepatic porto systemic shunt$ or transjugular intrahepatic portacaval shunt$ or transjugular intrahepatic porta systemic shunt$ or transjugular intrahepatic portasystemic shunt$ or transjugular intrahepatic shunt$ or transjugular intrahepatic stent$ or TIPS or TIPSS).ti,ab,ot,hw. (29,852)
-
or/39-56 (96,920)
-
38 and 57 (897)
-
3 or 58 (919)
-
exp animals/ not humans/ (4,540,224)
-
59 not 60 (894).
MEDLINE 805.
MEDLINE Epub Ahead of Print 18.
MEDLINE In-Process & Other Non-Indexed Citations 71.
MEDLINE Daily Update 0.
PubMed (National Library of Medicine)
Date range searched: inception to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
-
#41 (#39 AND #40) 255
-
#40 pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb] 3,121,488
-
#39 (#4 OR #38) 3451
-
#38 (#26 AND #37) 3428
-
#37 (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36) 176,154
-
#36 “ Portasystemic Shunt, Transjugular Intrahepatic”[Mesh] OR “transjugular intrahepatic portosystemic shunt”[tiab] OR “transjugular intrahepatic porto systemic shunt”[tiab] OR “transjugular intrahepatic portacaval shunt”[tiab] OR “transjugular intrahepatic porta systemic shunt”[tiab] OR “transjugular intrahepatic portasystemic shunt”[tiab] OR “transjugular intrahepatic shunt”[tiab OR “transjugular intrahepatic stent*”[tiab] OR TIPS[tiab] OR TIPSS[tiab] 29,035
-
#35 (platelet*[tiab] OR thrombocyt*[tiab]) AND (produc*[tiab] OR formation[tiab] OR stimulat*[tiab]) 71,046
-
#34 “ Thrombopoiesis”[Mesh] OR thrombopoiesi*[tiab] OR thrombocytopoies*[tiab] OR megakaryocytopoies*[tiab] 2712
-
#33 “ Megakaryocytes”[Mesh] OR (megakaryocyte*[tiab] OR karyocyte*[tiab]) AND (stimul*[tiab] OR maturat*[tiab] OR produc*[tiab]) 4666
-
#32 (spleen[tiab] OR splenic[tiab] OR “eria lienalis”[tiab] OR lineal[tiab]) AND (embolisation[tiab] OR embolization[tiab] OR embolism[tiab] OR embolus[tiab] OR thrombus[tiab] OR embolotherap*[tiab] OR “therapautic occlusion”[tiab]) 2234
-
#31 “ Splenic Artery”[Mesh] AND “Embolization, Therapeutic”[Mesh] 683
-
#30 “ Splenectomy”[Mesh] OR splenectom*[tiab] OR (spleen[tiab] AND (resect*[tiab] OR remov*[tiab] OR surg*[tiab])) 38,387
-
#29 “ Platelet Transfusion”[Mesh] OR ((platelet*[tiab] OR thrombocyt*[tiab]) AND (transfus*[tiab] OR infus*[tiab] OR administ*[tiab])) 47,154
-
#28 eltrombopag[tiab] OR promacta[tiab] OR revolade[tiab] OR “SB 497115”[tiab] OR SB497115[tiab] OR romiplostim[tiab] OR nplate[tiab] OR remiplistim[tiab] OR “amg 531”[tiab] OR amg531[tiab] OR promegapoietin[tiab] 825
-
#27 “ Receptors, Thrombopoietin”[Mesh] OR (thrombopoietin*[tiab] OR c-Mpl[tiab]) AND (agonist*[tiab] OR agent*[tiab] OR mimetic*[tiab] OR receptor*[tiab]) 1980
-
#26 (#15 AND #25) 11,827
-
#25 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) 649,767
-
#24 (liver*[tiab] OR hepatic[tiab] OR intrahepatic[tiab]) AND carcinoma*[tiab] 75,099
-
#23 haemochromatosis[tiab] OR hemochromatosis[tiab] OR “bronze diabetes”[tiab] OR “bronze diabetic”[tiab] OR “recklinghausen applebaum”[tiab] OR siderochromatosis[tiab] OR “primary biliary cholangitis”[tiab] OR hepatocarcinoma[tiab] OR hepatoma*[tiab] 40,197
-
#22 (liver*[tiab] OR hepatic[tiab] OR intrahepatic[tiab]) AND inflam*[tiab] 57,427
-
#21 (hepatitis[tiab] OR hepatopath*[tiab]) AND (chronic[tiab] OR acute[tiab] OR persistent[tiab] OR “long standing”[tiab] OR “long term”[tiab] OR recurr*[tiab]) 91,895
-
#20 (fibrosis[tiab] OR fibroses[tiab] OR scar*[tiab]) AND (liver*[tiab] OR hepatic[tiab]) 40,403
-
#19 chronic[tiab] AND “destructive cholangitis”[tiab] 118
-
#18 cirrhosis[tiab] OR cirrhosis[tiab] OR cirrhotic[tiab] 95,558
-
#17 “ liver disease”[tiab] OR “liver diseases”[tiab] OR “hepatic disease”[tiab] OR “hepatic diseases”[tiab] OR “intrahepatic disease”[tiab] OR “intrahepatic diseases”[tiab] OR “liver disorder”[tiab] OR “liver disorders”[tiab] OR “hepatic disorder”[tiab] OR “hepatic disorders”[tiab] OR “intrahepatic disorder”[tiab] OR “intrahepatic disorders”[tiab] OR “liver lesion”[tiab] OR “liver lesions”[tiab] OR “hepatic lesion”[tiab] OR “hepatic lesions”[tiab] OR “intrahepatic lesion”[tiab] OR “intrahepatic lesions”[tiab] 108,675
-
#16 “ Liver Diseases”[Mesh] 521,434
-
#15 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) 188,201
-
#14 (platelet*[tiab] OR thrombocyte*[tiab]) AND (defici*[tiab] OR reduc*[tiab] OR low[tiab] OR lower[tiab] OR lowest[tiab] OR few[tiab] OR fewer[tiab] OR fewest[tiab] OR decrease[tiab] OR decreases[tiab] OR decreased[tiab] OR defective[tiab] OR destruc*[tiab] OR destroy*[tiab]) 99,513
-
#13 “ immunodeficiency 2” OR immunodeficiency2 OR Imd2 46
-
#12 Moschcowitz[tiab] OR werlhof[tiab] OR “Wiskott-Aldrich Syndrome”[Mesh] OR (wiskott[tiab] AND Aldrich[tiab]) 2664
-
#11 (haemolytic[tiab] OR hemolytic[tiab]) AND (anaemi*[tiab] OR anemi*[tiab]) AND (microangiopath*[tiab]) 1765
-
#10 (hemolysis[tiab] OR haemolysis[tiab]) AND liver[tiab] AND platelet*[tiab] 1247
-
#9 “ HELLP Syndrome”[Mesh] OR “HELLP syndrome” OR “HELLP syndromes” 2583
-
#8 (thrombotic[tiab] AND microangiopath*[tiab]) OR “hemolytic uremic” OR “haemolytic uremic” OR gasser*[tiab] 12,074
-
#7 “ jacobsen syndrome” OR “paris trousseau” OR “kasabach merritt” OR “May Hegglin” OR hemangioma[tiab] OR haemangioma[tiab] 17,717
-
#6 (11q[tiab] OR 11q23[tiab]) AND (disorder*[tiab] OR syndrome*[tiab] OR delet*[tiab] OR Jacobsen[tiab]) 1605
-
#5 “ Thrombocytopenia”[Mesh] OR thrombocytopeni*[tiab] OR thrombocytopaeni*[tiab] OR thrombopeni*[tiab] OR thrombopaeni*[tiab] OR macrothrombocytopeni*[tiab] OR macrothrombocytopaeni*[tiab] 73,938
-
#4 (#2 OR #3) 47
-
#3 lusutrombopag OR mulpleta OR “S 888711” OR S888711 14
-
#2 avatrombopag OR doptelet OR “AKR 501” OR AKR501 OR “AS 1670542” OR AS1670542 OR “E 5501” OR E5501 OR “oralE 5501” OR oralE5501 OR “YM 477” OR YM477 34.
EMBASE (via Ovid)
Date range searched: 1974 to week 3 2019.
Date searched: 24 January 2019.
Search strategy
-
avatrombopag/ (64)
-
(avatrombopag or doptelet or AKR 501 or AKR501 or AS 1670542 or AS1670542 or E 5501 or E5501 or oralE 5501 or oralE5501 or YM 477 or YM477 or 570406-98-3 or 677007-74-8).af. (135)
-
lusutrombopag/ (33)
-
(lusutrombopag or mulpleta or S 888711 or S888711 or 1110766-97-6).af. (33)
-
or/1-4 (163)
-
exp thrombocytopenia/ (157,171)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot. (87,986)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot. (1015)
-
(jacobsen adj3 syndrome$).ti,ab,ot. (187)
-
paris trousseau.ti,ab,ot. (49)
-
kasabach merritt.ti,ab,ot. (793)
-
(hemangioma or haemangioma).ti,ab,ot. (18,275)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot. (5177)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot. (7454)
-
gasser$.ti,ab,ot. (1885)
-
(HELLP adj2 syndrome$).ti,ab,ot. (3305)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot. (11)
-
May Hegglin.ti,ab,ot. (262)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot. (2048)
-
moschcowitz.ti,ab,ot. (93)
-
werlhof.ti,ab,ot. (55)
-
(wiskott and aldrich).ti,ab,ot. (2815)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot. (71)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot. (33,439)
-
or/6-24 (221,567)
-
chronic liver disease/ or liver disease/ or liver cirrhosis/ or liver fibrosis/ or chronic hepatitis/ (244,905)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$)).ti,ab,ot. (170,572)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab,ot. (134,378)
-
((chronic adj3 nonsuppurative destructive cholangitis) or (chronic adj3 non suppurative destructive cholangitis)).ti,ab,ot. (126)
-
((fibrosis or fibroses or scar$) adj3 (liver$ or hepat$)).ti,ab,ot. (38,165)
-
((hepatitis or hepatopath$) adj3 (chronic or acute or persistent or long stand$ or long term or recurr$)).ti,ab,ot. (93,566)
-
((liver$ or hepat$ or intrahepat$) adj3 inflam$).ti,ab,ot. (20,905)
-
(haemochromatosis or hemochromatosis or bronze$ diabet$ or recklinghausen applebaum or siderochromatosis).ti,ab,ot. (9700)
-
primary biliary cholangitis.ti,ab,ot. (1046)
-
liver cell carcinoma/ (136,789)
-
((liver$ or hepat$ or intrahepat$) adj3 carcinoma$).ti,ab,ot. (122,282)
-
(hepatocarcinoma or hepatoma$).ti,ab,ot. (35,186)
-
or/26-37 (532,951)
-
25 and 38 (13,778)
-
thrombopoietin receptor/ (1769)
-
((thrombopoietin$ or c-Mpl) adj3 (agonist$ or agent$ or mimetic$ or receptor$)).ti,ab,ot. (2199)
-
eltrombopag/ (1783)
-
(eltrombopag or promacta or revolade or SB 497115 or SB497115 or 496775-61-2).ti,ab,ot,hw,rn,tn. (1834)
-
romiplostim/ (1552)
-
(romiplostim or nplate or remiplistim or amg 531 or amg531 or 267639-76-9).ti,ab,ot,hw,rn,tn. (1698)
-
promegapoietin.ti,ab,ot,hw,rn,tn,dj. (25)
-
thrombocyte transfusion/ (17,075)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$)).ti,ab,ot. (13,882)
-
splenectomy/ (32,248)
-
(splenectom$ or (spleen adj2 (resect$ or remov$ or surg$))).ti,ab,ot. (27,238)
-
spleen artery/ and exp artificial embolism/ (457)
-
((spleen or splenic or eria lienalis or lienal) adj3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap$ or therap$ occlus$)).ti,ab,ot. (1536)
-
megakaryocyte/ and (stimulation/ or cell maturation/) (1079)
-
((megakaryocyte$ or karyocyte$) adj3 (stimul$ or maturat$ or produc$)).ti,ab,ot. (1555)
-
thrombocytopoiesis/ (4137)
-
(thrombopoiesi$ or thrombocytopoies$ or megakaryocytopoies$).ti,ab,ot. (2708)
-
((platelet$ or thrombocyt$) adj3 (produc$ or formation or stimulat$)).ti,ab,ot. (20,991)
-
transjugular intrahepatic portosystemic shunt/ (3426)
-
(transjugular intrahepatic portosystemic shunt$ or transjugular intrahepatic porto systemic shunt$ or transjugular intrahepatic portacaval shunt$ or transjugular intrahepatic porta systemic shunt$ or transjugular intrahepatic portasystemic shunt$ or transjugular intrahepatic shunt$ or transjugular intrahepatic stent$ or TIPS).ti,ab,ot. (35,802)
-
or/40-59 (124,052)
-
39 and 60 (1558)
-
5 or 61 (1651)
-
animal/ or animal experiment/ (3,692,962)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot. (4,424,329)
-
63 or 64 (5,722,776)
-
exp human/ or human experiment/ (19,263,219)
-
65 not (65 and 66) (4,428,740)
-
62 not 67 (1614).
Cochrane Database of Systematic Reviews (Cochrane Library: Wiley)
Date range searched: Cochrane Central Register of Controlled Trials (Cochrane Library: Wiley) – issue 1 of 12, January 2019.
Date searched: 24 January 2019.
Search strategy
-
#1 avatrombopag or doptelet or “AKR 501” or AKR501 or “AS 1670542” or AS1670542 or “E 5501” or E5501 or “oralE 5501” or oralE5501 or “YM 477” or YM477 47
-
#2 lusutrombopag or mulpleta or “S 888711” or S888711 11
-
#3 #1 or #2 58
-
#4 MeSH descriptor: [Thrombocytopenia] explode all trees 1121
-
#5 (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*):ti,ab,kw 7871
-
#6 ((11q or 11q23) NEAR/3 (disorder* or syndrome* or delet* or jacobsen)):ti,ab,kw 42
-
#7 (jacobsen NEAR/3 syndrome*):ti,ab,kw 0
-
#8 “paris trousseau” 2
-
#9 “kasabach merritt” 4
-
#10 (hemangioma or haemangioma):ti,ab,kw 298
-
#11 (thrombotic NEAR/2 (microangiopath* or micro angiopath*)):ti,ab,kw 70
-
#12 (hemolytic uremic or haemolytic uremic) 135
-
#13 (gasser*):ti,ab,kw 100
-
#14 MeSH descriptor: [HELLP Syndrome] this term only 45
-
#15 (HELLP NEAR/2 syndrome*):ti,ab,kw 130
-
#16 ((hemolysis or haemolysis) NEAR/3 platelet*):ti,ab,kw 9
-
#17 “May Hegglin” 0
-
#18 ((haemolytic or hemolytic) NEAR/2 (anaemi* or anemi*) NEAR/2 (microangiopathic or micro angiopathic)):ti,ab,kw 16
-
#19 (moschcowitz):ti,ab,kw 1
-
#20 (werlhof):ti,ab,kw 0
-
#21 MeSH descriptor: [Wiskott-Aldrich Syndrome] this term only 6
-
#22 (wiskott and aldrich):ti,ab,kw 24
-
#23 (“immunodeficiency 2” or immunodeficiency2 or Imd2):ti,ab,kw 1
-
#24 ((platelet* or thrombocyte*) NEAR/3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)):ti,ab,kw 2416
-
#25 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 10,523
-
#26 MeSH descriptor: [Liver Diseases] explode all trees 13,186
-
#27 ((liver* or hepat* or intrahepat*) NEAR/2 (disease* or disorder* or lesion*)):ti,ab,kw 7716
-
#28 (cirrhosis or cirrhoses or cirrhotic):ti,ab,kw 8338
-
#29 (chronic NEAR/3 destructive cholangitis):ti,ab,kw 1
-
#30 ((fibrosis or fibroses) NEAR/3 (liver* or hepat*)):ti,ab,kw 1583
-
#31 ((hepatitis or hepatopath*) NEAR/3 (chronic or acute or persistent or long stand* or long term or recurr*)):ti,ab,kw 9152
-
#32 ((liver or hepat* or intrahepat*) NEAR/3 inflam*):ti,ab,kw 663
-
#33 (haemochromatosis or hemochromatosis or bronze* diabet* or recklinghausen applebaum or siderochromatosis):ti,ab,kw 96
-
#34 primary biliary cholangitis:ti,ab,kw 287
-
#35 ((liver* or hepat* or intrahepat*) NEAR/3 carcinoma*):ti,ab,kw 3866
-
#36 (hepatocarcinoma or hepatoma*):ti,ab,kw 172
-
#37 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 27,420
-
#38 #25 and #37 787
-
#39 MeSH descriptor: [Receptors, Thrombopoietin] this term only 45
-
#40 ((thrombopoietin* or c-Mpl or mpl) NEAR/3 (agonist* or agent* or mimetic* or receptor*)):ti,ab,kw 196
-
#41 (eltrombopag or promacta or revolade or “SB 497115” or SB497115):ti,ab,kw 198
-
#42 (romiplostim or nplate or remiplistim or “amg 531” or amg531):ti,ab,kw 157
-
#43 promegapoietin 0
-
#44 MeSH descriptor: [Platelet Transfusion] this term only 300
-
#45 ((platelet* or thrombocyt*) NEAR/3 (transfus* or infus* or administ*)):ti,ab,kw 3034
-
#46 MeSH descriptor: [Splenectomy] this term only 176
-
#47 (splenectom* or (spleen NEAR/2 (resect* or remov* or surg*))):ti,ab,kw 617
-
#48 MeSH descriptor: [Splenic Artery] this term only 18
-
#49 ((spleen or splenic or eria lienalis or lienal) NEAR/3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap* or “therap* occlus*”)):ti,ab,kw 38
-
#50 MeSH descriptor: [Megakaryocytes] this term only 28
-
#51 ((megakaryocyte* or karyocyte*) NEAR/3 (stimul* or maturat* or produc*)):ti,ab,kw 27
-
#52 MeSH descriptor: [Thrombopoiesis] this term only 8
-
#53 (thrombopoiesi* or thrombocytopoies* or megakaryocytopoies*):ti,ab,kw 89
-
#54 ((platelet* or thrombocyt*) NEAR/3 (produc* or formation or stimulat*)):ti,ab,kw 848
-
#55 MeSH descriptor: [Portasystemic Shunt, Transjugular Intrahepatic] this term only 94
-
#56 (“transjugular intrahepatic portosystemic shunt*” or “transjugular intrahepatic porto systemic shunt*” or “transjugular intrahepatic portacaval shunt*” or “transjugular intrahepatic porta systemic shunt*” or “transjugular intrahepatic portasystemic shunt*” or “transjugular intrahepatic shunt*” or “transjugular intrahepatic stent*” or TIPS or TIPSS):ti,ab,kw 1028
-
#57 #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 5620
-
#58 #38 and #57 110
-
#59 #3 or #58 146.
Cochrane Database of Systematic Reviews 8.
Cochrane Central Register of Controlled Trials 138.
Kleijnen Systematic Reviews Evidence (www.ksrevidence.com): database last updated 24 January 2019
Date range searched: 2012 to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
| # | Query | Results |
|---|---|---|
| 1 | avatrombopag OR doptelet OR “AKR 501” OR AKR501 OR “AS 1670542” OR AS1670542 OR “E 5501” OR E5501 OR “oralE 5501” OR oralE5501 OR “YM 477” OR YM477 OR lusutrombopag OR mulpleta OR “S 888711” OR S888711 in All text | – |
| 2 | thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* in All text | 461 |
| 3 | (11q OR 11q23) AND (disorder* OR syndrome* OR delet* OR Jacobsen) in All text | – |
| 4 | “jacobsen syndrome” OR “paris trousseau” OR “kasabach merritt” OR “May Hegglin” OR hemangioma OR haemangioma in All text | 42 |
| 5 | (thrombotic AND microangiopath*) OR “hemolytic uremic” OR “haemolytic uremic” OR gasser* OR “HELLP syndrome” OR “HELLP syndromes” in All text | 46 |
| 6 | (hemolysis OR haemolysis) AND liver AND platelet* in All text | 10 |
| 7 | (haemolytic OR hemolytic) AND (anaemi* OR anemi*) AND (microangiopath*) in All text | 1 |
| 8 | Moschcowitz OR werlhof OR (wiskott AND Aldrich) in All text | – |
| 9 | “immunodeficiency 2” OR immunodeficiency2 OR Imd2 in All text | – |
| 10 | (platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*) in All text | 540 |
| 11 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 1027 |
| 12 | “liver disease” OR “liver diseases” OR “hepatic disease” OR “hepatic diseases” OR “intrahepatic disease” OR “intrahepatic diseases” OR “liver disorder” OR “liver disorders” OR “hepatic disorder” OR “hepatic disorders” OR “intrahepatic disorder” OR “intrahepatic disorders” OR “liver lesion” OR “liver lesions” OR “hepatic lesion” OR “hepatic lesions” OR “intrahepatic lesion” OR “intrahepatic lesions” OR cirrhosis OR cirrhosis OR cirrhotic in All text | 994 |
| 13 | chronic AND “destructive cholangitis” in All text | – |
| 14 | (fibrosis OR fibroses OR scar*) AND (liver* OR hepatic) in All text | 256 |
| 15 | (hepatitis OR hepatopath*) AND (chronic OR acute OR persistent OR “long standing” OR “long term” OR recurr*) in All text | 488 |
| 16 | (liver* OR hepatic OR intrahepatic) AND inflam* in All text | 165 |
| 17 | haemochromatosis OR hemochromatosis OR “bronze diabetes” OR “bronze diabetic” OR “recklinghausen applebaum” OR siderochromatosis OR “primary biliary cholangitis” OR hepatocarcinoma OR hepatoma* in All text | 29 |
| 18 | (liver* OR hepatic OR intrahepatic) AND carcinoma* in All text | 664 |
| 19 | #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 | 1885 |
| 20 | #11 AND #19 | 68 |
| 21 | #1 OR #20 | 68 |
Database last updated 24 January 2019, 13:06.
Epistemonikos (www.epistemonikos.org/en/)
Date range searched: up to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
Title/Abstract: avatrombopag OR doptelet OR “AKR 501” OR AKR501 OR “AS 1670542” OR AS1670542 OR “E 5501” OR E5501 OR “oralE 5501” OR oralE5501 OR “YM 477” OR YM477 OR lusutrombopag OR mulpleta OR “S 888711” OR S888711
OR
Title/Abstract: (thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni*) AND (“liver* disease*” OR “hepatic disease*” OR “liver* disorder*” OR “hepatic disorder*” OR “liver* lesion*” OR “hepatic lesion*” OR cirrho* OR fibros* OR “liver* carcinoma*” OR “hepatic carcinoma*”)
OR
Title/Abstract: ((platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*)) AND (“liver* disease*” OR “hepatic disease*” OR “liver* disorder*” OR “hepatic disorder*” OR “liver* lesion*” OR “hepatic lesion*” OR cirrho* OR fibros* OR “liver* carcinoma*” OR “hepatic carcinoma*”).
Records retrieved: 212.
Database of Abstracts of Reviews of Effects (www.crd.york.ac.uk/CRDWeb/)
Date ranges searched:
Health Technology Assessment database – up to 31 March 2018.*
NHS Economic Evaluation Database – up to 31 March 2015.*
Date searched: 24 January 2019.
*Database of Abstracts of Reviews of Effects and NHS Economic Evaluation Database have ceased; records were published until 31 March 2015. HTA database records were added until 31 March 2018; updating and addition of new records will resume on the International Network of Agencies for Health Technology Assessment platform.
Search strategy
-
(avatrombopag or doptelet or AKR 501 or AKR501 or AS 1670542 or AS1670542 or E 5501 or E5501 or oralE 5501 or oralE5501 or YM 477 or YM477 or 570406-98-3) 2
-
(lusutrombopag or mulpleta or S 888711 or S888711 or 1110766-97-6) 0
-
1 OR #2 2
-
MeSH DESCRIPTOR Thrombocytopenia EXPLODE ALL TREES 107
-
(thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) 369
-
(11q or 11q23) 0
-
(jacobsen near3 syndrome*) 0
-
(paris trousseau) 0
-
(kasabach merritt) 1
-
(hemangioma or haemangioma) 34
-
(thrombotic near2 (microangiopath* or micro angiopath*)) 0
-
(hemolytic uremic or haemolytic uremic) 14
-
(gasser*) 4
-
MeSH DESCRIPTOR HELLP Syndrome EXPLODE ALL TREES 5
-
(HELLP near2 syndrome*) 11
-
((hemolysis or haemolysis) near2 liver near2 platelet*) 2
-
(May Hegglin) 0
-
((haemolytic or hemolytic) near (anaemi* or anemi*)) 18
-
(microangiopath* near thrombotic) 0
-
(moschcowitz or werlhof) 0
-
MeSH DESCRIPTOR Wiskott-Aldrich Syndrome EXPLODE ALL TREES 0
-
(wiskott and Aldrich) 5
-
(immunodeficiency 2 or immunodeficiency2 or Imd2) 1
-
((platelet* or thrombocyte*) near3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) 24
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 467
-
MeSH DESCRIPTOR Liver Diseases EXPLODE ALL TREES 1983
-
((liver or hepat* or intrahepat*) near (disease* or disorder* or lesion*)) 723
-
(cirrhosis or cirrhoses or cirrhotic) 643
-
(chronic near3 cholangitis) 1
-
((fibrosis or fibroses or scar*) near3 (liver* or hepat*)) 49
-
((hepatitis or hepatopath*) near3 (chronic or acute or persistent or long stand* or long term or recurr*)) 547
-
((liver* or hepat* or intrahepat*) near3 inflam*) 20
-
(haemochromatosis or hemochromatosis or bronze* diabet* or recklinghausen applebaum or siderochromatosis) 37
-
(primary biliary cholangitis) 1
-
((liver* or hepat* or intrahepat*) near3 carcinoma*) 516
-
(hepatocarcinoma or hepatoma*) 14
-
#26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 2427
-
#25 AND #37 36
-
#3 OR #38 37.
Database of Abstracts of Reviews of Effects 19.
Health Technology Assessment database 7.
NHS Economic Evaluation Database 11.
PROSPERO (www.crd.york.ac.uk/PROSPERO/)
Date range searched: up to 24 January 2019.
Date searched: 24 January 2019.
-
#1 avatrombopag or doptelet or “AKR 501 “ or AKR501 or “AS 1670542 “ or AS1670542 or “E 5501 “ or E5501 or “oralE 5501 “ or oralE5501 or “YM 477 “ or YM477 or lusutrombopag or mulpleta or “S 888711 “ or S888711 3
-
#2 thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* 177
-
#3 (platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*) 363
-
#4 #2 OR #3 478
-
#5 “liver* disease*” OR “hepatic disease*” OR “liver* disorder*” OR “hepatic disorder*” OR “liver* lesion*” OR “hepatic lesion*” OR cirrho* OR fibros* OR “liver* carcinoma*” OR “hepatic carcinoma*” 1205
-
#6 #4 AND #5 37
-
#7 #1 OR #6 39.
Science Citation Index Expanded (Web of Science)
Date range searched: 1988 to 23 January 2019.
Date searched: 24 January 2019.
Search strategy
| # 38 | 722 | #1 or #37 |
| # 37 | 687 | #25 and #36 |
| # 36 | 211,185 | #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 |
| # 35 | 170,937 | TS=(“transjugular intrahepatic portosystemic shunt*” or “transjugular intrahepatic porto systemic shunt*” or “transjugular intrahepatic portacaval shunt*” or “transjugular intrahepatic portal systemic shunt*” or “transjugular intrahepatic portasystemic shunt*” or “transjugular intrahepatic shunt*” or “transjugular intrahepatic stent*” or TIPS or TIPSS) |
| # 34 | 15,958 | TS=((platelet* or thrombocyt*) NEAR/3 (produc* or formation or stimulat*)) |
| # 33 | 2359 | TS=(thrombopoiesi* or thrombocytopoies* or megakaryocytopoies*) |
| # 32 | 1088 | TS=((megakaryocyte* or karyocyte*) NEAR/3 (stimul* or maturat* or produc*)) |
| # 31 | 983 | TS=((spleen or splenic or “eria lienalis” or lienal) NEAR/3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap* or “therap* occlus*”)) |
| # 30 | 13,388 | TS=(splenectom* or (spleen NEAR/2 (resect* or remov* or surg*))) |
| # 29 | 7879 | TS=((platelet* or thrombocyt*) NEAR/3 (transfus* or infus* or administ*)) |
| # 28 | 780 | TS=(romiplostim or nplate or remiplistim or “amg 531” or amg531 or promegapoietin) |
| # 27 | 882 | TS=(eltrombopag or promacta or revolade or “SB 497115” or SB497115) |
| # 26 | 1591 | TS=((thrombopoietin* or c-Mpl) NEAR/3 (agonist* or agent* or mimetic* or receptor*)) |
| # 25 | 4437 | #16 and #24 |
| # 24 | 367,240 | #17 or #18 or #19 or #20 or #21 or #22 or #23 |
| # 23 | 148,666 | TS=(“primary biliary cholangitis”) or TS=((liver or hepat* or intrahepat*) NEAR/3 carcinoma*) or TS= (hepatocarcinoma or hepatoma*) |
| # 22 | 9840 | TS=(haemochromatosis or hemochromatosis or “bronze* diabet*” or “recklinghausen applebaum” or siderochromatosis) |
| # 21 | 16,207 | TS=((liver* or hepat* or intrahepat*) NEAR/3 inflam*) |
| # 20 | 73,241 | TS=((hepatitis or hepatopath*) NEAR/3 (chronic or acute or persistent or “long stand*” or “long term” or recurr*)) |
| # 19 | 29,320 | TS=((fibrosis or fibroses or scar*) NEAR/3 (liver* or hepat*)) |
| # 18 | 96,017 | TS=(cirrhosis or cirrhoses or cirrhotic) or TS= (chronic NEAR/3 “destructive cholangitis”) |
| # 17 | 121,928 | TS=((liver* or hepat* or intrahepat*) NEAR/2 (disease* or disorder* or lesion*)) |
| # 16 | 98,158 | #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 |
| # 15 | 20,790 | TS=((platelet* or thrombocyte*) NEAR/3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) |
| # 14 | 3306 | TS=(werlhof) or TS=(wiskott and aldrich) or TS=(“immunodeficiency 2” or immunodeficiency2 or Imd2) |
| # 13 | 48 | TS=(moschcowitz) |
| # 12 | 870 | TS=((haemolytic or hemolytic) NEAR/2 (anaemi* or anemi*) NEAR/2 (microangiopathic or “micro angiopathic”)) |
| # 11 | 170 | TS=(“May Hegglin”) |
| # 10 | 272 | TS=((hemolysis or haemolysis) NEAR/2 liver NEAR/2 platelet*) |
| # 9 | 3797 | TS=(gasser*) or TS=(HELLP NEAR/2 syndrome*) |
| # 8 | 10,671 | TS=(“hemolytic uremic” or “haemolytic uremic”) |
| # 7 | 3876 | TS=(thrombotic NEAR/2 (microangiopath* or “micro angiopath*”)) |
| # 6 | 11,949 | TS=(hemangioma or haemangioma) |
| # 5 | 703 | TS=(“kasabach merritt”) |
| # 4 | 189 | TS=(jacobsen NEAR/3 syndrome*) OR TS=(“paris trousseau” NEAR/3 syndrome*) |
| # 3 | 643 | TS=((11q or 11q23) NEAR/3 (disorder* or syndrome* or delet* or jacobsen)) |
| # 2 | 53,278 | TS=(thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) |
| # 1 | 56 | TS=(avatrombopag or doptelet or “AKR 501” or AKR501 or “AS 1670542” or AS1670542 or “E 5501” or E5501 or “oralE 5501” or oralE5501 or “YM 477” or YM477) or TS=(lusutrombopag or mulpleta or “S 888711” or S888711) |
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1982 to 23 January 2019.
Date searched: 24 January 2019.
Search strategy
| S1 | avatrombopag or doptelet or “AKR 501” or AKR501 or “AS 1670542” or AS1670542 or “E 5501” or E5501 or “oralE 5501” or oralE5501 or “YM 477” or lusutrombopag or mulpleta or “S 888711” or S888711 | 15 |
| S2 | (MH “Thrombocytopenia+”) | 5320 |
| S3 | TI (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) OR AB (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) | 7424 |
| S4 | TI ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) OR AB ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) | 33 |
| S5 | TI (jacobsen N3 syndrome*) OR AB (jacobsen N3 syndrome*) | 8 |
| S6 | TI (“paris trousseau” or “kasabach merritt” or “May Hegglin”) OR AB (“paris trousseau” or “kasabach merritt” or “May Hegglin”) | 101 |
| S7 | TI (hemangioma or haemangioma) OR AB (hemangioma or haemangioma) | 2028 |
| S8 | TI (thrombotic N2 (microangiopath* or “micro angiopath*”)) or AB (thrombotic N2 (microangiopath* or “micro angiopath*”)) | 536 |
| S9 | TI (“hemolytic uremic” or “haemolytic uremic” or gasser*) or AB (“hemolytic uremic” or “haemolytic uremic” or gasser*) | 824 |
| S10 | (MH “HELLP Syndrome”) | 476 |
| S11 | TI (HELLP N2 syndrome*) or AB (HELLP N2 syndrome*) | 438 |
| S12 | TI ((hemolysis or haemolysis) N2 liver N2 platelet*) or AB ((hemolysis or haemolysis) N2 liver N2 platelet*) | 78 |
| S13 | TI ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) or AB ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) | 159 |
| S14 | TI ((microangiopath* or micro angiopath*) N2 thrombotic) or AB ((microangiopath* or micro angiopath*) N2 thrombotic) | 536 |
| S15 | TI (moschcowitz or werlhof or (wiskott and Aldrich)) or AB (moschcowitz or werlhof or (wiskott and Aldrich)) | 93 |
| S16 | (MH “Wiskott-Aldrich Syndrome”) | 52 |
| S17 | TI (“immunodeficiency 2” or immunodeficiency2 or Imd2) or AB (“immunodeficiency 2” or immunodeficiency2 or Imd2) | 1 |
| S18 | TI ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) or AB ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) | 2419 |
| S19 | S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 | 14,324 |
| S20 | (MH “Liver Diseases+”) | 55,452 |
| S21 | TI ((liver* or hepat* or intrahepat*) N2 (disease* or disorder* or lesion*)) OR AB ((liver* or hepat* or intrahepat*) N2 (disease* or disorder* or lesion*)) | 14,234 |
| S22 | TI (cirrhosis or cirrhoses or cirrhotic) or AB (cirrhosis or cirrhoses or cirrhotic) | 7845 |
| S23 | TI (chronic N3 destructive cholangitis) or AB (chronic N3 destructive cholangitis) | 3 |
| S24 | TI ((fibrosis or fibroses or scar*) N3 (liver* or hepat*)) or AB ((fibrosis or fibroses or scar*) N3 (liver* or hepat*)) | 2587 |
| S25 | TI ((hepatitis or hepatopath*) N3 (chronic or acute or persistent or “long stand*” or “long term” or recurr*)) or AB ((hepatitis or hepatopath*) N3 (chronic or acute or persistent or “long stand*” or “long term” or recurr*)) | 6144 |
| S26 | TI ((liver* or hepat* or intrahepat*) N3 inflam*) or AB ((liver* or hepat* or intrahepat*) N3 inflam*) | 1639 |
| S27 | TI (haemochromatosis or hemochromatosis or “bronze* diabet*” or “recklinghausen applebaum” or siderochromatosis or “primary biliary cholangitis”) or AB (haemochromatosis or hemochromatosis or “bronze* diabet*” or “recklinghausen applebaum” or siderochromatosis or “primary biliary cholangitis”) | 813 |
| S28 | TI ((liver* or hepat* or intrahepat*) N3 carcinoma*) or AB ((liver* or hepat* or intrahepat*) N3 carcinoma*) | 9387 |
| S29 | TI (hepatocarcinoma or hepatoma*) or AB (hepatocarcinoma or hepatoma*) | 799 |
| S30 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 | 66,144 |
| S31 | S19 and S30 | 972 |
| S32 | TI ((thrombopoietin* or c-Mpl) N3 (agonist* or agent* or mimetic* or receptor*)) or AB ((thrombopoietin* or c-Mpl) N3 (agonist* or agent* or mimetic* or receptor)) | 184 |
| S33 | TI (eltrombopag or promacta or revolade or “SB 497115” or SB497115) or AB (eltrombopag or promacta or revolade or “SB 497115” or SB497115) | 171 |
| S34 | TI (romiplostim or nplate or remiplistim or “amg 531” or amg531 or promegapoietin) or AB (romiplostim or nplate or remiplistim or “amg 531” or amg531 or promegapoietin) | 146 |
| S35 | (MH “Platelet Transfusion”) | 1182 |
| S36 | TI ((platelet* or thrombocyt*) N3 (transfus* or infus* or administ*)) or AB ((platelet* or thrombocyt*) N3 (transfus* or infus* or administ*)) | 1250 |
| S37 | (MH “Splenectomy”) | 1354 |
| S38 | TI (splenectom* or (spleen N3 (resect* or remov* or surg*))) or AB (splenectom* or (spleen N3 (resect* or remov* or surg*))) | 1636 |
| S39 | (MH “Splenic Artery”) AND (MH “Embolization, Therapeutic+”) | 155 |
| S40 | TI ((spleen or splenic or “eria lienalis “ or lienal) N3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap* or therap* occlus*)) or AB ((spleen or splenic or “eria lienalis “ or lienal) N3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap* or therap* occlus*)) | 234 |
| S41 | TI ((megakaryocyte* or karyocyte*) N3 (stimul* or maturat* or produc*)) or AB ((megakaryocyte* or karyocyte*) N3 (stimul* or maturat* or produc*)) | 28 |
| S42 | TI (thrombopoiesi* or thrombocytopoies* or megakaryocytopoies*) or AB (thrombopoiesi* or thrombocytopoies* or megakaryocytopoies*) | 67 |
| S43 | TI ((platelet* or thrombocyt*) N3 (produc* or formation or stimulat*)) or AB ((platelet* or thrombocyt*) N3 (produc* or formation or stimulat*)) | 962 |
| S44 | (MH “Portasystemic Shunt, Surgical”) | 895 |
| S45 | TI (“transjugular intrahepatic portosystemic shunt*” or “transjugular intrahepatic porto systemic shunt*” or “transjugular intrahepatic portacaval shunt*” or “transjugular intrahepatic porta systemic shunt*” or “transjugular intrahepatic portasystemic shunt*” or “transjugular intrahepatic shunt*” or “transjugular intrahepatic stent*” or TIPS or TIPSS) or AB (“transjugular intrahepatic portosystemic shunt*” or “transjugular intrahepatic porto systemic shunt*” or “transjugular intrahepatic portacaval shunt*” or “transjugular intrahepatic porta systemic shunt*” or “transjugular intrahepatic portasystemic shunt*” or “transjugular intrahepatic shunt*” or “transjugular intrahepatic stent*” or TIPS or TIPSS) | 22,430 |
| S46 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 28,031 |
| S47 | S31 and S46 | 113 |
| S48 | S1 or S47 | 122 |
Latin American and Caribbean Health Sciences (http://lilacs.bvsalud.org/en/)
Date range searched: 1982 to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
((MH:c15.378.140.855 OR MH:c15.378.100.100.970 OR thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* OR trombocitopeni* OR ((platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*))) AND (MH:C06.552 or “liver disease” OR “liver diseases” OR “hepatic disease” OR “hepatic diseases” OR “intrahepatic disease” OR “intrahepatic diseases” OR “liver disorder” OR “liver disorders” OR “hepatic disorder” OR “hepatic disorders” OR “intrahepatic disorder” OR “intrahepatic disorders” OR “liver lesion” OR “liver lesions” OR “hepatic lesion” OR “hepatic lesions” OR “intrahepatic lesion” OR “intrahepatic lesions” OR hepatopatias OR cirrhosis OR cirrhoses OR cirrhotic OR cirrose OR cirrosis OR ((liver$ OR hepatic OR intrahepatic) AND carcinoma$))) OR (avatrombopag OR doptelet OR “AKR 501” OR akr501 OR “AS 1670542” OR as1670542 OR “E 5501” OR e5501 OR “oralE 5501” OR orale5501 OR “YM 477” OR ym477 OR lusutrombopag OR mulpleta OR “S 888711” OR s888711).
Search limited to non-MEDLINE databases:
-
LILACS (89)
-
IBECS (45)
-
BINACIS (13)
-
CUMED (4)
-
MedCarib (4)
-
LIS -Health Information Locator (1)
-
Index Psychology – Theses (1).
Northern Light Life Sciences Conference Abstracts (via Ovid)
Date range searched: 2010–19 week 2.
Date searched: 24 January 2019.
Search strategy
-
(avatrombopag or doptelet or AKR 501 or AKR501 or AS 1670542 or AS1670542 or E 5501 or E5501 or oralE 5501 or oralE5501 or YM 477 or YM477).af. (15)
-
(lusutrombopag or mulpleta or S 888711 or S888711 or 1110766-97-6).af. (10)
-
1 or 2 (25)
-
exp thrombocytopenia/ (19,173)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,hw. (18,543)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,hw. (132)
-
(jacobsen adj3 syndrome$).ti,ab,hw. (41)
-
(paris trousseau or kasabach merritt or hemangioma or haemangioma).ti,ab,hw. (2487)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,hw. (1515)
-
(hemolytic uremic or haemolytic uremic or gasser$).ti,ab,hw. (643)
-
hellp syndrome/ (410)
-
(HELLP adj2 syndrome$).ti,ab,hw. (415)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,hw. (0)
-
May Hegglin.ti,ab,hw. (10)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,hw. (77)
-
(moschcowitz or werlhof or (wiskott and Aldrich)).ti,ab,hw. (468)
-
wiskott-aldrich syndrome/ (460)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,hw. (0)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,hw. (1916)
-
or/4-19 (24,421)
-
exp Liver Diseases/ (70,505)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$)).ti,ab,hw. (27,653)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab,hw. (14,624)
-
(chronic adj3 destructive cholangitis).ti,ab,hw. (3)
-
((fibrosis or fibroses or scar$) adj3 (liver$ or hepat$)).ti,ab,hw. (4585)
-
((hepatitis or hepatopath$) adj3 (chronic or acute or persistent or long stand$ or long term or recurr$)).ti,ab,hw. (8107)
-
((liver$ or hepat$ or intrahepat$) adj3 inflam$).ti,ab,hw. (1780)
-
(haemochromatosis or hemochromatosis or bronze$ diabet$ or recklinghausen applebaum or siderochromatosis).ti,ab,hw. (1151)
-
primary biliary cholangitis.ti,ab,hw. (230)
-
((liver$ or hepat$ or intrahepat$) adj3 carcinoma$).ti,ab,hw. (13,730)
-
(hepatocarcinoma or hepatoma$).ti,ab,hw. (900)
-
or/21-31 (89,117)
-
20 and 32 (2415)
-
thrombopoietin/ (1145)
-
((thrombopoietin$ or c-Mpl) adj3 (agonist$ or agent$ or mimetic$ or receptor$)).ti,ab,hw. (206)
-
(eltrombopag or promacta or revolade or SB 497115 or SB497115 or 496775-61-2).ti,ab,hw. (279)
-
(romiplostim or nplate or remiplistim or amg 531 or amg531 or 267639-76-9).ti,ab,hw. (256)
-
promegapoietin.ti,ab,hw. (0)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$)).ti,ab,hw. (896)
-
(splenectom$ or (spleen adj3 (resect$ or remov$ or surg$))).ti,ab,hw. (1139)
-
((spleen or splenic or eria lienalis or lienal) adj3 (embolisation or embolization or embolism or embolus or thrombus or embolotherap$ or therap$ occlus$)).ti,ab,hw. (141)
-
megakaryocytes/ (2226)
-
((megakaryocyte$ or karyocyte$) adj3 (stimul$ or maturat$ or produc$)).ti,ab,hw. (72)
-
(thrombopoiesi$ or thrombocytopoies$ or megakaryocytopoies$).ti,ab,hw. (114)
-
((platelet$ or thrombocyt$) adj3 (produc$ or formation or stimulat$)).ti,ab,hw. (944)
-
(transjugular intrahepatic portosystemic shunt$ or transjugular intrahepatic porto systemic shunt$ or transjugular intrahepatic portacaval shunt$ or transjugular intrahepatic porta systemic shunt$ or transjugular intrahepatic portasystemic shunt$ or transjugular intrahepatic shunt$ or transjugular intrahepatic stent$ or TIPS or TIPSS).ti,ab,hw. (2278)
-
or/34-46 (8073)
-
33 and 47 (221)
-
3 or 48 (227).
Transfusion Evidence Library (www.transfusionevidencelibrary.com/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
(avatrombopag OR doptelet OR lusutrombopag OR mulpleta) OR ((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* OR (platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*)) AND (“liver disease*” OR “hepatic disease*” OR “liver disorder*” OR “hepatic disorder*” OR “liver lesion*” OR “hepatic lesion*” OR cirrhosis OR cirrhosis OR cirrhotic OR “liver* carcinoma*” OR “hepatic carcinoma*”)).
Records retrieved: 40.
RePEc (http://repec.org/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
IDEAS search interface.
(avatrombopag | doptelet | lusutrombopag | mulpleta | thrombocytopenia | thrombocytopenic | thrombocytopaenia | thrombocytopaenic | thrombopenia | thrombopenic | thrombopaenia | thrombopaenic).
Records retrieved: 14.
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/search/advanced)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
(avatrombopag OR doptelet OR “AKR 501” OR AKR501 OR “AS 1670542” OR AS1670542 OR “E 5501” OR E5501 OR “oralE 5501” OR oralE5501 OR “YM 477” OR YM477 OR lusutrombopag OR mulpleta OR “S 888711” OR S888711) OR ((thrombocytopenia OR thrombocytopenic OR thrombocytopaenia OR thrombocytopaenic OR thrombopenia OR thrombopenic OR thrombopaenia OR thrombopaenic OR macrothrombocytopenia OR macrothrombocytopenic OR macrothrombocytopaenia OR macrothrombocytopaenic) AND (liver OR hepatic OR intrahepatic OR cirrhosis OR cirrhoses OR cirrhotic)).
319 studies found.
World Health Organization International Clinical Trials Register Portfolio (www.who.int/ictrp/search/en/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Advanced search option
| Search | Results |
|---|---|
| Intervention: avatrombopag OR doptelet OR AKR 501 OR AKR501 OR AS 1670542 OR AS1670542 OR E 5501 OR E5501 OR oralE 5501 OR oralE5501 OR YM 477 OR YM477 OR lusutrombopag OR mulpleta OR S 888711 OR S888711 | (49 records for) 20 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: thrombopoietin receptor OR thrombopoietin agonist OR thrombopoietin agent |
(25 records for) 25 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* Intervention: eltrombopag OR promacta OR revolade or SB 497115 or SB497115 or 496775-61-2 |
(234 records for) 97 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* Intervention: romiplostim OR nplate OR remiplistim OR amg 531 OR amg531 OR 267639-76-9 OR promegapoietin |
(140 records for) 56 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: platelet transfusion OR platelet infusion OR platelet administration OR thrombocyt* transfusion OR thrombocyt* infusion OR thrombocyt* administration |
(15 records for) 14 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: splenectomy OR spleen resection OR spleen remove OR spleen surgery |
(4 records for) 4 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: embolisation OR embolism OR thrombus |
(1 record for) 1 trial found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: megakaryocyte OR karyocyte |
(1 record for) 1 trial found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: thrombopoiesis OR thrombocytopoies OR megakaryocytopoies |
(0 records for) 0 trials found |
|
Condition: thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaenia* Intervention: platelet production OR thrombocyt* production OR platelet formation OR thrombocyt* formation OR platelet stimulation OR thrombocyt* stimulation |
(0 records for) 0 trials found |
| Total | 218 |
| Total after deduplication | 207 |
US Food and Drug Administration (www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Drugs@FDA searched
| Drug name | Results |
|---|---|
| doptelet (avatrombopag) | 1 |
| mulpleta (lusutrombopag) | 1 |
| promacta (eltrombopag) | 1 |
| nplate (romiplostim) | 1 |
| promegapoietin | 0 |
| Total | 4 |
European Medicines Agency (www.ema.europa.eu)
Date range searched: up to 23 January 2019.
Date searched 23 January 2019.
Search strategy
| Medicines; search; EPARs | EPARs |
|---|---|
| doptelet (avatrombopag) | 0 |
| mulpleta (lusutrombopag) | 0 |
| revolade (eltrombopag, promacta) | 1 |
| nplate (romiplostim) | 1 |
| promegapoietin | 0 |
| Total | 2 |
OAIster (http://oaister.worldcat.org)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
(avatrombopag OR doptelet OR lusutrombopag OR mulpleta) OR ((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND (liver* OR hepat*) AND (thrombopoietin* receptor* OR thrombopoietin* agonist* OR thrombopoietin* agent* OR eltrombopag OR promacta OR revolade OR romiplostim OR nplate OR platelet transfus* OR platelet infus* OR platelet admin* OR thrombocyt* transf* OR thrombocyt* infus* OR thrombocyt* admin* OR splenectom* OR spleen resect* OR spleen remov* OR spleen surger* OR emboli* OR thrombus OR megakaryocyte* OR karyocyte* OR thrombopoiesis OR thrombocytopoies OR megakaryocytopoies OR platelet produc* OR thrombocyt* produc* OR platelet forma* OR thrombocyt* forma* OR platelet stimul* OR thrombocyt* stimul*)).
Records retrieved: 37.
OpenGrey (www.opengrey.eu/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
(avatrombopag OR doptelet OR lusutrombopag OR mulpleta) OR ((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND ((thrombopoietin* NEAR receptor*) OR (thrombopoietin* NEAR agonist*) OR (thrombopoietin* NEAR agent*) OR eltrombopag OR promacta OR revolade OR romiplostim OR nplate OR (platelet NEAR transfus*) OR (platelet NEAR infus*) OR (platelet NEAR admin*) OR (thrombocyt* NEAR transf*) OR (thrombocyt* NEAR infus*) OR (thrombocyt* NEAR admin*) OR splenectom* OR (spleen NEAR resect*) OR (spleen NEAR remov*) OR (spleen NEAR surger*) OR emboli* OR thrombus OR megakaryocyte* OR karyocyte* OR thrombopoiesis OR thrombocytopoies OR megakaryocytopoies OR (platelet NEAR produc*) OR (thrombocyt* NEAR produc*) OR (platelet NEAR forma*) OR (thrombocyt* NEAR forma*) OR (platelet NEAR stimul*) OR (thrombocyt* NEAR stimul*)).
Records retrieved: 41.
Copac (https://copac.jisc.ac.uk/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
-
Keyword: avatrombopag
-
Keyword: doptelet
-
Keyword: lusutrombopag
-
Keyword: mulpleta
-
Keyword: thrombocytopeni* liver* thrombopoietin*
-
Keyword: thrombocytopaeni* liver* thrombopoietin*
-
Keyword: thrombopeni* liver* thrombopoietin*
-
Keyword: thrombopaeni* liver* thrombopoietin*
-
Keyword: thrombocytopeni* hepati* thrombopoietin*
-
Keyword: thrombocytopaeni* hepati* thrombopoietin*
-
Keyword: thrombopeni* hepati* thrombopoietin*
-
Keyword: thrombopaeni* hepati* thrombopoietin*
-
Keyword: thrombocytopeni* liver* eltrombopag
-
Keyword: thrombocytopaeni* liver* eltrombopag
-
Keyword: thrombopeni* liver* eltrombopag
-
Keyword: thrombopaeni* liver* eltrombopag
-
Keyword: thrombocytopeni* hepati* eltrombopag
-
Keyword: thrombocytopaeni* hepati* eltrombopag
-
Keyword: thrombopeni* hepati* eltrombopag
-
Keyword: thrombopaeni* hepati* eltrombopag
-
Keyword: thrombocytopeni* liver* romiplostim
-
Keyword: thrombocytopaeni* liver* romiplostim
-
Keyword: thrombopeni* liver* romiplostim
-
Keyword: thrombopaeni* liver* romiplostim
-
Keyword: thrombocytopeni* hepati* romiplostim
-
Keyword: thrombocytopaeni* hepati* romiplostim
-
Keyword: thrombopeni* hepati* romiplostim
-
Keyword: thrombopaeni* hepati* romiplostim
-
Keyword: thrombocytopeni* liver* “platelet transfus*"
-
Keyword: thrombocytopaeni* liver* “platelet transfus*"
-
Keyword: thrombopeni* liver* “platelet transfus*"
-
Keyword: thrombopaeni* liver* “platelet transfus*"
-
Keyword: thrombocytopeni* hepati* “platelet transfus*"
-
Keyword: thrombocytopaeni* hepati* “platelet transfus*"
-
Keyword: thrombopeni* hepati* “platelet transfus*"
-
Keyword: thrombopaeni* hepati* “platelet transfus*"
-
Keyword: thrombocytopeni* liver* splenectom*
-
Keyword: thrombocytopaeni* liver* splenectom*
-
Keyword: thrombopeni* liver* splenectom*
-
Keyword: thrombopaeni* liver* splenectom*
-
Keyword: thrombocytopeni* hepati* splenectom*
-
Keyword: thrombocytopaeni* hepati* splenectom*
-
Keyword: thrombopeni* hepati* splenectom*
-
Keyword: thrombopaeni* hepati* splenectom*
-
Keyword: thrombocytopeni* liver* “splenic emboli*"
-
Keyword: thrombocytopaeni* liver* “splenic emboli*"
-
Keyword: thrombopeni* liver* “splenic emboli*"
-
Keyword: thrombopaeni* liver* “splenic emboli*"
-
Keyword: thrombocytopeni* hepati* “splenic emboli*"
-
Keyword: thrombocytopaeni* hepati* “splenic emboli*"
-
Keyword: thrombopeni* hepati* “splenic emboli*"
-
Keyword: thrombopaeni* hepati* “splenic emboli*"
-
Keyword: thrombocytopeni* liver* megakaryocyte*
-
Keyword: thrombocytopaeni* liver* megakaryocyte*
-
Keyword: thrombopeni* liver* megakaryocyte*
-
Keyword: thrombopaeni* liver* megakaryocyte*
-
Keyword: thrombocytopeni* hepati* megakaryocyte*
-
Keyword: thrombocytopaeni* hepati* megakaryocyte*
-
Keyword: thrombopeni* hepati* megakaryocyte*
-
Keyword: thrombopaeni* hepati* megakaryocyte*.
Records retrieved: 90.
Utilities/health-related quality-of-life search strategies
| Database/resource | Host | Date range | Results | Date searched |
|---|---|---|---|---|
| MEDLINE | Ovid | 1946 to week 3 January 2019 | 569 | 24 January 2019 |
| MEDLINE Epub Ahead of Print; MEDLINE In-Process & Other Non-Indexed Citations; MEDLINE Daily Update | Ovid | 23 January 2019 | 26 | 24 January 2019 |
| PubMed | National Library of Medicine | Up to 24 January 2019 | 35 | 24 January 2019 |
| EMBASE | Ovid | 1974 to week 3 2019 | 863 | 24 January 2019 |
| HTA database | www.crd.york.ac.uk/CRDWeb/ | Up to 31 March 2015 | 70 | 24 January 2019 |
| NHS EED | www.crd.york.ac.uk/CRDWeb/ | Up to 31 March 2018 | 110 | 24 January 2019 |
| Science Citation Index Expanded (SCI) | Web of Science | 1988 to 23 January 2019 | 422 | 24 January 2019 |
| CINAHL | EBSCOhost | 1982 to 23 January 2019 | 260 | 24 January 2019 |
| Latin American and Caribbean Health Sciences (LILACS) | http://lilacs.bvsalud.org/en/ | 1982 to 24 January 2019 | 837 | 24 January 2019 |
| Northern Light Life Sciences Conference Abstracts | Ovid | 2010–19/week 2 | 63 | 24 January 2019 |
| CEA Registry | www.cearegistry.org | Up to 23 January 2019 | 18 | 23 January 2019 |
| ScHARR Health Utilities Database | www.scharrhud.org/ | Up to 23 January 2019 | 0 | 23 January 2019 |
| OAIster | http://oaister.worldcat.org | Up to 23 January 2019 | 73 | 23 January 2019 |
| OpenGrey | www.opengrey.eu/ | Up to 23 January 2019 | 1 | 23 January 2019 |
| Copac | https://copac.jisc.ac.uk/ | Up to 23 January 2019 | 104 | 23 January 2019 |
| Total records retrieved | 3451 | |||
| Duplicate records removed | 1022 | |||
| Total records to screen | 2429 | |||
MEDLINE (via Ovid): 1946 to week 3 January 2019
Date ranges searched:
MEDLINE Epub Ahead of Print (via Ovid): 22 January 2019.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid): 23 January 2019.
MEDLINE Daily Update (via Ovid): 22 January 2019.
Date searched: 24 January 2019.
Search strategy
-
quality-adjusted life years/ or quality of life/ (179,815)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (23,334)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (1938)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (5044)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (745)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (386)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (488)
-
"health related quality of life".ti,ab,ot. (37,648)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (11,042)
-
"assessment of quality of life".ti,ab,ot. (1664)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (9022)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (17,843)
-
(hye or hyes).ti,ab,ot. (63)
-
health$ year$ equivalent$.ti,ab,ot. (40)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (1339)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (817)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (3371)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (12,572)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (6642)
-
15d.ti,ab,ot. (1625)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (373)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (10,844)
-
(utilities or disutili$).ti,ab,ot. (6548)
-
(CLDQ or Chronic Liver Disease Questionnaire$).ti,ab,ot,hw. (161)
-
(LDSI or Liver Disease Symptom Index$).ti,ab,ot,hw. (18)
-
(LDQOL or Liver Disease Quality of Life Questionnaire$).ti,ab,ot,hw. (26)
-
(EORTC QLQ-HCC18 or EORTC QLQ-LMC21).ti,ab,ot,hw. (13)
-
(PLD-Q or Polycystic Liver Disease Questionnaire$).ti,ab,ot,hw. (5)
-
or/1-28 (228,242)
-
animals/ not (animals/ and humans/) (4,507,390)
-
29 not 30 (226,165)
-
letter.pt. (1,013,622)
-
editorial.pt. (479,604)
-
historical article.pt. (349,760)
-
or/32-34 (1,824,832)
-
31 not 35 (217,667)
-
exp Thrombocytopenia/ (45,457)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot,hw. (69,081)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot,hw. (574)
-
(jacobsen adj3 syndrome$).ti,ab,ot,hw. (129)
-
paris trousseau.ti,ab,ot,hw. (30)
-
kasabach merritt.ti,ab,ot,hw. (704)
-
(hemangioma or haemangioma).ti,ab,ot,hw. (32,339)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot,hw. (3354)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot,hw. (7663)
-
gasser$.ti,ab,ot,hw. (1689)
-
HELLP Syndrome/ (1709)
-
(HELLP adj2 syndrome$).ti,ab,ot,hw. (2561)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot,hw. (7)
-
May Hegglin.ti,ab,ot,hw. (221)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot,hw. (1411)
-
moschcowitz.ti,ab,ot,hw. (107)
-
werlhof.ti,ab,ot,hw. (120)
-
Wiskott-Aldrich Syndrome/ (1428)
-
(wiskott and Aldrich).ti,ab,ot,hw. (3312)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot,hw. (44)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot,hw. (22,231)
-
or/37-57 (132,417)
-
36 and 58 (595).
MEDLINE 569.
MEDLINE Epub Ahead of Print 4.
MEDLINE In-Process & Other Non-Indexed Citations 22.
MEDLINE Daily Update 0.
Health-related quality-of-life free-text terms based on figure 4 in Common Free-text Terms for Electronic Database Searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values From the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011).
PubMed (National Library of Medicine)
Date range searched: up to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
-
#31 #29 AND #30 35
-
#30 pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb] 3,121,488
-
#29 #17 AND #28 827
-
#28 (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) 188,201
-
#27 (platelet*[tiab] OR thrombocyte*[tiab]) AND (defici*[tiab] OR reduc*[tiab] OR low[tiab] OR lower[tiab] OR lowest[tiab] OR few[tiab] OR fewer[tiab] OR fewest[tiab] OR decrease[tiab] OR decreases[tiab] OR decreased[tiab] OR defective[tiab] OR destruc*[tiab] OR destroy*[tiab]) 99,513
-
#26 “immunodeficiency 2” OR immunodeficiency2 OR Imd2 46
-
#25 Moschcowitz[tiab] OR werlhof[tiab] OR “Wiskott-Aldrich Syndrome”[Mesh] OR (wiskott[tiab] AND Aldrich[tiab]) 2664
-
#24 (haemolytic[tiab] OR hemolytic[tiab]) AND (anaemi*[tiab] OR anemi*[tiab]) AND (microangiopath*[tiab]) 1765
-
#23 (hemolysis[tiab] OR haemolysis[tiab]) AND liver[tiab] AND platelet*[tiab] 1247
-
#22 “HELLP Syndrome”[Mesh] OR “HELLP syndrome” OR “HELLP syndromes” 2583
-
#21 (thrombotic[tiab] AND microangiopath*[tiab]) OR “hemolytic uremic” OR “haemolytic uremic” OR gasser*[tiab] 12,074
-
#20 “jacobsen syndrome” OR “paris trousseau” OR “kasabach merritt” OR “May Hegglin” OR hemangioma[tiab] OR haemangioma[tiab] 17,717
-
#19 (11q[tiab] OR 11q23[tiab]) AND (disorder*[tiab] OR syndrome*[tiab] OR delet*[tiab] OR Jacobsen[tiab]) 1605
-
#18 “Thrombocytopenia”[Mesh] OR thrombocytopeni*[tiab] OR thrombocytopaeni*[tiab] OR thrombopeni*[tiab] OR thrombopaeni*[tiab] OR macrothrombocytopeni*[tiab] OR macrothrombocytopaeni*[tiab] 73,938
-
#17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) 222,519
-
#16 CLDQ[tiab] OR “Chronic Liver Disease Questionnaire”[tiab] OR “Chronic Liver Disease Questionnaires”[tiab] OR LDSI[tiab] OR “Liver Disease Symptom Index”[tiab] OR “Liver Disease Symptom Indexes”[tiab] OR LDQOL[tiab] OR “Liver Disease Quality of Life Questionnaire”[tiab] OR “Liver Disease Quality of Life Questionnaires”[tiab] OR “EORTC QLQ-HCC18”[tiab] OR “EORTC QLQ-LMC21”[tiab] OR PLD-Q[tiab] OR “Polycystic Liver Disease Questionnaire”[tiab] OR “Polycystic Liver Disease Questionnaires”[tiab] 214
-
#15 utilities[tiab] OR disutili*[tiab] 6591
-
#14 HSUV*[tiab] OR “health state* value*”[tiab] OR “health state* preference*”[tiab] OR HSPV*[tiab] 135
-
#13 QALY*[tiab] OR DALY*[tiab] OR HALY*[tiab] OR YHL[tiab] OR HYES[tiab] OR YPLL[tiab] OR YHLL[tiab] OR qald*[tiab] OR qale*[tiab] OR qtime*[tiab] OR AQoL*[tiab] OR timetradeoff[tiab] OR “time tradeoff”[tiab] OR “time trade-off”[tiab] OR “time trade off”[tiab] OR TTO[tiab] OR “standard gamble”[tiab] OR “willingness to pay”[tiab] OR 15d[tiab] 18,990
-
#12 “Disability adjusted life”[tiab] OR “Disability-adjusted life”[tiab] OR “health adjusted life”[tiab] OR “health-adjusted life”[tiab] OR “years of healthy life”[tiab] OR “healthy years equivalent”[tiab] OR “years of potential life lost”[tiab] OR “years of health life lost”[tiab] 3319
-
#11 “quality time”[tiab] OR qwb[tiab] OR “quality of well being”[tiab] OR “quality of wellbeing”[tiab] OR “index of wellbeing”[tiab] OR “index of well being”[tiab] 556
-
#10 hui[tiab] OR hui1[tiab] OR hui2[tiab] OR hui3[tiab] OR hui4[tiab] OR hui-4[tiab] OR hui-1[tiab] OR hui-2[tiab] OR hui-3[tiab] 1335
-
#9 euroqol[tiab] OR “euro qol”[tiab] OR eq5d[tiab] OR “eq 5d”[ tiab] OR hql[tiab] OR hrql[tiab] OR hqol[tiab] OR “h qol”[tiab] OR hrqol[tiab] OR “hr qol”[tiab] OR hye[tiab] OR hyes[tiab] or “health year equivalent”[tiab] OR “health years equivalent”[tiab] 25,124
-
#8 “health related quality of life”[tiab] OR “quality adjusted life”[tiab] OR “quality-adjusted-life”[tiab] OR “assessment of quality of life”[tiab] 49,632
-
#7 sf8[tiab] OR “sf 8”[tiab] OR sf-8[tiab] OR “short form 8”[tiab] OR “shortform 8”[tiab] OR “sf eight”[tiab] OR sfeight[tiab] OR “shortform eight”[tiab] OR “short form eight”[tiab] 501
-
#6 sf20[tiab] OR “sf 20”[tiab] OR sf-20[tiab] OR “short form 20”[tiab] OR “shortform 20”[tiab] OR “sf twenty”[tiab] OR sftwenty[tiab] OR “shortform twenty”[tiab] OR “short form twenty”[tiab] 377
-
#5 sf6D[tiab] OR “sf 6D”[tiab] OR sf-6D[tiab] OR “short form 6D”[tiab] OR “shortform 6D”[tiab] OR “sf six D”[tiab] OR sfsixD[tiab] OR “shortform six D”[tiab] OR “short form six D”[tiab] 748
-
#4 sf12[tiab] OR “sf 12”[tiab] OR sf-12[tiab] OR “short form 12”[tiab] OR “shortform 12”[tiab] OR “sf twelve”[tiab] OR sftwelve[tiab] OR “shortform twelve”[tiab] OR “short form twelve”[tiab] 5072
-
#3 sf6[tiab] or “sf 6”[tiab] OR “sf-6”[tiab] OR “short form 6”[tiab] OR “shortform 6”[tiab] OR “sf six”[tiab] OR sfsix[tiab] OR “shortform six”[tiab] OR “short form six”[tiab] 1917
-
#2 sf36[tiab] OR “sf 36”[tiab] OR sf-36[tiab] OR “short form 36”[tiab] OR “shortform 36”[tiab] OR “sf thirtysix”[tiab] OR “sf thirty six”[tiab] OR “shortform thirtysix”[tiab] OR “shortform thirty six”[tiab] OR “short form thirty six”[tiab] OR “short form thirtysix”[tiab] OR “short form thirty six”[tiab] 23,445
-
#1 (“Quality-Adjusted Life Years”[Mesh]) OR “Quality of Life”[Mesh]) 179,608.
EMBASE (via Ovid)
Date range searched: 1974 to week 3 2019.
Date searched: 24 January 2019.
Search strategy
-
quality adjusted life year/ or quality of life index/ (25,499)
-
Short Form 12/ or Short Form 20/ or Short Form 36/ or Short Form 8/ (29,766)
-
"International Classification of Functioning, Disability and Health"/ or “ferrans and powers quality of life index"/ or “gastrointestinal quality of life index"/ (2998)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (37,386)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (2074)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (8180)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (1355)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (412)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (819)
-
“health related quality of life”.ti,ab,ot. (54,017)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (16,849)
-
“assessment of quality of life”.ti,ab,ot. (2629)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (16,871)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (28,883)
-
(hye or hyes).ti,ab,ot. (119)
-
health$ year$ equivalent$.ti,ab,ot. (40)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (2812)
-
(quality time or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or index of well being).ti,ab,ot,hw. (1083)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (4037)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (21,565)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (10,142)
-
15d.ti,ab,ot. (2352)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (539)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (17,247)
-
(utilities or disutili$).ti,ab,ot. (10,644)
-
(CLDQ or Chronic Liver Disease Questionnaire$).ti,ab,ot,hw. (343)
-
(LDSI or Liver Disease Symptom Index$).ti,ab,ot,hw. (32)
-
(LDQOL or Liver Disease Quality of Life Questionnaire$).ti,ab,ot,hw. (51)
-
(EORTC QLQ-HCC18 or EORTC QLQ-LMC21).ti,ab,ot,hw. (23)
-
(PLD-Q or Polycystic Liver Disease Questionnaire$).ti,ab,ot,hw. (9)
-
or/1-30 (166,039)
-
animal/ or animal experiment/ (3,692,962)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,355,627)
-
or/32-33 (6,355,627)
-
exp human/ or human experiment/ (19,263,219)
-
34 not (34 and 35) (4,905,535)
-
31 not 36 (163,378)
-
letter.pt. (1,054,787)
-
editorial.pt. (594,151)
-
note.pt. (740,957)
-
or/38-40 (2,389,895)
-
37 not 41 (158,841)
-
exp thrombocytopenia/ (157,171)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot. (87,986)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot. (1015)
-
(jacobsen adj3 syndrome$).ti,ab,ot. (187)
-
paris trousseau.ti,ab,ot. (49)
-
kasabach merritt.ti,ab,ot. (793)
-
(hemangioma or haemangioma).ti,ab,ot. (18,275)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot. (5177)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot. (7454)
-
gasser$.ti,ab,ot. (1885)
-
(HELLP adj2 syndrome$).ti,ab,ot. (3305)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot. (11)
-
May Hegglin.ti,ab,ot. (262)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot. (2048)
-
moschcowitz.ti,ab,ot. (93)
-
werlhof.ti,ab,ot. (55)
-
(wiskott and aldrich).ti,ab,ot. (2815)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot. (71)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot. (33,439)
-
or/43-61 (221,567)
-
42 and 62 (863).
Health-related quality-of-life free-text terms based on figure 4 in Common Free-text Terms for Electronic Database Searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values From the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011).
Health Technology Assessment database (www.crd.york.ac.uk/CRDWeb/) up to 31 March 2018; NHS Economic Evaluation Database up to 31 March 2015
Date searched: 24 January 2019.
Search strategy
-
MeSH DESCRIPTOR Thrombocytopenia EXPLODE ALL TREES 107
-
(thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) 369
-
(11q or 11q23) 0
-
(jacobsen near3 syndrome*) 0
-
(paris trousseau) 0
-
(kasabach merritt) 1
-
(hemangioma or haemangioma) 34
-
(thrombotic near2 (microangiopath* or micro angiopath*)) 0
-
(hemolytic uremic or haemolytic uremic) 14
-
(gasser*) 4
-
MeSH DESCRIPTOR HELLP Syndrome EXPLODE ALL TREES 5
-
(HELLP near2 syndrome*) 11
-
((hemolysis or haemolysis) near2 liver near2 platelet*) 2
-
(May Hegglin) 0
-
((haemolytic or hemolytic) near (anaemi* or anemi*)) 18
-
(microangiopath* near thrombotic) 0
-
(moschcowitz or werlhof) 0
-
MeSH DESCRIPTOR Wiskott-Aldrich Syndrome EXPLODE ALL TREES 0
-
(wiskott and Aldrich) 1
-
(immunodeficiency 2 or immunodeficiency2 or Imd2) 1
-
((platelet* or thrombocyte*) near3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) 24.
Health Technology Assessment database 70.
NHS Economic Evaluation Databases 110.
Science Citation Index Expanded (Web of Science)
Date range searched: 1988 to 23 January 2019.
Date searched: 24 January 2019.
Search strategy
| # 34 | 422 | #15 and #33 |
| # 33 | 149,819 | #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 |
| # 32 | 206 | TS=(CLDQ or “Chronic Liver Disease Questionnaire*” or LDSI or “Liver Disease Symptom Index*” or LDQOL or “Liver Disease Quality of Life Questionnaire*” or “EORTC QLQ-HCC18” or “EORTC QLQ-LMC21” or PLD-Q or “Polycystic Liver Disease Questionnaire*”) |
| # 31 | 46,426 | TI=(utilit*) or TS=(disutili*) |
| # 30 | 15,981 | TS=(utilit* NEAR/3 (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*)) |
| # 29 | 431 | TS=(HSUV* or “health state* value*” or “health state* preference*” or HSPV*) |
| # 28 | 11,538 | TS=(timetradeoff or “time tradeoff” or “time trade-off” or “time trade off” or TTO or “Standard gamble*” or “willingness to pay”) |
| # 27 | 12,299 | TS=(QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL*) |
| # 26 | 2703 | TS=(“Disability adjusted life” or “Disability-adjusted life” or “health adjusted life” or “health-adjusted life” or “years of healthy life” or “healthy years equivalent” or “years of potential life lost” or “years of health life lost”) |
| # 25 | 846 | TS=(“quality time” or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or “index of well being”) |
| # 24 | 16,492 | TS=(hql or hrql or hqol or “h qol” or hrqol or “hr qol” or hye or hyes or “health* year* equivalent*”) |
| # 23 | 10,202 | TS=((“assessment of quality of life”) or euroqol or “euro qol” or eq5d or “eq 5d”) |
| # 22 | 47,488 | TS=(“health related quality of life” or “Quality adjusted life” or “Quality-adjusted-life”) |
| # 21 | 443 | TS=(sf8 or “sf 8” or sf-8 or “short form 8” or “shortform 8” or “sf eight” or sfeight or “shortform eight” or “short form eight”) |
| # 20 | 255 | TS=(sf20 or “sf 20” or sf-20 or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty”) |
| # 19 | 886 | TS=(sf6D or “sf 6D” or sf-6D or “short form 6D” or “shortform 6D” or “sf six D” or sfsixD or “shortform six D” or “short form six D”) |
| # 18 | 4401 | TS=(sf12 or “sf 12” or “sf-12” or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve”) |
| # 17 | 9091 | TS=(sf6 or “sf 6” or sf-6 or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six”) |
| # 16 | 23,500 | TS=(sf36 or “sf 36 “ or sf-36 or “short form 36 “ or “shortform 36 “ or “sf thirtysix “ or “sf thirty six “ or “shortform thirtysix “ or “shortform thirty six “ or “short form thirty six “ or “short form thirtysix “ or “short form thirty six”) |
| # 15 | 98,158 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 |
| # 14 | 20,790 | TS=((platelet* or thrombocyte*) NEAR/3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) |
| # 13 | 3306 | TS=(werlhof) or TS=(wiskott and aldrich) or TS=(“immunodeficiency 2” or immunodeficiency2 or Imd2) |
| # 12 | 48 | TS=(moschcowitz) |
| # 11 | 870 | TS=((haemolytic or hemolytic) NEAR/2 (anaemi* or anemi*) NEAR/2 (microangiopathic or “micro angiopathic”)) |
| # 10 | 170 | TS=(“May Hegglin”) |
| # 9 | 272 | TS=((hemolysis or haemolysis) NEAR/2 liver NEAR/2 platelet*) |
| # 8 | 3797 | TS=(gasser*) or TS=(HELLP NEAR/2 syndrome*) |
| # 7 | 10,671 | TS=(“hemolytic uremic” or “haemolytic uremic”) |
| # 6 | 3876 | TS=(thrombotic NEAR/2 (microangiopath* or “micro angiopath*”)) |
| # 5 | 11,949 | TS=(hemangioma or haemangioma) |
| # 4 | 703 | TS=(“kasabach merritt”) |
| # 3 | 189 | TS=(jacobsen NEAR/3 syndrome*) OR TS=(“paris trousseau” NEAR/3 syndrome*) |
| # 2 | 643 | TS=((11q or 11q23) NEAR/3 (disorder* or syndrome* or delet* or jacobsen)) |
| # 1 | 53,278 | TS=(thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) |
Health-related quality-of-life free-text terms based on figure 4 in Common Free-text Terms for Electronic Database Searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values From the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011).
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1982 to 23 January 2019.
Date searched: 24 January 2019.
Search strategy
| S1 | (MH “Thrombocytopenia+”) | 5320 |
| S2 | TI (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) OR AB (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) | 7424 |
| S3 | TI ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) OR AB ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) | 33 |
| S4 | TI (jacobsen N3 syndrome*) OR AB (jacobsen N3 syndrome*) | 8 |
| S5 | TI (“paris trousseau” or “kasabach merritt” or “May Hegglin”) OR AB (“paris trousseau” or “kasabach merritt” or “May Hegglin”) | 101 |
| S6 | TI (hemangioma or haemangioma) OR AB (hemangioma or haemangioma) | 2028 |
| S7 | TI (thrombotic N2 (microangiopath* or “micro angiopath*”)) or AB (thrombotic N2 (microangiopath* or “micro angiopath*”)) | 536 |
| S8 | TI (“hemolytic uremic” or “haemolytic uremic” or gasser*) or AB (“hemolytic uremic” or “haemolytic uremic” or gasser*) | 824 |
| S9 | (MH “HELLP Syndrome”) | 476 |
| S10 | TI (HELLP N2 syndrome*) or AB (HELLP N2 syndrome*) | 438 |
| S11 | TI ((hemolysis or haemolysis) N2 liver N2 platelet*) or AB ((hemolysis or haemolysis) N2 liver N2 platelet*) | 78 |
| S12 | TI ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) or AB ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) | 159 |
| S13 | TI ((microangiopath* or micro angiopath*) N2 thrombotic) or AB ((microangiopath* or micro angiopath*) N2 thrombotic) | 536 |
| S14 | TI (moschcowitz or werlhof or (wiskott and Aldrich)) or AB (moschcowitz or werlhof or (wiskott and Aldrich)) | 93 |
| S15 | (MH “Wiskott-Aldrich Syndrome”) | 52 |
| S16 | TI (“immunodeficiency 2” or immunodeficiency2 or Imd2) or AB (“immunodeficiency 2” or immunodeficiency2 or Imd2) | 1 |
| S17 | TI ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) or AB ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) | 2419 |
| S18 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 14,324 |
| S19 | (MH “Quality-Adjusted Life Years”) OR (MH “Quality of Life+”) | 100,220 |
| S20 | TI (sf36 or “sf 36” or sf-36 or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”) or AB (sf36 or “sf 36” or sf-36 or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six”) | 8163 |
| S21 | TI (“health related quality of life” or “Quality adjusted life” or “Quality-adjusted-life” or “assessment of quality of life”) or AB (“health related quality of life” or “Quality adjusted life” or “Quality-adjusted-life” or “assessment of quality of life”) | 21,631 |
| S22 | TI (euroqol or “euro qol” or eq5d or “eq 5d” or hql or hrql or hqol or “h qol” or hrqol or “hr qol” or hye or hyes or “health* year* equivalent*”) or AB (euroqol or “euro qol” or eq5d or “eq 5d” or hql or hrql or hqol or “h qol” or hrqol or “hr qol” or hye or hyes or “health* year* equivalent*”) | 8536 |
| S23 | TI (“quality time” or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or “index of well being”) or AB (“quality time” or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or “index of well being”) | 373 |
| S24 | TI (“Disability adjusted life” or “Disability-adjusted life” or “health adjusted life or health-adjusted life” or “years of healthy life” or “healthy years equivalent” or “years of potential life lost” or “years of health life lost” or QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL*) or AB (“Disability adjusted life” or “Disability-adjusted life” or “health adjusted life or health-adjusted life” or “years of healthy life” or “healthy years equivalent” or “years of potential life lost” or “years of health life lost” or QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL*) | 4707 |
| S25 | TI (timetradeoff or “time tradeoff” or “time trade-off” or “time trade off” or TTO or “Standard gamble*” or “willingness to pay” or HSUV* or “health state* value*” or “health state* preference*” or HSPV*) or AB (timetradeoff or “time tradeoff” or “time trade-off” or “time trade off” or TTO or “Standard gamble*” or “willingness to pay” or HSUV* or “health state* value*” or “health state* preference*” or HSPV*) | 2360 |
| S26 | TI (utilit* N3 (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*)) or AB (utilit* N3 (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*)) | 4802 |
| S27 | TI (utilities or disutili*) or AB (utilities or disutili*) | 30,817 |
| S28 | TI (CLDQ or “Chronic Liver Disease Questionnaire*” or LDSI or “Liver Disease Symptom Index*” or LDQOL or “Liver Disease Quality of Life Questionnaire*” or “EORTC QLQ-HCC18” or “EORTC QLQ-LMC21” or PLD-Q or “Polycystic Liver Disease Questionnaire*”) or AB (CLDQ or “Chronic Liver Disease Questionnaire*” or LDSI or “Liver Disease Symptom Index*” or LDQOL or “Liver Disease Quality of Life Questionnaire*” or “EORTC QLQ-HCC18” or “EORTC QLQ-LMC21” or PLD-Q or “Polycystic Liver Disease Questionnaire*”) | 53 |
| S29 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 | 140,204 |
| S30 | S18 AND S29 | 260 |
Health-related quality-of-life free-text terms based on figure 4 in Common Free-text Terms for Electronic Database Searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values From the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011).
Latin American and Caribbean Health Sciences (http://lilacs.bvsalud.org/en/)
Date range searched: 1982 to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
(MH:c15.378.140.855 OR MH:c15.378.100.100.970 OR thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* OR trombocitopeni* OR ((platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*)) AND (MH:I01.800 OR MH:K01.752.400.750 OR MH:N06.850.505.400.425.837 OR MH:SP4.011.077.593 OR “Quality of Life” OR “Calidad de Vida” OR “Qualidade de Vida” OR MH:E05.318.740.100.500.700 OR MH:N01.224.935.530.700 OR MH:SP5.006.052.168.144 OR “Quality-Adjusted Life” OR “Años de Vida Ajustados por Calidad de Vida” OR “Anos de Vida Ajustados por Qualidade de Vida” OR euroqol OR “euro qo"l OR eq5d OR “eq 5d” OR “Disability adjusted life” OR “health adjusted life” OR QALY* OR DALY* OR timetradeoff OR “time tradeoff” OR “Standard gamble*” OR “willingness to pay” OR utility OR utilities or disutili*)).
Search limited to non-MEDLINE databases:
-
LILACS (444)
-
IBECS (317)
-
BINACIS (36)
-
BBO – Dentistry (30)
-
CUMED (18)
-
MedCarib (14)
-
BDENF – Nursing (1).
Northern Light Life Sciences Conference Abstracts (via Ovid)
Date range searched: 2010–19/week 2.
Date searched: 24 January 2019.
Search strategy
-
exp thrombocytopenia/ (19,173)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,hw. (18,543)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,hw. (132)
-
(jacobsen adj3 syndrome$).ti,ab,hw. (41)
-
(paris trousseau or kasabach merritt or hemangioma or haemangioma).ti,ab,hw. (2487)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,hw. (1515)
-
(hemolytic uremic or haemolytic uremic or gasser$).ti,ab,hw. (643)
-
hellp syndrome/ (410)
-
(HELLP adj2 syndrome$).ti,ab,hw. (415)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,hw. (0)
-
May Hegglin.ti,ab,hw. (10)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,hw. (77)
-
(moschcowitz or werlhof or (wiskott and Aldrich)).ti,ab,hw. (468)
-
wiskott-aldrich syndrome/ (460)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,hw. (0)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,hw. (1916)
-
or/1-16 (24,421)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,hw. (1251)
-
"health related quality of life".ti,ab,hw. (5026)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,hw. (313)
-
"assessment of quality of life".ti,ab,hw. (178)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,hw. (1122)
-
(hql or hrql or hqol or h qol or hrqol or hr qol or hye or hyes).ti,ab,hw. (5101)
-
health$ year$ equivalent$.ti,ab,hw. (0)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,hw. (47)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,hw. (99)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,hw. (1738)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,hw. (829)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,hw. (48)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,hw. (1620)
-
(utilities or disutili$).ti,ab,hw. (647)
-
(CLDQ or Chronic Liver Disease Questionnaire$).ti,ab,hw. (24)
-
(LDSI or Liver Disease Symptom Index$).ti,ab,hw. (2)
-
(LDQOL or Liver Disease Quality of Life Questionnaire$).ti,ab,hw. (1)
-
(EORTC QLQ-HCC18 or EORTC QLQ-LMC21).ti,ab,hw. (0)
-
(PLD-Q or Polycystic Liver Disease Questionnaire$).ti,ab,hw. (2)
-
or/18-36 (13,027)
-
17 and 37 (63).
Health-related quality-of-life free-text terms based on figure 4 in Common Free-text Terms for Electronic Database Searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values From the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011).
Cost-effectiveness Analysis Registry (www.cearegistry.org)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
-
avatrombopag
-
doptelet
-
lusutrombopag
-
mulpleta
-
thrombocytopenia
-
thrombocytopenic
-
thrombocytopaenia
-
thrombocytopaenic.
Records retrieved: 18.
ScHARR Health Utilities Database (www.scharrhud.org/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
| Search terms | Results |
|---|---|
| avatrombopag OR doptelet OR lusutrombopag | 0 |
| mulpleta OR thrombocytopenia OR thrombocytopenic | 0 |
| thrombocytopaenia OR thrombocytopaenic | 0 |
| Total | 0 |
OAIster (http://oaister.worldcat.org)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND (quality of life OR quality-adjusted life OR QALY* OR DALY* OR euroqol OR euro qol OR eq5d OR eq 5d OR health* year* equivalent* OR timetradeoff OR time tradeoff OR utility OR utilities OR disutili*)).
Records retrieved: 73.
OpenGrey (www.opengrey.eu/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND (quality of life OR quality-adjusted life OR QALY* OR DALY* OR euroqol OR euro qol OR eq5d OR eq 5d OR health* year* equivalent* OR timetradeoff OR time tradeoff OR utility OR utilities OR disutili*)).
Records retrieved: 1.
Copac (https://copac.jisc.ac.uk/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
-
keyword: thrombocytopeni* “quality of life”
-
keyword: thrombocytopaeni* “quality of life”
-
keyword: thrombopeni* “quality of life”
-
keyword: thrombopaeni* “quality of life”
-
keyword: thrombocytopeni* “quality adjusted life”
-
keyword: thrombocytopaeni* “quality adjusted life”
-
keyword: thrombopeni* “quality adjusted life”
-
keyword: thrombopaeni* “quality adjusted life”
-
keyword: thrombocytopeni* QALY*
-
keyword: thrombocytopaeni* QALY*
-
keyword: thrombopeni* QALY*
-
keyword: thrombopaeni* QALY*
-
keyword: thrombocytopeni* euroqol
-
keyword: thrombocytopaeni* euroqol
-
keyword: thrombopeni* euroqol
-
keyword: thrombopaeni* euroqol
-
keyword: thrombocytopeni* eq5d
-
keyword: thrombocytopaeni* eq5d
-
keyword: thrombopeni* eq5d
-
keyword: thrombopaeni* eq5d
-
keyword: thrombocytopeni* utilit*
-
keyword: thrombocytopaeni* utilit*
-
keyword: thrombopeni* utilit*
-
keyword: thrombopaeni* utilit*
-
keyword: thrombocytopeni* disutilit*
-
keyword: thrombocytopaeni* disutilit*
-
keyword: thrombopeni* disutilit*
-
keyword: thrombopaeni* disutilit*.
Records retrieved: 104.
Resource use/costs search strategies
| Database/resource | Host | Date range | Results (n) | Date searched |
|---|---|---|---|---|
| MEDLINE | Ovid | 1946 to week 3 January 2019 | 1260 | 24 January 2019 |
| MEDLINE Epub Ahead of Print; MEDLINE In-Process & Other Non-Indexed Citations; MEDLINE Daily Update | Ovid | 23 January 2019 | 159 | 24 January 2019 |
| PubMed | National Library of Medicine | Up to 24 January 2019 | 163 | 24 January 2019 |
| EMBASE | Ovid | 1974 to week 3 2019 | 4838 | 24 January 2019 |
| Science Citation Index Expanded | Web of Science | 1988 to 23 January 2019 | 1197 | 24 January 2019 |
| CINAHL | EBSCOhost | 1982 to 23 January 2019 | 337 | 24 January 2019 |
| Latin American and Caribbean Health Sciences | http://lilacs.bvsalud.org/en/ | 1982 to 24 January 2019 | 458 | 24 January 2019 |
| Northern Light Life Sciences Conference Abstracts | Ovid | 2010–19/week 2 | 226 | 24 January 2019 |
| OAIster | http://oaister.worldcat.org | Up to 23 January 2019 | 34 | 23 January 2019 |
| OpenGrey | www.opengrey.eu/ | Up to 23 January 2019 | 0 | 23 January 2019 |
| Copac | https://copac.jisc.ac.uk/ | Up to 23 January 2019 | 67 | 23 January 2019 |
| ISPOR | www.ispor.org | Up to 23 January 2019 | 70 | 23 January 2019 |
| HTAi | https://htai.org/ | Up to 23 January 2019 | 0 | 23 January 2019 |
| Total records retrieved | 8809 | |||
| Duplicate records removed | 3451 | |||
| Total records to screen | 5358 | |||
MEDLINE (via Ovid): 1946–week 3 January 2019
Date ranges searched:
MEDLINE Epub Ahead of Print (via Ovid) – 22 January 2019.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) – 23 January 2019.
MEDLINE Daily Update (via Ovid) – 22 January 2019.
Date searched: 24 January 2019.
Search strategy
-
exp Employment/ (80,218)
-
exp Work/ (59,092)
-
Efficiency/ (13,088)
-
Absenteeism/ (8634)
-
“Cost of Illness”/ or exp Cost Control/ or Budgets/ or Hospital Costs/ or Health Care Costs/ (102,801)
-
“Length of Stay”/ (79,691)
-
((employment or employed or employee$ or unemployment or unemployed) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,ot. (2131)
-
(productivity adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,ot. (2775)
-
((long standing or longstanding or long term or longterm or permanent or employee$) adj2 (absence$ or absent$ or ill$ or sick$ or disab$)).ti,ab,ot,hw. (9797)
-
llsi.ti,ab,ot. (14)
-
(cost$ adj2 (illness or disease$ or sickness$)).ti,ab,ot. (4481)
-
(burden$ adj2 (disease$ or illness or sickness$)).ti,ab,ot,hw. (22,023)
-
((social or societ$ or work$ or employe$ or business$ or communit$ or famil$ or carer$ or caregiver$) adj3 (burden$ or consequenc$ or impact$ or problem$ or productivity or sickness or impairment$)).ti,ab,ot,hw. (90,909)
-
((allowance or status or long-term or pension$ or benefit$) adj2 disab$).ti,ab,ot,hw. (11,403)
-
((unable or inability or incapacit$ or incapab$) adj3 work).ti,ab,ot,hw. (1720)
-
budget$ impact$.ti,ab,ot,hw. (1322)
-
budget$ implicat$.ti,ab,ot,hw. (62)
-
(cost$ saving or cost$ savings or cost$ saved).ti,ab,ot. (17,139)
-
(cost$ adj2 contain$).ti,ab,ot. (6659)
-
(cost$ adj2 audit$).ti,ab,ot. (127)
-
resource$ use$.ti,ab,ot,hw. (9087)
-
resource$ utili$.ti,ab,ot,hw. (9019)
-
resource$ usage.ti,ab,ot,hw. (347)
-
(length adj2 stay$).ti,ab,ot,hw. (105,746)
-
(hospital$ adj2 stay$).ti,ab,ot,hw. (79,212)
-
(duration adj2 stay$).ti,ab,ot,hw. (3195)
-
extended stay$.ti,ab,ot,hw. (179)
-
prolonged stay$.ti,ab,ot,hw. (838)
-
((hospitali?ation or hospitali?ed or hospital) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$ or budget$)).ti,ab,ot. (20,300)
-
(economic consequenc$ or cost consequenc$).ti,ab,ot. (3699)
-
or/1-30 (543,481)
-
exp Thrombocytopenia/ (45,457)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot,hw. (69,081)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot,hw. (574)
-
(jacobsen adj3 syndrome$).ti,ab,ot,hw. (129)
-
paris trousseau.ti,ab,ot,hw. (30)
-
kasabach merritt.ti,ab,ot,hw. (704)
-
(hemangioma or haemangioma).ti,ab,ot,hw. (32,339)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot,hw. (3354)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot,hw. (7663)
-
gasser$.ti,ab,ot,hw. (1689)
-
HELLP Syndrome/ (1709)
-
(HELLP adj2 syndrome$).ti,ab,ot,hw. (2561)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot,hw. (7)
-
May Hegglin.ti,ab,ot,hw. (221)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot,hw. (1411)
-
moschcowitz.ti,ab,ot,hw. (107)
-
werlhof.ti,ab,ot,hw. (120)
-
Wiskott-Aldrich Syndrome/ (1428)
-
(wiskott and Aldrich).ti,ab,ot,hw. (3312)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot,hw. (44)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot,hw. (22,231)
-
or/32-52 (132,417)
-
31 and 53 (1429)
-
exp animals/ not humans/ (4,540,224)
-
54 not 55 (1419).
MEDLINE 1260.
MEDLINE Epub Ahead of Print 23.
MEDLINE In-Process & Other Non-Indexed Citations 135.
MEDLINE Daily Update 1.
PubMed (via National Library of Medicine)
Date range searched: up to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
-
#28 #26 AND #27 163
-
#27 pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb] 3,121,488
-
#26 #11 AND #25 2144
-
#25 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) 551,151
-
#24 “length of stay”[tiab] OR “hospital stay”[tiab] OR “hospital cost”[tiab] OR “hospital costs”[tiab] OR “hospital expenditure”[tiab] OR “hospital budget”[tiab] OR “hospital budgets”[tiab] OR “economic consequence”[tiab] OR “economic consequences”[tiab] OR “cost consequence”[tiab] OR “cost consequences”[tiab] 118,299
-
#23 “resource use”[tiab] OR “resource utilise”[tiab] OR “resource utilize”[tiab] OR “resource utility”[tiab] OR “resource usage”[tiab] 7846
-
#22 “cost saving”[tiab] OR “cost savings”[tiab] OR “cost saved”[tiab] OR “costs saved”[tiab] OR “cost contain”[tiab] OR “cost contained”[tiab] OR “cost containment”[tiab] OR “cost audit”[tiab] 22,036
-
#21 “budget impact”[tiab] OR “budget impacts”[tiab] OR “budget implication”[tiab] OR “budget implications”[tiab] 1245
-
#20 (unable[tiab] OR inability[tiab] OR incapacity[tiab] OR incapable[tiab]) AND work[tiab] 9494
-
#19 “disability allowance”[tiab] OR “disability benefit”[tiab] OR “disability benefits”[tiab] 865
-
#18 (social[tiab] OR societ*[tiab] OR work*[tiab] OR community[tiab] OR family[tiab] OR carer*[tiab] OR caregiver*[tiab]) AND burden*[tiab] 55,842
-
#17 “cost of illness”[tiab] OR “cost of disease”[tiab] OR “cost of sickness”[tiab] OR “burden of illness”[tiab] OR “burden of disease”[tiab] OR “burden of sickness”[tiab] 11,376
-
#16 absentee*[tiab] OR “long term illness”[tiab] OR “longterm illness”[tiab] OR “long term sick”[tiab] OR “longterm sick”[tiab] OR “long term sickness”[tiab] OR “longterm sickness”[tiab] OR “long term disabled”[tiab] OR “longterm disabled”[tiab] OR “long term disability”[tiab] OR “longterm disability”[tiab] 9106
-
#15 employment[tiab] OR employee[tiab] OR unemployment[tiab] OR unemployed[tiab] 76,820
-
#14 “Length of Stay”[Mesh] 79,696
-
#13 “Cost of Illness”[Mesh] OR “Cost Control”[Mesh] OR “Budgets”[Mesh] OR “Hospital Costs”[Mesh] OR “Health Care Costs”[Mesh] 116,564
-
#12 “Employment”[Mesh] OR “Work”[Mesh] OR “Efficiency”[Mesh] OR “Absenteeism”[Mesh] 168,671
-
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) 188,201
-
#10 (platelet*[tiab] OR thrombocyte*[tiab]) AND (defici*[tiab] OR reduc*[tiab] OR low[tiab] OR lower[tiab] OR lowest[tiab] OR few[tiab] OR fewer[tiab] OR fewest[tiab] OR decrease[tiab] OR decreases[tiab] OR decreased[tiab] OR defective[tiab] OR destruc*[tiab] OR destroy*[tiab]) 99,513
-
#9 “immunodeficiency 2” OR immunodeficiency2 OR Imd2 46
-
#8 Moschcowitz[tiab] OR werlhof[tiab] OR “Wiskott-Aldrich Syndrome”[Mesh] OR (wiskott[tiab] AND Aldrich[tiab]) 2664
-
#7 (haemolytic[tiab] OR hemolytic[tiab]) AND (anaemi*[tiab] OR anemi*[tiab]) AND (microangiopath*[tiab]) 1765
-
#6 (hemolysis[tiab] OR haemolysis[tiab]) AND liver[tiab] AND platelet*[tiab] 1247
-
#5 “HELLP Syndrome”[Mesh] OR “HELLP syndrome” OR “HELLP syndromes” 2583
-
#4 (thrombotic[tiab] AND microangiopath*[tiab]) OR “hemolytic uremic” OR “haemolytic uremic” OR gasser*[tiab] 12,074
-
#3 “jacobsen syndrome” OR “paris trousseau” OR “kasabach merritt” OR “May Hegglin” OR hemangioma[tiab] OR haemangioma[tiab] 17,717
-
#2 (11q[tiab] OR 11q23[tiab]) AND (disorder*[tiab] OR syndrome*[tiab] OR delet*[tiab] OR Jacobsen[tiab]) 1605
-
#1 (“Thrombocytopenia”[Mesh] OR thrombocytopeni*[tiab] OR thrombocytopaeni*[tiab] OR thrombopeni*[tiab] OR thrombopaeni*[tiab] OR macrothrombocytopeni*[tiab] OR macrothrombocytopaeni*[tiab]) 73,938.
EMBASE (via Ovid)
Date range searched: 1974 to week 3 2019.
Date searched: 24 January 2019.
Search strategy
-
exp employment/ (82,835)
-
exp work/ (322,925)
-
“cost of illness”/ or cost control/ or hospital cost/ or budget/ or health care cost/ (271,582)
-
“length of stay”/ (159,635)
-
((employment or employed or employee$ or unemployment or unemployed) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,ot. (2669)
-
(productivity adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,ot. (3897)
-
((long standing or longstanding or long term or longterm or permanent or employee$) adj2 (absence$ or absent$ or ill$ or sick$ or disab$)).ti,ab,ot. (13,272)
-
llsi.ti,ab,ot. (16)
-
(cost$ adj2 (illness or disease$ or sickness$)).ti,ab,ot. (6727)
-
(burden$ adj2 (disease$ or illness or sickness$)).ti,ab,ot. (33,235)
-
((social or societ$ or work$ or employe$ or business$ or communit$ or famil$ or carer$ or caregiver$) adj3 (burden$ or consequenc$ or impact$ or problem$ or productivity or sickness or impairment$)).ti,ab,ot. (111,968)
-
((allowance or status or long-term or pension$ or benefit$) adj2 disab$).ti,ab,ot. (17,909)
-
((unable or inability or incapacit$ or incapab$) adj3 work).ti,ab,ot. (2444)
-
budget$ impact$.ti,ab,ot. (3571)
-
budget$ implicat$.ti,ab,ot. (87)
-
(cost$ saving or cost$ savings or cost$ saved).ti,ab,ot. (28,279)
-
(cost$ adj2 contain$).ti,ab,ot. (8302)
-
(cost$ adj2 audit$).ti,ab,ot. (208)
-
resource$ use$.ti,ab,ot. (13,699)
-
resource$ utili$.ti,ab,ot. (16,372)
-
resource$ usage.ti,ab,ot. (500)
-
(length adj2 stay$).ti,ab,ot. (89,167)
-
(hospital$ adj2 stay$).ti,ab,ot. (129,616)
-
(duration adj2 stay$).ti,ab,ot. (4967)
-
extended stay$.ti,ab,ot. (269)
-
prolonged stay$.ti,ab,ot. (1306)
-
((hospitali?ation or hospitali?ed or hospital) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$ or budget$)).ti,ab,ot. (31,590)
-
(economic consequenc$ or cost consequenc$).ti,ab,ot. (4997)
-
or/1-28 (1,048,603)
-
exp thrombocytopenia/ (157,171)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,ot. (87,986)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,ot. (1015)
-
(jacobsen adj3 syndrome$).ti,ab,ot. (187)
-
paris trousseau.ti,ab,ot. (49)
-
kasabach merritt.ti,ab,ot. (793)
-
(hemangioma or haemangioma).ti,ab,ot. (18,275)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,ot. (5177)
-
(hemolytic uremic or haemolytic uremic).ti,ab,ot. (7454)
-
gasser$.ti,ab,ot. (1885)
-
(HELLP adj2 syndrome$).ti,ab,ot. (3305)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,ot. (11)
-
May Hegglin.ti,ab,ot. (262)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,ot. (2048)
-
moschcowitz.ti,ab,ot. (93)
-
werlhof.ti,ab,ot. (55)
-
(wiskott and aldrich).ti,ab,ot. (2815)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,ot. (71)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,ot. (33,439)
-
or/30-48 (221,567)
-
animal/ or animal experiment/ (3,692,962)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot. (4,424,329)
-
50 or 51 (5,722,776)
-
exp human/ or human experiment/ (19,263,219)
-
52 not (52 and 53) (4,428,740)
-
29 and 49 (4872)
-
55 not 54 (4838).
Science Citation Index Expanded (via Web of Science)
Date range searched: 1988–23 January 2019.
Date searched: 24 January 2019.
Search strategy
| # 32 | 1197 | #15 AND #31 |
| # 31 | 317,316 | #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 |
| # 30 | 4262 | TS=(“economic consequenc*” or “cost consequenc*”) |
| # 29 | 19,538 | TS=((hospitalisation or hospitalization or hospitalised or hospitalized or hospital) NEAR/3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure* or budget*)) |
| # 28 | 98,595 | TS=((length NEAR/2 stay*) or (hospital* NEAR/2 stay*) or (duration NEAR/2 stay*) or “extended stay*” or “prolonged stay*”) |
| # 27 | 30,484 | TS=(“resource* use*” or “resource* utili*” or “resource* usage”) |
| # 26 | 4197 | TS=((cost* NEAR/2 contain*) or (cost* NEAR/2 audit*)) |
| # 25 | 19,854 | TS=(“cost* saving” or “cost* savings” or “cost* saved”) |
| # 24 | 2054 | TS=(“budget* impact*” OR “budget* implicat*”) |
| # 23 | 1173 | TS=((unable or inability or incapacit* or incapab*) NEAR/3 work) |
| # 22 | 10,217 | TS=((allowance or status or long-term or pension* or benefit*) NEAR/2 disab*) |
| # 21 | 106,170 | TS=((social or societ* or work* or employe* or business* or communit* or famil* or carer* or caregiver*) NEAR/3 (burden* or consequenc* or impact* or problem* or productivity or sickness or impairment*)) |
| # 20 | 25,333 | TS=(burden* NEAR/2 (disease* or illness or sickness*)) |
| # 19 | 6982 | TS=(cost* NEAR/2 (illness or disease* or sickness*)) |
| # 18 | 8744 | TS=((“long standing” or longstanding or “long term” or longterm or permanent or employee*) NEAR/2 (absence* or absent* or ill* or sick* or disab*)) |
| # 17 | 5598 | TS=(productivity NEAR/3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) |
| # 16 | 4719 | TS=((employment or employed or employee* or unemployment or unemployed) NEAR/3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) |
| # 15 | 98,158 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 |
| # 14 | 20,790 | TS=((platelet* or thrombocyte*) NEAR/3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) |
| # 13 | 3306 | TS=(werlhof) or TS=(wiskott and aldrich) or TS=(“immunodeficiency 2” or immunodeficiency2 or Imd2) |
| # 12 | 48 | TS=(moschcowitz) |
| # 11 | 870 | TS=((haemolytic or hemolytic) NEAR/2 (anaemi* or anemi*) NEAR/2 (microangiopathic or “micro angiopathic”)) |
| # 10 | 170 | TS=(“May Hegglin”) |
| # 9 | 272 | TS=((hemolysis or haemolysis) NEAR/2 liver NEAR/2 platelet*) |
| # 8 | 3797 | TS=(gasser*) or TS=(HELLP NEAR/2 syndrome*) |
| # 7 | 10,671 | TS=(“hemolytic uremic” or “haemolytic uremic”) |
| # 6 | 3876 | TS=(thrombotic NEAR/2 (microangiopath* or “micro angiopath*”)) |
| # 5 | 11,949 | TS=(hemangioma or haemangioma) |
| # 4 | 703 | TS=(“kasabach merritt”) |
| # 3 | 189 | TS=(jacobsen NEAR/3 syndrome*) OR TS=(“paris trousseau” NEAR/3 syndrome*) |
| # 2 | 643 | TS=((11q or 11q23) NEAR/3 (disorder* or syndrome* or delet* or jacobsen)) |
| # 1 | 53,278 | TS=(thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) |
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1982–23 January 2019.
Date searched: 24 January 2019.
Search strategy
| S1 | (MH “Thrombocytopenia+”) | 5320 |
| S2 | TI (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) OR AB (thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*) | 7424 |
| S3 | TI ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) OR AB ((11q or 11q23) N3 (disorder* or syndrome* or delet* or jacobsen)) | 33 |
| S4 | TI (jacobsen N3 syndrome*) OR AB (jacobsen N3 syndrome*) | 8 |
| S5 | TI (“paris trousseau” or “kasabach merritt” or “May Hegglin”) OR AB (“paris trousseau” or “kasabach merritt” or “May Hegglin”) | 101 |
| S6 | TI (hemangioma or haemangioma) OR AB (hemangioma or haemangioma) | 2028 |
| S7 | TI (thrombotic N2 (microangiopath* or “micro angiopath*”)) or AB (thrombotic N2 (microangiopath* or “micro angiopath*”)) | 536 |
| S8 | TI (“hemolytic uremic” or “haemolytic uremic” or gasser*) or AB (“hemolytic uremic” or “haemolytic uremic” or gasser*) | 824 |
| S9 | (MH “HELLP Syndrome”) | 476 |
| S10 | TI (HELLP N2 syndrome*) or AB (HELLP N2 syndrome*) | 438 |
| S11 | TI ((hemolysis or haemolysis) N2 liver N2 platelet*) or AB ((hemolysis or haemolysis) N2 liver N2 platelet*) | 78 |
| S12 | TI ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) or AB ((haemolytic or hemolytic) N2 (anaemi* or anemi*) N2 (microangiopathic or micro angiopathic)) | 159 |
| S13 | TI ((microangiopath* or micro angiopath*) N2 thrombotic) or AB ((microangiopath* or micro angiopath*) N2 thrombotic) | 536 |
| S14 | TI (moschcowitz or werlhof or (wiskott and Aldrich)) or AB (moschcowitz or werlhof or (wiskott and Aldrich)) | 93 |
| S15 | (MH “Wiskott-Aldrich Syndrome”) | 52 |
| S16 | TI (“immunodeficiency 2” or immunodeficiency2 or Imd2) or AB (“immunodeficiency 2” or immunodeficiency2 or Imd2) | 1 |
| S17 | TI ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) or AB ((platelet* or thrombocyte*) N3 (defici* or reduc* or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc* or destroy*)) | 2419 |
| S18 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 14,324 |
| S19 | (MH “Employment+”) | 41,279 |
| S20 | (MH “Work+”) | 5848 |
| S21 | (MH “Absenteeism”) | 4010 |
| S22 | (MH “Health Care Costs+”) | 48,268 |
| S23 | (MH “Caregiver Burden”) | 8374 |
| S24 | (MH “Health Facility Costs”) | 3920 |
| S25 | (MH “Budgets”) | 8929 |
| S26 | (MH “Cost Control+”) | 19,262 |
| S27 | (MH “Length of Stay”) | 34,378 |
| S28 | TI ((employment or employed or employee* or unemployment or unemployed) N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) or AB ((employment or employed or employee* or unemployment or unemployed) N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) | 1289 |
| S29 | TI (productivity N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) or AB (productivity N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure*)) | 1193 |
| S30 | TI ((long standing or longstanding or long term or longterm or permanent or employee*) N2 (absence* or absent* or ill* or sick* or disab*)) or AB ((long standing or longstanding or long term or longterm or permanent or employee*) N2 (absence* or absent* or ill* or sick* or disab*)) | 4533 |
| S31 | TI (cost* N2 (illness or disease* or sickness*)) or AB (cost* N2 (illness or disease* or sickness*)) | 2269 |
| S32 | TI (burden* N2 (disease* or illness or sickness*)) or AB (burden* N2 (disease* or illness or sickness*)) | 9253 |
| S33 | TI ((social or societ* or work* or employe* or business* or communit* or famil* or carer* or caregiver*) N3 (burden* or consequenc* or impact* or problem* or productivity or sickness or impairment*)) or AB ((social or societ* or work* or employe* or business* or communit* or famil* or carer* or caregiver*) N3 (burden* or consequenc* or impact* or problem* or productivity or sickness or impairment*)) | 43,091 |
| S34 | TI ((allowance or status or long-term or pension* or benefit*) N2 disab*) or AB ((allowance or status or long-term or pension* or benefit*) N2 disab*) | 4849 |
| S35 | TI ((unable or inability or incapacit* or incapab*) N3 work) or AB ((unable or inability or incapacit* or incapab*) N3 work) | 534 |
| S36 | TI (“budget* impact*” OR “budget* implicat*”) or AB (“budget* impact*” OR “budget* implicat*”) | 650 |
| S37 | TI (“cost* saving” or “cost* savings” or “cost* saved”) or AB (“cost* saving” or “cost* savings” or “cost* saved”) | 6473 |
| S38 | TI ((cost* N2 contain*) or (cost* N2 audit*)) or AB ((cost* N2 contain*) or (cost* N2 audit*)) | 2241 |
| S39 | TI (“resource* use*” or “resource* utili*” or “resource* usage”) or AB (“resource* use*” or “resource* utili*” or “resource* usage”) | 6674 |
| S40 | TI ((length N2 stay*) or (hospital* N2 stay*) or (duration N2 stay*) or “extended stay*” or “prolonged stay*”) or AB ((length N2 stay*) or (hospital* N2 stay*) or (duration N2 stay*) or “extended stay*” or “prolonged stay*”) | 38,550 |
| S41 | TI ((hospitalisation or hospitalization or hospitalised or hospitalized or hospital) N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure* or budget*)) or AB ((hospitalisation or hospitalization or hospitalised or hospitalized or hospital) N3 (economic* or cost or costs or costly or costing or price or prices or pricing or expenditure* or budget*)) | 8953 |
| S42 | TI (“economic consequenc*” or “cost consequenc*”) or AB (“economic consequenc*” or “cost consequenc*”) | 1030 |
| S43 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 | 243,749 |
| S44 | S18 AND S43 | 337 |
Latin American and Caribbean Health Sciences
Date range searched: 1982 to 24 January 2019.
Date searched: 24 January 2019.
Search strategy
((MH:c15.378.140.855 OR MH:c15.378.100.100.970 OR thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni* OR macrothrombocytopeni* OR macrothrombocytopaeni* OR trombocitopeni* OR ((platelet* OR thrombocyte*) AND (defici* OR reduc* OR low OR lower OR lowest OR few OR fewer OR fewest OR decrease OR decreases OR decreased OR defective OR destruc* OR destroy*))) AND (MH:N03.219.151.165 OR MH:N03.219.151.400 OR MH: N01.824.245 OR MH:F02.784.692.107 OR MH:I03.946 OR MH:E02.760.400.480 OR “cost of illness” OR “burden of illness” OR “cost saving” OR “cost savings” OR “cost saved” OR “budget impact” OR “resource use” OR “resource utilisation” OR “resource utilization” OR “resource utility” OR “resource usage” OR “costo de enfermedad” OR “efeitos psicossociais da doença” OR “length of stay” OR “hospital stay” OR “tiempo de internación” OR “tempo de internação” OR “health care cost” OR “health care costs” OR “costos de la atención en salud” OR “custos de cuidados de saúde” OR “hospital cost” OR “hospital costs” OR “hospital expenditure” OR “hospital expenditures” OR “economic consequence” OR “economic consequences” OR “cost consequence” OR “cost consequences” OR employment OR employed OR employee* OR unemployment OR unemployed OR empleo OR emprego OR work OR trabajo OR trabalho OR absenteeism OR absentismo OR absenteísmo OR carer* OR caregiver*)).
Search limited to non-MEDLINE databases:
-
LILACS (301)
-
IBECS (106)
-
BINACIS (25)
-
BBO – Dentistry (22)
-
CUMED (17)
-
MedCarib (3)
-
BDENF – Nursing (2)
-
BRISA/RedTESA (2)
-
Coleciona SUS (2).
Northern Light Life Sciences Conference Abstracts (via Ovid)
Date range searched: 2010–19/week 2.
Date searched: 24 January 2019.
Search strategy
-
exp thrombocytopenia/ (19,173)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab,hw. (18,543)
-
((11q or 11q23) adj3 (disorder$ or syndrome$ or delet$ or jacobsen)).ti,ab,hw. (132)
-
(jacobsen adj3 syndrome$).ti,ab,hw. (41)
-
(paris trousseau or kasabach merritt or hemangioma or haemangioma).ti,ab,hw. (2487)
-
(thrombotic adj2 (microangiopath$ or micro angiopath$)).ti,ab,hw. (1515)
-
(hemolytic uremic or haemolytic uremic or gasser$).ti,ab,hw. (643)
-
hellp syndrome/ (410)
-
(HELLP adj2 syndrome$).ti,ab,hw. (415)
-
((hemolysis or haemolysis) adj2 liver adj2 platelet$).ti,ab,hw. (0)
-
May Hegglin.ti,ab,hw. (10)
-
((haemolytic or hemolytic) adj2 (anaemi$ or anemi$) adj2 (microangiopathic or micro angiopathic)).ti,ab,hw. (77)
-
(moschcowitz or werlhof or (wiskott and Aldrich)).ti,ab,hw. (468)
-
wiskott-aldrich syndrome/ (460)
-
(immunodeficiency 2 or immunodeficiency2 or Imd2).ti,ab,hw. (0)
-
((platelet$ or thrombocyte$) adj3 (defici$ or reduc$ or low or lower or lowest or few or fewer or fewest or decrease or decreases or decreased or defective or destruc$ or destroy$)).ti,ab,hw. (1916)
-
or/1-16 (24,421)
-
((employment or employed or employee$ or unemployment or unemployed) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,hw. (121)
-
(productivity adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$)).ti,ab,hw. (248)
-
((long standing or longstanding or long term or longterm or permanent or employee$) adj2 (absence$ or absent$ or ill$ or sick$ or disab$)).ti,ab,hw. (623)
-
(cost$ adj2 (illness or disease$ or sickness$)).ti,ab,hw. (592)
-
(burden$ adj2 (disease$ or illness or sickness$)).ti,ab,hw. (3836)
-
((social or societ$ or work$ or employe$ or business$ or communit$ or famil$ or carer$ or caregiver$) adj3 (burden$ or consequenc$ or impact$ or problem$ or productivity or sickness or impairment$)).ti,ab,hw. (7569)
-
((allowance or status or long-term or pension$ or benefit$) adj2 disab$).ti,ab,hw. (802)
-
((unable or inability or incapacit$ or incapab$) adj3 work).ti,ab,hw. (59)
-
(budget$ impact$ or budget$ implicat$).ti,ab,hw. (1171)
-
(cost$ saving or cost$ savings or cost$ saved or (cost$ adj2 contain$) or (cost$ adj2 audit$)).ti,ab,hw. (4768)
-
(resource$ use$ or resource$ utili$ or resource$ usage).ti,ab,hw. (4055)
-
((length or hospital$ or duration) adj2 stay$).ti,ab,hw. (11,980)
-
(extended stay$ or prolonged stay$).ti,ab,hw. (94)
-
((hospitali?ation or hospitali?ed or hospital) adj3 (economic$ or cost or costs or costly or costing or price or prices or pricing or expenditure$ or budget$)).ti,ab,hw. (2579)
-
(economic consequenc$ or cost consequenc$).ti,ab,hw. (318)
-
or/18-32 (35,882)
-
17 and 33 (226).
OAIster (http://oaister.worldcat.org)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND (cost of illness OR burden of illness OR cost saving* OR resource use OR resource usage OR length of stay OR hospital stay OR health care cost OR health care costs OR hospital cost* OR economic consequence* OR cost consequence* OR employment OR employed OR employee* OR unemployment OR unemployed OR absenteeism OR carer* OR caregiver*)).
Records retrieved: 34.
OpenGrey (www.opengrey.eu/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
((thrombocytopeni* OR thrombocytopaeni* OR thrombopeni* OR thrombopaeni*) AND (cost of illness OR burden of illness OR cost saving* OR resource use OR resource usage OR length of stay OR hospital stay OR health care cost OR health care costs OR hospital cost* OR economic consequence* OR cost consequence* OR employment OR employed OR employee* OR unemployment OR unemployed OR absenteeism OR carer* OR caregiver*)).
Records retrieved: 0.
Copac (https://copac.jisc.ac.uk/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
-
keyword: thrombocytopeni* “cost of illness”
-
keyword: thrombocytopaeni* “cost of illness”
-
keyword: thrombopeni* “cost of illness”
-
keyword: thrombopaeni* “cost of illness”
-
keyword: thrombocytopeni* “burden of illness”
-
keyword: thrombocytopaeni* “burden of illness”
-
keyword: thrombopeni* “burden of illness”
-
keyword: thrombopaeni* “burden of illness”
-
keyword: thrombocytopeni* “resource use”
-
keyword: thrombocytopaeni* “resource use”
-
keyword: thrombopeni* “resource use”
-
keyword: thrombopaeni* “resource use”
-
keyword: thrombocytopeni*; Title words: cost
-
keyword: thrombocytopeni*; Title words: costs
-
keyword: thrombocytopaeni*; Title words: cost
-
keyword: thrombocytopaeni*; Title words: costs
-
keyword: thrombopeni*; Title words: cost
-
keyword: thrombopeni*; Title words: costs
-
keyword: thrombopaeni*; Title words: cost
-
keyword: thrombopaeni*; Title words: costs
-
keyword: thrombocytopeni*; Title words: economic
-
keyword: thrombocytopeni*; Title words: economics
-
keyword: thrombocytopaeni*; Title words: economic
-
keyword: thrombocytopaeni*; Title words: economics
-
keyword: thrombopeni*; Title words: economic
-
keyword: thrombopeni*; Title words: economics
-
keyword: thrombopaeni*; Title words: economic
-
keyword: thrombopaeni*; Title words: economics
-
keyword: thrombocytopeni* “length of stay”
-
keyword: thrombocytopaeni* “length of stay”
-
keyword: thrombopeni* “length of stay”
-
keyword: thrombopaeni* “length of stay”
-
keyword: thrombocytopeni* “hospital stay”
-
keyword: thrombocytopaeni* “hospital stay”
-
keyword: thrombopeni* “hospital stay”
-
keyword: thrombopaeni* “hospital stay”
-
keyword: thrombocytopeni* “hospital cost”
-
keyword: thrombocytopaeni* “hospital cost”
-
keyword: thrombopeni* “hospital cost”
-
keyword: thrombopaeni* “hospital cost”
-
keyword: thrombocytopeni* “hospital costs”
-
keyword: thrombocytopaeni* “hospital costs”
-
keyword: thrombopeni* “hospital costs”
-
keyword: thrombopaeni* “hospital costs”
-
keyword: thrombocytopeni* carer*
-
keyword: thrombocytopaeni* carer*
-
keyword: thrombopeni* carer*
-
keyword: thrombopaeni* carer*
-
keyword: thrombocytopeni* caregiver*
-
keyword: thrombocytopaeni* caregiver*
-
keyword: thrombopeni* caregiver*
-
keyword: thrombopaeni* caregiver*.
Records retrieved: 67.
ISPOR (www.ispor.org/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
| General website search | Results |
|---|---|
| avatrombopag OR doptelet | 0 |
| lusutrombopag OR mulpleta | 0 |
| thrombocytopenia OR thrombocytopenic OR thrombocytopaenia OR thrombocytopaenic OR thrombopenia OR thrombopenic OR thrombopaenia OR thrombopaenic | 27 |
| Total | 27 |
| Scientific Presentations Database search; keyword search | |
| avatrombopag | 0 |
| doptelet | – |
| lusutrombopag | 0 |
| mulpleta | – |
| Titles: thrombocytopenia | 44 |
| Titles: thrombocytopenic | 22 |
| Titles: thrombocytopaenia | 0 |
| Titles: thrombocytopaenic | 0 |
| Titles: thrombopenia | 0 |
| Titles: thrombopenic | 0 |
| Titles: thrombopaenia | 0 |
| Titles: thrombopaenic | 0 |
| Total | 66 |
| Overall total | 93 |
| Total after removal of duplicate records | 70 |
HTAi (https://htai.org/)
Date range searched: up to 23 January 2019.
Date searched: 23 January 2019.
Search strategy
-
avatrombopag
-
doptelet
-
lusutrombopag
-
mulpleta
-
thrombocytopenia
-
thrombocytopenic
-
thrombocytopaenia
-
thrombocytopaenic
-
thrombopenia
-
thrombopenic
-
thrombopaenia
-
thrombopaenic.
Records retrieved: 0.
Economic model: search strategies
Supplementary literature searches were conducted to identify data to help populate the economic model. The search strategies were developed pragmatically, using a targeted rather than an extensive approach. Limits included focused subject headings, restricted proximity, precise free-text terms, fewer databases and date limits.
PubMed search for National Institute for Health Research Health Technology Assessment reports with similar economic models
PubMed (via National Library of Medicine): up to 11 April 2019.
Date searched: 11 April 2019.
Search strategy
-
#16 Search (#14 AND #15) 42
-
#15 Search “Health Technol Assess"[jour] 1233
-
#14 Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #12 OR #13) 763,896
-
#13 Search “platelet transfusion"[tiab] OR “thrombocyte transfusion"[tiab] OR “blood transfusion"[tiab] 40,906
-
#12 Search “Platelet Transfusion"[Mesh] 6869
-
#10 Search (liver*[tiab] OR hepatic[tiab] OR intrahepatic[tiab]) AND carcinoma*[tiab] 76,177
-
#9 Search (haemochromatosis[tiab] OR hemochromatosis[tiab] OR “bronze diabetes"[tiab] OR “bronze diabetic"[tiab] OR “recklinghausen applebaum"[tiab] OR siderochromatosis[tiab] OR “primary biliary cholangitis"[tiab] OR hepatocarcinoma[tiab] OR hepatoma*[tiab]) 40,459
-
#8 Search (liver*[tiab] OR hepatic[tiab] OR intrahepatic[tiab]) AND inflam*[tiab] 58,570
-
#7 Search (hepatitis[tiab] OR hepatopath*[tiab]) AND (chronic[tiab] OR acute[tiab] OR persistent[tiab] OR “long standing"[tiab] OR “long term"[tiab] OR recurr*[tiab]) 92,789
-
#6 Search ((fibrosis[tiab] OR fibroses[tiab] OR scar*[tiab]) AND (liver*[tiab] OR hepatic[tiab])) 41,152
-
#5 Search chronic[tiab] AND “destructive cholangitis"[tiab] 118
-
#4 Search cirrhosis[tiab] OR cirrhosis[tiab] OR cirrhotic[tiab] 96,549
-
#3 Search “liver disease"[tiab] OR “liver diseases"[tiab] OR “hepatic disease"[tiab] OR “hepatic diseases"[tiab] OR “intrahepatic disease"[tiab] OR “intrahepatic diseases"[tiab] OR “liver disorder"[tiab] OR “liver disorders"[tiab] OR “hepatic disorder"[tiab] OR “hepatic disorders"[tiab] OR “intrahepatic disorder"[tiab] OR “intrahepatic disorders"[tiab] OR “liver lesion"[tiab] OR “liver lesions"[tiab] OR “hepatic lesion"[tiab] OR “hepatic lesions"[tiab] OR “intrahepatic lesion"[tiab] OR “intrahepatic lesions"[tiab] 110,351
-
#2 Search “Liver Diseases"[Mesh] 525,899
-
#1 Search ((“Thrombocytopenia"[Mesh] OR thrombocytopeni*[tiab] OR thrombocytopaeni*[tiab] OR thrombopeni*[tiab] OR thrombopaeni*[tiab] OR macrothrombocytopeni*[tiab] OR macrothrombocytopaeni*[tiab])) 74,587.
Literature searches to identify rates of procedures with bleeding risk in patients with chronic liver disease
MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily Update
Date range searched: 1946 to 17 May 2019.
Date searched: 20 May 2019.
Search strategy
-
exp *Liver Diseases/ and exp Chronic Disease/ (14,897)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$ or failure$) adj2 (chronic or refractory or unmanageab$ or uncontrol$ or resistant or persist$ or intractable$ or recurren$ or sustained or permanent$ or unremitting or unrelenting or continual$ or continuous$ or constant$ or unending or unceasing)).ti,ab. (23,997)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab. (93,496)
-
((fibrosis or fibroses or scar$) adj2 (liver$ or hepat$ or intrahepat$)).ti,ab. (21,311)
-
or/1-4 (130,417)
-
exp Specialties, Surgical/sn, td [Statistics & Numerical Data, Trends] (13,407)
-
exp Surgical Procedures, Operative/sn, td [Statistics & Numerical Data, Trends] (105,017)
-
exp Liver Diseases/sn [Statistics & Numerical Data] (185)
-
Paracentesis/sn, td or Thoracentesis/ or exp Endoscopy, Gastrointestinal/sn, td or Bronchoscopy/sn, td or Chemoembolization, Therapeutic/sn, td or Portasystemic Shunt, Transjugular Intrahepatic/sn, td or Oral Surgical Procedures/sn, td or Biliary Tract Surgical Procedures/sn, td or Nephrotomy/ or Radiofrequency Ablation/sn, td or Catheter Ablation/sn, td or Laparoscopy/sn, td (8036)
-
((paracentesis or paracenteses) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (303)
-
((thoracentesis or thoracenteses or thoracocentesis or thoracocenteses or pleurocentesis or pleurocenteses) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (232)
-
((endoscop$ or enteroscop$) adj2 (gastrointestinal or balloon$ or push or mucosal or submucosal) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (486)
-
(bronchoscop$ adj2 (gastrointestinal or balloon$ or push or mucosal or submucosal) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (4)
-
((ethanol or alcohol) adj2 (ablation or inject$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (242)
-
(chemoemboli?ati$ adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (261)
-
((vascular or cardiac or cardiovascular or heart or blood vessel$) adj2 (catheteri?ation or catherteri?ed) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (735)
-
((transjugular intrahepatic portosystemic shunt$ or transjugular intrahepatic porto systemic shunt$ or transjugular intrahepatic portacaval shunt$ or transjugular intrahepatic porta systemic shunt$ or transjugular intrahepatic portasystemic shunt$ or transjugular intrahepatic shunt$ or transjugular intrahepatic stent$ or TIPS) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (770)
-
((dental or tooth or teeth or molar) adj2 (surg$ or operat$ or reoperat$ or soldering or inlay or preparation or pulp extirpation or extraction$ or amputation or resect$ or removal or remove or reimplant$ or replantat$ or reinclusion or extract$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (673)
-
((bile or biliary or gall bladder or gallbladder) adj2 (surg$ or operat$ or reoperat$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (253)
-
((nephrostom$ or nephrotom$ or pyelostom$ or pyelotom$ or kidney incision$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (132)
-
((catheter$ or radiofrequency or radio frequency or electric$) adj2 ablation$ adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (1881)
-
(laparoscop$ or celioscop$ or peritoneoscop$ or pelvic endoscop$ or peritoneoscop$ or videolaparoscop$ or laparoendoscop$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (5125)
-
or/6-22 (126,330)
-
((surg$ or operat$ or reoperat$ or procedure$ or radiosurg$ or microsurg$ or perioperat$ or intraoperat$ or perisurg$ or intrasurg$ or postoperat$ or postsurg$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (217,981)
-
exp *Hemorrhage/ and exp *Risk/ (355)
-
*Blood Loss, Surgical/ (6090)
-
*postoperative hemorrhage/ (5616)
-
(bleeding or blood loss or blood losses or haemorrhage$ or hemorrhage$).ti,ab. (374,472)
-
or/25-28 (376,698)
-
24 and 29 (23,560)
-
5 and (23 or 30) (1796)
-
exp animals/ not humans/ (4,580,930)
-
(comment or editorial or historical article or letter).pt. (2,057,682)
-
31 not (32 or 33) (1757)
-
limit 34 to yr="2009 -Current” (795)
-
"cost of illness"/ or health care costs/ (58,162)
-
((cost$ or burden$) adj2 (illness or disease$ or sickness$ or health care or healthcare)).ti,ab. (56,342)
-
36 or 37 (103,028)
-
exp *General Surgery/ or (surg$ or operat$ or reoperat$ or procedure$ or radiosurg$ or microsurg$ or perioperat$ or intraoperat$ or perisurg$ or intrasurg$ or postoperat$ or postsurg$).ti,ab. (3,262,613)
-
5 and 38 and 39 (82)
-
40 not (32 or 33) (81)
-
limit 41 to yr=“2009 -Current” (59)
-
35 or 42 (845).
EMBASE (via Ovid)
Date range searched: 1974 to week 20 2019.
Date searched: 20 May 2019.
Search strategy
-
*chronic liver disease/ or *liver cirrhosis/ or *liver fibrosis/ or *chronic hepatitis/ (78,147)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$ or failure$) adj2 (chronic or refractory or unmanageab$ or uncontrol$ or resistant or persist$ or intractable$ or recurren$ or sustained or permanent$ or unremitting or unrelenting or continual$ or continuous$ or constant$ or unending or unceasing)).ti,ab. (36,615)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab. (136,515)
-
((fibrosis or fibroses or scar$) adj2 (liver$ or hepat$ or intrahepat$)).ti,ab. (34,839)
-
or/1-4 (196,772)
-
(exp *surgery/ or elective surgery/ or chronic liver disease/dm, su) and (statistics/ or trend study/ or reoperation/ or frequency/) (70,352)
-
(exp liver surgery/ or paracentesis/ or thoracocentesis/ or gastrointestinal endoscopy/ or bronchoscopy/ or ablation therapy/ or chemoembolization/ or blood vessel catheterisation/ or transjugular intrahepatic portosystemic shunt/ or exp dental procedure/ or biliary tract surgery/ or exp nephrostomy/ or nephrostomy tube/ or radiofrequency ablation/ or catheter ablation/ or exp laparoscopy/) and (statistics/ or trend study/ or reoperation/ or frequency/) (16,383)
-
((surg$ or operat$ or reoperat$ or procedure$ or radiosurg$ or microsurg$ or perioperat$ or intraoperat$ or perisurg$ or intrasurg$ or postoperat$ or postsurg$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (311,322)
-
((paracentesis or paracenteses) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (585)
-
((thoracentesis or thoracenteses or thoracocentesis or thoracocenteses or pleurocentesis or pleurocenteses) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (477)
-
((endoscop$ or enteroscop$) adj2 (gastrointestinal or balloon$ or push or mucosal or submucosal) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (900)
-
(bronchoscop$ adj2 (gastrointestinal or balloon$ or push or mucosal or submucosal) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (7)
-
((ethanol or alcohol) adj2 (ablation or inject$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (327)
-
(chemoemboli?ati$ adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (380)
-
((vascular or cardiac or cardiovascular or heart or blood vessel$) adj2 (catheteri?ation or catherteri?ed) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (1206)
-
((transjugular intrahepatic portosystemic shunt$ or transjugular intrahepatic porto systemic shunt$ or transjugular intrahepatic portacaval shunt$ or transjugular intrahepatic porta systemic shunt$ or transjugular intrahepatic portasystemic shunt$ or transjugular intrahepatic shunt$ or transjugular intrahepatic stent$ or TIPS) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (1053)
-
((dental or tooth or teeth or molar) adj2 (surg$ or operat$ or reoperat$ or soldering or inlay or preparation or pulp extirpation or extraction$ or amputation or resect$ or removal or remove or reimplant$ or replantat$ or reinclusion or extract$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (742)
-
((bile or biliary or gall bladder or gallbladder) adj2 (surg$ or operat$ or reoperat$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (326)
-
((nephrostom$ or nephrotom$ or pyelostom$ or pyelotom$ or kidney incision$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (220)
-
((catheter$ or radiofrequency or radio frequency or electric$) adj2 ablation$ adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (3513)
-
((laparoscop$ or celioscop$ or peritoneoscop$ or pelvic endoscop$ or peritoneoscop$ or videolaparoscop$ or laparoendoscop$) adj3 (rate or rates or occurrence or reoccurrence or frequen$ or repeat$ or pattern$ or trend$ or episode$ or prevalence or incidence or quantity or amount$ or number or numbers or life time or lifetime or long term or longterm or subsequent$ or repetition$ or reoperat$ or re-operate$ or readmiss$ or readmit$)).ti,ab. (8370)
-
or/9-21 (17,952)
-
exp *bleeding/ or operative blood loss/ or postoperative hemorrhage/ (287,515)
-
(bleeding or blood loss or blood losses or haemorrhage$ or hemorrhage$).ti,ab. (545,372)
-
23 or 24 (660,304)
-
(or/6-8) and 25 (46,987)
-
5 and (22 or 26) (1909)
-
animal/ or animal experiment/ (3,761,876)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot. (4,490,245)
-
28 or 29 (5,807,883)
-
exp human/ or human experiment/ (19,651,633)
-
30 not (30 and 31) (4,495,226)
-
27 not 32 (1898)
-
(editorial or letter or note).pt. (2,417,131)
-
conference$.pt,st,so. (4,205,445)
-
33 not (34 or 35) (1124)
-
“cost of illness”/ or disease burden/ (27,606)
-
exp *health care cost/ (62,402)
-
((cost$ or burden$) adj2 (illness or disease$ or sickness$ or health care or healthcare)).ti,ab,ot. (85,720)
-
or/37-39 (158,152)
-
exp *surgery/ or (surg$ or operat$ or reoperat$ or procedure$ or radiosurg$ or microsurg$ or perioperat$ or intraoperat$ or perisurg$ or intrasurg$ or postoperat$ or postsurg$).ti,ab. (5,124,738)
-
5 and 40 and 41 (210)
-
42 not (32 or 34 or 35) (97)
-
36 or 43 (1215)
-
limit 44 to yr="2009 -Current” (589).
NHS Economic Evaluation Database (www.crd.york.ac.uk/CRDWeb/) up to 31 March 2015; Health Technology Assessment database up to 31 March 2018
Date searched: 20 May 2019.
Search strategy
-
MeSH DESCRIPTOR Liver Diseases EXPLODE ALL TREES 1983
-
(((liver or hepat* or intrahepat*) near (disease* or disorder* or lesion*))) 723
-
((cirrhosis or cirrhoses or cirrhotic)) 643
-
(((fibrosis or fibroses or scar*) near3 (liver* or hepat*))) 49
-
(((hepatitis or hepatopath*) near3 (chronic or acute or persistent or long stand* or long term or recurr*))) 547
-
#1 OR #2 OR #3 OR #4 OR #5 2378
-
MeSH DESCRIPTOR General Surgery EXPLODE ALL TREES 61
-
MeSH DESCRIPTOR Reoperation EXPLODE ALL TREES 483
-
MeSH DESCRIPTOR Surgical Procedures, Operative EXPLODE ALL TREES 16,709
-
((surg* or operat* or reoperat* or procedure* or radiosurg* or microsurg* or perioperat* or intraoperat* or perisurg* or intrasurg* or postoperat* or postsurg*)) 23,205
-
#7 OR #8 OR #9 OR #10 27,484
-
#6 AND #11 886
-
* IN NHSEED FROM 2009 TO 2019 8219
-
#12 AND #13 84
-
* IN HTA FROM 2009 TO 2019 8591
-
#12 AND #15 43.
Cost-effectiveness Analysis Registry (www.cearegistry.org)
Date range searched: up to 20 May 2019.
Date searched: 20 May 2019.
Search strategy
-
chronic liver.
13 records retrieved.
ScHARR Health Utilities Database (www.scharrhud.org/)
Date range searched: up to 20 May 2019.
Date searched: 20 May 2019.
Search strategy
-
liver* or hepat* or intrahepat*.
15 records retrieved.
Literature searches to identify UK mortality associated with platelet transfusion
MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily Update (via Ovid)
Date range searched: 1946 to 24 May 2019.
Date searched: 28 May 2019.
Search strategy
-
Platelet Transfusion/ (6911)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$ or transfer$)).ti,ab. (8619)
-
1 or 2 (12,763)
-
exp Mortality/ or exp Death/ (487,368)
-
(mortalit$ or death or deaths or dead or died or fatal$ or decease$).ti,ab. (1,560,525)
-
4 or 5 (1,794,102)
-
exp United Kingdom/ (352,811)
-
(britain or united kingdom or uk or england or scotland or ireland or wales or english or scottish or irish or welsh).ti,ab,in. (1,680,163)
-
7 or 8 (1,873,549)
-
3 and 6 and 9 (162)
-
exp animals/ not humans/ (4,583,131)
-
10 not 11 (160)
-
(comment or editorial or historical article or letter).pt. (2,059,990)
-
12 not 13 (158)
-
limit 14 to yr=“2009 -Current” (93).
EMBASE (via Ovid)
Date range searched: 1974 to week 21 2019.
Date searched: 28 May 2019.
Search strategy
-
thrombocyte transfusion/ (17,434)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$ or transfer$)).ti,ab. (14,612)
-
1 or 2 (24,063)
-
exp mortality/ or exp death/ (1,512,465)
-
(mortalit$ or death or deaths or dead or died or fatal$ or decease$).ti,ab. (2,194,505)
-
4 or 5 (2,630,028)
-
exp United Kingdom/ or exp British citizen/ (401,362)
-
(britain or united kingdom or uk or england or scotland or ireland or wales or english or scottish or irish or welsh).ti,ab,in. (2,978,485)
-
7 or 8 (3,130,072)
-
3 and 6 and 9 (647)
-
animal/ or animal experiment/ (3,766,632)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot. (4,495,229)
-
11 or 12 (5,814,073)
-
exp human/ or human experiment/ (19,680,703)
-
13 not (13 and 14) (4,499,942)
-
(editorial or letter or note or (“conference abstract” or “conference review”)).pt. or conference$.so,st. (5,886,982)
-
10 not (15 or 16) (449)
-
limit 17 to yr=“2009 -Current” (295).
Literature searches to identify platelet transfusion refractoriness studies
MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily Update (via Ovid)
Date range searched: 1946 to 24 May 2019.
Date searched: 28 May 2019.
Search strategy
-
2 ((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$ or transfer$)).ti,ab. (8619)
-
3 1 or 2 (12,763)
-
4 (refractor$ or resistan$).ti,ab. (1,031,160)
-
5 3 and 4 (1180)
-
6 exp animals/ not humans/ (4,583,131)
-
7 5 not 6 (1108)
-
8 (comment or editorial or historical article or letter).pt. (2,059,990)
-
9 7 not 8 (1078)
-
10 limit 9 to yr="2009 -Current” (367).
EMBASE (via Ovid)
Date range searched: 1974 to week 21 2019.
Date searched: 28 May 2019.
Search strategy
-
*thrombocyte transfusion/ (3846)
-
((platelet$ or thrombocyt$) adj3 (transfus$ or infus$ or administ$ or transfer$)).ti,ab. (14,612)
-
1 or 2 (15,782)
-
(refractor$ or resistan$).ti,ab. (1,316,064)
-
3 and 4 (2192)
-
platelet refractoriness.dq. (18)
-
refractory thrombocytopenia/ (298)
-
or/5-7 (2437)
-
animal/ or animal experiment/ (3,766,632)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot. (4,495,229)
-
9 or 10 (5,814,073)
-
exp human/ or human experiment/ (19,680,703)
-
11 not (11 and 12) (4,499,942)
-
(editorial or letter or note or (“conference abstract” or “conference review”)).pt. or conference$.so,st. (5,886,982)
-
8 not (13 or 14) (1253)
-
limit 15 to yr="2009 -Current” (489).
Literature searches to identify chronic liver disease/thrombocytopenia cost of illness studies
MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily Update (via Ovid)
Date range searched: 1946 to 28 May 2019.
Date searched: 28 May 2019.
Search strategy
-
exp *Liver Diseases/ and exp Chronic Disease/ (14,897)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$ or failure$) adj2 (chronic or refractory or unmanageab$ or uncontrol$ or resistant or persist$ or intractable$ or recurren$ or sustained or permanent$ or unremitting or unrelenting or continual$ or continuous$ or constant$ or unending or unceasing)).ti,ab. (24,065)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab. (93,760)
-
((fibrosis or fibroses or scar$) adj2 (liver$ or hepat$ or intrahepat$)).ti,ab. (21,382)
-
or/1-4 (130,775)
-
exp *Thrombocytopenia/ (33,008)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab. (59,374)
-
6 or 7 (67,504)
-
"Cost of Illness"/ (25,073)
-
((cost$ or burden$) adj2 illness).ti,ab. (3967)
-
9 or 10 (27,504)
-
(5 or 8) and 11 (201)
-
limit 12 to yr=“2009 -Current” (149).
EMBASE (via Ovid)
Date range searched: 1974 to week 21 2019.
Date searched: 29 May 2019.
Search strategy
-
*chronic liver disease/ or *liver cirrhosis/ or *liver fibrosis/ or *chronic hepatitis/ (78,218)
-
((liver$ or hepat$ or intrahepat$) adj2 (disease$ or disorder$ or lesion$ or failure$) adj2 (chronic or refractory or unmanageab$ or uncontrol$ or resistant or persist$ or intractable$ or recurren$ or sustained or permanent$ or unremitting or unrelenting or continual$ or continuous$ or constant$ or unending or unceasing)).ti,ab. (36,672)
-
(cirrhosis or cirrhoses or cirrhotic).ti,ab. (136,679)
-
((fibrosis or fibroses or scar$) adj2 (liver$ or hepat$ or intrahepat$)).ti,ab. (34,905)
-
or/1-4 (197,020)
-
exp *thrombocytopenia/ (42,771)
-
(thrombocytopeni$ or thrombocytopaeni$ or thrombopeni$ or thrombopaeni$ or macrothrombocytopeni$ or macrothrombocytopaeni$).ti,ab. (90,374)
-
6 or 7 (100,725)
-
*“cost of illness”/ (5068)
-
((cost$ or burden$) adj2 illness).ti,ab. (5954)
-
9 or 10 (10,092)
-
(5 or 8) and 11 (104)
-
limit 12 to yr="2009 -Current” (90).
NHS Economic Evaluation Database (via CRD) (www.crd.york.ac.uk/CRDWeb/)
Date range searched: up to 31 March 2015.
Date searched: 29 May 2019.
Search strategy
-
MeSH DESCRIPTOR Cost of Illness EXPLODE ALL TREES 673
-
(“cost of illness”) IN NHSEED 667
-
#1 OR #2 725
-
MeSH DESCRIPTOR Liver Diseases EXPLODE ALL TREES 1983
-
(((liver or hepat* or intrahepat*) near (disease* or disorder* or lesion*))) IN NHSEED 221
-
((cirrhosis or cirrhoses or cirrhotic)) IN NHSEED 259
-
(((fibrosis or fibroses or scar*) near3 (liver* or hepat*))) IN NHSEED 9
-
#4 OR #5 OR #6 OR #7 2098
-
MeSH DESCRIPTOR Thrombocytopenia EXPLODE ALL TREES 107
-
((thrombocytopeni* or thrombocytopaeni* or thrombopeni* or thrombopaeni* or macrothrombocytopeni* or macrothrombocytopaeni*)) IN NHSEED 93
-
#9 OR #10 170
-
(#3 AND (#8 OR #11)) IN NHSEED FROM 2009 TO 2019 9.
Citation searches
Date searched: 23 May 2019.
| Included papers | SCI | GS | PM |
|---|---|---|---|
| Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018;155:705–18 | 13 | 19 | 4 |
| Terrault NA, Hassanein T, Howell CD, Joshi S, Lake J, Sher L, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol 2014;61:1253–9 | 23 | 30 | 8 |
| Hidaka H, Kurosaki M, Tanaka H, Kudo M, Abiru S, Igura T, et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol 2019;17:1192–200 | 2 | 5 | 1 |
| Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, Katsube T, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol 2019;54:171–81 | 4 | 9 | 2 |
| Brown RS, Imawari M, Izumi N, Osaki Y, Bentley R, Baykal T, et al. Lusutrombopag reliably increases platelet counts for up to 3 weeks in chronic liver disease patients with thrombocytopenia undergoing invasive procedures regardless of baseline platelet counts: results from two phase 3 trials. Hepatology 2018;68(Suppl. 1):1178A–9A | – | 0 | – |
| Brown RS, Imawari M, Izumi N, Osaki Y, Ochiai T, Kano T, et al. Lusutrombopag is a safe and efficacious treatment option for thrombocytopenia in patients with chronic liver disease undergoing invasive procedures: a pooled analysis of two phase 3 trials. Hepatology 2018;68(Suppl. 1):1148A | – | – | – |
| Caldwell S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Shah N. Characterization of baseline thrombopoietin levels in patients with chronic liver disease: results from 2 pooled clinical studies in patients with thrombocytopenia and liver disease. Hepatology 2018;68(Suppl. 1):487A–8A | – | 0 | – |
| Alkhouri N, Imawari M, Izumi N, Osaki Y, Ochiai T, Bentley R, et al. Use of the thrombopoietin receptor agonist lusutrombopag for management of thrombocytopenia in patients with hepatocellular carcinoma undergoing planned invasive procedures. Hepatology 2018;68(Suppl. 1):553A–4A | – | 0 | – |
| Poordad F, Allen LF, Aggarwal K, Vredenburg M, Alkhouri N. Superiority of avatrombopag to placebo in increasing platelet counts and reducing platelet transfusions in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures: pooled analysis of 2 randomized phase 3 studies. Res Pract Thromb Haemost 2018;2(Suppl. 1):10 | – | – | – |
| Poordad F, Allen L, Aggarwal K, Vredenburg M, Tian W, Terrault N. Exploratory analyses of the efficacy of avatrombopag versus placebo from 2 phase 3 studies using alternate baseline platelet count cohorts and an alternate secondary efficacy endpoint. Res Pract Thromb Haemost 2018;2(Suppl. 1):9 | – | – | – |
| Sammy S, Allen LF, Aggarwal K, Vredenburg M, Terrault N. Consistent efficacy of avatrombopag placebo in patients with thrombocytopenia and chronic liver disease undergoing procedures across various disease severities and etiologies. J Hepatol 2018;68(Suppl. 1):752 | – | 0 | – |
| Sammy S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Tian W, et al. Efficacy of avatrombopag compared with placebo across various mean baseline platelet count subgroups-pooled data from 2 phase 3 studies. J Hepatol 2018;68(Suppl. 1):751 | – | 0 | – |
| Reau NS, Sammy S, Allen LF, Aggarwal K, Vredenburg M, Kim WR. Avatrombopag decreases need for platelet transfusion in patients chronic liver disease and thrombocytopenia undergoing medical procedures with low to high associated bleeding risks. J Hepatol 2018;68(Suppl. 1):751 | – | 0 | – |
| Afdhal N, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Platelet response to lusutrombopag, a thrombopoietin receptor agonist, in patients with chronic liver disease and thrombocytopenia undergoing non-emergency invasive procedures: results from a phase 3 randomized, double-blind, placebo-controlled study. Blood 2017;130(Suppl. 1):abstract 291 | – | 4 | – |
| Frelinger AL, Koganov ES, Forde EE, Carmichael SL, Michelson AD. Avatrombopag, a novel thrombopoietin receptor agonist, increases platelet counts without increasing platelet activation in patients with thrombocytopenia due to chronic liver disease. Blood 2017;130(Suppl. 1):abstract 290 | – | 1 | – |
| Terrault N, Kuter DJ, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Superiority of avatrombopag to placebo in increasing platelet counts in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures: results from 2, phase 3 randomized studies. Blood 2017;130(Suppl. 1):abstract 18 | – | 3 | – |
| Peck-Radosavljevic M, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Lusutrombopag for treatment of thrombocytopenia in patients with chronic liver disease who are undergoing non-emergency invasive procedures: results from an international phase 3, randomized, double-blind, placebo-controlled study (L-PLUS 2). United European Gastroenterol J 2017;5:1145 | – | – | – |
| Izumi N, Osaki Y, Yamamoto K, Kurokawa M, Tanaka K, Kano T, et al. A phase 3, randomized, double-blind, placebo-controlled study of lusutrombopag for thrombocytopenia in patients with chronic liver disease undergoing elective invasive procedures in Japan (L-PLUS 1). Hepatology 2015;62:1397A–8A | 1 | 4 | – |
| Terrault N, Bibbiani F, Chen YC, Izumi N, Kayali Z, Soto JRL, et al. Superiority of avatrombopag (AVA) to placebo (PBO) for the treatment of chronic liver disease (CLD)-associated thrombocytopenia (TCP) in patients undergoing scheduled procedures: results of 2 randomized, PBO-controlled phase 3 studies. Hepatology 2017;66(Suppl. 1):124A–5A | 1 | 0 | – |
| Izumi N, Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, et al. Once-daily oral lusutrombopag, alternative to platelet transfusion in thrombocytopenic patients with chronic liver disease undergoing radiofrequency ablation: results from a phase 2B, randomized, double-blind study. J Hepatol 2014;60(Suppl. 1):386 | 2 | 3 | – |
| Terrault N, Hassanein T, Joshi S, Lake JR, Sher LS, Vargas HE, et al. Once-daily oral avatrombopag (E5501) prior to elective surgical or diagnostic procedures in patients with chronic liver disease and thrombocytopenia: results from a phase 2, randomized, double-blind, placebo-controlled study (Study 202). Hepatology 2012;56(Suppl. 1):253A–4A | – | 0 | – |
| Poordad F, Vredenburg M, Allen LF, Aggarwal K, Alkhouri N. Superiority of avatrombopag to placebo in increasing platelet counts and reducing platelet transfusions in patients with chronic liver disease-associated thrombocytopenia undergoing scheduled procedures-pooled analysis of 2 randomized phase 3 studies. Gastroenterology 2018;154:S529 | – | 0 | – |
| Saab S, Allen LF, Aggarwal K, Vredenburg M, Terrault N. Consistent efficacy of avatrombopag placebo in patients with thrombocytopenia and chronic liver disease undergoing procedures across various liver disease severities and etiologies. Gastroenterology 2018;154:S1247–8 | – | – | – |
| Saab S, Alkhouri N, Allen LF, Aggarwal K, Vredenburg M, Tian W. Efficacy of avatrombopag compared with placebo across various mean baseline platelet count subgroups: pooled data from 2 phase 3 studies. Gastroenterology 2018;154:S1249 | – | – | – |
| Vredenburg M, Reau N, Allen LF, Aggarwal K, Poordad F. Consistent efficacy of avatrombopag over placebo in the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures across demographic subgroups: pooled results of two phase 3 studies. Gastroenterology 2018;154:S532 | – | 0 | – |
| Afdhal NH, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, et al. Lusutrombopag for treatment of thrombocytopenia in patients with chronic liver disease who are undergoing non-emergency invasive procedures: results from an international phase 3, randomized, double-blind, placebo-controlled study (L-PLUS 2). Hepatology 2017;66:1254A | – | 0 | – |
| Shionogi Inc. Safety and Efficacy Study of Lusutrombopag for Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Elective Invasive Procedures (L-PLUS 2). 2015–17. URL:https://ClinicalTrials.gov/show/NCT02389621 (cited 23 January 2019) | – | – | – |
| Eisai Inc. Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing an Elective Procedure. 2013–17. URL: https://ClinicalTrials.gov/show/NCT01976104 (cited 23 January 2019) | – | – | – |
| Eisai Inc. Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing an Elective Procedure. 2014–17. URL: https://ClinicalTrials.gov/show/NCT01972529 (cited 23 January 2019) | – | – | – |
| Eisai Inc. Once-Daily Oral Avatrombopag Tablets Used in Participants with Chronic Liver Diseases and Thrombocytopenia Prior to Elective Surgical or Diagnostic Procedures. 2009–11. URL: https://ClinicalTrials.gov/show/NCT00914927 (cited 23 January 2019) | – | – | – |
| Eisai Co Ltd. Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing an Elective Procedure. JPRN-Japiccti-142746. 2014. URL: www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId%20=%20JapicCTI-142746 (accessed 23 January 2019) | – | – | – |
| Center for Drug Evaluation and Research, US FDA. Doptelet/Avatrombopag. Other Review(s). 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000OtherR.pdf (accessed 23 January 2019) | – | – | – |
| Center for Drug Evaluation and Research, US FDA. Doptelet (Avatrombopag). Drug Approval Package. 2018. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000TOC.cfm (accessed 23 January 2019) | – | – | – |
| Center for Drug Evaluation and Research, US FDA. Mulpleta (Lusutrombopag). Multi-Discipline Review/Summary. 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210923Orig1s000MultidisciplineR.pdf (accessed 23 January 2019) | – | – | – |
| Total | 46 | 78 | 15 |
| Combined total | 139 | ||
| Combined total after removal of duplicates | 59 | ||
Appendix 2 Table of excluded studies with rationale
This is not intended to be an exhaustive list of every study examining the intervention. However, it was intended to include studies that passed the first screening but on closer inspection were not deemed relevant and/or valid. This includes studies provided in company/sponsor submissions.
| Reason for exclusion | Reference |
|---|---|
| Population | Afdhal N, Giannini E, Tayyab GN, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag in chronic liver disease patients with thrombocytopenia undergoing an elective invasive procedure: results from ELEVATE, a randomised clinical trial. J Hepatol 2010;52(Suppl. 1):460 |
| Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med 2012;367:716–24 | |
| Allen R, Bryden P, Grotzinger KM, Stapelkamp C, Woods B. Cost-effectiveness of eltrombopag versus romiplostim for the treatment of chronic immune thrombocytopenia in England and Wales. Value Health 2016;19:614–22 | |
| Berg T, Riordan S, Karamanolis D, Garcia-Samaniego J, Porayko M, Campbell F, et al. ENABLE-ALL: safety and efficacy of eltrombopag in thrombocytopenic hepatitis C virus-infected patients with cirrhosis who withdrew from the ENABLE-1&2 studies. Hepatol Int 2014;8(Suppl. 1):172–3 | |
| Lopez-Plaza I, Weissfeld J, Triulzi DJ. The cost-effectiveness of reducing donor exposures with single-donor versus pooled random-donor platelets. Transfusion 1999;39:925–32 | |
| Intervention | Afdhal N, Dusheiko G, Giannini EG, Chen PJ, Han KH, Moshin A, et al. Final results of ENABLE 1, a phase 3, multicenter study of eltrombopag as an adjunct for antiviral treatment of hepatitis C virus-related chronic liver disease associated with thrombocytopenia. Hepatology 2011;54(Suppl. 1):1427A–8A |
| Afdhal NH, McHutchison JG, Shiffman ML, Rodriguez-Torres M, Dusheiko GM, Sigal S. Eltrombopag raises platelet counts in two weeks in patients with HCV and significant thrombocytopenia. Hepatology 2007;46(Suppl. 1):252A | |
| Ata RMA. The efficacy of eltrombopag in improving thrombocytopenia in patients with chronic liver disease: a meta analysis. Hepatol Int 2013;7(Suppl. 1):541 | |
| Botros Y, Hafez HA, Fouad R, El Negoly M, Shiha G, Waked I, et al. The effect of eltrombopag (Promecta) on thrombocytopenia in Egyptian patients with chronic hepatitis C. J Gastroenterol Hepatol Res 2016;5:2088–92 | |
| Chen P-J, Han K-H, Dusheiko GM, Campbell FM, Vasey SY, Patwardhan R, et al. Eltrombopag as a Supportive Agent to Enable Antiviral Therapy in East Asian Patients with Thrombocytopenia and Hepatitis C Virus. Paper presented at APASL Liver Week 2013, 6–10 June 2013, Singapore | |
| Dusheiko G, Afdhal N, Giannini EG, Chen PJ, Han KH, Rodriguez-Torres M, et al. Results of ENABLE 2, a phase 3, multicenter study of eltrombopag and peginterferon alfa-2B treatment in patients with hepatitis C and thrombocytopenia. J Hepatol 2012;56(Suppl. 2):27 | |
| Dusheiko G, Afdhal NH, Giannini E, Chen PJ, Han KH, Kamel YM, et al. Final results of open-label treatment with eltrombopag during ENABLE 1: a study of eltrombopag as an adjunct for antiviral treatment of hepatitis C virus associated with thrombocytopenia. Blood 2011;118:abstract no. 2232 | |
| Eltrombopag (Revolade) and thrombocytopenia in patients with hepatitis C. Hepatotoxic drug; more harms than benefits. Prescrire Int 2015;24:208–9 | |
| Giannini E, Dusheiko G, Afdhal N, Chen P, Han K, Mostafa Kamel Y, et al. Eltrombopag raises platelet counts prior to antiviral therapy in patients with chronic hepatitis C virus infection associated with thrombocytopenia. Haematologica 2012;97(Suppl. 1):251 | |
| GlaxoSmithKline Pharmaceuticals Ltd. TPL104054: Eltrombopag to Reduce the Need for Platelet Transfusion in Participants with Chronic Liver Disease and Thrombocytopenia Undergoing Elective Invasive Procedures. (ELEVATE). 2009. URL: www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=730 (accessed 23 January 2019) | |
| GlaxoSmithKline SA España. Estudio Aleatorizado, Doble Ciego, Controlado Con Placebo, Multicéntrico Para Evaluar La Seguridad Y Eficacia De Eltrombopag Para Reducir La Necesidad De Transfusión De Plaquetas En Sujetos Trombocitopénicos Con Enfermedad Hepática Crónica Que Se Van A Someter A Un Procedimiento Invasivo Programado. 2008. URL: www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2007-005851-40 (accessed 23 January 2019) | |
| Koganov ES, Carmichael SL, Forde EE, Frelinger AL, Michelson AD. Platelet function in thrombocytopenic patients with chronic liver disease. Blood 2017;130(Suppl. 1):abstract no. 2314 | |
| Provan D, Saleh M, Goodison S, Rafi R, Stone N, Hamilton JM, et al. The safety profile of eltrombopag, a novel oral platelet growth factor, in thrombocytopenic patients and healthy participants. J Clin Oncol 2006;24(Suppl.):18596 | |
| Comparator | GlaxoSmithKline Pharmaceuticals Ltd. Eltrombopag to Reduce the Need for Platelet Transfusion in Participants with Chronic Liver Disease and Thrombocytopenia Undergoing Elective Invasive Procedures. 2008–9. URL: https://ClinicalTrials.gov/show/NCT00678587 (cited 23 January 2019) |
| Outcomes | Dova Pharmaceuticals. Avatrombopag for the Treatment of Thrombocytopenia in Adults with Chronic Liver Disease Undergoing a Procedure. 2018. URL: https://ClinicalTrials.gov/show/NCT03554759 (cited 23 January 2019) |
| No extractable outcomes | Afdhal NH, Theodore D. Eltrombopag for thrombocytopenic patients with chronic HCV infection. Reply. Gastroenterology 2014;147:255–6 |
| Center for Drug Evaluation and Research, US FDA. Mulpleta (Lusutrombopag). Other Review(s). 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210923Orig1s000OtherR.pdf (accessed 23 January 2019) | |
| Dova Pharmaceuticals. Avatrombopag for the Treatment of Thrombocytopenia in Adults Scheduled for a Surgical Procedure. 2018. URL: https://ClinicalTrials.gov/show/NCT03326843 (cited 23 January 2019) | |
| Eisai Co. Ltd. A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Once-Daily Oral Avatrombopag in Japanese Participants with Chronic Liver Diseases and Thrombocytopenia. 2014–15. URL: https://ClinicalTrials.gov/show/NCT02227693 (cited 23 January 2019) | |
| Gordon S, Allen LF, Aggarwal K, Vredenburg M, Tian W, Alkhouri N. Body Mass Index Does Not Impact the Efficacy of Avatrombopag in Increasing Platelet Counts and Reducing Platelet Transfusions or Rescue Procedures for Bleeding in Cirrhotic Patients with Thrombocytopenia. Paper presented at American College of Gastroenterology Annual Meeting, 5–10 October 2018, Philadelphia, PA, USA | |
| Katsube T, Shimizu R, Fukuhara T, Kano T, Wajima T. Pharmacokinetic/pharmacodynamic modeling and simulation of lusutrombopag, a novel thrombopoietin receptor agonist, for treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures. United Eur Gastroenterol J 2018;6:A71 | |
| Liu X, Liu Y, Li Y. TPO Receptor Agonist for Patients with Thrombocytopenia and Chronic Liver Disease. 2018. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42018085313 (accessed 23 May 2019) | |
| Poordad F, Dalal MR, Grotzinger K, Shetty S. Medical resource utilization in chronic liver disease patients with thrombocytopenia. Gastroenterology 2007;132:A824 | |
| Poordad F, Loo N, Han X, Aggarwal K. Burden of platelet transfusions in chronic liver disease patients with thrombocytopenia: a case–control study. J Manag Care Spec Pharm 2018;24:S32–3 | |
| Poordad FF, Dalal MR, Grotzinger KM. Prevalence and medical resource utilization in HCV patients with thrombocytopenia. Gastroenterology 2008;134:A834 | |
| Qi X, De Stefano V, Guo X, Fan D. Thrombopoietin receptor agonists significantly increase the risk of portal vein thrombosis in liver diseases: meta-analysis of RCTs. Thromb Haemost 2015;113:1378–80 | |
| Romano F, Ruggeri M, Coretti S, Giannini EG, Sacchini D, Annicchiarico BE, et al. Economic assessment of eltrombopag in the treatment of thrombocytopenia in Italy. Value Health 2015;18:A626 | |
| Schelfhout J, Kauf T. A decision analysis model exploring the results of a phase II trial of eltrombopag for patients with chronic hepatitis C, cirrhosis and thrombocytopenia. Value Health 2011;14:A62 | |
| Tokyo Medical University. Comparison Between Lusutrombopag and Effectiveness of the Platelet Blood Transfusion. 2018. URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R0,00037394 (accessed 23 January 2019) | |
| Study type | Bussel JB. Avatrombopag. Br J Haematol 2018;183:342–3 |
| Center for Drug Evaluation and Research, US FDA. Doptelet (Avatrombopag). Proprietary Name Review(s). 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000NameR.pdf (accessed 23 January 2019) | |
| Center for Drug Evaluation and Research, US FDA. Mulpleta (Lusutrombopag). Drug Approval Package. 2018. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210923Orig1s000TOC.cfm (accessed 23 January 2019) | |
| Center for Drug Evaluation and Research, US FDA. Mulpleta (Lusutrombopag). Proprietary Name Review(s). 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210923Orig1s000NameR.pdf (accessed 23 January 2019) | |
| Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med 2009;60:193–206 | |
| Li B, Ji YJ, Shao Q, Zhu Z, Ji D, Li F, et al. Comparative efficacy and cost effectiveness of splenectomy and thrombopoietin prior to peginterferon and ribavirin therapy with compensatory cirrhosis associated with hepatitis C and thrombocytopenia. Experimental Ther 2015;10:2180–6 | |
| Mondelli MU. Eltrombopag: an effective remedy for thrombocytopaenia? J Hepatol 2008;48:1030–2 | |
| NIHR Horizon Scanning Centre (NIHR HSC). Avatrombopag for Thrombocytopenia in Chronic Liver Disease Prior to Surgery. 2014. URL: www.io.nihr.ac.uk/report/avatrombopag-for-thrombocytopenia-in-chronic-liver-disease-prior-to-surgery/ (accessed 24 January 2019) | |
| Qureshi K, Patel S, Meillier A. The use of thrombopoietin receptor agonists for correction of thrombocytopenia prior to elective procedures in chronic liver diseases: review of current evidence. Int J Hepatol 2016;2016:1802932 | |
| Ronge R. [Eltrombopag for the treatment thrombocytopenia in patients with cirrhosis associated with hepatitis C?] Z Gastroenterol 2008;46:246 | |
| Thrombocytopoenia – avatrombopag. Manufacturing Chemist 2012;83:24 | |
| Study size | Takada H, Izumi N, Kurosaki M, Itakura J, Tsuchiya K, Nakanishi H, et al. Real world experience of lusutrombopag for thrombocytopenia in patients with liver cirrhosis. J Hepatol 2018;68(Suppl. 1):467–8 |
Appendix 3 Table of serious adverse events
| Serious adverse event | Study authors, year | Trial name | NCT/other trial number | Lower/upper platelets (per µl) | Arm name | Follow-up time point (weeks) | Patients with event (n) | Patients analysed (N or NR) | Patients with event or NR (%) |
|---|---|---|---|---|---|---|---|---|---|
| Abdominal pain | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Abdominal pain – lower | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Abdominal pain – upper | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Acute kidney injury | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 | |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Acute myocardial infarction | Terrault et al., 201837 | ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 70 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 43 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 57 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 1 | 33 | 3.0 | |||||
| Acute respiratory failure | Terrault et al., 201837 | ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 70 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 43 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 57 | 1.8 | ||||
| Placebo 40 mg | NR/unclear | 0 | 33 | 0.0 | |||||
| Anaemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 | |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Anaphylactic transfusion reaction | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Ascites | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Asthma | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 48 | 0.0 |
| Placebo | NR/unclear | 1 | 48 | 2.1 | |||||
| Azotaemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Cardiac arrest | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Cardiac ventricular thrombosis | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Cellulitis | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Chronic hepatic failure | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Circulatory collapse | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Clostridium difficile infection | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Clostridium test positive | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Coma hepatic | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Dehydration | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Diarrhoea | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 1 | 32 | 3.1 | |||||
| Encephalopathy | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Epistaxis | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Fluid retention | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Gastrointestinal haemorrhage | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Generalised oedema | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Haematemesis | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 70 | 1.4 | ||
| Placebo 60 mg | NR/unclear | 0 | 43 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 57 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 33 | 0.0 | |||||
| Haemorrhagic anaemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Hepatic cirrhosis | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Hepatic encephalopathy | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 2 | 107 | 1.9 | |||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 | |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 70 | 0.0 | ||
| Placebo 60 mg | NR/unclear | 1 | 43 | 2.3 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 57 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 33 | 0.0 | |||||
| Hepatocellular carcinoma | Peck-Radosavljevic 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 2 | 107 | 1.9 | |||||
| Hyperkalaemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Hypertensive crisis | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Hypokalaemia | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Hyponatraemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Hypotension | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Ileus paralytic | Terrault et al., 201837 | ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 70 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 43 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 57 | 1.8 | ||||
| Placebo 40 mg | NR/unclear | 0 | 33 | 0.0 | |||||
| Multiorgan failure | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Multiple organ dysfunction syndrome | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| ADAPT-2 | NCT01976104 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 70 | 0.0 | ||
| Placebo 60 mg | NR/unclear | 0 | 43 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 57 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 1 | 33 | 3.0 | |||||
| Muscle spasms | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Myalgia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Nausea | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 | |||||
| Oesophageal varices haemorrhage | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 48 | 0.0 |
| Placebo | NR/unclear | 1 | 48 | 2.1 | |||||
| Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 | |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Platelet count decreased | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Pneumonia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| PVT | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 48 | 2.1 |
| Placebo | NR/unclear | 0 | 48 | 0.0 | |||||
| Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 | |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Post-procedural haemorrhage | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Postoperative fever/plural effusion | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 48 | 0.0 |
| Placebo | NR/unclear | 1 | 48 | 2.1 | |||||
| Procedural haemorrhage | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Procedural pain | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Pyrexia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 1 | 48 | 2.1 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 1 | 32 | 3.1 | |||||
| Sepsis | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Splenic haemorrhage | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Splenic infarction | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Splenomegaly | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Stress polycythaemia | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Syncope | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Transfusion reaction | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 3 | 48 | 6.3 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Upper gastrointestinal haemorrhage | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Urinary tract infection | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 0 | 89 | 0.0 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 1 | 58 | 1.7 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Urticaria | Hidaka et al., 201839 | L-PLUS 1 | JapicCTI-132323 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 48 | 0.0 |
| Placebo | NR/unclear | 1 | 48 | 2.1 | |||||
| Vertigo | Terrault et al., 201837 | ADAPT-1 | NCT01972529 | < 40,000 | Avatrombopag 60 mg | NR/unclear | 1 | 89 | 1.1 |
| Placebo 60 mg | NR/unclear | 0 | 48 | 0.0 | |||||
| 40,000–< 50,000 | Avatrombopag 40 mg | NR/unclear | 0 | 58 | 0.0 | ||||
| Placebo 40 mg | NR/unclear | 0 | 32 | 0.0 | |||||
| Vessel perforation | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 1 | 107 | 0.9 |
| Placebo | NR/unclear | 0 | 107 | 0.0 | |||||
| Vomiting | Peck-Radosavljevic et al., 201954 | L-PLUS 2 | NCT02389621 | < 50,000 | Lusutrombopag | NR/unclear | 0 | 107 | 0.0 |
| Placebo | NR/unclear | 1 | 107 | 0.9 |
Appendix 4 Forest plots of each intervention compared with placebo
FIGURE 9.
Proportion of participants who required neither platelet transfusion prior to the primary invasive procedure nor rescue therapy for bleeding from randomisation (risk ratio scale). IV, instrumental variable.

FIGURE 10.
Proportion of participants who required no platelet transfusion prior to the primary invasive procedure (risk ratio scale). IV, instrumental variable.
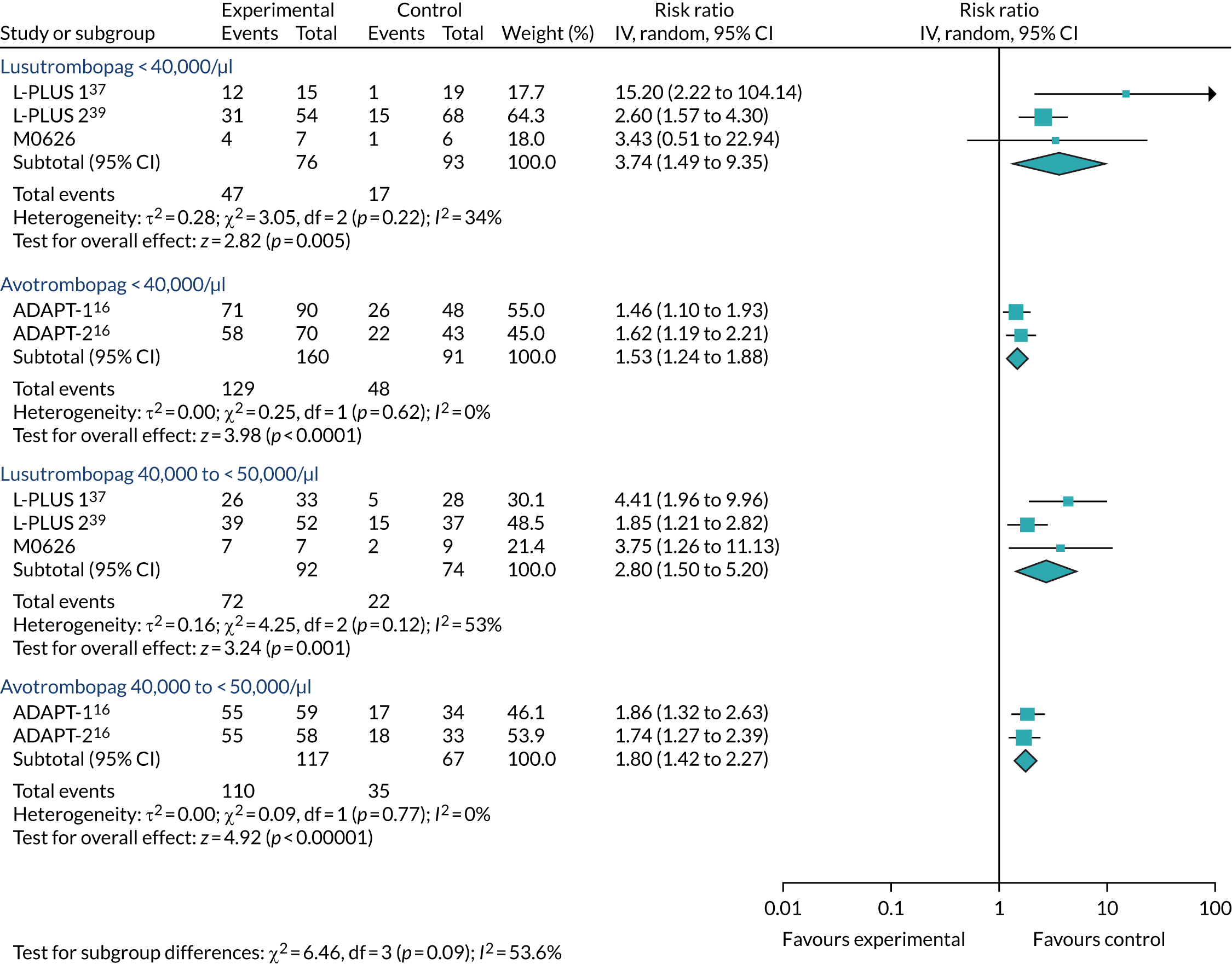
FIGURE 11.
Proportion of participants who required no rescue therapy for bleeding (risk ratio scale). IV, instrumental variable.
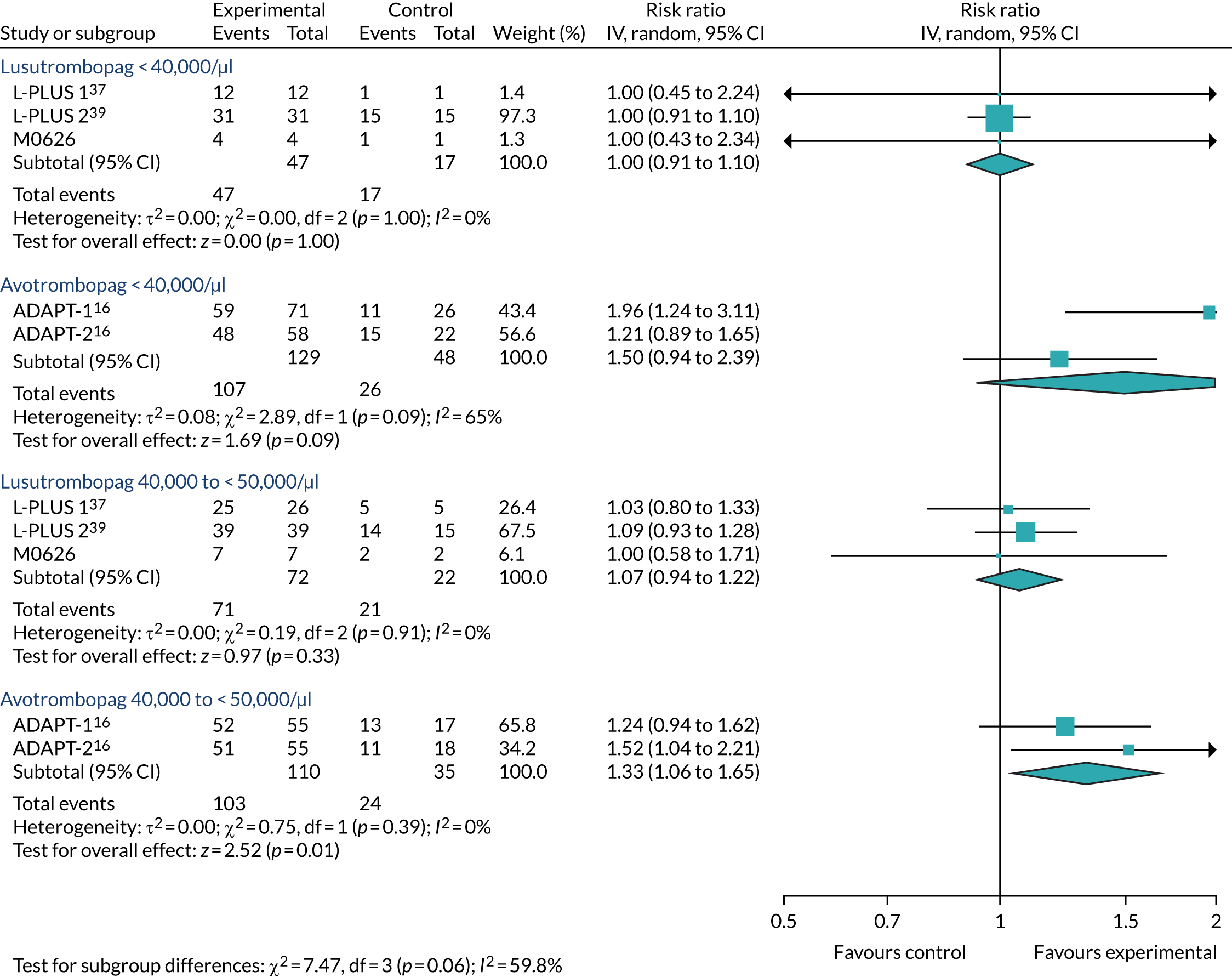
FIGURE 12.
Proportion of participants who required neither platelet transfusion prior to the primary invasive procedure nor rescue therapy for bleeding from randomisation (OR scale). IV, instrumental variable.
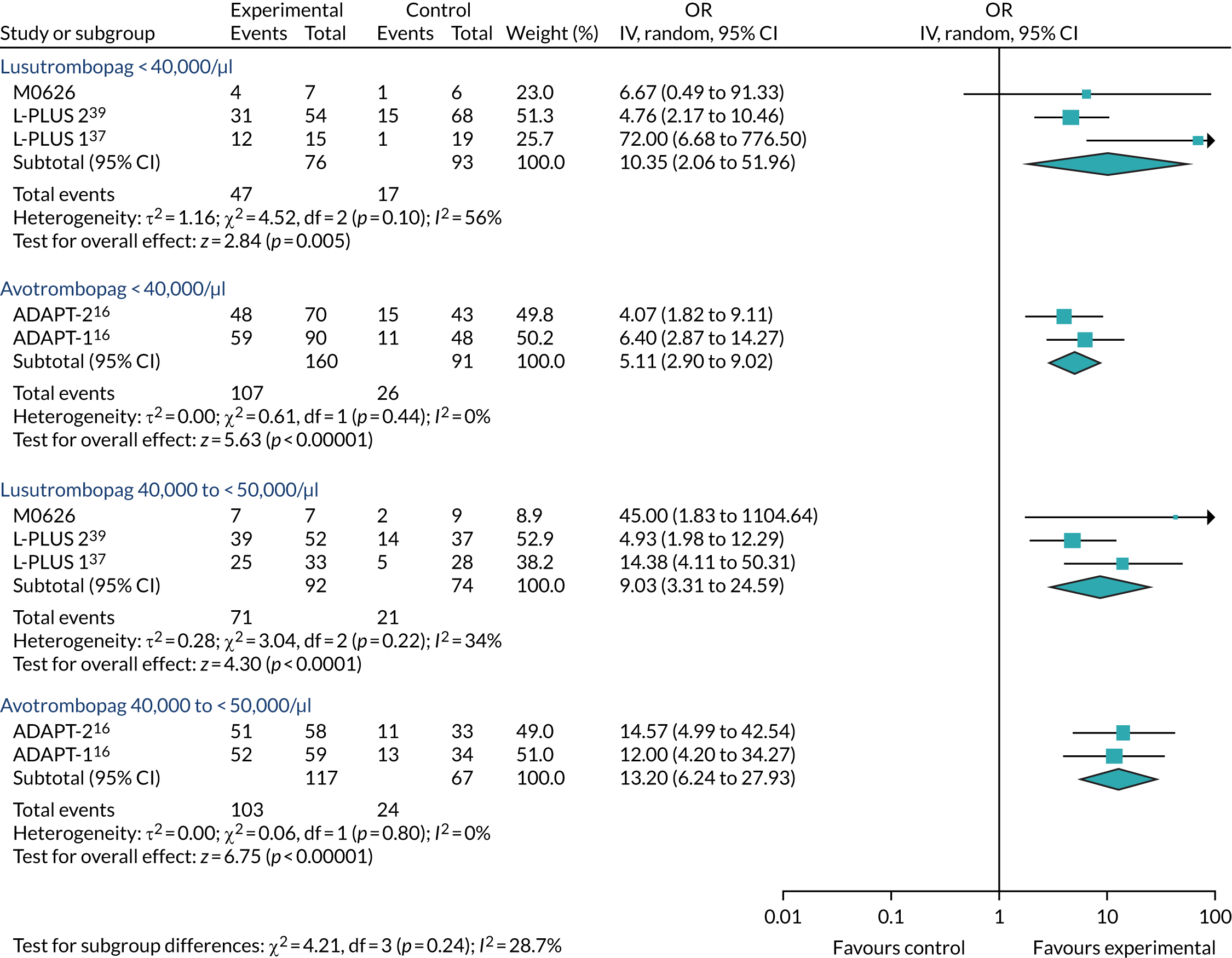
FIGURE 13.
Proportion of participants who required no platelet transfusion prior to the primary invasive procedure (OR scale). IV, instrumental variable.
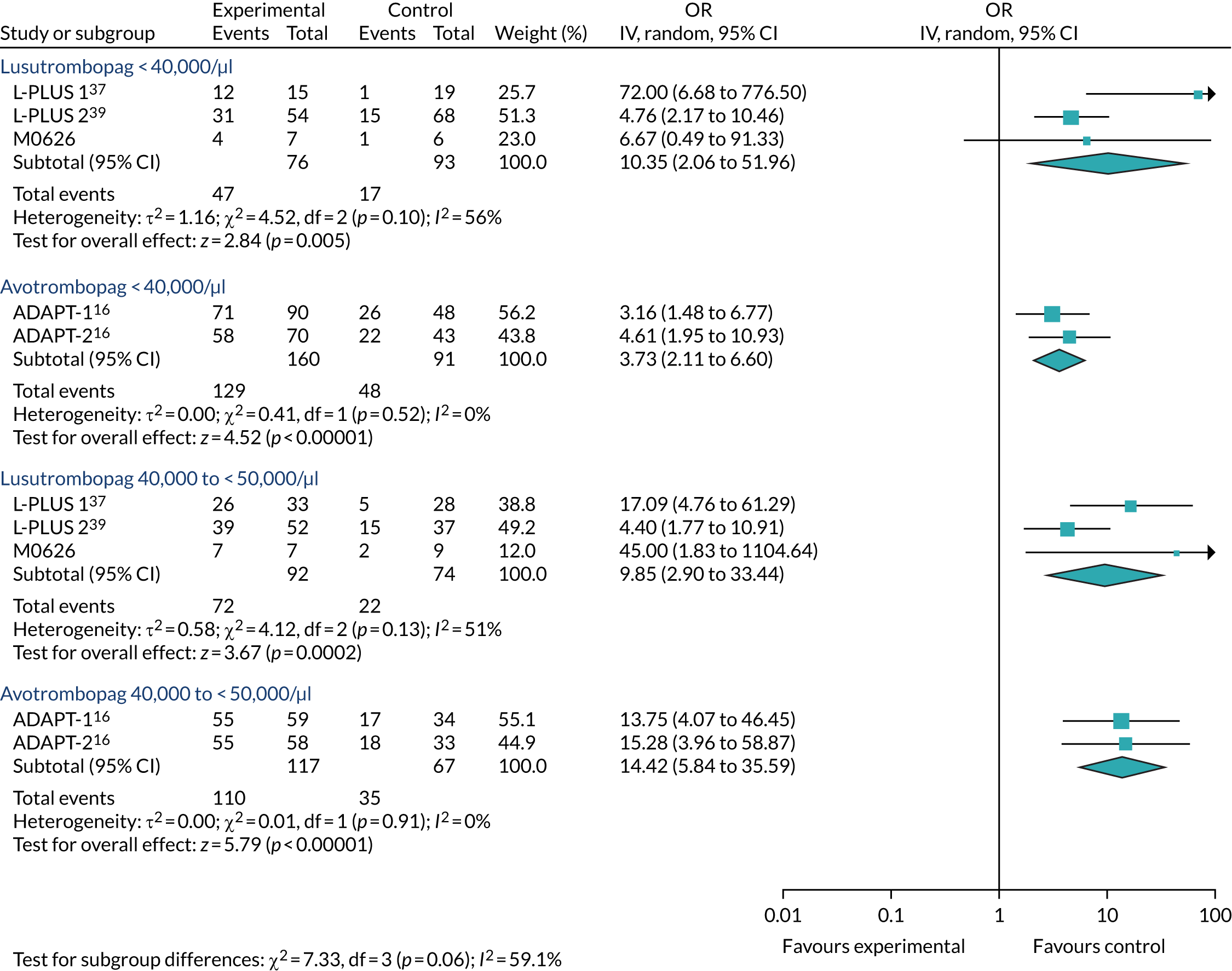
FIGURE 14.
Proportion of participants who required no rescue therapy for bleeding (OR scale). IV, instrumental variable.

Appendix 5 Details of the Bayesian meta-analysis
WinBUGS code for the meta-analysis of the baseline arms for absolute effects (e.g. placebo arm baseline proportions of patients who had no platelet transfusion prior to surgery among patients who had a platelet count of < 40,000/µl).
-
# Binomial likelihood, logit link
-
# Baseline random effects model
-
model{ # *** PROGRAM STARTS
-
for (i in 1:ns){ # LOOP THROUGH STUDIES
-
r[i] ∼ dbin(p[i],n[i]) # Likelihood
-
logit(p[i]) <- mu[i] # Log-odds of response
-
mu[i] ∼ dnorm(m,tau.m) # Random effects model
-
}
-
mu.new ∼ dnorm(m,tau.m) # predictive dist. (log-odds)
-
m ∼ dnorm(0,.0001) # vague prior for mean
-
var.m <- 1/tau.m # between-trial variance
-
tau.m <- pow(sd.m,-2) # between-trial precision = (1/between-trial variance)
-
sd.m ∼ dunif(0,5) # vague prior for between-trial SD
-
#sd.m <- dunif(0,0.5) #less vague prior for between-trial SD for circumventing numerical instability in the presence of zero cells
-
#tau.m ∼ dgamma(0.001,0.001) #gamma distributed prior
-
#sd.m <- sqrt(var.m) #gamma distributed prior
-
logit(R) <- m # posterior probability of response
-
logit(R.new) <- mu.new # predictive probability of response
-
}
-
#Data
-
list(ns=5) # ns=number of studies
-
#in sparse networks or several trials having zero cells, correction by adding 0.5 to the numerator and 1 to the denominator can be applied.
-
r[] n[] # Study ID
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
END.
WinBUGS code for the random-effects meta-analysis to obtain the binomial probabilities to be used in the electronic model (e.g. treatment-specific proportions of patients who had no platelet transfusion prior to surgery among patients who had a platelet count of < 40,000/µl).
-
# Binomial likelihood, logit link
-
# Random effects model for multi-arm trials
-
model{ # *** PROGRAM STARTS
-
for(i in 1:ns){ # LOOP THROUGH STUDIES
-
w[i,1] <- 0 # adjustment for multi-arm trials is zero for control arm
-
delta[i,1] <- 0 # treatment effect is zero for control arm
-
mu[i] ∼ dnorm(0,.0001) # vague priors for all trial baselines
-
for (k in 1:na[i]) { # LOOP THROUGH ARMS
-
r[i,k] ∼ dbin(p[i,k],n[i,k]) # binomial likelihood
-
logit(p[i,k]) <- mu[i] + delta[i,k] # model for linear predictor
-
rhat[i,k] <- p[i,k] * n[i,k] # expected value of the numerators
-
#Deviance contribution
-
dev[i,k] <- 2 * (r[i,k] * (log(r[i,k])-log(rhat[i,k]))
-
+ (n[i,k]-r[i,k]) * (log(n[i,k]-r[i,k]) - log(n[i,k]-rhat[i,k]))) }
-
# summed residual deviance contribution for this trial
-
resdev[i] <- sum(dev[i,1:na[i]])
-
for (k in 2:na[i]) { # LOOP THROUGH ARMS
-
# trial-specific LOR distributions
-
delta[i,k] ∼ dnorm(md[i,k],taud[i,k])
-
# mean of LOR distributions (with multi-arm trial correction)
-
md[i,k] <- d[t[i,k]] - d[t[i,1]] + sw[i,k]
-
# precision of LOR distributions (with multi-arm trial correction)
-
taud[i,k] <- tau *2*(k-1)/k
-
# adjustment for multi-arm RCTs
-
w[i,k] <- (delta[i,k] - d[t[i,k]] + d[t[i,1]])
-
# cumulative adjustment for multi-arm trials
-
sw[i,k] <- sum(w[i,1:k-1])/(k-1)
-
}
-
}
-
totresdev <- sum(resdev[]) # Total Residual Deviance
-
d[1]<-0 # treatment effect is zero for reference treatment
-
# vague priors for treatment effects
-
for (k in 2:nt){ d[k] ∼ dnorm(0,.0001) }
-
sd ∼ dunif(0,5) # vague prior for between-trial SD
-
#sd.m <- dunif(0,0.5) #less vague prior for between-trial SD for circumventing numerical instability in the presence of zero cells
-
tau <- pow(sd,-2) # between-trial precision = (1/between-trial variance)
-
# Provide estimates of treatment effects T[k] on the natural (probability) scale
-
# Given a Mean Effect, meanA, for 'standard' treatment A,
-
# with precision (1/variance) precA
-
A ∼ dnorm(meanA,precA)
-
for (k in 1:nt) { logit(T[k]) <- A + d[k]}
-
} # *** PROGRAM ENDS
-
#Data
-
# ns= number of studies; nt=number of treatments; meanA and precA are obtained from meta-analysis of the baseline arms for absolute effects
-
#in sparse networks or several trials having zero cells, correction by adding 0.5 to the numerator and 1 to the denominator can be applied.
-
list(ns=5, nt=3, meanA=-0.9979, precA=1.140) #RE of all 5 RCTs
-
r[,1] n[,1] r[,2] n[,2] t[,1] t[,2] na[] # Study ID
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
END.
WinBUGS code for the fixed-effects meta-analysis to obtain the binomial probabilities to be used in the electronic model (e.g. treatment-specific proportions of patients who had no platelet transfusion prior to surgery among patients who had a platelet count of < 40,000/µl).
-
# Binomial likelihood, logit link
-
# Fixed effects model
-
model{ # *** PROGRAM STARTS
-
for(i in 1:ns){ # LOOP THROUGH STUDIES
-
mu[i] ∼ dnorm(0,.0001) # vague priors for all trial baselines
-
for (k in 1:na[i]) { # LOOP THROUGH ARMS
-
r[i,k] ∼ dbin(p[i,k],n[i,k]) # binomial likelihood
-
logit(p[i,k]) <- mu[i] + d[t[i,k]] - d[t[i,1]] # model for linear predictor
-
rhat[i,k] <- p[i,k] * n[i,k] # expected value of the numerators
-
dev[i,k] <- 2 * (r[i,k] * (log(r[i,k])-log(rhat[i,k])) #Deviance contribution
-
+ (n[i,k]-r[i,k]) * (log(n[i,k]-r[i,k]) - log(n[i,k]-rhat[i,k])))
-
}
-
resdev[i] <- sum(dev[i,1:na[i]]) # summed residual deviance contribution for this trial
-
}
-
totresdev <- sum(resdev[]) #Total Residual Deviance
-
d[1]<-0 # treatment effect is zero for reference treatment
-
for (k in 2:nt){ d[k] ∼ dnorm(0,.0001) } # vague priors for treatment effects
-
# Provide estimates of treatment effects T[k] on the natural (probability) scale
-
# Given a Mean Effect, meanA, for ‘standard’ treatment A,
-
# with precision (1/variance) precA
-
A ∼ dnorm(meanA,precA)
-
for (k in 1:nt) { logit(T[k]) <- A + d[k] }
-
} # *** PROGRAM ENDS
-
#Data
-
# ns= number of studies; nt=number of treatments; meanA and precA are obtained from meta-analysis of the baseline arms for absolute effects
-
#in sparse networks or several trials having zero cells, correction by adding 0.5 to the numerator and 1 to the denominator can be applied.
-
list(ns=5, nt=3, meanA=-0.9979, precA=1.140) #FE of all 5 RCTs
-
r[,1] n[,1] r[,2] n[,2] t[,1] t[,2] na[] # Study ID
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
Confidential information has been removed
-
END.
WinBUGS output for the fixed-effects and random-effects meta-analyses conducted to obtain the binomial probabilities to be used in the electronic model (e.g. treatment-specific proportions of patients who had no platelet transfusion prior to surgery among patients who had a platelet count of < 40,000/µl).
Random effects
| Node | Mean | SD | MC error | 2.50% | Median | 97.50% | Start | Sample |
|---|---|---|---|---|---|---|---|---|
| T[1] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| T[2] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| T[3] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| d[2] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| d[3] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| tau | 227.7 | 9209 | 117.2 | 0.05228 | 0.5646 | 94.85 | 30001 | 100000 |
| sd | 1.588 | 1.111 | 0.01214 | 0.1027 | 1.331 | 4.373 | 30001 | 100000 |
| totresdev | 10.29 | 4.432 | 0.02536 | 3.43 | 9.685 | 20.57 | 30001 | 100000 |
| Dbar | Dhat | pD | DIC | |
|---|---|---|---|---|
| r | 45.268 | 35.598 | 9.67 | 5.49E+01 |
| total | 45.268 | 35.598 | 9.67 | 5.49E+01 |
Random effects with empirically observed priors [tau2∼lognormal(–2.13,1.582)] obtained from Turner et al.115
| Node | Mean | SD | MC error | 2.50% | Median | 97.50% | Start | Sample |
|---|---|---|---|---|---|---|---|---|
| T[1] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| T[2] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| T[3] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| d[2] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| d[3] | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 30001 | 100000 |
| tau | 24.3 | 146.1 | 0.8111 | 0.4684 | 6.061 | 155.4 | 30001 | 100000 |
| sd | 0.3883 | 0.6752 | 0.007158 | 0.006436 | 0.165 | 2.135 | 30001 | 100000 |
| totresdev | 11.54 | 4.408 | 0.02504 | 4.269 | 11.05 | 21.53 | 30001 | 100000 |
| Dbar | Dhat | pD | DIC | |
|---|---|---|---|---|
| r | 46.525 | 38.079 | 8.447 | 54.972 |
| total | 46.525 | 38.079 | 8.447 | 54.972 |
Fixed effects
| Node | Mean | SD | MC error | 2.5% | Median | 97.5% | Start | sample |
|---|---|---|---|---|---|---|---|---|
| T[1] | 0.3855 | 0.1279 | 3.98E-01 | 0.1629 | 0.3773 | 0.6522 | 30001 | 100000 |
| T[2] | 0.8288 | 0.09496 | 3.69E-01 | 0.5942 | 0.8484 | 0.9559 | 30001 | 100000 |
| T[3] | 0.8855 | 0.07547 | 4.11E-01 | 0.6912 | 0.9039 | 0.9767 | 30001 | 100000 |
| d[2] | 2.226 | 0.3734 | 0.002102 | 1.5120 | 2.2190 | 2.9750 | 30001 | 100000 |
| d[3] | 2.752 | 0.479 | 0.004095 | 1.8650 | 2.7310 | 3.7450 | 30001 | 100000 |
| totresdev | 13.79 | 3.82E+ 00 | 0.0161 | 8.37 | 13.11 | 23.04 | 30001 | 100000 |
| Dbar | Dhat | pD | DIC | |
|---|---|---|---|---|
| r | 45.268 | 38.195 | 7.073 | 5.23E+ 01 |
| total | 45.268 | 38.195 | 7.073 | 5.23E+ 01 |
Appendix 6 Probabilistic sensitivity analysis parameters
Parameters varied in probabilistic sensitivity analyses on general characteristics, efficacy, mortality and safety.
| Parameter varied in probabilistic sensitivity analysis | Condition/comparison | Trials/subgroup | Base value (SE) | Distribution (α, β) | 95% CI |
|---|---|---|---|---|---|
| General | |||||
| Age (years) | All trials and conditions pooled | 58.55 (0.39) | N (58.55, 0.39) | 57.8 to 59.3 | |
| Proportion male | 62.68% | B (487, 290) | 59.25% to 66.04% | ||
| Proportion Child–Pugh A | 57.46% (0.11) | Conditional beta distribution | |||
| Proportion Child–Pugh B | 38.93% (0.08) | ||||
| Proportion Child–Pugh C | 3.611% (0.01) | ||||
| Efficacy | |||||
| Proportion not receiving platelet transfusion prior to elective invasive procedure | Avatrombopag | < 40,000/µl | 0.571 | WinBUGS CODA | |
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.306 | ||||
| Avatrombopag | 40,000–< 50,000/µl | 0.899 | |||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.388 | ||||
| Proportion requiring rescue therapy | Avatrombopag | < 40,000/µl | 0.077 | ||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.180 | ||||
| Avatrombopag | 40,000–< 50,000/µl | 0.040 | |||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.178 | ||||
| Proportion on whom procedure not performed | Lusutrombopag | Pooled (L-PLUS 254 only) | 0.056 (0.000) | B (3, 49) | 0.01 to 0.13 |
| Placebo | 0.084 (0.000) | B (3, 34) | 0.02 to 0.19 | ||
| Mortality | |||||
| Due to platelet transfusion | Assumed to be the same for all patients in all subgroups | 4.6 × 10–6 | B (4.60, 999,995.40) | 1.4 × 10–6 to 9.7 × 10–6 | |
| Due to surgery | 0.019 (0.077) | B (0.04, 2.17) | 0.00 to 0.25 | ||
| Safety | |||||
| Number of ATDs per transfusion | Assumed the same for all patients (based on all patients in all lusutrombopag trials pooled) | 1.11 (0.22) | N (0.68, 1.55) | 0.68 to 1.55 | |
| Transfusion AE percentage | |||||
| Pneumonia | 3.95 × 10–5 | B (25.00, 632,861.39) | 2.6 × 10–5 to 5.6 × 10–05 | ||
| FAHR (major) | 7.38 × 10–5 | B (25.00, 338,559.21) | 4.8 × 10–5 to 1.05 × 10–4 | ||
| Bacteria | 6.34 × 10–8 | B (25.00, 394,026,225.00) | 4.1 × 10–8 to 9.1 × 10–8 | ||
| HAV | 6.34 × 10–8 | B (25.00, 394,026,225.00) | 4.1 × 10–8 to 9.1 × 10–8 | ||
| HBV | 6.3 × 10–8 | B (25.00, 394,026,225.00) | 4.1 × 10–8 to 9.1 × 10–8 | ||
| HEV | 6.34 × 10–7 | B (25.00, 39,402,577.50) | 4.1 × 10–7 to 9.1 × 10–7 | ||
| Parvovirus | 6.34 × 10–8 | B (25.00, 394,026,225.00) | 4.1 × 10–8 to 9.1 × 10–8 | ||
| Proportion experiencing bleeding | Avatrombopag | < 40,000/µl | 0.044 | WinBUGS CODA | |
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.014 | ||||
| Avatrombopag | 40,000–< 50,000/µl | 0.021 | |||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.033 | ||||
| Proportion experiencing PVT | Avatrombopag | < 40,000/µl | 0.012 | ||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.015 | ||||
| Avatrombopag | 40,000–< 50,000/µl | 0.002 | |||
| Lusutrombopag | Confidential information has been removed | ||||
| Placebo | 0.014 | ||||
| Parameter varied in probabilistic sensitivity analysis | Base value (SE) | Distribution (α, β) | 95% CI | ||
|---|---|---|---|---|---|
| Utilities and disutilities | |||||
| Utility | |||||
| CLD82 | 0.54 (0.051) | B (51.86, 43.56) | 0.44 to 0.64 | ||
| CLD99 | 0.80 (0.007) | B (2372.01, 589.30) | 0.79 to 0.82 | ||
| Disutility | |||||
| Transfusion-related reaction, NICE83 | 0.10 (0.02) | B (22.50, 202.50) | 0.06 to 0.14 | ||
| PVT84 | 0.03 (0.01) | B (24.28, 812.79) | 0.02 to 0.04 | ||
| Major bleed84 | 0.40 (0.08) | B (15.08, 22.90) | 0.25 to 0.55 | ||
| Minor bleed84 | 0.12 (0.02) | B (21.95, 157.97) | 0.08 to 0.17 | ||
| Duration | |||||
| Transfusion-related reaction | 4.00 (0.80) | Γ (25.00, 0.16) | 2.59 to 5.20 | ||
| PVT | 1.00 (0.20) | Γ (25.00, 0.04) | 0.65 to 1.30 | ||
| Major bleed | 1.00 (0.20) | Γ (25.00, 0.04) | 0.65 to 1.30 | ||
| Minor bleed | 1.00 (0.20) | Γ (25.00, 0.04) | 0.65 to 1.30 | ||
| Proportion of major bleeds | 0.30 (0.06) | B (17.50, 40.83) | 0.19 to 0.42 | ||
| Proportion of patients with transfusion-related reaction | 0.00 (0.00) | B (25.00, 218826.45) | 0.00 to 0.00 | ||
| Disutility | |||||
| Transfusion-related acute lung injury | 0.40 (0.08) | B (15.00, 22.50) | 0.25 to 0.56 | ||
| HAV | 0.03 (0.01) | B (24.25, 784.08) | 0.02 to 0.04 | ||
| HBV | 0.16 (0.03) | B (21.00, 110.25) | 0.10 to 0.23 | ||
| HCV | 0.46 (0.09) | B (13.50, 15.85) | 0.29 to 0.64 | ||
| HIV | 0.50 (0.10) | B (12.50, 12.50) | 0.31 to 0.69 | ||
| Parvovirus B19 (P-B19) | 0.03 (0.01) | B (24.25, 784.08) | 0.02 to 0.04 | ||
| Prion disease | 0.00 (0.00) | B (0.00, 0.00) | 0.00 to 0.00 | ||
| Severe allergic reactions | 0.40 (0.08) | B (15.00, 22.50) | 0.25 to 0.56 | ||
| Costs | |||||
| Platelet transfusion | |||||
| Day case86 | £499.20 (£99.84) | Γ (25.00, 19.97) | £323.05 to £649.26 | ||
| Elective inpatient86 | £971.06 (£194.21) | Γ (25.00, 38.84) | £628.42 to £1262.97 | ||
| Initial83 | £57.72 (£11.54) | Γ (25.00, 2.31) | £37.35 to £75.07 | ||
| Units83 | £230.39 (£46.08) | Γ (25.00, 9.22) | £149.10 to £299.65 | ||
| Follow-up83 | £262.00 (£52.40) | Γ (25.00, 10.48) | £169.55 to £340.76 | ||
| Administration cost of first unit102 | £61.37 (£12.27) | Γ (25.00, 2.45) | £39.72 to £79.82 | ||
| Administration cost of subsequent units102 | £40.31 (£8.06) | Γ (25.00, 1.61) | £26.09 to £52.43 | ||
| Apheresis103 | £219.30 (£43.86) | Γ (25.00, 8.77) | £141.92 to £285.22 | ||
| Number of platelet transfusions prior to surgery | < 40,000 | Avatrombopag | 1.00 (£0.20) | Γ (25.00, 0.04) | 0.70 to 1.40 |
| Lusutrombopag | Confidential information has been removed | Γ (25.00, 0.04) | 0.73 to 1.46 | ||
| Placebo (all trials pooled) | 1.12 (0.22) | Γ (25.00, 0.04) | 0.73 to 1.46 | ||
| Placebo (avatrombopag trials pooled) | 1.12 (0.22) | Γ (25.00, 0.04) | 0.72 to 1.45 | ||
| Placebo (lusutrombopag trials pooled) | 1.12 (0.22) | Γ (25.00, 0.04) | 0.65 to 1.30 | ||
| 40,000–< 50,000 | Avatrombopag | 1.00 (0.20) | Γ (25.00, 0.04) | 0.65 to 1.30 | |
| Lusutrombopag | Confidential information has been removed | Γ (25.00, 0.04) | 0.72 to 1.44 | ||
| Placebo (all trials pooled) | 1.11 (0.22) | Γ (25.00, 0.05) | 0.74 to 1.48 | ||
| Placebo (avatrombopag trials pooled) | 1.06 (0.21) | Γ (25.00, 0.04) | 0.69 to 1.38 | ||
| Placebo (lusutrombopag trials pooled) | 1.14 (0.23) | Γ (25.00, 0.04) | 0.65 to 1.30 | ||
| Adverse event cost, PVT | £958.95 (£191.79) | Γ (25.00, 38.36) | £620.58 to £1247.22 | ||
| Study M0626 (proportion of patients)79 | |||||
| Percutaneous RFA79 | Confidential information has been removed | The frequency and unit costs of the surgeries from each trial are sampled using beta distribution for the proportions (using the event and non-event numbers) and gamma distribution for the unit cost of the surgeries, assuming a SE/mean ratio of 0.2 | |||
| Endoscopic variceal ligation79 | Confidential information has been removed | ||||
| Endoscopic injection sclerotherapy79 | Confidential information has been removed | ||||
| Transcatheter arterial chemoembolisation79 | Confidential information has been removed | ||||
| Liver biopsy79 | Confidential information has been removed | ||||
| Dental extraction79 | Confidential information has been removed | ||||
| Vascular catheterisation79 | Confidential information has been removed | ||||
| Argon plasma coagulation79 | Confidential information has been removed | ||||
| Percutaneous ethanol injection therapy79 | Confidential information has been removed | ||||
| Endoscopy with/without polypectomy/biopsy79 | Confidential information has been removed | ||||
| Percutaneous RFA/microwave coagulation therapy79 | Confidential information has been removed | ||||
| Paracentesis79 | Confidential information has been removed | ||||
| Other liver procedures79 | Confidential information has been removed | ||||
| Other gastrointestinal procedures79 | Confidential information has been removed | ||||
| Others79 | Confidential information has been removed | ||||
| L PLUS 1 (proportion of patients)39 | |||||
| Percutaneous RFA39 | Confidential information has been removed | ||||
| Endoscopic variceal ligation39 | Confidential information has been removed | ||||
| Endoscopic injection sclerotherapy39 | Confidential information has been removed | ||||
| Transcatheter arterial chemoembolisation39 | Confidential information has been removed | ||||
| Liver biopsy39 | Confidential information has been removed | ||||
| Dental extraction39 | Confidential information has been removed | ||||
| Vascular catheterisation39 | Confidential information has been removed | ||||
| Argon plasma coagulation39 | Confidential information has been removed | ||||
| Percutaneous ethanol injection therapy39 | Confidential information has been removed | ||||
| Endoscopy with/without polypectomy/biopsy39 | Confidential information has been removed | ||||
| Percutaneous RFA/microwave coagulation therapy39 | Confidential information has been removed | ||||
| Paracentesis39 | Confidential information has been removed | ||||
| Other liver procedures39 | Confidential information has been removed | ||||
| Other gastrointestinal procedures39 | Confidential information has been removed | ||||
| Others39 | Confidential information has been removed | ||||
| L-PLUS 2 (proportion of patients)54 | |||||
| Percutaneous RFA54 | Confidential information has been removed | ||||
| Endoscopic variceal ligation54 | Confidential information has been removed | ||||
| Endoscopic injection sclerotherapy54 | Confidential information has been removed | ||||
| Transcatheter arterial chemoembolisation54 | Confidential information has been removed | ||||
| Liver biopsy54 | Confidential information has been removed | ||||
| Dental extraction54 | Confidential information has been removed | ||||
| Vascular catheterisation54 | Confidential information has been removed | ||||
| Argon plasma coagulation54 | Confidential information has been removed | ||||
| Percutaneous ethanol injection therapy54 | Confidential information has been removed | ||||
| Endoscopy with/without polypectomy/biopsy54 | Confidential information has been removed | ||||
| Percutaneous RFA/microwave coagulation therapy54 | Confidential information has been removed | ||||
| Paracentesis54 | Confidential information has been removed | ||||
| Other liver procedures54 | Confidential information has been removed | ||||
| Other gastrointestinal procedures54 | Confidential information has been removed | ||||
| Others54 | Confidential information has been removed | ||||
| ADAPT (proportion of patients)37 | |||||
| Percutaneous RFA37 | Confidential information has been removed | ||||
| Endoscopic variceal ligation37 | Confidential information has been removed | ||||
| Endoscopic injection sclerotherapy37 | Confidential information has been removed | ||||
| Transcatheter arterial chemoembolisation37 | Confidential information has been removed | ||||
| Liver biopsy37 | Confidential information has been removed | ||||
| Dental extraction37 | Confidential information has been removed | ||||
| Vascular catheterisation37 | Confidential information has been removed | ||||
| Argon plasma coagulation37 | Confidential information has been removed | ||||
| Percutaneous ethanol injection therapy37 | Confidential information has been removed | ||||
| Endoscopy with/without polypectomy/biopsy37 | Confidential information has been removed | ||||
| Percutaneous RFA/microwave coagulation therapy37 | Confidential information has been removed | ||||
| Paracentesis37 | Confidential information has been removed | ||||
| Other liver procedures37 | Confidential information has been removed | ||||
| Other gastrointestinal procedures37 | Confidential information has been removed | ||||
| Cost (£) | |||||
| Percutaneous RFA | 2309.03 (461.81) | ||||
| Endoscopic variceal ligation | 4202.11 (840.42) | ||||
| Endoscopic injection sclerotherapy | 2410.75 (482.15) | ||||
| Transcatheter arterial chemoembolisation | 2921.50 (584.30) | ||||
| Liver biopsy | 1546.72 (309.34) | ||||
| Dental extraction | 680.04 (136.01) | ||||
| Vascular catheterisation | 1125.62 (225.12) | ||||
| Argon plasma coagulation | 4202.11 (840.42) | ||||
| Percutaneous ethanol injection therapy | 2921.50 (584.30) | ||||
| Endoscopy w/wo polypectomy/biopsy | 1213.27 (242.65) | ||||
| Percutaneous RFA/microwave coagulation therapy | 2309.03 (461.81) | ||||
| Paracentesis | 1090.43 (218.09) | ||||
| Other liver procedures | 2921.50 (584.30) | ||||
| Other gastrointestinal procedures | 4202.11 (840.42) | ||||
| Others | 2309.03 (461.81) | ||||
| Pneumonia | 2640.00 (527.93) | ||||
| FAHR (major) | 1134.00 (226.85) | ||||
| Bacteria | 2024.00 (404.79) | ||||
| HAV | 6488.00 (1297.60) | ||||
| HBV | 8971.00 (1794.20) | ||||
| HEV | 6488.00 (1297.60) | ||||
| Parvovirus | 1095.00 (219.00) | ||||
Appendix 7 Cost-effectiveness scenario analyses
Drug prices
Given that the AG does not have a price for avatrombopag, and given that when both treatments have such a small impact on total QALYs the costs become very important, some scenarios around the pricing of avatrombopag were thought to be of value. In this scenario analysis, the prices of avatrombopag were lowered, in increments of 10%, by 10–80% from the assumed price of (confidential information has been removed). The results in Table 38 show that these drug price reductions slowly reduce the incremental costs and ICER comparing avatrombopag with no TPO-RA. At a (confidential information has been removed) price reduction, avatrombopag 40 mg dominates no TPO-RA in the 40,000 < 50,000/µl subgroup and the ICER is under the NICE threshold for avatrombopag 60 mg in the < 40,000/µl subgroup.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug price | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Confidential information has been removed (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 561.00 | 0.0001 | 5,954,692.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 481.00 | 0.0001 | 5,105,486.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 401.00 | 0.0001 | 4,256,281.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 321.00 | 0.0001 | 3,407,075.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 241.00 | 0.0001 | 2,557,869.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 161.00 | 0.0001 | 1,708,664.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 81.00 | 0.0001 | 859,458.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 1.00 | 0.0001 | 10,252.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Drug price | Lusutrombopag | Avatrombopag 40 mg | Placebo | Lusutrombopag vs. placebo | Avatrombopag 40 mg vs. placebo | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Confidential information has been removed (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 553.00 | 0.0004 | 1,336,283.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 473.00 | 0.0004 | 1,143,006.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 393.00 | 0.0004 | 949,729.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 313.00 | 0.0004 | 756,452.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 233.00 | 0.0004 | 563,174.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 153.00 | 0.0004 | 369,897.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 73.00 | 0.0004 | 176,620.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | –7.00 | 0.0004 | Dominates |
Number of adult therapeutic doses per platelet transfusion
Given the uncertainty surrounding the number of ATDs per platelet transfusion, scenarios involving this variable are important. As shown in Table 39, the assumption of one ATD per transfusion results in the highest ICER, as this results in the lowest cost for platelet transfusion and therefore the biggest incremental cost difference between the treatments and no TPO-RA. The Shionogi base case of three ATDs per transfusion (equivalent to treating ATDs as the assumed units in the Shionogi model) provides the lowest ICER compared with no TPO-RA. However, none of the assumed numbers of ATDs results in a cost-effective option, with the ICER of £631,735 for avatrombopag 40 mg compared no TPO-RA being the lowest ICER observed among these scenarios.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of ATDs | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 611.00 | 0.0002 | 3,537,235.00 | 656.00 | 0.0001 | 6,962,585.00 |
| Confidential information has been removed (AG BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 440.00 | 0.0002 | 2,544,402.00 | 526.00 | 0.0001 | 5,585,808.00 |
| 3 (Sh BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 268.00 | 0.0002 | 1,551,568.00 | 397.00 | 0.0001 | 4,209,031.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Number of ATDs | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 643.00 | 0.0000 | 87,422,995,623.00 | 656.00 | 0.0004 | 1,584,466.00 |
| Confidential information has been removed (AG BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Confidential information has been removed | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 481.00 | 0.0000 | 65,449,720,055.00 | 459.00 | 0.0004 | 1,108,100.00 |
| 3 (Sh BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 320.00 | 0.0000 | 43,476,444,487.00 | 261.00 | 0.0004 | 631,735.00 |
Cost of platelet transfusion
The AG also adjusted the costs of platelet transfusion. The AG base-case cost of £313.83 was replaced by two values calculated by Shionogi in its model. The scenario price of £517.28, based on the HRG codes for single plasma exchange or other intravenous blood transfusion and the Shionogi base-case value of £812.61, assuming 3 units per transfusion, both resulted in lower ICERs than the AG base case (Table 40). However, none reduced the ICER sufficiently to be considered cost-effective, with the lowest ICER being £620,415 for avatrombopag 40 mg compared with lusutrombopag.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost of PT (£) | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 313.83 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 517.28 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 458.00 | 0.0002 | 2,649,449.00 | 540.00 | 0.0001 | 5,731,478.00 |
| 812.61 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 264.00 | 0.0002 | 1,527,976.00 | 393.00 | 0.0001 | 4,176,316.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Cost of PT (£) | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 313.83 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 517.28 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 498.00 | 0.0000 | 67,774,610,741.00 | 480.00 | 0.0004 | 1,158,502.00 |
| 812.61 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 316.00 | 0.0000 | 42,954,304,853.00 | 257.00 | 0.0004 | 620,415.00 |
Cost of rescue therapy
In the Shionogi model, it was assumed that, in clinical practice, rescue therapy would consist of an additional platelet transfusion. The AG noted that this assumption was not matched by the data presented by the companies, which showed that other methods of rescue were also used by clinicians. However, in the face of uncertainty surrounding what would actually be given in the UK, the AG cost of platelet transfusion of £313.83 was used in the base case. The AG clinical expert stated that he would consider giving a combination of platelet transfusion, clotting factors and tranexamic acid. The cost of this combination was used as an alternative, at a value of £370.73. The remaining alternative value was based on the Shionogi base-case cost of platelet transfusion of £812.61. As shown in Table 41, increasing the cost of rescue therapy decreased the ICER, but not sufficiently to make any of the comparisons with no TPO-RA cost-effective.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost of rescue (£) | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 313.83 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 370.73 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 586.00 | 0.0002 | 3,388,557.00 | 634.00 | 0.0001 | 6,728,367.00 |
| 812.61 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 540.00 | 0.0002 | 3,122,610.00 | 579.00 | 0.0001 | 6,141,783.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Cost of rescue (£) | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. No TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 313.83 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 370.73 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 619.00 | 0.0000 | 84,223,078,121.00 | 624.00 | 0.0004 | 1,507,873.00 |
| 812.61 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 581.00 | 0.0000 | 79,040,824,307.00 | 554.00 | 0.0004 | 1,339,450.00 |
Inclusion of grade 2 bleeding adverse events
The direction and magnitude of the impact on the ICER of the inclusion of grade 2 bleeding events varied depending on which treatment had the highest probability of bleeding, as can be seen in Table 42. In the < 40,000/µl subgroup, avatrombopag patients had the highest probability of bleeding. Including grade 2 events increased the ICER dramatically. A large impact on the ICER was also seen with lusutrombopag, which resulted in the highest bleeding probability in the 40,000–< 50,000/µl subgroup, with the inclusion of grade 2 events decreasing the ICER substantially. However, in the remaining two comparisons, the inclusion of grade 2 bleeding events had little impact on the ICER.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bleed events | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Grade 3+ (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Grade 2+ | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | Confidential information has been removed | 3.3625 | 592.00 | 0.0002 | 3,321,286.00 | 641.00 | 0.0000 | 14,285,918.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Bleed events | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Grade 3+ (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Grade 2+ | Confidential information has been removed | 3.3624 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | –0.0001 | Dominated | 633.00 | 0.0004 | 1,463,076.00 |
Probability of requiring platelet transfusion, estimated from international trials only
Using the probability of platelet transfusion estimated from international trials only does not have a substantial impact on the ICER, as shown in Table 43. The direction of the impact varies, with the ICER decreasing slightly for the comparison between avatrombopag 60 mg and no TPO-RA, but increasing for all other comparisons with no TPO-RA.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probability of PT | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| All trials (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| International trials | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 650.00 | 0.0002 | 3,821,767.00 | 640.00 | 0.0001 | 6,796,147.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Probability of PT | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| All trials (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| International trials | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 661.00 | –0.0000 | Dominated | 638.00 | 0.0004 | 1,561,315.00 |
Efficacy input from fixed-effects model
As can be seen in Table 44, ICERs are very similar between the fixed-effect and random-effects model for all comparisons.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost of elective invasive procedure | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. No TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Random effects (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Fixed effects | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 615.00 | 0.0002 | 3,580,458.00 | 640.00 | 0.0001 | 6,791,874.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Cost of elective invasive procedure | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. No TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| All trials (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Fixed effects | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 78,479,066,324.00 | 636.00 | 0.0004 | 1,553,910.00 |
Literature source for long-term Child–Pugh grade-specific mortality
Although using the UKMi data as the source of long-term mortality estimation substantially reduces the QALYs gained in all treatment groups, the incremental QALYs remain very similar, as shown in Table 45. Therefore, the choice of long-term mortality data source has little impact on the ICER.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLD mortality | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| D’Amico et al.78 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| UKMi | Confidential information has been removed | 2.2304 | Confidential information has been removed | 2.2303 | Confidential information has been removed | 2.2302 | 592.00 | 0.0002 | 3,484,979.00 | 641.00 | 0.0001 | 6,960,183.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| CLD mortality | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| D’Amico et al.78 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| UKMi | Confidential information has been removed | 2.2302 | Confidential information has been removed | 2.2306 | Confidential information has been removed | 2.2302 | 624.00 | –0.0000 | Dominated | 633.00 | 0.0004 | 1,543,029.00 |
Under reporting factor for serious hazards of transfusion platelet transfusion-specific mortality
To test the potential impact of under-reporting of deaths platelet transfusion on the model results, under-reporting factors of 10 and 50 (corresponding to incidences of platelet transfusion-related deaths of 0.00046% and 0.023%, respectively) were tested in scenario analyses. As can be seen in Table 46, these increases in platelet transfusion-related mortality did substantially decrease the ICER. However, the under-reporting factor of 50 was chosen as a particularly extreme value and it is unlikely that incidences would in fact be this high.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjustment | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Unadjusted (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 10 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | Confidential information has been removed | 3.3624 | 592.00 | 0.0003 | 2,329,181.00 | 641.00 | 0.0001 | 4,276,706.00 |
| 50 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3622 | Confidential information has been removed | 3.3618 | 592.00 | 0.0006 | 962,453.00 | 641.00 | 0.0004 | 1,613,356.00 |
| Platelet count 40,000/µl–< 50,000/µl Subgroup | ||||||||||||
| Adjustment | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Unadjusted (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 10 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3624 | 624.00 | 0.00007561 | 8,253,003.00 | 633.00 | 0.0005 | 1,243,840.00 |
| 50 | Confidential information has been removed | 3.3623 | Confidential information has been removed | 3.3628 | Confidential information has been removed | 3.3619 | 624.00 | 0.0004 | 1,515,978.00 | 633.00 | 0.0009 | 679,613.00 |
Alternative method for calculating surgery-related mortality
As can be seen in Table 47, using the alternative posterior distribution method for calculating pooled surgery-related mortality from the trial data increased QALYs gained by all groups by approximately 0.042 QALYs but did not change the incremental QALYs, as the same surgery-related mortality applies to all patients and all patients in the model are assumed to eventually receive their surgery. Therefore, the ICER remained unchanged.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery mortality | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Binomial likelihood with predictive dist (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Posterior dist | Confidential information has been removed | 3.4050 | Confidential information has been removed | 3.4049 | Confidential information has been removed | 3.4048 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Surgery mortality | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Binomial likelihood with predictive dist (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Posterior dist | Confidential information has been removed | 3.4048 | Confidential information has been removed | 3.4052 | Confidential information has been removed | 3.4048 | 624.00 | 0.0000 | 84,890,371,846.00 | 633.00 | 0.0004 | 1,529,560.00 |
Alternative literature source for baseline chronic liver disease utility
As shown in Table 48, using the Scalone et al. 99 baseline utility value of 0.801, compared with the base-case value of 0.544, increased the QALYs gained by all groups by approximately 1.5 QALYs and resulted in slightly lower ICERs in all comparisons with no TPO-RA. The biggest impact was seen for lusutrombopag compared with no TPO-RA in the 40,000–< 50,000/µl subgroup, with the ICER approximately halving; however, this could be expected as this is the comparison with by far the fewest incremental QALYs, and therefore an increase (even a small one) makes a large impact on the very large ICER.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Utility | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Sullivan et al.82 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| Scalone et al.99 | Confidential information has been removed | 4.9559 | Confidential information has been removed | 4.9558 | Confidential information has been removed | 4.9557 | 592.00 | 0.0002 | 3,340,250.00 | 641.00 | 0.0001 | 6,598,656.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Utility | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| Sullivan et al.82 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| Scalone et al.99 | Confidential information has been removed | 4.9557 | Confidential information has been removed | 4.9561 | Confidential information has been removed | 4.9557 | 624.00 | 0.0000 | 156,520,686.00 | 633.00 | 0.0004 | 1,511,287.00 |
Alternative literature source for bleeding disutility
The AG could not find any alternative literature sources for the disutility of major bleeds. Therefore, the base-case value was increased and decreased by 25%. The direction of the impact of changes to the bleeding disutility value on the ICER varied depending on which treatment had the highest probability of bleeding, as can be seen in Table 49. In the < 40,000/µl subgroup, avatrombopag patients had the highest probability of bleeding. Therefore, decreasing the disutility for a major bleed decreased the ICER. The same was seen for lusutrombopag, which resulted in the highest bleeding probability in the 40,000–< 50,000/µl subgroup. However, in the remaining two comparisons, increasing the disutility decreased the ICER. However, changes in the ICER were never large enough to change the cost-effectiveness decision.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disutility bleed | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.397 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 0.298 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,459,576.00 | 641.00 | 0.0001 | 5,755,569.00 |
| 0.496 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,386,800.00 | 641.00 | 0.0001 | 8,319,164.00 |
| Platelet count 40,000/µl–< 50,000/µl subgroup | ||||||||||||
| Disutility bleed | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.397 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 0.298 | Confidential information has been removed | 3.3626 | Confidential information has been removed | 3.3630 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 16,349,327.00 | 633.00 | 0.0004 | 1,554,120.00 |
| 0.496 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | Dominated | 633.00 | 0.0004 | 1,505,764.00 |
Alternative literature source for portal vein thrombosis disutility
The AG could not find any alternative literature sources for the disutility of PVT. Therefore, the base-case value was increased and decreased by 25%. In all cases, decreasing the disutility increased the ICER and vice versa. However, the impact was small for all comparisons, as shown in Table 50.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disutility PVT | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.029 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 0.022 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,429,543.00 | 641.00 | 0.0001 | 6,837,935.00 |
| 0.036 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,416,086.00 | 641.00 | 0.0001 | 6,770,198.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Disutility PVT | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.029 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 0.022 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3630 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 248,437,463.00 | 633.00 | 0.0004 | 1,494,367.00 |
| 0.036 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | Dominated | 633.00 | 0.0004 | 1,566,450.00 |
Alternative literature source for transfusion-related adverse event disutilities
Increasing the disutility from 0.1 to 0.17 decreased the ICER marginally in all cases, as can be seen in Table 51. However, the impact of the change was small in all cases.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disutility PT AEs | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.1 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 0.17 (van Eerd) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,415,869.00 | 641.00 | 0.0001 | 6,786,757.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Disutility PT AEs | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.1 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 0.17 (van Eerd) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 1,877,500,949.00 | 633.00 | 0.0004 | 1,528,052.00 |
Alternative values for planned elective inpatient procedure delay disutility and duration
The ICER is very sensitive to the choice of elective invasive procedure delay disutility and duration, as shown in Table 52. A 0 disutility results in dominated ICERs for avatrombopag 60 mg compared with no TPO-RA in the < 40,000/µl subgroup, dominated ICERs for both treatments compared with no TPO-RA in the 40,000–< 50,000/µl subgroup and an ICER > £30,000,000 for the remaining comparison with no TPO-RA in the < 40,000/µl subgroup. Doubling the disutility to 0.144 provides substantially lower ICERs, but they are still not low enough for the treatments to be considered cost-effective.
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elective invasive procedure delay disutility | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0 | Confidential information has been removed | 3.3631 | Confidential information has been removed | 3.3630 | Confidential information has been removed | 3.3630 | 592.00 | 0.0000 | 32,339,613.00 | 641.00 | –0.0001 | Dominated |
| 0.036, 4 weeks | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3628 | Confidential information has been removed | 3.3628 | 592.00 | 0.0001 | 6,190,414.00 | 641.00 | 0.0000 | 37,853,996.00 |
| 0.072, 4 weeks (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 0.144, 4 weeks | Confidential information has been removed | 3.3624 | Confidential information has been removed | 3.3624 | Confidential information has been removed | 3.3621 | 592.00 | 0.0003 | 1,807,028.00 | 641.00 | 0.0002 | 2,576,727.00 |
| 0.072, 6 weeks | Confidential information has been removed | 3.3626 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3623 | 592.00 | 0.0003 | 2,365,315.00 | 641.00 | 0.0002 | 3,737,872.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Elective invasive procedure delay disutility | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0 | Confidential information has been removed | 3.3628 | Confidential information has been removed | 3.3630 | Confidential information has been removed | 3.3630 | 624.00 | –0.0002 | Dominated | 633.00 | 0.0000 | Dominated |
| 0.036, 4 weeks | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3630 | Confidential information has been removed | 3.3628 | 624.00 | –0.0001 | Dominated | 633.00 | 0.0002 | 3,081,487.00 |
| 0.072, 4 weeks (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.0000 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 0.144, 4 weeks | Confidential information has been removed | 3.3622 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3621 | 624.00 | 0.0002 | 4,037,573.00 | 633.00 | 0.0008 | 762,014.00 |
| 0.072, 6 weeks | Confidential information has been removed | 3.3624 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3623 | 624.00 | 0.0001 | 8,074,763.00 | 633.00 | 0.0006 | 1,017,245.00 |
Cost of planned elective inpatient procedure cancellation
The addition of the sunk cost for elective invasive procedure cancellation of £566.05 assumed by Shionogi in its base-case model did not have a substantial impact on the results (Table 53).
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunk cost (£) | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.00 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 566.05 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 576.00 | 0.0002 | 3,331,101.00 | 625.00 | 0.0001 | 6,635,655.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Sunk cost (£) | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0.00 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.00000001 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 566.05 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 608.00 | 0.00000001 | 82,734,247,223.00 | 617.00 | 0.0004 | 1,491,268.00 |
Proportion of patient requiring platelet transfusion hospitalised the day before planned elective inpatient procedure
In this scenario the AG tested the assumption that a proportion of those patients requiring platelet transfusion to raise their platelet count prior to surgery would be hospitalised the day before surgery to receive the transfusion. The cost of this extra day was taken from the NHS Reference Costs 2017/18,86 which provided a cost of £431.11 for an excess inpatient hospital day. This cost was multiplied by the relevant proportion of patients in each scenario and added to the elective invasive procedure costs of those patients who received platelet transfusion prior to elective invasive procedure in each treatment arm. The results show that this scenario does not have a substantial impact on results, even when an extra day of hospitalisation is included for all patients who receive platelet transfusion (Table 54).
| Platelet count < 40,000/µl subgroup | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion pre-hospitalised for PT (%) | Lusutrombopag | Avatrombopag 60 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 60 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0 (BC) | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 592.00 | 0.0002 | 3,422,801.00 | 641.00 | 0.0001 | 6,803,898.00 |
| 25 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 542.00 | 0.0002 | 3,133,602.00 | 612.00 | 0.0001 | 6,500,260.00 |
| 50 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 492.00 | 0.0002 | 2,844,403.00 | 584.00 | 0.0001 | 6,196,623.00 |
| 100 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3627 | Confidential information has been removed | 3.3626 | 392.00 | 0.0002 | 2,266,004.00 | 527.00 | 0.0001 | 5,589,349.00 |
| Platelet count 40,000–< 50,000/µl subgroup | ||||||||||||
| Proportion re-hospitalised for PT (%) | Lusutrombopag | Avatrombopag 40 mg | No TPO-RA | Lusutrombopag vs. no TPO-RA | Avatrombopag 40 mg vs. no TPO-RA | |||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| 0 (BC) | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 624.00 | 0.00000001 | 84,890,361,589.00 | 633.00 | 0.0004 | 1,529,560.00 |
| 25 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 576.00 | 0.00000001 | 78,347,697,323.00 | 578.00 | 0.0004 | 1,396,493.00 |
| 50 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 528.00 | 0.00000001 | 71,805,033,057.00 | 523.00 | 0.0004 | 1,263,425.00 |
| 100 | Confidential information has been removed | 3.3625 | Confidential information has been removed | 3.3629 | Confidential information has been removed | 3.3625 | 432.00 | 0.00000001 | 58,719,704,526.00 | 413.00 | 0.0004 | 997,291.00 |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AG
- assessment group
- ATD
- adult therapeutic dose
- CC
- complication
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CLD
- chronic liver disease
- CRD
- Centre for Reviews and Dissemination
- CSR
- clinical study report
- EMA
- European Medicines Agency
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FAHR
- febrile, allergic, hypotensive reactions
- FDA
- Food and Drug Administration
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICD-9-CM
- International Classification of Diseases, Ninth Edition, Clinical Modification
- ICER
- incremental cost-effectiveness ratio
- JAPIC
- Japic Clinical Trials Information
- KSR
- Kleijnen Systematic Reviews
- L-PLUS
- Lusutrombopag for the Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures
- L-PLUS 2
- Lusutrombopag for the Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures 2
- MCMC
- Markov chain Monte Carlo
- MeSH
- medical subject heading
- NHSBT
- NHS Blood and Transplant
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PVT
- portal vein thrombosis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SE
- standard error
- SHOT
- serious hazards of transfusion
- TAD
- transfusion-associated dyspnoea
- TPO-RA
- thrombopoietin receptor agonist
- UKMi
- UK Medicines Information
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential removed and replaced by the statement ‘confidential information (or) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for and research are based on all the considered in the original full NICE report.
