Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/45/01. The contractual start date was in December 2016. The draft report began editorial review in April 2019 and was accepted for publication in October 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Van den Bruel et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Acute infections in children are one of the most common problems in general practice and are associated with considerable burden on NHS resources. Nearly 40% of parents with children aged 6–17 months consult a health-care professional when their child has a high temperature. 1 In the UK, acute infections result in four consultations per person-year in children aged < 1 year, and 1.3 consultations per person-year in children aged 1–15 years. 2 Febrile illness accounts for 20% of all visits to the paediatric emergency department. 3
Guidelines recommend the measurement of temperature in each child presenting with fever symptoms using either electronic axillary thermometers or infrared tympanic thermometers in children aged ≥ 4 weeks. 4 However, axillary thermometers require health-care professionals to undress the child and hold the thermometer in the axilla for at least 30 seconds. 5 Infrared tympanic thermometers are easier to use, but may be inaccurate owing to ear wax or insufficient straightening of the ear canal. 6 Non-contact infrared thermometers (NCITs) convert measurements of the intensity of infrared radiation emitted by the body into temperature readings. The non-contact approach offers potential advantages, including reduced child discomfort or distress, measurement without interrupting sleep, minimal risk of cross-infection and no requirement for additional disposable probe covers. 7
This study was set up in response to a primary care diagnostic technology update by our group, as part of our Horizon Scan programme within the National Institute for Health Research (NIHR) Diagnostic Evidence Cooperative Oxford. The results of the review were published in November 2013 as an open-access publication on our website8 and published in the British Journal of General Practice by Wang et al. 7 All Horizon Scans are conducted according to standardised methods. The search strategy includes systematic searches of MEDLINE, EMBASE, MEDION, The Cochrane Library, TRIP and NHS Evidence, using tailored search strategies for each topic and limiting to English-language publications. For guidelines, we searched the National Institute for Health and Care Excellence (NICE)’s, the Scottish Intercollegiate Guidelines Network (SIGN)’s and the relevant professional bodies’ websites. We searched for supplementary information on manufacturer/trade websites and through web search engines. When assessing publications for inclusion in reports, we used critical appraisal tools developed by the Centre for Evidence Based Medicine, which are available at www.cebm.net/index.aspx?o=1157 (accessed December 2019). Reports are reviewed and edited by at least two general practitioners (GPs) and a pathology commissioner, and health economics sections are written by a health economist.
The report on non-contact thermometers summarised evidence on seven different non-contact thermometers in different price ranges. The Thermofocus (Tecnimed, Varese, Italy) was the most extensively studied thermometer (five out of six studies) for agreement with mercury-in-glass,9,10 electronic rectal,11,12 electronic axillary,12,13 electronic oral,12 infrared tympanic13 and temporal artery thermometers. 13 Some studies had more than one comparison. Agreement was found to be moderate to low. In addition, four studies were identified that assessed non-contact thermometer accuracy in detecting fever. Fever was defined as a temperature reading of ≥ 38 °C, measured by a mercury-in-glass thermometer axillary9 or rectally,10 a tympanic thermometer14 or an electronic thermometer used in the axilla, orally or rectally. 12 These studies found that non-contact thermometers had high sensitivity and specificity for detecting fever, with sensitivities ranging from 77% to 97% and specificities ranging from 75% to 97%. There was also evidence based on one study that children found the non-contact thermometers more acceptable than a mercury-in-glass axillary thermometer.
To our knowledge, there have been no other systematic reviews on non-contact thermometers apart from our Horizon Scan review. 7 However, there are several systematic reviews on other thermometer types, all highlighting the poor performance of electronic axillary and infrared tympanic thermometers compared with core body temperature measurements. 6,16,17 Compared with central thermometry, which ideally is measured via a pulmonary artery catheter, measurements using a tympanic membrane, temporal artery and axillary thermometer were all found to have limits of agreement wider than 0.5 °C in children in a 2015 systematic review including 75 studies. 16 In contrast, rectal measurements were found to have much lower limits of agreement of –0.24 to 0.04 °C.
Specifically comparing rectal with axillary readings using the same device, Craig et al. 17 found in their systematic review of 37 papers with > 5000 children that the mean axillary temperature was always lower than the mean rectal temperature, and the difference was larger for electronic thermometers than for mercury-in-glass thermometers [i.e. the pooled mean difference of rectal minus axillary temperature was 0.25 °C (95% limits of agreement –0.15 to 0.65 °C) for mercury thermometers and 0.85 °C (95% limits of agreement –0.19 to 1.90 °C) for electronic thermometers]. 17 In another systematic review6 including 44 papers with almost 6000 children, the same authors compared tympanic thermometers with rectal temperature using mercury, electronic or indwelling probe thermometers. The pooled mean difference between rectal and tympanic readings was 0.29 °C (95% limits of agreement –0.74 to 1.32 °C).
Reports of agreement between NCITs and conventional thermometers have been variable. Only one study has reported comparable data for the Thermofocus brand compared with an axillary measurement using a mercury-in-glass thermometer department9 recruiting 251 children aged between 1 month and 18 months in emergency clinics, paediatric outpatient, and one primary care centre. They found an overall mean difference of 0.07 °C and limits of agreement of –0.62 °C (95% CI –0.67 to –0.47 °C) and 0.76 °C (95% CI 0.61 to 0.91 °C).
However, comparisons with infrared tympanic thermometers reported larger mean differences of –0.38 °C [95% confidence interval (CI) –1.47 to 0.70 °C]18 and 2.34 °C (95% CI 0.26 to 4.42 °C). 14 Comparisons with rectal thermometry have also yielded variable results, with mean differences reported of 0.02910 and 0.34 °C. 19 Different devices have been shown to perform differently: closer agreement is reported with the Thermofocus than with the Beurer. 20
Finally, although NCITs are mostly reported to have high sensitivity and specificity in detecting a fever of ≥ 38 °C using conventional thermometry,9,10,12,14 sensitivity was estimated as only 27%19 and 12%20 in two studies.
In conclusion, agreement between non-contact thermometers and other methods of thermometry has been found to be variable and highly dependent on the thermometer with which the NCITs are compared. In addition to the lack of clear conclusions from existing studies, there is a lack of generalisability of these data to primary care settings as most previous studies were conducted in paediatric inpatient populations14,19,20 or mixed hospital ambulatory care and ward settings. 10,13 Furthermore, NCITs have been mainly compared with thermometers that are not currently recommended for use in children, including rectal10,11,19,20 and mercury-in-glass axillary thermometers. 9
Understanding the performance of NCITs in a primary care paediatric population compared with infrared tympanic and electronic axillary thermometers could allow introduction of this new technology into routine practice. We evaluated the agreement between two NCIT models at different price points versus electronic axillary and infrared tympanic thermometers in children who present with acute illness in primary care.
Chapter 2 Methods
This was a cross-sectional method agreement study with a nested qualitative study. The study ran from April 2017 to August 2018.
Children aged up to 5 years with an acute illness of a maximum of 14 days presenting to a general practice or an out-of-hours service were eligible for inclusion. Children for whom acute trauma was the main reason for presentation, who were clinically unstable, who had already been enrolled in the study or whose parents were unable to understand trial material in English were excluded from the study.
The study ran in nine general practices and one out-of-hours centre in Oxfordshire (UK). All potentially eligible children and their parents were consecutively approached by a research assistant from the study team in the waiting room for possible recruitment to the study. There were 11 research assistants involved in the study, all of whom had been trained in study procedures by the research team, which included an off-site explanation and dry run of study procedures, and on-site shadowing. Parents were handed patient information sheets to explain the goal of the study, the risks and the procedures. After verbal informed consent was obtained, temperature measurements were conducted either prior to or after the child’s appointment with the GP. The research assistant recorded demographic information (including household composition, parental age and ethnicity) and baseline information (including prior fever medication use, parental impression of fever and fever duration) for each child.
The child had their temperature measured using four different thermometers:
-
electronic axillary – Welch Allyn SureTemp (Welch Allyn®, USA)
-
infrared tympanic – Braun Thermoscan (Braun GmbH, Kronberg, Germany)
-
NCIT 1 – Thermofocus 0800 (Tecnimed Srl, Varese, Italy)
-
NCIT 2 – Firhealth Forehead Thermometer (Firhealth, Shenzhen, China).
The Thermofocus NCIT was included as it was the most extensively evaluated thermometer in other settings. The Firhealth device was included as an example of a cheaper NCIT that was easily available for purchase. At the time of writing this report, the Thermofocus was priced at around £80.00 and the Firhealth at around £30.00. The Thermofocus 0800H5 by Thermofocus is a certified medical device, Food and Drug Administration (FDA) approved and Conformité Européenne (CE) marked. It is a NCIT that measures body temperature by pointing the thermometer at the centre of the forehead. The optimal distance between the forehead and the thermometer is determined by a light-emitting diode (LED) system that emits two light beams. As the thermometer is moved closer to the forehead, at the right distance (approximately 3 cm) the two beams converge to a single red dot. Releasing the button until the lights flash will result in a temperature reading. A manual calibration system is provided with the thermometer to improve accurate readings. No extensive training is needed to operate the device and a comprehensive user manual is provided with the device.
The Firhealth Forehead Thermometer by Firhealth is a FDA-approved, CE-marked NCIT that works in essentially the same way as the Thermofocus. To measure temperature, the thermometer probe is pointed at the forehead at a distance of 1–5 cm. After pressing the ‘Measure’ button, the device bleeps and displays the temperature reading on the liquid-crystal display (LCD) screen.
The comparator thermometers (i.e. the electronic axillary and infrared tympanic thermometer) were chosen because these are recommended by NICE for use in acutely ill children. Children < 4 weeks of age did not have the tympanic thermometer measurement as per NICE recommendation; as a result, we opted for the electronic axillary thermometer for our primary outcome. The order in which the thermometers were used was randomised by our statistician prior to the study start for each participant (using a random number generator: www.random.org).
Measurements were performed consecutively in the shortest time frame possible, and no medication or drinks were administered between measurements. Once the four primary measurements were complete, a second measurement was taken with each NCIT to evaluate reproducibility. We also recorded failed measurements due to lack of co-operation of the child after three attempts, mechanical issues (operational or technological failure) and clinically implausible readings (based on researcher’s assessment).
Children’s reaction to the different measurements was recorded by the Patient Discomfort Scale21 as assessed by their parent(s). Children aged 4 or 5 years also completed the Wong–Baker FACES® Pain Rating Scale (Wong–Baker FACES® Foundation, Oklahoma City, OK, USA), which scores pain from 0 to 10 in 2-point increments. 22 Parents were asked to score the acceptability of each thermometer on a visual analogue scale by indicating their acceptability of each thermometer on a scale of 0–10 cm, and rank the thermometers by preference. To avoid bias, parents and children were blinded to the temperature measurements until they rated their acceptability.
Sample size
The sample size calculation was based on the desired accuracy of the limits of agreement. 23 Assuming an accuracy of ±0.075 °C for the 95% CIs of the limits of agreement (and the standard deviation of the agreement between temperatures measured by non-contact and electronic axillary thermometers would be 0.5 °C, as documented in previous studies9–12,14), a minimum sample size of 533 participants would have been required. We revised this sample size on 14 May 2018. Based on the data already available, the standard deviation for our primary analysis (Thermofocus non-contact thermometer vs. electronic axillary thermometer) was 0.65 °C. Using this standard deviation, the original sample size of 533 children would give us 0.10 °C accuracy for each limit of agreement (rather than 0.075 °C as anticipated). A reduced sample size of 400 children would give us 0.11 °C accuracy. Considering that thermometers measure temperatures only to the closest 0.1 °C, we felt that this would be sufficient as the rounding would make the two estimates equivalent. This change was discussed and approved by the funder. Secondary outcomes of agreement between the other thermometer types have been estimated with the same precision.
Protocol changes
Other than the change in sample size, there have been no changes to the protocol after the start of patient recruitment.
Analyses
Statistical methods focus on the agreement between thermometers, the accuracy of detecting fever and failure rates. All children contributed data to each analysis, when available.
Our primary outcome is the agreement between the Thermofocus NCIT and the electronic axillary thermometer. Analyses of agreement were conducted based on Bland–Altman plots, which provide an indication of bias and limits of agreement between the measurements. Exact 95% CIs around this estimate have been calculated.
Diagnostic accuracy for detecting fever (temperature of ≥ 38 °C measured by the electronic axillary thermometer) was analysed by calculating sensitivity, specificity, predictive values and likelihood ratios, with 95% CIs. Other analyses, such as failure rates, are reported as proportions.
The scores on the visual analogue scale and the Patient Discomfort Scale have been analysed using non-parametric techniques resulting in median acceptability [and interquartile ranges (IQRs)] for each thermometer. Thermometer preference was analysed via chi-squared goodness-of-fit testing.
Qualitative data collection and analysis
Recruitment
Parents were purposively sampled from those consenting to contact to achieve maximum variation in gender, age of parent, age of child, ethnicity and number of other children in the household. Recruitment continued until the research team agreed that data saturation had been achieved and sufficient explanation for the categories generated was reached.
Data collection
Interviews were conducted by telephone (n = 20) or face to face (n = 1), according to participant preference, from May 2017 to June 2018. All participants gave written or recorded verbal informed consent prior to the interview.
Interviews were conducted separately by two researchers trained in qualitative methodology; Elizabeth Morris (a female clinical researcher and salaried GP) and Fatene Abakar Ismail (a female research assistant). Continuity was ensured by regular discussion between researchers, and review of transcripts and topic guides by the team (EM, FAI, GHa and MG).
Interviews were semistructured and followed a flexible topic guide developed by the research team and patient and public involvement (PPI) panel. Content of the topic guide was informed by the primary aims of the study, the existing literature on parents’ experiences of childhood fever24–28 and the expertise of the research team. The topic guide was reviewed iteratively by the research team and evolved in response to emerging themes and ongoing PPI. In particular, the attribute of ‘accuracy’ emerged as an early theme and was incorporated into the topic guide to allow further detailed exploration. Interviews lasted 10–15 minutes, on average, and were audio-recorded, transcribed verbatim by a transcription company and checked against the original recording by the research team.
The study was approved by the South Central – Berkshire Research Ethics Committee (reference 17/SC/0068).
Data analysis
Data analysis followed a thematic approach. Following transcription, the researchers undertook familiarisation with the data, open coding and subsequent inductive reasoning to identify salient categories and relationships between emerging themes derived from the data. Data and codes were then checked by two researchers (EM and MG), with the assistance of NVivo (version 11; QSR International, Warrington, UK) qualitative data analysis software. Interpretation of the codes and themes was developed in discussion with the wider research team.
Chapter 3 Results
We recruited 401 children with a median age of 1.62 years (IQR 0.79–3.39 years), 203 (50.62%) of whom were boys. Most children were of white British ethnicity (69.83%). Approximately 30% of children were feverish at the time of inclusion and 33% were given fever medication in the previous 6 hours. Patient characteristics are listed in Table 1.
| Variable | Summary statistic |
|---|---|
| Age (years), median (IQR) | 1.62 (0.79–3.39) |
| Gender (boys), n (%) | 203 (50.62) |
| Ethnicity, n (%) | |
| White British | 280 (69.83) |
| White other | 38 (9.48) |
| Mixed | 27 (6.73) |
| Pakistani | 21 (5.24) |
| Other Asian | 11 (2.74) |
| African | 8 (2.00) |
| Indian | 5 (1.25) |
| Chinese | 5 (1.25) |
| Bangladeshi | 4 (1.00) |
| Caribbean | 1 (0.25) |
| Black British | 1 (0.25) |
| Mother’s age (years), median (IQR) | 32 (29–36) |
| Number of siblings, median (IQR) | 1 (0–1) |
| Parent believed child to be febrile at point of assessment, n (%) | 119 (29.75) |
| Fever medication in past 6 hours, n (%) | 134 (33.50) |
| Fever duration (days), median (IQR) | 1.5 (0.5–3) |
| Illness duration (days), median (IQR) | 3 (2–7) |
| Setting, n (%) | |
| Out-of-hours primary care | 34 (8.48) |
| In-hours primary care | 367 (91.52) |
There were 396 temperature readings with the Thermofocus non-contact thermometer (first measurement), 399 for the Firhealth non-contact thermometer (first measurement), 376 for the electronic axillary thermometer and 390 for the tympanic thermometer. Second measurements with the Thermofocus and Firhealth non-contact thermometers resulted in 395 and 397 readings, respectively.
The Bland–Altman plot for our primary outcome, which is the agreement between the Thermofocus non-contact thermometer (first measurement) and the electronic axillary thermometer, is presented in Figure 1. The mean difference between axillary and non-contact was –0.14 °C (95% CI –0.21 to –0.06 °C), with the lower limit of agreement being –1.57 °C (95% CI –1.69 to –1.44 °C) and the upper limit being 1.29 °C (95% CI 1.16 to 1.42 °C). This means that in 95 out of 100 cases we would expect the difference between the axillary and the non-contact thermometer to range between 1.57 °C lower and 1.29 °C higher than the average of the non-contact and axillary measurement of temperature.
FIGURE 1.
Bland–Altman plot for agreement between the Thermofocus non-contact thermometer and the electronic axillary thermometer. Reproduced with permission from Hayward et al. 15

The mean difference between the second non-contact thermometer (Firhealth, first measurement) and the electronic axillary thermometer was –0.16 °C (95% CI –0.23 to –0.09 °C); the lower limit of agreement was –1.54 °C (95% CI –1.66 to –1.41 °C) and the upper limit was 1.22 °C (95% CI 1.10 to 1.34 °C) (Figure 2).
FIGURE 2.
Bland–Altman plot for agreement between the Firhealth non-contact thermometer and the electronic axillary thermometer. Reproduced with permission from Hayward et al. 15

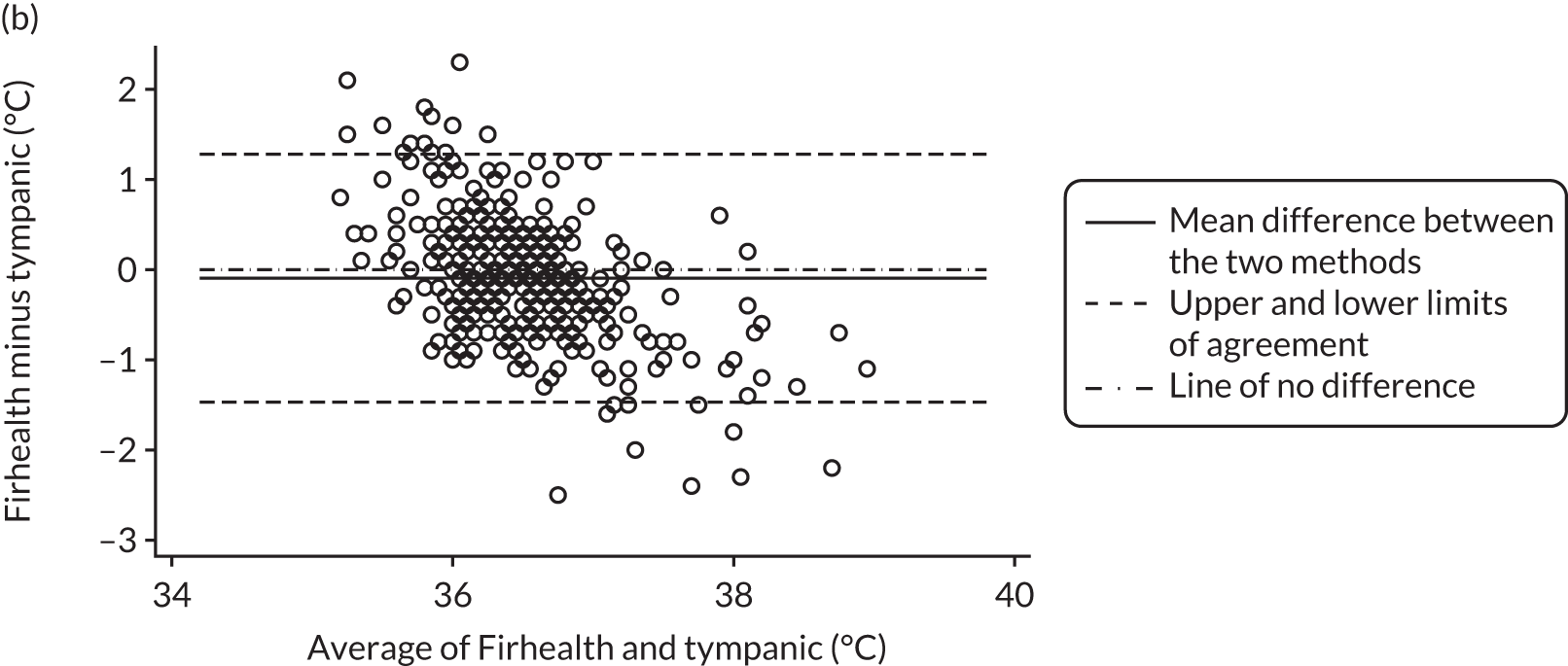
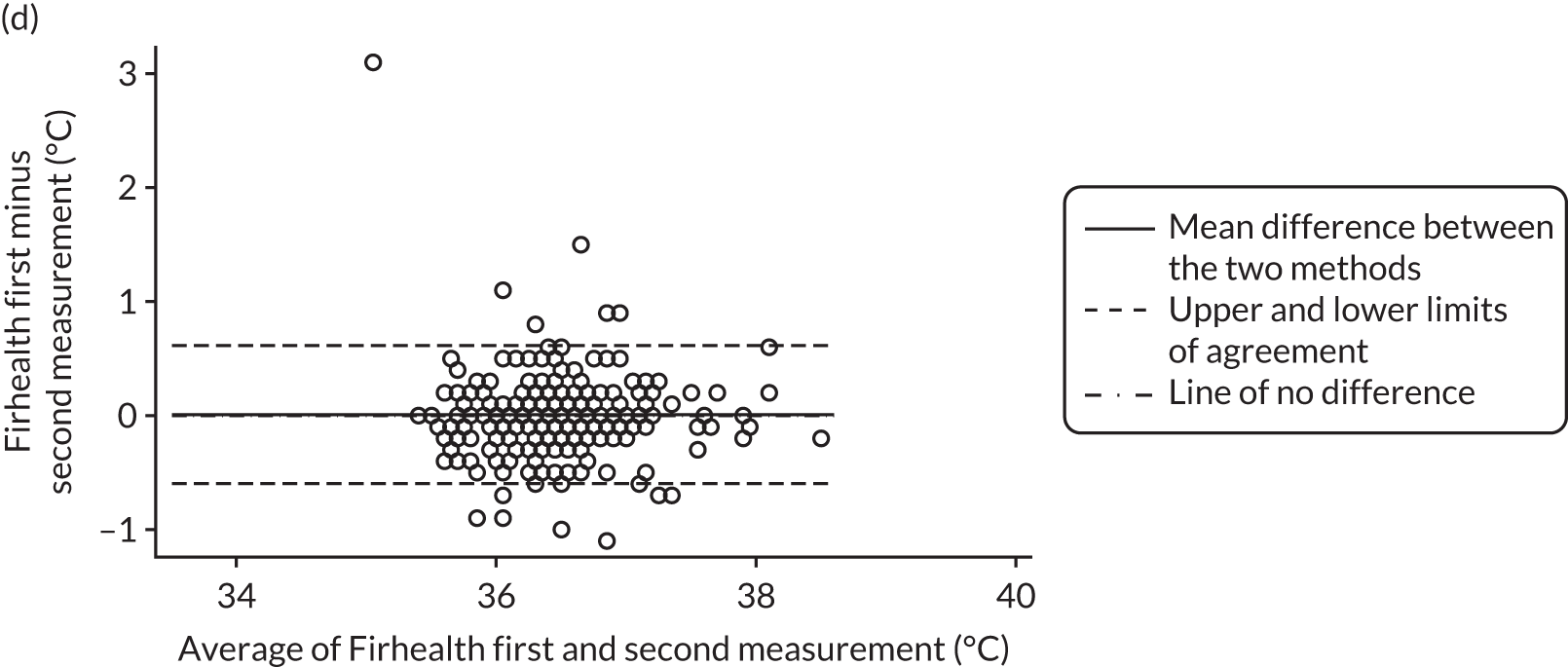
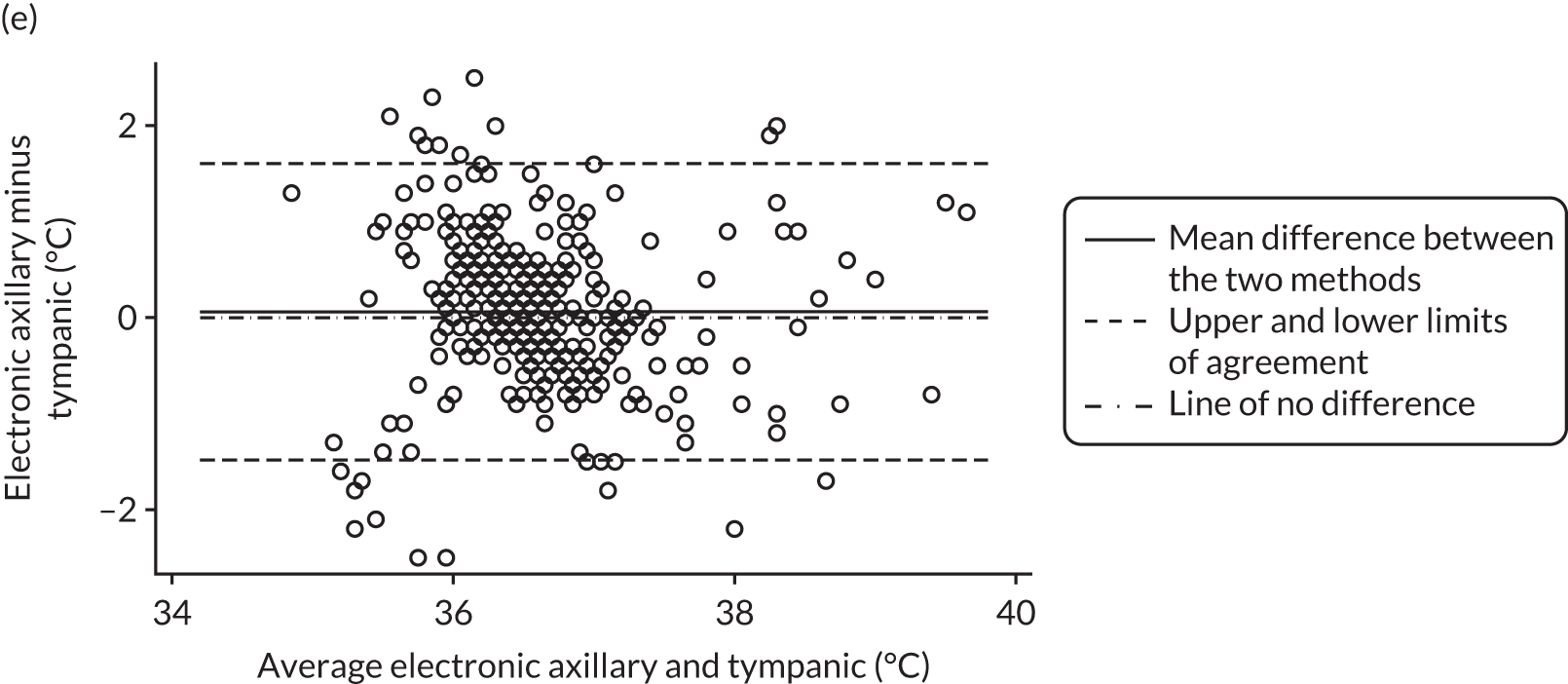
All method comparison results, including non-contact versus tympanic and non-contact first and second measurements, are summarised in Table 2. Accompanying plots are available in Appendix 2. When assessed against the tympanic thermometer, agreement was similarly dependent on average temperature.
| Comparison | Mean difference and 95% CI (°C) | Lower limit of agreement and 95% CI (°C) | Upper limit of agreement and 95% CI (°C) |
|---|---|---|---|
| Thermofocus NCIT minus electronic axillary, n = 371 | –0.14 (–0.21 to –0.06) | –1.57 (–1.69 to –1.44) | 1.29 (1.16 to 1.42) |
| Firhealth NCIT minus electronic axillary, n = 374 | –0.16 (–0.23 to –0.09) | –1.54 (–1.66 to –1.41) | 1.22 (1.10 to 1.34) |
| Thermofocus NCIT minus tympanic, n = 384 | –0.10 (–0.17 to –0.03) | –1.55 (–1.68 to –1.42) | 1.35 (1.22 to 1.48) |
| Firhealth NCIT minus tympanic, n = 387 | –0.10 (–0.17 to –0.03) | –1.47 (–1.59 to –1.35) | 1.28 (1.16 to 1.40) |
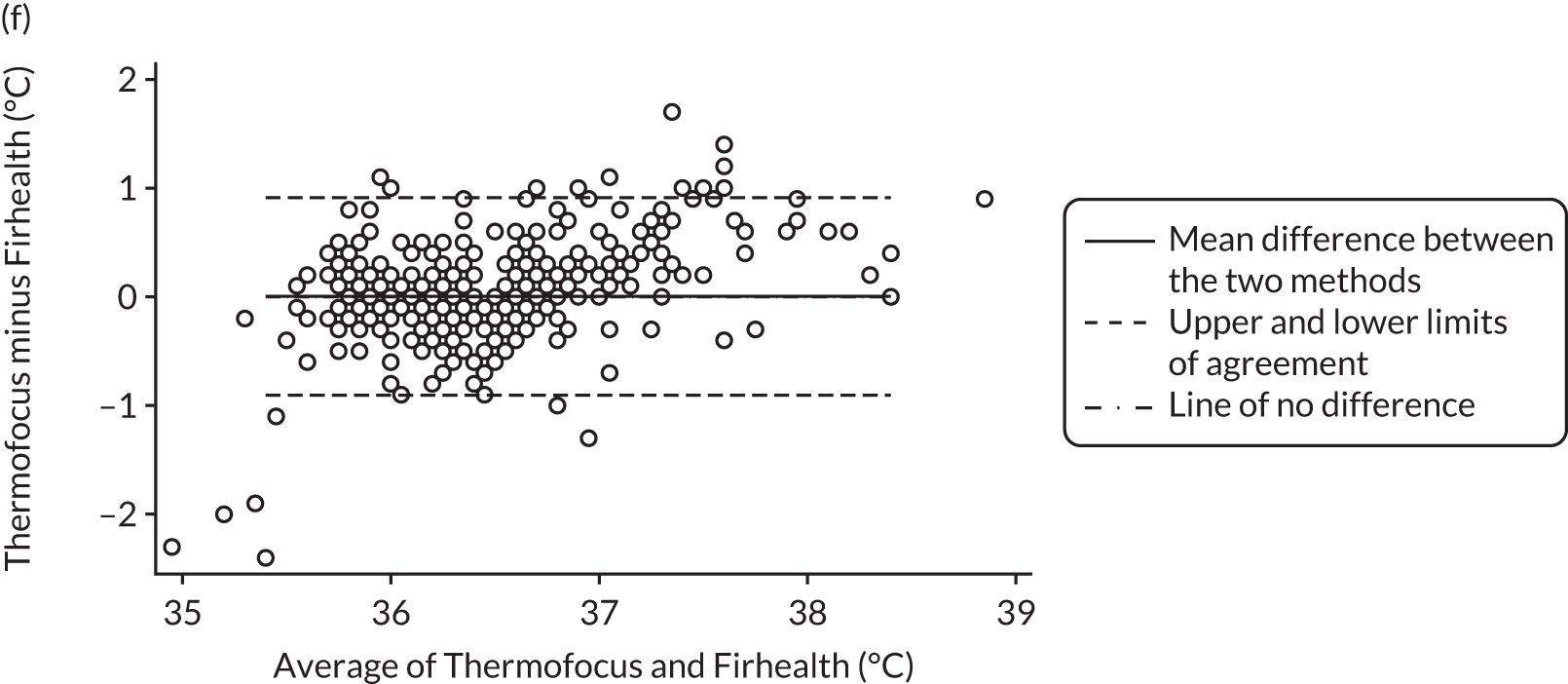
| Thermofocus NCIT minus Firhealth NCIT, n = 395 | 0.00 (–0.04 to 0.05) | –0.90 (–0.98 to –0.82) | 0.91 (0.83 to 0.99) |
| Electronic axillary minus infrared tympanic, n = 365 | 0.06 (–0.02 to 0.14) | –1.49 (–1.63 to –1.34) | 1.61 (1.47 to 1.75) |
| Thermofocus NCIT first minus second reading, n = 395 | –0.04 (–0.07 to –0.01) | –0.56 (–0.60 to –0.51) | 0.47 (0.43 to 0.52) |
| Firhealth non-contact first minus second reading, n = 396 | 0.01 (–0.02 to 0.04) | –0.60 (–0.65 to –0.54) | 0.61 (0.56 to 0.67) |
We calculated accuracy of non-contact thermometers for diagnosing fever, defined as a temperature of ≥ 38 °C by electronic axillary measurement. Prevalence of fever thus defined was 4.26% (95% CI 2.45% to 6.82%). Sensitivity and specificity for the Thermofocus non-contact thermometer were 66.7% (95% CI 38.4% to 88.2%) and 98.0% (95% CI 96.0% to 99.2%), respectively. For the Firhealth thermometer, sensitivity was 12.5% (95% CI 1.6% to 38.3%) and specificity was 99.4% (95% CI 98.0% to 99.9%). The sensitivity of the other standard thermometer, the tympanic thermometer, was moderate (62.5%, 95% CI 35.4% to 84.8%). Table 3 shows further details.
| Comparison | Thermofocus | Firhealth | Tympanic |
|---|---|---|---|
| Sensitivity, % (95% CI) | 66.7 (38.4 to 88.2) | 12.5 (1.6 to 38.3) | 62.5 (35.4 to 84.8) |
| Specificity, % (95% CI) | 98.0 (96.0 to 99.2) | 99.4 (98.0 to 99.9) | 96.0 (93.4 to 97.8) |
| Positive predictive value, % (95% CI) | 58.8 (32.9 to 81.6) | 50.0 (6.8 to 93.2) | 41.7 (22.1 to 63.4) |
| Negative predictive value, % (95% CI) | 98.6 (96.7 to 99.5) | 96.2 (93.7 to 97.9) | 98.2 (96.2 to 99.4) |
| Likelihood ratio + (95% CI) | 33.9 (15.0 to 76.7) | 22.4 (3.4 to 148.8) | 15.6 (8.2 to 29.5) |
| Likelihood ratio – (95% CI) | 0.34 (0.17 to 0.70) | 0.88 (0.73 to 1.06) | 0.39 (0.21 to 0.74) |
| Absolute numbers (true positive, false positive, false negative, true negative) | 10, 5, 7, 349 | 2, 14, 2, 356 | 10, 6, 14, 335 |
The number of attempts for each thermometer that were required to obtain a valid reading and the number of failed readings are listed in Table 4.
| Comparison | Thermofocus non-contact, n (%) | Firhealth non-contact, n (%) | Electronic axillary, n (%) | Tympanic, n (%) |
|---|---|---|---|---|
| One attempt required | 382 (95.3) | 390 (97.3) | 363 (90.5) | 364 (90.8) |
| Two attempts required | 10 (2.5) | 8 (2.0) | 11 (2.7) | 15 (3.7) |
| Three attempts required | 4 (1.0) | 1 (0.2) | 2 (0.5) | 9 (2.2) |
| No reading | ||||
| Technical error: thermometer not activating | 3 (0.8) | 1 (0.2) | 0 (0.0) | 0 (0.0) |
| Technical error: other | 1 (0.2) | 0 (0.0) | 7 (1.7) | 3 (0.7) |
| Lack of co-operation of the child | 1 (0.2) | 0 (0.0) | 16 (4.0) | 5 (1.2) |
| Reason not specified | 0 (0.0) | 1 (0.2) | 2 (0.5) | 0 (0.0) |
| Thermometer unsuitable (child aged < 4 weeks) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.2) |
| Total | 401 | 401 | 401 | 401 |
Acceptability of the thermometers
Children (n = 69) who were aged 4 or 5 years completed the Wong–Baker FACES® Pain Rating Scale. The median score was 0 (IQR 0–0) for both NCITs, and 0 (IQR 0–2) for the electronic axillary and tympanic thermometers.
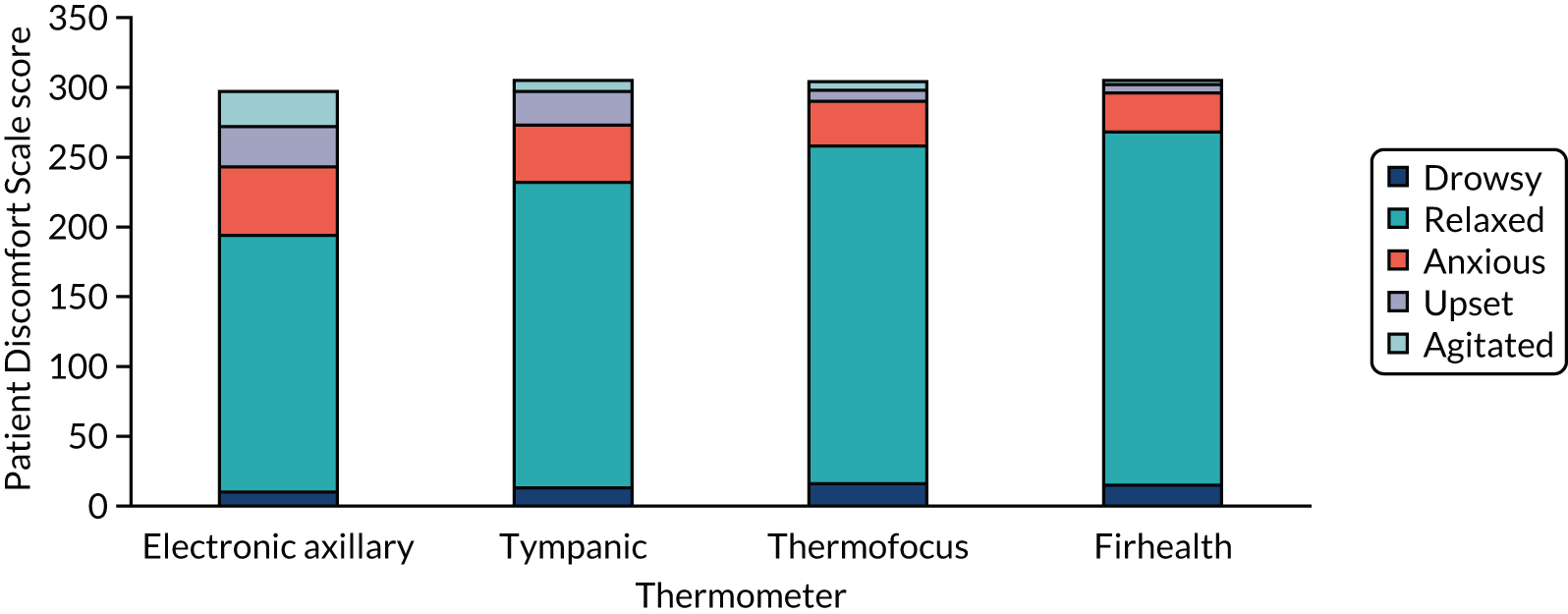
Overall, most children were rated by their parents as relaxed during temperature measurements with each thermometer. Figure 3 displays the distribution of the Patient Discomfort Scale for all four thermometers for the children in whom this was assessed (axillary, n = 297; tympanic, n = 305; Thermofocus, n = 304; Firhealth, n = 305). The median for each thermometer was 2, although the IQR was slightly larger for the axillary thermometer than for the other thermometers: Thermofocus median 2 (IQR 2–2), Firhealth median 2 (IQR 2–2), electronic axillary median 2 (IQR 2–3) and infrared tympanic median 2 (IQR 2–2).
FIGURE 3.
Results of the Patient Discomfort Scale.
Parental acceptability as assessed by a visual analogue scale was highest for the Firhealth non-contact thermometer (median 9 cm, IQR 7.6–9.5 cm), followed by the Thermofocus non-contact thermometer (median 8.5 cm, IQR 6.9–9.4 cm), the tympanic thermometer (median 7.6 cm, IQR 5.5–9 cm) and, finally, the electronic axillary thermometer (median 5 cm, IQR 2.1–7.6 cm).
When asked to rank the four thermometers by preference, with 1 indicating most preferred and 4 indicating least preferred, most parents preferred the Firhealth non-contact thermometer (median 2, IQR 1–2), followed by the Thermofocus (median 2, IQR 1–3), the tympanic thermometer (median 3, IQR 2–3) and, finally, the electronic axillary thermometer (median 4, IQR 3–4). However, these differences were not statistically significant (Friedman p = 1.00).
Qualitative interviews
A total of 65% of the participants in the METRIC study consented to be approached for the nested qualitative interviews. Interviews were conducted with 21 parents who had been purposively sampled to achieve a maximum variation sample, and recruitment continued until data saturation was achieved. The characteristics of 21 parents who participated in the interviews are described in the Appendix 1, Table 6. The main themes concerning the individual device attributes are summarised in Table 5, and described below.
Electronic axillary thermometer
Parents’ experiences with the axillary thermometers were described using negative language in almost all cases, with only two participants – both older parents – expressing neutral feelings towards them (’He was fine, he was quite happy with those’; i11, mother, white British, aged 31–40 years, one child).
A commonly described concern was the child’s perceived discomfort during use of the axillary thermometer, with more than half of respondents describing their child as appearing ‘uncomfortable’ during its use. Some participants felt this to be a more physical discomfort for the child, whereas, for others, the appearance of the device contributed to the child’s negative experience:
He freaked out at that one . . . Because he thought it was a needle . . . so he thought he was going to have an injection.
i8, mother, white British, aged 31–40 years, two children
Barriers to the convenience and practicality of the procedure required to measure the temperature with this device were also frequently raised by parents, with some feeling that having to undress the child to use the axillary thermometer was impractical for the parent and contributed to the child’s discomfort when they were already feeling unwell:
The armpit kind of seemed impractical . . . you have to undress your kid to jam a big metal stick under his arm.
i1, father, white British, two children
However, despite these experiences, several parents still expressed trust that if it was the medically recommended thermometer to use, they would be willing to persevere because the inconvenience or discomfort was temporary:
From a practical and user point of it, you know, it just seemed impractical, but if it’s the best then so be it, you know, makes no, there was no harm.
i1, father, white British, two children
Infrared tympanic thermometer
The parent descriptions of their child’s experience of temperature measurement with tympanic thermometers was more neutral than with the axillary thermometers, demonstrating more acceptance of the milder discomfort or displeasure displayed by their children in this context:
Yes she was used to that, she said it would be like a [um] that facial expression like very curious, what is that. But not really rigid not really in distress, no not at all.
i13, mother, aged > 40 years, one child
Several parents related this fact to the thermometer being the one their child was used to from being used in the home environment. However, a commonly emerging theme regarding the tympanic thermometer was parent concern about user-introduced variability, and that their skills in using it in the home environment might be affecting the results:
I’m sure if I got it, if I was trained I might know better but I’m never sure if I’ve put it far enough into her ear or not far enough in or, I mean I’d always had a normal reading from it but I’m not confident that I wouldn’t miss something by not being able to use it perfectly.
i17, mother, white British, aged 31–40 years, one child
You feel like there are so many ways that you can get it into their ear and you don’t know whether it needs to be really deep and like properly in or, you know, it comes against the part of the ear you don’t know whether it’s been an accurate reading or if you don’t have any background, medical background you don’t really know which, you know, if there are many ways in which it goes in is more effective or what. When you’re stressed and worried there’s lots of various things to worry about.
i6, mother, white British, aged 31–40 years, one child
Non-contact infrared thermometers
In comparison with the tympanic and axillary thermometers, the Thermofocus infrared thermometer was felt to provide feedback on ‘correct’ usage with its visible light feature, and on interpretation of the results with symbols, which were described as positive and reassuring components by several participants:
With the blue one there was a light, I thought that was quite helpful so you knew [um] exactly the distance you needed to be . . . I guess that also helps with the accuracy of reading because you know you’re taking it the right distance.
i15, mother, white British, aged 31–40 years, one child
The blue one had like a smiley face didn’t it so just kind of symbols rather than numbers is that right? I think I much preferred that because even though I would count myself as a fairly [um] knowledgeable person, when she’s unwell I’m a bit stressed I can’t then remember what counts as a temperature.
i6, mother, white British, aged 31–40 years, one child
Practicality and convenience of the procedure for parents and children emerged again as a theme, but was felt here to be a positive feature of the NCITs. The fact that the child might not even be aware of the temperature measurement being taken improved the experience from both the perspective of the parent and their perception of the child’s experience:
For ease, the forehead one where you don’t even touch them is an easy one isn’t it because . . . like you could do it while they’re asleep, you know, from a practical point of view that would probably be the easiest. Like I said it’s less intrusive [um] I think it’s a bit more flexible because you can do it when they’re asleep.
i1, father, white British, two children
It was as if nothing was happening he was just sitting on my lap, he was perfectly calm, so they were, I was quite impressed with those actually, I didn’t know they existed.
i10, father, white British, one child, with history of fever this episode
However, parents were, in general, less aware of this newer type of non-contact thermometer and expressed a wish for reassurance or guidance that they would be reliable, given their significant differences when compared with more traditional methods of temperature measurement:
I think it’s mainly because you’re kind of used to the [um] the touch the temperature when you get, when it’s touched the body and you know, like that physical contact for 1 or 2 minutes, so if you [um] kind of or use anything that is very different from the usual way of doing things then it always comes to your mind is this really reliable.
i14, father, aged 31–40 years, two children, with history of fever this episode
| Thermometer | Device attributes | Comments |
|---|---|---|
| Axillary |
Discomfort Impracticality Appearance |
Predominantly negative terminology used to describe the parental and child experience |
| Tympanic |
Familiarity Neutral convenience and comfort |
More neutral vocabulary and terminology used to describe the parental and child experience |
| NCIT |
Convenience Practicality Non-invasive Safety |
Most positive terminology used regarding ease and practicality of use; however, reassurance needed regarding the safety of newer technology |
Chapter 4 Patient and public involvement
The established PPI panel for the NIHR Diagnostic Evidence Cooperative Oxford agreed to adopt this study in to its portfolio of projects. We have discussed this project with our panel (which includes two mothers of children aged < 5 years) from inception until reporting of the final results.
The panel had access to the Department of Primary Care Health Sciences’ information technology and library facilities, and training opportunities were identified and offered to the group. They were reimbursed for their time, travel and childcare expenses.
The PPI panel provided feedback on the relevance of the topic itself, provided feedback on the study materials (particularly the patient information leaflets and acceptability ratings by children and parents), commented on the topic guide for the qualitative interviews with the parents and provided feedback on our sample size revision. In addition, we sought their advice on acceptable limits of agreement between thermometry devices, and discussed our emerging themes on the acceptability of the thermometer types from our qualitative interviews. We also asked for advice on how to disseminate our findings to a lay audience.
We discussed the initial study proposal with the NIHR Diagnostic Evidence Cooperative Oxford PPI panel and with a team of nursery nurses. They saw clear advantages to a non-contact thermometry device, particularly in eliminating the need for expensive and inconvenient disposable covers. One panel member reported that she owned a NCIT and doubted its accuracy; it provided lower readings than other thermometers. They felt that the study question was timely and important for parents as well as GPs. The issue of reproducibility of readings was raised and, in response, we have expanded our objectives to include an evaluation of NCIT reproducibility. They suggested that the cost of the Thermofocus could be prohibitive to adoption into primary care and nursery environments and were enthusiastic about the idea of including a cheaper comparison brand. The panel suggested modifications to the study flow to allow parents to remain in the GP waiting room for the study, or to participate after their GP appointment should they find this more convenient. They reviewed and clarified our Plain English summary.
The proposed change in sample size was discussed, in particular, with the two parents with young children who have commented on the study previously. Both parents felt that the change would still leave us with an acceptable amount of precision. One had tried all three types on her toddler and commented that the intermeasurement variability of one particular thermometer was far higher than this for a single thermometer (e.g. when measured in both ears by a tympanic thermometer). All acknowledged the need to balance finances and time with precision and thought that this was an acceptable compromise. One felt that it was important that we were transparent about this change and the rationale in our write-up. One felt that 400 was still a very large number of children to recruit to a study of this type.
We discussed the content of the topic guide with the PPI panel, and initial results from its pilot use; we discussed how to add the emerging theme of accuracy into the topic guide, and the panel made particular recommendations to explore parents’ feelings about cost of thermometers (and what might be acceptable to parents) in our qualitative interviews.
The PPI panel has also reviewed our final results and were very enthusiastic about them. The PPI panel felt that an article for the general media should definitely be written. The PPI panel commented that they and most of their friends owned any combination of the thermometers used in the study. The PPI panel commented that given the purchase price of these thermometers, it would be great if people were informed about their performance so that they could consider whether or not/what to purchase.
The PPI panel also suggested the potential to distribute leaflets (giving the study outcomes) through nurseries, schools, posters in GP surgeries (flash-up screens in waiting rooms) and even letters to parents at schools, although it realised that this may be quite ambitious and possibly not feasible.
Chapter 5 Discussion
Summary of the main findings
When comparing non-contact thermometers with currently recommended thermometers in young children (with a maximum age of 5 years), the mean difference between thermometers was not very large but the upper and lower limits of agreement were > 1 °C for the comparison with both the electronic axillary and the infrared tympanic thermometer. As a high temperature is considered to be a red flag for serious infections, this means that in a substantial number of children the differences between temperature readings could affect clinical decision-making. 4 The limits of agreement also surpass the commonly accepted limits of agreement of 0.5 °C. 29
This matters because high temperatures, especially of ≥ 40 °C, are associated with an increased risk of serious infection that can have very adverse consequences if left undetected and untreated. 30 In our study, two children with a temperature reading of at least 40 °C with the axillary thermometer had temperature readings of 38.2 and 39.3 °C with the Thermofocus non-contact thermometer, and 36.5 and 38.4 °C with the Firhealth non-contact thermometer. As is the nature of method comparison studies where there are no assumptions on which of the methods is the reference standard, it is impossible to say whether this pattern is caused by the non-contact thermometers or the axillary or tympanic thermometers.
We found that reproducibility of measurements with the non-contact thermometers was good, with very small differences between the first and the second reading of each non-contact thermometer. In addition, there were very small differences between both non-contact thermometers with the upper and lower limits of agreement being < 1 °C. Fewer attempts and fewer failures were reported for the non-contact thermometers than for the axillary or tympanic thermometers.
Parental satisfaction and child discomfort appear to be better for the non-contact thermometers but the majority rated all devices as acceptable. Based on their ranking, discomfort, pain score and acceptability, parents and children least preferred the axillary thermometer. This type of thermometer also resulted in the most failed readings. The Firhealth non-contact thermometer was most liked by parents and associated with the lowest discomfort for children. In the interviews, parents confirmed that they preferred the practicality and comfort of non-contact thermometers. In addition, they mostly used negative language when talking about the axillary thermometer, but they also indicated that they would still use it if this type of thermometer was recommended by clinicians.
Strengths and limitations
We recruited 401 children who attended GP settings with an acute illness, making this one of the larger studies to assess peripheral thermometers in routine clinical settings. We also employed a mixed-methods approach, combining and integrating quantitative and qualitative analyses. We randomised the order in which thermometers were used and compared two new types with two currently recommended types.
There are some limitations to our study. First, uncertainty exists over the accuracy of the currently recommended thermometers that we included as comparisons (i.e. the electronic axillary thermometer and infrared tympanic thermometer). In a recent systematic review,16 it was found that neither method met acceptable limits of agreement compared with core temperature measurements. This conclusion is reinforced by our comparison of the axillary and tympanic thermometers, which also showed wide limits of agreement of > 1.5 °C, which is well beyond the 0.5 °C threshold of acceptability. This, in turn, makes it challenging to interpret our findings. Second, we recruited fewer children who were febrile at the time of measurement than we had anticipated, meaning that the CIs around our estimate of sensitivity are wider than we would have liked. In an attempt to avoid this, our research team approached consecutive children arriving at GP waiting rooms, but the parents who agreed to participate may have been those with children who they felt were less unwell, a component of which could have been fever. Finally, the temperature measurements were performed by study personnel who inevitably developed expertise in using the equipment, which means that failure rates and accuracy may differ from what would be expected among practice staff or parents. However, all of the methods were simple to use and could be used frequently in most primary care settings, meaning that expertise would be likely to rapidly develop.
We encountered some difficulties in recruiting a sufficient number of patients in the time provided, which resulted in a loss of power. Recruitment problems were partly due to the complexity of the protocol, which required research assistants to go into the practices for recruitment rather than relying on opportunistic recruitment by practice staff. Research assistant time was further restrained by our study budget, meaning that hours of recruitment were ultimately limited. In addition, as children could not be entered in the study more than once, we reached saturation in practices that had collaborated in the study from the start, meaning that new practices had to be recruited that were increasingly further away.
Comparison with existing literature
We found a mean difference of –0.14 and –0.16 °C between NCITs and electronic axillary thermometers, which is within the range demonstrated by other studies comparing NCITs with electronic axillary, infrared tympanic and rectal thermometers. A study in 50 hospitalised children up to 14 years of age found a difference of –0.38 °C between electronic axillary and non-contact thermometers, with limits of agreement of –1.47 to 0.70 °C. 18 Another study in hospitalised children aged up to 17 years (n = 294) compared a tympanic thermometer (AccuSystem Genius2; Covidien, Dublin, Republic of Ireland), a NCIT (ThermoFlash Contactless; Visiomed Group SA, Paris, France) and a temporal artery scan thermometer (Exergen; Exergen, Watertown, MA, USA) with an electronic thermometer used rectally (Filac 3000; Covidien, Dublin, Republic of Ireland), and found that the mean difference between the non-contact and rectal thermometer was 0.34 °C (limits of agreement –0.92 to 1.60 °C). 19 Finally, Teran et al. 10 compared a non-contact thermometer (Thermofocus) and a temporal artery thermometer (Exergen), which is also infrared but requires minimal skin contact, with a mercury-in-glass thermometer used rectally in 500 children aged up to 48 months, half of whom were hospitalised and the other half presenting at the emergency room. They found a mean difference between the rectal reading and the non-contact reading of 0.029 °C (limits of agreement –0.50 to 0.58 °C). The study by Chiappini et al. 9 showed greater agreement between the Thermofocus and an axillary measurement, but they used a mercury-in-glass thermometer for the axillary temperature reading. They also used the average of two axillary temperature readings and three non-contact temperature readings.
The underestimation of fever was also reported in three studies: one comparing the Thermofocus NCIT with rectal thermometry in 200 children attending the emergency department,20 another of 294 paediatric inpatients comparing the ThermoFlash NCIT with rectal thermometry as described above19 and another of 119 healthy newborns comparing the Thermofocus with an electronic axillary thermometer and tympanic thermometer. 31 This trend was also noted in a systematic review of temporal artery thermometers, which also use infrared technology, compared with other methods. 29
Implications for research and practice
In children aged ≤ 5 years, the temperature measured by non-contact thermometers can differ from measurements with an electronic axillary or infrared tympanic thermometer by > 1 °C, and the agreement appears to be affected by the average temperature. Given the uncertainty over the accuracy of currently recommended thermometers, it is hard to draw firm conclusions about the likely impact on practice if non-contact thermometers were introduced as standard care. If we assume that electronic axillary thermometry is an accurate reference standard, then the moderate agreement between non-contact thermometers and an electronic axillary reading, and low sensitivity for fever mean that primary care clinicians should be cautious in using this technology.
However, we also found wide limits of agreement between electronic axillary and infrared tympanic thermometers, both of which are advocated in guidance. Furthermore, parents were more positive about the benefits of non-contact thermometers than about the electronic axillary and infrared tympanic thermometers, as evidenced by both rating scales and qualitative interviews, and there was good agreement between repeated measurements, which suggested that multiple attempts would not be required.
Therefore, clinicians need to be cautious about the accuracy of any peripheral thermometry approach and ensure that the management decisions they make are using these data as part of a holistic assessment. There is clear potential for technological innovation in this field to develop novel, more accurate methods of peripheral thermometry to support clinical decision-making. Once clinical evidence is more firmly established, an economic evaluation balancing clinical effectiveness with costs could be useful.
Acknowledgements
Contributions of authors
Professor Ann Van den Bruel (Associate Professor and GP) and Professor Gail Hayward (https://orcid.org/0000-0003-0852-627X) (Associate Professor and GP) conceived the study, provided oversight of study processes and drafted the manuscript. Ann Van den Bruel was responsible for submitting the final report.
Professor Jan Verbakel (Assistant Professor and GP) contributed to protocol development, interpretation of the findings and drafting of the manuscript.
Dr Kay Wang (https://orcid.org/0000-0002-7195-1730) (NIHR Postdoctoral Fellow and GP) contributed to protocol development, interpretation of the findings and drafting of the manuscript.
Dr Susannah Fleming (https://orcid.org/0000-0001-7205-2051) (Quantitative Researcher) contributed to protocol development, in particular the sample size calculations and analyses plan, interpretation of the findings and drafting of the manuscript.
Dr Gea Holtman (https://orcid.org/0000-0001-6579-767X) (Quantitative Researcher) contributed to data collection, interpretation of the findings and drafting of the manuscript.
Dr Margaret Glogowska (https://orcid.org/0000-0001-8029-1052) (Senior Health Services Researcher) and Elizabeth Morris (https://orcid.org/0000-0002-9913-6041) (NIHR In-practice Fellow and GP) contributed to protocol development, in particular the nested qualitative study, analysed the qualitative data, interpreted the findings and drafted the manuscript.
Mr George Edwards (https://orcid.org/0000-0002-0048-4178) (Research Assistant) contributed to data collection, data management, analyses and drafting of the manuscript.
Dr Fatene Abakar Ismail (https://orcid.org/0000-0002-1463-7980) (Trial Manager) contributed to protocol development, data collection and drafting of the manuscript.
Ms Kathryn Curtis (https://orcid.org/0000-0001-5367-9182) (Research Assistant) contributed to data collection, interpretation of the findings and drafting of the manuscript.
Dr James Goetz (https://orcid.org/0000-0003-2439-8116), Dr Grace Barnes (https://orcid.org/0000-0003-4296-1689), Dr Ralitsa Slivkova (https://orcid.org/0000-0002-2810-2266), Miss Charlotte Nesbitt (https://orcid.org/0000-0002-2873-153X), Dr Suhail Aslam, (https://orcid.org/0000-0003-4205-814X), Dr Ealish Swift (https://orcid.org/0000-0001-6884-4881) and Dr Harriet Williams (https://orcid.org/0000-0002-8009-9236) (medical students) contributed to data collection, interpretation of the findings and drafting of the manuscript.
Publication
Hayward G, Verbakel JY, Ismail FA, Edwards G, Wang K, Fleming S, et al. Non-contact infrared versus axillary and tympanic thermometers in children attending primary care: a mixed-methods study of accuracy and acceptability. Br J Gen Pract 2020;70:e236–44.[Corrigendum published in Br J Gen Pract 2020;70:486.]
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Hay AD, Heron J, Ness A. ALSPAC study team . The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract 2005;22:367-74. https://doi.org/10.1093/fampra/cmi035.
- Fleming DM, Smith GE, Charlton JR, Charlton J, Nicoll A. Impact of infections on primary care – greater than expected. Commun Dis Public Health 2002;5:7-12.
- Armon K, MacFaul R, Hemingway P, Werneke U, Stephenson T. The impact of presenting problem based guidelines for children with medical problems in an accident and emergency department. Arch Dis Child 2004;89:159-64. https://doi.org/10.1136/adc.2002.024406.
- National Institute for Health and Care Excellence . Fever in Under 5s: Assessment and Initial Management. NICE Guidelines [CG160] n.d. www.nice.org.uk/guidance/cg160 (accessed 26 June 2019).
- Omron® . Omron® 20-Second Flexible Digital Thermometer Model # MC-206 Instructions n.d. www.manualslib.com/manual/504879/Omron-Mc-206.html (accessed 26 June 2019).
- Craig JV, Lancaster GA, Taylor S, Williamson PR, Smyth RL. Infrared ear thermometry compared with rectal thermometry in children: a systematic review. Lancet 2002;360:603-9. https://doi.org/10.1016/S0140-6736(02)09783-0.
- Wang K, Gill P, Wolstenholme J, Price CP, Heneghan C, Thompson M, et al. Non-contact infrared thermometers for measuring temperature in children: primary care diagnostic technology update. Br J Gen Pract 2014;64:e681-3. https://doi.org/10.3399/bjgp14X682045.
- National Institute for Health Research . Horizon Scanning Reports n.d. www.community.healthcare.mic.nihr.ac.uk/reports-and-resources/horizon-scanning-reports (accessed December 2019).
- Chiappini E, Sollai S, Longhi R, Morandini L, Laghi A, Osio CE, et al. Performance of non-contact infrared thermometer for detecting febrile children in hospital and ambulatory settings. J Clin Nurs 2011;20:1311-18. https://doi.org/10.1111/j.1365-2702.2010.03565.x.
- Teran CG, Torrez-Llanos J, Teran-Miranda TE, Balderrama C, Shah NS, Villarroel P. Clinical accuracy of a non-contact infrared skin thermometer in paediatric practice. Child Care Health Dev 2012;38:471-6. https://doi.org/10.1111/j.1365-2214.2011.01264.x.
- Fortuna EL, Carney MM, Macy M, Stanley RM, Younger JG, Bradin SA. Accuracy of non-contact infrared thermometry versus rectal thermometry in young children evaluated in the emergency department for fever. J Emerg Nurs 2010;36:101-4. https://doi.org/10.1016/j.jen.2009.07.017.
- Selent MU, Molinari NM, Baxter A, Nguyen AV, Siegelson H, Brown CM, et al. Mass screening for fever in children: a comparison of 3 infrared thermal detection systems. Pediatr Emerg Care 2013;29:305-13. https://doi.org/10.1097/PEC.0b013e3182854465.
- Osio CE, Carnelli V. Comparative study of body temperature measured with a non-contact infrared thermometer versus conventional devices. The first Italian study on 90 pediatric patients. Minerva Pediatr 2007;59:327-36.
- Ng KG, Wong ST, Lim SM, Goh Z. Evaluation of the Cadi ThermoSENSOR wireless skin-contact thermometer against ear and axillary temperatures in children. J Pediatr Nurs 2010;25:176-86. https://doi.org/10.1016/j.pedn.2008.12.002.
- Hayward G, Verbakel JY, Ismail FA, Edwards G, Wang K, Fleming S, et al. Non-contact infrared versus axillary and tympanic thermometers in children attending primary care: a mixed-methods study of accuracy and acceptability. Br J Gen Pract 2020;70:e236-44.
- Niven DJ, Gaudet JE, Laupland KB, Mrklas KJ, Roberts DJ, Stelfox HT. Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Ann Intern Med 2015;163:768-77. https://doi.org/10.7326/M15-1150.
- Craig JV, Lancaster GA, Williamson PR, Smyth RL. Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ 2000;320:1174-8. https://doi.org/10.1136/bmj.320.7243.1174.
- Apa H, Gözmen S, Bayram N, Çatkoğlu A, Devrim F, Karaarslan U, et al. Clinical accuracy of tympanic thermometer and noncontact infrared skin thermometer in pediatric practice: an alternative for axillary digital thermometer. Pediatr Emerg Care 2013;29:992-7. https://doi.org/10.1097/PEC.0b013e3182a2d419.
- Allegaert K, Casteels K, van Gorp I, Bogaert G. Tympanic, infrared skin, and temporal artery scan thermometers compared with rectal measurement in children: a real-life assessment. Curr Ther Res Clin Exp 2014;76:34-8. https://doi.org/10.1016/j.curtheres.2013.11.005.
- Paes BF, Vermeulen K, Brohet RM, van der Ploeg T, de Winter JP. Accuracy of tympanic and infrared skin thermometers in children. Arch Dis Child 2010;95:974-8. https://doi.org/10.1136/adc.2010.185801.
- Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med 2001;155:376-81. https://doi.org/10.1001/archpedi.155.3.376.
- Hockenberry M, Wilson D, Winkelstein M. Wong’s Essentials of Pediatric Nursing. St. Louis, MO: Mosby; 2005.
- Bland JM, Altman DG. Agreed statistics: measurement method comparison. Anesthesiology 2012;116:182-5. https://doi.org/10.1097/ALN.0b013e31823d7784.
- Kelly M, McCarthy S, O’Sullivan R, Shiely F, Larkin P, Brenner M, et al. Drivers for inappropriate fever management in children: a systematic review. Int J Clin Pharm 2016;38:761-70. https://doi.org/10.1007/s11096-016-0333-2.
- Kelly M, Sahm LJ, Shiely F, O’Sullivan R, McGillicuddy A, McCarthy S. Parental knowledge, attitudes and beliefs regarding fever in children: an interview study. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-3224-5.
- de Bont EG, Loonen N, Hendrix DA, Lepot JM, Dinant GJ, Cals JW. Childhood fever: a qualitative study on parents’ expectations and experiences during general practice out-of-hours care consultations. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0348-0.
- Walsh A, Edwards H, Fraser J. Influences on parents’ fever management: beliefs, experiences and information sources. J Clin Nurs 2007;16:2331-40. https://doi.org/10.1111/j.1365-2702.2006.01890.x.
- Kai J. What worries parents when their preschool children are acutely ill, and why: a qualitative study. BMJ 1996;313:983-6. https://doi.org/10.1136/bmj.313.7063.983.
- Geijer H, Udumyan R, Lohse G, Nilsagård Y. Temperature measurements with a temporal scanner: systematic review and meta-analysis. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009509.
- Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D. European Research Network on Recognising Serious Infection investigators . Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 2010;375:834-45. https://doi.org/10.1016/S0140-6736(09)62000-6.
- Sollai S, Dani C, Berti E, Fancelli C, Galli L, de Martino M, et al. Performance of a non-contact infrared thermometer in healthy newborns. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-008695.
Appendix 1 Participant characteristics
| Characteristics | Number |
|---|---|
| Parental characteristics | |
| Gender | |
| Male | 6 |
| Female | 15 |
| Age (years) | |
| 20–30 | 3 |
| 31–40 | 11 |
| > 40 | 3 |
| White British ethnicity | |
| Yes | 15 |
| No | 6 |
| Case characteristics | |
| Age of child | Range 4 months–5 years |
| History of fever this episode | |
| Yes | 6 |
| No | 15 |
| Number of children in household | |
| 1 | 12 |
| 2 | 9 |
Appendix 2 Bland–Altman plots
FIGURE 4.
Bland–Altman plots of agreement for the other comparisons. Agreement between (a) Thermofocus and tympanic; (b) Firhealth and tympanic; (c) Thermofocus first and second measurements; (d) Firhealth first and second measurements; (e) electronic axillary and tympanic; and (f) Thermofocus and Firhealth.


List of abbreviations
- CE
- Conformité Européenne
- CI
- confidence interval
- FDA
- Food and Drug Administration
- GP
- general practitioner
- IQR
- interquartile range
- NCIT
- non-contact infrared thermometer
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PPI
- patient and public involvement
