Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/26/05. The contractual start date was in January 2014. The draft report began editorial review in December 2019 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Rodgers et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced from Rodgers et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have also been reproduced from Rodgers et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
In 2011, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme commissioned a call for proposals3 to determine the clinical effectiveness of robot-assisted training for upper limb recovery following a stroke: are robot-assisted training devices clinically effective for upper limb disability in post-stroke patients?
-
Technology: robotics or ‘electromechanical devices’. Researchers should justify the choice of machine, using the patient group and setting to inform their decision.
-
Patient group: post-stroke adults with moderate/severe paretic upper limb impairment. Researchers to define and justify which time point in the patient pathway.
-
Setting: community or hospital based.
-
Control or comparator treatment: treatment as usual (researchers to justify choice of control).
-
Design: three-arm efficacy randomised controlled trial (RCT): (1) treatment as usual, (2) enhanced physiotherapy and (3) robotic device. Researchers should undertake simple modelling of costs (comprehensive cost-effectiveness evaluation is not required).
-
Important outcomes: hand function, arm function and costs (including societal).
-
Other outcomes: rate of recovery, adverse events (pain or musculoskeletal injury), activities of daily living (ADL) and quality of life.
-
Minimum duration of follow-up: 6 months.
This report describes the findings of the Robot-Assisted Training for the Upper Limb after Stroke (RATULS) trial, which was commissioned to undertake this work.
Problems after stroke
Stroke is the commonest cause of complex disability in the UK. 4 The potential consequences of stroke are broad and include problems with mobility, arm and hand function, speech, cognition, vision, swallowing, continence, mood, pain and fatigue. 5 There are 1.2 million stroke survivors in the UK and > 100,000 people have a stroke each year. 6 Almost two-thirds of stroke survivors leave hospital with a disability. 6
Upper limb problems after stroke
Upper limb problems frequently occur following stroke, comprising loss of movement, co-ordination, sensation and dexterity, which lead to difficulties with everyday activities. Approximately 80% of people with acute stroke have upper limb motor impairment, and, of those with limited arm function early after stroke, 50% still experience problems after 4 years. 7 The strongest predictor of recovery is severity of initial neurological deficit; patients with severe initial upper limb impairment are unlikely to fully recover their arm function, with a clear impact on their quality of life. 8 Patients report that loss of arm function is one of the most distressing long-term consequences of stroke. Improving upper limb function has been identified as a research priority by stroke survivors, carers and clinicians. 9
Repetitive functional task practice to improve upper limb recovery
At the time of designing the RATULS trial, a systematic review7 reported that treatments that focused on increased intensity and repetitive task-specific practice showed promise for improving motor recovery. Since then, the body of evidence has increased, with a 2014 Cochrane overview of systematic reviews10 reporting moderate-quality Grading of Recommendations Assessment, Development and Evaluation evidence showing that arm function following a stroke can be improved by the provision of at least 20 extra hours of repetitive task training. In addition, a 2016 Cochrane review11 found that repetitive functional task practice for stroke patients was associated with improved arm function [standardised mean difference (SMD) 0.25, 95% confidence interval (CI) 0.01 to 0.49], hand function (SMD 0.25, 95% CI 0.00 to 0.51) and ADL (SMD 0.28, 95% CI 0.10 to 0.45).
Robot-assisted training for upper limb recovery
First proposed in the late 1980s, robot-assisted training enables stroke patients with moderate or severe upper limb impairment to perform repetitive tasks in a highly consistent manner, tailored to their motor abilities. At the time of designing the RATULS trial, the most recent (2008) Cochrane review12 of electromechanical and robot-assisted arm training after stroke reported outcomes from 328 patients who participated in 11 trials. The largest study had 55 participants13 and four of the 11 studies evaluated the Massachusetts Institute of Technology (MIT)-Manus robotic gym system (InMotion commercial version, Interactive Motion Technologies, Inc., Watertown, MA, USA). 13–16 The systematic review12 reported improvements with electromechanical and robot-assisted arm training in terms of arm motor function (SMD 0.68, 95% CI 0.24 to 1.11) and motor strength (SMD 1.03, 95% CI 0.29 to 1.78), but no improvement in ADL (SMD 0.29, 95% CI –0.47 to 1.06).
In 2010, the Veterans Affairs (VA) RCT was published evaluating the MIT-Manus in four centres in the USA. 17 This trial was the largest trial of robot-assisted training at the time, recruiting 127 patients with moderate or severe upper limb impairment who were ≥ 6 months after stroke. Participants were randomised to receive robot-assisted training, intensive comparison therapy or usual care. Robot-assisted training and intensive comparison therapy both consisted of 36 sessions, each lasting 1 hour, over 12 weeks. The trial found that robot-assisted training did not improve upper limb motor function at 12 weeks compared with intensive therapy or usual care (the primary outcome). However, participants who received robot-assisted training had significantly better results at 12 weeks on the Stroke Impact Scale (SIS) than those who received usual care. In secondary analyses, improvements were seen in motor function for those who received robot-assisted training compared with those who received usual care, but not compared with those who received intensive comparison therapy, at 36 weeks. The added costs of delivering robot-assisted training or intensive comparison therapy were compensated for by the fact that the health-care costs were lower than for usual care. 18
The Robot-Assisted Training for the Upper Limb after Stroke trial
The RATULS trial evaluated robot-assisted training using the MIT-Manus robotic gym system. This was compared with an upper limb therapy programme [named the enhanced upper limb therapy (EULT) programme] of the same frequency and duration, and with usual post-stroke care. Robot-assisted training and the EULT programme involved repetitive task practice and were provided in addition to usual care.
Chapter 2 Methods
Parts of this chapter have been reproduced from Rodgers et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial aim and objectives
Aim
The aim was to determine whether or not robot-assisted training with the MIT-Manus robotic gym system improved upper limb function post stroke.
Objectives
-
To determine whether or not robot-assisted training improved upper limb function post stroke, compared with an EULT programme or usual care.
-
To determine whether or not robot-assisted training improved upper limb impairment, ADL and quality of life, compared with an EULT programme or usual care.
-
To model the costs of robot-assisted training, compared with an EULT programme or usual care.
-
To seek the views and experiences of patients and health service professionals about the upper limb rehabilitation that they received or provided, and about factors affecting the implementation of the trial.
-
To explore:
-
the time pattern of upper limb recovery of participants in each treatment group
-
the impact of the severity of baseline upper limb function and time since stroke on the effectiveness of the interventions.
-
Trial design
The RATULS trial was a three-group, pragmatic, observer-blind, multicentre RCT with an embedded economic analysis and a process evaluation. Participants were randomised to receive one of the following: robot-assisted training (in addition to usual NHS care), an EULT programme (in addition to usual NHS care) or usual NHS care in accordance with local clinical practice.
Trial setting
The trial was conducted in four NHS centres in the UK. Each centre comprised a hub site, which was a stroke service in an NHS hospital (Queen’s Hospital, Barking, Havering and Redbridge University Hospitals NHS Trust; Northwick Park Hospital, London North West University Healthcare NHS Trust; Queen Elizabeth University Hospital, NHS Greater Glasgow and Clyde; and North Tyneside General Hospital, Northumbria Healthcare NHS Foundation Trust) with a MIT-Manus robotic gym system, plus several spoke sites (a total of 18), which were stroke services in adjacent NHS trusts and community services.
Participants who were randomised to receive robot-assisted training or EULT travelled to a hub stroke unit to receive these interventions. Participants who were randomised to usual care were treated by their local stroke service.
Trial participants
Adults with a first-ever stroke who fulfilled the following criteria were eligible to participate in the RATULS trial.
Inclusion criteria
-
Age ≥ 18 years.
-
Clinical diagnosis of stroke (cerebral infarction, primary intracerebral haemorrhage, subarachnoid haemorrhage).
-
Between 1 week and 5 years since stroke.
-
Moderate or severe upper limb functional limitation [i.e. Action Research Arm Test (ARAT) score of 0–39]19 due to stroke.
-
Able to provide consent to take part in the trial and to comply with the requirements of the protocol.
Exclusion criteria
-
More than one stroke (patients who had had a previous transient ischaemic attack could be invited to participate).
-
Other current significant impairment of the upper limb affected by stroke (e.g. fixed contracture, frozen shoulder, severe arthritis, recent fracture).
-
Diagnosis likely to interfere with rehabilitation or outcome assessments (e.g. registered blind).
-
Previous use of the MIT-Manus robotic gym system or other arm rehabilitation robot.
-
Current participation in a rehabilitation trial evaluating upper limb rehabilitation after stroke.
-
Previous enrolment in the RATULS trial.
Case ascertainment, recruitment and consent
Participants were recruited from both incident and prevalent stroke populations. Participants were sought from a number of settings in primary and secondary care, including stroke units, outpatient clinics, day hospitals, community rehabilitation services and general practices. The trial aimed to recruit similar numbers of participants within 3 months of stroke, > 3–12 months after stroke and > 12 months to 5 years after stroke.
Potential participants from secondary care
In secondary care, potential participants were identified by local clinicians and/or staff from the NIHR Local Clinical Research Network (LCRN). Staff approached potentially eligible patients, discussed the trial and provided a trial information leaflet. After allowing sufficient time for the information to be considered, staff asked the patient if they were potentially interested in taking part in the trial.
Potential participants were also identified from hospital stroke discharge summaries/clinic letters. If this method was used, potential participants were approached by letter. A short RATULS trial leaflet, a patient information sheet, a RATULS trial reply slip and a pre-paid envelope were enclosed with the letter. Interested patients could make contact by telephone or by return of the RATULS trial reply slip. Following a few short questions to confirm potential eligibility, a face-to-face appointment for further discussion was arranged, if appropriate.
Potential participants from primary care
To identify potential participants from primary care, general practices performed a database search using the trial inclusion/exclusion criteria. A general practitioner (GP) screened the list of potentially eligible participants to approve the issue of an invitation letter. This letter was accompanied by the same information that was sent to individuals identified from secondary care records. The invitation letter detailed the main trial eligibility criteria and asked interested patients to contact their trial centre for further information. Following a few short telephone questions to confirm potential eligibility, a face-to-face appointment for further discussion was arranged, if appropriate.
Potential participants from other sources
Local community stroke clubs and day centres were also given information about the trial. In addition, some individuals heard about the trial from press releases or saw information about the trial on a poster or a RATULS trial leaflet. Interested individuals were able to contact the trial centres directly for further information about the trial.
Consent
Individuals who were interested and potentially eligible to take part in the trial were given an appointment for further discussion and consent. This was conducted by a local trial co-ordinator or NIHR LCRN staff. Written informed consent was obtained if a patient wished to take part in the RATULS trial.
Screening assessment
Once informed consent was obtained, a screening assessment was performed by the local trial centre co-ordinator or NIHR LCRN staff. The following data were collected: demography, stroke details, comorbidity and upper limb function (ARAT score19,20). If a patient fulfilled the trial inclusion and exclusion criteria, the local trial co-ordinator/NIHR LCRN staff proceeded with the baseline assessment. If it was not possible to complete the baseline assessment on the same day as the screening assessment, eligibility for the trial was reconfirmed on the day of the baseline assessment.
Baseline assessment
The following baseline data were collected: stroke severity (measured using the National Institutes of Health Stroke Scale21), cognitive function (measured using the Montreal Cognitive Assessment22), language skills (measured using the Sheffield Screening Test for Acquired Language Disorders23), upper limb impairment [measured using the Fugl-Meyer Assessment (FMA)20,24 (motor and sensory arm sections)], ADL (measured using the Barthel ADL Index25), quality of life [measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)26], upper limb pain (measured using a numeric rating scale27) and current upper limb rehabilitation treatments.
In addition, patients were given a self-completion questionnaire containing pre-trial resource use questions [an adaption of the Client Services Receipt Inventory (CSRI)28–30].
Randomisation
Randomisation was conducted by the local trial co-ordinator/NIHR LCRN staff following completion of the baseline assessment. A central independent web-based service, hosted by Newcastle University Clinical Trials Unit, was used. Participants were stratified according to trial centre, time since stroke (< 3 months, 3–12 months, > 12 months) and severity of upper limb functional limitation (ARAT score categories:19 0–7, 8–13, 14–19 and 20–39), and randomised, using permuted block sequences, 1 : 1 : 1 to receive robot-assisted training, EULT or usual care. The sequences were prepared by an independent statistician prior to the start of enrolment.
Randomisation groups
Robot-assisted training using the MIT-Manus robotic gym system
This was delivered using the MIT-Manus robotic gym, which was specifically designed for clinical rehabilitation applications. 31–33
The robot-assisted training programme consisted of 45 minutes of face-to-face therapy during a 1-hour session, 3 days per week for 12 weeks, in addition to usual care. A detailed description of the robot-assisted training programme is provided in Chapter 4, and a completed Template for Intervention Description and Replication (TIDieR) checklist34 is provided in Appendix 1, Table 27. The MIT-Manus robotic gym recorded data on the robot-assisted training sessions content.
The enhanced upper limb therapy programme
The EULT programme aimed to match the frequency and duration of the robot-assisted training programme sessions. It was developed from the upper limb therapy programmes used in the NIHR HTA Botulinum Toxin for the Upper Limb after Stroke (BoTULS) trial35–37 and the Repetitive Arm Functional Tasks after Stroke (RAFTAS) project. 38 Using the principles of person-centred goal-setting and repetitive functional task practice, the EULT programme aimed to drive neuroplasticity and motor recovery after stroke.
The EULT programme consisted of 45 minutes of face-to-face therapy during a 1-hour session, 3 days per week for 12 weeks, in addition to usual care. A detailed description of the EULT programme is provided in Chapter 5, and a completed TIDieR checklist34 is provided in Appendix 1, Table 27. Therapists recorded data on the content of EULT sessions.
Usual care
Defining usual care is a challenge for any stroke rehabilitation trial. One of the current National Institute for Health and Care Excellence (NICE) quality standards is that:
Patients with stroke should be offered a minimum of 45 minutes of each appropriate therapy that is required, for a minimum of five days a week, at a level that enables the patient to meet their rehabilitation goals for as long as they are continuing to benefit from therapy and as long as they are able to tolerate it.
NICE39
For most stroke services, this is aspirational, and the majority of patients do not receive this intensity, particularly after discharge from hospital or early supported discharge services. 40 Patients with chronic stroke are unlikely to receive ongoing rehabilitation in the longer term. Most services do not regularly review patients to address unmet rehabilitation needs beyond 1 year.
Participants in all three randomisation groups received a trial ‘arm rehabilitation therapy log’ in which they were asked to record any ‘usual’ upper limb rehabilitation that they received during the course of the trial. Periodic text messages were sent to remind participants about completion of the rehabilitation logs. In addition, participants in all three randomisation groups received regular trial newsletters, which included requests to complete the rehabilitation logs.
Outcome measures
Primary outcome
The primary outcome was upper limb functional recovery ‘success’ (assessed using ARAT)19 at 3 months post randomisation (at the end of intervention period). The ARAT assesses upper limb function by scoring the ability to complete a range of functional tasks. The scale is made up of 19 items in four subscales (grasp, grip, pinch and gross movement), which are rated on a four-point ordinal scale ranging from zero (can perform no part of the test) to three (performs test normally). 19
The definition of upper limb functional recovery ‘success’ differed depending on baseline severity:
-
ARAT baseline score of 0–7; ‘success’ is an improvement of ≥ 3 points
-
ARAT baseline score of 8–13; ‘success’ is an improvement of ≥ 4 points
-
ARAT baseline score of 14–19; ‘success’ is an improvement of ≥ 5 points
-
ARAT baseline score of 20–39; ‘success’ is an improvement of ≥ 6 points.
A stepped approach was used because, although the minimal clinically important difference (MCID) for the ARAT is 10% of its range (6 points41), a smaller treatment effect may be clinically beneficial in those with severe initial upper limb functional limitation, who are likely to improve less than those with more moderate limitation.
Secondary outcomes
Secondary outcomes at 3 and 6 months were upper limb function (measured using the ARAT19) ‘success’ (at 6 months); total ARAT score; upper limb impairment (measured using the FMA24); ADL (measured using the Barthel ADL Index25); quality of life (measured using the SIS42 version 3.0); upper limb pain (measured using a numeric rating scale27); resource use costs (an adaptation of the CSRI28,43); and quality-adjusted life-years (QALYs), derived from the EQ-5D-5L. 26
Upper limb functional recovery ‘success’ at 6 months
The same success criteria based on the baseline ARAT19 score used at 3 months were used to define upper limb functional recovery ‘success’ at 6 months.
Total Action Research Arm Test score
A secondary analysis of ARAT used the numeric score, which ranges from 0 to 57. 19 A higher score indicates better upper limb function.
Action Research Arm Test subscales
The original plan was to analyse the subscales as numeric scores, and to compare means, but after database lock for the final data set, it was realised that the data distribution for subscales was U-shaped and almost binary, as participants mostly scored zero or full marks on each subscale. The clinical team felt that a better approach was to create a binary variable for each ARAT subscale. For this, if a participant scored 2 (‘completed test, but takes abnormally long time, or has great difficulty’) or 3 (‘performs test normally’) for any of the items in that subscale, that participant was classified as ‘could complete at least one task’ for that subscale. Conversely, if a participant scored 0 (‘can perform no part of the test’) or 1 (‘performs test partially’) for all parts of the subscale, that participant was classified as ‘could not complete one task’ for that subscale. The rationale was that a comparison should be made between participants who could complete a task fully and those who could not.
The Fugl-Meyer Assessment total upper-extremity score
The FMA total upper-extremity score (score 0–126) assesses upper limb impairment by incorporating the motor, sensory, range of motion and joint pain subscales. 24 Each item is scored on a 3-point ordinal scale (0–2 points per item):
-
Motor function. This has 24 items, which include scoring of the active movement of the shoulder, elbow, forearm, wrist and hand, and co-ordination and speed. The score for this subscale ranges from 0 to 66.
-
Sensation. This has six items and the score for this subscale ranges from 0 to 12.
-
Range of motion and joint pain. This has 12 items, which are scored for each range of motion and joint pain. The score for this subscale ranges from 0 to 48.
A higher FMA total upper-extremity score indicates less upper limb impairment.
Barthel Activities of Daily Living Index
The Barthel ADL Index consists of 10 items (bowels, bladder, grooming, toilet use, feeding, transfer, mobility, dressing, stairs and bathing), and is calculated by summing up the individual items and ranges from 0 to 20. 25 A higher score indicates a better ability to complete ADL independently.
The Stroke Impact Scale
The SIS is a self-completion stroke-specific questionnaire to measure quality of life. 42 There are 59 items investigating nine dimensions (strength, hand function, mobility, ADL, emotion, memory, communication, social participation and stroke recovery). Each item was scored on a 5-point scale (1–5). The dimension and domain scores range from 0 to 100. A higher score implies a better quality of life.
Upper limb pain
Upper limb pain was measured using a numeric rating scale on which participants were asked to rate their upper limb pain, with zero being no pain at all and 10 being ‘as painful as it could have been’. 27
The EuroQol-5 Dimensions, five-level version, quality-adjusted life-years and resource use
Participant responses to the EQ-5D-5L44 questionnaire, completed at baseline and at 3 and 6 months, were used to derive QALYs. Utility values were estimated from the responses using health state utility scores based on the UK population tariff,45,46 and mapped back to the EuroQol-5 Dimensions, three-level version, valuation set. 47 Costs incurred by the NHS and Personal Social Services (PSS) were collected via the adapted CSRI28–30 resource use questionnaires completed at baseline and at 6 months.
Undertaking outcome assessments
Outcomes were assessed at 3 months (± 7 days) and 6 months (± 7 days) following randomisation, and were undertaken in two stages.
Stage 1 was a self-completion postal questionnaire consisting of the SIS42 (at 3 and 6 months) and the adapted CSRI28–30 resource use questions (at 6 months only).
Stage 2 was a face-to-face assessment with a researcher who was masked to the randomisation group. The following data were collected: ARAT,19 FMA24 (total upper-extremity score), Barthel ADL Index,25 EQ-5D-5L,26 upper limb pain27 and adverse events. At the end of the 6-month stage 2 assessment, participants were given a further self-completion questionnaire and were asked to return this by post. This questionnaire contained time and travel resource use questions. 48,49
Staff training
All staff received trial-specific training at the start of their involvement, with refresher training provided throughout the trial. Staff performing trial assessments (screening, baseline, outcomes) and delivering trial interventions received training in these aspects. In addition, manuals describing delivery of the interventions and instructions on how to perform the assessments were provided. Video demonstrations of the ARAT and FMA were also prepared and made available for ongoing reference.
Masking
Owing to the nature of the interventions, it was not possible to mask participants or treating therapists to treatment allocation. It was intended that stage 2 outcome assessments would be conducted by a researcher masked to treatment allocation. At each outcome assessment, the researcher was asked to record whether or not they had unintentionally become aware of treatment allocation in conversation with a participant.
Trial withdrawal
No specific withdrawal criteria were preset. Participants could withdraw from the trial at any time and for any reason. A reason for withdrawal was sought, but participants could withdraw without providing an explanation.
Investigators, GPs, stroke physicians and therapists could also withdraw participants from the trial at any time if they felt that it was no longer in their interest to continue, for example because of intercurrent illness or adverse events. Participants were informed that data collected prior to withdrawal would be used in the trial analysis, unless consent for this was specifically withdrawn. Participants who wished to receive no further intervention were not withdrawn from trial follow-up unless they specifically requested this.
Safety evaluation
The safety of robot-assisted training, EULT and usual care was evaluated by examining the occurrence of all adverse events and serious adverse events (SAEs) in accordance with the National Research Ethics Service guidance for non-Clinical Trials of an Investigational Medicinal Product. 50
All adverse events were captured by including the following question in the outcome pro formas: ‘are there any new medical problems since the last study assessment?’.
Events considered to be SAEs were subsequently documented on a separate trial SAE form, and a causality and expectedness assessment was performed. As trial investigators or other members of the research team could become aware of SAEs at times other than at outcome assessment appointments, the SAE form was also used to directly capture these events.
The standard definition for a SAE was used. 51 A SAE is an untoward occurrence that:
-
results in death
-
is life-threatening
-
necessitates hospitalisation, or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect
-
is otherwise considered medically significant by the investigator.
Data management
Trial data were collected on trial-specific paper case record forms, and subsequently entered onto an online database by trial administrators at participating centres.
Sample size
The target sample size was 762 participants (254 participants per group). Responses from 216 participants in each randomisation group would provide 80% power (significance level of 1.7% because of multiple comparisons) to detect a 15% difference in upper limb function ‘success’ between each of the three pairs of treatments (robot-assisted training, EULT and usual care). The target was inflated for 15% attrition, subsequent to protocol publication. The baseline estimate of success was estimated as 30% from the BoTULS trial,35,36 and a difference of between 45% and 30% corresponds to an odds ratio of 1.9.
Statistical analysis of primary and secondary outcome data
Analysis populations
All analyses were done in the intention-to-treat (ITT) population of all participants, in the group to which they were assigned, who did not have missing data after simple imputation.
A secondary analysis comparing the primary outcome robot-assisted training and EULT was carried out on a per-protocol (PP) analysis set, which removed participants who did not receive at least 20 sessions of therapy. This was based on evidence that 20 hours of repetitive task training improves upper limb function. 10 No definition of PP could be made for the usual care group, so no comparisons were undertaken for this group.
The main analysis of the primary outcome was on all participants; however, we performed two sensitivity analyses. The first was based on participants who completed their assessment visits within 3 months ± 14 days and 6 months ± 28 days. We have taken 3 months to be 91 days, and 6 months to be 183 days, post randomisation date. The second sensitivity analysis excluded participants from the analysis if they had a zero ARAT score at baseline, as such patients have a poor prognosis for recovery of arm function. 52
Missing data in the measurement scales
The pattern and extent of missing observations in all scales was examined to investigate both the extent of missing data and whether it was missing at random or was informative. The number (percentage) of participants for whom a whole or partial measure was missing was reported by measure.
Apart from the SIS, for which we followed the specific scale developer’s rules,42 where no more than 20% of questions were missing or uninterpretable on specific scales, the score was calculated by using the median value of the respondent-specific completed responses on the rest of the scale to replace the missing items (i.e. simple imputation). 53
We planned that multiple imputation techniques would be considered if > 20% of participants had missing values for the primary outcome, but these techniques were not necessary.
Analysis of primary outcome
The primary outcome was ‘success’ at 3 months based on the ARAT score. 19
The primary outcome of ‘success’ at 3 months is reported descriptively by randomisation group and overall as a proportion.
Logistic regression was used to compare ‘success’ between the three randomisation groups at 3 months, adjusting for time since stroke, baseline upper limb function (ARAT score) and trial centre. A two-level logistic model (trial centre and participants) was used. We adjusted the coverage of the CIs to account for the three paired comparisons between the randomisation groups. Because the trial was powered on a significance level of 1.67%, we used 98.33% (100% – 1.67%) as the CI coverage. We considered the trial centre as a fixed effect. We explored the possibility that participants in a trial centre may be more alike in the treatment randomisation groups, owing to participants sharing therapists in the robot-assisted training and EULT groups, but not in the usual care group. This is called partial nesting, but accounting for this did not improve model fit; therefore, we did not include this in the models reported here. 54–56
Analysis of secondary outcomes
The secondary outcomes included upper limb functional recovery ‘success’ at 6 months, the ARAT total score19 (numeric rather than ‘success’), ARAT subscales,19 upper limb impairment (FMA total upper-extremity score24), ADL (Barthel ADL Index25), quality of life (SIS42) and upper limb pain (numeric rating scale27) at 3 and 6 months.
The secondary outcome of ‘success’ at 6 months was analysed as described for the primary outcome, and similarly for the binary version of the ARAT subscales.
All numeric secondary outcomes are reported at baseline (if collected) and at 3 and 6 months descriptively by randomisation group as means and standard deviations (SDs). The secondary outcomes were analysed at 3 and 6 months using linear regression, adjusting for time since stroke, baseline score and trial centre. The baseline score used to adjust the analysis was the same scale; for example, the baseline ARAT score was used to adjust for baseline in the model for the ARAT. The SIS was not collected at baseline; therefore, no baseline score adjustment could be made. We adjusted the coverage of the CIs to account for the three paired comparisons between the randomisation groups. Because the trial was powered on a significance level of 1.67%, we have used 98.33% (100% – 1.67%) as the CI coverage. We presented bias-corrected and accelerated CIs (100,000 bootstrap intervals) because of the distribution of the data.
Exploratory subgroup analysis
The protocol included a subgroup analysis to consider the relationship between the severity of baseline upper limb functional limitation and time since stroke on the effectiveness of the interventions. The subgroup analyses examined trial centre and age, and are listed below. There was not sufficient power to perform any formal subgroup analyses, so all analyses must be considered exploratory.
We performed subgroup analysis for the numeric ARAT score at 3 months across the following subgroups:
-
time since stroke (< 3 months, 3–12 months, > 12 months)
-
baseline ARAT score (0, 1–7, 8–13, 14–19, 20–39, 40–57)
-
trial centre
-
age (< 55 years, 55–70 years, > 70 years).
When preparing the detailed statistical analysis plan, two further subgroup analyses were prespecified. These were for the FMA motor score and the Barthel ADL Index at 3 months, but only for the time since stroke categories. CIs were calculated using bias-corrected and accelerated bootstrap CIs (100,000 samples).
Descriptive analysis of the relationship between treatment received and total Action Research Arm Test score
The total ARAT score at 3 and 6 months was plotted against the number of sessions attended, total duration of therapy received, total number of therapy repetitions (EULT group only) and total robot movement attempts (robot-assisted training group only) for the two intervention randomisation groups. Zero was assumed for duration, therapy task repetitions and movement attempts for participants who did not attend an appointment.
Safety
The proportion of participants with at least one SAE was reported by randomisation group and the difference in proportions (98.33% CI) was calculated. The median number of SAEs per participant was also calculated and the distribution of SAEs was compared across groups using the Mann–Whitney U-test. The above was also completed for adverse events at 3 and 6 months.
Economic analysis
The economic evaluation consisted of a microcosting analysis, a within-trial economic evaluation and a longer-term economic model. 57 Methods are described in Chapter 8.
Parallel process evaluation
A two-stage process evaluation was conducted to understand (1) participants’ and health service professionals’ experiences of robot-assisted training, EULT and usual care and (2) factors affecting the implementation of the trial within and across trial sites. Methods are described in Chapter 7.
Ethics and regulatory issues
The trial sponsor was Newcastle upon Tyne Hospitals NHS Foundation Trust. The trial was conducted in accordance with research governance framework for health and social care58 and good clinical practice. Ethics approval was granted by the National Research Ethics Committee Sunderland (reference number 13/NE/0274). Local NHS approvals were obtained from all participating NHS organisations. Monitoring of trial conduct and data collection was performed by regular visits to all participating trial centres. The trial was managed by a co-ordinating centre based at Newcastle University, Newcastle upon Tyne, UK. An independent Data Monitoring Committee and Trial Steering Committee were in place for the duration of the project.
Amendments made to the trial after it commenced
-
Modification of the postal invitation procedure for potential participants by letter (from both primary and secondary care). When the trial commenced, potential participants who were invited by letter were asked to telephone their local trial team if they would like more information. The response rate to these invitations was low; it was suggested that this was because the letter and information sheet were too long, and that people may prefer to respond in writing initially. The letter was revised, a short summary leaflet designed and a postal reply slip created. These revised documents were then used for postal invitations. In addition, the option to re-contact potential participants who did not respond to the initial invitation letter was introduced. This was by follow-up letter or telephone call, according to local preference.
-
Introduction of reminders to complete the trial arm rehabilitation logs. To attempt to promote completion of the trial arm rehabilitation logs, regular reminders via text message were introduced.
-
Provision of trial newsletters. Regular newsletters were introduced for participants during their time in the trial. This was in response to noting that attrition was higher than anticipated, and aimed to encourage retention in the trial.
-
Modification of the parallel process evaluation. Several changes were made to the parallel process evaluation:
-
The initial protocol included interviewing a larger proportion of participants in the robot-assisted training group. However, the need for equal data collection in both the robot-assisted training and EULT groups became apparent, owing to their equivalent complexity. Therefore, the requirement to interview more robot-assisted training group participants was removed.
-
The participant interview time points for the second period of data collection (August 2016 to April 2017) were changed from twice during the first 3 months to once during the first 3 months and once at around 6 months. This change was informed by the analysis of data collected in the first period of data collection (February to June 2016). It allowed continued exploration of views and experiences of treatments, but also additional exploration of the impact of therapy following the end of treatment, plus participants’ experiences of trial participation (e.g. completing trial documentation and outcome assessments).
-
A review of the quantitative outcome assessment data (ARAT score at baseline and at 3 and 6 months) was added to the process evaluation for some participants. Comparison of quantitative data with participants’ subjective experiences captured in the interview data aimed to inform interpretation of the trial.
-
The range of health service professionals to be interviewed was broadened. Rather than interviewing only health service professionals who delivered therapy to RATULS trial participants (as per the original trial protocol), other staff who were involved in the trial were included in interviews. These included principal investigators, NIHR LCRN staff, local site trial co-ordinators and trial administrators. This allowed more information to be captured about factors affecting the implementation of the trial.
-
-
Revision of recruitment target. In the original trial protocol, the target sample size was 720 participants, which included 10% attrition for the primary outcome. As the trial proceeded, it became apparent that dropout was > 10%, and nearer to 15%. The target sample size was therefore increased to 762 participants, to allow for 15% attrition.
Chapter 3 Randomised controlled trial results
Parts of this chapter have been reproduced from Rodgers et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Participant recruitment and randomisation
Between 14 April 2014 and 30 April 2018, 770 participants were recruited from the four trial centres, and were randomised as follows: robot-assisted training, 257 participants; EULT, 259 participants; and usual care, 254 participants.
Original predicted, revised predicted and actual cumulative recruitment are shown in Figure 1. Recruitment predictions were revised in July 2016, when target recruitment was increased to 762 participants. Monthly recruitment by trial centre is tabulated in Appendix 2, Table 28.
FIGURE 1.
Predicted and actual cumulative recruitment. The initial recruitment target was 720 participants, to allow for 10% attrition, but the sample size was increased to 762 participants to allow for 15% attrition.

Participant retention and follow-up
Participant flow through the trial is shown in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 2).
FIGURE 2.
The CONSORT flow diagram.
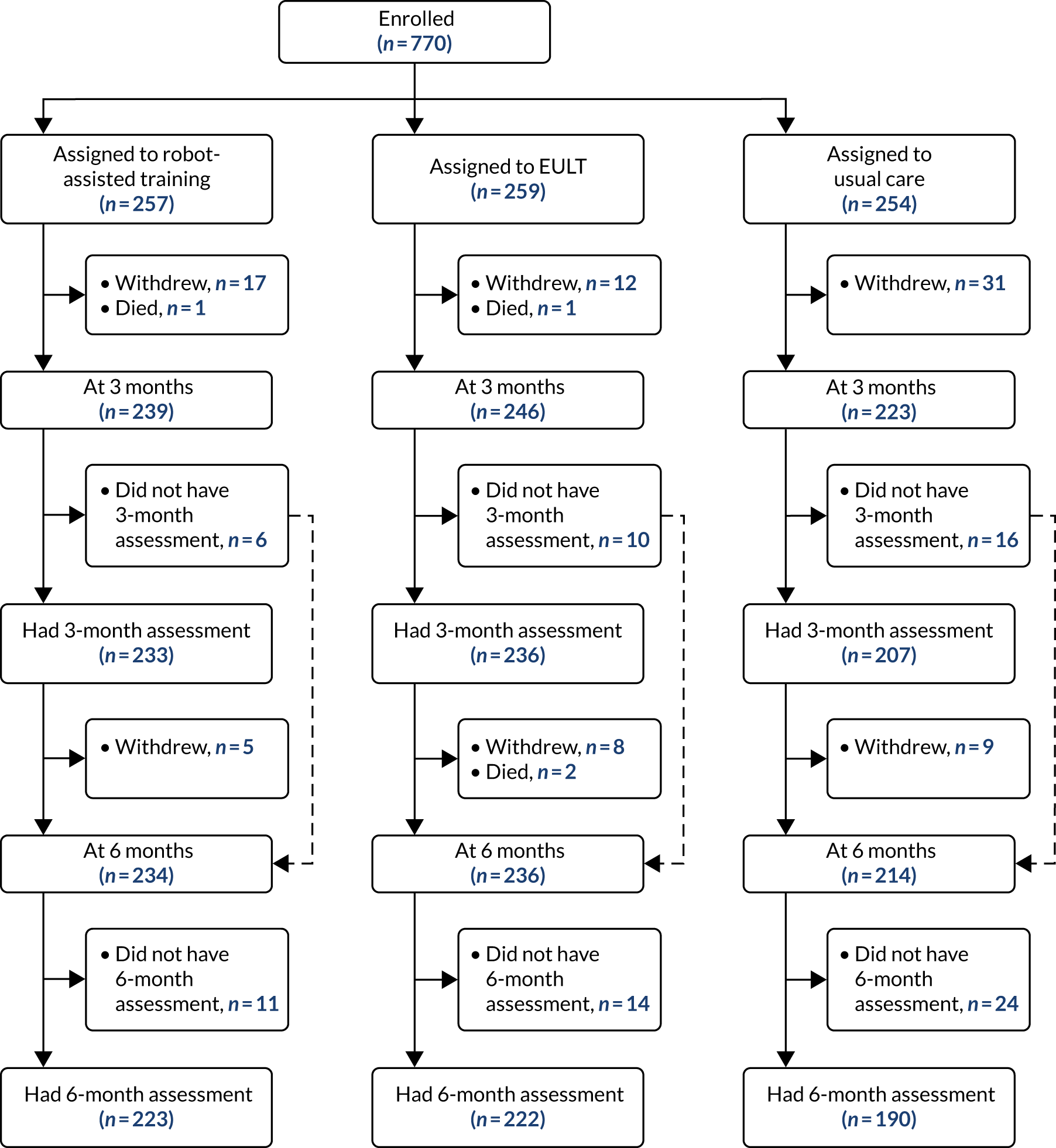
Of the 257 participants randomised to robot-assisted training, 233 (91%) completed a 3-month assessment and 223 (87%) completed a 6-month assessment. Of the 259 participants randomised to EULT, 236 (91%) completed a 3-month assessment and 222 (86%) completed a 6-month assessment. Of the 254 participants randomised to usual care, 207 (81%) completed 3-month assessment and 190 (75%) completed a 6-month assessment. There was a lower follow-up rate in the usual care group and, therefore, differential attrition between randomisation groups. Seven participants did not complete the ARAT at their 3-month assessment, but remained in the trial.
The numbers of participants who did not have assessments, and the reasons why, are reported in Appendix 2, Table 29. There were 60 withdrawals by 3 months, and a further 22 withdrawals by 6 months. The main reasons given for withdrawal were not wanting to be randomised to usual care [19/60 (32%) by 3 months], the burden of participation [19/60 (32%) by 3 months and 4/22 (18%) by 6 months] and being unwell [10/60 (17%) by 3 months and 6/22 (27%) by 6 months]. There were two deaths before 3 months and a further two deaths before 6 months. Assessments were not conducted for a further 32 people at 3 months and 49 people at 6 months. The main reasons for this were non-response to attempts to arrange the assessment [14/32 (44%) at 3 months and 37/49 (76%) at 6 months], missing the assessment and non-response to attempts to re-arrange [6/32 (19%) at 3 months and 5/49 (10%) at 6 months], and being unwell and unable to complete the assessment [8/32 (25%) at 3 months and 4/49 (8%) at 6 months].
Timing of participant outcome assessments
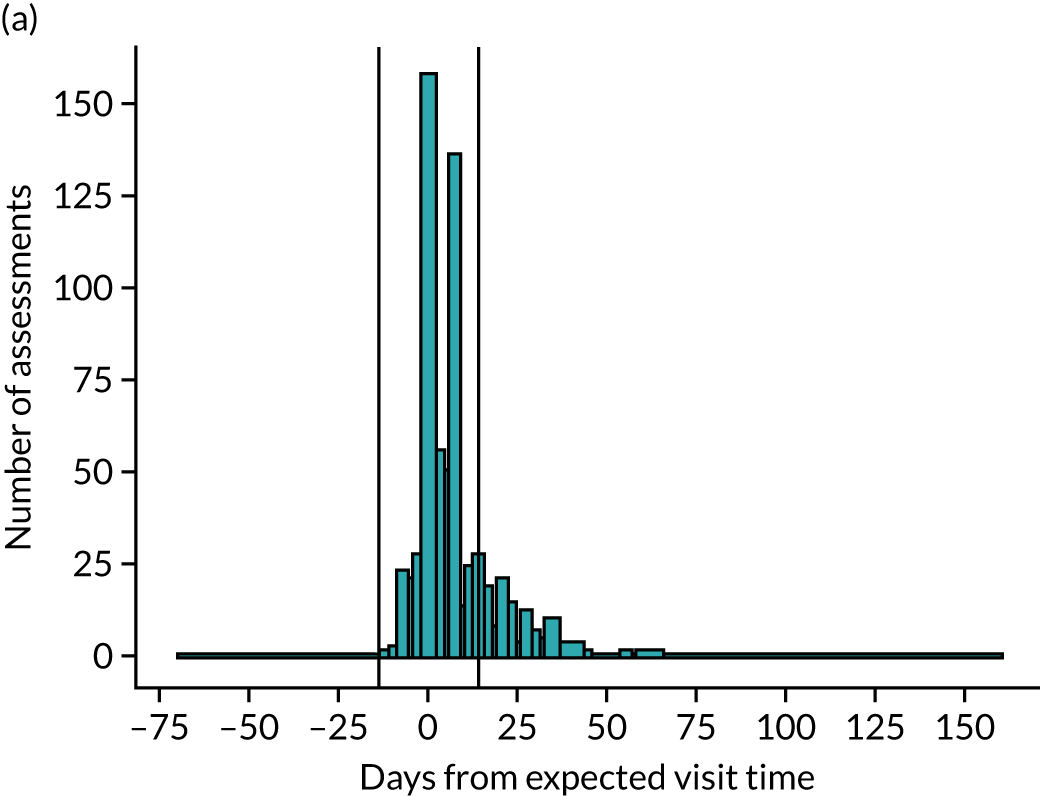
Of the 676 assessments scheduled for 3 months, 545 (81%) were carried out at 3 months ± 14 days; the median time from randomisation was 96 days [interquartile range (IQR) 91–102 days]. Of the 635 assessments scheduled for 6 months, 575 (91%) were carried out at 6 months ± 28 days; the median time from randomisation was 187 days (IQR 182–193 days) (Figure 3).
FIGURE 3.
Compliance with time of assessments at (a) 3 months and (b) 6 months. The vertical lines indicate the allowable window of time within which the assessment could take place (± 14 days for 3 months and ± 28 days for 6 months).

Missing data in the measurement scales
There were very few data missing across the scales, and simple imputation did not add many participants to the analyses. For example, the ARAT total score was fully completed for 668 out of 708 (94%) of expected participants at 3 months; after simple imputation, only one more participant was included, as this participant was the only one with missing data who had < 20% of items missing. The number (percentage) of participants with no missing data on the measurement scales is reported by measure and time point in Appendix 2, Tables 30–32. These tables also show how many participants were included after simple imputation. As < 20% of participants were missing data on the primary outcome after simple imputation, multiple imputation techniques were not used.
Participant baseline characteristics
Baseline demographics and stroke characteristics are shown in Tables 1 and 2; they appear balanced across groups. The mean age of participants was 61 years (SD 13.5 years), and 468 out of 770 (60.8%) participants were men. Most participants had experienced a cerebral infarction [613/770 (79.6%)]. The median time from stroke to randomisation was 240 days (IQR 109–549 days), and participants had a mean ARAT score of 8.4 points (SD 11.8 points), a mean FMA motor score of 18.1 points (SD 13.7 points) and a mean Barthel ADL Index score of 14.4 points (SD 3.9 points). Typically, the ARAT score was low [median 3 points (IQR 0–11 points)] and the distribution was positively skewed. The upper limb rehabilitation treatments that were being received at baseline are given in Appendix 2, Table 33. A total of 409 out of 768 (53.3%) participants were receiving physiotherapy and/or occupational therapy at the time of randomisation.
| Characteristic | Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total (N = 770) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 156 (60.7) | 159 (61.4) | 153 (60.2) | 468 (60.8) |
| Female | 101 (39.3) | 100 (38.6) | 101 (39.8) | 302 (39.2) |
| Age at randomisation (years), mean (SD) | 59.9 (13.5) | 59.4 (14.3) | 62.5 (12.5) | 60.6 (13.5) |
| Time from stroke to randomisation (days), median (IQR) | 233 (102–549) | 258 (115–546) | 242 (107–549) | 240 (109–549) |
| Time from stroke to randomisation (months), n (%) | ||||
| < 3 | 57 (22.2) | 46 (17.8) | 58 (22.8) | 161 (20.9) |
| 3–12 | 105 (40.9) | 117 (45.2) | 106 (41.7) | 328 (42.6) |
| > 12 | 95 (37.0) | 96 (37.1) | 90 (35.4) | 281 (36.5) |
| Arm affected by stroke, n (%) | ||||
| Right | 112 (43.6) | 116 (44.8) | 113 (44.5) | 341 (44.3) |
| Left | 145 (56.4) | 143 (55.2) | 141 (55.5) | 429 (55.7) |
| Stroke type, n (%) | ||||
| Cerebral infarction | 197 (76.7) | 202 (78.0) | 214 (84.3) | 613 (79.6) |
| Primary intracerebral haemorrhage | 58 (22.6) | 56 (21.6) | 38 (15.0) | 152 (19.7) |
| Subarachnoid haemorrhage | 2 (0.8) | 1 (0.4) | 2 (0.8) | 5 (0.6) |
| NIHSS total score (0–42) | n = 255 | n = 259 | n = 254 | n = 768 |
| Median (IQR) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 5.0 (4.0–7.0) | 5.0 (3.0–7.0) |
| Montreal Cognitive Assessment (0–30) | n = 248 | n = 250 | n = 242 | n = 740 |
| Median (IQR) | 24.0 (19.0–27.0) | 24.0 (19.0–27.0) | 24.0 (19.0–27.0) | 24.0 (19.0–27.0) |
| Sheffield Screening Test for Acquired Language Disorders | ||||
| Receptive skills score (0–11) | n = 255 | n = 258 | n = 254 | n = 767 |
| Median (IQR) | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) |
| Expressive skills score (0–9) | n = 251 | n = 258 | n = 254 | n = 763 |
| Median (IQR) | 11.0 (9.0–11.0) | 11.0 (10.0–11.0) | 11.0 (9.0–11.0) | 11.0 (9.0–11.0) |
| Total score (0–20) | n = 251 | n = 258 | n = 254 | n = 763 |
| Median (IQR) | 19.0 (16.0–20.0) | 19.0 (17.0–20.0) | 19.0 (17.0–20.0) | 19.0 (17.0–20.0) |
| Handedness,a n (%) | n = 255 | n = 259 | n = 254 | n = 768 |
| Right | 221 (86.7) | 223 (86.1) | 228 (89.8) | 672 (87.5) |
| Left | 34 (13.3) | 35 (13.5) | 25 (9.8) | 94 (12.2) |
| Ambidextrous | 0 (0.0) | 1 (0.4) | 1 (0.4) | 2 (0.3) |
| Dominant hand affected by stroke, n (%) | n = 255 | n = 259 | n = 254 | n = 768 |
| No (including ambidextrous) | 141 (55.3) | 131 (50.6) | 132 (52.0) | 404 (52.6) |
| Yes | 114 (44.7) | 128 (49.4) | 122 (48.0) | 364 (47.4) |
| Upper limb measure | Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total (N = 770) |
|---|---|---|---|---|
| ARAT (0–57) | n = 256 | n = 259 | n = 254 | n = 769 |
| ARAT total score | ||||
| Mean (SD) | 8.5 (11.9) | 8.7 (11.9) | 8.1 (11.5) | 8.4 (11.8) |
| Median (IQR) | 3.0 (0–12) | 3.0 (0–13) | 3.0 (0–11) | 3.0 (0–11) |
| ARAT total score category (0–57), n (%) | n = 256 | n = 259 | n = 254 | n = 769 |
| 0–7 | 178 (69.5) | 175 (67.6) | 173 (68.1) | 526 (68.4) |
| 8–13 | 18 (7.0) | 22 (8.5) | 23 (9.1) | 63 (8.2) |
| 14–19 | 13 (5.1) | 9 (3.5) | 13 (5.1) | 35 (4.6) |
| 20–39 | 47 (18.4) | 53 (20.5) | 45 (17.7) | 145 (18.9) |
| FMA | ||||
| Motor function score (0–66) | n = 255 | n = 259 | n = 254 | n = 768 |
| Mean (SD) | 18.0 (13.1) | 18.2 (14.1) | 18.2 (13.9) | 18.1 (13.7) |
| Range of motion and joint pain score (0–48) | n = 254 | n = 259 | n = 254 | n = 767 |
| Mean (SD) | 41.5 (6.1) | 41.5 (6.2) | 41.0 (6.6) | 41.3 (6.3) |
| Sensation score (0–12) | n = 253 | n = 258 | n = 252 | n = 763 |
| Mean (SD) | 9.4 (3.2) | 9.4 (3.3) | 9.8 (2.9) | 9.5 (3.1) |
| Total upper extremity score (0–126) | n = 254 | n = 259 | n = 254 | n = 767 |
| Mean (SD) | 68.9 (16.5) | 69.0 (17.9) | 68.9 (17.4) | 68.9 (17.3) |
| Barthel ADL Index (0–20) | n = 255 | n = 259 | n = 254 | n = 768 |
| Mean (SD) | 14.5 (3.8) | 14.3 (4.0) | 14.4 (3.9) | 14.4 (3.9) |
| Numeric pain scale (0–10) | n = 253 | n = 259 | n = 254 | n = 766 |
| Mean (SD) | 2.9 (3.2) | 2.7 (3.0) | 2.6 (3.1) | 2.7 (3.1) |
Primary outcome
The proportion of participants who achieved upper limb functional recovery ‘success’ at 3 months in the ITT analysis was 103 out of 232 (44%) in the robot-assisted training group, 118 out of 234 (50%) in the EULT group and 85 out of 203 (42%) in the usual care group (Table 3 and Figure 4). There was little evidence of a statistically significant difference in the incidence of ‘success’ at 3 months when comparing the robot-assisted training group with the usual care group [adjusted odds ratio (aOR) 1.2, 98.33% CI 0.7 to 2.0], the EULT group with the usual care group (aOR 1.5, 98.33% CI 0.9 to 2.5), or the robot-assisted training group with the EULT group (aOR 0.8, 98.33% CI 0.5 to 1.3). Although the unadjusted success rate was 8% higher in the EULT group than in the usual care group, the trial was powered to detect a difference of 15% in success rates between groups. The results of both sensitivity analyses and the PP analysis were consistent with the ITT analysis (see Table 3).
| Outcome | Robot-assisted training | EULT | Usual care | OR (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training vs. usual carea | EULT vs. usual carea | Robot-assisted training vs. EULTa | ||||||||||
| N | n (%) | N | n (%) | N | n (%) | Unadjusted | Adjustedb | Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
| Primary outcome, 3 months | ||||||||||||
| ITT | 232 | 103 (44.4) | 234 | 118 (50.4) | 203 | 85 (41.9) | 1.1 (0.7 to 1.8) | 1.2 (0.7 to 2.0) | 1.4 (0.9 to 2.2) | 1.5 (0.9 to 2.5) | 0.8 (0.5 to 1.2) | 0.8 (0.5 to 1.3) |
| Sensitivity analysis 1c | 197 | 89 (45.2) | 187 | 94 (50.3) | 157 | 66 (42.0) | 1.1 (0.7 to 1.9) | 1.1 (0.6 to 2.0) | 1.4 (0.8 to 2.3) | 1.4 (0.8 to 2.4) | 0.8 (0.5 to 1.3) | 0.8 (0.5 to 1.4) |
| Sensitivity analysis 2d | 148 | 71 (48.0) | 145 | 81 (55.9) | 116 | 56 (48.3) | 1.0 (0.5 to 1.8) | 1.1 (0.6 to 2.2) | 1.4 (0.7 to 2.5) | 1.6 (0.8 to 3.1) | 0.7 (0.4 to 1.3) | 0.7 (0.4 to 1.4) |
| PP | 222 | 97 (43.7) | 219 | 112 (51.1) | NA | NA | NA | NA | NA | NA | 0.7 (0.5 to 1.2) | 0.7 (0.4 to 1.2) |
| Secondary outcomes, 6 months | ||||||||||||
| ITT | 221 | 103 (46.6) | 218 | 118 (54.1) | 185 | 81 (43.8) | 1.1 (0.7 to 1.8) | 1.2 (0.7 to 2.1) | 1.5 (0.9 to 2.5) | 1.6 (0.9 to 2.8) | 0.7 (0.5 to 1.2) | 0.8 (0.5 to 1.3) |
| Sensitivity analysis 1c | 200 | 91 (45.5) | 203 | 110 (54.2) | 163 | 71 (43.6) | 1.1 (0.7 to 1.8) | 1.3 (0.7 to 2.2) | 1.5 (0.9 to 2.5) | 1.6 (0.9 to 2.9) | 0.7 (0.4 to 1.1) | 0.8 (0.4 to 1.3) |
| Sensitivity analysis 2d | 139 | 72 (51.8) | 136 | 84 (61.8) | 109 | 55 (50.5) | 1.1 (0.6 to 1.9) | 1.3 (0.6 to 2.8) | 1.6 (0.9 to 3.0) | 1.9 (0.9 to 4.1) | 0.7 (0.4 to 1.2) | 0.7 (0.3 to 1.4) |
| PP | 213 | 97 (45.5) | 206 | 113 (54.9) | NA | NA | NA | NA | NA | NA | 0.7 (0.4 to 1.1) | 0.7 (0.4 to 1.2) |
FIGURE 4.
Upper limb functional recovery ‘success’ at 3 and 6 months for robot-assisted training, EULT and usual care. (a) The proportion of participants achieving upper limb functional recovery ‘success’ by randomisation group; and (b) pairwise comparisons of group upper limb functional recovery ‘success’ presented as ORs (98.33% CIs) (ITT population). RT, robot-assisted training; UC, usual care.
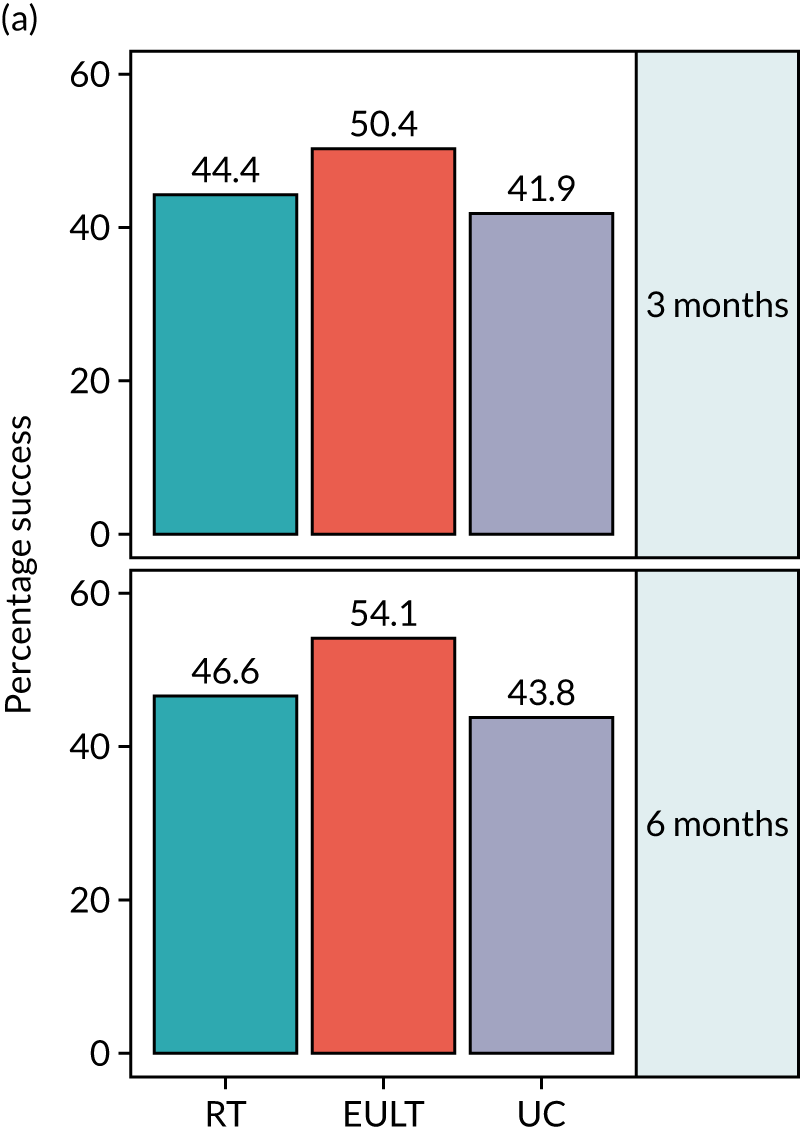
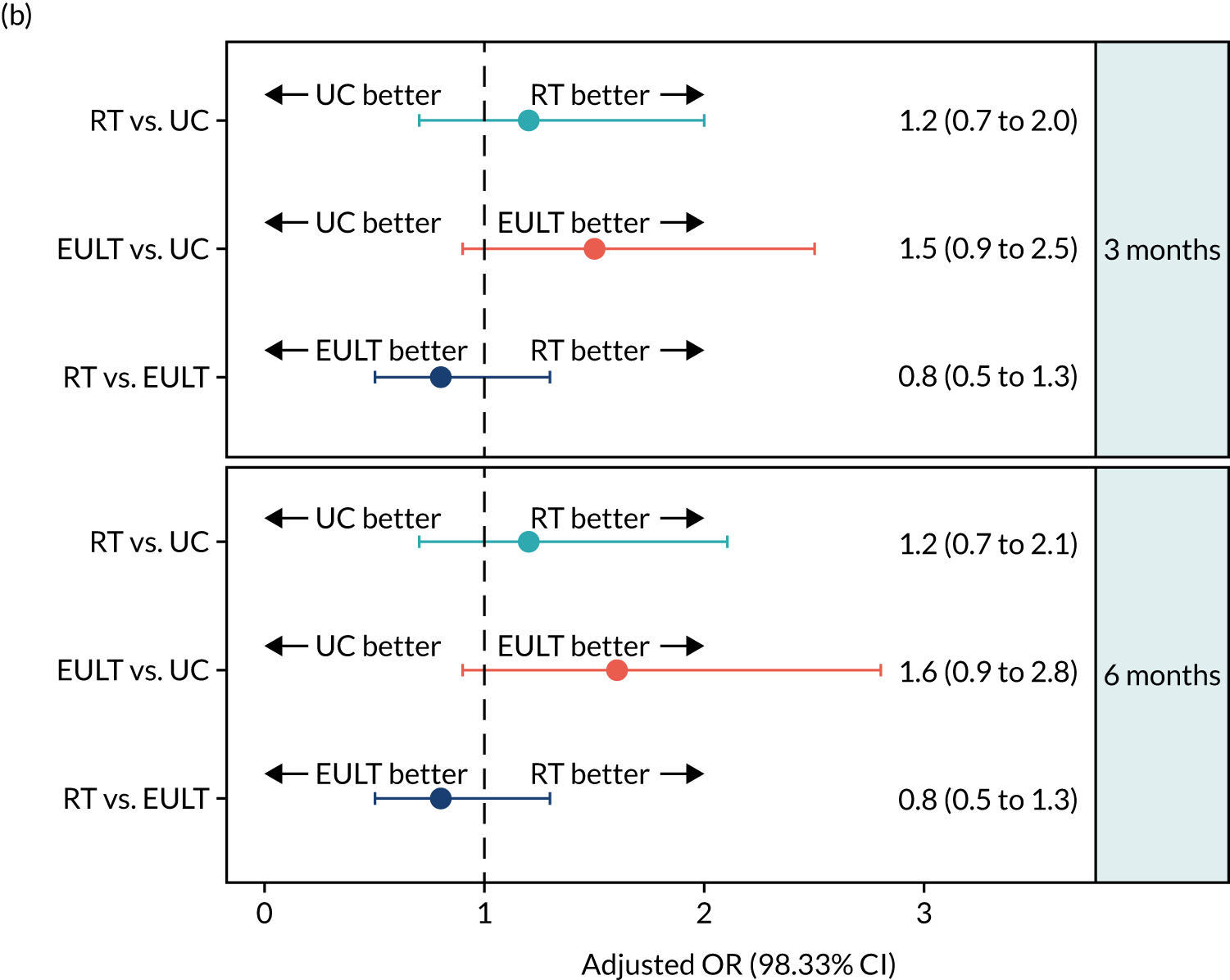
The secondary outcome results of upper limb functional recovery ‘success’ at 6 months are shown in Table 3 and Figure 4. The number of participants who achieved upper limb functional recovery ‘success’ at 6 months in the ITT analysis was 103 out of 221 (47%) in the robot-assisted training group, 118 out of 218 (54%) in the EULT group and 81 out of 185 (44%) in the usual care group; all groups showed slight improvement from the 3-month assessment. The unadjusted success rate was 10% higher in the EULT group than in the usual care group, but there was little evidence of a difference in the incidence of ‘success’ at 6 months when comparing the robot-assisted training group with the usual care group (aOR 1.2, 98.33% CI 0.7 to 2.1), the EULT group with the usual care group (aOR 1.6, 98.33% CI 0.9 to 2.8) or the robot-assisted training group with the EULT group (aOR 0.8, 98.33% CI 0.5 to 1.3). The results of both sensitivity analyses and the PP analysis were consistent with the ITT analysis (see Table 3).
Secondary outcomes
Robot-assisted training versus usual care
Action Research Arm Test total score
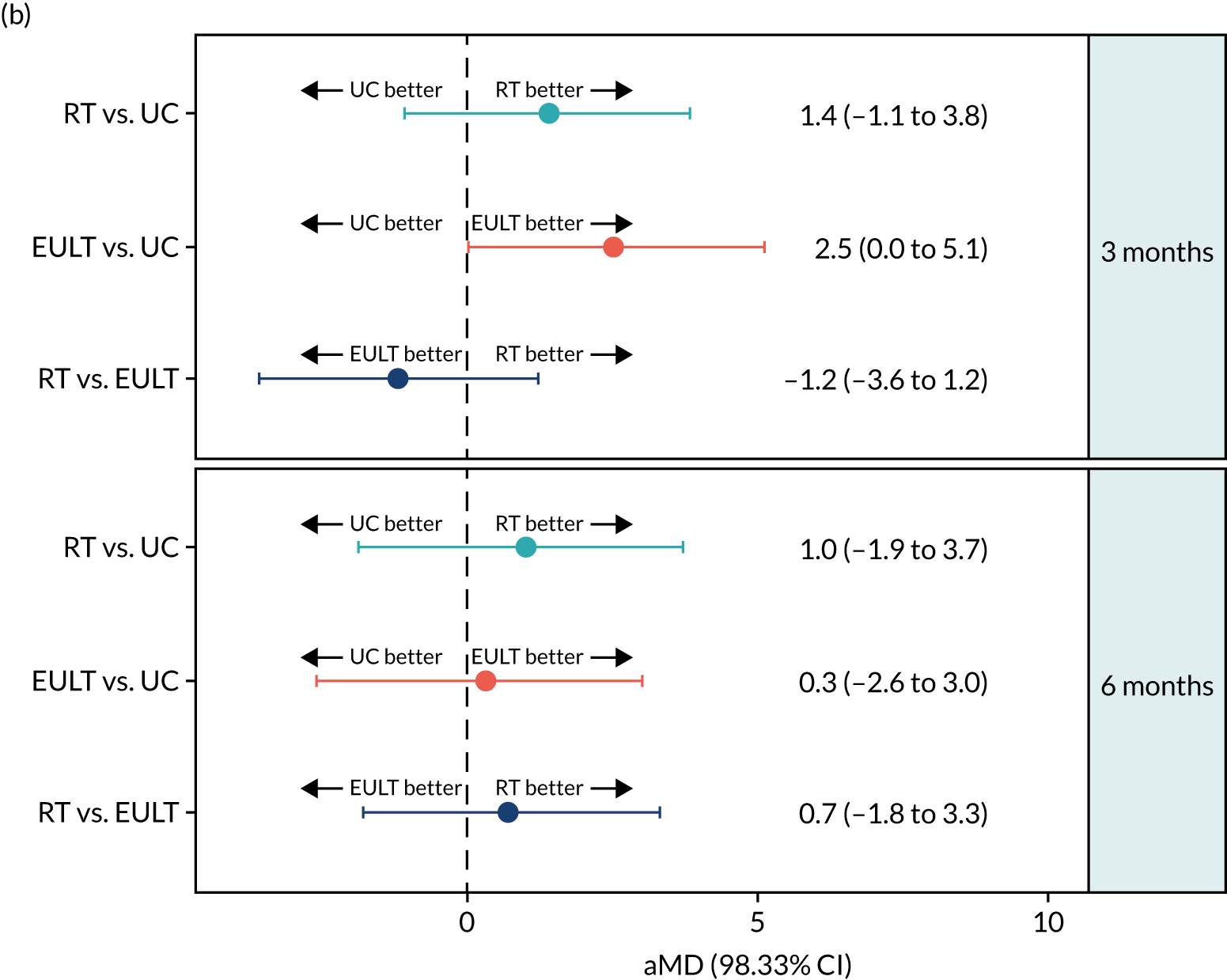
In the robot-assisted training group, the mean ARAT total scores were 8.5 points at baseline, 15.5 points at 3 months and 16.5 points at 6 months. For the usual care group, the mean ARAT total scores were 8.1 points at baseline, 14.2 points at 3 months and 16.4 points at 6 months. There was little evidence of a difference in the ARAT total score between these groups at 3 months [adjusted mean difference (aMD) 1.4, 98.33% CI –1.1 to 3.8] and 6 months (aMD 1.0, 98.33% CI –1.9 to 3.7) (Table 4 and Figure 5). The MCID for the ARAT total score is 6 points,59 so the changes in mean ARAT scores between baseline and 3 months were consistent with a clinically meaningful difference on this scale for both groups, but the differences between 3 and 6 months, and between groups at 3 and 6 months, were not. The results of the ARAT subscales are given in Appendix 3, Table 34.
| Time point | Robot-assisted training | EULT | Usual care | Mean difference (98.33% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | |||||||||||||
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Baseline | 256 | 8.5 (11.9) | 3 (0–12) | 259 | 8.7 (11.9) | 3 (0–13) | 254 | 8.1 (11.5) | 3 (0–11) | NA | NA | NA | NA | NA | NA |
| 3 months | 232 | 15.5 (19.1) | 5 (0–28) | 234 | 17.3 (20.1) | 5 (1–33) | 203 | 14.2 (19.6) | 3 (0–22) | 1.2 (–3.3 to 5.7) | 1.4 (–1.1 to 3.8) | 3.0 (–1.6 to 7.5) | 2.5 (0.0b to 5.1) | –1.8 (–6.2 to 2.6) | –1.2 (–3.6 to 1.2) |
| 6 months | 221 | 16.5 (19.7) | 4 (0–29) | 218 | 17.2 (19.9) | 5 (0–36) | 185 | 16.4 (21.3) | 3 (0–34) | 0.1 (–4.8 to 5.0) | 1.0 (–1.9 to 3.7) | 0.8 (–4.3 to 5.6) | 0.3 (–2.6 to 3.0) | –0.6 (–5.2 to 3.9) | 0.7 (–1.8 to 3.3) |
FIGURE 5.
The ARAT total score at baseline and at 3 and 6 months for robot-assisted training, EULT and usual care. (a) The ARAT total score by randomisation group; and (b) pairwise comparisons of the ARAT total score presented as mean differences (98.33% CIs). RT, robot-assisted training; UC, usual care.

Fugl-Meyer Assessment total upper-extremity score
A similar pattern was seen in the mean FMA total upper-extremity scores, which were 68.9 points at baseline, 76.6 points at 3 months and 78.2 points at 6 months in the robot-assisted training group. By comparison, they were 68.9 points at baseline, 74.2 points at 3 months and 77.9 points at 6 months in the usual care group. There was little evidence of a difference between these groups on this scale at 3 months (aMD 3.1, 98.33% CI –0.0 to 6.5) or at 6 months (aMD 1.6, 98.33% CI –1.8 to 5.2) (Table 5). There is no published MCID for the FMA total upper-extremity score.
| FMA and time point | Robot-assisted training | EULT | Usual care | Mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | ||||||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Total upper-extremity score (0–126) | ||||||||||||
| Baseline | 254 | 68.9 (16.5) | 259 | 69.0 (17.9) | 254 | 68.9 (17.4) | ||||||
| 3 months | 232 | 76.6 (22.1) | 234 | 77.8 (22.8) | 202 | 74.2 (23.6) | 2.4 (–2.9 to 7.6) | 3.1 (–0.0 to 6.5) | 3.6 (–1.8 to 8.9) | 3.7 (0.5 to 6.8) | –1.3 (–6.2 to 3.7) | –0.5 (–3.4 to 2.6) |
| 6 months | 221 | 78.2 (22.8) | 218 | 79.4 (24.1) | 186 | 77.9 (23.2) | 0.3 (–5.2 to 5.8) | 1.6 (–1.8 to 5.2) | 1.5 (–4.3 to 7.0) | 1.8 (–1.8 to 5.3) | –1.1 (–6.5 to 4.2) | –0.2 (–3.6 to 3.4) |
| Motor function (0–66) | ||||||||||||
| Baseline | 255 | 18.0 (13.1) | 259 | 18.2 (14.1) | 254 | 18.2 (13.9) | ||||||
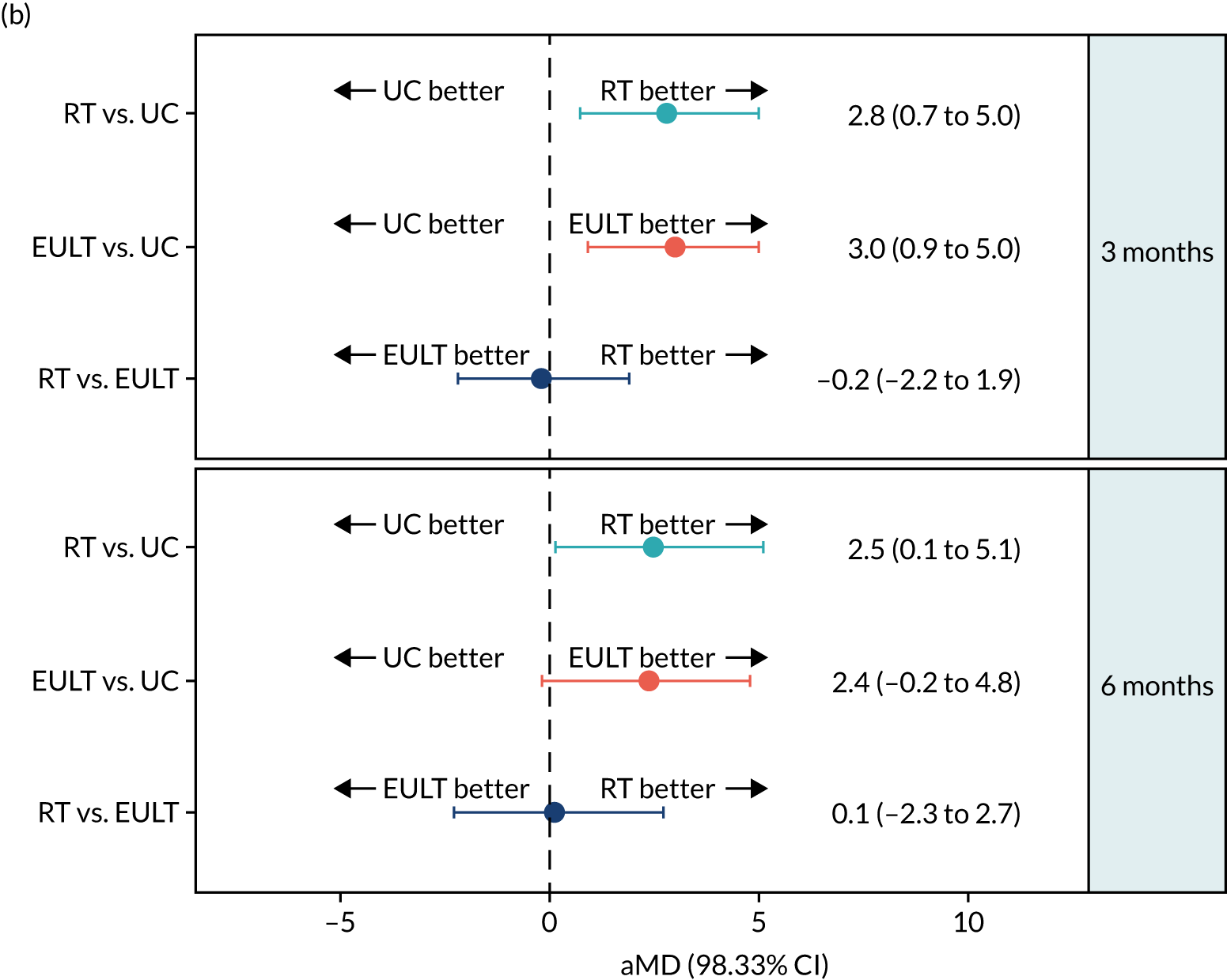
| 3 months | 232 | 26.2 (17.7) | 234 | 27.1 (18.3) | 202 | 24.2 (18.4) | 2.0 (–2.2 to 6.2) | 2.8 (0.7 to 5.0) | 2.9 (–1.3 to 7.1) | 3.0 (0.9 to 5.0) | –0.9 (–4.8 to 3.1) | –0.2 (–2.2 to 1.9) |
| 6 months | 221 | 27.2 (18.4) | 217 | 28.2 (19.3) | 186 | 26.3 (18.9) | 0.9 (–3.6 to 5.3) | 2.5 (0.1 to 5.1) | 1.9 (–2.7 to 6.4) | 2.4 (–0.2 to 4.8) | –1.0 (–5.3 to 3.3) | 0.1 (–2.3 to 2.7) |
| Range of motion and joint pain (0–48) | ||||||||||||
| Baseline | 254 | 41.5 (6.1) | 259 | 41.5 (6.2) | 254 | 41.0 (6.6) | ||||||
| 3 months | 232 | 40.8 (6.8) | 234 | 41.2 (6.2) | 204 | 40.0 (8.0) | ||||||
| 6 months | 221 | 41.5 (6.1) | 218 | 41.6 (5.9) | 186 | 41.6 (6.2) | ||||||
| Sensation (0–12) | ||||||||||||
| Baseline | 253 | 9.4 (3.2) | 258 | 9.4 (3.3) | 252 | 9.8 (2.9) | ||||||
| 3 months | 231 | 9.6 (3.1) | 234 | 9.5 (3.0) | 202 | 9.9 (2.9) | ||||||
| 6 months | 221 | 9.5 (3.0) | 218 | 9.6 (2.9) | 186 | 10.0 (2.7) | ||||||
Fugl-Meyer Assessment motor function subscale
This pattern of improvement was also seen for the FMA motor function subscale. For the robot-assisted training group, the mean scores were 18.0 points at baseline, 26.2 points at 3 months and 27.2 points at 6 months. In comparison, for the usual care group the mean scores were 18.2 points at baseline, 24.2 points at 3 months and 26.3 points at 6 months. The robot-assisted training group had a higher average FMA motor function subscale score than the usual care group at 3 months (aMD 2.8, 98.33% CI 0.7 to 5.0), and this difference was maintained at 6 months (aMD 2.5, 98.33% CI 0.1 to 5.1) (Figure 6; see also Table 5). The MCID for this subscale is 4 points for acute stroke patients and 5.25 points for chronic stroke patients, which makes it difficult to interpret the results from a mixed patient group. 59 Further details and descriptive statistics for the ‘range of motion and joint pain’ and ‘sensation’ subscales are given in Table 5.
FIGURE 6.
The FMA motor function subscale score at baseline and at 3 and 6 months for robot-assisted training, EULT and usual care. (a) The FMA motor function subscale score by randomisation group; and (b) pairwise comparisons of the FMA motor function subscale score presented as mean differences (98.33% CIs). RT, robot-assisted training; UC, usual care.

Barthel Activities of Daily Living Index
There was little change in the Barthel ADL Index score over time. In the robot-assisted training group, the mean was 14.5, 15.5 and 15.6 points at baseline, 3 months and 6 months, respectively. This was similar to the usual care group, in which the mean was 14.4, 15.3 and 15.3 points at baseline, 3 months and 6 months, respectively. There was little evidence of a difference between these groups on this scale at 3 months (aMD 0.2, 98.33% CI –0.4 to 0.8) or at 6 months (aMD 0.5, 98.33% CI –0.1 to 1.1) (Table 6). The MCID on this scale is 1.85 units,59 and so the changes in the mean Barthel ADL Index scores between baseline, 3 months and 6 months were not large enough to be consistent with a clinically meaningful difference on this scale for both groups (i.e. robot-assisted training and usual care), nor were the comparisons between group means at 3 or 6 months.
| Time point | Robot-assisted training | EULT | Usual care | Mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | ||||||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Barthel ADL Index (0–20) | ||||||||||||
| Baseline | 255 | 14.5 (3.8) | 259 | 14.3 (4.0) | 254 | 14.4 (3.9) | ||||||
| 3 months | 233 | 15.5 (3.4) | 236 | 15.9 (3.4) | 207 | 15.3 (3.8) | 0.2 (–0.6 to 1.0) | 0.2 (–0.4 to 0.8) | 0.6 (–0.2 to 1.5) | 0.7 (0.2 to 1.2) | –0.5 (–1.2 to 0.3) | –0.5 (–1.0 to –0.0) |
| 6 months | 223 | 15.6 (3.4) | 222 | 16.0 (3.5) | 190 | 15.3 (3.7) | 0.3 (–0.5 to 1.1) | 0.5 (–0.1 to 1.1) | 0.6 (–0.2 to 1.5) | 0.9 (0.3 to 1.5) | –0.4 (–1.2 to 0.4) | –0.4 (–1.0 to 0.1) |
Stroke Impact Scale
There was little change over time on any SIS subscale. The mean scores on the ADL subscale were 50.8 points at 3 months and 50.4 points at 6 months in the robot-assisted training group, but they were 50.8 and 51.9 points at 3 and 6 months, respectively, in the usual care group. The results were consistent with the Barthel ADL Index, in that they showed little evidence of a difference between these groups at 3 months (aMD 0.7, 98.33% CI –4.2 to 5.8) or at 6 months (aMD –0.6, 98.33% CI –5.6 to 4.5) (Table 7). The MCID on this SIS subscale is 5.9 units,59 so the differences between groups were not consistent with a clinically meaningful difference.
| SIS subscale and time point | Robot-assisted training | EULT | Usual care | Mean difference (98.33% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | |||||||||||||
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| ADL (0–100) | |||||||||||||||
| 3 months | 220 | 50.8 (22.5) | 48 (33–70) | 223 | 55.9 (19.8) | 55 (40–70) | 194 | 50.8 (21.1) | 50 (33–65) | 0.0b (–5.1 to 5.2) | 0.7 (–4.2 to 5.8) | 5.1 (0.3 to 9.9) | 5.6 (0.9 to 10.2) | –5.1 (–10.0 to –0.3) | –4.8 (–9.5 to –0.1) |
| 6 months | 212 | 50.4 (22.3) | 50 (33–68) | 216 | 52.5 (22.3) | 50 (38–68) | 179 | 51.9 (21.3) | 53 (35–70) | –1.4 (–6.7 to 3.9) | –0.6 (–5.6 to 4.5) | 0.6 (–4.7 to 5.8) | 1.1 (–3.9 to 6.1) | –2.0 (–7.2 to 3.1) | –1.7 (–6.7 to 3.3) |
| Hand function (0–100) | |||||||||||||||
| 3 months | 219 | 15.5 (24.4) | 0 (0–20) | 223 | 21.4 (27.9) | 5 (0–40) | 193 | 14.3 (22.9) | 0 (0–25) | 1.3 (–4.3 to 6.8) | 2.6 (–2.6 to 7.9) | 7.2 (1.1 to 13.1) | 7.9 (2.2 to 13.5) | –5.9 (–11.9 to 0.1) | –5.4 (–10.9 to 0.2) |
| 6 months | 213 | 15.7 (25.2) | 0 (0–25) | 216 | 18.4 (26.2) | 0 (0–35) | 179 | 14.8 (23.5) | 0 (0–25) | 0.9 (–5.0 to 6.8) | 1.9 (–3.6 to 7.5) | 3.6 (–2.4 to 9.5) | 3.9 (–1.8 to 9.6) | –2.7 (–8.6 to 3.3) | –2.0 (–7.4 to 3.6) |
| Mobility (0–100) | |||||||||||||||
| 3 months | 219 | 61.6 (25.1) | 64 (44–83) | 223 | 66.4 (23.2) | 69 (50–86) | 192 | 61.0 (24.6) | 61 (46–81) | 0.6 (–5.3 to 6.5) | 1.3 (–4.3 to 6.9) | 5.4 (–0.1 to 11.1) | 5.8 (0.4 to 11.2) | –4.8 (–10.4 to 0.7) | –4.5 (–9.9 to 0.9) |
| 6 months | 213 | 61.7 (24.8) | 64 (44–83) | 216 | 63.9 (23.7) | 68 (47–83) | 180 | 62.9 (23.8) | 65 (44–83) | –1.2 (–7.1 to 4.7) | –0.5 (–6.0 to 5.1) | 1.1 (–4.6 to 6.8) | 1.4 (–4.1 to 6.8) | –2.3 (–7.9 to 3.3) | –1.9 (–7.3 to 3.5) |
| Social participation (0–100) | |||||||||||||||
| 3 months | 217 | 47.7 (24.7) | 47 (29–66) | 221 | 51.7 (23.0) | 53 (38–69) | 193 | 47.1 (23.7) | 47 (31–63) | 0.6 (–5.1 to 6.3) | 0.5 (–5.2 to 6.2) | 4.6 (–0.9 to 10.1) | 4.7 (–0.7 to 10.1) | –4.0 (–9.5 to 1.5) | –4.2 (–9.6 to 1.3) |
| 6 months | 210 | 47.0 (25.9) | 44 (25–66) | 216 | 50.2 (24.4) | 50 (31–69) | 179 | 49.2 (23.8) | 50 (31–63) | –2.2 (–8.3 to 3.9) | –2.2 (–8.2 to 3.9) | 1.0 (–4.8 to 6.9) | 1.2 (–4.7 to 7.0) | –3.3 (–9.1 to 2.6) | –3.4 (–9.2 to 2.4) |
| Strength (0–100) | |||||||||||||||
| 3 months | 220 | 43.8 (20.4) | 44 (31–56) | 220 | 48.1 (22.2) | 50 (31–63) | 193 | 40.1 (19.1) | 38 (25–50) | ||||||
| 6 months | 213 | 40.9 (21.7) | 38 (25–56) | 216 | 44.3 (22.7) | 44 (25–63) | 176 | 40.3 (21.6) | 38 (25–56) | ||||||
| Emotion (0–100) | |||||||||||||||
| 3 months | 220 | 68.3 (18.2) | 69 (56–82) | 222 | 69.9 (16.5) | 69 (58–83) | 194 | 67.4 (20.5) | 67 (53–86) | ||||||
| 6 months | 211 | 66.9 (18.3) | 67 (53–81) | 216 | 68.3 (18.8) | 69 (56–83) | 179 | 67.4 (19.7) | 69 (56–83) | ||||||
| Memory (0–100) | |||||||||||||||
| 3 months | 219 | 76.0 (22.4) | 82 (61–96) | 222 | 80.3 (18.8) | 86 (68–96) | 194 | 76.8 (22.2) | 82 (61–96) | ||||||
| 6 months | 213 | 74.6 (22.7) | 79 (58–93) | 215 | 77.4 (21.2) | 82 (64–96) | 178 | 77.8 (23.4) | 86 (64–96) | ||||||
| Communication (0–100) | |||||||||||||||
| 3 months | 220 | 80.1 (23.4) | 89 (68–100) | 222 | 85.3 (20.5) | 96 (79–100) | 194 | 80.9 (24.3) | 93 (68–100) | ||||||
| 6 months | 213 | 79.3 (21.7) | 86 (68–100) | 216 | 83.7 (22.7) | 96 (75–100) | 179 | 82.2 (22.5) | 93 (71–100) | ||||||
| Stroke recovery (0–100) | |||||||||||||||
| 3 months | 217 | 47.9 (18.9) | 50 (30–60) | 223 | 50.3 (18.9) | 50 (40–60) | 190 | 45.2 (19.9) | 50 (30–60) | ||||||
| 6 months | 213 | 51.4 (20.4) | 50 (35–70) | 215 | 50.9 (21.5) | 50 (35–70) | 180 | 48.5 (20.3) | 50 (33–60) | ||||||
The mean scores on the SIS hand function subscale were 15.5 points at 3 months and 15.7 points at 6 months in the robot-assisted training group, but were 14.3 and 14.8 points at 3 and 6 months, respectively, in the usual care group. There was also little evidence of a difference in hand function between groups at 3 months (aMD 2.6, 98.33% CI –2.6 to 7.9) or 6 months (aMD 1.9, 98.33% CI –3.6 to 7.5). The MCID on this SIS subscale is 17.8 units,59 so the results were not consistent with a clinically meaningful difference.
The mean scores on the SIS mobility subscale were 61.6 points at 3 months and 61.7 points at 6 months in the robot-assisted training group, but were 61.0 and 62.9 points at 3 and 6 months, respectively, in the usual care group. As before, there was little evidence of a difference between groups for mobility at 3 months (aMD 1.3, 98.33% CI –4.3 to 6.9) or 6 months (aMD –0.5, 98.33% CI –6.0 to 5.1). However, the MCID on this SIS subscale is 4.5 units,59 which was included in these CIs, so a clinically meaningful difference between groups cannot be ruled out.
The mean scores on the SIS social participation subscale were 47.7 points at 3 months and 47.0 points at 6 months in the robot-assisted training group, but were 47.1 and 49.2 points at 3 and 6 months, respectively, in the usual care group. There was also little evidence of a difference at 3 months (aMD 0.5, 98.33% CI –5.2 to 6.2) or 6 months (aMD –2.2, 98.33% CI –8.2 to 3.9) between the robot-assisted training group and the usual care group, as measured by the SIS. More details and descriptive statistics for strength, emotion, memory, communication and stroke recovery are given in Table 7.
Robot-assisted training versus enhanced upper limb therapy
Action Research Arm Test total score
Both groups showed an improvement in ARAT total score from baseline to 3 months, but little improvement thereafter. In the robot-assisted training group, the mean ARAT total score was 8.5 points at baseline, 15.5 points at 3 months and 16.5 points at 6 months. In comparison, the mean ARAT total score for the EULT group was 8.7 points at baseline, 17.3 points at 3 months and 17.2 points at 6 months. There was little evidence of a difference in the ARAT total score between these groups at 3 months (aMD –1.2, 98.33% CI –3.6 to 1.2) or at 6 months (aMD 0.7, 98.33% CI –1.8 to 3.3) (see Table 4 and Figure 5). The MCID for ARAT total score is 6 points,59 so the changes in mean ARAT scores between baseline and 3 months were consistent with a clinically meaningful difference on this scale for both groups, but those between 3 and 6 months, and between groups at 3 and 6 months, were not. The results of the ARAT subscales are given in Appendix 3, Table 34.
Fugl-Meyer Assessment total upper-extremity score
There was a similar pattern in the FMA total upper-extremity score. The mean FMA total scores were 68.9 points at baseline, 76.6 points at 3 months and 78.2 points at 6 months in the robot-assisted training group, and were 69.0, 77.8 and 79.4 points at baseline, 3 months and 6 months, respectively, for the EULT group. There was little evidence of a difference between these groups on this scale at 3 months (aMD –0.5, 98.33% CI –3.4 to 2.6) or 6 months (aMD –0.2, 98.33% CI –3.6 to 3.4) (see Table 5). There is no published MCID for the FMA total upper-extremity score.
Fugl-Meyer Assessment motor function subscale
The FMA motor function subscale scores also showed a similar pattern. The mean score changed from 18.0 points at baseline to 26.2 points at 3 months and 27.2 points at 6 months in the robot-assisted training group, and was 18.2, 27.1 and 28.2 points at baseline, 3 months and 6 months, respectively, in the EULT group. There was little evidence of a difference in this subscale between the two groups at 3 months (aMD –0.2, 98.33% CI –2.2 to 1.9) or 6 months (aMD 0.1, 98.33% CI –2.3 to 2.7). The MCID for this subscale is 4 points for acute stroke patients and 5.25 points for chronic stroke patients59 (see Table 5 and Figure 6). Further details and descriptive statistics for ‘range of motion and joint pain’ and ‘sensation’ subscales are given in Table 5.
Barthel Activities of Daily Living Index
There was little change in the Barthel ADL Index score over time. In the robot-assisted training group, the mean score was 14.5 points at baseline, 15.5 points at 3 months and 15.6 points at 6 months. In comparison, the mean scores for the EULT group were 14.3, 15.9 and 16.0 points at baseline, 3 months and 6 months, respectively. There was limited evidence that the EULT group participants fared better than the robot-assisted training group participants when comparing ADL at 3 months (aMD –0.5, 98.33% CI –1.0 to –0.0), but there was no difference at 6 months (aMD –0.4, 98.33% CI –1.0 to 0.1) (see Table 6). The MCID on this scale is 1.85 units,59 and so the changes in mean Barthel ADL Index scores between baseline, 3 months and 6 months were not large enough to be consistent with a clinically meaningful difference on this scale for both groups, nor were the comparisons between group mean scores at 3 or 6 months.
Stroke Impact Scale
The mean scores on the SIS ADL subscale were 50.8 points at 3 months and 50.4 points at 6 months in the robot-assisted training group, but were 55.9 and 52.5 points at 3 and 6 months, respectively, in the EULT group. The scores of the ADL subscale of the SIS were consistent with the Barthel ADL Index scores, in that they showed evidence that EULT group participants fared better than the robot-assisted training group participants at 3 months (aMD –4.8, 98.33% CI –9.5 to –0.1), but there was no difference at 6 months (aMD –1.7, 98.33% CI –6.7 to 3.3) (see Table 7). The MCID on this scale is 5.9 units,59 which was included in the CI; therefore, a difference of this size cannot be ruled out at both time points.
The mean scores on the SIS hand function subscale were 15.5 points at 3 months and 15.7 points at 6 months in the robot-assisted training group, but were 21.4 and 18.4 points at 3 and 6 months, respectively, in the EULT group. There was little evidence of a difference between the robot-assisted training group and the EULT group in hand function at 3 months (aMD –5.4, 98.33% CI –10.9 to 0.2) or 6 months (aMD –2.0, 98.33% CI –7.4 to 3.6). The MCID on this scale is 17.8 units,59 so the results were not consistent with a clinically meaningful difference (see Table 7).
The mean scores on the SIS mobility subscale were 61.6 points at 3 months and 61.7 points at 6 months in the robot-assisted training group, but were 66.4 and 63.9 points at 3 and 6 months, respectively, in the EULT group. Similarly, there was little evidence of a difference in mobility at 3 months (aMD –4.5, 98.33% CI –9.9 to 0.9) or 6 months (aMD –1.9, 98.33% CI –7.3 to 3.5). The MCID on this scale is 4.5 units,59 which was included in these CIs, so a clinically meaningful difference cannot be ruled out.
Finally, the mean scores on the SIS social participation subscale were 47.7 points at 3 months and 47.0 points at 6 months in the robot-assisted training group, but were 51.7 and 50.2 points at 3 and 6 months, respectively, in the EULT group. There was little evidence of a difference in social participation scores at 3 months (aMD –4.2, 98.33% CI –9.6 to 1.3) or 6 months (aMD –3.4, 98.33% CI –9.2 to 2.4). More details and descriptive statistics for strength, emotion, memory, communication and stroke recovery subscales are given in Table 7.
Enhanced upper limb therapy versus usual care
Action Research Arm Test total score
In the EULT group, the mean ARAT total score was 8.7 points at baseline, 17.3 points at 3 months and 17.2 points at 6 months. In comparison, the mean ARAT total score for the usual care group was 8.1, 14.2 and 16.4 points at baseline, 3 months and 6 months, respectively. The EULT group participants had better upper limb function than the usual care group participants at 3 months (aMD 2.5, 98.33% CI 0.0 to 5.1), but this difference was not maintained at 6 months (aMD 0.3, 98.33% CI –2.6 to 3.0) (see Table 4 and Figure 5). The MCID for ARAT total score is 6 points,59 so the changes in mean ARAT scores between baseline and 3 months were consistent with a clinically meaningful difference on this scale for both groups, but the changes between 3 and 6 months, and between groups at 3 and 6 months, were not. The results of the ARAT subscales are given in Appendix 3, Table 34.
Fugl-Meyer Assessment total upper-extremity score
In the EULT group, the mean FMA total upper-extremity score was 69.0 points at baseline, 77.8 points at 3 months and 79.4 points at 6 months. In comparison, the mean scores for the usual care group were 68.9, 74.2 and 77.9 points at baseline, 3 months and 6 months, respectively. The EULT group participants had less upper limb impairment than the usual care group participants for the total score at 3 months (aMD 3.7, 98.33% CI 0.5 to 6.8), but this difference was not maintained at 6 months (aMD 1.8, 98.33% CI –1.8 to 5.3) (see Table 5). There is no published MCID for the FMA total upper-extremity score.
Fugl-Meyer Assessment motor function subscale
In the EULT group, the mean score for the FMA motor function subscale was 18.2 points at baseline, 27.1 points at 3 months and 28.2 points at 6 months. In comparison, the scores for the usual care group were 18.2, 24.2 and 26.3 points at baseline, 3 months and 6 months, respectively. The EULT group had a higher average FMA motor function subscale score than the usual care group at 3 months (aMD 3.0, 98.33% CI 0.9 to 5.0), but this difference was not maintained at 6 months (aMD 2.4, 98.33% CI –0.2 to 4.8) (see Table 5 and Figure 6). The MCID for this subscale is 4 points for acute stroke patients and 5.25 points for chronic stroke patients, which makes it difficult to interpret the results from a mixed patient group. 59 Further details and descriptive statistics for ‘range of motion and joint pain’ and ‘sensation’ subscales are given in Table 5.
Barthel Activities of Daily Living Index
In the EULT group, the mean scores for the Barthel ADL Index were 14.3 points at baseline, 15.9 points at 3 months and 16.0 points at 6 months. In comparison, the mean scores for the usual care group were 14.4, 15.3 and 15.3 points at baseline, 3 months and 6 months, respectively. The EULT group performed better than the usual care group when comparing ADL at 3 months (aMD 0.7, 98.33% CI 0.2 to 1.2) and at 6 months (aMD 0.9, 98.33% CI 0.3 to 1.5) (see Table 6). The MCID on this scale is 1.85 units,59 and so the changes in the mean Barthel ADL Index scores between baseline, 3 months and 6 months were not large enough to be consistent with a clinically meaningful difference on this scale for both groups, nor were the comparisons between group mean scores at 3 and 6 months.
Stroke Impact Scale
The mean scores on the SIS ADL subscale were 55.9 points at 3 months and 52.5 points at 6 months in the EULT group, but were 50.8 and 51.9 points at 3 and 6 months, respectively, in the usual care group. When comparing ADL, the results were consistent with the Barthel ADL Index, in that they showed evidence that EULT participants scored better than usual care participants (aMD 5.6, 98.33% CI 0.9 to 10.2) at 3 months. This difference was not maintained at 6 months (aMD 1.1, 98.33% CI –3.9 to 6.1) (see Table 7). The MCID on this scale is 5.9 units,59 which is included in the CI; therefore, a difference of this size between groups cannot be ruled out at both time points.
The mean scores on the SIS hand function subscale were 21.4 points at 3 months and 18.4 points at 6 months in the EULT group, but were 14.3 and 14.8 points at 3 and 6 months, respectively, in the usual care group. There was evidence that the EULT group scored better than the usual care group at 3 months (aMD 7.9, 98.33% CI 2.2 to 13.5), but not at 6 months (aMD 3.9, 98.33% CI –1.8 to 9.6). The MCID on this scale is 17.8 units,59 so the results were not consistent with a clinically meaningful difference.
The mean scores on the SIS mobility subscale were 66.4 points at 3 months and 63.9 points at 6 months in the EULT group, compared with 61.0 and 62.9 points at 3 and 6 months, respectively, in the usual care group. There was evidence that the EULT group scored better than the usual care group at 3 months (aMD 5.8, 98.33% CI 0.4 to 11.2), but not at 6 months (aMD 1.4, 98.33% CI –4.1 to 6.8). The MCID on this scale is 4.5 units,59 which was included in these CIs, so a clinically meaningful difference cannot be ruled out.
The mean scores on the SIS social participation subscale were 51.7 points at 3 months and 50.2 points at 6 months in the EULT group, compared with 47.1 and 49.2 points at 3 and 6 months, respectively, in the usual care group. There was little evidence of a difference between the EULT and usual care groups at 3 months (aMD 4.7, 98.33% CI –0.7 to 10.1) or 6 months (aMD 1.2, 98.33% CI –4.7 to 7.0). Descriptive statistics for strength, emotion, memory, communication and stroke recovery are given in Table 7.
Upper limb pain
Upper limb pain scores varied little over time, or between groups. The proportion of participants reporting upper limb pain in the arm affected by stroke at 3 months was 46% in the robot-assisted training group, 43% in the EULT group and 51% in the usual care group. The mean numeric pain score at 3 months was 2.5 points in the robot-assisted training group, 2.2 points in the EULT group and 2.7 points in the usual care group (Table 8). There was little evidence of a difference at 3 months between the robot-assisted training group and the usual care group (aMD –0.2, 98.33% CI –0.9 to 0.4), between the EULT group and the usual care group (aMD –0.6, 98.33% CI –1.2 to 0.1) or between the robot-assisted training group and the EULT group (aMD 0.3, 98.33% CI –0.3 to 0.9).
| Numeric rating scale and time point | Robot-assisted training | EULT | Usual care | Mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | ||||||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Upper limb pain (0–10)b | ||||||||||||
| Baseline | 253 | 2.9 (3.2) | 259 | 2.7 (3.0) | 254 | 2.6 (3.1) | ||||||
| 3 months | 232 | 2.5 (3.2) | 236 | 2.2 (2.9) | 206 | 2.7 (3.2) | –0.2 (–0.9 to 0.5) | –0.2 (–0.9 to 0.4) | –0.5 (–1.3 to 0.2) | –0.6 (–1.2 to 0.1) | 0.4 (–0.3 to 1.0) | 0.3 (–0.3 to 0.9) |
| 6 months | 223 | 2.5 (3.2) | 221 | 2.5 (3.2) | 190 | 2.0 (2.9) | 0.5 (–0.2 to 1.2) | 0.4 (–0.3 to 1.1) | 0.5 (–0.3 to 1.2) | 0.4 (–0.3 to 1.1) | 0.0c (–0.7 to 0.8) | 0.0c (–0.7 to 0.7) |
The proportion of participants reporting pain at 6 months was 44% in the robot-assisted training group, 45% in the EULT group and 39% in the usual care group. The mean numeric pain score at 6 months was 2.5 points in the robot-assisted training group, 2.5 points in the EULT group and 2.0 points in the usual care group. There was little evidence of a difference at 6 months between the robot-assisted training group and the usual care group (aMD 0.4, 98.33% CI –0.3 to 1.1), between the EULT group and the usual care group (aMD 0.4, 98.33% CI –0.3 to 1.1) or between the robot-assisted training group and the EULT group (aMD 0.0, 98.33% CI –0.7 to 0.7).
Pre-planned subgroup and exploratory analyses
Exploratory subgroup analysis
There was little evidence of a difference in the mean ARAT total score between pairs of randomisation groups when looking at the subgroups of participants for time since stroke, baseline ARAT score, trial centre and age (Table 9 and Figure 7). This was also the case for the FMA motor function subscale score (Table 10 and Figure 8a–c) and the Barthel ADL Index score (Table 11 and Figure 8d–f), which were examined by the time since stroke categories. Note that, by splitting the data across subgroups, the sample size is reduced for any comparison, so the CIs are wide. The CI includes results that are often consistent with a clinically meaningful difference (as per the MCID) on the scales, sometimes in both directions.
| Subgroup | Robot-assisted training | EULT | Usual care | Unadjusted mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | |
| Time since stroke | ||||||||||||
| < 3 months | 47 | 25.6 (22.2) | 23 (3–48) | 39 | 34.2 (23.9) | 46 (5–57) | 50 | 22.4 (24.3) | 7 (0–48) | 3.1 (–8.2 to 14.4) | 11.8 (–0.9 to 23.5) | –8.7 (–20.2 to 3.6) |
| 3–12 months | 94 | 16.2 (19.8) | 4 (0–29) | 110 | 16.5 (19.1) | 5 (3–30) | 77 | 13.4 (18.5) | 3 (0–21) | 2.8 (–4.4 to 9.7) | 3.1 (–3.9 to 9.4) | –0.3 (–6.6 to 6.3) |
| > 12 months | 91 | 9.5 (13.6) | 3 (0–11) | 85 | 10.5 (14.6) | 3 (0–15) | 76 | 9.6 (15.4) | 3 (0–8) | –0.2 (–6.0 to 5.0) | 0.8 (–5.0 to 6.3) | –1.0 (–6.2 to 4.0) |
| Baseline ARAT score | ||||||||||||
| 0 | 84 | 5.5 (12.1) | 0 (0–4) | 89 | 5.1 (12.0) | 0 (0–3) | 87 | 3.4 (10.6) | 0 (0–3) | 2.1 (–2.1 to 6.3) | 1.7 (–2.3 to 5.9) | 0.4 (–4.1 to 4.8) |
| 1–7 | 77 | 6.5 (10.3) | 3 (1–7) | 69 | 10.3 (15.0) | 4 (3–10) | 51 | 7.7 (13.1) | 3 (0–6) | –1.1 (–7.3 to 3.5) | 2.6 (–3.8 to 8.6) | –3.7 (–9.3 to 1.0) |
| 8–13 | 16 | 22.3 (15.6) | 19 (11–29) | 21 | 24.1 (14.7) | 23 (14–29) | 20 | 17.5 (13.7) | 16 (8–22) | 4.8 (–6.6 to 17.0) | 6.6 (–4.5 to 16.7) | –1.8 (–13.0 to 11.1) |
| 14–19 | 12 | 32.8 (14.2) | 29 (24–43) | 7 | 38.9 (14.2) | 38 (32–49) | 11 | 34.2 (22.0) | 33 (14–55) | –1.3 (–18.7 to 18.1) | 4.7 (–16.1 to 24.7) | –6.0 (–20.3 to 12.7) |
| 20–39 | 43 | 43.6 (11.0) | 45 (34–54) | 48 | 43.7 (11.9) | 47 (33–54) | 34 | 43.5 (12.3) | 46 (36–53) | 0.1 (–6.0 to 6.9) | 0.2 (–6.1 to 6.9) | –0.1 (–5.7 to 5.7) |
| Trial centre | ||||||||||||
| 1 | 65 | 16.2 (19.6) | 5 (1–29) | 70 | 18.7 (19.9) | 7 (3–33) | 57 | 16.2 (21.5) | 4 (0–39) | 0.1 (–9.0 to 8.7) | 2.5 (–6.6 to 11.1) | –2.4 (–10.5 to 5.7) |
| 2 | 76 | 12.3 (18.2) | 3 (0–17) | 72 | 13.6 (20.0) | 1 (0–25) | 68 | 9.6 (17.0) | 0 (0–10) | 2.7 (–4.4 to 9.5) | 4.0 (–3.6 to 11.5) | –1.3 (–8.9 to 6.1) |
| 3 | 34 | 19.7 (22.9) | 6 (0–45) | 28 | 26.1 (23.8) | 20 (3–54) | 30 | 24.1 (24.0) | 18 (0–48) | –4.4 (–18.3 to 9.7) | 2.0 (–12.9 to 17.0) | –6.4 (–20.6 to 7.9) |
| 4 | 57 | 16.3 (16.9) | 6 (3–28) | 64 | 16.0 (17.9) | 4 (3–32) | 48 | 12.3 (15.4) | 5 (3–16) | 4.0 (–3.9 to 11.3) | 3.7 (–4.1 to 11.0) | 0.2 (–7.3 to 7.9) |
| Age | ||||||||||||
| < 55 years | 81 | 17.0 (20.8) | 5 (1–29) | 90 | 17.0 (19.8) | 5 (3–30) | 53 | 12.0 (19.1) | 3 (0–14) | 5.0 (–3.9 to 12.9) | 5.0 (–3.7 to 12.5) | 0.0b (–7.3 to 7.5) |
| 55–70 years | 97 | 14.3 (17.9) | 5 (0–26) | 84 | 16.6 (20.9) | 3 (0–34) | 83 | 11.3 (17.6) | 3 (0–15) | 3.1 (–3.5 to 9.3) | 5.4 (–1.9 to 12.4) | –2.3 (–9.3 to 4.6) |
| > 70 years | 54 | 15.2 (18.6) | 5 (0–28) | 60 | 18.6 (19.9) | 9 (2–34) | 67 | 19.7 (21.4) | 6 (0–39) | –4.5 (–13.0 to 4.3) | –1.1 (–9.7 to 7.8) | –3.4 (–11.9 to 5.2) |
FIGURE 7.
Forest plots for the subgroup analysis of the unadjusted ARAT score by time since stroke, baseline ARAT score, trial centre and age categories, comparing (a) robot-assisted training and usual care; (b) EULT and usual care; and (c) robot-assisted training and EULT. RT, robot-assisted training; UC, usual care.


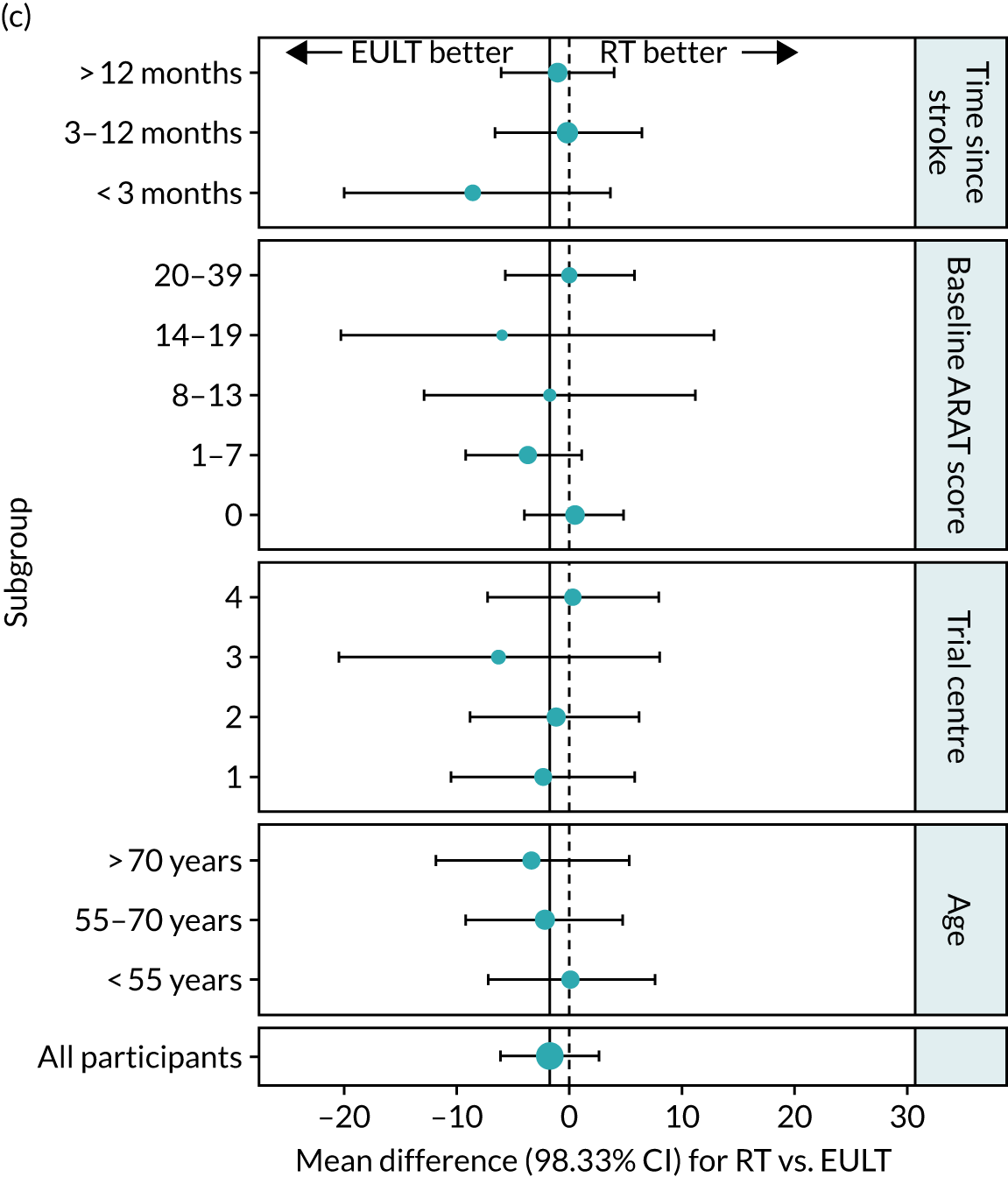
| Time since stroke | Robot-assisted training | EULT | Usual care | Unadjusted mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | |
| < 3 months | 47 | 36.0 (19.8) | 37 (16–55) | 39 | 38.8 (21.0) | 42 (21–58) | 50 | 30.3 (22.3) | 26 (6–51) | 5.7 (–4.6 to 15.9) | 8.5 (–2.7 to 19.2) | –2.8 (–13.1 to 7.9) |
| 3–12 months | 94 | 26.4 (17.8) | 23 (10–41) | 110 | 26.4 (17.6) | 23 (11–42) | 76 | 24.0 (18.4) | 17 (9–40) | 2.3 (–4.5 to 8.9) | 2.4 (–4.2 to 8.6) | –0.0 (–5.9 to 6.0) |
| > 12 months | 91 | 21.1 (14.2) | 19 (8–30) | 85 | 22.7 (15.7) | 20 (10–32) | 76 | 20.4 (14.2) | 18 (8–29) | 0.7 (–4.7 to 5.9) | 2.3 (–3.3 to 8.0) | –1.6 (–7.1 to 3.7) |
FIGURE 8.
Forest plots for the subgroup analysis of the unadjusted FMA motor function subscale score and Barthel ADL Index by time since stroke categories. (a) FMA, robot-assisted training vs. usual care; (b) FMA, EULT vs. usual care; (c) FMA, robot-assisted training vs. EULT; (d) Barthel ADL Index, robot-assisted training vs. usual care; (e) Barthel ADL Index, EULT vs. usual care; and (f) Barthel ADL Index, robot-assisted training vs. EULT. RT, robot-assisted training; UC, usual care.





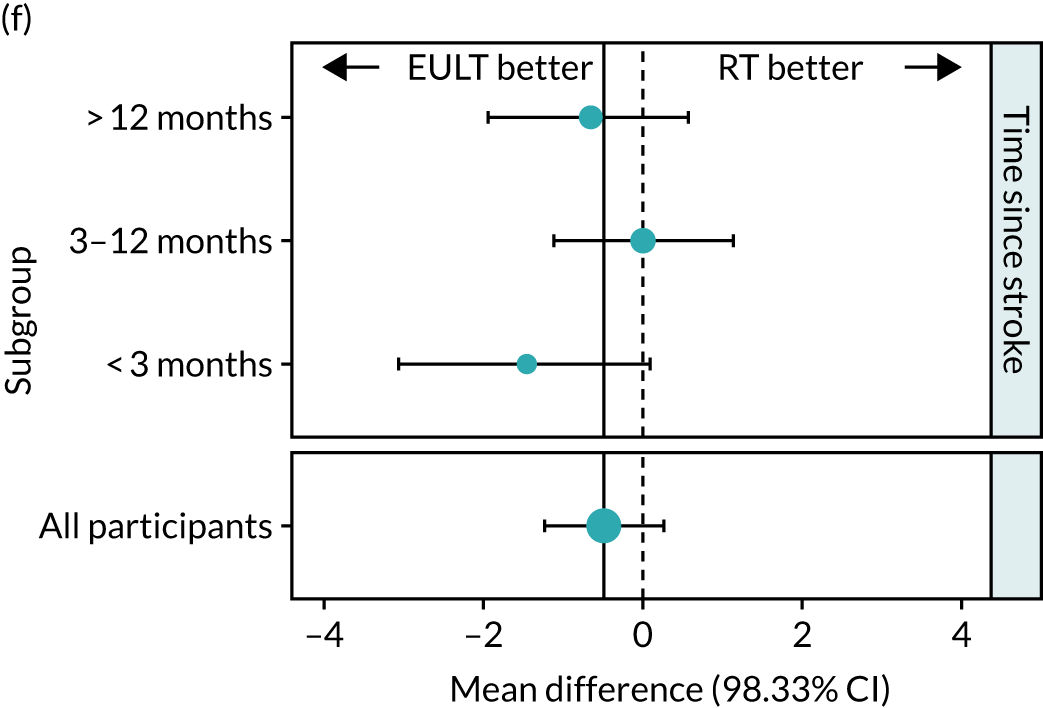
| Time since stroke | Robot-assisted training | EULT | Usual care | Unadjusted mean difference (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Robot-assisted training – usual care | EULT – usual care | Robot-assisted training – EULT | |
| < 3 months | 47 | 15.8 (3.3) | 17 (15–18) | 39 | 17.3 (2.8) | 18 (15–20) | 50 | 15.7 (4.1) | 17 (13–19) | 0.1 (–1.7 to 2.0) | 1.6 (–0.1 to 3.4) | –1.5 (–3.1 to 0.1) |
| 3–12 months | 95 | 15.6 (3.1) | 16 (14–18) | 110 | 15.6 (3.6) | 16 (14–18) | 80 | 14.9 (3.9) | 16 (13–18) | 0.7 (–0.5 to 2.0) | 0.7 (–0.6 to 2.0) | 0.0b (–1.1 to 1.2) |
| > 12 months | 91 | 15.2 (3.8) | 16 (13–18) | 87 | 15.8 (3.2) | 17 (14–18) | 77 | 15.4 (3.5) | 16 (13–18) | –0.2 (–1.6 to 1.1) | 0.4 (–0.9 to 1.7) | –0.6 (–1.9 to 0.6) |
Descriptive analysis of the relationship between treatment received and total Action Research Arm Test score
Descriptive analysis between treatment received and total ARAT score was completed for the two intervention groups. These data are shown in Chapters 4 and 5.
Masking of treatment allocation
At 3 months, the researchers conducting the outcome assessments reported that they became aware of the treatment group allocation for 101 out of 676 (15%) participants with non-missing data (see Appendix 4, Table 35). The rate in the robot-assisted training group, at 21%, was almost twice that in the EULT group (12%) and the usual care group (12%). The researcher was correct in 86 out of 94 (91%) cases; for 58 out of 101 (57%) cases, the researcher reported that the treatment was known before the outcome assessment was completed (see Appendix 4, Table 36). Becoming aware of the treatment group before outcome assessment was more common in the intervention groups than in the usual care group [robot-assisted training group, 34/50 (68%); EULT group, 15/26 (58%); and usual care group, 9/25 (36%)]. The results were similar at 6 months: the researchers thought that they knew the allocation of 88 out of the 633 (14%) assessments that were undertaken, and awareness of the treatment group before the outcome assessment was completed was also similar across the groups [robot-assisted training group, 22/39 (56%); EULT group, 15/25 (60%); and usual care group, 15/24 (63%)] (see Appendix 4, Tables 37 and 38).
Participant safety data
Serious adverse events
Serious adverse events were reported for 39 out of 257 (15.2%) participants in the robot-assisted training group (a total of 43 events), for 33 out of 259 (12.7%) participants in the EULT group (a total of 42 events) and for 20 out of 254 (7.9%) participants in the usual care group (a total of 29 events). The number of events per participant is shown in Table 12.
| Number of SAEs | Participants, n (%) | ||
|---|---|---|---|
| Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | |
| 0 | 218 (84.8) | 226 (87.3) | 234 (92.1) |
| 1 | 35 (13.6) | 28 (10.8) | 14 (5.5) |
| 2 | 4 (1.6) | 3 (1.2) | 3 (1.2) |
| 3 | 0 (0) | 1 (0.4) | 3 (1.2) |
| 4 | 0 (0) | 0 (0.0) | 0 (0) |
| 5 | 0 (0) | 1 (0.4) | 0 (0) |
| Total events | 43 | 42 | 29 |
The number of participants with at least one SAE during the trial was 39 out of 257 (15.2%) in the robot-assisted training group, 33 out of 259 (13.7%) in the EULT group and 20 out of 254 (7.9%) in the usual care group. The difference in proportions between the robot-assisted training and usual care groups was 7% (98.33% CI 0.2% to 14%); for the EULT and usual care groups, it was 5% (98.33% CI –2% to 12%), and for the robot-assisted training and EULT groups it was 2% (98.33% CI –5% to 10%). From these results, we can see that there is evidence of a larger proportion of participants in the robot-assisted training group than in the usual care group reporting SAEs. There was little evidence of a difference between the EULT and usual care groups, or between the robot-assisted training and EULT groups.
The median number of SAEs was 0 (IQR 0–0) across all three groups. The p-values from Mann–Whitney U-tests were 0.013 when comparing robot-assisted training with usual care, 0.08 when comparing EULT with usual care and 0.4 when comparing robot-assisted training with EULT, which indicate that there is evidence of a statistically significant difference between the robot-assisted training and usual care groups (with SAEs being slightly less common in the usual care group), but not between the EULT and usual care groups, or between the robot-assisted training and EULT groups (significance level = 0.05/3 = 0.0167).
Details of reported SAEs are shown in Appendix 5, Table 39. The most common reason for an event to be reported as a SAE was because it resulted in hospitalisation (robot-assisted training group, n = 32; EULT group, n = 37; and usual care group, n = 25). None of the SAEs was considered to be related to the trial intervention. Six events resulted in death (robot-assisted training group, n = 1; EULT group, n = 4; and usual care group, n = 1). Note that, in the CONSORT flow diagram (see Figure 2), four participants are reported to be deceased: one participant in the robot-assisted training group and three participants in the EULT group. For two participants (EULT group, n = 1; usual care group, n = 1), a SAE was initially reported during their involvement in the trial, but the date of death from this event was after their involvement ceased.
A summary of the SAEs that resulted in death is presented in Appendix 5, Table 40. A summary of all other SAEs is presented in Appendix 5, Table 41.
Non-serious adverse events
At 3 months, 38 out of 233 (16.3%) participants in the robot-assisted training group and 37 out of 236 (15.7%) participants in the EULT group in whom an assessment was carried out reported at least one non-serious adverse event. For the usual care group, this number was 35 out of 207 (16.9%) participants. At 6 months, 42 out of 223 (18.8%) participants in the robot-assisted training group and 46 out of 222 (20.7%) participants in the EULT group in whom an assessment was carried out reported at least one non-serious adverse event. For the usual care group, this was 27 out of 190 (14.2%) participants. There was little evidence of a difference in the proportion of participants with at least one adverse event between the pairs of groups. The median number of adverse events was 0 (IQR 0–0) for all groups at both time points. Full details of all non-serious events are shown in Appendix 5, Tables 42–44.
Chapter 4 The robot-assisted training programme
Introduction
This chapter includes a description of the robot-assisted training programme and details of delivery fidelity.
The robot-assisted training programme
Robot-assisted training used a MIT-Manus robotic gym system, which was available at each of the four trial hub sites. The MIT-Manus robotic gym comprises three components: a shoulder–elbow (InMotionARM robot), a wrist (InMotionWRIST robot) and a hand attachment (InMotionHAND robot). The shoulder–elbow and wrist components are standalone robot units, whereas the hand component is an attachment that fits onto the shoulder–elbow unit. The training programme was designed to be progressive and challenging. Over the course of the programme, all participants progressed from exercises that involved gross movements (e.g. shoulder–elbow movement) to exercises that involved more complex movements (e.g. hand movement). A detailed description of the robot-assisted training programme is provided in the following paragraph, and a completed TIDieR checklist34 is provided in Appendix 1, Table 27.
The robot-assisted training programme aimed to provide therapy sessions three times per week for 12 weeks, predominantly in an outpatient setting (36 sessions in total). This was divided into 18 sessions on the shoulder–elbow (± hand attachment) component and 18 sessions on the wrist robot component. A session lasted for up to 1 hour, which included 45 minutes of face-to-face therapy for each individual participant (the aim was to provide 27 hours of face-to-face therapy in total). Transport was arranged by the local trial co-ordinator if required. A senior therapist (physiotherapist or occupational therapist) undertook the initial training session on each robot unit (shoulder–elbow and wrist), and at the session in which the hand attachment was introduced. The remaining therapy sessions were designed to be delivered by a therapy assistant.
The 12-week robot-assisted training programme was divided into three consecutive blocks (Figure 9).
FIGURE 9.
Summary of the robot-assisted training programme.
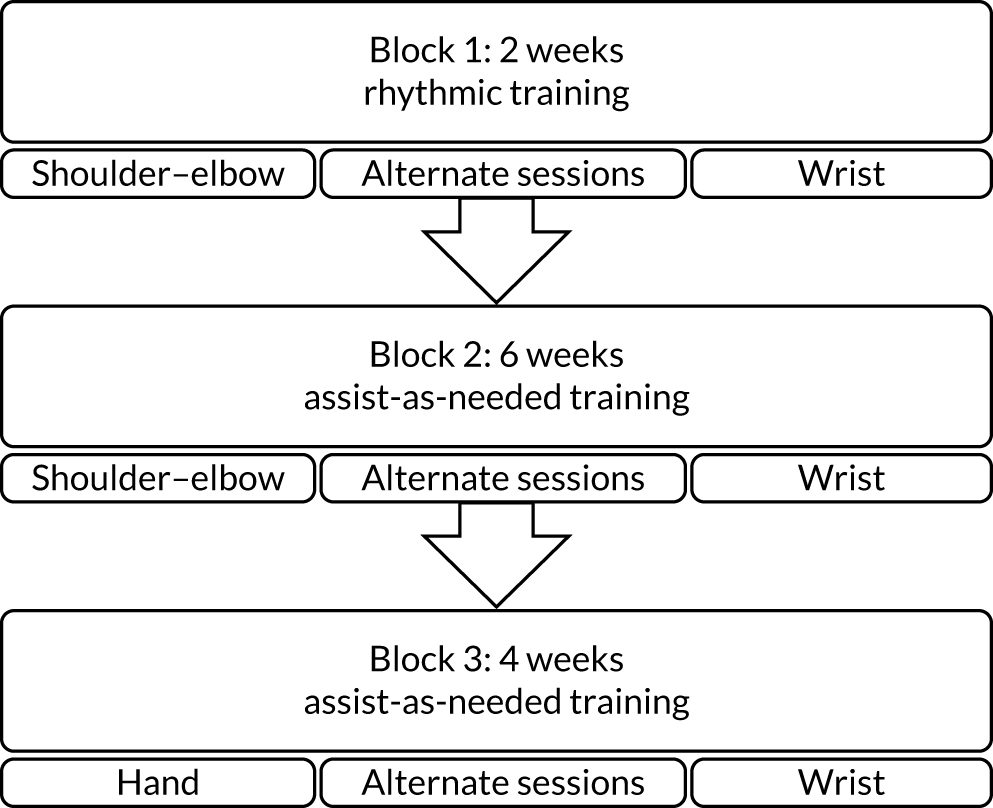
Block 1 lasted for 2 weeks; the shoulder–elbow and wrist robot components were used on alternate training sessions for three sessions each. Participants used the robot’s ‘playback protocol’, whereby the arm (placed in the robot arm support) was moved rhythmically towards the flashing red targets that appear sequentially on a clock face on the robot computer screen (Figure 10). This block allowed participants to become gradually accustomed to robot-assisted training.
FIGURE 10.
Robot-assisted training clock face exercise. The small white circle is the cursor the patient attempts to move; the large white circle is the target.

Block 2 lasted for 6 weeks; the shoulder–elbow and wrist robot components were used on alternate training sessions for nine sessions each. Participants used the ‘adaptive protocol’, whereby the arm (placed in the robot arm support) was ‘assisted as needed’ to reach the flashing red targets appearing sequentially on the clock face.
For the final 4 weeks (block 3), the shoulder–elbow and wrist robot units were used on alternate training sessions for six sessions each. In this block, if a participant was physically able to grasp the hand attachment, then the hand attachment was introduced. If a participant was physically unable to grasp the hand attachment, they continued the programme on the shoulder–elbow robot and did not use the hand attachment. Participants who used the hand attachment in this block used the ‘adaptive protocol’, whereby the arm is ‘assisted as needed’ by a robot to complete the exercises. On the wrist robot, and if a participant was unable to use the hand attachment on the shoulder–elbow robot, the ‘random protocol’ was used. The ‘random protocol’ was ‘assisted as needed’ as participants attempt to move towards flashing red targets on the clock face, but this time targets were presented in random order.
In addition to the robot training exercises described above, the robot-assisted training programme also involved regular ‘robot evaluations’ whereby kinematics (i.e. related to the movement pattern) and kinetics (i.e. related to the causes of movement) were measured by the robot. These were completed every 2 weeks.
The robot-assisted training programme was prescriptive, in that every session had a defined number and type of exercises that a participant should complete. Each exercise comprised a prespecified number of movement attempts. Movement attempts were ‘assisted’ for playback, adaptive and random protocols and ‘unassisted’ for robot evaluations.
The total number of potential movement attempts differed per session, as this depended on the robot (shoulder–elbow robot alone, shoulder–elbow robot with hand attachment, or wrist robot) and the training block. If a participant was able to use the hand attachment, the total number of possible shoulder–elbow robot movement attempts over the 18 sessions was 20,060 (a mean of 1114 movement attempts per session). For those participants who were unable to use the hand attachment, the number of possible movement attempts was 19,496 (a mean of 1083 movement attempts per session). There was no variation in training for the wrist robot; therefore, for each participant, the total number possible of wrist robot movement attempts over 18 sessions was 19,196 (a mean of 1066 movement attempts per session). Overall, the number of possible movement attempts for the robot-assisted training programme was 38,692 for those participants unable to use the hand attachment and 39,256 for those able to use the hand attachment (a mean 1075 and 1090 movement attempts per session, respectively).
All therapy movement attempts were ‘assisted’. The total possible number of ‘assisted’ movement attempts for each participant on each robot was 17,280 over the 18 sessions (a total of 34,560 over the 36 sessions and a mean of 960 movement attempts per session).
Robot evaluations were ‘unassisted’. The total possible number of ‘unassisted’ robot movement attempts for each participant was 2216 (for those unable to use the hand attachment) or 2780 (for those able to use the hand attachment) for the shoulder–elbow robot over the 18 sessions. The total possible number of ‘unassisted’ movement attempts for each participant on the wrist robot was 1916 over the 18 sessions. Over the 36 sessions, this equated to a total target of 4132 (for those unable to use the hand attachment) or 4696 (for those able to use the hand attachment) ‘unassisted’ movement attempts.
Training was provided to senior therapists and therapy assistants who delivered the robot-assisted training programme with updates throughout the trial. Six manuals provided guidance on intervention delivery. 60 The first described the purpose, principles and structure of the robot-assisted training programme, as well as staff roles and responsibilities; the second provided instructions on how to operate the MIT-Manus robotic gym system. The remaining manuals provided guidance on how to conduct the robot-assisted training programme on each robot component, with accompanying step-by-step flow charts to guide trial staff.
Recording the robot-assisted training programme
Details from all sessions were recorded on the trial robots. This included the number of times a participant used a trial robot, a participant’s duration on the robot and the number and type of exercises practised. The anonymised data from the trial robots were uploaded to the Newcastle University server. Data about non-attendance were recorded on the trial database.
Analysis of robot data
Descriptive analyses of numeric data were undertaken and presented as mean (SD) or median (IQR), as appropriate. Categorical data are presented as n (%).
Fidelity of the robot-assisted training programme
A total of 257 participants were randomised to receive robot-assisted training. Two participants withdrew from the trial before receiving any robot-assisted training, as they felt that it was too much of a burden. Data for the remaining 255 (99%) participants were available.
A total of 255 out of 257 (99%) participants received at least one robot-assisted training session, with 254 out of 257 (99%) of participants receiving one or more shoulder–elbow robot-assisted training sessions and 243 out of 257 (95%) participants receiving one or more wrist robot-assisted training sessions. Of the 254 participants who received training on the shoulder–elbow robot, 156 (61%) received training using the hand attachment.
Overall, 8026 out of a possible 9252 sessions (87%) were attended by participants. The number of sessions attended for the wrist robot was lower [3812/4626 (82%)] than the number of shoulder–elbow sessions attended [4214/4626 (91%)]. The median number of robot-assisted training sessions received per participant was 35 (IQR 31–36 sessions), with a median of 18 sessions (IQR 16–18 session) on the shoulder–elbow robot and 18 sessions (IQR 14–18 sessions) on the wrist robot. The most common reason for lack of attendance at sessions was because participants withdrew from the trial [429/1226 (35%) sessions that were not attended]. Details of reasons for all missed sessions can be found in Appendix 6, Table 45.
The median total duration of training (time on robot) per participant over the 12-week intervention period was 23 hours and 30 minutes (IQR 19 hours and 13 minutes to 25 hours and 47 minutes). The median total duration of training per participant was similar on both robots [11 hours and 40 minutes (IQR 9 hours and 54 minutes to 12 hours and 54 minutes) on the shoulder–elbow robot and 11 hours 56 minutes (IQR 9 hours and 35 minutes to 13 hours and 14 minutes) on the wrist].
The median duration of face-to-face training in each attended session was 41 minutes (IQR 35–47 minutes). Similarly, the median duration of face-to-face training in each attended session was similar for both robots [40 minutes (IQR 34–46 minutes) on the shoulder–elbow robot and 42 minutes (IQR 36–48 minutes) on the wrist robot].
In terms of movement attempts, participants achieved 15,002 out of 20,060 (75%) movement attempts on the shoulder–elbow robot with the hand attachment, 11,141 out of 19,496 (57%) on the shoulder–elbow robot without the hand attachment and 13,460 out of 19,196 (70%) on the wrist robot. Over the entire robot-assisted training programme, the median total number of movement attempts per participant was 27,407 (IQR 22,900–32,187 movement attempts). There was a median of 808 movement attempts (IQR 668–1040 movement attempts) per participant, per attended session. The numbers of movement attempts achieved for the shoulder–elbow robot (with and without the hand attachment), the wrist robot and overall are shown in Appendix 6, Table 46.
Movement attempts by baseline Action Research Arm Test score and time since stroke
The impact of baseline ARAT score and time since stroke on the number of assisted, unassisted and total movement attempts was investigated. The predefined categories of baseline ARAT score of 0–7 and 20–39 were used, but, owing to the small numbers of participants randomised with baseline ARAT scores in the ranges of 8–13 or 14–19, these two categories were combined. For time since stroke, the pre-defined categories of < 3 months, 3–12 months and > 12 months were used.
Participants with the low baseline ARAT scores (0–7) achieved the lowest median total number of movement attempts [25,368 (IQR 21,662–29,153)]. Participants with baseline ARAT score in the ranges of 8–19 and 20–39 achieved median total movement attempts of 32,187 (IQR 27,745–36,624) and 33,358 (IQR 29,704–38,348), respectively.
Time since stroke did not have an impact on the number of movement attempts achieved, with all subgroups achieving a similar number. Participants for whom it was < 3 months since their stroke achieved a median of 28,359 movement attempts (IQR 18,611–35,070 movement attempts), compared with a median of 26,706 movement attempts (IQR 23,319–32,163 movement attempts) for those for whom it was 3–12 months since their stroke, and a median of 27,412 movement attempts (IQR 23,660–30,061 movement attempts) for participants for whom it was > 12 months since their stroke. Appendix 6, Table 47, shows the number of assisted, unassisted and overall movement attempts by baseline ARAT score and time since stroke for the shoulder–elbow and wrist robots.
Descriptive analysis of the relationship between treatment received and total Action Research Arm Test score
The total ARAT score at 3 and 6 months was plotted against the number of sessions attended, total duration of therapy received and total robot movement attempts. For participants who did not attend an appointment, zero duration and zero movement attempts were assumed. There is little evidence of an association between total ARAT score and amount of treatment received (Figure 11).
FIGURE 11.
Scatterplots for the robot-assisted training group for ARAT total score at 3 and 6 months. (a) Number of sessions attended vs. ARAT score at 3 months; (b) total time on robot vs. ARAT score at 3 months; (c) total robot movement attempts vs. ARAT score at 3 months; (d) number of sessions attended vs. ARAT score at 6 months; (e) total time on robot vs. ARAT score at 6 months; and (f) total robot movement attempts vs. ARAT score at 6 months.
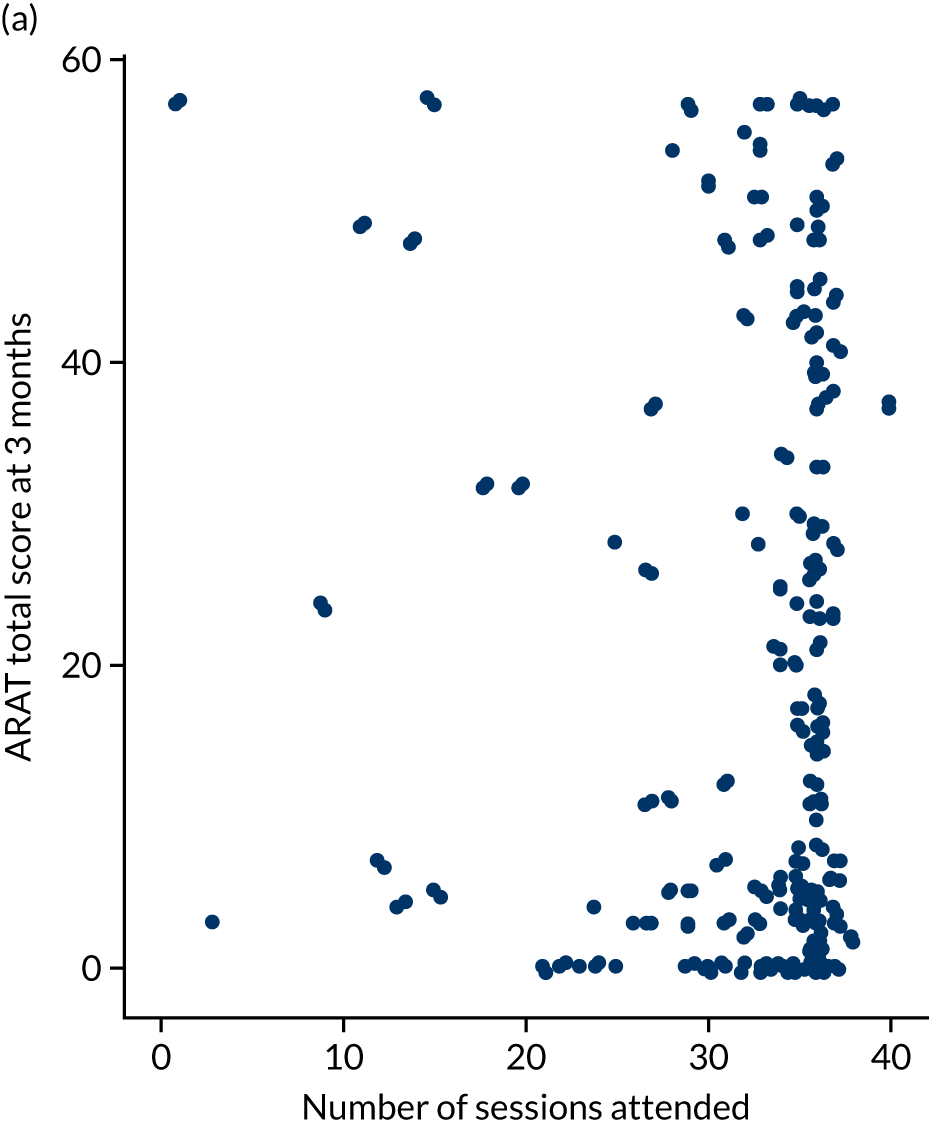
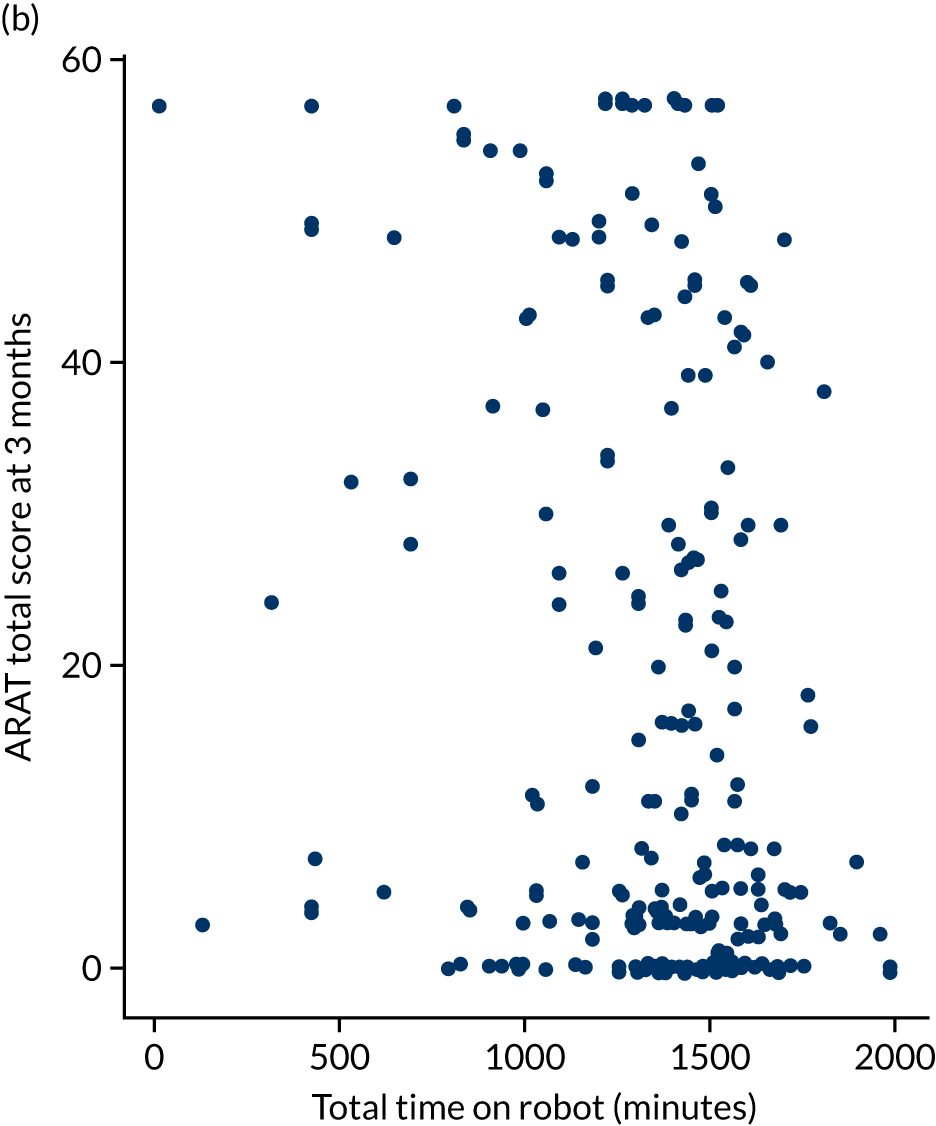

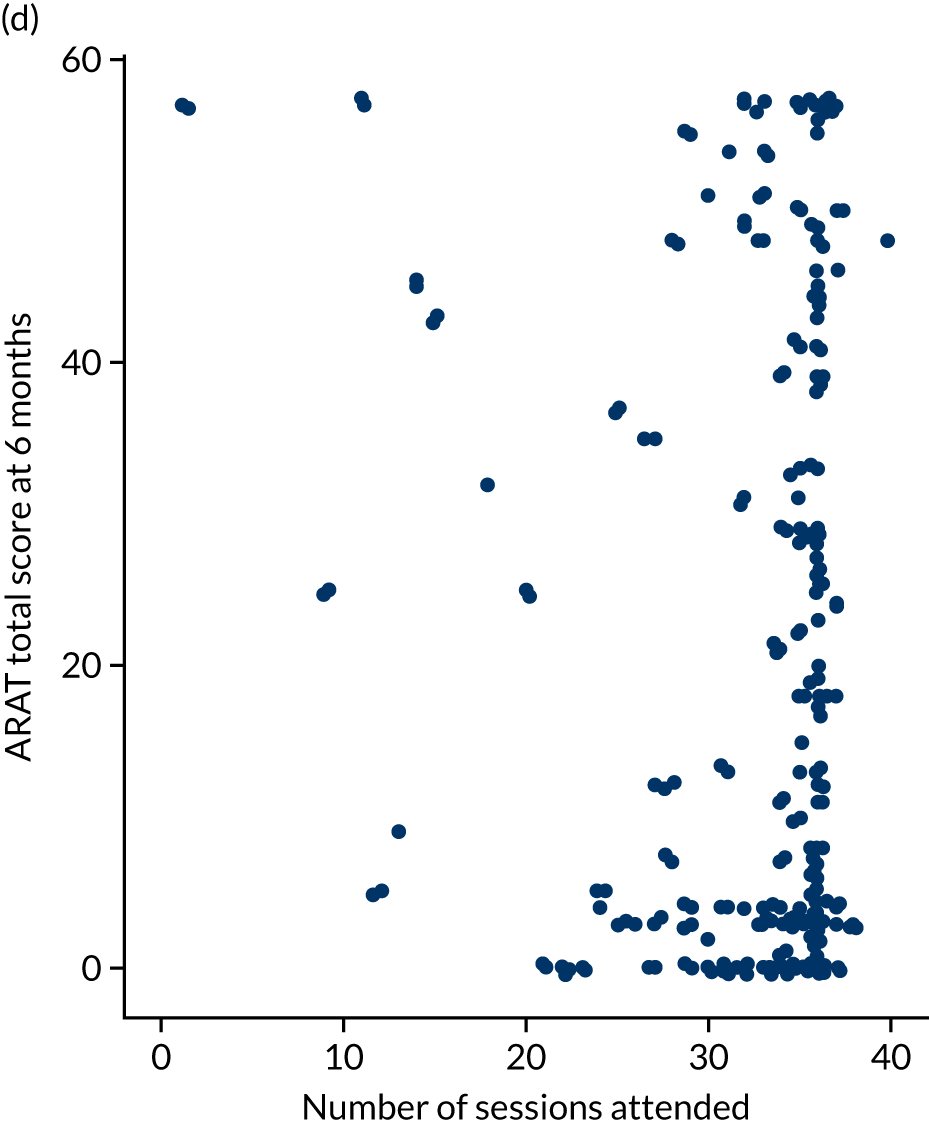

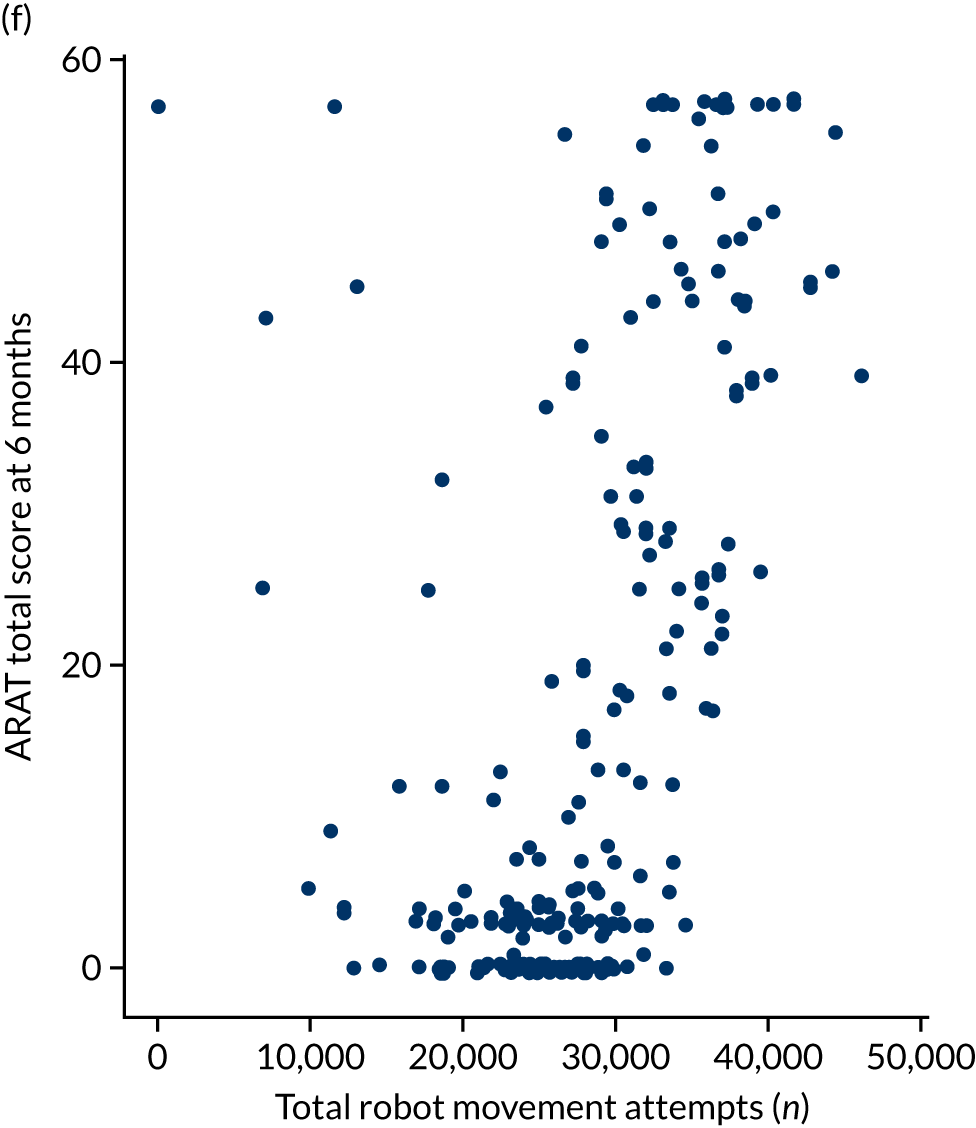
Chapter 5 The enhanced upper limb therapy programme
Parts of this chapter have been reproduced from Bosomworth et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter includes a description of the EULT programme and details of delivery fidelity, dose, goals selected and goal achievement.
The enhanced upper limb therapy programme
The EULT programme consisted of repetitive functional task practice, directed towards achieving participant-centred goals. The aim of the EULT programme was to promote long-term improvement in arm function that was useful to individual participants by focusing on their personal goals, through an intervention that was engaging and challenging, yet achievable. The intervention structure and principles were standardised, while its application allowed tailoring to individuals’ goals and abilities. A detailed description of the EULT programme is provided in this chapter, and a completed TIDieR checklist34 is provided in Appendix 1, Table 27.
To ensure that this comparator was matched to the robot-assisted training in terms of frequency and duration, the EULT programme aimed to provide therapy sessions three times per week for 12 weeks, predominantly in an outpatient setting (36 sessions in total). A session lasted for up to 1 hour, which included 45 minutes of face-to-face therapy for each individual participant (the aim was 27 hours of face-to-face therapy in total). Transport was arranged by the local trial co-ordinator if required. A senior therapist (physiotherapist or occupational therapist) undertook the initial therapy session and reviewed the participant every 4 weeks. The remaining therapy sessions were designed to be delivered by a therapy assistant.
At the start of their first session, participants were invited to identify personally relevant goals; these were not prespecified, other than that they should involve a functional task involving the affected arm. Participants were advised to select no more than four goals at each review session (up to 12 across the EULT programme), based on previous work. 62 Goals from previous arm rehabilitation studies were prepared as examples to facilitate goal selection. 35–38 The goal selection process was not formalised, but was undertaken according to each senior therapist’s clinical judgement. In each session, participants practised functional activities to work towards their goals. Activities could involve whole- or part-task practice, at the discretion of the senior therapist. Whole-task activity practice consisted of practising all of the components of the task in sequence. Part-task activity practice consisted of practising a specific part of a task. Part-task practice was undertaken when a participant had difficulty with a specific part of a task, as it enabled them to concentrate on this particular aspect while working towards completing the task as a whole. The order in which activities were practised, and the time spent on each, were at the discretion of the therapist and participant. Participants were encouraged to undertake as many repetitions as possible in each session. There was no set target for the number of repetitions.
At the start of each session, participants undertook a brief ‘warm-up’, consisting of focusing attention on their affected arm, gently stretching soft tissues and mobilising joints to stimulate sensation and proprioception, before commencing practice, which focused on an individual’s goals.
At therapy sessions 12 (end of week 4) and 24 (end of week 8), progress towards goals was reviewed with a senior therapist. If a participant had achieved a goal, a new goal and a new activity to practise were selected. If a participant found a goal or activity too challenging, or experienced other problems, an alternative goal and activity were chosen. At the final therapy session, practice continued and progress towards goals was reviewed with a senior therapist. Part of the session was also dedicated to providing feedback to the participant about progress over the course of the programme and advice about maintaining arm function in the longer term.
Training was provided to the senior therapists and therapy assistants who delivered the EULT programme, with updates throughout the trial. In addition, three manuals provided guidance on intervention delivery. 60 The first described the purpose, principles and structure of the EULT programme, as well as staff roles and responsibilities; the second provided guidance on how to structure each session (including assessment, warm-up and stretching, demonstration and education, progression, monitoring compensatory movements and feedback). The third manual provided examples of soft tissue stretches prior to task practice, and an overview of commonly selected goals, with accompanying step-by-step flow charts to guide and progress practice of functional tasks, each with whole-task and part-task options.
Recording the enhanced upper limb therapy programme
The following were recorded: session attendance, duration of session, duration of face-to-face therapy during a session, number of repetitions per task practised in each session, goals selected and type of task practice (whole or part task), and goal achievement. In terms of task repetitions, for whole-task practice, completion of the whole task counted as one task repetition, that is from the start position to a ‘return to the start position’ or to completion of the task (if different from the start position). For part-task practice, completion of the component of the part task counted as one task repetition.
Details about the goals selected were recorded at the initial therapy session and at the 4- and 8-week review sessions by the senior therapists. Information about whether or not a participant had achieved their goals (recorded as ‘yes’/‘no’, according to the senior therapist’s clinical judgement) was recorded at the 4-, 8- and 12-week review sessions. A formal goal attainment scale was not used.
Analysis of enhanced upper limb therapy data
Descriptive analyses of numeric data were undertaken and are presented as mean (SD) or median (IQR), as appropriate. Categorical data are presented as n (%).
Recorded goal descriptions were reviewed by a research physiotherapist and retrospectively coded into the following categories, as defined by the Canadian Occupational Performance Measure (COPM):63 self-care, productivity and leisure, and their respective subcategories and activity categories. An additional ‘other’ category was developed for when the described goal did not fit into one of the three COPM categories. The ‘other’ subcategories were coded as generic pick up/grasp/reach/place object; range of movement; upper limb strengthening; weight-bearing; and, when there was insufficient information to code a goal, unclassified.
Fidelity and dose of the enhanced upper limb therapy programme
A total of 259 RATULS trial participants were randomised to receive the EULT programme across the four trial centres. Data for all 259 participants were uploaded to the trial database.
Overall, 7877 of the 9324 (84%) total possible sessions were attended. A median of 34 sessions (IQR 29–36 sessions), out of the possible 36, were attended, per participant. The most common reason for lack of attendance at sessions was being unwell [406/1447 (28%) sessions that were not attended]. Reasons for all missed sessions can be found in Appendix 6, Table 45. Participants attended 941 out of 1036 (91%) possible senior therapist review sessions, with a median of four reviews (IQR 4–4 reviews) per participant. The overall median time spent at therapy sessions by each participant was 30 hours and 32 minutes (IQR 24 hours and 42 minutes to 34 hours and 5 minutes). The overall median duration of face-to-face therapy at the sessions was 24 hours and 40 minutes (IQR 20 hours and 24 minutes to 26 hours and 15 minutes) per participant. The median duration of each session was 60 minutes (IQR 45–60 minutes). The median duration of face-to-face therapy in each session was 45 minutes (IQR 45–45 minutes).
To further describe dose, the number of task repetitions was recorded. A median of 127 (IQR 70–190) task repetitions were achieved per participant per session attended. Overall, a median of 4121 (IQR 2395–5727) task repetitions per participant were achieved across the EULT programme.
Goal selection and goal achievement
A median of 12 goals (IQR 9–12 goals) were selected per participant during the 12-week EULT programme; the overall total was 2664 goals. Table 13 shows the goal choices and goal achievement in the EULT programme. Further detail about the activities practised is given in Appendix 6, Table 48. The most popular category of goal choice was ‘self-care’ [1449/2664 (54%)], followed by the ‘other’ category [661/2664 (25%)], ‘productivity’ [374/2664 (14%)] and ‘leisure’ [180/2664 (7%)]. In the ‘self-care’ category, the subcategory of ‘personal care’ was the most frequently selected, with 1283 out of 1449 (89%) goals. Of the 1283 personal care goals, the majority [622 (48%)] related to eating whereas 254 out of 1283 (20%) related to dressing. All 374 goals in the productivity category related to household management, with ‘cleaning’ and ‘cooking’ being chosen for 206 (55%) goals and 125 (33%) goals, respectively. In the leisure category, the most popular subcategory was ‘socialisation’ [93/180 (52%)], with the activity of ‘correspondence’ being chosen for 92 out of 93 (99%) of socialisation goals.
| Category | Goals in category, n/N (%) | Subcategory | Goals in subcategory, n/N (%) | ||
|---|---|---|---|---|---|
| Set | Achieveda | Set | Achieveda | ||
| Leisure | 180/2664 (7) | 109/171 (64) | Active recreation | 16/180 (9) | 10/14 (71) |
| Quiet recreation | 71/180 (39) | 41/69 (59) | |||
| Socialisation | 93/180 (52) | 58/88 (66) | |||
| Productivity | 374/2664 (14) | 211/351 (60) | Household management | 374/374 (100) | 211/351 (60) |
| Paid/unpaid work | 0/374 (0) | – | |||
| Play/school | 0/374 (0) | – | |||
| Self-care | 1449/2664 (54) | 640/1354 (47) | Community management | 25/1449 (2) | 19/23 (83) |
| Functional mobility | 141/1449 (10) | 65/136 (48) | |||
| Personal care | 1283/1449 (89) | 556/1195 (47) | |||
| Other | 661/2664 (25) | 327/625 (52) | Pick up/grasp/reach/place object | 163/661 (25) | 84/155 (54) |
| Range of movement | 413/661 (62) | 196/390 (50) | |||
| Upper limb strengthening | 8/661 (1) | 7/7 (100) | |||
| Unclassified | 26/661 (4) | 15/24 (63) | |||
| Weight-bearing | 51/661 (8) | 25/49 (51) | |||
Despite the specification that goals should involve a functional task using the arm, the ‘other’ category predominantly comprised impairment-based upper limb goals, with ‘range of movement’ being the most commonly chosen category [413/661 (62%)]. Range of movement was the second most commonly chosen goal overall. A total of 163 out of 661 (25%) goals in the ‘other’ category comprised generic reach, grasp, pick-and-place activities; although these were functional, they were not specified sufficiently to be allocated to any particular COPM category. There was insufficient information to subcategorise 26 out of 661 (4%) of the ‘other’ goals.
In terms of type of practice, participants predominantly used whole-task practice to work towards their goals [2213/2664 (83%) whole task, 451/2664 (17%) part task].
Of the 2664 goals selected, goal achievement data were recorded for 2501 (94%) goals. In total, 1287 out of 2501 (51%) goals were achieved, ranging between 47% and 100% for each COPM subcategory (see Table 13). A median of 5 goals (IQR 2–7 goals) were achieved per participant. Of the three most commonly chosen goals, 234 out of 587 (40%) related to eating were achieved, 196 out of 390 (50%) related to range of movement were achieved and 141 out of 242 (58%) related to dressing were achieved.
Goal selection and goal achievement by baseline Action Research Arm Test score and time since stroke
The impact of baseline ARAT score and time since stroke on goal selection and goal achievement was investigated. The predefined categories of baseline ARAT scores of 0–7 and 20–39 were used; however, because of the small numbers of participants randomised with baseline ARAT scores in the ranges of 8–13 and 14–19, these two subgroups were combined. For time since stroke, the predefined categories of < 3 months, 3–12 months and > 12 months were used.
For all subgroups, the most commonly selected goal was self-care; however, goal achievement varied according to baseline ARAT score and time since stroke (see Appendix 6, Table 49). Participants who had the lowest baseline ARAT score (0–7) had the lowest goal achievement, with 684 out of 1637 (42%) goals being achieved. For participants with a low baseline ARAT score, goals that related to personal care, range of movement and functional mobility had low achievement rates. Participants who had ARAT scores in the ranges of 8–19 or 20–39 had goal achievements of 201 out of 318 (63%) and 402 out of 546 (74%) goals, respectively. In terms of time since stroke, participants who were recruited > 1 year after stroke had the lowest goal achievement, with 336 out of 882 (38%) of goals being achieved. Those participants who had the lowest baseline ARAT score (0–7) and who were recruited > 1 year after stroke had the lowest goal achievement, with only 167 out of 616 (27%) goals being achieved. Participants randomised within between 3 and 12 months of their stroke achieved 616 out of 1176 (52%) goals, whereas those recruited < 3 months after stroke had the highest goal achievement, with 335 out of 443 (76%) goals being achieved.
Descriptive analysis of the relationship between treatment received and total Action Research Arm Test score
The total ARAT score at 3 and 6 months was plotted against the number of sessions attended, total duration of therapy received and total number of therapy repetitions. For participants who did not attend an appointment, zero duration and zero therapy repetitions were assumed. There is little evidence of an association between total ARAT score and amount of treatment received (Figure 12).
FIGURE 12.
Scatterplots for the EULT group for ARAT total score at 3 and 6 months. (a) ARAT score at 3 months vs. number of sessions attended; (b) ARAT score at 3 months vs. total session duration; (c) ARAT score at 3 months vs. total therapy duration; (d) ARAT score at 3 months vs. total number of repetitions; (e) ARAT score at 6 months vs. number of sessions attended; (f) ARAT score at 6 months vs. total session duration; (g) ARAT score at 6 months vs. total therapy duration; (h) ARAT score at 6 months vs. total number of repetitions.
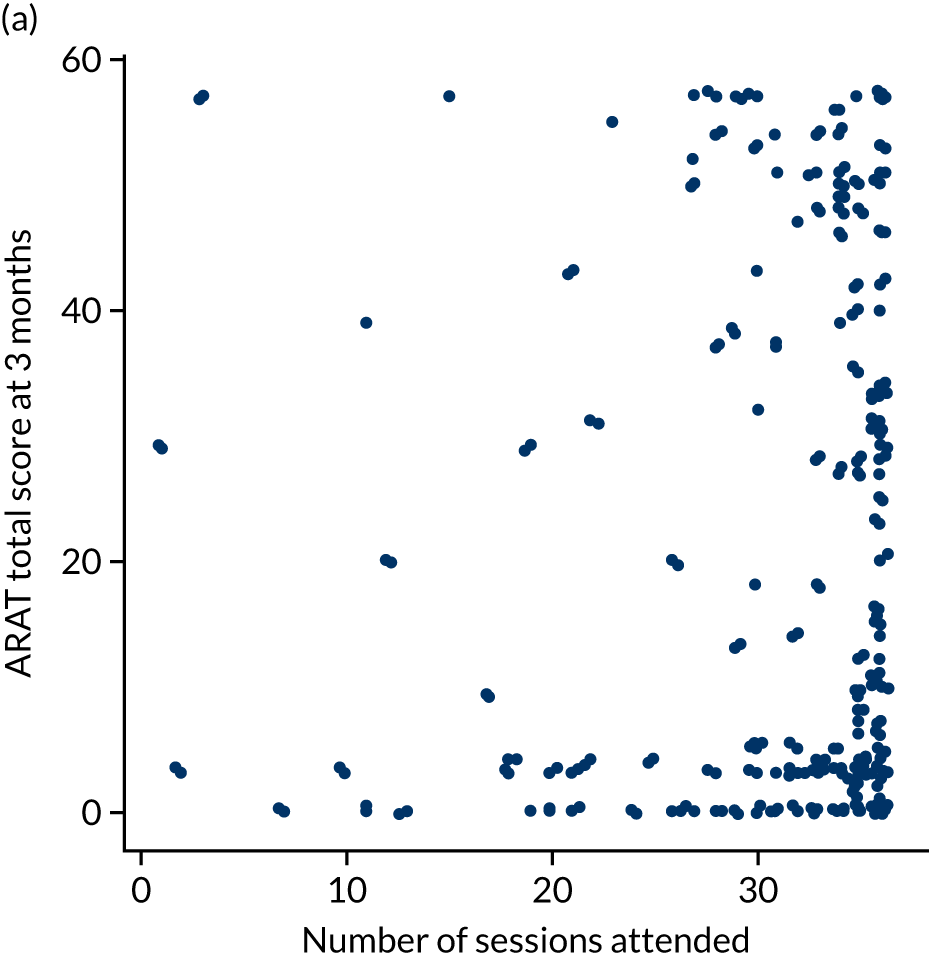
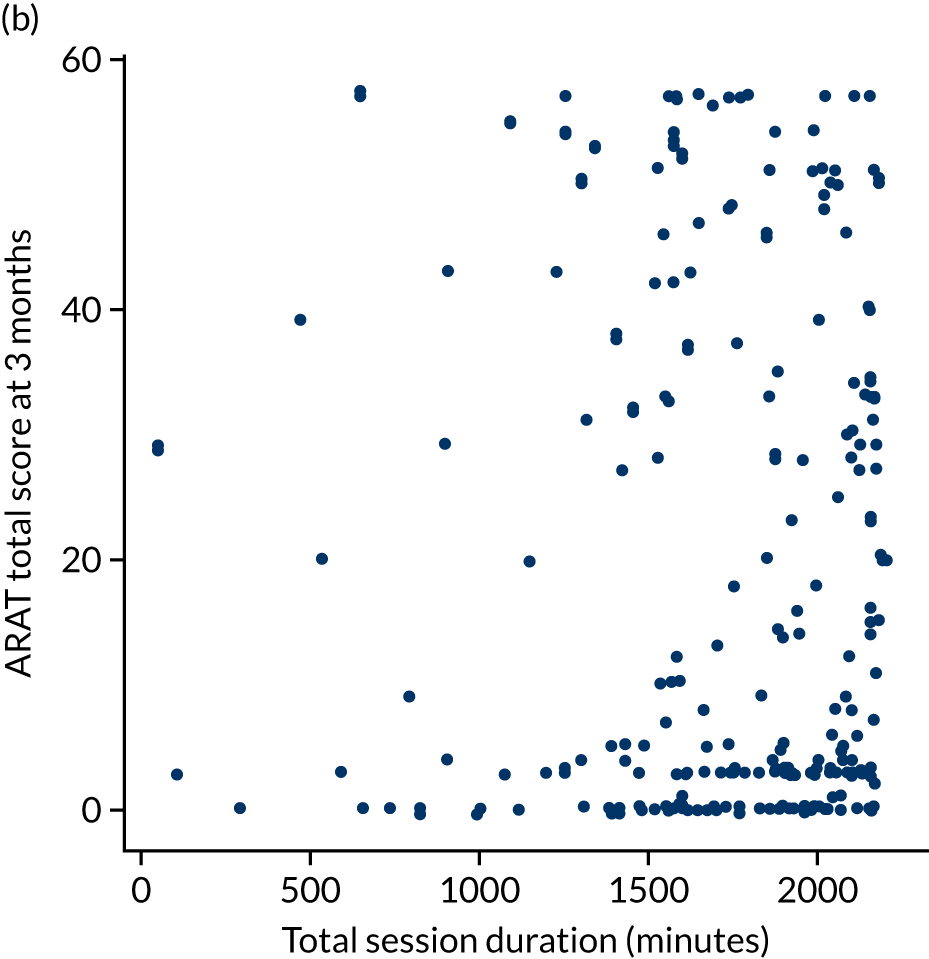


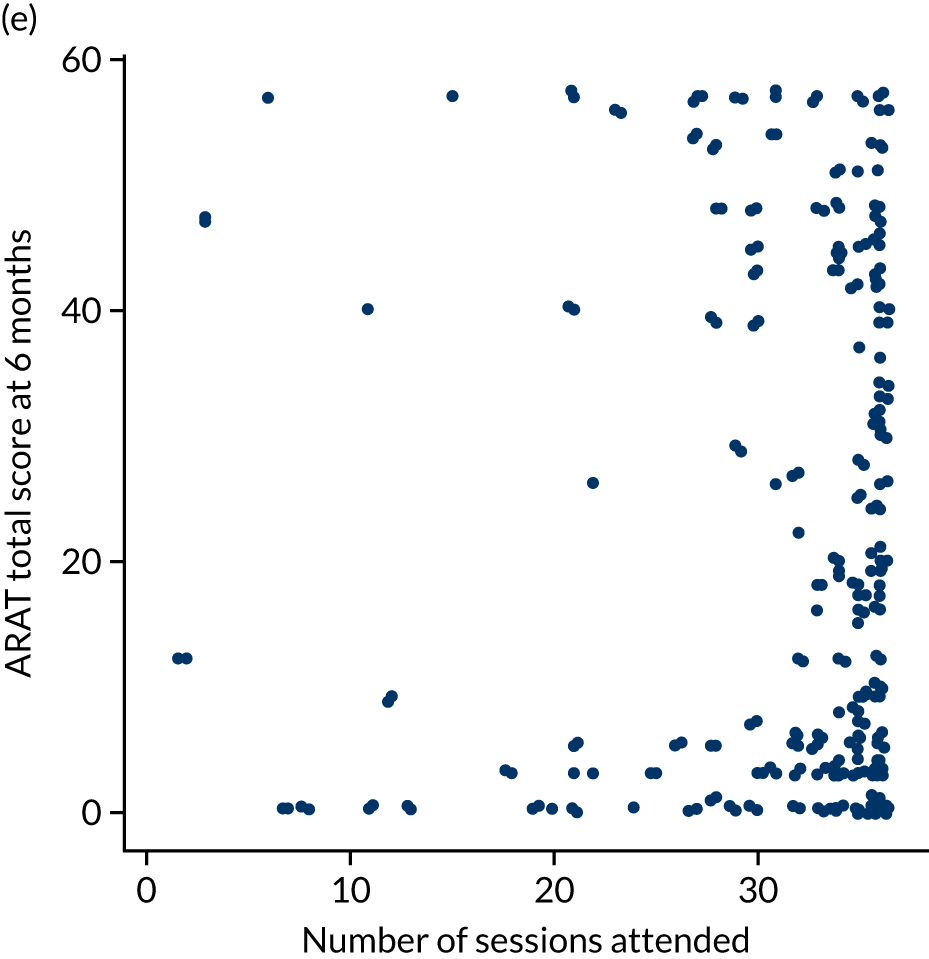
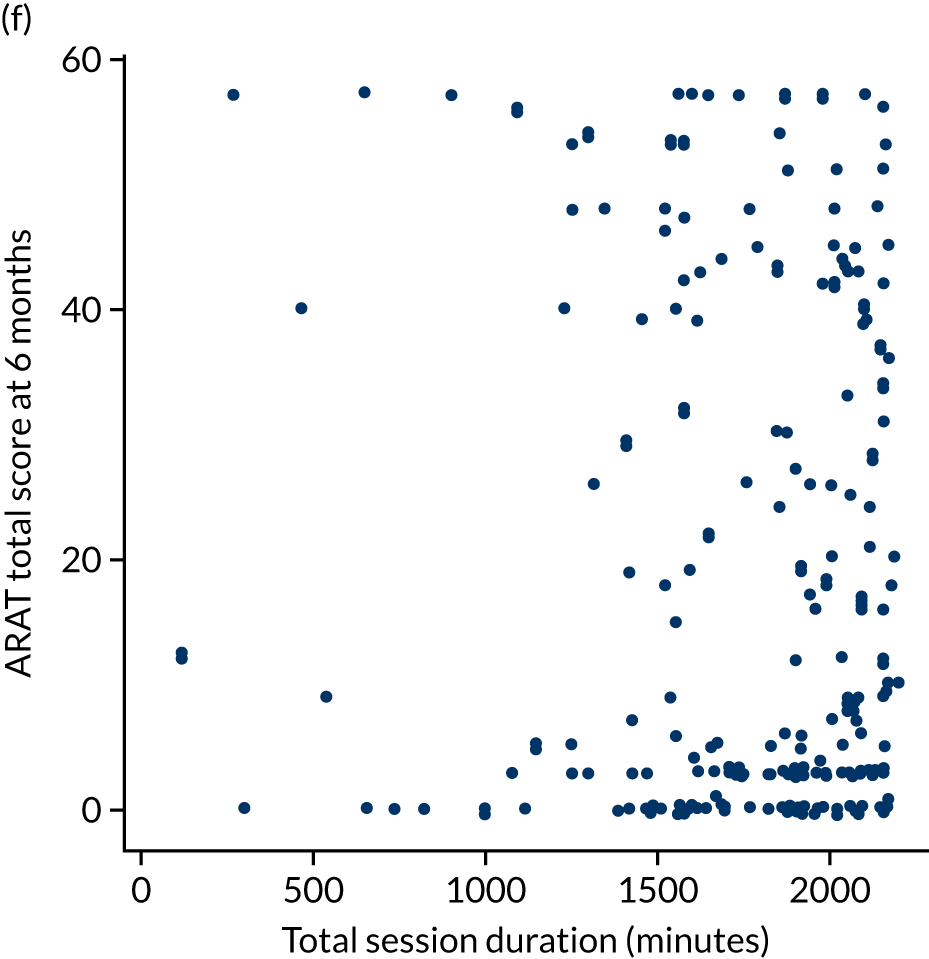
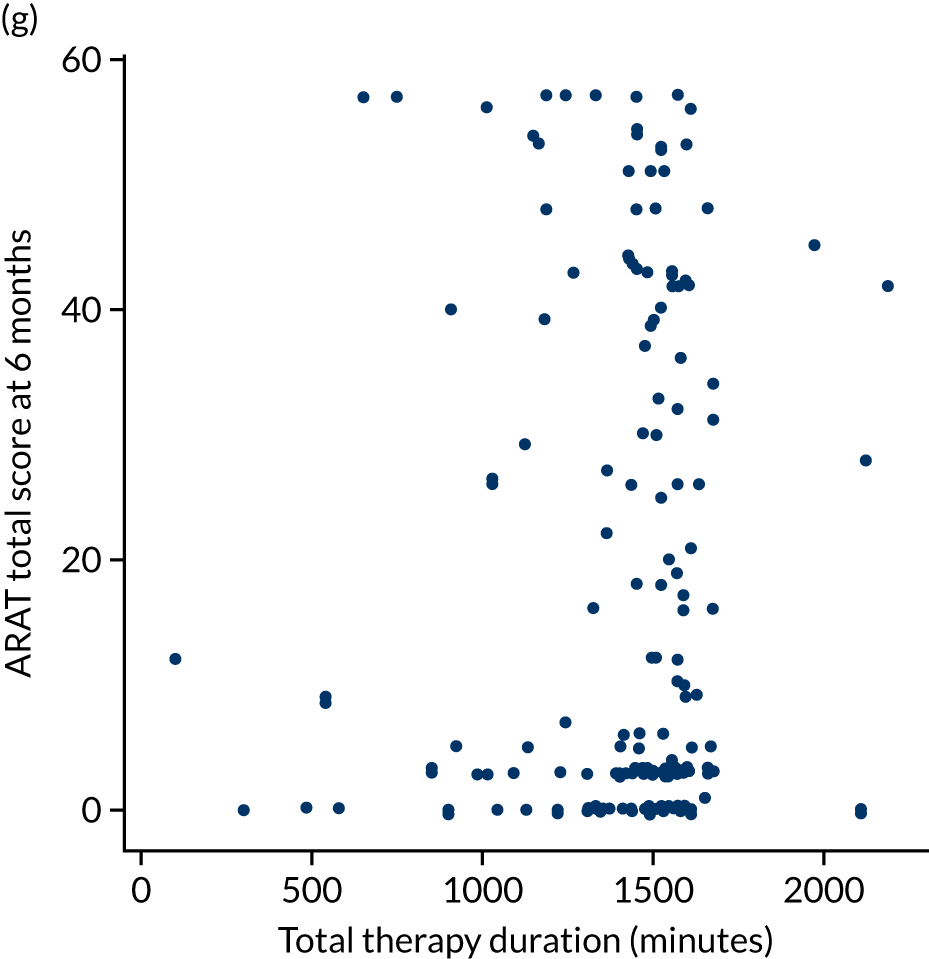

Chapter 6 Usual post-stroke care: arm rehabilitation therapy logs
Introduction
It was intended that all randomisation groups should continue to receive usual post-stroke care during participation in the trial. Trial interventions (robot-assisted training and the EULT programme) were delivered in addition to usual care. Trial involvement and/or randomisation group should not have had an effect on the usual care received. This chapter provides a description of the usual post-stroke care that trial participants reported that they received for their arm or hand, during their time in the trial.
What is usual care in the NHS?
The current NICE quality standard39 states that stroke patients in hospital or in the community should receive a minimum of 45 minutes of therapy, 5 days per week, and that this therapy should continue in the longer term, depending on a patient’s need. However, it is widely accepted that this is not met, especially once patients are discharged from hospital or early supported discharge services. 40 Furthermore, this guidance relates to all therapy; it does not distinguish upper limb rehabilitation from other aspects of rehabilitation. There is no specific standard about the amount or content of upper limb rehabilitation that should be provided post stroke.
Recording information about usual care arm therapy
All RATULS trial participants were given trial-specific arm rehabilitation therapy logs, and were asked to record any upper limb rehabilitation, other than the robot-assisted training or the EULT programme, that they received during the course of the trial. Two arm rehabilitation therapy logs were given to each participant: the log for trial weeks 1–12 (the intervention period) was given to participants at the baseline assessment and the log for trial weeks 13–24 (the follow-up period) was given to participants at the 3-month outcome assessment. The logs were collected from participants at the 3- and 6-month outcome assessment visits.
The bespoke self-completion logs were intended to be completed weekly. If a participant received upper limb therapy that was not a trial intervention, they were asked to record the frequency, duration and the type of therapy received. For type of therapy, participants, with help from their therapists, were asked to select from the following categories: passive stretching, range of motion, functional strengthening, weight-bearing through hand, trunk control, repetitive task-specific practice, personal ADL, extended ADL or other. Participants were also asked to record whether or not they undertook any self-practice upper limb exercises.
Usual care received by each randomisation group
Completion of the arm rehabilitation therapy logs
Of the 676 participants who attended a 3-month assessment, 445 (66%) returned a therapy log. There was little variation in the return rate of the arm rehabilitation therapy logs between randomisation groups, with 158 out of 233 (68%) in the robot-assisted training group, 159 out of 236 (67%) in the EULT group and 128 out of 207 (62%) in the usual care group returning their arm rehabilitation therapy log for trial weeks 1–12 (the intervention period).
Of the 635 participants who completed the 6-month assessment, 345 (54%) returned a therapy log for trial weeks 13–24 (the follow-up period): 118 out of 223 (53%) in the robot-assisted training group, 113 out of 222 (51%) in EULT group and 114 out of 190 (60%) in the usual care group.
Although some logs were returned, this did not mean that data were complete. Table 14 reports the number of weeks that participants provided some data in the arm rehabilitation logs. Data completeness was comparable for all randomisation groups for both time periods.
| Variable | Participants | |||||
|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | ||||
| Weeks 1–12 (N = 158) | Weeks 13–24 (N = 118) | Weeks 1–12 (N = 159) | Weeks 13–24 (N = 113) | Weeks 1–12 (N = 128) | Weeks 13–24 (N = 114) | |
| Number of weeks data were provided for, n (%) | ||||||
| 1 | 0 (0) | 1 (< 1) | 2 (1) | 0 (0) | 0 (0) | 1 (< 1) |
| 2 | 0 (0) | 0 (0) | 1 (< 1) | 0 (0) | 1 (< 1) | 0 (0) |
| 3 | 0 (0) | 1 (< 1) | 1 (< 1) | 0 (0) | 0 (0) | 1 (< 1) |
| 4 | 1 (< 1) | 0 (0) | 0 (0) | 1 (< 1) | 1 (< 1) | 0 (0) |
| 5 | 1 (< 1) | 1 (< 1) | 0 (0) | 1 (< 1) | 2 (2) | 0 (0) |
| 6 | 2 (1) | 0 (0) | 2 (1) | 1 (< 1) | 0 (0) | 0 (0) |
| 7 | 1 (< 1) | 0 (0) | 0 (0) | 1 (< 1) | 2 (2) | 1 (< 1) |
| 8 | 2 (1) | 2 (2) | 1 (< 1) | 0 (0) | 1 (< 1) | 1 (< 1) |
| 9 | 1 (< 1) | 4 (3) | 0 (0) | 0 (0) | 1 (< 1) | 3 (3) |
| 10 | 3 (2) | 0 (0) | 5 (3) | 4 (4) | 2 (2) | 2 (2) |
| 11 | 5 (3) | 2 (2) | 11 (7) | 2 (2) | 4 (3) | 9 (8) |
| 12 | 142 (89) | 107 (91) | 136 (86) | 103 (91) | 114 (89) | 96 (84) |
| Number of weeks therapy data was provided for each participant who returned their therapy log, median (IQR) | 12 (12–12) | 12 (12–12) | 12 (12–12) | 12 (12–12) | 12 (12–12) | 12 (12–12) |
| Participants who returned their therapy log, n/N (%) | 158/233 (68) | 118/223 (53) | 159/236 (67) | 113/222 (50) | 128/207 (62) | 114/190 (60) |
Content of usual care therapy received
Number of weeks therapy was received
In the arm rehabilitation therapy logs, each participant was asked each week, ‘have you had any therapy for your arm or hand in the last 7 days?’. During the intervention period, answers to this question were received for 1830 out of 1896 (97%) weeks in the robot-assisted training group, 1830 out of 1908 (96%) weeks in EULT group and 1476 out of 1536 (96%) weeks in the usual care group. For weeks 13–24, data were available about the number of weeks for which therapy was received for 1355 out of 1416 (96%) weeks in the robot-assisted training group, 1296 out of 1356 (96%) weeks in EULT group and 1317 out of 1356 (96%) weeks in the usual care group. Table 15 gives the number of weeks that participants who returned their log stated that they received therapy for their arm or hand outside the RATULS trial. When participants did not respond to this question, it was assumed that no therapy was received.
| Variable | Participants | |||||
|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | ||||
| Weeks 1–12 (N = 158) (1896 weeks) | Weeks 13–24 (N = 118) (1416 weeks) | Weeks 1–12 (N = 159) (1908 weeks) | Weeks 13–24 (N = 113) (1356 weeks) | Weeks 1–12 (N = 128) (1536 weeks) | Weeks 13–24 (N = 114) (1368 weeks) | |
| Number of weeks participants stated that they received usual care therapy for their arm or hand, n (%) | ||||||
| 0 | 93 (59) | 75 (64) | 83 (52) | 56 (50) | 53 (41) | 61 (54) |
| 1 | 2 (1) | 4 (3) | 9 (6) | 6 (5) | 3 (2) | 5 (4) |
| 2 | 4 (3) | 5 (4) | 8 (5) | 2 (2) | 2 (2) | 2 (2) |
| 3 | 3 (2) | 3 (3) | 5 (3) | 9 (8) | 2 (2) | 8 (7) |
| 4 | 4 (3) | 6 (5) | 1 (< 1) | 6 (5) | 6 (5) | 4 (4) |
| 5 | 2 (1) | 1 (< 1) | 3 (2) | 4 (4) | 5 (4) | 2 (2) |
| 6 | 2 (1) | 1 (< 1) | 6 (4) | 3 (3) | 4 (3) | 2 (2) |
| 7 | 9 (6) | 1 (< 1) | 2 (1) | 2 (2) | 7 (6) | 3 (3) |
| 8 | 5 (3) | 0 (0) | 8 (5) | 3 (3) | 3 (2) | 3 (3) |
| 9 | 7 (4) | 1 (< 1) | 3 (2) | 1 (< 1) | 5 (4) | 3 (3) |
| 10 | 4 (3) | 3 (3) | 5 (3) | 4 (4) | 3 (2) | 0 (0) |
| 11 | 5 (3) | 4 (3) | 8 (5) | 1 (< 1) | 8 (6) | 5 (4) |
| 12 | 18 (11) | 14 (12) | 18 (11) | 16 (14) | 27 (21) | 16 (14) |
| Number of weeks participants stated they received usual care therapy for their arm or hand, median (IQR) | 0 (0–7) | 0 (0–4) | 0 (0–8) | 1 (0–6) | 4 (0–11) | 0 (0–7) |
| Participants who stated that they received therapy for their arm or hand during the 12-week period, n/N (%) | 65/158 (41) | 43/118 (36) | 76/159 (48) | 57/113 (50) | 75/128 (59) | 53/114 (47) |
For weeks 1–12, 65 out of 158 (41%) participants in the robot-assisted training group, 76 out of 159 (48%) in the EULT group and 75 out of 128 (59%) in the usual care group stated that they received some usual care therapy for their arm or hand. However, only 18 out of 158 (11%) participants in the robot-assisted training group, 18 out of 159 (11%) in EULT group and 27 out of 128 (21%) in the usual care group stated that some usual care arm therapy was received for each of the 12 weeks. The usual care group participants reported receiving usual care therapy for their arm or hand more often than participants in the other two groups, with usual care arm therapy being received for a median of 4 weeks (IQR 0–11 weeks), compared with a median of 0 weeks for both the robot-assisted training and EULT groups (IQR 0–7 weeks and IQR 0–8 weeks, respectively).
For weeks 13–24, 43 out of 118 (36%) participants in the robot-assisted training group, 57 out of 113 (50%) in the EULT group and 53 out of 114 (47%) in the usual care group stated that they received some usual care arm therapy for their arm or hand. The number of participants who stated that therapy was received for all 12 weeks was similar in all groups: 14 out of 118 (12%) in the robot-assisted training group, 16 out of 113 (14%) in the EULT group and 16 out of 114 (14%) in the usual care group.
Amount of time that usual care arm therapy was received
If a participant received usual care arm therapy, they were asked to report the amount of time spent on this therapy each week. Across all randomisation groups, 216 out of 445 (49%) participants recorded that they received some usual care arm therapy during weeks 1–12. A total of 153 out of 345 (44%) participants recorded that they received some usual care arm therapy during weeks 13–24. For weeks 1–12, 213 out of 216 (93%) participants provided data for both the number of days and the amount of time spent on usual care arm therapy per week. For weeks 13–24, data were available for 133 out of 153 (86%) participants about the number of days and for 134 out of 153 (87%) participants for the amount of time spent on usual care arm therapy per week. However, in all groups, and for both time periods, the median number of days per week on which usual care arm therapy was provided was 0 days (IQR 0–1 days). Similarly, the median amount of time spent on usual care arm therapy per week for all groups at both time points was 0 minutes; see Appendix 6, Table 50.
Type of therapy received
Of those participants who recorded that they had received usual care arm therapy in weeks 1–12, 64 out of 65 (99%) in the robot-assisted training group, 75 out of 76 (99%) in the EULT group and 75 out of 75 (100%) in the usual care group gave further information about the type of therapy received. For weeks 13–24, 42 out of 43 (98%) participants in the robot-assisted training group, 56 out of 57 (98%) in the EULT group and 49 out of 53 (93%) in the usual care group gave further information on the type of therapy received. Table 16 gives the type of therapy that these participants received and details for how many weeks each type of therapy was undertaken over the 12-week intervention period and the 12-week follow-up period.
| Type of therapy | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|
| Weeks 1–12 (N = 524 weeks) (64 participants) | Weeks 13–24 (N = 313 weeks) (42 participants) | Weeks 1–12 (N = 533 weeks) (75 participants) | Weeks 13–24 (N = 375 weeks) (56 participants) | Weeks 1–12 (N = 645 weeks) (75 participants) | Weeks 13–24 (N = 373 weeks) (49 participants) | |
| Passive stretching | ||||||
| Participants who received this type of therapy, n/N (%) | 60/64 (94) | 35/42 (83) | 63/75 (84) | 50/56 (89) | 66/75 (88) | 42/49 (86) |
| Weeks this type of therapy was undertaken, median (IQR) | 7 (2–11) | 4 (1–11) | 6 (1–10) | 3 (1–11) | 7 (3–12) | 6 (2–11) |
| Improving range of motion | ||||||
| Participants who received this type of therapy, n/N (%) | 56/64 (86) | 33/42 (79) | 57/75 (76) | 48/56 (86) | 66/75 (88) | 37/49 (76) |
| Weeks this type of therapy was undertaken, median (IQR) | 7 (2–10) | 4 (1–9) | 5 (1–10) | 4 (1–9) | 7 (3–11) | 4 (1–10) |
| Functional strengthening | ||||||
| Participants who received this type of therapy, n/N (%) | 44/64 (69) | 27/42 (64) | 48/75 (64) | 36/56 (64) | 51/75 (68) | 31/49 (63) |
| Weeks this type of therapy was undertaken, median (IQR) | 3.5 (0–7) | 1 (0–4) | 1 (0–7) | 4 (0–8) | 2 (0–8) | 2 (0–7) |
| Weight-bearing through hand | ||||||
| Participants who received this type of therapy, n/N (%) | 38/64 (59) | 18/42 (43) | 41/75 (55) | 29/56 (52) | 45/75 (60) | 24/49 (49) |
| Weeks this type of therapy was undertaken, median (IQR) | 1.5 (0–7) | 0 (0–2) | 1 (0–6) | 1 (0–4) | 2 (0–4) | 0 (0–3) |
| Trunk control | ||||||
| Participants who received this type of therapy, n/N (%) | 32/64 (50) | 16/42 (38) | 32/75 (43) | 25/56 (45) | 35/75 (47) | 24/49 (49) |
| Weeks this type of therapy was undertaken, median (IQR) | 0.5 (0–5) | 0 (0–2) | 0 (0–4) | 0 (0–3) | 0 (0–4) | 0 (0–4) |
| Repetitive task-specific practice | ||||||
| Participants who received this type of therapy, n/N (%) | 43/64 (67) | 22/42 (52) | 42/75 (56) | 29/56 (52) | 48/75 (64) | 25/49 (51) |
| Weeks this type of therapy was undertaken, median (IQR) | 2.5 (0–7) | 1 (0–5) | 1 (0–8) | 1 (0–5) | 2 (0–7) | 1 (0–5) |
| Personal ADL | ||||||
| Participants who received this type of therapy, n/N (%) | 33/64 (52) | 16/42 (38) | 34/75 (45) | 24/56 (43) | 38/75 (51) | 18/49 (37) |
| Weeks this type of therapy was undertaken, median (IQR) | 1 (0–7) | 0 (0–1) | 0 (0–7) | 0 (0–4) | 1 (0–5) | 0 (0–4) |
| Extended ADL | ||||||
| Participants who received this type of therapy, n/N (%) | 25/64 (39) | 8/42 (19) | 23/75 (31) | 14/56 (25) | 21/75 (28) | 16/49 (33) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–3) | 0 (0–0) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) |
| Other | ||||||
| Participants who received this type of therapy, n/N (%) | 17/64 (27) | 14/42 (33) | 27/75 (36) | 22/56 (39) | 29/75 (39) | 21/49 (43) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–3) | 0 (0–6) |
For both time periods, there was little variation between the groups in the type of therapy undertaken; the most common type of therapy received was passive stretching. For weeks 1–12, passive stretching was undertaken for a median of 7 weeks (IQR 2–11 weeks) for the robot-assisted training group, 6 weeks (IQR 1–10 weeks) for the EULT group and 7 weeks (IQR 3–12 weeks) for the usual care group. For weeks 13–24, although it was still the most common type of therapy received, the number of weeks during which passive stretching was undertaken was reduced to a median of 4 weeks (IQR 1–11 weeks) for the robot-assisted training group, 3 weeks (IQR 1–11 weeks) for the EULT group and 6 weeks (IQR 2–11 weeks) for the usual care group for this time period.
The type of therapy received least was activities relating to extended ADL (e.g. cooking, cleaning). During weeks 1–12, activities relating to extended ADL were undertaken for a median of 0 weeks in all randomisation groups (IQR 0–3 weeks in the robot-assisted training group, IQR 0–2 weeks in the EULT group and IQR 0–1 weeks in the usual care group). Similar results were obtained for weeks 13–24, with this type of therapy being received for a median of 0 weeks in all randomisation groups (IQR 0–0 weeks in the robot-assisted training group, IQR 0–1 weeks in the EULT group and IQR 0–2 weeks in the usual care group). Details of the ‘other’ therapy specified by participants can be found in Appendix 6, Table 51.
Number of weeks self-practice was undertaken
In addition to the question about usual care arm therapy received, each week participants were asked to respond yes/no to the following question: ‘Have you undertaken any self-practice exercises for your arm or hand in the last 7 days?’.
For weeks 1–12, answers to this question were available for 1828 out of 1896 (96%) weeks for the robot-assisted training group, 1823 out of 1908 (96%) weeks for the EULT group and 1451 out of 1536 (94%) weeks for the usual care group. For weeks 13–24, responses were available for 1336 out of 1416 (94%) weeks for the robot-assisted training group, 1304 out of 1356 (96%) weeks for the EULT group and 1305 out of 1356 (96%) weeks for the usual care group. Table 17 gives the number of weeks that participants who returned their log stated that they completed any self-practice for their arm or hand. When participants did not respond to the question about self-practice, it was assumed that no self-practice was undertaken in those weeks.
| Participants | ||||||
|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | ||||
| Weeks 1–12 (N = 158 participants) (1896 weeks) | Weeks 13–24 (N = 118 participants) (1416 weeks) | Weeks 1–12 (N = 159 participants) (1908 weeks) | Weeks 13–24 (N = 113 participants) (1356 weeks) | Weeks 1–12 (N = 128 participants) (1536 weeks) | Weeks 13–24 (N = 114 participants) (1368 weeks) | |
| Number of weeks data were provided for, n (%) | ||||||
| 0 | 22 (14) | 11 (9) | 7 (4) | 6 (5) | 8 (6) | 7 (6) |
| 1 | 0 (0) | 4 (3) | 2 (1) | 0 (0) | 0 (0) | 1 (< 1) |
| 2 | 0 (0) | 0 (0) | 3 (2) | 3 (3) | 1 (< 1) | 1 (< 1) |
| 3 | 3 (2) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) |
| 4 | 5 (3) | 2 (3) | 3 (2) | 0 (0) | 1 (< 1) | 0 (0) |
| 5 | 3 (2) | 0 (0) | 0 (0) | 1 (< 1) | 5 (4) | 1 (< 1) |
| 6 | 5 (3) | 3 (3) | 3 (2) | 3 (3) | 0 (0) | 2 (2) |
| 7 | 1 (< 1) | 2 (2) | 3 (2) | 4 (4) | 3 (2) | 2 (2) |
| 8 | 2 (1) | 2 (2) | 3 (2) | 3 (3) | 4 (3) | 1 (< 1) |
| 9 | 2 (1) | 6 (5) | 7 (4) | 2 (2) | 5 (4) | 5 (4) |
| 10 | 7 (4) | 2 (2) | 9 (6) | 5 (4) | 8 (6) | 5 (4) |
| 11 | 17 (11) | 11 (9) | 20 (13) | 9 (8) | 11 (9) | 7 (6) |
| 12 | 91 (58) | 73 (62) | 99 (62) | 77 (68) | 82 (64) | 81 (71) |
| Number of weeks participants stated they completed self-practice for their arm or hand, median (IQR) | 12 (8–12) | 12 (9–12) | 12 (10–12) | 12 (11–12) | 12 (10–12) | 12 (11–12) |
| Participants who stated that they had completed self-practice for their arm or hand during the 12-week period, n (%) | 136 (86) | 107 (91) | 152 (96) | 107 (95) | 120 (94) | 107 (94) |
The amount of reported self-practice was high for both time periods. For weeks 1–12, 136 out of 158 (86%) participants in the robot-assisted training group, 152 out of 159 (96%) in the EULT group and 120 out of 128 (94%) in the usual care group stated that they completed self-practice at some stage during this time. For all groups, self-practice was undertaken for a median of 12 weeks (IQR 8–12 weeks for the robot-assisted training group, IQR 10–12 weeks for the EULT group and IQR 10–12 weeks for the usual care group). For weeks 13–24, self-practice was undertaken for a median of 12 weeks for all groups (IQR 9–12 weeks for the robot-assisted training group, IQR 11–12 weeks for the EULT group and IQR 11–12 weeks for the usual care group), with 107 out of 118 (91%) participants in the robot-assisted training group, 107 out of 113 (95%) in the EULT group and 107 out of 114 (94%) in the usual care group stating that they completed self-practice at some stage during this time.
Usual care arm therapy according to baseline arm function and time since stroke
In clinical practice, the intensity of therapy usually decreases as time since stroke increases, and varies according to severity of the upper limb neurological deficit. Therefore, to describe the therapy received by participants in the usual care arm, the arm rehabilitation log data were analysed according to participants’ baseline ARAT scores and time since stroke. The prespecified categories of baseline ARAT score of 0–7 and 20–39 were used; however, because of the small numbers of participants randomised with baseline ARAT scores in the ranges 8–13 and 14–19, these two categories were combined. For time since stroke, the prespecified categories of < 3 months, 3–12 months and > 12 months were used.
Completion of the arm rehabilitation therapy logs
The return rate of the arm rehabilitation therapy log varied according to a participant’s baseline ARAT score and time since stroke. Appendix 6, Tables 52 and 53, show the arm rehabilitation log data analysed according to participants’ baseline ARAT scores and time since stroke over trial weeks 1–12 and 13–24, respectively. When participants did not respond to the question, it was assumed that no usual care arm therapy/self-practice was undertaken in those weeks.
For those participants who attended a 3-month assessment, the lowest return rate was 9 out of 20 (45%) in the subgroup with a baseline ARAT score of between 8 and 19, and who were < 3 months post stroke. The subgroup with the highest return rate was those participants who had a baseline ARAT score of between 0 and 7 and were < 3 months post stroke [66/89 (74%)].
Fewer arm rehabilitation logs were returned at 6 months. However, there was still variation in the return rate between subgroups. For those participants who attended the 6-month assessment, the lowest return rate was 14 out of 30 (47%) in the subgroup with a baseline ARAT score of between 8 and 19 and who were > 12 months post stroke. The subgroup that had the highest return rate was those participants who had a baseline ARAT score of between 20 and 39 and who were > 3 months post stroke [19/31 (61%)].
Content of usual care therapy received
Number of weeks therapy was received
Regardless of a participant’s baseline ARAT score, those participants who were > 12 months post stroke received the least therapy, with a total of 48 out of 166 (29%) of these participants stating that they received usual care arm therapy in weeks 1–12; this proportion was 44 out of 122 (36%) participants in weeks 13–24.
Time spent receiving usual care therapy
For weeks 1–12, participants who had a baseline ARAT score of between 8 and 19 and who were < 3 months post stroke received the most usual care arm therapy. They received therapy on a median of 1 day per week (IQR 0–4 days) and a median of 80 minutes therapy time per week (IQR 0–180 minutes). For all subgroups during weeks 13–24, the median number of days per week on which usual care arm therapy was received was zero, with a median therapy time of zero minutes per week.
Type of therapy received
With the exception of the subgroup with a baseline ARAT score of between 20 and 39, the most common type of therapy received was passive stretching (> 80% of cases) over the intervention period. For those with a baseline ARAT score of between 20 and 39 and who were < 3 months post stroke, functional strength training, repetitive task-specific practice and personal ADL were equally as popular, being received in 10 out of 13 (77%) cases. For the follow-up period, passive stretching was the most common type of therapy received. Appendix 6, Tables 54 and 55, shows the type of therapy received according to a participant’s baseline ARAT score and time since stroke over trial weeks 1–12 and 13–24.
Number of weeks self-practice was undertaken
Levels of self-practice were high for both time periods and for all subgroups. For weeks 1–12, 408 out of 445 (92%) participants stated that self-practice was undertaken; for weeks 13–24, 321 out of 345 (93%) participants stated that self-practice was undertaken. There was minimal variation in the amount of self-practice undertaken between subgroups (see Appendix 6, Table 53).
Chapter 7 Qualitative process evaluation
Introduction
Process evaluation can contribute to the interpretation of the results of a RCT of a complex intervention. 64 Trial designs tend to prioritise the objective assessment of clinical outcomes, leaving the process of change (the space between the provision of the intervention resources and the outcomes) unclear. 34,65,66 Process evaluation can lead to an understanding of participants’ experiences and responses to trial interventions (acceptability), reflection on the mechanisms of impact of the intervention(s) and descriptions of the range of individual and contextual factors that affect how the interventions are delivered during the trial. It can also inform how interventions may need to be adapted and/or delivered in routine practice, after the trial, if proven efficacious.
Qualitative reviews and meta-syntheses of patient experiences of recovery after stroke demonstrate the multifaceted impact that stroke can have on a survivor, on their life after stroke and trajectories of recovery. 67–69 Rehabilitation for stroke patients is, therefore, a complex intervention. Often, interventions are multicomponent, target different domains, use multiple outcome measures and depend on engagement from both the participant and the health service professional delivering the treatment.
In line with the UK Medical Research Council’s guidance for the design and evaluation of complex interventions,70 a qualitative process evaluation was conducted to explore the experiences and delivery of two complex interventions (robot-assisted training and EULT) [both described in detail in Chapters 4 and 5, and using the TIDieR framework34 (see Appendix 1, Table 27)] and usual care for upper limb rehabilitation following stroke.
The objectives of this qualitative study were to:
-
seek the views and experiences of patients and health service professionals about the upper limb rehabilitation that they have received or provided
-
seek the views of patients and health service professionals about the factors that affected the implementation of the trial.
Qualitative process evaluation design and methods
The study design comprised semistructured interviews with both patients and health service professionals, conducted during two time periods (phases) in the trial (Figure 13). During phase 1, patients who were randomised to receive an intervention were interviewed at baseline and at 3 months post randomisation (at the end of the intervention period). During phase 2, different patients were interviewed at 3 months (post intervention) and 6 months (follow-up) post randomisation. Interviews took place in two phases to minimise the likelihood that reporting of the experiences of participants was unduly shaped by factors relating to delivery of the trial at the time of interviewing, which can change over the course of the trial, and to enable an iterative process of data collection and analysis between phases, providing scope to follow up initial findings in greater depth and/or explore questions not targeted in interviews from phase 1. The phase 1 interim analysis also informed phase 2; instead of interviewing patients at the beginning and end of therapy, we interviewed them at the end of therapy and 3 months after completing therapy (6 months post randomisation).This provided a post-intervention period of 3 months during which patients could reflect on any perceived impacts of therapy, before we interviewed them.
FIGURE 13.
Qualitative process evaluation study design. a, Some health service professionals were interviewed twice (n = 5).

In both phases, patients who were randomised to receive a trial intervention (robot-assisted training or EULT) were interviewed twice. This was to allow for exploration of changes in patients’ expectations and experiences at different points during the therapy programme (and to provide more detailed follow-up of emerging key issues at a participant’s second interview). In combination, the two data collection phases allowed us to collect qualitative data corresponding to the three assessment time points in the trial (baseline, 3 months and 6 months), without interviewing any patient more than twice.
Patients from the usual care group were interviewed only once to provide insight into the type of therapy received outside the trial context. Different health service professionals were interviewed up to two times in total, across phases 1 and 2.
Theoretical framework
This qualitative study was informed by normalisation process theory (NPT). 71 NPT explains the work undertaken by people to embed and implement new processes or technologies into routine practice. This study used NPT to develop an understanding of how the RATULS trial was delivered, through informing both data collection (interview topic guide) and analysis.
Study participants and recruitment
Patients and health service professionals were recruited from all open trial centres: the three trial centres that had commenced trial recruitment for phase 1 (by February 2016) and all four trial centres for phase 2 (from October 2016).
Patient study group
All participants in the trial were eligible to participate in the qualitative study. Selection for the qualitative study was based on the timing of qualitative data collection in each of the two phases and the timing of patients’ therapy programmes (see Figure 13). In phase 1, participants who had just been randomised to the trial were invited to undertake a first interview during the first 4 weeks of therapy. In phase 2, participants who were currently receiving therapy were invited to undertake a first interview on completion of their therapy. The local trial co-ordinators or administrator were asked to invite patients meeting these criteria, by providing them with an invitation letter and an expression of interest form. Patients who returned their expression of interest form were contacted by the researcher (NA) who further explained the study, ascertained whether or not the individual wanted to participate and arranged a time and date for their first interview to take place. Interviews took place at a patient’s home or at their local RATULS trial centre, according to patient preference. Written informed consent was taken on the day of the initial interview and covered participation for both interviews. Participants were clearly informed that they could withdraw from the qualitative study at any point, without affecting their participation in the trial.
It was not possible to purposively select participants on the basis of patient characteristics in a trial centre, because of the need for clustering interviews around specific time points in a patient’s therapy programme, and to be able to conduct enough interviews in a single fieldwork trip to a region away from the researcher’s base.
Health service professional study group
Health service professional interviewees primarily included the local principal investigators, trial administrators and co-ordinators, and senior therapists and therapy assistants who delivered the interventions. A small number of therapists who provided usual care, but who did not deliver the RATULS trial interventions, were also interviewed. Trial co-ordinators at each site identified the appropriate health service professionals and invited them to participate in the study. The trial co-ordinator provided the contact details of potential participants to the researcher (NA), who then contacted the professional and explained the study, and, if agreed by the participant, arranged a time and date for the interview to take place. Written informed consent was taken on the day of interview.
Data collection: semistructured interviews
Patient interviews were conducted using semistructured interview guides that were developed and adapted for robot-assisted training, EULT and usual care patient groups. These guides were also adapted for the first and second interviews, as appropriate to the time points being covered in each of the study phases. The topics covered in the interviews are detailed in Appendix 7, Table 56. The topic guides for professionals were informed by NPT, but were not rigidly structured around it. Patient topic guides focused more on therapy experiences. For the robot-assisted training and EULT groups, phase 1 data collection aimed to capture patients’ experiences of their stroke and rehabilitation to date, initial expectations of therapy in the RATULS trial, their experiences of participating in their allocated treatment, and their perceptions of the benefits and impacts of their allocated therapy. Based on phase 1 findings, the phase 2 interview guide was extended to explore maintenance of perceived impacts and experiences of participating in, and completing, the RATULS trial outcome assessments.
For usual care patients, in both study phases, interviews focused on their experiences of stroke rehabilitation and life pre stroke, expectations and hopes for recovery, and their experiences of taking part in the RATULS trial (including allocation to usual care).
In health service professional interviews, current provision of stroke rehabilitation was explored, including what worked well, what did not work well or could be improved (for patients) and perceptions of how the RATULS trial might advance research and practice in stroke rehabilitation. Professionals’ views of the trial interventions were explored, based on experience for those who delivered the interventions, and following provision of a description of the therapies for professionals who were not delivering the trial interventions. For those health service professionals who delivered robot-assisted training and EULT, views about how the interventions might be delivered in routine practice were also explored.
Data management and analysis
Interviews were transcribed verbatim. Transcripts were checked for completion and accuracy by the researcher who conducted the interview (NA), and anonymised prior to sharing with the research team for analysis. NVivo (QSR International, Warrington, UK) was used to facilitate data coding and management. Thematic analysis72 was conducted by two researchers (NA and TF) initially, informed by the framework approach. 73 Both researchers independently read a selection of transcripts from interviews from across the three trial randomisation groups (patient participants), and across trial centres (professional participants) to code data and identify emergent themes. The researchers subsequently met to discuss their coding, share interpretations of the data and jointly develop a coding framework for application to subsequent transcripts. A tentative thematic framework was developed based on these discussions, then applied to a number of further transcripts to categorise the data and test its applicability. The framework was refined as necessary through regular discussion between the two process evaluation researchers (NA and TF), and with wider project team members as appropriate, and applied to all transcripts. Coded data were summarised in data matrices (participant by theme) to facilitate a within- and cross-site comparison. This process was undertaken separately for the data sets relating to patient interviews and to professional participant interviews.
An additional exploratory analysis was undertaken with the quantitative data from two interview participants from phase 2, to explore any correspondence between improvements (or otherwise) in arm function as reported verbally during the qualitative interviews (at 3 and 6 months), with recorded assessments for those patients on the ARAT,19 which was the primary outcome of the RATULS trial. From each therapy group (robot-assisted training and EULT), the patients who made the greatest gain in ARAT score across the trial (from baseline to 6 months) were selected for this analysis. We chose to focus these case studies on highest number of ARAT points gained, as these participants seemed most likely to have experienced noticeable changes that might be described in their qualitative interviews. These case studies are therefore intended to be illustrative of improvement, in patients categorised as having a successful trial outcome, as experienced subjectively, rather than representative of improvement of all participants (which is beyond the scope of the analysis).
Results
Recruitment of participants to the qualitative study
A total of 79 patient and health service professional participants were interviewed across the two study phases, resulting in 115 interviews (Table 18). The 44 patients who were interviewed represented all of the patients who returned an expression of interest form for the interview study (and subsequently consented to participate). This included 12 patients from the usual care randomisation group (interviewed once only). All but one of the 32 patients from an intervention group were interviewed twice: one patient was not able to be contacted for their second interview. Therefore, in total, 75 interviews were conducted with patients in phase 1 (n = 39 participants) and phase 2 (n = 36 participants). Across the phases, time 1 (initial) interviews were a mean of 49 minutes’ duration (range 25–45 minutes), whereas time 2 (repeat) interviews were a mean of 43 minutes’ duration (range 14–88 minutes).
| Process evaluation phase (n interviews) | ||
|---|---|---|
| 1 | 2 | |
| First patient interviews |
|
|
| Second patient interviews |
|
|
| Professional interviews |
|
|
| Total interviews | 55 | 60 |
The key descriptive information about participants for guiding interpretation of results is provided in Table 19. In the results section, patient participants will be referred to using the pseudonyms presented in Table 19. These pseudonyms are used to attribute data excerpts, alongside their age, time since stroke and randomisation group. For professionals, general professional category only is provided, to preserve participants’ anonymity.
| Phase | Pseudonym | Age (years) | Sex | Time since stroke at first interview | Randomisation group |
|---|---|---|---|---|---|
| 1 | Henry | 67 | Male | 4 years | Robot-assisted training |
| 1 | Michael | 74 | Male | 17 months | Robot-assisted training |
| 1 | Alexander | 66 | Male | 1 month | EULT |
| 1 | Josie | 66 | Female | 1 month | Robot-assisted training |
| 1 | Sarah | 46 | Female | 8 months | EULT |
| 1 | Louise | 68 | Female | 10 months | Robot-assisted training |
| 1 | Ian | 53 | Male | 10 months | EULT |
| 1 | David | 65 | Male | 23 months | Robot-assisted training |
| 1 | John | 38 | Male | 4 years | EULT |
| 1 | Sally | 66 | Female | 14 months | EULT |
| 1 | Edward | 65 | Male | 1 month | EULT |
| 1 | Jenny | 56 | Female | 14 months | EULT |
| 1 | Isla | 52 | Female | 7 months | Robot-assisted training |
| 1 | Stephen | 82 | Male | 10 months | Robot-assisted training |
| 1 | Timothy | 52 | Male | 2 years | EULT |
| 1 | Sebastian | 62 | Male | 1 year | EULT |
| 2 | Lisa | 69 | Female | 4 months | EULT |
| 2 | Gerard | 66 | Male | 5 months | EULT |
| 2 | Keith | 79 | Male | 8 months | Robot-assisted training |
| 2 | Jim | 47 | Male | 15 months | EULT |
| 2 | Colin | 68 | Male | 20 months | Robot-assisted training |
| 2 | Suzanne | 44 | Female | 4 months | EULT |
| 2 | Kevin | 55 | Male | 7 months | Robot-assisted training |
| 2 | Jack | 58 | Male | 3 months | EULT |
| 2 | Christopher | 75 | Male | 3 months | EULT |
| 2 | Jane | 47 | Female | 2 months | Robot-assisted training |
| 2 | Lynne | 52 | Female | 9 months | Robot-assisted training |
| 2 | Liam | 48 | Male | 2 years | Robot-assisted training |
| 2 | Graham | 68 | Male | 4 years | Robot-assisted training |
| 2 | Peter | 37 | Male | 7 months | EULT |
| 2 | Amanda | 64 | Female | 1 year | EULT |
| 2 | Susie | 70 | Female | 22 months | EULT |
| 1 | Nicola | 70 | Female | 14 months | Usual care |
| 1 | Alison | 29 | Female | 5 years | Usual care |
| 1 | Clive | 73 | Male | 4 months | Usual care |
| 1 | Micky | 47 | Male | 6 months | Usual care |
| 1 | Harry | 62 | Male | 6 months | Usual care |
| 1 | Elaine | 81 | Female | 5 months | Usual care |
| 1 | Andrew | 75 | Male | 2 months | Usual care |
| 2 | Irene | 62 | Female | 5 months | Usual care |
| 2 | Glenn | 72 | Male | 4 months | Usual care |
| 2 | Joshua | 66 | Male | 5 months | Usual care |
| 2 | Matthew | 84 | Male | 1 month | Usual care |
| 2 | Gillian | 64 | Female | 2 weeks | Usual care |
In total, 35 health service professionals were interviewed. Of those involved in the delivery of the trial interventions (n = 14), five were physiotherapists, three were occupational therapists and six were therapy assistants. Members of the research staff who were interviewed (n = 13) included the local principal investigators, trial administrators, trial co-ordinators and the outcome assessors. An additional seven therapists working in stroke rehabilitation at the trial centres, who did not provide the RATULS trial intervention therapies, were interviewed: four physiotherapists, two occupational therapists and one therapy assistant. As some staff (n = 5) were interviewed in both phases of the project, a total of 40 interviews were conducted. The number of interviews per trial centre ranged from 4 to 12. To protect anonymity of professional participants, a breakdown of professional categories by trial centre is not provided (owing to small numbers), and information about trial centre is omitted.
Presentation of results
Results are reported in relation to three issues: patients’ experiences of robot-assisted training and EULT, patients’ experiences of participating in the usual care randomisation group and factors affecting the trial and the provision of robot-assisted training and EULT (drawing primarily on professional participants’ data).
Patients’ experiences of robot-assisted training and enhanced upper limb therapy
In this section, we describe the intervention participants’ experiences in terms of treatment acceptability (attendance, intensity and duration), participation challenges, physical and functional impacts, and social and psychological impacts, and we present case studies of participants who made large gains on the ARAT.
Treatment acceptability
Attendance
Patients who were randomised to receive one of the two RATULS trial interventions were invited to attend treatment sessions three times per week, for 12 weeks. All patients interviewed travelled to their treatment from their home. Travel was funded by the RATULS trial and organised by local staff (when necessary). The majority of participants reported using this support, and considered it an important facilitator of trial participation. For example, when asked about the difficulties of participating in the RATULS trial, a patient responded:
Maybe just getting there, but they had that boxed off because they had a taxi firm who used to send a taxi over. [. . .] That’s never been a problem for me, but I think if they took that aspect of it away, it would be really difficult.
Sarah, aged 46 years, 8 months post stroke, EULT
Other patients reported having a long commute to their treatment; for example, one patient drove an hour each way, which they found tiring. Therefore, logistical considerations, such as the allocation of treatment time (morning or afternoon) and ‘break’ days by trial centre staff, were also reported to be important considerations that facilitated participation. Furthermore, some patients reported missing treatment sessions because of other therapy appointments or commitments:
Well, that was difficult [organising appointments] because you had to work round with some of them [other therapy appointments], and the other thing was that my mum has got Alzheimer’s and she stays with my sister at the moment, but sometimes I have to look after her, so I had to cancel some of the sessions and then go back.
Jack, aged 58 years, 3 months post stroke, EULT
Flexibility in the delivery of the treatment programmes enabled this participant and others (in exceptional cases) to complete their missed sessions by slightly extending the duration of the treatment programme.
In summary, the transport and logistical support offered as part of the trial and flexibility in delivery of the treatment programmes facilitated participation in, and compliance with, the RATULS trial treatments.
Intensity and duration
Both RATULS trial interventions were designed to provide repetitive practice in sessions that lasted up to 1 hour. Patients participating in both robot-assisted training and EULT reported that the sessions could be physically and mentally tiring:
When I’m doing an exercise for the first time, then I’m using more of my mind, so to speak. You’re not doing remembered things; you’re having to re-train the muscle to pick up a comb and so forth, so to start with, it’s more tiring. As you become more used to it, more skilled, then it becomes, ‘Ah, yes, I can get on with that’.
Ian, aged 53 years, 10 months post stroke, EULT
This patient describes how the physical effort of practising functional tasks (e.g. picking up a comb) was also mentally tiring, as more cognitive processing was required, particularly in the early stages of treatment. In comparison with the functional focus in the EULT programme, in robot-assisted training, task practice was determined by a cursor on the screen. This type of activity was reported to be mentally and physically tiring also:
Working on the robotic machine and trying to concentrate on doing it properly is quite [tiring] – I was surprised how physically tiring the mental effort was.
Stephen, aged 82 years, 10 months post stroke, robot-assisted training
Patients discussed the fact that that robot-assisted training was particularly challenging when the cursor moved randomly, rather than predictably, as they were unable to pre-empt and prepare for the movement:
Then they became random, which was a wee bit more taxing on your brain.
Kevin, aged 55 years, 7 months post stroke, robot-assisted training
Some patients linked their levels of fatigue during treatment to the effects of their stroke, as described by this patient:
When I first started I still wasn’t very well, which I didn’t realise, because it was just exhausting, absolutely exhausting. And I had given myself huge expectations, I wanted to do well, I wanted to be the best there was.
Isla, aged 52 years, 7 months post stroke, robot-assisted training
This patient explained that, for them, participation in the treatment was ‘absolutely exhausting’ as they were still in the early stages of recovery post stroke. However, despite the physical and mental challenge, in general, patients appeared motivated to support their recovery through participation in such intensive treatments:
At the end of the day, I was only after a result in my arm. That was all I thought about before I went in ’til I came back out. When I was sitting ready to go in I would say, ‘Right, I’m going in here and I’m going to do the best I can possibly do for my hand today’. That was always in my mind.
Kevin, aged 55 years, 7 months post stroke, robot-assisted training
All stroke survivors who were interviewed completed their 12-week programme, with the exception of one (in the robot-assisted training group) who withdrew because of back pain, which made transferring from their wheelchair to the treatment chair difficult.
In regards to treatment duration, some participants reported that they felt that they had made all the progress that they could by the end of their treatment:
It was enough for me [treatment duration] and it was enough for [husband], I think, for driving there. But I can’t say if I had done anything more . . . if I went for 15 weeks, would it . . . I don’t think it would have done. It’s the [botulinum toxin] that’s done this bit.
Louise, 68 years, 10 months post stroke, robot-assisted training
In comparison with this participant, others felt that extending treatment duration would support further recovery; for example, an individual in the EULT programme commented:
I thought I was just getting into it and it ended. I thought I was coming on and then it stops.
John, aged 38 years, 4 years post stroke, EULT
Similarly, a patient randomised to receive robot-assisted training noted:
Yes, definitely [they feel they would have continued to progress if treatment had continued] because on different weeks we would discover new movements that were becoming available to me.
Liam, aged 48 years, 2 years post stroke, robot-assisted training
Others noted that extending treatment duration could support a ‘better goal’, and would help people who might need longer to achieve their rehabilitation goals. Furthermore, some patients thought that continued support was beneficial regardless of functional improvement:
The therapy they gave us was stretching and exercises and that, and that was good for the pain and the stiffness. So if it had continued, that would have been great.
Sarah, aged 46 years, 8 months post stroke, EULT
In summary, patients found both robot-assisted training and EULT physically and mentally challenging, but were motivated to complete their treatment to support their recovery. However, equally relevant to patients in both therapy groups, individuals had different rehabilitation needs and some felt that extending the treatment, or combining it with another type of intervention, may have been beneficial to support their rehabilitation and recovery journey over the longer term.
Participation challenges
The majority of patients interviewed completed their treatment programme. However, some reported challenges with participating in their therapies. For example, some people became frustrated when they were unable to complete a task:
I found that one [the wrist attachment] more tiring because I was trying [. . .] And she was saying, ‘Don’t try too hard. Just do what you can do, and we can see what . . .’ But I found that a bit frustrating, because I couldn’t get it, you know.
Colin, aged 68 years, 20 months post stroke, robot-assisted training
Similarly, a patient in the EULT programme commented:
I was more frustrated than tired at some of the things that I couldn’t do. Like, you wanted to lift it and lob it across the room and both the physio[therapist] and the OT [occupational therapist] knew you were getting to a stage where, ‘This is not working. She’s just not doing it,’ and they just changed it to something else. Just changed the task for that day. They didn’t stop it all together. You always went back to it the next time you came.
Jenny, aged 56 years, 14 months post stroke, EULT
The patients described how their frustration was alleviated with help from the therapy staff, who recommended taking a break, switching to a different task and trying again at their next therapy session. For one participant, in the EULT programme, not being able to complete a task ‘correctly’ was exacerbated by their confusion over the purpose of the repetitive functional task practice:
Honestly, I don’t think it was explained enough about the point-scoring and whether you did it wrong or right. I think you did it wrong, and the mentality is you get fed up with it because you’re doing it wrong or you weren’t completing it right, or you’re taking too many clicks to do it and then you thought ‘well what’s the point in asking me to do this if I can’t do it, get somebody else to do it’.
Jack, aged 58 years, 3 months post stroke, EULT
Having a meaningful and achievable goal was highlighted by another patient, who felt that a new goal (putting on a jacket) had been ‘tacked on at the end’ (Sarah, aged 46 years, 8 months post stroke, EULT) of their treatment, as they had not been able to achieve any of their original goals.
Alongside upper limb impairment, other post-stroke problems were reported to affect engagement in therapy. For example, in the robot-assisted training programme, having to follow the cursor on the computer screen caused difficulty for a patient with visual impairment; they explained:
When the ball went to 8:45 or 8:50, I didn’t see it, but now I know it’s there because I’m catching on to where the ball’s going, I suppose you get used to it: ‘oh, it’s going over there next’. You know it’s going to go left after a while and you’ve got to scan.
Louise, aged 68 years, 10 months post stroke, robot-assisted training
This patient described learning to scan the screen to observe the ball when it was moving into the area of their visual field loss. This individual also described the impact of not recognising whether or not their hand and wrist were moving correctly to follow the cursor on-screen, potentially because of sensory/proprioceptive impairment. This patient’s husband accompanied them to their treatment and explained developing a strategy whereby they called out ‘ceiling, floor, ceiling’, to help the participant move in the appropriate direction.
In summary, although patients represented a motivated group, engagement in either type of therapy could be difficult and frustrating if they were unable to complete the agreed tasks. Furthermore, challenges that related to specific treatments were reported, including lack of clarity in the goal-setting process in EULT, and the impact of visual and cognitive impairments on completing the robot-assisted training activities. These challenges were managed with support from the therapy staff and family, or by the patients themselves, using strategies such as taking breaks and tailoring goals.
Physical and functional impacts
The primary aim of the robot-assisted training and EULT was to improve the upper limb function of participants. Across both treatment programmes, the patients interviewed reported experiencing physical and functional benefits, as one patient in the robot-assisted training programme explained:
Well, folding clothes up, I can use both hands. Making the bed, I can use both hands. Dusting I don’t, I just use the left hand, and writing I need to use the left hand.
Josie, aged 66 years, 1 month post stroke, robot-assisted training
This patient highlights how she was now able to use two hands to perform some household tasks, whereas she used her unaffected hand and arm only for other activities that required more complex motor control.
A patient in the EULT programme reported:
This is a bit funny, but, before I came here, I had to get somebody to shave under my arms. I can do that myself now. I can dry my hair and straighten my hair, I can do that myself using two hands instead of just one hand, instead of getting someone else to do it.
Jenny, aged 56 years, 14 months post stroke, EULT
For this person, participation in the EULT programme had enabled her to regain independence in personal tasks for which she had previously required support. This patient also explained that the EULT programme was an opportunity to relearn how to use her affected hand to button a shirt, as ‘I had just completely forgotten what this hand did.’ (Jenny, aged 56 years, 14 months post stroke, EULT).
In other cases, physical impacts were linked to improved motor function (rather than ADL), for example:
I am able to move it [my arm] more, mobility. Not necessarily function, although in terms of the tasks, when I am there, doing the sessions, it works. I can just feel, and others can see, the ability to move it more than I could before.
Amanda, aged 64 years, 1 year post stroke, EULT
Similarly, other patients reported changes in strength and sensation, being more aware of their affected side, and reduced pain and stiffness, all of which were considered benefits of the interventions even when they did not translate into improvement in ADL. However, in terms of satisfaction with progress, although some patients reported being happy with the improvement made, others revealed a more complex picture:
It’s a really tough question because we all want the silver bullet, don’t we? We all want to come out with a fully functional bad arm. So, in that context, I wasn’t satisfied, but, in the slightly more realistic context of ‘It’s neurological rehab[ilitation] that you were trying to deal with’, then, as far as the trial is concerned, having come from absolutely no movement, to some functional use of my arm, then yes pretty . . . Like I say, it depends where you start off, going in.
Liam, aged 48 years, 2 years post stroke, robot-assisted training
This patient described how he compared his progress against his pre-stroke ability of having a fully functional arm; however, he did accept that this expectation was unrealistic. Similarly, another patient explained that he was happy with the progress that he had made while recognising that, for him, there was still work to be done:
It’s got me to a level that I had some doubt I would get to, so I think it’s been beneficial in that way. It has achieved what it was designed to do, I believe, to a large extent, to make life easier for me, and it has, it’s done it. As I say, still recognising that I’ve got some way to go.
Alexander, aged 68 years, 1 month post stroke, EULT
Specifically, in the robot-assisted training programme, some patients discussed that, although the robot-assisted training provided exercises for the shoulder, elbow and wrist, dexterity and finger movement was not a focus of the therapy:
It’s brought your arm and wrist on. You’re probably expecting more from the fingers.
I certainly was [. . .] But then I didn’t know what you had to do before I went.
But it is for the upper arm really, isn’t it?
This patient further reported that she had received botulinum toxin to support recovery of her hand movement, and commented ‘if I could just get maybe, one-to-one physio[therapy] and get these fingers working, I’ll be topped up’.
In summary, patients reported beneficial impacts on sensory and motor function, as well as pain, across both treatment groups, with EULT in particular offering an opportunity for participants to ‘relearn’ how to perform specific tasks of value to them. Even so, although patients were generally satisfied with the impacts experienced, not all therapy goals were fully achieved. Some participants discussed their expectation for continued progress and, in some cases, the need to access alternate treatments to those provided in the RATULS trial to support their recovery.
Social and psychological impacts
The interview schedule explored whether or not patients enjoyed the RATULS trial therapy programmes. In response, they discussed social and psychological benefits of attending the treatments. For example, a patient in the EULT programme discussed that she enjoyed interacting with the staff:
The staff are all lovely, they’re really nice, it’s good that you get a laugh, they’re all so friendly.
Jenny, aged 56 years, 14 months post stroke, EULT
The social benefits were not limited to EULT, as a patient in the robot-assisted training programme commented:
I can talk to the girls. I socialise. They give me company.
Jane, aged 47 years, 2 months post stroke, robot-assisted training
Patients also described socialising with fellow participants, with whom they could share experiences. For some patients, attending treatment was enjoyable as it simply gave them ‘hope’ for improvement:
Yes, I enjoyed coming in. [. . .] that was always in the back of my mind, ‘This next session I’m going to put 110% into and hopefully something will happen’. It was just hope.
Kevin, aged 55 years, 7 months post stroke, robot-assisted training
Often, the RATULS trial offered an opportunity for further rehabilitation to patients who had been discharged from NHS rehabilitation services. Another benefit of taking part in the trial, reported by some patients, was increased independence, as a patient explained:
I think [. . .] for me, because I was travelling such a long way, [it] had other benefits as well as . . . I knew I was going in for primarily upper limb work, but I was driving [. . .] The fact that I was staying away from home obviously has helped, in terms of making me a bit more independent, and being aware of being able to be independent again.
Liam, aged 48 years, 2 years post stroke, robot-assisted training
This patient drove themselves to their treatment, whereas others discussed travelling alone on public transport, or by taxi (organised by the trial centre staff) without a family member, all of which were reported to increase their confidence in their ability to act independently. Finally, participating in a RATULS trial therapy programme was said to provide a sense of purpose in a patient’s day, ‘a reason to get up and get dressed for’, as a patient explained:
You know, if I was to go over, if I was to go there, and at the end of these 3 months, if I haven’t achieved anything, from a psychological perspective, then that was a winner, because that is exactly what I needed at that time.
Isla, aged 52 years, 7 months post stroke, robot-assisted training
Although important to most people, having a sense of purpose was particularly significant to Isla, who described having a busy work and social life prior to her stroke.
In summary, the RATULS trial focused on improvement in upper limb function; however, attending the treatment sessions was reported to have social and psychological impacts that may have benefited the recovery process of participants in both the EULT and robot-assisted training groups.
Case studies of patients making greatest gains on the Action Research Arm Test
In phase 2, patients had their second interview at 6 months post randomisation, allowing exploration of maintenance of any improvements, progress or deterioration at around 3 months after finishing therapy. Overall, of the 15 patients interviewed at both the 3- and 6-month time points, 13 felt that they had maintained the physical and functional changes described at the time of their first interview. Only one patient (robot-assisted training) felt that their arm function had deteriorated since completing their treatment programme, despite continuing to exercise their affected arm. Here, we describe in more detail the self-reported improvements of two phase 2 patients (one from each treatment group) who made the highest gains in ARAT scores across the trial (Table 20).
| Participant | Group | Age (years) | Time from stroke to randomisation (months) | ARAT score (points) | ||
|---|---|---|---|---|---|---|
| Baseline | 3-month assessment | 6-month assessment | ||||
| Jack | EULT | 58 | 3 | 26 | 54 | 57 |
| Jane | Robot-assisted training | 47 | 2 | 28 | 55 | 57 |
The two participants (Jack and Jane) shared some characteristics. They were younger than the average RATULS trial participant (mean age 62.5 years), had moderate rather than severe reduction in upper limb function at their baseline assessment and were recruited early after their stroke. By 3 months, Jack and Jane’s ARAT scores had improved by 28 and 27 points, respectively, and both had an ARAT score of 57 (the maximum score) at 6 months.
On entering the trial, Jack reported that ‘[I] couldn’t open my hand or my fingers and my hand was very sore, it was just, like, a lead weight.’ He discussed the fact that, through participation in EULT, he was shown useful ‘techniques’ (e.g. how to button a shirt and tie shoelaces), and that his hand had been strengthened through the exercise. However, he found participation in EULT ‘repetitive’ and felt that the purpose of the repetitions was not fully explained to him. Further to this, he discussed, ‘they [the EULT exercises] were useful but some of them weren’t relevant to what I felt I should have been focusing on’ and explained the impact on his motivation to participate in the therapy: ‘If you’re doing something you’re not liking, you totally turn against it and you’re like, “that’s finished, I still can’t do it and there’s no point” ’.
This experience highlights the importance of involving patients in the goal-setting process, so that they are clear about, and happy with, the goals that they are working to achieve. Jack discussed, ‘one [goal] I was thinking of that could have been included was some keyboard stuff that would be relevant to what I’m doing.’ Using a computer keyboard was a skill that was relevant to his office-based job (pre stroke) and he felt that incorporating it as a goal would have been more useful to him than buttoning a shirt (a goal selected from examples provided to him at the start of his therapy). However, Jack did explain that cooking-based goals (tasks personal to him) were introduced at his request later on in the therapy. Despite improvement in his arm, Jack discussed that he had not been able to return to his office-based job:
I don’t know if I could actually manage that [returning to work]. [. . .] I feel a lot better and it seems to be improving all the time, but I still have the fatigue later on in the day, that kind of thing. It’s like that, that it’s not possible, because everybody I know has asked about my hand itself, and it’s not just the hand, it’s other things. I went to one of the doctors who specialises in strokes for talking, just like for the mind side of it, how it affects your mind and stuff and how you can deal with that, and that was very useful.
Jack was uncertain whether or not he would ever return to his previous work, but explained that a number of other stroke-related problems (e.g. fatigue and psychological impacts), for which he was seeking help, were preventing his participation in this activity, highlighting the complex and multifaceted nature of post-stroke recovery.
Jane participated in the robot-assisted training programme. On entering the trial, Jane reported that she felt that she had a good range of movement in her arm, and had been independent in her ADL since returning home from hospital. Jane commented that her hand and fingers were her main focus for improvement and that she had volunteered for the trial in order to improve as quickly as possible. In terms of participation in the robot-assisted training, Jane described:
Sometimes you feel like playing the games, really. When it is very easy when you reach something, you can enjoy it, but when it is too tiring, I feel my brain was hot that time, sweaty. It’s been a long journey, sitting there and start this.
Jane travelled independently (through choice) on public transport to the treatment, and, like some others, described that robot-assisted training was tiring after the long commute. However, Jane also reported that she felt that the commute was beneficial for her mobility and independence; robot-assisted training provided stimulation for the mind as well as the arm, and she enjoyed the social interaction that attending the treatment provided. Even so, Jane expressed some disappointment with the treatment that she had received:
The only thing is the robot doesn’t do your finger [. . .] It doesn’t train your finger, only the wrist, big joint, nothing to do with the delicate things [. . .] When I came here I really wanted that.
Jane felt that robot-assisted training did not support recovery of dexterity, which was what she was particularly keen to improve. Jane did report improvement in her fingers, but was unsure whether or not to attribute it to the robot-assisted training:
My finger can open now after the robot. We don’t know if it’s because of the robot or it is natural really. I also do some acupuncture as well. It’s all combination. My finger, big improvement really.
Further to this, Jane was the only participant to report a deterioration in her arm movement:
Before the robot, my arm fully, I can lift it above my head. I can do lots of things. After robot, because of the pain, I can’t do anything.
Jane attributed her deterioration to completing her treatment sessions as quickly as possible using her shoulder instead of her wrist, in some activities, to move the cursor on the screen. In relation to this issue, Jane discussed the importance of having professional guidance available during the treatment:
So [the professional] can say you didn’t use your wrist, [you] use your shoulder. They can tell that, yes, straightaway, and have rest and do this. Do properly. Do the quality job not the quantity. I doing quantity job really. I just want to finish this, finish and finish and finish and a good figure [score]. That’s wrong.
Jane felt that more professional instruction should be provided to promote ‘quality’ of movement over speed of task completion and attempting to achieve a good score. Reassuringly, at Jane’s second interview (around the time of her 6-month follow-up assessment), she reported that she had been able to regain her shoulder movement through participation in a different (non-RATULS trial), therapist-based, rehabilitation programme:
It’s fixed by [additional upper limb rehabilitation]. They gave me twice, I think, just physio[therapy]. They did this [lifting arm] and something, and it’s gone.
Therefore, although Jane represents a successful outcome in terms of the RATULS trial, from her perspective, the treatment had a limited beneficial impact and was also attributed to a deterioration in her ability to move her arm.
In summary, although these two patients improved their ARAT scores the most out of those interviewed in phase 2, their self-reported experiences of recovery highlight that the impact of therapy is more complex than the ARAT scores indicate. These participants were considered to have a successful outcome, and, although they reported improvements in their upper limb function, a deterioration in range of movement due to pain was attributed to the robot-assisted training, whereas, in the EULT programme, the participant wanted to practise tasks personalised to them and more relevant to their daily life. These case studies are illustrative, rather than representative of all patients’ experiences, but demonstrate that functional improvement is only one component of recovery from stroke.
Experiences of participants allocated to usual care
Twelve participants who were randomised to receive usual care were interviewed. All had been discharged from hospital; four were receiving ongoing rehabilitation from community-based NHS services, one of whom was supplementing these services with a private physiotherapist. Two participants were accessing ongoing physiotherapy solely through privately funded services. The most intensive rehabilitation received was by Irene (aged 62 years, 5 months post stroke), who reported that she was visited three times a week by a community stroke team. This team was providing therapy to improve Irene’s mobility and the use of her upper limb. The community team had also provided speech and language therapy, which had ended when Irene felt that she was no longer making progress. Although the rehabilitation received by Irene was comparable to either of the RATULS trial therapies in terms of number of visits (three times a week), the focus was not solely on the upper limb.
In comparison with Irene, the least intensive therapy received was by Gillian (aged 64 years, 2 weeks post stroke), who described attending one outpatient group at her local hospital every 3 weeks, where she exercised her upper limb under the supervision of a physiotherapist.
The type and amount of upper limb rehabilitation received by usual care participants varied. Other types of therapy undertaken at the time of the RATULS trial included mirror therapy, electrical stimulation and botulinum toxin. From this small number of interviews, we can conclude that, although it is unlikely that the other therapies and treatments were comparable to the RATULS trial therapy programmes in the targeting of the upper limb, some participants in the usual care group of the trial engaged in (NHS-provided and non-NHS-provided) activities to support their recovery after stroke.
Unsurprisingly, participants randomised to usual care expressed disappointment, particularly as they had had a 66% chance of receiving additional treatment, and because of the length of the screening process, which they felt had built their expectations of receiving treatment:
Then I was disappointed when, well my husband was as well. I don’t think we could quite believe it and, you know, we’d been through all that and why didn’t we just get told that in January instead of waiting all those weeks, ‘cause that was a long time to wait.
Nicola, aged 70 years, 14 months post stroke, usual care
It should be noted that two patients interviewed (Alison, aged 29 years, 5 years post stroke; and Clive, aged 79 years, 4 months post stroke) reported that they were confused by the three randomisation groups and thought that they would be receiving an intervention in time. These findings were reported back to the trial centre teams, who called the participants to provide clarification on what allocation to the usual care group entailed.
Factors affecting implementation of the Robot-Assisted Training for the Upper Limb after Stroke trial
The RATULS trial was complex, evaluating two forms of upper limb rehabilitation; 770 participants, across four trial centres, were recruited over a period of 49 months.
This section focuses on the processes that contributed to the successful delivery and completion of the trial, from the perspective of therapists and trial staff involved in delivering the RATULS trial. We include here, also, reference to professionals’ views about delivery of the different therapies themselves. The results are framed around the constructs of NPT. 71 NPT proposes that the embedding of a practice or intervention, in other words ‘becoming normalised’, is dependent on four kinds of work that must be undertaken: coherence (ways in which an intervention ‘makes sense’ and has clear purpose and objective), cognitive participation (willingness and ability to commit the time and energy needed to make it work), collective action (the resources, arrangements, skills, etc. required to make an intervention work) and reflexive monitoring (formal and informal mechanisms for appraising the process and outcomes of an intervention).
Coherence: did the Robot-Assisted Training for the Upper Limb after Stroke trial make sense to those involved?
Staff involved in the RATULS trial had a clear and (mostly) shared sense of understanding about what the trial aimed to achieve and what was involved for participants. Professionals who were interviewed attributed this to the early engagement of local staff and teams across the trial centres when the trial was being planned, for example prior to submission of the funding application. They felt that this enabled participating professionals to develop an understanding of the roles and experience needed to support successful trial delivery before commencement of the trial. As illustrated by the following comment from a trial co-ordinator, the complexity of the proposed trial was anticipated at the outset:
I involved the Head of Therapies as well and we, sort of, got together and we were just thinking first of all about, ‘Can we do this? Can we actually do this? Is it feasible? Do we have the patients? Can we support it financially?’ The result of those thoughts really was, ‘Yes, we can. We think we can’.
These very early discussions included the chief investigator (who was also the principal investigator at one trial centre), senior research associate, principal investigators at trial centres 2 and 3, and other key staff working at these trial centres. This early engagement was key to the successful running of the RATULS trial, as explained by a trial co-ordinator:
Do you know what is different about this study? From the very, very beginning I felt that I was involved in the setting up of the study. I felt that the trial team were engaging with me and involving me in setting up the study and that was important. [. . .] That’s why I feel that it’s like my baby, because of that I think.
This coherence-focused ‘groundwork’ involved engaging with therapy teams and other departments to ensure that the trial centres had the necessary resources, including financial, therapy and an appropriate patient population, to support the trial. Interviewees reported that, for the majority of trial centres, those involved were able to make realistic judgements about the feasibility of delivering the proposed trial. This ‘coherence work’ facilitated engagement and collaborative ‘troubleshooting’ throughout the duration of the trial.
Cognitive participation: how did those involved engage with the trial?
Jointly establishing the purpose, focus and feasibility of the RATULS trial at the outset facilitated cognitive participation, which refers to the organised participation of individuals in the work of implementing the trial, and their continued commitment to project delivery. Like many trials, participant recruitment was challenging, and required co-ordinated investment in promoting recruitment to meet the trial recruitment targets. 74 Sustained commitment towards the common goals of co-ordinating and delivering the trial was evident:
I sent a lot of e-mails out to [staff supporting the trial in the trial centre], with some quite clear, concise information, and I re-sent it, with any difficulties we were having, every couple of months. I went to visit a couple of hospitals: [city hospital name] and [community] hospital. I spoke to the community stroke team, went up to the acute wards and the rehabilitation wards on a regular basis, just asking the therapists if they had any patients, and made sure that they knew how to pass names on.
Senior therapist
I think having worked with the Stroke Research Network I knew a lot of the girls on the different sites. So that was an advantage to start with. [. . .] I don’t think you can be successful without those networks really. It’s something you’ve got to work at. Although you might do it initially to get them set up as a site, then you can’t just leave it there. You have to keep working at that because people do forget and they do have other trials.
Trial co-ordinator
From the trial management perspective, trial centre staff described a number of key activities that were undertaken to maximise and maintain staff engagement across the duration of the trial. These included routine updates about the trial from the co-ordinating centre, with information about recruitment and data quality at each trial centre, and regular communication and face-to-face meetings between all trial centres and the co-ordinating centre.
The senior therapists at each trial centre, who were occupational therapists or physiotherapists, were central to initiating and maintaining engagement with the trial processes. These professionals were responsible for supporting the therapy assistants in the delivery of the trial treatment programmes.
Each trial centre consisted of a hub hospital, which contained the MIT-Manus robotic gym, and spoke sites, which referred potential participants to the trial for screening. Spoke sites could be NHS trusts, community stroke teams or primary care. The trial was thus founded on solid working relationships across hub and spoke sites, and ongoing investment in sustaining those links.
Collective action: how was the work of the trial accomplished?
Trial interventions and assessments were mostly achieved as intended, according to the therapy and trial protocols, respectively. How closely therapy was delivered according to the therapy protocols was influenced by a number of factors. In this section, we outline ways in which patients and professionals worked together during therapy. The concepts of interactional workability, skill-set workability and contextual integration (that form elements of collective action) organise this section.
Interactional workability: getting the work of the trial to fit with existing practice
Here, interactional workability refers to the ease with which participants can incorporate the tasks required of the trial as part of their work, including therapy delivery. 71 There were logistical issues relating to participant recruitment that needed to be considered. In some cases, lack of availability of a hoist in the room with the robot meant that some patients were unable to participate in the trial, and were therefore not randomised. With experience, solutions (e.g. checking with a patient before arrival) were identified and adopted. If a patient had too much upper limb pain or muscle tone, they were also not recruited at that time, but they could be put on a review list, so that, if their condition changed, they could be recruited at a later date.
A number of process-related factors that affected the delivery of therapy programmes were also described. In relation to robot-assisted training, technical factors, particularly breakdown of machines or attachments, were reported to have some impact on therapy delivery. Machine breakdowns meant that individuals could not use certain attachments for the robot on occasion. In addition, the hand attachment was not able to be used by all participants, as some were unable to open their hand sufficiently to grasp the attachment. In such instances, therapy staff described that providing robotic therapy could be frustrating. In addition, some staff referred to differences in the degree of flexibility that the therapies offered in terms of working with patients to achieve a better therapy outcome:
I suppose with the robot, you perhaps don’t always get the same quality of someone’s movement, because they’re quite intent, you know, on moving the robot arm, however way they can, but perhaps not in the best pattern of movement. But, whereas in the enhanced, we’ve got a bit more control over how they, sort of, complete the task, perhaps.
Senior therapist
Patient-related factors that influenced the delivery of robot-assisted training, EULT or outcome assessments included the following: missed treatment sessions, missed outcome assessments, frustration experienced by some participants on being unable to make the arm movements required and participants having difficulty assessing their own progress (particularly in the robot-assisted training group). Therapists reported that, relative to robot-assisted training, EULT offered more opportunities for tailoring the intervention to participants’ personal goals and abilities:
If you do enhanced therapy, you can just be more focused, on sort, of practising exercises that they find important. For example, I don’t know, using a key board, or I had a patient who liked, before a stroke, liked knitting or, so you can make it a bit more personalised.
Therapy assistant
Trial staff used a number of approaches to maximise adherence to trial processes. Trial staff responded to challenges relating to recruitment of participants by improving the telephone screening process to gain a better understanding of patient eligibility for the trial by taking into account their practical and other needs (e.g. requiring a hoist). To maximise retention, trial co-ordinators shared their experiences to improve the explanations of trial processes during the consent procedure. The trial team/staff working on the trial maximised recruitment and retention by, when appropriate, completing assessments over the telephone, visiting patients at home and having contact about appointments made by staff with whom they had already developed a relationship. Offers of appointment/referral for a patient to see a consultant were made when appropriate.
Skill-set workability: aligning skills and knowledge to trial delivery
Skill-set workability refers to the compatibility between the requirements of the trial and the skills and knowledge of those working in the trial. 71 The RATULS trial co-ordinating centre team provided training, including a set of detailed manuals, for all the health service professionals involved in the delivery of the trial. This training included how to conduct the trial according to the trial protocol; how to deliver the interventions; and how to conduct the screening, baseline and outcome assessments. Most staff involved in delivering the trial had previous experience in either therapy delivery within or outside the context of stroke care, or performing outcome assessments for stroke research studies. In regards to the EULT delivery, a senior therapist commented:
You’re just learning about the protocol itself that [. . .] you set four treatment goals and normally in, in clinical practice you would set goals, but you might not set four goals and you might not review them every 4 weeks.
Senior therapist
For EULT, the therapy protocol, with additional structure (to set up to four goals, and timing of reviews) is what appeared to distinguish it from what they might provide in an NHS setting. Regarding robot-assisted training, some professionals were more apprehensive, as they had no experience of delivering this type of treatment:
I think that was the thing that probably worried me more than the enhanced therapy, was the fact that this was something that was unknown to me. That actually turned out to be much, much simpler than I expected it to be, once you get the basics of what you’re doing, switching everything on.
Therapy assistant
Most professionals explained that they quickly became familiar with robot-assisted training, and, with experience, it was considered ‘less labour intensive’ for themselves as therapists than traditional therapy. The professionals’ main responsibilities were to ensure that the patient was positioned appropriately in the orthotic support, and to oversee their progress.
The outcome assessors were generally research nurses who were familiar with performing some of the outcomes measures. However, they were not initially familiar with the ARAT and FMA scales. In addition to initial and refresher training, advice was occasionally provided by trial therapists to help the research nurses with interpretation of the terminology used in the measures.
Contextual integration: how were the resources organised to support delivery?
Contextual integration refers to the extent to which the tasks involved in delivering the trial are supported by the organisation in which the work takes place. 71 Although the resources allocated to each trial centre to deliver the RATULS trial were equivalent, there was scope at a local level for trial centres to organise their team structure to suit their local organisation’s structures and processes. For example, in both robot-assisted training and EULT, four review sessions with a senior therapist were allocated for an initial patient assessment when beginning the programmes and review of patient progress throughout the programmes. These sessions were always performed by a senior therapist (physiotherapist or occupational therapist). However, at two trial centres, the senior therapists were allocated directly to the RATULS trial – it constituted their main role. The other two trial centres used NHS therapists who had allocated time to perform the initial patient assessment and reviews, alongside treating other NHS patients. Involving several NHS therapists who had other responsibilities, rather than trial-specific therapists, created some challenges in trial delivery:
Most of the therapists who were supposed to be doing [RATULS treatments] were on the rotation, the ring rotation. So when that comes, you won’t be just [working in the] stroke department. It will be up other departments, wherever you are going to allow them or give them the time to come and see the patients, which we anticipated will be a little bit of a problem, as we found out.
Trial co-ordinator
The therapists who were interviewed treated stroke patients as their main clinical role, and they also reported rotating between different specialties. These practices made allocating time to support delivery of the RATULS trial therapy programmes challenging, particularly if they were rotated into non-stroke services, where managers who were initially unaware of the trial may have been less accommodating with therapists’ time.
Having dedicated administrative staff, as was the case in two trial centres with dedicated senior therapists, was also key to smooth delivery of the trial. In these trial centres, the administrators acted as a central point of contact for patients, professionals and the trial team, and supported the trial in many ways. This allowed senior therapists and therapy assistants to dedicate their time to treatment delivery. In other trial centres, administrative duties were allocated to the therapy assistants. Two people covered this role in one trial centre, whereas, in another centre, an individual therapy assistant was responsible for these tasks. The therapy assistant at this trial centre reported:
You do need, like, a dedicated team specifically just to RATULS. [. . .] I think you need at least three people working on this study specifically, to just obtain the results, especially if you’ve got other studies going on as well, you’d need to have a proper dedicated team so that I can recruit that many and to have the right trained staff to do it and then, you know, also to do the data entries and everything else.
Therapy assistant
At this trial centre, researchers attached to the RATULS trial (a trial co-ordinator and research manager) worked across multiple projects, and the senior therapists were from the stroke service. This therapy assistant was the professional who dedicated the most time to the trial; for this reason, they were responsible for delivering the treatments (under supervision from the senior therapists), organising travel on a patient’s behalf and inputting the assessment data into the central database. The therapy assistant commented that recruiting to target would make delivering the RATULS trial treatments at that trial centre unmanageable.
In summary, to achieve collective action in delivering the trial and its therapies, flexibility and adaptation, both therapy- and outcome assessment-related, were sometimes necessary. Therapists reported that delivering the components of the EULT programme according to the trial protocol did not represent much change to their normal therapy practice. Although robot-assisted training was a bigger change for them, and could be frustrating at times when machines or attachments were unavailable, once trained, they were usually able to deliver therapy satisfactorily. Despite this, EULT was seen to offer more flexibility in terms of meeting the rehabilitation goals of their patients. Insights into these experiences allow anticipation for what adjustments and flexibility may be required for successful delivery of the therapies in routine care.
Reflexive monitoring: how was the trial kept on track?
Throughout the trial, processes were continually monitored, and adapted if appropriate. In terms of supporting skills in undertaking trial assessments, this ongoing monitoring and support was key from the perspective of one of the therapy assistants:
Because I was so new at the time, I couldn’t think of the questions to ask because I was still trying to assess and it wasn’t until I got more and more settled in that I had more and more questions to ask, but it was nice, I could just e-mail [co-ordinating centre] and it was quick and easy to just get that cleared up.
Therapy assistant
The RATULS trial co-ordinating centre was responsible for ongoing monitoring of recruitment and data quality across trial centres. It also provided ongoing support to ensure that professionals across sites were undertaking the trial as per protocol, for example co-ordinating centre staff visited sites regularly and organised annual meetings attended by representatives of all trial centre teams to share learning and experiences, and to refresh therapy and assessment procedures as part of ongoing training. This ongoing work of assessing and responding to challenges as they emerged facilitated successful delivery of the trial.
Discussion
This qualitative study was designed to help illuminate the complexities of the RATULS trial and its therapy programmes, through in-depth exploration of patients’ and professionals’ experiences of participating in the trial and of providing robot-assisted training and the EULT programme. Rehabilitation requires the active participation of patients and professionals; therefore, any intervention’s success or failure is partly shaped by their experiences and their engagement with the therapies provided. Participant experiences provide important insight into the acceptability of the treatment, and challenges in intervention delivery that can inform future implementers and decision-makers, and facilitate the interpretation of the trial results.
Our study reports that robot-assisted training and EULT were generally acceptable to patients. Both interventions were physically and mentally challenging and, in some cases, frustrating for patients, when they struggled to complete a task. Some of these challenges were related to severity of the stroke in general (e.g. fatigue, mobility issues and cognitive impairment), and of arm impairment in particular, including sensory and proprioceptive impairment, as well as participants’ expectations of themselves and the intervention. Support from RATULS trial staff, and the patients’ families in trying to overcome these challenges was appreciated. Some patients expressed a wish for therapy to be extended beyond 12 weeks to support their achievement of goals for their upper limb and ongoing recovery more generally.
The RATULS trial reported that robot-assisted training and EULT did not improve upper limb function (the primary outcome) after stroke, compared with usual care. However, improvement in impairment was seen for both robot-assisted therapy and EULT, compared with usual care. Additional improvements in ADL and mobility were found in the EULT group. This study explored patients’ self-reported impacts of therapies. Patients who received either treatment reported physical improvements related to their upper limb, and many reported that these improvements were maintained at 6 months. However, some of the self-reported changes (e.g. better arm positioning, improved sensation and reduced stiffness) would not have been captured by the trial outcome measures, and indicate a potential mismatch (or limited focus) of objective assessments, relative to qualitative exploration of impacts of trialled interventions. This mismatch has been reported previously and highlights the importance of capturing participants’ own experiences of intervention impacts. 75 In addition, although patients generally expressed some level of satisfaction with physical improvements resulting from therapy, they self-assessed their progress with reference to their more general hopes and expectations for complete recovery, which were focused on regaining ‘before stroke’ capabilities and functioning.
A more detailed analysis of two phase 2 interviewees’ (who achieved a ‘successful’ outcome according to the RATULS trial criteria) experiences of therapy impact indicated that improvement is more complex than assessment scores indicate. There is a need to ensure that patient and practitioner understandings of therapy interventions are aligned as much as possible. Our findings highlight the importance of what ‘clinically assessed improvement’ means to patients, in terms of functionality and practical gains, and in terms of what impacts of therapy they experience as valuable. This point was further emphasised, as participants reported a range of positive impacts that crossed social and psychological domains, for example social engagement and increased confidence that arose from taking part in a trial (but these did not seem to be associated with the intervention content per se). These are additional benefits of therapy that are important for patients in their recovery process, but that would not be expected to be captured in measures included in a trial.
Although the RATULS trial demonstrated improvement in impairment following robot-assisted training, those who received EULT additionally showed improvement in ADL. This was not surprising, given the specificity of learning. In robot-assisted training, movements were stereotypical, identical and did not involve everyday objects, and movement patterns were tightly controlled by the device. In contrast, health-care professionals reported that the EULT programme offered more scope for working on a range of activities that linked to patients’ personal goals, and they also felt that there was more opportunity to monitor and facilitate motor control in this type of treatment. Activities could be varied, tailored and progressed, and feedback could be used selectively; all of these are components for optimising skill acquisition and transfer. Patients in the EULT group indicated that they valued some of the functional goals they had achieved.
Some participants (in both intervention groups) were engaging in other treatments (e.g. botulinum toxin, other forms of physiotherapy and other physical rehabilitation) outside the RATULS trial provision, as were some participants in the usual care group. This was allowed in the protocol. Furthermore, some participants in both groups expressed a need for therapy to be extended beyond 12 weeks, to support their rehabilitation potential in their upper limb and more general recovery. This resonates with the findings of a qualitative study of Australian stroke survivors’ (n = 19) and spouses’ (n = 9) perceptions of upper limb recovery after stroke, that emphasised survivors’ approach as one of ‘keeping the door open’ – maintaining hope for and continuing to work towards improvement. 76
Although broader conclusions about the therapies cannot be made for patients collectively on the basis of specific examples, our findings indicate that rehabilitation for upper limb recovery is complex, with the arm often being only part of the picture and patients engaging in multiple treatments (when possible) to achieve their rehabilitation potential. Effective engagement in upper limb therapy (both robot-assisted training and EULT) is likely to be influenced by factors related to stroke, such as fatigue, cognition and communication abilities, as well as emotional factors, motivation, self-efficacy and social support. These factors also influence other aspects of a patient’s life that constitute rehabilitation, such as participation in social activity and return to work. It must also be emphasised that, even for those indicating good improvement from upper limb therapy, the end of treatment does not mean the end of the recovery journey.
The second objective of this qualitative study was to identify factors affecting the implementation of the trial, and the trial therapies, increasingly an important focus in process evaluation of complex interventions. 64 It is important to understand two different objects of implementation here: the trial in its broader sense (which includes the therapies and delivery contexts, in combination with trial processes) and the therapies themselves, which may be subsequently provided in routine service provision in the absence of the trial. We used NPT to present the results about implementation of the trial and the RATULS trial therapies in that context. 71 The findings indicated that successful delivery of the trial required continuous investment in NPT framed ‘work’ to maintain commitment, engagement and reflection/adaptation to some of the trial processes, to achieve the tasks (primarily recruitment, therapy delivery and data collection) required for the RATULS trial.
Early engagement of trial centres in planning the trial enabled development of understanding of the trial and of the therapies being delivered (shared by most trial centres) (coherence), which facilitated engagement by staff (cognitive participation). In terms of achieving the work of the trial (collective action), allocation of therapy staff (dedicated RATULS therapists when possible) was highly facilitative, as was a level of flexibility in therapy provision and organisation (e.g. allowing patients to express preferences for days of therapy). The level of support, monitoring and feedback from the co-ordinating centre throughout the trial (regarding recruitment, completion of assessments, etc.) (reflexive monitoring) ensured ongoing engagement of sites, staff and patients.
Findings suggest that both robot-assisted training and EULT could be delivered successfully in routine service provision, if adequately resourced and optimally organised to allow for reasonable flexibility in delivery. Apart from some frustrations expressed by staff in relation to robot equipment failures, both therapies were acceptable to staff after adequate training in the treatment protocols.
Methodological reflections indicate key strengths, and some limitations, of this qualitative process evaluation. This is a large qualitative study of a multicentre trial, providing experiential accounts of trial participation and delivery from staff and patients across multiple time points during a patient’s trajectory through the trial. Conducting the interviews in two phases allowed some iterative development of fieldwork focus, based on issues arising during the trial (and from phase 1). This approach also enabled coverage of three different time points during therapy (commencement, completion and maintenance), while limiting the number of interviews for individual patients; in particular, the extent to which patients felt that they had been able to maintain any impacts at 6 months (3 months after therapy completion) could be explored in phase 2. As a qualitative process evaluation study, the absence of an observational component to supplement (or triangulate) interview findings may be a limitation, in that we have focused on participants’ accounts of therapy rather than having directly observed the sessions they spoke of. Observational methods can be valuable, but also intrusive to those involved. If we had taken this approach in the RATULS trial, it may have affected therapy negatively, for limited benefit in data collection. Other data were collected during the trial to document therapy provision, which can be used for some level of data triangulation, and these suggest that therapy was generally delivered as intended. The findings from the subset of trial participants who took part in the qualitative process evaluation have highlighted a range of experiences, and differing levels of engagement in therapy, that were able to be achieved by patients with different needs, and at different points in their recovery process after stroke. This suggests that adequate variability of participants in the trial was achieved.
Overall, the RATULS trial participants’ accounts of stroke were strongly aligned with the existing qualitative literature concerning the multifaceted impacts of stroke,68,69,77 variable nature of recovery over time76,78,79 and expression of hopes and expectations concerning fuller recovery over time. 76,80 Participating in the RATULS trial was viewed by patients as an opportunity to extend or supplement their recovery process, particularly in relation to upper limb rehabilitation. Some participants expressed disappointment on being allocated to the usual care group. Participants who received robot-assisted training and EULT tended to be highly motivated and engaged well with their therapies, and reported a range of benefits that represented improvements.
From the perspective of professional staff who delivered the RATULS therapies, both therapies could be delivered to patients as a 12-week programme, providing the necessary resources were available and staff engagement was high, and provided there was scope to make adjustments to therapy delivery to accommodate patient, therapist, equipment and organisation factors affecting therapy provision. Provision of both robot-assisted training and EULT in routine care (beyond the RATULS trial) may be more flexible in terms of delivery and organisation than was possible in the context of the trial, in which adherence to therapy and trial protocols were of paramount importance. Routine provision of these therapy treatments may differ, however, in ways that affect efficacy, and should be further explored in this context.
Conclusion
Participants in both the robot-assisted training and EULT groups generally found the therapies to be acceptable, and were able to complete their therapy programmes. Not surprisingly, some challenges were experienced by some participants relating to their upper limb capabilities and other stroke-related limitations that affected their engagement in the programmes. Participants reported a range of benefits from participating in either therapy programme, and many reported (self-judged) maintenance of some of the benefits at 6 months.
Delivering the RATULS trial required continuous investment of effort by trial centres and the co-ordinating centre, with high levels of engagement from the staff involved. At times, flexibility and adaptation of some of the processes, within the constraints of the trial protocol, were necessary to support continued engagement of patients and professionals. The findings of this qualitative process evaluation can be used to inform the delivery of robot-assisted training and the EULT programme for upper limb rehabilitation, for service provision beyond the RATULS trial.
Chapter 8 Health economic evaluation
Introduction
The health economic evaluation undertaken as part of the RATULS trial assessed the relative cost-effectiveness of the robot-assisted training using the MIT-Manus robotic gym, compared with the EULT programme and usual care. This evaluation included a within-trial analysis and an extrapolation of the results to estimate costs and outcomes beyond the time frame of the trial.
The analyses took the perspective of the UK NHS and PSS. A wider societal perspective was also explored by analysing the costs borne by participants and their carers. These costs included out-of-pocket expenses incurred for hospital admissions, GP visits and outpatient appointments and time away from usual activities such as work, study or caring responsibilities.
This chapter includes the main results of the within-trial economic evaluation and the extrapolation exercise beyond the trial time frame. For the within-trial analysis, the following outcome measures are reported:
-
NHS and PSS resource use
-
health-care costs per participant to the NHS and PSS over 6 months for each area of resource use
-
utility scores derived from responses to the EQ-5D-5L26 questionnaire at baseline and at 3 and 6 months post randomisation
-
average QALYs per participant at 6 months post randomisation
-
incremental cost-effectiveness ratios (ICERs)
-
cost-effectiveness acceptability curves (CEACs) to assess the probability of each of the interventions being considered most cost-effective at different willingness-to-pay (WTP) thresholds for a gained QALY.
For the extrapolation exercise, the following outcome measures are reported
-
average health-care costs per participant at 12 months post randomisation
-
average QALYs per participant at 12 months post randomisation
-
ICERs and CEACs derived by extrapolating costs and QALYs from the data observed during the trial.
Results are also reported for the prespecified per-protocol and sensitivity analyses. The sensitivity analyses explored uncertainty surrounding the level of resource use and the assumed lifespan of the MIT-Manus robotic gym.
A stochastic sensitivity analysis was carried out for all analyses to address potential variance in the outcome measures.
Methods
The economic analysis was conducted following best-practice guidelines conforming to the Consolidated Health Economic Evaluation Reporting Standards. 81
Baseline resource use
A health service utilisation questionnaire was developed, adapted from the CSRI28–30 and administered at baseline. Participants were asked about the care received in the 3 months before they joined the trial. It included questions relating to residential care and nursing home stays, visits to accident and emergency (A&E) departments, outpatient appointments, day patient appointments, overnight hospital stays and the use of GP and nursing services. To limit the response burden on participants, data were not collected on the use of therapy services, medications or community-based health care and social services.
This information was used to control for any imbalance in participant resource use at baseline.
Follow-up resource use
Information on resource use from baseline to 6 months’ follow-up was collected to derive the total mean cost per participant for each randomised group.
NHS and Personal Social Services resource use
The main within-trial economic analysis considered costs to the NHS and the PSS, which were measured at the 6-month follow-up. Data were collected via a health service utilisation questionnaire, which was completed by participants at the 6-month follow-up. Participants were asked about the care they received in the previous 6 months. The resource use areas considered were primary care, therapy services, secondary care NHS services, social care, stays at residential and nursing home facilities, the use of social services and community-based health care, and prescribed medications.
Costs to the participant and caregiver
Data on participant- and carer-related costs were collected via a time and travel questionnaire distributed at the 6-month follow-up assessment. This questionnaire was completed by the participants and their carers, who provided details about their out-of-pocket expenses relating to their most recent GP and secondary care appointments. This included travel time, time spent at appointments, mileage, parking and other transport-related costs. Details relating to the main activities that participants and carers would otherwise be doing were also provided.
Derivation of costs from NHS and Personal Social Services perspective
Individual patient-level data on resource use of the NHS and PSS were collected via the health service utilisation questionnaire. Intervention costs were based on information received from the trial and procurement NHS teams. These data were combined with unit costs obtained from routine data sources such as the data collected by the Personal Social Services Research Unit82 and the 2017/18 national schedule of reference costs. 83 The costs considered were presented in 2018 Great British pounds and categorised as follows:
-
intervention costs
-
follow-up costs
-
primary care
-
therapy and community-based services
-
secondary care
-
residential and nursing home care
-
social services
-
medication costs.
-
Intervention costs
Participants recruited to the trial could be allocated to one of two distinct interventions: robot-assisted training or the EULT programme. As per protocol, both interventions had the same duration and staff component as shown in Appendix 8, Table 57. The full duration of each therapy session was 60 minutes, including 45 minutes of face-to-face therapy. In addition to staff time, capital costs were calculated for robot-assisted training using the ‘equivalent annual cost’. 57 This methodology is described in Appendix 8.
Primary care costs
Consultations with GPs, nurses and calls to NHS Direct (now NHS 111) were all classified as primary care costs. The health service utilisation questionnaire allowed participants to state whether consultations had taken place at home, at the health centre or by telephone. Unit costs were obtained from Curtis and Burns. 82 (see Appendix 8, Table 58). An average cost per participant for each randomisation group was then calculated.
Therapy and community-based services costs
Therapy services were classified as physiotherapy, occupational therapy, and speech and language therapy (see Appendix 8, Table 59). Participants were asked to state whether these appointments took place at the hospital, the GP’s surgery, their own home, or elsewhere. Appointments with community-based health professionals (health visitor, geriatrician, psychiatrist, chiropodist, optician and pharmacist) were also collected. It was assumed that all therapy services reported by participants were NHS funded and excluded any private consultations.
Secondary care costs
The type of secondary costs captured in the health service utilisation questionnaire included:
-
Emergency visits to A&E departments.
-
Overnight stays in hospital. When reporting their overnight hospital stay, participants were also asked whether this followed an emergency (A&E) or general ward admission. They were also asked how long they stayed in hospital. Both of these factors influenced which unit costs were applied. The cost of stays of ≥ 22 days was based on excess bed-days beyond 21 days following the criteria used to define long-stay patients in acute hospitals by NHS England. 84
-
Planned outpatient appointments not resulting in an overnight stay.
-
Planned day patient appointments. Participants were asked about the approximate length of their hospital stay as a day patient (either half or full days), which determined which unit cost was applied.
Appendix 8, Table 60, provides details on unit costs and the different categories captured for secondary care use.
Residential and nursing home care costs
Stays in nursing and residential care facilities captured the participants’ residential and nursing home stays for the whole duration of the trial. Costs for this cost category were derived from Curtis and Burns82 and are outlined in Appendix 8, Table 61.
Social services costs
Social services were categorised as home help received by participants in the areas of personal care, household tasks or daily shopping (see Appendix 8, Table 62). The questionnaire also captured whether participants were in receipt of Meals on Wheels. To minimise recall bias, participants were asked to record the services received in the preceding week. This information was then multiplied by 24 (assuming 4 weeks in a month) to estimate the resource use for the full 6 months.
Medication costs
The cost of prescribed medication was included in the trial. Participants were asked to state the medication name, dosage, frequency, and start and end dates. When all information was available, the unit cost was taken from the British National Formulary (BNF)85 and multiplied by the total units prescribed for the stated period. If dosage was missing or incorrect, the BNF standard recommendation for the patient group was used. Prescription cost analysis86 was used in those cases for which it was impossible to ascertain the intake duration or the format prescribed. In this case, it was assumed that the participant had been issued one prescription for the most commonly prescribed format (e.g. dose, mode of intake) for the 6-month period.
Assessment of effects
The QALY was used in the cost-effectiveness analysis as the health outcome, as it is recommended by NICE. 87 QALYs were used to assess the health gain of participants over the 6-month trial period.
Estimation of quality-adjusted life-years
Health status was measured by the completion of the EQ-5D-5L questionnaire by participants at baseline and at 3 and 6 months post randomisation. Data from the EQ-5D-5L questionnaires were then used to derive utilities and QALYs for the cost–utility analysis.
Following NICE guidelines,88 responses to the EQ-5D-5L questionnaire were mapped to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), descriptive system to generate the utility values. These utility values have a range of –0.594 (worse than death) to 1 (perfect health). The resulting QALYs were calculated, applying the area-under-the-curve approach,89 using the utility values obtained at baseline and at 3 and 6 months post randomisation.
Dealing with missing data
The base-case cost-effectiveness analysis included only those participants for whom we had some data on costs and baseline EQ-5D-5L. Because data were found to be missing at random, multiple imputation was used to estimate the missing utility values at 3 and 6 months. Truncated normal regression was applied, controlling for age, sex and baseline ARAT score, to generate 10 different utility scores at each time point for each participant. The average value of the 10 iterations was taken and imputed in place of the missing utility value.
Because data on intervention costs were available for participants in each of two intervention groups, the only participants with missing total costs belonged to the usual care group. A sensitivity analysis was subsequently conducted to explore the effect on cost-effectiveness of applying multiple imputation to missing total costs, controlling for age, sex and baseline ARAT score.
Cost-effectiveness analysis
The base-case economic analysis takes the form of a cost-effectiveness analysis, with QALYs gained at 6 months as the primary economic analysis outcome measure. Costs included in the analysis comprised the intervention costs for the robot-assisted training and EULT programme groups and health service use over the 6-month follow-up period for all randomised groups. All participants reporting at least some costs (NHS, PSS or intervention) associated with their participation in the trial were included in the analysis.
Time and travel costs have been analysed but were not included in the base-case analysis, as this took an NHS and PSS perspective. These have been summarised and are described separately in Appendix 8, Time and travel analysis. The differences in costs and effects between the randomisation groups were calculated, formally tested for statistically significant differences using the ttest command in Stata® 15 (StataCorp LP, College Station, TX, USA), and presented in the form of an ICER. The ICER is defined as the difference in costs divided by the difference in effects (in this case QALYs) between two alternatives:
where C is costs.
A regression analysis on costs and outcomes was conducted using seemingly unrelated regression. 90 This approach involves the estimation of two linear regressions with their own dependent variable for costs and QALYs and a different set of explanatory variables. Randomisation group, trial centre and time since stroke were used as explanatory variables for both costs and QALYs. In addition, baseline utility score was used as an explanatory variable for the QALY equation and total baseline costs were added as an explanatory variable to the costs equation. This analysis was conducted in Stata 15 using the seemingly unrelated regression command sureg.
Results from the regression analysis were used to calculate the incremental cost per QALY gained for the comparison between the randomised groups at 6 months. Pairwise comparisons of the interventions were decided according to their cost (i.e. the least costly intervention was compared with the next least costly). If a comparator was both more costly and less effective than the others (i.e. it is dominated), then this comparator was dropped from any further cost-effectiveness comparisons.
Sensitivity analysis
Stochastic sensitivity analysis
The imprecision surrounding the estimates of costs, effects and cost-effectiveness was assessed by conducting a non-parametric stochastic sensitivity analysis. This technique allows for the comparison of arithmetic means without making assumptions about the distribution of costs and QALYs.
This approach involved drawing bootstrapped samples, with replacement, of the mean costs and mean QALYs from the original trial data. This process was repeated 1000 times, calculating new mean costs and mean QALYs. The number of replications was increased until the results were stable. The new values generated from the bootstrapping exercise were used to calculate the differences in costs and effects between groups.
The information generated from bootstrapping was then combined with a range of WTP values (£0, £10,000, £20,000, £30,000 and £50,000) per QALY gained. This generated a CEAC, which graphically represented and quantified the probability of each of the interventions being cost-effective at each of the prespecified WTP values.
Deterministic sensitivity analysis
A deterministic sensitivity analysis was carried out to explore any uncertainties surrounding the level of resource use and their impact on the cost-effectiveness of the interventions. No formal testing to detect differences across groups was conducted as part of the sensitivity analysis. The following areas of uncertainty were explored:
-
Extreme value analysis – this scenario assumed that the reason participants had not completed information on health-care resource use was because no health-care costs had been incurred. The total costs for these individuals was hence changed to zero, and the impact of this change in cost-effectiveness was assessed.
-
Multiple imputation of total missing costs – the possibility that those participants with missing total costs may have incurred some costs was also considered. Once the missing information analysis on costs established that information was missing at random, truncated normal regression was applied using age, sex and baseline ARAT score as covariates to generate 10 different total mean costs for each participant. After exploring the total cost distribution for each randomisation group, the upper limit was set at £25,000. The average value of the 10 iterations was taken and imputed in place of the missing value.
-
Increased useful life of the MIT-Manus robotic gym (7 years) – after seeking expert opinion, the effect on costs, QALYs and cost-effectiveness was explored in the event that the useful life of the robot could be extended from 5 to 7 years.
-
Exclusion of physiotherapy visits – participants were asked to record the physiotherapy sessions received during the 6-month follow-up period. To account for the risk of potentially ‘double-counting’ the therapy sessions received by participants as part of the trial, the sensitivity analysis explored how excluding the physiotherapy sessions recorded in the health service utilisation questionnaire would affect the results. All physiotherapy visits were excluded for those participants allocated to both the robot-assisted training and the EULT programme groups.
These analyses were combined with stochastic sensitivity analyses to minimise any uncertainty surrounding the ICER.
Subgroup analysis
To explore the impact of time since stroke on the cost-effectiveness of the interventions, an exploratory analysis was conducted for the subgroups outlined in the protocol. Three subgroups were prespecified (< 3 months, 3–12 months and > 12 months). No formal testing to detect differences across randomisation groups was included in the analysis.
Per-protocol analysis
A secondary cost-effectiveness analysis was conducted: removing from the data set those participants who did not receive at least 20 sessions of therapy in the robot-assisted training and the EULT programme groups. The cut-off point of 20 sessions is based on clinical evidence that 20 hours of therapy leads to improvements on functional outcome. 10 No formal testing to detect differences across groups was conducted as part of the per-protocol analysis.
Economic model
An economic model was originally planned in order to consider the long-term costs and utilities that each group of participants would probably experience over the course of their lifetime. The design and type of the economic model was contingent on the results of the within-trial analysis and, hence, the cost-effectiveness of the proposed interventions. Having examined the cost-effectiveness analysis (CEA) base-case results, the modelling exercise was designed to extrapolate the mean QALYs at 6 months to 12 months based on the results of the trial. It was assumed that participants across all groups maintained the same utility levels reported at the 6-month follow-up point.
Intervention costs and therapy-related costs (physiotherapy, occupational therapy, and speech and language therapy) were assumed not to continue beyond the 6-month trial period. However, all other levels of health-care resource use were assumed to remain constant and continue for an additional 6 months.
The differences in mean costs and effects between the randomisation groups were calculated, with differences across groups not being formally tested. The adjusted ICER at 12 months was derived after regression analysis on costs and outcomes; this was conducted using seemingly unrelated regression. 90 The same explanatory variables used in the base-case CEA were included. Choice of pairwise comparisons was decided according to their cost. As in the base-case CEA, if a comparator was both more costly and less effective than the others, it was dropped from any further cost-effectiveness comparisons.
A stochastic sensitivity analysis was conducted, drawing bootstrapped samples of mean costs and mean QALYs 1000 times. Results from this analysis were then combined with the same WTP values as used in the base-case CEA, generating a CEAC that represented the probability of the interventions being cost-effective at each WTP value.
Results
Response rates
The response rates relating to participant-completed health economics questionnaires are outlined in Appendix 8, Table 63. A progressive loss to follow-up was identified over the duration of the trial. The pattern of non-response was similar across the intervention groups; however, the loss to follow-up was more pronounced in the usual care group in both responses to the health service utilisation and quality-of-life questionnaires. Completion of resource use data at baseline was 96% across all randomisation groups. Overall, this decreased to 79% at 6 months, with 70% of participants in the usual care group completing the health service utilisation questionnaire.
Full completion of EQ-5D-5L at baseline was accomplished by 99% of participants. Completion rate decreased to 88% at 3 months and 82% at 6 months. The highest number of non-respondents belonged to the usual care group (see Appendix 8, Table 63).
Resource use
This section describes the use of health-care resources incurred by the three randomisation groups at both baseline and the 6-month follow-up. Mean and median resource use, SDs and IQRs have been reported. The information has been presented in two separate ways. First, the data were summarised for those participants who reported that they had accessed health-care services (Table 21 and Appendix 8, Table 64). Second, a summary (Table 22 and Appendix 8, Table 65) was created for all randomised groups at each time point to examine the overall mean (SD) and median (IQR) values.
| Area of resource utilisation | Randomisation group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | |||||||
| Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | |
| GP | |||||||||
| Surgery | 124 | 2.98 (4.63) | 2.00 (1.00–3.50) | 120 | 2.59 (2.16) | 2.00 (1.00–3.00) | 106 | 2.90 (2.09) | 2.00 (1.00–4.00) |
| Home | 33 | 2.33 (1.73) | 2.00 (1.00–3.00) | 33 | 1.75 (0.93) | 2.00 (1.00–2.00) | 27 | 2.48 (2.48) | 2.00 (1.00–3.00) |
| Telephone | 51 | 2.17 (1.60) | 2.00 (1.00–3.00) | 43 | 1.91 (1.27) | 1.00 (1.00–2.00) | 29 | 1.75 (0.99) | 1.00 (1.00–2.00) |
| Nurse | |||||||||
| Surgery | 62 | 1.98 (1.89) | 1.00 (1.00–2.00) | 53 | 1.96 (1.67) | 1.00 (1.00–2.00) | 49 | 3.10 (10.10) | 1.00 (1.00–2.00) |
| Home | 25 | 3.92 (4.74) | 2.00 (1.00–4.00) | 32 | 3.00 (3.26) | 2.00 (1.00–3.50) | 28 | 32.75 (107.52) | 1.00 (1.00–5.00) |
| Telephone | 8 | 2.12 (2.10) | 1.00 (1.00–3.00) | 7 | 1.57 (0.78) | 1.00 (1.00–2.00) | 5 | 1.4 (0.55) | 1.00 (1.00–2.00) |
| NHS Direct | 17 | 1.35 (0.86) | 1.00 (1.00–1.00) | 14 | 1.28 (0.61) | 1.00 (1.00–1.00) | 3 | 2.00 (1.73) | 1.00 (1.00–4.00) |
| Physiotherapy | |||||||||
| Hospital | 57 | 8.03 (9.55) | 4.00 (3.00–8.00) | 62 | 10.18 (14.81) | 4.50 (2.00–10.00) | 41 | 11.04 (12.60) | 6.00 (4.00–11.00) |
| Home | 49 | 11.55 (14.92) | 6.00 (3.00–20.00) | 57 | 11.24 (12.02) | 7.00 (3.00–16.00) | 48 | 15.40 (18.25) | 9.50 (4.50–20.00) |
| General practice surgery | 6 | 4.67 (5.64) | 2.50 (2.00–4.00) | 14 | 6.00 (7.38) | 3.00 (2.00–5.00) | 8 | 11.00 (15.45) | 7.00 (2.00–10.50) |
| Elsewhere | 11 | 4.36 (4.94) | 2.00 (1.00–10.00) | 6 | 7.50 (8.69) | 4.00 (2.00–10.00) | 9 | 12.00 (17.68) | 3.00 (1.00–12.00) |
| Occupational therapy | |||||||||
| Hospital | 22 | 6.13 (7.95) | 2.50 (1.00–6.00) | 20 | 13.00 (20.41) | 5.50 (1.50–15.50) | 13 | 7.61 (11.02) | 2.00 (1.00–6.00) |
| Home | 43 | 7.60 (9.86) | 3.00 (2.00–8.00) | 37 | 7.19 (7.88) | 4.00 (2.00–10.00) | 45 | 7.51 (12.71) | 3.00 (2.00–7.00) |
| General practice surgery | 1 | 1.00 (–) | 1.00 (1.00–1.00) | 1 | 2.00 (–) | 2.00 (2.00–2.00) | 1 | 3.00 (–) | 3.00 (3.00–3.00) |
| Elsewhere | 3 | 11.66 (5.77) | 15.00 (5.00–15.00) | 0 | – (–) | – (–) | 0 | – (–) | – (–) |
| Speech and language therapy | |||||||||
| Hospital | 16 | 7.56 (7.34) | 4.00 (2.50–12.50) | 18 | 7.22 (10.80) | 2.00 (1.00–12.00) | 14 | 11.57 (15.20) | 7.00 (1.00–12.00) |
| Home | 20 | 4.95 (4.58) | 3.00 (2.00–7.00) | 17 | 8.00 (12.04) | 4.00 (2.00–10.00) | 21 | 18.57 (41.83) | 6.00 (2.00–18.00) |
| General practice surgery | 0 | – (–) | – (–) | 2 | 3.50 (3.53) | 3.50 (1.00–6.00) | 3 | 1.00 (0.00) | 1.00 (1.00–1.00) |
| Elsewhere | 3 | 3.33 (1.52) | 3.00 (2.00–5.00) | 1 | 10.00 (–) | 10.00 (10.00–10.00) | 1 | 6.00 (–) | 6.00 (6.00–6.00) |
| A&E visits | 45 | 1.58 (0.92) | 1.00 (1.00–2.00) | 49 | 1.63 (1.48) | 1.00 (1.00–2.00) | 30 | 1.40 (1.16) | 1.00 (1.00–1.00) |
| Outpatient appointments | 110 | 3.16 (3.78) | 2.00 (1.00–4.00) | 100 | 3.06 (3.60) | 2.00 (1.00–4.00) | 83 | 3.14 (5.76) | 2.00 (1.00–3.00) |
| Hospital nights | |||||||||
| Admitted via A&E | 20 | 8.45 (13.64) | 3.00 (2.00–8.00) | 27 | 14.59 (34.48) | 5.00 (1.00–10.00) | 15 | 8.26 (9.74) | 3.00 (1.00–18.00) |
| Not admitted via A&E | 7 | 7.71 (16.48) | 1.00 (1.00–3.00) | 5 | 1.20 (0.45) | 1.00 (1.00–1.00) | 6 | 7.50 (7.06) | 5.50 (1.00–14.00) |
| Day patient treatment | |||||||||
| Half day | 12 | 1.42 (0.51) | 1.00 (1.00–2.00) | 13 | 1.54 (1.13) | 1.00 (1.00–1.00) | 7 | 1.43 (0.79) | 1.00 (1.00 – 2.00) |
| Full day | 6 | 1.00 (0.00) | 1.00 (1.00–1.00) | 4 | 1.25 (0.50) | 1.00 (1.00–1.50) | 1 | 1.00 (–) | 1.00 (1.00–1.00) |
| Residential care | 5 | 74.40 (68.00) | 69.00 (30.00–90.00) | 5 | 89.80 (75.87) | 105.00 (16.00–136.00) | 5 | 49.40 (45.82) | 40.00 (7.00–90.00) |
| Nursing home | 0 | – (–) | – (–) | 1 | 180 (0.00) | 180.00 (180.00–180.00) | 3 | 181.67 (0.57) | 182.00 (181.00–182.00) |
| Meals on Wheels | 1 | 4.00 (–) | 4.00 (4.00–4.00) | 2 | 4.50 (3.53) | 4.50 (2.00–7.00) | 2 | 7.5 (9.19) | 7.50 (1.00–14.00) |
| Home help | |||||||||
| Personal care | 48 | 12.70 (8.79) | 8.5 (7.00–21.00) | 43 | 14.83 (9.75) | 12.00 (7.00 – 28.00) | 38 | 14.45 (7.92) | 14.00 (7.00–21.00) |
| Household tasks | 21 | 7.47 (7.52) | 7.00 (1.00–14.00) | 18 | 8.05 (11.10) | 2.00 (1.00–7.00) | 18 | 10.28 (10.53) | 7.00 (1.00–21.00) |
| Shopping | 8 | 3.00 (2.56) | 1.5 (1.00–5.50) | 7 | 2.14 (2.19) | 1.00 (1.00–2.00) | 7 | 2.85 (2.85) | 1.00 (1.00–7.00) |
| Health visitor | 5 | 2.20 (2.17) | 1.00 (1.00–2.00) | 6 | 3.17 (4.40) | 1.00 (1.00–3.00) | 3 | 2.00 (1.00) | 2.00 (1.00–3.00) |
| Geriatrician | 2 | 3.50 (3.53) | 3.50 (1.00–6.00) | 3 | 1.67 (1.15) | 1.00 (1.00–3.00) | 0 | – (–) | – (–) |
| Psychiatrist | 10 | 2.70 (2.11) | 2.00 (1.00–3.00) | 6 | 2.67 (2.06) | 2.00 (1.00–4.00) | 2 | 1.00 (0.00) | 1.00 (1.00–1.00) |
| Psychologist | 23 | 4.09 (5.00) | 3.00 (1.00–4.00) | 18 | 3.28 (2.37) | 3.00 (1.00–4.00) | 15 | 5.13 (6.66) | 2.00 (1.00–6.00) |
| Chiropodist | 50 | 2.32 (1.35) | 2.00 (1.00–3.00) | 38 | 2.16 (1.57) | 2.00 (1.00–3.00) | 39 | 1.97 (1.28) | 2.00 (1.00–2.00) |
| Optician | 41 | 1.26 (0.55) | 1.00 (1.00–1.00) | 42 | 1.17 (0.58) | 1.00 (1.00–1.00) | 42 | 1.33 (0.57) | 1.00 (1.00–2.00) |
| Pharmacist | 34 | 4.12 (4.46) | 3.00 (1.00–6.00) | 27 | 4.59 (4.69) | 3.00 (1.00–6.00) | 31 | 6.55 (8.52) | 5.00 (2.00–6.00) |
| Area of resource utilisation | Randomisation group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | |||||||
| Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | |
| GP | |||||||||
| Surgery | 205 | 1.80 (3.88) | 1.00 (0.00–2.00) | 208 | 1.49 (2.08) | 1.00 (0.00–2.00) | 168 | 1.83 (2.17) | 1.00 (0.00–3.00) |
| Home | 209 | 0.37 (1.08) | 0.00 (0.00–0.00) | 213 | 0.27 (0.73) | 0.00 (0.00–0.00) | 174 | 0.38 (1.29) | 0.00 (0.00–0.00) |
| Telephone | 207 | 0.53 (1.22) | 0.00 (0.00–0.00) | 207 | 0.39 (0.96) | 0.00 (0.00–0.00) | 172 | 0.30 (0.77) | 0.00 (0.00–0.00) |
| Nurse | |||||||||
| Surgery | 203 | 0.61 (1.39) | 0.00 (0.00–1.00) | 210 | 0.49 (1.19) | 0.00 (0.00–1.00) | 169 | 0.90 (5.58) | 0.00 (0.00–1.00) |
| Home | 206 | 0.47 (2.07) | 0.00 (0.00–0.00) | 213 | 0.45 (1.65) | 0.00 (0.00–0.00) | 170 | 5.39 (44.63) | 0.00 (0.00–0.00) |
| Telephone | 211 | 0.08 (0.56) | 0.00 (0.00–0.00) | 212 | 0.52 (0.31) | 0.00 (0.00–0.00) | 174 | 0.04 (0.25) | 0.00 (0.00–0.00) |
| NHS Direct | 211 | 0.11 (0.44) | 0.00 (0.00–0.00) | 213 | 0.08 (0.35) | 0.00 (0.00–0.00) | 174 | 0.03 (0.32) | 0.00 (0.00–0.00) |
| Physiotherapy | |||||||||
| Hospital | 210 | 2.18 (6.10) | 0.00 (0.00–1.00) | 211 | 2.99 (9.23) | 0.00 (0.00–2.00) | 171 | 2.64 (7.72) | 0.00 (0.00–0.00) |
| Home | 205 | 2.76 (8.76) | 0.00 (0.00–0.00) | 207 | 3.10 (8.04) | 0.00 (0.00–1.00) | 170 | 4.35 (11.87) | 0.00 (0.00–2.00) |
| General practice surgery | 213 | 0.13 (1.16) | 0.00 (0.00–0.00) | 212 | 0.40 (2.63) | 0.00 (0.00–0.00) | 176 | 0.50 (3.85) | 0.00 (0.00–0.00) |
| Elsewhere | 212 | 0.23 (1.45) | 0.00 (0.00–0.00) | 213 | 0.21 (1.82) | 0.00 (0.00–0.00) | 175 | 0.62 (4.63) | 0.00 (0.00–0.00) |
| Occupational therapy | |||||||||
| Hospital | 211 | 0.64 (3.14) | 0.00 (0.00–0.00) | 211 | 1.23 (7.23) | 0.00 (0.00–0.00) | 174 | 0.57 (3.53) | 0.00 (0.00–0.00) |
| Home | 210 | 1.56 (5.39) | 0.00 (0.00–0.00) | 209 | 1.27 (4.28) | 0.00 (0.00–0.00) | 173 | 1.95 (7.23) | 0.00 (0.00–1.00) |
| General practice surgery | 213 | 0.00 (0.07) | 0.00 (0.00–0.00) | 211 | 0.01 (0.14) | 0.00 (0.00–0.00) | 176 | 0.02 (0.23) | 0.00 (0.00–0.00) |
| Elsewhere | 212 | 0.16 (1.49) | 0.00 (0.00–0.00) | 211 | 0.00 (0.00) | 0.00 (0.00–0.00) | 176 | 0.00 (0.00) | 0.00 (0.00–0.00) |
| Speech and language therapy | |||||||||
| Hospital | 213 | 0.57 (2.79) | 0.00 (0.00–0.00) | 213 | 0.65 (3.74) | 0.00 (0.00–0.00) | 173 | 0.94 (5.24) | 0.00 (0.00–0.00) |
| Home | 210 | 0.47 (2.00) | 0.00 (0.00–0.00) | 212 | 0.64 (3.97) | 0.00 (0.00–0.00) | 175 | 2.22 (15.42) | 0.00 (0.00–0.00) |
| General practice surgery | 212 | 0.00 (0.00) | 0.00 (0.00–0.00) | 213 | 0.03 (0.42) | 0.00 (0.00–0.00) | 176 | 0.02 (0.13) | 0.00 (0.00–0.00) |
| Elsewhere | 213 | 0.05 (0.42) | 0.00 (0.00–0.00) | 213 | 0.47 (0.68) | 0.00 (0.00–0.00) | 175 | 0.03 (0.45) | 0.00 (0.00–0.00) |
| A&E visits | 213 | 0.33 (0.77) | 0.00 (0.00–0.00) | 214 | 0.37 (0.98) | 0.00 (0.00 – 0.00) | 178 | 0.24 (0.70) | 0.00 (0.00 – 0.00) |
| Outpatient appointments | 212 | 1.64 (3.14) | 1.00 (0.00–2.00) | 215 | 1.42 (2.88) | 0.00 (0.00–2.00) | 176 | 1.48 (4.24) | 0.00 (0.00–2.00) |
| Hospital nights | |||||||||
| Admitted via A&E | 213 | 0.79 (4.77) | 0.00 (0.00–0.00) | 215 | 1.83 (12.95) | 0.00 (0.00–0.00) | 176 | 0.70 (3.59) | 0.00 (0.00–0.00) |
| Not admitted via A&E | 213 | 0.28 (3.09) | 0.00 (0.00–0.00) | 215 | 0.03 (0.19) | 0.00 (0.00–0.00) | 176 | 0.25 (1.81) | 0.00 (0.00–0.00) |
| Day patient treatment | |||||||||
| Half day | 205 | 0.08 (0.35) | 0.00 (0.00–0.00) | 210 | 0.09 (0.46) | 0.00 (0.00–0.00) | 175 | 0.06 (0.32) | 0.00 (0.00–0.00) |
| Full day | 199 | 0.03 (0.17) | 0.00 (0.00–0.00) | 201 | 0.025 (0.18) | 0.00 (0.00–0.00) | 169 | 0.06 (0.07) | 0.00 (0.00–0.00) |
| Residential care | 213 | 1.75 (14.65) | 0.00 (0.00–0.00) | 215 | 2.08 (17.07) | 0.00 (0.00–0.00) | 177 | 1.39 (10.72) | 0.00 (0.00–0.00) |
| Nursing home | 213 | 0.00 (0.00) | 0.00 (0.00–0.00) | 216 | 0.83 (12.24) | 0.00 (0.00–0.00) | 177 | 3.08 (23.51) | 0.00 (0.00–0.00) |
| Meals on Wheels | 213 | 0.02 (0.27) | 0.00 (0.00–0.00) | 213 | 0.04 (0.49) | 0.00 (0.00–0.00) | 177 | 0.08 (1.05) | 0.00 (0.00–0.00) |
| Home help | |||||||||
| Personal care | 211 | 2.89 (6.77) | 0.00 (0.00–0.00) | 210 | 3.04 (7.42) | 0.00 (0.00–0.00) | 173 | 3.17 (7.03) | 0.00 (0.00–0.00) |
| Household tasks | 213 | 0.74 (3.21) | 0.00 (0.00–0.00) | 212 | 0.68 (3.87) | 0.00 (0.00–0.00) | 175 | 1.06 (4.54) | 0.00 (0.00–0.00) |
| Shopping | 213 | 0.11 (0.74) | 0.00 (0.00–0.00) | 212 | 0.07 (0.53) | 0.00 (0.00–0.00) | 176 | 0.11 (0.76) | 0.00 (0.00–0.00) |
| Health visitor | 212 | 0.05 (0.45) | 0.00 (0.00–0.00) | 213 | 0.09 (0.85) | 0.00 (0.00–0.00) | 177 | 0.03 (0.28) | 0.00 (0.00–0.00) |
| Geriatrician | 212 | 0.03 (0.42) | 0.00 (0.00–0.00) | 213 | 0.02 (0.23) | 0.00 (0.00–0.00) | 177 | 0.00 (0.00) | 0.00 (0.00–0.00) |
| Psychiatrist | 212 | 0.13 (0.72) | 0.00 (0.00–0.00) | 213 | 0.75 (0.54) | 0.00 (0.00–0.00) | 175 | 0.01 (0.11) | 0.00 (0.00–0.00) |
| Psychologist | 209 | 0.45 (2.07) | 0.00 (0.00–0.00) | 211 | 0.28 (1.14) | 0.00 (0.00–0.00) | 176 | 0.44 (2.37) | 0.00 (0.00–0.00) |
| Chiropodist | 210 | 0.55 (1.19) | 0.00 (0.00–0.00) | 210 | 0.39 (1.06) | 0.00 (0.00–0.00) | 173 | 0.44 (1.02) | 0.00 (0.00–0.00) |
| Optician | 210 | 0.25 (0.56) | 0.00 (0.00–0.00) | 212 | 0.23 (0.53) | 0.00 (0.00–0.00) | 174 | 0.32 (0.64) | 0.00 (0.00–0.00) |
| Pharmacist | 211 | 0.66 (2.33) | 0.00 (0.00–0.00) | 207 | 0.60 (2.28) | 0.00 (0.00–0.00) | 174 | 1.16 (4.35) | 0.00 (0.00–0.00) |
Missing data were excluded from the analysis. No formal testing was conducted to detect statistically significant differences in resource use between randomisation groups.
Resource use at baseline
The information collected at baseline captured the resource use recorded by participants in the 3 months prior to being randomised to the trial. Response rates were high across all randomisation groups. Appendix 8, Table 64, shows the summary statistics for those participants who reported to have accessed health-care services. Key differences are described below; for full details of access to services, see Appendix 8, Response rates and health-care resource use.
For those who accessed GP and nursing services, there was no substantial variation between randomisation groups. The only exception was the average number of home nurse visits received by participants, which was higher in the usual care group. Large SDs indicate that there was considerable variation in the number of nurse home visits for this group, with some individuals using services very frequently, thereby inflating the mean.
Resource use in secondary care and residential and nursing home facilities was characterised by a substantial variability in the level of resource use, as shown by the high SDs for each of the randomisation groups. This was particularly noticeable in the number of nights in hospital reported by participants admitted to hospital through either A&E or a non-emergency admission. For those participants staying in residential care and nursing homes facilities, high mean and SD values were reported, indicating large variation between individuals, with long-term residents inflating the mean across all groups.
Descriptive statistics for the randomisation groups as a whole are described in Appendix 8, Table 65. This shows similar mean levels of resource use across all groups in primary care. Participants in the robot-assisted training group reported the highest mean number of hospital nights after A&E admission, followed by EULT and usual care.
Resource use at 6 months post randomisation
Participants reported on the level of health-care resource use over the 6 months of the trial. The type of health care accessed by participants included primary, therapy and secondary services provided by the NHS; stays at residential and nursing home facilities; and use of social services and community-based health care. Table 21 summarises the level of resource use for those accessing NHS and PSS services.
General practitioner consultations and outpatient hospital visits were the most common health-care services accessed by participants across all groups. This was followed by nurse consultations and physiotherapy appointments.
Reported use of secondary care services was characterised by a high variability in all categories except for day patient treatment and A&E visits. The highest mean values across all groups were found in the reported number of overnight hospital stays. The mean number of nights spent in hospital after admission via A&E was highest in the EULT group, followed by the robot-assisted training and usual care groups. When admission to hospital was not via A&E, the robot-assisted training group displayed the highest mean number of nights in hospital, followed by the EULT and usual care groups.
The use of community-based health care and social services was similar across all randomisation groups. The reported use of a community pharmacist, however, suggests the highest variability among all participants, with high SDs calculated for all groups. Table 22 shows that, overall, mean values were similar across all randomisation groups for most areas of health-care resource use. The main differences between groups were reported in the therapy services received, with the usual care group participants being the highest users of home physiotherapy and speech and language therapy services.
Participants were also asked to report additional NHS and social services received during the trial that were not captured by predefined questions in the questionnaire. The resource use and unit costs of these additional services is summarised in Appendix 8, Tables 70–73.
Health-care costs
Cost information at baseline was used to adjust for any imbalance in resources and costs after randomisation (see Appendix 8).
The costs collected at 6 months post randomisation were used to calculate incremental mean costs to ascertain the cost-effectiveness of the interventions. Table 23 summarises the total costs per participant for each cost category, as well as the mean total cost per randomisation group. The highest mean costs per participant were associated with the use of social care services, which included stays in residential and nursing home facilities and care assistance received at home. The average cost per participant was higher in the usual care group in all categories except in secondary care and other NHS and social services used by the participants. The addition of the intervention costs, however, reversed this trend, making the mean cost per participant highest in the group receiving robot-assisted training. Large SDs indicate that there was substantial variation in resource use between individuals across all randomisation groups.
| Area of resource utilisation | Randomisation group | |||||
|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Intervention costs | 2872 (0) | 2872 (2872–2872) | 1399 (0) | 1399 (1399–1399) | – (–) | – (–) |
| Primary care costs and community-based health care (including therapy services) | 743 (1031) | 322 (96–748) | 777 (1262) | 326 (94–904) | 1078 (1813) | 514 (145–1194) |
| Social care | 1410 (3146) | 0 (0–322) | 1541 (3943) | 0 (0–0) | 1890 (4281) | 0 (0–1612) |
| Secondary care | 733 (2247) | 142 (0–704) | 988 (4486) | 142 (0–566) | 668 (1880) | 138 (0–138) |
| Medication costs | 149 (302) | 53 (26–139) | 154 (273) | 75 (27–186) | 198 (347) | 81 (32–220) |
| Other NHS and social services | 727 (983) | 339 (172–904) | 790 (946) | 525 (186–860) | 307 (406) | 171 (128–256) |
| Deceased participants | 0 (0) | 0 (0–0) | 13,953 (4516) | 13,451 (9709–18,700) | – (–) | – (–) |
| Mean total cost | 5387 (4054) | 3778 (2962–5854) | 4451 (6033) | 2245 (1596–4442) | 3785 (5437) | 1302 (503–4598) |
| Mean difference between usual care and robot-assisted training (95% CI); p-value | –1601 (–2496 to –706); < 0.001 | |||||
| Mean difference between usual care and EULT (95% CI); p-value | –665 (–1774 to 444); 0.239 | |||||
The mean difference in total costs between the usual care and robot-assisted training groups was statistically significant, with usual care being, on average, £1601 less costly than robot-assisted training (p < 0.001).
Quality-of-life outcomes
The utility scores derived from responses to the EQ-5D-5L questionnaires are shown in Table 24. Response rates for the robot-assisted training and EULT randomisation groups were similar at all time points. The number of responses for the usual care group was noticeably lower both at 3 and 6 months. The utility values were similar across all groups. The highest mean utility increment occurs between baseline and 3 months across all groups. The highest mean QALY was reported for the EULT group (0.23), with the robot-assisted training and usual care groups yielding an equal mean QALY gained of 0.21 at 6 months. The mean difference in QALYs between each of the intervention groups (robot-assisted training and EULT) and usual care was very small and was not statistically significant, with the 95% CIs crossing zero.
| Robot-assisted training | EULT | Usual care | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | |
| Utility scores | |||||||||
| Baseline EQ-5D-5L | 254 | 0.36 (0.26) | 0.38 (0.17–0.56) | 259 | 0.39 (0.25) | 0.41 (0.20–0.57) | 254 | 0.37 (0.26) | 0.39 (0.18–0.58) |
| 3-month EQ-5D-5L | 232 | 0.45 (0.27) | 0.51 (0.26–0.67) | 236 | 0.48 (0.24) | 0.55 (0.32–0.67) | 207 | 0.42 (0.29) | 0.42 (0.21–0.64) |
| 6-month EQ-5D-5L | 223 | 0.46 (0.29) | 0.54 (0.22–0.71) | 222 | 0.50 (0.27) | 0.57 (0.32–0.72) | 190 | 0.46 (0.27) | 0.53 (0.25–0.66) |
| QALYs at 6 months after multiple imputation | 254 | 0.21 (0.12) | 0.22 (0.14–0.30) | 259 | 0.23 (0.10) | 0.24 (0.16–0.30) | 254 | 0.21 (0.11) | 0.22 (0.15–0.28) |
| Mean difference in QALYs between usual care and robot-assisted (95% CI); p-value | 0.00 (–0.20 to 0.20); 0.995 | ||||||||
| Mean difference in QALYs between usual care and EULT (95% CI); p-value | –0.02 (–0.35 to 0.00); 0.080 | ||||||||
Results from the within-trial economic evaluation
Incremental cost-effectiveness analysis
The unadjusted CEA shows that usual care was less costly than robot-assisted training and EULT, but that usual care was less effective than EULT. There was no evidence of a difference in QALYs between groups (Table 25). The deterministic results from the adjusted analysis show an ICER of £74,100 for the EULT group, compared with usual care. Robot-assisted training was dominated by EULT as it was, on average, both more costly and less effective, although this was not statistically significant.
| Randomisation group | Unadjusted mean (98.33% CI) | Adjusted incremental (98.33% CI) | Adjusted ICER (£) | Probability that each therapy is cost-effective at WTP of | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALY | QALY | Cost (£) | £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||
| Usual care | 3785 (2801 to 4770) | 0.21 (0.19 to 0.23) | – | 0.90 | 0.85 | 0.81 | 0.74 | 0.62 | ||
| EULT | 4451 (3548 to 5354) | 0.23 (0.21 to 0.24) | 0.010 (–0.005 to 0.025) | 741 (–461 to 1943) | 74,100 | 0.10 | 0.15 | 0.19 | 0.26 | 0.38 |
| Robot-assisted training | 5387 (4777 to 5996) | 0.21 (0.19 to 0.23) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
The results of the deterministic analysis do not reflect the statistical imprecision surrounding estimates of cost-effectiveness. This is provided by the stochastic sensitivity analysis. The results from this sensitivity analysis (Figure 14) suggest that there was approximately a 20% chance that EULT was cost-effective at a £20,000 WTP threshold value, and the probability that EULT was cost-effective is never > 40% over the range of WTP values considered. Robot-assisted training has a 0% chance of being cost-effective at all the WTP values considered in the analysis.
FIGURE 14.
Cost-effectiveness acceptability curve (base-case analysis): adjusted bootstrapped replications for CEA.
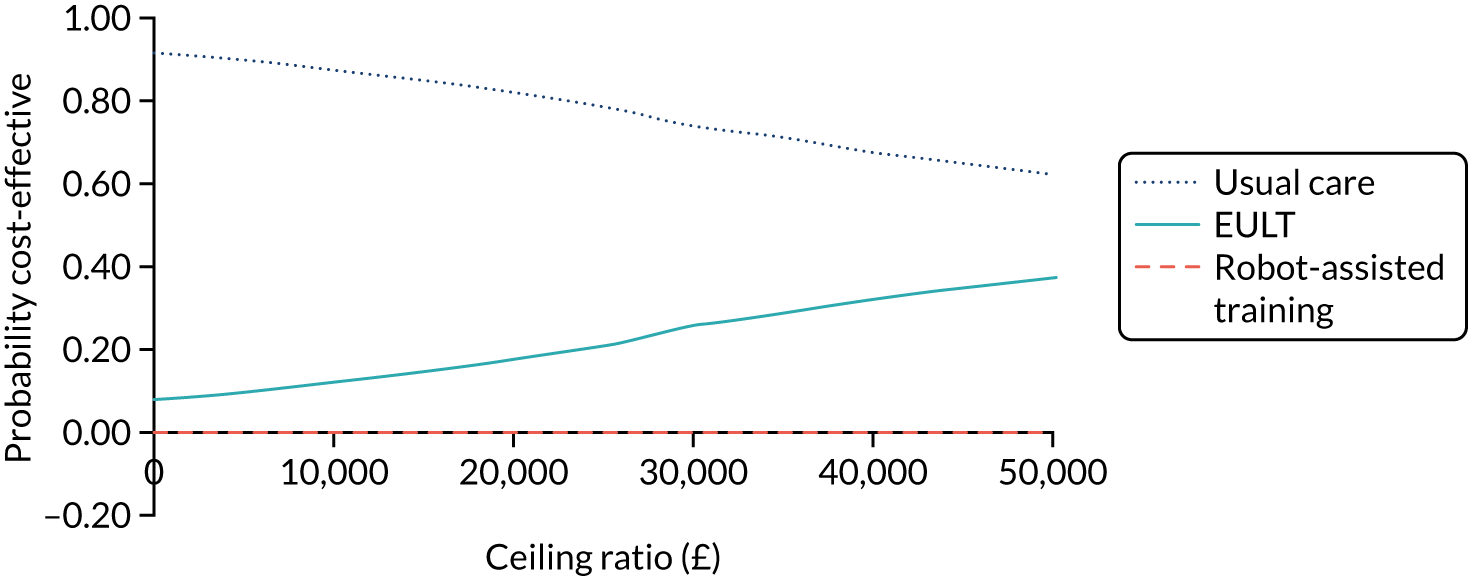
Additional graphical representation of the bootstrapped incremental costs and QALYs for EULT and robot-assisted training, compared with usual care, can be seen in Figures 15 and 16. Figure 15 represents a comparison between EULT and usual care, showing the bootstrapped incremental costs and QALYs, taking usual care as the reference point. As seen in Table 25, the majority of bootstrapped values show that EULT was more costly and more effective than usual care. The majority of these bootstrapped points fall above the £20,000 WTP threshold line, confirming that EULT has a low probability of being cost-effective except when society’s WTP for a QALY is very high.
FIGURE 15.
Incremental costs and QALYs for EULT in relation to usual care.
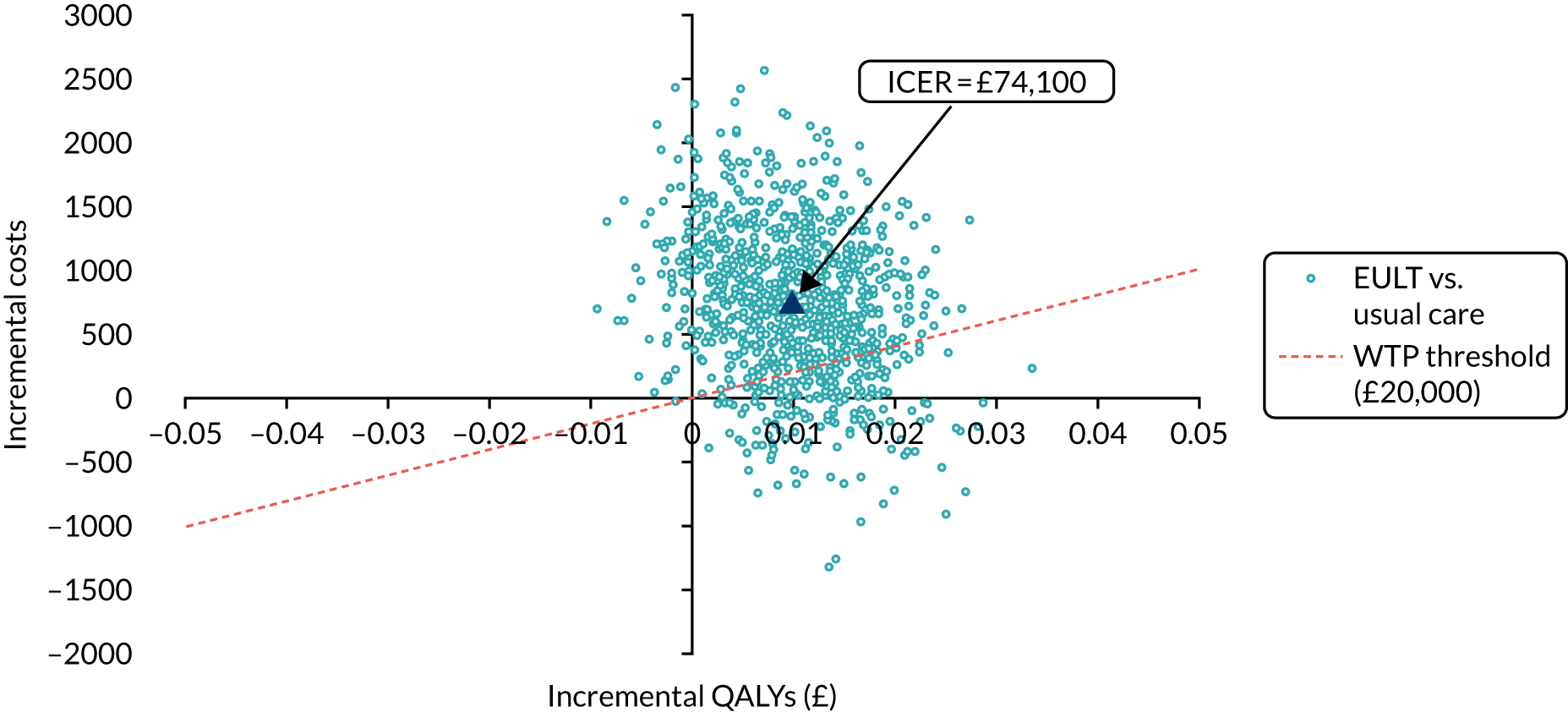
FIGURE 16.
Incremental costs and QALYs for robot-assisted training in relation to usual care.

For completeness, we have also graphically represented the bootstrapped incremental costs and QALYs for robot-assisted training compared with usual care. Figure 16 shows how the majority of bootstrapped values for robot-assisted training confirm that it was, on average, both more costly and as effective as usual care. A very small number of bootstrapped values fall below the £20,000 WTP threshold line, confirming that the probability of robot-assisted training being cost-effective is very close to zero, even when society’s WTP for a QALY is higher than might normally be considered to be the case.
Deterministic sensitivity analysis
The results of each scenario explored in the deterministic sensitivity analysis are detailed in Appendix 8, Deterministic sensitivity analysis. Table 26 provides a summary of the main findings. Robot-assisted training remains dominated in all the scenarios explored.
| Sensitivity analysis | Incremental (98.33% CI)a | ICER (£) | Probability of EULT being considered cost-effective at a WTP of £20,000 | |
|---|---|---|---|---|
| Cost (£) | QALY | |||
| Extreme analysis | 1892 (823 to 2961) | 0.011 (–0.002 to 0.024) | 172,000 | 0.00 |
| Multiple imputation | 550 (–503 to 1604) | 0.011 (–0.002 to 0.024) | 50,000 | 0.27 |
| Useful life of robot extended to 7 years | 741 (–460 to 1943) | 0.010 (–0.005 to 0.025) | 74,100 | 0.19 |
| Physiotherapy sessions excluded | 486 (–702 to 1674) | 0.010 (–0.005 to 0.025) | 48,600 | 0.34 |
When missing costs were changed to zero, the resulting ICER between EULT and usual care increased to £172,000 (see Appendix 8, Tables 74 and 75, and Figure 17). This increase was to be expected because all participants with missing total costs belonged to the usual care group. By imputing zero for missing costs, the mean costs for the usual care participants were reduced; hence, the ICER increased when compared with EULT (see Appendix 8, Tables 76 and 77, and Figure 18).
Results from extending the life of the robotic gym are shown in Appendix 8, Tables 78 and 79, and Figure 19. The lowest ICER resulted from the exclusion of physiotherapy sessions from the analysis (£48,600) (see Appendix 8, Tables 80 and 81, and Figure 20).
Subgroup analysis
The results from the adjusted and unadjusted subgroup analysis are outlined in Appendix 8, Subgroup analysis. Adjusted and unadjusted results suggest that robot-assisted training was, on average, more costly and less effective than EULT across all subgroups considered. The ICER, therefore, relates to the comparison between EULT and usual care at all times. The highest ICER (£126,143) relates to those participants who, at randomisation, had experienced a stroke between 1 and 5 years ago. The subgroup of participants who were < 3 months post stroke at randomisation had the lowest ICER (£31,400). The stochastic sensitivity analysis for this group suggests that EULT has a 41% probability of being cost-effective at the £20,000 WTP threshold.
The subgroup analysis results seem to mimic those found in the base-case economic evaluation. The results, however, need to be interpreted with caution because of the reduced sample size in each subgroup.
Per-protocol analysis
Results from the per-protocol analysis are reported in Appendix 8, Per-protocol analysis. Both the unadjusted and adjusted results suggest that robot-assisted training is, on average, more costly and less effective than EULT, and more costly and slightly more effective than usual care.
The resulting ICER (£68,000) relates to the comparison of EULT with usual care. The stochastic sensitivity analysis suggests that EULT has a 17% probability of being cost-effective at the £20,000 WTP threshold.
Results from the economic model
The development of the model was contingent on the CEA results. At the current £20,000 WTP threshold, usual care had the highest probability of being cost-effective (81%) at 6 months. The economic model explored the impact on the results if the data were extrapolated beyond the time frame of the trial (see Appendix 8, Tables 94–96, and Figure 24).
The unadjusted analysis shows that mean total costs per participant were lowest in the EULT group (£6892), followed by usual care (£6916) and robot-assisted training (£7538). The assumption that utility scores at 12 months equalled those reported at 6 months resulted in the mean QALY results mimicking those obtained in the base-case CEA. Participants in the EULT group had the highest mean QALYs at 12 months (0.48), followed by usual care (0.47) and robot-assisted training (0.44). These results indicate that there is no evidence of a difference in mean QALYs between groups at 12 months.
The adjusted CEA, however, shows that usual care was £128 less costly than EULT. Even though the point estimate of the unadjusted analysis showed that EULT was the least costly option, this is no longer the case when the CEA controls for baseline costs. These increased costs in EULT are then translated into a £6095 ICER for the comparison between EULT and usual care; however there is only a 55% probability of EULT being considered cost-effective compared with usual care at the £20,000 WTP value.
The base-case CEA results from the within-trial analysis precluded the need for a more complex economic model at this stage. An important limitation of the economic model is that untested assumptions (based on trial data) on both costs and QALYs have been made. Although costs may be estimated, there is no evidence that utilities will remain constant between 6 and 12 months. This means that cost-effectiveness from the extrapolated results must be interpreted with caution.
Changes to the mode of delivery of EULT and robot-assisted training may affect both costs and QALYs, and could be explored in further research. However, effectiveness data on changes to EULT or robot-assisted training delivery are not currently available and no sensible parameter values could have been used to inform the model.
Discussion
The main strength of this economic evaluation is that it was conducted alongside a rigorously run pragmatic RCT and followed guidelines for best practice throughout. 87,91 As a result, the economic evaluation was based on individual patient data collected during the trial and benefited from small numbers of missing health-care resource and quality-of-life data. Completion of baseline questionnaires was close to 100% across all randomisation groups. Although response rates for the health service utilisation questionnaire remained high at 6 months, at 79% overall, this decreased to 70% for participants in the usual care group. This number of missing responses is, however, to be expected. To assess the impact of the missing data, multiple imputation methods were applied in a sensitivity analysis. The cost-effectiveness results obtained using the imputed variables were consistent with those from the base-case analysis.
Self-reported quality-of-life data were collected at three points during the trial using the EQ-5D-5L questionnaire. 26 This enabled us to accurately measure QALY gains for participants across all groups using the preference-based instrument recommended by NICE. 88 One important strength is that assessing quality of life in this way will enable cost-effectiveness comparisons not only for this patient group, but across different disease areas. Following NICE guidance,88 responses to the EQ-5D-5L questionnaire were mapped to the EQ-5D-3L descriptive system to generate the utility values.
A noteworthy limitation of the economic evaluation is associated with the time frame of the trial. The within-trial economic evaluation assessed the cost-effectiveness of the interventions at 6 months. A longer-term perspective would have been achieved through the development of the long-term full economic model that was originally planned. However, given the results of the trial and the dominant status of usual care, the development of this type of model was no longer considered appropriate. As an alternative, a shorter-term model was conducted to derive cost-effectiveness results at 12 months. The results, however, need to be interpreted with caution because of the restricted assumptions made on both costs and utility values.
The economic evaluation fills a significant evidence gap, as no economic evaluation comparing robot-assisted training with usual care had been conducted in the UK NHS setting before, to our knowledge. A scoping review found little evidence of cost-effectiveness studies in the UK, and the only economic evaluation assessing the cost-effectiveness of robot therapy was US based. 18 Differences in the health-care systems between both countries mean that, unlike our economic evaluation, the results from this US study18 lack generalisability to the UK setting. Furthermore, there were differences in the therapy received by the randomisation groups, which reduces the grounds for comparability with the care received in the NHS.
The use of multiple sites across the UK contributed to the generalisability of the economic evaluation. The analysis controlled for differences in trial centres, hence minimising the chance of obtaining biased results from differences in costs and effects driven by location.
Overall, our economic evaluation was designed and conducted following NICE’s guidance for best practice,87,91 which resulted in robust and generalisable results.
The results from the economic evaluation create opportunities for further research. In particular, further analyses could explore the potential effect on both costs and QALYs of reconfigurations to the delivery of EULT and robot-assisted training.
Conclusion
The CEA results suggest that, on average, robot-assisted training was more costly than both EULT and usual care, and that robot-assisted training was less effective than EULT and as effective as usual care. EULT was, on average, more effective and more costly than usual care in both the adjusted and unadjusted CEA. The balance of probabilities favoured usual care as the preferred upper limb rehabilitation therapy over the range of WTP values considered for the participants included in the trial. Focusing on society’s WTP for a QALY, the stochastic analysis suggests that the balance of probabilities does not favour EULT as being cost-effective over any of the WTP values considered, despite EULT being more effective than the other randomisation groups.
A subgroup analysis in which the participants were categorised according to time since stroke did not change the direction of the cost-effectiveness results.
The lowest ICER (£48,600) between EULT and usual care in the sensitivity analysis was reported when the costs of physiotherapy sessions were excluded. Extrapolating within-trial results to 12 months produced the lowest ICER (£6095) for the comparison between EULT and usual care overall; however, high uncertainty surrounds the assumptions made on costs and utilities for the 12-month period. Further studies are needed to confirm if these results hold true.
Chapter 9 Discussion
Parts of this chapter have been reproduced from Rodgers et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have been reproduced from Rodgers et al. 92 Reprinted from The Lancet, 395, Rodgers H, Bosomworth H, van Wijck F, Krebs HI, Shaw L, Usual care: the big but unmanaged problem of rehabilitation evidence – authors’ reply, 337–8, 2020, with permission from Elsevier.
Parts of this chapter have been reproduced from Bosomworth et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Key findings
Primary outcome: achievement of prespecified improvement in upper limb function at 3 months
All groups showed improvement in upper limb function from baseline to 3 months. Upper limb functional recovery ‘success’ at 3 months was achieved by 44% of participants who received robot-assisted training, by 50% of participants who received the EULT programme and by 42% of participants who received usual care. However, we found little evidence of a difference in improvement of upper limb function at 3 months between stroke patients who were randomised to receive robot-assisted training, patients who were randomised to receive an EULT programme of goal-orientated repetitive functional task practice and patients who were randomised to receive usual care. The aOR comparing robot-assisted training, using the MIT-Manus robotic gym, with usual care was 1.2 (98.33% CI 0.7 to 2.0). The aOR comparing the EULT programme with usual care was 1.5 (98.33% CI 0.9 to 2.5). The aOR comparing the EULT programme with robot-assisted training was 0.8 (98.33% CI 0.5 to 1.3).
Secondary outcomes: upper limb function, upper limb impairment, activities of daily living, quality of life and upper limb pain
The improvements seen in upper limb function from baseline to 3 months were maintained at 6 months by all randomisation groups. Upper limb functional recovery ‘success’ at 6 months was achieved by 47% of participants who received robot-assisted training, by 54% of participants who received the EULT programme and by 44% of participants who received usual care. There were no statistically significant differences between the randomisation groups for ARAT ‘success’ at 6 months.
Although there was no statistically significant difference between the EULT programme and usual care in upper limb functional recovery ‘success’, the absolute difference was 8% at 3 months and 10% at 6 months, in favour of the EULT programme. These differences may be considered important by some patients and clinicians, but the trial was designed to detect a 15% difference between groups.
Of the many pre-planned comparisons of secondary outcomes, some indicated differences that might be clinically important, as the MCID was within the 98.33% CI. Robot-assisted training improved upper limb impairment, as measured by the FMA motor subscale at 3 months, when compared with usual care; this difference was maintained at 6 months. However, this improvement did not translate into improvement in upper limb function, ADL or quality of life. Indeed, robot-assisted training participants performed less well in ADL (Barthel ADL Index and SIS ADL subscale) at 3 months than those who received the EULT programme.
Enhanced upper limb therapy led to clinically important and statistically significant improvements in upper limb impairment, when compared with usual care at 3 months. EULT also led to clinically important and statistically significant improvements in mobility and ADL, as measured by the SIS, when compared with usual care at this time point. Statistically significant differences were found in favour of EULT in ADL (Barthel ADL Index) and hand function, but these were not felt to be clinically important. No clinically important differences were found between EULT and usual care, or robot-assisted training and EULT, at 6 months on any outcome measure.
There was no difference in the proportion of participants reporting upper limb pain between randomisation groups.
Subgroup analyses
No significant differences were found between the randomisation groups for upper limb function measured by the mean ARAT score at 3 months for any of the prespecified exploratory analyses: time since stroke, baseline ARAT score, trial centre and age. However, CIs were wide because of the smaller sample size of the subgroups. No significant differences in upper limb impairment or ADL were found between randomisation groups according to time since stroke.
Safety
More participants in the robot-assisted training group and the EULT group (15% and 13%, respectively) had SAEs than those who received usual care (8%). None was attributable to an intervention and the differences are probably because of a reporting bias, as participants who received an intervention had regular contact with RATULS trial teams, and were therefore more likely to make them aware of these events.
Health economic evaluation
The cost per participant over the 6-month trial duration was £5387 for those randomised to robot-assisted training, £4451 for those randomised to the EULT programme and £3785 for those randomised to usual care. Neither robot-assisted training nor EULT, when delivered with a 1 : 1 patient-to-therapist ratio, would be considered cost-effective at the UK current level of WTP for a QALY (£20,000–30,000).
Robot Assisted Training for the Upper Limb after Stroke trial results in the context of other studies
Robot-assisted training
The results of a 2017 systematic review93 of the effects of robot-assisted therapy for the upper limb after stroke (38 trials, 1206 participants) are consistent with the results of the RATULS trial. This review concluded that ‘effects on motor control are small and specific to the joints targeted by robot-assisted training, whereas no generalisation is found to improvements in upper limb capacity’. 93
However, the 2018 Cochrane systematic review94 of electromechanical and robot-assisted arm training (45 trials, 1619 participants) reported significantly improved ADL scores (SMD 0.31, 95% CI 0.09 to 0.50) and arm function (SMD 0.32, 95% CI 0.18 to 0.46) at the end of the intervention period. Although this review94 reported improvement in arm function, the widely used FMA motor subscale, a measure of upper limb impairment, was classified as an arm function measure in the meta-analysis. Other measures of arm function were the Chedoke–McMaster Stroke Assessment and the Wolf Motor Function Test. In addition, the clinical importance of these differences is unclear as statistically significant results are not discussed in relation to MCIDs. The review authors recommend that the results should be treated with caution because of study heterogeneity and variation in the quality of the included studies.
Another systematic review95 (10 trials, 362 participants) found no difference in arm impairment or arm function when robot-assisted training was compared with the same intensity and duration of conventional therapy.
The VA robotics trial17 is the largest trial (n = 127) included in the Cochrane review, and the RATULS trial used the same intensity and duration of training. Participants were > 6 months post stroke and had a FMA total upper-extremity score of between 7 and 38 at baseline. In the VA robotics trial,17 robot-assisted training improved upper limb impairment (FMA motor subscale24), compared with usual care, and the impairment advantage did translate into significant upper limb functional improvements (Wolf Motor Function Test96) and benefits in the SIS. 42
The VA robotics trial17 reported that the average total cost of treatment over a 12-week intervention period (the same duration and intensity of the RATULS trial) was US$5152 for robot-assisted training, and US$7382 for intensive comparison therapy (p = 0.001). 18 In the VA robotics trial,17 the base-case analysis assumed that the MIT-Manus robotic gym was used by two patients simultaneously. At 36 weeks, the total health-care costs were comparable for all three randomisation groups.
The REM_AVC trial97 was reported subsequent to the 2018 Cochrane review. 94 This trial randomised 218 participants to receive robot-assisted training with an Armeo Spring device or self-rehabilitation of the same frequency and duration, and found no difference between randomisation groups on the FMA. 97
Enhanced upper limb therapy programme
The EULT programme, based on goal-orientated repetitive functional task practice, resulted in some potentially clinically important benefits over usual care and improvements in upper limb impairments, which translated into improvements in ADL. These results support some of the conclusions of the 2016 Cochrane review11 (33 trials, 1853 participants) that found that repetitive functional task practice improved arm function (SMD 0.25, 95% CI 0.01 to 0.49), ADL (SMD 0.28, 95% CI 0.10 to 0.45) and hand function (SMD 0.25, 95% CI 0.00 to 0.51). The measures of arm function were Motor Assessment Scale – upper limb component; ARAT; Frenchay Arm Test; Wolf Motor Function Test; Functional Test of the Hemiparetic Upper Extremity; Box and Block Test; and Southern Motor Group Assessment. This review did not include the GRASP trial (103 participants), which found significant improvements in upper limb function, grip strength and upper limb use in daily activities in favour of the intervention. 98 The GRASP was a 4-week, largely self-administered, repetitive upper limb supplementary programme; participants were randomised at a mean of 21 days post stroke and had a mean FMA score of 40. Participants in the RATULS trial had considerably more severe upper limb impairment than those in the GRASP trial; therefore, the findings cannot be compared.
Mechanisms of action of robot-assisted training and enhanced upper limb therapy
Theories of neuroplasticity and motor learning99,100 support an approach to rehabilitation based on repetitive practice of tasks. 101,102 Both interventions were, therefore, underpinned by established theories of motor learning, including the stages of learning103 and motor programme theory,100,104 to structure the learning processes. Evidence from translational studies105,106 further supports the use of progressive, repetitive training protocols. Further information can be found in the RATULS interventions TIDieR table (see Appendix 1, Table 27).
It is important to consider why the improvements in impairment seen with robot-assisted training in the RATULS trial did not translate into improved function. The RATULS trial provided integrated training with all three components of the MIT-Manus robotic gym (shoulder–elbow robot, wrist robot and hand attachment), which adapt to participants’ abilities (providing more/less assistance as required). The robot-assisted training programme did not include grip or pinch activities. Participants trained specific movements of the affected arm in a spatially controlled manner, but it is possible that these did not resemble movements in everyday activities and, therefore, improvements in motor control (as shown on the FMA) did not transfer into ADL. Furthermore, there may have been a lack of guidance for participants about making the best use of any improvement in impairment in day-to-day activities. These suggestions are supported by data from the qualitative study, in which some interviewees commented that robot-assisted training focused on achieving gross arm movements rather than delicate tasks.
A trial107 published in 2019 (45 participants) randomised participants to receive robot-assisted training using the MIT-Manus robotic gym or robot-assisted training plus therapist-assisted transition-to-task training. Both interventions were provided for 1 hour, three times per week, for 12 weeks. There was no difference in the primary outcome: FMA score at 12 weeks. However, participants who received robot-assisted training plus therapist-assisted transition-to-task training showed a statistically significant improvement in motor performance (as measured by the Wolf Motor Function Test96) and hand function (as measured by the SIS42) at 12 weeks, compared with those who received robot-assisted training alone.
Many daily activities involve both upper limbs, including the hands, in bilateral (e.g. opening a drawer) or bimanual (e.g. making a sandwich) co-ordinated action. Furthermore, daily activities (e.g. buttoning a shirt) often involve a range of objects that require different types of grasp and/or manipulation. Such activities involve diverse and complex patterns of behaviour involving sensory, perceptual, cognitive and motor functions. This could explain why robot-assisted training resulted in a less favourable outcome in self-reported ADL than the EULT programme, in which training specifically focused on complex daily activities and functional tasks. The improvement in mobility seen following EULT would also support this theory, as the EULT programme included tasks involving the upper limbs in balance and sit-to-stand activities, which were not components of robot-assisted training. The EULT programme may also have addressed learned non-use of the affected arm by encouraging participants to use their arm in day-to-day activities; one participant who was interviewed in the qualitative study stated ‘I had completely forgotten what this hand did’.
The results of the RATULS trial provide a further example of the specificity of learning. 100 This is also supported by the results of the NIHR HTA Clinical and cost-effectiveness of aphasia computer treatment versus usual stimulation or attention control long term post-stroke (Big CACTUS) RCT,108 which found that, although patients with dysphasia due to stroke who undertook a self-managed programme of computer exercises showed improved word-finding abilities, this did not translate into improvement in general conversation ability.
The Robot-Assisted Training for the Upper Limb after Stroke trial interventions
Development and content of robot-assisted training and the enhanced upper limb therapy programme
We have reported the development, content and delivery of robot-assisted training and the EULT programme in accordance with international recommendations, enabling them to be replicated in further research and/or clinical practice. 34,109,110
There was robust development of the robot-assisted training and EULT programmes. Experts in both robot-assisted training and EULT were involved in designing and delivering the training programmes. Robot-assisted training was provided at the same frequency and duration as intended in the VA Robotics trial,17 which also evaluated MIT-Manus robotic gym. The intensive comparative therapy in the VA Robotics trial17 sought to replicate the form and intensity of upper limb movements provided by the MIT-Manus. A strength of the RATULS trial is that robot-assisted training was compared with an upper limb therapy programme, based on current evidence and best practice, and provided at the same frequency and duration.
The robot-assisted training programme was prescriptive in that every session had a defined number and types of exercises a participant should complete. Although the MIT-Manus was able to assist as needed, it employed a single approach independent of the phase of recovery or impairment level, that is the programme was not tailored to the individual. In contrast, there was scope for tailoring in the EULT programme, as it was based on individual goals selected by the participant and their therapist. The therapists were also able to tailor the specifics of the activity practised [including the nature and purpose of the task, any object(s) involved, specific joint movement(s), type and scheduling of practice, and environmental conditions] to the ability of the participant. In clinical practice, there would probably be further tailoring of the interventions.
Delivery of robot-assisted training and the enhanced upper limb therapy programme
Robot-assisted training was provided at a central hub, as this is the model that would probably be used in clinical practice. The EULT programme was provided as a centralised rather than a local service for logistical reasons.
Training programmes and manuals were provided to ensure that the interventions could be delivered as per protocol. There was regular contact between those who provided the intervention and the RATULS trial co-ordinating centre team to monitor progress and to address any queries. The qualitative process evaluation found that therapists who provided robot-assisted training and the EULT programme felt well supported throughout the trial and were able to provide the 12-week programmes as intended.
We have demonstrated that it is possible to report details about the interventions received by participants within a large multicentre randomised controlled rehabilitation trial. Intervention delivery was monitored throughout the trial, which contributed to high levels of fidelity.
Although the amount of therapy time is frequently reported in RCTs, information about how this time is spent is poorly reported. 50,111 Robot-assisted training participants undertook a median of 808 movement attempts per session attended, which demonstrates the intensity of treatment that this therapy can achieve. EULT participants achieved a median of 127 repetitions per session attended. Movement attempts and repetitions are different units of measurement and they are not comparable; therefore, we are not able to make direct comparisons about the intensity of repetitive practice between robot-assisted training and the EULT programme using these measures.
Goal-setting is currently considered to be integral to best practice in stroke rehabilitation and is recommended in national clinical guidelines;39 however, the evidence that this approach improves clinical outcomes is limited and there appears to be no consensus about the optimum approach. 112,113 It was decided not to use a formal goal-setting method [e.g. the COPM63 or the Goal-setting and Action Planning (G-AP) framework114] in the RATULS trial, as it is not widely used in most UK centres. 115
In the EULT programme, the three most commonly chosen goals were eating, improving range of movement and dressing. The selection of range of movement as a goal is likely to reflect the level of severe arm impairment in the trial population, and the difficulty of setting functional goals for these participants. Although goal achievement varied, the overall proportion was low (51%). However, participants who were < 3 months after stroke achieved the majority of their goals.
Goal selection in the EULT programme may have been overoptimistic, particularly for those with severe arm activity limitation, which has a poor prognosis, and for those who were ≥ 3 months after stroke, when spontaneous recovery tends to slow down. 52 A key component of therapy is managing expectations. Some patients with severe impairment and little chance of upper limb recovery may wish to select aspirational rather than achievable goals, which may motivate, but may also lead to unrealistic expectations and difficulty adjusting to the residual consequences of stroke.
Usual care
The RATULS trial was a pragmatic trial; therefore, usual care was chosen as a comparator group. Obtaining accurate information about the usual care that patients receive is a challenge to this type of study, and the assumption that usual care is uniformly provided is rarely true. 116 Although there are UK guidelines about the amount of therapy that a stroke patient should receive, they refer to contact time with a specific type of therapist (e.g. physiotherapist) rather than specifying the focus (e.g. upper limb) or intensity of the intervention itself. 117
Usual care records are not standardised and, often, basic information about content and dose is not recorded. Collecting data about the upper limb therapy provided by a large number of individuals from a range of services and organisations (stroke units, early supported discharge teams, community rehabilitation teams, outpatient therapists from hub and spoke sites), many of whom were not directly involved in the RATULS trial, was not feasible. 2 In addition, some patients arranged private rehabilitation. Therefore, participants were asked to record the usual care they received and to seek help if needed from their therapist(s) to complete their log books. However, this approach has limitations, including data completeness. Although the number of participants who returned a therapy log was low (66% at 3 months), there was little variation between randomisation groups for the number of log books returned or the completeness of the logs returned, for example of those returned, > 80% had all 12 weeks completed.
The usual care logs in the RATULS trial indicated that the most common type of therapy undertaken was passive stretching, whereas the least common was practising extended ADL. This finding resonates to some extent with findings from a recent survey of upper limb treatment reported by physiotherapists and occupational therapists across the UK. 118 This survey reported that, although there was considerable variation in treatments provided for stroke patients with severe upper limb deficits, the most common were range of movement exercises, mirror-box therapy and functional electrical stimulation. For those with less severe upper limb deficit, most interventions comprised active, task-specific practice. In terms of dose, upper limb therapy was reportedly provided for an average of 29 minutes, three times per week. 118 However, this amount exceeds that of observational studies of upper limb treatment. 76,111,119
Recording, measuring and monitoring the treatment received by individuals, and reporting of the rehabilitation services provided, should be integral to routine clinical practice and should not be seen as an added burden. 92 If this was achieved, these data could be available to research teams (with appropriate information governance), and would greatly facilitate the reporting of usual care in rehabilitation trials.
Disappointment about group allocation may have resulted in some usual care participants seeking or being provided with additional therapy, or increasing the amount of self-practice exercises undertaken, thereby introducing a competitive therapy bias. We did consider offering either robot-assisted training or EULT to usual care participants following the 6-month outcome assessment, but this was not feasible.
Methodological considerations
To our knowledge, the RATULS trial is the largest trial of upper limb robot-assisted rehabilitation undertaken to date. The RATULS trial is the first multicentre trial with adequate statistical power to compare robot-assisted training with both an evidence-based therapy programme of the same frequency and duration and with usual care.
Trial setting
The model of a central hub stroke service, where the MIT-Manus gym was based, supported by surrounding hospital and community services, enabled the RATULS trial to recruit to target. A recruitment rate of four participants per trial centre per month was achievable. Trial participants were willing to travel to a central hub to take part in the RATULS trial. Transport by taxi to and from therapy sessions and outcome assessments was provided if required, and this may have contributed to the high level of attendance. The qualitative process evaluation supports this view.
Participants
We recruited participants from both the incident and prevalent populations of stroke patients. We initially asked clinicians at hub and spoke sites to complete a screening log, but, as the trial progressed, we abandoned the screening log as this became a disincentive for clinical teams to refer potential participants. Stroke services, therapy services and self-referral were the main routes of referral.
Acute, subacute and chronic stroke patients were included, as there was no evidence from the literature on repetitive task training that suggested excluding patients at any stage of recovery. 10,120 Hebbian and non-Hebbian learning processes foster experience-dependent plasticity, and this process may continue for several years post stroke. 10,121,122 In addition, the subgroup analyses of time since stroke in the Cochrane systematic reviews11,94 of robot-assisted training and repetitive task training have found no evidence of an influence of time post stroke on functional outcomes, although the low quality of the evidence should be acknowledged. We felt that the interventions were likely to be offered to patients at all stages of recovery from stroke in clinical practice, as the current Royal College of Physicians’ National Clinical Guideline for Stroke117 suggest that patients should receive therapy for as long as they are continuing to benefit from it. Subgroup analyses of the RATULS trial showed no clear effect of time on the relative effectiveness of the interventions, thereby supporting previously published evidence and guidelines. 10,11,94,117,120–122
The RATULS trial participants were younger (60 years) than the average stroke population in the UK (75 years), and the proportion of males was higher (61% vs. 50%). 40 Participants who are recruited to stroke trials are usually younger and more likely to be male than the general stroke population. 123
It is likely that the pragmatic inclusion criteria led to some participants who had little prospect of recovery being recruited: 68% had severe upper limb functional limitation (ARAT score 0–7), which has a poor prognosis for recovery. 8 At the time the trial was designed, literature had been published about predictors (including biomarkers) of motor recovery after stroke. 124 Methods for testing the integrity of the corticospinal tract, thought to be a predictor for upper limb recovery after stroke, involve use of transcranial magnetic stimulation-induced motor-evoked potentials or magnetic resonance imaging. As this was a pragmatic trial and these methods are not used in routine clinical practice in the UK, biomarkers for testing corticomotor structure and function were not used in this trial. Currently, these biomarkers are not used in NHS clinical practice.
Sample size
In determining the sample size of the RATULS trial, we estimated that 30% participants who were randomised to receive usual care would achieve the primary outcome of upper limb functional recovery ‘success’ at 3 months. This figure was derived from data from the NIHR HTA BoTULS trial, which evaluated the role of treating upper limb spasticity due to stroke with botulinum toxin type A. 35,36 The proportion of usual care participants who achieved upper limb functional recovery ‘success’ in the RATULS trial at 3 months was higher than expected, at 42%. The reason why usual care participants improved more than anticipated is unclear. Most spontaneous upper limb recovery from stroke occurs within the first 6 months and there is little measurable recovery beyond 12 months. Part of the explanation may be that a lower proportion of RATULS trial participants (36%) were recruited beyond 1 year after stroke than in the BoTULS trial (45%). Differences in baseline ARAT scores between trials are unlikely to explain the increased upper limb functional recovery ‘success’ rate: in the RATULS trial, the median baseline ARAT score was 3 points (IQR 0–11 points), and in the BoTULS trial, it was 3 points (IQR 3–16 points).
Dose of therapy
It has been suggested that the dose of face-to-face therapy provided to robot-assisted training and EULT participants was too low, and this may explain why we did not demonstrate any significant benefit on the primary outcome when compared with usual care. 125,126 The robot-assisted training dose in this trial was based on the VA study. 17 It was felt that the VA study results were promising in terms of efficacy and efficiency and that the experiences of that trial should be built on. Therefore, the same number of sessions (36 sessions of 45 minutes of face-to-face therapy) were provided. At the time of designing the trial, there was no justification for increasing the amount of additional therapy and an increase would have surpassed the limit possible to provide in the NHS. The EULT intervention was dose-matched to the robot-assisted training intervention (in terms of total therapy time, session duration and frequency).
In relation to the optimum therapy dose, there is a lack of clarity in the literature about how dose is measured. 127 Dose is a multifactorial concept that includes the frequency, intensity, duration and timing of an intervention. Often, studies of dose in rehabilitation trials focus only on therapy time. However, in the RATULS trial, we have been able to report the number of sessions attended, session duration, duration of face-to-face therapy and the number of repetitions of tasks undertaken.
There is ongoing debate about the optimum amount of additional therapy time needed to improve arm function after stroke. A 2014 meta-analysis10 found strong evidence that an additional 17 hours of physiotherapy significantly improved a range of outcomes, including arm function, basic ADL and quality of life after stroke. We are not aware of dose-finding studies for therapy time of robot-assisted training. A 2016 Cochrane systematic review11 reported that repetitive task training significantly improved arm and hand function, but there were no significant differences in outcomes between a dose of at least 20 hours and smaller doses. A 2016 meta-analysis128 found a trend towards a positive relationship between therapy dose and activity, with findings suggesting that a 240% increase in usual care duration was required for a significant likelihood that activity limitations would improve. However, this meta-analysis did not report findings for arm rehabilitation in isolation. A number of more recent RCTs of upper limb therapy post stroke have also been neutral for their primary outcome. 129–134 These trials planned to deliver between 10 and 45 hours of additional upper limb therapy, with four trials aiming to deliver an additional ≥ 30 hours. 129,130,132,134
Three studies135–137 have reported benefits of high amounts of therapy time in upper limb therapy for chronic stroke patients. However, none of these studies had a lower dose or usual care comparator. An arm rehabilitation programme comprising 90 hours of repetitive task practice, in addition to other modalities, found clinically and statistically significant improvements in upper limb impairment and activity limitation. 137 Two small studies135,136 provided upper limb repetitive practice for 300 hours to patients with severe arm impairment, and reported benefits with this intensity of treatment. Although these results are interesting, these interventions require further evaluation in robust, adequately powered RCTs to demonstrate their feasibility, clinical effectiveness and cost-effectiveness.
To date, results from studies that aimed to determine the optimum dose in terms of repetitions have been inconsistent and inconclusive. A Phase II single-blind, randomised, repetition dose–response study evaluated a task-specific upper limb rehabilitation intervention comprising up to 32 hours of practice for stroke survivors at least 6 months after stroke with mild to moderate arm impairment. 138 Eighty-five participants were randomised to undertake either 3200, 6400, 9600 or individualised maximum repetitions. All groups improved, but there was no evidence of a repetition dose–response relationship.
Outcomes
The outcome measures used in the RATULS trial are widely used in stroke rehabilitation trials and have been shown to be valid, reliable and sensitive to change,139 although the ARAT has several psychometric properties (e.g. floor and ceiling effects) that would benefit from further investigation. 140 However, the primary outcome, derived from the ARAT score, was developed specifically for the RATULS trial and further validation is needed. A stepped approach was used because, although the MCID for the ARAT is 10% of its range (6 points41), a smaller treatment effect may be clinically beneficial in those with severe initial upper limb functional limitation, who are likely to improve less than those with more moderate limitation. The Lancet editorial about the main results of the RATULS trial supports the choice of primary outcome measure: ‘Although not used previously, this approach aimed to more sensitively capture meaningful change relative to the patient’s starting point, which makes sense but needs further validation’. 125
In 2017, the Stroke Recovery and Research Roundtable139 recommended outcome measures to be included in stroke recovery trials for each domain of the World Health Organization’s (WHO’s) International Classification of Functioning Disability and Health (ICF). 141 These included the ARAT to measure upper limb activity limitation,19 the FMA to measure body function and structure,24 and the EuroQol-5 Dimensions (EQ-5D) to measure quality of life. 44 All of these measures were included in the RATULS trial. The consensus recommendation also included using the modified Rankin Scale142 to measure global disability, which was not used in the RATULS trial. No consensus was reached about measures of participation. Another expert panel has recommended outcome measures, including the ARAT,19 FMA24 and SIS,42 to be used in motor function intervention trials. 143
We have reported and interpreted our finding against published MCIDs, regardless of the robustness of the evidence underpinning these values for stroke patients. 59 Lack of methodological robustness in studies to determine MCIDs and lack of patient and carer involvement in determining meaningful differences can lead to methodological or interpretation problems with clinical trials. 143–145 We recommend that a consensus about reporting research to identify MCIDs is developed, which could then be incorporated into Enhancing the QUAlity and Transparency Of health Research (EQUATOR) guidance. 146
Strengths, weaknesses and sources of bias
The RATULS trial findings are robust with a low risk of bias. The RATULS trial was undertaken and reported in accordance with current best practice. 146 The trial was conducted in line with a published protocol, and the statistical analysis plan and health economics analysis plan were finalised prior to data lock. All outcomes were analysed and reported according to these prespecified plans. One of the strengths of the trial is the completeness and high quality of the data (with the exception of the usual care therapy logs).
We have used the terms of impairment, upper limb function and ADL throughout this report because these terms were used in the original NIHR commissioning brief and the RATULS trial protocol, rather than WHO’s ICF. 141
Randomisation was by an independent web-based service with allocation concealment. It was not possible to mask trial participants to their randomisation group allocation and therapists who delivered robot-assisted training and the EULT programme were not masked to the intervention group. The same therapists and therapy assistants delivered both interventions at each trial centre. Outcome assessments were intended to be collected by a researcher who was masked to treatment allocation. However, outcome assessors reported that they were unmasked for 15% of outcome assessments at 3 months and 14% of outcome assessments at 6 months.
Overall attrition rates were acceptable, but the attrition rate was higher in the usual care group (19%) than in the robot-assisted training and EULT groups (both 9%), and differential attrition is a potential source of bias. Most of the withdrawals before 3 months in the usual care group were due to disappointment with treatment allocation.
The strengths and weaknesses of the process evaluation and economic evaluation have been discussed in Chapters 7 and 8, respectively.
Patient and public involvement
Our involvement with patient groups, clinical experience and research has consistently shown that patients feel that stroke rehabilitation focuses on mobility rather than recovery of upper limb function. Improving upper limb function has been identified as the fourth most important rehabilitation research priority by stroke survivors, carers and clinicians. 9
The Guidance for Reporting Involvement of Patients and Public 2147 was published in 2017, towards the end of the RATULS trial recruitment period; therefore, we were unable to be fully compliant. We have not systematically recorded how patient and carer involvement enhanced the research process throughout the trial.
Stroke survivors were involved in developing the outline and full NIHR proposal. At the grant application stage, the trial was presented to the NIHR Stroke Research Network (SRN) Lay Member Panel and comments from the panel were incorporated into the application. Input was sought from the North East SRN Patient and Carer Panel for the design and content of trial documents, including the trial information sheets and consent forms, and to help to develop resources to train staff who undertook trial assessments. Stroke survivors contributed to the development of the intervention programmes used in the RATULS trial and ran through the programme with researchers. The programmes were adjusted following their feedback.
One of the co-applicants is a stroke survivor and contributed to design, delivery and reporting of this work. One of the members of the Trial Steering Committee had had a stroke and gave a patient perspective about the trial.
Trial participants were sent newsletters throughout the trial and were sent a letter that included a lay summary of the results of the trial, following publication of the main results in The Lancet. 1 The results of the trial have been presented and discussed with the NIHR Clinical Research Network: Stroke North East Patient and Carer Panel (formerly the SRN Panel).
Chapter 10 Conclusion
Parts of this chapter have been reproduced from Rodgers et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The RATULS trial did not find evidence that a robot-assisted training programme using the MIT-Manus robotic gym, as implemented in this trial, improved upper limb function following a stroke when compared with an EULT programme based on goal-orientated repetitive functional task practice of the same frequency and duration, or with usual care.
Some differences in secondary outcome measures suggest potential benefits for robot-assisted training and the EULT programme that may have implications for clinical practice and future research: robot-assisted training led to improvement in upper limb impairment, compared with usual care; the EULT programme led to improvements in upper limb impairment, mobility and ADL, compared with usual care. Both interventions were acceptable to participants and therapists. Neither robot-assisted training nor the EULT programme as provided in the RATULS trial (1 : 1 patient-to-therapist ratio) were cost-effective at the current UK WTP per QALY (£20,000–30,000).
Implications for health and social care
The results of the RATULS trial do not support the routine use of robot-assisted training using the MIT-Manus robotic gym, as implemented in this trial, for patients with moderate or severe upper limb functional limitation due to stroke.
The RATULS trial provides some evidence of the potential benefits of the EULT programme, although, as delivered in this trial, it is unlikely to be cost-effective. Delivering EULT as group/classroom therapy would lead to a reduction in costs, which has the potential to make EULT a cost-effective intervention. However, the impact of this change on clinical effectiveness and QALYs is unknown and should be explored.
Recommendations for further research
The RATULS trial has demonstrated that a large multicentre trial to evaluate robot-assisted rehabilitation, which includes a clear description of the interventions received and high levels of intervention fidelity, is achievable. An editorial in The Lancet entitled ‘Robot-assisted training after stroke: RATULS advances science’125 was supportive of the trial, and we suggest that future rehabilitation trials could build on our experience.
Future trials may wish to stratify patients into groups with differing probabilities of upper limb recovery using techniques such as shoulder abduction and finger extension52 and/or advanced neuroimaging and transcranial magnetic stimulation to improve the targeting of therapies towards participants with the potential to respond. 148 Ongoing biomarker research may help in patient selection or treatment monitoring of future stroke rehabilitation trials. 110 Future trials may also wish to consider having a patient-reported outcome measure and one of the standard activity scales as co-primary outcome measures. 75 Future trials should be aligned with recommendations made by the Stroke Recovery and Rehabilitation Roundtable. 139,149
Recording, measuring and monitoring the treatment received are important components of research, clinical practice and audit. By following checklists and guidance to describe research interventions, the content of rehabilitation interventions evaluated in clinical trials could be accurately reported. 34,110,150 Unfortunately, collecting standardised minimum data about the usual care received remains a challenge in many settings, especially when it is provided by many services in a single study centre. 92 There needs to be a culture change in the recording and reporting of usual care in clinical practice to enable key data to be used to inform clinical practice and research.
The dose of therapy is an important component of any stroke rehabilitation trial. Therapy dose is a multifactorial concept. 127 Dosage can include the number of sessions attended, session frequency and duration, duration of face-to-face therapy, and the number of repetitions of tasks undertaken. Equally important is reporting of the type of therapeutic activities undertaken, and the way these are progressed. There is converging evidence that more therapy may result in better outcomes,50 but, in future, adequately powered dose-finding studies of promising interventions, tailored to targeted subgroups, are needed, that also take into account potential cost-effectiveness.
Despite the neutral primary outcome of the RATULS trial, robot-assisted training in upper limb rehabilitation after stroke still has potential. Further research is needed to determine how improvement in upper limb impairment seen with the MIT-Manus gym can be translated into meaningful improvement in upper limb function and ADL. For example, trials could be designed whereby robot-assisted training could be utilised at the start of an intervention session as a ‘primer’,151 with the aim to focus attention on the affected arm, assist movement and generate sensory, proprioceptive and visual feedback. The EULT intervention could then be utilised (in the same session or after a number of robotic sessions) to enable translation of improved motor control into meaningful, daily activities. 107,152
Robotics is a rapidly advancing field and a number of other innovative devices are available, some of which may be suitable for home use. In some parts of the world, the clinical use of robots has preceded evidence of effectiveness. It is important that there is partnership between developments in bioengineering, neuroscience and clinical evaluation to optimise improvement in patient outcomes and care.
Acknowledgements
We would like to thank the following for their contribution to this research project:
-
participants in this trial
-
staff at the RATULS trial NHS trial centres –
-
Glasgow: Jen Alexander, Elizabeth Colquhoun, Ozlem Dincarslan, Wendy Jackson, Pamela MacKenzie, Karen Shields and staff at adjacent sites
-
North Tyneside: Rebecca Davidson, Judith Murdy, Victoria Riddell, Anna Smith, Leanne Smith, Gail Storey, Marie Twentyman, Helen Walker, Rebecca Watson and staff at adjacent sites
-
Northwick Park: Robert Ballantine, Daniel Brooks, Aberami Chandrakumar, Angela Chamberlain, Anette David, Mushiya Mpelembue and staff at adjacent sites
-
Romford: Evelyne Burssens, Karen Dunne, Janice Hastings, Louise Hinkins, Sam King and staff at adjacent sites
-
-
staff who left the trial centre teams prior to the end of the trial
-
staff at Newcastle University who assisted with the delivery of the trial: Michael Adams, Clare Bowes and Deborah Jones
-
Leanne Smith for her analysis of the EULT goal data
-
Alastair Dodsworth for his work to develop the ARAT training programme
-
Jenni Hislop for her input into the health economics analysis plan
-
Data Monitoring and Ethics Committee members: John Bamford (chairperson), Richard McManus, Rebecca Palmer, Philip Rowe and Christopher Weir
-
Trial Steering Committee members: Jennifer Burr (chairperson), Jane Burridge, Nikola Sprigg, Sarah Tyson and Judith Williamson
-
staff at Biosense Medical Ltd (Chelmsford, UK) for their technical support
-
Brenda Hyland-Miller for staff training and support
-
the NIHR SRN and NIHR Clinical Research Network: Stroke for their support and encouragement
-
Jim Mackey for his support throughout.
Contributions of authors
Professor Helen Rodgers (https://orcid.org/0000-0003-3433-4175) (Professor of Stroke Care) was the chief investigator and lead grant holder. She was involved in the study design and delivery, interpretation of data and drafting the report.
Dr Helen Bosomworth (https://orcid.org/0000-0002-4670-8827) (Research Associate) was a researcher working on the project who managed the project. She was involved in the study design and delivery, interpretation of data, analysis of intervention content data and drafting the report.
Dr Hermano I Krebs (https://orcid.org/0000-0002-9317-0900) (Principal Research Scientist and Lecturer) was a co-investigator, involved in the study design and delivery, design of the robot-assisted training programme, interpretation of data and drafting the report.
Professor Frederike van Wijck (https://orcid.org/0000-0003-0855-799X) (Professor in Neurological Rehabilitation) was a co-investigator, involved in the study design and delivery, design of the EULT programme, interpretation of data and drafting the report.
Ms Denise Howel (https://orcid.org/0000-0002-0033-548X) (Senior Lecturer) was a co-investigator and the senior statistician. She was involved in the study design and delivery, interpretation of data and drafting the report, and supervised the main patient and carer analyses.
Dr Nina Wilson (https://orcid.org/0000-0001-5908-1720) [Research Associate (statistician)] was the study statistician who conducted the main data analyses and contributed to the drafting of Chapter 3.
Professor Tracy Finch (https://orcid.org/0000-0001-8647-735X) (Professor of Healthcare & Implementation Science) was a co-investigator and lead for the parallel process evaluation. She was involved in the study design, delivery, interpretation of data and drafting the report, and supervised the process evaluation.
Dr Natasha Alvarado (https://orcid.org/0000-0001-9422-4483) (Research Fellow) was a researcher working on the project who undertook the parallel process evaluation data collection and analyses, and drafted Chapter 7.
Dr Laura Ternent (https://orcid.org/0000-0001-7056-298X) (Senior Lecturer in Health Economics) was a co-investigator and senior study health economist. She was involved in the study design and delivery, interpretation of data and drafting the report, and supervised the health economic analyses.
Dr Cristina Fernandez-Garcia (https://orcid.org/0000-0002-7113-225X) (Health Economist and Bioethicist) was the health economist who conducted the main data analyses, and contributed to the drafting of Chapter 8.
Mrs Lydia Aird (https://orcid.org/0000-0002-0578-9309) (Specialist Physiotherapist in Stroke) was a co-investigator and was involved in the study design and delivery, interpretation of data and drafting the report.
Dr Sreeman Andole (https://orcid.org/0000-0001-8356-9320) (Clinical and Research Lead in Stroke) was a co-investigator and was involved in the study design and delivery.
Dr David L Cohen (https://orcid.org/0000-0001-7318-8567) (Consultant Physician) was a co-investigator and was involved in the study delivery, interpretation of data and drafting the report.
Professor Jesse Dawson (https://orcid.org/0000-0001-7532-2475) (Professor of Stroke Medicine and Consultant Physician) was a co-investigator and was involved in the study design and delivery, interpretation of data and drafting the report.
Professor Gary A Ford (https://orcid.org/0000-0001-8719-4968) (Professor of Clinical Pharmacology) was a co-investigator and was involved in the study design and delivery, interpretation of data and drafting the report.
Dr Richard Francis (https://orcid.org/0000-0002-6568-1295) (Research Associate) was a researcher working on the project who contributed to data management and analyses during the study delivery period.
Mr Steven Hogg (Stroke Survivor) was a co-investigator and is a service user; he was involved in the study design and delivery, and interpretation of data.
Dr Niall Hughes (https://orcid.org/0000-0001-5555-6442) (Consultant Physician) was a co-investigator and involved in the study design and delivery, interpretation of data and drafting the report.
Dr Christopher I Price (https://orcid.org/0000-0003-3566-3157) (Reader) was a co-investigator and involved in the study design and delivery, interpretation of data and drafting the report.
Professor Duncan L Turner (https://orcid.org/0000-0001-8916-4025) (Professor of Neurorehabilitation Sciences) was a co-investigator and was involved in the study design and delivery, interpretation of data and drafting the report.
Professor Luke Vale (https://orcid.org/0000-0001-8574-8429) (Health Foundation Chair in Health Economics) was a co-investigator and senior study health economist. He was involved in the study design, delivery, interpretation of data and drafting the report, and supervised the health economic analyses.
Professor Scott Wilkes (https://orcid.org/0000-0003-2949-7711) (Professor of General Practice and Primary Care) was a co-investigator and involved in the study design, delivery, interpretation of data and drafting the report.
Dr Lisa Shaw (https://orcid.org/0000-0002-3435-9519) (Principal Research Associate) was a co-investigator and managed the project. She was involved in the study design and delivery, interpretation of data and led the drafting of the report.
Publications
Rodgers H, Shaw L, Bosomworth H, Aird L, Alvarado N, Andole S, et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): study protocol for a randomised controlled trial. Trials 2017;18(1):340.
Rodgers H, Bosomworth H, Krebs HI, Van Wijck F, Howel D, Wilson N, et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394:51–62.
Rodgers H, Bosomworth H, Van Wijck F, Krebs HI, Shaw L. Usual care: the big but unmanaged problem of rehabilitation evidence – authors’ reply. Lancet 2020;395:337–8.
Bosomworth H, Rodgers H, Shaw L, Smith L, Aird L, Howel D, et al. Evaluation of the enhanced upper limb therapy programme within the Robot-Assisted Training for the Upper Limb after Stroke (RATULS) trial: descriptive analysis of intervention fidelity, goal selection and goal achievement. Clin Rehabil 2020; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review. A data-sharing agreement will need to be signed by data requestors.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Rodgers H, Bosomworth H, Krebs HI, Van Wijck F, Howel D, Wilson N, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394:51-62. https://doi.org/10.1016/S0140-6736(19)31055-4.
- Rodgers H, Shaw L, Bosomworth H, Aird L, Alvarado N, Andole S, et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2083-4.
- National Institute for Health Research (NIHR) . RATULS: Robot Assisted Training for the Upper Limb After Stroke n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/112605/#/ (accessed April 2011).
- Adamson J, Beswick A, Ebrahim C. Is stroke the most common cause of disability?. J Stroke Cerebrovasc Dis 2004;13:171-7. https://doi.org/10.1016/j.jstrokecerebrovasdis.2004.06.003.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398-403. https://doi.org/10.1161/STROKEAHA.110.598839.
- Stroke Association . State of the Nation 2018. www.stroke.org.uk/sites/default/files/state_of_the_nation_2018.pdf (accessed 5 September 2019).
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009;8:741-54. https://doi.org/10.1016/S1474-4422(09)70150-4.
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probablity of regaining dexterity in the flaccid upper limb. Stroke 2003;34:181-218. https://doi.org/10.1161/01.STR.0000087172.16305.CD.
- Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke – consensus from stroke survivors, caregivers, and health professionals. Int J Stroke 2014;9:313-20. https://doi.org/10.1111/j.1747-4949.2012.00942.x.
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD010820.pub2.
- French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD006073.pub3.
- Mehrholz J, Platz T, Kugler J, Pohl M. Electromechanical and robot-assisted arm training for improving arm function and activities of daily living after stroke. Cochrane Database Syst Rev 2008;4. https://doi.org/10.1002/14651858.CD006876.pub2.
- Volpe BT, Krebs HI, Hogan N, Edelstein OTR L, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology 2000;54:1938-44. https://doi.org/10.1212/wnl.54.10.1938.
- Daly JJ, Hogan N, Perepezko EM, Krebs HI, Rogers JM, Goyal KS, et al. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev 2005;42:723-36. https://doi.org/10.1682/jrrd.2005.02.0048.
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil 2003;84:477-82. https://doi.org/10.1053/apmr.2003.50110.
- Volpe BT, Lynch D, Rykman-Berland A, Ferraro M, Galgano M, Hogan N, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair 2008;22:305-10. https://doi.org/10.1177/1545968307311102.
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010;362:1772-83. https://doi.org/10.1056/NEJMoa0911341.
- Wagner TH, Lo AC, Peduzzi P, Bravata DM, Huang GD, Krebs HI, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke 2011;42:2630-2. https://doi.org/10.1161/STROKEAHA.110.606442.
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981;4:483-92. https://doi.org/10.1097/00004356-198112000-00001.
- Platz T, Pinkowski C, Van Wijck F, Johnson G. ARM: Arm Rehabilitation Measurement. Manual for Performance and Scoring of the Fugl-Meyer Test (Arm Section), Action Research Arm Test and the Box-and-Block Test. Baden-Baden: Deutscher Wissenschafts-Verlag (DWV); 2005.
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.STR.20.7.864.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Al-Khawaja I, Wade DT, Collin CF. Bedside screening for aphasia: a comparison of two methods. J Neurol 1996;243:201-4. https://doi.org/10.1007/BF02444015.
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13-31.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5. https://doi.org/10.1037/t02366-000.
- Williams A, Kind P, Brooks R, Rabin R. EQ-5D Concepts and Methods: A Development History. Dordrecht: Springer; 2005.
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994;56:217-26. https://doi.org/10.1016/0304-3959(94)90097-3.
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328. https://doi.org/10.1136/bmj.328.7448.1102.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Forster A, Young J, Kalra L, Smithard D, Knapp M, Patel A, et al. A Cluster Randomised Controlled Trial of a Structured Training Programme for Caregivers of In-patients after Stroke (protocol). Leeds: University of Leeds; 2006.
- Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 1998;6:75-87. https://doi.org/10.1109/86.662623.
- Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, et al. Robot-aided neurorehabilitation: a robot for wrist rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2007;15:327-35. https://doi.org/10.1109/TNSRE.2007.903899.
- Masia L, Krebs HI, Cappa P, Hogan N. Design and characterization of hand module for whole-arm rehabilitation following stroke. IEEE ASME Trans Mechatron 2007;12:399-407. https://doi.org/10.1109/TMECH.2007.901928.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Shaw L, Rodgers H, Price C, Van Wijck F, Shackley P, Steen N, et al. BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14260.
- Shaw LC, Price CI, Van Wijck FM, Shackley P, Steen N, Barnes MP, et al. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) trial: effect on impairment, activity limitation, and pain. Stroke 2011;42:1371-9. https://doi.org/10.1161/STROKEAHA.110.582197.
- Van Wijck F. Skill Acquisition in People With Chronic Upper Limb Spasticity After Stroke 2006.
- Brkic L, Shaw L, Van Wijck F, Francis R, Price C, Forster A, et al. Repetitive arm functional tasks after stroke (RAFTAS): a pilot randomised controlled trial. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0088-5.
- National Institute for Health and Care Excellence . Stroke Rehabilitation in Adults: Clinicial Guideline 2013. www.nice.org.uk/guidance/cg162/resources/stroke-rehabilitation-in-adults-pdf-35109688408261 (accessed 5 September 2019).
- Sentinel Stroke National Audit Programme, King’s College London . Home n.d. www.strokeaudit.org/ (accessed 5 September 2019).
- van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke 1999;30:2369-75. https://doi.org/10.1161/01.str.30.11.2369.
- Duncan PW, Bode RK, Min Lai S, Perera S. Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil 2003;84:950-63. https://doi.org/10.1016/S0003-9993(03)00035-2.
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet 2013;382:2069-76. https://doi.org/10.1016/S0140-6736(13)61603-7.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dündar Y, et al. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12120.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): two parallel randomised controlled trials. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15240.
- Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose–response relationships in stroke rehabilitation. Stroke 2014;45:2053-8. https://doi.org/10.1161/STROKEAHA.114.004695.
- Health Research Authority . Safety Reporting (Research Other Than Clinical Trials of Investigational Medical Products (CTIMPs)) for UK Health Departments’ Research Ethics Service (RES) n.d. www.hra.nhs.uk/documents/1911/Accessible_other_research_safety_progress_reports_procedural_table.pdf (accessed 15 July 2020).
- Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. EPOS Investigators. Stroke 2010;41:745-50. https://doi.org/10.1161/STROKEAHA.109.572065.
- Peyre H, Leplège A, Coste J. Missing data methods for dealing with missing items in quality of life questionnaires. A comparison by simulation of personal mean score, full information maximum likelihood, multiple imputation, and hot deck techniques applied to the SF-36 in the French 2003 decennial health survey. Qual Life Res 2011;20:287-300. https://doi.org/10.1007/s11136-010-9740-3.
- Roberts C. The implications of variation in outcome between health professionals for the design and analysis of randomized controlled trials. Stat Med 1999;18:2605-15. https://doi.org/10.1002/(SICI)1097-0258(19991015)18:19%3C2605::AID-SIM237%3E3.0.CO;2-N.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. https://doi.org/10.1191/1740774505cn076oa.
- Roberts C, Walwyn R. Design and analysis of non-pharmacological treatment trials with multiple therapists per patient. Stat Med 2013;32:81-98. https://doi.org/10.1002/sim.5521.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Research Governance Framework for Health and Social Care. London: Department of Health and Social Care; 2005.
- Shirley Ryan AbilityLab . Rehabilitation Measures Database n.d. www.sralab.org/rehabilitation-measures (accessed 17 April 2019).
- Newcastle University . Robot Assisted Training for the Upper Limb After Stroke n.d. https://research.ncl.ac.uk/ratuls/forhealthcareprofessionals/ (accessed 5 September 2019).
- Bosomworth H, Rodgers H, Shaw L, Smith L, Aird L, Howel D, et al. Evaluation of the enhanced upper limb therapy programme within the Robot-Assisted Training for the Upper Limb after Stroke trial: descriptive analysis of intervention fidelity, goal selection and goal achievement [published online ahead of print 11 September 2020]. Clin Rehabil 2020.
- Cunningham P, Turton AJ, Van Wijck F, Van Vliet P. Task-specific reach-to-grasp training after stroke: development and description of a home-based intervention. Clin Rehabil 2016;30:731-40. https://doi.org/10.1177/0269215515603438.
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther 1990;57:82-7. https://doi.org/10.1177/000841749005700207.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Dijkers MP. An end to the black box of rehabilitation?. Arch Phys Med Rehabil 2019;100:144-5. https://doi.org/10.1016/j.apmr.2018.09.108.
- Walker MF, Hoffmann TC, Brady MC, Dean CM, Eng JJ, Farrin AJ, et al. Improving the development, monitoring and reporting of stroke rehabilitation research: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12:472-9. https://doi.org/10.1177/1747493017711815.
- McKevitt C, Redfern J, Mold F, Wolfe C. Qualitative studies of stroke: a systematic review. Stroke 2004;35:1499-505. https://doi.org/10.1161/01.STR.0000127532.64840.36.
- Sarre S, Redlich C, Tinker A, Sadler E, Bhalla A, McKevitt C. A systematic review of qualitative studies on adjusting after stroke: lessons for the study of resilience. Disabil Rehabil 2014;36:716-26. https://doi.org/10.3109/09638288.2013.814724.
- Lou S, Carstensen K, Jørgensen CR, Nielsen CP. Stroke patients’ and informal carers’ experiences with life after stroke: an overview of qualitative systematic reviews. Disabil Rehabil 2017;39:301-13. https://doi.org/10.3109/09638288.2016.1140836.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage Publications Ltd; 2004.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Duncan Millar J, Van Wijck F, Pollock A, Ali M. Outcome measures in post-stroke arm rehabilitation trials: do existing measures capture outcomes that are important to stroke survivors, carers, and clinicians?. Clin Rehabil 2019;33:737-49. https://doi.org/10.1177/0269215518823248.
- Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors’ perspective. Disabil Rehabil 2005;27:1213-23. https://doi.org/10.1080/09638280500075717.
- Poltawski L, Allison R, Briscoe S, Freeman J, Kilbride C, Neal D, et al. Assessing the impact of upper limb disability following stroke: a qualitative enquiry using internet-based personal accounts of stroke survivors. Disabil Rehabil 2016;38:945-51. https://doi.org/10.3109/09638288.2015.1068383.
- Taule T, Råheim M. Life changed existentially: a qualitative study of experiences at 6–8 months after mild stroke. Disabil Rehabil 2014;36:2107-19. https://doi.org/10.3109/09638288.2014.904448.
- Shannon RL, Forster A, Hawkins RJ. A qualitative exploration of self-reported unmet need one year after stroke. Disabil Rehabil 2016;38:2000-7. https://doi.org/10.3109/09638288.2015.1107784.
- Atler K. The experiences of everyday activities post-stroke. Disabil Rehabil 2016;38:781-8. https://doi.org/10.3109/09638288.2015.1061603.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017 2017.
- Department of Health and Social Care . NHS Reference Costs 2017 18 2018.
- NHS England, NHS Improvement . Long-Stay Patients Methodology 2018.
- Joint Formulary Committee . British National Formulary 2018.
- NHS Digital . Prescription Cost Analysis – England 2018.
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual. Chapter 4: Incorporating Economic Evaluation 2014.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set 2017.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3100.
- Fiebig DG, Baltagi BH. A Companion to Theoretical Econometrics. Oxford: Blackwell Publishing Ltd; 2001.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Rodgers H, Bosomworth H, Van Wijck F, Krebs HI, Shaw L. Usual care: the big but unmanaged problem of rehabilitation evidence – authors’ reply. Lancet 2020;395:337-8. https://doi.org/10.1016/S0140-6736(19)32543-7.
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair 2017;31:107-21. https://doi.org/10.1177/1545968316666957.
- Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2018;9. https://doi.org/10.1002/14651858.CD006876.pub5.
- Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehabil Res Dev 2012;49:479-96. https://doi.org/10.1682/jrrd.2010.10.0210.
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001;32:1635-9. https://doi.org/10.1161/01.str.32.7.1635.
- Rémy-Néris O, Médée B, Bensmail D, Daveluy W, Benaim C, Froger J, et al. Rehabilitation robotics of the upper limb after stroke. The REM_AVC trial. Ann Phys Rehabil Med 2018;61. https://doi.org/10.1016/j.rehab.2018.05.045.
- Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke 2009;40:2123-8. https://doi.org/10.1161/STROKEAHA.108.544585.
- Cramer SC. An overview of therapies to promote repair of the brain after stroke. Head Neck 2011;33:5-7. https://doi.org/10.1002/hed.21840.
- Schmidt RA. Motor Control and Learning: A Behavioural Emphasis. Champaign, IL: Human Kinetics; 1988.
- Johansson BB. Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurol Scand 2011;123:147-59. https://doi.org/10.1111/j.1600-0404.2010.01417.x.
- Carey LM. Stroke Rehabilitation. Insights from Neuroscience and Imaging. Oxford: Oxford University Press; 2012.
- Fitts P, Posner M. Human Performance. Belmont, CA: Brooks and Cole; 1967.
- Schmidt R, Lee T. Motor Learning and Performance. Champaign, IL: Human Kinetics Europe Ltd; 2014.
- Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair 2010;24:620-35. https://doi.org/10.1177/1545968310361957.
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 1996;75:2144-9. https://doi.org/10.1152/jn.1996.75.5.2144.
- Conroy SS, Wittenberg GF, Krebs HI, Zhan M, Bever CT, Whitall J. Robot-assisted arm training in chronic stroke: addition of transition-to-task practice. Neurorehabil Neural Repair 2019;33:751-61. https://doi.org/10.1177/1545968319862558.
- Palmer R, Dimairo M, Cooper C, Enderby P, Brady M, Bowen A, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (Big CACTUS): a multicentre, single-blinded, randomised controlled trial. Lancet Neurol 2019;18:821-33. https://doi.org/10.1016/S1474-4422(19)30192-9.
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke 2017;12:444-50. https://doi.org/10.1177/1747493017711816.
- Cramer SC, Wolf SL, Adams HP, Chen D, Dromerick AW, Dunning K, et al. Stroke recovery and rehabilitation research: issues, opportunities, and the National Institutes of Health StrokeNet. Stroke 2017;48:813-19. https://doi.org/10.1161/STROKEAHA.116.015501.
- Hayward KS, Brauer SG. Dose of arm activity training during acute and subacute rehabilitation post stroke: a systematic review of the literature. Clin Rehabil 2015;29:1234-43. https://doi.org/10.1177/0269215514565395.
- Levack WM, Weatherall M, Hay-Smith EJ, Dean SG, McPherson K, Siegert RJ. Goal setting and strategies to enhance goal pursuit for adults with acquired disability participating in rehabilitation. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD009727.pub2.
- Sugavanam T, Mead G, Bulley C, Donaghy M, Van Wijck F. The effects and experiences of goal setting in stroke rehabilitation - a systematic review. Disabil Rehabil 2013;35:177-90. https://doi.org/10.3109/09638288.2012.690501.
- Scobbie L, Dixon D, Wyke S. Goal setting and action planning in the rehabilitation setting: development of a theoretically informed practice framework. Clin Rehabil 2011;25:468-82. https://doi.org/10.1177/0269215510389198.
- Plant S, Tyson SF. A multicentre study of how goal-setting is practised during inpatient stroke rehabilitation. Clin Rehabil 2018;32:263-72. https://doi.org/10.1177/0269215517719485.
- Negrini S, Arienti C, Kiekens C. Usual care: the big but unmanaged problem of rehabilitation evidence. Lancet 2020;395. https://doi.org/10.1016/S0140-6736(19)32553-X.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2016.
- Stockley R, Peel R, Jarvis K, Connell L. Current therapy for the upper limb after stroke: a cross-sectional survey of UK therapists. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-030262.
- de Jong LD, Van Wijck F, Stewart RE, Geurts ACH, Dijkstra PU. Content of conventional therapy for the severely affected arm during subacute rehabilitation after stroke: an analysis of physiotherapy and occupational therapy practice. Physiother Res Int 2018;23. https://doi.org/10.1002/pri.1683.
- Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0087987.
- Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restor Neurol Neurosci 2013;31:707-22. https://doi.org/10.3233/RNN-130332.
- Hebb D. The Organization of Behavior. New York, NY: Wiley; 1949.
- Tsivgoulis G, Katsanos AH, Caso V. Under-representation of women in stroke randomized controlled trials: inadvertent selection bias leading to suboptimal conclusions. Ther Adv Neurol Disord 2017;10:241-4. https://doi.org/10.1177/1756285617699588.
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170-80. https://doi.org/10.1093/brain/awl333.
- Bernhardt J, Mehrholz J. Robotic-assisted training after stroke: RATULS advances science. Lancet 2019;394:6-8. https://doi.org/10.1016/S0140-6736(19)31156-0.
- Ward NS, Kelly K, Brander F. An expert opinion: upper limb rehabilitation after stroke. Adv Clin Neurosci Rehabil 2019;18:20-2.
- Brunner I, Skouen JS, Hofstad H, Aßmuss J, Becker F, Pallesen H, et al. Is upper limb virtual reality training more intensive than conventional training for patients in the subacute phase after stroke? An analysis of treatment intensity and content. BMC Neurol 2016;16. https://doi.org/10.1186/s12883-016-0740-y.
- Schneider EJ, Lannin NA, Ada L, Schmidt J. Increasing the amount of usual rehabilitation improves activity after stroke: a systematic review. J Physiother 2016;62:182-7. https://doi.org/10.1016/j.jphys.2016.08.006.
- Adie K, Schofield C, Berrow M, Wingham J, Humfryes J, Pritchard C, et al. Does the use of Nintendo Wii Sports™ improve arm function? Trial of Wii™ in stroke: a randomized controlled trial and economics analysis. Clin Rehabil 2017;31:173-85. https://doi.org/10.1177/0269215516637893.
- Brunner I, Skouen JS, Hofstad H, Aßmus J, Becker F, Sanders AM, et al. Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES): a multicenter RCT. Neurology 2017;89:2413-21. https://doi.org/10.1212/WNL.0000000000004744.
- Kwakkel G, Winters C, van Wegen EE, Nijland RH, van Kuijk AA, Visser-Meily A, et al. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the EXPLICIT-Stroke randomized clinical trial. Neurorehabil Neural Repair 2016;30:804-16. https://doi.org/10.1177/1545968315624784.
- Pomeroy VM, Hunter SM, Johansen-Berg H, Ward NS, Kennedy N, Chandler E, et al. Functional strength training versus movement performance therapy for upper limb motor recovery early after stroke: a RCT. Efficacy Mech Eval 2018;5. https://doi.org/10.3310/eme05030.
- Saposnik G, Cohen LG, Mamdani M, Pooyania S, Ploughman M, Cheung D, et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre, single-blind, controlled trial. Lancet Neurol 2016;15:1019-27. https://doi.org/10.1016/S1474-4422(16)30121-1.
- Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA 2016;315:571-81. https://doi.org/10.1001/jama.2016.0276.
- Daly JJ, McCabe JP, Holcomb J, Monkiewicz M, Gansen J, Pundik S. Long-dose intensive therapy is necessary for strong, clinically significant, upper limb functional gains and retained gains in severe/moderate chronic stroke. Neurorehabil Neural Repair 2019;33:523-37. https://doi.org/10.1177/1545968319846120.
- McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2015;96:981-90. https://doi.org/10.1016/j.apmr.2014.10.022.
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry 2019;90:498-506. https://doi.org/10.1136/jnnp-2018-319954.
- Lang CE, Strube MJ, Bland MD, Waddell KJ, Cherry-Allen KM, Nudo RJ, et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: a Phase II, single-blind, randomized, controlled trial. Ann Neurol 2016;80:342-54. https://doi.org/10.1002/ana.24734.
- Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12:451-61. https://doi.org/10.1177/1747493017711813.
- Pike S, Lannin NA, Wales K, Cusick A. A systematic review of the psychometric properties of the Action Research Arm Test in neurorehabilitation. Aust Occup Ther J 2018;65:449-71. https://doi.org/10.1111/1440-1630.12527.
- World Health Organization . International Classification of Functioning, Disability and Health n.d. www.who.int/classifications/icf/en/ (accessed 21 October 2019).
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.str.19.5.604.
- Bushnell C, Bettger JP, Cockroft KM, Cramer SC, Edelen MO, Hanley D, et al. Chronic stroke outcome measures for motor function intervention trials: expert panel recommendations. Circ Cardiovasc Qual Outcomes 2015;8:163-9. https://doi.org/10.1161/CIRCOUTCOMES.115.002098.
- Bernstein JA, Mauger DT. The Minimally Clinically Important Difference (MCID): what difference does it make?. J Allergy Clin Immunol Pract 2016;4:689-90. https://doi.org/10.1016/j.jaip.2016.04.022.
- Cook CE. Clinimetrics corner: the Minimal Clinically Important Change Score (MCID): a necessary pretense. J Man Manip Ther 2008;16:E82-3. https://doi.org/10.1179/jmt.2008.16.4.82E.
- Equator Network . Enhancing the QUAlity and Transparency Of Health Research n.d. www.equator-network.org/ (accessed 21 October 2019).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017;4:811-20. https://doi.org/10.1002/acn3.488.
- Bernhardt J, Hayward KS, Dancause N, Lannin NA, Ward NS, Nudo RJ, et al. A stroke recovery trial development framework: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2019;14:792-80. https://doi.org/10.1177/1747493019879657.
- Van Stan JH, Dijkers MP, Whyte J, Hart T, Turkstra LS, Zanca JM, et al. The Rehabilitation treatment specification system: implications for improvements in research design, reporting, replication, and synthesis. Arch Phys Med Rehabil 2019;100:146-55. https://doi.org/10.1016/j.apmr.2018.09.112.
- Pomeroy V, Aglioti SM, Mark VW, McFarland D, Stinear C, Wolf SL, et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair 2011;25:33-4. https://doi.org/10.1177/1545968311410942.
- Hung CS, Hsieh YW, Wu CY, Lin YT, Lin KC, Chen CL. The effects of combination of robot-assisted therapy with task-specific or impairment-oriented training on motor function and quality of life in chronic stroke. PM&Amp;R 2016;8:721-9. https://doi.org/10.1016/j.pmrj.2016.01.008.
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke 2007;38:840-5. https://doi.org/10.1161/01.STR.0000247943.12887.d2.
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996;272:1791-4. https://doi.org/10.1126/science.272.5269.1791.
- Bizzi E, Accornero N, Chapple W, Hogan N. Posture control and trajectory formation during arm movement. J Neurosci 1984;4:2738-44. https://doi.org/10.1523/JNEUROSCI.04-11-02738.1984.
- Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc Natl Acad Sci USA 1999;96:4645-9. https://doi.org/10.1073/pnas.96.8.4645.
- Hogan N, Sternad D. Dynamic primitives of motor behavior. Biol Cybern 2012;106:727-39. https://doi.org/10.1007/s00422-012-0527-1.
- Levy-Tzedek S, Krebs HI, Song D, Hogan N, Poizner H. Non-monotonicity on a spatio-temporally defined cyclic task: evidence of two movement types?. Exp Brain Res 2010;202:733-46. https://doi.org/10.1007/s00221-010-2176-8.
- Schaal S, Sternad D, Osu R, Kawato M. Rhythmic arm movement is not discrete. Nat Neurosci 2004;7:1136-43. https://doi.org/10.1038/nn1322.
- Lee H, Rouse EJ, Krebs HI. Summary of human ankle mechanical impedance during walking. IEEE J Transl Eng Health Med 2016;4. https://doi.org/10.1109/JTEHM.2016.2601613.
- Susko T, Swaminathan K, Krebs HI. MIT-Skywalker: A novel gait neurorehabilitation robot for stroke and cerebral palsy. IEEE Trans Neural Syst Rehabil Eng 2016;24:1089-99. https://doi.org/10.1109/TNSRE.2016.2533492.
- Shumway-Cook A, Woollacott M. Motor Control: Translating Research into Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- NHS Employers . NHS Terms and Conditions (AfC) Pay Scales - Hourly 2018. https://www.nhsemployers.org/pay-pensions-and-reward/agenda-for-change/pay-scales-1819/hourly-1819 (accessed 1 December 2018).
- Bank of England . Inflation Calculator 2018. https://www.bankofengland.co.uk/monetary-policy/inflation/inflation-calculator (accessed 1 December 2018).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis LA. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- The University of Sheffield . Evaluation of NHS 111 Pilot Sites – Final Report 2012.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
Appendix 1 Description of the Robot-Assisted Training for the Upper Limb after Stroke trial interventions using the Template for Intervention Description and Replication checklist
| Item | Intervention group | |
|---|---|---|
| Robot-assisted training | EULT | |
| 1. Brief name: ‘State the intervention name’ | Robot-assisted training | EULT |
| 2. Why: ‘What is the scientific basis of the intervention in terms of the rationale, theory or goal of the intervention?’ | The rationale for repetitive training is based on converging theories and evidence from the following domains: motor control and learning, neuroplasticity, translational research and clinical trials | |
| The goal of both the robot-assisted training and the EULT programme was to improve arm function. This can be conceptualised, at the behavioural level, as a process of motor learning, which has been described as ‘a set of processes associated with practice/experience leading to relatively permanent changes in the capability for producing skilled action’100. Both interventions were therefore underpinned by established theories of motor learning, including the stages of learning103 and motor programme theory100,104 to structure the learning processes. At the neuronal level, learning involves Hebbian122 and non-Hebbian processes that foster experience-dependent plasticity.101,121,153,154 Evidence from translational studies105,106 further supports the use of progressive, repetitive training protocols. Finally, clinical trial evidence supports the use of repetitive functional task practice for upper limb recovery10,120 | ||
| Rationale for robot-assisted training: robot-assisted training enables repetitive tasks to be undertaken in a highly consistent and controllable manner. Robot-assisted training is based on the virtual trajectory hypothesis155 and the conjecture that movement is composed by primitives of movement, in particular, that movement is a composition of discrete (submovements),156 rhythmic (oscillations)157–159 and mechanical impedance.160,161 A Cochrane systematic review94 of electromechanical and robot-assisted arm training after stroke reported outcomes from a total of 1619 patients who participated in 45 trials. Improvements in arm function (SMD 0.32, 95% CI 0.18 to 0.46) and ADL (SMD 0.31, 95% CI 0.09 to 0.50) were found in patients who received this treatment.94 Studies were heterogeneous and varied in their quality | Rationale for EULT: the EULT programme was based on the motor-learning theory described above, as well as the established specificity of learning principle, which purports that practice is most effective if it resembles the task and environment of the target activity as closely as possible.104 The converging theory and evidence referred to above, supplemented by literature on clinical applications thereof,102,162 informed the focus, structure and progression of the skill acquisition process of the EULT programme | |
Essential elements:
|
Essential elements:
|
|
| 3. What materials: ‘What information/materials were provided to participants or the intervention providers in order to deliver the intervention? Information on where to access these materials should also be provided’ |
|
|
| 4. What (procedures): ‘What were the activities and/or procedures needed to deliver the intervention?’ |
|
|
| 5. Who provided: ‘What was the expertise and background of the intervention providers and was any intervention-specific training required?’ |
|
|
| 6. How: ‘What was the mode of delivery of the intervention (e.g. face to face, individually or in group)?’ | 1 : 1 face-to-face delivery | 1 : 1 face-to-face delivery |
| 7. Where: ‘In which location was the intervention provided (e.g. outpatients, hospital or patients own home)?’ | NHS hospital facilities: dedicated therapy room | NHS hospital facilities: therapy gym or dedicated therapy room |
| 8. When and how much: ‘What was the planned amount of intervention delivered in terms of the number of sessions, over what time period, the duration of sessions and the intensity/dose of delivery?’ | The robot-assisted training programme was provided for up to 45 minutes per day, 3 days per week, for 12 weeks (a total of 36 therapy sessions), in addition to usual NHS care. One hour was allowed for each therapy session to facilitate preparation and set-up | The EULT programme was provided for up to 45 minutes per day, 3 days per week, for 12 weeks (a total of 36 therapy sessions), in addition to usual NHS care. One hour was allowed for each therapy session to facilitate preparation and set-up |
| 9. Tailoring: ‘Was the intervention designed so that it was able to be personalised or adapted to an individual?’ |
|
The EULT programme was based on patient-centred goals. Up to four goals could be chosen. A senior therapist assessed/reviewed each participant at baseline and at 4, 8 and 12 weeks, and planned/adjusted the programme according to progress. The therapists were also able to tailor the specifics of each activity practised to the ability of the participant, taking into consideration a range of upper limb parameters (i.e. sensation and proprioception, range of motion, strengths and co-ordination), other functions (including sitting balance, visuo-spatial awareness, vision), as well as the person’s cognitive and emotional status, communication skills and level of motivation |
| 10. Modifications: ‘Were there any modifications/changes made to the intervention during the study and, if so, what were these changes?’ | There were no modifications to the interventions | |
| 11. How well (planned): ‘What were the methods used to assess, maintain or improve intervention adherence and fidelity during the study?’ |
|
|
| 12. How well (actual): ‘Was the intervention was delivered as intended?’ | See Chapter 4 for further information | See Chapter 5 for further information |
Appendix 2 Recruitment, withdrawal and missing data
| Month and year | Number of participants recruited | ||||
|---|---|---|---|---|---|
| North Tyneside | Glasgow | Romford | Northwick Park | Total | |
| April 2014 | 3 | 3 | x | x | 6 |
| May 2014 | 4 | 6 | 0 | x | 10 |
| June 2014 | 5 | 5 | 6 | x | 16 |
| July 2014 | 7 | 4 | 6 | x | 17 |
| August 2014 | 4 | 4 | 5 | x | 13 |
| September 2014 | 5 | 6 | 6 | x | 17 |
| October 2014 | 3 | 4 | 7 | x | 14 |
| November 2014 | 8 | 2 | 6 | x | 16 |
| December 2014 | 3 | 0 | 4 | x | 7 |
| January 2015 | 8 | 8 | 6 | x | 22 |
| February 2015 | 2 | 6 | 5 | x | 13 |
| March 2015 | 1 | 4 | 7 | x | 12 |
| April 2015 | 8 | 5 | 6 | 2 | 21 |
| May 2015 | 5 | 3 | 6 | 5 | 19 |
| June 2015 | 5 | 4 | 5 | 3 | 17 |
| July 2015 | 5 | 2 | 4 | 4 | 15 |
| August 2015 | 6 | 4 | 3 | 0a | 13 |
| September 2015 | 9 | 4 | 6 | 0a | 19 |
| October 2015 | 3 | 4 | 6 | 1 | 14 |
| November 2015 | 2 | 6 | 5 | 0 | 13 |
| December 2015 | 4 | 0 | 3 | 3 | 10 |
| January 2016 | 5 | 5 | 7 | 3 | 20 |
| February 2016 | 2 | 6 | 4 | 6 | 18 |
| March 2016 | 3 | 6 | 7 | 1 | 17 |
| April 2016 | 3 | 4 | 5 | 8 | 20 |
| May 2016 | 5 | 7 | 4 | 5 | 21 |
| June 2016 | 2 | 4 | 2 | 1 | 9 |
| July 2016 | 5 | 4 | 2 | 5 | 16 |
| August 2016 | 6 | 9 | 6 | 4 | 25 |
| September 2016 | 8 | 6 | 5 | 4 | 23 |
| October 2016 | 5 | 5 | 6 | 4 | 20 |
| November 2016 | 7 | 2 | 6 | 6 | 21 |
| December 2016 | 2 | 1 | 3 | 1 | 7 |
| January 2017 | 9 | 8 | 5 | 3 | 25 |
| February 2017 | 3 | 7 | 6 | 3 | 19 |
| March 2017 | 4 | 3 | 8 | 2 | 17 |
| April 2017 | 6 | 3 | 4 | 2 | 15 |
| May 2017 | 6 | 7 | 6 | 5 | 24 |
| June 2017 | 5 | 7 | 6 | 6 | 24 |
| July 2017 | 3 | 2 | x | 6 | 11 |
| August 2017 | 6 | 9 | x | 1 | 16 |
| September 2017 | 6 | 2 | x | 4 | 12 |
| October 2017 | 5 | 5 | x | 2 | 12 |
| November 2017 | 6 | 4 | x | 3 | 13 |
| December 2017 | 2 | 1 | x | 2 | 5 |
| January 2018 | 6 | 8 | x | 2 | 16 |
| February 2018 | 6 | 2 | x | 1 | 9 |
| March 2018 | 6 | 6 | x | 2 | 14 |
| April 2018 | 5 | 5 | x | 7 | 17 |
| Total | 237 | 222 | 194 | 117 | 770 |
| Reason for withdrawal/assessment not conducted | Participants (n) | |||
|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | Total | |
| At 3 months | ||||
| Assessment not conducted | 6 | 10 | 16 | 32 |
| Participant did not attend agreed scheduled appointment and did not respond to attempts to rearrange | 2 | 2 | 3 | 7 |
| Participant did not respond to telephone/e-mail/postal attempts to arrange assessment | 2 | 5 | 7 | 14 |
| Participant had a change in personal circumstances and was unable to attend assessment | 1 | 0 | 1 | 2 |
| Participant refused to complete assessment – reason unknown | 0 | 1 | 0 | 1 |
| Participant unwell and unable to do assessment | 1 | 2 | 5 | 8 |
| Died | 1 | 1 | 0 | 2 |
| Withdrawn | 17 | 12 | 31 | 60 |
| Participant did not wish to take part, as randomised to usual care | 0 | 0 | 20 | 20 |
| Participant experienced pain during therapy sessions | 0 | 1 | 0 | 1 |
| Participant experienced upper limb pain | 1 | 1 | 0 | 2 |
| Participant found to be ineligible, diagnosis not stroke | 1 | 0 | 1 | 2 |
| Participant had a change in personal circumstances and was unable to continue participation in the trial | 2 | 0 | 0 | 2 |
| Participant moved out of trial area | 1 | 1 | 0 | 2 |
| Participant refused to complete assessment – reason unknown | 0 | 1 | 0 | 1 |
| Participant refused to continue participation in the trial as they did not feel any improvement in arm | 1 | 0 | 0 | 1 |
| Participant refused to continue participation in the trial as they felt that it was too much of a burden | 4 | 6 | 9 | 19 |
| Participant unwell and unable to continue participation in the trial | 7 | 2 | 1 | 10 |
| Total number of withdrawals/assessment not conducted at 3 months | 24 | 23 | 47 | 94 |
| At 6 months | ||||
| Assessment not conducted | 11 | 14 | 24 | 49 |
| Participant did not attend agreed scheduled appointment and did not respond to attempts to rearrange | 2 | 1 | 2 | 5 |
| Participant did not respond to telephone/e-mail/postal attempts to arrange assessment | 6 | 12 | 19 | 37 |
| Participant had a change in personal circumstances and was unable to attend assessment | 1 | 0 | 0 | 1 |
| Participant paperwork was lost | 1 | 0 | 0 | 1 |
| Participant refused to complete assessment – reason unknown | 0 | 0 | 1 | 1 |
| Participant unwell and unable to do assessment | 1 | 1 | 2 | 4 |
| Died | 0 | 2 | 0 | 2 |
| Died before 3-month assessment | 1 | 1 | 0 | 2 |
| Withdrawn | 5 | 8 | 9 | 22 |
| Participant behaviour was inappropriate | 0 | 1 | 0 | 1 |
| Participant had a change in personal circumstances and was unable to continue participation in the trial | 0 | 0 | 1 | 1 |
| Participant had problems with transport arrangements to assessment | 0 | 0 | 1 | 1 |
| Participant moved out of trial area | 0 | 1 | 0 | 1 |
| Participant refused to continue participation in the trial (reason unknown) | 2 | 3 | 1 | 6 |
| Participant refused to continue participation in the trial because they did not feel any improvement in arm | 0 | 1 | 0 | 1 |
| Participant refused to continue participation in the trial, as they felt that it was too much of a burden | 0 | 1 | 3 | 4 |
| Participant unhappy with randomisation group | 0 | 0 | 1 | 1 |
| Participant unwell and unable to continue participation in the trial | 3 | 1 | 2 | 6 |
| Withdrawn before the 3-month assessment | 17 | 12 | 31 | 60 |
| Total number of withdrawals/assessment not conducted at 6 months | 34 | 37 | 64 | 135 |
| Measure | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number possible to imputea (% of expected) | |
| ARAT | |||||||||
| Grasp | 257 | 256 (99.6) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Grip | 257 | 256 (99.6) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Pinch | 257 | 256 (99.6) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Gross movement | 257 | 256 (99.6) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| ARAT total score | 257 | 256 (99.6) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Montreal Cognitive Assessment | 257 | 248 (96.5) | 259 | 250 (96.5) | 254 | 242 (95.3) | |||
| Sheffield Screening Test for Acquired Language Disorders | |||||||||
| Receptive skills score | 257 | 255 (99.2) | 259 | 258 (99.6) | 254 | 254 (100.0) | |||
| Expressive skills score | 257 | 251 (97.7) | 259 | 258 (99.6) | 254 | 254 (100.0) | |||
| Total score | 257 | 251 (97.7) | 259 | 258 (99.6) | 254 | 254 (100.0) | |||
| NIHSS | 257 | 253 (98.4) | 255 (99.2) | 259 | 259 (100.0) | 254 | 252 (99.2) | 254 (100.0) | |
| FMA | |||||||||
| Motor function score | 257 | 255 (99.2) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Range of motion and joint pain score | 257 | 254 (98.8) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Sensation score | 257 | 253 (98.4) | 259 | 258 (99.6) | 254 | 252 (99.2) | |||
| Total upper-extremity score | 257 | 253 (98.4) | 254 (98.8) | 259 | 258 (99.6) | 259 (100.0) | 254 | 252 (99.2) | 254 (100.0) |
| Barthel ADL Index | 257 | 255 (99.2) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Numerical Pain Scaleb | 257 | 253 (98.4) | 259 | 259 (100.0) | 254 | 254 (100.0) | |||
| Measure | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number possible to imputea (% of expected) | |
| ARAT | |||||||||
| Grasp | 239 | 232 (97.1) | 246 | 234 (95.1) | 223 | 203 (91.0) | |||
| Grip | 239 | 232 (97.1) | 246 | 234 (95.1) | 223 | 203 (91.0) | |||
| Pinch | 239 | 232 (97.1) | 246 | 234 (95.1) | 223 | 203 (91.0) | |||
| Gross movement | 239 | 232 (97.1) | 246 | 234 (95.1) | 223 | 202 (90.6) | |||
| ARAT total score | 239 | 232 (97.1) | 246 | 234 (95.1) | 223 | 202 (90.6) | 203 (91.0) | ||
| FMA | |||||||||
| Motor function score | 239 | 229 (95.8) | 232 (97.1) | 246 | 233 (94.7) | 234 (95.1) | 223 | 201 (90.1) | 202 (90.6) |
| Range of motion and joint pain score | 239 | 231 (96.7) | 232 (97.1) | 246 | 234 (95.1) | 223 | 204 (91.5) | ||
| Sensation score | 239 | 231 (96.7) | 246 | 234 (95.1) | 223 | 200 (89.7) | 202 (90.6) | ||
| Total upper-extremity score | 239 | 227 (95.0) | 232 (97.1) | 246 | 233 (94.7) | 234 (95.1) | 199 (89.2) | 202 (90.6) | |
| Barthel ADL Index | 239 | 233 (97.5) | 246 | 236 (95.9) | 223 | 207 (92.8) | |||
| SIS | |||||||||
| Strength | 239 | 217 (90.8) | 220 (92.1) | 246 | 219 (89.0) | 220 (89.4) | 223 | 193 (86.5) | |
| Hand function | 239 | 218 (91.2) | 219 (91.6) | 246 | 219 (89.0) | 223 (90.7) | 223 | 192 (86.1) | 193 (86.5) |
| Mobility | 239 | 217 (90.8) | 219 (91.6) | 246 | 216 (87.8) | 223 (90.7) | 223 | 184 (82.5) | 192 (86.1) |
| ADL | 239 | 212 (88.7) | 220 (92.1) | 246 | 215 (87.4) | 223 (90.7) | 223 | 191 (85.7) | 194 (87.0) |
| Emotion | 239 | 213 (89.1) | 220 (92.1) | 246 | 215 (87.4) | 222 (90.2) | 223 | 188 (84.3) | 194 (87.0) |
| Memory | 239 | 217 (90.8) | 219 (91.6) | 246 | 219 (89.0) | 222 (90.2) | 223 | 191 (85.7) | 194 (87.0) |
| Communication | 239 | 216 (90.4) | 220 (92.1) | 246 | 220 (89.4) | 222 (90.2) | 223 | 193 (86.5) | 194 (87.0) |
| Social participation | 239 | 183 (76.6) | 217 (90.8) | 246 | 176 (71.5) | 221 (89.8) | 223 | 148 (66.4) | 193 (86.5) |
| Stroke recoveryb | 239 | 217 (90.8) | 246 | 223 (90.7) | 223 | 190 (85.2) | |||
| Numerical Pain Scaleb | 239 | 232 (97.1) | 246 | 236 (95.9) | 223 | 206 (92.4) | |||
| Measure | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number after imputationa (% of expected) | Number expected | Number with complete-case data (% of expected) | Number possible to imputea (% of expected) | |
| ARAT | |||||||||
| Grasp | 234 | 221 (94.4) | 236 | 218 (92.4) | 214 | 185 (86.4) | |||
| Grip | 234 | 221 (94.4) | 236 | 218 (92.4) | 214 | 185 (86.4) | |||
| Pinch | 234 | 221 (94.4) | 236 | 218 (92.4) | 214 | 185 (86.4) | |||
| Gross movement | 234 | 221 (94.4) | 236 | 218 (92.4) | 214 | 185 (86.4) | |||
| ARAT total score | 234 | 221 (94.4) | 236 | 218 (92.4) | 214 | 185 (86.4) | |||
| FMA | |||||||||
| Motor function score | 234 | 219 (93.6) | 221 (94.4) | 236 | 217 (91.9) | 214 | 185 (86.4) | 186 (86.9) | |
| Range of motion and joint pain score | 234 | 220 (94.0) | 221 (94.4) | 236 | 218 (92.4) | 214 | 186 (86.9) | ||
| Sensation score | 234 | 220 (94.0) | 221 (94.4) | 236 | 218 (92.4) | 214 | 186 (86.9) | ||
| Total upper-extremity score | 234 | 218 (93.2) | 221 (94.4) | 236 | 217 (91.9) | 218 (92.4) | 214 | 185 (86.4) | 186 (86.9) |
| Barthel ADL Index | 234 | 223 (95.3) | 236 | 222 (94.1) | 214 | 190 (88.8) | |||
| SIS | |||||||||
| Strength | 234 | 210 (89.7) | 213 (91.0) | 236 | 215 (91.1) | 216 (91.5) | 214 | 175 (81.8) | 176 (82.2) |
| Hand function | 234 | 210 (89.7) | 213 (91.0) | 236 | 214 (90.7) | 216 (91.5) | 214 | 177 (82.7) | 179 (83.6) |
| Mobility | 234 | 208 (88.9) | 213 (91.0) | 236 | 213 (90.3) | 216 (91.5) | 214 | 174 (81.3) | 180 (84.1) |
| ADL | 234 | 204 (87.2) | 212 (90.6) | 236 | 206 (87.3) | 216 (91.5) | 214 | 173 (80.8) | 179 (83.6) |
| Emotion | 234 | 203 (86.8) | 211 (90.2) | 236 | 210 (89.0) | 216 (91.5) | 214 | 170 (79.4) | 179 (83.6) |
| Memory | 234 | 210 (89.7) | 213 (91.0) | 236 | 212 (89.8) | 215 (91.1) | 214 | 175 (81.8) | 178 (83.2) |
| Communication | 234 | 209 (89.3) | 213 (91.0) | 236 | 213 (90.3) | 216 (91.5) | 214 | 174 (81.3) | 179 (83.6) |
| Social participation | 234 | 180 (76.9) | 210 (89.7) | 236 | 179 (75.8) | 216 (91.5) | 214 | 150 (70.1) | 179 (83.6) |
| Stroke recoveryb | 234 | 213 (91.0) | 236 | 215 (91.1) | 214 | 180 (84.1) | |||
| Numerical Pain Scaleb | 234 | 223 (95.3) | 236 | 221 (93.6) | 214 | 190 (88.8) | |||
| Upper limb rehabilitation treatment | Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total (N = 770) |
|---|---|---|---|---|
| Physiotherapy/occupational therapy (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 127 (49.8) | 115 (44.4) | 117 (46.1) | 359 (46.7) |
| Yes, n (%) | 128 (50.2) | 144 (55.6) | 137 (53.9) | 409 (53.3) |
| Splints (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 181 (71.0) | 184 (71.0) | 180 (70.9) | 545 (71.0) |
| Yes, n (%) | 74 (29.0) | 75 (29.0) | 74 (29.1) | 223 (29.0) |
| Shoulder brace (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 225 (88.2) | 235 (90.7) | 220 (86.6) | 680 (88.5) |
| Yes, n (%) | 30 (11.8) | 24 (9.3) | 34 (13.4) | 88 (11.5) |
| TENS (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 235 (92.2) | 241 (93.1) | 237 (93.3) | 713 (92.8) |
| Yes, n (%) | 20 (7.8) | 18 (6.9) | 17 (6.7) | 55 (7.2) |
| Antispasticity medication (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 223 (87.5) | 213 (82.2) | 213 (83.9) | 649 (84.5) |
| Yes, n (%) | 32 (12.5) | 46 (17.8) | 41 (16.1) | 119 (15.5) |
| Botulinum toxin (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 234 (91.8) | 228 (88.0) | 234 (92.1) | 696 (90.6) |
| Yes, n (%) | 21 (8.2) | 31 (12.0) | 19 (7.5) | 71 (9.2) |
| Unknown, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.1) |
| Other upper limb rehabilitation (N) | 255 | 259 | 254 | 768 |
| No, n (%) | 244 (95.7) | 241 (93.1) | 242 (95.3) | 727 (94.7) |
| Yes, n (%) | 11 (4.3) | 18 (6.9) | 12 (4.7) | 41 (5.3) |
Appendix 3 Analysis of the Action Research Arm Test subscales
Robot-assisted training versus usual care
Both groups showed an improvement from baseline to 3 months, but little improvement thereafter, with little difference between groups. In the robot-assisted training group, the proportion of participants who could complete at least one task on the gross movement subscale was 33% at baseline, 43% at 3 months and 47% at 6 months. For the usual care group, these proportions were 31%, 39% and 41% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in the proportions for gross movement between these groups at 3 months (aOR 1.3, 98.33% CI 0.7 to 2.5) or 6 months (aOR 1.7, 98.33% CI 0.9 to 3.2) (Table 34).
| ARAT subscale | Time point | Robot-assisted training | EULT | Usual care | OR (98.33% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot-assisted training vs. usual care | EULT vs. usual care | Robot-assisted training vs. EULT | |||||||||||
| N | n (%) | N | n (%) | N | n (%) | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | ||
| Gross movement | Baseline | 256 | 85 (33.2) | 259 | 94 (36.3) | 254 | 78 (30.7) | NA | NA | NA | NA | NA | NA |
| 3 months | 232 | 100 (43.1) | 234 | 110 (47.0) | 203 | 80 (39.4) | 1.2 (0.7 to 1.9) | 1.3 (0.7 to 2.5) | 1.4 (0.9 to 2.2) | 1.4 (0.8 to 2.6) | 0.9 (0.5 to 1.3) | 1.0 (0.5 to 1.7) | |
| 6 months | 221 | 103 (46.6) | 218 | 107 (49.1) | 185 | 76 (41.1) | 1.3 (0.8 to 2.0) | 1.7 (0.9 to 3.2) | 1.4 (0.9 to 2.2) | 1.5 (0.8 to 2.9) | 0.9 (0.6 to 1.4) | 1.1 (0.6 to 2.0) | |
| Grasp | Baseline | 256 | 55 (21.5) | 259 | 58 (22.4) | 254 | 43 (16.9) | NA | NA | NA | NA | NA | NA |
| 3 months | 232 | 77 (33.2) | 234 | 83 (35.5) | 203 | 55 (27.1) | 1.3 (0.8 to 2.2) | 1.6 (0.7 to 3.2) | 1.5 (0.9 to 2.4) | 1.6 (0.8 to 3.4) | 0.9 (0.6 to 1.4) | 0.9 (0.5 to 1.8) | |
| 6 months | 221 | 83 (37.6) | 218 | 75 (34.4) | 185 | 57 (30.8) | 1.4 (0.8 to 2.2) | 1.8 (0.9 to 3.8) | 1.2 (0.7 to 2.0) | 1.0 (0.5 to 2.2) | 1.1 (0.7 to 1.8) | 1.8 (0.9 to 3.5) | |
| Grip | Baseline | 256 | 61 (23.8) | 259 | 58 (22.4) | 254 | 55 (21.7) | NA | NA | NA | NA | NA | NA |
| 3 months | 232 | 85 (36.6) | 234 | 91 (38.9) | 203 | 62 (30.5) | 1.3 (0.8 to 2.1) | 1.9 (0.9 to 4.0) | 1.4 (0.9 to 2.4) | 2.4 (1.1 to 5.1) | 0.9 (0.6 to 1.4) | 0.8 (0.4 to 1.5) | |
| 6 months | 221 | 79 (35.7) | 218 | 90 (41.3) | 185 | 65 (35.1) | 1.0 (0.6 to 1.7) | 1.2 (0.5 to 2.4) | 1.3 (0.8 to 2.1) | 1.8 (0.8 to 3.7) | 0.8 (0.5 to 1.3) | 0.7 (0.3 to 1.3) | |
| Pinch | Baseline | 256 | 29 (11.3) | 259 | 32 (12.4) | 254 | 30 (11.8) | NA | NA | NA | NA | NA | NA |
| 3 months | 232 | 54 (23.3) | 234 | 58 (24.8) | 203 | 42 (20.7) | 1.2 (0.7 to 2.0) | 1.6 (0.8 to 3.3) | 1.3 (0.7 to 2.2) | 1.6 (0.8 to 3.3) | 0.9 (0.5 to 1.5) | 1.0 (0.5 to 1.9) | |
| 6 months | 221 | 53 (24.0) | 218 | 58 (26.6) | 185 | 49 (26.5) | 0.9 (0.5 to 1.5) | 1.0 (0.5 to 2.1) | 1.0 (0.6 to 1.7) | 1.1 (0.6 to 2.3) | 0.9 (0.5 to 1.5) | 0.9 (0.5 to 1.8) | |
In the robot-assisted training group, the proportion of respondents who could complete at least one task on the grasp subscale was 21% at baseline, 33% at 3 months and 38% at 6 months. For the usual care group, these proportions were 17%, 27% and 31% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for grasp between these groups at 3 months (aOR 1.6, 98.33% CI 0.7 to 3.2) or 6 months (aOR 1.8, 98.33% CI 0.9 to 3.8) (see Table 34).
In the robot-assisted training group, the proportion of participants who could complete at least one task on the grip subscale was 24% at baseline, 37% at 3 months and 36% at 6 months. For the usual care group, these proportions were 22%, 31% and 35% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for grip between these groups at 3 months (aOR 1.9, 98.33% CI 0.9 to 4.0) or 6 months (aOR 1.2, 98.33% CI 0.5 to 2.4) (see Table 34).
In the robot-assisted training group, the proportion of participants who could complete at least one task on the pinch subscale was 11% at baseline, 23% at 3 months and 24% at 6 months. For the usual care group, these proportions were 12%, 21% and 26% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for pinch between these groups at 3 months (aOR 1.6, 98.33% CI 0.8 to 3.3) or 6 months (aOR 1.0, 98.33% CI 0.5 to 2.1) (see Table 34).
Robot-assisted training verses enhanced upper limb therapy
Both groups showed an improvement from baseline to 3 months, but little improvement thereafter, with small differences between groups. In the robot-assisted training group, the proportion of respondents who could complete at least one task on the gross movement subscale was 33% at baseline, 43% at 3 months and 47% at 6 months. In the EULT group, these proportions were 36%, 47% and 49% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for gross movement between these groups at 3 months (aOR 1.0, 98.33% CI 0.5 to 1.7) or 6 months (aOR 1.1, 98.33% CI 0.6 to 2.0) (see Table 34).
In the robot-assisted training group, the proportion of respondents who could complete at least one task on the grasp subscale was 21% at baseline, 33% at 3 months and 38% at 6 months. In the EULT group, these proportions were 22%, 35% and 34% at baseline, 3 months and 6 months, respectively. There was little evidence of a statistically significant difference in this proportion for grasp between these groups at 3 months (aOR 0.9, 98.33% CI 0.5 to 1.8) or 6 months (aOR 1.8, 98.33% CI 0.9 to 3.5) (see Table 34).
In the robot-assisted training group, the proportion of respondents who could complete at least one task on the grip subscale was 24% at baseline, 37% at 3 months and 36% at 6 months. In the EULT group, these proportions were 22% at baseline, 39% at 3 months and 41% at 6 months. There was little evidence of a difference in this proportion for grip between these groups at 3 months (aOR 0.8, 98.33% CI 0.4 to 1.5) or 6 months (aOR 0.7, 98.33% CI 0.3 to 1.3) (see Table 34).
In the robot-assisted training group, the proportion of respondents who could complete at least one task on the pinch subscale was 11% at baseline, 23% at 3 months and 24% at 6 months. In the EULT group, these proportions were 12% at baseline, 25% at 3 months and 27% at 6 months. There was little evidence of a difference in this proportion for pinch between these groups at 3 months (aOR 1.0, 98.33% CI 0.5 to 1.9) or 6 months (aOR 0.9, 98.33% CI 0.5 to 1.8) (see Table 34).
Enhanced upper limb therapy versus usual care
Both groups showed an improvement from baseline to 3 months, but little improvement thereafter, with occasional small differences between groups. In the EULT group, the proportion of respondents who could complete at least one task on the gross movement subscale was 36% at baseline, 47% at 3 months and 49% at 6 months. In the usual care group, these proportions were 31%, 39% and 41% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this rate for gross movement between these groups at 3 months (aOR 1.4, 98.33% CI 0.8 to 2.6) or 6 months (aOR 1.5, 98.33% CI 0.8 to 2.9) (see Table 34).
In the EULT group, the proportion of respondents who could complete at least one task on the grasp subscale was 22% at baseline, 35% at 3 months and 34% at 6 months. For the usual care group, these proportions were 17%, 27% and 31% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for grasp between these groups at 3 months (aOR 1.6, 98.33% CI 0.8 to 3.4) or 6 months (aOR 1.0, 98.33% CI 0.5 to 2.2) (see Table 34).
In the EULT group, the proportion of respondents who could complete at least one task on the grip subscale was 22% at baseline, 39% at 3 months and 41% at 6 months. In the usual care group, these proportions were 22%, 31% and 35% at baseline, 3 months and 6 months, respectively. There was evidence of a difference in the ARAT grip subscale at 3 months (aOR 2.4, 98.33% CI 1.1 to 5.1); however, this was not present at 6 months (aOR 1.8, 98.33% CI 0.8 to 3.7) (see Table 34).
In the EULT group, the proportion of respondents who could complete at least one task on the pinch subscale was 12% at baseline, 25% at 3 months and 27% at 6 months. In the usual care group, these proportions were 12%, 21% and 26% at baseline, 3 months and 6 months, respectively. There was little evidence of a difference in this proportion for pinch in the EULT group compared with the usual care group at 3 months (aOR 1.6, 98.33% CI 0.8 to 3.3) or at 6 months (aOR 1.1, 98.33% CI 0.6 to 2.3) (see Table 34).
Appendix 4 Masking data
| Did you know the participant’s treatment group? | Robot-assisted training (N = 239) | EULT (N = 246) | Usual care (N = 223) | Overall (N = 708) |
|---|---|---|---|---|
| n | 233 | 236 | 207 | 676 |
| No, n (%) | 183 (78.5) | 210 (89.0) | 182 (87.9) | 575 (85.1) |
| Yes, n (%) | 50 (21.5) | 26 (11.0) | 25 (12.1) | 101 (14.9) |
| Question | Robot-assisted training (N = 50) | EULT (N = 26) | Usual care (N = 25) | Overall (N = 101) |
|---|---|---|---|---|
| Which group did you think they were in? (n) | 47 | 23 | 24 | 94 |
| Robot-assisted training, n (%) | 44 (94) | 1 (4) | 0 (0) | 45 (48) |
| EULT, n (%) | 1 (2) | 21 (91) | 3 (13) | 25 (27) |
| Usual care, n (%) | 2 (4) | 1 (4) | 21 (88) | 24 (26) |
| Did the group given match the patient’s actual group? (n) | 47 | 23 | 24 | 94 |
| No, n (%) | 3 (6) | 2 (9) | 3 (13) | 8 (9) |
| Yes, n (%) | 44 (94) | 21 (91) | 21 (88) | 86 (91) |
| Was treatment known before the outcome assessment? (n) | 50 | 26 | 25 | 101 |
| No, n (%) | 16 (32.0) | 11 (42.0) | 16 (64.0) | 43 (42.6) |
| Yes, n (%) | 34 (68.0) | 15 (58.0) | 9 (36.0) | 58 (57.4) |
| Did you know the treatment group before starting the outcome assessment because you werea (n) | ||||
| Involved in randomisation | 34 | 15 | 9 | 58 |
| No, n (%) | 24 (71) | 3 (20) | 2 (22) | 29 (50) |
| Yes, n (%) | 10 (29) | 12 (80) | 7 (78) | 29 (50) |
| Involved in provision of robot-assisted training or EULT (n) | 34 | 15 | 9 | 58 |
| No, n (%) | 13 (38) | 15 (100) | 7 (78) | 35 (60) |
| Yes, n (%) | 21 (62) | 0 (0) | 2 (22) | 23 (40) |
| Involved in/aware of treatment discussions (n) | 34 | 15 | 9 | 58 |
| No, n (%) | 22 (65) | 3 (20) | 7 (78) | 32 (55) |
| Yes, n (%) | 12 (35) | 12 (80) | 2 (22) | 26 (45) |
| Involved in arranging transport (n) | 34 | 15 | 9 | 58 |
| No, n (%) | 33 (97) | 14 (93) | 9 (100) | 56 (97) |
| Yes, n (%) | 1 (3) | 1 (7) | 0 (0) | 2 (3) |
| Other (n) | 34 | 15 | 9 | 58 |
| No, n (%) | 32 (94) | 13 (87) | 9 (100) | 54 (93) |
| Yes, n (%) | 2 (6) | 2 (13) | 0 (0) | 4 (7) |
| Did you become aware of the treatment group during the outcome assessment becausea | ||||
| The participant made a comment (n) | 16 | 11 | 16 | 43 |
| Yes, n (%) | 16 (100) | 11 (100) | 16 (100) | 43 (100) |
| Other (n) | 50 | 25 | 25 | 100 |
| No, n (%) | 50 (100) | 25 (100) | 25 (100) | 100 (100) |
| Did you know the participant’s treatment group? | Robot-assisted training (N = 234) | EULT (N = 236) | Usual care (N = 214) | Overall (N = 684) |
|---|---|---|---|---|
| n | 223 | 220 | 190 | 633 |
| No, n (%) | 184 (82.5) | 195 (88.6) | 166 (87.4) | 545 (86.1) |
| Yes, n (%) | 39 (17.5) | 25 (11.4) | 24 (12.6) | 88 (13.9) |
| Question | Robot-assisted training (N = 39) | EULT (N = 25) | Usual care (N = 24) | Overall (N = 88) |
|---|---|---|---|---|
| Which group were they in? (n) | 39 | 24 | 24 | 87 |
| Robot-assisted training, n (%) | 36 (92) | 3 (13) | 0 (0) | 39 (45) |
| EULT, n (%) | 3 (8) | 19 (79) | 2 (8) | 24 (28) |
| Usual care, n (%) | 0 (0) | 2 (8) | 22 (92) | 24 (28) |
| Does the group given match the patient’s group? (n) | 39 | 24 | 24 | 87 |
| No, n (%) | 3 (8) | 5 (21) | 2 (8) | 10 (11) |
| Yes, n (%) | 36 (92) | 19 (79) | 22 (92) | 77 (89) |
| Was treatment known before the outcome assessment? (n) | 39 | 25 | 24 | 88 |
| No, n (%) | 17 (44) | 10 (40) | 9 (38) | 36 (41) |
| Yes, n (%) | 22 (56) | 15 (60) | 15 (63) | 52 (59) |
| Did you know the treatment group before starting the outcome assessment because you werea | ||||
| Involved in randomisation (n) | 22 | 15 | 15 | 52 |
| No, n (%) | 13 (59) | 4 (27) | 3 (20) | 20 (38) |
| Yes, n (%) | 9 (41) | 11 (73) | 12 (80) | 32 (62) |
| Involved in provision of robot-assisted training or EULT (n) | 22 | 15 | 15 | 52 |
| No, n (%) | 9 (41) | 11 (73) | 15 (100) | 35 (67) |
| Yes, n (%) | 13 (59) | 4 (27) | 0 (0) | 17 (33) |
| Involved in/aware of treatment discussions (n) | 22 | 15 | 15 | 52 |
| No, n (%) | 12 (55) | 4 (27) | 12 (80) | 28 (54) |
| Yes, n (%) | 10 (45) | 11 (73) | 3 (20) | 24 (46) |
| Involved in arranging transport (n) | 22 | 15 | 15 | 52 |
| No, n (%) | 22 (100) | 15 (100) | 14 (93) | 51 (98) |
| Yes, n (%) | 0 (0) | 0 (0) | 1 (7) | 1 (2) |
| Other (n) | 22 | 15 | 15 | 52 |
| No, n (%) | 19 (86) | 12 (80) | 13 (87) | 44 (85) |
| Yes, n (%) | 3 (14) | 3 (20) | 2 (13) | 8 (15) |
| Did you become aware of the treatment group during the outcome assessment becausea | ||||
| The participant made a comment (n) | 17 | 10 | 9 | 36 |
| No, n (%) | 1 (6) | 0 (0) | 0 (0) | 1 (3) |
| Yes, n (%) | 16 (94) | 10 (100) | 9 (100) | 35 (97) |
| Other (n) | 38 | 25 | 24 | 87 |
| No, n (%) | 38 (100) | 25 (100) | 24 (100) | 87 (100) |
Appendix 5 Patient safety data
| Reason | Participants experiencing SAEs, n (%) | ||
|---|---|---|---|
| Robot-assisted training (N = 43) | EULT (N = 42) | Usual care (N = 29) | |
| Event resulted in deatha | 1 (2.3) | 4 (9.5) | 1 (3.4) |
| Event was life-threatening | 3 (7.0) | 0 (0.0) | 0 (0.0) |
| Event resulted in admission to hospital or prolongation of hospitalisation | 32 (74.4) | 37 (88.1) | 25 (86.2) |
| Event resulted in persistent or significant disability or incapacity | 1 (2.3) | 0 (0.0) | 3 (10.3) |
| Event was ‘otherwise considered significant’ by the investigator | 6 (14.0) | 1 (2.4) | 0 (0.0) |
| SAE | Robot-assisted training | EULT | Usual care |
|---|---|---|---|
| Neurological | 0 | 1 | 1 |
| Glioblastoma multiformea | 0 | 0 | 1 |
| New strokea | 0 | 1 | 0 |
| Respiratory | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Urinary tract | 0 | 2 | 0 |
| Metastatic renal carcinoma | 0 | 1 | 0 |
| Urosepsis | 0 | 1 | 0 |
| Miscellaneous | 1 | 0 | 0 |
| Suicide | 1 | 0 | 0 |
| Total | 1 | 4 | 1 |
| SAE | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|
| Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | |
| Cardiovascular | 5 | 5 | 2 | 2 | 0 | 0 |
| Aortic aneurysm rupture | 1 | 0 | 0 | |||
| Elective carotid endarterectomy | 0 | 1 | 0 | |||
| Hypotension | 1 | 0 | 0 | |||
| Non-ST-elevation myocardial infarction | 1 | 0 | 0 | |||
| Paroxysmal atrial fibrillation | 1 | 0 | 0 | |||
| Possible angina | 0 | 1 | 0 | |||
| Vasovagal collapse | 1 | 0 | 0 | |||
| Endocrine | 0 | 0 | 1 | 1 | 1 | 1 |
| Amiodarone-induced thyrotoxicosis | 0 | 1 | 0 | |||
| Hyperglycaemia | 0 | 0 | 1 | |||
| Ear, nose and throat | 0 | 0 | 1 | 1 | 0 | 0 |
| Elective vocal cord medialisation | 0 | 1 | 0 | |||
| Gastrointestinal | 4 | 4 | 6 | 7 | 1 | 1 |
| Bleeding oesophageal ulcers | 0 | 1 | 0 | |||
| Elective endoscopy for diarrhoea | 1 | 0 | 0 | |||
| Elective hernia repair | 1 | 0 | 0 | |||
| Elective transoesophageal echo | 0 | 0 | 1 | |||
| Gallstone pancreatitis | 0 | 2 | 0 | |||
| Gallstones | 0 | 1 | 0 | |||
| Gastritis | 0 | 1 | 0 | |||
| Pancreatitis | 1 | 0 | 0 | |||
| Perirectal bleeding | 0 | 1 | 0 | |||
| Small-bowel obstruction | 0 | 1 | 0 | |||
| Typhoid | 1 | 0 | 0 | |||
| Gynaecological | 0 | 0 | 1 | 1 | 0 | 0 |
| Elective hysteroscopy | 0 | 1 | 0 | |||
| Musculoskeletal | 4 | 4 | 6 | 6 | 3 | 3 |
| Arthritis | 0 | 1 | 0 | |||
| Elective toe fusion | 1 | 0 | 0 | |||
| Fracture – clavicle | 0 | 0 | 1 | |||
| Fracture – humerus | 1 | 0 | 0 | |||
| Fracture – neck of femur | 0 | 2 | 1 | |||
| Fracture – rib | 0 | 1 | 0 | |||
| Fracture – wrist | 1 | 0 | 0 | |||
| Lumbar L3 or L4 disc prolapse | 1 | 0 | 0 | |||
| Muscle spasm – leg/back | 0 | 1 | 0 | |||
| Prosthetic hip replacement | 0 | 1 | 0 | |||
| Worsening rheumatoid arthritis | 0 | 0 | 1 | |||
| Neurological | 14 | 14 | 5 | 5 | 7 | 7 |
| Decompensation of stroke | 0 | 1 | 1 | |||
| Elective cranioplasty | 1 | 0 | 0 | |||
| Headache due to intracranial air from cranioplasty | 1 | 0 | 0 | |||
| Likely new stroke | 0 | 0 | 1 | |||
| New stroke | 2 | 1 | 1 | |||
| Non-convulsive status epilepticus | 1 | 0 | 0 | |||
| Seizure | 8 | 3 | 3 | |||
| Traumatic left subdural haematoma | 1 | 0 | 0 | |||
| Worsening stroke symptoms from focal seizure | 0 | 0 | 1 | |||
| Respiratory | 4 | 4 | 3 | 3 | 6 | 7 |
| Bronchitis | 0 | 1 | 0 | |||
| Elective admission for investigation of sleep apnoea | 1 | 0 | 0 | |||
| Influenza | 0 | 0 | 1 | |||
| Parapneumonic effusion | 0 | 0 | 1 | |||
| Pneumonia | 2 | 0 | 4 | |||
| Pulmonary emboli | 1 | 2 | 0 | |||
| Pulmonary oedema | 0 | 0 | 1 | |||
| Urinary tract | 3 | 3 | 3 | 4 | 3 | 3 |
| Elective biopsy of bladder lesion | 0 | 0 | 1 | |||
| Epididymo-orchitis | 0 | 0 | 1 | |||
| Hyperkalaemia and renal acidosis | 0 | 1 | 0 | |||
| Urinary retention | 0 | 1 | 1 | |||
| Urinary tract infection/urosepsis | 3 | 2 | 0 | |||
| Miscellaneous | 8 | 8 | 8 | 8 | 5 | 6 |
| Abdominal pain (cause unknown) | 1 | 0 | 0 | |||
| Acute kidney injury (cause unknown) | 1 | 0 | 0 | |||
| Deep-vein thrombosis and chest infection | 0 | 1 | 0 | |||
| Diarrhoea (cause unknown) | 0 | 1 | 1 | |||
| Diarrhoea and acute kidney injury (cause unknown) | 0 | 0 | 1 | |||
| Diarrhoea and nausea secondary to pembrolizumab | 0 | 0 | 1 | |||
| Elective axillary lipoma excision | 0 | 0 | 1 | |||
| Infected cranioplasty | 0 | 0 | 1 | |||
| Fall (mechanical) | 1 | 0 | 0 | |||
| Fall (possible post stroke seizure) | 0 | 1 | 0 | |||
| Head injury | 1 | 0 | 0 | |||
| Infected foot following injury | 0 | 1 | 0 | |||
| Intoxication and pneumonia | 0 | 1 | 0 | |||
| Likely viral illness | 1 | 0 | 0 | |||
| Pneumonia and seizure | 0 | 0 | 1 | |||
| Pyrexia (cause unknown) | 1 | 0 | 0 | |||
| Scald/burn | 0 | 1 | 0 | |||
| Transient difficulty walking (cause unknown) | 0 | 1 | 0 | |||
| Urosepsis and infective endocarditis | 1 | 0 | 0 | |||
| Vertigo | 0 | 1 | 0 | |||
| Worsening stroke symptoms (cause unknown) | 1 | 0 | 0 | |||
| Number of adverse events per participant | Participants, n (%) | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | |||||
| Robot-assisted training (N = 233) | EULT (N = 236) | Usual care (N = 207) | Robot-assisted training (N = 223) | EULT (N = 222) | Usual care (N = 190) | |
| 0 | 195 (83.7) | 199 (84.3) | 172 (83.1) | 181 (81.2) | 176 (79.3) | 163 (85.8) |
| 1 | 33 (14.2) | 34 (14.4) | 32 (15.5) | 40 (17.9) | 41 (18.5) | 23 (12.1) |
| 2 | 3 (1.3) | 3 (1.3) | 3 (1.4) | 2 (0.9) | 3 (1.4) | 4 (2.1) |
| 3 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.9) | 0 (0.0) |
| 4 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total events (n) | 46 | 40 | 38 | 44 | 53 | 31 |
At 3 months, 38 out of 233 (16.3%) participants in the robot-assisted training group and 37 out of 236 (15.7%) participants in the EULT group in whom an assessment was carried out reported at least one non-serious adverse event. For the usual care group, this was 35 out of 207 (16.9%) participants. The difference in proportions between the robot-assisted training and usual care groups was –1% (98.33% CI –10% to 8%); for the EULT and usual care groups, the difference was –1% (98.33% CI –10% to 8%); and for the robot-assisted training and EULT groups, the difference was 1% (98.33% CI –8% to 9%). There was little evidence of a difference in the proportion of participants with at least one adverse event between the pairs of groups. The median number of adverse events was 0 (IQR 0–0). The p-values from the Mann–Whitney U-tests were 0.9 when comparing robot-assisted training with usual care, 0.7 when comparing EULT with usual care and 0.8 when comparing robot-assisted training with EULT, which indicates that there was little evidence of a difference between the pairs of groups.
At 6 months, 42 out of 223 (18.8%) participants in the robot-assisted training group and 46 out of 222 (20.7%) participants in the EULT group in whom an assessment was carried out reported at least one non-serious adverse event. For the usual care group, this was 27 out of 190 (14.2%) participants. The difference in proportions between the robot-assisted training and usual care groups was 5% (98.33% CI –5% to 14%); for the EULT and usual care groups, the difference was 7% (98.33% CI –3% to 16%); and for the robot-assisted training and EULT groups, the difference was –2% (98.33% CI –11% to 8%). There was little evidence of a difference in the proportion of participants with at least one adverse event between the pairs of groups. The median number of adverse events was 0 (IQR 0–0) for all groups. The p-values from the Mann–Whitney U-tests were 0.2 when comparing robot-assisted training with usual care, 0.1 when comparing EULT with usual care and 0.6 when comparing robot-assisted training with EULT, which indicate little evidence of a difference between the pairs of groups.
| Adverse event | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|
| Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | |
| Cardiovascular | 0 | 0 | 0 | 0 | 2 | 2 |
| Heart palpitations | 0 | 0 | 1 | |||
| Pacemaker fitting | 0 | 0 | 1 | |||
| Dental | 1 | 1 | 0 | 0 | 0 | 0 |
| Tooth infection | 1 | 0 | 0 | |||
| Dermatological | 1 | 1 | 0 | 0 | 3 | 3 |
| Blisters on foot | 0 | 0 | 1 | |||
| Cyst on leg | 0 | 0 | 1 | |||
| Eczema | 1 | 0 | 0 | |||
| Leg ulcer | 0 | 0 | 1 | |||
| Endocrine | 2 | 2 | 2 | 2 | 1 | 1 |
| Diabetes mellitus | 2 | 1 | 1 | |||
| Hyperglycaemia | 0 | 1 | 0 | |||
| Ear, nose and throat | 1 | 1 | 0 | 0 | 2 | 2 |
| Ear infection | 1 | 0 | 0 | |||
| Oral thrush | 0 | 0 | 1 | |||
| Possible ear infection | 0 | 0 | 1 | |||
| Eye | 0 | 0 | 1 | 1 | 1 | 1 |
| Problems with eyes | 0 | 1 | 0 | |||
| Stye removal | 0 | 0 | 1 | |||
| Gastrointestinal | 3 | 3 | 5 | 5 | 2 | 2 |
| Abdominal pain and diarrhoea | 0 | 1 | 0 | |||
| Abnormal liver function | 0 | 1 | 0 | |||
| Constipation | 1 | 0 | 0 | |||
| Diarrhoea | 0 | 0 | 2 | |||
| Hernia | 0 | 2 | 0 | |||
| Infection at percutaneous endoscopic gastrostomy | 0 | 1 | 0 | |||
| Reflux | 1 | 0 | 0 | |||
| Scope for assessment of banding | 1 | 0 | 0 | |||
| Gynaecological | 0 | 0 | 1 | 1 | 0 | 0 |
| Fibroid | 0 | 1 | 0 | |||
| Musculoskeletal | 9 | 9 | 5 | 6 | 5 | 5 |
| Arthritis – knee | 0 | 0 | 1 | |||
| Foot problems | 1 | 0 | 0 | |||
| Fracture – ankle | 0 | 1 | 0 | |||
| Fracture – foot | 0 | 1 | 0 | |||
| Fracture – shoulder | 1 | 1 | 0 | |||
| Frozen shoulder | 3 | 1 | 0 | |||
| Osteoporosis | 1 | 0 | 0 | |||
| Podiatry referral | 1 | 0 | 0 | |||
| Possible arthritis | 0 | 0 | 1 | |||
| Shoulder pain | 2 | 1 | 1 | |||
| Tendonitis in arm | 0 | 0 | 1 | |||
| Trapped nerves in shoulder | 0 | 0 | 1 | |||
| Upper limb pain | 0 | 1 | 0 | |||
| Neurological | 1 | 1 | 5 | 5 | 5 | 5 |
| Memory loss | 0 | 0 | 1 | |||
| Neuropathic pain | 0 | 1 | 0 | |||
| Seizure(s) | 1 | 4 | 4 | |||
| Psychiatric | 2 | 2 | 3 | 3 | 2 | 2 |
| Depression | 2 | 3 | 1 | |||
| Panic attacks | 0 | 0 | 1 | |||
| Respiratory | 3 | 3 | 6 | 6 | 2 | 2 |
| Chest infection | 2 | 5 | 2 | |||
| Possible new diagnosis of chronic obstructive pulmonary disease | 0 | 1 | 0 | |||
| Cough | 1 | 0 | 0 | |||
| Urinary tract | 3 | 3 | 1 | 1 | 5 | 5 |
| Bladder infections | 0 | 0 | 1 | |||
| Haematuria | 1 | 0 | 0 | |||
| Incontinence | 0 | 0 | 2 | |||
| Urinary frequency | 0 | 0 | 1 | |||
| Urinary retention | 1 | 0 | 0 | |||
| Urinary tract infection | 1 | 1 | 0 | |||
| Water retention | 0 | 0 | 1 | |||
| Miscellaneous | 19 | 20 | 10 | 10 | 8 | 8 |
| Abscess on back | 1 | 0 | 0 | |||
| Allergic reaction | 0 | 1 | 0 | |||
| Blackout | 1 | 0 | 0 | |||
| Bone flap reinserted (on head) | 1 | 0 | 0 | |||
| Commenced on new medication for blood pressure and bladder control | 1 | 0 | 0 | |||
| Change in drugs | 0 | 0 | 1 | |||
| Curling toes | 1 | 0 | 0 | |||
| Dog bite | 0 | 0 | 1 | |||
| Double vision | 1 | 0 | 0 | |||
| Drug side effects | 2 | 1 | 0 | |||
| Fall | 6 | 2 | 2 | |||
| High cholesterol | 0 | 0 | 1 | |||
| Joint/muscle/back pain | 2 | 3 | 0 | |||
| Lightheaded | 0 | 1 | 0 | |||
| No details provided | 0 | 0 | 1 | |||
| Swallowing problems | 0 | 1 | 1 | |||
| Swelling of foot | 0 | 0 | 1 | |||
| Swelling of legs | 1 | 0 | 0 | |||
| Swollen ankles | 2 | 0 | 0 | |||
| Toenail removal | 1 | 0 | 0 | |||
| Vertigo | 0 | 1 | 0 | |||
| Adverse event | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|
| Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | Participants who experienced the event (n) | Total number of events | |
| Cardiovascular | 2 | 2 | 0 | 0 | 2 | 2 |
| High blood pressure | 0 | 0 | 1 | |||
| Hypotension | 2 | 0 | 0 | |||
| Palpitations | 0 | 0 | 1 | |||
| Dermatological | 2 | 2 | 6 | 6 | 2 | 2 |
| Eczema | 0 | 1 | 0 | |||
| Forehead lesion (cause unknown) | 0 | 0 | 1 | |||
| Itch | 1 | 1 | 0 | |||
| Infected toe | 0 | 0 | 0 | |||
| Leg rash | 0 | 1 | 0 | |||
| Leg ulcers | 0 | 2 | 0 | |||
| Lesion on face removed | 0 | 1 | 0 | |||
| Rash | 1 | 0 | 1 | |||
| Ear | 0 | 0 | 2 | 2 | 0 | 0 |
| Ear pain | 0 | 1 | 0 | |||
| Ears syringed | 0 | 1 | 0 | |||
| Endocrine | 1 | 1 | 2 | 2 | 0 | 0 |
| Diabetes mellitus | 1 | 0 | 0 | |||
| Menopause symptoms | 0 | 1 | 0 | |||
| Thyroid eye disease | 0 | 1 | 0 | |||
| Ear, nose and throat | 1 | 1 | 1 | 1 | 0 | 0 |
| Oral thrush | 0 | 1 | 0 | |||
| Potential throat cancer | 1 | 0 | 0 | |||
| Eye | 0 | 0 | 0 | 0 | 2 | 2 |
| Cataracts | 0 | 0 | 1 | |||
| Ophthalmologist review – no further information | 0 | 0 | 1 | |||
| Gastrointestinal | 5 | 5 | 3 | 3 | 2 | 2 |
| Constipation | 1 | 0 | 0 | |||
| Diarrhoea | 0 | 0 | 1 | |||
| Diarrhoea and vomiting | 0 | 1 | 0 | |||
| Gastroscopy | 1 | 0 | 0 | |||
| Haemorrhoids | 1 | 0 | 0 | |||
| Hernia | 1 | 1 | 0 | |||
| Irritable bowel syndrome | 0 | 0 | 1 | |||
| Piles | 1 | 0 | 0 | |||
| Rectal bleeding | 0 | 1 | 0 | |||
| Haematological | 1 | 1 | 1 | 1 | 0 | 0 |
| Low blood count | 1 | 1 | 0 | |||
| Musculoskeletal | 5 | 5 | 7 | 7 | 5 | 5 |
| Arthritis | 1 | 0 | 1 | |||
| Fracture – fingers | 1 | 1 | 0 | |||
| Fracture – foot | 1 | 0 | 1 | |||
| Fracture – humerus | 0 | 1 | 0 | |||
| Fracture – knuckles | 0 | 1 | 0 | |||
| Fracture – leg | 0 | 0 | 1 | |||
| Fracture – rib | 0 | 1 | 0 | |||
| Fracture – wrist | 1 | 0 | 0 | |||
| Frozen shoulder | 0 | 2 | 0 | |||
| Injured hand in car door | 1 | 0 | 0 | |||
| Orthotic appointments | 0 | 0 | 1 | |||
| Polymyalgia | 0 | 1 | 0 | |||
| Pre-operative assessment for knee replacement | 0 | 0 | 1 | |||
| Neurological | 5 | 5 | 5 | 5 | 4 | 4 |
| Foot drop | 1 | 0 | 0 | |||
| Migraine | 0 | 1 | 0 | |||
| Nerve impingement | 0 | 1 | 0 | |||
| Neuropathy | 0 | 0 | 1 | |||
| Pins and needles in affected arm | 1 | 0 | 0 | |||
| Seizure(s) | 3 | 3 | 3 | |||
| Psychiatric | 2 | 2 | 2 | 2 | 1 | 1 |
| Depression | 1 | 1 | 0 | |||
| Panic attacks | 0 | 1 | 0 | |||
| Psychiatric assessment – no further information | 1 | 0 | 0 | |||
| Stress | 0 | 0 | 1 | |||
| Respiratory | 2 | 2 | 6 | 6 | 1 | 1 |
| Asbestosis | 1 | 0 | 0 | |||
| Chest infection | 1 | 1 | 0 | |||
| Chronic obstructive pulmonary disease | 0 | 2 | 0 | |||
| Cough | 0 | 0 | 1 | |||
| Coughing blood | 0 | 1 | 0 | |||
| Pneumonia | 0 | 1 | 0 | |||
| Shortness of breath | 0 | 1 | 0 | |||
| Urinary tract | 3 | 3 | 4 | 4 | 0 | 0 |
| Incontinence | 1 | 0 | 0 | |||
| Urinary tract infection | 2 | 4 | 0 | |||
| Miscellaneous | 15 | 15 | 13 | 14 | 11 | 12 |
| Bloating and uncomfortable | 0 | 0 | 1 | |||
| Chest pain (no cause documented) | 1 | 0 | 0 | |||
| Collapse | 0 | 1 | 0 | |||
| Cut finger | 1 | 0 | 0 | |||
| Dizziness | 0 | 1 | 0 | |||
| Double vision | 1 | 0 | 0 | |||
| Drug side effects | 1 | 0 | 0 | |||
| Fall | 3 | 2 | 4 | |||
| Headaches | 1 | 0 | 0 | |||
| Head injury | 1 | 1 | 0 | |||
| Ingrowing toenail | 2 | 0 | 1 | |||
| Joint/muscle/back pain | 2 | 6 | 5 | |||
| No details given | 0 | 1 | 0 | |||
| Restless leg syndrome | 0 | 1 | 0 | |||
| Swallowing problems | 1 | 0 | 1 | |||
| Swelling of legs | 0 | 1 | 0 | |||
| Unwell (no further details) | 1 | 0 | 0 | |||
Appendix 6 Randomisation group data
| Reason for missed session | Robot-assisted training | EULT | ||
|---|---|---|---|---|
| Number of participants | Number of missed sessions | Number of participants | Number of missed sessions | |
| Adverse weather conditions | 0 | 0 | 3 | 5 |
| Did not attend (reason unknown) | 23 | 132 | 23 | 96 |
| Fatigue | 3 | 15 | 5 | 14 |
| Participant being discharged from hospital at time of therapy | 1 | 1 | 6 | 9 |
| Participant at work | 0 | 0 | 3 | 4 |
| Participant cancelled session (reason unknown) | 0 | 0 | 7 | 27 |
| Participant cancelled session (family emergency) | 2 | 2 | 2 | 5 |
| Participant could not tolerate three sessions per week | 3 | 31 | 1 | 23 |
| Participant could not tolerate the wrist robot | 3 | 41 | 0 | 0 |
| Participant died | 1 | 32 | 0 | 0 |
| Participant forgot appointment | 2 | 2 | 9 | 10 |
| Participant had another appointment | 29 | 69 | 54 | 115 |
| Participant moved area | 1 | 8 | 1 | 35 |
| Participant on holiday | 6 | 15 | 21 | 117 |
| Participant overslept | 0 | 0 | 2 | 3 |
| Participant unhappy with randomisation group | 0 | 0 | 1 | 31 |
| Participant unwell | 52 | 341 | 67 | 406 |
| Participant unable to leave house because of access problems | 1 | 1 | 2 | 26 |
| Participant working away from home | 0 | 0 | 2 | 50 |
| Personal circumstances | 5 | 54 | 12 | 88 |
| Problems with transport to appointment | 14 | 22 | 32 | 70 |
| Robot out of order | 1 | 1 | 0 | 0 |
| Shoulder pain | 5 | 62 | 2 | 4 |
| Therapy slot unavailable (e.g. bank holiday) | 7 | 14 | 20 | 27 |
| Withdrew | 15 | 429 | 11 | 282 |
| Variable | Number of shoulder–elbow movement attempts per training session | Total number of shoulder–elbow movement attempts | Number of shoulder–elbow movement attempts per training session (no hand attachment) | Total number of shoulder–elbow movement attempts (no hand attachment) | Number of wrist movement attempts per training session | Total number of wrist movement attempts | Overall total number of movement attempts per training session | Overall total number of movement attempts |
|---|---|---|---|---|---|---|---|---|
| Participants (n) | 156 | 156 | 98 | 98 | 243 | 243 | 255 | 255 |
| Median (IQR) | 840 (672–1024) | 15,002 (13,065–17,007) | 804 (488–1024) | 11,141 (6751–14,583) | 796 (668–1132) | 13,460 (11,236–16,220) | 808 (668–1040) | 27,407 (22,900–32,187) |
| Target movement attempts achieved, n/N (%) | 840/1114 (75) | 15,002/20,060 (75) | 804/1083 (74) | 11,141/19,496 (57) | 796/1066 (75) | 13,460/19,196 (70) | 808/1090 (74) | 27,407/39,256 (70) |
| Baseline ARAT score (n participants) | Time since stroke (n participants) | Value | Number of assisted shoulder-elbow movement attempts | Number of unassisted shoulder-elbow movement attempts | Total number of shoulder-elbow movement attempts | Number of assisted wrist movement attempts | Number of unassisted wrist movement attempts | Total number of wrist movement attempts | Overall number of assisted movement attempts | Overall number of unassisted movement attempts | Overall total number of movement attempts |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARAT score of 0–7 (n = 177) | ≤ 3 months (n = 38) | n | 37 | 37 | 37 | 35 | 35 | 35 | 38 | 38 | 38 |
| Median (IQR) | 11,666 (7056–14,025) | 1682 (1012–2119) | 13,520 (8230–16,166) | 11,640 (8672–14,200) | 1084 (592–1768) | 12,988 (9508–15,536) | 21,475 (16,344–27,794) | 2522 (1583–3795) | 24,436 (18,367–31,228) | ||
| > 3–12 months (n = 68) | n | 68 | 68 | 68 | 66 | 66 | 66 | 68 | 68 | 68 | |
| Median (IQR) | 10,734 (9016–13,065) | 1775 (1390–2124) | 12,378 (10,535–14,952) | 11,296 (9578–13,094) | 1648 (1014–1873) | 12,948 (10,845–14,809) | 21,660 (19,443–25,194) | 3333 (2221–3951) | 25,386 (21,710–28,998) | ||
| > 12 months (n = 71) | n | 71 | 71 | 71 | 69 | 69 | 69 | 71 | 71 | 71 | |
| Median (IQR) | 11,538 (9888–12,960) | 1910 (1608–2288) | 13,719 (11,566–15,066) | 10,760 (9672–12,080) | 1672 (1386–1844) | 12,341 (10,982–13,820) | 22,016 (20,020–24,776) | 3516 (3024–3995) | 25,628 (23,108–28,836) | ||
| ARAT score of 8–19 (n = 31) | ≤ 3 months (n = 6) | n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Median (IQR) | 14,681 (10,173–16,268) | 2264 (1656–2388) | 17,223 (12,193–18,790) | 17,688 (13,558–18,640) | 2106 (775–2216) | 19,794 (14,333–20,935) | 32,881 (25,873–33,426) | 4761 (2561–4831) | 37,411 (29,219–37,958) | ||
| > 3–12 months (n = 14) | n | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
| Median (IQR) | 13,048 (11,760–14,364) | 2143 (1760–2492) | 15,442 (13,733–16,324) | 14,192 (11,944–16,960) | 1778 (1521–1984) | 15,966 (13,432–19,142) | 28,060 (23,686–31,710) | 3995 (3462–4404) | 32,028 (27,341–35,827) | ||
| > 12 months (n = 11) | n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | |
| Median (IQR) | 12,880 (10,722–14,497) | 2316 (1956–2420) | 15,285 (13,064–16,917) | 13,560 (7552–16,380) | 1928 (1508–2088) | 15,592 (8788–18,364) | 27,120 (22,754–29,260) | 4252 (3968–4420) | 31,540 (26,966–33,593) | ||
| ARAT score of 20–39 (n = 47) | ≤ 3 months (n = 13) | n | 12 | 13 | 13 | 10 | 10 | 10 | 12 | 13 | 13 |
| Median (IQR) | 14,747 (10,804–16,772) | 2234 (1304–2596) | 17,101 (10,712–19,152) | 16,968 (14,006–19,848) | 1744 (1056–2216) | 19,054 (15,595–21,926) | 30,975 (26,288–35,092) | 3821 (2430–4612) | 33,723 (23,908–38,637) | ||
| > 3–12 months (n = 22) | n | 22 | 22 | 22 | 20 | 20 | 20 | 22 | 22 | 22 | |
| Median (IQR) | 13,560 (10,245–15,553) | 2037 (1612–2511) | 15,744 (11,837–17,628) | 15,316 (12,604–19,274) | 2048 (1545–2171) | 17,388 (14,557–21,267) | 27,980 (22,670–34,418) | 3966 (3049–4677) | 31,909 (26,171–38,463) | ||
| > 12 months (n = 12) | n | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| Median (IQR) | 14,841 (11,870–17,745) | 2176 (1928–2316) | 17,442 (14,138–20,152) | 15,328 (13,138–19,800) | 1998 (1829–2215) | 17,404 (14,981–21,942) | 29,695 (27,638–34,352) | 4365 (3978–4730) | 33,871 (31,556–39,015) |
| Category | Number of goals set in category/total number of goals set (%) | Number of goals achieved in category/number of goals set in category (for which there is achievement data) (%)a | Subcategory | Number of goals set in subcategory/total number of goals set in category (%) | Number of goals achieved in subcategory/number of goals set in subcategory (for which there is achievement data) (%)a | Activity category | Number of goals set in activity category/total number of goals set in subcategory (%) | Number of goals achieved in activity category/number of goals set in activity category (for which there is achievement data) (%)a |
|---|---|---|---|---|---|---|---|---|
| Leisure | 180/2664 (7) | 109/171 (64) | Active recreation | 16/180 (9) | 10/14 (71) | Outings | 0/16 (0) | 0/0 (0) |
| Sports | 16/16 (100) | 10/14 (71) | ||||||
| Travel | 0/16 (0) | 0/0 (0) | ||||||
| Quiet recreation | 71/180 (39) | 41/69 (59) | Crafts | 4/70 (6) | 3/4 (75) | |||
| Hobbies | 63/70 (90) | 35/62 (56) | ||||||
| Reading | 3/70 (4) | 3/3 (100) | ||||||
| Socialisation | 93/180 (52) | 58/88 (66) | Correspondence | 92/93 (99) | 57/87 (66) | |||
| Parties | 0/93 (0) | 0/0 (0) | ||||||
| Telephone calls | 0/93 (0) | 0/0 (0) | ||||||
| Visiting | 1/93 (1) | 1/1 (100) | ||||||
| Productivity | 374/2664 (14) | 211/351 (60) | Household management | 374/374 (100) | 211/351 (60) | Cleaning | 206/374 (55) | 100/189 (53) |
| Cooking | 125/374 (33) | 81/121 (67) | ||||||
| DIY | 11/374 (3) | 9/11 (82) | ||||||
| Laundry | 19/374 (5) | 13/19 (68) | ||||||
| Using light switches | 13/374 (3) | 8/11 (73) | ||||||
| Paid/unpaid work | 0/374 (0) | N/A | Finding/keeping a job | 0/0 (0) | 0/0 (0) | |||
| Volunteering | 0/0 (0) | 0/0 (0) | ||||||
| Play/school | 0/374 (0) | N/A | Homework | 0/0 (0) | 0/0 (0) | |||
| Play skills | 0/0 (0) | 0/0 (0) | ||||||
| Self-care | 1449/2664 (54) | 640/1354 (47) | Community management | 25/1449 (2) | 19/23 (83) | Finances | 19/25 (76) | 16/17 (94) |
| Shopping | 0/25 (0) | 0/0 (0) | ||||||
| Transportation | 6/25 (24) | 3/6 (5) | ||||||
| Functional mobility | 141/1449 (10) | 65/136 (48) | Indoor | 42/141 (30) | 24/40 (60) | |||
| Outdoor | 7/141 (5) | 4/7 (57) | ||||||
| Transfers | 92/141 (65) | 37/89 (42) | ||||||
| Personal care | 1283/1449 (89) | 556/1195 (47) | Bathing | 166/1283 (13) | 79/150 (53) | |||
| Child care | 4/1283 (< 1) | 0/3 (0) | ||||||
| Dressing | 254/1283 (20) | 0/1 (0) | ||||||
| Eating | 622/1283 (48) | 234/587 (40) | ||||||
| Grooming | 74/1283 (6) | 32/69 (46) | ||||||
| Hygiene | 161/1283 (13) | 70/142 (49) | ||||||
| Medication | 1/1283 (< 1) | 0/1 (0) | ||||||
| Other | 661/2664 (25) | 327/625 (52) | Pick up/grasp/reach/place object | 163/661 (25) | 84/155 (54) | N/A | ||
| Range of movement | 413/661 (62) | 196/390 (50) | ||||||
| Upper limb strengthening | 8/661 (1) | 7/7 (100) | ||||||
| Unclassified | 26/661 (4) | 15/24 (63) | ||||||
| Weight-bearing | 51/661 (8) | 25/49 (51) | ||||||
| Baseline ARAT score (n participants) | Time since stroke (n participants) | Number of goals set for each time since stroke subgroup/total number of goals set for ARAT subgroup (%) | Number of goals achieved in each time since stroke subgroup/number of goals set for each time since stroke subgroup (for which there is achievement data) (%)a | Category | Number of goals set in category for that subgroup/total number of goals set for that subgroup (%) | Number of goals achieved in category for that subgroup/number of goals set in category for that subgroup (for which there is achievement data) (%)a |
|---|---|---|---|---|---|---|
| 0–7 (n = 175) | < 3 months (n = 26) | 262/1759 (15) | 175/240 (73) | Leisure | 18/262 (15) | 17/18 (76) |
| Productivity | 22/262 (8) | 15/22 (68) | ||||
| Self-care | 118/262 (45) | 76/107 (71) | ||||
| Other | 104/262 (40) | 71/94 (76) | ||||
| 3–12 months (n = 79) | 823/1759 (47) | 342/781 (44) | Leisure | 27/823 (3) | 11/26 (42) | |
| Productivity | 142/823 (17) | 75/133 (56) | ||||
| Self-care | 480/823 (58) | 178/454 (39) | ||||
| Other | 174/823 (21) | 78/168 (46) | ||||
| > 12 months (n = 70) | 674/1759 (38) | 167/616 (27) | Leisure | 16/674 (2) | 2/14 (14) | |
| Productivity | 91/674 (17) | 29/80 (36) | ||||
| Self-care | 361/674 (54) | 77/332 (23) | ||||
| Other | 206/674 (31) | 59/190 (31) | ||||
| 8–19 (n = 31) | < 3 months (n = 5) | 48/333 (14) | 36/41 (88) | Leisure | 3/48 (6) | 3/3 (100) |
| Productivity | 6/48 (13) | 4/5 (80) | ||||
| Self-care | 24/48 (50) | 16/19 (84) | ||||
| Other | 15/48 (31) | 13/14 (93) | ||||
| 3–12 months (n = 13) | 136/333 (41) | 72/128 (56) | Leisure | 8/136 (6) | 3/7 (43) | |
| Productivity | 10/136 (7) | 8/10 (80) | ||||
| Self-care | 95/136 (70) | 46/89 (52) | ||||
| Other | 23/136 (17) | 15/22 (68) | ||||
| > 12 months (n = 13) | 149/333 (45) | 93/149 (62) | Leisure | 10/149 (7) | 7/10 (70) | |
| Productivity | 19/149 (13) | 16/19 (84) | ||||
| Self-care | 78/149 (52) | 45/78 (58) | ||||
| Other | 42/149 (28) | 25/42 (60) | ||||
| 20–39 (n = 53) | < 3 months (n = 15) | 168/572 (29) | 124/162 (77) | Leisure | 29/168 (17) | 26/27 (96) |
| Productivity | 26/168 (15) | 17/25 (68) | ||||
| Self-care | 77/168 (46) | 54/74 (73) | ||||
| Other | 36/168 (21) | 27/36 (75) | ||||
| 3–12 months (n = 25) | 274/572 (48) | 202/267 (76) | Leisure | 61/274 (22) | 38/59 (64) | |
| Productivity | 48/274 (18) | 39/47 (83) | ||||
| Self-care | 134/274 (49) | 130/104 (80) | ||||
| Other | 31/274 (11) | 21/31 (68) | ||||
| > 12 months (n = 13) | 130/572 (23) | 76/117 (65) | Leisure | 8/130 (6) | 6/8 (75) | |
| Productivity | 10/130 (8) | 8/10 (80) | ||||
| Self-care | 82/130 (63) | 44/71 (62) | ||||
| Other | 30/130 (23) | 18/28 (64) |
| Question | Value | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|---|
| Weeks 1–12 (N = 1896 weeks) (N = 158 participants) | Weeks 13–24 (N = 1416 weeks) (N = 118 participants) | Weeks 1–12 (N = 1908 weeks) (N = 159 participants) | Weeks 13–24 (N = 1356 weeks) (N = 113 participants) | Weeks 1–12 (N = 1536 weeks) (N = 128 participants) | Weeks 13–24 (N = 1368 weeks) (N = 114 participants) | ||
| On how many days per week have participants received therapy for their arm or hand? | n | 1884 weeks (157 participants) | 1412 weeks (110 participants) | 1881 weeks (158 participants) | 1353 weeks (110 participants) | 1529 weeks (127 participants) | 1367 weeks (106 participants) |
| Median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | |
| How much time (minutes) per week has been spent in therapy for their arm or hand? | n | 1884 weeks (157 participants) | 1401 weeks (109 participants) | 1883 weeks (158 participants) | 1350 weeks (110 participants) | 1519 weeks (127 participants) | 1367 weeks (106 participants) |
| Median (IQR) | 0 (0–20) | 0 (0–0) | 0 (0–30) | 0 (0–30) | 0 (0–60) | 0 (0–30) | |
| ‘Other’ therapy | Participants, n (%) | |||||
|---|---|---|---|---|---|---|
| Robot-assisted training | EULT | Usual care | ||||
| Weeks 1–12 (N = 524 weeks) (N = 64 participants) | Weeks 13–24 (N = 313 weeks) (N = 42 participants) | Weeks 1–12 (N = 533 weeks) (N = 75 participants) | Weeks 13–24 (N = 375 weeks) (N = 56 participants) | Weeks 1–12 (N = 645 weeks) (N = 75 participants) | Weeks 13–24 (N = 373 weeks) (N = 49 participants) | |
| Acupuncture | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Aquafit | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Balance work | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (7) | 0 (0) |
| Bobath | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Botulinum toxin injection | 1 (2) | 0 (0) | 1 (1) | 3 (4) | 0 (0) | 1 (1) |
| Compression splint | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Co-ordination | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Core stability | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Driving | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Electrical stimulation | 3 (5) | 2 (3) | 5 (7) | 3 (4) | 6 (8) | 3 (4) |
| Exercise – non-specific | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 2 (3) | 2 (3) |
| Fatigue/cognitive management strategies | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gait re-education | 2 (3) | 0 (0) | 3 (4) | 0 (0) | 2 (3) | 1 (1) |
| Guitar and keyboard practice | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Gym | 2 (3) | 4 (6) | 2 (3) | 0 (0) | 0 (0) | 1 (1) |
| Hand putty | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hydrotherapy | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Knitting | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Massage | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (1) |
| Mirror box | 2 (3) | 0 (0) | 2 (3) | 0 (0) | 2 (3) | 1 (1) |
| Physiotherapy session | 1 (3) | 3 (5) | 2 (3) | 1 (1) | 3 (4) | 1 (1) |
| Playing piano | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Playing tennis | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proprioceptive neuromuscular facilitation | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Putting on ankle brace | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Reaching work | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Reflexology | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| Reiki | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rowing machine and/or pulleys | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Saebo (Saebo, Inc., Charlotte, NC, USA) | 1 (3) | 0 (0) | 2 (3) | 2 (3) | 2 (3) | 3 (4) |
| Sensory work | 2 (3) | 1 (2) | 1 (1) | 1 (1) | 2 (3) | 0 (0) |
| Soft tissue release | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
| Specific muscle strengthening | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Splinting (application) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Splint made | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Strengthening (non-specific) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Suspension therapy | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Swimming | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Technology | 1 (2) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| TheraBand (TheraBand, Akron, OH, USA) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TheraBand and putty | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Uria splint | 3 (5) | 1 (2) | 1 (1) | 1 (1) | 2 (3) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) |
| Various activities (not specified) | 0 (0) | 0 (0) | 3 (4) | 1 (1) | 1 (1) | 3 (4) |
| Visualisation | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Weight-bearing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Writing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| Baseline ARAT score (n participants) | Time since stroke at baseline (n participants) | Participants who, n/N (%) | Number of weeks participants stated that they received therapy for their arm or hand, median (IQR); n participants | On how many days per week did participants receive therapy for their arm or hand, median (IQR); n weeks | How much time (minutes) per week has been spent in therapy for their arm or hand, median (IQR); n weeks | Participants who stated that they had completed self-practice for their arm or hand during the 12-week period, n/N (%) | Number of weeks participants stated that they completed self-practice for their arm or hand, median (IQR); n participants | ||
|---|---|---|---|---|---|---|---|---|---|
| Attended the 3-month assessment | Returned their arm rehabilitation log | Stated that they received therapy for their arm or hand during the 12-week period | |||||||
| 0–7 (n = 526) | < 3 months (n = 105) | 89/105 (85) | 66/89 (74) | 51/66 (49) | 10 (2–12); 66 participants | 1 (0–4); 790 weeks | 40 (0–135); 790 weeks | 60/66 (91) | 12 (10–12); 66 participants |
| 3–12 months (n = 232) | 201/232 (99) | 132/201 (66) | 70/132 (53) | 1 (0–9); 132 participants | 0 (0–1); 1582 weeks | 0 (0–35); 1573 weeks | 123/132 (93) | 12 (11–12); 132 participants | |
| > 12 months (n = 189) | 172/189 (91) | 120/172 (70) | 37/120 (31) | 0 (0–3); 120 participants | 0 (0–0); 1432 weeks | 0 (0–0); 1433 weeks | 109/120 (91) | 12 (9–12); 120 participants | |
| 8–19 (n = 98) | < 3 months (n = 23) | 20/23 (87) | 9/20 (45) | 7/9 (78) | 12 (1–12); 9 participants | 1 (0–4); 108 weeks | 80 (0–180); 108 weeks | 8/9 (89) | 12 (12–12); 9 participants |
| 3–12 months (n = 40) | 34/40 (85) | 18/34 (53) | 6/18 (33) | 0 (0–7); 18 participants | 0 (0–0); 216 weeks | 0 (0–0); 216 weeks | 15/18 (83) | 12 (12–12); 18 participants | |
| > 12 months (n = 35) | 33/35 (94) | 22/33 (66) | 6/22 (27) | 0 (0–3); 22 participants | 0 (0–0); 261 weeks | 0 (0–0); 261 weeks | 19/22 (86) | 12 (8–12); 22 participants | |
| 20–39 (n = 145) | < 3 months (n = 39) | 32/39 (82) | 20/32 (63) | 14/20 (70) | 7 (0–8); 20 participants | 0 (0–2); 222 weeks | 0 (0–71); 220 weeks | 20/20 (100) | 12 (11–12); 20 participants |
| 3–12 months (n = 70) | 61/70 (87) | 34/61 (56) | 20/34 (59) | 2 (0–8); 34 participants | 0 (0–1); 404 weeks | 0 (0–56); 406 weeks | 33/34 (97) | 12 (8–12); 34 participants | |
| > 12 months (n = 36) | 34/36 (94) | 24/34 (71) | 5/24 (21) | 0 (0–0); 24 participants | 0 (0–0); 279 weeks | 0 (0–0); 279 weeks | 21/24 (88) | 11 (4–12); 24 participants | |
| Baseline ARAT score (n participants) | Time since stroke at baseline (n participants) | Participants who, n/N (%) | Number of weeks participants stated that they received therapy for their arm or hand, median (IQR); n participants | On how many days per week did participants receive therapy for their arm or hand, median (IQR); n weeks | How much time (minutes) per week has been spent in therapy for their arm or hand, median (IQR); n weeks | Participants who stated that they had completed self-practice for their arm or hand during the 12-week period, n/N (%) | Number of weeks participants stated that they completed self-practice for their arm or hand, median (IQR); n participants | ||
|---|---|---|---|---|---|---|---|---|---|
| Attended the 6-month assessment | Returned their arm rehabilitation log | Stated that they received therapy for their arm or hand during the 12-week period | |||||||
| 0–7 (n = 526) | < 3 months (n = 105) | 80/105 (76) | 45/80 (56) | 32/45 (71) | 3 (0–7); 45 participants | 0 (0–1); 539 weeks | 0 (0–45); 539 weeks | 41/45 (91) | 12 (8–12); 45 participants |
| 3–12 months (n = 232) | 190/232 (82) | 103/190 (54) | 45/103 (44) | 0 (0–8); 103 participants | 0 (0–1); 1233 weeks | 0 (0–30); 1233 weeks | 98/103 (95) | 12 (11–12); 103 participants | |
| > 12 months (n = 189) | 163/189 (86) | 89/163 (55) | 35/89 (39) | 0 (0–9); 89 participants | 0 (0–1); 1068 weeks | 0 (0–25); 1068 weeks | 79/89 (89) | 12 (7–12); 89 participants | |
| 8–19 (n = 98) | < 3 months (n = 23) | 21/23 (91) | 10/21 (48) | 3/10 (30) | 0 (0–3); 10 participants | 0 (0–0); 120 weeks | 0 (0–0); 120 weeks | 8/10 (80) | 11 (7–12); 10 participants |
| 3–12 months (n = 40) | 33/40 (83) | 19/33 (58) | 6/19 (32) | 0 (0–3); 19 participants | 0 (0–0); 228 weeks | 0 (0–0); 227 weeks | 18/19 (95) | 12 (12–12); 19 participants | |
| > 12 months (n = 35) | 30/35 (86) | 14/30 (47) | 4/14 (29) | 0 (0–6); 14 participants | 0 (0–0); 168 weeks | 0 (0–0); 168 weeks | 14/14 (100) | 12 (8–12); 14 participants | |
| 20–39 (n = 145) | < 3 months (n = 39) | 30/39 (77) | 17/30 (57) | 9/17 (53) | 1 (0–5); 17 participants | 0 (0–0); 204 weeks | 0 (0–0); 204 weeks | 17/17 (100) | 12 (12–12); 17 participants |
| 3–12 months (n = 70) | 57/70 (81) | 29/57 (51) | 14/29 (48) | 0 (0–4); 29 participants | 0 (0–0); 345 weeks | 0 (0–0); 346 weeks | 28/29 (97) | 12 (12–12); 29 participants | |
| > 12 months (n = 36) | 31/36 (86) | 19/31 (61) | 5/19 (26) | 0 (0–4); 19 participants | 0 (0–0); 227 weeks | 0 (0–0); 213 weeks | 18/19 (95) | 12 (9–12); 19 participants | |
| Type of therapy | Baseline ARAT score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–7 (n = 157) | 8–19 (n = 19) | 20–39 (n = 38) | |||||||
| < 3 months (N = 51) | 3–12 months (N = 70) | > 12 months (N = 36) | < 3 months (N = 7) | 3–12 months (N = 6) | > 12 months (N = 6) | < 3 months (N = 13) | 3–12 months (N = 20) | > 12 months (N = 5) | |
| Passive stretching | |||||||||
| Participants who received this type of therapy, n/N (%) | 48/51 (94) | 63/70 (90) | 29/36 (81) | 7/7 (100) | 6/6 (100) | 6/6 (100) | 9/13 (69) | 17/20 (85) | 4/5 (80) |
| Weeks this type of therapy was undertaken, median (IQR) | 10 (4–12) | 7 (2–10) | 6 (1–10) | 10 (4–10) | 5 (1–10) | 7 (4–10) | 1 (0–7) | 3 (1–9) | 5 (2–8) |
| Improving range of motion | |||||||||
| Participants who received this type of therapy, n/N (%) | 49/51 (96) | 59/70 (84) | 25/36 (69) | 6/7 (86) | 5/6 (83) | 5/6 (83) | 9/13 (69) | 17/20 (85) | 4/5 (80) |
| Weeks this type of therapy was undertaken, median (IQR) | 10 (5–12) | 6 (2–10) | 5 (0–9) | 7 (1–12) | 7 (1–10) | 7 (3–10) | 4 (0–8) | 5 (2–8) | 4 (1–7) |
| Functional strengthening | |||||||||
| Participants who received this type of therapy, n/N (%) | 35/51 (69) | 41/70 (59) | 21/36 (58) | 6/7 (86) | 4/6 (67) | 5/6 (83) | 10/13 (77) | 18/20 (90) | 3/5 (60) |
| Weeks this type of therapy was undertaken, median (IQR) | 4 (0–9) | 1 (0–7) | 1 (0–6) | 6 (1–12) | 8 (0–11) | 7 (1–10) | 2 (1–6) | 4 (1–7) | 2 (0–5) |
| Weight-bearing through hand | |||||||||
| Participants who received this type of therapy, n/N (%) | 34/51 (67) | 40/70 (57) | 17/36 (47) | 3/7 (43) | 1/6 (17) | 5/6 (83) | 6/13 (46) | 13/20 (70) | 4/5 (80) |
| Weeks this type of therapy was undertaken, median (IQR) | 3 (0–10) | 1 (0–5) | 0 (0–4) | 0 (0–6) | 0 (0–0) | 6 (2–10) | 0 (0–4) | 2 (0–4) | 3 (1–4) |
| Trunk control | |||||||||
| Participants who received this type of therapy, n/N (%) | 30/51 (59) | 31/70 (44) | 15/36 (42) | 3/7 (43) | 1/6 (17) | 4/6 (67) | 5/13 (38) | 7/20 (35) | 3/5 (60) |
| Weeks this type of therapy was undertaken, median (IQR) | 1 (0–6) | 0 (0–6) | 0 (0–4) | 0 (0–7) | 0 (0–1) | 2 (0–7) | 0 (0–3) | 0 (0–4) | 2 (0–4) |
| Repetitive task-specific practice | |||||||||
| Participants who received this type of therapy, n/N (%) | 36/51 (71) | 37/70 (53) | 22/36 (61) | 6/7 (86) | 2/6 (33) | 4/6 (67) | 10/13 (77) | 13/20 (65) | 3/5 (60) |
| Weeks this type of therapy was undertaken, median (IQR) | 5 (0–10) | 1 (0–5) | 2 (0–9) | 11 (1–12) | 0 (0–5) | 6 (0–9) | 2 (1–7) | 2 (0–6) | 2 (0–3) |
| Personal ADL | |||||||||
| Participants who received this type of therapy, n/N (%) | 30/51 (59) | 29/70 (41) | 21/36 (58) | 4/7 (57) | 3/6 (50) | 3/6 (50) | 10/13 (77) | 12/20 (60) | 2/5 (40) |
| Weeks this type of therapy was undertaken, median (IQR) | 5 (0–12) | 0 (0–3) | 0 (0–2) | 1 (0–12) | 1 (0–1) | 2 (0–7) | 3 (1–6) | 2 (0–3) | 0 (0–5) |
| Extended ADL | |||||||||
| Participants who received this type of therapy, n/N (%) | 24/51 (47) | 16/70 (23) | 6/36 (17) | 2/7 (29) | 2/6 (33) | 3/6 (50) | 6/13 (46) | 8/20 (40) | 2/5 (40) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–5) | 0 (0–0) | 0 (0–0) | 0 (0–4) | 0 (0–2) | 1 (0–5) | 0 (0–4) | 0 (0–3) | 0 (0–4) |
| Other | |||||||||
| Participants who received this type of therapy, n/N (%) | 12/51 (24) | 27/70 (39) | 21/36 (58) | 1/7 (14) | 2/6 (33) | 5/6 (83) | 4/13 (31) | 4/20 (20) | 1/5 (20) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–0) | 0 (0–2) | 0 (0–6) | 0 (0–0) | 0 (0–5) | 6 (1–10) | 0 (0–3) | 0 (0–0) | 0 (0–1) |
| Type of therapy | Baseline ARAT score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–7 (n = 157) | 8–19 (n = 19) | 20–39 (n = 38) | |||||||
| < 3 months (N = 51) | 3–12 months (N = 70) | > 12 months (N = 36) | < 3 months (N = 7) | 3–12 months (N = 6) | > 12 months (N = 6) | < 3 months (N = 13) | 3–12 months (N = 20) | > 12 months (N = 5) | |
| Passive stretching | |||||||||
| Participants who received this type of therapy, n/N (%) | 28/31 (90) | 36/43 (84) | 29/33 (89) | 3/3 (100) | 5/6 (83) | 3/4 (75) | 8/9 (89) | 12/14 (86) | 3/4 (75) |
| Weeks this type of therapy was undertaken, median (IQR) | 3 (2–7) | 7 (2–12) | 6 (1–12) | 4 | 2 (1–6) | 9 (2–11) | 4 (1–9) | 2 (2–5) | 3 (1–7) |
| Improving range of motion | |||||||||
| Participants who received this type of therapy, n/N (%) | 28/31 (90) | 36/43 (84) | 22/33 (67) | 2/3 (67) | 4/6 (67) | 3/4 (75) | 7/9 (78) | 13/14 (93) | 3/4 (75) |
| Weeks this type of therapy was undertaken, median (IQR) | 4 (2–8) | 5 (2–11) | 3 (0–10) | 2 | 3 (0–6) | 5 (1–9) | 4 (1–9) | 3 (2–10) | 5 (1–7) |
| Functional strengthening | |||||||||
| Participants who received this type of therapy, n/N (%) | 23/31 (74) | 25/43 (58) | 19/33 (58) | 2/3 (67) | 3/6 (50) | 4/4 (100) | 6/9 (67) | 9/14 (64) | 3/4 (75) |
| Weeks this type of therapy was undertaken, median (IQR) | 3 (0–6) | 2 (0–8) | 1 (0–4) | 2 | 1 (0–3) | 6 (3–9) | 3 (0–8) | 2 (0–5) | 6 (1–10) |
| Weight-bearing through hand | |||||||||
| Participants who received this type of therapy, n/N (%) | 14/31 (45) | 21/43 (43) | 15/33 (45) | 1/3 (33) | 3/6 (50) | 3/4 (75) | 5/9 (56) | 6/14 (43) | 3/4 (75) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–3) | 0 (0–4) | 0 (0–4) | 0 | 1 (0–2) | 9 (5–11) | 3 (0–7) | 3 (0–3) | 3 (1–9) |
| Trunk control | |||||||||
| Participants who received this type of therapy, n/N (%) | 14/31 (45) | 14/43 (33) | 17/33 (52) | 2/3 (67) | 2/6 (33) | 4/4 (100) | 4/9 (44) | 5/14 (36) | 3/4 (75) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–2) | 0 (0–3) | 1 (0–7) | 0 | 0 (0–1) | 9 (5–11) | 0 (0–4) | 0 (0–1) | 2 (0–3) |
| Repetitive task-specific practice | |||||||||
| Participants who received this type of therapy, n/N (%) | 18/31 (58) | 23/43 (53) | 14/33 (42) | 2/3 (67) | 3/6 (50) | 4/4 (100) | 4/9 (44) | 6/14 (43) | 2/4 (50) |
| Weeks this type of therapy was undertaken, median (IQR) | 2 (0–6) | 1 (0–5) | 0 (0–3) | 1 | 0 (0–5) | 6 (3–8) | 0 (0–5) | 0 (0–6) | 3 (0–9) |
| Personal ADL | |||||||||
| Participants who received this type of therapy, n/N (%) | 15/31 (48) | 15/43 (35) | 10/33 (30) | 1/3 (33) | 3/6 (50) | 2/4 (50) | 4/9 (44) | 6/14 (43) | 2/4 (50) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–4) | 0 (0–3) | 0 (0–1) | 0 | 1 (0–2) | 3 (0–8) | 0 (0–7) | 0 (0–4) | 0 (0–6) |
| Extended ADL | |||||||||
| Participants who received this type of therapy, n/N (%) | 10/31 (32) | 10/43 (23) | 7/33 (21) | 1/3 (33) | 1/6 (17) | 1/4 (25) | 2/9 (22) | 4/14 (29) | 2/4 (50) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–2) | 0 (0–0) | 0 (0–0) | 0 | 0 (0–1) | 0 (0–6) | 0 (0–3) | 0 (0–1) | 3 (0–5) |
| Other | |||||||||
| Participants who received this type of therapy, n/N (%) | 7/31 (23) | 19/43 (44) | 18/33 (55) | 1/3 (33) | 2/6 (33) | 3/4 (75) | 1/9 (11) | 4/14 (29) | 2/4 (50) |
| Weeks this type of therapy was undertaken, median (IQR) | 0 (0–0) | 0 (0–3) | 1 (0–9) | 0 | 0 (0–2) | 4 (0–6) | 0 (0–0) | 0 (0–1) | 1 (0–9) |
Appendix 7 Qualitative parallel process evaluation supplementary data
| Start of therapy (baseline) | End of therapy (3 months post randomisation) | Three months post therapy (6 months post randomisation) | |
|---|---|---|---|
| Robot-assisted training and EULT | Phase 1 only:
|
Phase 1 interviews:
|
Phase 2 only: recap since first interview, including –
|
Phase 2 interviews: as for phase 1, first interview with additional questioning on –
|
|||
| Usual care topic guides | Phase 1 interviews:
|
||
Phase 2 interviews: as above, with additional probing about study processes –
|
|||
| Professionals – RATULS interventions | Phase 1 interviews:
|
||
Phase 2 interviews: as for phase 1, with more detailed probing on the conduct and delivery of the trial –
|
|||
| Repeated interviews: followed similar topic list, focusing on changes since previous interview and omitting questions that are not required again | |||
| Professionals – usual care | Both phase 1 and phase 2:
|
||
Appendix 8 Health economic evaluation supplementary data
Unit costs
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| Therapy assistant – band 3 mid-point | 1 hour | 25.70 | aCurtis and Burns 201782 | Page 203 |
|
| Senior therapist – band 6 mid-point | 15 minutes | 11.25 | aCurtis and Burns 201782 | Page 203 |
|
| Administrator – band 3 mid-point | 5 minutes | 0.79 | NHS Employers’ Agenda for Change payscales 2018163 |
|
| Item | Unit | Cost (£) | Source and comments | Page |
|---|---|---|---|---|
| GP consultations | ||||
| GP visit at surgery | 9.22 minutes per appointment | 38.00 | 162 | |
| GP home visit | 23.4 minutes per appointment | 96.44 |
|
|
| GP home visit travel time | 6 miles per visit | 3.36 | 176 | |
| GP telephone consultation | 4 minutes | 14.80 | 164 | |
| Nurse consultations | ||||
| Nurse visit at surgery | 15.5 minutes | 10.85 | ||
| Nurse home visit | 25 minutes | 17.50 |
|
|
| Nurse home visit travel time | 6 miles | 3.36 | 176 | |
| Nurse telephone consultation | 6.56 minutes | 7.90 | 164 | |
| Other | ||||
| NHS Direct/NHS 111 | Per call | 12.26 | aUniversity of Sheffield. 2012167 | 174 |
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| Physiotherapy | |||||
| Hospital appointment | Per appointment | 55 | National schedule of reference costs – year 2017–1883 | Code 650 | Total outpatient attendances (physiotherapy). Total cost column |
| Home appointment | Per 1-hour appointment | 36 | aCurtis and Burns 2014168 | Page 179 | No information for home visits. Hourly cost (including training) for community physiotherapist |
| Appointment at general practice surgery | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 health-care professional salary cost per working hour |
| Appointment elsewhere | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 scientific and professional salary cost per working hour |
| Occupational therapy | |||||
| Hospital appointment | Per appointment | 73 | National schedule of reference costs – year 2017–1883 | Code 651 | Total outpatient attendances (physiotherapy). Total cost column |
| Home appointment | Per 1-hour appointment | 45 | aCurtis and Burns 201782 | Page 177 | Based on the hourly cost (including training) of community occupational therapist |
| Appointment at general practice surgery | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 scientific and professional salary cost per working hour |
| Appointment elsewhere | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 scientific and professional salary cost per working hour |
| Speech and language therapy | |||||
| Hospital appointment | Per appointment | 104 | National schedule of reference costs – year 2017–1883 | Code 652 | Total outpatient attendances (speech and language therapy). Total cost column |
| Home appointment | Per 1-hour appointment | 36 | aCurtis and Burns 2014168 | Page 181 | No information for home visits. Hourly cost (including training) for community speech and language therapist |
| Appointment at general practice surgery | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 scientific and professional salary cost per working hour |
| Appointment elsewhere | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 155 | Based on a band 5 scientific and professional salary cost per working hour |
| Community-based health-care professionals | |||||
| Health visitor | Per 1-hour appointment | 76 | aCurtis and Burns 2015165 | Page 171 | Hourly cost (including qualification) per hour of patient-related work. It includes travel cost/transport costs, training course conferences, etc. |
| Geriatrician | Per 1-hour appointment | 87 | aCurtis and Burns 201782 | Pages 154 and 155 | Assumed to be consultant level at band 8c – hourly rate |
| Psychiatrist | Per consultation | 157 | National schedule of reference costs – year 2017–1883 | Code 722 | Assumed to be non-admitted face-to-face attendance to liaison psychiatry consultation |
| Psychologist | Per 1-hour appointment | 138 | aCurtis and Burns 2014168 | Page 183 | Hourly rate per hour of client contact of a clinical psychologist in the community |
| Chiropodist | Per 1-hour appointment | 32 | aCurtis and Burns 2014168 | Page 182 | Hourly rate for a community chiropodist/podiatrist |
| Optician | Per 1-hour appointment | 33 | aCurtis and Burns 201782 | Page 154 and 155, and www.nhsemployers.org/your-workforce/pay-and-reward/job-evaluation/national-job-profiles/allied-health-professionals (accessed 5 December 2018) | Assumed band 5 scientific and professional (optometrist) – hourly rate |
| Pharmacist | Per 1-hour appointment | 71 | aCurtis and Burns 2014168 | Page 184 | Hourly rate for direct patient contact for a community pharmacist |
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| A&E visit | Per visit | 138 | National schedule of reference costs – year 2017–1883 | Code T01NA – T04NA | Weighted average of all A&E visits (non-stay) |
| Outpatient appointment | Per visit | 137 | aCurtis and Burns 201782 | Page 110 | Weighted average of all hospital outpatients attendances |
| Hospital overnight stay (A&E admission) | Per night | 450 | National schedule of reference costs – year 2017–1883 | Code AA35A – AA35F | Average cost for all non-elective stroke-related hospital stay (up to 21 days) |
| Hospital overnight stay (A&E admission) | Per night | 317 | National schedule of reference costs – year 2017–1883 | Code AA35A – AA35F (non-elective: excess bed-days) | Average cost for all non-elective stroke-related excess bed-days (> 21 days) |
| Hospital overnight stay (Non-A&E admission) | Per night | 437 | National schedule of reference costs – year 2017–1883 | Code AA35A – AA35F (elective inpatient) | Average cost for all elective stroke-related hospital stay (up to 21 days) |
| Hospital overnight stay (Non-A&E admission) | Per night | 337 | National schedule of reference costs – year 2017–1883 | Code AA35A – AA35F (elective: excess bed-days) | Elective: excess bed-days (EL_XS) |
| Day patient treatment | Per half day | 140 | National schedule of reference costs – year 2017–1883 | Code OPROC | Weighted average of all outpatient procedures |
| Day patient treatment | Per full day | 742 | National schedule of reference costs – year 2017–1883 | Code AA22C – YR67B | Weighted average of all day cases |
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| Residential care | Per week | 813 | aCurtis and Burns 201782 | Page 35 | Mean cost per week of local authority own-provision residential care for older people |
| Nursing home | Per week | 762 | aCurtis and Burns 201782 | Page 33 | Mean cost per week made out of standard NHS nursing care contribution to PSS expenditure |
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| Meals on wheels | Per meal | 6.60 | aCurtis and Burns 2013166 | Page 127 | Information available only on the PSSRU 2012/13 average cost of £6.60 for the local authority and £5.00 for the independent sector |
| Home help: personal care | Per hour | 26 | aCurtis and Burns 201782 | Page 37 | Average standard hourly rate for services provided in-house. Duration of home visit assumed to be 30 minutes |
| Home help: shopping | Per hour | 26 | aCurtis and Burns 201782 | Page 37 | Average standard hourly rate for services provided in-house. Duration of average visit assumed to be 30 minutes |
| Home help: household tasks | Per hour | 26 | aCurtis and Burns 201782 | Page 37 | Average standard hourly rate for services provided in-house. Duration of home visit assumed to be 30 minutes. |
Response rates and health-care resource use
| Data response rates | Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total participants (N = 770) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Health service utilisation (fully or partially completed) | ||||||||
| Baseline | 248 | 96 | 254 | 98 | 240 | 94 | 742 | 96 |
| 6 months | 214 | 83 | 219 | 85 | 178 | 70 | 611 | 79 |
| EQ-5D-5L (fully completed) | ||||||||
| Baseline | 254 | 99 | 259 | 100 | 254 | 100 | 767 | 99 |
| 3 months | 232 | 90 | 236 | 91 | 207 | 81 | 675 | 88 |
| 6 months | 223 | 87 | 222 | 86 | 190 | 75 | 635 | 82 |
| Area of resource utilisation | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | |
| GP surgery | 104 | 2.34 (2.52) | 2.00 (1.00–3.00) | 129 | 1.51 (0.88) | 1.00 (1.00–2.00) | 107 | 1.79 (1.04) | 2.00 (1.00–2.00) |
| GP home | 35 | 1.31 (0.90) | 1.00 (1.00–1.00) | 27 | 1.78 (2.45) | 1.00 (1.00–1.00) | 34 | 1.44 (0.82) | 1.00 (1.00–2.00) |
| GP telephone | 38 | 1.74 (1.13) | 1.00 (1.00–2.00) | 41 | 1.46 (0.81) | 1.00 (1.00–2.00) | 35 | 1.34 (0.64) | 1.00 (1.00–2.00) |
| Nurse surgery | 42 | 1.36 (1.01) | 1.00 (1.00–1.00) | 49 | 1.45 (1.31) | 1.00 (1.00–1.00) | 48 | 1.46 (1.01) | 1.00 (1.00–1.50) |
| Nurse home | 34 | 2.56 (3.12) | 1.00 (1.00–2.00) | 32 | 3.00 (6.68) | 1.00 (1.00–2.50) | 37 | 11.03 (24.70) | 2.00 (1.00–4.00) |
| Nurse telephone | 11 | 1.91 (1.44) | 1.00 (1.00–3.00) | 4 | 1.75 (1.50) | 1.00 (1.00–2.50) | 6 | 1.33 (0.81) | 1.00 (1.00–1.00) |
| NHS Direct | 19 | 1.05 (0.23) | 1.00 (1.00–1.00) | 14 | 1.07 (0.27) | 1.00 (1.00–1.00) | 10 | 1.40 (0.97) | 1.00 (1.00–1.00) |
| A&E visits | 69 | 1.24 (0.82) | 1.00 (1.00–1.00) | 60 | 1.28 (1.21) | 1.00 (1.00–1.00) | 53 | 1.23 (0.54) | 1.00 (1.00–1.00) |
| Outpatient appointments | 113 | 2.26 (2.40) | 2.00 (1.00–3.00) | 109 | 1.98 (1.75) | 1.00 (1.00–2.00) | 98 | 2.31 (2.43) | 1.00 (1.00–2.00) |
| Hospital nights after being admitted via A&E | 66 | 26.5 (29.27) | 15.5 (3.00–37.00) | 62 | 23.87 (21.68) | 20.5 (7.00–36.00) | 55 | 39.36 (27.58) | 41.00 (14.00–60.00) |
| Hospital nights, not admitted via A&E | 3 | 74.00 (16.37) | 70.00 (60.00–92.00) | 9 | 27.89 (37.22) | 5.00 (2.00–61.00) | 10 | 30.30 (30.90) | 26.00 (3.00–45.00) |
| Day patient treatment (half day) | 4 | 1.25 (0.50) | 1.00 (1.00–1.50) | 6 | 1.50 (0.55) | 1.50 (1.00–2.00) | 8 | 1.75 (1.16) | 2.50 (1.00–2.50) |
| Day patient treatment (full day) | 10 | 4.10 (9.80) | 1.00 (1.00–1.00) | 6 | 8.5 (11.66) | 1.00 (1.00–22.00) | 4 | 1.00 (0.00) | 1.00 (1.00–1.00) |
| Residential care | 2 | 36.00 (43.84) | 36.00 (5.00–67.00) | 2 | 90.5 (0.70) | 90.5 (90.00–91.00) | 3 | 71.66 (31.75) | 90.00 (35.00–90.00) |
| Nursing home | 3 | 60.33 (51.33) | 90.00 (1.00–90.00) | 4 | 26.75 (42.58) | 8.00 (1.50–52.00) | 4 | 45.50 (30.34) | 90.50 (60.00–91.00) |
| Area of resource utilisation | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | Users (n)a | Mean (SD) | Median (IQR) | |
| GP surgery | 244 | 0.99 (2.01) | 0.00 (0.00–1.00) | 251 | 0.78 (0.99) | 1.00 (0.00–1.00) | 238 | 0.81 (1.13) | 0.00 (0.00–1.00) |
| GP home | 247 | 0.19 (0.57) | 0.00 (0.00–0.00) | 250 | 0.19 (0.97) | 0.00 (0.00–0.00) | 236 | 0.21 (0.59) | 0.00 (0.00–0.00) |
| GP telephone | 244 | 0.27 (0.77) | 0.00 (0.00–0.00) | 251 | 0.24 (0.63) | 0.00 (0.00–0.00) | 239 | 0.20 (0.53) | 0.00 (0.00–0.00) |
| Nurse surgery | 243 | 0.23 (0.66) | 0.00 (0.00–0.00) | 249 | 0.28 (0.81) | 0.00 (0.00–0.00) | 240 | 0.29 (0.74) | 0.00 (0.00–0.00) |
| Nurse home | 246 | 0.35 (1.45) | 0.00 (0.00–0.00) | 249 | 0.38 (2.57) | 0.00 (0.00–0.00) | 237 | 1.72 (10.45) | 0.00 (0.00–0.00) |
| Nurse telephone | 246 | 0.85 (0.49) | 0.00 (0.00–0.00) | 251 | 0.03 (0.27) | 0.00 (0.00–0.00) | 239 | 0.03 (0.24) | 0.00 (0.00–0.00) |
| NHS Direct | 244 | 0.08 (0.29) | 0.00 (0.00–0.00) | 251 | 0.06 (0.25) | 0.00 (0.00–0.00) | 240 | 0.06 (0.34) | 0.00 (0.00–0.00) |
| A&E visits | 246 | 0.35 (0.70) | 0.00 (0.00–1.00) | 253 | 0.30 (0.80) | 0.00 (0.00–0.00) | 238 | 0.27 (0.57) | 0.00 (0.00–0.00) |
| Outpatient appointments | 248 | 1.02 (1.97) | 0.00 (0.00–1.00) | 253 | 0.85 (1.50) | 0.00 (0.00–1.00) | 240 | 0.94 (1.92) | 0.00 (0.00–1.00) |
| Hospital nights after being admitted via A&E | 248 | 7.05 (19.05) | 0.00 (0.00–1.00) | 253 | 5.84 (14.82) | 0.00 (0.00–0.00) | 239 | 3.05 (21.17) | 0.00 (0.00–0.00) |
| Hospital nights, not admitted via A&E | 248 | 0.99 (8.36) | 0.00 (0.00–0.00) | 2.53 | 0.99 (8.41) | 0.00 (0.00–0.00) | 239 | 1.45 (8.78) | 0.00 (0.00–0.00) |
| Day patient treatment (half day) | 238 | 0.02 (0.17) | 0.00 (0.00–0.00) | 247 | 0.04 (0.24) | 0.00 (0.00–0.00) | 236 | 0.06 (0.37) | 0.00 (0.00–0.00) |
| Day patient treatment (full day) | 244 | 0.17 (2.05) | 0.00 (0.00–0.00) | 247 | 0.21 (2.11) | 0.00 (0.00–0.00) | 232 | 0.017 (0.13) | 0.00 (0.00–0.00) |
| Residential care | 248 | 0.29 (4.26) | 0.00 (0.00–0.00) | 254 | 0.71 (8.01) | 0.00 (0.00–0.00) | 240 | 0.90 (8.49) | 0.00 (0.00–0.00) |
| Nursing home | 248 | 0.73 (8.06) | 0.00 (0.00–0.00) | 254 | 0.42 (5.71) | 0.00 (0.00–0.00) | 239 | 1.26 (10.28) | 0.00 (0.00–0.00) |
Allocation of capital costs
The MIT-Manus robotic gym costs included purchased capital investment and maintenance fees. Costs associated with the institution’s estate and facilities were included in the salary costs of the staff delivering the therapy. No additional storage facilities were identified as the robotic gyms were installed in the therapy room. The allocation of these capital costs was conducted following the ‘equivalent annual cost’57 methodology. This method allowed us to convert the initial capital cost into an annual sum that equals the resources invested plus their opportunity cost.
The equivalent annual cost of each robot session was calculated under the following assumptions:
-
Robot usage: 35 average number of sessions per week (seven sessions held on an 8-hour day).
-
Weeks per year that the MIT-Manus robotic gym system is in use: 52 weeks.
-
Useful lifespan of the MIT-Manus robotic gym system is 5 years.
-
Training costs are not included as they are not considered to drive any differences in costs between randomisation groups.
-
The capital cost of the robotic gym was spread over its lifespan (5 years).
-
A discount factor of 3.5% was applied to account for the individual’s time preference for costs to be incurred later rather than sooner. This follows guidance for best practice.
The following table illustrates this method, incorporating the initial purchasing cost of £1,000,000, and £15,000 annual fees.
Equivalent annual cost or equivalent annuity
| Year | Discount factor at 3.5%a | Equivalent annual cost of £263,084b (£) |
|---|---|---|
| 1 | 0.9662 | 272,292 |
| 2 | 0.9335 | 138,487 |
| 3 | 0.9019 | 93,904 |
| 4 | 0.8714 | 71,625 |
| 5 | 0.8420 | 58,268 |
| Cost of robot per session | Cost (£) |
|---|---|
| Capital | |
| Opportunity cost of the capital (£58,268 × 5) | 291,340 |
| Annual cost of robotic gym | 58,268 |
| Annual cost of robotic gym per week (assume 52 weeks) | 1121 |
| Cost of robot per session – assuming an average of seven sessions per day | 32 |
| Maintenance | |
| Annual maintenance costs | 16,234 |
| Maintenance costs per week (52 weeks) | 312 |
| Maintenance costs per session (35 sessions in 1 week) | 8.92 |
| Total robotic gym cost per session (capital + maintenance) | 41.00 |
Time and travel analysis
Methods
Participants were asked to complete a time and travel questionnaire relating to their most recent health-care appointments over the previous 6 months. The questionnaire was divided into four distinct sections covering hospital admissions, outpatient appointments, GP visits and personal help or care. Information on work status and income was also collected to quantify the monetary cost of time spent at health-care appointments.
The questionnaire captured data on the type of health-care appointment, mode and cost of transport, mileage and length of journey. In addition, participants reported whether or not they had been accompanied by a relative or carer, the time they had spent at the appointment and the type of activity themselves or their relative/carer would have been doing instead.
Response rates
Table 66 summarises the responses rates for the different sections of the time and travel questionnaire by randomised group.
| Area of resource utilisation | Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total (N = 770) | ||||
|---|---|---|---|---|---|---|---|---|
| Answered (n) | % of total n | Answered (n) | % of total n | Answered (n) | % of total n | Answered (n) | % of total n | |
| Hospital admission | 35 | 14 | 37 | 14 | 33 | 13 | 105 | 14 |
| Outpatient appointments | 145 | 57 | 134 | 52 | 114 | 45 | 393 | 51 |
| GP visits | 146 | 57 | 150 | 58 | 122 | 48 | 418 | 54 |
| Personal help | 120 | 47 | 134 | 52 | 108 | 42 | 362 | 47 |
Responses to each of the sections were low across all randomisation groups. Information relating to hospital admission was poorly completed, with only 14% of participants, overall, providing any information. This percentage was higher when participants were asked about their outpatient appointments, GP visits and the personal help received, with responses rates increasing to 51%, 54% and 47%, respectively.
The number of participants completing all sections of the questionnaire was much lower (Table 67). Only 6% of all participants taking part in the study completed all sections of the questionnaire, with those allocated to robot-assisted training having the lowest response rate (5%). However, not all sections were required to be completed by participants.
| Robot-assisted training (N = 257) | EULT (N = 259) | Usual care (N = 254) | Total (N = 770) | ||||
|---|---|---|---|---|---|---|---|
| Answered (n) | % of total n | Answered (n) | % of total n | Answered (n) | % of total n | Answered (n) | % of total n |
| 13 | 5 | 18 | 7 | 16 | 6 | 47 | 6 |
Owing to these low responses, a full economic analysis of the costs incurred by participants and carers was deemed to be inappropriate. However, a further descriptive analysis to identify any potential variation in health-care visits that might influence differences in time and travel costs between groups was conducted, and is summarised in Tables 68 and 69.
Results
To ascertain whether time and travel costs may have been incurred by participants, a descriptive analysis of health-care visits as reported in the health service utilisation questionnaire was conducted. The focus was on those health-care visits that required participants to travel to hospital or the general practice surgery, as detailed in Table 68.
| Number of visits/appointments | Robot-assisted training | EULT | Usual care | |||
|---|---|---|---|---|---|---|
| Total (n) | Mean (SD) | Total (n) | Mean (SD) | Total (n) | Mean (SD) | |
| A&E visits | 71 | 0.33 (0.76) | 80 | 0.37 (0.98) | 42 | 0.24 (0.71) |
| Outpatient appointments | 348 | 1.36 (2.93) | 306 | 1.19 (2.69) | 261 | 1.05 (1.05) |
| Hospital admission via A&E | 24 | 1.26 (0.56) | 29 | 1.07 (0.27) | 18 | 1.20 (0.56) |
| Hospital admission not via A&E | 8 | 1.00 (0.00) | 5 | 1.00 (0.00) | 10 | 1.66 (1.21) |
| Day patient treatment (half day) | 17 | 0.08 (0.35) | 20 | 0.09 (0.09) | 10 | 0.06 (0.32) |
| Day patient treatment (full day) | 6 | 0.03 (0.17) | 5 | 0.02 (0.18) | 1 | 0.01 (0.08) |
| GP at surgery | 370 | 1.80 (3.87) | 311 | 1.50 (2.08) | 308 | 1.83 (2.17) |
| Nurse at general practice surgery | 123 | 0.61 (1.39) | 104 | 0.49 (1.19) | 152 | 0.89 (5.58) |
| Therapy visits at general practice surgery | 29 | 0.13 (1.16) | 93 | 0.43 (2.40) | 94 | 0.53 (3.86) |
Robot-assisted training participants reported the highest number of outpatient appointments and GP visits. Participants in the EULT group reported the highest number of full-day patient visits and A&E visits/admissions. Responses from the usual care group indicated that they were the heaviest users of therapy services and nurse consultations at the general practice services.
The information outlined in Table 68 was further aggregated into secondary care (excluding A&E), secondary care (including A&E) and primary care appointments. It was assumed that all secondary care admissions (excluding A&E) incurred similar costs to participants and carers, as would all health-care visits taking place at the general practice services. A&E visits and admissions may be less reliant on public transport and involve higher use of ambulance, private vehicles or taxi travel.
A one-way analysis-of-variance test was conducted to determine whether or not differences in the numbers of visits/appointments between randomisation groups were statistically significant. The p-values reported in Table 69 show that no statistically significant differences between randomisation groups were detected for any of the analysed categories (secondary care excluding A&E, A&E visits and admissions, and primary care appointments at general practice surgery). It is expected that a complete time and travel analysis would not change the cost-effectiveness results, with usual care still expected to yield the lowest travel cost due to the low number of secondary care visits and A&E admissions.
| Number of visits/appointments | Robot-assisted training | EULT | Usual care | One-way ANOVA analysis (p-value) | |||
|---|---|---|---|---|---|---|---|
| Total | Mean (SD) | Total | Mean (SD) | Total | Mean (SD) | ||
| Secondary care (excluding A&E visits and admissions)a | 379 | 1.48 (3.00) | 336 | 1.31 (2.81) | 282 | 1.14 (3.68) | 0.478 |
| Primary care appointments at general practice surgeryb | 522 | 2.45 (4.60) | 508 | 2.36 (3.91) | 554 | 3.15 (7.30) | 0.296 |
| Hospital visits and admission via A&E | 95 | 0.44 (1.02) | 109 | 0.50 (1.14) | 60 | 0.33 (0.94) | 0.279 |
Other NHS and social services reported
| Item | Users (n) | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|---|
| Robot-assisted training (n = 13) | ||||||
| Brain operation: surgery to correct malformation | 1 | Per hospital stay | 576 | National schedule of reference costs – year 2017–1883 | Codes AA24C to AA24H | Weighted average of all brain tumours or cerebral cysts (short stay) |
| Dietitian | 1 | Per visit | 86 | National schedule of reference costs – year 2017–1883 | Code A03 | Community Health Services |
| Ear, nose and throat | 1 | Per visit | 105 | National schedule of reference costs – year 2017–1883 | Code 120 | Outpatient attendance – consultant-led unit cost |
| Early Supported Discharge Team | 1 | Per visit | 91 | National schedule of reference costs – year 2017–1883 | Code SCRT | Unit price for Stroke Community Rehabilitation Teams |
| Emergency outpatient dental treatment | 1 | Per visit | 97 | National schedule of reference costs – year 2017–1883 | Code M01C | Emergency dental service, attendance |
| Social worker | 2 | Per hour | 82 | aCurtis and Burns 201782* | Page 174 | Hourly cost relating to patient-related work of a social worker |
| Spasticity clinic | 1 | Per visit | 91 | National schedule of reference costs – year 2017–1883 | Code SCRT | Unit price for Stroke Community Rehabilitation Teams |
| Splint fitted | 1 | Per procedure | 140 | National schedule of reference costs – year 2017–1883 | Code OPROC | Weighted average for outpatients procedures |
| Upper limb rehabilitation | 1 | Per visit | 91 | National schedule of reference costs – year 2017–1883 | Code SCRT | Unit price for Stroke Community Rehabilitation Teams |
| Stroke specialist | 1 | Per visit | 226 | National schedule of reference costs – year 2017–1883 | Code WF01A/328 | Outpatient attendance – consultant-led unit cost |
| STARRS | 1 | Per visit | 91 | National schedule of reference costs – year 2017–1883 | Code SCRT | Unit price for Stroke Community Rehabilitation Teams |
| Item | Users (n) | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|---|
| EULT (n = 11) | ||||||
| Acupuncture | 1 | Per FCE | 246 | National schedule of reference costs – year 2017–1883 | Code AB23Z | Unit price for acupuncture for pain management |
| Adult care service | 1 | Per hour | 82 | aCurtis and Burns 201782 | Page 174 | Hourly cost relating to patient-related work of a social worker |
| Anticoagulant clinic | 2 | Per visit | 49 | National schedule of reference costs – year 2017–1883 | Code WF01A/324 | Average unit cost for anticoagulant service |
| Arthritis special nurse | 1 | Per visit | 28 | National schedule of reference costs – year 2017–1883 | Code N07AF | Unit cost for one-to-one specialist nurse (arthritis) taken from Community Health Services section |
| Care provided morning and night | 1 | Per 30-minute visit | 13 | www.nhsemployers.org/pay-pensions-and-reward/agenda-for-change/pay-scales-1819/hourly-1819 | N/A | Home help personal 2 × 30 minutes. Each visit assumed to last 30 minutes |
| Dentist | 1 | Per hour | 127 | aCurtis and Burns 201782 | Page 165 | Unit price per hour of patient contact (NHS dentist – performer – only) |
| Gynaecologist | 1 | Per visit | 144 | National schedule of reference costs – year 2017–1883 | Code 502 | Outpatient attendance – consultant-led unit cost |
| Keyworker from CPN | 1 | Per hour | 44 | aCurtis and Burns 201782 | Page 185 | Community psychiatric nurse. Unit price for NHS Community Mental Health Teams |
| Orthotics | 2 | Per visit | 86 | National schedule of reference costs – year 2017–1883 | Code 658 | Outpatient attendance – consultant-led unit cost |
| Podiatrist | 1 | Per visit | 62 | National schedule of reference costs – year 2017–1883 | Code 653 | Outpatient attendance – consultant-led unit cost |
| Sleep therapy | 1 | Per FCE | 625 | National schedule of reference costs – year 2017–1883 | Code AA43A-B/DZ18D-G | Weighted average short-stay sleep therapies |
| Warfarin clinic at Renfrew Health Centre | 1 | Per visit | 91 | National schedule of reference costs – year 2017–1883 | Code SCRT | Unit price for Stroke Community Rehabilitation Teams |
| Wigton Community Hospital – physiotherapy and to help with walking | 1 | Per visit | 55 | National schedule of reference costs – year 2017–1883 | Code 650 | Unit cost physiotherapy – hospital outpatient attendance data |
| Counsellor | 1 | Per hour | 82 | aCurtis and Burns 201782 | Page 174 | Hourly cost relating to patient-related work of a social worker |
| Item | Users (n) | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|---|
| Usual care (n = 11) | ||||||
| Audiology | 1 | Per visit | 94 | National schedule of reference costs – year 2017–1883 | Code 84 | Outpatient attendance – consultant-led unit cost |
| Dentist | 1 | Per hour | 127 | aCurtis and Burns 201782 | Page 165 | Unit price per hour of patient contact (NHS dentist – performer – only) |
| Diabetic podiatrist | 1 | Per visit | 62 | National schedule of reference costs – year 2017–1883 | Code 653 | Outpatient attendance – consultant-led unit cost |
| Hearing assessment | 2 | Per FCE | 128 | National schedule of reference costs – year 2017–1883 | Code CA37B | Audiometry or hearing assessment (≥ 19 years) |
| Orthotics | 1 | Per visit | 86 | National schedule of reference costs – year 2017–1883 | Code 658 | Outpatient attendance – consultant-led unit cost |
| Neurologist | 1 | Per visit | 171 | National schedule of reference costs – year 2017–1883 | Code 400 | Outpatient attendance – consultant-led unit cost |
| Talking changes | 1 | Per visit | 44 | aCurtis and Burns 201782 | Page 185 | Unit price for NHS Community Mental Health Teams |
| Social services | 2 | Per hour | 82 | aCurtis and Burns 201782 | Page 174 | Hourly cost relating to patient-related work of a social worker |
| Eye hospital | 1 | Per visit | 125 | National schedule of reference costs – year 2017–1883 | Code 662 | Outpatient attendance – consultant-led unit cost |
Health-care costs per participant at baseline
| Resource use | Robot-assisted training | EULT | Usual care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | |
| Primary care costs | 247 | 74 (10) | 39 (0–103) | 252 | 66 (12) | 39 (0–79) | 240 | 96 (246) | 39 (0–101) |
| Social care | 248 | 117 (1039) | 0 (0–0) | 254 | 133 (1153) | 0 (0–0) | 240 | 249 (1530) | 0 (0–0) |
| Secondary care | 248 | 3369 (7659) | 142 (0–1032) | 254 | 2986 (6537) | 142 (0–1270) | 240 | 4130 (8183) | 142 (0–1610) |
| Total average cost | 248 | 3559 (7667) | 295 (78–1261) | 254 | 3184 (6429) | 239 (50–1524) | 240 | 4475 (8370) | 267 (50–3456) |
Deterministic sensitivity analysis
A deterministic sensitivity analysis was carried out to explore any uncertainty surrounding the level of resource use and its impact on the cost-effectiveness of the interventions. The following areas of uncertainty were explored:
-
missing resource total cost assumed to be zero
-
multiple imputation of total missing costs
-
increased useful life of robot (7 years)
-
exclusion of physiotherapy visits.
A stochastic sensitivity analysis was carried out in each scenario to address potential variance in the outcome measures resulting from the deterministic analysis. Bootstrapping techniques were employed to minimise any uncertainty surrounding the incremental cost-effectiveness ratio.
The results of the stochastic sensitivity analysis are presented through CEACs with various threshold values of the WTP for a QALY. The base-case unadjusted results are presented alongside in each of the costs summary tables for ease of comparison.
Extreme sensitivity analysis
The base-case analysis left all missing total cost information as missing. This section explores the effect on the CEA results of changing this missing information to zero.
Because all instances of missing information occurred in the usual care group, the mean cost for this group decreased (Table 74). As the mean QALYs remain the same, this has affected the resulting ICER between EULT and usual care in the adjusted analysis, increasing it to £172,000 (see Table 75).
| Robot-assisted training (n = 257) | EULT (n = 259) | Usual care (n = 254) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| Total cost per participant (£) at 6 months (missing costs changed to zero) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6032) | 2545 (1596–4442) | 2652 (4868) | 617 (0–2682) |
| Total cost per participant (£) at 6 months (base-case results) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6033) | 2245 (1596–4442) | 3785 (5437) | 1302 (503–4598) |
The mean costs for the robot-assisted training group appear to be higher than those reported in the usual care group, and robot-assisted training remains dominated by EULT.
After bootstrapping the results, usual care had almost a 100% probability of being cost-effective at all WTP values considered (Table 75 and Figure 17).
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 240) | 2653 (1916 to 3389) | – | 0.21 (0.19 to 0.23) | – | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | |
| EULT (n = 254) | 4451 (3548 to 5354) | 1892 (823 to 2961) | 0.23 (0.21 to 0.24) | 0.011 (–0.002 to 0.024) | 172,000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Robot-assisted training (n = 247) | 5387 (4777 to 5996) | – | 0.21 (0.19 to 0.23) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
FIGURE 17.
Cost-effectiveness acceptability curve (extreme sensitivity analysis).
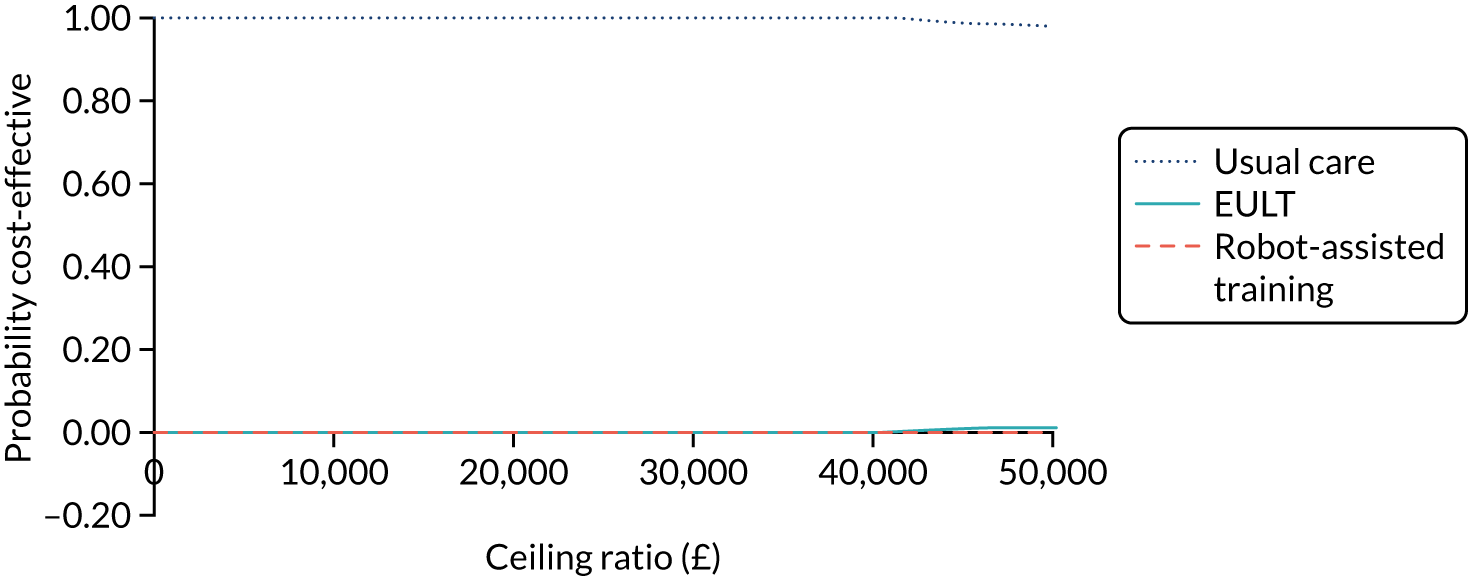
Multiple imputation of missing total costs
Multiple imputation was used to deal with the missing data relating to total costs. Once the missing information analysis on costs established that information was missing at random, truncated normal regression was applied using age, sex and baseline ARAT score as covariates to generate 10 different total mean costs for each participant. After exploring the total cost distribution for each randomised group, the upper limit was set at £25,000. The average value of the 10 iterations was taken and imputed in place of the missing value.
The results in Table 76 show that the mean costs of usual care increased, compared with the base case, although usual care remains the least costly option. This increase in mean costs is to be expected, as all the missing total costs related to participants in the usual care group. Consequently, the resulting ICER from the comparison between EULT and usual care decreased to £50,000 (Table 77). The probability of EULT being cost-effective at £50,000 is 51%.
| Robot-assisted training (n = 257) | EULT (n = 259) | Usual care (n = 254) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| Total cost per participant (£) at 6 months (multiple imputation) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6032) | 2545 (1596–4442) | 4019 (4624) | 2807 (795–2808) |
| Total cost per participant (£) at 6 months (base-case results) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6033) | 2245 (1596–4442) | 3785 (5437) | 1302 (503–4598) |
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 240) | 4019 (3320 to 4718) | – | 0.21 (0.19 to 0.23) | – | 0.87 | 0.81 | 0.73 | 0.63 | 0.49 | |
| EULT (n = 254) | 4451 (3548 to 5354) | 550 (–503 to 1604) | 0.23 (0.21 to 0.24) | 0.011 (–0.002 to 0.024) | 50,000 | 0.13 | 0.19 | 0.27 | 0.37 | 0.51 |
| Robot-assisted training (n = 247) | 5387 (4777 to 5996) | – | 0.21 (0.19 to 0.23) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
Robot-assisted training remains dominated by EULT as it is, on average, more costly and less effective than usual care.
FIGURE 18.
Cost-effectiveness acceptability curve: multiple imputation of missing total costs.

Useful life of robot
The effects on costs, QALYs and cost-effectiveness were explored in the event that the useful life of the robotic gym could be extended from 5 to 7 years. Extending the life of the robotic gym resulted in a reduction of the mean capital costs per patient and, hence, a lower mean total cost for the robot-assisted training group (Table 78) than in the base-case analysis.
| Robot-assisted training (n = 257) | EULT (n = 259) | Usual care (n = 178) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| Total cost per participant (£) at 6 months (extended life of robot) | |||||
| 5085 (4054) | 3476 (2660–5552) | 4451 (6032) | 2245 (1595–4442) | 3785 (5437) | 1302 (503–4598) |
| Total cost per participant (£) at 6 months (base-case results) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6033) | 2245 (1596–4442) | 3785 (5437) | 1302 (503–4598) |
An increase in the useful life of the robotic gym did not move the robot-assisted training away from its dominated status in the cost-effectiveness analysis. There was no evidence of a difference in mean total costs between randomisation groups because all CIs overlap.
As no changes have been made to the mean costs of usual care and EULT, the resulting adjusted ICER is the same as that in the base-case analysis (£74,100). The probability of robot-assisted training being cost-effective (Table 79 and Figure 19) is similar to that of the base-case analysis (see Table 25).
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALY (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 171) | 3785 (2801 to 4770) | – | 0.21 (0.19 to 0.23) | – | 0.90 | 0.85 | 0.80 | 0.73 | 0.62 | |
| EULT (n = 254) | 4451 (3548 to 5354) | 741 (–460 to 1943) | 0.23 (0.21 to 0.24) | 0.010 (–0.005 to 0.025) | 74,100 | 0.10 | 0.15 | 0.19 | 0.26 | 0.37 |
| Robot-assisted training (n = 247) | 5084 (4476 to 5694) | – | 0.21 (0.19 to 0.23) | Dominated by EULT | 0 | 0.00 | 0.01 | 0.01 | 0.01 | |
FIGURE 19.
Cost-effectiveness results: useful life of robot extended to 7 years (n = 672).

Exclusion of physiotherapy sessions
To account for the risk of potential double-counting, the sensitivity analysis explored how excluding the physiotherapy sessions recorded in the health service utilisation questionnaire would affect the results. All physiotherapy visits were excluded for those participants allocated to the robot-assisted training and EULT groups.
The exclusion of physiotherapy sessions resulted in lower mean costs for both the robot-assisted training and EULT groups (Table 80). Despite this reduction, robot-assisted training remains dominated by EULT, with usual care still remaining the least costly option. Nonetheless, there was no evidence of a difference in costs between randomisation groups.
| Robot-assisted training (n = 257) | EULT (n = 259) | Usual care (n = 178) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| Total cost per participant (£) at 6 months – (physiotherapy sessions excluded) | |||||
| 5191 (3971) | 3497 (2943–5537) | 4202 (5942) | 2159 (1521–3972) | 3785.47 (5437) | 1302 (503–4598) |
| Total cost per participant (£) at 6 months (base-case results) | |||||
| 5387 (4054) | 3778 (2962–5854) | 4451 (6033) | 2245 (1596–4442) | 3785 (5437) | 1302 (503–4598) |
The reduction in the mean total costs for the EULT group led to a decrease in the resulting ICER, to £48,600 (Table 81). The results plotted on the CEAC (Figure 20) show that, although usual care remains cost-effective at all WTP levels, EULT has a 51% probability of being cost-effective at the £50,000 WTP value.
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALY (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 171) | 3785 (2801 to 4770) | – | 0.21 (0.19 to 0.23) | – | 0.80 | 0.75 | 0.66 | 0.61 | 0.49 | |
| EULT (n = 254) | 4201 (3312 to 5091) | 486 (–702 to 1674) | 0.23 (0.21 to 0.24) | 0.010 (–0.005 to 0.025) | 48,600 | 0.20 | 0.25 | 0.34 | 0.39 | 0.51 |
| Robot-assisted training (n = 247) | 5191 (4595 to 5788) | – | 0.21 (0.19 to 0.23) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
FIGURE 20.
Cost-effectiveness acceptability curve: physiotherapy sessions excluded.
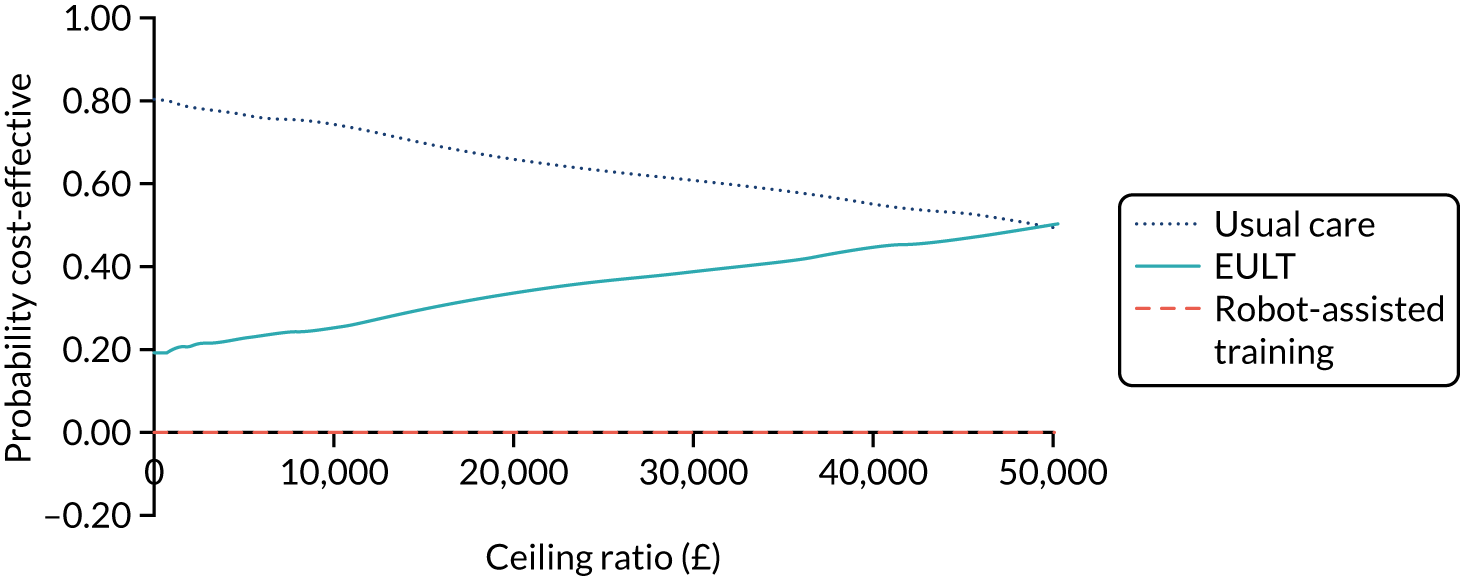
Subgroup analysis
Time since stroke: subgroup analysis
The mean total costs for each group are outlined in Table 82. The participants who were randomised 3–12 months post stroke have higher associated mean costs. Mean QALYs at 6 months (Table 83) are the same across all groups.
| < 3 months (n = 140) | 3–12 months (n = 294) | > 12months (n = 260) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 4382 (4429) | 2873 (1880–5458) | 5301 (6114) | 3151 (1783–6638) | 3995 (4475) | 2887 (1506–4489) |
| < 3 months (n = 161) | 3–12 months (n = 294) | > 12months (n = 260) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.22 (0.11) | 0.23 (0.16–0.30) | 0.22 (0.11) | 0.23 (0.14–0.30) | 0.22 (0.12) | 0.23 (0.15–0.31) |
Time since stroke (< 3 months post stroke)
This subgroup analysis includes all the participants who were randomised to the trial < 3 months post stroke. The mean costs and QALYs were analysed for this subgroup and are summarised in Tables 84 and 85. Total mean costs were highest for participants in the robot-assisted training group, and mean QALYs were highest in the EULT group. Usual care remained the lowest-cost therapy, with the lowest mean QALYs per participant.
| Robot-assisted training (n = 57) | EULT (n = 46) | Usual care (n = 37) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 5485 (4734) | 3865 (2979–5989) | 3863 (3645) | 2604 (1835–4460) | 3328 (4568) | 1159 (552–2873) |
| Robot-assisted training (n = 57) | EULT (n = 46) | Usual care (n = 37) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.22 (0.10) | 0.22 (0.16–0.30) | 0.24 (0.11) | 0.24 (0.19–0.31) | 0.21 (0.11) | 0.23 (0.17–0.27) |
Robot-assisted training remained dominated by EULT. However, the adjusted ICER between usual care and EULT was much lower than the one reported in the base-case analysis (£31,400 vs. £74,100). EULT is increasingly likely to become cost-effective as WTP values increase (at least over the range shown), with EULT having a 50% chance of being cost-effective at the £30,000 threshold value (Table 86 and Figure 21). Robot-assisted training had virtually zero probability of being considered cost-effective over the range of WTP values shown.
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALY (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 35) | 3328 (1443 to 5213) | – | 0.21 (0.17 to 0.25) | – | 0.77 | 0.69 | 0.59 | 0.50 | 0.39 | |
| EULT (n = 45) | 3863 (2527 to 5199) | 628 (–1650 to 2905) | 0.24 (0.20 to 0.28) | 0.020 (–0.01 to 0.06) | 31,400 | 0.22 | 0.31 | 0.41 | 0.50 | 0.61 |
| Robot-assisted training (n = 53) | 5485 (3938 to 7032) | – | 0.22 (0.19 to 0.25) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
FIGURE 21.
Cost-effectiveness results: time since stroke (< 3 months post stroke) (n = 133).
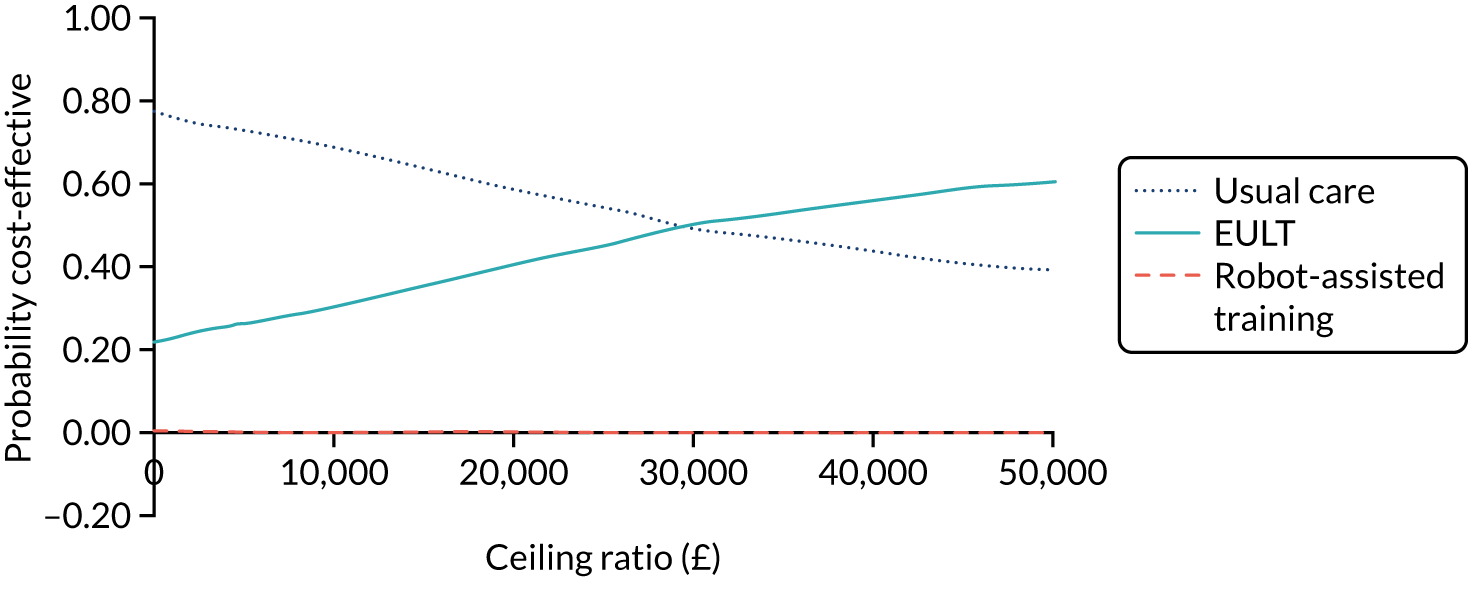
Time since stroke (3–12 months post stroke)
The effect on cost-effectiveness of being 3–12 months post stroke at randomisation was included in this analysis. Overall, mean costs remained highest for the robot-assisted training group (Table 87), whereas the highest mean QALY per participant was associated with EULT (Table 88). The delivery of robot-assisted training sessions was associated with higher costs and lower QALYs; hence, it was dominated by the usual care and EULT groups. The adjusted cost-effectiveness comparison between usual care and EULT yielded an ICER of £79,400, with usual care being cost-effective at all WTP values presented (Table 89 and Figure 22).
| Robot-assisted training (n = 105) | EULT (n = 117) | Usual care (n = 72) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 5790 (4198) | 3975 (2925–7103) | 5084 (7528) | 2463 (1556–4680) | 4943 (5937) | 2692 (833–6635) |
| Robot-assisted training (n = 104) | EULT (n = 117) | Usual care (n = 106) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.20 (0.12) | 0.22 (0.12–0.30) | 0.23 (0.09) | 0.24 (0.16–0.29) | 0.21 (0.11) | 0.23 (0.14–0.30) |
| Randomisation group | Cost (£) (98.33% CI) | Incremental cost (£) (98.33% CI)a | QALYs (98.33% CI) | Incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 69) | 4943 (3228 to 6658) | – | 0.21 (0.19 to 0.24) | – | 0.64 | 0.61 | 0.59 | 0.57 | 0.52 | |
| EULT (n = 115) | 5084 (3393 to 6774) | 397 (–1816 to 2609) | 0.23 (0.20 to 0.25) | 0.005 (–0.01 to 0.03) | 79,400 | 0.33 | 0.36 | 0.37 | 0.39 | 0.43 |
| Robot-assisted training (n = 101) | 5790 (4793 to 6786) | – | 0.20 (0.18 to 0.23) | Dominated by usual care and EULT | 0.03 | 0.03 | 0.04 | 0.04 | 0.05 | |
FIGURE 22.
Cost-effectiveness acceptability curve: time since stroke (3–12 months post stroke).

Time since stroke (> 12 months post stroke)
Participants who were > 12 months post stroke at randomisation were included in this analysis. As in the previous subgroup analyses, the robot-assisted training group had the highest mean costs (Table 90). Mean QALYs were, on average, lowest in participants in the usual care group (Table 91).
| Robot-assisted training (n = 95) | EULT (n = 96) | Usual care (n = 69) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 4882 (3385) | 3676 (3005–5377) | 3961 (4735) | 2136 (1529–3960) | 2823 (5160) | 1007 (231–2579) |
| Robot-assisted training (n = 95) | EULT (n = 96) | Usual care (n = 90) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.22 (0.13) | 0.24 (0.11–0.30) | 0.23 (0.11) | 0.23 (0.15–0.31) | 0.21 (0.12) | 0.21 (0.13–0.31) |
The cost-effectiveness comparison remained between the usual care and EULT groups because robot-assisted training is dominated by EULT. The resulting ICER of £126,143 was higher than ICERs from the previous subgroup and base-case analyses, with usual care being cost-effective at all WTP thresholds (Table 92 and Figure 23).
| Randomisation group | Cost (£) (98.33% CI) | Incremental cost (£) (98.33% CI)b | QALYs (98.33% CI) | Incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 67) | 2823 (1299 to 4348) | – | 0.21 (0.18 to 0.24) | – | 0.89 | 0.87 | 0.84 | 0.80 | 0.72 | |
| EULT (n = 94) | 3961 (2783 to 5138) | 883 (–677 to 2444) | 0.23 (0.20 to 0.25) | 0.007 (–0.01 to 0.03) | 126,143 | 0.11 | 0.12 | 0.15 | 0.19 | 0.24 |
| Robot-assisted training (n = 93) | 4822 (4036 to 5728) | – | 0.22 (0.18 to 0.25) | Dominated by EULT | 0.00 | 0.00 | 0.01 | 0.01 | 0.04 | |
FIGURE 23.
Cost-effectiveness acceptability curve: time since stroke (> 12 months post stroke).
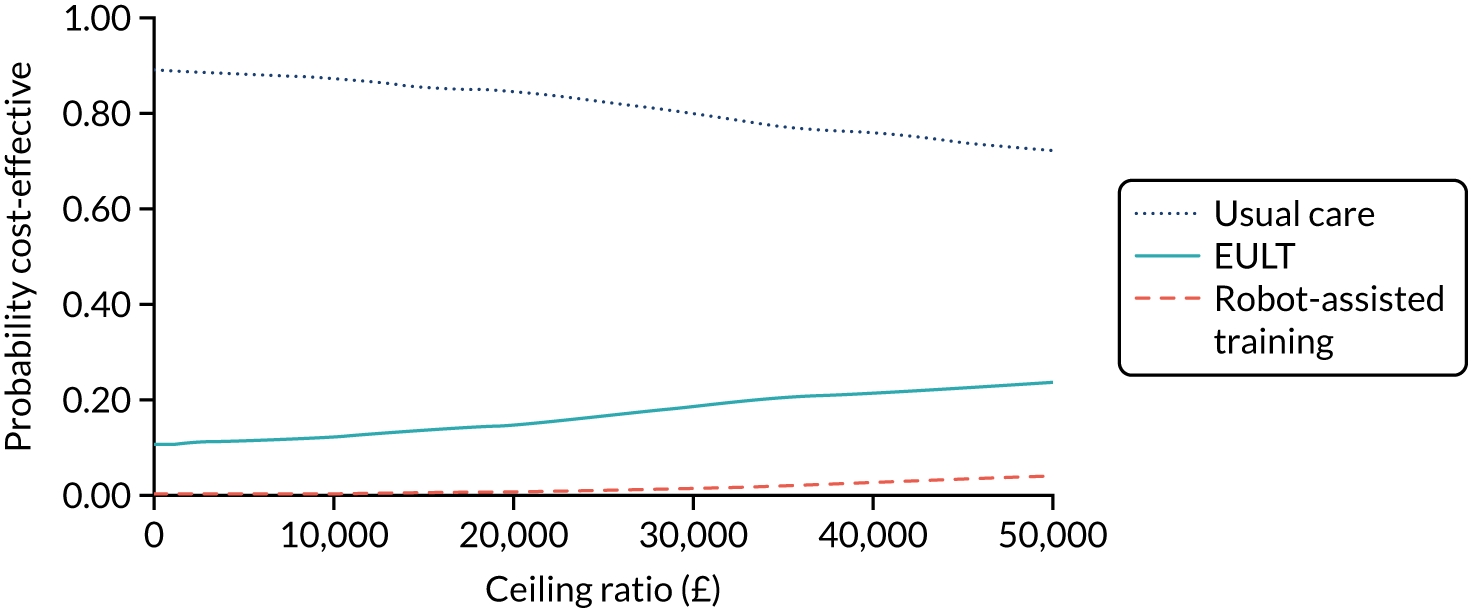
Per-protocol analysis
A total of 58 participants were identified as not having received at least 20 sessions of therapy and were therefore removed from the data set, reducing the sample size to 712. Thirty participants had been allocated to the EULT group and 28 to the robot-assisted training group.
Results from the cost-effectiveness analysis for the per-protocol analysis (Table 93) show how the unadjusted mean costs for both the robot-assisted training and EULT groups increased slightly compared with the base-case analysis (see Table 25). The unadjusted mean QALYs for the robot-assisted training group also increased, and they were higher than those reported for the usual care group (0.22 vs. 0.21). Usual care remains the least costly option, followed by EULT and robot-assisted training. The calculated ICER compares usual care with EULT. The resulting ICER is £68,000, compared with £74,100 ICER in the base-case analysis. The probability of EULT being cost-effective was never > 40% over the range of WTP values presented.
| Randomisation group | Unadjusted analysis, cost (£) (98.33% CI) | Adjusted analysis, incremental cost (£) (98.33% CI)a | Unadjusted analysis, QALYs (98.33% CI) | Adjusted analysis, incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 171) | 3785 (2801 to 4770) | – | 0.21 (0.19 to 0.23) | – | 0.92 | 0.88 | 0.83 | 0.75 | 0.61 | |
| EULT (n = 227) | 4551 (3596 to 5501) | 816 (–421 to 2054) | 0.23 (0.21 to 0.25) | 0.012 (–0.004 to 0.028) | 68,000 | 0.08 | 0.12 | 0.17 | 0.25 | 0.39 |
| Robot-assisted training (n = 221) | 5595 (4929 to 6261) | – | 0.22 (0.20 to 0.24) | Dominated by EULT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
Model results
| Robot-assisted training (n = 257) | EULT (n = 259) | Usual care (n = 178) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 7538 (7901) | 4245 (3048 to 8162) | 6892 (11,644) | 2951 (1738 to 6254) | 6916 (10,568) | 2260 (835 to 7284) |
| Robot-assisted training (n = 254) | EULT (n = 259) | Usual care (n = 254) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.44 (0.25) | 0.47 (0.28–0.63) | 0.48 (0.23) | 0.53 (0.35–0.63) | 0.45 (0.22) | 0.47 (0.31–0.59) |
| Randomisation group | Cost (£) (98.33% CI) | Incremental cost (£) (98.33% CI)a | QALYs (98.33% CI) | Incremental QALYs (98.33% CI)a | ICER (£) | Probability of each therapy being considered cost-effective at different threshold values for society’s WTP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Usual care (n = 67) | 6916 (5003 to 8830) | – | 0.45 (0.41 to 0.48) | – | 0.48 | 0.42 | 0.35 | 0.29 | 0.23 | |
| EULT (n = 94) | 6892 (5149 to 8635) | 128 (–2460 to 2204) | 0.48 (0.44 to 0.51) | 0.021 (–0.06 to 0.20) | 6095 | 0.40 | 0.48 | 0.55 | 0.62 | 0.69 |
| Robot-assisted training (n = 93) | 7538 (6350 to 8725) | – | 0.44 (0.40 to 0.48) | Dominated by EULT and usual care | 0.12 | 0.10 | 0.10 | 0.09 | 0.08 | |
FIGURE 24.
Cost-effectiveness results: model.
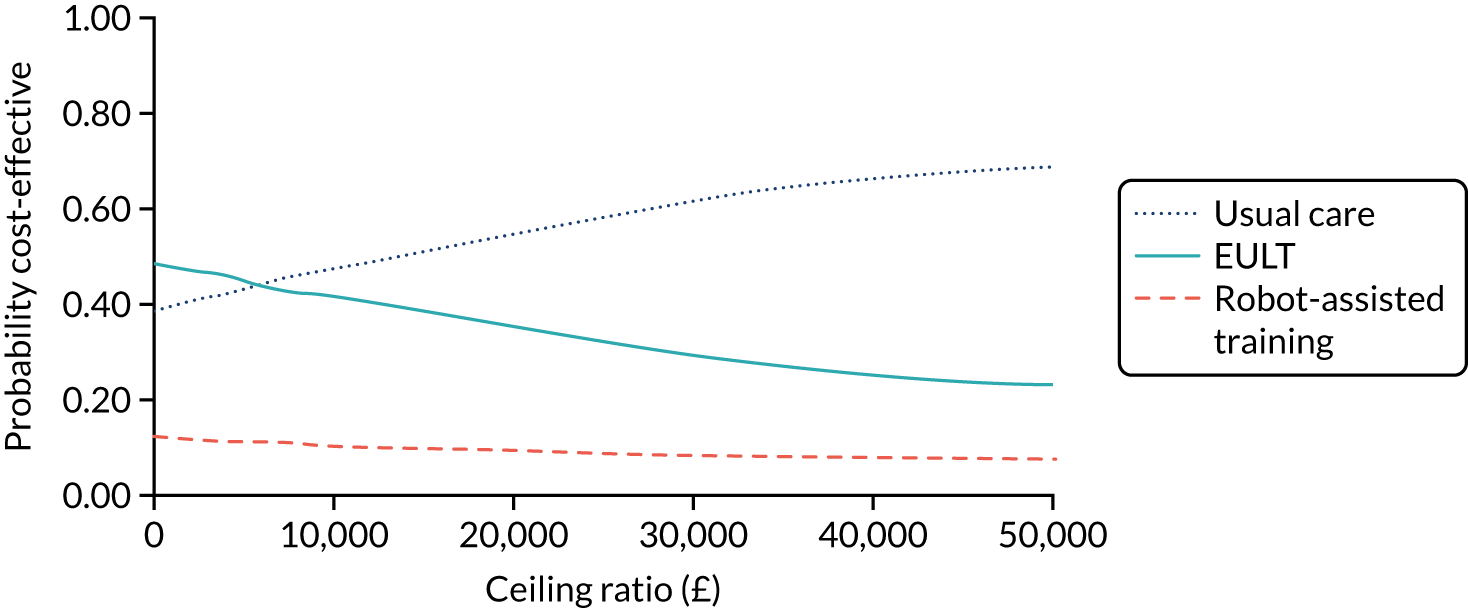
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- aMD
- adjusted mean difference
- aOR
- adjusted odds ratio
- ARAT
- Action Research Arm Test
- BNF
- British National Formulary
- BoTULS
- Botulinum Toxin for the Upper Limb after Stroke
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPM
- Canadian Occupational Performance Measure
- CSRI
- Client Services Receipt Inventory
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EULT
- enhanced upper limb therapy
- FMA
- Fugl-Meyer Assessment
- GP
- general practitioner
- GRASP
- Graded Repetitive Arm Supplementary Program
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- IQR
- interquartile range
- ITT
- intention to treat
- LCRN
- Local Clinical Research Network
- MCID
- minimal clinically important difference
- MIT
- Massachusetts Institute of Technology
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PP
- per protocol
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RATULS
- Robot-Assisted Training for the Upper Limb after Stroke
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SIS
- Stroke Impact Scale
- SMD
- standardised mean difference
- SRN
- Stroke Research Network
- TIDieR
- Template for Intervention Description and Replication
- VA
- Veterans Affairs
- WHO
- World Health Organization
- WTP
- willingness to pay
